Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/109/01. The protocol was agreed in February 2011. The assessment report began editorial review in February 2013 and was accepted for publication in July 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Colquitt et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
This technology assessment has been undertaken on the request of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme to inform the National Institute for Health and Care Excellence (NICE) appraisal Implantable Cardioverter Defibrillators for the Treatment of Arrhythmias and Cardiac Resynchronisation Therapy for the Treatment of Heart Failure (Review of TA95 and TA120). 1
Description of the underlying health problem
This assessment encompasses people at risk of sudden cardiac death (SCD) as a result of ventricular arrhythmias (abnormal heart rhythms) and people with heart failure (HF) as a result of left ventricular systolic dysfunction (LVSD) and cardiac dyssynchrony. For the purposes of this assessment, and in line with the NICE scope,1 three populations are considered:
-
people at increased risk of SCD as a result of ventricular arrhythmias despite receiving optimal pharmacological therapy (OPT)
-
people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT
-
people with both conditions described above.
In practice, however, these are not distinct populations and there is considerable overlap between the groups, such that people with HF from LVSD are at risk of SCD from ventricular arrhythmia.
Sudden cardiac death
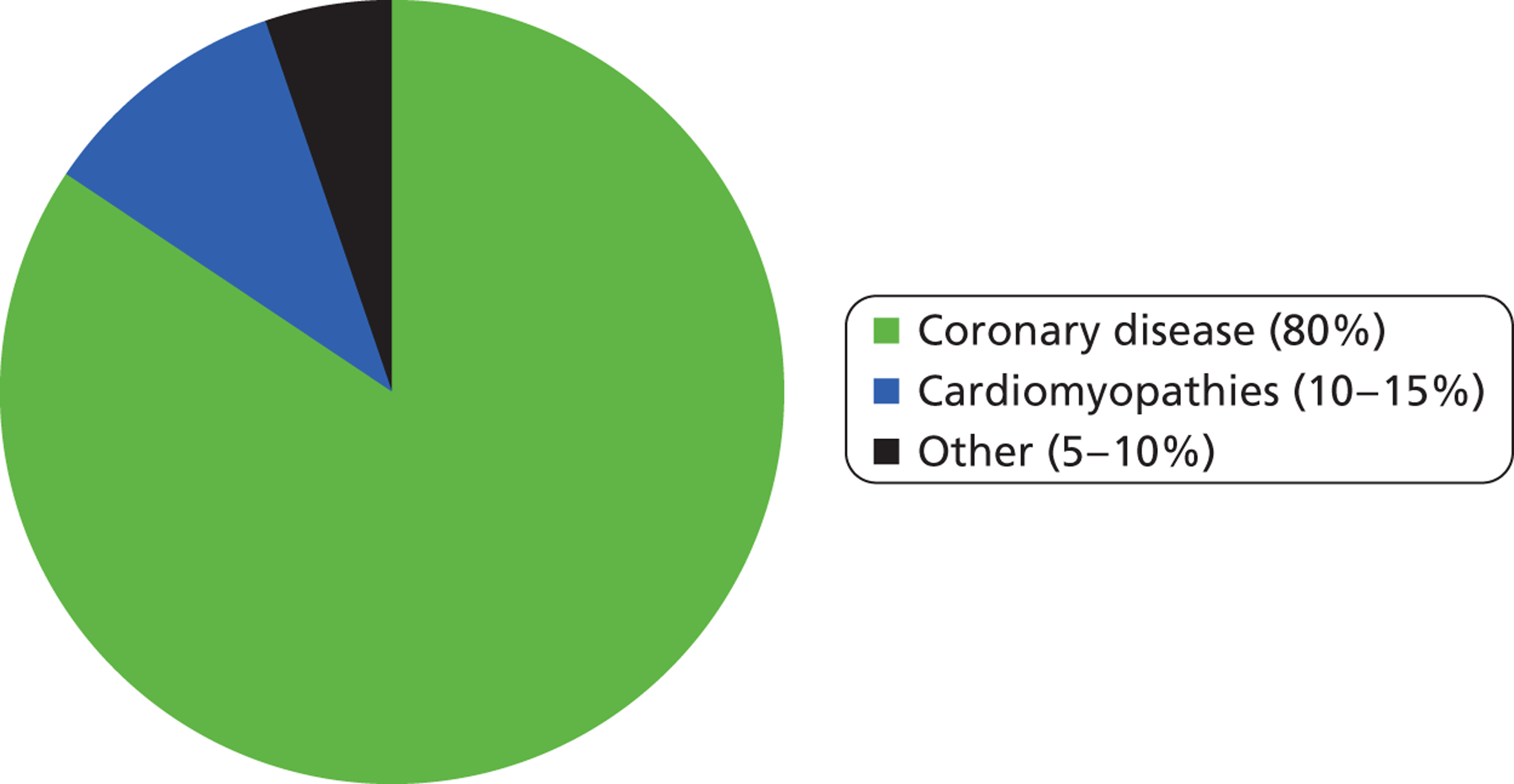
The widely accepted definition of SCD is a sudden and unexpected death from cardiac causes within an hour of the onset of symptoms. 2 Coronary heart disease (CHD) (narrowing or blocking of the coronary arteries) is the most common clinical finding associated with SCD, with about 80% of such deaths linked to this condition (Figure 1). CHD causes SCD mainly because it can lead to ventricular tachycardia (VT), which is an abnormally fast heart rhythm originating in one of the ventricles, and ventricular fibrillation (VF), which is an unco-ordinated and erratic contraction of the heart muscle of the ventricles. Patients with cardiomyopathies (diseases of heart muscle) account for a further 10–15% of cases of SCD and there is likely to be significant overlap between this group and those with CHD (i.e. some patients will have both conditions). The remaining 5–10% of SCD cases are associated with other disorders, either structurally abnormal congenital cardiac conditions or structurally normal but electrically abnormal hearts. 3
Deaths in England and Wales from CHD in 2010 numbered 140,301 (Table 1). It is thought that approximately 50% of all CHD-related deaths are SCDs. 6 The cause of SCD is frequently VT or VF, but may also be due to asystole (cessation of electrical activity in the heart) or causes other than arrhythmias (e.g. ischaemia)8,9 Commonly, VT develops initially followed by degeneration to VF, which then leads to the development of asystole. 10 According to guidelines of the American College of Cardiology, the American Heart Association and the European Society of Cardiology for the management of patients with ventricular arrhythmias and the prevention of SCD,7 VF is the rhythm recorded at the time of sudden cardiac arrest in 75–80% of cases. There is evidence that the incidence of VT/VF events has declined over time, perhaps reflecting an impact of treatment strategies targeted at coronary artery disease. 11–14
| Cause of death | Total | Men | Women |
|---|---|---|---|
| aCHD4 | 140,301 | 81,405 | 58,896 |
| SCDb | 70,151 | 40,703 | 29,448 |
| VFc | 52,613–56,121 | 30,527–32,562 | 22,086–23,558 |
People known to be at risk of SCD include those who have experienced a previous event that they survived, such as life-threatening arrhythmia (accounting for 5–10% of SCDs), haemodynamic abnormalities including HF (7–15% of SCDs) and acute coronary syndromes such as myocardial infarction (MI) and angina pectoris (≤ 20% of SCDs). 6 However, in ≥ 30% of SCDs, CHD had not been previously diagnosed in the patient, and in one-third of SCDs the patients were known to have cardiac disease but were considered to be at low risk for SCD. 6
A recent systematic review of 67 studies worldwide15 estimated that the average survival rate for adults following an out-of-hospital cardiac arrest was 7%. Depending on the clinical scenario, a small proportion of people who do survive a first life-threatening cardiac episode may remain at high risk of further episodes (e.g. if VF is due to left ventricular dysfunction). Secondary prevention (prevention of an additional life-threatening event) may therefore be required. When appropriate treatment and secondary preventative strategies are implemented, recent studies have reported 5-year survival ranging from 69% to 100%,16,17 although these may overestimate survival. It is important to recognise the multiple causes of the electrical process of VF, as not all patients with VF will be amenable to implantable cardiac defibrillator (ICD) therapy. For example, VF or VT occurring as a primary electrical process in Brugada syndrome would be expected to respond well to ICD therapy, whereas VF due to massive heart damage in a major acute MI may not. Deciding on the rational use of ICD therapy can be complex, as the risk of arrhythmic death and therefore the potential benefit from ICD therapy varies between pathologies (e.g. ischaemic heart disease, non-ischaemic cardiomyopathy or electrical disease) and also with the progression of the disease (e.g. the impact of ICD may vary depending on the time after an MI that the therapy is started).
Preventing a first life-threatening event (primary prevention of SCD) is challenging because it requires identifying people with a sufficient level of risk for primary prevention to be appropriate. There are multiple risk factors for SCD, which include increasing age, hereditary factors, being in the top 10% of risk for coronary atherogenesis, the presence of inflammatory markers (e.g. C-reactive protein), hypertension, left ventricular hypertrophy, intraventricular conduction abnormalities [e.g. left bundle branch block (LBBB)], obesity, diabetes and lifestyle factors (e.g. smoking, excessive alcohol consumption, lack of physical activity, social and economic stressors). 7 Currently no optimal strategy for risk stratification exists. 18
Heart failure
Heart failure is a clinical syndrome characterised by symptoms (breathlessness and fatigue) and signs (fluid retention) caused by failure of the heart to pump adequately. It is usually a chronic condition predominantly affecting people aged > 50 years and has a poor prognosis. 19 Coronary artery disease (ischaemic heart disease) has been identified as the most common cause of HF in two UK studies. 20,21 Other causes of HF are LVSD, hypertension, valve disease, atrial fibrillation or flutter, cardiomyopathy (either hypertrophic or restrictive) or cor pulmonale (pulmonary heart disease). The cause of HF was unknown in approximately one-third of cases in the two UK studies. 20,21 The NICE scope for this appraisal1 focuses on HF that is a result of LVSD. LVSD is an impairment in the ability of the left ventricle to pump blood into the circulation during contraction (systole). 19
The prognosis for HF patients is poor, with deterioration in quality of life (QoL) and reduced life expectancy. 19 In addition, HF patients may also be at risk of SCD. Patients with HF and LVSD from the Echocardiographic Heart of England Screening Study (ECHOES) cohort had a 5-year survival rate of 53%,22 and 3.8% of the deaths that occurred among those with HF and LVSD were sudden deaths,22 although SCD may be underestimated in this study. The 10-year survival in this study for those with HF and LVSD was 27.4%. 23 The severity of HF graded according to the New York Heart Association (NYHA) classification system is an indicator of prognosis. 24–27 This system has four classes to which patients can be assigned, with severity increasing with class number from I to IV (Table 2); however, it is worth noting that clinicians may differ in the way that they interpret and assign these classes. 28
| Class | Comfort at rest? | Limitation to physical activity? | Effect of physical activity |
|---|---|---|---|
| I | Yes | None | No undue fatigue, palpitations, dyspnoea or angina pain |
| II | Yes | Slight | Ordinary physical activity can result in fatigue, palpitations, dyspnoea or angina pain |
| III | Yes | Marked | Less than ordinary activity causes fatigue, palpitations, dyspnoea or angina pain |
| IV | May have HF or angina symptoms even at rest | Always | Unable to carry out any physical activity without new or increasing discomfort |
The most recent estimates for the incidence of HF in the UK come from the General Practice Research Database (GPRD). 29 In 2009 these data indicated that the incidence of HF was higher in Wales (men 44.6 and women 24.9 per 100,000 person-years) than in England (men 37.5 and women 23.0 per 100,000 person-years). The incidence of HF increased with age, being highest in those aged > 75 years (e.g. in England, men 326.0 and women 256.2 per 100,000 person-years), and incidence rates are higher in men than in women at all ages. From these data and those for Scotland and Northern Ireland, it has been estimated that there are > 27,000 new cases of HF in the UK each year. 29
The corresponding estimates for the prevalence of HF in the UK derived from the GPRD29 are similar in England and Wales (for all ages in men: 0.9% in England and 1.0% in Wales; for all ages in women: 0.7% in England and Wales). In total, this corresponds to almost 160,000 cases in England and Wales in 2009. Data from the ECHOES cohort have indicated that, of the total number of HF cases identified, approximately 50% have HF with LVSD. 22 Applying this proportion to the prevalence data for England and Wales from the GRPD would suggest that there were approximately 80,000 cases of HF with LVSD in 2009.
Description of the technology under assessment
The current technology assessment concerns specific types of cardiac implantable electronic devices for the prophylaxis and/or treatment of conduction system disease that use one or more of the following approaches to restore normal heart rhythm:
-
‘pacing’ – a series of low-voltage electrical impulses delivered at a fast rate to correct the heart rhythm
-
cardioversion’ – one or more small electric shocks delivered to the heart to restore a normal rhythm
-
‘defibrillation’ – one or more large electric shocks delivered to the heart to restore a normal rhythm.
Cardiac resynchronisation therapy (CRT) devices are a specific type of cardiac pacemaker that have three conducting leads (connected to the right atrium and both ventricles) and are used to correct inconsistency of the heartbeat between the right and left sides of the heart (dyssynchrony), referred to as biventricular pacing. These devices are known as CRT-pacers (CRT-Ps) (or biventricular pacers).
Implantable cardioverter defibrillators are used to provide cardioversion and/or defibrillation shocks to correct more serious dysfunction of the heart rhythm, including VT, VF and asystole, any one of which may be associated with SCD. ‘Single chamber’ ICDs have a single conducting lead connected only to the right ventricle; ‘dual chamber’ ICDs have two leads connected to the right atrium and the right ventricle. In addition to their cardioversion and defibrillation ability, modern ICDs provide the functionality of a standard pacemaker to treat slow heart rhythms (if necessary) by pacing the right-hand chamber(s) of the heart.
Modern types of CRT device may combine the functionality of both a CRT-P and an ICD and these are referred to as CRT-defibrillators (CRT-Ds).
Cardiac resynchronisation therapy is aimed at a specific subset of the HF population with evidence of delayed left ventricular activation (as manifest by prolongation of the QRS complex). Because this population is a priori at risk of arrhythmic death, CRT can be combined with an ICD. ICDs and CRT-D devices are appropriate for patients with a high risk of SCD, whereas CRT-P devices are appropriate in patients with less serious cardiac arrhythmias. However, as noted earlier, heart disease is a complex and progressive condition and patients who are initially implanted with a CRT-P may subsequently develop heart disease and be at risk of SCD, and an upgrade from a CRT-P to a CRT-D or an ICD may be appropriate. 30
Although they may differ in function, CRT and ICD devices are similar in size and structure – about the size of a pocket watch (capacity 30–40 ml, weight around 70 g, thickness approximately 13 mm) – and consist of a battery-powered pulse generator controlled by a microcomputer. They are implanted under the skin, typically just below the collar bone on the left or right side of the chest, and (depending on the device type) have one or more leads (tiny wires) that are routed through veins to the heart’s chambers for sensing electrical activity and for providing the corrective pacing, cardioversion and/or defibrillation impulses. Modern CRT and ICD devices store a record of the heart’s electrical activity and contain a wireless transmitter/receiver to enable the device to be programmed and interrogated from an external computer using wireless telemetry. Readings from a device may be transmitted by telephone, enabling the cardiologist to remotely check the performance of the device while the patient is at home.
Early devices were implanted using the transthoracic method, but current CRT and ICD devices are placed under the skin in the pectoral region with transvenous insertion of the leads into the heart under local anaesthesia, using high-resolution X-ray angiography to guide the placing of the leads. The procedure for primary prevention typically requires a maximum of a 1-night stay in hospital. For secondary prevention the length of stay will depend on any underlying health problems. The longevity of CRT and ICD devices is limited by their battery life, which is in the range of 4–7 years, depending on a number of factors including the pacing mode, pacing percentage and capacitor recharge interval. 31–33 Replacement of batteries alone is not feasible, so when the battery is due for renewal the pulse generator unit has to be replaced, in a minor surgical procedure. When possible the connecting leads are left in situ and only the generator unit itself is replaced, although eventually one or more of the connecting leads may also require replacement.
Modern devices can be specifically programmed to deliver resynchronisation pacing independently to the atria and ventricles of the heart to maximise synchronisation. The devices can also be programmed according to which of the heart’s chambers they monitor (sense) to detect existing electrical activity. The ability of CRT and ICD devices to recognise different types of arrhythmia may enable them to deliver more appropriate therapy, in particular lessening the incidence of inappropriate shocks. Several coding systems (typically comprising three to five letters) have been developed to indicate the programmed pacing/sensing modes. A widely used code developed by the Heart Rhythm Society (HRS) and the British Pacing and Electrophysiology Group (BPEG) consists of three letters to describe the pacing chamber [atrium, A; ventricle, V; or dual (i.e. both), D], three letters to describe the sensed chamber (A, V or D) and a further three letters to describe whether pacing is inhibited (I) or triggered (T) in response to the sensed beat or, if dual pacing and sensing are programmed, whether dual (D) inhibition and triggering (for the different chambers) occurs. As an example, the code ‘VVI’ would indicate ventricular pacing (shocks are delivered to the ventricle), ventricular sensing (electrical activity is monitored in the ventricle) and that pacing is inhibited if an electrical beat is sensed in the ventricle. To illustrate a more complex example, the code ‘DDD’ would indicate a device programmed for dual-chamber pacing and sensing. In this case the atrium would be stimulated if sinus bradycardia is detected. Both atrium and ventricle would be stimulated if bradycardia exists independently in both chambers. If heart block exists with normal sinus function the ventricle would be paced in synchrony with the atrium and, if sinus rhythm exists, pacing would be totally inhibited.
The most recent development in cardiac implantable electronic devices is the subcutaneous ICD (S-ICD), which was approved by the US Food and Drug Administration (FDA) in April 2012. The S-ICD is positioned just under the skin, outside the rib cage, and can be implanted under local anaesthesia. The electronics and batteries of the S-ICD enable it to deliver enough energy to defibrillate the heart without the need for a connecting lead to the heart, which avoids lead-related complications including the risk of dangerous infections (other potential procedural complications are considered below). A disadvantage of the S-ICD, however, is that it cannot provide long-term pacing. A RCT comparing S-ICD with transvenous ICD (ClinicalTrials.gov identifier NCT01296022)34 is currently under way and is due to complete in March 2015 and a registry study of S-ICD (ClinicalTrials.gov identifier NCT01085435)35 is due to complete in December 2016.
Potential procedural complications
The most challenging technical aspect of a CRT device implantation is the optimal placement of the third lead in the coronary sinus vein. The final position of the left ventricular pacing lead depends on the anatomy of the cardiac venous system, as well as the performance and stability of the pacing lead and the need to avoid phrenic nerve stimulation. 36 The left phrenic nerve (which sends signals between the brain and the diaphragm) may be stimulated by the left ventricular pacing lead, causing uncomfortable diaphragmatic twitch, which could prevent optimal left ventricular lead placement and can hinder left ventricular stimulation. Phrenic nerve stimulation occurs in around 20% of patients with bipolar leads. 37 A recent systematic review of implantation-related complications in 11 ICD and seven CRT trials suggests that the most common complications include coronary vein dissection (1.3%) and coronary vein perforation (1.3%), with coronary vein-related complications occurring in only 2.0% of patients. 38 This low rate is attributed to the growing experience of physicians combined with technical progress. The overall incidence of lead dislodgement for non-thoracotomy ICDs was 1.8%, with higher rates of lead dislodgement in the CRT trials, which varied from 2.9% to 10.6%. The reported overall rate of leads dislodged during and after 3095 successful implantations was 5.9%. A recent study in the USA,39 which was based on the National Cardiovascular Data Registry, found that, after adjusting for diagnostic test results and comorbidities, dual-chamber ICDs were associated with a 40% greater odds of procedural complications and a 45% greater odds of mortality than single-chamber ICDs, illustrating a greater risk of procedural complications with the more complex types of ICD device. Another recent study in the USA40 examined 16-year trends from 1993 to 2008 in the incidence of infections related to cardiac implantable electronic devices, based on data from the National Inpatient Sample (NIS). There has been a marked increase in infection incidence, notably since 2004, and this has been associated with an increase in in-hospital mortality and increased treatment costs. The reasons for the increased incidence of device-related infections are unclear, but could be related to the increased use of ICD and CRT devices relative to traditional pacemakers. Because of the demands placed on the battery, the longevity of ICD and CRT devices is lower than that of traditional pacemakers, and the need for more frequent surgical replacement of ICD and CRT devices might at least in part explain why the number of device-related infections has increased. 40
Setting, cost and equipment
Cardiac resynchronisation therapy and ICD device implants are carried out in local hospital or cardiac centres and can take from 1 to 3 hours depending on the type of device. Implantation of biventricular or resynchronisation devices is more complicated and takes longer than implantation of other ICDs. Implantation procedures are usually performed by senior cardiologists with specialist training in the technique, supported by cardiac technicians and nurses. Follow-up visits for patients can be as often as every 3–12 months, requiring support from senior cardiologists, cardiac nurses and technicians. According to the HRS/European Heart Rhythm Association (EHRA) Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices,41 whereas neither direct nor remote monitoring follow-up visits should be longer than 12 months, 6-monthly follow-up for ICD and CRT-D devices is recommended. The increasing complexity of devices could impact on the time needed for follow-up visits.
The average cost of the devices, including leads, has been estimated at £9692 for the ICD device, £3411 for CRT-P and £12,293 for CRT-D (see Chapter 5, Parameters common to all populations, and see Table 109 for further details). In addition to the cost of the device itself, high-quality digital X-ray equipment is necessary for coronary sinus angiography and positioning of the left ventricular pacing lead, as well as an external ICD programmer (a telemetry computer commercially produced and marketed for use with the device41) to enable the cardiologist to adjust the settings of the ICD after surgery or at follow-up visits as required.
Management of the disease
Existing guidelines for SCD and HF include NICE guidance on ICDs for arrhythmias42 and CRT for HF,43 and a NICE clinical guideline on the management of chronic HF. 44 Guidelines on the use of CRT have also been published by the European Society of Cardiology,45 the Heart Failure Society of America46 and the American College of Cardiology Foundation and the American Heart Association. 47 A 10-year National Service Framework for Coronary Heart Disease was published by the UK Department of Health in 2000,48 but this did not make specific recommendations on the use of CRT or ICD devices and is now out of date. Given the absence of a national framework, Heart Rhythm UK has recently developed standards for the implantation and follow-up of CRT devices. 49
Sudden cardiac death
Diagnosis of sudden cardiac death
As SCD can happen without warning, it is important for general practitioners and secondary care providers to be aware of risk factors so that patients at high risk of SCD can be identified and referred for cardiac evaluation. A range of diagnostic tests may be used to identify risk of SCD. An electrocardiogram (ECG) can detect abnormalities in the heart’s electrical activity and may reveal evidence of heart damage from CHD, or signs of a previous or current heart attack. Electrophysiological testing is sometimes used to identify the origins of an arrhythmia and programmed electrical stimulation (PES) of the heart may be used to stimulate the heart to induce the arrhythmia. An electrophysiological or PES study may be used before implantation of an ICD to confirm the need for an ICD or for diagnostic work-up. Other tests that may be used to identify SCD risk include ultrasound echocardiography and cardiac magnetic resonance imaging (to image or film different parts or the whole of the heart), blood tests (to check concentrations of chemicals involved in heart function, e.g. potassium and magnesium) and cardiac catheterisation (e.g. if blood samples from within the heart are required, or to inject dye for angiographic studies).
Implantable devices for sudden cardiac death
Ventricular arrhythmias, particularly sustained VT and VF, are life-threatening events. For patients who meet specified treatment criteria, the NICE guidance issued in 2006 [technology appraisal (TA)9542] recommends that ICD (or CRT-D) therapy is recommended for primary prevention (prevention of a first life-threatening arrhythmic event) and secondary prevention (prevention of an additional life-threatening event in survivors of sudden cardiac events or patients with recurrent unstable rhythms) of SCD. Patients with sustained ventricular arrhythmias associated with haemodynamic compromise in the presence of LVSD should be considered for ICD therapy after reversible factors are addressed. Patients with LVSD and who have recently had a MI or patients who have a cardiac condition that is associated with a high risk of sudden death should also be considered for ICD therapy in addition to OPT. OPT (as described below) is used as an adjunct or provided for those patients for whom an ICD would not be appropriate (e.g. those with a severely limited prognosis).
Specific recommendations of the NICE guidance42 (which does not cover non-ischaemic dilated cardiomyopathy) are that ICDs may be used as primary prevention for patients who have a history of previous (≤ 4 weeks) MI and either left ventricular dysfunction with a left ventricular ejection fraction (LVEF) < 35% (no worse than NYHA class III) and non-sustained VT on Holter (24-hour ECG) monitoring and inducible VT on electrophysiological testing or left ventricular dysfunction with a LVEF of < 30% (no worse than NYHA class III) and a QRS duration of ≥ 120 milliseconds; or who have a familial cardiac condition with a high risk of sudden death, including long QT syndrome, hypertrophic cardiomyopathy, Brugada syndrome or arrhythmogenic right ventricular dysplasia, or have undergone surgical repair of congenital heart disease.
Implantable cardioverter defibrillators as secondary prevention for arrhythmias are recommended for individuals who present, in the absence of a treatable cause, with one of the following: survived a cardiac arrest due to either VT or VF; spontaneous sustained VT causing syncope or significant haemodynamic compromise; sustained VT without syncope or cardiac arrest and who have an associated reduction in ejection fraction (LVEF < 35%) (no worse than NYHA class III). 42
Optimal pharmacological therapy for sudden cardiac death
Chronic prophylactic antiarrhythmic drug (AAD) therapy is aimed at suppressing the development of arrhythmias in patients at high risk of SCD. The class III drugs such as amiodarone are used for specific indications. These drugs may enhance the maintenance of sinus rhythm but cannot terminate an arrhythmia once it is initiated. A meta-analysis based on 8522 patients from 15 trials found that amiodarone reduced the risk of SCD by 29% and cardiovascular death (CVD) by 18% in patients at risk of SCD. 50 However, amiodarone therapy was neutral with respect to all-cause mortality and was associated with a high discontinuation rate and significant end-organ adverse reactions including hepatic, pulmonary and thyroid toxicity, with a two- and fivefold increased risk of pulmonary and thyroid toxicity respectively50 Other drugs that may be included in the OPT of SCD are angiotensin-converting enzyme (ACE) inhibitors (recommended for all patients with LVSD to improve ventricular geometry and function), aldosterone receptor antagonists (for people resistant to other drug therapy) and beta-blockers (to reverse ventricular remodelling) among others. 51
Heart failure
Diagnosis of heart failure
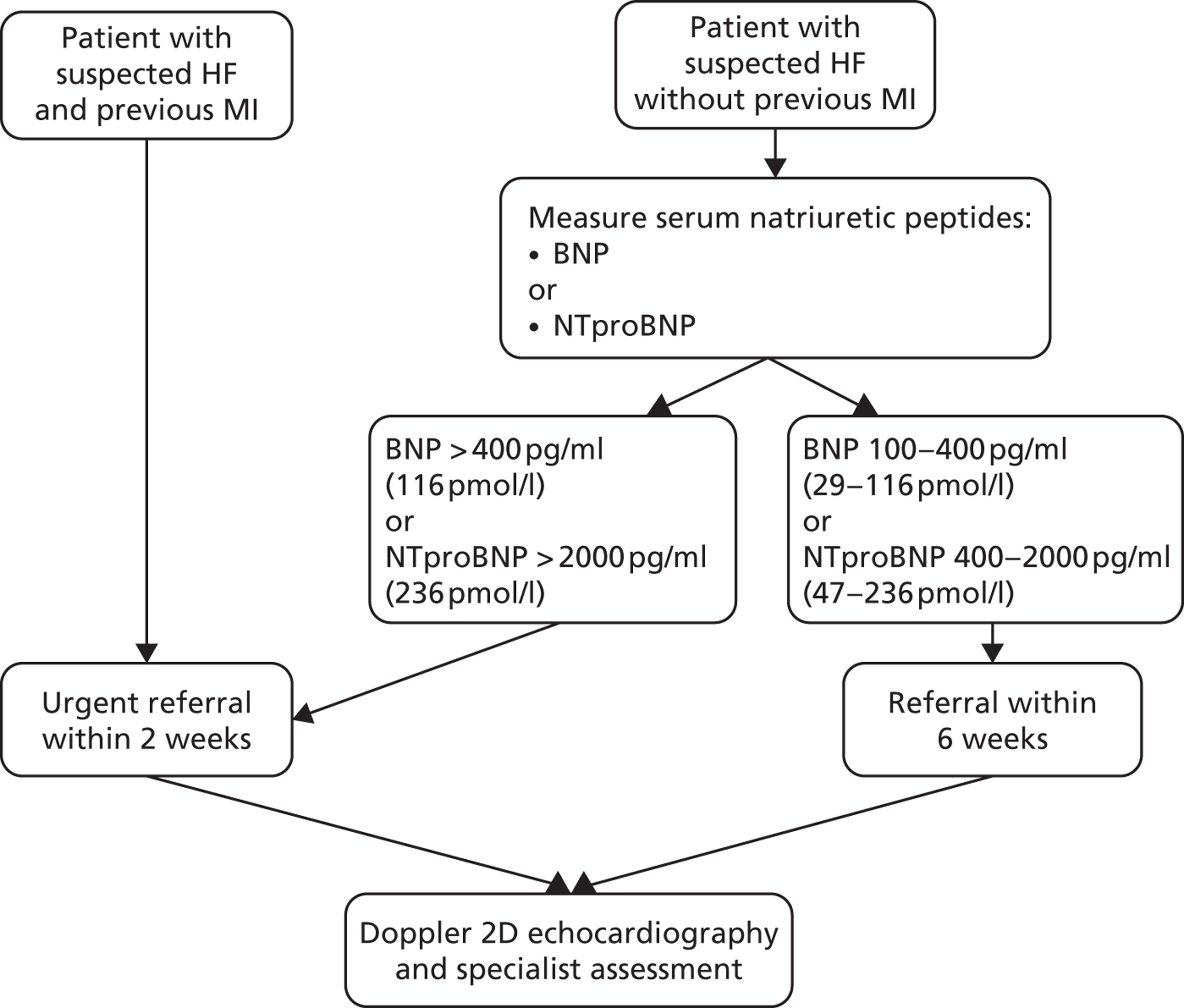
The NICE clinical guideline CG108, Chronic Heart Failure: Management of Chronic Heart Failure in Adults in Primary and Secondary Care,44 provides a diagnostic pathway for HF, the key elements of which are shown in Figure 2. Serum natriuretic peptides (SNPs; protein substances secreted by the wall of the heart when it is stretched or under increased pressure) should be measured in people with suspected HF without MI, although the guideline cautions that levels of SNPs can be reduced by certain conditions (e.g. obesity) or treatments (e.g. diuretics, ACE inhibitors, beta-blockers). Conversely, other conditions [e.g. left ventricular hypertropy, renal dysfunction, chronic obstructive pulmonary disease (COPD)] can cause high levels of SNPs. Therefore, an ECG and other tests (e.g. chest radiography, blood tests, urinalysis, spirometry) may be required to evaluate other possible diagnoses. Transthoracic Doppler two-dimensional echocardiography is used to assess the function (systolic and diastolic) of the left ventricle, to detect intracardiac shunts and to exclude important valve disease. If a poor image is obtained, other imaging methods (e.g. radionuclide angiography, cardiac magnetic resonance imaging or transoesophageal Doppler two-dimensional echocardiography) can be considered.
FIGURE 2.
Key elements in the NICE HF guideline diagnostic pathway. 52 BNP, B-type natriuretic peptide; NTproBNP, N-terminal pro-B-type natriuretic peptide.
Management of heart failure
A patient presenting with the typical signs and symptoms of HF should receive specialist assessment including echocardiography. 44 If HF is diagnosed the goals of treatment are to reduce mortality and improve the health outcome of the patient. In clinical practice, pharmacological agents are routinely used as the first-line therapy in managing HF44 (details of OPT for HF are given in Optimal pharmacological therapy for heart failure).
In addition to drug therapy, according to the NICE clinical guideline,44 individuals should be encouraged to participate in exercise-based cardiac rehabilitation (including a psychological and educational component), to give up smoking if applicable or be referred to a smoking cessation service, and to abstain from alcohol consumption if they have alcohol-related HF. Similarly, the European Society of Cardiology recommends that individuals with HF should be enrolled in a multidisciplinary care management programme. 53
Implantable devices for heart failure
As the severity of HF symptoms increases, a patient’s symptoms may no longer be controlled by OPT or lifestyle changes. There are multiple syndromes associated with HF that could predispose patients to the need for further intervention. In patients with HF, the existence of a modifiable risk factor such as arrhythmias may constitute a rationale for the use of multiple interventions. The NICE pathway for chronic HF52 indicates that, when symptoms are not controlled by OPT, treatment with CRT-P or CRT-D can be considered for patients meeting specific criteria.
Current NICE guidance issued in 2007 (TA12043) recommends CRT-P as a treatment option for individuals with HF who fulfil all of the following criteria: are currently experiencing or have recently experienced NYHA class III–IV symptoms; are in sinus rhythm – either with a QRS duration of ≥ 150 milliseconds estimated by standard ECG or with a QRS duration of 120–149 milliseconds estimated by ECG and mechanical dyssynchrony that is confirmed by echocardiography; have a LVEF of ≤ 35%; are receiving OPT. CRT-D may be considered for individuals who fulfil the criteria for implantation of a CRT-P device and who also separately fulfil the criteria for the use of an ICD device (see Implantable devices for sudden cardiac death).
Comments received from a clinical expert indicate that CRT is increasingly being considered for people without symptoms with the aim of improving prognosis by modifying the natural history of HF. Another interventional procedure that may be considered for patients with severe refractory symptoms is cardiac transplant. For those awaiting a donor heart, short-term circulatory support with a left ventricular assist device may be indicated. 54
Optimal pharmacological therapy for heart failure
Optimal medical drug therapy for HF can include ACE inhibitors, diuretics (for the relief of congestive symptoms and fluid retention), beta-blockers, aldosterone antagonists, digoxin (if symptoms continue despite the use of ACE inhibitors), amiodarone, anticoagulants (to reduce the risk of stroke), aspirin (to reduce the risk of vascular events), statins (to reduce the risk of MI and stroke), inotropic agents (to stimulate the heart muscle) and calcium channel blockers (for comorbid hypertension and angina).
The NICE 2010 clinical guideline44 suggests that medical drug therapy for HF has two aims – first, to improve morbidity (by reducing symptoms, improving exercise tolerance, reducing hospital admissions and improving QoL) and, second, to improve prognosis (by reducing all-cause mortality or HF-related mortality). According to the guideline, first-line treatment should include both ACE inhibitors and beta-blockers licensed for HF for all individuals with HF due to LVSD.
If an individual remains symptomatic despite optimal therapy with an ACE inhibitor and a beta-blocker, second-line treatment recommendations are to add one of the following: an aldosterone antagonist licensed for HF [especially if the patient has moderate to severe HF (NYHA class III–IV) or has had an MI within the past month] or an angiotensin II receptor antagonist (also known as an angiotensin receptor blocker or ARB) licensed for HF [especially if the patient has mild to moderate HF (NYHA class II–III)] or hydralazine in combination with nitrate [especially if the patient is of African or Caribbean origin and has moderate to severe HF (NYHA class III–IV)]. 44
Pharmacological recommendations for all types of HF include diuretics, calcium channel blockers, amiodarone, anticoagulants, aspirin and inotropic agents (such as dobutamine, milrinone or enoximone). ACE inhibitor therapy should not be initiated in individuals with a clinical suspicion of haemodynamically significant valve disease. 44
Current service provision
Current service provision is difficult to ascertain as the most recent audits of the use of CRT devices and ICDs in England and Wales55,56 suggest that there is considerable regional variation in implant rates. There is also a lack of information on patient referral patterns for the receipt of resynchronisation and defibrillation devices in the NHS. 57
The National Heart Failure Audit April 2010–March 201158 did not capture any information on the use of CRT devices or ICDs, but recommended that such data should be collected in future audits.
The most recent study to have reported the use of CRT devices and ICDs was the Cardiac Rhythm Management: UK National Clinical Audit 2010,55 which compared the rates of implantation of bradycardia pacemakers, ICDs and CRT devices during 2000–10 in comparison with national targets (a recent update of the audit56 provides additional data for January–December 2011 but is an interim version pending final publication). The audit collected data from 28 cardiac networks (regional groups of hospitals providing implants of pacemakers, CRT devices and ICDs) in England. There is clearly wide regional variation in the rates of implantation, with some cardiovascular networks having achieved or exceeded national target implant rates during 2010 and other networks not (Table 3). However, there is some debate about what the national targets should be. For example, a target of 100 ICD implants per million patients per annum has been proposed55 but other estimates that assume adherence to published guidelines suggest that the annual implant rate for ICDs should be higher, between 105 and 504 per million patients. 57 The wide regional variation in implant rates appears to suggest underuse in those regions with low implant rates. 57 The audit55 noted that the ratio of CRT-P implants to CRT-D implants and the ratio of ICD to CRT-D implants were highly variable among the cardiac networks in England, but it is not possible to determine the extent to which this variation reflects differences in local clinical practice and/or differences between patient populations. A study of ICD referral patterns in a single cardiac network in southern England57 found that implant rates were higher in areas where the local hospital was a regional cardiac centre compared with district general hospitals (with or without a device specialist), suggesting that some of the observed regional variation may reflect the structure of cardiac networks (the number and type of hospitals they include) and their patient referral pathways. 57 The discrepancy observed within the study of cardiac networks was greatest with respect to the use of ICDs for coronary artery disease primary prevention indications, and the authors suggested that this most likely reflects underuse of the therapy in the district hospitals rather than overuse in the regional cardiac centre. 57 A related study in the same cardiac network retrospectively investigated the management of ICD-implanted patients who developed HF. 59 Such patients may potentially benefit by being upgraded from an ICD to a CRT device. However, only a low proportion of these patients were found to have received an upgrade, raising the question of whether a CRT device might have been a more appropriate initial choice than an ICD for this patient subgroup. 59
| Device type | Averagea (range) no. of implants per million patients, adjusted for age and sex | National target (no. of implants per million patients, adjusted for age and sex) |
|---|---|---|
| ICD | 72 (34–131) | 100 |
| All CRT devices (CRT-P + CRT-D) | 114 (68–182) | 130 |
| All defibrillator devices (ICD + CRT-D) | 131 (81–197) | Not reported |
The audit55 reported data on the types of physiological pacing that were employed and also some data on the presenting symptoms and ECG patterns in patients with implants. As there is substantial overlap in the indications for resynchronisation and defibrillation devices,59 the choice of clinicians between ICD, CRT-D and CRT-P devices may in some cases have been arbitrary,55 and the audit did not discriminate between all of the possible pacing and defibrillation modes that can be programmed in modern implantable devices. Overall, in England during 2010, an ICD was the device type employed most frequently for syncope/cardiac arrest with VT/VF; CRT-D devices were the most frequent type implanted for HF with VT/VF; and CRT-P devices were the most frequent type employed in patients who had HF without VT/VF. Both CRT-D and ICD devices, but rarely CRT-P devices, were used for prophylaxis (Table 4). All device types were implanted more often in men than in women (80.1% of ICD, 83.4% of CRT-D and 68.4% of CRT-P devices were implanted in men). In 2011, a much higher proportion of CRT-D devices were implanted for primary prevention than for secondary prevention (78.3% vs. 21.7% respectively), although the proportions of ICDs implanted for primary and secondary prevention were similar (48.3% and 51.4% respectively). 55
| Presenting symptom and ECG | ICD (%) | CRT-D (%) | CRT-P (%) | Total (rounded) (%) |
|---|---|---|---|---|
| Syncope/cardiac arrest and VT/VF | 79.3 | 20.4 | 0.2 | 100 |
| HF and VT/VF | 29.8 | 68.2 | 1.9 | 100 |
| HF and any rhythm except VT/VF | 3.9 | 20.6 | 75.5 | 100 |
| Prophylactic (no symptoms) – all presenting ECGs | 48.5 | 48.8 | 2.7 | 100 |
The demand for device implants will increase because of a growing ageing population. In addition, there are increasing demands to expand the use of CRT devices, that is, to include individuals with NYHA class I–II symptoms, an ejection fraction of < 30% and a QRS interval wider than 130 milliseconds. This will increase the burden on existing services within cardiology, as well as raising the importance of device costs. The UK National Clinical Audit55 confirms that there has been a substantial increase in the number of CRT and ICD devices implanted in England and Wales during 2000–10. The interim update of the audit56 suggests, however, that, although more ICDs per million patients were implanted in England in 2011 than in 2010, the rate of increase has slowed and, overall, the total number of CRT implants per million patients was similar during 2010 and 2011.
In addition to the variation within the UK (see Table 3), there is considerable variation in the utilisation of implantable defibrillators across Europe,55 and ICD/CRT-D implant rates are considerably higher in the USA than in Europe. 60 The UK has approximately 0.7 ICD implant centres per million population, which is lower than in France, Germany, Italy and the USA. 60 It has been suggested that lower utilisation rates may reflect three main factors: a shortage of implant centres and electrophysiologists; poorly developed referral strategies/care pathways; and problems with specialist health-care investment. 60 The recently collected data55,60 suggest that systematic planning of ICD services is lacking in the UK, with underutilisation of CRT and ICD devices, although it is unclear if this impacts on the equality of service provision.
Chapter 2 Definition of the decision problem
This chapter states the key factors that will be addressed by this assessment and defines the scope of the assessment in terms of these key factors in line with the definitions provided in the NICE scope. 61 This assessment updates and expands on two previous technology assessment reports (TARs), The Clinical and Cost-Effectiveness of Implantable Cardioverter Defibrillators: a Systematic Review62 (which itself was an update of a TAR published in 200063) and The Clinical Effectiveness and Cost-Effectiveness of Cardiac Resynchronisation (Biventricular Pacing) for Heart Failure: Systematic Review and Economic Model. 64 The key differences between the present assessment and the previous assessments are outlined below and summarised in Appendix 1.
Decision problem
The interventions included within the scope of this assessment are ICD, CRT-P and CRT-D devices, each in addition to OPT.
Three populations are defined by the NICE scope:61
-
people at increased risk of SCD as a result of ventricular arrhythmias despite OPT
-
people with HF as a result of LVSD and cardiac dyssynchrony despite OPT
-
people with both conditions described above.
The first group, people at risk of SCD as a result of ventricular arrhythmias, includes and expands on the population considered in the previous ICD TAR. 62 For the present assessment this population is not restricted by NYHA classification and there is no specified cut-off for LVEF. The second group, people with HF as a result of LVSD and cardiac dyssynchrony, includes and expands on the population considered in the previous CRT TAR. 64 As in the previous TAR, this population is not restricted by NYHA classification in the present assessment, but unlike the previous TAR there is no specified cut-off for LVEF. The third group, people with both conditions, was not considered in the previous TARs. 62,64 People with cardiomyopathy are not excluded from consideration in this assessment.
Although the three populations are considered separately within the report for the purposes of this assessment, it is acknowledged that in practice these are not distinct groupings and there is considerable overlap between the groups: people with HF due to LVSD are at risk of SCD from ventricular arrhythmia.
The NICE scope61 did not indicate whether any subgroups of patients were of interest. No subgroups were predefined in the earlier guidance (TA9542), but subgroup analyses were reported in some included studies by LVEF, QRS duration and history of HF requiring treatment. Subgroups that were thought to be of interest in TA12043 and were therefore predefined were age, atrial fibrillation, NYHA class, degree of LVSD, degree of dyssynchrony and ischaemic and non-ischaemic HF. Relevant subgroups for the current assessment may also include renal failure. If sufficient evidence is available, consideration will be given to these subgroups.
The relevant comparisons for this assessment are as follows:
-
for people at increased risk of SCD as a result of ventricular arrhythmias despite OPT, ICD will be compared with standard care (OPT without ICD)
-
for people with HF as a result of LVSD and cardiac dyssynchrony despite OPT, CRT-P and CRT-D will be compared with each other or with standard care (OPT without CRT)
-
for people with both conditions described above, CRT-D will be compared with ICD, CRT-P or standard care (OPT alone).
The clinical outcomes of interest include mortality (including progressive HF mortality, non-HF mortality, all-cause mortality and SCD), health-related quality of life (HRQoL), symptoms and complications related to tachyarrhythmias and/or HF, HF hospitalisations, change in NYHA class, change in LVEF, and adverse effects of treatment. Outcomes for the assessment of cost-effectiveness will include direct costs based on estimates of health-care resources associated with the interventions as well as consequences of the interventions, such as treatment of adverse events.
Overall aims and objectives of the assessment
The aims of this health technology assessment are threefold:
-
to assess the clinical effectiveness and cost-effectiveness of ICDs in addition to OPT for the treatment of people who are at increased risk of SCD as a result of ventricular arrhythmias despite receiving OPT
-
to assess the clinical effectiveness and cost-effectiveness of CRT-P or CRT-D in addition to OPT for the treatment of people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT
-
to assess the clinical effectiveness and cost-effectiveness of CRT-D in addition to OPT for the treatment of people who have an increased risk of both SCD as a result of ventricular arrhythmias and HF as a result of LVSD and cardiac dyssynchrony despite OPT.
Chapter 3 Methods for the systematic reviews of clinical effectiveness and cost-effectiveness
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness were described in the research protocol, which was sent to the advisory group and to NICE for comment. Although helpful comments were received relating to the general content of the research protocol, there were none that identified specific problems with the methodology of the review. The methods outlined in the protocol are briefly summarised below.
Identification of studies
A search strategy was developed, tested and refined by an experienced information scientist. The strategy identified clinical effectiveness studies of ICDs for arrhythmias and CRT for the treatment of HF. Additional search strategies identified studies reporting on the cost-effectiveness of ICDs and CRT, and studies reporting on the epidemiology and natural history of arrhythmias and HF. Searches to inform cost-effectiveness modelling were also conducted. Sources of information and search terms are provided in Appendix 2. The most recent search was carried out in November 2012.
The following electronic databases were searched: The Cochrane Library including the Cochrane Database of Systematic Reviews (CDSR), the Cochrane Central Register of Controlled Trials, the Centre for Reviews and Dissemination (CRD) (University of York) Database of Abstracts of Reviews of Effectiveness (DARE), the NHS Economic Evaluation Database (NHS EED) and the Health Technology Assessment (HTA) database; MEDLINE (Ovid); EMBASE (Ovid); MEDLINE In-Process and Other Non-Indexed Citations (Ovid); Web of Science with Conference Proceedings: Science Citation Index Expanded (SCIE) and Conference Proceedings Citation Index – Science (CPCI) (ISI Web of Knowledge); Biosis Previews (ISI Web of Knowledge); Zetoc (Mimas); NIHR Clinical Research Network Portfolio; ClinicalTrials.gov; and Current Controlled Trials. Searches were carried out from database inception to the present for studies in the English language. Searches were limited to randomised controlled trials (RCTs) for the assessment of clinical effectiveness and to full economic evaluations for the assessment of cost-effectiveness. Bibliographies of retrieved papers and the manufacturers’ submission (MS) to NICE were assessed for relevant studies that met the inclusion criteria, and the expert advisory group was contacted to identify additional published and unpublished evidence.
Inclusion and exclusion criteria
The inclusion criteria for population, interventions and comparators are summarised in Table 5.
| Population | People at increased risk of SCD as a result of ventricular arrhythmias despite OPT | People with HF as a result of LVSD and cardiac dyssynchrony despite OPT | People with both conditions described to the left |
| Interventions | ICD in addition to OPT | CRT-P or CRT-D in addition to OPT | CRT-D in addition to OPT |
| Comparators | Standard care (OPT without ICD) | CRT-P vs. CRT-D; standard care (OPT without CRT) | ICDs; CRT-P; standard care (OPT alone) |
Population
-
People at increased risk of SCD as a result of ventricular arrhythmias despite OPT.
-
People with HF as a result of LVSD and cardiac dyssynchrony despite OPT.
-
People with both conditions described above.
Left ventricular systolic dysfunction was defined as a reduced LVEF using the cut-off provided by the publications (an arbitrary cut-off was not imposed by this review). Similarly, cardiac dyssynchrony was as defined by the publications, usually a prolonged QRS interval. Trials clearly stating that participants had a reduced LVEF, cardiac dyssynchrony and an indication for an ICD were considered as having both conditions.
Interventions
The interventions under consideration for each patient group are:
-
for people at increased risk of SCD: ICDs in addition to OPT
-
for people with HF: CRT-P or CRT-D in addition to OPT
-
for people with both conditions: CRT-D in addition to OPT.
Comparators
The comparators under consideration for each patient group are:
-
for people at increased risk of SCD: standard care (OPT without ICD)
-
for people with HF: CRT-P or CRT-D were compared with each other; standard care (OPT without CRT)
-
for people with both conditions: ICDs; CRT-P; standard care (OPT alone).
When screening studies for inclusion it became apparent that the pharmacological therapy in some of the older studies might not be considered optimal by current standards. After consultation with NICE and clinical experts, it was decided that trials in which the pharmacological therapy in either the intervention arm or the comparator arm was not optimal (i.e. was not current best practice based on clinical opinion) would be included in the systematic review.
Outcomes
Studies must have included one or more of the following outcome measures to be eligible for inclusion in this review:
-
mortality (including progressive HF mortality, non-HF mortality, all-cause mortality and SCD)
-
adverse effects of treatment
-
HRQoL
-
symptoms and complications related to tachyarrhythmias and/or HF
-
HF hospitalisations
-
change in NYHA class
-
change in LVEF.
Study design
-
For the systematic review of clinical effectiveness, only RCTs were eligible.
-
Studies published as abstracts or conference presentations from 2010 onwards were included only if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
-
Systematic reviews of the clinical effectiveness of ICDs and CRT were used as a source of references.
-
For the systematic review of cost-effectiveness, studies were included only if they reported the results of full economic evaluations [cost-effectiveness analyses (reporting cost per life-year gained), cost–utility analyses or cost–benefit analyses].
-
For the systematic review of QoL, primary studies or QoL data collected as part of a trial using the European Quality of Life-5 Dimensions (EQ-5D) (not visual analogue scale), and specified by NYHA class for people with HF, were included.
-
Non-English-language studies were excluded.
Screening and data extraction process
Studies were selected for inclusion in the systematic review of clinical effectiveness through a two-stage process using the criteria defined earlier. The titles and abstracts of studies identified by the search strategy were screened by two reviewers to identify all citations that potentially met the inclusion criteria. Full papers of potentially relevant studies were retrieved and assessed by two independent reviewers using a standardised eligibility form. Full papers or abstracts describing the same study were linked together, with the article reporting key outcomes designated as the primary publication. Data from included studies were extracted by one reviewer using a standardised data extraction form and checked by a second reviewer. At each stage, any disagreements were resolved by discussion, with the involvement of a third reviewer when necessary.
Titles and abstracts identified by the search strategies for the systematic reviews of cost-effectiveness and QoL were assessed for potential eligibility by two health economists using predetermined inclusion criteria. Full papers were assessed for inclusion by two reviewers.
Critical appraisal
The risk of bias of the clinical effectiveness studies was assessed according to criteria devised by The Cochrane Collaboration. 65 Criteria were applied by one reviewer and checked by a second reviewer, with differences in opinion resolved by consensus and by consultation with a third reviewer if necessary. Economic evaluations were appraised using criteria based on those recommended by Drummond and Jefferson,66 the requirements of the NICE reference case67 and the suggested guideline for good practice in decision-analytic modelling by Philips and colleagues68 (see Appendix 3). Published studies carried out from the UK NHS and Personal Social Services (PSS) perspective were examined in more detail.
Method of data synthesis
Clinical effectiveness data were synthesised through a narrative review with tabulation of the results of included studies. When data were of sufficient quality and homogeneity, meta-analysis of the clinical effectiveness studies was performed to estimate the risk ratio (RR) and 95% confidence intervals (CIs) for relevant outcomes. The random-effects method was used. Meta-analysis was performed using Review Manager 5 (RevMan; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Statistical heterogeneity was assessed using the chi-squared test and degrees of freedom (df), and the I2 statistic. When standard deviations (SDs) were not presented in the published papers, these were calculated from the available statistics [CIs, standard errors (SEs) or p-values]. 65 A minority of papers reported median values with 95% CIs; in these cases, rather than omitting the trial from a meta-analysis, it was assumed that the data were symmetrical (and so the median would be similar to the mean value) and the median was used directly in the meta-analysis.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 4 Clinical effectiveness
Overall quantity of evidence identified
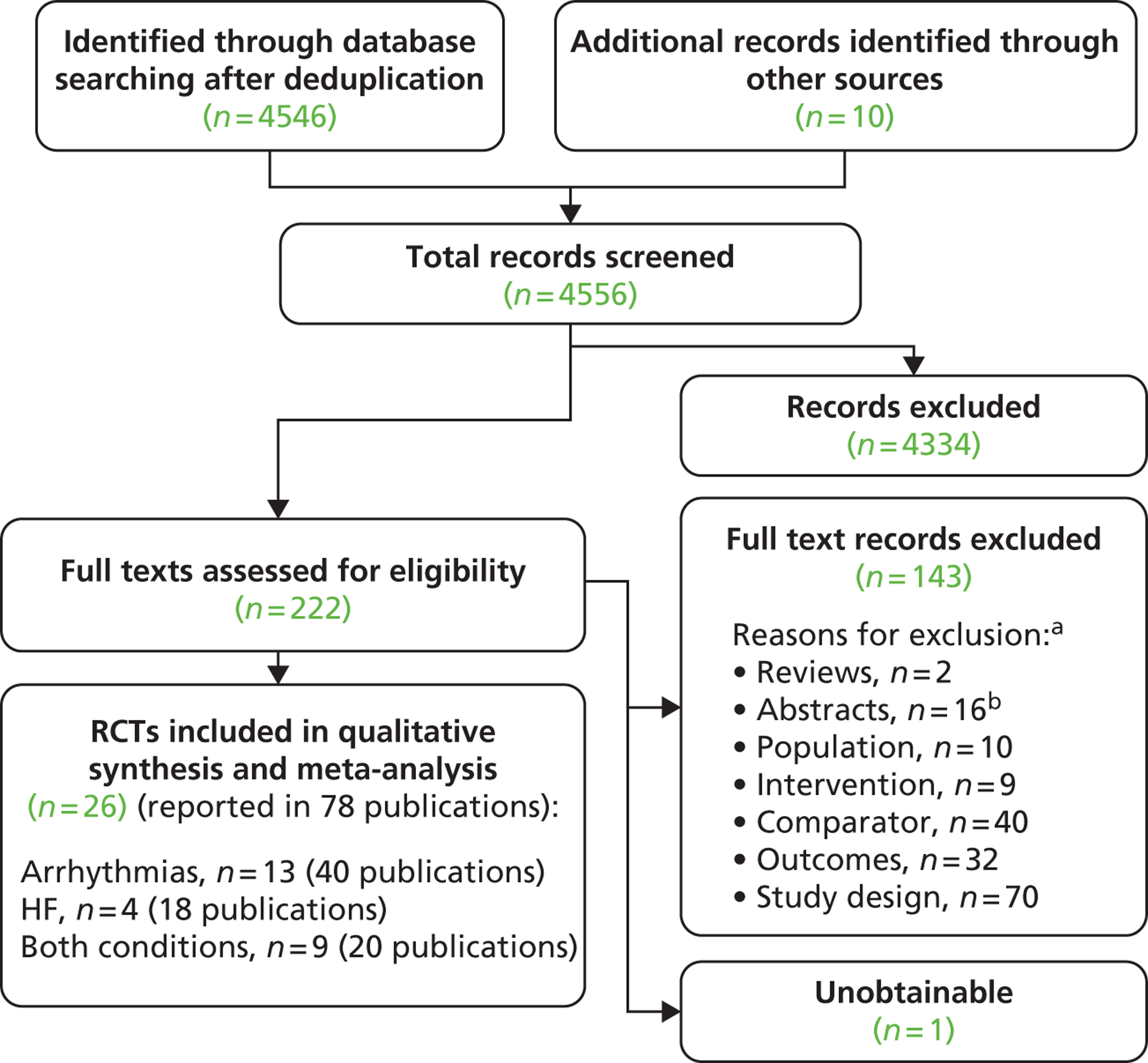
Searches identified a total of 4556 references after deduplication and full texts of 222 references were retrieved after screening titles and abstracts. The number of references excluded at each stage of the systematic review is shown in Figure 3. Selected references that were retrieved but later excluded are listed in Appendix 4 with reasons for exclusion. Papers were often excluded for more than one reason, with the most common reason being study design (70 papers), followed by comparator (40 papers) and outcomes (32 papers). Although not formally assessed, the level of agreement between reviewers for screening was considered good.
FIGURE 3.
Flow chart of identification of studies. a, Studies could be excluded for more than one reason; b, 16 of the abstracts/conference presentations were published from 2010 onwards (see Appendix 4) and were excluded as there were insufficient details included to allow an appraisal of the methodology and an assessment of the results as per the protocol.
Searches identified five relevant trials in progress, summaries of which can be found in Appendix 5.
Twenty-six eligible RCTs were identified (Table 6); many of these trials were reported in several publications (a total of 78 papers). Thirteen RCTs were considered to involve people at increased risk of SCD as a result of ventricular arrhythmias (see People at risk of sudden cardiac death as a result of ventricular arrhythmias), four trials were considered to involve people with HF as a result of LVSD and cardiac dyssynchrony (see People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony) and nine RCTs were considered to involve people with both of these conditions (see People with both conditions). Further details on the quantity and quality of research for each of these populations are described in the following sections.
| Study | Publicationa |
|---|---|
| People at increased risk of SCD as a result of ventricular arrhythmias | |
| AMIOVIRT | Strickberger et al. 2003,69 Wijetunga and Strickberger 200370 |
| AVID | AVID investigators 199771 and 1999,72 Hallstrom 1995,73 Schron et al. 200274 |
| CABG Patch | Bigger 1997,75 CABG Patch Trial Investigators and Coordinators 1993,76 Bigger et al. 199877 and 1999,78 Spotnitz et al. 1998,79 Namerow et al. 199980 |
| CASH | Kuck et al. 2000 81 |
| CAT | Bänsch et al. 2002,82 German Dilated Cardiomyopathy Study investigators 199283 |
| CIDS | Connolly et al. 200084 and 1993,85 Sheldon et al. 2000,86 Irvine et al. 2002,87 Bokhari et al. 200488 |
| DEBUT | Nademanee et al. 2003 89 |
| DEFINITE | Kadish et al. 200490 and 2000,91 Schaechter et al. 2003,92 Ellenbogen et al. 2006,93 Passman et al. 200794 |
| DINAMIT | Hohnloser et al. 200495 and 200096 |
| IRIS | Steinbeck et al. 200997 and 200498 |
| MADIT I | Moss et al. 1996,99 MADIT Executive Committee 1991100 |
| MADIT II | Moss et al. 2002101 and 1999,102 Greenberg et al. 2004,103 Noyes et al. 2007104 |
| SCD-HeFT | Bardy et al. 2005,105 Mitchell et al. 2008,106 Mark et al. 2008,107 Packer et al. 2009108 |
| People with HF as a result of LVSD and cardiac dyssynchrony | |
| CARE-HF | Cleland et al. 2005,109 2001,110 2006,111 2007112 and 2009,113 Gras et al. 2007,36 Gervais et al. 2009,114 Ghio et al. 2009115 |
| COMPANION | Bristow et al. 2004116 and 2000,117 US Food and Drug Administration 2004,118 Carson et al. 2005,119 Anand et al. 2009120 |
| MIRACLE | Abraham et al. 2002121 and 2000,122 US Food and Drug Administration 2001,123 St John Sutton et al. 2003124 |
| MUSTIC | Cazeau et al. 2001 125 |
| People with both conditions described above | |
| CONTAK-CD | Higgins et al. 2003,126 Saxon et al. 1999,127 Lozano et al. 2000,128 US Food and Drug Administration 2002129 |
| MADIT-CRT | Moss et al. 2009130 and 2005,131 Solomon et al. 2010,132 Goldenberg et al. 2011,133,134 Arshad et al. 2011135 |
| MIRACLE ICD | Young et al. 2003 136 |
| MIRACLE ICD II | Abraham et al. 2004 137 |
| Piccirillo 2006 | Piccirillo et al. 2006 138 |
| Pinter 2009 | Pinter et al. 2009 139 |
| RAFT | Tang et al. 2010140 and 2009141 |
| RethinQ | Beshai et al. 2007,142 Beshai and Grimm 2007143 |
| RHYTHM ICD | US Food and Drug Administration 2004144 and 2005145 |
People at risk of sudden cardiac death as a result of ventricular arrhythmias
Quantity and quality of research available
Eleven of the 13 RCTs included reported their findings in more than one paper; a summary of the included papers for each trial can be seen in Table 7. Seven of these RCTs plus one additional RCT [the Multicenter Unsustained Tachycardia Trial (MUSTT)146] were included in the 2005 TAR,62 as can be seen in Table 7. One further RCT [the Midlands Trial of Empirical Amiodarone versus Electrophysiology-Guided Interventions and Implantable Cardioverter-Defibrillators (MAVERIC)147] was noted in the 2005 TAR62 as in progress at that time. The interventions in the MUSTT146 and MAVERIC147 trials did not meet the scope of the present review; however, as these were included in the previous TARs62,63 they are discussed in Subgroup analyses reported by included randomised controlled trials. A list of other excluded studies can be seen in Appendix 4.
| Study | 2005 TAR62 (reason for exclusion) | Present TAR (participants) | Publicationa |
|---|---|---|---|
| Secondary prevention | |||
| AVID | Included | Included (cardiac arrest) | AVID investigators 199771 and 1999,72 Hallstrom 1995,73 Schron et al. 200274 |
| CASH | Included | Included (cardiac arrest) | Kuck et al. 2000 81 |
| CIDS | Included | Included (cardiac arrest) | Connolly et al. 200084 and 1993,85 Sheldon et al. 2000,86 Irvine et al. 2002,87 Bokhari et al. 200488 |
| DEBUT | Excluded (participants) | Included (sudden unexpected death syndrome) | Nademanee et al. 2003 89 |
| Primary prevention | |||
| DINAMIT | In progress | Included (early post MI) | Hohnloser et al. 200495 and 200096 |
| IRIS | New | Included (early post MI) | Steinbeck et al. 200997 and 200498 |
| MADIT I | Included | Included (remote from MI) | Moss et al. 1996,99 MADIT Executive Committee 1991100 |
| MADIT II | Included | Included (remote from MI) | Moss et al. 2002101 and 1999,102 Greenberg et al. 2004,103 Noyes et al. 2007104 |
| AMIOVIRT | Excluded (participants) | Included (cardiomyopathy) | Strickberger et al. 2003,69 Wijetunga and Strickberger 200370 |
| CAT | Included | Included (cardiomyopathy) | Bänsch et al. 2002,82 German Dilated Cardiomyopathy Study investigators 199283 |
| DEFINITE | Excluded (participants) | Included (cardiomyopathy) | Kadish et al. 200490 and 2000,91 Schaechter et al. 2003,92 Ellenbogen et al. 2006,93 Passman et al. 200794 |
| CABG Patch | Included | Included (need for CABG) | Bigger 1997,75 CABG Patch Trial Investigators and Coordinators 1993;76 Bigger et al. 199877 and 1999,78 Spotnitz et al. 1998,79 Namerow et al. 199980 |
| MUSTT | Included | Excluded because of intervention | Buxton et al. 1999,146 Lee et al. 2002148 |
| SCD-HeFT | In progress, in NICE TA9542 | Included (HF) | Bardy et al. 2005,105 Mitchell et al. 2008,106 Mark et al. 2008,107 Packer et al. 2009108 |
The RCTs used different criteria to identify groups at ‘high risk’ of SCD from ventricular arrhythmia. The Antiarrhythmics Versus Implantable Defibrillators (AVID),71 Cardiac Arrest Study Hamburg (CASH),81 Canadian Implantable Defibrillator Study (CIDS)84 and Defibrillator versus Beta-Blockers for Unexplained Death in Thailand (DEBUT)89 trials included people who had had a previous ventricular arrhythmia or who had been resuscitated from cardiac arrest. Four studies included people with either a recent MI [Defibrillator in Acute Myocardial Infarction Trial (DINAMIT)95 and the Immediate Risk Stratification Improves Survival (IRIS) trial97] or a MI > 3–4 weeks before study entry [Multicenter Automatic Defibrillator Implantation Trial I (MADIT I),99 MADIT II101]. The Amiodarone Versus Implantable Cardioverter-Defibrillator Randomized Trial (AMIOVIRT),69 Cardiomyopathy Trial (CAT)82 and Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE)90 trial included people with cardiomyopathy. The Coronary Artery Bypass Graft Patch (CABG Patch) trial75 recruited patients scheduled for coronary artery bypass graft surgery and at high risk for sudden death, and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)105 recruited a broad population of patients with mild to moderate HF. The results will be discussed according to the ‘high-risk’ group of the participants.
Characteristics of the included studies
Study characteristics are summarised in Tables 8–10 and participant characteristics are summarised in Tables 11–13. Additional details can be found in Appendix 7.
| Parameter | AVID71 | CASH81 | CIDS84 | DEBUT89 |
|---|---|---|---|---|
| Study design | RCT | RCT | RCT | RCT (pilot and main study) |
| Target population | Resuscitated from near-fatal VF; or symptomatic sustained VT with haemodynamic compromise | Resuscitated from cardiac arrest secondary to documented sustained ventricular arrhythmia | Previous sustained ventricular arrhythmia | SUDS survivors or probable survivors |
| Intervention | ICD + medical therapy | ICD + medical therapy | ICD + AAD for symptomatic VT | ICD + beta-blocker or amiodarone if frequent shocks |
| Comparator | AAD + medical therapy | AAD (amiodarone or metoprolol) + medical therapy | Amiodarone + AAD for symptomatic VT | Beta-blocker (long-acting propranolol); other beta-blockers if intolerable side effects |
| Country (no. of centres) | USA (53), Canada (3) | Germany (multicentre, number unclear) | Canada (19), Australia (3), USA (2) | Thailand (unclear) |
| Sample size (randomised) | 1016 | 288 | 659 | Pilot 20, main trial 66 |
| Length of follow-up | Mean 18.2 (SD 12.2) months | Mean 57 (SD 34) months | Mean 3 years | Maximum 3 years |
| Key inclusion criteria | VF, VT with syncope or VT without syncope but with ejection fraction ≤ 0.40 and systolic blood pressure < 80 mmHg; chest pain or near syncope.73 If patients underwent revascularisation their ejection fraction had to be ≤ 0.40 | Not reported. Rate was the only criterion selected for detection of a sustained ventricular arrhythmia | Any of following in the absence of either recent acute MI (≤ 72 hours) or electrolyte imbalance: documented VF; out-of-hospital cardiac arrest requiring defibrillation or cardioversion; documented, sustained VT causing syncope; other documented sustained VT at a rate ≥ 150 bpm causing presyncope or angina in a patient with a LVEF ≤ 35%; or unmonitored syncope with subsequent documentation of either spontaneous VT ≥ 10 seconds or sustained (≥ 30 seconds) monomorphic VT induced by programmed ventricular stimulation | SUDS survivor: a healthy subject without structural heart disease who had survived unexpected VF or cardiac arrest after successful resuscitation Probable SUDS survivor: a subject without structural heart disease who experienced symptoms indicative of the clinical presentation of SUDs, especially during sleep. ECG abnormalities showing RBBB-like pattern with ST elevation in right precordial leads and inducible VT/VF in electrophysiological testing |
| Parameter | DINAMIT95 | IRIS97 | MADIT I99 | MADIT II101 |
|---|---|---|---|---|
| Target population | Recent MI (6–40 days); reduced LVEF and impaired cardiac autonomic function | Recent MI (≤ 31 days) and predefined markers of elevated risk | Previous MI and left ventricular dysfunction | High-risk cardiac patients with previous MI and advanced left ventricular dysfunction |
| Study design | RCT | RCT | RCT | RCT |
| Intervention | ICD + OPT | ICD + OPT | ICD + conventional medical therapy | ICD + conventional medical therapy |
| Comparator | OPT | OPT | Conventional medical therapy | Conventional medical therapy |
| Country (no. of centres) | Canada (25), Germany (21), France, (8), UK (4), Poland (4), Slovakia (2), Austria (2), Sweden (2), USA (2), the Czech Republic (1), Switzerland (1), Italy (1) | Austria, the Czech Republic, Germany, Hungary, Poland, the Russian Federation, Slovakia (total 92) | USA (30), Europe (2) | USA (71), Europe (5) |
| Sample size | 674 | 898 | 196 | 1232 |
| Length of follow-up | Mean 30 (SD 13) months | Average 37 (range 0 to 106) months | Average 27 (range < 1 to 60) months | Average 20 months (range 6 days to 53 months) |
| Key inclusion criteria | Recent MI (6–40 days previously); LVEF ≤ 0.35; SD of normal-to-normal RR intervals of ≤ 70 milliseconds or a mean R–R interval of ≤ 750 milliseconds (heart rate ≥ 80 bpm) over a 24-hour period as assessed by 24-hour Holter monitoring performed at least 3 days after the infarction | Predefined markers of elevated risk – at least one of heart rate ≥ 90 bpm on first available ECG (within 48 hours of MI) and LVEF ≤ 40% (on one of days 5–31 after the MI); non-sustained VT of three or more consecutive ventricular premature beats during Holter ECG monitoring, with a heart rate ≥ 150 bpm (on days 5–31) | NYHA class I, II or III; LVEF ≤ 0.35; Q-wave or enzyme-positive MI > 3 weeks before entry; a documented episode of asymptomatic, unsustained VT unrelated to an acute MI; no indications for CABG or coronary angioplasty within past 3 months; sustained VT or fibrillation reproducibly induced and not suppressed after the intravenous administration of procainamide (or equivalent) | LVEF ≤ 0.30 in last 3 months; MI > 1 month before study entry |
| Parameter | Cardiomyopathy | CABG surgery | HF | ||
|---|---|---|---|---|---|
| AMIOVIRT69 | CAT82 | DEFINITE90 | CABG Patch75 | SCD-HeFT105 | |
| Target population | Non-ischaemic (DCM) and asymptomatic non-sustained VT | Recent-onset idiopathic DCM and impaired LVEF and without documented symptomatic VT | Non-ischaemic cardiomyopathy and moderate to severe left ventricular dysfunction | Patients scheduled for CABG surgery and at risk for sudden death (LVEF < 0.36 and abnormalities on ECG) | Broad population of patients with mild to moderate HF |
| Study design | RCT | RCT (pilot) | RCT | RCT | RCT |
| Intervention | ICD + OPT | ICD + OPT | ICD + OPT | ICD + OPT | ICD + OPT |
| Comparator | Amiodarone + OPT | OPT | OPTa | OPT; no specific therapy for ventricular arrhythmia | Amiodarone or placebo (two groups) + OPT |
| Country (no. of centres) | USA (10) | Germany (15) | USA (44), Israel (4) | USA (35), Germany (2) | USA (99%), Canada, New Zealand (total 148) |
| Sample size | 103 | 104 | 458 | 900 | 2521 |
| Length of follow-up | Mean 2 (SD 1.3) years | 2 years | Mean 29 (SD 14.4) months | Mean 32 months | Median 45.5 (range 24 to 72.6) months |
| Key inclusion criteria | Non-ischaemic DCM (left ventricular dysfunction in the absence of, or disproportionate to the severity of, CAD); LVEF ≤ 0.35; asymptomatic non-sustained VT; NYHA class I–III | NYHA class II or III; LVEF ≤ 30%; aged 18–70 years; symptomatic DCM ≤ 9 months | LVEF < 36%; presence of ambient arrhythmias; history of symptomatic HF; presence of non-ischaemic DCM | Scheduled for CABG surgery; LVEF < 0.36; marker of arrhythmia: abnormalities on ECG | NYHA class II or III; chronic, stable CHF from ischaemic or non-ischaemic causes; LVEF ≤ 35%; ischaemic CHF defined as LVSD associated with marked stenosis or a documented history of MI; non-ischaemic CHF defined as LVSD without marked stenosis |
| Parameter | AVID71 | CASH81 | CIDS84 | DEBUT (pilot trial)89 | DEBUT (main trial)89 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD | AAD | ICD | AAD | ICD | AAD | ICD | Beta-blocker | ICD | Beta-blocker | ||
| Amiodarone | Metoprolol | ||||||||||
| Sample size, n | 507 | 509 | 99 | 92 | 97 | 328 | 331 | 10 | 10 | 37 | 29 |
| Age (years), mean (SD) or [SEM] | 65 (11) | 65 (10) | 58 (11) | 59 (10) | 56 (11) | 63.3. (9.2) | 63.8 (9.9) | 44 [11] | 48 [15] | 40 [11] | 40 [14] |
| Sex, % male | 78 | 81 | 79 | 82 | 79 | 85.4 | 83.7 | 100 | 100 | 95 | 100 |
| Index arrhythmia VF, % | 44.6 | 45.0 | 84a | 45.1b | 50.1b | 70 | 60 | 24.3 | 37.9 | ||
| Index arrhythmia VT, % | 55.4 | 55.0 | 16a | 39.7b | 37.5b | 0 | 0 | 5.4 | 6.9 | ||
| Ischaemic heart disease, % | 81 | 81 | 73 | 77 | 70 | 82.9 | 82.2 | NR | NR | NR | NR |
| Dilated cardiomyopathy, % | NR | NR | 12 | 10 | 14 | 8.5 | 10.6 | NR | NR | NR | NR |
| Previous MI, % | 67 | 67 | NR | NR | NR | 77.1 | 75.8 | NR | NR | NR | NR |
| No CHF, % | 45 | 40 | 0 | 0 | 0 | 51.2 | 49.5 | 0 | 0 | 0 | 0 |
| NYHA I, % | 48 | 48 | 23 | 25 | 32 | 37.8 | 39.9 | 100 | 100 | 100 | 100 |
| NYHA II, % | 59 | 57 | 55 | 0 | 0 | 0 | 0 | ||||
| NYHA III, % | 7 | 12 | 18 | 18 | 13 | 11.0 | 10.6 | 0 | 0 | 0 | 0 |
| NYHA IV, % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| LVEF, mean (SD) or [SEM] | 0.32 (0.13) | 0.31 (0.13) | 0.46 (0.19) | 0.44 (0.17) | 0.47 (0.17) | 34.3 (14.5) | 33.3 (14.1) | 67 [12] | 69 [6] | 66 [10] | 67 [7] |
| Heart rate (bpm) | 77 (18) | 78 (17) | 81 (17) | 80 (17) | 76 (16) | NR | NR | 67 [12] | 64 [7] | 64 [11] | 66 [12] |
| QT interval (milliseconds), mean (SD) or [SEM] | 441 (40) | 445 (39) | 437 (42) | 430 (51) | 430 (48) | NR | NR | 396 [51] | 387 [31] | 404 [43] | 394 [31] |
| QRS interval (milliseconds), mean (SD) or [SEM] | 116 (26) | 117 (26) | NR | NR | NR | NR | NR | 98 [29] | 92 [12] | 99 [30] | 95 [16] |
| BBB (unspecified), % | 23 | 25 | 17 | 23 | 19 | NR | NR | NR | NR | NR | NR |
| Parameter | DINAMIT95 | IRIS97 | MADIT I99 | MADIT II101 | ||||
|---|---|---|---|---|---|---|---|---|
| ICD | OPT | ICD | OPT | ICD | OPT | ICD | OPT | |
| Sample size, n | 332 | 342 | 445 | 453 | 95 | 101 | 742 | 490 |
| Age (years), mean (SD) | 61.5 (10.9) | 62.1 (10.6) | 62.8 (10.5) | 62.4 (10.6) | 62 (9) | 64 (9) | 64 (10) | 65 (10) |
| Sex, % male | 75.9 | 76.6 | 77.5 | 75.9 | 92 | 92 | 84 | 85 |
| Arrhythmia, % | NR | NR | NSVT 22.2 | NSVT 24.1 | VT 100 | VT 100 | NR | NR |
| NYHA I, % | 13.5 | 12.0 | 28a | 37 | 33 | 35 | 39 | |
| NYHA II, % | 60.9 | 58.7 | 60a | 63 | 67 | 35 | 34 | |
| NYHA III, % | 25.6 | 29.3 | 12a | 25 | 23 | |||
| NYHA IV, % | 0 | 0 | 0.1a | 0 | 0 | 5 | 4 | |
| LVEF (%), mean (SD) | 28 (5) | 28 (5) | 34.6 (9.3) | 34.5 (9.4) | 27 (7) | 25 (7) | 23 (5) | 23 (6) |
| QRS interval (milliseconds), mean (SD) | 107 (24) | 105 (23) | NR | NR | NR | NR | 50% ≥ 120 milliseconds | 51% ≥ 120 milliseconds |
| LBBB/RBBB, % | NR | NR | 10.1/NR | 6.4/NR | 7/NR | 8/NR | 19/9 | 18/7 |
| Parameter | Cardiomyopathy | CABG surgery | HF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMIOVIRT69 | CAT82 | DEFINITE90 | CABG Patch75 | SCD-HeFT105 | |||||||
| ICD | Amiodarone | ICD | Control | ICD + OPT | OPT | ICD | Control | ICD | Amiodarone | Placebo | |
| Sample size, n | 51 | 52 | 50 | 54 | 229 | 229 | 446 | 454 | 829 | 845 | 847 |
| Age (years), mean (SD) or [range] | 58 (11) | 60 (12) | 52 (12) | 52 (10) | 58.4 [20.3–83.9] | 58.1 [21.8–78.7] | 64 (9) | 63 (9) | 60.1 [51.9–69.2]a | 60.4 [51.7–68.3]a | 59.7 [51.2–67.8]a |
| Sex, % male | 67 | 74 | 86 | 74 | 72.5 | 69.9 | 86.5 | 82.2 | 77 | 76 | 77 |
| Index arrhythmia, % | NSVT 100 | NSVT 100 | NSVT 53.1 | NSVT 58.0 | NSVT 22.3, PVCs 9.2, both 68.6 | NSVT 22.7, PVCs 9.6, both 67.7 | NR | NR | NSVT 25 | NSVT 23 | NSVT 21 |
| Ischaemic heart disease, %b | 4.9 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Duration of cardiomyopathy, mean (SD) or [median, range] | 2.9 (4.0) years | 3.5 (3.9) years | [3.0 months] | [2.5 months] | [2.39, 0.00–21.33 yearsc] | [3.27, 0.0–38.5 yearsc] | |||||
| NYHA class, % | |||||||||||
| I | 18 | 13 | 0 | 0 | 25.3 | 17.9 | NR | NR | 0 | ||
| II | 64 | 63 | 66.7 | 64.1 | 54.2 | 60.7 | 71 | 74 | 70 | ||
| III | 16 | 24 | 33.3 | 35.8 | 20.5 | 21.4 | 30 | ||||
| IV | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR | 0 | ||
| LVEF (%), mean (SD) or [range] | 22 (10) | 23 (8) | 24 (6) | 25 (8) | 20.9 [7–35] | 21.8 [10–35] | 27 (6) | 27 (6) | 24.0 [19.0–30.0]a | 25.0 [20.0–30.0]a | 25.0 [20.0–30.0]a |
| QRS interval (milliseconds), mean (SD) or [range] | NR | NR | 102 (29) | 114 (29) | 114.7 [78–196] | 115.5 [79–192] | 71%d | 74%d | NR | NR | NR |
| LBBB/RBBB, % | 16/42 | 8/53 | 24/2 | 37/0 | 19.7/3.5 | 19.7/3.1 | 10/NR | 12/NR | NR | NR | NR |
Intervention and comparators
The NICE scope and systematic review protocol defined the intervention for this group of people as ‘ICDs in addition to OPT’ and the comparator as ‘standard care (OPT without an ICD)’. Concepts of OPT have changed over time and OPT varies depending on the population (e.g. previous VF, post MI, HF), making a standard definition of OPT difficult. Standards of reporting have also changed, making it difficult in some instances to be clear what participants have received. As a consequence it was decided, and agreed with NICE, to include studies that compared ICDs (with or without OPT) with the different types of medical therapy, reporting the details of the pharmacological therapy used. The studies included were eligible on all other selection criteria.
The trials of people with previous VF or cardiac arrest compared ICDs with AADs, including either amiodarone or a beta-blocker (sotalol) (AVID71), amiodarone or a beta-blocker (metoprolol) in separate groups (CASH81) or amiodarone (CIDS84), or with a beta-blocker (propranolol) (DEBUT89). Use of other medication was permitted in these trials. AVID71 permitted the use of aspirin, beta-blockers and ACE inhibitors when clinically appropriate in both groups. CASH81 reported concurrent therapies at discharge (see Pharmacological therapy for further details of pharmacological therapy received by participants in all included trials). CIDS84 stated that AADs could be used in both groups to control supraventricular or non-sustained VTs that were symptomatic or might cause discharge of the ICD. DEBUT89 permitted other beta-blocking agents or amiodarone if intolerable side effects developed from propranolol or if frequent shocks from recurrent VF occurred, but did not provide additional data.
Trials of people with recent (IRIS,97 DINAMIT95) or remote (MADIT I,99 MADIT II)101 MI compared ICDs + OPT with OPT, although the pharmacological therapy in MADIT may not be considered optimal by current standards.
The trials of people with cardiomyopathy compared ICDs + OPT with amiodarone + OPT (AMIOVIRT69) or ICDs + OPT with OPT (CAT,82 DEFINITE90).
The CABG Patch trial75 included people scheduled for CABG surgery and compared ICDs + OPT with OPT (the trial protocol prohibited use of AADs for asymptomatic ventricular arrhythmias), although the pharmacological therapy may not be considered optimal by current standards. The ICDs used in this trial were epicardial defibrillators, mostly committed devices (i.e. they deliver a shock even if the arrhythmia stops before the end of charging) that were not capable of storing electrograms.
The SCD-HeFT trial105 was a three-arm trial comparing ICDs, amiodarone and placebo in a broad population of patients with mild-to moderate HF. All participants received OPT.
Participants
The DEBUT trial89 differed notably from the other three trials (AVID,71 CASH81 and CIDS84) of people resuscitated from cardiac arrest as participants in the DEBUT trial89 were survivors or probable survivors (symptoms indicative of the clinical presentation) of sudden unexplained death syndrome (SUDS) with otherwise normal hearts. All participants in the DEBUT study89 were of Thai origin and were similar to people with Brugada syndrome (a genetic disorder characterised by abnormal ECG findings and increased risk of cardiac death); as such the trial findings should also apply to this group of people.
The majority of participants in the AVID,71 CASH81 and CIDS84 trials had ischaemic heart disease (70–83%). A small proportion of those in the CASH81 and CIDS84 trials had dilated cardiomyopathy. Two-thirds of participants in the AVID trial71 and around three-quarters of those in the CIDS trial84 had a previous MI.
All participants in the CASH81 and DEBUT89 trials, 60% in the AVID trial71 and 50% in the CIDS trial84 had congestive heart failure (CHF). The majority (approximately 87%) of people in the CASH trial81 had NYHA class I or class II HF, whereas about 40% of those in the CIDS trial84 and half of those in the AVID trial71 fell into these categories. Only 10–11% of participants in the AVID71 and CIDS84 trials had moderate to severe HF (NYHA class III and IV), whereas 16% of people in the CASH trial81 had NYHA class III HF and none had NYHA class IV HF. Mean LVEF was higher in the CASH trial81 (46%) than in the AVID trial71 (32%) or the CIDS trial84 (34%), suggesting that there may have been a disproportionate representation of relatively healthy participants in the CASH trial. 81 The mean QT interval ranged from 387 milliseconds (DEBUT89) to 445 milliseconds (AVID). 71
The participants in the DEBUT trial89 were younger (mean age 40–48 years) than those in the other three trials (mean age 56–65 years) and all had NYHA class I HF. LVEF was higher in the DEBUT trial89 (mean LVEF 66–69%) than in the AVID,71 CASH81 and CIDS84 trials.
The MADIT I99 and MADIT II101 trials included people who had had a MI > 3 weeks or > 1 month previously. Participants in MADIT I99 were also required to have a LVEF of ≤ 35%, whereas the MADIT II trial101 required advanced left ventricular dysfunction (LVEF ≤ 30%). The DINAMIT95 and IRIS97 trials recruited participants with a recent MI (within 6–40 days and 5–31 days respectively). DINAMIT95 required participants to have a LVEF of ≤ 35% and a SD of normal-to-normal R–R intervals of ≤ 70 milliseconds or a mean R–R interval of ≤ 750 milliseconds (heart rate ≥ 80 beats per minute) over 24 hours. The IRIS trial97 included people with at least one of the following markers of risk: heart rate ≥ 90 beats per minute on the first available ECG and LVEF ≤ 40%; or non-sustained ventricular tachycardia (NSVT) of ≤ 3 consecutive ventricular premature beats during Holter ECG monitoring with a heart rate of ≥ 150 beats per minute.
The DINAMIT trial95 had the greatest majority of participants in NYHA class II or III (around 88%); the corresponding percentages in the IRIS,97 MADIT I99 and MADIT II101 trials were 27%, 63–67% and 60% respectively. The trials had either no or very few participants in NYHA class IV. Mean LVEF ranged from 23%101 to 35%,97 reflecting the different inclusion criteria of the studies.
The mean age of the participants in these trials was similar, ranging from 61.5 years in the DINAMIT trial95 to 65 years in MADIT II. 101 The majority of participants (from 76% in DINAMIT95 to 92% in MADIT I99) were men.
The AMIOVIRT69 and DEFINITE90 trials recruited people with non-ischaemic dilated cardiomyopathy, NSVT and a LVEF of ≤ 35%. CAT82 enrolled people with recent-onset (< 9 months) idiopathic dilated cardiomyopathy and a LVEF of ≤ 30%, but without documented symptomatic ventricular arrhythmias. Note that despite participants not having suffered ventricular arrhythmias, the low LVEF indicates a risk of ventricular arrhythmias and SCD and CAT82 was therefore judged eligible for inclusion in this review. Also, NSVT was identified with Holter ECG in over half of participants at baseline.
The majority of participants in these trials were in NYHA class II or III, with none in NYHA class IV. The AMIOVIRT69 (13–18%) and DEFINITE90 (18–25%) trials included more people in NYHA class I than the CAT trial,82 as this was an exclusion criteria of CAT. 82 Despite the lower cut-off for LVEF for inclusion in CAT,82 the mean LVEF at baseline was similar or slightly higher than in the other two trials (CAT82 24–25%, AMIOVIRT69 22–23%, DEFINITE90 21–22%). The mean QRS interval was similar between CAT82 [ICD 102 (SD 29) milliseconds, OPT 114 (SD 29) milliseconds] and DEFINITE90 [115 (range 78–196) milliseconds], although the measures of variance suggest that some participants had cardiac dyssynchrony.
Participants in CAT82 had a median duration of symptoms of just 3 months, compared with around 3 years in AMIOVIRT69 and DEFINITE. 90 The participants in CAT82 were also slightly younger (mean age 52 years) than in AMIOVIRT69 (mean age 59 years) or DEFINITE90 (mean age 58 years). The majority of participants (approximately 71% in AMIOVIRT69 and DEFINITE90 and 80% in CAT82) were men.
Participants in CABG Patch75 were scheduled for CABG surgery and at risk for SCD (LVEF < 36%) with abnormalities on ECG. People with a history of sustained VT or VF were excluded. The majority of participants (71–74%) were in NYHA class II or III with a mean LVEF of 27%. Most participants (83%) had had a previous MI. Mean age was about 64 years and 82–87% of participants were men.
SCD-HeFT105 included a broad population of people with mild to moderate HF from ischaemic or non-ischaemic causes and a LVEF of ≤ 35%. Ischaemic CHF was defined as LVSD associated with a ≥ 75% narrowing of at least one of three major coronary arteries (marked stenosis) or a documented history of MI. Non-ischaemic CHF was defined as LVSD without marked stenosis. Overall, 70% of participants were in NYHA class II and 30% were in class III. Median LVEF was 24–25% and less than one-quarter of participants had NSVT. The median age was 60 years and most participants (77%) were men.
Pharmacological therapy
Tables 14 and 15 display medication at hospital discharge.
| Medication | Cardiac arrest (secondary prevention) | Recent MI | Remote MI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVID71 | CASH81 | CIDS84 | DINAMIT95 | IRIS97 | MADIT I99a | MADIT II101b | |||||||||
| ICD | AAD | ICD | Amiodarone | Metoprolol | ICD | Amiodarone | ICD | OPT | ICD | OPT | ICD | OPT | ICD | OPT | |
| Sample size, n | 497 | 496 | 99 | 92 | 97 | 328 | 331 | 332 | 342 | 445 | 453 | 93 | 93 | 742 | 490 |
| ACE inhibitor, % | 68.8 | 68.2 | 45.5 | 43.5 | 41.2 | 94.9 | 94.4 | 90.9 | 91.1 | 60 | 55 | 68 | 72 | ||
| Antiarrhythmic, % | 13.4 | 17.4 | |||||||||||||
| Amiodarone | 1.8 | 95.8 | 0 | 97.8 | 0 | 2 | 74 | 13 | 10 | ||||||
| Other AAD | 4.2 | 1.2 | |||||||||||||
| Class I antiarrhythmic | 5.5 | 2.4 | 12 | 10 | 3 | 2 | |||||||||
| Anticoagulants and antiplatelets (%) | 92.2 | 92.1 | 96.1 | 95.8 | |||||||||||
| Acetylsalicylic acid (aspirin) | 60.7 | 59.2 | 57.6 | 44.6 | 41.2 | ||||||||||
| Warfarin | 21.9 | 34.8 | 9.1 | 6.5 | 9.3 | ||||||||||
| Beta-blockers, % | 42.3 | 16.5 | 33.5c | 21.4c | 87.0 | 86.5 | 97.1 | 95.3 | 26 | 8 | 70 | 70 | |||
| Metoprolol | 0 | 0 | 99.0 | ||||||||||||
| Sotalol | 0.2 | 2.8 | 19.8 | 1.5 | 1 | 7 | |||||||||
| Beta-blockers or sotalol | 27 | 15 | |||||||||||||
| Calcium channel blockers, % | 18.4 | 12.1 | 26.3 | 16.3 | 12.4 | 9 | 9 | ||||||||
| Diuretics, % | 48.2 | 50.7 | 33.3 | 27.2 | 30.9 | 53 | 52 | 72 | 81 | ||||||
| Nitrates, % | 36.4 | 37.0 | 29.3 | 29.3 | 24.7 | ||||||||||
| Other antihypertensive agents, % | 7.6 | 8.8 | |||||||||||||
| Digitalis, % | 46.8 | 40.6 | 26.3 | 25.0 | 15.5 | 58 | 38 | 57 | 57 | ||||||
| Digoxin, % | 29.6 | 22.7 | |||||||||||||
| Lipid-lowering agents, % | 13.2 | 11.5 | 76.8 | 79.5 | |||||||||||
| Statins, % | 91.6 | 91.5 | 67 | 64 | |||||||||||
| Medication | Cardiomyopathy | CABG surgery | HF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aAMIOVIRT69 | CAT82 | DEFINITE90 | CABG Patch75 | bSCD-HeFT105 | |||||||
| ICD | Amiodarone | ICD | OPT | ICD | OPT | ICD | OPT | ICD | Amiodarone | Placebo | |
| Sample size, n | 51 | 52 | 50 | 54 | 229 | 229 | 430 | 442 | 829 | 845 | 847 |
| ACE inhibitor, % | 90 | 81 | 94.0 | 98.1 | 83.8 | 87.3 | 54.7 | 53.8 | 83 | 87 | 85 |
| ACE inhibitor/ARB, % | 94 | 97 | 98 | ||||||||
| ARB, % | 13.5 | 8.7 | 14 | 14 | 16 | ||||||
| Amiodarone, % | 3.9 | 6.6 | 3.7 | 3.2 | |||||||
| Class I antiarrhythmic, % | 16.7 | 12.0 | |||||||||
| Anticoagulants, % | 15.3 | 14.7 | |||||||||
| Antiplatelets, % | 82.8 | 85.1 | |||||||||
| Acetylsalicylic acid (aspirin) | 58 | 55 | 56 | ||||||||
| Warfarin | 24.0 | 35.2 | 32 | 37 | 33 | ||||||
| Beta-blockers, % | 53 | 50 | 4.0 | 3.7 | 85.6 | 84.3 | 69 | 69 | 69 | ||
| Carvedilol | 56.3 | 58.5 | |||||||||
| Metoprolol | 25.8 | 18.8 | |||||||||
| Sotalol | 0.5 | 0.2 | |||||||||
| Other | 3.5 | 7.0 | 17.9 | 24.0 | |||||||
| Calcium channel blockers, % | 16.0 | 7.4 | 10.5 | 7.0 | |||||||
| Diuretics, % | 71 | 67 | 88.0 | 85.2 | 87.3 | 86.0 | 57.2 | 47.1 | |||
| Loop | 82 | 82 | 82 | ||||||||
| Potassium sparing | 20 | 21 | 19 | ||||||||
| Thiazide | 8 | 6 | 7 | ||||||||
| Spironolactone | 20 | 19 | |||||||||
| Nitrates, % | 32.0 | 25.9 | 9.2 | 13.1 | 8.1 | 8.1 | |||||
| Digitalis, % | 68.6 | 64.5 | |||||||||
| Digoxin, % | 71 | 67 | 41.5 | 42.4 | 67 | 73 | 70 | ||||
| Lipid-lowering agents, % | 9.5 | 8.4 | |||||||||
| Statins, % | 38 | 40 | 38 | ||||||||
Two-thirds of participants in the AVID trial71 were receiving ACE inhibitors. Only 6% of the ICD group received AADs at discharge. Beta-blockers were more common among the ICD group (42.3%) than among the AAD group (16.5%) (p < 0.001), which may have resulted in some bias towards ICD. Aspirin was received by around 60% of participants in the AVID trial71 and warfarin was received by a greater proportion of participants in the AAD arm (35%) than in the ICD arm (22%). Half of the participants in the AVID trial71 received diuretics, around 37% received nitrates and 12% (AAD arm) and 18% (ICD arm) received calcium channel blockers. Digitalis was received by 41% (AAD arm) and 47% (ICD arm) of participants (p = 0.04). The pharmacological therapy provided in the AVID trial71 would have been considered optimal at the time that the trial was conducted, although current standards would include less digitalis and more ACE inhibitors and beta-blocker therapy.
Less than half of participants in the CASH trial81 received ACE inhibitors at hospital discharge. The ICD and metoprolol groups did not receive any AADs, and the ICD and amiodarone groups did not receive any beta-blockers. Aspirin was received by around 60% of participants in the ICD group, but by fewer participants in the amiodarone (45%) and metoprolol (41%) arms. Less than 10% of participants in the CASH trial81 received warfarin, less than one-third received diuretics, around 30% received nitrates and 12% (metoprolol arm) to 26% (ICD arm) received calcium channel blockers. Digitalis was received by 15% (metoprolol arm) to 26% (ICD arm) of participants. The pharmacological therapy provided in the CASH trial81 would have been considered optimal at the time that the trial was conducted. However, beta-blocker treatment was an active comparator in this trial and was not used with ICDs, which may have resulted in bias against the ICD. ACE inhibitor use is low in this trial but the patients did not have indications for these at the time that the trial was undertaken.
None of the participants in the CIDS trial84 received ACE inhibitors at hospital discharge. Class I antiarrhythmics were received by just 2.4% (amiodarone arm) and 5.5% (ICD arm) of participants. A greater proportion of the ICD group than the amiodarone group received the beta-blocker sotalol (19.8% vs. 1.5%), beta-blockers other than solatol (33.5% vs. 21.4%) and digoxin (29.6% vs. 22.7%). No other drugs were reported. The pharmacological therapy provided in the CIDS trial84 would not be considered optimal by current standards and the higher use of beta-blockers in the ICD group may bias the trial in favour of ICDs.
Medication at hospital discharge is not reported in the DEBUT trial;89 however, use of beta-blockers was low in the ICD group (8/47 in main trial and pilot study combined).
Both groups in the DINAMIT trial95 were given ‘best conventional medical therapy’. ACE inhibitors were taken by around 95% of participants at baseline, antiplatelet agents by 92%, beta-blockers by 87% and lipid-lowering agents by 78%. The IRIS trial97 had a similarly high usage of ACE inhibitors (91%), antiplatelet agents (96%), beta-blockers (96%) and statins (92%). Antiarrhythmics (mainly amiodarone) were taken by a small proportion of participants (ICD arm 13.4% vs. OPT arm 17.4%, p = 0.11). Pharmacological therapy is considered optimal by current standards in both the DINAMIT trial95 and the IRIS trial. 97
The MADIT I trial99 presents data on drug use at 1 month (see Table 14) and last contact (see Appendix 7). Usage of ACE inhibitors (ICD arm 60%, medical therapy arm 55%) and beta-blockers (beta-blockers or sotalol: ICD arm 27%, medical therapy arm 15%) was low in this trial at 1 month and beta-blocker use was not balanced between the groups. Three-quarters of the medical therapy group received amiodarone at 1 month compared with 2% of the ICD group, but use of class I antiarrythmics was similar (ICD arm 12% vs. medical therapy arm 10%). At 1 month, 56% of the ICD patients and 8% of the medical therapy patients had no antiarrhythmic medication. Approximately half of the participants were receiving diuretics. Digitalis use was high by current standards (ICD arm 58%, medical therapy arm 38%). The pharmacological therapy provided in the MADIT I trial99 would not be considered optimal by current standards.
The MADIT II trial101 did not report medication at discharge but presented medication at last contact, which was a mean of 18 months (ICD arm) and 17 months (OPT arm) from enrolment. About 70% of participants received ACE inhibitors, about 10–13% received amiodarone and 2–3% received class I AADs. Beta-blockers were taken by 70% of participants, diuretics by 72% of the ICD group and 81% of the OPT group, digitalis by 57% of participants and statins by about two-thirds of participants. The pharmacological therapy provided in the MADIT II trial101 would be considered optimal by current standards.
The AMIOVIRT trial69 reports that OPT was encouraged in both the ICD group and the amiodarone group. Therapy at discharge was not reported but concomitant drug therapy was presented (see Table 15), with no statistically significant differences between the groups. A high proportion (81–90%) of participants received ACE inhibitors and approximately half received beta-blockers. Over two-thirds received diuretics and/or digoxin and one-fifth received spironolactone. Beta-blocker use is slightly lower in this trial than in current standards, but the pharmacological therapy is close to optimal.
About 96% of participants took ACE inhibitors at baseline in CAT,82 but beta-blocker use was low (4% of participants). Diuretics were taken by the majority of participants (85–88%), warfarin was received by 24–35% of participants, nitrates were taken by 26–32% of participants and calcium channel blockers were taken by 7–16% of participants. Observed differences between the groups were not statistically significant. Although acceptable at the time, the pharmacological therapy in this trial would not be considered optimal by current standards because of the low beta-blocker use.
Optimal pharmacological therapy was described for both groups in the DEFINITE trial. 90 A high proportion (about 86%) of participants received ACE inhibitors and a small proportion (8.7–13.5%) received ARBs. Beta-blockers were taken by 85%, diuretics by 87% and digoxin by 42%. A small proportion of each group received amiodarone (ICD arm 3.9%, OPT arm 6.6%) and nitrates (ICD arm 9.2%, OPT arm 13.1%). The pharmacological therapy in this trial would be considered optimal by current standards.
ACE inhibitors were taken by over half of the participants in the CABG Patch trial. 75 In total, 63.3% of the ICD group and 65.2% of the control group received no oral AADs. Class I antiarrythmics were taken by 16.7% (ICD arm) and 12.0% (OPT arm) of participants, amiodarone by 3.7% (ICD arm) and 3.2% (OPT arm) and beta-blockers (other than sotalol) by 17.9% (ICD arm) and 24% (OPT arm). There is an excess of AAD use in the ICD arm, which may paradoxically offset some of the ICD benefit. The majority of participants received antiplatelet drugs (84%), two-thirds received digitalis and around half received diuretics (47–57%). The pharmacological therapy provided in this trial would have been considered optimal at the time that the trial was conducted but use is low by current standards.
A high proportion (94–98%) of participants in SCD-HeFT105 were taking ACE inhibitors or an ARB at enrolment. Beta-blockers were taken by 69% of participants, digoxin by about 70%, aspirin by about 56%, warfarin by about 35% and statins by about 40%. Most (82%) received loop diuretics and 20% received potassium-sparing diuretics and a minority received thiazide (7%). This trial also reported medication at last follow-up, for which there was a statistically significant (p < 0.001) difference in beta-blocker use between groups (ICD arm 82%, amiodarone arm 72%, placebo arm 79%) (see Appendix 7). The pharmacological therapy in this trial would be considered optimal by current standards.
Outcomes
All-cause mortality was the primary outcome in all 13 trials in people at risk of SCD from ventricular arrhythmias. 69,71,78,81,82,84,89,90,95,97,99,101,105 Secondary outcomes tended to focus on other measures of mortality or survival. Ten RCTs assessed total cardiac deaths,69,72,78,82,84,95,97,99,103,108 13 RCTs assessed sudden cardiac and arrhythmic deaths,69,72,78,81,82,84,89,90,95,97,99,103,108 11 RCTs assessed cardiac non-arrhythmic deaths,69,72,78,82,84,90,95,97,99,103,108 10 RCTs assessed other non-cardiac causes of death,69,72,78,82,84,95,97,99,103,108 five RCTs assessed cumulative mortality75,84,90,97,105 and four RCTs assessed survival. 69,71,81,82 Other secondary outcome measures included heart hospitalisations (two RCTs71,101), symptoms and complications related to arrhythmias (three RCTs69,82,103), QoL (seven RCTs69,74,80,87,94,104,107) and adverse events (13 RCTs69,71,75,81,82,84,89,90,95,97,99,101,105).
Setting
The AVID,71 CASH81 and CIDS84 trials were multicentre studies, with the majority of the centres in the USA71 or Canada84 or in Germany. 81 The DEBUT study89 was conducted in Thailand but the number of centres was not reported. The number of participants ranged from 66 (DEBUT main study89) to 1016 (AVID71). The DEBUT trial89 also included a pilot study in which 20 participants were randomised. Length of follow-up ranged from a mean of 18.2 months (SD 12.2 months) in the AVID trial71 to 57 months (SD 34 months) in the CASH trial. 81
The DINAMIT,95 IRIS,97 MADIT I99 and MADIT II101 trials were multicentre studies. The majority of centres for the DINAMIT trial95 were in Canada, Germany and Europe (four UK centres) and the IRIS trial97 was conducted in Europe (not the UK) and the Russian Federation. The majority of centres for the MADIT I99 and MADIT II101 trials were in the USA. Sample size ranged from 196 (MADIT I99) to 1232 (MADIT II101). Mean follow-up ranged from 20 months in the MADIT II trial101 to 37 months in the IRIS trial. 97
The AMIOVIRT69 and DEFINITE90 trials were multicentre studies with the majority of centres in the USA, whereas CAT82 was a multicentre study conducted in Germany. Sample size was relatively small in AMIOVIRT69 and CAT82 (103 and 104 participants randomised respectively), with CAT82 designed as a pilot study. The DEFINITE trial90 randomised 458 participants. The trials had similar lengths of follow-up: a mean of 2 years in the AMIOVIRT69 and CAT82 trials and a mean of 2.4 years in the DEFINITE trial. 90
The CABG Patch trial75 was a multicentre study conducted primarily in the USA, with 900 participants randomised. Mean follow-up was 32 months. SCD-HeFT105 was also a multicentre study conducted mainly in the USA, with 2521 participants randomised. Median follow-up was 45.5 months.
Risk of bias
The risk of bias in the included trials is summarised in Table 16 and further details for each trial can be found in the data extraction tables in Appendix 7. All 13 trials were unclear on risk of bias associated with randomisation. In fact eight trials did not report details of either randomisation or allocation concealment; therefore, the risk of selection bias (differences between known and unknown baseline characteristics of the groups) is unclear. Five trials (CIDS,84 MADIT I,99 IRIS,97 DINAMIT,95 CABG Patch75) did not report the randomisation method, although sufficient details were reported to establish that the allocation sequence was adequately concealed and they were judged to have a low risk of selection bias.
| Domain | Judgement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVID71 | CASH81 | CIDS84 | DEBUT89 | IRIS97 | DINAMIT95 | MADIT I99 | MADIT II101 | CAT82 | AMIOVIRT69 | DEFINITE90 | CABG Patch75 | SCD-HeFT105 | |
| Selection bias | |||||||||||||
| Random sequence generation | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Allocation concealment | Unclear | Unclear | Low | Unclear | Low | Low | Low | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| Performance bias | |||||||||||||
| Blinding of participants, personnel | High | High | High | High | High | High | High | High | High | High | High | High | High |
| Detection bias | |||||||||||||
| Blinding of outcome assessment | Low,a highb | Low | Low,a highb | Low | Low | Low | Low | Low,a highb | Low | Low,a highb | Low,a highb | Low,a highb | Low,a highb |
| Attrition bias | |||||||||||||
| Incomplete outcome data addressed | Low,a highb | Low | Unclear | Unclear | Low | Low | Low | Low | Unclear | Low | Low | Low,a highb | Low,a unclearb |
| Reporting bias | |||||||||||||
| Selective reporting | Low | Low | High | Low | High | High | Low | Unclear | High | Low | High | Unclear | Low |
| Other bias | |||||||||||||
| Other sources | Low | Unclear | Low | Low | Low | High | Low | Low | Low | Low | Low | Low | Low |
It was not possible to blind participants and personnel (health-care providers) in these trials as one group received surgery. This could bias the results because of differences in behaviours across intervention groups or differences in the care provided, such as administration of co-interventions. The trials were therefore judged to have a high risk of performance bias. Cause of death was determined or reviewed by a committee blinded to treatment group in the AVID,71 DEFINITE,90 DINAMIT, 95 AMIOVIRT,69 IRIS97 and SCD-HeFT105 trials. Outcome assessors were not blinded in the other trials but mortality was judged unlikely to be influenced by lack of blinding and so the trials were considered to have a low risk of detection bias for this outcome. Unblinded trials reporting QoL69,71,75,84,90,101,105 were judged to have a high risk of detection bias for this outcome.
Risk of attrition bias (differences between groups in withdrawals from the study) was low in seven of the trials69,81,90,95,97,99,101 and unclear in three trials. 82,84,89 In the AVID,71 CABG Patch75 and SCD-HeFT105 trials, risk of attrition bias was judged to be low for mortality but high or unclear for QoL outcomes.
Risk of selective reporting bias (differences between reported and unreported findings) was considered to be low in six studies. 69,71,81,89,99,105 Five studies listed outcomes in a protocol or methods section that were not then reported. 82,84,90,95,97 Risk of selective reporting bias was unclear in two studies (MADIT II,101 CABG Patch75).
Risk of other sources of bias was judged to be high in DINAMIT95 as block randomisation in an unblinded trial can lead to prediction of allocation. The authors of the CASH study81 note that centres were reluctant to enrol patients for potential ICD therapy in the early phase of the study and to deny ICD therapy in the late phase of the study. The effect of this is unclear. Seven of the trials were stopped early;69,71,75,82,89,99,101 however, simulation evidence suggests that inclusion of stopped-early trials in meta-analyses does not lead to substantial bias. 65
Methodological comments
Similarity of groups at baseline
Although it was evident that there were differences between the 13 trials in the types of participants included (see earlier section on participants), within the trials participants appeared generally to be well balanced at baseline. However, some differences were evident. In the IRIS97 trial the ICD group had a higher proportion than the OPT group of people with LBBB (10.1% vs. 6.4%, p = 0.05) and diabetes mellitus (37.2% vs. 30.2%, p = 0.03). The CAT82 trial found a higher occurrence of bradycardias among the OPT group (18.8%) than among the ICD group (2.1%, p = 0.015). The DEFINITE90 trial noted that the OPT group (3.27 years) had a significantly longer mean duration of HF than the ICD + OPT group (2.39 years) (p = 0.04).
Sample size
All 13 trials included a calculation of sample size or statistical power based on the primary outcome measure of all-cause mortality. The CIDS (n = 659),84 DINAMIT (n = 674),95 DEFINITE (n = 458),90 CABG-Patch (n = 900)75 and SCD-HeFT (n = 2521)105 trials appeared to be adequately powered to detect a difference in all-cause mortality. In contrast, the CASH (n = 288),81 DEBUT (n = 66),89 MADIT II (n = 1232)101 and CAT (n = 104)82 trials were thought to be underpowered based on reported sample size calculations. Five trials were stopped early because they achieved an a priori stopping rule concerning crossing of efficacy boundaries [AVID (n = 1016),71 MADIT I (n = 196),99 MADIT II (n = 1232)101] or because interim analysis showed low event rates, which meant that further recruitment would not achieve adequate statistical power [AMIOVIRT (n = 103),69 CAT (n = 104)82]. Because of lower than anticipated mortality in the IRIS trial,97 an increase in sample size (n = 900) was recommended by the data and safety monitoring board.
Other issues
The CASH trial81 was designed as a four-arm trial (ICD, amiodarone, metoprolol, propafenone); however, the propafenone arm was terminated early after the interim analysis had been carried out. The DEBUT trial89 reports the results of a pilot study and the main trial, although both were small.
During the course of the MADIT I trial,99 a change was made from transthoracic to transvenous leads. The authors of this trial note that this altered the type of patient referred for entry to the trial.
Funding
The AVID71 and CIDS84 trials received funding from the National Heart, Lung, and Blood Institute and the Medical Research Council of Canada respectively. All of the other RCTs69,75,81,82,89,90,95,97,99,101,105 received some or all of their funding from the ICD manufacturers, which may represent a potential conflict of interests.
Assessment of effectiveness
All-cause mortality
All 13 trials comparing the use of ICDs with the use of AADs in people at increased risk of SCD from ventricular arrhythmias reported measures of all-cause mortality as their primary outcome measure. 69,71,75,81,82,84,89,90,95,97,99,101,105 Four trials71,81,84,89 assessed the use of ICDs compared with the use of AADs in people at increased risk of SCD from previous ventricular arrhythmias. All four trials showed beneficial effects on crude mortality rates for those receiving an ICD, although only the AVID trial71 (ICD arm 15.8%, AAD arm 24.0%, p < 0.012, follow-up 18.2 months) and the main DEBUT trial89 (ICD arm 0%, AAD arm 13.8%, p < 0.02, follow-up 3 years) found statistically significant differences. A separate pilot study for the DEBUT trial89 had previously shown no significant difference between the ICD arm and the AAD arm (ICD arm 0%, AAD arm 30.0%, p = 0.07, follow-up maximum 3 years). In the other two studies differences were either not statistically significant or not assessed. The CASH trial81 reported an all-cause mortality rate of 36.4% for the ICD group compared with 44.4% for the AAD group (p-value not stated, follow-up 57 months). The CIDS trial84 reported a crude mortality rate of 25.3% for the ICD group and 29.6% for the AAD group over the 3-year follow-up, equating to an annual crude mortality rate of 8.3% for the ICD group and 10.2% for the AAD group, a risk ratio reduction (RRR) of 19.7% (95% CI –7.7% to 40.0%, p = 0.142) (Table 17). A meta-analysis of the four studies (including the DEBUT pilot study89) using a random-effects model showed a statistically significant benefit for ICDs compared with AADs with a RR of 0.75 (95% CI 0.61 to 0.93, p = 0.010), with limited heterogeneity (χ2 = 5.89, df = 4, I2 = 32%) (Figure 4).
| Study | Follow-up | ICD, n/N (%) [rate/year, %] | OPT, n/N (%) [rate/year, %] | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| Cardiac arrest | |||||
| AVID71 | Mean 18.2 (SD 12.2) months | 80/507 (15.8, ± 95% CI 12.6 to 19) | AAD: 122/509 (24.0, ± 95% CI 20.3 to 27.7) | < 0.012 | |
| CASH81 | 57 (SD 34) months | 36/99 (36.4, 95% CI 26.9 to 46.6)a | Amiodarone: 40/92 (43.5, 95% CI 33.2 to 54.2)a | ||
| Metoprolol: 44/97 (45.4, 95% CI 35.2 to 55.8)a | |||||
| Both:b 84/189 (44.4, 95% CI 37.2 to 51.8)a | |||||
| CIDS84c | Mean 3 years | 83/328 (25.3) [8.3] | Amiodarone: 98/331 (29.6) [10.2] | RRR 19.7 | –7.7 to 40.0, 0.142 |
| DEBUT89 pilot study | Max. 3 years after randomisation | 0/10 (0) | Propranolol: 3/10 (30.0) | 0.07 | |
| DEBUT89 main study | 3 years | 0/37 (0) | Propranolol: 4/29 (13.8) | 0.02 | |
| Early post MI | |||||
| DINAMIT95 | Average 30 (SD 13) months | 62/332 (18.7) [7.5] | 58/342 (17.0) [6.9] | HR 1.08 | 0.76 to 1.55, 0.66 |
| IRIS97 | Average 37 months | 116/445 (26.1) | 117/453 (25.8) | HR 1.04 | 0.81 to 1.35, 0.15 |
| Remote from MI | |||||
| MADIT I99 | Average 27 months | 15/95 (15.8) | 39/101 (38.6) | HR 0.46 | 0.26 to 0.82, 0.009 |
| MADIT II101 | Average 20 months | 105/742 (14.2) | 97/490 (19.8) | HR 0.69 | 0.51 to 0.93, 0.016 |
| Cardiomyopathy | |||||
| AMIOVIRT69 | Mean 2.0 (SD 1.3) years | 6/51 (11.8) | Amiodarone + OPT: 7/52 (13.5) | 0.8 | |
| CAT82 | 1 year (primary end point) | 4/50 (8.0) | 2/54 (3.7) | 0.3672 | |
| Mean 5.5 (SD 2.2) years | 13/50 (26.0) | 17/54 (31.5) | |||
| DEFINITE90 | Mean 29.0 (SD 14.4) months | 28/229 (12.2) | 40/229 (17.5) | HR 0.65 | 0.40 to 1.06, 0.08 |
| Scheduled for CABG | |||||
| CABG Patch78 | Mean 32 (SD 16) months | 102/446 (22.9) | 96/454 (21.1) | ||
| HF | |||||
| SCD-HeFT105 | Median for surviving patients 45.5 (range 24–72.6) months | 182/829 (22.0) | Amiodarone + OPT:b 240/845 (28.4) | ||
| Placebo + OPT:b 244/847 (28.8) | HR 0.77d | 0.62 to 0.96,e 0.007 | |||
FIGURE 4.
All-cause mortality.
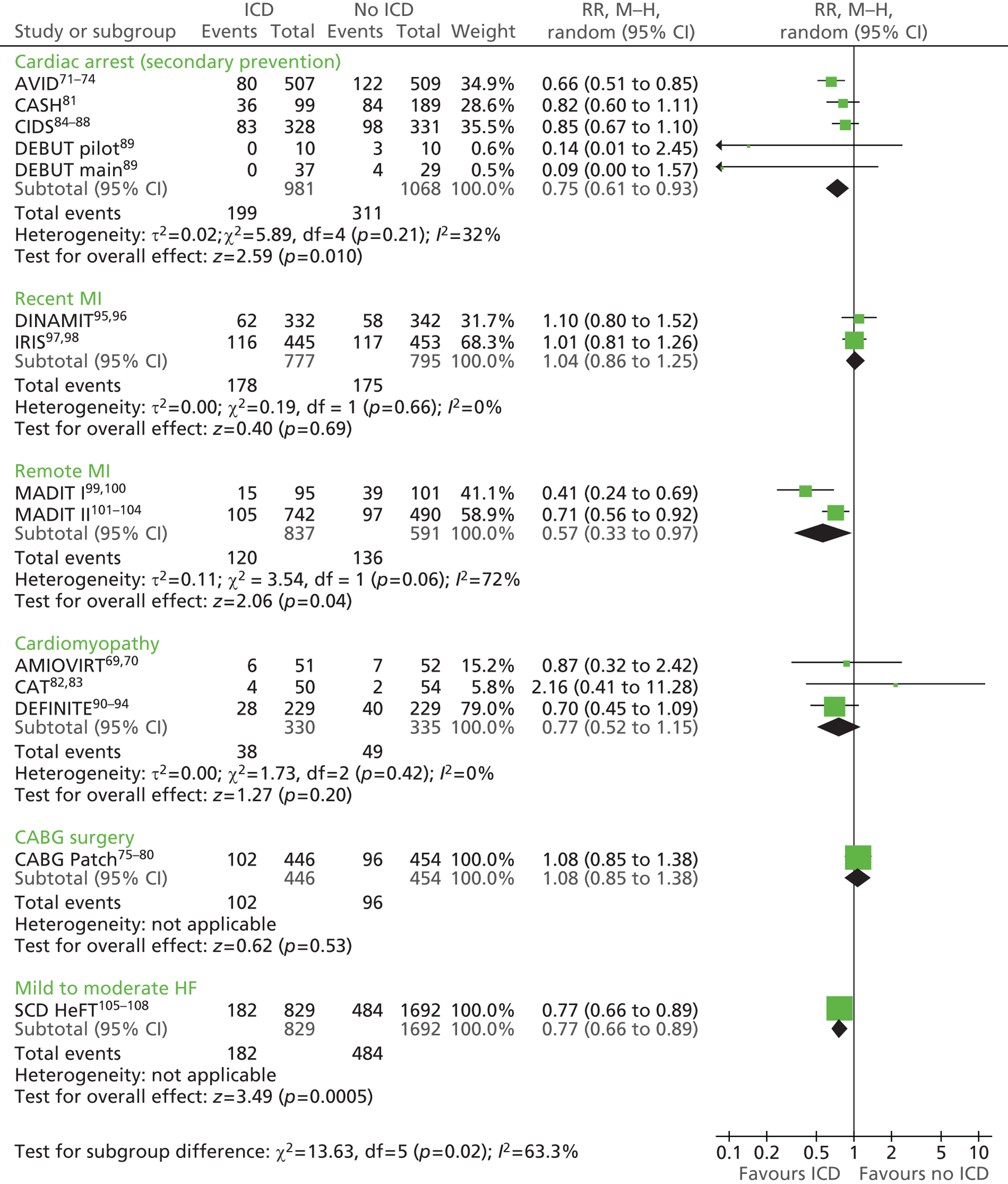
Of the nine trials69,75,82,90,95,97,99,101,105,146 including people who had not suffered a life-threatening arrhythmia but who were at increased risk, three showed statistically significant benefits for all-cause mortality in the ICD + OPT group compared with the different comparators (see Table 17). In the MADIT I trial,99 15.8% of participants receiving an ICD + OPT died compared with 38.6% of participants receiving OPT (mean follow-up 27 months), equating to a hazard ratio (HR) of 0.46 (95% CI 0.26 to 0.82, p = 0.009) (see Table 17). The MADIT II trial101 also found significant benefits, with 14.2% of those with an ICD + OPT dying compared with 19.8% of those who received OPT only (mean follow-up 20 months), a HR of 0.69 (95% CI 0.51 to 0.93, p = 0.016). Post-trial follow-up in the MADIT II study101 found continued benefit of ICDs at 8 years (HR 0.66, 95% CI 0.56 to 0.78, p = 0.001); analysis was undertaken on an efficacy basis by including data on crossovers and validated in an intention-to-treat (ITT) analysis. 149 The SCD-HeFT trial,105 which had a longer follow-up period (mean 45.5 months), reported that 22.0% of people who received an ICD + OPT died compared with 28.4% of those receiving amiodarone + OPT and 28.8% of those receiving placebo + OPT. HRs showed that the difference between the ICD + OPT group and the placebo + OPT group was statistically significant (HR 0.77, 97.5% CI 0.62 to 0.96, p = 0.007), whereas that between the amiodarone + OPT group and the placebo + OPT group was not statistically significant (HR 1.06, 97.5% CI 0.86 to 1.30, p = 0.53). 105 A meta-analysis of the two MADIT trials99,101 using a random-effects model showed a statistically significant benefit for those receiving ICDs + OPT compared with OPT alone, with a RR of 0.57 (95% CI 0.33 to 0.97, p = 0.04), although there was some apparent heterogeneity (χ2 = 3.54, df = 1, I2 = 72%), which may reflect differences in disease severity (see Figure 4).
The other six trials, which included people with cardiomyopathy,69,82,90 in the early period post MI95,97 or scheduled for a CABG,78 found no statistically significant differences between groups for all-cause mortality. The AMIOVIRT trial69 reported all-cause mortality after a mean follow-up of 2 years, finding that 11.8% of those with an ICD + OPT had died compared with 13.5% of those receiving amiodarone + OPT (p = 0.8). The CAT trial82 reported all-cause mortality at 1 year, showing no significant difference between groups (ICD + OPT 8% vs. OPT 3.7%, p = 0.3672). Longer mean follow-up to 5.5 years showed a limited difference between groups, with 26% of the ICD + OPT group and 31.5% of the OPT group dying (p-value not stated). The DEFINITE trial90 found that 12.2% of participants receiving an ICD + OPT and 17.5% of those receiving OPT had died at a mean follow-up of 29 months, a HR of 0.65 (95% CI 0.40 to 1.06, p = 0.08) (see Table 17). Combining these three cardiomyopathy trials using random-effects meta-analysis confirmed that there was no significant difference between the treatments, with a RR of 0.77 (95% CI 0.52 to 1.15, p = 0.20), with no heterogeneity (χ2 = 1.73, df = 2, I2 = 0%) (see Figure 4). The effect of combining the three cardiomyopathy trials with the non-ischaemic CHF subgroup of the SCD-HeFT trial105 is assessed in Subgroup analyses reported by included randomised controlled trials. The DINAMIT95 and IRIS97 trials assessed the effects of ICDs + OPT compared with OPT in those who were in the early period post MI. The DINAMIT trial95 reported that 18.7% of participants receiving an ICD + OPT and 17.0% of those receiving OPT had died by 30 months’ follow-up, resulting in a HR of 1.08 (95% CI 0.76 to 1.55, p = 0.66). Similarly, the IRIS trial97 found no significant difference in all-cause mortality between the ICD + OPT group (26.1%) and the OPT group (25.8%), reflected in a HR of 1.04 (95% CI 0.81 to 1.35, p = 0.15). Meta-analysis of the DINAMIT95 and IRIS97 trials confirmed that there was no significant difference between the treatments, with a RR of 1.04 (95% CI 0.86 to 1.25, p = 0.69), with no heterogeneity (χ2 = 0.19, df = 1, I2 = 0%) (see Figure 4). The CABG Patch trial,78 which included people who were scheduled for a CABG, reported a mortality rate of 22.9% for those receiving an ICD + OPT compared with 21.2% for those receiving OPT (p-value not stated), a RR of 1.08 (95% CI 0.85 to 1.38, p = 0.53) (see Figure 4).
Total cardiac deaths
Only two trials in people at increased risk of SCD due to previous ventricular arrhythmias, specifically the AVID72 and CIDS84 trials, assessed the effects of ICDs compared with AADs on total cardiac deaths (Table 18). Although both studies found lower crude rates for those receiving an ICD, neither reported whether the effect was statistically significant (AVID:72 ICD 12.4%, AAD 18.5%, p-value not stated; CIDS:84 ICD 20.4%, AAD 25.1%; p-value not stated). In addition, the CIDS trial84 found no statistically significant difference between the interventions with regard to annual crude mortality rates (ICD 6.7%, AAD 8.6%, RRR 23.4%, 95% CI –5.7 to 44.5, p = 0.104). However, a meta-analysis of the two studies using a random-effects model showed that ICDs had a statistically significant effect compared with AADs, with a RR of 0.74 (95% CI 0.61 to 0.91, p = 0.004) and no apparent heterogeneity (χ2 = 0.84, df = 1, I2 = 0%) (Figure 5).
| Study | Follow-up | ICD, n/N (%) [rate/year, %] | OPT, n/N (%) [rate/year, %] | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| Cardiac arrest | |||||
| AVID72 | Mean 18.2 (SD 12.2) months | 63/507 (12.4) | AAD: 94/509 (18.5) | ||
| CIDS84 | Mean 3 years | 67/328 (20.4) [6.7] | Amiodarone: 83/331 (25.1) [8.6] | RRR 23.4 | –5.7 to 44.5, 1.04 |
| Early post MI | |||||
| DINAMIT95 | Average 30 (SD 13) months | 46/332 (13.9) | 49/342 (14.3) | ||
| IRIS97 | Average 37 months | 95/445 (21.3) | 99/453 (21.9) | ||
| Remote from MI | |||||
| MADIT I99 | Average 27 months | 11/95 (11.6) | 27/101 (26.7) | ||
| MADIT II103 | Average 20 months | 79/742 (10.6) | 80/490 (16.3) | < 0.01 | |
| Cardiomyopathy | |||||
| AMIOVIRT69 | Mean 2.0 (SD 1.3) years | 4/51 (7.8) | Amiodarone + OPT: 5/52 (9.6) | ||
| CAT82 | 1 year (primary end point) | 4/50 (8.0) | 0/54 (0) | ||
| Scheduled for CABG | |||||
| CABG Patch78 | Mean 32 (SD 16) months | 76/446 (17.0) | 79/454 (17.4) | HR 0.97 | 0.71 to 1.33, 0.84 |
| HF | |||||
| SCD-HeFT108 | Median for surviving patients 45.5 (range 24 to 72.6) months | 122/829 (14.7) | Amiodarone + OPT: 162/845 (19.2), placebo + OPT: 167/847 (19.7) | HR 0.76 | 0.60 to 0.95, 0.018 |
FIGURE 5.
Total cardiac deaths.
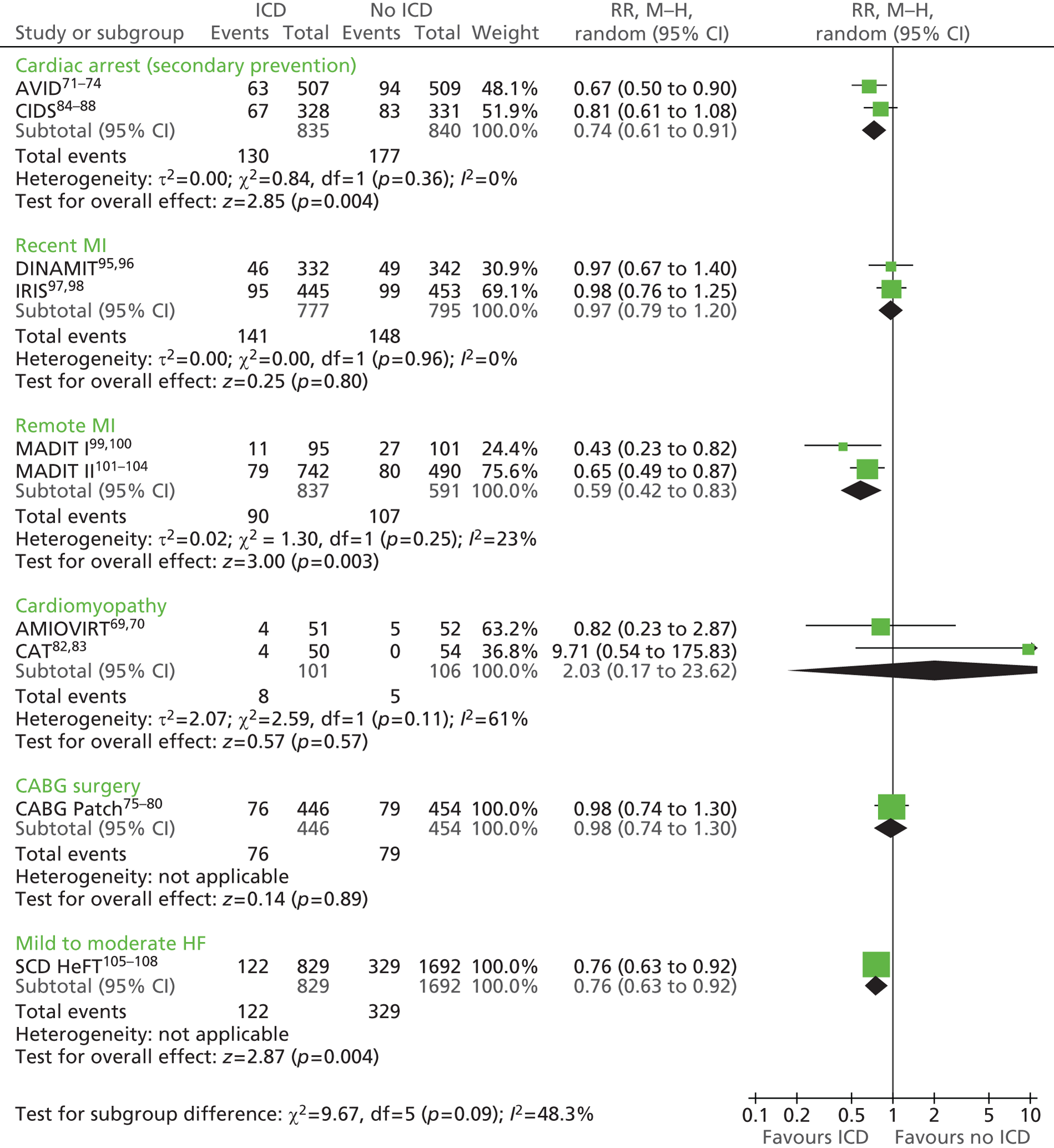
Eight trials69,78,82,95,97,99,101,108 in people who had not suffered a life-threatening arrhythmia but who were at increased risk assessed the effects of ICDs + OPT compared with either OPT, amiodarone + OPT or placebo + OPT on total cardiac deaths (see Table 18). Of these, only the MADIT II trial,103 which included people remote from MI (ICD + OPT 10.6%, OPT 16.3%, p < 0.01), and the SCD-HeFT trial,108 which included people with mild to moderate HF (ICD + OPT 14.7%, placebo + OPT 19.7%, amiodarone + OPT 19.2%; HR 0.76, 95% CI 0.60 to 0.95, p = 0.018), found statistically significant benefit for those receiving an ICD + OPT. A similar difference was identified in the MADIT I trial,99 which included people remote from MI (ICD + OPT 11.6%, OPT 26.7%); however, statistical significance was not stated. A meta-analysis of the MADIT I99 and II103 trials using a random-effects model showed a statistically significant benefit for ICDs + OPT, with a RR of 0.59 (95% CI 0.42 to 0.83, p = 0.003) and limited heterogeneity (χ2 = 1.3, df = 1, I2 = 23%) (see Figure 5).
The DINAMIT95 (ICD + OPT 13.9%, OPT 14.3%, p-value not stated) and IRIS97 (ICD + OPT 21.3%, OPT 21.9%, p-value not stated) trials, which included those with a recent MI, the AMIOVIRT trial,69 which included those with cardiomyopathy (ICD + OPT 7.8%, amiodarone + OPT 9.6%, p-value not stated) and the CABG Patch trial,78 which included people scheduled for a CABG (ICD + OPT 17.0%, OPT 17.4%; HR 0.97, 95% CI 0.71 to 1.33, p = 0.84) found limited differences in total cardiac deaths between those receiving ICD + OPT and those receiving either OPT or amiodarone + OPT (see Table 18). In contrast, the CAT trial,82 which included people with cardiomyopathy, reported higher total cardiac mortality among those receiving an ICD + OPT than among those receiving OPT (ICD + OPT 8%, OPT 0%), although the statistical significance was not stated. When these trials were meta-analysed by patient group using random-effects models, the lack of any statistically significant benefit was evident. Combining the DINAMIT95 and IRIS97 trials produced a RR of 0.97 (95% CI 0.79 to 1.20, p = 0.8) with no apparent heterogeneity (χ2 = 0, df = 1, I2 = 0%) (see Figure 5). The meta-analysis of the AMIOVIRT69 and CAT82 trials resulted in a RR of 2.03 (95% CI 0.17 to 23.62, p = 0.57) with some moderate heterogeneity (χ2 = 2.59, df = 1, I2 = 61%) (see Figure 5).
Sudden cardiac death/arrhythmic deaths
Sudden cardiac and arrhythmic death rates were lower among people receiving an ICD than among those receiving AADs in the four trials72,81,84,89 that included people at increased risk of SCD as a result of previous ventricular arrhythmias (Table 19). Both the CASH81 [ICD 13.0%, 95% CI 7.9 to 19.6; AAD (either amiodarone or metoprolol) 32.8%, 95% CI 27.2 to 41.8] and DEBUT89 (ICD 0%; AAD 13.8%) trials reported lower rates of SCD for those receiving an ICD than for those receiving AADs, although only the CASH trial81 showed a statistically significant difference. Similarly, the AVID72 and CIDS84 studies showed benefit for people receiving an ICD compared with AADs with regard to crude rate of arrhythmic deaths (AVID:72 ICD 4.7%, AAD 10.8%; CIDS84: ICD 9.2%, AAD 13.0%), although neither demonstrated a statistically significant difference. The CIDS trial84 also showed no statistically significant difference when comparing the interventions for annual crude mortality rate [ICD 3.0%, AAD 4.5%, RRR 32.8%; 95% CI –7.2 to 57.8, p = 0.094]. Combining the four studies through a random-effects meta-analysis showed a statistically significant benefit for the ICD group compared with the AAD group, with a RR of 0.49 (95% CI 0.34 to 0.69, p < 0.0001) and limited heterogeneity (χ2 = 5.47, df = 4, I2 = 27%) (Figure 6).
| Study | Follow-up | ICD, n/N (%) [rate/year, %] | OPT, n/N (%) [rate/year, %] | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| Cardiac arrest | |||||
| AVID72 | Mean 18.2 (SD 12.2) months | 24/507 (4.7) | AAD: 55/509 (10.8) | ||
| CASH81 | 57 (SD 34) months | 13/99 (13.1, 95% CI 7.9 to 19.6)a | Amiodarone: 27/92 (29.3, CI 19.4 to 40.8)b | ||
| Metoprolol: 34/97 (35.1, CI 25.2 to 48.8)b | |||||
| Both: 62/189 (32.8, CI 27.2 to 41.8)a | |||||
| CIDS84 | Mean 3 years | 30/328 (9.1) [3.0] | Amiodarone: 43/331 (13.0) [4.5] | RRR 32.8% | –7.2 to 57.8, 0.094 |
| DEBUT89 pilot study | Max. 3 years after randomisation | 0/10 (0) | Propranolol: 3/10 (30.0) | ||
| DEBUT89 main study | 3 years | 0/37 (0) | Propranolol: 4/29 (13.8) | ||
| Early post MI | |||||
| DINAMIT95 | Average 30 (SD 13) months | 12/332 (3.6) [1.5] | OPT: 29/342 (8.5) [3.5] | HR 0.42 | 0.22 to 0.83, 0.009 |
| IRIS97 | Average 37 months | 27/445 (6.1) | OPT: 60/453 (13.2) | HR 0.55 | 0.31 to 1.00, 0.049 |
| Remote from MI | |||||
| MADIT I99 | Average 27 months | 3/95 (3.2) | OPT: 13/101 (12.9) | ||
| MADIT II103 | Average 20 months | 28/742 (3.8) | OPT: 49/490 (10.0) | < 0.01 | |
| Cardiomyopathy | |||||
| AMIOVIRT69 | Mean 2.0 (SD 1.3) years | 1/51 (2.0) | Amiodarone + OPT: 2/52 (3.8) | 0.7 | |
| CAT82 | 1 year (primary end point) | 0/50 (0) | OPT: 0/54 (0) | ||
| DEFINITE90 | Mean 29.0 (SD 14.4) months | 3/229 (1.3) | OPT: 14/229 (6.1) | HR 0.20 | 0.06 to 0.71, 0.006 |
| Scheduled for CABG | |||||
| CABG Patch78 | Mean 32 (SD 16) months | 15/446 (3.4) | OPT: 28/454 (6.2) | 0.55 | 0.29 to 1.03, 0.06 |
| HF | |||||
| SCD-HeFT108 | Median for surviving patients 45.5 (range 24 to 72.6) months | 38/829 (4.6) | Amiodarone + OPT: 80/845 (9.5) | ||
| Placebo + OPT: 98/847 (11.6) | |||||
FIGURE 6.
Sudden cardiac deaths/arrhythmic deaths.
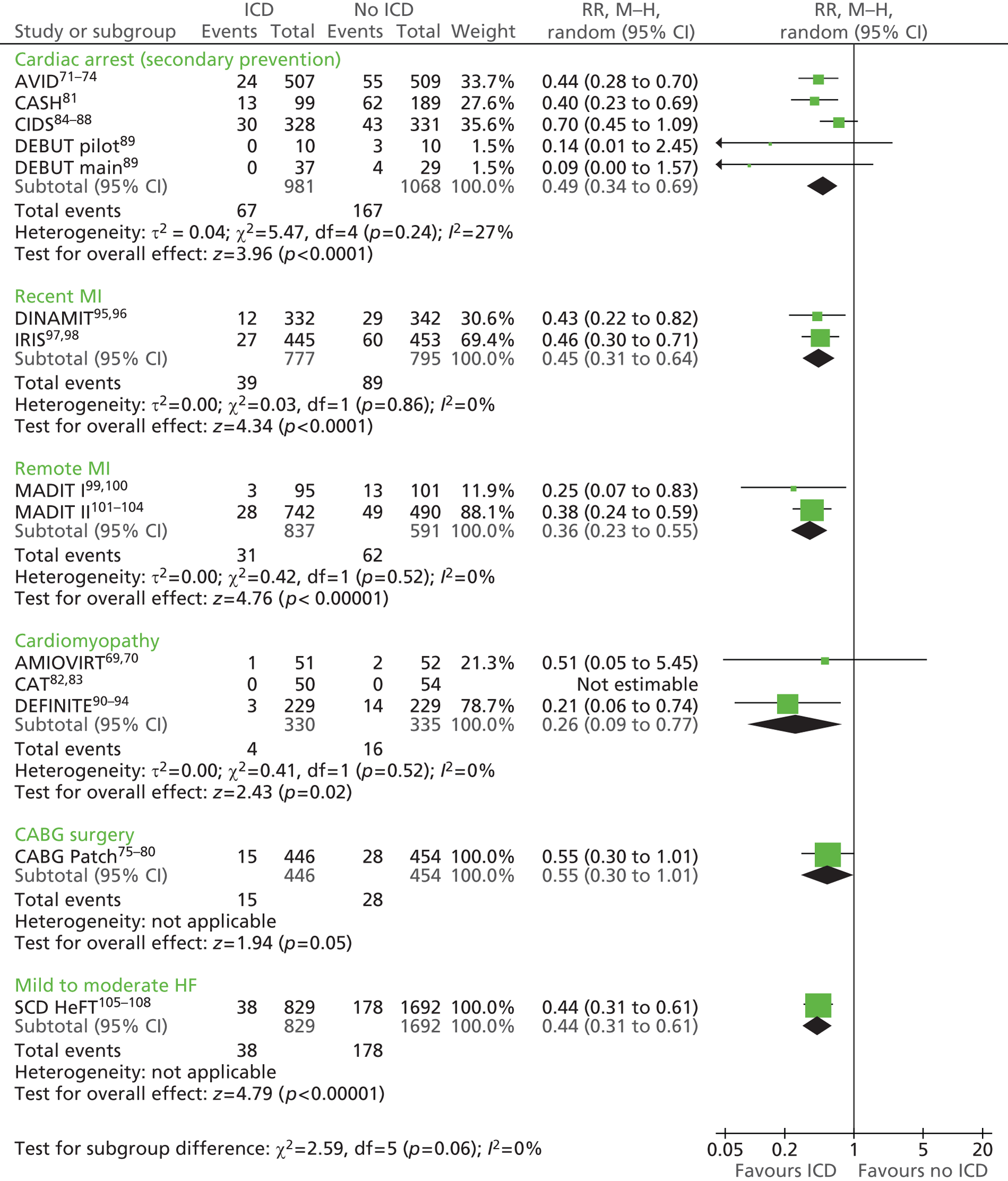
All nine trials69,78,82,90,95,97,99,103,108 that included people who had not suffered a life-threatening arrhythmia but who were at increased risk reported sudden cardiac or arrhythmic death as an outcome (see Table 19). Although eight of the trials69,78,90,95,97,99,103,108 showed benefit for those receiving an ICD + OPT compared with OPT, amiodarone + OPT or placebo + OPT, only four90,95,97,103 identified showed a statistically significant effect. The DINAMIT95 and IRIS97 trials highlighted the benefits of ICDs + OPT compared with OPT for people who had had a recent MI, reporting HRs of 0.42 (95% CI 0.22 to 0.83, p = 0.009) and 0.55 (95% CI 0.31 to 1.00, p = 0.049) respectively (see Table 19). When meta-analysed, a combined RR of 0.45 (95% CI 0.31 to 0.64, p < 0.0001) resulted, with no heterogeneity reported (χ2 = 0.03, df = 1, I2 = 0%) (see Figure 6).
The MADIT I99 (ICD + OPT 3.2%, OPT 12.9%, p-value not stated) and MADIT II103 (ICD + OPT 3.8%, OPT 10.0%, p < 0.01) trials, which included people remote from MI, showed lower rates of sudden cardiac or arrhythmic death among those receiving an ICD + OPT than among those receiving OPT. Meta-analysis using a random-effects model showed a significant benefit for ICDs + OPT with a RR of 0.36 (95% CI 0.23 to 0.55, p < 0.00001) and no heterogeneity (χ2 = 0.42, df = 1, I2 = 0%) (see Figure 6).
The AMIOVIRT,69 CAT82 and DEFINITE90 trials, which included people with cardiomyopathy, reported differing outcomes. The DEFINITE trial90 found that significantly fewer people who received an ICD + OPT (1.3%) died from sudden cardiac or arrhythmic death than those receiving OPT (6.1%), reflected in a HR of 0.20 (95% CI 0.06 to 0.71, p = 0.006) (see Table 19). Although the AMIOVIRT trial69 also found benefit for those receiving an ICD + OPT (2.0%) compared with those receiving amiodarone + OPT (3.8%), the benefit was not statistically significant (p = 0.7). The CAT trial82 reported no sudden cardiac or arrhythmic deaths in either the ICD + OPT group or the OPT group. A random-effects meta-analysis of the three trials showed an overall statistically significant benefit for participants who received an ICD + OPT compared with the comparator treatment, with a RR of 0.26 (95% CI 0.09 to 0.77, p = 0.02) and no heterogeneity (χ2 = 0.41, df = 1, I2 = 0%) (see Figure 6).
The CABG Patch trial,78 which included people who were scheduled for CABG surgery, reported lower rates of sudden cardiac and arrhythmic death in the ICD + OPT group (3.4%) than in the OPT (6.2%), although the difference was marginally insignificant (HR 0.55, 95% CI 0.29 to 1.03, p = 0.06) (see Table 19). In contrast, the SCD-HeFT trial108 found significantly lower sudden cardiac or arrhythmic mortality in the group receiving an ICD + OPT (4.6%) than in the group receiving amiodarone + OPT (9.5%) or the group receiving placebo + OPT (11.6%), with a RR of 0.44 (95% CI 0.31 to 0.61, p < 0.00001) (see Figure 6).
Non-arrhythmic cardiac deaths
Two trials72,84 that included people at increased risk of SCD because of previous ventricular arrhythmias reported rates of non-arrhythmic deaths. The AVID72 and CIDS84 trials assessed the effects of ICDs compared with the effects of AADs on crude non-arrhythmic cardiac deaths, with neither stating whether there was any statistically significant benefit (AVID72: ICDs 7.7%, AAD 7.7%; CIDS84: ICDs 11.3%, AAD 12.1%) (Table 20). The CIDS trial84 also reported annual crude mortality rates (ICDs 3.7%, AAD 4.2%), which resulted in a non-significant RRR of 13.5% (95% CI –35.4 to 44.7, p = 0.526). A random-effects meta-analysis confirmed the lack of a statistically significant difference between the groups, reporting a RR of 0.97 (95% CI 0.72 to 1.31, p = 0.83) and no heterogeneity (χ2 = 0.06, df = 1, I2 = 0%) (Figure 7).
| Study | Follow-up | ICD, n/N (%) [rate/year, %] | OPT, n/N (%) [rate/year, %] | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| AVID72 | Mean 18.2 (SD 12.2) months | 39/507 (7.7) | AAD: 39/509 (7.7) | ||
| CIDS84 | Mean 3 years | 37/328 (11.3) [3.7] | Amiodarone: 40/331 (12.1) [4.2] | RRR 13.5% | –35.4 to 44.7, 0.526 |
| Early post MI | |||||
| DINAMIT95 | Average 30 (SD 13) months | 34/332 (10.2) [4.1] | 20/342 (5.8) [2.4] | HR 1.72 | 0.99 to 2.99, 0.05 |
| IRIS97 | Average 37 months | 68/445 (15.3) | 39/453 (8.6) | HR 1.92 | 1.29 to 2.84, 0.001 |
| Remote from MI | |||||
| MADIT I99 | Average 27 months | 7/95 (7.4) | 13/101 (12.9) | ||
| MADIT II103 | Average 20 months | 43/742 (5.8) | 21/490 (4.3) | ||
| Cardiomyopathy | |||||
| AMIOVIRT69 | Mean 2.0 (SD 1.3) years | 3/51 (5.9) | Amiodarone + OPT: 3/52 (5.8) | 0.7 | |
| CAT82 | 1 year (primary end point) | 4/50 (8.0) | 0/54 (0) | ||
| DEFINITE90 | Mean 29.0 (SD 14.4) months | 9a/229 (3.9) | 11a/229 (4.8) | ||
| Scheduled for CABG | |||||
| CABG Patch78 | Mean 32 (SD 16) months | 57/446 (12.8) | 46/454 (10.1) | HR 1.24 | 0.84 to 1.84, 0.28 |
| HF | |||||
| SCD-HeFT108 | Median for surviving patients 45.5 (range 24 to 72.6) months | 81/829 (9.8) | Amiodarone + OPT: 77/845 (9.1), placebo + OPT: 68/847 (8.0) | ||
FIGURE 7.
Non-arrhythmic cardiac deaths.
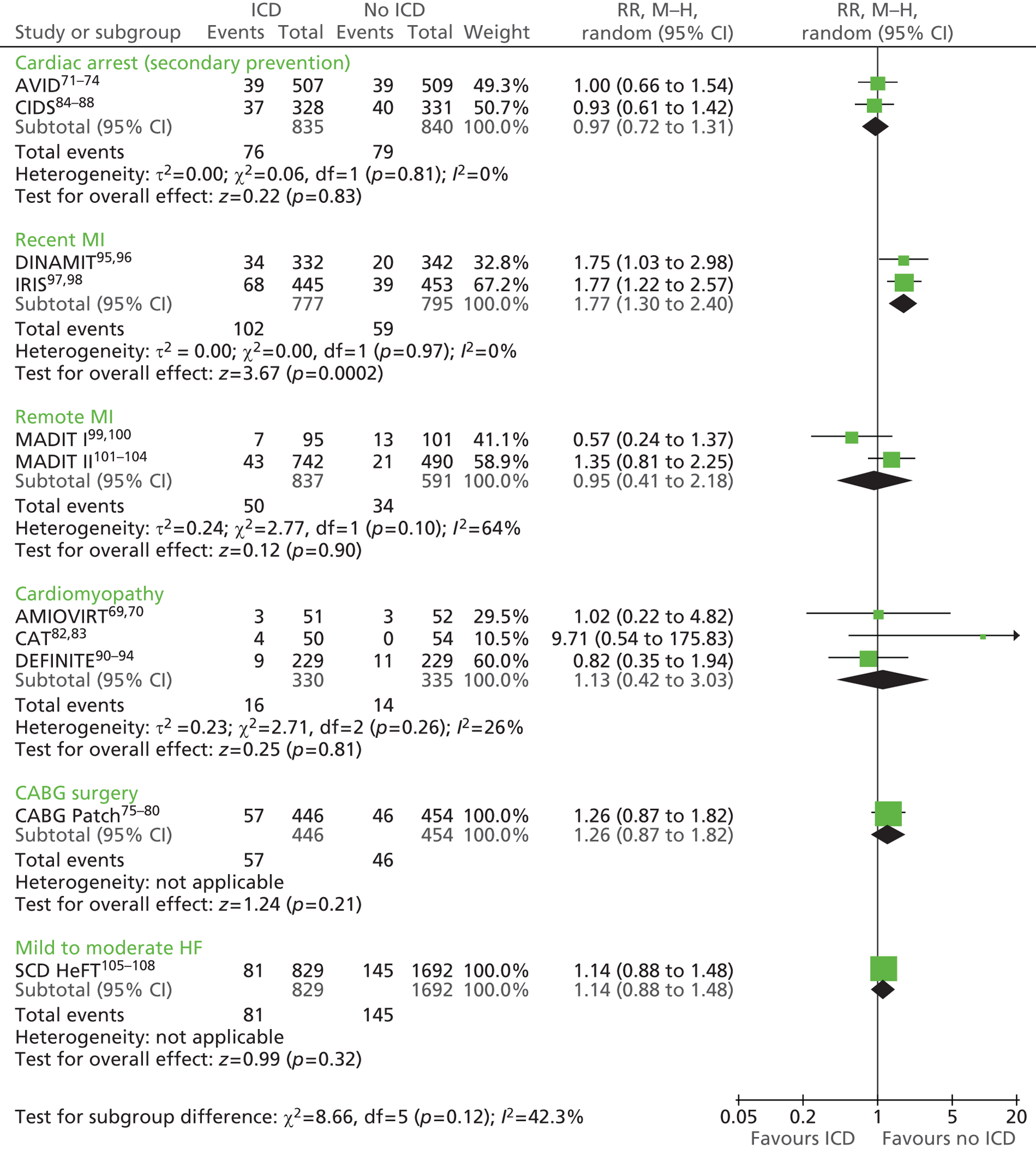
Implantable cardiac defibrillator + OPT appeared to have a limited effect on the occurrence of non-arrhythmic cardiac deaths compared with OPT, amiodarone + OPT or placebo + OPT in people who had not suffered a life-threatening arrhythmia but who were at increased risk (see Table 20). In people who had had a recent MI, the DINAMIT95 and IRIS trials97 found a statistically significant benefit for those receiving OPT only compared with those receiving an ICD + OPT, reporting HRs of 1.72 (95% CI 0.99 to 2.99, p = 0.05) and 1.92 (95% CI 1.29 to 2.84, p = 0.001) respectively. Combining the studies using a random-effects meta-analysis confirmed the statistically significant benefit for people receiving OPT, with a RR of 1.77 (95% CI 1.30 to 2.40, p = 0.0002) and no apparent heterogeneity (χ2 = 0, df = 1, I2 = 0%) (see Figure 7).
The effect of the different interventions on non-arrhythmic cardiac deaths in other patient subgroups was more equivocal. The MADIT I99 and MADIT II103 trials, which included people remote from MI, reported contrasting mortality rates (MADIT I:99 ICD + OPT 7.4%, OPT 12.9%; MADIT II:103 ICD + OPT 5.8%, OPT 4.3%). Meta-analysing these data using a random-effects model showed no statistically significant difference between the ICD + OPT group and the OPT group (RR 0.95, 95% CI 0.41 to 2.18, p = 0.9; χ2 = 2.77, df = 1, I2 = 64%) (see Figure 7). Similar variation was reported by the three trials assessing non-arrhythmic cardiac deaths among people with cardiomyopathy. The AMIOVIRT69 (ICD + OPT 5.9%, amiodarone + OPT 5.8%), CAT82 (ICD + OPT 8%, OPT 0%) and DEFINITE90 (ICD + OPT 3.9%, OPT 4.8%) trials reported differing mortality rates; when these data were meta-analysed there were no statistically significant differences between the groups (RR 1.13, 95% CI 0.42 to 3.03, p = 0.81; χ2 = 2.71, df = 2, I2 = 26%) (see Figure 7). Similarly, the CABG Patch trial,78 which included those who were scheduled for CABG surgery (RR 1.26, 95% CI 0.87 to 1.82, p = 0.21), and the SCD-HeFT trial,108 which included those with mild to moderate HF (RR 1.14, 95% CI 0.88 to 1.48, p = 0.32) found no statistically significant differences between the groups (see Figure 7).
Other causes of death: non-cardiac deaths
Two trials72,84 in people at increased risk of SCD because of previous ventricular arrhythmias assessed non-cardiac causes of death as an outcome (Table 21). The AVID72 and CIDS84 trials found no statistically significant difference between ICDs and AADs for the outcome of other non-cardiac causes of death (AVID:72 ICD 3.4%, AAD 5.5%, RR 1.78, 95% CI 0.98 to 3.26, p = 0.053; CIDS:84 non-cardiac vascular: ICD 0.9%, AAD 0.6%, RRR –36.6%, 95% CI –719.8 to 77.2, p = 0.732; non-vascular: ICD 4.0%, AAD 3.9%, RRR 4.5%, 95% CI –106.1 to 55.7, p = 0.908) (see Table 21), reflected in a random-effects meta-analysis (RR 0.79, 95% CI 0.45 to 1.37, p = 0.40; χ2 = 1.51, df = 1, I2 = 34%) (Figure 8). The CIDS trial84 presented annual crude death rates for the ICD and AAD groups for non-cardiac vascular (ICD 0.3%, AAD 0.2%) and non-vascular (ICD 1.3%, AAD 1.4%) causes,84 finding limited differences.
| Study | Outcome and follow-up | ICD, n/N (%) [rate/year, %] | OPT, n/N (%) [rate/year, %] | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| Cardiac arrest | |||||
| AVID72 | Mean 18.2 (SD 12.2) months | 17/507 (3.4) | AAD: 28/509a (5.5) | RR 1.78 | 0.98 to 3.26, 0.053 |
| CIDS84 | Non-cardiac vascular deaths, mean 3 years | 3/328 (0.9) [0.3] | Amiodarone: 2/331 (0.6) [0.2] | RRR –36.6% | –719.8 to 77.2, 0.732 |
| Non-vascular deaths, mean 3 years | 13/328 (4.0) [1.3] | 13/331 (3.9) [1.4] | RRR 4.5% | –106.1 to 55.7, 0.908 | |
| Early post MI | |||||
| DINAMIT95 | Non-cardiac vascular deaths, average 30 (SD 13) months | 5/332 (1.5) [0.6] | 3/342 (0.9) [0.4] | HR 1.69 | 0.40 to 7.06, 0.47 |
| Non vascular deaths, average 30 (SD 13) months | 11/332 (3.3) [1.3] | 6/342 (1.8) [0.7] | HR 1.85 | 0.68 to 5.01, 0.22 | |
| IRIS97 | Average 37 months | 21/445 (4.7) | 18/453 (4.0) | HR 1.23 | 0.51 |
| Remote from MI | |||||
| MADIT I99 | Non-cardiac deaths, average 27 months | 4/95 (4.2) | 6/101 (5.9) | ||
| Unknown (cardiac or non-cardiac deaths), average 27 months | 0/95 (0) | 6/101 (5.9) | |||
| MADIT II103 | Non-cardiac deaths, average 20 months | 22/742 (3.0) | 12/490 (2.4) | ||
| Unknown (cardiac or non-cardiac deaths), average 20 months | 4/742 (0.5) | 5/490 (1.0) | |||
| Cardiomyopathy | |||||
| AMIOVIRT69 | Mean 2.0 (SD 1.3) years | 2/51 (3.9) | Amiodarone + OPT: 2/52 (3.8) | 0.9 | |
| CAT82 | 1 year (primary end point) | 0/50 (0) | 2/54 (3.7) | ||
| Scheduled for CABG | |||||
| CABG Patch78 | Non-cardiac deaths, mean 32 (SD 16) months | 25/446 (5.6) | 17/454 (3.7) | HR 1.49 | 0.80 to 2.76, 21 |
| Unknown deaths | 1/446 (0.2) | 0/454 (0) | |||
| HF | |||||
| SCD-HeFT108 | Non-cardiac deaths, median for surviving patients 45.5 (range 24–72.6) months | 48/829 (5.8) | Amiodarone + OPT: 54/845 (6.4) | ||
| Placebo + OPT: 53/847 (6.3) | HR 0.80b | 0.57 to 1.12, NS | |||
| Unknown deaths, median for surviving patients 45.5 (range 24–72.6) months | 12/829 (1.4) | Amiodarone + OPT: 24/845 (2.8) | NS | ||
| Placebo + OPT 24/847 (2.8) | |||||
FIGURE 8.
Other causes of death: non-cardiac deaths.
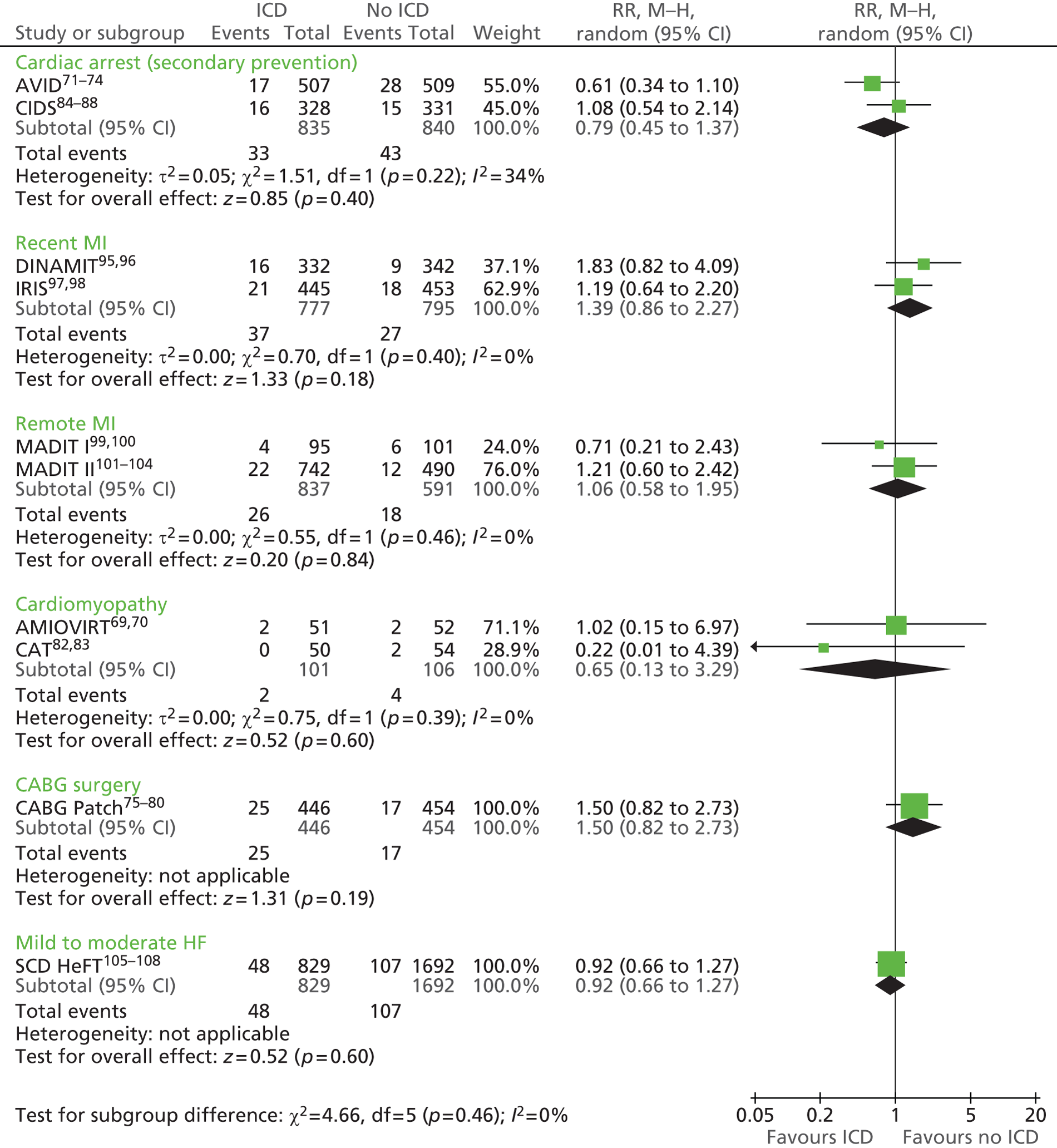
Eight trials69,78,82,95,97,99,103,108 in people who had not suffered a life-threatening arrhythmia but who were at increased risk assessed the effects of ICDs + OPT on other non-cardiac causes of death compared with the different comparator treatments, finding no statistically significant benefit (see Table 21). Meta-analyses using random-effects models of the DINAMIT95 and IRIS97 trials in people with a recent MI (RR 1.39, 95% CI 0.86 to 2.27, p = 0.18; χ2 = 0.70, df = 1, I2 = 0%), the MADIT I99 and MADIT II103 trials in people remote from MI (RR 1.06, 95% CI 0.58 to 1.95, p = 0.84; χ2 = 0.55, df = 1, I2 = 0%) and the AMIOVIRT69 and CAT82 trials in people with cardiomyopathy (RR 0.65, 95% CI 0.13 to 3.29, p = 0.60; χ2 = 0.75, df = 1, I2 = 0%) all found no statistically significant effects (see Figure 8). Similarly, the CABG Patch trial78 in people who were scheduled for CABG surgery (RR 1.50, 95% CI 0.82 to 2.73, p = 0.19) and the SCD-HeFT108 trial, which included people with mild-to moderate HF (RR 0.92, 95% CI 0.66 to 1.27, p = 0.60), reported no statistically significant differences between groups in deaths from other non-cardiac causes (see Figure 8).
Cumulative mortality
The cumulative mortality risk for both total and arrhythmic mortality was assessed annually for up to 3 years’ follow-up in the CIDS trial in people at increased risk of sudden cardiac death as a result of previous ventricular arrhythmias. 84 Rates were consistently lower for those receiving an ICD compared with those receiving AADs, with a RRR for total mortality of 15.4% in year 1, 29.7% in year 2 and 13.7% in year 3 and a RRR for arrhythmic mortality of 29.9% in year 1, 31.4% in year 2 and 17.8% in year 3 (Table 22).
| Study | Outcome | ICD | OPT | Effect |
|---|---|---|---|---|
| Cardiac arrest | ||||
| CIDS84 | Cumulative risks over time, total mortality, % | Amiodarone: | ||
| 1 year | 9.46 | 11.18 | ARR 1.72, RRR 15.4 | |
| 2 years | 14.75 | 20.97 | ARR 6.22, RRR 29.7 | |
| 3 years | 23.32 | 27.03 | ARR 3.71, RRR 13.7 | |
| Cumulative risks over time, arrhythmic mortality, % | ||||
| 1 year | 4.37 | 6.23 | ARR 1.86, RRR 29.9 | |
| 2 years | 6.68 | 9.74 | ARR 3.06, RRR 31.4 | |
| 3 years | 9.77 | 11.88 | ARR 2.11, RRR 17.8 | |
| Cardiomyopathy | ||||
| DEFINITE90 | All-cause mortality rate at 1 year, % | 2.6 | 6.2 | |
| All-cause mortality rate at 2 years, % | 7.9 | 14.1 | ||
| Early post MI | ||||
| IRIS97 | Cumulative 1-year death rate, %a | 10.6 | 12.5 | |
| Cumulative 2-year death rate, %a | 15.4 | 18.2 | ||
| Cumulative 3-year death rate, %a | 22.4 | 22.9 | ||
| Scheduled for CABG | ||||
| CABG Patch75 | Actuarial mortality by 4 years’ follow-up, % | 27 | 24 | p-value 0.64 |
| HR for death per unit time | 1.07 (95% CI 0.81 to 1.42) | |||
| HF | ||||
| SCD-HeFT105 | Kaplan–Meier estimates of death from any cause, 5-year event rate | 0.289 | Amiodarone + OPT: 0.340 | |
| Placebo + OPT: 0.361 | ||||
Four trials in people who had not suffered a life-threatening arrhythmia but who were at increased risk reported other mortality outcomes. 75,90,97,105 The IRIS trial97 in people with a recent MI presented cumulative death rates annually up to 3 years (see Table 22). Although this trial found lower mortality rates for those receiving an ICD + OPT (year 1 10.6%, year 2 15.4%, year 3 22.4%) than for those receiving OPT (year 1 12.5%, year 2 18.2%, year 3 22.9%), the differences were not found to be statistically significant (p = 0.76). Similarly, the DEFINITE trial,90 which included people with cardiomyopathy (year 1: ICD + OPT 2.6%, OPT 6.2%; year 2: ICD + OPT 7.9%, OPT 14.1%), and the SCD-HeFT trial,105 which included people with mild to moderate HF (Kaplan–Meier estimate, 5 years: ICD + OPT 0.289; amiodarone + OPT 0.340; placebo + OPT 0.361), also reported lower all-cause mortality following implantation of an ICD (p-values not stated). In contrast, the CABG Patch trial,75 which included people scheduled for CABG surgery, reported higher actuarial mortality at 4 years’ follow-up in those receiving an ICD + OPT (27%) than in those receiving OPT (24%), although the difference was not statistically significant (HR 1.07, 95% CI 0.81 to 1.42, p = 0.64) (see Table 22).
Survival
Differences in mortality were reflected in the survival outcomes reported by the AVID71,72 and CASH81 trials in people at increased risk of SCD as a result of previous ventricular arrhythmias. The AVID trial reported statistically significant differences in overall survival during the 3 years of follow-up (p < 0.02),71 survival free of cardiac death at 2 years (p = 0.0042)72 and survival to arrhythmic death at 2 years (p = 0.0002),72 favouring ICDs compared with AAD (Table 23). Survival free of non-arrhythmic cardiac death did not differ significantly between those receiving ICDs and those receiving AADs (p = 0.8039). 72 Despite the CASH trial81 finding benefits for ICDs compared with AADs for overall survival (HR 0.766, p = 0.081) and survival free of cardiac arrest (HR 0.481, p = 0.072), the differences were not statistically significant. In contrast, the CASH trial81 did report a significant benefit for survival free of sudden death for people who received an ICD compared with those who received AADs (HR 0.423, p = 0.005). The DEBUT trial89 reported a mean survival time for the AAD group of 26.2 [standard error of the mean (SEM) 1.4] months (no deaths in the ICD group).
| Study | Outcome and follow-up | ICD, n/N (%) | OPT, n/N (%) | p-value |
|---|---|---|---|---|
| Cardiac arrest | ||||
| AVID71 | Overall survival, %, mean 18.2 (SD 12.2) months | AAD: | < 0.02 | |
| 1 year | 89.3 | 82.3 | ||
| 2 years | 81.6 | 74.7 | ||
| 3 years | 75.4 | 64.1 | ||
| aSurvival free of cardiac death, %72 | 0.0042 | |||
| At 1 year | 90.9 | 85.1 | ||
| At 2 years | 85.0 | 81.2 | ||
| bSurvival to arrhythmic death, %72 | 0.0002 | |||
| At 1 year | 96.6 | 91.9 | ||
| At 2 years | 94.2 | 89.1 | ||
| Survival free of non-arrhythmic cardiac deathc | Presented in figure only | Presented in figure only | 0.8039 | |
| CASH81 | 57 (SD 34) months | AAD: | ||
| Overall survival, ICD vs. amiodarone/metoprolol | HR 0.766 | 97.5% CI upper bound 1.112, 0.081 | ||
| Survival free of sudden death, ICD vs. amiodarone/metoprolol | HR 0.423 | 97.5% CI upper bound 0.721, 0.005 | ||
| Survival free of cardiac arrest, ICD vs. amiodarone/metoprolol | HR 0.481 | 97.5% CI upper bound 1.338, 0.072 | ||
| DEBUT89 main study | 3 years | |||
| Mean (SEM) survival (months) | 26.2 (1.4) | |||
| Cardiomyopathy | ||||
| AMIOVIRT69 | Survival rate, % | 0.8d | ||
| 1 year | 96 | Amiodarone + OPT: 90 | ||
| 3 years | 88 | Amiodarone + OPT: 87 | ||
| Arrhythmia-free survival rate, % | 0.1e | |||
| 1 year | 78 | 82 | ||
| 3 years | 63 | 73 | ||
| CAT82 | Cumulative survival, % | 0.554 | ||
| 2 years | 92 | 93 | ||
| 4 years | 86 | 80 | ||
| 6 years | 73 | 68 | ||
Only the AMIOVIRT69 and CAT82 trials, which included people with cardiomyopathy, reported survival (see Table 23). The AMIOVIRT trial69 presented overall and arrhythmia-free survival rates for the ICD + OPT group and the amiodarone + OPT group at 1 and 3 years’ follow-up, showing no statistically significant difference (overall survival p = 0.1, arrhythmia-free survival p = 0.8). The CAT trial82 presented cumulative survival data for the ICD + OPT group and the OPT group for up to 6 years’ follow-up, finding no statistically significant difference between the groups (p = 0.554) (see Table 23).
Heart failure hospitalisations
Only the AVID study,71 which included people at increased risk of SCD because of previous ventricular arrhythmias, reported the proportion of patients rehospitalised annually for up to 3 years’ follow-up. Significantly higher rates were reported for the ICD group than for the AAD group (p = 0.04) (Table 24). For both groups, the rehospitalisation rate was > 55% at year 1, > 65% at year 2 and > 75% at year 3.
| Study | Outcome | ICD | OPT | p-value |
|---|---|---|---|---|
| Cardiac arrest | ||||
| AVID71 | % of patients rehospitalised (patients at risk N = 1011) | 0.04 | ||
| At 1 year | 59.5 | 55.6 | ||
| At 2 years | 74.8 | 64.7 | ||
| At 3 years | 83.3 | 75.5 | ||
| Remote from MI | ||||
| MADIT II101 | Hospitalisation because of HF, n (%) | 148 (19.9) | 73 (14.9) | |
| Patients hospitalised per 1000 months of active follow-up | 11.3 | 9.4 | 0.09 | |
The MADIT II trial,101 which included people remote from MI, reported the proportion of hospitalisations because of HF (ICD + OPT 19.9%, OPT 14.9%, p-value not stated) and the number of patients hospitalised per 1000 months of follow-up (ICD + OPT 11.3, OPT 9.4, p = 0.09), with higher rates among those receiving an ICD + OPT (see Table 24).
Symptoms/complications related to arrhythmias
The CAT82 and AMIOVIRT69 trials, which included people with cardiomyopathy, reported the occurrence of syncope. Some 12% of people with an ICD + OPT had syncope during VTs in the CAT trial82 and 3.9% of ICD + OPT patients and 5.8% of amiodarone + OPT patients had syncope in the AMIOVIRT study69 (Table 25). The MADIT II trial,103 which included people remote from MI, reported the number of adverse cardiac events in the week before SCD (ICD + OPT 28, OPT 49), with comparable rates of syncope and angina pectoris (4% for both), lower rates of MI for the ICD + OPT group (ICD + OPT 4%, OPT 10%) and higher rates of ventricular arrhythmia (ICD + OPT 25%, OPT 10%) and CHF (ICD + OPT 43%, OPT 16%) for the ICD + OPT group.
| Study | Outcome | ICD | OPT | Effect (HR) |
|---|---|---|---|---|
| Cardiomyopathy | ||||
| CAT82 | Syncope during VT, n/N (%) | 6/50 (12) | ||
| AMIOVIRT69 | Syncope, % | 3.9a | 5.8 | 0.7 |
| Remote from MI | ||||
| MADIT II103 | Adverse cardiac events in week before SCD, n | 28 | 49 | |
| Syncope, % | 4 | 4 | ||
| Angina pectoris, % | 4 | 4 | ||
| MI, % | 4 | 10 | ||
| Ventricular arrhythmia, % | 25 | 10 | ||
| CHF, % | 43 | 16 | ||
Quality of life
Two trials in people at increased risk of SCD because of previous ventricular arrhythmias, the AVID74 and CIDS87 trials, reported results from substudies using a range of generic and condition-specific measures of QoL (Table 26). The AVID trial74 assessed QoL using the Short Form questionnaire-36 items (SF-36) physical component summary (PCS) and mental component summary (MCS), the 46-item patient concerns checklist and the cardiac version of the QL index. Follow-up was for 12 months and assessments were made of the impact of adverse symptoms and ICD shocks. Comparison of PCS scores at baseline and 12 months’ follow-up showed no statistically significant differences between the ICD group and the AAD group (baseline: ICD 37.4, AAD 36.5, p = 0.3; 12 months: ICD 40.0, AAD 38.0, p = 0.3). In contrast, the ICD group had a lower (worse) mean score on the MCS at baseline than the AAD group, which was statistically significant (p = 0.006), although any difference had disappeared by 12 months’ follow-up. Scores on the patient concerns checklist did not differ significantly between the ICD group and the AAD group at baseline (ICD 15.9, AAD 16.2, p = 0.06) or at 12 months’ follow-up (p = 0.1). On the QL index the scores for the ICD and AAD groups were similar at baseline (ICD 22.1, AAD 21.9, p-value not stated) and at 12 months’ follow-up (scores and p-values not stated).
| Study | Outcome and follow-up | Intervention | Comparator(s) | 95% CI, p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac arrest (secondary prevention) | ||||||||||
| AVID74 | 1 year | (n = 416) | AAD (n = 384) | |||||||
| SF-36 PCS score, mean (SD) | ||||||||||
| Baseline | 37.4 (10.9) | 36.5 (11.2) | 0.3 | |||||||
| 12 months | 40 (10.5) a | 38 (17) a | ||||||||
| SF-36 MCS score, mean (SD) | ||||||||||
| Baseline | 45.9 (11.8) | 47.5 (11.5) | 0.006 | |||||||
| 12 months | 49 (16.5) a | 48 (17) a | ||||||||
| Patient concerns checklist, mean (SD) | ||||||||||
| Baseline | 15.9 (8.6) | 16.2 (8.9) | 0.06 | |||||||
| 12 months | NR | NR | 0.1 | |||||||
| QL index, mean (SD) | ||||||||||
| Baseline | 22.1 (4.9) | 21.9 (5.0) | ||||||||
| Impact of adverse symptoms on QoLb | ||||||||||
| SF-36 PCS score | –2.25 (–3.32 to –1.18), p < 0.001 | –1.64 (–2.89 to –0.41), p = 0.009 | ||||||||
| SF-36 MCS score | –2.32 (–3.76 to –0.88), p = 0.002 | –0.51 (–1.97 to 0.94), p = 0.5 | ||||||||
| Patient concerns | 1.84 (0.91 to 2.76), p < 0.001 | 0.91 (0.07 to 1.75), p = 0.03 | ||||||||
| Impact of ICD shocks on QoLb | ||||||||||
| SF-36 PCS score | –1.45 (–2.74 to –0.18), p = 0.03 | |||||||||
| SF-36 MCS score | –1.82 (–3.56 to –0.08), p = 0.04 | |||||||||
| Patient concerns | 2.15 (1.07 to 3.23), p < 0.001 | |||||||||
| CIDS87 | (n = 86) | Amiodarone (n = 92) | Time by group p-value | |||||||
| Domains of MHI, mean (SD) | ||||||||||
| Total indexc | ||||||||||
| Baseline | 173.2 (25.5) | 180.4 (27.8) | ||||||||
| 6 months | 183.1 (30.2) | 180.2 (31.1) | ||||||||
| 12 months | 184.3 (27.9) | 178.3 (28.7) | 0.001 | |||||||
| Psychological distressd | ||||||||||
| Baseline | 51.3 (14.1) | 47.8 (16.5) | ||||||||
| 6 months | 45.1 (17.6) | 47.6 (18.3) | ||||||||
| 12 months | 43.4 (15.9) | 48.8 (16.8) | 0.001 | |||||||
| Psychological well-beingc | ||||||||||
| Baseline | 58.5 (12.7) | 62.2 (12.3) | ||||||||
| 6 months | 62.2 (13.4) | 61.8 (14.1) | ||||||||
| 12 months | 61.7 (13.2) | 61.3 (13.3) | 0.03 | |||||||
| Domains of Nottingham Health Profile, mean (SD) | ||||||||||
| Energy leveld | (n = 83) | (n = 88) | ||||||||
| Baseline | 27.5 (32.2) | 24.4 (32.4) | ||||||||
| 6 months | 18.6 (30.1) | 27.8 (32.1) | ||||||||
| 12 months | 17.7 (26.1) | 36.8 (37.3) | 0.0001 | |||||||
| Physical mobility | (n = 84) | (n = 90) | ||||||||
| Baseline | 10.9 (12.0) | 13.2 (20.5) | ||||||||
| 6 months | 10.5 (13.7) | 15.1 (19.2) | ||||||||
| 12 months | 9.1 (13.6) | 17.7 (19.2) | 0.002 | |||||||
| Social isolationd | (n = 81) | (n = 88) | ||||||||
| Baseline | 8.5 (15.4) | 9.9 (17.7) | ||||||||
| 6 months | 9.8 (18.6) | 12.2 (22.4) | ||||||||
| 12 months | 8.5 (18.4) | 11.1 (22.6) | 0.9 | |||||||
| Emotional reactionsd | (n = 76) | (n = 86) | ||||||||
| Baseline | 17.3 (18.1) | 14.3 (20.1) | ||||||||
| 6 months | 11.1 (18.2) | 15.3 (22.4) | ||||||||
| 12 months | 8.3 (16.6) | 14.5 (19.6) | 0.002 | |||||||
| Paind | (n = 83) | (n = 90) | ||||||||
| Baseline | 4.4 (7.9) | 7.5 (15.1) | ||||||||
| 6 months | 7.5 (17.1) | 6.3 (13.6) | ||||||||
| 12 months | 4.5 (9.9) | 8.2 (15.4) | 0.52 | |||||||
| Sleep disturbanced | (n = 78) | (n = 88) | ||||||||
| Baseline | 31.4 (27.4) | 29.6 (31.5) | ||||||||
| 6 months | 25.0 (29.7) | 30.8 (31.0) | ||||||||
| 12 months | 23.9 (29.4) | 30.2 (32.4) | 0.02 | |||||||
| Life impairmentd | (n = 78) | (n = 83) | ||||||||
| Baseline | 2.0 (1.9) | 1.6 (1.7) | ||||||||
| 6 months | 1.6 (1.8) | 1.9 (1.9) | ||||||||
| 12 months | 1.6 (1.3) | 1.8 (1.9) | 0.005 | |||||||
| Effects of ICD shocks on HRQoL scores 87 | ICDs: no shocks (n = 66) | ICDs: 1–4 shocks (n = 27) | ICDs: ≥ 5 shocks (n = 15) | Amiodarone (n = 95) | Between-group p-value | |||||
| Domains of MHI, mean (SD) | ||||||||||
| Total indexc | ||||||||||
| Baseline | 175.9 (26.5) | 171.7 (22.7) | 171.2 (32.0) | 177.9 (27.1) | ||||||
| 12 months | 186.2 (26.9)e,f | 186.6 (21.7)e,f | 168.8 (41.2) | 175.6 (29.2) | 0.001 | |||||
| Within-group p-value | 0.001 | 0.001 | 0.725 | |||||||
| Psychological distressd | ||||||||||
| Baseline | 50.2 (15.2) | 50.8 (12.3) | 51.9 (18.1) | 49.8 (16.3) | ||||||
| 12 months | 42.5 (15.3)e,f | 41.4 (11.7)e,f | 52.7 (25.2) | 50.9 (17.5) | 0.001 | |||||
| Within-group p-value | 0.001 | 0.001 | 0.833 | |||||||
| Psychological well-beingc | ||||||||||
| Baseline | 60.1 (12.5) | 56.6 (11.6) | 57.1 (15.0) | 61.7 (12.0) | ||||||
| 12 months | 62.8 (13.1) | 62.1 (10.9)f | 55.6 (16.8) | 60.6 (13.3) | 0.02 | |||||
| Within-group p-value | 0.074 | 0.004 | 0.642 | |||||||
| Domains of NHP, mean (SD) | ||||||||||
| Energy leveld | (n = 64) | (n = 27) | (n = 15) | (n = 90) | ||||||
| Baseline | 28.6 (32.5) | 28.5 (30.5) | 22.6 (34.2) | 24.3 (30.8) | ||||||
| 12 months | 19.5 (27.1)e | 24.8 (33.4)e | 23.5 (29.5) | 37.0 (37.6) | 0.003 | |||||
| Within-group p-value | 0.02 | 0.115 | 0.859 | |||||||
| Physical mobilityd | (n = 65) | (n = 27) | (n = 15) | (n = 93) | ||||||
| Baseline | 13.1 (15.0) | 12.4 (10.2) | 7.1 (9.8) | 13.18 (20.1) | ||||||
| 12 months | 9.3 (12.4)e | 15.5 (17.3) | 8.0 (13.3) | 17.2 (19.1) | 0.02 | |||||
| Within-group p-value | 0.05 | 0.638 | 0.747 | |||||||
| Social isolationd | (n = 66) | (n = 27) | (n = 15) | (n = 92) | ||||||
| Baseline | 10.6 (16.7) | 4.3 (9.2) | 8.9 (16.1) | 11.8 (18.5) | ||||||
| 12 months | 8.8 (19.5) | 6.4 (15.5) | 12.8 (23.9) | 12.5 (23.0) | 0.57 | |||||
| Within-group p-value | 0.03 | 0.991 | 0.817 | |||||||
| Emotional reactionsd | (n = 61) | (n = 27) | (n = 14) | (n = 90) | ||||||
| Baseline | 16.2 (17.4) | 16.3 (17.1) | 21.6 (21.1) | 16.3 (19.8) | ||||||
| 12 months | 7.1 (14.6)e,f | 6.8 (10.2)e | 22.0 (31.0) | 15.9 (20.3) | 0.001 | |||||
| Within-group p-value | 0.001 | 0.02 | 0.886 | |||||||
| Paind | (n = 66) | (n = 27) | (n = 15) | (n = 92) | ||||||
| Baseline | 6.8 (11.8) | 4.0 (8.5) | 5.3 (8.3) | 8.5 (15.6) | ||||||
| 12 months | 6.4 (14.7) | 5.4 (11.7) | 5.5 (7.1) | 7.7 (14.5) | 0.71 | |||||
| Within-group p-value | 0.086 | 0.710 | 0.721 | |||||||
| Sleep disturbanced | (n = 62) | (n = 27) | (n = 14) | (n = 89) | ||||||
| Baseline | 30.0 (26.9) | 36.3 (31.4) | 27.3 (27.1) | 30.4 (30.5) | ||||||
| 12 months | 22.1 (28.1) | 29.1 (33.9) | 34.6 (35.4) | 30.1 (33.6) | 0.3 | |||||
| Within-group p-value | 0.002 | 0.042 | 0.680 | |||||||
| Lifestyle impairmentd | (n = 65) | (n = 26) | (n = 14) | (n = 82) | ||||||
| Baseline | 2.0 (2.0) | 2.4 (1.9) | 2.2 (1.9) | 1.7 (1.6) | ||||||
| 12 months | 1.3 (1.5)e | 1.4 (1.5)e | 1.4 (1.6) | 1.9 (1.9) | 0.03 | |||||
| Within-group p-value | 0.061 | 0.033 | 0.334 | |||||||
| Remote from MI | ||||||||||
| MADIT II104 | HUI3 scores while alive, 36 months | (n = 658) | (n = 431) | |||||||
| Baseline mean | 0.637 | 0.646 | ||||||||
| Baseline overall mean score including deathg | 0.637 | 0.646 | ||||||||
| Year 1, proportion alive | 0.93 | 0.903 | ||||||||
| Mean | 0.627 | 0.659 | ||||||||
| Mean annual changeh | –0.019 | –0.012 | ||||||||
| Overall mean score including deathg | 0.584 | 0.595 | ||||||||
| Year 2, proportion alive | 0.846 | 0.792 | ||||||||
| Mean | 0.622 | 0.667 | ||||||||
| Mean annual changeh | –0.027i | –0.011 | ||||||||
| Overall mean score including deathg | 0.526 | 0.529 | ||||||||
| Year 3, proportion alive | 0.767 | 0.667 | ||||||||
| Mean | 0.601 | 0.678 | ||||||||
| Mean annual changeh | –0.019j | –0.013 | ||||||||
| Overall mean score including deathg | 0.461 | 0.452 | ||||||||
| Cardiomyopathy | ||||||||||
| AMIOVIRT69 | 1 year | (n = 51) | Amiodarone + OPT (n = 52) | |||||||
| QWBS, mean (SD) | 74 (19) | 70 (22) | 0.5k | |||||||
| State–Trait Anxiety Inventory, mean (SD) | 61 (17) | 67 (20) | 0.4k | |||||||
| DEFINITE94 | (n = 227) | (n = 226) | ||||||||
| SF-12 | ||||||||||
| Long-term MCS scores | 0.89 | |||||||||
| Long-term PCS scores | NS | |||||||||
| Long-term MLWHFQ subscale scores | NS | |||||||||
| Scheduled for CABG | ||||||||||
| CABG Patch80 | 6 months | (n = 262) | (n = 228) | p-valuel | ||||||
| HRQoL, mean (SD) | ||||||||||
| Perception of health | ||||||||||
| General health status | 54.8 (22.9) | 58.3 (23.6) | NS | |||||||
| Perception of health transitionm | 2.4 (1.2) | 2.1 (1.2) | 0.030 | |||||||
| Physical limitations | 41.7 (42.3) | 49.2 (42.8) | 0.055 | |||||||
| Bodily pain | 57.4 (24.6) | 58.8 (24.8) | NS | |||||||
| Ability to function | ||||||||||
| Employment status | 0.25 (0.4) | 0.29 (0.5) | NS | |||||||
| Physical role functioning | 58.3 (27.5) | 61.8 (28.3) | NS | |||||||
| Emotional role functioning | 55.4 (43.4) | 67.3 (39.9) | 0.003 | |||||||
| Social functioning | 70.5 (27.2) | 70.8 (26.4) | NS | |||||||
| Psychological well-being | ||||||||||
| Mental health | 72.5 (18.3) | 77.2 (17.0) | 0.004 | |||||||
| Satisfaction with appearance | 6.0 (1.3) | 6.3 (1.1) | 0.008 | |||||||
| Satisfaction with scar | 7.0 (1.2) | 7.2 (1.1) | 0.040 | |||||||
| Received a shock prior to completing the 6-month QoL instrument, n/N (%) | 101/262 (38.5) | |||||||||
| ICD device did not fire (n = 161) | ICD device fired (n = 101) | OPT (n = 228) | OPT vs. ICD fired (95% CI)n | |||||||
| HRQoL, mean (SD) | ||||||||||
| Perception of health | ||||||||||
| General health status | 56.6 (23.3) | 52.1 (22.1) | 58.3 (23.6) | NS | ||||||
| Perception of health transitionm | 2.3 (1.2) | 2.5 (1.3) | 2.1 (1.2) | (–0.73 to –0.01)o | ||||||
| Physical limitations | 44.8 (42.9) | 36.8 (41.1) | 49.2 (42.8) | (0.31 to 24.6)p | ||||||
| Bodily pain | 57.8 (24.1) | 56.8 (25.3) | 58.8 (24.8) | NS | ||||||
| Ability to function | ||||||||||
| Employment status | 0.30 (0.5) | 0.18 (0.4) | 0.29 (0.5) | NS | ||||||
| Physical role functioning | 61.5 (27.5) | 53.2 (27.0) | 61.8 (28.3) | (0.7 to 16.6) | ||||||
| Emotional role functioning | 59.5 (43.4) | 49.1 (42.8) | 67.3 (39.9) | (6.2 to 30.1) | ||||||
| Social functioning | 71.6 (26.9) | 68.8 (27.7) | 70.8 (26.4) | NS | ||||||
| Psychological well-being | ||||||||||
| Mental health | 73.6 (43.4) | 70.6 (18.5) | 77.2 (17.0) | (1.5 to 11.6) | ||||||
| Satisfaction with appearance | 6.0 (1.3) | 6.0 (1.4) | 6.3 (1.1) | (–0.01 to 0.71) | ||||||
| Satisfaction with scar | 7.0 (1.2) | 7.1 (1.2) | 7.2 (1.1) | NS | ||||||
| Rate of rehospitalisation prior to completing the 6-month QoL instrument (%) | 36.0 | 55.5 | 33.8 | |||||||
| HF | ||||||||||
| SCD-HeFT107 | ICD + OPT (n = 816) | Amiodarone + OPT (n = 830), placebo + OPT (n = 833) | Difference (95% CI)q | |||||||
| DASI, mean score (SD) | ||||||||||
| Baseline | (n = 814) 24.6 (13.6) | (n = 825) 25.3 (14.1), (n = 829) 24.9 (14.1) | –0.34 (–1.68 to 1.00) | |||||||
| 3 months | (n = 766) 26.9 (14.1) | (n = 756) 26.2 (14.7), (n = 768) 26.2 (14.3) | –0.69 (–0.73 to 2.11) | |||||||
| 12 months | (n = 734) 26.8 (14.4) | (n = 676) 26.1 (14.5), (n = 697) 26.6 (14.8) | 0.16 (–1.35 to 1.68) | |||||||
| 30 months | (n = 665) 26.8 (14.3) | (n = 575) 27.1 (15.3), (n = 585) 25.9 (15.3) | 0.89 (–0.75 to 2.53) | |||||||
| ICD + OPT (n = 816) | Amiodarone + OPT (n = 830), placebo + OPT (n = 833) | Difference (95% CI),q p-value | ||||||||
| MHI-5 | ||||||||||
| Baseline | (n = 814) 71.7 (20.5) | (n = 827) 72.1 (20.1), (n = 830) 70.0 (21.4) | 1.64 (–0.39 to 3.67) | |||||||
| 3 months | (n = 764) 74.4 (19.3) | (n = 759) 72.9 (20.6), (n = 767) 71.3 (21.5) | 3.15 (1.10 to 5.19), ≤0.05 | |||||||
| 12 months | (n = 734) 74.5 (18.9) | (n = 674) 72.9 (20.5), (n = 693) 70.9 (21.5) | 3.68 (1.58 to 5.78), ≤0.05 | |||||||
| 30 months | (n = 654) 72.2 (19.1) | (n = 560) 73.2 (20.3), (n = 564) 71.0 (21.7) | 1.24 (–1.06 to 3.53) | |||||||
| ICD + OPT | Placebo + OPT | p-value | ||||||||
| MLWHFQ, median | ||||||||||
| Baseline | 41 | 43 | 0.77 | |||||||
| 3 months | 30 | 36 | 0.006 | |||||||
| 12 months | 32 | 36 | 0.07 | |||||||
| 30 months | 32 | 36 | 0.05 | |||||||
| ICD + OPT | Placebo + OPT | p-value | ||||||||
| Global health status, median | ||||||||||
| 3 months | 75 | 70 | 0.002 | |||||||
| 12 months | 75 | 70 | 0.05 | |||||||
| 30 months | 70 | 70 | 0.18 | |||||||
| ICD + OPT (n = 816) | p-value | |||||||||
| Received shock within 1 month before a scheduled QoL assessment (n = 49) | No shock | |||||||||
| SF-36 score, mean change | ||||||||||
| General health perceptions | –6.3 | 3.4 | 0.002 | |||||||
| Physical function | –8 | 10.9 | < 0.001 | |||||||
| Emotional function | –11 | 4.5 | 0.02 | |||||||
| Social function | –5.3 | 4.6 | 0.009 | |||||||
| Self-related health | –3.2 | 6.6 | 0.009 | |||||||
The effects of adverse symptoms and ICD shocks were assessed in the AVID trial74 using PCS scores, MCS scores and patient concerns using multivariate analysis including age, sex, race, index arrhythmia, ejection fraction, history of HF and use of beta-blockers at hospital discharge (see Table 26). Adverse symptoms led to a statistically significant worsening of PCS scores (p < 0.001), MCS scores (p = 0.002) and patient concern scores (p < 0.001) for the ICD group and PCS scores (p = 0.009) and patient concern scores (p = 0.03) for the AAD group. The occurrence of ICD shocks had a similar adverse effect on QoL, with statistically significant worsening of PCS scores (p = 0.03), MCS scores (p = 0.04) and patient concern scores (p < 0.001).
A substudy of the CIDS trial87 reported the effects of ICDs and AADs on three domains of the Mental Health Inventory (MHI) and seven domains of the Nottingham Health Profile (NHP), with an additional assessment of the consequences of ICD shocks on these measures (see Table 26). At 12 months’ follow-up the ICD group had shown a significantly greater improvement than the AAD group on the MHI domains of ‘total index’ (p = 0.001), ‘psychological distress’ (p = 0.001) and ‘psychological well-being’ (p = 0.03) and the NHP domains of ‘energy level’ (p = 0.0001), ‘physical mobility’ (p = 0.002), ‘emotional reactions’ (p = 0.002), ‘sleep disturbance’ (p = 0.02) and ‘lifestyle impairment’ (p = 0.005). It was notable that none of the domains on the MHI and the NHP improved for the AAD group between baseline and 12 months’ follow-up, with the domains of ‘energy level’ and ‘physical mobility’ deteriorating.
The effects of ICD shocks on QoL were assessed in the CIDS trial87 on the different domains of the MHI and the NHP through univariate comparisons between groups in terms of the numbers of shocks (i.e. ICD no shocks, ICD one to four shocks, ICD five or more shocks and AAD group without an ICD) (see Table 26). It was evident that the ICD five or more shocks group, like the AAD group without an ICD, did not experience the significant improvements in QoL that were reported by the ICD groups with less than five shocks. At 12 months’ follow-up the ICD five or more shocks group scored significantly worse (p < 0.05) than both the ICD no shocks group and the ICD one to four shocks group on the MHI ‘total index’ and ‘psychological distress’ domains, than the ICD one to four shocks group on the MHI ‘psychological well-being‘ domain and than the ICD no shocks group on the NHP ‘emotional reactions’ domain. Although the ICD five or more shocks group did not differ significantly from the AAD group without an ICD on any of the MHI and NHP domains, the ICD no shocks and ICD one to four shocks groups had significantly better (p < 0.05) QoL than the AAD group without an ICD on the MHI ‘total index’ and ‘psychological distress’ domains and the NHP ‘energy level’, ‘physical mobility’ (ICD no shocks only), ‘emotional reactions’ and ‘lifestyle impairment’ domains.
Five trials69,80,94,104,107 in people who had not suffered a life-threatening arrhythmia but who were at increased risk assessed QoL. The MADIT II trial104 assessed QoL in those remote from their MI through the Health Utilities Index 3 (HUI3), reporting the mean score, mean annual change and overall mean score (including death) for those alive at assessment annually to 3 years’ follow-up (see Table 26). The mean annual change in HUI3 scores showed a worsening in HRQoL for the ICD + OPT group compared with the OPT group annually, with a statistically significantly change in years 2 (p = 0.05) and 3 (p = 0.10). 104 Despite these changes, comparison of the HUI3 scores for the different interventions showed that they were not significantly different during follow-up, even when mortality was taken into account (valuing death as 0). 104
The AMIOVIRT study69 in people with cardiomyopathy assessed changes in QoL using the Quality of Well-Being Scale (QWBS) and the State–Trait Anxiety Inventory (STAI) (see Table 26). Comparison of the ICD + OPT group with the amiodarone + OPT group at 1 year of follow-up showed no statistically significant difference between the groups for well-being on the QWBS (p = 0.5) or for anxiety on the STAI (p = 0.4). Although the DEFINITE trial94 in people with cardiomyopathy assessed QoL using the Short Form questionnaire-12 items (SF-12) MCS and PCS and the Minnesota Living with Heart Failure Questionnaire (MLWHFQ), stating that no statistically significant differences were found between the ICD + OPT group and the OPT group, no data were reported.
The CABG Patch trial80 in people scheduled for a CABG assessed HRQoL using measures of perception of health, ability to function and psychological well-being at 6 months’ follow-up (see Table 26). On all measures of HRQoL the group receiving OPT reported a higher QoL than the ICD + OPT group, with statistically significant differences for the measures of perception of health transition (p = 0.030), emotional role function (p = 0.003), mental health (p = 0.004), satisfaction with appearance (p = 0.008) and satisfaction with scar (p = 0.040). 80 With 38.5% of people with an ICD + OPT having received a shock in the 6 months before completing the QoL instrument, the CABG Patch trial80 assessed the effects on QoL scores. On 10 of the 12 measures the OPT group had a higher QoL than the ICD + OPT group when the device either fired or did not fire. The scores for the ICD + OPT group when the device did not fire were similar to those of the OPT group, with no statistically significant differences (p-values not stated). In contrast, the ICD + OPT group when the device did fire had a lower QoL, with statistically significant differences (p = 0.05) for perception of health transition, physical limitations, physical role functioning, emotional role functioning, mental health and satisfaction with appearance.
The SCD-HeFT trial107 in people with HF reported QoL through a comparison of the Duke Activity Status Index (DASI), the Mental Health Inventory 5 (MHI-5), the MLWHFQ and the global health status of the ICD + OPT, amiodarone + OPT and placebo + OPT groups at baseline and 3, 12 and 30 months’ follow-up (see Table 26). The effects on QoL were compared between those experiencing shocks and those not receiving a shock in the ICD + OPT group using the SF-36. Using the DASI there were no clinical (4-point difference) or statistically significant differences in median or mean scores between the groups at baseline and 3, 12 and 30 months. On the MHI-5, outcomes were more equivocal. Although the differences in the median and mean scores comparing the ICD + OPT group and amiodarone + OPT group separately with the placebo + OPT group were below clinically meaningful levels (i.e. 5-point difference), some were statistically significant. Comparison of the median scores showed that the ICD + OPT group had significantly better scores than the placebo + OPT group at 3 months (p = 0.01) and 12 months (p = 0.003). By 30 months the scores for the ICD + OPT group had declined to baseline levels. Similarly, the mean scores for the ICD + OPT group differed significantly from those for the placebo + OPT group at 3 and 12 months (p = 0.05). Although the amiodarone + OPT group had a significantly higher MHI-5 score at baseline than the placebo + OPT group (p = 0.05), these differences disappeared during subsequent follow-up.
Similar improvements for the ICD + OPT group were reported on the MLWHFQ in the SCD-HeFT trial,107 resulting in significantly better scores for the ICD + OPT group than for the placebo + OPT group at 3 (p = 0.006) and 30 (p = 0.05) months (see Table 26). However, these differences were thought to be clinically insignificant (5-point change). In contrast, a comparison using a time trade-off utility measure showed that the health status of the ICD + OPT group and the placebo + OPT group declined from baseline with no statistically significant difference at 30 months’ follow-up (p = 0.18).
The effects of ICD shocks on QoL were assessed in the SCD-HeFT trial using the SF-36 (see Table 26). 107 A comparison of the changes in scores for those who had received a shock within 1 month of a scheduled QoL assessment and those who had not received a shock showed a significant decrease in the QoL of those who received a shock with regard to their relative perceptions of general health (p = 0.002), physical function (p < 0.001), emotional function (p = 0.02), social function (p = 0.009) and self-related health (p = 0.009). 107
Adverse events
All four trials71,81,84,89 comparing the use of ICDs with AADs in people at increased risk of SCD because of previous ventricular arrhythmias reported adverse events (Table 27). Reported adverse events differed between the trials, limiting comparisons. Only the total number of adverse events and mortality rates were compared between the interventions in the DEBUT trial89 and the AVID71 and CASH81 trials respectively. The DEBUT trial89 reported that 29.7% of the ICD group and 13.8% of the AAD group suffered adverse events (p-value not stated). The AVID trial71 compared deaths within 30 days of initiation of therapy or by hospital discharge if 30 days after therapy began, finding no statistically significant difference between the ICD group (2.4%) and the AAD group (3.5%) (p = 0.27). In contrast, the CASH trial81 found significantly (p = 0.029) higher mortality rates during the perioperative period for the ICD group (5.1%) than for the AAD group (1.1%). The only other comparison between interventions was in the AVID trial,71 finding that the use of thyroid replacement medication was higher for the AAD group at year 1 (10.0%) and year 2 (16.0%) than in the ICD group (1.0% years 1 and 2) (p-value not stated).
| Study | Outcome and follow-up | ICD, n/N (%) | OPT, n/N (%) | p-value | |
|---|---|---|---|---|---|
| Cardiac arrest (secondary prevention) | |||||
| AVID71 | Non-fatal torsade de pointes VT | 1/509 (0.2) | |||
| Suspected pulmonary toxicity, % | |||||
| At 1 year | 3 | ||||
| At 2 years | 5 | ||||
| Death from pulmonary toxicity | 1/509 (0.2) | ||||
| Thyroid replacement medication, % | |||||
| At 1 year | 1 | 10 | |||
| At 2 years | 1 | 16 | |||
| Death within 30 days of initiation of therapya | 12/507 (2.4) | 18/509 (3.5) | 0.27 | ||
| Bleeding requiring reoperation or transfusion | 6/507 (1.2) | ||||
| Serious haematoma | 13/507 (2.6) | ||||
| Infection | 10/507 (2.0) | ||||
| Pneumothorax | 8/507 (1.6) | ||||
| Cardiac perforation | 1/507 (0.2) | ||||
| Early dislodgement or migration of leads | 3/507 (0.6) | ||||
| Unsuccessful first attempt at ICD implantation without thoracotomy | 5/507 (1.0) | ||||
| Overall rate of non-fatal complications of implantation, % | 5.7 | ||||
| CASH81 | Amiodarone | Metoprolol | |||
| Drug-related pulmonary toxicity | 0/92 (0) | ||||
| Hyperthyroidism | 3/92 (3.3) | ||||
| Drug discontinuation required | 9/92 (9.8) | 10/97 (10.3) | |||
| Perioperative deaths or, for drug arms, deaths within the same time frame | All ICDs 5/99 (5.1) [epicardial ICDs 3/55 (5.4), endocardial ICDs 2/44 (4.5)] | AADs: 2/189 (1.1) [amiodarone 2/92 (2.2), metoprolol 0/97 (0)] | 0.029 | ||
| Other complications | |||||
| Infection | 3/99 (3.0) (explantation required for two) | ||||
| Haematoma or seroma | 6/99 (6.1) | ||||
| Pericardial effusion | 1/99 (1.0) | ||||
| Pleural effusion | 3/99 (3.0) | ||||
| Pneumothorax | 1/99 (1.0) | ||||
| Dislodgement or migration of system leads | 3/99 (3.0) | ||||
| Device dysfunction | 5/99 (5.1) | ||||
| Overall complication rate, % | 23.0 (including an explantation rate of 2.1) | ||||
| CIDS84 | 30-day mortality in implanted patients (n = 310) | ||||
| In patients with thoracotomy (n = 33) | 1/33 (3.0) | ||||
| In patients with non-thoracotomy lead system (n = 277) | 1/277 (0.4) | ||||
| ICD permanently or temporarily explanted because of infection, heart transplantation or patient preference | 16/310 (5.2) | ||||
| Adverse experiences ever reported | |||||
| Pulmonary infiltrate | 18/331 (5.7) (1.9% per year)b | ||||
| Visual symptoms (blurred, halo or decreased) | 48/331 (14.5) | ||||
| Bradycardia | 10/331 (3.0) | ||||
| Skin discolouration | 21/331 (6.3) | ||||
| Photosensitivity | 34/331 (10.3) | ||||
| Ataxia | 97/331 (17.2)b | ||||
| Tremor | 91/331 (15.4)b | ||||
| Insomnia | 64/331 (19.3) | ||||
| Peripheral neuropathy | 1/331 (0.3) | ||||
| ICD product discomfort | 25/328 (7.6) | ||||
| ICD malfunction | 2/328 (0.6) | ||||
| ICD pocket infection | 15/328 (4.6) (1.4% per year) | ||||
| ICD dislodgement/fracture | 8/328 (2.4) | ||||
| DEBUT (pilot study)89 | Operative mortality | 0/0 (0) | |||
| Adverse effects | 2/10 (20.0) | ||||
| Defibrillation discharges caused by supraventricular tachycardia or sinus tachycardia | 1/10 (10.0) | ||||
| T-wave oversensing | 0/0 (0) | ||||
| ICD replaced because of insulation break | 1/10 (10.0) | ||||
| DEBUT (main study)89 | Operative mortality | 0/0 (0) | |||
| Adverse effects | 11/37 (29.7) | 4/29 (13.8) | |||
| Minor complications, corrected by reprogramming devices without major intervention | |||||
| Defibrillation discharges caused by supraventricular tachycardia or sinus tachycardia | 7/37 (19.0) | ||||
| T-wave oversensing | 3/37 (8.1) | ||||
| Pocket erosion requiring removal of ICD | 1/37 (2.7) | ||||
| Side-effects in beta-blocker group | |||||
| Impotence/decrease in libido | 1/29 (3.4) | ||||
| Fatigue | 1/29 (3.4) | ||||
| Profound bradycardia | 1/29 (3.4) | ||||
| Hypotension plus central nervous system side effect | 1/29 (3.4) | ||||
| Early post MI | |||||
| DINAMIT95 | Number of deaths related to device implantation | 0/310 (0) | |||
| In-hospital device-related complications | 25/310 (8.1) | ||||
| IRIS97 | Died within 30 days of implantation | 7/415 (1.7) (n = 4 MI, n = 3 HF) | |||
| Died within 30 days of randomisation | 9/415 (2.2) | 11/453 (2.4) | |||
| Number of ICDs actually implanted | 415 | 39 (median 7.6 months after randomisation) | |||
| Inserted lead entangled in tricuspid valve, removed surgically | 1/415 (0.2) | ||||
| ICD explanted or permanently deactivated during follow-up (median 6.8 months after implantation) | 14/415 (3.4) | ||||
| Clinically significant complications requiring hospitalisation, surgical correction or intravenous drug administration | 65/415 (15.7), 76 complications | ||||
| Up to 30 days after implantation | 19/415 (4.6) | ||||
| During follow-up | 48/415 (11.6) | ||||
| Lead-related problems requiring surgical revision (included in the above complications) | 10/415 (2.4) (four had lead replacements) | ||||
| Remote from MI | |||||
| MADIT I99 | Operative deaths in the first 30 days | 0/95 (0) | 0/101 (0) | ||
| Hypotension | 0/95 (0) | 1/101 (1.0) | |||
| Syncope | 1/95 (1.1) | 5/101 (5.0) | |||
| Hypothyroidism | 0/95 (0) | 1/101 (1.0) | |||
| Sinus bradycardia | 3/95 (3.2) | 3/101 (3.0) | |||
| Pulmonary fibrosis | 0/95 (0) | 3/101 (3.0) | |||
| Pulmonary embolism | 1/95 (1.1) | 1/101 (1.0) | |||
| Atrial fibrillation | 4/95 (4.2) | 0/101 (0) | |||
| Pneumothorax | 2/95 (2.1) | 0/101 (0) | |||
| Bleeding | 1/95 (1.1) | 0/101 (0) | |||
| Venous thrombosis | 1/95 (1.1) | 0/101 (0) | |||
| Surgical infection | 2/95 (2.1) | 0/101 (0) | |||
| Problems with defibrillator lead | 7/95 (7.4) | 0/101 (0) | |||
| Malfunction of defibrillator generator | 3/95 (3.2) | 2/101 (2.0) | |||
| Total no. of patients with adverse events | 19/95 (20.0) | 12/101 (11.9) | |||
| MADIT II101 | Adverse effects of treatment, death during implantation | 0/742 (0) | |||
| Lead problems | 13/742 (1.8) | ||||
| Non-fatal infections requiring surgical intervention | 5/742 (0.7) | ||||
| Cardiomyopathy | |||||
| AMIOVIRT69 | Discontinued amiodarone because of adverse effects, mean follow-up 17.8 (SD 13.3) months | 25/52 (48.1) | |||
| CAT82 | Complications caused by ICD therapy | ||||
| Death within 30 days of ICD implantation | 0/50 (0) | ||||
| Device dislocation and bleeding requiring revision | 2/50 (4.0) | ||||
| Electrode dislocation requiring revision | 2/50 (4.0) | ||||
| Complications in 24 months of follow-up | 10 in seven patients | ||||
| Electrode dislocation and sensing/isolation defects | 7/50 (14.0) | ||||
| Infection with total device replacement | 2/50 (4.0) | ||||
| Perforation | 1/50 (2.0) | ||||
| DEFINITE90 | Complications during implantation of ICD | 3/229 (1.3) | |||
| Haemothorax | 1/229 (0.4) | ||||
| Pneumothorax | 1/229 (0.4) | ||||
| Cardiac tamponade | 1/229 (0.4) | ||||
| Procedure-related deaths | 0/229 (0) | ||||
| Complications during follow-up | 10/229 (4.4) | ||||
| Lead dislodgement or fracture | 6/229 (2.6) | ||||
| Venous thrombosis | 3/229 (1.3) | ||||
| Infection | 1/229 (0.4) | ||||
| Receipt of ICD upgrade during follow-up | 13/229 (5.7) | ||||
| Dual chamber ICD because of development of sinus node dysfunction | 2/229 (0.9) | ||||
| Biventricular devices for NYHA class III or IV HF and prolonged QRS interval | 11/229 (4.8) | ||||
| Scheduled for CABG | |||||
| CABG Patch75 | Death in the first 30 days after randomisation | 24/446 (5.4) | 20/454 (4.4) | 0.60 | |
| Postoperative complications | |||||
| MI | 18c/446 (4.0) | 16c/454 (3.5) | |||
| Sustained VT | 26c/446 (5.8) | 31c/454 (6.8) | |||
| VF | 15c/446 (3.4) | 24c/454 (5.3) | |||
| Bradycardia | 13c/446 (2.9) | 20c/454 (4.4) | |||
| Atrial fibrillation | 102c/446 (22.9) | 94c/454 (20.7) | |||
| Shock | 41c/446 (9.2) | 34c/454 (7.5) | |||
| New or more severe HF | 70c/446 (15.7) | 57c/454 (12.6) | |||
| Conduction defect | 63c/446 (14.1) | 66c/454 (14.5) | |||
| Residual central nervous system deficit | 16c/446 (3.6) | 9c/454 (2.0) | |||
| Bleeding treated with surgery | 22c/446 (4.9) | 14c/454 (3.1) | |||
| Postpericardiotomy syndrome | 4c/446 (0.9) | 3c/454 (0.7) | |||
| Deep sternal wound infection | 12c/446 (2.7) | 2c/454 (0.4) | 0.01 < p < 0.05 | ||
| Infection at wound or catheter site | 55c/446 (12.3) | 27c/454 (5.9) | 0.01 < p < 0.05 | ||
| Pneumonia | 38c/446 (8.5) | 18c/454 (4.0) | 0.01 < p < 0.05 | ||
| Other infection | 28c/446 (6.3) | 15c/454 (3.3) | |||
| Renal failure | 30c/446 (6.7) | 22c/454 (4.8) | |||
| Events during long-term follow-up | |||||
| Angina pectoris | 120c/446 (27.0) | 125c/454 (27.5) | |||
| MI | 2c/446 (0.5) | 19c/454 (4.2) | 0.01 < p < 0.05 | ||
| New or worsening HF | 190c/446 (42.5) | 193c/454 (42.5) | |||
| Ventricular arrhythmias | 87c/446 (19.4) | 65c/454 (14.3) | |||
| Atrial fibrillation | 66c/446 (14.7) | 46c/454 (10.1) | |||
| Hospitalisation | 274c/446 (61.4) | 251c/454 (55.2) | |||
| Repeat CABG surgery | 0/446 (0.0) | 3c/454 (0.7) | |||
| PTCA or atherectomy | 13c/446 (2.9) | 10c/454 (2.1) | |||
| Permanent cardiac pacemaker | 13c/446 (2.9) | 22c/454 (4.9) | |||
| ICD removed | 40/446 (9.0) | ||||
| Infection | 19/446 (4.3) | ||||
| ICD reached end of service period and not replaced | 5/446 (1.1) | ||||
| Patient request | 5/446 (1.1) | ||||
| HF | |||||
| SCD-HeFT105 | (n = 829) | Amiodarone + OPT (n = 845), placebo + OPT (n = 847) | |||
| Implantation was unsuccessful | 1/829 (0.1) | ||||
| ICD removed during follow-up | 32/829 (3.9) | ||||
| Clinically significant ICD complications, %d | |||||
| At time of implantation | 5 | ||||
| Later in the course of follow-up | 9 | ||||
| Increased tremor (amiodarone vs. placebo) at time of last follow-up, % | 4 | ||||
| Increased hypothyroidism (amiodarone vs. placebo) at time of last follow-up, % | 6 | ||||
Analysis of the adverse events reported for the ICD groups in the four trials71,81,84,89 showed that these tended to be limited in occurrence (see Table 27). The most frequent were those related to the placement and operation of the device itself, including defibrillation discharges caused by superventricular tachycardia or sinus tachycardia (19.0%);89 T-wave oversensing (8.1%);89 ICD product discomfort (7.6%);84 ICD permanently or temporarily explanted because of infection, heart transplantation or patient preference (5.2%);84 device dysfunction (5.1%);81 pocket erosion requiring removal of the ICD (2.7%);89 dislodgement or migration of system leads (3.0%);81 ICD dislodgement/fracture (2.4%);84 bleeding requiring reoperation or transfusion (1.2%);71 and unsuccessful first attempt at ICD implantation without thoracotomy (1.0%). 71 Other adverse events included haematoma or seroma (6.1%);81 serious haematoma (2.6%);71 pleural effusion (3.0%);81 infection (2.0–4.6%);71,84 and pneumothorax (1.6%). 71
Adverse events reported for the AAD groups differed between the four trials (see Table 27). 71,81,84,89 The CIDs trial84 found that > 10% of people receiving amiodarone reported insomnia (19.3%), ataxia (17.2%), tremor (15.4%), visual symptoms (14.5%) or photosensitivity (10.3%). Other adverse events reported in the CIDs trial84 included skin discolouration (6.3%) and pulmonary infiltrate (5.7%). In the CASH trial81 10% of people receiving amiodarone (9.8%) or metoprolol (10.3%) had to discontinue drug treatment. The AVID trial71 reported that 5% of the AAD group had suspected pulmonary toxicity at 2 years. Other adverse events reported by the AVID,71 CASH81 and DEBUT89 trials affected < 5% of participants (see Table 27).
All nine trials69,75,82,90,95,97,99,101,105 comparing ICDs + OPT with the differing comparator treatments in people who had not suffered a life-threatening arrhythmia but who were at increased risk reported adverse events, with six trials69,82,90,95,97,101 focused predominantly on those related to the placement of ICDs (see Table 27). The type of adverse events reported differed between the trials, making comparisons difficult. Adverse events were thought to affect between 5%105 and 61%75 of people receiving an ICD, depending on the definition of an adverse event or complication and the period of follow-up. Only three trials75,99,105 reported adverse events for the different comparator treatments, with rates varying from 11.9% to 55%.
Mortality rates associated with implantation of an ICD appeared low, with no deaths reported by four trials82,95,99,101 and crude death rates ranging from 1.6% to 5.4% in the IRIS97 and CABG Patch75 trials respectively. Deaths among those receiving the comparator treatments were reported only in the CABG Patch trial,75 with a crude death rate for the OPT group of 4.4%.
Lead-, electrode- or defibrillator generator-related problems were reported in five trials,82,90,97,99,101 affecting between 1.8% and 14.0% of people. In the IRIS trial,97 these led to a surgical revision rate of 2.4%. Surgical or device-related infections were reported in four trials,75,82,90,99 affecting between 0.4% and 12.3% of people in the ICD group. A further three trials81,82,101 reported infection leading to surgical intervention or device removal/replacement, which occurred in 0.7–4% of people.
Other non-device-specific adverse events were reported by four trials. 75,82,90,99 In the MADIT I99 and SCD-HeFT75 trials only syncope (5%) and hypothyroidism (6%) affected ≥ 5% of people in the comparator groups. The CABG Patch trial75 reported adverse events in the postoperative period and during long-term follow-up for both the ICD + OPT group and the OPT group, focusing predominantly on changes in underlying cardiac conditions. In the postoperative period the CABG Patch trial75 reported event rates of ≥ 5% for the ICD + OPT group and ≥ 4% for the OPT group for atrial fibrillation (ICD + OPT 22.9%, OPT 20.7%), new or severe HF (ICD + OPT 15.7%, OPT 12.6%), conduction defect (ICD + OPT 14.1%, OPT 14.5%), sustained VT (ICD + OPT 5.8%, OPT 6.8%), shock (ICD + OPT 9.2%, OPT 7.5%), pneumonia (ICD + OPT 8.5%, OPT 4.0%) and renal failure (ICD + OPT 6.7%, OPT 4.8%). 75 Events during long-term follow-up that affected ≥ 5% of the ICD + OPT group and the OPT group included new or worsening HF (ICD + OPT 42.5%, OPT 42.5%), angina pectoris (ICD + OPT 27.0%, OPT 27.5%), ventricular arrhythmias (ICD + OPT 19.4%, OPT 14.3%) and atrial fibrillation (ICD + OPT 14.7%, OPT 10.1%).
Subgroup analyses reported by included randomised controlled trials
Six trials71,75,90,97,103,105 reported prespecified subgroup analyses, although it should be noted that the trials were not powered to detect differences in subgroups.
The report of the AVID trial,71 which included people at increased risk of SCD because of previous ventricular arrhythmias, presented in a figure four prespecified subgroup analyses for all-cause mortality (age, LVEF, cause of arrhythmia and qualifying arrhythmia). No subgroup differed significantly from the others or the overall population. For most of the subgroups the 95% CIs crossed 1.0, apart from those for LVEF ≤ 35%, cause of arrhythmia coronary artery disease and VF rhythm, which favoured ICD. Subgroup analyses for the index arrhythmia were also reported (baseline: VF n = 455; VT n = 561). 72 ICDs improved survival free of arrhythmic death for people whose presenting arrhythmia was VT (p = 0.025) or VF (p = 0.0019). For non-arrhythmic cardiac death there were no statistically significant differences in survival between the ICD group and the AAD group for people presenting with either VT (p = 0.72) or VF (p = 0.98).
The IRIS trial,97 which included people in the early period post MI, prespecified 13 subgroup analyses for all-cause mortality, nine of which were presented in a figure [age, sex, CHF on admission, criterion of inclusion (for definitions see Appendix 7), ST-elevation MI, early reperfusion for ST-elevation MI, number of vessels, smoking and NYHA class at discharge] and four of which were not presented but described as similar in the two study groups (diabetes, hypertension, lipid abnormalities and number of risk factors). For most of the subgroups the 95% CIs crossed 1.0, apart from those for thrombolytic therapy for early reperfusion for ST-elevation MI (favoured control, data in figure only) and left main artery (favoured ICD, data in figure only).
In people remote from their MI, the MADIT II trial103 reported prespecified subgroup analyses for all-cause mortality using baseline characteristics, five of which were presented in a figure only (age, sex, ejection fraction, NYHA class or QRS interval) and seven of which were not presented (hypertension, diabetes, LBBB, atrial fibrillation, the interval since the most recent MI, type of ICD, and blood urea nitrogen level). The HRs in all of the subgroups were similar, with no statistically significant interactions.
The DEFINITE trial,90 which included people with cardiomyopathy, presented six prespecified subgroup analyses for all-cause mortality in a figure only (age, sex, LVEF, QRS interval, NHYA class and history of atrial fibrillation). None of the differences between subgroups were statistically significant. For most of the subgroups the 95% CIs crossed 1.0, apart from those for men (RR 0.49, 95% CI 0.27 to 0.90, p = 0.018), NYHA class III (RR 0.37, 95% CI 0.15 to 0.90, p = 0.02) and LVEF ≥ 20% (favoured ICD, data in figure only).
The CABG Patch trial,75 which included people who were scheduled for CABG surgery, evaluated 10 prespecified subgroups (age, sex, HF, NYHA class, LVEF, diabetes mellitus, QRS complex duration, use of ACE inhibitors, use of class I or class III AADs and use of beta-adrenergic-blocking drugs). HRs for the ICD group compared with the control group were found to be similar among the subgroups for all-cause mortality (data not reported).
The SCD-HeFT trial, which included people with mild to moderate HF, reported prespecified subgroup analyses for all-cause mortality105 and cause of death108 according to cause of CHF (ischaemic or non-ischaemic) and NYHA class (II or III) and for all-cause mortality according to race. 106 Table 28 presents the results for ICDs compared with placebo; subgroup results for the comparisons between amiodarone and placebo are reported in Appendix 7.
| Subgroup and outcome | ICD vs. placebo HR (95% CI), p-value |
|---|---|
| Ischaemic CHF | |
| All-cause mortality105 | 0.79 (0.60 to 1.04a), 0.05 |
| Cause of death108 | |
| Cardiac | 0.80 (0.60 to 1.05) |
| Sudden tachyarrhythmic | 0.43 (0.27 to 0.67) |
| HF | 1.11 (0.74 to 1.67) |
| Non-cardiac | 0.79 (0.50 to 1.22) |
| Non-ischaemic CHF | |
| All-cause mortality105 | 0.73 (0.50 to 1.07a), 0.06 |
| Cause of death108 | |
| Cardiac | 0.68 (0.44 to 1.03) |
| Sudden tachyarrhythmic | 0.34 (0.17 to 0.70) |
| HF | 1.21 (0.67 to 2.18) |
| Non-cardiac | 0.81 (0.48 to 1.37) |
| NYHA class II | |
| All-cause mortality105 | 0.54 (0.40 to 0.74a), < 0.001 |
| Cause of death108 | |
| Cardiac | 0.50 (0.36 to 0.70) |
| Sudden tachyarrhythmic | 0.26 (0.15 to 0.44) |
| HF | 0.93 (0.56 to 1.54) |
| Non-cardiac | 0.63 (0.40 to 0.99) |
| NYHA class III | |
| All-cause mortality105 | 1.16 (0.84 to 1.61a), 0.30 |
| Cause of death108 | |
| Cardiac | 1.17 (0.84 to 1.64) |
| Sudden tachyarrhythmic | 0.73 (0.41 to 1.29) |
| HF | 1.34 (0.86 to 2.09) |
| Non-cardiac | 1.10 (0.66 to 1.85) |
| Race African American | |
| All-cause mortality106 | 0.65 (95% CI 0.43 to 0.99) |
| Race white | |
| All-cause mortality106 | 0.73 (95% CI 0.58 to 0.90) |
There was no significant interaction between ICD therapy and the cause of CHF for all-cause mortality (p = 0.68). 105 The HRs for those with ischaemic and non-ischaemic CHF were 0.79 (97.5% CI 0.60 to 1.04, p = 0.05) and 0.73 (97.5% CI 0.50 to 1.07, p = 0.06) respectively. Similarly, there was no significant interaction between ICD therapy and the cause of CHF for each of the specified modes of death108 (see Table 28). A significant reduction in sudden death presumed to be ventricular tachyarrhythmic was found for both ischaemic (HR 0.43, 95% CI 0.27 to 0.67) and non-ischaemic (HR 0.34, 95% CI 0.17 to 0.70) causes of CHF, whereas no significant reduction in other modes of death was found for either subgroup (see Table 28).
There was a statistically significant interaction between ICD therapy and NYHA class (p < 0.001). 105 Compared with placebo, ICDs reduced the risk of death in people in NYHA class II (HR 0.54, 97.5% CI 0.40 to 0.74, p < 0.001), but not in those in NYHA class III (HR 1.16, 97.5% CI 0.84 to 1.61, p = 0.30). The interaction between ICD therapy and NYHA class was statistically significant for cardiac mortality (p = 0.0004) and sudden death presumed to be ventricular tachyarrhythmic (p = 0.0091), but not for HF (p = 0.29) or non-cardiac (p = 0.11) deaths. 108 ICD therapy reduced the risk of cardiac mortality (HR 0.50, 95% CI 0.36 to 0.70) and sudden tachyarrhythmic death (HR 0.26, 95% CI 0.15 to 0.44) in people in NYHA class II, but not in those in NYHA class III (HR 1.17, 95% CI 0.84 to 1.64, and HR 0.73, 95% CI 0.41 to 1.29 respectively).
There was no significant interaction between ICD therapy and race (p = 0.53); ICD therapy reduced the risk of death in both racial groups (African American: HR 0.65, 95% CI 0.43 to 0.99; white: HR 0.73 95% CI 0.58 to 0.90). 106
Combining data from the SCD-HeFT105 non-ischaemic CHF subgroup with data from the three cardiomyopathy trials (AMIOVIRT,69 CAT,82 DEFINITE90) was considered appropriate by clinical experts. SCD-HeFT105 did not report the number of events for all-cause mortality occurring in each of the ischaemic and non-ischaemic subgroups; therefore, these were estimated by reviewers and data from the non-ischaemic subgroup were combined in a meta-analysis (Figure 9). The SCD-HeFT non-ischaemic subgroup strongly influenced the analysis and a statistically significant effect in favour of ICD therapy with no statistical heterogeneity was found (RR 0.74, 95% CI 0.58 to 0.93, p = 0.01). This is in contrast to the non-significant result of the meta-analysis of the three cardiomyopathy trials alone (see Figure 4).
FIGURE 9.
All-cause mortality, cardiomyopathy RCTs and SCD-HeFT non-ischaemic CHF subgroup.
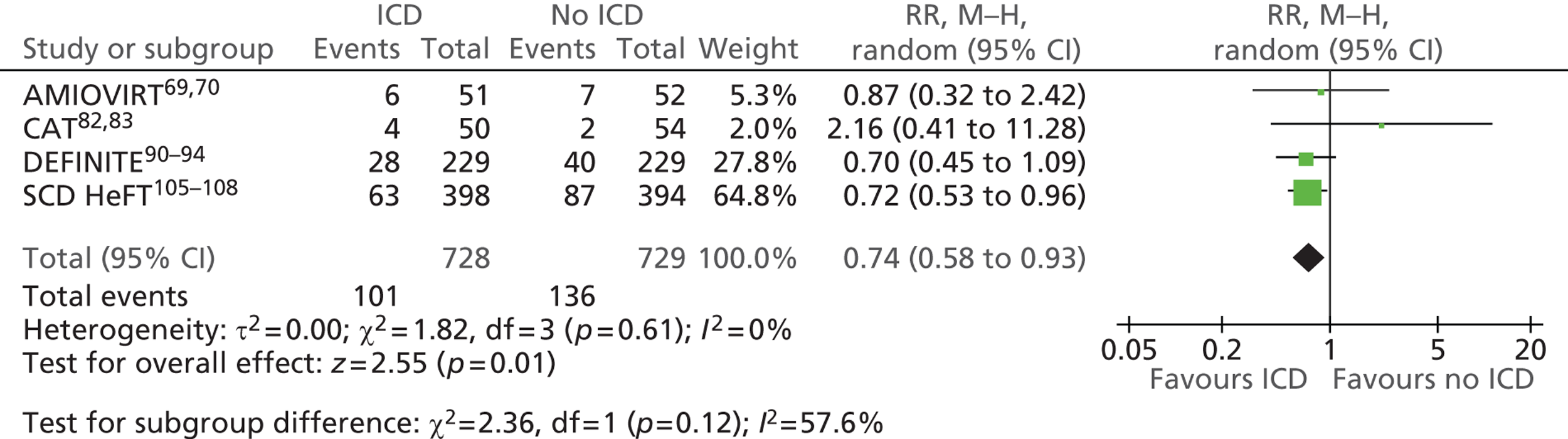
Other relevant trials
Two trials146,147 were excluded as the intervention did not meet the scope of the present review (many participants in the intervention arm did not receive an ICD); however, these trials presented subgroup data comparing ICD therapy with no ICD therapy that may be considered relevant. The MUSTT146 and MAVERIC147 trials have not undergone formal data extraction and quality assessment but the data are presented here for information.
The MUSTT study was included in the previous TARs62,63 although the authors noted that it did not meet their inclusion criteria if strictly applied (in that randomisation determined electrophysiology-guided therapy not ICD therapy). The authors also state that caution should be used when assessing the results as the study did not randomise participants to drug therapy or ICD and has the potential for bias and confounding of results. 62
The MUSTT study was designed to test the hypothesis that electrophysiology-guided antiarrhythmic therapy reduces SCD. People with sustained, monomorphic VT induced by any method of stimulation and those with sustained polymorphic VT (including ventricular flutter and fibrillation) induced by one or two extra stimuli were randomly assigned in equal numbers to receive either antiarrhythmic therapy guided by the results of electrophysiological testing or no antiarrhythmic therapy. ICD therapy could be recommended for people randomised to electrophysiological testing after at least one unsuccessful drug test. Median follow-up was 39 months. Beta-blocker use was significantly higher in the no therapy group (electrophysiological testing 29%, no therapy 51%, p = 0.001).
All-cause mortality was significantly reduced in the ICD group compared with the electrophysiology-guided therapy without a defibrillator group (RR 0.42, 95% CI 0.29 to 0.61, p < 0.001) and the no therapy group (RR 0.49, 95% CI 0.35 to 0.69, p < 0.001). 146 The overall mortality rate at 5 years was 24% among patients who received a defibrillator and 55% among those who did not.
The risk of death from cardiac arrest or arrhythmia was significantly reduced in patients who received an ICD compared with those receiving electrophysiology-guided therapy without a defibrillator (RR 0.24, 95% CI 0.13 to 0.43, p < 0.001) and those receiving no therapy (RR 0.28, 95% CI 0.16 to 0.49, p < 0.001). 146
The MAVERIC trial was in progress at the time of the previous TAR. 62 This multicentre UK study was designed to test the possibility of prospectively identifying, using electrophysiological testing, patients who would benefit most from ICD therapy in the context of the secondary prevention of SCD. Survivors of sustained VT, VF or SCD were randomised to electrophysiology-guided interventions (AADs, coronary revascularisation and ICD therapy) or empirical amiodarone therapy, with prestratification for haemodynamic status at the index event. Median follow-up was 60 months.
Subgroup analysis was presented for ICD recipients compared with non-ICD recipients, regardless of allocated treatment. As with the MUSTT trial, these results must be viewed with caution because of the lack of randomisation and the possibility of bias and confounding. An ICD was received by 31 of 108 (29%) patients randomised to electrophysiological testing [14/60 (23%) patients haemodynamically stable and 17/48 (35%) patients haemodynamically unstable at the index event] and 5 of 106 (5%) patients randomised to amiodarone [4/62 (6%) patients haemodynamically stable and 1/44 (2%) patients haemodynamically unstable at the index event]. ICD recipients were significantly younger [62.7 (SD 9.0) years vs. 68.1 (SD 9.8) years, p = 0.002] and less likely to have diabetes (5.3% vs. 18.8%, p = 0.042) than non-ICD recipients; other baseline characteristic were similar.
Survival was significantly better in ICD recipients than in non-ICD recipients [HR 0.54, 0.30 to 0.97 (definition of interval not stated), p = 0.0391]. Comparisons between ICD recipients and non-ICD recipients were also presented separately for haemodynamically stable patients [HR 0.71, 0.29 to 1.75 (definition of interval not stated), p = 0.4537] and haemodynamically unstable patients [HR 0.42, 0.20 to 0.92 (definition of interval not stated), p = 0.0299] at the index event. Multivariate analysis of factors affecting survival found that ICD implantation was associated with a non-statistically significant reduction in the risk of death [OR 0.43, 0.17 to 1.11 (definition of interval not stated), p = 0.080].
Summary of clinical effectiveness: people at risk of sudden cardiac death as a result of ventricular arrhythmias
-
A total of 13 RCTs were included that compared ICDs with medical therapy in people at risk of SCD because of arrhythmias. The trials were synthesised according to the criteria that they used to identify people at risk of SCD.
-
Risk of bias – As it was not possible to blind participants and personnel in these trials, they were judged to have a high risk of performance bias. Trials were judged to have a low risk of detection bias as assessment of mortality is unlikely to be influenced by lack of blinding; however, the risk of detection bias is high for QoL outcomes. Five trials were judged to have a low risk of selection bias, but this was unclear in eight trials because of inadequate reporting.
Ventricular arrhythmia/cardiac arrest (secondary prevention)
-
Four RCTs compared the effectiveness of ICDs and AADs. Average length of follow-up ranged from 18 months to 57 months and sample size ranged from 66 to 1016. The proportion of participants with CHF differed in the trials. In two trials 100% of participants had CHF, with > 80% in NYHA classes I and II. In the other two trials between 60% and 90% had CHF with approximately 50% in both trials in NYHA classes I and II. LVEF also varied, ranging from 30% to 70% across all four studies.
-
All four RCTs assessed all-cause mortality as the primary outcome measure, which when combined through meta-analysis showed a statistically significant benefit for ICDs compared with AADs (RR 0.75, 95% CI 0.61 to 0.93, p = 0.01). Differences were found in the four RCTs for the outcome of sudden cardiac/arrhythmic deaths, with a statistically significant benefit for ICDs compared with AADs when combined through meta-analysis (RR 0.49, 95% CI 0.34 to 0.69, p < 0.0001).
-
Meta-analysis of two trials showed a statistically significant benefit for ICDs compared with AAD for the outcome of total cardiac deaths (RR 0.74, 95% CI 0.61 to 0.91, p = 0.004); however, no differences were found for the outcomes of non-arrhythmic cardiac deaths (RR 0.97, 95% CI 0.72 to 1.31, p = 0.83) or other non-cardiac causes of death (RR 0.79, 95% CI 0.45 to 1.37, p = 0.40). Two RCTs reported different measures of survival, finding a statistically significant benefit for ICDs compared with AADs for overall survival at 3 years (difference 11%, p < 0.02), survival free of cardiac death at 2 years (difference 4%, p = 0.004), survival to arrhythmic death at 2 years in one trial (difference 5%, p = 0.0002) and survival free of sudden death at 57 months in the other trial (HR 0.423, p = 0.005). One RCT found lower cumulative mortality annually over 3 years’ follow-up with ICDs (difference: year 1 14.5%, year 2 1.7%, year 3 4.1%).
-
Two RCTs assessed QoL through separate substudies using a range of measures. In one RCT there were no significant between-group differences at follow-up. A second RCT found that QoL improved significantly in the ICD group on three domains of the MHI and five domains of the NHP, whereas there were no changes in the OPT group. In this trial the QoL of those experiencing five or more ICD shocks did not differ significantly from that of the OPT group when analysed using the MHI and the NHP. The no shocks and one to four shocks ICD groups showed significant improvements on the MHI and NHP compared with the OPT group.
-
One trial reported prespecified subgroup analyses for all-cause mortality. The subgroups for age, LVEF, cause of arrhythmia and qualifying arrhythmia did not differ significantly from each other or the overall population for all-cause mortality.
People with a recent myocardial infarction (within 6–41 days or ≤ 31 days)
-
Two RCTs compared ICDs + OPT with OPT. Length of follow-up was 30 or 37 months and sample size ranged from 674 to 898. About 60% of participants in both trials were in NYHA class II, but the majority of the remaining participants had NYHA class III symptoms in one trial and NYHA class I symptoms in the other trial. Similarly, mean LVEF differed between the studies (28% and 35%), reflecting different eligibility criteria.
-
Meta-analysis of the two trials found no difference between the groups in all-cause mortality (RR 1.04, 95% CI 0.86 to 1.25, p = 0.69), total cardiac deaths (RR 0.97, 95% CI 0.79 to 1.20, p = 0.8) and non-cardiac deaths (RR 1.39, 95% CI 0.86 to 2.27, p = 0.18). Those who received an ICD + OPT had a lower risk of SCD (RR 0.45, 95% CI 0.31 to 0.64, p < 0.0001) but a higher risk of non-arrhythmic cardiac death (RR 1.77, 95% CI 1.30 to 2.40, p = 0.0002). One trial reporting cumulative mortality found no statistically significant difference between the groups. QoL was not reported.
-
One trial reported prespecified subgroup analyses for all cause-mortality. No significant differences were found for the 13 prespecified subgroups.
People with remote myocardial infarction (> 3 weeks or > 1 month previously)
-
Two RCTs compared ICDs + OPT with OPT, although the pharmacological therapy in one of these may not be considered optimal by current standards. Average length of follow-up was 27 and 20 months and sample size was 196 and 1232 respectively. About two-thirds of participants had NYHA class II or III symptoms and one-third had NYHA class I symptoms. Mean LVEF differed between the studies (about 26% and 23%), reflecting different eligibility criteria.
-
Meta-analysis of the two trials found a reduction in all-cause mortality (RR 0.57, 95% CI 0.33 to 0.97, p = 0.04), total cardiac deaths (RR 0.59, 95% CI 0.42 to 0.83, p = 0.003) and SCD (RR 0.36, 95% CI 0.23 to 0.55, p < 0.00001) in the ICD + OPT group compared with the OPT group. There was no difference in non-arrhythmic cardiac death (RR 0.95, 95% CI 0.41 to 2.18, p = 0.9) or non-cardiac death (RR 1.06, 95% CI 0.58 to 1.95, p = 0.84) between the groups. One trial reporting hospitalisations found a higher rate per 1000 months’ follow-up among those who received an ICD (11.3 vs. 9.4, p = 0.09), with higher HF hospitalisations (19.9% vs. 14.9%, p-value not reported).
-
In one trial that assessed QoL using the HUI3, scores were lower in the ICD + OPT group than in the OPT group at baseline. Differences between the groups were not statistically significant at 3 years’ follow-up.
-
One trial reported prespecified subgroup analyses for all-cause mortality. The HRs in all 12 of the subgroups were similar, with no statistically significant interactions.
People with non-ischaemic or idiopathic dilated cardiomyopathy
-
Three RCTs compared ICD + OPT with OPT or ICD + OPT with amiodarone + OPT. Mean follow-up was between 24 months (two RCTs) and 29 months and sample size ranged from 103 to 458. One trial enrolled people with recent onset of disease. Over half to two-thirds of participants were in NYHA class II; in one trial the remaining participants were in NYHA class III, but in two trials around 15–21% were in NYHA class I. Mean LVEF ranged from 21% to 25%.
-
Meta-analysis found no significant difference between ICDs and OPT or amiodarone in all-cause mortality (RR 0.77, 95% CI 0.52 to 1.15, p = 0.20), total cardiac deaths (RR 2.03, 95% CI 0.17 to 23.62, p = 0.57), non-arrhythmic cardiac death (RR 1.13, 95% CI 0.42 to 3.03, p = 0.81) or non-cardiac death (RR 0.65, 95% CI 0.13 to 3.29, p = 0.60). However a reduction was found in rate of SCDs (RR 0.26, 95% CI 0.09 to 0.77, p = 0.02) with ICDs.
-
Two trials reported no significant difference in survival between groups.
-
Two trials reported no significant differences in QoL, assessed using the QWBS and STAI or the SF-12 MCS and PCS and MLWHFQ.
-
One trial reported six prespecified subgroup analyses for all-cause mortality. None of the differences between subgroups was statistically significant.
-
Meta-analysis of the three cardiomyopathy trials and the non-ischaemic CHF subgroup of the SCD-HeFT trial found a statistically significant reduction in all-cause mortality (RR 0.74, 95% CI 0.58 to 0.93, p = 0.01) with ICDs compared with OPT or amiodarone.
People scheduled for coronary artery bypass graft surgery
-
One trial compared ICD + OPT with OPT, although the pharmacological therapy would not be considered optimal by current standards. Mean follow-up was 32 months and 900 participants were randomised. The majority of participants were in NYHA class II or III and mean LVEF was 27%.
-
No significant difference was found between groups in all-cause mortality (RR 1.08, 95% CI 0.85 to 1.38, p = 0.53), total cardiac deaths (HR 0.97, 95% CI 0.71 to 1.33, p = 0.84), non-arrhythmic cardiac death (HR 1.24, 95% CI 0.84 to 1.84, p = 0.28), non-cardiac death (RR 1.50, 95% CI 0.82 to 2.73, p = 0.19) or actuarial mortality at 4 years’ follow-up (HR 1.07, 95% CI 0.81 to 1.42, p = 0.64). The rate of SCD was lower in the ICD group but this did not reach statistical significance (HR 0.55, 95% CI 0.29 to 1.03, p = 0.06).
-
HRQoL was higher among those receiving OPT than among those receiving an ICD + OPT for all measures and this was statistically significant for some perception of health transition, emotional role function, mental health, satisfaction with appearance and satisfaction with scar.
-
HRs for the ICD group compared with the control group for all-cause mortality were found to be similar among 10 prespecified subgroups.
A broad population of people with mild to moderate heart failure
-
One three-arm trial compared ICDs, amiodarone and placebo; all participants received OPT. Mean follow-up was 46 months and 2521 participants were randomised. Over two-thirds of participants were in NYHA class II, with the remaining participants in NYHA class III. Mean LVEF was 25%.
-
All-cause mortality was significantly lower in the ICD + OPT group than in the placebo + OPT group (HR 0.77, 97.5% CI 0.62 to 0.96, p = 0.007). A significant reduction in total cardiac deaths (HR 0.76, 95% CI 0.60 to 0.95, p = 0.018) and SCD (compared with the placebo and amiodarone groups combined, RR 0.44, 95% CI 0.31 to 0.61, p < 0.00001) in favour of ICD was also found. There was no statistically significant difference between the ICD group and the placebo and amiodarone groups combined in the number of non-arrhythmic cardiac deaths (RR 1.14, 95% CI 0.88 to 1.48, p = 0.32) or deaths from non-cardiac causes (RR 0.92, 95% CI 0.66 to 1.27, p = 0.60).
-
Little difference was found in QoL assessed using the DASI. Statistically significant differences in MHI scores and global health status at 3 and 12 months were not maintained at 30 months, and the difference in MHI score was not clinically meaningful. A significant decrease in perceptions of QoL was found using the SF-36 among people who had received an ICD shock within the previous month compared with those who had not received a shock.
-
There was no interaction between ICD therapy (p = 0.68) and the cause of CHF (ischaemic or non-ischaemic) for all-cause mortality or other specified modes of death. There was a statistically significant interaction between ICD therapy and NYHA class: compared with placebo, ICDs reduced the risk of all-cause mortality, cardiac mortality and sudden death presumed to be ventricular tachyarrhythmic in people with NYHA class II, but not in those with NYHA class III. The interaction between ICD therapy and NYHA class was not statistically significant for HF (p = 0.29) or non-cardiac (p = 0.11) deaths.
Adverse events
-
Adverse events were reported by all four RCTs that included those with previous ventricular arrhythmias. Up to 30% of people in the ICD groups reported adverse events, with most related to the placement and operation of the device. Rates in the OPT group appeared lower.
-
The nine RCTs that included people who had not suffered a life-threating arrhythmia reported adverse event rates in the ICD group of between 5% and 61%, depending on the definition of adverse event and length of follow-up. Adverse event rates for the comparator treatment group were between 11.9% and 55% in the three RCTs reporting this. Lead-, electrode- or defibrillator generator-related problems affected 1.8–14% of people in the five trials that reported this.
People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony
Quantity and quality of research available
Four RCTs109,116,121,125 comparing CRT-P and OPT in people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT met the inclusion criteria. In addition, one of these RCTs, the Comparison of Medical Therapy, Pacing, and Defibrillation in Patients with Left Ventricular Systolic Dysfunction (COMPANION) trial,116 compared CRT-P and CRT-D with OPT.
Three of the trials reported their findings in more than one paper; a summary of the included papers for each trial can be seen in Table 29. All of these studies were included in the 2007 TAR,64 which also included the RCT of the CONTAK-CD device. 126 This trial is discussed later in People with both conditions.
| Study | Publicationa |
|---|---|
| CARE-HF | Cleland et al. 2005,109 2001,110 2006,111 2008112 and 2009,113 Gras et al. 2007,36 Gervais et al. 2009,114 Ghio et al. 2009115 |
| COMPANION | Bristow et al. 2004116 and 2000,117 US Food and Drug Administration 2004,118 Carson et al. 2005,119 Anand et al. 2009120 |
| MIRACLE | Abraham et al. 2002121 and 2000,122 US Food and Drug Administration 2001,123 St John Sutton et al. 2003124 |
| MUSTIC | Cazeau et al. 2001 125 |
Characteristics of the included studies
Study characteristics are summarised in Table 30 and participant characteristics are summarised in Table 31. Further details can be found in the data extraction forms in Appendix 8.
| Parameter | CARE-HF109 | COMPANION116 | MIRACLE121 | MUSTIC125 |
|---|---|---|---|---|
| Study design | RCT | RCT | RCT | Randomised crossover trial |
| Target population | NYHA class III or IV as a result of LVSD and cardiac dyssynchrony | Advanced chronic HF and intraventricular conduction delays | Moderate to severe HF | Severe HF and major intraventricular delay |
| Intervention | CRT-P + medical therapy | CRT-P or CRT-D and OPT | CRT-P on and OPT | CRT-P on and OPT |
| Comparator | Standard medical therapy | OPT | CRT-P off and OPT | CRT-P off and OPT |
| Country (no. of centres) | Europe (82) (including France, Germany, Italy, Switzerland and the UK) | USA (128) | USA and Canada (45) | Europe (15) (France, Germany, Italy, Sweden, Switzerland and the UK) |
| Sample size (randomised) | 813 | 1520 | 453 | 58 |
| Length of follow-up | Mean 29.4 months (mean 37.4 months with 8-month extension) | Primary end point, median 11.9–16.2 months | 6 months | 3 months |
| Key inclusion criteria | HF for ≥ 6 weeks; NYHA class III or IV despite standard pharmacological therapy; LVEF ≤ 35%; LVEDD ≥ 30 mm;a QRS interval ≥ 120 milliseconds;b aortic pre-ejection delay> 140 milliseconds, interventricular mechanical delay > 40 milliseconds, delayed activation of posterolateral left ventricular wall | Sinus rhythm; NYHA class III or IV; LVEF ≤ 35%; LVEDD ≥ 60 mm; QRS ≥ 120 milliseconds; PR interval > 150 milliseconds | HF due to ischaemic or non-ischaemic cardiomyopathy for > 1 month; NYHA class III or IV; LVEF ≤ 35%; LVEDD ≥ 55 mm; QRS interval ≥ 130 milliseconds; 6-minute walk distance ≤ 450 m | Severe HF due to idiopathic or ischaemic LVSD; sinus rhythm; NYHA class III for ≥ 1 month whilst on OPT; LVEF < 35%; LVEDD > 60 mm; QRS interval > 150 milliseconds; No standard indication for a pacemaker |
Intervention and comparators
In the Multicenter InSync Randomized Clinical Evaluation (MIRACLE)121 and Multisite Stimulation in Cardiomyopathies (MUSTIC)125 trials, all participants were implanted with a CRT-P device and pacing was inactivated in the control group. Participants in the CArdiac REsynchronization in Heart Failure (CARE-HF)109 and COMPANION116 trials received either a device + OPT or OPT only. Pharmacological therapy in all four trials would be considered optimal by current standards.
Participants
The trials included people with NYHA class III or IV HF, with the majority of participants in NYHA class III (ranging from 82% in CARE-HF109 to 100% in MUSTIC125). All of the trials included participants with LVEF < 35%; average LVEF was about 22% in the MIRACLE121 and COMPANION trials116 and 25% in the CARE-HF trial. 109
The trials differed in their eligibility criteria with regard to the QRS interval, with the CARE-HF109 and COMPANION116 trials requiring a QRS interval of ≥ 120 milliseconds, the MIRACLE trial121 requiring a QRS interval of ≥ 130 milliseconds and the MUSTIC trial125 requiring a QR interval of > 150 milliseconds. This is reflected in the average QRS interval at baseline in these studies, with the longest average QRS interval seen in the MUSTIC trial125 (see Table 31). When reported, the proportion of participants with ischaemic heart disease ranged from 36% (CARE-HF109) to 59% (COMPANION116).
| Parameter | CARE-HF109 | COMPANION116 | MIRACLE121 | MUSTIC125 | |||||
|---|---|---|---|---|---|---|---|---|---|
| CRT-P | OPT | CRT-P | CRT-D | OPT | CRT-P on | CRT-P off | CRT-P on | CRT-P off | |
| Sample size, n | 409 | 404 | 617 | 595 | 308 | 228 | 225 | 29 | 29 |
| Age (years), mean (SD) | 67 (60–73)a | 66 (59–72)a | 67b | 66b | 68b | 63.9 (10.7) | 64.7 (11.2) | 64 (11) | 64 (8) |
| Sex, % male | 74 | 73 | 67 | 67 | 69 | 68 | 68 | 66 | 83 |
| Ischaemic heart disease, % | 40 | 36 | 54 | 55 | 59 | 50 | 58 | ||
| Dilated cardiomyopathy, % | 43 | 48 | |||||||
| NYHA class, % | |||||||||
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| III | 94 | 93 | 87 | 86 | 82 | 90 | 91 | 100 | 100 |
| IV | 6 | 7 | 13 | 14 | 18 | 10 | 9 | 0 | 0 |
| LVEF (%), mean (SD) | 25b | 25b | 20b | 22b | 22b | 21.8 (6.3) | 21.6 (6.2) | ||
| QRS interval (milliseconds), mean (SD) | 160b (152–180)a | 160b (152–180)a | 160b | 160b | 158b | 167 (21) | 165 (20) | 172 (22) | 175 (19) |
| LBBB/RBBB, % | 69/12 | 73/10 | 70/9 | ||||||
| 6-minute walk test (m), mean | 274b | 258b | 244b | 305 | 291 | 354 (110) | 346 (111) | ||
| Peak VO2 (ml/kg/minute), mean (SD) | 14.0 | 13.7 | 13.5 (8.4) | 14.1 (4.6) | |||||
| Heart rate (bpm), mean (SD) | 69b | 70b | 72b | 72b | 72b | 73 (13) | 75 (13) | 75 (12) | 75 (14) |
The mean age of the participants in the studies was similar, ranging from around 64 years in the MIRACLE121 and MUSTIC125 trials to 68 years in the COMPANION trial116 (see Table 31). The majority of participants were men (73% and 74% in the CARE-HF trial arms,109 67%, 67% and 69% in the three COMPANION trial arms,116 68% in both of the MIRACLE trial arms121 and 66% and 83% in the MUSTIC trial arms125).
Pharmacological therapy
Optimal pharmacological therapy was used in all of the trials (Table 32). At least 90% of all participants received ACE inhibitors or ARBs. Less than one-third (28%) of participants used beta-blockers in the MUSTIC study,125 between 55% and 62% used beta-blockers in the MIRACLE trial,121 between 66% and 68% used beta-blockers in the COMPANION trial116 and between 70% and 74% used beta-blockers in the CARE-HF trial. 109 Spironolactone use was not reported by the MIRACLE study121 but was 22% in the MUSTIC trial,125 and between 53% and 55% in the COMPANION trial109 and between 54% and 59% in the CARE-HF trial. 109 Less than half of the participants in the CARE-HF trial109 used diuretics, with around 94% of participants in the other studies using them. Both the CARE-HF trial109 and the MUSTIC trial125 reported that less than half of the participants used digoxin, and around one-third of participants in the MUSTIC trial125 used amiodarone. In the MIRACLE trial121 around three-quarters of participants used digitalis medication.
| Medication | CARE-HF109 | COMPANION116 | MIRACLE121 | MUSTIC125 | |||||
|---|---|---|---|---|---|---|---|---|---|
| CRT-P | OPT | CRT-P | CRT-D | OPT | CRT-P on | CRT-P off | CRT-P on | CRT-P off | |
| Sample size, n | 409 | 404 | 617 | 595 | 308 | 228 | 225 | 67a | |
| Aldosterone antagonist (spironolactone), % | 54 | 59 | 53 | 55 | 55 | 22 | |||
| Amiodarone, % | 31 | ||||||||
| ACE inhibitor, % | 70 | 69 | 69 | ||||||
| ACE inhibitor or ARB, % | 95 | 95 | 89 | 90 | 89 | 93 | 90 | 96 | |
| Beta-blockers, % | 70 | 74 | 68 | 68 | 66 | 62 | 55 | 28 | |
| Digitalis, % | 78 | 79 | |||||||
| Diuretic, % | 94 | 94 | 93 | 94 | |||||
| Loop diuretic, % | 43 | 44 | 94 | 97 | |||||
| Digoxin, % | 40 | 45 | 48 | ||||||
Outcomes
Although all four trials reported all-cause mortality, it was not a primary outcome. The primary outcome of two trials was a composite end point: all-cause mortality and all-cause hospitalisation in the COMPANION trial116 and all-cause mortality and unplanned hospitalisation for a major cardiovascular event in the CARE-HF trial. 109 Composite outcomes can be seen in the data extraction forms (see Appendix 8) but have not been discussed in this report. The primary outcome of the MIRACLE121 and MUSTIC125 trials was distance walked in 6 minutes; changes in NYHA class and QoL were also primary outcomes in the MIRACLE trial. 125
All four trials reported mortality from SCD. In addition, the COMPANION116 and MUSTIC125 trials reported total cardiac deaths and the CARE-HF109 and COMPANION116 trials reported death from HF. HF hospitalisation was reported by all four trials. The CARE-HF,109 MIRACLE121 and MUSTIC125 trials reported details on worsening HF whereas arrhythmias were reported by the CARE-HF109 and MUSTIC125 trials. All trials except for the MUSTIC trial125 reported change in NYHA class, but only the CARE-HF109 and MIRACLE121 trials reported changes in LVEF. HRQoL and adverse events were reported by all trials.
Setting
All four studies were multicentre trials, with the number of centres ranging from 15 (MUSTIC125) to 128 (COMPANION116). The CARE-HF109 and MUSTIC125 trials were undertaken in Europe, with both including centres in the UK. The COMPANION study116 was undertaken in the USA whereas the MIRACLE121 trial included centres in the USA and Canada.
The MUSTIC study125 used a randomised crossover design, with 3 months’ follow-up for each of the two crossover periods. The length of follow-up for the MIRACLE study121 was 6 months. The mean length of follow-up in the CARE-HF study109 was 29.4 months, plus an 8-month extension (total mean follow-up 37.4 months). The COMPANION trial116 reported a median follow-up for the composite end point of 11.9 months for OPT, 15.7 months for CRT-D and 16.2 months for CRT-P. Median follow-up for mortality was also reported as 14.8 months for OPT, 16.0 months for CRT-D and 16.5 months for CRT-P.
Risk of bias
Details of the risk of bias for each study can be found in the data extraction tables in Appendix 8, with a summary in Table 33.
| Domain | Judgement | |||
|---|---|---|---|---|
| CARE-HF109 | COMPANION116 | MIRACLE121 | MUSTIC125 | |
| Selection bias | ||||
| Random sequence generation | Low | Unclear | Unclear | Unclear |
| Allocation concealment | Low | Unclear | Unclear | Unclear |
| Performance bias | ||||
| Blinding of participants and personnel | High | High | Low | High |
| Detection bias | ||||
| Blinding of outcome assessment | Compositea – low; secondaryb – high or unclear | Low | Low | High |
| Attrition bias | ||||
| Incomplete outcome data addressed | Compositea and echocardiographic outcomes – low; left ventricular remodelling outcomes – unclear | Low | Unclear | Low |
| Reporting bias | ||||
| Selective reporting | Low | Low | High | High |
| Other bias | ||||
| Other sources of bias | Low | Low | Low | High |
Because of a lack of reported details on randomisation methods and allocation concealment methods, the risk of selection bias for the COMPANION,116 MIRACLE121 and MUSTIC125 trials was unclear. The risk of selection bias was low in the CARE-HF trial. 109
The MIRACLE trial121 appeared to be at low risk of performance and detection bias, with both patients and physician unaware of treatment assignment (CRT-P on or off). The MUSTIC trial125 was at high risk of performance and detection bias, with only participants blinded to the treatment order (CRT-P on or off). Both the CARE-HF trial109 and the COMPANION trial116 were unblinded trials, placing them at high risk of performance bias. For detection bias, the CARE-HF trial109 was judged to be at low risk of bias for the composite end point of mortality and hospitalisation, using an end-points committee unaware of treatment assignment. However, without blinding, the trial was at high risk of detection bias for echocardiographic outcomes. The risk of detection bias for adverse events was unclear, with some adverse events classified by the end-points committee but others by an unblinded independent expert. The risk of detection bias in the COMPANION trial116 was low, with a steering committee and end-points committee unaware of treatment assignment.
Both the COMPANION trial116 and the MUSTIC trial125 were at low risk of attrition bias. The MUSTIC trial125 reported both numbers and reasons for withdrawals, whereas the COMPANION trial116 censored data in the ITT analysis for participants who withdrew and for whom data could not be obtained. The CARE-HF trial109 also reported ITT analyses and was at low risk of bias for mortality, hospitalisation and echocardiographic outcomes; however, the risk of bias for QoL and left ventricular reverse remodelling was unclear because of unexplained differences in numbers. The risk of attrition bias in the MIRACLE study121 was unclear for both primary and secondary outcomes. Although ITT analysis was used and attrition reported, the low numbers reported for the primary outcome of NYHA class and differences in sample size between the primary outcome and the secondary outcome were unexplained. Both the CARE-HF trial109 and the COMPANION study116 were at low risk of selective reporting bias. For both studies the protocol or rationale/design papers have been published and there was no evidence of missing outcomes. However, the MIRACLE121 and MUSTIC125 trials were at high risk of selective reporting bias. The MIRACLE trial121 assessed change in NYHA class but failed to report the data, and the MUSTIC trial125 included the SF-36 in the study protocol122 but did not report any data.
There was an additional risk of bias in the MUSTIC trial125 because of the use of block randomisation without blinding. However, the use of the crossover design appears appropriate.
Methodological comments
Similarity of groups at baseline
The groups in the four studies were generally well balanced at baseline.
Sample size
All four of the trials included a statistical power calculation. The CARE-HF,109 MIRACLE121 and MUSTIC125 trials appeared to be adequately powered to detect a difference in the relevant primary outcome measure. The MUSTIC trial125 randomised 58 participants, the MIRACLE trial121 randomised 453 participants and the CARE-HF trial109 randomised 813 participants. The COMPANION trial116 was stopped early when pre-established boundaries had been crossed, with 1520 participants randomised and 1000 primary end points already or almost met. The trial was designed with 2200 participants to detect a reduction of 25% in the primary end point.
Crossovers
By the end of the extension period in the CARE-HF trial,109 24% of participants in the OPT group had a CRT device implanted and activated and 2% of participants in the CRT-P treatment arm received a CRT-D device. The MIRACLE trial121 reported that 4% of participants crossed over from OPT to the CRT-P treatment group, but reported no details for the CRT-P treatment group. The COMPANION trial120 reported that, out of 78 cardiac procedures in the OPT group, 33 (42%) were for CRT implants. In addition, this trial reported that there were substantial withdrawals in the OPT group (26%) to receive commercially available implants, whereas the withdrawal rates in the CRT-P and CRT-D groups were 6% and 7% respectively. ITT analysis was performed in the trials.
Other issues
Studies differed in the timing of implantation, baseline evaluation and randomisation. Two studies randomised participants before implantation. In the CARE-HF study109 baseline measures were taken before randomisation and implantation, whereas in the COMPANION study116 randomisation occurred before implantation but baseline measures were taken 1 week after successful implantation. The remaining two studies (MIRACLE121 and MUSTIC125) randomised participants after implantation. In the MIRACLE study121 baseline measures were taken before implantation and randomisation whereas in the MUSTIC study125 baseline measures were taken after randomisation, which occurred 2 weeks after implantation. Thus, only those participants with a successful implantation underwent randomisation in both studies, limiting the generalisability of these studies. These differences may affect comparability between the studies.
The MUSTIC trial125 does not report all outcomes for both crossover periods. In addition, 10 participants did not complete both crossover periods (including five who did not complete the first period). The COMPANION trial116 had substantial withdrawals from the OPT group (see Crossovers).
Funding
All four trials received funding grants from the device manufacturers, with three trials funded by Medtronic109,121,125 and one by the Guidant Corporation. 116 In addition, three of the trials, the MIRACLE,121 MUSTIC125 and CARE-HF109 trials, reported conflicts of interests, as some/all authors were consultants or investigators for, or employees of, the company providing the funding. Both the CARE-HF trial109 and the COMPANION trial116 stated that sponsors had no role in data analysis, whereas the MIRACLE trial121 stated that sponsors placed no restrictions or limitation on the investigators performing the data analyses.
Assessment of effectiveness
All-cause mortality
All four studies reported all-cause mortality (Table 34), although it was not the primary outcome of the trials.
| Study | Follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | First 90 days of trial | 12/409 (2.9) | 15/404 (3.7) | ||
| 29.4 monthsa | 82/409 (20.0) | 120/404 (29.7) | HR 0.64 | 0.48 to 0.85, < 0.002 | |
| a37.4 months111 | 101/409 (24.7) | 154/404 (38.1) | HR 0.60 | 0.47 to 0.77, < 0.0001 | |
| Mortality rate 1 year,111 % | 9.7 | 12.6 | |||
| Mortality rate 2 year, % | 18 | 25.1 | |||
| Mortality rate 3 year, % | 23.6 | 35.1 | |||
| MIRACLE121 | 6 months | 12/228 (5.3) | 16/225 (7.1) | HR 0.73 | 0.34 to 1.54, 0.40 |
| MUSTIC125 | 6 months | First period: 1/29 (3.4b), second period: 2/29 (6.9b) | First period: 0/29 (0), second period: 0/29 (0) | RR 7.00b | 0.37 to 132.56, 0.19b |
| CRT-P, n/N (%) | OPT, n/N (%) | ||||
| COMPANION116 | CRT-P 16.5 months, OPT 14.8 monthsc | 131/617 (21.2) | 77/308 (25.0) | ||
| 12-month rate | 93b/617 (15) | 59b/308 (19) | HR 0.76 | 0.58 to 1.01, 0.059 | |
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| CRT-D 16.0 months, OPT 14.8 monthsc | 105/595 (17.6) | 77/308 (25.0) | RR 0.71b | 0.54 to 0.92, 0.009b | |
| 12-month rate | 71b/595 (12) | 59b/308 (19) | HR 0.64 | 0.48 to 0.86, 0.003 | |
| CRT-P, n/N (%) | CRT-D, n/N (%) | ||||
| CRT-P 16.5 months, CRT-D 16.0 monthsc | 131/617 (21) | 105/595 (18) | RR 1.20b | 0.96 to 1.52, 0.12b |
CRT-P compared with optimal pharmacological therapy
The CARE-HF trial109 reported a statistically significant difference in all-cause mortality between the groups after a mean follow-up of 37.4 months, which included an 8-month extension period (CRT-P 24.7% vs. OPT 38.1%, HR 0.60, 95% CI 0.47 to 0.77, p < 0.0001). Mortality rates at year 3 were 11.5 percentage points lower for the CRT-P group (CRT-P 23.6% vs. OPT 35.1%), although no statistical comparison was reported. After completion of the CARE-HF trial, long-term follow-up of people who survived and reconsented (343 of 813 originally enrolled) found that the effect of CRT persisted (HR 0.77, 95% CI 0.63 to 0.93, p = 0.007), despite implantation of CRT devices in > 95% of those originally assigned to the control group (ITT analysis undertaken, with participants remaining in their assigned group regardless of subsequent treatment). 150 In contrast, the MIRACLE trial121 found no statistically significant difference in all-cause mortality between the groups after 6 months’ follow-up (CRT-P 5.3% vs. OPT 7.1%, HR 0.73, 95% CI 0.34 to 1.54, p = 0.40), and the difference in the 12-month all-cause mortality rate between the CRT-P and OPT groups in the COMPANION trial116 did not reach statistical significance (CRT-P 15% vs. OPT 19%, HR 0.76, 95% CI 0.58 to 1.01, p = 0.059). The MUSTIC trial125 reported one death in the first crossover period (1/29, 3.4%) and two in the second crossover period (2/29, 6.9%) among those receiving CRT-P and none during the OPT period. No statistical comparison was reported.
The studies were considered sufficiently similar to combine in a meta-analysis (Figure 10). For meta-analysis of the MUSTIC crossover trial,125 all deaths in those receiving CRT-P or OPT from both crossover periods were included. This method provides a conservative analysis, with the study being underweighted rather than overweighted. 65 There was evidence of moderate statistical heterogeneity between the studies (χ2 = 4.99, df = 3, I2 = 40%). The RR for CRT-P compared with OPT for all-cause mortality using the random-effects method was 0.75 (95% CI 0.58 to 0.96, p = 0.02) (see Figure 10). Excluding the MUSTIC trial125 from the meta-analysis had little effect (RR 0.73, 95% CI 0.60 to 0.89, p = 0.002).
FIGURE 10.
All-cause mortality: CRT-P vs. OPT.
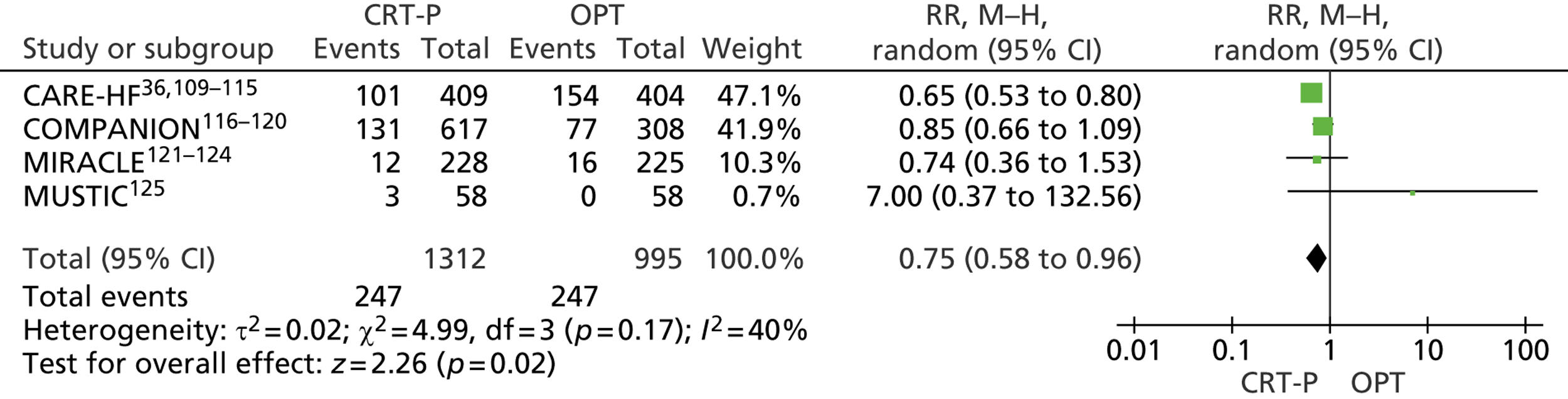
CRT-D compared with optimal pharmacological therapy
The COMPANION trial116 found a statistically significant reduction in mortality with CRT-D at 12 months (CRT-D 12% vs. OPT 19%, HR 0.64, 95% CI 0.48 to 0.86, p = 0.003), giving a reduction in risk of 36% for all-cause mortality.
CRT-P pacer compared with CRT-D
The COMPANION trial116 included three treatment arms (CRT-P, CRT-D and OPT). The difference in all-cause mortality between the CRT-P group (21%) and the CRT-D group (18%) was not statistically significant (RR 1.20, 95% CI 0.96 to 1.52, p = 0.12). However, all comparisons between CRT-P and CRT-D should be treated with caution as the trial was not powered for this comparison.
Total cardiac deaths
Both the COMPANION trial119 and the MUSTIC trial125 reported total cardiac deaths.
CRT-P compared with optimal pharmacological therapy
The COMPANION trial119 found no statistically significant difference in total cardiac deaths between CRT-P and OPT (CRT-P 17.7% vs. OPT 18.8%, p = 0.334), with a median follow-up of 16.5 months for CRT-P and 14.8 months for OPT (RR 0.94, 95% CI 0.70 to 1.25, p = 0.66) (Table 35). The three deaths that occurred in the MUSTIC trial125 were from cardiac causes, with no significant differences between treatment arms (CRT-P 5.2% vs. 0% OPT, RR 7.00, 95% CI 0.37 to 132.56, p = 0.19).
| Study | Follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| MUSTIC125 | 6 months | First period: 1/29 (3.4a), second period: 2/29 (6.9a) | First period 0/29 (0), second period 0/29 (0) | RR 7.00a | 0.37 to 132.56, 0.19a |
| COMPANION119 | CRT-P 16.5 months, OPT 14.8 monthsb | 109/617 (17.7c) | 58d/308 (18.8) | RR 0.94a | 0.70 to 1.25, 0.66a (0.334e) |
| % of deaths | 83.2 | 75.3 | |||
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| CRT-D 16.0 months, OPT 14.8 monthsb | 76/595 (12.8) | 58d/308 (18.8) | RR 0.68a | 0.50 to 0.93, 0.02a (0.006e) | |
| % of deaths | 72.4 | 75.3 | |||
| CRT-P, n/N (%) | CRT-D, n/N (%) | ||||
| CRT-P 16.5 months, CRT-D 16.0 monthsb | 109/617 (17.7c) | 76/595 (12.8) | RR 1.38a | 1.06 to 1.81, 0.02a | |
| % of deaths | 83.2 | 72.4 |
CRT-D compared with optimal pharmacological therapy
The COMPANION trial119 found that the number of cardiac deaths was statistically significantly lower in the CRT-D group than in the OPT group (12.8% vs. 18.8% respectively, p = 0.006), with a median follow-up of 16.0 months for CRT-D and 14.8 months for OPT (RR 0.68, 95% CI 0.50 to 0.93, p = 0.02) (see Table 35).
CRT-P compared with CRT-D
The number of cardiac deaths in the COMPANION trial119 was statistically significantly higher in the CRT-P group than in the CRT-D (RR 1.38, 95% CI 1.06 to 1.81, p = 0.02). However, all comparisons between CRT-P and CRT-D should be treated with caution as the trial was not powered for this comparison.
Heart failure deaths
Both the CARE-HF trial109 and the COMPANION trial119 reported mortality from HF.
CRT-P compared with optimal pharmacological therapy
The CARE-HF trial109 found that mortality attributed to worsening HF was statistically significantly lower in the CRT-P group than in the OPT group (around 9% vs. 16% respectively), with a risk reduction of 45% (HR 0.55, 95% CI 0.37 to 0.82, p = 0.003) at 37.4 months’ follow-up. The risk of HF was reported to be 3.0% per annum for those receiving CRT-P compared with 5.1% per annum for those receiving OPT. The COMPANION trial119 found no statistically significant differences between those receiving CRT-P and those receiving OPT (8.6% vs. 11.0% respectively, HR 0.71, 95% CI 0.46 to 1.09, p = 0.112), with follow-up of 16.5 months for those receiving CRT-P and 14.8 months for those receiving OPT (Table 36).
| Study | Follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | 29.4 monthsa | 33/409 (8.1) | 56/404 (13.9) | RR 0.58 | 0.39 to 0.87, 0.009 |
| a37.4 months (with extension)111 | 38/409 (9.3) | 64/404 (15.8) | HR 0.55 | 0.37 to 0.82, 0.003 | |
| Per annum (%) | 3.0 | 5.1 | |||
| COMPANION119 | CRT-P 16.5 months, OPT 14.8 monthsb | 53/617 (8.6) | 34/308 (11.0) | HR 0.71 | 0.46 to 1.09, 0.112 |
| % of deaths | 40.5 | 44.2 | |||
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| CRT-D 16.0 months, OPT 14.8 monthsb | 52/595 (8.7) | 34/308 (11.0) | HR 0.73 | 0.47 to 1.11, 0.143 | |
| % of deaths | 49.5 | 44.2 | |||
| CRT-P, n/N (%) | CRT-D, n/N (%) | ||||
| CRT-P 16.5 months, CRT-D 16.0 monthsb | 53/617 (8.6) | 52/595 (8.7) | RR 0.98c | 0.68 to 1.42, 0.93c | |
| % of deaths | 40.5 | 49.5 |
The studies were considered sufficiently similar to combine in a meta-analysis. There was no evidence of statistical heterogeneity between the studies (χ2 = 0.99, df = 1, I2 = 0%). The random-effects RR for HF deaths for the comparison between CRT-P and OPT was 0.67 (95% CI 0.51 to 0.88, p = 0.004) (Figure 11).
FIGURE 11.
Heart failure deaths: CRT-P vs. OPT.
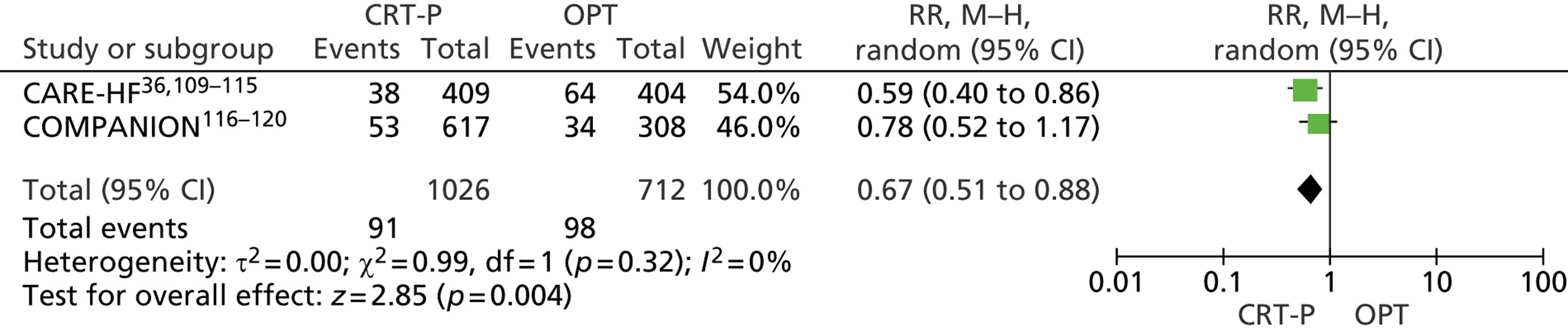
CRT-D compared with optimal pharmacological therapy
The COMPANION trial119 found no statistically significant difference in HF deaths between CRT-D (8.7%) and OPT (11.0%), with a HR of 0.73 (95% CI 0.47 to 1.11, p = 0.143) at 16.0 months’ follow-up for those receiving CRT-D and 14.8 months’ follow-up for those receiving OPT (see Table 36).
CRT-P compared with CRT-D
The HF death rates in the CRT-P and CRT-D groups in the COMPANION trial119 were similar (8.6% vs. 8.7% respectively), with a RR of 0.98 (95% CI 0.68 to 1.42, p = 0.93).
Sudden cardiac death
All trials reported SCDs, although there were uncertainties within the MIRACLE trial data. 121
CRT-P compared with optimal pharmacological therapy
The CARE-HF trial109 found the rate of SCDs to be statistically significantly lower in the CRT-P group than in the OPT group (7.8% vs. 13.4% respectively, HR 0.54, 95% CI 0.35 to 0.84, p = 0.005) at a mean follow-up of 37.4 months. The proportion of SCDs per year was reported to be 2.5% for those receiving CRT-P and 4.3% for those receiving OPT. There were two reported SCDs in the MUSTIC trial,125 one (1/29, 3.4%) in the first crossover period (after 26 days of active pacing) and one (1/29, 3.4%) in the second crossover period (2 hours after switching from inactive to active pacing). No statistical comparison was reported. CRT-P failed to reduce the risk of SCD in the COMPANION trial,119 with more sudden deaths in the group receiving CRT-P than in the group receiving OPT (7.8% vs. 5.8% respectively; HR 1.21, 95% CI 0.70 to 2.07, p = 0.485) at 16.5 months’ follow-up for those receiving CRT-P and 14.8 months’ follow-up for those receiving OPT. The study also reported the proportion of deaths classified as SCD as 36.6% for those receiving CRT-P and 23.4% for those receiving OPT (Table 37).
| Study | Follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | 29.4 monthsa | 29/409 (7.1) | 38/404 (9.4) | RR 0.75b | 0.47 to 1.20, 0.23b |
| a37.4 months111 | 32/409 (7.8) | 54/404 (13.4) | HR 0.54 | 0.35 to 0.84, 0.005 | |
| Per annum (%) | 2.5 | 4.3 | |||
| MUSTIC125 | 6 months | First crossover: 1/29 (3.4b), second crossover: 1/29 (3.4b) | First crossover: 0/29 (0), second crossover: 0/29 (0) | RR 5.00b | 0.25 to 99.82, 0.29b |
| COMPANION119 | CRT-P 16.5 months, OPT 14.8 monthsc | 48/617 (7.8) | 18/308 (5.8) | HR 1.21 | 0.70 to 2.07, 0.485 |
| % of deaths | 36.6 | 23.4 | |||
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| CRT-D 16.0 months, OPT 14.8 monthsc | 17/595 (2.9) | 18/308 (5.8) | HR 0.44 | 0.23 to 0.86, 0.020 | |
| % of deaths | 16.2 | 23.4 | |||
| CRT-P, n/N (%) | CRT-D, n/N (%) | ||||
| CRT-P 16.5 months, CTR-D 16.0 monthsc | 48/617 (7.8) | 17/595 (2.9) | RR 2.72b | 1.58 to 4.68, 0.0003b | |
| % of deaths | 36.6 | 16.2 |
Meta-analysis of the three trials found evidence of substantial statistical heterogeneity between the studies (χ2 = 7.22, df = 2, I2 = 72%). Differences in the rates of SCD between CRT-P and OPT were not statistically significant, with a random-effects RR of 0.97 (95% CI 0.44 to 2.14, p = 0.94) (Figure 12).
FIGURE 12.
Sudden cardiac death: CRT-P vs. OPT.
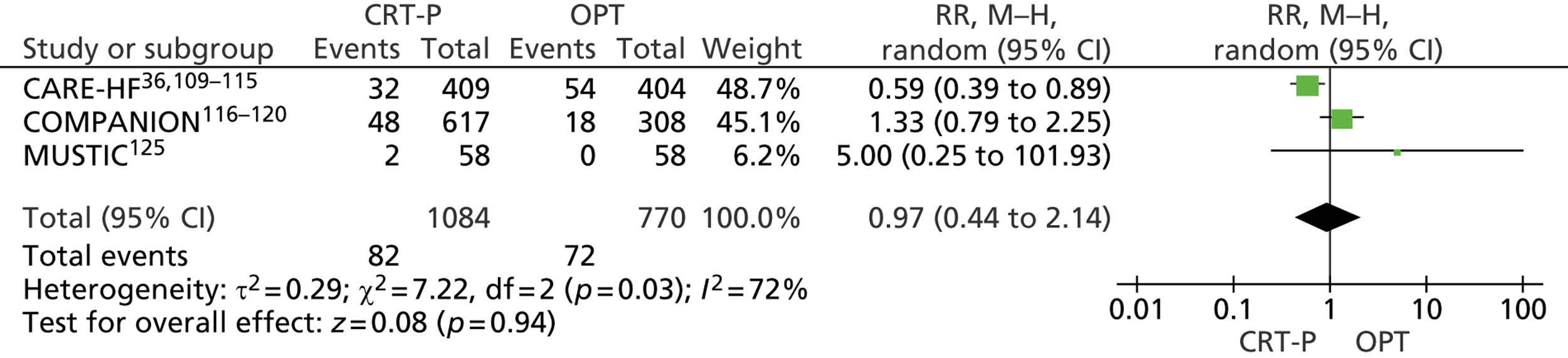
The FDA report123 associated with the MIRACLE trial reported the numbers of SCDs in each arm at 9 months’ follow-up (CRT-P n = 7, OPT n = 5) (the main publication121 reported outcomes at 6 months); however, the numbers in each arm were not reported and the total sample size in the FDA report (n = 536) differed from the number randomised in the main publication (n = 453). 121 If the sample size in each arm is assumed to be the same as the main publication, the RR for the trial is 1.38 (95% CI 0.45 to 4.29). Combining these data with the CARE-HF, COMPANION and MUSTIC data in a meta-analysis gives an overall RR of 1.02 (95% CI 0.54 to 1.94).
CRT-D compared with optimal pharmacological therapy
The COMPANION trial119 found the rate of SCD to be statistically significantly lower in the group receiving CRT-D than in the group receiving OPT (2.9% vs. 5.8% respectively), with a HR of 0.44 (95% CI 0.23 to 0.86, p = 0.020) at 16.0 months’ follow-up for those receiving CRT-D and 14.8 months’ follow-up for those receiving OPT.
CRT-P compared with CRT-D
In the COMPANION trial119 the rate of SCD was statistically significantly higher in the group receiving CRT-P than in the group receiving CRT-D (7.8% vs. 2.9% respectively; RR 2.72, 95% CI 1.58 to 4.68, p = 0.0003). However, all comparisons between CRT-P and CRT-D should be treated with caution as the trial was not powered for this comparison.
Other causes of death
The COMPANION trial119 found no statistically significant difference in the number of non-cardiac deaths between those receiving CRT-P and those receiving OPT (p = 0.122) or between those receiving CRT-D and those receiving OPT (p = 0.717). The numbers of vascular, non-cardiac and unknown deaths appear to be similar between those receiving CRT-P and those receiving CRT-D (Table 38).
| Study | Outcome and follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| COMPANION119 | Vascular deaths, CRT-P 16.5 months, OPT 14.8 monthsa | 5/617 (0.8) | 0 | ||
| % of deaths | 3.8 | ||||
| Non-cardiac deaths | 14/617 (2.3) | 11/308 (3.6) | 0.122 | ||
| % of deaths | 10.7 | 14.3 | |||
| Unknown deaths | 3/617 (0.5) | 8/308 (2.6) | |||
| % of deaths | 2.3 | 10.4 | |||
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| Vascular deaths, CRT-D 16.0, OPT 14.8 monthsa | 3/595 (0.5) | 0 | |||
| % of deaths | 2.8 | ||||
| Non-cardiac deaths | 21/595 (3.5) | 11/308 (3.6) | 0.717 | ||
| % of deaths | 10.7 | 14.3 | |||
| Unknown deaths | 5/595 (0.8) | 8/308 (2.6) | |||
| % of deaths | 4.8 | 10.4 |
Hospitalisations because of heart failure
All four trials reported hospitalisations because of HF. Additional hospitalisation outcomes reported by the trials, including cardiac and non-cardiac hospitalisations, are summarised in Appendix 6.
Number of people hospitalised because of heart failure
The CARE-HF trial109 found that fewer people were hospitalised because of HF in the CRT-P group (CRT-P 17.9% vs. OPT 32.9%; HR 0.48, 95% CI 0.36 to 0.64, p < 0.001) at 29.4 months’ mean follow-up. Similar results were found in the MIRACLE trial121 at 6 months’ follow-up (CRT-P 7.9% vs. OPT 15.1%, HR 0.50, 95% CI 0.28 to 0.88, p = 0.02) and in the COMPANION trial116 at 16.2 months’ follow-up for CRT-P and 11.9 months’ follow-up for OPT (CRT-P 29% vs. OPT 36%, RR 0.80, 95% CI 0.66 to 0.97, p = 0.02) (Table 39). In the MUSTIC trial,125 hospitalisations related to decompensated HF were lower in the group receiving CRT-P (CRT-P 10.3% vs. OPT 31.0%), but this failed to reach statistical significance (RR 0.33, 95% CI 0.10 to 1.11, p < 0.07).
| Study | Outcome and follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | Unplanned hospitalisation with worsening HF, 29.4 monthsa | 72/409 (17.6) | 133/404 (32.9) | HR 0.48 | 0.36 to 0.64, < 0.001 |
| MIRACLE121 | Hospitalisation for worsening HF, 6 months | 18/228 (7.9) | 34/225 (15.1) | HR 0.50 | 0.28 to 0.88, 0.02 |
| MUSTIC125 | Hospital admission because of decompensated HF, 3 monthsb | 3/29 (10.3) | 9/29 (31.0) | RR 0.33c | 0.10 to 1.11, 0.07c,d |
| COMPANION116 | Hospitalised one or more times with HF, CRT-P 16.2 months, OPT 11.9 monthse | 179/617 (29) | 112/308 (36) | RR 0.80c | 0.66 to 0.97, 0.02c |
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| Hospitalised one or more times with HF, CRT-D 15.7 months, OPT 11.9 monthse | 166/595 (28) | 112/308 (36) | RR 0.77c | 0.63 to 0.93, 0.008c |
The trials were combined in meta-analysis; however, the MUSTIC trial125 reported data for the first crossover period only. There was evidence of substantial statistical heterogeneity between the studies (χ2 = 8.50, df = 3, I2 = 65%), but the direction of effect is consistent. The RR of hospitalisation because of HF for CRT-P compared with OPT was 0.61 (95% CI 0.44 to 0.83, p = 0.002), giving a RRR for hospitalisation related to HF with CRT-P of 39% (Figure 13).
FIGURE 13.
Hospitalisations related to HF: CRT-P vs. OPT.
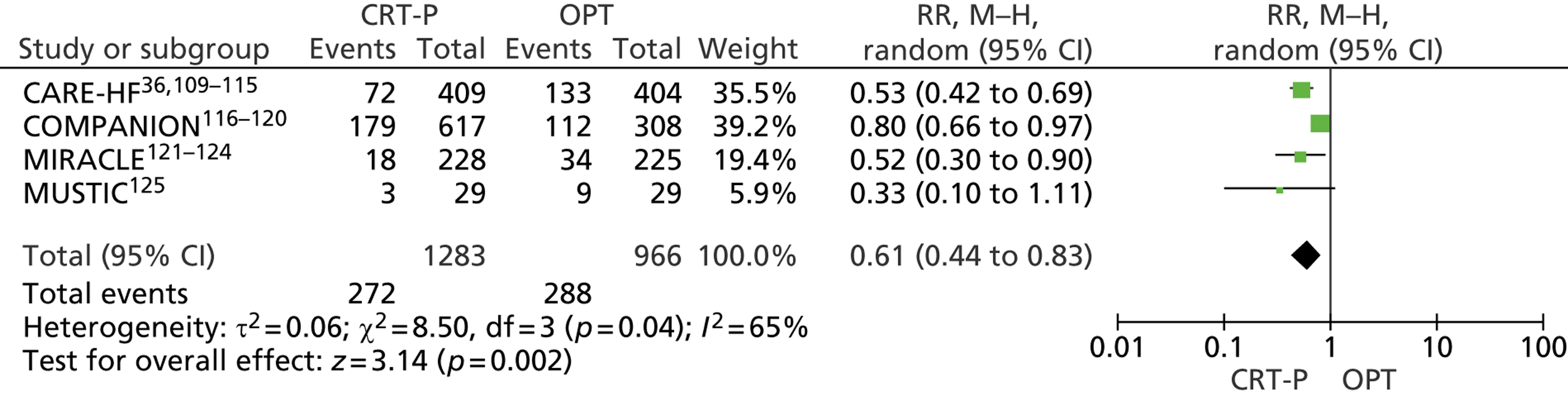
In the COMPANION trial119 there were significantly fewer people admitted to hospital with HF in the CRT-D group than in the OPT group (28% vs. 36% respectively), with a RR of 0.77 (95% CI 0.63 to 0.93, p = 0.008) at a median follow-up of 15.7 months for those receiving CTR-D and 11.9 months for those receiving OPT.
The COMPANION trial report116 states that no significant differences were found in any of the end points between those receiving CRT-P and those receiving CRT-D. In addition, the proportions of people hospitalised at least once with HF were similar in the two groups (28% vs. 29% respectively).
Number of hospitalisation events for heart failure
The CARE-HF,109 COMPANION120 and MIRACLE121 trials reported the number of hospitalisation events and/or number of days hospitalised because of HF. The CARE-HF trial109 reported the number of unplanned hospitalisations of patients because of worsening HF. The COMPANION trial120 reported the number of admissions, the percentage of total admissions and the average number of days hospitalised per patient year of follow-up, whereas the MIRACLE trial121 reported the total number of days hospitalised because of HF (Table 40).
| Study | Outcome and follow-up | CRT-P | OPT | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | Hospitalisation events, 29.4 monthsa | 122 | 252 | ||
| MIRACLE121 | Total no. of days hospitalised, 6 months | 83 | 363 | ||
| No. of hospitalisations | 25 | 50 | |||
| COMPANION120 | CRT-P 16.2 months, OPT 11.9 monthsb | ||||
| No. of admissions (% of total admissions) | 329 (33) | 235 (46) | |||
| Average no. of admissions per patient year of follow-up | 0.41 | 0.73 | |||
| Average days per patient year of follow-up (average length of stay per admission) | 3.6 (8.6) | 5.9 (8.2) | |||
| CRT-D | OPT | ||||
| CRT-D 15.7 months, OPT 11.9 monthsb | |||||
| No. of admissions (% of total admissions) | 333 (36) | 235 (46) | |||
| Average no. of admissions per patient year of follow-up | 0.43 | 0.73 | |||
| Average days per patient year of follow-up (average length of stay per admission) | 3.8 (8.8) | 5.9 (8.2) |
In the CARE-HF trial,109 the 72 participants in the CRT-P group (n = 409) who were hospitalised with worsening HF had a total of 122 hospitalisations, compared with a total of 252 hospitalisations for 133 patients in the OPT group (n = 404). In the COMPANION trial,120 33% of the total admissions were due to HF among patients receiving CRT-P compared with 46% of the total admissions among patients receiving OPT, at a median 16.2 months’ follow-up for those with CRT-P and median 11.9 months’ follow-up for those with OPT. The average number of admissions per patient year of follow-up was also lower in the group receiving CRT-P (CRT-P 0.41 vs. OPT 0.73). The average length of stay per admission was similar between the treatment groups (CRT-P 8.6 days vs. OPT 8.2 days). Similarly, the MIRACLE trial121 found that the total number of days hospitalised because of HF was lower in the CRT-P group than in the OPT group at 6 months’ follow-up (83 days vs. 363 days respectively), but no statistical comparison was reported. However, hospitalisation occurred twice as often in those receiving OPT (OPT 50 events vs. CRT-P 25 events) (see Table 40).
The rate of events was calculated for each trial (no. of events/N × follow-up) and combined in a meta-analysis using the inverse variance method. Although statistical heterogeneity was present (χ2 = 28.27, df = 3, I2 = 89%), the direction of the effect was fairly consistent (Figure 14). A significant reduction in the rate of HF hospitalisations was found in the CRT-P group (RR 0.58, 95% CI 0.35 to 0.96, p = 0.03).
FIGURE 14.
Number of hospitalisations because of HF: CRT-P vs. OPT.
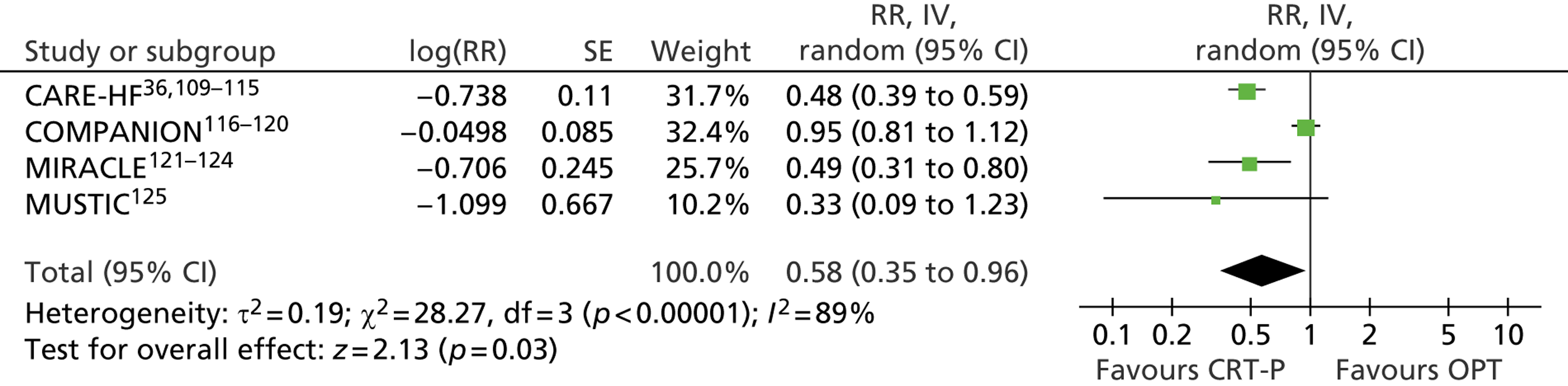
In the COMPANION trial120 the proportion of admissions that were related to HF was lower in the CRT-D group (CRT-P 36% vs. OPT 46%), at a median of 15.7 months’ follow-up for those receiving CRT-P and 11.9 months’ follow-up for those receiving OPT. The average number of admissions per patient year of follow-up was lower in those receiving CRT-D (CRT-D 0.43 vs. OPT 0.73). The average length of stay per admission was similar in both treatment groups (CRT-D 8.8 days vs. OPT 8.2 days) (see Table 40).
The COMPANION trial120 found that there were no significant differences between those receiving CRT-P and those receiving CRT-D for any of the hospitalisation end points; in addition, the proportion of admissions that were related to HF was similar between the groups (33% vs. 36% respectively). This was reflected in both the average number of admissions per patient year of follow-up (CRT-P 0.41 vs. CRT-D 0.43) and the average length of stay per admission (CRT-P 8.6 days vs. 8.8 CRT-D days) (see Table 40).
Arrhythmias
The CARE-HF trial109 reported atrial arrhythmias or ectopy whereas the MUSTIC trial125 reported decompensation due to persistent atrial fibrillation. Because of the different outcome measures used in the two trials, data were not pooled. No comparisons between CRT-D and OPT or between CRT-P and CRT-D were reported.
CRT-P compared with optimal pharmacological therapy
In the CARE-HF trial,109 the risk of arrhythmias or ectopy was significantly higher in the CRT-P group than in the OPT group (15.6% vs. 10.1% respectively; RR 1.54, 95% CI 1.07 to 2.23, p = 0.02). One case of decompensation due to persistent atrial fibrillation occurred in the OPT treatment group during the first crossover period of the MUSTIC trial125 (RR 0.33, 95% CI 0.01 to 8.02, p = 0.50) (Table 41).
| Study | Outcome and follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | Atrial arrhythmias or ectopy, 29.4 monthsa | 64/409 (15.6) | 41/404 (10.1) | RR 1.54b | 1.07 to 2.23, 0.02b |
| MUSTIC125 | Decompensation due to persistent atrial fibrillation, 6 months | First period: 0/29, second period: 0/29 | First period: 1/29 (3.4), second period: 0/29 | RR 0.33b | 0.01 to 8.02, 0.50b |
Worsening heart failure
Three of the trials reported data on worsening HF (not defined by NYHA class), but outcome definitions differed.
CRT-P compared with optimal pharmacological therapy
In the CARE-HF trial,109 fewer people receiving CRT-P experienced worsening HF than those receiving OPT (CRT-P 46.7% vs. OPT 64.9%; RR 0.72, 95% CI 0.63 to 0.82, p < 0.001) (Table 42). In the MIRACLE trial,121 there were fewer people with HF requiring intravenous diuretics (CRT-P 5.7% vs. OPT 10.7%; HR 0.51, 95% CI 0.26 to 1.00, p = 0.05), vasodilators or positive inotropic agents (CRT-P 2.6% vs. OPT 6.2%; HR 0.41, 95% CI 0.16 to 1.08, p = 0.06) or medication for HF (CRT-P 7.0% vs. OPT 15.6%; HR 0.43, 95% CI 0.24 to 0.77, p = 0.004) in the CRT-P group than in the OPT group (see Table 42). The MUSTIC trial125 reported one case of severe decompensation in the CRT-P off group, leading to a premature switch to active pacing (RR 0.33, 95% CI 0.01 to 8.02, p = 0.50). Despite the different definitions used by the trials, the risk of worsening HF was reduced with CRT-P when the trials were combined in a meta-analysis (RR 0.71, 95% CI 0.63 to 0.80, p < 0.00001) (Figure 15). No significant statistical heterogeneity was observed.
| Study | Outcome and follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | Worsening HF, 29.4 monthsa | 191/409 (46.7) | 263/405 (64.9) | RR 0.72b | 0.63 to 0.82,b < 0.001 |
| MIRACLE121 | HF requiring intravenous medication, 6 months | ||||
| Diuretic agents | 13/228 (5.7) | 24/225 (10.7) | HR 0.51 | 0.26 to 1.00, 0.05 | |
| Vasodilators or positive inotropic agents | 6/228 (2.6) | 14/225 (6.2) | HR 0.41 | 0.16 to 1.08, 0.06 | |
| Medication for HF | 16/228 (7.0) | 35/225 (15.6) | HR 0.43 | 0.24 to 0.77, 0.004 | |
| MUSTIC125 | Severe decompensation, 6 months | First period: 0/29 (0), second period: 0/29 (0) | First period: 1/29 (3.4), second period: 0/29 (0) | RR 0.33b | 0.01 to 8.02, 0.50b |
FIGURE 15.
Worsening HF: CRT-P vs. OPT.
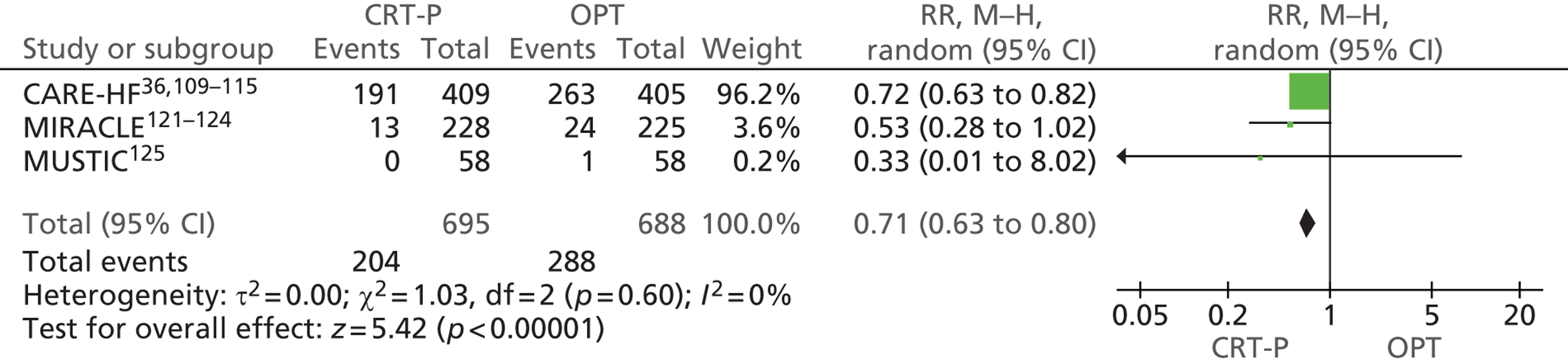
Change in New York Heart Association class
The CARE-HF,109 COMPANION116 and MIRACLE121 trials reported improvement in NYHA class. The three trials included people in NYHA classes III and IV at baseline. The CARE-HF trial109 reported NYHA class at 18 months and mean NYHA class at 90 days; the MIRACLE trial121 reported improvements in NYHA class at 6 months; and the COMPANION trial116 reported NYHA class at 3 and 6 months. NYHA class was one of three reported primary end points in the MIRACLE trial. 121
CRT-P compared with optimal pharmacological therapy
All three trials reported a statistically significant greater proportion of participants with improvement in NYHA class with CRT-P than with OPT (Table 43). The CARE-HF trial109 also reported an improvement in mean NYHA class with CRT-P [CRT-P 2.1 (SD 1.0) vs. OPT 2.7 (SD 0.9), p < 0.001]. There was no evidence of statistical heterogeneity between the studies when the data were pooled in a random-effects meta-analysis (χ2 = 70, df = 2, I2 = 0%) (Figure 16). The pooled data from all three trials showed an increase in the proportion of people with an improvement of one or more NYHA class in the CRT-P group compared with the OPT group (RR 1.68, 95% CI 1.52 to 1.86, p < 0.00001).
| Study | Outcome and follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CARE-HF109 | NYHA class, 18 months | ||||
| Class I | 105/409 (25.7) | 39/404 (9.7) | RR 1.67a,b | 1.44 to 1.93, < 0.00001a,b | |
| Class II | 150/409 (36.7) | 112/404 (27.7) | |||
| Class III or IV | 80/409 (19.6) | 152/404 (37.6) | |||
| NYHA class at 90 days, mean (SD) | 2.1 (1.0) | 2.7 (0.9) | MD 0.6 | 0.4 to 0.7, < 0.001 | |
| MIRACLE121 | 6 months | ||||
| Improved by two or more classes | 34/211 (16) | 12/196 (6) | RR 1.80b | 1.47 to 2.20, < 0.00001b | |
| Improved by one class | 109/211 (52) | 62/196 (32) | |||
| No change | 64/211 (30) | 115/196 (59) | |||
| Worsened | 4/211 (2) | 7/196 (4) | |||
| COMPANION116 | Improvement in NYHA class symptoms | ||||
| 3 months | 320c/551 (58) | 58c/242 (24) | < 0.001 | ||
| 6 months | 298c/489 (61) | 76b/199 (38) | RR 1.60b | 1.32 to 1.93, < 0.00001b,d | |
| CRT-D | OPT | ||||
| 3 months | 299c/543 (55) | 58c/242 (24) | < 0.001 | ||
| 6 months | 283c/497 (57) | 76c/199 (38) | RR 2.14b | 2.14 to 1.53, < 0.00001b,d | |
| CRT-P | CRT-D | ||||
| 3 months | 320c/551 (58) | 299c/543 (55) | |||
| 6 months | 298c/489 (61) | 283c/497 (57) | RR 0.93b | 0.84 to 1.04, 0.20b | |
FIGURE 16.
Participants with improvement by one or more NYHA class: CRT-P vs. OPT.
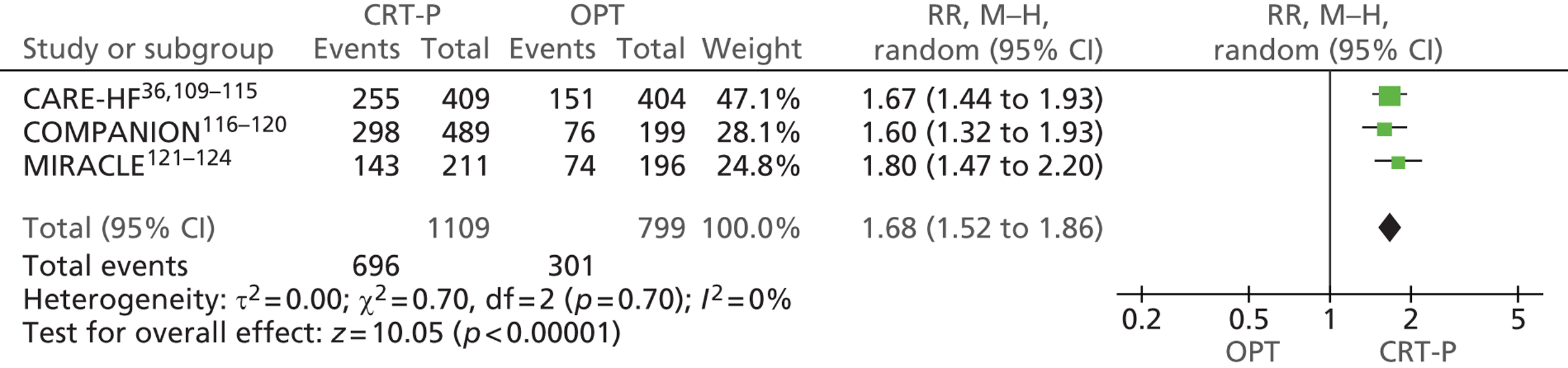
CRT-D compared with optimal pharmacological therapy
In the COMPANION trial,116 the proportion of people with an improvement in NYHA class was statistically significantly greater in the CRT-D group than in the OPT group at both 3 months (CRT-D 55% vs. OPT 24%, p < 0.001) and 6 months (CRT-D 57% vs. OPT 38%, p < 0.001) (see Table 43).
CRT-P compared with CRT-D
In the COMPANION trial116 the proportion of people with an improvement in NYHA class was similar between the CRT-P group and the CRT-D group at both 3 months (58% vs. 55% respectively) and 6 months (61% vs. 57% respectively; RR 0.93, 95% CI 0.84 to 1.04, p = 0.20) (see Table 43). However, this comparison should be treated with caution as the trial was not powered for this comparison.
Change in left ventricular ejaculation fraction
Only one trial reported LVEF. The MIRACLE trial121 reported absolute change in median LVEF at 6 months for those receiving CRT-P and those receiving OPT. No comparisons between CRT-D and OPT or between CRT-P and CRT-D were reported.
CRT-P compared with optimal pharmacological therapy
The MIRACLE trial121 reported an improvement in median LVEF with CRT-P (+4.6, 95% CI 3.2 to 6.4), but LVEF decreased with OPT (–0.2, 95% CI –1.0 to 1.5). The difference between the two changes was statistically significant at 6 months’ follow-up (p < 0.001).
Exercise capacity
The COMPANION trial116 reported the mean increase in 6-minute walk distance at 3 and 6 months, whereas the MIRACLE trial121 reported the median change from baseline in 6-minute walk distance and median change in total exercise time. Change in 6-minute walk distance was one of three primary end points in this trial. The MUSTIC trial125 reported mean distance walked in 6 minutes at 3 months (Table 44). The CARE-HF trial109 did not report 6-minute walk distance. Only two trials reported change in peak oxygen consumption. The MIRACLE trial121 reported the median change in oxygen consumption (VO2) and the MUSTIC trial125 reported mean VO2 (Table 45). No comparisons between CRT-D and OPT or between CRT-P and CRT-D were reported.
| Study | Outcome and follow-up | CRT-P | OPT | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| MIRACLE121 | 6 months | ||||
| Change in 6-minute walk distance (m), median (95% CI, SD) | +39 (26 to 54, 103.9a) (n = 214) | +10 (0 to 25, 89.2a) (n = 198) | 0.005 | ||
| Change in total exercise time (seconds), median (95% CI) | +81 (62 to 119) (n = 159) | +19 (–1 to 47) (n = 146) | 0.001 | ||
| MUSTIC125 | Distance walked in 6 minutes (m), mean (SD) | ||||
| Group 1 (CRT-P on, CTR-P off) (n = 22) | 384.1 (78.9) | 336.1 (128.3) | |||
| Group 2 (CRT-P off, CRT-P on) (n = 24) | 412.9 (116.9) | 316.2 (141.8) | |||
| Both groups (n = 46) | 399.2 (100.5) | 325.7 (134.4) | < 0.001 | ||
| COMPANION116 | Change in 6-minute walk distance (m), mean (SD) | ||||
| 3 months | 33 (99) (n = 422) | 9 (84) (n = 170) | < 0.001 | ||
| 6 months | 40 (96) (n = 373) | 1 (93) (n = 142) | < 0.001 | ||
| CRT-D | OPT | ||||
| Change in 6-minute walk distance (m), mean (SD) | |||||
| 3 months | 44 (109) (n = 420) | 9 (84) (n = 170) | < 0.001 | ||
| 6 months | 46 (98) (n = 378) | 1 (93) (n = 142) | < 0.001 | ||
| CRT-P | CRT-D | ||||
| Change in 6-minute walk, m, mean change (SD) | |||||
| 3 months | 33 (99) (n = 422) | 44 (109) (n = 420) | |||
| 6 months | 40 (96) (n = 373) | 46 (98) (n = 378) | MD –6.0a | –19.87 to 7.87, 0.40a | |
| Study | Outcome and follow-up | CRT-P | OPT | Effect | p-value |
|---|---|---|---|---|---|
| MIRACLE121 | Change in VO2 (ml/kg/minute), median (95% CI), 6 months | +1.1 (0.6 to 1.7) (n = 158) | +0.2 (–0.2 to 0.8) (n = 145) | 0.009 | |
| MUSTIC125 | VO2 (ml/kg/minute), mean (SD), 3 months | ||||
| Group 1 (CRT-P on, CTR-P off) (n = 18) | 15.9 (5.8) | 15.3 (5.9) | |||
| Group 2 (CRT-P off, CRT-P on) (n = 20) | 16.4 (3.6) | 14.8 (3.9) | |||
| Both groups (n = 38) | 16.2 (4.7) | 15 (4.9) | 0.029 | ||
CRT-P compared with optimal pharmacological therapy
In all three trials, the distance walked in 6 minutes was statistically significantly greater for the CRT-P group than the OPT group (see Table 44). In the MIRACLE trial,121 the CRT-P group also had a superior outcome for change in total exercise time (CRT-P 81 seconds vs. OPT 19 seconds, p = 0.001).
The trials were combined in a meta-analysis. For meta-analysis of the MUSTIC crossover trial,125 data were combined from both periods. This method provides a conservative analysis, with the study being underweighted rather than overweighted. 65 Trials reporting change values and final values were included in separate subgroups. There was some evidence of statistical heterogeneity between the studies with the inclusion of the MUSTIC trial125 (χ2 = 2.93, df = 2, I2 = 32%). The improvement in distance walked in 6 minutes was statistically significantly greater for those receiving CRT-P than for those receiving OPT [mean difference (MD) 38.14, 95% CI 21.74 to 54.54, p < 0.00001] (Figure 17).
FIGURE 17.
Change in 6-minute walk distance at 6 months.
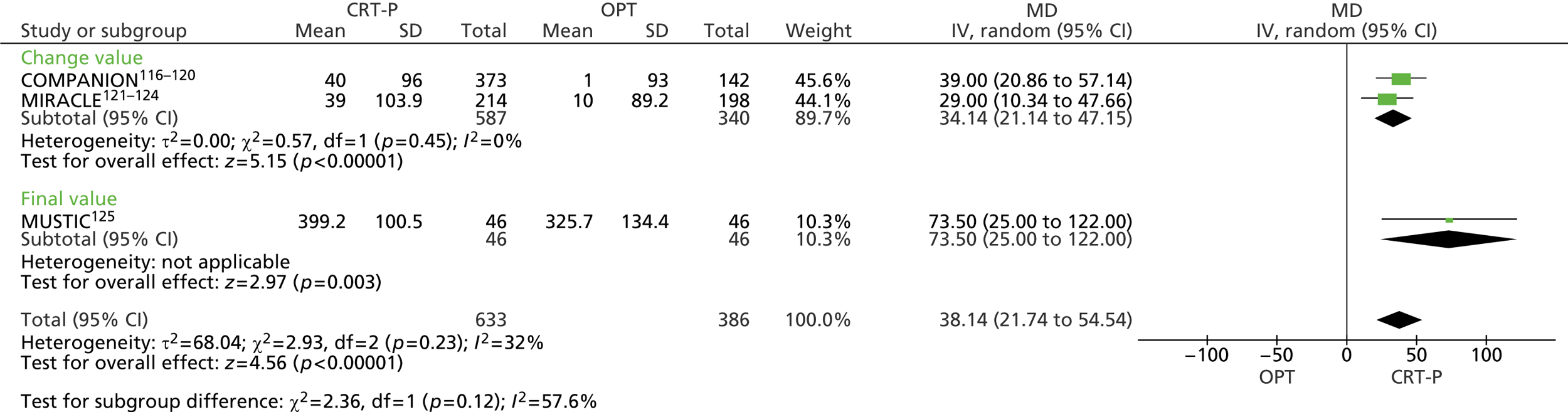
The MIRACLE trial121 reported statistically significantly greater improvements in VO2 with CRT-P than with OPT (+1.1 units vs. +0.2 units respectively, p = 0.009). In the MUSTIC trial,125 the authors combined the data from both crossover periods for the statistical analysis, which demonstrated a significantly greater VO2 in those receiving CRT-P (CRT-P 16.2 units vs. OPT 15 units, p = 0.029).
CRT-D compared with optimal pharmacological therapy
In the COMPANION trial,116 the improvement in 6-minute walk distance was statistically significantly greater with CRT-D than with OPT at 3 months (44 m vs. 9 m respectively, p < 0.001) and 6 months (46 m vs. 1 m respectively, p < 0.001).
CRT-D compared with CRT-P
There were no statistically significant differences in 6-minute walk distance between those receiving CRT-D and those receiving CRT-P (MD –6.0, 95% CI –19.87 to 7.87, p = 0.40). However, all comparisons between CRT-P and CRT-D should be treated with caution, as the trial was not powered for this comparison.
Quality of life
All four studies reported change in QoL assessed using the MLWHFQ. Change in MLWHFQ score was the primary outcome in the MUSTIC trial. 125 The CARE-HF trial113 also reported EQ-5D scores, mean quality-adjusted life-years (QALYs) and mean life-years (Table 46).
| Study | Outcome and follow-up | CRT-P | OPT | MD (95% CI), p-value |
|---|---|---|---|---|
| CARE-HF113 | QALYs, mean (95% CI) | (n = 409) | (n = 404) | |
| 3 months | 0.16 (0.15 to 0.16) | 0.15 (0.14 to 0.15) | 0.01 (0.001 to 0.018), 0.285 | |
| 18 months | 0.95 (0.91 to 0.99) | 0.82 (0.78 to 0.86) | 0.13 (0.07 to 0.018), < 0.0001 | |
| End of study, mean 37.4 months | 1.45 (1.38 to 1.53) | 1.22 (1.15 to 1.29) | 0.23 (0.13 to 0.33), < 0.0001 | |
| Life-years, mean (95% CI) | ||||
| 3 months | 0.241 (0.238 to 0.244) | 0.241 (0.238 to 0.244) | 0.0003 (–0.004 to 0.0045), 0.90 | |
| 18 months | 1.37 (1.34 to 1.40) | 1.33 (1.29 to 1.37) | 0.04 (–0.01 to 0.09), 0.13 | |
| End of study, mean 37.4 months | 2.07 (1.99 to 2.15) | 1.96 (1.88 to 2.05) | 0.10 (–0.01 to 0.22), 0.07a | |
| EQ-5D, mean (95% CI) | ||||
| Baseline | 0.60 (0.58 to 0.63) | 0.60 (0.57 to 0.63) | – | |
| 90 days109 | 0.70 (SD 28) | 0.63 (SD 0.29) | 0.08 (0.04 to 0.12), 0.001 | |
| 3 months | 0.69 (0.66 to 0.72) | 0.61 (0.59 to 0.64) | 0.08 (0.04 to 0.11), < 0.0001 | |
| 18 months | 0.61 (0.58 to 0.64) | 0.51 (0.48 to 0.54) | 0.10 (0.06 to 0.15), < 0.0001 | |
| End of study, mean 37.4 months | 0.56 (0.52 to 0.59) | 0.43 (0.39 to 0.46) | 0.13 (0.08 to 0.18), < 0.0001b | |
| MLWHFQ,c mean (95% CI) | ||||
| Baseline | 44.6 (42.5 to 46.7) | 43.7 (41.5 to 45.8) | – | |
| 90 days109 | 31 (SD 22) | 40 (SD 22) | –10 (–8 to –12), < 0.001 | |
| 3 months | 30.1 (27.9 to 32.3) | 38.9 (36.6 to 41.2) | –10.6 (–8.1 to –13.1), < 0.0001d | |
| 18 months | 28.4 (26.2 to 30.5) | 36.0 (33.5 to 38.5) | –10.7 (–7.6 to –13.8), < 0.0001d | |
| End of study, mean 37.4 months | 27.2 (24.9–29.5) (SD 23.7) | 35.1 (32.6–37.6) (SD 25.6) | –10.1 (–6.8 to –13.3), < 0.0001d | |
| MIRACLE121 | Change in MLWHFQ scorec | (n = 213) | (n = 193) | |
| 6 months, median (95% CI), SD | –18 (–22 to –12), 37 | –9 (–12 to –5), 24.7 | 0.001 | |
| MUSTIC125 | MLWHFQ score,c mean (SD) | |||
| Group 1 (CRT-P on, CRT-P off) (n = 23) | 33.3 (22) | 42.6 (20.9) | ||
| Group 2 (CRT-P off, CRT-P on) (n = 22) | 25.7 (20.4) | 44.0 (25) | ||
| Both groups (n = 45) | 29.6 (21.3) | 43.2 (22.8) | < 0.001 | |
| COMPANION116 | MLWHFQ (% increase), mean (SD) | |||
| 3 months | –24 (27) (n = 510) | –9 (21) (n = 243) | < 0.001 | |
| 6 months | –25 (26) (n = 460) | –12 (23) (n = 207) | < 0.001 | |
| CRT-D | OPT | |||
| 3 months | –24 (28) (n = 514) | –9 (21) (n = 243) | < 0.001 | |
| 6 months | –26 (28) (n = 478) | –12 (23) (n = 207) | < 0.001 | |
| CRT-P | CRT-D | |||
| 3 months | –24 (27) (n = 510) | –24 (28) (n = 514) | ||
| 6 months | –25 (26) (n = 460) | –26 (28) (n = 478) | 1.00 (2.46 to 4.46), 0.57e | |
CRT-P compared with optimal pharmacological therapy
All four trials showed statistically significant improvements in MLWHFQ scores in the CRT-P group compared with the OPT group (lower scores indicate better QoL). The trials were combined in a meta-analysis. The COMPANION trial116 and the MIRACLE trial121 reported mean change in MLWHFQ score from baseline whereas the CARE-HF trial113 and the MUSTIC trial125 reported final mean values. The MUSTIC trial125 reported data per crossover period and combined data for both crossover periods (Figure 18).
FIGURE 18.
Change in MLWHFQ scores.
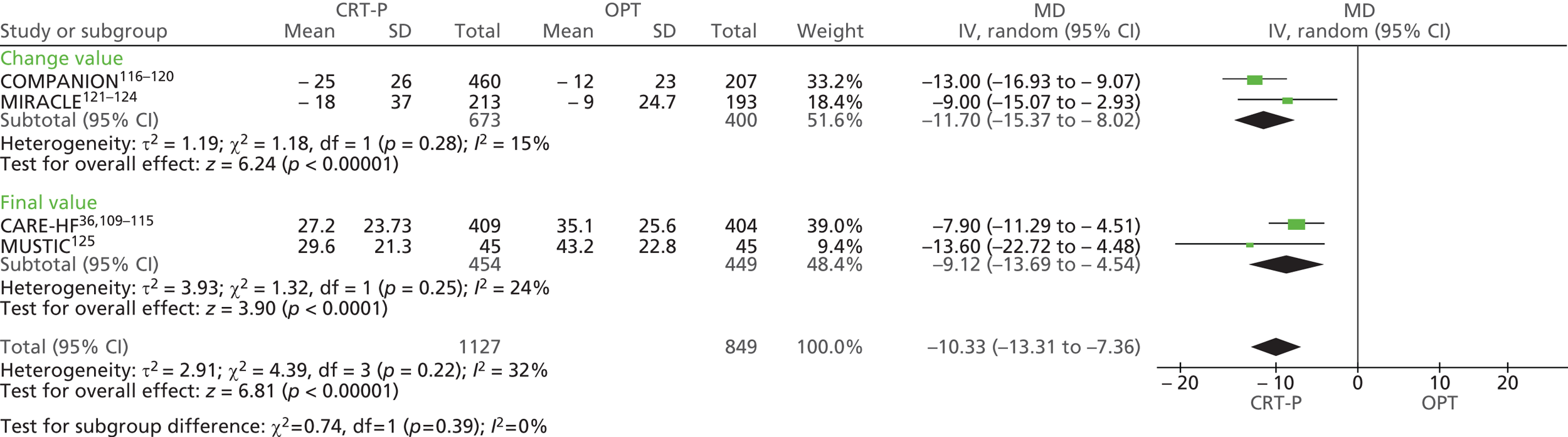
For meta-analysis of the MUSTIC crossover trial,125 the combined data from both crossover periods were included, as this method provides a conservative analysis, with the study being underweighted rather than overweighted. 65 There was some evidence of statistical heterogeneity between the studies (χ2 = 4.39, df = 3, I2 = 32%), but the direction of effect was consistent. The MD was –10.33 (95% CI –13.31 to –7.36) and MLWHFQ scores were statistically significantly lower in the CRT-P group than in the OPT group (p = 0.00001), indicating improved QoL.
Other QoL measures with statistically significant improvements in the CARE-HF trial113 were the EQ-5D and QALYs. The mean value of the EQ-5D was statistically significantly higher in the CRT-P group at each follow-up (90 days: CRT-P 0.70 vs. OPT 0.63, p < 0.001; 3 months: CRT-P 0.69 vs. OPT 0.61, p < 0.0001; 18 months: CRT-P 0.61 vs. OPT 0.51, p < 0.0001; end of study CRT-P 0.56 vs. OPT 0.43, p < 0.0001), although scores appeared to be lower by the end of the study (37.4 months) than at baseline in both treatment arms. The mean QALYs were statistically significantly higher in the CRT-P group at 18 months (CRT-P 0.95 vs. OPT 0.82, p < 0.0001) and at the end of the study (CRT-P 1.45 vs. OPT 1.22, p < 0.0001).
CRT-D compared with optimal pharmacological therapy
The reduction in MLWHFQ score, indicating improved QoL, in the COMPANION trial116 was statistically significantly greater in the CRT-D group at both 3 months (CRT-D –24 vs. OPT –9, p < 0.001) and 6 months (CRT-D –26 vs. OPT –12, p < 0.001).
CRT-P compared with CRT-D
In the COMPANION trial,116 improvements in MLWHFQ scores were similar in the CRT-P group and the CRT-D group at 6 months (–25 vs. –26 respectively, MD 1.00, 95% CI –2.46 to 4.46, p = 0.57).
Adverse events
Reporting of adverse events was limited, as can be seen in Tables 47 and 48. All participants in the MIRACLE trial121 and the MUSTIC trial125 were implanted with a CRT-P device, with pacing inactive in the control (OPT) group. Both trials randomised only those people who had a successful implantation, although the MIRACLE trial121 also reported adverse events for all enrolled participants (including 71 participants who were part of a pilot phase and not included in the effectiveness results) (see Table 47).
| Study | Adverse events | CRT device, n/N (%) |
|---|---|---|
| MIRACLE121 (enrolled n = 571; successfully implanted n = 528; randomised n = 453: CRT-P n = 228, OPT n = 225) | All participants undergoing implantation (n = 571) | |
| Unsuccessful implantation | 43/571 (7.5) | |
| Complete heart block requiring permanent cardiac pacing | 2/571 (0.4) | |
| Death from clinical events during implant procedure (progressive hypotension; asytole) | 2/571 (0.4) | |
| Coronary sinus dissection | 23/571 (4.0) | |
| Cardiac vein or coronary sinus perforationa | 12/571 (2.1) | |
| Participants who had successful implantation (n = 528) | ||
| Left ventricular lead repositioned | 20/528 (3.8) | |
| Left ventricular lead replaced | 10/528 (1.9) | |
| Pacemaker-related infection requiring explantation | 7/528 (1.3) | |
| Hospitalised for repositioning/replacement of left ventricular lead | ||
| CRT-P on | 11/228 (4.8) | |
| CRT-P off | 3/225 (1.3) | |
| MUSTIC125 (enrolled n = 67; randomised n = 58: CRT-P on, CRT-P off n = 29; CRT-P off, CRT-P on n = 29) | Unsuccessful implantation | 5/64 (7.8) |
| Early lead dislodgement | 8/58 (13.8) | |
| CRT-P on | ||
| Uncorrectable loss of left ventricular pacing efficacy | 2/58 (3.4) | |
| Decompensation attributed to rapidly progressive aortic stenosis | 1/58 (1.7) | |
| CRT-P off | ||
| Severe decompensating leading to a premature switch to active pacing | 1/58 (1.7) | |
| Decompensation due to persistent atrial fibrillation | 1/58 (1.7) | |
| Study | Adverse events | CRT-P, n/N (%) | OPT, n/N (%) | RR (95% CI), p-value |
|---|---|---|---|---|
| CARE-HF109 [enrolled and randomised n = 813: CRT-P n = 409, OPT n = 404 (CRT-P off)] | Unsuccessful implantation | 19/409 (4.6) | ||
| Device-related death | ||||
| HF aggravated by lead displacement | 1/409 (0.2) | |||
| Septicaemia after receiving a device | 1/404 (0.2) | |||
| Most common adverse device- or procedure-related events | ||||
| Lead displacement | 24/409 (5.9) | |||
| Coronary sinus dissection | 10/409 (2.4) | |||
| Pocket erosion | 8/409 (2.0) | |||
| Pneumothorax | 6/409 (1.5) | |||
| Device-related infection | 3/409 (0.7) | |||
| COMPANION116 (enrolled and randomised n = 1520: CRT-P n = 617, CRT-D n = 595, OPT n = 308) | Unsuccessful implantation | 78/617 (12.6) | ||
| Deaths from procedural complications | 5/615 (0.8) | |||
| Mortality rate 30 days after randomisation | 6b/617 (1.0) | 4b/308 (1.3) | 0.34 | |
| Moderate or severe adverse event from any cause | 407b/617 (66) | 188b/308 (61) | 0.15 | |
| Moderate or severe adverse event related to implantation procedure | 62b/617 (10) | |||
| Coronary venous dissection | 2b/617 (0.3) | |||
| Coronary venous perforation | 7b/617 (1.1) | |||
| Coronary venous tamponade | 3b/617 (0.5) | |||
| CRT-D, n/N (%) | OPT, n/N (%) | |||
| Unsuccessful implantation | 54/595 (9.1) | |||
| Deaths from procedural complications | 3/595 (0.5) | |||
| Mortality rate 30 days after randomisation | 11b/595 (1.8) | 4/308 (1.3) | 0.97 | |
| Moderate or severe adverse event from any cause | 411b/595 (69) | 188/308 (61) | 0.03 | |
| Moderate or severe adverse event related to implantation procedure | 48b/595 (8) | |||
| Coronary venous dissection | 3b/595 (0.5) | |||
| Coronary venous perforation | 5b/595 (0.8) | |||
| Coronary venous tamponade | 2b/595 (0.3) | |||
| CRT-P, n/N (%) | CRT-D, n/N (%) | |||
| Mortality rate 30 days after randomisation | 6b/617 (1.0) | 11b/595 (1.8) | 0.53 (0.20, 1.41), 0.20b | |
| Moderate or severe adverse event from any cause | 407b/617 (66) | 411b/595 (69) | 0.95 (0.88, 1.03), 0.25b |
The CARE-HF109 and COMPANION116 trials randomised participants to receive either a CRT-P (or CRT-D) device or OPT only (see Table 48). However, the CARE-HF109 trial limited reporting of adverse events to device-related complications. Only the COMPANION trial116 reported any statistical comparison between CRT-P or CRT-D and OPT for adverse events.
Between 4.6%109 and 12.6%116 of device implantations were unsuccessful in the trials (see Tables 47 and 48). Death from adverse clinical events during the implantation procedure occurred among 0.4% of all participants in the MIRACLE trial,121 whereas in the COMPANION trial116 0.8% of CRT-P recipients and 0.5% of CRT-D recipients died from procedural complications. The mortality rate 30 days after randomisation was not statistically significantly different between the OPT group (1.2%), the CRT-P group (1.0%, p = 0.34) and the CRT-D group (1.8%, p = 0.97),116 or between CRT-P and CRT-D (RR 0.53, 95% CI 0.20 to 4.41, p = 0.2). Device-related deaths occurred among 0.2% of participants randomised to CRT-P in the CARE-HF trial109 and among 0.2% of those randomised to OPT (after receiving a device), although the time period was not reported.
Moderate or severe adverse events related to the implantation procedure occurred in 10% of the CRT-P group and 8% of the CRT-D group in the COMPANION trial. 116 The most commonly reported adverse events were coronary sinus/venous dissection (0.3% CRT-P, 0.5% CRT-D;116 4.0%;121 2.4%109) or perforation (1.1% CRT-P, 0.8% CRT-D;116 2.1%121) and lead-related events (6%;109,121 13.8%125). In the MIRACLE trial,121 hospitalisation for repositioning or replacement of the left ventricular lead was more frequent in those in the CRT-P on group (4.8%) than those in the CRT-P off group (1.3%).
In the COMPANION trial,116 the proportion of moderate or severe adverse events from any cause was statistically significantly higher in the CRT-D group than in the OPT group (69% vs. 61% respectively, p = 0.03), but there was no statistically significant difference between the CRT-P group and the OPT group (66% vs. 61% respectively, p = 0.15) or between the CRT-P group and the CRT-D group (RR 0.95, 95% CI 0.88 to 1.03, p = 0.25). The CARE-HF trial109 reported that the frequency of respiratory tract infections, hypotension, falls or syncope, acute coronary syndromes, renal dysfunction, ventricular arrhythmias or ectopy, and neurological events was similar in the CRT-P and OPT only groups.
Subgroup analyses reported by included randomised control trials
Only the CARE-HF trial109 presented subgroup analyses that were clearly predefined (Tables 49 and 50). The trial reported LVEF in people with or without ischaemic heart disease. A statistically significant interaction between CRT-P and aetiology was found (p = 0.003), whereby people with non-ischaemic heart disease experienced a greater change in LVEF (see Table 49).
| Study | Outcome and follow-up | CRT-P | OPT | p-value | ||
|---|---|---|---|---|---|---|
| IHD (n = 168) | non-IHD (n = 197) | IHD (n = 135) | non-IHD (n = 235) | |||
| CARE-HF115 | LVEF (%) at baseline, median (IQR) | 25 (22 to 29) | 24 (21 to 29) | 26 (22 to 30) | 24 (21 to 29) | 0.1867 (IHD vs. non-IHD) |
| Mean (SD) change in LVEF at 18 months (%)a | 6.1 (1.2) | 10.9 (1.5) | 1.3 (0.7) | 2.4 (1.7) | 0.003 for interaction between CRT and aetiology | |
| Study | Subgroup | Patients with event/total no. of patientsa | HR (95% CI) |
|---|---|---|---|
| CARE-HF109 | Overall with primary end point | 383/813 | 0.63 (0.51 to 0.77) |
| Age (years)b | |||
| < 66.4 | 163/406 | 0.55 (0.40 to 0.75) | |
| ≥ 66.4 | 220/407 | 0.68 (0.52 to 0.89) | |
| Sex | |||
| Male | 290/597 | 0.62 (0.49 to 0.79) | |
| Female | 93/215 | 0.64 (0.42 to 0.97) | |
| NYHA class | |||
| III | 349/763 | 0.64 (0.52 to 0.80) | |
| IV | 34/50 | 0.50 (0.25 to 1.01) | |
| Dilated cardiomyopathy | |||
| No | 238/443 | 0.68 (0.53 to 0.88) | |
| Yes | 145/370 | 0.51 (0.36 to 0.73) | |
| Systolic blood pressure (mmHg)b | |||
| < 117 | 208/401 | 0.60 (0.46 to 0.80) | |
| ≥ 117 | 170/402 | 0.66 (0.48 to 0.89) | |
| NT-proBNP (pg/ml)c | |||
| < 214.5 | 122/366 | 0.53 (0.36 to 0.76) | |
| ≥ 214.5 | 224/366 | 0.70 (0.54 to 0.91) | |
| Ejection fraction (%)b | |||
| < 24.7 | 205/372 | 0.65 (0.49 to 0.86) | |
| ≥ 24.7 | 152/373 | 0.62 (0.44 to 0.85) | |
| End-systolic volume index (ml/m2)b | |||
| < 119.2 | 156/366 | 0.71 (0.52 to 0.98) | |
| ≥ 119.2 | 193/366 | 0.54 (0.40 to 0.73) | |
| QRS interval (milliseconds) | |||
| < 160 | 152/290 | 0.74 (0.54 to 1.02) | |
| ≥ 160 | 222/505 | 0.60 (0.46 to 0.79) | |
| Interventricular mechanical delay (milliseconds)b | |||
| < 49.2 | 199/367 | 0.77 (0.58 to 1.02) | |
| ≥ 49.2 | 147/368 | 0.50 (0.36 to 0.70) | |
| Mitral regurgitation area (cm2)b | |||
| < 0.218 | 114/302 | 0.86 (0.60 to 1.25) | |
| ≥ 0.218 | 175/303 | 0.56 (0.41 to 0.75) | |
| Glomerular filtration rate (ml/minute/1.73 m2)b | |||
| < 60.3 | 196/369 | 0.67 (0.50 to 0.89) | |
| ≥ 60.3 | 142/370 | 0.57 (0.40 to 0.80) | |
| Beta-blockers | |||
| No | 131/227 | 0.72 (0.51 to 1.02) | |
| Yes | 252/586 | 0.59 (0.46 to 0.76) | |
| Spironolactone | |||
| No | 166/356 | 0.58 (0.43 to 0.79) | |
| Yes | 217/457 | 0.67 (0.51 to 0.88) | |
| Loop diuretics | |||
| < 80 mg of furosemide or equivalent | 181/461 | 0.56 (0.42 to 0.76) | |
| ≥ 80 mg of furosemide or equivalent | 202/352 | 0.69 (0.53 to 0.92) | |
| Digoxin | |||
| No | 218/467 | 0.66 (0.50 to 0.86) | |
| Yes | 165/346 | 0.59 (0.43 to 0.81) | |
The effect of CRT-P on the composite end point (death from any cause or unplanned hospitalisation for a major cardiovascular event) in predefined subgroups with analysis stratified for NYHA class (except the subgroup analysis of NYHA class) can be seen in Table 50. The overall effect of CRT-P on the composite end point was a HR of 0.63 (95% CI 0.51 to 0.77) and there was little difference in this outcome for any of the predefined subgroups.
Summary of clinical effectiveness: people with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony
-
Four RCTs, with a combined total of 2844 participants, comparing CRT-P (and CRT-D in one trial) with OPT in people with HF as a result of LVSD and cardiac dyssynchrony were included. The trial comparing CRT-P and CRT-D with OPT randomised participants to each of the three groups but did not perform a direct comparison between CRT-D and CRT-P.
-
There was some risk of bias in the trials in relation to performance, detection and reporting bias, although risk was unclear in some cases because of inadequate reporting.
-
Length of follow-up in the trials varied and included 3 months, 6 months, a median of 11.9–15.7 months and a mean of 37.4 months (including an extension period). Sample size ranged from 58 to 1520 participants. The majority of participants had NYHA class III symptoms; the remaining few had NYHA class IV symptoms.
CRT-P compared with optimal pharmacological therapy
-
Meta-analysis found that CRT-P significantly reduced the risk of all-cause mortality (four trials; RR 0.75, 95% CI 0.58 to 0.96, p = 0.02), HF deaths (two trials; RR 0.67, 95% CI 0.51 to 0.88, p = 0.004) and HF hospitalisations (four trials; RR 0.61, 95% CI 0.44 to 0.83, p = 0.002).
-
Combining three RCTs in a meta-analysis demonstrated no significant difference in number of SCDs between the groups (RR 0.97, 95% CI 0.44 to 2.14, p = 0.94). One RCT (COMPANION) reported no statistically significant difference in total cardiac deaths (CRT-P 17.7% vs. OPT 18.8%, p = 0.334) or non-cardiac deaths (CRT-P 2.3% vs. OPT 3.6%, p = 0.122).
-
More people receiving CRT-P had an improvement of one or more NYHA class (RR 1.68, 95% CI 1.52 to 1.86, p < 0.00001) in the three trials reporting this outcome.
-
One RCT reported change in LVEF, showing a statistically significant improvement in LVEF with CRT-P compared with OPT (4.6% vs. –0.2%, p < 0.001) at 6 months.
-
There was a greater improvement in exercise capacity with CRT-P, as measured by the distance walked in 6 minutes (meta-analysis of three trials; change from baseline or final values, MD 38.14 m, 95% CI 21.74 to 54.54 m, p < 0.00001). A statistically significant improvement in peak oxygen consumption was also reported by two of the RCTs.
-
All four RCTs found statistically significant improvements in QoL (MLWHFQ) in the CRT-P group (change scores or final values, MD –10.33, 95% CI –13.31 to –7.36). One trial (CARE-HF) also reported statistically significant improvements in the CRT-P group in the EQ-5D (MD 0.13, 95% CI 0.08 to 0.18, p < 0.0001) and in QALYs (MD 0.23, 95% CI 0.13 to 0.33, p < 0.00001) at the end of the study (mean 37.4 months).
-
One trial reported prespecified subgroup analysis. A significant interaction between CRT-P and aetiology was found, whereby people with non-ischaemic heart disease had a greater change in LVEF. There was little difference in the effect of CRT-P on the composite outcome (death from any cause or unplanned hospitalisation for a major cardiovascular event) for 16 predefined subgroups.
CRT-D compared with optimal pharmacological therapy
-
One trial compared CRT-D with OPT. All-cause mortality (HR 0.64, 95% CI 0.48 to 0.86, p = 0.003), total cardiac deaths (RR 0.68, 95% CI 0.50 to 0.93, p = 0.02), SCDs (HR 0.44, 95% CI 0.23 to 0.86, p = 0.02) and HF hospitalisations (RR 0.77, 95% CI 0.63 to 0.93, p = 0.008) were reduced with CRT-D compared with OPT.
-
There were no significant differences in HF deaths (HR 0.73, 95% CI 0.47 to 1.11, p = 0.143) or non-cardiac deaths (CRT-D 2.3% vs. OPT 3.6%, p = 0.717) between the groups.
-
The proportions of people with an improvement of one or more NYHA class (CRT-D 57% vs. OPT 38%, p < 0.001), improvement in exercise capacity (change in 6-minute walk distance: CRT-D 46 m vs. OPT 1 m, p < 0.001) and improvement in QoL (MLWHFQ) score (CRT-D –26 vs. OPT –12, p < 0.001) at 6 months were statistically significantly greater in the CRT-D group.
CRT-P compared with CRT-D
-
One three-arm trial compared both CRT-P and CRT-D with OPT, but the trial was not powered for a statistical comparison between CRT-P and CRT-D. Statistical comparisons between CRT-P and CRT-D have been undertaken for the purposes of this review but should be viewed with caution.
-
Total cardiac deaths (RR 1.38, 95% CI 1.06 to 1.81, p = 0.02) and SCDs (RR 2.72, 95% CI 1.58 to 4.68, p = 0.0003) were higher in the CRT-P group than in the CRT-D group. All-cause mortality (RR 1.20, 95% CI 0.96 to 1.52, p = 0.12), HF deaths (RR 0.98, 95% CI 0.68 to 1.42, p = 0.93) and HF hospitalisations (28% vs. 29%) were similar in the CRT-P group and the CRT-D group.
-
Changes in NYHA class, exercise capacity and QoL were similar in the CRT-P group and the CRT-D group.
Adverse events
-
Two trials randomised people with successful implantation only. The other two trials reported device-related deaths of between 0.2% and 0.8% for those receiving CRT-P and 0.5% for those receiving CRT-D. Moderate or severe adverse events related to the implantation procedure were reported in 10% of the CRT-P group and 8% of the CRT-D group in one trial, with 13% and 9% of CRT-P and CRT-D implantations, respectively, unsuccessful. Moderate or severe adverse events from any cause were more common among those receiving CRT-D than among those receiving OPT (CRT-D 69%, CRT-P 66%, OPT 61%; CRT-D vs. OPT p = 0.03, CRT-P vs. OPT p = 0.15). Reported complications included lead displacements, infections and coronary sinus dissections.
People with both conditions
Quantity and quality of research available
Nine RCTs comparing CRT-D and ICDs in people at risk of SCD as a result of ventricular arrhythmias and with HF as a result of LVSD and cardiac dyssynchrony met the inclusion criteria. Five of these trials reported their findings in more than one paper; a summary of the included papers for each trial can be seen in Table 51.
| Study | Publicationa |
|---|---|
| CONTAK-CD | Higgins et al. 2003,126 Lozano et al. 2000,128 US Food and Drug Administration 2002,129 Saxon et al. 1999127 |
| MADIT-CRT | Moss et al. 2009130 and 2005,131 Solomon et al. 2010,132 Goldenberg et al. 2011,134,145 Arshad et al. 2011135 |
| MIRACLE ICD | Young et al. 2003 136 |
| MIRACLE ICD II | Abraham et al. 2004 137 |
| Piccirillo 2006 | Piccirillo et al. 2006 138 |
| Pinter 2009 | Pinter et al. 2009 139 |
| RAFT | Tang et al. 2010140 and 2009141 |
| RethinQ | Beshai et al. 2007,142 Beshai and Grimm 2007143 |
| RHYTHM ICD | US Food and Drug Administration 2004,144 US Food and Drug Administration 2005145 |
One of these studies (CONTAK-CD126) was included in the 2007 TAR on CRT;64 however, participants in the CONTAK-CD trial126 were required to have VT as an indication for ICD and defibrillating capacity was available to the control group and it is therefore discussed here rather than in the previous section.
No trials comparing CRT-D with OPT or CRT-D with CRT-P were identified for this population.
Characteristics of the included studies
Study characteristics are summarised in Table 52 and participant characteristics are summarised in Table 53. Further details can be found in the data extraction forms in Appendix 9.
| Parameter | CONTAK-CD126 | MADIT-CRT130 | MIRACLE ICD136 | MIRACLE ICD II137 | Piccirillo138 | Pinter139 | RAFT140 | RethinQ142 | Rhythm ICD144 |
|---|---|---|---|---|---|---|---|---|---|
| Study design | Crossover/parallel RCT | RCT | RCT | RCT | RCT | RCT | RCT | RCT | RCT |
| Intervention | CRT-D + OPT | CRT-D + OPT | CRT-D + OPT | CRT-D + OPT | CRT-D | CRT-D | CRT-D + OPT | CRT-D + OPT | CRT-D |
| Comparator | CRT off + OPT | ICD + OPT | CRT off + OPT | CRT off + OPT | ICD | CRT off + OPT | ICD + OPT | CRT off + OPT | CRT off + OPT |
| Country (no. of centres) | USA (47) | USA (88), Canada (2), Europe (20) | USA, Canada (63) | USA, Canada (63) | Italy (1) | Canada (7) | Canada (24), Europe and Turkey (8), Australia (2) | USA (34) | Unclear (50) |
| Sample size randomised | 490 | 1820 | 369 | 186 | 31 | 72 | 1798 | 172 | 179 |
| Length of follow-up | Max. 6 months | Average 2.4 years | 6 months | 6 months | 1 year | 6 months | Mean 40 (SD 20) months | 6 months | Average 12.1 (3.4) months |
| Key inclusion criteria | Intraventricular conduction delay and malignant VT/VF; NYHA classes II–IV; LVEF ≤ 35%; QRS interval ≥ 120 milliseconds; conventional indications for an ICD | Ischaemic or non-ischaemic CM; NYHA class I or II; LVEF ≤ 30%; ≥ QRS interval ≥ 130 milliseconds; sinus rhythm; met guideline indication for ICD therapy | CHF; stable drug regimen for ≥ 1 month; NYHA class III or IV; LVEF ≤ 35%; ≥QRS interval ≥ 130 milliseconds; LVEDD ≥ 55 mm; cardiac arrest due to VT or VF | Chronic HF; NYHA class II; LVEF ≤ 35%; ≥QRS interval ≥ 130 milliseconds; LVEDD ≥ 55 mm; indication for ICD therapy | Chronic HF secondary to ischaemic DCM; LVEF ≤ 35%; QRS interval > 120 milliseconds; sinus rhythm; prophylactic treatment with an ICD or CRT-D | Symptoms of dyspnoea or fatigue on climbing one or two flights or 6-MWD ≤ 450 m; ≥ 2 weeks drugs;a LVEF ≤ 35%; QRS interval > 120 milliseconds; sinus rhythm; high risk of sudden death and eligible for an ICD | Ischaemic or non-ischaemic causes; OPT; NYHA class II or III; LVEF ≤ 30%; QRS interval ≥ 120 or paced ≥ 200 milliseconds; sinus rhythm or permanent atrial fibrillation;b planned ICD implantation, primary or secondary prevention | Ischaemic or non-ischaemic CM; narrow QRS interval; intraventricular dyssynchrony; OPT; NYHA class III; LVEF ≤ 35%; QRS interval < 130 milliseconds; approved indication for an ICD | Symptomatic HF for ≥ 6 months; ≥ 90 of days OPT; NYHA class III or IV; LVEF ≤ 35%; QRS interval ≥ 150 milliseconds; ICD indication for VT |
| Parameter | CONTAK-CD126 | MADIT-CRT130 | MIRACLE ICD136 | MIRACLE ICD II137 | Piccirillo138 | Pinter139 | RAFT140 | RethinQ142 | Rhythm ICD144 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | |
| Sample size, n | 245 | 245 | 1089 | 731 | 187 | 182 | 85 | 101 | 16 | 15 | 36 | 36 | 894 | 904 | 87 | 85 | 119 | 59 |
| Age (years), mean (SD) | 66 (11) | 66 (11) | 65 (11) | 64 (11) | 66.6 (11.3) | 67.6 (9.2) | 63.0 (12.8) | 63.1 (12.1) | 65 (4) | 65 (8) | 66.3 (8.6) | 66.1 (8.8) | 66.1 (9.3) | 66.2 (9.4) | 60 (12) | 58 (14) | NR | NR |
| Sex, % male | 85 | 83 | 74.7 | 75.6 | 75.9 | 77.5 | 88.2 | 90.1 | 81 | 80 | 77.8 | 80.6 | 84.8 | 81.0 | 71 | 58 | NR | NR |
| IHD, % | 67 | 71 | 55 | 55 | 64.0 | 75.8 | 55.3 | 58.4 | 100 | 100 | 77.8 | 80.6 | 68.7 | 64.9 | 54 | 51 | NR | NR |
| NYHA class, % | ||||||||||||||||||
| I | 0 | 0 | 14.0 | 15.5 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR | 0 | 0 | 0 | 0 | 0.8 | 3.4 |
| II | 32 | 33 | 86 | 84.5 | 0 | 0 | 100 | 100 | 0 | 0 | NR | NR | 79.2 | 80.8 | 0 | 0 | 5.0 | 6.8 |
| III | 60 | 57 | 0 | 0 | 88.2 | 89.6 | 0 | 0 | 31.3 | 33.3 | NR | NR | 20.8 | 19.2 | 100 | 99a | 87.4 | 84.7 |
| IV | 8 | 10 | 0 | 0 | 11.8 | 10.4 | 0 | 0 | 68.8 | 66.7 | NR | NR | 0 | 0 | 0 | 0 | 6.7 | 5.1 |
| LVEF (%), mean (SD) | 21 (7) | 22 (7) | 24 (5) | 24 (5) | 24.2 (6.5) | 23.9 (6.0) | 24.4 (6.6) | 24.6 (6.7) | 23 (4) | 22 (8) | 21.2 (7.9)b | 24.0 (8.3)b | 22.6 (5.4) | 22.6 (5.1) | 25 (5) | 26 (6) | 25.6 (8.3) | 23.3 (6.4) |
| QRS interval (milliseconds) | ||||||||||||||||||
| Mean (SD) | 160 (27) | 156 (26) | 165 (22) | 162 (22) | 166 (25) | 165 (23) | 160 (4) | 159 (8) | NR | NR | 157 (23.6) | 158.3 (24.0) | 107 (12) | 106 (13) | 169 (16) | 167 (15) | ||
| ≥ 150, % | 64.2 | 65.1 | ||||||||||||||||
| < 120, % | 76 | 71 | ||||||||||||||||
| ≥ 120, % | 24 | 29 | ||||||||||||||||
| LBBB/RBBB, % | 54/14 | 55/12 | 70/13 | 71/13 | NR/13 | NR/13 | NR/12 | NR/21 | 73/8 | 71/10 | ||||||||
Intervention and comparators
The participants in six of these trials126,136,137,139,142,144 were implanted with a device that could provide both CRT and ICD therapy, and the devices in the comparator groups provided back-up ventricular pacing and active ICD therapy only (CRT off). In three of the trials130,138,140 the comparator group received an ICD only device. Participants in both groups of all trials also received OPT (discussed further in Pharmacological therapy).
Participants
Participants in eight of these studies were required to have guideline indications for ICD therapy (see Table 52). Piccirillo and colleagues138 state that the participants were undergoing prophylactic treatment with the ICD or CRT-D. Pinter and colleagues139 enrolled people who ‘were not candidates for CRT therapy based on guidelines at the time of the study’ (p. 1510); however, such patients would now be considered to have a conventional indication for CRT.
The trials differed in their eligibility criteria for severity of HF (see Table 52). The majority of participants in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT),130 the Multicenter InSync ICD II Randomized Clinical Evaluation (MIRACLE ICD II)137 and the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT)140 were in NYHA class II; in the CONTAK-CD RCT,126 the Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD),136 the Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS (RethinQ) trial142 and the Resynchronization for the HemodYnamic Treatment for Heart failure Management Implantable Cardioverter Defibrillator (RHYTHM ICD) trial144 the majority of participants were in NYHA class III; and the majority of participants in the study by Piccirillo and colleagues138 were in NYHA class IV (see Table 53). NYHA class was not reported by Pinter and colleagues139 although the eligibility criteria required mild to moderate HF. The proportion of participants with ischaemic heart disease varied between the trials, from around 52% (RethinQ142) to 100% (Piccirillo and colleagues138). The RethinQ trial142 enrolled people with ischaemic or non-ischaemic cardiomyopathy and the study by Piccirillo and colleagues138 enrolled people with ischaemic dilated cardiomyopathy.
The RethinQ trial142 differed from the other trials in the criteria used to define cardiac dyssynchrony. Conventionally, a wide QRS interval indicates electrical dyssynchrony. This trial, however, recruited people with a narrow QRS interval (< 130 milliseconds) and evidence of mechanical dyssynchrony on echocardiography. Mean QRS interval in this trial was about 107 milliseconds and approximately one-quarter of participants had a QRS duration of ≥ 120 milliseconds.
Mean QRS interval in the other eight trials, when reported, ranged from 156 milliseconds (CONTAK-CD126) to 169 milliseconds (RHYTHM ICD144). Pinter and colleagues139 did not report baseline QRS duration but required a minimum duration of 120 milliseconds for study eligibility. The Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT trial)130 required participants to have a QRS duration of at least 130 milliseconds and reported that around 65% of participants had a QRS interval of ≥ 150 milliseconds at baseline. Mean LVEF ranged from 21% (CONTAK-CD126) to 26% (RethinQ142).
The mean age of the participants in the trials was similar, ranging from 63 years (MIRACLE ICD II137) to 67 years (MIRACLE ICD136). The majority of participants (from 75% in MADIT-CRT130 to 90% in MIRACLE ICD II137) were men.
Pharmacological therapy
Table 54 displays medication at baseline. The majority of participants in all studies received ACE inhibitors and/or ARBs, although the proportion receiving beta-blockers varied between studies. Less than half of participants in the CONTAK-CD study,126 around 60% of participants in the MIRACLE ICD136 and MIRACLE ICD II137 trials and around 80–95% of participants in the MADIT-CRT,130 Piccirillo and colleagues,138 RAFT,140 RethinQ142 and RHYTHM ICD144 trials received beta-blockers. AAD use also varied between the studies: around 33–35% of participants in the MIRACLE ICD II study,137 33–42% of participants in the MIRACLE ICD study,136 less than one-quarter of participants in the RHYTHM ICD trial,144 around 15% of participants in the RAFT trial,140 8–12% in the RethinQ trial142 and around 7% in the MADIT-CRT trial130 were receiving AADs. Pharmacological therapy in each of these trials would be considered optimal or close to optimal by current standards, although beta-blocker use in the MIRACLE ICD trials was slightly low.
| Medication | CONTAK-CD126 | MADIT-CRT130 | MIRACLE ICD136 | MIRACLE ICD II137 | Piccirillo138 | RAFT140 | RethinQ142 | Rhythm ICD144 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | CRT-D | ICD | |
| Sample size, n | 245 | 245 | 1089 | 731 | 187 | 182 | 85 | 101 | 16 | 15 | 894 | 904 | 87 | 85 | 119 | 59 |
| ACE inhibitor, % | 77.0 | 77.0 | 92.5 | 89.0 | 97.6 | 95.0 | 100 | 100 | ||||||||
| ACE inhibitor/substitutes/ARB, % | 86 | 89 | 96.1 | 97.1 | 89 | 91 | 71.4 | 74.6 | ||||||||
| ARB, % | 20.8 | 20.2 | 20.2 | 16.9 | ||||||||||||
| AADs, % | 42.3 | 33.0 | 35.3 | 32.7 | 8 | 12 | 24.4 | 22.0 | ||||||||
| Amiodarone | 7.2 | 7.0 | 15.7 | 13.7 | ||||||||||||
| Other AAD | 1.3 | 0.9 | ||||||||||||||
| Class I AAD | 1.1 | 0.4 | ||||||||||||||
| Anticoagulants and antiplatelets, % | 85.7 | 81.4 | ||||||||||||||
| Acetylsalicylic acid (aspirin) | 100 | 93 | 65.3 | 68.8 | ||||||||||||
| Clopidogrel | 15.0 | 16.0 | ||||||||||||||
| Warfarin | 34.7 | 33.0 | ||||||||||||||
| Beta-blockers, % | 48 | 46 | 93.3 | 93.2 | 62.0 | 58.2 | 63.5 | 63.4 | 90.4 | 89.0 | 97 | 93 | 79.8 | 88.1 | ||
| Biskoprolol | 13 | 7 | ||||||||||||||
| Carvedilol | 81 | 80 | ||||||||||||||
| Calcium channel blockers, % | 11.3 | 9.2 | 9.2 | 15.3 | ||||||||||||
| Diuretics, % | 88 | 83 | 75.7 | 72.9 | 93.1 | 94.5 | 87.1 | 80.2 | 84.7 | 83.6 | 84 | 87 | 86.6 | 91.5 | ||
| Furosemide | 100 | 100 | ||||||||||||||
| Aldosterone antagonist | 32.3 | 30.9 | ||||||||||||||
| Spironolactone | 56 | 67 | 41.6 | 41.8 | ||||||||||||
| Nitrates, % | 32.8 | 39.0 | ||||||||||||||
| Positive inotropics/glycoside, % | 61.3 | 66.1 | ||||||||||||||
| Digitalis | 26.7 | 24.2 | ||||||||||||||
| Digoxin | 69 | 68 | 75 | 73 | ||||||||||||
| Statins, % | 67.5 | 67.2 | 67.9 | 68.4 | ||||||||||||
Key outcomes
The primary outcomes differed between the trials. All nine trials reported all-cause mortality but none as a primary outcome. Also reported were total cardiac deaths (seven trials126,137–140,142,144), death from HF (four trials126,137–139), SCD (six trials126,136–138,142,144) and death from other causes (six trials126,137–139,142,144). Three trials126,138,140 reported hospitalisation because of HF, six trials126,136–138,142,144 reported NYHA class and eight trials126,130,136–139,142,144 reported LVEF. Six trials126,136,137,139,142,144 reported exercise capacity assessed by the 6-minute walk test and/or peak oxygen consumption, and QoL assessed by the MLWHFQ. The primary outcome of three trials126,130,140 was a composite outcome; these can be seen in the data extraction forms in Appendix 9 but have not been presented here.
Setting
Other than the single-centre study by Piccirillo and colleagues,138 the trials were multicentre with the majority of the centres in the USA and Canada. Only one of the studies130 had a centre in the UK.
The number of participants randomised ranged from 31138 to 1820. 130 The length of follow-up was 6 months in the CONTAK-CD,126 MIRACE ICD,136 MIRACLE ICD II,137 Pinter and colleagues139 and RethinQ142 studies, 12 months in the Piccirillo and colleagues139 and RHYTHM ICD144 studies and an average of 2.4 years in the MADIT-CRT study130 and 40 months in the RAFT study. 140
Risk of bias
The risk of bias in the included studies is summarised in Table 55 and further details for each study can be found in the data extraction tables in Appendix 9. Only three of the studies136,137,142 were at low risk of selection bias. The MADIT-CRT study130 did not report the randomisation method used, although sufficient details were reported to establish that the allocation sequence was adequately concealed. The remaining studies did not report details of the randomisation method or allocation sequence concealment; therefore, the risk of selection bias is unclear.
| Domain | Judgement | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CONTAK-CD126 | MADIT-CRT130 | MIRACLE ICD136 | MIRACLE ICD II137 | Piccirillo138 | Pinter139 | RAFT140 | RethinQ142 | Rhythm ICD144 | |
| Selection bias | |||||||||
| Random sequence generation | Unclear | Unclear | Low | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Allocation concealment | Unclear | Low | Low | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Performance bias | |||||||||
| Blinding of participants and personnel | Low | High | Low | Low | High | Low | Low | Unclear | Unclear |
| Detection bias | |||||||||
| Blinding of outcome assessment | Low | High | Low | Low | High | Low | Low | Low | Unclear |
| Attrition bias | |||||||||
| Incomplete outcome data addressed | Primary – low; other – high | Survival – low; other – high | Primary – unclear; other – high | Unclear | Low | Low | Low | Primarya – low; other – high | Low |
| Reporting bias | |||||||||
| Selective reporting | Low | Low | Low | Low | Low | Low | High | Low | Unclear |
| Other bias | |||||||||
| Other sources of bias | Unclear | Low | Unclear | Low | Low | Low | Low | Low | Unclear |
There is a high risk of performance bias and detection bias in the MADIT-CRT study;130 treating physicians were aware of study group assignments, and diagnosis of HF and decisions about therapy or hospital admission were made by physicians aware of assignments, although members of the mortality and HF committees were unaware of study group assignments. Details of blinding of participants and personnel were not reported by Piccirillo and colleagues138 and, although spectral recording assessment was blinded, details of blinding of other outcomes were not reported. The RethinQ142 and RHYTHM ICD144 studies are described as ‘double blind’ but further details such as who was blinded and how this was maintained were not reported. However, outcome assessors were unaware of treatment assignment in the RethinQ trial. 142 There was a low risk of performance bias and detection bias in the other studies. 126,136,137,139,140
Risk of attrition bias in the CONTAK-CD trial126 was low for the primary outcome but high for other outcomes. MADIT-CRT130 was judged to have a low risk of bias for survival but a high risk of bias for ventricular remodelling outcomes. Risk of attrition bias was unclear for primary outcomes and high for secondary outcomes in MIRACLE ICD136 and unclear in MIRACLE ICD II. 137 The RethinQ trial142 was judged to have a low risk of attrition bias for primary and secondary outcomes but a high risk of bias for additional outcomes when missing data were not accounted for. The other studies138–140,144 had a low risk of attrition bias.
The RAFT study140 was considered to have a high risk of selective reporting bias as outcomes included in the protocol (e.g. QoL) were not reported in the trial publication. However, it is noted that this was a recent study and data may have been published after the completion of this report. The RHYTHM ICD study report was available only from the FDA website and does not appear to have been published in a journal. It is not clear whether selected outcomes have been presented to meet the needs of the FDA approval process. The other studies126,130,136–139,142 were judged to have a low risk of selective reporting bias.
The risks of other sources of bias were unclear in three studies. The study design, primary outcome measure and length of follow-up were changed during the course of the CONTAK-CD study,126 but the potential for these issues to introduce bias into the results is unknown. Because of a lack of details in the RHYTHM ICD report,144 the risk of other sources of bias is unclear. The sponsors (Medtronic Inc.) of the MIRACLE ICD study136 appear to have been involved in all aspects of the study, although the risk of bias from this is unclear. The other studies130,137–140,142 were judged to have a low risk of bias.
Methodological comments
Similarity of groups at baseline
The groups were generally well balanced at baseline (see Table 53). However, the ICD group of the MIRACLE ICD study136 had a higher proportion of participants with ischaemic heart disease. In the RHYTHM ICD study,144 the ICD group performed significantly better in the exercise test for peak VO2 (a primary outcome) and included a lower proportion of men, although the authors state that none of the differences was significant (statistical analysis not presented).
Sample size
Four of the trials were adequately powered to show a difference in their primary outcome(s). These were the MIRACLE ICD trial136 (a difference in NYHA class of 0.75, in QoL of 13 points or in 6-minute walk distance of 50 m), the trial by Pinter and colleagues139 (a 12% decrease in end-systolic volume), the RAFT study140 (a 25% relative reduction in the composite outcome) and the RethinQ trial142 (a difference of 23% in the proportion of patients who achieved the primary end point).
The actual event rate observed in the CONTAK-CD trial126 was approximately half that expected in the original study design and consequently the authors state that the study was not adequately powered to detect a statistically significant difference in HF events. The MADIT-CRT study130 was stopped on the recommendation of the independent data and safety monitoring board when the monitoring statistic reached the prespecified efficacy boundary. The study was then unblinded and analyses were limited to events occurring before trial termination. The MIRACLE ICD study136 was not powered to detect a morbidity or mortality difference. The study by Piccirillo and colleagues138 was a small study of 31 participants. The study report does not include details of a sample size calculation, and mortality and NYHA were not primary outcomes and therefore it is assumed that the trial was not powered to detect these outcomes. The MIRACLE ICD II137 and RHYTHM ICD144 studies do not report sample size calculations.
Crossovers
Crossovers between groups were reported by six of the trials. Crossover from the ICD group to the CRT-D group occurred in 2.8%139–12.4%130 of participants, the most common reason being for HF events (Table 56). Crossover from the CRT-D group to the ICD group occurred in 0%142–7.5%130 of participants, most commonly because of difficulties with the left ventricular/CRT pacing lead (see Table 56).
| Study | CRT-D, n/N (%) | ICD, n/N (%) |
|---|---|---|
| MADIT-CRT130 | 82/1089 (7.5) (technical difficulties positioning CRT pacing lead) | 91/731 (12.4) (30 before reaching an end point, 61 after HF event) |
| MIRACLE ICD136 | 10/187 (5) (two ventricular lead dislodgement, two diaphragmatic stimulation, six programming errors) | 14/182 (8) (11 worsening HF, two bradycardia, one programming error) |
| MIRACLE ICD II137 | 2/85 (2) (left ventricular lead dislodgement in one patient and diaphragmatic stimulation in biventricular and right ventricular pacing modes in one patient) | 5/101 (5) (bradycardia in three patients, centre error in one patient and pacemaker dependency after atrioventricular node ablation for atrial flutter in one patient) |
| Pinter139 | 1/36 (2.8) (late left ventricular capture failure) | 1/36 (2.8) (worsening CHF) |
| RAFT140 | Not reported | 96/904 (10.6) (36 before primary outcome, 60 after HF hospitalisation) |
| RethinQ142 | 0/87 (0) | 3/85 (3.5) (because of worsening HF) |
Other issues
There were some differences between studies in the timing of implantation, baseline evaluation and randomisation. The MADIT-CRT,130 Piccirillo and colleagues138 and RAFT140 studies randomised participants before or at the time of implantation. The CONTAK-CD trial126 implanted the device first because of the immediate need for ICD therapy and then programmed the randomised therapy after a minimum 30-day period with no CRT, during which time investigators were permitted to optimise pharmacological therapy.
The other studies136,137,139,142,144 randomised only those participants who were successfully implanted. In the MIRACLE ICD study136 randomisation occurred within 7 days of successful implantation; in the study by Pinter and colleagues139 participants were randomly assigned following completion of baseline procedures 14–28 days post implant; and in the RethinQ142 and RHYTHM ICD144 studies baseline evaluation occurred 14 days post implant, followed by randomisation.
The study design of the CONTAK-CD trial126 was modified because of regulatory concerns about morbidity and mortality associated with CRT and the length of follow-up in the randomised mode. This meant that the design changed from a randomised crossover design with crossover to occur after 3 months of randomised therapy (Phase I) to a parallel RCT design with 6 months of follow-up (Phase II). Data from both phases are reported.
The study by Piccarillo and colleagues138 was a small study that aimed to assess whether spectral indexes obtained by power spectral analysis of heart rate variability could predict malignant ventricular arrhythmias in patients. These data are beyond the scope of this report and have not been included. The study also reported mortality and NYHA class although these were not specified as primary or secondary outcomes.
The RAFT study140 initially enrolled both NYHA class II and NYHA class III patients; however, after a protocol revision in February 2006 the study enrolled only NYHA class II patients. Primary and secondary outcomes for patients with NYHA class II or NYHA class III HF were therefore analysed separately.
The RHYTHM ICD study144 has not been published in a journal. Data have been extracted from the FDA report but limited methodological details are reported.
Assessment of effectiveness
All-cause mortality
All nine trials reported data on all-cause mortality, although only two130,140 compared events between groups statistically (Table 57). The MADIT-CRT study130 found no statistically significant difference in all-cause mortality after an average follow-up of 2.4 years (CRT-D 6.8% vs. ICD 7.3%, HR 1.00, 95% CI 0.69 to 1.44, p = 0.99), whereas the RAFT study140 found a statistically significant reduction in mortality with CRT-D (CRT-D 20.8% vs. ICD 26.1%, HR 0.75, 95% CI 0.62 to 0.91, p = 0.003). Analysis of the remaining trials (CONTAK-CD:126 CRT-D 4.5% vs. ICD 6.5%, RR 0.69, 95% CI 0.33 to 1.45, p = 0.33; MIRACLE ICD:136 CRT-D 7.5% vs. ICD 8.2%, RR 0.91, 95% CI 0.45 to 1.83, p = 0.79; MIRACLE ICD II:137 CRT-D 2.4% vs. ICD 2.0%, RR 1.19, 95% CI 0.17 to 8.26, p = 0.86; Piccirillo and colleagues:138 CRT-D 0% vs. ICD 0%; Pinter and colleagues:139 CRT-D 2.8% vs. ICD 2.8%, RR 1.00, 95% CI 0.07 to 15.38, p = 1.00; RethinQ:142 CRT-D 5.7% vs. ICD 1.2%, RR 4.89, 95% CI 0.58 to 40.95, p = 0.14; RHYTHM ICD:144 CRT-D 10.8% vs. ICD 7.0%, RR 1.55, 95% CI 0.44 to 5.44, p = 0.49) demonstrated no statistically significant difference in all-cause mortality between devices in each of the trials. Length of follow-up was up to 6 months in six of the studies,126,136,137,139,142,144 12 months in the study by Piccirillo and colleagues138 and an average of 28.8 months in the MADIT-CRT study130 and 40 months in the RAFT study. 140
| Study | Follow-up (months) | CRT-D n/N (%) | ICD n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD126 | 3–6 | 11/245 (4.5) | 16/245 (6.5) | RR 0.69a | 0.33 to 1.45,a 0.33 |
| MADIT-CRT130 | Average 2.4 years | 74/1089 (6.8) | 53/731 (7.3) | HR 1.00 | 0.69 to 1.44, 0.99 |
| MIRACLE ICD136 | 6 | 14/187 (7.5) | 15/182 (8.2) | RR 0.91a | 0.45 to 1.83, 0.79a |
| MIRACLE ICD II137 | 6 | 2/85 (2.4) | 2/101 (2.0) | RR 1.19a | 0.17 to 8.26, 0.86a |
| Piccirillo138 | 12 | 0/16 (0) | 0/15 (0) | ||
| Pinter139 | 6 | 1/36 (2.8) | 1/36 (2.8) | RR 1.00a | 0.07 to 15.38, 1.00a |
| RAFT140 | Mean 40 (SD 20) | 186/894 (20.8) | 236/904 (26.1) | HR 0.75 | 0.62 to 0.91, 0.003 |
| RethinQ142 | 6 | 5/87 (5.7) | 1/85 (1.2) | RR 4.89a | 0.58 to 40.95, 0.14a |
| RHYTHM ICD144 | 6 | 9/83 (10.8) | 3/43 (7.0) | RR 1.55a | 0.44 to 5.44, 0.49a |
The trials were considered sufficiently similar to combine in a random-effects meta-analysis and were grouped according to the NYHA class of the majority of the participants in each trial. There was no evidence of significant statistical heterogeneity between the studies (χ2 = 4.82, df = 7, I2 = 0%). Note that the study by Piccirillo and colleagues138 was not estimable within the meta-analysis as zero events were observed in both groups. The RR for CRT-D compared with ICD was 0.84 (95% CI 0.73 to 0.96, p = 0.01) (Figure 19), giving a RRR of 16% with CRT-D for all-cause mortality. The results were strongly influenced by the large RAFT study140 with 40 months’ follow-up and when this study was removed from the analysis the results were no longer statistically significant (RR 0.95, 95% CI 0.72 to 1.24, p = 0.69).
FIGURE 19.
All-cause mortality.
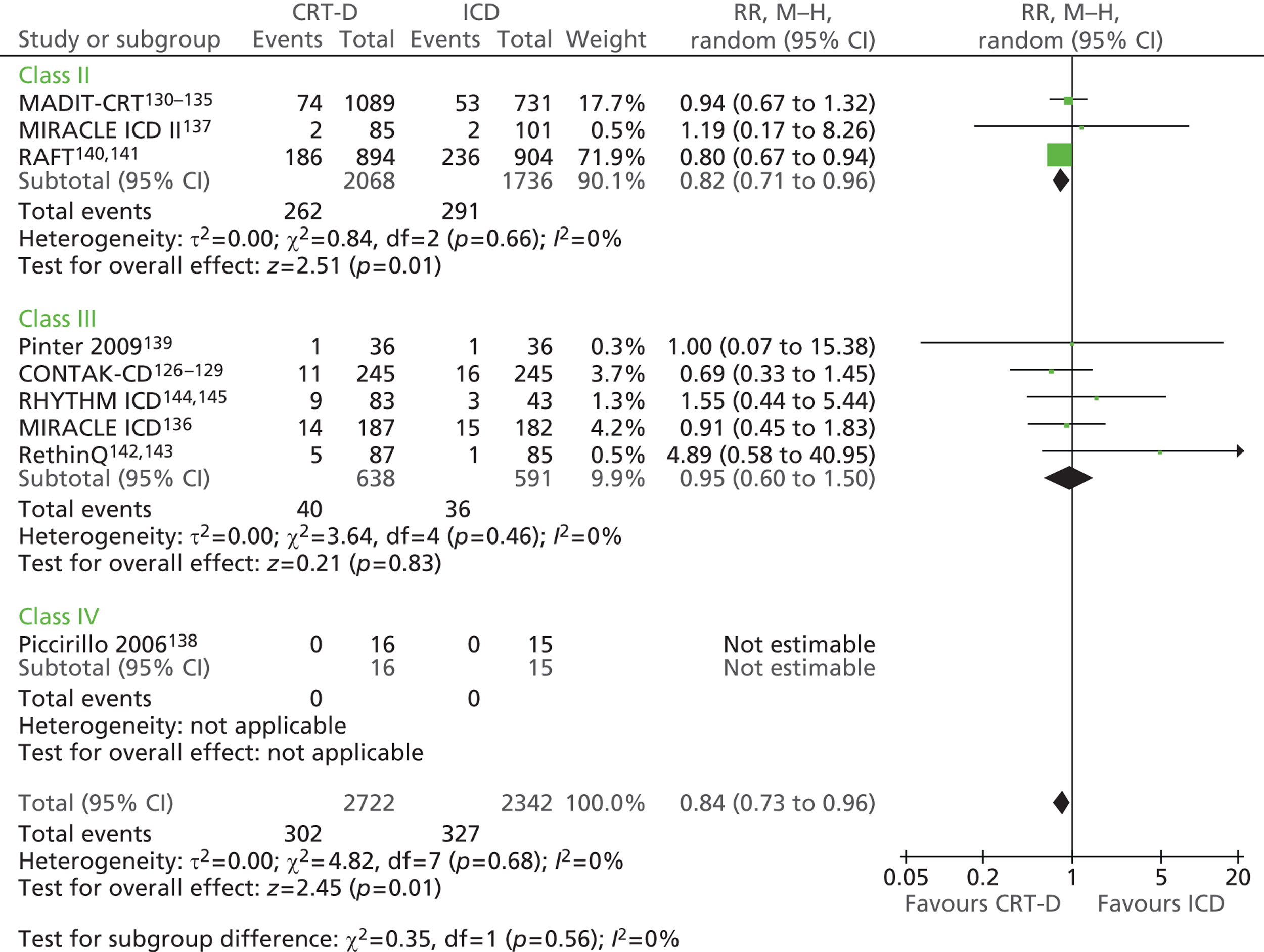
Total cardiac deaths
Seven trials reported data on total cardiac deaths, although only one of these compared events between groups statistically (Table 58). The RAFT study140 found that CRT-D was associated with a statistically significant reduction in cardiac deaths (CRT-D 14.5% vs. ICD 17.9%, HR 0.76, 95% CI 0.60 to 0.96, p = 0.02). When these trials were combined in a meta-analysis (random effects) the overall RR was 0.82 (95% CI 0.67 to 1.00, p = 0.05) in favour of CRT-D (Figure 20). There was no statistically significant heterogeneity (χ2 = 2.38, df = 5, I2 = 0%). Again these results were strongly influenced by the large RAFT study140 and when this was omitted from the analysis there was little difference between the interventions (RR 0.92, 95% CI 0.44 to 1.92, p = 0.83).
| Study | Follow-up (months) | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD126 | 3–6 | 7/245 (2.9) | 10/245 (4.1) | RR 0.70a | 0.27 to 1.81a |
| MIRACLE ICD II137 | 6 | 2/85 (2.4) | 2/101 (2.0) | RR 1.19a | 0.17 to 8.26a |
| Piccirillo138 | 12 | 0/16 (0) | 0/15 (0) | ||
| Pinter139 | 6 | 1/36 (2.8) | 1/36 (2.8) | RR 1.00a | 0.07 to 15.38a |
| RAFT140 | Mean 40 (SD 20) | 130/894 (14.5) | 162/904 (17.9) | HR 0.76 | 0.60 to 0.96, 0.02 |
| RethinQ142 | 6 | 4/87 (4.6) | 1/85 (1.2) | RR 3.91a | 0.45 to 34.26a |
| RHYTHM ICD144 | 6 | 1/83 (1.2) | 1/43 (2.3) | RR 0.52a | 0.03 to 8.08a |
FIGURE 20.
Total cardiac deaths.
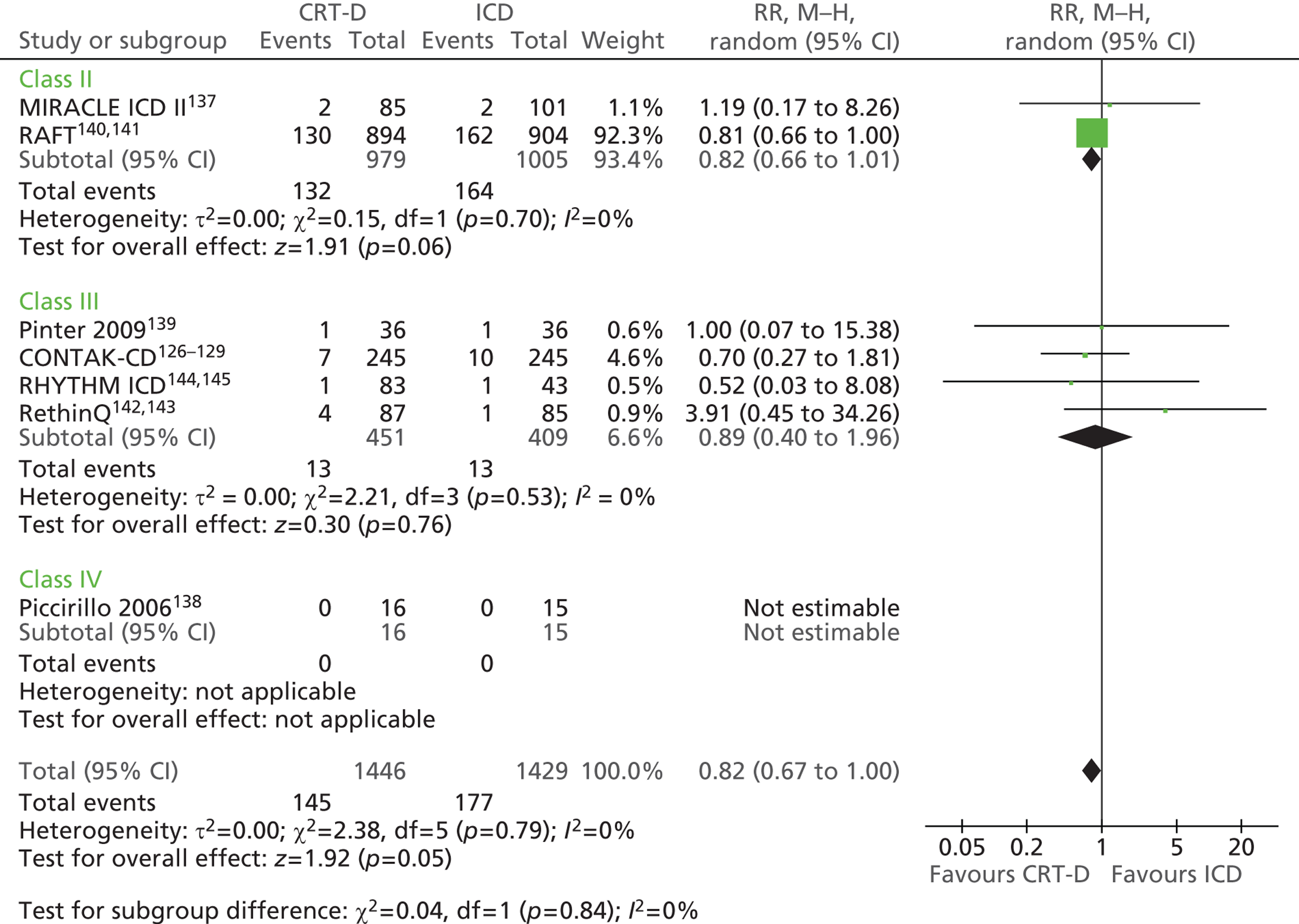
Heart failure deaths
There were no deaths from HF in the MIRACLE ICD II study137 of people with mild NYHA class II HF or in the small study by Piccirillo and colleagues138 of people in NYHA class IV or III. The CONTAK-CD study,126 in which the majority of participants had NYHA class III or II HF, reported deaths from HF in 1.6% and 3.7% of the CRT-D and ICD groups respectively. Two (2.3%) people in the CRT-D group and one person (1.2%) in the ICD group of the RethinQ trial142 died from HF (Table 59). Combining these trials in a random-effects meta-analysis gave an overall RR of 0.64 (95% CI 0.18 to 2.22, p = 0.48) (Figure 21).
| Study | Follow-up (months) | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD126 | 3–6 | 4/245 (1.6) | 9/245 (3.7) | RR 0.44a | 0.14 to 1.42, 0.17a |
| MIRACLE ICD II137 | 6 | 0/85 (0) | 0/101 (0) | ||
| Piccirillo138 | 12 | 0/16 (0) | 0/15 (0) | ||
| RethinQ142 | 6 | 2/87 (2.3) | 1/85 (1.2) | RR 1.95a | 0.18 to 21.15, 0.58a |
FIGURE 21.
Heart failure deaths.
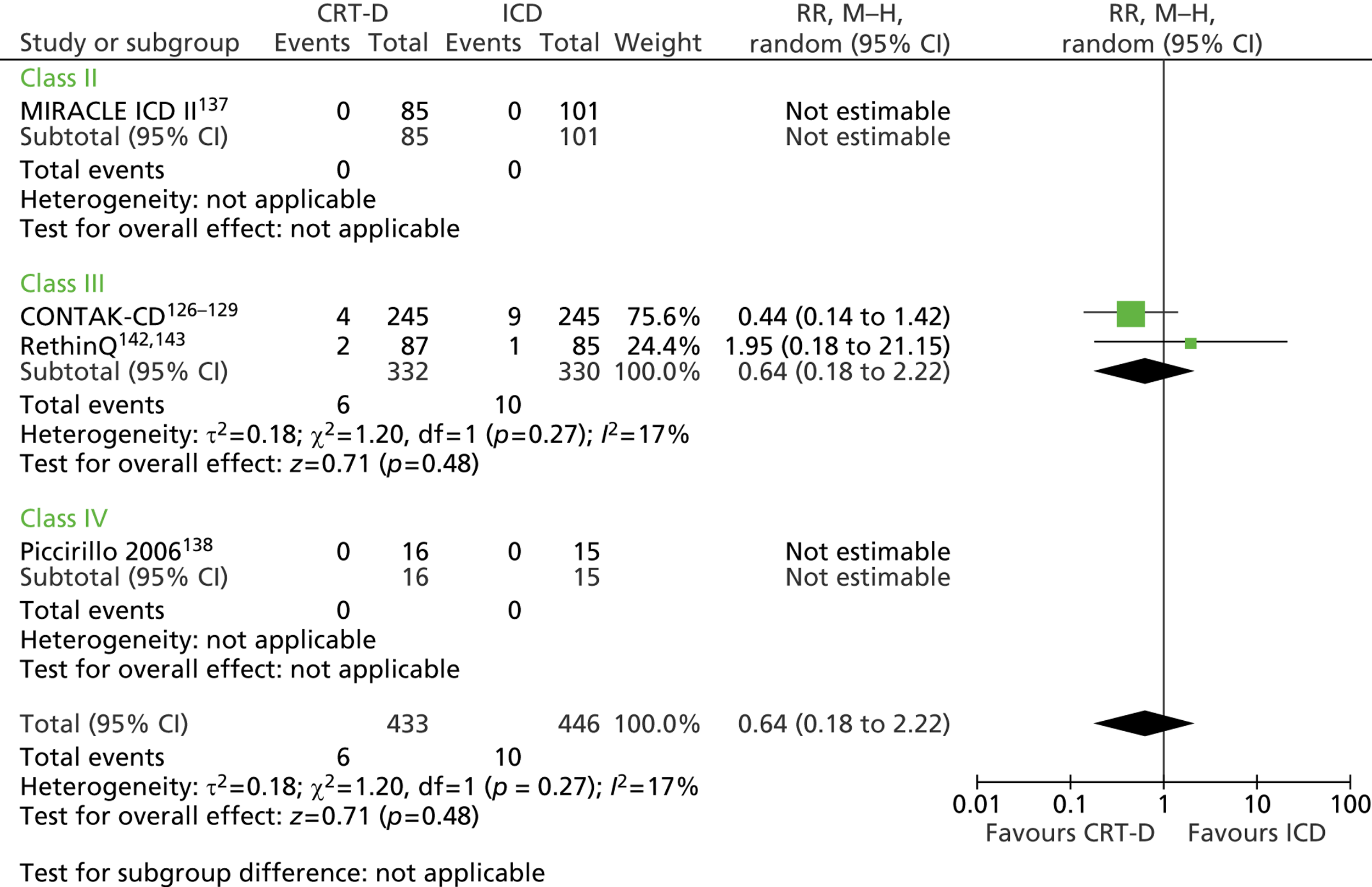
Sudden cardiac death
Six trials reported data on SCD (Table 60). No SCDs occurred in the small study by Piccirillo and colleagues138 or in the RethinQ142 or RHYTHM ICD144 studies. Combining the other three trials129,136,137 in a meta-analysis gives an overall RR of 1.45 (95% CI 0.43 to 4.92, p = 0.55), with no important statistical heterogeneity (χ2 = 0.61, df = 2, I2 = 0) (Figure 22).
| Study | Follow-up (months) | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD129 | 3–6 | 1/245 (0.4) | 0/245 (0) | RR 3.00 | 0.12 to 73.28, 0.5a |
| MIRACLE ICD136 | 6 | 3/187 (1.6) | 3/182 (1.6) | RR 0.97 | 0.2 to 4.76, 0.97a |
| MIRACLE ICD II137 | 6 | 2/85 (2.4) | 1/101 (1.0) | RR 2.38 | 0.22 to 25.76, 0.48a |
| Piccirillo138 | 12 | 0/16 (0) | 0/15 (0) | ||
| RethinQ143 | 6 | 0/87 (0) | 0/85 (0) | ||
| RHYTHM ICD144 | 6 | 0/83 (0) | 0/43 (0) |
FIGURE 22.
Sudden cardiac deaths.
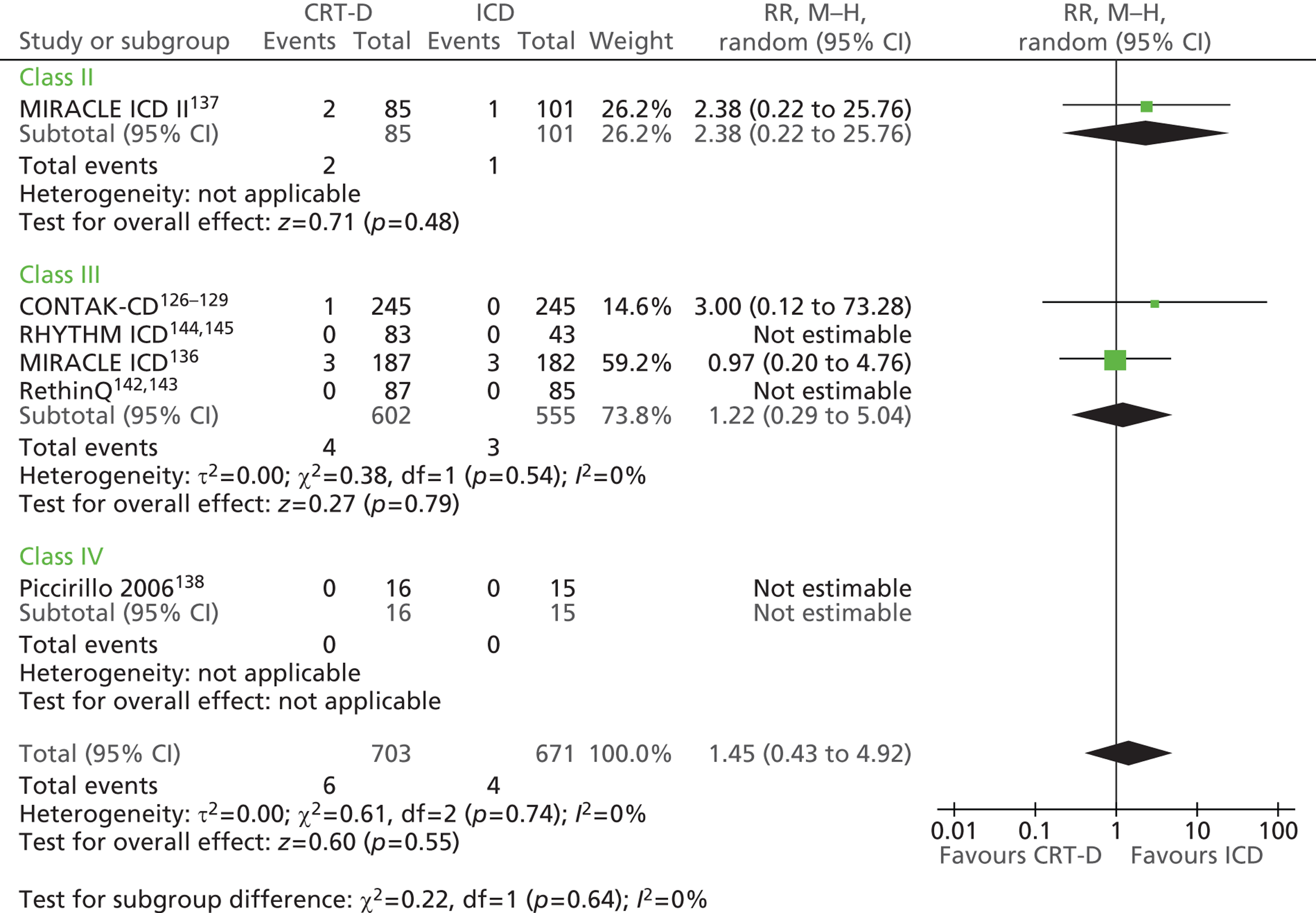
Other causes of death
Deaths from non-cardiac causes were reported in the CONTAK-CD trial129 (CRT-D 0.8% vs. ICD 1.2%) and the RHYTHM ICD study144 (CRT-D 8.4% vs. ICD 4.7%). One (1.1%) death of unknown cause occurred in the CRT-D group of the RethinQ trial. 142 No deaths from non-cardiac causes occurred in the studies by Piccirillo and colleagues138 or Pinter and colleagues139 (Table 61).
| Study | Follow-up (months) | Cause of death | CRT-D, n/N (%) | ICD, n/N (%) |
|---|---|---|---|---|
| CONTAK-CD129 | 3–6 | Cardiac (not pump failure or arrhythmic) | 2/245 (0.8) | 1/245 (0.4) |
| Non-cardiac | 2/245 (0.8) | 3/245 (1.2) | ||
| Unknown | 2/245 (0.8) | 3/245 (1.2) | ||
| MIRACLE ICD II137 | 6 | MI with cardiogenic shock | 0/85 (0) | 1/101 (1.0) |
| Piccirillo138 | 12 | Non-cardiac | 0/16 (0) | 0/15 (0) |
| Pinter139 | 6 | Non-cardiac | 0/36 (0) | 0/36 (0) |
| RethinQ142 | 6 | Unknown | 1/87 (1.1) | 0/85 (0) |
| Unknown cardiac | 1/87 (1.1) | 0/85 (0) | ||
| RHYTHM ICD144 | 6 | Cardiac non-arrhythmic | 1/83 (1.2) | 1/43 (2.3) |
| Cardiac unknown | 0/83 (0) | 0/43 (0) | ||
| Non-cardiac | 7/83 (8.4) | 2/43 (4.7) | ||
| Unknown | 1/83 (1.2) | 0/43 (0) |
Survival
No statistically significant difference in 6-month cumulative survival was found in the MIRACLE ICD study136 (CRT-D 92.4% vs. ICD 92.2%, p = 0.96) or the RethinQ study142 (CRT-D 94.2% vs. ICD 98.8%, p = 0.11), or in cumulative freedom from death caused by worsening HF in the RethinQ study142 (CRT-D 97.7% vs. 98.9%, p = 0.58) (Table 62). The probability of event-free survival at 5 years was 57.6% in the CRT-D group and 48.7% in the ICD group of the RAFT study;140 statistical significance was not reported.
| Study | Outcome and follow-up | CRT-D | ICD | p-value |
|---|---|---|---|---|
| MIRACLE ICD136 | 6-month cumulative survival (95% CI), % | 92.4 (87.5 to 95.4) | 92.2 (87.2 to 95.3) | 0.96 |
| RAFT140 | Probability of event-free survival at 5 years, % | 57.6 | 48.7 | |
| 5-year actuarial rate of death, % | 28.6 | 34.6 | ||
| RethinQ142 | Cumulative overall survival at 6 months (95% CI), % | 94.2 (86.7 to 97.6) | 98.8 (91.9 to 99.8) | 0.11 |
| Cumulative freedom from death caused by worsening HF (95% CI), % | 97.7 (91.1 to 99.4) | 98.9 (91.9 to 99.8) | 0.58 |
Hospitalisations related to heart failure
The CONTAK-CD,126 Piccirillo and colleagues138 and RAFT140 studies reported hospitalisations related to HF (Table 63); the MIRACLE ICD,136 Pinter and colleagues139 and RAFT140 studies reported all-cause hospitalisations (see Appendix 6). The RAFT study140 found a statistically significant reduction in hospitalisations for HF in the CRT-D group (CRT-D 19.5% vs. ICD 26.1%, HR 0.68, 95% CI 0.56 to 0.83, p < 0.001). The CONTAK-CD study126 reported that 13.1% of the CRT-D group were hospitalised because of HF compared with 15.9% of the ICD group. Two people (13.3%) with an ICD and none of the CRT-D group were hospitalised because of HF in the small study by Piccirillo and colleagues. 138 When the studies were combined in a random-effects meta-analysis, CRT-D reduced the RR of HF hospitalisation by 25% compared with ICD therapy (RR 0.75, 95% CI 0.64 to 0.88, p = 0.0005) (Figure 23).
| Study | Outcome and follow-up | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD126 | At least one HF hospitalisation, 6 months | 32/245 (13.1) | 39/245 (15.9) | RR 0.82a | 0.53 to 1.26, 0.37a |
| Piccirillo138 | Hospitalisation because of worsening HF, | 0/16 (0) | 2/15 (13.3) | RR 0.19a | 0.01 to 3.63, 0.27a |
| RAFT140 | Hospitalisation for HF, mean 40 (SD 20) months | 174/894 (19.5) | 236/904 (26.1) | HR 0.68 | 0.56 to 0.83, < 0.001 |
FIGURE 23.
Heart failure hospitalisations.
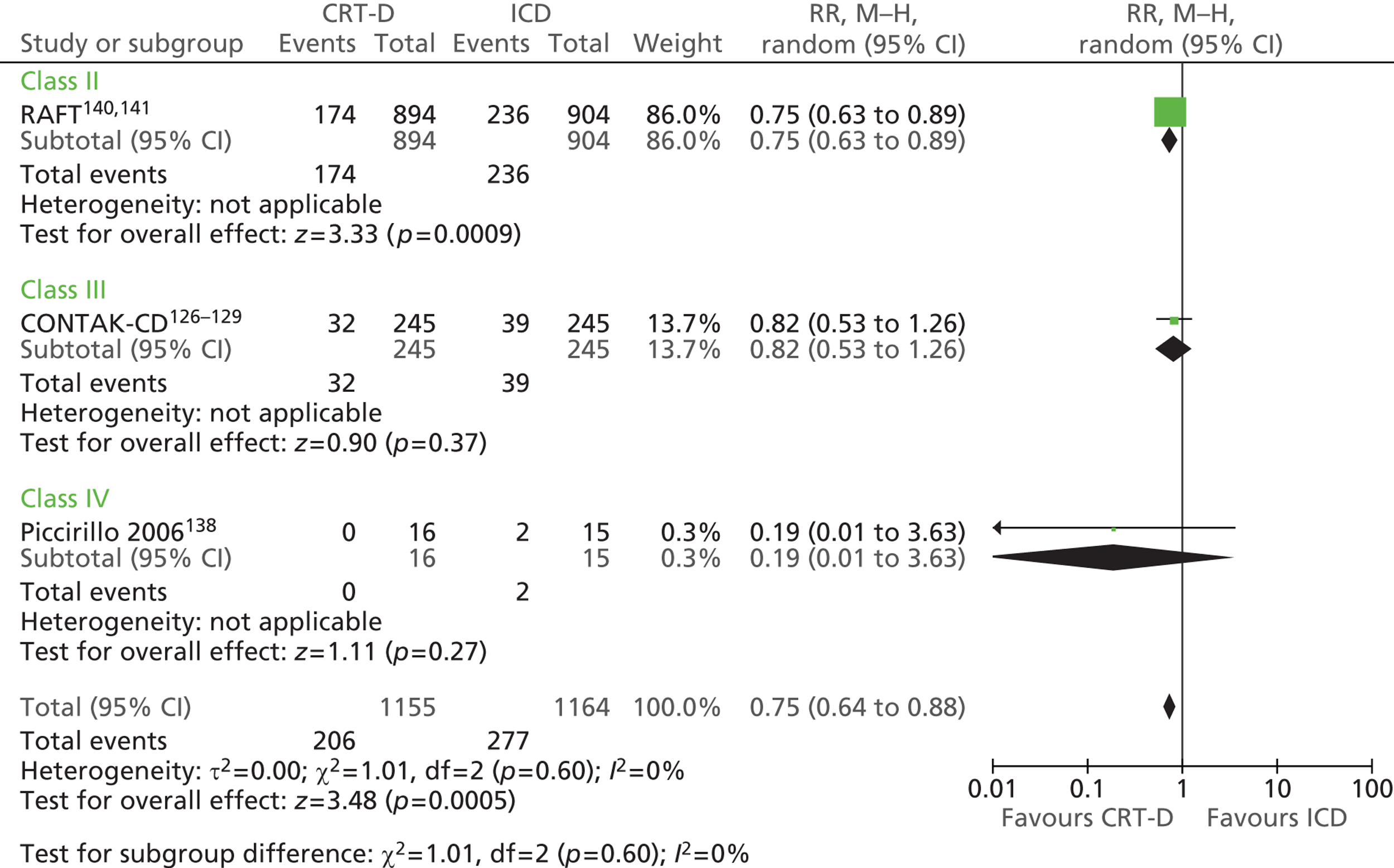
Arrhythmias
The number of participants experiencing at least one episode of VT or VF can be seen in Table 64. The proportions appear similar between groups. Random-effects meta-analysis demonstrated no statistically significant difference between the groups in the number of people experiencing at least one arrhythmia (RR 0.90, 95% CI 0.71 to 1.14, p = 0.38) (Figure 24).
| Study | Outcome and follow-up | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD126 | At least one VT/VF event, 6 months | 36/245 (14.7) | 39/245 (15.9) | RR 0.92a | 0.61 to 1.40, 0.71a |
| MIRACLE ICD136 | At least one spontaneous episode of VT or VF, 6 months | 42/187 (22) | 47/182 (26) | RR 0.87a | 0.61 to 1.25, 0.45,a 0.47b |
| MIRACLE ICD II137 | At least one appropriately detected spontaneous episode of VT or VF, 6 months | 19/85 (22) | 26/101 (26) | RR 0.87a | 0.52 to 1.46, 0.59,a 0.61b |
| Pinter139 | VT event requiring therapy from the device, 6 months | 7/36 (19.4) | 6/36 (16.7) | RR 1.17a | 0.43 to 3.13, 0.76,a NSb |
FIGURE 24.
Arrhythmias.
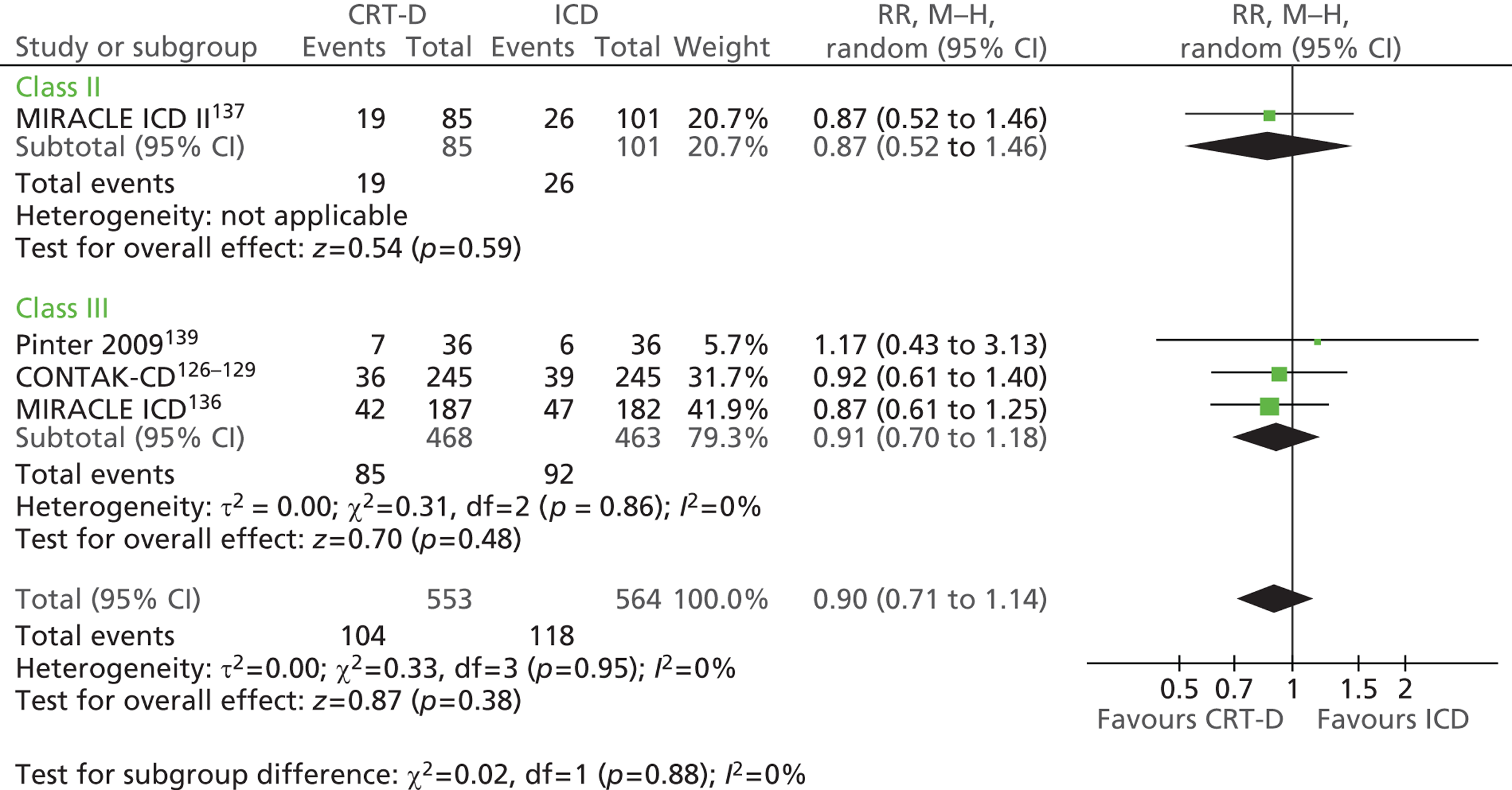
New York Heart Association class
Six trials reported change in NYHA class; three reported mean or median change and three reported the number of participants who improved. The MIRACLE ICD,136 MIRACLE ICD II137 and RHYTHM ICD144 trials reported a statistically significant improvement in mean or median NYHA class among people receiving CRT-D compared with people receiving and ICD (Table 65). Combining these studies in a random-effects meta-analysis gives a MD of –0.19 (95% CI –0.34 to –0.05, p = 0.008), although note that the MIRACLE ICD136 trial is not estimable (Figure 25). A significantly greater proportion of the CRT-D group improved by one class or more in the RethinQ trial142 (CRT-D 54% vs. ICD 29%, p = 0.006), and the majority (81%) of the participants in the CRT-D group in the study by Piccirillo and colleagues138 showed an improvement in NYHA class, compared with only 7% of those in the ICD group (see Table 65); however, there is some uncertainty surrounding these data because of a discrepancy in reporting in the paper (see Appendix 9). In the CONTAK-CD trial126 there was no statistically significant difference in the number of people who showed an improvement in NYHA class. Substantial heterogeneity was evident when these studies were combined in a random-effects meta-analysis (χ2 = 8.57, df = 2, I2 = 77%) and, although the direction of effect favoured CRT-D, this was not statistically significant (RR 1.81, 95% CI 0.91 to 3.60, p = 0.09) (Figure 26).
| Study | Outcome and follow-up | CRT-D, n/N (%) | ICD, n/N (%) | p-value |
|---|---|---|---|---|
| CONTAK-CD126 | 6 months | |||
| Improved by two classes | 12a/109 (11) | 2a/116 (2) | ||
| Improved by one class | 27a/109 (25) | 35a/116 (30) | 0.1 | |
| No change | 56a/109 (51) | 59a/116 (51) | ||
| Worsened | 14a/109 (13) | 20a/116 (17) | ||
| MIRACLE ICD136 | Change in NYHA class, 6 months | (n = 165) median –1 (95% CI –1 to –1, SD 0) | (n = 162) median 0 (95% CI –1 to 0, SD 3.2) | 0.007 |
| MIRACLE ICD II137 | Change in NYHA class, 6 months | (n = 82) mean –0.18 (SD 0.61) | (n = 98) mean 0.01 (SD 0.63) | 0.05 |
| Piccirillo138 | 12 months | |||
| Improved by two classesb | 5/16 (31.3) | 0/15 (0) | ||
| Improved by one classb | 8/16 (50.0) | 1/15 (6.7) | ||
| No changeb | 3/16 (18.8) | 11/15 (73.3) | ||
| Worsenedb | 0/16 (0) | 3/15 (20.0) | ||
| RethinQ142 | 6 months | |||
| Improved by one or more class | 41/76 (54) | 23/80 (29) | 0.006 | |
| No change | 31/76 (41) | 51/80 (64) | ||
| Worsened | 4/76 (5) | 6/80 (8) | ||
| RHYTHM ICD144 | Change in NYHA class, 6 months | (n = 83) mean –0.48 (SD 0.65) | (n = 43) mean –0.28 (SD 0.63) | 0.048 |
FIGURE 25.
Change in NYHA class.
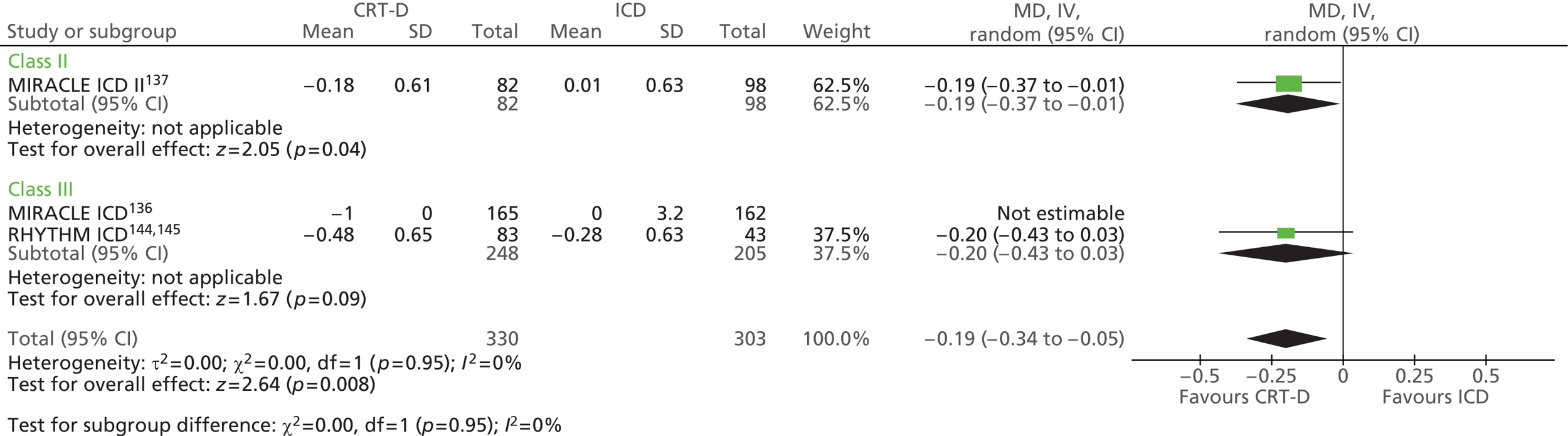
FIGURE 26.
Proportion of people with improvement in NYHA class.
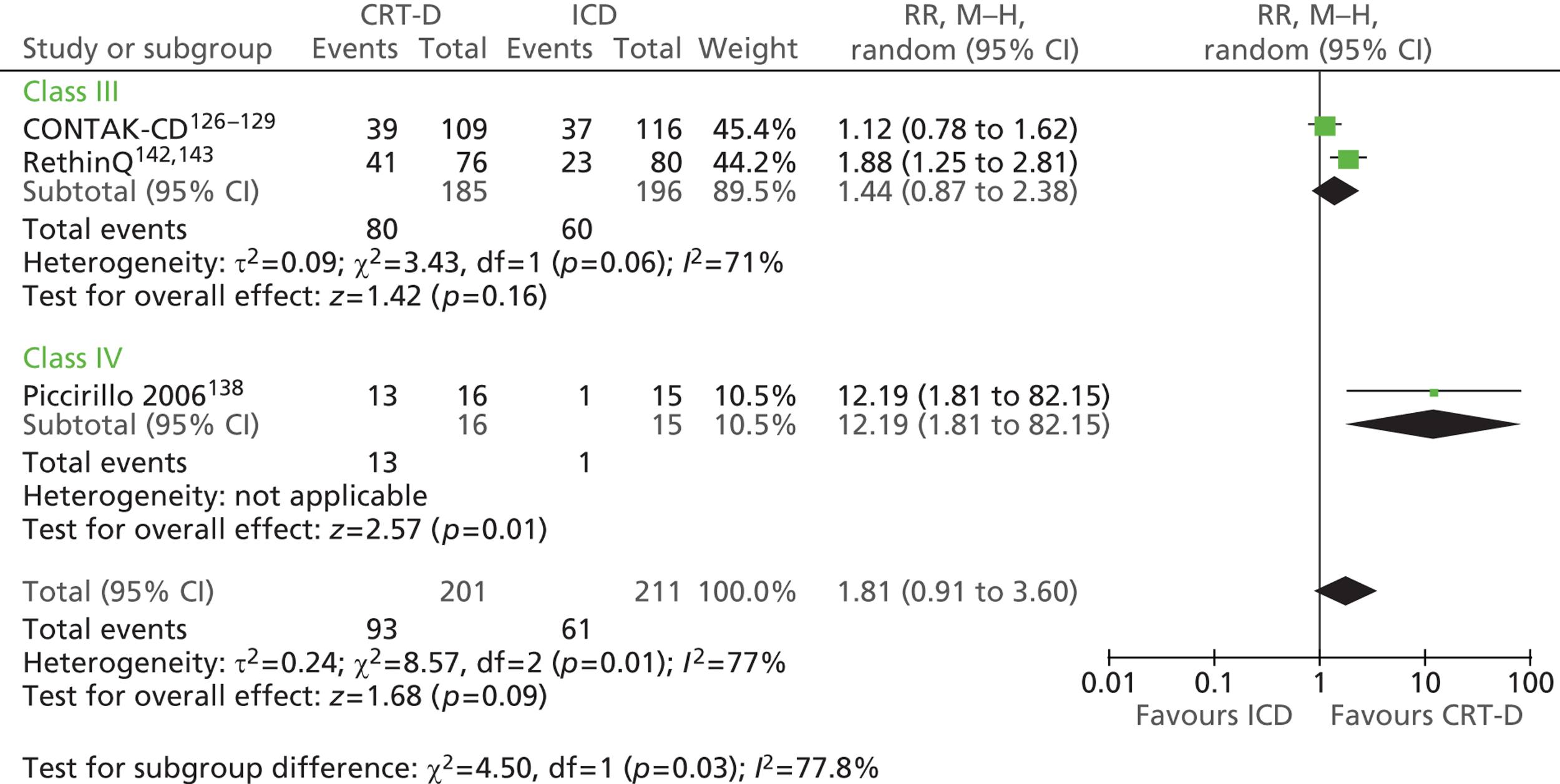
Worsening heart failure
The MADIT-CRT trial130 reported a statistically significant reduction in the number of people experiencing a non-fatal HF event in the CRT-D group compared with the ICD group (CRT-D 13.9% vs. ICD 22.8%, HR 0.59, 95% CI 0.47 to 0.74, p < 0.001). Fewer HF events requiring intravenous therapy occurred in the CRT-D group (24 events in 16.1% of patients) than in the ICD group (41 events in 22.3% of patients) in the RethinQ trial. 142 Worsening HF (other than that defined by change in NYHA class; see previous section) was not reported by the other trials.
Left ventricular ejection fraction
Three126,130,137 of the eight trials reporting LVEF described a statistically significant improvement in mean LVEF among people receiving CRT-D compared with those receiving an ICD, whereas three136,139,142 trials reported no statistically significant difference between the groups in change from baseline (Table 66). The study by Piccirillo and colleagues138 and the RHYTHM ICD study144 did not provide a statistical comparison. Combining the trials in a meta-analysis showed a statistically significant improvement in LVEF in the CRT-D group compared with the ICD group (MD 2.15, 95% CI 0.45 to 3.86, p = 0.01) (Figure 27). There is substantial statistical heterogeneity (χ2 = 21.12, df = 7, I2 = 67%); however, the direction of the effect is fairly consistent between studies.
| Study | Outcome and follow-up | CRT-D | ICD | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| CONTAK-CD126 | Change in LVEF (%), 6 months | (n = 222) mean 5.1 (SE 0.7; SD 10.4a) | (n = 216) mean 2.8 (SE 0.7; SD 10.3a) | MD 2.30b | 0.36 to 4.24, 0.02,b 0.020c |
| MADIT-CRT130 | Change in LVEF (%), average 2.4 years | (n = 746) mean 11 (SD 44.6a) | (n = 620) mean 3 (SD 44.6a) | MD 8.00b | 3.25 to 12.57, 0.001,b < 0.001c |
| MIRACLE ICD136 | Change in LVEF (%), 6 months | (n = 132) median 1.2 (95% CI 1.2 to 4.1; SD 8.4a) | (n = 133) median 1.7 (95% CI 0.7 to 2.4; SD 5.0a) | MD –0.50b | –2.17 to 1.17, 0.56,b 0.12c |
| MIRACLE ICD II137 | Change in LVEF (%), 6 months | (n = 68) mean 3.8 (SD 8.0) | (n = 85) mean 0.8 (SD 6.2) | MD 3.00b | 0.69 to 5.31, 0.01,b 0.02c |
| Piccirillo138 | LVEF (%) at 12 months | (n = 16) mean 28 (SD 4) | (n = 15) mean 22 (SD 8) | MD 6.00b | 1.50 to 10.50, 0.009b |
| Pinter139 | Change in LVEF (%), 6 months | ||||
| Measured by MUGA | (n = 36) mean 1.7 (SD 5.4) | (n = 36) mean 0.6 (SD 6.8) | NS | ||
| Measured by ECG | (n = 36) mean 3.9 (SD 8.9) | (n = 36) mean 1.9 (SD 6.8) | MD 2.00b | –1.66 to 5.66, 0.28,b NSc | |
| RethinQ142 | Change in LVEF (%), 6 months | (n = 68) median 1.2 (95% CI –0.4 to 4.4; SD 9.9a) | (n = 74) median 2.0 (95% CI 0.3 to 4.2; SD 4.2a) | MD 0.80b | 3.83 to 2.23, 0.61,b 0.83c |
| RHYTHM ICD144 | Change in LVEF (%), 6 months | (n = 83) mean 4.3 (SD 9.9) | (n = 43) mean 2.9 (SD 6.2) | MD 1.4b | –1.42 to 4.22, 0.33b |
FIGURE 27.
Change in LVEF.
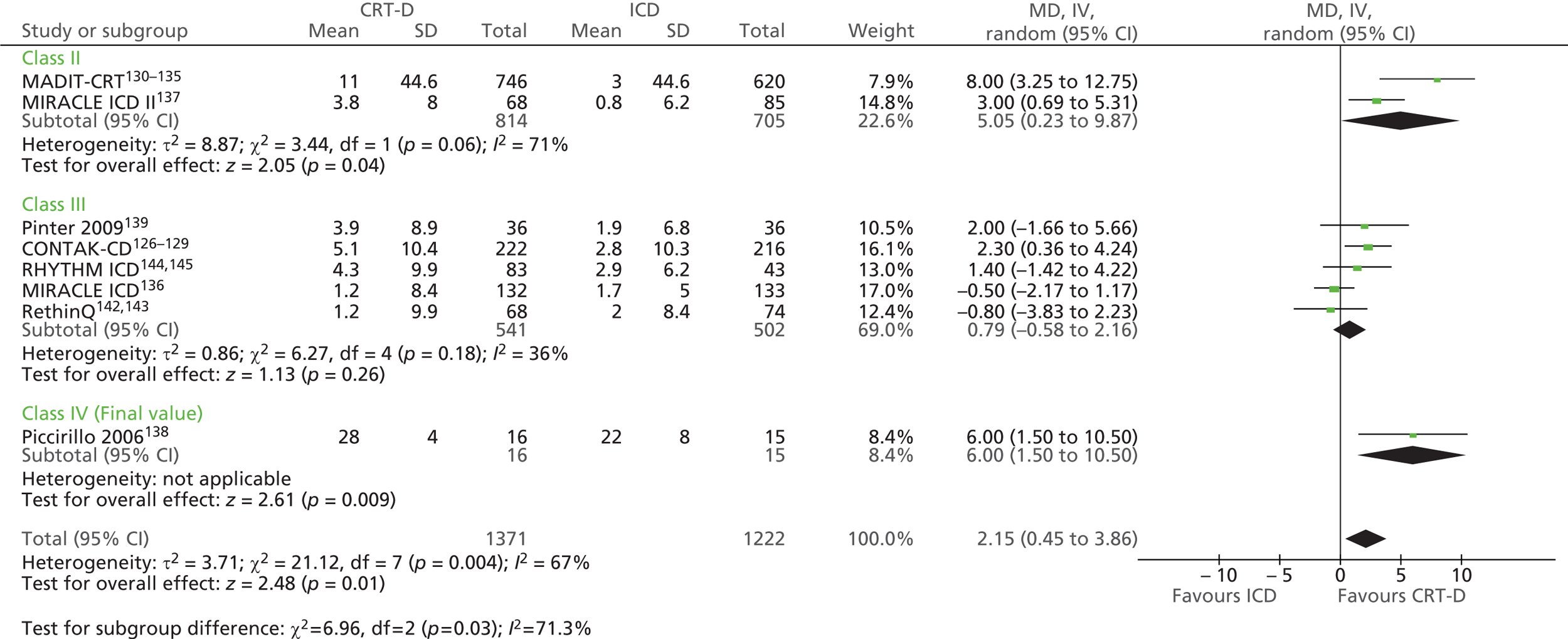
Exercise capacity
Exercise capacity was reported by six of the eight trials, with six studies measuring distance walked in 6 minutes and two trials measuring exercise duration, five trials measuring peak VO2 and one trial reporting the proportion of participants with an increase of at least 1.0 ml/kg body weight/minute in peak oxygen consumption (Table 67). The CONTAK-CD trial126 found improvements in both peak VO2 and distance walked in 6 minutes, which were statistically significantly greater in the CRT-D group than in the ICD group. The MIRACLE ICD136 and RHYTHM ICD144 trials found statistically significant improvements in peak VO2 but not distance walked in 6 minutes; the MIRACLE ICD136 trial also found significant improvements in exercise duration in favour of CRT-D. The MIRACLE ICD II trial137 (mild HF) found no statistically significant differences in change in peak VO2 or exercise duration, but found a significant improvement in ventilatory response to exercise with CRT-D compared with ICD. The RethinQ trial142 found no statistically significant differences in distance walked in 6 minutes or the proportion of participants with an increase of at least 1.0 ml/kg body weight/minute in peak VO2. There was no statistically significant difference in the change in 6 minute-walk distance in the study by Pinter and colleagues. 139
| Study | Outcome and follow-up | CRT-D | ICD | p-value |
|---|---|---|---|---|
| CONTAK-CD126 | Change in peak VO2 (ml/kg/minute), 3–6 months | (n = 216) mean 0.8 (SE 0.3; SD 4.4a) | (n = 201) mean 0.0 (SE 0.3; SD 4.3a) | 0.03 |
| Change in 6-minute walk distance (m), 3–6 months | (n = 224) mean 35 (SE 7; SD 104.8a) | (n = 220) mean 15 (SE 7; SD 103.8a) | 0.043 | |
| MIRACLE ICD136 | Change in 6-minute walk distance (m), 6 months | (n = 152) median 55 (95% CI 44 to 79) (SD 109.2a) | (n = 153) median 53 (95% CI 43 to 75) (SD 100.2a) | 0.36 |
| Change in peak VO2 (ml/kg/minute), 6 months | (n = 120) median 1.1 (95% CI 0.7 to 1.6) (SD 2.5a) | (n = 121) median 0.1 (95% CI –0.1 to 0.8) (SD 2.5a) | 0.04 | |
| Change in exercise duration (seconds), 6 months | (n = 120) median 55.5 (95% CI 30 to 79) (SD 135.5a) | (n = 123) median –11 (95% CI –55 to 12) (SD 187.7a) | < 0.001 | |
| MIRACLE ICD II137 | Change in peak VO2 (ml/kg/minute), 6 months | (n = 66) mean 0.5 (SD 3.2) | (n = 79) mean 0.2 (SD 3.2) | 0.87 |
| Change in exercise duration (seconds), 6 months | (n = 66) mean 42 (SD 167) | (n = 79) mean 37 (SD 186) | 0.56 | |
| Change in VE/VCO2 (ml/minute), 6 months | (n = 66) mean –1.8 (SD 6.2) | (n = 78) mean 0.5 (SD 5.2) | 0.01 | |
| Change in 6-minute walk distance (m), 6 months | (n = 78) mean 38 (SD 109) | (n = 93) mean 33 (SD 98) | 0.59 | |
| Pinter139 | Change in 6-minute walk distance (m), 6 monthsb | (n = 36) mean 53.3 (SD 113.3) | (n = 36) mean 27.3 (SD 71.1) | NS |
| RethinQ142 | Change in peak VO2 (ml/kg/minute), 6 months | (n = 76) median 0.4 (95% CI –0.6 to 1.2) (SD 3.9a) | (n = 80) median 0.5 (95% CI –0.3 to 1.1) (SD 3.1a) | |
| Peak VO2, increase ≥ 1.0 ml/kg/minute, n/N (%), 6 months | (n = 76) 35/76 (46) | (n = 80) 33/80 (41) | 0.63 | |
| Change in 6-minute walk distance (m), 6 months | (n = 75) median 26 (95% CI 0 to 46) (SD 100a) | (n = 79) median 6 (95% CI –17 to 30) (SD 104.9a) | 0.23 | |
| RHYTHM ICD144 | Change in peak VO2 (ml/kg/minute), 6 months | (n = 83) mean 0.52 (SD 2.5) | (n = 43) mean –1.41 (SD 4.6) | 0.001 |
| Change in 6-minute walk distance (m), 6 months | (n = 83) mean 13 (SD 74) | (n = 43) mean –15 (SD 142) | 0.07 |
Meta-analysis of these trials demonstrated that the change from baseline in peak VO2 (MD 0.75 ml/kg/minute, 95% CI 0.23 to 1.27 ml/kg/minute, p = 0.005) (Figure 28) and distance walked in 6 minutes (MD 14.5 m, 95% CI 2.9 to 26.1 m, p = 0.01) (Figure 29) were statistically significantly greater in the CRT-D group than in the ICD group. There was little statistical heterogeneity in these studies and, although the MIRACLE ICD136 and RethinQ142 trials report medians and not means, the difference remains statistically significant when these studies are omitted.
FIGURE 28.
Change in peak VO2.
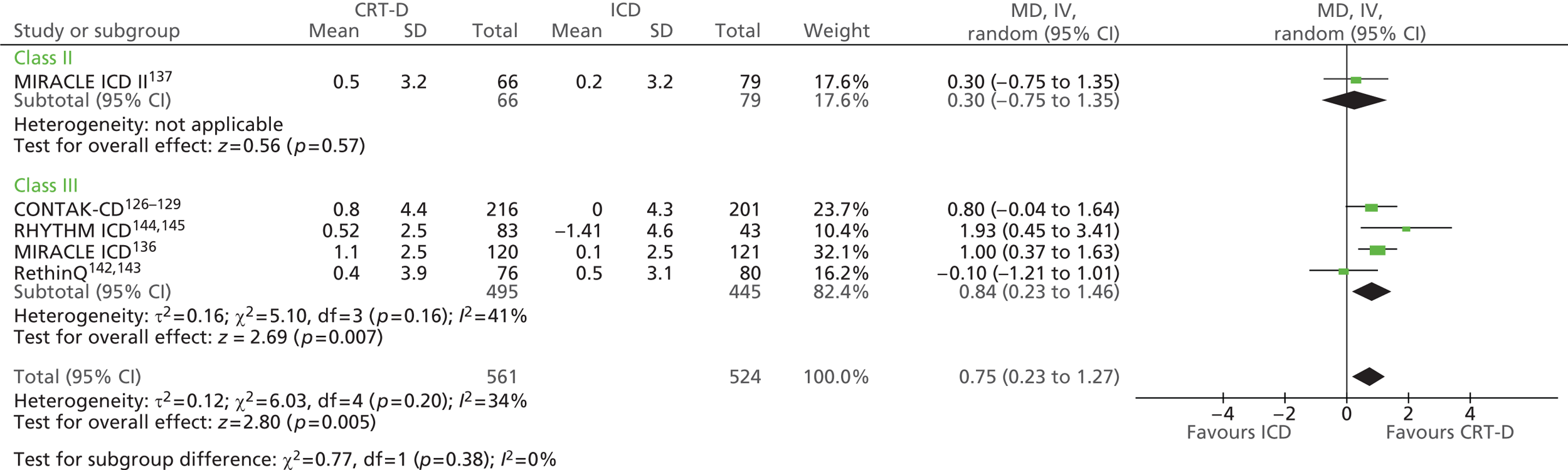
FIGURE 29.
Change in 6-minute walk distance.
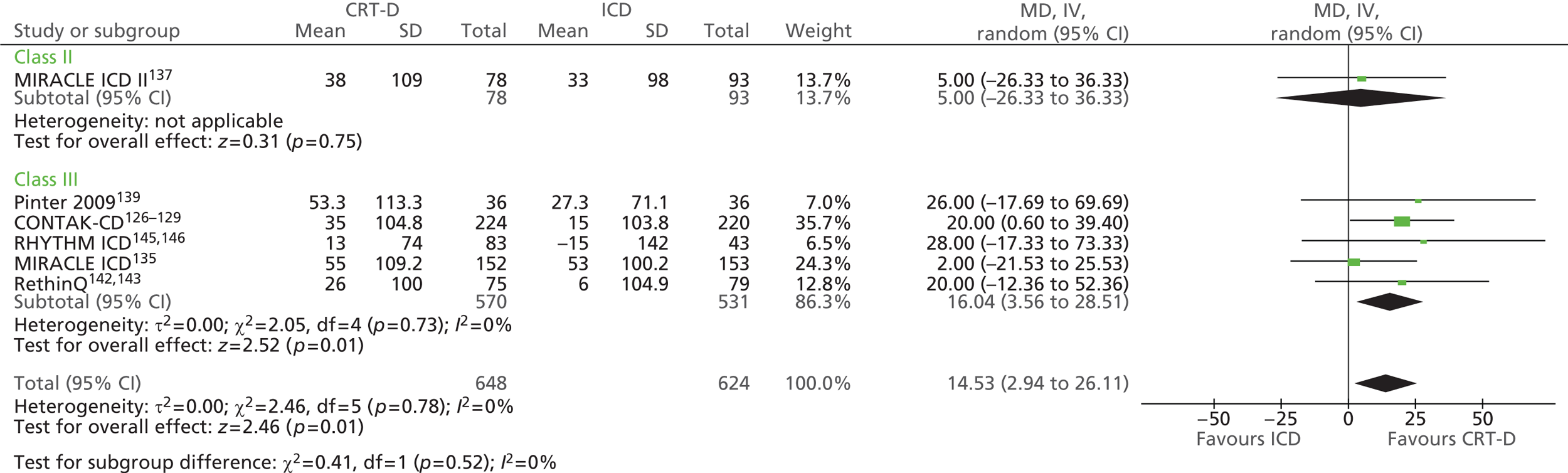
Quality of life
Six126,136,137,139,142,144 of the eight trials reported change in QoL at 6 months, assessed using the MLWHFQ (Table 68). An improvement in QoL score was seen with CRT-D when the trials were pooled (MD –6.9, 95% CI –10.4 to –3.4, p = 0.0001) (Figure 30). Pinter and colleagues139 also reported the DASI, the one-item Global Visual Analogue Scale and the SF-36. Comparisons of baseline to 6-month changes were statistically significantly different for the general health component of the SF-36 only [CRT-D –5.8 (SD 14.9) vs. ICD –5.8 (SD 13.6), p = 0.02].
| Study | Outcomea and follow-up | CRT-D | ICD | p-value |
|---|---|---|---|---|
| CONTAK-CD126 | Change in MLWHFQ score, 6 months | (n = 234) mean –7 (SE 2) (SD 30.6b) | (n = 255) mean 5 (SE 2) (SD 31.9b) | 0.39c |
| MIRACLE ICD136 | Change in MLWHFQ score, 6 months | (n = 162) median –17.5 (95% CI –21 to –14) (SD 22.6b) | (n = 157) median –11 (95% CI –16 to –7) (SD 28.5b) | 0.02 |
| MIRACLE ICD II137 | Change in MLWHFQ score, 6 months | (n = 81) mean –13.3 (SD 25.1) | (n = 96) mean –10.7 (SD 21.7) | 0.49 |
| Pinter139 | Change in score, 6 monthsd | |||
| DASI | (n = 36) mean 4.63 (SD 9.20) | (n = 36) mean 1.08 (SD 7.02) | NS | |
| Global Visual Analogue Scale | (n = 36) mean –0.07 (SD 2.22) | (n = 36) mean –0.17 (SD 1.64) | NS | |
| MLWHFQ, 6 months | ||||
| Total score | (n = 36) mean –7.8 (SD 20.1) | (n = 36) mean –0.2 (SD 13.5) | NS | |
| Physical dimension | (n = 36) mean –5.0 (SD 12.4) | (n = 36) mean –0.6 (SD 7.9) | NS | |
| Emotional dimension | (n = 36) mean –1.3 (SD 5.0) | (n = 36) mean 0.3 (SD 3.4) | NS | |
| SF-36, change to 6 monthsd | ||||
| Physical functioning | (n = 36) mean 11.2 (SD 24.2) | (n = 36) mean 6.3 (SD 21.2) | NS | |
| Role physical | (n = 36) mean 19.6 (SD 43.2) | (n = 36) mean 21.6 (SD 38.1) | NS | |
| Bodily pain | (n = 36) mean –3.3 (SD 16.6) | (n = 36) mean –2.3 (SD 13.1) | NS | |
| General health | (n = 36) mean –5.8 (SD 14.9) | (n = 36) mean –5.8 (SD 13.6) | 0.02 | |
| PCS | (n = 36) mean 1.4 (SD 6.4) | (n = 36) mean 1.3 (SD 4.8) | NS | |
| Vitality | (n = 36) mean 4.7 (SD 22.7) | (n = 36) mean 2.6 (SD 15.7) | NS | |
| Social functioning | (n = 36) mean 12.5 (SD 23.3) | (n = 36) mean 5.4 (SD 32.6) | NS | |
| Role emotional | (n = 36) mean 29.5 (SD 48.4) | (n = 36) mean 3.3 (SD 48.2) | NS | |
| Mental health | (n = 36) mean 4.5 (SD 14.5) | (n = 36) mean 0.1 (SD 21.8) | NS | |
| MCS | (n = 36) mean 5.1 (SD 10.1) | (n = 36) mean 0.5 (SD 12.4) | NS | |
| RethinQ142 | Change in MLWHFQ score, 6 months | (n = 76) median –8 (95% CI –10 to –1) (SD 19.7b) | (n = 80) median –7 (95% CI –11 to 3) (SD 31.5b) | 0.91 |
| RHYTHM ICD144 | Change in MLWHFQ score, 6 months | (n = 83) mean –7.8 (SD 22) | (n = 43) mean 3.4 (SD 31) | 0.009 |
FIGURE 30.
Change in MLWHFQ score.
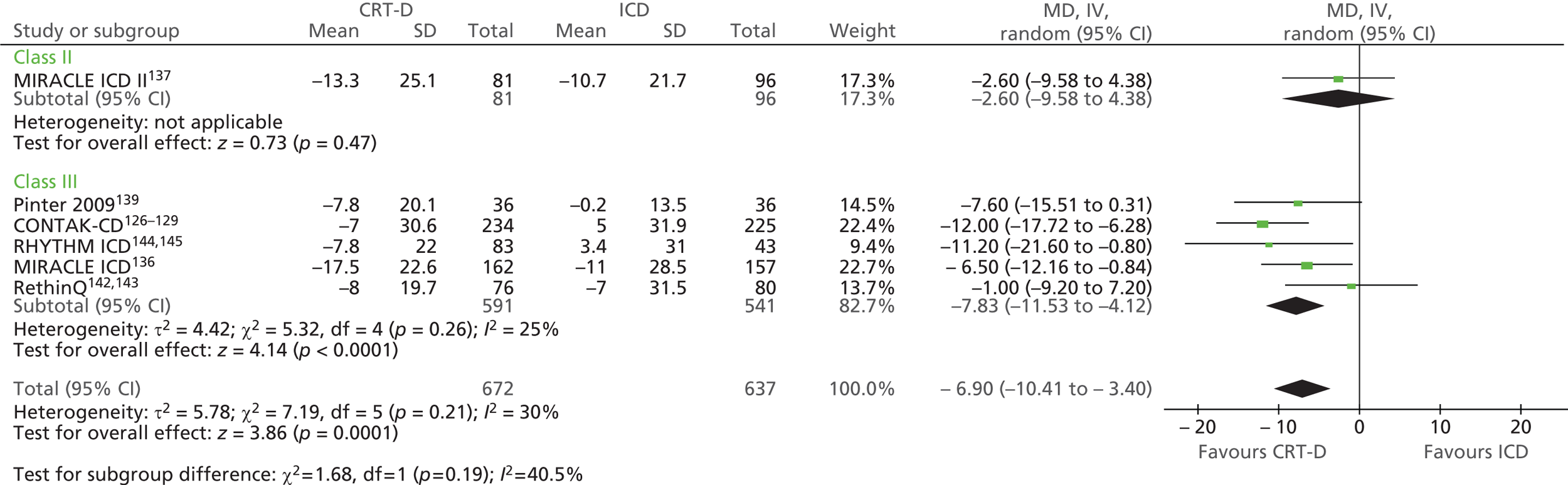
Adverse events
As described earlier, three130,138,140 of the trials compared CRT-D and ICD devices whereas all participants in the six remaining trials126,136,137,139,142,144 were implanted with a device that could provide both CRT and ICD therapy (CRT off in the comparator group). Differences in adverse events relating to the CRT-D device can therefore be assessed only in the former three trials and, of these, only the MADIT-CRT130 and RAFT140 trials provided adverse event data.
Reporting of adverse events by the included trials was limited and inconsistent. As can be seen in Table 69, in some of the trials the number of participants randomised differed from the number of people enrolled and who had implantation attempted, as in six of the trials only those with successful implantation were randomised. However, adverse event data were reported for all participants who underwent implantation or attempted implantation in the CONTAK-CD,126 MADIT-CRT,130 MIRACLE ICD,136 MIRACLE ICD II,137 RAFT140 and RHYTHM ICD144 studies. The MIRACLE ICD136 and MIRACLE ICD II137 studies also reported total complications for those with successful implants.
| CONTAK CD126 | MADIT-CRT130 | MIRACLE ICD136 | MIRACLE ICD II137 | Piccirillo138 | Pinter139 | RAFT140 | RethinQ142 | RHYTHM ICD144 | |
|---|---|---|---|---|---|---|---|---|---|
| Enrolled, n | 581 | 1820 | 429 | 222 | 1798 | 250 | 205 | ||
| Attempted implantation, n | 567 | Uncleara | 429 | 210 | 90 | Unclearb | 250c | 205 | |
| Implanted, n/N (%) | 501/567 (88.4) | 1790/1820 (98.4)d | 379/429 (88.3)e | 191/210 (91.0)e | 75/90 (83.3)e | 1787/1798 (99.4)f | Unclearc | 182/205 (88.8)e | |
| Randomised, n | 490 | 1820 | 369 | 186 | 31 | 72 | 1798 | 172 | 179 |
| Only successful implants randomised? | Yes | No | Yes | Yes | Unclear | Yes | No | Yes | Yes |
| Efficacy analysis, n | 490 | 1820 | 369 | 186 | 31 | 72 | 1798 | 156 | 126 |
Five125,136,137,142,144 of the trials using the same device in all participants, that is, CRT on compared with CRT off, reported adverse events for both interventions combined (Table 70). The MIRACLE ICD trial136 also reported adverse events separately for the CRT on and CRT off groups, as did the MADIT-CRT130 and RAFT140 trials for the CRT-D and ICD groups (Table 71). Adverse events were not reported in the study by Pinter and colleagues,139 and Piccirillo and colleagues138 stated that there no major complications following implantation but provided no further information.
| Study | Adverse events | n/N (%) (95% CI) |
|---|---|---|
| CONTAK-CD126,129 | ||
| Attempted implantation (n = 567) | Operative mortality | 12/567 (2.1) (0.9 to 3.3) |
| Overall lead-related adverse event rate | 75/517a (14.5) (11.5 to 17.5) | |
| Severe device-related events | 7/567 (1.2) | |
| Device-related complications (occurring in > 1% of patients): infections | 7/517a (1.4) | |
| MIRACLE ICD136 | ||
| Attempted implantation (n = 429) | Experienced complication from implant to hospital discharge | 120/429 (28) (159 complications) |
| Complication related to left ventricular lead | 37/159 (23% of complications) (included 15 coronary sinus dissections and four cardiac perforations) | |
| HF decompensation | 6/429 (received intravenous medication) | |
| Heart block | 3/429 (required bradycardia pacing support) | |
| Muscle stimulation | 4/429 (required either lead repositioning or replacement) | |
| Pericardial effusion | 2/429 (treated with a pericardiocentesis) | |
| Pericarditis | 1/429 (received intravenous medication) | |
| Haemo/pneumothorax | 3/429 (placement of chest tube) | |
| VT and VF | 5/429 (three received external defibrillation, two received intravenous medication) | |
| Elevated pacing thresholds or loss of capture | 7/429 (six received lead repositioning, one had set screw tightened in connector block) | |
| Died within 30 days of latest implant attempt | 5/429 (1.2) | |
| Successful implantation (n = 379) | From hospital discharge to the 6-month follow-up, total complications | 175/379 (46) (398 complications) |
| MIRACLE ICD II137 | ||
| Attempted implantation (n = 210) | Died (before randomisation) | 1/210 |
| From implant to hospital discharge | 46/210 (22) (56 complications) | |
| Complications related to placement of left ventricular lead | 19/56 (34% of complications) (including three coronary sinus dissections, three cardiac perforations and five lead dislodgements) | |
| Failed initial implant attemptb | 23/210 | |
| Successful implantation (n = 191)b | From hospital discharge to 6 months | 66/191 (35) (109 complications) |
| Complications related to left ventricular lead | 19/109 (17) (including 11 lead dislodgements, one cardiac perforation, three with diaphragmatic muscle stimulation and four elevated pacing thresholds) | |
| RethinQ142 | ||
| Randomised patients (n = 172) | Lead dislodgement | 13/172 (7.6) |
| Involving left ventricular lead | 5/172 (2.9) | |
| Infection | 6/172 (3.5) | |
| Bleeding or haematoma | 2/172 (1.2) | |
| Loss of pacemaker lead capture | 2/172 (1.2) | |
| Phrenic nerve stimulation | 3/172 (1.7) | |
| Deep venous thrombosis | 3/172 (1.7) | |
| Pneumothorax | 2/172 (1.2) | |
| Pericarditis | 2/172 (1.2) | |
| Coronary sinus perforation | 1/172 (0.6) | |
| RHYTHM ICD144 | ||
| Enrolled patients (n = 205), average 12.1 (SD 3.4) patient-months’ follow-up | Death (before randomisation or unsuccessful implant) | 5/205 (2.4) |
| Total complications (adverse events requiring invasive intervention) | 21/205 (10.2) (29 events) | |
| Coronary sinus perforation/dissection | 2 (1.0) (two events) | |
| Diaphragmatic/phrenic nerve stimulation | 3 (1.5) (three events) | |
| Lead dislodgement or migration | 8 (3.9) (nine events) | |
| Bleeding/haematoma | 6 (2.9) (six events) | |
| Blood clot/thrombosis | 1 (0.5) (one event) | |
| High defibrillation/cardioversion requirements | 2 (1.0) (two events) | |
| Infection | 1 (0.5) (one event) | |
| Noise on EGM post shock (non-SJM right ventricular lead) | 1 (0.5) (one event) | |
| Pneumothorax | 2 (1.0) (two events) | |
| Retained foreign body (surgical sponge) | 1 (0.5) (one event) | |
| Elevated pacing threshold – left ventricular lead | 1 (0.5) (one event) | |
| Total observations (adverse events managed without invasive intervention) | 57 (27.8) (68 events) | |
| Asystolic episode during left ventricular lead placement | 1 (0.5) (one event) | |
| Bleeding/haematoma | 10 (4.9) (10 events) | |
| Blood clot/thrombosis | 2 (1.0) (two events) | |
| Coronary sinus perforation/dissection | 6 (2.9) (six events) | |
| Diaphragmatic/phrenic nerve stimulation – left ventricular lead | 10 (4.9) (10 events) | |
| Diaphragmatic/phrenic nerve stimulation – right ventricular lead | 2 (1.0) (two events) | |
| Elevated pacing thresholds – left ventricular lead | 10 (4.9) (10 events) | |
| Elevated pacing thresholds – right ventricular lead | 2 (1.0) (two events) | |
| Heart block at implant | 2 (1.0) (two events) | |
| High defibrillation/cardioversion requirements | 1 (0.5) (one event) | |
| Hypotension requiring ventilator support | 1 (0.5) (one event) | |
| Inappropriate therapy for SVT | 10 (4.9) (13 events) | |
| Infection | 3 (1.5) (three events) | |
| Possible pulmonary embolism | 1 (0.5) (one event) | |
| T-wave sensing | 2 (1.0) (three events) | |
| Pocket inflammation/seroma | 1 (0.5) (one event) | |
| Left ventricular lead-related complications at 6 months | 11/155 (7.1) patients, 13 complications | |
| Epic HF system-related complications at 6 months | 13/182 (7.1) patients, 16 complications | |
| Total adverse events (29 complications and 68 observations) | 70 patients, 97 events | |
| Enrolled patients (n = 205), average 15.1 (SD 4.1) patient-months’ follow-up | Total complicationsc | 22/205 (10.7) (31events) |
| Lead dislodgement or migration | 9 (4.4) (10 events) | |
| Infection | 2 (1.0) (two events) | |
| Total observationsc | 59 (28.8) (76 events) | |
| Diaphragmatic/phrenic nerve stimulation – left ventricular lead | 14 (6.8) (14 events) | |
| Elevated pacing thresholds – left ventricular lead | 12 (5.9) (12 events) | |
| Inappropriate therapy for SVT | 11 (5.4) (14 events) | |
| Infection | 4 (2.0) (four events) | |
| Study | Adverse event | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|---|
| MADIT-CRT130 | |||||
| Enrolled and randomised (n = 1820; CRT-D n = 1089, ICD n = 731) | Death in hospital after device implantation | 1/1089 (pulmonary embolus) | 0/731 | ||
| Serious adverse events within 30 days of implantation | |||||
| Pneumothorax | (1.7) | (0.8) | |||
| Infection | (1.1) | (0.7) | |||
| Pocket haematoma requiring evacuation | (3.3) | (2.5) | |||
| Coronary venous dissection with pericardial effusion during CRT-ICD implantation | 5/1089 (0.5) | NA | |||
| Left ventricular coronary vein lead repositioned during first 30 days | 44/1089 (4.0) | NA | |||
| Frequency of serious device-related adverse events during long-term follow-up after the first 30 days | 4.5 per 100 device-months | 5.2 per 100 device-months | |||
| Removal of device | 14/1089 (1.3) | 5/731 (0.7) | |||
| MIRACLE ICD136 | |||||
| CRT on, n/N (%) | CRT off, n/N (%) | ||||
| Successful implantation and randomised (n = 369; CRT-D n = 187, CRT-off n = 182) | Complications after hospital discharge to 6 months | ||||
| Left ventricular lead-related complication | 20 (11) (21 events) | 13 (7) (14 events) | |||
| ICD system related | 9 (5) (9 events) | 13 (8) (14 events) | |||
| Procedure related | 10 (5) (10 events) | 11 (6) (13 events) | |||
| HF decompensation | 36 (19) (63 events) | 40 (22) (71 events) | |||
| Other | 45 (24) (81 events) | 44 (24) (74 events) | |||
| Total | 88 (47) (184 events) | 80 (44) (186 events) | |||
| RAFT140 | |||||
| CRT-D, n/N (%) | ICD, n/N (%) | ||||
| Implanted (n = 1787; CRT-D n = 888, ICD n = 899) | Death from worsening HF within 24 hours of implantation | 0/888 | 1/899 (0.1) | ||
| Device-related hospitalisation | 179/888 (20) | 110/899 (12.2) | HR 1.68 | 1.32 to 2.13, < 0.001 | |
| Adverse events within 30 days of implantationa | 124/888 (14.0) | 58/899 (6.5) | < 0.001 | ||
| Haemothorax or pneumothorax | 11/888 (1.2) | 8/899 (0.9) | 0.47 | ||
| Device pocket haematoma requiring intervention | 14/888 (1.6) | 11/899 (1.2) | 0.53 | ||
| Device pocket infection requiring intervention | 21/888 (2.4) | 16/899 (1.8) | 0.39 | ||
| Lead dislodgement requiring intervention | 61/888 (6.9) | 20/899 (2.2) | 0.0001 | ||
| Device-pocket problems requiring revision | 4/888 (0.5) | 1/899 (0.1) | 0.22 | ||
| Coronary sinus dissection | 11/888 (1.2) | 0/899 (0) | 0.0004 | ||
| Tamponade | 2/888 (0.2) | 2/899 (0.2) | 1 | ||
Between 83.3% and 99.4% of people undergoing an implantation attempt received an implanted device (see Table 69). Four of these studies136,137,139,144 clearly described the implantations as successful (83.3–91%).
Perioperative deaths occurred in between 0.1% (MADIT-CRT130) and 2.4% (RHYTHM ICD144) of participants (see Tables 70 and 71), although it is not clear whether or not the time period of reporting is consistent between studies. Lead-related complications with CRT-D were experienced by around 7% of participants in three trials,140,142,144 and the overall lead-related adverse event rate was 14.5% in the CONTAK-CD trial. 126 The MIRACLE ICD136 and MIRACLE ICD II137 trials reported the proportion of complications that were related to the left ventricular lead before hospital discharge (23% of 159 complications and 34% of 56 complications respectively). In total, 4% of people receiving CRT-D in the MADIT-CRT trial130 had the left ventricular lead repositioned during the first 30 days (see Table 71).
The RAFT trial140 compared adverse events statistically between the CRT-D group and the ICD group (see Table 71). The rate of device- or implantation-related complications within 30 days of implantation was significantly higher in the CRT-D group than in the ICD group (CRT-D 13.3% vs. ICD 6.8%, p < 0.001), as were the rates of device-related hospitalisations (CRT-D 20% vs. ICD 12.2%, HR 1.68, 95% CI 1.32 to 2.13, p < 0.001), lead dislodgement requiring intervention (CRT-D 6.9% vs. ICD 2.2%) and coronary sinus dissection (CRT-D 1.2% vs. ICD 0%). After the first 30 days, the MADIT-CRT trial130 reported 4.5 (CRT-D group) and 5.2 (ICD group) serious device-related adverse events per 100 device-months.
Subgroup analyses reported by included randomised control trials
Three trials reported prespecified subgroup analysis. The MADIT-CRT trial130 presented prespecified stratified analysis according to ischaemic or non-ischaemic cardiomyopathy classification. A similar benefit from CRT-D was found in those with ischaemic or non-ischaemic cardiomyopathy (Table 72). Subgroup analysis of risk of death or HF according to selected clinical characteristics found that CRT-D was associated with a greater benefit in people with a QRS duration of ≥ 150 milliseconds than in those with a QRS duration of < 150 milliseconds (p = 0.001 for interaction), and with a greater benefit in women than in men (p = 0.01 for interaction). There were no statistically significant interactions for the other subgroups (age, NYHA class, LVEF, left ventricular end-diastolic volume and left ventricular end-systolic volume) (see Table 72). Additional analysis stratified by men and women reported in a secondary publication135 is presented in Table 73 and shows that women achieved significantly better results from CRT-D than men.
| Subgroup | CRT-ICD | ICD only | Effect | 95% CI, p-value |
|---|---|---|---|---|
| Patients with ischaemic cardiomyopathy (NYHA class I or II) | ||||
| (n = 598) | (n = 401) | |||
| Death from any cause or non-fatal HF event, n/N (%) | 122/598 (20.4) | 117/401 (29.2) | HR 0.67 | 0.52 to 0.88, 0.003 |
| HF events only, n/N (%) | 96/598 (16.1) | 105/401 (26.2) | HR 0.58 | 0.44 to 0.78, < 0.001 |
| Death at any time, n/N (%) | 53/598 (8.9) | 35/401 (8.7) | HR 1.06 | 0.68 to 1.64, 0.80 |
| Patients with non-ischaemic cardiomyopathy (NYHA class I or II) | ||||
| (n = 491) | (n = 330) | |||
| Death from any cause or non-fatal HF event, n (%) | 65 (13.2) | 68 (20.6) | HR 0.62 | 0.44 to 0.89, 0.01 |
| HF events only, n (%) | 55 (11.2) | 62 (18.8) | HR 0.59 | 0.41 to 0.87, 0.01 |
| Death at any time, n (%) | 21 (4.3) | 18 (5.5) | HR 0.87 | 0.44 to 1.70, 0.68 |
| Risk of death or HF according to selected clinical characteristics | ||||
| No. of events/no. of patients | Effect | 95% CI, p-value for interaction | ||
| Age (years) | ||||
| < 65 years | 142/852 | HR 0.80a | ||
| ≥ 65 years | 230/968 | HR 0.60a | ||
| Sex | ||||
| Male | 294/1367 | HR 0.76 | 0.59 to 0.97 | |
| Female | 78/453 | HR 0.37 | 0.22 to 0.61, 0.01 | |
| NYHA class | ||||
| Ischaemic I | 53/265 | HR 0.76a | ||
| Ischaemic II | 186/734 | HR 0.62a | ||
| Non-ischaemic II | 133/821 | HR 0.60a | ||
| QRS duration (milliseconds) | ||||
| < 150 | 147/645 | HR 1.06 | 0.74 to 1.52 | |
| ≥ 150 | 225/1175 | HR 0.48 | 0.37 to 0.64, 0.001 | |
| LVEF (%) | ||||
| ≤ 25 | 101/646 | HR 0.70a | ||
| > 25 | 271/1174 | HR 0.60a | ||
| LVEDV (ml) | ||||
| ≤ 240 | 184/828 | HR 0.70a | ||
| > 240 | 184/969 | HR 0.62a | ||
| LVESV (ml) | ||||
| ≤ 170 | 190/835 | HR 0.66a | ||
| > 170 | 178/962 | HR 0.70a | ||
| All patients | 372/1820 | HR 0.66 | ||
| Outcome | Women (n = 453) | Men (n = 1367) | p-value for interaction | ||
|---|---|---|---|---|---|
| CRT-D | ICD | CRT-D | ICD | ||
| HF or death (primary end point),n/N (%) | 29/275 (11) | 51/178 (29) | 159/814 (20) | 137/553 (25) | |
| CRT-D vs. ICD HR 0.31, 95% CI 0.19 to 0.50, p < 0.001 | CRT-D vs. ICD HR 0.72, 95% CI 0.57 to 0.92, p < 0.01 | < 0.01 | |||
| HF only | n = 73 events, CRT-D vs. ICD HR 0.30, 95% CI 0.18 to 0.50, p < 0.001 | n = 249 events, CRT-D vs. ICD HR 0.65, 95% CI 0.50 to 0.84, p = 0.001 | < 0.01 | ||
| Death at any time | n = 20 events, CRT-D vs. ICD HR 0.28, 95% CI 0.10 to 0.79, p = 0.02 | n = 107 events, CRT-D vs. ICD HR 1.05, 95% CI 0.70 to 1.57, p = 0.83 | < 0.03 | ||
The RAFT trial140 reported an analysis of 11 prespecified subgroups (Table 74) and presented outcomes separately for the NYHA class II and III subgroups (Table 75). CRT-D and ICD were associated with a similar reduction in the composite primary outcome of death or hospitalisation for HF (p = 0.91 for interaction), death from any cause and hospitalisation for HF for NYHA classes II and III. A statistically significant interaction was found between treatment and QRS duration (p = 0.003), with CRT-D more effective in people with an intrinsic QRS duration of ≥ 150 milliseconds (HR 0.59, 95% CI 0.48 to 0.73) than in those with an intrinsic QRS duration of < 150 milliseconds (HR 0.99, 95% CI 0.77 to 1.27, p = 0.002 for interaction) or those with a paced QRS duration of ≥200 milliseconds (HR 1.07, 95% CI 0.63 to 1.84, p = 0.03 for interaction). A statistically significant interaction (p = 0.046) between treatment and QRS morphological type was also found, with CRT-D more effective in people with LBBB than in those with non-specific intraventricular conduction delay.
| Subgroup | HR (95% CI) | p-value for interaction |
|---|---|---|
| Age: < 65 years vs. ≥ 65 years | 0.75 | |
| Sex: male vs. female | 0.09 | |
| NYHA class: II vs. III | 0.91 | |
| Underlying heart disease: ischaemic vs. non-ischaemic | 0.90 | |
| QRS duration | ||
| Intrinsic QRS < 150 milliseconds vs. | 0.99 (0.77 to 1.27) | 0.003,a 0.002,b 0.003c |
| Intrinsic QRS ≥ 150 milliseconds vs. | 0.59 (0.48 to 0.73) | |
| Paced QRS ≥ 200 milliseconds | 1.07 (0.63 to 1.84) | |
| LVEF (%): < 20 vs. ≥ 20 | 0.05 | |
| QRS morphological features: RBBB vs. LBBB vs. NIVCD vs. paced | 0.046 | |
| Atrial rhythm: permanent atrial fibrillations or flutter vs. sinus or atrial paced | 0.14 | |
| Diabetes: yes vs. no | 0.22 | |
| Hypertension: yes vs. no | 0.84 | |
| Estimated GFR (ml/minute/1.73 m2): < 60 vs. ≥ 60 | 0.70 | |
| Subgroup | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI, p-value |
|---|---|---|---|---|
| NYHA class II | ||||
| (n = 708) | (n = 730) | |||
| Primary outcome: death or hospitalisation for HF | 193/708 (27.3) | 253/730 (34.7) | HR 0.73 | 0.61 to 0.88, 0.001 |
| Secondary outcomes | ||||
| Death from any cause | 110/708 (15.5) | 154/730 (21.1) | HR 0.71 | 0.56 to 0.91, 0.006 |
| Death from cardiovascular cause | 74/708 (10.5) | 100/730 (13.7) | HR 0.73 | 0.54 to 0.99, 0.04 |
| Hospitalisation for HF | 115/708 (16.2) | 159/730 (21.8) | HR 0.70 | 0.55 to 0.89, 0.003 |
| NYHA class III | ||||
| (n = 186) | (n = 174) | |||
| Primary outcome: death or hospitalisation for HF | 104/186 (55.9) | 111/174 (63.8) | HR 0.76 | 0.58 to 0.99, 0.04 |
| Secondary outcomes | ||||
| Death from any cause | 76/186 (40.9) | 82/174 (47.1) | HR 0.79 | 0.58 to 1.08, 0.14 |
| Death from cardiovascular cause | 56/186 (30.1) | 62/174 (35.6) | HR 0.77 | 0.54 to 1.10, 0.15 |
| Hospitalisation for HF | 59/186 (31.7) | 77/174 (44.3) | HR 0.63 | 0.45 to 0.88, 0.006 |
The RethinQ trial142 presented prespecified stratified analysis according to QRS interval (≥ 120 milliseconds or < 120 milliseconds) and cardiomyopathy classification (ischaemic or non-ischaemic) (Table 76). A statistically significant improvement in the proportion of people with an increase of at least 1 ml/kg body weight/minute in peak oxygen consumption was found with CRT-D for people with a QRS interval of ≥ 120 milliseconds (58.9% vs. 19.7%, p = 0.02), but not for those with a QRS interval of < 120 milliseconds (42.2% vs. 51.2%, p = 0.45). There was a statistically significant increase in the proportion with an improvement in NYHA class with CRT-D for both a QRS interval ≥ 120 milliseconds (70.7% vs. 28.0%, p = 0.01) and a QRS interval < 120 milliseconds (49.4 vs. 29.3%, p = 0.04). There was no statistically significant difference between CRT-D and ICD in QoL or distance walked in 6 minutes for either QRS interval subgroup. Analysis stratified by ischaemic or non-ischaemic cardiomyopathy classification reflected the results for the whole group for peak oxygen consumption, NYHA class and QoL. However, a statistically significant difference between CRT-D and ICD in change in distance walked in 6 minutes was found for those with non-ischaemic cardiomyopathy (55.0 m vs. 2.5 m, p = 0.01), but not for those with ischaemic cardiomyopathy (4.2 m vs. 5.8 m, p = 0.57).
| Subgroup | CRT-D on + OPT (QRS ≥ 120 milliseconds, n = 17; QRS < 120 milliseconds, n = 59) | ICD + OPT (QRS ≥ 120 milliseconds, n = 25; QRS < 120 milliseconds, n = 55) | p-value |
|---|---|---|---|
| QRS interval at 6 monthsa | |||
| Peak oxygen consumption, increase of ≥ 1 ml/kg/minute | |||
| QRS ≥ 120 milliseconds | 58.9 | 19.7 | 0.02 |
| QRS < 120 milliseconds | 42.2 | 51.2 | 0.45 |
| Proportion of patients improved by one or more NYHA class | |||
| QRS ≥ 120 milliseconds | 70.7 | 28.0 | 0.01 |
| QRS < 120 milliseconds | 49.4 | 29.3 | 0.04 |
| QoL, median change (%) | |||
| QRS ≥ 120 milliseconds | 0 | –3.7 | 0.24 |
| QRS < 120 milliseconds | –8.9 | –7.0 | 0.63 |
| 6-minute walk distance (m), median change | |||
| QRS ≥ 120 milliseconds | 0.0 | –19.1 | 0.86 |
| QRS < 120 milliseconds | 33.7 | 10.3 | 0.31 |
| CRT-D on + OPT (ischaemic, n = 40; non-ischaemic, n = 36) | ICD+OPT (ischaemic, n = 41; non-ischaemic, n = 39) | p-value | |
| Cardiomyopathy classification at 6 monthsa | |||
| Peak oxygen consumption, increase of ≥ 1 ml/kg/minute | |||
| Ischaemic | 40.0 | 44.2 | 0.82 |
| Non-ischaemic | 52.6 | 38.4 | 0.25 |
| Proportion of patients improved by one or more NYHA class | |||
| Ischaemic | 55.3 | 29.5 | 0.02 |
| Non-ischaemic | 53.2 | 28.4 | 0.04 |
| QoL, median change (%) | |||
| Ischaemic | –5.9 | –3.6 | 0.68 |
| Non-ischaemic | –10.6 | –6.5 | 0.60 |
| 6-minute walk distance (m), median change | |||
| Ischaemic | 4.2 | 5.8 | 0.57 |
| Non-ischaemic | 55.0 | 2.5 | 0.01 |
Summary of clinical effectiveness: people with both conditions
-
Nine RCTs were included comparing CRT-D with ICD in people both at risk of SCD as a result of ventricular arrhythmias and with HF as a result of LVSD and cardiac dyssynchrony.
-
No RCTs comparing CRT-D with OPT or CRT-D with CRT-P were identified for this population.
-
The risk of bias was low in some of the trials but unclear in others because of inadequate reporting.
-
Length of follow-up was 6 months in five trials, 1 year in two trials and an average of 2.4 years and 3.3 years in the remaining trials. Sample size ranged from 31 to 1820 participants.
-
The trials differed in their eligibility criteria for HF; the majority of participants were in NYHA class II in three trials, in NYHA class III in four trials, described as ‘mild to moderate’ in one trial and in NYHA class IV in one trial. One trial differed from the others in the criteria used to define cardiac dyssynchrony, recruiting people with a narrow QRS interval (< 130 milliseconds) and evidence of mechanical dyssynchrony on ECG. Trials were similar in other key characteristics. LVEF ranged from 21% to 26%.
-
Meta-analysis found that CRT-D reduced the risk of all-cause mortality (eight RCTs; RR 0.84, 95% CI 0.73 to 0.96, p = 0.01) and total cardiac deaths (six RCTs; RR 0.82, 95% CI 0.67 to 1.00, p = 0.05). These results were strongly influenced by the large RAFT trial, which included people with mild to moderate HF despite OPT, a LVEF ≤ 30% from ischaemic or non-ischaemic causes, a wide QRS interval and planned ICD implantation for indicated primary or secondary prevention of SCD.
-
Fewer trials reported HF deaths or SCDs separately, and there were no HF deaths or SCDs in some of these trials. Combining three RCTs in a meta-analysis found little difference in the rate of SCD between the CRT-D group and the ICD group (RR 1.45, 95% CI 0.43 to 4.92, p = 0.55).
-
The RAFT trial found a statistically significant reduction in the rate of HF hospitalisations with CRT-D. Two small trials found no significant difference between the groups for this outcome. Combining these trials in a meta-analysis demonstrated that CRT-D reduced the RR of hospitalisation by 25% compared with ICD (RR 0.75, 95% CI 0.64 to 0.88, p = 0.0005).
-
Meta-analysis of four trials found no statistically significant difference between the groups in the proportion of people experiencing at least one episode of VT or VF (RR 0.90, 95% CI 0.71 to 1.14, p = 0.38).
-
An improvement in NYHA class was found with CRT-D among two trials reporting mean or median change (MD –0.19, 95% CI –0.34 to –0.05, p = 0.008). The results were more heterogeneous among the three trials reporting the proportion of people who improved by one or more NYHA class: two trials found a statistically significant improvement with CRT-D but one trial found no difference between the groups (meta-analysis RR 1.81, 95% CI 0.91 to 3.60, p = 0.09).
-
There was substantial statistical heterogeneity in LVEF among the trials, although the direction of effect was fairly consistent. Meta-analysis found a significant improvement in LVEF with CRT-D compared with ICD (eight RCTs; MD 2.15%, 95% CI 0.45% to 3.86%, p = 0.01).
-
There was a greater improvement in exercise capacity in the CRT-D group than in the ICD group, as demonstrated by change from baseline in peak VO2 (five RCTs; MD 0.75, 95% CI 0.23 to 1.27, p = 0.005) and 6-minute walk distance (six RCTs, MD 14.5 m, 95% CI 2.9 to 26.1 m, p = 0.01).
-
An improvement in QoL (MLWHFQ) score was seen with CRT-D when six trials were pooled in a meta-analysis (MD –6.9, 95% CI –10.4 to –3.4, p = 0.0001). One trial reporting other measures of QoL (DASI, one-item Global Visual Analogue Scale and SF-36) found that differences between the groups in baseline to 6-month changes were statistically significant for the general health component of the SF-36 only.
-
Reporting of adverse events was inconsistent between the trials. The large RAFT trial found that the rate of device- or implantation-related complications within 30 days of implantation was significantly higher in the CRT-D group than in the ICD group (13.3% vs. 6.8%, p < 0.001), as was the rate of device-related hospitalisations (20% vs. 12.2%, HR 1.68, 95% CI 1.32 to 2.13, p < 0.001).
-
Three trials reported prespecified subgroup analysis. Two trials reported that CRT-D was associated with a greater benefit in people with a QRS duration of ≥ 150 milliseconds than in those with a QRS duration of < 150 milliseconds, and the third trial found a significant increase in the proportion of people with an improvement in peak oxygen uptake among those with a QRS interval of ≥ 120 milliseconds but not among those with a QRS interval of < 120 milliseconds. CRT-D was associated with greater benefit in women than in men (one trial) and with greater benefit in people with LBBB than in those with non-specific intraventricular conduction delay (one trial). One trial found a statistically significant improvement with CRT-D for distance walked in 6 minutes for those with non-ischaemic cardiomyopathy (55.0 m vs. 2.5 m, p = 0.01) but not for those with ischaemic cardiomyopathy (4.2 m vs. 5.8 m, p = 0.57). Other evaluated subgroups showed no statistically significant effects.
Summary of Southampton Health Technology Assessments Centre’s peer review of clinical effectiveness in the Association of British Healthcare Industries joint submission
A joint report on behalf of Biotronik UK, Boston Scientific, Medtronic UK, Sorin Group and St Jude Medical was submitted by the Association of British Healthcare Industries (ABHI) to NICE. 151 The clinical effectiveness evidence presented in this MS has been briefly appraised (see Appendix 10). The MS also presented individual patient data (IPD) network meta-analysis (NMA) (see following section) and an economic model (see Chapter 5, Review of the manufacturers’ submission).
A systematic review of clinical effectiveness was undertaken in the MS. 151 Details of the searches were reported and the search strategies were supplied. Details and results of studies included in the systematic review were tabulated. Risk of bias was assessed, although no narrative discussion of risk of bias was provided.
The inclusion criteria for the MS systematic review differed from those in the NICE scope61 and the results were not presented according to the population groups defined in the NICE scope. As a result of this, the MS and the Southampton Health Technology Assessments Centre’s (SHTAC) systematic reviews differ in the evidence included (see Appendix 10).
The MS does not explicitly report the conclusions from the systematic review of clinical effectiveness in the main body of the submission. The executive summary states that ‘there is a large body of RCT evidence confirming the efficacy and safety of ICD, CRT-P and CRT-D in patients with HF’ (p. 4);151 however, there is no comment regarding the comparative effectiveness of the interventions for each of the populations defined in the NICE scope. Further conclusions are presented in the MS based on the IPD NMA, which is discussed in the following section.
Individual patient data network meta-analysis: a critical appraisal
The joint submission from the manufacturers presents an IPD NMA using meta-regression to assess the effectiveness of ICDs, CRT-P and CRT-D in the different subgroups of people who have HF. 151 The intention was for the IPD NMA to inform the cost-effectiveness model produced on behalf of the manufacturers. As such, it focuses on the outcomes of all-cause mortality, all-cause hospitalisation and HRQoL. In undertaking the IPD NMA, the MS recognises the heterogeneous nature of patients with HF and the likelihood that the interventions may have differing effects. It also changes the focus of the assessment from an evaluation of the effectiveness of the devices for specific subgroups of patients as identified in the scope for the NICE appraisal, to trying to establish which subgroups of patients the different devices appear to benefit. Inevitably, these may not be the same groups. With limited published evidence on the effectiveness of devices in different patient subgroups with HF, the availability of IPD from the manufacturers makes a NMA meta-regression possible and justified.
This section presents a critical appraisal of the IPD NMA using a structured approach (see Appendix 10). It provides an assessment of the appropriateness of the methods used and of the results and conclusions presented.
Methods
Network of evidence
The systematic review of clinical effectiveness reported in the MS included a comprehensive and transparent search strategy, the criteria and reasons for study selection, extraction of baseline data on patient characteristics and study outcomes, quality assessment of studies and the process followed to complete these stages. The studies identified in the systematic review provided the basis for developing the network of evidence for the IPD NMA. However, the IPD NMA included only a subset of those studies identified in the systematic review for which the manufacturers provided IPD (13 of 22 trials; 95% of patients from the evidence network). Also, the evidence network excluded seven trials71,75,81,84,89,95,97 identified by the SHTAC systematic review (see earlier in this chapter). The extent of the evidence base for the NMA varied for the different outcomes assessed, with 13 trials (n = 12,638) for all-cause mortality, 11 trials for all-cause hospitalisation (n = uncertain as it refers to studies not included in the NMA) and three trials (n = 4432) for HRQoL. The MS outlines reasons for excluding specific studies from the overall evidence network, the approach taken to allocating trials to different comparisons and the basis for handling data (i.e. separating or aggregating trial arms or phases) from the trials. The effects of a more limited evidence base and the manipulation of data are discussed. For all-cause mortality, NMAs were produced to compare outcomes using aggregate data from all trials in the network with outcomes using data from the trials included in the IPD only, finding no significant differences. Similar comparisons were not produced for the other outcomes.
Issues relating to differences in the 13 IPD trials were also considered. The effects of length of follow-up, trial crossover, missing data and data handling were discussed in the MS, particularly with relation to all-cause mortality. Length of follow-up was restricted to that specified in trial protocols (commercial-in-confidence information has been removed) to limit the effects of trial crossover at the longest follow-up time (commercial-in-confidence information has been removed). Missing data for the covariables appeared limited (commercial-in-confidence information has been removed), with data imputed through multiple imputations when necessary (details provided in appendix 6 of the MS151). The covariables used to capture baseline risk and treatment effect modifiers in the NMA were outlined for the different outcomes assessed, with the rationale for their inclusion and for any data manipulation (i.e. continuous to categorical) discussed.
Statistical analysis
The IPD NMA adopted a multivariate approach through meta-regression to assess the effects of the different interventions on HF patients for the outcomes of all-cause mortality, all-cause hospitalisation and HRQoL, taking into account the impact of different patient characteristics. Although different types of regression were used for analysing the three outcomes, all analyses followed a similar two-stage approach. First, a baseline rate was estimated for each outcome independent of the treatment effects of the devices. This used the pooled data from the relevant IPD trials for all patients randomised to OPT (i.e. all IPD trials assessing the specific outcome irrespective of the device assessed), which was the comparator treatment for the appraisal. Second, device-specific treatment effects were estimated using all available data from the relevant IPD trials (i.e. trials focusing on the specific outcome for all of the interventions compared). In both stages of the analyses, patient characteristics were included as covariables to incorporate baseline risk and treatment effect modifiers. This allowed subgroup-specific treatment effects to be estimated and provided the opportunity to identify groups of patients for whom the treatment provided significant benefit. In using a NMA approach, all interventions included can be compared relative to each other, when direct and indirect evidence is available. This is important in the current assessment when direct evidence may be limited (e.g. CRT-D vs. CRT-P and CRT-D vs. OPT). However, it is important to note that the findings of NMA may be affected by limitations in the network of evidence, whether direct or indirect evidence, as will be evident from the appraisal of the NMA.
For the analysis of all-cause mortality, a parametric survival analysis was undertaken to generate estimates of baseline mortality for all patients randomised to OPT (n = 3477). Several parametric distributions were used (i.e. exponential, Gompertz, log-logistic, log-normal and Weibull) in models both with and without covariables (i.e. patient characteristics) to ascertain which provided the most realistic predictions of survival. It also allowed the effects of covariables to be considered and, when necessary, the approach to their inclusion to be altered (e.g. age as a time-dependent covariable). The MS states that these were assessed through visual comparisons of the fitted and Kaplan–Meier survival curves within the trial follow-up, visual review of the extrapolations and of the shape of the instantaneous hazard over time, the Akaike information criterion (AIC), Cox–Snell residuals, tests of the acceptability of the proportional hazards assumption or accelerated failure time assumption, comparison against external data and review by clinical experts. Although these methods appear appropriate, the MS presents only the AIC statistics, a Kaplan–Meier plot for the Weibull model (distribution selected for the analyses) showing risk quintiles and an assessment of the proportional hazards assumption. As such, it is not possible to comment with certainty whether or not the approach was suitable. IPD NMAs using meta-regression were undertaken to estimate the relative treatment effects (i.e. HRs) of the different devices compared with each other and with OPT, taking account of factors that may influence their effectiveness (i.e. covariables). An initial set of NMAs excluding the covariables were conducted at the aggregate level (i.e. trial). This allowed a comparison of the unadjusted efficacy estimates from the NMAs with those produced by pairwise meta-analyses from aggregate trial data and with the individual trial estimates. This enabled an assessment of whether the IPD NMA appeared representative or whether differences existed that required further examination. It also provided an opportunity to assess the type of analysis that should be undertaken (i.e. fixed vs. random effects). Although the MS reports that caterpillar plots, Brooks–Gelman–Rubin statistics, autocorrelation and deviance information criteria (DIC) were assessed, only the DIC are reported. A second set of analyses, incorporating the covariables from the IPD, were estimated using fixed-effects models. These analyses used the Cox proportional hazards approach and were stratified by study to allow the baseline hazard for each study to be independent. A rationale for using fixed-effects models and for the selection of covariables is presented and appeared appropriate. The MS states that proportional hazards tests and Schoenfeld residual-based tests were used to assess the models; however, these are not reported.
The analysis of all-cause hospitalisation focused on the expected number of events per month and the expected number of days per month spent in hospital (excluding events in the 60 days post randomisation as these were accounted for separately in the MS economic model). The analysis used a negative binomial regression model (NBRM) to estimate both the baseline hospitalisation rate for patients on OPT and the effect of the different treatments on hospitalisation rates. The modelling approach was decided through a comparison with Poisson regression using measures of goodness of fit [i.e. Bayesian information criterion (BIC), AIC and two times log-likelihood score] and the covariates were incorporated into the analyses through a stepwise process (included at a significance level of p = 0.05). Limited data availability meant that some categorical variables were pooled (e.g. NYHA) and for some subgroups estimates were either not calculated or considered unreliable. In such cases, adjustments were made and justifications provided. Although limited information on the specific elements of the process is provided, comparisons are made with previous evaluations when available. It is evident from the analysis that it is likely that the limited evidence base affects the results and, although adjustments are made, uncertainty remains.
Health-related quality of life was assessed using the EQ-5D. UK age- and gender-specific utilities152 were adjusted using disease- and treatment-specific decrements/increments estimated from the three IPD trials reporting EQ-5D data and were varied over time. Baseline HRQoL taking account of disease severity was estimated using the NBRM, following a similar procedure to that for all-cause hospitalisation (justification for approach is provided). Prior to the analysis the raw data had been transformed as they appeared skewed (commercial-in-confidence information has been removed). Derived values were checked against population norms and trial-specific values to ascertain whether clinically plausible, reflecting the uncertainties resulting from the limited IPD available. The impact of treatment on HRQoL was estimated using the MD from baseline to first follow-up (assumed as 180 days). With only three studies in the evidence network (n = 3736), observations were limited for ICDs and CRT-D and were skewed by NYHA groups. This weakened evidence network affected the regression analysis, producing counterintuitive results. Exploratory analysis using MLWHFQ data at 6 months, the MS systematic review of clinical effectiveness and a correction for a placebo effect was used to adjust the estimates for use in the MS cost-effectiveness model. Duration of benefit was estimated by comparing the mean device value with that for OPT and judging when no further difference occurred. Justification is provided for the decisions made.
Although it is not possible to provide a detailed critique of each stage in the three analyses (given the partial reporting of the exploratory and confirmatory analyses undertaken) or to replicate the NMA as the IPD remains unpublished, the steps taken seem appropriate and the results presented appear reasonable given the note of caution provided in the MS throughout all three analyses.
Results
All-cause mortality
The baseline Weibull survival model for patients randomised to OPT was shown, through Kaplan–Meier curves, to differentiate between patients with varying risk profiles and to demonstrate the heterogeneity in the IPD population. Predicted survival rates were reported to (commercial-in-confidence information has been removed). The baseline risk model was used in the MS cost-effectiveness model for the baseline survival curve (see Table 37, p. 121, in the MS151). Covariables included in the model with a statistically significant effect were age, sex, ischaemic aetiology, LVEF, NYHA class (I/II, III/IV) and QRS duration (< 120 milliseconds, ≥ 120 milliseconds).
Exploratory NMA models without the covariables were fitted for the different comparisons of the interventions using the trials identified in the evidence network (13 trials, 12,638 patients). These showed limited differences in the HRs for fixed- and random-effects models and for IPD compared with aggregate data for all trials in the network and for the pairwise meta-analyses. As such, it was considered appropriate to use the IPD for the NMA and to use fixed-effects models. The fixed-effects IPD NMA without the covariables estimated the HRs compared with (commercial-in-confidence information has been removed) for CRT-D, (commercial-in-confidence information has been removed) for CRT-P and (commercial-in-confidence information has been removed) for ICDs. HRs were presented for CRT-D compared with CRT-P (commercial-in-confidence information has been removed) and for CRT-D compared with ICD (commercial-in-confidence information has been removed). The MS states that proportional hazards tests showed that the benefits were maintained over time [global p-value for device terms (commercial-in-confidence information has been removed)].
Univariate analyses and multivariate stepwise selection procedures were used to explore the covariables for inclusion in the final NMA model as treatment effect modifiers. Rationales were provided for the covariables included for the different comparisons made. The final NMA model was used in the cost-effectiveness model presented in the MS (see table 39, p. 132, in the MS151). The final NMA model was used to show the predicted treatment effect for different subgroups, presented as HRs with CIs (assumed to be 95% CIs although not stated in the MS) (Table 77). Importantly, the MS warns that the analysis presented is ‘inherently more uncertain than the analysis without covariables’ and that ‘caution should be taken not to over-interpret individual subgroups since anomalies may arise as a result of patient level characteristics not accounted for’ (p. 130). 151 This is particularly important in relating the broad conclusions made to the results presented in the MS. The analyses highlighted that age, sex, QRS duration and LBBB pattern were significant predictors of benefit from the different devices.
| QRS (milliseconds) | Device | Sex and age group | |||
|---|---|---|---|---|---|
| Male < 60 years | Male ≥ 60 years | Female < 60 years | Female ≥ 60 years | ||
| Non-LBBB | |||||
| < 120 | ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ≥ 120 to < 150 | ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| ≥ 150 | ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| LBBB | |||||
| ≥ 120 to < 150 | ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| ≥ 150 | ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | |
It is evident from the forest plots presented in the MS (see figure 19, pp. 133–4151) and from HRs presented in Table 77 that for the majority of subgroups the devices provide some benefit for all-cause mortality compared with OPT (49 of 52 comparisons). However, the benefit provided by the devices is rarely statistically significant (14 of 52 comparisons show significant benefit; four of 52 comparisons are of borderline significance) and, as indicated in the MS, should be considered with some caution. Despite this, it is possible to highlight the main findings for the different subgroups for which the benefit is statistically significant or on the margins of statistical significance. ICDs provided a statistically significant benefit compared with OPT for men aged < 60 years irrespective of QRS duration or LBBB status and were marginally insignificant for both men aged ≥ 60 years and women aged < 60 years with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds and without LBBB. CRT-D benefited a wider group of patients compared with OPT. Benefits that were statistically significant or on the margins of statistical significance were reported for men and women of all ages with a QRS duration of ≥ 150 milliseconds and for women of all ages with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds. In contrast, CRT-P had a statistically significant effect only for women aged ≥ 60 years with a QRS duration of ≥ 150 milliseconds and with LBBB.
Following submission of this report we were informed of an error in the ABHI submission that had led to incomplete accounting of the covariance between the model parameters. Correcting the error resulted in a narrowing of the CIs around the HRs for the comparisons with OPT. As SHTAC did not have access to the IPD analyses, the error cannot be verified. Although this increased the number of comparisons for which there was a statistically significant benefit (28/52), the groups identified differed little from those that were shown to benefit significantly or that were on the margins of statistical significance in the previous SHTAC assessment. In the reanalysis ICDs were shown to provide a statistically significant benefit for all men irrespective of age, QRS duration or LBBB status and for women aged < 60 years with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds and without LBBB. CRT-D benefited a wider group of patients. Benefits that were statistically significant or on the margins of statistical significance were reported for men and women of all ages with a QRS duration ≥ 120 milliseconds and with or without LBBB. In contrast, CRT-P had a statistically significant effect only for men and women aged ≥ 60 years with a QRS duration of ≥ 150 milliseconds and with LBBB.
All-cause hospitalisation
The baseline regression model (see table 40, p. 139, in the MS151) for patients randomised to OPT produced monthly probabilities of hospitalisation for the different subgroups (Table 78). These were used for the baseline assessment. When data allowed, treatment effects were estimated using a process similar to a fixed-effects NMA (see table 42, p. 142, in the MS151) and are presented in Table 79. Limited data meant that estimates could not be provided for some groups (i.e. ICD NYHA class IV and CRT-P NYHA class I/II) and are thought unreliable for others (i.e. CRT-D NYHA classes III and IV). Alternative values have been put forward in the MS with justifications (see Table 79), which appear reasonable. The effects of the devices on all-cause hospitalisations were translated into monthly transition probabilities (Tables 80–82), which were used in the economic model presented in the MS.
| NYHA class I/II | NYHA class III | NYHA class IV | |
|---|---|---|---|
| Non-ischaemic aetiology | |||
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | |||
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Derived value | Value used in model | Justification | |
|---|---|---|---|
| ICD | |||
| NYHA class I/II | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis clinically plausible |
| NYHA class III | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis clinically plausible |
| NYHA class IV | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Device not assessed in this patient group |
| CRT-P | |||
| NYHA class I/II | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Device not assessed in this patient group |
| NYHA class III | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis clinically plausible |
| NYHA class IV | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis clinically plausible |
| CRT-D | |||
| NYHA class I/II | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis clinically plausible |
| NYHA class III | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis not clinically plausible. Assumed same as CRT-P-value given common component (CRT) |
| NYHA class IV | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis not clinically plausible. Assumed same as CRT-P-value given common component (CRT) |
| NYHA class I/II | NYHA class III | NYHA class IV | |
|---|---|---|---|
| Non-ischaemic aetiology | |||
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | NA |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | NA |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | NA |
| Ischaemic aetiology | |||
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | NA |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | NA |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | NA |
| NYHA class I/II | NYHA class III | NYHA class IV | |
|---|---|---|---|
| Non-ischaemic aetiology | |||
| QRS < 120 milliseconds | NA | NA | NA |
| QRS 120–149 milliseconds | NA | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | NA | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | |||
| QRS < 120 milliseconds | NA | NA | NA |
| QRS 120–149 milliseconds | NA | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | NA | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| NYHA class I/II | NYHA class III | NYHA class IV | |
|---|---|---|---|
| Non-ischaemic aetiology | |||
| QRS < 120 milliseconds | NA | NA | NA |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | |||
| QRS < 120 milliseconds | NA | NA | NA |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
Health-related quality of life
The NBRM (see table 52, p. 152, in the MS151) for patients randomised to OPT was used to generate baseline results for the different subgroups (Table 83). Given the limitations of the data set used, the estimates were checked with population norms and with the mean values from the three trials included in the IPD. Although variations were evident, the MS stated that they were felt to be within acceptable tolerance levels. Treatment effects on HRQoL were estimated as mean change from baseline using the IPD (Table 84). As several estimates appeared counterintuitive, reflecting the limited and skewed data available, the MS adjusted the values based on IPD analysis of MLWHFQ 6-month data and a systematic review (see Table 84). As a result, the MS suggests that caution should be used when interpreting the results. Validation of the adjusted values provided in the MS is difficult because of the lack of published evidence; as such, the increments presented should be viewed with caution. (Commercial-in-confidence information has been removed) and so this was applied in the economic model presented in the MS.
| NYHA class | Sex | Decrements from unity | ||
|---|---|---|---|---|
| Population norm | Derived | Disease-specific componenta | ||
| Non-ischaemic aetiology | ||||
| I/II | Male | 0.2100 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| I/II | Female | 0.2098 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| III | Male | 0.2100 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| III | Female | 0.2098 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| IV | Male | 0.2100 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| IV | Female | 0.2098 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | ||||
| I/II | Male | 0.2100 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| I/II | Female | 0.2098 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| III | Male | 0.2100 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| III | Female | 0.2098 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| IV | Male | 0.2100 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| IV | Female | 0.2098 | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| IPD analysis | Economic model | Justification for value used in economic model | ||
|---|---|---|---|---|
| n | Utility value (mean, SE)a | Utility valueb | ||
| NYHA class I/II | ||||
| OPT | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | No clinical reason why person already receiving OPT would have a change in utility |
| ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Value derived from IPD analysis (commercial-in-confidence information has been removed). Systematic review suggests ICDs have a positive impact |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Cost-effectiveness results not generated for this treatment option |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Value derived from IPD analysis (commercial-in-confidence information has been removed). Systematic review and MLWHFQ analysis suggest that CRT-D has a positive impact |
| NYHA class III | ||||
| OPT | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | No clinical reason why person already receiving OPT would have a change in utility |
| ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Results from IPD analysis not significantly different from zero. Literature review suggests that ICDs have no benefit in this group |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Value derived from IPD analysis (commercial-in-confidence information has been removed). Literature review and MLWHFQ analysis suggest that CRT-P has a benefit in this group |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Assumed same as CRT-P as not thought clinically different. IPD results derived from small patient numbers. Literature review and MLWHFQ analysis suggest that CRT-D has a benefit in this group |
| NYHA class IV | ||||
| OPT | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | No clinical reason why person already receiving OPT would have a change in utility |
| ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Cost-effectiveness results not generated for this treatment option |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Not enough information available. Assumed same as for NYHA class III. Analysis of MLWHFQ data supports this assumption |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Not enough information available. Assumed same as for NYHA class III. Analysis of MLWHFQ data supports this assumption |
Discussion
The MS presented an IPD NMA using meta-regression to assess the effectiveness of ICDs, CRT-P and CRT-D for different subgroups of people with HF. As part of the NMA, the MS used a systematic review to identify the network of evidence for which IPD were available. It provided an outline of the methods used in the systematic review and in the different stages of the NMA. The effects of different decisions were discussed and comparisons made, although analyses used to underpin many decisions were not presented. Limitations in the underlying IPD and uncertainties in the analyses were outlined, with the MS suggesting caution when interpreting and using the results. Importantly, the IPD NMA presented in the MS did not take account of the subgroups identified by the scope for the NICE appraisal. 61 Instead, it looked for subgroups of HF patients for whom the different devices appeared to have some benefit. Although challenging in terms of developing guidance, it reflects the opinion of part of the clinical community. Given the lack of published evidence on subgroups of HF patients, the IPD NMA provides a useful source of evidence. However, it should be used cautiously given the uncertainties in the methods used in the NMA, the limitations in the evidence base (weak and imbalanced data), the assumptions used and the adjustments made to some counterintuitive results, and the possibility that some of the findings may be the result of chance.
All-cause mortality
Fixed-effects IPD NMA without covariables showed that CRT-D, CRT-P and ICDs provided a statistically significant benefit compared with OPT for all-cause mortality. Comparison of CRT-D with both CRT-P and ICDs showed a statistically significant benefit for CRT-D. These results appeared appropriate when compared with the original trial results and the pairwise meta-analyses undertaken in the SHTAC systematic review (see earlier in this chapter) and the MS. When including covariates to identify subgroups that benefited from the different devices, the outcomes were less clear and the MS advises that the results should be interpreted with caution. It was evident that all of the devices appeared beneficial compared with OPT; however, rarely were differences statistically significant. CRT-D appeared to have a statistically significant benefit for people of all ages with a QRS duration of ≥ 150 milliseconds and for women of all ages with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds. Although CRT-D showed benefit for men of all ages, its effects were marginally insignificant. ICDs appeared to have a statistically significant benefit for men aged < 60 years for all QRS durations and for men aged ≥ 60 years with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds and without LBBB. CRT-P showed a statistically significant benefit only for women with a QRS duration of ≥ 150 milliseconds and with LBBB.
All-cause hospitalisations
Estimates of the effects of the different devices on all-cause hospitalisations showed that all were beneficial. ICDs reduced hospitalisations in people in NYHA groups I–III (commercial-in-confidence information has been removed) and CRD-P in NYHA groups III and IV (commercial-in-confidence information has been removed). Estimates for CRT-D suggested a constant effect for all NYHA groups (commercial-in-confidence information has been removed) and so were adjusted in the MS to reflect those of CRT-P.
Health-related quality of life
Baseline estimates of HRQoL from the IPD using the EQ-5D showed that patients in NYHA class I/II had similar values to population norms, whereas patients in NYHA classes III and IV had values that were progressively lower. Treatment estimates were counterintuitive, reflecting the limited IPD available. As a consequence, adjustments were made which assumed that CRT-P and CRT–D had the same effect on EQ-5D values and ICDs had an effect on NYHA class I/II only. Benefits were thought to last for a fixed period of (commercial-in-confidence information has been removed).
Chapter 5 Economic analysis
The aim of this section is to assess the cost-effectiveness of:
-
ICDs in addition to OPT for the treatment of people who are at increased risk of SCD as a result of ventricular arrhythmias despite receiving OPT
-
CRT-P or CRT-D in addition to OPT for the treatment of people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT
-
CRT-D in addition to OPT for the treatment of people with both conditions.
The economic analysis comprises:
-
a systematic review of the literature on the cost-effectiveness of ICDs for people at risk of SCD and CRT for people with HF
-
a systematic review of studies of the HRQoL of people at risk of SCD or with HF
-
a review of the MS to NICE151
-
an independent economic model and cost-effectiveness evaluation (the SHTAC model).
Systematic review of existing cost-effectiveness evidence
A systematic review of the literature was conducted to summarise the existing evidence on the cost-effectiveness of ICDs for the treatment of arrhythmias and CRT for the treatment of HF. The quality of the included publications was assessed and those of relevance to the UK are discussed in greater detail in terms of the methodology used and the potential generalisability of their results.
The methods and inclusion criteria considered for this review of economic evaluations are presented in Chapter 3 and details of the search strategy are documented in Appendix 2. Given the volume of studies meeting the inclusion criteria, data extraction was undertaken as follows: for studies included in previous assessments, data extraction was derived from these reports and checked against original publications; for newly identified evidence, data were extracted directly from the original publications.
Quantity and quality of research available
The literature searches identified 1410 studies that potentially met the inclusion criteria set out in Chapter 3 (see Inclusion and exclusion criteria). From screening titles and abstracts, 1334 publications were excluded and 76 were retrieved for full screening. Of these, 22 did not meet the inclusion criteria:
-
six were found not to be full economic evaluations
-
six were abstracts (five from 2010 and 2011 and one study treated as an abstract as it did not report sufficient details for inclusion)
-
three references were unobtainable and thus did not provide sufficient details for inclusion
-
three had a different comparator from that specified in the research protocol
-
two had a different population from that specified in the research protocol
-
one had a different intervention from that specified in the research protocol
-
one was a non-English-language report.
A list of relevant excluded studies is provided in Appendix 11.
A total of 54 papers met the inclusion criteria. 63,64,149,153–203 Three studies were each reported in two publications; therefore, 51 separate economic evaluations were included in this review. A flow chart describing the identification of the included studies is provided in Figure 31.
FIGURE 31.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness.
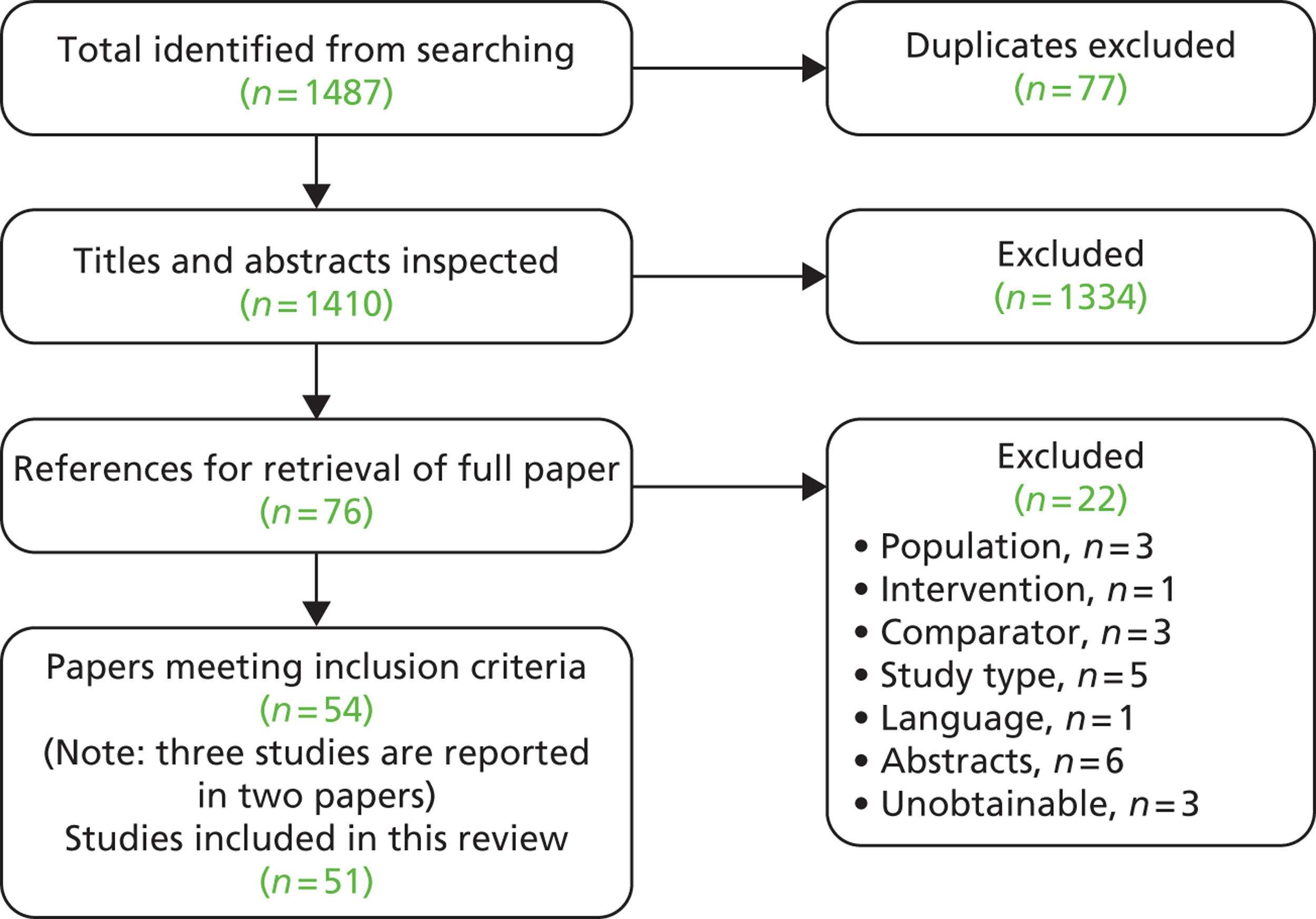
The included economic evaluations were categorised according to the type of the interventions that they assessed. Thirty-six63,149,153–186 of the included studies assessed ICDs and 1764,155,172,187–200 assessed CRT. Two155,172 of these studies assessed both ICDs and CRT; details of these two studies have been included within both the ICD and the CRT sections.
Economic evaluations of implantable cardiac defibrillators
Most of the economic evaluations identified in the systematic review were for the use of ICDs in patients at increased risk of SCD. Table 85 provides an overview of these studies.
| Study | Country | Population | Study type | Main source of effectiveness data | Authors’ conclusion (ICER) |
|---|---|---|---|---|---|
| Al-Khatib et al. 2005154 | USA | Adults with a history of MI and a LVEF ≤ 30% | Survival | MADIT II101 | Cost-effective (US$50,500 per LYG) |
| Bertoldi et al. 2011155 | Brazil | HF NYHA class II, III or IV; EF ≤ 35% | Markov | Meta-analysis of trials | Marginally cost-effective (INT$32,663 per QALY) |
| Buxton et al. 2006153 | UK | Secondary prevention patients at risk of SCD with previous CA or VT | Markov | Observational data and CIDS84 | Not cost-effective (£76,139 per QALY) |
| Caro et al. 2007156 | UK and France | HF NYHA class II or III; LV dysfunction ≤ 35% | DES | SCD-HeFT105 | Cost-effective (cost–benefit ratio 0.17 UK) |
| Chan et al. 2006157 | USA | Ischaemic heart disease and LVEF ≤ 30% | Markov | MADIT II101 | Not cost-effective in all MADIT II patients (US$55,800 per QALY); risk stratification with MTWA improves cost-effectiveness (US$48,800 per QALY) |
| Chan et al. 2009158 | USA | Cardiomyopathy (EF ≤ 35%) and no previous VA | Markov | Prospective cohort | Cost-effective for high-risk groups (US$70,881 per QALY) |
| Chen and Hay 2004159 | USA | Newly diagnosed HF NYHA class II or III | Markov | Not stated | Not cost-effective (US$97,863 per QALY) |
| Cowie et al. 2009160 | Belgium | LVEF ≤ 35%; HF NYHA class II or III; or previous MI | Markov | AMIOVIRT,69 CAT,82 DEFINITE,90 MADIT I,99 MADIT II,101 SCD-HeFT105 | Cost-effective (€29,530 per QALY) |
| Deniz et al. 2009161 | Canada | HF NYHA class II or II; LV dysfunction ≤ 35% | DES | SCD-HeFT105 | Cost-effective (cost–benefit ratio of 0.05) |
| Feingold et al. 2010162 | USA | Children (10–15 years) with dilated cardiomyopathy and HF | Markov | Paediatric cardiology prospective studies | Not cost-effective (US$281,622 per QALY) |
| Filion et al. 2009163 | Canada | Severe LV dysfunction at risk of SCD | Markov | Meta-analysis of trials | Not cost-effective (CAD$108,900 per QALY) |
| Gandjour et al. 2011164 | Germany | EF ≤ 30% or< 1 month after MI | Markov | MADIT II101 | Unclear (€44,736 per QALY) |
| Goldenberg et al. 2005165 | USA | Inherited cardiac disorders with high risk of SCD; patients aged 10–75 years | Survival | Several sources | Cost-effective in selected high-risk patients with inherited cardiac disorders because of gained productivity over lifetime (US$3328–600,000 per QALY) |
| Kupersmith et al. 1995167 | USA | High-risk patients with VT/VF with ICD implant from 1980–7 | Markov | Retrospective study with historical control subjects | Cost-effective (epicardial ICD US$31,100 per LYG; endocardial ICD US$25,700 per LYG) |
| Kuppermann et al. 1990166 | USA | CA survivors, not associated with MI, and persistent VT/VF | Decision tree + Markov | Several ICD case series | Cost-effective (US$15,600–29,600 per LYG) |
| Larsen et al. 1992168 | USA | Patients with sustained VT/VF | Markov | Case series of ICD patients | Cost-effective (US$29,244 per LYG) |
| Larsen et al. 2002169 | USA | EF ≤ 40%; sustained VT or resuscitated from CA | Trial | AVID71 | Moderately cost-effective (US$66,677 per LYG) |
| Mark et al. 2006170 | USA | HF NYHA class II or III; LV dysfunction ≤ 35% | Trial | SCD-HeFT105 | Cost-effective (US$41,530 per QALY) |
| McGregor and Chen 2004171 | Canada | Adults with a history of MI and a LVEF ≤ 30% | Markov | MADIT II101 | Unclear (CAD$47,458 per LYG) |
| Medical Services Advisory Committee 2006172 | Australia | Adults with a history of MI and a LVEF ≤ 30%; or HF NYHA class II or III and LV dysfunction ≤ 35% | Decision tree | SCD-HeFT,105 COMPANION116 | Cost-effective in patients with moderate to severe symptoms of CHF (AUS$39,885 per LYG) |
| Mushlin et al. 1998173 | Germany and USA | Adults with a history of MI and a LVEF ≤ 30% | Trial | MADIT99 | Cost-effective in selected high-risk patients (US$27,000 per LYG) |
| Neyt et al. 2008174 | Belgium | HF NYHA class II or II; LV dysfunction ≤ 35% | Markov | SCD-HeFT105 | Not cost-effective (€132,100 per QALY) |
| O’Brien et al. 1992175 | UK | Patients at high risk of SCD | Simple calculation model | ICD case series | Cost-effective (£15,400 per LYG) |
| Owens et al. 1997176 | USA | CA survivors at high risk of SCD | Markov | CASH,81 MADIT99 | Cost-effective for high-risk groups (US$74,400 per QALY) |
| Owens et al. 2002177 | USA | Patients at risk of SCD (trial characteristics) | Markov | MADIT,99 AVID,71 CIDS, CASH,81 MUSTT,146 CABG Patch75 | Cost-effective in high-risk groups (US$54,700 per QALY) |
| Parkes et al. 200063 | UK | Patients at risk of SCD from arrhythmias | Survival calculation | AVID71 | Unclear (£40,500–87,000 per LYG) |
| Ribeiro et al. 2010178,201 | Brazil | HF NYHA class II and III; LVEF ≤ 35% | Markov | Several sources; scenario with MADIT I99 | Not cost-effective (R$68,318 per QALY) |
| Sanders et al. 2001179 | USA | Patients with MI who did not have sustained VA | Markov | Range of ICD efficacies evaluated | Unclear (US$71,800 per QALY –US$557,900 per QALY for moderate efficacy and EF < 0.3 to EF > 0.4) |
| Sanders et al. 2004180 | USA | Adults with a history of MI and a LVEF ≤ 30% | Markov | MADIT II101 | Cost-effective (US$50,900 per QALY) |
| Sanders et al. 2005181 | USA | Patients at risk of SCD (trial characteristics) | Markov | MADIT,99 CABG Patch,75 MUSTT,146 MADIT II,101 DEFINITE,90 DINAMIT,95 COMPANION,116 SCD-HeFT105 | Cost-effective in selected high-risk patients (US$34,000–70,200 per QALY) |
| Sanders et al. 2010182 | USA | Patients with LV dysfunction | Markov | MADIT,99 MADIT II,101 DEFINITE,90 MUSTT,146 SCD-HeFT105 | Unclear, varies widely among trials (US$37,031–138,458 per QALY) |
| Sheldon et al. 2001183 and O’Brien et al. 2001202 | Canada | Secondary prevention patients at risk of SCD with previous CA or VT | Trial | CIDS84 | Not cost-effective (CAD$213,543 per LYG) but more attractive in patients with at least two risk factors for SCD (CAD$65,195 per LYG) |
| Wang et al. 2008149 | Japan | Brugada syndrome with abnormal heart | Markov | Several trials including DEBUT89 | Cost-effective (US$14,667 per QALY) |
| Weiss et al. 2002184 | USA | VT or VF | Retrospective cohort study | Unclear (US$78,400 per LYG) | |
| You et al. 2007185 | Canada | Hypertrophic cardiomyopathy at risk of SCD (no previous CA) | Markov | ICD registries and cohort studies | Cost-effective (US$19,400 per QALY) |
| Zwanziger et al. 2006186 | USA | Adults with a history of MI and a LVEF ≤ 30% | Trial | MADIT II101 | Not cost-effective for trial, 3.5 years time horizon (US$235,000 per LYG) |
Nineteen economic evaluations were conducted in the USA,154,157–159,162,165–170,176,177,179–182,184,186 five in Canada,161,163,171,183,185 three in the UK,63,153,175 three elsewhere in Europe,160,164,174 two in Brazil155,178 and one each in Australia172 and Japan. 149 Two studies were conducted in two countries (one in the UK and France156 and one in Germany and the USA173). Study type was predominantly cost–utility analysis149,153,155,157–160,162–165,170,174,176–182,185 and cost-effectiveness analysis63,154,166–169,171–173,175,183,184,186 with two cost–benefit analyses. 156,161 Most studies used a Markov model149,153,155,157–160,162–164,166–168,171,174,176–182,185 with five studies using a trial-based analysis169,170,173,183,186 and the remaining studies using a variety of methods. Most studies used a long-term time horizon of > 20 years,149,153–155,157–160,162,164–166,168,172,174–182,185 six studies had a short time horizon of < 7 years63,156,161,167,169,173 and six studies had a medium time horizon of between 8 and 19 years. 163,170,171,183,184,186 Fourteen studies were based on a single trial63,154,156,157,161,164,169–171,173,174,180,183,186 with the MADIT II trial101 (six studies154,157,164,171,180,186) and the SCD-HeFT trial105 (four studies156,161,170,174) the most commonly used. Ten studies used more than one trial, through meta-analysis, systematic review or different trial populations,149,153,155,160,163,172,176,177,181,182 eleven studies used other sources of evidence to model the intervention effect158,162,165–168,175,178,179,184,185 and one study did not state the source of data. 159 Almost half of the studies (n = 15) reported that ICDs were cost-effective,149,154–156,160,161,166–170,172,175,180,185 with an additional six finding ICDs cost-effective for high-risk groups according to study definitions. 158,165,173,176,177,181 Nine studies did not find ICDs cost-effective153,157,159,162,163,174,178,183,186 and six studies were unclear whether ICDs were cost effective or not. 63,164,171,179,182,184
The judgements of the methodological quality assessment of the studies on ICDs are summarised in Table 86. The studies vary in their quality and relevance to the UK NHS. As already described, many studies were conducted in countries outside the UK and it is unclear how generalisable their results are to the UK NHS. Generally, the later studies are of higher quality. Earlier studies were less likely to include QALYs, long-term life horizons or all relevant costs and consequences.
| Study | Decision problem relevant to the UK | Setting comparable to the UK | Appropriate methodology | Relevant costs and consequences | Data inputs justified | QALYs measured | Appropriate time horizon | Discounting | Incremental analysis | Sensitivity analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Al-Khatib et al. 2005154 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Bertoldi et al. 2011155 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Buxton et al. 2006153 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Caro et al. 2007156 | Y | Y | Y | ? | Y | N | N | Y | Y | Y |
| Chan et al. 2006157 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Chan et al. 2009158 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Chen and Hay 2004159 | Y | N | Y | ? | ? | Y | Y | Y | Y | Y |
| Cowie et al. 2009160 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Deniz et al. 2009161 | Y | N | Y | ? | Y | N | N | Y | Y | Y |
| Feingold et al. 2010162 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Fillion et al. 2009163 | Y | N | Y | ? | Y | Y | ? | Y | Y | Y |
| Gandjour et al. 2011164 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Goldenberg et al. 2005165 | Y | N | ? | ? | N | Y | Y | Y | Y | Y |
| Kupersmith et al. 1995167 | Y | N | Y | ? | Y | N | Y | Y | Y | Y |
| Kuppermann et al. 1990166 | Y | N | Y | N | N | N | N | Y | N | Y |
| Larsen et al. 1992168 | Y | N | Y | ? | Y | N | Y | Y | Y | Y |
| Larsen et al. 2002169 | Y | N | Y | ? | Y | N | ? | Y | Y | Y |
| Mark et al. 2006170 | Y | N | Y | ? | Y | Y | ? | Y | Y | Y |
| McGregor and Chen 2004171 | Y | N | ? | ? | Y | N | ? | Y | Y | Y |
| Medical Services Advisory Committee 2006172 | Y | N | Y | Y | Y | N | Y | N | Y | Y |
| Mushlin et al. 1998173 | Y | N | Y | N | ? | N | ? | Y | Y | Y |
| Neyt et al. 2008174 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| O’Brien et al. 1992175 | Y | Y | Y | N | ? | N | Y | Y | N | Y |
| Owens et al. 1997176 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Owens et al. 2002177 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Parkes et al. 200063 | Y | Y | ? | N | Y | Y | N | N | Y | Y |
| Ribeiro et al. 2010178,201 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Sanders et al. 2001179 | Y | N | Y | N | Y | Y | Y | Y | Y | Y |
| Sanders et al. 2004180 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Sanders et al. 2005181 | Y | N | Y | ? | N | Y | Y | Y | Y | Y |
| Sanders et al. 2010182 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Sheldon et al. 2001183 and O’Brien et al. 2001202 | Y | N | Y | N | Y | N | ? | Y | Y | Y |
| Wang et al. 2008149 | Y | N | Y | ? | N | Y | Y | Y | Y | Y |
| Weiss et al. 2002184 | Y | N | Y | ? | N | N | ? | Y | Y | N |
| You et al. 2007185 | Y | N | Y | ? | ? | Y | Y | Y | Y | Y |
| Zwanziger et al. 2006186 | Y | N | Y | ? | Y | N | ? | Y | Y | Y |
Five studies153,155,160,178,182 were considered to be of high methodological quality by meeting all or all but one (‘Setting comparable to the UK’) of the recognised criteria. 38,66 Of these, only one study153 was conducted for a UK setting and perspective and is considered of most relevance. However, it should be noted that this study, published in 2006, used data from patients mostly implanted before 2002 and therefore may not be generalisable to current practice. We describe this study in more detail in the following section.
Buxton and colleagues153
Buxton and colleagues153 developed a Markov model to estimate the cost-effectiveness of ICDs compared with AAD treatment in the UK in secondary prevention patients at risk of SCD (see Appendix 12 for data extraction). The economic evaluation was part of a wider study of the clinical characteristics, survival, QoL and costs of ICD patients in the UK. The model combined patient data from two major UK implanting centres with data from three published RCTs. 71,81,84 The Markov model had daily cycles and eight states: out of hospital (well); in hospital: arrhythmic, other cardiac, other non-cardiac, ICD maintenance, ICD replacement and amiodarone problems; and death.
UK-specific survival and admission rates were estimated from the UK sampled observational data for ICD patients, with data from the Canadian ICD trial84 used to estimate the relative survival and admission rates between ICD and amiodarone patients. The review of clinical characteristics included 535 UK patients implanted between 1991 and 2002. Mean actuarial survival at 1, 3 and 5 years was 92%, 86% and 71% respectively.
A cross-sectional survey collected HRQoL data using various QoL measures, including the EQ-5D, from a sample of 229 patients. The levels of most of the HRQoL measures were lower in the cohort than in the UK general population. There was no evidence of a change in QoL with time from implantation although length of follow-up is not clear. Patients who had suffered ICD shocks had significantly poorer HRQoL. Most patients nevertheless expressed a high level of satisfaction with ICD therapy. Based on the HRQoL data, the model base case assumes a constant utility value of 0.75 for all patients. Sensitivity analyses used utility estimates of 0.75 for ICD patients with 0.65 for patients receiving AADs and 0.83 for ICD patients with 0.80 for patients receiving AADs.
Buxton and colleagues153 collected resource and cost data for 211 patients from Papworth NHS Trust and 167 patients from Liverpool NHS Trust. In addition to the costs of the implantation, post-discharge costs (tests, medications and follow-up consultation) and costs of additional hospitalisations were also calculated. The mean initial cost of implantation showed little variation between centres or between earlier and more recent implants, and the model assumed a cost of £16,402 for the ICD device (with leads) and an implantation cost of £23,608 (device cost, implantation cost, associated tests and hospital stay).
Buxton and colleagues153 concluded that the benefit from an ICD may not be sufficient to make the technology cost-effective in the UK. The mean incremental cost-effectiveness ratio (ICER) for an average UK patient over a 20-year time horizon was £76,139 per QALY gained. Cost-effectiveness was most favourable for men aged > 70 years with a LVEF of < 35%. Patients with a LVEF of < 35% had an ICER of £72,000 per QALY over 20 years. Extrapolating over the lifetime of the patients with a low LVEF gave an ICER of £48,372 per QALY. A reduction in the cost of the implant/replacement and improvements in the reliability of ICDs (repair/replacement of 3% per patient-year instead of 6% in the base case) would reduce the ICER to £35,500 per QALY.
As noted earlier, this study used costs and resources associated with patients implanted between 1991 and 2002, which may not reflect current practice and could mean that the ICERs reported are no longer appropriate. The other high-quality studies, all published since this study was published and for slightly different populations and different settings, present a range of conclusions about the cost-effectiveness of ICDs, from not cost-effective178 to uncertain if cost-effective,182 marginally cost-effective155 and cost-effective. 160
Economic evaluations of cardiac resynchronisation therapy
Seventeen economic evaluations of the use of CRT concern patients with HF. 64,155,172,187–200 Table 87 provides an overview of these studies. Four studies were conducted in the UK64,189,190,198 with six conducted elsewhere in Europe. 187,188,191,193,196,199 Two studies were carried out in Australia,172,195 two in the USA,192,197 and one each in Canada,194 Brazil155 and Argentina. 200 The study type was mostly cost–utility analysis (n = 16) with one cost-effectiveness analysis. 172 Most studies used a Markov model (n = 11),64,155,187,188,193,194,196–200 with six studies using other methodology172,190–192,195 and one using trial-based analysis. 189 Twelve studies used a long-term time horizon of > 20 years64,155,172,188,189,191,194–198,200 and five studies had a short time horizon of < 8 years. 187,190,192,193,199 Eight studies were based on a single trial, with the CARE-HF109 (five studies188–191,198) and COMPANION116 (three studies172,192,196) trials the most commonly used. Five studies used more than one trial, through meta-analysis, systematic review or different trial populations155,194,195,197,200 and four studies used other sources of evidence to model the intervention effect. 64,130,193,199 The majority of the studies (n = 15) reported that CRT was cost-effective. 64,155,172,187–193,195,196,198–200 Two studies (conducted in the USA197 and Canada194) in patients in NYHA class III and with a prolonged QRS duration were uncertain whether or not CRT was cost-effective.
| Study | Country | Population | Study type | Main source of effectiveness data | Authors’ conclusion (ICER) |
|---|---|---|---|---|---|
| CRT-P vs. OPT | |||||
| Banz 2005187 | Germany | Patients with HF | Markov | Several publications and expert opinion | Cost-effective (€36,600 per QALY) |
| Bertoldi et al. 2011155 | Brazil | HF NYHA class II, III or IV; EF ≤ 35% | Markov | Meta-analyses | Cost-effective (INT$15,723 per QALY) |
| Blomstrom et al. 2008191 | Denmark, Finland, Sweden | HF NYHA class III or IV; LVEF < 35% | Survival | CARE-HF109 | Cost-effective (Denmark €4759 per QALY; Finland €3571 per QALY; Sweden €6493 per QALY) |
| Bond et al. 2009203 and Fox et al. 200764 | UK | HF NYHA class III or IV; LVEF < 35%; QRS > 120 milliseconds | Markov | Systematic review and other published sources | Cost-effective (£16,738 per QALY) |
| Callejo et al. 2010188 | Spain | HF NYHA class III or IV; LVEF < 35% | Markov | CARE-HF109 | Cost-effective (€28,612 per QALY) |
| Calvert et al. 2005189 | UK | HF NYHA class III or IV; LVEF < 35% | Trial-based | CARE-HF109 | Cost-effective (€19,319 per QALY) |
| Caro et al. 2006190 | UK | HF NYHA class III or IV; LVEF < 35% | DES | CARE-HF109 | Cost-effective (£15,247 per QALY) |
| Feldman et al. 2005192 | USA | HF NYHA class III or IV; LVEF ≤ 35%; QRS > 120 milliseconds | Survival | COMPANION116 | Cost-effective (US$19,600 per QALY) |
| Heerey et al. 2006193 | Ireland | HF NYHA class III or IV; QRS interval > 130 milliseconds | Markov | Retrospective cohort study | Cost-effective (dominant) |
| McAlister et al. 2004194 | Canada | HF NYHA class III and prolonged QRS duration | Markov | Systematic review (nine RCTs: MIRACLE,121 MIRACLE ICD,136 PATH-CHF,204 COMPANION,116 MUSTIC-SR,125 MUSTIC-AF,205 Garrigue et al.,206 CONTAK-CD,126 RD-CHF207) | Uncertain (US$90,700 per QALY) |
| Medical Services Advisory Committee 2006195 | Australia | HF NYHA class III or IV; LVEF < 35% | Decision tree | CARE-HF,109 MIRACLE121 | Cost-effective for patients with moderate to severe chronic HF (NYHA classes III and IV) (Aus$12,257 per QALY for a public hospital) |
| Neyt et al. 2011196 | Belgium | HF NYHA class III or IV; LVEF ≤ 35%; QRS interval > 120 milliseconds | Markov | COMPANION116 | Cost-effective (€11,200 per QALY) |
| Nichol et al. 2004197 | USA | HF NYHA class III and prolonged QRS duration | Markov | MUSTIC-SR,125 MUSTIC-AF,205 Path-CHF,204 CONTAK-CD,126 MIRACLE,121 MIRACLE ICD,136 COMPANION,116 Garrigue et al.,206 RD-CHF207 | Uncertain (US$107,800 per QALY) |
| Poggia et al. 2012200 | Argentina | HF NYHA class I or II; LVEF ≤ 40%; QRS interval ≥ 120 milliseconds | Markov | Meta-analysis of REVERSE,208 MADIT-CRT,209 RAFT140 | Cost-effective (INT$34,185 per QALY) |
| Yao et al. 2007198 | UK | HF NYHA class III or IV; LVEF < 35% | Markov | CARE-HF109 | Cost-effective (€7538 per QALY) |
| CRT-D vs. OPT | |||||
| Aidelsburger et al. 2008199 | Germany | HF NYHA class III or IV | Markov | COMPANION116 and Banz187 | May be cost-effective for NYHA classes III and IV depending on device longevity (€88,143 per QALY) |
| Feldman et al. 2005192 | USA | HF NYHA class III or IV; LVEF ≤ 35%; QRS > 120 milliseconds | Survival | COMPANION116 | Cost-effective (US$43,000 per QALY) |
| Medical Services Advisory Committee 2006172 | Australia | HF NYHA class III or IV; LVEF ≤ 35%; QRS > 120 milliseconds | Decision tree | COMPANION116 | Cost-effective for patients with CHF NYHA III or IV, sinus rhythm, LVEF ≤ 35% and a QRS duration ≥ 120 milliseconds despite OPT (Aus$22,944/LYG for a public hospital) |
| Yao et al. 2007198 | UK | HF NYHA class III or IV; LVEF < 35% | Markov | CARE-HF109 | Cost-effective at WTP of €44,100 per QALY |
| CRT-D vs. CRT-P | |||||
| Bertoldi et al. 2011155 | Brazil | HF NYHA class II, III or IV; EF ≤ 35% | Markov | Meta-analyses | Not cost-effective (INT$84,345 per QALY) |
| Bond et al. 2009203 and Fox et al. 200764 | UK | HF NYHA class III or IV; LVEF < 35%; QRS interval > 120 milliseconds | Markov | Systematic review and other published sources | Not cost-effective (£40,160 per QALY) |
| Callejo et al. 2010188 | Spain | HF NYHA class III or IV; LVEF < 35% | Markov | CARE-HF109 | Not cost-effective (€53,547 per QALY) |
| Neyt et al. 2011196 | Belgium | HF NYHA class III or IV; LVEF ≤ 35%; QRS interval > 120 milliseconds | Markov | COMPANION116 | Not cost-effective (€57,000 per QALY) |
| Yao et al. 2007198 | UK | HF NYHA class III or IV; LVEF < 35% | Markov | CARE-HF109 | Cost-effective (€18,017 per QALY) |
| CRT-D vs. ICD | |||||
| Bertoldi et al. 2011155 | Brazil | HF NYHA class II, III or IV; EF ≤ 35% | Markov | Meta-analyses | Marginally cost-effective (INT$36,940 per QALY) |
The judgements of the methodological quality assessment of the studies on CRT are summarised in Table 88. The studies vary in their quality and relevance to the UK NHS. As mentioned earlier, some studies are conducted in countries outside the UK and it is unclear how generalisable their results are to the UK NHS. The studies have been conducted in the last 10 years and generally are of fairly high quality. However, some studies have used a short time horizon and some have not included justification for the selection of effectiveness data sources or details of all costs and consequences. For one study the focus was patients with mild HF, which may limit relevance to the UK.
| Study | Decision problem relevant to the UK | Setting comparable to the UK | Appropriate methodology | Relevant costs and consequences | Data inputs justified | QALYs measured | Appropriate time horizon | Discounting | Incremental analysis | Sensitivity analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| CRT-P vs. OPT | ||||||||||
| Banz 2005187 | Y | N | Y | Y | Y | Y | N | N | Y | Y |
| Bertoldi et al. 2011155 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Blomstrom et al. 2008191 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Bond et al. 2009203 and Fox et al. 200764 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Callejo et al. 2010188 | Y | ? | Y | Y | Y | Y | Y | Y | Y | Y |
| Calvert et al. 2005189 | Y | Y | Y | ? | Y | Y | Y | Y | Y | Y |
| Caro et al. 2006190 | Y | Y | Y | ? | Y | Y | ? | Y | Y | Y |
| Feldman et al. 2005192 | Y | N | Y | ? | Y | Y | N | Y | Y | Y |
| Heerey et al. 2006193 | Y | N | Y | Y | ? | Y | N | Y | Y | Y |
| McAlister et al. 2004194 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Medical Services Advisory Committee 2006195 | Y | N | Y | ? | Y | Y | Y | Y | Y | Y |
| Neyt et al. 2011196 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Nichol et al. 2004197 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Poggia et al. 2012200 | ? | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Yao et al. 2007198 | Y | Y | Y | ? | ? | Y | Y | Y | Y | Y |
| CRT-D vs. OPT | ||||||||||
| Aidelsburger et al. 2008199 | Y | N | Y | Y | Y | Y | N | Y | Y | Y |
| Feldman et al. 2005192 | Y | N | Y | ? | Y | Y | N | Y | Y | Y |
| Medical Services Advisory Committee 2006172 | Y | N | Y | Y | Y | N | Y | N | Y | Y |
| Yao et al. 2007198 | Y | Y | Y | ? | ? | Y | Y | Y | Y | Y |
| CRT-D vs. CRT-P | ||||||||||
| Bertoldi et al. 2011155 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Bond et al. 2009203 and Fox et al. 200764 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Callejo et al. 2010188 | Y | ? | Y | Y | Y | Y | Y | Y | Y | Y |
| Neyt et al. 2011196 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Yao et al. 2007198 | Y | Y | Y | ? | ? | Y | Y | Y | Y | Y |
| CRT-D vs. ICD | ||||||||||
| Bertoldi et al. 2011155 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
Six studies64,155,188,194,196,197 were considered to be of high methodological quality by meeting all or all but one (‘Setting comparable to the UK’) of the recognised criteria. 38,66 Of these, one study,64 conducted for a UK setting, is considered to be of most relevance. We describe this study in more detail in the following section.
Fox and colleagues64
Fox and colleagues64 (also reported in Bond and colleagues203) developed a Markov model to compare CRT-P and CRT-D with OPT in patients with HF in the UK (see Appendix 12 for data extraction). The model followed a mixed-age cohort of people (start age from 30 to 90 years) with HF (NYHA class III and IV) because of LVSD (with LVEF ≤ 35%) and electrical dyssynchrony (QRS duration > 120 milliseconds) over their lifetime. A cycle length of 4 weeks was used and a lifetime time horizon.
The model had the following health states: surgery (original implant, upgrade, routine maintenance), postoperative complication, stable with device, stable with OPT, infection (CRT or ICD related), hospitalised (HF, HF and heart transplant) and death (sudden cardiac cause, HF, non-cardiac related).
The baseline population mortality in the OPT arm was taken from the CARE-HF trial109 as this was a large UK-based trial. The mortality benefit of CRT over time was calculated using the survival curve from the OPT group in the CARE-HF trial with the pooled HR, estimated in their systematic review of the clinical effectiveness of cardiac resynchronisation in HF. The model used QoL estimates related to NYHA class (class I 0.93 and class II 0.78 from Kirsch and McGuire;210 class III 0.61 and class IV 0.44 from Calvert and colleagues211) and utility for hospitalisation with HF (0.57 from McAllister and colleagues194). Patients were distributed across NYHA classes according to the data from the CARE-HF trial at baseline, 90 days and 18 months. The costs of the devices were obtained from a sample of 61 NHS ‘buying units’ (either individual health service trusts or purchasing consortia of trusts) during 2004 and 2005. Costing year and currency for the analysis were 2005 and UK pounds, except for drug costs, which were 2006 and UK pounds.
Compared with OPT, the model base-case analysis estimated that CRT-P conferred an additional 0.70 QALYs for an additional £11,630 per person, giving an estimated ICER of £16,735 per QALY gained for a mixed age cohort (range £14,630–20,333). 64,203 CRT-D compared with CRT-P conferred an additional 0.29 QALYs for an additional £11,689 per QALY, giving an ICER of £40,160 per QALY for a mixed-age cohort (range £26,645–59,391). Sensitivity analyses showed that, in comparison to CRT-P, CRT–D devices were most likely to be cost-effective when implanted in younger individuals and in those with a high risk of SCD.
Of the other five high-quality studies, the three studies155,188,196 with the patient group most comparable to that of Fox and colleagues64 also found that CRT-P was cost-effective when compared with OPT, whereas the other two studies were uncertain about cost-effectiveness. 194,197 Three of these high-quality studies155,188,196 also compared CRT-D with CRT-P and found it not to be cost-effective.
Summary of published economic evaluations
-
A systematic review of the cost-effectiveness of ICDs for the treatment of arrhythmias and CRT for the treatment of HF identified 51 studies (36 studies of ICDs63,149,153–186 and 17 of CRT64,155,172,187–200). Two studies55,172 analysed the cost-effectiveness of both ICD and CRT.
-
The evaluations were published between 1990 and 2012 and the majority were conducted in North America, but there were also several UK studies.
-
Most of the evaluations employed state transition models to estimate long-term outcomes extrapolated from short-term outcomes in the trials. Time horizons varied between 3 years and lifetime.
-
Many of the studies were based on a single trial, with the MADIT II and SCD-HeFT trials the most common ICD trials and the CARE-HF and COMPANION trials the most common CRT trials. There were also several evaluations that used results from systematic reviews and meta-analyses of different combinations of trials.
-
Almost half of the studies reported that ICDs were cost-effective, with the others either finding that ICDs were cost-effective only in high-risk groups or were not cost-effective or being uncertain about cost-effectiveness. Five studies153,155,160,178,182 were considered to be of high methodological quality; these studies report different conclusions about cost-effectiveness. Of these, only one study was conducted for a UK setting and perspective and is considered to be of most relevance. 153 This study reported a mean ICER of £76,139 per QALY gained for an average UK secondary prevention patient over a 20-year time horizon and therefore concluded that the benefit from ICDs may not be sufficient to make the technology cost-effective as used in the UK (in 2006). However, these results may not be applicable to current UK practice as some data used in the model came from patients implanted between 1990 and 2002, which is now out of date.
-
Almost all studies reported that CRT was cost-effective, with only two studies194,197 being uncertain whether CRT was cost-effective. Six studies64,155,188,194,196,197 were considered to be of high methodological quality, two of which were the studies reporting uncertainty about cost-effectiveness. 194,197 One of the high-quality studies64 was conducted for a UK setting and is considered to be of most relevance to the UK NHS. This study estimated an ICER of £16,735 per QALY gained for CRT-P compared with OPT and an ICER of £40,160 per QALY gained for CRT-D compared with CRT-P. The authors concluded that CRT-D is not cost-effective for left ventricular dysfunction and that CRT alone is the most cost-effective option in the population of patients evaluated (NYHA class III and IV with LVEF ≤ 35% and QRS duration > 120 milliseconds). CRT-D is more likely to be cost-effective in subgroups of younger patients or those with a high risk of SCD who would qualify for CRT.
-
Two of the included economic evaluations analysed both CRT and ICD, neither of which was conducted in the UK. 155,172 Both found that ICD was cost-effective compared with OPT, one172 found that CRT-D was cost-effective compared with OPT and the other155 found that CRT-D was marginally cost-effective compared with ICD.
Systematic review of health-related quality-of-life studies
A systematic review was undertaken to assess the HRQoL of people eligible for ICD or CRT devices. The aims of the review were to provide data to populate the lifetime economic model with utilities to calculate QALYs and to provide estimates of HRQoL by NYHA class for those with HF.
For adults, the NICE preferred measure of HRQoL is the EQ-5D212 and this was used in the previous ICD153 and CRT64 TARs. We were interested in HRQoL data of similar or better quality than that used in previous studies and therefore filtered the results of our searches to include studies using the EQ-5D (index not visual analogue scale). The search strategies used are described in Appendix 2. The inclusion and exclusion criteria for the review are shown in Chapter 3 (see Inclusion and exclusion criteria).
The search strategy identified 6696 references, which after filtering for the EQ-5D resulted in 218 potentially relevant papers. Titles and abstracts were screened and the full texts of 22 papers were retrieved for further inspection. After examining the retrieved papers, six studies met the inclusion criteria. 27,153,211,213–215 A summary of the selection process and the reasons for exclusion are presented in Figure 32. Most studies were excluded because they did not use the EQ-5D or did not report it in the required format. A list of the excluded studies is provided in Appendix 13.
FIGURE 32.
Flow chart of identification of studies for inclusion in the review of HRQoL.
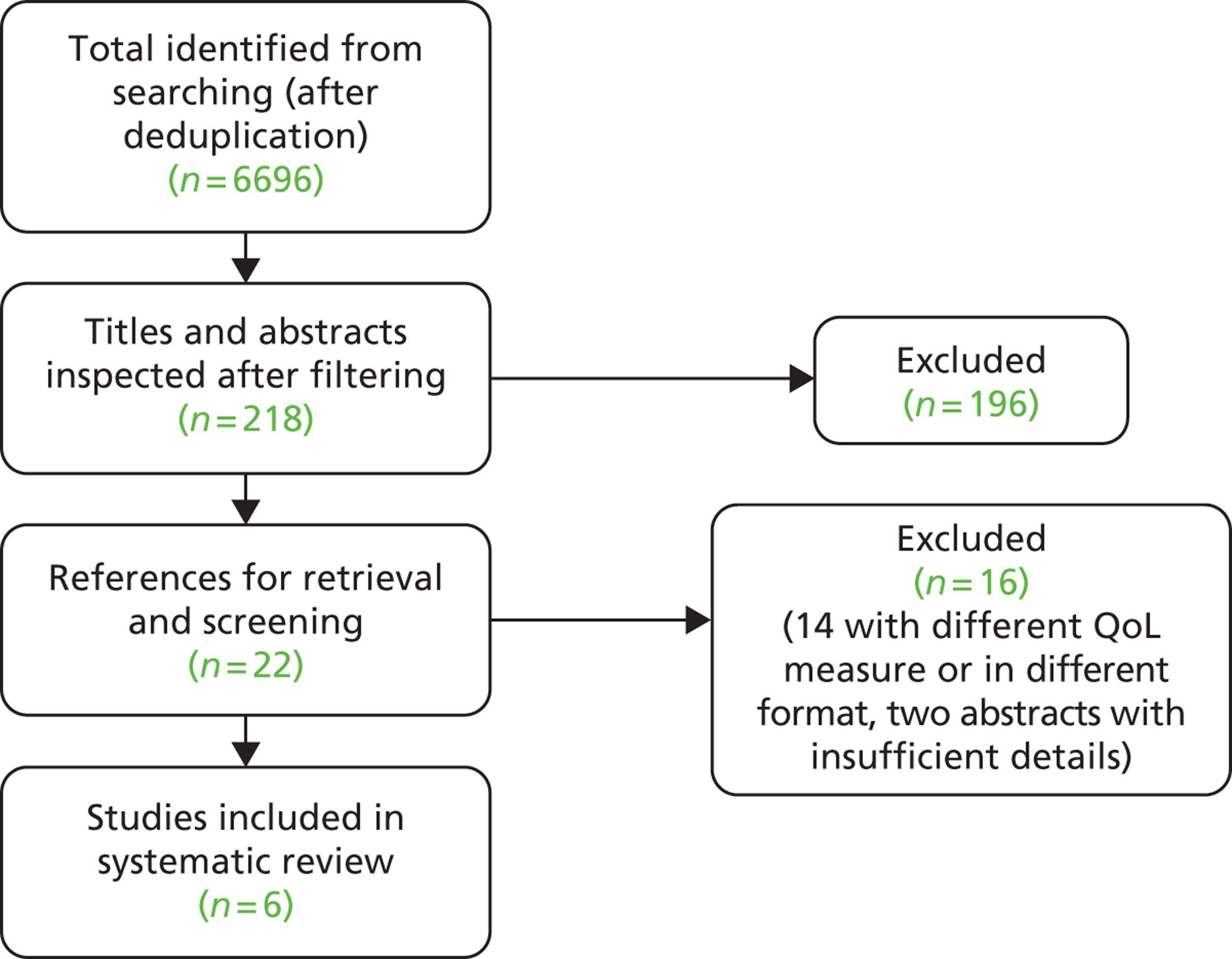
Health-related quality of life was assessed using the EQ-5D in four studies of patients with HF27,211,213,214 and two studies153,215 of patients who had received an ICD (Table 89). Three studies were cohort studies153,213,215 and three studies were observational analyses based on RCTs. 27,211,214
| Study | Country | Study type | Study population | Patient characteristics | QoL instrument and methodology | Results |
|---|---|---|---|---|---|---|
| Buxton et al. 2006153 | UK | Retrospective cohort study | 229 patients who had received an ICD | Mean age 60 years, 81% male; NYHA class ≥ III 26% | EQ-5D using UK population preferences | Mean EQ-5D was reported by time since ICD implantation (up to ≥ 6 years) and ranged from 0.69 to 0.78 |
| Calvert et al. 2005211 | UK | CARE-HF RCT109 | 813 patients with chronic HF because of LVSD and dyssynchrony | Mean age 65 years; 74% male; NYHA class III 94%, class IV 6% | EQ-5D using UK population preferences | Mean EQ-5D: 0.60 (95% CI 0.58 to 0.62). NYHA class III 0.61, class IV 0.44 |
| Eurich et al. 2006213 | USA/Canada | Cohort study | 298 patients with HF with LVSD | Mean age 60 years; male 75%; NYHA class I 11%, class II 43%, class III 41%, class IV 4% | EQ-5D with UK scoring at baseline and after 6 weeks | Mean EQ-5D: 0.66 (SD 0.26). Mean EQ-5D at 6 weeks: 0.71 (SD 0.22) for those with no change in NYHA |
| Gohler et al. 2009214 | USA | EPHESUS RCT214 | 1395 patients with chronic HF after acute MI | Mean age 64 years; male 71%; patient origin: US 31%, Europe 52%, Latin America 14% | EQ-5D weighted by the appropriate preference weight based on the subject’s origin | Mean EQ-5D by NYHA class: I 0.855 (95% CI 0.845 to 0.864), II 0.771 (95% CI 0.761 to 0.781), III 0.673 (95% CI 0.727 to 0.765), IV 0.532 (0.480 to 0.584) |
| Groeneveld et al. 2007215 | USA | Cohort study | Patients who had previously received ICD therapy for primary (n = 45) and secondary (n = 75) prevention | Mean age 60 years; male 73%; years since ICD implantation 2 | EQ-5D (country of population preferences not reported) | Median EQ-5D score: primary prevention: 0.84 (IQR 0.77, 1), secondary prevention: 0.84 (IQR 0.78–1) |
| Holland et al. 201027 | UK | Cohort analysis within HeartMed RCT216 | 293 patients with HF following emergency hospital admission | Mean age 77 years; male 64%; SA NYHA class I/II 33%, class III 34%, class IV 33% | EQ-5D using UK population preferences at baseline and 6 months’ follow-up | Mean baseline EQ-5D for SA NYHA class: I/II 0.72 (SD 0.25), III 0.53 (SD 0.32), IV 0.47 (SD 0.35). Mean 6-month EQ-5D for SA NYHA: I/II 0.6 (SD 0.25), III 0.38 (SD 0.32), IV 0.34 (SD 0.35) |
Buxton and colleagues153 conducted a retrospective postal survey of patients in the UK who had received an ICD between 1991 and 2002 as part of a wider review of ICD therapy. Based on the responses from 229 patients, they analysed the effect of time since implantation and age on HRQoL. Their analyses showed that there was no evidence that the time since implantation affects HRQoL substantially over time, with values similar at 1 year (0.78) and at > 6 years (0.77). However, there are limitations with the type of study used (cross-sectional survey) and results should be viewed with caution.
Groeneveld and colleagues215 measured and compared HRQoL among primary and secondary prevention ICD recipients in the USA. They recruited 120 patients undergoing clinical evaluation at cardiac electrophysiology clinics who had previously received an ICD. The average duration since ICD implantation was 2 years. The authors found no difference between the EQ-5D values of primary and secondary patients, with health state utility values of 0.84 for both groups. They concluded that the QoL in patients with ICDs was similar to that of similarly aged adults in the general population. This study also had limitations in terms of methodology because of the convenience sampling technique used.
Calvert and colleagues211 investigated the HRQoL of 813 patients with chronic HF because of LVSD and dyssynchrony (NYHA class III or IV) in the CARE-HF RCT109 in the UK. CARE-HF was a trial to investigate the effects of CRT-P on the mortality and morbidity of patients already receiving OPT. Baseline EQ-5D data were collected for 740 patients primarily (94%) in NYHA class III. The authors found that the mean baseline health state utility value was 0.6 and that HF had an important impact on all aspects of QoL, which was independent of age. A limitation of the study was that patients were not a random sample of patients with HF but were patients enrolled in a study who were already receiving OPT.
Eurich and colleagues213 compared several HRQoL measures in 298 people with HF. Patients were recruited across 14 medical centre outpatient departments in the USA and Canada. HRQoL was assessed at baseline and at 6 weeks. EQ-5D health state valuations were completed for both UK and US population valuations. Mean EQ-5D (UK valuation) was 0.66 at baseline and 0.71 at 6 weeks for those with no change in NYHA status (70% of patients). This was a cohort study that evaluated the random changes observed in HF patients in the outpatient setting with no specific intervention during the follow-up period.
Gohler and colleagues214 estimated utilities for NYHA classification and number of cardiovascular rehospitalisations for patients with chronic HF after acute MI in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) RCT. The EPHESUS trial was a multicentre RCT that investigated the effect of the aldosterone antagonist eplerenone. HRQoL was investigated in a subset of 1395 patients at months 0, 3, 6, 12 and 18 using the EQ-5D. The health state utility values were weighted by the appropriate preference weight based on a subject’s specific region of origin (USA 31%, Western Europe 52%, Latin America 14%). The study used univariate and multivariate linear regression analyses with independent variables for NYHA classification, number of cardiovascular hospitalisations between study intake and the follow-up time point, age, sex and cardiovascular morbidities. In univariate analyses, utilities associated with NYHA class were 0.85 for class I, 0.77 for class II, 0.67 for class III and 0.53 for class IV.
Holland and colleagues27 conducted a cohort analysis within the HeartMed RCT. A total of 293 adults with HF were included from three large district general hospitals in the UK after an emergency admission and were followed up for 6 months. The analysis aimed to test whether patients’ self-assigned NYHA class at baseline predicted outcomes. Patients classified themselves into one of four self-assigned NYHA classes using a questionnaire that described their functional status. Mean baseline EQ-5D scores were 0.72, 0.53 and 0.47 for self-assigned NYHA classes I/II, III and IV, respectively, and mean 6-month EQ-5D scores were 0.6, 0.38 and 0.34 respectively. The authors concluded that HF patients’ own assessment of their NYHA class is a predictor of outcomes in HF, in the same way as clinician-assigned NYHA classes are a predictor of outcomes; however, the study was limited by there being no clinician assessment to compare with patients’ own assessment.
Both studies in patients who had received an ICD had methodological limitations, with a key one being the selection of participants, who were a small number of volunteers attending a single defibrillator clinic in the USA215 and survey respondents at two centres in the UK. 153 This may have biased the results by not including patients who were representative of elsewhere with different experiences. However, in the absence of more rigorous information these studies supply some information of relevance. One of the studies suggests that there is no difference between the EQ-5D scores of primary and secondary prevention patients and that QoL for ICD patients was similar to that of the general population of similar age,215 and the other shows no evidence that QoL changes over time since implant. 153
Four cohort studies reported utility estimates for HF patients, with two conducted in the UK27,153 and two in the USA. 213,214 Patient characteristics were generally similar across studies in terms of sex and age, except for one study27 in which the mean age was greater (77 years compared with 60–65 years). The severity of HF as measured by NYHA class differed between the studies, with the percentage of NYHA class III participants ranging from 34%27 to 94%. 211 Mean baseline EQ-5D scores were similar in the two studies that reported this (0.60211 and 0.66213). Three studies reported mean baseline EQ-5D score by NYHA class. Mean baseline EQ-5D scores for NYHA class III were 0.61,211 0.63214 and 0.53 in the study in which patients self-assigned NYHA class. 27 For NYHA class IV, mean baseline EQ-5D scores were 0.44,211 0.53214 and 0.47. 27 Overall, the results suggest that HF has a significant effect on HRQoL. One study reports random changes in utility after 6 weeks in patients with no change in NYHA class213 and another, which used a self-assigned NYHA classification, showed decreased EQ-5D scores in each NYHA class after 6 months. 27
Summary of the health-related quality-of-life review
-
The systematic review found six relevant HRQoL studies that measured EQ-5D in HF,27,153,211,213–215 stratified by NYHA class, or that reported on patients who had previously received an ICD.
-
Two studies153,215 were conducted in patients who had received an ICD, one in the UK153 of patients at two hospitals who were implanted between 1991 and 2002 and who responded to a postal questionnaire and one215 of volunteers attending a defibrillator clinic in the USA.
-
The UK ICD study reported that the mean EQ-5D score did not change with time after implantation (mean EQ-5D score ranged from 0.69 to 0.78 for the years up to ≥ 6 years since implantation). The US study reported no difference between the EQ-5D scores of primary and secondary prevention patients (median EQ-5D score 0.84) and that QoL for ICD patients was similar to that of the general population.
-
Four cohort studies reported EQ-5D scores in HF, two127,153 in the UK (one of which was based on the CARE-HF trial) and two213,214 in the USA (one based on the EPHESUS trial).
-
Two studies reported similar mean baseline EQ-5D scores of 0.60 (UK RCT-based study211) and 0.66 (US cohort study213).
-
Three studies27,211,214 reported mean baseline EQ-5D score by NYHA class. Mean baseline EQ-5D scores for NYHA class III were 0.61211 and 0.53214 (UK studies) and 0.63 (US study). 214 The lowest value was reported in the study27 in which patients self-assigned NYHA class. Mean baseline EQ-5D scores for NYHA class IV were 0.44211 and 0.4727 (UK studies) and 0.53214 (US study).
-
One US study213 reported random changes in utility after 6 weeks in patients with no change in NYHA class and one UK study27 (which used a self-assigned NYHA classification) showed decreased EQ-5D scores in each NYHA class after 6 months.
-
Overall, the results show decreased EQ-5D scores in HF compared with those of the general population, particularly in NYHA classes III and IV.
Review of the manufacturers’ submission
As described in Chapter 4 (see Summary of Southampton Health Technology Assessments Centre’s peer review of clinical effectiveness in the Association of British Healthcare Industries joint submission), one MS consisting of a written report and an electronic model supporting the reported cost-effectiveness analyses was submitted to NICE. 151 Further details on the submission and a discussion of the clinical data reviewed and presented can be found in Chapter 4 and Appendix 10.
The review of the economic assessment within the MS consists of a brief overview of the cost-effectiveness analysis, including the approach taken to modelling disease progression and the effects of treatment, followed by a critical appraisal of the cost-effectiveness analysis.
Review of the cost-effectiveness analysis in the manufacturers’ submission
A structured data extraction form was used to guide the review of the MS (see Appendix 10),151 jointly submitted by the ABHI on behalf of Biotronik, Boston Scientific, Medtronic, Sorin and St Jude Medical. The submission includes a review of published clinical effectiveness studies of OPT, ICD, CRT-P and CRT-D for the treatment of cardiac arrhythmias and HF, a NMA of IPD and a report of an economic evaluation undertaken for the NICE multiple technology appraisal process.
The cost-effectiveness analysis uses a survival-based model to estimate the relative cost-effectiveness of OPT, ICD, CRT-P and CRT-D (compared with each other) in 48 subgroups of patients. IPD from 12,638 patients from 13 RCTs were used to inform the economic model. All individuals are adults with HF, with a LVEF ≤ 35% and/or at risk of SCD. This heterogeneous group of patients was split into 48 subgroups according to their NYHA class, QRS duration, LBBB status and aetiology of heart disease, and cost-effectiveness results are reported for each subgroup.
The perspective adopted for the economic evaluation is that of the UK NHS and PSS. General UK population utilities were used at baseline to which disease-specific decrements were applied. The impact of each intervention on HRQoL was incorporated as an intervention-specific increment. These estimates were derived from published sources and IPD from the trials included in the systematic review of clinical effectiveness studies in the MS.
For each subgroup, cost-effectiveness results were presented per intervention as incremental cost per QALY relative to the intervention immediately less effective.
The interventions compared in the MS consist of those included in the NICE scope. 61 However, not all of them were included as comparators for all patient subgroups in the MS, as no patients were identified for the following combinations:
-
ICD excluded for NYHA class IV
-
CRT-P excluded for NYHA class I/II and QRS duration < 120 milliseconds
-
CRT-D excluded for QRS duration < 120 milliseconds.
Clinical advice indicated that these exclusions are reasonable.
Modelling approach
A cohort survival model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) with two states for alive and dead. Death is modelled using a series of covariate-based regression equations for baseline risk and treatment effect using long-term IPD. Based on the numbers of patients alive, the model also estimates the numbers of patients hospitalised in each cycle. The model had monthly cycles and a lifetime time horizon. Costs and health benefits in the model were discounted at 3.5%.
The baseline probability of death is for patients who receive OPT but no device, based on a range of clinical covariates. These probabilities are used in combination with device-specific treatment effects, derived from the NMAs. For the model baseline survival curve, a Weibull distribution was used with the parameters of the risk model shown in Appendix 10. A similar approach is taken to estimate the probability of all-cause hospitalisation. HRQoL utilities are applied to patients in the model according to their treatment and clinical characteristics.
The model does not include short-term device-related adverse events as the costing approach used to derive total implant costs covers additional costs such as those of short-term adverse events.
Results were generated in a two-stage process. In the first, cost and QALY estimates were derived for all relevant comparators for all 4992 patient profiles [four NYHA classes, two aetiology status groups (ischaemic/non-ischaemic), three QRS categories, four LVEF categories, LBBB status (yes/no), two gender groups, 13 age categories]. In the second stage, results were aggregated over LVEF and age and gender categories, reducing the subgroups to 48, defined by NYHA class, QRS duration, LBBB status and aetiology.
Assumptions
The following additional assumptions are made in the model:
-
The effects of treatment on HRQoL diminish over time. The model assumes that the benefit observed at 6 months is maintained for up to 5 years and thereafter begins to recede in a linear manner over the time period from 5 to 10 years. After 10 years an individual with a device will have no additional HRQoL benefit over an identical person receiving OPT.
-
HRQoL increments were assumed to be associated with device implantation.
-
Reduction in all-cause hospitalisation varied according to the device implanted and NYHA class of the patient.
Estimation of effectiveness
The clinical effectiveness estimates were based on a NMA of IPD from 13 clinical trials (12,638 patients, followed up for up to 7.5 years). The clinical trials were CARE-HF,109 COMPANION,116 CONTAK-CD,126 DEFINITE,90 MADIT,99 MADIT II,103 MADIT-CRT,130 MIRACLE,121 MIRACLE ICD,136 RAFT,140 RethinQ,142 REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE)208 and SCD-HeFT. 105 These trials were identified through a systematic review of the clinical effectiveness of all of the interventions. A further nine trials69,82,125,138,139,144,241,244 were also identified in the review but IPD were not available for these trials (see Chapter 4 and Appendix 10 for further discussion on the clinical effectiveness data included in the MS).
The NMA enabled the combination of trials that compared different sets of treatments within a single analysis, and also allowed the available direct and indirect evidence to be used to inform comparisons between possible treatments. The analysis assessed the outcomes of all-cause mortality, all-cause hospitalisation and HRQoL, using the results to inform the economic model developed as part of the MS. A critique of the IPD NMA is presented later in this chapter.
The IPD NMA showed that ICDs, CRT-D and CRT-P were significantly more effective than OPT for people with HF when assessed for all-cause mortality, with CRT-D also providing statistically significant benefit compared with ICDs and CRT-P. The results of the analysis of those subgroups that benefited from the different interventions compared with OPT were less clear. CRT-D had a statistically significant benefit for all people with a QRS ≥ duration of ≥ 150 milliseconds and all women with a QRS ≥ duration from ≥ 120 milliseconds to < 150 milliseconds and a marginally insignificant effect for all men with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds. ICDs had a significant benefit for men aged < 60 years and for men aged ≥ 60 years with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds and without LBBB. CRT-P had a significant benefit for women with a QRS duration ≥ 150 milliseconds and LBBB. The NMA found that CRT-D had the strongest effect on all-cause mortality (commercial-in-confidence information has been removed). Treatment effects for the individual devices were also statistically significant (commercial-in-confidence information has been removed).
All devices reduced the rate of all-cause hospitalisations compared with OPT, with rates decreasing for NYHA classes I–III with ICDs (commercial-in-confidence information has been removed), for NYHA classes III (commercial-in-confidence information has been removed) and IV (commercial-in-confidence information has been removed) with CRT-P and for all NYHA classes with CRT-D (commercial-in-confidence information has been removed). HRQoL was assessed using the EQ-5D, showing counterintuitive results for the effects of treatment. Adjustments were made assuming that CRT-P and CRT-D would have the same effects and that ICDs would have an effect only on NYHA classes I and II. Benefits were thought to last for (commercial-in-confidence information has been removed) years.
UK device longevity estimates were derived from NHS data from the Central Cardiac Audit Database (CCAD)217 on all implants with verified life status from 2000 to 2011 (∼ 40,000 implants). The MS considers that these device longevity estimates represent the best currently available estimates as CCAD contains data on a large number of implants and it is run by the NHS Information Centre. Device-specific median survival estimates were obtained by fitting Weibull curves to the data. The Weibull curve was chosen as it is commonly used to model such data and it was considered a good fit (in terms of both within-data accuracy and long-term predictive plausibility). Median time to device failure in the model was 7.1 years for ICDs, 10.4 years for CRT-P and 5.8 years for CRT-D. The methodology used by the manufacturers to estimate device longevity is commonly used; however, clinical advice indicated that these figures seem to be overestimated.
Critical appraisal of the cost-effectiveness analysis in the manufacturers’ submission
The MS was appraised for methodological quality and generalisability to the UK NHS using a checklist adapted from the NICE reference case requirements67 and the Philips and colleagues’ checklist. 68 Overall, the submission meets all of the requirements for methodological quality and generalisability except that it did not provide evidence that the economic model had been validated, and the model assumptions were not listed and justified. Table 90 provides a summary of the critical appraisal of the MS.
| No. | Item | MS | Comments |
|---|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Yes | |
| 2 | Is the comparator routinely used in the UK NHS? | Yes | |
| 3 | Is the patient group in the study similar to those of interest in the UK NHS? | Yes | |
| 4 | Is the health-care system comparable to that in the UK? | Yes | |
| 5 | Is the setting comparable to that in the UK? | Yes | |
| 6 | Is the perspective of the model clearly stated? | Yes | |
| 7 | Is the study type appropriate? | Yes | |
| 8 | Is the modelling methodology appropriate? | Yes | |
| 9 | Is the model structure described and does it reflect the disease process? | Yes | |
| 10 | Are assumptions about the model structure listed and justified? | No | |
| 11 | Are the data inputs for the model described and justified? | Yes | |
| 12 | Is the effectiveness of the intervention established based on a systematic review? | Yes | |
| 13 | Are health benefits measured in QALYs? | Yes | |
| 14 | Are health benefits measured using a standardised and validated generic instrument? | Yes | |
| 15 | Are the resource costs described and justified? | Yes | |
| 16 | Have the costs and outcomes been discounted? | Yes | |
| 17 | Has uncertainty been assessed? | Yes | Limited to few parameters |
| 18 | Has the model been validated? | ? | Limited reporting of validation |
The model structure is consistent with the currently accepted theory of HF and ventricular arrhythmia. The MS does not describe the sources of evidence used to develop and inform the model structure but provides a brief justification for the choice of evidence (related to the large amount of IPD being available). The MS also does not include a review of economic evaluations of the scoped interventions and comparators. Other structures could have been adopted, but the fundamental features of the condition and the impact of the interventions seem to be captured. Adverse effects of treatment, such as perioperative complications, were not explicitly incorporated in the model. The model was populated with data from the systematic review of clinical effectiveness studies in the MS. A monthly cycle length and a lifetime horizon were appropriately used, and Weibull models were used to extrapolate all-cause mortality beyond trial duration. There is no reference to the internal validation of the model in the MS. Overall, the model results make intuitive sense and the conclusions seem valid. In addition, a comparison has been made between the results of the MS and results generated in previous appraisals and reasons have been given for any differences.
Estimation of quality-adjusted life-years
The approach taken for HRQoL was (1) to estimate UK-specific age and gender population utilities, (2) derive disease-specific decrements using IPD EQ-5D data and (3) derive treatment-specific increments associated with each device at first follow-up visit by NYHA class.
UK-specific age and gender population utilities were taken from a study by Kind and colleagues152 of 3395 individuals resident in the UK. Disease-specific decrements were taken from the CARE-HF,109 MADIT-CRT209 and RAFT140 trials. For the impact of treatment, the utility increments were calculated as the difference between baseline and first follow-up period. The health state utility values used in the model are presented in the data extraction form in Appendix 10.
The health state utility values used are derived from the patient-level EQ-5D data. The MS reports that some of the results were highly counterintuitive given the nature of the underlying disease and the interventions, for example the results for CRT-D for NYHA class III/IV showed a utility decrement, in contrast to those for CRT-P. The MS has dealt with these inconsistencies in the patient-level data by using several assumptions: CRT-D is assumed to have the same utility increment as CRT-P for NYHA class III/IV, ICDs are assumed to have (commercial-in-confidence information has been removed) for NYHA class III. ICDs are associated with a utility increment of (commercial-in-confidence information has been removed) for NYHA class I/II. CRT-D has a utility increment of (commercial-in-confidence information has been removed) for NYHA class I/II and (commercial-in-confidence information has been removed) for NYHA class III/IV. These values for ICDs and CRT-P were derived from the IPD analysis after subtracting the OPT NYHA class III value (commercial-in-confidence information has been removed). The values used for CRT-P were of a similar magnitude to those reported in the CARE-HF study, which gave a utility increment of 0.1 at 18 months after implantation compared with OPT patients.
In the model, the HRQoL benefit observed at 6 months is maintained up to 5 years and thereafter begins to recede in a linear manner over a time period of 5–10 years. After 10 years the model assumed that the individual with a CRT or an ICD device will have no additional HRQoL benefit over an identical person receiving OPT.
The MS does not report a systematic review of HRQoL studies. A review of utility values used in previous economic evaluations is reported but no details of how these were obtained are provided. The MS approach differs from that of most previous models (including those of Buxton and colleagues153 and Fox and colleagues64), in which no benefit from the interventions was assumed. However, the device-specific increments used in the MS are similar to those used in some of the previous models. 177,192,196 The impact on HRQoL of treatment-related adverse events (such as infection and perioperative complications), considered in previous models, was not included in the MS.
Estimation of costs
The resource use accounted for in the MS included device-related resources, medication and resources related to disease progression. IPD from the trials were used to estimate the mean number of all-cause hospitalisation events per month and the mean number of days hospitalised per month. Hospital costs were derived from the NHS reference costs218 and combined with the average mean length of stay. The HF hospitalisation event cost was £2295 and the non-HF hospitalisation event cost was £2448.
Device costs were sourced from the average selling prices obtained from the manufacturers via the ABHI. These prices are an aggregate across all sponsors (manufacturers) for ICD, CRT-P and CRT-D devices and leads sold in the UK to the NHS. The implantation costs were taken from the Healthcare Resource Group (HRG) tariff values. 218 Device-related infection costs were derived by inflating values in the previous TAR on CRT64 to £3139. Device costs, with implantation costs, were £15,248, £8281 and £17,849 for ICDs, CRT-P and CRT-D respectively. Further device costs are shown in Appendix 10.
The manufacturers assumed that an OPT regimen is taken by all patients for HF treatment, regardless of whether or not they receive a device in addition, and the drug cost allocated in any given month to each patient alive is based on his or her baseline NYHA class. The proportions of patients using a range of HF medications, by NYHA class, were derived from a combination of the clinical studies identified in the systematic review and expert opinion. The recommended daily dose for each commonly used drug was sourced from the British National Formulary (BNF). 219 The total cost of treatment per 1-month cycle was £14.28 for NYHA class I and between £22.13 and £22.30 for NYHA classes II–IV.
Overall, the derivation of costs and assumptions presented in the MS seems appropriate and consistent with previous approaches. However, specific searches for resource use or cost studies in the UK are not reported in the MS, and the impact of changes to the values and assumptions used was not analysed in the MS. The estimates in the model seem to cover the relevant resource use, including complications, non-HF hospitalisations and outpatient visits.
Cost-effectiveness results
The base-case deterministic results are presented for 48 subgroups defined by NYHA class, QRS duration, LBBB status and aetiology, but not for the population as a whole or according to the population groups scoped by NICE, and it is unclear how these results could be aggregated.
The base-case results can be found in the data extraction form (see Appendix 10) and are summarised in Table 91. The MS provides limited reporting of the results and sensitivity analyses. Generally, only the ICERs are presented for each of the base-case results, rather than a more detailed breakdown of costs and QALYs, and incremental costs and QALYs between competing interventions. For the base case, fully aggregated results with reporting of total costs and QALYs are presented only for subgroups of NYHA III class patients comparing CRT-D with OPT. Overall, the results show that for most subgroups there is at least one device with an ICER of < £30,000 per QALY and that, in some cases, a different device might be cost-effective if a £20,000 per QALY threshold is considered.
| HF severity | QRS duration (milliseconds) | Results summary |
|---|---|---|
| NYHA class I/II | < 120 | The ICERs for ICD vs. OPT are < £25,200 per QALY gained |
| 120–149 | ICD is a cost-effective treatment optiona (ICER < £17,000 per QALY) for patients with no LBBB. For CRT-D, all ICERs are < £25,000 per QALY gained in LBBB patients (£20,608–24,343) | |
| ≥ 150 | CRT-D is cost-effective treatmenta with an ICER of < £28,000 per QALY gained for all options | |
| NYHA class III | < 120 | ICD vs. OPT generates ICERs of < £30,000 per QALY |
| 120–149 | CRT-P is cost-effective.a CRT-D generates ICERs between £23,900 and £27,400 per QALY gained relative to CRT-P | |
| ≥ 150 | CRT-P is cost-effective vs. OPT (ICER < £20,000 per QALY). Compared with CRT-P, CRT-D generates ICERs of < £30,000 per QALY gained. ICD is either dominated or extendedly dominated | |
| NYHA class IV | < 120 | No comparative analysis was possible in this patient group as no patients were identified for this combination |
| ≥ 120 | For CRT-P compared with OPT, all ICERs are close to or < £20,000 per QALY gained. For the comparison between CRT-D and CRT-P, all ICERs are > £30,000 per QALY gained |
The manufacturers conclude that, in many cases in which there are small differences in cost-effectiveness between devices and high uncertainty as to which is the preferred device, NICE recommendations should allow for clinical flexibility.
The MS explores model uncertainty through deterministic and probabilistic sensitivity analyses, with most deterministic sensitivity analyses reported in the MS consisting of scenario analyses. Not all forms of uncertainty were explored, only uncertainty associated with a few methodological assumptions. The MS does not report the ranges used for the sensitivity analyses, only the different scenarios tested, and does not identify the model parameters with the greatest influence on the results. The MS does not report the assessment of uncertainty associated with resource use and cost parameters, and structural assumptions have not been tested. For instance, a scenario of reduced device longevity was not analysed nor one assuming no HRQoL benefit from the interventions.
The following scenarios were tested in sensitivity analyses: removal of treatment effect tapering (mortality and HRQoL), use of alternative NYHA-based IPD results and increase in device longevity. The base case assumed that treatment effects on mortality or HRQoL are not constant but diminish over time. When constant treatment effects for mortality and HRQoL were explored, ICERs in all patient groups were lower than in the base case.
According to the MS, there may be a lower mortality treatment effect in patients in NYHA class IV than in patients in NYHA classes I/II/III for CRT-D. The economic model was run using the estimated all-cause mortality treatment effects based on the grouping of NYHA class IV compared with NYHA class I–III patients. This analysis results in CRT-D becoming dominated in all NYHA class IV groups. The ICERs for all other groups are lower than in the base case. Device longevity was investigated by increasing time to device failure by 10%. There were only minimal changes to the cost-effectiveness results.
Probabilistic sensitivity analyses were conducted for a few subgroups, selected to reflect the baseline characteristics of participants in the MADIT-CRT trial, but no overall population analysis was performed. Because of the complexity of patient-level heterogeneity, the MS reported that a full probabilistic sensitivity analysis would take several months to execute. Results were presented graphically for four subgroups, men and women with and without LBBB, for patients of 65 years, NYHA class II, ischaemic, QRS duration > 150 milliseconds and LVEF between 20% and 25% patients. For these subgroups, CRT-D and OPT showed a similar probability of being cost-effective at a willingness-to-pay (WTP) threshold of £20,000 per QALY. The manufacturers concluded that the results suggested that the deterministic and probabilistic sensitivity analyses were broadly aligned.
The MS does not provide any details of the variables included in the probabilistic sensitivity analysis, such as mean values, distributions and variability of those variables. Credible intervals for the mean ICERs of the most cost-effective intervention were not reported either. It is therefore not clear whether the methods of assessment of parameter uncertainty are appropriate and whether the estimates of variation in the probabilistic sensitivity analysis are appropriate to reflect uncertainty in parameter estimates.
The MS compares the cost-effectiveness estimates with those produced in the previous appraisals of CRT in patients with NYHA class III/IV HF developed by Fox and colleagues64 and the review of ICDs in primary prevention. 153 The estimates from the MS model are markedly lower than those that were generated in the models developed for TA9542 and TA120. 43 The following reasons are given for the differences: real-time reduction in production costs, increases in device longevity compared with values used in previous models, better estimates of the impact of treatment on mortality and better understanding of the impact of treatment on HRQoL.
Summary of the Association of British Healthcare Industries submission
-
The ABHI submission was jointed submitted by the ABHI on behalf of five manufacturers.
-
The submission includes a NMA of IPD from over 12,000 patients and 13 RCTs.
-
The ABHI economic model is a survival model, based on IPD data according to patient clinical characteristics.
-
The model compared ICDs, CRT-P and CRT-D with OPT.
-
The model met all but two of the criteria for methodological quality.
-
The cost-effectiveness results are presented in the submission for subgroups according to NYHA class, QRS duration, LBBB status and aetiology.
-
The cost-effectiveness results do not directly address questions posed in the scope from NICE as it is unclear how the subgroups selected relate to the groups scoped by NICE.
-
Overall, ABHI’s results show that for most subgroups there is at least one device with an ICER of < £30,000 per QALY gained and in some cases a different device might have an ICER of < £20,000 per QALY gained.
Critique of the Association of British Healthcare Industries submission
The ABHI economic model is a cohort survival model with survival based on a series of covariate-based regression equations. The model includes the costs and HRQoL of associated events related to hospitalisation and device implantation. The general approach taken by the manufacturer seems reasonable and the model structure is consistent with the current understanding of HF and ventricular arrhythmia. Generally, the model meets most criteria for methodological quality, although there is limited reporting in the MS on the sources of evidence used to develop and inform the model structure, the assumptions used in the model have not been fully reported and explained and there is no evidence given in the MS for internal validation of the model.
The manufacturers’ joint submission presented an IPD NMA to assess the effectiveness of the different interventions for people with HF. It used meta-regression, allowing the effects of various patient characteristics on treatment outcomes to be assessed and any subgroups who may benefit differently to be identified. The analysis assessed the outcomes of all-cause mortality, all-cause hospitalisation and HRQoL, using the results to inform the economic model developed as part of the MS. As an appraisal of the IPD NMA is presented in Chapter 4 (see Individual patient data network meta-analysis: a critical appraisal), this section provides a brief summary of the limitations and findings that are relevant to the economic model produced as part of the MS.
The data sources used to populate the model for effectiveness are based on IPD data from over 12,000 patients and 13 RCTs and are of high quality; as stated by the MS151 this ‘represent[s] the first analysis of its kind and magnitude’ (p. 2). Although the NMA appeared to follow established methods and had access to unpublished IPD, aspects of the reporting of the analysis and apparent limitations in the data meant that there was uncertainty in the findings presented. Despite the IPD including 13 of the 22 trials (95% of patients) in the evidence network, data appeared limited given the covariables included (i.e. number of variables and subcategories) and the lack of data for specific outcomes assessed. As a consequence, the MS suggests that the analyses for all-cause mortality which include treatment effect modifiers (i.e. subgroups) should be interpreted cautiously, and it makes adjustments to counterintuitive results in the analyses of all-cause hospitalisations and HRQoL. The methods used in the NMA are discussed; however, the exploratory and confirmatory analyses used to determine the approach taken are not fully reported. Inevitably, these may affect the results and, although some comparisons are made with other evidence, a degree of uncertainty remains. Importantly, the IPD NMA has a different focus from that identified in the scope for the NICE appraisal. 61 Rather than assessing the effectiveness of the technologies in specific groups of patients, it tries to identify which patients the different technologies benefit. As these groups may not be the same, it is difficult to use the findings to address the original decision problem.
The assumptions made over costing and resource use are similar to the approach used by Fox and colleagues64 and are consistent with current clinical practice. However, specific searches for resource use or cost studies in the UK are not reported in the MS, and the impact of changes to the values and assumptions used was not analysed in the MS. The estimates in the model seem to cover the relevant resource use, including complications, non-HF hospitalisations and outpatient visits. In addition, the sources used appear reasonable. The UK device longevity estimates are based on all available implant data from CCAD and, as stated by the manufacturer, represent the best device longevity estimates currently available.
The MS does not report a systematic review of HRQoL studies. A review of utility values used in previous economic evaluations is reported but no details of how these were obtained are provided. The MS approach differs from that of most previous models (including those of Buxton and colleagues153 and Fox and colleagues64), in which no benefit from the interventions was assumed. However, the approach appears reasonable and intuitive and the device-specific increments used in the MS are similar to those used in some of the previous models177,192,196 and are of a similar magnitude to those reported in the CARE-HF study. 109
The model presents results according to subgroups defined by the manufacturers (NYHA class, QRS duration, LBBB status and aetiology) and it is not clear how subgroups defined in the MS relate to the populations scoped by NICE. Furthermore, the results have not been aggregated across subgroups and it is unclear how the results compare with those from previously developed economic models. Uncertainty is not comprehensively assessed in the MS as the sensitivity analyses presented are limited to a few scenarios. The methodology used in the MS for the probabilistic sensitivity analysis is not described in sufficient detail to determine whether or not joint parameter uncertainty was properly assessed.
Independent economic evaluation
Statement of the decision problem and perspective for the cost-effectiveness analysis
In accordance with the NICE scope,61 we developed an economic model to estimate the cost-effectiveness of:
-
ICDs for people at risk of SCD as a result of ventricular arrhythmias compared with standard care without an ICD
-
CRT-P or CRT-D for people with HF as a result of LVSD and cardiac dyssynchrony compared with each other and with standard care without CRT
-
CRT-D for people with both conditions compared with CRT-P, ICDs and OPT.
The perspective of the analysis was that of the NHS and PSS. A 3.5% rate was used to discount future health gains and costs.
Strategies and comparators
The scope for the appraisal as defined by NICE61 stated that the interventions to be considered are ICDs for patients at risk of SCD and CRT for patients with HF as a result of LVSD and cardiac dyssynchrony, alongside standard care (also referred to as OPT).
The scoped population groups are eligible for different interventions and comparators; hence, the cost-effectiveness analyses were performed specifically for each population group. Table 92 presents the relevant comparisons for each group, as per the scope61 developed by NICE for this assessment.
-
For people at increased risk of SCD as a result of ventricular arrhythmias despite OPT, an ICD with OPT will be compared with standard care (OPT without an ICD).
-
For people with HF as a result of LVSD and cardiac dyssynchrony despite OPT, CRT-P and CRT-D (both with OPT) will be compared with each other or with standard care (OPT without CRT).
-
For people with both conditions described above, CRT-D with OPT will be compared with an ICD with OPT, CRT-P with OPT or standard care (OPT alone).
| Population | Comparisons | |
|---|---|---|
| Intervention | Comparator | |
| Population 1 | ICD + OPT | OPT |
| Population 2 | CRT-P + OPT | OPT |
| CRT-D + OPT | OPT | |
| CRT-P + OPT | CRT-D + OPT | |
| Population 3 | CRT-D + OPT | OPT |
| CRT-D + OPT | CRT-P + OPT | |
| CRT-D + OPT | ICD + OPT | |
Methods for the economic analysis
Model type and rationale for the model structure
All-cause mortality, SCD, HF mortality and death from other causes were key outcomes in the clinical trials reviewed in Chapter 4. Secondary outcomes included hospitalisation from HF, NYHA class and QoL. To estimate the impact of changes in these outcomes we required an appropriate model of disease progression and its effect on patient HRQoL. We conducted a systematic search of the literature to identify source material on the natural history, epidemiology and treatment of SCD and HF (see Appendix 2). References identified by these searches, along with previous economic evaluations reviewed earlier (see Systematic review of existing cost-effectiveness evidence), informed the development of a Markov state transition model.
A Markov model developed in Microsoft Excel (2010) was used to simulate disease progression in a cohort of patients, who move between distinct health states over their lifetime. The probability of being in a given health state or moving to a different one (experiencing an event) is calculated repeatedly over 4-weekly cycles. Disease progression varies according to the characteristics of the population group and the care pathway that they follow. Each care pathway represents a distinct possible sequence of interventions. As patients are modelled moving between health states over a lifetime, the respective health outcomes and costs can be estimated for a given population following each care pathway. Utility values for the several health states modelled were used to estimate the benefit of each intervention in terms of QALYs.
The adaptation of the model developed by Fox and colleagues64 for TA12043 was found appropriate for the analysis of the cost-effectiveness of ICDs for the treatment of arrhythmias and the cost-effectiveness of CRT devices for the treatment of HF. For patients with HF as a result of LVSD and cardiac dyssynchrony considered as candidates for CRT, we based the pathways on those included in the model developed for TA120. 64 For patients at increased risk of SCD as a result of ventricular arrhythmias we adapted the pathways based on our review of previous models developed for this population and expert opinion. Further details on the development of the model can be found in Appendix 14.
Our model structure is similar to that of the model developed by Fox and colleagues. 64 The key events modelled were hospitalisation because of HF or arrhythmias, transplantation, surgical failure, death, perioperative complications of the implant procedure, routine device replacement, lead displacement, infections and device upgrades.
Figure 33 provides a general schematic of the health states that patients can experience and the possible transitions from one health state to another. Patients being managed with OPT enter the model in the stable health state of the OPT submodel, whereas patients undergoing management with a device enter in the implant surgery state and will typically transition to stable in the device submodel.
FIGURE 33.
General schematic of the model.
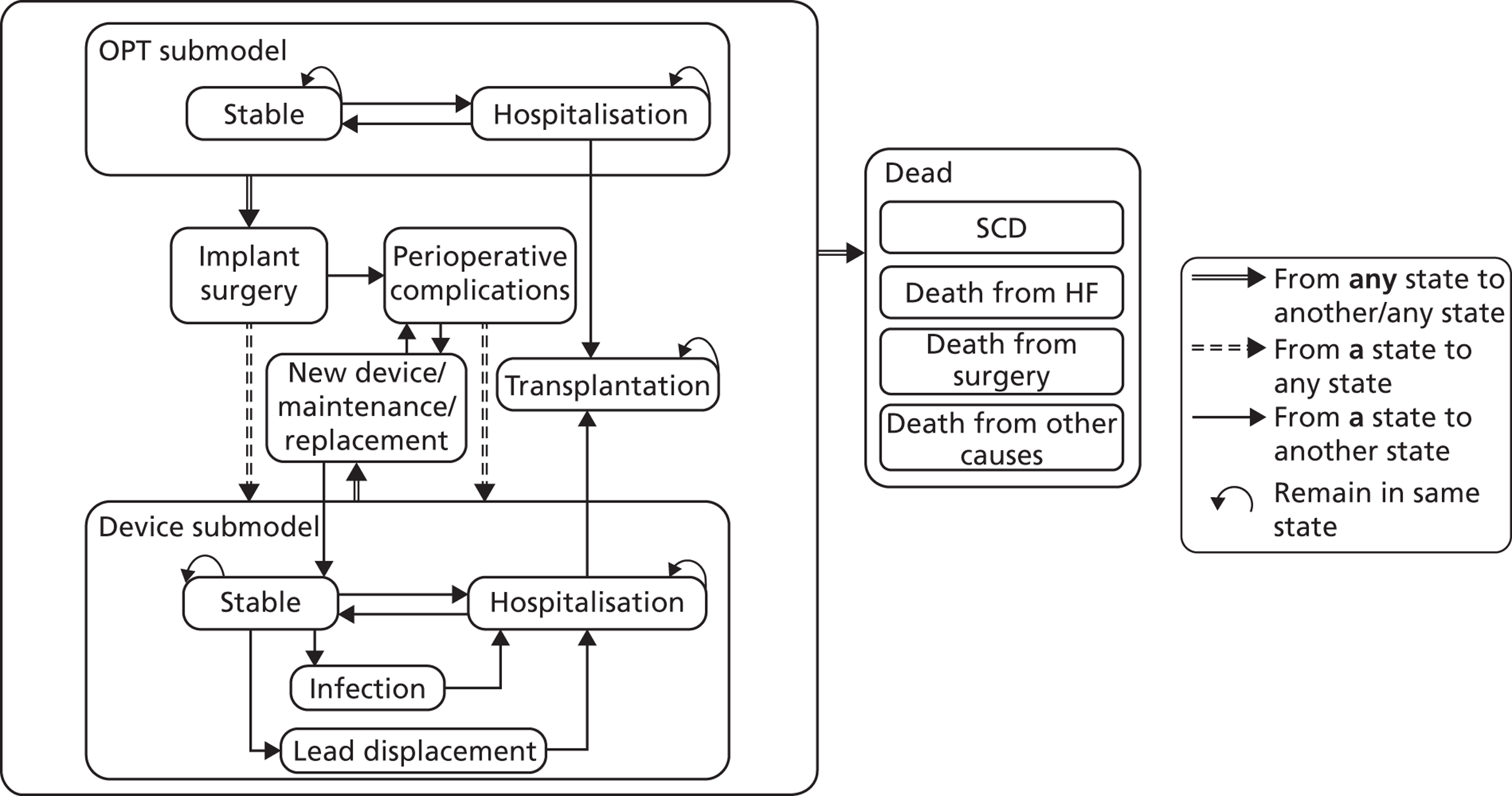
Patients in a stable health state (either receiving OPT or with a device) can remain stable, be hospitalised because of HF or arrhythmia or die from a variety of causes. In addition, patients in a stable health state with a device may experience device-related adverse events (infection or lead displacement/failure) or may require maintenance/replacement of their current device. Patients who are hospitalised because of HF may be referred for heart transplantation. Patients in any of the live health states (stable, hospitalised and transplanted) can die from arrhythmia (SCD), HF or any other cause (cardiac or non-cardiac). Transitions between health states vary according to the population group and the treatment received.
The model structure was developed to reflect the management of patients under current clinical practice, consisting of a simplistic approximation of the clinically plausible care pathways. Therefore, the model allows patients initially managed with OPT or CRT-P to have a device implanted or upgrade to a different device according to disease progression.
The model output obtained with this approach is intended to capture the impact of all treatments received by the patient over a lifetime, instead of only those of the treatment initially allocated, providing a more realistic estimation of the consequences of the adoption of a particular technology as initial treatment.
Relevant patient populations
The baseline cohorts modelled for the economic analysis consist of the three population groups who were identified in the scope developed by NICE61 for this assessment:
-
patients at increased risk of SCD as a result of ventricular arrhythmias despite receiving OPT
-
patients with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT
-
patients with both conditions.
Baseline characteristics (age, sex and, when relevant, proportion in each NYHA class) for the modelled cohorts were based on values reported in relevant clinical trials providing data to populate the model.
Treatment pathways
Population 1: patients at increased risk of sudden cardiac death as a result of ventricular arrhythmias despite receiving optimum pharmacological therapy
The treatment pathways modelled for each cohort of population 1 patients are shown in Table 93.
| Cohort | Treatment | |
|---|---|---|
| First | Second | |
| OPT | OPT | ICD + OPT |
| ICD + OPT | ICD + OPT | – |
Patients enter this arm of the model undergoing ICD implantation surgery. Patients undergoing surgery experience a risk of procedure-related death. Those who survive surgery and have a successful implantation can become stable with the device or be hospitalised because of HF, perioperative complications (including mechanical failures as well as operative complications such as haematoma or pneumothorax), lead displacement, infection or battery failure. Patients who experience unsuccessful implantation are referred for reimplantation and are subject to the same risks of surgical failure and any complications, such as surgical complications, infection or lead displacement, as those who attempt implantation for the first time.
Stable ICD patients can be hospitalised because of HF, severe arrhythmias, lead displacement, infection or battery failure. ICD patients who are hospitalised may continue to be hospitalised, return to the stable with ICD state after treatment or be referred for heart transplantation (if hospitalised for HF). Stable ICD patients are also subject to periodic battery replacement. As with initial implant surgery and reimplantation, these routine replacement procedures expose the patient to a risk of procedure-related death, perioperative complications and unsuccessful implantation.
In this arm, patients enter the model in a stable health state in which they are treated with OPT to prevent major ventricular arrhythmia. Stable OPT patients can remain stable, be hospitalised because of HF or be hospitalised because of major arrhythmia and therefore referred for ICD implantation. Hospitalised patients can return to the stable health state after treatment, be referred for ICD implantation (if hospitalised for major arrhythmia) or be referred for transplantation (if hospitalised for HF). Patients referred for ICD implantation are assumed to follow the same pathway described above for the cohort who enter the model receiving an ICD + OPT and to be subject to the same risk of events.
Being an adaptation of the economic model developed by Fox and colleagues64 for TA120,43 our model relies on some of the assumptions underlying Fox and colleagues’ model that were validated by clinical advice:
-
patients being managed with OPT alone who experience hospitalisation because of non-fatal arrhythmia are assumed to be referred to and undergo ICD implantation
-
patients receiving OPT and hospitalised because of HF who experience a serious arrhythmic event are assumed to be implanted with an ICD and to become stable with the device or be hospitalised because of HF, perioperative complications, lead displacement or infection in the following cycle.
For modelling simplicity and given the exceptional nature of some events, some assumptions underlying our model were incorporated following clinical advice:
-
patients with lead displacements are assumed to have no risk of surgical failure as these interventions do not require a new device
-
patients with an unsuccessful implantation are assumed to have reimplantation attempted in the following cycle
-
patients undergoing reimplantation are assumed to be subject to the same risks of events as those who attempt implantation for the first time
-
the model assumes no risk of return to management with OPT alone because of unsuccessful ICD implantation.
Population 2: patients with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony despite receiving optimum pharmacological therapy
Table 94 summarises the treatment pathways modelled for each cohort of population 2 patients.
| Cohort | Treatment | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| OPT | OPT | CRT-P + OPT | CRT-D + OPT | ICD + OPT |
| OPT | CRT-D + OPT | ICD + OPT | OPT | |
| CRT-P | CRT-P + OPT | CRT-D + OPT | ICD + OPT | OPT |
| CRT-P + OPT | OPT | CRT-P + OPT | CRT-D + OPT | |
| CRT-P + OPT | OPT | CRT-D + OPT | ICD + OPT | |
| CRT-D | CRT-D + OPT | ICD + OPT | OPT | CRT-P + OPT |
| CRT-D + OPT | ICD + OPT | OPT | CRT-D + OPT | |
Patients enter the model in a stable health state being treated with OPT to prevent HF. Stable OPT patients may remain stable or be hospitalised because of HF or severe arrhythmia. OPT patients who are hospitalised may return to the stable health state with OPT after treatment or be referred for CRT-P implantation, CRT-D implantation or transplantation. Patients referred for CRT devices follow a similar pathway to those described below for patients entering the model undergoing CRT-P or CRT-D implantation.
Patients with HF enter the model undergoing CRT-P implantation surgery. They may experience procedure-related mortality or survive the implantation procedure. Patients who survive the procedure may have successful or unsuccessful implantation. Patients with a successful CRT-P implantation may experience perioperative complications, lead displacement, infection and hospitalisation because of HF or severe arrhythmia; those who do not experience any of these events transition to the stable state with CRT-P alongside OPT. Patients who have an unsuccessful CRT-P implantation may return to the OPT stable health state or be hospitalised because of HF or severe arrhythmia, and then progress onwards according to the pathway described above for patients receiving OPT alone.
Stable CRT-P patients may be hospitalised if they experience HF, lead displacement, infection or battery failure. CRT-P patients who are hospitalised may return to the stable with CRT-P after treatment state, remain hospitalised, be referred for an upgrade to CRT-D if they experience serious arrhythmias or be referred for a heart transplant if they experience worsening HF.
Patients with HF enter the model undergoing CRT-D implantation surgery. Similar to patients who enter the model undergoing CRT-P implantation surgery, those who receive CRT-D may die from surgery or survive the implantation procedure. Patients who survive with a successful CRT-D implantation may experience perioperative complications, lead displacement, infection and hospitalisation because of HF or severe arrhythmia; those who do not experience any of these events transition to the stable state with CRT-D alongside OPT.
Patients who survive an unsuccessful CRT-D implantation are assumed to undergo an ICD implantation. These patients may die from ICD implantation surgery. Those who survive ICD implantation and have a successful implantation can become stable with the device or be hospitalised because of HF or severe arrhythmia, perioperative complications, lead displacement, infection or battery failure. Those with an unsuccessful ICD implantation are assumed to be managed with OPT alone and follow the pathway described earlier for population 2 receiving OPT.
Patients who are stable with CRT-D alongside OPT can be hospitalised if they experience HF or severe arrhythmia, lead displacement, infection or battery failure. CRT-D patients who are hospitalised may return to the stable with CRT-D after treatment state, remain hospitalised or be referred for a heart transplant if they experience worsening HF.
Some of the assumptions underlying our model for population 2 derive from the adaptation of the economic model developed by Fox and colleagues64 for TA12043 following clinical validation:
-
patients with CRT-P who experience a serious arrhythmic event are assumed to be referred for CRT-D implantation
-
patients who survive an unsuccessful CRT-P implantation are assumed to return to being managed with OPT alone
-
patients who are hospitalised because of HF and who are referred for a device upgrade are assumed to be implanted and become stable with the device or to be hospitalised because of HF, perioperative complications, lead displacement or infection in the following cycle.
Other assumptions were incorporated according to clinical advice:
-
patients who survive an unsuccessful CRT-D implantation are assumed to undergo an ICD implantation
-
for consistency with an unsuccessful CRT-P implantation, patients who survive an unsuccessful ICD implantation are assumed to return to being managed with OPT alone.
Population 3: patients with both conditions
For population 3, four cohorts were modelled receiving initially CRT-D + OPT, CRT-P + OPT, ICD + OPT or OPT alone. All of these strategies allow for subsequent device implants and upgrades. The treatment pathways modelled for each cohort of population 3 patients are presented in Table 95.
| Cohort | Treatment | |||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| OPT | OPT | CRT-D + OPT | ICD + OPT | OPT |
| OPT | CRT-P + OPT | CRT-D + OPT | ICD + OPT | |
| ICD + OPT | ICD + OPT | CRT-D + OPT | ICD + OPT | OPT |
| ICD + OPT | OPT | CRT-D + OPT | ICD + OPT | |
| ICD + OPT | OPT | CRT-P + OPT | CRT-D + OPT | |
| CRT-P + OPT | CRT-P + OPT | CRT-D + OPT | ICD + OPT | OPT |
| CRT-P + OPT | OPT | CRT-D + OPT | ICD + OPT | |
| CRT-P + OPT | OPT | CRT-P + OPT | CRT-D + OPT | |
| CRT-D + OPT | CRT-D + OPT | ICD + OPT | OPT | CRT-P + OPT |
| CRT-D + OPT | ICD + OPT | OPT | CRT-D + OPT | |
Patients with both conditions who enter the model undergoing CRT-D implantation surgery follow a pathway which is similar to that described earlier for population 2 receiving CRT-D + OPT. Patients who survive an unsuccessful CRT-D implantation are also assumed to undergo an ICD implantation. However, patients with an ICD who become hospitalised because of HF are referred for CRT-D reimplantation.
Patients with both conditions who enter the model undergoing CRT-P implantation surgery follow a similar pathway to that of population 2 receiving CRT-P + OPT described earlier.
Patients enter this arm of the model undergoing ICD implantation surgery. Those who survive with a successful ICD implantation can become stable with the device or be hospitalised because of HF, a serious arrhythmic event, perioperative complications, lead displacement, infection or battery failure. Those hospitalised for HF are upgraded to a CRT-D implant. Those with an unsuccessful ICD implantation are assumed to be managed with OPT alone and follow the pathway described below for those receiving OPT.
Patients with both conditions who enter the model being managed with OPT alone may remain stable with OPT or be hospitalised because of HF or severe arrhythmia. Patients hospitalised for HF may return to the stable health state with OPT after treatment or be referred for CRT-P implantation, CRT-D implantation or transplantation. OPT patients who are hospitalised because of serious arrhythmias are referred for CRT-D implantation. Patients referred for CRT devices follow a similar pathway to those described above for population 3 patients entering the model receiving CRT-P + OPT or CRT-D + OPT.
Some of the assumptions underlying the model of Fox and colleagues,64 which were validated by clinical advice, were used in our model:
-
patients being managed with OPT alone who experience a serious arrhythmic event are assumed to be referred for CRT-D implantation
-
patients with CRT-P who experience a serious arrhythmia are assumed to be referred for CRT-D implantation
-
patients with an ICD who are hospitalised because of HF are assumed to be referred for a CRT-D
-
patients who are hospitalised because of HF and who are referred for a device upgrade are assumed to be implanted and to become stable with the device or be hospitalised because of HF, perioperative complications, lead displacement or infection in the following cycle.
Clinical experts confirmed the reasonability of other assumptions used in our model:
-
patients who survive an unsuccessful CRT-D implantation are assumed to undergo an ICD implantation
-
for consistency with an unsuccessful CRT-P implantation, patients who survive an unsuccessful ICD implantation are assumed to return to being managed with OPT alone.
Pathways common to all populations
For each population modelled, patients being managed with a device can be in hospital because of perioperative complications, lead displacement, routine device replacement or infection. The pathways subsequent to each of these events are common to all populations:
-
Perioperative complications: patients with perioperative complications can become stable with the device or continue to be hospitalised because of HF, lead displacement, battery failure or infection.
-
Heart failure: patients hospitalised because of HF can return to the stable state with the device, continue to be hospitalised because of HF, experience a device-related infection or a lead displacement or be referred for a heart transplant. Concerning populations 2 and 3 exclusively, patients receiving CRT-P who are hospitalised because of HF can be referred for an upgrade to CRT-D if they experience a major arrhythmia or need a routine device replacement.
-
Lead displacement: patients experiencing lead displacement will undergo surgery to replace the lead(s) and are assumed to be subject to the same risks of surgical death, surgical failure and any complications as for an initial implantation.
-
Routine device replacement: patients will undergo re-surgery to replace the device because of battery failure. Devices are assumed to work for a fixed period and all patients stable with the device at the end of that period are assumed to have a new device fitted.
-
Infection: to treat a device-related infection, patients will undergo explantation of the device, treatment for the infection and reimplantation of a new device. These patients are assumed to have the same risks of surgical death, surgical failure and any complications as for an initial implantation.
Model assumptions common to all populations
As the models developed for each population follow a similar structure, the following assumptions are common to all of them:
-
patients can die from any health state in the model
-
patients in health states involving a surgical procedure can also die from surgery
-
the probability of death post transplant is assumed to be lower than the probability of death for non-transplanted patients, except in the first cycle
-
only patients who are hospitalised because of HF are assumed to be at risk of a heart transplant
-
patients referred for transplantation are assumed to remain in this health state until they die
-
patients hospitalised because of HF while being managed with OPT are assumed to have a null probability of remaining hospitalised because of HF in the following cycle
-
patients hospitalised because of perioperative complications are assumed to have no risk of surgical death or surgical failure
-
all patients undergoing surgery (as a result of the initial implantation, a reattempt at implantation, routine device replacement or infection) are assumed to have the same risk of surgical failure.
Discounting
In accordance with current NICE guidance,67 future costs and benefits were discounted at a rate of 3.5%. The impact of discounting using rates of 0% and 6% were explored in sensitivity analysis.
Presentation of the results for the base-case analyses
We report the findings on the cost-effectiveness of interventions based on analysis of cohorts of patients having the age and sex characteristics discussed earlier. For population 1 (people at increased risk of SCD as a result of ventricular arrhythmias despite OPT), comparisons for ICD + OPT are made against OPT. For population 2 (people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT), comparisons for CRT-P + OPT are made against OPT and comparisons for CRT-D + OPT are made against CRT-P + OPT and OPT. For population 3 (people with both conditions), comparisons for CRT-D + OPT are made against OPT, ICD + OPT and CRT-P + OPT.
The base-case results are reported in terms of estimated costs and QALYs accrued for each intervention, as well as incremental costs and QALYs gained for each comparison.
Assessment of uncertainty
Deterministic sensitivity analysis is used to address particular areas of uncertainty in the model related to model structure, methodological assumptions and parameters around which there is considerable uncertainty or which may be expected, a priori, to have a disproportionate impact on the study results. The purpose of this analysis is to identify clearly the impact of this uncertainty and to test the robustness of the cost-effectiveness results to variation in structural assumptions and parameter inputs.
Parameter uncertainty is addressed using probabilistic sensitivity analysis. 220 Probability distributions are assigned to the point estimates used in the base-case analysis and values from these distributions are sampled during the probabilistic analysis. The derivation of point estimates for state transitions, costs and health state utilities is described in the following section. Appendix 15 reports the variables included in the probabilistic sensitivity analysis, the form of distribution used for sampling and the parameters of the distribution.
Data sources and parameter estimates
Population 1: patients at increased risk of sudden cardiac death as a result of ventricular arrhythmias despite optimum pharmacological therapy
Effectiveness data
Survival estimates over time for use in the model were derived from data reported for the relevant trials included in our systematic review. Three trials with the longest reported follow-up (AVID,71 MADIT II101 and SCD-HeFT105) were included in this analysis. According to the evidence found in Chapter 4 (see People at risk of sudden cardiac death as a result of ventricular arrhythmias), patients who survived cardiac arrest or sustained VT are likely to be those for whom ICDs have consistently shown benefit. As the AVID trial71 was the largest trial found for this population, the results of this trial were used for our base-case analysis of patients at increased risk of SCD as a result of ventricular arrhythmia. MADIT II101 was the trial with largest number of patients with remote MI and was considered representative of a relevant group who might benefit from ICDs for primary prevention of SCD. Similarly, the results from the SCD-HeFT trial105 were used to inform a subgroup analysis of patients with mild to moderate HF with an indication for an ICD. An additional subgroup analysis was conducted for patients with cardiomyopathy using as a baseline the all-cause mortality reported for the SCD-HeFT105 subgroup of patients with non-ischaemic CHF in the placebo arm.
Kaplan–Meier curves for overall survival for the OPT arms (the control groups) of the relevant trials were used to derive the baseline mortality risk of patients receiving OPT in the population 1 model. Parametric models were fitted to these curves to derive approximate hazard functions and were assessed visually. Those showing better goodness of fit were used to estimate survival beyond trial follow-up. Hence, baseline time-dependent transition probabilities for transition to the all-cause mortality health state for the model OPT arm were calculated from the estimated hazard functions. 220 For patients receiving ICD + OPT, death transition probabilities were estimated by applying the RRs estimated for ICD + OPT in our systematic review of clinical effectiveness (see Chapter 4, People at risk of sudden cardiac death as a result of ventricular arrhythmias, All-cause mortality) to the baseline transition probabilities of the OPT arm.
Weibull approximations were fitted to the Kaplan–Meier curve for overall survival of patients from the AVID trial,71 the MADIT II trial101 and the SCD-HeFT trial. 105 Details of the regression analyses and comparison between the regression results and the observed survival in these trials are shown in Appendix 15. The Weibull distribution is defined according to two parameters: the scale parameter (λ) and the shape parameter (γ). These parameters were fitted using linear regression of transformations of the Kaplan–Meier estimates (see Appendix 15 for further details). To do this, scanned images of the Kaplan–Meier curves were imported in Engauge software (Engauge Digitizer – Digitizing software version 4.1; see http://digitizer.sourceforge.net/) and the extracted data points were then exported to Microsoft Excel for further analysis. Table 96 shows the parameters of the Weibull functions used in the model to estimate time-dependent mortality for the OPT arm of the population 1 model.
| Parameter | Mean (SE) | |||
|---|---|---|---|---|
| AVID71 (R2 = 0.994) | MADIT II101 (R2 = 0.9903) | SCD-HeFT105 (R2 = 0.993) | SCD-HeFT105 non-ischaemic CHF subgroup (R2 = 0.985) | |
| ln(λ) | –3.380 (0.026) | –4.628 (0.047) | –5.288 (0.039) | –4.821 (0.037) |
| γ | 0.696 (0.009) | 1.007 (0.017) | 1.083 (0.011) | 0.883 (0.011) |
The effect of an ICD compared with OPT on all-cause mortality of patients at increased risk of SCD is captured in the model by the RRs reported in Chapter 4 (see People at risk of sudden cardiac death as a result of ventricular arrhythmias, All-cause mortality). For the base-case analysis (secondary prevention of cardiac arrest), the pooled RR of 0.75 (95% CI 0.61 to 0.93) was used. For the subgroup analysis of patients with remote MI, a pooled RR from the MADIT I99 and MADIT II103 trials of 0.57 (95% CI 0.33 to 0.97) was used. The SCD-HeFT105 RR of 0.77 (95% CI 0.66 to 0.89) was used for the subgroup of patients with mild to moderate HF, and a pooled RR of 0.74 (95% CI 0.58 to 0.93) was used for patients with cardiomyopathy (derived from the SCD-HeFT105 non-ischaemic CHF subgroup and the three cardiomyopathy trials69,82,90).
MADIT II is the only RCT included in our systematic review (see Chapter 4, People at risk of sudden cardiac death as a result of ventricular arrhythmias, Assessment of effectiveness) reporting HF hospitalisations for patients at increased risk of SCD. The number of admissions per total number of trial participants (221 out of 1232 patients in the OPT and ICD arms) is reported for a 20-month follow-up period. The model accounts therefore for a risk of hospitalisation for HF of 0.0082 (95% CI 0 to 0.0202) per cycle for patients at risk of SCD being managed with OPT or ICD therapy, assuming that ICD therapy has no effect on HF hospitalisations.
The number of hospitalisations because of non-fatal arrhythmia is not reported by the trials included in our systematic review for population 1 (see Chapter 4, People at risk of sudden cardiac death as a result of ventricular arrhythmias, Assessment of effectiveness) and the number of patients who experienced arrhythmic events that is reported by some of the included trials is small. Following clinical advice, in our model the baseline probability that a patient at increased risk of SCD managed with OPT will be hospitalised for a non-fatal arrhythmia is assumed to be the same as that for patients with HF (0.0075, 95% CI 0.0002 to 0.0148), derived from the number of events in both arms (OPT and CRT-P) of the MIRACLE trial. 121 The sensitivity of the cost-effectiveness results to this assumption is explored later in this chapter (see Results of the independent economic analysis, Population 1: patients at increased risk of sudden cardiac death as a result of ventricular arrhythmias despite optimal pharmacological therapy) with a scenario analysis using the risk of ventricular arrhythmia for population 3 patients.
Patients being managed with OPT who experience hospitalisation because of non-fatal arrhythmias are assumed to be referred for ICD implantation (estimation described earlier). Patients hospitalised because of HF while being managed with OPT alone are assumed to be subject to a probability of being referred for ICD implantation of 0.0018 (95% CI 0 to 0.0059), the same as that for population 2 patients in the CARE-HF trial109 OPT arm who were referred for CRT-D implantation (see Data sources and parameter estimates, Population 2: patients with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony despite receiving optimum pharmacological therapy).
Adverse events in patients being managed with ICDs were categorised into those occurring at the time of implantation (or during the initial inpatient stay) and a set of longer-term adverse events that could occur around the time of implantation and during all subsequent cycles. The former set of adverse events includes procedure-related mortality, surgical complications and implant failure whereas the latter set includes lead displacements, infections and device malfunctions and dislodgements. As noted in the systematic review (see sections on adverse events in Chapter 4), reporting of individual adverse events in the included trials is limited.
Most trials of patients at increased risk of SCD in which surgical death was included explicitly as an outcome (MADIT II,103 DEFINITE,90 DINAMIT,95 DEBUT89) report no deaths related to the implantation procedure, with only the CASH trial reporting 5/99 perioperative deaths. A pooled probability of procedure-related death of 0.003 (95% CI 0 to 0.055) was used in the base-case analysis, based on five procedure-related deaths among 1449 patients.
Two trials included in our systematic review of clinical effectiveness report implant failure as an outcome of the ICD implantation procedure. This is taken to indicate a failure to achieve the required outcome, rather than mechanical failure of the device or failure/dislodgements of leads (which are reported separately). The AVID trial71 reports unsuccessful initial implant in approximately 1% of patients (5/507) in the defibrillator arm of the trial, corresponding to a probability of implant failure of 0.0098 (95% CI 0 to 0.0962). The SCD-HeFT trial108 reports a lower proportion of patients with unsuccessful implantation (1/829 patients). However, it is not clear whether this was failure of the initial implantation or followed revision of the initial implant procedure. The systematic review of RCTs and observational studies by Ezekowitz and colleagues221 reports a probability of implant failure of 0.011 (95% CI 0.009 to 0.013), which was used in the model.
Given the inconsistent reporting of perioperative and postoperative complications related to use of ICDs among the trials included in our systematic review, estimates from the systematic review of RCTs and observational studies by Ezekowitz and colleagues221 were used in the model. Table 97 presents the probabilities used for each type of event.
| Event | Riska | 95% CI |
|---|---|---|
| Perioperative complication | ||
| Mechanical complication | 0.053 | 0.046 to 0.062 |
| Postoperative complications | ||
| Lead problems | 0.0012 | 0.0010 to 0.0014 |
| Infections | 0.0005 | 0.0004 to 0.0006 |
Epidemiological data
The distribution of patients at increased risk of SCD by NYHA class was sourced from the baseline distribution of participants in the trials selected for our base case and alternative patient group analyses: the AVID trial71 for the secondary prevention of SCD and the MADIT II101 and SCD-HeFT105 trials for the primary prevention of SCD (Table 98).
| NYHA class | AVID71 | MADIT II101 | SCD-HeFT105 | |||
|---|---|---|---|---|---|---|
| AAD | ICD | OPT | ICD | OPT | ICD | |
| No HF, % | 45 | 40 | 0 | 0 | 0 | 0 |
| I, % | 48 | 48 | 39 | 35 | 0 | 0 |
| II, % | 48 | 48 | 34 | 35 | 70 | 70 |
| III, % | 7 | 12 | 23 | 25 | 30 | 30 |
| IV, % | 7 | 12 | 4 | 5 | 0 | 0 |
A summary of the clinical variables in the model for population 1 is provided in Table 99.
| Parameter type | Parameter | Source estimate | Distribution | |||
|---|---|---|---|---|---|---|
| Mean | SE | LL | UL | |||
| All-cause mortality | ln(λ) | –3.381 | 0.0257 | –3.431 | –3.330 | Normal |
| γ | 0.696 | 0.0092 | 0.678 | 0.714 | Normal | |
| HR ICD | 0.75 | 0.0816 | 0.61 | 0.93 | Log-normal | |
| All-cause mortality by age | HR 18–59 | 0.62 | 0.0459 | 0.54 | 0.72 | Log-normal |
| HR 75+ | 1.41 | 0.0051 | 1.40 | 1.42 | Log-normal | |
| Death as a result of surgery | DFS_ICD | 0.0034 | 0.0262 | 0 | 0.0548 | Normal |
| Probability of surgical death from transplantation | DFS_TRP | 0.122 | 0.007 | 0.109 | 0.136 | Normal |
| Event probabilities (per cycle) | ||||||
| Hospitalisation for HF | OPT | 0.0082 | 0.0061 | 0 | 0.0201 | Beta |
| RR ICD | 1 | 0.1 | 0.804 | 1.196 | Beta | |
| Probability of transplant following HF hospitalisation | HF_TRP | 0.0014 | 0.0025 | 0 | 0.0062 | Beta |
| Non-fatal arrhythmia requiring hospitalisation | HA_OPT | 0.0075 | 0.0037 | 0.00016 | 0.0148 | Beta |
| HA_ICD | 0.0075 | 0.0037 | 0.00016 | 0.0148 | Beta | |
| Probability of surgical failure | SF_ICD | 0.011 | 0.001 | 0.009 | 0.013 | Beta |
| Device replacement interval | ln(λ) | –15.784 | 0.203 | –16.182 | –15.385 | Normal |
| γ | 1.942 | 0.0273 | 1.889 | 1.996 | Normal | |
| Upgrade after HF hospitalisation | OPT to ICD | 0.0018 | 0.002 | 0 to | 0.0059 | Beta |
Population 2: patients with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony despite receiving optimum pharmacological therapy
Effectiveness data
Following the approach of Fox and colleagues,64 the population 2 model accounts for cardiac mortality (SCD and morality from worsening HF) and non-cardiac mortality.
The CARE-HF trial111 is the trial with the longest follow-up period (mean 37.4 months) of those included in the clinical effectiveness review for people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT. The CARE-HF trial reports survival curves for SCD and death from worsening HF; hence, baseline time-dependent probabilities of SCD and death from HF were derived from CARE-HF survival curves in the control group. The methodology used to derive baseline mortality is described in the previous section and further details can be found in Appendix 15.
Weibull approximations were fitted to the Kaplan–Meier curves for SCD and death from worsening HF in patients from the CARE-HF trial. The scale (λ) and the shape (γ) parameters that define the Weibull models used for the estimation of SCD and HF deaths for the OPT arm are shown in Table 100. Time-dependent death probabilities for population 2 patients receiving devices (CRT-P, CRT-D or ICD) were then derived by applying device-specific HRs or RRs to the baseline probabilities (OPT arm).
| Parameter | Mean | 95% CI |
|---|---|---|
| SCD | ||
| ln(λ) | –6.069 | –6.173 to –5.964 |
| γ | 1.140 | 1.107 to 1.173 |
| HF | ||
| ln(λ) | –6.115 | –6.256 to –5.974 |
| γ | 1.223 | 1.179 to 1.266 |
The relative effect of CRT-P on HF deaths was obtained from the meta-analysis in Chapter 4 (see People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony, Assessment of effectiveness, Heart failure deaths) (encompassing the CARE-HF109 and COMPANION116 trials; RR 0.67; 95% CI 0.51 to 0.88). That for CRT-D patients was sourced from the COMPANION trial116 (HR 0.73, 95% CI 0.47 to 1.11). The estimate for the RR of SCD for CRT-P patients obtained in the meta-analysis in Chapter 4 (see People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony, Assessment of effectiveness, Sudden cardiac death) (pooled from the CARE-HF,109 COMPANION116 and MUSTIC125 trials ) is 0.97 (95% CI 0.44 to 2.14). Given its wide 95% CI, a RR of 1 was used in our economic model and this estimate was assumed to range between the mean estimates of RR reported in the most relevant trials (0.54 from CARE-HF109 and 1.13 from the COMPANION116). The RR of SCD for CRT-D patients was sourced from the COMPANION trial116 (HR 0.44, 95% CI 0.23 to 0.86).
For population 2 patients who were using an ICD because of CRT-D implant failure, the RRs for SCD and death from worsening HF were sourced from the SCD-HeFT trial. 108 This was considered to be the most representative study from the systematic review of ICDs, as it included a broad population of patients with mild to moderate HF. A RR of 1.14 (95% CI 0.88 to 1.48) is reported for non-arrhythmic cardiac death (assumed to be that due to HF) and 0.44 (95% CI 0.31 to 0.61) for SCD. Considering that population 2 patients are expected to be at higher risk of death from HF and lower risk of SCD than the SCD-HeFT participants (population 1), these parameters were subject to sensitivity analysis (see Results of the independent economic analysis, Population 2).
Non-cardiac-related death rates were derived from the 2010 mortality statistics for England and Wales from the Office for National Statistics. 4 All deaths not allocated an International Classification of Diseases, 10th edition,5 code I00–I52 (for heart disease) were included. Table 101 shows the non-cardiac death rates by age used in the model for population 2. Proportions of UK patients with HF were estimated based on the 2011 statistics for incidence of HF by gender reported by the British Heart Foundation. 222
| Age group (years) | Probability of non-cardiac death per cycle |
|---|---|
| 15–24 | 0.000027 |
| 25–34 | 0.000045 |
| 35–44 | 0.000088 |
| 45–54 | 0.000177 |
| 55–64 | 0.000449 |
| 65–74 | 0.001084 |
| 75–84 | 0.002896 |
| ≥ 85 | 0.008566 |
The hospitalisation baseline risk estimate (0.037, 95% CI 0.025 to 0.049) was pooled from the number of events reported for the OPT arm in the relevant trials included in the systematic review of clinical effectiveness: CARE-HF109 (252/404 events in 29.4 months), MIRACLE121 (50/225 patients in 6 months), MUSTIC125 (9/29 events in 3 months) and COMPANION116 (235/308 events in 11.9 months).
The RR of hospitalisation because of HF for patients receiving CRT-P compared with those receiving OPT was estimated to be 0.58 (95% CI 0.35 to 0.96), pooling risks from the CARE-HF,109 COMPANION,116 MIRACLE121 and MUSTIC125 trials as described in Chapter 4 (see People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony, Assessment of effectiveness). The COMPANION trial116 reports a RR of 0.77 (95% CI 0.63 to 0.93; p = 0.008) for patients receiving CRT-D compared with those receiving OPT. As per Fox and colleagues,64 the risk of hospitalisation because of HF for patients with an ICD was assumed to be the same as for patients receiving OPT (RR = 1).
Fox and colleagues64 report using the number of severe arrhythmic events reported in the MIRACLE trial121 (26/532 participants) to estimate the risk of hospitalisation for non-fatal arrhythmic events. Considering the 6-month follow-up of the trial, this corresponds to a rate of 0.0977 events per patient-year and a 0.0075 (95% CI 0.0002 to 0.0148) probability of experiencing an arrhythmic event per cycle. This probability was assumed to be the same for patients being managed with OPT and for patients receiving CRT-P. Given the lack of evidence on hospitalisation for arrhythmia for population 2 patients receiving CRT-D or an ICD, these patients have been assumed to be at the same risk as those being managed with CRT-P or OPT alone.
Adverse events occurring in patients being managed with CRT were categorised in a similar way to those occurring with ICDs, that is, into those occurring at the time of implantation or initial inpatient stay (procedure-related deaths, implant failures and perioperative complications) and longer-term adverse events (lead displacements, infections and device malfunctions).
The probability of death related to the surgical procedure for CRT implantation was derived from the number of events reported in patients randomised to the CRT-arm in the trials included in our systematic review of clinical effectiveness. The CARE-HF trial109 reported one death in 409 patients, the MIRACLE trial121 one death in 571 patients, the MUSTIC tial125 one death in 64 patients and the COMPANION trial116 five deaths in 617 patients. A probability of procedure-related death of 0.048 (95% CI 0.0015 to 0.0081) per cycle is therefore considered in the model for CRT-P. The COMPANION trial116 also reports three procedure-related deaths out of 595 patients in the CRT-D arm, which corresponds to a probability of procedure-related death of 0.005 (95% CI 0 to 0.0107) per cycle.
The probability of implant failure for patients who attempt CRT implantation was derived from the relevant trials included in the systematic review. A pooled probability of implant failure of 0.084 (95% CI 0.070 to 0.097) per cycle was estimated for patients undergoing CRT-P implantation from four trials: CARE-HF109 (19/409), MIRACLE121 (43/571), MUSTIC125 (5/64) and COMPANION116 (78/617). The COMPANION trial116 reports 54 implant failures in 595 patients undergoing CRT-D implantation; thus, a probability of implant failure of 0.087 (95% CI 0.064 to 0.109) per cycle is used in the model for CRT-D.
Given the limited and heterogeneous reporting of surgical complications related to CRT implantation among the trials included in our systematic review, the probability of patients having an operative complication from a CRT implant was sourced from Fox and colleagues,64 who report a pooled risk of complications from the CARE-HF,109 MIRACLE,121 MUSTIC125 and CONTAK-CD126 trials and both CRT arms of the COMPANION trial. 116 A probability of perioperative complications of 0.1063 (SE = mean/10) was used for both CRT-P and CRT-D.
Three trials included in the systematic review of clinical effectiveness reported the number of lead-related complications that occurred with CRT-P during the follow-up periods: CARE-HF109 (24/409), MIRACLE121 (30/571) and MUSTIC125 (8/58). These were used to estimate a pooled risk of lead displacement of 0.0037 (95% CI 0.0004 to 0.0071) for use in the model for patients being managed with CRT-P or CRT-D.
A probability of 0.0006 (95% CI 0 to 0.002) for device-related infections in patients being managed with CRT-P was derived from the relevant trials included in the systematic review of clinical effectiveness that explicitly reported this outcome: CARE-HF109 (3/409 in 29.4 months) and MIRACLE121 (7/528 in 6 months). For CRT-D, a probability of infection of 0.0006 (95% CI 0 to 0.0015) was derived similarly, using the events reported in the CONTAK-CD126 (7/517 in 6 months), RethinQ142 (6/172 in 6 months), RHYTHM ICD144 (4/205 in 15.1 months), MADIT-CRT130 (12/1089 in 28.8 months) and RAFT140 (21/888 in 40 months) trials.
Following hospitalisation, patients being managed with OPT can be referred for CRT-P or CRT-D implantation, whereas patients being managed with CRT-P can be referred for CRT-D implantation. The probabilities of device upgrade after hospitalisation were derived from the CARE-HF trial,111 assuming that the upgrades reported occurred after hospitalisation due to HF. For the OPT arm (n = 404), the CARE-HF trial111 reports 43 upgrades to CRT-P and 23 upgrades to CRT-D in 29.4 months of follow-up, whereas in the CRT-P arm (n = 409) eight patients upgraded to CRT-D. This corresponds to a probability of 0.0033 (95% CI 0 to 0.009) for upgrading from OPT to CRT-P, 0.0018 (95% CI 0 to 0.0059) for upgrading from OPT to CRT-D and 0.0006 (95% CI 0 to 0.003) for upgrading from CRT-P to CRT-D.
Clinical advice indicated that patients with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT would upgrade to an ICD only in case of failure of CRT-D implantation, which can be estimated by multiplying the probability of upgrading from OPT to CRT-D (0.001, 95% CI 0 to 0.003) by the probability of CRT-D implant failure (0.087, 95% CI 0.064 to 0.109).
For population 2 patients who end up receiving an ICD, our model considers the same data for ICD-related adverse events reported earlier for population 1.
Epidemiological data
The distribution of HF patients by NYHA class used (Table 102) is the same as that used in the previous model by Fox and colleagues,64 who derived the distribution of patients per NYHA class at baseline and 90 days from the CARE-HF trial109 and the conference proceedings of the Brescia study by Curnis and colleagues. 223
| NYHA class | Mean (%) | LL (%) | UL (%) |
|---|---|---|---|
| OPT | |||
| Proportion at baseline | |||
| IIIa | 93.8 | 75.42 | 100.00 |
| IVb | 6.2 | 4.98 | 7.42 |
| Proportion at 90 days | |||
| Ia | 10.1 | 8.12 | 12.08 |
| IIa | 29.9 | 24.04 | 35.76 |
| III | 54.8 | 44.06 | 65.54 |
| IV | 5.2 | 4.18 | 6.22 |
| Proportion at 18 months | |||
| Ic | 12.7 | 10.21 | 15.19 |
| IIa | 37.3 | 29.99 | 44.61 |
| III | 45.7 | 36.74 | 54.66 |
| IV | 4.3 | 3.46 | 5.14 |
| CRT/ICDd | |||
| Proportion at baselinee | |||
| III | 93.8 | 75.42 | 100.00 |
| IV | 6.2 | 4.98 | 7.42 |
| Proportion at 90 days | |||
| Ia | 29.5 | 23.72 | 35.28 |
| IIa | 41.5 | 33.37 | 49.63 |
| III | 27.2 | 21.87 | 32.53 |
| IV | 1.8 | 1.45 | 2.15 |
| Proportion at 18 months | |||
| Ic | 31.5 | 25.33 | 37.67 |
| II | 44.4 | 35.70 | 53.10 |
| III | 22.5 | 18.09 | 26.91 |
| IV | 1.5 | 1.21 | 1.79 |
A summary of the clinical variables in the model for population 2 is shown in Table 103.
| Parameter type | Parameter | Source estimate | Distribution | |||
|---|---|---|---|---|---|---|
| Mean | SE | LL | UL | |||
| Death from HF, age 65–74 years, OPT | ln(λ) | –6.115 | 0.070 | –6.253 | –5.977 | Normal |
| γ | 1.223 | 0.022 | 1.180 | 1.265 | Normal | |
| HR CRT-P | 0.67 | 0.094 | 0.51 | 0.88 | Log-normal | |
| HR CRT-D | 0.73 | 0.163 | 0.47 | 1.11 | Log-normal | |
| HR ICD | 1.14 | 0.153 | 0.88 | 1.48 | Log-normal | |
| Post-transplant mortality | RR TRP | 0.35 | 0.035 | 0.281 | 0.419 | Log-normal |
| SCD | ln(λ) | –6.069 | 0.053 | –6.173 | –5.964 | Normal |
| γ | 1.140 | 0.017 | 1.107 | 1.173 | Normal | |
| HR CRT-P | 1.00 | 0.1505 | 0.54 | 1.13 | Log-normal | |
| HR CRT-D | 0.44 | 0.1607 | 0.23 | 0.86 | Log-normal | |
| HR ICD | 0.44 | 0.0765 | 0.31 | 0.61 | Log-normal | |
| All-cause mortality RR by age (years) | 18–64 | 0.62 | 0.05 | 0.54 | 0.72 | Log-normal |
| 75+ | 1.41 | 0.01 | 1.40 | 1.42 | Log-normal | |
| Event probabilities (per cycle) | ||||||
| Surgical mortality | ICD | 0.003 | 0.026 | 0.000 | 0.055 | Beta |
| CRT-P | 0.005 | 0.002 | 0.001 | 0.008 | Beta | |
| CRT-D | 0.005 | 0.003 | 0.000 | 0.011 | Beta | |
| TRP | 0.122 | 0.007 | 0.109 | 0.136 | Beta | |
| Hospitalisation for HF | OPT | 0.037 | 0.006 | 0.025 | 0.049 | Beta |
| RR ICD | 1 | 0.1 | 0.804 | 1.196 | Beta | |
| RR CRT-P | 0.58 | 0.1556 | 0.35 | 0.96 | Beta | |
| RR CRT-D | 0.77 | 0.0765 | 0.63 | 0.93 | Beta | |
| Transplant following HF hospitalisation | TRP | 0.001 | 0.002 | 0.000 | 0.006 | Beta |
| Non-fatal arrhythmia requiring hospitalisation | OPT | 0.007 | 0.004 | 0.000 | 0.015 | Beta |
| ICD | 0.007 | 0.004 | 0.000 | 0.015 | Beta | |
| CRT-P | 0.007 | 0.004 | 0.000 | 0.015 | Beta | |
| CRT-D | 0.007 | 0.004 | 0.000 | 0.015 | Beta | |
| Probability of upgrade after HF hospitalisation | OPT to ICD | 0 | 0 | 0 | 0 | Beta |
| OPT to CRT-P | 0.003 | 0.003 | 0.000 | 0.009 | Beta | |
| OPT to CRT-D | 0.002 | 0.002 | 0.000 | 0.006 | Beta | |
| CRT-P to CRT-D | 0.001 | 0.001 | 0.000 | 0.003 | Beta | |
| Surgical failure | ICD | 0.011 | 0.001 | 0.009 | 0.013 | Beta |
| CRT-P | 0.084 | 0.007 | 0.070 | 0.097 | Beta | |
| CRT-D | 0.087 | 0.012 | 0.064 | 0.109 | Beta | |
Population 3: patients with both conditions
Effectiveness data
Estimates of survival over time were derived from Kaplan–Meier curves reported for relevant trials included in the systematic review. The two largest trials reporting the longest follow-up periods and comparing events between groups statistically (MADIT-CRT130 and RAFT140) were included in this analysis. As reported in Chapter 4, length of follow-up was an average of 28.8 months in the MADIT-CRT trial130 and 40 months in the RAFT trial. 140 Survival estimates from the trial with the longest follow-up (RAFT) were used for the base-case analysis and those from MADIT-CRT were used in scenario analysis.
Both trials report Kaplan–Meier curves for all-cause mortality for CRT-D + OPT and ICD + OPT. As CRT-D + OPT was the intervention scoped by NICE for population 3,61 we used its mortality estimates as the baseline for this population and used HRs and RRs to derive all-cause mortality for patients receiving OPT alone, ICD + OPT and CRT-P + OPT.
The methodology used to derive baseline mortality is similar to that described earlier for populations 1 and 2; further details can be found in Appendix 15. Table 104 presents the parameters of the Weibull models obtained using data from the RAFT140 and MADIT-CRT trials. 130
| Parameter | Mean | 95% CI |
|---|---|---|
| RAFT140 | ||
| ICD-CRT arm (R2 = 0.9894) | ||
| ln(λ) | –6.334 | –6.467 to –6.202 |
| γ | 1.243 | 1.20 to 1.27 |
| MADIT–CRT130 | ||
| Men, CRT-D arm (R2 = 0.989) | ||
| ln(λ) | –6.935 | –7.005 to –6.865 |
| γ | 1.287 | 1.266 to 1.308 |
The risk of all-cause mortality for patients with an ICD relative to those receiving CRT-D was derived from the pooled RR of 0.84 (95% CI 0.73 to 0.96) estimated in Chapter 4 (see People with both conditions, Assessment of effectiveness, All-cause mortality) for CRT-D compared with ICD therapy. A RR of 1.19 (95% CI 1.04 to 1.37) for ICD therapy compared with CRT-D was used to estimate all-cause mortality in the ICD arm.
In the systematic review of clinical effectiveness studies of people with both conditions, only RCTs comparing CRT-D with ICD therapy were found. However, the COMPANION trial116 reports the HR for all-cause mortality for patients with HF as a result of LVSD and cardiac dyssynchrony, from which we derived the HR of 1.56 (95% CI 1.16 to 2.08) for OPT compared with CRT-D, assuming that the same relative effect would be expected in population 3.
Given the lack of RCTs in people with both conditions directly comparing CRT-P with CRT-D or assessing interventions other than CRT-D or ICDs, we used the evidence available on the clinical effectiveness of CRT-P and CRT-D in patients with HF as a result of LVSD and cardiac dyssynchrony. The only trial comparing CRT-P with CRT-D was the COMPANION trial. 116 A non-statistically significant RR of 1.20 (95% CI 0.96 to 1.52) for all-cause mortality was reported for CRT-P compared with CRT-D. However, the COMPANION trial116 was not powered for this comparison. Considering the lack of robust evidence for this comparison, the risk of all-cause mortality for patients with CRT-P was assumed to be the same as for those with CRT-D (RR = 1). This assumption was subject to sensitivity analysis by varying the parameter between the assigned upper and lower limits of the 95% CI (0.80 to 1.20).
The trials included in the systematic review of clinical effectiveness (see Chapter 4, People with both conditions, Assessment of effectiveness, Hospitalisations related to heart failure) do not report the number of hospitalisations because of HF. Instead, the CONTAK-CD,126 Piccirillo and colleagues138 and RAFT140 trials report the number of patients receiving CRT-D hospitalised for HF (at least once during the trial). In 6 months of follow-up, the CONTAK-CD trial126 reported that 32 of 245 patients in the CRT-D arm were hospitalised; Piccirillo and colleagues138 reported that none of 16 patients in the CRT-D arm, who were followed for 12 months, were hospitalised; and the RAFT trial140 reported that 174 of 894 patients in the CRT-D arm were hospitalised during the 40 months of follow-up. The number of patients experiencing at least one hospitalisation during the follow-up period of the trials provides a minimum number of hospitalisations from which we derived a baseline risk of hospitalisation because of HF (probability of event occurring 0.0077, 95% CI 0.0027 to 0.0128). Given that our model is likely to be underestimating the total number of hospitalisations, and consequently the resource use involved, the probability of hospitalisation because of HF was subject to sensitivity analysis (see Results of the independent economic analysis).
The RR of hospitalisation because of HF for patients with an ICD compared with those receiving CRT-D was estimated to be 1.33 (95% CI 1.14 to 1.56), the reverse of the RR of 0.75 (95% CI 0.64 to 0.88) obtained in Chapter 4 (see People with both conditions, Assessment of effectiveness, Hospitalisations related to heart failure) by pooling risks from the CONTAK-CD,126 Piccirillo and colleagues138 and RAFT140 trials.
The COMPANION trial116 reports no significant differences in hospitalisations because of HF between CRT-P and CRT-D for patients with HF (see Chapter 4, People with heart failure, Assessment of effectiveness, Hospitalisations because of heart failure). Hence, assuming that no significant differences would be expected either in patients with both conditions (at risk of SCD as a result of ventricular arrhythmias and with HF as a result of LVSD and cardiac dyssynchrony), the risk of hospitalisation because of HF estimated for CRT-D (0.0077) was used for CRT-P (RR = 1).
Evidence on the RR of hospitalisation for HF in patients receiving OPT compared with CRT-D was found only for patients with HF (population 2). The COMPANION trial116 reported a statistically significant difference in HF hospital admissions per patient between the CRT-D arm and the OPT arm (0.43 vs. 0.73 admissions per patient-year respectively). The RR estimated for hospitalisations because of HF for OPT compared with CRT-D was 1.67 (95% CI 1.51 to 1.86, p < 0.00001).
The baseline risk of hospitalisation for arrhythmia used in the model (probability of event occurring 0.029, 95% CI 0.015 to 0.042) was derived from trials included in the systematic review of clinical effectiveness (see Chapter 4, People with both conditions, Assessment of effectiveness) reporting the number of patients receiving CRT-D who experienced at least one episode of VF: MIRACLE ICD136 (42/187), MICACLE ICD II137 (19/85), CONTAK-CD126 (36/245) and the trial by Pinter and colleagues139 (7/36). Similar to the estimation of hospitalisations for HF, our model is likely to be underestimating the total number of hospitalisations for arrhythmic events and the value used was therefore subject to sensitivity analysis (see Results of the independent economic evaluation).
The meta-analysis (see Chapter 4, People with both conditions, Assessment of effectiveness) found a non-statistically significant difference between CRT-D and ICD in the number of patients experiencing at least one arrhythmic event (RR 0.90, 95% CI 0.71 to 1.14, p = 0.38). Hence, the inverse RR of 1.11 (95% CI 0.88 to 1.41) for ICD compared with CRT-D was used in the model.
No evidence to derive a measure of relative effect was found for hospitalisations for arrhythmia comparing CRT-P or OPT with CRT-D. The COMPANION trial116 states that hospitalisations because of other cardiac causes were not significantly different between the OPT group and the CRT group. Therefore, our model assumes that the risk of hospitalisation because of arrhythmia for patients managed with OPT alone or CRT-P is the same as that for patients managed with CRT-D (RR = 1).
Given the inconsistent reporting and lack of clear definitions of device-related adverse events reported in the relevant trials included in the systematic review of clinical effectiveness for people with both conditions (population 3), our model assumes the same risks for population 3 as for population 2 (people with HF).
The RAFT trial140 reported the number of patients by NYHA class at baseline (Table 105). No evidence on the effect of the devices on HF progression was found; hence, the model assumes no effect on patient distribution by NYHA class. An alternative scenario was created to explore the impact of accounting for the potential benefit of CRT devices for population 3, assuming that 50% of patients with a CRT device improve by one NYHA class at 6 months of treatment (see Results of the independent economic analysis).
| NYHA class | Proportion at baseline, n (%) | |
|---|---|---|
| ICD (n = 904) | CRT-D (n = 894) | |
| II | 730 (80.8) | 708 (79.2) |
| III | 174 (19.2) | 186 (20.8) |
A summary of the clinical variables in the model for population 3 is provided in Table 106.
| Parameter type | Parameter | Source estimate | Distribution | |||
|---|---|---|---|---|---|---|
| Mean | SE | LL | UL | |||
| All-cause mortality, baseline – CRT-D | ln(λ) | –6.334 | 0.068 | –6.467 | –6.202 | Normal |
| γ | 1.234 | 0.018 | 1.199 | 1.270 | Normal | |
| HR CRT-P | 1 | 0.100 | 0.804 | 1.196 | Log-normal | |
| HR ICD | 1.190 | 0.084 | 1.042 | 1.370 | Log-normal | |
| HR OPT | 1.563 | 0.235 | 1.163 | 2.083 | Log-normal | |
| All-cause mortality RR by age (years) | 18–64 | 0.621 | 0.046 | 0.54 | 0.72 | Log-normal |
| 75+ | 1.410 | 0.005 | 1.4 | 1.42 | Log-normal | |
| Event probabilities (per cycle) | ||||||
| Hospitalisation for HF | CRT-D | 0.008 | 0.003 | 0.003 | 0.013 | Beta |
| RR ICD | 1.333 | 0.133 | 1.136 | 1.563 | Log-normal | |
| RR CRT-P | 1 | 0.1000 | 0.804 | 1.196 | Log-normal | |
| RR OPT | 1.67 | 0.0893 | 1.51 | 1.86 | Log-normal | |
| Non-fatal arrhythmia requiring hospitalisation | CRT- D | 0.029 | 0.007 | 0.015 | 0.042 | Log-normal |
| RR ICD | 1.111 | 0.111 | 0.880 | 1.410 | Log-normal | |
| RR CRT-P | 1 | 0.1 | 0.804 | 1.196 | Log-normal | |
| RR OPT | 1 | 0.1 | 0.804 | 1.196 | Log-normal | |
| Probability of upgrade after HF hospitalisation | OPT to ICD | 0.002 | 0.002 | 0 | 0.006 | Beta |
| OPT to CRT-P | 0.003 | 0.003 | 0 | 0.009 | Beta | |
| OPT to CRT-D | 0.002 | 0.002 | 0 | 0.006 | Beta | |
| CRT-P to CRT-D | 0.001 | 0.001 | 0 | 0.003 | Beta | |
| ICD to CRT-D | 0.007 | 0.003 | 0.001 | 0.013 | Beta | |
| Surgical mortality | ICD | 0.003 | 0.026 | 0 | 0.055 | Beta |
| CRT-P | 0.005 | 0.002 | 0.001 | 0.008 | Beta | |
| CRT-D | 0.005 | 0.003 | 0 | 0.011 | Beta | |
| Surgical failure | ICD | 0.011 | 0.001 | 0.009 | 0.013 | Beta |
| CRT-P | 0.084 | 0.007 | 0.070 | 0.097 | Beta | |
| CRT-D | 0.087 | 0.012 | 0.064 | 0.109 | Beta | |
| Device lifetime | ICD ln(λ) | –15.784 | 0.203 | –16.182 | –15.385 | Normal |
| ICD γ | 1.943 | 0.027 | 1.889 | 1.996 | Normal | |
| CRT-P ln(λ) | –14.222 | 0.242 | –14.697 | –13.747 | Normal | |
| CRT-P γ | 1.677 | 0.032 | 1.613 | 1.740 | Normal | |
| CRT-D ln(λ) | –15.465 | 0.273 | –16 | –14.931 | Normal | |
| CRT-D γ | 1.935 | 0.036 | 1.863 | 2.006 | Normal | |
Parameters common to all populations
Age-related mortality
The variation of risk of death according to age was incorporated in our model using the same estimates as those used by Fox and colleagues,64 who derived the RR of death from the publication by Shahar and colleagues. 224 The RR of death for patients aged < 65 years compared with those aged 65–74 years is 0.62 (95% CI 0.54 to 0.72). For those aged ≥ 75 years compared with those aged 65–74 years the RR is 1.41 (95% CI 1.40 to 1.42).
Distribution of patients eligible for implantable cardiac defibrillator and cardiac resynchronisation therapy by age
The distribution of heart device implants by age was derived from a report commissioned by the British Cardiovascular Society, the British Heart Foundation and the Cardio & Vascular Coalition on access to cardiac care in the UK. 225 Table 107 shows the estimated distribution of implanted devices by age.
| Age group (years) | ICDs, % | CRTs, % | ICDs/CRTs, % |
|---|---|---|---|
| 0–34 | 5.9 | 1.5 | 3.8 |
| 35–44 | 6.4 | 2.4 | 4.5 |
| 45–54 | 13.0 | 9.7 | 11.4 |
| 55–64 | 22.6 | 21.7 | 22.1 |
| 65–74 | 30.9 | 36.7 | 33.7 |
| 75–84 | 19.8 | 25.3 | 22.5 |
| 85+ | 1.4 | 2.7 | 2.0 |
| Total | 100.0 | 100.0 | 100.0 |
The distribution of patients with ICD implants was deemed to be a good proxy for population 1 patients at increased risk of SCD, whereas the distribution of CRT implants was used for population 2 patients with HF. For population 3 with both conditions, the distribution of both ICD and CRT devices was used in the model.
Heart transplantation
The model takes into account that patients subject to heart transplantation have a procedure-related risk of death of 12.2% (95% CI 10.9% to 13.6%), the 30-day mortality rate estimated by the UK Cardiothoracic Transplant Audit226 from data for all patients transplanted between 1995 and 2011.
The risk of death post transplantation was incorporated using the estimate derived by Fox and colleagues. 64 The RR of death from all causes for patients who had a heart transplant (0.35) was derived from the median survival estimates reported by Hussey and colleagues227 for UK patients receiving a heart transplant (10.6 years) and those on OPT (3.7 years).
Abraham and colleagues121 report two heart transplants in 532 participants in the MIRACLE trial. As in Fox and colleagues,64 for population 2 we assumed that these patients were referred for transplantation after hospitalisation for HF, estimating a 0.0014 (95% CI 0 to 0.0062) probability of transplantation per cycle for patients hospitalised for HF.
Given the paucity of data regarding the number of transplants after hospitalisation for HF in the trials for populations 1 and 3, our model assumes the same risk as that for patients with HF (population 2).
Health-related quality of life
Utility values for the several health states modelled were used to estimate the benefit of each intervention in terms of QALYs. Overall, the HRQoL of patients in stable health states was modelled to vary according to their NYHA class. A specific utility value was used for hospitalisation and decrements were applied to health states involving surgery (including for initial device implantation, device-related complications and device replacement) or infection.
The utility values by NYHA class used in the model (Table 108) were from one study214 (described earlier in the systematic review of HRQoL studies) that reported utility values for all NYHA classes.
| Health state | NYHA class | Utility value (95% CI) | Source |
|---|---|---|---|
| Stable | I | 0.855 (0.845 to 0.864) | Gohler et al.214 |
| II | 0.771 (0.761 to 0.781) | ||
| III | 0.673 (0.727 to 0.765) | ||
| IV | 0.532 (0.48 to 0.584) | ||
| Hospitalisation and heart transplantation | 0.57 | Holland et al.27 | |
| Decrement due to surgery | 0.05 | Assumption64 | |
| Decrement due to infection | 0.1 | Assumption64 |
One observational analysis within the UK27 was also included in the systematic review. Holland and colleagues27 reported utility estimates per NYHA class at baseline in patients with HF following emergency hospital admission, estimating an average score of 0.57 (see Table 108). This utility value is similar to that estimated by McAlister and colleagues194 and used in Fox and colleagues’ model. 64 Our model also assumed that the proportion of time hospitalised was on average one-quarter of the month.
As in Fox and colleagues’ model,64 utility estimates for transplantation were assumed to be similar to those for hospitalised patients and post-transplanted patients were assumed to have a similar HRQoL to NYHA class I patients.
None of the studies found in the systematic review reported the impact of surgery or infection on the QoL of patients eligible for ICD or CRT. As per Fox and colleagues,64 decrements of 0.05 for the impact of surgery and of 0.1 for infection were assumed.
One study153 reporting utilities for UK patients at increased risk of SCD as a result of ventricular arrhythmias was included in the systematic review of HRQoL studies described earlier. Buxton and colleagues153 concluded that there was no evidence that self-reported HRQoL changes substantially over time. Therefore, we assumed that the NYHA class of modelled patients was constant over the modelled time horizon. The distribution of patients by NYHA class reported at baseline in the relevant trials for population 1 was used in our model in combination with utility values by NYHA class from Gohler and colleagues214 (see Table 108) to estimate a NYHA class-weighted average utility value.
Buxton and colleagues153 also found that patients who had suffered inappropriate ICD shocks had significantly lower HRQoL, reporting mean utility values of 0.7 and 0.8 for patients with at least one inappropriate shock and with no shocks respectively. Patients who experience inappropriate shocks are expected to be hospitalised and have their HRQoL affected. The impact of inappropriate shocks on HRQoL and costs was implicitly accounted for in our economic model through the hospitalisation rates used. Following clinical advice, we incorporated the probability of hospitalisation for severe arrhythmia in patients with an ICD. Although there is limited evidence on hospitalisations for severe arrhythmia in the trials included in our systematic review for population 1, we assumed that patients with an ICD are as likely to be hospitalised for non-fatal arrhythmia as patients being managed with OPT alone (see Data sources and parameter estimates, Population 1).
For population 2, the impact of CRT on the HRQoL of patients with HF over time was captured in the model by changes in the distribution of patients with HF by NYHA class derived from the relevant trials (see Data sources and parameter estimates, Population 2, Distribution of patients per New York Heart Association class). Given that evidence of the impact on the distribution of patients by NYHA class was available only for population 2 patients receiving CRT-P or OPT alone, the model assumed the same effect for any CRT device and ICDs were assumed to have the same impact as OPT alone.
For population 3, robust evidence of the effect of the devices on HF progression was not found; hence, CRT and ICD devices were assumed to have no impact on the distribution of patients by NYHA class over time (i.e. this distribution was assumed constant). The distribution of patients by NYHA class reported in the relevant trials for the CRT-D and ICD arms at baseline (see Data sources and parameter estimates, Population 3) was applied to patients receiving CRT-P and OPT alone, respectively, in the model. As both arms of the trial show a similar distribution (approximately 80% and 20% for NYHA classes II and III respectively), the model assumes similar utility values for patients receiving CRT, an ICD or OPT alone (e.g. 0.75 for patients who are stable with therapy). Therefore, this base-case approach might be underestimating the benefit of CRT devices for HRQoL in population 3. To estimate the impact of accounting for this potential benefit of CRT devices on the cost-effectiveness results for population 3, an alternative approach was adopted in the scenario analysis (see Results of the independent economic analysis), assuming that 50% of patients with a CRT device improve by one NYHA class at 6 months of treatment.
Utility values by NYHA class from Gohler and colleagues214 were then used to estimate NYHA class-weighted average utility values for patients for all populations. Table 108 summarises the utility values used in our model and their sources.
Resource use and costs
Resource use and cost estimation aimed to cost all relevant resources consumed in the care of patients in the three populations being studied. Similar to the previous model for the assessment of CRT devices,64 the resources considered in the current model include medication and resources involved in device implantation, device-related complications and maintenance, hospitalisation for HF or severe arrhythmia and heart transplantation.
The economic model estimates resource use associated with each intervention based on event rates and patient transition probabilities among the different health states. Unit costs associated with each resource used are then applied for estimation of the total cost per intervention.
The device-related costs used in the economic model (Table 109) correspond to the estimates provided in the MS. These were derived from average selling prices aggregated across all manufacturers for ICD, CRT-P and CRT-D devices and for leads sold in the UK to the NHS.
| Device component | Mean cost (£) | Lower value (£)a | Upper value (£)a |
|---|---|---|---|
| Whole system | |||
| CRT-P | 3411 | 2742 | 4080 |
| CRT-D | 12,293 | 9884 | 14,702 |
| ICD | 9692 | 7792 | 11,592 |
| Leadsb | |||
| CRT-P | 811 | 652 | 970 |
| CRT-D | 541 | 435 | 647 |
| ICD | 543 | 437 | 649 |
| Battery | |||
| CRT-P | 2600 | 2090 | 3110 |
| CRT-D | 11,752 | 9449 | 14,055 |
| ICD | 9149 | 7356 | 10,942 |
Estimates of device longevity were also sourced from the ABHI joint submission, which reports the Kaplan–Meier plots of time to device replacement derived from data submitted to CCAD. Estimates of mean time to replacement were derived from the reported survival functions for use in the model. Table 110 presents the parameters of the Weibull approximations obtained for each device type and the respective mean lifetimes. Clinical advice indicated that the longevity of the devices might be overestimated; hence, these parameters were subject to sensitivity analysis and a scenario of shorter device longevity was explored (see Results of the independent economic analysis).
| Parameter | Mean | 95% CI |
|---|---|---|
| ICDs | ||
| ln(λ) | –15.784 | –16.182 to –15.385 |
| γ | 1.943 | 1.889 to 1.996 |
| Device longevity (years) | 8.20 | 12.76 to 5.40 |
| CRT-P | ||
| ln(λ) | –14.222 | –13.747 to –14.697 |
| γ | 1.677 | 1.613 to 1.74 |
| Device longevity (years) | 11.81 | 22.22 to 6.58 |
| CRT-D | ||
| ln(λ) | –15.465 | –16.000 to –14.931 |
| γ | 1.935 | 1.863 to 2.006 |
| Device longevity (years) | 7.19 | 13.05 to 4.14 |
Costs associated with device implantation, complications or maintenance were sourced from the 2012–13 UK NHS tariff,229 whereas the costs of hospitalisations and transplantation were derived from the 2010–11 NHS Reference Costs (NHS trusts and primary care trusts combined HRG data). 218
Table 111 presents the procedure costs used in the economic model. Only elective care estimates were used to derive the mean cost of device-related procedures. For HRGs concerning non-device-related procedures, the mean cost was estimated as a weighted average of the national average unit costs reported for elective and long-stay non-elective care. Lower and upper values of all procedure costs were derived from the 2010–11 NHS reference costs218 as a weighted average of the lower and upper quartile unit costs reported for elective and long-stay non-elective care.
| Procedure | Mean cost (£) | Lower value (£) | Upper value (£) | Source |
|---|---|---|---|---|
| Device-related procedures | ||||
| Implantation, reimplantation and lead displacement/replacement | ||||
| CRT-P | 4870 | 3356 | 7816 | UK tariff 2012–13229 elective EA07Z and MS151a |
| CRT-D | 5556 | 5363 | 18,267 | UK tariff 2012–13229 elective EA12Z |
| ICD | 5556 | 5363 | 18,267 | UK tariff 2012–13229 elective EA12Z |
| Explant | ||||
| CRT-P | 2748 | 2153 | 4542 | UK tariff 2012–13229 elective EA39Z |
| CRT-D | 2748 | 2153 | 4542 | UK tariff 2012–13229 elective EA39Z |
| ICD | 2748 | 2153 | 4542 | UK tariff 2012–13229 elective EA39Z |
| Battery failure/device replacement | ||||
| CRT-P | 2748 | 2153 | 4542 | UK tariff 2012–13229 elective EA39Z |
| CRT-D | 5556 | 5363 | 18,267 | UK tariff 2012–13229 elective EA12Zb |
| ICD | 5556 | 5363 | 18,267 | UK tariff 2012–13229 elective EA12Zc |
| Hospitalisation | ||||
| HF | 2308 | 1669 | 2578 | NHS Reference Costs 2010–11218 EB03H/EB03I |
| Arrhythmia | 1372 | 922 | 1601 | NHS Reference Costs 2010–11218 EB07H/EB07I |
| Heart transplant | 35,606 | 21,449 | 43,315 | NHS Reference Costs 2010–11218 EA02Z |
The economic model developed for the current assessment accounts for hospitalisation for HF and hospitalisation for severe arrhythmia. According to Fox and colleagues,64 fewer resources are expected to be used to manage hospitalised patients with a device than hospitalised patients receiving OPT. Thus, the conservative approach of assuming the same resource use for all groups was taken. The costs associated with the management of hospitalisation for HF and arrhythmia were derived from the 2010–11 NHS Reference Costs218 and are presented in Table 111.
The HRG codes EB03H and EB03I refer to HF or shock events with or without complications respectively; hence, a weighted average of the national average unit costs reported for each HRG was estimated including both elective and long-stay non-elective care. Similarly, EB07H and EB07I concern arrhythmia or conduction disorders with or without complications. The cost of hospitalisation for arrhythmia was estimated in the same way as the cost of hospitalisation for HF.
The cost of a heart transplant was estimated as a weighted average of the national average unit costs reported for elective and long-stay non-elective care (HRG code EA02Z).
Device implantation involves a surgical procedure and device-related resources; hence, the costs of a whole system and of the implantation procedure (see Tables 109 and 111) were included. The HRG code specific for ICD implantation is EA12Z and the code for biventricular resynchronisation therapy procedures is EA07Z. The CRT-D implantation cost was assumed to be the same as that for ICD implantation (a conservative approach was taken given the higher cost of EA12Z than EA07Z).
Device upgrades and routine/maintenance replacements were assumed to be similar in terms of resource use and costs to the initial implantation.
The resources used for managing operative complications were also accounted for in the economic model. The definition of operative complications and the detail of their reporting varied among the RCTs included in our systematic review of clinical effectiveness. Therefore, the rates of operative complications were sourced from the RAFT trial,140 a large RCT of patients who are at risk of SCD as a result of ventricular arrhythmia and with HF as a result of LVSD and cardiac dyssynchrony, managed with CRT-D or ICD devices. For the estimation of an average cost of operative complications, we assumed these to be a combination of lead displacements, infections and device-related problems requiring intervention or device substitution. Thus, the cost of operative complications was estimated as a weighted average of these events using the proportions presented in Table 112 for each device type.
| Complications | CRT, n (%) | ICD, n (%) |
|---|---|---|
| Device-related problems requiring replacementa | 4 (4) | 1 (2) |
| Complications requiring interventionb | 75 (75) | 31 (65) |
| Infections | 21 (21) | 16 (33) |
| Total | 100 | 48 |
The unit cost estimation for lead displacements, infections and device malfunctions is described in the following section. The unit cost for complications requiring intervention was assumed to be that of lead displacements, and device-related problems requiring replacement were assumed to cost as much as an initial implant.
Management of device-related problems requires a different approach according to each type of event, as different components of the device may need replacement or adjustment and different lengths of hospital admission might be necessary. Fox and colleagues64 considered lead displacement or failure, lead infection and battery replacement or failure to be the most frequent device-related complications. All types of devices (ICD and CRT) are assumed to have the same types of problems and these are assumed to require similar management regardless of device type. Only costs (device and procedural) are expected to differ according to the type of device.
Managing a lead displacement/failure occurrence is assumed to require a surgical intervention to adjust or replace the lead, which is expected to use similar resources to those used for initial implantation. For cost estimation, the cost of the leads and of implantation surgery were considered.
The treatment of lead infections usually requires surgery for explant of the infected device, a prolonged hospital stay to control the infection, a post-discharge outpatient visit to confirm the absence of infection and the implantation of a new system. The resource use and costs involved in the treatment of infections are provided in Table 113.
| Item | Mean (£) | Lower limit (£) | Upper limit (£) | Source |
|---|---|---|---|---|
| Explant cost | 2748 | 2153 | 4542 | UK tariff 2012–13229 elective EA39Z |
| Extra bed-day cost | 316 | 190 | 370 | NHS Reference Costs 2010–11218 EA39Z |
| Length of stay (days) | 4.43 | 2.65 | 7.12 | NHS Reference Costs 2010–11218 EA39Z |
| Outpatient visit cost | 123 | 94 | 148 | NHS Reference Costs 2010–11218 – Service 320 – Cardiology |
| Total cost of infectiona | ||||
| CRT-P | 12,553 | 7285 | 15,265 | |
| CRT-D | 21,580 | 17,202 | 38,966 | |
| ICD | 18,977 | 15,109 | 35,853 | |
The HRG code EA39Z includes procedures for removal of the cardiac pacemaker system and it was applied as the explant cost for all types of devices. Mean length of stay was derived as a weighted average of the length of stay reported for elective and long-stay non-elective care. The lower limit corresponds to an average length of stay for elective care, whereas the upper limit is the average length of stay for long-stay non-elective care. The cost of each additional bed-day was derived from the excess bed-day national average unit costs for elective and long-stay non-elective care for explants (EA39Z). The post-discharge outpatient visit cost was assumed to be a weighted average of those reported for single and multiprofessional visits of Service 320 – Cardiology under non-admitted face-to-face consultant-led follow-up attendance (TPCTCLFUSFF and TPCTCLFUMFF).
Battery replacement or failure and device malfunctions are assumed in the model to require a short admission to hospital to replace the device. As the battery is part of the generator unit of the device, its replacement is implied. Following the approach of Fox and colleagues,64 the cost of the procedure for battery replacement for an ICD was assumed to be the same as the cost of initial implantation (HRG code EA12Z), whereas that for CRT-P was assumed to the same as the cost of device explant (HRG code EA39Z). Clinical advice indicated that the cost of the procedure for battery replacement for CRT-D should be the same as that for an ICD.
Device-related total costs
Table 114 summarises the device-related total costs used in the economic model. These include the costs of device components and procedure by event.
| Event | Mean cost (£) | Lower value (£) | Upper value (£) | Components |
|---|---|---|---|---|
| Initial implant and reimplantation | ||||
| CRT-P | 8281 | 6098 | 11,895 | Whole system and implantation costs |
| CRT-D | 17,849 | 15,246 | 32,969 | |
| ICD | 15,248 | 13,155 | 29,858 | |
| Lead displacement/replacement | ||||
| CRT-P | 5681 | 4008 | 8786 | Lead and initial implantation costs |
| CRT-D | 6097 | 5798 | 18,914 | |
| ICD | 6099 | 5799 | 18,916 | |
| Battery failure/replacement | ||||
| CRT-P | 5348 | 3884 | 6974 | Generator and battery replacement costs (EA39Z) |
| CRT-D | 17,308 | 14,811 | 32,322 | Generator and battery replacement costs (EA12Z) |
| ICD | 14,705 | 12,718 | 29,209 | |
| Infection | ||||
| CRT-P | 12,553 | 7285 | 15,265 | Includes explant, reimplantation, extra bed-days and outpatient visits |
| CRT-D | 21,580 | 17,202 | 38,966 | |
| ICD | 18,977 | 15,109 | 35,853 | |
| Operative complicationsa | ||||
| CRT-P | 4884 | 2442 | 9768 | Includes device-related problems requiring replacement (initial implantation cost), complications requiring intervention (lead replacement cost) and infections (infection cost) |
| CRT-D | 6634 | 3317 | 13,268 | |
| ICD | 3432 | 1716 | 6864 | |
Drug costs
Patients with HF being managed with a device or with OPT alone receive a combination of drugs of several classes for this condition according to their NYHA class. The approach for estimation of drug use by NYHA class and of costs is similar to that taken by Fox and colleagues64 and the MS,151 in which a given proportion of patients in each NYHA class is assumed to consume a selected range of drugs. The drugs, daily doses and proportions chosen for our base-case analysis are those reported in the MS,151 based on the systematic review and expert opinion. These are presented in Table 115.
| Drug (mg/day) | Proportion of patients by NYHA class, % | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Atorvastatin (10) | 20 | 20 | 20 | 20 |
| Simvastatin (20) | 55 | 55 | 55 | 55 |
| Warfarin (1) | 10 | 15 | 25 | 40 |
| Clopidogrel (75) | 15 | 15 | 15 | 15 |
| Ramipril (10) | 90 | 90 | 90 | 90 |
| Carvedilol (25) | 85 | 85 | 75 | 70 |
| Spironolactone (25) | 0 | 30 | 30 | 30 |
| Digoxin (125)a | 5 | 25 | 25 | 25 |
| Furosemide (60) | 75 | 80 | 90 | 95 |
| Eplerenone (25) | 0 | 30 | 30 | 30 |
Unit costs for the selected drugs were derived from BNF 61. 219 The 4-week cycle cost was assumed to be that of the 28-tablet pack (assuming one tablet per day) for all drugs except furosemide, for which the cost of three packs of 28 tablets (20 mg) was used. The drug cost by NYHA class is presented in Table 116. The cost of OPT for population 1 patients without HF was assumed to be the same as that for NYHA class I patients.
| Drug (mg/day) | Cost (£) per 4 weeks by NYHA class | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Atorvastatin (10) | 0.38 | 0.38 | 0.38 | 0.38 |
| Simvastatin (20) | 0.50 | 0.50 | 0.50 | 0.50 |
| Warfarin (1) | 0.09 | 0.13 | 0.21 | 0.34 |
| Clopidogrel (75) | 0.35 | 0.35 | 0.35 | 0.35 |
| Ramipril (10) | 1.25 | 1.25 | 1.25 | 1.25 |
| Carvedilol (25) | 1.37 | 1.37 | 1.21 | 1.13 |
| Spironolactone (25) | 0 | 0.43 | 0.43 | 0.43 |
| Digoxin (125)a | 0.05 | 0.25 | 0.25 | 0.25 |
| Furosemide (60) | 1.8 | 1.92 | 2.16 | 2.28 |
| Eplerenone (25) | 0 | 12.82 | 12.82 | 12.82 |
| Total | 5.78 | 19.39 | 19.56 | 19.73 |
Results of the independent economic analysis
Population 1: patients at increased risk of sudden cardiac death as a result of ventricular arrhythmias despite optimal pharmacological therapy
Base-case analysis: implantable cardiac defibrillators for secondary prevention of sudden cardiac death
The AVID trial71 provided the estimates for all-cause mortality and the distribution of patients by NYHA class used for our base-case analysis of patients at increased risk of SCD as a result of ventricular arrhythmias, as it was the largest trial of patients who were resuscitated from near-fatal VF or symptomatic sustained VT with hemodynamic compromise. Appendix 15 presents all variables used in the model for the base-case analysis. The estimated base-case results for a mixed-gender cohort of 65-year-old patients are reported in Table 117 in terms of estimated costs and QALYs accrued for patients managed with OPT or ICD, as well as incremental costs and QALYs gained with ICD + OPT compared with OPT.
| Intervention | Cost (£) | Life-years | QALYs | Incremental cost (£) | Incremental life-years | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|---|---|
| OPT | 15,890 | 7.32 | 5.95 | – | – | – | – |
| ICD + OPT | 31,382 | 8.25 | 6.75 | 15,492 | 0.93 | 0.80 | 19,479 |
A gain of 0.80 QALYs (equivalent to 290 days in full health) is estimated for the addition of ICD to the management of patients at increased risk of SCD with OPT, at an incremental cost of £15,492 and an ICER of £19,479 per QALY gained.
The costs and QALYs estimated for each intervention are plotted in Figure 34.
FIGURE 34.
Cost-effectiveness plane: population 1.
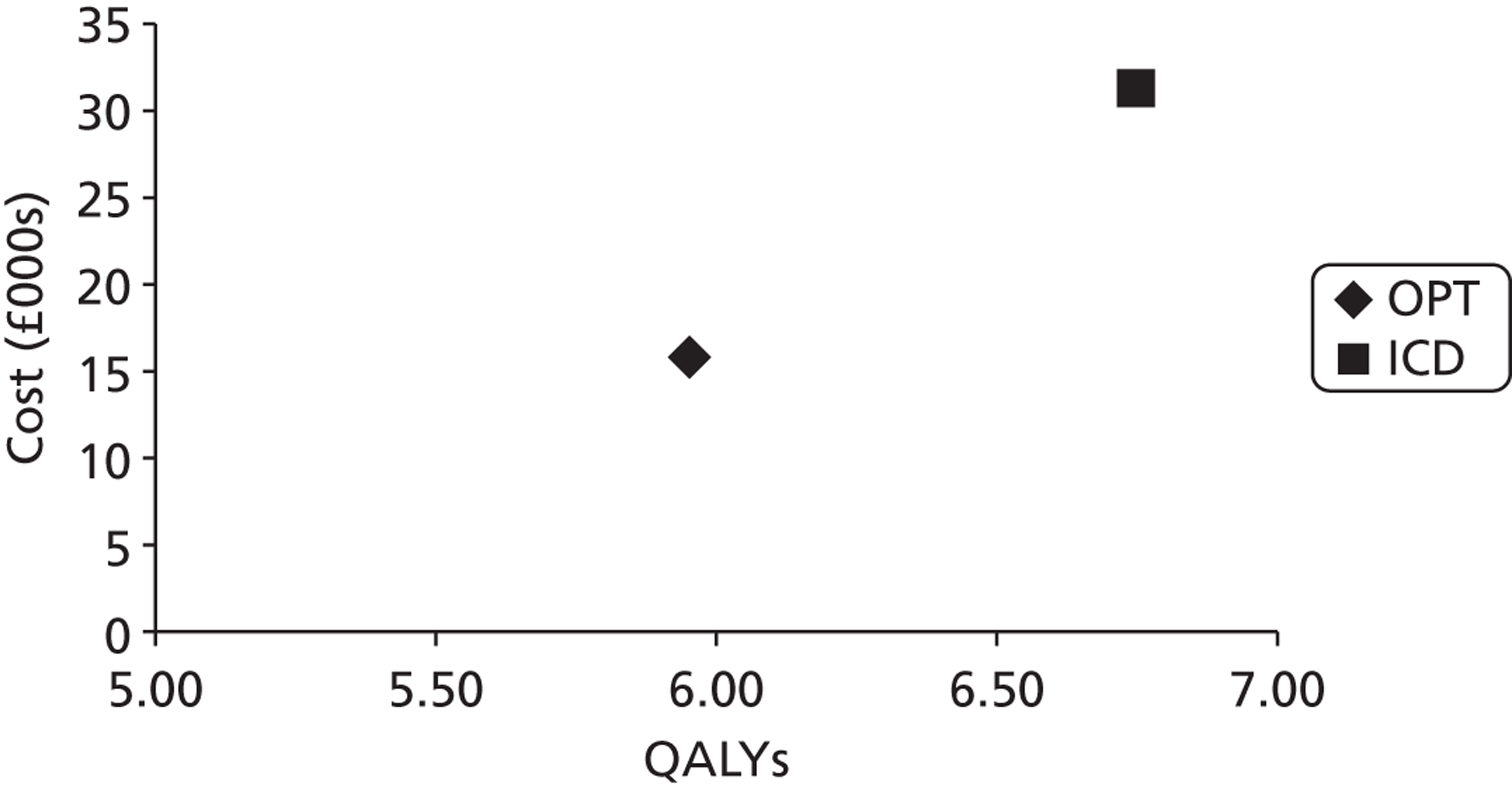
Overall survival estimated in the model was compared with that reported in the relevant trials (see Appendix 16 for details).
The number of major events estimated in the economic model for the base-case analysis is presented in Table 118 for both strategies being compared for population 1. Initially managing patients with OPT alone is estimated to lead to 454 ICD implants in patients hospitalised for a serious arrhythmic event and patients who are referred for an ICD following hospitalisation for HF. As the number of implanted patients in the OPT alone arm is much smaller than the number in the ICD + OPT arm, less replacements and complications requiring a new device are estimated for this arm. The risks of hospitalisation because of HF and arrhythmia are similar for patients being managed with OPT alone and patients being managed with ICD + OPT; thus, the numbers of these events are similar between arms as well.
| Event | Strategy | |
|---|---|---|
| OPT | ICD + OPT | |
| Initial implant | 0 | 1000 |
| Upgradeb | 454 | 0 |
| Implant reattemptc | 10 | 22 |
| Hospitalisation | 1966 | 2244 |
| Routine replacement | 541 | 921 |
| Postoperative complications | 58 | 114 |
| Lead displacement | 77 | 171 |
| Infection | 32 | 71 |
| Total no. of devicesd | 1037 | 2014 |
The percentage of time spent in the main health state categories by an average patient for each strategy is presented in Table 119. Patients in both arms spend most of their time in the stable with therapy health state, and the proportions were similar between arms. A reduced proportion of time was then spent in the device-related intervention and hospitalisation health states.
| Health state category | % of remaining life | |
|---|---|---|
| OPT | ICD + OPT | |
| Stable with therapy | 97.61 | 96.50 |
| OPT | 47.78 | 0.00 |
| ICD | 49.83 | 96.50 |
| Hospitalisation | 1.19 | 1.55 |
| Implant surgery | 0.37 | 0.71 |
| Routine replacement | 0.43 | 0.63 |
| Postoperative complications | 0.06 | 0.12 |
| Lead displacement | 0.05 | 0.08 |
| Infection | 0.03 | 0.05 |
| Device-related interventionsa | 0.93 | 1.59 |
Deterministic sensitivity analysis
Deterministic sensitivity analyses was undertaken to explore the effect of uncertainty related to key parameters and methodological and structural assumptions on the cost-effectiveness results. Scenario analyses were performed to explore modelling relevant population groups as well as using alternative utility estimates to derive QALYs. Univariate sensitivity analyses were also conducted on parameters expected a priori to be influential on results.
Cost-effectiveness results were estimated for a scenario of a mixed-age and mixed-gender cohort of patients eligible for ICD for the secondary prevention of SCD. The distribution of ICD implants by age in the UK reported by the British Cardiovascular Society, the British Heart Foundation and the Cardio and Vascular Coalition225 was used as a proxy for the distribution of patients at increased risk of SCD as a result of ventricular arrhythmia. Age-dependent variables in the population 1 model were those that determined all-cause mortality (baseline risk and RR of death by age group). Table 120 shows the results for the mixed-age cohort and per age group.
| Starting age (years) | OPT costs (£) | ICD costs (£) | OPT QALYs | ICD QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| 30 | 27,207 | 43,410 | 9.74 | 10.69 | 17,083 |
| 40 | 25,982 | 41,968 | 9.33 | 10.23 | 17,856 |
| 50 | 23,535 | 39,238 | 8.54 | 9.35 | 19,228 |
| 60 | 16,947 | 32,673 | 6.29 | 7.15 | 18,182 |
| 70 | 14,268 | 29,361 | 5.41 | 6.12 | 21,298 |
| 80 | 9681 | 24,129 | 3.85 | 4.36 | 28,211 |
| 90 | 5382 | 18,232 | 2.40 | 2.45 | 288,611 |
| Mixed | 16,559 | 31,838 | 6.17 | 6.91 | 24,967 |
Overall, the ICER increases with age as the QALY gain with ICD + OPT decreases compared with OPT alone as the decrement in incremental benefits from treatment over time is steeper than that for incremental costs. The ICER of £24,967 per QALY gained for the mixed-age cohort shows that ICD + OPT is within the WTP range of £20,000–30,000 per QALY gained.
The MADIT II trial101 was the trial with the largest number of patients with a remote MI and was considered representative of a relevant group who might benefit from ICD therapy for the primary prevention of SCD. Cost-effectiveness results for the subgroup analysis of patients with a remote MI, using MADIT II all-cause mortality for a cohort of 64-year-old patients and a pooled RR of 0.57 (effect of ICD + OPT on all-cause mortality relative to OPT), are presented in Table 121.
| Intervention | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|
| OPT | 14,783 | 6.77 | 5.17 | – |
| ICD + OPT | 31,583 | 8.36 | 6.35 | 14,231 |
An increment of 1.18 QALYs per patient is estimated using ICD + OPT for the primary prevention of SCD at an additional cost of £16,800. The health benefit estimated from using ICD + OPT for the primary prevention of SCD in patients remote from their MI instead of OPT alone is greater than that for secondary prevention, in accordance with the lower pooled RR (0.57) estimated for patients with a remote MI than that used in the base-case analysis (RR = 0.75). The estimated ICER for this patient group is £14,231 per QALY gained.
The all-cause mortality rate in the placebo arm, the RR for ICDs of 0.77 (95% CI 0.66 to 0.89) and the distribution of patients by NYHA class from the SCD-HeFT trial105 were used to inform an analysis of 60-year-old patients with mild to moderate HF with an indication for an ICD. Table 122 shows the cost-effectiveness results for this subgroup analysis.
| Intervention | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|
| OPT | 17,760 | 7.84 | 5.79 | – |
| ICD | 32,416 | 8.51 | 6.28 | 29,756 |
An additional benefit of 0.49 QALYs (approximately 180 days in full health) is estimated for ICD + OPT for the primary prevention of SCD in patients with mild to moderate HF at an additional cost of £14,655 compared with OPT alone. The estimated ICER for this subgroup of patients (£29,756 per QALY gained) is just below the WTP threshold of £30,000 per QALY gained.
The two cohorts initially managed with OPT alone or ICD + OPT for the primary prevention of SCD showed higher costs and slightly longer life expectancy than in the base-case analysis (secondary prevention of SCD). However, given the greater severity of HF in these patients (see distribution by NYHA class in Data sources and parameter estimates, Population 1), both cohorts gained fewer QALYs than secondary prevention patients (base-case analysis).
The all-cause mortality rate reported for the SCD-HeFT105 subgroup of patients with non-ischaemic CHF in the placebo arm was used as the baseline mortality rate for a subgroup analysis of 60-year-old patients with cardiomyopathy. The mortality preventative effect of ICDs was incorporated using a pooled RR of 0.74 (95% CI 0.58 to 0.93) from the non-ischaemic subgroup of the SCD-HeFT,105 AMIOVIRT,69 CAT82 and DEFINITE90 trials. The SCD-HeFT105 distribution of patients by NYHA class was also used. Table 123 reports the estimated cost-effectiveness results for this subgroup.
| Intervention | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|
| OPT | 24,845 | 10.59 | 7.83 | – |
| ICD | 40,218 | 11.39 | 8.42 | 26,028 |
The primary prevention of SCD with ICD + OPT in patients with cardiomyopathy is expected to cost £15,373 more than initial prevention with OPT alone and subsequent implantations for an incremental benefit of 0.59 QALYs (216 days in full health). Compared with the base case (secondary prevention of SCD), both treatment strategies for patients with cardiomyopathy have a higher cost and provide a greater benefit (about £9000 more for 1.67 or 1.88 QALYs more with ICD + OPT or OPT alone respectively) over a lifetime. The ICER estimated for the cardiomyopathy subgroup is £26,028 per QALY.
Table 124 shows the results of the univariate sensitivity analyses conducted on key inputs in the model, allowing the estimation of their impact on the cost-effectiveness results. The range used for most parameters was their 95% CI.
| ∫ Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 15,492 | 0.80 | 19,479 |
| Structural parameters | |||||
| Time horizon | Lifetime | AVID trial follow-up (3 years)71 | 13,330 | 0.09 | 141,235 |
| Discount rates of costs and benefits (%) | 3.5, 3.5 | 0, 0 | 16,836 | 1.18 | 14,271 |
| 6, 1.5 | 14,908 | 0.99 | 15,069 | ||
| Survival and HRs | |||||
| Baseline all-cause mortality, ln(λ), γ | –3.381, 0.696 | –3.431, 0.678 | 15,496 | 0.78 | 19,854 |
| –0.330, 0.714 | 15,449 | 0.80 | 19,416 | ||
| All-cause mortality HR (ICDs) | 0.75 | 0.61 | 17,126 | 1.37 | 12,480 |
| 0.93 | 13,772 | 0.18 | 78,268 | ||
| Age-related RR of death, > 75 years | 1.41 | 1 | 15,551 | 0.81 | 19,241 |
| 2 | 15,367 | 0.76 | 20,137 | ||
| Event probabilities | |||||
| Risk of hospitalisation for HF (OPT) | 0.008 | 0 | 15,251 | 0.79 | 19,197 |
| 0.020 | 15,869 | 0.80 | 19,920 | ||
| RR of hospitalisation for HF (ICDs) | 1 | 0.804 | 15,262 | 0.80 | 19,184 |
| 1.196 | 15,723 | 0.80 | 19,773 | ||
| Risk of implantation following HF hospitalisation | 0.002 | 0 | 15,506 | 0.80 | 19,484 |
| 0.006 | 15,461 | 0.79 | 19,466 | ||
| Risk of surgical death (ICDs) | 0.003 | 0 | 15,491 | 0.82 | 18,950 |
| 0.055 | 15,507 | 0.48 | 32,605 | ||
| Risk of surgical death (transplant) | 0.122 | 0.109 | 15,492 | 0.80 | 19,476 |
| 0.136 | 15,492 | 0.80 | 19,481 | ||
| Risk of surgical failure | 0.011 | 0.009 | 15,464 | 0.80 | 19,442 |
| 0.013 | 15,521 | 0.80 | 19,516 | ||
| Risk of perioperative complications | 0.053 | 0.046 | 15,469 | 0.80 | 19,448 |
| 0.062 | 15,523 | 0.80 | 19,518 | ||
| Risk of lead infections | 0.0005 | 0.0004 | 15,371 | 0.80 | 19,321 |
| 0.0006 | 15,614 | 0.80 | 19,636 | ||
| Risk of lead displacements | 0.0012 | 0.001 | 15,415 | 0.80 | 19,372 |
| 0.0014 | 15,570 | 0.80 | 19,585 | ||
| Device lifetime, ln(λ), γ | –15.78, 1.94 (∼ 8 years) | –16.182, 1.889 (∼13 years) | 13,158 | 0.80 | 16,456 |
| –15.385, 1.996 (∼5 years) | 19,467 | 0.79 | 24,706 | ||
The univariate sensitivity analyses for the structural parameters did not result in large changes to the ICER, apart from that for the model time horizon. The only analysis that increased the ICER to > £30,000 per QALY gained was that in which the time horizon was shortened to the survival follow-up period reported in the AVID trial71 (as very few health benefits are accrued over that time period compared with the incremental cost of ICD implantation).
Among the mortality-related estimates, the model results showed particular sensitivity to the HR for all-cause mortality associated with the ICD + OPT arm, more than tripling to £78,268 per QALY gained when the upper limit of the HR (0.93) was used.
The event-related estimates that had the greatest impact on the ICER were the risk of surgical death during ICD implantation and the device lifetime. When the risk of death from ICD surgery was varied according to the 95% CI the ICER ranged from £18,950 to £32,605 per QALY gained, and when the device lifetime was changed from 8 years to 13 years or 5 years the ICER ranged from £16,456 to £24,706 per QALY gained respectively.
There is limited reporting of the number of hospitalisations for non-fatal arrhythmia in the trials included in our systematic review for population 1 (patients at increased risk of SCD). Following clinical advice, our base-case analysis assumes the same risk as that for patients with HF (probability of event occurring 0.0075, 95% CI 0.0002 to 0.0148), derived from the MIRACLE trial. 121 As this is likely to be an underestimate of the risk for population 1 patients, a scenario analysis was conducted using the risk of hospitalisation for ventricular arrhythmia for population 3 patients with an ICD (also at increased risk of SCD as a result of ventricular arrhythmia).
In the population 3 model, the risk of hospitalisation because of arrhythmia for patients with an ICD is 0.032 (probability of event occurring; 95% CI 0.017 to 0.046), obtained by applying the pooled RR of 1.11 to the baseline risk of patients managed with CRT-D (0.029) derived in Chapter 4 (see People with both conditions, Assessment of effectiveness, Arrhythmias). For the population 1 scenario, the risk of hospitalisation because of arrhythmia was assumed to be 0.032 for patients with an ICD and for patients being managed with OPT alone. Table 125 summarises the cost-effectiveness results for this scenario. Compared with the base-case analysis, a slightly lower ICER (£18,185 per QALY gained) is estimated using a higher risk of hospitalisation for arrhythmia, as the OPT arm shows a substantial gain in QALYs compared with the ICD + OPT arm, despite the greater increase in cost.
| Intervention | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|
| OPT | 29,759 | 7.78 | 6.34 | – |
| ICD | 37,120 | 8.26 | 6.74 | 18,185 |
In the base-case analysis, NYHA class-weighted average utility estimates of 0.81 and 0.82 were used for the OPT arm and the ICD arm, respectively, using the distribution of patients per NYHA class in the AVID trial. 71 A scenario analysis was conducted using a mean utility estimate of 0.75 irrespective of NYHA class and treatment arm as per Buxton and colleagues. 153 This lower average utility value led to an estimated 0.69 QALY gain (instead of the 0.80 QALY gain estimated for the base case). Therefore, the ICER of ICD + OPT compared with OPT alone for the secondary prevention of SCD increased to £22,372 per QALY gained.
All device-related costs (i.e. costs associated with implantation, perioperative complications, treatment of lead displacement, infection and device replacement) were varied to the lower and upper limits of their 95% CI, for example the ICD implantation cost was reduced by 14% to £13,155. For this scenario the ICER ranged from £16,888 to £37,832 per QALY gained.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was performed for the base case to estimate the impact of joint parameter uncertainty on the model’s cost-effectiveness results. Appendix 15 reports the variables (mean values and CIs) included in the probabilistic sensitivity analysis, the form of distribution used for sampling and the parameters of the distribution. Probabilistic sensitivity analysis results of 10,000 iterations are presented in Figure 35 in terms of cost and QALYs for each strategy. The probabilistic mean ICER is £20,479 per QALY gained [interquartile range (IQR) £9857 to £61,685 per QALY gained].
FIGURE 35.
Cost-effectiveness scatterplot: population 1.
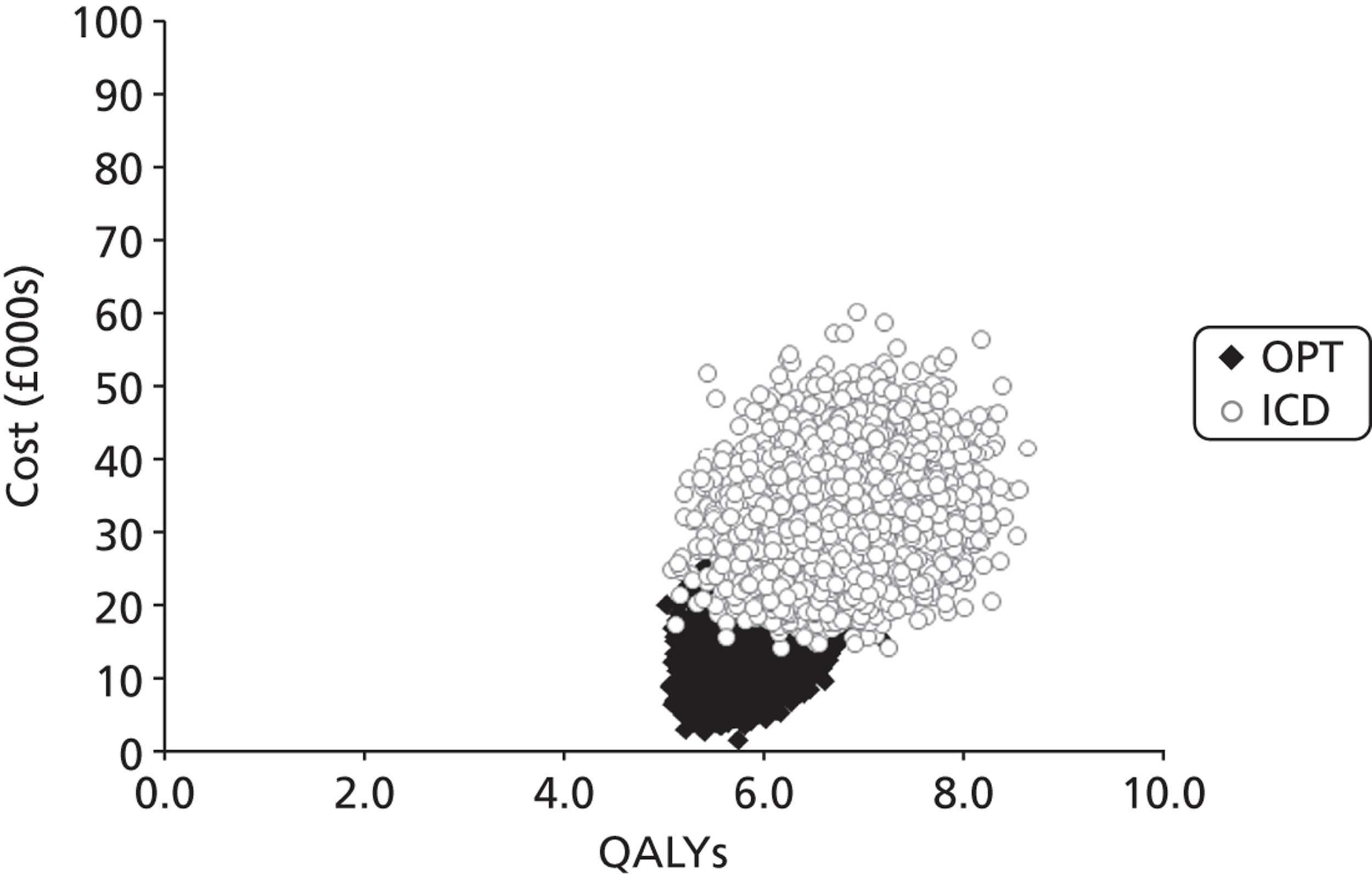
Figure 36 shows the variation in the probability of cost-effectiveness for both interventions as the WTP threshold increases from £0 to £50,000 per QALY gained. The addition of ICD to OPT for SCD secondary prevention has a 51% probability of being cost-effective at a WTP threshold of £20,000 per QALY gained and a 82% probability of being cost-effective at a WTP threshold of £30,000 per QALY gained.
FIGURE 36.
Cost-effectiveness acceptability curve: population 1.
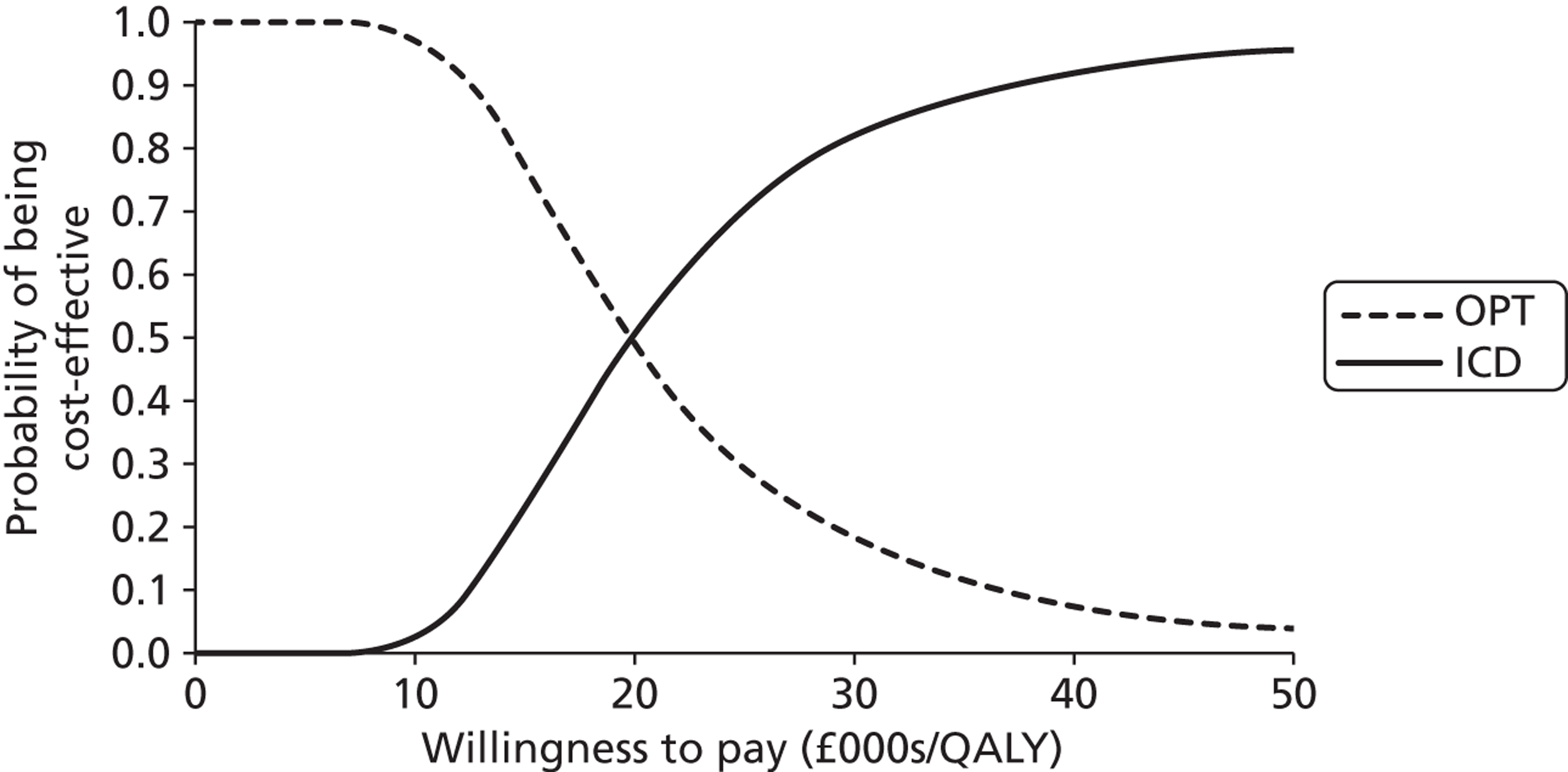
Population 2: patients with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony despite receiving optimum pharmacological therapy
People with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT were modelled receiving initially OPT alone or CRT-P or CRT-D alongside OPT. This allowed the estimation of the relative cost-effectiveness of these treatment strategies, and results for the comparisons specified in the NICE scope61 (CRT-P + OPT vs. OPT, CRT-D + OPT vs. OPT, and CRT-D + OPT vs. CRT-P + OPT) are given in this section.
Base-case analysis
For our base-case analysis, a 70-year-old mixed-gender cohort of patients with HF was modelled receiving the relevant treatment strategies. Table 126 presents the estimated discounted costs, life-years and QALYs accrued for patients managed with OPT, CRT-P + OPT or CRT-D + OPT as well as the incremental cost per QALY gained for the relevant comparisons.
| Strategy | Cost (£) | Life-years | QALYs | Incremental cost (£) | Incremental life-years | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|---|---|
| Vs. next best optiona | |||||||
| OPT | 7615 | 4.86 | 3.48 | – | – | – | – |
| CRT-P + OPT | 26,460 | 5.51 | 4.17 | 18,845 | 0.66 | 0.68 | 27,584 |
| CRT-D + OPT | 38,163 | 7.21 | 4.58 | 11,703 | 1.69 | 0.41 | 28,420 |
| Vs. OPT | |||||||
| CRT-D + OPT | 38,163 | 7.21 | 4.58 | 30,548 | 2.35 | 1.09 | 27,899 |
The ICERs for initial management with CRT-P or CRT-D alongside OPT compared with initial management with OPT alone were similar (£27,584 and £27,899 per QALY gained respectively). The addition of CRT-P to OPT results in a gain of 0.68 QALYs at a cost of £18,845 compared with OPT, and the addition of CRT-D to OPT yields a gain of 1.09 QALYs at a cost of £30,548 compared with OPT. CRT-D + OPT was more costly (by £11,703) and more effective (resulting in an increase of 0.41 QALYs) than CRT-P + OPT, resulting in an ICER of £28,420 per QALY gained compared with CRT-P + OPT. The costs and QALYs estimated for each intervention are plotted in Figure 37.
FIGURE 37.
Cost-effectiveness plane: population 2.
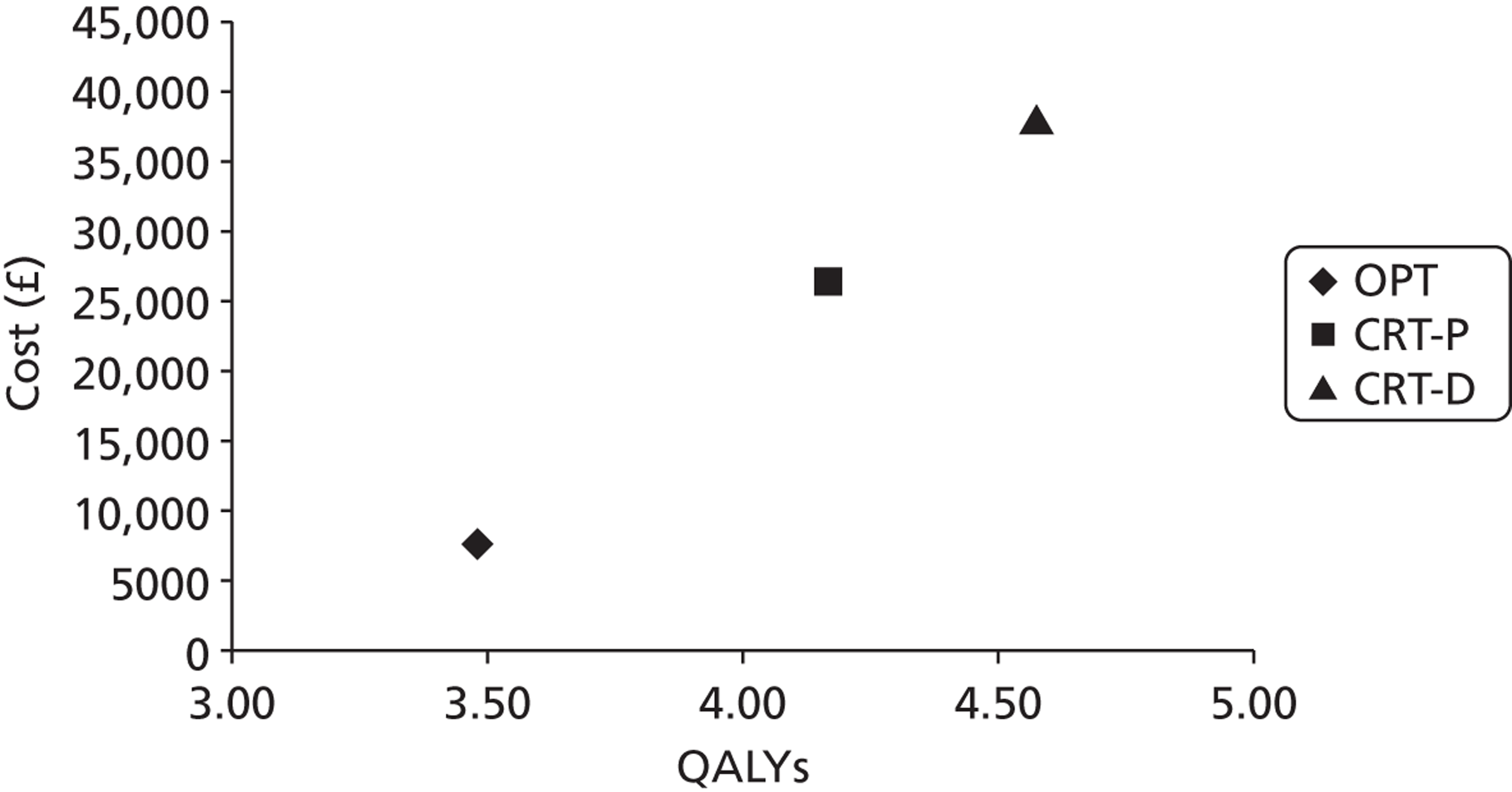
Heart failure deaths and SCDs estimated in the model were compared with those reported in the CARE-HF trial109 (see Appendix 16 for details).
The percentage of time spent in the main health states by an average patient in each strategy is presented in Table 127. Patients in each strategy spent most time in the stable with therapy health state. The cohort initially managed with OPT alone spent slightly more time in the stable with therapy health state, but it is also the strategy with the highest proportion of remaining life spent in hospital. The CRT cohorts spent slightly less time hospitalised; however, they spent more time in the device-related intervention health state (i.e. because of implant surgery, postoperative complications, routine upgrades, lead displacements and infections). About 27% of the lifetime of patients initially managed with CRT-P + OPT was spent stable with a CRT-D device as result of the upgrade.
| Health state category | % of remaining life | ||
|---|---|---|---|
| OPT | CRT-P + OPT | CRT-D + OPT | |
| Stable with therapy | 95.15 | 94.17 | 93.44 |
| OPT | 93.85 | 7.90 | 0.15 |
| CRT-P | 0.54 | 55.86 | 0 |
| CRT-D | 0.67 | 26.86 | 83.06 |
| ICD | 0.09 | 3.54 | 10.24 |
| Hospitalisation | 4.22 | 2.80 | 3.63 |
| OPT | 4.18 | 0.36 | 0.01 |
| CRT-P | 0.01 | 1.26 | 0 |
| CRT-D | 0.03 | 1.02 | 3.14 |
| ICD | 0.00 | 0.17 | 0.48 |
| Implant surgery | 0.03 | 1.70 | 1.24 |
| Routine replacement | 0.01 | 0.32 | 0.56 |
| Lead displacement | 0.00 | 0.33 | 0.34 |
| Postoperative complications | 0.00 | 0.25 | 0.22 |
| Infection | 0.00 | 0.06 | 0.06 |
| Device-related interventionsa | 0.05 | 2.65 | 2.42 |
Table 128 shows the number of events for each cohort of population 2 patients. The cohorts initially managed with CRT alongside OPT (CRT-P + OPT or CRT-D + OPT) are estimated to require a similar total number of devices (comprising initial implants, upgrades, infections and replacements) over a lifetime. Although the CRT-P + OPT group required fewer device replacements than the CRT-D + OPT group given the longer lifetime of CRT-P, more upgrades were needed than in the CRT-D + OPT arm. The 228 ICDs reported as upgrades from CRT-D in the CRT-D + OPT strategy in Table 128 are assumed to be successful ICD implants after CRT-D implant failures.
| Event | Strategy | ||
|---|---|---|---|
| OPT | CRT-P + OPT | CRT-D + OPT | |
| Initial implant | 0 | 1000 | 1000 |
| ICD | 0 | 0 | 0 |
| CRT-P | 0 | 1000 | 0 |
| CRT-D | 0 | 0 | 1000 |
| Hospitalisation | 3043 | 2349 | 3385 |
| OPT | 3013 | 299 | 6 |
| CRT-P | 9 | 1057 | 0 |
| CRT-D | 18 | 854 | 2929 |
| ICD | 3 | 140 | 450 |
| Upgrade | 20 | 421 | 156 |
| ICD | 1 | 58 | 156 |
| CRT-P | 10 | 1 | 0 |
| CRT-D | 8 | 362 | 0 |
| Surgical complications | 3 | 208 | 204 |
| ICD | 0 | 5 | 13 |
| CRT-P | 1 | 132 | 0 |
| CRT-D | 2 | 71 | 191 |
| Lead displacement | 3 | 275 | 315 |
| ICD | 0 | 4 | 12 |
| CRT-P | 2 | 183 | 0 |
| CRT-D | 2 | 88 | 303 |
| Infection | 0.6 | 46.3 | 55.7 |
| ICD | 0.0 | 1.6 | 5.1 |
| CRT-P | 0.3 | 29.9 | 0.0 |
| CRT-D | 0.3 | 14.8 | 50.7 |
| Replacement | 6.6 | 269.3 | 523.9 |
| ICD | 0.7 | 29.6 | 66.7 |
| CRT-P | 1.1 | 32.6 | 0.0 |
| CRT-D | 4.8 | 207.2 | 457.2 |
| Total no. of devicesb | 27 | 1737 | 1736 |
| ICD | 2 | 89 | 228 |
| CRT-P | 11 | 1063 | 0 |
| CRT-D | 14 | 584 | 1508 |
Deterministic sensitivity analysis
The effect of uncertainty related to key parameters and methodological and structural assumptions on the cost-effectiveness results was explored through subgroup, univariate and scenario analyses.
Cost-effectiveness results were estimated for a scenario of a mixed-age and mixed-gender cohort of patients with HF. The distribution of patients with HF by age group reported by Cowie and colleagues20 was used, and the proportion of men with HF was derived from the prevalence of HF per sex in the UK from British Heart Foundation Statistics. 29 Age-dependent variables in the population 2 model were those that determined SCD, HF death, other-cause mortality and RR of death by age group.
The model results for different starting ages are detailed in Table 129. These results show that the ICER increases non-linearly with age and the ICERs of the three comparisons are consistently similar among age groups. For most age groups, CRT-P + OPT compared with OPT alone is the strategy with the lowest ICER and CRT-D + OPT compared with CRT-P + OPT is the strategy with the highest ICER. The exception is for 80-year-old patients, for whom the opposite is estimated to occur, as CRT-D + OPT compared with CRT-P + OPT shows a smaller QALY gain (0.33) at a lower cost (£10,757) than that estimated for CRT-P + OPT relative to OPT alone (0.49 QALYs gained at a cost of £16,000).
| Starting age (years) | Strategy | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. OPT | ICER (£/QALY gained) vs. CRT-P + OPT |
|---|---|---|---|---|---|---|
| 30 | OPT | 12,614 | 7.98 | 5.77 | – | – |
| CRT-P + OPT | 40,482 | 9.30 | 7.05 | 21,678 | – | |
| CRT-D + OPT | 54,997 | 15.65 | 7.69 | 22,065 | 22,848 | |
| 40 | OPT | 12,419 | 7.80 | 5.63 | – | – |
| CRT-P + OPT | 39,572 | 9.00 | 6.82 | 22,870 | – | |
| CRT-D + OPT | 53,849 | 13.44 | 7.40 | 23,413 | 24,519 | |
| 50 | OPT | 11,862 | 7.47 | 5.39 | – | – |
| CRT-P + OPT | 37,713 | 8.51 | 6.45 | 24,444 | – | |
| CRT-D + OPT | 51,531 | 12.17 | 6.97 | 25,106 | 26,447 | |
| 60 | OPT | 10,081 | 6.39 | 4.60 | – | – |
| CRT-P + OPT | 32,755 | 7.22 | 5.47 | 26,029 | – | |
| CRT-D + OPT | 45,486 | 9.76 | 5.91 | 26,953 | 28,771 | |
| 70 | OPT | 7615 | 4.86 | 3.48 | – | – |
| CRT-P + OPT | 26,460 | 5.51 | 4.17 | 27,584 | – | |
| CRT-D + OPT | 38,163 | 7.21 | 4.58 | 27,899 | 28,420 | |
| 80 | OPT | 5882 | 3.77 | 2.69 | – | – |
| CRT-P + OPT | 21,882 | 4.23 | 3.18 | 32,656 | – | |
| CRT-D + OPT | 32,639 | 5.33 | 3.52 | 32,598 | 32,511 | |
| 90 | OPT | 4075 | 2.64 | 1.87 | – | – |
| CRT-P + OPT | 16,509 | 2.78 | 2.08 | 61,057 | – | |
| CRT-D + OPT | 25,261 | 3.15 | 2.20 | 64,917 | 71,322 | |
| Mixed | OPT | 8218 | 5.23 | 3.75 | – | – |
| CRT-P + OPT | 28,016 | 5.91 | 4.47 | 28,928 | – | |
| CRT-D + OPT | 39,932 | 7.93 | 4.88 | 29,416 | 30,321 |
Tables 130–132 present the results of the deterministic sensitivity analyses of the most influential parameters for each of the relevant comparisons (i.e. those that when varied between the 95% CI limits caused a variation > £10,000 per QALY in the ICER). The other parameters were varied but had a smaller impact on the results.
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 18,845 | 0.68 | 27,584 |
| Risk of hospitalisation for non-fatal arrhythmia (CRT-P) | 0.0075 | 0.0002 | 8765 | 0.56 | 15,780 |
| 0.0148 | 24,169 | 0.76 | 31,978 | ||
| RR of HF death (CRT-P) | 0.67 | 0.51 | 19,575 | 0.84 | 23,307 |
| 0.88 | 17,993 | 0.50 | 36,019 | ||
| RR of HF death (CRT-D) | 0.73 | 0.47 | 19,788 | 0.84 | 23,522 |
| 1.11 | 17,836 | 0.51 | 34,720 | ||
| RR of SCD (CRT-P) | 1 | 0.54 | 20,471 | 1.03 | 19,825 |
| 1.13 | 18,443 | 0.60 | 30,925 |
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 30,548 | 1.09 | 27,899 |
| RR of HF death (CRT-D) | 0.73 | 0.47 | 33,541 | 1.62 | 20,671 |
| 1.11 | 27,381 | 0.53 | 52,082 | ||
| RR of SCD (CRT-D) | 0.44 | 0.23 | 32,147 | 1.38 | 23,283 |
| 0.86 | 27,962 | 0.63 | 44,659 | ||
| Device lifetime (CRT-D), ln(λ), γ | –15.465, 1.935 (∼7 years) | –16.000, 1.863 (∼13 years) | 25,309 | 1.12 | 22,643 |
| –14.931, 2.006 (∼4 years) | 39,322 | 1.05 | 37,363 |
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 11,703 | 0.41 | 28,420 |
| RR of HF death (CRT-D) | 0.73 | 0.47 | 13,754 | 0.78 | 17,602 |
| 1.11 | 9545 | 0.01 | 793,839 | ||
| RR of SCD (CRT-P) | 1 | 0.54 | 10,063 | 0.06 | 169,196 |
| 1.13 | 12,108 | 0.50 | 24,250 | ||
| RR of SCD (CRT-D) | 0.44 | 0.23 | 12,817 | 0.62 | 20,180 |
| 0.86 | 9912 | 0.08 | 129,220 | ||
| Device lifetime (CRT-D), ln(λ), γ | –15.465, 1.935 (∼7 years) | –16, 1.863 (∼13 years) | 8608 | 0.43 | 20,238 |
| –14.931, 2.006 (∼4 years) | 17,811 | 0.38 | 46,640 | ||
| RR of HF death (CRT-P) | 0.67 | 0.51 | 10,966 | 0.25 | 43,231 |
| 0.88 | 12,563 | 0.60 | 21,042 | ||
| Risk of hospitalisation for non-fatal arrhythmia (CRT-P) | 0.0075 | 0.0002 | 21,857 | 0.54 | 40,450 |
| 0.0148 | 6335 | 0.34 | 18,707 | ||
| Baseline mortality from HF, ln(λ), γ | –6.115, 1.223 | –6.253, 1.180 | 12,546 | 0.52 | 24,157 |
| –5.977, 1.265 | 10,864 | 0.31 | 35,220 | ||
| Baseline mortality from SCD, ln(λ), γ | –6.069, 1.140 | –6.173, 1.107 | 11,460 | 0.33 | 34,318 |
| –5.964, 1.173 | 11,924 | 0.49 | 24,316 |
Table 130 shows that the risk of hospitalisation for a serious arrhythmic event for HF patients managed with CRT-P, the RRs of HF death for patients managed with CRT-P and CRT-D, and the RR of SCD for HF patients managed with CRT-P have the most impact on the cost-effectiveness results for the comparison between CRT-P + OPT and OPT alone as initial treatment.
The results for this comparison are particularly sensitive to the risk of hospitalisation for non-fatal arrhythmia for HF patients managed with CRT-P, as the ICER decreases to £15,780 per QALY gained when the lower limit of the 95% CI of the estimate is used. On the other hand, the ICER rises to £31,978 per QALY gained when the upper limit of the 95% CI is used, as the cost of the CRT-P + OPT cohort increases substantially whereas that for the OPT alone cohort stays the same. Patients being managed with CRT-P who are hospitalised because of arrhythmias are assumed to be referred for CRT-D implantation. The cost increment for the CRT-P cohort is hence accompanied by a small health gain.
The RR of SCD for patients managed with CRT-P was varied between the RRs reported in the CARE-HF109 and COMPANION116 trials, as these indicate a relative effect in opposite directions. The ICER for CRT-P + OPT compared with OPT alone decreases to £19,825 per QALY gained when the RR of SCD for patients managed with CRT-P from the CARE-HF trial109 (0.54) is used, that is, when CRT-P is assumed to considerably reduce the risk of SCD. A cost of £30,925 per QALY gained is estimated when the RR from the COMPANION trial116 (1.13) is used, a scenario in which CRT-P would increase the risk of SCD.
Generally, the results for the addition of CRT-D to OPT were robust to the variation of most of the parameters (see Table 131) compared with the results for the other two comparisons (CRT-P + OPT vs. OPT and CRT-D + OPT vs. CRT-P + OPT). They were mainly sensitive to the RR of HF death and the RR of SCD for patients managed with CRT-D, and to the lifetime of the CRT-D device, confirming that the cost-effectiveness of the addition of CRT-D to OPT is determined by the survival benefit associated with this device. The most influential parameter for this comparison was the RR of HF death associated with CRT-D (0.73). Varying the RR between 0.47 and 1.11 resulted in the ICER ranging between £20,671 and £52,082, respectively, a difference of £31,411. When the upper limit of this estimate is considered (1.11), the preventative benefit of CRT-D for HF death disappears and the ICER for CRT-D + OPT compared with OPT alone rises to > £50,000 per QALY gained.
The results for the comparison between CRT-D and CRT-P alongside OPT were the most sensitive to the variation of individual parameters, with eight parameters that made the ICER range by > £10,000 (see Table 132). The most influential parameter for this comparison was the RR of HF death for patients managed with CRT-D compared with OPT alone, followed by the RRs of SCD for both CRT-D and CRT-P devices relative to OPT alone.
The estimate of the RR of HF death for patients managed with CRT-D was sourced from the COMPANION trial116 (RR 0.73, 95% CI 0.47 to 1.11). When a higher risk of HF death for CRT-D than for OPT alone is assumed (RR = 1.11), the incremental benefit of CRT-D + OPT is almost null relative to CRT-P + OPT (0.01), resulting in an extremely high ICER.
The ICER for CRT-D + OPT compared with CRT-P + OPT also becomes extremely high when the RR of SCD for patients managed with CRT-P is changed to the lowest limit. The pooled RR of SCD for CRT-P patients of 0.97 (95% CI 0.44 to 2.14) was obtained in the meta-analysis reported in Chapter 4 (see People with heart failure, Assessment of effectiveness, Sudden cardiac death). Given its wide 95% CI, a RR of 1 was used in the model and this value was varied in the sensitivity analysis between the mean estimates of RR reported in the most relevant trials (0.54 from the CARE-HF trial109 and 1.13 from the COMPANION trial). 116 Under the CARE-HF trial scenario, the preventative effect of CRT-P on SCD becomes higher than that of CRT-D, that is, the incremental benefit of CRT-D + OPT relative to CRT-P + OPT (0.06) is much smaller than in the base case (0.41).
Similarly, if the RR of SCD for patients managed with CRT-D is increased to 0.86 (the upper limit of its 95% CI, sourced from the COMPANION trial),116 only 0.08 incremental QALYs are estimated for CRT-D + OPT compared with CRT-P + OPT, resulting in a particularly high ICER.
Varying the life expectancy of the CRT-D device, the RR of HF death for patients managed with CRT-P and the risk of hospitalisation for severe arrhythmia for patients managed with CRT-P also had a substantial influence on the ICER, making it range by > £20,000. The ICER for CRT-D + OPT compared with CRT-P + OPT decreased substantially when a longer device lifetime was used (13 years) for CRT-D, the RR of HF death with CRT-P was increased or the risk of hospitalisation for arrhythmia with CRT-P was higher.
Overall, the ICERs for the comparisons relevant for population 2 are sensitive mainly to survival-related parameters that determine the incremental benefit of the devices for patient survival, such as the RRs of SCD and HF death for CRT-P and CRT-D, the risk of hospitalisation because of arrhythmia for CRT-P and the lifetime of the device for CRT-D. Device lifetime was also influential because of the incremental costs incurred if a device needs replacing more frequently.
Clinical advice indicated that the device longevity estimates used in the base-case analysis could be overestimated, particularly for CRT-P. Table 133 presents the device lifetime estimates used in the previous model by Fox and colleagues64 and those used in the current model.
| Device | Lifetime (years) | |
|---|---|---|
| Fox et al.,64 mean | SHTAC, mean (95% CI) | |
| ICD | 5.0 | 8.2 (5.4 to 12.8) |
| CRT-D | 5.5 | 7.2 (4.1 to 13.1) |
| CRT-P | 6.5 | 11.8 (6.6 to 22.2) |
A scenario analysis was conducted using the mean device lifetime estimates used by Fox and colleagues. 64 The results for this scenario are presented in Table 134. Compared with the base-case analysis, higher costs are estimated for CRT-D and CRT-P alongside OPT because of the shorter device lifetime (by approximately £4500 and £2000 respectively). Also, slightly fewer QALYs (–0.02) are estimated to be accrued than in the base-case analysis as patients are estimated to spend more time in the device-related interventions health state and less time stable with therapy.
| Strategy | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. OPT | ICER (£/QALY gained) vs. CRT-P + OPT |
|---|---|---|---|---|---|
| OPT | 7652 | 4.86 | 3.48 | – | – |
| CRT-P + OPT | 28,555 | 5.50 | 4.15 | 31,334 | – |
| CRT-D + OPT | 42,627 | 7.18 | 4.56 | 32,505 | 34,416 |
A scenario using the utility estimates from the study by Fox and colleagues64 (presented in Table 135) was explored. The utility estimates used in the base-case analysis can be found in Table 108.
| Health state | Mean utility value | Source |
|---|---|---|
| NYHA class I | 0.93 | Kirsch and McGuire 2000210 |
| NYHA class II | 0.78 | Kirsch and McGuire 2000210 |
| NYHA class III | 0.61 | Calvert et al.211 |
| NYHA class IV | 0.44 | Calvert et al.211 |
| Hospitalisation and transplantation | 0.57 | McAllister et al.194 |
| Decrement from surgery | 0.05 | Assumption |
| Decrement from infection | 0.1 | Assumption |
Table 136 shows the cost-effectiveness results for this scenario, with the same costs per strategy as those estimated for the base-case analysis. In this scenario, fewer QALYs (–0.09) were estimated for OPT alone and more QALYs were estimated for the CRT strategies (+0.04 and +0.05 for CRT-P and CRT-D respectively). The lower ICERs presented in this scenario for the comparisons between CRT-P and CRT-D and OPT alone are explained by the greater differences in QALYs gained among strategies than in the base-case analysis. As both CRT cohorts had similar QALY increments in this scenario, the ICER for CRT-D compared with CRT-P in this scenario (£27,893 per QALY gained) does not differ as much from the ICER in the base case (£28,420 per QALY gained).
| Intervention | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. OPT | ICER (£/QALY gained) vs. CRT-P + OPT |
|---|---|---|---|---|---|
| OPT | 7615 | 4.86 | 3.39 | – | – |
| CRT-P + OPT | 26,460 | 5.51 | 4.21 | 22,892 | – |
| CRT-D + OPT | 38,163 | 7.21 | 4.63 | 24,580 | 27,893 |
All device-related costs (including those associated with implantation, perioperative complications, treatment of lead displacement, infection and device replacement) were varied as a group to the lower and upper limits of their 95% CIs (see Table 114). The ICER ranged from £20,977 to £48,486 per QALY gained for CRT-P + OPT compared with OPT, from £23,652 to £53,556 per QALY gained for CRT-D + OPT compared with OPT, and from £28,090 to £61,967 per QALY gained for CRT-D + OPT compared with CRT-P + OPT. Considering a willing-to-pay threshold of £30,000 per QALY gained, when the upper limits of the device-related costs are used, both CRT strategies become non-cost-effective compared with OPT alone, and CRT-D + OPT becomes non-cost-effective compared with CRT-P + OPT. The scenario using the lower limits of the device-related costs resulted in a reduction in costs of > £4500 for both CRT strategies and of < £100 for OPT alone. Thus, the ICERs for the comparisons between the CRT devices and OPT alone are reduced much more substantially than the ICER for the comparison between CRT-D and CRT-P.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was performed for the base case to estimate the impact of joint parameter uncertainty on the model’s cost-effectiveness results. Appendix 15 reports the variables (mean values and CIs) included in the probabilistic sensitivity analysis, the form of distribution used for sampling and the parameters of the distribution. Table 137 reports the estimated probabilistic results of 10,000 iterations in terms of costs and QALYs for each strategy and their relative cost-effectiveness.
| Strategy | Cost (£) | QALYs | ICER (£/QALY gained) vs. OPT (IQR) | ICER (£/QALY gained) vs. CRT-P + OPT (IQR) |
|---|---|---|---|---|
| OPT | 7604 | 3.48 | – | – |
| CRT-P + OPT | 25,874 | 4.14 | 27,434 (16,314 to 47,527) | – |
| CRT-D + OPT | 38,156 | 4.56 | 28,158 (17,431 to 49,839) | 27,899 (–175 to 159,172) |
The probabilistic results are consistent with the deterministic base-case analysis. Both CRT-P + OPT and CRT-D + OPT have ICERs of < £30,000 per QALY gained compared with initial management with OPT alone, as well as CRT-D + OPT compared with CRT-P + OPT. The IQR estimated for the probabilistic ICER for the comparison between CRT-D + OPT and CRT-P + OPT reflects the overlap in model results for CRT-P and CRT-D.
The probabilistic sensitivity analysis results are presented in Figure 38 in terms of incremental costs and QALYs, showing their dispersion on the cost-effectiveness scatterplot and the partial overlap of the cost-effectiveness results for the three strategies, particularly between CRT-P and CRT-D.
FIGURE 38.
Cost-effectiveness scatterplot: population 2.
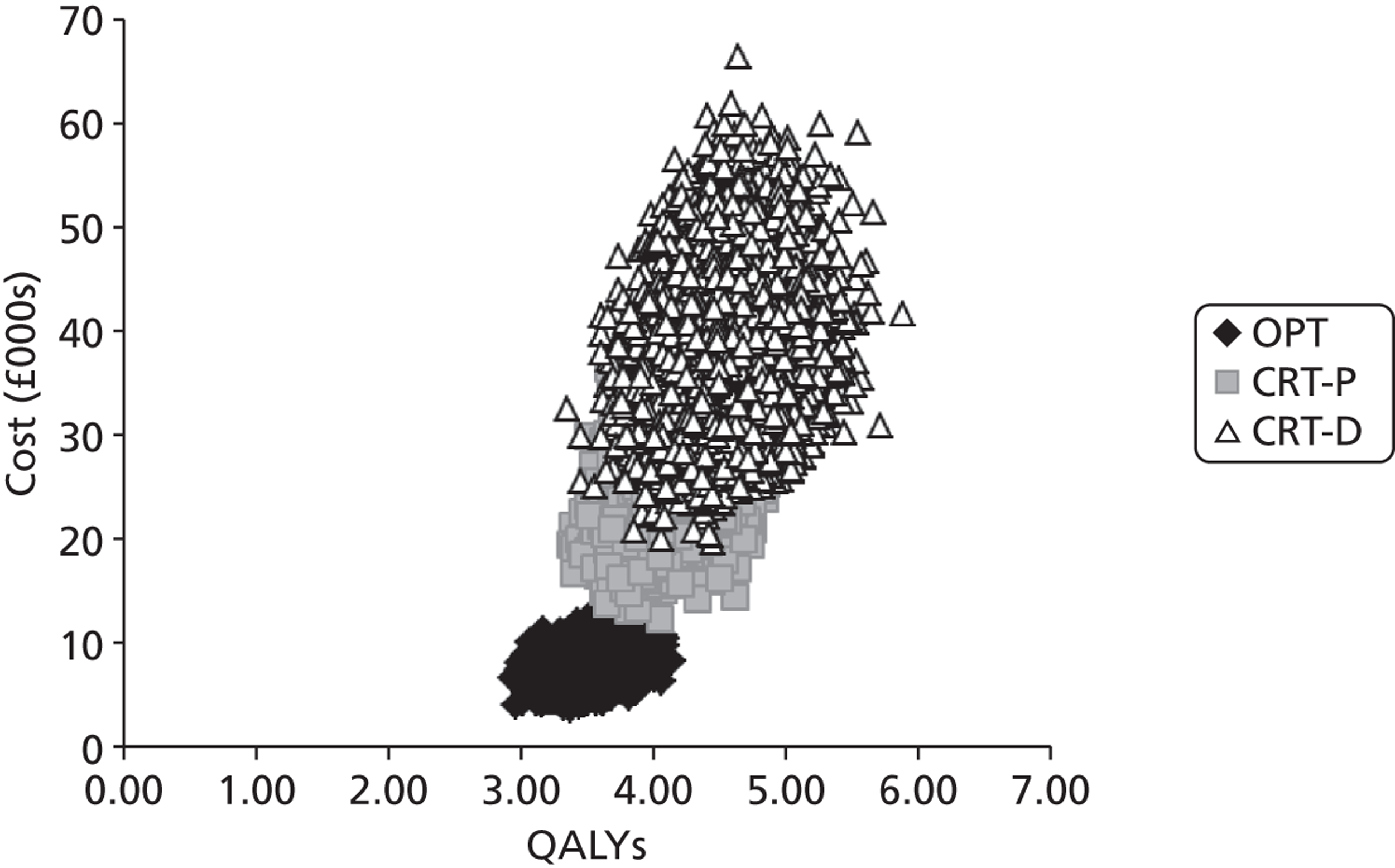
Figure 39 shows the variation in the probability of the three treatment strategies being cost-effective as the WTP threshold increases from £0 to £50,000 per QALY gained. At a WTP threshold of £20,000 per QALY gained, the probability of OPT alone (with subsequent upgrades), CRT-P + OPT and CRT-D + OPT being cost-effective is 83%, 9% and 8% respectively. Above a WTP threshold of £28,000 per QALY gained, the intervention with the highest probability of being cost effective is CRT-D + OPT (38%). At a WTP threshold of £30,000 per QALY gained, CRT-D + OPT and CRT-P + OPT have a 46% and 31% probability of being cost-effective, respectively, whereas OPT alone has a 23% probability of being cost-effective.
FIGURE 39.
Cost-effectiveness acceptability curve: population 2.
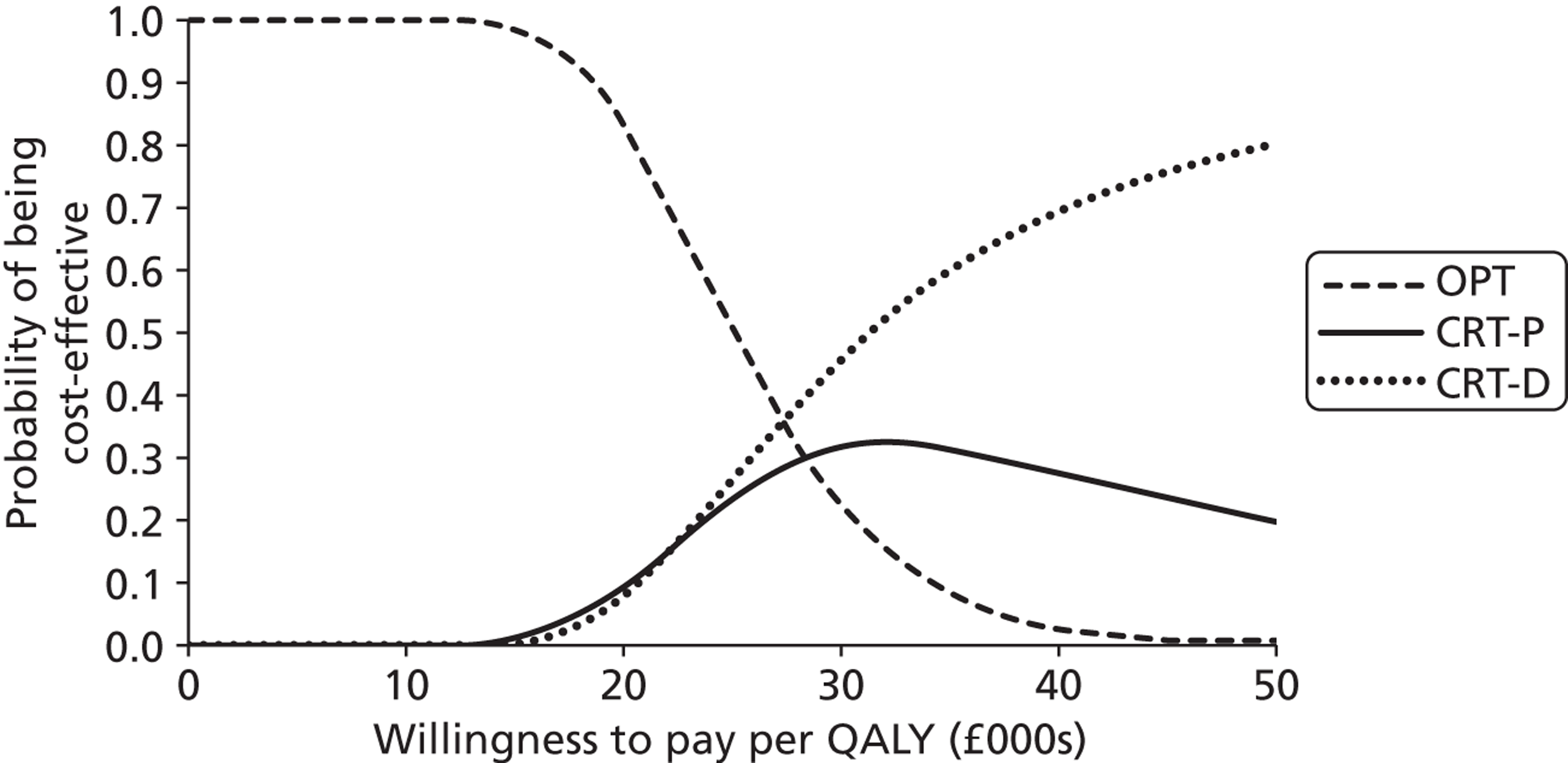
Population 3: people with both conditions
Patients with both conditions were modelled receiving initially OPT alone, ICD + OPT, CRT-P + OPT or CRT-D + OPT, to estimate the relative cost-effectiveness of these four treatment strategies. The relevant comparisons for this population are therefore CRT-D + OPT compared with OPT alone (allowing for subsequent device implantations) or CRT-P or ICD alongside OPT.
Base-case analysis
The RAFT trial140 provided the estimates for all-cause mortality and the distribution of patients by NYHA class used for our base-case analysis for population 3. Table 138 presents the estimated discounted costs, life-years and QALYs gained for each strategy, as well as the ICERs for the relevant comparisons.
| Strategy | Cost (£) | Life-years | QALYs | Incremental cost (£) | Incremental life-years | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|---|---|
| Vs. next best optiona | |||||||
| ICD + OPT | 39,719 | 7.45 | 5.57 | – | – | – | – |
| OPT | 40,006 | 7.59 | 5.67 | 287 | 0.14 | 0.10 | 2824 |
| CRT-P + OPT | 51,202 | 7.96 | 5.94 | 11,196 | 0.37 | 0.27 | Dominated |
| CRT-D + OPT | 50,911 | 8.01 | 5.98 | 10,906 | 0.42 | 0.31 | 35,193 |
| Vs. ICD + OPT | |||||||
| CRT-P + OPT | 51,202 | 7.96 | 5.94 | 11,483 | 0.51 | 0.37 | Dominated |
| CRT-D + OPT | 50,911 | 8.01 | 5.98 | 11,193 | 0.56 | 0.41 | 27,195 |
The initial management of population 3 patients with ICD + OPT is estimated to be the least costly and least effective strategy. Initial management with OPT alone (followed by subsequent device implants as necessary) had a similar estimated cost (£287 more) to that of ICD + OPT and resulted in 0.10 more QALYs. Thus, each additional QALY gained with OPT alone is estimated to cost £2824 more. A significant proportion of population 3 patients initially managed with OPT alone are estimated to be referred for CRT-D over their lifetime. These patients will therefore benefit from lower risks of death and hospitalisation for HF than patients receiving ICD + OPT. They are estimated to spend more time stable with therapy and to have a slightly higher QALY gain (0.10) than those managed with ICD + OPT.
Similar costs and QALYs are estimated for the CRT-P + OPT and CRT-D + OPT strategies. As a marginally higher cost and slightly fewer QALYs are estimated for CRT-P + OPT than for CRT-D + OPT, CRT-P + OPT is dominated by CRT-D + OPT.
Compared with OPT alone, every additional QALY gained with CRT-D + OPT costs £35,193 more. CRT-D + OPT compared with ICD + OPT has an ICER of £27,195 per QALY gained.
The costs and QALYs gained per strategy are presented graphically in Figure 40, in which the proximity between the CRT strategies and the proximity between the OPT alone and ICD + OPT strategies is noticeable.
FIGURE 40.
Cost-effectiveness plane for population 3.
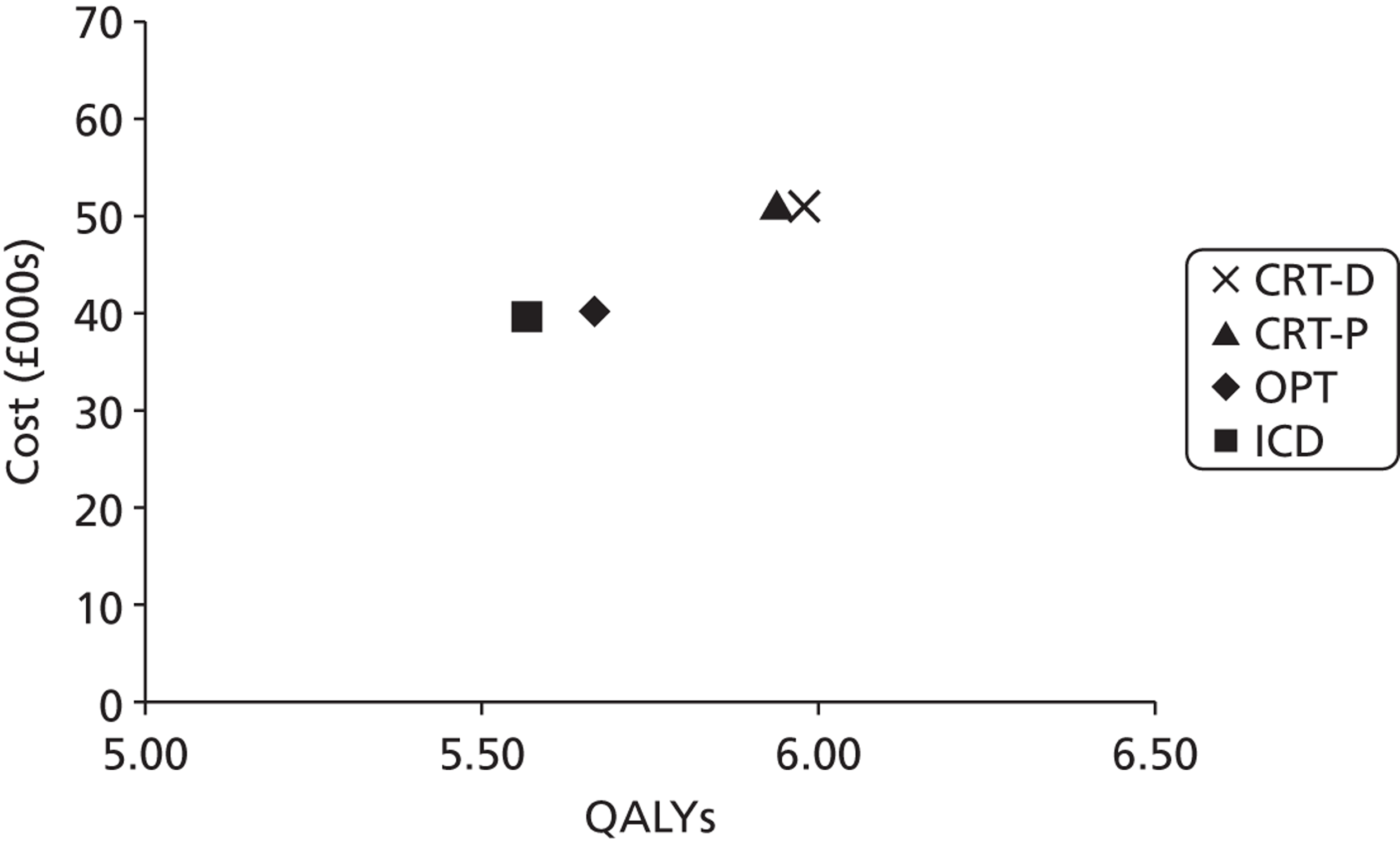
The overall survival estimated in the model was compared with that reported in the relevant trials (see Appendix 16 for details).
The percentage of time spent in the main health state categories by an average patient for each strategy is presented in Table 139. All strategies being compared show similar occupancies for the stable with therapy health state (most of the patient’s lifetime) or the device-related interventions health state (implant surgery, postoperative complications, routine replacements, lead displacements and infections). The model also estimates small differences between strategies in the time spent in hospital.
| Health state categories | % of remaining life | |||
|---|---|---|---|---|
| OPT | ICD | CRT-P | CRT-D | |
| Stable with therapy | 94.32 | 93.28 | 93.53 | 93.33 |
| OPT | 22.68 | 0.42 | 1.99 | 0.07 |
| ICD | 10.52 | 89.70 | 10.44 | 13.00 |
| CRT-P | 0.03 | 0 | 20.59 | 0 |
| CRT-D | 61.10 | 3.15 | 60.50 | 80.26 |
| Hospitalisation | 3.07 | 4.08 | 2.95 | 3.62 |
| Implant surgery | 0.78 | 0.87 | 1.54 | 0.91 |
| ICD | 0.13 | 0.84 | 0.13 | 0.15 |
| CRT-P | 0.00 | 0 | 0.76 | 0 |
| CRT-D | 0.65 | 0.04 | 0.65 | 0.76 |
| Routine replacement | 0.66 | 0.54 | 0.67 | 0.70 |
| Lead displacement | 0.25 | 0.13 | 0.33 | 0.33 |
| Postoperative complications | 0.17 | 0.09 | 0.26 | 0.20 |
| Infection | 0.05 | 0.05 | 0.06 | 0.06 |
| Device-related interventionsa | 1.90 | 1.67 | 2.85 | 2.19 |
The numbers of the most relevant events estimated for each arm of the population 3 model are presented in Table 140. The cohort of patients initially managed with OPT alone is estimated to receive 1850 implants (1552 CRT-D, 297 ICD and one CRT-P), of which 820 are estimated to be associated with routine replacements according to the estimated battery lifetime. In the cohort initially implanted with an ICD, 47 are expected to upgrade to CRT-D and nine are expected to receive an ICD subsequently because of CRT-D implant failure. Both strategies in which the defibrillator function is implanted initially (ICD + OPT and CRT-D + OPT) involve fewer device upgrades, with the reported ICD upgrades resulting from CRT-D implant failure.
| Event | Strategy | |||
|---|---|---|---|---|
| OPT | ICD | CRT-P | CRT-D | |
| Initial implant | 0 | 1000 | 1000 | 1000 |
| ICD | 0 | 1000 | 0 | 0 |
| CRT-P | 0 | 0 | 1000 | 0 |
| CRT-D | 0 | 0 | 0 | 1000 |
| Hospitalisation | 5446 | 4957 | 4797 | 4790 |
| OPT | 1171 | 21 | 110 | 4 |
| ICD | 578 | 4776 | 603 | 757 |
| CRT-P | 808 | 15 | 1072 | 3 |
| CRT-D | 2889 | 144 | 3012 | 4025 |
| Upgrade | 974 | 56 | 1025 | 203 |
| ICD | 160 | 9 | 169 | 195 |
| CRT-P | 1 | 0 | 0 | 0 |
| CRT-D | 812 | 47 | 856 | 8 |
| Surgical complications | 212 | 107 | 343 | 259 |
| ICD | 17 | 96 | 17 | 20 |
| CRT-P | 0 | 0 | 119 | 0 |
| CRT-D | 196 | 11 | 206 | 239 |
| Lead displacement | 313 | 151 | 432 | 435 |
| ICD | 17 | 137 | 17 | 22 |
| CRT-P | 0 | 0 | 106 | 0 |
| CRT-D | 296 | 15 | 309 | 413 |
| Infection | 57 | 59 | 76 | 78 |
| ICD | 7 | 57 | 7 | 9 |
| CRT-P | 0 | 0 | 17 | 0 |
| CRT-D | 50 | 2 | 52 | 69 |
| Replacement | 820 | 647 | 874 | 919 |
| ICD | 130 | 609 | 137 | 148 |
| CRT-P | 0 | 0 | 4 | 0 |
| CRT-D | 690 | 38 | 733 | 771 |
| Number of devicesb | 1850 | 1762 | 2974 | 2201 |
| ICD | 297 | 1674 | 313 | 353 |
| CRT-P | 1 | 0 | 1021 | 0 |
| CRT-D | 1552 | 88 | 1640 | 1848 |
Deterministic sensitivity analysis
Cost-effectiveness results were estimated for a scenario of a mixed-age and mixed-gender cohort of population 3 patients. The distribution of ICD and CRT implants by age in the UK reported by the British Cardiovascular Society, the British Heart Foundation and the Cardio & Vascular Coalition225 was used as a proxy for the distribution of population 3 patients. Age-dependent variables in the population 3 model were those that determined all-cause mortality (baseline risk and RR of death by age group).
Table 141 summarises the model results for different starting ages. For most age groups, ICD + OPT is the strategy with the least estimated benefit. Compared with the next best option or with ICD + OPT in most age groups, OPT alone is the strategy with the lowest ICER, CRT-P + OPT is dominated and CRT-D + OPT is that with the highest ICER.
| Starting age (years) | Strategy | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. next best optiona | ICER (£/QALY gained) vs. ICD + OPT |
|---|---|---|---|---|---|---|
| 30 | OPT | 55,173 | 10.67 | 7.98 | 18,499 | 18,499 |
| ICD + OPT | 51,560 | 10.40 | 7.78 | – | – | |
| CRT-P + OPT | 66,193 | 11.01 | 8.23 | Dominated | Dominated | |
| CRT-D + OPT | 65,417 | 11.08 | 8.28 | 34,236 | 28,020 | |
| 40 | OPT | 54,428 | 10.51 | 7.86 | 19,094 | 19,904 |
| ICD + OPT | 51,012 | 10.26 | 7.68 | – | – | |
| CRT-P + OPT | 65,414 | 10.84 | 8.10 | Dominated | Dominated | |
| CRT-D + OPT | 64,637 | 10.90 | 8.15 | 34,872 | 28,887 | |
| 50 | OPT | 52,222 | 10.02 | 7.49 | 19,708 | 19,708 |
| ICD + OPT | 49,165 | 9.81 | 7.34 | – | – | |
| CRT-P + OPT | 63,107 | 10.34 | 7.72 | Dominated | Dominated | |
| CRT-D + OPT | 62,323 | 10.40 | 7.77 | 36,881 | 30,672 | |
| 60 | OPT | 46,012 | 8.77 | 6.55 | 13,715 | 13,715 |
| ICD + OPT | 43,844 | 8.55 | 6.39 | – | – | |
| CRT-P + OPT | 56,718 | 9.05 | 6.75 | Dominated | Dominated | |
| CRT-D + OPT | 55,951 | 9.09 | 6.79 | 41,328 | 30,376 | |
| 70 | OPT | 38,026 | 7.21 | 5.38 | 835 | 835 |
| ICD + OPT | 37,938 | 7.06 | 5.27 | – | – | |
| CRT-P + OPT | 49,140 | 7.56 | 5.64 | Dominated | Dominated | |
| CRT-D + OPT | 48,856 | 7.61 | 5.68 | 36,625 | 27,196 | |
| 80 | OPT | 33,517 | 6.35 | 4.74 | Dominant | Dominant |
| ICD + OPT | 35,152 | 6.30 | 4.70 | – | – | |
| CRT-P + OPT | 44,963 | 6.77 | 5.05 | Extendedly dominated | Extendedly dominated | |
| CRT-D + OPT | 45,139 | 6.82 | 5.08 | 33,761 | 26,092 | |
| 90 | OPT | 29,415 | 5.37 | 4.00 | – | Extendedly dominated |
| ICD + OPT | 32,257 | 5.42 | 4.05 | Extendedly dominated | – | |
| CRT-P + OPT | 40,388 | 5.68 | 4.23 | Extendedly dominated | Extendedly dominated | |
| CRT-D + OPT | 40,599 | 5.72 | 4.26 | 43,438 | 38,916 | |
| Mixed | OPT | 41,612 | 7.92 | 5.92 | 6489 | 6489 |
| ICD + OPT | 40,888 | 7.77 | 5.81 | – | – | |
| CRT-P + OPT | 52,673 | 8.27 | 6.17 | Dominated | Dominated | |
| CRT-D + OPT | 52,297 | 8.32 | 6.21 | 36,697 | 28,330 |
All-cause mortality reported for men in the CRT-D arm of the MADIT-CRT trial130 and the respective HR for ICD therapy for the whole population of the MADIT-CRT trial (1.00, 95% CI 0.69 to 1.44) were used as an alternative scenario to the outcomes from the RAFT trial140 used in the base-case analysis. Table 142 summarises the cost-effectiveness results for this scenario.
| Strategy | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. next best optiona |
|---|---|---|---|---|
| OPT | 49,908 | 9.59 | 7.17 | – |
| CRT-P + OPT | 60,736 | 9.89 | 7.39 | Dominated |
| CRT-D + OPT | 60,051 | 9.97 | 7.45 | Dominated |
| ICD + OPT | 49,957 | 10.01 | 7.49 | 154 |
Generally, most strategies became more costly and yielded a greater health benefit in this scenario than in the base case. OPT alone (and subsequent device implants) is the least costly and least effective strategy in this scenario. ICD + OPT is slightly more costly but yields a greater benefit than OPT alone. As CRT-P + OPT and CRT-D + OPT are less effective than ICD + OPT and much more costly, both CRT strategies are dominated by ICD + OPT compared with OPT alone. Therefore, the results obtained with the MADIT-CRT trial data indicate that ICD + OPT is the most cost-effective strategy, with an ICER of £154 per QALY gained compared with OPT alone.
As the MADIT-CRT trial found no statistically significant difference in all-cause mortality between the ICD arm and the CRT-D arm, for this scenario the model assumed the same risk of death for the ICD and CRT-D cohorts. A similar benefit was therefore estimated for the ICD + OPT and CRT-D + OPT strategies (the 0.04 difference in QALYs gained is because less time is spent in the device-related interventions health state in the ICD + OPT cohort than in the CRT-D + OPT cohort). A much lower cost was estimated for ICD + OPT than for CRT-D + OPT as the first is estimated to involve fewer device upgrades and replacements.
Comprehensive univariate sensitivity analyses were performed on the parameters informing the population 3 model. Tables 143–146 present the sensitivity analysis results for the most influential parameters (i.e. those that when varied between the 95% CI limits caused a variation of > £20,000 per QALY in the ICER) for each of the relevant comparisons: CRT-D + OPT compared with OPT alone (allowing for subsequent device implantations), CRT-D + OPT compared with CRT-P + OPT and CRT-D + OPT compared with ICD + OPT.
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 10,906 | 0.31 | 35,193 |
| RR of all-cause mortality (OPT) | 1.563 | 1.163 | 9109 | 0.07 | 124,733 |
| 2.083 | 12,972 | 0.58 | 22,240 | ||
| Time horizon | Lifetime | CRT-D lifetime (7 years) | 9347 | 0.15 | 63,837 |
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 11,193 | 0.41 | 27,195 |
| RR of all-cause mortality (ICD) | 1.19 | 1.04 | 9407 | 0.07 | 127,299 |
| 1.37 | 12,981 | 0.75 | 17,262 | ||
| Device lifetime (CRT-D), ln(λ), γ | –15.465, 1.935 (7 years) | –16.000, 1.863 (13 years) | 3841 | 0.44 | 8784 |
| –14.931, 2.006 (4 years) | 22,019 | 0.37 | 59,421 | ||
| Device lifetime (ICD), ln(λ), γ | –15.78 1.94 (∼8 years) | –16.182, 1.889 (∼13 years) | 14,285 | 0.41 | 35,034 |
| –15.385, 1.996 (∼5 years) | 5951 | 0.42 | 14,218 |
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | –291 | 0.04 | Dominant |
| Baseline risk of hospitalisation for non-fatal arrhythmia (CRT-D) | 0.0285 | 0.0146 | 3993 | 0.04 | 93,501 |
| 0.0424 | –1823 | 0.04 | Dominant | ||
| Device lifetime (CRT-D), ln(λ), γ | –15.465, 1.935 (∼7 years) |
–16, 1.863 (∼13 years) |
–866 | 0.04 | Dominant |
| –14.931, 2.006 (∼4 years) |
1840 | 0.03 | 58,794 | ||
| RR of hospitalisation for non-fatal arrhythmia (CRT-P) | 1 | 0.80 | 1374 | 0.04 | 38,915 |
| 1.20 | –1457 | 0.04 | Dominant | ||
| Risk of lead displacement (CRT-D) | 0.004 | 0.0004 | –926 | 0.05 | Dominant |
| 0.0071 | 313 | 0.03 | 9393 | ||
| RR of all-cause mortality (OPT) | 1.563 | 1.163 | –460 | 0.02 | Dominant |
| 2.083 | –97 | 0.07 | Dominant | ||
| Discount rates of costs and benefits (%) | 3.5, 3.5 | 0, 0 | –1054 | 0.05 | Dominant |
| 6, 1.5 | 207 | 0.05 | 4370 | ||
| Risk of surgical mortality with CRT-P | 0.0048 | 0.0015 | –450 | 0.02 | Dominant |
| 0.0081 | –131 | 0.06 | Dominant | ||
| Risk of lead infections (CRT-D) | 0.0006 | 0 | –659 | 0.04 | Dominant |
| 0.0015 | 243 | 0.04 | 6432 | ||
| Risk of lead displacement (CRT-P) | 0.0037 | 0.0004 | 188 | 0.03 | 5513 |
| 0.0071 | –764 | 0.04 | Dominant | ||
| Time horizon | Lifetime | CRT-D lifetime (7 years ) | –613 | 0.02 | Dominant |
| Parameter | Base-case value | DSA value | Incremental cost (£) | Incremental QALYs | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| Base case | – | – | 287 | 0.10 | 2824 |
| Time horizon | Lifetime | CRT-D lifetime (7 years) | –4395 | –0.05 | 94,341 |
| Device lifetime (CRT-D), ln(λ), γ | –15.465, 1.935 (∼7 years) |
–16, 1.863 (∼13 years) |
–6129 | 0.12 | Dominant |
| –14.931, 2.006 (∼4 years) |
8653 | 0.07 | 123,385 | ||
| Device lifetime (ICD), ln(λ), γ | –15.78, 1.94 (∼ 8 years) |
–16.182, 1.889 (∼13 years) |
3505 | 0.10 | 35,868 |
| –15.385, 1.996 (∼5 years) |
–5086 | 0.11 | Dominant | ||
| Baseline risk of hospitalisation for non-fatal arrhythmia (CRT-D) | 0.0285 | 0.0146 | –4565 | –0.09 | 49,987 |
| 0.0424 | 2086 | 0.19 | 10,896 | ||
| RR of hospitalisation for non-fatal arrhythmia (OPT) | 1 | 0.8 | –1978 | 0.04 | Dominant |
| 1.2 | 1923 | 0.15 | 13,107 | ||
| RR of hospitalisation for non-fatal arrhythmia (ICD) | 1.11 | 0.88 | 2330 | 0.10 | 22,346 |
| 1.41 | –2334 | 0.10 | Dominant | ||
| Baseline risk of all-cause mortality (CRT-D), ln(λ), γ | –6.334, 1.234 | –6.467, 1.198 | 2047 | 0.14 | 14,124 |
| –6.202, 1.270 | –1092 | 0.06 | Dominant | ||
| Risk of lead displacement (CRT-D) | 0.0037 | 0.0004 | –1083 | 0.11 | Dominant |
| 0.0071 | 1600 | 0.09 | 17,916 | ||
| Discount rates of costs and benefits (%) | 3.5, 3.5 | 0, 0 | 3183 | 0.22 | 14,529 |
| 6, 1.5 | –1212 | 0.16 | Dominant |
The cost-effectiveness results for the comparison between initial treatment with CRT-D + OPT and initial treatment with OPT alone (see Table 143) were quite robust to the variation of the parameters in the model, with only two parameters varying the ICER by > £20,000. The comparison between CRT-D + OPT and OPT alone showed great sensitivity to the RR of all-cause mortality for the OPT alone arm. The ICER for CRT-D + OPT decreased to £22,240 per QALY gained when a greater risk of death is assumed for OPT than for CRT-D + OPT (because of the incremental QALY gain with the latter). When a shorter time horizon was considered (assuming the same as the lifetime of the CRT-D device), less benefit from CRT-D + OPT relative to OPT alone was accrued and therefore the ICER increased.
Table 144 shows the univariate sensitivity analysis results for CRT-D + OPT compared with ICD + OPT. The most influential parameters for this comparison were the RR of all-cause mortality for patients managed with an ICD and the lifetime of the CRT-D and ICD devices.
Assuming a lower RR of death for patients managed with an ICD would substantially increase the ICER for CRT-D + OPT compared with ICD + OPT, as there is a very small QALY gain (0.07). Also, assuming a 4-year device lifetime for the CRT-D device would almost double the ICER for CRT-D + OTP compared with ICD + OPT.
Varying the lifetime of the ICD device also had a substantial impact on the incremental cost of CRT-D compared with ICD. When the ICD was assumed to have a longer lifetime (13 years), a higher incremental cost for CRT-D was estimated and this strategy became non-cost-effective (ICER £35,034 per QALY gained). The opposite happened when the ICD was assumed to have a lifetime of 5 years (alongside the 7-year lifetime of the CRT-D device).
Table 145 shows the univariate sensitivity analysis for CRT-D + OPT compared with CRT-P + OPT, with 10 parameters that made the ICER range by > £20,000. As the estimated costs and benefits of these strategies are so similar, the comparison between CRT-D + OPT and CRT-P + OPT is sensitive to the variation of more parameters. Overall, this comparison showed greater sensitivity to parameters related to the preventative effect of the devices on arrhythmia (baseline risk of hospitalisation for arrhythmia with CRT-D and RR of hospitalisation for arrhythmia of CRT-P) and the lifetime of the CRT-D device.
For the base-case analysis, the baseline risk of hospitalisation for arrhythmia for patients managed with CRT-D (0.0285) was derived from the relevant trials included in the systematic review. As no evidence on hospitalisation for arrhythmia was found for the comparison between CRT-P and CRT-D, the risk for patients managed with CRT-P was assumed to be the same as that for CRT-D, given that clinical advice suggested that population 3 patients are likely to be hospitalised for arrhythmia irrespective of whether or not they have a device with a defibrillator function implanted. When a lower baseline risk of hospitalisation for arrhythmia is used, the ICER for CRT-D + OPT compared with CRT-P + OPT increases significantly as the incremental cost of CRT-D is estimated to increase with no additional benefit. Under this scenario, all strategies show a reduction in the estimated costs; however, the costs of strategies without a defibrillator function (CRT-P and OPT alone) are reduced by more (about £10,000 less) than the cost of those with a defibrillator function (CRT-D and ICD), which incur costs of about £5000 less than in the base case. When the RR of hospitalisation for arrhythmia for patients managed with CRT-P is assumed to be less than the baseline risk, the cost of the CRT-P + OPT strategy decreases and this strategy is no longer dominated by CRT-D + OPT.
As for the previous comparison of two strategies both involving initial treatment with a device, varying the lifetime of the CRT-D device had a great impact on the ICER for the comparison between CRT-D + OPT and CRT-P + OPT. The incremental cost associated with a 4-year time period for replacement led to an ICER of £58,794 per QALY gained.
The comparison between OPT and ICD + OPT was also sensitive to many parameters (see Table 146), given that the estimated costs and QALYs for these strategies were very similar. It showed particular sensitivity to the time horizon, the lifetime of the CRT-D and ICD devices, the baseline risk of hospitalisation for non-fatal arrhythmia (CRT-D) and the RR of hospitalisation for non-fatal arrhythmia (OPT and ICD).
Assuming a shorter time horizon resulted in a substantial increase in the ICER for the comparison between OPT alone and ICD + OPT, as the first strategy showed a cost saving associated with a very small reduction in the health benefits accrued. When the 8-year ICD lifetime was assumed as the time horizon for the model, there was an increase in the incremental cost for OPT alone and less benefit compared with ICD + OPT. This increase in incremental cost with OPT alone is mainly a result of referrals for CRT-D implants because of severe arrhythmic events.
A substantial increase in the incremental cost for OPT alone compared with ICD + OPT is also estimated when CRT-D devices are assumed to require replacement every 4 years, associated with a small reduction in incremental QALYs compared with the base case, resulting in an ICER of £123,385 per QALY gained. When the lifetime of the ICD device is assumed to be longer than in the base case (13 years), the incremental cost of OPT increases but the same incremental benefit is estimated relative to the base case.
The baseline risk of hospitalisation for arrhythmia and the relative effects of the alternative treatments also had noticeable impacts on the comparison between OPT alone and ICD + OPT. With a lower baseline risk of hospitalisation, the estimated costs and QALYs for all strategies decreased (strategies without a defibrillator function have a greater reduction in costs than those with a defibrillator function) compared with the base case. Mainly because of fewer referrals for CRT-D implants, OPT alone (followed by the subsequent implants) was the strategy that was the most cost saving relative to the base case and also the one with the greatest loss of QALYs accrued, hence the high ICER estimated for it compared with ICD + OPT when a lower baseline risk of hospitalisation for severe arrhythmia was used. The ICER for OPT alone compared with ICD + OPT also increases when the RR of hospitalisation for arrhythmia is assumed to be higher for OPT or lower for ICD + OPT, as the additional cost associated with OPT increases substantially (and the additional benefit rises slightly or does not change respectively).
Table 147 presents the parameters that result in a change in the most cost-effective strategy as theirvalue is varied between their 95% CI limits. These relate mainly to the longevity of the devices with a defibrillator function (these have a shorter estimated lifetime relative to that of CRT-P), the RR of all-cause mortality for ICD and OPT, the baseline risk of hospitalisation for arrhythmia for CRT-D and the RR of hospitalisation for arrhythmia for ICD, and the discount rates for costs and benefits.
| Parameter | Base-case value | DSA value | Most cost-effective strategy at £20,000/QALY | Most cost-effective strategy at £30,000/QALY |
|---|---|---|---|---|
| Base case | – | – | OPT | OPT |
| Time horizon | Lifetime | 8 years (ICD lifetime) | ICD + OPT | ICD + OPT |
| Device lifetime (CRT-D), ln(λ), γ | –15.465, 1.935 (∼7 years) | UL: –14.934, 2.006 (∼4 years) | ICD + OPT | ICD + OPT |
| Device lifetime (ICD), ln(λ), γ | –15.784, 1.943 (∼8 years) | LL: –16.182, 1.889 (∼13 years) | ICD + OPT | ICD + OPT |
| RR of all-cause mortality (ICD) | 1.19 | LL: 1.04 | ICD + OPT | ICD + OPT |
| RR of all-cause mortality (OPT) | 1.563 | UL: 2.08 | ICD + OPT | CRT-D + OPT |
| Discount rates of costs and benefits (%) | 3.5, 3.5 | 0, 0 | OPT | CRT-D + OPT |
| 6, 1.5 | OPT | CRT-D + OPT | ||
| Baseline risk of hospitalisation for arrhythmia (CRT-D) | 0.029 | LL: 0.015 | OPT | CRT-P + OPT |
| RR of hospitalisation for arrhythmia with ICD | 1.11 | LL: 0.88 | ICD + OPT | OPT |
Overall, ICD + OPT becomes the most cost-effective strategy at a WTP threshold of £20,000 per QALY gained when an 8-year time horizon (the lifetime of an ICD device), a shorter CRT-D device lifetime (approximately 4 years), a longer ICD device lifetime (approximately 13 years), a lower RR of all-cause mortality for patients managed with ICD (RR = 1.04), a higher RR of all-cause mortality for patients managed with OPT (RR = 2.08) or a lower RR of hospitalisation for arrhythmia for patients managed with ICD (RR = 0.88) is used.
Under a scenario of not discounting future costs and benefits or of discounting future costs at a higher rate (6%) than future benefits (1.5%), CRT-D + OPT would become the most cost-effective strategy at a WTP threshold of £30,000 per QALY gained (ICERs of £25,602 and £29,650 per QALY gained, respectively, compared with OPT alone). If a higher RR of all-cause mortality for patients being managed with OPT compared with those being managed with CRT-D is used (RR = 2.08), CRT-D becomes the optimal strategy at a WTP threshold of £30,000 per QALY gained, with an ICER of £22,240 per QALY gained.
The strategy of CRT-P + OPT became the most cost-effective strategy at a WTP threshold of £30,000 per QALY gained when the lower limit of the baseline risk of hospitalisation for arrhythmia for patients managed with CRT-D was used (ICER of £26,200 per QALY gained compared with OPT alone).
Clinical advice indicated that device longevity estimates in the base-case analysis could be overestimated. A scenario analysis using the mean device lifetime estimates used by Fox and colleagues64 (see Table 133) was conducted and the results are presented in Table 148. In this scenario, initial management with OPT alone (and subsequent upgrades) was less costly and more effective than with ICD + OPT (i.e. OPT alone dominated ICD + OPT). CRT-P + OPT is more costly and more effective than OPT alone; however, the ICER for CRT-P + OPT compared with OPT alone is higher (£43,274 per QALY gained) than that for CRT-D + OPT compared with OPT alone (£39,318 per QALY gained). CRT-P + OPT is therefore extendedly dominated by CRT-D + OPT compared with OPT alone. Compared with ICD + OPT, CRT-D + OPT has an ICER of £23,690 per QALY gained and CRT-P + OPT is also extendedly dominated in this case.
| Strategy | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. next best optiona | ICER (£/QALY gained) vs. ICD + OPT |
|---|---|---|---|---|---|
| ICD + OPT | 47,068 | 7.44 | 5.56 | – | – |
| OPT | 44,567 | 7.57 | 5.65 | Dominant | – |
| CRT-P + OPT | 56,135 | 7.94 | 5.92 | Extendedly dominated | Extendedly dominated |
| CRT-D + OPT | 56,601 | 7.99 | 5.96 | 39,318 | 23,690 |
The population 3 base-case analysis is based on the conservative assumption that CRT devices have no impact on the distribution of patients by NYHA class over time. This scenario analysis assumes similar HF progression as used in the population 2 model. The population 2 model assumes a given initial distribution of patients by NYHA class (initially more severe than that in the population 3 model). At 9 months and 18 months, different distributions by NYHA class (derived from the CARE-HF trial109 and the BRESCIA study223) are assumed, capturing the effect of CRT on patients’ HRQoL.
Table 149 shows the cost-effectiveness results for this scenario. The results show an ICER of £27,396 per QALY gained for CRT-D + OPT compared with OPT alone, similar to that seen in the population 2 model (£27,899 per QALY gained).
| Strategy | Cost (£) | Life-years | QALYs | Incremental | ICER (£/QALY gained) | ||
|---|---|---|---|---|---|---|---|
| Cost (£) | Life-years | QALYs | |||||
| Vs. next best optiona | |||||||
| ICD + OPT | 39,719 | 7.45 | 5.37 | – | – | – | – |
| OPT | 40,006 | 7.59 | 5.68 | 287 | 0.14 | 0.31 | 936 |
| CRT-P + OPT | 51,202 | 7.96 | 6.04 | 11,196 | 0.37 | 0.36 | Dominated |
| CRT-D + OPT | 50,911 | 8.01 | 6.08 | 10,906 | 0.42 | 0.40 | 27,396 |
A scenario using the utility estimates used by Fox and colleagues64 (presented in Table 135) was explored. Table 150 shows the cost-effectiveness results for this scenario. Using the same utility values as Fox and colleagues did not effect the model results significantly (a reduction of 0.02 QALYs for OPT alone and 0.03 QALYs for all of the strategies beginning with implantation of a device). The ICERs obtained with this scenario are similar to those in the base-case analysis.
| Strategy | Cost (£) | Life-years | QALYs | ICER (£/QALY gained) vs. next best optiona | ICER (£/QALY gained) vs. ICD + OPT |
|---|---|---|---|---|---|
| ICD + OPT | 39,719 | 7.45 | 5.55 | – | – |
| OPT | 40,006 | 7.59 | 5.64 | 3,033 | – |
| CRT-P + OPT | 51,202 | 7.96 | 5.91 | Dominated | Dominated |
| CRT-D + OPT | 50,911 | 8.01 | 5.95 | 35,515 | 27,859 |
All relevant comparisons showed great sensitivity to costs when these were varied as a group between the lower and upper limits of their 95% CIs (see Table 114). When all costs were varied, the range in the ICER was > £25,000 per QALY gained for all relevant comparisons except for OPT compared with ICD + OPT, which showed a small variation. The ICER ranged from £22,271 to £50,824 per QALY gained for CRT-D + OPT compared with ICD + OPT, from £13,829 to £43,853 per QALY gained for CRT-D + OPT compared with CRT-P + OPT, and from £28,200 to £60,864 for CRT-D + OPT compared with OPT alone.
Under a scenario using the upper limit of all costs, ICD + OPT and OPT alone are the most cost-effective strategies at a WTP threshold of £20,000 and £30,000 per QALY gained respectively. When the lower limit of all costs (including device-related costs, health state costs and pharmacological therapy costs) is used, the most cost-effective strategy at a threshold of £30,000 per QALY gained is CRT-D + OPT.
Probabilistic sensitivity analysis
Table 151 reports the base-case probabilistic sensitivity analysis for population 3. Appendix 15 reports the variables (mean values and CIs) included in the probabilistic sensitivity analysis and the form of distribution used for sampling and the parameters of the distribution. Overall, the probabilistic results are consistent with the deterministic results. The results show that an additional QALY gained with OPT alone is estimated to cost £13,053 more than with ICD + OPT. The estimated ICER for CRT-D + OPT compared with OPT alone is £34,988 per QALY gained. Compared with ICD + OPT, the ICER for CRT-D + OPT is £23,133 per QALY gained.
| Strategy | Cost (£) | QALYs | ICER (£/QALY gained) vs. next best optiona (IQR) | ICER (£/QALY gained) vs. ICD + OPT (IQR) |
|---|---|---|---|---|
| ICD + OPT | 44,310 | 5.58 | – | – |
| OPT | 38,732 | 5.63 | 13,053 (–515,869 to 471,462) | – |
| CRT-P + OPT | 51,286 | 5.94 | Extendedly dominated | Extendedly dominated |
| CRT-D + OPT | 51,690 | 5.98 | 34,988 (–191,681 to 264,108) | 23,133 (–196,334 to 222,149) |
The probabilistic sensitivity analysis results of 10,000 iterations are presented in Figure 41 in terms of average costs and QALYs, showing the overlap of the results for the different strategies on the scatterplot.
FIGURE 41.
Cost-effectiveness scatterplot: population 3.
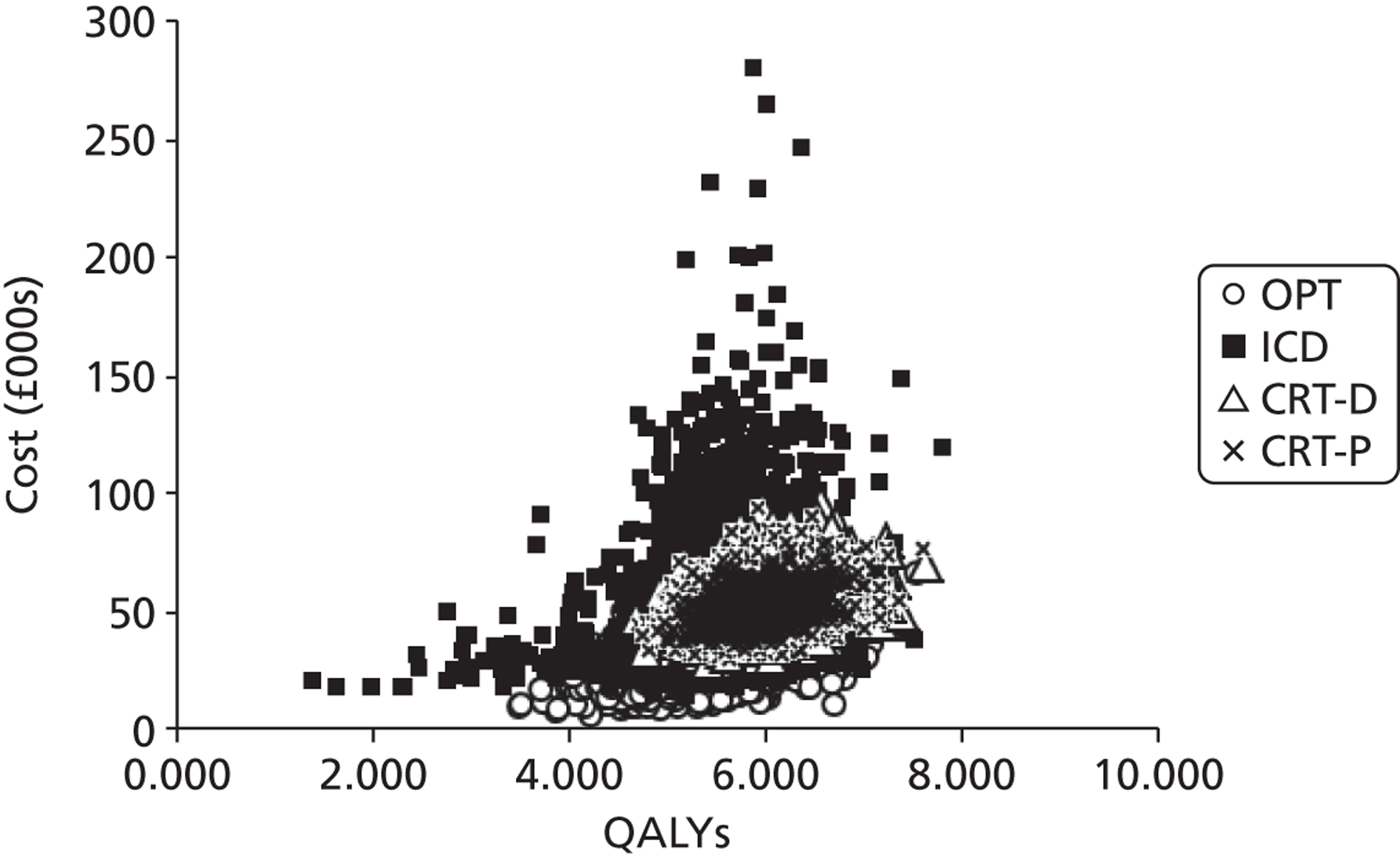
Figure 42 shows the variation in the probability of being cost-effective for the different treatment strategies as the WTP threshold increases from £0 to £50,000 per QALY gained. At a WTP threshold of £20,000 per QALY gained, the probability of OPT alone, ICD + OPT, CRT-D + OPT and CRT-P + OPT being cost-effective is 57%, 37%, 3% and 3% respectively. Above a WTP of £42,000 per QALY gained, the intervention with the highest probability of being cost effective is CRT-D + OPT (31%). At a WTP threshold of £30,000 per QALY gained, OPT alone, ICD + OPT, CRT-D + OPT and CRT-P + OPT have a probability of being cost-effective of 44%, 31%, 15% and 10% respectively.
FIGURE 42.
Cost-effectiveness acceptability curve: population 3.
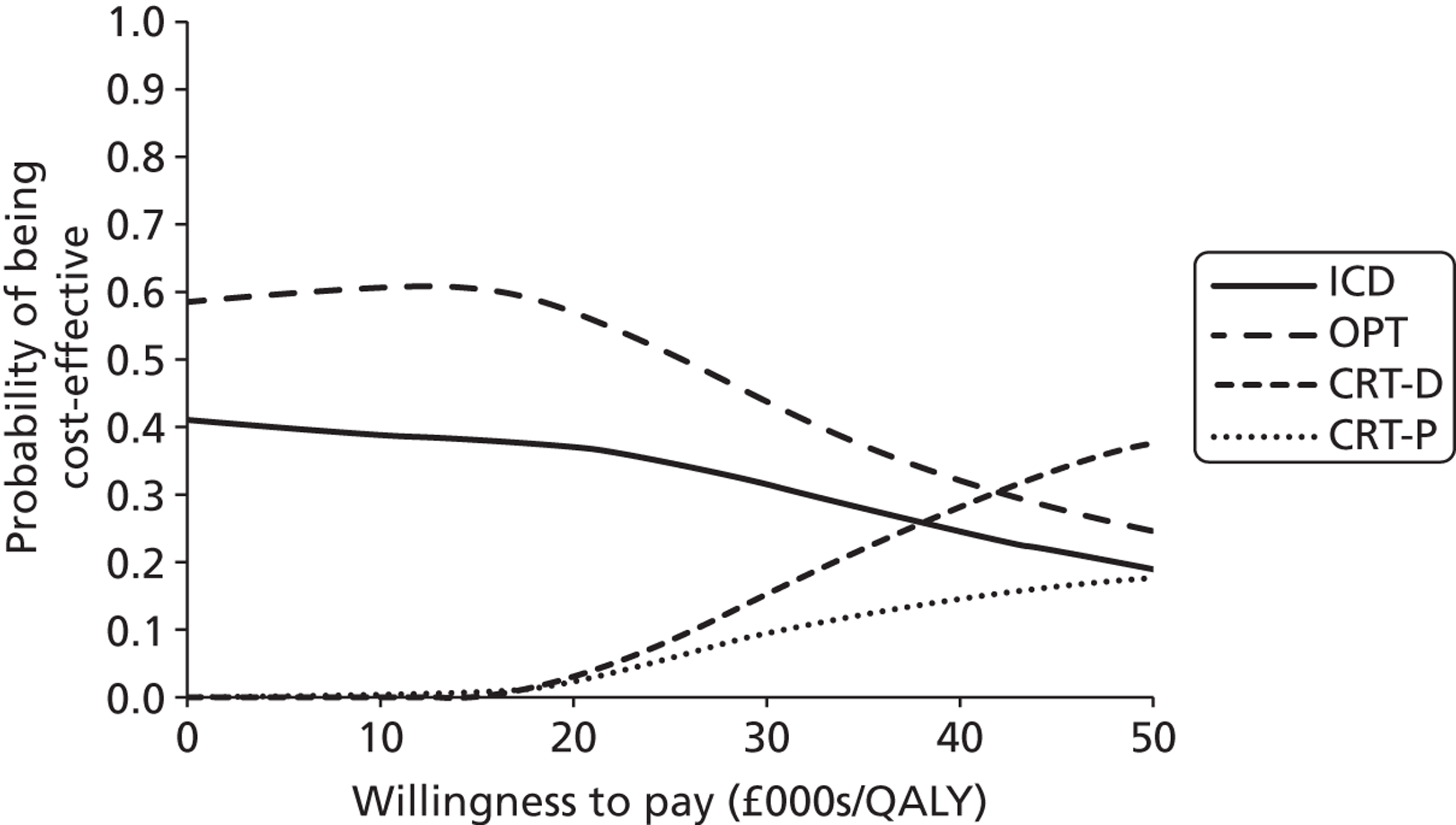
Summary of the independent economic evaluation
Population 1
-
The addition of ICD to OPT for the secondary prevention of SCD has an ICER of £19,479 per QALY gained compared with OPT alone. Its probability of being cost-effective at a WTP of £20,000 and £30,000 per QALY gained is 51% and 82% respectively.
-
The ICER for the mixed-age cohort is slightly higher (£24,967 per QALY gained) as it increased with age and 52% of these patients are expected to be aged > 65 years.
-
Subgroup analysis using MADIT II trial data shows that ICD + OPT is cost-effective (ICER of £14,231 per QALY gained) for the primary prevention of SCD in patients with remote MI.
-
For the SCD-HeFT trial data (patients with mild to moderate HF), the estimated ICER for ICD +OPT is £29,756 per QALY gained compared with OPT alone.
-
For patients with non-ischaemic cardiomyopathy the ICER was £26,028 per QALY gained.
-
The parameters that have the greatest impact on the ICER were the time horizon, the HR for all-cause mortality associated with the ICD + OPT arm, the risk of surgical death during ICD implantation and the lifetime of the device.
Population 2
-
The addition of CRT-P to OPT (in the initial stage of management of HF) resulted in an ICER of £27,584 per QALY gained compared with initial management with OPT alone (allowing for subsequent implants). Similarly, initial implantation of a CRT-D device alongside OPT resulted in an ICER of £27,899 per QALY gained compared with OPT alone. When comparing CRT-D + OPT with CRT-P + OPT, a slightly higher ICER was estimated (£28,420 per QALY gained).
-
At a WTP of £20,000 per QALY gained, initial management with OPT alone followed by implantation of the clinically necessary devices is the strategy with the highest probability of being cost-effective (81%). Above a WTP of £28,000 per QALY, the strategy with the highest probability of being cost effective is CRT-D + OPT (38%).
-
The incremental cost-effectiveness results for the comparisons relevant for population 2 seem to be sensitive mainly to device-related costs and to parameters that determine the incremental benefit of the devices for patient survival, such as the RRs of SCD and HF death for patients managed with CRT-P. The lifetime of the CRT-D device was also particularly influential because of the incremental costs incurred when it became shorter.
-
In a scenario assuming the upper limit estimates of device-related costs or the lower limit estimates for the longevity of all devices, both CRT-P + OPT and CRT-D + OPT became non-cost-effective compared with initial management with OPT alone (followed by the subsequent upgrades).
Population 3
-
In the base case the most cost-effective strategy for people with both conditions at a WTP of £20,000–30,000 per QALY is initial management with OPT alone (followed by device implantation and subsequent upgrades as necessary). Both strategies including initial implantation of a CRT device have ICERs that are greater than the WTP range of £20,000–30,000 per QALY gained compared with OPT alone (CRT-D £35,193 per QALY gained; CRT-P £41,414 per QALY gained). Costs and QALYs for CRT-D and CRT-P are similar.
-
CRT-D + OPT is cost-effective compared with ICD + OPT at a WTP of £30,000 per QALY (ICER of £27,195 per QALY gained).
-
At a WTP of £30,000 per QALY, OPT alone, ICD + OPT, CRT-D + OPT and CRT-P + OPT have a probability of being cost-effective of 44%, 31%, 15% and 10% respectively. Above a WTP of £42,000 per QALY, the intervention with the highest probability of being cost effective is CRT-D + OPT (31%).
-
In an alternative scenario analysis using MADIT-CRT trial data, CRT-P and CRT-D are dominated by ICD + OPT, which is the most cost-effective strategy (ICER of £154 per QALY gained vs. OPT).
-
Overall, the relative cost-effectiveness of the strategies compared for population 3 was most sensitive to costs and the lifetime of the CRT-D device. The risk of all-cause mortality for OPT relative to CRT-D was the most influential parameter for the comparison between CRT-D + OPT and OPT alone (followed by the subsequent updates). Similarly, the preventative effect on all-cause mortality estimated for ICD therapy was particularly important for the comparison between CRT-D + OPT and ICD + OPT. The preventative effect of the devices on hospitalisation for arrhythmia, as well as the longevity of the CRT-D device, were particularly prominent for the comparison between CRT-D + OPT and CRT-P + OPT. The most influential parameters for the comparison between OPT alone (and subsequent device implantations) and ICD + OPT were the lifetime of the CRT-D and ICD devices and the risk of hospitalisation for arrhythmia associated with CRT-D, ICD and OPT.
Chapter 6 Assessment of factors relevant to the NHS and other parties
Factors relevant to service provision
The possible extension of indications for ICD and CRT devices is likely to lead to an increase in the demand for their use. This will have the potential to impact on the organisation and provision of the service and the cost of the service to the NHS. The implications are likely to be greater given the recognition that current rates of implantation of the devices remain below national targets in the UK (see Table 3), there are regional variations in utilisation55 and there is a growing ageing population that is likely to place additional pressure on the service. Any development will need to take account of the reasons for the low implantation rates as well as increasing provision for any extension of the indications. Factors thought to underlie the low implantation rates include a shortage of implantation centres and electrophysiologists, poorly developed referral strategies/care pathways and problems with specialist health-care investment. 60 Further expansion to accommodate any additional development in the service would necessitate an increase in appropriately trained cardiologists, associated clinical staff and technicians, and properly equipped implantation centres. Access to service provision and location of services are issues for consideration.
Factors relevant to patients and carers
The sudden death of a wage earner results in costs to his or her relatives that are difficult to quantify but are important nonetheless. With an ICD, individuals and their families feel reassured. The improvements associated with CRT are expected to lessen the impact of HF on the lives of individuals and their families.
Chapter 7 Discussion
Statement of principal findings
Clinical effectiveness
People at risk of sudden cardiac death: implantable cardiac defibrillators compared with optimum pharmacological therapy
Thirteen RCTs were included that compared ICDs with medical therapy, four RCTs in people at increased risk of SCD because of previous ventricular arrhythmias (secondary prevention) and nine RCTs in people who have not suffered a life-threatening arrhythmia but who are at risk (primary prevention). Risk of bias was noted in the RCTs, specifically performance bias because of lack of blinding, detection bias with regard to QoL outcomes and possible selection bias because of inadequate reporting. Length of follow-up varied from 18 to 57 months in the four RCTs on secondary prevention and from 20 to 37 months in the nine RCTs on primary prevention. Sample size ranged from 66 to 1016 in the four RCTs on secondary prevention and from 103 to 2521 in the nine RCTs on primary prevention. Most participants suffered from CHF, with 50–80% of those in the secondary prevention RCTs in NYHA classes I and II and 50–66% in the primary prevention RCTs in NYHA class II or II/III. LVEF varied from 30% to 70% in the secondary prevention RCTs and from 22% to 35% in the primary prevention RCTs. The studies were synthesised according to the criteria that they used to identify people at risk of SCD.
Ventricular arrhythmia/cardiac arrest (secondary prevention)
Four RCTs compared ICDs with AAD. Meta-analysis found that ICDs significantly reduced the risk of all-cause mortality (four RCTs; RR 0.75, 95% CI 0.61 to 0.93; p = 0.01), SCD (four RCTs; RR 0.49, 95% CI 0.34 to 0.69; p < 0.001) and total cardiac deaths (two RCTs; RR 0.74, 95% CI 0.61 to 0.91; p = 0.004). No significant differences were found between ICDs and AAD for non-arrhythmic cardiac deaths (two RCTs; RR 0.97, 95% CI 0.72 to 1.31; p = 0.83) or other non-cardiac causes of death (two RCTs; RR 0.79, 95% CI 0.45 to 1.37; p = 0.40). Two RCTs reported significant benefits for ICDs compared with AAD for overall survival at 3 years (difference 11%; p < 0.02), survival free of cardiac death at 2 years (difference 4%; p = 0.004), survival from arrhythmic death at 2 years (difference 5%, p = 0.0002) and survival free of sudden death at 57 months (HR 0.423; p = 0.005). In terms of QoL, one RCT found significant improvements in the SF-36 PCS and MCS and patient concerns checklist for both groups up to the 1-year follow-up, with no significant between-group differences. Using the MHI and NHP, another RCT showed benefits for ICDs but not OPT at 1 year of follow-up. Both RCTs showed a worsening QoL with increasing numbers of shocks. Prespecified subgroup analyses for age, LVEF, cause of arrhythmia and qualifying arrhythmia demonstrated no significant difference from each other or the overall population for all-cause mortality.
One RCT (DEBUT) was included in the present review in addition to those included in the previous TAR. 62 The population in this trial, SUDS survivors, differed from those of the other RCTs. Despite this difference, the results from the present review concur with those of the previous review. 62
People with a recent myocardial infarction (within 6–41 days or ≤ 31 days)
Two RCTs compared ICD + OPT with OPT. Meta-analysis of two trials found no difference between the groups for all-cause mortality (RR 1.04, 95% CI 0.86 to 1.25; p = 0.69), total cardiac deaths (RR 0.97, 95% CI 0.79 to 1.20; p = 0.8) and non-cardiac deaths (RR 1.39, 95% CI 0.86 to 2.27; p = 0.18). People with an ICD + OPT had a lower risk of SCD (RR 0.45, 95% CI 0.31 to 0.64; p < 0.0001) but a higher risk of non-arrhythmic cardiac death (RR 1.77, 95% CI 1.30 to 2.40; p = 0.0002) than people receiving OPT alone. One trial reporting cumulative mortality found no statistically significant difference between groups. QoL was not reported. One trial reported no significant differences for 13 prespecified subgroups (age, sex, CHF on admission, criterion of inclusion, ST-elevation MI, early reperfusion for ST-elevation MI, number of vessels, smoking and NYHA class at discharge, diabetes, hypertension, lipid abnormalities, number of risk factors) for all-cause mortality.
These trials were not included in the previous TAR. 62
People with remote myocardial infarction (> 3 weeks or > 1 month previously)
Meta-analysis of the two trials in this group found a reduction in all-cause mortality (RR 0.57, 95% CI 0.33 to 0.97; p = 0.04), total cardiac deaths (RR 0.59, 95% CI 0.42 to 0.83; p = 0.003) and SCDs (RR 0.36, 95% CI 0.23 to 0.55; p < 0.00001) with ICD + OPT compared with OPT. There was no difference between the groups in non-arrhythmic cardiac deaths (RR 0.95, 95% CI 0.41 to 2.18; p = 0.1) or non-cardiac deaths (RR 1.06, 95% CI 0.58 to 1.95; p = 0.84). One trial reporting hospitalisations found higher rates per 1000 months’ follow-up among people receiving an ICD (11.3 vs. 9.4; p = 0.09), with higher HF hospitalisations (19.9% vs. 14.9%; p-value not reported). One trial assessed QoL using the HUI3, finding a worsening QoL for both the ICD + OPT group and the OPT group annually over 3 years, with no statistically significant differences. One trial reported prespecified subgroup analyses for all-cause mortality. The HRs in all 12 of the subgroups (age, sex, ejection fraction, NYHA class or QRS interval, hypertension, diabetes, LBBB, atrial fibrillation, the interval since the most recent MI, type of ICD, and blood urea nitrogen) were similar, with no statistically significant interactions.
Both of these trials were included in the previous TAR62 and no additional RCTs in this population were identified in the present review.
People with non-ischaemic or idiopathic dilated cardiomyopathy
Three RCTs compared ICD + OPT with OPT or ICD + OPT with amiodarone + OPT. Meta-analysis found no significant difference between the groups in all-cause mortality (RR 0.77, 95% CI 0.52 to 1.15; p = 0.20), total cardiac deaths (RR 2.03, 95% CI 0.17 to 23.62; p = 0.57), non-arrhythmic cardiac deaths (RR 1.13, 95% CI 0.42 to 3.03; p = 0.81) or non-cardiac deaths (RR 0.65, 95% CI 0.13 to 3.29; p = 0.60). However, a statistically significant reduction was found in SCDs (RR 0.26, 95% CI 0.09 to 0.77; p = 0.02) with ICD therapy. No statistically significant differences were found for measures of survival or QoL using the QWBS, STAI, SF-12 MCS or PCS and MLWHFQ. One trial reported six prespecified subgroup analyses for all-cause mortality (age, sex, LVEF, QRS interval, NHYA class and history of atrial fibrillation). None of the differences between subgroups was statistically significant
An additional meta-analysis was undertaken on the advice of clinical experts, combining data on all-cause mortality from the non-ischaemic CHF subgroup of the SCD-HeFT trial with data from the three cardiomyopathy trials. The SCD-HeFT non-ischaemic subgroup strongly influenced the analysis and a statistically significant effect in favour of ICDs with no statistical heterogeneity was found for all-cause mortality (RR 0.74, 95% CI 0.58 to 0.93; p = 0.01).
Only one of the three cardiomyopathy RCTs (CAT) was included in the previous TAR;62 the other two RCTs (AMIOVIRT, DEFINITE) were excluded from the previous TAR62 on the basis of the populations included in the trials. There were no SCDs in either group in the CAT trial. However, the inclusion of the comparatively large DEFINITE trial in the present review strongly influences the results, demonstrating a significant reduction in SCDs with ICDs in people with non-ischaemic cardiomyopathy and moderate to severe left ventricular dysfunction.
People scheduled for coronary artery bypass graft surgery
No significant difference between groups was found in all-cause mortality (RR 1.08, 95% CI 0.85 to 1.38; p = 0.53), total cardiac deaths (HR 0.97, 95% CI 0.71 to 1.33; p = 0.84), non-arrhythmic cardiac deaths (RR 1.26, 95% CI 0.87 to 1.82; p = 0.21), non-cardiac deaths (RR 1.50, 95% CI 0.82 to 2.73; p = 0.19) or actuarial mortality at 4 years’ follow-up (HR 1.07, 95% CI 0.81 to 1.42; p = 0.64) in one trial. Rates of SCD were lower in the ICD group but this did not reach statistical significance (HR 0.55, 95% CI 0.29 to 1.03; p = 0.06). HRQoL was higher among people receiving OPT for all measures and this was statistically significant for some: perception of health transition, emotional role function, mental health, satisfaction with appearance and satisfaction with scar. HRs for ICDs compared with the control for all-cause mortality were found to be similar among 10 prespecified subgroups (age, sex, HF, NYHA class, LVEF, diabetes mellitus, QRS complex duration, use of ACE inhibitors, use of class I or class III AADs and use of beta-adrenergic-blocking drugs).
This trial was included in the previous TAR62 and no additional RCTs in this population were identified in the present review.
People with mild to moderate heart failure
All-cause mortality was significantly lower in the ICD + OPT group than in the placebo + OPT group (HR 0.77, 97.5% CI 0.62 to 0.96; p = 0.007) in one trial. A significant reduction in total cardiac deaths (HR 0.76, 95% CI 0.60 to 0.95; p < 0.018) and SCDs (compared with the placebo and amiodarone groups combined; RR 0.44, 95% CI 0.31 to 0.61; p < 0.00001) was also found for ICDs. There was no statistically significant difference in non-arrhythmic cardiac deaths (RR 1.14, 95% CI 0.88 to 1.48; p = 0.32) or deaths from non-cardiac causes (RR 0.92, 95% CI 0.66 to 1.27; p = 0.60) between the ICD + OPT group and the placebo and amiodarone groups combined. QoL was assessed using the DASI, MHI and global health status measures, with either a limited difference or no long-term difference between the interventions. ICD shock resulted in a significant decrease in QoL. Prespecified subgroup analyses found no interaction between ICD therapy (p = 0.68) and the cause of CHF (ischaemic or non-ischaemic) for all-cause mortality, cardiac deaths, sudden deaths presumed to be ventricular tachyarrhythmic, HF deaths or non-cardiac deaths. There was a statistically significant interaction between ICD therapy and NYHA class, with ICDs reducing the risk of all-cause mortality, cardiac mortality and sudden death presumed to be ventricular tachyarrhythmic in people in NYHA class II but not in those in NYHA class III. The interaction between ICD therapy and NYHA class was not statistically significant for HF or non-cardiac deaths.
This trial was in progress at the time of the previous TAR. 62
All four RCTs of people with previous ventricular arrhythmias reported adverse events, showing higher rates for the ICD groups (up to 30%), with most related to the placement and operation of the device. The nine primary prevention RCTs reported adverse event rates of between 5% and 61% for people with an ICD, depending on the definition of adverse event and length of follow-up. Adverse event rates for the comparator treatment were between 12% and 55% in the three RCTs reporting this. Lead-, electrode- or defibrillator generator-related problems affected 1.8–14% of people in five trials.
People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony: CRT-P or CRT-D compared with each other or with optimum pharmacological therapy
Four RCTs were included that compared CRT-P with OPT in people with HF as a result of LVSD and cardiac dyssynchrony. One of these RCTs included a third arm (CRT-D). No other RCTs comparing CRT-P with OPT or with CRT-D were identified. There was some risk of bias in the trials, although the risk of bias was unclear in some cases because of inadequate reporting. Length of follow-up in the four RCTs varied: 3 months, 6 months, a median of 11.9–15.7 months and a mean of 37.4 months including an extension period, respectively. Sample size ranged from 58 to 1520 participants. The majority of participants had NYHA class III symptoms; the remaining few had NYHA class IV symptoms. The eligibility cut-off for LVEF was ≤ 35% in the trials, with an average baseline LVEF of 22–25% where this was reported. QRS interval was required to be ≥ 120 milliseconds (two trials), ≥ 130 milliseconds or > 150 milliseconds. The average baseline QRS interval was between 160 milliseconds and 175 milliseconds. Where reported, the proportion of participants with ischaemic heart disease varied from around 40% to around 60%.
CRT-P compared with optimum pharmacological therapy
Meta-analysis found that CRT-P reduced the risk of all-cause mortality (RR 0.75, 95% CI 0.58 to 0.96, p = 0.02), HF deaths (RR 0.67, 95% CI 0.51 to 0.88, p = 0.004) and HF hospitalisations (RR 0.61, 95% CI 0.44 to 0.83, p = 0.002). Combining three RCTs in a meta-analysis demonstrated no significant difference in SCDs (RR 0.97, 95% CI 0.44 to 2.14, p = 0.94). One RCT reported no statistically significant difference in total cardiac deaths (CRT-P 17.7% vs. OPT 18.8%, p = 0.334) or non-cardiac deaths (CRT-P 2.3% vs. OPT 3.6%, p = 0.122).
More people receiving CRT-P had an improvement of one or more NYHA class (RR 1.68, 95% CI 1.52 to 1.86, p < 0.00001). One RCT reported change in LVEF and reported a statistically significant improvement with CRT-P compared with OPT (4.6% vs. –0.2%, p < 0.001) at 6 months. There was a greater improvement in exercise capacity with CRT-P, as measured by the distance walked in 6 minutes (meta-analysis of three trials; change from baseline or final values: MD 38.14 m, 95% CI 21.74 to 54.54 m, p < 0.00001). A statistically significant improvement in VO2max was also reported by two of these RCTs. All four RCTs found statistically significant improvements in QoL (using the MLWHFQ) with CRT-P (change from baseline or final values: MD –10.33, 95% CI –13.31 to –7.36). One trial also reported a statistically significant improvement in EQ-5D score and increased QALYs with CRT-P.
One trial reported prespecified subgroup analysis. A significant interaction between CRT-P and aetiology was found, with people with non-ischaemic heart disease having a greater change in LVEF. There was little difference in the effect of CRT-P on the composite outcome (death from any cause or unplanned hospitalisation for a major cardiovascular event) for 16 predefined subgroups (age, sex, NHYA class, dilated cardiomyopathy, systolic blood pressure, N-terminal pro-B-type natriuretic peptide, ejection fraction, end-systolic volume index, QRS interval, interventricular mechanical delay, mitral regurgitation area, glomerular filtration rate, beta-blocker use, spironolactone use, loop diuretics use, digoxin use).
CRT-D compared with optimum pharmacological therapy
One (three-arm) trial compared CRT-D with OPT. All-cause mortality (HR 0.64, 95% CI 0.48 to 0.86; p = 0.003), total cardiac deaths (RR 0.68, 95% CI 0.50 to 0.93; p = 0.02), SCDs (HR 0.44, 95% CI 0.23 to 0.86; p = 0.02) and HF hospitalisations (RR 0.77, 95% CI 0.63 to 0.93; p = 0.008) were reduced with CRT-D compared with OPT. There was no significant difference in HF deaths (HR 0.73, 95% CI 0.47 to 1.11; p = 0.143) or non-cardiac deaths (CRT-D 2.3% vs. OPT 3.6%; p = 0.717) between the CRT-D group and the OPT group. The proportion of people with an improvement of one or more NYHA class (57% vs. 38%; p < 0.001) and the improvements in exercise capacity [change in 6-minute walk distance: 46 m (SD 98 m) vs. 1 m (SD 93 m); p < 0.001] and QoL score (using the MLWHFQ) [–26 (SD 28) vs. –12 (SD 23); p < 0.001] were statistically significantly greater with CRT-D than with OPT.
CRT-P compared with CRT-D
One three-arm trial compared both CRT-P and CRT-D with OPT, but the trial was not powered for a statistical comparison of CRT-P with CRT-D. Direct statistical comparisons between CRT-P and CRT-D have been undertaken for the purposes of this review but should be viewed with caution.
Total cardiac deaths (RR 1.38, 95% CI 1.06 to 1.81; p = 0.02) and SCDs (RR 2.72, 95% CI 1.58 to 4.68; p = 0.0003) were higher with CRT-P than with CRT-D. All-cause mortality (RR 1.20, 95% CI 0.96 to 1.52; p = 0.12), HF deaths (RR 0.98, 95% CI 0.68 to 1.42; p = 0.93) and HF hospitalisations (28% vs. 29%) were similar between the CRT-P group and the CRT-D group. Changes in NYHA class, exercise capacity and QoL were also similar for CRT-P and CRT-D.
Two trials randomised people with successful implantation only. The other two trials reported a rate of device-related deaths of between 0.2% and 0.8% for those receiving CRT-P and 0.5% for those receiving CRT-D. The rate of moderate or severe adverse events related to the implantation procedure was reported by one trial as 10% for those receiving CRT-P and 8% for those receiving CRT-D, with 13% and 9% of CRT-P and CRT-D implantations being unsuccessful respectively. Moderate or severe adverse events from any cause were more common among those receiving CRT-D than among those receiving OPT (CRT-D 69%, CRT-P 66%, OPT 61%; CRT-D vs. OPT p = 0.03, CRT-P vs. OPT p = 0.15). Reported complications included lead displacements, infections and coronary sinus dissections.
No trials in addition to those included in the previous TAR64 were identified. However, one trial (CONTAK-CD) that was included in the previous report was not included in this section of the present report as the population, intervention and comparator were more appropriately considered in the section on people with both conditions. Despite this difference, the results from the present review concur with those of the previous review. 64
People with both conditions: CRT-D compared with optimum pharmacological therapy, CRT-P or implantable cardiac defibrillator
Nine RCTs were included that compared CRT-D with ICD in people at risk of SCD as a result of ventricular arrhythmias and with HF as a result of LVSD and cardiac dyssynchrony. No RCTs comparing CRT-D with OPT or with CRT-P were identified for this population. The risk of bias was low in some of the included trials but was unclear in others because of inadequate reporting. Length of follow-up was 6 months in five trials, 1 year in two trials and an average of 2.4 years and 3.3 years in the remaining two trials. Sample size ranged from 31 to 1820 participants. The trials differed in their eligibility criteria for HF: the majority of participants were in NYHA class II in three trials, in NYHA class III in four trials, described as having mild to moderate HF in one trial (NYHA class not reported) and in NYHA class IV in one trial. The eligibility cut-off for LVEF was ≤ 35% in seven trials and ≤ 30% in two trials, with a mean LVEF at baseline of between 21% and 26%. One trial (RethinQ) differed from the others in the criteria used to define cardiac dyssynchrony, recruiting people with a narrow QRS interval (< 130 milliseconds) and evidence of mechanical dyssynchrony on ECG. Of the other trials, the QRS interval was ≥ 120 milliseconds (four trials), ≥ 130 milliseconds (three trials) or ≥ 150 milliseconds (one trial). The mean QRS interval at baseline was 107 milliseconds in one trial (RethinQ) and between 156 milliseconds and 169 milliseconds in the remaining trials where reported. The proportion of participants with ischaemic heart disease varied from just over 50% to 100%.
Meta-analysis found that CRT-D reduced the risk of all-cause mortality (RR 0.84, 95% CI 0.73 to 0.96; p = 0.01), total cardiac deaths (RR 0.82, 95% CI 0.67 to 1.00; p = 0.05) and HF hospitalisations (RR 0.75, 95% CI 0.64 to 0.88; p = 0.0005) compared with ICD therapy. Fewer trials reported HF deaths or SCDs separately, and no HF deaths or SCDs occurred in some of these trials. Combining three RCTs in a meta-analysis found little difference in SCDs between the CRT-D group and the ICD group (RR 1.45, 95% CI 0.43 to 4.92; p = 0.55).
Meta-analysis of four trials found no statistically significant difference between groups in the proportion of people experiencing at least one episode of VT or VF (RR 0.90, 95% CI 0.71 to 1.14; p = 0.38). An improvement in average NYHA class (MD –0.19, 95% CI –0.34 to –0.05; p = 0.008) and in the proportion of people who improved by one or more NYHA class (RR 1.81, 95% CI 0.91 to 3.60; p = 0.09) and in average LVEF (MD 2.15%, 95% CI 0.45 to 3.86%; p = 0.01), left ventricular end-diastolic volume (MD –19.7 ml, 95% CI –32.1 to –7.3 ml; p < 0.0.002) and left ventricular end-systolic volume (MD –20.9 ml, 95% CI –32.9 to –8.8 ml; p < 0.0007) was found with CRT-D. There was no overall difference in end-diastolic diameter (MD –0.29 mm, 95% CI –1.67 to 1.08 mm; p = 0.67) or end-systolic diameter (MD –1.88 mm, 95% CI –4.39 to 0.62 mm; p = 0.14). Substantial statistical heterogeneity was present for these outcomes and some trials reported median values, which may indicate skewed data. One trial of people with moderate to severe HF found a significantly greater reduction in QRS interval with CRT-D than with ICD (–20 milliseconds vs. 0 milliseconds; p < 0.001). The QRS interval was similar in the CRT-D and ICD groups in two trials of people with mild or mild to moderate HF.
There was a greater improvement in exercise capacity (change in peak VO2: MD 0.75 ml/kg/minute, 95% CI 0.23 to 1.27 ml/kg/minute; p = 0.005; change in 6-minute walk distance: MD 14.5 m, 95% CI 2.9 to 26.1 m; p = 0.01) and QoL (change in MLWHFQ score: MD –6.9, 95% CI –10.4 to –3.4; p = 0.0001) with CRT-D than with ICD. One small trial of people with mild to moderate HF reporting other measures of QoL (DASI, one-item Global Visual Analogue Scale and SF-36) found that the comparisons of baseline to 6-month changes were statistically significantly different for the general health component of the SF-36 only.
When the large RAFT trial contributed data to the meta-analyses, the results were strongly influenced by it. The RAFT trial included people with mild to moderate HF despite receiving OPT, a LVEF of ≤ 30% from ischaemic or non-ischaemic causes, a wide QRS interval and planned ICD implantation for indicated primary or secondary prevention of SCD.
The extent of reporting of adverse events varied between the trials. Some trials reported adverse events for all people undergoing implantation attempts, but only randomised people who had a successful implant. Only three trials reported adverse events according to the device received. The large RAFT trial reported adverse events for all implanted participants and found that the rate of device- or implantation-related complications within 30 days of implantation was significantly higher in the CRT-D group than in the ICD group (13.3% vs. 6.8%; p < 0.001), as was the rate of device-related hospitalisations (20% vs. 12.2%, HR 1.68, 95% CI 1.32 to 2.13; p < 0.001).
Three trials reported prespecified subgroup analyses. Two trials reported that CRT-D was associated with a greater benefit in people with a QRS duration of ≥ 150 milliseconds than in those with a QRS duration of < 150 milliseconds; the third trial found a significant increase in the proportion of people with an improvement in peak oxygen uptake among those with a QRS duration of ≥ 120 milliseconds but not among those with a QRS duration of < 120 milliseconds. CRT-D was associated with a greater benefit in women than in men (one trial) and with a greater benefit in people with LBBB than in those with non-specific intraventricular conduction delay (one trial). One trial found a statistically significant improvement with CRT-D in distance walked in 6 minutes for those with non-ischaemic cardiomyopathy (55.0 m vs. 2.5 m; p = 0.01) but not for those with ischaemic cardiomyopathy (4.2 m vs. 5.8 m; p = 0.57). Other evaluated subgroups showed no statistically significant effects.
This evidence (apart from the CONTAK-CD trial) has not been previously evaluated in a TAR. 62,64
Summary of the industry-submitted individual patient data network meta-analysis
The MS reported an IPD NMA that assessed the effectiveness of ICDs, CRT-P and CRT-D compared with OPT for people with HF. As people with HF vary considerably, the NMA aimed to identify subgroups who may benefit from the different interventions. The NMA assessed the outcomes of all-cause mortality, all-cause hospitalisation and HRQoL, with the findings informing the economic model presented in the MS. The focus of the NMA differed from that specified in the scope for the appraisal, trying to establish which subgroups may benefit from the interventions rather than assessing their effectiveness in the groups identified in the original decision problem.
The NMA was based on a network of evidence identified from a systematic review presented in the MS. It included 13 of 22 trials (95% of patients in the network) from the network for which IPD were available. The network excluded seven RCTs identified in this report. The evidence base for the different outcomes varied (all-cause mortality: 13 trials, all-cause hospitalisation: 11 trials and HRQoL: three trials), resulting in limited and, on occasions, skewed data that affected the results of the NMA. The MS outlined the methods followed in the different stages of the NMA; however, it did not provide comprehensive results from each stage to allow a full appraisal of the decisions made and their effect on the results. The IPD NMA used meta-regression to assess the clinical effectiveness of the different interventions, allowing the impact of different patient characteristics to be taken into account in the analysis (i.e. baseline risks and treatment modifiers). The NMA followed a two stage process: first, baseline rates were estimated for patients randomised to the comparator treatment of OPT independent of treatment effects; second, device-specific treatment effects were estimated from relevant IPD trials to allow comparison with the baseline rates. Baseline risk and treatment effect modifiers (i.e. patient characteristics) were included in both stages to allow subgroups to be identified. When possible, the MS assessed the validity of the results against other evidence, making adjustments when considered necessary because of counterintuitive results or a lack of data.
The results of the NMA showed that there was a benefit for people receiving a device compared with OPT for the three outcomes; however, the extent of the benefit and the subgroups most affected remained uncertain. Fixed-effects NMA without the covariables for all-cause mortality estimated HRs that showed a statistically significant benefit for all devices compared with OPT (commercial-in-confidence information has been removed). HRs showed a statistically significant benefit for CRT-D compared with CRT-P (commercial-in-confidence information has been removed) and ICD (commercial-in-confidence information has been removed). NMA models including covariables (treatment modifiers) reported findings that were more equivocal and the MS states that they should be interpreted with caution. Although HRs showed that all devices appeared to have a beneficial effect compared with OPT, rarely were the differences statistically significant. CRT-D appeared to have a statistically significant effect for people with QRS duration of ≥ 150 milliseconds. It also had an effect for people with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds, which was statistically significant for women and marginally insignificant for men. ICDs had a statistically significant benefit for men aged < 60 years and men aged ≥ 60 years with a QRS duration from ≥ 120 milliseconds to < 150 milliseconds and without LBBB. CRT-P provided a statistically significant effect for women with a QRS duration of ≥ 150 milliseconds and LBBB. Similar benefits from all devices when compared with OPT were shown for all-cause hospitalisations, although limited data meant that some comparisons were not possible. All-cause hospitalisations were reduced in people in NYHA classes I–III receiving an ICD (commercial-in-confidence information has been removed), in NYHA classes III and IV with CRT-P (commercial-in-confidence information has been removed) and in all NYHA groups with CRT-D (commercial-in-confidence information has been removed). Results for HRQoL were less clear because of the scarcity of data available for the NMA. Although the use of the devices led to improvements in EQ-5D values, some comparisons could not be made and others produced counterintuitive results. As a consequence, the MS adjusted values to show that ICDs had benefit for people in NYHA class I/II and that CRT-P and CRT-D had the same effect for people in NYHA classes III and IV. Given that most utility values were changed and that limited comparisons can be made with other evidence, these data should be interpreted with caution.
The IPD NMA provides an opportunity to undertake a more detail analysis of the effectiveness of ICDs, CRT-P and CRT-D in relation to the comparator treatment of OPT, evaluating the benefits for specific groups of people with HF. Unfortunately, limitations in the data available and lack of detail concerning the methods used render the findings uncertain. It is clear that all of the devices are beneficial compared with OPT for all-cause mortality. They also appear to have benefit for the outcomes of all-cause hospitalisation and HRQoL, although the extent of the effect is less clear. However, the benefits for specific subgroups remain unclear. When some benefits are shown, the warnings in the MS concerning the analysis cause some concern. In addition, the subgroups identified in the NMA differ from those outlined in the scope for the appraisal, making translation of the results between them difficult.
Cost-effectiveness
Summary of previously published economic evaluations
The systematic review of the cost-effectiveness of ICDs for the treatment of arrhythmia and of CRT for treatment of HF identified 51 studies (36 studies of ICDs and 17 of CRT). Most of the evaluations employed state transition models to estimate long-term outcomes extrapolated from short-term outcomes in trials. Almost half of the studies reported that ICDs were cost-effective, with the remaining studies finding that ICDs were cost effective only in high-risk groups or were not-cost effective or that it was uncertain whether they were cost-effective. One high-quality study was conducted for a UK setting and perspective and reported a mean ICER for ICDs compared with AADs for an average UK secondary prevention patient over a 20-year time horizon of £76,139 per QALY gained. However, these results may not be applicable to current UK practice as some data used in the model are now out of date. Almost all studies reported that CRT was cost-effective, with only two studies uncertain whether it was cost-effective. One high-quality study was conducted for a UK setting and estimated an ICER of £16,735 per QALY gained for CRT-P compared with OPT and an ICER of £40,160 per QALY gained for CRT-D compared with CRT-P.
Summary of the systematic review of quality-of-life studies
The systematic review found six relevant HRQoL studies that measured EQ-5D in HF, stratified by NYHA class, or that reported on patients who had previously received an ICD. Two studies were conducted in patients who had received an ICD. One study of UK patients who responded to a postal questionnaire found that mean EQ-5D score did not change with time after implant; the other study of volunteers attending a defibrillator clinic in the USA reported no difference between the EQ-5D scores of primary and secondary prevention patients and that QoL for ICD patients was similar to that of the general population. Four cohort studies reported EQ-5D scores in HF, with baseline scores ranging from 0.44 to 0.66 depending on NYHA classification. Overall, the results show decreased EQ-5D scores in HF compared with those of the general population, particularly in NYHA classes III and IV.
Summary of the industry-submitted economic evaluation
One submission was received from ABHI. The general approach taken in the MS seems reasonable, with the model structure consistent with the current understanding of HF and ventricular arrhythmia. Assumptions over costing are also consistent with current clinical practice. However, there is limited reporting in the MS on some sources of evidence used in the model. Uncertainty is not comprehensively assessed as the sensitivity analyses presented are limited to few scenarios and the methodology used for the probabilistic sensitivity analysis is not described in sufficient detail to determine whether or not joint parameter uncertainty was properly assessed. The cost-effectiveness results presented in the submission (according to subgroups specified by ABHI) do not directly address questions posed in NICE’s scope,61 as it is unclear how the subgroups selected relate to the groups scoped by NICE. Overall, the results show that for most subgroups there is at least one device with an ICER of < £30,000 per QALY gained, and in some cases a different device might have an ICER of < £20,000 per QALY gained.
Summary of the independent economic model
We developed an independent state transition model based on that created by Fox and colleagues64 for TA120. 43 The care pathways and assumptions have been adapted according to new evidence and clinical advice to allow for the assessment of the cost-effectiveness of ICDs, CRT-P and CRT-D for people at risk of SCD as a result of ventricular arrhythmias and/or HF as a result of LVSD and cardiac dyssynchrony.
People at risk of sudden cardiac death
The current economic model indicates that the initial management of patients at increased risk of SCD with an ICD alongside OPT is a cost-effective strategy compared with initial treatment with OPT alone (ICER £19,479 per QALY). The use of ICDs for the secondary prevention of SCD had a 51% and 82% probability of being cost-effective at a WTP of £20,000 and £30,000 per QALY gained respectively. ICDs were also estimated as being cost-effective (within the WTP range of £20,000–30,000 per QALY gained) for the primary prevention subgroups analysed (people with remote MI, a broad population with mild to moderate HF, and non-ischaemic cardiomyopathy patients). The parameters with the greatest impact on the cost-effectiveness results were the time horizon, the HR for all-cause mortality associated with the ICD + OPT arm, the risk of surgical death during ICD implantation and the lifetime of the device.
People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony
For patients with HF as a result of LVSD and cardiac dyssynchrony, the base-case analysis found that the addition of either CRT-P or CRT-D to OPT (in the initial stage of management of HF) may be considered cost-effective at a WTP of £30,000 compared with OPT alone (allowing for subsequent device implantation), with ICERs of £27,584 per QALY and £27,899 per QALY respectively. The use of CRT-D + OPT compared with CRT-P + OPT was also likely to be cost-effective (ICER £28,420 per QALY). At a WTP of £20,000 per QALY, initial management with OPT alone (followed by the clinically necessary device implants) was the strategy with the highest probability of being cost-effective (81%). Above a WTP of £28,000 per QALY, the strategy with the highest probability of being cost effective was CRT-D + OPT (38%). At £30,000 per QALY, CRT-D + OPT and CRT-P + OPT had a 46% and 31% probability of being cost-effective, respectively, whereas OPT alone had a 23% probability of being cost-effective.
The parameters with the most influence on the model results for the comparison between CRT-P and OPT were the risk of hospitalisation for a serious arrhythmic event for patients receiving CRT-P, the risk of HF death for both patients receiving CRT-P and patients receiving CRT-D and the risk of SCD for patients receiving CRT-P. The results of the comparison between CRT-D and OPT were most influenced by the risks of HF death and SCD death in CRT-D patients and the lifetime of the device. The results of the comparison between CRT-D and CRT-P were the most sensitive to the variation of individual parameters, with eight parameters causing the ICER to range by > £10,000, the most influential being the risk of HF death for CRT-D patients and the risk of SCD for both CRT-D and CRT-P patients.
People with both conditions
The base-case analysis found that the most cost-effective strategy for people with both conditions at a WTP of £20,000–30,000 per QALY was initial management with OPT alone (followed by device implantation and subsequent upgrades as necessary), with an ICER of £2824 per QALY compared with ICD + OPT (the least costly and least effective strategy). Costs and QALYs for CRT-D + OPT and CRT-P + OPT were similar. CRT-D had an ICER of < £30,000 when compared with ICD + OPT (ICER £27,195 per QALY) but not when compared with initial management with OPT alone (ICER £35,193 per QALY). At a WTP of £30,000 per QALY, OPT alone, ICD + OPT, CRT-D + OPT and CRT-P + OPT had a 44%, 31%, 15% and 10% probability of being cost-effective respectively. Above a WTP of £42,000 per QALY, the intervention with the highest probability of being cost effective was CRT-D + OPT (31%).
However, the results differ when using an alternative scenario from the MADIT-CRT trial. In this case, ICD + OPT is slightly more costly but yields a greater benefit than OPT alone. As CRT-P + OPT and CRT-D + OPT are less effective than ICD + OPT and much more costly, both CRT strategies are dominated by ICD + OPT compared with OPT alone. Therefore, the results obtained with the MADIT-CRT data indicate that ICD + OPT is the most cost-effective strategy, with an ICER of £154 per QALY gained compared with OPT alone. The cost-effectiveness results for the comparison between CRT-D + OPT and ICD + OPT were quite robust to the variation of input parameters. The most influential parameters for this comparison were the RR of all-cause mortality for ICD patients and the lifetime of the CRT-D and ICD devices.
Exploration of differences in results between population 2 and population 3
In response to comments querying the face validity of the results for population 3 compared with population 2, the differences were explored.
The baseline mortality risk for population 2 was higher than that for population 3, although the RR improvement with CRT-P compared with OPT was similar in the two populations. In the original analyses there was a greater benefit in terms of survival for population 2 than for population 3 because of the high numbers of crossovers to CRT-D in the OPT group in population 3. A new scenario for population 3 was conducted using a higher baseline risk, similar to the risk of all-cause mortality for population 2. All-cause mortality for population 3 was 50% higher (all-cause mortality yearly probability: OPT 0.105, CRT-D 0.065), giving a similar ICER to the baseline ICER (£34,964 vs. £35,193).
The approach to modelling changes in QoL also differed between population 2 and population 3. The population 2 model assumed a given initial distribution of patients by NYHA class (initially more severe than that in the population 3 model). At 9 and 18 months, different distributions by NYHA class (derived from the CARE-HF and Brescia studies) were assumed to capture the effect of CRT on patients HRQoL. In the population 3 model, the HRQoL of patients was kept constant over time, assuming an initial distribution of patients per NYHA class as reported for the RAFT trial at baseline. The effect of these differences is that there is more QoL benefit in population 2 than in population 3 for patients receiving CRT-D. It is likely that these differences in HF progression explain some of the differences between the results for populations 2 and 3. A scenario analysis was undertaken assuming that population 3 has the same HF progression as population 2. This resulted in an ICER of £27,396 per QALY gained for CRT-D compared with OPT, similar to that in the population 2 model (£27,899 per QALY).
Strengths and limitations of the assessment
Strengths
This review has the following strengths:
-
It is independent of any vested interest.
-
It has been undertaken following the principles for conducting a systematic review. The methods were set out in a research protocol that defined the research question, inclusion criteria, quality criteria, data extraction process and methods to be employed at different stages of the review.
-
A multidisciplinary advisory group has informed the review from its initiation. The research protocol was informed by comments received from the advisory group and the advisory group has reviewed and commented on the final report.
-
The review brings together within one assessment report the most up-to-date evidence for the clinical effectiveness and cost-effectiveness of ICDs, CRT-P and CRT-D for people at risk of SCD as a result of ventricular arrhythmias and/or HF as a result of LVSD and cardiac dyssynchrony. This evidence has been critically appraised and presented in a consistent and transparent manner.
-
An economic model has been developed de novo following recognised guidelines and systematic searches have been conducted to identify data for the economic model. The main results have been summarised and presented.
Limitations
In contrast, this assessment also has certain limitations.
Limitations of the included trials
-
Randomised patients with successful implantation may overestimate the benefits and underestimate adverse effects.
-
Trials have not been conducted in the UK and may not be generalisable.
-
The time horizon of the included trials may be inadequate.
-
Blinding of participants and health-care providers is impossible in trials that compare devices and drugs; however, it is important to acknowledge the bias that may occur as a result of this. It would be possible to blind outcome assessors in these trials.
-
The definition of OPT has changed over time; therefore, the pharmacological therapy used in some of the included trials would not be considered optimal by current standards.
Limitations of the systematic review of clinical effectiveness
-
Three populations were defined by the NICE scope;61 however, there are no accepted a priori criteria that could be used to categorise trials. Clinical experts were consulted to allocate trials to the population groups and pragmatic decisions were taken to allocate trials and ensure that all relevant RCT evidence comparing eligible interventions and comparators was included.
-
A decision was made to also include trials in which medical therapy would not be considered optimal by current standards. Pharmacological therapy varied between the trials and was described in detail.
-
The MUSTT and MAVERIC trials were excluded from the systematic review as the intervention did not meet the scope of the review (many participants in the intervention arm did not receive an ICD); however, these trials presented subgroup data for the comparison between ICD therapy and no ICD therapy. These trials were not subjected to formal data extraction and quality assessment but were presented for information.
-
Significant statistical heterogeneity was shown between trials for some outcomes; therefore, the pooled data should be viewed with caution. Some trials reported median values and CIs rather than mean values. Median values are similar to mean values when the distribution of data is symmetrical and so can be used directly in the meta-analyses. 65 However, means and medians can be very different from each other if the data are skewed. The use of median values in some of the meta-analyses may have contributed to statistical heterogeneity.
-
The review included only subgroup analyses specified a priori by the trials. However, subgroup analysis lacks statistical power and may be misleading, for example because of problems of multiplicity. Subgroup analyses should therefore be viewed with caution.
Limitations of the independent economic model
The independent model for the current appraisal was developed to address the decision problem specified in the NICE scope for the appraisal61 and followed recommended guidance provided in the NICE Guide to the Methods of Technology Appraisal. 67 It was based on an adaptation of a model structure used in the previous appraisal of CRT for HF (TA12043), developed by Fox and colleagues,64 providing a consistent approach and comparability. Despite following recognised guidance on developing economic models,67,68 the evaluation has some limitations:
-
As the independent model was based on an adaptation of a model developed by Fox and colleagues,64 it relies on some of the same assumptions made with regard to the structure of the model. These relate to the referral of patients receiving particular treatment options, whether the comparator or an intervention, to receive an alternative intervention following occurrence of a particular event (e.g. a non-fatal arrhythmia for a patient on OPT or a serious arrhythmic event for a patient on CRT-P or an unsuccessful CRT-P implantation). As these were validated by Fox and colleagues by clinical advice and considered during previous appraisals, it was felt that they were of limited concern.
-
Additional structural assumptions were made with regard to the risks and timing of the reimplantation of devices, alternative options for those patients with unsuccessful implantation, and perioperative complications, surgical failure, heart transplantation and death. As with the assumptions in the model by Fox and colleagues,64 these were incorporated following clinical advice.
-
Survival estimates over time for the model were derived from relevant trials with the longest follow-up. These were identified in the systematic review of clinical effectiveness produced for this assessment. Given the heterogeneous nature of the studies included, it is possible that the studies used in the analysis did not encompass the differences in the patient groups. To limit any possible effects, base-case and subgroup analyses were carried out to try and encompass the different patients included. Also, follow-up varied (range 18–45.5 months) in the different studies used, affecting the extent to which survival curves had to be extrapolated.
-
Parameter values for the clinical effectiveness of the interventions were sourced, where possible, from the systematic review undertaken for this assessment. Unfortunately, limitations in the evidence base meant that some parameters either were not available for the specific populations being modelled or were presented in a single study that may not have encompassed the inherent variability in heterogeneous patient populations being assessed (e.g. hospitalisation rates, complications). When necessary, parameter values were obtained from studies in other population groups included within the appraisal or from other studies or sources outside of the systematic review. These were assumed to be representative.
-
The evidence base for patients who had both HF and an increased risk of SCD (population 3) was limited, with most studies assessing CRT-D or ICDs. In particular, the lack of a direct comparison between CRT-P and CRT-D meant that evidence had to be used from studies on the clinical effectiveness of CRT-P and CRT-D in patients with HF as a result of LVSD and cardiac dyssynchrony (population 2).
-
The availability of HRQoL data varied for the effects of the different devices and for additional procedures or adverse events. Baseline utility values were available by NYHA class. Data were not identified for the effects of transplantation, surgery or infections and assumptions were made following those used by Fox and colleagues. 64 Device-related utility values were assessed through their effect on changes in the distribution of patients by NYHA class. Data were available only for patients receiving CRT-P or OPT alone for population 2 and so the effects of CRT-D were assumed to be the same as for CRT-P devices. Robust evidence on HRQoL was not found for population 3 and so CRT and ICD devices were assumed to have no impact on utility and baseline values were maintained. These assumptions may underestimate the benefits of the devices for HRQoL.
-
Resource use and costs were obtained from routinely published sources. As some costs were not specifically identified in the routine sources, assumptions were made. These included the costs of the implantation of the devices, the costs of upgrades and routine replacements, the costs of operative complications and device-related complications and drug costs. Alternative data were sourced from Fox and colleagues,64 the MS and clinical advice.
-
The model structure allows patients initially managed with OPT or CRT-P to have a device upgraded to a different device according to disease progression. The result of this assumption is that there are a large number of upgrades in some population arms. This is most evident in population 3. These upgrades occur in patients who experience hospitalisation because of non-fatal arrhythmia (and then undergo ICD/CRT-D implantation) and are based on the previous modelling structure in the study by Fox and colleagues. 64
When limitations have arisen in the evaluation, these have been identified in the report. Assumptions made or data identified from alternative sources have been checked through clinical advice, and the effects parameters thought to be influential on the results have been assessed through sensitivity analyses.
Comparison of the independent economic evaluation with other evaluations
For patients in the UK at increased risk of SCD, Buxton and colleagues153 estimated an ICER of £76,139 per QALY gained for ICD + OPT compared with OPT for the secondary prevention of SCD over a 20-year time horizon. As some data used in the model are now out of date, these results may not be applicable to current UK practice and may not be comparable with the results of the current model. Different modelling structures and different data inputs were used in the current model, as well as different approaches to estimate HRQoL. Both models estimated similar utility values for the OPT and ICD + OPT cohorts; however, the average utility values estimated in the current model for OPT alone (0.81) and ICD + OPT (0.82) are higher than the 0.75 assumed for both arms by Buxton and colleagues. Scenario analysis using the same average utility values as used by Buxton and colleagues153 resulted in an ICER of £22,372 per QALY gained for ICD + OPT compared with initial management with OPT alone for the secondary prevention of SCD.
For patients with HF, Fox and colleagues64 estimated ICERs of £16,735 per QALY gained for CRT-P compared with OPT, £22,231 per QALY gained for CRT-D compared with OPT and £40,160 per QALY gained for CRT-D compared with CRT-P. The current model estimates a slightly higher cost and QALY gain for all strategies. However, the estimated incremental benefit of CRT-P compared with OPT is less than that in the previous model and is associated with a higher incremental cost; hence, an ICER of £25,779 per QALY gained is estimated for CRT-P compared with OPT. As a greater incremental benefit is estimated with CRT-D compared with CRT-P at a similar cost, a smaller ICER (£24, 943 per QALY) is estimated for CRT-D compared with CRT-P. The same incremental benefit is estimated for CRT-D compared with OPT but the current model estimates a higher incremental cost for CRT-D; thus, a higher ICER (£27,899 per QALY) is estimated for CRT-D compared with OPT.
The differences in results between models can be explained by using updated costs, different estimates of the lifetime of the devices, a different set of utilities by NYHA class and structural differences between models (such as referring patients being managed with OPT alone for CRT-P implantation in case of hospitalisation for HF, instead of ICD, or for CRT-D following hospitalisation for arrhythmia). Using the same utility values as in the model of Fox and colleagues64 increases the incremental benefit of both CRT-P and CRT-D compared with OPT and with each other and therefore reduces the ICERs to £22,892 per QALY gained for CRT-P compared with OPT, £24,580 per QALY gained for CRT-D compared with OPT and £27,893 per QALY gained for CRT-D compared with CRT-P. The scenario using the same device lifetime estimates as in the study by Fox and colleagues64 resulted in higher ICERs for CRT devices compared with OPT because of higher costs and slightly fewer QALYs estimated for both CRT-D + OPT and CRT-P + OPT.
The joint economic evaluation submitted by ABHI151 concluded that for most subgroups there is at least one device with an ICER of < £30,000 per QALY gained and in some cases a different device might have an ICER of < £20,000 per QALY gained. The general approach taken in the MS seems reasonable as the model structure is consistent with the current understanding of HF and ventricular arrhythmia and the assumptions over costing are also consistent with current clinical practice. However, the cost-effectiveness results presented in the MS (according to subgroups specified by ABHI) do not directly address questions posed in NICE’s scope,61 as it is unclear how the subgroups selected relate to the groups scoped by NICE. The independent economic model was developed to address NICE’s scope and was based on the published clinical evidence and on previously published evaluations. Hence, a different modelling approach was taken and the limited data available did not allow for the analysis of the subgroups defined by ABHI. It is therefore unclear how the cost-effectiveness results of the current model compare with those from the MS.
Other recent systematic reviews/meta-analyses
Huang and colleagues230 presented a meta-analysis comparing CRT-D with no CRT-D (CRT-P, ICD or OPT) and found that all-cause mortality was reduced in CRT-D patients. However, three of the trials included were not RCTs. Subgroup analysis comparing CRT-D with ICD therapy is also presented but includes only three of the nine relevant trials identified by the current review. Without the large RAFT trial, the meta-analysis by Huang and colleagues230 found no significant difference in all-cause mortality between CRT-D and ICD therapy. Al-Majed and colleagues231 assessed CRT in people with advanced HF and those with less symptomatic disease. The inclusion criteria for their systematic review differed from those in the present review (eligible comparators were inactive pacing, right or left ventricular pacing alone and ICD therapy); therefore, there are some differences in the trials included in the meta-analyses and the results are not directly comparable. The meta-analyses found that CRT-D reduced all-cause mortality and HF hospitalisations in subgroups with NYHA class I/II and class III/IV symptoms. Functional outcomes were improved in people with NYHA class III/V but not class I/II symptoms. A systematic review and meta-analysis by Wells and colleagues232 compared CRT-D with ICD therapy or OPT and conducted subgroup analysis for NYHA class. All-cause mortality was reduced with CRT-D compared with ICD therapy or OPT. Compared with ICD therapy, CRT-D reduced all-cause mortality for people with NYHA class I or II but not class III or IV symptoms. The differences in effects for the NYHA class subgroups between these the two meta-analyses231,232 are due to the different comparators and trials included. A meta-analysis by Bertoldi and colleagues233 also found a significant reduction in all-cause mortality with CRT-P compared with OPT and with CRT-D compared with ICD therapy, despite including slightly different trials.
Uncertainties
-
No new evidence comparing CRT-P and CRT-D devices was identified; therefore, the relative clinical effectiveness and cost-effectiveness of the devices in people with HF as a result of LVSD and cardiac dyssynchrony, with or without an established indication for an ICD, remains uncertain.
-
No robust evidence was identified on the effect of CRT and ICD devices on HF progression in people with both conditions.
-
No evidence was found on the RR of hospitalisation because of arrhythmia for CRT-P devices compared with CRT-D devices in people with both conditions; hence, CRT devices were assumed to have the same preventative effect on severe arrhythmia. New evidence would reduce the uncertainty associated with this parameter, to which the comparison between CRT-D + OPT and CRT-P + OPT showed particularly sensitivity.
-
Utility data were not identified for patients with both conditions or for patients receiving CRT-D or an ICD. Also, no utility decrements were found for the effects of transplantation, surgery or infections.
-
Routine cost data were not available for the implantation of devices, upgrades and routine device replacements or for operative complications.
Chapter 8 Conclusions
Implications for service provision
Implantable cardioverter defibrillators were found to reduce all-cause mortality in people who were at increased risk of SCD as a result of ventricular arrhythmias, in which increased risk was defined as previous ventricular arrhythmias/cardiac arrest, MI > 3 weeks previously, non-ischaemic cardiomyopathy (depending on the data included) or ischaemic or non-ischaemic CHF and a LVEF of ≤ 35%. No benefit from an ICD was found in people who were scheduled for CABG surgery. A significant reduction in SCD was found in people with a recent MI, but there was no difference in all-cause mortality. No significant differences between prespecified subgroups were reported by most of the trials reporting these. The addition of ICD to OPT was cost-effective at a WTP threshold of £30,000 for all of the scenarios modelled, and at a WTP threshold of £20,000 in some cases.
Cardiac resynchronisation therapy – pacer and CRT-D both reduced the risk of mortality and HF hospitalisations in people with HF as a result of LVSD and cardiac dyssynchrony when compared with OPT. Improvements in NYHA class, exercise capacity and QoL were also found with both devices. The risk of SCD was lower with CRT-D than with CRT-P, but other outcomes, including all-cause mortality, were similar between the devices. Both CRT-P and CRT-D had ICERs of < £30,000 per QALY gained compared with OPT, as did the comparison between CRT-D and CRT-P.
Compared with ICD, CRT-D reduced the risk of all-cause mortality and HF hospitalisation in people with both conditions. An improvement in LVEF, exercise capacity and QoL was also found with CRT-D compared with ICD. Device or implantation complications were more common with CRT-D. The costs and QALYs for CRT-D and CRT-P were similar. The ICER was < £30,000 per QALY for the comparison of CRT-D + OPT with ICD + OPT (unless no difference in all-cause mortality was assumed) but not for the comparison with initial management with OPT alone.
The conclusions should be considered in light of the limitations of this evaluation, such as the approach of allocating heterogeneous trials to three population groups and the uncertainties in the economic evaluation.
Suggested research priorities
One three-arm trial comparing CRT-D and CRT-P with OPT in people with HF as a result of LVSD and cardiac dyssynchrony was identified by the systematic review. The trial was not designed to directly compare CRT-D and CRT-P and no additional trials of this comparison were identified. Furthermore, the trial excluded people meeting the general indications for an ICD. A RCT comparing CRT-D and CRT-P in people with HF as a result of LVSD and cardiac dyssynchrony is required, for both those with and those without an ICD indication.
The evidence base for ICD therapy in cardiomyopathy is limited. A trial is needed into the benefits of ICDs for non-ischaemic cardiomyopathy in the absence of dyssynchrony.
Acknowledgements
We would like to thank members of our advisory group panel who provided expert advice and comments on the protocol, the map of eligible trials, the clinical pathways and/or a draft of this report: Dr R Anderson, Associate Professor of Health Economics and Evaluation and Deputy Director, Peninsula Technology Assessment Group (PenTAG), University of Exeter; Professor AJ Camm, Professor of Clinical Cardiology, St George’s Hospital Medical School, London; J Fearnley, Head of Strategic Operations, Arrhythmia Alliance, the Heart Rhythm Charity; Dr A Morley-Davies, Consultant Cardiologist/Electrophysiologist, University Hospital of North Staffordshire; Dr C Pepper, Consultant Cardiologist, Leeds General Infirmary; Dr D Todd, Liverpool Heart and Chest Hospital NHS Foundation Trust.
We are also grateful to Karen Welch, Information Specialist, SHTAC, University of Southampton, for generating and running the literature searches; Geoff Frampton, Research Fellow, SHTAC, University of Southampton, for contributing to the background section; Jeremy Jones, Principal Research Fellow in Health Economics, SHTAC, University of Southampton, for contributing to the development of the protocol and economic evaluation; and Jonathan Shepherd, Principal Research Fellow, SHTAC, University of Southampton, for reviewing a draft of this report.
The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the HTA programme, the NIHR, the NHS or the Department of Health. Any errors are the responsibility of the authors.
Contributions of authors
Jill L Colquitt (senior research fellow) co-ordinated the project, developed the research protocol, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted and edited the final report.
Diana Mendes (research fellow) developed the protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, developed the economic evaluation and drafted the report.
Andrew J Clegg (Professor of Health Services Research/Director of SHTAC) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report and acted as guarantor for the project.
Petra Harris (research fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted the report.
Keith Cooper (senior research fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, developed the economic evaluation and drafted the report.
Joanna Picot (senior research fellow) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies and contributed to the background section.
Jackie Bryant (principal research fellow) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Implantable Cardioverter Defibrillators for the Treatment of Arrhythmias and Cardiac Resynchronisation Therapy for the Treatment of Heart Failure (Review of TA95 and TA120). Final Scope. London: NICE; 2011.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: a Textbook of Cardiovascular Medicine. Philadelphia, PA: Elsevier Saunders; 2012.
- Chugh S, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008;51:213-28. http://dx.doi.org/10.1016/j.pcad.2008.06.003.
- Office for National Statistics . Deaths Registered in England and Wales in 2010 by Cause 2011. www.ons.gov.uk/ons/dcp171778_239518.pdf (accessed 23 January 2014).
- World Health Organization . International Classification of Diseases, 10th Edition. n.d. www.who.int/classifications/icd/en/ (accessed April 2014).
- Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation 2012;125:1043-52. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.023846.
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 2006;48:e247-346. http://dx.doi.org/10.1016/j.jacc.2006.07.010.
- Pratt CM, Greenway PS, Schoenfeld MH, Hibben ML, Reiffel JA. Exploration of the precision of classifying sudden cardiac death. Implications for the interpretation of clinical trials. Circulation 1996;93:519-24. http://dx.doi.org/10.1161/01.CIR.93.3.519.
- Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation 2008;118:1497-518. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.189375.
- Lopshire JC, Zipes DP. Sudden cardiac death: better understanding of risks, mechanisms, and treatment. Circulation 2006;114:1134-6. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.647933.
- Youngquist ST, Kaji AH, Niemann JT. Beta-blocker use and the changing epidemiology of out-of-hospital cardiac arrest rhythms. Resuscitation 2008;76:376-80. http://dx.doi.org/10.1016/j.resuscitation.2007.08.022.
- Polentini MS, Pirrallo RG, McGill W. The changing incidence of ventricular fibrillation in Milwaukee, Wisconsin (1992–2002). Prehosp Emerg Care 2006;10:52-60. http://dx.doi.org/10.1080/10903120500366961.
- Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA 2002;288:3008-13. http://dx.doi.org/10.1001/jama.288.23.3008.
- Bunch TJ, White RD. Trends in treated ventricular fibrillation in out-of-hospital cardiac arrest: ischemic compared to non-ischemic heart disease. Resuscitation 2005;67:51-4. http://dx.doi.org/10.1016/j.resuscitation.2005.04.015.
- Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81:1479-87. http://dx.doi.org/10.1016/j.resuscitation.2010.08.006.
- Bunch TJ, Kottke TE, Lopez-Jimenez F, Mahapatra S, Elesber AA, White RD. A comparative analysis of short- and long-term outcomes after ventricular fibrillation out-of-hospital cardiac arrest in patients with ischemic and nonischemic heart disease. Am J Cardiol 2006;98:857-60. http://dx.doi.org/10.1016/j.amjcard.2006.04.023.
- Dumas F, Rea TD. Long-term prognosis following resuscitation from out-of-hospital cardiac arrest: role of aetiology and presenting arrest rhythm. Resuscitation 2012;83:1001-5. http://dx.doi.org/10.1016/j.resuscitation.2012.01.029.
- Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, et al. Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation 2011;123:2423-30. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.959734.
- Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137-46. http://dx.doi.org/10.1136/hrt.2003.025270.
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J 1999;20:421-8. http://dx.doi.org/10.1053/euhj.1998.1280.
- Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 2001;22:228-36. http://dx.doi.org/10.1053/euhj.2000.2289.
- Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J 2007;28:1128-34. http://dx.doi.org/10.1093/eurheartj/ehm102.
- Taylor CJ, Roalfe AK, Iles R, Hobbs FD. Ten-year prognosis of heart failure in the community: follow-up data from the Echocardiographic Heart of England Screening (ECHOES) study. Eur J Heart Fail 2012;14:176-84. http://dx.doi.org/10.1093/eurjhf/hfr170.
- Madsen BK, Hansen JF, Stokholm KH, Brons J, Husum D, Mortensen LS. Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J 1994;15:303-10.
- Bouvy ML, Heerdink ER, Leufkens HG, Hoes AW. Predicting mortality in patients with heart failure: a pragmatic approach. Heart 2003;89:605-9. http://dx.doi.org/10.1136/heart.89.6.605.
- Ahmed A, Aronow WS, Fleg JL. Predictors of mortality and hospitalization in women with heart failure in the Digitalis Investigation Group trial. Am J Ther 2006;13:325-31. http://dx.doi.org/10.1097/00045391-200607000-00009.
- Holland R, Rechel B, Stepien K, Harvey I, Brooksby I. Patients’ self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail 2010;16:150-6. http://dx.doi.org/10.1016/j.cardfail.2009.08.010.
- Miller-Davis C, Marden S, Leidy NK. The New York Heart Association classes and functional status: what are we really measuring?. Heart Lung 2006;35:217-24. http://dx.doi.org/10.1016/j.hrtlng.2006.01.003.
- Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M. London: British Heart Foundation; 2010.
- Boehmer JP. CRT only or CRT plus ICD?. Eur Heart J 2004;6:D83-7. http://dx.doi.org/10.1016/j.ehjsup.2004.05.003.
- Schaer BA, Koller MT, Sticherling C, Altmann D, Joerg L, Osswald S. Longevity of implantable cardioverter-defibrillators, influencing factors, and comparison to industry-projected longevity. Heart Rhythm 2009;6:1737-43. http://dx.doi.org/10.1016/j.hrthm.2009.09.013.
- Horlbeck FW, Mellert F, Kreuz J, Nickenig G, Schwab JO. Real-world data on the lifespan of implantable cardioverter-defibrillators depending on manufacturers and the amount of ventricular pacing. J Cardiovasc Electrophysiol 2012;23:1336-42. http://dx.doi.org/10.1111/j.1540-8167.2012.02408.x.
- Hauser R, Hayes D, Parsonnet V, Furman S, Epstein A, Hayes J, et al. Feasibility and initial results of an internet-based pacemaker and ICD pulse generator and lead registry. Pacing Clin Electrophysiol 2001;24:82-7. http://dx.doi.org/10.1046/j.1460-9592.2001.00082.x.
- Olde NLR, Knops RE, Bardy GH, Blaauw Y, Boersma LV, Bos JS, et al. Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J 2012;163:753-60. http://dx.doi.org/10.1016/j.ahj.2012.02.012.
- Pedersen SS, Lambiase P, Boersma LV, Murgatroyd F, Johansen JB, Reeve H, et al. Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD: design and rationale of the EFFORTLESS S-ICD Registry. Pacing Clin Electrophysiol 2012;35:574-9. http://dx.doi.org/10.1111/j.1540-8159.2012.03337.x.
- Gras D, Bocker D, Lunati M, Wellens HJ, Calvert M, Freemantle N, et al. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace 2007;9:516-22. http://dx.doi.org/10.1093/europace/eum080.
- Biffi M, Moschini C, Bertini M, Saporito D, Ziacchi M, Diemberger I, et al. Phrenic stimulation: a challenge for cardiac resynchronization therapy. Circ Arrhythm Electrophysiol 2009;2:402-10. http://dx.doi.org/10.1161/CIRCEP.108.836254.
- van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ, van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol 2011;58:995-1000. http://dx.doi.org/10.1016/j.jacc.2011.06.007.
- Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR Implantable Cardioverter-Defibrillator Registry. J Am Coll Cardiol 2011;58:1007-13. http://dx.doi.org/10.1016/j.jacc.2011.04.039.
- Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States. J Am Coll Cardiol 2011;58:1001-6. http://dx.doi.org/10.1016/j.jacc.2011.04.033.
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Europace 2008;10:707-25. http://dx.doi.org/10.1093/europace/eun122.
- Implantable Cardioverter Defibrillators for Arrhythmias (TA95). London: NICE; 2006.
- Cardiac Resynchronisation Therapy for the Treatment of Heart Failure (TA120). London: NICE; 2007.
- National Institute for Health and Clinical Excellence . Chronic Heart Failure: Management of Chronic Heart Failure in Adults in Primary and Secondary Care 2010. http://publications.nice.org.uk/chronic-heart-failure-cg108 (accessed 23 January 2014).
- Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology . Guidelines for cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2007;28:2256-95. http://dx.doi.org/10.1093/eurheartj/ehm305.
- Stevenson WG, Hernandez AF, Carson PE, Fang JC, Katz SD, Spertus JA, et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America guideline committee. J Card Fail 2012;18:94-106. http://dx.doi.org/10.1016/j.cardfail.2011.12.004.
- Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NAM, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:1297-313. http://dx.doi.org/10.1016/j.jacc.2012.07.009.
- Coronary Heart Disease: National Service Framework for Coronary Heart Disease – Modern Standards and Service Models. London: Department of Health; 2000.
- Standards for Implantation and Follow-Up of Cardiac Rhythm Management Devices. Heart Rhythm UK; 2011.
- Piccini JP, Berger JS, O’Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J 2009;30:1245-53. http://dx.doi.org/10.1093/eurheartj/ehp100.
- Lane RE, Cowie MR, Chow AWC. Prediction and prevention of sudden cardiac death in heart failure. Heart 2005;91:674-80. http://dx.doi.org/10.1136/hrt.2003.025254.
- NICE Pathway: Chronic Heart Failure. London: NICE; 2010.
- McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J 2012;33:1787-847. http://dx.doi.org/10.1093/eurheartj/ehs104.
- Short-Term Circulatory Support with Left Ventricular Assist Devices as a Bridge to Cardiac Transplantation or Recovery. London: NICE; 2006.
- Cunningham D, Charles R, Cunningham M, de Lange A. Cardiac Rhythm Management: UK National Clinical Audit 2010. London: National Institute for Cardiovascular Outcomes Research; 2012.
- Cunningham D, Charles R, Cunningham M, Whittaker T. Cardiac Rhythm Management: UK National Clinical Audit Report. London: National Institute for Cardiovascular Outcomes Research; 2012.
- Scott PA, Turner NG, Chung A, Morgan JM, Roberts PR. Varying implantable cardioverter defibrillator referral patterns from implanting and non-implanting hospitals. Europace 2009;11:1048-51. http://dx.doi.org/10.1093/europace/eup145.
- McDonagh T, Standing M, Cleland J, Mitchell P, Dargie H, Cunningham D, et al. National Heart Failure Audit April 2010–March 2011. London: National Institute for Cardiovascular Outcomes Research; 2012.
- Scott PA, Whittaker A, Zeb M, Watts E, Yue AM, Roberts PR, et al. Rates of upgrade of ICD recipients to CRT in clinical practice and the potential impact of the more liberal use of CRT at initial implant. Pacing Clin Electrophysiol 2012;35:73-80. http://dx.doi.org/10.1111/j.1540-8159.2011.03255.x.
- Camm JA, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the ‘enigma’?. Europace 2010;12:1063-9. http://dx.doi.org/10.1093/europace/euq282.
- National Institute for Health and Care Excellence . Arrhythmias – ICDs &Amp; Heart Failure – Cardiac Resynchronisation: Final Scope n.d. www.nice.org.uk/guidance/ta314/resources/arrythmias-icds-heart-failure-cardiac-resynchronisation-final-scope2 (accessed 19 September 2012).
- Bryant J, Brodin H, Loveman E, Payne E, Clegg A. The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review. Health Technol Assess 2005;9.
- Parkes J, Bryant J, Milne R. Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review. Health Technol Assess 2000;4.
- Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess 2007;11.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) 2011. www.cochrane-handbook.org (accessed February 2014).
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Guide to the Methods of Technology Appraisal, 2013. London: NICE; 2013.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Strickberger SA, Hummel JD, Bartlett TG, Frumin HI, Schuger CD, Beau SL, et al. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia – AMIOVIRT. J Am Coll Cardiol 2003;41:1707-12. http://dx.doi.org/10.1016/S0735-1097(03)00297-3.
- Wijetunga M, Strickberger SA. Amiodarone Versus Implantable Defibrillator Randomized Trial. Amiodarone versus Implantable Defibrillator (AMIOVIRT): background, rationale, design, methods, results and implications. Card Electrophysiol Rev 2003;7:452-6. http://dx.doi.org/10.1023/B:CEPR.0000023158.52511.76.
- AVID investigators . A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997;337:1576-83. http://dx.doi.org/10.1056/NEJM199711273372202.
- AVID investigators. Causes of death in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial . J Am Coll Cardiol 1999;34:1552-9. http://dx.doi.org/10.1016/S0735-1097(99)00376-9.
- Hallstrom AP. Antiarrhythmics Versus Implantable Defibrillators (AVID) – rationale, design, and methods. Am J Cardiol 1995;75:470-5. http://dx.doi.org/10.1016/S0002-9149(99)80583-9.
- Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation 2002;105:589-94. http://dx.doi.org/10.1161/hc0502.103330.
- Bigger JT. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. Coronary Artery Bypass Graft (CABG) Patch trial investigators. N Engl J Med 1997;337:1569-75. http://dx.doi.org/10.1056/NEJM199711273372201.
- The Coronary Artery Bypass Graft (CABG) Patch trial . The CABG Patch trial investigators and coordinators. Prog Cardiovasc Dis 1993;36:97-114.
- Bigger JT, Parides MK, Rolnitzky LM, Meier P, Levin B, Egan DA. Changes in sample size and length of follow-up to maintain power in the Coronary Artery Bypass Graft (CABG) Patch trial. Control Clin Trials 1998;19:1-14. http://dx.doi.org/10.1016/S0197-2456(97)00124-4.
- Bigger JT, Whang W, Rottman JN, Kleiger RE, Gottlieb CD, Namerow PB, et al. Mechanisms of death in the CABG Patch trial – a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation 1999;99:1416-21. http://dx.doi.org/10.1161/01.CIR.99.11.1416.
- Spotnitz HM, Herre JM, Raza ST, Hammon JW, Baker LD, Fitzgerald DM, et al. Effect of implantable cardioverter-defibrillator implantation on surgical morbidity in the CABG Patch trial. Surgical investigators of the Coronary Artery Bypass Graft Patch trial. Circulation 1998;98:II77-80.
- Namerow PB, Firth BR, Heywood GM, Windle JR, Parides MK. Quality-of-life six months after CABG surgery in patients randomized to ICD versus no ICD therapy: findings from the CABG Patch trial. Pacing Clin Electrophysiol 1999;22:1305-13. http://dx.doi.org/10.1111/j.1540-8159.1999.tb00623.x.
- Kuck K-H, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748-54. http://dx.doi.org/10.1161/01.CIR.102.7.748.
- Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation 2002;105:1453-8. http://dx.doi.org/10.1161/01.CIR.0000012350.99718.AD.
- German Dilated Cardiomyopathy Study investigators . Prospective studies assessing prophylactic therapy in high risk patients: the German Dilated CardioMyopathy Study (GDCMS) – study design. Pacing Clin Electrophysiol 1992;15:697-700. http://dx.doi.org/10.1111/j.1540-8159.1992.tb05166.x.
- Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, et al. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000;101:1297-302. http://dx.doi.org/10.1161/01.CIR.101.11.1297.
- Connolly SJ, Gent M, Roberts RS, Dorian P, Green MS, Klein GJ, et al. Canadian Implantable Defibrillator Study (CIDS): study design and organization. CIDS Co-Investigators. Am J Cardiol 1993;72:103-8. http://dx.doi.org/10.1016/0002-9149(93)90972-F.
- Sheldon R, Connolly S, Krahn A, Roberts R, Gent M, Gardner M. Identification of patients most likely to benefit from implantable cardioverter-defibrillator therapy: the Canadian Implantable Defibrillator Study. Circulation 2000;101:1660-4. http://dx.doi.org/10.1161/01.CIR.101.14.1660.
- Irvine J, Dorian P, Baker B, O’Brien BJ, Roberts R, Gent M, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9. http://dx.doi.org/10.1067/mhj.2002.124049.
- Bokhari F, Newman D, Greene M, Korley V, Mangat I, Dorian P. Long-term comparison of the implantable cardioverter defibrillator versus amiodarone: eleven-year follow-up of a subset of patients in the Canadian Implantable Defibrillator Study (CIDS). Circulation 2004;110:112-16. http://dx.doi.org/10.1161/01.CIR.0000134957.51747.6E.
- Nademanee K, Veerakul G, Mower M, Likittanasombat K, Krittayapong R, Bhuripanyo K, et al. Defibrillator Versus beta-Blockers for Unexplained Death in Thailand (DEBUT): a randomized clinical trial. Circulation 2003;107:2221-6. http://dx.doi.org/10.1161/01.CIR.0000066319.56234.C8.
- Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151-8. http://dx.doi.org/10.1056/NEJMoa033088.
- Kadish A, Quigg R, Schaechter A, Anderson KP, Estes M, Levine J. Defibrillators in nonischemic cardiomyopathy treatment evaluation. Pacing Clin Electrophysiol 2000;23:338-43. http://dx.doi.org/10.1111/j.1540-8159.2000.tb06759.x.
- Schaechter A, Kadish AH. DEFibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation. DEFibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE). Card Electrophysiol Rev 2003;7:457-62. http://dx.doi.org/10.1023/B:CEPR.0000023162.45506.c4.
- Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy?. Circulation 2006;113:776-82. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.561571.
- Passman R, Subacius H, Ruo B, Schaechter A, Howard A, Sears SF, et al. Implantable cardioverter defibrillators and quality of life: results from the defibrillators in nonischemic cardiomyopathy treatment evaluation study. Arch Intern Med 2007;167:2226-32. http://dx.doi.org/10.1001/archinte.167.20.2226.
- Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004;351:2481-8. http://dx.doi.org/10.1056/NEJMoa041489.
- Hohnloser SH, Connolly SJ, Kuck KH, Dorian P, Fain E, Hampton JR, et al. The Defibrillator in Acute Myocardial Infarction Trial (DINAMIT): study protocol. Am Heart J 2000;140:735-9. http://dx.doi.org/10.1067/mhj.2000.110088.
- Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427-36. http://dx.doi.org/10.1056/NEJMoa0901889.
- Steinbeck G, Andresen D, Senges J, Hoffmann E, Seidl K, Brachmann J, et al. Immediate Risk-Stratification Improves Survival (IRIS): study protocol. Europace 2004;6:392-9. http://dx.doi.org/10.1016/j.eupc.2004.04.008.
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation trial investigators. N Engl J Med 1996;335:1933-40. http://dx.doi.org/10.1056/NEJM199612263352601.
- MADIT Executive Committee . Multicenter Automatic Defibrillator Implantation Trial (MADIT): design and clinical protocol. MADIT Executive Committee. Pacing Clin Electrophysiol 1991;14:920-7. http://dx.doi.org/10.1111/j.1540-8159.1991.tb04136.x.
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83. http://dx.doi.org/10.1056/NEJMoa013474.
- Moss AJ, Cannom DS, Daubert JP, Hall WJ, Higgins SL, Klein H, et al. Multicenter Automatic Defibrillator Implantation Trial II (MADIT II): design and clinical protocol. Ann Noninvasive Electrocardiol 1999;4:83-91. http://dx.doi.org/10.1111/j.1542-474X.1999.tb00369.x.
- Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML, et al. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II). J Am Coll Cardiol 2004;43:1459-65. http://dx.doi.org/10.1016/j.jacc.2003.11.038.
- Noyes K, Corona E, Zwanziger J, Hall J, Zhao HW, Wang HK, et al. Health-related quality of life consequences of implantable cardioverter defibrillators – results from MADIT II. Med Care 2007;45:377-85. http://dx.doi.org/10.1097/01.mlr.0000257142.12600.c1.
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225-37. http://dx.doi.org/10.1056/NEJMoa043399.
- Mitchell JE, Hellkamp AS, Mark DB, Anderson J, Poole JE, Lee KL, et al. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008;155:501-6. http://dx.doi.org/10.1016/j.ahj.2007.10.022.
- Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, Davidson-Ray L, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med 2008;359:999-1008. http://dx.doi.org/10.1056/NEJMoa0706719.
- Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation 2009;120:2170-6. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.853689.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49. http://dx.doi.org/10.1056/NEJMoa050496.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The CARE-HF study (CArdiac REsynchronisation in Heart Failure study): rationale, design and end-points. Eur J Heart Fail 2001;3:481-9. http://dx.doi.org/10.1016/S1388-9842(01)00176-3.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J 2006;27:1928-32. http://dx.doi.org/10.1093/eurheartj/ehl099.
- Cleland J, Freemantle N, Ghio S, Fruhwald F, Shankar A, Marijanowski M, et al. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response: a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. J Am Coll Cardiol 2008;52:438-45. http://dx.doi.org/10.1016/j.jacc.2008.04.036.
- Cleland JG, Calvert MJ, Verboven Y, Freemantle N. Effects of cardiac resynchronization therapy on long-term quality of life: an analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J 2009;157:457-66. http://dx.doi.org/10.1016/j.ahj.2008.11.006.
- Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail 2009;11:699-705. http://dx.doi.org/10.1093/eurjhf/hfp074.
- Ghio S, Freemantle N, Scelsi L, Serio A, Magrini G, Pasotti M, et al. Long-term left ventricular reverse remodelling with cardiac resynchronization therapy: results from the CARE-HF trial. Eur J Heart Fail 2009;11:480-8. http://dx.doi.org/10.1093/eurjhf/hfp034.
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140-50. http://dx.doi.org/10.1056/NEJMoa032423.
- Bristow MR, Feldman AM, Saxon LA. Heart failure management using implantable devices for ventricular resynchronization: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial. COMPANION Steering Committee and COMPANION Clinical Investigators. J Card Fail 2000;6:276-85. http://dx.doi.org/10.1054/jcaf.2000.9501.
- Summary of Safety and Effectiveness Data. P010012/SO26. Washington, DC: US Food and Drug Administration; 2004.
- Carson P, Anand I, O’Connor C, Jaski B, Steinberg J, Lwin A, et al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol 2005;46:2329-34. http://dx.doi.org/10.1016/j.jacc.2005.09.016.
- Anand IS, Carson P, Galle E, Song R, Boehmer J, Ghali JK, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation 2009;119:969-77. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.793273.
- Abraham WT, Fisher WG, Smith AL, DeLurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53. http://dx.doi.org/10.1056/NEJMoa013168.
- Abraham WT. Rationale and design of a randomized clinical trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with advanced heart failure: the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). J Card Fail 2000;6:369-80. http://dx.doi.org/10.1054/jcaf.2000.20841.
- Summary of Safety and Effectiveness. P010015. Washington, DC: US Food and Drug Administration; 2001.
- St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003;107:1985-90. http://dx.doi.org/10.1161/01.CIR.0000065226.24159.E9.
- Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001;344:873-80. http://dx.doi.org/10.1056/NEJM200103223441202.
- Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 2003;42:1454-9. http://dx.doi.org/10.1016/S0735-1097(03)01042-8.
- Saxon LA, Boehmer JP, Hummel J, Kacet S, De Marco T, Naccarelli G, et al. Biventricular pacing in patients with congestive heart failure: two prospective randomized trials. The VIGOR CHF and VENTAK CHF investigators. Am J Cardiol 1999;83:120-3. http://dx.doi.org/10.1016/S0002-9149(98)01012-1.
- Lozano I, Bocchiardo M, Achtelik M, Gaita F, Trappe HJ, Daoud E, et al. Impact of biventricular pacing on mortality in a randomized crossover study of patients with heart failure and ventricular arrhythmias. Pacing Clin Electrophysiol 2000;23:1711-12. http://dx.doi.org/10.1111/j.1540-8159.2000.tb07001.x.
- Summary of Safety and Effectiveness. Original PMA P010012. Washington, DC: US Food and Drug Administration; 2002.
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329-38. http://dx.doi.org/10.1056/NEJMoa0906431.
- Moss AJ, Brown MW, Cannom DS, Daubert JP, Estes M, Foster E, et al. Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT): design and clinical protocol. Ann Noninvasive Electrocardiol 2005;10:34-43. http://dx.doi.org/10.1111/j.1542-474X.2005.00073.x.
- Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: Multicenter Automatic Defibrillator Implantation Trial: Cardiac Resynchronization Therapy. Circulation 2010;122:985-92. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.955039.
- Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, et al. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;124:1527-36. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.014324.
- Goldenberg I, Hall WJ, Beck CA, Moss AJ, Barsheshet A, McNitt S, et al. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011;58:729-37. http://dx.doi.org/10.1016/j.jacc.2011.04.024.
- Arshad A, Moss AJ, Foster E, Padeletti L, Barsheshet A, Goldenberg I, et al. Cardiac resynchronization therapy is more effective in women than in men: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol 2011;57:813-20. http://dx.doi.org/10.1016/j.jacc.2010.06.061.
- Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA 2003;289:2685-94. http://dx.doi.org/10.1001/jama.289.20.2685.
- Abraham WT, Young JB, Leon AR, Adler S, Bank AJ, Hall SA, et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation 2004;110:2864-8. http://dx.doi.org/10.1161/01.CIR.0000146336.92331.D1.
- Piccirillo G, Magri D, di Carlo S, De Laurentis T, Torrini A, Matera S, et al. Influence of cardiac-resynchronization therapy on heart rate and blood pressure variability: 1-year follow-up. Eur J Heart Fail 2006;8:716-22. http://dx.doi.org/10.1016/j.ejheart.2006.01.008.
- Pinter A, Mangat I, Korley V, Connolly S, Connors S, Gardner M, et al. Assessment of resynchronization therapy on functional status and quality of life in patients requiring an implantable defibrillator. Pacing Clin Electrophysiol 2009;32:1509-19. http://dx.doi.org/10.1111/j.1540-8159.2009.02543.x.
- Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385-95. http://dx.doi.org/10.1056/NEJMoa1009540.
- Tang AS, Wells GA, Arnold M, Connolly S, Hohnloser S, Nichol G, et al. Resynchronization/defibrillation for ambulatory heart failure trial: rationale and trial design. Curr Opin Cardiol 2009;24:1-8. http://dx.doi.org/10.1097/HCO.0b013e32831bc63b.
- Beshai JF, Grimm RA, Nagueh SF, Baker JH, Beau SL, Greenberg SM, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 2007;357:2461-71. http://dx.doi.org/10.1056/NEJMoa0706695.
- Beshai JF, Grimm R. The resynchronization therapy in narrow QRS study (RethinQ study): methods and protocol design. J Interv Card Electrophysiol 2007;19:149-55. http://dx.doi.org/10.1007/s10840-007-9156-3.
- Summary of Safety and Effectiveness Data. P030054. Washington, DC: US Food and Drug Administration; 2004.
- Summary of Safety and Effectiveness Data. P030054_S10. Washington, DC: US Food and Drug Administration; 2005.
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial investigators. N Engl J Med 1999;341:1882-90. http://dx.doi.org/10.1056/NEJM199912163412503.
- Lau EW, Griffith MJ, Pathmanathan RK, Ng GA, Clune MM, Cooper J, et al. The Midlands Trial of Empirical Amiodarone versus Electrophysiology-Guided Interventions and Implantable Cardioverter-Defibrillators (MAVERIC): a multi-centre prospective randomised clinical trial on the secondary prevention of sudden cardiac death. Europace 2004;6:257-66. http://dx.doi.org/10.1016/j.eupc.2004.03.009.
- Lee KL, Hafley G, Fisher JD, Gold MR, Prystowsky EN, Talajic M, et al. Effect of implantable defibrillators on arrhythmic events and mortality in the multicenter unsustained tachycardia trial. Circulation 2002;106:233-8. http://dx.doi.org/10.1161/01.CIR.0000021920.73149.C3.
- Wang K, Yamauchi K, Li P, Kato H, Kobayashi M, Kato K, et al. Cost-effectiveness of implantable cardioverter-defibrillators in Brugada syndrome treatment. J Med Syst 2008;32:51-7. http://dx.doi.org/10.1007/s10916-007-9107-7.
- Cleland JG, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, et al. Long-term mortality with cardiac resynchronization therapy in the Cardiac Resynchronization-Heart Failure (CARE-HF) trial. Eur J Heart Fail 2012;14:628-34. http://dx.doi.org/10.1093/eurjhf/hfs055.
- Implantable Cardioverter Defibrillators for the Treatment of Arrhythmias and Cardiac Resynchronisation Therapy for the Treatment of Heart Failure (Review of TA95 and TA120). London: ABHI; 2012.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: University of York, Centre for Health Economics; 1999.
- Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al. A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context. Health Technol Assess 2006;10.
- Al-Khatib SM, Anstrom KJ, Eisenstein EL, Peterson ED, Jollis JG, Mark DB, et al. Clinical and economic implications of the Multicenter Automatic Defibrillator Implantation Trial-II. Ann Intern Med 2005;142:593-600. http://dx.doi.org/10.7326/0003-4819-142-8-200504190-00007.
- Bertoldi EG, Rohde LE, Zimerman LI, Pimentel M, Polanczyk CA. Cost-effectiveness of cardiac resynchronization therapy in patients with heart failure: the perspective of a middle-income country’s public health system. Int J Cardiol 2011;163:309-15. http://dx.doi.org/10.1016/j.ijcard.2011.06.046.
- Caro JJ, Ward A, Deniz HB, O’Brien JA, Ehreth JL. Cost–benefit analysis of preventing sudden cardiac deaths with an implantable cardioverter defibrillator versus amiodarone. Value Health 2007;10:13-22. http://dx.doi.org/10.1111/j.1524-4733.2006.00140.x.
- Chan PS, Stein K, Chow T, Fendrick M, Bigger JT, Vijan S. Cost-effectiveness of a microvolt T-wave alternans screening strategy for implantable cardioverter-defibrillator placement in the MADIT-II-eligible population. J Am Coll Cardiol 2006;48:112-21. http://dx.doi.org/10.1016/j.jacc.2006.02.051.
- Chan PS, Nallamothu BK, Spertus JA, Masoudi FA, Bartone C, Kereiakes DJ, et al. Impact of age and medical comorbidity on the effectiveness of implantable cardioverter-defibrillators for primary prevention. Circ Cardiovasc Qual Outcomes 2009;2:16-24. http://dx.doi.org/10.1161/CIRCOUTCOMES.108.807123.
- Chen L, Hay JW. Cost-effectiveness of primary implanted cardioverter defibrillator for sudden death prevention in congestive heart failure. Cardiovasc Drugs Ther 2004;18:161-70. http://dx.doi.org/10.1023/B:CARD.0000029034.65769.f7.
- Cowie MR, Marshall D, Drummond M, Ferko N, Maschio M, Ekman M, et al. Lifetime cost-effectiveness of prophylactic implantation of a cardioverter defibrillator in patients with reduced left ventricular systolic function: results of Markov modelling in a European population. Europace 2009;11:716-26. http://dx.doi.org/10.1093/europace/eup068.
- Deniz HB, Ward A, Jaime CJ, Alvarez P, Sadri H. Cost–benefit analysis of primary prevention of sudden cardiac death with an implantable cardioverter defibrillator versus amiodarone in Canada. Curr Med Res Opin 2009;25:617-26. http://dx.doi.org/10.1185/03007990802695037.
- Feingold B, Arora G, Webber SA, Smith KJ. Cost-effectiveness of implantable cardioverter-defibrillators in children with dilated cardiomyopathy. J Card Fail 2010;16:734-41. http://dx.doi.org/10.1016/j.cardfail.2010.04.009.
- Filion KB, Xie X, van der Avoort CJ, Dendukuri N, Brophy JM. Microvolt T-wave alternans and the selective use of implantable cardioverter defibrillators for primary prevention: a cost-effectiveness study. Int J Technol Assess Health Care 2009;25:151-60. http://dx.doi.org/10.1017/S0266462309090205.
- Gandjour A, Holler A, Dipl GO, Adarkwah CC. Cost-effectiveness of implantable defibrillators after myocardial infarction based on 8-year follow-up data (MADIT II). Value Health 2011;14:812-17. http://dx.doi.org/10.1016/j.jval.2011.02.1180.
- Goldenberg I, Moss AJ, Maron BJ, Dick AW, Zareba W. Cost-effectiveness of implanted defibrillators in young people with inherited cardiac arrhythmias. Ann Noninvasive Electrocardiol 2005;10. http://dx.doi.org/10.1111/j.1542-474X.2005.00070.x.
- Kuppermann M, Luce BR, McGovern B, Podrid PJ, Bigger JT, Ruskin JN. An analysis of the cost effectiveness of the implantable defibrillator. Circulation 1990;81:91-100. http://dx.doi.org/10.1161/01.CIR.81.1.91.
- Kupersmith J, Hogan A, Guerrero P, Gardiner J, Mellits ED, Baumgardner R, et al. Evaluating and improving the cost-effectiveness of the implantable cardioverter-defibrillator. Am Heart J 1995;130:507-15. http://dx.doi.org/10.1016/0002-8703(95)90359-3.
- Larsen GC, Manolis AS, Sonnenberg FA, Beshansky JR, Estes NA, Pauker SG. Cost-effectiveness of the implantable cardioverter-defibrillator: effect of improved battery life and comparison with amiodarone therapy. J Am Coll Cardiol 1992;19:1323-34. http://dx.doi.org/10.1016/0735-1097(92)90341-J.
- Larsen G, Hallstrom A, McAnulty J, Pinski S, Olarte A, Sullivan S, et al. Cost-effectiveness of the implantable cardioverter-defibrillator versus antiarrhythmic drugs in survivors of serious ventricular tachyarrhythmias: results of the Antiarrhythmics Versus Implantable Defibrillators (AVID) economic analysis substudy. Circulation 2002;105:2049-57. http://dx.doi.org/10.1161/01.CIR.0000015504.57641.D0.
- Mark DB, Nelson CL, Anstrom KJ, Al Khatib SM, Tsiatis AA, Cowper PA, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation 2006;114:135-42. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.581884.
- McGregor M, Chen J. Should the implantable cardiac defibrillator be used for primary prevention of sudden death? A review of the issues relevant to hospital decision making. Can J Cardiol 2004;20:1199-204.
- Implantable Cardioverter Defibrillators for Prevention of Sudden Cardiac Death. Canberra: Australian Government Department of Health and Ageing; 2006.
- Mushlin AI, Hall WJ, Zwanziger J, Gajary E, Andrews M, Marron R, et al. The cost-effectiveness of automatic implantable cardiac defibrillators: results from MADIT. Circulation 1998;97:2129-35. http://dx.doi.org/10.1161/01.CIR.97.21.2129.
- Neyt M, Thiry N, Ramaekers D, Van Brabandt H. Cost effectiveness of implantable cardioverter-defibrillators for primary prevention in a Belgian context. Appl Health Econ Health Policy 2008;6:67-80. http://dx.doi.org/10.2165/00148365-200806010-00006.
- O’Brien BJ, Buxton MJ, Rushby JA. Cost effectiveness of the implantable cardioverter defibrillator: a preliminary analysis. Br Heart J 1992;68:241-5. http://dx.doi.org/10.1136/hrt.68.8.241.
- Owens DK, Sanders GD, Harris RA, McDonald KM, Heidenreich PA, Dembitzer AD, et al. Cost-effectiveness of implantable cardioverter defibrillators relative to amiodarone for prevention of sudden cardiac death. Ann Intern Med 1997;126:1-12. http://dx.doi.org/10.7326/0003-4819-126-1-199701010-00001.
- Owens DK, Sanders GD, Heidenreich PA, McDonald KM, Hlatky MA. Effect of risk stratification on cost-effectiveness of the implantable cardioverter defibrillator. Am Heart J 2002;144:440-8. http://dx.doi.org/10.1067/mhj.2002.125501.
- Ribeiro RA, Stella SF, Zimerman LI, Pimentel M, Rohde LE, Polanczyk CA. Cost-effectiveness of implantable cardioverter defibrillators in Brazil in the public and private sectors. Arq Bras Cardiol 2010;95:577-86. http://dx.doi.org/10.1590/S0066-782X2010005000134.
- Sanders GD, Hlatky MA, Every NR, McDonald KM, Heidenreich PA, Parsons LS, et al. Potential cost-effectiveness of prophylactic use of the implantable cardioverter defibrillator or amiodarone after myocardial infarction. Ann Intern Med 2001;135:870-83. http://dx.doi.org/10.7326/0003-4819-135-10-200111200-00007.
- Sanders GD, Hlatky MA, Owens DK. Special report: cost-effectiveness of implantable cardioverter defibrillators in a MADIT-II population. Technol Eval Cent Assess Program Exec Summ 2004;19:1-22.
- Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med 2005;353:1471-80. http://dx.doi.org/10.1056/NEJMsa051989.
- Sanders GD, Kong MH, Al-Khatib SM, Peterson ED. Cost-effectiveness of implantable cardioverter defibrillators in patients ≥ 65 years of age. Am Heart J 2010;160:122-31. http://dx.doi.org/10.1016/j.ahj.2010.04.021.
- Sheldon R, O’Brien BJ, Blackhouse G, Goeree R, Mitchell B, Klein G, et al. Effect of clinical risk stratification on cost-effectiveness of the implantable cardioverter-defibrillator: the Canadian implantable defibrillator study. Circulation 2001;104:1622-6. http://dx.doi.org/10.1161/hc3901.096720.
- Weiss JP, Saynina O, McDonald KM, McClellan MB, Hlatky MA. Effectiveness and cost-effectiveness of implantable cardioverter defibrillators in the treatment of ventricular arrhythmias among Medicare beneficiaries. Am J Med 2002;112:519-27. http://dx.doi.org/10.1016/S0002-9343(02)01078-1.
- You JJ, Woo A, Ko DT, Cameron DA, Mihailovic A, Krahn M. Life expectancy gains and cost-effectiveness of implantable cardioverter/defibrillators for the primary prevention of sudden cardiac death in patients with hypertrophic cardiomyopathy. Am Heart J 2007;154:899-907. http://dx.doi.org/10.1016/j.ahj.2007.06.026.
- Zwanziger J, Hall WJ, Dick AW, Zhao H, Mushlin AI, Hahn RM, et al. The cost effectiveness of implantable cardioverter-defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol 2006;47:2310-18. http://dx.doi.org/10.1016/j.jacc.2006.03.032.
- Banz K. Cardiac resynchronization therapy (CRT) in heart failure – a model to assess the economic value of this new medical technology. Value Health 2005;8:128-39. http://dx.doi.org/10.1111/j.1524-4733.2005.03092.x.
- Callejo D, Guerra M, Hernandez-Madrid A, Blasco JA. Economic assessment of cardiac resynchronization therapy. Rev Esp Cardiol 2010;63:1235-43. http://dx.doi.org/10.1016/S0300-8932(10)70293-1.
- Calvert MJ, Freemantle N, Yao G, Cleland JG, Billingham L, Daubert JC, et al. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J 2005;26:2681-8. http://dx.doi.org/10.1093/eurheartj/ehi662.
- Caro JJ, Guo S, Ward A, Chalil S, Malik F, Leyva F. Modelling the economic and health consequences of cardiac resynchronization therapy in the UK. Curr Med Res Opin 2006;22:1171-9. http://dx.doi.org/10.1185/030079906X112516.
- Blomstrom P, Ekman M, Lundqvist CB, Calvert MJ, Freemantle N, Lonnerholm S, et al. Cost effectiveness of cardiac resynchronization therapy in the Nordic region: an analysis based on the CARE-HF trial. Eur J Heart Fail 2008;10:869-77. http://dx.doi.org/10.1016/j.ejheart.2008.06.018.
- Feldman AM, de Lissovoy G, Bristow MR, Saxon LA, De Marco T, Kass DA, et al. Cost effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol 2005;46:2311-21. http://dx.doi.org/10.1016/j.jacc.2005.08.033.
- Heerey A, Lauer M, Alsolaiman F, Czerr J, James K. Cost effectiveness of biventricular pacemakers in heart failure patients. Am J Cardiovasc Drugs 2006;6:129-37. http://dx.doi.org/10.2165/00129784-200606020-00007.
- McAlister F, Ezekowitz J, Wiebe N, Rowe B, Spooner C, Crumley E, et al. Rockville, MD: Agency for Healthcare Research; 2004.
- Cardiac Resynchronisation Therapy for Severe Heart Failure. Canberra: Australian Government Department of Health and Ageing; 2006.
- Neyt M, Stroobandt S, Obyn C, Camberlin C, Devriese S, De Laet C, et al. Cost-effectiveness of cardiac resynchronisation therapy for patients with moderate-to-severe heart failure: a lifetime Markov model. BMJ Open 2011;1. http://dx.doi.org/10.1136/bmjopen-2011-000276.
- Nichol G, Kaul P, Huszti E, Bridges JF. Cost-effectiveness of cardiac resynchronization therapy in patients with symptomatic heart failure. Ann Intern Med 2004;141:343-51. http://dx.doi.org/10.7326/0003-4819-141-5-200409070-00102.
- Yao G, Freemantle N, Calvert MJ, Bryan S, Daubert JC, Cleland JG. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J 2007;28:42-51. http://dx.doi.org/10.1093/eurheartj/ehl382.
- Aidelsburger P, Grabein K, Klauss V, Wasem J. Cost-effectiveness of cardiac resynchronization therapy in combination with an implantable cardioverter defibrillator (CRT-D) for the treatment of chronic heart failure from a German health care system perspective. Clin Res Cardiol 2008;97:89-97. http://dx.doi.org/10.1007/s00392-007-0586-9.
- Poggio R, Augustovsky F, Caporale J, Irazola V, Miriuka S. Cost-effectiveness of cardiac resynchronization therapy: perspective from Argentina. Int J Technol Assess Health Care 2012;28:429-35. http://dx.doi.org/10.1017/S0266462312000505.
- Ribeiro RA, Stella SF, Camey SA, Zimerman LI, Pimentel M, Rohde LE, et al. Cost-effectiveness of implantable cardioverter-defibrillators in Brazil: primary prevention analysis in the public sector. Value Health 2010;13:160-8. http://dx.doi.org/10.1111/j.1524-4733.2009.00608.x.
- O’Brien BJ, Connolly SJ, Goeree R, Blackhouse G, Willan A, Yee R, et al. Cost-effectiveness of the implantable cardioverter-defibrillator: results from the Canadian Implantable Defibrillator Study (CIDS). Circulation 2001;103:1416-21. http://dx.doi.org/10.1161/01.CIR.103.10.1416.
- Bond M, Mealing S, Anderson R, Dean J, Stein K, Taylor RS. Is combined resynchronisation and implantable defibrillator therapy a cost-effective option for left ventricular dysfunction?. Int J Cardiol 2009;137:206-15. http://dx.doi.org/10.1016/j.ijcard.2008.05.073.
- Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39:2026-33. http://dx.doi.org/10.1016/S0735-1097(02)01895-8.
- Leclercq C, Walker S, Linde C, Clementy J, Marshall AJ, Ritter P, et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J 2002;23:1780-7. http://dx.doi.org/10.1053/euhj.2002.3232.
- Garrigue S, Bordachar P, Reuter S, Jais P, Haissaguerre M, Clementy J. Comparison of permanent left ventricular and biventricular pacing in patients with heart failure and chronic atrial fibrillation: a prospective hemodynamic study. Card Electrophysiol Rev 2003;7:315-24. http://dx.doi.org/10.1023/B:CEPR.0000023167.11038.8f.
- Leclerq C, Cazeau S, Lellouche D, . Upgrading from Right Ventricular Pacing to Biventricular Pacing in Previously Paced Patients with Advanced Heart Failure: a Randomized Controlled Study (the RD-CHF Trial) n.d.
- Linde C, Gold M, Abraham WT, Daubert J-C. Rationale and design of a randomized controlled trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with asymptomatic left ventricular dysfunction with previous symptoms or mild heart failure – The REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. American Heart Journal 2006;151:288-94. http://dx.doi.org/10.1016/j.ahj.2005.03.002.
- Moss A. MADIT-CRT: the Multicentre Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy. Eur J Heart Fail 2009;11:1217-19.
- Kirsch J, McGuire A. Establishing health state valuations for disease-specific states: an example from heart disease. Health Econ 2000;9:149-58. http://dx.doi.org/10.1002/(SICI)1099-1050(200003)9:2<149::AID-HEC501>3.0.CO;2-N.
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail 2005;7:243-51. http://dx.doi.org/10.1016/j.ejheart.2005.01.012.
- Guide to the Single Technology Appraisal Process. London: NICE; 2009.
- Eurich DT, Johnson JA, Reid KJ, Spertus JA. Assessing responsiveness of generic and specific health related quality of life measures in heart failure. Health Qual Life Outcomes 2006;4. http://dx.doi.org/10.1186/1477-7525-4-89.
- Gohler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, et al. Utility estimates for decision-analytic modeling in chronic heart failure – health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185-7. http://dx.doi.org/10.1111/j.1524-4733.2008.00425.x.
- Groeneveld PW, Matta MA, Suh JJ, Yang F, Shea JA. Quality of life among implantable cardioverter-defibrillator recipients in the primary prevention therapeutic era. Pacing Clin Electrophysiol 2007;30:463-71. http://dx.doi.org/10.1111/j.1540-8159.2007.00694.x.
- Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, et al. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39164.568183.AE.
- Public Health England . Central Cardiac Audit Database (CCAD) n.d. http://www.sepho.org.uk/viewResource.aspx?id=6584 (accessed April 2014).
- Department of Health . NHS Reference Costs 2010–2011 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_122803 (accessed 23 January 2014).
- British National Formulary. London: BMA and RPS; 2011.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Ezekowitz JA, Armstrong PW, McAlister FA. Implantable cardioverter defibrillators in primary and secondary prevention: a systematic review of randomized, controlled trials. Ann Intern Med 2003;138:445-52. http://dx.doi.org/10.7326/0003-4819-138-6-200303180-00007.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J. Coronary Heart Disease Statistics: 2012 Edition. London: British Heart Foundation; 2012.
- Curnis A, Caprari F, Mascioli G, Bontempi L, Scivales A, Bianchetti F, et al. Milan: Springer-Verlag Italia; 2003.
- Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail 2004;10:374-9. http://dx.doi.org/10.1016/j.cardfail.2004.02.003.
- Access to Cardiac Care in the UK: A Report on Recent Trends, Variations in Access & Future Need. Oxford: British Cardiac Society and the British Heart Foundation; 2009.
- UK Cardiothoracic Transplant Audit, Annual Report. London: Clinical Effectiveness Unit, Royal College of Surgeons of England, and Statistics and Clinical Audit, NHS Blood and Transplant; 2011.
- Hussey JC, Bond ZC, Collett D, Wallwork J. Long term patient survival for heart transplant recipients in the UK n.d. www.organdonation.nhs.uk/statistics/presentations/pdfs/bts_2004_long-term_pt_survival_%20heart.pdf (accessed 23 January 2014).
- Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer 2006;42:2867-75. http://dx.doi.org/10.1016/j.ejca.2006.08.010.
- Department of Health . NHS UK Tariff 2012–13 2012. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_132654 (accessed 23 January 2014).
- Huang Y, Wu W, Cao Y, Qu N. All cause mortality of cardiac resynchronization therapy with implantable cardioverter defibrillator: a meta-analysis of randomized controlled trials. Int J Cardiol 2010;145:413-17. http://dx.doi.org/10.1016/j.ijcard.2010.05.016.
- Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med 2011;154:401-12. http://dx.doi.org/10.7326/0003-4819-154-6-201103150-00313.
- Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S, et al. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ 2011;183:421-9. http://dx.doi.org/10.1503/cmaj.101685.
- Bertoldi EG, Polanczyk CA, Cunha V, Ziegelmann PK, Beck-da-Silva L, Rohde LE. Mortality reduction of cardiac resynchronization and implantable cardioverter-defibrillator therapy in heart failure: an updated meta-analysis. Does recent evidence change the standard of care?. Journal of Card Fail 2011;17:860-6. http://dx.doi.org/10.1016/j.cardfail.2011.06.372.
- Kron J, Herre J, Renfroe EG, Rizo-Patron C, Raitt M, Halperin B, et al. Lead- and device-related complications in the antiarrhythmics versus implantable defibrillators trial. Am Heart J 2001;141:92-8. http://dx.doi.org/10.1067/mhj.2001.111261.
- Kron J. Clinical significance of device-related complications in clinical trials and implications for future trials: insights from the Antiarrhytmics Versus Implantable Defibrillators (AVID) trial. Card Electrophysiol Rev 2003;7:473-8. http://dx.doi.org/10.1023/B:CEPR.0000023163.67758.b1.
- Klein RC, Raitt MH, Wilkoff BL, Beckman KJ, Coromilas J, Wyse DG, et al. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J Cardiovasc Electrophysiol 2003;14:940-8. http://dx.doi.org/10.1046/j.1540-8167.2003.01554.x.
- Curtis AB, Cannom DS, Bigger JT, DiMarco JP, Estes NA, Steinman RC, et al. Baseline characteristics of patients in the Coronary Artery Bypass Graft (CABG) Patch trial. Am Heart J 1997;134:787-98. http://dx.doi.org/10.1016/S0002-8703(97)80001-4.
- Siebels J, Cappato R, Ruppel R, Schneider MA, Kuck KH. ICD versus drugs in cardiac arrest survivors: preliminary results of the Cardiac Arrest Study Hamburg. Pacing Clin Electrophysiol 1993;16:552-8. http://dx.doi.org/10.1111/j.1540-8159.1993.tb01624.x.
- Siebels J, Cappato R, Ruppel R, Schneider MA, Kuck KH. Preliminary results of the Cardiac Arrest Study Hamburg (CASH). Am J Cardiol 1993;72:109-13. http://dx.doi.org/10.1016/0002-9149(93)90973-G.
- Siebels J, Kuck KH. Implantable cardioverter defibrillator compared with antiarrhythmic drug treatment in cardiac arrest survivors (the Cardiac Arrest Study Hamburg). Am Heart J 1994;127:1139-44. http://dx.doi.org/10.1016/0002-8703(94)90101-5.
- Foley PW, Patel K, Irwin N, Sanderson JE, Frenneaux MP, Smith RE, et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study. Heart 2011;97:1041-7. http://dx.doi.org/10.1136/hrt.2010.208355.
- Linde C, Gold M, Abraham WT, Daubert JC. REVERSE Study Group . Baseline characteristics of patients randomized in the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Congest Heart Fail 2008;14:66-74. http://dx.doi.org/10.1111/j.1751-7133.2008.07613.x.
- Linde C, Abraham WT, Gold MR, St John SM, Ghio S, Daubert C, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834-43. http://dx.doi.org/10.1016/j.jacc.2008.08.027.
- US Food and Drug Administration . Summary of Safety and Effectiveness Data (SSED) 2005. www.fda.gov/ohrms/dockets/dockets/05m0289/05m-0289-aav0001-03-SSED-vol1.pdf (accessed April 2014).
Appendix 1 Comparison of inclusion criteria in previous and present technology assessment reports
| Parameter | ICD TAR62 | CRT TAR64 | Present TAR |
|---|---|---|---|
| Population | Adults at high risk of SCD as a result of arrhythmia: ‘Secondary prevention’: (i) cardiac arrest as a result of either VT or VF; (ii) spontaneous sustained VT causing syncope or significant haemodynamic compromise; (iii) sustained VT without syncope/cardiac arrest, and who have an associated reduction in LVEF (< 35%) but who are no worse than NYHA class III
|
People with HF (any NYHA class) as a result of LVSD with evidence of cardiac dyssynchrony (QRS duration > 120 milliseconds) and LVSD (LVEF ≤ 35%) | People at increased risk of SCD as a result of ventricular arrhythmias despite OPT; people with HF as a result of LVSD and cardiac dyssynchrony despite OPT; people with both conditions described above |
| Intervention | ICD | CRT-P or CRT-D | ICD, CRT-P, CRT-D |
| Comparator | AAD or placebo/control | OPT alone, CRT-P vs. CRT-D | OPT, CRT-P vs. CRT-D, CRT-D vs. ICD |
| Outcomes | Mortality, QoL, adverse effects | Mortality, number of people with HF hospitalisations, exercise capacity, NYHA class, number with adverse effects, QoL | Mortality, adverse effects, QoL, symptoms and complications related to tachyarrhythmias and/or HF, HF hospitalisations, change in NYHA class, change in LVEF |
Appendix 2 Sources of information, including databases searched and search terms
All databases searched for the systematic reviews of clinical effectiveness and cost-effectiveness are presented in the following table. Searches were updated in November 2012.
| Database searched | Clinical effectiveness searches | Cost effectiveness and QoL searches |
|---|---|---|
| Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) | All available years | |
| Cochrane Database of Systematic Reviews (CDSR, The Cochrane Library) | All available years | |
| Database of Abstracts of Reviews of Effects (DARE, CRD) | All available years | All available years |
| EMBASE | All available years | 1990–2011 |
| HTA database (CRD) | All available years | All available years |
| MEDLINE (Ovid) | All available years | All available years |
| MEDLINE In-Process & Other Non-Indexed Citations (MEIP) | Searched 13 November 2012 | Searched 13 November 2012 |
| NHS Economic Evaluation Database (NHS EED, CRD) | All available years | |
| Web of Science (Science Citation Index Expanded and Conference Proceedings) | All available years | All available years |
| Biosis Previews (ISI Web of Knowledge) | All available years | All available years |
| Zetoc (Mimas) | 1990–2012 | |
| Searched for ongoing trials | ||
| NIHR Clinical Research Network (NIHR CRN Portfolio, formally UKCRN website) Current Controlled Trials (CCT) ClinicalTrials.gov World Health Organization International Clinical Trials Registry Platform (ICTRP) |
||
The MEDLINE search strategy (presented in the following section) for the systematic review of clinical effectiveness was adjusted as necessary for the other electronic databases for both the clinical effectiveness and the cost effectiveness (including QoL information) searches. Search strategies for the systematic review are available from the authors on request. Citations identified by the searches were added to a Reference Manager database (version 12; Thomson ResearchSoft, San Francisco, CA, USA).
MEDLINE search strategy
-
Defibrillators, Implantable/ (9092)
-
(implant* adj2 (defibrilat* or defibrillat*)).tw. (7371)
-
ICDs.tw. (1750)
-
(S-ICD or S-ICDS).mp. (10)
-
subcutaneous ICD*1.tw. (14)
-
(implant* adj5 ICD*1).tw. (3365)
-
(CRT or CRT-D or CRT-P).mp. (5381)
-
dual chamber ICD.tw. (100)
-
single chamber ICD.tw. (33)
-
resynch* therap*.tw. (2776)
-
((heart or cardiac or myocardial or coronary) adj2 (resynch* or depolari* or repolari*)).tw. (4300)
-
(atriobiventricular adj10 pac*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] (13)
-
(atriobiventricular adj10 stimulat*).mp. (1)
-
BVP.tw. (166)
-
(biventricular adj10 pac*).mp. (1222)
-
(biventricular adj10 stimulat*).mp. (149)
-
(cardiover* or “cardio-ver*” or cardioconver* or “cardio-conver*” or “cardio conver*”).tw. (10,472)
-
or/1-17 (23,443)
-
exp arrhythmia/ (149,057)
-
Tachycardia, Ventricular/ or Arrhythmias, Cardiac/ or Tachycardia/ or Ventricular Fibrillation/ (79,877)
-
Atrial Fibrillation/ (27,947)
-
Heart Ventricles/bs, in [Blood Supply, Injuries] (878)
-
exp Ventricular Dysfunction, Left/ (18,010)
-
exp cardiomyopathy, dilated/ (11,764)
-
ventricula* remodel*.tw. (2958)
-
bundle-branch block/ (6995)
-
Heart Failure/ (73,266)
-
exp heart failure, congestive/ (74,453)
-
Death, Sudden, Cardiac/ (9241)
-
Heart Arrest/ (20,135)
-
(ventricul* adj2 (tachycardia* or fibril* or arrhythmia*)).tw. (34,555)
-
((heart or cardiac or myocardial or coronary) adj2 (failur* or arrest* or sudden)).tw. (116,912)
-
((cardiac or ventricular or intraventricular) adj5 asynchron*).tw. (438)
-
((cardiac or ventricular or intraventricular) adj5 dyssynchron*).tw. (844)
-
tachyarrhythmia*.tw. (6663)
-
“abnormal heart rhythm*”.tw. (37)
-
(“unexpected death” or “sudden death”).tw. (16,602)
-
(cardiomyopathy or cardiomyopathies).tw. (38,422)
-
Myocardial Infarction/ (128,452)
-
“heart attack*”.tw. (3218)
-
Long QT Syndrome/ (4998)
-
Syncope/ (8267)
-
(syncope adj2 (cardiogenic or heart or cardiac or myocardial)).tw. (519)
-
(atrial adj2 (fibril* or flutter*)).tw. (30,606)
-
( “sudden cardiac death” or “sudden arrhythmic death”).tw. (7232)
-
“unstable heart rhythm*”.tw. (2)
-
“left ventricular systolic dysfunction”.tw. (1601)
-
((reduced or reduction or impair*) adj2 left ventricular ejection fraction).tw. (572)
-
LVSD.tw. (238)
-
((heart or cardiac or myocardial) adj2 dysfunction*).tw. (10,374)
-
exp cardiomyopathies/ (64,726)
-
Brugada syndrome.tw. (1352)
-
arrhythmogenic right ventricular dysplasia.tw. (777)
-
ARVD.tw. (378)
-
(surg* adj5 “congenital heart disease”).tw. (1327)
-
((familial or genetic or inherited) adj “heart disease”).tw. (53)
-
(“heart failure” or “cardiac failure” or “ventricula*1 failure”).tw. (93,943)
-
Heart Defects, Congenital/su [Surgery] (12,194)
-
Heart Conduction System/ (26,125)
-
exp Cardiac Pacing, Artificial/ (18,111)
-
exp Pacemaker, Artificial/ (21,156)
-
exp Heart-Assist Devices/ (6947)
-
or/19-62 (502,075)
-
18 and 63 (17,567)
-
Randomized Controlled Trials as Topic/ (75,979)
-
randomized controlled trial.pt. (315,877)
-
controlled clinical trial.pt. (83,182)
-
Controlled Clinical Trial/ (83,182)
-
random allocation/ (72,622)
-
Double-Blind Method/ (111,942)
-
Single-Blind Method/ (15,496)
-
(random* adj2 allocat*).tw. (16,697)
-
placebo*.tw. (131,568)
-
((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. (109,548)
-
Research Design/ (64,180)
-
((random* or control*) adj5 (trial* or stud*)).tw. (414,902)
-
random*.tw. (534,613)
-
exp Placebos/ (30,269)
-
Meta-Analysis/ (30,726)
-
meta analysis.pt. (30,726)
-
meta analys*.tw. (34,905)
-
(systematic adj2 (review* or overview*)).tw. (30,123)
-
Technology Assessment, Biomedical/ (7447)
-
or/65-83 (1,030,489)
-
64 and 84 (2873)
-
(comment or editorial or letter).pt. (1,090,861)
-
85 not 86 (2728)
-
limit 87 to english language (2501)
-
limit 88 to (cats or cattle or chick embryo or dogs or goats or guinea pigs or hamsters or horses or mice or rabbits or rats or sheep or swine) (94)
-
patient*.tw. (3,739,049)
-
89 not 90 (68)
-
88 not 91 (2433)
Reference lists
The reference lists of retrieved articles were examined for additional studies.
Other searches
The expert advisory group was contacted to obtain information about additional references and any ongoing studies.
British societies and conferences (sources checked November 2012)
Heart Rhythm in press articles
Heart Rhythm Society conferences 2010–12
Appendix 3 Economic evaluation checklist
| No. | Item | Study | Comments |
|---|---|---|---|
| 1 | Is there a clear statement of the decision problem? | ||
| 2 | Is the comparator routinely used in the UK NHS? | ||
| 3 | Is the patient group in the study similar to those of interest in the UK NHS? | ||
| 4 | Is the health-care system comparable to that of the UK? | ||
| 5 | Is the setting comparable to that of the UK? | ||
| 6 | Is the perspective of the model clearly stated? | ||
| 7 | Is the study type appropriate? | ||
| 8 | Is the modelling methodology appropriate? | ||
| 9 | Is the model structure described and does it reflect the disease process? | ||
| 10 | Are assumptions about model structure listed and justified? | ||
| 11 | Are the data inputs for the model described and justified? | ||
| 12 | Is the effectiveness of the intervention established based on a systematic review? | ||
| 13 | Are health benefits measured in QALYs? | ||
| 14 | Are health benefits measured using a standardised and validated generic instrument? | ||
| 15 | Are the resource costs described and justified? | ||
| 16 | Have the costs and outcomes been discounted? | ||
| 17 | Has uncertainty been assessed? | ||
| 18 | Has the model been validated? |
Appendix 4 List of excluded clinical effectiveness studies and recent abstracts
Excluded studies and reasons for exclusion
Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation 2003;108:266–9. [Reason for exclusion: outcomes.]
Alonso C, Ritter P, Leclercq C, Mabo P, Bailleul C, Daubert JC, et al. Effects of cardiac resynchronization therapy on heart rate variability in patients with chronic systolic heart failure and intraventricular conduction delay. Am J Cardiol 2003;91:1144–7. [Reason for exclusion: outcomes and study design.]
Aranda JM Jr, Conti JB, Johnson JW, Petersen-Stejskal S, Curtis AB. Cardiac resynchronization therapy in patients with heart failure and conduction abnormalities other than left bundle-branch block: analysis of the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Clin Cardiol 2004;27:678–82. [Reason for exclusion: study design.]
Are implantable cardioverter-defibrillators or drugs more effective in prolonging life? The Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial Executive Committee. Am J Cardiol 1997;79:661–3. [Reason for exclusion: patient group, intervention, outcomes and study design.]
Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39:2026–33. [Reason for exclusion: comparator.]
Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier AR, et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol 2003;42:2109–16. [Reason for exclusion: comparator.]
Auricchio A, Metra M, Gasparini M, Lamp B, Klersy C, Curnis A, et al. Long-term survival of patients with heart failure and ventricular conduction delay treated with cardiac resynchronization therapy. Am J Cardiol 2007;99:232–8. [Reason for exclusion: population, comparator and study design.]
Barsheshet A, Moss AJ, McNitt S, Jons C, Glikson M, Klein HU, et al. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm 2011;8:212–18. [Reason for exclusion: study design.]
Barsheshet A, Wang PJ, Moss AJ, Solomon SD, Al-Ahmad A, McNitt S, et al. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011;57:2416–23. [Reason for exclusion: study design.]
Beshai JF. Resynchronization therapy in patients with narrow QRS (RethinQ). ACC Cardiosource Rev J 2007;16:30. [Reason for exclusion: abstract (insufficient details).]
Beshai JF, Daubert J-C. RethinQ (the Resynchronization Therapy in Normal QRS Study). Clin Cardiol 2008;31:89–90. [Reason for exclusion: abstract (insufficient details).]
Beshai JF, Truong Q. Resynchronization therapy in patients with narrow QRS (RethinQ). ACC Cardiosource Rev J 2008;17:44. [Reason for exclusion: study design.]
Birnie DH, Ha A, Higginson L, Green M, Thibault B, Wells G, et al. Importance of QRS duration and morphology in determining response to cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). 64th Annual Meeting of the Canadian Cardiovascular Society, Vancouver, Canada, 22–26 October 2011. [Reason for exclusion: outcomes.]
Boerrigter G, Costello-Boerrigter LC, Abraham WT, Sutton MG, Heublein DM, Kruger KM, et al. Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J Card Fail 2008;14:539–46. [Reason for exclusion: study design.]
Brachmann J, Freigang K, Saggau W. Coronary Artery Bypass Graft Patch trial. Pacing Clin Electrophysiol 1993;16:571–5. [Reason for exclusion: comparator and outcomes.]
Breithardt G. MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy): cardiac resynchronization therapy towards early management of heart failure. Eur Heart J 2009;30:2551–3. [Reason for exclusion: outcomes and study design.]
Brenyo A, Link MS, Barsheshet A, Moss AJ, Zareba W, Wang PJ, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011;58:1682–9. [Reason for exclusion: outcomes.]
Brodine WN, Tung RT, Lee JK, Hockstad ES, Moss AJ, Zareba W, et al. Effects of beta-blockers on implantable cardioverter defibrillator therapy and survival in the patients with ischemic cardiomyopathy (from the Multicenter Automatic Defibrillator Implantation Trial-II). Am J Cardiol 2005;96:691–5. [Reason for exclusion: comparator and study design.]
Brodsky MA, McAnulty J, Zipes DP, Baessler C, Hallstrom AP, AVID investigators. A history of heart failure predicts arrhythmia treatment efficacy: data from the Antiarrythmics Versus Implantable Defibrillators (AVID) study. Am Heart J 2006;152:724–30. [Reason for exclusion: study design.]
Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial investigators. N Engl J Med 1999;341:1882–90. [Erratum published in N Engl J Med 2000;342:1300.] [Reason for exclusion: intervention and comparator (although the study is excluded, some details of the study are discussed in the report.)]
Campbell P, Bourgoun M, Shah A, Foster E, Brown MW, Moss AJ, et al. Effect of baseline right ventricular function on outcomes after CRT: an analysis of the MADIT-CRT population. J Am Coll Cardiol 2011;57(Suppl. 14):E205. [Reason for exclusion: outcomes.]
Campbell P, Takeuchi M, Bourgoun M, McNitt S, Goldenberg I, Zareba W, et al. Relationship between change in ventricular size and function and BNP in patients undergoing CRT therapy: MADIT-CRT. J Card Fail 2011;17(Suppl. 8):S57. [Reason for exclusion: abstract (insufficient details).]
Cappato R, Boczor S, Kuck KH; CASH investigators. Response to programmed ventricular stimulation and clinical outcome in cardiac arrest survivors receiving randomised assignment to implantable cardioverter defibrillator or antiarrhythmic drug therapy. Eur Heart J 2004;25:642–9. [Reason for exclusion: study design.]
Cardiomyopathy trial. The Cardiomyopathy Trial Investigators. Pacing Clin Electrophysiol 1993;16:576–81. [Reason for exclusion: outcomes.]
Cawley PJ, Al-Khatib SM. Amiodarone versus implantable cardioverter defibrillator for asymptomatic nonsustained ventricular tachycardia in nonischemic dilated cardiomyopathy. Am Heart J 2004;147:790–1. [Reason for exclusion: intervention, comparator, outcomes and study design.]
Chung ES, Menon SG, Weiss R, Schloss EJ, Chow T, Kereiakes DJ, et al. Feasibility of biventricular pacing in patients with recent myocardial infarction: impact on ventricular remodeling. Congest Heart Fail 2007;13:9–15. [Reason for exclusion: population.]
Chung ES, Mazur W, Menon SG, Schloss EJ, Chow T, Kereiakes DJ. Peri-infarct pacing with CRT in the early postinfarct phase to attenuate long-term remodeling. J Cardiovasc Transl Res 2009;2:126–9. [Reason for exclusion: outcomes.]
Chung ES, Dan D, Solomon SD, Bank AJ, Pastore J, Iyer A, et al. Effect of peri-infarct pacing early after myocardial infarction: results of the prevention of myocardial enlargement and dilatation post myocardial infarction study. Circ Heart Fail 2010;3:650–8. [Reason for exclusion: population.]
Cleland JGF. New results from the CARE-HF programme. ESC Congress Report 2005, p. 13. URL: www.escardio.org/congresses/esc_congress_2005/Documents/ESC-Congress-HotLines-and-CTU-Reports-2005.pdf (accessed January 2014). [Reason for exclusion: abstract (insufficient details).]
Cleland JG, Ghosh J, Freemantle N. Can cardiac-resynchronization therapy reduce mortality in patients suffering from advanced chronic heart failure? Nat Clin Pract Cardiovasc Med 2004;1:10–11. [Reason for exclusion: Outcome and study design.]
Cleland JG, Ghosh J, Freemantle N, Kaye GC, Nasir M, Clark AL, et al. Clinical trials update and cumulative meta-analyses from the American College of Cardiology: WATCH, SCD-HeFT, DINAMIT, CASINO, INSPIRE, STRATUS-US, RIO-LIPIDS and cardiac resynchronisation therapy in heart failure. Eur J Heart Fail 2004;6:501–8. [Reason for exclusion: study design (review).]
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Baseline characteristics of patients recruited into the CARE-HF study. Eur J Heart Fail 2005;7:205–14. [Reason for exclusion: outcomes.]
Cleland JG, Freemantle N, Daubert JC, Toff WD, Leisch F, Tavazzi L. Long-term effect of cardiac resynchronisation in patients reporting mild symptoms of heart failure: a report from the CARE-HF study. Heart 2008;94:278–83. [Reason for exclusion: study design.]
Curtis AB, Cannom DS, Bigger JT Jr, DiMarco JP, Estes NA III, Steinman RC, et al. Baseline characteristics of patients in the coronary artery bypass graft (CABG) Patch trial. Am Heart J 1997;134:787–98. [Reason for exclusion: outcomes.]
Cygankiewicz I, Gillespie J, Zareba W, Brown MW, Goldenberg I, Klein H, et al. Predictors of long-term mortality in Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) patients with implantable cardioverter-defibrillators. Heart Rhythm 2009;6:468–73. [Reason for exclusion: comparator and study design.]
Cygankiewicz I, McNitt S, Thomsen PEB, Kautzner J, Moss AJ, Zareba W. Heart rate turbulence predicts heart failure events in MADIT-CRT patients. 64th Annual Meeting of the Canadian Cardiovascular Society, Vancouver, Canada, 22–26 October 2011. [Reason for exclusion: comparator, outcomes and study design.]
Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II. J Am Coll Cardiol 2008;51:1357–65. [Reason for exclusion: comparator and study design.]
Daubert C, Gold MR, Abraham WT, Ghio S, Hassager C, Goode G, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol 2009;54:1837–46. [Reason for exclusion: population and intervention.]
De Marco T, Wolfel E, Feldman AM, Lowes B, Higginbotham MB, Ghali JK, et al. Impact of cardiac resynchronization therapy on exercise performance, functional capacity, and quality of life in systolic heart failure with QRS prolongation: COMPANION trial sub-study. J Card Fail 2008;14:9–18. [Reason for exclusion: intervention.]
Domanski MJ, Sakseena S, Epstein AE, Hallstrom AP, Brodsky MA, Kim S, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. AVID Investigators. Antiarrhythmics Versus Implantable Defibrillators. J Am Coll Cardiol 1999;34:1090–5. [Reason for exclusion: study design.]
Domanski MJ, Epstein A, Hallstrom A, Saksena S, Zipes DP. Survival of antiarrhythmic or implantable cardioverter defibrillator treated patients with varying degrees of left ventricular dysfunction who survived malignant ventricular arrhythmias. J Cardiovasc Electrophysiol 2002;13:580–3. [Reason for exclusion: study design (comparator).]
Dorian P, Hohnloser SH, Thorpe KE, Roberts RS, Kuck KH, Gent M, et al. Mechanisms underlying the lack of effect of implantable cardioverter-defibrillator therapy on mortality in high-risk patients with recent myocardial infarction: insights from the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT). Circulation 2010;122:2645–52. [Reason for exclusion: study design.]
Filho MM, Pedrosa AA, Costa R, Nishioka SA, Siqueira SF, Tamaki WT, et al. Biventricular pacing improves clinical behavior and reduces prevalence of ventricular arrhythmia in patients with heart failure. Arq Bras Cardiol 2002;78:110–13. [Reason for exclusion: comparator.]
Foley PW, Patel K, Irwin N, Sanderson JE, Frenneaux MP, Smith RE, et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study. Heart 2011;97:1041–7. [Reason for exclusion: abstract.]
Foster E, Solomon SD, McNitt S, Heintze J, Vogt J, Almendral J, et al. MADIT CRT: who are the super responders to cardiac resynchronisation therapy? Europace 2010;12:i50. [Reason for exclusion: comparator.]
Freudenberger RS, Hellkamp AS, Halperin JL, Poole J, Anderson J, Johnson G, et al. Risk of thromboembolism in heart failure: an analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation 2007;115:2637–41. [Reason for exclusion: population and design.]
Giorgberidze I, Saksena S, Krol RB, Munsif AN, Kolettis T, Mathew P, et al. Risk stratification and clinical outcome of minimally symptomatic and asymptomatic patients with nonsustained ventricular tachycardia and coronary disease: a prospective single-center study. Am J Cardiol 1997;80:3–9F. [Reason for exclusion: intervention and study design.]
Gold MR, Daubert C, Sutton MSJ, Ghio S, Abraham WT, Linde C. Left ventricular reverse remodeling predicts mortality: results from the reverse study. J Am Coll Cardiol 2011;57(14 Suppl.):E11. [Reason for exclusion: population.]
Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2010;122:1265–71. [Reason for exclusion: study design.]
Goscinska-Bis K, Bis J, Krejca M, Ulczok R, Szmagala P, Bochenek A, et al. Totally epicardial cardiac resynchronization therapy system implantation in patients with heart failure undergoing CABG. Eur J Heart Fail 2008;10:498–506. [Reason for exclusion: intervention.]
Gould PA, Kong G, Kalff V, Duffy SJ, Taylor AJ, Kelly MJ, et al. Improvement in cardiac adrenergic function post biventricular pacing for heart failure. Europace 2007;9:751–6. [Reason for exclusion: population.]
Gradaus R, Seidl K, Korte T, Himmrich E, Wieneke H, Schuchert A, et al. Reduction of ventricular tachyarrhythmia by treatment of atrial fibrillation in ICD patients with dual-chamber implantable cardioverter/defibrillators capable of atrial therapy delivery: the REVERT-AF Study. Europace 2007;9:534–9. [Reason for exclusion: comparator.]
Hallstrom AP, McAnulty JH, Wilkoff BL, Follmann D, Raitt MH, Carlson MD, et al. Patients at lower risk of arrhythmia recurrence: a subgroup in whom implantable defibrillators may not offer benefit. Antiarrhythmics Versus Implantable Defibrillator (AVID) trial investigators. J Am Coll Cardiol 2001;37:1093–9. [Reason for exclusion: study design.]
Healey JS, Hohnloser SH, Exner DV, Birnie DH, Philippon F, Basta M, et al. Does cardiac resynchronization therapy improve outcomes in patients with chronic atrial tachyarrhythmias? Results from the resynchronization for ambulatory heart failure trial (RAFT). Can J Cardiol 2011;27(Suppl. 5):S335. [Reason for exclusion: abstract.]
Higgins SL, Daubert JL, Akhtar M. Who are the MADIT patients? Multicenter Automatic Defibrillator Implantation Trial. Am J Cardiol 1997;80:42–6F. [Reason for exclusion: comparator, outcomes and study design.]
Hoppe UC, Casares JM, Eiskjaer H, Hagemann A, Cleland JG, Freemantle N, et al. Effect of cardiac resynchronization on the incidence of atrial fibrillation in patients with severe heart failure. Circulation 2006;114:18–25. [Reason for exclusion: study design.]
Huang DT, Sesselberg HW, McNitt S, Noyes K, Andrews ML, Hall WJ, et al. Improved survival associated with prophylactic implantable defibrillators in elderly patients with prior myocardial infarction and depressed ventricular function: a MADIT-II substudy. J Cardiovasc Electrophysiol 2007;18:833–8. [Reason for exclusion: study design.]
Jiménez-Candil J, Arenal A, García-Alberola A, Ortiz M, del Castillo S, Fernández-Portales J, et al. Fast ventricular tachycardias in patients with implantable cardioverter-defibrillators: efficacy and safety of antitachycardia pacing. A prospective and randomized study. J Am Coll Cardiol 2005;45:460–1. [Reason for exclusion: comparator and study design.]
Kadish A, Schaechter A, Subacius H, Thattassery E, Sanders W, Anderson KP, et al. Patients with recently diagnosed nonischemic cardiomyopathy benefit from implantable cardioverter-defibrillators. J Am Coll Cardiol 2006;47:2477–82. [Reason for exclusion: study design.]
Kadish AH, Bello D, Finn JP, Bonow RO, Schaechter A, Subacius H, et al. Rationale and design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol 2009;20:982–7. [Reason for exclusion: outcomes.]
Klein RC, Raitt MH, Wilkoff BL, Beckman KJ, Coromilas J, Wyse DG, et al. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J Cardiovasc Electrophysiol 2003;14:940–8. [Reason for exclusion: comparator and study design.]
Knight BP, Desai A, Coman J, Faddis M, Yong P. Long-term retention of cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:72–7. [Reason for exclusion: comparator and study design.]
Kron J. Clinical significance of device-related complications in clinical trials and implications for future trials: insights from the Antiarrhytmics Versus Implantable Defibrillators (AVID) trial. Card Electrophysiol Rev 2003;7:473–8. [Reason for exclusion: comparator and study design.]
Kron J, Herre J, Renfroe EG, Rizo-Patron C, Raitt M, Halperin B, et al. Lead- and device-related complications in the Antiarrhythmics Versus Implantable Defibrillators trial. Am Heart J 2001;141:92–8. [Reason for exclusion: comparator and study design.]
Lau EW, Griffith MJ, Pathmanathan RK, Ng GA, Clune MM, Cooper J, et al. The Midlands Trial of Empirical Amiodarone versus Electrophysiology-Guided Interventions and Implantable Cardioverter-Defibrillators (MAVERIC): a multi-centre prospective randomised clinical trial on the secondary prevention of sudden cardiac death. Europace 2004;6:257–66. [Reason for exclusion: intervention (although the study is excluded, some details of the study are discussed in the report).]
Laveneziana P, O’Donnell DE, Ofir D, Agostoni P, Padeletti L, Ricciardi G, et al. Effect of biventricular pacing on ventilatory and perceptual responses to exercise in patients with stable chronic heart failure. J Appl Physiol 2009;106:1574–83. [Reason for exclusion: outcomes.]
Leclercq C, Walker S, Linde C, Clementy J, Marshall AJ, Ritter P, et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J 2002;23:1780–7. [Reason for exclusion: comparator.]
Lee KL, Hafley G, Fisher JD, Gold MR, Prystowsky EN, Talajic M, et al. Effect of implantable defibrillators on arrhythmic events and mortality in the multicenter unsustained tachycardia trial. Circulation 2002;106:233–8. [Reason for exclusion: intervention and comparator (although the study is excluded, some details of the study are discussed in the report).]
Leon AR, Abraham WT, Curtis AB, Daubert JP, Fisher WG, Gurley J, et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol 2005;46:2348–56. [Reason for exclusion: study design (review).]
Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation In Cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002;40:111–18. [Reason for exclusion: comparator and study design.]
Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert JC. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am J Cardiol 2003;91:1090–5. [Reason for exclusion: comparator and study design.]
Linde C, Gold M, Abraham WT, Daubert JC; REVERSE study group. Rationale and design of a randomized controlled trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with asymptomatic left ventricular dysfunction with previous symptoms or mild heart failure – the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Am Heart J 2006;151:288–94. [Reason for exclusion: population, intervention and outcomes.]
Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834–43. [Reason for exclusion: population and intervention.]
Linde C, Gold M, Abraham WT, Daubert JC; REVERSE study group. Baseline characteristics of patients randomized in the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Congest Heart Fail 2008;14:66–74. [Reason for exclusion: population, intervention and outcomes.]
Linde C, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, et al. The influence of left ventricular ejection fraction on the extent of reverse remodeling by cardiac resynchronization therapy in mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. J Am Coll Cardiol 2009;53(Suppl. 10):A183. [Reason for exclusion: population, intervention and outcomes.]
Linde C, Leman R, Daubert C, Abraham WT, Gold MR. Cardiac resynchronization therapy in mild heart failure: is there is difference in outcome between NYHA class I versus II? Results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) trial. J Am Coll Cardiol 2009;53(Suppl. 10):A188. [Reason for exclusion: abstract (insufficient details).]
Linde C, Abraham WT, Gold MR, Daubert C; REVERSE study group. Cardiac resynchronization therapy in asymptomatic or mildly symptomatic heart failure patients in relation to etiology: results from the REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) study. J Am Coll Cardiol 2010;56:1826–31. [Reason for exclusion: population and intervention.]
Lindenfeld J, Feldman AM, Saxon L, Boehmer J, Carson P, Ghali JK, et al. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation 2007;115:204–12. [Reason for exclusion: study design.]
Link MS, Moss AJ, Goldenburg I, Viskin S, Delnoy PP, Kutz A, et al. Prognostic implications of supraventricular tachycardia in CRT-D treated patients: the MADIT-CRT experience. Europace 2010;12(Suppl. 1):i136. [Reason for exclusion: comparator and outcomes.]
Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ. Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circ Heart Fail 2011;4:332–8. [Reason for exclusion: study design.]
Mathew J, Katz R, Dixit S, Gerstenfeld EP, Sutton MS, Gold MR, et al. Kidney disease and cardiac remodeling in patients with cardiac resynchronization therapy: results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Heart Rhythm 2011;8(Suppl. 1):S496. [Reason for exclusion: abstract.]
Maynard C. Rehospitalization in surviving patients of out-of-hospital ventricular fibrillation (the CASCADE study). Cardiac Arrest in Seattle: Conventional Amiodarone Drug Evaluation. Am J Cardiol 1993;72:1295–300. [Reason for exclusion: intervention and comparator.]
Moore HJ, Fletcher RD, Platt MD, Boineau R, Anderson J, Johnson GW, et al. SCD-heft: non-sustained ventricular tachycardia on baseline Holter monitor association with appropriate implantable cardioverter defibrillator therapy for ventricular tachycardia and ventricular fibrillation. Heart Rhythm 2011;8(Suppl. 1):S138. [Reason for exclusion: comparator and study design.]
Moss AJ. Update on MADIT: the Multicenter Automatic Defibrillator Implantation Trial. The long QT interval syndrome. Am J Cardiol 1997;79:16–19. [Reason for exclusion: study design.]
Moss AJ. Background, outcome, and clinical implications of the Multicenter Automatic Defibrillator Implantation Trial (MADIT). Am J Cardiol 1997;80:28–32F. [Reason for exclusion: study design.]
Moss AJ, Fadl Y, Zareba W, Cannom DS, Hall WJ; Defibrillator Implantation Trial Research Group. Survival benefit with an implanted defibrillator in relation to mortality risk in chronic coronary heart disease. Am J Cardiol 2001;88:516–20. [Reason for exclusion: study design.]
Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004;110:3760–5. [Reason for exclusion: comparator, outcomes and study design.]
Moss AJ. MADIT-CRT: the Multicentre Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy. Eur J Heart Fail 2009;11:1217–19. [Reason for exclusion: study design.]
Noyes K, Corona E, Veazie P, Dick AW, Zhao H, Moss AJ. Examination of the effect of implantable cardioverter-defibrillators on health-related quality of life: based on results from the Multicenter Automatic Defibrillator Trial-II. Am J Cardiovasc Drugs 2009;9:393–400. [Reason for exclusion: study design.]
Olshansky B, Wood F, Hellkamp AS, Poole JE, Anderson J, Johnson GW, et al. Where patients with mild to moderate heart failure die: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2007;153:1089–94. [Reason for exclusion: outcomes.]
Piccini JP, Al-Khatib SM, Hellkamp AS, Anstrom KJ, Poole JE, Mark DB, et al. Mortality benefits from implantable cardioverter-defibrillator therapy are not restricted to patients with remote myocardial infarction: an analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Heart Rhythm 2011;8:393–400. [Reason for exclusion: study design.]
Piepoli MF, Villani GQ, Corra U, Aschieri D, Rusticali G. Time course of effects of cardiac resynchronization therapy in chronic heart failure: benefits in patients with preserved exercise capacity. Pacing Clin Electrophysiol 2008;31:701–8. [Reason for exclusion: intervention.]
Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol 2007;18:181–4. [Reason for exclusion: study design.]
Piotrowicz K, Noyes K, Lyness JM, McNitt S, Andrews ML, Dick A, et al. Physical functioning and mental well-being in association with health outcome in patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial II. Eur Heart J 2007;28:601–7. [Reason for exclusion: study design.]
Raitt MH, Klein RC, Wyse DG, Wilkoff BL, Beckman K, Epstein AE, et al. Comparison of arrhythmia recurrence in patients presenting with ventricular fibrillation versus ventricular tachycardia in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. Am J Cardiol 2003;91:812–16. [Reason for exclusion: comparator, outcomes and study design.]
Raviele A, Bongiorni MG, Brignole M, Cappato R, Capucci A, Gaita F, et al. Early EPS/ICD strategy in survivors of acute myocardial infarction with severe left ventricular dysfunction on optimal beta-blocker treatment. The BEta-blocker STrategy plus ICD trial. Europace 2005;7:327–37. [Reason for exclusion: comparator.]
Saxon LA. The MIRACLE trial: an electrophysiologist’s perspective. J Card Fail 2002;8:202–3. [Reason for exclusion: outcomes and study design.]
Saxon LA, Bristow MR, De Marco T, Krueger SK. Procedural outcomes and device performance in the COMPANION trial of resynchronization therapy for heart failure. Circulation 2004;110(Suppl. S):443. [Reason for exclusion: abstract (insufficient details).]
Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. Circulation 2006;114:2766–72. [Reason for exclusion: study design.]
Sesselberg HW, Moss AJ, McNitt S, Zareba W, Daubert JP, Andrews ML, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm 2007;4:1395–402. [Reason for exclusion: comparator and study design.]
Siebels J, Cappato R, Ruppel R, Schneider MA, Kuck KH. Preliminary results of the Cardiac Arrest Study Hamburg (CASH). CASH Investigators. Am J Cardiol 1993;72:109–13F. [Reason for exclusion: comparator.]
Siebels J, Cappato R, Ruppel R, Schneider MA, Kuck KH. ICD versus drugs in cardiac arrest survivors: preliminary results of the Cardiac Arrest Study Hamburg. Pacing Clin Electrophysiol 1993;16:552–8. [Reason for exclusion: comparator.]
Siebels J, Kuck KH. Implantable cardioverter defibrillator compared with antiarrhythmic drug treatment in cardiac arrest survivors (the Cardiac Arrest Study Hamburg). Am Heart J 1994;127:1139–44. [Reason for exclusion: comparator.]
Singh SN, Poole J, Anderson J, Hellkamp AS, Karasik P, Mark DB, et al. Role of amiodarone or implantable cardioverter/defibrillator in patients with atrial fibrillation and heart failure. Am Heart J 2006;152:974.e7–11. [Reason for exclusion: study design.]
St John Sutton M, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 2006;113:266–72. [Reason for exclusion: comparator and study design.]
St John Sutton M. Cardiac resynchronization therapy and reduced risk of death and nonfatal heart failure events. Curr Heart Fail Rep 2009;6:211–12. [Reason for exclusion: study design.]
St John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, et al. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation 2009;120:1858–65. [Reason for exclusion: population and intervention.]
Steinberg JS, Beckman K, Greene HL, Marinchak R, Klein RC, Greer SG, et al. Follow-up of patients with unexplained syncope and inducible ventricular tachyarrhythmias: analysis of the AVID registry and an AVID substudy. Antiarrhythmics Versus Implantable Defibrillators. J Cardiovasc Electrophysiol 2001;12:996–1001. [Reason for exclusion: study design.]
Steinberg JS, Martins J, Sadanandan S, Goldner B, Menchavez E, Domanski M, et al. Antiarrhythmic drug use in the implantable defibrillator arm of the Antiarrhythmics Versus Implantable Defibrillators (AVID) study. Am Heart J 2001;142:520–9. [Reason for exclusion: comparator and study design.]
Steinberg JS, Fischer A, Wang P, Schuger C, Daubert J, McNitt S, et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol 2005;16:359–65. [Reason for exclusion: comparator and study design.]
Steinberg JS, Joshi S, Schron EB, Powell J, Hallstrom A, McBurnie M. Psychosocial status predicts mortality in patients with life-threatening ventricular arrhythmias. Heart Rhythm 2008;5:361–5. [Reason for exclusion: intervention, comparator and study design.]
Stellbrink C, Auricchio A, Butter C, Sack S, Vogt J, Bocker D, et al. Pacing Therapies in Congestive Heart Failure II study. Am J Cardiol 2000;86:138–43K. [Reason for exclusion: population and outcomes.]
Stellbrink C, Morgan J, Schalij M, Padeletti L, Brugada J, Boccanelli S, et al. Cardiac resynchronization therapy benefits patients with or without ICD indication – results from the prospective European multicenter PACMAN trial. Circulation 2004;110(Suppl. S):726. [Reason for exclusion: abstract.]
Sze E, Moss AJ, McNitt S, Barsheshet A, Andrews ML, Zareba W, et al. Risk factors for recurrent heart failure events in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II). J Cardiovasc Electrophysiol 2010;21:1217–23. [Reason for exclusion: study design.]
Tang AS, Wells GA, Talajic M, Arnold O, Sheldon R, Connolly S, et al. The Resynchronization/Defibrillation for Ambulatory Heart Failure Trial (RAFT). Circulation 2010;122:2216. [Reason for exclusion: outcomes.]
US Food and Drug Administration. Summary of Safety and Effectiveness Data. P030035/S3. Washington, DC: US Food and Drug Administration; 2005. [Reason for exclusion: FDA report with insufficient details (no baseline characteristics).]
Vorobiof G, Goldenberg I, Moss AJ, Zareba W, McNitt S. Effectiveness of the implantable cardioverter defibrillator in blacks versus whites (from MADIT-II). Am J Cardiol 2006;98:1383–6. [Reason for exclusion: study design.]
Wever EF, Hauer RN, van Capelle FL, Tijssen JG, Crijns HJ, Algra A, et al. Randomized study of implantable defibrillator as first-choice therapy versus conventional strategy in postinfarct sudden death survivors. Circulation 1995;91:2195–203. [Reason for exclusion: comparator.]
Wever EF, Hauer RN, Schrijvers G, van Capelle FJ, Tijssen JG, Crijns HJ, et al. Cost-effectiveness of implantable defibrillator as first-choice therapy versus electrophysiologically guided, tiered strategy in postinfarct sudden death survivors. A randomized study. Circulation 1996;93:489–96. [Reason for exclusion: comparator.]
Wever EFD, Ramanna H, Hauer RNW, Robles de Medina EO. Cardioverter-defibrillator implantation: better first-choice strategy for postinfarction cardiac arrest survivors. Cardiol Rev 1996;13:28–33. [Reason for exclusion: comparator.]
Whang W, Bigger JT Jr. Diabetes and outcomes of coronary artery bypass graft surgery in patients with severe left ventricular dysfunction: results from the CABG Patch trial database. The CABG Patch Trial Investigators and Coordinators. J Am Coll Cardiol 2000;36:1166–72. [Erratum published in J Am Coll Cardiol 2001;37:2012.] [Reason for exclusion: study design.]
Wijetunga M, Strickberger SA. Comparison of therapies for nonischemic dilated cardiomyopathy. Cardiol Rev 2004;21:18–20. [Reason for exclusion: study design.]
Wittenberg SM, Cook JR, Hall WJ, McNitt S, Zareba W, Moss AJ, et al. Comparison of efficacy of implanted cardioverter-defibrillator in patients with versus without diabetes mellitus. Am J Cardiol 2005;96:417–19. [Reason for exclusion: study design.]
Woo GW, Petersen-Stejskal S, Johnson JW, Conti JB, Aranda JA Jr, Curtis AB. Ventricular reverse remodeling and 6-month outcomes in patients receiving cardiac resynchronization therapy: analysis of the MIRACLE study. J Interv Card Electrophysiol 2005;12:107–13. [Reason for exclusion: study design.]
Zareba W, Piotrowicz K, McNitt S, Moss AJ; MADIT II Investigators. Implantable cardioverter-defibrillator efficacy in patients with heart failure and left ventricular dysfunction (from the MADIT II population). Am J Cardiol 2005;95:1487–91. [Reason for exclusion: study design.]
Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;123:1061–72. [Reason for exclusion: study design.]
References unobtainable from The British Library
Gold MR, Linde C, Abraham WT, Gardiwal A, Daubert JC. The impact of cardiac resynchronization therapy on the incidence of ventricular arrhythmias in mild heart failure. Heart Rhythm 2011;8:679–84.
Recent abstract or conference presentations excluded because of insufficient details to allow appraisal of methodology and assessment of results
Abraham W, Gras D, Birgersdotter-Green U, Calo L, Clyne C, Klein N, et al. Response to cardiac resynchronization therapy varies with gender: sub-analysis from the FREEDOM trial. Europace 2011; Abstract P1163.
Arnold JM, Newton G, Mielniczuk L, Talajic M, Yee R, Wells GA, et al. Cardiac resynchronization therapy is an effective therapy for patients with impaired renal function. Circulation 2011;124:A16054.
Bardy G, Lee K, Mark D, Poole J, Fishbein D, Boineau R, et al. Long term follow-up in the Sudden Cardiac Death Heart Failure Trial (SCD-HEFT). Heart Rhythm 2012;9:1579.
Birnie D, Ha A, Higginson L, Green M, Thibault B, Wells G, et al. Importance of ECG morphology in determining response to cardiac resynchronization therapy: results from Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Can J Cardiol 2011;27(Suppl. 5):S242–3.
Chapa D, Thomas SA, Friedmann E. Results of PFOS trial. Heart Lung 2010;39:352.
Daubert JP, Hranitzky PM, McNitt S, Klein HU, Gold MR, Wilber DJ, et al. MADIT-CRT: resynchronization patients experience more complications than ICD-only patients but outcomes are not significantly impacted. Circulation 2011;124:A9364.
Diab I, Kamdar R, Hunter R, Berriman T, Abrams D, Dhinoja M, et al. Value of echocardiographic mechanical dyssynchrony assessment in selecting patients for cardiac resynchronization therapy. J Interv Card Electrophysiol 2010;27:182.
Gillis AM, Kerr C, Philippon F, Newton G, Talajic M, Froeschl M, et al. Impact of CRT on hospitalizations in the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Heart Rhythm 2011;8(Suppl. 1):S494.
Healey J, et al. Effect of cardiac resynchronization on the development of atrial fibrillation: a report from the Resynchronization for Ambulatory Heart Failure Trial (RAFT). Heart Rhythm 2012;9(Suppl. 1):S371.
Jamerson D, Tomkins C, McNitt S, Polonsky B, Zareba W, Moss A. Early procedure-related adverse events by gender in MADIT-CRT. J Am Coll Cardiol 2012;59(Suppl. 1):E919.
Parkash R, Thibault B, Sterns L, Sapp JL, Krahn A, Talajic M, et al. The fidelis lead fracture occurs more frequently in patients with cardiac resynchronization therapy. Report from the device committee of the resynchronization/defibrillation for ambulatory heart failure (RAFT) study. Heart Rhythm 2011;8(Suppl. 1):S121–2.
Romanov A, Pokushalov E, Prohorova D, Cherniavsky A, Shabanov V, Goscinska-Bis K, et al. Coronary artery bypass grafting with concomitant cardiac resynchronization therapy in patients with ischemic heart failure: Results from a multicenter study. Europace 2010;12(Suppl. 1):i50.
Talajic M, Gillis AM, Healey JS, Mitchell LB, Sapp JL, Tung S, et al. Effect of CRT on mode of death in patients with heart failure receiving an ICD: Results from the resynchronization-defibrillation for ambulatory heart failure trial (RAFT). Heart Rhythm 2011;8(Suppl. 1):S63.
Talajic M, Yetisir E, Mitchell LB, Luce M, Theoret-Patrick P, Wells GA, et al. Long-term device-related adverse events after cardiac resynchronization therapy: insights from the resynchronization-defibrillation for ambulatory heart failure trial (RAFT). Circulation 2011;124:A13983.
Talajic M, Yetisir E, Mitchell LB, Luce M, Theoret-Patrick P, Wells GA, et al. Adverse events associated with cardiac resynchronization therapy (CRT): Insights from the resynchronization-defibrillation for ambulatory heart failure trial (RAFT). Can J Cardiol 2011;27(Suppl.):S243–4.
Thibault B, Ducharme A, Harel F, White M, O’Meara E, Roy D, et al. Resynchronization therapy does not help heart failure patients with a QRS duration < 120 ms. Eur Heart J 2011;32(Suppl. 1):148.
Appendix 5 Ongoing trials
Five relevant trials in progress were identified by the searches:
-
ICD2 (Implantable Cardioverter Defibrillator in Dialysis patients) trial – ‘A prospective randomised controlled trial to evaluate the prevention of sudden cardiac death using implantable cardioverter defibrillators in dialysis patients’ (ISRCTN20479861). This trial aims to determine whether or not ICD therapy in dialysis patients aged 55–80 years will result in a significant reduction in sudden cardiac (arrhythmic) death rates compared with no ICD therapy. This is a multicentre RCT carried out in the Netherlands. Start date: 1 April 2007; end date: 1 April 2017. Funding: Biotronik Nederland BV.
-
DANISH (Efficacy of ICD in Patients with Non-Ischemic Systolic Heart Failure) trial – ‘Efficacy of implantable cardioverter defibrillator in patients with non-ischemic systolic heart failure on mortality’ (NCT00542945 and NCT00541268). The comparator is OPT only. This is a multicentre RCT carried out in Denmark. Start date: December 2007; end date: December 2012. Funding: not stated.
-
REFINE-ICD (Risk Estimation Following Infarction, Noninvasive Evaluation) trial – ‘Efficacy of implantable defibrillator therapy after a myocardial infarction’ (NCT00673842). This trial aims to determine whether prophylactic ICD therapy reduces mortality in MI survivors with better-preserved left ventricular function compared with standard medical care and standard post-MI treatment. This is a multicentre RCT carried out in Canada. Start date: March 2011; end date: February 2018. Funding: not stated but collaborators are Alberta Innovation and Science, Medtronic and GE Healthcare.
-
EchoCRT (Echocardiography Guided Cardiac Resynchronization Therapy) trial (NCT00683696). This trial aims to evaluate the effects of CRT-D on mortality and morbidity of patients with HF as a result of LVSD, already receiving OPT, with a narrow QRS width and echocardiographic evidence of ventricular dyssynchrony, compared with OPT only and CRT-D off. This is an international multicentre RCT (including Australia, Austria, Belgium, Canada, the Czech Republic, Denmark, France, Germany, Israel, Italy, the Netherlands, Poland, Portugal, Spain, Switzerland, the UK and the USA). Start date: August 2008; end date: December 2012. Funding: Biotronik, Inc.
-
ADOPT trial – ‘Assessment of efficacies of cardiac resynchronization therapies (CRT-P/D) for heart failure patients in China’ (ChiCTR-TRC-09000574). This trial aims to evaluate whether CRT-P/D in addition to OPT can further reduce mortality, improve congestive HF symptoms and enhance QoL compared with OPT alone in Chinese congestive HF patients. This is a multicentre RCT carried out in China. Start date: October 2008; end date: December 2012. Funding: Medtronik, Inc.
Appendix 6 Hospitalisations: total, cardiac and non-cardiac
People with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony
Number of patients hospitalised
The CARE-HF trial109 reported unplanned hospitalisations for a major cardiovascular event and this was the primary outcome of the study. In addition, the study reported mean number of days in hospital by 3 months, mean days in hospital after 3 months and mean days in hospital overall during the entire study (median 29.6 months). The COMPANION trial120 reported data for all hospital admissions, cardiac admissions and non-cardiac admissions.
CRT-P compared with optimum pharmacological therapy
In the CARE-HF trial109 there were statistically significantly fewer unplanned hospitalisations for a major cardiovascular event with CRT-P than with OPT (31% vs. 46% respectively; HR 0.61, 95% CI 0.49 to 0.77; p < 0.001). The mean number of days in hospital overall was also lower with CRT-P than with OPT, but no statistical comparisons for these outcomes were reported (Table 152). Similarly, in the COMPANION trial,120 the rates of all hospital admissions (CRT-P 63% vs. OPT 65%; p = 0.02) and cardiac admissions (CRT-P 49% vs. OPT 53%; p < 0.01) were both statistically significantly lower with CRT-P than with OPT. However, the rate of non-cardiac hospital admissions was higher with CRT-P than with OPT (36% vs. 27% respectively), but no statistical comparison was reported.
| Study | Outcome and follow-up | CRT-P, n/N (%) | OPT, n/N (%) | Effect | 95% CI; p-value |
|---|---|---|---|---|---|
| CARE-HF109 | Major cardiovascular event, 29.4 monthsa | 125/409 (31) | 184/404 (46) | HR 0.61 | 0.49 to 0.77, < 0.001 |
| Mean days in hospital by 3 months | 7.5, median 4 (IQR 2–8) | 3.4, median 0 (IQR 0–1) | – | – | |
| Days in hospital after 3 months | 222 | 384 | – | – | |
| Mean days in hospital overall during entire study (reported as median of 29.6 months) | 20.7, median 9 (IQR 4–26) | 22.4, median 9 (IQR 0–31) | – | – | |
| MIRACLE121 | Hospitalisations unrelated to HF or function of left ventricular lead | 37/228 (16.2) | 33/225 (14.7) | – | – |
| COMPANION120b | All admissions, CRT-P 16.2 months, OPT 11.9 monthsc | 388/617(63) | 199/308 (65) | – | 0.02 |
| Cardiac admissions | 301/617 (49) | 164/308 (53) | – | < 0.01 | |
| Non-cardiac admissions | 222/617 (36) | 84/308 (27) | – | – | |
| CRT-D, n/N (%) | OPT, n/N (%) | ||||
| All admissions, CRT-D 15.7 months, OPT 11.9 monthsc | 372/595 (63) | 199/308 (65) | – | 0.03 | |
| Cardiac admissions | 284/595 (48) | 164/308 (53) | – | < 0.01 | |
| Non-cardiac admissions | 207/595 (35) | 84/308 (27) | – |
CRT-D compared with optimum pharmacological therapy
All hospital admissions (CRT-D 63% vs. OPT 65%; p = 0.03) and cardiac hospital admissions (CRT-D 48% vs. OPT 53%; p < 0.01) were statistically significantly lower with CRT-D than with OPT in the COMPANION trial (see Table 152). 120 However, non-cardiac hospital admissions were higher with CRT-D than with OPT (35% vs. 27% respectively), but no statistical comparison was reported.
Number of events/days of admission
CRT-P compared with optimum pharmacological therapy
The CARE-HF trial109 reported 222 unplanned hospitalisations for a major cardiovascular event in the CRT-P group (n = 409) and 384 in the OPT group (n = 404) (Table 153). The COMPANION trial120 found statistically significantly fewer admissions per patient-year for a cardiac procedure for those receiving CRT-P (CRT-P 0.13 vs. OPT 0.24; p < 0.01). The number of average admissions per patient-year of follow-up was lower for those receiving CRT-P (CRT-P 1.25 vs. OPT 1.59). The average number of hospital days per patient-year of follow-up was also lower for CRT-P (CRT-P 8.3 vs. OPT 11.0), with the average length of hospital stay per admission similar for both treatment groups (CRT-P 6.7 days vs. OPT 6.9 days). The average number of hospital admissions per patient-year of follow-up was lower with CRT-P for cardiac causes (CRT-P 0.79 vs. OPT 1.20) but higher for non-cardiac causes (CRT-P 0.46 vs. OPT 0.39 admissions). Average number of hospital days per patient-year of follow-up for cardiac (CRT-P 5.2 vs. OPT 8.1) and non-cardiac (CRT-P 3.2 vs. OPT 2.8) causes, and average length of stay per hospital admission for cardiac (CRT-P 6.5 vs. OPT 6.8 days) and non-cardiac (CRT-P 6.9 vs. OPT 7.1 days) causes were similar between treatment groups.
| Study | Outcome and follow-up | CRT-P | OPT | Effect | 95% CI; p-value |
|---|---|---|---|---|---|
| CARE-HF109 | No. of unplanned hospitalisations for a major cardiovascular event, 29.4 monthsa | 222 | 384 | – | – |
| COMPANION120b | No. of admissions (% of total admissions), no. of average admissions per patient-year of follow-up; CRT-P 16.2 months, OPT 11.9 monthsa | ||||
| All admissions | 993 (n/a), 1.25 | 516 (n/a), 1.59 | – | – | |
| Cardiac | 628 (63), 0.79 | 338 (75), 1.20 | – | – | |
| Non-cardiac | 365 (37), 0.46 | 126 (24), 0.39 | – | – | |
| Average hospital days per patient-year of follow-up (average length of stay per admission in days); CRT-P 16.2 months, OPT 11.9 monthsa | |||||
| All admissions | 8.3 (6.7) | 11.0 (6.9) | – | – | |
| Cardiac | 5.2 (6.5) | 8.1 (6.8) | – | – | |
| Non-cardiac | 3.2 (6.9) | 2.8 (7.1) | – | – | |
| No. of admissions per patient-year for a cardiac procedure | 0.13 | 0.24 | – | < 0.01 | |
| CRT-D | OPT | ||||
| No. of admissions (% of total admissions), no. of average admissions per patient-year of follow-up; CRT-D 15.7 months, OPT 11.9 monthsa | |||||
| All admissions | 919 (n/a) 1.20 | 516 (n/a) 1.59 | – | – | |
| Cardiac | 580 (63) 0.76 | 338 (75) 1.20 | – | – | |
| Non-cardiac | 339 (37) 0.44 | 126 (24) 0.39 | – | NS | |
| Average hospital days per patient-year of follow-up (average length of stay per admission in days); CRT-D 15.7 months, OPT 11.9 monthsa | |||||
| All admissions | 8.6 (7.2) | 11.0 (6.9) | – | – | |
| Cardiac | 5.5 (7.2) | 8.1 (6.8) | – | – | |
| Non-cardiac | 3.8 (8.8) | 2.8 (7.1) | – | – | |
| No. of admissions per patient-year for a cardiac procedure | 0.09 | 0.24 | – | < 0.01 | |
CRT-D compared with optimum pharmacological therapy
The COMPANION trial120 reported statistically significantly fewer hospital admissions per patient-year for a cardiac procedure in those receiving CRT-D (CRT-D 0.09 vs. 0.24 OPT; p < 0.01). The number of average admissions per patient-year of follow-up was lower in those receiving CRT-D (CRT-D 1.20 vs. 1.59 OPT). The average number of hospital days per patient-year of follow-up was also lower in those receiving CRT-D (8.6 days vs. 11.0 days OPT), with the average length of hospital stay per admission similar for both treatment groups (CRT-D 7.2 days vs. OPT 6.9 days). Those receiving CRT-D had fewer average hospital admissions per patient-year of follow-up for cardiac causes (CRT-D 0.76 vs. OPT 1.20), but more admissions for non-cardiac causes (CRT-D 0.44 vs. OPT 0.39). Average hospital days per patient-year of follow-up for cardiac (CRT-D 5.5 days vs. OPT 8.1 days) and non-cardiac (CRT-D 3.8 days vs. OPT 2.8 days) causes, and average length of stay per hospital admission for cardiac (CRT-D 7.2 days vs. OPT 6.8 days) and non-cardiac (CRT-D 8.8 days vs. OPT 7.1 days) causes were similar for both treatment groups (see Table 153).
CRT-P compared with CRT-D
The authors of the COMPANION study120 state that no significant differences were found in any of the hospitalisation end points for CRT-P vs. CRT-D, but statistics were not reported.
People with both conditions
The RAFT study140 reported that a similar proportion of participants (about 56%) in each group were hospitalised at least once (Table 154), and the majority were hospitalised for a cardiac cause (CRT-D 47.3%, ICD 44.7%; p = 0.56). All-cause hospitalisations were also similar in the MIRACLE ICD study,136 although the mean length of stay was slightly reduced with CRT-D [mean 4.8 days (SD 4.9 days) vs. mean 5.4 days (SD 4.7 days); p = 0.06]. All-cause hospitalisations were slightly lower with CRT-D in the Pinter study139 (30.6% vs. 36.1%).
| Study | Outcome and follow-up | CRT-D, n/N (%) | ICD, n/N (%) | Effect | 95% CI; p-value |
|---|---|---|---|---|---|
| MIRACLE ICD136 | Hospitalisation, 6 months | 85/187 (45.5) | 78/182 (42.9) | – | – |
| Length of hospital stay (days), mean (SD) | 4.8 (4.9) | 5.4 (4.7) | – | 0.06 | |
| Pinter139 | Patients hospitalised, 6 months | 11/36a (30.6) | 13/36a (36.1) | – | – |
| RAFT140 | One or more hospitalisations during follow-up (mostly cardiovascular reasons), 40 (SD 20) monthsb | 509/894 (56.9) | 509/904 (56.3) | – | – |
| Hospitalisation: cardiac cause | 423/894 (47.3) | 404/904 (44.7) | HR 1.04 | 0.56 |
Appendix 7 Data extraction: people at risk of sudden cardiac death as a result of ventricular arrhythmias
Amiodarone Versus Implantable Cardioverter-Defibrillator Randomized Trial (AMIOVIRT)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Strickberger et al. 2003,69 Wijetunga and Strickberger, 200370 Study design: RCT Country: USA No. of centres: 10 Funding: unrestricted research grant from the guidant corporation |
Intervention: ICD + OPT (ICDs were inserted using conventional non-thoracotomy techniques) Comparator: Amiodarone + OPT (dose: 800 mg/day for first week, 400 mg/day for 1 year and then 300 mg/day) Other interventions used: OPT with ACE inhibitors, beta-blockers and potassium-sparing diuretics was strongly encouraged and attempted throughout the duration of the study for both groups |
Indication for treatment: NIDCM and asymptomatic NSVT No. of randomised participants: 103; ICD: 51 OPT: 52 Inclusion criteria: age ≥ 18 years; NIDCM (left ventricular dysfunction in the absence of, or disproportionate to the severity of, coronary artery disease); LVEF ≤ 0.35; asymptomatic NSVT (three or more consecutive ventricular premature depolarisations with a rate of > 100 beats per minute, lasting < 30 seconds and not associated with symptoms of cerebral hypofusion); NYHA class I–III Exclusion criteria: syncope; pregnancy; a contraindication to amiodarone or defibrillator therapy or concomitant therapy with a class I AAD; or NIDCM diagnosed within 6 months70 |
Primary outcome: total mortality Secondary outcomes: SCD, non-SCD, non-cardiac death, syncope, arrhythmia-free survival, QoL and costs Method of assessing outcomes: stored ECGs and all available clinical data were used to determine the appropriateness of ICD therapy. Causes of death were determined by an events committee, with each of the three members independently evaluating all information available regarding each death. Differences in the cause of death were adjudicated and a consensus reached QoL: completed by patients both at the time of randomisation and during follow-up visits:
Cost analysis: in- and outpatient costs for the 24 patients based on University of Michigan health system for 1 year starting at study entry (not data extracted) Amiodarone group: assessed for thyroid function and aspartate and alanine transaminase plasma levels; chest radiograph obtained at baseline and every 4 months during follow-up; serum concentrations of amiodarone and desethylamiodarone were obtained 4 months and 1 year after initiation of treatment (until 30 June 2001) ICD group: defibrillator follow-up was performed every 4 months, including evaluation of stored ECGs and sensing and pacing functions Definitions: arrhythmia-free survival: freedom from death, syncope, appropriate ICD therapy and sustained VT or VF Length of follow-up: mean duration 2.0 years (SD 1.3 years, range 0.1–4.8 years); ICD 2.2 years (SD 1.2 years); amiodarone 1.8 years (SD 1.4 years), p = 0.4 Recruitment: August 1996–September 2000 |
Participant characteristics
| Characteristic | ICD (n = 51) | Amiodarone (n = 52) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 58 (11) | 60 (12) | 0.5 |
| Sex, % male | 67 | 74 | 0.3 |
| Ethnicity | NR | NR | |
| NYHA classification, % | 0.9 | ||
| I | 18 | 13 | |
| II | 64 | 63 | |
| III | 16 | 24 | |
| LVEF, mean (SD) | 0.22 (0.10) | 0.23 (0.08) | 0.5 |
| Heart rate (bpm), mean (SD) | 80 (17) | 78 (14) | 0.7 |
| RBBB, % | 16 | 8 | 0.2 |
| LBBB, % | 42 | 53 | 0.3 |
| Electrophysiology findings | |||
| No. of beats of NSVT, mean (SD) | 8 (7) | 12 (21) | 0.2 |
| NSVT (bpm), mean (SD) | 160 (27) | 151 (20) | 0.4 |
| NSVT identified, % | 0.7 | ||
| ECG | 6 | 8 | |
| Event monitor | 26 | 29 | |
| Holter monitor | 6 | 2 | |
| Hospital telemetry | 62 | 61 | |
| Current pharmacological therapy | NR | NR | |
| Duration of NIDCM (years), mean (SD) | 2.9 (4.0) | 3.5 (3.9) | 0.6 |
| CAD > 70%,a n/N (%) | 2/41 (4.9) | 3/27 (11.0) | 0.3 |
| Cardiac history | |||
| Previous treatment | NR | NR | |
| Comorbidities | |||
| Diabetes mellitus, % | 31 | 36 | 0.6 |
| Hypertension, % | 58 | 67 | 0.4 |
| QWBS, mean (SD) | 67 (15) | 70 (17) | 0.5 |
| STAI, mean (SD) | 75 (25) | 79 (21) | 0.5 |
Results
| Outcome | ICD (n = 51) | Amiodarone (n = 52) | p-value |
|---|---|---|---|
| Primary outcome total mortality, n (%) | 6 (11.8) | 7 (13.5) | 0.8 |
| Secondary outcomes | |||
| Cardiac deaths, n (%) | 4 (67) | 5 (71) | 0.9 |
| SCD | 1 (25) | 2 (40) | 0.7 |
| Non-SCD | 3 (75) | 3 (60) | 0.7 |
| Survival rate, % | |||
| At 1 year | 96 | 90 | 0.8 |
| At 3 years | 88 | 87 | |
| Arrhythmia-free survival rate, % | |||
| At 1 year | 78 | 82 | 0.1 |
| At 3 years | 63 | 73 | |
| Non-cardiac death, n (%) | 2 (33) | 2 (29) | 0.9 |
| Cardiac transplant, n (%) | 1 (2) | 2 (4) | 0.8 |
| Syncope, % | 3.9a | 5.8 | 0.7 |
| HRQoL | |||
| QWBS 1 year, mean (SD) | 74 (19) | 70 (22) | 0.5b |
| STAI 1 year, mean (SD) | 61 (17) | 67 (20) | 0.4b |
Concomitant drug therapy at last follow-up
| Drug therapy | ICD (n = 51) | Amiodarone (n = 52) | p-value |
|---|---|---|---|
| Beta-blocker, % | 53 | 50 | 0.5 |
| ACE inhibitor, % | 90 | 81 | 0.4 |
| Digoxin, % | 71 | 67 | 0.5 |
| Diuretic, % | 71 | 67 | 0.5 |
| Spironolactone, % | 20 | 19 | 0.9 |
Adverse effects of treatment
| 25 patients discontinued amiodarone because of adverse side effects (mean 17.8, SD 13.3, range 1.2–43.8 months)a |
-
Allocation to treatment groups: randomisation was stratified by centre (patients who refused study participation were followed in a voluntary registry).
-
Blinding: unblinded trial. Assessors for causes of death were blinded (independent events review committee) and all references to amiodarone or ICD therapy were removed from the reviewed documents (including the death certificate, other relevant medical records and interviews with family members).
-
Comparability of treatment groups: There were no statistically significant differences at baseline between the treatment groups.
-
Method of data analysis: patients who underwent cardiac transplantation were censored from data analysis beginning on the day of transplantation. All analyses were based on ITT. Primary and secondary end points were compared between the two groups with a log-rank test, and survival curves were constructed using Kaplan–Meier methods. Continuous variables are expressed as mean ± 1 SD and were compared using the Student’s t-test, except for comparisons between baseline and 1-year QoL scores within the two study groups, which were compared with a paired t-test. A chi-squared or Fisher’s exact test was used to compare nominal variables. A p-value of < 0.05 was considered statistically significant. A data safety monitoring board evaluated the results every 10 deaths. Prospectively determined stopping rules consisted of a mortality difference at a significance level of < 0.025, or a significance level of > 0.025 (90% power) based on a power calculation conditional on holding outcomes stable and assuming enrolment of 600 patients. At the first interim analysis in September 2000, the study enrolment was discontinued because the prospective stopping rule for the inability to demonstrate statistical significance was reached.
-
Sample size/power calculation: during the anticipated follow-up duration of 2 years, the expected total mortality rates were 20% in the amiodarone group and 10% in the ICD group. An 80% power to identify a reduction in total mortality from 20% to 10% was calculated to require 219 patients in each group (p < 0.05, two-sided t-test).
-
Attrition/dropout: states that no patients were lost to follow-up. Amiodarone: crossover from amiodarone to ICD (n = 8): near-syncope with documented VT (n = 2), cardiac arrest (n = 2) or amiodarone intolerance (n = 4); ICD insertion (months): mean 26.1 (SD 16.9) after study entry. ICD patients also receiving amiodarone (n = 11): frequent appropriate defibrillator therapies (n = 1; 200 mg/day, SD 0 mg/day), atrial fibrillation (n = 8; 200 mg/day, SD 0 mg/day), other reasons (n = 2; 150 mg/day, SD 71 mg/day).
-
Generalisability: only to patients with NIDCM and asymptomatic NSVT.
-
Outcome measures: appear appropriate.
-
Intercentre variability: not reported.
-
Conflict of interests: none reported, but supported by grant from Guidant Corporation.
NIDCM, non-ischaemic dilated cardiomyopathy.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Randomly assigned and stratified by centre, but no details of sequence generation |
| Allocation concealment | Unclear | Not reported |
| Performance bias | ||
| Blinding of participants and personnel | ||
| Mortality | High | No blinding |
| QoL | High | May be influenced by lack of blinding |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality | Low | Members of independent events review committee assessing causes of death were blinded |
| QoL | High | May be influenced by lack of blinding |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | States that all analyses were based on ITT; no patients lost to follow-up |
| Reporting bias | ||
| Selective reporting | Low | No study protocol available but results for specified primary and secondary outcomes were reported |
| Other bias | ||
| Other sources of bias | Low | |
Antiarrhythmics Versus Implantable Defibrillators (AVID) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| AVID investigators 1997,71 1999,72 Hallstrom 1995,73 Schron et al. 200274 Study design: RCT Countries: USA and Canada No. of centres: 56 (53 USA, three Canada) Funding: National Heart, Lung, and Blood Institute, Bethesda, MD; contract N01-HC-25117 |
Intervention: ICDs; investigators chose any ‘state-of-the-art’ ICD meeting prespecified criteria Comparator: best contemporary AADs. Consideration of the use of sotalol left to physician judgement. If patients eligible for sotalol a second randomisation assigned them to either amiodarone (doses determined empirically) or sotalol (guided by electrophysiological testing, Holter monitoring or both) Other interventions used: aspirin, beta-blockers and ACE inhibitors when clinically appropriate |
Indication for treatment: resuscitated from near-fatal VF; or symptomatic sustained VT with hemodynamic compromise No. of randomised participants: 1016; ICD: 507 (93% non-thoracotomy lead system, 5% epicardial system, 2% no device implanted), AAD: 509 [356 began immediate treatment with amiodarone; remaining 153 randomised to amiodarone (n = 79) or sotalol (n = 74)] QoL substudy:74 800; ICD: 416, AAD: 384 Inclusion criteria: VF, VT with syncope or VT without syncope but with ejection fraction ≤ 0.40 and systolic blood pressure < 80 mmHg, chest pain or near syncope.73 If patients underwent revascularisation their ejection fraction had to be ≤ 0.40 Exclusion criteria: contraindication to amiodarone or ICD therapy, transient or correctable cause identified for the arrhythmia, CABG or percutaneous transluminal coronary angioplasty planned and ejection fraction > 0.40, left ventricular aneurysm surgery planned or performed since index event, recent amiodarone exposure (definition provided), long QT syndrome, atrial fibrillation or other supraventricular arrhythmia requiring class I or III antiarrhythmic agents, bradycardia or heart block without permanent pacemaker, NYHA class IV HF, life expectancy < 1 year73 |
Primary outcome: overall mortality Secondary outcomes: cost, QoL Other: ICD shock, sustained arrhythmia, syncope Method of assessing outcomes: patients evaluated every 3 months and at the time of events. Cause of death reviewed by events committee QoL substudy74 at baseline (before randomisation) and 3, 6 and 12 months after randomisation:
Defibrillator shocks categorised as appropriate or inappropriate on the basis of clinical presentation, R–R intervals and ECGs Length of follow-up: mean 18.2 (SD 12.2) months. For QoL substudy74 follow-up was 1 year Recruitment: 1 June 1993 to7 April 1997 |
Main study
Participant characteristics
| Characteristic | ICD (n = 507) | AAD (n = 509) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 65 (11) | 65 (10) | |
| Sex, % male | 78 | 81 | |
| Ethnicity, % white | 87 | 86 | |
| Index arrhythmia VF, n | 226 | 229 | |
| Index arrhythmia sustained VT, n | 281 | 280 | |
| CHF at enrolment, % | |||
| No CHF | 45 | 40 | |
| NYHA class I or II | 48 | 48 | |
| NYHA class IIIa | 7 | 12 | |
| Angina at enrolment, % | |||
| No angina | 64 | 65 | |
| CCS class I or II | 34 | 33 | |
| CCS class III | 2 | 2 | |
| LVEF, mean (SD) | 0.32 (0.13) | 0.31 (0.13) | |
| Median time from index event to measurement (days) | 3 | 3 | |
| Findings on baseline ECGb | |||
| Heart rate (bpm), mean (SD) | 77 (18) | 78 (17) | |
| PR interval (milliseconds), mean (SD) | 178 (37) | 183 (37) | |
| QRS complex (milliseconds), mean (SD) | 116 (26) | 117 (26) | |
| Corrected QT interval (milliseconds), mean SD | 441 (40) | 445 (39) | |
| Paced, % | 3 | 4 | |
| Bundle branch block, % | 23 | 25 | |
| Clinical history before index arrhythmia, % | |||
| Atrial fibrillation or fluttera | 21 | 26 | |
| VF | 5 | 5 | |
| VT | 14 | 15 | |
| Unexplained syncope | 11 | 15 | |
| Coronary artery disease | 81 | 81 | |
| MI | 67 | 67 | |
| CHF | 46 | 47 | |
| Hypertension | 55 | 56 | |
| Diabetes | 25 | 24 | |
| Angina | 48 | 50 | |
| Peripheral vascular disease | 16 | 15 | |
| AAD therapy | 16 | 15 | |
| Coronary revascularisation during hospitalisation for the index arrhythmia, % | 10 | 12 | |
| ICD (n = 497) | AAD (n = 496) | ||
| Therapy at discharge, %c | |||
| ICD | 98.6 | 1.4 | |
| Amiodarone | 1.8 | 95.8 | |
| Sotalol | 0.2 | 2.8 | |
| Beta-blocker | 42.3 | 16.5 | < 0.001d |
| Calcium channel blocker | 18.4 | 12.1 | |
| Both beta-blocker and calcium channel blocker | 5.3 | 2.4 | |
| Digitalis | 46.8 | 40.6 | 0.04d |
| Diuretic agent | 48.2 | 50.7 | |
| Other AAD | 4.2 | 1.2 | |
| ACE inhibitor | 68.8 | 68.2 | |
| Nitrate | 36.4 | 37.0 | |
| Other antihypertensive agent | 7.6 | 8.8 | |
| Lipid-lowering agent | 13.2 | 11.5 | |
| Acetylsalicylic acid (aspirin) | 60.7 | 59.2 | |
| Warfarin | 21.9 | 34.8 | |
Results
| Outcome | ICD (n = 507) | AAD (n = 509) | p-value | ||
|---|---|---|---|---|---|
| Deaths, n/N | 80/507 | 122/509 | < 0.012 | ||
| Cause of death, n72 | |||||
| Cardiac death | 63 | 94 | |||
| Arrhythmic | 24 | 55 | |||
| Non-arrhythmic | 39 | 39 | |||
| Non cardiac death | 17 | 28 (three attributed to pulmonary toxicity from amiodarone) | 0.053; RR 1.78 (95% CI 0.98 to 3.26) | ||
| Crude death rate (±95% CI) over mean follow-up of 18.2 (SD 12.2) months, % | 15.8 (±3.2) | 24.0 (±3.7) | |||
| Survival free of cardiac death72 (non-cardiac deaths censored), % | 0.0042 | ||||
| At 1 year | 90.9 | 85.1 | |||
| At 2 years | 85.0 | 81.2 | |||
| Survival to arrhythmic death72 (non-cardiac and non-arrhythmic deaths censored), % | 0.0002 | ||||
| At 1 year | 96.6 | 91.9 | |||
| At 2 years | 94.2 | 89.1 | |||
| Survival free of non-arrhythmic cardiac death (non-cardiac and arrhythmic deaths censored) | Presented in figure only | Presented in figure only | 0.8039 | ||
| Overall survival through the course of study, % | < 0.02 in favour of ICD | ||||
| Patients surviving at 1 year | 89.3 | 82.3 | |||
| Patients surviving at 2 year | 81.6 | 74.7 | |||
| Patients surviving at 3 year | 75.4 | 64.1 | |||
| Cumulative % of patients with any activation of the ICD (antitachycardia pacing or shock) | Index VFa | Index VTa | < 0.001 for VT vs. VF | ||
| At 3 months | 15 | 36 | |||
| At 1 year | 39 | 68 | |||
| At 2 years | 53 | 81 | |||
| At 3 years | 69 | 85 | |||
| % of patients rehospitalised (denominator n = 1011) | 0.04 | ||||
| At 1 year | 59.5 | 55.6 | |||
| At 2 years | 74.8 | 64.7 | |||
| At 3 years | 83.3 | 75.5 | |||
| Change in NYHA class | NR | NR | |||
| Change in LVEF | NR | NR | |||
| Exercise capacity outcomes | NR | NR | |||
| Crossover rate, % | |||||
| At 1 year | 17.7 | 12.6 | < 0.001 | ||
| At 2 years | 25.7 | 18.9 | |||
| At 3 years | 33.7 | 24.3 | |||
| Therapy at follow-up, % | 12 months (n = 338) | 24 months (n = 171) | 12 months (n = 306) | 24 months (n = 162) | |
| ICD | 97.9 | 95.7 | 9.5 | 9.8 | |
| Amiodarone | 8.3 | 9.3 | 84.7 | 82.4 | |
| Sotalol | 1.8 | 3.1 | 5.8 | 8.5 | |
| Beta-blocker | 38.1 | 39.4 | 11.0 | 10.1 | |
| Calcium channel blocker | 22.9 | 19.4 | 16.6 | 14.1 | |
| Both beta-blocker and calcium channel blocker | 6.8 | 5.6 | 2.1 | 0.7 | |
| Digitalis | 45.8 | 44.4 | 37.9 | 32.3 | |
| Diuretic agent | 56.0 | 56.9 | 59.3 | 56.4 | |
| Other AAD | 7.1 | 10.0 | 3.8 | 4.0 | |
| ACE inhibitor | 68.4 | 68.1 | 65.5 | 63.1 | |
| Nitrate | 29.1 | 28.1 | 27.9 | 29.5 | |
| Other antihypertensive agent | 9.0 | 10.0 | 9.4 | 6.1 | |
| Lipid-lowering agent | 19.5 | 23.1 | 17.2 | 19.5 | |
| Acetylsalicylic acid (aspirin) | 55.4 | 62.5 | 55.4 | 56.4 | |
| Warfarinftsa | 24.8 | 22.5 | 35.4 | 30.2 | |
Adverse effects of treatment
| Adverse effect | ICD | Amiodarone | Sotalol | p-value |
|---|---|---|---|---|
| Non-fatal torsade de pointes VT, n | 1 | |||
| Suspected pulmonary toxicity in patients treated with amiodarone, % | ||||
| At 1 year | 3 | |||
| At 2 years | 5 | |||
| Death from pulmonary toxicity, n | 1 | |||
| Thyroid replacement medication, % | ||||
| At 1 year | 1 | 10 | ||
| At 2 years | 1 | 16 | ||
| Death within 30 days of initiation of therapy, n/N (%)a | 12/507 (2.4) | 18/509 (3.5) | 0.27 | |
| Bleeding requiring reoperation or transfusion, n | 6 | |||
| Serious haematoma, n | 13 | |||
| Infection, n | 10 | |||
| Pneumothorax, n | 8 | |||
| Cardiac perforation, n | 1 | |||
| Early dislodgement or migration of leads, n | 3 | |||
| Unsuccessful first attempt at ICD implantation without thoracotomy, n | 5b | |||
| Overall rate of non-fatal complications of implantation, % (reported in discussion) | 5.7 | |||
Subgroup data71
| Subgroup | HR | 95% CI | p-value |
|---|---|---|---|
| Age (years) | |||
| < 60 | 0.57 | 0.31 to 1.05 | |
| 60–69 | 0.63 | 0.38 to 1.04 | |
| ≥ 70 | 0.67 | 0.44 to 1.00 | |
| LVEF | |||
| > 0.35 | 0.86 | 0.47 to 1.61 | |
| ≤ 0.35 | 0.57 | 0.41 to 0.79 | |
| Cause of arrhythmia | |||
| Coronary artery disease | 0.62 | 0.46 to 0.86 | |
| Other | 0.62 | 0.28 to 1.35 | |
| Rhythm | |||
| VF | 0.57 | 0.38 to 0.86 | |
| VT | 0.68 | 0.46 to 1.02 | |
| Overall | 0.62 | 0.47 to 0.83 | |
Subgroup data72
| Outcomes | Index arrhythmia VF (n = 455 at baseline) | Index arrhythmia VT (n = 561 at baseline) | p-value |
|---|---|---|---|
| Survival free of arrhythmic death | Improved by the ICD for patients whose presenting arrhythmia was VT (p = 0.025) or VF, with twice as many deaths in the AAD group (p = 0.0019). Survival curves presented but not extracted | ||
| Non-arrhythmic cardiac death | No difference in survival between ICD and AAD groups in patients with either VT (p = 0.72) or VF (p = 0.98) | ||
Quality-of-life substudy74
Participant characteristics
| Characteristic | ICD (n = 416) | AAD (n = 384) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 64.3 (10.5) | 64.7 (10.1) | 0.5 |
| Sex, % male | 81.3 | 80.5 | 0.8 |
| Ethnicity, % white | 89.7 | 88.0 | 0.5 |
| Live with spouse partner, % | 72.6 | 70.6 | 0.5 |
| High-school graduate, % | 74.0 | 74.5 | 0.9 |
| Index arrhythmia VF, % | 43.5 | 42.4 | 0.8 |
| LVEF, mean (SD) | 0.33 (0.13) | 0.32 (0.14) | 0.6 |
| History of HF, % | 44.5 | 41.1 | 0.3 |
| Discharge beta-blocker use, % | 43.0 | 16.4 | < 0.001 |
Results
| Outcome | ICD (n = 416) | AAD (n = 384) | p-value |
|---|---|---|---|
| SF-36 PCS score, mean (SD) | |||
| Baseline | 37.4 (10.9) | 36.5 (11.2) | 0.3 |
| 12 months | 40 (10.5) | 38 (17) | |
| SF-36 MCS score, mean (SD) | |||
| Baseline | 45.9 (11.8) | 47.5 (11.5) | 0.006 |
| 12 months | 49 (16.5) | 48 (17) | |
| Patient concerns checklist, mean (SD) | |||
| Baseline | 15.9 (8.6) | 16.2 (8.9) | 0.06 |
| Follow-up | NR | NR | 0.1 |
| QoL index baseline, mean (SD) | 22.1 (4.9) | 21.9 (5.0) | Similar at baseline and follow-up |
| Impact of adverse symptoms on QoLa | |||
| SF-36 PCS score | –2.25 (–3.32, –1.18), p < 0.001 | –1.64 (–2.89, –0.41), p = 0.009 | |
| SF-36 MCS score | –2.32 (–3.76, –0.88), p = 0.002 | –0.51 (–1.97, 0.94), p = 0.5 | |
| Patient concerns | 1.84 (0.91, 2.76), p < 0.001 | 0.91 (0.07, 1.75), p = 0.03 | |
| Impact of ICD shocks on QoLa,b | |||
| SF-36 PCS score | –1.45 (–2.74, –0.18), p = 0.03 | ||
| SF-36 MCS score | –1.82 (–3.56, –0.08), p = 0.04 | ||
| Patient concerns | 2.15 (1.07, 3.23), p < 0.001 | ||
| ICD shocksb | |||
| Experienced one or more shocks during first year of follow-up, n/N (%) | 144/373 (39) | ||
| Experienced one or two shocks | 71/144 (49) | ||
| Experienced three or more shocks | 73/144 (51) | ||
| Proportion of shocks considered appropriate, % | 94 | ||
-
Allocation to treatment groups: stratified by clinical site and index arrhythmia. 73 AAD group subrandomised to empirical amiodarone or Holter-/electrophysiology-guided sotalol (if no contraindications to sotalol, otherwise assigned to amiodarone). 71
-
Blinding: not stated but presume unblinded because only one group received an ICD and implantation of this requires an operation. The primary end point of overall mortality not likely to be affected by bias. Cause of death analysis was blinded. All references to therapy with either ICD or AAD were removed from medical records sent to the clinical trial centre. In addition, ‘sham blinding’ was performed to try and mimic the removal of items that would have been deleted if the patient had been randomised to the alternative arm. The committee judging cause of death knew that sham blinding could occur.
-
Comparability of treatment groups: described as similar except for history of atrial fibrillation or flutter and NYHA class III HF. Also, more patients were taking beta-blockers (p < 0.001) and slightly more were taking digitalis (p = 0.04) in the ICD group at discharge than in the AAD group (see footnote d in Participant characteristics). Adjusting for the difference in beta-blocker use in the Cox regression analysis slightly reduced the estimated beneficial effect of ICD on survival (unadjusted HR for ICD vs. AAD 0.62, adjusted HR 0.67). In the QoL substudy baseline characteristics were similar except that patients in the ICD group were more often discharged with beta-blocker therapy.
-
Method of data analysis: the null hypothesis was that there was no difference in overall mortality between therapy with an ICD and AAD therapy. Analysis was by ITT for overall mortality, QoL and costs;73 however, it is clear from the numbers reported that for other outcomes analysis was not by ITT. Significance was based on a two-sided alpha level of 0.05 for comparisons of survival distributions. At the end of the pilot phase sequential data monitoring was performed every 6 months. Criteria for termination of the study were based on an O’Brien–Fleming spending function, which requires a substantial difference between treatment groups to stop the study early (referenced). Subgroup analyses were to be specified early in the course of the second phase (after the pilot phase with the first 200 participants), and the intention was to limit severely the numbers of a priori subgroup analyses. 73 Two subgroup analyses are specified: index arrhythmia (VF vs. VT) and cardiac substrate (coronary artery disease vs. cardiomyopathy). In the QoL substudy74 both appropriate and inappropriate shocks were included in the analysis. Because follow-up QoL values cannot be reliably defined for patients who die before reassessment the primary analyses were limited to patients who survived for 1 year after randomisation. Secondary sensitivity analyses included all QoL substudy participants. A chi-squared test or t-test was used for pairwise comparisons. Generalised estimating equations were used to model change in QoL scores over time to account for correlation of individual values and to deal with missing follow-up data. Separate models were used for PCS, MCS and patient concerns checklist scores. Models were adjusted for baseline characteristics of age, sex, race, living alone vs. with a spouse or partner, index arrhythmia, ejection fraction, history of HF and beta-blocker use to assess the independent relationship of variables with QoL. All analyses were ITT and p ≤ 0.05 was considered significant.
-
Sample size/power calculation: a sample size of 1200 patients was estimated, assuming an average follow-up of 2.6 years and an event rate of 40% in the AAD group at 4 years, to detect a 30% decrease in mortality. The data and safety monitoring board recommended stopping the trial on 7 April 1997 when analysis revealed that the difference in the primary outcome variable between the two groups had crossed the statistical boundary for early termination of the study (1016 patients had been randomised).
-
Attrition/dropout: in 2% of the ICD group no device was implanted. In the AAD group 13/74 patients assigned to sotalol had adequate suppression of arrhythmia and were receiving sotalol at discharge. The remaining 61/74 patients randomised to sotalol received amiodarone (n = 58), another AAD (n = 1) or an ICD (n = 2). 25.7% of ICD group and 18.9% of AAD group crossed over to the other therapy by 24 months. The crossover rate was higher among those initially assigned to therapy with an ICD (p < 0.001). States that rates of crossover did not compromise the power of the study and that most crossovers occurred because arrhythmia recurred, rather than because of intolerance to either drugs or devices.
QoL substudy:74 of the 1016 participants randomised in the main study, 905 (89%) completed at least one QoL assessment in the first year of follow-up and most of these (800/905, 88%) survived for 1 year and were included in the analyses of QoL (n = 416 in the ICD group and n = 384 in the AAD group). Complete QoL data were available for most patients at each time point; more data were missing at later compared with earlier assessments. Most (49%) incomplete data were missing because collection fell outside the specified time period. Details reported (not extracted) for whole study (but not for treatment groups).
General comments-
Generalisability: in the discussion of the paper it is noted that data in the AVID registry show that the clinical characteristics of patients included in the trial were similar to those who were not included and therefore the AVID study authors believed that the population studied was representative of the general population of patients who are resuscitated from VF or who have symptomatic, sustained VT.
QoL substudy:74 there were differences between the 905 participants who completed at least one QoL assessment and those in the trial as a whole. QoL substudy participants were younger on average (65 vs. 68 years) and more likely to be male (81% vs. 70%), white (88% vs. 70%) and living with a spouse or partner (71% vs. 51%) and to have graduated from high school (73% vs. 42%) than 111 non-participants. Also reports differences between those who died in the first year vs. those who survived.
-
Outcome measures: appear appropriate. For the QoL substudy74 definitions and categorisation of symptoms provided.
-
Intercentre variability: not discussed.
-
Conflict of interests: no conflicts of interest statement made.
-
Other: a registry was maintained for all patients who qualified for the study but did not undergo randomisation to compare the randomised and non-randomised patients. The registry also followed patients with VF or VT who were not eligible for randomisation. Data on long-term mortality among the non-randomised patients could be obtained from the National Death Index.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | ‘Allocation is stratified by clinical site and index arrhythmia (ventricular fibrillation or ventricular tachycardia)’.73 No other information provided |
| Allocation concealment | Unclear | No information provided |
| Performance bias | ||
| Blinding of participants and personnel | High | Not explicitly stated but presume unblinded (because only one of the two groups received an ICD). QoL self-assessment by participants at risk of bias because of knowledge of intervention received |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Overall mortality and cause of death | Low | For overall mortality outcome risk of bias likely to be low in an unblinded study. Committee judging causes of death were blinded to the participant group |
| QoL | High | |
| Attrition bias | ||
| Incomplete outcome data addressed – overall mortality | Low | ‘Analysis was performed according to the intention-to-treat principle’.71 Although there were crossovers between groups, no dropouts are recorded in the paper |
| Incomplete outcome data addressed – QoL | High | The QoL substudy did not include all randomised participants and there were some differences between those completing the QoL substudy and the whole trial population. In addition, data from those who completed the baseline QoL assessment but died within a year could not be included in the QoL assessment, which may be another source of bias |
| Reporting bias | ||
| Selective reporting | Low | Paper available describing rationale, design and methods for the study |
| Other bias | ||
| Other sources of bias | Low | |
Coronary Artery Bypass Graft (CABG) Patch trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Bigger et al. 1997,75–78 Namerow et al. 1999,80 Spotnitz et al. 199879 Study design: RCT Countries: USA and Germany No. of centres: 37 (USA 35, Germany two) Funding: National Heart Lung and Blood Institute grants HL-48120 and HL-48159 and a grant from Guidant Corporation/CPI, St Paul, MN |
Intervention: ICD: epicardial defibrillator. Leads and pulse generators provided by Guidant Corporation/CPI (St Paul, MN). Most were committed devices (i.e. deliver a shock even if the arrhythmia stops before the end of charging) that were not capable of storing ECGs Comparator: control group, OPT (subject to caveats described below). No defibrillator therapy75 and no specific therapy for ventricular arrhythmias237 Other interventions used: ICD group: the protocol prohibited the use of AADs for asymptomatic ventricular arrhythmias and specified that patients without contraindications should be treated with aspirin. Clinical advice has indicated that, although the drug therapy received was lower than current standards (especially for statin use) for a trial conducted at this time, it would have been considered OPT |
Indication for treatment: patients scheduled for CABG surgery and at risk for sudden death (LVEF < 0.36 and abnormalities on an ECG). Prophylactic No. of randomised participants: 900; ICD: 446, control: 454 Inclusion criteria: scheduled for CABG surgery, < 80 years old, LVEF < 0.36, marker of arrhythmia: abnormalities on an ECG (duration filtered QRS complex ≥ 114 milliseconds; root mean square voltage in the terminal 40 milliseconds of the QRS complex < 20 µV; or duration of the terminal filtered QRS complex at < 40 µV > 38 milliseconds) Exclusion criteria: history of VT or VF, diabetes mellitus with poor blood glucose control or recurrent infections, previous or concomitant aortic or mitral valve surgery, concomitant cerebrovascular surgery, serum creatinine > 3 mg/dl (265 mmol/l), emergency coronary bypass surgery, non-cardiovascular condition with expected survival < 2 years, inability to attend follow-up visits |
Primary outcome: mortality Secondary outcomes: not explicitly stated but QoL and adverse events reported Method of assessing outcomes: follow-up visits every 3 months QoL study:80 single assessment at 6 months included (1) seven of the subscales of the SF-36: general health, physical functioning, physical role functioning, bodily pain, social functioning, emotional role functioning, mental health; for each subscale a raw score is transformed to a 0–100 scale; (2) health transition variable with five response categories (higher score represents perception that heath status has become worse); (3) items on employment status and body image (two two-item scales: satisfaction with appearance and satisfaction with scar; higher scores = greater satisfaction) Length of follow-up: mean of 32 months Recruitment: pilot study from 14 August 1990, full-scale study from 1993. Final enrolment 5 February 1996.80 Study data reported on 30 April 1997 for main trial publication75 |
Participant characteristics
| Characteristica | ICD (n = 446) | Control (n = 454) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 64 (9) | 63 (9) | |
| Sex, male/female, n | 386/60 | 373/81 | |
| Ethnicity, %80 | NS | ||
| White | 88 | 86 | |
| African American | 7 | 10 | |
| Other | 5 | 4 | |
| LVEF, mean (SD) | 0.27 (0.06) | 0.27 (0.06) | |
| Heart rate (bpm), mean (SD) | 79 (15) | 79 (14) | |
| Findings on 12-lead ECG, % | |||
| Duration of QRS complex > 100 milliseconds | 71 | 74 | |
| LBBB | 10 | 12 | |
| Q-wave MI | 52 | 53 | |
| Cardiovascular history, % | |||
| Cigarette smoking at any time | 79 | 76 | |
| Angina pectoris | 76 | 76 | |
| MI | 83 | 82 | |
| Two or more previous MIs | 30 | 33 | |
| HF | 51 | 49 | |
| Treatment for HF | 49 | 47 | |
| NYHA functional class II or III | 71 | 74 | |
| Treatment for hypertension | 54 | 52 | |
| Diabetes mellitus | 36 | 40 | |
| Diabetes treated with insulin | 17 | 20 | |
| Treatment for ventricular arrhythmias | 7 | 7 | |
| PTCA or atherectomy | 11 | 11 | |
| CABG surgery | 12 | 10 | |
| Electronic cardiac pacemaker | 2 | 2 | |
| Systolic blood pressure (mmHg), mean (SD) | 126 (19) | 123 (19) | |
| Pulmonary rales, % | 20 | 25 | |
| S3 gallop, % | 14 | 11 | |
| Left ventricular end-diastolic pressure (mmHg), mean (SD) | 21 (10) | 22 (10) | |
| Findings on coronary angiography, % | |||
| One-vessel disease | 8 | 9 | |
| Two-vessel disease | 36 | 36 | |
| Three-vessel disease | 55 | 55 | |
| ICD (n = 430) | Control (n = 442) | ||
| Drug therapy at hospital discharge, % of patientsb | |||
| Oral AADs | |||
| None | 63.3 | 65.2 | |
| Class I drugs | 16.7 | 12.0 | |
| Amiodarone | 3.7 | 3.2 | |
| Sotalol | 0.5 | 0.2 | |
| Beta-blockers (not sotalol) | 17.9 | 24.0 | |
| ACE inhibitors | 54.7 | 53.8 | |
| Diuretics | 57.2 | 47.1 | |
| Digitalis | 68.6 | 64.5 | |
| Nitrates | 8.1 | 8.1 | |
| Calcium channel blockers | 10.5 | 7.0 | |
| Antiplatelet drugs | 82.8 | 85.1 | |
| Oral anticoagulants | 15.3 | 14.7 | |
| Lipid-lowering drugs | 9.5 | 8.4 | |
Results
| Outcome | ICD (n = 446) | Control (n = 454) | p-value | ||
|---|---|---|---|---|---|
| Deaths in the first 30 days after randomisation, n (%, calculated by reviewer) | 24 (5.4) | 20 (4.4) | 0.60 | ||
| aDeaths during mean (SD) follow-up of 32 (16) months,78 n | 102 | 96 | |||
| Mechanism of death,78 n/N (%) | |||||
| Cardiac | 76/102 (74.5) | 79/96 (82.3) | |||
| Primary arrhythmic | 13/102 (12.7) | 22/96 (22.9) | Arrhythmic deaths 15% vs. 29%, χ2 = 5.10, p = 0.024 | ||
| Secondary arrhythmic | 2/102 (2) | 6/96 (6.3) | |||
| Non-arrhythmic, cardiac | 57/102 (55.9) | 46/96 (47.9) | |||
| Myocardial pump failure | 30/102 (29.4) | 23/96 (24.0) | χ2 = 0.75, p = 0.358 | ||
| Cardiac procedure | 27/102 (26.5) | 23/96 (24.0) | |||
| Unwitnessed, cardiac | 0 | 2/96 (2.1) | |||
| Uncertain, cardiac | 4/102 (3.9) | 3/96 (3.1) | |||
| Non cardiac | 25/102 (24.5) | 17/96 (17.7) | |||
| Unknown | 1/102 (1.0) | 0 | |||
| RR (95% CI) of cause-specific death by treatment assignment78 | |||||
| Cardiac | 0.97 (0.71 to 1.33) | 0.84 | |||
| Arrhythmic | 0.55 (0.29 to 1.03) | 0.06 | |||
| Non-arrhythmic, cardiac | 1.24 (0.84 to 1.84) | 0.28 | |||
| Myocardial pump failure | 1.28 (0.74 to 2.22) | 0.37 | |||
| Procedure death | 1.20 (0.69 to 2.10) | 0.52 | |||
| Non-cardiac | 1.49 (0.80 to 2.76) | 0.21 | |||
| Total | 1.07 (0.81 to 1.42) | 0.63 | |||
| Actuarial mortality by 4 years’ follow-up (%) | 27 | 24 | 0.64 | ||
| HR (95% CI) for death per unit time | 1.07 (0.81 to 1.42) | ||||
| HR (95% CI) from Cox regression model stratified by clinical centre and LVEF | 1.02 (0.76 to 1.35) | ||||
| HR (95% CI) from Cox model beginning 30 days after randomization | 1.03 (0.75 to 1.41) | ||||
| Received a shock within 1 year of ICD implantation (actuarial incidence presented in a figure), % | 50 | ||||
| Received a shock within 2 years of ICD implantation (actuarial incidence presented in a figure), % | 57 | ||||
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |||
| HF hospitalizations | NR | NR | |||
| Change in NYHA class | NR | NR | |||
| Change in LVEF | NR | NR | |||
| Exercise capacity outcomes (e.g. 6-minute walk distance, total exercise time, peak VO2) | NR | NR | |||
| 3 months (n = 403) | 1 year (n = 374) | 3 months (n = 411) | 1 year (n = 373) | ||
| Drug therapy after CABG, %b | |||||
| Oral AADs | |||||
| None | 70.7 | 70.3 | 70.1 | 72.9 | |
| Class I drugs | 8.2 | 7.5 | 5.8 | 4.8 | |
| Amiodarone | 4.2 | 6.1 | 3.6 | 2.9 | |
| Sotalol | 1.0 | 0.8 | 0.5 | 0.5 | |
| Beta-blockers (not sotalol) | 16.4 | 16.0 | 21.7 | 19.8 | |
| ACE inhibitors | 60.3 | 64.2 | 63.7 | 67.8 | |
| Diuretics | 61.3 | 64.7 | 57.2 | 55.2 | |
| Digitalis | 70.7 | 70.6 | 62.5 | 60.1 | |
| Nitrates | 10.9 | 15.8 | 12.2 | 16.9 | |
| Calcium channel blockers | 9.2 | 12.0 | 7.1 | 9.7 | |
| Antiplatelet drugs | 78.2 | 79.1 | 83.7 | 82.6 | |
| Oral anticoagulants | 20.6 | 20.1 | 16.8 | 16.6 | |
| Lipid-lowering drugs | 12.9 | 23.0 | 13.4 | 23.3 | |
Quality-of-life outcomes
| Outcome | ICD (n = 262) | Control (n = 228) | p-valuea | |
|---|---|---|---|---|
| HRQoL at 6 months, mean (SD)80 | ||||
| Perception of health | ||||
| General health status | 54.8 (22.9) | 58.3 (23.6) | NS | |
| Perception of health transitionb | 2.4 (1.2) | 2.1 (1.2) | 0.030 | |
| Physical limitations | 41.7 (42.3) | 49.2 (42.8) | 0.055 | |
| Bodily pain | 57.4 (24.6) | 58.8 (24.8) | NS | |
| Ability to function | ||||
| Employment status | 0.25 (0.4) | 0.29 (0.5) | NS | |
| Physical role functioning | 58.3 (27.5) | 61.8 (28.3) | NS | |
| Emotional role functioning | 55.4 (43.4) | 67.3 (39.9) | 0.003 | |
| Social functioning | 70.5 (27.2) | 70.8 (26.4) | NS | |
| Psychological well-being | ||||
| Mental health | 72.5 (18.3) | 77.2 (17.0) | 0.004 | |
| Satisfaction with appearance | 6.0 (1.3) | 6.3 (1.1) | 0.008 | |
| Satisfaction with scar | 7.0 (1.2) | 7.2 (1.1) | 0.040 | |
| Received a shock before completing the 6-month QoL instrument, n/N (%) | 101/262 (38.5) | |||
| ICD device did not fire (n = 161) | ICD device fired (n = 101) | Control (n = 228) | Control vs. ICD fired (95% CI)c | |
| HRQoL at 6 months, mean (SD)80 | ||||
| Perception of health | ||||
| General health status | 56.6 (23.3) | 52.1 (22.1) | 58.3 (23.6) | NS |
| Perception of health transitionb | 2.3 (1.2) | 2.5 (1.3) | 2.1 (1.2) | –0.73 to –0.01d |
| Physical limitations | 44.8 (42.9) | 36.8 (41.1) | 49.2 (42.8) | 0.31 to 24.6e |
| Bodily pain | 57.8 (24.1) | 56.8 (25.3) | 58.8 (24.8) | NS |
| Ability to function | ||||
| Employment status | 0.30 (0.5) | 0.18 (0.4) | 0.29 (0.5) | NS |
| Physical role functioning | 61.5 (27.5) | 53.2 (27.0) | 61.8 (28.3) | 0.7 to 16.6 |
| Emotional role functioning | 59.5 (43.4) | 49.1 (42.8) | 67.3 (39.9) | 6.2 to 30.1 |
| Social functioning | 71.6 (26.9) | 68.8 (27.7) | 70.8 (26.4) | NS |
| Psychological well-being | ||||
| Mental health | 73.6 (43.4) | 70.6 (18.5) | 77.2 (17.0) | 1.5 to 11.6 |
| Satisfaction with appearance | 6.0 (1.3) | 6.0 (1.4) | 6.3 (1.1) | –0.01 to 0.71 |
| Satisfaction with scar | 7.0 (1.2) | 7.1 (1.2) | 7.2 (1.1) | NS |
| Rate of rehospitalisation before date of 6-month QoL assessment (%) | 36.0 | 55.5 | 33.8 | |
| ICDs explanted before completing 6-month QoL assessment, n/N | 12/262 | |||
| At patient request | 1 | |||
| Because of infection | 8 | |||
| Other reason | 3 | |||
Adverse effects of treatment
| Adverse effect | ICD (n = 446) | Control (n = 454) | p-valuea |
|---|---|---|---|
| Postoperative complications, % | |||
| MI | 4.0 | 3.5 | |
| Sustained VT | 5.8 | 6.8 | |
| VF | 3.4 | 5.3 | |
| Bradycardia | 2.9 | 4.4 | |
| Atrial fibrillation | 22.9 | 20.7 | |
| Shock | 9.2 | 7.5 | |
| New or more severe HF | 15.7 | 12.6 | |
| Conduction defect | 14.1 | 14.5 | |
| Residual central nervous system deficit | 3.6 | 2.0 | |
| Bleeding treated with surgery | 4.9 | 3.1 | |
| Postpericardiotomy syndrome | 0.9 | 0.7 | |
| Deep sternal wound infection | 2.7 | 0.4 | 0.01 < p < 0.05 |
| Infection at wound or catheter site | 12.3 | 5.9 | 0.01 < p < 0.05 |
| Pneumonia | 8.5 | 4.0 | 0.01 < p < 0.05 |
| Other infection | 6.3 | 3.3 | |
| Renal failure | 6.7 | 4.8 | |
| Events during long-term follow-up, % | |||
| Angina pectoris | 27.0 | 27.5 | |
| MI | 0.5 | 4.2 | 0.01 < p < 0.05 |
| New or worsening HF | 42.5 | 42.5 | |
| Ventricular arrhythmias | 19.4 | 14.3 | |
| Atrial fibrillation | 14.7 | 10.1 | |
| Hospitalisation | 61.4 | 55.2 | |
| Repeat CABG surgery | 0.0 | 0.7 | |
| PTCA or atherectomy | 2.9 | 2.1 | |
| Permanent cardiac pacemaker | 2.9 | 4.9 | |
| ICD removed, nb | 40 | ||
| Infection | 19 | ||
| ICD reached end of service period and not replaced | 5 | ||
| Patient request | 5 | ||
-
Allocation to treatment groups: two independent randomisation schedules were set up for each hospital, one for patients with a LVEF ≤ 0.2 and the other for those with a LVEF of 0.21–0.35. Randomisation therefore stratified by LVEF and also by centre. 76 Patients randomly assigned to ICD or control group within randomly permuted blocks. Randomisation took place in the operating room after completion of CABG surgery and patients were on partial cardiopulmonary bypass. The attending surgeon had the option not to have the patient randomly assigned if he or she thought that implanting and testing an ICD in the patient was too risky. Assignment supplied by data co-ordinating centre in opaque envelopes sealed with a validating label.
-
Blinding: no blinding; states that the nature of the intervention precluded the blinding of investigators or patients.
-
Comparability of treatment groups: states that baseline characteristics of the two study groups were similar. There was no baseline assessment for QoL because informed consent was obtained just hours before surgery, which made it impossible to obtain preoperative QoL data.
-
Method of data analysis: data were reviewed by an independent data and safety monitoring board. Four interim analyses were scheduled and performed. These were based on sequential monitoring procedures for the groups, with prospective stopping rules defined by a Lan–DeMets boundary with an O’Brien–Fleming spending function. Cumulative survival curves were estimated by the Kaplan–Meier method. Cox proportional hazards regression models were used to estimate HRs. Log-rank tests, stratified according to LVEF and clinical centre, were used to test hypotheses about between-group differences. Secondary analyses (also based on Cox models) examined survival after surgery and treatment interactions for prespecified subgroups. Ten prospectively selected covariates [age, sex, presence/absence of HF, NYHA functional class, LVEF, presence/absence of diabetes, duration of QRS complex (> 100 milliseconds or ≤ 100 milliseconds), use of ACE inhibitors, use of class I or class III AADs and use of beta-adrenergic blocking drugs] were evaluated for their interaction with the effect of ICD on risk of death. All analyses used the ITT principle. The last of the four interim analyses of mortality data was on 2 April 1997; 76% of the anticipated information was available. This fourth analysis showed no difference between the ICD group and the control group and a negligible chance that a difference would ever be found. The board therefore recommended that the data on the primary end point be reported as of 30 April 1997 while the trial continued to pursue its secondary objectives.
-
QoL substudy:80 Comparisons of scales based on t-tests. Analysis of variance models were used to test for differences in QoL scales between three groups: (1) control, (2) ICD – device did not fire and (3) ICD – device did fire. If a significant difference was found between the three groups based on an F-test, subsequent pairwise comparisons of each group to the others were made adopting Tukey’s method to maintain an overall 0.05 type 1 error probability. There was no correction or testing of the several scales from the QoL instrument. All tests were two-tailed.
-
Sample size/power calculation: design ensured that the study had a power of > 80% to detect a difference of 26% in mortality between the groups, a difference that corresponded to a 40% reduction in the hazard rate for death from all causes in the ICD group compared with the control group (allowing for anticipated crossovers). Originally the protocol was for 800 patients to be recruited and monitored for a minimum of 2 years. Many would have needed their ICD pulse generators to be replaced during follow-up. However, a clarification of the Medicare reimbursement policy for investigational use of devices caused a protocol change which meant that ICDs would not be replaced at the end of service life because of battery depletion. This change would have decreased the average follow-up time and statistical power. Mortality was also lower than expected in the control group. Therefore, in October 1994 the data and safety monitoring board recommended that power be restored by increasing recruitment from 800 to 900 patients and lengthening the minimum follow-up to 42 months (which is the average service time of a Ventak P pulse generator). ICDs with battery depletion before 39 months were replaced. 77
-
Attrition/drop-out: of 1422 eligible patients, 1055 (74%) signed a consent form. Of these, 155 were not randomised (n = 67 found to meet one or more criteria for exclusion between enrolment and randomisation, n = 88 not randomised because surgeon decided intraoperative events made ICD implantation too risky). There were 70 crossovers during follow-up: 18 control group patients had an ICD implanted; 12 patients assigned to the ICD group did not receive one because of death or hemodynamic instability in the operating room; 40 ICD group patients had the ICD removed (see Adverse events). At 42 months the cumulative rate of crossover to the control group was 10% and the cumulative rate of crossover to the ICD group was < 5%. QoL substudy:29 Of the 900 participants randomised in the main study, only 719 were expected to complete the 6-month QoL instrument [study authors presumed that death (43%), language difficulties (19%) (those whose first language was not English were not expected to complete the instrument) and completing 6 months of follow-up (38%) prior to the development of the QoL instrument would cause some participants to be unable to contribute data]. Of the 719 expected to have completed the instrument, 490 did so (68% of those expected, 54% of total trial population). A comparison of the characteristics of those who completed vs. those who did not complete the instrument is presented (not data extracted). This showed that completers differed by race, educational attainment, occupational attainment and randomisation group (higher rate of completion in ICD group).
-
Other: QoL substudy:80 ICD patients were recommended not to participate in the enrolling centre’s ICD support group meetings because their ICDs had been placed prophylactically and therefore they differed from those receiving ICDs for conventional reasons. It was anticipated that the meeting might cause trial participants to become confused and anxious.
-
Generalisability: this study found that the study population did not benefit from an ICD. In the discussion section of the paper75 the authors indicate that they enrolled a high proportion of eligible patients from a well-characterised population. However, mortality in this population differed from that in the AVID71 and MADIT99,101 trials and this leads the study authors to conclude that there must be differences between the enrolled populations. The authors speculate that the indicator for arrhythmia used may be the important factor and that the occurrence of either natural or induced sustained ventricular arrhythmias is a better marker for an at-risk population than abnormalities on a signal-averaged ECG, as was used in this study. Revascularisation may be another factor contributing to differences between this and other studies. The QoL part of the study80 notes that the ICDs in this study were older generation, which were larger and more intrusive than current devices. Thus, outcomes on satisfaction with appearance may not apply to new generation devices. In addition, the QoL findings are based on English-speaking, predominantly white male participants and so the results may not be generalisable to other groups, and other differences between those who did and did not complete the QoL study may also impact on generalisability.
-
Outcome measures: appear appropriate although not all (e.g. QoL outcomes) were ITT.
-
Intercentre variability: not discussed.
-
Conflict of interests: not explicitly stated. The leads and pulse generators were provided by the device manufacturer, Guidant Corporation/CPI, who also provided part of the grant funding for the study.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | States ‘randomised’ and also mentions ‘randomly permuted blocks’ but no detail about how randomisation schedule was set up |
| Allocation concealment | Low | Central allocation, opaque sealed envelopes |
| Performance bias | ||
| Blinding of participants and personnel | High | ‘The nature of the intervention precluded the blinding of investigators or patients’75 |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality | Low | ‘The nature of the intervention precluded the blinding of investigators or patients’.75 Death unlikely to be influenced by lack of blinding |
| QoL | High | |
| Attrition bias | ||
| Mortality | Low | States analysed according to the ITT principle. Methods for handling censored data not described but bias unlikely, particularly as no significant difference between groups and trial was expecting to find one |
| QoL | High | Not all participants contributed data; those who did differed from those who did not and there was a higher rate of completion in the ICD group |
| Reporting bias | ||
| Selective reporting | Unclear | Protocol76 states primary outcome and lists 11 of the secondary outcomes but does not indicate how many secondary outcomes there would be overall. Most outcomes appear to have been reported |
| Other bias | ||
| Other sources of bias | Low | |
Cardiac Arrest Study Hamburg (CASH)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Kuck et al. 200081 Study design: RCT Country: Germany No. of centres: multicentre but number of centres not reported Funding: supported by a grant from CPI/Guidant Corporation and ASTRA GmbH |
Intervention: ICD Cardiac Pacemakers, Inc. devices were used (Ventak AID, Ventak AICD, Ventak P, Ventak PRx, Ventak Mini). From recruitment start to June 1991 participants received an epicardial device (n = 55). From July 1991 participants received an endocardial device (n = 44). If patients required surgical revascularisation, implantation of an epicardial or endocardial device was performed at the time of or 7–15 (mean 10 ± 3) days after CABG surgery respectively Comparator: AAD, either amiodarone or metoprolol (propafenone arm originally included but eliminated). Amiodarone oral loading dose of 1000 mg/day for 7 days, followed by maintenance dose of 200–600 mg/day. Metoprolol initiated at 12.5–25 mg/day and increased within 7–14 days to a maximum of 200 mg/day if tolerated. Details reported for propafenone (study arm terminated early as a result of interim analysis) in other publications238–240 – excluded comparator Other interventions used: concurrent therapies at discharge reported (see below) but doses not provided |
Indication for treatment: patients resuscitated from cardiac arrest secondary to documented sustained ventricular arrhythmias. Index arrhythmia VF in 293/349 (84%) patients and VT in 56/349 (16%) patients (entire group before termination of propafenone arm) No. of randomised participants: 349, but this dropped to 288 after termination of the propafenone arm; ICD: 99, amiodarone: 92, metoprolol: 97. Some evidence for error in participant numbers and/or missing data. Details in methodological comments Inclusion criteria: not reported. Rate was the only criterion selected for detection of a sustained ventricular arrhythmia Exclusion criteria: cardiac arrest occurred within 72 hours of an acute MI, cardiac surgery, electrolyte abnormalities or proarrhythmic drug effect |
Primary outcome: all-cause mortality Secondary outcomes: sudden death, recurrence of cardiac arrest at 2-year follow-up Method of assessing outcomes: evaluations at 2, 4, 6, 12, 18 and 24 months then every 12 months thereafter. Sudden death defined as death within 1 hour of the onset of symptoms or an unwitnessed death. Cardiac arrest defined as sudden circulatory collapse requiring resuscitation Length of follow-up: minimum of 2 years, study terminated March 1998. Mean 57 (SD 34) months Recruitment: from March 1987 to March 1992 (propafenone arm terminated early) or to 1996 (remaining study arms) |
Participant characteristics
| Characteristic | ICD (n = 99) | Amiodarone (n = 92) | Metoprolol (n = 97) | p-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 58 (11) | 59 (10) | 56 (11) | |
| Sex, % male | 79 | 82 | 79 | |
| Ethnicity | NR | NR | NR | |
| Underlying disease, % | ||||
| Coronary artery disease | 73 | 77 | 70 | |
| Dilated cardiomyopathy | 12 | 10 | 14 | |
| Others | 6 | 2 | 5 | |
| No heart disease | 9 | 11 | 11 | |
| CHF at enrolment, % | ||||
| NYHA class I | 23 | 25 | 32 | |
| NYHA class II | 59 | 57 | 55 | |
| NYHA class II (drug arms combined) | 56 | |||
| NYHA class III | 18 | 18 | 13 | |
| LVEF, mean (SD) | 0.46 (0.19) | 0.44 (0.17) | 0.47 (0.17) | |
| 0.46 (0.17) | ||||
| Heart rate (bpm), mean (SD) | 81 (17) | 80 (17) | 76 (16) | |
| Findings on baseline ECG | ||||
| Corrected QT interval (milliseconds), mean (SD) | 437 (42) | 430 (51) | 430 (48) | |
| Bundle branch block, % | 17 | 23 | 19 | |
| Concurrent therapies at discharge, n | ||||
| ICD | 99 | 0 | 0 | |
| Amiodarone | 0 | 90 | 0 | |
| Metoprolol | 0 | 0 | 96 | |
| Digitalis | 26 | 23 | 15 | |
| Diuretic agents | 33 | 25 | 30 | |
| Nitrates | 29 | 27 | 24 | |
| Calcium channel blockers | 26 | 15 | 12 | |
| ACE inhibitors | 45 | 40 | 40 | |
| Acetylsalicylic acid (aspirin) | 57 | 41 | 40 | |
| Warfarin | 9 | 6 | 9 | |
| Coronary revascularisation during hospitalisation after index event, % | 19 | 21 | ||
| Cardiac history | NR | NR | NR | |
| Previous treatment | NR | NR | NR | |
| Comorbidities | NR | NR | NR | |
| Exposure time to primary events (months) | 4767.36 | 4169.41 | 5078.40 | |
Results
| Outcome | ICD (n = 99) | Amiodarone (n = 92) | Metoprolol (n = 97) | p-value |
|---|---|---|---|---|
| Crude death rate during mean (SD) follow-up of 57 (34) months, % (CIa) | 36.4 (26.9 to 46.6) | 44.4 (37.2 to 51.8) | ||
| 43.5 (33.2 to 54.2) | 45.4 (35.2 to 55.8) | 0.845b | ||
| Overall survival (ICD vs. antiarrhythmic therapy) | HR 0.766 (97.5% CI upper bound 1.112);c survival curve presented but not data extracted | 0.081d | ||
| Crude sudden death rate, % (CIa) | 13.0 (7.9 to 19.6) | 33.0 (27.2 to 41.8) | ||
| 29.5 (19.4 to 40.8) | 35.1 (25.2 to 48.8) | 0.467b | ||
| Survival free of sudden death (ICD vs. antiarrhythmic therapy) | HR 0.423 (97.5% CI upper bound 0.721); survival curve presented but not data extracted | 0.005d | ||
| Crude rate of non-fatal cardiac arrest, % (CIa) | 11.1 (6.9 to 16.5) | 19.5 (12.2 to 25.6) | ||
| Survival free of cardiac arrest (ICD vs. antiarrhythmic therapy) | HR 0.481 (97.5% CI upper bound 1.338); no survival curve presented | 0.072d | ||
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | NR | |
| HRQoL | NR | NR | NR | |
| HF hospitalisations | NR | NR | NR | |
| Change in NYHA class | NR | NR | NR | |
| Change in LVEF fraction | NR | NR | NR | |
| Exercise capacity outcomes | NR | NR | NR | |
Adverse effects of treatment
| Adverse effect | ICD (n = 99) | Amiodarone (n = 92) | Metoprolol (n = 97) | p-value |
|---|---|---|---|---|
| Drug-related pulmonary toxicity, n | 0 | NR | ||
| Hyperthyroidism, n (%) | 3 (3.3) | |||
| Drug discontinuation required, n (%) | 9 (9.8) | 10 (10.3) | ||
| Perioperative death or, for drug arms, deaths within the same time frame, n (%) | 5 (5.1); 3 (5.4) epicardial ICD, 2 (4.5) endocardial ICD | 2 (1.1) | p = 0.029 | |
| 2 | 0 | |||
| Other complications, n | ||||
| Infection | 3 (explantation required for 2) | |||
| Haematoma or seroma | 6 | |||
| Pericardial effusion | 1 | |||
| Pleural effusion | 3 | |||
| Pneumothorax | 1 | |||
| Dislodgement or migration of system leads | 3 | |||
| Device dysfunction | 5 | |||
| Overall complication rate, % | 23.0 (including an explantation rate of 2.1%) | |||
-
Allocation to treatment groups: randomisation ratio ICD : AAD = 1 : 3 (ICD : amiodarone : metoprolol : propafenone = 1 : 1 : 1 : 1). All patients assigned to the AAD arm underwent repeat predischarge 24-hour Holter monitoring, PES and exercise testing. Response to serial drug testing did not affect the therapy assignment obtained by randomisation.
-
Blinding: not reported.
-
Comparability of treatment groups: described as similar in the two treatment groups (ICD and AAD) but data presented separately for amiodarone and metoprolol groups. Baseline characteristics were not reported for the suspended propafenone arm.
-
Method of data analysis: analysis by ITT. An interim analysis was required by the safety monitoring board in March 1992 because of the unexpectedly long recruitment time and subsequent data in the literature showing life-threatening proarrhythmic effects by class Ic antiarrhythmic agents. The aim of this analysis was to prevent further patients being assigned to a possibly harmful treatment. However, as no precautions had been stated concerning multiple group comparisons and multiple looks into the data at the study start the interim analysis meant that the overall significance level for comparisons of the ICD group with each of the three drug groups was adjusted according to Bonferroni inequality. Time to clinical events (i.e. mortality, sudden death, cardiac arrest recurrence) for ICD vs. AAD was analysed using the Kaplan–Meier method. Cumulative survival functions were compared using the log-rank (Mantel–Cox) test. The Cox proportional regression model was used for calculation of HRs with the patients groups as randomised (ITT).
-
Sample size/power calculation: based on an assumption that ICDs would in the worst case be as effective as AADs. The alpha-level for comparison of survival distributions between the ICD and drug arms was based on a one-sided test; the significance test was at a 0.025 level. Design had a power of 80% to detect a difference of 19 percentage points in 2-year mortality rates between the two arms (50% expected mortality rate in patients assigned to the drug arm, 31% in the ICD arm). Sample size of 390 with a 1 : 3 (ICD : drug therapy) ratio for randomisation estimated to be sufficient. States that the 19.6% 2-year all-cause mortality rate observed in the amiodarone and metoprolol groups was less than half the mortality rate used to calculate the trial sample size, thus rendering the trial underpowered to test the working hypothesis. Note that data were presented and analysed separately for the two drugs and it is unclear whether the study was powered for this.
-
Attrition/dropout: three participants are unaccounted for from the description of numbers of participants. Overall, 349 included (293 VF + 56 VT) but 58 receiving propafenone were eliminated from the trial after an interim analysis found a higher all-cause mortality rate in this arm. This should leave 291 participants; however, it is stated that 288 remained in the continuing three study arms. Two in the amiodarone group refused to start drug therapy (table 2 in the paper indicates that these are included among the 92 in the amiodarone group). During follow-up six (6.1%) patients in the ICD arm and 11 (5.8%) in the drug arm crossed over or added the other therapy by 24 months. Three (3.0%) patients in the ICD arm and none of those assigned to amiodarone received beta-blockers during follow-up.
-
Generalisability: the study authors suggest that the mean LVEF for the whole study population (0.46) suggests that there may have been a disproportionate representation of relatively healthy patients in the trial. The effect of this on the generalisability of the results to more typical patients is unclear but the authors suggest that the benefit of ICD therapy may have been underestimated in the trial
-
Outcome measures: appear appropriate.
-
Intercentre variability: unclear as the number of centres and their characteristics not reported. The discussion section of the paper does note as a limitation the small number of participating centres and their reluctance to enrol patients for potential ICD therapy in the early phase of the study and to deny ICD therapy in the late phase of the study.
-
Conflict of interests: not stated.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | No information provided |
| Allocation concealment | Unclear | No information provided |
| Performance bias | ||
| Blinding of participants and personnel | High | No information provided, assume none |
| Detection bias | ||
| Blinding of outcome assessment | Low | No information provided but mortality unlikely to be influenced by lack of blinding |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | ‘For calculation of hazard ratios, the Cox proportional regression model was used with the patients grouped as randomised (intention to treat)’81 Crossovers or addition of the other treatment was similar in the two groups (ICD 6.1%, AAD 5.8%) |
| Reporting bias | ||
| Selective reporting | Low | The study protocol is not available but primary and secondary outcomes are specified and defined. The outcomes are the outcomes expected |
| Other bias | ||
| Other sources of bias | Unclear | Study authors note that centres were reluctant to enrol patients for potential ICD therapy in the early phase of the study and to deny ICD therapy in the late phase of the study. It is not clear whether or not this could have introduced any bias |
Cardiomyopathy Trial (CAT)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Bänsch et al. 2002,82 German Dilated Cardiomyopathy Study investigators 199283 Study design: RCT (pilot phase) Country: Germany No. of centres: 15 Funding: grant from Guidant Corporation, Giessen, Germany |
Intervention: ICD + OPT. Transvenous electrode systems (Endotak, Cardiac Pacemakers, Inc.). Pulse generators Ventak P2, P3, PrX II, CPI. Defibrillation threshold of < 20 J mandatory. VT zone with detection rate of 200 bpm programmed for all patients. All shocks programmed to maximum output 30 J. Pacemaker rate 40 bpm Comparator: OPT Other interventions used: both groups received pharmacological treatment throughout the trial (details in Participant characteristics). No changes in ACE inhibitor, digitalis and diuretic medications between baseline and 2-year follow-up were documented |
Indication for treatment: recent-onset idiopathic dilated cardiomyopathy (DCM) and impaired LVEF and without documented symptomatic VT No. of randomised participants: 104; ICD: 50, control: 54 Inclusion criteria: NYHA class II or III, LVEF ≤ 30%, LVEDD not reported, QRS interval not reported, aged 18–70 years, symptomatic DCM ≤ 9 months Exclusion criteria: coronary artery disease (coronary stenosis > 70%), previous history of MI, myocarditis or excessive alcohol consumption, symptomatic bradycardia, VT, VF, on heart transplant list, significant valvular disease, hypertrophic or restricted cardiomyopathy, NYHA class I or IV, mentally unable to understand protocol |
Primary outcomes: all-cause mortality at 1 year Secondary outcomes: heart transplantation, cardiac mortality (sudden and non-sudden cardiac death), sustained VT (adequate ICD therapy), symptomatic ventricular tachyarrhythmias requiring antiarrhythmic treatment, complications Method of assessing outcomes: visits every 3 months and encouraged to make additional visit if the first shock, cluster of shocks or syncope had occurred. ECGs stored on devices Length of follow-up: 2 years Recruitment: 1991–7 |
Participant characteristics
| Characteristic | ICD (n = 50) | Control (n = 54) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 52 (12) | 52 (10) | NS |
| Sex, male/female, n | 43/7 | 40/14 | NS |
| Ethnicity | NR | NR | |
| NYHA class, % | |||
| I | 66.7 | 64.1 | NS |
| III | 33.3 | 35.8 | |
| Duration of symptoms (months), median | 3.0 | 2.5 | NS |
| LVEF (%), mean (SD) | 24 (6) | 25 (8) | NS |
| Heart rate | NR | NR | |
| Echocardiographya | |||
| Left ventricular end-diastolic volume (mm), mean (SD) | 69 (7) | 69 (8) | NS |
| Left ventricular end-systolic volume (mm), mean (SD) | 58 (9) | 59 (10) | NS |
| ECG rhythm, % | NS | ||
| Sinus | 79.6 | 86.8 | |
| Atrial fibrillation/flutterb | 20.4 | 11.3 | |
| Paced | 0 | 1.9 | |
| QRS morphology, % | NS | ||
| Normal | 72.9 | 55.1 | |
| Not normal | 27.1 | 44.9 | |
| LBBB | 84.6 | 81.8 | |
| RBBB | 7.7 | 0 | |
| Other or undefined bundle branch block | 7.7 | 18.2 | |
| QRS widthc (milliseconds), mean (SD) | 102 (29) | 114 (29) | NS |
| Patients with NSVT, % | 53.1 | 58.0 | NS |
| Median duration of NSVT (seconds) (25th–75th percentile) | 5 (3.0–6.5) | 3.5 (2.3–6.0) | NS |
| Rate of NSVT (bpm), mean (SD) | 175 (39) | 157 (23) | NS |
| Bradycardias, % | 2.1 | 18.8 | 0.015 |
| Sinoatrial block | 0 | 4.2 | |
| Atrioventricular block | 2.1 | 14.6 | NS |
| Inducible VT, % | 6.1 | 0 | NS |
| Inducible VF, % | 16.0 | 3.7 | NS |
| Current pharmacological therapy, % | |||
| Beta-blocker | 4.0 | 3.7 | NS |
| Calcium antagonist | 16.0 | 7.4 | NS |
| Digitalis | 86.0 | 75.9 | NS |
| Diuretics | 88.0 | 85.2 | NS |
| Nitrates | 32.0 | 25.9 | NS |
| ACE inhibitor | 94.0 | 98.1 | NS |
| Warfarin | 24.0 | 35.2 | NS |
| Cardiac history | NR | NR | |
| Previous treatment | NR | NR | |
| Comorbidities | NR | NR | |
| Follow-up (months) (per protocol), mean (SD) | 22.7 (4.5) | 22.9 (4.2) | NS |
| Follow-up (years) (per August 2000), mean (SD) | 5.7 (2.2) | 5.2 (2.1) | NS |
Results
| Outcome | ICD (n = 50) | Control (n = 54) | p-value |
|---|---|---|---|
| All-cause mortality after 1 year (primary end point),a n | 4 (all cardiac) | 2 (both non-cardiac)b | 0.3672 |
| All-cause mortality after mean (SD) 5.5 (2.2) years’ follow-up, n | 13 | 17 | |
| Cumulative survival, % | |||
| 2 year | 92 | 93 | 0.554 |
| 4 years | 86 | 80 | |
| 6 years | 73 | 68 | |
| HRQoL | NR | NR | |
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | NR | NR | |
| Change in LVEF | NR | NR | |
| Exercise capacity outcomes (e.g. 6-minute walk distance, total exercise time, peak VO2) | NR | NR | |
| Received adequate therapy from ICD for VTs > 200 bpm, n | 11 | NA | |
| Syncope during VT, n | 6 | ||
Adverse effects of treatment
| Adverse effect | ICD (n = 50) | Control (n = 54) | p-value |
|---|---|---|---|
| Complications caused by ICD therapy | |||
| Deaths within 30 days of ICD implantation, n | 0 | ||
| Device dislocation and bleeding requiring revision, n | 2 | ||
| Electrode dislocation requiring revision, n | 2 | ||
| Complications in 24 months of follow-up | 10 in 7 patients | ||
| Electrode dislocation and sensing/isolation defects, n | 7 | ||
| Infection with total device replacement, n | 2 | ||
| Perforation, n | 1 | ||
-
Allocation to treatment groups: random assignment performed centrally. Closed envelopes with the assigned study group were sent to each centre. Envelopes opened when a patient was enrolled.
-
Blinding: none reported so presume no blinding.
-
Comparability of treatment groups: no differences between groups except for bradycardias caused by sinus arrest and atrioventricular block I and II (Wenckebach), which were more common in the control group (18.8%) than the ICD group (2.1%) (p = 0.015) during Holter monitoring. Any other differences observed between groups were not statistically significant.
-
Method of data analysis: no statement made regarding whether analysis ITT or not. Blind interim analysis after inclusion of 100 patients at 1 year of follow-up was planned because of considerable variation in the all-cause mortality rate in different studies that had informed the sample size calculation. Interim analysis conducted in 1997 showed overall 1-year mortality rate of only 5.6% (well below the assumed 30%). As difference between the groups was only 2.6%, randomisation was stopped (as per protocol) and scheduled follow-up of 2 years completed by randomised patients. Survival rates presented as Kaplan–Meier curves and compared with log-rank statistics. Cox proportional regression models calculated to estimate prognostic relevance of patient characteristics. Data described by mean (SD) if normally distributed or otherwise by median (25%–75% percentiles). Quantitative comparisons between groups performed using two-sided analysis using Mann–Whitney exact test; qualitative characteristics compared using the exact Fisher chi-squared test.
-
Sample size/power calculation: all-cause mortality rate assumed to be 30% in the first year with 40% of deaths being sudden. On this assumption 1348 patients had to be enrolled to show a 1-year survival benefit of 6% for ICD treatment, with a power of 80% and a probability value of 0.05.
-
Attrition/dropout: no details reported.
-
Generalisability: as the trial was stopped because of futility after 1 year because of the low event rate, results are not likely to be generalisable.
-
Outcome measures: appear appropriate although the secondary outcome of heart transplantation was not commented on.
-
Intercentre variability: not commented on.
-
Conflict of interests: no statement other than support was by a grant from Guidant Corporation.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | States ‘were randomly assigned’ but no further description |
| Allocation concealment | Unclear | Envelopes used but does not state whether these were opaque and sequentially numbered |
| Performance bias | ||
| Blinding of participants and personnel | High | Blinding unlikely |
| Detection bias | ||
| Blinding of outcome assessment | Low | Blinding unlikely but the outcome of all-cause mortality is unlikely to be affected |
| Attrition bias | ||
| Incomplete outcome data addressed | Unclear | No details reported regarding attrition |
| Reporting bias | ||
| Selective reporting | High | Incidence of heart transplantation specified as a secondary outcome but no reporting on this |
| Other bias | ||
| Other sources of bias | Low | |
Canadian Implantable Defibrillator Study (CIDS)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Connolly et al. 199385 and 2000,84 Irvine et al. 2002,87 Sheldon et al. 200086 (no additional data extracted), Bokhari et al. 200488 Study design: RCT Countries: Canada, Australia, USA No. of centres: Canada: 19, Australia: 3, USA: 2 Funding: Medical Research Council of Canada |
Intervention: ICD. Implant criteria met with three consecutive successful defibrillations at ≥ 10 J below maximum device output. Either thoracotomy or non-thoracotomy lead systems used Comparator: amiodarone ≥ 1200 mg/day for ≥ 1 week in hospital, ≥ 400 mg/day for ≥ 10 weeks then ≥ 300 mg/day. Dose could be lowered to a minimum of 200 mg/day for intolerable side effects Other interventions used: AADs could be used in both groups to control supraventricular or NSVTs that were symptomatic or might cause discharge of the ICD |
Indication for treatment: previous sustained ventricular arrhythmia No. of randomised participants: ICD randomised: 328, ICD received implant: 310, amiodarone: 331. For QOL: 317 randomised and eligible, 287 survived to 12 months, 178 had data at 6 and 12 months Inclusion criteria: any of following in the absence of either recent acute MI (≤ 72 hours) or electrolyte imbalance: documented VF; out-of-hospital cardiac arrest requiring defibrillation or cardioversion; documented sustained VT causing syncope; other documented sustained VT at a rate ≥ 150 bpm causing presyncope or angina in a patient with a LVEF ≤ 35%; or unmonitored syncope with subsequent documentation of either spontaneous VT ≥ 10 seconds or sustained (≥ 30 seconds) monomorphic VT induced by programmed ventricular stimulation. Ventricular tachyarrhythmias induced in laboratory met criteria if patient had previous spontaneous documented sustained VT and the induced arrhythmia was monomorphic sustained VT Exclusion criteria: amiodarone or ICD not considered appropriate, excessive perioperative risk for ICD implantation, previous amiodarone therapy for ≥ 6 weeks, non-arrhythmic medical condition making 1-year survival unlikely, long QT syndrome |
Primary outcomes: death from any cause Secondary outcomes: arrhythmic death (based on clinical classification of cardiac deaths by Hinkle and Thaler (reference provided), QoL,87 side effects, arrhythmia recurrence Method of assessing outcomes: 2 and 6 months after randomisation then every 6 months. All deaths adjudicated by an external validation committee not blinded to treatment QoL study:87 emotional functioning: Rand Corporations 38-item MHI; HRQoL: NHP. Assessed in hospital before or just after randomisation (people after randomisation may have started therapy), then by mailed questionnaire at 2, 6 and 12 months Length of follow-up: ICDs: mean 3.0 years; amiodarone: mean 2.9 years For long-term follow-up of subset of patients from one centre:88 follow-up until April 2002, mean 5.6 (SD 2.6) years, median 5.92 (range 0.08–11.08) years Recruitment: October 1990–January 1997 |
Participant characteristics
| Characteristic | ICD (n = 328) | Amiodarone (n = 331) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 63.3. (9.2) | 63.8 (9.9) | |
| Sex, % male | 85.4 | 83.7 | |
| Ethnicity | NR | NR | |
| Index arrhythmia, % | |||
| VF or cardiac arrest | 45.1 | 50.1 | |
| VT with syncope | 15.9 | 10.6 | |
| Other VT | 23.8 | 26.9 | |
| Unmonitored syncope | 15.2 | 12.4 | |
| Primary cardiac diagnosis, % | |||
| Ischaemic heart disease with MI | 75.6 | 73.1 | |
| Ischaemic heart disease without MI | 7.3 | 9.1 | |
| Dilated cardiomyopathy | 8.5 | 10.6 | |
| Valvular heart disease | 1.2 | 3.0 | |
| Other heart disease | 3.7 | 2.4 | |
| No heart disease | 3.7 | 1.8 | |
| CHF, % | |||
| None | 51.2 | 49.5 | |
| NYHA class I or II | 37.8 | 39.9 | |
| NYHA class III or IV | 11.0 | 10.6 | |
| LVEF (%), mean (SD) | 34.3 (14.5) | 33.3 (14.1) | |
| LVEF < 20%, % | 11.3 | 13.3 | |
| Heart rate | NR | NR | |
| Baseline electrophysiological study | |||
| Ever done, % | 62.2 | 62.8 | |
| Inducible VT or VF, n/N (%) | 154/204 (75.7) | 147/208 (70.7) | |
| Coronary angiography, % | |||
| Ever done | 75.6 | 78.2 | |
| Three-vessel disease | 19.0 | 18.9 | |
| Chest radiography, % | |||
| Interstitial abnormality (document on previous standard chest radiography report) | 15.5 | 17.6 | |
| Other abnormality | 31.4 | 34.6 | |
| Current pharmacological therapy | NR | NR | |
| Cardiac history, % | |||
| Angina pectoris | 51.2 | 57.1 | |
| MI | 77.1 | 75.8 | |
| CABG surgery | 31.4 | 28.1 | |
| Previous treatment | NR | NR | |
| Medical conditions, % | |||
| Liver disorder | 1.5 | 2.7 | |
| Respiratory disease | 17.5 | 17.8 | |
| Thyroid disease | 5.8 | 3.9 | |
Results
| Outcome | ICDs (n = 328) | Amiodarone (n = 331) | RRRa (95% CI) (%), p-value |
|---|---|---|---|
| 30-day mortality in implanted patients (n = 310), n/N (%) | |||
| Patients with thoracotomy (n = 33) | 1/33 (3.3) | ||
| Patients with non-thoracotomy lead system (n = 277) | 1/277 (0.36) | ||
| Outcome event rate summary, no. of events [rate/year (%)] | |||
| All-cause mortality | 83 (8.3) | 98 (10.2) | 19.7 (–7.7 to 40.0), 0.142 |
| Arrhythmic death | 30 (3.0) | 43 (4.5) | 32.8 (–7.2 to 57.8), 0.094 |
| Other cardiac death | 37 (3.7) | 40 (4.2) | 13.5 (–35.4 to 44.7), 0.526 |
| Non-cardiac vascular death | 3 (0.3) | 2 (0.2) | –36.6 (–719.8 to 77.2), 0.732 |
| Non-vascular death | 13 (1.3) | 13 (1.4) | 4.5 (–106.1 to 55.7), 0.908 |
| Total cardiac death | (6.7) | (8.6) | 23.4 (–5.7 to 44.5), 1.04 |
| ARR, RRR (%) | |||
| Cumulative risks over time, % | |||
| Total mortality | |||
| 1 year | 9.46 | 11.18 | 1.72, 15.4 |
| 2 years | 14.75 | 20.97 | 6.22, 29.7 |
| 3 years | 23.32 | 27.03 | 3.71, 13.7 |
| Arrhythmic mortality | |||
| 1 year | 4.37 | 6.23 | 1.86, 29.9 |
| 2 years | 6.68 | 9.74 | 3.06, 31.4 |
| 3 years | 9.77 | 11.88 | 2.11, 17.8 |
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | NR | NR | |
| Change in LVEF | NR | NR | |
| Exercise capacity outcomes | NR | NR | |
| Concomitant antiarrythmic medications, % patients | |||
| Beta-blocker (other than sotalol) | |||
| Hospital discharge | 33.5 | 21.4 | |
| 1 year | 37.0 | 21.2 | |
| 3 years | 33.3 | 19.0 | |
| 5 years | 29.6 | 22.4 | |
| Sotalol | |||
| Hospital discharge | 19.8 | 1.5 | |
| 1 year | 21.5 | 2.5 | |
| 3 years | 23.3 | 4.9 | |
| 5 years | 24.1 | 4.1 | |
| Digoxin | |||
| Hospital discharge | 29.6 | 22.7 | |
| 1 year | 34.5 | 21.9 | |
| 3 years | 34.7 | 22.5 | |
| 5 years | 33.3. | 24.5 | |
| Class I AAD (any Vaughan Williams class I) | |||
| Hospital discharge | 5.5 | 2.4 | |
| 1 year | 8.4 | 2.8 | |
| 3 years | 10.0 | 2.1 | |
| 5 years | 9.3 | 2.0 | |
Health-related quality of life87
| QoL measure | ICD (n = 86) | Amiodarone (n = 92) | Time by group p-value (ANOVA) |
|---|---|---|---|
| Domains of MHI, mean (SD) | |||
| Total indexa | |||
| Baseline | 173.2 (25.5) | 180.4 (27.8) | |
| 6 months | 183.1 (30.2) | 180.2 (31.1) | |
| 12 months | 184.3 (27.9) | 178.3 (28.7) | 0.001 |
| Psychological distressb | |||
| Baseline | 51.3 (14.1) | 47.8 (16.5) | |
| 6 months | 45.1 (17.6) | 47.6 (18.3) | |
| 12 months | 43.4 (15.9) | 48.8 (16.8) | 0.001 |
| Psychological well-beinga | |||
| Baseline | 58.5 (12.7) | 62.2 (12.3) | |
| 6 months | 62.2 (13.4) | 61.8 (14.1) | |
| 12 months | 61.7 (13.2) | 61.3 (13.3) | 0.03 |
| Domains of NHP, mean (SD) | |||
| Energy levelb | (n = 83) | (n = 88) | |
| Baseline | 27.5 (32.2) | 24.4 (32.4) | |
| 6 months | 18.6 (30.1) | 27.8 (32.1) | |
| 12 months | 17.7 (26.1) | 36.8 (37.3) | 0.0001 |
| Physical mobilityb | (n = 84) | (n = 90) | |
| Baseline | 10.9 (12.0) | 13.2 (20.5) | |
| 6 months | 10.5 (13.7) | 15.1 (19.2) | |
| 12 months | 9.1 (13.6) | 17.7 (19.2) | 0.002 |
| Social isolationb | (n = 81) | (n = 88) | |
| Baseline | 8.5 (15.4) | 9.9 (17.7) | |
| 6 months | 9.8 (18.6) | 12.2 (22.4) | |
| 12 months | 8.5 (18.4) | 11.1 (22.6) | 0.9 |
| Emotional reactionsb | (n = 76) | (n = 86) | |
| Baseline | 17.3 (18.1) | 14.3 (20.1) | |
| 6 months | 11.1 (18.2) | 15.3 (22.4) | |
| 12 months | 8.3 (16.6) | 14.5 (19.6) | 0.002 |
| Painb | (n = 83) | (n = 90) | |
| Baseline | 4.4 (7.9) | 7.5 (15.1) | |
| 6 months | 7.5 (17.1) | 6.3 (13.6) | |
| 12 months | 4.5 (9.9) | 8.2 (15.4) | 0.52 |
| Sleep disturbanceb | (n = 78) | (n = 88) | |
| Baseline | 31.4 (27.4) | 29.6 (31.5) | |
| 6 months | 25.0 (29.7) | 30.8 (31.0) | |
| 12 months | 23.9 (29.4) | 30.2 (32.4) | 0.02 |
| Life impairmentb | (n = 78) | (n = 83) | |
| Baseline | 2.0 (1.9) | 1.6 (1.7) | |
| 6 months | 1.6 (1.8) | 1.9 (1.9) | |
| 12 months | 1.6 (1.3) | 1.8 (1.9) | 0.005 |
Effect of implantable cardiac defibrillator shocks on Mental Health Inventory and Nottingham Health Profile scores87
| QoL measure | ICDs, no shocks (n = 66) | ICDs, one to four shocks (n = 27) | ICDs, five or more shocks (n = 15) | Amiodarone, no ICD (n = 95) | Between-group p-value |
|---|---|---|---|---|---|
| Domains of MHI, mean (SD) | |||||
| Total indexa | |||||
| Baseline | 175.9 (26.5) | 171.7 (22.7) | 171.2 (32.0) | 177.9 (27.1) | |
| 12-month follow-up | 186.2 (26.9)b,c | 186.6 (21.7)b,c | 168.8 (41.2) | 175.6 (29.2) | 0.001 |
| Within-group p-value | 0.001 | 0.001 | 0.725 | ||
| Psychological distressd | |||||
| Baseline | 50.2 (15.2) | 50.8 (12.3) | 51.9 (18.1) | 49.8 (16.3) | |
| 12-month follow-up | 42.5 (15.3)b,c | 41.4 (11.7)b,c | 52.7 (25.2) | 50.9 (17.5) | 0.001 |
| Within-group p-value | 0.001 | 0.001 | 0.833 | ||
| Psychological well-beinga | |||||
| Baseline | 60.1 (12.5) | 56.6 (11.6) | 57.1 (15.0) | 61.7 (12.0) | |
| 12-month follow-up | 62.8 (13.1) | 62.1 (10.9)c | 55.6 (16.8) | 60.6 (13.3) | 0.02 |
| Within-group p-value | 0.074 | 0.004 | 0.642 | ||
| Domains of NHP, mean (SD) | |||||
| Energy leveld | (n = 64) | (n = 27) | (n = 15) | (n = 90) | |
| Baseline | 28.6 (32.5) | 28.5 (30.5) | 22.6 (34.2) | 24.3 (30.8) | |
| 12-month follow-up | 19.5 (27.1)b | 24.8 (33.4)b | 23.5 (29.5) | 37.0 (37.6) | 0.003 |
| Within-group p-value | 0.02 | 0.115 | 0.859 | ||
| Physical mobilityd | (n = 65) | (n = 27) | (n = 15) | (n = 93) | |
| Baseline | 13.1 (15.0) | 12.4 (10.2) | 7.1 (9.8) | 13.18 (20.1) | |
| 12-month follow-up | 9.3 (12.4)b | 15.5 (17.3) | 8.0 (13.3) | 17.2 (19.1) | 0.02 |
| Within-group p-value | 0.05 | 0.638 | 0.747 | ||
| Social isolationd | (n = 66) | (n = 27) | (n = 15) | (n = 92) | |
| Baseline | 10.6 (16.7) | 4.3 (9.2) | 8.9 (16.1) | 11.8 (18.5) | |
| 12-month follow-up | 8.8 (19.5) | 6.4 (15.5) | 12.8 (23.9) | 12.5 (23.0) | 0.57 |
| Within-group p-value | 0.03 | 0.991 | 0.817 | ||
| Emotional reactionsd | (n = 61) | (n = 27) | (n = 14) | (n = 90) | |
| Baseline | 16.2 (17.4) | 16.3 (17.1) | 21.6 (21.1) | 16.3 (19.8) | |
| 12-month follow-up | 7.1 (14.6)b,c | 6.8 (10.2)b | 22.0 (31.0) | 15.9 (20.3) | 0.001 |
| Within-group p-value | 0.001 | 0.02 | 0.886 | ||
| Paind | (n = 66) | (n = 27) | (n = 15) | (n = 92) | |
| Baseline | 6.8 (11.8) | 4.0 (8.5) | 5.3 (8.3) | 8.5 (15.6) | |
| 12-month follow-up | 6.4 (14.7) | 5.4 (11.7) | 5.5 (7.1) | 7.7 (14.5) | 0.71 |
| Within-group p-value | 0.086 | 0.710 | 0.721 | ||
| Sleep disturbanced | (n = 62) | (n = 27) | (n = 14) | (n = 89) | |
| Baseline | 30.0 (26.9) | 36.3 (31.4) | 27.3 (27.1) | 30.4 (30.5) | |
| 12-month follow-up | 22.1 (28.1) | 29.1 (33.9) | 34.6 (35.4) | 30.1 (33.6) | 0.3 |
| Within-group p-value | 0.002 | 0.042 | 0.680 | ||
| Lifestyle impairmentd | (n = 65) | (n = 26) | (n = 14) | (n = 82) | |
| Baseline | 2.0 (2.0) | 2.4 (1.9) | 2.2 (1.9) | 1.7 (1.6) | |
| 12-month follow-up | 1.3 (1.5)b | 1.4 (1.5)b | 1.4 (1.6) | 1.9 (1.9) | 0.03 |
| Within-group p-value | 0.061 | 0.033 | 0.334 | ||
Adverse effects of treatment
| Adverse effect | ICD (n = 328) | Amiodarone (n = 331) | p-value |
|---|---|---|---|
| ICD permanently or temporarily explanted because of infection, heart transplantation or patient preference, n/N | 16/310 | ||
| Adverse experiences ever reported, n/N (%) | |||
| Pulmonary infiltrate | 18/331 (5.7) (1.9% per year)a | ||
| Visual symptoms (blurred, halo or decreased) | 48/331 (14.5) | ||
| Bradycardia | 10/331 (3.0) | ||
| Skin discolouration | 21/331 (6.3) | ||
| Photosensitivity | 34/331 (10.3) | ||
| Ataxia | 97/331 (17.2)a | ||
| Tremor | 91/331 (15.4)a | ||
| Insomnia | 64/331 (19.3) | ||
| Peripheral neuropathy | 1/331 (0.3) | ||
| ICD product discomfort, n/N (%) | 25/328 (7.6) | ||
| ICD malfunction, n/N (%) | 2/328 (0.6) | ||
| ICD pocket infection, n/N (%) | 15/328 (4.6) (1.4% per year) | ||
| ICD dislodgement/fracture, n/N (%) | 8/328 (2.4) | ||
Long-term follow-up of subset of patients from one centre88
Participant characteristics
| Characteristic | ICD (n = 60) | Amiodarone (n = 60) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 64 (9.2) | 64 (8.7) | NS |
| Sex, male, n (%) | 50 (83) | 50 (83) | NS |
| Index arrhythmia, % | |||
| VF | 18 | 27 | NS |
| VT | 35 | 23 | 0.044 |
| Syncope/inducible VT, % | 7 | 10 | NS |
| History of MI, n (%) | 36 (60) | 31 (52) | NS |
| Coronary artery disease, n (%) | 48 (80) | 48 (80) | NS |
| NYHA class I or II, n (%) | 57 (95) | 57 (95) | NS |
| NYHA class III or IV, n (%) | 3 (5) | 3 (5) | NS |
| LVEF (%), mean (SD) | 33.9 (12.5) | 32.1 (11.1) | NS |
| CABG surgery, n (%) | 19 (32) | 22 (37) | NS |
| Percutaneous coronary intervention, n (%) | 4 (7) | 2 (3) | NS |
| Beta-blockers, n (%) | 23 (38) | 21 (35) | NS |
| Diabetes mellitus, n (%) | 7 (12) | 11 (18) | NS |
| Hypertension, n (%) | 13 (22) | 14 (23) | NS |
Results
| Outcome | ICD (n = 60) | Amiodarone (n = 60) | p-value |
|---|---|---|---|
| Total deaths, n (%) | 16 (27) | 28 (47) | 0.0231 |
| Total mortality per year, % | 2.8 | 5.5 | HR 2.011 (95% CI 1.087 to 3.721, p = 0.0261)a |
| Presumed arrhythmic death, % | 2 | 12 | 0.049 |
| Cardiac death, % | 8 | 11 | |
| Vascular death, % | 1 | 1 | |
| Non-cardiac death, % | 5 | 4 | |
| Symptomatic non-fatal arrhythmia recurrence, n | 12 |
Adverse effects of treatment
| Adverse effect | ICD (n = 60) | Amiodarone (n = 60) | p-value |
|---|---|---|---|
| Side effects related to amiodarone, n patients (%) | 49 (82) | ||
| Side effects requiring dose reduction or discontinuation, n patients (%) | 30 (50) | ||
| Serious adverse effects requiring discontinuation, n patients | 13 | ||
| Severe side effects requiring permanent removal of the ICD and crossover to amiodarone | 0 | ||
| Procedures performed in addition to initial implants, n procedures | 68 | ||
| Defibrillators replaced | 50 | ||
| Battery end of life | 41 | ||
| Pocket infections | 3 | ||
| Other reasons | 6 | ||
| Leads replaced | 18 | ||
| Lead fracture | 16 | ||
| Lead failure/dislodgement | 2 | ||
| Patients undergoing two or more procedures to replace device or change a lead (up to seven procedures, details reported), n | 41 | ||
| Perioperative death, n | 0 | ||
| Pneumothorax, n | 1 | ||
| Deep-vein thrombosis, n | 1 | ||
| Pocket haematoma postoperatively, n | 1 | ||
| ICD turned off at patients request because of terminal cancer | 2 | ||
| Inappropriate therapy, n (%) | 30 (50) |
-
Allocation to treatment groups: central randomisation was stratified by clinical centre and LVEF (≤ 35% and > 35%).
-
Blinding: ‘all deaths adjudicated by an External Validation Committee whose members had no other affiliation to study. Despite best efforts, it was not always possible to blind Committee to treatment allocation’. 84
-
Comparability of treatment groups: described as well-balanced.
-
Method of data analysis: states analysis based on the ITT principle. Study planned as a one-sided comparison with the hypothesis that ICDs would be superior to amiodarone. Two-sided statistics presented in response to review process. Cumulative mortality summarised as Kaplan–Meier survival curves. Curves compared using Mantel–Haenszel test incorporating stratification for LVEF. Cox’s proportional hazards method used to adjust for imbalances in baseline prognostic risk and to investigate potential subgroup effects. External safety and efficacy monitoring committee reviewed the unblinded study data every 6 months for safety and did three formal interim analyses of efficacy with the intention of stopping the study early in favour of ICD if one-sided p ≤ 0.001. For QoL study,87 analysis of variance with repeated measures used. Significant time changes and group effects followed up by means of post-hoc tests (Tukey honestly significant difference test). Scores on the NHP were normalised by use of a log plus 1 transformation. Effects of the number of ICD shocks on QoL were assessed using analysis of covariance. Analysis based on the ITT principle.
-
Sample size/power calculation: study originally designed with a primary outcome of arrhythmic death; this was changed in 1993 to all-cause mortality because of concerns that the ICD might prevent some arrhythmic deaths but, because of completing risks, have little effect on overall survival. This change led to an increase in the patient enrolment target from 400 to 650 patients, which provided 90% power to detect a relative reduction in all-cause mortality of 33% with the ICD with an anticipated 3-year mortality rate of 30% on amiodarone. Crossover rates of 5% per year for both treatment groups were anticipated. QoL study87 was conducted with the original 400 patients only because of cost. Of these, 317 spoke English; participation rate was 79%. In the QoL study, 9/92 receiving amiodarone received an ICD and 14/86 with an ICD received amiodarone by 12 months. The long-term follow-up of a subset of patients from one centre would not be adequately powered. 88
-
Attrition/dropout: of the entire trial population, 328 were randomised to the ICD group and 310 (94.5%) received an ICD. Of the 18 who did not receive an ICD, seven died in hospital awaiting ICD surgery and 10 decided against an ICD (patient or physician) after randomisation; in addition, there was one technical problem. A total of 16 patients had their ICD explanted permanently or temporarily because of infection, heart transplantation or patient preference; 52/331 (15.7%) patients randomised to amiodarone received an ICD. For QoL,87 of the original 400 participants, 317 spoke English; participation rate was 79%. Of the 317 recruited, 287 (90.5%) were alive at the 12-month assessment; 22/287 (7.7%) were missing the baseline QoL assessment (11 from each group) and 127/287 (44%) were missing data at one of the follow-up assessments (63 amiodarone, 64 ICD). Missing baseline date were replaced by the mean for the variable across both treatment groups and 2-month data were excluded, resulting in a sample of 178/287 (62.0%) participants with 6- and 12-month data. In total, 9/92 in the amiodarone group received an ICD within the first 12months and 14/86 in the ICD group were taking amiodarone at 12 months. For the subset of patients from a single centre87 it is stated that follow-up was complete in the ICD group and 3/60 patients were lost to follow-up in the amiodarone group. In the amiodarone group 19/60 crossed over to the ICD group because of adverse events (n = 12) or arrhythmia recurrence (n = 7). For those with an ICD, 26/60 were receiving amiodarone during follow-up. 88
-
Generalisability: included people with VF, sustained VT or unmonitored syncope likely because of VT. Most participants from centres in Canada.
-
Outcome measures: mortality, QoL and adverse events only.
-
Intercentre variability: not reported.
-
Conflict of interests: not stated. Amiodarone supplied by Wyeth-Ayerst Pharmaceuticals, Ltd.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | ‘Central randomisation was stratified by clinical centre and LVEF (≤ 35% and > 35%)’.84 Method not stated |
| Allocation concealment | Low | ‘Central randomisation’.84 No further details given but assume allocation concealed by central allocation |
| Performance bias | ||
| Blinding of participants and personnel | High | No details reported but assume participants and personnel not blinded |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality | Low | ‘All deaths adjudicated by an External Validation Committee whose members had no other affiliation to study. Despite best efforts, it was not always possible to blind Committee to treatment allocation’.84 Mortality unlikely to be influenced by lack of blinding |
| QoL | High | |
| Attrition bias | ||
| Incomplete outcome data addressed | Unclear | Changes to intervention reported but missing data not reported. Crossover rates higher than anticipated in planned analysis. For QoL subgroup, missing data did not differ between treatment groups |
| Reporting bias | ||
| Selective reporting | High | Study design paper published,85 which specifies secondary outcome events: ‘nonfatal recurrence of ventricular fibrillation or sustained ventricular tachycardia causing syncope or cardiac arrest requiring cardioversion or defibrillator, other than by an ICD’. Publication of these outcomes for the whole group not identified by the systematic review |
| Other bias | ||
| Other sources of bias | Low | |
Defibrillator versus Beta-Blockers for Unexplained Death in Thailand (DEBUT) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Nademanee et al. 200389 Study design: RCT; pilot study and main study Country: Thailand No. of centres: not reported Funding: Grant-in-Aid from Cardiac Rhythm Management and Guidant Corporation, St Paul, MN |
Intervention: ICD (Guidant Corporation, St Paul, MN) Comparator: beta-blockade (long-acting propranolol 40 mg/day up to 160 mg/day) Other interventions used: other beta-blocking agents or amiodarone permitted if intolerable side effects of propranolol or if frequent shocks from recurrent VF developed |
Indication for treatment: SUDS survivors or probable survivors No. of randomised participants: pilot study: 20 (ICD: 10, beta-blockers: 10); main study: 66 (ICD 37, beta-blockers: 29) (155 screened, 88 not randomised, one randomised but refused ICD) Inclusion criteria: SUDS survivor defined as a healthy subject without structural heart disease who had survived unexpected VF or cardiac arrest after successful resuscitation. Probable SUDS survivor defined as a subject without structural heart disease who experienced symptoms indicative of the clinical presentation of SUDs, especially during sleep, including agonal respiration, transient episodes of stress, abnormal respiration associated with grasping and groaning, syncope or seizure-like symptoms. ECG abnormalities showing RBBB-like pattern with ST elevation in right precordial leads and inducible VT/VF in electrophysiological testing Exclusion criteria: no further detail |
Primary outcome: death from all causes Secondary outcomes: recurrent VT/VF or cardiac arrest Method of assessing outcomes: first month and at 3-month intervals Length of follow-up: maximum 3 years after randomisation. Median follow-up not reported Recruitment: Pilot study January 1995 to April 1997; main study May 1997 to December 2000 (trial terminated by data safety monitoring board) |
Participant characteristics
Pilot study
| Characteristic | ICD (n = 10) | Beta-blocker (n = 10) | p-value |
|---|---|---|---|
| Age (years), mean (SEM) | 44 (11) | 48 (15) | 0.63 |
| Sex, male, n (%) | 10 (100) | 10 (100) | |
| Ethnicity | NR | NR | |
| SUDS survivors, n | 8 | 6 | |
| Probable SUDS survivors, n | 2 | 4 | |
| NYHA class I, n (%) | 10 (100) | 10 (100) | |
| LVEF (%), mean (SEM) | 67 (12) | 69 (6) | 0.66 |
| RVEF (%), mean (SEM) | 60 (8) | 58 (8) | 0.76 |
| Received CPR, n | 9 | 6 | 0.30 |
| Received defibrillation, n | 8 | 5 | 0.35 |
| Symptoms during index event, n | |||
| Loss of consciousness, intervention | 8 | 6 | 0.63 |
| Loss of consciousness, spontaneous recovery | 2 | 3 | 0.99 |
| Near syncope | 0 | 1 | 0.99 |
| Agonal respiration during sleep | 0 | 0 | |
| Seizure | 0 | 0 | |
| Difficult to arouse with signs of distress | 0 | 0 | |
| Rhythm at time of recording, n | 0.10 | ||
| VF | 7 | 6 | |
| VT | 0 | 0 | |
| Unknown or not documented | 0 | 4 | |
| ECG abnormalities manifesting as RBBB and ST elevation at the precordial lead (V1–V3), n (%) | NR | NR | |
| Heart rate (bpm), mean (SEM) | 67 (12) | 64 (7) | |
| PR interval (milliseconds), mean (SEM) | 166 (26) | 169 (30) | |
| QRS interval (milliseconds), mean (SEM) | 98 (29) | 92 (12) | |
| QT interval (milliseconds), mean (SEM) | 396 (51) | 387 (31) | |
| Induced VF (≥ 300 bpm), n (%) | 1 (13) | 1 (10) | |
| Induced polymorphic VT (≤ 300 bpm), n (%) | 4 (50) | 8 (80) | |
| Non-inducible VF/VT, n (%) | 3 (37) | 1 (10) | |
| Electrophysiological study not carried out, n | 2 | 0 | |
| Atrio-His conduction time (milliseconds), mean (SEM) | 94 (10) | 94 (12) | |
| His-Purkinje conduction time (milliseconds), mean (SEM) | 58 (18) | 54 (3) | |
| Signal-averaging ECG performed, n (%) | 5 | 8 | |
| Positive | 4 (80) | 4 (50) | |
| Negative | 1 (20) | 4 (50) | |
Main study
| Characteristic | ICD (n = 37) | Beta-blocker (n = 29) | |
|---|---|---|---|
| Age (years), mean (SEM) | 40 (11) | 40 (14) | 0.95 |
| Sex, male, n (%) | 35 (95) | 29 (100) | 0.5 |
| Ethnicity | NR | NR | |
| SUDS survivors, n | 22 | 20 | |
| Probable SUDS survivors, n | 15 | 9 | |
| NYHA class I, n (%) | 37 (100) | 28 (100)a | |
| LVEF (%), mean (SEM) | 66 (10) | 67 (7) | 0.55 |
| RVEF (%), mean (SEM) | 62 (13) | 60 (8) | 0.6 |
| Received CPR, n | 26 | 20 | 0.92 |
| Received defibrillation, n | 17 | 18 | 0.17 |
| Symptoms during index event, n | |||
| Loss of consciousness, intervention | 26 | 21 | 0.85 |
| Loss of consciousness, spontaneous recovery | 5 | 4 | 0.99 |
| Near syncope | 2 | 1 | 0.99 |
| Agonal respiration during sleep | 3 | 3 | 0.99 |
| Seizure | 0 | 5 | 0.01 |
| Difficult to arouse with signs of distress | 2 | 4 | 0.67 |
| Rhythm at time of recording, n | 0.74 | ||
| VF | 9 | 11 | |
| VT | 2 | 2 | |
| Unknown or not documented | 26 | 16 | |
| ECG abnormalities manifesting as RBBB and ST elevation at the precordial lead (V1–V3), n (%) | 23 (62) | 16 (55) | |
| Heart rate (bpm), mean (SEM) | 64 (11) | 66 (12) | 0.48 |
| PR interval (milliseconds), mean (SEM) | 180 (98) | 163 (27) | 0.48 |
| QRS interval (milliseconds), mean (SEM) | 99 (30) | 95 (16) | 0.43 |
| QT interval (milliseconds), mean (SEM) | 404 (43) | 394 (31) | 0.33 |
| Induced VF (≥ 300 bpm), n (%) | 8 (22) | 8 (30) | 0.70 |
| Induced polymorphic VT (≤ 300 bpm), n (%) | 15 (40) | 11 (41) | |
| Non-inducible VF/VT, n (%) | 14 (38) | 8 (30) | |
| Electrophysiological study not carried out | 0 | 2 | |
| Atrio-His conduction time (milliseconds), mean (SEM) | 100 (22) | 96 (22) | 0.58 |
| His-Purkinje conduction time (milliseconds), mean (SEM) | 51 (8) | 49 (11) | 0.47 |
| Signal-averaging ECG performed, n (%) | 29 | 21 | 0.74 |
| Positive | 11 (38) | 7 (33) | |
| Negative | 18 (62) | 14 (67) | |
Results
Pilot study
| Outcome | ICD (n = 10) | Beta-blocker (n = 10) | p-value |
|---|---|---|---|
| Died before main trial, n | 1 | ||
| Death during follow-up, n | 0 | 3 (2 SUDS survivors, 1 probable SUDS survivor) at 5.4, 11.8 at 24.6 months | 0.07 |
| Multiple VF episodes successfully treated by ICD, n | 5 |
Adverse effects of treatment
| Adverse effect | ICD (n = 10) | Beta-blocker (n = 10) | p-value |
|---|---|---|---|
| Operative mortality, n | 0 | ||
| Adverse effects, n/N (%) | 2/10 (20) | ||
| Defibrillation discharges caused by supraventricular tachycardia or sinus tachycardia | 1 | ||
| T-wave oversensing | 0 | ||
| ICD replaced because of insulation break, n | 1 |
Main study
| Outcome | ICD (n = 37) | Beta-blocker (n = 29) | p-value |
|---|---|---|---|
| Mortality during 3-year follow-up, n (%) | 0 | 4 (14) | 0.02 |
| Annual death rate | 0 | About 10% | |
| Survival (months), mean (SEM) | 26.2 (1.4) | ||
| Recurrent VF (effectively treated by ICD), n (%) | 7 (19) |
Adverse effects of treatment
| Adverse effect | ICD (n = 37) | Beta-blocker (n = 29) | p-value |
|---|---|---|---|
| Operative mortality | 0 | ||
| Adverse effects, n/N (%) | 11/37 (30) | 4 (14) | |
| Minor complications, corrected by reprogramming devices without major intervention, n | |||
| Defibrillation discharges caused by supraventricular tachycardia or sinus tachycardia | 7 | ||
| T-wave oversensing | 3 | ||
| Pocket erosion requiring removal of ICD, n | 1 | ||
| Side-effects in beta-blocker group, n | |||
| Impotence/decrease in libido | 1 | ||
| Fatigue | 1 | ||
| Profound bradycardia | 1 | ||
| Hypotension plus central nervous system side effect | 1 | ||
Pilot and main study combined
| Outcome | ICD (n = 47) | Beta-blocker (n = 39) | p-value |
|---|---|---|---|
| Sudden death, n | 0 | 7 | |
| Multiple VF episodes and defibrillation shocks, n | 12 | ||
| Annual rate of VF episodes or sudden death (%) | 20 | 10 |
-
Allocation to treatment groups: randomisation stratified by SUDS survivor vs. probable SUDS survivor.
-
Blinding: not reported.
-
Comparability of treatment groups: groups similar.
-
Method of data analysis: interim analyses planned after half of patients and three-quarters of patients had been randomised. Trial planned to be stopped after first interim analysis if survival analysis was p < 0.005 and after second analysis if p < 0.006. Final statistical analysis at the 0.048 significance level. Trial stopped at first interim analysis by data safety monitoring board even though analysis did not reach level of significance, based on cumulative weight of all evidence gained from data (including pilot study) that ICDs were superior. Baseline characteristics compared and any significantly different factors were used as covariates in subsequent analysis. States ITT analysis used to contrast mortality rates and used Kaplan–Meier methods for calculating survival curves, log-rank method for comparing survival curves and Cox regression methods for comparing survival curves adjusting for covariates found to be different between treatment arms.
-
Sample size/power calculation: from the pilot study it was estimated that 114 patients needed to be randomised, based on an expected annual mortality rate of 20% for the SUDS population. Assuming that the annual mortality rate would be reduced 10-fold (i.e. by up to 2%) in the ICD arm, 57 patients per treatment arm were required to produce the expected difference at 80% power and 0.05 two-sided significance level. Note that only 66 patients were randomised. The annual death rate in the beta-blocker arm was about 10%, half that used for the sample size calculation.
-
Attrition/dropout: in total, 155 people were screened, 64 had probable SUDS, either non-inducible or unclear marker, 10 refused to be enrolled, one was randomised to the ICD group but refused, two preferred ICD treatment, five had brain anoxic encephalopathy, six had presence of heart disease and one entered after the trial was stopped. Attrition/dropout after randomisation not reported. Not clear if all 66 participants were followed for 3 years.
-
Generalisability: small trial stopped early. Population differs significantly from that in other trials as participants are survivors of sudden unexplained death with otherwise normal hearts with no HF. All participants were of Thai origin, mostly men. Participants similar to those with Brugada syndrome (a genetic disorder characterised by abnormal ECG findings and increased risk of SCD); study findings should also apply to this group of people.
-
OPT used: the use of beta-blockers is low in the ICD group (exact numbers in main trial not clear, but 8/47 in the main trial and pilot study combined). The study used an active comparator.
-
Outcome measures: limited to death from all causes, VT/VF episodes and adverse events.
-
Intercentre variability: not reported.
-
Conflict of interests: not stated. Supported by grant-in-aid from Cardiac Rhythm Management and Guidant Corporation, St Paul, MN.
-
Other: paper reports the results of a pilot study and main study.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Details not reported |
| Allocation concealment | Unclear | Details not reported |
| Performance bias | ||
| Blinding of participants and personnel | High | Not reported but unlikely to be blinded because of surgical intervention in one arm |
| Detection bias | ||
| Blinding of outcome assessment | Low | Not reported but assessment of mortality unlikely to be influenced by lack of blinding |
| Attrition bias | ||
| Incomplete outcome data addressed | Unclear | States ITT analysis but loss to follow-up not reported. Follow-up for maximum 3 years; not clear how many participants followed for this length of time |
| Reporting bias | ||
| Selective reporting | Low | |
| Other bias | ||
| Other sources of bias | Low | |
Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Kadish et al. 200091 and 2004,90 Schaechter et al. 200392 Ellenbogen et al. 2006,93 Passman et al. 200794 Study design: RCT Countries: USA and Israel No. of centres: 48 (USA 44, Israel 4) Funding: St Jude Medical |
Intervention: ICD + standard oral medical therapy for HF (OPT). Single chamber device. Programmed to back up VVI pacing at a rate of 40 bpm and to detect VF at a rate of 180 bpm Comparator: OPT. Medical therapy in both groups for HF included ACE inhibitors unless contraindicated (then hydralazine, nitrates or ARBs) and beta-blocker therapy (unless not tolerated) with carvedilol. Doses of ACE inhibitors and beta-blockers adjusted to recommended levels for HF patients or to highest tolerated doses. Digoxin and diuretics used when necessary to manage clinical symptoms. Use of AADs (e.g. amiodarone) discouraged but allowed for some patients with symptomatic atrial fibrillation or supraventricular arrhythmias. No other AADs used Other interventions used: none reported |
Indication for treatment: non-ischaemic cardiomyopathy and moderate to severe left ventricular dysfunction No. of randomised participants: 458; ICD + OPT: 229, OPT: 229 Inclusion criteria: NYHA class not reported, LVEF < 36%, LVEDD not reported, QRS interval not reported, presence of ambient arrhythmias (episode of non-sustained VT 3–15 beats at a rate of > 20 bpm or an average of at least 10 premature ventricular complexes per hour on 24-hour Holter monitoring), history of symptomatic HF, presence of non-ischaemic dilated cardiomyopathy, absence of clinically significant coronary artery disease, age 21–80 years93 Exclusion criteria: NYHA class IV, not a candidate for an ICD, electrophysiological testing within the last 3 months, permanent pacemaker, cardiac transplantation appeared imminent, familial cardiomyopathy associated with sudden death, acute myocarditis, congenital heart disease |
Primary outcome: death from any cause Secondary outcomes: sudden death from arrhythmia, QoL94 Method of assessing outcomes: at 3-month intervals. Cause of death used Epstein classification; therefore, patients with progressive symptomatic deterioration of pump failure who died from terminal VF were not considered to have had sudden death from arrhythmia. ICD shocks assessed at each follow-up or when indicated by symptoms.94 QoL assessed with self-administered SF-12 and the MLWHFQ at baseline, 1 month after randomisation and every 3 months thereafter (to 63 months)94 Length of follow-up: duration computed from randomisation to death or to the date of the 68th death for those who did not die. Mean (SD) 29.0 (14.4) months Recruitment: July 1998 to June 2002 |
Participant characteristics
| Characteristica | ICD + OPT (n = 229) | OPT (n = 229) | p-value |
|---|---|---|---|
| Age (years), mean (range) | 58.4 (20.3–83.9) | 58.1 (21.8–78.7) | |
| Sex male, n (%) | 166 (72.5) | 160 (69.9) | |
| Self-reported ethnicity, n (%) | |||
| White | 154 (67.2) | 154 (67.2) | |
| Black | 59 (25.8) | 59 (25.8) | |
| Hispanic | 13 (5.7) | 13 (5.7) | |
| Pacific Islander | 1 (0.4) | 0 | |
| Asian | 0 | 1 (0.4) | |
| Other | 2 (0.9) | 2 (0.9) | |
| Qualifying arrhythmia, n (%) | |||
| NSVT only | 51 (22.3) | 52 (22.7) | |
| PVCs only | 21 (9.2) | 22 (9.6) | |
| NSVT and PVCs | 157 (68.6) | 155 (67.7) | |
| Severity of disease, e.g. NYHA classification | |||
| NYHA class I, n (%) | 58 (25.3) | 41 (17.9) | |
| NYHA class II, n (%) | 124 (54.2) | 139 (60.7) | |
| NYHA class III, n (%) | 47 (20.5) | 49 (21.4) | |
| LVEF (%), mean (range) | 20.9 (7–35) | 21.8 (10–35) | |
| Heart rate | NR | NR | |
| QRS interval (milliseconds), mean (range) | 114.7 (78–196) | 115.5 (79–192) | |
| LBBB, n (%) | 45 (19.7) | 45 (19.7) | |
| RBBB, n (%) | 8 (3.5) | 7 (3.1) | |
| Pharmacological therapy, n (%) | |||
| ACE inhibitor | 192 (83.8) | 200 (87.3) | |
| Beta-blocker | 196 (85.6) | 193 (84.3) | |
| Carvedilol | 129 (56.3) | 134 (58.5) | |
| Metoprolol | 59 (25.8) | 43 (18.8) | |
| Other | 8 (3.5) | 16 (7.0) | |
| Diuretic | 200 (87.3) | 197 (86.0) | |
| ARB | 31 (13.5) | 20 (8.7) | |
| Amiodarone | 9 (3.9) | 15 (6.6) | |
| Digoxin | 95 (41.5) | 97 (42.4) | |
| Nitrate | 21 (9.2) | 30 (13.1) | |
| Duration of HF (years), mean (range) | 2.39 (0.0–21.33) | 3.27 (0.0–38.5) | 0.04 |
| History of diabetes, n (%) | 52 (22.7) | 53 (23.1) | |
| History of atrial fibrillation, n (%) | 52 (22.7) | 60 (26.2) | |
| Distance walked in 6 minutes (m), mean (range) | 311.2 (29–1143) | 328.3 (18–1317) | |
| ICD + OPT (n = 227) | OPT (n = 226) | ||
| HRQoL94a | |||
| Physical score (MLWHFQ), mean (SD) | 20 (12) | 20 (12) | 0.98 |
| Emotional score (MLWHFQ), mean (SD) | 11 (8) | 10 (8) | 0.59 |
| PCS (SF-12), mean (SD) | 37 (11) | 38 (10) | 0.47 |
| MCS (SF-12), mean (SD) | 45 (11) | 47 (11) | 0.14 |
Results
| Outcome | ICD + OPT (n = 229) | OPT (n = 229) | HR (95% CI) p-value |
|---|---|---|---|
| All-cause mortality, n | 28 | 40 | 0.65 (0.40 to 1.06),a 0.08 |
| All-cause mortality rate at 1 year, % | 2.6 | 6.2 | |
| All-cause mortality rate at 2 years, % | 7.9 | 14.1 | |
| Sudden death from arrhythmia, n | 3 | 14 | 0.20 (0.06 to 0.71), 0.006 |
| Death from HF, n | 9 | 11 | |
| Receipt of appropriate ICD shocksb | 41 patients, 91 shocks | ||
| Receipt of inappropriate ICD shocksb | 49 patients | ||
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | NR | NR | |
| Change in LVEF | NR | NR | |
| Exercise capacity outcomes (e.g. 6-minute walk distance, total exercise time, peak VO2) | NR | NR | |
| ICD + OPT (n = 227) | OPT (n = 226) | p-value | |
| HRQoL94 | |||
| Long-term MCS score | 0.89 | ||
| Long-term PCS score | NS, p-value not reported | ||
| Long-term MLWHFQ subscale score | NS, p-value not reported | ||
Adverse effects of treatment
| Adverse effect | ICD + OPT (n = 229) | OPT (n = 229) | p-value |
|---|---|---|---|
| Complications during implantation of ICD,a n (%) | 3 (1.3) | ||
| Haemothorax | 1 | ||
| Pneumothorax | 1 | ||
| Cardiac tamponade | 1 | ||
| Procedure-related deaths, n | 0 | ||
| Complications during follow-up, n (%) | 10 (4.4) | ||
| Lead dislodgement or fracture | 6 | ||
| Venous thrombosis | 3 | ||
| Infection | 1 | ||
| Receipt of ICD upgrade during follow-up, n | 13 | ||
| Dual chamber ICD because of development of sinus node dysfunction | 2 | ||
| Biventricular device for NYHA class III or IV HF and prolonged QRS interval | 11 |
Prespecified subgroup analyses
| Subgroup analysis | RR (95% CI) | p-value |
|---|---|---|
| RR of death from any cause after receipt of ICD in comparison to OPT | ||
| For men | 0.49 (0.27 to 0.90) | 0.018 |
| For NYHA class III HF patients | 0.37 (0.15 to 0.90) | 0.02 |
-
Allocation to treatment groups: randomisation stratified by centre and by the use or non-use of amiodarone for supraventricular arrhythmias.
-
Blinding: cause of death determined by an events committee unaware of patients’ treatment assignments. Blinding process included editing information from progress notes or laboratory reports that could have identified the presence of an ICD.
-
Comparability of treatment groups: similar apart from duration of HF [ICD + OPT mean 2.39 (range 0.0–21.33) years, OPT mean 3.27 (range 0.0–38.5) years, p = 0.04].
-
Method of data analysis: all analyses ITT. Data collection and analysis independently performed at Northwestern University, IL. Interim analyses performed after 22, 34, 45, 50 and 56 deaths. Critical values for interim and final analyses assumed an O’Brien–Fleming type of spending function. For patient safety, stopping boundaries were defined in favour of the null hypothesis of no effect of the ICD on the risk of death at each interim analysis. No boundaries were crossed at any of the five interim analyses so the report presents the final analysis results at the time of the 68th death. The p-value for significance in the final analysis was 0.041 on the basis of a two-sided test. Baseline characteristics were compared using two-sample t-tests for continuous variables and chi-square tests for categorical variables. Log-rank test was used to compare Kaplan–Meier survival curves. Cox proportional hazards model was used to adjust for covariates and to estimate the HR for death and corresponding 95% CI in the ICD group vs. the OPT group. Data for patients receiving a heart transplant were censored at the time of transplantation. All reported p-values are two-tailed. QoL outcomes were compared using hierarchical linear regression. QoL analyses were controlled for baseline differences and predetermined characteristics (sex, age, NYHA class, ethnicity, ejection fraction, duration of HF, history of atrial fibrillation). Covariates were entered into and removed from the model stepwise at the group level with α = 0.05 and α = 0.10 as the criterion for entry and removal respectively. 94
-
Sample size/power calculation: designed to have statistical power of 85% based on a one-sided test. Two-year mortality rate of 15% assumed in the comparator group and 7.5 in the ICD group with enrolment of 458 patients and 56 deaths. To report results with the use of two-sided tests and 85% statistical power, follow-up was extended to include 68 deaths.
-
Attrition/dropout: prespecified criteria meant that the OPT group patients received an ICD if they had a cardiac arrest or an episode of unexplained syncope consistent with the occurrence of an arrhythmic event. Overall, 23 (10%) of the OPT group received an ICD during follow-up, primarily for this reason (no further details provided). Two ICD group participants declined implantation of the device after randomisation. Additionally, one patient had the ICD explanted and one had the device inactivated. All four were included in the ICD group (ITT analysis). In the QoL analysis, missing months of data were treated following a full information restricted maximum likelihood estimation approach. 94 The QoL analysis excluded five patients who did not provide any data (two from the ICD group, three from the OPT group). QoL data were missing from one or two visits for 130 patients, and 178 patients had missing QoL data from more than two visits. States no relationship between QoL and varying length of follow-up or dropping out of the study. No significant differences between complete and incomplete QoL data by patient age, sex or NYHA class but patients without missing data are more likely to be white and have a better ejection fraction and less likely to have diabetes than those with missing data (all p < 0.05). Those with complete data were more likely to report a better baseline QoL. No interactions between data completeness and treatment group (p = 0.2).
-
Generalisability: focus was on primary prevention of sudden death in patients with non-ischaemic cardiomyopathy and moderate to severe left ventricular dysfunction. Results unlikely to be generalisable to higher-risk groups, e.g. secondary prevention of sudden death.
-
Outcome measures: appear appropriate.
-
Intercentre variability: randomisation stratified by centre but no comments regarding intercentre variability.
-
Conflict of interests: states study sponsor did not have access to the data. Three of the authors had received fees from one or more of Medtronic, Guidant Corporation and St Jude Medical.
-
Other: included after receiving advice from experts who indicated that it was similar to the AMIOVERT trial investigating whether ICDs reduce mortality in a high-risk population with cardiomyopathy and no coronary disease. Note that mean QRS interval is < 120 milliseconds in each group so on average no cardiac dyssynchrony.
Criteria for assessment of risk of bias in randomised controlled trials65
| Judgementa | Support for judgement | |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | No details about sequence generation |
| Allocation concealment | Unclear | No details reported |
| Performance bias | ||
| Blinding of participants and personnel | High | Not reported |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality | Low | Events committee determining cause of death blinded |
| QoL | High | |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | ITT analysis and attrition for each group reported with reasons |
| Reporting bias | ||
| Selective reporting | High | A cost analysis is listed in both papers reporting on study design and organisation91,92 but no cost outcomes are reported in the identified papers |
| Other bias | ||
| Other sources of bias | Low | |
Defibrillator in Acute Myocardial Infarction Trial (DINAMIT)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Hohnloser et al. 200096 and 200495 Study design: RCT Countries: 12 countries worldwide No. of centres: 73 (Canada 25, Germany 21, UK 4, Slovakia 2, Poland 4, France 8, Czech Republic 1, Austria 2, Switzerland 1, Sweden 2, Italy 1, USA 2) Funding: Supported by a grant from St Jude Medical |
Intervention: ICD + OPT (supplied by St Jude Medical). Single-chamber ICD implanted within 1 week of randomisation. Implanted leads were required to achieve an R wave of < 4.9 mV, a pacing threshold of > 2.1 V at 0.5 milliseconds and a defibrillation threshold with a safety margin of at least 10 J. Postoperatively, the ICD was set to detect VT and VF. The detection rate for tachycardia was set at ≥ 175 bpm per minute for ≥ 16 beats. The device was programmed to deliver all discharges at maximal output in the VF zone (≥ 200 bpm). Bradycardia pacing was programmed for activation at a minimum of 40 bpm. Antitachycardia pacing within the VT zone (175–200 bpm) could be activated to deliver four bursts of six to 10 beats beginning at 81% of the tachycardia cycle length, with 10-milliseconds decrements between bursts Comparator: OPT (best conventional medical therapy) Other interventions used: Best conventional medical therapy. Investigators were encouraged to treat all study patients with ACE inhibitors, beta-blockers, aspirin and lipid-lowering drugs as appropriate (reasons for not giving these medications were documented) |
Indication for treatment: Recent MI (within 6–40 days), reduced LVEF and impaired cardiac autonomic function No. of randomised participants: 674; ICD: 332, OPT: 342 Inclusion criteria: Age 18–80 years, recent MI (within 6–40 days), LVEF ≤ 0.35, SD of normal-to-normal R–R intervals of ≤ 70 milliseconds or a mean R–R interval of ≤ 750 milliseconds (HR ≥ 80 bpm) over a 24-hour period as assessed by 24-hour Holter monitoring performed at least 3 days after the infarction Exclusion criteria: CHF or NYHA class IV at time of randomisation, non-cardiac disease that limited life expectancy, CABG performed since the qualifying infarction or planned to be performed within 4 weeks of randomisation, three-vessel percutaneous coronary intervention performed since the qualifying infarction, name on a waiting list for a heart transplant, current ongoing ICD therapy, previous implantation of a permanent pacemaker, requirement for an ICD (i.e. sustained VT or VF > 48 hours after the qualifying infarction), low probability that the study ICD could be implanted within 7 days of randomisation, expected poor compliance with the protocol |
Primary outcome: Death from any cause Secondary outcome: Death from cardiac arrhythmia Method of assessing outcomes: Cause of death ascertained by local investigators and documentation based on information obtained from witnesses, family members, death certificates, hospital records and autopsy reports when available, not from ICD telemetry. All deaths were reviewed by a committee and classification of each death was agreed based on clinical circumstances of death and not ICD information. Deaths were classified as either arrhythmic or non-arrhythmic in nature (based on criteria from Hinkle and Thaler, reference provided). Follow-up visits were scheduled at 3 and 6 months after randomisation and at 6-monthly intervals thereafter. Follow-up ended in September 2003, about 15 months after last patient was recruited Length of follow-up: Mean follow-up 30 (SD 13) months, maximum 4 years from randomisation Recruitment: April 1998–June 2002 |
Participant characteristics
| Characteristic | ICD (n = 332)a | OPT (n = 342)a | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 61.5 (10.9) | 62.1 (10.6) | NR |
| Sex male, n (%) | 252 (75.9) | 262 (76.6) | NR |
| Ethnicity | NR | NR | |
| CHF with index MI, n (%) | 156 (47.0) | 167 (48.8) | NR |
| NYHA class I | 21 (13.5) | 20 (12.0) | NR |
| NYHA class II | 95 (60.9) | 98 (58.7) | NR |
| NYHA class III | 40 (25.6) | 49 (29.3) | NR |
| LVEF, mean (SD) | 0.28 (0.05) | 0.28 (0.05) | NR |
| Heart rate | NR | NR | |
| Electrophysiology | |||
| QRS duration (milliseconds), mean (SD) | 107 (24) | 105 (23) | NR |
| Peak creatine kinase (U/l), mean (SD) | 2329 (3837) | 2138 (2349) | NR |
| New Q-wave infarction, n (%) | 240 (72.3) | 256 (74.9) | NR |
| SD of normal-to-normal RR intervals (milliseconds), mean (SD) | 61 (21) | 61 (22) | NR |
| 24-hour RR interval (milliseconds), mean (SD) | 745 (106) | 747 (105) | NR |
| Beta-blockers, n (%) | 289 (87.0) | 296 (86.5) | NR |
| ACE inhibitors, n (%) | 315 (94.9) | 323 (94.4) | NR |
| Antiplatelet agents, n (%) | 306 (92.2) | 315 (92.1) | NR |
| Lipid-lowering agents, n (%) | 255 (76.8) | 272 (79.5) | NR |
| Cardiac history, n (%) | |||
| Previous MI | 123 (37.0) | 111 (32.5) | NR |
| Previous CABG | 25 (7.5) | 24 (7.0) | NR |
| Previous PTCA | 49 (14.8) | 38 (11.1) | NR |
| Location of index MI, n (%) | |||
| Anterior | 239 (72.0) | 247 (72.2) | NR |
| Other | 93 (28.0) | 95 (27.8) | NR |
| In-hospital therapy for MI, n (%) | |||
| Any | 208 (62.7) | 212 (62.0) | NR |
| PTCA only | 87 (26.2) | 92 (26.9) | NR |
| Thrombolysis only | 88 (26.5) | 76 (22.2) | NR |
| Both PTCA and thrombolysis | 33 (9.9) | 44 (12.9) | NR |
| None | 115 (34.6) | 111 (32.5) | NR |
| Unknown | 9 (2.7) | 19 (5.6) | NR |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 102 (30.7) | 98 (28.7) | NR |
| Hypertension | 155 (46.7) | 154 (45.0) | NR |
Results
| Outcome | ICD (n = 332) | OPT (n = 342) | HR (95% CI),a p-valueb |
|---|---|---|---|
| Mortality rate, n [rate (%/year]c,d | |||
| Primary outcome: death from any cause | 62 (7.5) | 58 (6.9) | 1.08 (0.76 to 1.55), 0.66 |
| Secondary outcome: death from arrhythmia | 12 (1.5) | 29 (3.5) | 0.42 (0.22 to 0.83), 0.009 |
| Secondary outcome: non-arrhythmic causes | 50 (6.1) | 29 (3.5) | 1.75 (1.11 to 2.76), 0.02 |
| Cardiac, non-arrhythmic | 34 (4.1) | 20 (2.4) | 1.72 (0.99 to 2.99), 0.05 |
| Vascular, non-cardiac | 5 (0.6) | 3 (0.4) | 1.69 (0.40 to 7.06), 0.47 |
| Non-vascular | 11 (1.3) | 6 (0.7) | 1.85 (0.68 to 5.01), 0.22 |
| Percutaneous or surgical coronary revascularisation, n (%) | 33 (9.9) | 50 (14.6) | p = 0.08 |
| Prescribed amiodarone, n (%) | 27 (8.1) | 46 (13.5) | p = 0.04 |
Adverse effects of treatment
| Adverse effect | ICD (n = 332) |
|---|---|
| Death related to device implantation, n | 0 |
| In-hospital device-related complications,a n | 25/310 |
-
Allocation to treatment groups: central randomisation was performed at the study co-ordinating and methods centre. Patients were randomly assigned in a 1 : 1 ratio. The randomisation sequence was stratified according to centre and balanced within randomly varying blocks of two, four or six patients.
-
Blinding: unblinded study; blinding reported for independent review committee.
-
Comparability of treatment groups: described as well balanced for baseline clinical characteristics and early use of reperfusion therapy (states no significant differences). The ICD group had slightly higher percentages for previous MI and percutaneous transluminal coronary angioplasty (PTCA) and in-hospital therapy for ‘thrombolysis only’. The OPT group had slightly higher percentages for NYHA class III as well as in-hospital therapy for ‘both PTCA and thrombolysis’ and ‘unknown’. Average time from MI to randomisation was 18 days – similar between groups (no p-value reported). Amiodarone use was higher in the OPT group.
-
Method of data analysis: the primary study outcome was evaluated according to the ITT principle. The cumulative risks of death from any cause and from specific causes over time were estimated separately for each treatment group with the use of the Kaplan–Meier procedure and were compared between groups with the use of the Mantel–Haenszel test. A single interim analysis of efficacy was performed by an external safety and efficacy monitoring committee after 66deaths (about half the anticipated number) had occurred. A one-sided p-value of < 0.001 would have resulted in early termination of the study. Before unblinding, a decision was made to use two-sided statistical testing.
-
Sample size/power calculation: on the basis of mortality data from similar populations of patients, it was anticipated that the OPT group would have a 3-year mortality rate of 30.0% and that 40.0% of these deaths would be caused by arrhythmias. The net effect of preventing 80.0% of these deaths from arrhythmias with the use of an ICD would be to reduce the total mortality rate to 20.4%. Based on a one-sided test at an alpha level of 0.05, 525 patients would be required for the study to have 80% power to identify a difference between the groups. Because mortality rates were lower than expected during the study, the target enrolment was increased to 674 patients. States that it is unlikely that the similarity between the two groups in the rate of death from all causes represents a false-negative result because of an inadequate sample size.
-
Attrition/dropout: four patients in the OPT group had only partial follow-up data available. ICD received: 310/332; 20/332 patients refused ICD implantation, 2/332 died before receiving the ICD.
-
Generalisability: limited to high-risk patients with a recent MI, reduced LVEF and impaired cardiac autonomic function.
-
Outcome measures: limited to mortality. No adverse event data for the OPT group and limited adverse event data for the ICD group.
-
Intercentre variability: not reported.
-
Conflict of interests: Drs Hohnloser, Kuck, Dorian and Connolly are consultants to and have received lecture fees from St Jude Medical. Dr Fain is an employee of St Jude Medical. Data analysis was performed at the Hamilton Civic Hospitals Research Centre by two of the authors (Mr Roberts and Dr Gent). All investigators had full access to the data.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | The randomisation sequence was stratified according to centre and balanced within randomly varying blocks of two, four or six patients. No details of sequence generation |
| Allocation concealment | Low | Central randomisation |
| Performance bias | ||
| Blinding of participants and personnel | High | Described as an unblinded study |
| Detection bias | ||
| Blinding of outcome assessment | Low | Assessment of causes of death by unblinded local investigators, but all causes of deaths were reviewed by an independent blinded central validation committee |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Primary outcome was evaluated according to the ITT principle; unclear how partially missing follow-up data for four OPT patients were accounted for in relation to secondary outcomes |
| Reporting bias | ||
| Selective reporting | High | QoL mentioned in protocol but data not reported |
| Other bias | ||
| Other sources of bias | High | Block randomisation in unblinded trial can lead to prediction of allocation |
Immediate Risk Stratification Improves Survival (IRIS) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Steinbeck et al. 200498 and 200997 Study design: RCT Countries: Austria, the Czech Republic, Germany, Hungary, Poland, the Russian Federation, Slovakia No. of centres: 92 Funding: grants from Medtronic Bakken Research Center, AstraZeneca and R Becker |
Intervention: ICD + OPT. In total, 78% received Medtronic models of the GEM® family, 11% Micro Jewel II, 8% Maximo and 3% Marquis. A total of 81% were single-chamber ICDs. A Fidelis lead was used in 21% of patients. Protocol required two consecutive terminations of VF at 10 J below the maximum ICD output and VVI pacing at 40 bpm, with maximal shock energy turned on for treatment of VF (threshold ≥ 200 bpm) and treatment for VT turned off initially Comparator: OPT (not described further) Other interventions used: Not stated |
Indication for treatment: Recent MI (within ≤ 31days) and predefined markers of elevated risk No. of randomised participants: 898; ICD: 445, OPT: 453 Inclusion criteria: Predefined markers of elevated risk: at least one of heart rate ≥ 90 bpm on the first available ECG (within 48 hours of MI) and LVEF ≤ 40% (on one of days 5–31 after MI); NSVT of three or more consecutive ventricular premature beats during Holter ECG monitoring, with a heart rate of ≥ 150 bpm (on days 5–31) Exclusion criteria: Ventricular arrhythmia before the index MI or > 48 hours after the event and required treatment, NYHA class IV, interval > 31 days between the MI and presentation, no ECG within 48 hours of chest pain onset, indication for CABG surgery, psychiatric disorder, severe concomitant disease, history of poor compliance with treatment, current participation in another trial, unstable clinical condition |
Primary outcome: Overall mortality Secondary outcomes: SCD [death occurred within minutes of onset of acute symptoms, resulted from a documented cardiac arrhythmia or was not witnessed and occurred unexpectedly and without recognisable causes (e.g. during sleep)], non-SCD, non-cardiac death Method of assessing outcomes: 3 and 6 months after randomisation and then at 6-month intervals Length of follow-up: Average 37 (range 0–106) months Recruitment: June 1999–October 2007 |
Participant characteristics
| Characteristic | ICD (n = 445) | OPT (n = 453) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 62.8 (10.5) | 62.4 (10.6) | |
| Sex, male, n (%) | 345 (77.5) | 344 (75.9) | |
| Ethnicity | NR | NR | |
| Criteria for inclusion, n (%) | |||
| Criterion 1 only (heart rate and LVEF) | 299 (67.2) | 303 (66.9) | |
| Criterion 2 only (NSVT) | 99 (22.2) | 109 (24.1) | |
| Criteria 1 and 2 | 47 (10.6) | 41 (9.1) | |
| LVEF (%), mean (SD) | 34.6 (9.3) | 34.5 (9.4) | |
| Criterion 1 only | 32.2 (6.3) | 31.9 (6.7) | |
| Criterion 2 only | 45.9 (10.8) | 44.8 (11.0) | |
| Criteria 1 and 2 | 29.6 (7.0) | 31.4 (6.7) | |
| Heart rate | NR | NR | |
| Electrophysiology findings | NR | NR | |
| Medical therapy on admission n/N (%) | |||
| Antiplatelet agents | 438/443 (98.9) | 442/452 (97.8) | |
| Beta-blockers | 394/442 (89.1) | 388/453 (85.7) | |
| ACE inhibitors | 361/443 (81.5) | 373/453 (82.3) | |
| STEMI, n (%) | 341 (76.6) | 348 (76.8) | |
| Reperfusion in STEMI, n/N (%) | |||
| None | 43/340 (12.6) | 48/348 (13.8) | |
| PTCA | 243/340 (71.5) | 253/348 (72.7) | |
| Thrombolytic therapy, with or without PTCA | 54/340 (15.9) | 47/348 (13.5) | |
| Anterior wall MI, n/N (%) | 282/439 (64.2) | 300/449 (66.8) | |
| HF on admission n/N (%) | 197/444 (44.4) | 209/453 (46.1) | |
| Previous MI, n/N (%) | 77/444 (17.3) | 89/453 (19.6) | |
| Atrial fibrillation, n/N (%) | 60/445 (13.5) | 61/453 (13.5) | |
| LBBB, n/N (%) | 45/445 (10.1) | 29/453 (6.4) | 0.05 |
| Hypertension, n/N (%) | 296/444 (66.7) | 300/453 (66.2) | |
| Diabetes mellitus, n/N (%) | 165/444 (37.2) | 137/453 (30.2) | 0.03 |
| NYHA class at discharge (in 885 surviving patients), n (%) | |||
| I | 247 (28) | ||
| II | 531 (60) | ||
| III | 106 (12) | ||
| IV | 1 (0.1) | ||
| Discharge medication, % of patients | |||
| Antiplatelet agents | 96.1 | 95.8 | |
| Beta-blockers | 97.1 | 95.3 | |
| ACE inhibitors | 90.9 | 91.1 | |
| Statins | 91.6 | 91.5 | |
| AADs (mainly amiodarone) | 13.4 | 17.4 | 0.11 |
Results
| Outcome | ICD (n = 445) | OPT (n = 453) | HR (95% CI) (unadjusted), p-value |
|---|---|---|---|
| Cause of death,a n/n (%) | |||
| Any cause | 116/445 (26.1) | 117/453 (25.8) | 1.04 (0.81 to 1.35), 0.15 |
| SCD | 27/445 (6.1) | 60/453 (13.2) | 0.55 (0.31 to 1.00), 0.049 |
| Non-SCD | 68/445 (15.3) | 39/453 (8.6) | 1.92 (1.29 to 2.84), 0.001 |
| Non-cardiac death | 21/445 (4.7) | 18/453 (4.0) | 1.23, 0.51 |
| Cumulative 1-year death rate, %b | 10.6 | 12.5 | |
| Cumulative 2-year death rate, %b | 15.4 | 18.2 | |
| Cumulative 3-year death rate, %b | 22.4 | 22.9 | |
| HRQoL | NR | NR | |
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | NR | NR | |
| Change in LVEF | NR | NR | |
| Exercise capacity outcomes (e.g. 6-minute walk distance, total exercise time, peak VO2) | NR | NR | |
Adverse effects of treatment
| Adverse effect | ICD (n = 445) | OPT (n = 453) | p-value |
|---|---|---|---|
| No. of ICDs actually implanted | 415 | 39 (median 7.6 months after randomisation) | |
| Inserted lead entangled in tricuspid valve, removed surgically | 1/415 patients | ||
| ICD explanted or permanently deactivated during follow-up (median 6.8 months after implantation) | 14/415 patients | ||
| Clinically significant complications requiring hospitalisation, surgical correction or intravenous drug administration | 65/415 (15.7%) patients, 76 complications | ||
| Up to 30 days after implantation | 19 (4.6%) patients | ||
| During follow-up | 48 (11.6%) patients | ||
| Lead-related problems requiring surgical revision (included in the above complications) | 10 patients (four had lead replacements) | ||
| Died within 30 days of implantation, n | 7 (MI 4, HF 3) | ||
| Died within 30 days of randomisation, n | 9 | 11 |
-
Allocation to treatment groups: randomisation by the data co-ordinating centre with risk stratification to ensure a balanced number of patients with ST elevation and non-ST elevation MI between the ICD and control groups within these strata. 98 No further details on allocation.
-
Blinding: an adverse event committee unaware of treatment assignment classified deaths. An independent data co-ordinating centre undertook unblinding, data collection and statistical analysis.
-
Comparability of treatment groups: comparable for most characteristics.
-
Method of data analysis: primary analysis was ITT including all randomised patients with written informed consent obtained. Conducted by an independent data co-ordinating centre and independently repeated by one of the authors. Subdistribution hazard analyses performed using R software. Baseline comparisons were carried out using Fisher’s exact tests, chi-squared tests or Wilcoxon tests as appropriate. Cumulative risks of death estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Cumulative mortality by year and annual rates calculated using an inverse Kaplan–Meier analysis. Calculation of HRs and subgroup analysis performed on the basis of Cox proportional hazards models. Proportional hazards assumption tested on the basis of Schoenfeld residuals. Subgroup analyses (13 prespecified and one post hoc added for the effect of amiodarone) were performed one by one, with use of a corresponding interaction test for comparison of the treatment effect between subgroups. Causes of death were analysed on the basis of proportional subdistribution hazard models (as causes of death represent competing risks).
-
Sample size/power calculation: the 2-year survival rate was assumed to be 70.6% for the medical therapy group and 79.4% for the ICD group (RRR approximately 30% in the ICD group). Assumed two-sided alpha error of 5%, beta error of 20%, 30-month recruitment period and 2-year minimum follow-up. With a loss to follow-up of 1% per year and accounting for group sequential design the number of patients required in each group was 350. Recruitment time was more than doubled because the percentage of screened patients excluded was unexpectedly high. In December 2005 the data and safety monitoring board, because of lower than anticipated mortality, recommended increasing the required number of patients to 900 and extending follow-up until the last patient had been in the study for 1 year.
-
Attrition/dropout: 415/445 ICD group patients actually received an ICD: 14 withdrew consent; 11 refused ICD implantation; five died before implantation could take place. ICDs were removed in 15 patients and 39 in the OPT group were given ICDs.
-
Other: to increase recruitment two modifications to the protocol were made: (1) non-ST elevation MI included from June 2002; (2) qualifying heart rate on the first ECG was reduced from 100 bpm to 90 bpm from October 2004.
-
Generalisability: people within 31 days of a MI.
-
Outcome measures: appear appropriate.
-
Intercentre variability: not reported.
-
Conflict of interests: sponsors were informed of the trial outcome after the evaluation had been completed. Sponsors had an opportunity to review and provide comments on the predefined final analysis plan and the manuscript but did not have a role in study design, data analysis or the interpretation of results.
bpm, beats per minute.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Details not reported |
| Allocation concealment | Low | Randomisation by data co-ordinating centre |
| Performance bias | ||
| Blinding of participants and personnel | High | No blinding |
| Detection bias | ||
| Blinding of outcome assessment | Low | No blinding but outcomes not likely to be influenced (deaths classified by blinded committee) |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Primary analysis according to the ITT principle |
| Reporting bias | ||
| Selective reporting | High | Protocol paper98 indicates that the SF-36 will be used to determine QoL but this outcome is not reported |
| Other bias | ||
| Other sources of bias | Low | |
Multicenter Automatic Defibrillator Implantation Trial (MADIT) I
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Moss et al. 1996,99 MADIT Executive Committee 1991100 Study design: RCT Countries: USA and Europe No. of centres: 32 (USA 30, Europe 2) Funding: research grant from CPI/Guidant Corporation, St Paul, MN (also donated ICDs)100 |
Intervention: ICD + medical therapy. Pulse generators (monophasic n = 79; biphasic n = 11) and lead systems supplied by CPI/Guidant Corporation. Non-thoracotomy transvenous leads included in 1993. Late in the trial, a small number of patients had pulse generators with ECG storage implanted (number not reported). Defibrillators were implanted using standard techniques and testing was carried out during the implantation procedure (endeavoured to achieve defibrillation within a 10-J safety margin) Comparator: conventional medical therapy. Attending physician elected medical therapy and use of FDA-approved AADs in both groups Other interventions used: none reported |
Indication for treatment: previous MI and left ventricular dysfunction No. of randomised participants: 196; ICD: 95 (transthoracic stratum 45, transvenous stratum 50), OPT: 101 (transthoracic stratum 53, transvenous stratum 48). Total transthoracic stratum: 98, total transvenous stratum: 98 Crossovers: 16; ICD: 5 (no ICD fitted), deactivated ICD: 2, OPT: 11 (ICD fitted) Loss to follow up: ICD: 1, OPT: 2 Inclusion criteria: age 25–80 years, NYHA class I, II or III, LVEF ≤ 0.35, Q-wave or enzyme-positive MI > 3 weeks before entry, a documented episode of asymptomatic, unsustained VT (run of 3–30 ventricular ectopic beats at a rate > 120 bpm) unrelated to an acute MI, no indications for CABG surgery or coronary angioplasty within the past 3 months, sustained VT or VF reproducibly induced and not suppressed after the intravenous administration of procainamide (or equivalent) Exclusion criteria: previous cardiac arrest or VT causing syncope not associated with an acute MI, symptomatic hypotension while in a stable rhythm, MI within the past 3 weeks, CABG surgery within the past 2 months or coronary angioplasty within the past 3 months, non-contraceptive-taking women of childbearing age, advanced cerebrovascular disease, any condition other than cardiac disease associated with a reduced likelihood of survival for the duration of the trial, participating in other clinical trials |
Primary outcome: death from all causes Secondary outcomes: none specified Other outcomes reported: prevalence of medications, adverse events, impact of 11 preselected baseline characteristics and medication type on observed HR for overall mortality Method of assessing outcomes: causes of death: categorised as either cardiac or non-cardiac (Hinkle and Thaler classification, reference provided) by two people reviewing information on deaths on or before 24 March 1996. Cardiac causes further categorised into arrhythmic, non-arrhythmic or uncertain Follow-up visits: clinical evaluation, recorded use of medication, test of defibrillator; 1 month after randomisation, thereafter 3-monthly until trial was stopped. Final evaluation 1 month after end of trial Length of follow-up: < 1 month to 61 months (average 27 months). Average 37 months for earlier transthoracic stratum (n = 98), 16 months for later transvenous stratum (n = 98) Recruitment: 27 December 1990 |
Participant characteristics
| Characteristic | ICD (n = 95) | OPT (n = 101) | p-value |
|---|---|---|---|
| Age (years), mean (SD)a | 62 (9) | 64 (9) | NR |
| Sex, % male/femalea | 92/8 | 92/8 | NR |
| Ethnicity | NR | NR | |
| NYHA class II or III, %a | 63 | 67 | NR |
| Cardiac findings at enrolment, % | |||
| Pulmonary congestion (defined radiographically as mild, moderate or severe) | 18 | 20 | NR |
| Blood urea nitrogen > 25 mg/dl (8.92 mmol/l)a | 22 | 21 | NR |
| Cholesterol > 200 mg/dl (5.17 mmol/l) | 41 | 49 | NR |
| LBBB, %a | 7 | 8 | NR |
| LVEF, mean (SD)a | 0.27 (0.07) | 0.25 (0.07) | NR |
| Qualifying unsustained VT (no. of consecutive beats), mean (SD) | 10 (9) | 9 (10) | NR |
| Electrophysiology – initial induction, % | |||
| Monomorphic VT | 87 | 91 | NR |
| Polymorphic VT | 7 | 7 | NR |
| VF | 6 | 2 | NR |
| Electrophysiology – induction after antiarrhythmic challenge, % | |||
| Monomorphic VT | 92 | 94 | NR |
| Polymorphic VT | 7 | 5 | NR |
| VF | 1 | 1 | NR |
| Cardiac history, % | |||
| Two or more previous MIsa | 34 | 29 | NR |
| Treatment for ventricular arrhythmias | 42 | 35 | NR |
| Treatment for CHFa | 52 | 51 | NR |
| Treatment for hypertensiona | 48 | 35 | NR |
| CABG surgerya | 46 | 44 | NR |
| Coronary angioplasty | 17 | 27 | NR |
| Implanted pacemaker | 2 | 7 | NR |
| Interval of ≥ 6 months between most recent MI and enrolmenta | 75 | 76 | NR |
| Insulin-dependent diabetic, % | 7 | 5 | NR |
| Cigarette smoking (any time), % | 79 | 73 | NR |
Results
| Outcome | ICD (n = 95) | OPT (n = 101) | HR (95% CI), p-value |
|---|---|---|---|
| Mortality: cause of death, n | |||
| Cardiac cause | 11 | 27 | NR |
| Primary arrhythmia | 3 | 13 | NR |
| Non-arrhythmia | 7 | 13 | NR |
| Uncertain | 1 | 1 | NR |
| Non-cardiac cause | 4 | 6 | NR |
| Unknown cause | 0 | 6 | NR |
| Total | 15 | 39 | 0.46 (0.26 to 0.82), 0.009 |
Cardiac medication
| Medication | 1 montha | Last contactb | p-value | ||
|---|---|---|---|---|---|
| ICD (n = 93) | OPT (n = 93) | ICD (n = 86) | OPT (n = 82) | ||
| AADs, % | |||||
| Amiodarone | 2 | 74 | 7 | 45 | NR |
| Beta-blockers | 26 | 8 | 27 | 5 | NR |
| Class I antiarrhythmic agents | 12 | 10 | 11 | 11 | NR |
| Sotalol | 1 | 7 | 4 | 9 | NR |
| Beta-blockers or sotalol | 27 | 15 | 31 | 14 | NR |
| No antiarrhythmic medication | 56 | 8 | 44 | 23 | NR |
| Other cardiac medication, % | |||||
| ACE inhibitors | 60 | 55 | 57 | 51 | NR |
| Digitalis | 58 | 38 | 57 | 30 | NR |
| Diuretics | 53 | 52 | 52 | 47 | NR |
Adverse effects of treatment
| Adverse effect | ICD (n = 95)a | OPT (n = 101)a | p-value |
|---|---|---|---|
| Operative deaths in the first 30 days, n | 0 | 0 | |
| Hypotension, n | 0 | 1 | |
| Syncope, n | 1 | 5 | |
| Hypothyroidism, n | 0 | 1 | |
| Sinus bradycardia, n | 3 | 3 | |
| Pulmonary fibrosis, n | 0 | 3 | |
| Pulmonary embolism, n | 1 | 1 | |
| Atrial fibrillation, n | 4 | 0 | |
| Pneumothorax, n | 2 | 0 | |
| Bleeding, n | 1 | 0 | |
| Venous thrombosis, n | 1 | 0 | |
| Surgical infection, n | 2 | 0 | |
| Problems with defibrillator lead, n | 7 | 0 | |
| Malfunction of defibrillator generator, n | 3 | 2 | |
| Total no. of patients with adverse events | 19 | 12 |
-
Allocation to treatment groups: random assignment of eligible patients to either the ICD group or the OPT group within 30 days of completing the qualifying electrophysiological study. The randomisation scheme included stratification according to centre and the interval between the most recent MI and enrolment (< 6 months or ≥ 6 months). The random assignment was made by the co-ordinating centre and transmitted to the enrolling clinical centre by telephone (hard copy followed). 100 After March 1993 and once non-thoracotomy transvenous leads were approved at a centre, a new stratum consisting of patients assigned to transvenous ICD or OPT was initiated.
-
Blinding: the executive committee was unaware of the results of the study throughout the trial and revised the sequential design during the trial on two occasions.
-
Comparability of treatment groups: baseline characteristics between the two treatment groups described as similar (no statistical testing reported).
-
Method of data analysis: a triangular sequential design, modified for two-sided alternatives, was used with preset boundaries to permit termination of the trial if the efficacy or inefficacy of ICDs was established, or if there was evidence that there was no difference in outcome between the ICD group and the OPT group. Weekly data analysis was used, starting at the point at which 10 deaths had been reported. The trial was designed to be terminated when the path of the log-rank statistic, measuring imbalance between the survival curves for the two groups, crossed one of the preset termination boundaries (efficacy, inefficacy or no difference in outcome) of the sequential design. Because of the slow rate of enrolment from 12 November 1995 (before first enrolled patient had reached the fifth year of the study), patient data were censored for analytical purposes at 5 years, with subsequent follow-up information on such patients censored from the ongoing sequential analysis. Analyses were stratified according to the type of device (transthoracic or transvenous) and followed the ITT principle. All analyses and potential covariates were prespecified. After termination of the trial, sequential analysis methods were used to calculate a p-value and HR (median unbiased), along with a 95% CI based on the p-value function. Secondary analyses were performed with the Cox proportional hazards regression model, adjusted for relevant covariates. Separate Cox regression analyses were carried out in the transthoracic and transvenous strata, to determine whether the efficacy of defibrillators was similar in these two groups. Preselected baseline covariates and prescribed cardiac medications recorded at the 1-month clinic visit were evaluated in the Cox model to determine their effect on the risk of death per unit of time in the ICD group compared with that in the OPT group (the HR). Survival curves for patients assigned to ICD treatment and OPT treatment were determined according to the method of Kaplan and Meier (reference cited). However, a note in the text states that the HR, derived from the sequential design, takes into account the sequential stopping rule, but was not adjusted for covariates.
-
Sample size/power calculation: the trial was designed to have an 85% power to detect a 46% reduction in mortality rate among ICD patients compared with a postulated 2-year mortality rate of 30% among the patients randomly assigned to OPT, with a two-sided significance level of 0.05. After the introduction of transvenous leads (1 September 1993), the power requirement of the trial was increased from 85% to 90% so ‘as not to compromise the credibility of the study’.
-
Attrition/dropout: numbers lost to follow-up reported (ICD n = 1; OPT n = 2). Percentage of patients who completed the 1838 scheduled follow-up clinic visits was 92% for the ICD group and 86% for the OPT group. There were 16crossovers, 11 in the OPT group [adverse drug reaction n = 2, unexplained syncope n = 2, investigator concern about episodes of ventricular tachyarrhythmia n = 6 and aborted cardiac arrest (VF) n = 1] and five in the ICD group (high defibrillation threshold n = 1 and patient preference n = 4). Two patients had their defibrillators deactivated during the course of the trial.
-
Generalisability: authors acknowledge that the change to transvenous leads altered the type of patient referred for entry into the trial. Generalisability is limited to high-risk patients with CHD and left ventricular dysfunction, spontaneous asymptomatic unsustained VT and inducible and non-suppressible ventricular tachyarrhythmia on electrophysiological testing.
-
Outcome measures: appear appropriate although unclear if all ITT (cardiac medication).
-
Intercentre variability: not reported. However, an evaluation of the consistency of the beneficial effect of ICDs in eachof the two centres with the highest enrolment (n = 42 and n = 21) and comparison of the results in the high-enrolment centres with the results in the 30 low-enrolment centres (total n = 133) showed that the reduction in mortality with ICDs is similar among these groups (no statistical testing reported).
-
Conflict of interests: states that all investigators agreed in writing not to hold stock in CPI/Guidant Corporation or any other defibrillator-manufacturing company before study participation and to abide by the conflict of interest standards (reference cited).
-
Other: study officially stopped when the efficacy boundary of the sequential design was crossed (when 51 deaths were reported).
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | No details of randomisation procedure in either trial paper99 or protocol.100 Patients were ‘randomly assigned’ by clinical centre and chronology of the interval after a previous MI100 |
| Allocation concealment | Low | Random assignment provided to centres by telephone before receiving hard copy100 |
| Performance bias | ||
| Blinding of participants and personnel | High | Unblinded trial |
| Detection bias | ||
| Blinding of outcome assessment | Low | A two-member end point subcommittee independently reviewed information on the causes and circumstances of deaths and categorised them, but does not state blinded to allocation.99,100 Mortality unlikely to be influenced by lack of blinding |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Analyses ‘followed the ITT principle’. For the purpose of analysis, patients were not withdrawn from the trial and every effort was made to ascertain the occurrence or non-occurrence of the primary end point.100 Although not a primary outcome, it is unclear how missing data for type of medication (n = 10) were dealt with in the analysis |
| Reporting bias | ||
| Selective reporting | Low | Described outcomes reported. Protocol published100 |
| Other bias | ||
| Other sources of bias | Low | |
Multicenter Automatic Defibrillator Implantation Trial (MADIT) II
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Moss et al. 1999102 and 2002,101 Greenberg et al. 2004,103 Noyes et al. 2007104 Study design: RCT Countries: USA and Europe No. of centres: 76 (USA 71, Europe 5) Funding: research grant from Guidant Corporation, St Paul, MN, to the University of Rochester School of Medicine and Dentistry, NY |
Intervention: ICD + conventional medial therapy. Transvenous defibrillator systems (Guidant Corporation) and standard defibrillator implant techniques were used. ICD programming and prescribing medications were at the discretion of the patients’ physicians Comparator: conventional medical therapy (OPT). The appropriate use of beta-blockers, ACE inhibitors and lipid-lowering drugs was strongly encouraged in both study groups Other interventions used: none reported |
Indication for treatment: high-risk cardiac patients with previous MI and advanced left ventricular dysfunction No. of randomised participants: 1232; ICD: 742, OPT: 490 Crossovers: 54; ICD: 32 [no ICD fitted: 21 (2.8%); ICD removed: 11 (1.5%) (nine heart transplants)]; deactivated ICD: 12 (usually because of terminal illness); OPT: 22 (4.5%) ICD fitted Loss to follow-up: ICD: 2, OPT: 1 Inclusion criteria: age > 21 years, LVEF ≤ 0.30 in last 3 months (assessed by angiography, radionuclide scanning or ECG), MI > 1 month before study entry (documented by an abnormal Q wave on ECG, elevated cardiac enzyme levels on laboratory testing during hospitalisation for suspected MI, a fixed defect on thallium scanning or localised akinesis on ventriculography with evidence of obstructive coronary disease on angiography), frequent or repetitive ventricular ectopic beats during 24-hour Holter monitoring from July 1997 until 1 January 1998 (discontinued as majority of cases had such arrhythmias) Exclusion criteria: indication approved by the FDA for an ICD (and patients who met the MADIT I criteria for an ICD102), NYHA class IV at enrolment, undergone coronary revascularisation within the last 3 months, MI within the past month (evidenced by measurement of cardiac enzyme levels), advanced cerebrovascular disease, women of childbearing age not using medically prescribed contraception, any condition other than cardiac disease that was associated with a high likelihood of death during the trial, not willing to sign the consent form |
Primary outcome: all-cause mortality Secondary outcomes: adverse events, HRQoL, economic outcomes, incidence of SCD, incidence of cardiac death from progressive left ventricular failure Method of assessing outcomes: patients followed up 1 month post randomisation and at 3-monthly intervals. Causes of death were assessed using a modified version of the Hinkle–Thaler system (see General comments) Cause of death definitions:103 SCD (modified Hinkle–Thaler system): (1) Died suddenly and unexpectedly within 1 hour of cardiac symptoms in the absence of progressive cardiac deterioration; (2) died unexpectedly in bed during sleep; (3) died unexpectedly within 24 hours of last being seen alive. SCD subclassified into those with and those without symptoms of severe left ventricular dysfunction NYHA ≥ III HF Non-SCD: patients who died of progressive cardiac failure or patients who did not meet the time criteria for sudden death Progressive cardiac failure: unstable clinical progression of deteriorating pump function in the setting of active therapy, most often in an intensive care setting (patients with advanced HF in whom death was not anticipated as imminent were categorised as sudden death if their terminal event met the time criteria) SCD (clinical classification): Death within 1 hour of symptom onset – primary (without preceding symptoms) or secondary (complaint of chest pain during the hour before death). Marked ECG changes indicative of active MI were absent in any of the reviewed records Multiple cause category: presence of several medical problems in which CHD contributed to, but was not the dominant feature of, the mortality event HRQoL:104 HUI3 self-administered during face-to-face study visits at baseline and 3, 12, 24 and 36 months. Patients could complete the HUI3 at home and mail it back. HUI3 has eight attributes (vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain discomfort; –0.0371 = worse possible state, 0 = death, 1 = best possible health state) Length of follow-up: average 20 months (range 6 days to 53 months); HUI3: up to 36 months104 Recruitment: 11 July 1997–20 November 2001 |
Participant characteristics
| Characteristic | ICD (n = 742) | OPT (n = 490) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 64 (10) | 65 (10) | NR |
| Sex, % male/female | 84/16 | 85/15 | NR |
| Ethnicity | NR | NR | |
| Diagnosis | NR | NR | |
| NYHA functional class, %a | |||
| I | 35 | 39 | NR |
| II | 35 | 34 | NR |
| III | 25 | 23 | NR |
| IV | 5 | 4 | NR |
| LVEF (%), mean (SD) | 23 (5) | 23 (6) | NR |
| Heart rate | NR | NR | |
| Blood urea nitrogen > 25 mg/dl (8.92 mmol/l), % | 29 | 32 | NR |
| Atrial fibrillation, n | 9 | 8 | NR |
| QRS interval ≥ 120 milliseconds, n | 50 | 51 | NR |
| Non-specific conduction defect, n | 22 | 26 | NR |
| RBBB, n | 9 | 7 | NR |
| LBBB, n | 19 | 18 | NR |
| Medication at last contact, %b | |||
| Amiodarone | 13 | 10 | NR |
| ACE inhibitors | 68 | 72 | NR |
| Beta-blockers | 70 | 70 | NR |
| Calcium channel blockers | 9 | 9 | NR |
| Class I antiarrhythmic agents | 3 | 2 | NR |
| Digitalis | 57 | 57 | NR |
| Diuretics | 72 | 81 | NR |
| Lipid-lowering statin drugs | 67 | 64 | NR |
| Cardiac history | |||
| Interval of > 6 months between most recent MI and enrolment, % | 88 | 87 | NR |
| Previous treatment, % | |||
| Hypertension | 53 | 53 | NR |
| CABG surgery | 58 | 56 | NR |
| Coronary angioplasty | 45 | 42 | NR |
| Diabetes, % | 33 | 38 | NR |
| Current or former cigarette smoker, % | 80 | 82 | NR |
Baseline characteristics by subgroup103
| Characteristic | ICD | OPT | p-value | ||
|---|---|---|---|---|---|
| Alive (n = 637) | Dead (n = 105) | Alive (n = 393) | Dead (n = 97) | ||
| Age (years), mean (SD) | 64 (11) | 69 (9)a | 64 (10) | 68 (10)a | |
| Sex, % male | 84 | 82 | 86 | 84 | |
| NYHA functional class, %b | |||||
| I | 36 | 27a | 41 | 29a | |
| II | 37 | 27a | 36 | 27a | |
| III | 27 | 46a | 23 | 44a | |
| LVEF (%), mean (SD) | 23 (5) | 22 (6)a | 24 (5) | 23 (6)a | |
| Blood urea nitrogen > 25 mg/dl (8.92 mmol/l), % | 25 | 51a | 28 | 49a | |
| Atrial fibrillation, n | 8 | 12 | 7 | 16a | |
| QRS interval ≥ 12 seconds, n | 49 | 57 | 49 | 59 | |
| RBBB, n | 9 | 7 | 7 | 8 | |
| LBBB, n | 19 | 28 | 16 | 27 | |
| Previous treatment, % | |||||
| Hypertension | 53 | 54 | 53 | 55 | |
| CABG surgery | 58 | 59 | 56 | 56 | |
| Coronary angioplasty | 47 | 36 | 45 | 31 | |
| Cardiac history, % | |||||
| Interval of > 6 month between most recent MI and enrolment, % | 88 | 87 | 87 | 89 | |
| Diabetes, % | 32 | 34 | 36 | 43 | |
| Cardiac morbidity after enrolment | |||||
| Hospitalisation for HF, n | 20 | 60a | 15 | 41a | |
| MI, n | 4 | 20a | 4 | 15a | |
| Coronary revascularisation, n | 5 | 6 | 4 | 6 | |
Baseline health-related quality of life104
| HRQoL measure | ICD (n = 658) | OPT (n = 431) | p-value |
|---|---|---|---|
| HUI3 score, mean | 0.637 | 0.646 | > 0.10 |
| SF-12 PCS, mean | 36.293 | 36.444 | > 0.10 |
| SF-12 MCS, mean | 50.505 | 50.419 | > 0.10 |
| Hospitalised at baseline (%), mean | 14.7 | 10.9 | > 0.10 |
Results
| Outcome | ICD (n = 742) | OPT (n = 490) | HR (95% CI), p-value |
|---|---|---|---|
| Primary outcome: mortality, no. of deaths (%) | 105 (14.2) | 97 (19.8) | 0.69 (0.51 to 0.93), 0.016a 31% reduction in risk of death at any interval for ICD compared with OPT |
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | NR | NR | |
| Change in LVEF | NR | NR | |
| Exercise capacity outcomes (e.g. 6-minute walk distance, total exercise time, peak VO2) | NR | NR |
Subgroup analyses103
| Outcome | ICD (n = 105) | OPT (n = 97) | p-value |
|---|---|---|---|
| Cause of death by treatment group (modified Hinkle–Thaler scheme), n (%) | |||
| Cardiac death | |||
| SCD | 28 (27) | 49 (51) | p < 0.01 |
| Without severe left ventricular dysfunction | 18 | 34 | |
| With severe left ventricular dysfunction | 10 | 15 | |
| Non-SCD | 43 (41) | 21 (22) | p < 0.01 |
| Unclassified cardiac death | 8 (8) | 10 (10) | |
| Total cardiac death | 79 | 80 | |
| Non-cardiac death/non-coronary death | 22 (21) | 12 (12) | |
| Unknown/unclassified | 4 (4) | 5 (5) | |
| Nominal death rates, % (n/N) | |||
| Cardiac death rate | 10.6 (79/742) | 16.3 (80/490) | p < 0.01 |
| SCD rate | 3.8 (28/742) | 10.0 (49/490) | |
| Non-SCD rate | 5.8 (43/742) | 4.3 (21/490) | |
| Total all-cause mortality | 14.2 (105/742) | 19.8 (97/490) | |
| Clinical classification scheme, cause of death: cardiac death, n (%) | |||
| SCD | 24 (23) | 48 (49) | p < 0.01 |
| Primary arrhythmia (without preceding symptoms) | 22 | 41 | |
| Secondary arrhythmia (with chest pain symptoms) | 2 | 7 | |
| Primary mechanical | 40 (38) | 19 (20) | |
| Cardiac procedure | 1 | 1 | |
| Multiple causes | 8 (8) | 3 (3) | |
| Non-cardiac/non-coronary death | 22 (21) | 12 (12) | |
| Unknown/unclassified death | 10 (10) | 14 (10) | |
| Nominal death rate: cardiac rates, % (n/N) | |||
| Cardiac death | 9.8 (73/742) | 14.5 (71/490) | p < 0.01 |
| SCD | 3.2 (24/742) | 9.8 (48/490) | p < 0.01 |
| Primary mechanical cardiac death | 5.4 (40/742) | 3.9 (19/490) | |
| Total all-cause mortality | 14.2 (105/742) | 19.8 (97/490) | p < 0.01 |
| Nominal death rate out of hospitala | 3.8 (28/742) | 9.6 (47/490) | p < 0.01 |
| Nominal death rate in hospital | 5.7 (42/742) | 4.5 (22/490) | |
Health-related quality of life
| HUI3 scores while alive | ||||||||
|---|---|---|---|---|---|---|---|---|
| ICD (n = 658) | OPT (n = 431) | |||||||
| 0 | Year 1 | Year 2 | Year 3 | 0 | Year 1 | Year 2 | Year 3 | |
| Proportion alive | 0.93 | 0.846 | 0.767 | 0.903 | 0.792 | 0.667 | ||
| Mean score | 0.637 | 0.627 | 0.622 | 0.601 | 0.646 | 0.659 | 0.667 | 0.678 |
| Mean annual changea | –0.019 | –0.027b | –0.019c | –0.012 | –0.011 | –0.013 | ||
| Overall mean score including deathd | 0.637 | 0.584 | 0.526 | 0.461 | 0.646 | 0.595 | 0.529 | 0.452 |
Adverse effects of treatment
| Adverse effect | ICD (n = 742) | OPT (n = 490) | p-value |
|---|---|---|---|
| Death during implantation, n | 0 | ||
| Lead problems, n (%) | 13 (1.8) | ||
| Non-fatal infections requiring surgical intervention, n (%) | 5 (0.7) | ||
| Hospitalisation because of HF, n (%) | 148 (19.9) | 73 (14.9) | |
| Patients hospitalised per 1000 months of active follow-up | 11.3 | 9.4 | p = 0.09 |
| Adverse cardiac events in the week before SCD,103 (%) | (n = 28) | (n = 49) | |
| Syncope | 4 | 4 | |
| Angina pectoris | 4 | 4 | |
| MI | 4 | 10 | |
| Ventricular arrhythmia | 25 | 10 | |
| CHF | 43 | 16 |
-
Allocation to treatment groups: patients were randomly assigned by the co-ordinating centre in a 3 : 2 ratio to receive an ICD (60.2%) or OPT (39.8%) stratified by clinical centre.
-
Blinding: none reported. Authors state that information will be reported periodically to the independent safety monitoring subcommittee but kept confidential from investigators, executive committee and sponsors.
-
Comparability of treatment groups: authors state that baseline characteristics and prevalence of the use of various cardiac mediations at the time of the last follow-up visit were similar between the two groups but no p-values are reported.
-
Method of data analysis: analysis was performed according to the ITT principle. A triangular sequential design modified for two-sided alternatives and corrected for the lag in obtaining data accrued but not reported before the termination of the trial, for weekly monitoring, with preset boundaries to permit termination of the trial if ICD was found to be superior to, inferior to or equal to OPT was used. Secondary analyses were performed with the use of the Cox proportional hazards regression model. Survival curves were determined according to the Kaplan–Meier method, with comparisons of cumulative mortality based on logarithmic transformation. P-values were termed nominal when not adjusted for sequential monitoring. All p-values were two-tailed. Analyses used version 2.0 of the trial database, released on 16 January 2002. The trial was stopped on 20 November 2001 after analysis revealed that the difference in mortality between both groups had reached the prespecified efficacy boundary (p = 0.027). Subgroups were prespecified. Mortality events103 were based on version 3.0 of the database (released 26 July 2002), chi-squared statistics were used for categorical data, t-test were used for continuous variables (independent samples), the Kaplan–Meier method was used for cumulative survival curves and the log-rank method was used for statistical comparison of cumulative mortality. The Cox proportional hazards regression model was used to calculate the risk of SCD and non-SCD in the total population and in subgroups stratified by relevant baseline characteristics for patients randomised to ICD compared with OPT. Missing HUI3 scores104 were imputed using a multivariate fixed-effects model, regressing the difference between baseline score and a score for each subsequent visit on time, treatment, sex, age, death during the trial, death within 6 months of the HRQoL assessment, sudden death within 6 months of the HRQoL assessment, presence of diabetes, use of diuretics and having NYHA class II–IV symptoms.
-
Sample size/power calculation: the trial was designed to have 95% power to detect a 38% reduction in the 2-year mortality rate in the ICD group, given a postulated 2-year mortality rate of 19% among the OPT group with a two-sided significance level of 0.05. For proportional hazards modelling, power was maintained for a true HR of 0.63 after allowance for crossover. Originally it was estimated that 1200 patients (720 ICDs and 480 OPT) were needed. On 4 May 2001, the executive committee increased the enrolment goal to 1500 patients so that enrolment would be ongoing while data on outcomes were still accruing.
-
Attrition/dropout: the percentage of patients who completed the 8749 scheduled follow-up clinic visits was 97% for the ICD group and 94% for the OPT group [authors state that the status of three patients (two ICD, one OPT) at the termination of the trial is unknown]. Reasons for dropout not reported. HRQoL assessed in the European study centres (n = 109). Patients with missing data at baseline (n = 22) were excluded, as were patients with poor data quality (n = 12). Questionnaires returned after trial termination were also excluded (n = 8), but this number appears to have been accounted for as part of the number of patients with poor-quality data. In total, 8.5% of the HRQoL data were missing and summary reasons were provided.
-
Generalisability: limited to high-risk cardiac patients with a previous MI and advanced left ventricular dysfunction.
-
Outcome measures: appear appropriate.
-
Intercentre variability: not reported.
-
Conflict of interests: supported by a research grant from Guidant Corporation, St Paul, MN. Drs Cannom, Daubert and Higgins have given lectures sponsored by the grant provider (Guidant Corporation). Authors state that all investigators agreed to abide by the conflict-of-interest guidelines and that investigators had full access to the data and performed the analysis with no limitations imposed by the sponsor.
-
Other: ICD patients were not responsible for the incurred costs of the ICD, implantation or hospitalisation for the procedure.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Patients randomly assigned but no details of procedure |
| Allocation concealment | Unclear | Not reported |
| Performance bias | ||
| Blinding of participants and personnel | High | No blinding reported |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality | Low | No blinding reported. Data were independently reviewed but the committee was not blinded.103 Mortality unlikely to be influenced by lack of blinding |
| QoL | High | |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Analysis was performed according to the ITT principle. Missing HUI3 scores were imputed using a multivariate fixed-effects model (see Methodological comments) |
| Reporting bias | ||
| Selective reporting | Unclear | Apart from the primary end point, the protocol paper specifies only four secondary objectives (association of induced VT with ICD discharge rate; patients at risk of increased mortality according to prespecified Holter-recorded electrocardiological parameters at baseline; cost-effectiveness of ICDs; QoL) |
| Other bias | ||
| Other sources of bias | Low | No costs in relation to ICDs were incurred by patients |
Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Bardy et al. 2005,105 Packer et al. 2009,108 Michell et al. 2008,106 Mark et al. 2008107 Study design: RCT Countries: USA (99%107), Canada and New Zealand106 No. of centres: 148106 Funding: grants from the National Heart, Lung, and Blood Institute, the National Institutes of Health and Medtronic, Wyeth-Ayerst Laboratories and Knoll Pharmaceuticals |
Group 1: ICDs. Single chamber ICD (Medtronic, model 7223) programmed to shock only mode (to treat only rapid sustained VT or VF). Detection rate of ≥ 187 bpm. Antitachycardia pacing therapies not permitted Group 2: amiodarone. Dose partly based on weight. Loading dose of 800 mg daily for 1 week and then 400 mg daily for 3 weeks. Then, patients > 200 lb (90.9 kg) received 400 mg daily, patients 150–200 lb (68.2–90.9 kg) received 300 mg daily and patients < 150 lb (68.2 kg) received 200 mg daily. If a patient had bradycardia the loading or maintenance dose could be lowered Group 3: placebo, administered in the same way as amiodarone Other interventions used: all participants received optimal HF medical therapy.108 If clinically reasonable, all patients were required to receive treatment with a beta-blocker and an ACE inhibitor. When appropriate, participants received aldosterone, aspirin and statins105 |
Indication for treatment: Broad population of patients with mild to moderate HF No. of randomised participants: 2521; ICD: 829, amiodarone: 845, placebo:847 Inclusion criteria: NYHA class II or III chronic stable CHF from ischaemic or non-ischaemic causes, LVEF ≤ 35%, ≥ 18 years. Ischaemic CHF defined as LVSD associated with ≥ 75% narrowing of at least one of three major coronary arteries (marked stenosis) or a documented history of MI. Non-ischaemic CHF defined as LVSD without marked stenosis Exclusion criteria: None stated |
Primary outcomes: death from any cause. For the QoL study, the DASI and the SF-36 MHI-5 Secondary outcomes: other scales from the SF-36, number of ‘bed-days’ and ‘disability-days’, MLWHFQ, health status utility, global health status Method of assessing outcomes: every 3 months with alternating clinic visits and telephone calls. Data downloaded from ICD memory regularly at visits. Deaths were classified by an events committee. Cardiac deaths were subclassified as sudden death (VT, bradyarrhythmic, HF related, other cardiac causes). Non-cardiac deaths included stroke, peripheral arterial embolism, pulmonary embolism, aneurysm rupture, acute haemorrhage and non-vascular events (e.g. serious lung, liver, kidney or other organ failure, cancer and sepsis)108 QoL:107 measured by structured interviews at baseline (before randomisation) and at months 3, 12 and 30 (or at the end of study follow-up). Interviews at the time of scheduled clinic visit or by telephone if visit was missed. A short proxy form was used if patients were too ill, had a language barrier or were otherwise unable to participate in a full interview. The DASI reflects cardiac-specific physical functioning (score 0–58, higher scores indicate better function, a difference of ≥ 4 points is considered clinically significant). The SF-36 MHI-5 reflects psychological well-being (score 0–100, higher scores indicate better function). A clinically significant difference was approximated as one-quarter of 1 SD (5 points in this study). Other SF-36 scales were scored the same way ‘Bed-days’ were defined as the number of days spent in bed all or most of the day in the last 42 days. ‘Disability-days’ were defined as the number of days (excluding bed-days) that the patient cut down usual activities for health reasons MLWHFQ was scored from 0 to 105 (higher scores indicate worse function, clinically significant difference approximately 5 points) Health status utility [0 (dead)–1 (excellent)] was assessed using the time trade-off technique Global health was rated on a scale of 0 (dead)–100 (excellent health) with a 5-point difference (one-quarter of 1 SD) approximating clinical significance Length of follow-up: to 31 October 2003. Median follow-up for surviving patients 45.5 (range 24–72.6) months Recruitment: September 1997–July 2001 |
Participant characteristics
| Characteristic | ICD (n = 829) | Amiodarone (n = 845) | Placebo (n = 847) | p-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 60.1 (51.9 to 69.2) | 60.4 (51.7 to 68.3) | 59.7 (51.2 to 67.8) | |
| Sex, male, n (%)a | 639 (77) | 639 (76) | 655 (77) | |
| Non-white race, n (%) | 189 (23) | 196 (23) | 204 (24) | |
| LVEF (%), median (IQR) | 24.0 (19.0–30.0) | 25.0 (20.0–30.0) | 25.0 (20.0–30.0) | |
| Heart rate (bpm), median (IQR) | 74 (65 to 84) | 72 (64 to 82) | 73 (64 to 84) | |
| NSVT,b n (%) | 210 (25) | 193 (23) | 180 (21) | |
| Syncope, n (%) | 52 (6) | 54 (6) | 56 (7) | |
| Systolic blood pressure (mmHg), median (IQR) | 118 (104–131) | 118 (106–130) | 120 (108–132) | |
| Diastolic blood pressure (mmHg), median (IQR) | 70 (61–80) | 70 (62–80) | 70 (62–80) | |
| Medication use at enrolment, n (%) | ||||
| ACE inhibitor | 684 (83) | 731 (87) | 718 (85) | |
| ARB | 114 (14) | 118 (14) | 132 (16) | |
| ACE inhibitor or ARB | 783 (94) | 822 (97) | 827 (98) | |
| Beta-blocker | 576 (69) | 581 (69) | 581 (69) | |
| Diuretic | ||||
| Loop | 676 (82) | 696 (82) | 692 (82) | |
| Potassium sparing | 168 (20) | 174 (21) | 165 (19) | |
| Thiazide | 63 (8) | 52 (6) | 60 (7) | |
| Digoxin | 552 (67) | 614 (73) | 589 (70) | |
| Acetylsalicylic acid (aspirin) | 477 (58) | 461 (55) | 477 (56) | |
| Warfarin | 266 (32) | 310 (37) | 281 (33) | |
| Statin | 312 (38) | 334 (40) | 319 (38) | |
| Diabetes, n (%) | 253 (31) | 243 (29) | 271 (32) | |
| Pulmonary disease, n (%) | 175 (21) | 147 (17) | 158 (19) | |
| Hypercholesterolaemia, n (%)c | 431 (52) | 442 (52) | 456 (54) | |
| Hypertension, n (%) | 453 (55) | 469 (56) | 478 (56) | |
| Atrial fibrillation or flutter, n (%) | 141 (17) | 132 (16) | 117 (14) | |
Results
| Outcome | ICD (n = 829) | Amiodarone (n = 845) | Placebo (n = 847) | HR (95% CI), p-value |
|---|---|---|---|---|
| Mortality from any cause, n (%) | 182 (22) | 240 (28) | 244 (29) | HR amiodarone vs. placebo 1.06 (97.5% CI 0.86 to 1.30), 0.53; HR ICD vs. placebo 0.77 (97.5% CI 0.62 to 0.96), 0.007 |
| Kaplan–Meier estimate for death from any cause, 5-year event rate | 0.289 | 0.340 | 0.361 | |
| Cardiac deaths, n/no. of deaths (%)108 | 122/182 (67) | 162/240 (68) | 167/244 (68) | HR amiodarone vs. placebo 1.05 (0.85 to 1.31), NS; HR ICD vs. placebo 0.76 (0.60 to 0.95), 0.018 |
| Tachyarrhythmic | 37/182 (20) | 75/240 (31) | 95/244 (39) | HR amiodarone vs. placebo 0.84 (0.62 to 1.13), 0.25; HR ICD vs. placebo 0.40 CI 0.27 to 0.59), p < 0.001 |
| Bradyarrhythmic | 1/182 (< 1) | 5/240 (2) | 3/244 (1) | |
| HF | 72/182 (40) | 67/240 (28) | 66/244 (27) | HR amiodarone vs. placebo 1.14 (0.81 to 1.60), NS; HR ICD vs. placebo 1.14 (0.82 to 1.60), NS |
| Non-arrhythmic, non-HF | 9/182 (5) | 10/240 (4) | 2/244 (1) | |
| Cardiac but unable to classify further | 3/182 (2) | 5/240 (2) | 1/244 (< 1) | |
| Non-cardiac deaths, n/no. of deaths (%)108 | 48/182 (26) | 54/240 (23) | 53/244 (22) | HR amiodarone vs. placebo 1.10 (0.80 to 1.50), NS; HR ICD vs. placebo 0.80 (0.57 to 1.12), NS |
| Vascular | 11/182 (6) | 10/240 (4) | 12/244 (5) | |
| Non-vascular | 37/182 (20) | 44/240 (18) | 41/244 (17) | |
| Unknown deaths, n/no. of deaths (%)108 | 12/182 (7) | 24/240 (10) | 24/244 (10) | NS |
| Medication use at last follow-up, n (%) | (n = 822) | (n = 840) | (n = 838) | |
| ACE inhibitor | 576 (70) | 594 (71) | 619 (74) | |
| ARB | 144 (18) | 152 (18) | 145 (17) | |
| ACE inhibitor or ARB | 706 (86) | 718 (85) | 740 (88) | |
| Beta-blocker | 672 (82) | 605 (72) | 662 (79) | < 0.001 |
| Diuretic | ||||
| Loop | 649 (79) | 665 (79) | 674 (80) | |
| Potassium sparing | 261 (32) | 236 (28) | 278 (33) | |
| Thiazide | 80 (10) | 95 (11) | 88 (11) | |
| Digoxin | 512 (63) | 496 (59) | 524 (62) | |
| Acetylsalicylic acid (aspirin) | 449 (55) | 474 (56) | 451 (54) | |
| Warfarin | 279 (34) | 272 (32) | 300 (36) | |
| Statin | 395 (48) | 405 (48) | 387 (46) | |
| ICD shocks, n/N (%) | ||||
| Received for any cause, n/N (%) | 259/829 (31) | |||
| Received for rapid VT or VF, n/N (%) | 177/259 (68) | |||
| Annual rate of ICD shocks during 5-year follow up, % | 7.5 | |||
| Annual rate of appropriate shocks (sustained VT or VF) during 5-year follow-up, % | 5.1 | |||
Adverse effects of treatment
| Adverse effect | ICD (n = 829) | Amiodarone (n = 845) | Placebo (n = 847) | p-value |
|---|---|---|---|---|
| Implantation unsuccessful, n (%) | 1 (< 1) | |||
| ICD removed during follow-up, n (%) | 32 (4) | |||
| Clinically significant ICD complications,a % | ||||
| At the time of implantation | 5 | |||
| Later in the course of follow-up | 9 | |||
| At time of last follow-up, % | ||||
| Increased tremor | 4 (amiodarone vs. placebo) | 0.02 | ||
| Increased hypothyroidism | 6 (amiodarone vs. placebo) | < 0.001 | ||
Prespecified subgroup analyses105,106,108
| Outcome | ICD (n = 829) | Amiodarone (n = 845) | Placebo (n = 847) | HR (95% CI), p-value |
|---|---|---|---|---|
| Mortality from any cause – ischaemic CHF105 | HR amiodarone vs. placebo 1.05 (97.5% CI 0.81 to 1.36), 0.66; HR ICD vs. placebo 0.79 (97.5% CI 0.60 to 1.04), 0.05 | |||
| Kaplan–Meier estimates of mortality from any cause – 5-year event rate ischaemic CHF105 | 0.359 (n = 431) | 0.417 (n = 426) | 0.432 (n = 453) | |
| Cause of death, participants with ischaemic CHF108 | ||||
| Cardiac | HR amiodarone vs. placebo 0.96 (0.73 to 1.26); HR ICD vs. placebo 0.80 (0.60 to 1.05) | |||
| Sudden tachyarrhythmic | HR amiodarone vs. placebo 0.70 (0.48 to 1.03); HR ICD vs. placebo 0.43 (0.27 to 0.67) | |||
| HF | HR amiodarone vs. placebo 1.17 (0.78 to 1.77); HR ICD vs. placebo 1.11 (0.74 to 1.67) | |||
| Non-cardiac | HR amiodarone vs. placebo 1.21 (0.88 to 1.94); HR ICD vs. placebo 0.79 (0.50 to 1.22) | |||
| Mortality from any cause – non-ischaemic CHF105 | HR amiodarone vs. placebo 1.07 (97.5% CI 0.76 to 1.51), 0.65; HR ICD vs. placebo 0.73 (97.5% CI 0.50 to 1.07), 0.06 | |||
| Kaplan–Meier estimates of mortality from any cause – 5-year event rate non-ischaemic CHF105 | 0.214 (n = 398) | 0.258 (n = 419) | 0.279 (n = 394) | |
| Cause of death, participants with non-ischaemic CHF108 | ||||
| Cardiac | HR amiodarone vs. placebo 1.23 (0.85 to 1.77); HR ICD vs. placebo 0.68 (0.44 to 1.03) | |||
| Sudden tachyarrhythmic | HR amiodarone vs. placebo 1.13 (0.68 to 1.85); HR ICD vs. placebo 0.34 (0.17 to 0.70) | |||
| HF | HR amiodarone vs. placebo 1.06 (0.58 to 1.96) HR ICD vs. placebo 1.21 (0.67 to 2.18) | |||
| Non-cardiac | HR amiodarone vs. placebo 0.81 (0.48 to 1.36); HR ICD vs. placebo 0.81 (0.48 to 1.37) | |||
| Mortality from any cause – NYHA II105 | HR amiodarone vs. placebo 0.85 (97.5% CI 0.65 to 1.11), 0.17; HR ICD vs. placebo 0.54 (97.5% CI 0.40 to 0.74), < 0.001 | |||
| Kaplan–Meier estimates of mortality from any cause – 5-year event rate NYHA II105 | 0.201 (n = 566) | 0.264 (n = 601) | 0.320 (n = 594) | |
| Cause of death, participants with NYHA class II CHF108 | ||||
| Cardiac | HR amiodarone vs. placebo 0.88 (0.66 to 1.17); HR ICD vs. placebo 0.50 (0.36 to 0.70) | |||
| Sudden tachyarrhythmic | HR amiodarone vs. placebo 0.68 (0.47 to 0.99); HR ICD vs. placebo 0.26 (0.15 to 0.44) | |||
| HF | HR amiodarone vs. placebo 0.93 (0.56 to 1.54); HR ICD vs. placebo 0.93 (0.56 to 1.54) | |||
| Non-cardiac | HR amiodarone vs. placebo 0.79 (0.52 to 1.20); HR ICD vs. placebo 0.63 (0.40 to 0.99) | |||
| Mortality from any cause – NYHA III105 | HR amiodarone vs. placebo 1.44 (97.5% CI 1.05 to 1.97), 0.010; HR ICD vs. placebo 1.16 (97.5% CI 0.84 to 1.61), 0.30 | |||
| Kaplan–Meier estimates of mortality from any cause – 5-year event rate NYHA III105 | 0.484 (n = 263) | 0.528 (n = 244) | 0.456 (n = 253) | |
| Cause of death, participants with NYHA class III CHF108 | ||||
| Cardiac | HR amiodarone vs. placebo 1.33 (95% CI 0.95 to 1.86); HR ICD vs. placebo 1.17 (95% CI 0.84 to 1.64) | |||
| Sudden tachyarrhythmic | HR amiodarone vs. placebo 1.22 (95% CI 0.73 to 2.03); HR ICD vs. placebo 0.73 (95% CI 0.41 to 1.29) | |||
| HF | HR amiodarone vs. placebo 1.34 (95% CI 0.84 to 2.11); HR ICD vs. placebo 1.34 (95% CI 0.86 to 2.09) | |||
| Non-cardiac | HR amiodarone vs. placebo 1.68 (95% CI 1.03 to 2.73); HR ICD vs. placebo 1.10 (95% CI 0.66 to 1.85) | |||
Prespecified analysis by race106
| Outcome | ICD | Amiodarone | Placebo | |||
|---|---|---|---|---|---|---|
| AA 36% | White 33% | AA 30% | White 34% | AA 34% | White 33% | |
| Risk of death | HR ICD vs. placebo 0.65 (95% CI 0.43 to 0.99), p = not reported | HR ICD vs. placebo 0.73 (95% CI 0.58 to 0.90), p = not reported | HR amiodarone vs. placebo 1.08 (95% CI 0.71 to 1.64), p = not reported | HR amiodarone vs. placebo 1.11 (95% CI 0.90 to 1.37), p = not reported | ||
| ICD discharges | No significant difference observed between white and AA participants, HR 1.10 (95% CI 0.80 to 1.51), p = 0.56 | |||||
Quality-of-life study107
| Outcome | ICD (n = 816) | Amiodarone (n = 830) | Placebo (n = 833) | Difference (95% CI), p-value |
|---|---|---|---|---|
| DASI, mean score (SD) | ||||
| Baseline | 24.6 (13.6) (n = 814) | 25.3 (14.1) (n = 825) | 24.9 (14.1) (n = 829) | Amiodarone vs. placebo 0.44 (–0.92 to 1.80); ICD vs. placebo –0.34 (–1.68 to 1.00) |
| 3 months | 26.9 (14.1) (n = 766) | 26.2 (14.7) (n = 756) | 26.2 (14.3) (n = 768) | Amiodarone vs. placebo –0.01 (–1.47 to 1.45); ICD vs. placebo –0.69 (–0.73 to 2.11) |
| 12 months | 26.8 (14.4) (n = 734) | 26.1 (14.5) (n = 676) | 26.6 (14.8) (n = 697) | Amiodarone vs. placebo –0.58 (–2.14 to 0.97); ICD vs. placebo 0.16 (–1.35 to 1.68) |
| 30 months | 26.8 (14.3) (n = 665) | 27.1 (15.3) (n = 575) | 25.9 (15.3) (n = 585) | Amiodarone vs. placebo 1.20 (–0.56 to 2.96); ICD vs. placebo 0.89 (–0.75 to 2.53) |
| MHI-5, mean score (SD) | ||||
| Baseline | 71.7 (20.5) (n = 814) | 72.1 (20.1) (n = 827) | 70.0 (21.4) (n = 830) | Amiodarone vs. placebo 2.11 (0.11 to 4.11), ≤ 0.05; ICD vs. placebo 1.64 (–0.39 to 3.67) |
| 3 months | 74.4 (19.3) (n = 764) | 72.9 (20.6) (n = 759) | 71.3 (21.5) (n = 767) | Amiodarone vs. placebo 1.60 (–0.51 to 3.72); ICD vs. placebo 3.15 (1.10 to 5.19), ≤ 0.05 |
| 12 months | 74.5 (18.9) (n = 734) | 72.9 (20.5) (n = 674) | 70.9 (21.5) (n = 693) | Amiodarone vs. placebo 1.99 (–0.24 to 4.22); ICD vs. placebo 3.68 (1.58 to 5.78), ≤ 0.05 |
| 30 months | 72.2 (19.1) (n = 654) | 73.2 (20.3) (n = 560) | 71.0 (21.7) (n = 564) | Amiodarone vs. placebo 2.22 (–0.24 to 4.68); ICD vs. placebo 1.24 (–1.06 to 3.53) |
| MLWHFQ, median | ||||
| Baseline | 41 | NR | 43 | 0.77 |
| 3 months | 30 | NR | 36 | 0.006 |
| 12 months | 32 | NR | 36 | 0.07 |
| 30 months | 32 | NR | 36 | 0.05 |
| Global health status, median107 | ||||
| 3 months | 75 | 70 | 0.002 | |
| 12 months | 75 | 70 | 0.05 | |
| 30 months | 70 | 70 | 0.18 | |
Subgroup analyses: quality-of-life study107
| Outcome | ICD (n = 816) | p-value | |
|---|---|---|---|
| Received shocka (n = 49) | No shock | ||
| SF-36 score, mean changeb | |||
| General health perceptions | –6.3 | 3.4 | 0.002 |
| Physical function | –8 | 10.9 | < 0.001 |
| Emotional function | –11 | 4.5 | 0.02 |
| Social function | –5.3 | 4.6 | 0.009 |
| Self-related health | –3.2 | 6.6 | 0.009 |
-
Allocation to treatment groups: patients assigned to amiodarone or placebo began therapy as outpatients immediately after randomisation. ICD group patients received the device a median of 3 days after randomisation (IQR 2-5 days). Permuted-block randomisation was carried out, stratified by clinical site, cause of CHD (ischaemic vs. non-ischaemic) and NYHA class (II vs. III). Block size randomly chosen as 3 or 6.
-
Blinding: placebo and amiodarone administered in double-blind fashion. Wyeth-Ayerst Pharmaceuticals provided tablets that appeared identical. 105 The events committee that adjudicated deaths was blinded to treatment assignment (a nurse removed all information identifying randomised therapy assignment from reports). 108
-
Comparability of treatment groups: authors state that there were no significant differences between the groups at baseline. By the last follow-up visit there was a difference in use of beta-blockers (p < 0.001). The median dose of amiodarone and placebo was 300 mg/day 3 months after randomisation and remained so throughout the study. QoL study:107 selected baseline characteristics are reported and described as well balanced between the groups.
-
Method of data analysis: pairwise comparisons (amiodarone vs. placebo; ICD vs. placebo) performed according to the ITT principle. All statistical tests two-tailed. Cumulative mortality rates calculated using the Kaplan–Meier method. Event (or censoring) times measured from time of randomisation (time zero). Differences in mortality rates assessed using the log-rank test, with adjustment for NYHA class and cause of CHF. RRs expressed as HRs with 97.5% CIs (consistent with an alpha level of 0.025) are derived from the Cox proportional hazards model (however, 95% CIs are reported by Parker et al. 108). Cox model also used to test the significance of interactions between NYHA class and treatment, and between cause of CHF and treatment. Six interim analyses were performed and reviewed by the independent data and safety monitoring board using two-sided, symmetrical O’Brien–Fleming boundaries generated with the Lan–DeMets alpha-spending function approach to group sequential testing. Because of sequential testing the level of significance for each major treatment comparison at completion of the study was 0.023. Some patients may have had ICD discharges that were either not recorded or not reported to the ICD core laboratory, which would limit the ability to know the true rate of ICD events. QoL study:107 continuous data described with means (SD) and/or medians (25–75 percentiles). Categorical variables described with percentages. Pearson’s chi-squared test was used for categorical variable comparisons and the Wilcoxon rank-sum test was used for continuous variables. The Wilcoxon rank-sum test for changes in scores from the most recent QoL measurements before a shock occurred was used to compare patients who received a shock within the month preceding a QoL assessment with those who did not. The analysis was repeated with 2- and 12-month time frames. To account for potential bias as a result of the significant difference in mortality between the groups, an estimator for the survival average causal effect was applied in a sensitivity analysis. All reported p-values were two-sided and no adjustments were made for multiple testing.
-
Sample size/power calculation: based on the assumption that the placebo group would have an annual mortality rate of 10%. Powered at 90% to detect a 25% reduction in death from any cause with amiodarone or ICD therapy compared with placebo on the basis of an alpha level for each comparison of 0.025.
-
Attrition/dropout: vital status known for all 2521 patients at the time of the last scheduled follow-up visit. The non-compliance rate for study drug therapy (discontinuation of placebo or amiodarone for any period) was 27% (458 patients) – 22% of the placebo group (189/847 patients) and 32% of the amiodarone group (269/845 patients). Crossovers: 125 patients (7%) in the drug groups crossed over to open-label amiodarone (44 in the amiodarone group and 81 in the placebo group). In the ICD group 113/829 (14%) patients received open-label amiodarone during some part of the follow-up and 17/829 (2%) patients assigned to ICD therapy declined to undergo implantation. Crossover to some form of ICD therapy occurred in 188 patients (11%) in the drug groups during follow-up. Median time from randomisation to crossover was 26.7 months. QoL study:107 98% completed the baseline QoL questionnaires. At each follow-up 93–95% of eligible patients were included; overall, 95% of the questionnaires were collected. A total of 1.2% of patients declined to complete the questionnaires, 1.4% of the forms were judged incomplete and in 69/6268 (1.1%) interviews proxy forms were substituted for the full questionnaire.
-
Other: none of the 716 patients for whom defibrillation testing data were reported required more than a 30-J shock for defibrillation (the maximum device output).
-
Generalisability: broad population of patients with mild to moderate HF and no exclusions stated. However, the majority of the participants were American and the racial mix of participants differs to that likely in the UK.
-
Outcome measures: appear appropriate.
-
Intercentre variability: for the QoL study specific training was provided at each site to ensure standardisation of data collection. 107 No other details provided.
-
Conflict of interests: authors state that companies provided study drugs and ICDs free of charge and provided additional clinical and research funding. However, neither company had any role in the design, analysis or interpretation of the study.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | States permuted-block randomisation, stratified by clinical site, cause of CHD and NYHA class, with block size randomly chosen as 3 or 6. However, no details about generation of sequence |
| Allocation concealment | Unclear | No details provided |
| Performance bias | ||
| Blinding of participants and personnel | High | No blinding of ICD arm. QoL: risk of bias between ICD and non-ICD groups because of knowledge of intervention received |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality | Low | Events committee who adjudicated deaths was blinded to treatment group |
| QoL | High | QoL data obtained by structured interview; risk of bias between ICD and non-ICD groups because of knowledge of intervention received |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Mortality | Low | ITT analysis and vital status known for all patients at time of last visit |
| QoL | Unclear | Some explanation of missing data but not by treatment group |
| Reporting bias | ||
| Selective reporting | Low | Protocol not available but papers appear to report all of the expected and stated outcomes |
| Other bias | ||
| Other sources of bias | Low | |
Appendix 8 Data extraction: people with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony
Cardiac Resynchronization – Heart Failure (CARE-HF) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Cleland et al. 2001,110 2005,109 2006,111 2008112 and 2009,113 Gras et al. 2007,36 Gervais et al. 2009,114 Ghio et al. 2009115 Study design: RCT Countries: European countries including the UK, France, Germany, Switzerland and Italy109 No. of centres: 82109 Funding: supported by a grant from Medtronic |
Intervention: CRT-P + medical therapy (standard pharmacological therapy). CRT (Medtronic InSync or InSync III device) provided atrial-based, biventricular stimulation. Standard right ventricular and Attain (Medtronic) left ventricular leads. Back-up atrial pacing set at 60 bpm, interventricular delay set at zero, atrioventricular delay echocardiographically optimised109 Comparator: medical therapy (standard pharmacological therapy) only109 Other interventions used: none reported. Standard medications adjusted if needed at follow-up visits |
Indication for treatment: NYHA class III or IV from LVSD and cardiac dyssynchrony receiving standard pharmacological therapy109 No. of randomised participants: 813; CRT-P + medical therapy: 409, medical therapy alone: 404109 Inclusion criteria: NYHA class III or IV despite standard pharmacological therapy, LVEF ≤ 35%, LVEDD ≥ 30 mm (indexed to height), QRS interval ≥ 120 milliseconds (patients with QRS interval of 120–149 milliseconds required to meet two of three additional criteria for dyssynchrony: aortic pre-ejection delay > 140 milliseconds, interventricular mechanical delay > 40 milliseconds, delayed activation of posterolateral left ventricular wall), age ≥ 18 years, HF for ≥ 6 weeks109 Exclusion criteria: major cardiovascular event in previous 6 weeks, conventional indications for a pacemaker or an ICD, HF requiring continuous intravenous therapy, atrial arrhythmias109 |
Primary outcomes: composite of death from any cause or an unplanned hospitalisation for a major cardiovascular event (only first hospitalisation counted).109 For extension phase, death from any cause111 Secondary outcomes: death from any cause, composite of death from any cause and unplanned hospitalisation for HF, 90-day NYHA class, 90-day QoL.109 For extension phase, mode of death111 Method of assessing outcomes: assessment at baseline and 1, 3, 6, 9, 12 and 18 months, then at 6-month intervals. For QoL,113 assessment at baseline and 3 months, then disease-specific instrument only at 18 months and study end QoL: patient assessed using the disease-specific MLWHFQ (score range 0–105, higher score indicates lower QoL) and the generic EQ-5D (score range –0.594 to 1.0, lower score indicates lower QoL, negative scores considered worse than death) Length of follow-up: mean 29.4 months (range 18.0–44.7 months).109 For QoL,113 median 29.6 months (IQR 23.6–34.6 months). After 8-month extension phase, mean 37.4 months (range 26.1–52.6 months), median 37.6 months (IQR 31.5–42.5 months)111 Recruitment: January 2001–March 2003109 |
Participant characteristics109
| Characteristic | CRT-P + medical therapy (n = 409) | Medical therapy (n = 404) | p-value |
|---|---|---|---|
| Age (years), median (range) | 67 (60–73) | 66 (59–72) | |
| Sex, male, n (%) | 304 (74) | 293 (73) | |
| Ethnicity | NR | NR | |
| Dilated cardiomyopathy, n (%) | 177 (43) | 193 (48) | |
| Ischaemic heart disease, n (%) | 165 (40) | 144 (36) | |
| Heart disease of other causes, n (%) | 67 (16) | 67 (17) | |
| NYHA class IV, n (%) | 23 (6) | 27 (7) | |
| LVEF (%), median (range) | 25 (21–29) | 25 (22–29) | |
| QRS interval (milliseconds), median (range) | 160 (152–180) | 160 (152–180) | |
| Heart rate (bpm), median (range) | 69 (60–78) | 70 (61–78) | |
| Left ventricular end-systolic volume index (ml/m2), median (range) | 121 (92–151) | 117 (94–147) | |
| Interventricular mechanical delay (milliseconds), median (range) | 49 (32–67) | 50 (30–66) | |
| Mitral regurgitation area (cm2), median (range) | 0.21 (0.12–0.33) | 0.23 (0.11–0.34) | |
| Use of ACE inhibitor or ARB, n (%) | 387 (95) | 383 (95) | |
| Use of beta-blocker, n (%) | 288 (70) | 298 (74) | |
| Use of spironolactone, n (%) | 219 (54) | 238 (59) | |
| Use of high-dose loop diuretic, n (%) | 175 (43) | 177 (44) | |
| Use of digoxin, n (%) | 165 (40) | 181 (45) | |
| Systolic blood pressure (mmHg), median (range) | 110 (100–125) | 110 (100–125) | |
| Diastolic blood pressure (mmHg), median (range) | 70 (60–79) | 70 (60–80) | |
| N-terminal pro-brain natriuretic peptide (pg/ml), median (range) | 1920 (744–4288) | 1806 (719–3949) | |
| Glomerular filtration rate (ml/minute/1.73 m2), median (range) | 60 (46–73) | 61 (46–73) |
Results
| Outcome109 | CRT-P + medical therapy (n = 409) | Medical therapy (n = 404) | HR or difference in means (95% CI), p-value | ||
|---|---|---|---|---|---|
| Death or unplanned hospitalisation for a cardiovascular event (primary outcome), n/N (%) | 159/409 (39) | 224/404 (55) | HR 0.63 (0.51 to 0.77), < 0.001 | ||
| Unplanned hospitalisation for a cardiovascular event (primary outcome), n/N (%)a | 125/409 (31) | 184/404 (46) | HR 0.61 (0.49 to 0.77), < 0.001 | ||
| Death from any cause, n/N (%) | 82/409 (20) | 120/404 (30) | HR 0.64 (0.48 to 0.85), < 0.002 | ||
| Additional deaths during the extension phase, n111 | 19 | 34 | |||
| Deaths in main study + deaths in extension phase, n/N (%) | 101/409 (24.7), 7.9% per annum | 154/404 (38.1), 12.2% per annum | HR 0.60 (0.47 to 0.77), < 0.0001 | ||
| Principal cause of death, n/N deaths (%) | |||||
| Cardiovascular | 167/202 (83) | ||||
| Non-cardiovascular | 34/202 (17) | ||||
| Not classifiable | 1/202 (0.5) | ||||
| Death attributed to worsening HF, n/N deaths (%) | 33/82 (40) | 56/120 (47) | |||
| Deaths from HF in main study + extension phase, n111 | 38 (3.0% per annum) | 64 (5.1% per annum) | HR 0.55 (0.37 to 0.82), 0.003 | ||
| Death classified as sudden, n/N deaths (%) | 29/82 (35) | 38/120 (32) | |||
| Sudden deaths in the extension phase, n/N deaths111 | 3/19 | 16/34 | |||
| Sudden deaths in main study + extension phase, n111 | 32 (2.5% per annum) | 54 (4.3% per annum) | HR 0.54 (0.35 to 0.84), 0.005 | ||
| Mortality rate, % | |||||
| 1 year | 9.7 | 12.6 | |||
| 2 years | 18.0 | 25.1 | |||
| 3 years111 | 23.6 | 35.1 | |||
| Death from any cause or unplanned hospitalisation with worsening HF, n/N (%) | 118/409 (29) | 191/404 (47) | HR 0.54 (0.43 to 0.68), < 0.001 | ||
| Unplanned hospitalisation with worsening HF, n/N (%)a | 72/409 (18) | 133/404 (33) | HR 0.48 (0.36 to 0.64), < 0.001 | ||
| Deaths in the first 90 days, n | 12 | 15 | |||
| Heart transplantations, nb | |||||
| Emergency | 1 | 3 | |||
| Elective | 9 | 6 | |||
| MLWHFQ score, mean (SD) at 90 daysc | 31 (22) | 40 (22) | Difference in means –10 (–8 to –12), < 0.001 | ||
| EQ-5D score, mean (SD) at 90 daysc | 0.70 (0.28) | 0.63 (0.29) | Difference in means 0.08 (0.04 to 0.12), < 0.001 | ||
| NYHA class, mean (SD) at 90 days | 2.1 (1.0) | 2.7 (0.9) | Difference in means 0.6 (0.4 to 0.7), < 0.001 | ||
| NYHA class at 18 months, n | |||||
| Class I | 105 | 39 | |||
| Class II | 150 | 112 | |||
| Class III or IV | 80 | 152 | |||
| Difference in meansd (95% CI) | p-value | ||||
| LVEF (%) | |||||
| At 3 monthse | +3.7 (3.0 to 4.4) | < 0.001 | |||
| At 18 monthse | +6.9 (5.6 to 8.1) | < 0.001 | |||
| Heart rate (bpm) | |||||
| At 3 months | +1.1 (–1.2 to 3.4) | 0.33 | |||
| At 18 months | +1.0 (–1.5 to 3.6) | 0.43 | |||
| Systolic blood pressure (mmHg) | |||||
| At 3 months | +5.8 (3.5 to 8.2) | < 0.001 | |||
| At 18 months | +6.3 (3.6 to 8.9) | < 0.001 | |||
| Diastolic blood pressure (mmHg) | |||||
| At 3 months | +1.5 (0.1 to 2.9) | 0.03 | |||
| At 18 months | +1.3 (–1.8 to 4.4) | 0.42 | |||
| Interventricular mechanical delay (ms)e | |||||
| At 3 months | –21 (–25 to –18) | < 0.001 | |||
| At 18 months | –21 (–25 to –17) | < 0.001 | |||
| Left ventricular end-systolic volume index (ml/m2) | |||||
| At 3 months | –18.2 (–21.2 to –15.1) | < 0.001 | |||
| At 18 months | –26.0 (–31.5 to –20.4) | < 0.001 | |||
| Mitral regurgitation area (cm2) | |||||
| At 3 months | –0.051 (–0.073 to –0.028) | < 0.001 | |||
| At 18 months | –0.042 (–0.070 to –0.014) | 0.003 | |||
| N-terminal pro-brain natriuretic peptide (pg/ml) | |||||
| At 3 months | –225 (–705 to –255) | 0.36 | |||
| At 18 months | –1122 (–1815 to –429) | < 0.002 | |||
| IHD (n = 168) | Non-IHD (n = 197) | IHD (n = 135) | Non-IHD (n = 235) | p-value | |
| LEVF | |||||
| Baseline (%), median (IQR)115 | 25 (22 to 29) | 24 (21 to 29) | 26 (22 to 30) | 24 (21 to 29) | 0.1867 (IHD vs. non-IHD) |
| Mean (SD) change at 18 months from baseline (%)f | 6.1 (1.2) | 10.9 (1.5) | 1.3 (0.7) | 2.4 (1.7) | 0.003 for interaction between CRT and aetiology |
Quality-of-life results113
| Outcome | CRT-P + medical therapy (n = 409) | Medical therapy (n = 404) | MD (95% CI), p-value |
|---|---|---|---|
| Mean QALYs (95% CI) | |||
| 3 months | 0.16 (0.15 to 0.16) | 0.15 (0.14 to 0.15) | 0.01 (0.001 to 0.018), 0.285 |
| 18 months | 0.95 (0.91 to 0.99) | 0.82 (0.78 to 0.86) | 0.13 (0.07 to 0.018), < 0.0001 |
| End of study | 1.45 (1.38 to 1.53) | 1.22 (1.15 to 1.29) | 0.23 (0.13 to 0.33), < 0.0001 |
| Mean life-years (95% CI) | |||
| 3 months | 0.241 (0.238 to 0.244) | 0.241 (0.238 to 0.244) | 0.0003 (–0.004 to 0.0045), 0.90 |
| 18 months | 1.37 (1.34 to 1.40) | 1.33 (1.29 to 1.37) | 0.04 (–0.01 to 0.09), 0.13 |
| End of study | 2.07 (1.99 to 2.15) | 1.96 (1.88 to 2.05) | 0.10 (–0.01 to 0.22), 0.07a |
| EQ-5D score (95% CI) | |||
| Baseline | 0.60 (0.58 to 0.63) | 0.60 (0.57 to 0.63) | – |
| 3 months | 0.69 (0.66 to 0.72) | 0.61 (0.59 to 0.64) | 0.08 (0.04 to 0.11), < 0.0001 |
| 18 months | 0.61 (0.58 to 0.64) | 0.51 (0.48 to 0.54) | 0.10 (0.06 to 0.15), < 0.0001 |
| End of study | 0.56 (0.52 to 0.59) | 0.43 (0.39 to 0.46) | 0.13 (0.08 to 0.18), < 0.0001b |
| MLWHFQ score (95% CI) | |||
| Baseline | 44.6 (42.5 to 46.7) | 43.7 (41.5 to 45.8) | – |
| 3 months | 30.1 (27.9 to 32.3) | 38.9 (36.6 to 41.2) | –10.6 (–8.1 to –13.1), < 0.0001c |
| 18 months | 28.4 (26.2 to 30.5) | 36.0 (33.5 to 38.5) | –10.7 (–7.6 to –13.8), < 0.0001c |
| End of study | 27.2 (24.9 to 29.5) | 35.1 (32.6 to 37.6) | –10.1 (–6.8 to –13.3), < 0.0001c |
| Mean [median (IQR)] days in hospital by 3 months | 7.5 [4 (2–8)] | 3.4 [0 (0–1)] | |
| Days in hospital after 3 months | 222 | 384 | |
| Mean [median (IQR)] days in hospital overall during entire study (median 29.6 months) | 20.7 [9 (4–26)] | 22.4 [9 (0–31)] | |
Adverse effects of treatment109
| Adverse effect | CRT-P + medical therapy (n = 409) | Medical therapy (n = 404) | p-value |
|---|---|---|---|
| Device-related deaths, n | 1 (HF aggravated by lead displacement) | 1 (septicaemia after receiving a device) | |
| Most common adverse device- or procedure-related events, n patients | |||
| Lead displacement | 24 | ||
| Coronary sinus dissection | 10 | ||
| Pocket erosion | 8 | ||
| Pneumothorax | 6 | ||
| Device-related infection | 3 | ||
| Worsening HF, n patients | 191 | 263 | < 0.001 |
| Atrial arrhythmias or ectopy, n patients | 64 | 41 | 0.02 |
Subgroup analyses109
| Subgroup | Patients with event/total no. of patients | HR (95% CI) |
|---|---|---|
| Overall with primary end point | 383/813 | 0.63 (0.51 to 0.77) |
| Age (years)a | ||
| < 66.4 | 163/406 | 0.55 (0.40 to 0.75) |
| ≥ 66.4 | 220/407 | 0.68 (0.52 to 0.89) |
| Sex | ||
| Male | 290/597 | 0.62 (0.49 to 0.79) |
| Female | 93/215 | 0.64 (0.42 to 0.97) |
| NYHA class | ||
| III | 349/763 | 0.64 (0.52 to 0.80) |
| IV | 34/50 | 0.50 (0.25 to 1.01) |
| Dilated cardiomyopathy | ||
| No | 238/443 | 0.68 (0.53 to 0.88) |
| Yes | 145/370 | 0.51 (0.36 to 0.73) |
| Systolic blood pressure (mmHg)a | ||
| < 117 | 208/401 | 0.60 (0.46 to 0.80) |
| ≥ 117 mmHg | 170/402 | 0.66 (0.48 to 0.89) |
| N-terminal pro-brain natriuretic peptide (pg/ml) | ||
| < 214.5 | 122/366 | 0.53 (0.36 to 0.76) |
| ≥ 214.5 | 224/366 | 0.70 (0.54 to 0.91) |
| Ejection fraction (%)a | ||
| < 24.7 | 205/372 | 0.65 (0.49 to 0.86) |
| ≥ 24.7 | 152/373 | 0.62 (0.44 to 0.85) |
| Left ventricular end-systolic volume index (ml/m2)a | ||
| < 119.2 | 156/366 | 0.71 (0.52 to 0.98) |
| ≥ 119.2 | 193/366 | 0.54 (0.40 to 0.73) |
| QRS interval (milliseconds) | ||
| < 160 | 152/290 | 0.74 (0.54 to 1.02) |
| ≥ 160 | 222/505 | 0.60 (0.46 to 0.79) |
| Interventricular mechanical delay (milliseconds)a | ||
| < 49.2 | 199/367 | 0.77 (0.58 to 1.02) |
| ≥ 49.2 | 147/368 | 0.50 (0.36 to 0.70) |
| Mitral regurgitation area (cm2)a | ||
| < 0.218 | 114/302 | 0.86 (0.60 to 1.25) |
| ≥ 0.218 | 175/303 | 0.56 (0.41 to 0.75) |
| Glomerular filtration rate (ml/minute/1.73 m2)a | ||
| < 60.3 | 196/369 | 0.67 (0.50 to 0.89) |
| ≥ 60.3 | 142/370 | 0.57 (0.40 to 0.80) |
| Beta-blockers | ||
| No | 131/227 | 0.72 (0.51 to 1.02) |
| Yes | 252/586 | 0.59 (0.46 to 0.76) |
| Spironolactone | ||
| No | 166/356 | 0.58 (0.43 to 0.79) |
| Yes | 217/457 | 0.67 (0.51 to 0.88) |
| Loop diuretics | ||
| < 80 mg of furosemide or equivalent | 181/461 | 0.56 (0.42 to 0.76) |
| ≥ 80 mg of furosemide or equivalent | 202/352 | 0.69 (0.53 to 0.92) |
| Digoxin | ||
| No | 218/467 | 0.66 (0.50 to 0.86) |
| Yes | 165/346 | 0.59 (0.43 to 0.81) |
-
Allocation to treatment groups: randomisation stratified by NYHA class and carried out by an independent clinical research organisation (Quintiles, Dublin) using a minimisation procedure. 109
-
Blinding: not blinded;109 however, members of the end points committee (who classified all hospitalisations and some adverse events) were not aware of patients’ treatment assignments. Procedure- or device-related adverse events classified by an unblinded independent expert. 109
-
Comparability of treatment groups: baseline characteristics similar.
-
Method of data analysis: all prespecified analyses carried out according to the ITT principle. Time to event calculated using Kaplan–Meier method and analysed with Cox proportional hazard models (baseline NYHA as a covariate). Continuous data (including QoL113 and ECG outcomes115) analysed using mixed models that included baseline variables as patient-level covariates and study centres as random effects. Dichotomous outcomes analysed using non-linear mixed models with NYHA class as a patient-level covariate and study centres as random effects. Adverse event rates compared using Fisher’s exact test. Two planned interim analyses were conducted by the data and safety monitoring board with the use of non-symmetrical stopping rules. 109 Missing QoL scores were imputed using EQ-5D and MLWHFQ scores, sex, NYHA class, interventricular mechanical delay and mitral regurgitation at baseline. A score of zero was assigned at the time of patient death or time of heart transplantation. 113 QALYs calculated for each patient as the area under the curve estimated through linear interpolation of individual patient-level estimates of health utility based on EQ-5D scores at baseline, 3 and 18 months and the end of the study. 113
-
Sample size/power calculation: statistical power of 80% to identify a 14% relative reduction or a 5.7% point reduction in the rate of events (α = 0.025, 300 events predicted). 109
-
Attrition/dropout: of the 409 patients assigned to CRT-P, an attempt at implantation was made in 404. One patient died before the procedure and in the other four cases the patient or the investigator decided not to proceed with implantation. A CRT-P device was implanted and activated in 390 (95%) patients [six patients had an unplanned hospitalisation for cardiovascular reasons (reached primary end point) before the device was activated], and eight patients received CRT-D]. In 43 patients from the medical therapy group implantation of a CRT-P device was attempted, and in 23 patients implantation of a CRT-D device was attempted (both attempted in one patient). The device was activated in 50 patients. In 10 cases the device was programmed to provide standard pacemaker or ICD-only functions to avoid crossover. In the remaining five patients implantation was unsuccessful. In 19 patients (5%) the device was activated before the primary end point was reached; eight subsequently reached the primary end point (six died). Among the 31 patients who reached the primary end point before the device was activated, seven subsequently died. 109 At the end of the extension phase the survival of one participant in the medical therapy group was unknown. 111 During the extension phase four patients who had received a device in the main phase had it activated, and 41 additional patients had a CRT device implanted and activated. Therefore, at the end of the extension phase a total of 95/404 participants in the medical therapy group had received a CRT device and had it activated, of whom 22 (23.2%) had died. 111 In the paper reporting left ventricular reverse modelling outcomes,115 baseline ECGs were not analysable for 78 (10%) participants. Reasons for this were baseline data not received by the core ECG laboratory (n = 36), damaged video tape (n = 4) and poor-quality examination (n = 38).
-
Other: the extension phase was declared before study closure and without knowledge of the results. 111
-
Generalisability: included patients with LVSD and cardiac dyssynchrony who have moderate or severe HF and who are in sinus rhythm.
-
Outcome measures: appear appropriate.
-
Intercentre variability: not commented on but data analysis included study centres as random effects as noted in the method of data analysis, which presumably took this into account. 109
-
Conflict of interests: all of the authors had conflicts of interest, which are stated at the end of the report. 109 The sponsor had no access to the database and did not participate in the analysis of the results or the writing of the article.
Criteria for assessment of risk of bias in randomised controlled trials65
| Judgementa | Support for judgement | |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Low | Randomisation used a minimisation procedure |
| Allocation concealment | Low | Allocation by independent organisation |
| Performance bias | ||
| Blinding of participants and personnel | High | Unblinded trial |
| Detection bias | ||
| Blinding of outcome assessment | ||
| Mortality and hospitalisation | Low | End points committee not aware of patients’ treatment assignments |
| ECG outcomes | High | Unblinded trial. No indication that core laboratory quantifying these data were unaware of treatment assignment |
| Adverse events | Unclear | Some adverse events (not specified which) were classified by the end points committee who were unaware of patients’ treatment assignments but other procedure- or device-related adverse events were classified by an unblinded independent expert |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Mortality, hospitalisation, ECG outcomes | Low | Analyses according to the ITT principle. Crossovers reported |
| QoL | Unclear | Missing QoL scores imputed but amount of missing data not reported |
| Left ventricular reverse remodelling outcomes | Unclear | Not all participants were included because not all had a readable baseline ECG (10% missing). Authors state that clinical characteristics of groups were similar to those of the total trial population. Reasons for missing data not reported for each group, only overall, so not clear if reasons for missing data are similar between groups |
| Reporting bias | ||
| Selective reporting | Low | Rationale, design and end points paper available.110 Primary and secondary outcomes appear to have been reported as planned. Separate papers report outcomes109,111,113,115 |
| Other bias | ||
| Other sources of bias | Low | |
Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Bristow et al. 2000117 and 2004,116 Carson et al. 2005,119 US FDA 2004118 Anand et al. 2009120 Study design: RCT Country: USA No. of centres: 128 Funding: Guidant Corporation, St Paul, MN |
Intervention: OPT and either CRT-P (Guidant model 1241 Contak TR) or CRT-D (Guidant model 1823 Contak CD) Comparator: OPT – loop diuretics, ACE inhibitors, spironolactone and beta-blockers (unless not tolerated). Also permitted: booster diuretics, ARBs, digoxin, alternative vasodilators, calcium channel blockers Other interventions used: none reported |
Indication for treatment: advanced chronic HF and intraventricular conduction delays No. of randomised participants: 1520; CRT-P: 617, CRT-D: 595, OPT: 308 Inclusion criteria: NYHA class III or IV, QRS duration ≥ 120 milliseconds, PR interval > 150 milliseconds, LVEF ≤ 35%, OPT, LVEDD ≥ 60 mm, age ≥ 18 years, sinus rhythm Exclusion criteria: ICD indications, life expectancy < 6 months, chronic atrial tachyarrhythmias, indications for antibradycardia pacing, unexplained syncope, MI within 60 days of randomisation, uncontrolled blood pressure, surgically uncorrected primary valvular heart disease, progressive or unstable angina, pregnancy, hypertrophic obstructive cardiomyopathy, amyloid disease, tricuspid prosthesis, hospitalisation for HF > 4 hours in previous month117 |
Primary outcomes: all-cause mortality and all-cause hospitalisation (composite end point) Secondary outcomes: cardiac morbidity, all-cause mortality, cardiac hospitalisation, 6-minute walk distance, NYHA class before and after treatment, adverse events, HRQoL (MLWHFQ) Method of assessing outcomes: first events for hospitalisation related to cardiovascular causes or HF, use of outpatient intravenous medication and cause of death adjudicated by end points committee. Clinical evaluations at baseline, 1 week and 1 month, then 3-monthly117 Length of follow-up, median: primary end point: CRT-P 16.2 months (vs. OPT, p < 0.001); CRT-D 15.7 months (vs. OPT, p < 0.001); OPT 11.9 months. Mortality: CRT-P 16.5 months (vs. OPT, p < 0.028); CRT-D 16.0 months (vs. OPT, p < 0.129); OPT 14.8 months Recruitment: January 2000–December 2002 |
Participant characteristics (pre randomisation/implant)
| Characteristic | CRT-P (n = 617) | CRT-D (n = 595) | OPT (n = 308) | p-value |
|---|---|---|---|---|
| Age (years), median | 67 | 66 | 68 | |
| Sex, % male | 67 | 67 | 69 | |
| Ethnicity | NR | NR | NR | |
| Severity of HF, % | ||||
| NYHA class III | 87 | 86 | 82 | |
| NYHA class IVa | 13 | 14 | 18 | |
| QRS interval (ms), median | 160 | 160 | 158 | |
| LVEF, median | 0.20 | 0.22 | 0.22 | |
| LVEDD (mm), median | 68 | 67 | 67 | |
| Heart rate (bpm), median | 72 | 72 | 72 | |
| Blood pressure (mmHg), median | ||||
| Systolic | 110 | 112 | 112 | |
| Diastolic | 68 | 68 | 64 | |
| Ischaemic cardiomyopathy, % | 54 | 55 | 59 | |
| Pharmacological therapy, % | ||||
| Beta-blocker | 68 | 68 | 66 | |
| Spironolactone | 53 | 55 | 55 | |
| ACE inhibitor | 70 | 69 | 69 | |
| ACE inhibitor or ARB | 89 | 90 | 89 | |
| Loop diuretic | 94 | 97 | 94 | |
| Left branch bundle block, % | 69 | 73 | 70 | |
| Right branch bundle block, % | 12 | 10 | 9 | |
| Duration of HF (years), median | 3.7 | 3.5 | 3.6 | |
| 6-minute walk distance (m), median | 274 | 258 | 244 | |
| Diabetes, % | 39 | 41 | 45 | |
Results
| Outcome | CRT-P (n = 617) | CRT-D (n = 595) | OPT (n = 308) | HR (95% CI), p-value: OPT vs. CRT-P; OPT vs. CRT-D |
|---|---|---|---|---|
| Composite end point (all-cause mortality or hospitalisation) (primary end point)a | ||||
| Events during study, n | 414 | 390 | 216 | |
| 12-month event rate, % | 56 | 56 | 68 | 0.81 (0.69 to 0.96), 0.014; 0.80 (0.68 to 0.95), 0.010 |
| All-cause mortalitya | ||||
| Events during study, n/N (%) | 131/617 (21.2) | 105/595 (17.6) | 77/308 (25.0) | |
| 12-month event rate, % | 15 | 12 | 19 | 0.76 (0.58 to 1.01), 0.059; 0.64 (0.48 to 0.86), 0.003 |
| Death or hospitalisation from cardiovascular causesa | ||||
| Events during study, n | 338 | 312 | 188 | |
| 12-month event rate, % | 45 | 44 | 60 | 0.75 (0.63 to 0.90), 0.002; 0.72 (0.60 to 0.86), < 0.001 |
| Death or hospitalisation from HFa | ||||
| Events during study, n | 237 | 212 | 145 | |
| 12-month event rate, % | 31 | 29 | 45 | 0.66 (0.53 to 0.87), 0.002; 0.60 (0.49 to 0.75), < 0.001 |
| Cause of death,119 n (% of patients) [% of deaths] | ||||
| Cardiacb | 109 (17.1) [83.2] | 76 (12.8) [72.4] | 54 (18.8) [75.3] | p = 0.334; p = 0.006 |
| SCDb | 48 (7.8) [36.6] | 17 (2.9) [16.2] | 18 (5.8) [23.4] | 1.21 (0.70 to 2.07), 0.485; 0.44 (0.23 to 0.86), 0.020 |
| Pump failureb | 53 (8.6) [40.5] | 52 (8.7) [49.5] | 34 (11.0) [44.2] | 0.71 (0.46 to 1.09), 0.112; 0.73 (0.47 to 1.11), 0.143 |
| Ischaemic | 2 (0.3) [1.5] | 4 (0.7) [3.8] | 4 (1.3) [5.2] | |
| Cardiac procedure | 6 (1.0) [4.6] | 2 (0.3) [1.9] | 2 (0.6) [2.6] | |
| Other | 0 | 1 (0.2) [1.0] | 0 | |
| Vascular | 5 (0.8) [3.8] | 3 (0.5) [2.8] | 0 | |
| Non-cardiacb | 14 (2.3) [10.7] | 21 (3.5) [20.0] | 11 (3.6) [14.3] | p = 0.122; p = 0.717 |
| Unknown | 3 (0.5) [2.3] | 5 (0.8) [4.8] | 8 (2.6) [10.4] | |
| c,dHospital admissions120 | ||||
| Patients hospitalised at least once, n/N (%) | ||||
| All hospital admissions | 388/617 (63) | 372/595 (63) | 199/308 (65) | p = 0.02;e p = 0.03e |
| Cardiac | 301/617 (49) | 284/595 (48) | 164/308 (53) | p < 0.01;e p < 0.01e |
| HF | 179/617 (29) | 166/595 (28) | 112/308 (36) | p < 0.01;e p < 0.01e |
| Non-cardiac | 222/617 (36) | 207/595 (35) | 84/308 (27) | |
| No. of admissions (% of total admissions), no. of average admissions per patient-year of follow-up | ||||
| All hospital admissions | 993 (NA), 1.25 | 919 (NA), 1.20 | 516 (NA), 1.59 | |
| Cardiac | 628 (63), 0.79 | 580 (63), 0.76 | 338 (75), 1.20 | |
| HF | 329 (33), 0.41 | 333 (36), 0.43 | 235 (46), 0.73 | |
| Non-cardiac | 365 (37), 0.46 | 339 (37), 0.44 | 126 (24), 0.39 | |
| Hospitalisation time (days): average days per patient-year of follow-up (average length of stay per admission) | ||||
| All hospital admissions | 8.3 (6.7) | 8.6 (7.2) | 11.0 (6.9) | |
| Cardiac | 5.2 (6.5) | 5.5 (7.2) | 8.1 (6.8) | |
| HF | 3.6 (8.6) | 3.8 (8.8) | 5.9 (8.2) | |
| Non-cardiac | 3.2 (6.9) | 3.2 (7.2) | 2.8 (7.1) | NS |
| Cardiac procedure, number of hospital admissions per patient-yearf | 0.13 | 0.09 | 0.24 | p < 0.01 |
| CRT implants, n/N (% of procedures) | 33/78 (42) | |||
| Electrophysiological studies, n/N (% of procedures) | 13/78 (17) | |||
| Pacer/ICD implants, n/N (% of procedures) | 13/101 (13) | 10/78 (13) | ||
| Heart transplants, n/N (% of procedures) | 5/78 (6) | |||
| Other, n/N (% of procedures) | 15/78 (19) | |||
| Lead revision, n/N (% of procedures) | 42/101 (42) | 36/69 (52) | ||
| Increase in 6-minute walk distance (m), mean change (SD) | ||||
| 3 months | (n = 422) 33 (99) | (n = 420) 44 (109) | (n = 170) 9 (84) | p < 0.001; p < 0.001 |
| 6 months | (n = 373) 40 (96) | (n = 378) 46 (98) | (n = 142) 1 (93) | p < 0.001; p < 0.001 |
| Increase in QoL (%),g mean change (SD) | ||||
| 3 months | (n = 510) –24 (27) | (n = 514) –24 (28) | (n = 243) –9 (21) | p < 0.001; p < 0.001 |
| 6 months | (n = 460) –25 (26) | (n = 478) –26 (28) | (n = 207) –12 (23) | p < 0.001; p < 0.001 |
| Proportion of patients with improvement in NYHA class symptoms, % | ||||
| 3 months | (n = 551) 58 | (n = 543) 55 | (n = 242) 24 | p < 0.001; p < 0.001 |
| 6 months | (n = 489) 61 | (n = 497) 57 | (n = 199) 38 | p < 0.001; p < 0.001 |
| Duration of procedure (minutes), median (patients randomised after 1 July 2001) | (n = NR) 164 | (n = NR) 176 | ||
Adverse effects of treatment
| Adverse effect | CRT-P (n = 617) | CRT-D (n = 595) | OPT (n = 308) | p-value: CRT-P vs. OPT; CRT-D vs. OPT |
|---|---|---|---|---|
| Unsuccessful implantation, n/N (%) | 78/617 (13) | 54/595 (9) | ||
| Deaths from procedural complications, n/N (%) | 5/615 (0.8) | 3/595 (0.5) | ||
| Mortality rate 30 days after randomisation, % | 1.0 | 1.8 | 1.2 | 0.34; 0.97 |
| Moderate or severe adverse event from any cause, %a | 66 | 69 | 61 | 0.15; 0.03 |
| Moderate or severe adverse event related to implantation procedure, % | 10 | 8 | ||
| Coronary venous dissection | 0.3 | 0.5 | ||
| Coronary venous perforation | 1.1 | 0.8 | ||
| Coronary venous tamponade | 0.5 | 0.3 | ||
| Withdrawal rate, % | ||||
| For all patients | 6 | 7 | 26 | |
| For patients who had not reached the primary end point | 2 | 2 | 13 | |
-
Allocation to treatment groups: randomisation ratio 1 : 2 : 2 (OPT : CRT-P : CRT-D). Randomisation stratified by centre and beta-blocker use.
-
Blinding: patients, physicians, statisticians, data management group and safety and monitoring board not blinded. Steering committee, end points committee and sponsor were unaware of assignments.
-
Comparability of treatment groups: groups similar at baseline.
-
Method of data analysis: all analyses were carried out according to the ITT principle. Efficacy analyses were based on time to first event (unless otherwise stated), differences were determined using the log-rank statistic and time to event used the Kaplan–Meier method. Nominal p-values and p-values adjusted for sequential monitoring were reported. HRs were unadjusted for covariates, Wald chi-squared statistic used for subgroups. Baseline differences were evaluated using the Wilcoxon rank-sum test for continuous and ordered data and Pearson’s chi-squared test was used for categorical data.
-
Sample size/power calculation: trial designed with 2200 participants to detect a reduction of 25% in the primary end point and rate of death from any cause at an alpha value of 0.02 in the CRT-P group and 0.03 in the CRT-D group, each compared with OPT. With a target of 1000 primary events, the trial had statistical power of > 90% for the primary end point and 80% for the secondary end point. The trial was stopped early when pre-established boundaries had been crossed; 1520 participants had been randomised and 1000 primary end points already or almost met.
-
Attrition/dropout: substantial withdrawals from the OPT group (see Adverse effects of treatment) to receive commercially available implants, because of arrhythmia or HF. Patients contacted to consent to collection of data for the duration of the study; data censored if this information could not be obtained. Status for the primary end point through to the end of the study known for 91% of the OPT group and 99% of the other groups; data on mortality complete for 96% of the OPT group and 99% of the other groups.
-
Generalisability: people with advanced HF and increased QRS interval.
-
Outcome measures: authors state that the composite end point based on both mortality and hospitalisation was chosen to avoid the analytical difficulty encountered with competing risk: death precludes subsequent hospitalisation for chronic HF decompensation. 117 Demonstration of a favourable hospitalisation outcome may be offset by the inability to survive, and benefit of survival may be offset by incremental chronic HF morbidity requiring recurrent hospitalisations.
-
Intercentre variability: not reported.
-
Conflict of interests: authors state that sponsor had no role in data analysis.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Details not reported |
| Allocation concealment | Unclear | Details not reported |
| Performance bias | ||
| Blinding of participants and personnel | High | No blinding |
| Detection bias | ||
| Blinding of outcome assessment | Low | Steering committee and end points committee unaware of assignment. Outcomes objective and unlikely to be influenced |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | ITT analysis. Data censored for people who withdrew and for whom data could not be obtained |
| Reporting bias | ||
| Selective reporting | Low | Protocol published; no evidence of missing outcomes |
| Other bias | ||
| Other sources of bias | Low | |
Multicenter InSync Randomized Clinical Evaluation (MIRACLE)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Abraham et al. 2000122 and 2002,121 St John Sutton et al. 2003,124 US FDA 2001123 Study design: RCT Countries: USA and Canada No. of centres: 45 Funding: Medtronic, Inc., Minneapolis, MN |
Intervention: optimal medical therapy and CRT-P on: VDDa 30, InSync model 8040 (Medtronic), three pacing leads Comparator: optimal medical therapy and CRT-P off: VDI 30 (ventrical paced, atrial and ventricular sensed, no response to sensing), InSync model 8040 (Medtronic Inc.) Other interventions used: medication for HF for both groups kept constant |
Indication for treatment: moderate to severe HF and a prolonged QRS interval No. of randomised participants: 453; CRT-P on: 228, OPT: 225 Inclusion criteria: HF due to ischaemic or non-ischaemic cardiomyopathy for > 1 month, NYHA class III or IV, LVEF ≤ 35%, LVEDD ≥ 55 mm, QRS interval ≥ 130 milliseconds, age ≥ 18 years, 6-minute walk distance ≤ 450 m, optimal medical therapy121,122 Exclusion criteria: pacemaker or ICD, indication for or contraindication to cardiac pacing, cardiac or cerebral ischaemic event within ≤ 3 months, atrial fibrillation within ≤ 1 month, severe primary pulmonary disease, systolic blood pressure > 170 mmHg or < 80 mmHg, heart rate > 140 bpm, serum creatinine > 3.0 mg/dl, serum aminotransferase more than three times the upper limit of normal, unstable angina, acute MI or coronary surgery within ≤ 3 months, life expectancy < 6 months121,122 |
Primary outcomes: NYHA class, QoL, 6-minute walk distance Secondary outcomes: all-cause mortality, HF hospitalisations, exercise capacity (peak oxygen consumption, time on treadmill), LVEF, LVEDD, QRS duration, severity of mitral regurgitation, clinical composite response (improved, worsened or unchanged), an analysis of death or worsening HF (as safety variables), number of days spent in hospital Method of assessing outcomes: questionnaires at baseline and at 1, 3 and 6 months. Clinical events review committee adjudicated adverse events/end points122 Length of follow-up: 6 months Recruitment: November 1998–December 2000 |
Participant characteristics (pre randomisation and ≤ 7 days pre implantation)
| Characteristic | CRT-P (n = 228) | OPT (n = 225) |
|---|---|---|
| Age (years), mean (SD) | 63.9 (10.7) | 64.7 (11.2) |
| Sex, % male | 68 | 68 |
| Ethnicity, % white | 90 | 91 |
| Ischemia, % | 50 | 58 |
| NYHA class III, % | 90 | 91 |
| LVEF (%), mean (SD) | 21.8 (6.3) | 21.6 (6.2) |
| Duration of QRS interval (milliseconds), mean (SD) | 167 (21) | 165 (20) |
| Heart rate (bpm), mean (SD) | 73 (13) | 75 (13) |
| LVEDD (mm), mean (SD) | 70 (10) | 69 (10) |
| Area of mitral regurgitant jet (cm2), mean (SD) | 7.6 (6.4) | 7.2 (4.9) |
| Distance walked in 6 minutes (m), mean (SD) | 305 (85) | 291 (101) |
| MLWHFQ score,a mean (SD) | 59 (20) | 59 (21) |
| Total exercise time (seconds), mean (SD) | 484 (209) | 462 (217) |
| Peak oxygen consumption (ml/kg bodyweight/minute), mean (SD) | 14.0 (3.5) | 13.7 (3.8) |
| Systolic blood pressure (mmHg), mean (SD) | 114 (18) | 115 (18) |
| Diastolic blood pressure (mmHg), mean (SD) | 69 (10) | 68 (10) |
| Receiving digitalis, % | 78 | 79 |
| Receiving diuretic agents, % | 94 | 93 |
| Receiving ACE inhibitors or ARBs, % | 93 | 90 |
| Receiving beta-blockers, % | 62 | 55 |
Results
| Outcome (at 6 months) | CRT-P (n = 228) | OPT (n = 225) | HR (CI 95%), p-value |
|---|---|---|---|
| All-cause mortality, n/N | 12/228 | 16/225 | 0.73 (0.34 to 1.54), 0.40 |
| Hospitalisations for worsening HF | |||
| People, n/N | 18/228 | 34/225 | 0.50 (0.28 to 0.88), 0.02 |
| Events, n | 25 | 50 | |
| Total no. of days | 83 | 363 | |
| Death or worsening HF requiring hospitalisation, n/N | 28/228 | 44/225 | 0.60 (0.37 to 0.96), 0.03 |
| Death or worsening HF requiring hospitalisation or intravenous treatment, n/N | 36/228 | 55/225 | 0.61 (0.40 to 0.93), 0.02 |
| Worsening HF leading to use of intravenous, n/N | |||
| Diuretic agents | 13/228 | 24/225 | 0.51 (0.26 to 1.00), 0.05 |
| Vasodilators or positive inotropic agents | 6/228 | 14/225 | 0.41 (0.16 to 1.08), 0.06 |
| Medication for HF | 16/228 | 35/225 | 0.43 (0.24 to 0.77), 0.004 |
| Change in NYHA class (primary outcome), n/N (%) | p < 0.001 | ||
| Improved by two or more classes | 34/211 (16) | 12/196 (6) | |
| Improved by one class | 109/211 (52) | 62/196 (32) | |
| No change | 64/211 (30) | 115/196 (59) | |
| Worsened | 4/211 (2) | 7/196 (4) | |
| Change in distance walked in 6 minutes (m), median (95% CI) (primary outcome) | (n = 214) +39 (26 to 54) | (n = 198) +10 (0 to 25) | p = 0.005 |
| Change in MLWHFQ score, median (95% CI) (primary outcome) | (n = 213) –18 (–22 to –12) | (n = 193) –9 (–12 to –5) | p = 0.001 |
| Change in peak oxygen consumption (ml/kg/minute), median (95% CI) | (n = 158) +1.1 (0.6 to 1.7) | (n = 145) +0.2 (–0.2 to 0.8) | p = 0.009 |
| Change in total exercise time (seconds), median (95% CI) | (n = 159) +81 (62 to 119) | (n = 146) +19 (–1 to 47) | p = 0.001 |
| Absolute change in LVEF (%), median (95% CI) | (n = 155) +4.6 (3.2 to 6.4) | (n = 146) –0.2 (–1.0 to 1.5) | p < 0.001 |
| Change in LVEDD (mm), median (95% CI) | (n = 90) –3.5 (–6 to –1) | (n = 98) 0.0 (–1 to 2) | p < 0.001 |
| Change in area of mitral regurgitation jet (cm2), median (95% CI) | (n = 116) –2.7 (–4.0 to –2.1) | (n = 118) –0.5 (–1.1 to 0.0) | p < 0.001 |
| Change in QRS duration (ms), median (95% CI) | (n = 206) –20 (–20 to –12) | (n = 192) 0 (–10 to 0) | p < 0.001 |
| Clinical composite HF score | p < 0.001 | ||
| Improved, % | 67 | 39 | |
| Worsened, % | 16 | 27 | |
Adverse effects of treatment
| Adverse effect | CRT-P (n = 228) | OPT (n = 225) |
|---|---|---|
| Hospitalised for repositioning or replacement of left ventricular lead,a n | 11 | 3 |
| Hospitalisations not related to HF or function of left ventricular lead,a n | 37 | 33 |
| All participants undergoing implantation (n = 571) | ||
| Complete heart block requiring permanent cardiac pacing, n/N | 2/571 | |
| Death from progressive hypotension, n/N | 1/571 | |
| Asystole, resuscitated but died 1 month later, n/N | 1/571 | |
| Coronary sinus dissection, n/N (%) | 23/571 (4) | |
| Cardiac vein or coronary sinus perforation (three of these recovered and continued in the study), n/N (%) | 12/571 (2) | |
| Participants who underwent successful implantation (n = 528) | ||
| Left ventricular lead repositioned, n/N | 20/528 | |
| Left ventricular lead replaced, n/N | 10/528 | |
| Pacemaker-related infection requiring explantation, n/N | 7/528 | |
-
Allocation to treatment groups: randomisation in permuted blocks to ensure balance between groups within centres. Sealed envelopes used.
-
Blinding: patients and physicians treating them for HF and performing study evaluations were unaware of treatment assignments. An electrophysiologist who was not involved with clinical care opened a sealed envelope at the time of randomisation, programmed the device and performed all tests that could reveal the identity of the pacing mode.
-
Comparability of treatment groups: authors state that groups were similar with respect to all baseline characteristics.
-
Method of data analysis: authors state that all end points were analysed according to the ITT principle. For continuous variables, comparisons of changes from baseline to 6 months between groups were evaluated using the Wilcoxon rank-sum test. The chi-squared test was used for categorical end points. Only patients with data at baseline and 6 months were included in these analyses, but results were similar if patients with incomplete data were included, using the last value carried forward. Cumulative survival curves for the risk of a major clinical event used the Kaplan–Meier method and were tested for significance using the log-rank statistic. Cox proportional hazards regression models were used to estimate HRs.
-
Sample size/power calculation: sample size of 224 patients per group estimated on the basis of the assumption that the study would have 80% power (two-sided α = 0.0167) to detect a difference in NYHA class of 0.75, in QoL of 13 points or in distance walked in 6 minutes of 50 m.
-
Attrition/dropout: in total, 571 agreed to participate, with 528 successfully implanted and 43 not successfully implanted. Of those who were successfully implanted, two required cardiac pacing, two became clinically unstable, 71 were enrolled in the initial pilot phase and 453 were randomised to main study. OPT group: 24/225 did not complete the 6-month follow-up (16 died, two had a heart transplant, one had complications related to the device and five missed the 6-month visit). CRT-P group: 13/228 did not complete the 6-month follow-up (12 died and one had complications related to the device). No patient was lost to follow-up for the analysis of death or worsening HF. In total, 10/225 in the control group crossed over to the CRT-P group, seven because of worsening HF and three because of bradycardia.
-
Generalisability: only those successfully implanted underwent randomisation. Generalisability limited to people with moderate to severe HF and a prolonged QRS interval.
-
Outcome measures: clinical events review committee adjudicated with regard to adverse events/end points. QoL was assessed using a validated questionnaire.
-
Intercentre variability: not reported.
-
Conflict of interests: stated. some of the authors are consultants or investigators for, or employees of, Medtronic; one author was also on the advisory board of St Jude Medical. Authors state that investigators had full access to all data and performed analyses without restrictions or limitations from the sponsor.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Randomised in permuted blocks; further details not reported |
| Allocation concealment | Unclear | Sealed envelopes used but unclear if they were opaque and sequentially numbered |
| Performance bias | ||
| Blinding of participants and personnel | Low | Patients and physicians treating them for HF and performing study evaluations were unaware of treatment assignments |
| Detection bias | ||
| Blinding of outcome assessment | Low | Patients and physicians treating them for HF and performing study evaluations were unaware of treatment assignments |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Primary outcomes | Unclear | States ITT analysis used and attrition reported; also reports that analysis included last value carried forward analysis. However, numbers are low for NYHA class (primary outcome) without giving reasons why |
| Secondary outcomes | Unclear | Reasons for different sample sizes unclear |
| Reporting bias | ||
| Selective reporting | High | SF-36 is included in the protocol paper122 but results for this measure are not reported |
| Other bias | ||
| Other sources of bias | Low | |
Multisite Stimulation in Cardiomyopathies (MUSTIC) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Cazeau et al. 2001125 Study design: randomised crossover study Countries: Europe (France, Germany, Italy, Sweden, Switzerland, the UK) No. of centres: 15 Funding: ELA Recherche, Medtronic and the Swedish Heart and Lung Association and by a grant from the Swedish Medical Research Council |
Intervention: CRT-P on: atrioventricular (active) pacing [Chorum 7336 MSP (ELA Medical), and InSync 8040 (Medtronic Inc.)] Comparator: CRT-P off: ventricular inhibited (inactive) pacing at a basic rate of 40 bpm Other interventions used: no modification to medication other than adjustment of dose of diuretic permitted. OPT (n = 67): ACE inhibitors or equivalent 96%, diuretics 94%, digoxin 48%, amiodarone 31%, beta-blockers 28%, spirololactone 22% |
Indication for treatment: severe HF and major intraventricular delay but without standard indications for a pacemaker No. of enrolled participants: 67 No. of randomised participants: 58; group 1 (CRT-P on, CRT-P off): 29, group 2 (CRT-P off, CRT-P on): 29 Inclusion criteria: severe HF because of idiopathic or ischaemic LVSD, NYHA class III for ≥ 1 month whilst on OPT, LVEF < 35%, LVEDD > 60 mm; QRS interval > 150 milliseconds, in sinus rhythm, without a standard indication for a pacemaker Exclusion criteria: hypertrophic or restrictive cardiomyopathy, suspected acute myocarditis, correctable valvulopathy, acute coronary syndrome lasting < 3 months, coronary revascularisation during last 3 months or scheduled revascularisation, treatment-resistant hypertension, severe obstructive lung disease, inability to walk, life expectancy < 1 year not associated with cardiovascular disease, indication for an ICD |
Primary outcome: distance walked in 6 minutes Secondary outcomes: QoL, peak oxygen uptake, hospital admissions because of decompensated HF, patient preference, death Method of assessing outcomes: assessed at baseline (4 weeks before implantation), at randomisation (2 weeks after implantation) and at the end of each crossover phase. QoL measured using the MLWHFQ (total score 0–105, higher score indicates worse QoL). The 6-minute walk test was carried out according to Guyatt et al. and Lipkin et al. (references provided): two tests at each visit with an interval of at least 3 hours between them; the maximal difference between the two tests was 15% and the value recorded was the mean of the results of the two tests. Patient preference – at the end of the crossover phase patients were asked which 3-month period they preferred Length of follow-up: participants received the intervention and the comparator for 3 months each in random order Recruitment: March 1998–March 1999 |
Participant characteristics (at randomisation 2 weeks post implant)
| Characteristic | Group 1 (CRT-P on, CRT-P off) (n = 29) | Group 2 (CRT-P off, CRT-P on) (n = 29) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 64 (11) | 64 (8) | 0.91 |
| Sex, male, n/N | 19/29 | 24/29 | 0.13 |
| Ethnicity | NR | NR | |
| NYHA class III, % | 100 | 100 | |
| Weight (kg), mean (SD) | 79 (19) | 78 (16) | 0.97 |
| Distance walked in 6 minutes (m), mean (SD) | 354 (110) | 346 (111) | 0.82 |
| Peak VO2 (ml/kg of body weight/minute), mean (SD) | 13.5 (8.4) | 14.1 (4.6) | 0.41 |
| QoL score, mean (SD) | 48 (19) | 46 (25) | 0.66 |
| Heart rate (bpm), mean (SD) | 75 (12) | 75 (14) | 0.89 |
| QRS interval (milliseconds), mean (SD) | 172 (22) | 175 (19) | 0.48 |
Results
| Outcome | CRT-P on | CRT-P off | p-value |
|---|---|---|---|
| Mortality over 6-month period | |||
| First crossover period: sudden death after 26 days of active pacing | 1 | ||
| Second crossover period: acute MI few hours after premature switch to active pacing as a result of severe decompensation | 1 | ||
| Second crossover period: sudden death 2 hours after switching from inactive to active pacing | 1 | ||
| Distance walked in 6 minutes (m), mean (SD)a | |||
| Group 1 (CRT-P on, CRT-P off) (n = 22) | 384.1 (78.9) | 336.1 (128.3) | |
| Group 2 (CRT-P off, CRT-P on) (n = 24) | 412.9 (116.9) | 316.2 (141.8) | |
| Both groups (n = 46) | 399.2 (100.5) | 325.7 (134.4) | p < 0.001 |
| Peak VO2 (ml/kg of body weight/minute), mean (SD) | |||
| Group 1 (CRT-P on, CRT-P off) (n = 18) | 15.9 (5.8) | 15.3 (5.9) | |
| Group 2 (CRT-P off, CRT-P on) (n = 20) | 16.4 (3.6) | 14.8 (3.9) | |
| Both groups (n = 38) | 16.2 (4.7) | 15 (4.9) | p = 0.029 |
| QoL score, mean (SD) | |||
| Group 1 (CRT-P on, CRT-P off) (n = 23) | 33.3 (22) | 42.6 (20.9) | |
| Group 2 (CRT-P off, CRT-P on) (n = 22) | 25.7 (20.4) | 44 (25) | |
| Both groups (n = 45) | 29.6 (21.3) | 43.2 (22.8) | p < 0.001 |
| HF hospitalisations at 3 months (first crossover period only), n/N | 3/29 | 9/29 | p < 0.05 |
| Patient preference after 6 months (n = 48),b n/N (%) | 41/48 (85) | 2/48 (4) | p < 0.001 |
Adverse effects of treatment
| Adverse effect | CRT-P on | CRT-P off | p-value |
|---|---|---|---|
| Uncorrectable loss of left ventricular pacing efficacy, n | 2 | ||
| Severe decompensation leading to a premature switch to active pacing, n | 1 | ||
| Decompensation attributed to rapidly progressive aortic stenosis, n | 1 | ||
| Decompensation due to persistent atrial fibrillation, n | 1 |
-
Allocation to treatment groups: randomisation of order of treatment followed a block design with stratification according to study centre. Authors also state that patients were ‘randomly assigned to and equally distributed between the two study groups.
-
Blinding: described as single blind. Authors state that patients had no knowledge of the order of treatment but no details are provided.
-
Comparability of treatment groups: similar.
-
Method of data analysis: authors state that all analyses are based on the ITT principle; thus, all enrolled patients were included in the analysis but each efficacy end point could be assessed only in patients with no data missing after the completion of both crossover phases. Baseline characteristics were assessed using the chi-squared test for dichotomous variables and the Student’s t-test or Wilcoxon non-parametric test for quantitative or categorical variables. Responses obtained for all criteria assessing clinical efficacy were compared using the Wilcoxon test and according to a two-period and two-treatment (two-by-two) crossover design. Period and carry-over effects were checked before the efficacy of treatment was evaluated. Morbidity and mortality were compared during the first crossover period and were described for all other phases of the study. The stability of the results was assessed in a per-protocol analysis, which included only patients without any deviations from the protocol. The authors state that no significant carry-over and period effects were noted. Threshold of significance 0.05
-
Sample size/power calculation: on the basis of previous reports of mortality rates in NYHA class III, a 10% mortality rate at 6 months was estimated. A 10% failure rate of left ventricular lead implantation and a 20% rate of premature termination because of loss of left ventricular pacing efficacy of unstable HF was expected. A 10% increase in the distance walked in 6 minutes with active pacing was estimated. The total target sample needed was estimated to be 22 patients for a study with a 95% confidence level and 95% power. For the MLWHFQ score, a predicted 10% reduction with active pacing necessitated a 30-patient sample. Considering mortality and dropouts, 40 patients were needed
-
Attrition/dropout: three patients withdrew before implantation, two with unstable HF (one subsequently died) and one with a pre-existing indication for pacing. Implantation of a left ventricular lead was attempted in 64 patients. In six patients it was removed before randomisation, five because of failed implantation of the left ventricular lead and one because of sudden death while the device was inactive. A total of 10 patients did not complete two crossover periods: first crossover period: one withdrew consent at randomisation, two had uncorrectable loss of ventricular pacing efficacy, one switched from inactive to active pacing because of severe decompensation and one died suddenly after 26 days of active pacing; second crossover period: three had worsening HF (one had decompensation with active pacing, one had decompensation during inactive pacing), one died suddenly after switching to active pacing and one had lung cancer
-
Generalisability: patients were randomised 2 weeks after implantation. Only patients who were successfully implanted were randomised.
-
Outcome measures: appropriate but change in NYHA class not reported.
-
Intercentre variability: not reported.
-
Conflict of interests: part funded by ELA Recherche and Medtronic. Four authors are paid consultants of Medtronic or ELA Recherche and one author is an employee of ELA Recherche.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Details not reported |
| Allocation concealment | Unclear | Details not reported |
| Performance bias | ||
| Blinding of participants and personnel | High | Authors state that participants had no knowledge of the order of treatments, but not clear how this was maintained. Personnel not blinded; 6-minute walk test and QoL outcomes may be influenced by lack of blinding |
| Detection bias | ||
| Blinding of outcome assessment | High | States ‘single blind’ so assume only participants were blinded |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Numbers and reasons reported |
| Reporting bias | ||
| Selective reporting | High | Change in NYHA class assessed but data not reported |
| Other bias | ||
| Other sources of bias | High | Use of block randomisation without blinding means that it may be possible to predict future assignments. Crossover design appears appropriate |
Appendix 9 Data extraction: people with both conditions
CONTAK-CD trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Higgins et al. 2003,126 Lozano et al. 2000,128 US FDA 2002,129 Saxon et al. 1999127 Study design: crossover RCT in phase I; parallel RCT in phase II Country: USA (see General comments, Intercentre variability) No. of centres: 47 Funding: Guidant Corporation, St Paul, MN |
Intervention: CRT-D + OPT Comparator: ICD + OPT Devices were either Model 1822 Ventak CHF Automatic Implantable Cardioverter Defibrillator or Model 1283 Contak CD device (Guidant Corporation) Initially, the left ventricle was paced with a commercially available epicardial pace/sense lead. Later, a lead that could be placed transvenously using over-the-wire techniques in the coronary venous vasculature was introduced. A cardioversion/defibrillation lead was implanted in the right ventricle and a pace/sense lead was placed in the right atrium for this three-lead CRT system. Details of lead positioning are reported but have not been data extracted Randomised therapy programmed after a minimum 30-day period with no CRT. During this period investigators were permitted to optimise pharmacological therapy. OPT not defined Other interventions used: none stated |
Indication for treatment: patients with symptomatic HF, intraventricular conduction delay and malignant ventricular tachyarrhythmias (VT/VF) requiring therapy from an ICD No. of randomised participants: 490; CRT-D: 245, ICD: 245 Inclusion criteria: NYHA class II–IV, LVEF ≤ 35%, QRS interval ≥ 120 milliseconds, conventional indications for an ICD (American College of Cardiology/American Heart Association guidelines),126 age ≥ 18 years, symptomatic HF despite OPT (must include ACE inhibitors if tolerated)127 Exclusion criteria: atrial tachyarrhythmias or conventional indications for a permanent pacemaker,126 concomitant cardiac surgery, unable to undergo device implant, unable to comply with protocol and follow-up including exercise testing, life expectancy < 6 months because of other conditions, amyloid disease, hypertrophic obstructive cardiomyopathy, requires in-hospital continuous intravenous inotropes, use of pre-existing cardioversion/defibrillation leads other than those specified in the protocol, involved in other cardiovascular clinical investigations of active therapy or treatment127 |
Primary outcome: progression of HF, defined as a composite end point of all-cause mortality, hospitalisation for worsening HF, ventricular tachyarrhythmias requiring device therapy (initially the primary outcome was peak VO2 but this was changed when the study design was changed) Secondary outcomes: VO2, QoL, 6-minute walk distance, biventricular antitachycardia pacing efficacy, defibrillation therapy safety127 Method of assessing outcomes: VO2 assessed by cardiopulmonary exercise test.127 QoL measured using the MLWHFQ. A Heart Failure Events Committee adjudicated all deaths and hospitalisations. Operative mortality was defined as death from any cause within 30 days of the implant procedure Length of follow-up: maximum of 6 months (but some patients, presumed to be all those in phase I, only 3 months) Recruitment: February 1998–December 2000 |
Participant characteristics
| Participant characteristics | CRT-D (n = 245) | ICD (n = 245) | p-value |
|---|---|---|---|
| Age (years), mean (SD)a | 66 (11) | 66 (11) | |
| Sex, % male | 85 | 83 | |
| Ethnicity | NR | NR | |
| Aetiology ischaemic, % | 67 | 71 | |
| NYHA class, % | |||
| II | 32 | 33 | |
| III | 60 | 57 | |
| IV | 8 | 10 | |
| LVEF (%), mean (SD)a | 21 (7) | 22 (7) | |
| QRS interval (milliseconds), mean (SD)a | 160 (27) | 156 (26) | |
| Intraventricular conduction delay, % | |||
| LBBB | 54 | 55 | |
| Non-specific | 32 | 33 | |
| RBBB | 14 | 12 | |
| Diuretic, % | 88 | 83 | |
| ACE inhibitor/ARB, % | 86 | 89 | |
| Beta-blocker, % | 48 | 46 | |
| Digoxin, % | 69 | 68 | |
| Peak VO2 (ml/kg/minute), mean (SD)a | 13.8 (4.6) | 13.5 (3.8) | |
| QoL score, mean (SD)a | 44 (25) | 40 (23) | |
| 6-minute walk distance (m), mean (SD)a | 316 (119) | 320 (121) | |
| LVID in diastole (mm), mean (SD)a | 71 (11) | 70 (10) | |
| LVID in systole (mm), mean (SD)a | 59 (11) | 58 (11) | |
| Heart rate | NR | NR | |
| Cardiac history | NR | NR | |
| Previous treatment | NR | NR | |
| Comorbidities | NR | NR | |
Results
| Outcome | CRT-D (n = 245) | ICD (n = 245) | p-value |
|---|---|---|---|
| Progression of HF, n/N | 79/245 | 94/245 | 0.35 |
| Mortality | 11/245 | 16/245 | |
| HF hospitalisations (at least one) | 32/245 | 39/245 | |
| At least one VT/VF event | 36/245 | 39/245 | |
| All-cause mortality,a n | 109 | ||
| Death during the study treatment phase (detail by group below) | 27 | ||
| Death during the long-term follow-up phase | 70 | ||
| Cause of death, n/N (%) | |||
| Pump failure | 47/109 (43) | ||
| Non-cardiac | 21/109 (19) | ||
| Arrhythmic | 9/109 (8) | ||
| Ischaemic | 2/109 (2) | ||
| Cardiac in nature but unknown aetiology | 2/109 (2) | ||
| Insufficient information for independent events committee to be able to adjudicate | 28/109 (26) | ||
| Deaths during the study treatment phase,129 n/N (%) | 11/245 (4.5) | 16/245 (6.5) | |
| Cardiac, pump failure | 4/245 (1.6) | 9/245 (3.7) | |
| Cardiac, arrhythmic | 1/245 (0.4) | 0/245 (0) | |
| Cardiac, other | 2/245 (0.8) | 1/245 (0.4) | |
| Non-cardiac | 2/245 (0.8) | 3/245 (1.2) | |
| Unknown | 2/245 (0.8) | 3/245 (1.2) | |
| Total survival, % | |||
| At 1 year | 85 | ||
| At 2 years | 74 | ||
| At 3 years | 70 | ||
| Received appropriate treatment of ventricular tachyarrhythmias, n/N (%) | 36/245 (15) | 39/245 (16) | |
| VT alone | 25/245 (10) | 27/245 (11) | |
| VF alone | 7/245 (3) | 6/245 (2) | |
| VT and VF | 4/245 (2) | 6/245 (2) | |
| VT/VF episodes during therapy evaluation phase (excluding those with no episodes), median | 2.5 | 2 | |
| QoL score, mean change (SE)b | –7 (2) (n = 234) | 5 (2) (n = 225) | 0.39 |
| Change in NYHA class, % | (n = 109) | (n = 116) | |
| Improved by two classes | 11 | 2 | |
| Improved by one class | 25 | 30 | 0.10c |
| No change | 51 | 51 | |
| Worsened | 13 | 17 | |
| LVEF (%), mean change (SE)b | 5.1 (0.7) (n = 222) | 2.8 (0.7) (n = 216) | 0.020 |
| LVID in diastole (mm), mean change (SE)b | –3.4 (0.6) (n = 228) | –0.3 (0.6) (n = 219) | < 0.001 |
| LVID in systole (mm), mean change (SE)b | –4.0 (0.7) (n = 228) | –0.7 (0.7) (n = 219) | < 0.001 |
| Peak VO2 (ml/kg/minute), mean change (SE)b | 0.8 (0.3) (n = 216) | 0.0 (0.3) (n = 201) | 0.030 |
| 6-minute walk distance (m), mean change (SE)b | 35 (7) (n = 224) | 15 (7) (n = 220) | 0.043 |
Adverse effects of treatment
| Adverse effect | CRT-D and ICD | ||
|---|---|---|---|
| Operative mortality126,129 | 12/567 (2.1%) (95% CI 0.9% to 3.3%) | ||
| Causes of death for operative mortality,129 n | Implants (n = 501) | Attempts (n = 66) | Total (n = 567) |
| Total deaths | 10 | 2 | 12 |
| Cardiac: pump failure | 5 | 1 | 6 |
| Cardiac: arrhythmic | 2 | 1 | 3 |
| Non-cardiaca | 2 | 0 | 2 |
| Unknown | 1 | 0 | 1 |
| Overall lead-related adverse event rate | n = 75 (unique patients), 14.5% (95% CI 11.5% to 17.5%) | ||
| Lead-related, n/N | 53/448 | ||
| Procedure-related, n/N | 27/517 | ||
| Severe device-related events, n patients/N | 7/567 (1.2% with at least one event) | ||
| Telemetry difficulty; device explanted | 2 (0.4%, 95 CI 0.0% to 0.9%) | ||
| VT during cardiopulmonary exercise testing | 1 (0.2%, 95 CI 0.0% to 0.5%) | ||
| Coronary sinus perforation | 1 (0.2%, 95 CI 0.0% to 0.5%) | ||
| Inappropriate shock because of oversensing | 1 (0.2%, 95 CI 0.0% to 0.5%) | ||
| Lead dislodgement | 1 (0.2%, 95 CI 0.0% to 0.5%) | ||
| Anaphylaxis in association with use of pulmonary artery catheter | 1 (0.2%, 95 CI 0.0% to 0.5%) | ||
| Device-related complications (only those occurring in > 1% of patients) in all patients implanted (n = 448), n (%) | |||
| Loss of left ventricular capture | 31 (6.9) | ||
| Loss of right atrial capture | 7 (1.6) | ||
| Ventricular oversensing | 6 (1.3) | ||
| Extracardiac stimulation | 5 (1.1) | ||
| Device-related complications (only those occurring in > 1% of patients) in all patients attempted or implanted (n = 517), n (%) | |||
| Infections | 7 (1.4) | ||
-
Allocation to treatment groups: not described.
-
Blinding: double blind.
-
Comparability of treatment groups: groups are described as balanced with no statistically significant differences with respect to baseline characteristics (no statistical testing reported).
-
Method of data analysis: patients from phase I contributed data from a 3-month treatment phase and patients from phase II contributed data from a 6-month treatment phase for the analysis of the primary end point. The 3-month treatment phase correlates to the first study period (i.e. before any crossover). Cox proportional hazards models were fitted for the combination of events with the treatment effect adjusted for covariates chosen by the Heart Failure Events Committee before the primary end point analysis. The covariates included NYHA class, QRS interval, ischaemic aetiology, LVEF and bundle branch morphology. The Wei method (reference provided) was used to calculate a composite effect of the treatment and covariates. For continuous variables the longitudinal (repeated measures) analysis method (reference provided) was used to compare the difference in the sample means. This method accounted for the patterns of missing data and took full advantage of the correlation structure, and all of the data were used to estimate the model parameters. Model parameters were estimated using maximum likelihood. Values of p < 0.05 were considered to be significant for all tests. The events contributing to the composite primary end point appear to be analysed using the ITT principle. It is clear from the numbers reported for the secondary outcomes that analyses for change in QoL, NYHA class, LVEF, LVID in diastole and in systole, peak VO2 and 6-minute walk distance are not analysed using the ITT principle. No reasons are given for the missing data. The study authors do not comment on whether the alteration of the study design between phase I and phase II of the study was expected to have an impact on the methods of data analysis.
-
Sample size/power calculation: not described, although Higgins et al. 126 state that it was postulated that the therapy would reduce the events contributing to the composite primary end point by 25%. However, the actual event rate observed was approximately half that expected in the original study design and consequently the authors state that the study was not adequately powered to detect a statistically significant difference in HF events.
-
Attrition/dropout: initially 581 patients were enrolled (248 in phase I and 333 in phase II) but 14 either withdrew consent or were withdrawn by the investigator (found not to meet eligibility criteria) before an implant procedure and 66 did not receive the system being used in this trial because of the inability to place the coronary venous lead. These patients received a conventional ICD instead. Therefore, 501 were implanted (222 in phase I and 279 in phase II) with the intervention system. Of these, 448/501 (89%) received a transvenous system and 53/501 (11%) received a transthoracic system (phase I: 51, phase II: 2). Of the 501 patients implanted, 11 did not enter the randomised part of the study 30 days after the implant procedure [10 patients died (see Adverse effects) and one withdrew in the 30-day post-implant recovery period before the randomised therapy was programmed]. As noted above, not all analyses used the ITT principle and, when data are missing, no reasons for this are provided.
-
Other: (1) the study design was modified because of regulatory concerns about morbidity and mortality associated with CRT and the length of follow-up in the randomised mode of the initial design. This meant that the design changed from a crossover RCT design (crossover to occur after the first 3 months of randomised therapy) to a parallel RCT design with 6 months of follow-up in phase II. (2) During the course of the trial positive clinical trial results led to the widespread adoption of HF medications such as beta-blockers and spironolactone. There was also an evolution in HF management, focusing on increased outpatient surveillance. Both of these factors may have contributed to the reduction in the number of HF events expected. The improvement seen in many patients once medical management was optimised before randomisation also may have made it more difficult to show a benefit of treatment in healthier patients, and may have contributed to the reduction in statistical power to show improvement in those patients who remained in NYHA class III/IV despite optimal HF medication.
-
Generalisability: the authors point out that the results may not be generalisable to patients with chronic atrial fibrillation, chronotropic incompetence and sinus bradycardia. The study also only studied CRT delivered in an atrial synchronous manner (i.e. the VDD modea). Therefore, the effects of atrial pacing as well as adaptive-rate pacing delivered with the DDD(R) modesb are not known.
-
Outcome measures: appear to be appropriate; however, the reason(s) why the study sponsor decided to change the primary end point from peak VO2 to a composite HF outcome are not provided.
-
Intercentre variability: the key paper for this study126 and the summary of safety and effectiveness for the device used129 state that the centres were based in the USA. However, an earlier paper reporting on phase I of the study128 states that patients were enrolled from sites in the USA, Europe and Australia (number of centres not reported). Therefore, it is not clear whether all or only some of the trial centres involved in phase I contributed data to the key paper for the study.
-
Conflict of interests: not stated but note that the study sponsor (manufacturer of the device) chose to change the primary end point during the course of the study.
-
Other: the chief sources of information for this data extraction were the peer-reviewed publications of Higgins et al. ,126 Saxon et al. 127 and Lozano et al. 128 As operative mortality was the only adverse event reported by the key trial paper,126 the summary of safety and effectiveness129 submitted by the manufacturer, Guidant Corporation, to the FDA as part of its approvals process was used as a source of adverse event data.
LVID, left ventricular internal diameter.
The pacemaker senses atrial and ventricular activity, but paces only in the ventricle.
Dual-chamber pacing and sensing, both triggered and inhibited mode, with rate modulation (increases the patient’s heart rate in response to exercise).
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Study described as randomised controlled study but no further details provided |
| Allocation concealment | Unclear | No details provided |
| Performance bias | ||
| Blinding of participants and personnel | Low | Study described as double blind. ‘Both the patient and the heart failure specialist treating the patient are blinded to the pacing mode’127 |
| Detection bias | ||
| Blinding of outcome assessment | Low | Study described as double blind. ‘Both the patient and the heart failure specialist treating the patient are blinded to the pacing mode’127 ‘A Heart Failure Event Committee (HFEC) adjudicated all deaths and hospitalisations’.126 It is not clear whether this committee was blind to the pacing mode. However, these outcomes are unlikely to have been influenced by a lack of blinding |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Primary outcome: progression of HF (composite including mortality, HF hospitalisations and VT and VF events) | Low | From the data provided these analyses appear to account for all participants |
| Change in QoL, NYHA class, LVEF, LVID in diastole and systole, peak VO2 and 6-minute walk distance | High | It is clear from the numbers provided that there are missing data. No reasons for missing data are given |
| Reporting bias | ||
| Selective reporting | Low | A description of the study is available127 and the only outcome mentioned here that is missing from the published papers is blood laboratory tests. However, these are not likely to be a key outcome for this intervention |
| Other bias | ||
| Other sources of bias | Unclear | The study design and primary outcome measure were changed during the course of the study. The length of follow-up from phase I was 3 months whereas that from phase II was 6 months. The potential for these issues to introduce a bias into the results is unknown |
Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Moss et al. 2005131 and 2009,130 Solomon et al. 2010,132 Goldenberg et al. 2011,133,134 Arshad et al. 2011135 Study design: RCT Countries: USA, Canada and Europe No. of centres: text states 110: 88 in the USA, 2 in Canada and 20 in Europe (the Czech Republic 1, Denmark 1, France 1, Germany 4, Hungary 1, Italy 3, Israel 3, Poland 1, Spain 2, Switzerland 1, the Netherlands 3, UK 1). Inconsistency between numbers reported in the text and appendix Funding: supported by a research grant from Boston Scientific to the University of Rochester with funds distributed to the co-ordination and data centre, enrolling centres, core laboratories, committees and boards under subcontracts from the University of Rochester, NY |
Intervention: CRT-ICD. Programmed mode was DDD with lower rate of 40 bpm and hysteresis off Comparator: ICD only. Programmed pacing mode was VVI for single-chamber units and DDIa for dual-chamber units with lower rates of 40 bpm and hysteresis off in both single- and dual-chamber units. Commercially available transvenous devices (Boston Scientific) were used Other interventions used: OPT for HF131 |
Indication for treatment: mild cardiac symptoms, reduced ejection fraction and wide QRS complex. All met the guideline indication for ICD therapy No. of participants: 1820 (1271 in the USA, 22 in Canada, 527 in Europe); CRT-ICD: 1089, ICD only: 731 Inclusion criteria: NYHA class I or II, LVEF ≤ 30%, QRs interval ≥ 130 milliseconds, age ≥ 21 years with ischaemic cardiomyopathy (NYHA class I or II) or non-ischaemic cardiomyopathy (NYHA class II only), sinus rhythm, ejection fraction < 30% and prolonged intraventricular conduction with QRs duration of > 130 milliseconds; met guideline indication for ICD therapy Exclusion criteria: existing indication for CRT; implanted pacemaker, ICD or resynchronisation device; NYHA class III or IV symptoms; previous CABG surgery, percutaneous coronary intervention or an enzyme-positive MI up to 3 months before enrolment; NYHA class I with non-ischaemic cardiomyopathy; those with angiographic evidence of coronary disease who are candidates for coronary revascularisation and who are likely to undergo a procedure in the foreseeable future; second- or third-degree heart block; irreversible brain damage from pre-existing cerebral disease; women who are pregnant or planning to become pregnant; reversible non-ischaemic cardiomyopathy; chronic atrial fibrillation up to 1 month before enrolment; presence of other life-limiting disease, e.g. cancer; participating in other trials; unwilling to co-operate; living too distant from clinic for ease of follow-up visits; unlikely to be resident in the area for duration of the trial; unwilling to consent |
Primary outcomes: death or non-fatal HF events (whichever came first) Secondary outcomes: none reported Method of assessing outcomes: baseline 12-lead ECG and echocardiogram; baseline physical examination and 6-minute walk test. Two-dimensional echocardiography assessed changes in left ventricular volumes and ejection fraction between baseline and 1-year follow-up. Volumes were estimated by averaging those derived from the two-chamber and four-chamber views according to Simpson’s method (no reference provided). States that ejection fraction was calculated in the usual fashion (no further details or reference) Diagnosis of HF required signs and symptoms consistent with CHF that was responsive to intravenous decongestive therapy (outpatient basis) or an augmented decongestive regimen with oral or parenteral medication during inpatient hospital stay Clinical follow-up 1 month after randomisation and then at 3-month intervals until termination of the trial. Clinical and device testing carried out at each visit Length of follow-up: to trial termination. The trial was stopped on 22 June 2009. Average follow-up was 2.4 years Recruitment dates: 22 December 2004–23 April 2008 |
Participant characteristics
| Characteristic | CRT-ICD (n = 1089) | ICD (n = 731) |
|---|---|---|
| Age (years), mean (SD) | 65 (11) | 64 (11) |
| Sex, male, n (%) | 814 (74.7) | 553 (75.6) |
| Ethnicity, n/N (%) | ||
| White | 979/1083 (90.4) | 657/724 (90.7) |
| Black | 87/1083 (8.0) | 56/724 (7.7) |
| Other | 17/1083 (1.6) | 11/724 (1.5) |
| Cardiac history and NYHA class, n (%) | ||
| Ischaemic heart disease, class I | 152 (14.0) | 113 (15.5) |
| Ischaemic heart disease, class II | 446 (41.0) | 288 (39.4) |
| Non-ischaemic heart disease, class II | 491 (45.1) | 330 (45.1) |
| NYHA class III or IV > 3 months before enrolment, n (%) | 109 (10.0) | 73 (10.0) |
| Cardiac findings at enrolment | ||
| Blood pressure (mmHg), mean (SD) | ||
| Systolic | 124 (17) | 121 (18) |
| Diastolic | 72 (10) | 71 (10) |
| Blood urea nitrogen ≥ 26 mg/dl (9.3 mmol/l), n/N (%) | 260/1082 (24.0) | 177/721 (24.5) |
| Creatinine (mg/dl), mean (SD) | 1.2 (0.4) | 1.2 (0.4) |
| LBBB, n/N (%) | 761/1088 (69.9) | 520/729 (71.3) |
| RBBB, n/N (%) | 136/1088 (12.5) | 92/729 (12.6) |
| QRS duration ≥ 150 milliseconds, n (%) | 699 (64.2) | 476 (65.1) |
| LVEF, mean (SD) | 0.24 (0.05) | 0.24 (0.05) |
| 6-minute walk distance (m), mean (SD) | 359 (107) | 363 (108) |
| Heart rate | NR | NR |
| ECG or Doppler findings (ml), mean (SD) | ||
| Left ventricular end-diastolic volume | 245 (60) | 251 (65) |
| Left ventricular end-systolic volume | 175 (48) | 179 (53) |
| Medication, n (%) | ||
| Aldosterone antagonist | 352 (32.3) | 226 (30.9) |
| Amiodarone | 78 (7.2) | 51 (7.0) |
| ACE inhibitor | 839 (77.0) | 563 (77.0) |
| ARB | 227 (20.8) | 148 (20.2) |
| Beta-blocker | 1016 (93.3) | 681 (93.2) |
| Class I antiarrhythmic agent | 12 (1.1) | 3 (0.4) |
| Digitalis | 291 (26.7) | 177 (24.2) |
| Diuretic | 824 (75.7) | 533 (72.9) |
| Lipid-lowering statin | 735 (67.5) | 491 (67.2) |
| Previous treatment | NR | NR |
| Cardiac risk factors, n/N (%) | ||
| Treatment for hypertension | 691/1085 (63.7) | 461/730 (63.2) |
| Atrial fibrillation > 1 month before enrolment | 118/1063 (11.1) | 90/717 (12.6) |
| Diabetes mellitus | 329/1088 (30.2) | 223/729 (30.6) |
| Cigarette smoking | 122/1069 (11.4) | 92/717 (12.8) |
| Body mass index ≥ 30 kg/m2 | 385/1072 (35.9) | 263/723 (36.4) |
| Coronary bypass surgery | 317/1088 (29.1) | 208/730 (28.5) |
Results
| Outcome | CRT-ICD (n = 1089) | ICD only (n = 731) | HR (95% CI), p-value |
|---|---|---|---|
| Death from any cause or non-fatal HF event, n/N (%) | 187/1089 (17.2) | 185/731 (25.3) | 0.66 (0.52 to 0.84), 0.001 |
| Deaths | 36/1089 (3.3) | 18/731 (2.5) | NR |
| HF events only | 151/1089 (13.9) | 167/731 (22.8) | 0.59 (0.47 to 0.74), < 0.001 |
| HF events occurring in hospital, n/N | 136/151 | 140/167 | |
| HF events outside the hospital, n/N | 15/151 | 27/167 | |
| Death at any time,a n/N (%) | 74/1089 (6.8) | 53/731 (7.3) | 1.00 (0.69 to 1.44), 0.99 |
| HRQoL | NR | NR | |
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | NR | NR | |
| Left ventricular remodelling | |||
| Change in LVEF | 0.11 (n = 746) | 0.03 (n = 620) | < 0.001 |
| Left ventricular end-diastolic volume average changeb from baseline to 1 year (ml) | –52 (n = 746) | –15 (n = 620) | < 0.001 |
| Left ventricular end-systolic volume average changeb from baseline to 1 year (ml) | –57 (n = 746) | –18 (n = 620) | < 0.001 |
| Exercise capacity outcomes | NR | NR | |
Adverse effects of treatment
| Adverse effect | CRT-ICD (n = 1089) | ICD only (n = 731) |
|---|---|---|
| Death in hospital after device implantation, n | 1 (pulmonary embolus) | – |
| Serious adverse events in the 30 days after device implantation (% of patients) | ||
| Pneumothorax | 1.7 | 0.8 |
| Infection | 1.1 | 0.7 |
| Pocket haematoma requiring evacuation | 3.3 | 2.5 |
| Coronary venous dissection with pericardial effusion during CRT-D + ICD implantation, n (%) | 5 (0.5) | – |
| Left ventricular coronary vein lead repositioned during first 30 days, n (%) | 44 (4.0%) | – |
| Frequency of serious device-related adverse events during long-term follow-up after the first 30 days | 4.5 per 100 device-months | 5.2 per 100 device-months |
| Removal of device, n (%) | 14 (1.3) | 5 (0.7) |
Subgroup data
| Subgroup | CRT-ICD | ICD only | HR (95% CI), p-value |
|---|---|---|---|
| Patients with ischaemic cardiomyopathy (NYHA class I or II) | (n = 598) | (n = 401) | |
| Death from any cause or non-fatal HF event, n/N (%) | 122/598 (20.4) | 117/401 (29.2) | 0.67 (0.52 to 0.88), 0.003 |
| HF events only | 96/598 (16.1) | 105/401 (26.2) | 0.58 (0.44 to 0.78), < 0.001 |
| Death at any time, n/N (%) | 53/598 (8.9) | 35/401 (8.7) | 1.06 (0.68 to 1.64), 0.80 |
| Patients with non-ischaemic cardiomyopathy (NYHA class I or II) | (n = 491) | (n = 330) | |
| Death from any cause or non-fatal HF event, n (%) | 65 (13.2) | 68 (20.6) | 0.62 (0.44 to 0.89), 0.01 |
| HF events only | 55 (11.2) | 62 (18.8) | 0.59 (0.41 to 0.87), 0.01 |
| Death at any time, n (%) | 21 (4.3) | 18 (5.5) | 0.87 (0.44 to 1.70), 0.68 |
| No. of events/no. of patients | HR (95% CI), p-value | ||
| Risk of death or HF according to selected clinical characteristics | |||
| Age (years) | |||
| < 65 | 142/852 | 0.80a | |
| ≥ 65 | 230/968 | 0.60a | |
| Sex | |||
| Male | 294/1367 | 0.76 (0.59 to 0.97) | |
| Female | 78/453 | 0.37 (0.22 to 0.61), 0.01 for interaction | |
| NYHA class | |||
| Ischaemic I | 53/265 | 0.76a | |
| Ischaemic II | 186/734 | 0.62a | |
| Non-ischaemic II | 133/821 | 0.60a | |
| QRS duration (ms) | |||
| < 150 | 147/645 | 1.06 (0.74 to 1.52) | |
| ≥ 150 | 225/1175 | 0.48 (0.37 to 0.64), 0.001 for interaction | |
| LVEF (%) | |||
| ≤ 25 | 101/646 | 0.70a | |
| > 25 | 271/1174 | 0.60a | |
| Left ventricular end-diastolic volume (ml) | |||
| ≤ 240 | 184/828 | 0.70a | |
| > 240 | 184/969 | 0.62a | |
| Left ventricular end-systolic volume (ml) | |||
| ≤ 170 | 190/835 | 0.66a | |
| > 170 | 178/962 | 0.70a | |
| All patients | 372/1820 | 0.66 | |
Subgroup analysis by gender135
| Outcome | Women (n = 453) | Men (n = 1367) | p-value | ||
|---|---|---|---|---|---|
| CRT-D | ICD | CRT-D | ICD | ||
| HF or death (primary end point) | 29/275 (11%) | 51/178 (29%) | 159/814 (20%) | 137/553 (25%) | |
| CRT-D vs. ICD HR 0.31 (95% CI 0.19 to 0.50), p < 0.001 | CRT-D vs. ICD HR 0.72(95% CI 0.57 to 0.92), p < 0.01 | < 0.01 for interaction | |||
| HF only | n = 73 events CRT-D vs. ICD HR 0.30(95% CI 0.18 to 0.50), p < 0.001 |
n = 249 events CRT-D vs. ICD HR 0.65(95% CI 0.50 to 0.84), p = 0.001 |
< 0.01 for interaction | ||
| Death at any time | n = 20 events CRT-D vs. ICD HR 0.28(95% CI 0.10 to 0.79), p = 0.02 |
n = 107 events CRT-D vs. ICD HR 1.05 (95% CI 0.70 to 1.57), p = 0.83 |
< 0.03 for interaction | ||
-
Allocation to treatment groups: randomisation, in a 3 : 2 ratio to CRT-ICD or ICD only, was stratified according to clinical centre and ischaemic status with the use of an algorithm that ensured near balance in each stratum. Random assignment was made by the co-ordinating and data centre and transmitted to the enrolling clinical centre by logging on to a web-based automated program or by telephone with hard copy to follow. 131
-
Blinding: treating physicians were aware of study group assignments. Diagnosis of HF and decisions about therapy or hospital admission for patients with HF were made by physicians aware of study group assignments. Adjudication of end points was carried out by an independent mortality committee and a HF committee who was unaware of study group assignments, according to prespecified criteria.
-
Comparability of treatment groups: baseline characteristics and use of cardiac medications at enrolment are described as similar in the two groups.
-
Method of data analysis: ITT analysis (except for paired volume and ejection fraction studies). Event monitoring was prespecified and involved an independent data and safety monitoring board at up to 20 successive multiples of approximately 35 adjudicated events, precisely specified in terms of variance of the log-rank statistic, with topping boundaries specified for termination of the trial in favour of CRT-ICD therapy, in favour of ICD only therapy or for no significant difference. Analysis of the primary end point, based on the statistical log-rank test stratified according to study centre and ischaemic status, was used to evaluate statistical significance for the trial. A Cox proportional hazards regression model (similarly stratified) was used to estimate HRs. These analyses were adjusted for the group sequential stopping rule and incorporated late reported events that occurred before termination of the trial. Cox proportional hazards regression was used for additional primary analyses for HF alone, death at any time and evaluation of 10 prespecified categorical subgroups and treatment interactions. All p-values were two-tailed and were not adjusted for the stopping rule (except for the primary end point analysis). Absolute change in left ventricular volumes and the ejection fraction was evaluated with paired-sample t-tests in patients in each study group who had paired baseline and 12-month recordings. The trial was stopped on the recommendation of the independent data and safety monitoring board when the monitoring statistic reached the prespecified efficacy boundary. The study was then unblinded and analyses were limited to events occurring before trial termination. A plan for secondary analyses related to recurring HF events and a number of tertiary analyses was outlined. Of the tertiary analyses, only ECG changes at 1 year are reported. The authors state that some caution in the interpretation of the subgroup interactions is needed because of multiple testing but that, given the significance of the comparison, the chance of getting two or more false positives is small, and the analyses showed a relatively constant treatment effect over time.
-
Sample size/power calculation: a Wang–Tsiatis (Δ = 0.1 category) group sequential design (reference provided) was used with a power of 95% to detect a HR of 0.75 at a two-sided significance level of 0.05.
-
Attrition/dropout: in the CRT-ICD arm 11/1089 patients (1.0%) did not receive a device; in the ICD only arm 19/731 patients (2.6%) did not receive a device. Overall, implantation of a device was achieved in 98.4% of patients, with 95.4% receiving the device to which they had been assigned. During the trial, 173 crossovers occurred for the following reasons: in patients assigned to an ICD only, 91 (12.4%) received CRT-ICD (30 at discretion of the physician before reaching an end point and 61 after a HF event); in patients assigned to CRT-ICD, 82 (7.5%) received an ICD only because of technical difficulties (not further described) in positioning the CRT pacing lead in the coronary vein. During the trial, devices were also removed for a variety of reasons (as noted in the adverse effects section; reasons not provided). In the CRT-ICD group, 44 patients (4.0%) declined to continue participating in the study, were withdrawn by a physician or were lost to follow-up compared with 55 patients (7.5%) in the ICD only group. In total, 201 patients in the CRT-ICD group underwent the 1-year ECG evaluation with the CRT device switched off. These patients are not included in the paired volume and ejection fraction studies.
-
Generalisability: the study was designed to investigate the use of combined CRT-ICD in mildly symptomatic or asymptomatic patients and thus the results are unlikely to be transferable to more severe HF patients.
-
Outcome measures: the primary end point was a composite measure but the discussion section describes this as appropriate and widely used in HF trials. Other outcomes appear appropriate; however, not all were analysed according to the ITT principle.
-
Intercentre variability: authors state that no significant interaction effects were identified between the 37 centres with low enrolment (< 10 patients) and the remaining 73 centres with higher enrolment.
-
Conflict of interests: 11/14 authors named on the publication declared one or more potential conflicts of interest in the form of grant support, lecture fees, consulting fees or institutional fellowships from one or more company.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | No information provided |
| Allocation concealment | Low | ‘Random assignment made by the coordinating and data centre and transmitted to the enrolling clinical centre by logging on to a web-based automated program or by telephone with hard copy to follow’131 |
| Performance bias | ||
| Blinding of participants and personnel | High | ‘The treating physicians were aware of study group assignments’130 |
| Detection bias | ||
| Blinding of outcome assessment | High | ‘Members of the heart-failure adjudication committee were unaware of study-group assignments, but the investigators who decided on therapy or hospital admission for patients with heart failure were aware of such assignments’.130 Authors acknowledged that ‘it is possible that the investigators’ knowledge of study-group assignment contributed in some way to the lower frequency of HF in the CRT-ICD group’130 |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Survival/HF outcomes | Low | ‘Data analysis was performed according to the intention-to-treat principle’130 ‘For the purpose of analysis, subjects will not be censored at withdrawal, and every effort will be made to ascertain the occurrences or non-occurrence of the primary endpoints’131 |
| Ventricular remodelling outcomes | High | 201/1820 participants not included in the paired volume and ejection fraction studies |
| Reporting bias | ||
| Selective reporting | Low | Paper available describing design and clinical protocol. Outcomes of interest reported as expected131 |
| Other bias | ||
| Other sources of bias | Low | |
Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Young et al. 2003136 Study design: RCT Countries: USA and Canada No. of centres: Not stated; reviewer counted 63 listed Funding: Medtronic Inc. (manufacturer of the device used in the trial) |
Intervention: ICD-CRT + OPT Mode programmed that paced both ventricles simultaneously following atrial-sensed events at rate of ≤ 130/minute. ICD active. Atrial pacing occurred only for sinus rates < 35/minute Comparator: ICD + OPT Mode programmed that inhibited atrial or ventricular pacing unless intrinsic rate < 35/minute. ICD active. Participants implanted with Model 7272 InSync ICD (Medtronic Inc.), which can deliver atrial-synchronised biventricular pacing for cardiac resynchronisation, antitachycardia pacing through right ventricular or right ventricular and left ventricular leads, and cardioversion and defibrillation to treat ventricular tachyarrhythmias delivered through the right ventricular lead only Other interventions used: stable and appropriate drug regimen, which included an ACE inhibitor or ARB, if tolerated, for at least 1 month. A beta-blocker had to have been initiated at least 3 months before enrolment. Initiation of a beta-blocker was not permitted during the trial period |
Indication for treatment: moderate to severe HF, wide QRS interval, LVSD and an established indication for an ICD No. of participants: 369; ICD-CRT + OPT 187, ICD + OPT 182 Inclusion criteria: indications for an ICD: cardiac arrest (loss of consciousness) due to VT or VF without a transient reversible cause, patients with recurrent, poorly tolerated and sustained VT (spontaneously or induced); NYHA class III or IV CHF; LVEF ≤ 35%, LVEDD ≥ 55 mm; QRS interval ≥ 130 milliseconds; age ≥ 18 years; stable drug regimen for ≥ 1 month Exclusion criteria: life expectancy < 6 months; baseline 6-minute walk test > 450 m; bradycardia requiring pacemaker; unstable angina, MI, CABG, PTCA, cerebral vascular accident or transient ischaemic attack within previous 3 months; more than two infusions of inotropic drug per week; systolic blood pressure > 170 or < 80 mmHg; resting heart rate > 140 bpm; serum creatinine > 3.0 mg/dl; hepatic enzymes more than three times the upper limit of normal; severe lung disease; chronic atrial arrhythmias or cardioversion or paroxysmal atrial fibrillation within previous 1 month; heart transplant recipient; severe valvular heart disease |
Primary outcomes: NYHA class, QoL score, distance walked covered in 6 minutes Secondary outcomes: included peak VO2, treadmill exercise duration, LVEF, left ventricular end-systolic and end-diastolic volumes, LVEDD, severity of mitral regurgitation, QRS duration, neurohormone concentrations and a clinical composite response (worsened, improved or unchanged). Worsened defined as death, hospitalised because of worsening HF, permanently discontinued double-blind treatment (because of worsening HF, withdrawal of consent or other administrative reason), worsening NYHA class at LOCF or moderate to marked worsening of patient global assessment score at LOCF; improved defined as not worsened and demonstrated improvement in NYHA class at LOCF or a moderate to marked improvement in patient global assessment score at LOCF; unchanged defined as neither improved nor worsened Complication definition: a sign, symptom, illness or other medical event that was resolved invasively (penetrated the skin, parenteral fluids or drugs) or resulted in death or serious injury to patient; termination of a significant device function Method of assessing outcomes: visits at 1, 3 and 6 months. At each visit: interrogation of implanted device, QoL assessment, 6-minute walk distance, estimation of NYHA class and monitoring of drug regimen. At 6 month visit: ECG, cardiopulmonary exercise test and measurement of plasma neurohormones Length of follow-up: 6 months Recruitment: October 1999–August 2001 |
Participant characteristics
| Characteristic | ICD-CRT + OPT (n = 187) | ICD + OPT (n = 182) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 66.6 (11.3) | 67.6 (9.2) | |
| Gender, male, n (%) | 142 (75.9) | 141 (77.5) | |
| Ethnicity | NR | NR | |
| Underlying heart disease, n (%) | 0.02 | ||
| Ischaemic | 119 (64.0) | 138 (75.8) | |
| Non-ischaemic | 67 (36.0) | 48 (26.4) | |
| Indication for ICD, n (%) | |||
| Cardiac arrest | 17 (9) | 20 (11) | |
| Sustained VT | 71 (38) | 76 (42) | |
| Induced VF and sustained VT | 99 (53) | 85 (47) | |
| NYHA class, n (%) | |||
| III | 165 (88.2) | 163 (89.6) | |
| IV | 22 (11.8) | 19 (10.4) | |
| LVEF (%), mean (SD) | 24.2 (6.5) | 23.9 (6.0) | |
| Resting heart rate/minute, mean (SD) | 71.0 (12.4) | 71.3 (12.9) | |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 113 (18) | 114 (17) | |
| Diastolic | 66 (11) | 67 (10) | |
| QRS duration (milliseconds), mean (SD) | 165 (22) | 162 (22) | |
| Isolated right bundle branch block, n (%) | 25 (13) | 24 (13) | |
| LVEDD (mm), mean (SD) | 75.6 (9.6) | 76.7 (10.4) | |
| LVESD (mm), mean (SD) | 248 (93) | 240 (87) | |
| LVEDV (ml), mean (SD) | 322 (100) | 311 (96) | |
| Mitral regurgitation, average jet area (cm2), mean (SD) | 7.5 (5.9) | 7.3 (6.7) | |
| QoL life score, mean (SD) | 56.8 (22.6) | 55.2 (22.6) | |
| 6-minute walk distance (m), mean (SD) | 243 (129) | 243 (117) | |
| Peak VO2 (ml/kg/minute), mean (SD) | 13.3 (3.6) | 13.4 (3.8) | |
| Exercise duration (seconds), mean (SD) | 468 (205) | 506 (230) | |
| Baseline medications, n (%) | |||
| ACE inhibitor or ACE inhibitor substitute | 173 (92.5) | 162 (89.0) | |
| Antiarrhythmic | 79 (42.3) | 60 (33.0) | |
| Beta-locker | 116 (62.0) | 106 (58.2) | |
| Diuretic | 174 (93.1) | 172 (94.5) | |
Results
| Outcomes | ICD-CRT + OPT (n = 187) | ICD + OPT (n = 182) | Control vs. CRT p-value |
|---|---|---|---|
| No. of deaths during 6 months | 14 | 15 | NR |
| Sudden deaths | 3 | 3 | |
| 6-month cumulative survival, % | 92.4 (95% CI 87.5 to 95.4) | 92.2 (95% CI 87.2 to 95.3) | log-rank p = 0.96 |
| Primary outcomes (including all patients with data), median change (95% CI), n | |||
| Change in QoL scorea | –17.5 (–21 to –14), 162 | –11 (–16 to –7), 157 | 0.02 |
| Change in NYHA functional class | –1 (–1 to –1), 165 | 0 (–1 to 0), 162 | 0.007 |
| Change in 6-minute walk distance (m) | 55 (44 to 79), 152 | 53 (43 to 75), 153 | 0.36 |
| Primary LOCF analysis (excluding patients who died and those with either no baseline or no follow-up data at 1, 3 and 6 months), median change (95% CI), n | |||
| Change in QoL score | –17 (–21 to –13), 170 | –11 (–16 to –6), 163 | 0.01 |
| Change in NYHA functional class | –1 (–1 to –1), 171 | 0 (–1 to 0), 166 | 0.006 |
| Change in 6-minute walk distance (m) | 54.5 (40 to 75), 166 | 52 (40 to 74), 163 | 0.32 |
| Secondary outcomes, median change (95% CI), n | |||
| Cardiopulmonary exercise | |||
| Change in peak VO2 (ml/kg/minute) | 1.1 (0.7 to 1.6), 120 | 0.1 (–0.1 to 0.8), 121 | 0.04 |
| Change in exercise duration (seconds) | 55.5 (30 to 79), 120 | –11 (–55 to 12), 123 | < 0.001 |
| Echocardiographic left ventricular size and function | |||
| Change in end-diastolic volume (ml) | –19.9 (–39.7 to –6.3), 132 | –5.7 (–16.2 to 1.8), 133 | 0.06 |
| Change in end-systolic volume (ml) | –22.2 (–32.8 to –10.7), 132 | –8.2 (–19.1 to 0.6), 133 | 0.06 |
| Change in ejection fraction (absolute %) | 2.1 (1.2 to 4.1), 132 | 1.7 (0.7 to 2.4), 133 | 0.12 |
| Change in end-diastolic diameter (mm) | –0.1 (–0.3 to 0.1), 70 | –0.2 (–0.3 to 0), 67 | 0.81 |
| Change in end-systolic diameter (mm) | –0.1 (–0.4 to 0.1), 69 | –0.3 (–0.5 to –0.1), 65 | 0.53 |
| Change in mitral regurgitant jet area (mm) | –0.55 (–2.00 to 0), 130 | –0.33 (–0.85 to 0), 126 | 0.58 |
| Change in overall clinical status, n (%) | |||
| Improved | 98 (52.4) | 78 (42.9) | 0.07 |
| Unchanged | 28 (15.0) | 43 (23.6) | |
| Worsened | 61 (32.6) | 61 (33.5) | |
| Change in QRS duration (milliseconds) | –20 (–21 to –14), 162 | 0, n = 160 | < 0.001 |
| Changes in plasma neurohormones (pg/ml) | |||
| Brain natriuretic peptide | –50 (–163 to –6), 119 | –68 (–133 to –6), 121 | 0.77 |
| Dopamine | 0, n = 112 | 0, n = 117 | 0.37 |
| Norepinephrine (ng/dl) | 4 (–12 to 68), 113 | –17 (–54 to 49), 117 | 0.58 |
| Epinephrine | 0 (–4 to 0), 112 | –3 (–8 to 0), 115 | 0.05 |
| Big endothelin | –2.5 (–6.0 to 1.3), 110 | –1.8 (–3.7 to 0.9), 119 | 0.98 |
| Hospitalisations between randomisation and 6-month visit, n/N (%) | 85/187 (45.5) | 78/182 (42.9) | |
| Length of hospital stay (days), mean (SD) | 4.8 (4.9) | 5.4 (4.7) | 0.06 |
| Probability of hospitalisation for worsening HF or death from any cause, % | 25.7 (95% CI 19.6 to 32.3) | 25.9 (95% CI 19.8 to 32.5) | 0.69 |
| Risk of death or all cause hospitalisation, % | 47.4 (95% CI 40.0 to 54.4) | 48.3 (95% CI 40.6 to 55.6) | 0.88 |
| Experienced one or more spontaneous episode of VT or VF, n/N (%) | 42/187 (22) | 47/182 (26) | 0.47 |
| Episode not successfully terminated within interval determined by device criteria,b n/N (%) | 1/678 (0.1) | 4/233 (1.7) | |
| Appropriate ICD shocks | 89 events, 24/187 patients (13%) | 154 events, 26/182 patients (14%) | 0.76 |
| Inappropriate ICD shocks | 18 events, 8/187 patients (4%) | 59 events, 13/182 patients (7%) | 0.27 |
| Appropriate: only antitachycardia pacing used | 608 events, 33/187 patients (18%) | 229 events, 31/182 patients (17%) | 0.89 |
| Inappropriate: only antitachycardia pacing used | 35 events, 13/187 patients (7%) | 32 events, 8/182 patients (4%) | 0.37 |
| Therapy compliance | 94% ventricular paced for ≥ 90% of the time | 86% received no right ventricular pacing | |
Adverse effects of treatment
| Adverse effect | All patients undergoing implant attempt (n = 429) | |
|---|---|---|
| Experienced complication from implant to hospital dischargea | 120/429 patients (28%), 159 complications | |
| Complications related to left ventricular lead | 37/159 (23%) (including 15 coronary sinus dissections and 4 cardiac perforations) | |
| HF decompensation | 6 patients (received intravenous medication) | |
| Heart block | 3 patients (required bradycardia pacing support) | |
| Muscle stimulation | 4 patients (required either lead repositioning or replacement) | |
| Pericardial effusion | 2 patients (treated with a pericardiocentesis) | |
| Pericarditis | 1 patient (received intravenous medication) | |
| Haemo/pneumothorax | 3 patients (placement of chest tube) | |
| VT and VF | 5 patients (3 received external defibrillation, 2 intravenous medications) | |
| Elevated pacing thresholds or loss of capture | 7 patients (6 received lead repositioning, 1 set screw tightened in connector block) | |
| Died within 30 days of latest implant attemptb | 5/429 (1.2%) | |
| ICD-CRT + OPT (n = 187) | ICD + OPT (n = 182) | |
| For patients successfully implanted and randomised: complications after hospital discharge to 6-month follow-up | ||
| Left ventricular lead-related complication | 21 complications in 20 patients (11%) | 14 complications in 13 patients (7%) |
| ICD system related | 9 complications in 9 patients (5%) | 14 complications in 13 patients (8%) |
| Procedure related | 10 complications in 10 patients (5%) | 13 complications in 11 patients (6%) |
| HF decompensation | 63 complications in 36 patients (19%) | 71 complications in 40 patients (22%) |
| Other | 81 complications in 45 patients (24%) | 74 complications in 44 patients (22%) |
| Total | 184 complications in 88 patients (47%) | 186 complications in 80 patients (44%) |
| Crossed over to alternative treatment | 10 (5%) to ICD only (2 ventricular lead dislodgement, 2 diaphragmatic stimulation, 6 programming errors) | 14 (8%) to CRT (11 worsening HF, 2 bradycardia, 1 programming error) |
-
Allocation to treatment groups: randomisation occurred within 7 days of successful implantation but after a cardiopulmonary exercise test. Random assignment in blocked groups of four by centre (to balance CRT and control assignments at each centre). Random allocation sequence was generated by SAS software version 8.2 (SAS Institute Inc., Cary, NC, USA). Centres were unaware of randomisation method. Assignment provided to unblinded electrophysiology staff in consecutively numbered and opaque sealed envelopes opened at the time of randomisation.
-
Blinding: states double blind. Patients and physicians from HF team (not involved in programming of the device) remained unaware of assignment until after the 6-month visit. Blinded phase of study complete at 6 months. Independent core laboratories, unaware of assignment, interpreted data. Adverse events classified by clinical events review committee without knowledge of random assignment.
-
Comparability of treatment groups: states similar except that the control group had a higher percentage of participants with ischaemic heart disease.
-
Method of data analysis: only participants with both baseline and follow-up data were included in efficacy analyses. All randomised patients who underwent an implant attempt were included in the adverse event analysis. For all other analyses all randomised patients were included. States that all end points were analysed by ITT principle but this appears to conflict with earlier statement that both baseline and follow-up data were required for participants to be included in efficacy analyses. Data presented as median changes between baseline and 6-month follow-up. CIs for medians computed using a distribution-free approach. Mean values presented with SDs. Continuous variable (including NYHA) changes from baseline in control vs. CRT group and demographic characteristics compared using the Wilcoxon rank-sum test. Differences in distribution of categorical end points between the two groups compared with Fisher’s exact test. Survival curves constructed using the Kaplan–Meier method (time zero = date of implant) and differences between curves examined using the log-rank test statistic. CIs for survival computed on the log-log survival scale. Objectives considered reached for the three primary efficacy variables if differences between the groups for all three end points had p ≤ 0.05 or if two had p ≤ 0.025 or if one had p ≤ 0.017, using the Hochberg criterion. For secondary end points p < 0.05 was considered significant. All p-values were calculated using two-sided tests. Details of analysis that was not prespecified were not data extracted.
-
Sample size/power calculation: estimated based on the assumption that the study would have 80% power (two-sided α = 0.017) to detect a difference in NYHA class of 0.75, in QoL of 13 points or in 6-minute walk distance of 50 m. There were 112 patients per treatment group. Study was not powered to detect a morbidity or mortality difference.
-
Attrition/dropout: of 639 patients initially enrolled, 210 had mild HF symptoms and as per protocol were not included in this analysis; 60 NYHA class III or IV patients had an implant attempted but did not enter the randomised therapy phase. This left 369 patients included in the randomisation. In the CRT-D + OPT group 10 (5%) crossed over to ICD therapy only (these were included in the analysis), 14 died, six missed the 6-month follow-up and two received cardiac transplantation (these 22 not included in the analysis), leaving 165/187 included in the primary efficacy analysis. In the ICD + OPT group 14 (8%) crossed over to CRT (included in analysis), 15 died and five missed the 6-month follow-up (these 20 not included in the analysis), leaving 162/182 included in the primary efficacy analysis.
-
Generalisability: only those with successful implantation were randomised. Generalisable to those with moderate to severe HF; however, relatively short length of follow-up (6 months) and so results may not be generalisable over long time periods.
-
Outcome measures: appear appropriate but QoL tool not specified.
-
Intercentre variability: not commented on.
-
Conflict of interests: eight of the 11 listed authors made financial disclosures. The sponsor had responsibility for the initial study design, day-to-day study operations, data collection, data management and statistical analysis in conjunction with the co-principal investigators. The study sponsor also participated in preparation and review of the manuscript for accuracy. States that investigators performed analyses without restrictions or limitations from sponsors.
-
Other: except for ICD indication, patient inclusion criteria and study design were identical to those of the MIRACLE trial.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Low | Sequence generated by computer software |
| Allocation concealment | Low | Consecutively numbered and opaque sealed envelopes |
| Performance bias | ||
| Blinding of participants and personnel | Low | States double blind, participants and physicians blinded |
| Detection bias | ||
| Blinding of outcome assessment | Low | States double blind, use of independent lab and blinded committee |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Primary outcomes | Unclear | Numbers and reasons for missing data are given; appear balanced between groups for primary outcomes. Crossovers were included as assigned. However, denominator should be n = 165 for CRT and n = 162 for ICD but this is only the case for NYHA class and not for the other two primary outcomes QoL and 6-minute walk distance |
| Secondary outcomes | High | Amount of missing data varies between different secondary outcomes although appears to be a similar proportion in each group. Reasons not provided |
| Reporting bias | ||
| Selective reporting | Low | Protocol not available but expected outcomes reported |
| Other bias | ||
| Other sources of bias | Unclear | Study sponsor appears to have been involved in all aspects of the study |
Multicenter InSync ICD II Randomized Clinical Evaluation (MIRACLE ICD II)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Abraham et al. 2004138 Study design: RCT Countries: USA and Canada No. of centres: 63136 Funding: Medtronic, Inc., St Paul, MN |
Intervention: CRT-D + OPT Combined cardiac resynchronisation/ICD device (model 7272 InSync ICD, Medtronic), with three pacing leads: standard right atrial pacing lead, standard right ventricular pacing/defibrillation lead, and one of several left ventricular transvenous leads positioned in a distal cardiac vein via coronary sinus Device programmed to pace both ventricles after atrial sensed events at rates of ≤ 130 bpm. Atrial pacing occurred only for sinus rates of ≤ 35 bpm Comparator: ICD + OPT Active ICD therapy only. Device as above. Device programmed to inhibit atrial or ventricular pacing unless intrinsic rate was < 35 bpm Other interventions used: all appropriate treatments for HF, including diuretic, ACE inhibitor or ARB and usually digitalis and beta-blocker. Doses stable for ≥ 1 month except for beta-blocker, stable for 3 months |
Indication for treatment: mild NYHA class II HF symptoms, a wide QRS complex and an established indication for an ICD No. of participants: enrolled 222, implant attempt 210, successful implant 191, randomised 186; CRT-D 85, ICD 101 Completed study: CRT-D 82, ICD 98 Inclusion criteria: NYHA class II chronic HF, LVEF ≤ 35%, LVEDD ≥ 55 mm, QRS interval ≥ 130 milliseconds, indication for ICD Exclusion criteria: variety of medical reasons including having an indication for or contraindication to cardiac pacing |
Primary outcome: change in peak VO2 Secondary outcomes: ventilatory response to exercise [minute ventilation/minute carbon dioxide production (VE/VCO2)], NYHA class, QoL, 6-minute hall walk test, left ventricular volume, LVEF, composite clinical response (worsened, improved or unchanged) Method of assessing outcomes: 7 days before implantation: NYHA class, 6-minute hall walk test, QoL assessed using MLWHFQ, 2-dimensional Doppler ECG, plasma neurohormone concentrations, QRS interval assessed using 12-lead ECG; before randomisation: exercise capacity measured using baseline treadmill cardiopulmonary exercise test using modified Naughton protocol; at 1, 3 and 6 months: interrogation of CRT-ICD, QoL, 6-minute hall walk distance, NYHA class, monitor drug regimen, ECG, metabolic exercise testing, plasma neurohormone measurements at 6-month visit Adverse events: complication defined as a sign, symptom, illness or other medical event that was resolved invasively or resulted in death or serious injury; termination of a significant device function Length of follow-up: blinded phase of study completed at 6-month follow-up. CRT activated in control group Recruitment: July 2002 |
Participant characteristics
| Characteristic | CRT-ICD (n = 85) | ICD (n = 101) | p-value |
|---|---|---|---|
| Mean (SD) unless stated otherwise | |||
| Age (years) | 63.0 (12.8) | 63.1 (12.1) | |
| Gender, men, n (%) | 75 (88.2) | 91 (90.1) | |
| Ethnicity | NR | NR | |
| NYHA functional class II, n (%) | 85 (100) | 101 (100) | |
| LVEF (%) | 24.4 (6.6) | 24.6 (6.7) | |
| LVEDD (cm) | 7.6 (1.0) | 7.5 (1.0) | |
| LVESD (cm) | 6.5 (1.2) | 6.5 (1.2) | |
| LVEDV (cm3) | 337 (147) | 329 (108) | |
| LVESV (cm3) | 260 (134) | 252 (98) | |
| QRS duration (ms) | 166 (25) | 165 (23) | |
| Mitral regurgitation, average jet area (cm2) | 5.9 (6.0) | 6.1 (5.3) | |
| QoL score | 41.8 (25.1) | 39.8 (21.2) | |
| 6-min walk test (m) | 355 (125) | 383 (108) | |
| Peak VO2 (ml/kg/minute) | 16.4 (4.4) | 16.8 (5.0) | |
| VE/VCo2 (ml/minute) | 39.3 (9.7) | 38.7 (9.4) | |
| Exercise duration (seconds) | 647 (242) | 664 (228) | |
| Resting heart rate (bpm) | 69.7 (11.5) | 68.6 (12.3) | |
| Blood pressure (mmHg) | |||
| Systolic | 116.2 (15.8) | 116.8 (16.8) | |
| Diastolic | 69.9 (10.4) | 69.4 (10.2) | |
| Underlying heart disease, n (%) | |||
| Ischaemic | 47 (55.3) | 59 (58.4) | |
| Right bundle branch block, n (%) | 10 (11.8) | 21 (20.8) | |
| Baseline neurohormones (pg/ml) | |||
| Brain natriuretic peptide | 631 (909) | 538 (806) | |
| Norepinephrine | 413 (379) | 355 (249) | |
| Epinephrine | 29 (28) | 31 (28) | |
| Big endothelin | 15 (12) | 18 (15) | |
| Dopamine | 23 (51) | 12 (7) | |
| Baseline medications, n (%) | |||
| ACE inhibitor | 83 (97.6) | 96 (95.0) | |
| Antiarrhythmic | 30 (35.3) | 33 (32.7) | |
| Beta-blocker | 54 (63.5) | 64 (63.4) | |
| Diuretic | 74 (87.1) | 81 (80.2) | |
Results
| Outcomes | CRT-ICD (n = 85) | ICD (n = 101) | p-value |
|---|---|---|---|
| Mortality during 6-month follow-up, n | 2 (2 cardiac arrests) | 2 (1 cardiac arrest, 1 MI with cardiogenic shock) | |
| Change from baseline to 6 months, mean (SD), n | |||
| Change in QoL score | –13.3 (25.1), 81 | –10.7 (21.7), 96 | 0.49 |
| Symptoms and complications related to tachyarrhythmias and/or HF | NR | NR | |
| HF hospitalisations | NR | NR | |
| Change in NYHA class | –0.18 (0.61), 82 | 0.01 (0.63), 98 | 0.05 |
| Change in peak VO2 (ml/kg/minute) | 0.5 (3.2), 66 | 0.2 (3.2), 79 | 0.87 |
| Change in exercise duration (seconds) | 42 (167), 66 | 37 (186), 79 | 0.56 |
| Change in VE/VCO2, (ml/minute) | –1.8 (6.2), 66 | 0.5 (5.2), 78 | 0.01 |
| Change in 6-minute walk distance (m) | 38 (109), 78 | 33 (98), 93 | 0.59 |
| Echocardiographic left ventricular size and function | |||
| Change in LV end-diastolic volume (ml) | –41 (76), 69 | –16 (62), 85 | 0.04 |
| Change in LV end-systolic volume (ml) | –42 (77), 68 | –14 (57), 85 | 0.01 |
| Change in LVEF (absolute %) | 3.8 (8.0), 68 | 0.8 (6.2), 85 | 0.02 |
| Change in mitral regurgitant jet area (mm) | –1.7 (4.7), 62 | –1.0 (3.7), 84 | 0.25 |
| Change in overall clinical status, n (%) | |||
| Improved | 49 (58) | 36 (36) | 0.01 |
| Unchanged | 19 (22) | 34 (34) | (all) |
| Worsened | 17 (20) | 31 (31) | |
| Change in QRS duration | –9 (24), 78 | –9 (22), 95 | 0.97 |
| Changes in plasma neurohormones (pg/ml) | |||
| Brain natriuretic peptide | –195.2 (831.6), 64 | –96.3 (581.6), 71 | 0.81 |
| Dopamine | –10.3 (59.7), 60 | 5.5 (18.2), 71 | 0.26 |
| Norepinephrine | 10.1 (396.0), 60 | 63.3 (248.3), 71 | 0.86 |
| Epinephrine | –6.5 (34.2), 60 | –4.7 (25.8), 71 | 0.67 |
| Big endothelin | –2.3 (15.6), 61 | –4.3 (15.6), 70 | 0.69 |
| One or more appropriately detected, spontaneous episodes of VT or VF, n/N (%) | 19/85 (22) | 26/101 (26) | 0.61 |
| Percentage of inappropriately detected VT/VF | |||
| Treated | NR | NR | 0.21 |
| Shocked | NR | NR | 0.78 |
Adverse effects of treatment
| Adverse effect | |
|---|---|
| From implant to hospital discharge | 46/210 (22%) patients,a 56 complications |
| Complication related to placement of left ventricular lead | 19/56 (34%) (including 3 coronary sinus dissections, 3 cardiac perforations, 5 lead dislodgements) |
| Failed initial implant attempt | 23/210 |
| From hospital discharge to 6-month follow-up | 66/191 (35%) patients,b 109 complications |
| Complications related to left ventricular lead | 19/109 (17%) (including 11 lead dislodgements, 1 cardiac perforation, 3 diaphragmatic muscle stimulation, 4 elevated pacing threshold) |
-
Allocation to treatment groups: patients randomly assigned, in permuted groups for each centre. SAS software used to generate the random allocation sequence. Method of randomisation not disclosed to participating centres and was accomplished in blocked groups of four for each centre to ensure balance of CRT and control assignments at each participating institution. Randomisation occurred after a successful implant.
-
Blinding: patients and physicians treating for HF and performing study evaluations unaware of treatment assignment. At each site an electrophysiologist unblinded to treatment programmed the device and performed all tests that could reveal the identity of assigned mode. The clinical events review committee reviewed and classified adverse events without knowledge of the randomised assignment.
-
Comparability of treatment groups: states no statistically significant differences noted between the groups.
-
Method of data analysis: states that all end points were analysed according to ITT principle; results were assessed on the basis of the original treatment assignment. Changes in continuous variables, including NYHA class, from baseline to 6 months in the control group were compared with changes in the CRT group using the Wilcoxon rank-sum test. For categorical end points, compared differences in distribution of responses to treatment at 6 months using Fisher’s exact test, except for inappropriately detected VT/VF episodes, for which generalised estimating equation methods were used. p < 0.05 was considered statistically significant. All p-values were calculated using two-sided tests. States that in an analysis that was not prespecified, potential clinically relevant covariates were analysed by ANOVA with random assignment as independent variables; however data are not reported in the paper.
-
Sample size/power calculation: not reported.
-
Attrition/dropout: 222 participants were enrolled and implant was attempted in 210 (reasons for no implant not reported); 191 (91%) were successfully implanted (reasons for implant failure not reported): one patient died and four patients had left ventricular lead dislodgements that were not corrected. The remaining 186 patients were randomised. In each group two patients died and one missed the 6-month follow-up visit. Five ICD patients (5%) crossed over to CRT-D before 6 months (biventricular pacing was activated early because of bradycardia in three patients, there was a centre error in one patient and there was pacemaker dependency after atrioventricular node ablation for atrial flutter in one patient). Two CRT-D patients (2%) crossed over to no pacing (biventricular pacing was deactivated because of left ventricular lead dislodgement in one patient and there was diaphragmatic stimulation in biventricular and right ventricular pacing modes in one patient).
-
Generalisability: participants had mildly symptomatic class II HF. Exercise capacity was not moderately or severely impaired at baseline. Randomisation occurred after a successful implant; therefore, patients not representative of all patients eligible for CRT.
-
Outcome measures: appropriate but follow-up only 6 months. Composite outcome not defined in paper but reference provided. Adverse events not reported separately for treatment groups.
-
Intercentre variability: not reported.
-
Conflict of interests: study supported by Medtronic, Inc. Conflicts declared by authors, including receiving honoraria from and/or being a consultant and/or investigator for Medtronic, Guidant, St Jude Medical and/or GlaxoSmithKline and/or shareholder in Medtronic.
ANOVA, analysis of variance.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Low | ‘SAS software (SAS Institute) was used to generate the random allocation sequence’137 |
| Allocation concealment | Low | ‘Method of randomisation was not disclosed to participating centres’137 ‘At each site, an electrophysiologist unblinded to treatment opened a sealed envelope at the time of randomisation, programmed the device, and performed all tests that could reveal the identity of the assigned mode’137 |
| Performance bias | ||
| Blinding of participants and personnel | Low | ‘Neither the patients nor the physicians treating them were aware of the treatment assignment. At each site, an electrophysiologist unblinded to treatment opened a sealed envelope at the time of randomisation, programmed the device, and performed all tests that could reveal the identity of the assigned mode’137 ‘Clinical events review committee reviewed and classified adverse events without knowledge of randomised assignment’137 |
| Detection bias | ||
| Blinding of outcome assessment | Low | ‘Cardiopulmonary gas exchange analysis was done at a core laboratory with personnel blinded to CRT activation status’137 ‘Standard protocols used to perform echocardiograms and collect plasma neurohormones, Independent core laboratories, blinded to patient study assignment, interpreted the data’137 ‘Clinical events review committee reviewed and classified adverse events without knowledge of randomised assignment’137 |
| Attrition bias | ||
| Incomplete outcome data addressed | Unclear | States ITT but reports various patient numbers for each outcome. Not clear why these data are missing |
| Reporting bias | ||
| Selective reporting | Low | Protocol not available but expected outcomes reported. Analysis described in methods section not reported in the results section, but not relevant to this review as not prespecified |
| Other bias | ||
| Other sources of bias | Low | |
Piccirillo and colleagues study
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Piccirillo et al. 2006138 Study design: RCT Country: Italy No. of centres: one Funding: not reported |
Intervention: CRT-D Comparator: ICD Biventricular pacemaker (Guidant Corporation); the final pace setting was VDD with a lower rate well below the patient’s lowest intrinsic heart rate to maintain natural atrial tracking at rest (setting essential to allow power spectral analysis of heart rate variability) Both groups were taking standard medications for HF, including ramipril (2.5–10 mg/day) or losartan (50 mg/day), furosemide (25–250 mg/day), spironolactone (25–50 mg/day), carvedilol (6.25–50 mg/day) or bisoprolol (2.5–5 mg/day), digoxin (0.125 mg/day or 0.250 mg/day) and acetylsalicylic acid (100 mg/day) Other interventions used: none reported |
Indication for treatment: CHF (with low ejection fraction and prolonged QRS interval) secondary to ischaemic dilated cardiomyopathy No. of randomised participants: 31; CRT-D: 16, ICD: 15 Also reported data for healthy non-randomised control group (n = 12). Data not extracted Inclusion criteria: LVEF ≤ 35%, QRS interval > 120 milliseconds and sinus rhythm Exclusion criteria: malignancy, primary valve disease, frequent extrasystoles (more than one per minute), atrial fibrillation or other arrhythmias requiring a pacemaker (atrioventricular disturbances) or defibrillator for secondary prevention because of a history of malignant arrhythmias |
Primary/secondary outcome (not stated which): spectral indices based on power spectral analysis and changes in spectral indices (not data extracted). Also reported mortality and change in NYHA class Method of assessing outcomes: details of power spectral analysis and assessment of changes in spectral indices not data extracted. All ICD shocks assessed by three expert cardiologists to evaluate appropriateness Length of follow-up: 1 year Recruitment: not reported |
Participant characteristics
| Characteristic | CRT-D (n = 16) | ICD (n = 15) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 65 (4) | 65 (8) | |
| Sex, male/female, n | 13/3 | 12/3 | |
| Ethnicity | NR | NR | |
| NYHA class, n | |||
| III | 5 | 5 | |
| IV | 11 | 10 | |
| LVEF (%), mean (SD) | 23 (4) | 22 (8) | |
| QRS length (milliseconds), mean (SD) | 160 (4) | 159 (8) | |
| Heart rate (bpm), mean (SD) | 79 (4) | 81 (8) | |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 112 (12) | 109 (19) | |
| Diastolic | 68 (8) | 69 (11) | |
| Electrophysiology findings | |||
| End-systolic diameter (mm), mean (SD) | 60 (8) | 59 (8) | |
| End-diastolic diameter (mm), mean (SD) | 69 (4) | 70 (19) | |
| Current pharmacological therapy, n | |||
| Digoxin | 12 | 11 | |
| Ramipril | 16 | 15 | |
| Furosemide | 16 | 15 | |
| Spironolactone | 9 | 10 | |
| Carvedilol | 13 | 12 | |
| Biskoprolol | 2 | 1 | |
| Acetylsalicylic acid | 16 | 14 | |
| Cardiac history, n | |||
| Unstable symptoms of HF | 0 | 0 | |
| Hospitalisation | 0 | 0 | |
| Recent previous treatment, n | |||
| Coronary angioplasty | 0 | 0 | |
| Revascularisation procedures | 0 | 0 | |
| Change of therapy during the past 3 months, n | 0 | 0 | |
| Comorbidities | NR | NR | |
| Body mass index (kg/m2), mean (SD) | 26 (4) | 26 (4) | |
Results
| Outcome | CRT-D (n = 16) | ICD (n = 15) | p-value |
|---|---|---|---|
| Deaths, n | 0 | 0 | |
| HRQoL | NR | NR | |
| Received appropriate shocks, n | 2 | 4 | |
| Sustained VT | 1 | 3 | |
| Sustained VF | 1 | 1 | |
| Hospitalisation because of worsening CHF, n | 0 | 2 | |
| NYHA class after 12 months,a n | |||
| I | 1 | 0 | |
| II | 3a | 1 | |
| III | 6 | 1 | |
| IV | 6a | 13 | |
| LVEF (%),b mean | 28 | 22 | |
| Exercise capacity outcomes | NR | NR | |
| Heart rate (bpm), mean (SD) | 75 (4) | 76 (4) | |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 115 (4)c | 108 (11) | |
| Diastolic | 69 (4) | 70 (4) | |
| End-systolic diameter (mm), mean (SD) | 55 (4)c | 61 (4) | |
| End-diastolic diameter (mm), mean (SD) | 66 (8)c | 72 (11)c | |
| Change in diuretic medication, n | 5 reduced | 6 increased | |
Adverse effects of treatment
| Adverse effect | CRT-D (n = 16) | ICD (n = 15) | p-value |
|---|---|---|---|
| NR | NR |
-
Allocation to treatment groups: patients were randomly assigned in a 1 : 1 ratio to ICD or CRT-D.
-
Blinding: spectral recording assessment blinded (outcomes not extracted) but no other blinding reported.
-
Comparability of treatment groups: authors state that there were no significant differences in age, body mass index, sex distribution or blood pressure between the two CHF groups and the control group (no p-values reported; p-values were reported for the CHF groups vs. the control group but were not data extracted).
-
Method of data analysis: linear data expressed as mean ± SD. Non-linear data expressed as median (IQR). ITT analysis not reported. Baseline ICD and CRT-D group data before implantation compared with control group data. The data for the ICD and CRT-D groups were then compared at baseline and at 1 year. One-way analysis of variance was used to compare the general characteristics and other linear data between the study groups. The Kruskal–Wallis test and Mann–Whitney U test were used for non-normally distributed data. The Wilcoxon test was used for variables with a non-linear distribution. Event-free survival functions were estimated using the Kaplan–Meier method and differences between the curves were tested for significance using the log-rank statistic; RRs were computed using a Cox proportional hazards regression model. As spectral analysis outcomes were not extracted (because not specified for review), the methods for the analysis of these outcomes were also not extracted.
-
Sample size/power calculation: none reported.
-
Attrition/dropout: none; all patients completed the study.
-
Generalisability: sample size too small to generalise, but results would be limited to patients with post-ischaemic dilated cardiomyopathy, excluding primary dilated cardiomyopathy patients.
-
Outcome measures: extracted outcome measures appear appropriate.
-
Intercentre variability: not applicable, one centre only.
-
Conflict of interests: not reported.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Only states randomly assigned in a 1 : 1 ratio; no other details reported |
| Allocation concealment | Unclear | No details reported |
| Performance bias | ||
| Blinding of participants and personnel | High | No blinding reported |
| Detection bias | ||
| Blinding of outcome assessment | High | Assessment of spectral recordings blinded (outcomes not extracted), but no other blinding reported |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | No ITT analysis reported, but all data appear to have been reported and authors state that all patients completed the study |
| Reporting bias | ||
| Selective reporting | Low | No protocol available, but all stated outcomes were reported |
| Other bias | ||
| Other sources of bias | Low | |
Pinter and colleagues study
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Pinter et al. 2009139 Study design: RCT Country: Canada No. of centres: 7 Funding: Guidant Inc., Minneapolis, MN |
All patients: CONTAK CD CHF device, model 1823, or CONTAK RENEWAL heat failure device, model H135 (Guidant Corporation). Standard atrial pacing lead, ventricular defibrillator lead and Easytrak left ventricular pacing lead (Guidant Corporation) Intervention: CRT-D (CRT on). Pacing programmed to dual-chamber tracking pacing mode (DDD) with lower rate limit at 40 bpm and maximum tracking rate 20 bpm less than the tachycardia detect rate. Atrioventricular delay determined by a proprietary algorithm. Right ventricular and left ventricular pacing were simultaneous Comparator: ICD (CRT off). Dual-chamber non-tracking pacing mode (DDI) with 40 bpm back-up biventricular pacing Other interventions used: not reported but inclusion criteria state ≥ 2 weeks of treatment with maximal tolerated doses of ACE inhibitors or beta-blockers unless adverse effects or contraindicated |
Indication for treatment: Mild to moderate HF at high risk of sudden death and eligible for an ICD but not a candidate for CRT based on guidelines at the time of the study No. of randomised participants: 72; CRT-D: 36, ICD: 36 Inclusion criteria: HF: unequivocal symptoms of dyspnoea or fatigue on climbing two or fewer flights of stairs or a 6-minute walk distance ≤ 450 m; LVEF ≤ 35% within 6 months of implant; QRS interval > 120 milliseconds; ≥ 2 weeks of treatment with maximal tolerated doses of ACE inhibitors or beta-blockers unless adverse effects or contraindicated; age 18–80 years Exclusion criteria: pacing for symptomatic bradycardia; not in sinus rhythm; MI or unstable angina within 6 weeks; CABG surgery within 4 weeks; Canadian Cardiovascular Society class 3 or worse angina; typical RBBB morphology in lead V1; pregnant |
Primary outcome: LVESV change from baseline to 6 months Secondary outcomes: change in QoL, stroke volume, cardiac volume, mitral jet area, cardiac output, LVEF, serum B-type natriuretic peptide, average heart rate, SDANN. Also reports 6-minute walk distance, death and hospitalisations Method of assessing outcomes: at baseline and 6 months. LVESV measured by quantitative resting radionuclide angiogram (multigated acquisition scan), 6-minute walk test, 24-hour Holter monitoring for heart rate and SDANN. QoL measured with the MLWHFQ, SF-36, DASI and one-item Global Visual Analogue Scale Length of follow-up: 6 months Recruitment: not reported |
Participant characteristics
| Characteristic | CRT on (CRT-D) (n = 36) | CRT off (ICD) (n = 36) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 66.3 (8.6) | 66.1 (8.8) | NS |
| Sex, % male | 77.8 | 80.6 | NS |
| Ethnicity | NR | NR | |
| NYHA classification | NR | NR | |
| Left ventricular measurements by multigated acquisition scan, mean (SD) | |||
| LVESV (ml) | 242 (96) | 251 (147) | NS |
| LVEDV (ml) | 314 (108) | 335 (156) | NS |
| LVEF (%) | 24.2 (7.5) | 26.8 (8.4) | NS |
| Left ventricular measurements by ECG, mean (SD) | |||
| LVESV (ml) | 217 (72) | 213 (101) | NS |
| LVEDV (ml) | 270 (74) | 272 (106) | NS |
| LVEF (%) | 21.2 (7.9) | 24.0 (8.3) | NS |
| Heart rate (bpm) | 68.1 (12.3) | 63.6 (11.0) | NS |
| Blood pressure (mmHg), mean (SD) | |||
| Systolic | 113 (19.6) | 114.1 (20.8) | NS |
| Diastolic | 65.7 (10.0) | 65.2 (10.7) | NS |
| Current pharmacological therapy | NR | NR | |
| Cardiac history, % | |||
| Coronary artery disease | 77.8 | 80.6 | NS |
| Previous MI | 66.7 | 75.0 | NS |
| CABG surgery | 38.9 | 30.6 | NS |
| Coronary angioplasty | 8.3 | 22.2 | NS |
| Dilated cardiomyopathy | 16.7 | 8.33 | NS |
| Valvular disease | 16.7 | 8.33 | NS |
| Mitral regurgitation grade 2/3/4 | 9/11/1 | 7/5/1 | 0.09 |
| Atrial fibrillation | 16.7 | 5.6 | NS |
| Primary arrhythmia, % | |||
| Cardiac arrest | 25.0 | 16.7 | NS |
| Sustained VT | 58.3 | 55.5 | NS |
| Prophylactic ICD | 16.7 | 27.8 | NS |
| Hypertension, % | 11.1 | 22.2 | NS |
| Diabetes, % | 30.6 | 25.0 | NS |
| Serum creatinine (µmol/l), mean (SD) | 121 (42) | 114 (36) | NS |
| Assessment of functional status | |||
| 6-minute walk distance (m), mean (SD) | 314 (114) | 338 (110) | NS |
| DASI, mean (SD) | 11.3 (9.8) | 12.4 (9.3) | NS |
| Global Visual Analogue Scale, mean (SD) | 6.4 (2.0) | 6.5 (1.9) | NS |
| MLWHFQ, mean (SD) | |||
| Complete score | 42.3 (20.8) | 42.8 (24.9) | NS |
| Physical dimension | 20.1 (9.2) | 17.7 (9.8) | NS |
| Emotional dimension | 8.5 (6.4) | 9.1 (7.6) | NS |
| SF-36 health survey subscales, mean (SD) | |||
| Physical functioning | 46.7 (24.9) | 44.5 (26.5) | NS |
| Role physical | 14.0 (26.9) | 12.4 (23.9) | NS |
| Bodily pain | 93.0 (11.4) | 95.3 (11.0) | NS |
| General health | 59.4 (12.7) | 59.0 (9.6) | NS |
| Vitality | 43.9 (19.4) | 42.8 (25.2) | NS |
| Social functioning | 59.4 (27.1) | 61.7 (29.0) | NS |
| Role emotional | 46.7 (46.0) | 54.0 (47.5) | NS |
| Mental health | 65.3 (20.0) | 69.0 (22.9) | NS |
| SF-36 survey component scores, mean (SD) | |||
| PCS | 39.5 (5.7) | 39.1 (5.7) | NS |
| MCS | 43.7 (11.6) | 46.0 (13.7) | NS |
Results
| Outcomea | CRT on (CRT-D) (n = 36) | CRT off (ICD) (n = 36) | p-value |
|---|---|---|---|
| Deaths in 6 months’ follow-up from cardiac causes, n/N | 1/36 | 1/36 | |
| Left ventricular measurements by multigated acquisition scan, change from baseline to 6 monthsb | |||
| LVESV (ml) (primary outcome) | –7 (52) | –30 (47) | NS |
| LVEDV (ml) | –7 (61) | –34 (65) | NS |
| LVEF (%) | 1.7 (5.4) | 0.6 (6.8) | NS |
| Left ventricular measurements by ECG, change from baseline to 6 monthsb | |||
| LVESV (ml) | –21 (45) | –5 (22) | NS |
| LVEDV (ml) | –16 (44) | –13 (47) | NS |
| LVEF (%) | 3.9 (8.9) | 1.9 (6.8) | NS |
| Cardiac output measured by multigated acquisition scan (l/minute)b | |||
| Baseline | 4.5 (1.6) | 5.1 (1.9) | |
| 6 months | 4.8 (1.8) | 4.7 (1.8) | |
| Difference | 0.38 (1.5) | –0.56 (1.9) | 0.033 |
| Patients hospitalised (%)c | 30.6 | 36.1 | |
| Jugular venous pressure (cm) above the sternal angleb | |||
| Baseline | 2.1 (2.3) | 2.1 (2.1) | NS |
| 6 months | 2.9 (2.27) | 4.3 (2.5) | NR |
| B-type natriuretic peptide level (ng/l)b | |||
| Baseline | 198.7 (167.2) | 200.9 (208.7) | |
| 6 months | 119.4 (131.7) | 107.6 (99.4) | NS |
| SDANN (milliseconds) | |||
| Baseline | 83.2 (31.1) | 93.7 (29.4) | NS |
| 6 months | 83.0 (30.6) | 109.8 (41.5) | NR |
| Interventricular dyssynchrony (milliseconds) | |||
| Baseline | 40 (48) | 47 (36) | |
| 6 months | 13 (40) | 48 (34) | |
| Horizontal extent of the mitral regurgitation jet area (cm2)b | |||
| Baseline | 4.79 (3.06) | 3.58 (3.66) | |
| 6 months | 3.90 (3.65) | 3.00 (2.74) | |
| QRS duration (milliseconds)b | |||
| Baseline | 169.1 (22.8) | 159.5 (17.4) | |
| 6 months | 163.3 (24.3) | 163.8 (22.3) | |
| Ventricular tachyarrhythmia event requiring therapy from the device, n (%) | 7 (19.4) | 6 (16.7) | NS |
| No. of treated VT episodes per patient | 5.9 (6.1) | 3.4 (2.7) | NS |
| Assessment of functional status, change from baseline to 6 monthsb | |||
| 6-minute walk distance (m) | 53.3 (113.3) | 27.3 (71.1) | NS |
| DASI | 4.63 (9.20) | 1.08 (7.02) | NS |
| Global Visual Analogue Scale | –0.07 (2.22) | –0.17 (1.64) | NS |
| MLWHFQ | |||
| Total score | –7.8 (20.1) | –0.2 (13.5) | NS |
| Physical dimension | –5.0 (12.4) | –0.6 (7.9) | NS |
| Emotional dimension | –1.3 (5.0) | 0.3 (3.4) | NS |
| SF 36, change from baseline to 6 monthsb | |||
| Physical functioning | 11.2 (24.2) | 6.3 (21.2) | NS |
| Role physical | 19.6 (43.2) | 21.6 (38.1) | NS |
| Bodily pain | –3.3 (16.6) | –2.3 (13.1) | NS |
| General health | –5.8 (14.9) | –5.8 (13.6) | 0.02 |
| PCS | 1.4 (6.4) | 1.3 (4.8) | NS |
| Vitality | 4.7 (22.7) | 2.6 (15.7) | NS |
| Social functioning | 12.5 (23.3) | 5.4 (32.6) | NS |
| Role emotional | 29.5 (48.4) | 3.3 (48.2) | NS |
| Mental health | 4.5 (14.5) | 0.1 (21.8) | NS |
| MCS | 5.1 (10.1) | 0.5 (12.4) | NS |
Adverse effects of treatment
| Adverse effect | CRT on (CRT-D) (n = 36) | CRT off (ICD) (n = 36) | p-value |
|---|---|---|---|
| Not reported | |||
-
Allocation to treatment groups: all patients received a device. Left ventricular pacing was turned off in the immediate postoperative period. Patients were randomly assigned following completion of baseline procedures 14–28 days post implant.
-
Blinding: patients were blinded to treatment allocation. All post-implant study evaluations were performed by personnel blinded to treatment allocation.
-
Comparability of treatment groups: no significant differences, although there were more patients with significant mitral regurgitation in the CRT on group (p = 0.09).
-
Method of data analysis: primary end point analysed according to ITT principle. Data were analysed using the unpaired t-test, Wilcoxon signed-rank test and repeated measures analysis of variance as appropriate. The difference in change from baseline between groups and within groups was analysed using the Wilcoxon signed-rank test. For some outcomes data are compared within groups only and not between groups; these p-values have not been extracted.
-
Sample size/power calculation: allowing for 10% dropout or crossover rate, it was estimated that 70 patients had to be included to show a clinically meaningful 12% decrease in end-systolic volume with 80% power and a two-tailed alpha of 0.05.
-
Attrition/dropout: in total, 75/90 (83.3%) attempted implants were successful. Of these, two were not randomised because of device-related technical difficulties (double sensing) and one was not randomised because of worsening HF. Of the 72 randomised, five missed the 6-month visit (one from each group died from cardiac causes; two crossed over, one from the CRT off group to the CRT on group because of worsening CHF and one from the CRT on group to the CRT off group because of late left ventricular capture failure; and one in the CRT on group was too ill). Therefore, 67/72 (93%) completed the study (CRT on: 33; CRT-off: 34).
-
Generalisability: only those with successful implantation were randomised. This is a study of prophylactic CRT for patients with mild to moderate HF; patients did not meet guidelines for CRT at the time of the study but may meet indications for CRT by current standards.
-
Outcome measures: radionuclide angiography was selected for the measurement of the primary end point because of the assumption that it is more accurate than ECG in measuring left ventricular outcomes. NYHA class and adverse events were not reported.
-
Intercentre variability: not reported.
-
Conflict of interests: two authors have received honoraria and research funding from Guidant Corporation. The study was supported by an unrestricted educational grant from Guidant Corporation.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Details not reported |
| Allocation concealment | Unclear | Details not reported |
| Performance bias | ||
| Blinding of participants and personnel | Low | States that patients were blinded, although not clear how this was maintained |
| Detection bias | ||
| Blinding of outcome assessment | Low | States that all post-implant study evaluations were performed by personnel blinded to treatment allocation |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Attrition and crossovers reported. ITT analysis performed |
| Reporting bias | ||
| Selective reporting | Low | No protocol available but outcomes listed in the methods were reported on |
| Other bias | ||
| Other sources of bias | Low | |
Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT)
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Tang et al. 2009141 and 2010140 Study design: RCT Countries: Canada, Europe, Turkey and Australia No. of centres: 34 (Canada 24, Europe and Turkey 8, Australia 2) Funding: Canadian Institutes of Health Research. Medtronic of Canada (industry partner) provided funding and CRT components |
Intervention: ICD-CRT (commercially available transvenous leads and devices, Medtronic). Standard implantation technique. Programming standardised to maximise ventricular pacing Comparator: ICD. Programming standardised to minimise ventricular pacing Other interventions used: OPT for both groups: a beta-blocker, an ACE inhibitor or ARB, spironolactone, aspirin and statins when appropriate; uniform arrhythmia detection and therapy |
Indication for treatment: initially mild to moderate (NYHA class II or III) HF despite OPT, later restricted to NYHA class II HF with LVSD and a wide QRS complex No. of randomised participants: 1798; ICD-CRT: 894, ICD: 904 Inclusion criteria: NYHA class II or III (revised in February 2006 to class II only) symptoms despite receiving OPT, LVEF ≤ 30% from ischaemic or non-ischaemic causes, QRS interval ≥ 120 milliseconds or a paced QRS duration of ≥ 200 milliseconds, sinus rhythm or permanent atrial fibrillation or flutter with a controlled ventricular rate (≤ 60 bpm at rest and ≥ 90 bpm during a 6-minute walk test) or planned atrioventricular junction ablation after device implantation and planned ICD implantation for indicated primary or secondary prevention of SCD. Optimal HF pharmacological therapy141 Exclusion criteria: major coexisting illness; recent cardiovascular event protocol;141 life expectancy of < 1 year from non-cardiac cause; expected cardiac transplantation within 1 year (status 1); received intravenous inotropic agent in the last 4 days; acute coronary syndrome including MI can be included if the patient has had a previous MI with left ventricular dysfunction (LVEF ≤ 30%); in-hospital patients who have acute cardiac or non-cardiac illness that requires intensive care; uncorrected or uncorrectable primary valvular disease; restrictive, hypertrophic or reversible form of cardiomyopathy; severe primary pulmonary disease such as cor pulmonale; tricuspid prosthetic valve; patients with an existing ICD (patients with an existing pacemaker can be included if they satisfy all other inclusion/exclusion criteria); coronary revascularisation (CABG or percutaneous coronary intervention) < 1 month if previous LVEF > 30% (more recent revascularisations can be included if previous LVEF ≤ 30%); included in other clinical trial that will affect the objectives of this study; history of non-compliance with medical therapy; unable or unwilling to provide informed consent |
Primary outcome: composite outcome of death from any cause or HF leading to hospitalisation Secondary outcomes: death from any cause at any time during the study, death from any cardiovascular cause, and hospitalisation for HF among all patients (those with NYHA class II and class III HF at baseline) Method of assessing outcomes: hospitalisation for HF was defined as admission to a health-care facility lasting > 24 hours with symptoms of CHF and subsequent treatment for HF (admissions for other medical problems that then developed into HF in the hospital were not classified as hospitalisation for HF). An adjudication committee reviewed available documents and determined the cause of death and whether or not hospitalisations that lasted > 24 hours were due to the exacerbation of HF. All adverse events occurring within 30 days after ICD implantation were adjudicated as related to or unrelated to the ICD Follow-up visits 1 month after device implantation and then 6-monthly until ≥ 18 months until the end of the trial, with clinical assessment and device interrogation at each visit Length of follow-up: minimum of 18 months. Mean 40 (SD 20) months; mean follow-up for surviving patients 44 (SD 18) months Recruitment: January 2003–February 2009 |
Participant characteristics
| Characteristics | ICD–CRT (n = 894) | ICD (n = 904) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 66.1 (SD 9.3) | 66.2 (SD 9.4) | NR |
| Sex, male, n (%) | 758 (84.8) | 732 (81.0) | NR |
| Ethnicity | NR | NR | |
| NYHA class, n (%) | |||
| II | 708 (79.2) | 730 (80.8) | NR |
| III | 186 (20.8) | 174 (19.2) | NR |
| LVEF (%), mean (SD) | 22.6 (5.4) | 22.6 (5.1) | NR |
| Atrial rhythm, n (%) | |||
| Permanent atrial fibrillation or flutter | 114 (12.8) | 115 (12.7) | NR |
| Sinus or atrial paced | 780 (87.2) | 789 (87.3) | NR |
| QRS duration (ms) | |||
| Intrinsic | |||
| No. of patients | 826 | 837 | |
| Mean (SD) | 157 (23.6) | 158.3 (24.0) | NR |
| Paced | |||
| No. of patients | 68 | 67 | |
| Mean (SD) | 206.5 (24.0) | 210.3 (18.3) | NR |
| QRS morphological type, n (%) | |||
| RBBB | 68 (7.6) | 93 (10.3) | NR |
| LBBB | 652 (72.9) | 643 (71.1) | NR |
| Non-specific intraventricular conduction delay | 106 (11.9) | 101 (11.2) | NR |
| Ventricular paced | 68 (7.6) | 67 (7.4) | NR |
| Peripheral vascular disease, n (%) | 88 (9.8) | 90 (10.0) | NR |
| Underlying heart disease, n (%) | |||
| Ischaemic | 614 (68.7) | 587 (64.9) | NR |
| Non-ischaemic | 280 (31.3) | 317 (35.1) | NR |
| Hospitalisation for HF in last 6 months, n (%) | 238 (26.6) | 223 (24.7) | NR |
| Previous treatment, n (%) | |||
| Percutaneous coronary intervention | 220 (24.6) | 208 (23.0) | NR |
| CABG surgery | 293 (32.8) | 313 (34.6) | NR |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 293 (32.8) | 313 (34.6) | NR |
| Hypertension | 402 (45.0) | 397 (43.9) | NR |
| Current cigarette smoking, n (%) | 121 (13.5) | 127 (14.0) | NR |
| Medication, n (%) | |||
| Beta-blocker | 808 (90.4) | 805 (89.0) | NR |
| ACE inhibitor or ARB | 859 (96.1) | 878 (97.1) | NR |
| Spironolactone | 372 (41.6) | 378 (41.8) | NR |
| Digoxin | 301 (33.7) | 319 (35.3) | NR |
| Acetylsalicylic acid (aspirin) | 584 (65.3) | 622 (68.8) | NR |
| Warfarin | 310 (34.7) | 298 (33.0) | NR |
| Clopidogrel | 134 (15.0) | 145 (16.0) | NR |
| Statin | 607 (67.9) | 618 (68.4) | NR |
| Diuretic | 757 (84.7) | 756 (83.6) | NR |
| Calcium channel blocker | 101 (11.3) | 83 (9.2) | NR |
| Amiodarone | 140 (15.7) | 124 (13.7) | NR |
| Other AAD | 12 (1.3) | 8 (0.9) | NR |
| 6-minute walk test | |||
| No. of patients | 789 | 765 | |
| Distance (m), mean (SD) | 351.3 (106.7) | 354.9 (110.1) | NR |
| Estimated glomerular filtration rate | |||
| No. of patients | 885 | 897 | |
| %, mean (SD) | 59.5 (19.8) | 60.8 (21.9) | NR |
| Rate (ml/minute/1.73 m2), n (%) | |||
| < 30 | 57 (6.4) | 63 (7.0) | NR |
| 30–59 | 398 (45.0) | 383 (42.7) | NR |
| ≥ 60 | 430 (48.6) | 451 (50.3) | NR |
Results
| Outcome | ICD-CRT (n = 894) | ICD (n = 904) | HR (95% CI), p-value |
|---|---|---|---|
| All patients | |||
| Primary outcome: death or hospitalisation for HF, n/N (%) | 297/894 (33.2) | 364/904 (40.3) | 0.75 (0.64 to 0.87), < 0.001 |
| Secondary outcomes | |||
| Death from any cause, n/N (%) | 186/894 (20.8) | 236/904 (26.1) | 0.75 (0.62 to 0.91), 0.003 |
| Death from cardiovascular cause, n/N (%) | 130/894 (14.5) | 162/904 (17.9) | 0.76 (0.60 to 0.96), 0.02 |
| Hospitalisation for HF, n/N (%) | 174/894 (19.5) | 236/904 (26.1) | 0.68 (0.56 to 0.83), < 0.001 |
| Hospitalisation more than once during follow-up (mostly cardiovascular), n | 509 | 509 | NR |
| Hospitalisation: cardiac cause, n | 423 | 404 | HR 1.04, 0.56 |
| Probability of event-free survival at 5 years, % | 57.6 | 48.7 | NR |
| 5-year actuarial rate of death (%) | 28.6 | 34.6 | NR |
| ICD-CRT (n = 708) | ICD (n = 730) | ||
| Patients in NYHA class II | |||
| Primary outcome: death or hospitalisation for HF, n/N (%) | 193/708 (27.3) | 253/730 (21.1) | 0.73 (0.61 to 0.88), 0.001 |
| Secondary outcomes, n/N (%) | |||
| Death from any cause | 110/708 (15.5) | 154/730 (21.1) | 0.71 (0.56 to 0.91), 0.006 |
| Death from cardiovascular cause | 74/708 (10.5) | 100/730 (13.7) | 0.73 (0.54 to 0.99), 0.04 |
| Hospitalisation for HF | 115/708 (16.2) | 159/730 (21.8) | 0.70 (0.55 to 0.89), 0.003 |
| ICD-CRT (n = 186) | ICD (n = 174) | ||
| Patients in NYHA class III | |||
| Primary outcome: death or hospitalisation for HF, n/N (%) | 104/186 (55.9) | 111/174 (63.8) | 0.76 (0.58 to 0.99), 0.04 |
| Secondary outcomes, n/N (%) | |||
| Death from any cause | 76/186 (40.9) | 82/174 (47.1) | 0.79 (0.58 to 1.08), 0.14 |
| Death from cardiovascular cause | 56/186 (30.1) | 62/174 (35.6) | 0.77 (0.54 to 1.10), 0.15 |
| Hospitalisation for HF | 59/186 (31.7) | 77/174 (44.3) | 0.63 (0.45 to 0.88), 0.006 |
Adverse effects of treatment
| Adverse effect | ICD-CRT (n = 888) | ICD (n = 899) | HR (95% CI), p-value |
|---|---|---|---|
| Death from worsening HF within 24 hours of device implantation, n | 1 | ||
| Device-related hospitalisation, n (%) | 179 (20) | 110 (12.2) | 1.68 (1.32 to 2.13), < 0.001 |
| Number of device- or implantation-related complications during the first 30 days after device implantation, n/Na | 118/888 | 61/899 | – (–), < 0.001 |
| Adverse effects at 30 days after device implantation, n/Na | 124/888 | 58/899 | – (–), < 0.001 |
| Haemothorax or pneumothorax, n (%) | 11 (1.2) | 8 (0.9) | 0.47 (–), – |
| Device pocket haematoma requiring intervention, n (%) | 14 (1.6) | 11 (1.2) | 0.53 (–), – |
| Device pocket infection requiring intervention, n (%) | 21 (2.4) | 16 (1.8) | 0.39 (–), – |
| Lead dislodgement requiring intervention, n (%) | 61 (6.9) | 20 (2.2) | – (–), < 0.0001 |
| Device pocket problems requiring revision, n (%) | 4 (0.5) | 1 (0.1) | 0.22 (–), – |
| Coronary sinus dissection, n (%) | 11 (1.2) | 0 | 0.0004 (–), – |
| Tamponade, n (%) | 2 (0.23) | 2 (0.22) | 1 (–), – |
-
Allocation to treatment groups: random assignment in a 1 : 1 ratio and stratification according to clinical centre, atrial rhythm (atrial fibrillation or flutter or sinus atrial pacing) and planned implantation of a single- or dual-chamber ICD.
-
Blinding: described as double blind. Patients and general health-care providers (including the team responsible for HF management and reporting of clinical events) were blinded, as was the adjudication committee responsible for reviewing available documents and determining cause of death. Arrhythmia teams (physicians and caregivers) performing device implantation and device management were not blinded.
-
Comparability of treatment groups: authors state that baseline clinical characteristic are similar between the two groups.
-
Method of data analysis: all analyses were conducted according to the ITT principle. Survival analysis techniques were used to compare the two groups with respect to the primary outcome and principal secondary outcomes. Survival in each of the two groups was summarised with the use of Kaplan–Meier product limit estimates. Survival curves were compared using non-parametric log-rank tests. HRs and associated 95% CIs were calculated with the use of the Cox proportional hazards model. Primary and secondary outcomes for patients with NYHA class II or III HF were analysed separately as NYHA class III patients were enrolled only during the first part of the study, before the protocol was revised in February 2006 to include only NYHA class II patients. Cox proportional hazard models were used to test for interactions in the various planned subgroups. The protocol states that planned subgroup analyses would include AF compared with no AF and NYHA class II compared with class III (p. 16). 141 Chi-squared tests were used to compare the Kaplan–Meier (actuarial) rate of event-free survival at 5 years. The HR was used to calculate the number needed to treat to prevent one death or hospitalisation for HF in one patient. Underlying assumptions for these statistical procedures were assessed (in particular the proportional hazards assumption). Analyses were conducted with the use of SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
-
Sample size/power calculation: the study had a statistical power of 85% to detect a 25% relative reduction in the primary outcome, given a two-sided alpha value of 0.05 and taking into consideration the expected rate of loss to follow-up and crossover. 140 To detect a 20% RRR in the primary end point for CRT-ICD, given an alpha value of 0.05 (two-sided) and 90% power, a sample size of 1500 patients will be needed (750 per group). This calculation assumes an exponential survival with all patients followed to the primary end point or termination of the study, and allows for a 5% inability to implant the left ventricular lead (based on the most recent data indicating a 96% implant success rate in a worldwide registry) and a 3% crossover rate from the control group (ICD) to the experimental group (CRT-ICD). 141 This sample size will also be able to detect a 25% RRR in total mortality with the assumption of 11% annual mortality in the control group, given an alpha value of 0.05 (two-sided) and 80% power. 141
-
Attrition/dropout: ICD-CRT group: 888/894 (99.3%) received ICD-CRT; leads were successfully implanted in 841/888 (94.7%); 53/888 (60%) did not receive CRT (47 leads failed, six lead malfunctions); of those who did not undergo implantation, four died and two declined to participate (the patient or physician). ICD group: 899/904 (99.4%) received ICD; of those who did not undergo implantation, four declined to participate (patient or physician) and in one there was a lack of venous access. Crossover: ICD to ICD-CRT: 36 (4%) before the occurrence of a primary outcome and 60 (6.6%) after hospitalisation for HF. ICD-CRT group: eight withdrew, two were lost to follow-up; ICD group: four withdrew, one was lost to follow-up.
-
Other: (1) To increase recruitment to 34 patients per month, Medtronic sponsored the expansion to more centres in Europe and Turkey from the original 21 centres (Canada 21, Germany two, Australia two, New Zealand one; see protocol, p. 16). 141 However, no enrolment for the centre in New Zealand is reported. (2) Two planned interim analyses were conducted for the data and safety monitoring board (first planned with 33% enrolled and followed for 2 years; second planned with 66% enrolled and followed for 2 years141) and an O’Brien–Fleming alpha spending function was used to adjust the sample size for these interim analyses.
-
Generalisability: mild to moderate HF patients with LVSD and a wide QRS complex.
-
Outcome measures: appear appropriate.
-
Intercentre variability: not reported.
-
Conflict of interests: medtronic did not participate in the conduct of the trial, the reporting of the data or the decision to submit the manuscript for publication.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | Random assignment in a 1 : 1 ratio with stratification according to centre. No details on sequence generation |
| Allocation concealment | Unclear | No details reported |
| Performance bias | ||
| Blinding of participants and personnel | Low | Double blind. Patients and general health-care providers were blinded, but not device caregivers |
| Detection bias | ||
| Blinding of outcome assessment | Low | Members of the adjudication committee responsible for reviewing available documents and determining cause of death were blinded |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | ITT analysis; CONSORT (Consolidated Standards of Reporting Trials) flow chart (including numbers analysed) provided in an appendix |
| Reporting bias | ||
| Selective reporting | High | The protocol141 described ‘other outcomes’ (e.g. QoL), but no data for these were reported. However, this is a recent study and it is possible that further data will be published in the future |
| Other bias | ||
| Other sources of bias | Low | |
Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS (RethinQ) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| Beshai et al. 2007,142 Beshai and Grimm 2007143 Study design: RCT Country: USA No. of centres: 34 Funding: St Jude Medical |
Intervention: CRT-D on + OPT (CRT device: Epic HF or Atlas+ HF, St Jude Medical) with a standard right atrial, right ventricular defibrillator and left ventricular leads. Detection and treatment of tachyarrhythmias turned on143 Comparator: ICD + OPT (device as above). Detection and treatment of tachyarrhythmias turned on143 Other interventions used: OPT for both groups defined as a beta-blocker for a minimum of 90 days and an ACE inhibitor or ARB for a minimum of 30 days, unless contraindicated or not tolerated (for stable medical regimen no more than a 100% increase or a 50% decrease in dose). Also included: aldactone inhibitors, diuretics and cardiac glycosides (i.e. digoxin) as indicated. If intolerant to ACE inhibitors or ARBs or if contraindicated, alternate therapy as appropriate, including afterload reduction agents (e.g. hydralazine) combined with nitrates143 |
Indication for treatment: standard indication for an ICD (ischaemic or non-ischaemic cardiomyopathy and LVEF ≤ 35%), a narrow QRS interval and intraventricular mechanical dyssynchrony No. of randomised participants: 172; CRT-D on: 87, CRT-D off: 85 Inclusion criteria: NYHA class III caused by either ischaemic or non-ischaemic cardiomyopathy, LVEF ≤ 35%, QRS interval < 130 milliseconds, approved indication for an ICD, stable conventional medical regimen, evidence of mechanical dyssynchrony on ECG, able to complete exercise stress testing and a 6-minute walk test (limited only by cardiac fitness)143 Exclusion criteria: standard indication for cardiac pacing or previous treatment with CRT, standard bradycardic indication for pacing, continuous atrial fibrillation (lasting > 1 month) < 1 year before enrolment, cardioversion for atrial fibrillation in the past month, ability to walk > 450 m during the 6-minute walk test, NYHA class I, II or IV, symptomatic COPD, classification of status 1 for cardiac transplantation or consideration for transplantation in the next 6 months, recent MI, unstable angina, cardiac revascularisation (PTCA or CABG) within 40 days of enrolment, recent stroke or transient ischaemic attack within 3 months of enrolment, severe musculoskeletal disorder/s, pregnant or a planned pregnancy in the next 6 months, life expectancy of ≤ 6 months, age < 18 years143 |
Primary outcomes: proportion of patients with an increase of ≥ 1.0 ml/kg body weight/minute in peak oxygen consumption during cardiopulmonary exercise testing142 and survival from CRT-D system-related complications143 Secondary outcomes: QoL and NYHA class Method of assessing outcomes: baseline evaluation 14 days after successful implantation, including cardiopulmonary exercise testing (maximum exercise tolerance on treadmill/bicycle ergonometry measuring heart rate, minute ventilation, oxygen uptake and carbon dioxide output), NYHA class assessment, 6-minute walk distance, QoL evaluation (MLWHFQ, score from 0 to 105, higher score indicates poorer QoL), assessment of medication stability, ECG for optimisation of atrioventricular and interventricular delay and 12-lead ECG. Evaluation repeated at 6 months Mechanical dyssynchrony definition: an opposing wall delay of ≥ 65 milliseconds on tissue Doppler imaging or a mechanical dyssynchrony in the septal to posterior wall of ≥ 130 milliseconds on M-mode ECG Length of follow-up: 6 months Recruitment: August 2005–January 2007 |
Participant characteristics
| Characteristic | CRT-D on + OPT (n = 87) | ICD + OPT (n = 85) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 60 (12) | 58 (14) | |
| Sex, male, n (%) | 62 (71) | 49 (58) | |
| Ethnicity | NR | NR | |
| NYHA class III, n (%) | 87 (100) | 84 (99) | |
| LVEF (%), mean (SD) | 25 (5) | 26 (6) | |
| End-diastolic diameter (mm), mean (SD) | 66 (9) (n = 85) | 65 (9) (n = 84) | |
| End-systolic diameter (mm), mean (SD) | 56 (9) (n = 85) | 53 (9) (n = 84) | |
| End-diastolic volume (ml), mean (SD) | 216 (78) | 210 (75) | |
| End-systolic volume (ml), mean (SD) | 163 (65) | 156 (64) | |
| QRS interval (milliseconds), mean (SD) | 107 (12) | 106 (13) | |
| < 120 ms, n (%) | 66 (76) | 60 (71) | |
| ≥ 120 ms, n (%) | 21 (24) | 25 (29) | |
| Underlying heart disease, n (%) | |||
| Ischaemic | 47 (54) | 43 (51) | |
| Non-ischaemic | 40 (46) | 42 (49) | |
| Indication for ICD, n (%) | |||
| Primary prevention | 74 (85) | 73 (86) | |
| Secondary prevention | 13 (15) | 12 (14) | |
| Pre-ejection period (milliseconds), mean (SD) | 112 (21) (n = 86) | 112 (22) (n = 86) | |
| Interventricular mechanical delay (milliseconds), mean (SD) | 9 (28) (n = 85) | 8 (31) (n = 82) | |
| Intraventricular mechanical dyssynchrony (milliseconds), mean (SD)a | |||
| Septal to posterior wall | 106 (45) (n = 24) | 112 (51) (n = 33) | |
| Septal to lateral wall | 81 (39) (n = 85) | 86 (38) (n = 85) | |
| Anteroseptal to posterior wall | 78 (34) (n = 83) | 81 (45) (n = 81) | |
| Mitral regurgitation, n (%) | |||
| None or mild | 59 (68) | 55 (66) | |
| Moderate | 25 (29) | 23 (28) | |
| Severe | 3 (3) | 5 (6) | |
| Medication at baseline, n (%) | |||
| ACE inhibitor or substituteb | 77 (89) | 77 (91) | |
| Beta-blocker | 84 (97) | 79 (93) | |
| Diuretic | 73 (84) | 74 (87) | |
| AAD | 7 (8) | 10 (12) | |
| Peak oxygen consumption (ml/kg/minute), mean (SD) | 12.1 (3.3) | 12.4 (4.5) | |
| Exercise duration (minute), mean (SD) | 8.9 (3.0) | 9.0 (3.8) | |
| QoL (MLWHFQ) score, mean (SD) | 54 (24) | 57 (26) | |
| 6-minute walk distance (m), mean (SD) | 301 (94) | 297 (100) | |
Results
| Outcome | CRT-D on + OPT (n = 87) | ICD + OPT (n = 85) | p-value |
|---|---|---|---|
| Mortality before 6 months, n/N (%) | 5/87 (5.7) | 1/85 (1.2) | |
| Unknown cardiac causes | 2/87 (2.3) | ||
| Pump failure | 2/87 (2.3) | 1/85 (1.2) | |
| Unknown cause | 1/87 (1.2) | ||
| Mortality at 7 months, pump failure, n/N (%) | 1/85 (1.2)a | ||
| Cumulative overall survival at 6 months, % (95% CI) | 94.2 (86.7 to 97.6) | 98.8 (91.9 to 99.8) | 0.11 |
| Cumulative freedom from death caused by worsening HF, % (95% CI) | 97.7 (91.1 to 99.4) | 98.9 (91.9 to 99.8) | 0.58 |
| Change in peak VO2 | (n = 76) | (n = 80) | 0.63 |
| Median change (ml/kg/minute) (95% CI) | 0.4 (–0.6 to 1.2) | 0.5 (–0.3 to 1.1) | |
| Primary outcome: increase of ≥ 1.0 ml/kg/minute, n/N (%) | 35/76 (46) | 33/80 (41) | |
| Change in QoL (MLWHFQ) score | (n = 76) | (n = 80) | |
| Median change (95% CI) | –8 (–10 to –1) | –7 (–11 to 3) | 0.91 |
| Change in NYHA class, n/N (%) | (n = 76) | (n = 80) | 0.006 |
| Improved by one or more class | 41/76 (54) | 23/80 (29) | |
| No change | 31/76 (41) | 51/80 (64) | |
| Worsened | 4/76 (5) | 6/80 (8) | |
| Change in 6-minute walk distance (m) | (n = 75) | (n = 79) | |
| Median change (95% CI) | 26 (0 to 46) | 6 (–17 to 30) | 0.23 |
| Change in ejection fraction (%) | (n = 68) | (n = 74) | |
| Median change (95% CI) | 1.2 (–0.4 to 4.4) | 2.0 (0.3 to 4.2) | 0.83 |
| Change in end-diastolic volume (ml) | (n = 68) | (n = 74) | |
| Median change (95% CI) | –16 (–29 to –8) | –11 (–30 to –2) | 0.71 |
| Change in end-systolic volume (ml) | (n = 68) | (n = 74) | |
| Median change (95% CI) | –19 (–34 to –12) | –18 (–28 to –8) | 0.81 |
| Change in end-diastolic diameter (mm) | (n = 72) | (n = 77) | |
| Median change (95% CI) | 0 (–2 to 0) | –1 (–2 to 1) | 0.49 |
| Change in end-systolic diameter (mm) | (n = 72) | (n = 77) | |
| Median change (95% CI) | –1 (–3 to 0) | 0 (–2 to 2) | 0.34 |
| Change in degree of mitral regurgitation, n/N (%) | (n = 76) | (n = 80) | > 0.99 |
| Improved by one or more grade | 8/76 (11) | 9/80 (12) | |
| No change | 60/76 (81) | 61/80 (80) | |
| Worsened by one or more grade | 6/76 (8) | 6/80 (8) |
Adverse effects of treatment
| Adverse effect | CRT-D on + OPT (n = 87) | ICD + OPT (n = 85) | p-value |
|---|---|---|---|
| HF events requiring intravenous therapy | 24 events in 14/87 patients (16.1%) | 41 events in 19/85 patients (22.3%) | |
| Lead dislodgement, n/N (%) | 13/172 (7.6) | ||
| Left ventricular lead, n/N (%) | 5/172 (2.9) | ||
| Infection, n/N (%) | 6/172 (3.5) | ||
| Bleeding or haematoma, n/N (%) | 2/172 (1.2) | ||
| Loss of pacemaker lead capture, n/N (%) | 2/172 (1.2) | ||
| Phrenic nerve stimulation, n/N (%) | 3/172 (1.7) | ||
| Deep venous thrombosis, n/N (%) | 3/172 (1.7) | ||
| Pneumothorax, n/N (%) | 2/172 (1.2) | ||
| Pericarditis, n/N (%) | 2/172 (1.2) | ||
| Coronary sinus perforation, n/N (%) | 1/172 (0.6) | ||
Subgroup analysis
Subgroup analysis according to QRS interval at 6 monthsa
| Subgroup | CRT-D on + OPT (QRS ≥ 120 milliseconds, n = 17; QRS < 120 milliseconds, n = 59) | ICD + OPT (QRS ≥ 120 milliseconds, n = 25; QRS < 120 milliseconds, n = 55) | p-value |
|---|---|---|---|
| Peak VO2, proportion of patients with an increase of at least 1 ml/kg body weight/minute from baseline | |||
| QRS ≥ 120 milliseconds | 58.9 | 19.7 | 0.02 |
| QRS < 120 milliseconds | 42.2 | 51.2 | 0.45 |
| NYHA class, proportion of patients whose condition improved by at least one class from baseline | |||
| QRS ≥ 120 milliseconds | 70.7 | 28.0 | 0.01 |
| QRS < 120 milliseconds | 49.4 | 29.3 | 0.04 |
| QoL, median change from baseline (%) | |||
| QRS ≥ 120 milliseconds | 0 | –3.7 | 0.24 |
| QRS < 120 milliseconds | –8.9 | –7.0 | 0.63 |
| 6-minute walk distance, median change from baseline (m) | |||
| QRS ≥ 120 milliseconds | 0.0 | –19.1 | 0.76 |
| QRS < 120 milliseconds | 33.7 | 10.3 | 0.31 |
Subgroup analysis according to cardiomyopathy classification at 6 monthsa
| Subgroup | CRT-D on + OPT (ischaemic, n = 40; non-ischaemic, n = 36) | ICD + OPT (ischaemic, n = 41; non-ischaemic, n = 39) | p-value |
|---|---|---|---|
| Peak VO2, proportion of patients with an increase of at least 1 ml/kg body weight/minute from baseline | |||
| Ischaemic | 40.0 | 44.2 | 0.82 |
| Non-ischaemic | 52.6 | 38.4 | 0.25 |
| NYHA class, proportion of patients whose condition improved by at least one class from baseline | |||
| Ischaemic | 55.3 | 29.5 | 0.02 |
| Non-ischaemic | 53.2 | 28.4 | 0.04 |
| QoL, median change from baseline (%) | |||
| Ischaemic | –5.9 | –3.6 | 0.68 |
| Non-ischaemic | –10.6 | –6.5 | 0.60 |
| 6-minute walk distance, median change from baseline (m) | |||
| Ischaemic | 4.2 | 5.8 | 0.57 |
| Non-ischaemic | 55.0 | 2.5 | 0.01 |
-
Allocation to treatment groups: random assignment in a 1 : 1 ratio according to centre and stratified according to cardiomyopathy classification and QRS interval (< 120 milliseconds and ≥ 120 milliseconds) within each centre. Randomisation assignments were created in S-PLUS software (Insightful) and provided to site personnel (aware of study group assignments) with the use of an interactive voice-response system at the baseline visit. Participants were randomised after successful implantation and once all baseline evaluations were completed.
-
Blinding: study paper reports that trial was double blind but site personnel provided with randomisation assignments were aware of study group assignments. Site personnel unaware of study group assignments administered all evaluations at 6 months. Independent committees whose members were unaware of study group assignments and investigational centre adjudicated all deaths and adverse events.
-
Comparability of treatment groups: authors state that none of the differences between the groups was significant but no p-values were reported.
-
Method of data analysis: all end points were analysed according to the ITT principle. Secondary end points were each evaluated at a significance level of 0.025 and were considered significant only if the primary efficacy end point was met with the use of the gatekeeper method. All p-values were calculated with the use of a two-sided test. Survival curves were constructed according to the Kaplan–Meier method and the differences between curves were examined by the log-rank statistic. Data for all patients were censored at 196 days, the last day of the 6-month window for clinical visits. CIs for survival were computed on a log–log scale. For continuous variables, data are presented as median changes between baseline and 6 months. CIs for the median were computed with the use of a distribution-free approach. Comparisons of changes from baseline to 6 months between the CRT-D off (control) group and the CRT-D on group were evaluated for significance by the Wilcoxon rank-sum test. Mean (SD) values are presented. For categorical variables, differences in the distribution of responses to treatment at 6 months in the two groups were compared by Fisher’s exact test. CIs for proportions were computed by exact methods. The protocol specified that end point analyses be performed for patients with data available at 6 months and for those who died, withdrew or were unable to perform the evaluation at 6 months because of worsening HF. The last group of patients were included in the analysis with their worst values imputed as follows: 0 ml/kg/minute for peak VO2, a score of 105 on the QoL scale, NYHA class IV and 0 m for the 6-minute walk distance.
-
Sample size/power calculation: the study was powered to detect a difference of 23% in the proportion of patients who achieved the primary end point in the CRT-D on group compared with the CRT-D off group (control). The proportion who improved in the control group was assumed to be 25%. The sample size required to detect this difference with a statistical power of 80% at the 0.05 significance level was 76 patients in each group, with the use of Fisher’s exact test. On the basis of an attrition rate of 40%, the study required a total of 250 participants.
-
Attrition/dropout: total recruitment: 250, total randomised: 172 (four unsuccessful implantation, two deaths, three withdrawals, 69 did not meet inclusion criteria). CRT-D on: three died from other causes than HF, three withdrew for reasons other than worsening HF, three had < 6 months’ follow-up and two had no exercise test at follow-up; 76 participants were included in efficacy analyses, two died from HF. CRT-D off: four had < 6 months’ follow-up, one had no exercise test at 6 months; 80 participants were included in efficacy analyses, two died from HF and two did not have an exercise test because of worsening HF. Crossovers: three participants crossed from CRT-D off to CRT-D on because of worsening HF (included in the control group analysis); there were no crossovers from CRT-D on to CRT-D off.
-
Generalisability: limited to participants with successful implantation, a QRS interval < 130 milliseconds, NYHA class III symptoms and evidence of mechanical dyssynchrony [authors state that only 4% of patients were eligible to participate in the study solely on the basis of mechanical dyssynchrony in the septal to posterior wall of ≥ 130 milliseconds on M-mode ECG; 96% qualified on the basis of the tissue Doppler imaging criterion (i.e. an opposing wall delay of ≥ 65 milliseconds)].
-
Outcome measures: appear to be appropriate. The primary outcome measure was the proportion of patients with an increase of 1.0 ml/kg body weight/minute in peak oxygen consumption during cardiopulmonary exercise testing. The study was not powered for mortality.
-
Intercentre variability: not reported.
-
Conflict of interests: Drs Beshai, Grimm, Nagueh, Greenberg and Pires received lecture/consulting fees, support and/or grants from St Jude Medical, Medtronic, GE and/or Boston Scientific. The authors state that there was no other potential conflict of interest relevant to the publication and that investigators had full access to all data and performed analyses without restrictions or limitations from the sponsor.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Low | Random assignment in a 1 : 1 ratio according to centre and stratified according to the cardiomyopathy classification and the QRS interval within each centre. Randomisation assignments created in S-PLUS software (Insightful) |
| Allocation concealment | Low | Allocation provided to site personnel with the use of an interactive voice response system at the baseline visit |
| Performance bias | ||
| Blinding of participants and personnel | Unclear | Paper states that study was double blind but unclear who was blinded. Randomisation assignments were provided to site personnel, unclear if these personnel continued to be involved in the care of participants |
| Detection bias | ||
| Blinding of outcome assessment | Low | Site personnel conducting evaluations at 6 months were unaware of treatment assignments, as were independent committee members adjudicating all deaths and adverse events |
| Attrition bias | ||
| Incomplete outcome data addressed | ||
| Peak oxygen consumption (primary outcome), QoL, NYHA class, 6-minute walk distance, mortality before 6 months | Low | Paper states that all end points were analysed according to the ITT principle. The protocol specified that end point analyses be performed for patients with data available at 6 months and for those who died, withdrew or were unable to perform the evaluation at 6 months because of worsening HF. However, analysis was performed with 66 CRT-D on + OPT group participants and 80 ICD + OPT group participants, because of some participants not having completed a cardiopulmonary exercise test for reasons other than worsening HF. Numbers and reasons given |
| Other end points | High | Missing data; reasons not given |
| Reporting bias | ||
| Selective reporting | Low | All protocol outcomes reported |
| Other bias | ||
| Other sources of bias | Low | |
Resynchronization for the HemodYnamic Treatment for Heart failure Management Implantable Cardioverter Defibrillator (RHYTHM ICD) trial
| Reference and design | Intervention and comparator | Participants | Outcome measures |
|---|---|---|---|
| US Food and Drug Administration 2004144 and 2005145 Study design: RCT Country: not stated No. of centres: 50 Funding: not stated but presumed to be the device manufacturer, St Jude Medical |
Intervention: CRT-D (device: St Jude Medical Epic HF model V-338, maximum output 30 J, with Aescula left ventricular leads) Comparator: ICD Other interventions used: not stated |
Indication for treatment: patients indicated for ICD therapy with NYHA class III/IV HF and a prolonged QRS duration No. of randomised participants: 205 enrolled, 182 successful implants, 179 baseline visit; CRT-D: 119, ICD: 60 Inclusion criteria: LVEF ≤ 35%, QRS interval ≥ 150 ms, ICD indication for treatment of life-threatening VT, symptomatic HF for ≥ 6 months, NYHA class III or IV despite ≥ 90 days of appropriate pharmacological therapy, receiving OPT for CHF (including ACE inhibitor and beta-blocker as tolerated), stable for 30 days before enrolment, ability to complete a cardiopulmonary exercise stress test and 6-minute walk test, able to consent and comply with follow-up tests and evaluations Exclusion criteria: standard bradycardic indication for pacing, chronic atrial fibrillation (continuous atrial fibrillation lasting > I month) within 1 year or cardioversion for atrial fibrillation in the past month, able to walk > 450 m in the 6-minute walk test, NYHA class I or II, contraindication for an emergency thoracotomy, candidate for cardiac transplantation in the next 6 months, recent (within 1 month) MI, unstable angina or cardiac revascularisation, stroke or transient ischaemic attack in the last 3 months, severe musculoskeletal disorder(s), pregnancy, participation in other clinical investigations, life expectancy < 6 months |
Primary outcomes: left ventricular lead-related complications at 6 months, Epic HF system-related complications at 6 months, defibrillation system effectiveness: VF detection/redetection times, CRT efficacy (peak VO2) Secondary outcomes: improvement at 6 months in NYHA class, QoL (MLWHFQ) score and 6-minute walk test; Aescula left ventricular lead performance and lead pacing capture threshold Method of assessing outcomes: baseline visit approximately 2 weeks after implant. Follow-up at 1, 3 and 6 months. After 6 months crossover to CRT-D permitted and follow-up every 3 months Complications defined as adverse events that required invasive intervention. Observations defined as adverse events managed without invasive intervention (e.g. reprogramming of the pulse generator) Length of follow-up: average 12.1 months (SD 3.4 months), range 0.3–20.3 patient-months. Outcomes reported at 6 months Recruitment: July 2002–October 2003 |
Participant characteristics
| Participant characteristics | CRT-D (n = 119) | ICD (n = 59) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | NR | NR | |
| Sex | NR | NR | |
| Ethnicity | NR | NR | |
| NYHA class, n (%) | 0.61 | ||
| I | 1 (0.8) | 2 (3.4) | |
| II | 6 (5.0) | 4 (6.8) | |
| III | 104 (87.4) | 50 (84.7) | |
| IV | 8 (6.7) | 3 (5.1) | |
| LVEF (%), mean (SD), range | 25.6 (8.3), 9–48 | 23.3 (6.4), 11–43 | 0.07 |
| Heart rate | NR | NR | |
| QRS duration (milliseconds), mean (SD), range | 169 (16), 120–210 | 167 (15), 130–200 | 0.40 |
| Left ventricular end-diastolic dimension (mm), mean (SD), range | 66.2 (8.5), 44.7–85.9 | 66.0 (9.4), 50.1–84.2 | 0.88 |
| Left ventricular end-systolic dimension (mm), mean (SD), range | 57.1 (9.4), 37.1–76.2 | 56.9 (10.5), 37.9–78.2 | 0.93 |
| QoL score, mean (SD), range | 48 (24), 0–103 | 46 (24), 4–100 | 0.53 |
| 6-minute walk distance (m), mean (SD), range | 275 (103), 37–561 | 291 (89), 31–480 | 0.30 |
| Cardiopulmonary exercise test | |||
| Peak VO2 (ml/kg/minute), mean (SD), range | 10.8 (3.0), 4.3–26.9 | 12.3 (3.5), 6.0–23.1 | 0.006 |
| Exercise time (minutes), mean (SD), range | 8.0 (3.2), 0.7–16.5 | 8.9 (3.6), 2.3–19.8 | 0.08 |
| Baseline medication, n (%) | |||
| ACE inhibitors/substitutes | 85 (71.4) | 44 (74.6) | 0.79 |
| Beta-blockers | 95 (79.8) | 52 (88.1) | 0.24 |
| ARBs | 24 (20.2) | 10 (16.9) | 0.76 |
| Diuretics | 103 (86.6) | 54 (91.5) | 0.47 |
| Positive inotropics/glycoside | 73 (61.3) | 39 (66.1) | 0.65 |
| Nitrates | 39 (32.8) | 23 (39.0) | 0.51 |
| Anticoagulants and antiplatelets | 102 (85.7) | 48 (81.4) | 0.59 |
| Calcium channel blockers | 11 (9.2) | 9 (15.3) | 0.35 |
| AADs | 29 (24.4) | 13 (22.0) | 0.87 |
Results
| Outcome | CRT-D (n = 83) | ICD (n = 43) | p-value |
|---|---|---|---|
| Total deathsa at 6-month visit, average 12.1 (SD 3.4) patient-months of follow-up | 9 | 3 | |
| Cardiac arrhythmic | 0 | 0 | |
| Cardiac non-arrhythmic | 1 | 1 | |
| Cardiac unknown | 0 | 0 | |
| Non-cardiac | 7 | 2 | |
| Unknown | 1 | 0 | |
| Additional deaths after the 6-month visit,144 average 15.1 (SD 4.1) patient-months of follow-up | 4 | 1 | |
| Cardiac arrhythmic | 0 | 0 | |
| Cardiac non-arrhythmic | 1 | 0 | |
| Cardiac unknown | 1 | 0 | |
| Non-cardiac | 1 | 1 | |
| Unknown | 1 | 0 | |
| QoL score, mean (SD) | |||
| Baseline | 48.3 (24) | 42.0 (23) | |
| 6-month follow-up | 40.4 (22) | 45.4 (31) | |
| Change | –7.8 (22) | 3.4 (31) | 0.009 |
| NYHA class, mean (SD) | |||
| Baseline | 3.01 (0.33) | 2.86 (0.52) | |
| 6-month follow-up | 2.53 (0.69) | 2.58 (0.73) | |
| Change | –0.48 (0.65) | –0.28 (0.63) | 0.048 |
| Peak VO2b (ml/kg/minute), mean (SD) (primary outcome) | |||
| Baseline | 11.2 (3.0) | 12.8 (3.7) | |
| 6-month follow-up | 11.7 (3.2) | 11.4 (5.6) | |
| Change | 0.52 (2.5) | –1.41 (4.6) | 0.001 |
| Per-protocol analysis of change in peak VO2 (ml/kg/minute), mean (SD) at 6 months | (n = 85) 0.52 (2.5) | (n = 41) –1.47 (4.7) | 0.001 |
| 6-minute walk distance (m), mean (SD) | |||
| Baseline | 284 (105) | 298 (94) | |
| 6-month follow-up | 197 (122) | 283 (150) | |
| Change | 13 (74) | –15 (142) | 0.07 |
| Improvement in echocardiography parameters at 6 months, mean (SD) | (n = 82) | (n = 40) | |
| LVEDD (mm) | –4.3 (5.4) | –2.4 (6.5) | |
| LVESD (mm) | –4.6 (7.0) | –3.0 (6.4) | |
| LVEDV (ml) | –43 (69) | –37 (53) | |
| LVESV (ml) | –43 (58) | –36 (47) | |
| LVEF (%) | 4.3 (9.9) | 2.9 (6.2) | |
| MR (grade)c | –0.06 (0.74) | 0.10 (0.50) | |
| E/A wave point ratio | –0.08 (0.8) | –0.02 (1.2) | |
| Sphericity index | –0.02 (0.1) | 0.02 (0.1) | |
| Pre-ejection time (milliseconds) | –1.5 (52) | 7.3 (33) | |
| Intraventricular mechanical delay (milliseconds) | –14.5 (52) | –6.4 (48) | |
| Tei Index | –0.4 (0.8) | –0.05 (0.5) | |
| Contraction interval (milliseconds) | –94 (124) | –55 (103) | |
| Discontinuations and withdrawals (excluding withdrawals because of deaths and after unsuccessful implant), average of 15.1 (SD 4.1) patient-months of follow-up144 | |||
| System explant | n = 1,d day 1 after implant | ||
| Heart transplant | n = 1, 75 days after implant | ||
| At request of patient | n = 1, 28 days after implant; n = 1, 397 days after implant | ||
| At request of patient’s family | n = 1, 293 days after implant | ||
Adverse effects of treatment
| Adverse effect | Reported for the whole study group before randomisation (n = 205)a |
|---|---|
| Total complications, n patients (%), n events; average 12.1 (SD 3.4) patient-months of follow-up145 | 21 (10.2), 29 |
| Coronary sinus perforation/dissection | 2 (1.0), 2 |
| Diaphragmatic/phrenic nerve stimulation | 3 (1.5), 3 |
| Lead dislodgement or migration | 8 (3.9), 9 |
| Bleeding/haematomab | 6 (2.9), 6 |
| Blood clot/thrombosis | 1 (0.5), 1 |
| High defibrillation/cardioversion requirements | 2 (1.0), 2 |
| Infection | 1 (0.5), 1 |
| Noise on EGM post shock (non-SJM right ventricular lead)c | 1 (0.5), 1 |
| Pneumothorax | 2 (1.0), 2 |
| Retained foreign body (surgical sponge) | 1 (0.5), 1 |
| Elevated pacing threshold – left ventricular lead | 1 (0.5), 1 |
| Total observations, n patients (%), n events; average 12.1 (SD 3.4) patient-months of follow-up145 | 57 (27.8), 68 |
| Asystolic episode during left ventricular lead placement | 1 (0.5), 1 |
| Bleeding/haematomab | 10 (4.9), 10 |
| Blood clot/thrombosis | 2 (1.0), 2 |
| Coronary sinus perforation/dissection | 6 (2.9), 6 |
| Diaphragmatic/phrenic nerve stimulation – left ventricular lead | 10 (4.9), 10 |
| Diaphragmatic/phrenic nerve stimulation – right ventricular lead | 2 (1.0), 2 |
| Elevated pacing thresholds – left ventricular lead | 10 (4.9), 10 |
| Elevated pacing thresholds – right ventricular lead | 2 (1.0), 2 |
| Heart block at implant | 2 (1.0), 2 |
| High defibrillation/cardioversion requirements | 1 (0.5), 1 |
| Hypotension requiring ventilator support | 1 (0.5), 1 |
| Inappropriate therapy for SVT | 10 (4.9), 13 |
| Infection | 3 (1.5), 3 |
| pulmonary embolism | 1 (0.5), 1 |
| T-wave sensing | 2 (1.0), 3 |
| Pocket inflammation/seroma | 1 (0.5), 1 |
| Left ventricular lead-related complications at 6 months | 11/155 patients, 13 complications |
| Epic HF system-related complications at 6 months | 13/182 patients, 16 complications |
| Total complications, n patients (%), n events; average 15.1 (SD 4.1) patient-months of follow-up (only those complications with added data detailed below)144 | 22 (10.7), 31 |
| Lead dislodgement or migration | 9 (4.4), 10 |
| Infection | 2 (1.0), 2 |
| Total observations, n patients (%), n events; average 15.1 (SD 4.1) patient-months of follow-up (only those observations with added data detailed below)144 | 59 (28.8), 76 |
| Diaphragmatic/phrenic nerve stimulation – left ventricular lead | 14 (6.8), 14 |
| Elevated pacing thresholds – left ventricular lead | 12 (5.9), 12 |
| Inappropriate therapy for SVT | 11 (5.4), 14 |
| Infection | 4 (2.0), 4 |
-
Allocation to treatment groups: states randomised in a ratio of 2 : 1 (CRT-D : ICD).
-
Blinding: states double blind.
-
Comparability of treatment groups: report does not comment on this; groups appear broadly comparable – the only significant difference appears to be for peak VO2 for the exercise test for which the ICD group performed significantly better than the CRT-D group. Note that this measure is a primary outcome.
-
Method of data analysis: not stated. Analysed data set was smaller than the randomised data set because of attrition (see below).
-
Sample size/power calculation: not reported.
-
Attrition/dropout: in total, 17 (increasing to 22 with additional follow-up144) patients were withdrawn because of death (patients with unsuccessful implant, n = 3 deaths; death between implant and baseline visit, n = 2; death between baseline and 6-month visit, n = 8; death after 6-month visit, n = 4); 5/17 deaths were not attributed to a treatment group as they occurred in patients who did not have a successful implant (unrelated to implant procedure) or death occurred before the baseline visit and randomisation. Out of 205 enrolled patients, 23 implants were unsuccessful [unable to cannulate CS, n = 7; unable to obtain distal lead placement, n = 6; unable to obtain stable lead position, n = 3; high pacing thresholds, n = 3; CS dissection, n = 3; high defibrillation threshold, n = 1]. Therefore, 182 patients were successfully implanted; of these, one patient withdrew before baseline, and two (as noted above) died before the baseline visit, leaving 179 patients. One further patient attended the baseline visit but refused randomisation and baseline evaluations except for device interrogation and electrical measurements. Thus, baseline evaluations for 178 patients are presented. Of the 179 patients who attended for the baseline visit a flow chart shows that 119 were assigned to CRT-D and 60 were assigned to ICD. A further 36 in the CRT-D group were not included in the analysable patient group for the effectiveness analysis (one refused the baseline CPET, two were withdrawn, two could not complete the baseline/6-month CPET for non-cardiac reasons, six died, four had an invalid baseline/6-month CPET and 21 had < 6 months’ follow up) and 17 were not analysable in the ICD group (one refused the baseline CPET, two died, four had an invalid baseline/6-month CPET and 10 had < 6 months’ follow-up). Consequently, the analysed data set included 83 CRT-D participants and 43 ICD participants.
-
Generalisability: uncertain – no indication of age, sex or ethnicity of the participants. Country in which the trial took place also not reported. Patients had an indication for ICD therapy plus NYHA class III/IV HF and a prolonged QRS duration. Those with chronic atrial fibrillation were excluded. Baseline evaluation occurred 14 days post implant followed by randomisation; only those with successful implants were randomised.
-
Outcome measures: primarily this was a study of safety; effectiveness outcomes were on the whole secondary measures. Outcomes seem appropriate.
-
Intercentre variability: not commented on in the report.
-
Conflict of interests: not stated in the report but the study appears to have been funded and conducted by the device manufacturers.
CPET, cardiopulmonary exercise test; CS, coronary sinus.
Criteria for assessment of risk of bias in randomised controlled trials65
| Domain | Judgementa | Support for judgement |
|---|---|---|
| Selection bias | ||
| Random sequence generation | Unclear | No information provided |
| Allocation concealment | Unclear | No information provided |
| Performance bias | ||
| Blinding of participants and personnel | Unclear | States double blind but no detail about how this was achieved |
| Detection bias | ||
| Blinding of outcome assessment | Unclear | States double blind but no detail about how this was achieved |
| Attrition bias | ||
| Incomplete outcome data addressed | Low | Although there was a high degree of attrition this has been clearly documented and appears similar (numbers and reasons) between groups |
| Reporting bias | ||
| Selective reporting | Unclear | Report is a submission to the FDA and it is not clear whether or not only selected outcomes have been presented to meet the needs of the FDA approvals process |
| Other bias | ||
| Other sources of bias | Unclear | Because of a lack of details, e.g. methodological and details on patient characteristics, the risks of other sources of bias are unclear |
Appendix 10 Southampton Health Technology Assessments Centre’s peer review of the manufacturers’ submission
Comprehensiveness of ascertainment of published studies
Clinical effectiveness
The MS151 contains a systematic review of clinical effectiveness. In addition, a NMA of IPD is presented (see table in Southampton Health Technology Assessments Centre’s critical appraisal of the individual patient data network meta-analysis). The details and results of the studies included in the systematic review were tabulated. The risk of bias was also assessed and tabulated in appendix 3 of the MS but no narrative discussion of risk of bias was provided. The studies were not presented according to the population groups specified in the NICE scope,61 and the inclusion criteria for the systematic review and NMA differ from those of the NICE scope. The statement of the decision problem defines the population of interest as ‘adults with heart failure (NYHA I to IV) and LVEF ≤ 35%, and/or at risk of sudden cardiac death’ (p. 44). 151 The population inclusion criteria for the systematic review are defined as ‘adults with LVEF ≤ 40% or those who may not have (LVEF) ≤ 40% but are considered to be secondary prevention patients according to TA 95 criteria’ or ‘adults who have experienced prior myocardial infarction or coronary revascularisation; this must have occurred more than 45 days prior to enrolment’ (p. 51). 151 In addition, for the IPD NMA, the four interventions of interest (OPT, ICD, CRT-P and CRT-D) were not all included as comparators in all of the patient subgroups (for rationale see table 6, p. 45). 151 The MS states that this was either based on contraindication (e.g. CRT not being recommended for patients with a QRS duration of < 120 milliseconds) or on a paucity of IPD data (described as ‘proxy for non-use in routine clinical practice’). This differs from the NICE scope.
-
Were databases and dates of searches specified? Yes. Searches were conducted on 27 and 28 June 2011; no update searches were reported. The MS states that timelines initially provided by NICE to all technology sponsors were followed. MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched. The MS states that searches were restricted to the English language and a start publication date of 1990. Reference lists of full-text retrieved papers were also scanned.
-
Were search strategies supplied? Yes, search strategies for the three databases are presented in Appendix 1 of the MS. 151
-
Was enough detail provided for it to be reproducible? Yes.
-
Did the manufacturers search for/report on ongoing studies? No.
-
Did the manufacturers search for conference proceedings? No, there were no specific searches for conference abstracts and the MS states that abstracts were excluded from the assessment.
-
How much of the data is commercial-in-confidence/academic-in-confidence? There are no commercial-in-confidence/academic-in-confidence data in the systematic review but the vast majority of the IPD are marked commercial-in-confidence (no academic-in-confidence data).
Cost-effectiveness
The MS did not report any additional searches for cost-effectiveness studies.
Studies identified
-
Clinical trials (details): 22 RCTs reported in 46 publications (total records identified in the MS: 4749; total records identified by SHTAC: 4169), plus five trials (reported in 11 publications) of secondary prevention that were not data extracted.
-
Did any meet our inclusion criteria that we have not already included? No additional trials were identified in the MS. However, there are differences in the included/excluded trials:
-
People at risk of SCD – the MS did not describe or report data for secondary prevention studies (listed in appendix 4 of the MS151) and provided justification for this (reduction in implant costs, absence of new studies since TA95;42 in the MS it is stated that this patient group is believed to lie outside the scope of the current appraisal). SHTAC included four secondary prevention studies: AVID,71 CASH,81 CIDS84 and DEBUT. 89 Of the primary prevention trials, SHTAC included three trials that were not included in the MS: DINAMIT,95 IRIS97 and CABG Patch. 75 The MS excluded DINAMIT and IRIS for the ‘inappropriate population’ and one paper linked to the CABG Patch trial was excluded for its ‘end point’ although other papers from this trial were not mentioned.
-
People with HF – SHTAC excluded three of the trials included in the MS: (1) RESPOND (Resynchronization in Patients with Heart Failure and a Normal QRS Duration;241 participants did not have cardiac dyssynchrony), (2) REVERSE208,242,243 (mixed population receiving the interventions CRT-P or CRT-D with the comparators OPT or ICD and results not presented separately) and (3) VECTOR (Ventricular Resynchronization Therapy Randomized Trial;244 FDA report with insufficient information to allow the assessment of methods and results; no baseline characteristics reported).
-
The MS excluded ‘patients with familial cardiac conditions with a high risk of SCD, including long QT syndrome, hypertrophic cardiomyopathy, Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy, and following surgical repair of Tetralogy of Fallot’ (p. 54). 151 SHTAC did not exclude these patients and therefore included the DEBUT study. 89
-
A list of excluded studies with reasons for exclusion was provided in response to a request from SHTAC.
-
Clinical analysis
-
Any major differences in evidence reported? Despite the REVERSE trial including a mixed population, intervention (CRT-P or CRT-D) and comparators (OPT or ICD), the MS presents only patients randomised to CRT-D compared with ICD in tables for simplicity, and notes this on p. 55 of the MS. 151 The 22 trials are tabulated together and not according to the groups defined in the NICE scope. The narrative synthesis of results often does not refer to the different populations in the studies, for example those with cardiomyopathy or MI. The MS does not undertake meta-analyses of outcomes reported by studies included in the systematic review, but reports the meta-analyses undertaken by Fox and colleagues in 200764 and others.
-
Are the MS conclusions similar to those of the SHTAC review? The MS does not explicitly report the conclusions from the systematic review in the main body of the submission. The executive summary states that ‘there is a large body of RCT evidence confirming the efficacy and safety of ICD, CRT-P and CRT-D in patients with HF’ (p. 4). 151 There is no comment regarding the comparative effectiveness of the interventions for the NICE-defined populations. Further conclusions are presented based on the IPD NMA.
-
Any indirect comparisons? No indirect comparisons of the included studies were undertaken in the MS. However, the MS presents a NMA of IPD combining data from 13 of the 22 included studies.
-
Any differences in outcome measures? The MS reports the same outcome measures as the SHTAC review.
-
Any extra adverse event information? A narrative overview of adverse events in the included studies and information from previous meta-analyses is presented.
Interpretation
-
Does the interpretation of the clinical data match the analyses? The MS does not explicitly provide an interpretation of the systematic review. The interpretation of the IPD NMA is assessed below.
Questions
Southampton Health Technology Assessments Centre’s critical appraisal of the individual patient data network meta-analysis
| Appraisal criteria | Criteria met? |
|---|---|
| A. Conceptual basis | |
| 1. Is a justification given for conducting a mixed treatment comparison? | Yes. The MS correctly identifies that an IPD NMA would be beneficial in helping to understand the effects of ICDs, CRT-P and CRT-D on health outcomes for patients with HF. It is particularly important given the limited direct evidence for some comparisons. Also, it is helpful in identifying subgroups within a heterogeneous patient population, providing the opportunity to capture baseline risks and relative treatment effects. With published evidence at an aggregate level, the effectiveness for subgroups is not addressed by most trials and is inconsistently reported in others. Provision of confidential IPD by the manufacturers made such an analysis possible |
| B. Systematic processes | |
| 2. Is a comprehensive and transparent search strategy reported? | Yes. There was a comprehensive and transparent search strategy for the systematic review (not separate searches for the NMA) that provided the basis for the evidence network. The IPD was based on 14 RCTs from 22 trials included in the network of evidence from the systematic review (reported by the MS as 13 as two trials were combined). IPD were supplied by the manufacturers |
| 3. Are inclusion/exclusion criteria adequately reported? | Yes. RCTs for which IPD could be obtained were from the systematic review. The criteria do not strictly accord with the decision problem specified in the NICE scope for the appraisal (see Appendix 9 Comprehensiveness of ascertainment of published studies) |
| 4. Are the numbers of included/excluded studies from the mixed treatment comparison reported, with reasons for exclusions? | Yes. The number of trials excluded (13/22 RCTs, dated 1996–2010) and reasons for exclusion from the evidence network are reported. Justifications for exclusion include manufacturers’ IPD data not available (two studies); available data sets could not be reconciled with the published data (two studies); two manufacturer-sponsored studies that the systematic review searches failed to identify until after the database for the NMA had been assembled (VECTOR: started in 2000 and details published in a 2005 US FDA report;244 RESPOND: journal article published February 2011241); and two trials were not sponsored by the manufacturers contributing to the submission. In addition to these trials, SHTAC also included seven trials (DINAMIT,95 IRIS97 and CABG Patch75 and four secondary prevention RCTs71,81,84,89) that were not included in the MS. Although the excluded studies account for only 5.3% of the data (n = 712/13,350), it is unclear what impact their exclusion has on the results. A flow chart is presented for the systematic review and numbers excluded from the NMA are reported |
| 5. Is a visual representation of the data networks provided? | Yes. A visual network diagram was provided for the systematic review (section 4, p. 103, of the MS151). An explanation is provided for the way that the different trials were handled within the network. The REVERSE trial208 was treated as two trials [CRT-P and CRT-D, as well as split into EU and US populations because of the different protocol-specific duration of follow-up (24 months and 12 months respectively)]. The CONTAK-CD trial126 was also treated as two trials as the crossover design was changed to a 6-month parallel-group trial halfway through (phase 2). The MIRACLE ICD trial136 was combined with the MIRCALE ICD II trial137 as the MS states that these were effectively a single trial. In addition, the MS pooled the data from the amiodarone and placebo arms in the SCD HeFT trial105 |
| 6. Are the data from included studies extracted and tabulated? | Yes. Baseline information was presented in the systematic review for the individual trials (see tables 7–11, pp. 57–72151). A summary table for the IPD trials with combined participant baseline characteristics per device (table 35, p. 110151) is presented for comparison with UK summary data (table 36, p. 111151). The MS suggests that differences in NYHA class between the two tables are distorted because of previous NICE decisions about the devices, differences in the format that other data are presented in and high levels of missing data in the UK National Audit. The MS suggests that, despite this, the IPD is broadly reflective of the UK population. Comparison is further complicated by QRS data being presented as means (milliseconds) in the MS table but as percentages (prolonged) in the UK summary table. A cross-check with the original trial publications is not possible as this is based on a large database of IPD |
| 7. Is the quality of the included studies assessed? | Yes. All of the NMA trials were critically appraised in the systematic review. Risk of bias for all 22 studies is presented in Appendix 3 of the MS,151 but there is no discussion of this. No studies were excluded because of any potential risk of bias and the MS fails to address any of the issues arising from the assessment |
| C. Statistical analysis | |
| 8. Are the statistical procedures adequately described and executed? | No. Overall procedures used are reported, but specific details of the analyses for the outcomes of all-cause mortality, all-cause hospitalisation and HRQoL are omitted. This limits the opportunity to appraise the NMA. Published sources are referred to for the methods employed in statistical analysis Analysis of the three outcomes follows a similar two-stage approach, although different types of regression were used. First, baseline rates were estimated independent of treatment effect using pooled data from the IPD trials on OPT (the comparator). Second, device-specific treatment effects were estimated using relevant IPD trials measuring the specific outcome in question. Both stages used patient characteristics as covariables to incorporate baseline risk and treatment effect modifiers. This allowed subgroups of patients to be identified for whom the devices may have a differential effect All-cause mortality For all-cause mortality, a parametric survival analysis was undertaken to generate estimates of baseline mortality. Parametric distributions assessed included exponential, Gompertz, log-logistic, log-normal and Weibull. Covariables were assessed for inclusion and, when necessary, transformation undertaken (e.g. age as a time-dependent covariable). Models were assessed using fitted and Kaplan–Meier survival curves within trial follow-up, visual review of the extrapolations and of the shape of the instantaneous hazard over time, AIC, Cox–Snell residuals, tests of acceptability of the proportional hazards assumption or accelerated failure time assumption, comparison against external data and review by clinical experts. Results of the tests are not presented. The Weibull distributions formed the basis for the final baseline model IPD NMA using meta-regression was undertaken with and without covariables to estimate relative treatment effects (i.e. HRs), comparing devices and OPT. Comparisons were made between the NMA, pairwise meta-analyses and aggregate trial data to judge whether representative and the type of analyses that should be undertaken (see appendix 7151). The MS reports that caterpillar plots, Brooks–Gelman–Rubin statistics, autocorrelation and DIC were assessed, although few results are reported. Covariables were selected through univariate analyses, multivariate stepwise procedures and exploratory analyses. Final fixed-effects models using a Cox proportional hazards approach and stratified for study were estimated and assessed using proportional hazards tests (see appendix 8151) and Schoenfeld residual tests (not reported) All-cause hospitalisations The analysis focused on ‘expected number of events per month’ and ‘expected number of days per month spent in hospital’ (excluding events within 60 days post randomisation as these were included in the economic model). Negative binomial regression was used to estimate baseline rates for OPT patients and the effects of treatment for all devices. The approach was decided through measures of goodness of fit (i.e. BIC, AIC and two times log-likelihood score) and the covariates were incorporated into the analyses through a stepwise process (included at a significance level of p = 0.05), although details are not reported. Limited data resulted in pooling of some categorical variables (e.g. NYHA groups). Justifications were provided for decisions and comparisons are made with previous evaluations HRQoL HRQoL was assessed using the EQ-5D, adjusting UK age- and gender-specific utilities with disease- and treatment-specific decrements/increments estimated from the IPD trials reporting EQ-5D. Baseline HRQoL was estimated using a similar process to that for all-cause hospitalisation. Before analysis, raw data were transformed as they were skewed. Derived values were checked against population norms and trial values. Treatment impact was estimated through MDs from baseline to first follow-up (180 days). Limited and skewed data resulted in counterintuitive results so MLWHFQ 6-month IPD data and evidence from the systematic were used to adjust final values (justifications provided). Duration of effect was estimated when mean device vs. OPT values showed no difference |
| 9. Is there a sufficient discussion of heterogeneity? | The MS recognises the heterogeneous nature of the trials included in the IPD NMA. This is reflected in the approach taken – use of meta-regression to try to account for the variation, the process for including covariables and the presentation and the discussion of the results for different subgroups. There is some limited discussion of measures of goodness of fit associated with the NMA; however, this is not related specifically to taking account of heterogeneity. Some comparisons are made between the NMA, individual trial results and pairwise meta-analyses, highlighting differences related to heterogeneous studies |
| 10. Is the type of model used (i.e. fixed or random effects) reported and justified? | Yes. Comparisons of NMA results from IPD trials and all trials using both fixed- and random-effects models are reported and said to be broadly similar (p. 123151), although random-effects CIs are wider. The MS states for all-cause mortality that the DIC assessment of model fit supported the use of the fixed-effect model: all trials: fixed-effects DIC = 59.0 vs. random-effects DIC = 60.8; IPD trials: fixed-effects DIC = 1.4 vs. random-effects DIC = 3.0. Although modelling of all-cause hospitalisation and HRQoL used a fixed-effects approach and it is indicated that goodness of fit statistics were assessed, no data or discussion are presented |
| 11. Was sensitivity analysis conducted? | Yes, in relation to the covariables included in the baseline and treatment effect models through univariate and multivariate stepwise analyses (see appendix 9151). No sensitivity analyses were undertaken on the trials included or the quality of the studies |
| 12. Is any of the programming code used in the statistical programme provided? | The MS did not provide any programming codes used in the statistical programme |
| D. Presentation and interpretation of the evidence | |
| 13. Is there a tabulation/illustration of the results for each intervention and for each outcome? | Results are presented through a series of tabulations and illustrations, specifically: All-cause mortality Baseline model results were presented through Kaplan–Meier plots of parametric curves and tabulation of risk models. Treatment effects from the NMA were presented through forest plots for different devices and covariables and tabulation of the preferred model All-cause hospitalisation Baseline model results were presented through Kaplan–Meier plots and tabulation of the baseline risk model. Treatment effects from the NMA were presented through tabulation of the preferred model and effects on events per month by device HRQoL Outcomes are effect of disease severity on HRQoL at baseline, treatment effect on HRQoL, explorative analysis of change in MLWHFQ score at 6 months, HRQoL treatment benefit duration and addition IPD analyses (long-term MLWHFQ data from all studies and devices) – results were presented in tables, histograms and line graphs |
| 14. Is there a narrative commentary on the results? | Yes. The MS presents narrative comments on the results, putting them into the context of other research and providing comments on the main limitations [i.e. dichotomisation may miss some of the heterogeneity in response to therapy in the 120–150 milliseconds QRS category (p. 128151); lack of power in the analysis to detect modest effect modifiers (p. 137151)] or uncertainties [i.e. treatment effect beyond the included number of years (p. 137151)]. The MS provides a cautionary note regarding not overinterpreting individual subgroups as anomalies may arise as a result of participant-level characteristics not accounted for (p. 130151) |
| 15. Does the discussion of the results reflect the data presented? | The discussion of the results for the three outcomes does reflect the results presented and a warning is provided about the limitations of the IPD available and the analyses undertaken. The discussion also places the results in the context of other evidence |
| 16. Have the authors commented on how their results compare with other published studies (e.g. MTCs) and do they offer any explanation for discrepancies? | Partly. The MS comments on how some of the results compare with those of other reviews, meta-analyses and studies or with routinely collected data. It also undertakes additional analyses to check outcomes. In some instances the MS provides alternative values because of uncertainties in the results, providing justifications. Importantly, the MS recognises the limitations in the IPD and NMA undertaken, providing a note of caution |
| 17. Have the authors discussed whether or not there are any differences in effects between the direct evidence and the indirect evidence? | The MS reports that good concordance between pairwise meta-analysis and NMA suggests reasonable concordance between the indirect data and the direct data (p. 124151). Unable to establish if there were any discrepancies in the IPD data |
Southampton Health Technology Assessments Centre’s peer review of the economic evaluation within the manufacturers’ submission
Study characteristics
Reference
Association of British Healthcare Industries. Implantable Cardioverter Defibrillators for the Treatment of Arrhythmias and Cardiac Resynchronisation Therapy for the Treatment of Heart Failure (Review of TA95 and TA120). ABHI; 2012.
Health technology
ICD and CRT.
Interventions and comparators
Implantable cardioverter defibrillators and CRT for the treatment of cardiac arrhythmias and HF.
Was a no treatment/supportive care strategy included?
Optimal pharmacological therapy.
Describe interventions/strategies
As above.
Research question
For adults with HF and a LVEF ≤ 35%, and/or at risk of SCD, which patients should receive an ICD, a CRT-P or a CRT-D device, based on their clinical parameters.
Study type
Cost–utility analysis.
Study population
Adults with HF (NYHA classes I–IV) and a LVEF ≤ 35%, and/or at risk of SCD.
Institutional setting
Secondary care.
Country/currency
UK pounds.
Funding source
Biotronik, Boston Scientific, Medtronic, Sorin and St Jude Medical.
Analytical perspective
National Health Service and PSS.
Effectiveness
The clinical effectiveness estimates were based on a NMA of IPD from 13 clinical trials (12,638 patients, followed up for up to 7.5 years). The clinical trials were CARE-HF,109 COMPANION,116 CONTAK-CD,126 DEFINITE,90 MADIT I,99 MADIT II,101 MADIT-CRT,130 MIRACLE,121 MIRACLE ICD,136 RAFT,140 RethinQ,142 REVERSE208 and SCD-HeFT. 105 These trials were identified through a systematic review of clinical effectiveness for all of the interventions. A further nine trials were also identified in the review but IPD were not available for these trials.
The NMA enabled trials that compared different sets of treatments to be combined within a single analysis and direct and indirect evidence to be used to inform a comparison between possible treatments.
All-cause mortality
The NMA found CRT-D to have the strongest effect on all-cause mortality with a HR of (commercial-in-confidence information has been removed). Treatment effects for the individual devices were (commercial-in-confidence information has been removed).
The parameters used in the cost-effectiveness model are shown in Table 155. This table shows the predicted treatment effect for each subgroup.
| Variablea | HR | p-value |
|---|---|---|
| ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| LBBB | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Age ≥ 60 years | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Gender = male | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICD*QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICD*QRS ≥ 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICD*LBBB | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICD*Gender = male | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICD*Age ≥ 60 years | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTP*QRS ≥ 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTP*LBBB | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTP*Gender = male | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTP*Age ≥ 60 years | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTD*QRS ≥ 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTD*LBBB | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTD*Gender = male | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRTD*Age ≥ 60 years | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
All-cause hospitalisation
Across all NYHA classes, device therapy was associated with a reduction in admission rates. In NYHA classes I–III, ICDs were associated with a (commercial-in-confidence information has been removed) reduction in monthly admission rates and CRT with a (commercial-in-confidence information has been removed) reduction. The effect in NYHA class IV was even more pronounced, with CRT offering a (commercial-in-confidence information has been removed) reduction in monthly admission rates.
Intervention costs
Individual patient data from the trials were used to estimate the mean number of all-cause hospitalisation events per month and the mean number of days hospitalised per month. The hospital costs were derived from the NHS Reference Costs218 and combined with the average mean length of stay. The HF hospitalisation event cost was £2295 and the non-HF hospitalisation event cost was £2448.
Device costs were sourced from the average selling prices from the manufacturers via ABHI. These prices are an aggregate across all sponsors (manufacturers) for ICD, CRT-P and CRT-D devices and leads sold in the UK to the NHS. The implantation costs were taken from the HRG tariff values. 218 The device-related infection cost was derived by inflating the value in Fox and colleagues64 to £3139. Device costs, with implantation costs, are shown in Table 156.
| Item | Cost (£) | Components |
|---|---|---|
| Initial implant operation (ICD) | 15,248 | ABHI system costs (incl. leads) and UK tariff EA12Z |
| Initial implant operation (CRT-P) | 8281 | UK tariff E07Z |
| Initial implant operation (CRT-D) | 17,849 | ABHI system costs (incl. leads) and UK tariff EA12Z |
| Replacement (ICD) | 14,705 | ABHI system costs (excl. leads) and UK tariff EA12Z |
| Replacement (CRT-P) | 8281 | UK tariff E07Z |
| Replacement (CRT-D) | 17,308 | ABHI System costs (excl. leads) and UK tariff EA12Z |
| Device-related infection (ICD) | 18,964 | See section 5.5.3.3 in the MS151 |
| Device-related infection (CRT-P) | 12,541 | See section 5.5.3.3 in the MS151 |
| Device-related infection (CRT-D) | 21,568 | See section 5.5.3.3 in the MS151 |
| Battery replacement (ICD) | 12,004 | ABHI generator costs (excl. leads) and UK tariff EA39Z |
| Battery replacement (CRT-P) | 8381 | UK tariff |
| Battery replacement (CRT-D) | 14,672 | ABHI generator costs (excl. leads) and UK tariff EA39Z |
Medication costs
The cost of HF medication cost included for the patients in the model. The proportion of patients using a range of HF medications, by NYHA class, was derived through a systematic review and expert opinion. Common values are applied to all four interventions in each month of the model, on the basis of baseline NYHA values. Recommended doses and purchase costs of the medications were taken from the BNF. 219 The total cost of treatment per 1-month model cycle was £14.28 for NYHA class I and between £22.13 and £22.30 for NYHA classes II–IV.
Indirect costs
Not applicable.
Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
The approach taken for HRQoL was (1) to estimate UK-specific age- and gender-specific population utilities, (2) derive disease-specific decrements using IPD EQ-5D data and (3) derive treatment-specific increments associated with each device at first follow-up visit by NYHA class.
UK-specific age- and gender-specific population utilities were taken from a study of 3395 individuals resident in the UK. 152 Disease-specific decrements were taken from the CARE-HF,109 MADIT-CRT130 and RAFT140 trials. For the impact of treatment, the utility increment was calculated as the difference between baseline and the first follow-up period.
The HRQoL benefit observed at 6 months is maintained up to 5 years and thereafter begins to recede in a linear manner over the time period 5–10 years. After 10 years the model assumed that the individual with a CRT or ICD device will have no additional HRQoL benefit over an identical person receiving OPT.
List the utility values used in the evaluation
Individuals in NYHA class I/II have the same HRQoL as an age-equivalent member of the general public (Table 157). Patients in NYHA classes III and IV have extra decrements by sex and ischaemic aetiology (Table 158).
| Age band (years) | Male | Female |
|---|---|---|
| < 25 | 0.94 (0.12) | 0.94 (0.12) |
| 25–34 | 0.93 (0.16) | 0.93 (0.15) |
| 35–44 | 0.91 (0.17) | 0.91 (0.15) |
| 45–54 | 0.84 (0.27) | 0.85 (0.23) |
| 55–64 | 0.78 (0.28) | 0.81 (0.26) |
| 65–74 | 0.78 (0.28) | 0.78 (0.25) |
| 75+ | 0.75 (0.28) | 0.71 (0.27) |
| Covariable | Beta coefficient | SE | Z-score | e^β |
|---|---|---|---|---|
| NYHA = III | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| NYHA = IV | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Age | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Gender = male | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Constant | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Treatment | NYHA I/II | NYHA III | NYHA IV |
|---|---|---|---|
| OPT | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| ICD | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-P | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| CRT-D | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
Modelling
The model is a survival model with two states for alive and dead. Death is modelled through a series of covariate-based regression equations for baseline risk and treatment effect using long-term IPD. There is also a state for all-cause hospitalisation that is aligned to mortality.
The baseline probability of death is for patients who receive OPT but no device, based on a range of clinical covariates. These probabilities are used in combination with device-specific treatment effects, derived from the NMA. A similar approach is taken to estimate the probability of all-cause hospitalisation. HRQoL utility is applied to patients in the model according to their treatment and clinical characteristics.
The model does not include short-term device-related adverse events as the costing approach used to derive total implant costs covers additional costs such as short-term adverse events.
Results were generated in a two-stage process. In the first, both for patients with and without LBBB, cost and QALY estimates were derived for all relevant comparators for all 4992 patient profiles [four NYHA classes × two aetiology status (ischaemic/non-ischaemic) × three QRS categories × four LVEF categories × LBBB status (yes/no) × two gender groups × 13 age categories]. In the second stage, these were collapsed to 48 subgroups defined by NYHA class, QRS duration, LBBB status and aetiology. Results were aggregated over LVEF and age and gender categories.
Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text)
Mortality
For the model the baseline survival curve was derived using the following formulae:
where h(t) is the instantaneous hazard, S(t) is the survival curve, β are the coefficients on the covariables and X is the set of covariables (which can be time dependent).
| Variable | Coefficient | HR for prognostic variablea | p-value |
|---|---|---|---|
| Age (per year) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Male gender | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| NYHA class III | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| NYHA class IV | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS duration < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| LVEF > 20% and ≤ 25% | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| LVEF > 25% and ≤ 30% | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| LVEF > 30% | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| log(scale) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| log(shape) | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
All-cause hospitalisation
The derived monthly probabilities are shown in Table 161, using a starting age of 66 years.
| NYHA class I/II | NYHA class III | NYHA class IV | |
|---|---|---|---|
| Non-ischaemic aetiology | |||
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| Ischaemic aetiology | |||
| QRS < 120 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS 120–149 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
| QRS ≥ 150 milliseconds | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed | Commercial-in-confidence information has been removed |
Device lifetime
UK device longevity estimates were derived from an analysis of all implants with verified life status from 2000 to 2011 (∼40,000 implants). Device-specific median survival estimates were used to inform transition probabilities of device failure in the model. Median time to device failure in the model was 7.1 years for an ICD device, 10.4 years for a CRT-P device and 5.8 years for a CRT-D device.
What is the model time horizon?
Lifetime.
What discount rates have been applied in the model?
3.5% for costs and benefits.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
The model estimates the total lifetime QALYs for various patient subgroups, but these values are not presented in the report.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
The model estimates the total lifetime costs for various patient subgroups, but these values are not presented in the report.
Synthesis of costs and benefits
The results of the base-case deterministic cost-effectiveness analysis are presented for 48 subgroups defined by NYHA class, QRS duration, LBBB status and aetiology (24 subgroups for patients with LBBB and 24 subgroups for patients without) (Tables 162 and 163). All individuals are assumed to have a LVEF ≤ 35%. The authors stated that ischaemia did not substantively impact on cost-effectiveness and so the results presented are therefore applicable to both ischaemic and non-ischaemic patients
| NYHA class | Aetiology | QRS duration (milliseconds) | n | Cost-effectiveness sequence | ICER (£) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | First | Second | Third | Fourth | ||||
| I | Non-ischaemic | < 120 | 66 | OPT | ICD | NA | NA | Referent | 24,304 | NA | NA |
| I | Non-ischaemic | ≥ 120, < 150 | 11 | OPT | CRT-D | ICD | NA | Referent | Dominated | 16,619 | NA |
| I | Non-ischaemic | ≥ 150 | 8 | OPT | ICD | CRT-D | NA | Referent | 18,074 | 1,080,057 | NA |
| I | Ischaemic | < 120 | 272 | OPT | ICD | NA | NA | Referent | 24,016 | NA | NA |
| I | Ischaemic | ≥ 120, < 150 | 216 | OPT | CRT-D | ICD | NA | Referent | Dominated | 16,234 | NA |
| I | Ischaemic | ≥ 150 | 106 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 21,086 | NA |
| II | Non-ischaemic | < 120 | 710 | OPT | ICD | N/A | NA | Referent | £25,110 | NA | NA |
| II | Non-ischaemic | ≥ 120, < 150 | 232 | OPT | CRT-D | ICD | NA | Referent | Dominated | 17,016 | NA |
| II | Non-ischaemic | ≥ 150 | 141 | OPT | ICD | CRT-D | NA | Referent | 20,312 | 27,175 | NA |
| II | Ischaemic | < 120 | 788 | OPT | ICD | NA | NA | Referent | 23,884 | NA | NA |
| II | Ischaemic | ≥ 120, < 150 | 756 | OPT | CRT-D | ICD | NA | Referent | Dominated | 16,749 | NA |
| II | Ischaemic | ≥ 150 | 470 | OPT | ICD | CRT-D | NA | Referent | 20,697 | 22,777 | NA |
| III | Non-ischaemic | < 120 | 255 | OPT | ICD | NA | NA | Referent | 29,402 | NA | NA |
| III | Non-ischaemic | ≥ 120, < 150 | 150 | OPT | CRT-P | ICD | CRT-D | Referent | Extendedly dominated | 19,760 | 27,336 |
| III | Non-ischaemic | ≥ 150 | 109 | OPT | ICD | CRT-P | CRT-D | Referent | Dominated | 13,227 | 24,350 |
| III | Ischaemic | < 120 | 438 | OPT | ICD | NA | NA | Referent | 26,923 | NA | NA |
| III | Ischaemic | ≥ 120, < 150 | 426 | OPT | CRT-P | ICD | CRT-D | Referent | 19,670 | Extendedly dominated | 27,796 |
| III | Ischaemic | ≥ 150 | 192 | OPT | ICD | CRT-P | CRT-D | Referent | Dominated | 14,392 | 25,734 |
| IV | Non-ischaemic | < 120 | 5 | OPT | NA | NA | NA | Referent | NA | NA | NA |
| IV | Non-ischaemic | ≥ 120, < 150 | 12 | OPT | CRT-P | CRT-D | NA | Referent | 17,324 | 30,624 | NA |
| IV | Non-ischaemic | ≥ 150 | 9 | OPT | CRT-P | CRT-D | NA | Referent | 16,304 | 33,901 | NA |
| IV | Ischaemic | < 120 | 42 | OPT | NA | NA | NA | Referent | NA | NA | NA |
| IV | Ischaemic | ≥ 120, < 150 | 52 | OPT | CRT-P | CRT-D | NA | Referent | 24,366 | 43,500 | NA |
| IV | Ischaemic | ≥ 150 | 10 | OPT | CRT-P | CRT-D | NA | Referent | 18,065 | 37,802 | NA |
| NYHA class | Aetiology | QRS duration (milliseconds) | n | Cost-effectiveness sequence | ICER (£) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | First | Second | Third | Fourth | ||||
| I | Non-ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | N/A | NA | NA |
| I | Non-ischaemic | ≥ 120, < 150 | 21 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 21,021 | NA |
| I | Non-ischaemic | ≥ 150 | 33 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 18,118 | NA |
| I | Ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | N/A | NA | NA |
| I | Ischaemic | ≥ 120, < 150 | 76 | OPT | ICD | CRT-D | NA | Referent | 19,989 | 24,343 | NA |
| I | Ischaemic | ≥ 150 | 165 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 17,335 | NA |
| II | Non-ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | N/A | NA | NA |
| II | Non-ischaemic | ≥ 120, < 150 | 385 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 26,608 | NA |
| II | Non-ischaemic | ≥ 150 | 1308 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 17,794 | NA |
| II | Ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | N/A | NA | NA |
| II | Ischaemic | ≥ 120, < 150 | 477 | OPT | ICD | CRT-D | NA | Referent | 20,640 | 21,277 | NA |
| II | Ischaemic | ≥ 150 | 982 | OPT | ICD | CRT-D | NA | Referent | Extendedly dominated | 17,479 | NA |
| III | Non-ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | NA | NA | NA |
| III | Non-ischaemic | ≥ 120, < 150 | 189 | OPT | ICD | CRT-P | CRT-D | Referent | Dominated | 12,550 | 23,831 |
| III | Non-ischaemic | ≥ 150 | 775 | OPT | ICD | CRT-P | CRT-D | Referent | Dominated | 9798 | 27,592 |
| III | Ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | NA | NA | NA |
| III | Ischaemic | ≥ 120, < 150 | 355 | OPT | ICD | CRT-P | CRT-D | Referent | Dominated | 15,449 | 25,540 |
| III | Ischaemic | ≥ 150 | 773 | OPT | ICD | CRT-P | CRT-D | Referent | Dominated | 11,408 | 29,912 |
| IV | Non-ischaemic | < 120 | 0 | OPT | NA | NA | NA | Referent | NA | NA | NA |
| IV | Non-ischaemic | ≥ 120, < 150 seconds | 22 | OPT | CRT-P | CRT-D | NA | Referent | 14,715 | 31,920 | NA |
| IV | Non-ischaemic | ≥ 150 | 81 | OPT | CRT-P | CRT-D | NA | Referent | 12,076 | 35,660 | NA |
| IV | Ischaemic | < 120 seconds | 0 | OPT | NA | NA | NA | Referent | NA | NA | NA |
| IV | Ischaemic | ≥ 120, < 150 | 38 | OPT | CRT-P | CRT-D | NA | Referent | 23,340 | 41,695 | NA |
| IV | Ischaemic | ≥ 150 | 97 | OPT | CRT-P | CRT-D | NA | Referent | 17,722 | 46,445 | NA |
Summary of results
NYHA class I/II
-
QRS duration < 120 milliseconds: the ICERs for ICD compared with OPT are < £25,200 per QALY gained.
-
QRS duration 120–149 milliseconds: ICD is a cost-effective treatment option (ICER < £17,000 per QALY) for patients with no LBBB. For CRT-D, all ICERs are < £25,000 per QALY gained in LBBB patients (£20,608–24,343).
-
QRS duration ≥ 150 milliseconds: CRT-D is a cost-effective treatment with an ICER of < £28,000 per QALY for all options.
NYHA class III
-
QRS duration < 120 milliseconds: ICD compared with OPT generates ICERs of < £30,000 per QALY.
-
QRS duration 120–149 milliseconds: CRT-P is cost-effective. CRT-D generates ICERs of between £23,900 and £27,400 per QALY gained relative to CRT-P.
-
QRS duration > 150 milliseconds: CRT-P is cost-effective compared with OPT (ICER < £20,000 per QALY). Compared with CRT-P, CRT-D generates ICERs of < £30,000 per QALY gained. ICD is either dominated or extendedly dominated.
NYHA class IV
-
QRS duration < 120 milliseconds: no comparative analysis was possible in this patient group.
-
QRS duration ≥ 120 milliseconds: For CRT-P compared with OPT, all ICERs are close to or < £20,000 per QALY gained. For the comparison of CRT-D with CRT-P, all ICERs are > £30,000 per QALY gained.
The authors reported that, in many cases, there is little difference between the best and second best options (when viewed in terms of ICERs), and there may be other issues that clinicians wish to take into account; they conclude that there seems to be a reasonable case for building clinical flexibility into the recommendations in those cases in which the ICER differences between technologies are small and the uncertainty over which is the preferred device is high.
Give results of any statistical analysis of the results of the evaluation
Not applicable.
Was any sensitivity analysis performed?
Yes, deterministic sensitivity analysis.
What scenarios were tested in the sensitivity analysis?
The following scenarios were tested in sensitivity analyses: removal of treatment effect tapering (mortality and HRQoL), use of alternative NYHA-based IPD results and increase in device longevity.
Give a summary of the results of the sensitivity analysis
The following scenarios were tested in sensitivity analyses: removal of treatment effect tapering (mortality and HRQoL), use of alternative NYHA-based IPD results and increase in device longevity. The base case assumed that treatment effects on mortality or HRQoL are not constant but diminish over time. When constant treatment effects for mortality and HRQoL were explored, the ICERs in all patient groups were lower than in the base case.
According to the MS, there may be a lower mortality treatment effect of CRT-D in patients in NYHA class IV than in patients in NYHA classes I–III. The economic model was run using the estimated all-cause mortality treatment effects based on the grouping of NYHA class IV patients compared with NYHA class I–III patients. This analysis results in CRT-D becoming dominated in all NYHA class IV groups. The ICERs for all other groups are lower than in the base case.
Device longevity was investigated by increasing the time to device failure by 10%. This resulted in only minimal changes to the ICERs.
Conclusions/implications
Give a brief summary of the authors’ conclusions from their analysis
This analysis reconfirms the clinical and economic value of ICD, CRT-P and CRT-D in NYHA class I–IV HF patients.
What are the implications of the evaluation for practice?
The recommendations from this analysis would lead to a widening of the eligibility criteria for an ICD or a CRT device and consequently an increase in implant rates. The analysis estimates that the additional annual expenditure incurred by the NHS would range from £41.6M to £230.2M, depending on the choice of scenario and year of interest.
Southampton Health Technology Assessments Centre commentary
Selection of comparators
The interventions compared in the MS consist of those included in NICE’s scope. 1 However, not all of them were included as comparators for all patient subgroups:
-
ICD was excluded for NYHA class IV
-
CRT-P was excluded for NYHA class I/II and QRS duration < 120 milliseconds
-
CRT-D was excluded for QRS duration < 120 milliseconds.
These exclusions seem to conflict with NICE’s scope, for example some patients in the scoped population with HF and ventricular arrhythmia considered eligible for an ICD are likely to be in NYHA class IV.
Validity of estimate of measure of benefit
Device-specific increments seem similar to those in previous models but the magnitude of the HF-related decrements is not clear from the regression coefficients reported in the MS.
Validity of estimate of costs
Overall, the derivation of costs and assumptions presented in the MS seem appropriate and consistent with previous approaches. However, specific searches for resource use or cost studies in the UK are not reported and the impact of changes to the values and assumptions used was not analysed in the MS. The estimates in the model seem to cover the relevant resource use, including complications, non-HF hospitalisations and outpatient visits.
Appendix 11 List of excluded economic evaluations
Alcaraz A, Gonzalez ZJ, Augustovski F. Cost-effectiveness of implantable cardioverter-defibrillator in patients with risk factors for sudden death in Argentina. Value Health 2011;14(Suppl. 1):S33–8. [Reason for exclusion: language.]
Anderson MH, Camm AJ. Implications for present and future applications of the implantable cardioverter-defibrillator resulting from the use of a simple model of cost efficacy. Br Heart J 1993;69:83–92. [Reason for exclusion: no comparator.]
Bryant J, Brodin H, Loveman E, Clegg A. Clinical effectiveness and cost-effectiveness of implantable cardioverter defibrillators for arrhythmias: a systematic review and economic evaluation. Int J Technol Assess Health Care 2007;23:63–70. [Reason for exclusion: abstract has limited details.]
Feingold B, Arora G, Webber SA, Smith KJ. Cost-effectiveness of implantable cardioverter-defibrillators in children with dilated cardiomyopathy. J Card Fail 2010;16:734–41. [Reason for exclusion: population.]
Groarke J, Orfali N, Nolan P, Heerey A, Kasim S, Crowley J, et al. Cost effectiveness of implantable cardioverter defibrillator (ICD) therapy in clinical practice. Eur Heart J 2010;31(Suppl. 1):225. [Reason for exclusion: abstract.]
Groeneveld PW, Farmer SA, Suh JJ, Matta MA, Yang F. Outcomes and costs of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death among the elderly. Heart Rhythm 2008;5:646–53. [Reason for exclusion: no economic evaluation.]
Hauer RN, Derksen R, Wever EF. Can implantable cardioverter-defibrillator therapy reduce healthcare costs? Am J Cardiol 1996;78:134–9. [Reason for exclusion: comparator.]
Kutyifa V, Aidelsburger P, Schauer S, Merkely B, Klein H, Kuniss M, et al. Cost-effectiveness of cardiac resynchronization therapy in combination with an implantable cardioverter defibrillator in mild heart failure based on Markov modeling using UK cost approach in MADIT CRT. Eur Heart Jl 2012;33:896. [Reason for exclusion: abstract.]
L’Agence Nationale d’Accreditation d’Evaluation en Sante (ANAES). Implantable Cardioverter Defibrillators: Update. Paris: L’Agence Nationale d’Accreditation d’Evaluation en Sante (ANAES); 2001. [Reason for exclusion: no economic evaluation.]
Linde C, Mealing S, Hawkins N, Eaton J, Brown B, Daubert JC, et al. Cost-effectiveness of cardiac resynchronization therapy in patients with asymptomatic to mild heart failure: insights from the European cohort of the REVERSE (Resynchronization Reverses remodeling in Systolic Left Ventricular Dysfunction). Eur Heart J 2011;32:1631–9. [Reason for exclusion: population.]
Maniadakis N, Ekman M, Calvert MJ, Freemantle N, Karamalis M, Vardas P. Cost effectiveness of cardiac resynchronization therapy in Greece: an analysis based on the CArdiac REsychronization in Heart Failure trial. Europace 2011;13:1597–603. [Reason for exclusion: excluded in error.]
Medical Advisory Service. Internet-based device-assisted remote monitoring of cardiovascular implantable electronic devices. Ont Health Technol Assess Ser 2012;12(1). [Reason for exclusion: intervention.]
Mushlin AI, Zwanziger J, Gajary E, Andrews M, Marron R. Approach to cost-effectiveness assessment in the MADIT trial. Am J Cardiol 1997;80:F33–41. [Reason for exclusion: no economic evaluation.]
Neyt M, Stroobandt S, Obyn C, Camberlin C, Devriese S, De Laet C, et al. Cost-effectiveness of cardiac resynchronisation therapy for patients with moderate-to-severe heart failure. Value Health 2011;14:A253. [Reason for exclusion: abstract.]
Health Improvement Scotland. The Use of Cardiac Resynchronization Therapy (CRT) for Heart Failure. Evidence Note 10. URL: www.healthcareimprovementscotland.org/our_work/technologies_and_medicines/earlier_evidence_notes/evidence_note_10.aspx (accessed April 2014). [Reason for exclusion: no economic evaluation.]
Pons JM, Granados A. Implantable Cardioverter Defibrillator: Experience in Catalonia (1989–1995) and Elements of its Evaluation. Catalonia, Spain: Department of Health; 1997. [Reason for exclusion: unobtainable.]
Poggio R, Augustovsky F, Caporale J, Irazola V, Miriuka S. Cost-effectiveness of cardiac resynchronization therapy: perspective from Argentina. Int J Technol Assess Health Care 2012;28:429–35. [Reason for exclusion: population.]
Pozzolini A. Cost-effectiveness of ICD Therapy in the Prevention of Sudden Death in CAD and/or HF Patients. Milan: Springer-Verlag Italia; 2007. [Reason for exclusion: unobtainable.]
Shah P, Rongione A, Hewitt P, Rosner C, May C, Burton N, et al. Is cardiac resynchronization therapy a cost-effective strategy in patients whose ultimate destination is a left ventricular assist device? J Heart Lung Transplant 2012;31:S50–1. [Reason for exclusion: abstract.]
Taylor R. The Clinical and Cost Effectiveness of Biventricular Pacing for Patients with Severe Heart Failure. A West Midlands Health Technology Assessment Collaboration Report. Report no. 55. Birmingham: Department of Public Health and Epidemiology, University of Birmingham; 2005. [Reason for exclusion: no economic evaluation.]
Wells GA, Coyle D, Nichol G, Coyle K, Talajic M, Tang A. Cost effectiveness of cardiac resynchronization therapy (CRT) for mild to moderate heart failure. Can J Cardiol 2012;28(Suppl.):S419. [Reason for exclusion: unobtainable.]
Wever EF, Hauer RN, Schrijvers G, van Capelle FJ, Tijssen JG, Crijns HJ, et al. Cost-effectiveness of implantable defibrillator as first-choice therapy versus electrophysiologically guided, tiered strategy in postinfarct sudden death survivors. A randomized study. Circulation 1996;93:489–96. [Reason for exclusion: comparator.]
Appendix 12 Data extraction: cost-effectiveness
Buxton and colleagues 2006153
| Country | UK |
| Analysis type | Cost–utility analysis/cost-effectiveness analysis |
| Study type | Markov model |
| Perspective | UK NHS |
| Time horizon | 20 years |
| Discounting (rate) | Base-case discount rates were 6% for costs and 1.5% for benefits |
| Costing year, currency | 2001/2 prices, UK pounds |
| Population | Secondary prevention patients at risk of SCD with previously documented cardiac arrest or VT |
| Intervention(s), comparator(s) | ICD vs. OPT (amiodarone) |
| Intervention effect | Transition probabilities were estimated using IPD from the CIDS trial84 (for OPT patients) and UK sampled observational data (for ICD patients) |
| Health outcomes | A cross-sectional survey collected HRQoL data (using the NHP, SF-36, Hospital Anxiety and Depression questionnaire and EQ-5D) on a sample of 229 patients |
| Device cost | Cost of ICD (with leads) £16,402 |
| Results | Over a 20-year time horizon, the mean discounted incremental cost was £70,900. The mean discounted incremental gain was 1.24 years or 0.93 QALYs for ICD compared with OPT. The ICER for an average UK patient was £76,139 per QALY gained |
| Sensitivity analysis | Sensitivity analyses suggested that targeting those patients at greatest risk of SCD, through either age or poor LVEF, would increase the overall cost-effectiveness of ICD |
| Authors’ conclusions | The results suggest that ICDs, as currently applied in the UK, are not cost-effective by conventional standards |
| Reviewer’s comments | Sound UK study that included QoL and costing studies for ICD patients |
Quality assessment form for economic evaluations
| Item | Y/N/? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Y |
| 2. Is the setting comparable to the UK? | Y |
| 3. Is the analytical and modelling methodology appropriate? | Y |
| 4. Are all the relevant costs and consequences for each alternative identified? | Y |
| 5. Are the data inputs for the model described and justified? | Y |
| 6. Are health outcomes measured in QALYs? | Y |
| 7. Is the time horizon considered appropriate? | Y |
| 8. Are costs and outcomes discounted? | Y |
| 9. Is an incremental analysis performed? | Y |
| 10. Is uncertainty assessed? | Y |
Bond and colleagues 2009,203 derived from Fox and colleagues 200764
| Country | UK | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis type | Cost–utility analysis | |||||||||||||||||||||||||||||||||||||||||||||||||
| Study type | Markov model | |||||||||||||||||||||||||||||||||||||||||||||||||
| Perspective | UK NHS | |||||||||||||||||||||||||||||||||||||||||||||||||
| Time horizon | Lifetime | |||||||||||||||||||||||||||||||||||||||||||||||||
| Discounting (rate) | Costs and QALYs 3.5% | |||||||||||||||||||||||||||||||||||||||||||||||||
| Costing year, currency | 2005 UK pounds for all costs except drug costs (2006 UK pounds) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Population | A mixed-age cohort of patients with NYHA class III and IV HF, evidence of LVSD (LVEF ≤ 35%) and evidence of electrical dyssynchrony (QRS duration > 120 milliseconds) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention(s), comparator(s) | CRT vs. OPT;a CRT-Db vs. CRT; OPT vs. CRT vs. CRT–D | |||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention effectc | RR of death from HF with device: CRT and CRT-D: HR 0.68 (95% CI 0.46 to 0.98); ICD: HR 0.95 (95% CI 0.74 to 1.21) RR of sudden death with device: CRT: HR 0.75 (95% CI 0.45 to 1.18); CRT–D: HR 0.44 (95% CI 0.23 to 0.86); ICD: HR 0.37 (95% CI 0.27 to 0.50) |
|||||||||||||||||||||||||||||||||||||||||||||||||
| Health outcomes | Mean model survival was 4.7 years, 5.8 years and 6.2 years for OPT, CRT and CRT-D respectively. NYHA class-specific estimates of QoL were used to derive time-dependent utility estimates (derived from the CARE-HF trial109 and the study by Kirsch and McGuire,210 which used EQ-5D and UK population values) and utility of hospitalisation because of HF (from McAlister et al.194) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Device cost | Surgery to implant new system (includes cost of the device): CRT £5074; CRT-D £17,266; ICD £11,596 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Results | DiscountedMean cost (£)Mean QALYsIncremental cost (£)Incremental QALYsICER (£) (95% CI)p(CE) (%)OPT93673.10––––CRT20,9973.80––––CRT-D32,6874.09––––CRT vs. OPT––11,6300.7016,738 (14,630 to 20,333)91.3CRT-D vs. CRT––11,6890.2940,160 (26,645 to 59,391)26.3p(CE), probability of being cost-effective at a WTP threshold of £30,000 per QALY. | Discounted | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) (95% CI) | p(CE) (%) | OPT | 9367 | 3.10 | – | – | – | – | CRT | 20,997 | 3.80 | – | – | – | – | CRT-D | 32,687 | 4.09 | – | – | – | – | CRT vs. OPT | – | – | 11,630 | 0.70 | 16,738 (14,630 to 20,333) | 91.3 | CRT-D vs. CRT | – | – | 11,689 | 0.29 | 40,160 (26,645 to 59,391) | 26.3 | p(CE), probability of being cost-effective at a WTP threshold of £30,000 per QALY. | ||||||
| Discounted | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) (95% CI) | p(CE) (%) | ||||||||||||||||||||||||||||||||||||||||||||
| OPT | 9367 | 3.10 | – | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||
| CRT | 20,997 | 3.80 | – | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||
| CRT-D | 32,687 | 4.09 | – | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||
| CRT vs. OPT | – | – | 11,630 | 0.70 | 16,738 (14,630 to 20,333) | 91.3 | ||||||||||||||||||||||||||||||||||||||||||||
| CRT-D vs. CRT | – | – | 11,689 | 0.29 | 40,160 (26,645 to 59,391) | 26.3 | ||||||||||||||||||||||||||||||||||||||||||||
| p(CE), probability of being cost-effective at a WTP threshold of £30,000 per QALY. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Sensitivity analysis | Deterministic univariate and probabilistic sensitivity analyses were conducted. One-way sensitivity analyses show the sensitivity of the results to structural parameters, event probabilities and RRs. In comparison to CRT, CRT-D devices were most likely to be cost-effective when implanted in younger individuals and in those with a high risk of SCD. A cost-effectiveness probability frontier shows that CRT is most likely to be the most cost-effective option at WTP thresholds between £17,000 and £39,000. Above the WTP threshold of £40,000, CRT-D would be the option with the highest expected net benefit (approximately 50% probability of being cost-effective) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Authors’ conclusions | CRT-D is not cost-effective for LVSD. Instead, CRT alone remains the most cost-effective policy option in this population. CRT-D is more likely to be cost-effective in the subgroups of younger patients or those with a high risk of SCD who would qualify for CRT | |||||||||||||||||||||||||||||||||||||||||||||||||
| Reviewer’s comments | PenTAG’s cost–utility analysis in the UK setting using clinical effectiveness data from accompanying systematic review and meta-analysis of RCTs | |||||||||||||||||||||||||||||||||||||||||||||||||
Quality assessment form for economic evaluations
| Item | Y/N/? |
|---|---|
| 1. Is the decision problem (including interventions compared and patient group) relevant to the UK? | Y |
| 2. Is the setting comparable to the UK? | Y |
| 3. Is the analytical and modelling methodology appropriate? | Y |
| 4. Are all the relevant costs and consequences for each alternative identified? | Y |
| 5. Are the data inputs for the model described and justified? | Y |
| 6. Are health outcomes measured in QALYs? | Y |
| 7. Is the time horizon considered appropriate? | Y |
| 8. Are costs and outcomes discounted? | Y |
| 9. Is an incremental analysis performed? | Y |
| 10. Is uncertainty assessed? | Y |
Appendix 13 List of excluded quality-of-life studies
Almenar-Pertejo M, Almenar L, Martinez-Dolz L, Campos J, Galan J, Girones P, et al. Study on health-related quality of life in patients with advanced heart failure before and after transplantation. Transplant Proc 2006;38:2524–6. [Reason for exclusion: format of measure.]
Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005;7:411–17. [Reason for exclusion: format of measure.]
Austin J, Williams WR, Ross L, Hutchison S. Five-year follow-up findings from a randomized controlled trial of cardiac rehabilitation for heart failure. Eur J Cardiovasc Prev Rehabil 2008;15:162–7. [Reason for exclusion: format of measure.]
Austin J, Williams WR, Hutchison S. Multidisciplinary management of elderly patients with chronic heart failure: five year outcome measures in death and survivor groups. Eur J Cardiovasc Nurs 2009;8:34–9. [Reason for exclusion: format of measure.]
Cooper TJ, Dickstein K, Hasselberg N, Comin-Colet J, Filippatos G, Lainscak M, et al. Changes in symptom and quality-of-life assessments correlate strongly and consistently with changes in functional capacity in patients with heart failure. Eur J Heart Fail 2011;10(Suppl. 1):S162. [Reason for exclusion: abstract.]
de Rivas B, Permanyer-Miralda G, Brotons C, Aznar J, Sobreviela E. Health-related quality of life in unselected outpatients with heart failure across Spain in two different health care levels. Magnitude and determinants of impairment: the INCA study. Qual Life Res 2008;17:1229–38. [Reason for exclusion: Spanish tariff for EQ-5D.]
Flynn KE, Lin L, Ellis SJ, Russell SD, Spertus JA, Whellan DJ, et al. Outcomes, health policy, and managed care: relationships between patient-reported outcome measures and clinical measures in outpatients with heart failure. Am Heart J 2009;158:S64–71. [Reason for exclusion: EQ-5D VAS.]
Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3-year follow-up study assessing hospitalization and mortality. Eur J Heart Fail 2010;12:1002–8. [Reason for exclusion: format of measure.]
Kaplan RM, Tally S, Hays RD, Feeny D, Ganiats TG, Palta M, et al. Five preference-based indexes in cataract and heart failure patients were not equally responsive to change. J Clin Epidemiol 2011;64:497–506. [Reason for exclusion: format of measure.]
Kirsch J, McGuire A. Establishing health state valuations for disease specific states: an example from heart disease. Health Econ 2000;9:149–58. [Reason for exclusion: time trade-off measure.]
Kontodimopoulos N, Argiriou M, Theakos N, Niakas D. The impact of disease severity on EQ-5D and SF-6D utility discrepancies in chronic heart failure. Eur J Health Econ 2011;12:383–91. [Reason for exclusion: format of measure.]
Linde C, Mealing S, Hawkins N, Eaton J, Brown B, Daubert JC, et al. Cost-effectiveness of cardiac resynchronization therapy in patients with asymptomatic to mild heart failure: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction). Eur Heart J 2011;32:1631–9. [Reason for exclusion: utility not reported.]
Marti B, Delgado J, Oliva J, Llano M, Pascual P, Comin J, et al. Quality of life in chronic symptomatic heart failure patients in Spain. Value Health 2010;7:A363. [Reason for exclusion: abstract.]
Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005;150:707–15. [Reason for exclusion: format of measure.]
Spiraki C, Kaitelidou D, Papakonstantinou V, Prezerakos P, Maniadakis N. Health-related quality of life measurement in patients admitted with coronary heart disease and heart failure to a cardiology department of a secondary urban hospital in Greece. Hellenic J Cardiol 2008;49:241–7. [Reason for exclusion: format of measure.]
Sullivan MD, Newton K, Hecht J, Russo JE, Spertus JA. Depression and health status in elderly patients with heart failure: a 6-month prospective study in primary care. Am J Geriatr Cardiol 2004;13:252–60. [Reason for exclusion: uses EQ-5D VAS.]
Appendix 14 Development of Southampton Health Technology Assessments Centre model
From the review of published economic evaluations, the study by Fox and colleagues64 was found to be the most adequate for the derivation of a model that would allow the questions of the current assessment to be addressed, with the necessary adaptations to reflect current clinical practice.
The structure of Fox and colleagues’ model for the OPT arm was considered appropriate for the SHTAC population 1 model. The Fox and colleagues model structure for the OPT arm allowed patients initially managed with OPT alone to be upgraded to ICD + OPT in case of hospitalisation for arrhythmia (assumed direct referral to ICD implantation) or following hospitalisation for HF [given that in the CARE-HF trial109 a proportion of those in the OPT alone arm who were hospitalised for HF were then referred to a device with a defibrillation function (CRT-D), Fox and colleagues assumed the same probability for referral to an ICD]. Clinical advice to SHTAC indicated that referral from OPT alone to ICD implantation would occur in clinical practice only for population 1 patients. Fox and colleagues’ model structure, including health states modelled and transitions allowed, was therefore replicated for population 1.
According to clinical advice, population 2 patients would be referred to CRT-P implantation (given their HF severity) and, in case of also being at high risk of severe arrhythmia, could be referred to CRT-D implantation. Clinical opinion confirmed that Fox and colleagues’ model structure for the population 2 CRT-P arm was appropriate for the SHTAC population 2 model and that it could form the basis for modelling population 3.
Adaptations common to all models
Fox and colleagues64 used a specific set of transitions between health states for each of the treatment arms, that is, patients in each arm were eligible only for some of the available treatments (e.g. HF patients initially managed with OPT could upgrade only to ICD + OPT whereas those in the CRT-P arm could upgrade to CRT-D or ICD or explant and be managed with OPT alone). Following feedback from clinical experts, we decided that all treatment arms in each model should use the same transition matrix, allowing the modelled cohort of patients to start with the respective treatment and be referred for upgrades according to probability estimates derived from clinical trials included in SHTAC’s clinical effectiveness review (see Chapter 4).
For consistency with other surgical procedures and devices modelled and following clinical advice, the risk of death as a result of transplantation, the risk of hospitalisation for arrhythmia with ICD and CRT-D therapy, and the risk of lead displacement for ICD therapy were incorporated in the models for all populations.
The model developed by Fox and colleagues64 assumed that all patients being managed with a device would be subject to routine device replacement, including patients who were stable with the device, those having a device-related complication (perioperative, lead displacement or lead infection), those hospitalised because of HF with a device or those referred for an upgrade following hospitalisation because of HF. For modelling simplicity and acknowledging the risk of underestimation, in SHTAC’s model only patients who are stable with the device were subject to routine device replacement, assuming that this allows for a reasonable estimation of the number of replacements in the patient cohorts over a lifetime.
Model-specific adaptations
Surgical failure was incorporated in the model for population 1 for consistency with the models for populations 2 and 3. As clinical advice suggested that returning to management with OPT alone after unsuccessful ICD implantation would be very unlikely, the population 1 model assumes that patients who survive unsuccessful ICD implantation reattempt it the following cycle.
In accordance with the model developed by Fox and colleagues,64 in the models for populations 2 and 3, patients receiving CRT-P who experience lead infection or displacement were assumed to return to being managed with OPT alone if experiencing surgical failure. For consistency among health states, the risk of surgical failure was explicitly accounted for in all health states involving surgery (including first device implantation and routine device replacements). For model simplicity and consistency, patients who survive unsuccessful ICD implantation were also assumed to return to management with OPT alone. Those with unsuccessful CRT-D implantation were assumed to undergo ICD implantation.
The model structure for population 2 differs slightly from that in the study by Fox and colleagues. 64 The main variations relate to the transitions allowed for patients managed with OPT alone. In SHTAC’s model, patients with OPT alone who are hospitalised because of HF or severe arrhythmia can be referred to CRT-P or CRT-D implantation in the following cycle, according to the probabilities reported in the relevant trials. Patients can receive an ICD only following unsuccessful CRT-D implantation, as patients who survive unsuccessful CRT-D implantation are assumed to undergo ICD implantation.
The main adaptation introduced to Fox and colleagues’ model structure for population 3 relates to the referral of patients to CRT-D implantation. Patients being managed with OPT alone or CRT-P + OPT who are hospitalised because of a serious arrhythmic event are assumed to undergo CRT-D implantation in the same cycle. Those being managed with OPT alone because of unsuccessful CRT-P implantation can be referred to ICD implantation if they experience a life-threatening arrhythmia.
Appendix 15 Parameters included in the probabilistic sensitivity analyses
Parameter inputs for the population 1 model
| Parameter type | Parameter | Source estimate | Distribution | |||
|---|---|---|---|---|---|---|
| Mean | SE | LL | UL | |||
| All-cause mortality | ln(λ) | –3.381 | 0.0257 | –3.431 | –3.330 | Normal |
| γ | 0.696 | 0.0092 | 0.678 | 0.714 | Normal | |
| HR ICD | 0.75 | 0.0816 | 0.61 | 0.93 | Log-normal | |
| All causes multiplier | HR 18–59 | 0.62 | 0.0459 | 0.54 | 0.72 | Log-normal |
| HR 75+ | 1.41 | 0.0051 | 1.40 | 1.42 | ||
| Because of surgery | ICD | 0.0034 | 0.0262 | –0.0479 | 0.0548 | Normal |
| Probability of perioperative death | Transplant | 0.122 | 0.007 | 0.109 | 0.136 | Normal |
| Event probabilities (per cycle) | ||||||
| Hospitalisation for HF | OPT | 0.0082 | 0.0061 | –0.0036 | 0.0201 | Beta |
| RR ICD | 1 | 0.1 | 0.804 | 1.196 | ||
| Probability of transplant following HF hospitalisation | Transplant | 0.0014 | 0.0025 | –0.0034 | 0.0062 | Beta |
| Non-fatal arrhythmia requiring hospitalisation | OPT | 0.0075 | 0.0037 | 0.00016 | 0.0148 | Beta |
| ICD | 0.0075 | 0.0037 | 0.00016 | 0.0148 | ||
| Probability of surgical failure | ICD | 0.011 | 0.0441 | –0.07659 | 0.0962 | Beta |
| Device replacement interval | ln(λ) | –15.784 | 0.203 | –16.182 | –15.385 | Normal |
| γ | 1.942 | 0.0273 | 1.889 | 1.996 | ||
| Upgrade after HF hospitalisation | OPT to ICD | 0.0018 | 0.002 | –0.0023 | 0.0059 | Beta |
Parameter inputs for the population 2 model
| Parameter type | Parameter | Source estimate | LL | UL | Distribution | |
|---|---|---|---|---|---|---|
| Mean | SE | |||||
| Death from HF, age 65–74 years, OPT | ln(λ) | –6.115 | 0.070 | –6.253 | –5.977 | Normal |
| γ | 1.223 | 0.022 | 1.180 | 1.265 | Normal | |
| HR CRT-P | 0.67 | 0.094 | 0.51 | 0.88 | Log-normal | |
| HR CRT-D | 0.73 | 0.163 | 0.47 | 1.11 | Log-normal | |
| HR ICD | 1.14 | 0.153 | 0.88 | 1.48 | Log-normal | |
| Post-transplant mortality | RR Transplant | 0.35 | 0.035 | 0.281 | 0.419 | Log-normal |
| Death from SCD | ln(λ) | –6.069 | 0.053 | –6.173 | –5.964 | Normal |
| γ | 1.140 | 0.017 | 1.107 | 1.173 | Normal | |
| HR CRT-P | 1 | 0.1505 | 0.54 | 1.13 | Log-normal | |
| HR CRT-D | 0.44 | 0.1607 | 0.23 | 0.86 | Log-normal | |
| HR ICD | 0.44 | 0.0765 | 0.31 | 0.61 | Log-normal | |
| All-cause mortality RR by age (years) | 18–64 | 0.62 | 0.05 | 0.54 | 0.72 | Log-normal |
| 75+ | 1.41 | 0.01 | 1.4 | 1.42 | ||
| Event probabilities (per cycle) | ||||||
| Surgical mortality | ICD | 0.003 | 0.026 | 0.000 | 0.055 | Beta |
| CRT-P | 0.005 | 0.002 | 0.001 | 0.008 | ||
| CRT-D | 0.005 | 0.003 | 0.000 | 0.011 | ||
| Transplant | 0.122 | 0.007 | 0.109 | 0.136 | ||
| Hospitalisation for HF | OPT | 0.037 | 0.006 | 0.025 | 0.049 | Beta |
| RR ICD | 1 | 0.1 | 0.804 | 1.196 | ||
| RR CRT-P | 0.58 | 0.1556 | 0.35 | 0.96 | ||
| RR CRT-D | 0.77 | 0.0765 | 0.63 | 0.93 | ||
| Transplant following HF hospitalisation | Transplant | 0.001 | 0.002 | –0.003 | 0.006 | Beta |
| Non-fatal arrhythmia requiring hospitalisation | OPT | 0.007 | 0.004 | 0.000 | 0.015 | Beta |
| ICD | 0.007 | 0.004 | 0.000 | 0.015 | ||
| CRT-P | 0.007 | 0.004 | 0.000 | 0.015 | ||
| CRT-D | 0.007 | 0.004 | 0.000 | 0.015 | ||
| Probability of upgrade after HF hospitalisation | OPT to ICD | 0 | 0 | 0 | 0 | Beta |
| OPT to CRT-P | 0.003 | 0.003 | 0.000 | 0.009 | ||
| OPT to CRT-D | 0.002 | 0.002 | 0.000 | 0.006 | ||
| CRT-P to CRT-D | 0.001 | 0.001 | 0.000 | 0.003 | ||
| Surgical failure | ICD | 0.011 | 0.001 | 0.009 | 0.013 | Beta |
| CRT-P | 0.084 | 0.007 | 0.070 | 0.097 | ||
| CRT-D | 0.087 | 0.012 | 0.064 | 0.109 | ||
Parameter inputs for the population 3 model
| Parameter type | Parameter | Source estimate | LL | UL | Distribution | |
|---|---|---|---|---|---|---|
| Mean | SE | |||||
| All-cause mortality, baseline – CRT-D | ln(λ) | –6.334 | 0.068 | –6.467 | –6.202 | Normal |
| γ | 1.234 | 0.018 | 1.199 | 1.270 | Normal | |
| HR CRT-P | 1 | 0.100 | 0.804 | 1.196 | Log-normal | |
| HR ICD | 1.190 | 0.084 | 1.042 | 1.370 | Log-normal | |
| HR OPT | 1.563 | 0.235 | 1.163 | 2.083 | Log-normal | |
| All-cause mortality RR by age (years) | 18–64 | 0.621 | 0.046 | 0.54 | 0.72 | Log-normal |
| 75+ | 1.410 | 0.005 | 1.4 | 1.42 | ||
| Event probabilities (per cycle) | CRT-D | 0.008 | 0.003 | 0.003 | 0.013 | Beta |
| Hospitalisation for HF | RR ICD | 1.333 | 0.133 | 1.136 | 1.563 | Log-normal |
| RR CRT-P | 1 | 0.1000 | 0.804 | 1.196 | ||
| RR OPT | 1.67 | 0.0893 | 1.51 | 1.86 | ||
| Non-fatal arrhythmia requiring hospitalisation | CRT- D | 0.029 | 0.007 | 0.015 | 0.042 | Log-normal |
| ICD RR | 1.111 | 0.111 | 0.880 | 1.410 | ||
| CRT-P RR | 1 | 0.1 | 0.804 | 1.196 | ||
| OPT RR | 1 | 0.1 | 0.804 | 1.196 | ||
| Probability of upgrade after HF hospitalisation | OPT to ICD | 0.002 | 0.002 | 0 | 0.006 | Beta |
| OPT to CRT-P | 0.003 | 0.003 | 0 | 0.009 | ||
| OPT to CRT-D | 0.002 | 0.002 | 0 | 0.006 | ||
| CRT-P to CRT-D | 0.001 | 0.001 | 0 | 0.003 | ||
| ICD to CRT-D | 0.007 | 0.003 | 0.001 | 0.013 | ||
| Surgical mortality | ICD | 0.003 | 0.026 | 0 | 0.055 | Beta |
| CRT-P | 0.005 | 0.002 | 0.001 | 0.008 | ||
| CRT-D | 0.005 | 0.003 | 0 | 0.011 | ||
| Surgical failure | ICD | 0.011 | 0.001 | 0.009 | 0.013 | Beta |
| CRT-P | 0.084 | 0.007 | 0.070 | 0.097 | ||
| CRT-D | 0.087 | 0.012 | 0.064 | 0.109 | ||
| Device lifetime | ICD ln(λ) | –15.784 | 0.203 | –16.182 | –15.385 | Normal |
| CD γ | 1.943 | 0.027 | 1.889 | 1.996 | ||
| CRT-P ln(λ) | –14.222 | 0.242 | –14.697 | –13.747 | ||
| CRT-P γ | 1.677 | 0.032 | 1.613 | 1.740 | ||
| CRT-D ln(λ) | –15.465 | 0.273 | –16 | –14.931 | ||
| CRT-D γ | 1.935 | 0.036 | 1.863 | 2.006 | ||
For all populations: utilities
| Parameter type | Parameter | Mean | SE | UL | LL | Distribution |
|---|---|---|---|---|---|---|
| No HF | 0.855 | 0.0048 | 0.845 | 0.864 | Beta | |
| Per NYHA class | NYHA I | 0.855 | 0.0048 | 0.845 | 0.864 | Beta |
| NYHA II | 0.771 | 0.0051 | 0.761 | 0.781 | ||
| NYHA III | 0.673 | 0.0097 | 0.727 | 0.765 | ||
| NYHA IV | 0.532 | 0.0265 | 0.48 | 0.584 | ||
| HF hospitalisation | Hospitalisation with HF | 0.57 | 0.0570 | 0.458 | 0.682 | Beta |
| Utility decrement | Surgery | 0.05 | 0.0255 | 0 | 0.1 | Beta |
| Infection | 0.1 | 0.0255 | 0.05 | 0.15 | ||
| Proportion of month hospitalised for HF (%) | 25 | 0.0255 | 20 | 30 | Beta |
Costs and resource use
| Parameter type | Parameter | Mean (£) | SE (£) | UL (£) | LL (£) | Distribution |
|---|---|---|---|---|---|---|
| Implantation | CRT-P | 8281 | 1479 | 6098 | 11,895 | Gamma |
| CRT-D | 17,849 | 4521 | 15,246 | 32,969 | ||
| ICD | 15,248 | 4261 | 13,155 | 29,858 | ||
| Lead displacement/implantation failure | CRT-P | 5681 | 1219 | 4008 | 8786 | Gamma |
| CRT-D | 6097 | 3346 | 5798 | 18,914 | ||
| ICD | 6099 | 3346 | 5799 | 18,916 | ||
| Battery failure/device malfunction | CRT-P | 5348 | 788 | 3884 | 6974 | Gamma |
| CRT-D | 17,308 | 1704 | 14,811 | 32,322 | ||
| ICD | 14,705 | 4207 | 12,718 | 29,209 | ||
| Infection | CRT-P | 12,553 | 2036 | 7285 | 15,265 | Gamma |
| CRT-D | 21,580 | 5552 | 17,202 | 38,966 | ||
| ICD | 18,977 | 5292 | 15,109 | 35,853 | ||
| Operative complications | CRT-P | 4884 | 1869 | 2442 | 9768 | Gamma |
| CRT-D | 6634 | 2539 | 3317 | 13,268 | ||
| ICD | 3432 | 1313 | 1716 | 6864 | ||
| Non-elective hospitalisation | HF hospitalisation | 2308 | 232 | 1669 | 2578 | Gamma |
| Arrhythmia hospitalisation | 1372 | 173 | 922 | 1601 | ||
| Transplant | Heart transplant | 35,606 | 5578 | 21,449 | 43,315 | Gamma |
| Outpatient appointments, 6-monthly | Outpatient cardiology specialist follow-up | 123 | 14 | 94 | 148 | Gamma |
| OPT drugs, average monthly cost per class | NYHA class I | 5.78 | 2.21 | 2.89 | 11.56 | Gamma |
| NYHA class II | 19.39 | 7.42 | 9.695 | 38.78 | ||
| NYHA class III | 19.56 | 7.48 | 9.78 | 39.12 | ||
| NYHA class IV | 19.73 | 7.55 | 9.865 | 39.46 |
Appendix 16 Regression analyses for deriving model parameters
Kaplan–Meier curves for overall survival were used to derive approximate hazard functions using a Weibull distribution. Transition probabilities, used in the model, can be calculated from the estimated hazard functions. 220 The Weibull distribution is defined according to two parameters: the scale parameter (λ) and the shape parameter (γ). These parameters were fitted using linear regression of transformations of the Kaplan–Meier estimates. To do this, scanned images of the Kaplan–Meier curves were imported in Engauge software and the extracted data points were then exported to Microsoft Excel for further analysis.
For a Weibull distribution the survival function is given by:
with scale parameter λ and shape γ.
Taking the log of both sides gives:
Taking the log of both sides again gives:
which is a linear function and can be fit using least-squares methods to provide estimates of λ and γ.
Population 1
Table 164 shows the parameters derived for estimation of all-cause mortality for the OPT arm in the model for population 1.
| Parameter | Mean (SE) | |||
|---|---|---|---|---|
| AVID71 (R2 = 0.994) | MADIT II101 (R2 = 0.9903) | SCD-HeFT105 (R2 = 0.993) | SCD-HeFT,105 non-ischaemic CHF subgroup (R2 = 0.985) | |
| ln(λ) | –3.380 (0.026) | –4.628 (0.047) | –5.288 (0.039) | –4.821 (0.037) |
| γ | 0.696 (0.009) | 1.007 (0.017) | 1.083 (0.011) | 0.883 (0.011) |
Secondary prevention
Figure 43 shows the Weibull approximation fitted to the Kaplan–Meier curve for overall survival of patients in the AVID trial,71 who survived VF or sustained VT that had caused haemodynamic compromise. Goodness of fit can be inspected visually as well as being indicated by the R2 measure close to 1 (R2 = 0.994). The shape parameter (γ = 0.70) for the Weibull approximation for the AVID trial is < 1, indicating that the hazard rate decreases with time.
FIGURE 43.
Kaplan–Meier survival estimates and Weibull approximation for all-cause mortality: AVID trial population. 71
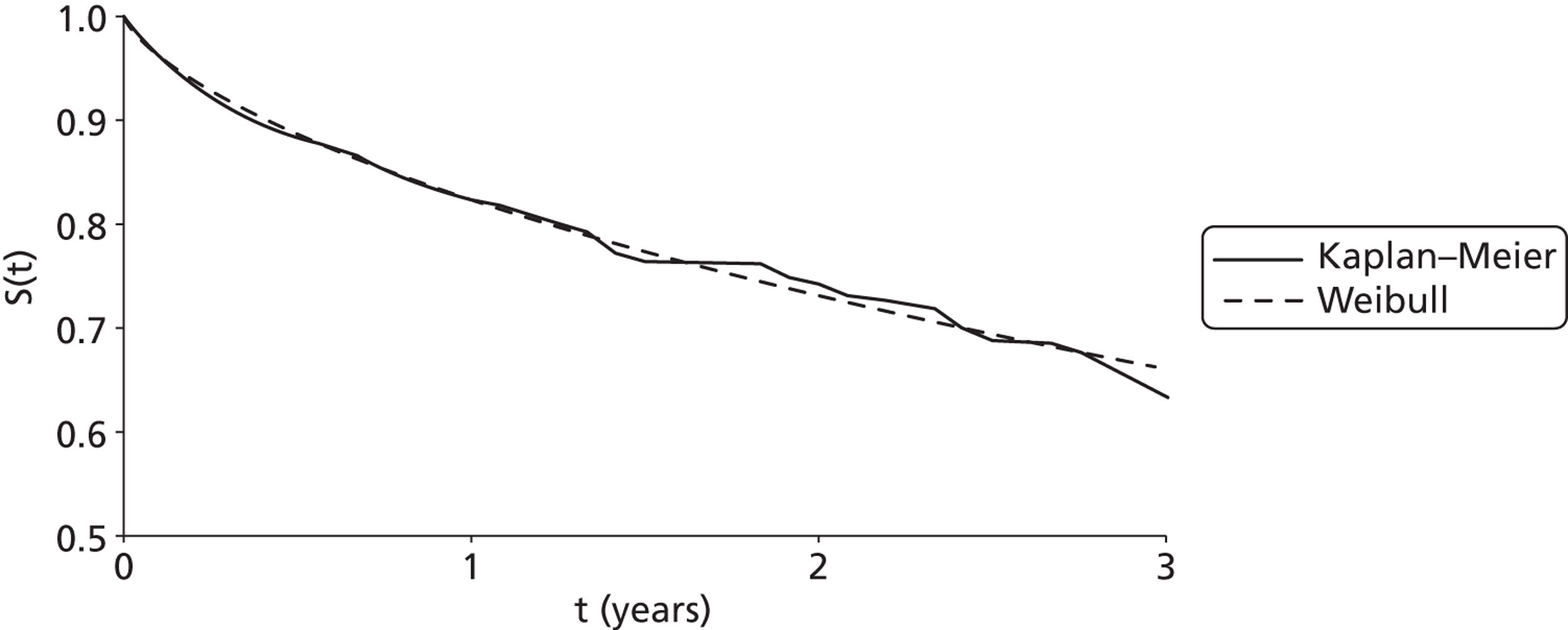
Primary prevention: remote myocardial infarction
Figure 44 illustrates the curve-fitting process for patients with remote MI and reduced LVEF using data extracted from the MADIT II trial,101 showing the fitted Weibull approximation. Visual inspection suggests that the curve fits the data well (R2 from the regression is 0.99). The shape parameter (γ = 1.01) is close to 1, which indicates that the distribution could potentially be reduced to the exponential form.
FIGURE 44.
Kaplan–Meier survival estimates and Weibull approximation for all-cause mortality in patients with remote MI and reduced LVEF: MADIT II trial population. 52
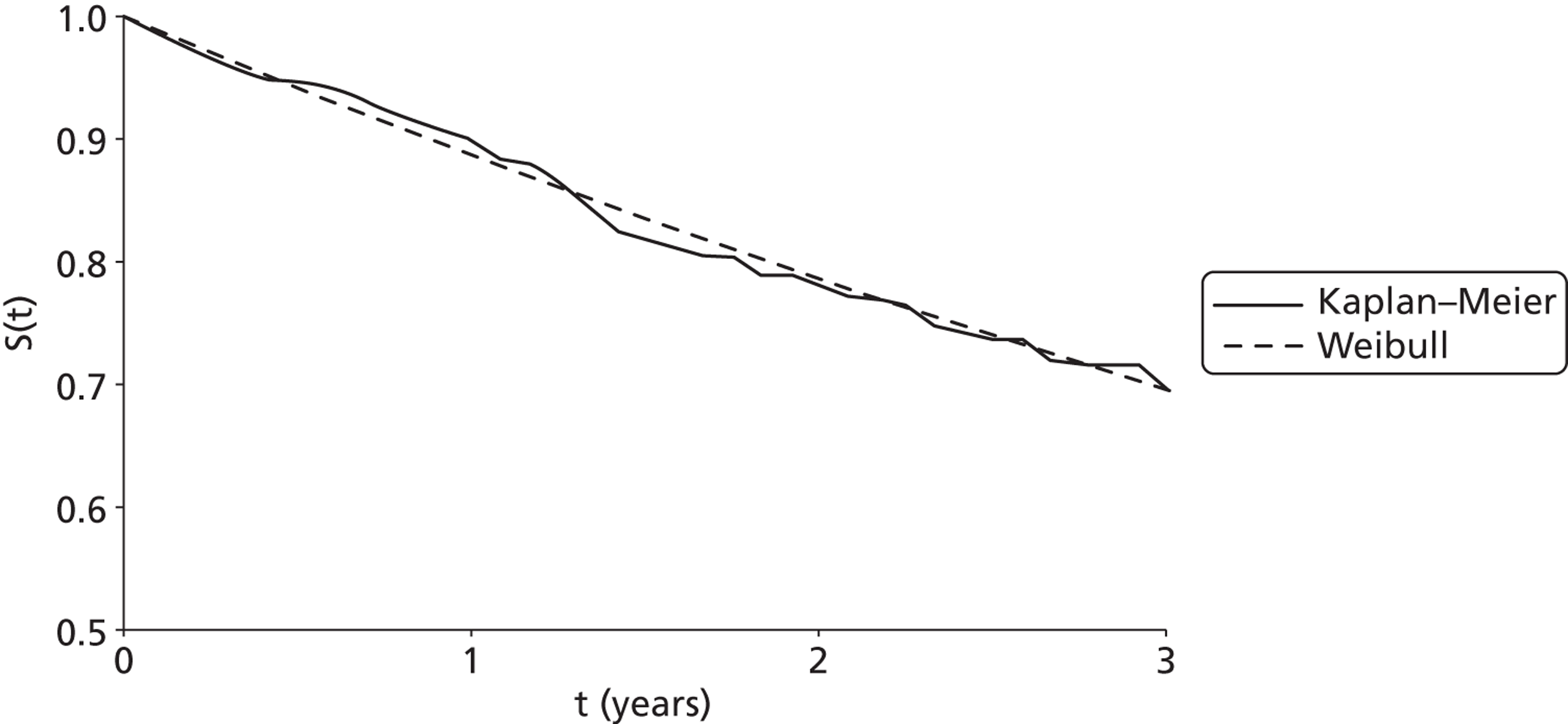
Primary prevention: mild to moderate heart failure
The Kaplan–Meier curve for overall survival of patients in the control group with mild to moderate HF at increased risk of SCD using data extracted from the SCD-HeFT trial105 is shown in Figure 45, as well as its derived Weibull approximation. The R2 of 0.993 confirms the goodness of fit of the Weibull model to the Kaplan–Meier curve of the trial. The shape parameter (γ = 1.08) is slightly above 1, indicating that the hazard rate slightly increases with time.
FIGURE 45.
Kaplan–Meier survival estimates and Weibull approximation for overall survival in patients with mild to moderate HF: SCD-HeFT trial population. 105
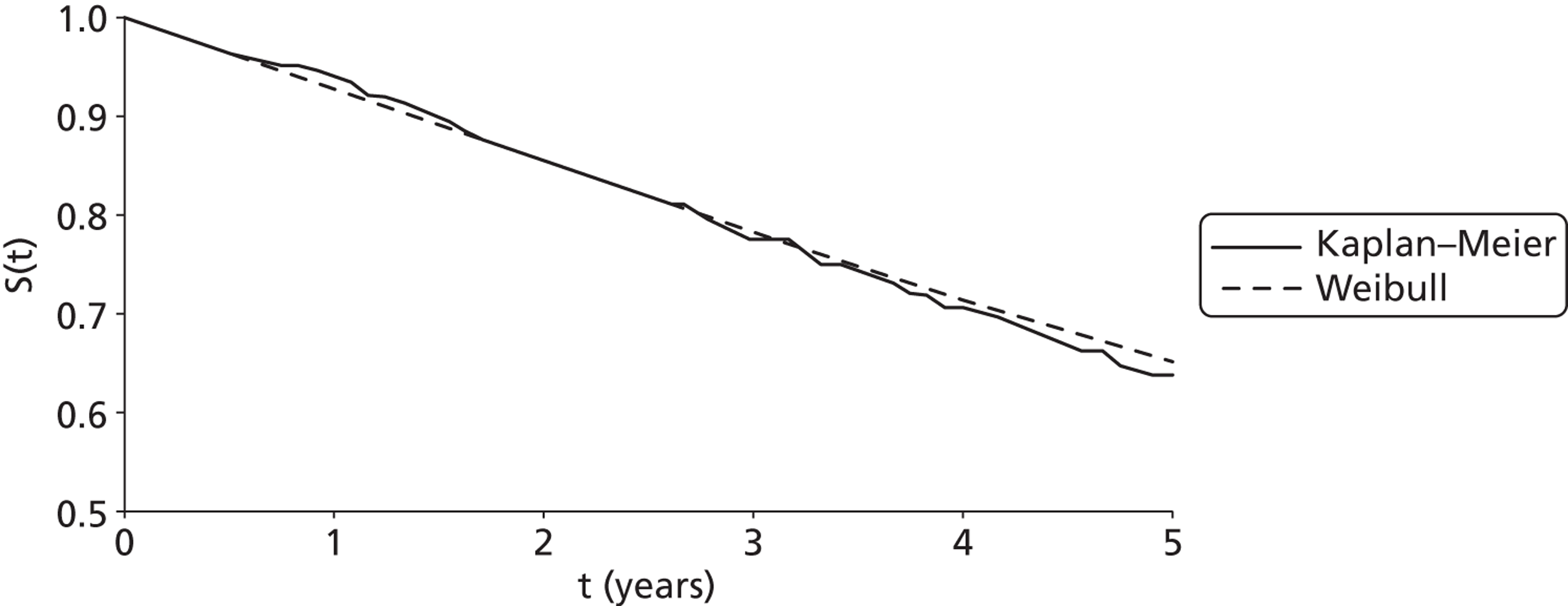
Primary prevention: cardiomyopathy
The SCD-HeFT trial105 reported all-cause mortality for the subgroup of patients with non-ischaemic CHF. The Kaplan–Meier curve for the placebo arm was used to derive the baseline mortality for the subgroup analysis of patients with cardiomyopathy (Figure 46). The R2 from the regression (0.99) and visual inspection of the Weibull approximation suggest that the model fits the Kaplan–Meier estimates well.
FIGURE 46.
Kaplan–Meier survival estimates and Weibull approximation for all-cause mortality in patients with non-ischaemic CHF: SCD-HeFT trial population. 105
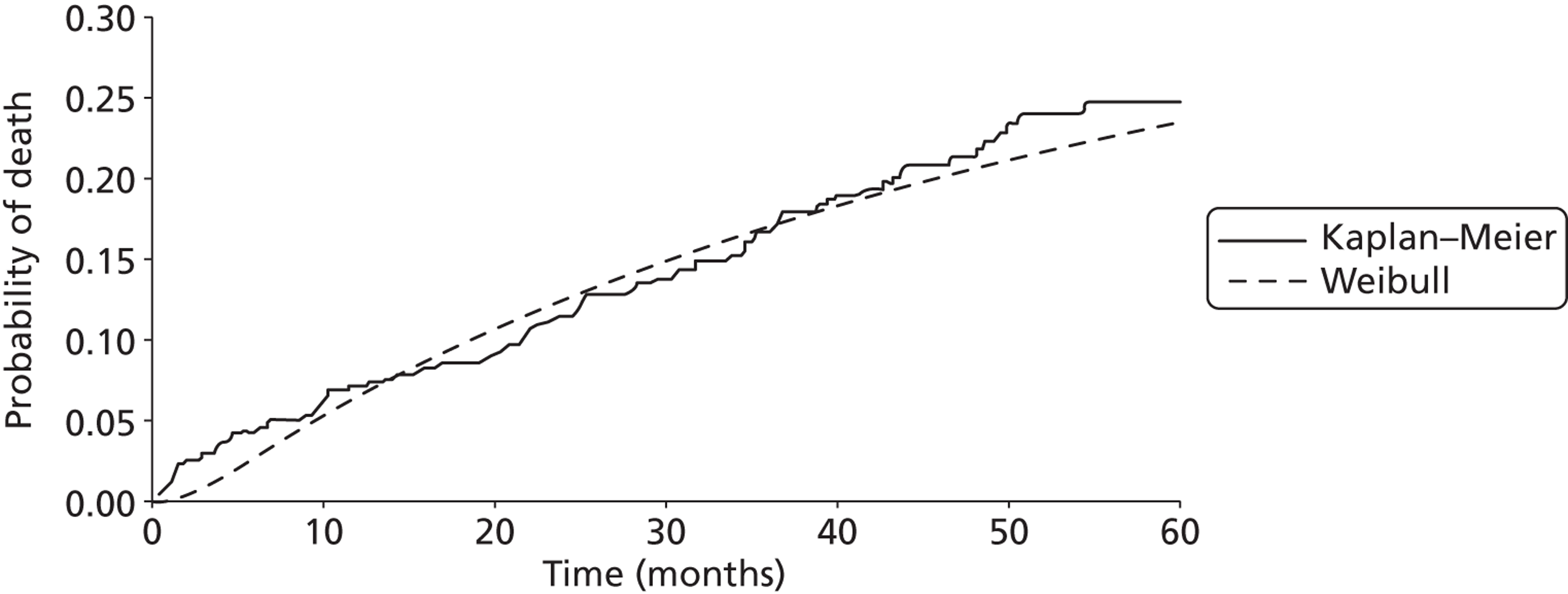
Table 165 provides a comparison between the observed survival reported at given years for each trial and the model predictions.
| Year | Study report | Weibull approximation | ||
|---|---|---|---|---|
| AAD | ICD | AAD | ICDa | |
| AVID71 (R2 = 0.994), λ = 0.0340, γ = 0.6962 | ||||
| 1 | 0.823 | 0.893 | 0.825 | 0.881 |
| 2 | 0.747 | 0.816 | 0.733 | 0.814 |
| 3 | 0.641 | 0.754 | 0.662 | 0.762 |
| Conventional medical therapy | ICD | Conventional medical therapy | ICDb | |
| MADIT II101 (R2 = 0.9903), λ = 0.0098, γ = 1.0068 | ||||
| 1 | 0.90 | 0.91 | 0.89 | 0.92 |
| 2 | 0.78 | 0.84 | 0.79 | 0.85 |
| 3 | 0.69 | 0.78 | 0.70 | 0.78 |
| Placeboc | ICDc | Placebo | ICDd | |
| SCD-HeFT105 (R2 = 0.993), λ = 0.0051, γ = 1.0831 | ||||
| 1 | 0.940 | 0.938 | 0.928 | 0.944 |
| 2 | 0.854 | 0.885 | 0.854 | 0.885 |
| 3 | 0.777 | 0.827 | 0.783 | 0.828 |
| 4 | 0.708 | 0.777 | 0.716 | 0.773 |
| 5 | 0.639 | 0.711 | 0.653 | 0.720 |
Population 2
Cardiac mortality
The CARE-HF trial111 is the trial with the longest follow-up period of those included in SHTAC’s clinical effectiveness review for people with HF as a result of LVSD and cardiac dyssynchrony despite receiving OPT. Hence, baseline time-dependent probabilities of SCD and death from worsening HF were derived from CARE-HF survival curves. 111 Table 166 shows the parameters derived for the estimation of SCD and HF deaths for the OPT arm.
| Parameter | Mean | 95% CI |
|---|---|---|
| SCD | ||
| ln(λ) | –6.069 | –6.173 to –5.964 |
| γ | 1.140 | 1.107 to 1.173 |
| HF | ||
| ln(λ) | –6.115 | –6.256 to –5.974 |
| γ | 1.223 | 1.179 to 1.266 |
Population 3
Mortality and relative risks
Estimates of survival over time were derived from Kaplan–Meier curves reported for relevant trials included in the systematic review. The two largest trials reporting the longest follow-up and comparing events between groups statistically (MADIT-CRT135 and RAFT140) were included in this analysis.
Kaplan–Meier curves for all-cause mortality were used to derive approximate hazard functions using a Weibull distribution. Parameters for the Weibull distribution were fit in Microsoft Excel using linear regression of transformations of the Kaplan–Meier estimates obtained using Engauge software. Table 167 presents the regression results using data extracted from both trials. 135,140
| Parameter | Mean | 95% CI |
|---|---|---|
| RAFT140 | ||
| ICD-CRT arm (R2 = 0.9894) | ||
| ln(λ) | –6.334 | –6.202 to –6.467 |
| γ | 1.243 | 1.20 to 1.27 |
| MADIT-CRT135 | ||
| Men, CRT-D arm (R2 = 0.989) | ||
| ln(λ) | –6.935 | –7.005 to –6.865 |
| γ | 1.287 | 1.266 to 1.308 |
The R2 statistics reported for the regressions in Table 167 confirm that the Weibull models fit the data well. Figure 47 shows the Weibull approximation to the Kaplan–Meier estimates obtained from the curve published for the ICD-CRT arm of the RAFT trial. 140 The γ value (1.24, 95% CI 1.20 to 1.27) is > 1, indicating that the probability of death increases over time.
FIGURE 47.
Weibull approximation to Kaplan–Meier survival for all-cause mortality of patients receiving CRT-D in the RAFT trial. 140
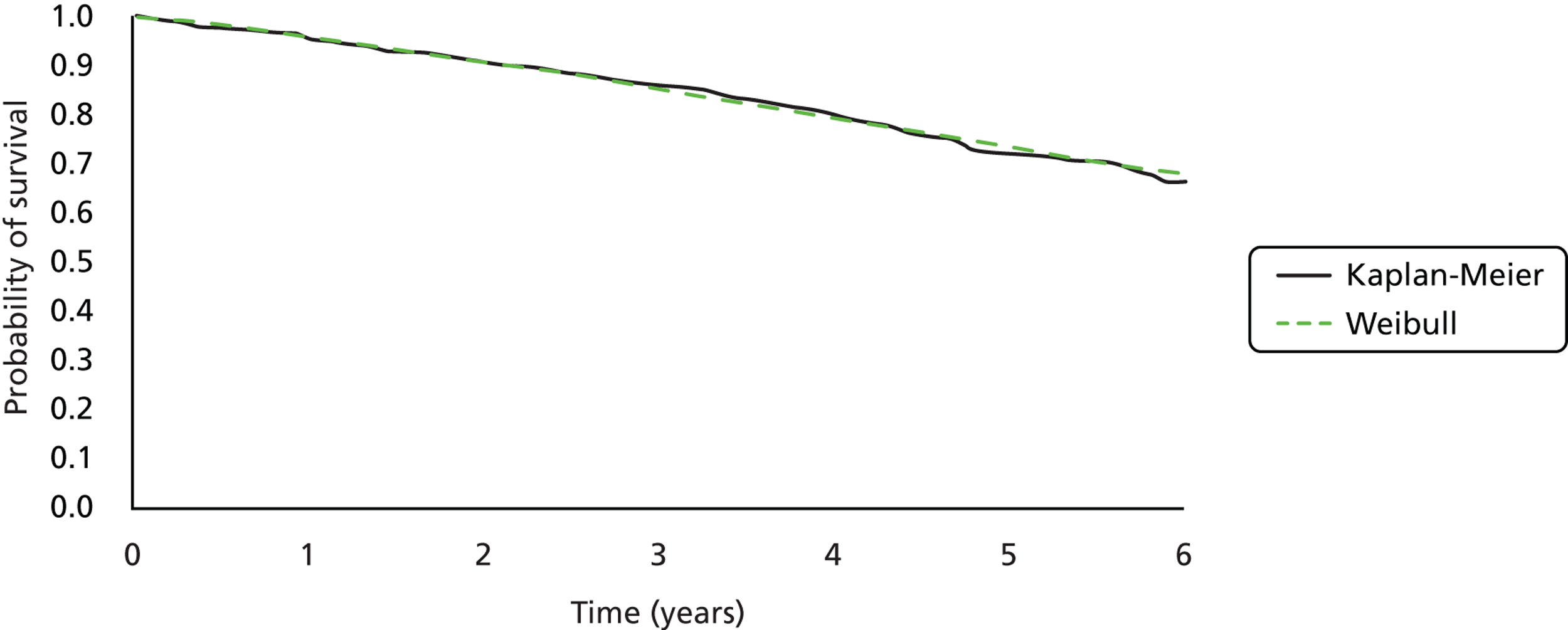
Table 168 provides a comparison between observed survival at times reported for the trials and model predictions.
| Year | Study report | Weibull approximation | ||
|---|---|---|---|---|
| ICD-CRTa | ICDa | ICD-CRT | ICDb | |
| RAFT140 | ||||
| 1 | 0.954 | 0.937 | 0.959 | 0.945 |
| 2 | 0.902 | 0.877 | 0.906 | 0.876 |
| 3 | 0.860 | 0.811 | 0.849 | 0.804 |
| 4 | 0.797 | 0.718 | 0.792 | 0.733 |
| 5 | 0.714c | 0.654c | 0.736 | 0.664 |
| 6 | 0.663 | 0.553 | 0.681 | 0.599 |
| CRT-Da | ICDa | CRT-D | ICDd | |
| MADIT-CRT men135 | ||||
| 1 | 0.974 | 0.976 | 0.974 | 0.975 |
| 2 | 0.946 | 0.939 | 0.938 | 0.941 |
| 3 | 0.889 | 0.929 | 0.897 | 0.901 |
| 4 | 0.855 | 0.851 | 0.854 | 0.858 |
Appendix 17 Validation of the independent economic model
Validation against the model developed by Fox and colleagues64
At an early stage of model development, the OPT arm of the model developed by Fox and colleagues64 for TA12043 was replicated. The OPT arm consisted of a cohort of patients with HF initially managed with OPT alone who are eligible for ICD implantation. Table 169 summarises the outputs of the original model and the replica in terms of life-years and respective discounted QALYs spent in each health state. The same state occupancy was obtained with both versions of the model.
| Health state | Life-years | Discounted QALYs | ||
|---|---|---|---|---|
| Fox and colleagues64 | Replica | Fox and colleagues64 | Replica | |
| Stable with OPT | 3.42 | 3.42 | 2.17 | 2.17 |
| Hospitalised with OPT | 0.13 | 0.13 | 0.08 | 0.08 |
| ICD implantation | 0.03 | 0.03 | 0.02 | 0.02 |
| Perioperative complications | 0.01 | 0.01 | 0.00 | 0.00 |
| Stable with ICD | 1.56 | 1.56 | 0.98 | 0.98 |
| Hospitalised with ICD | 0.06 | 0.06 | 0.04 | 0.04 |
| Device replacement | 0.02 | 0.02 | 0.01 | 0.01 |
| Device-related infection | 0.00 | 0.00 | 0.00 | 0.00 |
| Lead displacement | 0.00 | 0.00 | 0.00 | 0.00 |
| Transplanted | 0.03 | 0.03 | 0.02 | 0.02 |
| Total | 5.26 | 5.26 | 3.31 | 3.31 |
Having reproduced this model arm, the model was adapted according to clinical advice to reflect disease progression for the populations defined in the scope developed by NICE61 for this assessment.
Validation against trial data
Population 1
The model was validated against trial data for all-cause mortality from the AVID,71 MADIT II101 and SCD-Heft105 trials. The model used the all-cause mortality regression parameters calculated for these trials and the trial RR for ICDs, that is, 0.66 for AVID, 0.71 for MADIT II and 0.77 for SCD-HEFT. Figures 48–50 show the results from these analyses. The model-generated results show a good fit against the AVID trial71 data. The model results show a reasonable fit against the MADIT II and SCD-HeFT trial data, although the model appears to slightly underestimate the benefit of ICD compared with OPT and therefore may be a conservative fit.
FIGURE 48.
Overall survival curves for OPT and ICD compared with AVID trial data. 71 M, model; T, trial.
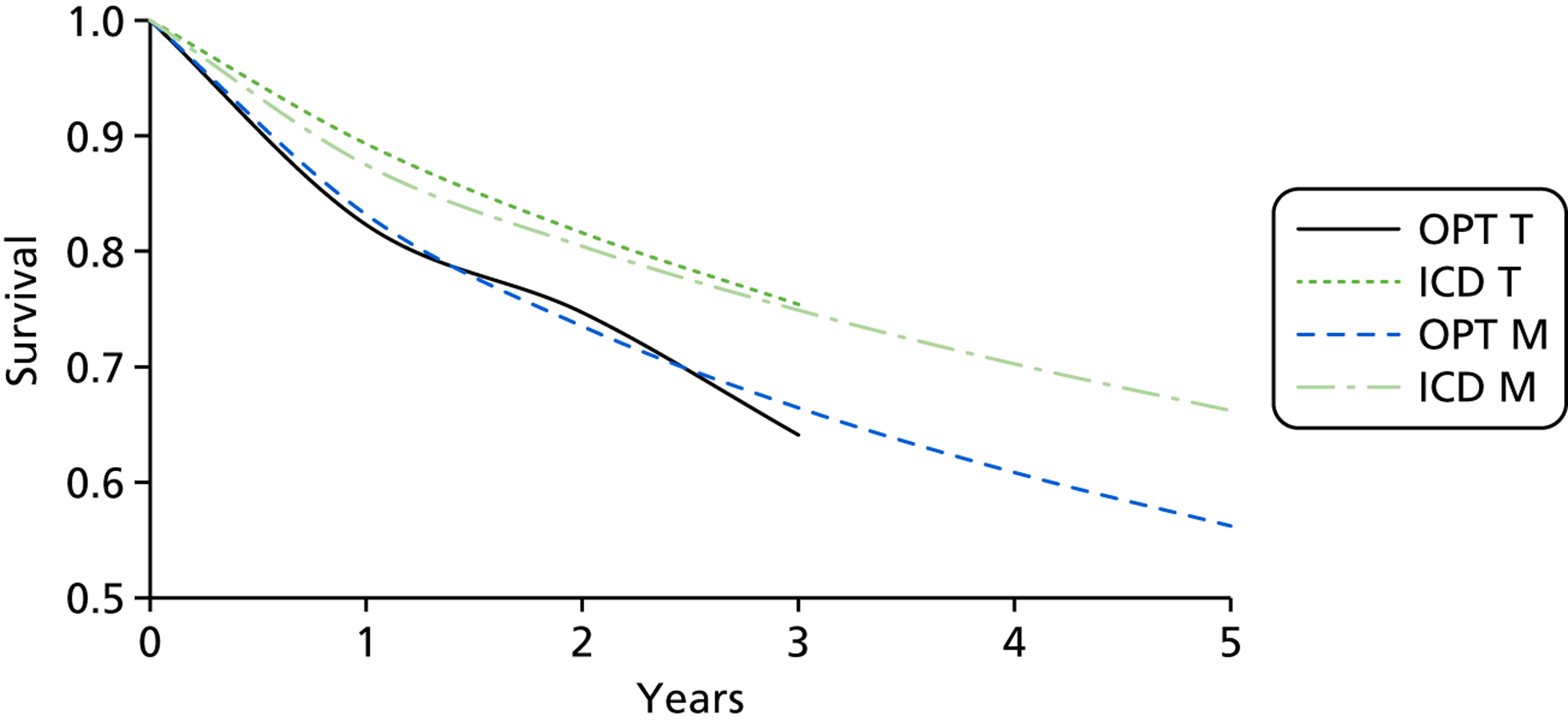
Population 2
The model was validated against the trial data for all-cause mortality from the CARE-HF trial. 111 The model used the SCD and HF mortality regression parameters calculated for this trial and the trial RR for ICD, that is 0.55 for HF and 0.54 for SCD. Figure 51 shows the results from this analysis. The model-generated results show a reasonable fit against the CARE-HF trial111 data, although the model underestimates all-cause mortality for the OPT arm. This is likely to be an underestimate of non-cardiac mortality for this group. The model results show a reasonable fit against the CRT arm from the CARE-HF trial, although the model appears to underestimate the benefit of CRT compared with OPT and therefore may be a conservative fit.
Population 3
The model was validated against the trial data for all-cause mortality from the RAFT trial. 140 The model used the all-cause mortality regression parameters calculated for this trial and the trial RR of 0.75 for CRT-D compared with ICD. Figure 52 shows the results from this analysis. The model-generated results show a good fit against the RAFT trial data.
Glossary
- CONTAK-CD
- Randomised controlled trial of the CONTAK-CD device.
- QRS interval
- An electrocardiogram trace pattern (comprising three electrocardiogram waves: Q, R and S) corresponding to the depolarisation of the right and left ventricles of the heart. The duration or ‘width’ of the QRS interval is an indicator of ventricular dyssynchrony.
- QT
- Q and T wave on an electrocardiogram.
List of abbreviations
- AAD
- antiarrhythmic drug
- ABHI
- Association of British Healthcare Industries
- ACE
- angiotensin-converting enzyme
- AIC
- Akaike information criterion
- AMIOVIRT
- Amiodarone Versus Implantable Cardioverter-Defibrillator Randomized Trial
- ARB
- angiotensin receptor blocker
- AVID
- Antiarrhythmics Versus Implantable Defibrillators
- BIC
- Bayesian information criterion
- BNF
- British National Formulary
- CABG Patch
- Coronary Artery Bypass Graft Patch
- CARE-HF
- CArdiac REsynchronization in Heart Failure
- CASH
- Cardiac Arrest Study Hamburg
- CAT
- Cardiomyopathy Trial
- CCAD
- Central Cardiac Audit Database
- CHD
- coronary heart disease
- CHF
- congestive heart failure
- CI
- confidence interval
- CIDS
- Canadian Implantable Defibrillator Study
- COMPANION
- Comparison of Medical Therapy, Pacing, and Defibrillation in Patients with Left Ventricular Systolic Dysfunction
- CONTAK-CD
- randomised controlled trial of the CONTAK-CD device
- COPD
- chronic obstructive pulmonary disease
- CRD
- Centre for Reviews and Dissemination
- CRT
- cardiac resynchronisation therapy
- CRT-D
- cardiac resynchronisation therapy – defibrillator
- CRT-P
- cardiac resynchronisation therapy – pacer
- CVD
- cardiovascular death
- DASI
- Duke Activity Status Index
- DEBUTE
- Defibrillator versus Beta-Blockers for Unexplained Death in Thailand
- DEFINITE
- Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation
- df
- degree of freedom
- DIC
- deviance information criteria
- DINAMIT
- Defibrillator in Acute Myocardial Infarction Trial
- ECG
- electrocardiogram
- ECHOES
- Echocardiographic Heart of England Screening Study
- EPHESUS
- Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- US Food and Drug Administration
- GPRD
- General Practice Research Database
- HF
- heart failure
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HRS
- Heart Rhythm Society
- HTA
- Health Technology Assessment
- HUI3
- Health Utilities Index 3
- ICD
- implantable cardioverter defibrillator
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- IQR
- interquartile range
- IRIS
- Immediate Risk Stratification Improves Survival
- ITT
- intention to treat
- LBBB
- left bundle branch block
- LVEDD
- left ventricular end-diastolic diameter
- LVEF
- left ventricular ejection fraction
- LVSD
- left ventricular systolic dysfunction
- MADIT
- Multicenter Automatic Defibrillator Implantation Trial
- MADIT-CRT
- Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy
- MAVERIC
- Midlands Trial of Empirical Amiodarone versus Electrophysiology-Guided Interventions and Implantable Cardioverter-Defibrillators
- MCS
- mental component summary
- MD
- mean difference
- MHI
- Mental Health Inventory
- MHI-5
- Mental Health Inventory 5
- MI
- myocardial infarction
- MIRACLE
- Multicenter InSync Randomized Clinical Evaluation
- MIRACLE ICD
- Multicenter InSync ICD Randomized Clinical Evaluation
- MLWHFQ
- Minnesota Living with Heart Failure Questionnaire
- MS
- manufacturers’ submission
- MUSTIC
- Multisite Stimulation in Cardiomyopathies
- MUSTT
- Multicenter Unsustained Tachycardia Trial
- NBRM
- negative binomial regression model
- NHP
- Nottingham Health Profile
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMA
- network meta-analysis
- NSVT
- non-sustained ventricular tachycardia
- NYHA
- New York Heart Association
- OPT
- optimal pharmacological therapy
- PCS
- physical component summary
- PES
- programmed electrical stimulation
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QWBS
- Quality of Well-Being Scale
- RAFT
- Resynchronization-Defibrillation for Ambulatory Heart Failure Trial
- RCT
- randomised controlled trial
- RESPOND
- Resynchronization in Patients with Heart Failure and a Normal QRS Duration
- RethinQ
- Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS
- REVERSE
- REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction
- RHYTHM ICD
- Resynchronization for the HemodYnamic Treatment for Heart failure Management Implantable Cardioverter Defibrillator
- RR
- risk ratio
- RRR
- risk ratio reduction
- SCD
- sudden cardiac death
- SCD-HeFT
- Sudden Cardiac Death in Heart Failure Trial
- SD
- standard deviation
- SE
- standard error
- SEM
- standard error of the mean
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SHTAC
- Southampton Health Technology Assessments Centre
- S-ICD
- subcutaneous implantable cardiac defibrillator
- SNP
- serum natriuretic peptide
- STAI
- State–Trait Anxiety Inventory
- SUDS
- sudden unexplained death syndrome
- TA
- technology appraisal
- TAR
- technology assessment report
- VECTOR
- Ventricular Resynchronization Therapy Randomized Trial
- VF
- ventricular fibrillation
- VO2
- oxygen consumption
- VT
- ventricular tachycardia
- WTP
- willingness to pay
This monograph is based on the technology assessment report produced for the National Institute for Health and Care Excellence (NICE). The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence data removed and replaced by the statement ‘commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.