Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/60/26. The contractual start date was in May 2009. The draft report began editorial review in December 2012 and was accepted for publication in November 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Ashby et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Venous leg ulcers
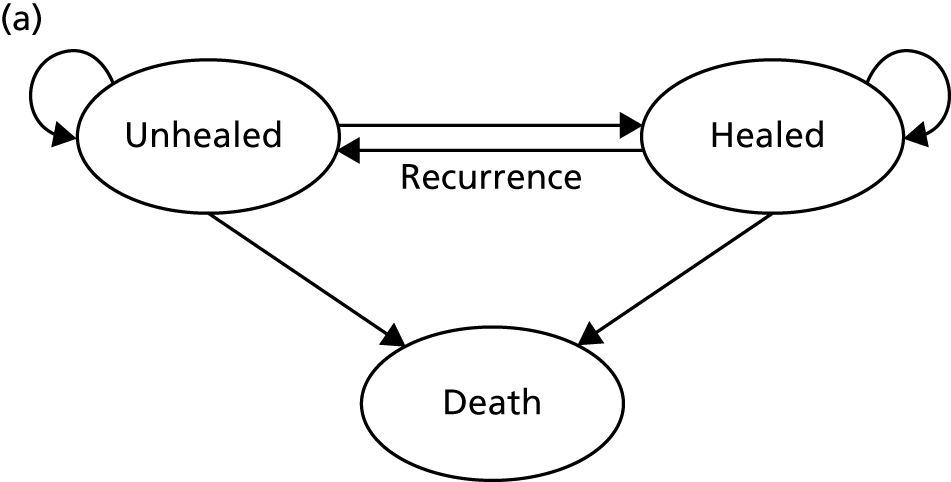
Venous leg ulcers are chronic wounds that generally occur within the gaiter region of the leg as a consequence of venous insufficiency. 1 The underlying venous insufficiency and associated venous hypertension are generally caused by venous valve dysfunction, deep vein occlusion or failure of the calf muscle pump. 2,3 Venous leg ulceration typically presents as repeated cycles of ulceration, healing and recurrence, with ulcers typically taking weeks or months to heal. 1,4,5 Once healed, 12-month recurrence rates have been estimated at between 18% and 28%. 6,7
Venous leg ulcers are distressing to patients, painful, prone to infection, malodorous and have a severe negative impact upon patients’ mobility and quality of life. 8,9 These wounds are one of the most prevalent chronic wound types in the UK, with an estimated point prevalence of 0.16%. 10 There is a progressive increase in venous leg ulceration with age and the annual UK prevalence in people of > 65 years is estimated at 1.7%. 11
Venous leg ulcers are costly to treat. In 2004, the Healthcare Commission estimated annual UK NHS leg ulcer treatment costs of £300–600M. 12 More recent data from UK venous leg ulcer studies indicates the annual cost of treating one venous leg ulcer episode to be approximately £1300,7,13 with treatment costs rising as ulcers increase in size and duration. 14,15 In the UK, most leg ulcer patients are treated in the community,16,17 and it is the nursing time that is associated with frequent treatment consultations that drives these high costs. 14,18 The significant morbidity, high prevalence and economic burden associated with the treatment of venous leg ulceration1 highlights the need to identify the most clinically effective and cost-effective treatments for these wounds.
Compression for venous leg ulcers
The management of venous leg ulcers aims to (1) provide a wound environment that supports healing while avoiding excess moisture and tissue maceration and (2) improve venous return. Although there are many types of wound dressings used in the management of venous leg ulcers, there is an absence of evidence for their relative effectiveness. 19 In contrast, evidence shows that compression is an effective treatment for venous leg ulcers, as is recommended by major UK clinical guidelines for first-line use. 20,21 Compression works by applying pressure to the leg, which may decrease vein diameter and improve valve function thus increasing the blood flow towards the heart and reducing venous reflux. 22,23 Compression is normally applied to the lower leg with the most pressure exerted at the ankle, gradually lessening towards the knee. 21
Compression can be applied in a number of ways, most commonly using bandages or hosiery (stockings); there are many different bandaging and hosiery systems available for use. The relative effectiveness of these various systems on ulcer healing is summarised in a detailed systematic review reporting 59 comparisons from a total of 4321 randomised controlled trial (RCT) participants. 19 The review concluded that multicomponent systems delivering ‘high’ compression (commonly recognised as delivering 40 mmHg at the ankle) are most effective in terms of healing venous leg ulcers.
Multicomponent compression bandage systems
In the UK, the most widely used multicomponent compression bandage system is the four-layer bandage (4LB). The short-stretch bandage (SSB) is also widely used. Both systems were compared in VenUS (Venous Ulcer Study) I, which randomised 387 participants with venous leg ulcers. 7 The study found that the 4LB significantly reduced time to healing [median survival time 92 days in the 4LB group compared with 126 days in the SSB group: adjusted hazard ratio (HR) 1.33, 95% confidence interval (CI) 1.05 to 1.67]. This study finding was supported in an individual patient data (IPD) analysis of all relevant RCTs,24 in which the 4LB was more effective in terms of ulcer healing than the short-stretch system (adjusted HR 1.31, 95% CI 1.09 to 1.58). Thus, the 4LB is considered a gold standard treatment for venous leg ulcers. However, it is important to note that good clinical outcomes from bandaging (in terms of ulcer healing) rely heavily on nurses’ application skills and patient concordance in wearing compression continuously. Application and concordance are a particular issue for the 4LB, which consists of a wool component plus three subsequent bandage components, making the final compression system time-consuming to apply and bulky. This bulk may impact on concordance by reducing mobility, making shoes difficult to wear and causing discomfort. 25
Compression hosiery
Compression hosiery for treating venous leg ulcers
Single-layer compression hosiery can be used to treat venous leg ulcers – there are three UK standard classes of hosiery that deliver increasing levels of compression: class I (14–17 mmHg); class II (18–24 mmHg) and class III (25–35 mmHg). Thus, no standard single hosiery achieves 40 mmHg compression at the ankle. Furthermore, the Class III stocking has been noted as being difficult to apply as it can be tight26,27 and, anecdotally, application can also be painful for patients. Recently, new two-layer compression hosiery systems (referred to hereafter as ‘HH’) have been marketed in the form of two stockings which, when worn simultaneously, are designed to provide a total of 40 mmHg compression. Furthermore, several of these stocking systems have been designed with a smooth first layer (or understocking) providing light compression over which a second overstocking (i.e. UK class II or III, depending on the understocking) slips on. In fact there may be increased potential for patients with sufficient mobility and dexterity (using newly marketed applicator devices) to remove and reapply the stockings themselves.
Five published RCTs28–32 have compared compression hosiery (minimum of 25 mmHg compression at the ankle) with compression bandaging for healing venous leg ulcers (Table 1).
| Author (year), country, n recruited | Details of hosiery group | Details of bandage groupa | Duration of follow-up | Proportion of participants with healed venous leg ulcers |
|---|---|---|---|---|
| Junger 2004,28 Germany and the Netherlands n = 121 |
Bauerfeind VenoTrain® ulcertec (Birmingham, UK) 39 mmHg |
SSB: Roselastic® S, Karl Otto Braun KG (Wolfstein, Germany) | 12 weeks | Hosiery = 48% (29/61) |
| Bandage = 32% (19/60) | ||||
| p = 0.01 | ||||
| Polignano 2004,29 Italy n = 56 |
ConvaTec SurePress® Comfort 35 mmHg |
SSB: Comprilan®, Smith & Nephew (Hull, UK) | 12 weeks | Hosiery = 44% (12/27) |
| Bandage = 17% (5/29) | ||||
| p = 0.03 | ||||
| Mariani 2008,30 Italy n = 60 |
Sigvaris® Ulcer X (Andover, UK) 39 mmHg |
SSB: (details not reported) | 16 weeks | Hosiery = 83% (25/30) |
| Bandage = 70% (21/30) | ||||
| p = 0.01 | ||||
| Taradaj 2009,31 Poland n = 80 |
Sigvaris® 702 (Andover, UK) 25–32 mmHg |
SSB: (details not reported) | 2 months | Hosiery = 37.5% (15/40) |
| Bandage = 12.5.0% (5/40) | ||||
| p ≤ 0.001 | ||||
| Finlayson 2012,32 Australia n = 103 |
Class III system (details not reported) 30–35 mmHg |
4LB (details not reported) | 24 weeks | Hosiery: 72% (31/43) |
| Four layer: 84% (38/45)b | ||||
| p = 0.24 |
Four of these RCTs compared compression hosiery with the SSB29–32 and one compared compression hosiery with the 4LB. 32 None of the compression hosiery products evaluated was the more recently developed HH systems.
Findings for the compression hosiery compared with SSB have been summarised in a recently updated systematic review. 19 Briefly, of the four RCTs,29–32 three were identified as being at high risk of bias and one at unclear risk of bias. Fixed-effects meta-analysis of ulcer-healed data from all four RCTs found that significantly more ulcers healed in the compression hosiery group than in the bandage group at between 2 and 4 months’ follow-up, although heterogeneity between studies was high [relative risk (RR) = 1.62, 95% CI 1.26 to 2.10, I2 = 60%]. A random-effects model also suggested that, on average, significantly more ulcers healed with compression hosiery (RR = 1.66, 95% CI 1.07 to 2.58).
One RCT32 has compared compression hosiery with the 4LB for healing venous leg ulcers. As this study32 was recently published and is not included in the updated systematic review it is summarised here. Finlayson et al. 32 compared a single class III compression stocking with the 4LB in 103 participants with venous leg ulcers. It is important to note that there may have been a difference in the level of compression received between trial groups, as the compression hosiery was recorded as delivering 30−35 mmHg, whereas the 4LB aims to deliver up to 40 mmHg of compression at the ankle.
Upon request, the author provided outcome data regarding ulcer healing for 98 participants for 24 weeks of follow-up. In total, 72% (31/43) of compression hosiery-treated ulcers healed by 24 weeks compared with 84% (38/45) of 4LB-treated ulcers [RR 0.85, 95% CI 0.68 to 1.07 (no statistically significant difference)]. However, the adjusted HR (4LB vs. compression hosiery) reported was 2.1 (95% CI 1.4 to 4.3), suggesting that those in the 4LB group had twice the hazard of healing compared with the hosiery group, with this finding being statistically significant.
Compression hosiery for preventing recurrence of venous leg ulcers
Venous leg ulcers occur as a result of underlying venous disease. Thus, once an ulcer has healed, if compression therapy ceases (resulting in the re-establishment of high venous pressures) then ulcer recurrence might be expected. Current guidelines state that ‘Below knee graduated compression hosiery is recommended to prevent recurrence of venous leg ulcer (sic) in patients where leg ulcer healing has been achieved’ (Scottish Intercollegiate Guidelines Network 2010, p. 4). 21 This guidance is predominantly based on three previous RCTs,33–35 summarised in a key review,36 where no RCT data were pooled. In summary, one RCT (n = 153) reported statistically significant evidence that offer of a class III compression stocking post healing prevented recurrence of venous leg ulcers compared with no compression at 6 months post healing (RR 0.46, 95% CI 0.27 to 0.76; p = 0.003). 33 However, the review authors judged this RCT as being at high risk of attrition bias. 36 Two further RCTs34,35 (n = 300 and n = 338) compared a UK class II stocking with a UK class III stocking in preventing venous ulcer recurrence; both studies were classed as being at possible high risk of bias owing to non-blinded outcome assessment for ulcer recurrence. Nelson et al. 35 reported that there was no evidence of a difference between the class II and III stockings in terms of recurrence at 5 years (RR = 0.82, 95% CI 0.61 to 1.12; p = 0.22). They also reported that compliance was higher with the class II stocking. Conversely, Milic et al. 34 reported that allocation to receive a class III stocking resulted in a significant reduction in ulcer recurrence when compared with a class II stocking, with no difference in compliance (RR = 0.57, 95% CI 0.39 to 0.81; p = 0.002).
Investigating compression treatments for venous leg ulcers
There is limited evidence for the effectiveness of HH in terms of ulcer healing. However if, because of its reduced bulk, this treatment can easily be worn with shoes and potentially enhance ankle/leg mobility and patient compliance while also delivering a standardised level of compression (as bandager skill is not an issue) and being easy to apply it may increase ulcer healing rates. Further potential benefits worth investigating are the opportunity for patients to self care, leading to a possible reduction in nurse consultations and the reusability of stockings compared with the disposable bandages. However, a pragmatic trial is required, as it is plausible that HH may not yield improvements if patients do not like the treatment, they remove and do not reapply the stockings, and/or the compression delivered does not endure sufficiently after washing and reapplying the stockings over several months.
At the time this study was commissioned, our community nurse coapplicants had identified this as an important area of clinical and economic uncertainty at a time when HH was being marketed as an alternative to bandaging.
Finally, as noted above (see Compression hosiery for preventing recurrence of venous leg ulcers), there is some evidence that the use of compression hosiery after healing prevents recurrence of venous leg ulcers. 37 In evaluating HH it seems relevant to investigate whether the offer of this treatment has any impact, post healing, on ulcer recurrence, for example if those wearing hosiery as a treatment carry on wearing it as a maintenance therapy.
Facilitating decision-making regarding compression treatments for treating venous leg ulcers
Although the 4LB and HH are compression treatments of relevance to decision-makers, there are several alternative compression systems available that nurses (and patients) may select, including the SSB system and the two-layer bandage (2LB) system (bottom component with cohesive bandage top component). It is therefore important that decision-makers consider all available evidence for all competing alternatives when making treatment decisions, not just the evidence from a single RCT. RCTs are designed to compare two or more alternative treatments; however, it is often not practical for one RCT to compare all available treatment options. This means that for a decision-maker the information provided by head-to-head trial comparisons can be limited and partial, i.e. we would like to know which treatment option is the most clinically effective and/or cost-effective from among all treatments of interest. The limitations of head-to-head trial comparisons can be overcome if available evidence from multiple RCTs can be considered collectively.
Thus, although a trial comparing any two compression treatments is very important, the value to clinical (and societal) decision-making of assessment using all trial findings from all relevant trials regarding the use of all high-compression treatments must also be recognised. However, the information from the trials must be further evidence synthesised together using formal methods such as mixed-treatment comparison (MTC) meta-analysis.
Mixed-treatment comparisons
Mixed-treatment comparison meta-analysis (also known as network meta-analysis) extends meta-analytic methods by enabling the simultaneous comparison of multiple interventions in a single model. 38,39
Mixed-treatment comparisons allow eligible RCTs to be included when they are linked by one or more common comparator(s) in a network of evidence. 40 As an example, assume three relevant treatments (A, B and C) have been evaluated in two RCTs: one comparing A with B, and another B with C. A network can be defined linking A to B to C. By linking RCT evidence, a consistent evidence base is created and derived relative treatment effect estimates may be informed by direct, indirect or both direct and indirect data. Indirect data can produce effectiveness estimates for pairs of treatments that have not been compared in head-to-head trials. In our example this means inferences over A vs. C can be generated using the indirect evidence available through the network.
In practice, where multiple RCTs inform multiple network links, the synthesis can become complex, as direct and indirect evidence contribute to estimating relative effectiveness for specific comparisons. However, by simultaneously using all (direct and indirect) evidence forming the network, uncertainty is appropriately considered for the comparisons of interest. Additionally, this approach provides an opportunity for formal assessment of consistency between direct and indirect evidence. In all cases, the validity of these methods depends on certain assumptions being held – some are common to standard pairwise meta-analysis (e.g. ‘similarity’ between included trials) and others relate to the ‘consistency’ between the direct and the indirect estimates, which can be assessed within the network. In the presence of heterogeneity, incorporating information on patients’ characteristics in the synthesis model has been shown to improve estimates and, by doing so, possibly resolve inconsistencies. 37,41,42
Decision-analytic modelling
Although MTCs are able to synthesise all RCT data, beyond trials, the relative cost-effectiveness of all relevant treatments for a condition can be investigated in a decision-analytic model. Though within-trial economic analysis offers the opportunity to evaluate cost-effectiveness in participants who contribute resource-use data alongside effectiveness data, such analyses are also limited by the scope of the RCT in which they sit. Decision-analytic models provide a structure within which both RCT and non-RCT evidence from a range of sources can be synthesised to describe a specific problem, and through this framework overall costs and effects can be estimated. The advantage of using this framework is that cost-effectiveness results can be based on all available evidence, across the full range of possible alternative interventions and clinical strategies over a relevant time horizon, and specific patient groups can be analysed separately.
Summary of main points
Venous leg ulcers are common chronic wounds that have a severe negative impact upon patients’ quality of life and health. The treatment of these wounds costs the NHS hundreds of millions of pounds per year. The application of compression is known to assist in the healing of venous leg ulceration. The 4LB (which delivers 40 mmHg of compression at the ankle) is the current gold standard treatment for healing venous leg ulcers and is recommended by major UK clinical guidelines for first-line use.
Two-layer hosiery has been designed to deliver the same amount of compression as the 4LB, with the potential advantages of being easier for patients to wear and apply. These factors may increase patient concordance with compression and thus improve ulcer-healing rates. There is some evidence from RCTs that compression hosiery may be effective in healing venous leg ulcers when compared with the SSB. 28–30 However, only one RCT32 has compared compression hosiery with the 4LB, although the compression hosiery used was not a two-layer system designed to deliver up to 40 mmHg at the ankle.
Report structure
This work is presented in four parts:
-
Part I relates to the conduct of a RCT comparing HH with the 4LB in people with venous leg ulcers.
-
Part II relates to the conduct of a MTC meta-analysis of high-compression treatments for venous leg ulcers.
-
Part III relates to the conduct of a decision-analytic model to assess the cost-effectiveness of high-compression treatments for venous leg ulcers.
-
Part IV is an overall discussion of the work presented.
Part I Venous leg Ulcer Study IV (VenUS IV) trial
We undertook a RCT to evaluate the clinical effectiveness and cost-effectiveness of HH in the treatment of venous leg ulcers.
Chapter 2 Research objectives
To compare the clinical effectiveness and cost-effectiveness of compression hosiery with the 4LB in terms of time to complete healing of venous leg ulcers, cost of treatment, health-related quality of life and participant concordance to treatment.
Primary objective
To compare the effects of compression hosiery with the 4LB on time to healing of the reference ulcer (the largest eligible venous leg ulcer).
Secondary objectives
To compare the:
-
cost-effectiveness of compression hosiery with the 4LB
-
effects of compression hosiery with the 4LB on the time to healing of the reference leg
-
effects of compression hosiery and the 4LB on health-related quality of life
-
effects of compression hosiery and the 4LB on participant concordance to treatment
-
effects of compression hosiery and the 4LB on reported adverse events
-
effects of compression hosiery and the 4LB on ulcer recurrence.
Chapter 3 Methods
Trial design
We conducted a pragmatic, multicentred, two-arm, parallel, open RCT with equal randomisation. Participants with venous leg ulcers were randomised (1 : 1) to receive either:
-
the 4LB (aiming to deliver 40 mmHg at the ankle), or
-
the HH (consisting of a two-layer compression stocking system aiming to deliver 40 mmHg at the ankle).
Approvals obtained
This study was approved by the University of York Health Sciences Research Governance Committee on 8 June 2009 and by the Northern and Yorkshire Research Ethics Committee (REC) on 26 September 2009 (REC reference number 09/H0903/25). The North East Yorkshire and Northern Lincolnshire Comprehensive Local Research Network completed their global checks on 21 October 2009; thereafter research management and governance approval was obtained for each trial centre (see Appendix 1). This trial was assigned the International Standard Randomised Controlled Trial Number of ISRCTN49373072.
Duration of follow-up
Planned participant follow-up was for a maximum of 12 months. However, when it became necessary to extend the trial recruitment phase the participants who were recruited during the final 12 months of recruitment (from 30 June 2012) were followed up for between 4 and 12 months.
Trial centres
The study was conducted by 34 study centres within England and Northern Ireland (see Appendix 2). Centres were recruited throughout the trial. Centres recruited participants from various sources, including community nurse teams/services, general practitioner (GP) practices, leg ulcer clinics (community and outpatient), tissue viability clinics/services and wound clinics.
Participant eligibility
Inclusion criteria
People for whom all of the following criteria applied:
-
At least one venous leg ulcer. A venous leg ulcer was defined as any break in the skin which had either (1) been present for > 6 weeks or (2) occurred in a person with a history of venous leg ulceration. Ulcers were considered purely venous if, clinically, no other aetiology was suspected. The ulcer was required to be venous in appearance (i.e. moist, shallow, of an irregular shape) and was to lie wholly or partially within the gaiter region of the leg.
-
An ankle–brachial pressure index (ABPI) of ≥ 0.8, taken within the previous 3 months.
-
Able and willing to tolerate high compression.
-
Aged ≥ 18 years.
Exclusion criteria
Potential participants were excluded if they fulfilled any of the following exclusion criteria:
-
An ABPI of > 1.20 (taken with the previous 3 months) and, in the treating nurses’ clinical judgement and/or according to local guidelines, the potential participant should not receive high compression.
-
A leg ulcer of non-venous aetiology (i.e. arterial).
-
Wound exudate levels too high for the use of HH (decision made according to the nurses’ clinical judgement).
-
Unwilling to give informed consent to participate within this trial.
-
Unable to give informed consent to participate within this trial.
-
Currently in another study evaluating leg ulcer therapies.
-
A known allergy to any trial product.
-
Gross leg oedema.
-
Previously been recruited into this trial.
-
Another reason that excluded them from participating within this trial (decision made according to the nurses’ clinical judgement).
Sample size
We estimated that 489 participants were required for Venous leg Ulcer Study IV (VenUS IV). The sample size calculation was based upon VenUS I,7 which assessed the clinical effectiveness and cost-effectiveness of the 4LB compared with the SSB in people with venous leg ulcers. In VenUS I,7 386 participants were recruited over 20 months from nine UK centres. The primary outcome measure was time to healing of the reference leg. The HR was 1.33 (95% CI 1.05 to 1.67) and the median survival times were 92 days for the 4LB group and 126 days for SSB.
For VenUS IV, the primary outcome was also time to healing. However, the aim was to estimate the size of the difference between the compression systems, rather than to look for a difference of any given size. In VenUS I,7 the upper 95% CI of 1.67 was a 25% increase in the point estimate of 1.33. Therefore, a trial with similar size, population, outcome and treatments was also expected to allow estimation of the HR to within 25%. Power calculations suggested that 400 participants, a median survival in the control group of 100 days and follow-up of one year would provide 90% power to detect an increase in median time to healing of 41 days and a decrease in the HR for healing to 0.72, or a decrease in median time to healing of 72 days and an increase in the HR to 1.42. Assuming 10% attrition, this meant 444 participants were required.
The original Venous Ulcer Study (VenUS I7) analysis treated centre as a fixed effect, after checking for a centre by treatment interaction, which was not significant. Using log area of the original ulcer as a covariate and mobility as a three-level factor, the standard error (SE) of the log HR for the treatment effect was 0.119, with centre as a fixed effect. However, for the purpose of the current sample size calculation, the possibility of centre effect was also considered by assuming robust SEs with centre as a cluster. This inflated the variance compared with a fixed-effects model. In this case the SE was 0.129 (making the treatment effect not significant; p = 0.07). The square of the ratio of these SEs was 1.19. Therefore, if centre effects in VenUS IV were similar to those in VenUS I7 then we estimated that the sample size would need to be increased by 19% to maintain the same power.
However, in terms of potential centre effects we noted that in VenUS I7 there were reasons to suspect that there would be variation in bandaging skill, with some centres having prior experience in at least one type of treatment being compared. In VenUS IV, the intervention (HH) did not require special skill to apply and all centres were expected to be experienced in use of the standard treatment (4LB), although some variation in its application was possible. Because of the differences between VenUS I7 and IV, we expected there to be less variation between centres in VenUS IV than in VenUS I. 7 Thus, in order to look for centre effects in the VenUS IV analyses, adjusting for them using robust SEs and accounting for the loss of power this may produce, we estimated that the sample size be inflated by 10% to 489 participants.
Recruitment into the trial
Patients were screened for eligibility by study research nurses or designated health-care professionals, according to the trial inclusion and exclusion criteria. Patients were screened from a wide range of sources: community nurse teams/service, GP practices, joint tissue viability/vascular clinics, leg ulcer clinics, outpatient clinics, outpatient leg ulcer clinics, tissue viability clinics/services, treatment room clinics and wound clinics. The reason(s) for a patient’s ineligibility were recorded (see Appendix 3). When a patient’s sole reason for exclusion was high wound exudate levels or gross leg oedema, nurses were encouraged to rescreen for trial eligibility once exudate levels or oedema had been managed. Decisions regarding the management of exudate and/or oedema were left to the discretion of the treating health professional.
Those patients who fulfilled all of the eligibility criteria were approached by the study research nurse and provided with both verbal and written information about the trial in a face-to-face meeting (see Appendix 4, Patient information sheet). Patients were then given adequate time to consider participation within the trial. Study research nurses then obtained voluntary verbal assent and full written consent from those patients who wished to enter the trial (see Appendix 4, Consent form).
Baseline assessment
After obtaining written consent and verbal assent from the participant, baseline data were collected by the study research nurse using the patient record form (see Appendix 5) and from the participant via the participant baseline questionnaire (see Appendix 6).
Participant details
Participants’ contact details (name, address, telephone numbers and e-mail address), date of birth and GP details (name of GP, name of GP surgery and address) were recorded.
Ulcer history and assessment
The most recent ABPI measurement and the date it was taken were recorded. Also recorded were the total number of ulcer episodes (n); time since development of the first ulcer (years and/or months); duration of the reference ulcer (years and/or months) and duration of oldest ulcer (years and/or months).
To measure ulcer area, a tracing of the reference ulcer (the largest eligible ulcer) and all other ulcers on the reference leg (the leg that contained the reference ulcer) was taken using a fine-nibbed marker pen on to a wound measurement grid composed of 1-cm2 squares (P12v2, ConvaTec, Uxbridge, Middlesex, UK). Ulcer area was determined by totalling the number of squares and/or partial squares on the grid contained within the traced ulcer area. At the end of the trial, ulcer area was calculated from the wound grid by the use of a software program (Mouseyes, version 3.1, Dr Robert John Taylor, Salford, UK). 43
All ulcers on both legs were drawn onto a leg diagram and the reference ulcer was clearly labelled using an identification code. The reference ulcer was coded as either R1 (located on the right leg) or L1 (located on the left leg). If there was more than one ulcer, the ulcers were coded according to descending area – the next largest as R2 (if located on the right leg) or L2 (if located on the left leg) and so on.
Participant mobility, anthropometry and glycated haemoglobin measurements
The level of participant mobility (walking and ankle mobility) was noted as was the participant’s height (feet/inches or centimetres), weight (stones/pounds or kilograms) and ankle circumference (centimetres). Body mass index (BMI, kg/m2) was calculated using the formula ‘weight (kg) divided by [height (m)]2’. If the participant was diabetic then their glycated haemoglobin (%) was recorded with the measurement date. However, as the trial progressed, some centres found it difficult to obtain this measurement. As it was not considered necessary for the trial analyses, the collection of this measurement was stopped following a decision by the Trial Management Group (TMG).
Current treatments received
Participants’ current treatment(s) for their venous leg ulcer(s) were recorded.
Treatment preference
Each participant’s trial treatment preference (i.e. no treatment preference, prefer to receive HH or prefer to receive the 4LB) was recorded.
Digital photographs
Study research nurses took a digital photograph of the reference ulcer and reference leg using a Nikon digital camera (Coolpix L20 or Coolpix L22, Nikon Corporation, Tokyo, Japan) according to the protocol developed for taking digital photographs during the trial (see Appendix 7). These anonymised photographs were uploaded onto an online management system and sent securely to the York Trials Unit (University of York).
Health-related quality of life/health utility
Participants completed a baseline questionnaire consisting of 12-Item Short Form Health Survey (SF-12) (Short Form questionnaire-12 items, version 2, standard recall, QualityMetric, Lincoln, RI, USA) and the EQ-5D™ (European Quality of Life-5 dimensions questionnaire, EuroQol Group, Rotterdam, The Netherlands).
The SF-12 measures eight health areas, which can be used to calculate a Physical Component Summary (PCS) score (based upon physical functioning, role limitations due to physical health, bodily pain and perceptions of general health) and a Mental Component Summary (MCS) score (based upon vitality, social functioning and role limitations owing to emotional health and mental health). The SF-12 is well validated in a variety of UK populations, including older people and leg ulcer patients. 44,45
The EQ-5D questionnaire measures five domains of health and provides an assessment of mobility, individual’s ability to self care, ability to perform usual activities, and evaluation of pain/discomfort and anxiety/depression. It is a widely recognised and validated generic measure of health utility and has been assessed for acceptability and validity in patients with venous leg ulcers,44,46–48 as well as being validated in other patient groups. 49–57
Ulcer-related pain
Participants were asked to rate the intensity of any leg ulcer-related pain that they had experienced in the previous 24 hours using the 21-point Box Scale (BS-21). The BS-21 pain scale was divided into units of five, and ranged from a value of 0 (no pain) to 100 (the worst pain imaginable).
Resource use
Participants provided details of any care received from the NHS within the past 3 months, recording the number of consultations the participants had with health professionals at different locations. Participants were asked to record details according to whether the consultation was related to their leg ulcer or a different reason.
Randomisation
Randomisation was carried out using an independent, remote telephone randomisation service (freephone telephone number), based at the York Trials Unit, or via an online randomisation service (URL: www.yorkrand.com/). Randomisation was conducted using a prevalidated computer program to ensure complete allocation concealment. Randomisation was stratified by ulcer duration (≤ 6 months or > 6 months) and ulcer area (≤ 5 cm2 and > 5 cm2) using permuted blocks (block sizes four and six), as these variables are known predictors of healing. 58 The computerised randomisation system was checked periodically during the trial following standard operating procedures.
The study research nurse called a freephone telephone number to speak to an operator independent to the trial at the York Trials Unit, or accessed the online randomisation service and provided details of the participant. The study research nurse was informed of the participant’s allocated trial treatment (HH or 4LB) and given a unique identification number, which was used to identify the participant throughout the trial.
Trial interventions
Control group
Participants in the control group were allocated to receive the 4LB (standard/usual care). Nurses were allowed to choose which 4LB system the participant should receive, as long as it was designed to deliver 40 mmHg at the ankle and adhered to recommendations shown in Table 2. Examples of key 4LB components are shown in Table 3. The treatment was applied according to standard practice, either by the participant’s usual treatment nurse or by a study research nurse.
| Ankle circumference | Layer 1 | Layer 2 | Layer 3 | Layer 4 |
|---|---|---|---|---|
| < 18 cm | Wool to make leg circumference a minimum of 18 cm | Crepe bandage | Class 3a bandage | Cohesive bandage, class 3b |
| 18–25 cm | Wool | Crepe bandage | Class 3a bandage | Cohesive bandage, class 3b |
| 25–30 cm | Wool | Class 3c bandage | Cohesive bandage | N/A |
| > 30 cm | Wool | Class 3a bandage | Class 3c bandage | Cohesive bandage, class 3b |
| Wool | K-Soft® (Urgo, Loughborough, UK); Profore® #1 (Smith & Nephew) |
| Light support bandage | K-Lite® (Urgo); Profore #2 (Smith & Nephew) |
| Class 3a bandage | K-Plus® (Urgo); K-Plus® Long (Urgo); Profore #3 (Smith & Nephew); Elset® (Mölnlycke, Dunstable, UK); CliniPlus (CliniSupplies, Harrow, UK) |
| Class 3b bandage | Ko-Flex® (Urgo); Profore #4 (Smith & Nephew); Coban® (3M, Bracknell, UK); Ultra Fast® (Robinsons, Worksop, UK) |
| Class 3c bandage | Mölnlycke Setopress®; Tensopress® (Smith & Nephew); Profore+® (Smith & Nephew) |
Intervention group
Participants in the intervention group were allocated to receive HH. This was a two-layer compression hosiery kit, consisting of an understocking (or liner) and an overstocking, worn over the understocking. The two stockings were designed to deliver sustained, graduated compression of up to 40 mmHg (and no less than 35 mmHg) at the ankle (Table 4).
| Brand | Understocking (mmHg) | Overstocking (mmHg) | Total compressiona (mmHg) | Made-to-measure service available |
|---|---|---|---|---|
| Carolon Multi-Layer Compression System (H&R Healthcare, Hull, UK) | 16–18 | 19–22 | 35–40 | No |
| Clini Duo40 (CliniSupplies) | 10 | 30 | 40 | No |
| Mediven Ulcer kit (medi UK, Hereford, UK) | 20 | 20 | 40 | Yes |
| Activa leg ulcer hosiery kit (Activa Healthcare, Burton-on-Trent, UK) | 10 | 25–35 | 35–45 | Yes |
| Jobst UlcerCARE (BSN medical, Hull, UK) | 10 | 30 | 40 | Yes |
| VenoTrain ulcertec (Bauerfeind UK) | 10 | 30 | 40 | No |
The size of the HH kit used depended upon the participant’s ankle circumference (cm), calf circumference (cm) and/or foot length (cm); nurses consulted product-specific measurement tables to ensure that the participants received the correctly sized kit. Made-to-measure HH was obtained for participants who require this. HH was applied and worn according to manufacturer’s instructions. Participants were told they could remove the overstocking only at night, if deemed necessary by the treating nurse.
Participants were expected to receive their allocated trial treatment immediately after randomisation. However, where a treatment was not available for immediate application (e.g. if a participant required a made-to-measure HH kit), the participant was treated with an appropriate treatment in the interim, as dictated by the treating nurse, until the allocated trial treatment became available. The interim treatment received was recorded in a treatment log (see Appendix 5, Treatment log: trial dressing log booklet).
Participant follow-up
Appendix 8 shows a summary flow chart of participant follow-up processes.
Participants were allocated their trial treatment and received this treatment until (1) they were no longer able to continue receiving their allocated trial treatment and instead changed to another treatment that replaced the allocated compression treatment (designated the ‘non-trial’ treatment); (2) their reference leg healed and treatment was no longer required; (3) they were lost to follow-up; or (4) they died.
During the trial, every nurse consultation for treatment of the reference leg was recorded in a treatment log until healing of the reference leg, along with location of consultations, and dressing(s) and treatment(s) applied (see Appendix 5, dressing log booklets).
Trial completion and exit
Participants were deemed to have exited the trial when they:
-
withdrew consent [wished to exit the trial (ulcer unhealed) and have no further data collected]
-
had been in the trial for 12 months post randomisation
-
had reached the end of the trial
-
died
-
were lost to follow-up
-
had another reason for exit, according to the clinical judgement of the study research nurse.
Nurses were required to complete a participant event form (see Appendix 5), giving the main reason for the participant’s exit from the trial. No further clinical data were collected from these participants and they were not sent any further participant questionnaires.
Rather than completely withdrawing from the trial, participants could opt to selectively withdraw from (1) clinical data collection (i.e. still remain in the trial and receive participant questionnaires only) or (2) receiving participant-completed questionnaires (i.e. still remain in the trial and allow clinical data collection to continue).
Measurement and verification of primary outcome measure
Time to healing of the reference ulcer (blinded)
The primary outcome measure of this study was time to healing of the reference ulcer. Healing was defined as complete epithelial cover in the absence of a scab (eschar) with no dressing required. When the treating nurse considered the reference ulcer to have healed, a digital photograph was taken of the healed reference ulcer site. Additional photographs were then taken of the same site, once per week for 4 weeks; photographs were taken regardless of whether the reference ulcer was considered to have recurred during this time period. Digital photographs were uploaded onto a secure server at the York Trials Unit using a secure online management system.
All digital images were assessed by two experienced Tissue Viability Specialist Nurses, who were blinded to treatment allocation. Independently, assessors determined whether they considered a participant’s reference ulcer to have healed, not healed or were unsure whether healing had occurred. When an ulcer was deemed to have healed, the appropriate date (based on date of photograph in which healing was recorded) was then taken to be the date of healing for the reference ulcer. A set of decision rules were made for resolving disagreements between assessors (see Appendix 9). In cases when one assessor considered the ulcer to have healed and the second assessor considered the ulcer unhealed, a third assessor was consulted, who determined whether healing had occurred.
Measurement and verification of secondary outcome measures
Time to healing of the reference ulcer (unblinded)
Although the primary outcome measure for this trial was time to healing of the reference ulcer (as determined by assessors blinded to treatment allocation), time to healing of the reference ulcer as determined by the treating nurse was used as a secondary outcome measure. When the treating nurse considered the reference ulcer to have healed, she/he telephoned the York Trials Unit randomisation line to report the date of healing.
Time to ulcer-free reference leg
Nurses reported the date on which they thought the reference leg was ulcer free, and this was reported by telephone to the York Trials Unit randomisation line; this information was also captured via the ulcer healed form (see Appendix 5).
Health-related quality of life/utility and resource use
Post randomisation, participants’ health-related quality of life and resource use were measured at 3, 6, 9 and 12 months using postal questionnaires. Each quarterly questionnaire was identical in content and contained the same health-related quality-of-life/utility instruments as the baseline questionnaire (EQ-5D and SF-12) and the same tools for measuring ulcer-related pain (BS-21 and verbal descriptor pain scales) and resource use (see Appendix 5).
Post-randomisation questionnaires were posted to participants from the York Trials Unit, along with a pre-addressed and prepaid envelope. Where necessary, reminder letters were sent by post to participants at 2 and 4 weeks had questionnaires not been returned. A systematic review assessing ways to increase participant response to postal questionnaires59 reported that response rates could be almost doubled by the use of monetary incentives (odds ratio 1.99, 95% CI 1.81 to 2.18) and could also be increased if the incentive was unconditional. Participants were therefore sent £5 with their final questionnaire and were informed that this was an unconditional token of appreciation for the time they had taken to complete questionnaires throughout the study.
Briefly, we also collected disease-specific health-related quality-of-life data using the VEINES quality-of-life questionnaire (VEINES-QOL) instrument at baseline and 4 months. These data were collected as part of a substudy that was aiming to conduct a validation study of this measure in patients with venous leg ulcers. This substudy is reported separately; however, we report summary data in this report for reference.
Participant concordance to treatment
In order to monitor treatment concordance and patterns of compression use, study nurses recorded in a treatment log when a participant was no longer receiving their allocated trial treatment but rather was receiving a non-trial treatment, i.e. change to a leg ulcer treatment that was different from that to which the participant was allocated at randomisation and the reason for this change (see Appendix 5, Trial dressing log booklet). Participants were also asked about their opinions and use of their allocated trial treatment via a treatment-specific 1-month participant questionnaire (see Appendix 6).
Ulcer recurrence
One month after the reference leg was reported as healed, nurses undertook a monthly assessment for ulcer recurrence (see Appendix 5, Monthly nurse assessment form); this monthly assessment continued until the participant had exited the trial. Where there had been a recurrence of venous leg ulceration on the reference leg, the date of recurrence was also recorded. To maximise data collection participants with a healed reference leg were also provided with a reference leg-specific postcard (see Appendix 6) to complete if they had a recurrence. This was then returned in a pre-addressed and postage-paid envelope to the York Trials Unit.
Adverse events
An adverse event is defined as any undesirable medical experience occurring to a participant, whether or not considered related to the trial treatment. 60 Adverse events were classified as serious (death, life- or limb-threatening event, hospitalisation required/prolonged, persistent or significant disability/incapacity or other medically important condition) or non-serious (all other adverse events). 60
Nurses were asked to report adverse events (see Appendix 5). The treating nurse was also required to make an assessment regarding the relationship between the adverse event and trial treatment (HH or 4LB) assessed by a set of decision rules (see Appendix 10). All adverse event forms were returned to the York Trials Unit. Serious adverse events (SAEs) were also reported directly to the York Trials Unit via a telephone call to the trial coordinator and/or via fax to the York Trials Unit. Nurses reviewed adverse events until they had resolved (see Appendix 5).
All SAEs were reported to the Trial Steering Committee (TSC), trial centre research and development offices and the North East Yorkshire and Northern Lincolnshire Comprehensive Local Research Network. The local REC was notified of any unexpected SAEs that were considered to be related to trial treatment. Expected events in this study population were defined using previous research data. Participants in previous VenUS trials have been generally elderly (mean ages of 71, 74 and 69 years, respectively, in VenUS I–III7,13,61) and VenUS IV participants were expected to be similar. As one might expect, people with venous leg ulceration have been shown to present with other co-morbidities, including hypertension, congestive heart failure and osteoarthritis;62 these and other medical conditions may require hospitalisation. A small percentage of participants would also be expected to die during the study, as reported in previous VenUS trials. 7,13,61
For analyses, all adverse events were reviewed by the TMG (blinded to allocation), which made the final decision regarding the relationship between an adverse event and trial group. Furthermore, all non-serious adverse events (NSAEs) that were considered to be definitely or probably related to trial treatment were reviewed by a tissue viability research nurse and categorised for analysis (Box 1).
-
Alternative non-trial treatment initiated by participant or another.
-
Bandage or hosiery failure.
-
Bandage or hosiery related pain and/or discomfort.
-
Dryness.
-
Excoriation.
-
Infection.
-
Maceration.
-
Medical event relating to leg.
-
Non-surgical hospitalisation related to leg ulceration.
-
Occurrence of new ulcer.
-
Skin damage.
-
Skin deterioration.
-
Surgical intervention to leg.
-
Ulcer deterioration.
-
Ulcer-related pain.
Clinical analyses
Analyses were conducted following the principles of intention to treat, with all events analysed according to the participant’s original randomised treatment allocation, irrespective of deviation based on non-concordance. Analyses were performed using Stata version 12 (StataCorp, College Station, TX, USA). Treatment effects are presented as HRs together with their 95% CIs; p-values of < 0.05 for two-tailed tests were taken to indicate statistical significance.
Baseline data
All categorical baseline variables were summarised as a frequency (n, %) by treatment group. Continuous variables were summarised using descriptive statistics [n, mean, standard deviation (SD), minimum, maximum, interquartile range (IQR) and median]. No formal statistical analyses were conducted.
Trial completion
Details of trial exit were taken from the Trial Exit form. When an exit form had not been completed, data were derived from the length of time a participant was in the study from randomisation to their last clinical follow-up documenting healing status, together with data on deaths.
Primary analyses
Time to healing of the reference ulcer (blinded)
The primary outcome was time to healing of the reference ulcer, measured in days from date of randomisation. A healed event and date of healing were obtained through central, independent, blinded assessment of digital photographs. The time to reference ulcer healing was right censored in participants who (1) withdrew from the study; (2) were lost to follow-up; (3) died; or (4) reached the end of trial follow-up.
For each interventional group, the distribution of time to ulcer healing was described using Kaplan–Meier survival curves. Treatment differences were evaluated using the Cox proportional hazards (CPH) model adjusted by baseline factors – ulcer area, ulcer duration and participant mobility7,61 – with shared centre frailty effects. 63–65 Ulcer area and duration were logarithmically transformed because these data, as in previous VenUS trials, were highly skewed. Participant mobility was a three-level factor (participant walks freely, walks with difficulty, is immobile).
The shared frailty effect is a random effect in the CPH model, which has a multiplicative effect on the hazard of healing. In this case, the frailties were modelled specific to each centre and thus described the degree of correlation between participants within centres. The need for a centre frailty effect in the model was assessed using a likelihood ratio test that evaluated whether the frailty variance was zero using a 50 : 50 mixture of chi-squared distributions. If a non-significant frailty effect was found then the CPH model would be fitted without centre frailty effects (but still adjusted for baseline factors).
An unadjusted analysis was also performed using the CPH model. Log-rank and Wilcoxon survival comparisons were made, and median time to healing was calculated with 95% CI for each treatment group.
For each CPH model, the proportional hazard assumption was evaluated through inspection of log–log plots and formally by a statistical test using Schoenfeld residuals. 66
Secondary analyses
Time to healing of the reference ulcer (non-blinded)
Time to healing of the reference ulcer, where healing was assessed by nurses during follow-up (i.e. non-blinded time to ulcer healing), was evaluated using the same methods and processes described for the primary analyses with an identical censoring strategy.
Time to ulcer-free reference leg
Time to an ulcer-free reference leg [the leg with the largest eligible ulcer (reference ulcer)] was defined as time from randomisation to the time when the participant was assessed as having an ulcer-free reference leg. The time to reference leg healing was right censored in participants who (1) withdrew from the study; (2) were lost to follow-up, (3) died; or (4) reached the end of trial follow-up – whichever came first. This outcome was analysed like the primary outcome with identical censoring strategy and adjustments.
Health-related quality of life
The SF-12 questionnaires were scored67 and the PCS and MCS scores were summarised with descriptive statistics (n, mean, SD, minimum, maximum, IQR and median) by treatment group at baseline, month 3, month 6, month 9 and month 12. As the PCS and MCS scores were measured longitudinally with time, the relationship between PCS or MCS scores with treatment was evaluated through a linear mixed model (LMM) to account for the dependence of PCS or MCS scores measured within the same participant as described by Fitzmaurice et al. 68 and Verbeke and Molenberghs. 69 In the LMM, the PCS or MCS score was adjusted by ulcer area, ulcer duration, time, centre and participant mobility. To assess whether there were differences between the treatments during time of follow-up, an interaction between treatment and time was tested for inclusion in the models.
The VEINES-QOL questionnaire was scored as T-score, with mean set to 50 and SD to 10. Each sample was standardised to itself, so that only comparisons within the sample are meaningful.
Leg ulcer-related pain
At the same time as completing the SF-12, participants were asked to rate the intensity of any leg ulcer-related pain that they had experienced in the previous 24 hours using a scale from 0 (no pain) to 100 (the worst pain imaginable) in steps of 5 (i.e. there were 20 categories to choose from). These data were presented descriptively as (n, mean, SD, minimum, maximum and median).
Participant use of compression treatments
In the HH group, data were presented regarding the proportion of participants at 1 month from randomisation:
-
wearing their allocated bandaging during the day in each of the four measured categories (everyday/most days/some days/never)
-
wearing one or two layers
-
applying the HH themselves or having it applied by a carer.
For the 4LB group, data were presented regarding the proportion of participants at 1 month from randomisation:
-
wearing their allocated bandaging in each of the four categories (everyday/most days/some days/never)
-
removing their bandages either never or at least once.
Additionally, participant use of treatment was summarised as the proportion of participants changing from their randomised treatment to another treatment (designated the non-trial treatment) within each treatment group.
Adverse events
It was envisaged that the total number of adverse events would be compared by treatment group, considering clustering by participant, using a random-effects Poisson regression model adjusting for ulcer area, ulcer duration, centre and participant mobility. However, if the variability in the data was found to be higher than that modelled (as had been noted in previous studies, VenUS II61 and VenUS III13) then a random-effects negative binomial regression model would be used, adjusting for the same covariates. If a large number of participants reported no adverse events (as found in VenUS III),13 a zero-inflated random-effects Poisson regression model or a zero-inflated random-effects negative binomial regression model70,71 would be fitted to the adverse events data, adjusting for the same covariates. This analytic strategy was repeated for NSAEs and SAEs separately.
Non-serious adverse events and SAEs were further summarised by classification and by treatment group. The blinded assessment of relationship of adverse event to treatment were summarised by treatment group. At the suggestion of the TSC, if the numbers of adverse events per participant were found to be small throughout follow-up then these data would also be analysed using a by random-effects logistic regression, with treatment differences compared adjusting for ulcer area, ulcer duration, centre and participant mobility.
Ulcer recurrence
The proportion of participants having a recurrence of an ulcer on the reference leg, post healing, was presented by treatment group. Time to recurrence was defined as time from an ulcer-free reference leg to date of recurrence, or censored in those who had healed with an ulcer-free reference leg until they were withdrawn from the study, died from any cause or reached the end of the study – whichever came first. This outcome was analysed in the same way as the other survival analyses using a CPH model adjusted for baseline ulcer area, ulcer duration and participant mobility, also testing for shared centre frailty effects. The distribution of time to recurrence from healing was described using Kaplan–Meier survival curves by treatment group.
Sensitivity analyses and handling of missing data
Survival data were assumed to be completely known (data collection was designed to be complete). Adverse event reporting was also assumed to be complete. Missing data on covariates included as adjusting factors in the statistical models were to be assumed missing at random. Sensitivity analyses were carried out:
-
for comparison of results with and without a centre random effect
-
where there were missing data on the covariates, comparison of results from the best model chosen in (1) with and without multiple imputation.
Economic analyses
The economic analysis was conducted in the form of cost-effectiveness and cost–utility analyses using IPD collected during the RCT. In the cost-effectiveness analysis, health benefits were measured as incremental ulcer-free days, whereas incremental quality-adjusted life years (QALYs) were used in the cost–utility analysis. The QALY combines survival and health-related quality of life into a single measure, thereby providing a common currency to enable comparisons across different health conditions and interventions.
The perspective of the economic analysis was that of the NHS and Personal Social Services (PSS) [as recommended by the National Institute for Health and Care Excellence (NICE)]. 72 The time horizon for the analysis was 12 months from the date of randomisation, which was also the maximum duration of follow-up; hence, neither costs nor health benefits (ulcer-free days and QALYs) were discounted. The analysis was conducted in Stata version 12.1.
Details of each constituent component of the economic analysis, i.e. health benefits and costs, and their estimation methods are discussed below (see Resource use and unit costs). This is followed by a description of the statistical methods used. Finally, we detail the methods implemented to presenting cost-effectiveness and cost–utility results and decision uncertainty.
Resource use and unit costs
The cost of resources used for each trial participant was calculated as the product of resources used during the trial follow-up period and the relevant unit costs. Three different elements of resource use were considered in the estimation of ulcer-related treatment costs:
-
use of allocated compression treatments during trial (compression hosiery, 4LB and any other compression treatments used, e.g. while waiting for the trial treatments to become available)
-
use of non-trial treatments (for participants who changed to a non-trial treatment)
-
health-care consultations (visits to/from health-care provider for ulcer-related reasons).
Other treatments, such as primary and secondary contact dressings or skin preparations, were assumed to be used equally across treatment groups and these resources were not included in the economic analyses. Unit costs for the compression treatments were based on the NHS prescription cost data for 2010–11,73 and all values are in British pound sterling (£).
Compression treatments
The number and type of compression treatments used in the trial, and thus costed, were taken from the treatment logs completed by a nurse at each ulcer-related consultation until healing of the reference leg.
For the HH group, data were recorded on the brand of compression hosiery kit from the following choices: mediven, Activa, VenoTrain ulcertec, Jobst and ‘other’. For the ‘other’ category of kits, the unit price was based on the type of kit specified in the text box. When the brand of kit was not available, we assumed it to be Activa, which was the most frequently used kit in this study. Also, the numbers of new understockings and overstockings were recorded where relevant. When only understockings (liners) were given/applied, the cost of liner-only packs was used. For the four-layer group, the type of product was recorded as either a kit or individual bandaging components and unit costs were applied accordingly (Table 5). Made-to-measure kits were costed appropriately. The treatment logs also recorded other treatments and procedures applied to the reference leg during the visit. These included alternate compression treatments used when the randomised treatment was not available for a short period. Delivery of all compression treatments (including made-to-measure hosiery) reported by the nurse in this question were costed and included in the cost analysis.
| Type of dressing | Cost | Source |
|---|---|---|
| HH | ||
| Mediven | Kit £30.43; liner only £16.30 | NHS prescription costs |
| Activa | Kit £21.56; liner only £16.12 | NHS prescription costs |
| Ulcertec | Kit £27.1 | NHS prescription costs |
| Jobst | Kit £29.95; liner only £18.09 | NHS prescription costs |
| Altipress 40, Altimed (Loughborough, UK) | Kit £13.89; liner only £10.99 | NHS prescription costs |
| Carolon | Kit £26.5; liner only £7.33 | NHS prescription costs |
| Clini Duo | Kit £13.07; liner only £10.34 | NHS prescription costs |
| Made to measure | Kit £40.09 | Cost provided by supplier (Actilymph, Activa) |
| Weighted mean cost | Kit £21.54; liner only £15.93 | NHS prescription costs |
| 4LB | ||
| K-Four, Urgo | £6.67 | NHS prescription costs |
| Profore | £8.80 | NHS prescription costs |
| System 4, Mölnlycke | £7.49 | NHS prescription costs |
| Ultra four, Robinson | £5.69 | NHS prescription costs |
| Weighted mean cost | £7.63 | NHS prescription costs |
| Individual components of 4LB | ||
| K-Soft, Urgo | £0.45 | NHS prescription costs |
| K-Lite, Urgo | £0.98 | |
| K-Plus, Urgo | £2.22 | |
| Ko-Flex, Urgo | £2.93 | |
| Profore #1 | £0.67 | |
| Profore #2 | £1.29 | |
| Profore #3 | £3.77 | |
| Profore #4 | £3.12 | |
| SSB | ||
| Actiban, Activa | £3.44 | NHS prescription costs |
| Actico, Activa | £3.22 | |
| Comprilan, Smith & Nephew | £3.25 | |
| Rosidal K®, Activa | £3.39 | |
| Silkolan, Urgo | £3.39 | |
| Weighted mean cost | £3.23 | |
| 2LB kits | ||
| ProGuide®, Smith & Nephew | £9.77 | NHS prescription costs |
| Coban | £8.08 | |
| K-Two, Urgo | £8.18 | |
| Weighted mean cost | £8.20 | |
| Other bandages | ||
| K-band, Urgo | £0.29 | NHS prescription costs |
| Three-layer reduced (or low) compression bandaging | £4.01 | |
If and when participants changed to a non-trial treatment details of any new therapy were recorded: the SSB, the 2LB, three-layer reduced compression bandaging, the 4LB, low-compression bandaging, high-compression bandaging, low-compression hosiery or other system. Nurses continued to record consultations and treatments delivered as before. Non-trial treatments were also costed.
Consultations with health-care providers
Data on the number of consultations with health-care providers were available from quarterly participant-reported questionnaires. Each participant recorded the following resource-use data, referring to the previous 3 months (participant-reported data): number of GP consultations at doctor’s surgery and at home; number of nurse consultations at doctor’s surgery and at home; number of hospital outpatient visits with a doctor or nurse; number of hospital day admissions for minor day procedure or day surgery; and number of hospital inpatient nights. The number of ulcer-related and non-ulcer-related health consultations were recorded separately and only the ulcer-related health consultations were included in the economic analysis.
A second source of data regarding the number of ulcer-related nurse consultations was available from treatment logs that were completed by nurses (nurse-reported data) (see Appendix 5). However, since this nurse-reported data capture only recorded consultations until the reference leg was healed, it did not count any consultations related to ulcer recurrence. Because of this, using nurse-reported data had the potential to underestimate the total number of ulcer-related consultations, thus participant-reported data were used as the base case. A sensitivity analysis was conducted that used number of nurse-reported consultations.
With regards to ulcer-related hospital inpatient stay, two sources of data for hospitalisations were interrogated: adverse events data and participant reported information.
The unit costs used to calculate the cost of health service consultations in the trial are summarised in Table 6.
| Parameter description | Value | Source |
|---|---|---|
| GP visit | ||
| Doctor’s surgery/clinic visit, cost per minute | £2.5 | PSSRU 2011 (GP unit cost per consultation minute, without qualification costs but including direct care staff costs) |
| Duration of clinic visit, minutes | 11.7 | As above |
| Home visit, cost per minute | £4.3 | As above |
| Duration of home visit, minutes | 23.4 | As above |
| Nurse visit | ||
| At doctor’s surgery, cost per minute | £0.7 | PSSRU 2011 (Nurse unit cost per consultation minute, without qualifications costs) |
| Duration of clinic visit, minutes: HH | 25.4 | VenUS IV |
| Duration of clinic visit, minutes: 4LB | 30.1 | VenUS IV |
| Nurse visit at home, cost per minute | £1.1 | VenUS III13 |
| Duration of home visit, minutes: HH | 34.2 | VenUS IV |
| Duration of home visit, minutes: 4LB | 36.2 | VenUS IV |
| Outpatient visit | ||
| Doctor (consultant or non-consultant) | £105.1 | NHS Reference Costs 2010–11.a Unit cost of face-to-face outpatient attendance (based on weighted average) |
| Nurse | £61.8 | As above |
| Hospital admission | ||
| Without overnight stay | £368.8 | NHS Reference Costs 2010–11.a Weighted average of day-case HRG data and outpatient procedures |
Health benefits
Mean time to healing
Incremental ulcer-free days (the measure of benefit in the cost-effectiveness analysis) were derived using the primary trial outcome, i.e. healing of the reference ulcer. Although time to healing is known for participants who heal during the trial period, non-healing participants are subject to censoring (as we do not know if and when their reference ulcer healed, just that it was not healed by a certain point). In a standard clinical analysis where not all participants have the event of interest in a given time period, the median time to event is the preferred summary statistic. However, for the economic analyses it is the mean time to healing that provides the most useful information to the decision-maker (on the expected health benefits associated with health technologies) and thus was used here. Methods to deal with censoring that allow a mean time to healing to be calculated are detailed in the section on statistical methods. Mean time to healing as an effectiveness outcome was reported as gain in ulcer-free days which is equal to the difference in mean time to healing between the HH and 4LB groups (same absolute value). However, it should be noted that data were not available on ulcer-free days beyond initial healing of the reference ulcer; hence, the impact of ulcer recurrence was not evaluated in this analysis.
Utility and quality-adjusted life-year scores
The outcome for the cost–utility analysis was the QALY measured over 12 months (maximum period of follow-up). The health state descriptor measure used was the EQ-5D, a widely recognised and validated descriptive system of health utility. 74,75 EQ-5D data were collected at baseline and at 3, 6, 9 and 12 months. The EQ-5D questionnaire has five questions, each relating to a different health dimension: mobility, self-care, ability to undertake usual activity, pain and anxiety/depression. Each question has three possible response levels: no problems, moderate problems and severe problems (the new five-response version of the EQ-5D was not available at the start of this study). Based on their combined answers to the EQ-5D questionnaire, participants could be classified as being in 1 of 243 possible health states. Each of these health states has an associated utility weight, which denotes the impact this state will have on health-related quality of life. Utility weights were calculated using an independent predefined algorithm obtained by the elicitation of societal preferences for the health dimensions in a random population sample through a time trade-off technique. 76 Perfect health has a weight of ‘1’, which decreases as health becomes impaired. In the case of participants who died, utility values were assigned a value of ‘0’.
For this study, given the quarterly timings for EQ-5D data collection, the study time horizon was partitioned in homogeneous subintervals and utility scores computed (from EQ-5D scores) for each 3-monthly time point. QALYs were estimated using time-weighted averages of the utility scores measured at the beginning and end of each interval. Hence, QALY for each quarter was equal to the product of the mean utility score during the interval and the duration of interval. 77
As with self-report resource-use data, participants were asked to continue providing quarterly EQ-5D data until the end of the study regardless of healing state. This is important because participants may have higher utility levels after healing in which case the benefit of treatment in reducing the time to healing will be reflected in higher total QALYs.
Statistical methods for within-trial economic analysis
Estimating mean time to healing, costs and quality-adjusted life-years
Not all study participants experienced healing during the study period; some were censored due to dropout from the study, loss to follow-up, death or conclusion of the study before healing occurs. Traditional statistical methods for survival analysis are based on the assumption that the reason for censoring is independent of the outcome – non-informative censoring. However, this assumption is not valid for cost data. 78 The primary reason is that individuals accrue costs at different rates: individuals in poor health may accumulate costs at higher rates – and in turn have higher cumulative costs at censoring time and event time – than those in better health. The cost at the time of censoring is thus informative of the latent cost at healing, even if censoring is independent of the survival time. 79 Therefore, using traditional methods of survival analysis to analyse cost data will produce biased results in presence of censoring; and the same can be demonstrated for QALYs.
To account for censoring, inverse probability weighting (IPW) was used in the estimation of mean time to healing, mean costs and mean QALYs. This method has been used in the literature80,81 and was previously used in VenUS II61 and III. 13 In the IPW approach, only the participants with the observed outcome of interest (for instance, observed healing data in the time-to-healing model) contribute to the analysis, and their contributions are inversely weighted by their probability of being observed. The censoring distribution is estimated through the Kaplan–Meier estimator.
Baseline covariates expected to influence cost and outcomes were included in the models. These covariates were the same as those used in the clinical analysis and included (log of) ulcer area, (log of) ulcer duration and participant mobility level; in addition, baseline utility was used in the QALY regression – discussed later. However, although the IPW method can be used non-parametrically, this approach does not allow for the covariate adjustment required here. Rather, linear regression methods with IPW weighting have been proposed in the literature. 80 Furthermore, as noted in the clinical analyses, there was reason to anticipate that there could be heterogeneity of treatment effects across centres, i.e. there may be a centre effect. To account for centre effect, LMMs have been suggested. 82 LMMs are characterised as containing both fixed effects (similar to standard linear regression coefficients) and random effects. Here, centre was treated as a random effect. This treatment of centre effect is equivalent to the shared frailty approach used in the clinical analysis, which assumes group-specific random effect (describing the degree of correlation of participants within centres).
Thus, in this analysis we specifically used IPW-weighted LMMs to estimate the difference in mean cost, mean ulcer-free days and mean QALYs. The overall error distribution in mixed effects models was assumed to be Gaussian. However, as the distribution of costs and outcomes may not follow parametric assumptions, the CIs were estimated using the non-parametric bootstrap method, which assumes that the empirical distribution of the data is an adequate representation of the true distribution of the data. 77
Cost-effectiveness analysis
Although the regression models above estimate the difference in costs and health benefits between treatment groups (and the statistical significance of this difference), for decision-making it is the expected value of this difference that is of interest. To assess incremental cost-effectiveness and cost utility, we compared the expected value of the mean difference in costs between trial groups to the expected value of the mean difference in the number of ulcer-free days and QALYs, respectively. There are four possible scenarios from such an analysis (shown using a cost-effectiveness plane in Figure 1). HH may be estimated to be (on average) more expensive and bring fewer health benefits than the 4LB (HH is dominated by the 4LB). In this case, the decision regarding preference of 4LB over HH seems straightforward, as HH is more costly and refers less benefit to patients.
FIGURE 1.
Cost-effectiveness plane showing cost and benefit differences between alternative treatments.
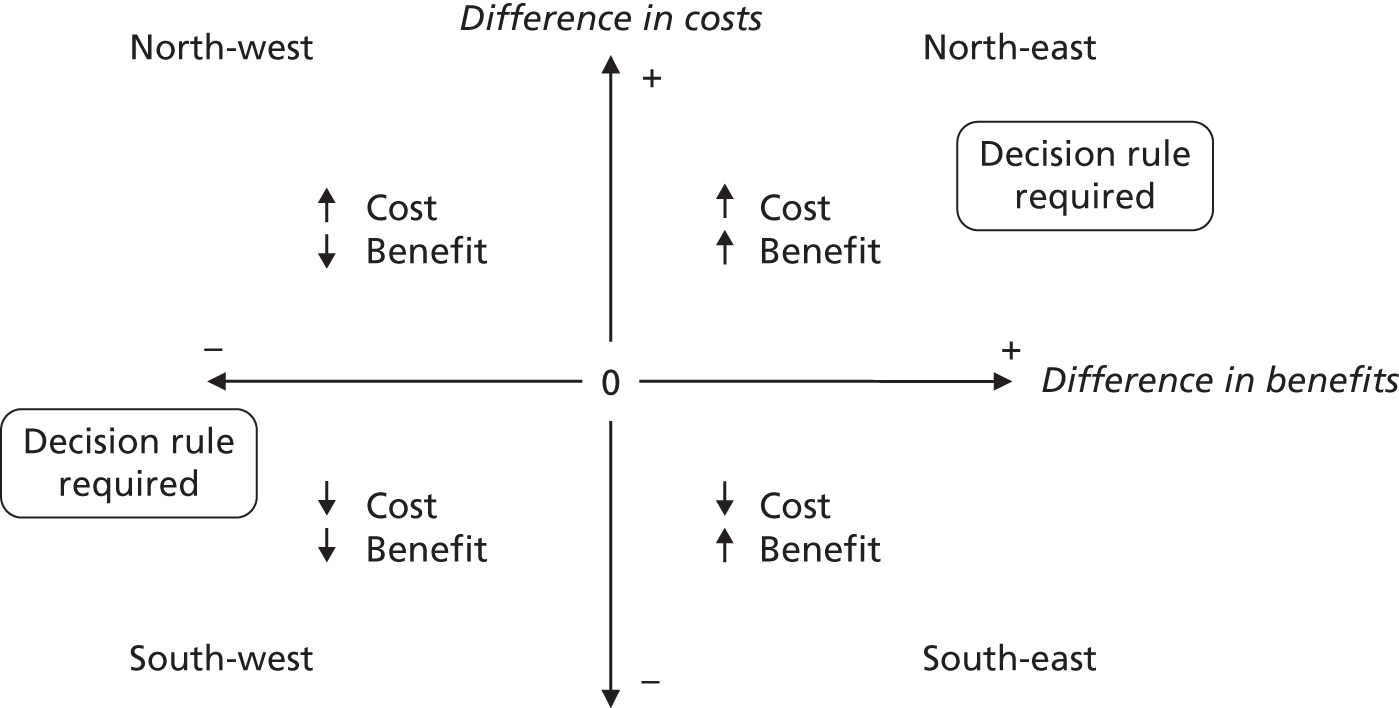
A similarly straightforward scenario is the reverse, i.e. if HH is expected to be less costly and more beneficial than the 4LB (HH dominates the 4LB). However, if HH were expected to be more costly and more beneficial than the 4LB, or less costly and less beneficial, it is necessary to evaluate whether the increased cost of the new intervention is worth the increased benefit, or if the reduced benefit associated with the new treatment is justified by the reduced costs.
To ascertain the cost-effectiveness of a health-care intervention relative to another in the absence of dominance, one needs to combines costs and health benefit in a single measure to which a decision rule regarding cost-effectiveness can be applied. The incremental cost-effectiveness ratio (ICER) is commonly used, and is the ratio of the mean difference in cost between alternative treatments being compared to the mean difference in health benefits:
where:
-
C1 = mean cost associated with the use of HH
-
C0 = mean cost associated with the use of the 4LB
-
B1 = mean health benefit associated with HH
-
B0 = mean health benefit associated with the 4LB.
The decision rule for cost-effectiveness on the basis of the ICER indicates that a treatment strategy can be considered cost-effective only if the decision-maker’s willingness to pay for an additional unit of health benefit (QALY, ulcer-free day) is greater (or equal) to the ICER. According to NICE, the willingness to pay for an additional QALY ranges between £20,000 and £30,000. 83 Therefore, if the result of cost–utility analysis (the estimated cost per QALY) is below this threshold, the intervention will be considered cost-effective. Caution is required when interpreting the cost-effectiveness results, as there is no established threshold for cost per ulcer-free day gained. Without this information we cannot determine whether or not the new intervention is cost-effective – a decision-maker interested in the results will be responsible for establishing the threshold.
Decision uncertainty assessment
All presented analyses are based on sampled data collected within this RCT. Thus, if this trial were repeated using a different study sample we would expect different mean incremental cost and benefit values to been observed, resulting in a different ICER estimate. Thus to fully understand inferences made from our data, the expected costs and benefits calculated must be estimated with uncertainty. Furthermore, although decision-makers can then decide on the provision of services, using expected cost-effectiveness findings in the presence of uncertainty, it is vital that the consequences of this uncertainty, and the extent to which it impacts on the adoption decision, should be investigated to inform whether further research is needed. 84
Confidence intervals
Uncertainty was assessed using a non-parametric bootstrap resampling technique. 77 The bootstrap technique samples (with replacement) from the observed cost and benefit pairs while maintaining the correlation structure between costs and benefits.
For each bootstrap resample, an IPW estimate of expected total mean costs, expected mean QALYs and expected ulcer-free days was calculated, which allowed computation of cost-effectiveness and cost–utility outcome replicates. The 95% CIs for the differential costs and QALYs were then calculated using bias-corrected non-parametric bootstrapping. 77
Cost-effectiveness acceptability curves
As discussed, in the presence of uncertainty it is important to evaluate the joint distribution of costs and benefits to inform decision uncertainty. This evaluation is often presented as the probability that an intervention is cost-effective when compared with an alternative treatment for predefined cost-effectiveness threshold values.
To explore decision uncertainty regarding the cost-effectiveness of HH, the joint distribution of mean cost and mean outcomes was evaluated using cost-effectiveness acceptability curves (CEACs) from the bootstrapped cost-effectiveness pairs. The CEAC expresses the likelihood that the cost-effectiveness estimate reflects a cost-effective intervention, based on the existing evidence. 85 The CEAC summarises – for every value of willingness-to-pay thresholds – the evidence in favour of the intervention being cost-effective. In this case, given the trial data, the CEAC for HH represents the probability of this therapy being cost-effective compared with the 4LB for a range of willingness-to-pay values for an ulcer-free day/QALY.
Subgroup analysis
A post hoc subgroup analysis was conducted to investigate whether total cost varied based on participant baseline characteristics; hence, cost per participant was evaluated based on age (≤ 60, 61–70, 71–80, > 80 years), BMI [underweight (< 18.5 kg/m2), normal healthy weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese classes I–II (30–39.9 kg/m2) obese class III (40+ kg/m2)86] and mobility (walks freely vs. walks with difficulty or immobile). To investigate potential interaction between treatment and baseline characteristics in the cost regression, separate interaction terms were included for treatment and age, BMI or mobility in the model used in the primary analysis (see Estimating mean time to healing, costs and quality-adjusted life-years, above). As in the primary analysis, an IPW-weighted mixed-effects model was used to evaluate costs at all quarterly time points at which cost data were collected. This post hoc analysis was initiated after unblinding of the primary results to the investigators and should therefore be regarded as exploratory in nature.
Sensitivity analysis
Nurse consultation recorded in treatment logs (nurse reported)
As highlighted previously, data on number of nurse consultations was available, as well as being self-reported by participants in quarterly questionnaires. This source of nurse consultation data was explored in a sensitivity analysis. Nurses also recorded the duration and location of nurse consultation. For costing purposes, the location of nurse consultations was simplified as either a home visit or health facility visit (unit cost assumed to be that of a nurse consultation at GP surgery). The duration of a consultation was calculated as the difference between the arrival and departure times recorded on the treatment log.
Other
Finally, if there were an imbalance in baseline costs between HH and the 4LB (based on the number of participant-reported health-care consultations in the 3 months before randomisation) then a further sensitivity analysis was planned using baseline cost as a covariate in the cost regression.
Chapter 4 Protocol changes
The following changes were made to the original trial protocol, after it was initially approved by the REC on 26 September 2009.
Inclusion and exclusion criteria
In the original protocol, patients were excluded if they had very bony prominences at risk of pressure damage. The rationale behind this was that participants who were treated with 4LBs would have a protective soft wool layer applied (as part of the 4LB system), whereas participants who received HH would not receive such padding. The TMG and TSC were concerned that this exclusion criterion could mean excluding patients who would benefit from the trial treatment. After consultation with clinician colleagues, and scrutinising the HH manufacturers’ guidelines (which do not mention the presence of bony prominences as a contraindication to wearing HH), it was concluded that the original risk of wearing HH was overestimated and patients with very bony prominences should not be excluded from the trial.
Patients with diabetes mellitus, whose blood sugar was not well controlled (unstable diabetes mellitus), were originally excluded from this trial. However, according to published literature,3 expert clinical opinion from a consultant vascular surgeon (who was also Independent Chair of the TSC) and guidelines from manufacturers of HH, participants with diabetes who present with a venous leg ulcer can be treated with high compression as long as their ABPI falls within normal limits. Patients with diabetes mellitus whose blood sugar was not well controlled were therefore eligible for inclusion within this trial, as long as nurses followed standard practice of regularly re-assessing diabetic patients for the presence of neuropathy, ischaemia or other indications that could make compression unsafe in this patient group.
During the course of the trial, the trial inclusion criterion regarding the ABPI measurement was altered. Originally, patients were included only if their ABPI was between ≥ 0.8 and < 1.20. Clinicians informed us that the use of the upper limit of 1.20 did not reflect current practice regarding compression use, and eligible patients were being unnecessarily excluded from this trial. Indeed, although a lower limit of 0.8 is widely recognised and cited in robust guidelines,21 there is no agreed upper ABPI at which high compression should not be delivered.
To investigate this in more detail, we surveyed to 27 trial centres regarding their practice. Of the 26 respondents, 24 (92%) said they would use high compression on patients with an ABPI of > 1.20. Clinicians said that, in practice, the decision to apply high-compression treatment to patients with an ABPI of > 1.20 would be dictated by clinical judgement and local guidelines. The exclusion criterion regarding ABPI was therefore altered to exclude patients with an ABPI of > 1.20 (taken within the last 3 months) only if in the nurse’s clinical judgement and/or according to local guidelines that patient should not receive high compression.
In order to capture all reasons for excluding patients from this trial, a new pre-trial exclusion was created. This allowed nurses to exclude those patients who met all of the inclusion criteria and none of the exclusion criteria but, in the clinical opinion of the nurse, could not be included in the trial for another reason. The same approach was used in VenUS III. 13
Treatments
In the trial protocol we specified that all compression hosiery kits must consist of a two-layer compression system delivering up to 40 mmHg at the ankle. The ConvaTec SurePress Comfort Pro compression hosiery kit did not fulfil these criteria, as it delivered a maximum of 35–36-mmHg compression. Moreover, it did not come with a self-applicator; therefore, application of this treatment could prove difficult for some trial participants. Therefore, this compression hosiery kit was removed from the list of hosiery kits that could be used during the trial.
Serious adverse events
Any unexpected SAEs considered to be related to the trial treatment were required to be reported to the REC. In order to define which events would fit this definition, the protocol needed to contain a description of those SAEs that would be expected but unrelated to the trial treatment in this study population. The protocol was therefore amended to provide a description of these events.
Gaiter region
During recruitment it became apparent that nurses were unsure if they could recruit patients with a venous leg ulcer on, or extending below, the submalleolar region (i.e. below the ankle). To clarify this, the wording in the protocol was changed to describe the gaiter region in which the venous leg ulcer must be present either fully or partially.
Data collection processes
We changed the protocol to reflect a change to the collection of healing data. Rather than report healing via the ulcer healed form (see Appendix 5), we also asked trial nurses to telephone the York Trials Unit when they considered the reference ulcer (and leg) to have healed. The operator who took the call entered the date(s) of healing into the Trial Management Database. This change ensured data were captured accurately and also acted as a prompt for trial nurses to receive electronic reminders to inform them of when post-healing photographs were due.
Recruitment and follow-up
Recruitment for this trial was due to start on 1 October 2009 and finish on 28 February 2011; this was to be followed by a 12-month follow-up period from 1 March 2011 to 29 February 2012. The trial was due to end on 30 June 2012. This was modified owing to slow recruitment (only 34% of target recruited for 31 October 2010).
Recruitment was initially extended by 8 months and planned to continue into the follow-up period. Recruitment was therefore due to end on 31 October 2011, with participant follow-up continuing until 29 February 2012. This meant that follow-up was reduced for those participants who were recruited towards the end of the proposed extended recruitment period. The recruitment period was revised again in November 2011 owing to poor recruitment (406/489 participants recruited by 31 November 2011, 83% of expected sample size). A 4-month extension to the study was approved by the National Institute of Health Research. This meant that recruitment continued until 29 February 2012, participant follow-up ended on 30 June 2012 and the trial ended on 31 October 2012. Consequently, some participants recruited at the end of the recruitment period were only followed up for a maximum of 4 months. Similar protocol amendments were made in VenUS II61 and III. 13
Participant questionnaires
In the original protocol, participants who complete their final 12-month questionnaire were sent £5 reimbursement for completion of this questionnaire. This was changed to include a £5 reimbursement for all participants upon completion of their final questionnaire, if that happened to be completed before 12 months. This change reflected the variations in final follow-up.
Chapter 5 Clinical results
Recruitment
Recruitment began in November 2009 and ceased in February 2011. In total, 3411 patients with leg ulcers were screened as potential participants and of these 457 (15.5%) were randomised. Over the course of the trial there were 34 participating UK centres (two centres recruited no participants): this recruitment was staggered with centres joining and leaving the trial over its course. The number of participants recruited per centre ranged from 1 to 55 (Figure 2). The rate of recruitment is shown in Figure 3. Further details of the recruiting centres are presented in Appendix 1.
FIGURE 2.
Participant recruitment by centre. PCT, primary care trust.
FIGURE 3.
Target vs. actual trial recruitment.
Of the 457 participants randomised, 230 were allocated to the HH group and 227 to the 4LB group. Three participants were excluded because they were found to be ineligible after randomisation, and thus the number of eligible, randomised participants was 454 (224 4LB, 230 HH). Only one participant (4LB) provided no follow-up beyond baseline. Therefore, 453 (223 4LB, 230 HH) contributed data to the analysis of the primary outcome.
The flow of participants through the trial is presented in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram87 (Figure 4). This shows the total number of participants screened for eligibility, randomised into each treatment group, post-randomisation exclusions and changes from allocated trial treatment.
FIGURE 4.
VenUS IV CONSORT flow diagram. a, Of the 667 participants who fulfilled more than one exclusion criterion, the following number of participants fulfilled two (n = 537); three (n = 107); four (n = 18); five (n = 4) or six (n = 1) exclusion criteria; b, further details on the 614 patients who were excluded on other clinical grounds: ulcer was not a venous leg ulcer (n = 203); ulcer healed or close to healing (n = 94); treatment preference (n = 62); other condition excluded patient from study (n = 53); unable to obtain ABPI measurement (n = 45); ulcer was not in gaiter region (n = 33); patient could not tolerate or apply trial treatments (n = 32); health-care provider decided venous leg ulcer treatment (n = 32); patient was referred elsewhere for treatment (n = 30); patient was non-compliant with treatment (n = 24); no reason given (n = 5); other reason (n = 1).
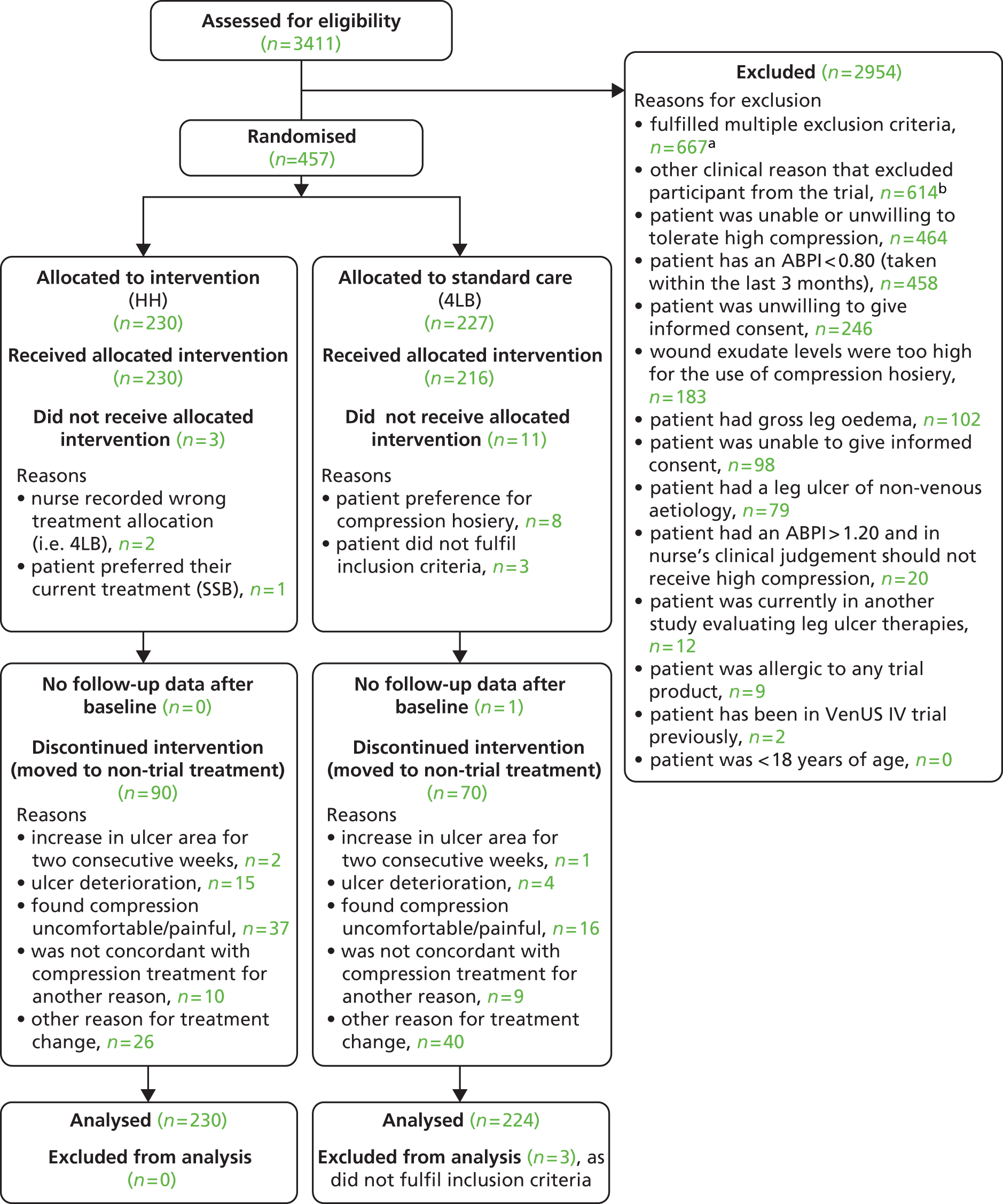
Baseline demographics and clinical characteristics
The baseline data are summarised in Tables 7–10 and show demographic and clinical characteristics to be fairly well balanced between the treatment groups. Table 7 summarises the participant characteristics. Overall, the trial had a similar proportion of male and female participants (50.7% and 49.3%, respectively). The mean age of participants was 69 years, with a range from 26.4 to 99.3 years. The mean participant BMI was 31 kg/m2 (obese) and 63.8% of participants had no mobility problems.
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Gender | |||
| Male | 117 (50.9%) | 113 (50.4%) | 230 (50.7%) |
| Female | 113 (49.1%) | 111 (49.6%) | 224 (49.3%) |
| Age | |||
| Mean (SD) | 68.3 (15.1) | 68.9 (13.8) | 68.6 (14.5) |
| Median (min., max.) | 71.1 (26.4, 96.7) | 71.1 (29.1, 99.3) | 71.1 (26.4, 99.3) |
| IQR (25–75%) | 59.2–79.9 | 62.0–78.7 | 60.4–79.4 |
| Missing | 0 | 0 | 0 |
| BMI (kg/m2) | |||
| Mean (SD) | 30.9 (7.9) | 31.2 (8.0) | 31.0 (8.0) |
| Median (min., max.) | 29.5 (16.2, 64.7) | 29.4 (17.7, 60.5) | 29.5 (16.2, 64.7) |
| IQR (25–75%) | 25.5–34.6 | 25.4–34.9 | 25.5–34.9 |
| Missing | 3 (1.3%) | 3 (1.3%) | 6 (1.3%) |
| Mobility | |||
| Participant walks freely | 139 (60.7%) | 150 (67.0%) | 289 (63.8%) |
| Participant walks with difficulty | 89 (38.9%) | 71 (31.7%) | 160 (35.3%) |
| Participant is immobile | 1 (0.4%) | 3 (1.3%) | 4 (0.9%) |
| Diabetic | |||
| Yes | 32 (13.9%) | 46 (20.5%) | 78 (17.2%) |
| No | 198 (86.1%) | 178 (79.5%) | 376 (82.8%) |
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Size of ulcer (cm2) | |||
| ≤ 5 cm2 | 156 (67.8%) | 149 (66.5%) | 305 (67.2%) |
| > 5 cm2 | 74 (32.2%) | 75 (33.5%) | 149 (32.8%) |
| Mean (SD) | 9.4 (15.4) | 9.3 (21.2) | 9.4 (18.5) |
| Median (min., max.) | 4.1 (0.1, 135.5) | 3.7 (0.1, 185.7) | 3.9 (0.1, 185.7) |
| IQR (25–75%) | 1.6–8.7 | 1.6–8.2 | 1.6–8.7 |
| Missing | 1 (0.4%) | 0 | 1 (0.2%) |
| Ulcer duration (months) | |||
| ≤ 6 months | 148 (64.4%) | 145 (64.7%) | 293 (64.5%) |
| > 6 months | 82 (35.7%) | 79 (35.3%) | 161 (35.5%) |
| Mean (SD) | 10.8 (20.0) | 12.3 (25.6) | 11.5 (22.9) |
| Median (min., max.) | 4.0 (1.0, 204.0) | 4.0 (1.0, 204.0) | 4.0 (1.0, 204.0) |
| IQR (25–75%) | 3.0–12.0 | 2.0–9.0 | 2.0–11.0 |
| Missing (%) | 1 (0.4%) | 2 (0.9%) | 3 (0.7%) |
| Total of ulceration episodes since first episode | |||
| Mean (SD) | 3.1 (6.0) | 2.7 (3.7) | 2.9 (5.0) |
| Median (min., max.) | 1.0 (0.0, 70.0) | 1.0 (0.0, 20.0) | 1.0 (0.0, 70.0) |
| IQR (25–75%) | (1.0–3.0) | (1.0–3.0) | (1.0–3.0) |
| Missing | 11 (4.8%) | 13 (5.8%) | 24 (5.3%) |
| Time since first ulcer (months) | |||
| Mean (SD) | 98.5 (155.4) | 96.2 (144.7) | 97.3 (150.1) |
| Median (min., max.) | 36.0 (1.0, 840.0) | 36.0 (0.0, 696.0) | 36.0 (0.0, 840.0) |
| IQR (25–75%) | 4.0–120.0 | 4.5–120.0 | 4.0–120.0 |
| Missing | 3 (1.3%) | 4 (1.8%) | 7 (1.5%) |
| Duration of oldest ulcer on reference leg (months) | |||
| Mean (SD) | 13.7 (37.5) | 12.1 (25.5) | 12.9 (32.1) |
| Median (min., max.) | 5.0 (1.0, 480.0) | 4.0 (0.0, 204.0) | 5.0 (0.0, 480.0) |
| IQR (25–75%) | 3.0–12.0 | 2.0–9.0 | 2.0–12.0 |
| Missing | 7 (3.0%) | 6 (2.7%) | 13 (2.9%) |
| Total number of ulcers per participant (reference leg) | |||
| Mean (SD) | 1.5 (1.0) | 1.6 (1.3) | 1.6 (1.2) |
| Median (min., max.) | 1.0 (1.0, 8.0) | 1.0 (1.0, 11.0) | 1.0 (1.0, 11.0) |
| IQR (25–75%) | 1.0–2.0 | 1.0–2.0 | 1.0–2.0 |
| Missing | 0 | 0 | 0 |
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Reference leg | |||
| Left | 135 (58.7%) | 121 (54.0%) | 256 (56.4%) |
| Right | 95 (41.3%) | 103 (46.0%) | 198 (43.6%) |
| Ankle circumference (cm) | |||
| Mean (SD) | 24.3 (3.0) | 24.7 (3.5) | 24.5 (3.3) |
| Median (min., max.) | 24.0 (18.0, 36.0) | 24.0 (12.0, 43.0) | 24.0 (12.0, 43.0) |
| IQR (25–75%) | 22.0–26.0 | 22.3–26.5 | 22.0–26.0 |
| Missing | 3 (1.3%) | 4 (1.8%) | 7 (1.5%) |
| Ankle mobility of reference leg | |||
| Participant has full range of motion | 159 (69.1%) | 154 (68.8%) | 313 (68.9%) |
| Reduced range of ankle motion | 65 (28.3%) | 67 (29.9%) | 132 (29.9%) |
| Participant’s ankle is fixed | 6 (2.6%) | 3 (1.3%) | 9 (2.0%) |
| ABPI | |||
| Mean (SD) | 1.1 (0.1) | 1.1 (0.1) | 1.1 (0.1) |
| Median (min., max.) | 1.1 (0.8, 1.5) | 1.1 (0.7, 1.5) | 1.1 (0.7, 1.5) |
| IQR (25–75%) | 1.0–1.2 | 1.0–1.2 | 1.0–1.2 |
| Missing | 3 (1.3%) | 9 (4.0%) | 12 (2.6%) |
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Preference | |||
| Compression hosiery | 108 (47.4%) | 112 (50.7%) | 220 (49.0%) |
| 4LB | 29 (12.7%) | 29 (13.1%) | 58 (12.9%) |
| No preference | 91 (39.9%) | 80 (36.2%) | 171 (38.1%) |
| Current treatments | |||
| 4LB | 119 (51.7%) | 102 (45.5%) | 221 (48.7%) |
| SSB | 15 (6.5%) | 14 (6.3%) | 29 (6.4%) |
| Compression hosiery | 18 (7.8%) | 13 (5.8%) | 31 (6.8%) |
| Other compression bandage | 31 (13.5%) | 38 (17.0%) | 69 (15.2%) |
| Not receiving compression | 37 (16.1%) | 41 (18.3%) | 78 (17.2%) |
| Other treatment | 5 (2.2%) | 8 (3.6%) | 13 (2.9%) |
Tables 8 and 9 summarise ulcer- and limb-related clinical characteristics. At randomisation participants were stratified by ulcer size (≤ 5 cm2 or > 5 cm2) and duration of the reference ulcer (≤ 6 months or > 6 months) and therefore these prognostic factors were balanced. In total, 67.2% of participants had ulcers of ≤ 5 cm2, and 64.5% had ulcers present for ≤ 6 months. The distribution of the actual size and duration of ulcers was positively skewed, with a small number of participants having very large or old ulcers. The number of ulcer episodes since the first occurrence was similar between groups with an overall median of 1 (range 0 to 70), indicating that for at least 50% of participants this was their second episode of leg ulceration. The mean ABPI of the reference limb (the leg with the reference ulcer) was 1.1, with a range from 0.7 to 1.5.
Table 10 shows a fair balance between treatment preferences at baseline by allocated treatment group, with 49% of participants preferring compression hosiery. However, only 6.8% of participants had a current treatment of compression hosiery before starting the trial, whereas 48.7% were being treated with the 4LB.
Trial completion and trial exit
Participants were followed up for up to 12 months, irrespective of whether they healed or not during this time. Data on reason for trial exit are presented in Table 11 by treatment group. This was returned for just over half of all participants, with the reasons for trial exit being fairly evenly balanced between groups. As these data were not returned for a large number of participants, status in terms of whether or not participants healed according to the trial nurses and the status of participants who left the study unhealed is also given in the table.
| Reasons | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Exit data (healed and unhealed) | |||
| Exit data not returned | 91 (39.6%) | 102 (45.5%) | 193 (42.5%) |
| Wishes to exit (ulcer unhealed) | 8 (3.5%) | 7 (3.1%) | 15 (3.3%) |
| Dieda | 7 (3.0%) | 3 (1.3%) | 10 (2.2%) |
| In trial for 12 months | 86 (37.4%) | 75 (33.5%) | 161 (35.5%) |
| Lost to follow-upb | 2 (0.9%) | 0 (0.0%) | 2 (0.4%) |
| Trial end reached | 31 (13.5%) | 35 (15.6%) | 66 (14.5%) |
| Other | 5 (2.2%) | 2 (0.9%) | 7 (1.5%) |
| Participant status (reference ulcer) | |||
| Reference ulcer healedc | 174 (75.7%) | 182 (81.3%) | 356 (78.4%) |
| Died (unhealed) | 5 (2.2%) | 2 (0.9%) | 7 (1.5%) |
| Exited (unhealed) | 8 (3.5%) | 7 (3.1%) | 15 (3.3%) |
| Lost to follow-up (unhealed) | 15 (6.5%) | 15 (6.7%) | 30 (6.6%) |
| Completed follow-up (unhealed)d | 28 (12.2%) | 18 (8.0%) | 46 (10.1%) |
Primary outcome: ulcer healing (blinded)
The primary outcome was time to healing of the reference ulcer, for which healing was judged from dated photographs by independent assessors who were blind to treatment allocation. The unadjusted analysis of time to healing used a log-rank test to compare the survivor functions of the treatment groups. There was no evidence of a difference between the treatment groups in time to ulcer healing [log-rank test statistic 0.23, degrees of freedom (df) = 1; p = 0.63] (Table 12). Figure 5 shows the Kaplan–Meier survival curves for each treatment.
| Characteristic | HH (n = 230) | 4LB (n = 223) |
|---|---|---|
| No. healing | 163 (70.9%) | 157 (70.4%) |
| Median time to healing (survival time in days) (95% CI) | 99 (84 to 126) | 98 (85 to 112) |
| HR from unadjusted Cox model (95% CI) | 0.95 (0.76 to 1.18) | |
| Log-rank test statistic, p-value | 0.23 (1 df), 0.63 | |
| Wilcoxon test statistic, p-value | 0.13 (1 df), 0.71 | |
FIGURE 5.
Kaplan–Meier plot of time to healing (blinded) by treatment group.
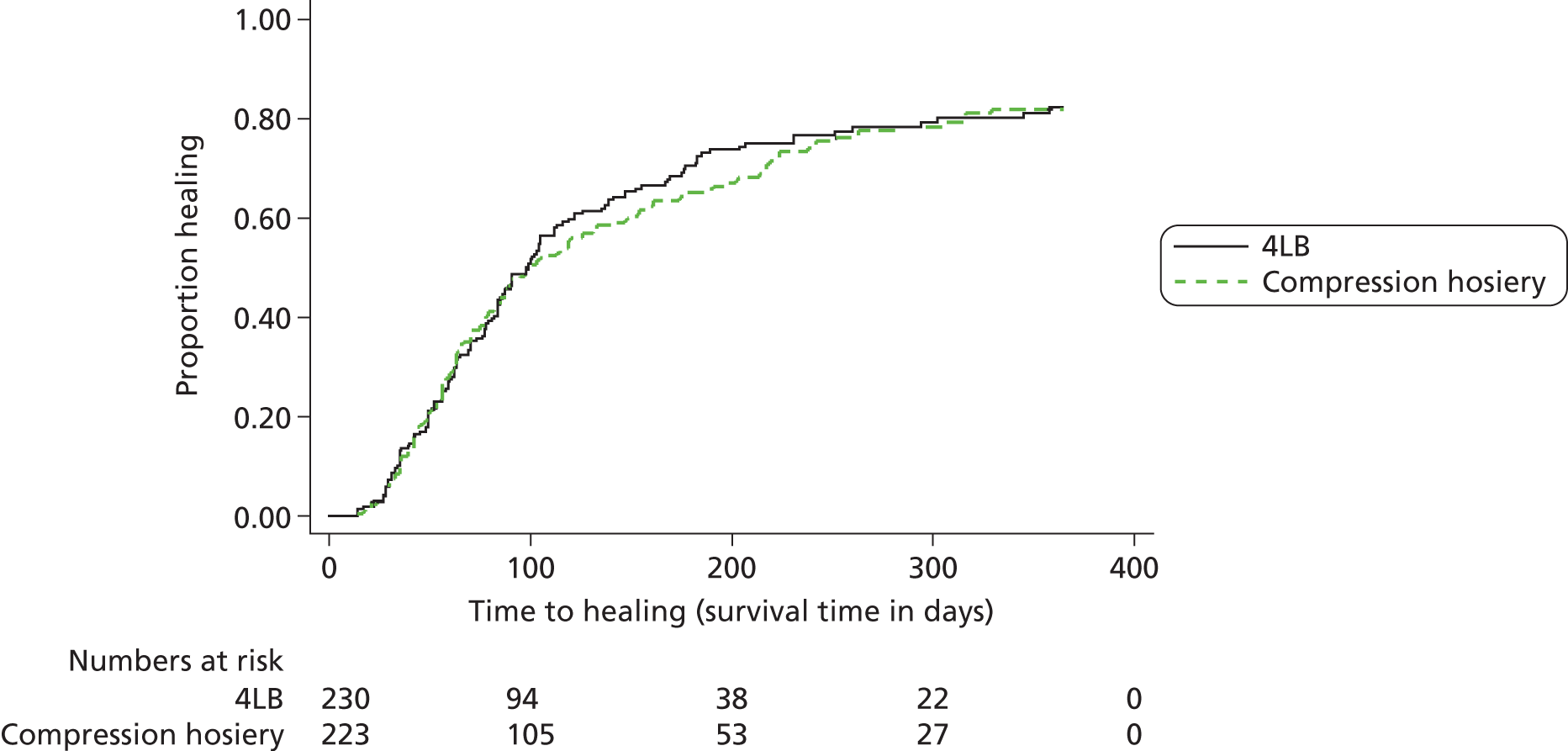
We then implemented a CPH model adjusted for baseline ulcer area, ulcer duration and participant mobility with shared centre frailty effects. A significant frailty effect for centre was shown, meaning the within-centre correlation needed to be accounted for and that this model was appropriate. The results, given in Table 13, do not demonstrate evidence of a treatment effect for HH compared with the 4LB in this adjusted model, with a HR of 0.99 (95% CI 0.79 to 1.25). Both baseline ulcer area and ulcer duration were included in the model after logarithmic transformation and were statistically significant predictors of time to healing (p < 0.001) with larger ulcers and those of a longer duration having a reduced risk of healing.
| Parameter | Estimate (SE) | HR (95% CI) | p-value |
|---|---|---|---|
| HH vs. 4LB | −0.01 (0.12) | 0.99 (0.79 to 1.25) | 0.96 |
| Log (area) | −0.31 (0.05) | 0.73 (0.66 to 0.81) | < 0.001a |
| Log (duration) | −0.51 (0.06) | 0.60 (0.53 to 0.68) | < 0.001a |
| Mobility | |||
| Participant walks freely | 0.00 | 1.00 | |
| Participant walks with difficulty | −0.13 (0.13) | 0.88 (0.68 to 1.13) | 0.31 |
| Participant is immobile | 0.67 (0.72) | 1.95 (0.47 to 8.05) | 0.36 |
| Theta | 0.06 (0.04) | ||
Further (post hoc) analyses were carried out to investigate the centre effect. First, the analysis was repeated excluding the 12 centres that had < 5 participants randomised. This produced very similar estimates of effect and the centre frailty remained significant (p = 0.01). Second, the analysis was repeated including a covariate that adjusted for the number of participants per centre. Again, similar results were observed, the centre effect for the overall model remained (p = 0.04) and there was no evidence of an association between centre size and time to healing (HR = 1.00, 95% CI 0.99 to 1.01; p = 0.57). In our next analysis, we repeated the primary analysis of time to healing stratified for each treatment group. In this case, area and duration of ulcer remained significantly associated with risk of healing. In the 4LB group, the test of significance of frailty parameter showed weak evidence of a centre effect (p = 0.08), whereas in the HH group there was no evidence of a centre effect (p = 0.20). In the design of VenUS IV there was a prior assertion that centre effect may be related to bandager skill and these results are not inconsistent with this assertion. However, it should be noted that these analyses were conducted in smaller groups of participants and may be underpowered to detect true differences.
In the primary analysis, visual inspection of the survival function and log–log plots indicated potential non-proportionality of hazards for the treatment groups; however, the test using Schoenfeld residuals was not statistically significant (p = 0.79 in the unadjusted model and p = 0.07 overall in the adjusted model). The only variable that did appear to violate the proportional hazards (PH) assumption was log(ulcer area). A sensitivity analyses was performed, which removed ‘ulcer area’ from the model and this did not alter the conclusions; the HR for HH compared with 4LB was 0.94 (95% CI 0.75 to 1.19).
Finally, because there was some change baseline imbalance in the proportion of people with diabetes in treatment arms, we repeated the primary analysis adjusting for diabetes (yes/no). Being diabetic was not found to be significantly associated with time to healing (p = 0.182), and it made very little difference to the overall treatment effect (HR = 1.01, 95% CI 0.80 to 1.26).
Ulcer healing (unblinded)
Time to healing as assessed by the nurses during the course of the trial was analysed using the same methods as those for the primary outcome. The unadjusted analyses are presented in Table 14. There was no significant centre frailty effect found (p = 0.25) and the models with and without centre frailty effects gave very similar results. In line with our analysis plan, we therefore present the model without centre frailty effects in Table 15. Again, there was no evidence of a treatment effect for HH compared with the 4LB in this adjusted model, with a HR of 0.91 (95% CI 0.73 to 1.12).
| Characteristic | HH (n = 230) | 4LB (n = 223) |
|---|---|---|
| No. healing | 174 (75.7%) | 182 (81.6%) |
| Median time to healing (survival time in days) (95% CI) | 84 (64 to 106) | 73 (62 to 88) |
| HR from unadjusted Cox model (95% CI) | 0.85 (0.69 to 1.05) | |
| Log-rank test statistic, p-value | 2.33 (1 df); p = 0.13 | |
| Wilcoxon test statistic, p-value | 1.39 (1 df); p = 0.24 | |
| Parameter | Estimate (SE) | HR (95% CI) | p-value |
|---|---|---|---|
| Compression hosiery vs. 4LB | −0.10 (0.11) | 0.91 (0.73 to 1.12) | 0.36 |
| Log (area) | −0.34 (0.05) | 0.71 (0.65 to 0.78) | < 0.001a |
| Log (duration) | −0.44 (0.05) | 0.65 (0.58 to 0.72) | < 0.001a |
| Mobility | |||
| Participant walks freely | 0.00 | 1.00 | |
| Participant walks with difficulty | −0.11 (0.12) | 0.90 (0.71 to 1.13) | 0.35 |
| Participant is immobile | 0.89 (0.59) | 2.44 (0.77 to 7.70) | 0.13 |
Healing of the reference leg
As the intention was for all participants to remain in the trial for up to 12 months, regardless of healing status, we were able to investigate time to healing of the reference leg. This assessment was unblinded and recorded by nurses during the trial. At the end of follow-up 343 participants’ (76.1%) reference legs were completely healed, representing 72.9% (167/229) in the two-layer group and 79.3% (176/222) in the 4LB group. Median time to reference leg healing was 84 days overall (95% CI 70 to 97 days): 77 days in the 4LB group (95% CI 63 to 92 days) and 91 days in the compression hosiery group (95% CI 70 to 126 days), log-rank test p = 0.12. There was no significant centre frailty effect found (p = 0.17) in the case of unblinded reference leg healing, and the models with and without centre frailty effects gave very similar results. The results are given in Table 16 and are consistent with those presented in Table 15 for unblinded reference ulcer healing.
| Parameter | Estimate (SE) | HR (95% CI) | p-value |
|---|---|---|---|
| HH vs. 4LB | −0.11 (0.11) | 0.90 (0.72 to 1.11) | 0.33 |
| Log (area) | −0.31 (0.05) | 0.73 (0.66 to 0.80) | < 0.001a |
| Log (duration) | −0.43 (0.06) | 0.65 (0.58 to 0.72) | < 0.001a |
| Mobility | |||
| Participant walks freely | 0.00 | 1.00 | |
| Participant walks with difficulty | −0.06 (0.12) | 0.94 (0.74 to 1.19) | 0.60 |
| Participant is immobile | 0.93 (0.59) | 2.53 (0.80 to 8.00) | 0.11 |
Health-related quality of life and leg ulcer-related pain
The SF-12 questionnaire was used to assess self-reported health-related quality of life at baseline, and months 3, 6, 9 and 12. Descriptive statistics of the PCS and MCS scores are presented in Tables 17 and 18 and Figures 6 and 7. In all cases the minimum, and worst, score possible was ‘0’ and the maximum ‘100’.
| Timeline/statistic | HH, n = 230 | 4LB, n = 224 | Overall, n = 454 |
|---|---|---|---|
| Baseline | 222 | 216 | 438 |
| Mean (SD) | 38.1 (10.8) | 38.8 (11.7) | 38.4 (11.2) |
| Median (range) | 38.5 (9.7–64.8) | 39.1 (11.9–62.1) | 39.0 (9.7–64.8) |
| Missing n (%) | 8 (3.5) | 8 (3.6) | 16 (3.5) |
| 3 months | 190 | 186 | 376 |
| Mean (SD) | 39.4 (12.0) | 38.2 (12.6) | 38.8 (12.3) |
| Median (range) | 40.2 (8.7–62.0) | 39.9 (6.0–59.5) | 40.2 (6.0–62.0) |
| Missing n (%) | 40 (17.4) | 38 (17.0%) | 78 (17.2%) |
| 6 months | 163 | 164 | 327 |
| Mean (SD) | 38.8 (11.7) | 37.8 (13.9) | 38.3 (12.9) |
| Median (range) | 39.6 (14.4–65.3) | 38.2 (8.7–60.5) | 39.5 (8.7–65.3) |
| Missing n (%) | 67 (29.1) | 60 (26.8) | 127 (28.0) |
| 9 months | 145 | 142 | 287 |
| Mean (SD) | 39.1 (12.6) | 39.2 (12.8) | 39.2 (12.7) |
| Median (range) | 39.6 (4.8–63.0) | 37.6 (16.0–61.2) | 39.5 (4.8–63.0) |
| Missing n (%) | 85 (37.0) | 82 (36.6) | 167 (36.8) |
| 12 months | 119 | 123 | 242 |
| Mean (SD) | 39.1 (11.9) | 38.5 (12.4) | 38.8 (12.1) |
| Median (range) | 39.4 (14.9–60.2) | 39.4 (11.9–59.9) | 39.4 (11.9–60.2) |
| Missing n (%) | 111 (48.3) | 101 (45.1) | 212 (46.7) |
| Timeline/statistic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Baseline | 222 | 216 | 438 |
| Mean (SD) | 48.6 (12.0) | 50.5 (10.4) | 49.6 (11.3) |
| Median (range) | 51.0 (9.1–70.9) | 52.7 (17.3–68.3) | 52.0 (9.1–70.9) |
| Missing n (%) | 8 (3.5) | 8 (3.6) | 16 (3.5) |
| 3 months | 190 | 186 | 376 |
| Mean (SD) | 49.1 (10.9) | 50.5 (11.2) | 49.8 (11.0) |
| Median (range) | 50.9 (18.0–67.7) | 53.0 (14.3–68.3) | 52.2 (14.3–68.3) |
| Missing n (%) | 40 (17.4) | 38 (17.0) | 78 (17.2) |
| 6 months | 163 | 164 | 327 |
| Mean (SD) | 51.1 (11.3) | 50.8 (10.5) | 50.9 (10.9) |
| Median (range) | 54.1 (15.9–72.1) | 54.0 (16.0–67.9) | 54.1 (15.9–72.1) |
| Missing n (%) | 67 (29.1) | 60 (26.8) | 127 (28.0) |
| 9 months | 145 | 142 | 287 |
| Mean (SD) | 49.8 (11.1) | 50.3 (10.1) | 50.1 (10.6) |
| Median (range) | 52.4 (17.6–70.1) | 52.4 (24.1–64.8) | 52.4 (17.6–70.1) |
| Missing n (%) | 85 (37.0) | 82 (36.7) | 167 (36.8) |
| 12 months | 119 | 123 | 242 |
| Mean (SD) | 49.3 (10.8) | 50.9 (11.6) | 50.1 (11.2) |
| Median (range) | 51.1 (17.3–68.3) | 54.0 (18.0–68.5) | 52.6 (17.3–68.5) |
| Missing n (%) | 111 (48.3) | 101 (45.1) | 212 (46.7) |
FIGURE 6.
Short Form questionnaire-12 items: MCS scores over time (mean and 95% CI).
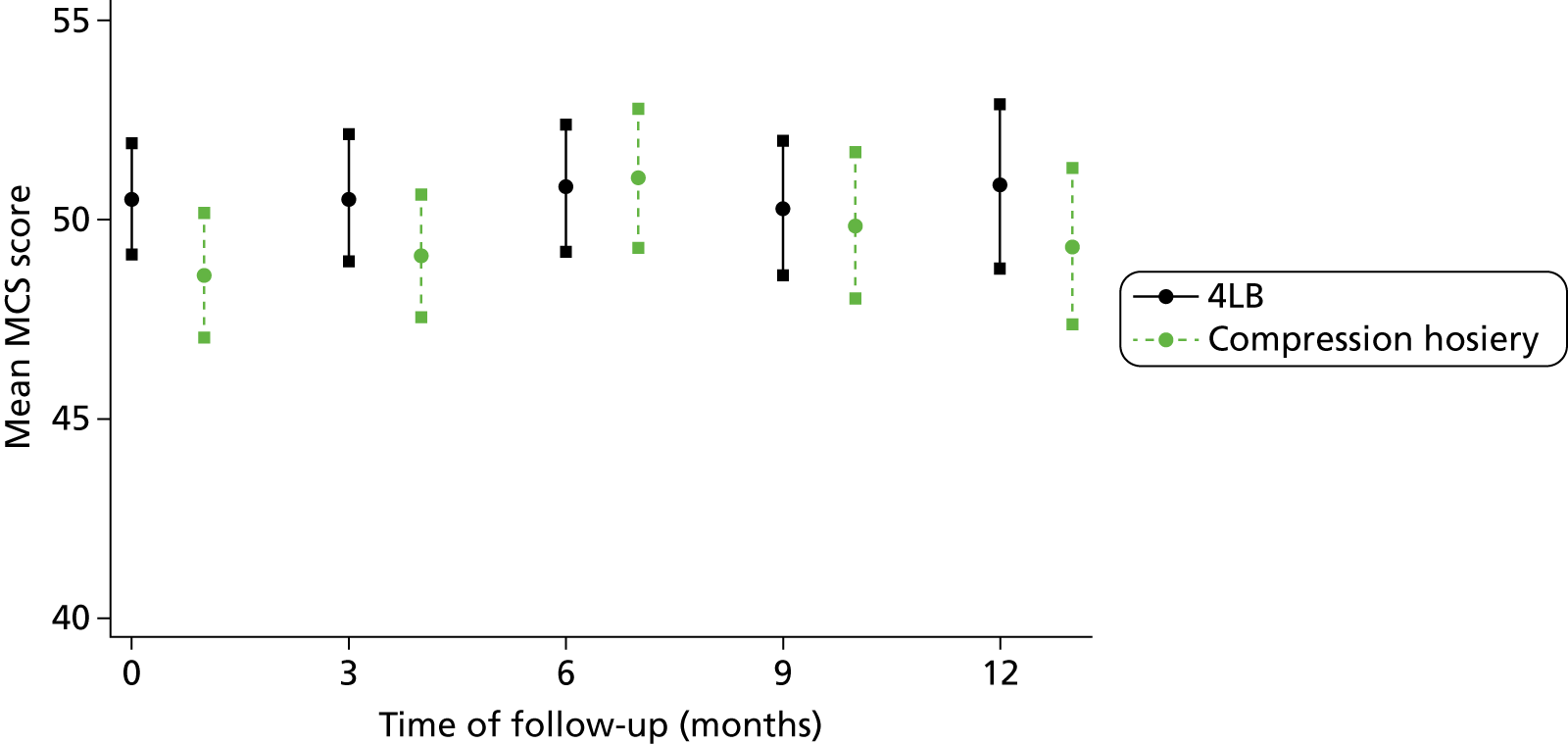
FIGURE 7.
Short Form questionnaire-12 items: PCS scores over time (mean and 95% CI).
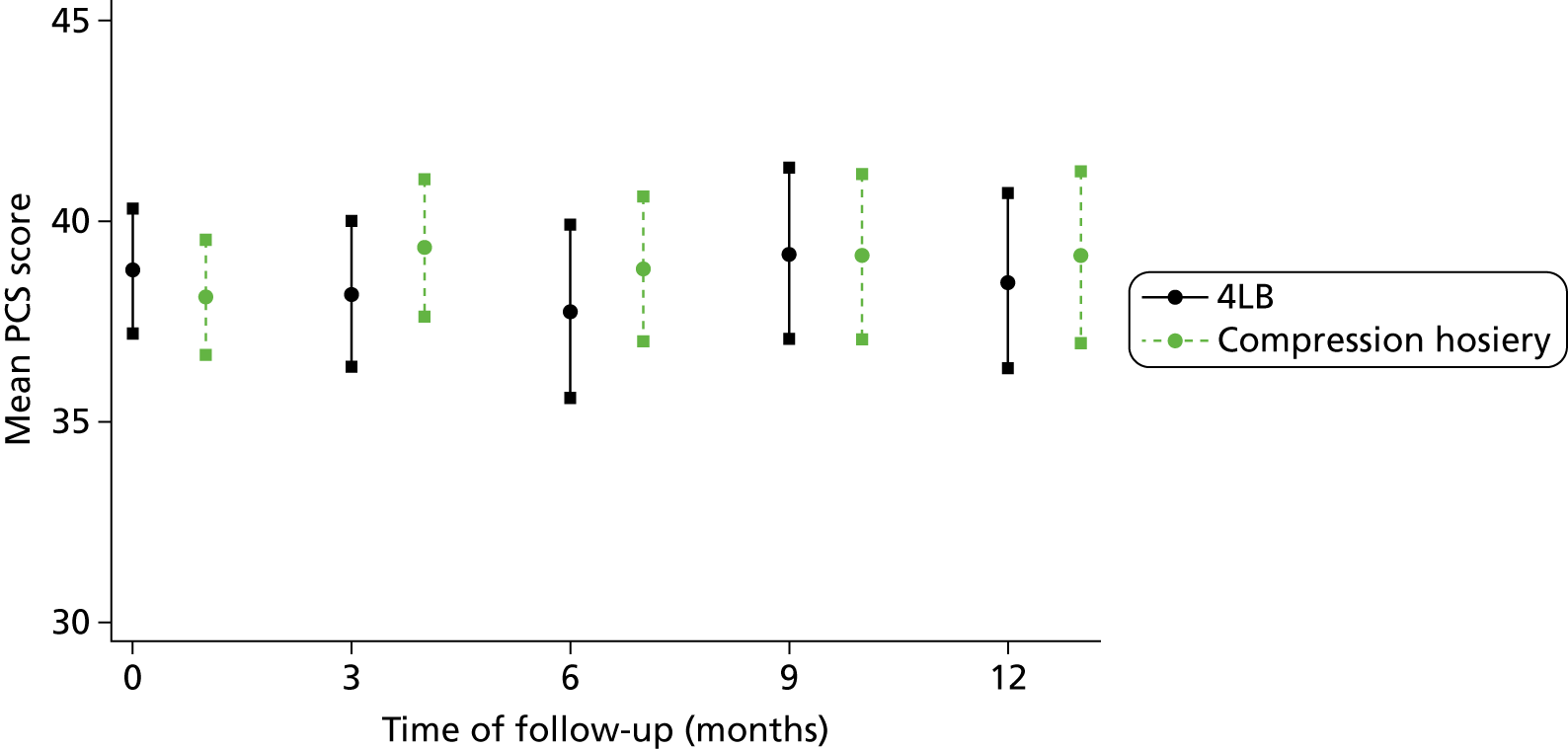
The mean baseline PCS score the of the trial study population was 38.4 (SD 11.2) and the mean baseline MCS score was 49.6 (SD 11.3). In general, the study population seemed to have a lower quality of life in terms of physical health but similar mental health compared with the US reference population sample who have a mean of 50 (SD 10) for both components of the SF-12, although the PCS score was similar to the SF-12 reference population for individuals aged > 75 years61 (mean 37.9, SD 11.16).
There was no evidence of a statistically significant relationship of PCS or MCS score with treatment over 12 months when investigated using LMMs, adjusting for baseline ulcer area, ulcer duration, participant mobility, centre, baseline scores (PCS and MCS scores, respectively) and time. Interaction between treatment and time was also investigated and this was not significant for the MCS model analyses (p = 0.45). For PCS, there was some evidence of significant interaction of treatment with time (p < 0.01). At 3 months those allocated to the HH had a significantly higher PCS score (suggesting better physical health) than those allocated to the 4LB after adjustments were taken into account. At the other time points, PCS score was also higher for the compression hosiery group but these differences were not statistically significant. In the PCS and MCS analyses, reduced participant mobility was associated with reduced PCS and MCS scores overall (p < 0.001 in both cases).
It should be noted that, due to the varying follow-up period, 151 participants randomised after 30 June 2011 had varying trial exit dates, ranging from 4 to 12 months post randomisation and this explains some of the missing health-related quality-of-life data. Taking this into account, in terms of missing SF-12 data, of the participants who healed, 39.1% in the HH group and 43.4% in the 4LB group did not return forms subsequent to healing up to their trial exit dates. For those participants who did not heal 32.1% in the HH group and 23.8% in the 4LB group had SF-12 missing at time of trial exit.
The mean (SD) VEINES-QOL scores at baseline were 49.8 (10.0) (n = 228) for compression hosiery and 50.2 (10.0) (n = 221) for 4LB, at 4 months they were 49.8 (9.9) (n = 167) and 50.2 (10.1) (n = 168), respectively. The estimated difference after 4 months, 4LB minus compression hosiery, was 0.0 scale points, (SE = 1.1, 95% CI−2.2 to 2.2; p = 1.0), adjusted for baseline VEINES-QOL score and clustering. Hence for VEINES-QOL the difference between treatment arms was estimated to be less than one-quarter of a SD.
Ulcer-related pain in the previous 24 hours was also measured at baseline, and months 3, 6, 9 and 12. Although these pain scores improved in both groups over time there was no evidence of difference in change in pain score between treatment groups (Table 19).
| Timeline/statistic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Baseline | 221 | 218 | 439 |
| Mean (SD) | 28.0 (27.1) | 35.5 (30.4) | 31.7 (29.0) |
| Median (range) | 20.0 (0.0 –100.0) | 30.0 (0.0 –100.0) | 25.0 (0.0 –100.0) |
| Missing, n (%) | 9 (3.9) | 6 (2.7) | 15 (3.3) |
| 3 months | 119 | 117 | 236 |
| Mean (SD) | 17.1 (22.2) | 21.5 (27.6) | 19.2 (25.1) |
| Median (range) | 5.0 (0.0 –90.0) | 10.0 (0.0 –100.0) | 10.0 (0.0 –100.0) |
| Missing, n (%) | 111 (48.3) | 107 (47.8) | 228 (50.2) |
| 6 months | 86 | 85 | 171 |
| Mean (SD) | 15.3 (21.8) | 22.5 (27.5) | 18.9 (25.0) |
| Median (range) | 5.0 (0.0 –100.0) | 15.0 (0.0 –100.0) | 5.0 (0.0 –100.0) |
| Missing, n (%) | 144 (62.6) | 139 (62.1) | 283 (62.3) |
| 9 months | 64 | 74 | 138 |
| Mean (SD) | 14.8 (22.3) | 19.4 (27.3) | 17.2 (25.1) |
| Median (range) | 5.0 (0.0 –85.0) | 5.0 (0.0 –95.0) | 5.0 (0.0 –95.0) |
| Missing, n (%) | 166 (72.2) | 150 (67.0) | 316 (69.6) |
| 12 months | 51 | 57 | 108 |
| Mean (SD) | 18.0 (24.6) | 18.6 (27.6) | 18.3 (26.1) |
| Median (range) | 5.0 (0.0 –95.0) | 5.0 (0.0 –100.0) | 5.0 (0.0 –100.0) |
| Missing, n (%) | 179 (77.8) | 167 (74.6) | 346 (76.2) |
Participant use of compression treatment
Table 20 details how participants recorded their use of the therapy 1 month from randomisation.
| Characteristic | HH (n = 193) | Characteristic | 4LB (n = 200) |
|---|---|---|---|
| Frequency of HH wearing during day | Frequency of 4LB wear | ||
| Every day | 159 (90.7%) | Every day | 188 (96.9%) |
| Most days | 4 (2.3%) | Most days | 0 (0.0%) |
| Some days | 6 (3.4%) | Some days | 2 (1.0%) |
| Did not wear | 6 (3.4%) | Not all | 4 (2.1%) |
| HH layer during the day | Removed 4LB yourself? | ||
| One layer | 24 (13.9%) | Yes | 41 (21.1%) |
| Two layers | 149 (86.1%) | No | 153 (78.9%) |
| Frequency of HH wear during night | – | – | |
| Every night | 127 (72.2%) | – | – |
| Most nights | 7 (4.0%) | – | – |
| Some nights | 6 (3.4%) | – | – |
| Did not wear | 36 (20.5%) | – | – |
| Layers of HH worn at night | – | – | |
| One layer | 58 (38.7%) | – | – |
| Two layers | 92 (61.3%) | – | – |
| Who normally applies HH? | – | – | |
| Nurse | 95 | – | – |
| Yourself | 85 | – | – |
| If yourself, HH easy to apply? | – | – | |
| Yes | 67 (55.4%) | – | – |
| No | 54 (44.6%) | – | – |
| If friend/relative, HH easy to apply? | – | – | |
| Yes | 9 (19.2%) | – | – |
| No | 38 (80.9%) | – | – |
| Did not wear my HH | 11 | – | – |
Details of change from allocated trial treatment were noted in the treatment logs completed by nurses at each visit until healing. In total, 152 (33.6%) participants were recorded as changing to a non-trial treatment before healing, with a higher proportion changing in the HH group than in the 4LB group [39.3% vs. 27.8%, χ2 = 6.69 (1 df) p = 0.01]. Reason for treatment change is given in Table 21.
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| Treatment change | 88 (38.3%) | 62 (27.8%) | 150 (33.0%) |
| Reason for change | |||
| Increase in ulcer area | 2 (2.2%) | 1 (1.6%) | 3 (2.0%) |
| Ulcer deterioration | 15 (16.7%) | 4 (6.5%) | 19 (12.5%) |
| Compression uncomfortable | 37 (41.1%) | 15 (24.2%) | 52 (34.2%) |
| Participant not concordant | 10 (11.1%) | 8 (12.9%) | 18 (11.8%) |
| Other | 24 (27.3%) | 34 (54.8%) | 58 (38.7%) |
Duration of treatment with allocated compression treatment
Time to treatment change was investigated in post hoc analyses and was defined as time from randomisation until date treatment change was recorded. There were 16 participants (6 HH, 10 4LB) noted to have had a change of treatment on the day of randomisation (11 participants never received their allocated treatment and five tried their allocated treatment but changed to another treatment on the same day of randomisation). Time to treatment change was investigated using a CPH regression, in which the 16 participants who changed treatment on the day of randomisation were given an arbitrary time to treatment change of 0.1 day (one-tenth of a day) and data were right censored in participants who (1) withdrew from the study; (2) were lost to follow-up; (3) died; (4) healed; or (5) reached the end of the trial – whichever came first. Median time to treatment change was 133 days in the HH group (95% CI 91 days, upper limit not estimable) and 213 days in the 4LB group (95% CI 182 days, upper limit not estimable). Treatment allocation, age and whether or not a participant had experienced a NSAE were included as covariates in this analysis. All of these factors were found to be associated with time to treatment change, with evidence of a shorter time to treatment change (from allocated trial treatment to non-trial treatment) in the HH group (HR 1.59, 95% CI 1.14 to 2.21; p = 0.005), those who were older (HR 1.02, 95% CI 1.00 to 1.03; p = 0.003) and those with at least one NSAE (HR 1.75, 95% CI 1.18 to 2.59; p = 0.005). The model was repeated including baseline ulcer area, duration, participant mobility and centre, but there was no evidence to suggest that these were associated with time to treatment change, and the associations between time to treatment change allocated treatment, age and NSAEs remained.
Adverse events
Adverse event data were collected by treating nursing staff. Nurses classified events SAE or NSAE and treatment related or non-treatment related. These data are described in Tables 22 and 23. In total, 300 participants had 895 adverse events. Of these, 9.5% were classed as serious.
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| SAEs | |||
| No. of participants with an SAE | 33 (14.3%) | 33 (14.7%) | 66 (14.5%) |
| No. of SAEs | 43 | 42 | 85 |
| Classification | |||
| Death | 9 (20.9%) | 3 (7.1%) | 12 (14.1%) |
| Life- or limb-threatening event | 1 (2.3%) | 2 (4.8%) | 3 (3.5%) |
| Hospitalisation required/prolonged | 32 (74.4%) | 32 (76.2) | 64 (75.3%) |
| Persistent or significant disability/incapacity | 1 (2.3%) | 0 (0%) | 1 (1.2%) |
| Other medically important condition | 7 (16.3%) | 9 (21.4%) | 16 (18.8%) |
| Outcome of event | |||
| Recovered fully | 6 (14.0%) | 11 (26.2%) | 17 (20.0%) |
| Recovered partially | 5 (11.6%) | 8 (19.1%) | 13 (15.3%) |
| Died | 9 (20.9%) | 3 (7.1%) | 12 (14.1%) |
| Ongoing | 22 (51.2%) | 17 (40.5%) | 39 (45.9%) |
| Missing | 1 (2.3%) | 3 (7.1%) | 4 (4.7%) |
| Relationship of SAE to treatment (blinded) | |||
| Unrelated | 27 (62.8%) | 28 (66.7%) | 55 (64.7%) |
| Unlikely to be related | 10 (23.3%) | 5 (11.9%) | 15 (17.7%) |
| Possibly related | 6 (14.0%) | 8 (19.1%) | 14 (16.5%) |
| Probably related | 0 (0%) | 1 (2.4%) | 1 (1.2%) |
| Definitely related | 0 (0%) | 0 (0%) | 0 (0%) |
| Not able to assess if related | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) |
| Characteristic | HH (n = 230) | 4LB (n = 224) | Overall (n = 454) |
|---|---|---|---|
| NSAEs | |||
| No. of participants with a NSAE | 154 (67.0%) | 130 (58.0%) | 284 (62.6%) |
| No. of NSAEs | 463 | 347 | 810 |
| Relationship of NSAE to treatment (blinded) | |||
| Unrelated | 62 (13.4%) | 50 (14.4%) | 112 (13.8%) |
| Unlikely to be related | 26 (5.6%) | 35 (10.1%) | 61 (7.5%) |
| Possibly related | 173 (37.4%) | 120 (34.6%) | 293 (36.2%) |
| Probably related | 85 (18.4%) | 72 (20.8%) | 157 (19.4%) |
| Definitely related | 108 (23.3%) | 59 (17.0%) | 167 (20.6%) |
| Not able to assess if related | 8 (1.7%) | 11 (3.2%) | 19 (2.4%) |
| Missing | 1 (0.2%) | 0 (0%) | 1 (0.1%) |
| Classification (definitely/probably related) | |||
| Alternative non-trial care initiated by participant or other | 8 (4.1%) | 4 (3.1%) | 12 (3.7%) |
| Bandage/hosiery failure | 47 (24.4%) | 23 (17.6%) | 70 (21.6%) |
| Bandage-/hosiery-related pain/discomfort | 39 (20.2%) | 27 (20.6%) | 66 (20.4%) |
| Dryness | 0 (0.0%) | 1 (0.8%) | 1 (0.3%) |
| Excoriation | 1 (0.5%) | 0 (0.0%) | 1 (0.3%) |
| Infection | 11 (5.7%) | 9 (6.9%) | 20 (6.2%) |
| Maceration | 2 (1.0%) | 0 (0.0%) | 2 (0.6%) |
| Medical event relating to leg | 0 (0.0%) | 1 (0.8%) | 1 (0.3%) |
| Occurrence of new ulcer | 26 (13.5%) | 30 (22.9%) | 56 (17.3%) |
| Skin damage | 27 (14.0%) | 16 (12.2%) | 43 (13.3%) |
| Skin deterioration | 8 (4.2%) | 6 (4.6%) | 14 (4.3%) |
| Ulcer deterioration | 17 (8.8%) | 13 (9.9%) | 30 (9.3%) |
| Ulcer-related pain | 7 (3.6%) | 1 (0.8%) | 8 (2.5%) |
| Total | 193 (100%) | 131 (100%) | 324 (100%) |
Serious adverse events
For SAEs, the numbers, classification and relationship to treatment overall were fairly well balanced between the treatment groups, with a slightly higher number of deaths reported in the HH group (see Table 22). There were no SAEs that were definitely related to treatment: only one was probably related (4LB group) and 16.5% of SAEs (14.0% HH, 19.1% 4LB) were possibly related. No participant had more than two SAEs reported during the course of study.
Non-serious adverse events
A higher proportion of participants in the HH group experienced one or more NSAEs compared with those allocated to the 4LB group [70.0% compared with 58.0%, χ2 = 3.86 (1 df); p = 0.050]. We also compared the total number of events experienced by participants (HH vs. 4LB) adjusting for the prognostic factors (baseline ulcer area, duration, participant mobility) and using robust SEs for centre effects in a zero-inflated negative binomial regression. There was no statistically significant difference between groups with a RR of 1.12 (95% CI 0.95 to 1.32). The proportion of events classified (by blinded assessors) as probably or definitely related to trial treatment was 41.7% in the HH group and 37.8% in the 4LB group. These probably or definitely related events were also classified into types of event, blind to treatment allocation. Of these events a higher number were classified as failure in the HH group, but more new ulcer occurrences were recorded in the 4LB group.
Recurrence
As participants were followed up beyond healing of the reference leg, it was possible to report recurrence of an ulcer in those participants who healed, up until the last date that the participant was involved in the study. In total, 65 recurrences on the reference leg were observed in the 343 participants who healed during the course of study. In the HH group, 14.4% (24/167) of participants with a healed reference leg were observed to recur compared with 23.3% (41/176) in the 4LB group. Time to recurrence was compared using a CPH model adjusting for baseline prognostic factors (ulcer area, duration, participant mobility), testing for shared centre frailty effects, using the same approach as the previous survival analyses. There was no significant centre frailty effect found (p = 0.38) in this analysis of time to recurrence from healing, and the models with and without centre frailty effects gave the same estimate for the treatment effect and very similar results for the adjusting factors. The results without centre frailty effects are given in Table 24. The results indicate a greater hazard of recurrence when allocated to the 4LB but with wide CIs and imprecision around the size of this effect, with a HR of 0.56 (95% CI 0.33 to 0.94, p = 0.026). The Kaplan–Meier survival curves by treatment group are given in Figure 8. Because of the limited number of recurrence events, median time to recurrence from healing could not be estimated.
FIGURE 8.
Kaplan–Meier plot of time to recurrence by treatment group.
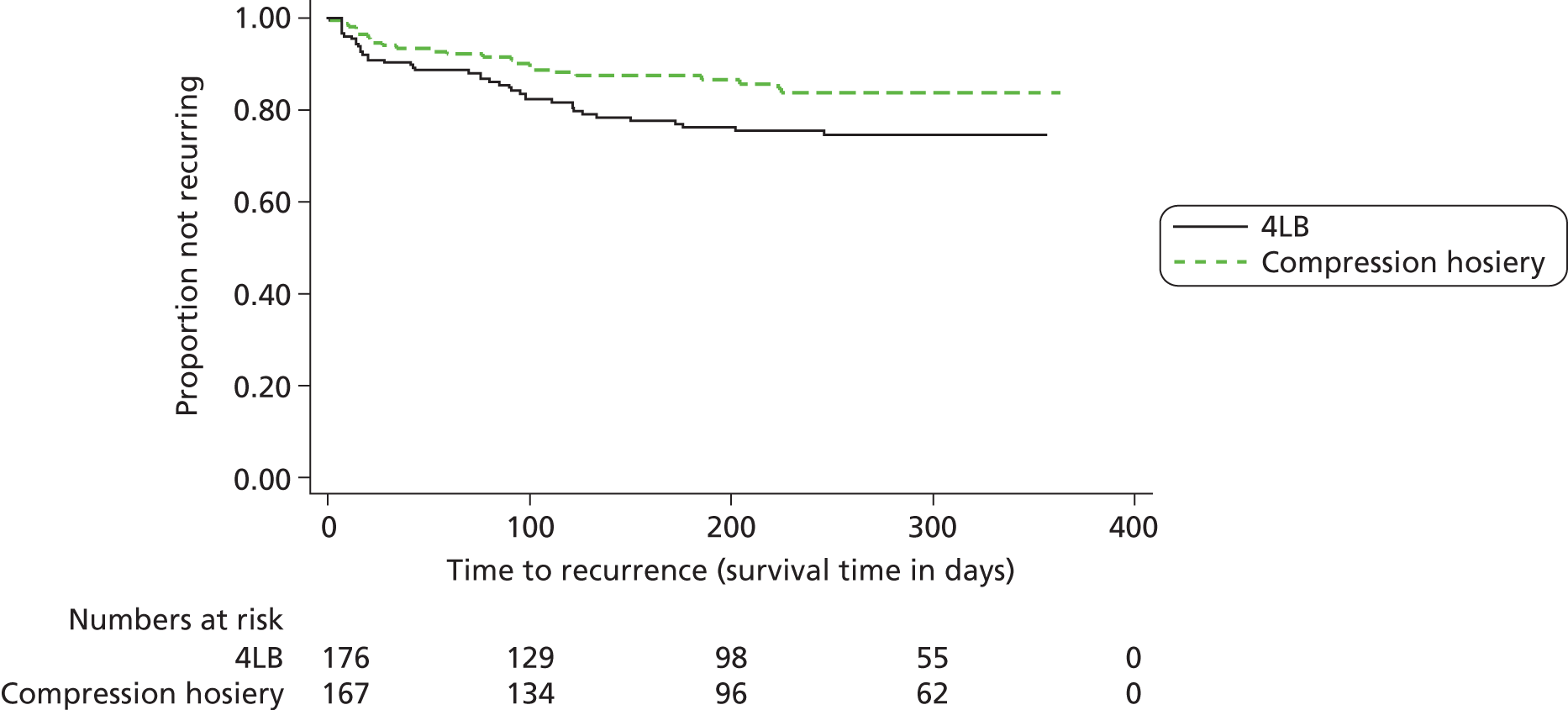
| Parameter | Estimate (SE) | HR (95% CI) | p-value |
|---|---|---|---|
| HH vs. 4LB | −0.58 (0.26) | 0.56 (0.33 to 0.94) | 0.03a |
| Log (area) | −0.08 (0.10) | 0.92 (0.76 to 1.12) | 0.42 |
| Log (duration) | 0.31 (0.12) | 1.36 (1.07 to 1.72) | 0.01a |
| Mobility | |||
| Participant walks freely | 0.00 | 1.00 | |
| Participant walks with difficulty | 0.75 (0.26) | 2.12 (1.26 to 3.56) | 0.004a |
| Participant is immobile | 1.23 (1.02) | 3.41 (0.46 to 25.02) | 0.23 |
In further exploratory post hoc analyses, we investigated the relationship between age, BMI and treatment change (from trial to non-trial treatment) with recurrence by adding these into the CPH model along with the other adjusting baseline covariates (ulcer area, ulcer duration and participant mobility). The association of allocated treatment with recurrence remained and there was no evidence of an association of age, BMI or treatment change with recurrence.
Proportional hazards, sensitivity analyses and multiple imputation
The PH assumption was tested for each survival analysis and none was found to be significant overall. However, there was evidence that the log transformation of ulcer area was not proportional within the adjusted models for each of the survival outcomes, except that of recurrence. Sensitivity analyses were carried out excluding the term from the model. This approach did not alter the interpretation of the results.
Analyses were repeated with and without centre effects. For all unblinded time-to-event outcomes (ulcer healing, leg healing and recurrence), the analyses with and without shared centre frailty effect gave similar results and the shared centre frailty effect did not need to be taken into account. However, for the primary outcome, for which ulcer healing was assessed blind to allocation, the centre effect was significant and therefore results are presented from the analyses that accounted for this. For adverse events, results were similar with and without centre; however, the prespecified analysis was to include random effects for centre and therefore these results were presented.
Data were assumed to be complete for time-to-event outcomes (ulcer healing, leg healing and recurrence) and for adverse events. Data were available for over 98% of adjusting covariates. Therefore, it was not necessary to repeat analyses using multiple imputation for these outcomes.
Summary of clinical findings
-
Median times to healing were 99 days (95% CI 84 to 126 days) for HH and 98 days (95% CI 85 to 112 days) for the 4LB. There was no evidence of a difference between HH and the 4LB in terms of ulcer healing with adjustment for baseline ulcer area, ulcer duration and participant mobility and centre included as a random effect (HR of 0.99, 95% CI 0.79 to 1.25; p = 0.96). Nor was there a difference in time to healing of the entire reference leg (p = 0.33).
-
Initial ulcer area and ulcer duration were both statistically significant predictors of time to healing.
-
There was no evidence of a statistically significant relationship of PCS or MCS score with treatment over time when investigated using LMMs, adjusting for baseline ulcer area, ulcer duration, participant mobility and centre.
-
In total, 39% of those allocated to HH moved from trial treatment to a replacement treatment (non-trial treatment) compared with 28% of those allocated to the 4LB. The median time to treatment change was 133 days in the HH group compared with 213 days in the 4LB group.
-
Although there was no evidence of a difference in the number of people reporting one or more SAEs between trial groups (14.4% HH, 14.7%, 4LB; p = 0.91), more participants in the HH group experienced one or more NSAEs compared with those allocated to the 4LB group (70.0% compared with 58.0%; p = 0.05). However, when results were investigated for the total number of events per person and adjustments made, there was no statistically significant difference in the number of adverse events between groups.
-
Post ulcer healing, fewer ulcer recurrences were observed in the HH group (14.4%) than in the 4LB group (23.3%). This difference was maintained and shown to be statistically significant in a survival analysis of time from healing of reference leg to recurrence (HR = 0.56, 95% CI 0.33 to 0.94; p = 0.026) when adjusted for baseline ulcer duration, ulcer area and participant mobility, both with and without shared centre frailty effects. Initial ulcer duration and the participant having reduced mobility were also statistically significant predictors of time to recurrence. It is important to note that as this analysis was undertaken in a subgroup (only those whose reference leg had healed) and does not represent all of those randomised, the advantages of unbiased analyses gained from randomisation are not maintained here.
Chapter 6 Economic results
A total of 454 participants were recruited: 230 were randomly allocated to receive HH and 224 to receive the 4LB. There were 63 participants (34 in HH and 29 in 4LB group) for whom self-reported resource-use data were not available at any time point (recorded as missing values throughout). The mean duration of follow-up for the self-reported cost and utility data was 9.83 months.
Resource use and costs
Compression treatments
Participants were allocated to their compression treatment at randomisation (the ‘trial treatment’) and received this treatment until they changed to another treatment (designated the ‘non-trial treatment’); their reference ulcer leg healed (i.e. treatment was not needed any more); or they were lost to follow-up or died.
Number of trial treatments received
During each treatment consultation, the existing compression treatment (if any) was checked, and if needed, new compression treatment/s were applied or given to the participant. The mean number of treatment applications per participant was 3.0 (SD = 3.87) in the HH group and 15.08 (SD = 17.98) in the 4LB group (Table 25). In the HH group a total of 662 compression treatments were applied/given in a total of 2627 nurse consultations, whereas in the 4LB group new compression treatments were applied/given in most of the 3453 recorded nurse consultations.
| No. of applications | HH (n = 230) | 4LB (n = 224) |
|---|---|---|
| Trial treatment applications per participant | ||
| Mean (SD) | 3.0 (3.87) | 15.08 (17.98) |
| Median (min. to max.) | 2 (0 to 31) | 9 (0 to 108) |
| n (%) | 221 (96.1) | 218 (97.3) |
| Other compression applications during trial treatment per participanta | ||
| Mean (SD) | 2.23 (4.89) | 0.55 (0.98) |
| Median (min. to max.) | 1 (0 to 52) | 0 (0 to 6) |
| n (%) | 221 (96.1) | 218 (97.3) |
| Non-trial applications in participant who switcheda | ||
| Mean (SD) | 20.2 (25.0) | 12.8 (19.0) |
| Median (min. to max.) | 11 (1 to 186) | 5.5 (1 to 112) |
| n (%) | 88 (38.3) | 54 (24.1) |
It is possible that, during ‘trial treatment’, participants received other treatments or procedures, including compression treatments, to the reference leg, for example because the allocated compression treatment was unavailable for a short period of time. A total of 226 participants received other compression treatments during their trial treatment period (151 in the HH group and 75 in the 4LB group); these were included in the cost analysis.
Number of non-trial treatments received
There were 2706 consultations in 148 participants classed as have moved from receiving trial treatment to receiving non-trial treatments (90 in the compression hosiery group and 58 in the 4LB group). The mean number of consultations for these participants during the non-trial treatment period was 18.3 (SD = 24.65); however, there were more non-trial treatments per participant recorded for those in the compression hosiery group who changed treatment (mean = 20.2; SD = 25.0) compared with the 4LB group (mean = 12.8; SD = 19.0).
Cost of compression treatments
The type and frequency of compression treatments given/applied to participants was reported by nurses until healing of the reference leg (Table 26: unadjusted costs). The mean costs of compression treatments during the trial treatment period per participant (including trial treatment and other compression treatments) were £99.0 (SD = 107.3) and £136.7 (SD = 157.4) for the hosiery and four-layer groups respectively. However, there were more non-trial treatments in the hosiery arm and the overall mean costs (including the trial and non-trial treatments) per participant were similar between treatment groups (£155.9 compared with £155.6). The mean estimated costs of trial compression treatment per application during the trial treatment phase were £27.89 (SD = £27.41, minimum–maximum = £7.63–646.8) for HH, and £8.55 (SD = 0.81, minimum–maximum = £0.29–8.84) for 4LB. Note that participants could be given more than one HH packs for self-application at home.
| Cost | HH (n = 230) | 4LB (n = 224) |
|---|---|---|
| Trial treatment (all compression treatments) | ||
| Mean (SD) | 99 (107.3) | 136.7 (157.4) |
| Median (min. to max.) | 64.7 (3.23–718.2) | 88.4 (3.23–933.6) |
| n (%) | 220 (95.7) | 218 (97.3) |
| Non-trial treatment | ||
| Mean (SD) | 151.1 (187.9) | 90.8 (126.7) |
| Median (min. to max.) | 80.2 (0.48–1095.5) | 43.1 (0.48–710.4) |
| n (%) | 88 (38.3) | 54 (24.1) |
| Total compression treatment (trial and non-trial treatments) | ||
| Mean (SD) | 155.9 (177.8) | 155.6 (175.5) |
| Median (min. to max.) | 93.3 (4.01–1480.4) | 97.1 (8.8–991.0) |
| n (%) | 225 (97.8) | 223 (99.6) |
Consultations with health-care providers
Consultations with health-care providers were reported by participants in quarterly questionnaires and these participant data were used in the base-case analysis. Participants reported both ulcer-related and non-ulcer-related consultations; however, only ulcer-related consultations were used in the economic analysis as differences across groups were expected only for ulcer-related resource use.
Participant self-reported data suggested that the number of ulcer-related consultations with health-care professionals was lower in the HH group in all categories of consultations except for the hospital day admissions, which were marginally higher in the HH group. The number of non-ulcer-related visits was similar across the treatment groups (Table 27).
| No. of visits | Related to ulcers | Not related to ulcers | ||
|---|---|---|---|---|
| HH (n = 230) | 4LB (n = 224) | HH (n = 230) | 4LB (n = 24) | |
| GP, surgery | ||||
| Mean (SD) | 0.9 (2.5) | 1.8 (4.8) | 3.4 (4.5) | 3.8 (6.7) |
| Median (min. to max.) | 0 (0–17) | 0 (0–35) | 2 (0–24) | 2 (0–54) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
| GP, home | ||||
| Mean (SD) | 0.1 (0.8) | 0.2 (1.2) | 0.3 (1.1) | 0.7 (3) |
| Median (min. to max.) | 0 (0–8) | 0 (0–12) | 0 (0–8) | 0 (0–33) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
| Outpatient doctor visits | ||||
| Mean (SD) | 0.5 (1.1) | 1.1 (5.2) | 2.1 (4.7) | 2.1 (5.5) |
| Median (min. to max.) | 0 (0–6) | 0 (0–60) | 0 (0–32) | 0 (0–43) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
| Nurse surgery visits | ||||
| Mean (SD) | 5.5 (11.6) | 9 (19) | 2.6 (6.8) | 3.1 (9.9) |
| Median (min. to max.) | 0 (0–88) | 1 (0–155) | 0 (0–80) | 1 (0–122) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
| Nurse home visits | ||||
| Mean (SD) | 5.9 (19.1) | 7.8 (17.5) | 1.5 (7.4) | 1.3 (6.1) |
| Median (min. to max.) | 0 (0–216) | 0 (0–112) | 0 (0–90) | 0 (0–60) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
| Outpatient nurse visits | ||||
| Mean (SD) | 7.8 (13.3) | 8.2 (13.4) | 2.8 (9) | 1.8 (6.2) |
| Median (min. to max.) | 0 (0–76) | 2 (0–77) | 0 (0–90) | 0 (0–44) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
| Hospital day admissions | ||||
| Mean (SD) | 0.5 (2.7) | 0.2 (1.2) | 0.6 (2.4) | 0.5 (1.8) |
| Median (min. to max.) | 0 (0–25) | 1.2 (0–11) | 0 (0–26) | 1.8 (0–18) |
| n (%) | 196 (85.2) | 195 (87.1) | 196 (85.2) | 195 (87.1) |
The proportion of consultations taking place in participant’s homes was similar between groups (29.3% for the HH group and 28.6% for the 4LB group). Doctor consultations accounted for a small proportion of the total number of consultations, and the number of such consultations was marginally lower in the HH group.
There were few hospital admissions (day cases and inpatient admissions). In total, 14 hospitalisations in the 4LB group and five in the HH group were reported by participants; therefore, as anticipated, there were small and potentially random differences in the number of stays that could result in large differences in costs and bias the results. Furthermore, evaluation of nurse-reported hospitalisation data revealed poor consistency with the participant-reported inpatient data (only 6/19 hospitalisations were also recorded in SAE forms by nurses and only one seemed to be related to leg ulcer based on the description of SAEs). We attribute these inconsistencies to the fact that participants may not easily be able to distinguish ulcer-related and unrelated hospital inpatient admissions. It was decided to exclude inpatient hospitalisation costs from the analysis.
Ulcer-related nurse consultations
The total number of participant-reported ulcer-related nurse consultations differed across treatment groups (mean of 19.2 consultations in the HH group and 25.0 in the 4LB group) (Table 28). Using this participant-reported data we calculated number of nurse consultations per week for the duration of follow-up. Again consultations with nurses were more frequent in the 4LB group (see Table 29).
| Ulcer-related nurse consultations | Participant reported | Nurse reported | ||
|---|---|---|---|---|
| HH (n = 230) | 4LB (n = 224) | HH (n = 230) | 4LB (n = 224) | |
| Total no. | ||||
| Mean (SD) | 19.2 (25) | 25.0 (29.1) | 19.67 (23.6) | 19.12 (22.24) |
| Median (min. to max.) | 12 (0–240) | 16 (0–196) | 11 (1–257) | 11.5 (0–135) |
| n (%) | 196 (85.2) | 195 (87.1) | 230 (100) | 223 (99.6) |
| Mean no. of visits per week | ||||
| Mean (SD) | 0.57 (0.7) | 0.69 (0.8) | 1.26 (0.84) | 1.31 (0.82) |
| Median (min. to max.) | 0.35 (0–5.0) | 0.47 (0–5.08) | 1.15 (0.15–7) | 1.17 (0.15–7) |
| n (%) | 196 (85.2) | 195 (87.1) | 228 (99.1) | 219 (97.7) |
Nurse-reported data on number of ulcer-related consultations are also presented for reference (see Table 28). The trial design specified that nurse-reported ulcer-related consultations were recorded only until the time of first healing of the reference leg. However, it is possible that this approach underestimated the number of consultations as it did not consider the impact of ulcer recurrence (hence participant-recorded data were used for the base-case analysis). Indeed, this view would explain the lower total mean number of nurse consultations in the four-layer group for nurse-reported data (the 4LB group had more ulcer recurrence than the HH group).
The difference between treatment groups in number of consultations per week was similar for nurse- and participant-reported data in that there were more nurse consultations per week in the 4LB group than in the HH group (see Table 28). However, the mean number of nurse consultations per week was lower in participant-reported data than in nurse-reported data. This is because the denominator in the nurse-reported consultations per visits was the duration until the last compression treatment was delivered. As the participant-reported data were collected at quarterly intervals, the duration denominator for consultations per week was always 12 weeks, even if the participant had healed after 6 weeks and did not have any further ulcer-related consultations.
Duration of nurse consultation
Data suggest that the duration of ulcer-related nurse consultations differed slightly between treatment groups (Table 29), this difference being more marked for clinic visits. A summary of the unadjusted ulcer-related costs of health-care provider consultations for each of the trial groups is presented in Table 30.
| Type of visit | Duration of nurse treatment visit (minutes), mean (SD) | |
|---|---|---|
| HH | 4LB | |
| All visits | 32.1 (16.3) | 32.9 (15.2) |
| Trial treatment visit | 30.9 (18.4) | 33.4 (15.5) |
| At a clinic | 25.4 (16.3) | 30.1 (12.9) |
| At home | 34.2 (20.3) | 36.2 (17.6) |
| Visits | HH | 4LB |
|---|---|---|
| Ulcer-related, GP surgery (£) | ||
| Mean (SD) | 25.1 (72.2) | 53.1 (139.7) |
| Median (min. to max.) | 0 (0–497.3) | 0 (0–1023.8) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Ulcer-related, GP home (£) | ||
| Mean (SD) | 14.9 (80.5) | 24.8 (121.4) |
| Median (min. to max.) | 0 (0–805) | 0 (0–1207.4) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Ulcer-related, outpatient department, with a doctor (£) | ||
| Mean (SD) | 47.7 (115.3) | 116.4 (547.8) |
| Median (min. to max.) | 0 (0–630.6) | 0 (0–6306) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Ulcer-related, nurse surgery (£) | ||
| Mean (SD) | 97.4 (205.7) | 189.1 (400.5) |
| Median (min. to max.) | 0 (0–1564.6) | 21.1 (0–3265.9) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Ulcer-related, nurse home (£) | ||
| Mean (SD) | 222.8 (719.8) | 311.4 (698.6) |
| Median (min. to max.) | 0 (0–8125.9) | 0 (0–4459.8) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Ulcer-related, outpatient department, with a nurse (£) | ||
| Mean (SD) | 484 (819.7) | 506.4 (830.5) |
| Median (min. to max.) | 0 (0–4696.8) | 123.6 (0–4758.6) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Ulcer-related, hospital day admissions (£) | ||
| Mean (SD) | 175 (1012.2) | 81.3 (441.1) |
| Median (min. to max.) | 0 (0–9220) | 0 (0–4056.8) |
| n (%) | 196 (85.2) | 195 (87.1) |
| Total cost over the follow-up period | ||
| Mean (SD) | £1066.9 (1681.1) | £1282.6 (1551.9) |
| Median (min. to max.) | £568.4 (0–14,872.2) | £775 (0–10,659.9) |
| n (%) | 196 (85.2) | 195 (87.1) |
Total costs
Baseline costs and quarterly estimates during the follow-up are presented in Table 31. In the base-case analysis, participant-reported data on number of ulcer-related consultations with health-care providers were combined with compression treatment costs (trial and non-trial treatments). The cost of nurse consultations was the major cost driver of total costs. As no imbalance was found in baseline costs, sensitivity analysis using baseline cost as a covariate was not required.
| Time/statistic | HH | 4LB |
|---|---|---|
| Baseline | ||
| Mean (SD) | 669.5 (710.1) | 687.3 (1022.6) |
| Median (min. to max.) | 480.7 (0–7546.6) | 488.3 (0–12,821) |
| n (%) | 228 (99.1) | 220 (98.2) |
| Months 0–3 | ||
| Mean (SD) | 588.2 (615.8) | 618.3 (551.1) |
| Median (min. to max.) | 430.8 (0–4477) | 564.6 (12.68 to 3746) |
| n (%) | 190 (82.6) | 192 (85.7) |
| Months 3–6 | ||
| Mean (SD) | 349.2 (702.1) | 410.5 (749.1) |
| Median (min. to max.) | 61.8 (0 to 5712.2) | 99.5 (0–6726.8) |
| n (%) | 162 (70.4) | 166 (74.1) |
| Months 6–9 | ||
| Mean (SD) | 278.5 (986.5) | 393.3 (1052.3) |
| Median (min. to max.) | 0 (0–10,793.8) | 21.1 (0–9256.6) |
| n (%) | 145 (63) | 144 (64.3) |
| Months 9–12 | ||
| Mean (SD) | 239 (576.2) | 298.5 (662.9) |
| Median (min. to max.) | 0 (0–4826.5) | 0 (0–4225) |
| n (%) | 122 (53) | 125 (55.8) |
| Total over 12 monthsa | ||
| Mean (SD) | 1222.1 (1787) | 1446.6 (1643.1) |
| Median (min. to max.) | 658 (0–15,483.4) | 918.3 (12.68–10,813.5) |
| n (%) | 196 (85.2) | 195 (87.1) |
To account for the censored nature of the data, mean differences in ulcer-related costs between treatments were estimated using IPW regression estimates of time to survival. The results of the base-case analysis show that those allocated to the HH group incurred, on average, £302.4 less per participant per year (95% bias corrected CI –£697.6 to £96.2) than with the 4LB group (Table 32). This difference was not statistically significant.
| Treatment group | Mean (£) | 95% bias corrected CI (£) |
|---|---|---|
| HH | 1492.9 | 1187.3 to 1954.3 |
| 4LB | 1795.3 | 1559.7 to 2185.0 |
| Difference | −302.4 | −697.6 to 96.2 |
Health benefits
Mean time to healing
On average, participants allocated to the HH group healed 1.4 days later than those allocated to the 4LB group. However, this difference was not statistically significant (95% bias corrected CI of the difference was from 36.8 days to –29.3 days) (Table 33).
| Treatment group | Mean (days) | 95% bias corrected CI (days) |
|---|---|---|
| 4LB | 129.2 | 98.1 to 142.6 |
| HH | 130.6 | 105.9 to 142.4 |
Utility and quality-adjusted life-years
Quarterly utility scores (per participant by treatment group) were calculated using EQ-5D responses, and quarterly QALYs were computed using time-weighted averages of the utility scores measured at the beginning and end of each interval. Quarterly utility scores are presented in Table 34 and unadjusted average QALYs per group described in Table 35 (note: only 41% of the sample had complete cases, i.e. utility scores available at all time points). There is a trend for an increase in quality of life over follow-up time for participants allocated to HH; however, this trend is less clear in the 4LB group.
| Time/statistic | HH | 4LB |
|---|---|---|
| Baseline | ||
| Mean (SD) | 0.618 (0.294) | 0.607 (0.306) |
| Median (min. to max.) | 0.725 (−0.074 to 1) | 0.71 (−0.358 to 1) |
| n | 219 | 215 |
| 3 months | ||
| Mean (SD) | 0.668 (0.289) | 0.675 (0.310) |
| Median (min. to max.) | 0.725 (−0.126 to 1) | 0.725 (−0.594 to 1) |
| n | 184 | 177 |
| 6 months | ||
| Mean (SD) | 0.696 (0.277) | 0.674 (0.309) |
| Median (min. to max.) | 0.727 (−0.181 to 1) | 0.726 (−0.239 to 1) |
| n | 157 | 158 |
| 9 months | ||
| Mean (SD) | 0.713 (0.261) | 0.646 (0.339) |
| Median (min. to max.) | 0.727 (−0.016 to 1) | 0.691 (−0.594 to 1) |
| n | 139 | 141 |
| 12 months | ||
| Mean (SD) | 0.714 (0.271) | 0.682 (0.303) |
| Median (min. to max.) | 0.71 (−0.239 to 1) | 0.727 (−0.239 to 1) |
| n | 113 | 120 |
| Timeline/statistic | HH | 4LB |
|---|---|---|
| 0–3 months | ||
| Mean (SD) | 0.161 (0.062) | 0.164 (0.065) |
| Median (min. to max.) | 0.177 (−0.011 to 0.25) | 0.181 (−0.071 to 0.25) |
| n | 176 | 172 |
| 3–6 months | ||
| Mean (SD) | 0.171 (0.067) | 0.172 (0.071) |
| Median (min. to max.) | 0.179 (−0.025 to 0.25) | 0.186 (−0.060 to 0.25) |
| n | 149 | 143 |
| 6–9 months | ||
| Mean (SD) | 0.178 (0.062) | 0.165 (0.076) |
| Median (min. to max.) | 0.182 (0.011 to 0.25) | 0.176 (−0.067 to 0.25) |
| n | 132 | 126 |
| 9–12 months | ||
| Mean (SD) | 0.180 (0.062) | 0.168 (0.072) |
| Median (min. to max.) | 0.179 (−0.025 to 0.25) | 0.179 (−0.060 to 0.25) |
| n | 106 | 110 |
| Annual (complete case analysis) | ||
| Mean (SD) | 0.705 (0.234) | 0.688 (0.234) |
| Median (min. to max.) | 0.746 (−0.066 to 1) | 0.714 (−0.232 to 1) |
| n | 95 | 89 |
After adjustment for baseline utility scores and stratification covariates, and after accounting for the censored nature of data, individuals in the HH group had, on average, more QALYs than individuals in the 4LB group [annual difference in QALYs of 0.034 (95% CI –0.0006 to 0.0778): Table 36].
| Treatment group | Mean QALYs (years) | 95% bias corrected CI (years) |
|---|---|---|
| HH | 0.685 | 0.665 to 0.716 |
| 4LB | 0.651 | 0.619 to 0.682 |
| Difference | 0.034 | −0.0006 to 0.0778 |
Cost-effectiveness and uncertainty
Our adjusted base-case analysis showed that participants randomised to receive HH for the treatment of venous leg ulcers had, on average, slightly more QALYs over the duration of the trial, but incurred lower costs than those participants allocated to receive the 4LB. Although these differences were not statistically significant at the conventional 5% significance level, the joint distribution of mean costs and mean QALYs suggest that HH is likely to be, on average, more effective and less costly than the 4LB over a 12-month time horizon, i.e. HH is the dominant treatment. If, based on these costs utility results, the NHS were to decide on these alternative treatments, HH should be recommended for use in patients with venous leg ulcers.
To investigate the uncertainty over the mean difference in costs and health benefits between trial groups, we used the incremental cost-effectiveness plane, where we graphically plotted the results of 1000 replicates of the non-parametric bootstrap of the mean difference in cost and QALYs. As Figure 9a shows, most of the cost and QALY pair replicates (i.e. 92%) fall in the bottom right (south-east) quadrant of the plane, suggesting that differential costs and QALY gains favour HH over the 4LB. The CEAC (see Figure 9b) confirms that, based on the cost–utility analysis, HH is cost-effective at the conventional thresholds of willingness-to-pay per QALY. This result is in line with the observed distribution of the cost-effectiveness scatterplot, which suggests that HH is less costly and is likely to produce more QALYs than the 4LB.
FIGURE 9.
Cost-effectiveness plane (a) and acceptability curve (b) for cost per QALY analysis (base case). The dashed line represents a probability of cost-effectiveness of 0.5.
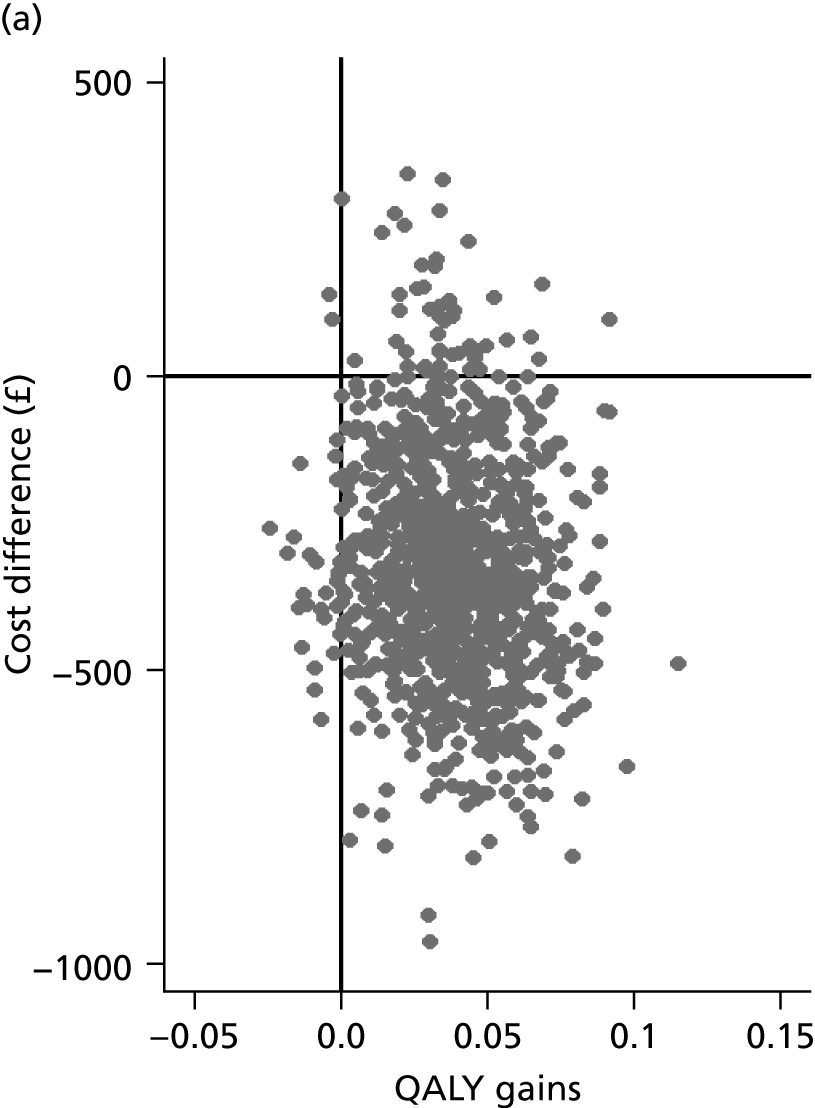
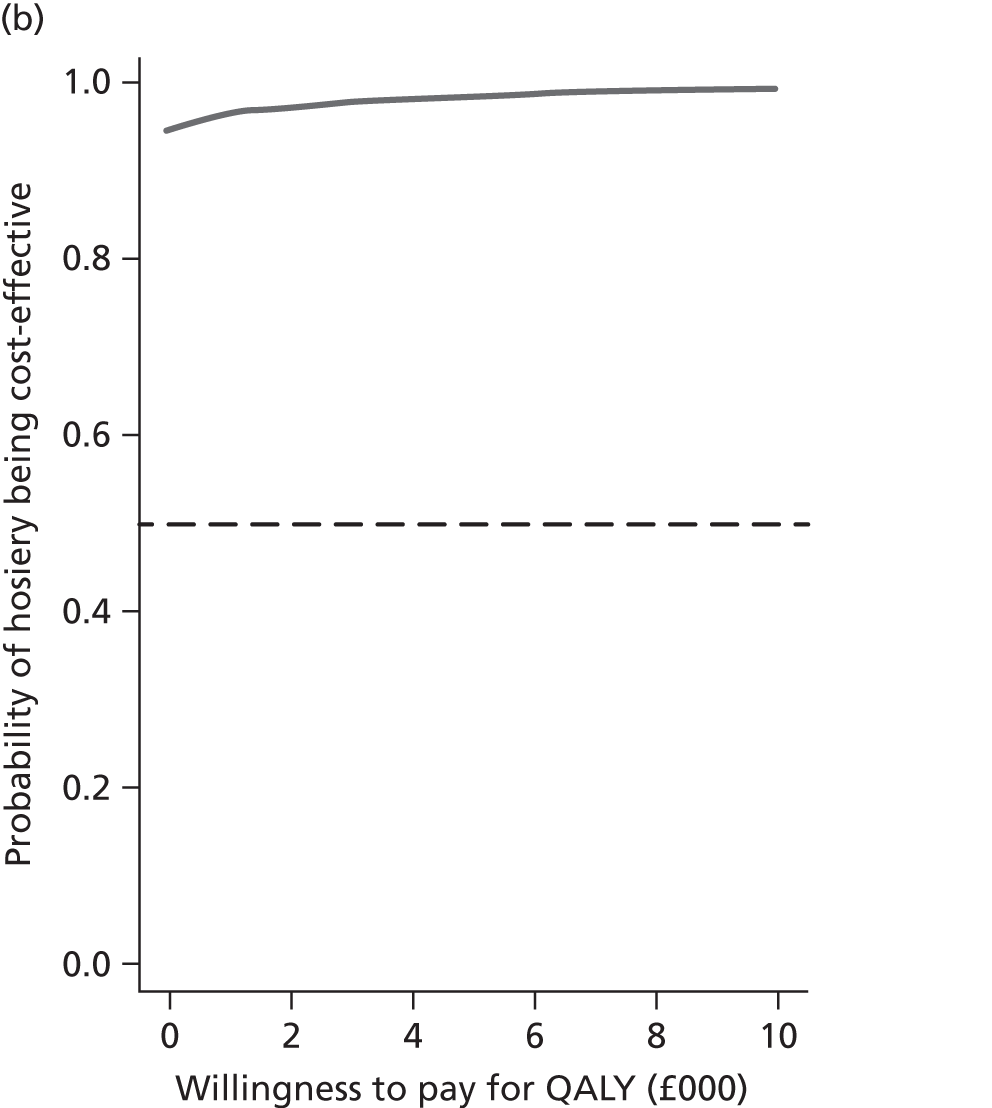
We also evaluated the mean number of ulcer-free days (based on time to first healing of the reference ulcer, and thus ignoring recurrence), which are slightly higher in the 4LB group, although this difference is highly uncertain and far from being statistically significant. This is consistent with the clinical trial findings from VenUS IV. However, the cost savings associated with HH remains the same as in the cost–utility analysis, i.e. £302 in favour of HH. In circumstances when the intervention is likely to be cheaper but is expected to be less effective than a comparator, we apply a decision rule to assess whether the intervention is cost-effective at different thresholds for willingness to pay; we do this by combining our estimates of differential costs and health benefits to estimate the ICER. The ICER associated with HH was estimated at £219.2 per ulcer-free day. As noted earlier, the observed difference in ulcer-free days is highly uncertain, which leads to high uncertainty on whether to adopt hosiery (probability of hosiery being cost-effective, rather than 4LB, is close to 50%: Figure 10); however, there is much more certainty around potential cost savings associated with HH (see Figure 10). More importantly, it should be reinforced that, unlike QALY assessments, the analysis of ulcer-free days was based on time to first healing of the reference ulcer, and ulcer recurrence was not considered in this health outcome measure. However, we know that recurrence was more common in the 4LB group. Thus, any relative benefit of lower recurrence rates in the HH group is not represented in this analysis.
FIGURE 10.
Cost-effectiveness plane and acceptability curve for cost per ulcer-free day analysis (base case). The dashed line represents a probability of cost-effectiveness of 0.5.
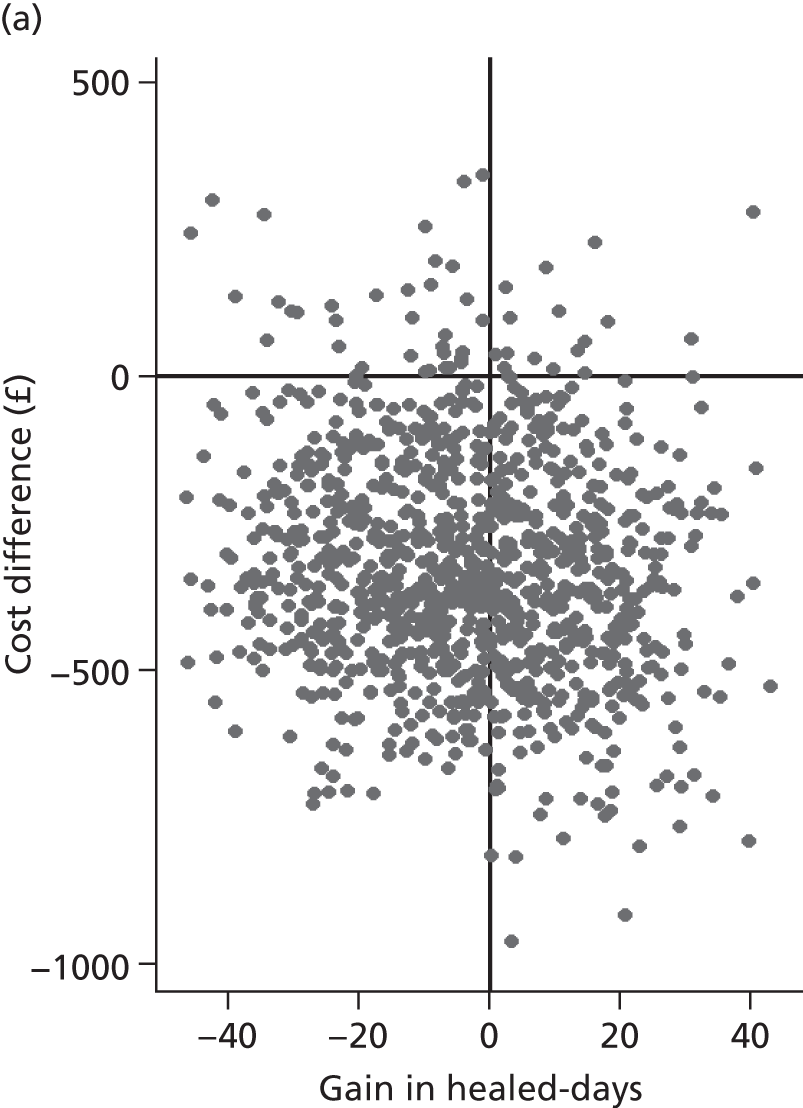
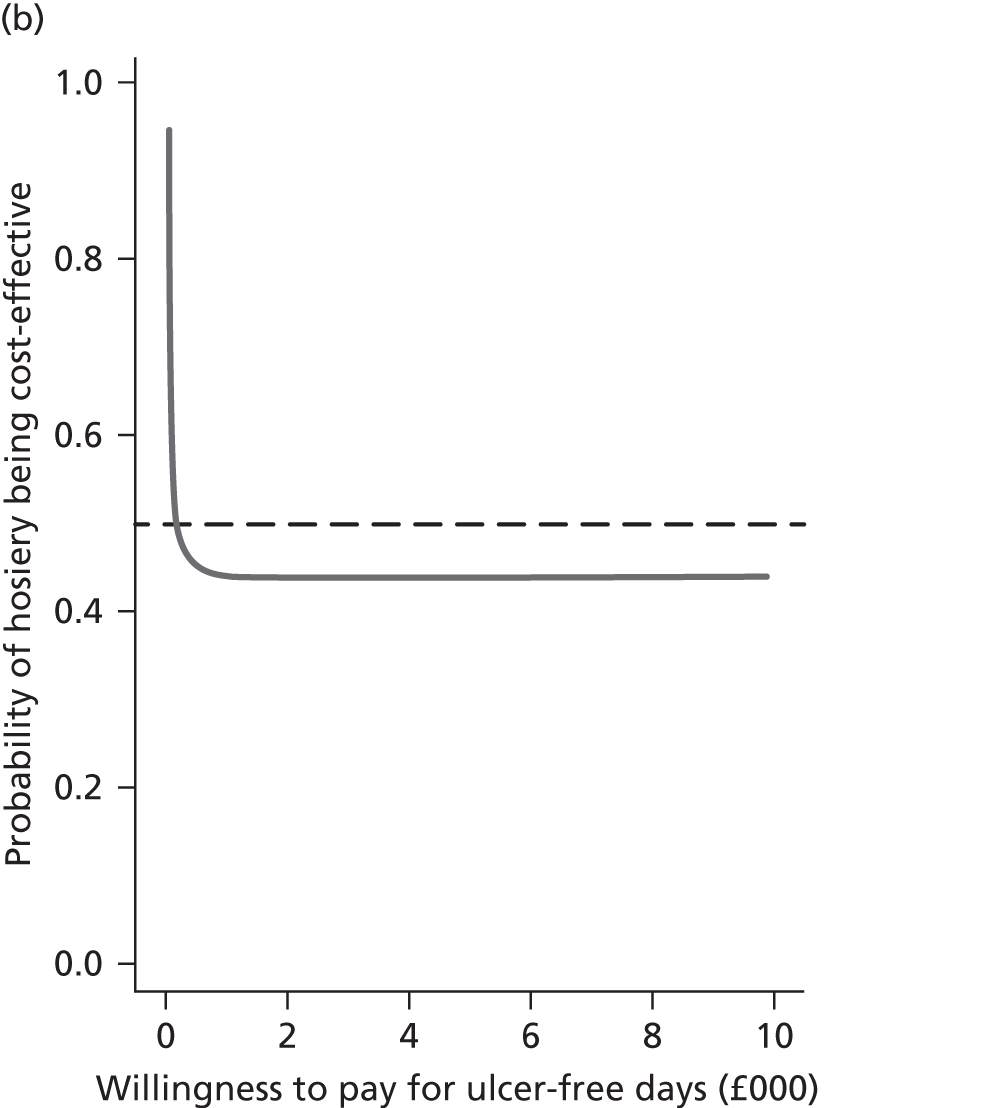
Sensitivity analysis
We investigated the impact of using nurse-reported data on ulcer-related consultations (rather than participant-reported) on the results. Unadjusted and adjusted total costs for this scenario are shown in Tables 37 and 38. The adjusted mean costs per participant per year for the HH group was £982.7 (95% bias corrected CI: £758.7 to 1310.3) compared with £1096.8 for the 4LB (95% bias corrected CI £909.4 to £1388.5). The difference in means between the two groups was –£114.1 (95% bias corrected CI –£418.4 to £182.3). The estimated difference in this scenario was lower than that in the base case.
| Timeline/statistic | HH (£) | 4LB (£) |
|---|---|---|
| Months 0–3 | ||
| Mean (SD) | 441.1 (484.3) | 443.2 (308.6) |
| Median (min. to max.) | 335.6 (38.79–4685.7) | 384 (29.87–2083.7) |
| n (%) | 230 (100) | 223 (99.6) |
| Months 3–6 | ||
| Mean (SD) | 220.1 (508.9) | 236.8 (447.2) |
| Median (min. to max.) | 88.9 (0–5139.9) | 48.6 (0–4687.4) |
| n (%) | 195 (84.8) | 190 (84.8) |
| Months 6–9 | ||
| Mean (SD) | 183.6 (801.6) | 242.7 (839.5) |
| Median (min. to max.) | 0 (0–9112.2) | 0 (0–7326.5) |
| n (%) | 159 (69.1) | 151 (67.4) |
| Months 9–12 | ||
| Mean (SD) | 159 (535.3) | 130.4 (300.6) |
| Median (min. to max.) | 0 (0–4968.7) | 0 (0–1868.1) |
| n (%) | 127 (55.2) | 132 (58.9) |
| Total over 12 months | ||
| Mean (SD) | 842.4 (1429.8) | 886.5 (1179.8) |
| Median (min. to max.) | 443.4 (39.34–12,019.1) | 504.8 (29.87–8705.4) |
| n (%) | 230 (100) | 223 (99.6) |
| Treatment group | Mean (£) | 95% bias corrected CI (£) |
|---|---|---|
| HH | 982.7 | 758.7 to 1310.3 |
| 4LB | 1096.8 | 909.4 to 1388.5 |
| Difference | −114.1 | −418.4 to 182.3 |
As only the costs were subjected to sensitivity analysis, the health benefit estimates were equivalent to base case. In terms of cost-effectiveness, results here were similar to those observed in the base-case analysis. HH was cost-effective compared with the 4LB, and is the dominant strategy in the cost–utility analysis. Based on the sensitivity analysis, the probability of HH being cost-effective compared with 4LBs is 98.2% at £20,000/QALY and 98.1% at £30,000/QALY.
Subgroup analysis and interaction effects with baseline characteristics
Tables 39–41 present total cost per participant based on age groups, BMI and participant mobility. The summary statistics suggest no indication of a relationship between cost and age or BMI; however, costs were found to be higher in less mobile participants than in those who walked freely. This confirms the rationale behind including mobility as a covariate in the primary analysis of cost.
| Age groups (years) | Mean | Median | SD | Min. | Max. | n |
|---|---|---|---|---|---|---|
| 0–60 | 1235.9 | 895.4 | 1296.6 | 0 | 7002.9 | 79 |
| 61–70 | 1124.4 | 633.9 | 1574.3 | 28.2 | 10,813.5 | 92 |
| 71–80 | 1576.3 | 943.6 | 2024.4 | 21.6 | 15,483.4 | 127 |
| > 80 | 1294.1 | 746.7 | 1700.3 | 12.7 | 10,943.1 | 93 |
| BMI category | Mean | Median | SD | Min. | Max. | n |
|---|---|---|---|---|---|---|
| Underweight | 565.8 | 606.3 | 351.7 | 151.5 | 899.1 | 4 |
| Normal weight | 1434.7 | 892.0 | 1832.6 | 0.0 | 10,943.1 | 86 |
| Overweight | 1149.6 | 717.6 | 1316.5 | 0 | 7232.9 | 113 |
| Obese classes I and II | 1529.4 | 799.1 | 2131.9 | 17.6 | 15,483.4 | 134 |
| Obese class III | 1162.3 | 884.1 | 1030.3 | 56.8 | 4477.0 | 49 |
| Mobility | Mean | Median | SD | Min. | Max. | n |
|---|---|---|---|---|---|---|
| Walks freely | 1125.2 | 723.8 | 1287.8 | 0 | 9522.7 | 253 |
| Walks with difficulty or immobile | 1717.0 | 1093.1 | 2263.9 | 0 | 15,483.4 | 138 |
We also investigated any potential interaction between baseline characteristics and treatment in cost regressions at each quarterly time point at which participant data were collected. There was no evidence of interaction between BMI and treatment or mobility and treatment in the cost regression (p > 0.05 at all quarterly time points). The interaction between age and treatment was significant at 9 months (p = 0.02); however, the interaction was not significant at other time points.
Summary of within-trial cost-effectiveness findings
-
The estimated mean annual per participant cost was £302.4 less for the HH group compared with the 4LB group; although this difference was not statistically significant (bias corrected 95% CI –£697.6 to £96.2). The main driver for this cost difference was the higher number of nurse consultations in the 4LB group.
-
On average, participants allocated to the HH group had higher QALYs than those allocated to the four-layer group [annual difference in adjusted QALYs of 0.034 (95% bias corrected CI −0.0006 to 0.0778)].
-
Participants allocated to HH incurred lower mean annual costs compared with the 4LB group and higher QALYs. Based on QALYs as the measure of benefit, HH has over a 95% probability of being the most cost-effective treatment based on this within-trial analyses.
-
The analysis using ulcer-free day as measure of benefit evaluated the treatments to have similar effectiveness (1.4 ulcer-free days difference in favour of 4LB), with wide uncertainty. Despite the lower expected costs associated with the use of HH, the uncertainty in effectiveness meant there was high uncertainty regarding its cost-effectiveness in this analysis. However, this analysis did not capture recurrence. Given that within VenUS IV there were fewer recurrences in the hosiery arm, the estimate of ulcer-free days in this analysis could have been biased against hosiery.
-
The sensitivity analysis based on nurse-reported resource use showed that the use of hosiery was still associated with lower costs than four-layer bandaging, although the cost difference was probably underestimated, as resource use during recurrence was not considered.
Chapter 7 Discussion
This is the first RCT to compare HH (designed to deliver 40 mmHg compression at the ankle), with the 4LB in the treatment of venous leg ulcers. As both treatments are in current clinical use in the UK and elsewhere, our findings are important for decision-makers – both as stand-alone RCT data and via subsequent incorporation into further evidence synthesis (see Parts II and III).
Clinical effectiveness
Ulcer healing
We found no evidence of a difference in time to ulcer healing between HH and the 4LB. The HR for healing was 0.99 (95% CI 0.79 to 1.25) meaning there is almost the same hazard (or ‘chance’) of healing in the HH group and the 4LB group. The CI indicates that HH may reduce the hazard (or ‘chance’) of healing by as much as 21% or increase it by as much as 25%.
This imprecision was observed despite this being one of the largest RCTs comparing compression treatments for venous leg ulcers ever conducted. 19 Although undertaking further large RCTs may reduce uncertainty around this treatment decision, it is important to consider the cost of investing in a further RCT in relation to the value collected data might have in improving decision-making. These issues will be investigated further in relation to high-compression treatments for venous leg ulceration in Part III of this report, where the amount of uncertainty around treatment effects will be investigated when all available data are synthesised and the value of further research considered.
The median time to healing in the 4LB group in VenUS IV was similar to that in VenUS I7 (99 days compared with 92 days, respectively) and both were pragmatic trials with wide inclusion criteria. However, the VenUS IV estimate was higher than the 70-day median time to healing reported for 4LB participants in the only other RCT comparing this treatment with HH. 32 However, this study excluded participants with clinical signs of infection so it may have evaluated a participant population with less-complex ulcers. Additionally, we note that Finlayson et al. 32 reported that the hazard of healing in the 4LB group was twice that of the HH group when their HH delivered a maximum of 35 mmHg compression at the ankle. Given trial findings from VenUS IV, one further interpretation may be that HH used to treat venous leg ulcers should aim to deliver 40 mmHg at the ankle in order to achieve similar time to healing as the 4LB.
As observed in VenUS I–III,7,13,61 as well as several other studies,88–90 we found that baseline ulcer area and duration were statistically significant predictors of time to healing (p ≤ 0.001). We also found that study centre is a significant predictor of ulcer healing, a finding that remained when adjusted for the number of participants per centre. As the application of compression bandaging requires skill, with the bandager relying almost entirely on technique for correct application, we postulated at the start of the study that a centre effect may be driven by differing bandaging skills between centres. This notion was supported by our tentative post hoc analysis, which suggested stronger evidence for a centre effect in the 4LB group than the HH group (which does not require the same skill level in application).
The decision to account for a potential centre effect in our sample size calculation was shown to be valid. We suggest that triallists planning future RCTs with a bandaging arm should consider the possibility of a centre effect and take this into account in sample size calculations and analyses.
Health-related quality of life
Changes in health-related quality of life over the duration of the trial were measured using the SF-12. At baseline the study population had a low quality of life in terms of physical health as observed in previous studies,7,13,61 whereas the MCS score was similar to population norms.
There was little change in the PCS and MSC scores over time in both the HH and the 4LB group of VenUS IV. The adjusted analysis showed slightly higher (all non-significant except at 3 months) scores in the HH group compared with the four-layer group, which agrees with the EQ-5D results reported in the cost-effectiveness analysis. There was a reduction in mean ulcer-related pain scores (over previous 24-hour period), during the trial but with no evidence of a difference between groups.
Although previous work has shown that the SF-12 is responsive to changes in the health-related quality of life of patients with venous leg ulcers upon ulcer healing,44 we note here that although 70% of trial participants had a reference ulcer heal during the study, there was no perceptible impact on mean PCS scores over time. It is important to acknowledge that it is people with a number of underlying physical comorbidities who are at increased risk of develop venous ulceration; these conditions include congestive heart failure, diabetes, peripheral vascular disease of the lower extremity and rheumatoid arthritis. 62
Adverse events
Although several adverse events were reported in VenUS IV, this had been anticipated given the older study population at risk of a number of comorbidities. 7,13,61 There was no statistically significant difference in the number of participants with one or more SAEs between trial groups, neither was there a difference in the total number of SAEs reported. Conversely, more participants in the HH group experienced one or more NSAEs compared with participants in the 4LB group (67.0% compared with 58.0%; p = 0.050); although again there was no evidence of a difference in the total number of NSAEs reported between each group (RR 1.12, 95% CI 0.95 to 1.32). Neither was there a difference in the total number of NSAEs recorded as being probably or definitely related to trial treatment (41.7% HH, 37.8% 4LB; p = 0.26).
Within the NSAEs that were probably or definitely related to trial treatment, the most common event across trial groups was bandage/hosiery failure (22%). It is not clear if these treatments were considered as failing from the perspective of the treating health professional or the participant themselves. A potential issue with the HH may have been difficulty in self-application, especially for those with grip and/or coordination problems, as the hosiery remain relatively tight and can require some dexterity to apply. Application aids are available but we did not record the level of their use in this trial.
Bandage- or hosiery-related pain and discomfort were also commonly reported as definitely or probably treatment-related NSAEs across trial groups (20%). Potential reasons for pain and/or discomfort with hosiery may have related to presence of friction via rubbing on bony prominences/discomfort caused by the hosiery top band and the lack of a padding layer as used in many bandaging systems. It is also possible that issues of discomfort could be related to the accuracy of measurement for hosiery wear. These issues would need to be investigated further.
Participant use of compression treatments
Although 61% of participants in the HH group remained on their allocated treatment, there was evidence that significantly more participants in this group changed from their trial treatment to a non-trial treatment compared with those allocated to the 4LB (where 72% remained on allocated treatment). A post hoc analysis showed that as well as being allocated to HH, a previous NSAE and age were also predictive of a shorter period on trial treatment. The most common specified reason given for the treatment change across groups was ‘compression uncomfortable’ and 71% of these reports were from the HH group. These data triangulate with the NSAE findings, further suggesting that, for some people, HH is not an optimal treatment, especially in terms of comfort.
The increased treatment change in the HH compared with the 4LB group was somewhat contradictory to what we had postulated before starting this RCT. Previously, reasons for patient non-compliance with compression bandages have been reported as pain, discomfort, application difficulty, discomfort with footwear and poor physical appearance of legs. 91 We suggested that HH might be more acceptable to venous leg ulcer patients because it is less bulky than bandages, as well as potentially easier to apply and less reliant on bandager skill. Indeed upon entry into the trial (but prior to randomisation) 50% of trial participants expressed a preference for HH over the 4LB (compared with 13% expressing a preference for four layer and 37% having no preference). In reality there may be people for whom HH is not an optimal treatment (e.g. in those who cannot apply it easily themselves).
Recurrence
In VenUS IV there was a statistically significant reduction in the chance of recurrence with HH compared with 4LB (HR 0.56, 95% CI 0.33 to 0.94; p = 0.026). This translates to a reduction in the chance of recurrence of ulceration for people allocated to HH of between 6% and 67%. This estimate was relatively imprecise as the sample size was limited to only those whose reference leg completely healed during the trial. Additionally, we acknowledge that this analysis was not of the population as randomised and thus must be interpreted with some caution. However, given that the same number of participants healed in these two groups, recurrence results would have been affected only if the characteristics of participants healing differed across the two groups, which is unlikely.
Although VenUS IV focused on HH as a treatment for healing ulcers, when planning the study we hypothesised that perhaps those who become used to wearing HH and healed were more likely to wear compression hosiery post healing (and perhaps more likely to stay in HH than those moving maintenance hosiery following treatment with a compression bandage) and thus have a reduced risk of recurrence. This hypothesis may potentially explain the results observed with those who found HH an agreeable (and effective) treatment and continuing to wear it. However, we did not collect data on use and adherence to maintenance compression treatments in this trial so could not investigate this hypothesis further. We also note that our protocol gave no guidance as to the provision of maintenance compression therapy and we assumed that standard procedures would be followed. We note there are currently limited data available on the proportion of people with a previous venous leg ulcer who are compliant with wearing compression hosiery as a maintenance treatment. A national audit conducted over 10 years ago reported that of patients with venous leg ulcers, on whom data were received, 88% had be given prophylactic compression hosiery (level of compression not reported). 92 However, the uptake, and use of these stockings was not assessed/reported.
Likewise, there are few data reporting how widely HH is currently used as a treatment for active venous leg ulceration. However, we know from VenUS IV that only 6.5% of trial participants were receiving compression hosiery as a treatment prior to entering the trial (compared with 49% receiving the 4LB). Although we acknowledge that these figures could be an underestimate (if people currently receiving HH did not enter the trial so as not to ‘risk’ randomisation to a non-hosiery treatment), if we accept the 6.5% figure as a guide to current HH use for the treatment of venous leg ulcers we can postulate that use is low. Yet, based on findings regarding recurrence here, even with the levels of treatment change recorded, HH may be the better treatment of choice for some patients in terms of reducing overall time spent with venous leg ulcers.
It is important to note that we do not suggest that there is no place for other compression therapies in the treatment of venous leg ulcers. By default those coming into this study would have been considered eligible for treatment with either of the trial compression therapies. There will be other patients, for example those with large, oedematous or awkwardly shaped legs, for whom experienced health professionals may select other compression therapies from the different options available.
Cost-effectiveness
To aid decision-making, it is important to assess the value for money – in terms of costs and benefits – that alternative treatments offer. We conducted a cost–utility analysis and a cost-effectiveness analysis – both utilising the cost data from ulcer-related health-care consultations and the number of ulcer-related treatments. The base-case analysis found that, on average, participants allocated to the HH group ‘cost’ £302 less (per participant) than those allocated to the 4LB group. The cost difference was driven by the reduction in nurse consultations in the HH group over the duration of the trial.
The cost–utility analysis used QALYs as the measure of benefit. QALYs are often the recommended measure of benefit for societal decision-makers, as they are generic and thus allow comparisons to be made across different treatments, conditions and patient populations. In this analysis, HH was shown to have marginally higher QALYs of 0.034 compared with the 4LB group. This QALY difference, despite being mathematically small, may represent a significant benefit in terms of health-related quality of life – it is equivalent to every participant receiving HH spending 36 more days in full health per year, rather than spending those days with a less optimal utility score (here assumed to be 0.65, the mean baseline utility score at trial entry).
The difference in QALYs between treatment groups, as measured by the EQ-5D, may be related to the increased recurrence rates in the 4LB group. Alternatively participants may have found HH to be less bulky and thus less limiting with regards footwear and mobility. The direction of the difference in QALYs is consistent with the small, predominantly, non-statistically significant difference in PCS scores (also favouring HH at all time points).
If we assume HH and 4LB to be equivalent in terms of effectiveness then HH should be recommended for use in this patient population because patients are expected to incur fewer costs with HH than when using the 4LB. However, despite the study reporting similar health benefits for both treatments, it was not designed as an equivalence trial and there is still some uncertainty associated with the clinical and cost results. Exploration of this uncertainty using joint uncertainty analysis of cost and QALY estimates showed there was a > 95% probability of HH being the most cost-effective treatment at willingness-to-pay thresholds of £20,000–30,000 (used by NICE).
We also conducted a cost-effectiveness analysis using the difference in time to initial healing of the reference ulcer as the outcome measure. The analysis found a small but highly uncertain difference of 1.4 ulcer-free days (fewer days in the hosiery group). However, this analysis did not consider recurrence events during the follow-up period, which were more common in the 4LB group. As a result, ulcer-free days were likely to be underestimated in the HH group in the cost-effectiveness analysis, and, as a consequence, this analysis has limited interpretability in the context of VenUS IV study findings. In contrast, the cost–utility analysis included utility data beyond initial healing and during recurrence, and this may partly explain why outcomes in cost–utility and cost-effectiveness analyses provide different results.
A sensitivity analysis considered the number of nurse-reported consultations (rather than participant reported) and showed that HH incurred costs of £114 less per participant than the 4LB. Although the cost analysis still favoured HH, this difference in cost was smaller than the base-case analysis, mainly because the nurse-reported data were recorded for only the initial healing phase and not the recurrence period. Consequently, the nurse-reported data are likely to underestimate the resource use, especially in the 4LB group. We recommend that future trial designs should record treatment-related data beyond the time of initial healing (i.e. during the entire duration of the study) to capture any relevant differences in costs and outcomes especially regarding recurrence.
The economic analysis concluded that, given the trial data available from VenUS IV, HH is highly likely to be cost-effective compared with the 4LB, even given the larger number of changes from trial treatment in the HH group. The cost-effectiveness of hosiery is mainly driven by reduced costs attributable to fewer nurse consultations, and also due to a small improvement in health-related quality of life (we hypothesise that these differences are related to the difference in recurrence observed). A further potential advantage of HH is that, as less variation in application skills is expected with HH, the choice of this treatment may reduce variation of treatment effect across centres (i.e. centre effect) and promote homogeneity of outcomes between participants.
Finally, when changing recommendations for treatments, the potential implementation costs in the NHS should also be considered. As HH is used in practice, we do not envisage significant implementation costs. However, additional efforts may be required to reduce any delays in making HH available when recommended (especially in made-to-measure cases).
Strength and limitations of the study
Sample size
As far as we are aware this is the largest, individually randomised RCT that has been undertaken to evaluate leg ulcer interventions, although we fell just short of our recruitment target of 489. Even so, it is important to note that we did obtain the precision around the point estimate that was planned for in our original sample size calculation.
Blinded outcome assessment
We were unable to conduct blinded outcome assessment of wounds in person for VenUS IV as the provision of a community-based blinded assessor across all trial centres would have been too resource intensive. Additionally, as described previously, the actual logistics of performing blinded outcome assessment would be challenging. 61 Thus, blinded outcome assessment was conducted centrally with two blinded assessors examining digital photographs taken monthly from baseline until healing, and then weekly from healing for 4 weeks. This frequency of data collection was implemented in this study for the first time and was based on our previous experience of the blinded outcome assessment processes used in VenUS II61 and III. 13
The use of sequential images from the healing period was found to work extremely well, as it allowed blinded outcome assessors to give a healed date subsequent to that given by the treating nurse if required. In VenUS IV we also implemented an electronic system for uploading pictures that helped to ensure that we received most photographs and had limited missing data.
A recent study examined data from 21 RCTs (4391 participants) that had conducted both blinded and unblinded assessment of their binary, subjective outcomes. The study reported that, on average, non-blinded outcome assessment exaggerated the odds ratio by 36% when compared with blinded outcome assessment. 93 However, this meta-epidemiological study included data from our previous study, VenUS II,61 which, like VenUS IV, found little difference in blinded and unblinded outcome assessments of healing. In VenUS IV, the unblinded assessment of ulcer healing resulted in slightly more ulcers recorded as healed, and the corresponding HR was slightly lower than the blinded assessment (HR 0.91, 95% CI 0.73 to 1.12). Thus there was still no statistically significant treatment effect although there was greater precision around the point estimate. Although giving similar results, blinded outcome assessment remains an important strength of our venous ulcer trials.
Attrition
Attrition was not an issue in analysis of the trial primary outcome (with 453/454 patients contributing at least some follow-up in the survival analysis and 6.5% of 2LB and 6.7% of four-layer hosiery patients being classified as lost to follow-up during the study), however, as with VenUS trials I–III;7,13,61 we observed a marked reduction in participant response rates for postal questionnaire containing SF-12, EQ-5D and resource-use data. This drop in response occurred despite using strategies that aimed to maximise response with the use of a £5 incentive, as well as reminders to return questionnaire. As in VenUS II,61 analysis suggested that participants were less likely to return questionnaires if they had not healed, meaning that we may have overestimated health-related quality-of-life estimates, although there was little change in these estimates during the trial. In non-healing participants there was some evidence of lower return rates in the HH group; it is unclear whether this might led to an overestimate of health-related quality of life in this group.
Generalisability of the results
VenUS IV was a pragmatic trial with broad eligibility criteria and a protocol that aimed to minimise the influence of the different service delivery models in operation across the participating centres (including outpatient clinics, general practice, district nursing teams, specialist community and tissue viability teams). Trial data for VenUS IV was collected from 32 centres across England and a centre in Northern Ireland. Of these centres there were 10 that recruited 72% of all participants. The results of this trial should therefore be highly generalisable to similar patient populations across the UK and probably to other areas of the world where care is delivered by nurses and where the clinical profiles of the patients are similar.
Part II Mixed-treatment comparison meta-analysis of high-compression treatments for venous leg ulcers
Chapter 8 Introduction
Part I provides evidence about the relative effectiveness of HH and the 4LB for healing venous leg ulcers. In practice, however, health professionals and patients have other compression treatments available to them which are thought to deliver compression of a similar magnitude (40 mmHg of compression at the ankle or high compression). Important competing alternatives to HH and the 4LB are:
-
The SSB An inelastic bandage system for which one to three rolls of bandage are applied over orthopaedic wool.
-
The zinc paste bandage An inelastic system consisting of a paste bandage, often with a support bandage on top.
-
The 2LB system Bottom layer with cohesive compression bandage.
From a decision-making perspective, using research evidence to inform the optimal treatment choice from all high-compression systems is important. However, this can be difficult when faced with several choices variously compared in a number of two-arm RCTs. Thus, to aid decision-making there is potential value in synthesising trial effectiveness data from VenUS IV with other relevant RCTs’ data within a MTC. MTCs are an extension of standard meta-analysis and allow RCT data for three or more relevant treatments to be linked in a network (via common comparators). Once linked, relative effects estimates can be produced for all included treatments – even where head-to-head trials for comparisons do not exist. However, it is important to note that although MTCs use the existing RCT evidence base to maximally inform clinical (and societal) decision-making (based on clinical effectiveness data), a number of assumptions are required. Fundamentally, a MTC assumes that features of included RCTs are homogeneous, as, theoretically, its results are considered equivalent to results from a single trial with a group for each treatment included in the network.
In this chapter all available RCTs evaluating high-compression treatments for venous leg ulcers were synthesised in a MTC with the aim of better informing decision-making regarding which treatment is likely to be most effective in terms of healing venous leg ulcers.
Chapter 9 Research objectives
To estimate the relative effectiveness of high-compression treatments for healing venous leg ulcers using all available RCT evidence.
To evaluate how the inclusion of evidence from VenUS IV (on the comparison of HH vs. the 4LB) informs estimate of treatment effects, consequent treatment recommendations and the uncertainty regarding these.
Chapter 10 Methods
Identification of relevant randomised controlled trials
The objectives of the MTC work were focused on compression systems aiming to deliver high compression (classed as ≥ 40 mmHg compression at the ankle) in the treatment of venous leg ulcers. However, at this identification stage all RCTs evaluating compression treatments [high and non-high compression (< 40 mmHg)] for venous leg ulcers were included so as to assess their potential contribution to the MTC network.
Randomised controlled trials were identified from the most recent version of an ongoing Cochrane review update available to us (search dates May 2012). 19 We did not conduct this review but rather utilised aspects of the search and results here. Given the recency and rigour of this Cochrane review, it was used as the sole source of RCTs for the MTC – thus we broadly adopted its eligibility criteria. 19 Please refer to the Cochrane review19 for additional information on each trial. However, as the primary outcome of the MTC was ulcer healing (time to healing and/or number of ulcers completely healed within a specific time period), RCTs from the source review were excluded if they did not report at least one of these outcomes. Ulcer healing (effectiveness) data were extracted from the source review directly as was information regarding treatment type, number of participants allocated to each treatment group and trial duration. The latter was assumed to be the trial follow-up time unless otherwise stated.
Description of available data
Initial classification
Data were obtained from 40 RCTs (Table 42; for further details see Appendix 11), but three were excluded as they did not report suitable endpoints for the MTC analysis (e.g. reduction in wound area reported rather than time to healing or proportion of participants healed). Of the remaining 37 RCTs, many evaluated well-established compression treatments that were recognised as aiming to deliver either high (≥ 40 mmHg) or non-high (< 40 mmHg) compression. However, a number evaluated ad hoc treatments, for example hybrids of compression components, with a compression level that we could not easily classify. Thus, to formally establish whether evaluated treatments aimed to deliver high compression (or not) an internationally recognised expert (Hugo Partsch) in compression systems classified each compression treatment, based on its component composition (Box 2) [see Table 42, ‘(2)’]. Subsequently, the expert classified each treatment as high or not high compression, based on the number of components, the initial pressure (reported or estimated by expert depending on detail report) and stiffness of the final treatment (estimated by expert). Among all available 82 treatment groups, 45 treatment groups were classified as high compression and 37 as non-high compression [see Table 42, ‘(I)’].
Type of treatment:
-
B = bandage
-
H = hosiery
-
V = Velcro device.
Details of layers:
-
a = adhesive
-
c = cohesive
-
e = elastic
-
i = inelastic
-
z = zinc paste.
In the case of multilayer bandaging, the first letter B denotes treatment type. Details of subsequent ‘active’ components are then recorded. Thus a 4LB classification starts with ‘B’ followed by details of the type of bandages, e.g. ‘Beec’ (two elastic and a cohesive bandage).
Hosiery is denoted as ‘H’ (HH for HH) and Velcro devices by ‘V’.
Note that some unusual compression treatments exist, e.g. the combinations of bandage and hosiery (BheH) or hosiery with Velcro device (HV).
Also note that some treatments of the same class (e.g. HH) could be classified as non-high compression or as high compression. These were here considered as unique treatments due to different level of compression they were judged to deliver.
Details on further grouping of high-compression systems of interest-
4LB = ‘Beec’ and ‘Beea’.
-
SSB = ‘Bii’, ‘Biic’ and ‘Bc’.
-
Paste = ‘Bzc’ and ‘Bza’.
-
Multicomponent hosiery = ‘HH’.
-
2LB = ‘Bic’.
Further grouping
Subsequently, where possible, treatments were further grouped into key categories consistent with current practice. For example treatments defined as ‘Beec’ and ‘Beea’ were grouped into the 4LB group. Similarly, ‘Bii’, ‘Biic’ and ‘Bc’ were all grouped into a SSB group; ‘Bzc’ and ‘Bza’ into a zinc paste group (referred to as ‘paste’); ‘HH’ grouped as ‘HH (two-layer: aiming to deliver 40 mmHg compression at the ankle)’ and ‘Bic’ grouped as the 2LB system (subcompression wadding and cohesive bandage). The remaining, generally ad hoc, systems were not grouped further. This classification process was checked with members of the TMG, cross-checked with the source review and judged as valid. After this further grouping process the evidence evaluated 24 unique treatments, 13 of which were classed as high compression (see Table 42, ‘3’).
| ID | Studies | n | High or not (1) | Initial classification (2) | Further grouping (3) |
|---|---|---|---|---|---|
| 2 | Cordts 199294 | 23 | Not | Bc | Bc |
| 20 | Not | Bz | Bz | ||
| 3 | Eriksson 198695 | 17 | High | Bza | Paste |
| 17 | Not | Be | Be | ||
| 6 | Nelson 200796 | 128 | Not | Ba | Ba |
| 117 | High | Beec | 4LB | ||
| 7 | Danielsen 199897 | 23 | Not | Be | Be |
| 20 | Not | Bi | Bi | ||
| 8 | Moody 199998 | 26 | Not | Bi | Bi |
| 26 | Not | Be | Be | ||
| 9 | Moffatt 200399 | 57 | High | Beec | 4LB |
| 52 | Not | Be | Be | ||
| 10 | Callam 1992100 | 65 | Not | Bee | Bee |
| 67 | High | Bic | 2LB | ||
| 11 | Gould 1998101 | 19 | Not | Bze | Bze |
| 20 | Not | Bzee | Bzee | ||
| 12 | Meyer 2002102 | 57 | Not | Bzee | Bzee |
| 55 | Not | Bzie | Bzie | ||
| 15 | Moffatt 1999103 | 115 | High | Beec | 4LB |
| 117 | High | Beec | 4LB | ||
| 16 | Vowden 2000104 | 50 | High | Beec | 4LB |
| 50 | High | Beea | 4LB | ||
| 49 | High | Beec | 4LB | ||
| 24 | Meyer 200325 | 64 | Not | Bzee | Bzee |
| 69 | High | Beec | 4LB | ||
| 27 | Hendricks 1985105 | 10 | Not | Bzee | Bzee |
| 14 | Not | H | H | ||
| 28 | Koksal 2003106 | 27 | Not | Bz | Bz |
| 26 | Not | H | H | ||
| 30 | Polignano 200429 | 29 | Not | Bi | Bi |
| 27 | Not | HH | HH | ||
| 31 | Junger 2004107 | 88 | Not | H | H |
| 90 | Not | Bi | Bi | ||
| 32 | Milic 2007108 | 75 | Not | BeH | BeH |
| 75 | High | Bee | Bee | ||
| 33 | Harley 2004109 | 16 | High | Beec | 4LB |
| 14 | Not | Be | Be | ||
| 35 | Mariani 200830 | 26 | Not | HH | HH |
| 30 | Not | Bi | Bi | ||
| 36 | Taradaj 200931 | 40 | Not | H | H |
| 40 | Not | Bi | Bi | ||
| 37 | Milic 2010110 | 42 | Not | BeH | BeH |
| 46 | Not | BeH | BeH | ||
| 43 | High | BeHe | BeHe | ||
| 38 | Brizzio 2010111 | 28 | Not | H | H |
| 27 | Not | Bi | Bi | ||
| 1 | Kralj 1996112 | 20 | High | Beec | 4LB |
| 20 | High | Ba | Ba | ||
| 5 | Colgan 1995113 | 10 | High | BzeaH | BzeaH |
| 10 | High | Beec | 4LB | ||
| 10 | Not | Be | Be | ||
| 13 | Duby 1993114 | 25 | High | Bii | SSB |
| 25 | High | Beec | 4LB | ||
| 26 | Not | Bzee | Bzee | ||
| 14 | Wilkinson 1997115 | 17 | High | Beec | 4LB |
| 18 | High | BHeH | BHeH | ||
| 17 | Scriven 1998116 | 32 | High | Beec | 4LB |
| 32 | High | Biic | SSB | ||
| 18 | Partsch 2001117 | 53 | High | Beec | 4LB |
| 59 | High | Bii | SSB | ||
| 19 | Ukat 2003118 | 44 | High | Beec | 4LB |
| 45 | High | Bii | SSB | ||
| 20 | Franks 2004119 | 74 | High | Beec | 4LB |
| 82 | High | Bc | SSB | ||
| 21 | Iglesias 20047 | 195 | High | Beec | 4LB |
| 192 | High | Bii | SSB | ||
| 23 | Polignano 2004120 | 39 | High | Beec | 4LB |
| 29 | High | Bzc | Paste | ||
| 26 | Blecken 2005121 | 12 | High | HV | HV |
| 12 | High | Beec | 4LB | ||
| 29 | Junger 200428 | 61 | High | HH | HH |
| 60 | High | Bii | SSB | ||
| 34 | Moffatt 2008122 | 42 | High | Beec | 4LB |
| 39 | High | Bic | 2LB | ||
| 39 | Szewczyk 2010123 | 15 | High | 4LB | 4LB |
| 16 | High | 2LB | 2LB | ||
| 40 | Wong 2012124 | 107 | High | 4LB | 4LB |
| 107 | High | SSB | SSB | ||
| Studies excluded (did not report suitable endpoints to contribute to analyses) | |||||
| 25 | DePalma 1999125 | 19 | Not | Bze | Bze |
| 19 | High | V | V | ||
| 4 | Travers 1992126 | 15 | High | Ba | Ba |
| 12 | Not | Bzee | Bzee | ||
| 22 | Knight 1996127 | 5 | High | Beec | 4LB |
| 5 | Not | Bz | Bz | ||
Formation of analytic sample
Although all included 37 RCTs (evaluating both high and non-high-compression treatments) formed a network of evidence (see Appendix 12), treatments not connected to the main network were excluded (‘BeHe’ and ‘Bee’). Additionally, given the focus of this MTC work on high-compression treatments, non-high-compression treatments were excluded unless they formed indirect links between high-compression treatments and thus contributed to inferences on these (i.e. ‘Bzee’, ‘H’, ‘Bi’, ‘Be’). These decisions resulted in the exclusion of 11 further RCTs, leaving an analytic sample for the MTC comprising 26 RCTs (Table 43).
| ID | Study | Final groups | Follow-up (weeks) | n | Duration (months) | Size | No. healed | Availability of evidence | Included in base-case analysis |
|---|---|---|---|---|---|---|---|---|---|
| 13 | Duby 1993114 | 4LB | 12 | 25 | 20.5 | 11.9 | 11 | AD | Yes |
| SSB | 12 | 25 | 26.7 | 13.1 | 10 | Yes | |||
| Bzee | 12 | 26 | 34.5 | 12.3 | 6 | No | |||
| 17 | Scriven 1998116 | 4LB | 52 | 32 | 13 | 13.3 | 17.6 | AD | Yes |
| SSB | 52 | 32 | 21 | 8.3 | 18.24 | Yes | |||
| 18 | Partsch 2001117 | 4LB | 16 | 53 | 1.25 | 1.5 | 33 | AD | Yes |
| SSB | 16 | 59 | 1 | 1.9 | 43 | Yes | |||
| 19 | Ukat 2003118 | 4LB | 12 | 44 | – | 17.7 | 13 | AD | Yes |
| SSB | 12 | 45 | – | 12.2 | 10 | Yes | |||
| 20 | Franks 2004119 | 4LB | 24 | 74 | 2 | 5 | 59 | AD | Yes |
| SSB | 24 | 82 | 2 | 3.5 | 62 | Yes | |||
| 29 | Junger 200428 | SSB | 12 | 60 | 5.57 | 5.95 | 19 | AD | Yes |
| HH | 12 | 61 | 4.14 | 5.62 | 29 | Yes | |||
| 1 | Kralj 1996112 | 4LB | 24 | 20 | 7.9 | 18.6 | 7 | AD | Yes |
| Ba | 24 | 20 | 6.9 | 17.2 | 8 | Yes | |||
| 23 | Polignano 2004120 | 4LB Paste |
24 | 39 | – | 10.1 | 29 | AD | Yes |
| 24 | 29 | – | 9.3 | 19 | Yes | ||||
| 14 | Wilkinson 1997115 | 4LB | 12 | 17 | – | 11.2 | 8 | AD | Yes |
| BHeH | 12 | 18 | – | 8.6 | 8 | Yes | |||
| 5 | Colgan 1995113 | 4LB | 12 | 10 | 9.3 | 27.5 | 6 | AD | Yes |
| BzeaH | 12 | 10 | 66.5 | 48.5 | 7 | Yes | |||
| Be | 12 | 10 | 53.5 | 42.8 | 2 | No | |||
| 26 | Blecken 2005121 | 4LB | 12 | 12 | – | 50.08 | 4 | AD | Yes |
| HV | 12 | 12 | – | 48.98 | 4 | Yes | |||
| 34 | Moffatt 2008122 | 4LB | 4 | 42 | 48.8 | 5.7 | 3 | AD | Yes |
| 2LB | 4 | 39 | 46.6 | 11.8 | 6 | Yes | |||
| 39 | Szewczyk 2010123 | 4LB | 12 | 15 | – | 6 | 9 | AD | Yes |
| 2LB | 12 | 16 | – | 5.3 | 10 | Yes | |||
| 40 | Wong 2012124 | 4LB | 24 | 107 | – | – | 72 | AD | Yes |
| SSB | 24 | 107 | – | – | 77 | Yes | |||
| 21 | Iglesias 20047 | 4LB | 52 | 195 | 3 | 3.81 | 107 | IPD | Yes |
| SSB | 52 | 192 | 3 | 3.82 | 86 | Yes | |||
| 3 | Eriksson 198695 | Paste | 12 | 17 | – | – | 7 | AD | No |
| Be | 12 | 17 | – | – | 9 | No | |||
| 7 | Danielsen 199897 | Be | 52 | 23 | 22.2 | 19.7 | 12 | AD | No |
| Bi | 52 | 20 | 27.8 | 16.5 | 3 | No | |||
| 8 | Moody 199998 | Bi | 12 | 26 | – | – | 8 | AD | No |
| Be | 12 | 26 | – | – | 8 | No | |||
| 9 | Moffatt 200399 | 4LB | 12 | 57 | 1.5 | – | 40 | AD | No |
| Be | 12 | 52 | 1.5 | – | 30 | No | |||
| 24 | Meyer 200325 | Bzee | 52 | 64 | 19.8 | – | 51 | AD | No |
| 4LB | 52 | 69 | 14.8 | – | 45 | No | |||
| 27 | Hendricks 1985105 | Bzee | 78 | 10 | 29.5 | 28.28 | 7 | AD | No |
| H | 78 | 14 | 11.9 | 45.35 | 10 | No | |||
| 31 | Junger 2004107 | H | 12 | 88 | 1.45 | 2.4 | 51 | AD | No |
| Bi | 12 | 90 | 1.5 | 2.4 | 51 | No | |||
| 33 | Harley 2004109 | 4LB | 30 | 16 | – | – | 13 | AD | No |
| Be | 30 | 14 | – | – | 8 | No | |||
| 36 | Taradaj 200931 | H | 8 | 40 | 30.54 | 20.57 | 15 | AD | No |
| Bi | 8 | 40 | 30.11 | 20.33 | 5 | No | |||
| 38 | Brizzio 2010128 | H | 24 | 28 | – | 13.1 | 14 | AD | No |
| Bi | 24 | 27 | – | 12.2 | 18 | No | |||
| 41 | VenUS IV | 4LB | 52 | 224 | 12.29 | 9.30 | 157 | IPD | Yes |
| HH | 52 | 230 | 10.82 | 9.41 | 163 | Yes |
Finally, although treatments ‘Ba’, ‘BzeaH’, ‘HV’ and ‘BHeH’ were classed as high compression, they were considered as ad hoc treatments that were not widely used in clinical practice. Because of this they were included in the MTC but their results are not reported here in detail.
Implementation of the mixed-treatment comparison
The evidence in the analytic data set (see Table 43) was organised into two MTC networks, as shown in Figure 11. One network included only treatments classified as aiming to deliver high compression (Figure 11a) and another where both high and non-high-compression treatments were included (Figure 11b). Given the focus of this work on high-compression treatments, the base-case analysis used the network with high-compression treatments only (see Figure 11a). This network encompassed five standard treatments (4LB, SSB, paste, HH and the 2LB) and four ad hoc systems (Ba, HV, BzeaH and BHeH) (see Figure 11a). This base-case network consisted of 32 groups (data points) from 16 trials (including VenUS IV). The scenario model was evaluated in a sensitivity analysis, which is detailed later.
FIGURE 11.
Network of RCTs. In the network, a unique treatment category is indicated by a circle: high compression = green circles, non-high-compression treatments = smaller grey circles. Arrows between circles indicate that these treatments had been compared in a trial [trials are identified using ‘( )’, numbered as in column ‘ID’ in Table 43]. (a) Base-case analysis; (b) scenario analysis.
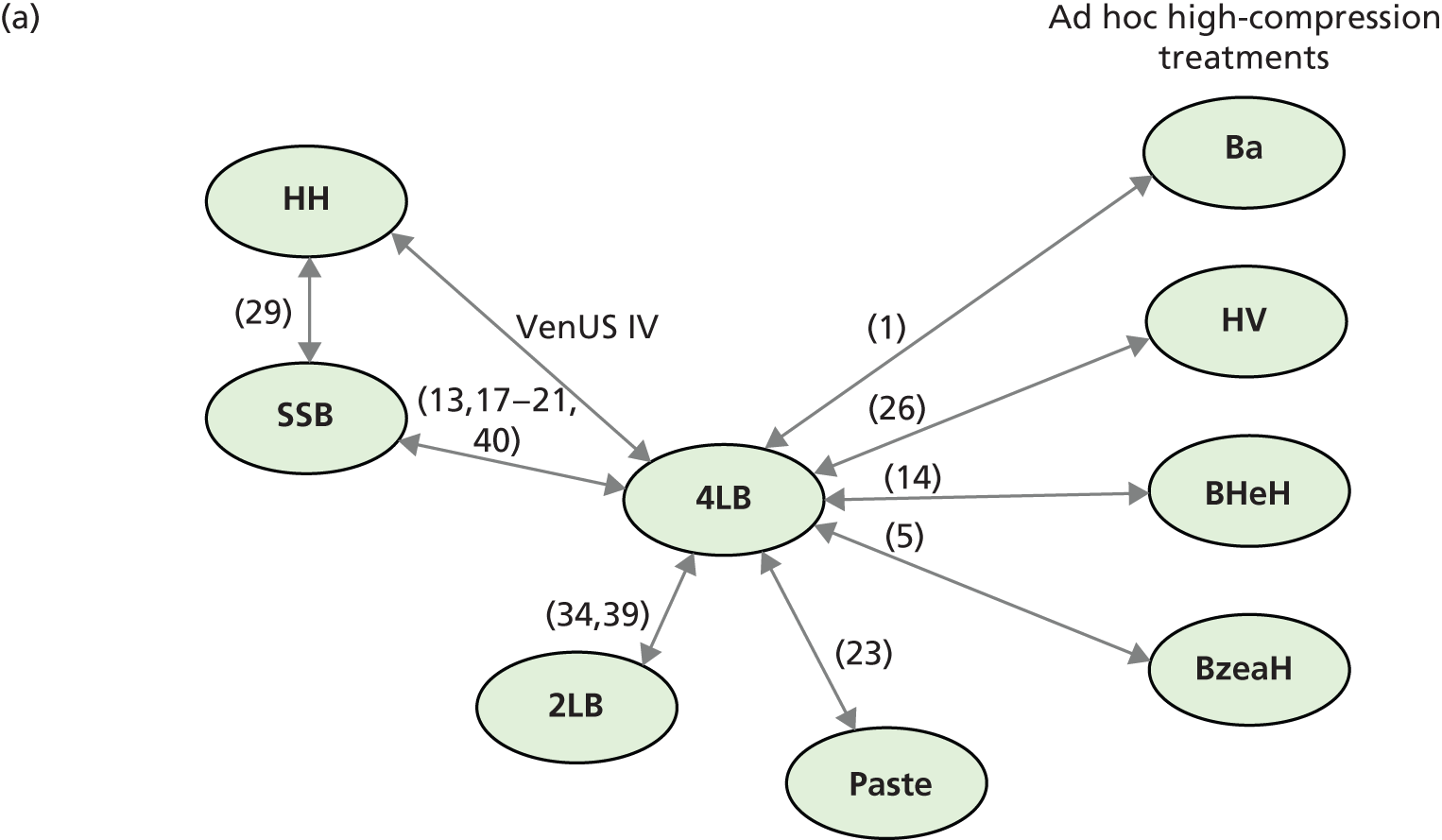
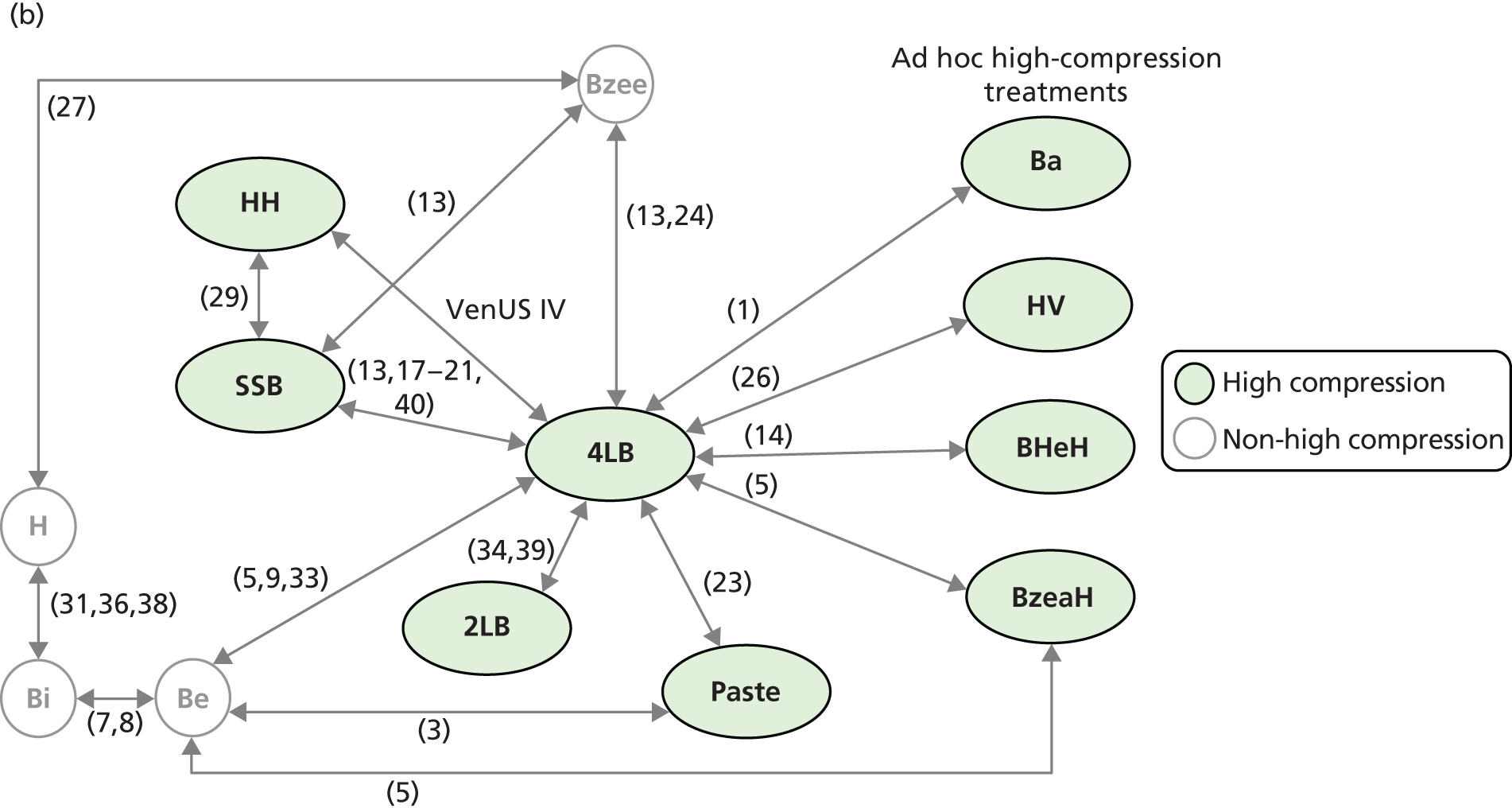
In this MTC the most populated comparison was the 4LB compared with SSB, which was informed by seven RCTs, six of which had aggregate (trial summary) data114,116–119,124 and one of which had IPD data. 7 The comparison between the 4LB and HH was also populated by IPD from VenUS IV. The link between the 2LB and 4LB systems was informed by two RCTs, and each of the remaining six comparisons in the evidence network of the base-case analysis were informed by aggregate information extracted from one trial in each case (see Figure 11a).
Although this base-case analysis included trial data from VenUS IV, the model was also run excluding these trial data to provide an insight into the contribution of VenUS IV data in the conclusions drawn from the MTC.
Statistical model for the data
For each included RCT, the most complete ulcer healing outcome data available were used in the MTCs: for two studies time to ulcer healing and time to censoring were available as IPD (VenUS I7 and VenUS IV); for the remaining RCTs, outcome data were reported as the number of healed ulcers by study group [aggregate data (AD)]. We maximally drew on all available data by statistically synthesising the AD with the IPD, extending the methodology described in Smith et al. 129 and Saramago et al. 130
Briefly, data were synthesised by assuming that the time to healing underlying both AD and the IPD was described by the same parametric distribution. For the IPD, where time to healing (under censoring) was observed for each RCT participant – these data could directly inform the distribution of the time to healing (the likelihood). For the AD, by assuming a binomial likelihood, the number of participants healed was used to inform the probability of participants being healed. In turn, the probability of participants being healed was related (algebraically) to the common distribution of time to healing taking into account the duration of follow-up in each study (analogous to the synthesis models defined by Soares et al. 131 This approach allowed all ulcer healing data (proportion of ulcers healed and time to ulcer healing) to be defined as time to ulcer healing thus the measure of effectiveness used to report MTC findings was the HR.
Had we only considered aggregate information (reducing the information on studies with available IPD to AD and synthesising this in the standard way with the remaining aggregate information), the synthesis would have been constrained to use of the exponential distribution, which imposes a constant hazard of healing over time (the hazard of healing at any instant is constant). The use of IPD here allowed more complex time-to-event distributions, namely the Weibull and Gompertz distributions, to be investigated. Both these distributions are flexible when compared with the exponential, in that they can reflect an increasing, decreasing or constant hazard of healing over time. 63 In addition to Weibull and Gompertz, we recognise that there is other time-to-event distributions such as the log-normal or the generalised gamma, which could potentially have provided a better fit to the time-to-healing data. However, given that these distributions do not allow the probability of healing over time to be expressed in a closed form (key to the approach proposed here for the joint synthesis of IPD and AD), we opted to use the Weibull distribution, as implementation issues prevented us from using the Gompertz distribution. Further work is required in this area to embrace these other modelling frameworks.
The parametric survival modelling of the IPD studies was implemented using regression analysis that allowed for baseline covariate adjustment. All relative treatment effects were presented as HRs with the 4LB arbitrarily chosen as reference treatment in all cases. As several of the treatment comparisons in the MTC network were populated by a single RCT (exceptions are SSB vs. 4LB and 2LB vs. 4LB), a fixed-effects modelling approach was used. In additional analysis (not reported here), we also considered the use of a random-effects model. We found no significant gains in quality of fit, and thus adopted the more parsimonious fixed-effects approach throughout. This is consistent with previous published work synthesising evidence on the comparison between SSB and 4LB,24 which also chose to undertake a fixed-effects approach because there was no significant evidence of between-study heterogeneity. The baseline covariates considered were participant mobility, duration and area of ulcer and centre, all available in VenUS I7 and VenUS IV. The effects of these covariates on the hazard of healing were assumed equal in both IPD studies (both studies were used to estimate the covariate effects). The effect of centre was described using a common frailty effect across the IPD trials.
Besides adjusting for baseline covariates, we tested the inclusion of interaction terms between alternative treatments and baseline ulcer area and duration simultaneously in the RCTs with IPD and aggregate outcome data – as described by Cooper et al. 41 and Saramago et al. 130 In considering treatment–effect interactions, the use of IPD allows individual patient covariate information to be modelled and, consequently, avoids potential ecological fallacy. 132 Interaction terms were not found to be important in explaining the hazard of healing over time in this case study and were excluded from the final model.
All analyses were conducted from a Bayesian perspective, using WinBUGS software version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK. URL: www.mrcbsu.cam.ac.uk/bugs/WinBUGS/contents.shtml). Alternative model specifications were implemented to assess the inclusion of baseline covariates and treatment–interaction terms. Goodness of fit was assessed using the deviance information criterion (DIC). 133 The DIC is a measure that balances fit and complexity, allowing parsimony to be considered in model choice. Alternative model specifications were used (see Appendix 12) and model fit was assessed using DIC. Results for the best fitting model only are presented along with 95% credible intervals (Crls), the Bayesian equivalent of CIs.
The treatment with the lowest HR estimated in the MTC (with respect to the reference compression system, the 4LB) is expected to have the highest effectiveness in healing venous leg ulcers. However, it is important to be fully aware of uncertainty around these estimates. Thus, in addition to presenting CrIs, for the selected model in the base-case analysis we explored uncertainty regarding treatment choice, presenting this as the probability that each compression system was the ‘best’ treatment in terms of being the most effective (when compared with all other evaluated treatments).
Quality assessment and consistency of evidence
As with other methods of evidence synthesis, the quality of the data included in the model must be reflected in conclusions made. Although there is no recognised system to undertake such quality assessment for MTC we have previously published a modified Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (we called this iGRADE) to allow us to assess and communicate the quality of MTC-derived evidence. 134 The iGRADE approach uses the five GRADE categories that allow the quality of evidence to be decreased, with focus of some categories altered to be relevant for MTC assessment (see Appendix 12 for further information). We conducted a cautious application of iGRADE (see Appendix 13) to the key estimates of this base-case analysis MTC, in which estimates could be graded as very low-quality evidence, low evidence, moderate evidence and high-quality evidence.
One of the dimensions assessed in the iGRADE is of evidence inconsistency, i.e. discrepancy between the direct and the indirect evidence in existing evidence loops. 135 We formally assessed for inconsistencies using the back calculation method suggested by Bucher et al. 136 Briefly, within an evidence loop, direct and indirect estimates of a pairwise treatment effect were compared against a null hypothesis that there would be no difference between them.
Sensitivity analysis
Inclusion of non-high-compression treatments in the mixed-treatment comparison
Despite findings from non-high-compression treatments being less relevant here, it is possible that the inclusion of data from RCTs evaluating these treatments could impact on inferences on the high-compression treatments of interest. For this reason a network of evidence with non-high-compression treatments was also considered as a secondary ‘scenario’ analysis (studies numbered 3, 7–9, 24, 27, 31, 33, 36, 38, as shown in the network diagram in Figure 11b). In addition to the nine high-compression treatments, the network consisted of four non-high-compression treatments (Bzee, H, Bi and Be), adding 10 further RCTs and 22 additional treatment groups to the network (where two of the additional RCTs were three armed).
This analysis tested the robustness of the evidence synthesis findings for the base-case analysis by assessing how any supplementary indirect evidence had an impact in the estimation of treatment effects for high-compression treatments.
The effectiveness of the two-layer bandage
Because the quality of RCT data driving the relative effectiveness of the 2LB to the 4LB was so low, we conducted a post hoc sensitivity analysis assuming that there was no difference in the effectiveness of these treatments.
Additional analyses
Presentation of mixed-treatment comparison results in terms of probability of healing over time
Mixed-treatment comparison results were initially presented using the HR. However, the hazard (and its ratio) can sometimes be a difficult measure to interpret, as it reflects the instantaneous risk of healing, which is a somewhat abstract (but mathematically useful) measure of the likelihood of healing. In order to aid the interpretation of the MTC, the hazard of healing findings was mathematically transformed to give the probability of participants healing over time for each of the high-compression systems of interest. The data were presented in this way to aid the interpretation of findings and the implications of these to clinical practice.
Uncertainty over the probability of healing with four-layer bandage (reference treatment)
As the 4LB group was a reference treatment in the MTC analyses, its estimates were not shown as uncertain. For completeness, we also presented uncertainty over the probability of healing in the 4LB group (taken from the MTC model as previously described).
Determinants of ulcer healing
Finally, for general interest to the field, and to aid analysis in Part III regarding the cost-effectiveness of subgroups of venous ulcer patients, we explored the implication adjustments made in the MTC for participant’s baseline characteristics (ulcer area, ulcer duration at baseline and mobility), which investigate whether these have an impact on healing. This analysis indicates which participants characteristics are expected to be associated with a higher, or lower, probability of healing over time. For the characteristics deemed relevant (those that showed statistically significant effects) we illustrated how the probability of healing over time was affected, using inferences obtained in the MTC (note that these parameters were informed by the IPD trials, VenUS I7 and VenUS IV).
Chapter 11 Results
Base-case analysis including VenUS IV data
Table 44 shows the HR estimates for each alternative high-compression treatments evaluated in existing studies, compared with the 4LB. Where HR > 1, the treatment is deemed more effective (higher hazard of healing) than the reference 4LB; where HR < 1, the treatment is deemed less effective than the 4LB and a HR of ‘1’ means that the treatments are deemed the same in terms of effectiveness. Generally, the network included small studies, which led to high imprecision around the point estimates for some relative treatment effects – particularly for some ad hoc high-compression treatments.
| HRs | Base-case analysis | Scenario analysis | |||||
|---|---|---|---|---|---|---|---|
| Including VenUS IV (1) | Excluding VenUS IV (2) | Including VenUS IV (3) | |||||
| HR | 95% CrI | HR | 95% CrI | HR | 95% CrI | ||
| Treatment effects (vs. 4LB) | SSB | 0.88 | 0.76 to 1.03 | 0.92 | 0.79 to 1.07 | 0.89 | 0.77 to 1.04 |
| HH | 1.05 | 0.85 to 1.29 | 1.57 | 0.87 to 2.90 | 1.05 | 0.85 to 1.29 | |
| Paste | 0.77 | 0.41 to 1.42 | 0.78 | 0.41 to 1.43 | 0.68 | 0.39 to 1.18 | |
| 2LB | 1.40 | 0.65 to 3.05 | 1.38 | 0.63 to 3.08 | 1.39 | 0.64 to 3.14 | |
| Ba | 1.19 | 0.43 to 3.47 | 1.20 | 0.41 to 3.43 | 1.22 | 0.42 to 3.50 | |
| BHeH | 0.93 | 0.34 to 2.62 | 0.93 | 0.34 to 2.61 | 0.93 | 0.33 to 2.61 | |
| BzeaH | 1.33 | 0.42 to 4.51 | 1.36 | 0.42 to 4.55 | 1.98 | 0.65 to 6.02 | |
| HV | 1.00 | 0.23 to 4.22 | 0.99 | 0.22 to 4.32 | 1.01 | 0.22 to 4.48 | |
| Baseline characteristics | Log(area) | 0.71 | 0.66 to 0.76 | 0.69 | 0.62 to 0.77 | 0.71 | 0.66 to 0.76 |
| Log(duration) | 0.92 | 0.90 to 0.94 | 0.94 | 0.92 to 0.96 | 0.92 | 0.90 to 0.94 | |
| Difficulty in walking | 0.71 | 0.60 to 0.85 | 0.71 | 0.55 to 0.91 | 0.72 | 0.60 to 0.85 | |
| Immobile | 0.67 | 0.23 to 1.52 | 0.51 | 0.11 to 1.45 | 0.66 | 0.22 to 1.48 | |
From the set of high-compression treatments of interest (4LB, SSB, HH, paste and 2LB), the 2LB (where a 2LB system was a subcompression wadding layer and cohesive bandage aiming to deliver 40 mmHg compression at the ankle) is expected to be most effective (2LB vs. 4LB HR: 1.40, 95% CrI 0.65 to 3.05). However, it is important to note that direct data for this estimate come from two small RCTs; consequently, uncertainty around this estimate is considerable. For this reason, the quality of the evidence is crucial in aiding the interpretation of these findings and is considered later.
The SSB is expected to be less effective than the 4LB (HR 0.88, 95% CrI 0.76 to 1.03); there is little uncertainty, although CrIs do include ‘1’. This is the most precise treatment comparison in the network. The HR estimated for the comparison of HH with the 4LB is close to ‘1’ (HR 1.05, 95% CrI 0.85 to 1.29); existing evidence does not suggest that the effectiveness of these treatments differs for treatment of venous leg ulcers in terms of ulcer healing.
Probability of treatments being best
An important feature of the Bayesian methodology used here is the ability to assess the probability that each relevant treatment is the best with respect to the primary endpoint (i.e. time to ulcer healing). When making recommendations on which treatment to use, this measure reflects the impact of uncertainty on the relative treatment effects. In the base-case analysis model (including VenUS IV data) the treatment associated with greater probability of healing was the 2LB (71.9%) (Table 45), reflecting the fairly high relative effect point estimate and the wide uncertainty around this from available evidence (see Table 44).
Quality assessment and consistency of evidence
Interpretation of the MTC evidence must consider also its quality. The overall quality of the evidence for each treatment comparison was assessed using the iGRADE approach. Table 46 shows the result of this assessment together with the effect estimates from comparison of the five main ulcer treatments.
| Compression type | 4LB | SSB | HH | Paste | 2LB |
|---|---|---|---|---|---|
| 4LB | 1 | ||||
| SSB | 0.885 H (0.76 to 1.03) | 1 | |||
| HH | 1.045 H (0.85 to 1.29) | 1.183 L (0.93 to 1.49) | 1 | ||
| Paste | 0.772 M (0.41 to 1.42) | 0.874 L (0.46 to 1.61) | 0.737 M (0.38 to 1.41) | 1 | |
| 2LB | 1.395 L (0.65 to 3.05) | 1.574 L (0.72 to 3.5) | 1.333 VL (0.6 to 3.02) | 1.819 VL (0.68 to 5) | 1 |
Although the MTC model finds that 2LB was associated with a higher hazard of healing than the other treatments, quality assessment shows that these findings were driven by direct, but low-quality, evidence encompassing two studies [see Table 42, study numbers 34122 and 39123 with unclear/high risk of bias (one study was particularly small, with 31 participants)]. Inferences on the 4LB compared with SSB were made using data from multiple RCTs; however, the results are driven by a high-quality study for which IPD were available7 and the quality classification for this estimate is high. Except for the two treatment comparisons informed by a high-quality study available in IPD format, estimates were considered to be of medium to very low quality.
In the network of evidence there was one closed loop through which both direct and indirect data informed relative treatment effect estimates. Thus, the existence of inconsistency was explored. There was no evidence of statistically significant discrepancies between the direct and the indirect data. However, we note, given the fairly high uncertainty in the evidence base and the obtained estimates, only large differences in direct and indirect data within the loop would have returned a statistically significant result.
Overall, the quality of the evidence limits the confidence that we have in some MTC findings. Although estimates for the 2LB suggest that it has the highest probability of being the most effective, these findings are supported by low- or very low-quality evidence. Estimates for the SSB and HH indicate that they have a fairly low probability of being the best; however, these estimates are informed by high-quality evidence.
Contribution of VenUS IV to the mixed-treatment comparison
The MTC model was also run excluding trial data from VenUS IV [see Table 44, (2)]. This shows that inferences on HH were altered by the inclusion of VenUS IV evidence. When VenUS IV data were ‘excluded’, estimates were driven by indirect evidence from small RCTs and the model suggested that HH was more effective than the 4LB. In this model, the treatment associated with the highest probability of ulcer healing was HH [58.4%, see Table 45, (2)]. The inclusion of trial data from VenUS IV, which found no evidence of a difference in ulcer healing between HH and the 4LB, reduces the uncertainty surrounding point estimates.
Further results (not shown) indicate that the hazard of healing was expected to decrease over time when VenUS IV data were excluded, implying that it was expected that ulcers were less likely to heal as time goes on. When including VenUS IV, the hazard of healing was expected to be approximately constant over time.
Sensitivity analysis
Inclusion of non-high-compression treatments in the mixed-treatment comparison
The results of the sensitivity analysis considering evidence on both high-compression and non-high-compression treatments [see Table 45, (3)] show that extending the network had a very limited effect, with the relative effect estimates of the main five high-compression treatments remaining similar (see Table 45, (3) vs. (1)].
Sensitivity analysis regarding the effectiveness of two-layer bandage
Because the quality of RCT data driving the relative effectiveness of the 2LB to the 4LB was so low we conducted a post hoc analysis, assuming that there was no difference in the effectiveness of these treatments. In this scenario the high-compression treatment with highest estimated probability of being best was HH, with approximately 67% probability of being best (the 4LB and the 2LB, together, would have 5.5% probability of being best).
Additional analyses
Presentation of mixed-treatment comparison results in terms of probability of healing over time
For ease of interpretation we also present MTC findings as the probability of healing over time (Figure 12). In Figure 12a, point estimates for the probability of healing are plotted showing that, at any time point, the 2LB is expected to be the most effective high-compression treatment and paste is the least effective one. The expected probability of healing for HH is equivalent to that of the 4LB.
FIGURE 12.
Reflecting uncertainty over relative treatment effects in the probability of healing over time for the five main high-compression ulcer treatments. (a) The expected probabilities of healing (point estimates) across time (25 months); (b) to (e) compare the expected values for 4LB with the healing probability (point estimates and uncertainty) of each of the other four high-compression treatments. Estimates reflect the average participant in the trial data from VenUS IV (mean ulcer area at baseline of 9.4 cm2 and ulcer duration at baseline of 11.5 months).
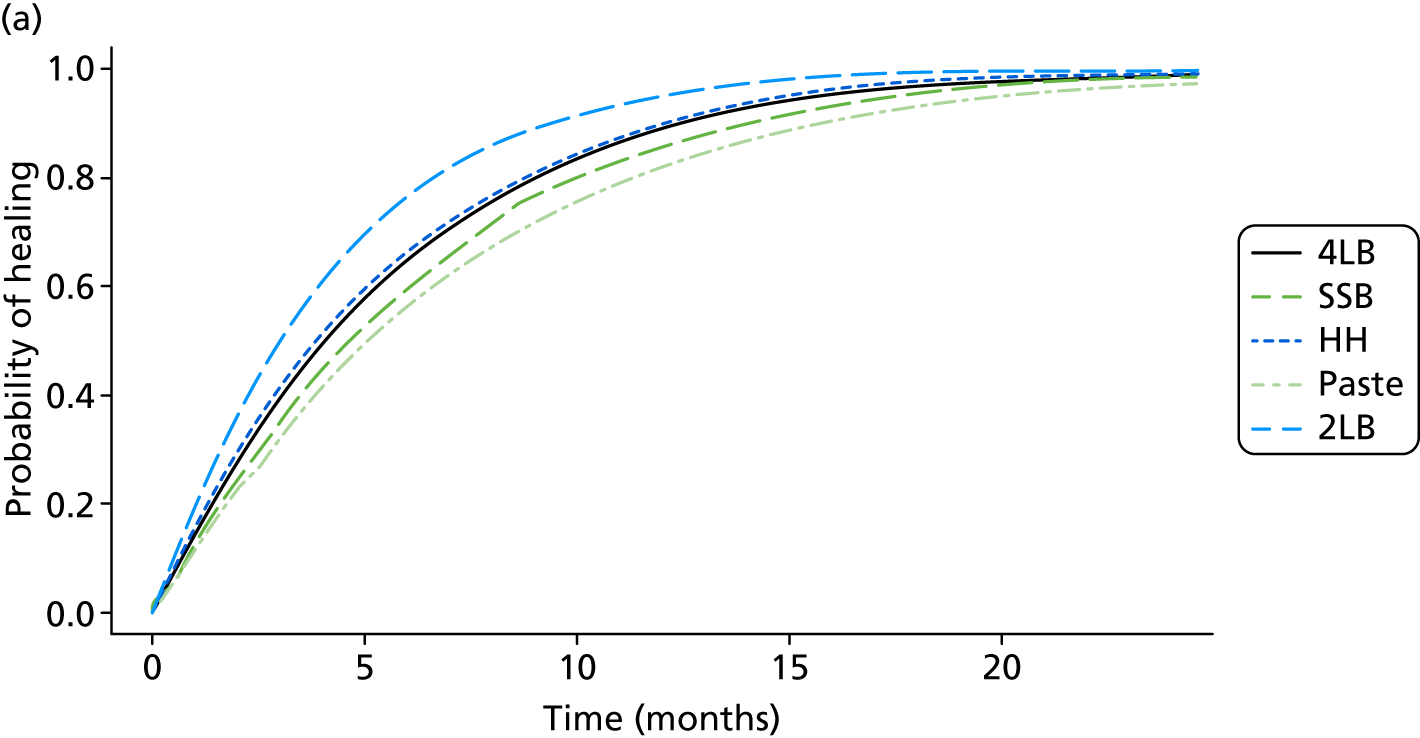
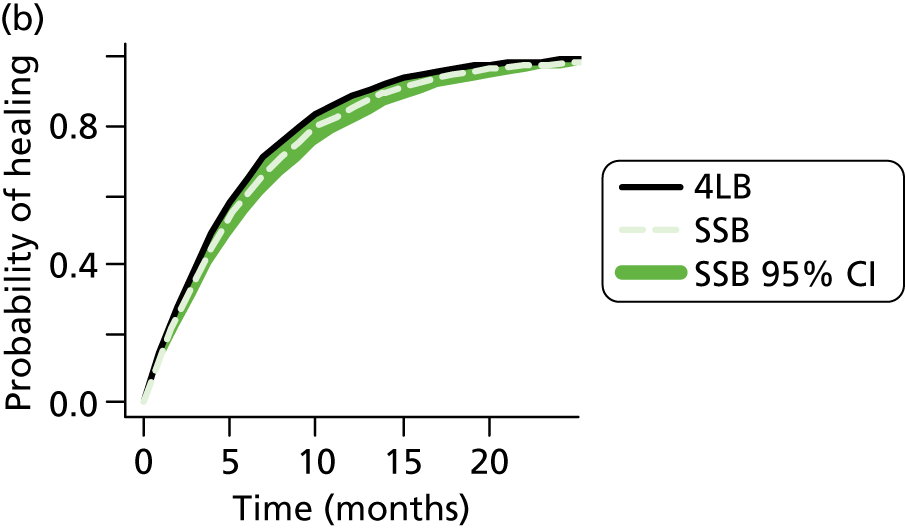
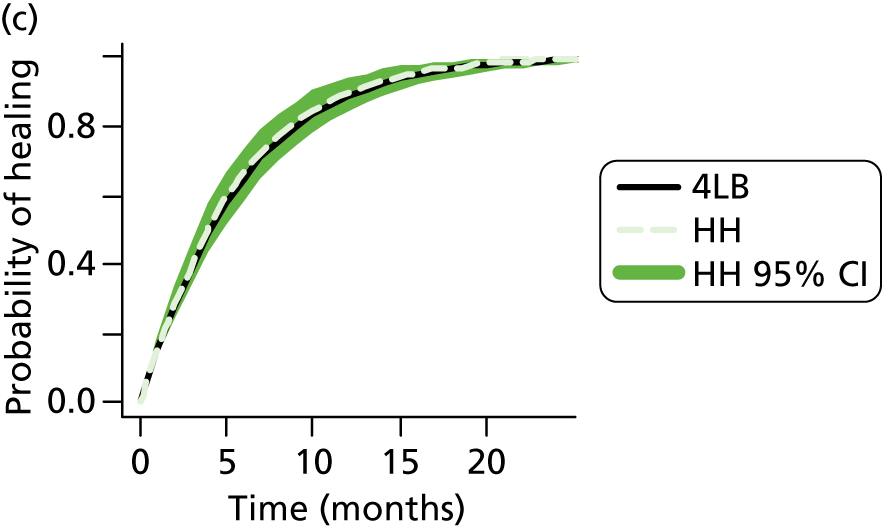
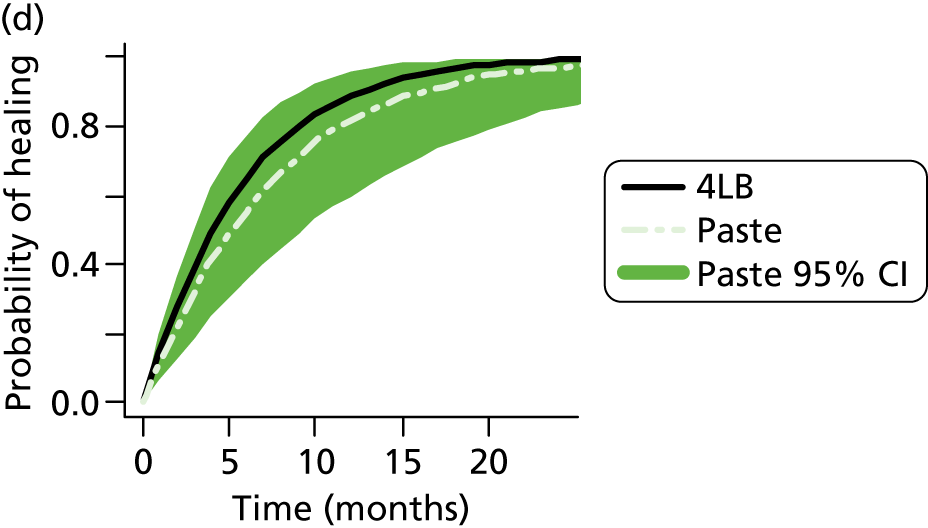
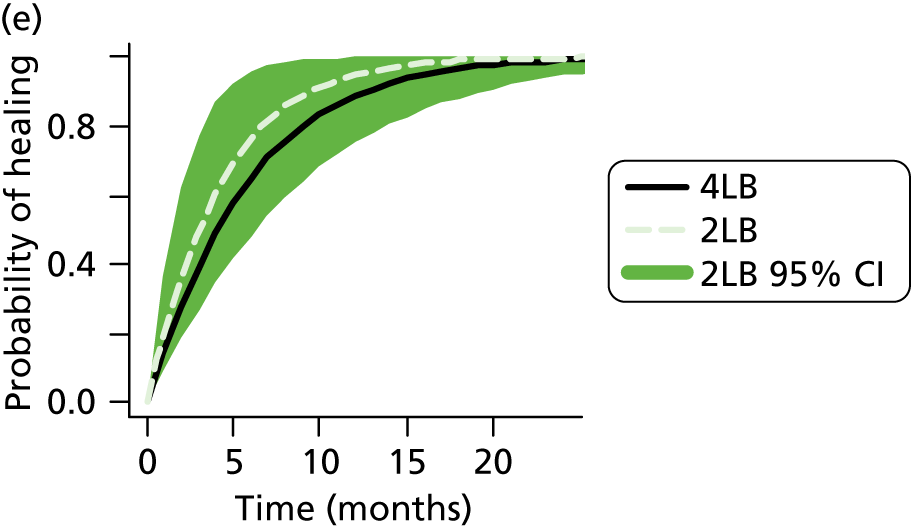
Parts (b) to (e) of Figure 12 illustrate the uncertainty around the probability of healing using 95% credibility bands (the equivalent to confidence bands) represented by shaded areas. Thus, for a given time point and with 95% confidence, current RCT evidence indicates that the probability of a cohort of participants healing lies somewhere in the shaded area. As the 4LB group was a reference treatment in the MTC analyses, its estimates were not shown as uncertain here. Parts (b) and (c) of Figure 12 show that uncertainty regarding the relative probability of healing with the SSB and HH is less marked than with other treatments – the confidence bands are narrower in parts (b) and (c) than in parts (d) and (e). The expected probability of healing with the 4LB is on the upper bound of the shaded area for SSB (reflecting the upper bound of the CrI for the HR of approximately ‘1’). For paste and the 2LB, uncertainty in their HRs translates into a large uncertainty in the probability of healing over time – the full line in parts (d) and (e) is inside the shaded areas, indicating that no evidence of a statistically significant difference from 4LB. In both these cases, estimated uncertainty is particularly large at between 5 and 20 months after treatment initiation.
Uncertainty over the probability of healing with four-layer bandage (reference treatment)
All findings to this point have been based on the relative treatment effects in relation to the 4LB, the common comparator. For this reason, 4LB estimates shown in the plots of Figure 12 were not uncertain. However, the synthesis models also evaluated uncertainty over the probability of healing in the 4LB group, controlling for centre effect and participants’ baseline characteristics. The estimated probability of healing associated with the 4LB over time (specifically for a participant with area and duration of ulcer equal to the mean values observed in VenUS IV) is represented in Figure 13 and uncertainty in the estimates is shown in the shaded area.
FIGURE 13.
Probability of healing across time (36 months) for the 4LB considering baseline heterogeneity. (For an average participant with a mean ulcer area at baseline of 9.4 cm2 and ulcer duration at baseline of 11.5 months.)
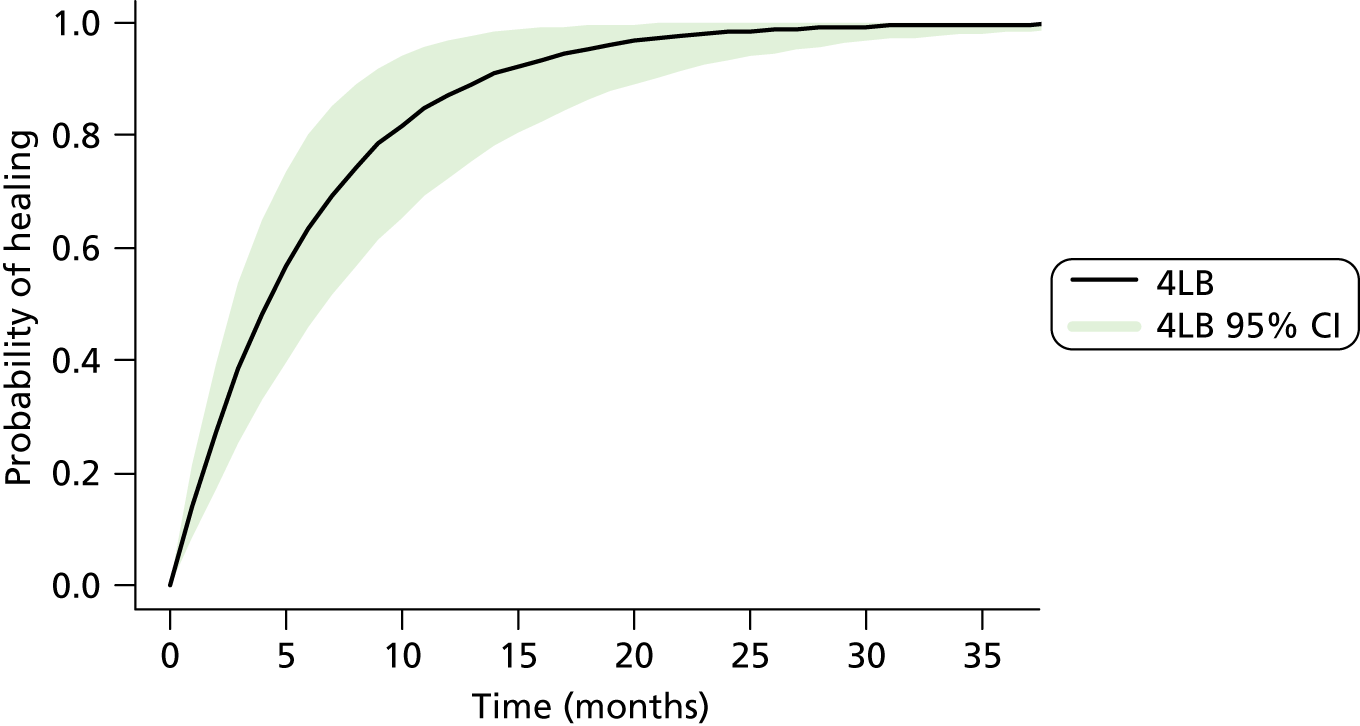
Determinants of ulcer healing
For completeness it is important to understand how patient characteristics might impact on venous ulcer healing and the models here provide an ideal opportunity to investigate this given the available baseline and healing data for VenUS I7 and IV. The base-case analysis model, including trial data from VenUS IV, suggests that no matter the compression treatment used, log baseline ulcer size and log ulcer duration are important predictors of ulcer healing time (HR of approximately 0.71 and 0.92, respectively, CrIs do not include 1 – Table 44), with increased time to ulcer healing as ulcer area and duration increase. Based on VenUS I7 and VenUS IV data, we found that an individual with a venous leg ulcer of one month’s duration (all remaining characteristics assumed at their average value, i.e. ulcer duration at baseline of 11.5 months approximately 36% possibility of having limited mobility and 0.1% of being immobile), who starts using the 4LB, is expected to heal within 12 months (with a probability of 0.92). If, instead, a patient has an ulcer of approximately 5cm2 and starts treatment with the 4LB, the probability that this ulcer heals within six months is 0.55. For all sizes or durations of the baseline ulcer considered, patients that started treatment with the 4LB are expected to have healed with probability of 0.88 or higher at 36 months. Figure 14 shows additional information on the impact of these two factors on the effect of treatment with the 4LB. Participant mobility was also seen to be a relevant factor to explain time to ulcer healing as participants with ‘difficulty in walking’ were associated with slower healing than those that ‘walk freely’ (HR of 0.72 in Table 44).
FIGURE 14.
Probability of healing across time (36 months) for the 4LB considering (a) different durations of ulcer at baseline and (b) different ulcer sizes at baseline. All remaining characteristics assumed at their average value.
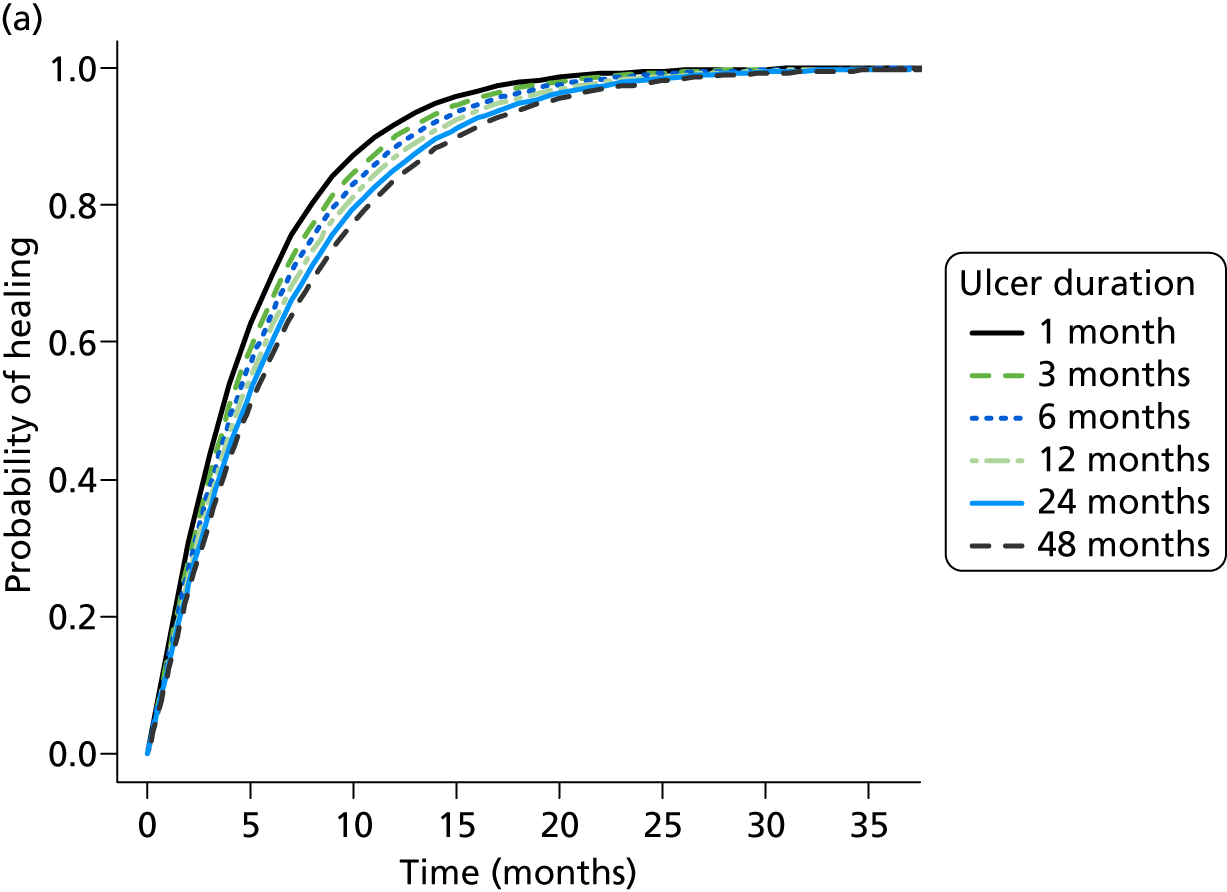
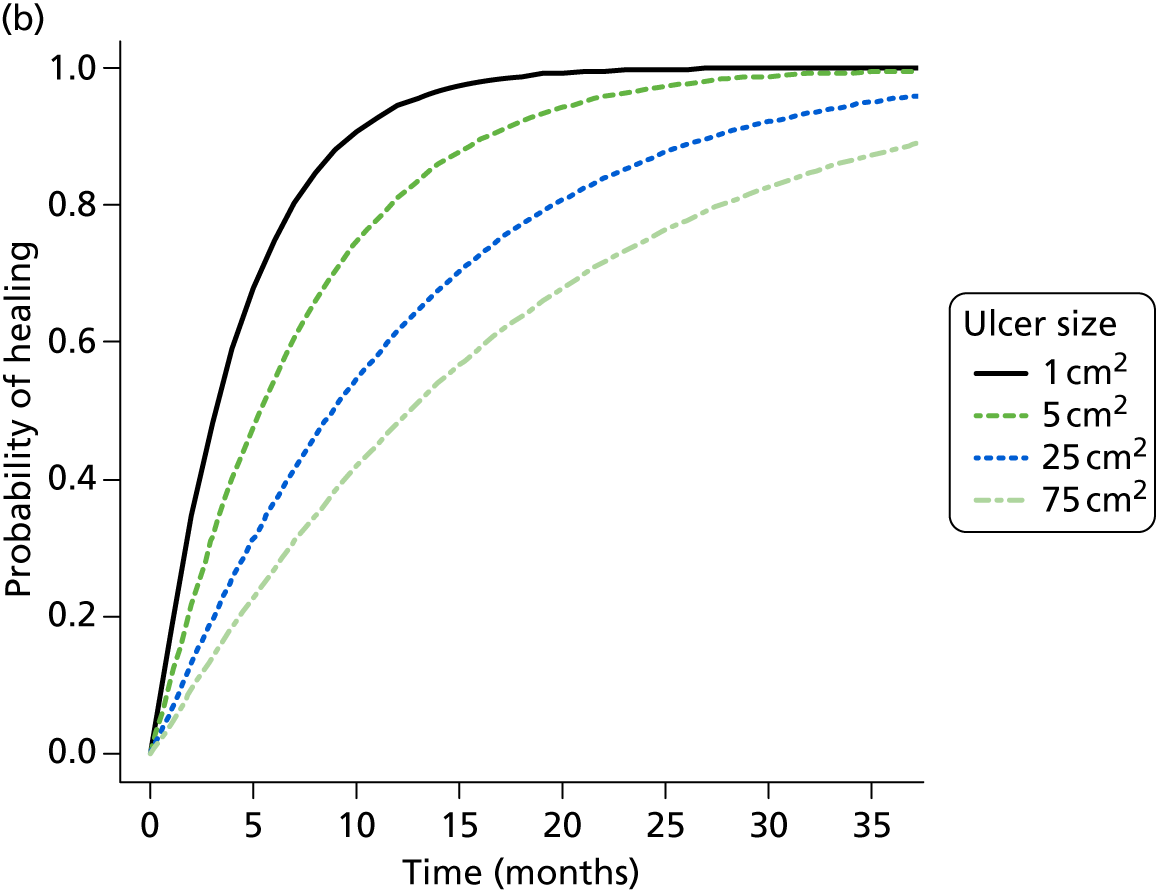
Chapter 12 Discussion
In this work, the evidence from RCTs regarding the effectiveness of alternative high-compression treatments for healing leg ulcers was synthesised using a MTC approach. This resulted in data for all relevant health technologies (4LB, SSB, HH, paste bandage and the 2LB) being evaluated simultaneously as a network of evidence. We note that the process used to classify treatments as high, and not high compression, was novel, and, given the limited information reported regarding compression treatments investigated in included RCTs, assumptions were required and the process had subjective elements. However, classification had excellent face validity given what are commonly recognised as ‘high’-compression treatments.
Relative effectiveness of high-compression treatments
The analyses showed that network-derived relative effectiveness estimates considered to be of high or medium quality (i.e. those from comparison of the 4LB with the SSB and the 4LB with the HH) are akin to the results of the individual VenUS studies (I and IV) that drive these findings. Although the evidence did not demonstrate a difference between HH and the 4LB, the SSB was expected to be less effective than both of these treatments.
The MTC estimates highlight the considerable uncertainty for many treatment comparisons, reflecting the fact that many of these were populated by small studies. However, although uncertainty in the evidence base was reflected in the wide CrIs, the effect of including low-quality studies was less transparent, and could not easily be adjusted for. Hence, we evaluated the quality of MTC-derived estimates using an exploratory approach based on GRADE. We acknowledge that this method has not been fully evaluated and that this is an area of ongoing work. However, based on this assessment it was clear the some relative effectiveness estimates were being driven by low- or very low-quality evidence (at a high risk of bias).
Given the evidence considered in the MTC, the treatment expected to be most effective was the 2LB system. However, this finding was driven by estimates judged as being of low or very low quality with large uncertainty. As with all models, the outputs from MTCs are limited by the type and quality of the data that informs them, hence here the 2LB findings should be interpreted with caution as demonstrated by our post hoc analysis, which suggests that were these systems to be considered to have the same effectiveness then the results of the model would favour HH.
Although the results of this analysis are based on the most complete synthesis of available RCT evidence, a key message for all decision-makers is the importance of having appropriately sized and robust RCTs available for evidence synthesis. The output of MTCs can be incredibly useful for clinical and policy decision-making; however, when important links are informed by poor-quality studies it is difficult to control for the potential bias. There are methods proposed in the literature for bias adjustment that down weigh the contribution of evidence with a high risk of bias,137–139 but more research is needed on how to generate the appropriate weights. Alternatively, evidence at high risk of bias could have been excluded from the analysis, in which case results would no longer reflect all available evidence. Also, in this MTC, although there are links informed by reasonable evidence, other links, informed by potentially biased estimates, are still required to maintain a network where there is relatively sparse data. Consequently, as with previous work,134 we suggest that, in addition to being a useful meta-analytical tool, exploration of MTC outputs can provide increased understanding regarding how study and data quality are driving the estimate of relative effectiveness across a clinical area.
Methodological approach
Within this work we applied a series of novel MTC models that synthesised time-to-event (healing) data while considering baseline participant-level covariates. The developed methodology also allowed IPD to be included alongside AD, thus allowing maximal use of data available. The time-to-event models implemented were fully parametric and the Weibull distribution was used; this allowed flexible assumptions over how the hazard of healing changes over time. It was not possible to apply alternative flexible distributions within this MTC model: the Gompertz distribution cannot be implemented with censoring within the software, and others, such as the log-logistic or the log-normal, do not allow the probability of healing over time to be expressed in a closed form, and hence impede the approach proposed here for the joint synthesis of IPD and AD (AD). In the different analyses implemented, vague or non-informative priors were used – the use of these is not expected to influence the interpretation of results.
By including evidence in IPD format we were able to model this evidence in a more complete way, for example it was possible to describe baseline hazard of healing over time. In doing so we followed some of the same conventions and assumptions as IPD meta-analysis (given the similarity of approach). For example, we assumed the shape parameter of the time-to-event distribution to be common across studies, or a common effect of baseline covariates on the hazard of healing. For this reason, analyses explored only covariates that were available in both individual level data sets: duration and size of the reference ulcer at baseline. Additionally, and considering the same variables, the inclusion of treatment interactions was explored. Within this example, these were not deemed important.
Part III Cost-effectiveness of high-compression treatments for venous leg ulcers
Chapter 13 Introduction
Part I of this VenUS IV report includes trial-based findings regarding the comparative cost-effectiveness of HH and the 4LB. However, because other high-compression options are available for the treatment of venous leg ulcers this pairwise comparison alone does not fully inform clinical and societal decision-making. Thus, in Part II we presented synthesis of all RCT evidence on the relative effectiveness (in terms of ulcer healing) of high-compression treatments for treating venous leg ulcers.
In Part III, we extend the VenUS IV analysis further by using a decision-analytic model to estimate the cost-effectiveness of all relevant high-compression treatments for venous leg ulcers. This approach utilises trial data from Part I and evidence synthesis data from Part II, as well as additional relevant evidence from the literature, ensuring that uncertainty over parameter values is assessed across all inputs. The advantages of extending the analysis, besides the value of considering cost-effectiveness and uncertainty for all relevant alternative treatments, are the ability to explicitly include other key health benefits (such as recurrence) in the estimation process while also extending the time frame of existing studies to evaluate longer-term effects.
In this section we report (1) our specification of the decision problem; (2) our review of previously published decision models to inform the structure of our model; (3) our searches for sources of evidence that could inform our model;140 (4) a detailed description of our final model and (5) the results of model implementation. All stages of work were discussed with the TMG for feedback on specific aspects of the analyses, including the model structure, data inputs and assumptions.
Specification of the decision problem
The study population was patients with (one or more) venous leg ulcers. The health technologies evaluated were the key treatments explored in Part II: the 4LB; the SSB; HH; the paste bandage and the 2LB (see Parts I and II for more complete definitions of these treatments). All of these treatments are used with the aim of healing venous leg ulcer(s); however, the costs associated with use of each treatment and their relative effectiveness may differ. We simultaneously assessed these two interrelated dimensions – the costs and benefits of each treatment – from the perspective of the NHS and PSS.
Chapter 14 Informing and structuring the decision model: review of existing economic modelling evidence
A review of cost-effectiveness models potentially relevant to this work was undertaken to help inform our final model structure.
This review aimed to (1) evaluate published decision-analytic models in the area of leg ulcers (regarding both high compression and other interventions) in order to identify structural assumptions and data sources potentially relevant to our own decision model; (2) highlight key areas of uncertainty and potential data gaps; and (3) identify key parameter inputs requiring additional systematic reviews and/or analyses of primary data.
Reviewing methods
A search was constructed by the Cochrane Wounds Group information specialist in conjunction with modelling specialists (see Appendix 13 for search strategy used). Three data sets were searched: MEDLINE, EMBASE and the NHS Economic Evaluation Database (NHS EED). Two reviewers (JD and LHC) independently screened resulting titles and abstracts against a set of predefined study eligibility criteria:
The study:
-
considered venous leg ulcers
-
included a full economic evaluation (i.e. include both costs and benefits)
-
used a model as a method to represent disease progression.
Any potentially eligible studies were obtained in full and were again screened against the eligibility criteria for inclusion by two reviewers (JD and LHC). A third reviewer (MS) resolved disagreements.
Data regarding model structure and specifications were then extracted from included studies. This included information on time horizon, perspective, model structure, model states, study populations and outcome of interest. Features of each reviewed model were then assessed for relevance to our decision problem. A summary of this assessment was presented as a narrative synthesis.
Review results
In total, 1038 title and abstract records were identified. After screening against the eligibility criteria, 19 studies were obtained as full papers, after further screening 10 studies were excluded (three of these were duplicates, two reported only costs and five did not involve decision modelling). Nine studies were therefore included in the review141–149 (Table 47).
| Study | Population | Treatments | Model design | Time horizon (weeks) | Key health states | Perspective | Outcome measure |
|---|---|---|---|---|---|---|---|
| Carr 1999141 | Patients with venous leg ulcers | Four-layer compression bandaging system (Profore) vs. usual care | Markov model | 52 | Healed, recurrence and unhealed ulcer | NHS | Cost per ulcer healed |
| Schonfeld 2000142 | Patients with hard-to-heal venous leg ulcers | Graftskin (Apligraf®, Novartis, Horsham, UK) vs. compression therapy (Unna’s boot) | Semi-Markov model | 52 | Healed, recurrence, unhealed ulcer and no therapy | Commercial health plan | Cost per ulcer healed |
| Korn 2002143 | Patients with history of venous stasis ulceration | Prophylaxis with compression stockings and patient education vs. no prophylaxis | Markov model | 12 | Compliant, non-compliant, healed and unhealed | USA community sector | Cost per QALY |
| Guest 2005144 | Patients with exuding venous leg ulcers | Carboxymethylcellulose vs. gauze dressing | Decision tree | 18 | Healed and unhealed | Sickness funds (Germany) plus community sector (USA) | Cost per ulcer healed |
| Scanlon 2005145 | Patients with critically colonised venous leg ulcers | Contreet® foam (Coloplast, Peterborough, UK) vs. Aquacel® AG (ConvaTec, Deeside, UK) vs. Actisorb® silver (Johnson & Johnson, Wokingham, UK) vs. Iodoflex® (Smith & Nephew, Hull, UK) | Markov model | 4 | Healed and unhealed | Societal perspective | Cost per percentage reduction in wound area |
| Iglesias and Claxton 2006146 | Patients with venous leg ulcers | Oral pentoxifilin 1200 mg/day plus usual wound care vs. placebo plus usual wound care | Markov model | 52 | Healed and unhealed | NHS | Cost per QALY |
| Clegg and Guest 2007147 | Elderly patients with chronic, non-healing wounds of > 6 months’ duration | Bioelectric stimulation therapy (Posifect®, Biofisica, Duluth, GA, USA) vs. standard care | Markov model | 16 | Improved, unchanged, worsened and healed | NHS | Cost per QALY |
| Marinel-lo-Roura 2007148 | Patients with venous leg ulcers | Compression therapy vs. compression therapy plus pentoxifilin 1200 mg/day | Decision tree | 24 | Healed and unhealed | Spanish Health Service | Cost per ulcer healed |
| Guest 2009149 | Patients with hard-to-heal venous leg ulcers | Amelogenin (Xelma®, Mölnlycke, Dunstable, UK) plus compression bandaging vs. compression bandaging | Markov model | 52 | Improved, unchanged, worsened, recurrence and healed | NHS | Cost per QALY |
The time horizons for the included models ranged from 4 weeks to 12 months. The perspectives used included payer’s, insurer’s, societal or health service (e.g. NHS). Of the nine studies,141–149 only four143,146–149 reported cost per QALY as the outcome measure; the remainder used more specific outcomes, such as cost per ulcer healed, cost per week in healed state, and cost associated with a prespecified reduction in wound area. Markov models were used in six141,143,145–147,149 out of the nine141–149 studies; however, the model structures varied.
In populating the models, various sources of information were used. Effectiveness parameters were most commonly informed by published evidence with some studies using results from individual trials. For cost parameters, most studies gathered information on resource use and unit costs separately with resource use informed/elicited from experts or based on findings from the literature. Studies that evaluated QALYs utilised published quality-of-life scores.
Description of models’ key characteristics
All decision models included ulcer healing as a key event (by means of defining an unhealed and healed health states). Of the nine included studies, five also considered other events such as ulcer recurrence,141 complications and adverse events,141 compliance/treatment change142 and degree of improvement of the ulcer. 147 From these studies we selected four model structures that could, potentially, have applied to our decision problem (Figure 15). Each structure was considered in more detail with key features outlined below.
Ulcer recurrence
The main goal of treating venous leg ulcers is ulcer healing. However, many (possibly most) ulcers recur post healing. Although the frequency and speed of recurrence may not be determined by initial ulcer treatment, quantifying recurrence is important, as the event may affect the level of health after treatment: i.e. health-related quality of life after first healing should consider the possibility of a recurrence, otherwise QALY gains of treatment may be overestimated. In decision models, recurrence was represented using a transition backwards from the health state ‘healed’ to ‘unhealed’ (see Figure 15a). 7 Alternatively, recurrence was assumed to be a distinct state (see Figure 15b), allowing for healing or death to occur at different rates after recurrence.
Adverse events/complications
Complications and severe adverse events related to treatments are often relevant for economic analyses owing to the relative high cost of negative events compared with other categories of resource use. One of the models reviewed,142 which evaluated a surgical intervention, set up an additional health state representing the discontinuation of treatment after occurrence of such events. In this way, the model allowed for distinct costs and healing (and/or death) rates in patients with severe adverse events/complications. Figure 15c presents an adapted diagrammatic representation of how a model considering both recurrence and adverse events could be specified.
Compliance
Compliance with venous leg ulcer treatments may vary. Compliance is here used to describe the degree to which a patient uses the treatment as prescribed, with variations mainly concern duration of treatment. Poor compliance with treatment may reduce or annul the effectiveness of a treatment. However, delivery of treatments to non-compliant patients may still incur costs. The model reported by Korn et al. 143 examines the impact of compliance with venous leg ulcer treatments. Here patients entering the model are classed as compliant or non-compliant and undergo distinct transition rates to reflect this. Following this design, the model could be expressed by the diagram in Figure 15d.
Final model structure and parameters
Given the decision problem at hand, it was decided that the most relevant model structure was Figure 15a, which was adopted (its structure is shown again in Figure 16 with further annotation).
FIGURE 16.
Decision model structure.
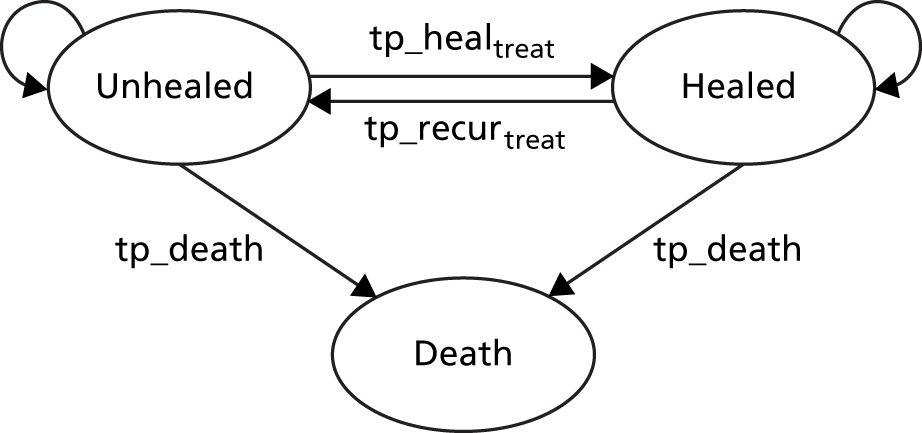
Key events in this model were healing and recurrence. Patients initiated the model in the unhealed state with the occurrence of healing tracked over time. Once healed, ulcers could recur over time. The model also considered a probability of patients dying over time, and defined death as a health state. Utility scores, resource use and costs were assigned to the length of stay in the different states, or to treatments, thus allowing evaluation of total costs incurred over time and quality adjustment of the lifetime estimated in the model. Table 48 presents a description of the parameters required to define the model.
| Parameter | Parameter description |
|---|---|
| Specification of patient population | |
| Ulcer duration | Duration in months of reference ulcer at start of treatment |
| Ulcer area | Size of the reference ulcer at start of treatment |
| Patient mobility | Patient mobility categorised: ‘walks freely’, ‘walks with difficulty’ and ‘immobile’ |
| Age | Age of the patient at start of treatment (years) |
| Transitions | |
| tp_healtreat | Transition probability from unhealed to healed |
| tp_recurtreat | Transition probability of having a recurrent ulcer, i.e. from healed to unhealed |
| tp_death | Transition probability of dying from any cause |
| Costs and resource use | |
| Costs while unhealed, not related to treatment | |
| Hospv | Average monthly ulcer-related hospital outpatients visits |
| Clinv | Number of ulcer-related doctor consultations per month |
| c_hospv | Cost of hospital visits |
| c_clinv | Cost of ulcer-related doctor consultations |
| Costs while unhealed, related to treatment | |
| T_durtreat | Duration of compression treatment with treat |
| nursevtreat | Average monthly number of ulcer-related nurse consultations while receiving treatment (treat) |
| c_nursev | Cost of a nurse consultation (excludes costs of treatments) |
| c_bandtreat | Cost of compression treatment (treat), per consultation |
| nursev_after | Average monthly number of ulcer-related nurse consultations while patient receives standard care |
| c_band_after | Cost of standard care (bandages and stockings) per consultation |
| Quality-of-life score | |
| u_decunh | EQ-5D scores for unhealed ulcer patients |
| u_pop | EQ-5D scores for healed leg ulcer patients |
We did not expect severe adverse events or complications to differ across high-compression treatments, thus the decision model did not consider that occurrence of adverse events affected transition to healing or death. However, the model did evaluate treatment-specific costs in which costs from adverse events were considered. Additionally, the selected decision model did not explicitly consider that compliance affected healing or recurrence – we knew from Part II that RCTs rarely reported compliance information clearly (the exception was trial data from VenUS IV). Instead, compliance was modelled implicitly (through the intention-to-treat analysis), and thus healing and recurrence rates depend on compliance within individual trials. However, the decision model did evaluate the effect of compliance on treatment costs by defining a ‘duration of treatment’ parameter that could differ among compression treatments. The impact and consequences of this parameter will be discussed in the final section of Part III.
Chapter 15 Literature searches for decision model input parameters
Based on the selected model, further literature searching was conducted to identify evidence on the following categories of model parameters: health-related quality of life/utility, costs and resource use, ulcer recurrence and mortality. Effectiveness (ulcer healing) evidence had been sought and synthesised separately (methods and results of this analysis are reported in Part II of this report).
Specially, in terms of further evidence, key data of interest were for (1) health-related quality of life/utility – data regarding possible changes to health-related quality of life/utility for a patient whose ulcer heals; (2) resource use – data regarding the costs incurred by patients with an unhealed ulcer; (3) recurrence – data regarding whether recurrence depends on the treatment received for the primary ulcer; whether recurrence is likely to increase, decrease or stay constant over time (from healing); any data regarding recurrence rate and whether recurrent ulcers differ from primary ulcers in terms of healing times or impact on death rates; and (4) mortality – evidence regarding a relationship between having an unhealed ulcer and increased mortality risk.
Searching methods and data extraction
Multiple searches were conducted by researchers at the Centre for Reviews and Dissemination using eight relevant databases (see Appendix 14). Two reviewers (JD and LHC) screened the abstracts independently against the eligibility criteria specified in Table 49. Disagreements were resolved by a third reviewer (MS). Abstracts that failed to meet the above criteria were excluded. The full papers of the selected studies were obtained (agreed by both reviewers) and rescreened for a final decision regarding inclusion. Again any disagreements were resolved by a third reviewer. Data were extracted on the parameters of interest and presented descriptively.
| Parameter type | Aim to identify evidence on | Inclusion criteria |
|---|---|---|
| Health-related quality of life/utility | Health-related quality-of-life scores of patients with venous leg ulcers both healed and unhealed | Studies were included if they:
|
| Costs and resource use | Costs/resource use by patients with an unhealed venous leg ulcer | Studies were included if they:
|
| Recurrence | Recurrence rates of patients with venous leg ulcer | Studies were included if:
|
| Mortality | Whether having a venous leg ulcer affects the patient’s mortality | Studies were included if they:
|
Results of the review
Health-related quality of life/utility
In total, 72 citations were initially identified and full papers were obtained for 33 of these after which a further 30 studies were excluded (see Appendix 15). The main characteristics of the three included studies44,48,150 are described in Table 50. The study by Walters et al. 48 reported the health-related quality-of-life results from a RCT that investigated the clinical effectiveness of community compared with home-based service in England. All trial participants received the 4LB and were asked to report their EQ-5D index score at 12 weeks and 12 months. The study also reported the proportion of healed and unhealed patients at 12 months. However, the analysis methods utilised was unclear from the RCT report and utility results across the 12-month assessment appeared inconsistent. We noted a bigger decrease in utility score at 3 months for healed participants compared with unhealed participants (suggesting decreased health-related quality of life), whereas overall a positive average utility gain per month up to 12 months was estimated for healed patients (Table 51). We did not contact the authors for further details. The two more recent studies were National Institute for Health Research-funded RCTs, one comparing the clinical effectiveness and cost-effectiveness of the 4LB with the SSB (VenUS I),7 and the other comparing the effects of silver-donating with non-silver low-adherent dressings in the treatment of venous leg ulcers (VULCAN trial). 150 All participants in the VULCAN trial150 wore multilayer compression bandage or hosiery over their dressing. Both studies reported EQ-5D scores separately for healed and unhealed ulcers, and could be used to establish an effect of ulcer healing on health-related quality of life (see Table 51). These results were used to inform the decision model, for which an increase in health-related quality-of-life score for a patient whose ulcer healed was estimated to be 0.00251 in the VULCAN trial150 and 0.11 in VenUS I7 (adjusted analysis).
| Study | Evaluated treatments | n | Instruments | Assessment time |
|---|---|---|---|---|
| Walters 199948 | 4LBs | 233 | EQ-5D/SF-36 | Baseline, 12 weeks, 12 months (healed vs. unhealed) |
| Iglesias 200544 | Four-layer vs. SSBs | 387 | EQ-5D/SF-36 | Baseline, every 3 months up to 1 year (healed vs. unhealed) |
| Michaels 2009150 | Multilayer compression bandages or hosiery | 213 | EQ-5D/SF-36 | Baseline, 1, 3, 6 and 12 months (healed vs. unhealed) |
| Study | Status | Baseline | 3 months | Mean gain per month | Disutility of patients with unhealed ulcers at 3 monthsa |
|---|---|---|---|---|---|
| Walters 199948 | Unhealed | 0.57, SD = 0.18, n = 233 | 0.55 | –0.03 | – |
| SD = 0.16 | SD = 0.13 | ||||
| n = 161 | n = 78 | ||||
| Healed | 0.53 | 0.02 | – | ||
| SD = 20 | SD = 0.14 | ||||
| n = 37 | n = 69 | ||||
| Iglesias 200544 | Unhealed | 0.62, SE = 0.02, n = 328 | 0.64 | – | 0.1100, SE = 0.02848 |
| SD = 0.02 | |||||
| n = 151 | |||||
| Healed | 0.75 | – | – | ||
| SD = 0.03 | |||||
| n = 110 | |||||
| Michaels 2009150 | Unhealed | 0.5792 | 0.6474 | – | 0.00251, SE = 0.04556 |
| n = 59 | |||||
| Healed | 0.6978 | 0.753 | – | – | |
| n = 987 |
Costs and resource use
In total, 72 citations were identified. After screening, 25 full papers were obtained. Following further screening, 16 studies were excluded; these were studies conducted outside of the UK and those studies that did not include information relevant for our model (see Appendix 16). Thus two studies informed our model for this parameter: VenUS I7 and VULCAN150 (details of these RCTs have been described elsewhere).
Based on the trial-level cost-effective analysis of VenUS IV (see Part I) nurse consultations for ‘unhealed’ participants was known to be a key cost driver; consequently, data relating to this cost component was of particular interest. However, of the two studies identified, only VenUS I7 assessed the number of visits for specific compression treatments, reporting a mean of 5.1 consultations per month in the 4LB arm and 6.0 in the SSB arm. No similar evidence was available for the paste and 2LB.
Ulcer recurrence
In total, 182 citations were identified. Of these, 147 were excluded and full papers were obtained for 35 studies. Upon further screening 29 studies were subsequently excluded for a variety of reasons (see Appendix 17): 17 studies were either not primary research or did not report recurrence results, eight studies were conducted outside of the UK, four studies evaluated surgical treatments (data from these were thus considered to potentially misrepresent recurrence rates in patients using compression), two studies assessed prevention strategies not used in current clinical practice, and four studies did not report whether participants were routinely offered the use of prevention compression and were considered too old to be able to provide data that was contemporary to VenUS IV regarding routine use of prevention treatments. It was for this last reason that VenUS I7 was excluded.
Details of the six included studies6,150,151–154 all relevant to the UK, are shown in Table 52 (all were RCTs). Three RCTs examined the effectiveness of surgical approaches (i.e. venous surgery and grafting) in preventing ulcer recurrence. Surgery is not commonly used in the UK as a treatment to heal venous ulcers thus we extracted data from only the control arms here (where participants received compression treatments). The proportion of participants with a recurred ulcer ranged from 30% at 6 months, 28–36% at 1 year and 56% at 4 years. The RCTs by Brooks et al. 152 and by Nelson et al. 153 investigated the effect of using hosiery after healing in preventing recurrence. Brooks et al. 151 suggest that a nurse education programme significantly improved ulcer recurrence rates but there was no association with the time spent in maintenance hosiery. Nelson et al. 153 found no evidence of a difference in recurrence rates between class 2 and class 3 compression hosiery. The final included study was the VULCAN RCT,150 in which all RCT participants were advised to wear hosiery after healing to prevent recurrence.
| Study (year) | Technology | n | Max. follow-up (months) | Recurrence rate |
|---|---|---|---|---|
| Barwell 20046 | Surgery | 428 | 14 | The 12-month recurrence rate for patients in the surgery arm vs. compression arm was 12% vs. 28% The 14-month recurrence rate for patients in the surgery arm vs. compression arm was 15% vs. 34% |
| Gohel 2007154 | Surgery | 500 | 48 | Ulcer recurrence rates at 4 years were significantly lower in the compression plus surgery group than in the compression group (31% vs. 56%) |
| Barwell 2000151 | Surgery | 669 | Up to 36 | Recurrence rates at 1, 2 and 3 years were 14%, 20% and 26%, respectively, for operated legs, and 28%, 30% and 44%, respectively, for non-operated legs |
| Brooks 2004152 | Prevention | 49 | 12 | There was only one leg ulcer recurrence (4%) in the experimental group compared with 15 in the control (36%) |
| Nelson 200635 | Prevention | 382 | 60 | Over 5 years, 107 patients (36%) had ulcer recurrence. Moderate- and high-compression hosiery did not differ for recurrence of venous ulceration (39% vs. 32%) |
| Michaels 2009150 | Compression treatment | 213 | 12 | A total of 24 patients had recurrent ulcers within 1 year: the recurrence rates were 11.6% (n = 11) for the intervention and 14.4% (n = 13) for the control dressings |
Although our decision model explicitly accounts for the possibility of a recurrence, it does not differentiate between primary ulcers and recurrent ulcers in terms of differential times to healing or impact on death rates. First, this was because we found no evidence of such an effect (the studies reviewed did not investigate whether risk of ulcer recurrence depended on the treatment received for the primary ulcer). Second, the population of interest within the current evaluation included people with venous leg ulceration that could be primary or recurrent.
Regarding the rate of recurrence, studies were heterogeneous and results varied significantly across studies. VULCAN,150 the most recent study, reported the lowest recurrence rate. We postulated that developments in the management of patients at risk of venous leg ulceration may have led to a reduction in recurrence rates in more recent years – thus, in addition to trial data from VenUS IV, we used only evidence from VULCAN149 to inform the decision model for recurrence data. Despite the evidence suggesting that the likelihood of recurrence may decrease as time from healing increases (see Barwell et al. ),151 the data available in the literature alone was insufficient to allow this issue to be considered explicitly.
Mortality
A total of 414 citations were identified (see Appendix 18). After initial screening, six studies were obtained in full;155–160 however, we were unable to retrieve one paper. 155 Of the remaining five studies,156–160 three were conducted in Sweden,154–159 one in Austria160 and one in the UK. 159 Subsequently, we focused on the UK study. 159 This was a RCT that evaluated the effect of a national community intervention programme on healing rates of chronic leg ulcer in Scotland.
In this cluster RCT, 3949 participants were registered and followed up using quarterly surveys over a 30-month period. Overall, there were 489 deaths and 65 amputations, corresponding to a quarterly rate of death of 3.7%. The study found no evidence of a difference between the two treatment groups – participants subject to a programme of nurse training that was specific for leg ulcer care (intervention), which included workshops, lecture material, and a range of dressing and bandaging material, among other items compared with no training (control).
Implications of additional evidence obtained for the decision model
The searches show that the data for key inputs for the decision model were sparse. Regarding health-related quality of life, although we identified some evidence regarding improved health-related quality of life upon ulcer healing (VenUS I7 and VULCAN150) it remains unclear how health-related quality-of-life changes over time for patients with unhealed ulcers. The same two RCTs provided estimates of the resource use associated with unhealed patients.
For ulcer recurrence, the literature suggests that the likelihood of an ulcer recurring decreases as time since healing increases. However, the data available were not sufficient to consider this explicitly in the decision model. Evidence on ulcer recurrence was heterogeneous. We note that clinical practice with respect to prevention of recurrence in venous leg ulcers has changed substantially over the last decade with the routine introduction of effective strategies such as the use maintenance therapies (i.e. hosiery after healing). It is not clear whether such changes explain the heterogeneity of recurrence figures across studies; however, we decided to consider evidence from the most recent study only. 150 There was no current evidence that having a venous leg ulcer directly affects (or not) mortality but there was some evidence that the population of patients with an ulcer may differ from the general population (matched by age and gender) that needed to be considered in the mortality rate.
The evidence gathered from the searches was further used to inform the decision model. How specifically this evidence was transformed (if needed) and included in the model is described in the following section.
Chapter 16 Cost-effectiveness analyses methods
Framework of analysis
The cost-effectiveness of alternative high-compression treatments for venous leg ulcers was evaluated using a decision model, where costs and benefits associated with patients’ use of treatments of interest were evaluated. The model structure is shown below (see Figure 17) and a list of parameters in Table 48. The decision model was implemented in the software package R, version 2.13.1 (The R Foundation for Statistical Computing, Vienna, Austria).
The model was defined using monthly cycles; this was deemed an appropriate duration reflecting the potential transitions between model states. The model considered a time horizon of 12 years, after which time it was anticipated that most individuals in the cohort would have healed (owing to the high mean age of the cohort at baseline, most patients were likely to have died before the end of the time horizon). Costs were evaluated from the perspective of the NHS and PSS, expressed in UK pounds sterling at 2011 prices. Both expected costs and outcomes were discounted using a 3.5% annual discount rate, in line with current NICE guidelines72 for technology assessment in the UK. The cost-effectiveness measure considered was ICER = ΔC/ΔE (where ΔC is the mean difference in costs, and ΔE is the mean difference in QALYs), or its reformulation, the net benefit (NB) measure. A full incremental analysis was carried out due to multiple treatments being assessed. The net monetary benefit (NMB) is defined as Tr × ΔE − ΔC and the new technology is accepted if NMB > 0, where Tr is the predefined threshold value used by NICE to establish value for money of health technologies in the NHS (i.e. willingness to pay). 72
The model was probabilistic, hence uncertainty around the expected value of the inputs and its impact on decision uncertainty was considered. In practice, uncertainty around each parameter was described using probability distributions and this uncertainty was then propagated through the model using Monte Carlo simulation (5000 iterations). Considerations regarding uncertainty surrounding a decision to reject a technology were based on CEACs, as in Part I. The CEAC is more informative than CIs, and has a natural Bayesian interpretation: it represents the probability of a treatment being cost-effective,85,161 estimated using the proportion of Monte Carlo samples that lie below a specific Tr.
Where uncertainty over an adoption decision based on existing information exists, the expected consequences of this uncertainty can be quantified using expected value of perfect information (EVPI) methods. 162 EVPI informs the decision-maker of the consequences for the health-care system (in £s) of the possibility of making the wrong decision, and informing the maximum value of conducting further research to reduce uncertainty and improve decision-making. 163 The EVPI should be considered for the total and future patient population who may gain from additional information over the expected lifetime of the technology – the population EVPI. The decision-maker should consider conducting the research only if the costs of the research are lower than the EVPI.
Scenario and subgroup analyses
The base-case model presents the results of the analysis including VenUS IV evidence. A further scenario was considered (without VenUS IV scenario) where trial data from VenUS IV was excluded from the model, thus allowing us to compare these results with the base case to assess the contribution of VenUS IV data to decision-making in the area.
Also, subgroup cost-effectiveness analyses were implemented. It is important to assess the cost-effectiveness of interventions by population subgroup, as an intervention may be cost-effective for one subgroup of the population and not for another. Thus, there may be population health gains from stratifying treatment decisions based on subgroup membership. This analysis explored (1) the average patient population with venous leg ulcers (no subgroups); (2) subgroups specified according to baseline duration of the reference ulcer, specifically at 1, 3, 6, 12, 24 and 48 months; and (3) subgroups specified according to the baseline area of the reference ulcer, specifically at 1, 5, 25 and 75 cm2.
Incorporating evidence into the model
Evidence from VenUS IV, Part II and studies identified in literature searches was used to populate the decision model. This section describes how the evidence was used in the decision model, specifically regarding the (1) specification of the patient population; (2) transitions from unhealed to healed; (3) transitions from healed to unhealed (ulcer recurrence); (4) transitions to death; and (5) costs and resource use and health-related quality of life/utilities.
Specification of the patient population
In Part II, we have seen that the probability of healing is determined by ulcer duration and area. It is intuitive that the probability of dying is determined by the age of the cohort. It is thus important to specify the key characteristics of our model population using values representative of venous leg ulcer patients. For the base case VenUS IV information was used, whereas the scenario analysis (without VenUS IV scenario) reflected participant characteristics observed in VenUS I. 7 Table 53 shows detailed information on the values assumed.
| Evidence from literature plus from VenUS IV (base-case analysis) | Evidence from literature (scenario analysis) | ||
|---|---|---|---|
| 1. Specification of patient population | |||
| Descriptive statistics from VenUS IV (n = 454), mean (SE) | Ulcer duration (months) = 11.54 (1.08) | Descriptive statistics from VenUS I7 (n = 397), mean (SE) | Ulcer duration (months) = 9.41 (0.53) |
| Ulcer area (cm2) = 9.35 (0.8676) | Ulcer area (cm2) = 10.22 (1.1830) | ||
| Patient mobility, %: | Patient mobility, %: | ||
| Walks freely = 63.80 | Walks freely = 62.14 | ||
| Walks with difficulty = 35.32 | Walks with difficulty = 37.08 | ||
| Immobile = 0.88 | Immobile = 0.78 | ||
| Age (years) = 68.61 (0.6791) | Age (years) = 71.59 (0.6714) | ||
| 2. Transition probabilities from unhealed to healed | |||
| Transition probabilities are based on a MTC synthesis model using Weibull regression of healing outcome data from multiple studies in the literature (including VenUS IV; see base-case MTC analysis in Chapter II), mean (SE) | βlog_ulcer_area = –0.34 (0.0362) | Transition probabilities are based on a MTC synthesis model using Weibull regression of healing outcome data from multiple studies in the literature (not including VenUS IV; see base-case MTC analysis in Chapter II), mean (SE) | βlog_ulcer_area = –0.37 (0.0562) |
| βlog_ulcer_duration = –0.08 (0.0104) | βlog_ulcer_duration = –0.06 (0.0110) | ||
| βmobility_difficult = –0.34 (0.0905) | βmobility_difficult = –0.35 (0.1308) | ||
| βimmobile = –0.44 (0.4847) | βimmobile = –0.74 (0.6545) | ||
| μVenUS IV = –3.34 (0.1390) | μVenUS I = –3.09 (0.2020) | ||
| βnew_centre = –0.004 (0.2269) | βnew_centre = –0.0001 (0.2863) | ||
| γ (ancillary parameter) = 1.07 (0.0320) | γ (ancillary parameter) = 0.92 (0.0412) | ||
| HRSSB = 0.89 (0.0671) HRHH = 1.05 (0.1122) HRpaste = 0.81 (0.2584) HR2LB = 1.51 (0.6264) |
HRSSB = 0.92 (0.0720) HRHH = 1.65 (0.5257) HRpaste = 0.82 (0.2629) HR2LB = 1.50 (0.6313) |
||
| 3. Transition probability from healed to unhealed (ulcer recurrence) | |||
| Hazard of recurrence modelled using VenUS IV data by a Gompertz survival regression, mean (SE) | β0: –2.80 (0.2460) βmobility_difficult: 0.77 (0.2595) βimmobile: 1.20 (1.0150) γ (ancillary parameter): –0.28 (0.0571) HRHH = 0.56 (0.1482) |
Constant rate of recurrence throughout time and the same for all treatments, VULCAN,164 mean (SE) | tp_recurr = 0.012 (3.412 × 10–5) |
| 4. Transition to death | |||
| Same as scenario analysis | From population statistics adjusted for mortality rate of leg ulcer patients from Brown et al. (2002)159 | Office for National Statistics (2011)165 | |
Transitions from unhealed to healed
Effectiveness evidence was synthesised in a MTC as described in Part II. The Weibull MTC model was used to define the transition probabilities to healing. The base case included IPD trial data from VenUS IV and the scenario analyses excluded it. The MTC results were incorporated into the decision model using the posterior samples or predictive distributions when a random effect across centres had been considered [extracted from the Convergence Diagnostic and Output Analysis WinBUGS output (CODA)].
Specifically, the probability of healing for patients starting treatment with the 4LB was specified according to the patient’s ‘baseline’ mobility status, ulcer area and duration. The mean values (section 1 of Table 53) and the regression coefficients estimated in the MTC (βlog_ulcer_area, βlog_ulcer_duration, βmobility_difficult, βimmobile, μ and βnew_centre) were linearly combined on the log scale to generate λ (representing the scale of the ulcer healing time distribution), which was used, in turn, to calculate the transition probability to healing according to the following formula:
where tpheal4LB(tu) is the transition probability for 4LB for each cycle (where the cycle length was 1 month, and thus the times at each cycle u were represented by tu) and the (ancillary) γ parameter defines the shape of the distribution. The relative effects of the remaining treatments compared with the 4LB were then applied to this baseline value as follows:
where HRi is the HR of treatment i in relation to the 4LB. Figure 17 illustrates the cumulative probability of ulcer healing with the 4LB in the model, for the base case (with trial data VenUS IV, reproducing Figure 15) and for the scenario analysis (without VenUS IV). Uncertainty in the estimates is shown in the shaded areas. As expected, for the majority of the time points considered, uncertainty is higher when excluding VenUS IV evidence than when including it.
FIGURE 17.
Probability of healing with the 4LB for the base-case and scenario analyses.
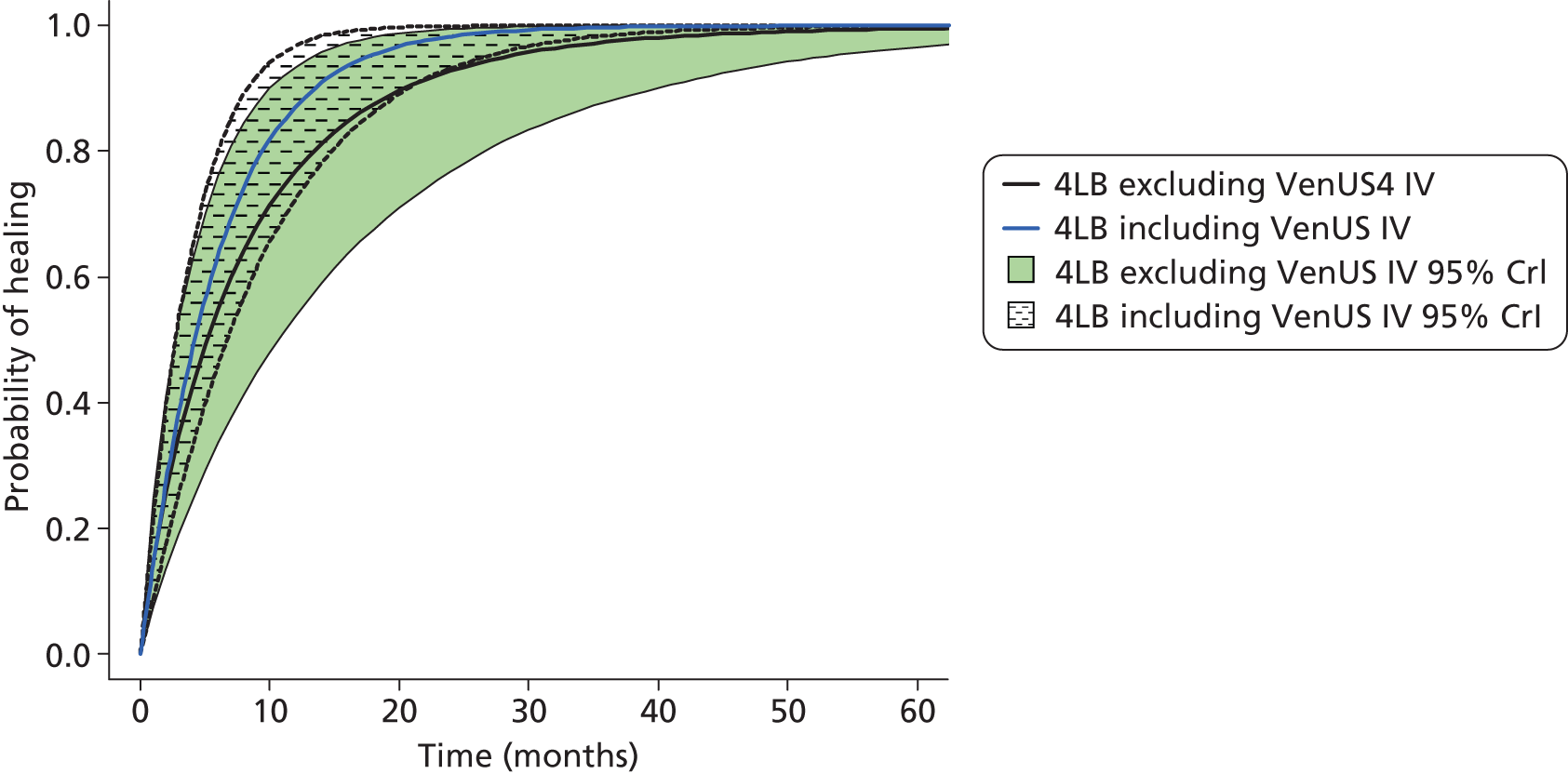
Transitions from healed to unhealed (ulcer recurrence)
For the base-case analysis, trial data from VenUS IV alone was used to inform this parameter, where approximately 19% of participants recurred during follow-up (65 out of 343). Additionally, differences were found between treatment arms with respect to recurrence (approximately 15.0% of participants allocated to the HH group had a recurrence event compared with 23.5% in participants allocated to the 4LB group). Given the availability of IPD, the probability of recurrence parameter was evaluated using time-to-event models. This analysis was parametric and we evaluated the following alternative distributions of time to event: exponential, Weibull, Gompertz, log-logistic, log-normal and gamma. The selection of the distribution was based on values of the Akaike information criterion (AIC),166 considering goodness of fit and penalising model complexity (Table 54). All regression models adjusted for baseline participant mobility (categorical variable) and baseline reference ulcer duration (months). Baseline ulcer area was removed from the model based on the lack of statistical significance.
| Time-to-event model | AIC |
|---|---|
| Exponential PH | 538.8 |
| Weibull PH | 520.9 |
| Gompertz | 510.7 |
| Log-logistic AFT | 517.5 |
| Log-normal AFT | 513.5 |
| Generalised gamma AFT | 513.8 |
The Gompertz regression was selected based on lower AIC value, which indicates a better fit (see Table 54). Figure 18 shows the cumulative probability of ulcer recurrence for the 4LB arm of VenUS IV, based on the selected model. This shows that, on average, 12 months after healing the probability of observing an ulcer recurrence was estimated to be approximately 27.0% in participants allocated to 4LB compared with 17.7% for participants allocated to HH. Again, uncertainty in the estimates is shown in the shaded areas.
FIGURE 18.
Probability of recurrence for the 4LB and hosiery from VenUS IV (base-case analysis).
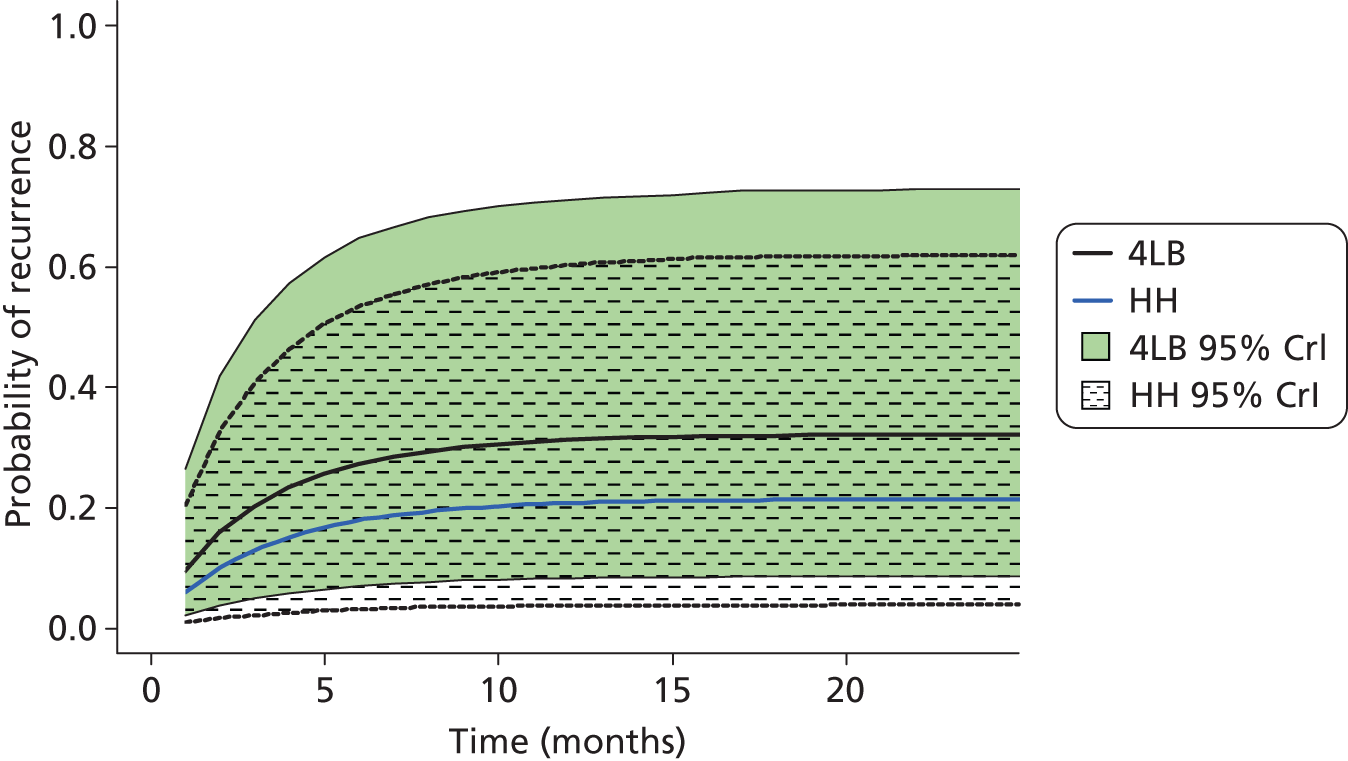
The distribution chosen to represent time to recurrence – the Gompertz distribution – assumes that the hazard of recurrence depends on time since healing. This time dependency was built into the decision model structure using tunnel states to relax the Markov assumption. Tunnel states are a modelling ‘trick’, for which one state is split into as many states as the number of cycles for which we wish to consider the time dependency. In this case, the initial healed state was transformed into 13 states: ‘healed 1’, ‘healed 2’, etc. Patients were allowed to remain in states for only one cycle (1 month), after which they either recurred (transit to the unhealed state) or died. In this way, the time patients spent healed was tracked and the probability of recurrence depended on this duration. If, after 12 months, patients had not recurred or died then they transitioned into a ‘healed’ state for which the probability of ulcer recurrence was assumed null. The transition probability for recurrence, tpirecurr, was defined using the parameter estimates in Table 54 using the following relation:
where λ is a scale parameter (a linear combination, on the log scale, of the average population characteristics and estimated coefficients: β0, βmobility_difficult, βimmobile), γ the shape parameter of the Gompertz distribution and ‘HR’ the HR for recurrence of treatment i relative to the 4LB (note that only HH was assumed to affect recurrence, thus all remaining compression treatments for which models were run were assigned a HR of ‘1’).
In the scenario analysis, evidence from the VULCAN trial150 was the source of recurrence data (and trial data from VenUS IV were not included). The VULCAN trial150 observed that 13% of participants recurred within 12 months (24 from a sample of 185 participants healed within the study). This information was used in the model by defining a transition probability for recurrence using:
where precurr represents the probability of recurrence observed in the study (0.13). In this scenario analysis; recurrence was not assumed to depend on treatments used for ulcer healing as the evidence from the review conducted did not indicate that such an effect might exist.
Transition to death
Most ulcer patients are elderly and they generally have more comorbidities than the average population. 7,13,61 Although the presence of an ulcer may not directly affect patients’ mortality, the characteristics of this population may imply a higher mortality rate that the general population. Transitions probabilities to the absorbing state (death) were informed by official population statistics165 and adjusted using the mortality ratio of a population of patients with ulcers (calculated from Brown et al.,159 taken to be 2.36 times higher than of the general population). Mortality was not assumed to depend on the treatments evaluated. The same information was used in both the base-case and scenario analyses.
Costs, resource use and health-related quality of life/utilities
Table 55 identifies the evidence used for costs and resource use and health-related quality of life for both the base-case and scenario analyses. Two main cost components were defined:
-
Costs dependent on health state (healed and unhealed) The model considered only ulcer-related costs, so once a patient had healed then no costs were incurred in the model. When patients had an ulcer, i.e. were in the unhealed health state, clinician and hospital consultations were considered (assumed to be not treatment dependent) (see Table 55).
-
Costs dependent on treatment Treatment costs were a function of the average number of nurse consultations per month for each patient, the cost of each of those nurse contacts and the cost of the treatment applied in each visit (see Table 5), i.e. mean number of nurse consultations × (cost of nurse visit + cost of applied treatment).
| Evidence from literature plus from VenUS IV (base-case analysis) | Evidence from literature (scenario analysis) | ||
|---|---|---|---|
| Costs and resource use | |||
| Depending on treatment | |||
| Treatment duration, VenUS IV, mean (SE) | Tdur4LB = 2.48 (0.1819) | Treatment duration | Tdur = while unhealed |
| TdurSSB = Tdur4LB | |||
| Tdur HH = 3.06 (0.2066) | |||
| Tdurpaste = Tdur4LB | |||
| Tdur2LB = Tdur4LB | |||
| No. of nurse visits, VenUS I7 and VenUS IV, mean (SE) | nursev4LB = 5.68 (0.2401) | No. of nurse visits, VenUS I, mean (SE) | nursev4LB = 5.10 (0.2335) nursevSSB = 6.00 (0.2952) nursevHH = nursev4LB nursevpaste = nursev4LB nursev2LB = nursev4LB |
| nursevSSB = 6.00 (0.2952) | |||
| nursevHH = 5.16 (0.1499) | |||
| nursevpaste = nursev4LB | |||
| nursev2LB = nursev4LB | |||
| Depending on health state (healed, unhealed) | |||
| No. of clinician visits, VenUS IV evidence, mean (SE) | clinv = 0.26 (0.1302) | No. of clinician visits, VenUS I, mean (SE) | clinv = 0.25 (0.1600) |
| No. of hospital visits, VenUS IV, mean (SE) | hospv = 0.12 (0.0498) | No. of hospital visits, VenUS I, mean (SE) | hospv = 0.35 (0.0800) |
| Health related quality-of-life scores | |||
| Population utility scores (u_pop), UK population norms per year of age adjusted for baseline utility values from VenUS IV | Kind et al.74 and VenUS IV | Population utility scores (u_pop), UK population norms per year of age adjusted for baseline utility values from Michaels et al. (2009)164 | Kind et al. (1996)74 and Michaels et al. (2009)164 |
| Utility decrement of patient with unhealed ulcer, VULCAN trial (Michaels et al. 2009),164 VenUS I (Iglesias et al. 2004)7 and VenUS IV, mean (SE) | u_decunh = 0.0820 (0.02143) | Utility decrement of patient with unhealed ulcer, VULCAN trial (Michaels et al. 2009),164 and VenUS I (Iglesias et al. 2004)7 mean (SE) | u_decunh = 0.0800 (0.02423) |
The mean number of nurse consultations while unhealed was derived from VenUS IV (see Table 28) in the base case and VenUS I11 in the scenario analysis. Given the scarcity of information on resource use for other compression treatments, in the ‘without VenUS IV’ scenario, the number of nurse consultations while on HH, paste and the 2LB was assumed to be the same as the 4LB. The number of nurse consultations for the SSB group was assumed to differ (based on data from VenUS I). In the base case, the number of nurse consultations while on the paste and the 2LB were again assumed to be the same as the 4LB owing to absence of information. Consultations for HH were informed by VenUS IV (see Table 28) and consultations for SSB were again informed by VenUS I.
The ‘without VenUS IV’ scenario considered that treatments of interest were provided to patients throughout the unhealed period. Evidence from VenUS IV, however, established that the same treatments are not used throughout an ulcer episode. As this is relevant for costs, it was considered in the base-case analysis by costing the treatment of interest for a period of time (Tdurtreat) after which ‘standard treatments’ were costed. These standard treatments were costed as an average of the compression treatments reported in VenUS IV after the allocated treatment was discontinued. In VenUS IV, duration of treatment was found to differ between the 4LB and HH. No evidence was available with respect to the duration of treatment for SSB, paste and 2LB. Thus duration of these treatments was assumed to be the same as the 4LB (see Table 55).
The health-related quality-of-life/utilities scores of healed patients were assumed to vary with time according to UK population utility score norms (per year of age) [Centre for Health Economics (CHE) report, 1999]. 167 These values were adjusted by baseline utility values from VULCAN150 for the ‘without VenUS IV’ scenario and for the base case, to reflect the fact that a patient population with venous leg ulcers is expected to have worse health than the general population.
Patients with unhealed ulcers were assigned a decrement in utility. In the scenario without VenUS IV, adjusted estimates at 12 weeks from VULCAN trial150,164 and VenUS I7 – 0.00251 and 0.11, respectively – were pooled. To pool, a weighted average of the study estimates was used, with the weights being the inverse of the variance for each of the study estimates (i.e. the larger the variance, the smaller the weight, and vice versa). The pooled value obtained for this scenario was 0.08 (SE = 0.024, Table 56). For the base case, VenUS IV was used in addition to the other two studies; the pooled statistic assumed a value of 0.082 (SE = 0.021), a similar value to that of the scenario analysis.
| Study/studies | Mean adjusted utility decrement at 3 months (SE) |
|---|---|
| Michaels 2009 (VULCAN trial)164 | 0.0025 (0.04556) |
| Iglesias 2004 (VenUS I trial)7 | 0.1100 (0.02848) |
| VenUS IV trial | 0.0894 (0.04504) |
| VULCAN164 trial + VenUS I7 (scenario analysis) | 0.0800 (0.02423) |
| VULCAN164 trial + VenUS I7 + VenUS IV trial (base-case analysis) | 0.0820 (0.02143) |
Specification of distributions for probabilistic analysis
To perform probabilistic analysis input parameters were represented as a probability distribution. The type of distribution was dictated by the nature, characteristics and method of estimation used to derive the evidence for each parameter (Table 57). Once the type of distribution was defined, the mean statistics and SE were used to define the distribution parameters using the method of moments. For the ‘without VenUS IV’ scenario analysis, the probability of ulcer recurrence was modelled through a standard beta distribution parameterised by the number of individuals that recurred given those healed at 3 months (see Table 57). Consultations with health-care professionals were represented by a normal distribution, parameterised using evidence in Table 55. Adjusted utility decrements for unhealed ulcer patients were represented by a standard beta distribution. Adjusted population utility scores per year of age and decrements due to having an unhealed ulcer were also modelled using normal distributions.
| Model input parameter | Distributional assumption | |
|---|---|---|
| Evidence from literature plus from VenUS IV (base-case analysis) | Evidence from literature (scenario analysis) | |
| 1. Specification of patient population | ||
| Descriptive statistics | Non-stochastic | Non-stochastic |
| 2. Transition probabilities from unhealed to healed | ||
| Transition probabilities (see Part II) | MCMC simulations used | MCMC simulations used |
| 3. Transition probability from healed to unhealed (ulcer recurrence) | ||
| Rate of recurrence (tp_recurr) | Multivariate normal distribution (mean estimates and variance/covariance matrix used) defined to characterise each of the Gompertz regression parameters | Beta (r_recur + 1, n_recur - r_recur + 1; see Table 53) |
| 4. Transitions to death | ||
| tp_death | Non-stochastic | |
| 5. costs and resource use and health-related quality of life/utilitiesa | ||
| tdurtreat | Normal distribution (see Table 55) | – |
| nursevtreat | Normal distribution (see Table 55) | Normal (Table 55) |
| clinv | Normal distribution (see Table 55) | Normal (Table 55) |
| hospv | Normal distribution (see Table 55) | Normal (Table 55) |
| u_decunh | Beta (see Tables 55 and 56) | Beta (see Tables 55 and 56) |
| u_pop | Normal distribution (CHE report, 1999167) | Normal (CHE report, 1999167) |
Chapter 17 Cost-effectiveness analyses: results
Base-case analysis
The base-case mean cost, benefits and ICERs for each of the evaluated compression therapies are shown in Table 58. The estimated mean costs for the 2LB (two-component systems, with a top component that is a cohesive bandage) were lower than those of other treatments, and its benefits were, on average, the highest. Thus, all remaining interventions were dominated by this treatment.
| Intervention | Benefits (QALYs) | Costs (£s) | Mean incremental benefits (QALYs) | Mean incremental costs (£s) | ICER (£s) | ||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| 2LB | 3.8595 | 3.329 to 4.3218 | 16,174.0 | 7565.5 to 31,534.3 | – | – | Dominant |
| HH | 3.8524 | 3.3619 to 4.3001 | 18,434.8 | 12,103.7 to 28,670.8 | –0.0072 | 2261 | Dominated |
| 4LB | 3.8061 | 3.278 to 4.2703 | 19,505.2 | 12,146.9 to 32,531.3 | –0.0534 | 3331 | Dominated |
| SSB | 3.7769 | 3.2245 to 4.291 | 20,847.7 | 12,882.4 to 34,495.6 | –0.0826 | 4674 | Dominated |
| Paste | 3.7322 | 3.1018 to 4.2343 | 23,196.1 | 12,154.7 to 40,226.3 | –0.1274 | 7022 | Dominated |
Figure 19 summarises the main cost-effectiveness results. The 2LB, identified by an inverted triangle in the figure, had the highest expected NBs. The 2LB system had the highest probability of being cost-effective (between 70% and 80%) for threshold values from £0 to £100,000, whereas the HH achieved the second highest probability of being cost-effective with values of between 20% and 30% (results not shown).
FIGURE 19.
Effectiveness vs. costs (a) and NMB vs. probability of being cost-effective (b) for base-case analysis (£20,000 threshold).
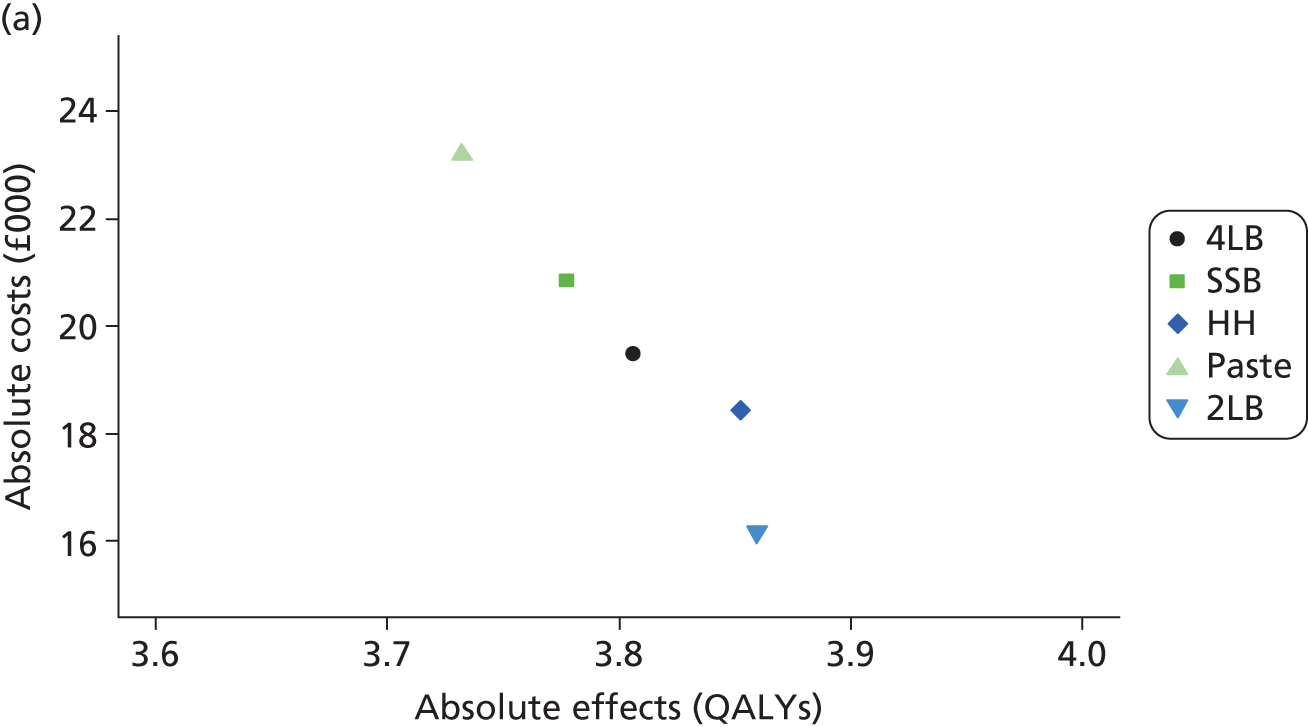
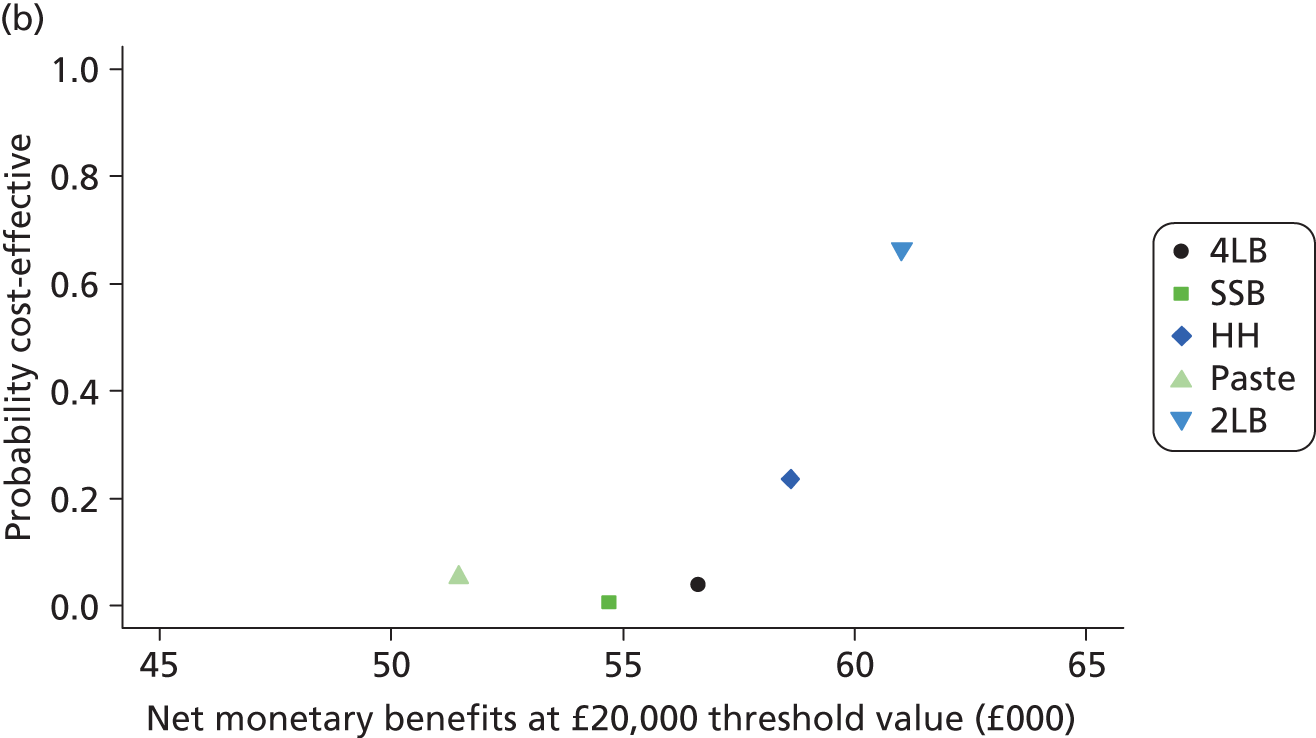
The estimated per-patient EVPI for the base case was approximately £1680 at a £20,000 threshold ratio (Table 59). Considering the high incidence of venous leg ulcers (estimated at 365,000 per year), the population EVPI was estimated to be approximately £2.9B for the same threshold value.
| Expected value of perfect information | ||||
|---|---|---|---|---|
| Cost-effectiveness threshold value (£) | Base-case analysis | Scenario analysis | ||
| Per patient | Population (× 1,000,000) | Per patient | Population (× 1,000,000) | |
| 20,000 | 1677 | 2866 | 3804 | 6501 |
| 30,000 | 1957 | 3346 | 4525 | 7734 |
| 50,000 | 2525 | 4315 | 6001 | 10,257 |
Contribution of VenUS IV to cost-effectiveness: the ‘without VenUS IV’ scenario
In this scenario analysis, the decision model was run without evidence from VenUS IV. The results indicated that, consistent with the base-case analysis, the 4LB, SSB and paste bandage were dominated by HH and the 2LB. Contrary to the base case, HH (rather than the 2LB) was expected to offer the highest mean QALYS (approximately 4.80 QALYs gained), but given its costs, HH was not deemed cost-effective at usual threshold values (£20,000 to £30,000).
The 2LB system had the highest probability of being cost-effective, but without the information from VenUS IV, uncertainty regarding the use of hosiery was higher (the probability of being cost-effective would be 36%, Figure 20). Irrespective of the threshold value considered, individual and population EVPI estimates for the scenario analysis were always higher than those estimated for the base-case analysis (see Table 59). This indicates that the supplementary information provided by VenUS IV was valuable in reducing the consequences of decision uncertainty.
FIGURE 20.
Effectiveness vs. costs (a) and NMB vs. probability of being cost-effective (b) for scenario analysis (£20,000 threshold).
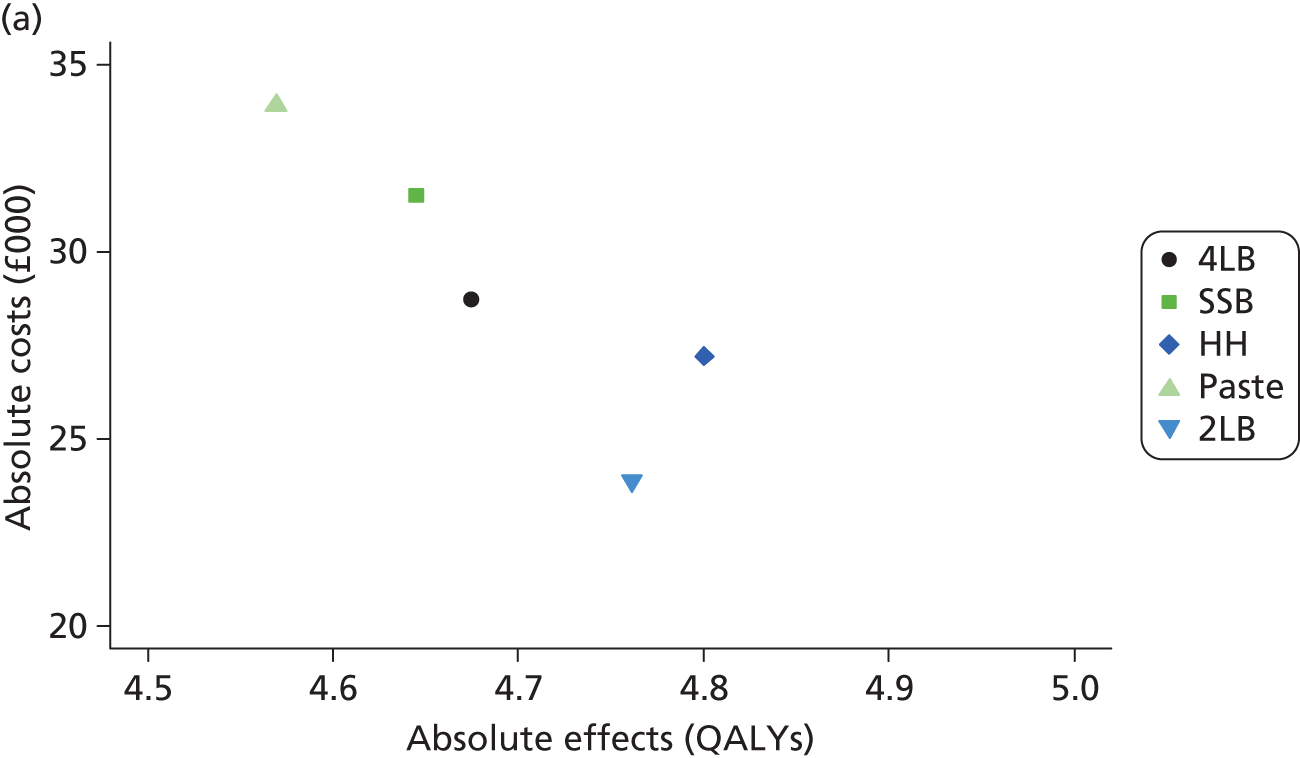
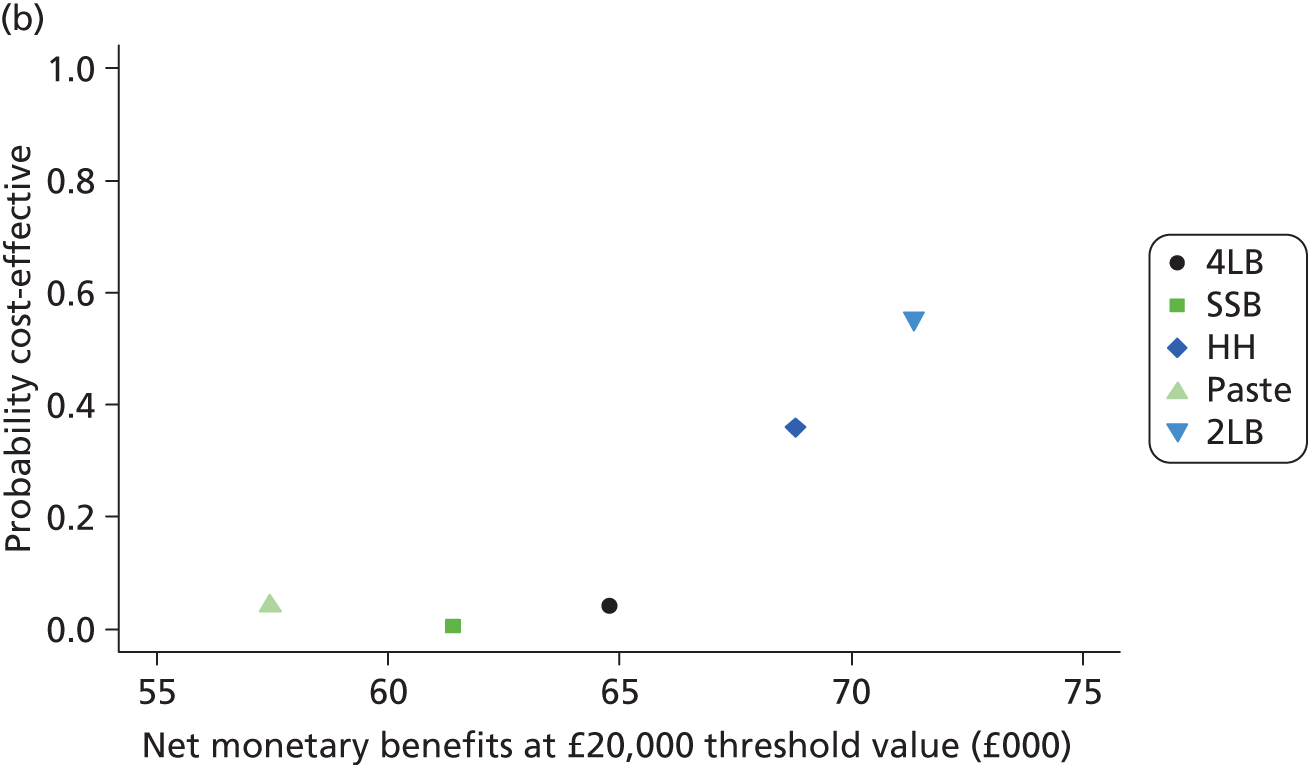
Subgroup cost-effectiveness analysis: the impact of determinants of ulcer healing and recurrence
With respect to the impact of baseline patient characteristics on the cost-effectiveness of treatments, the base-case model suggests that, for all baseline ulcer durations analysed, decisions would be the same as for the average patient population; i.e. the 2LB dominates all of the other treatment alternatives (Table 60). However, as ulcer duration increases, the benefits from HH are more evident. For an ulcer with 24 months’ duration HH is still not cost-effective, but its ICER is estimated at approximately £38,000 per QALY gained, a value closer to the range of £20,000–30,000, which NICE used to guide cost-effectiveness decision-making. The 2LB was the treatment of choice for the range of reference ulcer sizes analysed.
| Intervention | Baseline duration of reference ulcer (months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | 24 | ||||||
| ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | |
| 2LB | Dominant | 0.699 | Dominant | 0.692 | Dominant | 0.692 | Dominant | 0.662 | – | 0.591 |
| HH | Dominated | 0.188 | Dominated | 0.204 | Dominated | 0.204 | Dominated | 0.242 | £37,761 | 0.316 |
| 4LB | Dominated | 0.046 | Dominated | 0.040 | Dominated | 0.040 | Dominated | 0.038 | Dominated | 0.040 |
| SSB | Dominated | 0.007 | Dominated | 0.007 | Dominated | 0.007 | Dominated | 0.006 | Dominated | 0.006 |
| Paste | Dominated | 0.060 | Dominated | 0.057 | Dominated | 0.057 | Dominated | 0.053 | Dominated | 0.046 |
| Intervention | Baseline area of reference ulcer (cm2) | |||||
|---|---|---|---|---|---|---|
| 1 | 5 | 25 | ||||
| ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | |
| 2LB | Dominant | 0.672 | Dominant | 0.664 | Dominant | 0.656 |
| HH | Dominated | 0.216 | Dominated | 0.239 | Dominated | 0.254 |
| 4LB | Dominated | 0.047 | Dominated | 0.038 | Dominated | 0.033 |
| SSB | Dominated | 0.009 | Dominated | 0.006 | Dominated | 0.005 |
| Paste | Dominated | 0.056 | Dominated | 0.053 | Dominated | 0.052 |
Baseline ulcer area and duration determine two key components of health benefit in the decision model, ulcer healing and recurrence probabilities. In Table 61 it is shown how these two factors may concurrently influence the two model components.
| Area of reference ulcer (cm2) | Duration of reference ulcer (months) | No. (%) of patients (VenUS IV, n = 451) | Probability (%) of 4LB ulcer healing (12 months) | Probability (%) of 4LB ulcer recurrence (12 months) |
|---|---|---|---|---|
| 1 | 1 | 0–2 cm2 and 0–2 months: 20 (4.4) | 97.6 | 25.1 |
| 3 | 0–2 cm2 and 2–4 months: 54 (12) | 96.6 | 25.5 | |
| 6 | 0–2 cm2 and 4–8 months: 31 (6.9) | 95.9 | 27.2 | |
| 12 | 0–2 cm2 and 8–16 months: 28 (6.2) | 95.1 | 34.8 | |
| 24 | 0–2 cm2 and > 16 months: 13 (2.9) | 94.2 | 74.9 | |
| 5 | 1 | 2–8 cm2 and 0–2 months: 11 (2.4) | 88.2 | 25.1 |
| 3 | 2–8 cm2 and 2–4 months: 65 (14.4) | 85.8 | 25.5 | |
| 6 | 2–8 cm2 and 4–8 months: 46 (10.2) | 84.2 | 27.2 | |
| 12 | 2–8 cm2 and 8–16 months: 28 (6.2) | 82.5 | 34.8 | |
| 24 | 2–8 cm2 and > 16 months: 30 (6.7) | 80.7 | 74.9 | |
| 25 | 1 | > 8 cm2 and 0–2 months: 5 (1.1) | 70.9 | 25.1 |
| 3 | > 8 cm2 and 2–4 months: 40 (8.9) | 67.6 | 25.5 | |
| 6 | > 8 cm2 and 4–8 months: 36 (8) | 65.5 | 27.2 | |
| 12 | > 8 cm2 and 8–16 months: 16 (3.5) | 63.4 | 34.8 | |
| 24 | > 8 cm2 and > 16 months: 28 (6.2) | 61.3 | 74.9 |
Rather than looking at the impact of each subgroup specification individually, Table 62 shows how these specifications may simultaneously affect adoption decisions. For smaller ulcers and ulcers of short duration, the 2LB dominates all other comparators (with approximately 70% probability of being cost-effective at £20,000), but for reference ulcers of 5 cm2 and of longer duration HH is far from being considered cost-effective relative to the 2LB, with an estimated ICER of approximately £42,000 per QALY gained. If the reference ulcer is large in size and of long duration, HH is also not cost-effective relative to the 2LB, as decision-makers would need to be willing to pay approximately £95,000 per additional QALY.
| Intervention | Baseline characteristics of reference ulcer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 cm2 and 1 month | 1 cm2 and 3 months | 5 cm2 and 3 months | 5 cm2 and 24 months | 25 cm2 and 24 months | ||||||
| ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | ICER (3) | Prob. CE (£20K) | |
| 2LB | Dominant | 0.708 | Dominant | 0.708 | Dominant | 0.694 | – | 0.584 | – | 0.589 |
| HH | Dominated | 0.164 | Dominated | 0.168 | Dominated | 0.198 | £41,788 | 0.328 | 0.325 | |
| 4LB | Dominated | 0.056 | Dominated | 0.052 | Dominated | 0.043 | Dominated | 0.037 | Dominated | 0.035 |
| SSB | Dominated | 0.010 | Dominated | 0.010 | Dominated | 0.007 | Dominated | 0.006 | Dominated | 0.005 |
| Paste | Dominated | 0.062 | Dominated | 0.062 | Dominated | 0.058 | Dominated | 0.045 | Dominated | 0.045 |
Sensitivity analysis regarding the effectiveness of two-layer bandage
In Part II we saw that the current relative effectiveness estimates for the 2LB (in terms of healing) are low- or very low-quality estimates, in part due to the limited quality of the two direct RCTs driving these estimates. Thus in Part II an extra ‘post hoc’ sensitivity scenario was used, which considered equivalent effectiveness in healing for the 2LBs and 4LBs. Cascading these results here showed that, in this particular situation, HH becomes the cost-effective treatment, dominating the remaining health interventions. Figure 21 shows that, at a £20,000 ceiling ratio, HH is the treatment with highest probability of being cost-effective (approximately 60%) and the one that provides higher net gains.
FIGURE 21.
Net monetary benefit vs. probability of being cost-effective for the scenario in which healing effect of 2LB is equivalent to 4LB (£20,000 threshold).
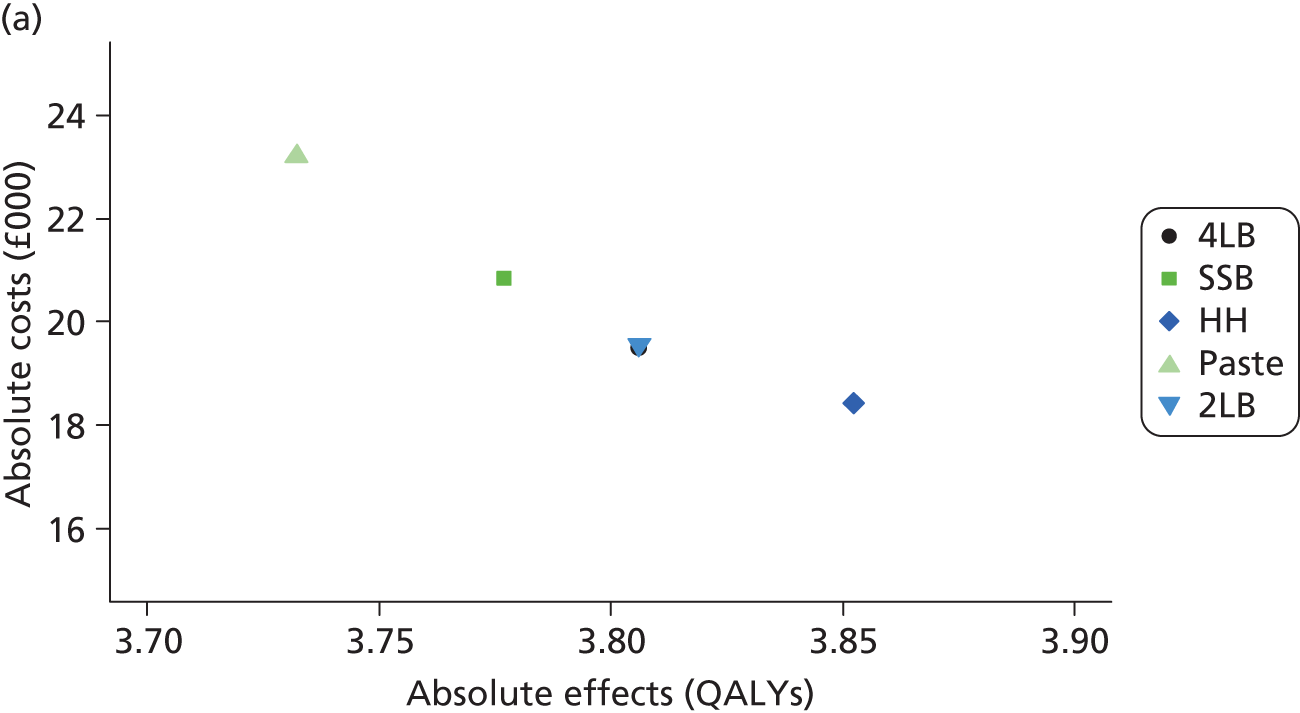
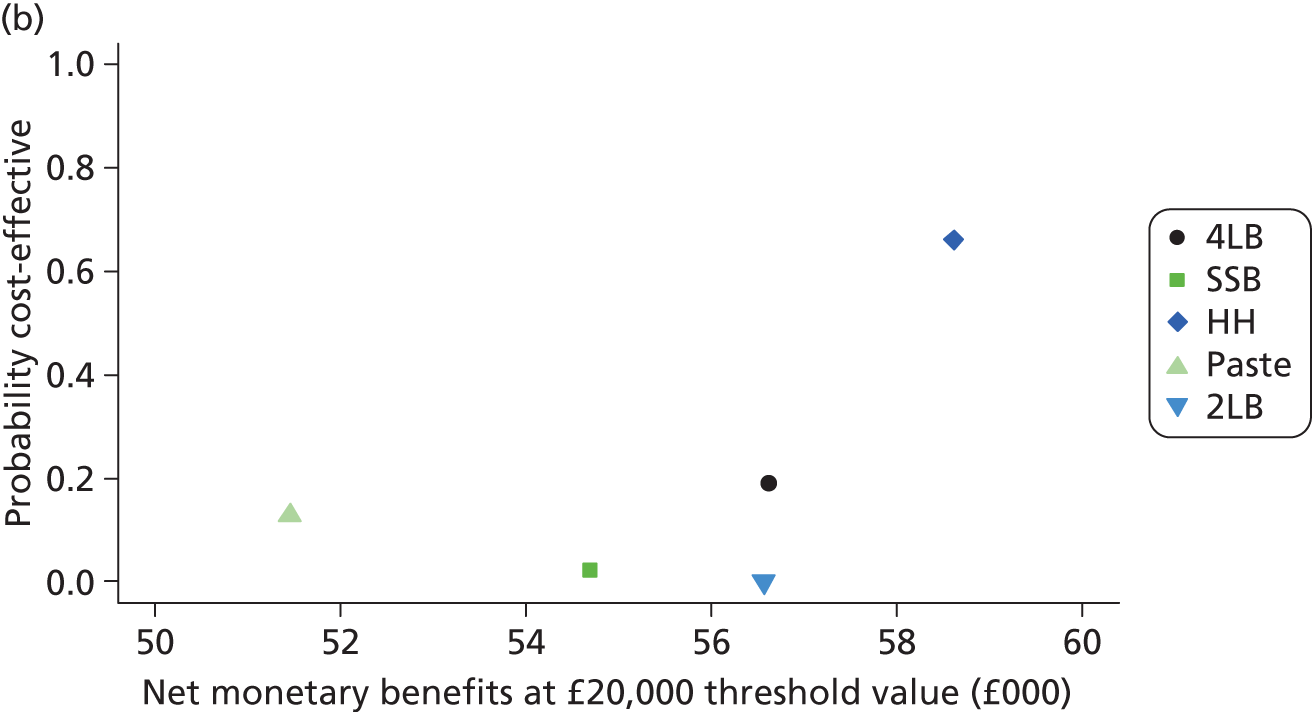
Chapter 18 Discussion
Assessing the cost-effectiveness of alternative high-compression treatments for venous leg ulcers is important in a health-care system operating under a fixed budget. This work extended the within-trial analyses presented in Part I by allowing other relevant alternatives (SSB, paste and two-layer system) to be considered and by allowing a longer time frame to be considered. Moreover, the decision model allows other health benefits, such as the effect of hosiery on recurrence, to be explicitly considered. To our knowledge this is the first decision model analysis undertaken for high-compression treatments.
Cost-effectiveness of high-compression treatments
Current evidence suggests that the 2LB is the most cost-effective compression system to treat venous leg ulcers (base-case analysis). However, this result is determined by ‘low-quality’ evidence on the relative effectiveness on healing of 2LB (see discussion section of Part II). Thus, we conducted an alternative scenario analysis, in which 2LB was assumed to be as effective as 4LB: the results demonstrated that 2LB is no longer cost-effective (actually, the probability of this treatment being cost-effective is close to zero in this scenario) and HH becomes cost-effective, as its benefits in terms of recurrence ‘compensate’ for its additional costs in relation to other available treatments such as the 4LB.
We also evaluated the cost-effectiveness results for subgroups of the population using the base-case analysis. We used determinants of healing and recurrence (baseline ulcer area and duration) to define such subgroups. This analysis showed that, under current evidence, 2LB was cost-effective in all subgroups. However, for patients with a longer duration of the ulcer and smaller area (e.g. ulcer size of 5 or 25 cm2 and 24 months’ ulcer duration), the probability of HH being cost-effective increases (to approximately 30%). However, in these cases, for HH to be adopted, decision-makers need to be ‘willing to pay’ or displace large amounts of funds.
Study strengths and limitations
In the assessment performed in this work, the evidence base on effectiveness was sparse, as many of the comparisons within the network contained only a small number of trials (see Part II). This led to high uncertainty in some effectiveness estimates which, in turn, were propagated throughout the decision model, affecting the estimates of costs and QALYs. Additional studies may be required to augment the evidence base and reduce uncertainty over decisions on the cost-effectiveness of alternative strategies in this area. Moreover, there were numerous decision model input parameters for which limited data existed. This was addressed by imposing conservative assumptions of equivalence between some compression treatments for some specific model parameters (e.g. treatment duration and nurse visits for 2LB were assumed equivalent to the 4LB).
A fully parametric distribution (i.e. Gompertz) was assumed to adequately represent the time to recurrence of healed ulcers. Other distributions were not attempted, as although the effect of a different distribution could affect absolute outcomes, it is less likely that incremental cost-effectiveness would be affected. Twelve tunnel states (representing 12 months) were incorporated in the decision model to reflect this time dependency after healing, after which the probability of recurrence was assumed null. This assumption was based on lack of evidence on recurrence after 12 months and the fact that it is known a priori that a proportion of patients do not recur. This null recurrence after 12 months’ assumption could have been relaxed by assuming a constant probability of recurrence, equal to one at 12 months, for all of the subsequent model cycles.
Value of further research
Given the uncertainty in the available data, and, consequently, in the decision regarding treatment choice, it is important to explore whether investing in further research to reduce such uncertainty is worthwhile. The maximum potential value of additional evidence was estimated to be approximately £2.9B at the population level (for a cost-effectiveness threshold value of £20,000 and considering the potential users of this technology, estimated at 365,000 per year). This represents the maximum value of further research that might resolve existing uncertainties. Because this value exceeds the likely costs of further investigation, additional research in this area is potentially worthwhile.
Part IV Overall discussion
Chapter 19 Overall discussion
Summary of findings from Parts I, II and III
Part I
Trial data from VenUS IV suggests that there is no evidence of a difference in time to ulcer healing for people treated with HH and the 4LB (HR of 0.99, 95% CI 0.79 to 1.25; p = 0.96). More participants in the HH group (39.3%) changed from their allocated trial treatment compared with the 4LB group (27.8%; p = 0.01) and although there was no statistically significant difference in the number of adverse events between groups – significantly more participants in the HH group reported one or more NSAEs during the trial (70.0% vs. 58.0%; p = 0.050). However, time to recurrence might be lower in those allocated to HH (HR 0.56, 95% CI 0.33 to 0.94; p = 0.026). Additionally, based on within-trial data alone, HH is very likely to be cost-effective compared with the 4LB owing to a reduction in the number of nurse consultations associated with hosiery and potentially, a reduction in recurrence.
Part II
When the evidence on effectiveness (in terms of healing of venous leg ulcers) was evaluated in the context of all other high-compression treatments (through a MTC meta-analysis), the two-layer compression bandage (two-component system with a top component that is a cohesive bandage) had the highest probability of ulcer healing (72%) compared with the HH (16% probability), paste bandage (6%), 4LB (6%) and SSB (0%). However, the evidence regarding the 2LB is categorised as low to very low quality. We are far more confident in the MTC’s finding that the SSB is less effective than both the 4LB and HH. This evidence is categorised as medium to high quality, partly owing to the presence of VenUS I7 and IV data.
Part III
When analyses were extended to include all potential health benefits and the costs incurred by patients with venous leg ulcers, results suggest that the 2LB system (two-component system with a top component that is a cohesive bandage) had the highest probability of being the most cost-effective high-compression treatment for venous leg ulcers. As in Part II, the low quality of the RCT data for the effects of the 2LB mean that considerable uncertainty remains. Balance must be sought between acknowledging the comprehensiveness of this model in terms of utilising all available evidence to inform decision-making and recognition of the limitation of available evidence regarding some treatments.
Conclusions
Trial data from VenUS IV found no evidence of a difference in venous ulcer healing between HH and four-layer bandaging. The results also suggested that HH may reduce ulcer recurrence rates when compared with the 4LB and be a cost-effective treatment. However, more people allocated HH changed to another treatment and more participants in the two-layer compression hosiery group reported NSAEs.
In additional analysis, when all high-compression treatments were considered, the 2LB had the highest probability of being clinically effective and cost-effective. However, the quality of the underlying evidence was sparse and poor overall.
Contribution of this study to the evidence
This RCT addressed an important clinical uncertainty in leg ulcer care. We also considered these new trial data in conjunction with those from all other relevant RCTs of high-compression treatments. Furthermore, we combined these data with all other evidence relevant to this decision problem in order to estimate the most cost-effective high-compression treatment.
Together, these three stages [(1) RCT; (2) synthesis of effectiveness evidence; and (3) cost-effectiveness modelling] provide the most comprehensive evidence up-to-date evidence regarding the relative clinical effectiveness and cost-effectiveness of high-compression treatments for active venous leg ulceration.
Implications for health care
The results of this study (which incorporates a RCT, a MTC and a cost-effectiveness model) suggest that hosiery is as effective as the 4LB in healing venous leg ulcers, although more patients may change from HH during the course of treatment thus it may not be the optimal treatment for all patients. Importantly, while wearing HH, participants were seen by nurses on fewer occasions and so this treatment was more cost-effective than the 4LB. Participants in the HH group also demonstrated lower rates of ulcer recurrence than those in the 4LB group; an interesting finding that we are not able to fully explain. It may be that patients who wear HH as an ulcer treatment are more likely to wear compression stockings for secondary prevention after healing (and may wear higher compression); we are unable to confirm this hypothesis with the trial data.
Two-layer hosiery is a cost-effective alternative to four-layer bandaging and can be considered as a treatment for those people with venous leg ulcers who are suitable for this treatment, for example if they or a carer are able to apply the hosiery. We have limited information regarding the practicality of using HH with different dressing types (in terms of ease of hosiery application while keeping specific dressings in place).
Although all current evidence suggests that two-layer compression bandaging may be an effective and cost-effective treatment for venous leg ulcers, this conclusion is associated with significant uncertainty as the existing evidence comprises small and low-quality trials.
Recommendations for future research
Analyses presented in this report demonstrate that VenUS IV was worthwhile, as it determined the value of HH in treating active venous ulceration. The value of further information analysis showed that the inclusion of VenUS IV considerably reduced the consequences of decision uncertainty.
The findings of Parts II and III emphasise the tentative nature of the evidence supporting the use of 2LB. As the 2LB is in current clinical use, even although there is uncertainty regarding its clinical effectiveness and cost-effectiveness, we suggest that important further research is required. This further research should focus on establishing the comparative effectiveness of the two-layer compression bandage with HH.
Acknowledgements
We would especially like to thank the participants who took part in this trial. Special thanks are also due to the Principal Investigators, research nurses and health-care professionals who screened and recruited patients into the study as well as collecting trial data and ensuring the success of the trial. We would also like to thanks Hugo Partsch for the expert advice he generously provided in this study and Dr Susan O’Meara for sharing data from her ongoing Cochrane review, as well as her advice and insights regarding compression treatments. Finally, we would like to extend our special thanks to Mr and Mrs Hopper for their commitment, enthusiasm and patience during this study.
The Principal Investigators (past and present) were:
Una Adderley, Jacqui Ashton, Lynne Atcheson, Pauline Beldon, Fiona Buckley, Clare Cattermole, Leanne Cook, Stephen Foley, Wyn Glencross, Lalit Gurnani, Gemma Hancock, Amardeep Heer, Valerie Henderson, Karen Johnson, Nicci Kimpton, Sara Kray, Julie Lambert, James Larcombe, Alison Layton, Martin Linton, Calum Lyon, Jeanette Milne, Nicky Morton, Sarah Pankhurst, Anne-Marie Perrin, Rachael Robinson, Terry Shipperley, Gillian Speight, Nikki Stubbs, Nick Taylor, Kathryn Vowden, Shernaz Walton, Nicola Whayman, Deborah Wickens, Anne Witherow and Amanda Youle.
The research nurses and co-investigators (past and present) were:
Shirley Aspin, Sue Atkinson, Olga Balazikova, Clare Barker, John Barker, Sue Barnes, Resty Bautista, Lucy Bellas, Jo-Anne Beresford, Kerry Brennan, Laura Brockway, Margaret Broome, Janet Brown, Kate Brown, Latitia Butler, Karen Clark, Louise Collins, Adele Collinson, Claire Cox, Sarah Davies, Jackie Dawson, Carolyn Devonport, Caroline Dixon, Janet Doolan, Kathryn Fairbrother, Annette Fendell, Adrienne Ford, Pam Freeman, Vicky Gilchrist, Annette Given, Ann Graham, Elizabeth Green, Jill Green, Andrea Hanson, Nicola Harding, Hayley Harvey, Sarah Hay, Elisabeth Hooker, Jane Horton, Louise Jones, Peter Jones, Helen Jung, Sarah Kavanagh, Jo Keevil, Debbie Kelly, Deborah Kemp, Melanie Kempster, Vicky Lam, Gayle Law, Claire Livingstone, Fiona Longstaff, Karen Lowes, Elizabeth Marshall, Janet McGowan, Jennifer Millar, Sharon Moon, Timothy Moss, Jennifer Mullan, Sarah Nicholson, Jackie Nye, Emma Openshaw, Donna O’Shea, Carol Owen, Cilla Page, Hannah Patten, Robert Penman, Kathryn Porter, Julie Poyzer, Susan Reading, Sarah Smith, Louise Snell, Barbara Stewart, June Tanner, Samantha Tapscott, Julie Thackray, Amanda Tomlinson, Debra Vickery, Ben Walker, Jane Walker, April Weaver, Nicola Whitfield, Andrea Whitton, Denise Williams, Lindsey Worstenholme, Emma Wright, Jane Young and Judith Young.
Nikki Stubbs, Gemma Hancock and Angie Oswald performed the blinded outcome assessment of healing of the ulcer photographs. Catherine Arundel measured the baseline ulcer tracings. Sue Collins checked clinical data received for completeness and posted out questionnaires to study participants. Ruth Foxlee, Kate Light and Melissa Harden conducted literature searches.
Contributions of authors
Rebecca L Ashby (research fellow, health sciences) coordinated the study.
Rhian Gabe (senior statistician, health sciences) performed the statistical analysis.
Shehzad Ali (research fellow, health economics) conducted the trial-based cost-effectiveness analysis.
Pedro Saramago (research fellow, health economics) conducted the MTC meta-analysis and cost-effectiveness analyses.
Ling-Hsiang Chuang (research fellow, health economics) contributed to the evidence synthesis and cost-effectiveness analysis.
Una Adderley (lecturer, health care) gave clinical input and advice during the trial, and contributed to the development of the grant application and trial protocol.
J Martin Bland (Professor, Health Statistics) oversaw the conduct of the analysis and contributed to the development of the grant application and trial protocol.
Nicky A Cullum (Professor, Nursing) was Chief Investigator 2009–12 and contributed to the development of the grant application and trial protocol.
Jo C Dumville (research fellow, health sciences) was Chief Investigator 2009–12 and contributed to the development of the grant application and trial protocol.
Cynthia P Iglesias (senior research fellow, health economics) contributed to the development of the grant application and trial protocol.
Arthur R Kang’ombe wrote the statistical analysis plan.
Marta O Soares (research fellow, health economics) oversaw and advised on all elements of the cost-effectiveness work, and contributed to the development of the grant application and trial protocol.
Nikki C Stubbs (clinical team leader, tissue viability) gave clinical input and advice during the trial, and contributed to the development of the grant application and trial protocol.
David J Torgerson (Professor, Trial Methodology) provided advice on trial conduct and contributed to the development of the grant application and trial protocol.
All authors were members of the Trial Management Group and prepared the final manuscript.
Trial Steering Committee members
Professor Ian Chetter (Chair), Dr Jenny Freeman (external member), Mrs Brenda King (external member), John Hopper (patient representative) and Doreen Hopper (patient representative). Other members (past and present) were: Una Adderley, Shehzad Ali, Rebecca Ashby, Jacqui Ashton, Martin Bland, Sue Collins, Ben Cross, Nicky Cullum, Jo Dumville, Rhian Gabe, Pedro Saramago, Cynthia Iglesias, Arthur Kang’ombe, Marta Soares, Nikki Stubbs, David Torgerson, Jude Watson, Anne Witherow and Gillian Worthy.
Owing to the low-risk nature of this trial (as both treatments being evaluated were being used routinely in clinical practice), it was not deemed necessary to have a separate Data Monitoring and Ethics Committee to oversee the trial. Instead, unblinded adverse events data, details of patients no longer receiving randomised treatments and details of post-randomised exclusions were presented by the trial coordinator (RA) and trial statisticians (AK, MB) to independent members of the TSC [Chair (IC), independent clinician (BK)and independent statistician (JF)] prior to TSC meetings. This decision was ratified by the Health Technology Assessment (HTA) programme and minutes of these meetings were sent to the HTA programme.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol 2001;44:401-21. http://dx.doi.org/10.1067/mjd.2001.111633.
- Gloviczki P, Gloviczki ML. Evidence on efficacy of treatments of venous ulcers and on prevention of ulcer recurrence. Perspect Vasc Surg Endovasc Ther 2009;21:259-68. http://dx.doi.org/10.1177/1531003510373660.
- Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. BMJ 2004;328:1358-62. http://dx.doi.org/10.1136/bmj.328.7452.1358.
- Abbade LP, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol 2005;44:449-56. http://dx.doi.org/10.1111/j.1365-4632.2004.02456.x.
- Maggisano R, Harrison AW. The Venous System. Toronto, ON: University of Toronto; 2004.
- Barwell JR, Davies CE, Deacon J, Harvey K, Minor J, Sassano A, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet 2004;363:1854-9. http://dx.doi.org/10.1016/S0140-6736(04)16353-8.
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technol Assess 2004;8.
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes 2007;5. http://dx.doi.org/10.1186/1477-7525-5-44.
- Persoon A, Heinen MM, van der Vleuten CJ, de Rooij MJ, van de Kerkhof PC, van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004;13:341-54. http://dx.doi.org/10.1046/j.1365-2702.2003.00859.x.
- Nelzen O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg 1994;81:182-7. http://dx.doi.org/10.1002/bjs.1800810206.
- Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381-6. http://dx.doi.org/10.1067/mjd.2002.121739.
- National Audit of the Management of Venous Leg Ulcers. London: Healthcare Commission; 2008.
- Watson JM, Kang’ombe AR, Soares MO, Chuang LH, Worthy G, Bland JM, et al. Use of weekly, low dose, high frequency ultrasound for hard to heal venous leg ulcers: the VenUS III randomised controlled trial. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d1092.
- Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair Regen 2005;13:13-8. http://dx.doi.org/10.1111/j.1067-1927.2005.130103.x.
- Morrell CJ, Walters SJ, Dixon S, Collins KA, Brereton LM, Peters J, et al. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ 1998;316:1487-91. http://dx.doi.org/10.1136/bmj.316.7143.1487.
- Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J (Clin Res Ed) 1985;290:1855-6. http://dx.doi.org/10.1136/bmj.290.6485.1855.
- Hickie S, Ross S, Bond C. A survey of the management of leg ulcers in primary care settings in Scotland. J Clin Nurs 1998;7:45-50. http://dx.doi.org/10.1046/j.1365-2702.1998.00124.x.
- Laing W. Chronic Venous Diseases of the Leg. London: Office of Health Economics; 1992.
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11. http://dx.doi.org/10.1002/14651858.CD000265.pub3.
- The Nursing Management of Venous Leg Ulcers. London: Royal College of Nursing; 2006.
- Management of Chronic Venous Leg Ulcers – A National Clinical Guideline. Edinburgh: SIGN; 2010.
- Understanding Compression Therapy. Frederiksberg: European Wound Management Association; 2004.
- Moffatt C. Compression Therapy in Practice. Trowbridge, Wiltshire: Cromwell Press; 2007.
- O’Meara S, Tierney J, Cullum N, Bland M, Cooper P, Franks P, et al. Four layer bandage compared with short stretch bandage for venous leg ulcers: systematic review and meta-analysis of randomised controlled trials with data from individual patients. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1344.
- Meyer FJ, McGuinness CL, Lagattolla NR, Eastham D, Burnand KG. Randomized clinical trial of three-layer paste and four-layer bandages for venous leg ulcers. Br J Surg 2003;90:934-40. http://dx.doi.org/10.1002/bjs.4173.
- Moffatt CJ, Dorman MC. Recurrence of leg ulcers within a community ulcer service. J Wound Care 1995;4:57-61.
- Johnson GV. Elastic stockings. BMJ (Clin Res Ed) 1988;296. http://dx.doi.org/10.1136/bmj.296.6623.720-e.
- Junger M, Wollina U, Kohnen R, Rabe E. Efficacy and tolerability of an ulcer compression stocking for therapy of chronic venous ulcer compared with a below-knee compression bandage: results from a prospective, randomized, multicentre trial. Curr Med Res Opin 2004;20:1613-23. http://dx.doi.org/10.1185/030079904X4086.
- Polignano R, Guarnera G, Bonadeo P. Evaluation of SurePress Comfort: a new compression system for the management of venous leg ulcers. J Wound Care 2004;13:387-91. http://dx.doi.org/10.12968/jowc.2004.13.9.26703.
- Mariani F, Mattaliano V, Mosti G, Gasbarro V, Bucalossi M, Blattler W, et al. The treatment of venous leg ulcers with a specifically designed compression stocking kit. Phlebologie 2008;37:191-7.
- Taradaj J, Franek A, Brzezinska-Wcislo L, Blaszczak E, Polak A. Randomized trial of medical compression stockings versus two-layer short-stretch bandaging in the management of venous leg ulcers. Phlebologie 2009;38:157-63.
- Finlayson KJ, Courtney MD, Gibb MA, O’Brien JA, Parker CN, Edwards HE. The effectiveness of a four-layer compression bandage system in comparison to Class 3 compression hosiery on healing and quality of life for patients with venous leg ulcers: a randomised controlled trial. Int Wound J 2014;11:21-7. http://dx.doi.org/10.1111/j.1742-481X.2012.01033.x.
- Vandongen YK, Stacey MC. Graduated compression elastic stockings reduce lipodermatosclerosis and ulcer recurrence. Phlebology 2000;15:33-7. http://dx.doi.org/10.1007/s005230070035.
- Milic DJ, Zivic SS, Bogdanovic DC, Pejic M, Jovanovic M. A randomized trial of class 2 and class 3 elastic compression in the prevention of recurrence of venous ulceration. J Vasc Surg 2010;51:797-8. http://dx.doi.org/10.1016/j.jvs.2009.11.033.
- Nelson EA, Harper DR, Prescott RJ, Gibson B, Brown D, Ruckley CV. Prevention of recurrence of venous ulceration: randomized controlled trial of class 2 and class 3 elastic compression. J Vasc Surg 2006;44:803-8. http://dx.doi.org/10.1016/j.jvs.2006.05.051.
- Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2012;8.
- Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol 2009;62:857-64. http://dx.doi.org/10.1016/j.jclinepi.2008.10.001.
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313-24. http://dx.doi.org/10.1002/sim.1201.
- Lu G, Ades A. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Sutton A, Ades A, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 2008;26:753-67. http://dx.doi.org/10.2165/00019053-200826090-00006.
- Cooper N, Sutton A, Morris D, Ades A, Welton N. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med 2009;28:1861-81. http://dx.doi.org/10.1002/sim.3594.
- Salanti G, Dias S, Welton NJ, Ades AE, Golfinopoulos V, Kyrgiou M, et al. Evaluating novel agent effects in multiple-treatments meta-regression. Stat Med 2010;29:2369-83.
- Taylor R. ‘Mouseyes’: an aid to wound measurement using an computer. J Wound Care 1997;6:123-6.
- Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705-18. http://dx.doi.org/10.1007/s11136-005-2751-9.
- Iglesias CP, Birks YF, Torgerson DJ. Improving the measurement of quality of life in older people: the York SF-12. QJM 2001;94:695-8. http://dx.doi.org/10.1093/qjmed/94.12.695.
- Loftus S. A longitudinal, quality of life study comparing four layer bandaging and superficial venous surgery for the treatment of venous leg ulcers. J Tissue Viabil 2001;11:14-9.
- Palfreyman S. Assessing the impact of venous ulceration on quality of life. Nurs Times 2008;104:34-7.
- Walters SJ, Morrell CJ, Dixon S. Measuring health-related quality of life in patients with venous leg ulcers. Qual Life Res 1999;8:327-36. http://dx.doi.org/10.1023/A:1008992006845.
- Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Validation of the EQ-5D in patients with a history of acute coronary syndrome. Curr Med Res Opin 2005;21:1209-16. http://dx.doi.org/10.1185/030079905X56349.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551-9. http://dx.doi.org/10.1093/rheumatology/36.5.551.
- Johnson JA, Pickard AS. Comparison of the EQ-5D and SF-12 health surveys in a general population survey in Alberta, Canada. Med Care 2000;38:115-21. http://dx.doi.org/10.1097/00005650-200001000-00013.
- Konig HH, Ulshofer A, Gregor M, von Tirpitz C, Reinshagen M, Adler G, et al. Validation of the EuroQol questionnaire in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2002;14:1205-15. http://dx.doi.org/10.1097/00042737-200211000-00008.
- Mahadeva S, Wee HL, Goh KL, Thumboo J. The EQ-5D (Euroqol) is a valid generic instrument for measuring quality of life in patients with dyspepsia. BMC Gastroenterol 2009;9. http://dx.doi.org/10.1186/1471-230X-9-20.
- Öster C, Willebrand M, Dyster-Aas J, Kildal M, Ekselius L. Validation of the EQ-5D questionnaire in burn injured adults. Burns 2009;35:723-32. http://dx.doi.org/10.1016/j.burns.2008.11.007.
- Prieto L, Sacristan JA, Hormaechea JA, Casado A, Badia X, Gomez JC. Psychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patients. Curr Med Res Opin 2004;20:827-35. http://dx.doi.org/10.1185/030079904125003674.
- Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart 2006;92:62-7. http://dx.doi.org/10.1136/hrt.2004.052787.
- van Agt HM, Essink-Bot ML, Krabbe PF, Bonsel GJ. Test-retest reliability of health state valuations collected with the EuroQol questionnaire. Soc Sci Med n.d.;39:1537-44.
- Margolis DJ, Berlin JA, Strom BL. Risk factors associated with the failure of a venous leg ulcer to heal. Arch Dermatol 1999;135:920-6. http://dx.doi.org/10.1001/archderm.135.8.920.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev 2007;2.
- ICH Expert Working Group . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline for Good Clinical Practice E6(R1) 1996. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf.
- Dumville JC, Worthy G, Bland JM, Cullum N, Dowson C, Iglesias C, et al. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b773 (accessed 1 November 2012).
- Margolis DJ, Knauss J, Bilker W. Medical conditions associated with venous leg ulcers. Br J Dermatol 2004;150:267-73. http://dx.doi.org/10.1111/j.1365-2133.2004.05773.x.
- Collett D. Modelling Survival Data in Medical Research. London: Chapman and Hall/CRC; 2003.
- Cox D. Analysis of Survival Data. London: Chapman and Hall/CRC; 1984.
- Kalbfleisch J, Prentice R. The Statistical Analysis of Failure Time. New York, NY: Wiley; 2002.
- Schoenfeld D. Partial residuals for the proportional hazards model. Biometrika 1982;69:51-5. http://dx.doi.org/10.1093/biomet/69.1.239.
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12v2® Health Survey (With a Supplement Documenting Version 1). Lincoln, RI: QualityMetric; 2002.
- Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004.
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000.
- Agresti A. Categorical Data Analysis. Hoboken, NJ: John Wiley; 2002.
- Erdman D, Jackson L, Sinko A. Zero-inflated Poisson and Sero-inflated Negative Binomial Models using the COUNTREG Procedure. San Antonio, Texas: SAS Institute; 2008.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Prescription Cost Analysis. London: NHS Information Centre; 2011.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. New York, NY: Lippincott-Raven; 1996.
- Brazier J. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- Dolan P, Gudex C, Kind P. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York, Centre for Health Economics; 1995.
- Glick H. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Lin DY, Etzioni R, Feuer EJ, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics 1997;53:419-34. http://dx.doi.org/10.2307/2533947.
- Etzioni RD, Feuer EJ, Sullivan SD, Lin D, Hu C, Ramsey SD. On the use of survival analysis techniques to estimate medical care costs. J Health Econ 1999;18:365-80. http://dx.doi.org/10.1016/S0167-6296(98)00056-3.
- Lin DY. Regression analysis of incomplete medical cost data. Stat Med 2003;22:1181-200. http://dx.doi.org/10.1002/sim.1377.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45. http://dx.doi.org/10.1002/sim.1794.
- Gomes M, Ng E, Grieve R, Nixon R, Carpenter J, Thompson S. Developing appropriate analytical methods for cost-effectiveness analyses that use data from cluster randomised trials. Med Decis Mak 2012;32:350-61. http://dx.doi.org/10.1177/0272989X11418372.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733-44. http://dx.doi.org/10.2165/00019053-200826090-00004.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Fenwick E, O’Brien B, Briggs A. Cost-effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- World Health Organization . BMI Classification 2012 n.d. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed 22 November 2012).
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987-91. http://dx.doi.org/10.1001/jama.285.15.1987.
- Skene AI, Smith JM, Doré CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992;305:1119-21. http://dx.doi.org/10.1136/bmj.305.6862.1119.
- Milic DJ, Zivic SS, Bogdanovic DC, Karanovic ND, Golubovic ZV. Risk factors related to the failure of venous leg ulcers to heal with compression treatment. J Vasc Surg 2009;49:1242-7. http://dx.doi.org/10.1016/j.jvs.2008.11.069.
- Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163-8. http://dx.doi.org/10.1111/j.1067-1927.2004.012207.x.
- Van Hecke A, Grypdonck M, Defloor T. A review of why patients with leg ulcers do not adhere to treatment. J Clin Nurs 2009;18:337-49. http://dx.doi.org/10.1111/j.1365-2702.2008.02575.x.
- Cheater F, Currie L, Harvey G, Liao X-h, Loftus-Hills A, Meldrum P, et al. The Management of Patients with Venous Leg Ulcers. London; 2001.
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e1119.
- Cordts P, Hanrahan L, Rodriguez A, Woodson J, LaMorte W, Menzoian J. A prospective, randomized trial of Unna’s boot versus Duoderm CGF hydroactive dressing plus compression in the management of venous leg ulcers. J Vasc Surg 1992;15:480-6. http://dx.doi.org/10.1016/0741-5214(92)90186-C.
- Eriksson G. Comparison of two occlusive bandages in the treatment of venous leg ulcers. Br J Dermatol 1986;114:227-30. http://dx.doi.org/10.1111/j.1365-2133.1986.tb02801.x.
- Nelson EA, Prescott RJ, Harper DR, Gibson B, Brown D, Ruckley CV. A factorial, randomized trial of pentoxifylline or placebo, four-layer or single-layer compression, and knitted viscose or hydrocolloid dressings for venous ulcers. J Vasc Surg 2007;45:134-41. http://dx.doi.org/10.1016/j.jvs.2006.09.043.
- Danielsen L, Madsen S, Henriksen L. Venous leg ulcer healing: A randomized prospective study of long-stretch versus short-stretch compression bandages. Phlebology 1998;13:59-63.
- Moody M. Comparison of Rosidal K and SurePress in the treatment of venous leg ulcers. Br J Nurs 1999;8:345-55. http://dx.doi.org/10.12968/bjon.1999.8.6.6660.
- Moffatt C, McCullagh L, O’Connor T, Doherty D, Hourican C, Stevens J, et al. Randomized trial of four-layer and two-layer bandage systems in the management of chronic venous ulceration. Wound Repair Regen 2003;11:166-71. http://dx.doi.org/10.1046/j.1524-475X.2003.11303.x.
- Callam M, Harper D, Dale J, Brown D, Gibson B, Prescott R, et al. Lothian and Forth Valley leg ulcer healing trial. Part 1: Elastic versus non-elastic bandaging in the treatment of chronic leg ulceration. Phlebology 1992;7:136-41.
- Gould D, Campbell S, Newton H, Duffelen P, Griffin M, Harding E. Setopress vs Elastocrepe in chronic venous ulceration. Br J Nurs 1998;7:66-73.
- Meyer F, Burnand K, Lagattolla N, Eastham D. Randomized clinical trial comparing the efficacy of two bandaging regimens in the treatment of venous leg ulcers. Br J Surg 2002;89:40-4. http://dx.doi.org/10.1046/j.0007-1323.2001.01936.x.
- Moffatt C, Simon D, Franks P, Connolly M, Fielden S, Groarke L, et al. Randomised trial comparing two four-layer bandage systems in the management of chronic leg ulceration. Phlebology 1999;14:139-42. http://dx.doi.org/10.1007/s005239970002.
- Vowden K, Mason A, Wilkinson D, Vowden P. Comparison of the healing rates and complications of three four-layer bandage regimens. J Wound Care 2000;9:269-72. http://dx.doi.org/10.12968/jowc.2000.9.6.25992.
- Hendricks WM, Swallow RT. Management of stasis leg ulcers with Unna boots versus elastic support stockings. J Am Acad Dermatol 1985;12:90-8. http://dx.doi.org/10.1016/S0190-9622(85)70015-1.
- Koksal C, Bozkurt AK. Combination of hydrocolloid dressing and medical compression stocking versus Unna’s boot for the treatment of venous leg ulcers. Swiss Med Wkly 2003;133:364-8.
- Junger M, Partsch H, Ramelet AA, Zuccarelli F. Efficacy of a ready-made tubular compression device versus short-stretch compression bandages in the treatment of venous leg ulcers. Wounds 2004;16:313-20.
- Milic DJ, Zivic SS, Bogdanovic DC, Perisic ZD, Milosevic ZD, Jankovic RJ, et al. A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers. J Vasc Surg 2007;46:750-5. http://dx.doi.org/10.1016/j.jvs.2007.04.062.
- Harley J, Harcourt D, Hutchinson B, McLean M, Long M. A comparative trial of long stretch compression bandage versus multi-layer compression bandage in the treatment of chronic venous ulcers. Prim Intent 2004;12:9-13.
- Milic D, Zivic S, Bogdanovic D, Jovanovic M, Jankovic R, Milosevic Z, et al. The influence of different sub-bandage pressure values on venous leg ulcers healing when treated with compression therapy. J Vasc Surg 2010;51:655-61. http://dx.doi.org/10.1016/j.jvs.2009.10.042.
- Brizzio E, Amsler F, Lun B, Blattler W. Comparison of low-strength compression stockings with bandages for the treatment of recalcitrant venous ulcers. J Vasc Surg 2010;51:410-16. http://dx.doi.org/10.1016/j.jvs.2009.08.048.
- Kralj B, Kosicek M. Randomised Comparative Trial of Single-Layer and Multi-Layer Bandages in the Treatment of Venous Leg Ulcer n.d.
- Colgan MP, Teevan M, McBride C, O’Sullivan L, Moore D, Shanik G. Cost Comparisons in the Management of Venous Ulceration n.d.
- Duby T, Hoffman D, Cameron J, Doblhoffbrown D, Cherry G, Ryan T. A randomized trial in the treatment of venous leg ulcers comparing short stretch bandages, 4 layer bandage system, and a long stretch paste bandage system. Wounds 1993;5:276-9.
- Wilkinson E, Buttfield S, Cooper S, Young E. Trial of two bandaging systems for chronic venous leg ulcers. J Wound Care 1997;6:339-40.
- Scriven J, Taylor L, Wood AJ, Bell PR, Naylor A, London N. A prospective randomised trial of four-layer versus short stretch compression bandages for the treatment of venous leg ulcers. Ann R Coll Surg Engl 1998;80:215-20.
- Partsch H, Damstra R, Tazelaar D, Schuller-Petrovic S, Velders A, De Rooij M, et al. Multicentre, randomised controlled trial of four-layer bandaging versus short-stretch bandaging in the treatment of venous leg ulcers. Vasa 2001;30:108-13. http://dx.doi.org/10.1024/0301-1526.30.2.108.
- Ukat A, Konig M, Vanscheidt W, Munter K. Short-stretch versus multilayer compression for venous leg ulcers: a comparison of healing rates. J Wound Care 2003;12:139-43. http://dx.doi.org/10.12968/jowc.2003.12.4.26490.
- Franks P, Moody M, Moffatt C, Martin R, Blewett R, Seymour E, et al. Randomized trial of cohesive short-stretch versus four-layer bandaging in the management of venous ulceration. Wound Repair Regen 2004;12:157-62. http://dx.doi.org/10.1111/j.1067-1927.2004.012206.x.
- Polignano R, Bonadeo P, Gasbarro S, Allegra C. A randomised controlled study of four-layer compression versus Unna’s Boot for venous ulcers. J Wound Care 2004;13:21-4. http://dx.doi.org/10.12968/jowc.2004.13.1.26563.
- Blecken S, Villavicencio J, Kao T. Comparison of elastic versus nonelastic compression in bilateral venous ulcers: a randomized trial. J Vasc Surg 2005;42:1150-5. http://dx.doi.org/10.1016/j.jvs.2005.08.015.
- Moffatt C, Edwards L, Collier M, Treadwell T, Miller M, Shafer L, et al. A randomised controlled 8-week crossover clinical evaluation of the 3M Coban 2 Layer Compression System versus Profore to evaluate the product performance in patients with venous leg ulcers. Int Wound J 2008;5:267-79. http://dx.doi.org/10.1111/j.1742-481X.2008.00487.x.
- Szewczyk MT, Jawien A, Cierzniakowska K, Cwajda-Bialasik J, Moscicka P. Comparison of the effectiveness of compression stockings and layer compression systems in venous ulceration treatment. Arch Med Sci 2010;6:793-9. http://dx.doi.org/10.5114/aoms.2010.17097.
- Wong I, Andriessen A, Charles H, Thompson D, Lee D, So W, et al. Randomized controlled trial comparing treatment outcome of two compression bandaging systems and standard care without compression in patients with venous leg ulcers. J Eur Acad Dermatol Venereol 2012;26:102-10. http://dx.doi.org/10.1111/j.1468-3083.2011.04327.x.
- DePalma RG, Kowallek D, Spence RK, Caprini JA, Nehler MR, Jensen J, et al. Comparison of costs and healing rates of two forms of compression in treating venous ulcers. Vasc Surg 1999;33:683-90. http://dx.doi.org/10.1177/153857449903300617.
- Travers JP, Dalziel KL, Makin GS. Assessment of a new one-layer adhesive bandaging method in maintaining prolonged limb compression and effects on venous ulcer healing. Phlebology 1992;7:59-63.
- Knight CA, McCulloch J. A Comparative Study Between Two Compression Systems in the Treatment of Venous Insufficiency in Leg Ulcers n.d.
- Brizzio E. Comparison of low-strength compression stockings with bandages for the treatment of recalcitrant venous ulcers: a randomized trial. Wound Repair Regen 2010;18.
- Smith CT, Williamson PR, Marson AG. An overview of methods and empirical comparison of aggregate data and individual. J Eval Clin Pract 2005;11:468-78. http://dx.doi.org/10.1111/j.1365-2753.2005.00559.x.
- Saramago P, Sutton AJ, Cooper NJ, Manca A. Mixed treatment comparisons using aggregate and individual participant level. Stat Med 2012;31:3516-36. http://dx.doi.org/10.1002/sim.5442.
- Soares MO, Dumville JC, Ades AE, Welton NJ. Treatment comparisons for decision making: facing the problems of sparse and few data. J R Stat Soc 2014;177:259-79. http://dx.doi.org/10.1111/rssa.12010.
- Piantadosi S, Byar DP, Green SB. The ecological fallacy. Am J Epidemiol 1988;127:893-904.
- Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol 2002;64:583-616. http://dx.doi.org/10.1111/1467-9868.00353.
- Dumville JC, Soares MO, O’Meara S, Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia 2012;55:1902-10. http://dx.doi.org/10.1007/s00125-012-2558-5.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. http://dx.doi.org/10.1002/sim.3767.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8.
- Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost-effectiveness modelling. Stat Med 2003;22:3687-709. http://dx.doi.org/10.1002/sim.1586.
- Turner RM, Spiegelhalter DJ, Smith GC, Thompson SG. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc 2009;172:21-47. http://dx.doi.org/10.1111/j.1467-985X.2008.00547.x.
- Welton NJ, Ades AE, Carlin JB, Altman DG, Sterne JAC. Models for potentially biased evidence in meta-analysis using empirically based priors. J R Stat Soc Ser A Stat Soc 2009;172:119-36. http://dx.doi.org/10.1111/j.1467-985X.2008.00548.x.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology. Pharmacoeconomics 2006;24:355-71. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Carr L, Philips Z, Posnett J. Comparative cost-effectiveness of four-layer bandaging in the treatment of venous leg ulceration. J Wound Care 1999;8:243-8. http://dx.doi.org/10.12968/jowc.1999.8.5.26361.
- Schonfeld WH, Villa KF, Fastenau JM, Mazonson PD, Falanga V. An economic assessment of Apligraf (Graftskin) for the treatment of hard-to-heal venous leg ulcers. Wound Repair Regen 2000;8:251-7. http://dx.doi.org/10.1046/j.1524-475x.2000.00251.x.
- Korn P, Patel S, Heller J, Deitch J, Krishnasastry K, Bush H, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg 2002;35:950-7. http://dx.doi.org/10.1067/mva.2002.121984.
- Guest J, Ruiz F, Mihai A, Lehman A. Cost effectiveness of using carboxymethylcellulose dressing compared with gauze in the management of exuding venous leg ulcers in Germany and the USA. Curr Med Res Opin 2005;21:81-92. http://dx.doi.org/10.1185/030079904X15219.
- Scanlon E, Karlsmark T, Leaper D, Carter K, Poulsen P, Hart-Hansen K, et al. Cost-effective faster wound healing with a sustained silver-releasing foam dressing in delayed healing leg ulcers: a health-economic analysis. Int Wound J 2005;2:150-60. http://dx.doi.org/10.1111/j.1742-4801.2005.00083.x.
- Iglesias C, Claxton K. Comprehensive decision-analytic model and Bayesian value-of-information analysis: pentoxifylline in the treatment of chronic venous leg ulcers. Pharmacoeconomics 2006;24:465-78. http://dx.doi.org/10.2165/00019053-200624050-00005.
- Clegg J, Guest J. Modelling the cost-utility of bio-electric stimulation therapy compared to standard care in the treatment of elderly patients with chronic non-healing wounds in the UK. Curr Med Res Opin 2007;23:871-83. http://dx.doi.org/10.1185/030079906X167705.
- Marinel-lo-Roura J, Iglesias-García C, Lorenzo-Ferrer J, Mercadal-Dalmau J, Lozano-Fernández de Pinedo R. Angiologia 2007;59:45-54. http://dx.doi.org/10.1016/S0003-3170(07)75026-5.
- Guest J, Nagy E, Sladkevicius E, Vowden P, Price P. Modelling the relative cost-effectiveness of amelogenin in non-healing venous leg ulcers. J Wound Care 2009;18:216-24. http://dx.doi.org/10.12968/jowc.2009.18.5.42176.
- Michaels JA, Campbell B, King B, Palfreyman SJ, Shackley P, Stevenson M. Randomized controlled trial and cost-effectiveness analysis of silver-donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br J Surg 2009;96:1147-56. http://dx.doi.org/10.1002/bjs.6786.
- Barwell J, Taylor M, Deacon J, Ghauri A, Wakely C, Phillips L, et al. Surgical correction of isolated superficial venous reflux reduces long-term recurrence rate in chronic venous leg ulcers. Eur J Vasc Endovasc Surg 2000;20:363-8. http://dx.doi.org/10.1016/S1078-5884(00)91196-1.
- Brooks J, Ersser S, Lloyd A, Ryan T. Nurse-led education sets out to improve patient concordance and prevent recurrence of leg ulcers. J Wound Care 2004;13:111-16. http://dx.doi.org/10.12968/jowc.2004.13.3.26585.
- Nelson E, Cullum N, Jones J. Venous leg ulcers. Clin Evid 2006;15:2607-26.
- Gohel M, Barwell J, Taylor M, Chant T, Foy C, Earnshaw J, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ 2007;335:83-7. http://dx.doi.org/10.1136/bmj.39216.542442.BE.
- Aydin C, Inan B, Teker M. Venous ulcer treatment methods. Heart Surg Forum 2011;14.
- Nelzen O, Bergqvist D, Lindhagen A. Long-term prognosis for patients with chronic leg ulcers: a prospective cohort study. Eur J Vasc Endovasc Surg 1997;13:500-8. http://dx.doi.org/10.1016/S1078-5884(97)80179-7.
- Ebbeskog B, Skonda E, Lindholm C, Grauers M, Ohman S. A follow-up study of leg ulcer patients in south Stockholm. J Wound Care 1999;8:170-4. http://dx.doi.org/10.12968/jowc.1999.8.4.25868.
- Lindholm C, Bergsten A, Berglund E. Chronic wounds and nursing care. J Wound Care 1999;8:5-10. http://dx.doi.org/10.12968/jowc.1999.8.1.25828.
- Brown A, Bums E, Chalmers L, Corcoran F, Dale J, Douglas S, et al. Effect of a national community intervention programme on healing rates of chronic leg ulcer: randomised controlled trial. Phlebology 2002;17:47-53. http://dx.doi.org/10.1007/BF02637185.
- Obermayer A, Gostl K, Partsch H, Benesch T. Venous reflux surgery promotes venous leg ulcer healing despite reduced Ankle Brachial Pressure Index. Int Angiol 2008;27:239-46.
- O’Hagan A, Stevens J, Montmartin J. Inference for the cost-effectiveness acceptability curve and cost-effectiveness ratio. Pharmacoeconomics 2000;17:339-49. http://dx.doi.org/10.2165/00019053-200017040-00004.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. http://dx.doi.org/10.1016/S0167-6296(98)00039-3.
- Michaels JA, Campbell WB, King BM, Macintyre J, Palfreyman SJ, Shackley P, et al. A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial. Health Technol Assess 2009;13.
- Office for National Statistics (ONS) . Mortality Statistics 2011 n.d. www.ons.gov.uk/ons/rel/vsob1/death-registrations-by-single-year-of-age/england-and-wales-2011/index.html (accessed 5 September 2012).
- Akaike H, Petrov BN, Csaki F. Information theory and the maximum likelihood principle n.d.
- Kind P, Hardman G, Macran S. York: Centre for Health Economics, University of York; 1999.
- Ware JE, Kosinski MMA, Turner DBM, Gandek BMS. How to Score Version 2 of the SF-12 Health Survey. Boston, Massachusetts: QualityMetric; 2005.
- Saris-Baglama RN, Dewey CJ, Chisholm GB, Plumb E, Kosinski M, Bjorner JB, et al. QualityMetric Health Outcomes™ Scoring Software 2.0. Washington, DC: QualityMetric; 2007.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using STATA. College Station, TX: STATA PRESS; 2008.
- Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman and Hall; 1997.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: University of Kent at Canterbury; 2009.
- Raikou M, McGuire A. Estimating medical costs under conditions of censoring. J Health Econ 2004;23:443-70. http://dx.doi.org/10.1016/j.jhealeco.2003.07.002.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. http://dx.doi.org/10.1080/01621459.1958.10501452.
- Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics 1997;53:419-34. http://dx.doi.org/10.2307/2533947.
- Hallstrom A, Sullivan SD. On estimating costs for economic evaluation in failure time studies. Med Care 1998;36:433-6. http://dx.doi.org/10.1097/00005650-199803000-00019.
- Royston P, Parmar MKB, Altman DG. Visualizing length of survival in time-to-event studies: a complement to Kaplan–Meier plots. J Natl Cancer Inst 2008;100:92-7. http://dx.doi.org/10.1093/jnci/djm265.
- Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika 2002;87:329-43. http://dx.doi.org/10.1093/biomet/87.2.329.
- O’Hagan A, Stevens JW. On estimators of medical costs with censored data. J Health Econ 2004;23:615-25. http://dx.doi.org/10.1016/j.jhealeco.2003.06.006.
- Zhao H, Bang H, Wang H, Pfeifer PE. On the equivalence of some medical cost estimators with censored data. Stat Med 2007;26:4520-30. http://dx.doi.org/10.1002/sim.2882.
- Lin DY. Linear regression of censored medical costs. Biostatistics 2000;1:35-47. http://dx.doi.org/10.1093/biostatistics/1.1.35.
- Thompson SG, Nixon RM. How sensitive are cost-effectiveness analyses to choice of parametric distributions?. Med Decis Mak 2005;25:416-23. http://dx.doi.org/10.1177/0272989X05276862.
- NHS Reference Costs 2009/2010. London: DH; 2011.
- British National Formulary. London: BMA and RPS; 2011.
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making?. Health Econ 2006;15:677-87. http://dx.doi.org/10.1002/hec.1093.
- Ades AE, Sculpher M, Sutton A, Abrams K, . Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1-19. http://dx.doi.org/10.2165/00019053-200624010-00001.
- Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. New York, NY: Chapman and Hall; 1995.
- Van Hout BA, Al MJ, Gordon GS, Rutten FFH. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. http://dx.doi.org/10.1002/hec.4730030505.
Appendix 1 Details of regulatory approvals obtained for each trial centre
| Centre name | Date approvals secured | Organisation that gave NHS RM&G approval | Time taken for approvals (days)a |
|---|---|---|---|
| Bolton | 4 May 2010 | Bolton PCT | 165 |
| Bradford | 13 May 2010 | Bradford Teaching Hospitals NHS Trust | 91 |
| Brightonb | 14 April 2010 | Sussex NHS Research Consortium | 160 |
| Cambridgeb | 10 November 2010 | NHS Cambridgeshire | 65 |
| Cornwall | 13 April 2010 | Cornwall and Isles of Scilly PCT | 98 |
| Danetre | 3 March 2011 | NHS Northamptonshire | 90 |
| Dereham | 19 October 2010 | Norfolk Community Health and Care | 12 |
| Diss | 22 October 2010 | Norfolk Community Health and Care | 2 |
| Epsom | 16 June 2010 | Epsom and St Helier University Hospitals NHS Trust | 118 |
| Hainault | 13 December 2010 | NHS Havering | 136 |
| Harrogate Hospital | 16 April 2010 | Harrogate and District NHS Foundation Trust | 92 |
| Harrogate (GP Buckley) | 4 May 2011 | NHS North Yorkshire and York | 20 |
| Harrogate (GP Robinson) | 22 June 2011 | NHS North Yorkshire and York | 72 |
| Harrogate (GP Taylor) | 6 May 2011 | NHS North Yorkshire and York | 25 |
| Hull | 11 January 2010 | Hull and East Yorkshire Hospitals NHS Trust | 160 |
| Kent | 2 March 2010 | Eastern and Coastal Kent Community Services | 19 |
| Kingston | 2 December 2009 | NHS Kingston | 97 |
| Lancashire | 15 January 2010 | NHS Central Lancashire | 37 |
| Latham house | 24 January 2011 | NHS Leicestershire County and Rutland | 26 |
| Leeds | 22 October 2009 | NHS Leeds | 113 |
| Mid Yorkshireb | 12 August 2010 | Mid Yorkshire Hospitals NHS Trust | 212 |
| Mowbray Square | 5 December 2011 | NHS North Yorkshire and York | 47 |
| Nantwich | 14 October 2011 | Central and Eastern Cheshire PCT | 81 |
| Northern Ireland | 14 January 2010 | Western Health and Social Care Trust | 79 |
| North Lancashire | 19 November 2010 | NHS North Lancashire | 37 |
| North Yorkshire | 14 December 2009 | North Yorkshire and York PCT | 102 |
| Northumberland | 18 April 2011 | Newcastle PCT | 46 |
| Norwich | 19 October 2010 | Norfolk Community Health and Care | 1 |
| Nottingham | 23 April 2010 | NHS Nottinghamshire County and NHS Nottinghamshire City | 218 |
| Sedgefield | 18 October 2010 | County Durham PCT | 7 |
| South of Tyne and Wear | 26 July 2010 | NHS South of Tyne and Wear | 12 |
| Suffolk | 8 December 2010 | NHS Suffolk | 9 |
| Whitby | 22 December 2010 | NHS North Yorkshire and York | 163 |
| York Hospital | 20 May 2010 | North Yorkshire and York PCT | 14 |
Appendix 2 Details of trial centres
| Centre name | Sources of recruitment | Date recruited | |
|---|---|---|---|
| First participant | Last participant | ||
| Bolton | CN team/service, leg ulcer clinics, tissue viability clinics, treatment room clinics | 1 June 2010 | 3 February 2012 |
| Bradford | Outpatient clinics | 17 June 2010 | 24 January 2012 |
| Brightona | CN teams/service, GP practices and leg ulcer clinics | 5 May 2010 | 28 February 2012 |
| Cambridgea | CN teams/service, GP practices and tissue viability clinics/services | 10 January 2011 | 3 October 2011 |
| Cornwall | CN teams/service, GP practice, tissue viability/vascular clinic and leg ulcer clinics | 18 June 2010 | 28 February 2012 |
| Danetre | GP practice | N/A | N/A |
| Dereham | Outpatient leg ulcer clinic | 21 December 2010 | 28 February 2012 |
| Diss | Leg ulcer clinics | 5 January 2011 | 23 February 2012 |
| Epsom and St Helier | Outpatient leg ulcer clinic | 9 July 2010 | 19 August 2011 |
| Hainault | Leg ulcer clinic | 15 March 2011 | 12 May 2011 |
| Harrogate Hospital | Outpatient leg ulcer clinic | 7 July 2010 | 10 October 2011 |
| Harrogateb | GP practice | 10 June 2011 | 27 July 2011 |
| Harrogatec | GP practice | 11 November 2011 | 11 November 2011 |
| Harrogated | GP practice | 17 June 2011 | 17 June 2011 |
| Hull | Outpatient leg ulcer clinics | 26 March 2010 | 12 April 2011 |
| Kent | GP practices and leg ulcer clinics | 2 June 2010 | 5 October 2011 |
| Kingston | Leg ulcer clinics | 18 October 2010 | 18 October 2010 |
| Lancashire | GP practices and leg ulcer clinics | 22 February 2010 | 27 February 2012 |
| Latham house | GP practice | 1 March 2011 | 5 December 2011 |
| Leeds | CN teams/service, GP practices, leg ulcer clinics and tissue viability clinics/service | 16 November 2009 | 17 February 2012 |
| Mid Yorkshirea | CN teams/service, plastic dressing clinics and vascular clinics | 1 September 2010 | 24 February 2012 |
| Mowbray Square | GP practice | 18 January 2012 | 18 January 2012 |
| Nantwich | GP practice | 17 November 2011 | 17 November 2011 |
| Northern Ireland | Outpatient leg ulcer clinics | 6 April 2011 | 22 February 2012 |
| North Lancashire | GP practice and wound clinics | 2 February 2011 | 2 February 2011 |
| North Yorkshire | CN teams/service, GP practices and tissue viability clinics/services | 30 April 2010 | 19 July 2011 |
| Northumberland | CN teams/service, leg ulcer clinics and wound clinics | 28 June 2011 | 29 February 2012 |
| Norwich | Outpatient leg ulcer clinic | 11 January 2011 | 8 February 2012 |
| Nottingham | CN teams/services and leg ulcer clinic | 24 June 2010 | 20 April 2011 |
| Sedgefield | GP practice | 21 June 2011 | 21 June 2011 |
| South of Tyne and Wear | CN teams/service, GP practices, leg ulcer clinics and tissue viability clinics/services | 9 November 2010 | 31 January 2012 |
| Suffolke | N/A | N/A | N/A |
| Whitby | GP practice | 10 January 2011 | 11 July 2011 |
| York Hospital | Outpatient clinic | 2 July 2010 | 25 November 2011 |
Appendix 3 Pre-trial screening form
Appendix 4 Patient information sheet and consent form
Patient information sheet
Consent form
Appendix 5 Data collection forms (forms completed by health-care professionals)
Patient record form
Treatment log: trial dressing log booklet
Treatment log: non-trial dressing log booklet
Participant event form
Serious adverse event form
Review of serious adverse event form
Non-serious adverse event form
Review of non-serious adverse event form
Ulcer healed form
Monthly nurse assessment form
Appendix 6 Data collection forms (questionnaires completed by participants)
Participant baseline questionnaire
Participant 1-month questionnaire: four-layer bandage
Participant 1-month questionnaire: compression hosiery
Participant 3-month questionnaire (same as 6-, 9- and 12-month questionnaires)
Participant postcard
Appendix 7 Digital photography protocol
Every digital photograph must include the colour reference target card, which includes a centimetre measuring scale and colour targets. The patient’s trial number and date must always be clearly written on the colour target card. Please make sure that the colour target card is included in the photograph otherwise the photograph cannot be used as data collected.
-
Please take two photographs at baseline of the reference ulcer and the reference leg.
-
Each month you will need to take a photograph of the reference ulcer.
-
Please take a photograph of the reference ulcer site when the reference ulcer is reported as healed. You will also need to take a photograph of the healed site once per week for 4 weeks.
Please always try to take a photograph of the reference leg ulcer from directly above the wound; if it is photographed at an angle then it may be difficult to assess the wound accurately.
In the case of circumferential wounds additional adjacent photographs may be required.
Every reasonable effort must be made to take all consecutive photographs from the same viewpoint and distance using the same camera and same zoom facility.
Please ensure that the ulcer and surrounding area are cleaned thoroughly before taking the photograph. This is to reduce the possibility of blinded assessors being able to predict the treatment received by the patient.
All consecutive views of the reference ulcer area to be photographed using the trial camera.
All cameras have been calibrated so they are standardised to the same specification – please do not change any of these settings at any time. All cameras have been calibrated to the same specification as follows: Easy Auto mode – the camera responds to the shooting conditions at the time and controls the majority of camera settings. White balance is automatically set and used to preserve the natural colours under types of lighting.
The flash is set to automatic.
All digital photographs to be kept confidential and secure for the duration of the trial. Patient confidentiality will be maintained throughout trial by the use of unique trial numbers.
No film, recording media or data to be manipulated or changed in any way with the intention of affecting the results of the trial.
All photographs taken during the trial will be uploaded on to the management database system. When you have uploaded the photograph, please delete it from the memory card. DO NOT COMPRESS PHOTOS – Upload them straight from the camera on to the management database.
All cameras are supplied with a guide and it might be helpful to read this before you start to use the camera.
Taking photographs: framing the picture
Switch the camera ON. You may need to set the date and time. Press the picture/scene button and choose the Easy Auto mode. Hold the camera steadily in both hands about 18 inches to 2 feet away from the patient. Zoom in using the optical zoom facility on the camera (press ‘T’), ensuring that the whole of the wound area and the colour reference target card are included in the picture.
When you press the ‘T’ button, a white oblong box will appear in the top of the viewfinder and a bar will move towards the line (two-thirds along the box) until it stops. Stop pressing the ‘T’ button at this stage and take your photograph. Press the ‘W’ button to zoom out. Depress the shutter button half way to focus the image, depress fully to take the photograph.
Once you have taken the photograph, press the picture playback button to assess the quality of the photograph. If the photo is of poor quality, please take another image(s).
Appendix 8 VenUS IV flow chart
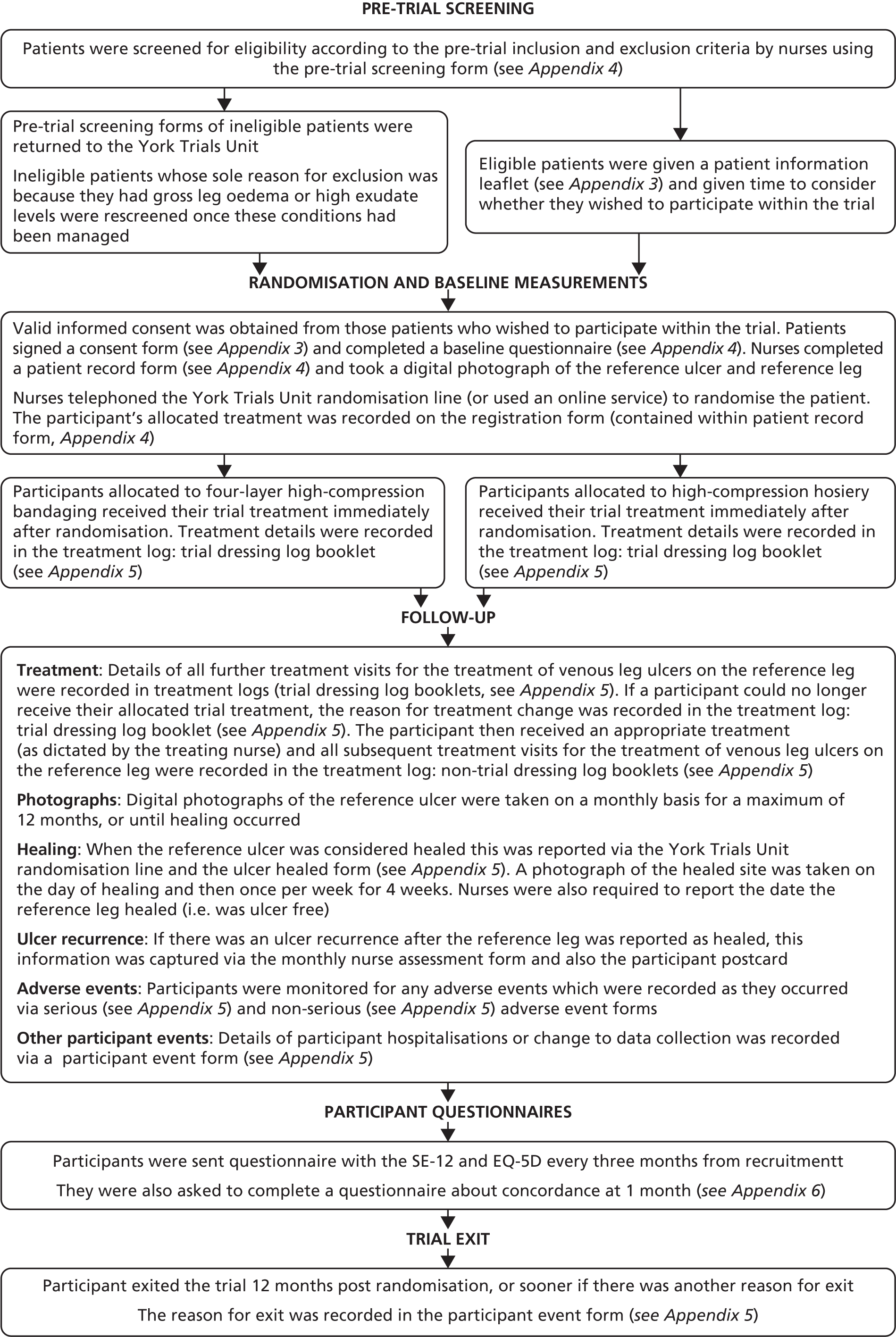
Appendix 9 Decision rules for verification of the primary outcome measure (healing of the reference ulcer)
-
If the two assessors disagree whether the reference ulcer has healed, there will be the following combinations with regards to healing: yes/no, yes/unsure, no/unsure.
-
If the two assessors say: ‘yes/unsure’ then we will say the ulcer has healed, using the date provided by the assessor who said ‘yes’.
-
If the two assessors say ‘no/unsure’ then we will say the ulcer has not healed.
-
If the two assessors say ‘unsure/unsure’ then we will say the ulcer has not healed.
-
If the two assessors say ‘yes/no’ then the third assessor will be consulted and will decide if the ulcer is healed or not. The third assessor’s decision will be final – the third assessor will decide if the ulcer has healed or not. If she/he is unsure whether the ulcer has healed, the ulcer will be considered unhealed.
-
-
If the two assessors agree that the ulcer is healed, but have chosen different dates of healing, we will take a consistently conservative approach and say that the longer time is the right time.
-
If no photographs of the reference ulcer are available, the unblinded date the treating nurse recorded will be used.
-
If the (treating) nurses say that the wound is healed and stop taking photographs but blinded assessors says the wound is not healed then we will consider the wound healed.
-
If photos are taken of a participant for 12 months and the date of healing occurs beyond 12 months post-randomisation, the participant will be regarded as unhealed at 12 months.
-
If an assessor says she/he is unsure whether a participant had healed, but provides a date of healing anyway, the date of healing will not be recorded. This is because we are recording dates of healing only if the assessor has selected the ‘yes’ option to indicate that the participant has healed.
-
Photos taken after a large interval of time has elapsed (i.e. ≥ 1 month) since the due date of the last healed photo (post-healed photo 4) will not be included in the blinded outcome assessment.
Appendix 10 Guidance for nurses regarding the relationship between a non-serious adverse event and trial treatment
Non-serious adverse events decision rules
An adverse event may be defined as any undesirable experience occurring in a participant, whether or not considered related to the treatment being used in the trial. Where the adverse event is not considered to be serious, the event is commonly referred to as a NSAE.
In VenUS IV, we ask that you diligently report both SAEs and NSAEs. When completing the NSAEs form, you will be asked if you think that the event that occurred is related to trial treatment. You can choose from the following options:
-
unrelated
-
unlikely to be related
-
possibly related
-
probably related
-
definitely related
-
not able to assess if related.
-
We now recommend that any non-serious event involving a venous leg ulcer should be considered to be, at the very least, possibly related to the trial treatment (bandages or stockings).
-
We also ask that you let us know if the event occurred on the reference or non-reference leg.
-
If there is an ulcer recurrence, please specify to which leg this applies.
We also provide the following guidance:
Classified as possibly related All other events involving the ulcer, ulcerated leg and/or skin, which are not probably or definitely related and are not the result of an accident (defined as a trip/fall/DIY related, not considered to be caused by the trial treatment), include ulcer recurrence and events caused by treatments other than 4LB or stockings. State whether the event relates to the reference or non-reference leg.
Classified as probably related Increase in ulcer size, increased pain, discolouration of treated leg, new ulceration to treated leg, blistering to a treated leg. State whether the event relates to the reference or non-reference leg.
Classified as definitely related Treatment-related skin trauma (i.e. skin damage attributable to the bandage or stocking), strong evidence that bandage or stocking caused the event. Nurse to state whether event relates to reference or non-reference leg.
Appendix 11 Data from randomised controlled trials used to inform the mixed-treatment comparison network
All available studies
| ID | Studies | Evaluated treatment | High or not | Partsch classification | Final classification | No. of patients | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|
| Studies including non-high-compression treatments | |||||||
| 2 | Cordts 199294 | Hydrocolloid dressing (Duoderm) plus cohesive elastic bandage (Coban) | Not | Bc | Bc | 23 | 12 |
| Unna’s boot (Dome-Paste, a zinc oxide and calamine impregnated bandage) | Not | Bz | Bz | 20 | 12 | ||
| 3 | Eriksson 198695 | Inner stocking impregnated with zinc oxide paste plus an outer elastic bandage (Tensoplast or Porelast Acryl) | High | Bza | Paste | 17 | 12 |
| Hydrocolloid dressing (Duoderm) plus elastic bandage (Wero) | Not | Be | Be | 17 | 12 | ||
| 4 | Travers 1992126 | Single-component system with elastic cohesive bandage (Panelast Acryl) applied from foot to below knee with 50% overlap | High | Ba | Ba | 15 | 7 |
| Three-component system applied from foot to below knee: zinc oxide and calamine paste bandage (Calaband); non-adhesive elastic bandage (Tensopress) applied with 50% overlap and 50% stretch; and elasticated tubular bandage (Tensogrip) | Not | Bzee | Bzee | 12 | 7 | ||
| 6 | Nelson 200796 | Single-layer bandage (hydrocolloid-lined, woven, elastomeric, adhesive bandage applied in a figure-of-eight technique from toe to knee) | Not | Ba | Ba+ | 128 | 24 |
| Original Charing Cross four-layer bandage comprising wool, crepe, Elset and Coban | High | Beec | 4LB | 117 | 24 | ||
| 7 | Danielsen 199897 | Lower leg padded with gauze then long-stretch, non-adhesive compression bandage (Setopress) applied in a spiral with 50% overlap and approximately 86% extension | Not | Be | Be | 23 | 52 |
| Lower leg padded with gauze then short-stretch, non-adhesive compression bandage (Comprilan) applied in a spiral with 50% overlap | Not | Bi | Bi | 20 | 52 | ||
| 8 | Moody 199998 | Undercast padding (Cellona) plus short-stretch compression bandage (Rosidal K) | Not | Bi | Bi | 26 | 12 |
| Undercast padding (SurePress padding) plus long-stretch compression bandage (SurePress bandage) | Not | Be | Be | 26 | 12 | ||
| 9 | Moffatt 200399 | Profore | High | Beec | 4LB | 57 | 12 |
| 2LB (Surepress) | Not | Be | Be | 52 | 12 | ||
| 10 | Callam 1992100 | Three-component compression system: orthopaedic wool (Soffban Natural), elastic bandage (Tensopress) and cotton-elastic graduated compression tubular support bandage (Tensoshape) | Not | Bee | Bee+ | 65 | 12 |
| Three-component compression system: orthopaedic wool (Soffban Natural), non-elastic cotton-elastic bandage (Elastocrepe) and non-elastic cotton Lycra cohesive bandage (Tensoplus Forte) | High | Bic | Bic | 67 | 12 | ||
| 11 | Gould 1998101 | Three-component compression system: medicated paste bandage, elastic bandage (Setopress) and elasticated viscose stockinette | Not | Bze | Bze | 19 | 15 |
| Three-component compression system: medicated paste bandage, cotton crepe bandage (Elastocrepe) and elasticated viscose stockinette | Not | Bzee | Bzee | 20 | 15 | ||
| 12 | Meyer 2002102 | Viscopaste bandage plus Tensopress (elastic bandage) plus Tensoshape (graduated cotton-elastic tubular retaining bandage) | Not | Bzee | Bzee | 57 | 40 |
| Viscopaste bandage plus Elastocrepe (inelastic bandage) plus Tensoshape (graduated cotton-elastic tubular retaining bandage) | Not | Bzie | Bzie | 55 | 40 | ||
| 15 | Moffatt 1999103 | Original Charing Cross four-layer bandage comprising wool, crepe, Elset and Coban | High | Beec | 4LB | 115 | 24 |
| Profore | High | Beec | 4LB | 117 | 24 | ||
| 16 | Vowden 2000104 | Original Charing Cross four-layer bandage system: orthopaedic wool (Soffban), crepe bandage (Smith & Nephew), elastic bandage (Elset, Seton Scholl) and elastic cohesive bandage (Coban) | High | Beec | 4LB | 50 | 20 |
| Modified Charing Cross four-layer bandage system: orthopaedic wool (Soffban), elastic bandage (K-Lite), elastic bandage (K-Plus, Parema) and adhesive elastic bandage (Coban) | High | Beea | 4LB | 50 | 20 | ||
| A four-layer bandage kit (Robinson Ultra Four): wound dressing, Sohfast, K-Lite, K-plus and Cohfast | High | Beec | 4LB | 49 | 20 | ||
| 22 | Knight 1996127 | Profore | High | Beec | 4LB | 5 | -- |
| Unna’s boot (described as a paste impregnated gauze compression dressing) | Not | Bz | Bz | 5 | -- | ||
| 24 | Meyer 200325 | Three-layer bandage: Steripaste bandage plus Setopress bandage plus Tubigrip bandage | Not | Bzee | Bzee | 64 | 52 |
| Four-layer bandage system: orthopaedic wool (Velband); crepe bandage; elastic bandage (Elset); and elastic cohesive bandage (Coban) | High | Beec | 4LB | 69 | 52 | ||
| 25 | DePalma 1999125 | Unna’s boot consisting of zinc oxide, glycerin and gelatin impregnated 10 cm × 9 m roller gauze bandage (Medicopaste) covered by an elastic Ace-type bandage | Not | Bze | Bze | 19 | 12 |
| Thera-Boot: a device consisting of a series of interlocking, non-elastic bands encircling the leg, and held in place by hook and loop fasteners plus a foot piece made of very low-stretch bands | High | V | V | 19 | 12 | ||
| 27 | Hendricks 1985105 | Unna’s Boot compression system: zinc oxide and calamine paste impregnated bandage (Dome-Paste); gauze bandage (Kerlix); and elastic bandage | Not | Bzee | Bzee | 10 | 78 |
| Open-toe, below-knee, elastic compression stocking (Futuro) | Not | H | H | 14 | 78 | ||
| 28 | Koksal 2003106 | Unna’s Boot containing calamine, zinc oxide, glycerine, sorbitol, gelatine and magnesium aluminium silicate | Not | Bz | Bz | 27 | 16 |
| Hydrocolloid dressing (Comfeel) plus class II elastic compression stocking | Not | H | H | 26 | 16 | ||
| 30 | Polignano 200429 | SSB (Comprilan) | Not | Bi | Bi | 29 | 12 |
| SurePress Comfort (two knee-high nylon and spandex stockings, which are latex free; a medium compression overstocking and light compression understocking) | Not | HH | HH+ | 27 | 12 | ||
| 31 | Junger 2004107 | Tubular compression device. The device was knitted, knee length, heel-less, and open toed; exerted graduated pressure, corresponding to class III compression stockings | Not | H | H | 88 | 12 |
| SSB (Rosidal K) | Not | Bi | Bi | 90 | 12 | ||
| 32 | Milic 2007108 | Cotton gauze without tension (50% overlap) plus cotton crepe bandage plus knee-length tubular compression device (Tubulcus) plus medium-stretch elastic compression bandage (Niva) | Not | BeH | BeH | 75 | 52 |
| Cotton gauze without tension (50% overlap) plus cotton crepe bandage plus two medium-stretch elastic compression bandages (Niva) | High | Bee | Bee | 75 | 52 | ||
| 33 | Harley 2004109 | Four-layer bandage system: orthopaedic wool; crepe bandage; elastic bandage (Elset); and elastic cohesive bandage (Coban) | High | Beec | 4LB | 16 | -- |
| Surepress: wool layer and long-stretch bandage by ConvaTec | Not | Be | Be | 14 | -- | ||
| 35 | Mariani 200830 | Two layers of stocking (outer layer is Sigvaris) | Not | HH | HH+ | 26 | 16 |
| SSB, applied with in two or more layers with spiral turns or turns at eight | Not | Bi | Bi | 30 | 16 | ||
| 36 | Taradaj 200931 | Medical compression stocking (Sigvaris 702) | Not | H | H | 40 | 8 |
| SSB | Not | Bi | Bi | 40 | 8 | ||
| 37 | Milic 2010110 | The first and second layers were cotton gauze without tension and cotton crepe bandage, and the third layer was tubular compression device (Tubulcus) | Not | BeH | BeH | 42 | 26 |
| Cotton gauze without tension (50% overlap) plus cotton crepe bandage plus knee-length tubular compression device (Tubulcus) plus medium-stretch elastic compression bandage (Niva) | Not | BeH | BeH | 46 | 26 | ||
| The first and second layers were cotton gauze without tension and cotton crepe bandage; the third layer was tubular compression device (Tubulcus) and the final layer was two-layer elastic bandage | High | BeHe | BeHe | 43 | 26 | ||
| 38 | Brizzio 2010128 | Open-toe elastic compression stocking (Sigvaris) | Not | H | H | 28 | 24 |
| SSB | Not | Bi | Bi | 27 | 24 | ||
| Studies including high-compression treatments | |||||||
| 1 | Kralj 1996112 | Profore | High | Beec | 4LB | 20 | 24 |
| Hydrocolloid dressing (Tegasorb) and single-layer inelastic bandage (Porelast) | High | Ba | Ba | 20 | 24 | ||
| 5 | Colgan 1995113 | Modified Unna’s boot, a compression system with four components: paste bandage; cotton crepe bandage (Elastocrepe); elastic adhesive bandage (Elastoplast); class II compression sock) | High | BzeaH | BzeaH | 10 | 12 |
| Profore | High | Beec | 4LB | 10 | 12 | ||
| Polyurethane foam dressing (Lyofoam dressing) plus elastic bandage (Setopress) | Not | Be | Be | 10 | 12 | ||
| 13 | Duby 1993114 | Short-stretch system: orthopaedic wool; two or more layers of SSB applied in counter rotating directions (Comprilan); and net covering (Tricofix) | High | Bii | SSB | 25 | 12 |
| Four-layer bandage system: orthopaedic wool; crepe bandage; elastic bandage (Elset); and elastic cohesive bandage (Coban) | High | Beec | 4LB | 25 | 12 | ||
| Paste-bandage system: zinc and ichthammol paste bandage (Icthopaste); cotton crepe bandage (Elastocrepe); and elastic tubular bandage (Tubigrip) | Not | Bzee | Bzee | 26 | 12 | ||
| 14 | Wilkinson 1997115 | Charing Cross four-layer bandage (Profore): dressing (Tricotex), orthopaedic wool (Soffban), crepe bandage, elastic bandage (Litepress) and cohesive elastic bandage (Coplus) | High | Beec | 4LB | 17 | 12 |
| Alternative four-layer bandage: dressing (Tricotex), elasticated viscose stockinette (Tubifast), elastic bandage (Setopress) and elasticated viscose stockinette (Tubifast) | High | BHeH | BHeH | 18 | 12 | ||
| 17 | Scriven 1998116 | Four-layer bandage system: orthopaedic wool (Velband); crepe bandage; elastic bandage (Elset); and elastic cohesive bandage (Coban) | High | Beec | 4LB | 32 | 52 |
| Short-stretch system: orthopaedic wool (Velband), 50% stretch and 50% overlap between turns (Rosidal K), and elastic cohesive bandage applied without stretch (Coban) | High | Biic | SSB | 32 | 52 | ||
| 18 | Partsch 2001117 | Profore | High | Beec | 4LB | 53 | 16 |
| SSB: orthopaedic padding plus two SSBs (Rosidal K) applied using the Putter technique | High | Bii | SSB | 59 | 16 | ||
| 19 | Ukat 2003118 | Profore | High | Beec | 4LB | 44 | 12 |
| SSB comprising two bandages 10 cm wide | High | Bii | SSB | 45 | 12 | ||
| 20 | Franks 2004119 | 4L bandage (Flexiban, Setocrepe, Elset, Coban) | High | Beec | 4LB | 74 | 24 |
| SSB (Flexiban, Actico) | High | Bc | SSB | 82 | 24 | ||
| 21 | Iglesias 20047 | Four-layer bandage: orthopaedic wool padding, crepe retention bandage, class 3A compression bandage and cohesive compression bandage, all applied with 50% overlap | High | Beec | 4LB | 195 | 52 |
| SSB: orthopaedic wool padding covered with one or two short-stretch compression bandages (Comprilan or Rosidal K), applied using spiral, figure-of-eight or modified Putter techniques | High | Bii | SSB | 192 | 52 | ||
| 23 | Polignano 2004120 | Profore | High | Beec | 4LB | 39 | 24 |
| Unna’s Boot comprising zinc oxide paste bandage (Viscopaste) elastic cohesive bandage (Tensoplast) | High | Bzc | Paste | 29 | 24 | ||
| 26 | Blecken 2005121 | Adjustable compression boot system: paraffin-impregnated gauze primary dressing (Aquafor); sterile absorbent gauze; pad cushion; surgical cotton stockinette; individually adjustable Velcro bands (CircAid); and elastic anklet (Medi) | High | HV | HV | 12 | 12 |
| Four-layer bandage: paraffin-impregnated gauze primary dressing (Aquafor); single layer of sterile absorbent gauze; 1-cm thick felt pad; thick gauze bandage (Kerlix); and elastic bandage | High | Beec | 4LB | 12 | 12 | ||
| 29 | Junger 200428 | U-Stocking (VenoTrain ulcertec) | High | HH | Hosiery | 61 | 12 |
| Two SSBs, wrapped around the leg in opposite directions from the metatarsophalangeal joint to the head of the fibula | High | Bii | SSB | 60 | 12 | ||
| 34 | Moffatt 2008122 | Four-layer compression bandage (Profore) | High | Beec | 4LB | 42 | 4 |
| Two-layer compression (Coban) | High | Bic | Bic | 39 | 4 | ||
| 39 | Szewczyk 2010123 | Elastic compression class II in the form of Maxis knee-length compression stockings (PPH Real) | High | 4LB | 4LB | 15 | 9 |
| Two-layer ProGuide (Smith & Nephew) compression (cotton wool and cotton band plus compression bandage) | High | 2LB | 2LB | 16 | 10 | ||
| 40 | Wong 2012124 | Four-layer compression bandaging (Profore; Smith & Nephew, Hull, UK) | High | 4LB | 4LB | 107 | 72 |
| Short-stretch compression bandaging (Rosidal sys; Lohmann and Rauscher GmbH and Co KG, Rengsdorf, Germany) | High | SSB | SSB | 107 | 77 | ||
| VenUS IV | Compression hosiery | High | 4LB | 4LB | 224 | 157 | |
| 4LB | High | HH | HH | 230 | 163 | ||
Appendix 12 Network of evidence
In the network, a unique treatment category is indicated by a circle: high compression = green circles, non-high-compression treatments = smaller unshaded. Arrows between circles indicate that these treatments had been compared in a trial [trials are identified using ‘( )’, numbered as in column ‘ID’ in Table 41].
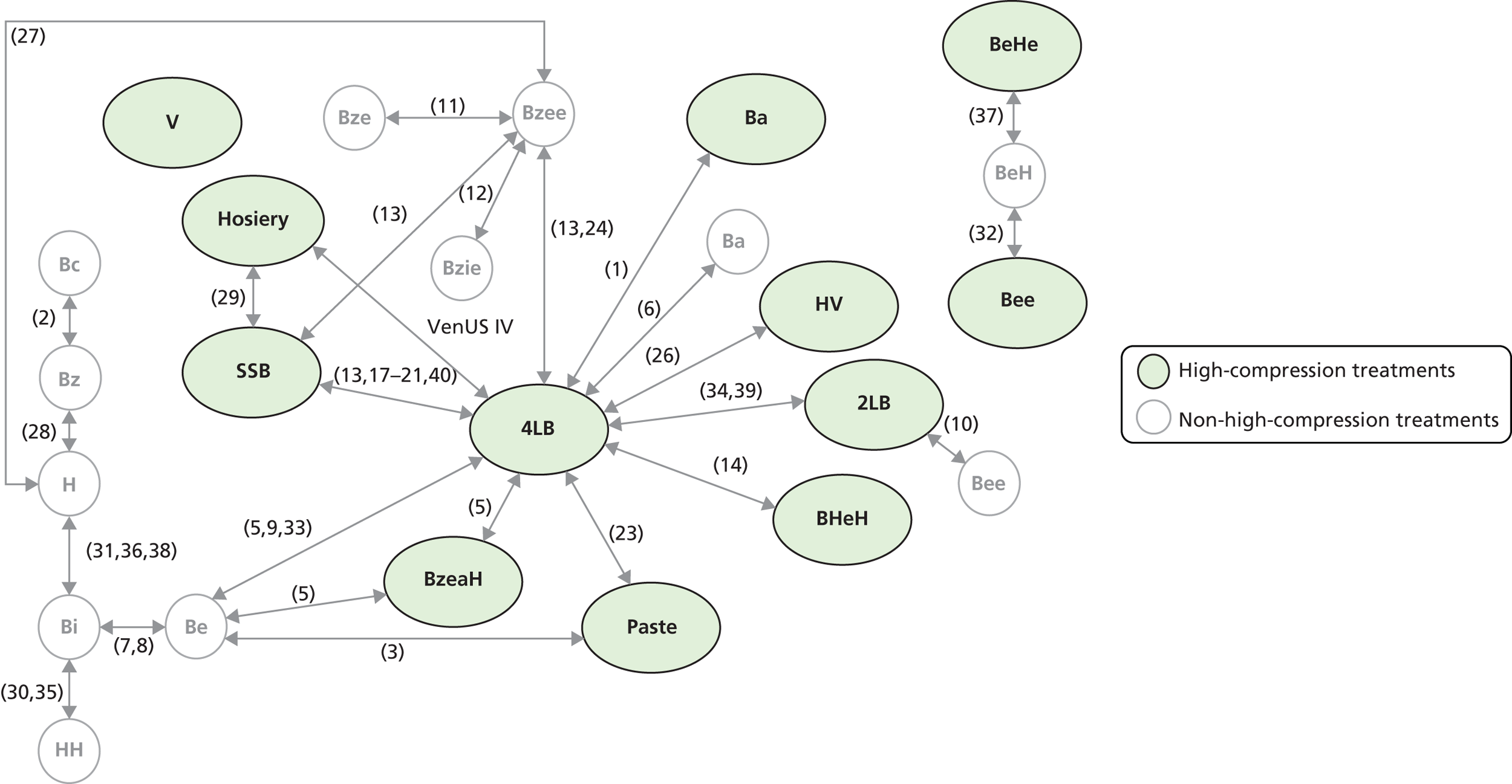
Appendix 13 Quality assessment of mixed-treatment comparison estimates using iGRADE: comparison with the GRADE tool
| GRADE category | GRADE definition and guidance | iGRADE category | iGRADE definitions and guidance | iGRADE issues |
|---|---|---|---|---|
| Limitations in design | Risk of bias
|
Limitations in design | Use GRADE limitations in design rating for DIRECT links to assess the MTC estimates to which these links clearly contributed No: GRADE limitations in design category recorded, as ‘no’ for all links identified as informing the MTC estimate Serious: GRADE limitations in design category recorded as serious for one or more links identified as informing the MTC estimate, but none identified as very serious Very serious: GRADE limitations in design category recorded as very serious for one or more links identified as informing the MTC estimate |
Qualitative assessment of risk of bias difficult for indirect evidence. When direct and indirect evidence are available, this assessment may be subjective |
| Inconsistency | Unexplained heterogeneity of results
|
Sensitivity of results | Judgement based on the impact of sensitivity analysis on the MTC network and thus estimates (e.g. removing each trial in which there are two or more informing a link, or sensitivity to alternative priors in random-effects analysis) No: No or small change in estimate and intervals Serious: Some notable change in estimate and intervals Very serious: Large change in estimate and intervals |
Does not address unexplained heterogeneity per se |
| Indirectness | Indirect comparison
|
Indirectness/Inconsistency: Within GRADE the term inconsistency is used to refer to unexplained heterogeneity. Within MTC inconsistency has meaning specific to agreement between direct and indirect data. Furthermore, in GRADE the presence of indirectness is taken as a reason to downgrade evidence; however, in the context of a MTC in which indirect data are expected and ideally adds value, such an approach does not make sense. Thus we merged these categories resulting in joint assessment of unexplained heterogeneity and/or assessment of inconsistency where possible | Define the type of data available for each MTC comparison as follows:
Serious: 2, 3 Very serious: N/A |
Assessment of heterogeneity based in DIRECT links is challenging Cannot always assess for inconsistencies |
| Imprecision | CIs around estimates of treatment effect
|
Imprecision | Judged by the size of CrI around ORs. As ORs were used to analyse data with relative high number of events a more conservative interval width used than would have been utilised were data presented using risk ratios No: uncertainty judged to be reasonable (upper interval < 2.5) Serious: judged to be inadequate (upper interval > 2.5 < 5) Very serious: (upper interval > 5) |
|
| Publication bias |
|
Publication bias | Use GRADE limitations in design rating for DIRECT links to assess the MTC estimates to which these links clearly contributed Unlikely: GRADE publication bias category recorded as unlikely for links identified as informing the MTC estimate Likely: Grade publication bias category recorded as likely for one or more links identified as informing the MTC estimate and none identified as very likely Very likely: for GRADE publication bias category recorded as very likely for one or more link identified as informing the MTC estimate |
Qualitative assessment of publication bias difficult for indirect evidence Again, in the presence of both direct and indirect evidence there is the need to consider potential publication bias in the indirect links, as well as the direct links informing the same comparison. Yet, outlined in the discussion of limitations, assessing potential bias in indirect comparison is complex. If, for example, AC is biased (missing studies) favouring A and BC is biased (missing studies) favouring B, then the AB indirect estimate will be unbiased if the bias in AC is similar to the bias in BC |
Appendix 14 Search on cost-effectiveness decision models
Search strategy
-
exp Stochastic Processes/ (11,513)
-
exp Models, Theoretical/ (681,838)
-
exp Models, Statistical/ (155,161)
-
exp Models, Economic/ (6040)
-
exp Monte Carlo Method/ (11,646)
-
exp Markov Chains/ (5630) ((stochastic or mathematical or statistical or theoretical or population or process or probabili* or simulat* or monte carlo or markov) adj model*).tw. (34,897) ((economic* or pharmacoeconomic* or decision* or cost*) adj model*).tw. (2041)
-
exp Economics, Medical/ (2817)
-
exp Health Care Costs/ (28,406)
-
exp “Costs and Cost Analysis”/ (89,053)
-
exp “Cost of Illness”/ (11,710) (cost-effective* or cost effective* or cost-utility or cost utility or cost-benefit or cost benefit or cost-minimi* or cost minimi*).tw. (42,080)
-
or/1-13 (801,777)
-
exp Leg Ulcer/ (8068) (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or (lower extremit* adj ulcer*) or crural ulcer* or ulcus cruris).tw. (3102)
-
or/15-16 (8640)
-
14 and 17 (675)
Appendix 15 Search on utilities
NHS Economic Evaluation Database (NHS EED), Issue 2 of 4, April 2011; Database of Abstracts of Reviews of Effects (DARE), Issue 2 of 4, April 2011; Health Technology Assessments (HTAs), Issue 2 of 4 Apr 2011; Cochrane Central Register of Controlled Trials (CENTRAL), Issue 2 of 4, April 2011; via The Cochrane Library; Wiley http://onlinelibrary.wiley.com/
Date of search: 24 June 2011.
Search strategy
#1 ((utilit* NEXT approach*) or (health NEXT gain) or hui or hui2 or hui3):ti,ab (145)
#2 ((health NEXT measurement* NEXT scale*) or (health NEXT measurement* NEXT questionnaire*)):ti,ab (4)
#3 ((standard NEXT gamble*) or (categor* NEXT scal*) or (linear NEXT scal*) or (linear NEXT analog*) or (visual NEXT scal*) or (magnitude NEXT estimat*)):ti,ab (828)
#4 ((time NEXT trade NEXT off*) or (rosser* NEXT classif*) or (rosser* NEXT matrix) or (rosser* NEXT distress*) or hrqol):ti,ab (747)
#5 ((index NEXT of NEXT wellbeing) or (quality NEXT of NEXT wellbeing) or qwb):ti,ab (30)
#6 ((multiattribute* NEXT health NEXT ind*) or (multi NEXT attribute* NEXT health NEXT ind*)):ti,ab (0)
#7 ((health NEXT utilit* NEXT index) or (health NEXT utilit* NEXT indices)):ti,ab (103)
#8 ((multiattribute* NEXT theor*) or (multi NEXT attribute* NEXT theor*) or (multiattribute* NEXT analys*) or (multi NEXT attribute* NEXT analys*)):ti,ab 0
#9 ((health NEXT utilit* NEXT scale*) or (classification NEXT of NEXT illness NEXT state*)):ti,ab (2)
#10 health NEXT state* NEXT utilit*:ti,ab (34)
#11 well NEXT year*:ti,ab (2)
#12 ((multiattribute* NEXT utilit*) or (multi NEXT attribute* NEXT utilit*)):ti,ab (9)
#13 health NEXT utilit* NEXT scale*:ti,ab (1)
#14 ((euro NEXT qual) or (euro NEXT qol) or eq5d or (eq NEXT 5d) or euroqual or euroqol):ti,ab (631)
#15 (qualy or qaly or qualys or qalys or (quality NEXT adjusted NEXT life NEXT year*)):ti,ab (697)
#16 willingness NEXT to NEXT pay:ti,ab (316)
#17 (hye or hyes or (health* NEXT year* NEXT equivalent*)):ti,ab (2)
#18 ((person NEXT trade NEXT off*) or (person NEXT tradeoff*) or (time NEXT tradeoff*) or (time NEXT trade NEXT off*)):ti,ab (122)
#19 theory NEXT utilit*:ti,ab (0)
#20 (sf36 or (sf NEXT 36)):ti,ab (1960)
#21 ((short NEXT form NEXT 36) or (shortform NEXT 36) or (sf NEXT thirtysix) or (sf NEXT thirty NEXT six) or (shortform NEXT thirtysix) or (shortform NEXT thirty NEXT six) or (short NEXT form NEXT thirtysix) or (short NEXT form NEXT thirty NEXT six)):ti,ab (843)
#22 ((sf NEXT 6d) or sf6d or (short NEXT form NEXT 6d) or (shortform NEXT 6d) or (sf NEXT six*) or (shortform NEXT six*) or (short NEXT form NEXT six*)):ti,ab (77)
#23 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22) (5064)
#24 MeSH descriptor Leg Ulcer, this term only (332)
#25 MeSH descriptor Varicose Ulcer, this term only (333)
#26 ((varicose or venous or leg or stasis or crural) NEXT ulcer*):ti,ab (1195)
#27 (ulcus NEXT cruris):ti,ab (11)
#28 (#24 OR #25 OR #26 OR #27) (1320)
#29 (#23 AND #28) (11)
MEDLINE In-Process & Other Non-Indexed Citations <June 23, 2011> and MEDLINE <1948 to June Week 3> via OvidSP http://ovidsp.ovid.com/
Date of search: 24 June 2011.
Search strategy
-
(utilit$ approach$ or health gain or hui or hui2 or hui3).ti,ab. (1126)
-
(health measurement$ scale$ or health measurement$ questionnaire$).ti,ab. (31)
-
(standard gamble$ or categor$ scal$ or linear scal$ or linear analog$ or visual scal$ or magnitude estimat$).ti,ab. (3795)
-
(time trade off$ or rosser$ classif$ or rosser$ matrix or rosser$ distress$ or hrqol).ti,ab. (5318)
-
(index of wellbeing or quality of wellbeing or qwb).ti,ab. (151)
-
(multiattribute$ health ind$ or multi attribute$ health ind$).ti,ab. (2)
-
(health utilit$ index or health utilit$ indices).ti,ab. (496)
-
(multiattribute$ theor$ or multi attribute$ theor$ or multiattribute$ analys$ or multi attribute$ analys$).ti,ab. (9)
-
(health utilit$ scale$ or classification of illness state$).ti,ab. (8)
-
health state$ utilit$.ti,ab. (180)
-
well year$.ti,ab. (20)
-
(multiattribute$ utilit$ or multi attribute$ utilit$).ti,ab. (156)
-
health utilit$ scale$.ti,ab. (7)
-
(euro qual or euro qol or eq5d or eq 5d or euroqual or euroqol).ti,ab. (2376)
-
(qualy or qaly or qualys or qalys or quality adjusted life year$).ti,ab. (4743)
-
willingness to pay.ti,ab. (1556)
-
(hye or hyes or health$ year$ equivalent$).ti,ab. (58)
-
(person trade off$ or person tradeoff$ or time tradeoff$ or time trade off$).ti,ab. (787)
-
theory utilit$.ti,ab. (6)
-
(sf36 or sf 36).ti,ab. (9903)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab. (4559)
-
(sf 6d or sf6d or short form 6d or shortform 6d or sf six$ or shortform six$ or short form six$).ti,ab. (254)
-
or/1-22 (27,228)
-
leg ulcer/ or varicose ulcer/ (9896)
-
(varicose ulcer$ or venous ulcer$ or leg ulcer$ or stasis ulcer$ or crural ulcer$ or ulcus cruris).ti,ab. (6381)
-
24 or 25 (11,292)
-
23 and 26 (45)
EMBASE<1980 to 2011 Week 24> via OvidSP http://ovidsp.ovid.com/
Date of search: 24 June 2011.
Search strategy
-
(utilit$ approach$ or health gain or hui or hui2 or hui3).ti,ab. (1300)
-
(health measurement$ scale$ or health measurement$ questionnaire$).ti,ab. (44)
-
(standard gamble$ or categor$ scal$ or linear scal$ or linear analog$ or visual scal$ or magnitude estimat$).ti,ab. (4017)
-
(time trade off$ or rosser$ classif$ or rosser$ matrix or rosser$ distress$ or hrqol).ti,ab. (6446)
-
(index of wellbeing or quality of wellbeing or qwb).ti,ab. (164)
-
(multiattribute$ health ind$ or multi attribute$ health ind$).ti,ab. (2)
-
(health utilit$ index or health utilit$ indices).ti,ab. (550)
-
(multiattribute$ theor$ or multi attribute$ theor$ or multiattribute$ analys$ or multi attribute$ analys$).ti,ab. (13)
-
(health utilit$ scale$ or classification of illness state$).ti,ab. (8)
-
health state$ utilit$.ti,ab. (231)
-
well year$.ti,ab. (23)
-
(multiattribute$ utilit$ or multi attribute$ utilit$).ti,ab. (184)
-
health utilit$ scale$.ti,ab. (7)
-
(euro qual or euro qol or eq5d or eq 5d or euroqual or euroqol).ti,ab. (3146)
-
(qualy or qaly or qualys or qalys or quality adjusted life year$).ti,ab. (5735)
-
willingness to pay.ti,ab. (1868)
-
(hye or hyes or health$ year$ equivalent$).ti,ab. (71)
-
(person trade off$ or person tradeoff$ or time tradeoff$ or time trade off$).ti,ab. (856)
-
theory utilit$.ti,ab. (7)
-
(sf36 or sf 36).ti,ab. (12,365)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab. (5051)
-
(sf 6d or sf6d or short form 6d or shortform 6d or sf six$ or shortform six$ or short form six$).ti,ab. (314)
-
or/1-22 (32,581)
-
leg ulcer/ (9588)
-
(varicose ulcer$ or venous ulcer$ or leg ulcer$ or stasis ulcer$ or crural ulcer$ or ulcus cruris).ti,ab. (7662)
-
24 or 25 (12,235)
-
23 and 26 (59)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost, inception to 17 June 2011
Date of search: 24 June 2011.
Search strategy
-
S28 S23 and S27 (22)
-
S27 S24 or S25 or S26 (3186)
-
S26 TI (“varicose ulcer*” or “venous ulcer*” or “leg ulcer*” or “stasis ulcer*” or “crural ulcer*” or “ulcus cruris” ) or AB ( “varicose ulcer*” or “venous ulcer*” or “leg ulcer*” or “stasis ulcer*” or “crural ulcer*” or “ulcus cruris”) (1989)
-
S25 (MH “Venous Ulcer”) (1223)
-
S24 (MH “Leg Ulcer”) (1911)
-
S23 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 (6687)
-
S22 TI ( “sf 6d” or sf6d or “short form 6d” or “shortform 6d” or “sf six*” or “shortform six*” or “short form six*” ) or AB ( “sf 6d” or sf6d or “short form 6d” or “shortform 6d” or “sf six*” or “shortform six*” or “short form six*” ) (73)
-
S21 TI ( “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirtysix” or “short form thirty six” ) or AB ( “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirtysix” or “short form thirty six” ) (1408)
-
S20 TI ( sf36 or “sf 36” ) or AB ( sf36 or “sf 36” ) (2823)
-
S19 TI “theory utilit*” or AB “theory utilit*” (3)
-
S18 TI ( “person trade off*” or “person tradeoff*” or “time tradeoff*” or “time trade off*” ) or AB ( “person trade off*” or “person tradeoff*” or “time tradeoff*” or “time trade off*” ) (132)
-
S17 TI ( hye or hyes or “health* year* equivalent*” ) or AB ( hye or hyes or “health* year* equivalent*” ) (5)
-
S16 TI “willingness to pay” or AB “willingness to pay” (301)_
-
S15 TI ( qualy or qaly or qualys or qalys or “quality adjusted life year*” ) or AB ( qualy or qaly or qualys or qalys or “quality adjusted life year*” ) (999)
-
S14 TI ( “euro qual” or “euro qol” or eq5d or “eq 5d” or euroqual or euroqol ) or AB ( “euro qual” or “euro qol” or eq5d or “eq 5d” or euroqual or euroqol ) (574)
-
S13 TI “health utilit* scale*” or AB “health utilit* scale*” (2)
-
S12 TI ( “multiattribute* utilit*” or “multi attribute* utilit*” ) or AB ( “multiattribute* utilit*” or “multi attribute* utilit*” ) (42)
-
S11 TI “well year*” or AB “well year*” (4)
-
S10 TI “health state* utilit*” or AB “health state* utilit*” (35)
-
S9 TI (“health utilit* scale*” or “classification of illness state*” ) or AB (“health utilit* scale*” or “classification of illness state*” ) (3)
-
S8 TI (“multiattribute* theor*” or “multi attribute* theor*” or “multiattribute* analys*” or “multi attribute* analys*” ) or AB (“multiattribute* theor*” or “multi attribute* theor*” or “multiattribute* analys*” or “multi attribute* analys*”) (3)
-
S7 TI (“health utilit* index” or “health utilit* indices”) or AB (“health utilit* index” or “health utilit* indices”) (133)
-
S6 TI (“multiattribute* health ind*” or “multi attribute* health ind*” ) or AB (“multiattribute* health ind*” or “multi attribute* health ind*”) (0)
-
S5 TI (“index of wellbeing” or “quality of wellbeing” or qwb ) or AB (“index of wellbeing” or “quality of wellbeing” or qwb ) (48)
-
S4 TI (“time trade off*” or “rosser* classif*” or “rosser* matrix” or “rosser* distress*” or hrqol ) or AB (“time trade off*” or “rosser* classif*” or “rosser* matrix” or “rosser* distress*” or hrqol ) (1333)
-
S3 TI (“standard gamble*” or “categor* scal*” or “linear scal*” or “linear analog*” or “visual scal*” or “magnitude estimat*” ) or AB (“standard gamble*” or “categor* scal*” or “linear scal*” or “linear analog*” or “visual scal*” or “magnitude estimat*”) (470)
-
S2 TI ( “health measurement* scale*” or “health measurement* questionnaire*” ) or AB ( “health measurement* scale*” or “health measurement*” questionnaire* ) (6)
-
S1 TI ( “utilit* approach*” or “health gain” or hui or hui2 or hui3 ) or AB ( “utilit* approach*” or “health gain” or hui or hui2 or hui3 ) (289)
British Nursing Index (BNI) and Archive <1985 to June 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 24 June 2011.
Search strategy
-
(utilit$ approach$ or health gain or hui or hui2 or hui3).ti,ab. (20)
-
(health measurement$ scale$ or health measurement$ questionnaire$).ti,ab. (3)
-
(standard gamble$ or categor$ scal$ or linear scal$ or linear analog$ or visual scal$ or magnitude estimat$).ti,ab. (11)
-
(time trade off$ or rosser$ classif$ or rosser$ matrix or rosser$ distress$ or hrqol).ti,ab. (55)
-
(index of wellbeing or quality of wellbeing or qwb).ti,ab. (0)
-
(multiattribute$ health ind$ or multi attribute$ health ind$).ti,ab. (0)
-
(health utilit$ index or health utilit$ indices).ti,ab. (2)
-
(multiattribute$ theor$ or multi attribute$ theor$ or multiattribute$ analys$ or multi attribute$ analys$).ti,ab. (0)
-
(health utilit$ scale$ or classification of illness state$).ti,ab. (0)
-
health state$ utilit$.ti,ab. (0)
-
well year$.ti,ab. (1)
-
(multiattribute$ utilit$ or multi attribute$ utilit$).ti,ab. (5)
-
health utilit$ scale$.ti,ab. (0)
-
(euro qual or euro qol or eq5d or eq 5d or euroqual or euroqol).ti,ab. (12)
-
(qualy or qaly or qualys or qalys or quality adjusted life year$).ti,ab. (65)
-
willingness to pay.ti,ab. (13)
-
(hye or hyes or health$ year$ equivalent$).ti,ab. (3)
-
(person trade off$ or person tradeoff$ or time tradeoff$ or time trade off$).ti,ab. (2)
-
theory utilit$.ti,ab. (1)
-
(sf36 or sf 36).ti,ab. (65)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab. (24)
-
(sf 6d or sf6d or short form 6d or shortform 6d or sf six$ or shortform six$ or short form six$).ti,ab. (0)
-
or/1-22 (262)
-
Leg Ulcers/ (1500)
-
(varicose ulcer$ or venous ulcer$ or leg ulcer$ or stasis ulcer$ or crural ulcer$ or ulcus cruris).ti,ab. (1375)
-
24 or 25 (1780)
-
23 and 26 (8)
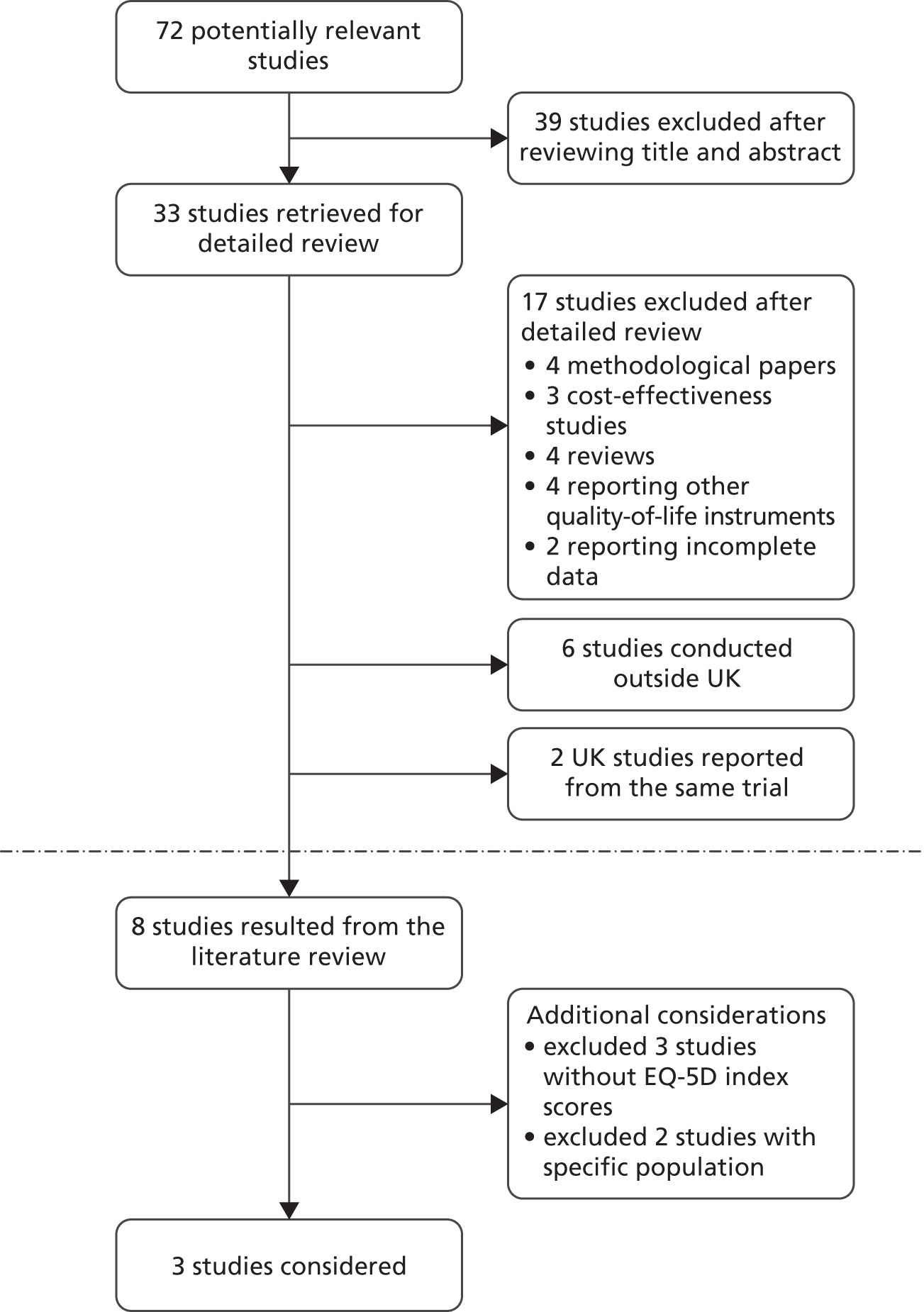
Appendix 16 Search on resource use
NHS Economic Evaluation Database (NHS EED), Issue 2 of 4, April 2011 via The Cochrane Library, Wiley http://onlinelibrary.wiley.com/
Date of search: 24 June 2011.
Search strategy
#1 MeSH descriptor Leg Ulcer, this term only (332)
#2 MeSH descriptor Varicose Ulcer, this term only (333)
#3 ((varicose or venous or leg or stasis or crural) NEXT ulcer*):ti,ab (1195)
#4 (ulcus NEXT cruris):ti,ab (11)
#5 (#1 OR #2 OR #3 OR #4) (1320)
#6 MeSH descriptor Compression Bandages explode all trees (98)
#7 (compression or bandag* or stocking* or hosiery or wrapp*):ti,ab (3213)
#8 (#6 OR #7) (3227)
#9 (#5 AND #8) (359)
#10 (#9), from 2000 to 2011 NHS EED only (9)
MEDLINE In-Process & Other Non-Indexed Citations (23 June 2011) and MEDLINE <1948 to June Week 3> via OvidSP http://ovidsp.ovid.com/
Date of search: 24 June 2011.
Search strategy
-
economics/ (26,064)
-
exp “costs and cost analysis”/ (157,130)
-
economics, dental/ (1829)
-
exp “economics, hospital”/ (17,217)
-
economics, medical/ (8404)
-
economics, nursing/ (3848)
-
economics, pharmaceutical/ (2238)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (359,832)
-
(expenditure$ not energy).ti,ab. (14,872)
-
value for money.ti,ab. (719)
-
budget$.ti,ab. (15,780)
-
or/1-11 (473,942)
-
((energy or oxygen) adj cost).ti,ab. (2442)
-
(metabolic adj cost).ti,ab. (638)
-
((energy or oxygen) adj expenditure).ti,ab. (13,676)
-
or/13-15 (16,123)
-
12 not 16 (470,219)
-
letter.pt. (733,090)
-
editorial.pt. (286,936)
-
historical article.pt. (275,453)
-
or/18-20 (1,282,649)
-
17 not 21 (445,614)
-
exp animals/ not humans/ (3,602,202)
-
22 not 23 (421,480)
-
Leg Ulcer/ (6902)
-
Varicose Ulcer/ (3428)
-
(varicose ulcer$ or venous ulcer$ or leg ulcer$ or stasis ulcer$ or crural ulcer$ or ulcus cruris).ti,ab. (6381)
-
25 or 26 or 27 (11,292)
-
exp Compression Bandages/ (704)
-
(compression or bandag$ or stocking$ or hosiery or wrapp$).ti,ab. (72,861)
-
29 or 30 (73,121)
-
24 and 28 and 31 (160)
-
limit 32 to (english language and yr = “2000 -Current”) (101)
EMBASE <1980 to 2011 Week 24> via OvidSP http://ovidsp.ovid.com/
Date of search: 24 June 2011.
Search strategy
-
Health Economics/ (30,145)
-
exp Economic Evaluation/ (166,705)
-
exp Health Care Cost/ (160,711)
-
PHARMACOECONOMICS/ (1872)
-
1 or 2 or 3 or 4 (302,726)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (428,178)
-
(expenditure$ not energy).ti,ab. (17,011)
-
(value adj2 money).ti,ab. (909)
-
budget$.ti,ab. (18,111)
-
6 or 7 or 8 or 9 (446,579)
-
5 or 10 (598,768)
-
letter.pt. (728,556)
-
editorial.pt. (372,044)
-
note.pt. (436,538)
-
12 or 13 or 14 (1,537,138)
-
11 not 15 (538,571)
-
(metabolic adj cost).ti,ab. (647)
-
((energy or oxygen) adj cost).ti,ab. (2519)
-
((energy or oxygen) adj expenditure).ti,ab. (14,940)
-
17 or 18 or 19 (17,444)
-
16 not 20 (534,623)
-
exp ANIMAL/ (1,615,378)
-
exp animal experiment/ (1,444,801)
-
Nonhuman/ (3,653,629)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4,026,868)
-
22 or 23 or 24 or 25 (5,824,661)
-
exp human/ (12,298,918)
-
exp human experiment/ (289,909)
-
27 or 28 (12,300,300)
-
26 not (26 and 29) (4,603,455)
-
21 not 30 (492,736)
-
leg ulcer/ (9588)
-
(varicose ulcer$ or venous ulcer$ or leg ulcer$ or stasis ulcer$ or crural ulcer$ or ulcus cruris).ti,ab. (7662)
-
32 or 33 (12,235)
-
exp compression therapy/ (5422)
-
compression bandage/ (534)
-
compression garment/ (1256)
-
(compression or bandag$ or stocking$ or hosiery or wrapp$).ti,ab. (80,270)
-
35 or 36 or 37 or 38 (83,688)
-
31 and 34 and 39 (270)
-
limit 40 to (english language and yr = “2000 -Current”) (179)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) <inception to 17 June 2011> via EBSCOhost
Date of search: 24 June 2011.
Search strategy
S29 S20 and S24 and S28 (66)
S28 S25 or S26 or S27 (7861)
S27 TI ( compression or bandag* or stocking* or hosiery or wrap* ) or AB ( compression or bandag* or stocking* or hosiery or wrap* ) (6788)
S26 (MH “Compression Garments”) (1049)
S25 (MH “Compression Therapy”) (1201)
S24 S21 or S22 or S23 (3186)
S23 TI (“varicose ulcer*” or “venous ulcer*” or “leg ulcer*” or “stasis ulcer*”
or “crural ulcer*” or “ulcus cruris” ) or AB ( “varicose ulcer*” or “venous ulcer*”
or “leg ulcer*” or “stasis ulcer*” or “crural ulcer*” or “ulcus cruris” ) (1989)
S22 (MH “Venous Ulcer”) (1223)
S21 (MH “Leg Ulcer”) (1911)
S20 S18 NOT S19 (90,676)
S19 MH “Animal Studies” (21,419)
S18 S13 NOT S17 (90,826)
S17 S14 or S15 or S16 (280,532)
S16 PT commentary (121,332)
S15 PT letter (106,150)
S14 PT editorial (123,921)
S13 S11 OR S12 (98,558)
S12 TI (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) OR AB (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) (67,795)
S11 S7 OR S10 (45,615)
S10 S8 OR S9 (11,490)
S9 MH “Health Resource Utilization” (6954)
S8 MH “Health Resource Allocation” (4784)
S7 S1 NOT S6 (38,036)
S6 S2 OR S3 or S4 OR S5 (349,628)
S5 MH “Business+” (52,830)
S4 MH “Financing, Organized+” (70,837)
S3 MH “Financial Support+” (223,713)
S2 MH “Financial Management+” (27,583)
S1 MH “Economics+” (354,431)
British Nursing Index and Archive <1985 to June 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 24 June 2011.
Search strategy
-
health economics/ (178)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (4144)
-
(value adj2 money).ti,ab. (52)
-
budget$.ti,ab. (357)
-
(expenditure$ not energy).ti,ab. (75)
-
1 or 2 or 3 or 4 or 5 (4598)
-
leg ulcers/ (1500)
-
(varicose ulcer$ or venous ulcer$ or leg ulcer$ or stasis ulcer$ or crural ulcer$ or ulcus cruris).ti,ab. (1375)
-
7 or 8 (1780)
-
dressings/ (1857)
-
(compression or bandag$ or stocking$ or hosiery or wrapp$).ti,ab. (755)
-
10 or 11 (2171)
-
6 and 9 and 12 (33)
-
limit 13 to yr = “2000 -Current” (29)
Flow chart of studies included in the literature review for resource use.
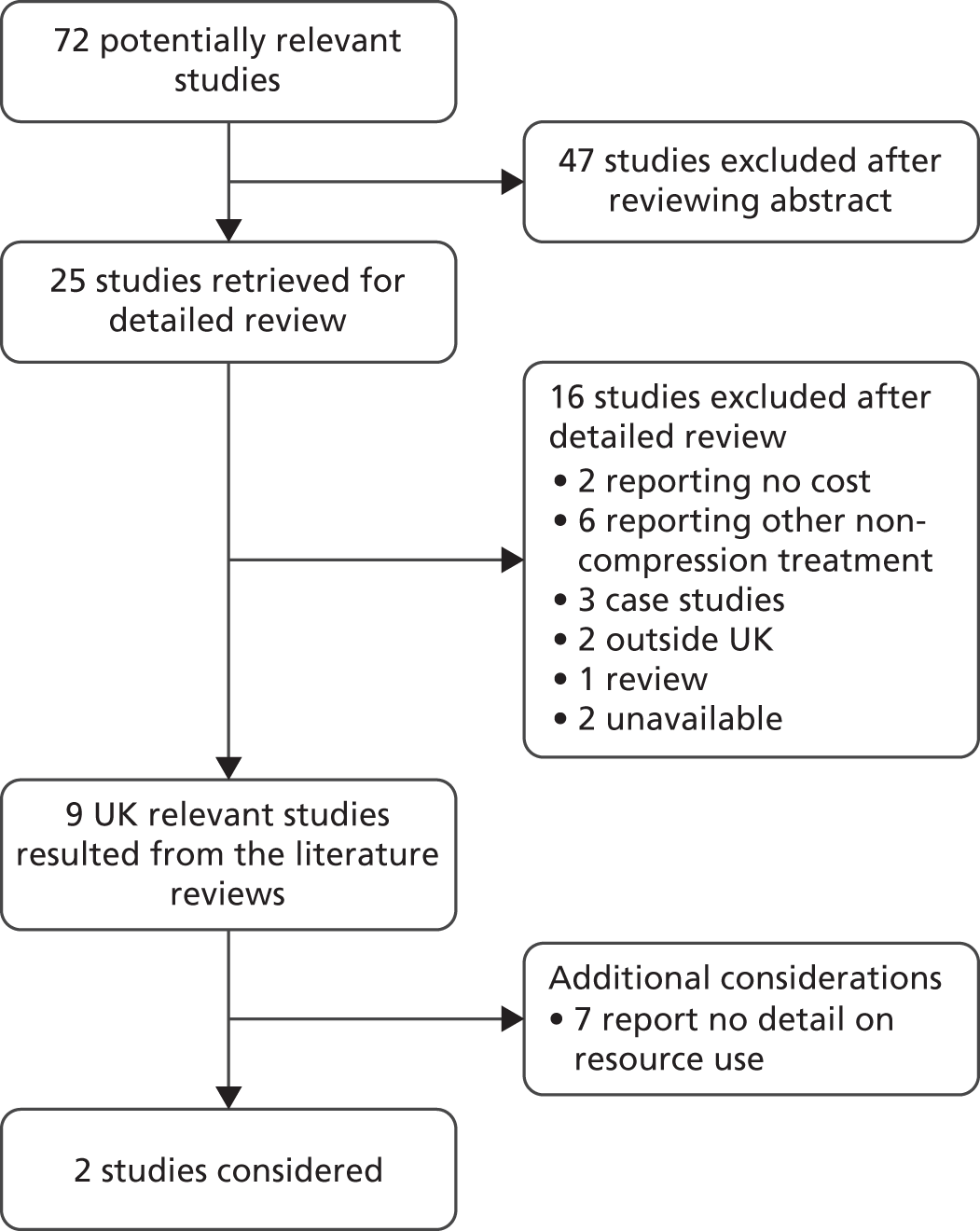
Appendix 17 Search on recurrence
Search limiters – English language and publication dates of 2000 onwards.
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 2 of 4, April 2011 via The Cochrane Library, Wiley http://onlinelibrary.wiley.com/
Date of search: 17 June 2011.
Search strategy
#1 MeSH descriptor Leg Ulcer, this term only with qualifier: MO (0)
#2 MeSH descriptor Varicose Ulcer, this term only with qualifier: MO (0)
#3 (#1 OR #2) (0)
#4 mortalit* (37,094)
#5 fatal or fatality or fatalities (3771)
#6 death (23,269)
#7 MeSH descriptor Mortality, this term only (372)
#8 MeSH descriptor Life Expectancy, this term only (489)
#9 life expectancy (1973)
#10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) (49,828)
#11 varicose NEXT ulcer* or venous NEXT ulcer* or leg NEXT ulcer* or stasis NEXT ulcer* or crural NEXT ulcer* or ulcus NEXT cruris (1417)
#12 MeSH descriptor Leg Ulcer, this term only (332)
#13 MeSH descriptor Varicose Ulcer, this term only (333)
#14 (#11 OR #12 OR #13) (1417)
#15 (#10 AND #14) (64)
#16 (#3 OR #15) (64)
Ovid MEDLINE(R) without revisions <1996 to June Week 2 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 17 June 2011.
Search strategy
-
leg ulcer/ or varicose ulcer/ (4044)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (3263)
-
or/1-2 (4700)
-
compression bandages/ (51)
-
stockings, compression/ (635)
-
(compression or bandag* or stocking* or hosiery or wrapp*).ti,ab. (39,597)
-
or/4-6 (39,854)
-
Recurrence/ (78,999)
-
Recurrence.ti,ab. (94,662)
-
reoccur*.ti. (26)
-
re-occur*.ti. (9)
-
reoccur*.ti,ab. (816)
-
re-occur*.ti,ab. (171)
-
or/8-13 (156,734)
-
3 and 7 and 14 (150)
-
15 (150)
-
limit 16 to (english language and yr = “2000 -Current”) (107)
MEDLINE In-Process & Other Non-Indexed Citations <28 June 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 29 June 2011.
Search strategy
-
leg ulcer/ or varicose ulcer/ (0)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (200)
-
or/1-2 (200)
-
compression bandages/ (0)
-
stockings, compression/ (0)
-
(compression or bandag* or stocking* or hosiery or wrapp*).ti,ab. (5486)
-
or/4-6 (5486)
-
Recurrence/ (0)
-
Recurrence.ti,ab. (6165)
-
reoccur*.ti. (3)
-
re-occur*.ti. (2)
-
reoccur*.ti,ab. (61)
-
re-occur*.ti,ab. (18)
-
or/8-13 (6236)
-
3 and 7 and 14 (8)
-
15 (8)
-
limit 16 to (english language and yr = “2000 -Current”) (7)
EMBASE <1996 to 2011 Week 23> via OvidSP
Date of search: 17 June 2011.
Search strategy
-
leg ulcer/ (5167)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (4615)
-
or/1-2 (6738)
-
compression bandage/ (531)
-
compression garment/ (1247)
-
(compression or bandag* or stocking* or hosiery or wrapp*).ti,ab. (52,472)
-
or/4-6 (53390)
-
Recurrence risk/ (19,647)
-
Recurrence.ti,ab. (127,067)
-
reoccur*.ti. (30)
-
re-occur*.ti. (11)
-
reoccur*.ti,ab. (1207)
-
re-occur*.ti,ab. (224)
-
or/8-13 (137,016)
-
3 and 7 and 14 (158)
-
15 (158)
-
limit 16 to (english language and yr = “2000 -Current”) (117)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost, inception to 17 June 2011
Date of search: 17 June 2011.
Search strategy
| S23 | s22 | Limiters – English Language; published date from: 1 January 2000 to 31 December 2011 Search modes – Boolean/phrase |
Interface – EBSCOhost Search screen – advanced search Database – CINAHL |
| S22 | S9 and S16 and S21 | Search modes – Boolean/phrase | |
| S21 | S17 or S18 or S19 or S20 | ||
| S20 | TI re-occur* or AB re-occur* | ||
| S19 | TI reoccur* or AB reoccur* | ||
| S18 | TI Recurrence or AB Recurrence | ||
| S17 | (MH “Recurrence”) | ||
| S16 | S10 or S11 or S12 or S13 or S14 or S15 | ||
| S15 | TI wrapp* or AB wrapp* | ||
| S14 | TI hosiery or AB hosiery | ||
| S13 | TI stocking* or AB stocking* | ||
| S12 | TI bandag* or AB bandag* | ||
| S11 | TI compression or AB compression | ||
| S10 | (MH “Compression Garments”) | ||
| S9 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 | ||
| S8 | TI “ulcus cruris” or AB “ulcus cruris” | ||
| S7 | TI “crural ulcer*” or AB “crural ulcer*” | ||
| S6 | TI “stasis ulcer*” or AB “stasis ulcer*” | ||
| S5 | TI “leg ulcer*” or AB “leg ulcer*” | ||
| S4 | TI “venous ulcer*” or AB “venous ulcer*” | ||
| S3 | TI “varicose ulcer*” or AB “varicose ulcer*” | ||
| S2 | (MH “Venous Ulcer”) | ||
| S1 | (MH “Leg Ulcer”) |
British Nursing Index (BNI) and Archive <1985 to June 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 17 June 2011.
Search strategy
-
leg ulcers/ (1500)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (1375)
-
or/1-2 (1780)
-
dressings/ (1857)
-
(compression or bandag* or stocking* or hosiery or wrapp*).ti,ab. (755)
-
or/4-5 (2171)
-
Recurrence.ti,ab. (183)
-
reoccur*.ti. (2)
-
re-occur*.ti. (0)
-
reoccur*.ti,ab. (4)
-
re-occur*.ti,ab. (0)
-
or/7-11 (186)
-
3 and 6 and 12 (24)
-
limit 13 to yr = “2000 -Current” (18)
Flow chart of studies included in the literature review for recurrence.
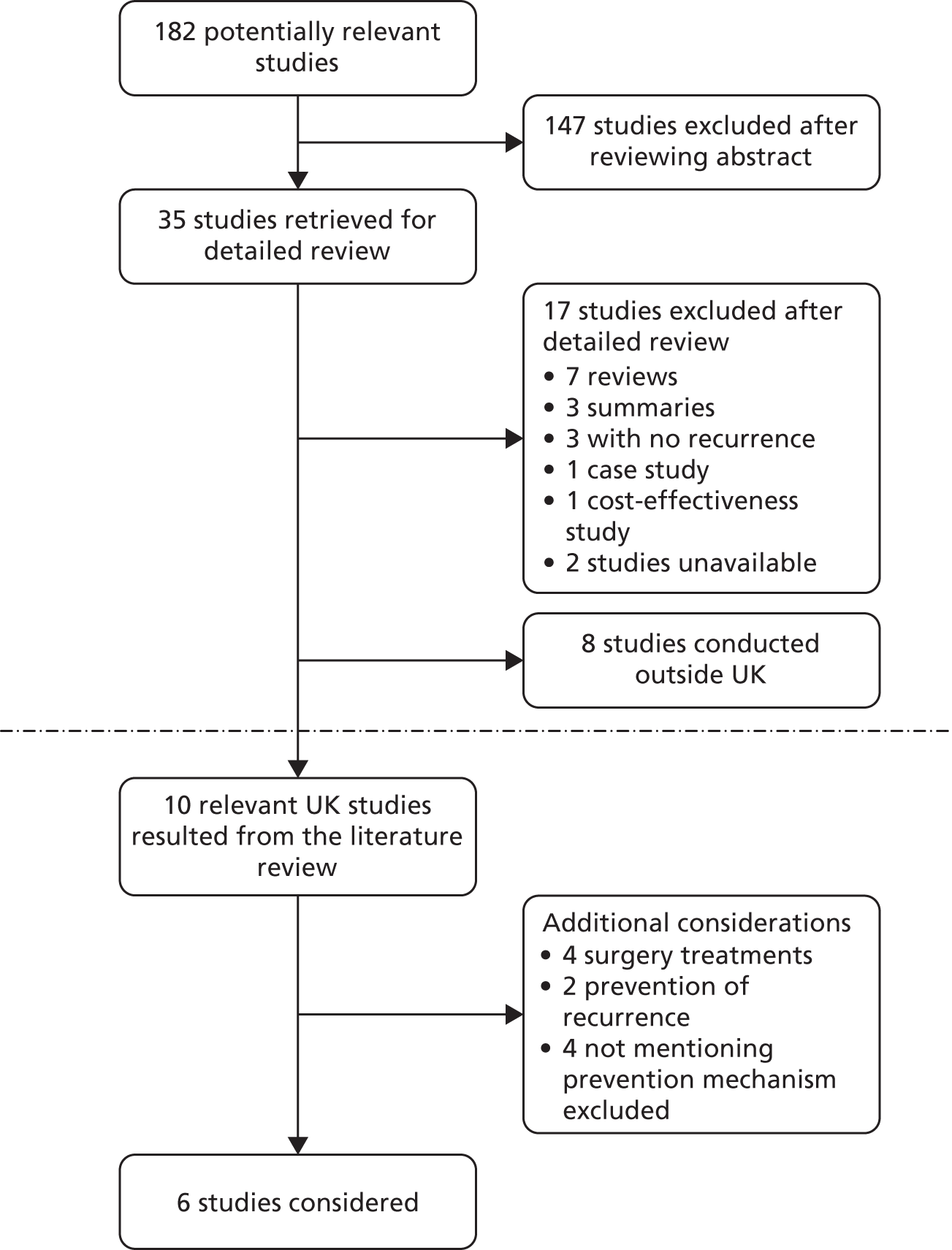
Appendix 18 Search on mortality
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 2 of 4, April 2011 via The Cochrane Library, Wiley http://onlinelibrary.wiley.com/
Date of search: 28 June 2011.
Search strategy
#1 MeSH descriptor Leg Ulcer, this term only with qualifier: MO (0)
#2 MeSH descriptor Varicose Ulcer, this term only with qualifier: MO (0)
#3 (#1 OR #2) (0)
#4 mortalit* (37,094)
#5 fatal or fatality or fatalities (3771)
#6 death (23,269)
#7 MeSH descriptor Mortality, this term only (372)
#8 MeSH descriptor Life Expectancy, this term only (489)
#9 life expectancy (1973)
#10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) (49,828)
#11 varicose NEXT ulcer* or venous NEXT ulcer* or leg NEXT ulcer* or stasis NEXT ulcer* or crural NEXT ulcer* or ulcus NEXT cruris (1417)
#12 MeSH descriptor Leg Ulcer, this term only (332)
#13 MeSH descriptor Varicose Ulcer, this term only (333)
#14 (#11 OR #12 OR #13) (1417)
#15 (#10 AND #14) (64)
#16 (#3 OR #15) (64)
Ovid MEDLINE(R) without revisions <1948 to June Week 3 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 28 June 2011.
Search strategy
-
Leg Ulcer/mo [Mortality] (21)
-
Varicose Ulcer/mo [Mortality] (4)
-
1 or 2 (25)
-
mortalit*.ti,ab. (349,434)
-
(fatal or fatality or fatalities).ti,ab. (81,658)
-
death.ti,ab. (361,068)
-
Mortality/ (30,881)
-
life expectancy.ti,ab. (14,583)
-
life expectancy/ (12,145)
-
or/4-9 (731,352)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (6183)
-
Leg Ulcer/ (6902)
-
Varicose Ulcer/ (3428)
-
or/11-13 (11094)
-
10 and 14 (228)
-
3 or 15 (239)
-
16 (239)
-
limit 17 to english language (196)
MEDLINE In-Process & Other Non-Indexed Citations <28 June 28 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 29 June 2011.
Search strategy
-
Leg Ulcer/mo [Mortality] (0)
-
Varicose Ulcer/mo [Mortality] (0)
-
1 or 2 (0)
-
mortalit*.ti,ab. (16,602)
-
(fatal or fatality or fatalities).ti,ab. (3556)
-
death.ti,ab. (15,616)
-
Mortality/ (4)
-
life expectancy.ti,ab. (657)
-
life expectancy/ (1)
-
or/4-9 (32836)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (200)
-
Leg Ulcer/ (0)
-
Varicose Ulcer/ (0)
-
or/11-13 (200)
-
10 and 14 (3)
-
3 or 15 (3)
-
16 (3)
-
limit 17 to english language (2)
EMBASE <1980 to 2011 Week 25> via OvidSP http://ovidsp.ovid.com/
Date of search: 28 June 2011.
Search strategy
-
leg ulcer/ (9596)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (7665)
-
1 or 2 (12244)
-
mortality/ (368517)
-
life expectancy/ (21572)
-
mortalit*.ti,ab. (419856)
-
(fatal or fatality or fatalities).ti,ab. (93807)
-
death.ti,ab. (419312)
-
life expectancy.ti,ab. (17088)
-
or/4-9 (996364)
-
3 and 10 (406)
-
limit 11 to english language (342)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost, inception to 17 June 2011
Date of search: 28 June 2011. A total of 31 records were retrieved.
Search strategy
| S21 | S3 or S20 | Search modes: Boolean/phrase | Interface: EBSCOhost Search screen: advanced search Database: CINAHL |
| S20 | S12 and S19 | ||
| S19 | S13 or S14 or S15 or S16 or S17 or S18 | ||
| S18 | TI “life expectancy” or AB “life expectancy” | ||
| S17 | (MH “Life Expectancy”) | ||
| S16 | (MH “Mortality”) | ||
| S15 | TI death or AB death | ||
| S14 | TI ( fatal or fatality or fatalities ) or AB (fatal or fatality or fatalities ) | ||
| S13 | TI mortalit* or AB mortalit* | ||
| S12 | S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 | ||
| S11 | TI “ulcus cruris” or AB “ulcus cruris” | ||
| S10 | TI “crural ulcer*” or AB “crural ulcer*” | ||
| S9 | TI “stasis ulcer*” or AB “stasis ulcer*” | ||
| S8 | TI “leg ulcer*” or AB “leg ulcer*” | ||
| S7 | TI “venous ulcer*” or AB “venous ulcer*” | ||
| S6 | TI “varicose ulcer*” or AB “varicose ulcer*” | ||
| S5 | (MH “Venous Ulcer”) | ||
| S4 | (MH “Leg Ulcer”) | ||
| S3 | S1 or S2 | ||
| S2 | (MH “Venous Ulcer/MO”) | ||
| S1 | (MH “Leg Ulcer/MO”) | ||
British Nursing Index (BNI) and Archive <1985 to June 2011> via OvidSP http://ovidsp.ovid.com/
Date of search: 28 June 2011. One record was retrieved.
Search strategy
-
leg ulcers/ (1500)
-
(varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris).ti,ab. (1375)
-
1 or 2 (1780)
-
mortalit*.ti,ab. (1317)
-
(fatal or fatality or fatalities).ti,ab. (151)
-
death.ti,ab. (2759)
-
Community Medicine/ (929)
-
life expectancy.ti,ab. (107)
-
or/4-8 (4946)
-
3 and 9 (1)
Flow chart of studies included in the literature review for mortality.
Appendix 19 VenUS IV statistical analysis plan: clinical analysis
Introduction
This is a randomised multicentred, pragmatic, two-armed controlled trial with equal randomisation to demonstrate the clinical effectiveness and cost-effectiveness of compression hosiery compared with 4LB. The purpose of this document is to provide the clinical statistical analysis plan for the study. The cost-effectiveness analysis plan is documented elsewhere.
Study objectives
Primary objective
The objective of this multicentred, pragmatic, two-armed, parallel RCT is to assess the clinical effectiveness and cost-effectiveness of compression hosiery compared with 4LB in terms of time to ulcer healing.
Secondary objectives
To compare:
-
time to ulcer-free reference leg between the patients with compression hosiery and those with 4LB
-
the longitudinal profile of health-related quality of life [collected at baseline then via postal survey at 3-monthly intervals (baseline, 3, 6, 9 and 12 months)] between the patients with compression hosiery and those with 4LB
-
the concordance to treatment (throughout trial) between the patients with compression hosiery and those with 4LB
-
ulcer recurrence rates between the patients with compression hosiery and those with 4LB
-
the number of adverse events (throughout the trial) between the patients with compression hosiery and those with 4LB.
Study design
Sample size calculation
The sample size estimation is based on VenUS I,7 a RCT that compared time to ulcer healing between two groups of patients [4LB vs. SSB, HR 1.33 (95% CI 1.05 to 1.67), median time to healing 92 days vs. 126 days]. The aim of VenUS IV is to estimate the size of the difference between the compression hosiery and 4LB rather than to look for a difference of any given size. Therefore, 400 patients (200 in each arm of the study) will allow us to detect a HR of either 0.72 or 1.41 between the two groups, with 90% power at the 5% significance level. Adjusting for centre variation and loss to follow-up of approximately 10%, the estimated sample size is about 489 in both intervention groups. Hence we anticipate recruiting 489 patients within duration of 17 months. These patients will be followed up for a maximum duration of 12 months.
Randomisation and blinding
Eligible patients will be randomised in a 1 : 1 ratio to either compression hosiery or 4LB. Randomisation will be stratified by baseline ulcer area (≤ 5 cm2 vs. > 5cm2) and baseline ulcer duration (≤ 6 months vs. > 6 months) using permuted blocks design so that patients with these characteristics are approximately equally distributed between the two intervention groups. Randomisation will be centralised and will be undertaken at York Trials Unit. This is an unblinded study. It is not possible to blind the nurses or participants to the treatment being received. The interventions have different modes of application and look different.
The assessment of the main outcome measure will be blinded. The status of an ulcer as healed will be established by two independent assessors using digital photographs of the reference leg ulcers, taken by the treating nurses at healing and then at weekly intervals after healing for 4 weeks.
Study outcomes and how they will be measured
Primary
The primary outcome is the time to healing of the largest eligible ulcer on the reference leg measured in days. For each patient in the study this will be measured as the duration from randomisation to the time when their reference leg ulcer heals, they withdraw from the study, they are lost to follow-up, they die from any cause or they exit the trial after 12 months – whichever event comes first.
The time to healing of the reference leg ulcer is blinded in the sense that it will be determined from photographs of the reference leg ulcer taken by the treating nurses when they feel the ulcer is healed. The blinded outcome assessment will be conducted by two blinded clinical experts. Where agreement is not reached between the reviewers, a third reviewer will be consulted, who will give a final decision. If no photographs are available, unblinded survival time taken from the treating nurse records will be used instead. In addition, if nurses say that the wound is healed and stop taking photographs but blinded assessors says the wound is not healed then we will consider the wound healed.
Secondary
-
Non-blinded time to reference leg ulcer healing This will be measured similarly as the main outcome, but the healing date will be determined by nurse decision and not based on blinded photographs.
-
Non-blinded time to an ulcer-free reference leg The reference leg is the leg with the largest eligible ulcer that will be followed during the study. This outcome will be the time from randomisation to the time when the patient either has an ulcer-free reference leg, withdrawn from the study, been lost to follow-up or died from any cause, or time of data analysis (end of study) – whichever comes first.
-
Health-related quality-of-life scores at baseline and months 3, 6, 9 and 12 These scores are physical scores (physical functioning, role physical, bodily pain and general health), mental scores (vitality, social functioning, role emotional and mental health), Physical Component Score and Mental Component Score. All of these scores will be measured by the SF-12 (version 2, standard recall) questionnaire168 and will be scored using the QualityMetric Health Outcomes™ Scoring Software 2.0. 169
-
Patient concordance to treatment This will be measured by (1) the proportion of patients changing from their randomised treatment to another treatment in the compression hosiery group compared with the 4LB group; (2) how often they wear compression hosiery during the day (everyday/most days/some days/did not wear compression hosiery); (3) how many layers the patient wears and whether the patient or carer applied the hosiery; (4) how often (everyday/most days/some days/not at all) patients with 4LB wear 4LB; and (5) the number of times that the patients removed their bandages. These outcomes are measured 1 month post randomisation.
-
The number of patients having the recurrence of a venous leg ulcer on the reference leg, in the two intervention groups This will be measured by self-reported ulcer recurrence events in the two groups and also reported by nurses in the monthly nurse assessment questionnaire.
-
The total number of each patient’s adverse events (SAEs) and NSAEs throughout the period of the trial This will be measured from nurse-reported adverse events forms.
Statistical methods
Pooling of data for analyses
The study will be conducted under a common protocol at each investigational site with the intention of pooling the data for analysis. Every effort will be made to promote consistency in study execution at each investigational site (study centre). Centres with very few participants will not be combined with other centres because we are not interested in the actual estimates of the centre effects but rather we would like to simply adjust for them. 170
Statistical analyses
All analyses will be conducted at the 5% significance level. Estimates and their 95% CIs will be constructed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and Stata version 11 (versions may change).
Primary analyses
The primary analysis will be performed as near to an intention to treat as we can. The primary analysis involves the primary outcome as defined above. More specifically, the time to reference leg ulcer healing will be right censored when any one of the following situations has occurred: (1) participant has withdrawn from the study; (2) subject is lost to follow-up; (3) subject has died from any causes; or (4) end of follow-up (12 months). For each intervention arm, the distribution of time to ulcer healing will be described using Kaplan–Meier survival estimates (Table 63). Treatment differences will be evaluated using the CPH model with shared centre frailty effects63–65 (Table 64). A shared frailty effect is a random effect in a CPH model that has a multiplicative effect on the hazard of healing. In this case the shared centre frailty effects will model the frailties as being specific to each centre hence describing the degree of correlation of patients within centres (subjects within a centre are correlated because they share a common frailty). From our experience with another study (VenUS III)13 patients from same centre are correlated, hence this ought to be adjusted for in this study with centre shared frailty effects. The need for a centre frailty effect will be evaluated via a likelihood ratio test that evaluates if the frailty variance is zero using a 50 : 50 mixture of chi-squared distributions. If there is a non-significant frailty effect then CPH model without centre frailty effects will be fitted instead to the data.
| Characteristic | Compression hosiery (n = xxx) | 4LB (n = xxx) |
|---|---|---|
| No. healing/total no. healed (%) | ||
| Unadjusted Kaplan–Meier estimates | ||
| 25% percentile of time to healing (days) (95% CI) | ||
| 50% percentile of time to healing (median) (days) (95% CI) | ||
| Log-rank test statistic, p-value | ||
| Wilcoxon test statistic, p-value | ||
| Parameter | Estimate (SE) | Hazard ratio (95% CI) | p-value | Test of PH assumption, p-value |
|---|---|---|---|---|
| Without centre frailty effect | ||||
| Compression hosiery vs. 4LB | ||||
| Log (area) | ||||
| Log (duration) | ||||
| Mobility | ||||
| Patient walks freely | ||||
| Patient walks with difficulty | ||||
| Patient is immobile | ||||
| Global test of PH assumption assumption | ||||
| With centre frailty effect | ||||
| Compression hosiery vs. 4LB | ||||
| Log (area) | ||||
| Log (duration) | ||||
| Mobility | ||||
| Patient walks freely | ||||
| Patient walks with difficulty | ||||
| Patient is immobile | ||||
| Theta | ||||
| Global test of PH assumption |
Given that it is not known whether the effectiveness of the treatment will decrease, increase or remain constant during follow-up, the proportional hazard assumption will be evaluated formally by a statistical test using Schoenfeld residuals. 66 Treatment effects will be adjusted by baseline characteristics (ulcer area, ulcer duration, centre and patient mobility). 7,61 Ulcer area and duration are continuous variables and will be logarithmically transformed. Centre will be included as a shared frailty effect and patient mobility as a three-level factor.
Secondary analyses
Many of the secondary analyses will be performed using the secondary outcomes as outlined in the ‘study outcomes and how they will be measured’ section. In all analyses centre will be treated as a random effect.
-
Time to event secondary endpoint (non-blinded time to ulcer healing) will be analysed like the primary outcome with identical censoring strategy. The CPH model with a shared centre frailty effect here will be further adjusted by the same covariates (e.g. ulcer area, ulcer duration, centre and patient mobility) as in the primary analysis. All other model building procedures will be the same as for the primary outcome (Tables 65 and 66).
-
Time to event secondary endpoint (non-blinded time to an ulcer free reference leg) will be analysed like the primary outcome with identical censoring strategy. Treatment effects will be adjusted by ulcer area, ulcer duration, centre and patient mobility. All other procedures are identical to the analysis of the primary outcome.
-
The continuous physical scores (physical functioning, role physical, bodily pain and general health), mental scores (vitality, social functioning, role, emotional and mental health), PCS and MCS will be summarised with descriptive statistics (n, mean, SD, minimum, maximum, IQR and median) by treatment group at baseline, and months 3, 6, 9 and 12 (Tables 67 and 68). As the PCS and MCS were measured longitudinally with time (baseline and months 3, 6, 9 and 12), the relationship between PCS or MCS with treatment will be evaluated through a LMM to account for the dependence of PCS or MCS measured within the same patient as described by Verbeke and Molenberghs and also Fitzmaurice et al. 68,69 In the LMM the PCS or MCS will be adjusted by ulcer area, ulcer duration, time, centre and patient mobility. To assess whether there are differences between the treatments during time of follow-up, an interaction between treatment and time will be tested for inclusion in the models.
-
Patient concordance to treatment will be analysed as follows: (1) the proportion of patients changing from their randomised treatment will be summarised by treatment randomised; (2) in compression hosiery, the frequency (%) of wearing compression hosiery will be summarised by the four categories (every day/most days/some days/did not wear compression hosiery); (3) the number of layers (one layer, two layers) will be summarised as frequency (%); similarly, the subject (nurse, patient) applying the stockings will be summarised by frequency (%), (4) for patients with 4LBs, the frequency of wearing a 4LB will be summarised by the four categories (every day/most days/some days/not at all); and (5) number of times (none, at least once) patients have removed any of the layers of bandages themselves will be summarised as frequency (%) (Table 69).
-
The recurrence of a leg ulcer post healing is a binary outcome (recurrence yes, recurrence no). This will be analysed using random-effects logistic regression70 adjusting for ulcer area, ulcer duration, centre, patient mobility and duration between healing and end of study to ascertain whether the recurrence rates are the same in the two treatment groups.
-
The total number of each patient’s adverse events is a count outcome. This will be analysed by a random-effects Poisson regression model adjusting for ulcer area, ulcer duration, centre and patient mobility. It is anticipated that the variability in the data will be higher than that modelled by the random-effects Poisson regression model (experience from previous studies, VenUS II and VenUS III). 13,61 In the event of such a scenario, a random-effects negative binomial regression model will be used adjusting for the same covariates. Also, from previous experience (VenUS III),13 there will be an excess of ‘zeros’ (a lot of patients without adverse events throughout the whole period of the study). In the event of this, a zero-inflated, random-effects Poisson regression model or a zero-inflated, random effects negative binomial regression model70,71 will be fitted to the adverse events data adjusting for the same covariates. This analysis will be repeated for NSAEs and SAEs separately (Table 70). If the numbers of adverse events per participant is very small throughout follow-up then the adverse events data will also be analysed by random-effects logistic regression. For each participant in the study a success will be defined as having at least one adverse event during follow-up and failure will be defined as having no adverse event during follow-up. Treatment differences will be compared adjusting for ulcer area, ulcer duration, centre and patient mobility (Table 71).
| Parameter | Estimate (SE) | Hazard ratio (95% CI) | p-value | Test of PH assumption, p-value |
|---|---|---|---|---|
| Without centre frailty effect | ||||
| Compression hosiery vs. 4LB | ||||
| Log (area) | ||||
| Log (duration) | ||||
| Mobility | ||||
| Patient walks freely | ||||
| Patient walks with difficulty | ||||
| Patient is immobile | ||||
| Global test of PH assumption | ||||
| With centre frailty effect | ||||
| Compression hosiery vs. 4LB | ||||
| Log (area) | ||||
| Log (duration) | ||||
| Mobility | ||||
| Patient walks freely | ||||
| Patient walks with difficulty | ||||
| Patient is immobile | ||||
| Theta | ||||
| Global test of PH assumption | ||||
| Parameter | Estimate (SE) | Hazard ratio (95% CI) | p-value | Test of PH assumption p-value |
|---|---|---|---|---|
| Without centre frailty effect | ||||
| Compression hosiery vs. 4LB | ||||
| Log (area) | ||||
| Log (duration) | ||||
| Mobility | ||||
| Patient walks freely | ||||
| Patient walks with difficulty | ||||
| Patient is immobile | ||||
| Global test of PH assumption | ||||
| With centre frailty effect | ||||
| Compression hosiery vs. 4LB | ||||
| Log (area) | ||||
| Log (duration) | ||||
| Mobility | ||||
| Patient walks freely | ||||
| Patient walks with difficulty | ||||
| Patient is immobile | ||||
| Theta | ||||
| Global test of PH assumption | ||||
| Timeline/statistic | PCSs | MCSs | ||||
|---|---|---|---|---|---|---|
| Compression hosiery (n = xxx) | 4LB (n = xxx) | Overall (N = xxx) | Compression hosiery (n = xxx) | 4LB (n = xxx) | Overall (N = xxx) | |
| Baseline | ||||||
| Mean (SD) | ||||||
| Median (min., max.) | ||||||
| IQR (25–75%) | ||||||
| Missing, n (%) | ||||||
| 3 months | ||||||
| Mean (SD) | ||||||
| Median (min., max.) | ||||||
| IQR (25–75%) | ||||||
| Missing, n (%) | ||||||
| 6 months | ||||||
| Mean (SD) | ||||||
| Median (min., max.) | ||||||
| IQR (25–75%) | ||||||
| Missing, n (%) | ||||||
| 9 months | ||||||
| Mean (SD) | ||||||
| Median (min., max.) | ||||||
| IQR (25–75%) | ||||||
| Missing, n (%) | ||||||
| 12 months | ||||||
| Mean (SD) | ||||||
| Median (min., max.) | ||||||
| IQR (25–75%) | ||||||
| Missing, n (%) | ||||||
| Timeline/statistic | Compression hosiery (n = xxx) | 4LB (n = xxx) | Overall (N = xxx) | Compression hosiery (n = xxx) | 4LB (n = xxx) | Overall (N = xxx) |
|---|---|---|---|---|---|---|
| Physical scores | Mental Scores | |||||
| Physical functioning | Vitality | |||||
| Baseline | ||||||
| 3 months | ||||||
| 6 months | ||||||
| 9 months | ||||||
| 12 months | ||||||
| Role physical | Social functioning | |||||
| Baseline | ||||||
| 3 months | ||||||
| 6 months | ||||||
| 9 months | ||||||
| 12 months | ||||||
| Bodily pain | Role emotional | |||||
| Baseline | ||||||
| 3 months | ||||||
| 6 months | ||||||
| 9 months | ||||||
| 12 months | ||||||
| General health | Mental health | |||||
| Baseline | ||||||
| 3 months | ||||||
| 6 months | ||||||
| 9 months | ||||||
| 12 months |
| Characteristic | Compression hosiery (n = xxx) | Characteristic | Four-layer high compression (n = xxx) |
|---|---|---|---|
| Frequency of stockings wearing during day | Frequency of 4LB wearing | ||
| Everyday | Everyday | ||
| Most days | Most days | ||
| Some days | Some days | ||
| Did not wear | Not all | ||
| Layers of stockings during the day | Ever removed 4LB yourself? | ||
| One layer | Yes | ||
| Two layers | No | ||
| Frequency of stockings wearing during night | |||
| Every night | – | – | |
| Most nights | – | – | |
| Some nights | – | – | |
| Did not wear | – | – | |
| Layers of stockings during the night | |||
| One layer | – | – | |
| Two layers | – | – | |
| Who normally applies compression stockings? | |||
| Nurse | – | – | |
| Yourself | – | – | |
| If yourself, stockings easy to apply? | |||
| Yes | – | – | |
| No | – | – | |
| If friend/relative, stockings easy to apply? | |||
| Yes | – | – | |
| No | – | – | |
| Did not wear my stockings | – | – | |
| Parameter | NSAEs | SAEs | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | p-value | 95% CI | Estimate (SE) | p-value | 95% CI | |
| Fixed effects | ||||||
| Intercept | ||||||
| Compression hosiery vs. 4LB | ||||||
| Log (area) | ||||||
| Log (duration) | ||||||
| Mobility | ||||||
| Patient walks freely | ||||||
| Patient walks with difficulty | ||||||
| Patient is immobile | ||||||
| Covariance parameters | ||||||
| Centre effect | ||||||
| Dispersion parameter | ||||||
| Estimate (SE) | (p-value) | 95% CI | – | – | – | |
| Fixed effects | ||||||
| Intercept | – | – | – | |||
| Compression hosiery vs. 4LB | – | – | – | |||
| Log (area) | – | – | – | |||
| Log (duration) | – | – | – | |||
| Mobility | – | – | – | |||
| Patient walks freely | – | – | – | |||
| Patient walks with difficulty | – | – | – | |||
| Patient is immobile | – | – | – | |||
| Covariance parameters | – | – | – | |||
| Centre effect | – | – | – | |||
| Dispersion parameter | – | – | – | |||
| Parameter | NSAEs | SAEs | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | Odds ratio (95% CI) | p-value | Estimate (SE) | Odds ratio (95% CI) | p-value | |
| Fixed effects | ||||||
| Compression hosiery vs. 4LB | ||||||
| Log (area) | ||||||
| Log (duration) | ||||||
| Mobility | ||||||
| Patient walks freely | ||||||
| Patient walks with difficulty | ||||||
| Patient is immobile | ||||||
| Random effects | ||||||
| Centre | ||||||
| Measurement error | ||||||
| Estimate (SE) | Odds ratio (95% CI) | p-value | – | – | – | |
| Fixed effects | – | – | – | |||
| Compression hosiery vs. 4LB | – | – | – | |||
| Log (area) | – | – | – | |||
| Log (duration) | – | – | – | |||
| Mobility | – | – | – | |||
| Patient walks freely | – | – | – | |||
| Patient walks with difficulty | – | – | – | |||
| Patient is immobile | – | – | – | |||
| Random effects | – | – | – | |||
| Centre | ||||||
| Measurement error | ||||||
Summary of baseline and follow-up characteristics
Baseline patient data
Categorical variables like gender, mobility, ankle mobility of reference leg and diabetes status will be summarised as frequency (%) by treatment group. Continuous variables, such as age, height, weight and BMI will be summarised with descriptive statistics (n, mean, SD, minimum, maximum, IQR and median) (Table 72).
| Characteristic | Compression hosiery (n = xxx) | Four-layer high compression (n = xxx) |
|---|---|---|
| Gender | ||
| Male | ||
| Female | ||
| Age | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Height | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Weight | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| BMI | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Mobility | ||
| Patient walks freely | ||
| Patient walks with difficulty | ||
| Patient is immobile | ||
| Ankle mobility of reference leg | ||
| Patient has full range of motion | ||
| Patient has reduced range of ankle motion | ||
| Patient’s ankle is fixed | ||
| Diabetic | ||
| Yes | ||
| No | ||
Baseline ulcer data
Categorical variables, such as baseline tracing, treatment preference, current treatments and baseline questionnaire completed will be summarised as frequency (%) by treatment group. Continuous variables, such as size of ulcer, ulcer duration and ankle circumference, will be summarised with descriptive statistics (n, mean, SD, minimum, maximum, IQR and median) (Table 73).
| Characteristic | Compression hosiery (n = xxx) | Four-layer high compression (n = xxx) |
|---|---|---|
| Size of ulcer | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Ulcer duration | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Ankle circumference | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Baseline tracing done | ||
| Yes | ||
| No | ||
| Preference | ||
| Compression hosiery (stockings) | ||
| Four-layer compression bandaging | ||
| No preference | ||
| Current treatments | ||
| Four-layer compression bandaging | ||
| Short stretch bandaging | ||
| Compression hosiery | ||
| Other compression bandaging | ||
| Not receiving compression | ||
| Other treatment | ||
| Baseline questionnaire completed | ||
| Yes | ||
| No | ||
Baseline reference limb data
Categorical variables like reference leg followed will be summarised as frequency (%) by treatment group. Continuous variables, such as ABPI, total number of ulceration episodes, duration since first ulcer, duration of reference ulcer, duration of oldest ulcer on reference leg and total number of ulcers per patient on the reference leg, will be summarised with descriptive statistics (n, mean, SD, minimum, maximum, IQR and median) (Table 74).
| Characteristic | Compression hosiery (n = xxx) | Four-layer high compression (n = xxx) |
|---|---|---|
| Reference leg | ||
| Left | ||
| Right | ||
| ABPI | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Total of ulceration episodes | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Duration since first ulcer | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Duration of reference ulcer (years) | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Duration of oldest ulcer on reference leg (years) | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
| Total no. of ulcers per patient (reference leg) | ||
| Mean (SD) | ||
| Median (min., max.) | ||
| IQR (25–75%) | ||
| Missing (%) | ||
Centre enrolment, healing rates and adverse events
Enrolment is stratified by investigational site (centre) and within each centre this will be summarised as frequency (%) by treatment received and overall. Within each centre the percentage healed and number of adverse events (SAES and NSAEs) will be summarised as frequency (%) (Table 75).
| Study centre | Compression hosiery (n = xxx) | 4LB (n = xxx) | Overall (N = xxx) | % Healed |
|---|---|---|---|---|
| Bolton | ||||
| Bradford | ||||
| Brighton | ||||
| Cambridge | ||||
| Cornwall | ||||
| Durham | ||||
| Epsom | ||||
| Harrogate | ||||
| Hull | ||||
| Kent | ||||
| Kingston | ||||
| Lancashire (Central) | ||||
| Lancashire (North) | ||||
| Leeds | ||||
| Mid Yorks and Wakefield | ||||
| Norfolk (Dereham) | ||||
| Norfolk (Diss) | ||||
| Norfolk (Norwich) | ||||
| N Ireland | ||||
| N Yorks | ||||
| Nottingham | ||||
| Redbridge | ||||
| Suffolk (Sudbury) | ||||
| South of Tyne and Wear | ||||
| Whitby | ||||
| York Hospital |
Description of adverse events
Serious adverse events are further classified as death, persistent or significant disability/incapacity, hospitalisation required/prolonged and other medically important condition. This will be summarised as frequency (%) by treatment group. The outcome of the serious adverse event and their relationship with treatment are categorical variables and will be summarised as frequency (%) by treatment received.
The relationship between non-serious adverse events and treatment received is a categorical variable and will be summarised by frequency (%) (Tables 76 and 77).
| Characteristic | Compression hosiery (n = xxx) | Four-layer high compression (n = xxx) |
|---|---|---|
| SAE | ||
| Classification | ||
| Death | ||
| Life- or limb-threatening event | ||
| Hospitalisation required/prolonged | ||
| Persistent or significant disability/incapacity | ||
| Other medically important condition | ||
| Outcome of event | ||
| Recovered fully | ||
| Recovered partially | ||
| Died | ||
| Ongoing | ||
| Relationship of adverse event to treatment (nurse’s classification) | ||
| Unrelated | ||
| Unlikely to be related | ||
| Possibly related | ||
| Probably related | ||
| Definitely related | ||
| Not able to assess if related | ||
| NSAE | ||
| Relationship of adverse event to treatment | ||
| Unrelated | ||
| Unlikely to be related | ||
| Possibly related | ||
| Probably related | ||
| Definitely related | ||
| Not able to assess if related |
| Characteristic | Compression hosiery (n = xxx) | Four-layer high compression (n = xxx) |
|---|---|---|
| Discomfort | ||
| Mean (SD) | ||
| Median (min, max) | ||
| IQR (25–75%) | ||
| Missing (%) |
Sensitivity analysis and missing data handling
Sensitivity analysis will be carried out in two ways, namely (1) comparing results with and without a centre random effect and (2) comparing results with and without multiple imputation of the best model chosen in ‘(1)’. Ten data sets will be generated for each multiple imputation procedure. This will be done with all of the statistical models fitted to the data.
In the comparison of results with and without a centre random effect, for the primary outcome (time to ulcer healing) and for the survival type secondary outcomes (the non-blinded time to ulcer healing and the non-blinded time to an ulcer free reference leg), a CPH model with a centre frailty effect will be compared with one without a centre frailty effect (centre effects completely ignored) to ascertain the changes in the treatment effect. For the other non-survival type secondary outcomes this will be addressed as follows: for the PCS or MCS, the LMM with and without a centre random effect, will be compared; for the recurrence of the leg ulcer post healing, the random-effects logistic regression, with and without a centre random effect, will be compared; for the number of adverse events, a zero-inflated Poisson regression model, with and without a centre random effect, will be compared.
Missing data will be estimated by multiple imputations. For this part of the sensitivity analysis it will be assumed that the data are multivariate and normally distributed, and the missing data are missing at random. 171 That is, the probability that an observation is missing can depend on the observed values of the individual but not on the missing variable values of the individual. We will also assume that the imputer’s model (model used to impute the missing values) is the same as the analyst’s model (model used to analyse the data). Therefore, multiple imputation will be conducted as follows. For the primary outcome (time to ulcer healing) and for the survival type secondary outcomes (the non-blinded time to ulcer healing and the non-blinded time to an ulcer-free reference leg), it will be further assumed that the outcomes and their censoring status is completely known (we have designed our data collection to have complete data on these outcomes) and the missing values for ulcer area, ulcer duration, centre and patient mobility will be imputed. For the other non-survival secondary outcomes, the imputation will be as follows. For the PCS or MCS, multiple imputations will be carried out on missing values (for PCS – ulcer area, ulcer duration, centre and patient mobility; for MCS – ulcer area, ulcer duration, centre and patient mobility separately). For the recurrence of the leg ulcer post healing, multiple imputation will be carried out on missing values (recurrence (yes/no), ulcer area, ulcer duration, centre and patient mobility). For the number of adverse events, multiple imputation will be carried out on missing values (NSAEs, SAEs, ulcer area, ulcer duration, centre and patient mobility). In all imputations, ulcer area and ulcer duration will be logarithmically transformed. Other outcomes (PCS, MCS, non serious adverse events, serious adverse events) will be logarithmically transformed if need be.
Separate tables of the results of sensitivity analyses will not be produced but the results will be commented on in the text, and emphasis placed on results that differ considerably between models with and without multiple imputations. Our primary results are those without multiple imputations, so in an event that there are differences between these results and those with multiple imputations, those without multiple imputations will be taken as the primary results. Nonetheless, the differences will be explained explicitly.
Appendix 20 VenUS IV statistical analysis plan: cost-effectiveness analysis
This document provides the economic analysis plan for the VenUS IV study, a RCT of high-compression hosiery (hosiery) compared with high-compression bandaging (4LB) in the treatment of venous leg ulcers.
The VenUS IV protocol specifies that a within-trial analysis – a cost-effectiveness analysis using trial data only – will be undertaken to assess the cost-effectiveness of hosiery compared with 4LB in study participants. Although within-trial analysis is considered to have high internal validity, there is a growing awareness that the findings from any RCT should not be considered in isolation but, in fact, are more valuable from a decision-making context when used to update existing evidence on all treatments of interest. Thus, in addition to conducting the trial-level analysis, a second, separate VenUS IV economic analysis will be conducted to assess the cost-effectiveness of all relevant high-compression devices. This second analysis will incorporate the findings from VenUS IV into the wider existing evidence base including other, alternative, high-compression treatments. Such an analysis requires the development of a decision-analytic model to accommodate multiple different information sources. This document will focus on the conduct of both analyses, in turn.
Within-trial analysis: outline of the analysis
The within-trial economic analysis will be performed using individual patient-level data from VenUS IV trial. The analytical approach will take the form of cost-effectiveness and cost–utility analyses. The difference between the two approaches, in the case of VenUS IV, lies in the outcome measure used, which will be ulcer healing for the cost-effectiveness analysis and QALYs for the cost–utility analysis. A QALY is defined as a year lived with full health, calculated by multiplying quality of life and length of life. This is discussed later in more detail. Based on trial evidence, incremental cost-effectiveness (or cost–utility) ratios will be calculated by taking a ratio of the difference in the mean costs and mean effects (or utility measure). Incremental NMB will be calculated by weighting the QALYs gained by the maximum willingness to pay for a QALY and subtracting the cost difference. 84
The economic analysis will be conducted using the perspective of the NHS and PSS. 172 The period of analysis is 12 months, which is the equal to the maximum period of patient follow-up in the trial. Hence, future costs and health outcomes will not be discounted. The analyses will be conducted using Stata version 10 or version 11.
Outcomes for the analysis
The outcome for the cost-effectiveness analysis is the same as the primary outcome of the clinical trial, i.e. the time to healing of the largest eligible ulcer on the reference leg, measured in days. The outcome for the cost–utility analysis is the quality-adjusted life-years over a period of 12 months, which is the maximum period of follow-up of patients. Patients who have healed before the end of the study will be followed up until the end of the study to collect utility (and resource use) data. This is important because patients may have higher utility levels after healing, in which case the benefit of treatment in reducing the time to healing will be reflected in higher total QALYs.
Analysis methods
The economic analysis will address methodological issues that are particularly relevant to the VenUS IV study. These include censoring of costs and outcomes data, potential cluster (centre) effect and competing risks, and ulcer recurrence. A brief description of the current literature on methods to deal with (some or all) the issues identified is presented below; the preferred approach is then discussed in detail.
Overview of methods
Censoring of costs and outcomes
The cost-effectiveness and cost–utility analyses will use patient-level information on healing and costs/utility accumulated over the period of the study. Participants whose ulcers have not healed at the end of follow-up, or who died or were lost to follow-up before healing took place, will be treated as censored observations. Those participants lost to follow-up (before or after healing) will also have incomplete information on costs/utility. Furthermore, when time to healing is observed, costs/utility data may be incomplete if patients are censored beyond the point of healing and before the end of follow-up period. Thus, censoring makes direct estimation of mean time to healing, mean costs and mean QALYs impossible for the two treatment arms. Below we review the methods used in the literature to address censored costs and outcomes.
Various methods have been proposed in the literature to address censoring of costs and outcomes in an economic analysis. Naive approaches include the use of a full sample estimator and an uncensored case estimator. The former approach is based on taking a simple mean of the observed costs and outcomes for all individuals in the sample, irrespective of whether individuals were censored or not. This approach is bound to underestimate total costs and QALYs as costs and outcomes beyond censoring are not observed. The uncensored case estimator uses data only from patients who were uncensored during the study interval. This approach is biased towards the costs and outcomes of patients with shorter time to event. Hence, the naive approaches are inappropriate for handling censored data. 173
A further potential approach is the use of standard survival analysis methods. These methods treat time to event as the random variable of interest, and censoring time as the respective censoring variable to estimate non-parametric Kaplan–Meier survival curves. 174 When analysing costs, the total cost incurred is the random variable of interest and cost at censoring time is the censoring variable. However, standard survival analyses assume independence of the response variable and the respective censoring variable, i.e. censoring is assumed to be non-informative. However, for costs and quality-of-life data this is usually not true. 175,176 The primary reason is that individuals accrue costs at different rates. For instance, individuals in poor health tend to accumulate costs at much higher rates and, in turn, have higher cumulative costs at censoring time and event time than those in better health. 80 Different rates of cost accumulation (resource use) lead to dependent censoring in costs with independent censoring in time. 13 Censoring costs are hence informative of the latent survival costs, even if censoring is completely independent of the survival time.
Another potential approach to address the challenge posed by censored data is to use imputation methods to estimate costs and QALYs beyond the observed period. Imputation would require fitting parametric survival curves with appropriate distribution functions to the data observed during the study period. 177 The approach is based on extrapolation of the modelled survival distribution into the future and assumes that the whole sample will eventually experience the event (i.e. heal) at the rate dictated by the fitted distribution. However, unrealistic survival times may be imputed particularly when a large proportion of survival times are censored. As a result, we do not consider this approach to be the most appropriate to address the censoring issue at hand.
Other approaches that are commonly used in the literature include non-parametric weighted mean estimators proposed separately by Lin et al. 175 and Bang and Tsiatis. 178 Both include alternative estimators for situations when patient cost histories are available (Lin1175 and Bang and Tsiatis-Partitioned178) and when only total costs are observed at the end of the individual’s observation period (Lin2175 and Bang and Tsiatis-Simple178). These estimators assume independence between time to event and censoring time but not between costs at different time points for a particular patient. They further assume that patients are not censored in the study because they have accumulated unusually high or low costs. Both Lin175 and Bang and Tsiatis estimators work by dividing the study period into k small intervals. Lin175 estimators then calculate the sum product of Kaplan–Meier probability of survival to the start of each interval k (Lin 1175) or the probability of experiencing the event during each interval k (Lin2175) and the mean cost during the interval. Lin1175 assumes that censoring occurs at only the endpoints of the intervals. This condition is a potential limitation of the estimator, which was subsequently addressed by Bang and Tsiatis estimators. Both Lin1175 and Lin2175 have been found to be consistent when censoring occurs either at the start or the end of an interval. 179
The Bang and Tsiatis estimators are based on IPW as proposed by Bang and Tsiatis. 178 Bang and Tsiatis-Simple178 uses cost information from uncensored individuals only, hence censoring is allowed to occur anywhere during the interval. It works by weighting each complete cost observation by the inverse of the probability of not being censored during the interval. Bang and Tsiatis178 also proposed the Bang and Tsiatis-Partitioned estimator178 to be used when complete cost histories are available.
Recent studies have demonstrated that Lin2175 and Bang and Tsiatis-Simple estimators178 are less efficient, more unstable and sensitive to the level of censoring than Lin1175 and Bang and Tsiatis-Partitioned estimators178 respectively. 173,179,180 However, when cost histories are not available, the Lin2175 175 and BT-Simple estimators178 represent the preferred approach. When cost histories are available, both Lin1175 and Bang and Tsiatis-Partitioned estimators178 are found to perform well. However, when these two estimators are compared, the Bang and Tsiatis-Partitioned estimator178 is preferred, as it is not restricted by the pattern of censoring distribution. 173
An important limitation of the non-parametric weighted estimators is that they do not directly allow for covariate adjustment. Hence, any imbalance in the baseline characteristics, including utility levels, cannot be controlled for using a non-parametric weighting approach alone. However, parametric applications of these estimators have been demonstrated in the literature. 81,181 Of particular note is Lin’s IPW estimator181 that uses IPW approach for each cost interval as used by both BT estimators. However, unlike BT estimators that are non-parametric in nature, Lin’s IPW estimator181 uses regression models to estimate the treatment effect while weighting the observations by IPW method to account for censored observations. This regression approach allows controlling for covariates. Lin’s first IPW estimator181 was applied to linear regression models. However, it is well known in the literature that cost and QALY distributions are usually skewed. 182 Lin80 later proposed the same IPW approach using generalised linear models (GLMs). GLM is a flexible generalisation of the ordinary least squares that allows distributions other than the Gaussian distribution to fit the empirical cost and outcome data.
Cluster (centre) effect
When patients in a study are recruited from multiple centres, there is a potential for heterogeneity of treatment effects that vary across centres. Gomes et al. 82 evaluated the relative performance of the following analytical methods for cost-effectiveness analysis of cluster randomised trials: seemingly unrelated regression (SUR) with and without robust standard errors, generalised estimating equation (GEE) with robust standard errors, a two-stage non-parametric bootstrap method and a multilevel model (MLM). The study found that SUR without robust standard errors performed poorly compared with the models that accounted for cluster effect. In case of fewer clusters (< 20), the GEE and SUR (with robust SE) performed badly. MLM was suggested to be the most appropriate method for a wide range of circumstances with clustered data.
Statistical methods for the analysis of VenUS IV data
Based on the review of statistical methods for the economic analysis of censored data, the following methods will be used for the cost-effectiveness and cost–utility analysis of VenUS IV.
The VenUS IV study is recruiting patients from multiple centres across the UK. The statistical and economic analysis need to be aware that there may be heterogeneity of treatment effects across centres. This may be due to variation in bandaging skills, as some centres may have more experience with the use of four-layer bandaging than others. Furthermore, there may be other unknown centre-level effects that may be correlated with the treatment effect. Hence, for the economic analysis of VenUS IV study, we will be using MLM with patient and centres specified as the two levels of the analysis. MLM can be specified within the GLM framework using multilevel GLM (MGLM). To deal with censored costs and outcomes in the study, we will use the IPW weighting method of Lin. 80 Hence, in summary, our preferred approach is to use inverse probability weighted multilevel generalised linear model (IPW-MGLM).
During the analysis, we will explore whether the cost and outcome equations can be programmed as a system of bivariate or trivariate equations to allow for error correlation between equations. The analysis will be conducted using Stata version 10.1 or a later version. In case of software limitations preventing such analyses, the required cost and outcomes equations will be estimated independently as univariate equations followed by estimation of uncertainty using non-parametric bootstrap method.
Although the primary event of interest in VenUS IV study is healing of the reference ulcer, other events may compete with the primary event of interest. The primary competing event in the current case is death, i.e. patients who die will not heal. Hence, death is a competing event and is also informative about the event of interest. However, based on previous VenUS studies, we know that death is a rare event within the study interval (two deaths reported by the end of April 2011). Hence, death can be treated as a censoring event as it was treated in the previous VenUS analyses. Similarly, recurrence of the healed ulcer will be recorded during the study period. We anticipate that the impact of recurrence of ulcer will be reflected via increased costs and reduced utility level.
Data validation
The within-trial economic analysis will use the validated data set produced for the statistical analysis of the RCT. Data on health services resource use and quality of life (EQ-5D) will be separately validated for the economic analysis. Further validation of the data will be undertaken as follows:
-
Participants for whom there are no cost and outcome data other than baseline will be considered as censored 1 day after randomisation.
-
Following data reporting validation, decisions will be made regarding queries about participant-reported health services resource-use data.
Missing data
Missing data (other than censoring) will be evaluated to assess the occurrence of specific patterns. If needed, missing data will be handled through the most adequate methodology in line with the statistical analysis plan of VenUS IV study. Missing data on covariates will also be evaluated and, if necessary, appropriate imputation methods will be used.
Costs
Analysis will be carried out from the perspective of the NHS and PSS. Data on resource use will be collected for the entire duration of the trial for each participant. Costs will be calculated for each trial participant as the product of resource units used and the relevant unit cost.
The following types of resource use will be used for cost estimation.
Costs of:
-
treatments applied (trial or non-trial treatments)
-
GP and nurse visits (cost/per average length of visit)
-
outpatient visit and hospital inpatient stay.
Information regarding resources equally probable to be used in the two arms of the trial will not be considered in the economic analysis, as their effect is expected to annul in the incremental analysis. 7
Individual participants’ resource use
Resource-use information will be available from study data record forms for each patient and will also be collected from patients at each visit during the treatment of venous leg ulcer. For each participant the relevant resource-use information will include:
-
The type and number of trial treatments (source of data: trial application booklets completed by treating health professional)
-
The type and number of non-trial treatments (trial application booklets: completed by treating health professional)
-
The number of nurse visits for leg ulcer treatment (application booklets completed by treating health professional)
-
Dressing types (application booklets completed by treating health professional)
-
Number of GP consultations at doctor’s surgery or home because of leg ulcer or other reasons (self-reported in quarterly questionnaires)
-
Number of nurse consultations at doctor’s surgery or home because of leg ulcer or other reasons (self-reported in quarterly questionnaires)
-
Number of doctor consultations in a hospital outpatient clinic or other location because of leg ulcer or other reasons (self-reported in quarterly questionnaires)
-
Number of nurse consultations in a hospital outpatient clinic or other location because of leg ulcer or other reasons (self-reported in quarterly questionnaires)
-
Number of hospital admissions without overnight stays because of leg ulcer or other reasons (self-reported and data collected from nurses will be compared to establish the most accurate)
-
Number of nights in hospital for inpatient stay because of leg ulcer or other reasons (self-reported and data collected from nurses will be compared to establish the most accurate)
-
Other costs over £5 in value, i.e. diagnostic tests will be costed on ad hoc basis.
Unit costs
The mid-year of the trial, i.e. 2011, will be used as the year of pricing. Unit costs associated with resource use in each treatment arm will be estimated based on the appropriate version of NHS reference costs database183 and Personal and Social Services Research database. 168 Unit costs of treatment products will be obtained from the appropriate edition of British National Formulary (BNF). 184 In case the information is not available in the BNF database, product costs will be obtained from the manufacturer.
Results
The results section will summarise the costs incurred and health benefits in terms of time to healing and individual level utilities for the two treatment arms. Cost-effectiveness and cost–utility analyses will be presented along with estimates of uncertainty. These are briefly discussed below.
Total costs
Descriptive statistics for cumulative costs will be summarised by trial arm at 3, 6, 9 and 12 months’ follow-up. Cost distributions will also be evaluated for total costs at 12 months. The IPW-MGLM regression (discussed above) will be used to estimate the mean cost difference between treatment arms while controlling for covariates and accounting for censoring.
Health benefits
Health benefits will be considered in two ways, one being the time required for the reference ulcer to heal (for cost-effectiveness analysis) and second being the change in individual-level utility estimates over the study period (for the cost–utility analysis).
Time to healing
Health benefit will be defined as the difference in mean time to healing of the reference leg ulcer. Death will be treated as a censoring event. As above, the IPW-MGLM regression will be used to estimate the mean difference in time to healing between treatment arms while controlling for covariates. The analysis will be conducted on an intention-to-treat basis.
Participant utilities
Changes in patient utility level will be measured using the EQ-5D questionnaire, which evaluates patient’s quality of life on the following five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The EQ-5D questionnaire will be administered at baseline, and also at 3, 6, 9 and 12 months, to assess utility level at each time point. All EQ-5D data will be rechecked to ensure that all scores are sensible. The utility scores will be weighted, based on the UK social tariffs obtained by elicitation of health preferences through time trade-off in a large sample of the general population. 176 Thus, EQ-5D scores will be used to estimate QALYs for each patient over the 12-month period and evaluated within the cost–utility analytical framework. Adjustment will be made for baseline EQ-5D data.
Mean EQ-5D scores will be presented for each follow-up time point by trial arm. Differences in the utility score at baseline must be considered to adjust QALY between group differences. As above, we will use IPW-MGLM approach to control for covariates and account for censoring.
Cost-effectiveness and cost–utility analyses
Cost-effectiveness of compression hosiery compared with compression bandaging will be expressed in terms of ICER for cost per ulcer-free day. Point estimate of the ICER will be computed as difference in costs and difference in time to healing for the two treatment arms, using the regression approach discussed above. Uncertainty around the costs and effects will be presented using incremental cost-effectiveness planes. To evaluate decision uncertainty cost-effectiveness acceptability curves will be presented, which will evaluate the probability of compression hosiery being more cost-effective than compression bandaging.
Cost–utility analysis will be conducted in a similar manner as the cost-effectiveness analysis with QALYs being the measure of health benefit.
Sensitivity analysis
Sensitivity analysis will be considered in line with the statistical analysis plan. Hence, first we will consider undertaking the trial-based economic analysis with and without the centre effect. Second, the analysis will also consider multiple imputation of model covariates. Further sensitivity analysis will also be considered, including investigating the impact of assumptions around resource use and unit costs.
Decision model
Trials are designed to compare two or more alternative treatments. In general, however, it is not practically possible for one experimental study to compare all available treatment options. This means that, for a decision-maker, the information provided by head-to-head trial comparisons can be limited and partial – they still need to know which treatment option is the most cost-effective one among all treatments of interest. This limitation of head-to-head trial comparison can be overcome if available evidence from multiple sources can be used. Decision-analytic models provide a structure within which evidence from a range of sources can be synthesised to describe a specific problem, and through this framework overall costs and effects can be estimated. The advantage of using this framework is that the cost-effectiveness results can be based in all available evidence, across the full range of possible alternative interventions and clinical strategies, over a relevant time horizon and for specific patient groups. 185
Decision problem
We will construct a decision-analytic model to allow estimation of the cost-effectiveness of all relevant high-compression treatments, including 4LB and hosiery, for venous leg ulcers. The analysis will include two health outcomes: a measure of ulcer healing and QALY. The choice of healing measure (e.g. time to healing or healing rate) will depend on data availability. As mentioned above, a QALY is defined as a year lived with full health, calculated by multiplying quality of life and length of life. Again, the choice of score for health-related quality of life (or utility) for QALY calculation – for instance scores measured by EQ-5D or Short Form questionnaire-6 Dimensions (SF-6D) – is subject to data availability. Cost will be presented in UK pounds sterling. The mid-year of the trial, i.e. 2011, will be used as the year of pricing. The time horizon will be defined based on data availability and if extrapolation is used relevant assumptions will be evaluated. The study population comprises patients aged > 18 years with at least one venous leg ulcer, and are able and willing to tolerate high compression. The analysis will be conducted based on the perspective of the NHS and PSS, and findings are expected to inform decision-makers on which treatment to adopt for use in the NHS, out of a set of alternatives.
Identifying the treatments of interest
Treatments considered within this evaluation should be those with the aim of achieving ‘high compression’, defined as ‘ankle sub-bandage pressure of 35–40 mmHg’. High compression is one of the key factors for healing venous leg ulcers. 19 The second consideration is that treatments of interest included in this analysis should be relevant to the UK decision-making context. However, treatments that are not currently used in general practice may ‘possibly’ be cost-effective and should be considered in the analysis.
We will identify compression treatments for venous leg ulcer from (1) the Cochrane review ‘Compression for venous leg ulcer’,19 in which all RCTs investigating the effectiveness of compression treatments for venous leg ulcer are recorded (planned update in 2011, these data will also be included); (2) the most recent BNF,184 in which all current practices in the UK are listed; and (3) clinical experts who might provide relevant alternative treatments to be considered for which no data exist. We will also check trial database records to check ongoing or planned evaluation of any new intervention types. Given that high compression is the main criterion for selecting treatments of interest, a survey will be conducted to establish what treatments identified in the above formulary and review aim to deliver ‘high compression’, as there are likely to be a number of ad hoc treatments about which we are not clear about. A group of experts, clinicians and nurses will be invited to give their opinions. The treatments of interest will be those treatments that have been classified as high compression. We will also work with clinicians to place individual treatments into meaningful treatment groups (i.e. 4LB, SSB, high-compression hosiery, etc.).
Defining the model structure
A systematic literature review will be conducted to identify published economic evaluations using decision analytic models regarding the treatment and/or prevention of venous leg ulcers. The purpose of the review is to provide information of the current developments in the field in terms of model structure and assumptions used in the analyses, and to prepare for potential issues that the project might encounter.
The systematic literature review will be conducted by two independent reviewers according to agreed screening criteria:
-
Does the study consider venous leg ulcers?
-
Is the study a full economic evaluation (i.e. includes costs and benefits)?
-
Does the study use any kind of model as a method to represent disease progression?
Only studies that meet all of the above criteria (agreed by both reviewers) will be extracted and reviewed. The information extracted will include treatment comparator, study population, model design, model assumption, effectiveness, utility, cost and uncertainty. The findings of this review will be used to inform the model structure.
Obtaining data to inform model
The model will be informed by a variety of parameters, such as data on effectiveness of over healing and utilities. Procedures to obtain data and to synthesise available evidence are detailed below.
Effectiveness
Rather than conduct a separate review of effectiveness data we will utilise the Cochrane Review19 – This review was conducted in 2009 and will be updated (in 2011) to include additional evidence of six recent studies. The aim of the review is to evaluate the clinical effectiveness of compression bandage or stocking systems in the treatment of venous leg ulceration. This review will serve as the major source of data on the effectiveness of alternative treatments (AD).
Evidence synthesis on effectiveness
At this stage, we know from the Cochrane review19 that multiple trials evaluating the clinical effectiveness of high-compression treatment on venous leg ulceration exist. We will use a MTC approach186 to estimate the relative effectiveness multiple treatments by synthesising the existing evidence base of trials. When trials have common comparators, this process allows relative effectiveness estimates to be made for treatments not compared in head-to-head trials (i.e. indirect evidence) while maintaining the randomisation of each trial. In this way, all available evidence can be used to estimate treatment effects for pairs of treatments that have, at least, indirect evidence (i.e. for comparisons that have not been examined in any trial). Currently, there is a lack of direct (head-to-head) evidence in the literature on the relative effectiveness of hosiery in relation to 4LB. Using an MTC approach, the relative effectiveness between hosiery and 4LB can be estimated based on available indirect evidence. The direct evidence will be provided once the VenUS IV is completed (details of the inclusion of VenUS IV data in the model are outlined below).
In defining the MTC model, we will aim to combine data from studies for which IPD is available to the authors (e.g. VenUS I)7 with aggregate data identified from studies in the Cochrane review. We also aim to incorporate (instead of exclude) trials that report alternative measures of healing (e.g. proportion of patients healed or time to healing), by appropriately modelling these within the MTC. Inferences will be obtained using Bayesian methods. If necessary, the sensitivity to prior distributions and the validity and consistency of the MTC will be explored. 187
Other model parameters
Other parameters possibly required for the model include utilities, resource use and cost parameters, and death rates. Current evidence for these parameters, for different disease states (as defined in the model) will be identified from the literature, if possible, we will work with the Centre for Reviews and Dissemination to conduct these searches. However, if it is not feasible to obtain all the information from the literature or if there is no evidence available then data extracted from the IPD sources (VenUS I7 and VenUS IV studies) may be used.
Methods of analysis
To simultaneously address several of the elements included in decision-analytic modelling, i.e. evidence synthesis based on MTC, estimation of other model inputs and evaluation of uncertainty, we will construct a comprehensive probabilistic decision-analytic model evaluated using Markov chain Monte Carlo simulation implemented in the specialist software WinBUGS. The model outputs will be the estimated expected mean costs, effectiveness, and QALYs associated with each alternative treatment. Estimated total costs and outcomes will be discounted properly according to the latest guidance of health technology appraisal.
Decision uncertainty
Uncertainty regarding cost-effectiveness will be evaluated using probabilistic sensitivity analysis, where inputs into the analysis are defined as probability distributions that reflect uncertainty. The estimated mean QALYs and costs associated with each treatment option will be combined with a feasible range of values for decision-makers’ willingness to pay (λ), to obtain distribution of net benefits at different levels of λ. The uncertainty surrounding the decision to adopt a given treatment option as a cost-effective treatment as different levels of willingness to pay will be represented in acceptability curves (CEACs). CEACs are a graphical representation of the probability of an intervention being cost-effective (on the vertical axis) for a range of willingness-to-pay values λ (on the horizontal axis) associated with the health outcome of interest. 188
Scenarios and sensitivity analysis
Scenario analyses Two scenarios will be investigated regarding the estimation of differential inputs (model parameters). First, all model inputs will be defined based on evidence collected from the literature, excluding the findings from VenUS IV (pre VenUS IV analysis). A second scenario will incorporate the finding from VenUS IV alongside all of the evidence from the literature as described above (post VenUS IV analysis). In the latter, the IPD data on effectiveness from VenUS IV will be incorporated in the existing MTC. Other parameters for which VenUS IV evidence is available will also inform the model, alongside existing evidence.
Sensitivity analysis The impact of assumptions undertaken in the analysis regarding the evidence over parameters or relating to the decision model (such as extrapolation) will be evaluated, if possible.
Value of further research
As part of this analysis we will conduct a value of information analysis. Uncertainty around treatment decisions means that in many cases there is always a chance that the ‘wrong’ decision will be made. 162 With estimates of probability of error and the opportunity cost of error, the expected cost of uncertainty or the expected opportunity loss surrounding the decisions can be calculated. This is also known as the EVPI and can be used to indicate whether further research is potentially worthwhile. It would also be useful to have indication of what type of additional evidence would be most valuable. Therefore the expect value of perfect information for parameters is calculated to identify those parameters for which more precise estimates would be most valuable.
List of abbreviations
- 2LB
- two-layer bandage
- 4LB
- four-layer bandage
- ABPI
- ankle–brachial pressure index
- AD
- aggregate data
- AIC
- Akaike information criterion
- BMI
- body mass index
- BS-21
- 21-point Box Scale
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPH
- Cox proportional hazards
- CrI
- credible interval
- df
- degrees of freedom
- DIC
- deviance information criterion
- EQ-5D
- European Quality of Life-5 Dimensions
- EVPI
- expected value of perfect information
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HH
- two-layer hosiery
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- IPW
- inverse probability weighting
- IQR
- interquartile range
- LMM
- linear mixed model
- MCS
- Mental Component Summary
- MTC
- mixed-treatment comparison
- NB
- net benefit
- NICE
- National Institute of Health and Care Excellence
- NMB
- net monetary benefit
- NSAE
- non-serious adverse event
- PCS
- Physical Component Summary
- PH
- proportional hazards
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SSB
- short-stretch bandage
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VEINES-QOL
- VEINES quality-of-life questionnaire
- VenUS
- Venous Ulcer Study
- VenUS IV
- Venous leg Ulcer Study IV