Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/12/01. The protocol was agreed in July 2012. The assessment report began editorial review in May 2013 and was accepted for publication in November 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Hartwell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the underlying health problem
Hepatitis C is a disease of the liver arising from the blood-borne hepatitis C virus (HCV). It is a slowly progressing disease which has two main phases of infection: acute and chronic. The period immediately after HCV infection is the acute phase. In some people the virus will be cleared spontaneously during this phase, with the remainder developing chronic infection. Chronic HCV, defined as infection persisting for more than 6 months, is the focus of this assessment.
Hepatitis C virus is a ribonucleic acid (RNA) virus which has six genetic variations, known as genotypes. There are six major HCV genotypes (genotype 1, 2, 3, etc.), and within these there are several subtypes (labelled a, b, c, etc.). The prevalence of the genotypes varies considerably between countries, with the most prevalent groups in England and Wales being genotypes 1 and 3 (representing at least 90% of infections). 1–3 Of these, genotype 3a is the most common with a prevalence of 39%, followed by genotype 1a with a prevalence of 22%. 3 Response to treatment is strongly influenced by HCV genotype (see Current service provision).
Chronic HCV infection can be categorised as mild, moderate or severe according to the extent of damage to the liver. This is based on both the level of fibrosis (scarring) in the liver and the degree of inflammation and destruction of liver cells (necroinflammation) (discussed further in Diagnosis and staging). Many children and young people with chronic HCV infection are asymptomatic although symptoms may occur later in the disease when liver damage has progressed.
Aetiology
Hepatitis C virus is acquired primarily through exposure to contaminated blood. In adults in the UK, the most common source of infection is through the sharing of injecting equipment in intravenous drug misuse. This accounts for around 90% of cases. 3 Other sources of HCV infection include needle stick injury, tattooing and body piercing, and treatment with contaminated blood products (prior to the introduction of blood screening in the mid-1980s). The risk of sexual transmission is thought to be low. 3
In children, mother-to-child (‘vertical’) transmission is the primary reason for HCV infection, with perinatal transmission being the most important route, and to a lesser extent, intrauterine transmission. 4,5 The rate of perinatal transmission from a HCV-infected mother to her child ranges from 2% to 5%. 6,7 Breast feeding does not appear to increase the risk of HCV transmission, even though HCV RNA may be detected in breast milk and colostrum. A number of factors may change the risk of mother-to-child transmission. There is an increased risk of transmission depending on the level of maternal viral load and whether or not the mother is also co-infected with human immunodeficiency virus (HIV). 8 A systematic review of 77 studies published in 2001 showed that the rate of mother-to-child transmission was in the region of 5% from women without HIV infection and 22.1% from women with HIV infection. 9
Epidemiology
Estimates based on laboratory surveillance by the Health Protection Agency (HPA)10 in the UK suggest that around 216,000 individuals were chronically infected with HCV in 2011. The prevalence of HCV in children of all ages is unclear and difficult to establish. The HPA report estimated that 26 children aged 1 year or below, 21 young people aged 1–14 years, and 439 people between the ages of 15 and 24 years were newly diagnosed with HCV in England in 2010. Among those aged 15–24 years, many cases of HCV will be acquired through injecting drug use, which often begins in late adolescence and early adulthood. 10
Published population-based studies range in their estimates, in part owing to many studies having small, and in some cases, unrepresentative samples (e.g. antenatal screening can be selective), and thus vertical transmission may be undetected in some. Estimates generally suggest that the prevalence of HCV in children in developed countries is between 0.1% and 0.4%. 4,7,11 In some populations this may exceed 10% (e.g. in some regions of Saudi Arabia and Africa). 7 Estimates of regional prevalence rates in pregnant women in the UK range from 0.19% to 0.43%. 4 The prevalence of HCV genotypes in children is thought to be similar to that in adults given that the majority are infected by vertical transmission. Studies have shown that genotypes 1, 2 and 3 are the most clinically relevant groups in children with HCV, while genotype 4 is less prevalent. 7
Progression and prognosis
The natural history of HCV acquired during childhood is not completely understood, although the age at onset of HCV infection is thought to be an important factor in the long-term outcome. In children, spontaneous viral clearance tends to occur early in the history of an infection and is more likely before the age of 4 years. 12 Once established, chronic HCV infection tends to persist into adult life, although the associated liver disease may be asymptomatic. 12 In vertical transmission, estimates suggest that somewhere between 2.4% and 55% of children will spontaneously clear the infection, with the cumulative probability of progression to chronic HCV being approximately 80%. 4,7,12 Caution is required in the interpretation of these data, however, as most of the studies that these estimates come from have small numbers of children, with different ages at acquisition of HCV and different comorbidities. 4 Spontaneous viral clearance is thought to be dependent on genotype, with children infected with genotype 3 having a higher likelihood of clearance than those with genotype 1. 7,12
Chronic HCV is a slowly progressing disease that usually develops over a number of years, often decades. The severity of chronic HCV relates to the duration of infection, meaning that progression to advanced disease is less likely in children than in adults. 11 A recent systematic review6 evaluated the outcomes of untreated HCV in children from population-based screening studies. Results from 25 studies including 733 people infected with HCV as children showed that of the 180 (25%) who underwent a liver biopsy as adults, only 4% had developed liver cirrhosis, with no other individuals developing any severe adverse outcomes. The authors concluded that the majority of people with disease acquired during childhood have a mild degree of hepatitis and fibrosis during childhood. No clear risk factors for severe adverse outcomes were identified in the studies reviewed. The review conclusions were limited by the relatively short follow-up periods in most of the studies included. 6 Other studies suggest that the rate of advanced liver fibrosis or cirrhosis seen on liver biopsy in children with chronic HCV infection is also relatively low, in the range of 2–6%. 7,11–15 According to clinical experts, no children have undergone liver transplantation because of chronic HCV infection in the UK. Despite the relatively innocuous nature of chronic HCV in children, clinicians believe that treatment during childhood is beneficial, as a definitive resolution of disease may be achieved in a subgroup of patients, treatment may reduce children’s social burden and factors may be more favourable for a response (e.g. a low viral load, less advanced disease). 7,16 In addition, clinical opinion suggests that children experience fewer side effects of treatment than adults.
Some differences in outcomes between vertically infected and parenterally infected children have been found. For example, in young children vertical transmission may be associated with higher levels of alanine aminotransferase (ALT), an enzyme that may be elevated in concentration if damage to liver cells has occurred. Overall, however, the mode of infection appears to have a relatively limited impact on outcomes, which reflect a slowly progressing disease. 17,18
Diagnosis and staging
The need for diagnostic testing in children is established by assessment of potential risk factors, such as HCV infection or drug use in the mother, or exposure to contaminated blood products or organ transplants. Diagnosis is undertaken using blood tests to detect HCV antibodies and HCV RNA. 4 Identification of HCV antibodies uses enzyme-linked immunosorbent assays or enhanced chemiluminescence tests, where test accuracy indices have been shown to be excellent. 19 In cases of suspected vertical transmission, this testing procedure should ideally be undertaken after the child is older than 18 months, because maternal antibodies can cross the placenta and persist for up to 18 months, leading to potentially unreliable test results. 16
A positive antibody test will be followed up with a test for the presence of HCV RNA in serum in order to determine active infection. 16,19 This is typically undertaken using a real-time polymerase chain reaction (PCR)-based test as these yield both sensitive and quantitative detection ranges. Recent clinical guidelines suggest that if undertaken in early infancy, a positive serum HCV RNA should be rechecked after 12 months of age to establish the presence of chronic HCV. 16 At this point, the determination of positive HCV RNA may indicate acute or chronic infection, and the clinician will use patient history of the timing between the test and the likely exposure to aid diagnosis. If a test for HCV antibodies is positive but a test for HCV RNA is negative, this could indicate a resolved infection, and testing would be repeated after 6 months for confirmation. 4
If chronic HCV infection is established, testing may be undertaken to establish the HCV genotype using a further PCR assay. In adults, evidence has shown that HCV infections with viruses of genotypes 2 or 3 are the most likely to resolve with therapy, whereas infections with viruses of genotypes 1a or 1b are less likely to respond. The determination of the genotype is therefore an important and useful means to establish treatment options including the timing and duration of treatments, and once this is known it may be followed up with a liver biopsy to determine the extent of any liver fibrosis. 16,19 In children infected with the more responsive genotypes 2 or 3, treatments may commence without the need to test for the extent of liver fibrosis because the benefits of treatment are likely to outweigh the risks. In those with genotypes 1a and 1b, however, the extent of liver fibrosis will be used to weigh up the benefits and risks of treating immediately versus waiting. 16 For some children who have been vertically infected the biopsy may be delayed until age 8–10 years, as evidence of the natural history shows that fibrosis is unlikely to occur until at least this age. 19
In children who have undergone a liver biopsy, chronic HCV infection may be classified as mild, moderate or severe based on histological appearance. To determine the severity, two components of the liver biopsy sample are assessed: fibrosis (scarring) and necroinflammation. 20 The extent of fibrosis is expressed as a ‘stage’ ranging from no fibrosis to cirrhosis in its severe form. Cirrhosis can progress from a compensated state, where the liver is still functioning despite the fibrosis, to a decompensated state where the functioning of the liver is seriously impaired. The extent of necroinflammation of the liver is expressed as a ‘grade’ of disease activity which relates to the rate at which the disease stage is changing. A weakness of the histological classification is that it does not differentiate the clinical process of decompensation (compensated or decompensated liver function can occur at the same stage of fibrosis or cirrhosis), so the fibrosis stage score may not necessarily increase as decompensation occurs. Inflammatory activity in the liver can increase or decrease, or remain constant, during the disease process. 20
There are a number of commonly used systems for classifying liver biopsy samples. The three most commonly used are the Knodell histological activity index (HAI), the Ishak revised HAI and the METAVIR system. Knodell and colleagues’ system21 comprises four components; one classifies the amount of fibrosis [scored from 0 (no fibrosis) to 4 (cirrhosis)], and three the extent of necroinflammation (periportal and/or bridging necrosis; intralobular degeneration; and portal inflammation, with a combined maximum score of 18). The maximum combined score is therefore 22, where higher scores reflect more severe disease.
A revision of the HAI, primarily for use as a research tool, was published in 1995 by Ishak and colleagues. 22 The revised system applies five components. Four of these measure components of necroinflammation grading – periportal or periseptal interface hepatitis, confluent necrosis, focal (spotty) lytic necrosis, apoptosis and focal inflammation, and portal inflammation – with a maximum score of 18. The fifth relates to fibrosis staging with a maximum score of 6. The maximum score is therefore 24.
The METAVIR system was developed specifically for use in HCV and again scores the fibrosis stage and the necroinflammation grade. 23 Fibrosis is scored from 0 to 4, from no fibrosis to cirrhosis or advanced scarring. Necroinflammation is scored on a scale of 0 (no histological activity) to 3 (severe activity). The maximum score is therefore 7. The METAVIR system is the most widely used system in clinical trials of antiviral treatment in chronic HCV and is considered to be the most validated instrument currently available. 20
Impact of disease
Many children infected with HCV appear to be clinically asymptomatic or show only mild, non-specific symptoms (e.g. fatigue, flu-like symptoms, nausea). 6,7,16 As mentioned above (see Progression and prognosis), a small proportion of patients with chronic HCV will develop significant liver disease during their childhood. A retrospective study of 246 patients on the UK HCV National Register Database13 found that when patients who were infected with HCV before the age of 16 years reached their late teens, some had started to show signs and symptoms of liver disease, including enlarged liver (43 patients), enlarged spleen (20 patients), visible blood vessel abnormalities (spider nevi) (four patients), abdominal fluid retention (ascites) (three patients), jaundice (three patients), bleeding oesophageal varicose veins (varices) (one patient) and itching (one patient). Many of the patients on the database had comorbidities and, overall, those who developed signs and symptoms of liver disease were found to be statistically significantly more likely to have had underlying medical conditions in addition to HCV infection. 13
Another study, based on medical records submitted by 12 paediatric and infectious diseases centres in Italy, investigated outcomes for 504 children who were infected with HCV before the age of 16 years but who did not have comorbid viral, autoimmune, metabolic or haematological disorders or malignancy. 12 The majority of children had non-specific, transient and mild symptoms at the time of diagnosis. However, six (1.8%) went on to develop signs and symptoms of advanced liver disease, including weakness (asthenia), nosebleed, itching, ascites and gastrointestinal bleeding, with a mean duration of HCV exposure from putative time of exposure to diagnosis of cirrhosis of 9.9 years. 12 However, these data should be interpreted with caution, as they are from retrospective studies where the methods of population selection and data capture are unclear. Transplant would be offered for children with end-stage disease with significant complications of cirrhosis, including variceal bleeding and refractory ascites, those with decompensated liver function (coagulopathy and encephalopathy) or those who develop hepatocellular carcinoma. However, these are rare in children with HCV infection without any other comorbidity and, as mentioned above, no children in the UK are thought to have undergone liver transplantation as a result of chronic HCV.
Evidence from adult populations suggests that chronic HCV infection eventually leads to impairment in quality of life (QoL), even in the absence of liver inflammation, with patients feeling unwell in terms of both their physical and mental health. 24,25 However, information on the impact of infection with chronic HCV on children’s QoL is very limited and it is difficult to draw clear conclusions from the available evidence. A small study on 19 HCV-infected children in Australia concluded that physical and psychosocial summary scores from validated self-reported and parent-reported questionnaires were significantly lower in infected than in non-infected children. Children reported reduced physical functioning but were otherwise less concerned than their parents about future health. 26 Another study on 114 treatment-naive HCV-infected children used validated questionnaires to elucidate the behavioural, emotional and cognitive functioning of the children and their caregivers. 27 Children with HCV had significantly lower cognitive functioning scores than a normative sample, and some caregivers were found to be highly distressed by their children’s medical circumstances, which limited family activities. However, the authors concluded that overall QoL was not impaired in children with chronic HCV infection. 27,28
In adults, chronic infection with HCV is recognised as a social stigma29 and it has been suggested that children chronically infected with HCV, and their families, experience the burden of social stigma,8 although to date this does not appear to have been analysed quantitatively. According to clinical experts consulted during this technology assessment, parents often carry immense guilt, especially mothers, if they have transmitted a HCV infection, and disclosing the diagnosis to their child can also be a huge burden. Children with HCV may experience stigma as a result of carrying an infection that they may later transmit; inappropriate segregation that can arise because of ignorance; and having a virus that may be perceived as related to negative social factors such as drug use and HIV.
Childhood infection with HCV has been estimated to increase the risk of liver-related death 26-fold. 30 There is a significant economic impact of paediatric HCV infection; projected 10-year costs associated with paediatric HCV infection in the USA (arising from the costs of screening, monitoring and treatment) have been estimated at $199–335M. 31 We are not aware of any cost data for the UK.
Current service provision
Treatment of chronic HCV is aimed at eradicating the virus and preventing related complications. Accordingly, the main goal of treatment is to clear HCV and achieve a sustained virological response (SVR), defined as undetectable HCV RNA in the serum at least 6 months after treatment ends. Successful treatment reduces the rate of progression of liver fibrosis and related complications and improves QoL for patients. Some baseline factors are known to be predictive of a greater likelihood of achieving a SVR, such as early virological response (EVR) [measured 12 weeks after therapy commencement and defined as a negative HCV RNA (complete EVR) or a minimum 2 log10 drop in HCV RNA levels (partial EVR)]. Other factors include having genotype 2 or 3 (as stated previously), mild disease and low viral load.
Beyond the age of 4 years, most children and young people with chronic HCV are unlikely to clear the virus spontaneously and should be assessed for antiviral treatment. It is recommended that children diagnosed with HCV are referred to, and managed in conjunction with, a paediatric hepatologist at one of three specialised paediatric hepatology centres in the UK:4 London, Birmingham or Leeds. Shared-care pathways are well established in the UK, with treatment and overall care delivered outside the three specialist centres at joint clinics. Specialist hepatology nurses are also involved, particularly in the administration of antiviral treatment.
Optimal therapy for children with chronic HCV is not clearly defined because of the lack of efficacy data in children. 4,11 Published National Institute for Health and Care Excellence (NICE) technology appraisals32–34 on the treatment of chronic HCV recommend treatment for any severity of disease but relate only to adults. There is currently no NICE guidance for the treatment of hepatitis C in patients younger than 18 years. The 2006 Scottish Intercollegiate Guidelines Network (SIGN) guidelines on the management of hepatitis C recommend that children with moderate or severe HCV should be considered for treatment with a combination of peginterferon and ribavirin (RBV), while the benefits of treatment for those with mild HCV should be weighed against the risk of treatment side effects. 35
In current clinical practice in the UK, all children over 4 years of age are considered for treatment, with selection not based on histological severity. Treatment is rarely given to children under the age of 4 years as they may still clear the virus spontaneously. At older ages, treatment may take into account school stage (e.g. avoiding school examination years) where possible. In those with mild disease, which is the majority, the decision to treat is based on genotype and the likelihood of response. Children with genotypes that respond more favourably to treatment (genotypes 2 or 3) are more likely to receive treatment, whereas those with genotypes 1 or 4 may receive a ‘watchful waiting’ approach, as long as there is no evidence of significant disease. Treatment of the minority who have severe disease is always considered more urgent, and treatment is more likely to be recommended. However, according to clinical expert opinion, it is rare for treatment of severe cases to be provided without considering the HCV genotype. Owing to the lack of current guidance, there may be variation in practice between the three specialist centres in the UK. At some centres, biopsy would not be used in patients with HCV genotypes 2 or 3 but may be considered for genotype 1 if it would help guide the treatment decision. For patients keen to be treated, biopsy would not be performed, whereas for patients preferring to wait, a biopsy may be deferred up to 10 years after infection.
For those children who are not treated, or where treatment is deferred, a best supportive care (BSC) approach is taken. A formal definition of BSC for children and young adults with chronic HCV is lacking. However, patients in this population are typically asymptomatic and it appears to be generally understood that BSC implies no active treatment. BSC may include watchful waiting, with 6-monthly reviews and monitoring of viral load and disease progression using blood tests for assessing HCV RNA or HCV antibodies, and ultrasound scans every 1–2 years. The definition of BSC as comprising no active treatment is consistent with the NICE scope and the manufacturers’ submissions (MSs) and is the definition employed in our economic analysis (see Chapter 5).
Two types of peginterferon alfa are available (see Description of technology under assessment), of which both are used in clinical practice in the UK, although the preferred form of the drug may vary between the treatment centres. The decision of when to treat is made on a case-by-case basis by the treating clinician in conjunction with the child or young person and/or his or her parent(s).
Description of technology under assessment
The intervention under review is dual therapy with peginterferon alfa and RBV. The peginterferons are cytokines whose mechanism of action is to assist the immune response by inhibiting viral replication. Two pharmacokinetically different forms36 are available: peginterferon alfa-2a (Pegasys®, Roche) and peginterferon alfa-2b [ViraferonPeg®, Merck Sharp & Dohme (MSD)]. RBV is a synthetic nucleoside analogue which is available in two primary forms, Copegus® (Roche) and Rebetol® (MSD). It is also available as a number of generic forms: Ribavirin BioPartners (BioPartners), Ribavirin Mylan (Generics UK) and Ribavirin Teva (Teva Pharma). At the time of writing, Copegus® was indicated for combination therapy only with peginterferon alfa-2a, while Rebetol® was indicated for combination therapy only with peginterferon alfa-2b.
Peginterferon alfa-2a was originally licensed in June 2002 and an extension to the licence to allow treatment in children and young people was granted in March 2013. In clinical practice, the dose used for children is 180 mcg/1.73 m2 body surface area (BSA), once weekly, administered subcutaneously (an injection beneath the skin). Peginterferon alfa-2b was originally licensed in May 2000, with the most recent extension to the licence for use in children granted in February 2012. The recommended dose for children is 60 mcg/m2 BSA, once weekly, administered subcutaneously. Treatment duration is recommended at 24 or 48 weeks dependent on genotype.
The two primary forms of RBV were licensed in November 2002 for Copegus® and May 1999 (oral tablets) and January 2005 (oral solution) for Rebetol®. The recommended dose of RBV is dependent on body weight and is 15 mg/kg/day for children and adolescents weighing < 47 kg. It is taken each day in two divided doses as an oral solution.
For peginterferon alfa-2b, the most recent therapeutic indication is the treatment of children and adolescents aged 3 years and older with chronic hepatitis C, without liver decompensation, who are positive for serum HCV RNA and who have not previously been treated. The licence for peginterferon alfa-2a is indicated for the same group of children and adolescents but for those aged 5 years and older. The marketing authorisations do not permit peginterferon monotherapy in this age group and treatment must be given in combination with RBV. Full details of the indications, dosages and duration of treatment are given in the summaries of product characteristics. 37–40
Clinical opinion suggests that, in the absence of any clear differences in clinical effectiveness, the choice of whether to use peginterferon alfa-2a or -2b may depend on whether or not the drug is licensed, how easy it is to accurately measure the dose (as dosing in children is weight based, requiring flexibility of dispensing) and local trust contracting arrangements (e.g. drug choice may be led by the adult service which treats a greater number of patients).
Chapter 2 Definition of the decision problem
This section states the key factors that will be addressed by this assessment in line with the definitions provided in the NICE scope.
There have been a number of technology appraisals (TAs) by NICE of peginterferon and RBV for the treatment of adults with chronic hepatitis C, addressing mild (TA10633) and moderate to severe (TA7532) HCV, with the most recent appraisal in 2010 focusing on specific patient subgroups that were affected by licence extensions (TA20034). All of these appraisals were supported by independent assessment reports conducted by Southampton Health Technology Assessments Centre (SHTAC). 20,41,42 Since publication of these three TAs, an additional extension to the licence for peginterferon alfa-2b has been granted, and an extension for peginterferon alfa-2a is undergoing consideration, to include those under the age of 18 years. The current health technology assessment (HTA) relates specifically to the treatment of children and young people.
The interventions included within the scope of this assessment are (1) peginterferon alfa-2a in combination with RBV; and (2) peginterferon alfa-2b in combination with RBV. The population as defined by the NICE scope is children and young people aged 3–17 years with chronic HCV, and encompasses all groups including those with HIV co-infection; all grades of severity of chronic HCV (mild, moderate and severe); and those who are treatment naive or, if appropriate, who have not responded and/or relapsed to previous treatments.
The relevant comparisons for this assessment are supportive care (including treatment without any form of interferon therapy) and the interventions compared with each other within their licensed indications. The outcomes under consideration include SVR (HCV RNA levels 6 months after treatment cessation), virological response to treatment (e.g. HCV RNA levels at treatment week 12 or at the end of treatment), biochemical response (changes in ALT levels), liver inflammation and fibrosis, mortality, adverse effects of treatment including effects on growth, and health-related quality of life (HRQoL). Fuller definitions of the outcomes are provided in Chapter 4, Assessment of effectiveness.
Overall aims and objectives of assessment
The aim of this HTA is to review the clinical effectiveness and cost-effectiveness of peginterferon alfa (-2a and -2b) in combination with RBV, within the licensed indications, for the treatment of chronic HCV in children and young people. The objectives are:
-
to undertake a systematic review of the clinical effectiveness and cost-effectiveness of peginterferon alfa in combination with RBV for children and young people with chronic HCV
-
to critique the MSs to NICE from Roche (peginterferon alfa-2a) and MSD (peginterferon alfa-2b) to identify the strengths and weaknesses of the respective submissions
-
to develop an economic model to establish the cost-effectiveness of peginterferon alfa in combination with RBV for children and young people with chronic HCV.
Chapter 3 Methods
The methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness were described a priori in a published research protocol. Peer-review comments were sought from our clinical advisory group as well as from NICE. Minor amendments were made as appropriate but no comments that identified specific problems with the methods of the review were received. The methods of the economic evaluation are detailed in Chapter 5.
Identification of studies for the systematic reviews of clinical effectiveness and cost-effectiveness
A search strategy was developed and refined by an experienced information specialist to identify all relevant studies investigating the two forms of peginterferon alfa with RBV in children and young people with chronic HCV. Separate searches were conducted to identify studies of clinical effectiveness, cost-effectiveness, resource use/costs, HRQoL and epidemiology. The search strategies are provided in Appendix 1. Searches for clinical effectiveness and cost-effectiveness literature were undertaken from database inception to November 2012. The searches were not restricted by study design or language. The strategies were applied to the following databases:
-
Cochrane Database of Systematic Reviews (CDSR)
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
Centre for Reviews and Dissemination (CRD) (University of York) databases: Database of Abstracts of Reviews of Effects (DARE), the NHS Economic Evaluation Database (NHS EED) and the HTA database
-
MEDLINE (Ovid)
-
EMBASE (Ovid)
-
PREMEDLINE In-Process & Other Non-Indexed Citations (Ovid)
-
Web of Science with Conference Proceedings: Science Citation Index Expanded (SCIE) and Conference Proceedings Citation Index – Science (CPCI) (ISI Web of Knowledge)
-
Bioscience Information Service (BIOSIS) Previews (ISI Web of Knowledge).
Bibliographies of retrieved papers were screened for relevant studies, and the MSs to NICE were assessed for any additional studies. Members of the advisory group who were contacted for advice and peer review were also asked to identify any additional published and unpublished references. All search results were downloaded into a Reference Manager database (Thomson ResearchSoft, San Francisco, CA, USA).
Other websites, including key hepatitis C websites and symposia, were also searched for completed or ongoing studies. These included ClinicalTrials.gov; Current Controlled Trials (CCT); UK Clinical Research Network Study Portfolio (UKCRN); HPA; Food and Drug Administration (FDA); Department of Health; Zetoc; Scirus; Hepatitis C Trust; World Hepatitis Alliance; British Association for the Study of the Liver (BASL); European Association for the Study of the Liver (EASL); British Liver Trust; British Society of Gastroenterology (BSG); Foundation for Liver Research; American Association for the Study of Liver Diseases (AASLD); Hepatitis C Scotland; Welsh Association for Gastroenterology and Endoscopy (WAGE); British Association for Liver Disease Nursing Forum (BASLNF); HIVandHepatitis.com; Cambridge Liver Symposium; and the British Viral Hepatitis Group (BVHG).
Inclusion process
Each reference identified by the clinical effectiveness search strategy was screened for potential eligibility on the basis of title and, where available, abstract, using the inclusion criteria detailed below. Screening was carried out independently by two reviewers and the full texts of potentially relevant studies were obtained for further assessment. Screening of full papers was performed in a two-stage process. Firstly, papers were screened according to the inclusion criteria for population, intervention and outcomes using an inclusion coding sheet (see Appendix 2). Papers that fulfilled these inclusion criteria were then screened on the basis of study design according to the hierarchy outlined below (see Study design). It was not anticipated that there would be much randomised controlled trial (RCT) evidence in this population group, and the two-stage process allowed an assessment of the different levels of evidence available while ensuring that all relevant studies were captured. Full papers were screened by one reviewer and checked by a second. At each stage, any disagreement between reviewers was resolved by discussion or involvement of a third reviewer where necessary.
Titles and abstracts identified by the cost-effectiveness search strategy were assessed for potential eligibility by two reviewers independently. Studies were only considered for inclusion if they reported the results of full economic evaluations (details below). Full papers of potentially relevant studies were retrieved and assessed for inclusion by two reviewers independently.
Inclusion and exclusion criteria
The following criteria reflect those stipulated in the final scope issued by NICE.
Population
Children and young people aged 3–17 years (peginterferon alfa-2b) or 5–17 years (peginterferon alfa-2a) with chronic HCV, without liver decompensation and who were positive for HCV RNA. All groups were considered, including:
-
people with HIV co-infection
-
people with all grades of severity of chronic hepatitis C (mild, moderate and severe)
-
people who were treatment naive or, if appropriate, those who had been previously treated but who relapsed or did not respond.
Interventions
-
Peginterferon alfa-2a in combination with RBV.
-
Peginterferon alfa-2b in combination with RBV.
Comparators
-
Best supportive care (e.g. symptomatic treatment, monitoring, treatment without any form of interferon therapy).
-
The interventions compared with each other within their licensed indications, i.e. peginterferon alfa-2a and RBV versus peginterferon alfa-2b and RBV.
Outcomes
Studies had to report SVR (defined as undetectable HCV RNA at least 6 months after treatment cessation). Studies could also include one or more of the following:
-
virological response to treatment (e.g. during treatment, end of treatment)
-
biochemical response (e.g. ALT)
-
liver inflammation and fibrosis
-
mortality
-
adverse effects of treatment, including effects on growth
-
HRQoL.
Study design
-
Randomised controlled trials were included if available. If no RCTs of relevance were identified, non-RCTs were considered for inclusion. Studies without a control group were only considered for inclusion in the absence of any controlled studies.
-
Studies published since 2007 as abstracts or conference presentations were only included if sufficient details were presented to allow an appraisal of the methodology and an assessment of results to be undertaken.
-
For the systematic review of cost-effectiveness, studies were only included if they reported the results of full economic evaluations [cost–utility analyses, cost-effectiveness analyses (reporting cost per life-year gained), cost–benefit analyses or cost–consequence analyses].
-
Systematic reviews were only used as a source of references.
-
Case series, case studies, narrative reviews, editorials and opinions were not included.
-
Only studies published in the English language were included.
Data extraction strategy
Data from included clinical effectiveness and cost-effectiveness studies were extracted by one reviewer using a standardised and piloted data extraction form. Extracted data were checked by a second reviewer with any discrepancies resolved by discussion or recourse to a third reviewer when necessary.
Critical appraisal strategy
The quality of the clinical effectiveness studies was assessed according to criteria based on those used by the CRD (University of York). 43 The quality of the included economic evaluations was assessed using a critical appraisal checklist based upon those proposed by Drummond and Jefferson44 and Philips and colleagues. 45 Quality criteria of the included studies were assessed by one reviewer, and checked for agreement by a second reviewer. Any disagreements were resolved by consensus or consultation with a third reviewer if necessary.
Method of data synthesis
Clinical effectiveness data were synthesised through a narrative review with tabulation of the results of included studies. Full data extraction forms of all the included studies can be found in Appendix 3. From a clinical effectiveness perspective, it was not considered appropriate to combine the studies in a meta-analysis, primarily because of study design and poor study quality, with the related uncertainties. There was also some heterogeneity between studies in patient characteristics (e.g. mode of HCV transmission, genotype mix and treatment history), all of which can have a potential impact on the virological response to treatment. However, it was necessary to calculate a weighted average of SVR and EVR to provide estimates for the economic model (see Chapter 5, Southampton Health Technology Assessments Centre’s data sources for further details).
Chapter 4 Clinical effectiveness
Quantity and quality of research available
Literature searches identified 1384 references, with a total of 811 after removal of duplicates. Following the initial screening of titles and abstracts, 750 were excluded because they did not meet the specified inclusion criteria, and the full text of 61 articles was retrieved. Of these, 36 were excluded and 25 were further reviewed for possible inclusion. These were articles that met all of the inclusion criteria but had other factors to consider (e.g. the age of the participants exceeded the upper or lower limit without separate reporting of age-relevant subgroups). As such, nine of these articles were excluded after further inspection, leaving 16 included publications (seven studies). The total number of published papers included at each stage of the systematic review is shown in the flow chart in Figure 1, and the list of retrieved studies (with reasons for exclusion) can be seen in Appendix 4. The most common reason for exclusion was wrong study population (many of the studies were in adults). A number of relevant abstracts were identified but were not included owing to the insufficient reporting of methods and/or baseline data.
FIGURE 1.
Flow chart for the identification of studies.
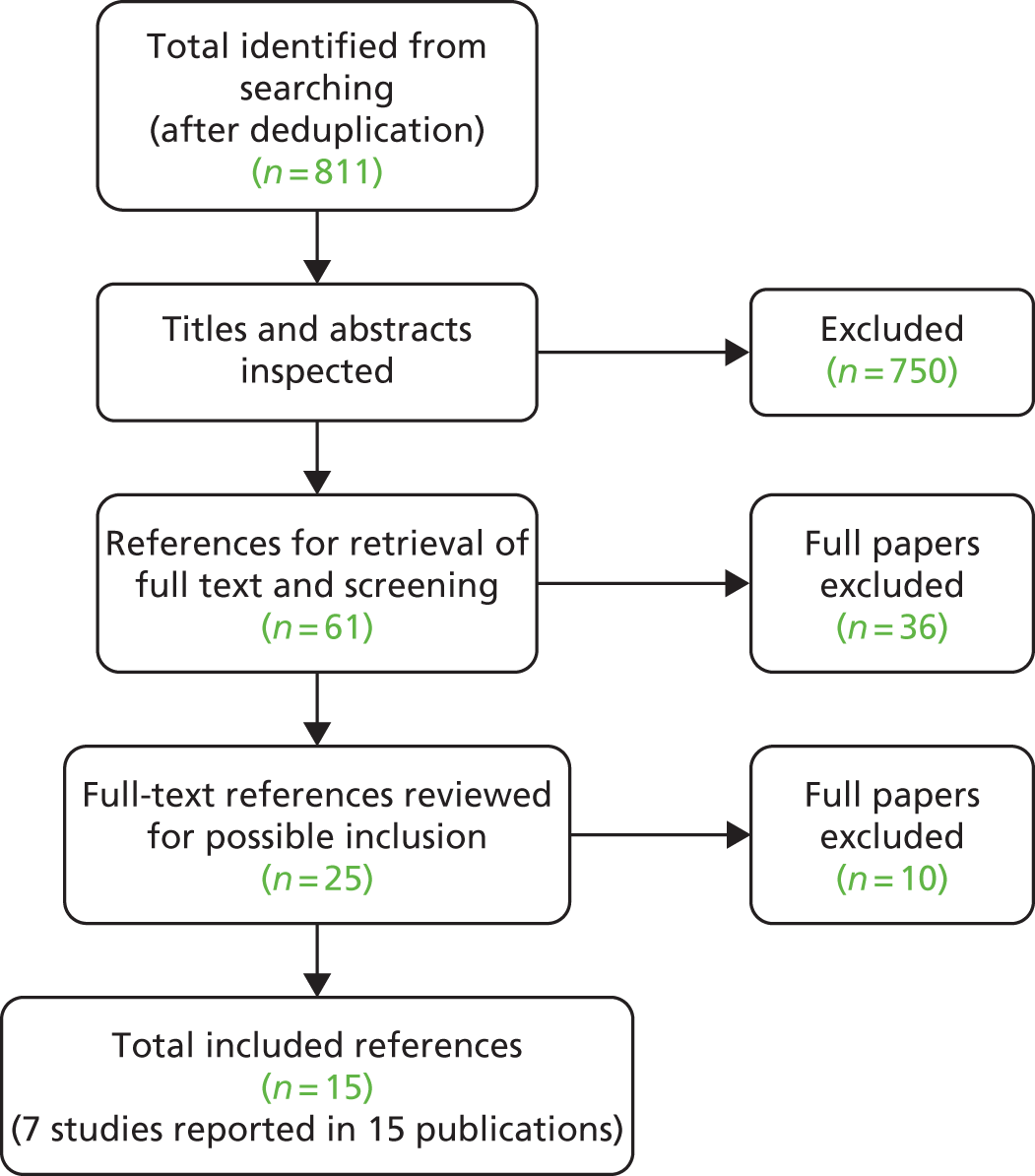
Fifteen publications describing seven studies met the inclusion criteria of the review. 28,46–59 The eight additional publications were either abstracts52,55,58 or articles that were linked28,49,50,53,54 (e.g. reporting additional outcomes) to the main studies. 46–48,51,56,57,59 All of the included studies were single-arm, uncontrolled cohort studies, with the exception of one (Schwarz and colleagues56), which was a RCT. This was the pivotal licence trial (known as PEDS-C) for peginterferon alfa-2a and RBV treatment in children and young people aged 5–18 years. The comparator arm in this trial was peginterferon monotherapy (peginterferon alfa-2a + placebo), which did not meet the inclusion criteria for the review (as based on the NICE scope for this appraisal60). Thus, data for the intervention arm only could be used, effectively treating this as a single-arm cohort study. One study47 provided few aggregate data but fulfilled the inclusion criteria and has been included. Caution is suggested in interpreting data from this study and this is reiterated in the results section. No studies were identified in children and young people with HIV co-infection.
The following section provides a description of the primary publications for the seven included studies46–48,51,56,57,59 (Table 1).
| Author | Peginterferon type |
|---|---|
| Schwarz et al., 201156 | Peginterferon alfa-2a |
| Sokal et al., 201057 | Peginterferon alfa-2a |
| Al Ali et al., 201046 | Peginterferon alfa-2b |
| Pawlowska et al., 201051 | Peginterferon alfa-2b |
| Wirth et al., 201059 | Peginterferon alfa-2b |
| Ghaffar et al., 200947 | Peginterferon alfa-2b |
| Jara et al., 200848 | Peginterferon alfa-2b |
Overview of the included studies
The key characteristics of the included studies are shown in Table 2. Two studies evaluated peginterferon alfa-2a and RBV,56,57 and five studies evaluated peginterferon alfa-2b and RBV. 46–48,51,59 The dose of peginterferon was administered subcutaneously once per week in all the studies, and was largely similar within peginterferon type. Peginterferon alfa-2a, given according to BSA, was similar in the two studies [180 µg/1.73m2/week56 (= 104 µg/m2/week) and 100 µg/m2/week57], both with a maximum dosage of 180 µg. The peginterferon alfa-2b dosage, given according to body weight, was 1.5 µg/kg/week in three studies,46,47,51 and Wirth and colleagues59 reported that the dose of 60 µg/m2/week used in their study was equivalent to the licensed dose for adults of 1.5 µg/kg/week. The study by Jara and colleagues48 used a lower dose of 1.0 µg/kg/week. RBV was administered orally at a dose of 15 mg/kg/day in all the studies, with the two peginterferon alfa-2a studies stating a maximum dosage of 1200 mg,57 or 1200 mg for body weight ≥ 75 kg and 1000 mg for body weight < 75 kg. 56 RBV is usually administered in two divided doses although this was explicitly stated in only three studies (one peginterferon alfa-2a,56 two peginterferon alfa-2b47,48).
| Study | Methods | Key inclusion criteria | Key patient characteristics | Outcomes |
|---|---|---|---|---|
| PEG α-2a + RBV | ||||
| Schwarz et al., 201156 + related publications28,49,53–55,61 |
Design: RCT (but treated as a single-cohort study) Number of centres: 11 Country: USA Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases; FDA; National Institutes of Health/National Centre for Research Resources. Additional support from Roche Interventions: PEG α-2a, 180 µg/1.73 m2 BSA/week (max. 180 µg) + RBV, 15 mg/kg/day (max. 1200 mg if ≥ 75 kg and 1000 mg if < 75 kg) Duration: 48 weeks Follow-up: 24 weeks post treatment No. of participants: 55 (single arm) |
Aged 5–18 years Chronic HCV infection (plasma HCV RNA on two tests ≥ 6 months apart) Chronic liver disease, as indicated by inflammation and/or fibrosis, consistent with chronic HCV infection on a liver biopsy obtained within the past 36 months; compensated liver disease (Child–Pugh Grade A) Haemoglobin values > 11 g/dl for females; > 12 g/dl for males Normal TSH Able to swallow a RBV/placebo tablet Signed informed consent from parent/legal guardian Excluded if co-infected with HIV or HBV, or previously treated with IFN or RBV |
Mean age: 10.7 years Male: n = 27 (49%) Treatment naive: 100% Mean duration of infection: 105 months Genotype 1: n = 45 (82%) Genotype 2: n = 4 (7%) Genotype 3: n = 6 (11%) Genotype 6: n = 0 Transmission:
HCV RNA ≥ 600,000 IU/ml: n = 32 (58%)a Mean ALT: 49 IU/l; > ULN, n = 32 (58%) Mean AST: 45 IU/l; > ULN, n = 28 (51%) HAI inflammation:
|
Primary outcome: SVR Secondary outcomes:
|
| Sokal et al., 201057 | Design: single-cohort study Number of centres: 6 Countries: Belgium, UK, Sweden, Brazil, Latvia Sponsor: stated funding was from the drug companies involved Interventions: PEG α-2a, 100 µg/m2 BSA/week (max. 180 µg) + RBV, 15 mg/kg/day (max. 1200 mg) Duration: 24 or 48 weeks according to genotype Follow-up: 24 weeks post treatment No. of participants: 65 |
Treatment-naive children and adolescents aged 6–17 years Positive anti-HCV serum antibodies and detectable serum HCV RNA Not limited by levels of serum aminotransferases, HCV genotype or mode of infection All patients presenting with hepatitis C were approached for inclusion Adequate contraception was compulsory (if applicable) Excluded if co-infected with HIV or HBV |
Mean age: not reported for whole group; 11.3 and 12.6 years for subgroups Male: n = 30 (46%) Treatment naive: 100% Duration of infection: not reported Genotype 1: n = 45 (69%) Genotype 2: n = 2 (3%) Genotype 3: n = 16 (25%) Genotype 4: n = 1 (2%) Genotype 5 or 6: n = 1 (2%) Transmission:
|
Primary outcome: SVR Secondary outcomes:
|
| PEG α-2b + RBV | ||||
| Al Ali et al., 201046 | Design: single-cohort study Number of centres: 1 Country: Kuwait Sponsor: none Interventions: PEG α-2b, 1.5 µg/kg/week + RBV, 15 mg/kg/day Duration: 48 weeks Follow-up: 24 weeks post treatment No. of participants: 12 |
Treatment-naive patients aged 14–17 years Detectable HCV RNA Genotype 4 Anti-HCV-positive liver biopsy findings consistent with the diagnosis of HCV, for whom a decision to treat was made Patients were included independent of mode of acquisition of infection, level of serum aminotransferases or serum HCV RNA viral load |
Mean age: 15.75 years Male: n = 8 (67%) Treatment naive: 100% Duration of infection: not reported Genotype 4: 100% Transmission:
Mean serum ALT: 91 IU/l (range 34–194 IU/l) Mean METAVIR histological grade: 1.67 (range 1–2) Mean METAVIR fibrosis score: 0.67 (range 0–3) |
Primary outcome: SVR Secondary outcomes:
|
| Pawlowska et al., 201051 + abstract52 |
Design: single-cohort study Number of centres: 1 Country: Poland Sponsor: states ‘departmental sources’ Interventions: PEG α-2b, 1.5 µg/kg/week + RBV, 15 mg/kg/day Duration: 48 weeks, although also states 24 or 48 weeks according to genotype Follow-up: 24 weeks post treatment No. of participants: 53 |
Children aged 8–17 years Chronic HCV diagnosed by the presence of serum HCV RNA and histopathological changes in the liver (by liver biopsy and ultrasound) Excluded if co-infected with HIV or HBV |
Mean age: 13.6 years (range 8–17 years) Male: n = 37 (70%) Treatment naive: n = 29 (54%); previously treated (PEG α-2b + RBV), n = 24 (46%) Mean duration of infection: 5.4 yearsb Genotype 1: n = 27 (50%) Genotype 3: n = 2 (4%) Genotype 4: n = 24 (46%) Transmission:
Fibrosis score (modified Scheuer scale):≤ stage 2, 100% Necroinflammatory score (modified Scheuer scale): ≤ stage 2, 100% |
Primary outcome: SVR Secondary outcomes:
|
| Wirth et al., 201059 + abstract58 |
Design: single-cohort study Number of centres: 22 Countries: Austria, France, Germany, Italy, Spain, Argentina, Chile, USA, Puerto Rico Sponsor: majority of authors received funding or were employed by Schering-Plough Interventions: PEG α-2b, 60 µg/m2 BSA/week + RBV, 15 mg/kg/day Duration: 24 or 48 weeks according to genotype and viral load Follow-up: 24 weeks post treatment No. of participants: 107 |
Children aged 3–17 years with previously untreated chronic HCV Evidence of fibrosis and/or inflammatory activity from liver biopsy was requested from all patients before enrolment; however, a waiver was permitted for children aged 3–11 years who had an elevated ALT in the year before screening Absolute neutrophil count ≥ 1500 mm3; platelet count ≥ 100,000 mm3; and haemoglobin levels ≥ 11 g/dl for girls and 12 g/dl for boys Excluded if co-infected with HIV or HBV |
Mean age: 10 years Male: n = 51 (48%) Treatment naive: 100% Mean duration of infection: 8.5 years Genotype 1: n = 72 (67%) Genotype 2: n = 15 (14%) Genotype 3: n = 15 (14%) Genotype 4: n = 5 (5%) Transmission:
Serum ALT: normal, n = 63 (59%); abnormal, n = 44 (41%) METAVIR inflammatory activity score: none, n = 6 (6%); mild, n = 47 (44%); moderate, n = 32 (30%); severe, n = 19 (18%); missing, n = 3 (3%) Also reports key characteristics within age groups |
Primary outcome: SVR Secondary outcomes:
|
| Ghaffar et al., 200947 | Design: single-cohort study Number of centres: 1 Country: Egypt Sponsor: stated ‘donations’ Interventions: PEG α-2b, 1.5 µg/kg/week + RBV, 15 mg/kg/day Duration: 52 weeks Follow-up: 12 months post treatment No. of participants: 7 |
Aged between 8 and 16 years, both genders Chronic HCV infection (positive antibodies with HCV RNA positivity and ALT/AST ≤ 1.5 times the ULN) Well-compensated liver disease, normal levels for haemoglobin, platelets, white blood cells, glucose, serum creatinine, normal thyroid profile and negative autoantibodies No co-infection with any other hepatotrophic virus or HIV |
Age range: 8–13 years Male: n = 5 (71%) Previously treated (IFN): n = 1 (14%); unclear for other six (possibly treatment naive) Mean duration of infection: unclear [4.5 years for two (29%); 12.7 years for remaining five (71%)] Genotype 4a: n = 1 (14%) Genotype 4b: n = 5 (71%) Genotype unknown (not tested): n = 1 (14%) Transmission:
Serum ALT range: 52–223 IU/l (median 77 IU/l) Serum AST range: 63–321 IU/l (median 76 IU/l) Fibrosis score: not reported for all participants |
Outcomes (not stated as primary or secondary):
|
| Jara et al., 200848 | Design: single-cohort study Number of centres: 1 Country: Spain Sponsor: not stated; Schering-Plough provided interventions and assistance for designing the study Interventions: PEG α-2b, 1.0 µg/kg/week + RBV, 15 mg/kg/day Duration: 24 or 48 weeks according to genotype Follow-up: at least 24 weeks post treatment No. of participants: 30 |
Aged between 3 and 16 years Chronic HCV, defined serum HCV RNA titres (> 50 IU/ml) for ≥ 3 years with continuous or intermittently elevated ALT values Non-responders to IFN α monotherapy eligible if they accounted for < 25% of the patient population Excluded if co-infected with HIV or non-HCV liver disease |
Mean age: 10 years (range 3.5–16 years) Male: not reported Treatment naive:n = 24 (80%); previously treated (IFN α monotherapy), n = 6 (20%) Duration of infection: not reported Genotype 1: n = 26 (87%) Genotype 3: n = 3 (10%) Genotype 4: n = 1 (3%) Transmission:
Mean serum ALT: 75 IU/l (range 29–232 IU/l) Mean serum AST: 52 IU/l (range 24–157 IU/l) Knodell fibrosis score: < 4, 58%; 4–7, 31%; ≥ 8, 10% |
Primary outcome: SVR Secondary outcomes: |
The duration of treatment was 48 weeks46,56 or 52 weeks47 in three studies (one peginterferon alfa-2a,56 two peginterferon alfa-2b46,47), whereas two studies (one peginterferon alfa-2a,57 one peginterferon alfa-2b48) treated participants for different durations according to genotype, which was generally 24 weeks for genotype 2 or 3 and 48 weeks for genotypes 1, 4, 5 or 6. The information provided by Pawlowska and colleagues51 on treatment duration was not clear. They reported a duration of 48 weeks for all participants while also reporting that participants received 24 or 48 weeks of treatment according to genotype (2 and 3 or 1 and 4, respectively). Wirth and colleagues59 also treated participants for different durations according to genotype but further divided those with genotype 3 according to baseline viral load, so that those with genotype 2 and those with genotype 3 and a low viral load received 24 weeks of therapy, and those with genotype 1 or 4 and genotype 3 with a high viral load received 48 weeks of therapy.
All of the included studies were relatively small. The trial by Wirth and colleagues (peginterferon alfa-2b)59 was the largest, recruiting 107 participants. The two peginterferon alfa-2a studies56,57 and one peginterferon alfa-2b study51 were similar in size, with 53–65 participants (although Schwarz and colleagues56 had n = 55 for the peginterferon and RBV arm, with a total study size of n = 114). The numbers of participants in the three smaller studies ranged from 7 to 30. 46–48
Five of the studies (both peginterferon alfa-2a,56,57 three peginterferon alfa-2b48,51,59) included participants with a mix of genotypes, although all included a higher proportion of participants with genotype 1 (range 50–87%), or genotypes 1 or 4 (range 71–96%) than the other genotype subgroups. Participants with genotypes 2 or 3 accounted for only 3–25% of the included populations across the studies. The remaining two studies, both evaluating peginterferon alfa-2b, included only participants with genotype 4 (Al Ali and colleagues46), or largely genotype 4 where six of seven participants had genotype 4 and one was unknown (not tested) (Ghaffar and colleagues47). Over half of the studies included treatment-naive populations, with four (both peginterferon alfa-2a,56,57 two peginterferon alfa-2b46,59) having 100% of participants not previously treated, and a fifth study (of peginterferon alfa-2b48) consisting largely of treatment-naive participants (80%). Ghaffar and colleagues47 stipulated that one of seven children was previously treated whereas the treatment history of the other six children was not reported (so it is unclear whether they were treatment naive or this was unknown). The study by Pawlowska and colleagues51 (peginterferon alfa-2b) was conducted in a mixed population of treatment-naive and previously treated (with non-pegylated interferon alfa-2b and RBV) participants in roughly equal proportions (54% and 46%, respectively).
The age ranges of children included in the peginterferon alfa-2a studies (5–18 years) and peginterferon alfa-2b studies (3–17 years) are within those of the anticipated licence for peginterferon alfa-2a and the existing licence for peginterferon alfa-2b. Three of the peginterferon alfa-2b studies focused on a narrower age range, 8–17 years, which excluded young children. 46,47,51 The mean age was approximately 10–11 years in four studies (both peginterferon alfa-2a,56,57 two peginterferon alfa-2b48,59) and older (14–16 years) in two peginterferon alfa-2b studies;46,51 one peginterferon alfa-2b study47 did not report mean age but the median was 10 years. The proportion of male participants was approximately half to two-thirds of the total population in all studies except one (peginterferon alfa-2b48) (not reported). Vertical transmission was the most common mode of infection in four studies (both peginterferon alfa-2a,56,57 two peginterferon alfa-2b48,59), accounting for nearly half the included population in one study57 and around 70% in the other three. 48,56,59 Parenteral transmission was the most common route in the other three peginterferon alfa-2b studies,46,47,51 ranging from 42% to 100%, and included infection via intravenous drug use, transfusion and other medical procedures. In four studies (both peginterferon alfa-2a,56,57 two peginterferon alfa-2b46,59) the mode of infection was unknown in 14–22% of participants.
Baseline HCV RNA levels across the seven included studies varied. In the two peginterferon alfa-2a studies, approximately two-thirds of participants had relatively high baseline HCV RNA levels [> 500,000 international units (IU)/ml57 or ≥ 600,000 IU/ml56], and one study of peginterferon alfa-2b46 also reported high baseline HCV RNA, with a mean of 780,000 IU/ml. Two other peginterferon alfa-2b studies51,59 reported similar proportions (range 40–55%) of participants with either high (> 500,000 or > 600,000 IU/ml) or low (< 500,000 or < 600,000 IU/ml) HCV RNA levels. In the study by Ghaffar and colleagues47 (peginterferon alfa-2b), most of the participants had low HCV RNA levels at baseline (median 145,000 IU/ml). The seventh study (peginterferon alfa-2b48) reported a mean HCV RNA of 5 log10 IU/ml, stating that 67% (20/30) of participants had a viral load of > 105 × IU/ml with only one patient having log10 viral load < 4.5. Fibrosis levels indicated mild liver disease in most or all of the population across the seven studies, although fibrosis was not reported for all the participants in the study by Ghaffar and colleagues. 47 According to clinical opinion, there is generally no single agreed definition of what constitutes a ‘high’ or ‘low’ viral load and the cut-off value is different between the two peginterferons.
Studies differed in the numbers of centres and countries that they included. Four (all peginterferon alfa-2b) were single-centre studies (Egypt,47 Kuwait,46 Poland51 and Spain48) while three (both peginterferon alfa-2a,56,57 one peginterferon alfa-2b59) were multicentre studies (ranging from 6 to 22 centres). Schwarz and colleagues recruited patients from 11 centres, all located in the USA,56 and the remaining two multicentre studies involved multiple centres in different countries. 57,59 Sokal and colleagues57 included six centres located in Brazil, the UK (Birmingham Children’s Hospital), Belgium, Latvia and Sweden, and Wirth and colleagues59 included 22 centres located in the USA, South America and Europe. For two studies (one peginterferon alfa-2a, one peginterferon alfa-2b), funding was either received from the drug companies involved,57 or the majority of the authors had received funding from, or were employed by, the drug manufacturer. 59 One study (peginterferon alfa-2b) did not state the funding source but did report that the drug manufacturer provided the interventions and ‘assistance for designing the study’,48 while a fourth (peginterferon alfa-2a) reported unspecified ‘additional support’ from the drug manufacturer as well as stating other sources. 56 The three remaining peginterferon alfa-2b studies either received no financial support46 or reported vaguely that their funding was from ‘donations’47 or ‘other departmental sources’. 51
All seven studies specified the patients’ age as an inclusion criterion and this ranged from 3 to 18 years (although the maximum age of included participants was 17 years). All studies required patients to have chronic HCV infection46–48,51,56,59 and/or detectable plasma HCV RNA,46–48,51,56,57 although only one study (peginterferon alfa-2b) specified a detection threshold of HCV RNA for inclusion (> 50 IU/ml48). Inflammation and/or fibrosis from liver biopsy or ultrasound investigations was specified as supporting evidence of liver disease in four studies (one peginterferon alfa-2a,56 three peginterferon alfa-2b46,51,59) and two studies (one peginterferon alfa-2a,56 one peginterferon alfa-2b47) explicitly stated that the liver disease should be compensated. Four studies specified either that treatment-naive patients were included (one peginterferon alfa-2a, two peginterferon alfa-2b)46,57,59 or that patients previously treated with interferon or RBV were excluded,56 whereas one study (peginterferon alfa-2b) permitted non-responders to interferon alfa monotherapy provided that they accounted for < 25% of the population. 48
Six studies excluded participants who were co-infected with HIV or hepatitis B (not reported in one peginterferon alfa-2b study47), and two studies47,48 also excluded those who were co-infected with any other non-HCV liver disease. Five studies (one peginterferon alfa-2a, four peginterferon alfa-2b) excluded those with thrombocytopaenia, anaemia and neutropenia – conditions that are consistent with decompensated liver disease or are made worse by taking peginterferon + RBV – by stipulating certain laboratory readings46,48,56,59 or specifying ‘normal levels’47 in their inclusion/exclusion criteria. Details of other inclusion/exclusion criteria specified by the studies can be found in the data extractions forms in Appendix 3.
Six of the seven studies specified that SVR was the primary outcome. 46,48,51,56,57,59 SVR may also have been the primary outcome in the remaining peginterferon alfa-2b study (Ghaffar and colleagues47), although this was not stated explicitly. In terms of secondary outcomes, EVR was reported by all seven studies46–48,51,56,57,59 (though not specifically stated as secondary by Ghaffar and colleagues47), and five studies (both peginterferon alfa-2a,56,57 three peginterferon alfa-2b46,47,51) reported an end-of-treatment virological response (EOT, abbreviated in some studies as ETR; the former is used hereafter for consistency). Other secondary virological outcomes reported were rapid virological response (RVR),48,56,59 predictors of viral response,48,51,56,57,59 virological response at 24 weeks,48 virological breakthrough51 and relapse. 51,56,59 Biochemical response was reported by two peginterferon alfa-2b studies. 47,59 Adverse events were reported by all seven studies. Four studies (one peginterferon alfa-2a57 and three peginterferon alfa-2b48,51,59) reported growth and only one study (peginterferon alfa-2a) reported QoL. 56
A summary of the included studies in terms of patient population, in line with the NICE scope, is given below:
-
Peginterferon alfa-2a/-2b Peginterferon alfa-2a, n = 2; peginterferon alfa-2b, n = 5.
-
Treatment naive/previously treated Treatment naive (100% of population), n = 4; mixed treatment (naive and previously treated), n = 2; unclear, n = 1.
-
Severity of chronic HCV Mild fibrosis (most or all of the population), n = 6; unclear, n = 1.
-
HIV co-infection n = 0.
Quality assessment of included studies
The methodological quality of reporting in the included studies was assessed using criteria based on guidance by the CRD at the University of York,43 and is shown in Table 3. The quality assessment criteria relate to various aspects of study design which may help to gauge the relative strengths and weaknesses of the individual studies. On the whole, the cohort studies were of generally poor quality, although the study by Schwarz and colleagues56 (peginterferon alfa-2a) fared better in its reporting of methodological details. This was a RCT, although as detailed previously, it is treated as a single-cohort study in this assessment.
| Quality criteria | Schwarz et al., 201156 | Sokal et al., 201057 | Wirth et al., 201059 | Pawlowska et al., 201051 | Al Ali et al., 201046 | Ghaffar et al., 200947 | Jara et al., 200848 |
|---|---|---|---|---|---|---|---|
| Selection criteria predefined | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Blinding of participants | Yes | N/A | N/A | N/A | N/A | N/A | N/A |
| More outcomes measured than reported | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Withdrawals and dropouts described | Yesa | Yes | Yes | NR | Yes | NR | Yes |
| Analysis accounts for missing data | Unclear | Unclear | Unclear | No | Unclear | N/Ab | No |
| If analysis accounts for missing data, were methods appropriate? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
All the studies specified their criteria for patient selection a priori, stating their inclusion and exclusion criteria to varying degrees of detail (although Ghaffar and colleagues47 did not specify any exclusion criteria). The lack of randomisation procedures (resulting from the single-arm study design of most studies) may mean there is a higher risk of bias. Given that six of the seven studies were uncontrolled cohort studies with only one intervention arm, the blinding of participants was not applicable. The seventh study, by Schwarz and colleagues,56 reported that participants (in both arms) and investigators were blinded as to whether they were receiving placebo or RBV in combination with peginterferon alfa-2a, with placebo/RBV tablets being supplied in the same dosing regimen.
For most of the studies it was unclear whether or not the authors measured more outcomes than they reported. Schwarz and colleagues56 (peginterferon alfa-2a) was the only study to clearly describe measuring more outcomes than were reported (in either the main paper56 or related publications28,49,50,53–55), stating that assessments of body composition and growth would be reported separately. (A recent publication62 reporting these outcomes did not meet the inclusion criteria for the review as combined results were reported for the peginterferon alfa-2a combination and monotherapy groups together). Pawlowska and colleagues51 reported that there were plans to assess growth 5 years after treatment cessation but there were no further details. Most of the studies provided adequate details of participant withdrawals and losses to follow-up, with the exception of two peginterferon alfa-2b studies47,51 where this information was not reported. However, four studies (one peginterferon alfa-2a,57 three peginterferon alfa-2b46,47,59) reported very little or no methodology relating to data analysis, and all seven studies were either not clear or did not report whether or not the statistical analysis accounted for any missing data.
Assessment of the generalisability of the studies is difficult owing to the single-cohort study design, poor methodological quality, and variation in the participant inclusion criteria and countries included, as well as other uncertainties.
Assessment of effectiveness
The included studies of the two forms of peginterferon alfa in the following section provide no evidence of a comparative nature, against either BSC or each other. It should be noted that these single-cohort studies reported few or no statistical analyses on the data. Therefore, the narratives reported in this section are based on observation of the data and should be interpreted with caution.
Sustained virological response
Sustained virological response was reported to be the primary outcome in six of the included studies, but not specifically stated as such in the seventh (Ghaffar and colleagues47). Results are reported in Table 4.
| Study | Treatment | SVR, % (n/N) |
|---|---|---|
| Schwarz et al., 201156 | PEG α-2a + RBV 48 weeks, n = 55 |
53, 95% CI 40 to 66 (29/55) |
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
66 (43/65)a (two ND) |
| Al Ali et al., 201046 | PEG α-2b + RBV 48 weeks, n = 12 |
75 (9/12) |
| Pawlowska et al., 201051 | PEG α-2b + RBV 24 or 48 weeks, n = 53 |
49 (26/53)b |
| Wirth et al., 201059 | PEG α-2b + RBV 24 or 48 weeks, n = 107 |
65 (70/107) |
| Ghaffar et al., 200947 | PEG α-2b + RBV 52 weeks, n = 7 |
29 (2/7) |
| Jara et al., 200848 | PEG α-2b + RBV 24 or 48 weeks, n = 30 |
50 (15/30) |
Sustained virological response was defined as undetectable serum HCV RNA 24 weeks after the end of treatment in six studies (both peginterferon alfa-2a studies56,57 and four peginterferon alfa-2b studies46,48,51,59) and 12 months after the end of treatment in one study (Ghaffar and colleagues47). Three studies specifically defined the lower limit of detection for attainment of SVR as 50 IU/ml,57 < 50 IU/ml46 or < 10 IU/ml56 (although the last is reported to be < 100 IU/ml in two related publications54,55). Quantitative and qualitative lower limits of detection of HCV RNA are reported by most of the other studies,48,51,59 ranging from 25 to 600 IU/ml, but it is not always clear which virological outcome they relate to (i.e. EVR, EOT or SVR). Details for individual studies can be seen in the data extraction forms in Appendix 3.
Peginterferon alfa-2a
Sustained virological response rates were similar in the two studies evaluating peginterferon alfa-2a,56,57 ranging from 53% to 66%. The longer-term follow-up of participants in the PEDS-C trial56 found that for those children who achieved an SVR who were followed up for 2 years [45/55 (82%)], durability of viral response was 100%.
Peginterferon alfa-2b
For those receiving peginterferon alfa-2b, SVR rates across five studies46–48,51,59 ranged from 29% to 75%. The two studies46,47 reporting the lowest and highest rates in this range had very small participant numbers, which may raise a question over the reliability of the data, and all, or most, participants in both were genotype 4 children. Excluding these two very small studies,46,47 the SVR rates in those receiving peginterferon alfa-2a appear comparable with those receiving peginterferon alfa-2b (range 49–65%).
It should be noted that the study by Jara and colleagues48 used a lower dose of peginterferon alfa-2b than the other studies (1.0 µg/kg/week vs. 1.5 µg/kg/week), but it is unclear whether or not this had an impact on the rate of SVR achieved.
Sustained virological response according to prognostic factors
It should be noted that there were some differences between studies in the SVR subgroups in terms of how different categories were defined (e.g. low/high viral load, abnormal ALT), and there were also inconsistencies in grouping different categories (e.g. genotypes, histology). These differences should be borne in mind when interpreting the results. Furthermore, numbers in some of the SVR subgroups were very small and are unlikely to be statistically powered, so results should be interpreted with caution.
Genotype
Sustained virological response rates according to HCV genotype were reported by both of the peginterferon alfa-2a studies56,57 and by three peginterferon alfa-2b studies. 48,51,59 These are shown in Table 5.
| Study | Treatment | SVR according to genotype | |
|---|---|---|---|
| Genotype | SVR, % (n/N) | ||
| Schwarz et al., 201156 | PEG α-2a + RBV 48 weeks, n = 55 |
Genotype 1 | 47, 95% CI 32 to 61 (21/45) |
| Genotypes 2–6a | 80, 95% CI 55 to 100 (8/10) | ||
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
Genotype 1, 4, 5 or 6b | 57 (27/47) (one ND) |
| Genotype 2 or 3 | 89 (16/18) (one ND) | ||
| Pawlowska et al., 201051 | PEG α-2b + RBV 24 or 48 weeks, n = 53 |
Genotype 1 | 48 (13/27) |
| Genotype 3 | 50 (1/2) | ||
| Genotype 4 | 50 (12/24) | ||
| Wirth et al., 201059 | PEG α-2b + RBV 24 or 48 weeks, n = 107 |
Genotype 1 | 53 (38/72) |
| Genotype 2 or 3 | 93 (28/30) | ||
| Genotype 4 | 80 (4/5) | ||
| Jara et al., 200848 | PEG α-2b + RBV 24 or 48 weeks, n = 30 |
Genotype 1 | 46 (12/26) |
| Genotype 3 | 100 (3/3) | ||
| Genotype 4 | 0 (0/1) | ||
Peginterferon alfa-2a
The PEDS-C study by Schwarz and colleagues56 grouped HCV genotypes into ‘genotype 1’ and ‘genotype 2–6’. This is slightly unusual as genotypes are generally grouped according to response to treatment, whereby genotypes 2 and 3 would be grouped together and genotypes 1, 4, 5 and 6 would be grouped together. However, in the peginterferon alfa-2a and RBV treatment arm of the PEDS-C trial (the only arm of PEDS-C considered in this review), there were no participants with genotypes 4, 5 or 6, so the ‘genotype 2–6’ group actually only consists of children with genotypes 2 and 3. Additionally, in the other peginterferon alfa-2a study by Sokal and colleagues,57 only 2 out of 65 (3%) participants had genotypes 4, 5 or 6, so the grouping of ‘genotype 1, 4, 5 or 6’ contained predominantly genotype 1 participants (and hence this group has been considered genotype 1 in the following section).
Response rates within each genotype group were similar across the two studies evaluating peginterferon alfa-2a. 56,57 SVRs for participants with genotype 1 ranged from 47% to 57%, while response rates for genotypes 2 and 3 were observed to be higher, ranging from 80% to 89%. Sokal and colleagues57 reported that the SVR rates were statistically significantly higher for those with genotype 2 or 3 than those with genotype 1, 4, 5 or 6 (89% vs. 57%, p < 0.01).
Peginterferon alfa-2b
Sustained virological responses for genotype 1 were similar across the three peginterferon alfa-2b studies, ranging from 46% to 53%. 48,51,59 Response rates for genotypes 2 and 3 were observed to be more variable and higher, with an overall range of 50–100%. However, one study grouped genotypes 2 and 3 together,59 while two studies48,51 reported only on genotype 3. The numbers of participants with genotype 3 in these two studies were very small (one of two51 and three of three48). SVRs for genotype 4 in the three peginterferon alfa-2b studies48,51,59 varied greatly, ranging from 0% to 80%; the number of participants as a proportion of the total study population in the genotype 4 subgroup was very small in two of these studies (1 of 3048 and 5 of 10759), which may explain some of the variation.
Pawlowska and colleagues51 examined differences in SVR rates but found no statistically significant difference in SVR between those with HCV genotypes 1 and 4 (48% vs. 50%), although no quantitative statistics or p-values were reported.
Viral load
Three studies (both the peginterferon alfa-2a studies56,57 and one peginterferon alfa-2b study59) reported SVR according to baseline viral load, stratified into low (< 500,000 or ≤ 600,000 IU/ml) or high (> 500,000 or ≥ 600,000 IU/ml) HCV RNA viral load (Table 6).
| Study | Treatment | SVR according to viral load | |
|---|---|---|---|
| HCV RNA (IU/ml) | SVR, % (n/N) | ||
| Schwarz et al., 201156 | PEG α-2a + RBV 48 weeks, n = 55 |
< 600,000 | 70 (16/23) |
| ≥ 600,000 | 50 (16/32) | ||
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
< 500,000 | 74 (17/23) |
| Genotype 2 or 3 | 90 (9/10) | ||
| Genotype 1, 4, 5 or 6 | 62 (8/13) | ||
| > 500,000 | 55 (22/40) | ||
| Genotype 2 or 3 | 100 (7/7) | ||
| Genotype 1, 4, 5 or 6 | 45 (15/33) | ||
| aWirth et al., 201059 | PEG α-2b + RBV 24 or 48 weeks, n = 107 |
≤ 600,000 | 79 (46/58)b |
| Genotype 1 | 72 (28/39)c | ||
| Genotype 2 or 3 | 94 (15/16) | ||
| Genotype 4 | 100 (3/3) | ||
| > 600,000 | 49 (22/45)b | ||
| Genotype 1 | 29 (9/31)c | ||
| Genotype 2 or 3 | 100 (13/13) | ||
| Genotype 4 | 0 (0/1) | ||
Peginterferon alfa-2a
By observation of values in the two studies, children with low baseline viral load appear to have achieved higher SVRs (range 70–74%) than those with a higher viral load at baseline (range 50–55%). Sokal and colleagues57 also reported SVRs according to both baseline viral load and genotype. The results appear to show that a greater proportion of children with genotype 2 or 3 achieved a SVR than those with genotype 1, 4, 5 or 6, regardless of viral load.
Peginterferon alfa-2b
The peginterferon alfa-2b study (Wirth and colleagues59) also found that children with low baseline viral load were more likely to achieve a SVR than those with a higher viral load at baseline (79% vs. 49%, respectively), based on observation of the data. When groups were further split by genotype, SVR rates were higher in children with genotype 2 or 3 (100%) than in those with genotype 1 or 4 (0–29%) in those with high viral load. For those with low baseline viraemia, SVRs were higher in children with genotype 2 or 3 (94%) than genotype 1 (72%), but lower than genotype 4 (100%). Wirth and colleagues59 reported that in genotype 1 patients, the SVR was statistically significantly higher in those with low baseline viral load than in those with high baseline viral load (72% vs. 29%, p = 0.0006).
Previous treatment history
Peginterferon alfa-2a
Both of the peginterferon alfa-2a studies56,57 evaluated only treatment-naive children and SVR results are reported in Table 4 and discussed previously.
Peginterferon alfa-2b
Two peginterferon alfa-2b studies48,51 that recruited both treatment-naive and previously treated participants reported SVR rates separately by treatment history (Table 7). In the study by Pawlowska and colleagues,51 approximately half of the children had been previously treated with non-pegylated interferon alfa-2b plus RBV for 12 months, 2–5 years earlier. One-fifth of the children in the study by Jara and colleagues48 had received treatment with non-pegylated interferon monotherapy 3–5 years earlier.
| Study | Treatment | SVR according to previous treatment | |
|---|---|---|---|
| Treatment history | SVR, % (n/N) | ||
| Pawlowska et al., 201051 | PEG α-2b + RBV 24 or 48 weeks, n = 53 |
Treatment naive | 62 (18/29)a |
| Genotype 1 | 62 (10/16) | ||
| Genotype 3 | 50 (1/2) | ||
| Genotype 4 | 72 (8/11) | ||
| Previously treated | 33 (8/24)a | ||
| Genotype 1 | 27 (3/11) | ||
| Genotype 3 | N/Ab | ||
| Genotype 4 | 30 (4/13) | ||
| Jara et al., 200848 | PEG α-2b + RBV 24 or 48 weeks, n = 30 |
Treatment naive | 55 (11/20)c |
| Previously treated | 17 (1/6)c | ||
As can be seen in Table 7, higher rates of SVR were achieved in participants who were treatment naive (55–62%) than in those who had been previously treated (17–33%). Pawlowska and colleagues51 also reported SVR rates further split by genotype group. Higher SVR rates were again observed in those who were treatment naive than in previously treated participants for both genotypes 1 and 4 (both genotype 3 participants were treatment naive). It should be noted that numerators in the genotype subgroups do not add up correctly to the total number of treatment-naive and previously treated participants, and also that participant numbers in these subgroups were small, and hence these results should be viewed with caution.
Baseline alanine aminotransferase levels
Three included studies reported SVR according to ALT levels at baseline, although none defined ‘normal’ or ‘abnormal’ levels per se. Results are shown in Table 8. As mentioned below (see Biochemical response), there appears to be no clear consensus on what would be considered ‘normal’ ranges of ALT concentrations in children and young adults.
| Study | Treatment | SVR according to ALT levels | |
|---|---|---|---|
| ALT | SVR, % (n/N) | ||
| Schwarz et al., 201156 | PEG α-2a + RBV 48 weeks, n = 55 |
Normal ALT | 70, 95% CI 51 to 88 (16/23) |
| ALT > ULN | 41, 95% CI 24 to 58 (13/32) | ||
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
Normal ALT | 80 (24/30) |
| Genotype 2 or 3 | 89 (8/9) | ||
| Genotype 1, 4, 5 or 6 | 89 (17/19) | ||
| Abnormal ALTa | 58 (19/33) | ||
| Genotype 2 or 3 | 100 (8/8) | ||
| Genotype 1, 4, 5 or 6 | 37b (10/27) | ||
| Wirth et al., 201059 | PEG α-2b + RBV 24 or 48 weeks, n = 107 |
Normal ALT | 67 (42/63)c |
| Genotype 1 | 56 (23/41) | ||
| Genotype 2 or 3 | 90 (18/20) | ||
| Genotype 4 | 50 (1/2) | ||
| Abnormal ALTa | 64 (28/44)c | ||
| Genotype 1 | 48 (15/31) | ||
| Genotype 2 or 3 | 100 (10/10) | ||
| Genotype 4 | 100 (3/3) | ||
Peginterferon alfa-2a
Both of the peginterferon alfa-2a studies56,57 reported SVR according to baseline ALT levels. For both studies, the rate of SVR was higher in those with normal ALT levels at baseline (range 70–80%) than in those whose baseline ALT levels were not normal (range 41–58%) (described as either abnormal57 or ALT > upper limit of normal56). Sokal and colleagues57 also reported results further split by genotype. For children with normal ALT at baseline, SVR was not affected by genotype. However, for children with abnormal ALT at baseline, those with the more favourable genotype 2 or 3 appeared to have a higher SVR rate than those with genotype 1, 4, 5 or 6 (largely genotype 1, as previously stated). Sokal and colleagues57 also reported that in children with genotype 1, 4, 5 or 6, the SVR was statistically significantly higher in those with normal baseline ALT than in those with abnormal baseline ALT [89% vs. 37% (although the text in the publication states 36%), p < 0.001].
Peginterferon alfa-2b
Wirth and colleagues59 was the only peginterferon alfa-2b study that reported SVR by baseline ALT levels (see Table 8). Unlike the two peginterferon alfa-2a studies, SVR appeared to be similar regardless of whether participants had normal or abnormal levels of ALT at baseline (67% and 64%, respectively). Results were further split by genotype. For children with normal ALT at baseline, those with the more favourable genotype 2 or 3 appeared to have a higher SVR rate than those with genotype 1 or 4. This was also the case for children with abnormal baseline ALT; here, those with genotype 2 or 3 had higher SVR rates than those with genotype 1, but not those with genotype 4. However, there were only three children in the latter subgroup, so these results should be interpreted with caution.
Liver histology
Peginterferon alfa-2a
Both the peginterferon alfa-2a studies56,57 reported SVR according to baseline liver histology (Table 9). Schwarz and colleagues56 reported SVRs according to fibrosis stage (none or stages 1–6) using the Ishak fibrosis classification system, as well as inflammation [minimal (grades 1–3) or mild–marked (grades 4–12)] using the Knodell HAI. Both are commonly used systems for classifying liver biopsy samples and determine the severity of HCV infection. A more detailed explanation of biopsy classification systems, and their comparability, is given in Chapter 1, Description of the underlying health problem. Sokal and colleagues57 reported SVRs according to whether or not there was fibrosis, but did not specify which fibrosis classification system was used, making direct comparisons difficult. Those with fibrosis were classified as F1 and F2 only, indicating mild liver disease.
| Study | Treatment | SVR according to liver histology | |
|---|---|---|---|
| Histological parameter | SVR, % (n/N) | ||
| Schwarz et al., 201156 | PEG α-2a + RBV 48 weeks, n = 55 |
Fibrosis stage | |
| None | 43, 95% CI 6 to 80 (3/7) | ||
| Stages 1–6 | 53, 95% CI 39 to 67 (25/47) | ||
| Inflammation HAI | |||
| Minimal (grades 1–3) | 43, 95% CI 23 to 64 (10/23) | ||
| Mild–marked (grades 4–12) | 58, 95% CI 41 to 75 (18/31) | ||
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
No fibrosis | 76 (25/33) |
| Genotype 2,3 | 100 (8/8) | ||
| Genotype 1, 4, 5, 6 | 68 (17/25) | ||
| Fibrosis | 60 (18/30) | ||
| Genotype 2,3 | 89 (8/9) | ||
| Genotype 1, 4, 5, 6 | 48 (10/21) | ||
There did not appear to be any impact of the degree of liver fibrosis on SVR rates. For children with no liver fibrosis at baseline, SVRs were 43% in one study56 compared with 76% in the second study,57 although it should be noted that there were only seven participants in this subgroup in the PEDS-C study. 56 In children with some degree of fibrosis, rates of SVR were more similar between the two studies (53%56 and 60%57). Sokal and colleagues57 further stratified SVRs by genotype, with SVR rates observed to be higher in those with genotype 2 or 3 than in those with genotype 1, 4, 5 or 6, regardless of the level of baseline fibrosis.
In the PEDS-C study by Schwarz and colleagues,56 rates of SVR appeared lower in children with a lower grade of disease activity than in those with mild–marked liver inflammation (43% vs. 58%), although it should be noted that confidence intervals for both groups were wide.
Peginterferon alfa-2b
No peginterferon alfa-2b studies reported SVR according to liver histology.
Multivariate analysis of predictors of sustained virological response
Only one study, PEDS-C,56 used a multivariate approach to explore factors predictive of SVR (based on a logistic model). However, the PEDS-C trial included data from a placebo monotherapy arm which is outside the scope of the current assessment. The following significant predictors of SVR were identified (for full results see the data extraction form in Appendix 3): female sex; non-maternal HCV transmission; genotype non-1; moderate or marked liver inflammation; absence of steatosis; and lower baseline levels of HCV RNA.
Virological response during treatment
All seven included studies reported virological response at various time points during treatment, including RVR, EVR and EOT. RVR is defined as a viral load that does not exceed a specified (although not standardised) limit after 4 weeks of therapy. EVR can be defined as complete EVR, which means HCV RNA is undetectable after 12 weeks, or partial EVR, which means virus is still detectable but there has been at least a 2 log10 drop compared with the baseline value.
Rapid virological response was reported by two studies (one peginterferon alfa-2a56 and one peginterferon alfa-2b48), although in the PEDS-C study56 it was defined as a lack of detectable HCV RNA at week 5 of treatment (rather than at week 4). Jara and colleagues48 reported the proportion of children with negative HCV RNA at week 4 of treatment, which we infer to be RVR, although this was not explicitly defined as such by the authors. All seven studies reported EVR. Three peginterferon alfa-2b studies did not specifically define EVR but reported undetectable47,59 or negative48 HCV RNA at week 12 of treatment, which we infer to be EVR. A fourth peginterferon alfa-2b study46 defined EVR as a HCV RNA level < 50 IU/ml at week 12 compared with baseline, while the two peginterferon alfa-2a studies defined EVR as a decrease of ≥ 2 log10 at week 12 compared with baseline. 56,57 Pawlowska and colleagues51 described EVR as ‘levels of HCV RNA viral load at week 12 of treatment’ but did not specify if levels had to be undetectable or reach a lower limit (they did, however, define these for the subcategories of complete and partial EVR – see the data extraction form in Appendix 3). Six of the included studies reported EOT, defined as undetectable HCV RNA at the end of treatment (48 weeks,56 52 weeks,47 or 24 or 48 weeks57)51 or HCV RNA < 50 IU/ml at week 48. 46 The sixth study (Wirth and colleagues59) reported EOT but did not provide a definition. Results can be seen in Table 10.
| Study | Treatment | Virological response | |
|---|---|---|---|
| Virological parameter | Proportion with response, % (n/N) | ||
| Schwarz et al., 201156 | PEG α-2a + RBV 48 weeks, n = 55 |
RVR (week 5) | 24 (13/55)a |
| EVR (week 12) | 59 (32/55)a | ||
| EOT (week 48) | 64b (35/55)a | ||
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
EVR (week 12) | 65 (42/65)c (3ND) |
| EOT (week 24 or 48) | 68 (44/65)c (2ND) | ||
| Al Ali et al., 201046 | PEG α-2b + RBV 48 weeks, n = 12 |
EVR (week 12) | 83 (10/12) |
| EOT (week 48) | 83 (10/12) | ||
| Pawlowska et al., 201051 | PEG α-2b + RBV 24 or 48 weeks, n = 53 |
EVR (week 12) | 77 (41/53) |
| EOT (week 48) | 66 (35/53) | ||
| Wirth et al., 201059 | PEG α-2b + RBV 24 or 48 weeks, n = 107 |
EVR (week 12) | 68 (73/107)d |
| EOT (week 24 or 48) | 70 (75/107)d | ||
| Ghaffar et al., 200947 | PEG α-2b + RBV 52 weeks, n = 7 |
EVR (week 12)e | 29 (2/7) |
| EOT (week 52) | 43 (3/7) | ||
| Jara et al., 200848 | PEG α-2b + RBV 24 or 48 weeks, n = 30 |
RVR (week 4)e | 3 (1/30) |
| EVR (week 12)e | 52 (15/29) | ||
Peginterferon alfa-2a
Only one peginterferon alfa-2a study (Schwarz and colleagues56) presented results for RVR. The proportion of participants who achieved a RVR (at week 5) was 24%. Both studies reported similar rates of EVR, ranging from 59% to 65%. Sokal and colleagues57 performed a statistical comparison between the genotype subgroups and found that children with genotype 2 or 3 achieved a significantly higher EVR than those with genotype 1, 4, 5 or 6 (83% vs. 57%, p < 0.05). EOT rates were also similar between the two peginterferon alfa-2a studies (64% at 48 weeks56 and 68% at 24 or 48 weeks57). Sokal and colleagues57 again reported a statistically significant difference between the genotype subgroups, whereby 94% of children with genotype 2 or 3 achieved an EOT compared with 57% of children with genotype 1, 4, 5 or 6 (p < 0.001).
Peginterferon alfa-2b
One peginterferon alfa-2b study (Jara and colleagues48) reported RVR, with only one participant (3%) achieving a RVR at week 4. This is lower than the rate reported in the peginterferon alfa-2a study. 56 EVR ranged from 52% to 83% across four peginterferon alfa-2b studies,46,48,51,59 while the very small study by Ghaffar and colleagues47 was an outlier reporting a much lower rate (29%). EOT rates were similar across three of the four peginterferon alfa-2b studies that reported them,46,51,59 with a range of 66% to 83%; again, Ghaffar and colleagues’ study47 was an outlier, reporting a lower EOT response rate (43% at week 52) compared with the other studies (66%51 and 83%46 at week 48, and 70% at week 24 or 4859).
Non-response and relapse
Five studies (both the peginterferon alfa-2a studies56,57 and three peginterferon alfa-2b studies46,48,51) reported the proportion of participants who did not respond to treatment, although a specific definition of non-response was not given by any of the studies. Five studies (one peginterferon alfa-2a study56 and four peginterferon alfa-2b studies46,48,51,59) reported data for participants who relapsed. Relapse was defined by three studies as the reappearance of HCV RNA (detectable HCV RNA at week 72,51 at last follow-up59 or after stopping therapy56) after previously having undetectable HCV RNA at the end of treatment. Two of the peginterferon alfa-2b studies reported data but did not specifically define relapse. 46,48 Results can be seen in Table 11.
| Study | Treatment | Non-response, % (n/N) | Relapse, % (n/N) |
|---|---|---|---|
| Schwarz et al., 201156 + related publication28 | PEG α-2a + RBV 48 weeks, n = 55 |
25 (14/55) | 17 (9/55)a |
| Sokal et al., 201057 | PEG α-2a + RBV 24 or 48 weeks, n = 65 |
12 (8/65)b | NR |
| Al Ali et al., 201046 | PEG α-2b + RBV 48 weeks, n = 12 |
17 (2/12) | 8 (1/12) |
| Pawlowska et al., 201051 + abstract52 | PEG α-2b + RBV 24 or 48 weeks, n = 53 |
51 (27/53) | 17 (9/53)c |
| Wirth et al., 201059 | PEG α-2b + RBV 24 or 48 weeks, n = 107 |
NR | 12 (9/72)d 8 (9/107)e |
| Jara et al., 200848 | PEG α-2b + RBV 24 or 48 weeks, n = 30 |
47 (14/30) | 3 (1/30) |
Peginterferon alfa-2a
Both peginterferon alfa-2a studies presented results for non-response,56,57 and this ranged from 12% to 25%. The proportion of participants with virological relapse was only reported in the PEDS-C study56 and was found to be 17%.
Peginterferon alfa-2b
Rates of non-response in the three peginterferon alfa-2b studies46,48,51 varied, with one study (Al Ali and colleagues46) reporting a rate of 17% which was similar to those reported in the two peginterferon alfa-2a studies. The authors46 stated that the two non-responders had baseline HCV RNA levels that were higher than those of most of the other patients, but do not provide any quantitative data to support this. The other two studies48,51 reported higher rates, ranging from 47% to 51%.
The proportion of participants with virological relapse reported in four peginterferon alfa-2b studies46,48,51,59 ranged from 3% to 17%. Wirth and colleagues59 stated that relapse only occurred in patients with genotype 1.
Biochemical response
Peginterferon alfa-2a
Neither of the two studies on peginterferon alfa-2a reported biochemical outcomes.
Peginterferon alfa-2b
Three of the five studies on peginterferon alfa-2b reported changes in liver enzyme concentrations in response to treatment. In the small study by Ghaffar and colleagues,47 median concentrations of serum ALT and aspartate aminotransferase (AST) had each declined to around 50% of their baseline values after 52 weeks (the statistical significance of these changes was not reported) (Table 12). Two larger studies48,59 mentioned changes in ALT but did not report absolute values of ALT concentrations. Wirth and colleagues59 reported that normalisation of ALT occurred in 34 of 44 patients (77%) who had elevated ALT at baseline. Jara and colleagues48 mentioned that 28 of 30 patients (93%) had elevated ALT levels at baseline; in 14 of 15 children (93%) who attained a SVR during the first month, ALT values normalised and remained normal throughout the treatment and follow-up.
| Study | Treatment | Biochemical responsea | |
|---|---|---|---|
| Baseline median, IU/l (range) | Post-treatment week 52 median, IU/l (range) | ||
| Ghaffar et al., 200947 | PEG α-2b + RBV 52 weeks, n = 7 |
ALT: 77 (52–223) AST: 76 (63–321) |
ALT: 39 (17–63) AST: 38 (20–69) |
The ranges of ALT and AST concentrations found in these studies are difficult to compare with what would be considered ‘normal’ ranges in clinical practice, as (based on clinical expert opinion) there are no universally agreed standard reference ranges for children, and the concentrations that would be considered ‘normal’ vary between laboratories and age groups.
Histological response
Peginterferon alfa-2a
Neither of the two studies on peginterferon alfa-2a reported histological outcomes.
Peginterferon alfa-2b
Only the small study by Ghaffar and colleagues47 reported changes in histology in response to treatment. However, results were only provided for four of the seven participants. Based on the HAI, three patients showed a small improvement relative to baseline and one patient exhibited fibrosis regression. As these results were based on a subgroup of a very small population, they should be interpreted with caution.
Quality of life
Peginterferon alfa-2a
Only the PEDS-C study56 reported changes in participants’ QoL, assessed using the Child Health Questionnaire (CHQ) – Parent Form 50. 27,28,53 PEDS-C also reported changes in participants’ behavioural and emotional functioning [using the Child Behaviour Checklist (CBCL)], depression [using the Children’s Depression Inventory (CDI)] and cognitive functioning [using the Behaviour Rating Inventory of Executive Function (BRIEF)], which may assist interpretation of QoL. 28,53 The CHQ, CBCL and BRIEF instruments were all completed by the child’s parent or guardian whereas the CDI was completed by the child.
The CHQ yielded two composite scores for physical health and psychosocial functioning, as well as scores for 11 different scales [physical functioning; role/social limitations (emotional, physical); general health; bodily pain/discomfort; parent impact (emotional, time); self-esteem; mental health; general behaviour; and family impact]. Scores ranged from 0 to 100, with higher scores reflecting better QoL. The CBCL yielded three composite scores for internalising, externalising and total behaviour problems, and eight clinical scales (anxious/depressed; withdrawn/depressed; somatic problems; social problems; thought problems; attention problems; rule-breaking behaviour; and aggressive behaviour). Higher scores reflect more behavioural or emotional problems, and scores ≥ 65 are considered indicative of clinically significant behaviour problems. For the CDI, a score ≥ 19 is considered indicative of possible clinical depression and for the BRIEF, a score ≥ 65 is considered indicative of clinical impairment in executive function. For each of these assessments, clinical decline was defined as a change in score > 1 standard deviation (SD) plus a change in score classification from no impairment at baseline to clinical impairment at follow-up. Clinical improvement was defined as a > 1-SD change in score plus a change in score classification from clinical impairment at baseline to no impairment at follow-up.
Most of the participants in the peginterferon alfa-2a and RBV arm of the PEDS-C trial (86–95%) showed no clinical changes in any of the measures of QoL, behaviour, depression or executive function after 24 weeks of treatment. The exception was mean CHQ physical summary scores, which declined significantly relative to baseline, indicating an overall worsening of the physical aspects of QoL; eight (15%) of the participants were classified as having experienced a clinically significant decline and no participants as having experienced a clinically significant improvement (Table 13). However, the authors noted that the mean CHQ scores at baseline and at 24 weeks were both within the ‘average range’ (not defined). Three participants (5%) exhibited a clinically significant decline in the depression score, with one being withdrawn from the study following a suicide gesture, but the majority of participants (95%) exhibited no clinical change in depression scores after 24 weeks.
| QoL outcome | Mean ± SD baseline score | Mean ± SD score at 24-week follow-up | Clinically significant improvement, % (n/N) | Clinically significant decline, % (n/N) | No clinical change, % (n/N) | p-value for changes in mean scores |
|---|---|---|---|---|---|---|
| CHQ physical summary | 52.1 ± 4.8 | 49.8 ± 7.5 | 0 | 15 (8/55) | 86 (47/55) | 0.013 (mean change 2.40 ± 6.8) |
| CHQ psychosocial summary | 52.1 ± 7.9 | 52.3 ± 10.2 | 5 (3/55) | 7 (4/55) | 88 (48/55) | NR |
| CBCL internalising | 52.4 ± 8.5 | 51.0 ± 11.0 | 4 (2/55) | 5 (3/55) | 91 (50/55) | NS |
| CBCL externalising | 50.4 ± 9.4 | 48.8 ± 10.3 | 2 (1/55) | 5 (3/55) | 93 (51/55) | NS |
| CBCL total behaviour problem | 51.5 ± 9.3 | 49.7 ± 10.2 | 2 (1/55) | 4 (2/55) | 95 (52/55) | NS |
| CDI total score | 5.9 ± 4.2 | 6.2 ± 5.6 | 0 | 5 (3/55) | 95 (52/55) | NS |
| BRIEF global executive composite | 53.5 ± 9.9 | 52.2 ± 10.1 | 5 (3/55) | 5 (3/55) | 90 (49/55) | NS |
Long-term follow-up of all children who completed 48 weeks of treatment revealed no statistical differences from baseline (p > 0.05) for any of the outcome measures after 1 or 2 years of follow-up. 28 Very few children had clinical elevations on the CBCL, none had a clinically high depression score at 1 year and only one child had a clinically elevated depression score at the 2-year follow-up assessment (no further data were presented28).
As well as presenting changes in QoL for all participants, the PEDS-C trial reported QoL for a subgroup of 41 participants who achieved a virological response at 24 weeks and continued on peginterferon alfa-2a and RBV for 48 weeks28 (see full data extraction form in Appendix 3). It should be noted that this subgroup is small and was likely not powered for subgroup analysis, so results should be interpreted with caution. Mean subgroup scores for QoL, behaviour, depression and executive function assessed at 48 weeks and 6 months decreased slightly but did not differ significantly from baseline (p > 0.05). Most of the children did not experience clinically significant changes in physical QoL (83%), internalising behaviours (95%), externalising behaviours (95%) or total behaviour problems (93%) during treatment. Seven participants experienced clinically significant changes in physical QoL. In two cases, scores had returned to baseline levels by the end of treatment. The remaining five participants experienced an early clinical decline that persisted through the end of treatment, but in three of these cases the scores had returned to baseline values by the 6-month post-treatment assessment.
Peginterferon alfa-2b
None of the five studies on peginterferon alfa-2b reported QoL outcomes.
Growth
Four studies (one peginterferon alfa-2a57 and three peginterferon alfa-2b48,51,59) reported whether or not their participants’ height and weight changed during treatment. These studies presented results for the overall study population, not separately for the subgroups of participants who received treatment for 24 or 48 weeks according to HCV genotype. Changes in growth were often presented only in a brief narrative in the publication text, without quantitative data, and relate to short-term follow-up.
Peginterferon alfa-2a
One of the two peginterferon alfa-2a studies (Sokal and colleagues57) reported changes in participants’ height and weight during treatment. The authors reported that baseline and follow-up z-scores for height were −0.4 ± 1.0 and −0.5 ± 1.1, and those for weight were −0.3 ± 0.9 and −0.3 ± 1.0. These changes in height and weight from baseline to follow-up were not statistically significant.
Peginterferon alfa-2b
Three of the five peginterferon alfa-2b studies48,51,59 reported changes in participants’ height and weight during treatment. Pawlowska and colleagues51 mentioned briefly that there was no influence on height at follow-up (24 weeks after treatment) or 2 years after follow-up. In the remaining two studies, growth rates decreased during treatment but subsequently recovered. Jara and colleagues48 observed that growth during the 48-week period was reduced in 85% of participants (22/26) by 1.6 cm compared with the growth velocity 50th percentile for age and sex (three participants had finished growth before therapy). Growth velocity was entirely normal in the 6-month period after the end of treatment; however, the modest decrease in height percentile observed during therapy was not recovered. Wirth and colleagues59 observed that 70% of participants (75/107) had a clearly inhibited growth velocity (< third percentile) during the treatment phase. Mean growth velocity was 2.47 ± 2.22 cm/year during treatment and increased to 5.73 ± 4.1 cm/year in the follow-up period. Mean height percentiles were 50.87 ± 28.89 in the treatment period and 44.25 ± 27.59 at the end of follow-up, with mean changes in the height percentile of −7.7 and 1.1 during the treatment and follow-up periods, respectively. The decrease in mean height percentile during treatment was greater in participants whose treatment duration was longer (n = 55, mean 334 days) than in those whose treatment duration was shorter (n = 52, mean 155 days) (−11.8 vs. −3.6); however, the statistical significance of these differences was not reported.
The three studies of peginterferon alfa-2b each reported that their participants lost weight during treatment, and they each classified weight loss as an adverse event. Jara and colleagues48 observed that 67% of participants (20/30) experienced weight loss, with 23% of the participants (7/30) losing more than 5% of their baseline weight, although weight gain occurred on cessation of treatment. Overall, body weight decreased by 4.8% by week 24 but returned to baseline values by week 48. 48 Pawlowska and colleagues51 observed that 43% of participants (23/53) experienced weight loss exceeding 10%, with the proportion being lower for treatment-naive children (34.5%, 10/29) than for those previously treated (54.2%, 13/24). Wirth and colleagues59 reported that 19% of participants (20/107) lost weight, with the mean weight percentiles being 56.57 ± 29.35 in the treatment period and 53.39 ± 29.51 at the end of follow-up. This gave mean changes in the weight percentiles of −15.5 and 12.3 during the treatment and follow-up periods, respectively.
Adverse events
Peginterferon alfa-2a
The incidence of dose discontinuation due to adverse events was reported by both studies of peginterferon alfa-2a and was relatively low, ranging from 3%57 to 7%56 (Table 14).
| Event | PEG α-2a + RBV: incidence of event, % (n/N) | PEG α-2b + RBV: incidence of event, % (n/N) | |||||
|---|---|---|---|---|---|---|---|
| Schwarz et al., 201156 (n = 55) | Sokal et al., 201057 (n = 65) | Al Ali et al., 201046 (n = 12) | Pawlowska et al., 201051 (n = 53) | Wirth et al., 201059 (n = 107) | Ghaffar et al., 200947 (n = 7) | Jara et al., 200848 (n = 30) | |
| Dose discontinuation | |||||||
| AE | 7 (4/55)a | 3 (2/65)b | NR | NR | 1 (1/107) | NR | 10 (3/30) |
| Other reasonc | NR | NR | NR | NR | 0 (0/107) | NR | NR |
| Dose modification | |||||||
| Any AE | 51 (28/55)d | 23 (15/65) | NR | NR | 25 (27/107) | 0 (0/7) | NR |
| Anaemia | 11 (6/55)d | 5 (3/65) | 33 (4/12) | 6 (3/53) | 7 (7/107)e | NR | 0 (0/30) |
| Neutropenia | NR | 17 (11/65) | NR | NR | 12 (13/107) | NR | 23 (7/30) |
| Weight/growth | NR | NR | NR | NR | 10 (11/107) | NR | NR |
| Other reason | 2 (1/55)d | 6 (4/65) | NR | NR | 24 (26/107) | NR | NR |
| Serious AE | 4 (2/55) | 6 (4/65) | NR | NR | 0 (0/107)f | NR | NR |
| Death | NR | NR | NR | NR | 0 (0/107) | NR | NR |
The incidence of dose modification for any adverse event was reported by both studies of peginterferon alfa-2a and ranged from 23%57 to 51%. 56 The most frequent specific events leading to dose modification were neutropenia, which was reported in one study only with an incidence of 17%,57 and anaemia, which was reported in both studies with incidence rates of 5%57 to 11%. 56 Dose modification was reported separately for different treatment durations and treatment drugs by Sokal and colleagues. 57 Dose reduction of peginterferon alfa-2a occurred in 22% of participants treated for 24 weeks and 23% of those treated for 48 weeks, while the incidence of dose reduction of RBV due to anaemia in these groups was 0% and 6%, respectively. This is suggestive of a slightly higher risk of RBV dose modification with longer treatment duration. These differences were not tested statistically.
Serious adverse events were defined differently in the two studies. They occurred at relatively low incidence rates of 4% (considered by the authors as possibly secondary to the drug therapy)56 and 6% (it is unclear whether or not these were related to the drug therapy). 57 No deaths were reported.
Both trials of peginterferon alfa-2a reported the incidence of specific adverse events (see full data extractions in Appendix 3 for more details). The most frequent adverse events reported were those typically associated with peginterferon and RBV, and included flu-like symptoms (54–91%), headache (45–62%), injection site reactions (14–45%), myalgia or arthralgia (12–36%), irritability (31–34%) and fatigue (27–34%). One study reported that gastrointestinal symptoms were relatively frequent (56%)56 while the other study reported a 38% incidence of abdominal pain. 57 The PEDS-C trial56 reported that ‘treatment led to significant declines in total white blood cell counts, absolute neutrophil counts and haemoglobin levels which returned to baseline when therapy stopped’ (data were presented in line graphs – not extracted here), but haematological adverse events were not reported by the other study,57 except where noted as reasons for dose discontinuation or modification (see Table 14). Owing to the single-cohort nature of the studies, the incidence rates of adverse events were not tested statistically.
Effects of treatment duration on adverse events were reported by Sokal and colleagues. 57 Thyroid hormone problems occurred in 15% of participants who were treated for 48 weeks but did not occur in any of those treated for 24 weeks. The statistical significance of this difference was not reported and the authors did not specify whether or not this was the only adverse event that differed between the treatment duration subgroups.
Peginterferon alfa-2b
The incidence of dose discontinuation due to adverse events was reported by two peginterferon alfa-2b studies and ranged from 1%59 to 10%. 48 This is similar to the incidence of dose discontinuation reported in the two studies of peginterferon alfa-2a (3% to 7%) (see Table 14).
The incidence of dose modification for any adverse event was not consistently reported in the studies of peginterferon alfa-2b. No dose modification occurred in the small study by Ghaffar and colleagues. 47 Wirth and colleagues59 reported that 25% of the participants (27/107) had a dose reduction or interruption for any adverse event; however, their data for dose modification due to specific adverse events suggest that 53% of the participants (57/107) actually experienced a dose modification (see Table 14). The most frequent specific events that led to dose modification were anaemia (0–33% of participants) and neutropenia (12–23% of participants, but only reported for two of the studies48,59). Wirth and colleagues59 provided separate results for dose modification by age subgroups, showing that older participants (aged 12–17 years) had a higher incidence of dose modification for any reason (35%) than younger participants (aged 3–11 years) (19%), although the difference was not tested statistically.
None of the studies of peginterferon alfa-2b explicitly defined any of their adverse events as serious or reported any deaths (although one study59 did mention that no life-threatening or treatment-related adverse events occurred).
All five studies of peginterferon alfa-2b reported the incidence of specific adverse events (see full data extractions in Appendix 3 for more details), although the types of event that were reported varied among the studies. The most commonly reported adverse events were the same as those observed in the studies of peginterferon alfa-2a. Flu-like symptoms and/or fever occurred in all the studies, affecting 66–100% of their participants. Other frequent adverse events reported were headache (45–67%48,51,59), anaemia (11–33%46,51,59), leukopenia (10–67%,46,51,59), neutropenia (17–33%46,48,59), myalgia and/or arthralgia (33–58%46,48,59) abdominal pain (21–43%48,51,59), injection site reactions (29–34%48,51,59) and nausea and/or vomiting (27–45%48,59). A limitation to interpreting these findings is that adverse events were not consistently reported in all of the studies and it is unclear how frequent these adverse events would have been in those studies which did not mention them. Owing to the single-cohort nature of the studies, the incidence rates of adverse events were not tested statistically.
Differences in the incidence of adverse events with age were reported by Wirth and colleagues, based on subgroups of participants who were aged 3–11 years and 12–17 years. 59 Adverse events that occurred with greater frequency in the older subgroup were blood and lymphatic disorders (30% vs. 9%), neutropenia (23% vs. 6%) and anaemia (10% vs. 4%). The statistical significance of these differences was not reported.
Differences in the incidence of adverse events between previously treated and treatment-naive participants were reported by Pawlowska and colleagues. 51 Adverse events that were more frequent in the previously treated subgroup were flu-like symptoms (79% vs. 55%), headache (67% vs. 28%), weight loss > 10% (54% vs. 35%), injection site local reaction (50% vs. 21%), abdominal pain (42% vs. 3%) and neurasthenia (29% vs. 14%). In contrast, thrombocytopenia was more frequent in the treatment-naive subgroup (21% vs. 8%). The statistical significance of these differences was not reported.
Summary of clinical effectiveness
-
Seven studies (two peginterferon alfa-2a and five peginterferon alfa-2b) were included; six were single-arm, uncontrolled cohort studies and one was a RCT for which only data for a single arm met the inclusion criteria. No studies were identified that compared peginterferon alfa with BSC, nor peginterferon alfa-2a with peginterferon alfa-2b.
-
The studies were relatively small (range 7–107 participants) and of generally poor quality, with a potentially high risk of bias (owing to the study design) and little reporting of data/statistical analysis. Therefore, caution is advised in the interpretation of results. The generalisability of the studies to a UK population of children and young people is uncertain.
-
Sustained virological response rates ranged from 53% to 66% for peginterferon alfa-2a and 29% to 75% for peginterferon alfa-2b. Excluding two studies with very small participant numbers resulted in a range of 49–65% for peginterferon alfa-2b.
-
For both peginterferon alfa-2a and peginterferon alfa-2b, children with genotype 2 or 3 appeared to have higher SVR rates than those with genotype 1 (two peginterferon alfa-2a and three peginterferon alfa-2b studies), and children with low viral load at baseline achieved higher SVR rates than those with high viral load in three studies (two peginterferon alfa-2a and one peginterferon alfa-2b). Where participants were of mixed treatment history (two peginterferon alfa-2b studies), children who were treatment naive were more likely to achieve a SVR than those who had been previously treated. The rate of SVR appeared higher in those with normal than in those with abnormal ALT levels at baseline in the two peginterferon alfa-2a studies, whereas in one peginterferon alfa-2b study SVR rates were very similar irrespective of ALT levels. There did not appear to be any impact of the degree of liver fibrosis on SVR rates in the two peginterferon alfa-2a studies that reported it. It should be noted that numbers of children in some of these subgroups were very small and none of the studies was powered for subgroup analysis, therefore results should be interpreted with caution. In five studies, children with genotype 2 or 3 appeared to have higher SVR rates than those with genotype 1.
-
Rates of non-response were variable, ranging from 12% to 25% (two peginterferon alfa-2a studies) and 17% to 51% (three peginterferon alfa-2b studies). A relapse rate of 17% was reported by one peginterferon alfa-2a study and a range of 3–17% across four peginterferon alfa-2b studies.
-
No conclusions can be drawn on the effect of treatment on biochemical response (normalisation of ALT levels) (three studies), or histological response (one study), as these were poorly and inconsistently reported.
-
In one peginterferon alfa-2a study, a clinically significant decline was reported in physical health (15% of children) and in the QoL depression score (5% of children) 24 weeks after starting treatment, but most children showed no clinical changes in any of the measures of QoL, behaviour, depression or executive function at 24 weeks. For children who completed 48 weeks of treatment, there were no statistical differences from baseline for any of the QoL outcome measures after 1 or 2 years of follow-up.
-
For one peginterferon alfa-2a study, there were no statistically significant changes in height nor weight from baseline to follow-up. For peginterferon alfa-2b (three studies), there was either no impact on height and weight, or rates decreased during treatment but recovered at the end of treatment or follow-up. The impact on growth was often presented only in a brief narrative so results are not reliable.
-
Although not consistently reported, the most frequently occurring adverse events were largely similar across all the studies and were typical of those associated with peginterferon and RBV. These included flu-like symptoms, headache, myalgia and/or arthralgia, gastrointestinal symptoms, injection site reactions, anaemia, leukopenia and neutropenia. Serious adverse events occurred at relatively low incidence rates of 4–6% in the two peginterferon alfa-2a studies that reported them.
-
The incidence of dose discontinuation due to adverse events was relatively low and ranged from 3% to 7% (two peginterferon alfa-2a studies) and 1% to 10% (two peginterferon alfa-2b studies). Dose modifications occurred at a rate of 23–51% in two peginterferon alfa-2a studies, while one small peginterferon alfa-2b study reported no modifications and one other was unclear because of inconsistent reporting. Adverse events leading to dose modification were usually anaemia and neutropenia.
Southampton Health Technology Assessments Centre’s review of clinical effectiveness in manufacturers’ submissions to the National Institute for Health and Care Excellence
Merck Sharp & Dohme: peginterferon alfa-2b and ribavirin
The MSD MS reported a systematic review of clinical effectiveness evidence that was conducted by an independent academic group. The bibliographic databases and search strategies were specified and the searches appear to be reproducible; the study selection, data extraction and quality assessment steps were reported. The MS included eight studies but only presented study characteristics for five of these. The studies included were five non-RCTs of peginterferon alfa-2b and RBV, as well as one RCT and two non-RCT studies of peginterferon alfa-2a and RBV. Of these eight studies in the MS, six met the inclusion criteria for the Assessment Group (AG) report (four on peginterferon alfa-2b46,48,51,59 and two on peginterferon alfa-2a and RBV56,57). One study of peginterferon alfa-2b and RBV63 included in the MS was excluded from our AG appraisal because the population age range exceeded the upper limit specified in the scope. The other study (peginterferon alfa-2a and RBV64) was excluded from our AG appraisal because of the intervention (participants received non-pegylated interferon before peginterferon). Conversely, the AG report includes a non-RCT study of peginterferon alfa-2b and RBV47 that was not included in the MS.
Sustained virological response rates in the MS are comparable with those seen in the clinical effectiveness section here, with only minor discrepancies noted in other virological outcomes (in two studies48,59). The MS reported briefly on growth inhibition and adverse events and presented results of meta-analyses which pooled data for SVR, EVR, relapse, discontinuation of treatment and selected adverse events. In addition to the five studies of peginterferon alfa-2b and RBV, some of the meta-analyses also included three studies of peginterferon alfa-2a and RBV. There appears to be moderate to substantial heterogeneity in these meta-analyses, according to the reported I2-values, but the MS does not provide guidance on interpretation.
Overall, the MSD MS analysis appears reasonably well conducted but the methods of meta-analysis were not reported and interpretation of the meta-analysis results, in light of the apparent study heterogeneity, is unclear. The MS seems to focus on comparing peginterferon alfa-2b and RBV against peginterferon alfa-2a and RBV, but it is unclear how the RCT and non-RCT evidence was combined in the meta-analysis. The MS concludes that both forms of peginterferon and RBV are clinically effective compared with BSC, with no clear differences indicated between the two forms.
Roche: peginterferon alfa-2a and ribavirin
Roche did not conduct a systematic review of clinical effectiveness evidence (bibliographic databases and search strategies were not specified and insufficient detail was given for the search for evidence to be reproducible). The MS provided results primarily from the PEDS-C trial, plus three other non-RCTs. The processes used for inclusion/exclusion screening, data extraction and quality assessment were not reported, nor were the study details or patient characteristics of the non-RCTs. Two of the four studies, including PEDS-C, are included in the present AG report. 56,57 The remaining two studies do not meet the inclusion criteria for the AG assessment because the population age range exceeded the upper limit specified in the scope,65 or the trial was retrospective with no details of peginterferon dose or treatment duration. 66
The MS reported comparative data for both arms of the PEDS-C trial, even though PEG monotherapy is outside the licence and scope. SVR rates in the MS are comparable with those seen in the clinical effectiveness section of the present report. Virological outcomes during treatment (RVR, EVR, EOT) and QoL were not reported in the MS, while data on body composition and growth were reported only from those studies that did not meet the inclusion criteria for the AG report. Subgroup analyses for HIV co-infected and retreated patients were included in the MS using an extrapolation study, carried out using data from four studies for which details are extremely limited. In addition, the numbers of participants are very small.
Overall, the Roche MS appears uncritical and does not provide an explicit interpretation of the clinical evidence. The MS concludes that the PEDS-C trial demonstrates efficacy of peginterferon alfa-2a and RBV over monotherapy (although this comparison is outside the scope of the appraisal). The MS states that there is no safety concern with regard to adverse events; however, only adverse event data from the PEDS-C trial were considered.
Ongoing studies
No ongoing studies were identified in searches.
Chapter 5 Economic analysis
The aim of this section is to assess the cost-effectiveness of peginterferon alfa and RBV in children and young people with chronic HCV.
The economic analysis comprises a systematic review of the literature on the cost-effectiveness of peginterferon alfa and RBV treatment; a systematic review of studies of the HRQoL of patients with chronic HCV; a review of the drug manufacturers’ submissions to NICE; and an independent economic model and cost-effectiveness evaluation (the SHTAC model).
Systematic review of existing cost-effectiveness evidence
A systematic review was undertaken in order to identify economic evaluations of peginterferon alfa treatment in children with chronic HCV. A total of 694 references were identified; one conference abstract67 was identified and retrieved and a further full paper was identified through ad hoc searches and retrieved (Figure 2). 68 Neither study met the criteria for inclusion (see Chapter 3, Inclusion and exclusion criteria) in the systematic review. The full paper68 investigated non-pegylated interferon treatments in children and was therefore excluded on grounds of the intervention. The conference abstract67 did not provide enough detail of methods or results to allow a critical appraisal. Therefore, neither of these studies has been formally quality assessed; however, they are summarised here in terms of the included patient groups, and the assumptions underpinning the economic evaluation, as they provide context for the present review.
FIGURE 2.
Flow chart for the identification of cost-effectiveness studies.
Mernagh and colleagues67 conducted an economic evaluation in Australia and present this in a conference abstract. Their evaluation was conducted in children receiving a single course of peginterferon alfa-2b. The treated patient group was compared with an untreated group. Limited information on the patient group is available; however, children and adolescents with a body weight of at least 27 kg were included as this reflects the lowest dosage allowed in Australia. 67 A lifetime Markov model is the basis for this cost–utility analysis. No detailed information is reported on the assumptions employed in the model. No sources are stated for the natural history, utility, cost or effectiveness inputs except that these were taken from published sources. It is therefore not possible to assess the relevance of this evaluation to the current decision problem. The authors conclude that treatment of HCV with peginterferon alfa-2b is a cost-effective treatment in children, regardless of age. The incremental cost-effectiveness ratio (ICER) presented was reported to be AU$2373 (approximately £1450) per quality-adjusted life-year (QALY) gained.
Sinha and colleagues68 compared a cohort of 10-year-olds with chronic HCV and no co-infection or comorbidity, receiving non-pegylated interferon for 6 months or 12 months, with no treatment. The perspective of the evaluation was societal and the analysis was undertaken on a US population basis. A decision tree model was employed for the treatment phase, from which children entered a Markov model in one of three states: non-responder, sustained response or no sustained response. 68 The authors state that the natural history of chronic HCV in children is a prolonged phase with no progression, which is delayed until adulthood. Therefore, a latent phase was built into the model, with no transition allowed from the children’s state of chronic HCV to more severe states. In the base case this latent phase was set at 15 years, and varied between 0 and 25 years in the sensitivity analysis. Furthermore, the authors state that there is evidence that a higher proportion of paediatric patients will have mild HCV than adults, and mild disease is associated with slower progression than severe disease. 68 Therefore, they have assumed that 90% of the cohort would progress at an annual rate of 1% to cirrhosis and that the remaining 10% would progress at a rate of 10%. After transition to cirrhosis, the rate of further complications is similar across the groups. SVR rates were taken from a pooled estimate of rates from five intervention studies; these were 58% for 6 months’ and 71% for 12 months’ treatment. Ranges around these were tested in sensitivity analyses. Discounting for costs and outcomes was at a rate of 3%, with sensitivity analysis varying this from 0% to 7%. In this study68 the alternatives of ‘no treatment’ and ‘treatment for six months’ with alfa interferon were both dominated by treatment with alfa interferon for 12 months. This strategy continued to dominate where the cohort age was adjusted to 5 and 15 years of age. 68
The two studies summarised in this section did not include any assumptions or data that were relevant for the development of the SHTAC economic model.
Systematic review of health-related quality-of-life studies
A systematic review was undertaken to assess the HRQoL of people with chronic HCV. The aim of the review was to provide data to populate the lifetime economic model with health state utility values to calculate QALYs. Specifically, the aim was to update previous searches for HRQoL in adults41 and complete full searches for studies in children. For adults, the preferred measure of HRQoL is the European Quality of Life-5 Dimensions (EQ-5D)69 and this was used in the previous studies of chronic HCV. We are interested in HRQoL data that are of similar or better quality than those used in previous studies and have therefore restricted our searches to those studies using the EQ-5D. For children, other preference-based generic measures were sufficient (Table 15). The search strategies used are described in Appendix 1. The inclusion and exclusion criteria for the review are shown in Table 15.
| Patients | Children and young people with chronic HCV (aged 3–17 years), including co-infection/previously treated/treatment naive Adults with chronic HCV including co-infection/previously treated/treatment naive (studies dated 2009 onwards) |
| Study design | Primary study or QoL collected as part of a trial In children: using generic, preference-based (VAS/TTO/SG) measures such as EQ-5D, SF-36/SF-6D, HUI In adults: using EQ-5D (not VAS) |
| Other | Abstracts excluded if insufficient data available for critical appraisal |
The search strategy identified 701 papers in adults and 123 papers in children that were potentially relevant. The titles and abstracts were screened, with the full text of nine and five papers retrieved for further inspection for adults and children, respectively. After checking the retrieved papers, one adult study met the inclusion criteria. 70 No studies in children were identified. A summary of the selection process and the reasons for exclusion are presented in Figure 3. For children, four studies were excluded because of incorrect QoL measure and one study was an abstract with insufficient detail for critical appraisal. A list of the excluded studies is shown in Appendix 5. An additional study meeting the inclusion criteria in adults was identified from the bibliography of another study. 71 This study had not been identified in the previous reviews of chronic HCV in adults. We therefore included this study in the present review.
FIGURE 3.
Flow chart of identified studies for HRQoL review in chronic HCV adults and children.
Bjornsson and colleagues70 investigated HRQoL in patients in different stages of chronic HCV-induced liver disease by comparing patients in the mild/moderate fibrosis stage with those with compensated and decompensated cirrhosis as well as those with SVR. Consecutive patients on regular follow-up were recruited in 16 outpatient clinics in nine different centres in Sweden. Patients were included if they had active or previous HCV infection and were excluded if they had previously undergone a liver transplantation or had life-threatening problems such as hepatocellular carcinoma (HCC). Patients with compensated cirrhosis and decompensated cirrhosis due to aetiologies other than HCV were recruited from a single centre. There were 339 chronic HCV patients (Table 16) and 133 non-HCV patients (data not shown here). The study assessed patient HRQoL using the Short Form questionnaire-36 items (SF-36) and EQ-5D questionnaires. The present review focuses on the EQ-5D data only.
| Indication/disease | Participants (n) | Median age, years (IQR) | Sex (%) | EQ-5D index value (SD) |
|---|---|---|---|---|
| Chronic HCV | 158 | 46 (13) | Male 62, female 38 | 0.811 (0.230) |
| Compensated cirrhosis | 76 | 52 (11) | Male 76, female 24 | 0.749 (0.212) |
| Decompensated cirrhosis | 53 | 55 (10) | Male 71, female 29 | 0.656 (0.266) |
| SVR | 52 | 51 (14) | Male 56, female 44 | 0.792 (0.209) |
Across the different cohorts the EQ-5D was shown to vary between 0.656 and 0.811 for decompensated cirrhosis and chronic HCV (mild/moderate fibrosis) respectively, indicating poorer HRQoL in those with decompensated cirrhosis (p < 0.001). The HRQoL in chronic HCV and SVR patients, as measured by the EQ-5D index value (Table 16), was similar to that of healthy controls from the Swedish population (reported in the study as being 0.819).
Chong and colleagues71 investigated the HRQoL of a cohort of 193 chronic HCV patients from Canada using a visual analogue scale (VAS), standard gamble (SG), Health Utilities Index (HUI) and the EQ-5D. The present review focuses on the data from the EQ-5D only. Consecutive patients in two outpatient centres were recruited and were categorised into seven defined groups based on the stage of their HCV. The different categories were no biopsy data, mild/moderate HCV, compensated cirrhosis, decompensated cirrhosis, HCC, transplant and SVR, as seen in Table 17. The number of participants in each group ranged from 9 to 44, and the mean age ranged from 44 to 63 years.
| Category | Participants (n) | Mean age, years (SE) | Sex (%) | Mean utility (95% CI) | Mean utility, Thompson Coon72 (95% CI) |
|---|---|---|---|---|---|
| No biopsy | 35 | 47 (2.1) | Male 51, female 49 | 0.73 (0.62 to 0.83) | – |
| Mild/moderate | 44 | 44 (1.5) | Male 73, female 27 | 0.76 (0.68 to 0.83) | – |
| Compensated cirrhosis | 24 | 57 (2.0) | Male 29, female 71 | 0.74 (0.66 to 0.83) | 0.75 (0.66 to 0.83) |
| Decompensated cirrhosis | 9 | 57 (3.9) | Male 67, female 33 | 0.66 (0.46 to 0.86) | 0.66 (0.46 to 0.86) |
| HCC | 15 | 63 (2.7) | Male 93, female 7 | 0.65 (0.44 to 0.86) | 0.64 (0.44 to 0.86) |
| Transplant | 30 | 54 (1.7) | Male 70, female 30 | 0.69 (0.62 to 0.77) | – |
| SVR | 36 | 48 (1.3) | Male 64, female 36 | 0.83 (0.77 to 0.90) | – |
The EQ-5D was seen to vary between 0.65 for HCC patients and 0.83 for those with a SVR following treatment with non-pegylated interferon and RBV. The authors compared the EQ-5D scores from each of the seven subgroups with the Canadian population norms [0.821, 95% confidence interval (CI) 0.810 to 0.832] and these were seen to be statistically significantly different, except for the SVR group. In this study71 the HRQoL was therefore reduced in all participants except those who had been successfully treated. Those with decompensated cirrhosis or HCC and those who had received a liver transplant had observably lower HRQoL; however, this was not statistically analysed. Since publication of the Chong and colleagues study71 the authors of a UK-based study72 have applied UK social preference weights to the individual patient data for the compensated cirrhosis, decompensated cirrhosis and HCC data, to produce EQ-5D scores of direct relevance to the UK population (Table 17).
Both of the included studies assessed the EQ-5D of adults with chronic HCV in different stages of the condition. Although the groups were not directly comparable, there were similarities within their case definitions. Some differences can be observed between the estimates from the two studies. In the chronic HCV/mild-to-moderate patients, the EQ-5D was seen to be higher in the Bjornsson and colleagues70 study than in the Chong and colleagues71 study (0.811 vs. 0.76). In the SVR groups of the two studies, the EQ-5D estimates were seen to be higher in the Chong and colleagues71 study than in the Bjornsson and colleagues70 study (0.83 vs. 0.79). Rates for compensated cirrhosis and decompensated cirrhosis were seen to be very similar despite the slightly different case definitions used between the two studies. Both of these studies had reasonable sample sizes, although the Bjornsson and colleagues70 study was larger, and there were fewer categories used in this study, which may, in part, explain the differences observed. Neither of these studies is directly generalisable to the UK population, and both were in adult populations only. Despite this, and in the absence of evidence in children, it would appear that these estimates are reasonably robust and update estimates previously applied in UK economic evaluations. As such, these will be applied in the present economic evaluation (see Southampton Health Technology Assessments Centre’s data sources for further details).
Review of evidence submissions from manufacturers to the National Institute for Health and Care Excellence
Merck Sharp & Dohme’s submission: cost-effectiveness analysis
Overview
The MSD submission to NICE consists of a written document (containing submitted evidence on the clinical effectiveness and a cost-effectiveness analysis) and a fully executable, electronic copy of the MSD economic model. The MS reports the total costs, the QALYs gained and the cost-effectiveness associated with the treatment of children and young people with chronic HCV with peginterferon alfa (-2a and -2b) and RBV compared with supportive care. The analysis was conducted from the perspective of the NHS and personal and social services (PSS) over a lifetime horizon. The results are presented for several age subgroups and for subgroups relating to HCV genotype.
The MS carried out targeted searches for cost-effectiveness studies for the treatment of paediatric HCV. It found two studies but neither study was relevant to the current decision problem.
Modelling approach
The cost-effectiveness model adopted for the MS is a state transition Markov model that is structurally similar to a published model previously used for adults with chronic HCV. 20,41 The manufacturer’s state transition diagram describing the health states within the model and the allowable transitions between these states is shown in Appendix 7. The model estimates the morbidity and costs resulting from progressive liver disease and treatment costs. It has a lifetime horizon (until the age of 100 years) with a cycle length of 1 year, except for the first year. The model consists of seven non-absorbing health states (SVR, mild HCV, moderate HCV, HCV with compensated cirrhosis, HCC, decompensated cirrhosis and liver transplant) and one absorbing health state of death.
In the first year patients receive treatment for 12, 24 or 48 weeks, depending on the stopping rule and patient genotype. For genotype 2 and 3 patients, the first year was split into two cycles: the first 24 weeks when all patients receive treatment, and the remaining weeks until the end of the year when patients either respond with a SVR or continue with treatment. For genotypes 1 and 4, the first year was split into three cycles: in the first 12 weeks all patients receive treatment, in weeks 13–48 patients remain on treatment only if an EVR was achieved, and in weeks 48–52 responders with a SVR discontinue treatment and those without a SVR continue.
In the absence of child-specific transition probabilities, the adult transition probabilities were used from previous technology appraisals for the treatment of chronic HCV in adults. 20,41 The same transition probabilities were used across all genotypes, in line with these appraisals.
Assumptions
The MS used most of the previous assumptions from the previous HTA model. 20,41 In addition, they stated the following assumptions:
-
The base-case analysis did not take into account spontaneous viral clearance.
-
It was assumed that the treatment would discontinue if an EVR (i.e. undetectable HCV RNA at treatment week 12) was not achieved at week 12.
-
Adult transition probabilities (except for the transitions from mild to moderate and from moderate to compensated cirrhosis), utility weights and health state costs (except for SVR state costs) were applied owing to the lack of data for paediatric patients.
Critical appraisal of model
The MSD MS was appraised for methodological quality and generalisability to the UK NHS using a checklist adapted from the NICE reference case requirements69 and Philips and colleagues’ checklist45 (see Appendix 8). The submission meets all of the requirements for methodological quality and generalisability, except that it did not provide any evidence that the economic model had been validated.
Estimation of effectiveness
The main treatment effect applied in the model is the SVR for treated patients, with the proportion of patients in each of the modelled populations achieving a SVR based on data from clinical trials conducted in the relevant patient populations. The effectiveness was derived from a systematic review of the literature for the efficacy of peginterferon alfa and RBV. The review identified eight clinical trials in paediatric patients. 46,48,51,56,57,59,63,64 A meta-analysis was then conducted to synthesise the data by genotype (see Chapter 4, Southampton Health Technology Assessments Centre’s review of clinical effectiveness in manufacturers’ submissions to the National Institute for Health and Care Excellence for limitations of this meta-analysis). The treatment efficacy estimates used in the model are shown in Table 18 and Appendix 7. The MS also uses EVR for genotypes 1 and 4, where the proportion of patients who achieve EVR is 0.64 and 0.61 for peginterferon alfa-2a and peginterferon alfa-2b, respectively.
| Genotypes | Treatment | SVR | ||
|---|---|---|---|---|
| Proportion | 95% CI | Distribution and parameters | ||
| 2 and 3 | PEG α-2a + RBV | 0.84 | 0.69 to 0.95 | Beta α = 24.82; β = 4.73 |
| PEG α-2b + RBV | 0.92 | 0.80 to 0.99 | Beta α = 27.90; β = 2.43 | |
| 1 and 4 | PEG α-2a + RBV | 0.52 | 0.42 to 0.62 | Beta α = 49.34; β = 45.55 |
| PEG α-2b + RBV | 0.51 | 0.45 to 0.58 | Beta α = 115.37; β = 110.85 | |
The trials identified and chosen differ slightly from those in the present clinical effectiveness systematic review. The reasons for the differences and a review of the clinical effectiveness data, presented in the MS, are given in Chapter 4, Southampton Health Technology Assessments Centre’s review of clinical effectiveness in manufacturers’ submissions to the National Institute for Health and Care Excellence.
Estimation of quality-adjusted life-years
Merck Sharp & Dohme conducted a systematic literature review on the HRQoL of children and young people with HCV that identified four studies; however, none of these were considered to be appropriate for use in the analysis. Adult values were identified as the most appropriate estimates. The utility weights were obtained from previous HTAs (see Appendix 7). 20,41 Utilities used in the MSD model can be seen below in Critique of the manufacturers’ submissions for Merck Sharp & Dohme and Roche, and in Appendix 7.
Estimation of costs
The costs included in the model consisted of treatment-related costs including drug acquisition costs, costs associated with treatment initiation and on-treatment and post-treatment monitoring, and health state costs. Costs were based upon previous HTAs,20,41 with adjustment to reflect the experience of a child or young person with HCV as advised by experts, and inflated to 2010–11 prices using the Hospital and Community Health Services (HCHS) Index. 73 Health state costs used were from the previous NICE technology assessments of the treatment of chronic HCV in adults (see Appendix 7). 20,41 Health state costs were inflated to 2010–11 prices using the HCHS Index. 73
Unit prices for the treatments were obtained from the British National Formulary (BNF) 63. 74 The dosages used were 180 µg/1.73 m2 per week for peginterferon alfa-2a, 60 µg/m2 per week for peginterferon alfa-2b and 15 mg/kg for RBV. The following assumptions were made in order to calculate the treatment cost:
-
RBV oral solution The number of bottles per month was rounded up to the nearest integer.
-
RBV capsule/tablet The number of capsules required per day was calculated based on the summary of product characteristics. No wastage was considered.
-
Peginterferon alfa-2a The number of syringes required per administration was rounded up to the nearest integer (for syringes of 135 or 180 µg).
-
Peginterferon alfa-2b The number of syringes required per administration was rounded up to the nearest integer (for syringes of 50, 80, 100, 120 or 150 µg).
The treatment cost of a course of peginterferon alfa in combination with RBV was:
-
genotypes 2 and 3 (24-week treatment)
-
age 3–4 years: £2400.00 on peginterferon alfa-2b
-
age 5–8 years: £3326.20 on peginterferon alfa-2a; £3180.42 on peginterferon alfa-2b
-
age 9–13 years: £3628.06 on peginterferon alfa-2a; £4370.16 on peginterferon alfa-2b
-
age 14–17 years: £4558.02 on peginterferon alfa-2a; £4554.80 on peginterferon alfa-2b
-
-
genotypes 1 and 4 (48-week treatment)
-
age 3–4 years: £4800.00 on peginterferon alfa-2b
-
age 5–8 years: £6652.40 on peginterferon alfa-2a; £6360.84 on peginterferon alfa-2b
-
age 9–13 years: £7256.12 on peginterferon alfa-2a; £8740.32 on peginterferon alfa-2b
-
age 14–17 years: £9116.03 on peginterferon alfa-2a; £9109.59 on peginterferon alfa-2b.
-
Treatment-related costs for treatment initiation and on-treatment and post-treatment monitoring can be seen in Critique of the manufacturers’ submissions for Merck Sharp & Dohme and Roche below, and in Appendix 7.
Cost-effectiveness results
The MS reports results by age group and genotype, in terms of total costs, life-years and QALYs. Table 19 shows the base-case results for all patients (aged 5–17 years). Patients receiving peginterferon alfa-2a, peginterferon alfa-2b and BSC accrued a total of 19.16, 19.24 and 16.77 discounted QALYs at a cost of £17,798, £17,526 and £22,750, respectively. Both combinations of peginterferon alfa and RBV dominated BSC in all patients (aged 5–17 years) and in age and genotype subgroup analyses. Peginterferon alfa-2b dominated peginterferon alfa-2a in all patients (aged 5–17 years) and in all subgroup analyses, except in patients aged 9–13 years and those with HCV of genotypes 1 and 4.
| Treatment | Vs. supportive care | ||||||
|---|---|---|---|---|---|---|---|
| Cost (£) | Life-years | QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) | |
| Supportive care | 22,750 | 56.15 | 16.77 | NA | NA | NA | NA |
| PEG α-2a | 17,798 | 63.84 | 19.16 | –4952 | 7.69 | 2.39 | Dominates |
| PEG α-2b | 17,526 | 64.09 | 19.24 | –5224 | 7.94 | 2.47 | Dominates |
The MS conducted deterministic sensitivity analyses (DSAs) around structural assumptions (time horizon, discount rates) and the model parameter values. The DSA results showed that peginterferon alfa-2b dominated BSC in nearly all analyses, except for time horizon and discount rates. The ICERs for peginterferon alfa-2b versus peginterferon alfa-2a were robust to variation in the model parameters, i.e. peginterferon alfa-2b dominated peginterferon alfa-2a for all analyses. The MS probabilistic sensitivity analyses (PSAs) showed that there is a 100% probability that peginterferon alfa-2a and peginterferon alfa-2b in combination with RBV are cost-effective. The cost-effectiveness plane for cost and QALYs for the treatments for the PSA are shown in Figure 4. The cost-effectiveness acceptability curve (CEAC) is shown in Figure 5.
FIGURE 4.
Merck Sharp & Dohme’s cost-effectiveness plane for all patients aged 5–17 years.
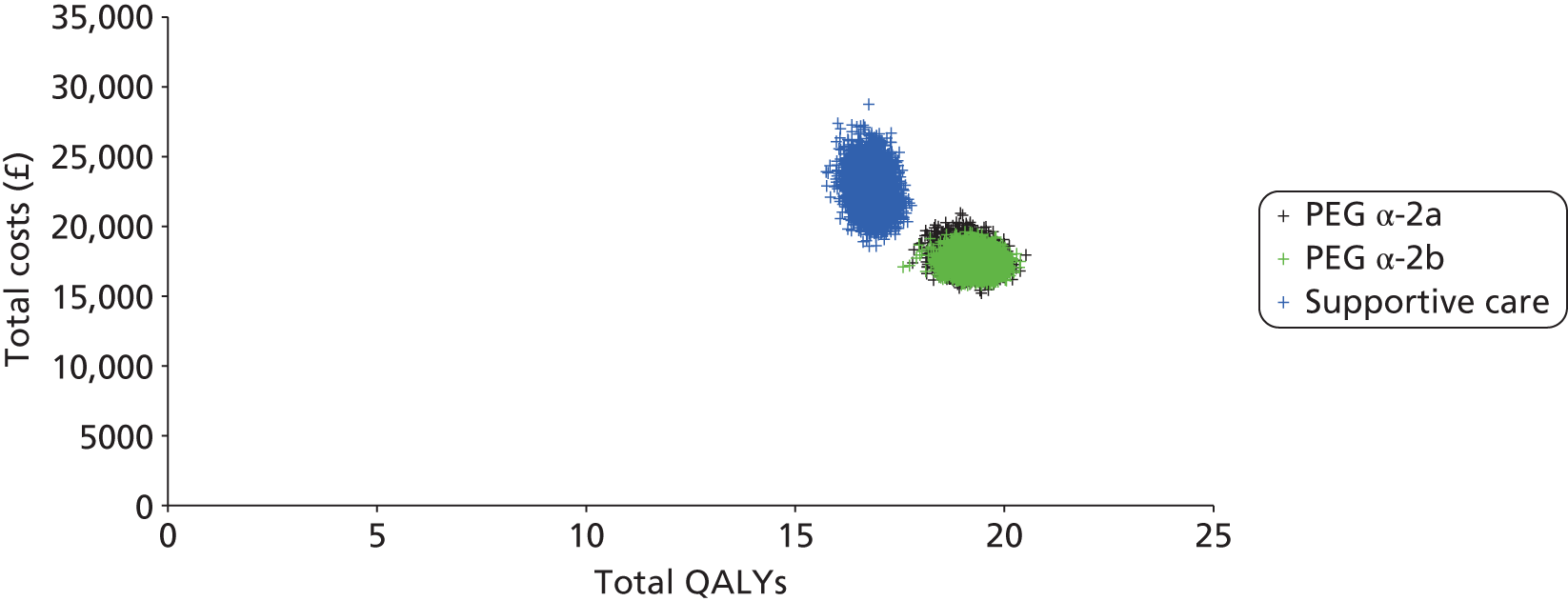
FIGURE 5.
Merck Sharp & Dohme’s CEAC for all patients aged 5–17 years.
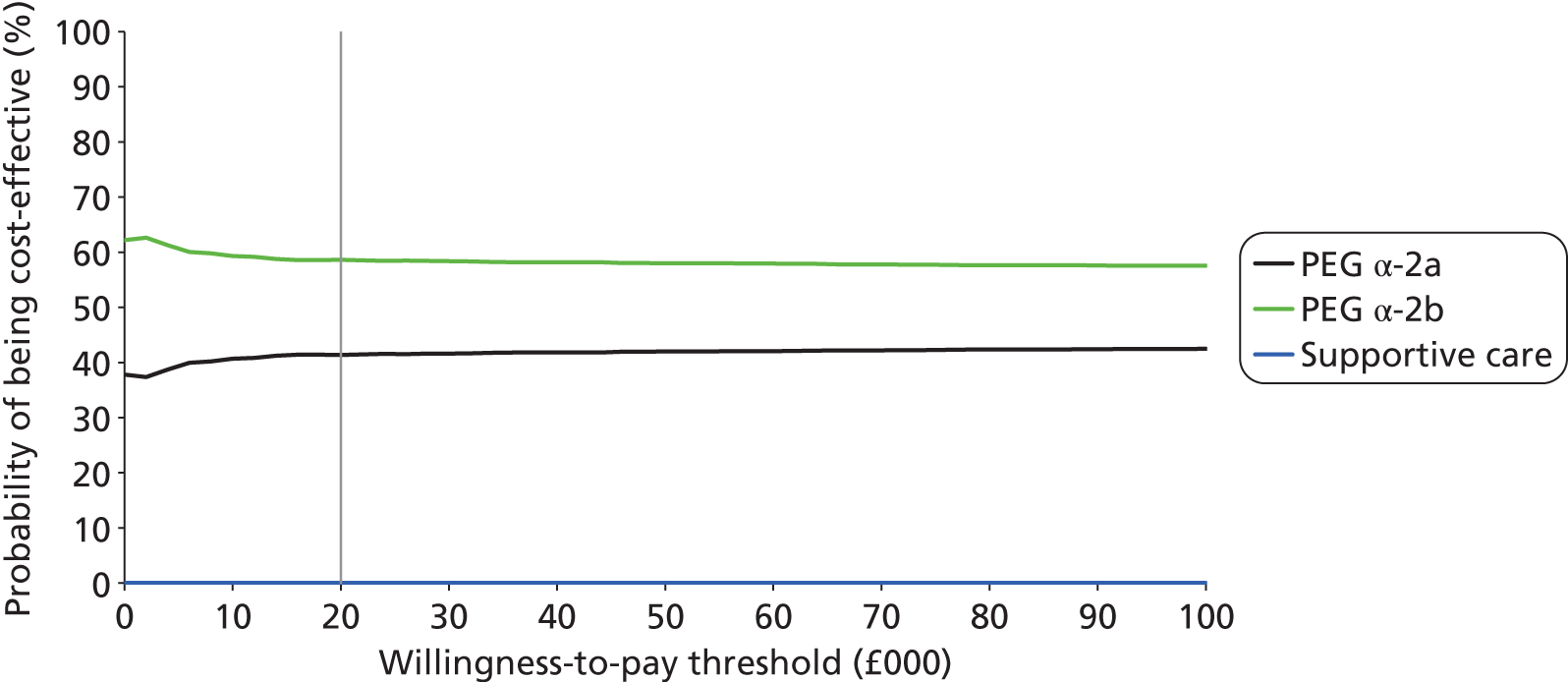
Summary of Merck Sharp & Dohme’s submission
-
The MSD model was based upon that developed in previous HTAs for chronic HCV in adults.
-
The submission met all but one criterion for methodological quality.
-
The model compared peginterferon alfa-2a with peginterferon alfa-2b and BSC.
-
Treatment efficacy was estimated for SVR as a weighted average of the eight clinical trials for peginterferon alfa-2a and peginterferon alfa-2b.
-
The base-case analysis did not take into account spontaneous viral clearance.
-
It was assumed that the treatment would be discontinued if an EVR was not achieved at week 12 for genotypes 1 and 4.
-
Adult transition probabilities (except for the transitions from mild to moderate and from moderate to compensated cirrhosis), utility weights and health state costs (except for SVR state costs) were applied to paediatric patients owing to the lack of data.
-
A lifetime horizon was used.
Roche’s submission to the National Institute for Health and Care Excellence: cost-effectiveness analysis
Overview
The Roche submission to NICE consists of a written document (containing submitted evidence on the clinical effectiveness and a cost-effectiveness analysis) and a fully executable, electronic copy of the Roche economic model. The MS reports the total costs, the QALYs gained and the cost-effectiveness associated with the treatment of children and young people with chronic HCV with peginterferon alfa-2a and RBV, compared with BSC. The analysis was conducted from the perspective of the NHS over a 30-year time horizon. The results are presented for a baseline population of children aged 11 years with chronic HCV who were treatment naive and had no co-infection.
Modelling approach
The cost-effectiveness model adopted for the MS is a Markov model that is structurally similar to a published model previously used for adults with chronic HCV. 20,41 The manufacturer’s state transition diagram, describing the health states within the model and the allowable transitions between these states, is shown in Appendix 7. The model estimates the morbidity and costs resulting from progressive liver disease and treatment costs. It has a time horizon of 30 years with a cycle length of 1 year. The MS comments that the time horizon chosen was considered long enough to capture important costs and effects arising from treatment, as the care pathway is difficult to predict for paediatric patients in whom initial treatment is unsuccessful. The base-case analysis considers treatment-naive patients, as reflected in four published clinical trials56,57,65,66 of peginterferon alfa-2a and RBV. The proportions of participants who enter with mild and moderate chronic HCV (88% and 12%, respectively) are based upon a weighted average of data from the four clinical trials. The model consists of seven non-absorbing health states (SVR, mild chronic HCV, moderate chronic HCV, chronic HCV with compensated cirrhosis, HCC, decompensated cirrhosis and liver transplant) and one absorbing health state of death.
The MS model included a probability of spontaneous SVR for untreated children with chronic HCV, based upon a rapid review conducted by Roche. The results of its rapid review suggested that the probability of spontaneous SVR may vary depending upon how and when the infection was acquired – through vertical transmission at birth or other means during infancy or childhood. From the clinical trial evidence identified,56,57,65,66 the MS used an average of 70% of patients with vertically acquired chronic HCV and 30% with non-vertically acquired chronic HCV. Those children with vertically transmitted chronic HCV were assumed to have spontaneous SVR within the first 5 years only, and had an annual probability of 2.37% during this time. Similarly, spontaneous SVR was assumed to occur only during the first 5 years of infection for non-vertically transmitted chronic HCV, with a probability of 1.65%.
Roche conducted a rapid review on the natural history of HCV acquired in childhood. Their review found that observational data from several studies suggest that chronic HCV acquired in childhood progresses more slowly than that acquired in adulthood. They estimated the transition probabilities using a study by Guido and colleagues,75 a multicentre retrospective study that analysed fibrosis progression and its related risk factors in paediatric chronic HCV. Guido and colleagues75 found that the mean disease duration for paediatric patients with compensated cirrhosis was almost 20 years. For the transitions to more severe health states, such as decompensated cirrhosis, HCC and liver transplantation, adult transition probabilities were used from previous HTAs of the treatment of chronic HCV in adults20,41 (see Appendix 7).
Assumptions
The MS used most of the assumptions from the previous HTA model. In addition, it stated the following assumptions:
-
Spontaneous viral clearance was included within the model for untreated patients.
-
No chronic HCV-related costs were assumed to accrue to patients achieving SVR.
Critical appraisal of model
The Roche MS was appraised for methodological quality and generalisability to the UK NHS using a checklist adapted from the NICE reference case requirements69 and the Philips and colleagues checklist45 (see Appendix 8). The submission meets all of the requirements for methodological quality and generalisability, except that it did not provide any evidence that the economic model had been validated (see Appendix 7).
Estimation of effectiveness
Treatment efficacy was estimated for SVR as a weighted average of the four clinical trials for peginterferon alfa-2a. 56,57,65,66 The weighted average SVR was 59% for genotypes 1, 4, 5 and 6 and 89% for genotypes 2 and 3 with 24 weeks’ treatment (Table 20; see also Appendix 7). The trials identified and chosen differ from those in the present clinical effectiveness review. The reasons for the differences and a review of the clinical effectiveness data, presented in the manufacturer submissions, are given in Chapter 4 (see Southampton Health Technology Assessments Centre’s review of clinical effectiveness in manufacturers’ submissions to the National Institute for Health and Care Excellence).
| Parameter | Genotypes 1/4/5/6 | Genotypes 2/3 (48 weeks’ treatment) | Genotypes 2/3 (24 weeks’ treatment) | ||
|---|---|---|---|---|---|
| SVR (%) | Dropout (%) | SVR (%) | Dropout (%) | SVR (%) | |
| Weighted average | 59 | 23 | 80 | 10 | 89 |
Estimation of quality-adjusted life-years
Roche conducted a systematic literature review on the HRQoL of children and young people with HCV which identified two partially applicable studies reporting utilities of children with chronic HCV. However, both were based on an expert’s time trade-off (TTO) values for adults with chronic HCV. Adult values were identified by the MS as the most appropriate estimates. The utility weights were obtained from previous HTAs. 20,41 Health state utility values were estimated in a stepwise fashion using a relative effect compared with a baseline utility for the general population. A utility multiplier for the health state was derived by comparing the utility in the literature with the utility of the general population with the same age and gender composition. These utility multipliers were then applied to baseline utilities.
For children under the age of 17 years the economic model applied a baseline utility of 0.95 based on a study by Saigal and colleagues. 76 For the healthy population aged 17 years and above, the model applied the utilities of adults derived using an algorithm developed by Ara and Brazier. 77 Utilities used in the Roche model can be seen in Critique of the manufacturers’ submissions for Merck Sharp & Dohme and Roche and in Appendix 7.
Estimation of costs
The model costs consisted of treatment-related costs, including drug acquisition costs, costs associated with treatment initiation and on-treatment and post-treatment monitoring, and health states costs. Unit prices for the treatments were obtained from BNF 63. 74 The doses used in the analysis were in line with the dosing schedule in the relevant clinical trials. Drug costs for peginterferon alfa-2a were calculated for a dosage of 180 µg/1.73 m2 BSA (maximum 180 µg) subcutaneously, once weekly. RBV (as Copegus®) was administered orally in a dose of 15 mg/kg body weight, twice daily (maximum 1200 mg/day for body weight ≥ 75 kg and 1000 mg/day for body weight < 75 kg). In the base case the estimated costs for 48 weeks of combination therapy are £8307.
No syringe sharing was assumed in the model, and for all treatments the most efficient vial/syringe to deliver the dose was assumed (i.e. that which produced the least wastage). In other words, if the dose for peginterferon alfa-2a was estimated to be 125 µg, then one 135-µg pre-filled syringe was used. Similarly, if the dose was estimated to be 137 µg, then the next larger syringe (180 µg) was used.
The economic model incorporated a costing protocol developed as part of a previously developed HTA report41 to estimate the appropriate evaluation, monitoring and surveillance cost. Health state costs used were from the previous NICE technology assessment of the treatment of chronic HCV in adults. 20,41 Costs were inflated to 2010–11 values using the HCHS Pay and Prices Index. 73
Treatment-related costs for treatment initiation and on-treatment and post-treatment monitoring can be seen below in Critique of the manufacturers’ submissions for Merck Sharp & Dohme and Roche, and in Appendix 7.
Cost-effectiveness results
The MS reports results by genotype, in terms of total costs, life-years and QALYs. Table 21 shows the base-case results for children aged 11 years. Treating genotype 1, 4 and 5 patients with peginterferon alfa-2a and RBV improved outcomes by 1.01 QALYs compared with BSC and cost an additional £3971, which gives an ICER of £3915. For genotypes 2 and 3, treatment for 24 weeks improved QALYs by 1.57 compared with BSC and cost £1864 less. For this group, peginterferon alfa-2a dominates no treatment.
| Treatment | Cost (£) | Life-years | QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|---|
| Genotypes 1, 4 and 5 | |||||||
| No treatment | 8199 | 18.47 | 14.20 | NA | NA | NA | NA |
| PEG α-2a | 12,170 | 18.56 | 15.21 | 3971 | 0.09 | 1.01 | 3915 |
| Genotypes 2 and 3 | |||||||
| No treatment | 8199 | 18.47 | 14.20 | NA | NA | NA | NA |
| PEG α-2a, 24 weeks | 6336 | 18.61 | 15.77 | –1864 | 0.14 | 1.57 | Dominates no treatment |
The MS performed one-way and two-way DSAs for the likelihood of SVR, time horizon, discounting, baseline cohort characteristics, rate of progression to cirrhosis, probability of SVR with treatment, health state costs, health state utilities and timing of treatment. The cost-effectiveness of peginterferon alfa-2a compared with BSC remains below £13,000 per QALY for all analyses. Model results are most sensitive to time horizon, rate of disease progression, probability of SVR with treatment, liver disease at entry and annual cost of achieving SVR.
In the PSA, for patients with genotypes 2 and 3 there is a 97.2% probability of 24 weeks of combination therapy being cost-effective compared with no treatment, at a willingness-to-pay (WTP) threshold of £20,000 per QALY. In patients with genotypes 1, 4 and 5 there is a 91.6% probability of this being cost-effective at a £20,000 per QALY threshold. The cost-effectiveness planes for cost and QALYs by genotype group are shown in Figure 6. The CEAC is shown in Figure 7.
FIGURE 6.
Roche’s scatterplots for (a) genotypes 1, 4 and 5; and (b) genotypes 2 and 3.
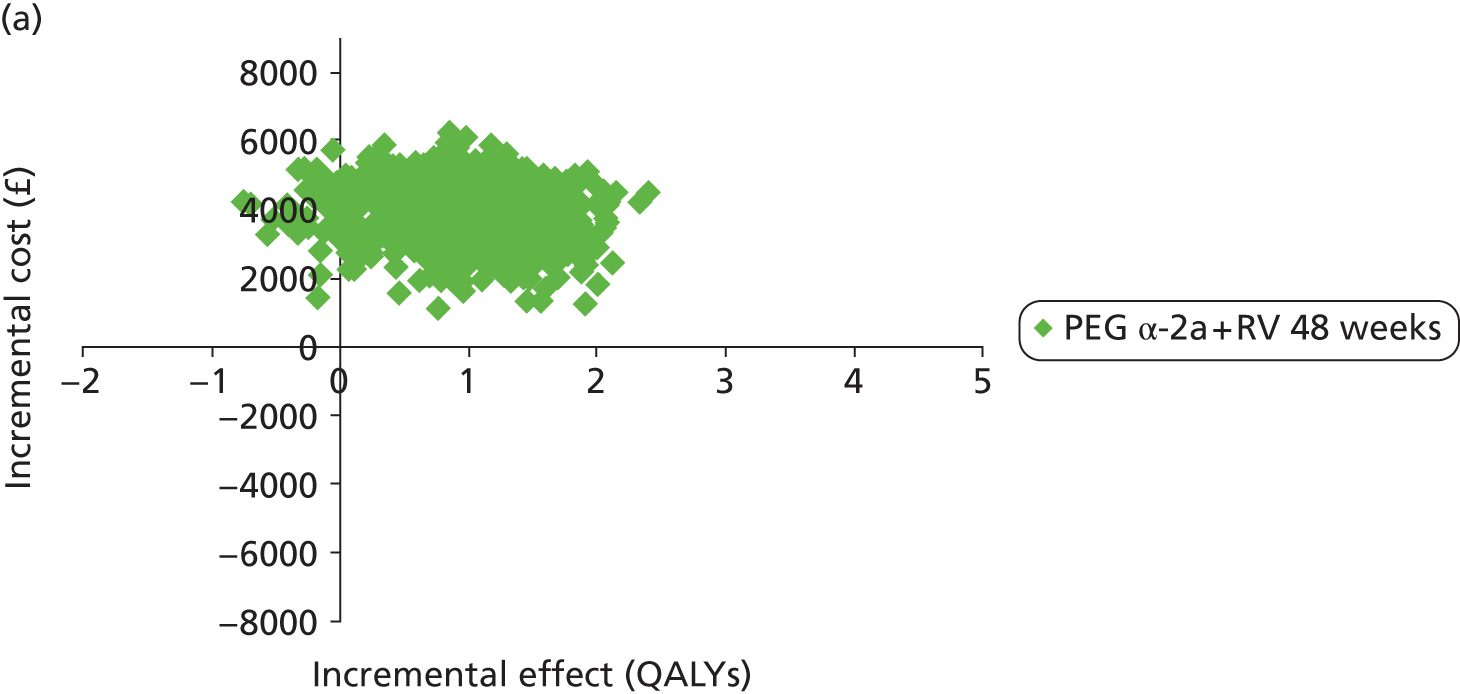
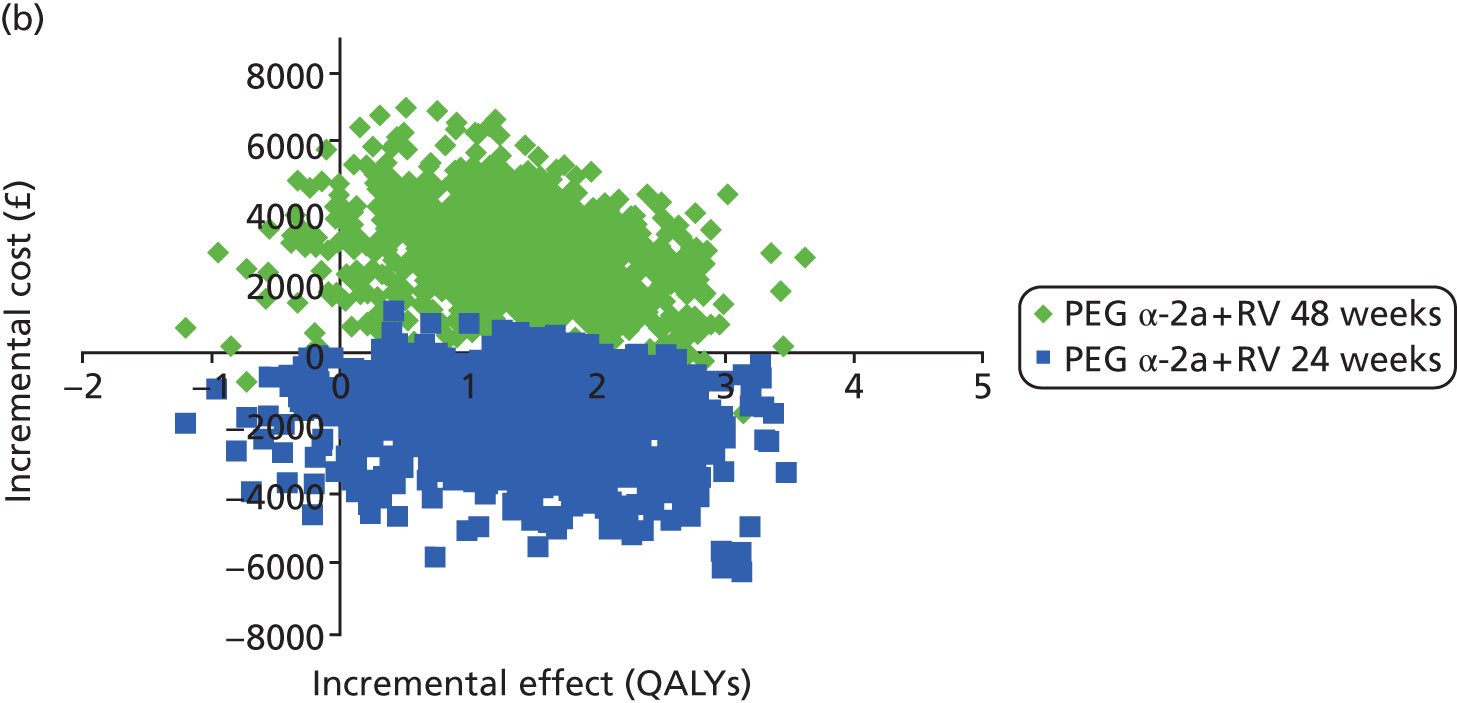
FIGURE 7.
Roche’s CEACs for (a) genotypes 1, 4 and 5; and (b) genotypes 2 and 3. CE, cost-effectiveness.
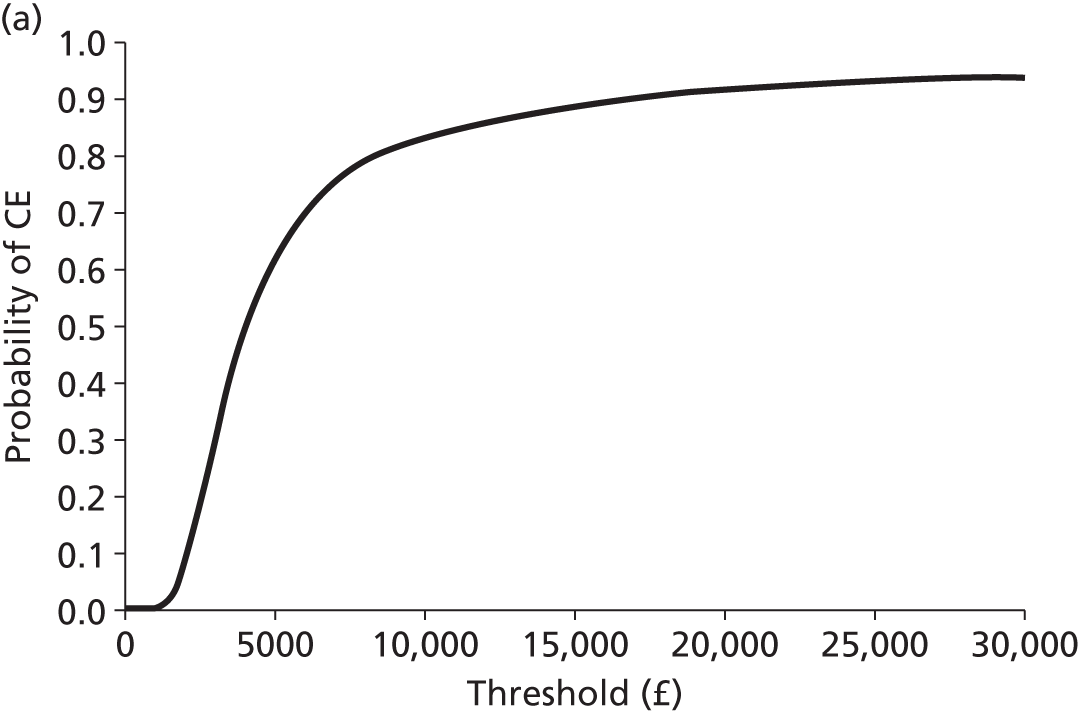
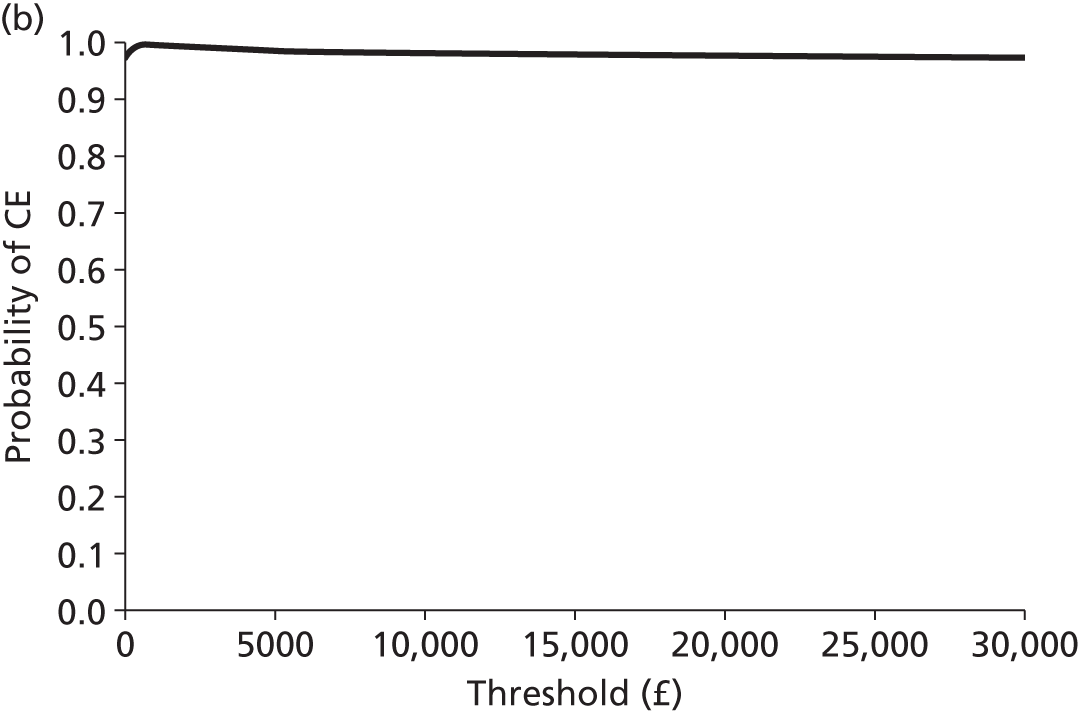
Summary of Roche’s submission
-
The Roche model is based upon that developed for previous HTAs of chronic HCV in adults.
-
The submission met all but one criterion for methodological quality.
-
The model compared peginterferon alfa-2a with BSC.
-
Treatment efficacy was estimated for SVR as a weighted average of four clinical trials for peginterferon alfa-2a.
-
Costs and utilities were based upon adult data from previous NICE appraisals.
-
Transition probabilities were based upon adult data, except for transitions between fibrosis health states, which was based upon a retrospective study in children.
-
A 30-year time horizon was used.
-
Spontaneous viral clearance was included within the model for untreated patients.
-
No chronic HCV-related costs were assumed to accrue to patients achieving SVR.
-
Health state utility values were calculated using a relative effect of utility found in the literature compared with a utility for the general population with the same age and gender composition. This method assumes that the relative difference in utility between health states is greater for children than for adults, which appears counterintuitive.
Critique of the manufacturers’ submissions for Merck Sharp & Dohme and Roche
Merck Sharp & Dohme and Roche used the state transition model applied in previous HTAs of peginterferon alfa treatments in adult populations. 20,41 The model structure was considered to be appropriate and was also used in the SHTAC analysis with minor changes to the classification of the health states ‘mild chronic HCV’ and ‘moderate chronic HCV’ (using the METAVIR fibrosis scale F0–F3 according to more recently published evidence).
In the absence of child-specific data, the MSD and Roche MSs have used adult data relating to transition probabilities, costs and utility values. It should be noted that within a long-term model, a large proportion of the time spent in the model would represent the patient’s life as an adult rather than a child, and that these values would therefore be appropriate for most of the duration of the model. In addition, these data were based upon previous HTAs which used a systematic approach. 20,41
As the majority of the patients started in mild chronic HCV, few of these would have been expected to progress to the more severe health states before they reach adulthood. Both the MSD and Roche MSs have conducted reviews to estimate the transition probabilities between the mild and moderate, and moderate and compensated cirrhosis health states. The probability of transition from mild to moderate HCV was the same for both MSs; for moderate HCV to compensated cirrhosis, transition probabilities varied from 0.0038 (MSD) to 0.021 (Roche). The transition probabilities differ from those used in the SHTAC model (a summary of these values can be seen in Table 22). The choice of transition probabilities used in the SHTAC model is discussed more fully in subsequent sections.
| From | To | MSD | Roche | SHTACa |
|---|---|---|---|---|
| Mild HCV | Moderate HCV | 0.014 | 0.014 | 0.025 |
| Moderate HCV | Compensated cirrhosis | 0.0038 | 0.021 | 0.014 |
The manufacturers differ in their choice of time horizon. MSD uses a lifetime horizon, whereas Roche uses a time horizon of 30 years and considers that this is long enough to capture important costs and effects arising from treatment. The appropriate approach for modelling treatments for chronic diseases that affect patients’ long-term prognoses, as recommended by NICE,69 is to use the lifetime horizon.
Roche assumes spontaneous SVR for children based upon its review of the literature, whereas MSD does not (as per the previous technology assessments20,41). However, it is noted that the probability of spontaneous SVR is small (< 2%) in the Roche submission and that it is unlikely to materially affect the cost-effectiveness results.
The health state utility values applied in the models of the two submissions can be seen in Table 23 and Appendix 7. It can be seen that the utility values applied for the mild and moderate HCV states, compensated and decompensated cirrhosis, HCC, liver transplant and post liver transplant, and the decrement applied during treatment, are the same in both submissions. These were all the utility values applied in the previous adult chronic HCV models with no adjustment made for the present population of children. 20,41 SVR from mild disease differs slightly between the two submissions (0.82 and 0.83 for MSD and Roche, respectively). Roche does not provide utilities for SVR from moderate disease or compensated cirrhosis, and it is unclear from the submission whether or not the utility weight used for the SVR from mild HCV was also used for these two health states.
| MSD health state | MSD utility weight | Roche health state | Roche utility weight |
|---|---|---|---|
| – | – | Healthy children (≤ 16 years old) | 0.95 |
| Mild HCV | 0.77 | Mild disease | 0.77 |
| Moderate HCV | 0.66 | Moderate disease | 0.66 |
| Compensated cirrhosis | 0.55 | Cirrhosis | 0.55 |
| SVR from mild HCV | 0.82 | SVR after mild disease | 0.83 |
| SVR from moderate HCV | 0.72 | – | – |
| SVR from compensated cirrhosis | 0.61 | – | – |
| Decompensated cirrhosis | 0.45 | Decompensated cirrhosis | 0.45 |
| HCC | 0.45 | HCC | 0.45 |
| Liver transplant | 0.45 | – | – |
| Post liver transplant | 0.67 | Post liver transplantation | 0.67 |
| Disutility due to adverse events | 0.11 | Treatment for mild diseasea | 0.66 |
| – | – | Treatment for moderate diseasea | 0.55 |
Health state costs used in the MSD and Roche submissions are shown in Table 24 and it can be seen that the majority of costs were the same across the two submissions. Further details of the 95% CIs, distributions and parameters for the MSD submission are given in Appendix 7.
| Health state | MSD annual costs, £ (2010–11) | Roche annual costs, £ (2010–11) |
|---|---|---|
| SVR from mild or moderate HCV | 132.18 | 0 |
| SVR from compensated cirrhosis | 191.11 | Not reported |
| Mild HCV | 178 | 178 |
| Moderate HCV | 926 | 926 |
| Compensated cirrhosis | 1469 | 1470 |
| Decompensated cirrhosis | 11,775 | 11,780 |
| HCC | 10,492 | 10,496 |
| Liver transplant | 47,495 | 47,513 |
| Post liver transplant | 1788 | 1789 |
Southampton Health Technology Assessments Centre’s economic evaluation
Overview
We developed a model to estimate the costs, benefits and cost-effectiveness of treatments for chronic HCV for children and young people. The scope for the appraisal, as issued by NICE, states that the interventions to be considered are:
-
peginterferon alfa-2a with RBV
-
peginterferon alfa-2b with RBV.
The comparator for these interventions is BSC, defined in the NICE scope as treatment without any form of interferon treatment, and one another. The perspective of the cost-effectiveness analysis is that of the NHS and PSS. The model estimates the lifelong costs and benefits from each treatment. The costs and benefits were discounted at 3.5% per year, as recommended by NICE. 69 The base price for the costs was taken from the most recently available data (2011–12). The intervention effect in terms of probability of SVR was derived from the systematic review of the clinical effectiveness reported in Chapter 4. The outcome of the economic evaluations is reported as the cost per QALY gained.
Model type and rationale for the model structure
The lifetime model of the natural history of chronic HCV aims to convert the principal outcome of interest in the clinical trials, that is, the probability of SVR, to long-term survival outcomes. To estimate the impact of this intermediate effect on final outcomes for patients, we required an appropriate model. No other models for chronic HCV in children were found and therefore the previous models for adults provide the best available peer-reviewed structure. We adapted our previously published models20,41 which were used in NICE guidance TA106 and TA200. 33,34 These models were developed for the progression of chronic HCV in adults. Where necessary, they have been modified to reflect the younger patient group in this analysis (discussed in full in subsequent sections). The model has a time horizon of 70 years, as this was considered long enough to capture all relevant costs and benefits, and a cycle length of 1 year.
The state transition diagram describing the health states within the model and the allowable transitions between these states is shown in Figure 8. For the current model, we have modified the structure to include health states for the fibrosis states (F0–F4), defined according to the METAVIR scoring system, instead of the previous health states of mild HCV, moderate HCV and compensated cirrhosis. This is based on more recent evidence on the progression of HCV by Thein and colleagues. 78 They conducted a systematic review of published prognostic rates to determine stage-specific fibrosis progression rates, based on a total of 111 studies of individuals with chronic HCV infection (n = 33,121). Although many of these studies had retrospective designs, the authors meta-analysed studies using both fixed and random effects, provided a number of sensitivity analyses and adjusted for covariates, and the results were reasonably robust to each of these. We therefore consider that these estimates are the most reliable data available. These data have allowed improvements to be made to the model, although at the present time not all chronic HCV data required for modelling fully complement these additional health states. Figure 8 shows nine health states. For clarity, mortality (an absorbing state) has not been included. In this diagram ellipses indicate health states and arrows indicate allowable transitions between health states.
FIGURE 8.
State transition diagram for SHTAC’s economic model. Shaded areas indicate those health states that lead to increased mortality risk compared with that of the general population.
The figure indicates that patients with chronic HCV (F0–F3) or compensated cirrhosis (F4) may have successful treatment (attain a SVR), remain in their current health state or progress to more severe stages of liver disease. The SVR state is assumed to be a permanent condition, with no spontaneous reactivation of HCV infection, although individuals are not immune to reinfection (this is outside the scope of the analysis). Individuals in the SVR health state are assumed to face the same mortality risks as the general population and face no greater risk of HCC than the general population.
For the utility values and health state costs, the previous health states of mild HCV and moderate HCV were used, where mild HCV relates to F0 and F1, and moderate HCV relates to F2 and F3. Targeted searches, undertaken as part of this assessment, did not identify any other new natural history evidence relating to progression or management of chronic HCV specific to children or young people. Utilities are associated with each health state and for the patient cohort the total number of QALYs is calculated. Patients on treatment with peginterferon alfa have a lower HRQoL than those not on treatment, owing to adverse events. This was assumed to be the same decrement for both treatments (see Health state values/utilities).
Patients with chronic HCV (F0–F4) face the same mortality risk as the general population. However, patients with decompensated liver disease or HCC and those who undergo liver transplantation face higher mortality rates, related to their stage of liver disease, than the general population.
Modelling assumptions
We included most of the assumptions from the previous HTA models. These were:
-
that the patient’s stage of disease [mild HCV (F0, F1), moderate HCV (F2, F3) and compensated cirrhosis (F4)] prior to treatment influences their subsequent risk of progressive liver disease, post-treatment surveillance and also HRQoL
-
that patients not exhibiting a SVR are expected to face the same risk of disease progression as untreated patients
-
that the same SVR applies for patients with mild or moderate HCV, and for those patients with compensated cirrhosis
-
that the model did not account for reinfection and onward transmission of HCV
-
that the possibility of HCC patients receiving a liver transplant was not considered on account of its rarity
-
that discontinuation due to adverse events was not accounted for as it is considered rare
-
that costs associated with the management of adverse events were not accounted for as they were unlikely to be substantial
-
that there is a reduction in utility while patients are being treated with peginterferon alfa and RBV.
In addition, we included the following assumptions after discussion with our expert advisors:
-
The base-case analysis assumed that no patients would have spontaneous SVR.
-
It was assumed that treatment would discontinue at 24 weeks if an EVR was not achieved at week 12 for genotype 1 or 4.
-
Adult transition probabilities, utility weights and health state costs were applied for paediatric patients owing to lack of data.
-
There is no change to parental utility values. Although there is some suggestion that parental QoL may be reduced, the evidence is not sufficient to be applied in the model.
Evaluation of uncertainty
The evaluation of the cost-effectiveness of treatment for chronic HCV in children is based on uncertain information about variables such as the clinical effectiveness, HRQoL and resource use. This uncertainty was evaluated using DSA, PSA and scenario analyses. One-way DSAs were conducted to evaluate the influence of individual parameters, model structure and assumptions on the robustness of the model (see Results of independent economic analysis).
Multiparameter uncertainty in the model was addressed using PSA (see Results of independent economic analysis). 79 In the PSA, probability distributions are assigned to the point estimates used in the base-case analysis. The model is run for 1000 iterations, with a different set of parameter values for each iteration, by sampling parameter values at random from their probability distributions. The uncertainty surrounding the cost-effectiveness of the treatment is represented on a CEAC according to the probability that the intervention will be cost-effective at a particular WTP threshold. Appendix 9 reports the parameters included in the PSA, the form of distribution used for sampling each parameter, and the upper and lower limits assumed for each variable.
Model validation
The SHTAC model was validated by checking the model structure, calculations and data inputs for technical correctness. The structure is similar to that used in previous HTAs20,41 but was redeveloped to include the health states F0–F4. The SHTAC model was checked for internal consistency against the MS economic models by running the SHTAC model with the inputs used in the MS models. The robustness of the model to changes in input values was tested using sensitivity analyses to ensure that any changes to the input values produced changes to the results of the expected direction and magnitude. Finally, the model results were compared with those from the MSs.
Southampton Health Technology Assessments Centre’s data sources
Baseline cohort of children with chronic hepatitis C virus
The baseline characteristics of the modelled populations were taken from the clinical trials used for the effectiveness of the peginterferon alfa treatments (see Chapter 4, Table 2). Table 25 shows the initial distribution of patients among fibrosis states based on the studies included in the clinical effectiveness review. 46–48,51,56,57,59
| Source | F0 (%) | F1 (%) | F2 (%) | F3 (%) | F4a (%) |
|---|---|---|---|---|---|
| All patients | 24.6 | 66.2 | 7.1 | 2.1 | 0.0 |
The included studies use a variety of measures to assess the degree of fibrosis in the participants at baseline. Where possible we have aligned these to relate to the model structure of F0–F4, and calculated weighted averages to generate the proportion of participants starting within each category. These can be seen in Table 25 and have been applied in the model. The distributions for F0 and F4 are the most reliable because all studies that reported fibrosis at baseline reported the proportion of participants without any fibrosis (F0) and with cirrhosis (F4). The remaining distributions are likely to be subject to some uncertainty because of the different measures used. However, the proportions appear to be in line with those estimated in a systematic review6 of the natural history of childhood chronic HCV (discussed in Chapter 1, Description of the underlying health problem), and those of a retrospective study identified on targeted searches of the natural history of childhood chronic HCV (Guido and colleagues75). Patients eligible for treatment with either peginterferon alfa-2a or -2b have a starting age of 11 years, based on the mean ages of those in the clinical trials (see Chapter 4, Assessment of effectiveness).
Natural history and effectiveness data
Table 26 reports the transition probabilities adopted in the natural history model for the economic evaluation. They represent the transition probabilities for the BSC comparator and are taken from previous HTAs,20,41 except for the transition between the chronic HCV health states. As described above, we have modified the structure to include health states for the fibrosis states (F0–F4), defined according to the METAVIR scoring system, instead of the previous health states of mild and moderate HCV and compensated cirrhosis. The transition probabilities for the transitions between these health states are taken from the random effects meta-analysis of studies included in the systematic review conducted by Thein and colleagues. 78 In addition to these probabilities, there will be a risk of progressing to death.
| Health state | Transition probabilitya | Source | |
|---|---|---|---|
| From | To | ||
| F0 | F1 | 0.117 (95% CI 0.104 to 0.130) | Thein et al.78 |
| F1 | F2 | 0.085 (95% CI 0.075 to 0.096) | Thein et al.78 |
| F2 | F3 | 0.120 (95% CI 0.109 to 0.133) | Thein et al.78 |
| F3 | Compensated cirrhosis (F4) | 0.116 (95% CI 0.104 to 0.129) | Thein et al.78 |
| Compensated cirrhosis (F4) | Decompensated cirrhosis | 0.039 (SE 0.010) | bFattovich et al.80 |
| HCC | 0.014 (SE 0.010) | bFattovich et al.80 | |
| Decompensated cirrhosis | HCC | 0.014 (SE 0.010) | bFattovich et al.80 |
| Liver transplant | 0.020 (SE 0.005) | bSiebert et al.81 | |
| Death | 0.130 (SE 0.010) | bFattovich et al.80 | |
| HCC | Death | 0.430 (SE 0.030) | bFattovich et al.80 |
| Liver transplantation | Death | Year 1 = 0.150 (SE 0.015) | cWright et al.82 |
| Year 2 = 0.057 (SE 0.005) | bSiebert et al.81 | ||
Our systematic review of economic evaluations identified one full cost-effectiveness study for treatments of chronic HCV in children. 68 Although not meeting the criteria for inclusion in the present review, as the treatments were not peginterferons, this study suggests that the natural history of chronic HCV in children has a prolonged phase, with progression delayed until adulthood. This was based on expert opinion, and therefore a latent phase of 15 years was built into the model, with no transitions to more severe disease health states allowed. Our targeted searches for natural history identified evidence that disagreed with this assumption. Guido and colleagues75 analysed fibrosis scores in 112 paediatric patients and found that the progression rate in children was consistent with that in adults. They concluded that disease progression was dependent on duration of HCV infection. It is unclear from the evidence presented whether or not this was independent of age. Based on the evidence that disease progression is dependent on duration of infection, we concluded that the most appropriate approach is to assume similar transition probabilities between fibrosis states in adults and children, as the starting age of children in the cohort is 11 years. We have therefore used the transition probabilities for adults for transitions between the more severe health states, which were taken from the reviews of natural history and/or economic evaluations used previously (see Table 26).
Table 27 reports the treatment effects (proportion of patients achieving SVR) that have been applied, in the model, to estimate the effectiveness of peginterferon alfa and RBV combination therapies in children, taken from studies included in the systematic review of clinical effectiveness (see Chapter 4).
| Intervention | Genotype | SVR (%) | 95% CI (%)a | EVR (%) | 95% CI (%)a | Source |
|---|---|---|---|---|---|---|
| PEG α-2a + RBV | Overall | 60.00 | 51.23 to 68.76 | 61.67 | 52.96 to 70.36 | Schwarz et al., 2011;56b Sokal et al., 201057 |
| 1 or 4 | 52.17 | 40.86 to 63.48 | 57.45 | 43.31 to 71.58 | ||
| 2 or 3 | 85.71 | 72.25 to 98.67 | 83.33 | 66.11 to 100.50 | ||
| PEG α-2b + RBV | Overall | 58.37 | 51.69 to 65.05 | 67.79 | 61.43 to 74.13 | Al Ali et al., 2010;46 Pawlowska et al., 2010;51 Wirth et al., 2010;59 Ghaffar et al., 2009;47c Jara et al., 200848 |
| 1 or 4 | 50.97 | 43.09 to 58.83 | 61.04 | 50.51 to 71.56 | ||
| 2 or 3 | 91.43 | 82.15 to 100.70 | 86.67 | 74.50 to 98.83 |
As discussed in Chapter 4, Quantity and quality of research available, no evidence of a comparative nature was identified. Studies included were all single-cohort designs. No head-to-head evidence of effectiveness was therefore available to be used in the economic evaluation of peginterferon alfa compared with BSC, or the evaluation of peginterferon alfa-2a compared with peginterferon alfa-2b. In addition, data were not suitable for formal indirect comparison owing to the lack of any comparators. A pragmatic approach was therefore taken to use the available evidence through an unadjusted indirect comparison. Caution is recommended in the interpretation of the economic evaluations because there is no means by which the similarity of the included studies can be assessed. For example, participant characteristics, the settings for the studies and the measurement of the endpoints are not controlled for as they would be in a RCT. Any differences observed in the results of the evaluation may therefore be misleading. Although the use of such an approach has an inherent risk of bias, it does provide an illustration of the likely estimate of cost-effectiveness given the constraints of the data available.
Estimates of the SVR and EVR for total populations and subgroup populations of genotypes 1 or 4 and 2 or 3 were estimated using a weighted average approach. Two studies provided data on SVR and EVR for peginterferon alfa-2a and five for peginterferon alfa-2b (see Chapter 4, Sustained virological response). Rates were pooled for the two treatments weighted by the sample size to provide an estimate and an estimated variance that could be used in the economic model. As can be seen in Table 27, the SVR estimates for the two treatments are similar (and CIs around these overlap), and so caution is required when interpreting the outcomes of the model where the point estimates suggest that one treatment is more effective than the other.
We assumed that no patients receiving BSC achieved spontaneous SVR, following guidance from our expert advisory group that spontaneous viral clearance after the age of 4 years is unlikely. In the absence of data, for the base-case analyses we have assumed that the same SVR applies for all patients with chronic HCV (F0–F4). This seems a reasonable assumption, given that most patients start in the mild hepatitis health states F0 and F1, and none start in the compensated cirrhosis state (F4). This assumption was also used in previous HTAs of adult chronic HCV. 41
The distribution of the HCV genotypes in the populations within the studies of peginterferon alfa-2a and peginterferon alfa-2b were different. Grouping these as either genotype 1 or 4, or genotype 2 or 3, and taking a weighted average approach, it can be seen that in the populations within the studies of peginterferon alfa-2a, 77% would be classed as genotype 1 or 4 and 23% as genotype 2 or 3. In the studies of peginterferon alfa-2b, the rates are 82% and 18% for genotype 1 or 4 and genotype 2 or 3 respectively. As evidence suggests that those with genotype 2 or 3 are more likely to respond to treatment, this would suggest that the treatment responses seen in the peginterferon alfa-2a studies may be better because of the distribution of the genotypes in the baseline populations. A scenario analysis which included adjusted genotype distributions was undertaken to test the effects of the different genotype distributions on the base case [see Treatment effectiveness (sustained virological response) of peginterferon alfa-2a versus peginterferon alfa-2b].
Health state values/utilities
As discussed previously (see Systematic review of health-related quality-of-life studies), our systematic review of HRQoL did not identify any studies that assessed HRQoL in children with chronic HCV. Two studies were identified that assessed HRQoL in adults, and in the absence of any health state utility values for children, we decided to derive our base-case health state utility values from these studies. One included study assessed 489 consecutive HCV patients attending outpatient clinics in Sweden. 70 The other assessed the utilities of 193 outpatients at various stages of chronic HCV progression in Canada. 71 The suitability of these data to the current decision problem, and the assumptions made to apply these data to the child population, are discussed below; however, these data are consistent with the NICE reference case69 for measuring and valuing health benefits. HRQoL measurements were undertaken using the EQ-5D, with HRQoL valued using a tariff derived in a general population. 83
In adult models for HCV undertaken in previous HTAs, utility values were taken from the UK mild chronic hepatitis C trial. 82 The studies by Bjornsson and colleagues70 and Chong and colleagues71 contained larger sample sizes in each health state category than the mild hepatitis C trial. 82 There was good agreement between the values in the Bjornsson and colleagues70 and Chong and colleagues71 studies. Furthermore, the values seen in these two studies are higher for all health states than those seen in the UK mild chronic hepatitis C trial. We considered that utility weights in children and young people would be expected to be higher than those in adults because comorbidities are known to be fewer in children and general population norms for HRQoL are lower in children. Therefore, we have used data from the studies by Bjornsson and colleagues70 and Chong and colleagues,71 rather than the mild hepatitis C trial. 82
The utility values from these studies were seen in Tables 16 and 17 and the utility values used in the SHTAC economic evaluation can be seen in Table 28. Based upon the study by Bjornsson and colleagues,70 we assumed that there was no difference in the health state utility values between the SVR and mild and moderate HCV (F0–F3) health states and that these were equal to the utility value for the general population. In the absence of data specifically for a moderate HCV disease state in the two included studies, we assumed that the utility values were the same as for the mild-to-moderate/chronic HCV state because HCV is often asymptomatic for longer in children and young people. For the analysis, we adopted the utility value for the general population for all these health states (0.82).
| Health state | Health state utility value | Decrement vs. SVR | Source |
|---|---|---|---|
| SVR (from mild disease) | 0.82 | – | aBjornsson et al.70 |
| Mild HCV (F0/F1) | 0.82 | – | aBjornsson et al.70 |
| Treatment for mild HCV (F0/F1) | 0.71 | 0.11 | bMild hepatitis C trial82 |
| Moderate HCV (F2/F3) | 0.82 | – | aBjornsson et al.70 |
| Treatment for moderate HCV (F2/F3) | 0.71 | 0.11 | bMild hepatitis C trial82 |
| Cirrhosis (F4) | 0.75 | 0.07 | Bjornsson et al.70 |
| Chong et al.71 | |||
| Decompensated cirrhosis | 0.66 | 0.16 | Bjornsson et al.70 |
| Chong et al.71 | |||
| HCC | 0.64 | 0.18 | Chong et al.71 |
| Liver transplantation | 0.69c | 0.13 | Ratcliffe et al.84 |
| Chong et al.71 | |||
| Post liver transplantation | 0.73 | 0.09 | Ratcliffe et al.84 |
The two included studies did not report HRQoL for populations undergoing treatment for mild or moderate HCV. We have therefore estimated this using the utility decrement observed between the untreated and treated participants in the UK mild hepatitis C trial and applied this to the utility value (0.82) used in the model for the mild and moderate disease state, assuming that this utility decrement for treatment with peginterferon alfa would be similar for adults and children. The decrement of 0.11 led to a utility of 0.71 for treated children with mild and moderate HCV. Utilities for cirrhosis, decompensated cirrhosis and HCC are taken from the two included studies. For liver transplantation, the estimate from the Chong and colleagues71 study corresponded with a value used in the previous SHTAC model in adults, which had been taken from a post-transplantation study by Ratcliffe and colleagues. 84 In the absence of any data on HRQoL in the post-liver transplantation population the utility value used in the previous HTAs20,41 for adult HCV (from Ratcliffe and colleagues84) was applied.
To take account of the change in HRQoL over patient lifetimes, we use age-related population norms for HRQoL. As stated above, we assume that the health state utility values for SVR (and HCV states F0–F3) would be the same as for the general population. For these health states, the health state utility values are taken from the study by Kind and colleagues,85 who developed age- and gender-specific UK EQ-5D population norms (Table 29). We fit these data to give the following:
where x is an individual’s age in years.
| Age band (years) | Male | Female |
|---|---|---|
| Under 25 | 0.94 | 0.94 |
| 25–34 | 0.93 | 0.93 |
| 35–44 | 0.91 | 0.91 |
| 45–54 | 0.84 | 0.85 |
| 55–64 | 0.78 | 0.81 |
| 65–74 | 0.78 | 0.78 |
| 75 + | 0.75 | 0.71 |
For all other health states in the model, the health state utility value is calculated by subtracting the health state decrement from the age-related health state utility value (see Table 28).
Cost data
Costs in the model include additional resource use, for example laboratory tests, diagnostic tests and outpatient visits (described as intervention costs), costs relating to the health states used in the model (health state costs) and costs relating to the treatments (drug costs).
Intervention costs
Protocols describing the frequency and intensity of monitoring of patients being treated with peginterferon alfa were developed for the previous assessment, based on clinical guidelines and discussion with hepatologists/specialist nurses at Southampton University Hospitals Trust, and are described in full in the previous HTAs. 20,41 Costs associated with these protocols were not applied to BSC in the model. The costs of patient management include initial evaluation, assessments of the suitability of treatment, clinical decision-making regarding choice of treatment and final tests prior to commencement of treatment. These costs have been uprated to 2011–12 values (from 2003–4 prices) using the HCHS Pay and Prices Index73 and are reported in Table 30. The costs used in the model were based upon adult costs, as costs for children were unavailable (a fuller discussion of the possible difference between adult and child costs is given in Chapter 7).
| On-treatment monitoring | Cost (£) |
|---|---|
| 12 weeks | 721 |
| 16 weeks | 869 |
| 24 weeks | 880 |
| 48 weeks | 1168 |
| 72 weeks | 1155 |
The stopping rules for treatment costs for peginterferon alfa have been based on advice from a clinical member of our advisory group, as follows:
-
Patients with genotype 1 or 4 receive 48 weeks’ treatment if they achieve an EVR.
-
Patients with genotype 1 or 4 receive 24 weeks’ treatment if they do not achieve an EVR.
-
Patients with genotype 2 or 3 receive 24 weeks’ treatment.
Health state costs
Targeted searches for health state costs for chronic HCV in children did not reveal any relevant studies and so costs were used from the previous HTAs for the treatment of chronic HCV in adults. 20,41 Health state costs for SVR, chronic HCV (F0–F3), compensated cirrhosis (F4), decompensated cirrhosis and HCC were taken from the observational study conducted during the UK mild HCV trial (Table 31). 82 Post-liver transplantation costs were taken from a Department of Health-funded study of the costs of liver transplantation. 84 Costs have been updated to 2011–12 costs using the HCHS Pay and Prices Index. 73 Costs for liver transplantation were taken from NHS reference costs for liver transplant (code ref. GA01C) for 2010. 86 The health state costs applied for SVR are only applied for the first year after treatment ends.
| Health state | Cost (£/year) |
|---|---|
| SVR | 346a |
| Mild chronic HCV (F0/F1) | 184a |
| Moderate chronic HCV (F2/F3) | 959a |
| Compensated cirrhosis (F4) | 1521a |
| Decompensated cirrhosis | 12,193a |
| HCC | 10,865a |
| Liver transplantation | 32,732b |
| Post liver transplantation | 1852c |
Treatment costs
In addition to the health state and health service costs, drug costs also need to be estimated. Drug unit costs were taken from BNF 64 (September 2012). 87 The average weight, height and BSA of children by age is shown in Table 32. BSA was estimated using the Dubois formula,88 as recommended by the BNF. 74
| Age (years) | Height (cm) | Weight (kg) | BSA (m2)a |
|---|---|---|---|
| 3 | 96 | 14 | 0.60 |
| 5 | 109 | 18 | 0.74 |
| 7 | 122 | 23 | 0.89 |
| 10 | 138 | 32 | 1.12 |
| 12 | 149 | 39 | 1.28 |
| 14 | 161 | 50 | 1.50 |
The corresponding prescribing costs for a child of age 11 years are shown in Table 33. Drug costs for peginterferon alfa-2a (Pegasys®) were calculated for a dosage of 100 µg/m2, administered by patients once per week, corresponding to a weekly cost of £107.76 for a 135-µg pen. The total drug cost for a 24-week course of treatment is £2586 and for 48 weeks is £5172. Peginterferon alfa-2a is used in combination with RBV (Copegus®) which had a weekly cost of £46.25, and a cost of £1110 and £2220 for 24 weeks and 48 weeks respectively. Drug costs for peginterferon alfa-2b (ViraferonPeg®) were calculated for a dosage of 60 µg/m2 per week. This corresponds to a weekly cost of £106.34. The total drug cost for a 24-week course of treatment is £2552 and for 48 weeks is £5104. Peginterferon alfa-2b is used in combination with RBV (Rebetol®) which had a weekly cost of £62.51, and a cost of £1500 and £3000 for 24 weeks and 48 weeks, respectively.
| Medication | Dose | Cost per week (£) | Brand name | Total cost (£) | Duration (weeks) |
|---|---|---|---|---|---|
| PEG α-2a | 100 µg/m2 per week | 107.76 | Pegasys® | 2586 | 24 |
| 5172 | 48 | ||||
| PEG α-2b | 60 µg/m2 per week | 106.34 | ViraferonPeg® | 2552 | 24 |
| 5104 | 48 | ||||
| RBV | 15 mg/kg per day | 46.25 | Copegus® | 1110 | 24 |
| 2220 | 48 | ||||
| RBV | 15 mg/kg per day | 62.51a | Rebetol® | 1500 | 24 |
| 3000 | 48 |
A discount rate of 3.5% was applied to future costs and benefits.
Results of independent economic analysis
This section reports the cost-effectiveness results for a cohort of 11-year-olds with chronic HCV, receiving either peginterferon alfa-2a or -2b (in combination with RBV), compared with BSC and with one another. The results for costs and QALYs are presented for each alternative.
The modelled, undiscounted duration in each health state for BSC, peginterferon alfa-2a and peginterferon alfa-2b are presented in Table 34. The results show increased survival for both treatments for chronic HCV when compared with BSC (47.5 years), and a slight survival advantage in patients receiving peginterferon alfa-2a (57.4 years, compared with 57.1 years in peginterferon alfa-2b).
| Health state | BSC (years) | PEG α-2a + RBV (years) | PEG α-2b + RBV (years) |
|---|---|---|---|
| SVR | 0.0 | 38.2 | 37.0 |
| F0–F3 | 28.6 | 11.5 | 12.1 |
| F4 | 14.5 | 5.9 | 6.2 |
| Decompensated cirrhosis | 3.1 | 1.3 | 1.3 |
| HCC | 0.6 | 0.2 | 0.2 |
| Post liver transplant | 0.7 | 0.3 | 0.3 |
| Total (undiscounted) | 47.5 | 57.4 | 57.1 |
The longer duration spent in more severe disease states by children in the BSC cohort is reflected in the health state costs for this group, presented in Table 35. Although this group does not incur treatment costs, the total undiscounted costs are substantially higher for this cohort than either of the treatment groups, particularly for the cirrhosis and decompensated cirrhosis health states. The additional long-term costs for the BSC cohort outweigh the treatment costs of peginterferon alfa-2a and -2b. The costs are slightly higher for peginterferon alfa-2b than peginterferon alfa-2a, for both the treatment and health state costs.
| Cost type | BSC (£/patient) | PEG α-2a + RBV (£/patient) | PEG α-2b + RBV (£/patient) |
|---|---|---|---|
| Treatment costs overall | 0 | 6481 | 7241 |
| Health state costs | |||
| SVR | 0 | 208 | 201 |
| F0–F3 | 17,684 | 7103 | 7456 |
| F4 | 21,985 | 8956 | 9390 |
| Decompensated cirrhosis | 38,250 | 15,667 | 16,420 |
| HCC | 6027 | 2462 | 2581 |
| Post liver transplant | 3159 | 1303 | 1365 |
| Total health state costs | 87,105 | 35,699 | 37,413 |
| Total costs per patient | 87,105 | 42,180 | 44,654 |
The base-case results, including discounted total and incremental costs, life-years, QALYs and the ICER for all genotypes, are reported in Table 36 for patient cohorts that contain all genotypes. Both peginterferon treatments have lower lifetime costs than BSC, with a reduction in costs of between £8874 (peginterferon alfa-2b) and £10,190 (peginterferon alfa-2a) over a patient lifetime. Furthermore, both treatments increase the lifetime life-years (by 1.82–1.88) and QALYS (by 1.66–1.72) compared with BSC. Treatment with peginterferon alfa-2a and -2b dominate BSC, that is, they have lower lifetime costs and are more effective.
| Treatment | Costs (£) | Life-years | QALYs | Vs. BSC | |||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) | ||||
| BSC | 29,245 | 22.75 | 20.53 | – | – | – | – |
| PEG α-2a | 19,055 | 24.64 | 22.25 | –10,190 | 1.88 | 1.72 | Dominates |
| PEG α-2b | 20,371 | 24.57 | 22.19 | –8874 | 1.82 | 1.66 | Dominates |
Base-case results for patients with genotype 1 or 4 only are presented in Table 37 and the results for genotype 2 or 3 are shown in Table 38. The treatment effect (SVR) for genotype 2 or 3 is better than for genotype 1 or 4, and consequently the results from the model reflect this. There are more additional QALYs accrued in the genotype 2 or 3 subgroup (2.52–2.68 QALYs) than in the genotype 1 or 4 subgroup (1.44–1.47 QALYs), and more reduction in cost in the genotype 2 or 3 subgroup (£17,414–£18,043) than in the genotype 1 or 4 subgroup (£6929–£7967).
| Treatment | Costs (£) | Life-years | QALYs | Vs. BSC | |||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) | ||||
| BSC | 29,245 | 22.75 | 20.53 | – | – | – | – |
| PEG α-2a | 21,278 | 24.39 | 22.00 | –7967 | 1.63 | 1.47 | Dominates |
| PEG α-2b | 22,316 | 24.35 | 21.97 | –6929 | 1.60 | 1.44 | Dominates |
| Treatment | Costs (£) | Life-years | QALYs | Vs. BSC | |||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) | ||||
| BSC | 29,245 | 22.75 | 20.53 | – | – | – | – |
| PEG α-2a | 11,831 | 25.45 | 23.05 | –17,414 | 2.70 | 2.52 | Dominates |
| PEG α-2b | 11,202 | 25.61 | 23.21 | –18,043 | 2.85 | 2.68 | Dominates |
The results for genotypes 1 or 4 and 2 or 3 are similar to the base-case results; both peginterferon treatment options had lower lifetime costs and were more effective than BSC, that is, they dominate BSC.
The base-case results for the comparison of peginterferon alfa-2a with peginterferon alfa-2b can be found in Table 39. The results, including discounted total and incremental costs, life-years, QALYs and ICERs, are shown for all genotypes, genotype 1 or 4 and genotype 2 or 3. In the overall population base case, peginterferon alfa-2a has slightly lower lifetime costs (£19,055 compared with £20,371) and is slightly more effective than peginterferon alfa-2b (22.25 QALYs compared with 22.19). This leads to peginterferon alfa-2b being dominated by peginterferon alfa-2a. In the genotype 1 or 4 subpopulation, a similar outcome can be observed, with peginterferon alfa-2b being dominated. However, for those with genotype 2 or 3, peginterferon alfa-2b has lower lifetime costs and is more effective, and therefore peginterferon alfa-2b dominates peginterferon alfa-2a. This apparent difference illustrates how marginal the differences between treatments are. As stated previously, the estimates of effectiveness were very similar based on the included clinical effectiveness studies. These effectiveness estimates predominately drive the differences in costs and outcomes of the two treatments within the model. This is further explored below (see Scenario analyses).
| Treatment | Costs (£) | Life-years | QALYs | Peginterferon alfa-2b vs. peginterferon alfa-2a | |||
|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) | ||||
| All genotypes | |||||||
| BSC | 29,245 | 22.75 | 20.53 | – | – | – | – |
| PEG α-2a | 19,055 | 24.64 | 22.25 | – | – | – | – |
| PEG α-2b | 20,371 | 24.57 | 22.19 | 1316 | –0.06 | –0.06 | Dominated |
| Genotype 1 or 4 | |||||||
| BSC | 29,245 | 22.75 | 20.53 | – | – | – | – |
| PEG α-2a | 21,278 | 24.39 | 22.00 | – | – | – | – |
| PEG α-2b | 22,316 | 24.35 | 21.97 | 1038 | –0.03 | –0.03 | Dominated |
| Genotype 2 or 3 | |||||||
| BSC | 29,245 | 22.75 | 20.53 | – | – | – | – |
| PEG α-2a | 11,831 | 25.45 | 23.05 | – | – | – | – |
| PEG α-2b | 11,202 | 25.61 | 23.21 | –629 | 0.16 | 0.15 | Dominates |
Notwithstanding the limitations of the evidence base, overall the base-case analyses suggest that peginterferon alfa-2a and peginterferon alfa-2b are cost-effective compared with BSC. Peginterferon alfa-2a is cost-effective compared with peginterferon alfa-2b in the overall population and the subgroup with genotype 1 or 4, but is not cost-effective compared with peginterferon alfa-2b in the subgroup with genotype 2 or 3.
Deterministic sensitivity analyses
One-way DSAs were performed by varying one parameter at a time from its base value, leaving all other variables unchanged. The sensitivity analysis investigated the effect of uncertainty around the model assumptions, structure and parameter values on the cost-effectiveness results, in order to highlight the most influential parameters.
Where possible, the parameters were varied according to the ranges of the CIs of these parameters, based on the published estimates or estimated by reviewers. Where these data were not available, an alternative suitable range was chosen. The same ranges were used in the DSAs and PSAs, and these are described in Appendix 9. Owing to the large number of parameters, some of the parameters were combined and varied together, rather than individually, for example the transition probabilities, health state costs and utility values.
The total costs of the cohort treated with peginterferon alfa are lower than the total costs of the cohort treated with BSC, and QALYs gained are higher (treatment is cheaper and more effective). In the DSA, some of the ICERs yielded would be negative, which can be difficult to interpret using traditional thresholds for assessing cost-effectiveness as they may appear counterintuitive. For the DSA, results are therefore represented in terms of incremental net benefit (INB), whereby one treatment is more cost-effective (using a WTP threshold of £20,000) than another if it has a higher net benefit. This approach was used previously in the HTAs of shortened treatment duration for hepatitis C41 and is explained in more detail in Appendix 10. The equation used to calculate INB is:
where Q is QALY and C is cost.
The results of the DSAs are shown in Tables 40–42 for peginterferon alfa-2a versus BSC, peginterferon alfa-2b versus BSC and peginterferon alfa-2b versus peginterferon alfa-2a, respectively. For all analyses for peginterferon alfa-2a or -2b compared with BSC, changes to the model parameters and assumptions do not affect the baseline results and BSC is dominated by peginterferon alfa-2a and -2b. The model results are most sensitive to changes in the discount rate, time horizon, treatment SVR and baseline fibrosis make-up of the cohort. Changes to the transition probabilities, utility values and health state costs have a relatively small effect on the model results.
| Parameter | PEG α-2a vs. BSC | ||||
|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | INB (£) | ICER (£/QALY) | ICER (£/QALY) | |
| Baseline | –10,190 | 1.72 | 44,616 | –5920 | Dominates |
| Time horizon 30 years | –3187 | 0.27 | 8631 | –11,709 | Dominates |
| Time horizon 90 years | –10,310 | 1.82 | 46,714 | –5664 | Dominates |
| Discount rate 0% | –44,925 | 8.93 | 223,550 | –5030 | Dominates |
| Discount rate 6% costs, 1.5% outcomes | –2499 | 4.30 | 88,515 | –581 | Dominates |
| Discount rate 1.5% costs, 1.5% outcomes | –23,966 | 4.30 | 109,982 | –5573 | Dominates |
| Discount rate 6% costs, 6% outcomes | –2499 | 0.58 | 14,060 | –4324 | Dominates |
| SVR for PEG α-2a 69% | –12,690 | 2.00 | 52,665 | –6349 | Dominates |
| SVR for PEG α-2a 51% | –7689 | 1.44 | 36,568 | –5325 | Dominates |
| SVR for PEG α-2b 65% | –10,190 | 1.72 | 44,616 | –5920 | Dominates |
| SVR for PEG α-2b 52% | –10,190 | 1.72 | 44,616 | –5920 | Dominates |
| Cohort 100% F0 | –6587 | 1.10 | 28,537 | –6002 | Dominates |
| Cohort 100% F2 | –17,242 | 2.86 | 74,428 | –6030 | Dominates |
| Cohort 100% F3 | –19,971 | 4.23 | 104,496 | –4725 | Dominates |
| Cohort 20% F4 | –12,794 | 2.73 | 67,441 | –4682 | Dominates |
| Starting age 5 years | –11,015 | 1.81 | 47,214 | –6086 | Dominates |
| Starting age 16 years | –8718 | 1.61 | 40,949 | –5410 | Dominates |
| Transition probabilities LCI | –9399 | 0.87 | 26,750 | –10,834 | Dominates |
| Transition probabilities UCI | –9491 | 2.68 | 63,090 | –3542 | Dominates |
| Utility values LCI | –10,190 | 1.88 | 47,776 | –5422 | Dominates |
| Utility values UCI | –10,190 | 1.57 | 41,685 | –6471 | Dominates |
| Utility values, HTA report | –10,190 | 3.14 | 72,896 | –3250 | Dominates |
| Health state costs LCI | –6423 | 1.72 | 40,850 | –3731 | Dominates |
| Health state costs UCI | –14,466 | 1.72 | 48,893 | –8404 | Dominates |
| Parameter | PEG α-2b vs. BSC | ||||
|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | INB (£) | ICER (£/QALY) | ICER (£/QALY) | |
| Baseline | –8874 | 1.66 | 42,068 | –5347 | Dominates |
| Time horizon 30 years | –2105 | 0.26 | 7282 | –8131 | Dominates |
| Time horizon 90 years | –8990 | 1.76 | 44,096 | –5122 | Dominates |
| Discount rate 0% | –42,451 | 8.63 | 215,036 | –4919 | Dominates |
| Discount rate 6% costs, 1.5% outcomes | –1440 | 4.15 | 84,503 | –347 | Dominates |
| Discount rate 1.5% costs, 1.5% outcomes | –22,191 | 4.15 | 105,255 | –5343 | Dominates |
| Discount rate 6% costs, 6% outcomes | –1440 | 0.55 | 12,530 | –2597 | Dominates |
| SVR for PEG α-2a 69% | –8874 | 1.66 | 42,068 | –5347 | Dominates |
| SVR for PEG α-2a 51% | –8874 | 1.66 | 42,068 | –5347 | Dominates |
| SVR for PEG α-2b 65% | –10,819 | 1.88 | 48,328 | –5769 | Dominates |
| SVR for PEG α-2b 52% | –7207 | 1.47 | 36,702 | –4887 | Dominates |
| Cohort 100% F0 | –5391 | 1.06 | 26,534 | –5100 | Dominates |
| Cohort 100% F2 | –15,691 | 2.75 | 70,789 | –5696 | Dominates |
| Cohort 100% F3 | –18,329 | 4.08 | 99,854 | –4496 | Dominates |
| Cohort 20% F4 | –11,391 | 2.64 | 64,141 | –4319 | Dominates |
| Starting age 5 years | –11,703 | 1.75 | 46,610 | –6705 | Dominates |
| Starting age 16 years | –7886 | 1.55 | 38,957 | –5076 | Dominates |
| Transition probabilities LCI | –8110 | 0.83 | 24,797 | –9720 | Dominates |
| Transition probabilities UCI | –8199 | 2.59 | 59,925 | –3170 | Dominates |
| Utility values LCI | –8874 | 1.81 | 45,100 | –4899 | Dominates |
| Utility values UCI | –8874 | 1.52 | 39,255 | –5842 | Dominates |
| Utility values, HTA report | –8874 | 3.03 | 69,414 | –2932 | Dominates |
| Health state costs LCI | –5233 | 1.66 | 38,426 | –3153 | Dominates |
| Health state costs LCI | –13,008 | 1.66 | 46,201 | –7837 | Dominates |
| Parameter | PEG α-2b vs. PEG α-2a | ||||
|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | INB (£) | ICER (£/QALY) | ICER (£/QALY) | |
| Baseline | 1316 | –0.06 | –2549 | –21,345 | Dominated |
| Time horizon 30 years | 1082 | –0.01 | –1349 | –81,135 | Dominated |
| Time horizon 90 years | 1320 | –0.06 | –2619 | –20,323 | Dominated |
| Discount rate 0% | 2474 | –0.30 | –8513 | –8191 | Dominated |
| Discount rate 6% costs, 1.5% outcomes | 1059 | –0.15 | –4012 | –7176 | Dominated |
| Discount rate 1.5% costs, 1.5% outcomes | 1775 | –0.15 | –4728 | –12,024 | Dominated |
| Discount rate 6% costs, 6% outcomes | 1059 | –0.02 | –1530 | –45,016 | Dominated |
| SVR for PEG α-2a 69% | 3816 | –0.34 | –10,597 | –11,256 | Dominated |
| SVR for PEG α-2a 51% | –1185 | 0.22 | 5500 | –5491 | Dominates |
| SVR for PEG α-2b 65% | –629 | 0.15 | 3711 | –4082 | Dominates |
| SVR for PEG α-2b 52% | 2983 | –0.25 | –7914 | –12,097 | Dominated |
| Cohort 100% F0 | 1196 | –0.04 | –2003 | –29,626 | Dominated |
| Cohort 100% F2 | 1551 | –0.10 | –3639 | –14,852 | Dominated |
| Cohort 100% F3 | 1642 | –0.15 | –4642 | –10,946 | Dominated |
| Cohort 20% F4 | 1403 | –0.09 | –3300 | –14,783 | Dominated |
| Starting age 5 years | –687 | –0.06 | –605 | 10,641 | 10,641 |
| Starting age 16 years | 831 | –0.06 | –1991 | –14,339 | Dominated |
| Transition probabilities LCI | 1289 | –0.03 | –1953 | –38,855 | Dominated |
| Transition probabilities UCI | 1292 | –0.09 | –3164 | –13,809 | Dominated |
| Utility values LCI | 1316 | –0.07 | –2676 | –19,345 | Dominated |
| Utility values UCI | 1316 | –0.06 | –2430 | –23,625 | Dominated |
| Utility values, HTA report | 1316 | –0.11 | –3482 | –12,151 | Dominated |
| Health state costs LCI | 1190 | –0.06 | –2423 | –19,308 | Dominated |
| Health state costs LCI | 1458 | –0.06 | –2691 | –23,657 | Dominated |
For the DSA for peginterferon alfa-2a versus BSC (Table 40), the ICERs varied between −£581 and −£11,709 (INB £8631–£223,550). The model results are most influenced by the discount rate chosen, and the incremental costs (−£2499 to −£44,925) and QALYs (0.58–8.93) vary considerably for changes to the discount rate. The previous NICE discount rate (6% costs, 1.5% benefits) produces a more favourable ICER than the current NICE discount rate (3.5% costs, 3.5% benefits). Using a shorter time horizon does not capture all the costs and health benefits. The total additional QALYs for peginterferon alfa-2a compared with BSC varies between 0.27 and 1.82 QALYs for the 30 and 90 years, respectively.
For the DSA for peginterferon alfa-2b versus BSC (Table 41), the ICERs varied between −£347 and −£9720 (INB £7282–£215,036). BSC remains dominated in each analysis. As for the results shown in Table 40 for peginterferon alfa-2a, the model results are also most influenced by the discount rate chosen and the time horizon (see Table 41).
The DSA for peginterferon alfa-2b versus peginterferon alfa-2a are shown in Table 42. The DSA should be treated with caution due to uncertainty around the relative treatment effect for SVR for peginterferon alfa-2b versus peginterferon alfa-2a. The model results are most sensitive to changes in the treatment effectiveness. Peginterferon alfa-2b continues to be dominated by peginterferon alfa-2a for all changes to the model parameters, except for changes to the SVR (peginterferon alfa-2a 51% or peginterferon alfa-2b 65%, shown in bold in Table 42) and the starting age of the cohort (age 5 years).
Overall, it appears that the results are robust to changes in the model assumptions, the structure and the input parameters.
Scenario analyses
In addition to the sensitivity analyses, three scenario analyses were undertaken to investigate the uncertainty around structural assumptions. The first investigated a range of estimates for the SVR, as those used in the base-case analysis were based on pooled estimates from cohort studies included in the review of clinical effectiveness and are therefore subject to uncertainty. The second varied the rate of progression of the cohort to the cirrhosis disease state and the final scenario assessed the impact of delaying treatment until adulthood (referred to as ‘watchful waiting’) rather than treating with peginterferon alfa during childhood.
(1) Treatment effectiveness (sustained virological response) of peginterferon alfa-2a versus peginterferon alfa-2b
For the base-case analysis (reported above in Table 39), the SVR for peginterferon alfa-2b is slightly lower (58%) than for peginterferon alfa-2a (60%) and this results in an increased cost of £1316 and reduced QALYs (−0.06). In this case, peginterferon alfa-2a dominates peginterferon alfa-2b (i.e. is the optimal treatment strategy).
Table 43 shows the cost-effectiveness results of peginterferon alfa-2b compared with peginterferon alfa-2a using a range of estimates of the SVR for peginterferon alfa-2b. These estimates of SVR for peginterferon alfa-2b are varied between 50% and 70%, while keeping the SVR estimate for peginterferon alfa-2a at 60% for all analyses.
| SVR (%) | PEG α-2b | PEG α-2b, incremental | ICER (£/QALY) | Optimal treatment | |||
|---|---|---|---|---|---|---|---|
| PEG α-2a | PEG α-2b | Cost (£) | QALYs | Cost (£) | QALYs | ||
| 60 | 58a | 20,371 | 22.19 | 1316 | –0.06 | Dominated | PEG α-2a |
| 60 | 50 | 22,594 | 21.94 | 3539 | –0.31 | Dominated | PEG α-2a |
| 60 | 55 | 21,205 | 22.10 | 2149 | –0.15 | Dominated | PEG α-2a |
| 60 | 60 | 19,815 | 22.25 | 760 | 0.00 | N/A | PEG α-2a |
| 60 | 65 | 18,426 | 22.40 | –629 | 0.15 | Dominates | PEG α-2b |
| 60 | 70 | 17,037 | 22.56 | –2018 | 0.31 | Dominates | PEG α-2b |
The cost-effectiveness of peginterferon alfa-2a compared with peginterferon alfa-2b is proportionate to the relative SVR of these treatments. For strategies where SVR for peginterferon alfa-2a is greater than or the same as that for peginterferon alfa-2b, peginterferon alfa-2a is the optimal treatment; conversely, where SVR for peginterferon alfa-2b is greater than that for peginterferon alfa-2a, peginterferon alfa-2b is the optimal treatment. This demonstrates the extent of the uncertainty in the base-case results.
As noted above, in the base-case analysis the SVR for peginterferon alfa-2b is slightly lower (58%) than for peginterferon alfa-2a (60%). However, there is uncertainty about the reliability of the relative SVR effect sizes between peginterferon alfa-2a and peginterferon alfa-2b, due to the poor quality of the studies and the lack of head-to-head trials. Furthermore, the SVR study estimates range from 53% to 66% in children treated with peginterferon alfa-2a and 49% to 65% for those treated with peginterferon alfa-2b when excluding the two studies with very small participant numbers, and this further demonstrates the variance around the estimates in the study samples.
The SVR for each treatment for each study is influenced by the population genotype distribution. As noted above, the distribution of the HCV genotypes in the populations within the studies of peginterferon alfa-2a and those of peginterferon alfa-2b were different. Within the studies of peginterferon alfa-2a, 77% would be classed as genotype 1 or 4 and 23% as genotype 2 or 3. In the studies of peginterferon alfa-2b the rates are 82% and 18% for genotype 1 or 4 and genotype 2 or 3, respectively. As evidence suggests that those with genotype 2 or 3 are more likely to respond to treatment, this would suggest that the treatment responses seen in the peginterferon alfa-2a studies may be better because of the distribution of the genotypes in the baseline populations. We adjusted the SVR treatment response for peginterferon alfa-2a and -2b by assuming the same genotype distributions for both treatments (80% genotype 1 or 4, 20% genotype 2 or 3). In this scenario, the SVR treatment response for both treatments was 59%; both treatment cohorts have the same total QALY (22.22) and peginterferon alfa-2a (£19,333) has a slightly lower cost than peginterferon alfa-2b (£20,093).
(2) Progression of hepatitis to cirrhosis
We conducted a scenario analysis varying the progression rate to cirrhosis (F4). In the base case the patients with BSC spent a mean duration of 28.65 years in the chronic HCV health states (F0–F3) (see Table 34). The base-case transition probabilities between the health states ranging between F0 (no cirrhosis) and F3 (compensated cirrhosis) were all in the region of 0.1 (see Table 26). For the scenario analysis we varied the transition probabilities between the fibrosis states from 0.05 to 0.30, with the same probability for transitions between each of these states (F0–F4).
Table 44 shows the effect of varying these transition probabilities on the comparison of peginterferon alfa-2a with BSC. The time spent in the chronic HCV health states varies between 10 and 48 years. For all analyses, the total costs for the BSC cohort are higher than those for the peginterferon alfa-2a group and the total QALYs are lower, i.e. peginterferon alfa-2a dominates BSC. Therefore, treatment with peginterferon alfa-2a is likely to be cost-effective with a greater or lesser degree of time spent in the chronic HCV health state. These analyses have been completed using treatment with peginterferon alfa-2a; however, the same would also be true for analyses completed using treatment with peginterferon alfa-2b.
| Transition probability | Time (years) | BSC | PEG α-2a | ICER (£/QALY) | ||
|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||
| Base case | 28.65 | 29,245 | 20.53 | 19,055 | 22.25 | PEG α-2a dominates |
| 0.05 | 48.57 | 19,797 | 22.65 | 15,091 | 23.16 | PEG α-2a dominates |
| 0.10 | 30.20 | 29,157 | 20.75 | 19,021 | 22.35 | PEG α-2a dominates |
| 0.15 | 20.69 | 34,597 | 19.25 | 21,290 | 21.71 | PEG α-2a dominates |
| 0.20 | 15.59 | 37,954 | 18.17 | 22,690 | 21.25 | PEG α-2a dominates |
| 0.30 | 10.42 | 41,859 | 16.78 | 24,321 | 20.65 | PEG α-2a dominates |
The natural history of chronic HCV acquired in infancy is not well understood and there is some uncertainty around the rate of progression to cirrhosis and more severe liver disease. Guido and colleagues75 estimated that the mean time from infection to cirrhosis was 28 years, based upon a median fibrosis progression rate of 0.142. They considered that this was consistent with the duration observed for adults of 30 years. The transition probabilities between fibrosis states estimated by Guido and colleagues were higher than the transition probabilities used in our model (from Thein and colleagues78), and so the duration in the chronic HCV states in our model was longer than that estimated by Guido and colleagues. 75 However, we note that the study by Thein and colleagues,78 although using an adult population, is a far larger study (n = 33,121) than the study by Guido and colleagues (n = 112). 75
(3) Watchful waiting
We investigated a scenario of treating patients with peginterferon alfa-2a as children (aged 11 years) compared with a ‘watchful waiting’ scenario where patients are treated only once they are adults (aged 18–30 years). The results are shown in Table 45. These show that strategies for watchful waiting cost more and are associated with reduced QALYs compared with treating as a child (i.e. strategy of treating children dominates watchful waiting). For example, treating adults aged 21 years would be associated with an increased cost of £3872 and decreased QALYs of 0.07 compared with treatment at age 11 years. The potential disbenefit of delaying treatment until age 30 years is even greater, with a reduction in QALYs of 0.45; owing to the progressive nature of the disease, many patients would have reached more severe disease by this age. These analyses have been completed using treatment with peginterferon alfa-2a; however, the same would also be true for analyses completed using treatment with peginterferon alfa-2b.
| Strategy | Cost (£) | Life-years | QALYs | Incremental cost (£) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|
| PEG α-2a | 19,055 | 24.64 | 22.25 | |||
| WW, treatment age 18 years | 21,959 | 24.62 | 22.22 | 2904 | –0.02 | –140,104 |
| WW, treatment age 21 years | 22,928 | 24.56 | 22.17 | 3872 | –0.07 | –53,662 |
| WW, treatment age 25 years | 24,476 | 24.43 | 22.04 | 5420 | –0.20 | –26,962 |
| WW, treatment age 30 years | 26,668 | 24.19 | 21.79 | 7612 | –0.45 | –17,095 |
Probabilistic sensitivity analysis
In addition to the DSA and scenario analyses, a PSA was undertaken. Parameters were sampled probabilistically from appropriate distributions; these included the proportions of children distributed across genotypes, the transition probabilities, the health state utilities and monitoring, health state and treatment costs. Details of the PSA and the distributions applied are reported in Appendix 9. One thousand simulations were run. A summary of the results from the PSA are shown in Table 46.
| Treatment | Costs, £ (IQR) | QALYs (IQR) | ICER (£/QALY) vs. BSC | ICER (£/QALY) vs. PEG α-2a |
|---|---|---|---|---|
| BSC | 29,689 (26,958–31,979) | 20.50 (20.1–21.0) | – | – |
| PEG α-2a | 19,226 (17,679–20,593) | 22.22 (22.0–22.5) | Dominates | – |
| PEG α-2b | 20,558 (19,202–21,777) | 22.16 (21.9–22.4) | Dominates | Dominated |
The PSA results closely reflect the deterministic base-case results; the total costs for both peginterferon alfa-2a and -2b (£19,226 and £20,558, respectively) are less than those incurred by patients receiving BSC (£29,689). Patients in the BSC group also accrue fewer QALYs than their counterparts in both treatment groups: 20.50 compared with 22.22 with peginterferon alfa-2a and 22.16 with peginterferon alfa-2b. Therefore, in the PSA peginterferon alfa-2a and -2b both dominate BSC. Peginterferon alfa-2a has lower lifetime costs and is more effective than peginterferon alfa-2b in this analysis, and dominates this treatment. As stated above (see Results of independent economic analysis), there is uncertainty around the reliability of the relative SVR effect size between peginterferon alfa-2a and -2b.
The scatterplots for cost and health outcomes of the treatment options in the PSA are shown in Figure 9. The CEAC is shown in Figure 10, and indicates that at all WTP thresholds, peginterferon alfa-2a has the highest probability of being cost-effective. At WTP thresholds of £20,000 and £30,000 per QALY, the probability of being cost-effective is 68% and 66% for peginterferon alfa-2a, and 32% and 34% for peginterferon alfa-2b, respectively.
FIGURE 9.
Scatterplot of the costs and health benefits for peginterferon alfa-2a, peginterferon alfa-2b and BSC.
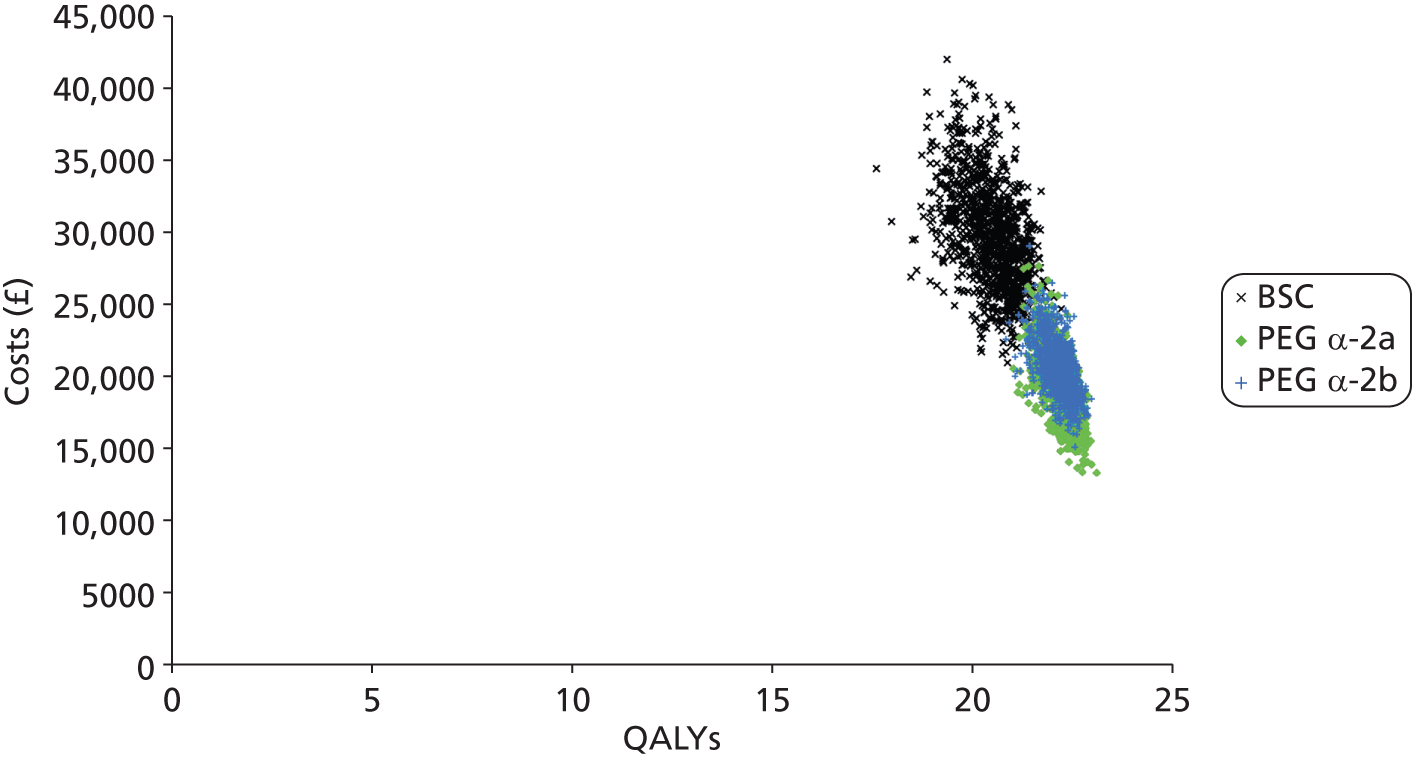
FIGURE 10.
Cost-effectiveness acceptability curve for the PSA results.
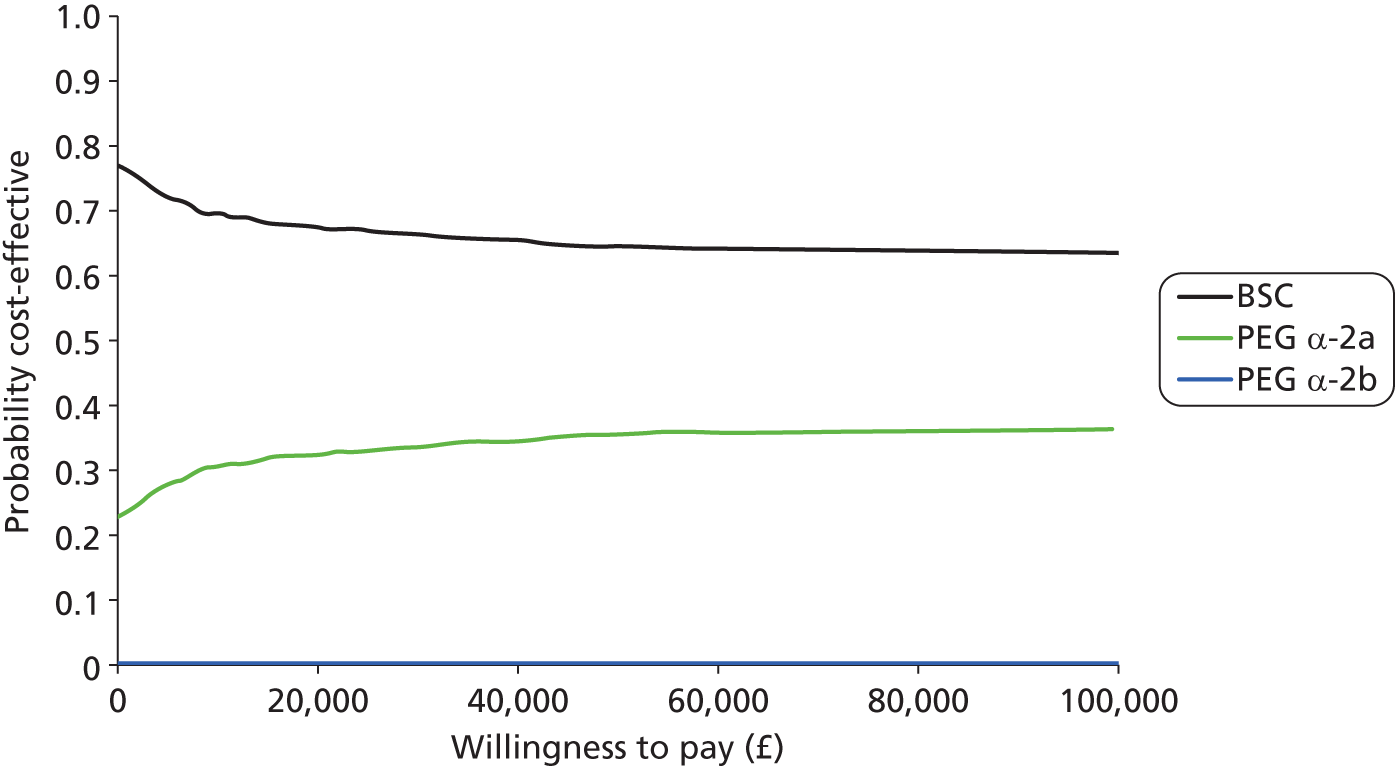
Comparison of the cost-effectiveness results from the manufacturers’ submissions and Southampton Health Technology Assessments Centre’s model
The results of the manufacturers’ and SHTAC’s economic analyses are summarised in Table 47. Roche did not present its results for an all-patient cohort; rather, it presented its analyses based on genotype groups. The results seen from the three analyses are similar with respect to the overall cost-effectiveness conclusions; that is, that peginterferon alfa-2a and -2b (both in combination with RBV) are dominant compared with BSC. MSD and SHTAC also present analyses of the comparison of peginterferon alfa-2a with peginterferon alfa-2b as per the NICE scope. These differ, with MSD suggesting that peginterferon alfa-2b dominates peginterferon alfa-2a whereas SHTAC suggests the opposite; however, the difference between the results is marginal.
| Result | Analysis | BSC | PEG α-2a | PEG α-2b |
|---|---|---|---|---|
| Total cost (£) | SHTAC | 29,245 | 19,055 | 20,371 |
| MSD, all patients | 22,750 | 17,798 | 17,526 | |
| Roche, genotypes 1 and 4 | 8199 | 12,170 | – | |
| Roche, genotypes 2 and 3 | 8199 | 6336 | – | |
| Total QALYs | SHTAC | 20.53 | 22.25 | 22.19 |
| MSD, all patients | 16.77 | 19.16 | 19.24 | |
| Roche, genotypes 1 and 4 | 14.20 | 15.21 | – | |
| Roche, genotypes 2 and 3 | 14.20 | 15.77 | – | |
| Incremental cost vs. BSC (£) | SHTAC | – | –10,190 | –8874 |
| MSD, all patients | – | –4952 | –5224 | |
| Roche, genotypes 1 and 4 | – | 3971 | – | |
| Roche, genotypes 2 and 3 | – | –1864 | – | |
| Incremental QALYs vs. BSC | SHTAC | – | 1.72 | 1.66 |
| MSD, all patients | – | 2.39 | 2.47 | |
| Roche, genotypes 1 and 4 | – | 1.01 | – | |
| Roche, genotypes 2 and 3 | – | 1.57 | – | |
| ICER vs. BSC (£/QALY) | SHTAC | – | Dominates | Dominates |
| MSD, all patients | – | Dominates | Dominates | |
| Roche, genotypes 1 and 4 | – | 3915 | – | |
| Roche, genotypes 2 and 3 | – | Dominates | – | |
| ICER PEG α-2b vs. PEG α-2a (£/QALY) | SHTAC | – | – | Dominated |
| MSD, all patients | – | – | Dominates | |
| Roche, genotypes 1 and 4 | – | – | – | |
| Roche, genotypes 2 and 3 | – | – | – |
The total costs vary widely between the analyses; for example, the cost of BSC varies between £8199 (Roche) and £29,245 (SHTAC). Roche uses a much shorter time horizon than the other two analyses, which largely explains these differences. The difference in costs between SHTAC and MSD (for BSC) is due to the length of time patients spend in the HCV health states; this is considerably shorter in the SHTAC analysis than in the MSD analysis, and therefore patients incur higher health-care costs. The incremental costs for peginterferon alfa-2a versus BSC vary between £3971 (Roche genotype 1) and −£10,190 (SHTAC).
There is also a wide range of total QALY estimates between the studies. For BSC, these vary between 14.2 (Roche) and 20.53 (SHTAC). The incremental QALYs vary between 1.01 (Roche genotype 1) and 2.39 (MSD). These differences are largely down to shorter time horizon (Roche) and the lower utility values used in the MSD than in the SHTAC analysis.
Summary of cost-effectiveness
-
A systematic search of the literature found no studies that met the inclusion criteria. Two cost-effectiveness studies in children were summarised: one abstract and one full economic evaluation for non-pegylated interferon treatment.
-
A systematic review of studies of QoL in children with chronic HCV did not identify any relevant studies. An update of searches for QoL in adults found one new study and one previously unidentified study that provided EQ-5D for patients with chronic HCV.
-
Two manufacturers submitted evidence to be considered for the appraisal:
-
MSD, the manufacturer of peginterferon alfa-2b, constructed a lifetime Markov model with a model structure based upon that developed for previous NICE appraisals for adults. The model used the effectiveness of the treatments from a meta-analysis of the clinical trials. The base-case results from the submission found that both combinations of peginterferon alfa dominated BSC in all age and genotype subgroups. There were small differences in costs and health outcomes between peginterferon alfa-2a and -2b. Peginterferon alfa-2b dominated peginterferon alfa-2a for most age and genotype subgroups.
-
Roche, the manufacturer of peginterferon alfa-2a, also constructed a Markov model based upon that developed for previous NICE appraisals for adults, with a time horizon of 30 years. The model used the effectiveness of peginterferon alfa-2a from a weighted average of the four clinical trials. The base-case results from the submission found that, compared with BSC, peginterferon alfa-2a is a cost-effective option for the treatment of paediatric HCV in genotype 1, 4 and 5 patients (ICER of £3914/QALY gained) and in genotype 2 and 3 patients (ICER dominates).
-
-
The authors of this report developed an independent Markov model, based upon that developed for previous NICE appraisals for adults, with a time horizon of 70 years. From this model, peginterferon alfa in combination with RBV was more effective and had lower lifetime costs than BSC. The costs were slightly lower for peginterferon alfa-2a compared with peginterferon alfa-2b and the QALYs slightly higher; thus, peginterferon alfa-2b was dominated by peginterferon alfa-2a. The DSAs showed that these conclusions for peginterferon alfa compared with BSC were robust for all changes to the structural assumptions and input parameters. Peginterferon alfa-2b dominates peginterferon alfa-2a for all sensitivity analyses except for changes to the SVR. The model results are most sensitive to changes in the discount rate, time horizon, treatment SVR and the starting fibrosis make-up of the cohort. According to the PSA, peginterferon alfa-2a has the greatest probability of being cost-effective at a WTP threshold of £30,000 per QALY.
Chapter 6 Assessment of factors relevant to the NHS and other parties
Peginterferon alfa-2a and -2b in combination with RBV are used in the treatment of adults with chronic HCV following previous NICE guidance,32–34 and our expert clinical advisors suggest that many children with chronic HCV are currently treated with peginterferon alfa and RBV. The small number of children with chronic HCV in the UK are generally referred to one of the three specialist paediatric hepatology centres, although they can be managed within shared care networks at local clinics. If there were to be wider access to treatment, there may be an impact in terms of resources, such as recruitment and training of specialist hepatology nurses, and additional input from general practitioners (GPs) and child psychology services.
Other than peginterferon alfa, there are currently no licensed agents available and hence limited treatment options for this population. Peginterferon alfa and RBV treatment may therefore be considered to be novel therapies for children and young people under 18 years of age who have not previously had access to treatment for chronic HCV. Clinicians are of the opinion that this is a rapidly changing treatment community with other, newer drugs in the pipeline, including non-interferon-based therapies such as protease inhibitors (see Chapter 8, Suggested research priorities). The emergence of newer treatment options may affect the uptake of peginterferon alfa and RBV therapy and, consequently, those with less favourable genotypes may prefer to wait for newer drugs.
We are not aware of any issues relating to equality.
Chapter 7 Discussion
Statement of principal findings
Clinical effectiveness
The results of seven studies evaluating peginterferon alfa treatment in children and young people were included in this systematic review. The evidence was limited to uncontrolled single-cohort studies (with one RCT treated as a single-cohort study) which had relatively small populations. Overall, the studies were of low methodological quality and, owing to the nature of the design, few statistical analyses were reported.
The studies varied in their population characteristics according to a number of factors, including HCV genotype mix, mean participant age, treatment history, mode of HCV transmission, baseline HCV RNA levels and different countries involved. Six studies excluded those co-infected with HIV or hepatitis B, three peginterferon alfa-2b studies46,47,51 specifically excluded younger children (< 8 years old), two48,51 (peginterferon alfa-2b) included a mix of treatment-naive and previously treated children (with a third study unclear47) and only one study57 (peginterferon alfa-2a) included UK participants. The design and quality of the studies, as well as other uncertainties, make assessment of the generalisability of the studies difficult and the variation in these factors may have implications for the interpretation of the findings.
The SVR rates in the included studies ranged from 53% to 66% in children treated with peginterferon alfa-2a and 29% to 75% in those treated with peginterferon alfa-2b, although the range was 49–65% if two studies with very small participant numbers46,47 are excluded. These rates are comparable with those seen in adults with chronic HCV (50–60%20). Observed patterns in the SVR subgroups (genotype 2 or 3, treatment naive, low viral load at baseline) also appear to be consistent with what is seen in adults, i.e. higher SVR rates are observed in these subgroups. However, the numbers of children in some of these subgroups were very small and none of the studies was powered for subgroup analysis; therefore, results should be interpreted with caution. Serious adverse events were defined differently in the studies and the numbers of data provided on adverse events varied considerably from one study to the next. However, overall the relatively mild adverse events typically associated with the use of peginterferon and RBV were frequent (e.g. flu-like symptoms were almost universal) and consistent with those in adults.
No studies were identified that directly compared peginterferon alfa-2a with peginterferon alfa-2b, and as any comparison would be by observation of data only, no conclusions can be drawn in the present review. Two recent (2010)89,90 meta-analyses of RCTs comparing peginterferon alfa-2a with peginterferon alfa-2b in adults both concluded that no recommendations could be made of one over the other. Whether or not the two peginterferon alfa forms are equally effective in children remains inconclusive.
Cost-effectiveness
Systematic searches did not identify any evidence that met the criteria for the systematic review of cost-effectiveness studies of peginterferon alfa treatments in children or HRQoL in children with chronic HCV. A small number of studies with some limited relevance were identified (although these did not meet the inclusion criteria) and were summarised for context to the cost-effectiveness analysis.
Two manufacturers submitted evidence for the cost-effectiveness of their respective treatments versus BSC, and one (MSD) also compared the cost-effectiveness of the two treatments. Both manufacturers based their model structure on one previously developed for NICE appraisals of these treatments in adults. There were differences, however, in the time horizon and the sources of the effectiveness data. MSD used a lifetime horizon and Roche a 30-year time horizon. Effectiveness data were sourced from a meta-analysis of single-cohort studies in the MSD model and a weighted average of single-cohort studies in the Roche model. Neither approach was assessed by the present review of clinical effectiveness to be robust, and these estimates should therefore be treated with caution. In the MSD model the base-case results found that both combinations of peginterferon alfa dominated BSC in all age and genotype subgroups. There were small differences in costs and health outcomes between peginterferon alfa-2a and peginterferon alfa-2b. Peginterferon alfa-2b dominated peginterferon alfa-2a for most age and genotype subgroups. In the Roche model the base-case results found that peginterferon alfa-2a is a cost-effective option for the treatment of paediatric HCV compared with BSC. Both submissions were assessed as meeting the majority of quality standards required of an economic evaluation.
An independent Markov model, based upon the previous SHTAC model, was undertaken by the assessment group to assess the cost-effectiveness of the two peginterferon alfa treatments in combination with RBV compared with BSC and one another, as per the NICE scope. A 70-year time horizon was used. Many of the assumptions used previously were employed, with the addition of assumptions that no children would have spontaneous viral clearance, that treatment would discontinue if an EVR was not achieved at week 12, and that adult transition probabilities, utility weights and health state costs could be applied for paediatric patients owing to lack of data. Effectiveness data were taken from the studies identified in the systematic review of clinical effectiveness, where a weighted average approach was used to establish the SVRs and EVRs. Utility data were taken from published sources although, as noted above, these were in adult populations. Results from this model, albeit based upon poor evidence, suggest that peginterferon alfa (-2a or -2b) in combination with RBV is more effective and has lower lifetime costs than BSC. Peginterferon alfa-2a has slightly lower lifetime costs and is more effective than peginterferon alfa-2b in the overall population and the subgroup with genotype 1 or 4, but not in the subgroup with genotype 2 or 3.
Strengths and limitations of the assessment
Strengths
The current technology assessment addresses a specific knowledge gap concerning the clinical effectiveness and cost-effectiveness of peginterferon alfa-2a and peginterferon alfa-2b in children and adolescents with chronic hepatitis C.
This technology assessment report has been undertaken by an independent evidence synthesis team free of any vested interest. The technology assessment addressed a clear question with a well-defined specific population, intervention, comparator and outcomes. An independent advisory group including clinical experts commented on the research protocol and the final report. The systematic review of clinical effectiveness followed standard principles of evidence synthesis recommended by the CRD to minimise bias. 43 The methods were set out a priori in a peer-reviewed research protocol that defined the research question, inclusion criteria, critical appraisal approach, data extraction process, and the methods of data synthesis to be used. The study selection step was conducted independently by two reviewers and all other steps were conducted by one reviewer and checked independently by a second reviewer, with all decisions recorded and transparently reported.
An economic model has been developed following recognised guidelines,44,45 and systematic searches have been conducted to identify data for the economic model. The searches, critical appraisal of the economic evidence, and development and reporting of the economic model were conducted by one reviewer and checked independently by a second reviewer.
Manufacturers’ submissions of evidence were systematically data-extracted and critically appraised by the review team using a standard health economic evaluation checklist (see Appendix 8) and, where appropriate, information from the submissions was used to inform the economic evaluation.
The model captures variability and uncertainty in effectiveness, costs and clinical practice using one-way DSA to evaluate the influence of individual parameters, model structure and assumptions on the robustness of the model. Multiparameter uncertainty was addressed using PSAs for a range of parameter distributions (see Appendix 9), while scenario analyses were used to explore differences between the treatments and the effect of varying the rate of progression to cirrhosis. Uncertainty around the cost-effectiveness of the interventions for different WTP thresholds is represented transparently in CEACs.
Limitations
Despite the extensive and systematic searches, relatively little clinical effectiveness evidence was found, with only two studies of peginterferon alfa-2a and five studies of peginterferon alfa-2b meeting the inclusion criteria for the systematic review. These were small studies, with only 7–107 participants. Although one study was a RCT, only one of its arms was relevant, meaning that no head-to-head comparisons of peginterferon alfa against BSC, or peginterferon alfa-2a against peginterferon alfa-2b were available. The available evidence thus relies solely on pre- to postintervention comparisons of outcomes within single cohorts of participants. On the whole, the studies were poorly reported, with high risk of bias, meaning that their results should be interpreted with caution. Although all studies reported the primary outcome (SVR), some other relevant outcomes were only reported in a few studies, meaning that no conclusions can be drawn regarding the effectiveness of peginterferon alfa-2a or -2b on biochemical response (normalisation of ALT levels), histological response (degree of inflammation, fibrosis or cirrhosis), QoL or growth (height or weight changes were reported in most studies but effects were not consistent).
Owing to heterogeneity among the studies in their participant characteristics and a lack of information on the variance of SVR estimates, quantitative meta-analysis to obtain pooled effect estimates was not appropriate. The smallest study (which had only seven participants and was not conducted in the UK) was an outlier in having a considerably lower SVR than the six other studies. With such small studies it can be difficult to determine whether they should be classed as a prospective cohort or case series (our inclusion criteria were conservative in order not to exclude potentially relevant evidence). The possible implications of including or excluding this study were considered as part of a structured narrative synthesis.
As noted previously, generalisability of the clinical effectiveness studies is difficult to determine. The generalisability to a UK paediatric population is uncertain and the results may also not be generalisable to participants with hepatic comorbidities, as the studies excluded participants with hepatitis B and HIV and, in some cases, other liver diseases. There were no studies identified in children and young people with HIV co-infection that met the inclusion criteria.
A systematic review of the literature for cost-effectiveness studies found none that met the inclusion criteria (two cost-effectiveness studies in children were identified, but one was reported superficially in an abstract only while the other, a full economic evaluation, included interferon rather than peginterferon treatment). A systematic review of studies of QoL in children with chronic HCV did not identify any relevant studies.
Parameters in the model (disease progression, utility and health state costs) have not been derived for the specific patient group in this assessment, i.e. children, as targeted searches undertaken for this review did not identify suitable data, and so parameter values have been taken for the adult population. It is uncertain how applicable these parameter values are. However, it should be noted that, as the model is a lifetime model, these parameter values are relevant during the adult years. There is also some degree of uncertainty over the intervention costs used, which include monitoring and outpatient costs. In the absence of clinical guidelines in children, these were based on the previous assessments in adults and uprated to current values. It is possible that the costs for the tertiary referral centres for children differ from the costs of secondary care in adults.
As there were no direct head-to-head comparisons of the interventions, a pragmatic approach was taken in the economic model to use the available SVR evidence through an unadjusted indirect comparison. Caution is recommended in the interpretation of the economic evaluation because, as no relevant RCTs were available, there is no means by which the similarity of the included studies can be assessed; however, it is generally recognised that the SVR of the control group is zero. The clinical effectiveness data for SVR that were included in the economic model are also limited by the small size and number of the primary research studies that met the inclusion criteria for the clinical effectiveness systematic review. Although the analysis approach has inherent risk of bias, the sensitivity and scenario analyses conducted provide an illustration of the likely estimate of the cost-effectiveness. Owing to the large number of parameters included in the sensitivity analyses, some parameters (e.g. for health state costs, transition probabilities and utility values) were combined and varied together rather than individually.
A potential limitation of the current evidence synthesis is that the searches for clinical effectiveness and cost-effectiveness data were limited to English-language publications. The approach can be justified as the context of the technology assessment is specifically the NHS in England and Wales. However, only one of the identified studies57 included any participants from the UK.
Uncertainties
Despite efforts to explore uncertainty in the evidence synthesis, some important uncertainties remain. There is uncertainty about the reliability of the relative SVR effect sizes between peginterferon alfa-2a and peginterferon alfa-2b, due to the poor quality of the studies and the lack of head-to-head trials. Variance around the estimates in the study samples is illustrated by SVR rates that range from 53% to 66% in children treated with peginterferon alfa-2a, and 49% to 65% in those treated with peginterferon alfa-2b when excluding two studies of peginterferon alfa-2b which had very small participant numbers (7 and 12 participants, respectively46,47). Some studies reported SVR rates in relation to prognostic factors (e.g. genotype or previous treatment history). However, these relationships were reported in few studies, and inevitably involved even smaller numbers of participants than were available for the primary outcome, so their reliability is uncertain.
The cost-effectiveness of peginterferon alfa-2a compared with peginterferon alfa-2b is proportionate to the relative SVR for these treatments. For strategies where SVR for peginterferon alfa-2a is greater than or the same as that for peginterferon alfa-2b, peginterferon alfa-2a is the optimal treatment; conversely, where SVR for peginterferon alfa-2b is greater than that for peginterferon alfa-2a, peginterferon alfa-2b is the optimal treatment. This demonstrates the extent of the uncertainty in the base-case results. The economic evaluation used a number of assumptions which had been discussed with our expert advisory group. We took a conservative approach to the assumption about stopping treatment in those with genotype 1 or 4 at 24 weeks if no EVR was obtained (at 12 weeks), because expert opinion was mixed. Finally, with regard to the parameters in the model, in the absence of data the model assumes that the treatment response is the same across all chronic HCV states (F0–F4); however, some uncertainty remains about this assumption.
The distribution of participants among the health states used in the model was based on a weighted average of the distribution of the participants in the studies included in the clinical effectiveness systematic review. It is uncertain how generalisable these populations are to the population of children with chronic HCV in the UK, and it is possible that in the economic model participants may be in more or less severe disease states than those seen in the UK.
Growth is an important outcome for assessing the effects of peginterferon alfa and RBV in children and adolescents with chronic HCV. The results appear to provide a weak indication that height and/or weight increases were reduced by peginterferon alfa-2b but not peginterferon alfa-2a, but this may not be reliable as only one peginterferon alfa-2a study reported this outcome and height/weight changes were not universal in the peginterferon alfa-2b studies.
Another outcome of particular interest in children and adolescents with chronic HCV is QoL. The experience of clinicians who informed the technology assessment suggests that children and adolescents may experience non-trivial QoL decrements associated with the stigma of the disease. However, there are very limited QoL data available for this population and only one of the included studies addressed this outcome, precluding any conclusions concerning the possible impact of peginterferon alfa on children and adolescents with chronic HCV. In the economic model, HRQoL was taken from studies in adults owing to a lack of data. It remains uncertain whether or not there is any impact on parent or carer’s QoL, and this has not been assessed in the cost-effectiveness model for the current assessment.
The scope of the current technology assessment is explicit about the age of the eligible population, namely children and adolescents aged 3–17 years. A number of primary research studies had populations that did not meet these criteria, as they included younger and/or older participants, and were therefore excluded (reasons for exclusion are reported in Appendix 4). It is unclear whether or not the inclusion of studies whose populations differed only marginally from the specified age range would have made any difference to the results. Although widening the age range might have allowed more evidence to be considered, it is difficult to determine at what point the age limits would become irrelevant to the decision problem. Also, deviation from the a priori-specified inclusion criteria may increase the risk of selection bias.
Chapter 8 Conclusions
Treatment of children and young people with peginterferon (alfa-2a or -2b) and RBV may be a viable option. Results from the independent Markov model suggest that peginterferon (alfa-2a or -2b) in combination with RBV is more effective and has lower lifetime costs than BSC. However, the available evidence is of poor quality.
Implications for service provision
A recommendation for treatment with peginterferon alfa and RBV in children and young people with chronic HCV could potentially cause difficulties with delivery of the service in terms of accessibility. There are currently only three specialised paediatric hepatology centres in the UK (located in London, Leeds and Birmingham), meaning that the nearest treatment centre could potentially be quite some distance away, with resultant implications of time off school and work (for parents). However, our clinical advisors affirm that shared care pathways are well established in the UK with treatment and overall care delivered outside the three specialist centres at joint clinics. The challenge of treating children and young people in more centres would be in making treatment accessible to all patients but with each centre treating enough patients to maintain expertise. Other implications of a recommendation for treatment that should be considered are the possible need for more clinical nurse specialists and the additional burden on GPs, haematologists and child psychology services as a result of managing adverse effects.
Suggested research priorities
The evidence included in this review comes from poor quality uncontrolled cohort studies that are relatively small and have uncertain generalisability to the UK population of children and young people with chronic HCV. Ideally, better quality evidence should come from well-designed RCTs, although there may be ethical issues with randomising children to placebo. Well-conducted, head-to-head RCTs of peginterferon alfa-2a and RBV versus peginterferon alfa-2b and RBV would provide the best evidence of the effectiveness of these treatments, but these are unlikely given the emergence of newer treatments. If larger cohort studies were to be carried out, they should be statistically powered for the various subgroups in whom treatment response varies (e.g. genotype, treatment history, baseline viral load, etc.), and should be conducted in participants who reflect the chronic HCV paediatric population in the UK. However, pragmatically it is acknowledged that in the absence of antenatal screening, only small numbers of children with HCV will be identified, and hence study samples remain small. The adverse effects of peginterferon alfa treatment on growth in children is a concern, as is the impact on HRQoL, but data in the included studies are sparse and short term. Longer-term, more robust data are required to ascertain the long-term impact of peginterferon alfa treatment on the growth and QoL of children and young people with chronic HCV. Research in this area would perhaps be the most valuable.
Acknowledgements
We would like to thank members of our advisory group panel who provided expert advice and comments on the protocol and/or a draft of this report:
Ms Catherine Arkley, Chief Executive Officer, Child Liver Disease Foundation, Birmingham.
Dr Suzanne Davison, Consultant Paediatric Hepatologist, Leeds Teaching Hospitals NHS Trust.
Professor Anil Dhawan, Professor of Paediatric Hepatology and Director of Paediatric Liver Centre, King’s College Hospital, London.
Professor Will Irving, Professor of Virology, Queen’s Medical Centre, Nottingham.
Dr Alec Miners, Lecturer in Health Economics, London School of Hygiene & Tropical Medicine.
Dr Kathryn Nash, Consultant Hepatologist, Southampton General Hospital.
We are also grateful to Karen Welch, Information Specialist, SHTAC, University of Southampton, for generating and running the literature searches and Jackie Bryant, Principal Research Fellow, SHTAC, University of Southampton, for reviewing a draft of this report.
Contributions of authors
Debbie Hartwell (Senior Research Fellow) developed the research protocol, contributed to the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report, and project managed the study.
Keith Cooper (Senior Research Fellow, Health Economics) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, developed the economic evaluation and drafted the report.
Geoff K Frampton (Research Fellow) contributed to the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence and drafted the report.
Louise Baxter (Research Fellow, Health Economics) assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, assisted in developing the economic evaluation and drafted the report.
Emma Loveman (Senior Research Fellow) contributed to developing the research protocol, drafted the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted the report and acted as guarantor for the project.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Thomson BJ, Finch RG. Hepatitis C virus infection. Clin Microbiol Infect 2005;11:86-94. http://dx.doi.org/10.1111/j.1469-0691.2004.01061.x.
- Flamm SL. Chronic hepatitis C virus infection. JAMA 2003;289:2413-17. http://dx.doi.org/10.1001/jama.289.18.2413.
- Hepatitis C in the UK: 2008 Report. London: Health Protection Agency Centre for Infections; 2008.
- Davison SM, Mieli-Vergani G, Sira J, Kelly DA. Perinatal hepatitis C virus infection: diagnosis and management. Arch Dis Child 2006;91:781-5. http://dx.doi.org/10.1136/adc.2005.081877.
- Wirth S, Kelly D, Sokal E, Socha P, Mieli-Vergani G, Dhawan A, et al. Guidance for clinical trials for children and adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 2011;52:233-7. http://dx.doi.org/10.1097/MPG.0b013e3181f6f09c.
- Robinson JL, Doucette K. The natural history of hepatitis C virus infection acquired during childhood. Liver Int 2012;32:258-70. http://dx.doi.org/10.1111/j.1478-3231.2011.02633.x.
- Wirth S. Current treatment options and response rates in children with chronic hepatitis C. World J Gastroenterol 2012;18:99-104. http://dx.doi.org/10.3748/wjg.v18.i2.99.
- Roy A, Schwarz K. Hepatitis C in Children. Oregon City, OR: Hepatitis C Choices, Caring Ambassadors Program, Inc.; 2008.
- Yeung LTF, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology 2001;34:223-9. http://dx.doi.org/10.1053/jhep.2001.25885.
- Hepatitis C in the UK: 2011 Report. London: Health Protection Agency; 2011.
- Hu J, Doucette K, Hartling L, Tjosvold L, Robinson J. Treatment of hepatitis C in children: a systematic review. PLoS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0011542.
- Bortolotti F, Verucchi G, Camma C, Cabibbo G, Zancan L, Indolfi G, et al. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology 2008;134:1900-7. http://dx.doi.org/10.1053/j.gastro.2008.02.082.
- Harris HE, Mieli-Vergani G, Kelly D, Davison S, Gibb DM, Ramsay ME, et al. A national sample of individuals who acquired hepatitis C virus infections in childhood or adolescence: risk factors for advanced disease. J Pediatr Gastroenterol Nutr 2007;45:335-41. http://dx.doi.org/10.1097/MPG.0b013e3180dc9337.
- Jara P, Resti M, Hierro L, Giacchino R, Barbera C, Zancan L, et al. Chronic hepatitis C infection in childhood: clinical patterns and evolution in 224 white children. Clin Infect Dis 2003;36:275-80. http://dx.doi.org/10.1086/345908.
- Rumbo C, Fawaz RL, Emre SH, Suchy FJ, Kerkar N, Morotti RA, et al. Hepatitis C in children: a quaternary referral center perspective. J Pediatr Gastroenterol Nutr 2006;43:209-16. http://dx.doi.org/10.1097/01.mpg.0000228117.52229.32.
- Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infancts, children and adolescents. JPGN 2012;54:838-55. http://dx.doi.org/10.1097/MPG.0b013e318258328d.
- Rerksuppaphol S, Hardikar W, Dore GJ. Long-term outcome of vertically acquired and post-transfusion hepatitis C infection in children. J Gastroenterol Hepatol 2004;19:1357-62. http://dx.doi.org/10.1111/j.1440-1746.2004.03463.x.
- England K, Thorne C, Harris H, Ramsay M, Newell ML. The impact of mode of acquisition on biological markers of paediatric hepatitis C virus infection. J Viral Hepatitis 2011;18:533-41. http://dx.doi.org/10.1111/j.1365-2893.2011.01128.x.
- Jhaveri R. Diagnosis and management of hepatitis C virus-infected children. Pediatr Infect Dis J 2011;30:983-5. http://dx.doi.org/10.1097/INF.0b013e318236ac37.
- Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007;11.
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431-5. http://dx.doi.org/10.1002/hep.1840010511.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. http://dx.doi.org/10.1016/0168-8278(95)80226-6.
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-93. http://dx.doi.org/10.1002/hep.510240201.
- Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998;27:209-12. http://dx.doi.org/10.1002/hep.510270132.
- Akobeng AK, Davison S. Quality of life of patients with chronic hepatitis C virus infection. J Pediatr Gastroenterol Nutr 2000;30:224-6. http://dx.doi.org/10.1097/00005176-200002000-00028.
- Nydegger A, Srivastava A, Wake M, Smith AL, Hardikar W. Health-related quality of life in children with hepatitis C acquired in the first year of life. J Gastroenterol Hepatol 2008;23:226-30. http://dx.doi.org/10.1111/j.1440-1746.2007.04859.x.
- Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr 2009;48:341-7. http://dx.doi.org/10.1097/MPG.0b013e318185998f.
- Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Peginterferon with or without ribavirin has minimal effect on quality of life, behavioral/emotional, and cognitive outcomes in children. Hepatology 2011;53:1468-75. http://dx.doi.org/10.1002/hep.24248.
- Zacks S, Beavers K, Theodore D, Dougherty K, Batey B, Shumaker J, et al. Social stigmatization and hepatitis C virus infection. J Clin Gastroenterol 2006;40:220-4. http://dx.doi.org/10.1097/00004836-200603000-00009.
- Omland LH, Krarup H, Jepsen P, Georgsen J, Harritshøj LH, Riisom K, et al. Mortality in patients with chronic and cleared hepatitis C viral infection: a nationwide cohort study. J Hepatol 2010;53:36-42. http://dx.doi.org/10.1016/j.jhep.2010.01.033.
- Jhaveri R, Grant W, Kauf TL, McHutchison J. The burden of hepatitis C virus infection in children: estimated direct medical costs over a 10-year period. J Pediatr 2006;148:353-8. http://dx.doi.org/10.1016/j.jpeds.2005.10.031.
- Interferon alfa (Pegylated and Non-pegylated) and Ribavirin for the Treatment of Chronic Hepatitis C. London: NICE; 2000.
- Peginterferon alfa and Ribavirin for the Treatment of Mild Chronic Hepatitis C. London: NICE; 2006.
- Peginterferon alfa and Ribavirin for the Treatment of Chronic Hepatitis C. London: NICE; 2010.
- Management of Hepatitis C. A National Clinical Guideline. Edinburgh: SIGN; 2006.
- Bruno R, Sacchi P, Ciappina V, Zochetti C, Patruno S, Maiocchi L, et al. Viral dynamics and pharmacokinetics of peginterferon alpha-2a and peginterferon alpha-2b in naive patients with chronic hepatitis c: a randomized, controlled study. Antiviral Ther 2004;9:491-7.
- Roche Products Limited . Summary of Product Characteristics – Peginterferon Alfa-2a (Pegasys) 2012. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000395/human_med_000974.jsp&mid=WC0b01ac058001d124 (accessed 21 May 2012).
- Merck Sharp & Dohme Limited . Summary of Product Characteristics – Peginterferon Alfa-2b (ViraferonPeg) 2012. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000329/human_med_001142.jsp&mid=WC0b01ac058001d124 (accessed 21 May 2012).
- Merck Sharp & Dohme Limited . Summary of Product Characteristics – Ribavirin (Rebetol). 2012. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000246/human_med_001017.jsp&mid=WC0b01ac058001d124 (accessed 21 May 2012).
- Roche Products Limited . Summary of Product Characteristics – Ribavirin (Copegus) 2012. www.medicines.org.uk/EMC/medicine/10081/SPC/Pegasys±135mcg±and±180mcg±±solution±for±injection±in±Pre-filled±Syringe±Pre-filled±Pen/ (accessed 21 May 2012).
- Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15170.
- Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon alpha 2a and 2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review. Health Technol Assess 2004;8.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: York Publishing Services Ltd; 2009.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Al Ali J, Owayed S, Al-Qabandi W, Husain K, Hasan F. Pegylated interferon alfa-2b plus ribavirin for the treatment of chronic hepatitis C genotype 4 in adolescents. Ann Hepatol 2010;9:156-60.
- Ghaffar TY, El Naghy S, El Sebaie H, El Monaiery M, Ghaffar AY. Pegylated alpha interferon 2B plus ribavirin in the treatment of HCV genotype 4 infection. Indian J Pediatr 2009;76:895-8. http://dx.doi.org/10.1007/s12098-009-0187-x.
- Jara P, Hierro L, de la Vega A, Diaz C, Camarena C, Frauca E, et al. Efficacy and safety of peginterferon-alpha2b and ribavirin combination therapy in children with chronic hepatitis C infection. Pediatr Infect Dis J 2008;27:142-8. http://dx.doi.org/10.1097/INF.0b013e318159836c.
- Murray KF, Rodrigue JR, Gonzalez-Peralta RP, Shepherd J, Barton BA, Robuck PR, et al. Design of the PEDS-C trial: pegylated interferon +/- ribavirin for children with chronic hepatitis C viral infection. Clin Trials 2007;4:661-73. http://dx.doi.org/10.1177/1740774507085445.
- Narkewicz MR, Rosenthal P, Schwarz KB, Drack A, Margolis T, Repka MX, et al. Ophthalmologic complications in children with chronic hepatitis C treated with pegylated interferon. J Pediatr Gastroenterol Nutr 2010;51:183-6. http://dx.doi.org/10.1097/MPG.0b013e3181b99cf0.
- Pawlowska M, Pilarczyk M, Halota W. Virologic response to treatment with Pegylated Interferon alfa-2b and Ribavirin for chronic hepatitis C in children. Med Sci Monit 2010;16:CR616-21.
- Pawlowska M, Pilarczyk M, Halota W, Jendryczka E. Virological response to treatment with pegylated interferon alfa-2b and ribavirin chronic hepatitis C in children. Hepatology 2008;48:855A-6A.
- Rodrigue JR, Balistreri WF, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Peginterferon alfa-2a with or without ribavirin results in minimal effect on quality of life, emotional, and cognitive outcomes: Results of the Peds-C trial. Hepatology 2009;50.
- Rosenthal P, Mohan P, Barton B, Robuck PR, Schwarz KB. Incidence of neutropenia and impact on sustained virological response (Svr) in children and adolescents with chronic hepatitis C treated with peginterferon with or without ribavirin: the Peds-C experience. Hepatology 2008;48:1031A-2A.
- Schwarz KB, Gonzalez-Peralta RP, Murray KF, Molleston JP, Haber B, Jonas MM, et al. Peginterferon with or without ribavirin for chronic hepatitis C in children and adolescents: final results of the Peds-C trial. Hepatology 2008;48.
- Schwarz KB, Gonzalez-Peralta RP, Murray KF, Molleston JP, Haber BA, Jonas MM, et al. The combination of ribavirin and peginterferon is superior to peginterferon and placebo for children and adolescents with chronic hepatitis C. Gastroenterology 2011;140:450-8. http://dx.doi.org/10.1053/j.gastro.2010.10.047.
- Sokal EM, Bourgois A, Stephenne X, Silveira T, Porta G, Gardovska D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in children and adolescents. J Hepatol 2010;52:827-31. http://dx.doi.org/10.1016/j.jhep.2010.01.028.
- Wirth S, Ribes-Koninckx C, Bortolotti F, Zancan L, Jara P, Shelton MM, et al. Children with HCV infection show high sustained virologic response rates on peginterferon alfa-2b plus ribavirin treatment. Hepatology 2008;48:392A-3A.
- Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol 2010;52:501-7. http://dx.doi.org/10.1016/j.jhep.2010.01.016.
- Peginterferon alfa and Ribavirin for the Treatment of Chronic Hepatitis C in Children and Young People [ID373]: Final Scope. London: NICE; 2012.
- Schwarz KB. PEDS-C: Pegylated Interferon + - Ribavirin for Children With Hepatitis C 2011. www.clinicaltrials.gov/ct2/show/NCT00100659?term=PEDS-C&rank=1 (accessed 21 May 2012).
- Jonas MM, Balistreri W, Gonzalez-Peralta RP, Haber B, Lobritto S, Mohan P, et al. Pegylated interferon for chronic hepatitis C in children affects growth and body composition: results from the pediatric study of hepatitis C (PEDS-C) trial. Hepatology 2012;56:523-31. http://dx.doi.org/10.1002/hep.25690.
- Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology 2005;41:1013-18. http://dx.doi.org/10.1002/hep.20661.
- Zhang HF, Yang XJ, Zhu SS, Dong Y, Chen DW, Jia WZ, et al. An open-label pilot study evaluating the efficacy and safety of peginterferon alfa-2a combined with ribavirin in children with chronic hepatitis C. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2005;19:185-7.
- Sluzewski W, Kowala-Piaskowska A, Wysocki J, Figlerowicz M, Gorczyca A, Halota W, et al. Treatment of chronic hepatitis C in children with pegylated interferon alpha2a and ribavirin – a multi-center study. Acta Poloniae Pharm 2012;69:319-26.
- Abdel-Hady MM, Bansal S, Davison S, Brown MP, Tizzard S, Mulla S, et al. UK experience of treatment of chronic viral hepatitis C in children and adolescents: predictors of viral response and quality of life. Hepatology 2010;52.
- Mernagh P, Norris S, Del CM. Anti-viral treatment of chronic hepatitis C in a paediatric population: a cost-effectiveness analysis. Value Health 2011;7. http://dx.doi.org/10.1016/j.jval.2011.08.935.
- Sinha M, Das A. Cost-effectivness analysis of different strategies of managment of chronic hepatitis C infection in children. Pediatr Infect Dis J 2000;19:23-30. http://dx.doi.org/10.1097/00006454-200001000-00006.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Bjornsson E, Verbaan H, Oksanen A, Fryden A, Johansson J, Friberg S, et al. Health-related quality of life in patients with different stages of liver disease induced by hepatitis C. Scand J Gastroenterol 2009;44:878-87. http://dx.doi.org/10.1080/00365520902898135.
- Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003;98:630-8. http://dx.doi.org/10.1111/j.1572-0241.2003.07332.x.
- Thompson-Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess 2007;11.
- Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU, University of Kent; 2010.
- British National Formulary. London: BMA and RPS; 2012.
- Guido M, Bortolotti F, Leandro G, Jara P, Hierro L, Larrauri J, et al. Fibrosis in chronic hepatitis C acquired in infancy: is it only a matter of time?. Am J Gastroenterol 2003;98:660-3. http://dx.doi.org/10.1111/j.1572-0241.2003.07293.x.
- Saigal S, Rosenbaum P, Stoskopf B, Hoult L, Furlong W, Feeny D, et al. Comprehensive assessment of the health status of extremely low birth weight children at eight years of age: comparison with a reference group. J Pediatr 1994;125:411-17. http://dx.doi.org/10.1016/S0022-3476(05)83288-3.
- Ara R, Brazier J. Populating an Economic Model with Health State Utility Values: Moving Towards Better Practice. Sheffield: University of Sheffield; 2009.
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-31. http://dx.doi.org/10.1002/hep.22375.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463-72. http://dx.doi.org/10.1053/gast.1997.v112.pm9024300.
- Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut 2003;52:425-32. http://dx.doi.org/10.1136/gut.52.3.425.
- Wright M, Grieve R, Roberts J, Main J, Thomas HC. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 2006;10.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl 2002;8:263-70. http://dx.doi.org/10.1053/jlts.2002.31345.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Department of Health . NHS Reference Costs 2010–2011 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_122803 (accessed 16 December 2011).
- British National Formulary. London: BMA and RPS; 2012.
- Wang Y, Moss J, Thisted R. Predictors of body surface area. J Clin Anesth 1992;4:4-10. http://dx.doi.org/10.1016/0952-8180(92)90111-D.
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51:1176-84. http://dx.doi.org/10.1002/hep.23504.
- Zhao S, Liu E, Chen P, Cheng D, Lu S, Yu Q, et al. A comparison of peginterferon alfa-2a and alfa-2b for treatment-naive patients with chronic hepatitis C virus: a meta-analysis of randomized trials. Clin Ther 2010;32:1565-77. http://dx.doi.org/10.1016/j.clinthera.2010.08.009.
- Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology 2008;47:836-43. http://dx.doi.org/10.1002/hep.22094.
- Peginterferon alfa and Ribavirin for the Treatment of Chronic Hepatitis C in Children and Young People Aged 3–17 Years: Manufacturer Submission of Evidence. London: NICE; 2012.
- Cesaro S, Bortolotti F, Petris MG, Brugiolo A, Guido M, Carli M. An updated follow-up of chronic hepatitis C after three decades of observation in pediatric patients cured of malignancy. Pediatr Blood Cancer 2010;55:108-12.
- Iorio R, Giannattasio A, Sepe A, Terracciano LM, Vecchione R, Vegnente A. Chronic hepatitis C in childhood: an 18-year experience. Clin Infect Dis 2005;41:1431-7. http://dx.doi.org/10.1086/497141.
- Fioredda F, Moser A, Bertoluzzo L, Lackner H, Giacchino R, La SM, et al. Natural course of HCV infection in childhood cancer survivors. Support Care Cancer 2010;18:1413-20. http://dx.doi.org/10.1007/s00520-009-0763-7.
- Kage M, Fujisawa T, Shiraki K, Tanaka T, Fujisawa T, Kimura A, et al. Pathology of chronic hepatitis C in children. Child Liver Study Group of Japan. Hepatology 1997;26:771-5. http://dx.doi.org/10.1002/hep.510260333.
- Peginterferon alfa-2a and Ribavirin for the Treatment of Paediatric Chronic Hepatitis C. Manufacturer Submission of Evidence. London: NICE; 2012.
- European Paediatric Hepatitis C Virus Network . A significant sex – but not elective cesarean section – effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis 2005;192:1872-9. http://dx.doi.org/10.1086/497695.
- Barshes NR, Udell IW, Lee TC, O’Mahony CA, Karpen SJ, Carter BA, et al. The natural history of hepatitis C virus in pediatric liver transplant recipients. Liver Transp 2006;12:1119-23. http://dx.doi.org/10.1002/lt.20793.
- Deaths Registered in England and Wales in 2010 By Cause. Statistical Bulletin. n.d.
Appendix 1 Search strategies
Clinical effectiveness searches
The following strategies were used to search MEDLINE (Ovid) and EMBASE (Ovid) from inception to November 2012. The strategies were translated into the other databases listed in Chapter 3, Identification of studies for the systematic reviews of clinical effectiveness and cost-effectiveness.
MEDLINE
-
Hepatitis C, Chronic/ (13,735)
-
(“hepatitis C” or HCV).tw. (48,770)
-
exp Hepatitis C/ (41,824)
-
Hepacivirus/ (20,518)
-
or/1-4 (56,080)
-
Ribavirin/ (7195)
-
(ribavirin* or copegus or rebetol or rebetron or rebretron or ribamide or ribamidil or ribamidyl or ribasphere or varazid or vilona or viramid or virazid or virazole or RibaPak).ti,ab,nm. (9111)
-
6 or 7 (9111)
-
(peginterferon$ or peg-ifn or peg-interferon$ or (pegylat$ adj3 interferon$) or peg$ or (polyethylene glycol adj3 interferon$) or ViraferonPeg or pegintron or "peg-intron” or Pegasys).mp. (29,312)
-
Interferon-Alfa/ or Interferons/ (40,682)
-
(“IFN alfa” or “IFN alpha” or IFNalfa or IFNalpha or “interferon alfa” or “interferon alpha").tw. (24,503)
-
10 or 11 (49,805)
-
Polyethylene Glycols/ (32,260)
-
12 and 13 (3545)
-
9 or 14 (29,368)
-
5 and 8 and 15 (3824)
-
exp Child/ (1,432,200)
-
Child, Preschool/ (694,089)
-
Adolescent/ (1,468,478)
-
(child* or toddler* or adolesc* or teenage* or youth* or pediatric* or paediatric*).tw. (945,660)
-
or/17-20 (2,424,786)
-
16 and 21 (325)
EMBASE
-
(hepatitis C or hcv).mp. (87,019)
-
exp Hepatitis C/ or exp Hepatitis C virus/ (75,723)
-
1 or 2 (87,019)
-
(peginterferon$ or peg-ifn or peg-interferon$ or (peg$ adj3 interferon$) or (polyethylene glycol adj3 interferon$) or Pegasys or pegintron or viraferonpeg).mp. (13,639)
-
peginterferon/ or peginterferon alpha2a/ or peginterferon alpha2b/ (11,347)
-
4 or 5 (13,639)
-
ribavirin/ (20,451)
-
(ribavirin* or copegus or rebetol or rebetron or rebretron or ribamide or ribamidil or ribamidyl or ribasphere or varazid or vilona or viramid or virazid or virazole or RibaPak).ti,ab,tn. (12,251)
-
7 or 8 (21,598)
-
3 and 6 and 9 (9778)
-
peginterferon alpha2a plus ribavirin/ or peginterferon alpha2b plus ribavirin/ (195)
-
3 and 11 (186)
-
10 or 12 (9809)
-
child/ (1,305,129)
-
preschool child/ (472,983)
-
adolescent/ (1,188,986)
-
(child$ or toddler$ or adolesc* or teenage* or youth* or pediatric* or paediatric*).tw. (1,347,475)
-
(preschool* or “pre-school*”).tw. (24,127)
-
or/14-18 (2,545,759)
-
13 and 19 (421)
-
limit 13 to (preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>) (210)
-
20 or 21 (422)
Cost-effectiveness searches
The following strategies were used to search MEDLINE (Ovid) and EMBASE (Ovid) from inception to November 2012. The strategies were translated into the other databases listed in Chapter 3, Identification of studies for the systematic reviews of clinical effectiveness and cost-effectiveness.
MEDLINE
-
Hepatitis C, Chronic/ (13,814)
-
(“hepatitis C” or HCV).tw. (48,925)
-
exp Hepatitis C/ (41,962)
-
Hepacivirus/ (20,593)
-
or/1-4 (56,255)
-
Ribavirin/ (7252)
-
(ribavirin* or copegus or rebetol or rebetron or rebretron or ribamide or ribamidil or ribamidyl or ribasphere or varazid or vilona or viramid or virazid or virazole or RibaPak).ti,ab,nm. (9176)
-
6 or 7 (9176)
-
(peginterferon$ or peg-ifn or peg-interferon$ or (pegylat$ adj3 interferon$) or peg$ or (polyethylene glycol adj3 interferon$) or ViraferonPeg or pegintron or “peg-intron” or Pegasys).mp. (29,508)
-
Interferon-Alfa/ or Interferons/ (40,773)
-
(“IFN alfa” or “IFN alpha” or IFNalfa or IFNalpha or “interferon alfa” or “interferon alpha”).tw. (24,561)
-
10 or 11 (49,919)
-
Polyethylene Glycols/ (32,457)
-
12 and 13 (3591)
-
9 or 14 (29,565)
-
5 and 8 and 15 (3879)
-
exp economics/ (456,096)
-
exp economics hospital/ (17,917)
-
exp economics pharmaceutical/ (2332)
-
exp economics nursing/ (3862)
-
exp economics medical/ (13,273)
-
exp “Costs and Cost Analysis”/ (164,677)
-
exp Cost Benefit Analysis/ (53,972)
-
exp models economic/ (8599)
-
(cost* adj2 (effective* or benefit* or utilit* or minim*)).tw. (73,771)
-
markov chains/ or monte carlo method/ (23,357)
-
(decision adj1 (tree* or analys* or model*)).tw. (6891)
-
exp health care costs/ (40,596)
-
or/17-28 (520,897)
-
16 and 29 (144)
-
(letter or editorial or comment or historical article).pt. (1,397,929)
-
30 not 31 (141)
EMBASE
-
(hepatitis C or hcv).mp. (88,182)
-
exp Hepatitis C/ or exp Hepatitis C virus/ (76,553)
-
1 or 2 (88,182)
-
(peginterferon$ or peg-ifn or peg-interferon$ or (peg$ adj3 interferon$) or (polyethylene glycol adj3 interferon$) or Pegasys or pegintron or viraferonpeg).mp. (13,980)
-
peginterferon/ or peginterferon alpha2a/ or peginterferon alpha2b/ (11,579)
-
4 or 5 (13,980)
-
ribavirin/ (20,743)
-
(ribavirin* or copegus or rebetol or rebetron or rebretron or ribamide or ribamidil or ribamidyl or ribasphere or varazid or vilona or viramid or virazid or virazole or RibaPak).ti,ab,tn. (12,561)
-
7 or 8 (21,934)
-
3 and 6 and 9 (10,038)
-
peginterferon alpha2a plus ribavirin/ or peginterferon alpha2b plus ribavirin/ (203)
-
3 and 11 (194)
-
10 or 12 (10,071)
-
*Health Economics/ (16,281)
-
*Economics/ (11,217)
-
monte carlo method/ (17,113)
-
(cost* or economic*).ti. (126,413)
-
markov.tw. (11,647)
-
"monte carlo".tw. (22,996)
-
(cost* adj2 (effective* or utili* or benefit* or minimi* or consequence* or analys* or saving* or breakdown* or estimate* or variable* or allocation* or control* or illness)).tw. (130,225)
-
(econom* or pharmacoeconomic* or “pharmaco economic*” or budget*).tw. (214,740)
-
cost/ (49,867)
-
cost minimization analysis/ (2073)
-
cost of illness/ (12,986)
-
cost utility analysis/ (4167)
-
drug cost/ (51,793)
-
health care cost/ (110,482)
-
economic evaluation/ (7191)
-
pharmacoeconomics/ (5675)
-
budget/ (17,530)
-
“resource use”.tw. (5040)
-
“resource utili”.tw. (1)
-
(decision adj1 (tree* or analys* or model*)).tw. (9480)
-
(“unit cost*” or “hospital cost*” or “health care cost*” or “healthcare cost*” or “medical cost*”).tw. (27,557)
-
(managed adj2 (care or clinical or network)).tw. (19,055)
-
(resource* adj1 allocat*).tw. (5901)
-
(resource* adj1 utili*).tw. (6341)
-
or/14-37 (594,150)
-
13 and 38 (533)
-
(cost and effective* and “hepatitis C”).ti. (175)
-
(cost and effective* and “hepatitis C”).ab. (608)
-
40 or 41 (646)
-
6 and 9 and 42 (208)
-
11 and 42 (4)
-
39 or 43 or 44 (543)
-
(comment or editorial or letter).pt. (1,197,206)
-
45 not 46 (507)
Health-related quality-of-life searches
The following strategies were used to search MEDLINE (Ovid) and EMBASE (Ovid) from 1947 to November 2012. The strategies were translated into the other databases listed in Chapter 3, Identification of studies for the systematic reviews of clinical effectiveness and cost-effectiveness.
MEDLINE
-
value of life/ (5222)
-
quality adjusted life year/ (5699)
-
quality adjusted life.ti,ab. (4572)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab. (3826)
-
disability adjusted life.ti,ab. (873)
-
daly$.ti,ab. (885)
-
health status indicators/ (17,959)
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirstysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (12,492)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (898)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve of sftwelve or shortform twelve or short form twelve).ti,ab. (1941)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. (18)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty of sftwenty or shortform twenty of short form twenty).ti,ab. (303)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (2707)
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab. (5603)
-
(hye or hyes).ti,ab. (52)
-
health$ year$ equivalent$.ti,ab. (36)
-
health utilit$.ab. (770)
-
(hui or hui1 or hui2 or hui3).ti,ab. (700)
-
disutil$.ti,ab. (169)
-
rosser.ti,ab. (72)
-
quality of well being.ti,ab. (297)
-
quality of wellbeing.ti,ab. (5)
-
qwb.ti,ab. (150)
-
willingness to pay.ti,ab. (1664)
-
standard gamble$.ti,ab. (580)
-
time trade off.ti,ab. (604)
-
time tradeoff.ti,ab. (191)
-
tto.ti,ab. (459)
-
(index adj2 well being).mp. (419)
-
(quality adj2 well being).mp. (736)
-
(health adj3 utilit$ ind$).mp. (531)
-
((multiattribute$ or multi attribute$) adj3 (health ind$ or theor$ or health state$ or utilit$ or analys$)).mp. (206)
-
quality adjusted life year$.mp. (7493)
-
(15D or 15 dimension$).mp. (1018)
-
(12D or 12 dimension$).mp. (314)
-
rating scale$.mp. (74,498)
-
linear scal$.mp. (480)
-
linear analog$.mp. (784)
-
visual analog$.mp. (24,844)
-
(categor$ adj2 scal$).mp. (1040)
-
or/1-40 (150,176)
-
(letter or editorial or comment).pt. (1,134,806)
-
41 not 42 (146,086)
-
Hepatitis C, Chronic/ (13,926)
-
(“hepatitis C” or HCV).tw. (49,230)
-
exp Hepatitis C/ (42,205)
-
Hepacivirus/ (20,742)
-
or/44-47 (56,582)
-
43 and 48 (441)
EMBASE
-
quality adjusted life year/ (9303)
-
quality adjusted life.ti,ab. (6565)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab. (6358)
-
disability adjusted life.ti,ab. (1139)
-
daly*.ti,ab. (1265)
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (17,835)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (1404)
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab. (2980)
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab. (31)
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab. (376)
-
(euroqol or “euro qol” or “eq5d” or “eq 5d”).ti,ab. (4495)
-
(hql or hqol or “h qol” or hrqol or “hr qol”).ti,ab. (8357)
-
(“hye” or “hyes”).ti,ab. (63)
-
health* year* equivalent*.ti,ab. (41)
-
health utilit*.ti,ab. (1192)
-
(hui or hui1 or hui2 or hui3).ti,ab. (969)
-
disutil*.ti,ab. (270)
-
rosser.ti,ab. (83)
-
quality of well being.ti,ab. (332)
-
quality of wellbeing.ti,ab. (18)
-
qwb.ti,ab. (170)
-
willingness to pay.ti,ab. (2513)
-
standard gamble*.ti,ab. (695)
-
time trade off.ti,ab. (804)
-
time tradeoff.ti,ab. (208)
-
tto.ti,ab. (698)
-
(index adj2 well being).mp. (568)
-
(quality adj2 well being).mp. (994)
-
(health adj3 util* adj ind*).mp. (888)
-
((multiattribute* or multi attribute*) adj3 (health ind* or theor* or health state* or util* or analys*)).mp. (299)
-
quality adjusted life year*.mp. (11,067)
-
health status indicator*.ti,ab. (327)
-
(15D or 15 dimension*).mp. (1511)
-
(12D or 12 dimension*).mp. (452)
-
"health related quality of living".ti,ab. (3)
-
"health related quality of life".ti,ab. (22,371)
-
rating scale*.mp. (115,178)
-
visual analog*.mp. (42,178)
-
(categor* adj scale*).mp. (702)
-
linear scal*.mp. (655)
-
linear analog*.mp. (962)
-
"quality of life".ti. (44,803)
-
or/1-42 (231,142)
-
(letter or editorial or comment).pt. (1,199,398)
-
43 not 44 (224,733)
-
(“hepatitis C” or HCV).tw. (70,769)
-
Hepatitis C/ or Hepatitis C virus/ (76,817)
-
hepacivirus.tw. (58)
-
or/46-48 (87,733)
-
45 and 49 (942)
Appendix 2 Inclusion criteria worksheet for systematic review of clinical effectiveness
Full paper inclusion coding
Author: Ref ID: Reviewer 1: Reviewer 2:
| Yes | No | Unclear | |
|---|---|---|---|
| Population | |||
| Children and young people with chronic hepatitis C (aged 3–17 years) | |||
| Compensated liver disease a | |||
| Treatment naive | |||
| Previously treated | |||
| Mixed treatment (treatment naive and previously treated) | |||
| Co-infection with HIV | |||
| Mixed age population (i.e. ≤ 17 years and ≥ 18 years) | |||
| Interventionb | |||
| Either: | |||
| Peginterferon alfa-2a + ribavirin | |||
| Peginterferon alfa-2b + ribavirin | |||
| Outcomes | |||
| Sustained virological response (SVR) | |||
| Can also include any of the following outcomes: | |||
| Virological response [e.g. during treatment (RVR, EVR), end of treatment (EOT)] | |||
| Biochemical response (e.g. % response, ALT levels) | |||
| Histological response (e.g. % response, liver inflammation and fibrosis) | |||
| Adverse effects (including effects on growth) | |||
| Mortality | |||
| QoL | |||
| Other | |||
| Study design | |||
| Randomised controlled trial (RCT) | |||
| Non-randomised controlled trial (CCT) | |||
| Cohort study – two groups | |||
| Cohort study – single arm | |||
| Systematic review | |||
| Other (specify) | |||
| Publication type | |||
| Full text paper | |||
| Conference abstract (published 2007 to 2012 only) | |||
| If the study is an abstract only, is there sufficient detail to be included? | |||
| Comparatorsb | |||
| Either: | |||
| Peginterferon alfa-2a + ribavirin | |||
| Peginterferon alfa-2b + ribavirin | |||
| Best supportive care (symptomatic treatment, monitoring, etc.) | |||
| Placebo | |||
| N/A or no comparator | |||
| Other (specify) | |||
| Include | Exclude | Unclear | |
| Reviewer 1 Decision | |||
| Reviewer 2 Decision | |||
| Final decision | |||
Appendix 3 Data extraction forms and critical appraisal
Studies of peginterferon alfa-2a: Schwarz and colleagues (2011)56
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Schwarz et al.56 + linked publications28,49,50,53–55,61 Year: 2011 (linked publications 2007–11) Study design: RCT but treated as single cohort Number of centres: 11 Country: USA Funding: Supported by the National Institute of Diabetes and Digestive and Kidney Diseases, the FDA and in part by the National Institutes of Health/National Centre for Research Resources. Additional support was provided by Roche |
Group 1 Drug 1: PEG α-2a Dose: 180 µg/1.73 m2 BSA/week subcutaneously (maximum 180 µg) Duration: 48 weeks Drug 2: RBV Dose: 15 mg/kg twice daily taken orally (maximum 1200 mg/day if ≥ 75 kg and 1000 mg if < 75 kg) Duration: 48 weeks Study design details: Multicentre RCT (n = 114) but data taken from one arm only as the comparator (PEG + placebo, i.e. PEG monotherapy) is outside the NICE scope Patients with detectable HCV RNA at 24 weeks were considered treatment failures; PEG + placebo treatment failures were offered open-label PEG + RBV for a further 48 weeks (unless HCV RNA remained positive) Dose reductions were made at three levels (for PEG α-2a) and reduced by half (for RBV) according to the extent of specific AEs. Details reported in supplementary tables |
Total numbers involved: n = 55 PEG + RBV Treatment naive: 100%61 Previous treatment: N/A HCV/HIV co-infection: No61 Duration of infection, mean (± SD): 105 ± 56 months Inclusion criteria: Aged 5–18 years; chronic HCV infection documented by the presence of HCV RNA in plasma on two tests at least 6 months apart; chronic liver disease, as indicated by inflammation and/or fibrosis, consistent with chronic HCV infection on a liver biopsy obtained within the past 36 months, not consistent with other known liver disease and not normal; compensated liver disease (Child–Pugh Grade A); haemoglobin values > 11 g/dl for females; > 12 g/dl for males; normal TSH; able to swallow a RBV/placebo tablet; signed informed consent from parent/legal guardian and willingness of parent/legal guardian to abide by the requirements of the study61 Exclusion criteria: Any prior treatment with IFN or RBV; receipt of any investigational drug or any systemic antiviral therapy < 6 weeks prior to the first dose of study drug (except for patients who have taken or are expected to require acyclovir for herpetic lesions); positive test at screening for anti-HAV IgM Ab, HBsAg, anti-HBc IgM Ab, or anti-HIV Ab; history or other evidence of bleeding from oesophageal varices or of a medical condition associated with chronic liver disease other than HCV; decompensated liver disease; history of autoimmune or immunologically mediated disease; absolute neutrophil count < 1500 cells/mm3, Hb < 11 g/dl for females and < 12 g/dl for males, WBC > 17.5 × 109/l, or platelet count < 90,000/mm3; serum creatinine level > 1.5 × ULN for age; major depression or a history of severe psychiatric disorder; chronic pulmonary or cardiac disease associated with functional limitation; thyroid disease poorly controlled on prescribed medications; poorly controlled diabetes as defined by HbA1c of > 8%; solid organ or bone marrow transplantation; coagulopathy (INR > 1.5); active or suspected cancer or a history of malignancy where the risk of recurrence is > 20% within 2 years; haemoglobinopathy; haemophilia; severe retinopathy; severe illness or any other conditions which would make the patient unsuitable for the study; sexually active females of child-bearing potential (defined as age ≥ 10 years) and sexually active males who are not practising two forms of effective contraception during treatment and during the 6 months after treatment has stopped; pregnancy or breastfeeding; males whose female partners are pregnant; active substance abuse; a sibling and/or any other child living in the same household or sharing the same primary caregiver enrolled in the study61 Age (years), mean (± SD): 10.7 ± 3.3 Age 5–11 years, n/N: 30/55 (55%) Age 12–17 years, n/N: 25/55 (46%) Gender male, n/N: 27/55 (49%) BMI z-scores, mean (± SD): 0.8 ± 1.0 Ethnic groups, n/N: Non-white: 12/55 (22%); Caucasian: 43/55 (78%)55 Mode of infection, n/N: Maternal–infant, 39/55 (71%); transfusion, 6/55 (11%); other (not specified), 10/55 (18%) (19% reported in paper) Genotypes, n/N: 1, 45/55 (82%); 2, 4/55 (7%); 3, 6/55 (11%); 4, NR; 6, 0 Total Childhood Depression Index raw score, mean (± SD): 5.9 ± 4.2 Sample attrition/dropout: No losses to follow-up (week 72) but therapy discontinued in four patients (reasons given). Seven (13%) lost to follow-up by the first annual visit rising to 10 (18%) by the second annual visit |
Primary outcomes: SVR Secondary outcomes: RVR, EVR, ETR; relapse; safety (AEs) and adherence, durability of response (at years 1 and 2), predictors of SVR; QoL, behavioural/emotional and cognitive functioning;28,53 autoantibodies and autoimmune disease (not data extracted); ophthalmologic effects50 (not data extracted). Also states body composition and growth were measured Length of follow-up: 24 weeks after treatment cessation; longer-term follow-up at 1 and 2 years Methods of assessing outcomes: Qualitative HCV RNA assessed with COBAS® AMPLICOR® HCV v2.0 (Roche) qualitative PCR with lower limit of detection of 60 IU/ml at baseline and weeks 24, 48 and 72. Quantitative HCV RNA assays performed at end of study on plasma stored at –80°C and thawed once. HCV RNA levels measured at entry and weeks 1, 3, 5, 12, 24, 48 and 72 using high throughput quantitative assay [COBAS® TaqMan® HCV Test v2.0 with High Pure System for Viral Nucleic Acid Extraction (Roche Molecular Systems, Pleasanton, CA)] with lower limit of quantification of 25 IU/ml and lower limit of detection of 10 IU/ml in EDTA plasma. HCV viral genotyping performed at entry using a line probe assay (Innogenetics, Ghent, Belgium) SVR defined as undetectable plasma HCV RNA (< 10 IU/ml) at least 24 weeks after treatment cessation (states < 100 IU/ml in two linked papers54,55) RVR defined as lack of detectable plasma HCV RNA at week 5 EVR defined as a decrease ≥ 2 log10 IU/ml at week 12 compared with baseline ETR response defined as no detectable plasma HCV RNA at the end of therapy Relapse defined as patients with an ETR response who became HCV RNA positive after stopping therapy Paediatric AIDS Toxicity Table used as a guide for grading severity of AEs. Medication compliance assessed by co-ordinators’ review of a medication diary completed by parents/guardians, and pill and vial counts by researchers or investigational pharmacists Knodell21 HAI and Ishak classification systems used for measurement of fibrosis and inflammation Measures of QoL, behavioural/emotional and cognitive functioning obtained at baseline and at 24 and 48 weeks, 6 months post treatment and at two subsequent annual visits. QoL assessed using the CHQ – Parent Form 50; the CBCL assessed behavioural functioning; the CDI assessed symptoms of depression; the BRIEF measured cognitive functioning. The CHQ, CBCL and BRIEF were completed by parents, CDI was completed by child28 |
| Participant characteristics/outcomes | Baseline | Post treatment |
|---|---|---|
| HCV RNA (log 10 IU/ml), mean ± SD | 6.2 ± 0.8 | 1.4a |
| HCV RNA ≥ 600,000 IU/ml, n / N (%) | 32/55 (58)b | NR |
| ALT (IU/l), mean ± SD | 49 ± 59 | NR |
| ALT > ULN, n / N (%) | 32/55 (58) | NR |
| AST (IU/l), mean ± SD | 45 ± 40 | NR |
| AST > ULN, n / N (%) | 28/55 (51) | NR |
| HAI (inflammation), n / N (%) | NR | |
| Minimal (1–3) | 23/54 (43) | |
| Mild (4–6) | 10/54 (19) | |
| Moderate (7–9) | 19/54 (35) | |
| Marked (10–12) | 2/54 (4) | |
| Steatosis, n / N (%) | NR | |
| None | 29/54 (54) | |
| Minimal (≤ 5% of tissue) | 21/54 (39) | |
| Mild (6–33% of tissue) | 4/54 (7) | |
| Fibrosis score, n / N (%) | NR | |
| None | 7/54 (13) | |
| Portal–periportal fibrosis (Ishak 1–2) | 43/54 (80) | |
| Bridging fibrosis (Ishak 3–4) | 4/54 (7) | |
| Cirrhosis (Ishak 5–6) | 0 |
| Outcomes | Baseline | Post treatment | p-value |
|---|---|---|---|
| Viral response, n/N (%, 95% CI) | |||
| RVR (week 5) | N/A | 13/55a (24) | N/A |
| EVR (week 12) | N/A | 32/55a (59) | N/A |
| Virological response at week 24 | N/A | 41/55a (75) | N/A |
| ETR (week 48) | N/A | 35/55a (64)b | N/A |
| SVR (week 72) | N/A | 29/55 (53, 40 to 66) | N/A |
| SVR according to baseline characteristics, n/N (%, 95% CI) | |||
| HCV RNA < 600,000 IU/ml | N/A | 16/23 (70) | N/A |
| HCV RNA ≥ 600,000 IU/ml | N/A | 16/32 (50) | N/A |
| Normal ALT | N/A | 16/23 (70, 51 to 88) | N/A |
| ALT > ULN | N/A | 13/32 (41, 24 to 58) | N/A |
| Genotype 1 | N/A | 21/45 (47, 32 to 61) | N/A |
| Genotype 2–6 | N/A | 8/10 (80, 55 to 100) | N/A |
| Inflammation HAI | N/A | N/A | |
| Minimal (1–3) | 10/23 (43, 23 to 64) | ||
| Mild to marked (4–12) | 18/31 (58, 41 to 75) | ||
| Fibrosis stage | N/A | N/A | |
| None | 3/7 (43, 6 to 80) | ||
| Stage 1–6 | 25/47 (53, 39 to 67) | ||
| Steatosis | N/A | N/A | |
| Present | 9/25 (36, 17 to 55) | ||
| Absent | 19/29 (66, 48 to 83) | ||
| Non-response | N/A | 14/55 (25)28 | N/A |
| Relapse | N/A | 9/55 (17)a | N/A |
| QoL outcomes at 24 weeks (n = 55)28 | Baseline, mean ± SD | Post treatment, mean ± SD | p-value |
|---|---|---|---|
| CHQ Physical summarya,b | 52.1 ± 4.8 | 49.8 ± 7.5 | Mean change 2.40 ± 6.8, p = 0.01353 |
| CHQ Psychosocial summary | 52.1 ± 7.9 | 52.3 ± 10.2 | NR |
| CBCL Internalisingc | 52.4 ± 8.5 | 51.0 ± 11.0 | p = ns53 |
| CBCL Externalisingc | 50.4 ± 9.4 | 48.8 ± 10.3 | p = ns53 |
| CBCL Total Behaviour Problemc | 51.5 ± 9.3 | 49.7 ± 10.2 | p = ns53 |
| CDI Total scorec | 5.9 ± 4.2 | 6.2 ± 5.6 | p = ns53 |
| BRIEF Global Executive Compositec | 53.5 ± 9.9 | 52.2 ± 10.1 | p = ns53 |
| Change in QoL at 24 weeks28 | Clinically significant improvement, n/N (%) | Clinically significant decline, n/N (%) | No clinical change, n/N (%) |
|---|---|---|---|
| CHQ Physical summary | 0 | 8/55 (15) | 47/55 (86) |
| CHQ Psychosocial summary | 3/55 (5) | 4/55 (7) | 48/55 (88) |
| CBCL Internalising | 2/55 (4) | 3/55 (5) | 50/55 (91) |
| CBCL Externalising | 1/55 (2) | 3/55 (5) | 51/55 (93) |
| CBCL Total Behaviour Problem | 1/55 (2) | 2/55 (4) | 52/55 (95) |
| CDI Total score | 0 | 3/55 (5) | 52/55 (95) |
| BRIEF Global Executive Composite | 3/55 (5) | 3/55 (5) | 49/55 (90) |
| QoL for those with virological response at 24 weeks (n = 41)28 | Baseline, mean ± SD | 24 weeks, mean ± SD | 48 weeks, mean ± SD | 6 months, mean ± SD |
|---|---|---|---|---|
| CHQ Physical summarya | 52.5 ± 4.2 | 49.3 ± 7.6 | 50.7 ± 8.0 | 51.9 ± 7.5 |
| CHQ Psychosocial summarya | 52.3 ± 8.1 | 52.0 ± 9.3 | 51.9 ± 8.4 | 52.9 ± 9.3 |
| CBCL Internalisinga | 53.9 ± 8.4 | 50.9 ± 11.3 | 49.7 ± 10.4 | 49.1 ± 10.8 |
| CBCL Externalisinga | 51.9 ± 9.0 | 49.9 ± 9.9 | 49.4 ± 9.5 | 48.5 ± 10.5 |
| CBCL Total Behaviour Problema | 52.8 ± 8.5 | 50.4 ± 10.1 | 50.0 ± 10.3 | 48.5 ± 11.9 |
| CDI Total scorea | 6.2 ± 4.4 | 6.1 ± 5.0 | 5.7 ± 3.8 | 4.7 ± 3.3 |
| BRIEF Global Executive Compositea | 53.1 ± 10.5 | 52.5 ± 9.7 | 52.4 ± 12.1 | 51.8 ± 11.1 |
| AEs | Baseline | n/N (%) |
|---|---|---|
| Dose discontinuation | N/A | 4/55 (7)a,b |
| Dose reduction | N/A | c28/55 (51)55 |
| PEG | 21/55 (38)c | |
| RBV | 14/55 (25)c | |
| Dose reduction for anaemia | N/A | 6/55 (11)54 |
| Dose reduction for thrombocytopenia | N/A | 1/55 (2)54 |
| Specific AEs | ||
| Flu-like symptoms | N/A | 50/55 (91) |
| Headache | N/A | 34/55 (62) |
| Gastrointestinal symptoms | N/A | 31/55 (56) |
| Injection site reactions | N/A | 25/55 (45) |
| Joint/muscle aches | N/A | 20/55 (36) |
| Irritability | N/A | 17/55 (31) |
| Fatigue | N/A | 15/55 (27) |
| Rash | N/A | 11/55 (20) |
| Itching | N/A | 8/55 (15) |
| Anorexia | N/A | 7/55 (13) |
| Trouble sleeping | N/A | 6/55 (11) |
| Depression | N/A | 2/55 (4) |
| Mortality | N/A | NR |
| Effects on growth | N/A | NRd |
Methodological comments
-
Allocation to treatment groups Randomisation allocation sequences were generated at a data co-ordinating centre (using a computer-generated randomisation scheme49) which determined treatment allocation in a 1 : 1 ratio. Randomisation was stratified by centre according to genotype (genotype 1 vs. non-1). Randomisations were blocked using random blocking factors of 2 and 4 owing to the relatively small sample size within each clinical site (≈ 10 participants for each of 11 sites) to best balance the groups. 49
-
Allocation concealment Allocation of each participant to treatment group was conveyed to the centres via a centralised telephone service.
-
Blinding Participants, families and investigators were blinded to treatment group. Placebo tablets were supplied in the same dosing regimen as RBV tablets. Does not report whether or not outcome assessors were blinded.
-
Analysis by intention to treat Intention-to-treat analysis noted for the RCT – all randomised subjects were included in the primary efficacy analysis.
-
Comparability of treatment groups at baseline Not applicable as only data from one arm (peginterferon + RBV) were used.
-
Method of data analysis A multivariate logistic model was constructed to predict SVR using baseline values and results of HCV RNA quantification at 12 weeks. Significance was assessed using a Wald chi-squared test comparing the maximum likelihood estimate for each parameter against zero. For ease of presenting odds ratios, continuous variables were dichotomised at their mean. SAS statistical software version 9.1.3 (SAS Institute Inc., Cary, NC, USA) was used for all analyses. Further details are reported by Murray and colleagues. 49 For QoL data, descriptive statistics were calculated to summarise medical, sociodemographic and outcome variables. Repeated measures analysis of variance (ANOVA) was used to assess treatment group and time effects on all outcomes. Clinical decline was operationalized as a > 1 SD change in score plus a change in score classification from impairment at baseline to no clinical impairment at 24 weeks; clinical improvement was defined as > 1 SD change in score plus a change in score classification from clinical impairment at baseline to no impairment at 24 weeks. To reduce the probability of type 1 error rate, analyses were initially performed only on the composite or summary scales of the outcome measures. If a statistically significant main or interaction effect emerged, differences on the individual scales were examined for the respective outcome measures. In the final set of analyses, Pearson correlation coefficients and t-tests were calculated to examine the relationship between sociodemographic characteristics and outcomes at the different time points. Owing to the large number of tests in this analysis cluster, p < 0.01 was considered as the level of statistical significance. PAWS (version 17.0) statistical software was used for all analyses. 28
-
Sample size/power analysis The RCT was designed to have a statistical power of 80% (standard chi-squared test of equality with two-sided α = 0.05) to detect an absolute difference of at least 25% in the proportion achieving SVR in the two treatment groups, adjusting for an estimated 15% dropout rate. 49 It was calculated that 56 patients in each study group were needed to detect a difference of between 25% and 35%. 49
-
Attrition/dropout None lost to follow-up (week 72) but therapy discontinued early in 4 out of 55 (7%); reasons stated. Seven (13%) lost to follow-up by the first annual visit, rising to 10 (18%) by the second annual visit.
General comments
-
Generalisability Western population, most commonly infected via vertical transmission, who are largely Caucasian with early-stage disease and HCV genotype 1.
-
Intercentre variability Not reported.
-
Conflict of interests Roche provided the drugs and supported the quantitative viral testing but had no role in the study design, oversight, analysis or interpretation. Thirteen of 17 authors have received support/grants from Roche and other pharmaceutical companies. One author is an employee of Roche Molecular Systems; the remaining three authors disclose no conflicts.
-
Other Probably the pivotal trial for peginterferon alfa-2a licence approval (Roche).
Quality criteria for assessment of randomised controlled trials
| Quality criterion | Judgement |
|---|---|
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Yes |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease, genotype, viral load? | N/A (used single-arm data) |
| 4. Were outcome assessors blinded to the treatment allocation? | NR |
| 5. (i) Were there any unexpected imbalances in dropouts between groups? (ii) If so, were they explained or adjusted for? |
N/A |
| 6. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Yes |
| 7. (i) Did the analysis include an intention-to-treat analysis? (ii) If so, was this defined? |
Yes Yes |
| 8. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Unclear |
Quality criteria for assessment of uncontrolled single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the treatment? | Yes |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Yesa |
| 4. Were withdrawals and dropouts completely described? | Yesb |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Unclearb |
Studies of peginterferon alfa-2a: Sokal and colleagues (2010)57
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Sokal et al.57 Year: 2010 (study 2003–5) Study design: Single cohort Number of centres: 6 Countries: Belgium, UK, Sweden, Brazil, Latvia Funding: Stated funding was from the drug companies involved; study was partially supported by a grant from Roche |
Group 1 Drug 1: PEG α-2a Dose: 100 µg/m2 (maximum 180 µg) once weekly by injection Drug 2: RBV Dose: 15 mg/kg/day (maximum 1200 mg) taken orally Duration: Both drugs 24 or 48 weeks according to genotype subgroups (see below) Patients were withdrawn from treatment if HCV PCR assay result was positive at week 24. Stepwise dose reduction was allowed in cases of AEs (dose steps reported according to severity), with return to initial doses if AEs were resolved Study design details: Single-cohort open-label study with patients treated according to genotype in two subgroups: Subgroup A: Genotype 2 or 3 treated for 24 weeks Subgroup B: Genotype 1, 4, 5 or 6 treated for 48 weeks Both subgroups received the same drugs at the same doses |
Total numbers involved: 65 Genotype subgroup A: 18 Genotype subgroup B: 47 Treatment naive: Yes (100%) Previous treatment: N/A HCV/HIV co-infection: 0 (0%) Duration of infection: NR Inclusion criteria: Treatment-naive children and adolescents aged 6–17 years with positive anti-HCV serum antibodies and detectable serum HCV RNA. Not limited by levels of serum aminotransferases, HCV genotype or mode of infection. All patients presenting with hepatitis C were approached for inclusion. Adequate contraception was compulsory (if applicable) Exclusion criteria: Pregnancy, anaemia (normal levels according to sex and age), decompensated liver disease, HIV or HBV infection, epilepsy, depression or other poorly controlled psychiatric disease, renal failure, retinopathy, alcohol or drug dependence, or other (unspecified) co-existing medical conditions Age (years), mean ± SD Overall population: NR Subgroup A: 11.3 ± 3.6 Subgroup B: 12.6 ± 3.6 Gender male, n (%) Overall population: 30 (46) Subgroup A: 9 (50) Subgroup B: 21 (45) Weight (kg), mean (± SD) Overall population: NR Subgroup A: 40.9 ± 3.8 Subgroup B: 43.8 ± 16.7 Ethnic groups, n (%) Overall population: White, 57 (88); black, 2 (3); Asian, 1 (2); other, 5 (8) Subgroup A: White, 17 (94); black, 0 (0); Asian, 0 (0); other, 1 (6) Subgroup B: White, 40 (85); black, 2 (4); Asian, 1 (2); other, 4 (9) Mode of infection, n (%) Overall population: Vertical, 30 (46); transfusion, 15 (23); other (medical procedure), 6 (9); unknown, 14 (22) Subgroup A: Vertical, 10 (56); transfusion, 7 (39); other (medical procedure), 0 (0); unknown, 1 (6) Subgroup B: Vertical, 20 (43); transfusion, 8 (17); other (medical procedure), 6 (13); unknown, 13 (28) Genotypes, n (%): 1, 45 (69); 2, 2 (3); 3, 16 (25); 4, 1 (2); 5 or 6, 1 (2) Sample attrition/dropout, n (%): 10 (15) all of whom were in subgroup B No virological response at week 24: 8 (12) Serious AE: 2 (3) Other violation: 0 (0) |
Primary outcomes: SVR Secondary outcomes: EVR; EOT; predictors of virological response; safety (AEs); growth Length of follow-up: All patients were followed for 24 weeks after cessation of therapy Methods of assessing outcomes: SVR defined as the absence of detectable HCV RNA at the end of the follow-up as measured by a PCR assay (COBAS AmplicorTM HCV test, v.2.0; Roche Laboratories) which has a lower limit of detection of 50 IU/ml EVR defined as the percentage of patients with at least a 2-log drop in HCV RNA levels at week 12 compared with baseline as measured by quantitative real-time PCR assay (COBAS AmplicorTM HCV test v2.0) ETR defined as the percentage of patients with non-detectable HCV RNA at the end of the treatment period (24 or 48 weeks according to the genotype subgroup) Liver fibrosis: classification system NR |
| Participant characteristics/outcomes | Baseline | Post treatment |
|---|---|---|
| HCV RNA (IU/ml), n (rounded %) | ||
| Overall population | ||
| < 500,000 | 23 (36) | NR |
| > 500,000 | 42 (65) | NR |
| Subgroup A | ||
| < 500,000 | 10 (56) | NR |
| > 500,000 | 8 (44) | NR |
| Subgroup B | ||
| < 500,000 | 13 (28) | NR |
| > 500,000 | 34 (72) | NR |
| Serum ALT (IU/l), mean ± SD | NRa | NR |
| Fibrosis score, n / N (%) | ||
| Overall population | ||
| No fibrosis | 34/65 (52) | NR |
| Fibrosis (total) | 30/65 (46) | NR |
| Grade F1 | 21/65 (32) | NR |
| Grade F2 | 9/65 (14) | NR |
| Subgroup A | ||
| No fibrosis | 8/18 (44) | NR |
| Fibrosis (total) | 10/18 (56) | NR |
| Grade F1 | 7/18 (39) | NR |
| Grade F2 | 3/18 (17) | NR |
| Subgroup Bb | ||
| No fibrosis | 26/47 (55) | NR |
| Fibrosis (total) | 20/47 (43) | NR |
| Grade F1 | 14/47 (30) | NR |
| Grade F2 | 6/47 (13) | NR |
| Necroinflammatory score, mean ± SD | NR | NR |
| Outcomes | Baseline | Post treatment | p-value |
|---|---|---|---|
| Viral response, % (n/N) | |||
| RVR (4 weeks) | N/A | NR | |
| EVR (12 weeks) | |||
| Overall population | N/A | 65 (42/65) (3 ND)a | See notesb |
| Subgroup A | N/A | 83 (15/18) (2 ND) | |
| Subgroup B | N/A | 57 (27/47) (1 ND) | p < 0.05 |
| EOT | |||
| Overall population | N/A | 68 (44/65) (2 ND)a | See notesb |
| Subgroup A | N/A | 94 (17/18) (1 ND) | |
| Subgroup B | N/A | 57 (27/47) (1 ND) | p < 0.001 |
| SVR (end of follow-up) | |||
| Overall population | N/A | 66 (43/65) (2 ND)a | See notesb |
| Subgroup A | N/A | 89 (16/18) (1 ND) | |
| Subgroup B | N/A | 57 (27/47) (1 ND) | p < 0.01 |
| SVR subgroup data, rounded % (n/N)c | |||
| SVR by EVR | |||
| Overall population | N/A | 85 (35/42)d | |
| Subgroup A | N/A | 93 (13/14) | |
| Subgroup B | N/A | 81 (22/27) | |
| SVR by no EVR | N/A | 30 (6/20) | |
| SVR by baseline viral load, overall population | |||
| < 5 × 105 IU/ml | N/A | 74 (17/23) | |
| > 5 × 105 IU/ml | N/A | 55 (22/40) | |
| SVR by baseline viral load, subgroup A | |||
| < 5 × 105 IU/ml | N/A | 90 (9/10) | |
| > 5 × 105 IU/ml | N/A | 100 (7/7) | |
| SVR by baseline viral load, subgroup B | |||
| < 5 × 105 IU/ml | N/A | 62 (8/13) | |
| > 5 × 105 IU/ml | N/A | 45 (15/33) | |
| SVR by ALT screening | |||
| Baseline ALT normal | N/A | 80 (24/30) | |
| Baseline ALT abnormal | N/A | 58 (19/33) | |
| SVR by histology | |||
| Baseline no fibrosis | N/A | 76 (25/33) | |
| Baseline fibrosis | N/A | 60 (18/30) | |
| SVR by genotype and ALT, subgroup A | |||
| Baseline ALT normal | N/A | 89 (8/9) | |
| Baseline ALT abnormal | N/A | 100 (8/8) | |
| SVR by genotype and ALT, subgroup B | |||
| Baseline ALT normal | N/A | 89 (17/19) | |
| Baseline ALT abnormal | N/A | 37 (10/27) | See notese |
| SVR by genotype and fibrosis, subgroup A | |||
| Baseline no fibrosis | N/A | 100 (8/8) | |
| Baseline fibrosis | N/A | 89 (8/9) | |
| SVR by genotype and fibrosis, subgroup B | |||
| Baseline no fibrosis | N/A | 68 (17/25) | |
| Baseline fibrosis | N/A | 48 (10/21) | |
| Other viral response outcomes | N/A | No additional outcomes reported | |
| Non-response, overall population | N/A | 12 (8/65) | |
| Non-response, subgroup A | N/A | 0 (0/18) | |
| Non-response, subgroup B | N/A | 17 (8/47) | |
| Quality-of-life outcomes | |||
| NR | NR | ||
| AEs, % (n/N) | |||
| Dose discontinuation for serious AEs (acute hepatitis, laboratory abnormality/thyreotoxicosis) | N/A | 3 (2/65) | |
| Dose discontinuation due to non-response at 24 weeks | Same numbers as for non-response above | Same numbers as for non-response above | |
| PEG dose reduction for AEf | |||
| Overall population | N/A | 23 (15/65) | |
| Subgroup A | N/A | 22 (4/18) | |
| Subgroup B | N/A | 23 (11/47) | |
| Overall population, by event | |||
| Neutropenia | N/A | 17 (11/65) | |
| Thrombocytopenia | N/A | 1.5 (1/65) | |
| Laboratory anomaly | N/A | 1.5 (1/65) | |
| Asthenia | N/A | 1.5 (1/65) | |
| Non-response to treatment | N/A | 1.5 (1/65) | |
| RBV dose reduction for AEf | |||
| Anaemia, overall population | N/A | 5 (3/65) | |
| Anaemia, subgroup A | N/A | 0 (0/18) | |
| Anaemia, subgroup B | N/A | 6 (3/47) | |
| Serious AEs | |||
| Acute hepatitis | N/A | 1.5 (1/65) | |
| Thyreotoxicosis | N/A | 1.5 (1/65) | |
| Urinary tract infection | N/A | 1.5 (1/65) | |
| Pulmonary hypertension | N/A | 1.5 (1/65) | |
| Specific AEs | |||
| Fever/flu-like symptoms | N/A | 54 (35/65) | |
| Headache | N/A | 45 (29/65) | |
| Abdominal pain | N/A | 38 (25/65) | |
| Fatigue | N/A | 34 (22/65) | |
| Irritability/depression/mood change (no suicidal ideation) | N/A | 34 (22/65) | |
| Dermatitis | N/A | 29 (19/65) | |
| Nausea/vomiting | N/A | 23 (15/65) | |
| Infection | N/A | 23 (15/65) | |
| Viral | N/A | 9 (6/65) | |
| Bacterial | N/A | 14 (9/65) | |
| Decreased appetite | N/A | 21.5 (14/65) | |
| Insomnia | N/A | 18 (12/65) | |
| Sore throat | N/A | 15 (10/65) | |
| Diarrhoea | N/A | 14 (9/65) | |
| Injection site pain/erythema/local infection | N/A | 14 (9/65) | |
| Dyspnoea | N/A | 11 (7/65) | |
| Thyroid hormone problems, overall population | N/A | 11 (7/65) | |
| Thyroid hormone problems, subgroup A | N/A | 0 (0/18) | |
| Thyroid hormone problems, subgroup B | N/A | 15 (7/47) | |
| Myalgia | N/A | 9 (6/65) | |
| Alopecia | N/A | 9 (6/65) | |
| Bleeding | N/A | 9 (6/65) | |
| Pruritus | N/A | 6 (4/65) | |
| Arthralgia | N/A | 3 (2/65) | |
| Enuresis/dysuria | N/A | 3 (2/65) | |
| Palpitations | N/A | 3 (2/65) | |
| Mortality, % (n/N) | |||
| N/A | NR (= none) | ||
| Effects on growth | |||
| Weight, z-score | –0.3 ± 0.9 | –0.3 ± 1.0 | Stated NS |
| Height, z-score | –0.4 ± 1.0 | –0.5 ± 1.1 | Stated NS |
Methodological comments
-
Allocation to treatment groups Not applicable; single-cohort study.
-
Allocation concealment Not applicable.
-
Blinding None (stated open label).
-
Analysis by intention to treat Not applicable; non-randomised study (stated that primary analysis had an ‘intent-to-treat’ approach but no further details reported).
-
Comparability of treatment groups at baseline Not applicable (the two subgroups were reported to be similar, except for pretreatment viral load which was higher in group B).
-
Method of data analysis Fisher’s exact test (misspelt) used but the groups being compared were not stated.
-
Sample size/power analysis Not reported.
-
Attrition/dropout Reported with reasons.
General comments
-
Generalisability Treatment-naive children and adolescents aged 6–17 years, genotypes 1–6, of predominantly white ethnicity, with positive anti-HCV serum antibodies and detectable serum HCV RNA. Not limited by levels of serum aminotransferases, HCV genotype or mode of infection. Duration of HCV infection unclear.
-
Intercentre variability Not reported.
-
Conflict of interests Funding was from the drug manufacturer (Roche).
Quality criteria for assessment of uncontrolled, single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | N/A |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Yes |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Unclear (test reported but not the groups being compared) |
Studies of peginterferon alfa-2b: Al Ali and colleagues (2010)46
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Al Ali et al.46 Year: 2010 (study dates NR) Study design: Single cohort Number of centres: One (not explicitly stated) Country: Kuwait Funding: Stated that no financial support was used for this work |
Group 1 Drug 1: PEG α-2b Dose: 1.5 µg/kg per week by subcutaneous injection Duration: 48 weeks Drug 2: RBV Dose: 15 mg/kg/day taken orally Duration: 48 weeks PEG α-2b was administered in the local primary care clinic by a registered nurse who documented compliance. Adherence to RBV ingestion was monitored by capsule count Stepwise reductions in treatments were permitted for management of adverse events and laboratory abnormalities (no further details reported) Study design details: Open-label uncontrolled single-cohort study described as a pilot study |
Total numbers involved: 12 Treatment naive: Yes (100%) Previous treatment: N/A HCV/HIV co-infection: NR Duration of infection: NR Inclusion criteria: Treatment-naive patients aged 14–17 years with detectable HCV RNA, genotype 4 and anti-HCV-positive liver biopsy findings consistent with the diagnosis of chronic hepatitis C, for whom a decision to treat was made. Patients were included independent of mode of acquisition of infection, level of serum aminotransferases or serum HCV RNA viral load Exclusion criteria: Clinical or biochemical evidence of hepatic decompensation, severe psychiatric disorders, haemoglobin < 100 g/l, white blood cell count < 2500/mm3, platelet counts < 70,000/mm3 and serum creatinine > 200 mmol/l Age (years), mean (range): 15.75 (14–17) Gender male, n (%): 8 (67) Weight or BMI: NR Ethnic groups, n (%): Not explicitly stated but implied Middle Eastern (study carried out in Kuwait) Mode of infection, n (%): Vertical, 2 (17); i.v. drug use, 2 (17); transfusion, 1 (8); other (dental procedures), 2 (17); unknown, 5 (42) Genotypes, n (%): 4, 12 (100) Sample attrition/dropout: 11 patients (92%) completed the study. The patient who dropped out developed type 1 diabetes mellitus |
Primary outcomes: SVR Secondary outcomes: EVR; EOT; adverse events Length of follow-up: 24 weeks post treatment Methods of assessing outcomes: SVR was defined as an undetectable (< 50 IU/ml) HCV RNA after 24 weeks of treatment-free follow-up (week 72) EVR was defined as HCV RNA < 50 IU/ml at week 12 of therapy EOT response was defined as HCV RNA < 50 IU/ml at week 48 Serum HCV RNA testing was performed using a qualitative PCR assay (Cobas Amplicor HCV Test v2.0) Liver histology was graded using the METAVIR scoring system (no details provided) |
| Participant characteristics/outcomes | Baseline | Post treatment |
|---|---|---|
| HCV RNA (× 10 6 IU/ml), mean (range) | 0.78 (0.23–1.80) | NR |
| Serum ALT (IU/l), mean (range) | 91 (34–194) | NR |
| Fibrosis score, mean (range) | ||
| METAVIR histological gradea | 1.67 (1–2) | NR |
| METAVIR fibrosis scorea | 0.67 (0–3) | NR |
| Necroinflammatory score, mean (± SD) | NR | NR |
| Outcomes | Baseline | Post treatmenta |
|---|---|---|
| Viral response, % (n/N) | ||
| RVR (4 weeks) | N/A | NR |
| EVR (12 weeks) | N/A | 83 (10/12) |
| EOT | N/A | 83 (10/12) |
| SVR (end of follow-up; week 72) | N/A | 75 (9/12) |
| SVR subgroup data, % (n/N) | N/A | NR |
| SVR by EVR | N/A | 100 (10/10) |
| Non-response | N/A | 17 (2/12)b |
| Relapse | N/A | 8 (1/12)c |
| Quality-of-life outcomes | ||
| NR | NR | |
| AEs, % (n/N) | ||
| Dose discontinuation for any AE | N/A | NRd |
| Dose discontinuation for other reason | N/A | NR |
| Dose reduction for any AE | N/A | NR |
| Dose reduction for anaemia | N/A | 33 (4/12)e |
| Dose reduction for neutropenia | N/A | NR |
| Dose reduction for other reason | N/A | NR |
| Specific AEs | ||
| Fever/flu-like symptoms | N/A | 100 (12/12) |
| Leukopenia | N/A | 67 (8/12)f |
| Myalgia | N/A | 58 (7/12) |
| Anaemia (haemoglobin < 10 g/dl) | N/A | 33 (4/12)e |
| Neutropenia | N/A | 17 (2/12) |
| Type 1 diabetes mellitus | N/A | 8 (1/12) |
| Hypothyroidism | N/A | 8 (1/12) |
| Insomnia | N/A | 8 (1/12) |
| Mortality, % (n/N) | ||
| NR | NR | |
| Effects on growth | ||
| NR | NR | |
Methodological comments
-
Allocation to treatment groups Not applicable.
-
Allocation concealment Not applicable.
-
Blinding None (stated open label).
-
Analysis by intention to treat Not applicable.
-
Comparability of treatment groups at baseline Not applicable.
-
Method of data analysis None reported; results presented narratively.
-
Sample size/power analysis Not reported.
-
Attrition/dropout Reported with reasons.
General comments
-
Generalisability Genotype 4 treatment-naive patients, likely of Middle Eastern ethnicity, with mild liver disease with low pretreatment viral load.
-
Intercentre variability Not applicable (single centre).
-
Conflict of interests Stated only that no funding was provided; no declaration of interests given in the paper.
Quality criteria for assessment of uncontrolled, single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to treatment? | N/A |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Yes |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Unclear (no formal analysis conducted) |
Studies of peginterferon alfa-2b: Pawlowska and colleagues (2010)51
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Pawlowska et al.51,52 Year: 2010 (abstract 2008) Study design: Single cohort Number of centres: One (not explicitly stated) Country: Poland Funding: States ‘departmental sources’ |
Group 1 Drug 1: PEG α-2b Dose: 1.5 µg/kg/week by subcutaneous injection Duration: 48 weeksa Drug 2: RBV Dose: 15 mg/kg/day taken orally Duration: 48 weeksa Study design details: Single uncontrolled cohort but children were ‘divided’ into two groups according to previous treatment (treatment naive and previously treated). Baseline characteristics and most results are presented for the whole cohort as well as separately for the two subgroups |
Total numbers involved: 53 (29 treatment naive; 24 previously treated) Treatment naive: n = 29 (54%) Previous treatment: n = 24 (46%) treated with IFN α-2b + RBV for 12 months, 2–5 years earlier (10 relapsers, 8 non-responders, 6 breakthroughs) HCV/HIV co-infection: None Duration of infection (years), mean ± SD: 5.4 ± 3.6 (4.12 ± 3.7 naive; 6.92 ± 2.8 treated); abstract reports 8.5 ± 4.652 Inclusion criteria: Children aged 8–17 years with chronic hepatitis C diagnosed by the presence of serum HCV RNA and histopathological changes in the liver. All children had a liver biopsy and ultrasound Exclusion criteria: Histological evidence of hepatocellular carcinoma, chronic liver disease other than chronic hepatitis C, co-infection with hepatitis B or HIV Age (years), mean ± SD (range): 13.6 ± 2.4 (8–17) Gender male, n (%): 37 (70) (20 naive, 17 treated) Ethnic groups, n (%): NR Mode of infection, n:b Vertical, 0; transfusion, 5 (1 naive, 4 treated); hospital-acquired, 53 (29 naive, 24 treated); surgical procedure, 16 (7 naive, 9 treated) Genotypes, n (%): 1, 27 (50) (16 naive, 11 treated); 2, 0; 3, 2 (4) (both naive); 4, 24 (46) (11 naive, 13 treated) Sample attrition/dropout: None reported |
Primary outcomes: SVR Secondary outcomes: EVR (partial and complete), EOT, relapse, breakthrough, non-response, predictors of virological response, adverse events, growth (very brief narrative only) Length of follow up: 24 weeks after end of treatment. SVR was also measured again 24 months after the initial SVR assessment (few data presented) and follow-up for assessment of growth is ongoing (plan is for 5 years) Methods of assessing outcomes: Blood samples to determine HCV RNA viral load and ALT activity performed at each clinic visit. Serum HCV RNA viral load determined at baseline, weeks 12 and 48 during treatment, and after 24 weeks of untreated follow-up by quantitative PCR assay [COBAS® AmpliPrep/COBAS TaqMan HCV Test (Roche, Geneva, Switzerland)] with a limit of detection of 43 IU/ml SVR defined as undetectable HCV RNA in serum 24 weeks after the end of treatment. EVR defined as HCV RNA viral load at week 12 of treatment; complete EVR = undetectable serum HCV RNA at week 12, partial EVR = a decrease in HCV RNA of > 2 logs relative to baseline EOT defined as undetectable serum HCV RNA at week 48 of treatment Relapse defined as appearance of HCV RNA at week 72 after undetectable serum HCV RNA at week 48 Histology classification system used: modified Scheuer scale HCV genotypes defined using the INNO-LiPA HCV II test (Innogenetics, Ghent, Belgium) Safety was monitored at each clinic visit by laboratory tests, physical examination and adverse events reported by the patient or guardian |
| Participant characteristics/outcomes | Baseline | Post treatment |
|---|---|---|
| HCV RNA (IU/ml), mean ± SD | ||
| Alla | 4.56 × 105 | NR |
| Naive | 4.35 ± 3.09 × 105 | NR |
| Treated | 5.16 ± 2.12 × 105 | NR |
| HCV RNA, n / N (%) b | ||
| All, < 500,000 IU/ml | 21/53 (40) | NR |
| All, > 500,000 IU/ml | 29/53 (55) | NR |
| Naive, < 500,000 IU/ml | 12/53 (23); 12/29 (41)c | NR |
| Naive, > 500,000 IU/ml | 15/53 (28); 15/29 (52)c | NR |
| Treated, < 500,000 IU/ml | 9/53 (17); 9/24 (38)c | NR |
| Treated, > 500,000 IU/ml | 14/53 (26); 14/24 (58)c | NR |
| Serum ALT (IU/l), mean ± SD | ||
| All | 45.8 ± 24.3 | NR |
| Naive | 48.0 ± 29.0 | NR |
| Treated | 43.0 ± 21.0 | NR |
| Fibrosis score, modified Scheuer scale stages 0 to 4 | ||
| All | ≤ stage 2 | NR |
| Necroinflammatory score, modified Scheuer scale grades 0 to 4 | ||
| All | ≤ grade 2 | NR |
| Outcomes | Baseline | Post treatmenta |
|---|---|---|
| Viral response, % (n/N) | ||
| RVR | N/A | NR |
| EVR b | ||
| All | N/A | 77.4 (41/53) |
| Naive | N/A | 86.2 (25/29) |
| Treated | N/A | 66.7 (16/24) |
| EOT | ||
| All | N/A | 66 (35/53) |
| Naive | N/A | 65 (19/29) |
| Treated | N/A | 66.7 (16/24) |
| SVR | ||
| All | N/A | 49.1 (26/53)c |
| Naive | N/A | 62.1 (18/29) |
| Treated | N/A | 33.3 (8/24) |
| SVR subgroup data by genotype, % (n/N) | ||
| Genotype 1 | ||
| All | N/A | 48 (13/27) |
| Naive | N/A | 62 (10/16) |
| Treated | N/A | 27 (3/11) |
| Genotype 3 (both naive) | N/A | 50 (1/2) |
| Genotype 4 | ||
| All | N/A | 50 (12/24) |
| Naive | N/A | 72 (8/11) |
| Treated | N/A | 30 (4/13) |
| Relapse, % (n/N) | ||
| All | N/A | 17.0 (9/53)d |
| Naive | N/A | 3.4 (1/29)d |
| Treated | N/A | 33.3 (8/24) |
| Breakthrough, % (n/N) | ||
| All | N/A | 11 (6/53) |
| Naive | N/A | 20 (6/29) |
| Treated | N/A | 0 |
| Non-response, % (n/N) | ||
| All | N/A | 50.9 (27/53) |
| Naive | N/A | 37.9 (11/29) |
| Treated | N/A | 66.7 (16/24) |
| Quality-of-life outcomes | ||
| N/A | NR | |
| AEs, % (n/N) | ||
| Dose discontinuation for any AE | N/A | NR |
| Dose discontinuation for other reason | N/A | NR |
| Dose reduction for any AE | N/A | NR |
| Dose reduction (RBV) for anaemia | ||
| All | N/A | 6 (3/53) |
| Naive | N/A | 10 (3/29) |
| Dose reduction for neutropenia | N/A | NR |
| Dose reduction for other reason | N/A | NR |
| Specific AEs, % (n/N)e | ||
| Flu-like syndrome | ||
| All | N/A | 66.0 (35/53) |
| Naive | N/A | 55.2 (16/29) |
| Treated | N/A | 79.2 (19/24) |
| Leukopenia | ||
| All | N/A | 64.2 (34/53) |
| Naive | N/A | 65.5 (19/29) |
| Treated | N/A | 62.5 (15/24) |
| Fever | ||
| All | N/A | 50.2 (27/53) |
| Naive | N/A | 55.2 (16/29) |
| Treated | N/A | 45.8 (11/24) |
| Headache | ||
| All | N/A | 45.3 (24/53) |
| Naive | N/A | 27.6 (8/29) |
| Treated | N/A | 66.7 (16/24) |
| Weight loss > 10% | ||
| All | N/A | 43.4 (23/53) |
| Naive | N/A | 34.5 (10/29) |
| Treated | N/A | 54.2 (13/24) |
| Local reaction | ||
| All | N/A | 34.0 (18/53) |
| Naive | N/A | 20.7 (6/29) |
| Treated | N/A | 50.0 (12/24) |
| Anaemia | ||
| All | N/A | 24.5 (13/53) |
| Naive | N/A | 24.1 (7/29) |
| Treated | N/A | 25.0 (6/24) |
| Abdominal pain | ||
| All | N/A | 20.8 (11/53) |
| Naive | N/A | 3.4 (1/29) |
| Treated | N/A | 41.7 (10/24) |
| Neurastheniaf | ||
| All | N/A | 20.8 (11/53) |
| Naive | N/A | 13.8 (4/29) |
| Treated | 29.2 (7/24) | |
| Hair loss | ||
| All | N/A | 20.8 (11/53) |
| Naive | N/A | 24.1 (7/29) |
| Treated | N/A | 16.7 (4/24) |
| Thrombocytopenia | ||
| All | N/A | 15.1 (8/53) |
| Naive | N/A | 20.7 (6/29) |
| Treated | N/A | 8.3 (2/24) |
| Mortality, % (n/N) | ||
| N/A | NR | |
| Effects on growthg | ||
| All | N/A | ‘No influence on height at follow-up or 2 years after follow-up’ |
Methodological comments
-
Allocation to treatment groups Not applicable.
-
Allocation concealment Not applicable.
-
Blinding None (stated open uncontrolled study).
-
Analysis by intention to treat Not applicable.
-
Comparability of treatment groups at baseline Not applicable. However, baseline characteristics were presented separately for the naive and treated subgroups (as well as the whole group); naive patients appeared to have lower baseline HCV RNA, shorter duration of infection and higher proportion with genotype 1, but no statistical comparisons were reported. All other characteristics appear comparable.
-
Method of data analysis Serum HCV RNA was analysed by descriptive statistics. Reports that means and SDs were calculated at the various time points during treatment and follow-up. Virological response outcomes were presented as proportions (n, %). States that the t-test, Mann–Whitney U-test and chi-squared test were used to compare ‘examined groups’ – unclear whether this refers to naive and treated groups or genotype groups, or those achieving/not achieving SVR. Few statistics are reported for the main outcomes. Children with genotype 3 were excluded from the statistical analyses.
-
Sample size/power analysis None reported.
-
Attrition/dropout None reported.
General comments
-
Generalisability Polish population, treatment-naive and previously treated mixture of patients, with largely hospital-acquired mode of infection. Fifty-five per cent have a high baseline HCV RNA viral load, approximately half are previously treated and most are genotype 1 or 4.
-
Intercentre variability Single-centre study.
-
Conflict of interests None reported.
Quality criteria for assessment of uncontrolled, single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the treatment? | N/A |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | NR |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
No |
Studies of peginterferon alfa-2b: Wirth and colleagues (2010)59
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Wirth et al.58,59 Year: 2010 (study dates NR) Study design: Single cohort Number of centres: 22 Countries: Austria, France, Germany, Italy, Spain, Argentina, Chile, USA, Puerto Rico Funding: 25 of the 32 authors received funding from the drug manufacturer (Schering-Plough); six authors were employed by the drug manufacturer; support for writing the manuscript was also provided by the drug manufacturer |
Group 1 Drug 1: PEG α-2b Dose: 60 µg/m2/week Drug 2: RBV Dose: 15 mg/kg/day Duration: Both drugs 24 or 48 weeks according to genotype and viral load (see subgroups below) Prespecified dose reduction and discontinuation criteria: PEG α-2b dose reduced if neutrophil count < 750/mm3 or platelet count < 70,000/mm3; RBV dose reduced if haemoglobin < 10 g/dl. Both drugs discontinued if neutrophil count < 500/mm3; platelet count < 50,000/mm3; or haemoglobin < 8.5 g/dl. Two-step dose reductions were used: PEG α-2b reduced initially to 40 µg/m2 weekly then if needed to 20 µg/m2. RBV reduced initially to 12 mg/kg/day then if needed to 8 mg/kg/day Study design details: Single cohort with two subgroups according to genotype and viral load Subgroup A (n = 27): Genotypes 2 and 3 with low baseline viral load (< 600,000 IU/ml) were treated for 24 weeks Subgroup B (n = 80): Genotypes 1, 3 and 4 with high baseline viral load (≥ 600,000 IU/ml) were treated for 48 weeks Trial registration numbers: NCT00104052 and NCT00761735 |
Total numbers involved: 107 (one to 12 patients per centre) Subgroup A: n = 27; subgroup B: n = 80 Baseline characteristics were presented for all patients and also separately for ages 3–11 years (n = 67) and 12–17 years (n = 40) Treatment naive: Yes (100%) Previous treatment: N/A HCV/HIV co-infection: No (100%) Duration of infection (years), mean ± SD Overall: 8.5 ± 4.2 Ages 3–11 years: 6.5 ± 2.5 Ages 12–17 years: 12.3 ± 4.1 Inclusion criteria: Children aged 3–17 years with previously untreated chronic hepatitis C; absolute neutrophil count ≥ 1500/mm3; platelet count ≥ 100,000/mm3; and haemoglobin levels ≥ 11 g/dl for girls and 12 g/dl for boys. Evidence of fibrosis and/or inflammatory activity from liver biopsy was requested from all patients before enrolment; however, a waiver was permitted for children aged 3–11 years who had an elevated ALT in the year before screening Exclusion criteria (stated as the ‘key’ exclusion criteria, which may indicate there were others): Decompensated liver disease; co-existing HBV or HIV infection; haemoglobinopathy; haemophilia; malignant or immunological diseases; neurological or psychiatric disorders; retinopathy; substance abuse; chronic cardiopulmonary disease; immunosuppressive treatment. Patients with body weight > 90 kg were also excluded Age (years), mean Overall: 10 Ages 3–11 years: 7 Ages 12–17 years: 14 Gender male, n (%) Overall: 51 (48) Ages 3–11 years: 27 (40) Ages 12–17 years: 24 (60) Weight or BMI: NR Ethnic group: white, n (%) Overall: 95 (89) Ages 3–11 years: 60 (90) Ages 12–17 years: 35 (88) Mode of infection, n (%) Overall: Vertical, 75 (70); parenteral/transfusion, 12 (11); sporadic/other (not specified), 20 (19) Ages 3–11 years: Vertical, 52 (78) parenteral/transfusion, 4 (6); sporadic/other (not specified), 11 (16) Ages 12–17 years: Vertical, 23 (58); parenteral/transfusion, 8 (20); sporadic/other (not specified), 9 (23) Genotypes, n (%) Overall: 1, 72 (67); 2, 15 (14); 3, 15 (14); 4, 5 (5) Ages 3–11 years: 1, 47 (70); 2, 6 (9); 3, 10 (15); 4, 4 (6) Ages 12–17 years: 1, 25 (63); 2, 9 (23); 3, 5 (13); 4, 1 (3) Sample attrition/dropout: Outcomes were reported for all patients who started therapy (n = 107). One patient discontinued therapy due to thrombocytopenia at 42 weeks |
Primary outcome: SVR Secondary outcomes: RVR, EVR, predictors of virological response, relapse, biochemical response, adverse events, growth Length of follow-up: 24 weeks after end of therapy. Patients with < 2 log10 drop in HCV RNA in week 12 or detectable HCV RNA at week 24 discontinued therapy and entered follow-up Methods of assessing outcomes: SVR was defined as undetectable HCV RNA 24 weeks after completion of therapy RVR was defined as undetectable HCV RNA at treatment week 4 EVR was defined as undetectable HCV RNA at treatment week 12 Relapse was defined as undetectable HCV RNA at the last treatment visit and detectable HCV RNA at the last follow-up visit Biochemical response was defined as normalisation of ALT levels among patients with elevated ALT at baseline Plasma HCV RNA was measured using a proprietary assay (TaqMan; Schering-Plough); lower limit of detection 125 IU/ml Liver biopsy slides were assessed using METAVIR fibrosis and activity scores Adverse events were graded as mild, moderate or severe |
| Participant characteristics/outcomes | Baseline | Post treatment |
|---|---|---|
| HCV RNA (IU/ml) | ||
| Overall | ||
| Geometric mean | 442,748 | NR |
| < 600,000, % (n/N) | 54 (58/107) | NR |
| > 600,000, % (n/N) | 42 (45/107) | NR |
| Missing, % (n/N) | 4 (4/107) | NR |
| Ages 3–11 years | ||
| Geometric mean | 398,107 | NR |
| < 600,000, % (n/N) | 57 (38/67) | NR |
| > 600,000, % (n/N) | 40 (27/67) | NR |
| Missing, % (n/N) | 3 (2/67) | NR |
| Ages 12–17 years | ||
| Geometric mean | 531,018 | NR |
| < 600,000, % (n/N) | 50 (20/40) | NR |
| > 600,000, % (n/N) | 45 (18/40) | NR |
| Missing, % (n/N) | 5 (2/40) | NR |
| Serum ALT (IU/l), % (n/N) | ||
| Overall | ||
| Normal | 59 (63/107) | NR |
| Abnormal | 41 (44/107) | NR |
| Ages 3–11 years | ||
| Normal | 55 (37/67) | NR |
| Abnormal | 45 (30/67) | NR |
| Ages 12–17 years | ||
| Normal | 65 (26/40) | NR |
| Abnormal | 35 (14/40) | NR |
| METAVIR fibrosis score,a % (n/N) | ||
| Overall | ||
| F0 | 12.5 (13/107) | NR |
| F1 | 82.2 (88/107)b | NR |
| F2 | 1.9 (2/107) | NR |
| F3 | 1.0 (1/107) | NR |
| Ages 3–11 years | ||
| F0 | 13.8 (9/67) | NR |
| F1 | 83.1 (56/67) | NR |
| F2 | 1.5 (1/67) | NR |
| F3 | 1.5 (1/67) | NR |
| Ages 12–17 years | ||
| F0 | 10.3 (4/40) | NR |
| F1 | 87.2 (35/40) | NR |
| F2 | 2.6 (1/40) | NR |
| F3 | 0.0 (0/40) | NR |
| METAVIR inflammatory activity score, % (n/N) | ||
| Overall | ||
| None | 6 (6/107) | NR |
| Mild | 44 (47/107) | NR |
| Moderate | 30 (32/107) | NR |
| Severe | 18 (19/107) | NR |
| Missing | 3 (3/107) | NR |
| Ages 3–11 years | ||
| None | 4 (3/67) | NR |
| Mild | 40 (27/67) | NR |
| Moderate | 33 (22/67) | NR |
| Severe | 19 (13/67) | NR |
| Missing | 3 (2/67) | NR |
| Ages 12–17 years | ||
| None | 8 (3/40) | NR |
| Mild | 50 (20/40) | NR |
| Moderate | 25 (10/40) | NR |
| Severe | 15 (6/40) | NR |
| Missing | 3 (1/40) | NR |
| Liver steatosis, % (n/N) | ||
| Overall | ||
| 0 | 71 (76/107) | NR |
| > 0% to ≤ 5% | 22 (24/107) | NR |
| > 5% to ≤ 32% | 4 (4/107) | NR |
| Missing | 3 (3/107) | NR |
| Ages 3–11 years | ||
| 0 | 69 (46/67) | NR |
| > 0% to ≤ 5% | 24 (16/67) | NR |
| > 5% to ≤ 32% | 4 (3/67) | NR |
| Missing | 3 (2/67) | NR |
| Ages 12–17 years | ||
| 0 | 75 (30/40) | NR |
| > 0% to ≤ 5% | 20 (8/40) | NR |
| > 5% to ≤ 32% | 3 (1/40) | NR |
| Missing | 3 (1/40) | NR |
| Outcomes | Baseline | Post treatment | p-value |
|---|---|---|---|
| Viral response | |||
| RVR (4 weeks) | N/A | NR | |
| EVR (12 weeks) by genotype, % ( n / N ) a | |||
| Overall population (n = 107) | N/A | 68 (73/107) | |
| Genotype 1 (n = 72) | N/A | 60 (43/72) | |
| Genotypes 2 and 3 (n = 30) | N/A | 87 (26/30) | |
| Genotype 4 (n = 5) | N/A | 80 (4/5) | |
| EOT by genotype, % ( n / N ) a | |||
| Overall population (n = 107) | N/A | 70 (75/107) | |
| Genotype 1 (n = 72) | N/A | 60 (43/72) | |
| Genotypes 2 and 3 (n = 30) | N/A | 93 (28/30) | |
| Genotype 4 (n = 5) | N/A | 80 (4/5) | |
| SVR (end of follow-up), % ( n / N ) | N/A | 65 (70/107) | |
| SVR subgroup data, % (reported for genotype 1 only; n = 72) | |||
| Patients with RVR achieving SVR | N/A | 89 | |
| Patients with EVR achieving SVR | N/A | 84 | |
| SVR by genotype, % ( n / N ) | p = 0.0005, but not stated which comparison this refers to | ||
| Genotype 1 | N/A | 53 (38/72) | |
| Genotypes 2 and 3 | N/A | 93 (28/30) | |
| Genotype 4 | N/A | 80 (4/5) | |
| SVR by genotype and baseline viral load, % ( n / N ) b | |||
| Genotype 1 (n = 72) | |||
| Low (≤ 600,000 IU/ml) | N/A | 72 (28/39) | p = 0.0006 |
| High (> 600,000 IU/ml) | N/A | 29 (9/31) | (Low vs. high) |
| Missing | N/A | 50 (1/2) | |
| Genotypes 2 and 3 (n = 30) | |||
| Low (≤ 600,000 IU/ml) | N/A | 94 (15/16) | |
| High (> 600,000 IU/ml) | N/A | 100 (13/13) | |
| Missing | N/A | 0 (0/1) | |
| Genotype 4 (n = 5) | |||
| Low (≤ 600,000 IU/ml) | N/A | 100 (3/3) | |
| High (> 600,000 IU/ml) | N/A | 0 (0/1) | |
| Missing | N/A | 100 (1/1) | |
| Other viral response outcomesc | |||
| SVR by genotype and age group, % ( n / N ) | |||
| Genotype 1 (n = 72) | |||
| Ages 3–11 years | N/A | 51 (24/47) | |
| Ages 12–17 years | N/A | 56 (14/25) | |
| Genotypes 2 and 3 (n = 30) | |||
| Ages 3–11 years | N/A | 88 (14/16) | |
| Ages 12–17 years | N/A | 100 (14/14) | |
| Genotype 4 (n = 5) | |||
| Ages 3–11 years | N/A | 75 (3/4) | |
| Ages 12–17 years | N/A | 100 (1/1) | |
| SVR by genotype and mode of infection, % ( n / N ) | |||
| Genotype 1 (n = 72) | |||
| Vertical | N/A | 50 (26/52) | |
| Transfusion/parenteral | N/A | 80 (4/5) | |
| Sporadic/other (not specified) | N/A | 53 (8/15) | |
| Genotypes 2 and 3 (n = 30) | |||
| Vertical | N/A | 95 (18/19) | |
| Transfusion/parenteral | N/A | 100 (6/6) | |
| Sporadic/other (not specified) | N/A | 80 (4/5) | |
| Genotype 4 (n = 5) | |||
| Vertical | N/A | 75 (3/4) | |
| Transfusion/parenteral | N/A | 100 (1/1) | |
| Sporadic/other (not specified) | N/A | N/A | |
| SVR by genotype and baseline ALT, % ( n / N ) d | |||
| Genotype 1 (n = 72) | |||
| Normal | N/A | 56 (23/41) | |
| Abnormal | N/A | 48 (15/31) | |
| Genotypes 2 and 3 (n = 30) | |||
| Normal | N/A | 90 (18/20) | |
| Abnormal | N/A | 100 (10/10) | |
| Genotype 4 (n = 5) | |||
| Normal | N/A | 50 (1/2) | |
| Abnormal | N/A | 100 (3/3) | |
| Non-response, % ( n / N ) | N/A | NR | |
| Relapse, by genotype, % | |||
| Genotype 1 | N/A | 12% (9/72) genotype 1 cohort, thus 8% (9/107) whole cohort | |
| Genotype 2 | N/A | 0 | |
| Genotype 3 | N/A | 0 | |
| Genotype 4 | N/A | 0 | |
| Quality-of-life outcomes | |||
| NR | NR | ||
| AEs | |||
| Dose discontinuation for AEs, % (n/N) | |||
| Thrombocytopenia (week 42) | N/A | 1 (1/107)e | |
| Other reason | N/A | 0 (0/107) | |
| Dose reduction for AEs, % (n/N) | |||
| Any AE, overall | N/A | 25 (27/107) | |
| Ages 3–11 years | N/A | 19 (13/67) | |
| Ages 12–17 years | N/A | 35 (14/40) | |
| Blood and lymphatic disorders, overall | N/A | 17 (18/107) | |
| Ages 3–11 years | N/A | 9 (6/67) | |
| Ages 12–17 years | N/A | 30 (12/40) | |
| Anaemia, overall | N/A | 7 (8/107)f | |
| Ages 3–11 years | N/A | 4 (3/67) | |
| Ages 12–17 years | N/A | 10 (4/40) | |
| Neutropenia, overall | N/A | 12 (13/107) | |
| Ages 3–11 years | N/A | 6 (4/67) | |
| Ages 12–17 years | N/A | 23 (9/40) | |
| Gastrointestinal disorders, overall | N/A | 2 (2/107) | |
| Ages 3–11 years | N/A | 3 (2/67) | |
| Ages 12–17 years | N/A | 0 (0/40) | |
| Diarrhoea, overall | N/A | 1 (1/107) | |
| Ages 3–11 years | N/A | 1 (1/67) | |
| Ages 12–17 years | N/A | 0 (0/40) | |
| Nausea, overall | N/A | 2 (2/107) | |
| Ages 3–11 years | N/A | 3 (2/67) | |
| Ages 12–17 years | N/A | 0 (0/40) | |
| Vomiting, overall | N/A | 1 (1/107) | |
| Ages 3–11 years | N/A | 1 (1/67) | |
| Ages 12–17 years | N/A | 0 (0/40) | |
| Fall, overall | N/A | 1 (1/107) | |
| Ages 3–11 years | N/A | 0 (0/67) | |
| Ages 12–17 years | N/A | 3 (1/40) | |
| Weight/growth decrease, overallg | N/A | 10 (11/107) | |
| Ages 3–11 years | N/A | 9 (6/67) | |
| Ages 12–17 years | N/A | 13 (5/40) | |
| Pruritic rash, overall | N/A | 1 (1/107) | |
| Ages 3–11 years | N/A | 0 (0/67) | |
| Ages 12–17 years | N/A | 3 (1/40) | |
| Specific AEs of ≥ 10% incidence, % (n/N)h | |||
| Any treatment-related AE | N/A | 100 (107/107)i | |
| Anaemia, overall | N/A | 11 (12/107) | |
| Ages 3–11 years | N/A | 6 (4/67) | |
| Ages 12–17 years | N/A | 20 (8/40) | |
| Leukopenia, overall | N/A | 10 (11/107) | |
| Ages 3–11 years | N/A | 11 (7/65)j | |
| Ages 12–17 years | N/A | 10 (4/40) | |
| Neutropenia, overall | N/A | 33 (35/107) | |
| Ages 3–11 years | N/A | 24 (16/67) | |
| Ages 12–17 years | N/A | 48 (19/40) | |
| Abdominal pain, overall | N/A | 21 (22/107) | |
| Ages 3–11 years | N/A | 25 (17/67) | |
| Ages 12–17 years | N/A | 13 (5/40) | |
| Upper gastrointestinal disorder, overall | N/A | 12 (13/107) | |
| Ages 3–11 years | N/A | 9 (6/67) | |
| Ages 12–17 years | N/A | 18 (7/40) | |
| Nausea, overall | N/A | 18 (19/107) | |
| Ages 3–11 years | N/A | 18 (12/67) | |
| Ages 12–17 years | N/A | 18 (7/40) | |
| Vomiting, overall | N/A | 27 (29/107) | |
| Ages 3–11 years | N/A | 40 (27/67) | |
| Ages 12–17 years | N/A | 5 (2/40) | |
| Asthenia, overall | N/A | 15 (16/107) | |
| Ages 3–11 years | N/A | 16 (11/67) | |
| Ages 12–17 years | N/A | 13 (5/40) | |
| Chills, overall | N/A | 21 (23/107) | |
| Ages 3–11 years | N/A | 27 (18/67) | |
| Ages 12–17 years | N/A | 13 (5/40) | |
| Fatigue, overall | N/A | 30 (32/107) | |
| Ages 3–11 years | N/A | 33 (22/67) | |
| Ages 12–17 years | N/A | 25 (10/40) | |
| Injection site erythema, overall | N/A | 29 (31/107) | |
| Ages 3–11 years | N/A | 27 (18/67) | |
| Ages 12–17 years | N/A | 33 (13/40) | |
| Irritability, overall | N/A | 14 (15/107) | |
| Ages 3–11 years | N/A | 15 (10/67) | |
| Ages 12–17 years | N/A | 13 (5/40) | |
| Fever, overall | N/A | 80 (86/107) | |
| Ages 3–11 years | N/A | 90 (60/67) | |
| Ages 12–17 years | N/A | 65 (26/40) | |
| Weight decrease, overall | N/A | 19 (20/107) | |
| Ages 3–11 years | N/A | 21 (14/67) | |
| Ages 12–17 years | N/A | 15 (6/40) | |
| Anorexia, overall | N/A | 29 (31/107) | |
| Ages 3–11 years | N/A | 37 (25/67) | |
| Ages 12–17 years | N/A | 15 (6/40) | |
| Decreased appetite, overall | N/A | 22 (24/107) | |
| Ages 3–11 years | N/A | 27 (18/67) | |
| Ages 12–17 years | N/A | 15 (6/40) | |
| Arthralgia, overall | N/A | 17 (18/107) | |
| Ages 3–11 years | N/A | 21 (14/67) | |
| Ages 12–17 years | N/A | 10 (4) | |
| Myalgia, overall | N/A | 17 (18/107) | |
| Ages 3–11 years | N/A | 21 (14/67) | |
| Ages 12–17 years | N/A | 10 (4) | |
| Dizziness, overall | N/A | 14 (15/107) | |
| Ages 3–11 years | N/A | 13 (9/67) | |
| Ages 12–17 years | N/A | 15 (6/40) | |
| Headache, overall | N/A | 62 (66/107) | |
| Ages 3–11 years | N/A | 66 (44/67) | |
| Ages 12–17 years | N/A | 55 (22/40) | |
| Alopecia, overall | N/A | 17 (18/107) | |
| Ages 3–11 years | N/A | 18 (12/67) | |
| Ages 12–17 years | N/A | 15 (6/40) | |
| Psychiatric or behavioural adverse events, % | |||
| N/A | 28 | ||
| Specific psychiatric/behavioural AEs reported by at least two patients, %k | |||
| Nervousness | N/A | 8 | |
| Agitation | N/A | 4 | |
| Aggression | N/A | 3 | |
| Mood alteration | N/A | 3 | |
| Anxiety | N/A | 3 | |
| Insomnia | N/A | 3 | |
| Restlessness | N/A | 3 | |
| Anger | N/A | 2 | |
| Depression | N/A | 2 | |
| Depressed mood | N/A | 2 | |
| Affect lability | N/A | 2 | |
| Clinical laboratory AEs, % (n/N) | |||
| ≥ 1 abnormal thyroid stimulating hormone value during treatment or follow-up | N/A | 23 (25/107) | |
| Clinical hypothyroidism | N/A | 3 (3/107) | |
| Mortality, % (n/N) | |||
| N/A | 0 (0/107) | ||
| Effects on growth | |||
| Clearly inhibited growth velocity < third percentile during treatment phase, % (n/N) | N/A | 70 (75/107) | |
| Growth velocity (cm/year), mean ± SD | |||
| Treatment period | N/A | 2.47 ± 2.22 | |
| Follow-up period | N/A | 5.73 ± 4.10 | |
| Mean (SD) height percentile, overall | 50.87 (28.89) | 44.25 (27.59)l | |
| Ages 3–11 years | 51.14 (28.07) | 42.32 (25.82)l | |
| Ages 12–17 years | 50.41 (30.57) | 47.49 (30.39)l | |
| Mean change in height percentile | |||
| Baseline to end of treatment | N/A | –7.7 | |
| During follow-up | N/A | 1.1 | |
| Mean (SD) weight percentile, overall | 56.57 (29.35) | 53.39 (29.51)l | |
| Ages 3–11 years | 54.84 (30.3) | 50.46 (30.33)l | |
| Ages 12–17 years | 59.47 (27.82) | 58.30 (27.76)l | |
| Mean change in weight percentile | |||
| Baseline to end of treatment | N/A | –15.5 | |
| During follow-up | N/A | 12.3 | |
Methodological comments
-
Allocation to treatment groups Not applicable.
-
Allocation concealment Not applicable.
-
Blinding None (stated open label).
-
Analysis by intention to treat Not applicable.
-
Comparability of treatment groups at baseline Not applicable.
-
Method of data analysis Statistical tests and comparisons not reported. Stated that carry-forward analysis was performed, which included patients who had missing HCV RNA data at 24 weeks after treatment but undetectable HCV RNA at 12 weeks after treatment as sustained responders.
-
Sample size/power analysis Not reported.
-
Attrition/dropout Reported with reasons.
General comments
-
Generalisability Treatment-naive patients of white ethnicity with body weight not exceeding 90 kg, with evidence of fibrosis, inflammation and/or elevated ALT but without concurrent hepatitis B virus or HIV infection.
-
Intercentre variability Not reported, other than the numbers of patients recruited at each of the 22 centres (in supplementary online material).
-
Conflict of interests All but one of the 32 authors received funding from or were employed by the drug manufacturer (Schering-Plough); the drug manufacturer also supported writing of the manuscript.
-
Other Note that eight of nine genotype 3 patients with high viral load received only 24 weeks of therapy, in contrast to the 48 weeks specified in the protocol for this group.
Quality criteria for assessment of uncontrolled, single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to treatment? | N/A (stated open label) |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Yes |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
Unclear (no formal analysis reported) |
Studies of peginterferon alfa-2b: Ghaffar and colleagues (2009)47
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Ghaffar et al.47 Year: 2009 Study design: Single-cohort study Number of centres: One Country: Egypt Funding: Stated to be by donations |
Group 1: n = 7 Drug 1: PEG α-2b Dose: 1.5 µg/kg once per week Duration: 52 weeks Drug 2: RBV Dose: 15 mg/kg daily in two doses Duration: As above Study design details: Very few aggregate data presented, is a case series in effect |
Total numbers involved: Seven Treatment naive: NR for six patients Previous treatment: One patient previously treated with IFN HCV/HIV co-infection: NR Duration of infection: Unclear (4.5 years for two, 12.7 years for remaining five) Inclusion criteria: Aged between 8 and 16 years, both sexes, chronic HCV infection (positive antibodies with HCV RNA positivity and ALT/AST ≤ 1.5 times ULN), well compensated liver disease, normal levels for haemoglobin, platelets, white blood cells, glucose, serum creatinine, normal thyroid profile and negative autoantibodies (anti-smooth muscle, antinuclear and anti-LKM). No co-infection with any other hepatotrophic virus or HIV Exclusion criteria: Not stated Age (years): Mean not provided, ranged from 8 to 13, median 10 years (calculated by reviewer) Gender male, n (%): 5 (71) Weight/BMI: NR Ethnic groups, n (%): NR Mode of infection, n (%): Vertical, 1 (14); parenteral, 5 (author definition) (71); transfusion, 0 (unless included above); vertical and parenteral, 1 (14) Genotypes, n (%): 4a, 1 (14); 4b, 5 (71); not tested, 1 (14) Sample attrition/dropout: Not stated, assumed none |
Primary outcomes: Not stated as primary or secondary: SVR, EVR (not specifically defined), ETR, serum HCV RNA, biochemical response (ALT, AST), side effects, bilirubin, blood count Secondary outcomes: See above Length of follow-up: 12 months after stopping treatment Methods of assessing outcomes: Serum HCV RNA was determined by Amplicor PCR (Roche Diagnostics). Genotype subtypes determined by restriction fragment length polymorphism ETR was defined as undetectable HCV RNA at the end of treatment SVR was defined as undetectable HCV RNA that persists during the entire 12 months post therapy Histology: classification system used was a modified Knodell–Ishak score |
| Participant characteristics/outcomes | Baseline | Post treatment (week 52) |
|---|---|---|
| HCV RNA (IU/ml) | Median: 145,000 Range: 74,000–758,000 |
Low viraemia, n = 4 Det,a n = 3 |
| Serum ALT (IU/l) | Median: 77 Range: 52–223 |
Median: 39 Range: 17–63 |
| Serum AST (IU/l) | Median: 76 Range: 63–321 |
Median: 38 Range: 20–69 |
| Fibrosis score, mean ± SD | See note below for four participantsb | See note below for four participantsb |
| Necroinflammatory score, mean ± SD | NR | NR |
| Outcomes | Baseline | Post treatment | p-value |
|---|---|---|---|
| Viral response, % (n/N) | |||
| RVR (4 weeks) | NR | NR | NR |
| EVRa (12 weeks) | 28.6 (2/7) | ||
| ETR (52 weeks) | N/A | 42.9 (3/7) | N/A |
| SVR (end of follow-up) | N/A | 28.6 (2/7) | N/A |
| SVR subgroup datab | N/A | NR | |
| Other viral response outcomes | N/A | NR | |
| Non-response | N/A | NR | |
| Relapse | N/A | NR | |
| Quality-of-life outcomes | |||
| N/A | NR | ||
| AEs, % (n/N) | |||
| Dose discontinuation for any AE | N/A | NR | |
| Dose discontinuation for other reason | N/A | NR | |
| Dose reduction for any AE | N/A | 0 | |
| Dose reduction for anaemia | N/A | NR | |
| Dose reduction for neutropenia | N/A | NR | |
| Dose reduction for other reason | N/A | NR | |
| Specific AEs | N/A | ||
| Flu-like symptoms | 100 (7/7) | ||
| Excessive hair loss | 14 (1/7) | ||
| Mild reduction in blood counts | 14 (1/7) | ||
| Behavioural change | 14 (1/7) | ||
| Mortality, % (n/N) | |||
| N/A | NR | ||
| Effects on growth | |||
| N/A | NR | ||
Methodological comments
-
Allocation to treatment groups Not applicable.
-
Allocation concealment Not applicable.
-
Blinding Stated is an open-label study.
-
Analysis by intention to treat Not applicable.
-
Comparability of treatment groups at baseline Not applicable.
-
Method of data analysis No aggregate data presented and no statistical analyses undertaken.
-
Sample size/power analysis Not applicable.
-
Attrition/dropout Not reported.
General comments
-
Generalisability Minimal data provided on patient demographics; study undertaken in Egypt, which may limit generalisability to the UK.
-
Intercentre variability Not applicable.
-
Conflict of interests Stated none declared.
Quality criteria for assessment of uncontrolled, single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | N/A |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | NR |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
N/A (no analysis) |
Studies of peginterferon alfa-2b: Jara and colleagues (2008)48
| Reference and design | Intervention | Participants | Outcome measures |
|---|---|---|---|
| Author: Jara et al.48 Year: 2008 Study design: Single-cohort study (pilot study) Number of centres: One Country: Spain Funding: Not stated, but Schering-Plough helped design the study and provided the treatments free of charge |
Drug 1: PEG α-2b subcutaneously Dose: 1.0 µg/kg/week Duration: 24 weeks for genotypes 2 and 3, 48 weeks for genotypes 1 and 4 Drug 2: RBV Dose: 15 mg/kg/day orally in two divided doses Duration: As above Discontinuation: Treatment discontinuation was considered at week 24 if serum HCV RNA titers remained detectable. In practice, therapy was maintained even if no viral clearance, provided drugs were tolerated Adjustments: PEG α-2b dose transiently decreased in those with a neutrophil count < 1.25 × 109 cells/ml to avoid neutropenia. PEG α-2b temporarily discontinued if neutrophil counts fell below 1.00 × 109 cells/ml and resumed once neutrophil counts exceeded 1.00 × 109 cells/ml Concurrent treatment: Oral vitamin E supplements Study design details: Two subgroups according to genotype and therefore duration of treatment (as above) |
Total numbers involved: 30 (subgroup genotypes 1 and 4, n = 27; genotype 3, n = 3) Treatment naive: n = 24 (80%) Previous treatment: Six patients (20%) treated with IFN-α monotherapy 3–5 years earlier HCV/HIV co-infection: None Duration of infection: Not stated Inclusion criteria: Aged between 3 and 16 years with chronic hepatitis C, defined serum HCV RNA titers (> 50 IU/ml) for ≥ 3 years with continuous or intermittently elevated ALT values. Non-responders to IFN-α monotherapy eligible if they accounted for < 25% of the patient population Histology: Knodell score 3 (range 1–8)a Exclusion criteria: Neutropenia (< 1000 × 109 cells/l), anaemia (haemoglobin < 10 g/dl), thrombocytopenia (< 150 × 109 cells/l) or decompensated liver disease. HIV and non-HCV liver disease. Comorbid medical conditions (e.g. moderate or severe depression, psychiatric conditions, seizures, renal insufficiency) that could compromise tolerability of the study drugs. Those testing positive for autoimmunity markers (antinuclear antibody, smooth muscle antibody, liver kidney microsomal antibody type 1) enrolled if other features did not suggest autoimmune hepatitis Age (years), mean (range): 10 (3.5–16.0) Gender male, n (%): Not stated Weight, kg: Assumed median 36 (range 13–67) Ethnic groups, n (%): Not stated Mode of infection, n (%): Vertical, 21 (70); parenteral, 9 (30) Genotypes, n (%): 1, 26 (87); 2, 0; 3, 3 (10); 4, 1 (3) Sample attrition/dropout: Two discontinued in the 48-week treatment group before 24 weeks (one hyperthyroidism, one high-grade fever). Five discontinued between 24 and 48 weeks of treatment (two because of lack of response; two because of breakthrough; one because of hyperthyroidism) |
Primary outcomes: SVR Secondary outcomes: RVR (not specifically defined), EVR (not specifically defined), virological response, predictors for SVR (not data extracted except previous treatment status), safety (adverse events), QoL and growth (reported very briefly in text) Length of follow-up: Followed up every 12 weeks for at least 24 weeks after the end of treatment Methods of assessing outcomes: SVR defined as undetectable HCV RNA 24 weeks after treatment cessation HCV RNA titers were measured with use of a PCR assay every month during the first 24 weeks of therapy, at 4- to 12-week intervals until completion of therapy, at end of treatment and each follow-up visit. Qualitative (COBAS Amplicor HCV Monitor v2.0: lower limit of detection ≥ 50 IU/ml) and quantitative (COBAS Amplicor HCV Monitor v2.0: lower limit of detection ≥ 600 IU/ml) analyses performed Histology classification system used: Knodell scoring system Safety assessed by clinical visits and laboratory tests, weekly for 4 weeks, then monthly until week 24, then at 4- to 12-week intervals until completion of therapy. Hospital admission for 48 hours after first administration to monitor reactions to therapy |
| Participant characteristics/outcomes | Baseline | Post treatment |
|---|---|---|
| HCV RNA (IU/ml), log10 mean (range) | 5 (3–6)a | NR |
| Serum ALT (IU/l), mean (range) | 75 (29–232)b | NR |
| Serum AST (IU/l), mean (range) | 52 (24–157) | NR |
| Fibrosis score | ||
| < 4 | 58% | NR |
| 4–7 | 31% | NR |
| ≥ 8 | 10% | NR |
| Cirrhosis | 0% | NR |
| Necroinflammatory score, mean ± SD | NR | NR |
| Outcomes | Baseline | Post treatment |
|---|---|---|
| Viral response, % (n/N) | ||
| RVRa (4 weeks) | N/A | 3 (1/30) |
| EVRb (12 weeks) | N/A | 52 (15/29) |
| Virological responsec (week 24) | N/A | 64 (18/28) |
| SVR (end of follow-up)d | N/A | 50 (15/30) |
| SVR subgroup datae | ||
| SVR by genotype | N/A | |
| Genotype 3 | 100 (3/3) | |
| Genotype 1 | 46 (12/26) | |
| Genotype 4 | 0 (0/1) | |
| SVR by previous treatment (n = 26)f | N/A | |
| Treatment naive | 55 (11/20) | |
| Retreated (non-response/relapse) | 17 (1/6) | |
| Non-response | N/A | 47 (14/30) |
| Relapse | N/A | 3 (1/30) |
| Quality-of-life outcomes | ||
| NR | NR | |
| AEs, % (n/N) | ||
| Dose discontinuation for any AE | N/A | n = 3 (1 high grade fever, 2 hyperthyroidism) |
| Dose discontinuation for other reason | N/A | NR |
| Dose reduction for any AE | N/A | NR |
| Dose reduction for anaemia | N/A | 0 |
| Dose reduction for neutropenia | N/A | 23 (7/30) |
| Dose reduction for other reason | N/A | NR |
| Specific AEs, % (n/N) | ||
| Flu-like, drug related | ||
| Fever | N/A | 100 (30/30) |
| Fatigue | N/A | 73 (22/30) |
| Myalgia | N/A | 33 (10/30) |
| Abdominal pain | N/A | 43 (13/30) |
| Nausea and vomiting | N/A | 27 (8/30) |
| Headache | N/A | 67 (20/30) |
| Injection site | ||
| Erythema | N/A | 33 (10/30) |
| Gastrointestinal | ||
| Decreased appetite | N/A | 77 (23/30) |
| Constipation | N/A | 10 (3/30) |
| Weightg | ||
| Weight loss | N/A | 67 (20/30) |
| Weight loss > 5% baseline | N/A | 23 (7/30) |
| Behaviour/neurological | ||
| Irritability | N/A | 33 (10/30)h |
| Dizziness | N/A | 23 (7/30)i |
| Anxiety | N/A | 7 (2/30) |
| Hair, skin, mucosae | ||
| Sore mouth | N/A | 43 (13/30) |
| Hair loss | N/A | 10 (3/30) |
| Nose bleeding | N/A | 10 (3/30) |
| Dry skin | N/A | 10 (3/30) |
| Pruritus | N/A | 7 (2/30) |
| Endocrine | ||
| Antithyroid antibodies | N/A | 13 (4/30) |
| Hyperthyroidism | N/A | 7 (2/30) |
| Transient high TSH or T4 | N/A | 20 (6/30) |
| Infections | ||
| Upper respiratory tract | N/A | 53 (16/30) |
| Gastrointestinal | N/A | 30 (9/30) |
| Skin | N/A | 13 (4/30) |
| Mortality, % (n/N) | ||
| N/A | NR | |
| Effects on growth, during 48 weeks (n/N) | ||
| Reduced by 1.6 cm compared with growth velocity 50th percentile for age and sexj | N/A | 22/26 (three fully grown) |
Methodological comments
-
Allocation to treatment groups Not applicable.
-
Allocation concealment Not applicable.
-
Blinding Not applicable.
-
Analysis by intention to treat Not applicable.
-
Comparability of treatment groups at baseline Not applicable.
-
Method of data analysis Statistical analysis of relationships between patient baseline characteristics and SVR by Fisher’s exact tests, and patient baseline characteristics and responder status by Student’s t-test.
-
Sample size/power analysis Stated was a pilot study.
-
Attrition/dropout Numbers and reasons provided.
General comments
-
Generalisability Limited data available on patient demographics, and small sample size with wide range of ages and mixture of routes of infection. Likely to be most generalisable to genotype 1 population.
-
Intercentre variability Not applicable.
-
Conflict of interests Not stated.
Quality criteria for assessment of uncontrolled, single-cohort studies
| Quality criterion | Judgement |
|---|---|
| 1. Were the patient selection criteria specified a priori? | Yes |
| 2. Was the participant blinded to the research question? | N/A |
| 3. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Unclear |
| 4. Were withdrawals and dropouts completely described? | Yes |
| 5. (i) Did the analysis account for missing data? (ii) If so, were the methods appropriate? |
No |
Appendix 4 Table of excluded studies of clinical effectiveness
Retrieved references for screening that were excluded (n = 45)
| Reference | Full paper/abstract | Exclusion criterion |
|---|---|---|
| Abdel-Aziz DH, Sabry NA, El-Sayed MH, El-Gazayerly ON. Efficacy and safety of pegylated interferon in children and adolescents infected with chronic hepatitis C: a preliminary study. J Pharm Prac 2011;24:203–10 | Paper | Outcome |
| Adiv OE, Zion N, Shaoul R. Pegylated interferon and ribavirin treatment for children with hepatitis C. J Pediatr Gastroenterol Nutr 2010;50:E161 | Abstract | Study design (retrospective) |
| Akram M, Idrees M, Zafar S, Hussain A, Butt S, Afzal S, et al. Effects of host and virus related factors on interferon-alpha + ribavirin and pegylated-interferon + ribavirin treatment outcomes in chronic hepatitis C patients. Virol J 2011;8:234 | Paper | Population (age) |
| Baker RD, Dee D, Baker SS. Response to pegylated interferon alpha-2b and ribavirin in children with chronic hepatitis C. J Clin Gastroenterol 2007;41:111–14 | Paper | Population (age) |
| Carey I, Pariante C, Bansal S, Subramaniam P, Tizzard S, Vergani D, et al. Psychiatric side effects of antiviral therapy with pegylated interferon and ribavirin are associated with poor response in children with chronic hepatitis C. Gut 2010;59:A43–4 | Abstracta | Insufficient detail in abstract |
| Carey I, Tizzard S, Bansal S, Subramaniam P, Vergani D, Mieli-Vergani G. Response to pegylated interferon + ribavirin in children with chronic hepatitis C is associated with more severe haematological toxicity and fewer neuropsychiatric symptoms. J Pediatr Gastroenterol Nutr 2010;50(Suppl. 2):E35–6 | Abstracta | Insufficient detail in abstract |
| Carey I, Bansal S, Mendes A, Subramaniam P, Cebecauerova D, Vergani D, et al. Low pre-treatment numbers of CD4+/PD-1+ lymphocytes and low HCV-specific IL-10 production during therapy with pegylated-interferon + ribavirin predict response in children with chronic hepatitis C. J Pediatr Gastroenterol Nutr 2010;50(Suppl. 2):E17 | Abstracta | Insufficient detail in abstract |
| Carey I, Bruce MJ, Bansal S, Tizzard S, Mendes A, Joshi D, et al. Genetic, virological and immunological pre-treatment predictors of therapy response to Peg-IFN/ribavirin in children with chronic hepatitis C. Hepatology 2011;54:469A–70A | Abstract | Insufficient detail in abstract |
| Carey I, Mendes A, Cebecauerova D, Bansal S, Subramaniam P, Tizzard S, et al. Low pre-treatment numbers of CD4+/PD1+ lymphocytes and low HCV-specific IL-10 production during therapy with pegylated-interferon plus ribavirin predict response in children with chronic hepatitis C. J Hepatol 2010;52:S266 | Abstracta | Insufficient detail in abstract |
| Carey I, Mendes A, Cebecauerova D, Bansal S, Subramaniam P, Tizzard S, et al. Low NK cell number, low HCV-specific IL-10 production and high CD56(bright) cell number predict response to pegylated-interferon/ribavirin therapy in chronic hepatitis C in children. J Hepatol 2010;52:S176–7 | Abstracta | Insufficient detail in abstract |
| Carey I, Mendes A, Bansal S, Subramaniam P, Longhi MS, Cebecauerova D, et al. Sharp decrease in HCV-specific interferon-gamma and IL-10 production during antiviral therapy with pegylated interferon and ribavirin predict sustained virological response in children with chronic hepatitis C. Hepatology 2009;50:634A | Abstracta | Insufficient detail in abstract |
| Carey I, Cebecauerova D, Bansal S, Subramaniam P, Hussain MJ, Mytilinaiou M, et al. Response to pegylated interferon/ribavirin in chronic hepatitis C in children is predicted by pre-treatment number of activated natural killer (NK) cells. Hepatology 2008;48:321A | Abstract | Insufficient detail in abstract |
| Carey I, Mytilinaiou M, Hussain M, Bansal S, Subramaniam P, Horner M, et al. HCV-specific production of IL-10 and IFN-gamma-inducible protein-10 (IP-10) levels predict treatment response to pegylated interferon and ribavirin in children with chronic hepatitis C infection. Hepatology 2007;46:279A | Abstract | Insufficient detail in abstract |
| Dusheiko G, Danta M. Can Peg-IFN alpha-2a plus ribavirin be used to treat patients with chronic hepatitis C and normal alanine aminotransferase levels? Nature Clin Prac Gastroenterol Hepatol 2005;2:130–1 | Abstract | Population |
| Etani Y, Ida S. Peginterferon alpha-2a, ribavirin and fluvastatin combination therapy for chronic hepatitis C in children and adolescents. Gastroenterology 2011;5:S457 | Abstract | Insufficient detail in abstract |
| Fattovich G, Baroni GS, Pasino M, Pierantonelli I, Covolo L, Ieluzzi D, et al. Post-load insulin resistance does not predict virological response to treatment of chronic hepatitis C patients without the metabolic syndrome. Digest Liver Dis 2012;44:419–25 | Paper | Population |
| Fransen van de Putte DE, Fischer K, Posthouwer D, Mauser-Bunschoten EP. The burden of HCV treatment in patients with inherited bleeding disorders. Haemophilia 2011;17:791–9 | Paper | Population |
| Fung J, Lai C-L, Hung I, Young J, Cheng C, Wong D, et al. Chronic hepatitis C virus genotype 6 infection: Response to pegylated interferon and ribavirin. J Infect Dis 2008;198:808–12 | Paper | Population |
| Garcia-Algar O, Garriga L, Molera C. Sustained viral response hematological markers during the treatment of chronic hepatitis C infection in children. Hepatitis Month 2012;12:1–2 | Paper | Design |
| Gehring S, Kullmer U, Koeppelmann S, Gerner P, Wintermeyer P, Wirth S. Prevalence of autoantibodies and the risk of autoimmune thyroid disease in children with chronic hepatitis C virus infection treated with interferon-alpha. World J Gastroenterol 2006;12:5787–92 | Paper | Population |
| Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology 2008;47:836–43 | Paper | Intervention |
| Graham CS, Wells A, Liu T, Sherman KE, Peters M, Chung RT, et al. Relationships between cellular immune responses and treatment outcomes with interferon and ribavirin in HIV/hepatitis C virus co-infection. AIDS 2006;20:345–51 | Paper | Population |
| Gramenzi A, Cursaro C, Margotti M, Balsano C, Spaziani A, Anticoli S, et al. Ketoprofen, peginterferon 2a and ribavirin for genotype 1 chronic hepatitis C: a phase II study. World J Gastroenterol 2009;15:5946–52 | Paper | Population |
| Hierro CL, Alvarez L, Andueza S, Gordo-Giralt R, Lledin D, Camarena C, et al. Influence of IL28B gene polymorphisms on sustained response to peginterferon plus ribavirin in children with chronic hepatitis. J Hepatol 2011;54(Suppl. 1):S524–5 | Abstract | Insufficient detail in abstract |
| Hoofnagle JH. Peds-C. Hepatology 2005;41:421 | Paper (one page) | Outcome |
| Inati A, Taher A, Ghorra S, Koussa S, Taha M, Aoun E, et al. Efficacy and tolerability of peginterferon alpha-2a with or without ribavirin in thalassaemia major patients with chronic hepatitis C virus infection. Br J Haematol 2005;130:644–6 | Paper | Population |
| Inui A, Komatsu H, Sogo T, Hashimoto T, Fujisawa T. Pegylated interferon-alpha2b and ribavirin combination therapy for pediatric patients with chronic hepatitis C. Acta Hepatol Japonica 2008;49:386–8 | Paper | Language |
| Jenke AC, Moser S, Orth V, Zilbauer M, Gerner P, Wirth S. Mutation frequency of NS5A in patients vertically infected with HCV genotype 1 predicts sustained virological response to peginterferon alfa-2b and ribavirin combination therapy. J Viral Hepatitis 2009;16:853–9 | Paper | Population |
| Jonas MM, Balistreri W, Gonzalez-Peralta RP, Haber B, Lobritto S, Mohan P, et al. Pegylated interferon for chronic hepatitis C in children affects growth and body composition: results from the pediatric study of hepatitis C (PEDS-C) trial. Hepatology 2012;56:523–31 | Paper | Intervention |
| Kowala-Piaskowska A, Mozer-Lisewska I, Figlerowicz M, Sluzewski W. Adverse effects during the treatment with pegylated interferon and ribavirin in children with chronic hepatitis C. Pharmacoepidemiol Drug Saf 2007;16:1095–103 | Paper | Population |
| Kowala-Piaskowska A, Sluzewski W, Figlerowicz M, Mozer-Lisewska I. Early virological response in children with chronic hepatitis C treated with pegylated interferon and ribavirin. Infection 2007;35:175–9 | Paper | Intervention |
| Kowala-Piaskowska A, Mozer-Lisewska I, Januszkiewicz-Lewandowska D, Michalak M, Zeromski J, Madalinski K, et al. RNA-HCV viral load in serum, peripheral blood mononuclear cells and liver in children with chronic hepatitis C. Acta Pol Pharm 2012;69:859–63 | Paper | Intervention |
| Michielsen P, Bottieau E, Van Vlierberghe H, Van Marck E, Vandemaele E, Denys M, et al. Treatment of chronic hepatitis C in patients with human immunodeficiency virus (HIV) with weekly peginterferon alpha-2b plus ribavirin: a multi-centred Belgian study. Acta Gastroenterol Belg 2009;72:389–93 | Paper | Population |
| Moghaddam MA, Zali MR, Andabili SHA, Derakhshan F, Miri SM, Alavian SM. High rate of virological response to peginterferon alpha-2a-ribavirin among non-cirrhotic Iranian hemophilia patients with chronic hepatitis C. Iranian Red Crescent Med J 2012;14:2 | Paper | Population |
| Mohan N, Ganeja V, Kaul D, Khanna V. PEG interferon alpha 2a (40kD) plus ribavirin treatment in thalassemic children and adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 2007;44:144 | Abstract | Insufficient detail in abstract |
| Nelson DR, Zeuzem S, Andreone P, Ferenci P, Herring R, Jensen DM, et al. Balapiravir plus peginterferon alfa-2a (40KD)/ribavirin in a randomized trial of hepatitis C genotype 1 patients. Ann Hepatol 2012;11:15–31 | Paper | Population |
| Osaki Y, Ueda Y, Marusawa H, Nakajima J, Kimura T, Kita R, et al. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol 2012;47:444–51 | Paper | Population |
| Pawlowska M, Halota W. Pegylated interferon alpha-2a and ribavirin in the treatment of children with chronic hepatitis C. Gastroenterology 2009;136:A839 | Abstract | Insufficient detail in abstract |
| Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr 2009;48:341–7 | Paper | Population |
| Sluzewski W, Kowala-Piaskowska A, Wysocki J, Figlerowicz M, Gorczyca A, Halota W, et al. Treatment of chronic hepatitis C in children with pegylated interferon alpha2a and ribavirin – a multi-center study. Acta Pol Pharm 2012;69:319–26 | Paper | Population |
| Sokal E, Bourgois A, Stephenne X, Silveira T, Porta G, Gardovska D, et al. Multicenter trial of peginterferon alfa-2A and ribavirin for paediatric chronic hepatitis C. J Pediatr Gastroenterol Nutr 2009;48(Suppl. 3):E13 | Abstract | Insufficient detail in abstract |
| Tabatabaei SV, Alavian SM, Behnava B, Keshvari M, Miri SM, Karimi EP, et al. Anti-HCV treatment of thalassemia major adolescents with peginterferon alfa-2a and ribavirin. Hepatol Int 2011; Hepatology International conference (source unclear) | Abstract | Insufficient detail in abstract |
| Tabatabaei SV, Alavian SM, Keshvari M, Behnava B, Miri SM, Elizee PK, et al. Low dose ribavirin for treatment of HCV infected thalassemia major patients; new indications for combination therapy. Hepatitis Month 2012;12:372–81 | Paper | Population |
| Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology 2005;41:1013–18 | Paper | Population |
| Zhang H. Preliminary observational study on efficacy and tolerability of peg-IFN on 151 pediatric and adolescent chronic hepatitis C patients. Hepatology 2009;50(Suppl. 4):759A–60A | Abstract | Intervention |
Appendix 5 Table of excluded studies for systematic review of health-related quality of life
Adults
| Reference | Reason for exclusion |
|---|---|
| Scalone L, Ciampichini R, Fagiuoli S, Gardini I, Del PA, Gaeta L, et al. Testing the performance of the newly developed version of the EQ-5D with 5 levels of severity: Application on a cohort of patients with chronic hepatic diseases. Value Health 2010;13:A240 | Abstract |
| Fagiuoli S, Scalone L, Ciampichini R, Fusco F, Gaeta L, Del PA, et al. Societal burden in patients with chronic hepatic diseases: The come study results. J Hepatol 2012;56(Suppl. 2):S11–12 | Abstract |
| Fagiuoli S, Scalone L, Ciampichini R, Fusco F, Gaeta L, Del PA, et al. Costs and quality of life in patients with liver transplantation. Liver Transplant 2012;18:S262 | Abstract |
| Fagiuoli S, Scalone L, Ciampichini R, Fusco F, Gaeta L, Del PA, et al. Costs and quality of life in patients with chronic hepatic diseases: The COME study results. Digest Liver Dis 2012;56:S11 | Abstract |
| Bauch PM, Sterling RK, Clement LM, Velez FF. Current evidence regarding the hepatitis C patient experience. Value Health 2010;13:3–A75 | Abstract |
| John-Baptiste A, Tomlinson G, Hsu P, Krajden M, Heathcote J, Laporte A, et al. Quality of life following antiviral therapy for chronic hepatitis C virus infection. Can J Gastroenterol 2009 | Abstract |
| John-Baptiste AA, Tomlinson G, Hsu PC, Krajden M, Heathcote EJ, Laporte A, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol 2009;104:2439–48 | Inappropriate QoL measure |
| Younossi Z, Aggarwal J, Martin M, Hernandez N, Donepudi M, Bayliss M, et al. Health-related quality-of-life among genotype 1 treatment-naive chronic hepatitis C patients receiving telaprevir combination treatment: Post-hoc analyses of data from the advance trial. J Hepatol 2012;56:S462–3 | Abstract |
Children
| Reference | Reason for exclusion |
|---|---|
| Akobeng AK, Davison S. Quality of life of patients with chronic hepatitis C virus infection. J Pediatr Gastroenterol Nutr 2000;30:224–6 | Inappropriate QoL measure |
| Hamer C. The impact of combination therapy with peginterferon alpha-2a and ribavirin on the energy intake and body weight of adult hepatitis C patients. J Hum Nutr Diet 2008;2:486–93 | Inappropriate QoL measure |
| Iorio R, Pensati P, Botta S, Moschella S, Impagliazzo N, Vajro P, et al. Side effects of alpha-interferon therapy and impact on health-related quality of life in children with chronic viral hepatitis. Pediatr Infect Dis J 1997;16:984–90 | Inappropriate QoL measure |
| Rodrigue JR, Balistreri W, Haber B, Jonas M, Mohan P, Molleston JP, et al. Impact of hepatitis C virus (HCV) infection in children: Quality of life, emotional, and cognitive outcomes. Hepatology 2006;44:437A–8A | Abstract |
| Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Peginterferon with or without ribavirin has minimal effect on quality of life, behavioral/emotional, and cognitive outcomes in children. Hepatology 2011;53:1468–75 | Inappropriate QoL measure |
Appendix 6 Health-related quality-of-life studies: data extraction forms
Reference (lead author, year, reference ID)
Bjornsson 200970
Study characteristics
Research question
What are the stated objectives of the study?
To determine the HRQoL in patients with different stages of HCV and to compare HRQoL in HCV cirrhosis with non-HCV-induced cirrhosis.
Describe the type of study and study design.
Observational study comparing six cohorts with different stages of HCV or non-HCV cirrhosis. Four are relevant to this review. Also included data from healthy controls.
Was the sample from (i) the general population, (ii) patients with the disease of interest, (iii) individuals with knowledge of the disease, (iv) other?
HCV patients attending regular follow-up in outpatient clinics in different centres.
What are the characteristics of the baseline cohort for the evaluation?
| Indication/disease | Chronic hepatitis Ca | Compensated cirrhosisb | Decompensated cirrhosisc | SVRd |
|---|---|---|---|---|
| Number | 158 | 76 | 53 | 52 |
| Median age, years (IQR) | 46 (13) | 52 (11) | 55 (10) | 51 (14) |
| Sex (%) | Male 62; female 38 | Male 76; female 24 | Male 71; female 29 | Male 56; female 44 |
| All patients | ||||
| QoL instrument | EQ-5D, SF-36 | |||
| Utility values (Yes/No) | Yes | |||
| Treatment effect, if reported | N/A | |||
Country/setting
What is the country and setting for the evaluation?
Sweden, outpatient centres (16 clinics in nine centres).
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single observational study.
Results
Summarise the results.
EQ-5D index: HCV 0.811 (SD 0.230), compensated cirrhosis 0.749 (SD 0.212), decompensated cirrhosis 0.656 (SD 0.266), SVR 0.792 (SD 0.209), healthy controls 0.819 (SD 0.217). SF-36 scores presented but not extracted as not relevant to the present economic model.
Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Yes.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
N/A
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
Impairment in HRQoL in patients with HCV was associated with the severity of liver disease, patients with decompensated cirrhosis exhibiting the highest impairment in HRQoL.
What are the implications of the study for the model?
The data provide an alternative source for the HRQoL parameters in the model.
Reference (lead author, year, reference ID)
Chong 200371
Study characteristics
Research question
What are the stated objectives of the study?
To elicit utilities directly from those infected with HCV along the entire clinical spectrum of the disease (see below for details of categories).
Describe the type of study and study design.
Observational cohort study.
Was the sample from (i) the general population, (ii) patients with the disease of interest, (iii) individuals with knowledge of the disease, (iv) other?
People with HCV attending an outpatient clinic.
What are the characteristics of the baseline cohort for the evaluation?
| Disease stage | No biopsya | Mild/moderate HCVb | Compensated cirrhosisc | Decompensated cirrhosisd | HCCe | Transplant | SVRf |
|---|---|---|---|---|---|---|---|
| Number | 35 | 44 | 24 | 9 | 15 | 30 | 36 |
| Mean age, years (SE) | 47 (2.1) | 44 (1.5) | 57 (2.0) | 57 (3.9) | 63 (2.7) | 54 (1.7) | 48 (1.3) |
| Sex, n (%)g | Male: 18 (51) Female: 17 (49) |
Male: 32 (73) Female: 12 (27) |
Male: 7 (29) Female: 17 (71)h |
Male: 6 (67) Female: 3 (33) |
Male: 14 (93) Female: 1 (7) |
Male: 21 (70) Female: 9 (30) |
Male: 23 (64) Female: 13 (36) |
| Ethnicity white, n (%)g | 26 (74) | 37 (84) | 20 (83) | 5 (55) | 7 (47) | 24 (80) | 29 (81) |
| QoL instrument | VAS; SG; HUI; EQ-5D. Only EQ-5D data extracted here as of relevance to economic model | ||||||
| Utility values (Yes/No) | Yes | ||||||
| Treatment effect | N/A | ||||||
Country/setting
What is the country and setting for the evaluation? Canada, outpatient centres.
Data sources
Effectiveness
Were the QoL data derived from a single (observational) study, a review/synthesis or combination of previous studies, expert opinion?
Single observational study. In addition, a publication by Thompson Coon et al. 72 applied UK social preference weights to the individual patient data for the compensated cirrhosis, decompensated cirrhosis and HCC data from the Chong study (see below).
Results
Summarise the results.
| Study | Mean utility (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| No biopsy | Mild/moderate HCV | Compensated cirrhosis | Decompensated cirrhosis | HCC | Transplant | SVR | |
| Chong | 0.73 (0.62 to 0.83) | 0.76 (0.68 to 0.83) | 0.74 (0.66 to 0.83) | 0.66 (0.46 to 0.86) | 0.65 (0.44 to 0.86) | 0.69 (0.62 to 0.77) | 0.83 (0.77 to 0.90) |
| Thompson Coon | – | – | 0.75 (0.66 to 0.83) | 0.66 (0.46 to 0.86) | 0.64 (0.44 to 0.86) | – | – |
Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Yes.
Mapping
If a model was used, describe the type of model (e.g. regression) or other conversion algorithm.
N/A.
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
QoL differences across the HCV spectrum are small; however, this is significantly diminished when compared with population norms.
What are the implications of the study for the model?
Provide alternative utility scores.
Appendix 7 Cost-effectiveness data extraction forms for manufacturers’ submissions
This record was compiled by SHTAC following the format used by the NHS CRD Economic Evaluation Database.
Study characteristics
1 Reference (lead author, year, reference ID)
MSD 2012. 92
1.1 Health technology
Peginterferon alfa-2b and RBV.
1.2 Interventions and comparators
What interventions/strategies were included?
Peginterferon alfa-2a and peginterferon alfa-2b in combination with RBV.
Was a no treatment/supportive care strategy included?
Best supportive care.
Describe interventions/strategies.
Peginterferon alfa-2a (Pegasys®) and RBV (Copegus®) and peginterferon alfa-2b (ViraferonPeg®) and RBV (Rebetol®).
1.3 Research question
What are the stated objectives of the evaluation?
To estimate the cost-effectiveness of peginterferon (alfa-2a and -2b) in combination with RBV for the treatment of chronic HCV in children and young people aged 3–17 years, compared with supportive care.
1.4 Study type
Cost-effectiveness/cost–utility/cost–benefit analysis? Cost–utility analysis.
1.5 Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
A baseline population of children and young people aged 5–17 years with chronic HCV who were treatment naive and had no HIV co-infection. It also included an additional analysis for 3- to 4-year-olds.
1.6 Institutional setting
Where is/are the intervention(s) being evaluated usually provided?
NHS secondary care.
1.7 Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
UK, £ price year: 2010–11.
1.8 Funding source
MSD.
1.9 Analytical perspective
What is the perspective adopted for the evaluation [health service, health and personal social services, third-party payer, societal (i.e. including costs borne by individuals and lost productivity)]?
NHS and PSS.
2 Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
Effectiveness derived from a systematic review of the literature for the efficacy of peginterferon alfa and RBV, in terms of EVR and SVR. The review identified eight clinical trials in paediatric patients. 46,48,51,56,57,59,63,64 A meta-analysis was then conducted to synthesise the data by genotype.
| Genotype | Treatment | EVR | SVR | ||||
|---|---|---|---|---|---|---|---|
| Proportion | 95% CI | Distribution and parameters | Proportion | 95% CI | Distribution and parameters | ||
| 2/3 | PEG α-2a | N/A | N/A | N/A | 0.84 | 0.69 to 0.95 | Beta α = 24.82; β = 4.73 |
| PEG α-2b | N/A | N/A | N/A | 0.92 | 0.80 to 0.99 | Beta α = 27.90; β = 2.43 |
|
| 1/4 | PEG α-2a | 0.64 | 0.51 to 0.76 | Beta α = 35.61; β = 20.03 |
0.52 | 0.42 to 0.62 | Beta α = 49.34; β = 45.55 |
| PEG α-2b | 0.61 | 0.48 to 0.74 | Beta α = 32.38; β = 20.70 |
0.51 | 0.45 to 0.58 | Beta α = 115.37; β = 110.85 |
|
3 Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
The costs consisted of treatment-related costs, including drug acquisition costs, cost associated with treatment initiation and on-treatment and post-treatment monitoring, and health states costs. Costs associated with treating adverse events were not considered in the model as they were unlikely to be substantial.
Unit prices for peginterferon alfa-2b, peginterferon alfa-2a and RBV were obtained from BNF 63. The dosages used were 180 µg/1.73 m2 per week for peginterferon alfa-2a, 60 µg/m2 per week for peginterferon alfa-2b and 15 mg/kg for RBV. The treatment cost of a course of peginterferon alfa in combination with RBV was:
-
genotypes 2/3 (24-week treatment)
-
age 3–4 years: £2400.00 on peginterferon alfa-2b
-
age 5–8 years: £3326.20 on peginterferon alfa-2a; £3180.42 on peginterferon alfa-2b
-
age 9–13 years: £3628.06 on peginterferon alfa-2a; £4370.16 on peginterferon alfa-2b
-
age 14–17 years: £4558.02 on peginterferon alfa-2a; £4554.80 on peginterferon alfa-2b
-
-
genotypes 1/4 (48-week treatment)
-
age 3–4 years: £4800.00 on peginterferon alfa-2b
-
age 5–8 years: £6652.40 on peginterferon alfa-2a; £6360.84 on peginterferon alfa-2b
-
age 9–13 years: £7256.12 on peginterferon alfa-2a; £8740.32 on peginterferon alfa-2b
-
age 14–17 years: £9116.03 on peginterferon alfa-2a; £9109.59 on peginterferon alfa-2b.
-
Patients incur costs associated with treatment initiation, and on-treatment and post-treatment monitoring. Costs were based upon a previous NICE technology assessment33,34 with adjustment to reflect the experience of a child or young person with HCV as advised by experts. Prices were inflated from 2003–4 to 2010–11 using the HCHS index.
| Time period | Parameter | Cost (by age group) | Notes |
|---|---|---|---|
| Treatment initiation | Initial evaluation | £355.23 | Applied to all patients |
| Investigation for treatment | 3–13 years: £775.72 14–17 years: £778.79 |
Applied to all treated patients (on PEG α) | |
| On-treatment monitoring | 12-week treatment | 3–13 years: £1328.20 14–17 years: £1340.48 |
Applied to all patients with HCV of genotype 1/4 who have not achieved an EVR (on PEG α) |
| 24-week treatment | 3–13 years: £1999.90 14–17 years: £2021.38 |
Applied to all patients with genotype 2/3 HCV (on PEG α) | |
| 48-week treatment | 3–13 years: £3329.38 14–17 years: £3357.00 |
Applied to all patients with HCV of genotype 1/4 who completed the treatment course (on PEG α) | |
| Post-treatment monitoring | Monitoring up to 24 weeks after treatment | 3–13 years: £249.51 14–17 years: £258.72 |
Applied to all patients who were treated (and who have achieved an EVR if genotype 1/4) and who have not progressed to HCC |
| Annual surveillance | £191.11/year | Applied to those who achieved a SVR and who have not progressed to HCC; the costs were applied from the year after treatment for 5 years for patients who had mild/moderate HCV and lifetime for patients who had cirrhotic HCV |
Health state costs were used from the previous NICE technology assessment of the treatment of chronic HCV in adults. 33,34 Health state costs were inflated to 2010–11 using the HCHS index.
| Health state | Annual costs (£, 2010–11) | 95% CI (£) | Distribution and parameters |
|---|---|---|---|
| SVR from mild or moderate HCV | 132.18 | 99 to 165 | N/A |
| SVR from compensated cirrhosis | 191.11 | 143 to 239 | N/A |
| Mild HCV | 178 | 109 to 247 | Gamma k = 25.70; θ = 5.37 |
| Moderate HCV | 926 | 733 to 1119 | Gamma k = 88.85; θ = 8.07 |
| Compensated cirrhosis | 1469 | 884 to 2054 | Gamma k = 24.23; θ = 46.96 |
| DC | 11,775 | 7930 to 15,620 | Gamma k = 36.03; θ = 253.13 |
| HCC | 10,492 | 5659 to 15,325 | Gamma k = 18.11; θ = 448.80 |
| Liver transplant | 47,495 | 33,748 to 61,242 | Gamma k = 89.75/13.78; θ = 304.50/686.42 |
| Post-liver transplant | 1788 | 890 to 2686 | Gamma k = 15.22; θ = 91.01 |
3.1 Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
None included.
4 Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
A systematic literature review on the HRQoL of children and young people with HCV identified six studies; however, none of these were appropriate to be used in the analysis. Adult values were identified as the most appropriate estimates. The utility weights were obtained from published NICE technology appraisals. 33,34
4.1 List the utility values used in the evaluation
| Health state | Utility weight | 95% CI | Distribution and parameters |
|---|---|---|---|
| Mild HCV | 0.77 | 0.74 to 0.80 | Beta α = 521.24; β = 155.9 |
| Moderate HCV | 0.66 | 0.60 to 0.72 | Beta α = 168.25; β = 86.67 |
| Compensated cirrhosis | 0.55 | 0.45 to 0.65 | Beta α = 47.10; β = 38.54 |
| SVR from mild HCV | 0.82 | 0.74 to 0.90 | Beta α = 65.87; β = 14.46 |
| SVR from moderate HCV | 0.72 | 0.62 to 0.82 | Beta α = 58.06; β = 22.58 |
| SVR from compensated cirrhosis | 0.61 | 0.51 to 0.71 | Beta α = 58.05; β = 37.11 |
| DC | 0.45 | 0.39 to 0.51 | Beta α = 123.75; β = 151.25 |
| HCC | 0.45 | 0.39 to 0.51 | Beta α = 123.75; β = 151.25 |
| Liver transplant | 0.45 | 0.39 to 0.51 | Beta α = 123.75; β = 151.25 |
| Post liver transplant | 0.67 | 0.57 to 0.77 | Beta α = 59.25; β = 29.19 |
| Disutility due to adverse events | 0.11 | N/A | N/A |
5 Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? List them if reported.
A state transition Markov model was developed based on the model structure used in the economic evaluation for TA200. 20,41 The model includes seven non-absorbing health states and an absorbing death state as shown in the figure:
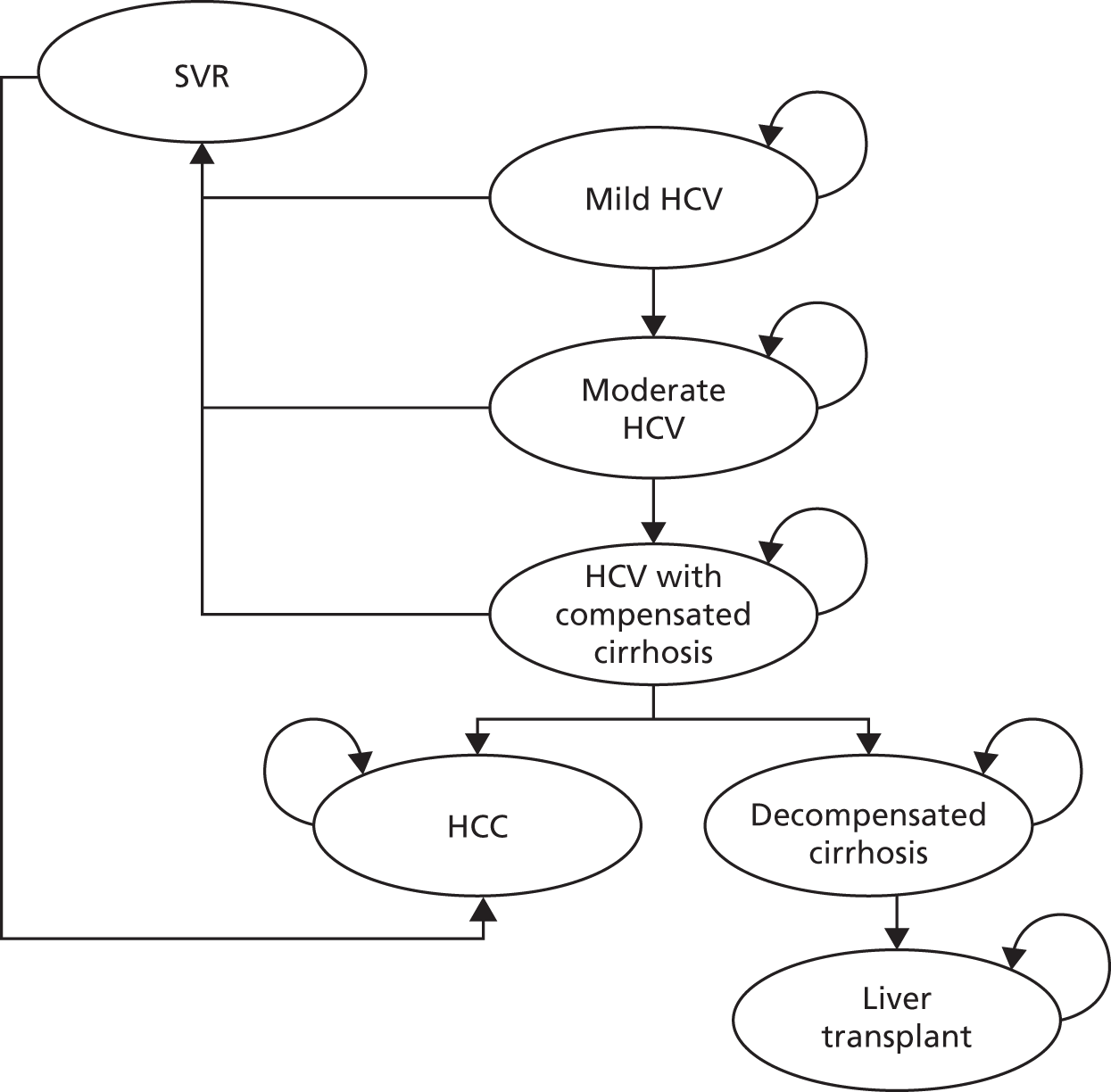
Patients enter the model with mild HCV, moderate HCV or compensated cirrhosis and are eligible to receive treatment in cycle 1. The cycle length of the model was 1 year, except for the first year. In the first year patients receive treatment for either 12, 24 or 48 weeks depending on the futility rule and genotype. For genotype 2 and 3 patients, the first year was split into two cycles:
-
The first 24 weeks, during which all patients receive treatment.
-
The following weeks until the end of the year, when patients who do not achieve SVR stay in the same HCV state or progress to a more severe disease state. Those who achieve SVR move to the corresponding SVR state.
For genotypes 1 and 4, the first year was split into three cycles:
-
The first 12 weeks, during which patients are assessed for the futility rule (EVR). Patients terminate treatment if they do not achieve EVR.
-
The following 36 weeks, when patients who did achieve EVR remain on treatment. Those who did not achieve EVR remain in the same HCV state or progress to a more severe disease state.
-
The following weeks until the end of the year, when patients who do not achieve SVR stay in the same HCV state or progress to a more severe disease state. Those who achieve SVR move to the corresponding SVR state.
Patients with SVR are considered to be ‘cured’ and at no further risk of more severe disease, except for those in the state SVR with compensated cirrhosis, who have an excess risk of developing HCC.
The mortality rates from all three SVR health states – mild HCV, moderate HCV and compensated cirrhosis – are assumed to be the same as for the general population. Patients with decompensated cirrhosis, HCC and those who receive a liver transplant face a higher risk of mortality than the general population.
Liver transplant is also split into two states: ‘liver transplant’ and ‘post liver transplant’. Patients remain in the liver transplant state for one cycle then transition to the post-liver transplant state where they either remain or transition to the death state.
In line with previous economic evaluations, the following assumptions were made:
-
the model did not account for reinfection and onward transmission of HCV
-
the possibility of HCC patients receiving a liver transplant was not considered because of its rarity.
A systematic review was undertaken for the natural history of HCV in children and young people. Data were extracted from the seven studies12,75,91,93–96 identified and then pooled to give an estimate for the annual transition probability between mild HCV and moderate HCV, and moderate HCV and compensated cirrhosis. The transition probabilities used in the model for all other transitions were the same as for the previous NICE appraisals. 33,34
5.1 Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text)
| Health state | Transition probability | 95% CI | Distribution and parameters | |
|---|---|---|---|---|
| From | To | |||
| SVR (CC) | SVR (CC) | # | # | # |
| HCC | 0.014 | 0.0000 to 0.0335 | Beta α = 1.93; β = 136.11 | |
| Mild HCV | Mild HCV | # | # | # |
| Moderate HCV | 0.0257a/0.025b | 0.0187 to 0.0348 | Beta α = 38.12; β = 1445.26 | |
| Moderate HCV | Moderate HCV | # | # | # |
| CC | 0.0038a/0.037b | 0.0018 to 0.0079 | Beta α = 5.94; β = 1556.36 | |
| CC | CC | # | # | # |
| DC | 0.039 | 0.0194 to 0.0586 | Beta α = 14.62; β = 360.17 | |
| HCC | 0.014 | 0.0000 to 0.0335 | Beta α = 1.93; β = 136.11 | |
| DC | DC | # | # | # |
| HCC | 0.014 | 0.0000 to 0.0335 | Beta α = 1.93; β = 136.11 | |
| Liver transplant | 0.020 | 0.0182 to 0.0418 | Beta α = 10.87; β = 532.58 | |
| Deathc | 0.130 | 0.1104 to 0.1496 | Beta α = 147.03; β = 983.97 | |
| HCC | HCC | # | # | # |
| Deathc | 0.430 | 0.3713 to 0.4887 | Beta α = 117.10; β = 155.23 | |
| Liver transplant | Liver transplant | # | # | # |
| Deathc | Year 1: 0.150; year 2+: 0.057 | Year 1: 0.1218 to 0.2982; year 2+: 0.0344 to 0.0796 | Year 1: beta α = 9.29; β = 52.67 Year 2+: beta α = 22.90; β = 378.88 |
|
5.2 What is the model time horizon?
Lifetime (until 100 years of age).
5.3 What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
An annual discount rate of 3.5% was applied to future costs and health outcomes.
5.4 List assumptions used in the model
-
The base-case analysis did not take into account spontaneous viral clearance.
-
The model did not account for reinfection and onward transmission of HCV.
-
The possibility of HCC patients receiving a liver transplant was not considered because of its rarity.
-
It was assumed that the treatment would discontinue if an EVR (i.e. undetectable HCV-RNA at treatment week 12) was not achieved at week 12.
-
Discontinuation due to adverse events was not accounted for as it is considered rare.
-
It was assumed that patients not achieving a SVR could experience disease progression from treatment initiation (genotypes 2 and 3) or from EVR (at week 12, genotypes 1 and 4).
-
Adult transition probabilities (except for the transitions from mild to moderate and from moderate to compensated cirrhosis), utility weights and health state costs (except for SVR state costs) were applied to paediatric patients owing to the lack of data.
-
Costs associated with the management of adverse events were not accounted for as they were unlikely to be substantial.
6 Results/analysis
What measure(s) of benefit were reported in the evaluation?
Cost per QALY gained. Results were presented for the whole group and also by age group and genotype.
6.1 Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
| Intervention | Total QALYs | ||
|---|---|---|---|
| All patients (5–17 years) | Genotype 2/3 | Genotype 1/4 | |
| Supportive care | 16.77 | 16.77 | 16.77 |
| PEG α-2a | 19.16 | 20.02 | 18.73 |
| PEG α-2b | 19.24 | 20.33 | 18.7 |
6.2 Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
| Intervention | Total costs (£) | ||
|---|---|---|---|
| All patients (5–17 years) | Genotype 2/3 | Genotype 1/4 | |
| Supportive care | 22,750 | 22,750 | 22,750 |
| PEG α-2a | 17,798 | 11,837 | 20,778 |
| PEG α-2b | 17,526 | 10,385 | 21,097 |
6.3 Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
| For all patients (5–17 years) | Vs. supportive care | |||
|---|---|---|---|---|
| Incremental costs | Incremental LYG | Incremental QALYs | ICER | |
| Supportive care | – | – | – | – |
| PEG α-2a | –£4952 | 7.69 | 2.39 | Dominates |
| PEG α-2b | –£5224 | 7.94 | 2.47 | Dominates |
| For all patients (5–17 years) | PEG α-2a vs. PEG α-2b | |||
|---|---|---|---|---|
| Incremental costs | Incremental LYG | Incremental QALYs | ICER | |
| PEG α-2a | – | – | – | – |
| PEG α-2b | –£271 | 0.24 | 0.08 | Dominates |
6.4 Give results of any statistical analysis of the results of the evaluation
None.
6.5 Was any sensitivity analysis performed? If yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]?
Deterministic and probabilistic sensitivity analyses.
6.6 What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
For DSA the following scenarios were tested: spontaneous viral clearance, time horizon, efficacy of peginterferon alfa-2a and -2b, transition probabilities, costs associated with treatment initiation, monitoring and health state costs, health state utility weights and disutility due to treatment, discount rates.
For PSA, the efficacy of peginterferon alfa-2a and -2b, transition probabilities, health states costs and health state utilities were included.
6.7 Give a summary of the results of the sensitivity analysis – did they differ substantially from the base-case analysis? If so, what were the suggested causes?
The DSA presented results for peginterferon alfa-2b versus peginterferon alfa-2a and versus BSC.
The DSA results showed that peginterferon alfa-2b dominated BSC in nearly all base-case analyses, except for time horizon and discount rates. The ICERs for peginterferon alfa-2b versus peginterferon alfa-2a were robust to variation in the model parameters, i.e. peginterferon alfa-2b dominated peginterferon alfa-2a for all analyses.
The results of all PSA showed that there is 100% certainty that peginterferon alfa-2a and peginterferon alfa-2b in combination with RBV are cost-effective compared with BSC.
7 Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
Peginterferon alfa-2a and -2b in combination with RBV are cost-effective treatment options for children and young people (aged 5–17 years) with HCV, compared with supportive care.
8 Southampton Health Technology Assessments Centre commentary
Selection of comparators: Appropriate.
Validity of estimate of measure of benefit:
Appropriate, based upon previous HTA reports.
Validity of estimate of costs:
Appropriate, based upon previous HTA reports.
This record was compiled by SHTAC following the format used by the NHS CRD Economic Evaluation Database.
Study characteristics
1 Reference (lead author, year, reference ID)
Roche 2012. 97
1.1 Health technology
Peginterferon alfa-2a and RBV.
1.2 Interventions and comparators
What interventions/strategies were included?
Peginterferon alfa-2a and RBV.
Was a no treatment/supportive care strategy included?
Best supportive care.
Describe interventions/strategies.
Peginterferon alfa-2a (Pegasys®) and RBV (Copegus®). Doses were 180 µg/1.73 m2 BSA subcutaneously once weekly for peginterferon and 15 mg/kg orally twice daily for RBV.
1.3 Research question
What are the stated objectives of the evaluation?
To estimate the cost-effectiveness of peginterferon in combination with RBV for the treatment of children and young people with HCV, compared with supportive care.
1.4 Study type
Cost-effectiveness/cost–utility/cost–benefit analysis?
Cost–utility analysis.
1.5 Study population
What definition was used for [condition]? What are the characteristics of the baseline cohort for the evaluation?
A baseline population of children aged 11 years with HCV, who were treatment naive and had no HIV co-infection. The proportion of patients that enter with mild and moderate disease is based upon a weighted average from the four named clinical trials.
1.6 Institutional setting
Where is/are the intervention(s) being evaluated usually provided?
NHS secondary care.
1.7 Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
UK, £, price year 2010–11.
1.8 Funding source
Roche.
1.9 Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third-party payer, societal [i.e. including costs borne by individuals and lost productivity])?
NHS and PSS.
2 Effectiveness
Were the effectiveness data derived from a single study, a review/synthesis of previous studies or expert opinion? Give the definition of treatment effect used in the evaluation. Give the size of the treatment effect used in the evaluation.
Treatment efficacy was estimated for SVR as a weighted average of the four clinical trials for peginterferon alfa-2a.
| Study | Genotypes 1/4/5/6 | Genotypes 2/3 (48 weeks of treatment) | Genotypes 2/3 (24 weeks of treatment) | ||
|---|---|---|---|---|---|
| SVR (%) | Dropout (%) | SVR (%) | Dropout (%) | SVR (%) | |
| Schwarz et al.56 | 47 | 29 | 80 | 10 | – |
| Sokal et al.57 | 57 | 17 | – | – | 89 |
| Sluzewski et al.65 | 78 | NR | – | – | 75 |
| Abdel-Hady et al.66 | 56 | NR | – | – | 90 |
| Weighted average | 59 | 23 | 80 | 10 | 89 |
Spontaneous SVR was included for the no treatment group based upon a rapid review of the literature by the manufacturer. The risk of spontaneous SVR differs depending on how the infection was acquired: through vertical transmission (VT) at birth or other means (non-VT) during infancy or childhood. The annual probability of spontaneous SVR was 1.65% for non-VT and 2.37% for VT, according to evidence from the European Paediatric Hepatitis C Virus Network (EPHCVN). Spontaneous SVR among non-VT occurs during the first 5 years of infection.
3 Intervention costs
Were the cost data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)? List the direct intervention costs and other direct costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used.
The costs consisted of treatment-related costs, including drug acquisition costs, costs associated with treatment initiation and on-treatment and post-treatment monitoring, and health states costs.
Unit prices for the treatments were obtained from BNF 63. The doses used in the analysis were in line with the dosing schedule in the relevant clinical trials. BSA was estimated using the Dubois formula. The MS estimated doses for different patient ages, and hence costs for these cohorts (see MS table 16).
Drug costs for peginterferon alfa-2a were calculated for a dosage of 180 µg/1.73 m2 BSA (maximum 180 µg) subcutaneously once weekly. RBV (as Copegus®) was administered in a dose of 15 mg/kg orally twice daily (maximum 1200 mg/day if ≥ 75 kg and 1000 mg if < 75 kg).
No syringe sharing was assumed in the model, and therefore wastage was included in the calculation of cost. For all treatments, the most efficient vial/syringe to deliver the dose was assumed (i.e. that which produced the least wastage). In other words, if the dose for peginterferon alfa-2a was estimated to be 125 µg, then one 135-µg pre-filled syringe was used. Similarly, if the dose was estimated to be 137 µg, then the next larger syringe (180 µg) was used.
In the base case, the estimated costs for 48 weeks of combination therapy are £8307.
| Cohort age (years) | Weekly dose, PEG α-2a (µg) | Daily dose, RBV (mg) | Weekly cost, PEG α-2a + RBV (£) |
|---|---|---|---|
| 5 | 83.27 | 311.13 | 95.82 |
| 6 | 91.42 | 352.30 | 134.92 |
| 7 | 100.69 | 404.00 | 138.90 |
| 8 | 107.53 | 440.19 | 141.69 |
| 9 | 117.29 | 498.96 | 146.22 |
| 10 | 125.71 | 550.11 | 150.16 |
| 11a | 137.42 | 631.27 | 173.06 |
| 12 | 150.58 | 728.37 | 180.54 |
| 13 | 159.56 | 787.98 | 185.14 |
| 14 | 171.29 | 880.58 | 192.28 |
| 15 | 176.45 | 925.58 | 195.75 |
| 16–24 | 180 | 1000 | 201.48 |
The economic model incorporates a costing protocol developed as part of a previously developed HTA report to estimate the appropriate evaluation, monitoring and surveillance cost. They inflated costs to 2010–11 values using the HCHS Pay and Prices Index. 73
In total, treatment monitoring costs were £564 for 24 weeks of treatment and £811 for 48 weeks of treatment.
Health state costs were used from the previous NICE technology assessments of the treatment of HCV in adults. 20,41 Health state costs were inflated to 2010–11 values using the HCHS index. 73
| Health state | Annual costs(£, 2010–11) |
|---|---|
| SVR | 0 |
| Mild HCV | 178 |
| Moderate HCV | 926 |
| Compensated cirrhosis | 1470 |
| DC | 11,780 |
| HCC | 10,496 |
| Liver transplant | 47,513 |
| Post liver transplant | 1789 |
3.1 Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
None included.
4 Health state valuations/utilities (if study uses quality-of-life adjustments to outcomes)
Were the utility data derived from a single (observational) study, a review/synthesis of previous studies or expert opinion? Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
A systematic literature review on the HRQoL of children and young people with HCV identified two partially applicable studies reporting utilites of children with chronic HCV, but both were based on an expert’s TTO values for adults with chronic HCV. Adult values were identified as the most appropriate estimates. The utility weights were obtained from published NICE technology appraisals. 20,41
Health state utilities were estimated in a stepwise fashion:
-
Baseline utilities for the general population were estimated.
-
A utility multiplier was derived by comparing the health state utility from the literature to the utility of the general population with the same age and gender composition.
-
Utility multipliers (from step 2) were applied to baseline utilities (from step 1) corresponding to the model cohort age and gender composition.
For children under the age of 17 years the economic model applied a baseline utility of 0.95, based on a study by Saigal and colleagues. 76 For the healthy population aged 17 years and above, they applied the utilities of adults derived using a model developed by Ara and Brazier. 77
4.1 List the utility values used in the evaluation
| Health state | Mean utility | Mean age (years) | % male | Source |
|---|---|---|---|---|
| Healthy children (≤ 16 years old) | 0.95 | – | – | Saigal et al.76 |
| SVR after mild disease | 0.83 | 39.8 | 52 | Wright et al.82 |
| Treatment for mild disease | 0.66 | |||
| Mild disease | 0.77 | |||
| Treatment for moderate disease | 0.55 | |||
| Moderate disease | 0.66 | |||
| Cirrhosis | 0.55 | |||
| Decompensated cirrhosis and HCC | 0.45 | |||
| Post liver transplantation | 0.67 |
5 Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original. What was the purpose of the model (i.e. why was a model required in this evaluation)? What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? List them if reported.
A state transition Markov model was developed based on the model structure used in the economic evaluation for TA200. 34,40 The model includes seven non-absorbing health states and an absorbing death state, as shown in the figure:
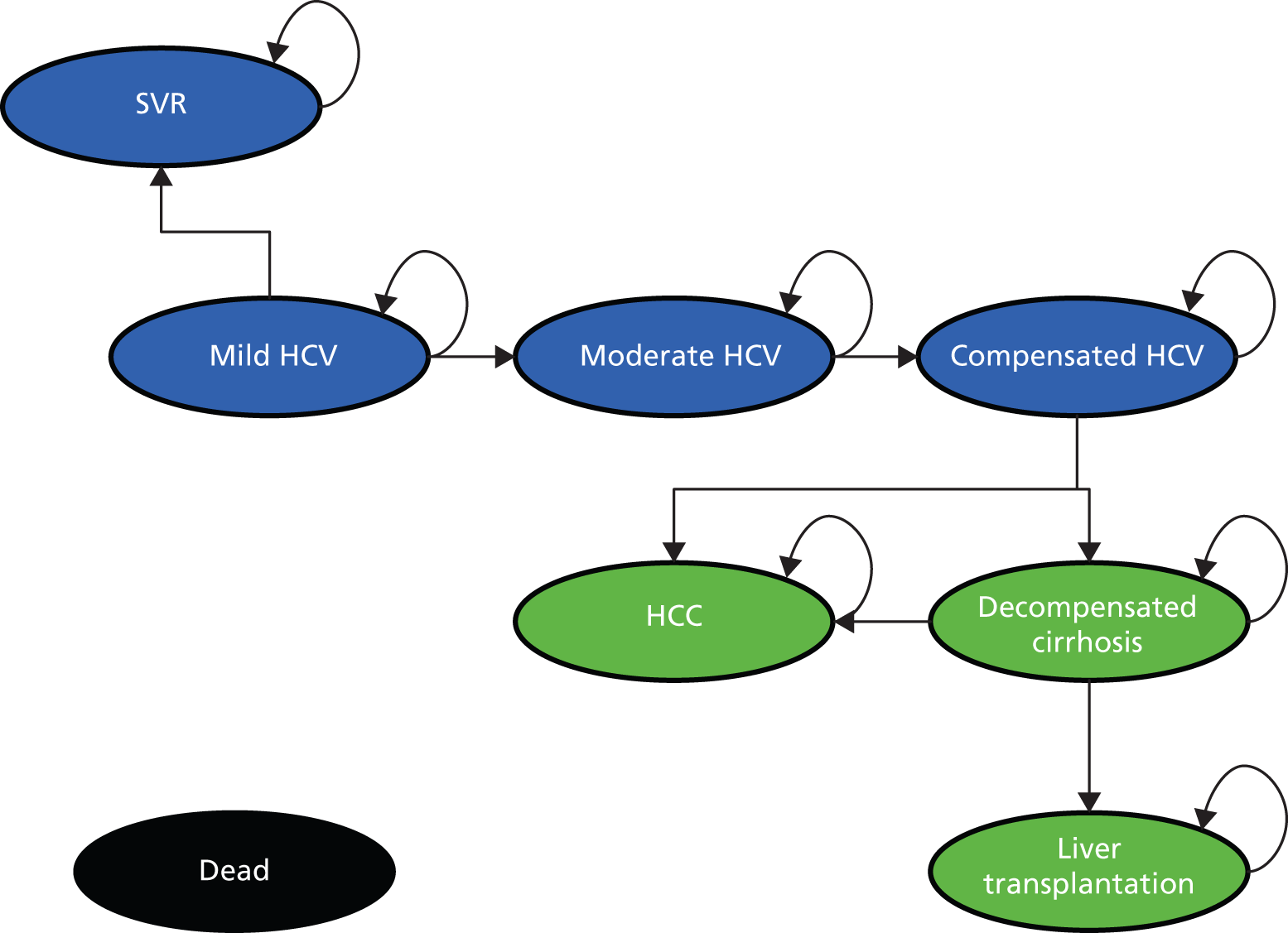
In the model, SVR is assumed to be a permanent condition (i.e. cure), with no spontaneous reactivation of HCV infection. The diagram indicates that in the absence of successful treatment or spontaneous clearance, patients with HCV may remain in their current health state or move on to increasingly severe stages of liver disease (decompensated cirrhosis, HCC and liver transplantation). Transitions to death can happen from any health state. Individuals in the blue health states (SVR, mild and moderate fibrosis and compensated cirrhosis) are assumed to face the same mortality risks as the general population; individuals in the green health states (decompensated cirrhosis, HCC and liver transplantation) face excess mortality risks attributable to chronic liver disease.
5.1 Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text)
| Parameters | Annual probability | Source |
|---|---|---|
| Spontaneous SVR: VT | 0.237 | European Paediatric Hepatitis C Virus Network 200598 |
| Spontaneous SVR: non-VT | 0.016 | Literature review |
| Mild to moderate disease | 0.014 | Guido et al.75 |
| Moderate disease to cirrhosis | 0.021 | Estimation based on Guido et al.75 and Wright et al.82 |
| Cirrhosis to decompensated cirrhosis | 0.040 | Wright et al.82 |
| Cirrhosis or decompensated cirrhosis to HCC | 0.014 | Wright et al.82 |
| Decompensated cirrhosis to death | 0.130 | Wright et al.82 |
| HCC to death | 0.430 | Wright et al.82 |
| Decompensated cirrhosis to liver transplant | 0.020 | Wright et al.82 |
| Liver transplant to death (year 1) | 0.160 | Barshes et al.99 |
| Liver transplant to death (subsequent years) | 0.038 | Barshes et al.99 |
| All-cause death | Time dependent | ONS 2011: UK life table100 |
5.2 What is the model time horizon?
Thirty years, which was considered long enough to capture important costs and effects arising from treatment.
5.3 What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
An annual discount rate of 3.5% was applied to future costs and benefits.
5.4 List assumptions used in the model
-
Efficacy and discontinuation data for patients with moderate HCV at baseline were not available from the literature; therefore, it was assumed that stage of fibrosis would not have an impact on the probability of SVR.
-
It was assumed that these adverse events may have an impact on HRQoL, but were unlikely to require additional resource use.
-
Based on interviews with clinical experts during the development of the economic model, no HCV-related costs were assumed to accrue to patients achieving SVR.
6 Results/analysis
What measure(s) of benefit were reported in the evaluation?
Cost per QALY gained. Results presented by genotype.
6.1 Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
Treating genotype 1, 4 and 5 patients with peginterferon alfa-2a and RBV improved outcomes by 1.01 QALYs compared with BSC. For genotypes 2 and 3, treatment for 24 weeks improved QALYs by 1.57 compared with BSC.
6.2 Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
Treating genotype 1, 4 and 5 patients with peginterferon alfa-2a and RBV cost an additional £3971 compared with BSC. For genotypes 2 and 3, treatment for 24 weeks cost £1834 less than BSC.
6.3 Synthesis of costs and benefits: are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
| Treatment | Cost (£) | Outcome (life-years) | Outcome (QALYs) | ICER (£/QALY gained) |
|---|---|---|---|---|
| Genotypes 1, 4 and 5 | ||||
| No treatment | 8199 | 18.47 | 14.20 | |
| PEG α-2a + RBV | 12,170 | 18.56 | 15.21 | |
| Incremental | 3971 | 0.09 | 1.01 | 3915 |
| Genotypes 2 and 3 | ||||
| No treatment | 8199 | 18.47 | 14.20 | |
| PEG α-2a + RBV, 24 weeks | 6336 | 18.61 | 15.77 | |
| PEG α-2a + RBV, 48 weeks | 11,010 | 18.60 | 15.61 | |
| Incrementals | ||||
| PEG α-2a + RBV, 24 weeks vs. no treatment | –1864 | 0.14 | 1.7 | Dominates no treatment |
| PEG α-2a + RBV, 24 weeks vs. 48 weeks | 4675 | –0.01 | –0.16 | Dominated by 24 weeks |
6.4 Give results of any statistical analysis of the results of the evaluation
None.
6.5 Was any sensitivity analysis performed? If yes, what type(s) [i.e. deterministic (one-way, two-way, etc.) or probabilistic]?
Deterministic and probabilistic sensitivity analyses.
6.6 What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
The following sensitivity analyses were performed: likelihood of spontaneous viral clearance, time horizon, discounting, age at entry to the model, distribution of fibrosis at entry to the model, rate of disease progression from mild to moderate fibrosis to compensated cirrhosis, probability of SVR with treatment, health state costs, health state utilities, timing of treatment.
6.7 Give a summary of the results of the sensitivity analysis – did they differ substantially from the base-case analysis? If so, what were the suggested causes?
The cost-effectiveness of peginterferon alfa-2a remains < £10,000 per QALY for all analyses, except for the use of a time horizon of 15 years (ICER of £12,010 per QALY for genotypes 1, 4 and 5). Model results are most sensitive to time horizon, the rate of disease progression, probability of SVR with treatment, the distribution of SVR with treatment, distribution of patients across liver disease stages at entry to the model and annual cost of achieving SVR.
In the PSA, for patients with genotypes 2 and 3 there is a 97.2% probability of 24 weeks of combination therapy being cost-effective compared with no treatment at a WTP threshold of £20,000 per QALY. In patients with genotypes 1, 4 and 5 there is a 91.6% probability of being cost-effective at a £20,000 per QALY threshold.
7 Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis.
Peginterferon alfa-2a in combination with RBV is a cost-effective treatment option for children and young people with HCV compared with best supportive care.
8 Southampton Health Technology Assessments Centre’s commentary
Selection of comparators:
Appropriate.
Validity of estimate of measure of benefit:
Appropriate, based upon previous HTA reports.
Validity of estimate of costs:
Appropriate, based upon previous HTA reports.
Appendix 8 Critical appraisal checklist of economic evaluation
The Roche and MSD MSs were appraised for methodological quality and generalisability to the UK NHS using a checklist adapted from the NICE reference case requirements,69 and the Philips and colleagues’ checklist. 45
| Item | MSD | Roche |
|---|---|---|
| 1. Is there a clear statement of the decision problem? | Yes | Yes |
| 2. Is the comparator routinely used in the UK NHS? | Yes | Yes |
| 3. Is the patient group in the study similar to those of interest in UK NHS? | Yes | Yes |
| 4. Is the health-care system comparable with the UK? | Yes | Yes |
| 5. Is the setting comparable with the UK? | Yes | Yes |
| 6. Is the perspective of the model clearly stated? | Yes | Yes |
| 7. Is the study type appropriate? | Yes | Yes |
| 8. Is the modelling methodology appropriate? | Yes | Yes |
| 9. Is the model structure described and does it reflect the disease process? | Yes | Yes |
| 10. Are assumptions about model structure listed and justified? | Yes | Yes |
| 11. Are the data inputs for the model described and justified? | Yes | Yes |
| 12. Is the effectiveness of the intervention established based on a systematic review? | Yes | Yes |
| 13. Are health benefits measured in QALYs? | Yes | Yes |
| 14. Are health benefits measured using a standardised and validated generic instrument? | Yes | Yes |
| 15. Are the resource costs described and justified? | Yes | Yes |
| 16. Have the costs and outcomes been discounted? | Yes | Yes |
| 17. Has uncertainty been assessed? | Yes | Yes |
| 18. Has the model been validated? | No | No |
Appendix 9 Probabilistic sensitivity analysis variables
Variables and probability distributions used in the probabilistic sensitivity analyses
| Name | Mean value | Standard error | Distribution | Alpha | Beta |
|---|---|---|---|---|---|
| Patient distribution | |||||
| Patients with genotypes 1 and 4 (PEG α-2a) | 0.77 | 0.05 | Beta | 53.8 | 16.1 |
| Patients with genotypes 2 and 3 (PEG α-2a) | 0.23 | 0.07 | Beta | 8.1 | 27.1 |
| Patients with genotypes 1 and 4 (PEG α-2b) | 0.82 | 0.04 | Beta | 74.8 | 16.4 |
| Patients with genotypes 2 and 3 (PEG α-2b) | 0.18 | 0.05 | Beta | 10.4 | 47.6 |
| Treatment effect | |||||
| EVR for genotypes 1 and 4 (PEG α-2a) | 0.57 | 0.05 | Beta | 49.2 | 37.1 |
| EVR for genotypes 1 and 4 (PEG α-2b) | 0.61 | 0.04 | Beta | 91.0 | 58.2 |
| SVR at age 11 years | 0.00 | ||||
| SVR for genotypes 1 and 4 (PEG α-2a) | 0.52 | 0.06 | Beta | 39.43 | 36.40 |
| SVR for genotypes 2 and 3 (PEG α-2a) | 0.86 | 0.07 | Beta | 22.91 | 3.73 |
| SVR overall (all genotypes) (PEG α-2a) | 0.60 | 0.06 | Beta | 42.21 | 28.14 |
| SVR for genotypes 1 and 4 (PEG α-2b) | 0.51 | 0.04 | Beta | 79.15 | 76.04 |
| SVR for genotypes 2 and 3 (PEG α-2b) | 0.91 | 0.05 | Beta | 32.83 | 3.25 |
| SVR overall (all genotypes) (PEG α-2b) | 0.58 | 0.03 | Beta | 121.64 | 88.09 |
| Transition probabilities | |||||
| F0 state to F1 | 0.12 | 0.01 | Beta | 274.62 | 2072.54 |
| F1 state to F2 | 0.09 | 0.01 | Beta | 230.28 | 2478.89 |
| F2 state to F3 | 0.12 | 0.01 | Beta | 337.99 | 2478.60 |
| F3 state to F4 | 0.12 | 0.01 | Beta | 292.30 | 2227.52 |
| F4 state to DC | 0.04 | 0.01 | Beta | 14.58 | 359.21 |
| F4 state to HCC | 0.01 | 0.01 | Beta | 1.92 | 135.12 |
| DC to HCC | 0.01 | 0.01 | Beta | 1.92 | 135.12 |
| DC to liver transplant | 0.02 | 0.01 | Beta | 15.66 | 767.34 |
| DC to death related to hepatitis C | 0.13 | 0.01 | Beta | 146.90 | 983.10 |
| HCC to death | 0.43 | 0.03 | Beta | 116.67 | 154.66 |
| Liver transplant to death | 0.15 | 0.02 | Beta | 84.85 | 480.82 |
| Post-transplant state to death | 0.06 | 0.01 | Beta | 122.50 | 2026.54 |
| Health state utility value | |||||
| Utility of SVR from mild disease | 0.82 | ||||
| Utility of F0/F1 | 0.82 | ||||
| Utility of F2/F3 | 0.82 | ||||
| Utility of patients in F0/F1 receiving treatment | 0.71 | 0.02 | Beta | 435.91 | 178.05 |
| Utility of patients in F2/F3 receiving treatment | 0.71 | 0.02 | Beta | 435.91 | 178.05 |
| Utility of patients in F4 | 0.75 | 0.02 | Beta | 237.05 | 79.02 |
| Utility of patients in F4 receiving treatment | 0.64 | 0.04 | Beta | 109.81 | 61.77 |
| Utility of patients with DC | 0.66 | 0.04 | Beta | 110.28 | 56.81 |
| Utility of patients with hepatocellular cancer | 0.64 | 0.10 | Beta | 14.74 | 8.29 |
| Utility of patients in post-liver transplant state | 0.69 | 0.03 | Beta | 163.30 | 73.37 |
| Utility of patients receiving liver transplant | 0.73 | 0.05 | Beta | 56.82 | 21.02 |
| Monitoring costs | |||||
| For patients receiving 12 weeks of treatment | £721 | £71 | Gamma | £102.77 | 7.02 |
| For patients receiving 16 weeks of treatment | £869 | £86 | Gamma | £102.79 | 8.45 |
| For patients receiving 24 weeks of treatment | £880 | £87 | Gamma | £102.70 | 8.57 |
| For patients receiving 48 weeks of treatment | £1168 | £115 | Gamma | £102.79 | 11.36 |
| For patients receiving 72 weeks of treatment | £1155 | £114 | Gamma | £102.87 | 11.23 |
| Health state cost | |||||
| Health state cost for SVR | £346 | £48 | Gamma | 51.42 | 6.73 |
| Health state cost for F0/F1 | £184 | £27 | Gamma | 45.69 | 4.03 |
| Health state cost for F2/F3 | £959 | £76 | Gamma | 158.93 | 6.03 |
| Health state cost for F4 | £1521 | £231 | Gamma | 43.29 | 35.13 |
| Health state cost for DC | £12,193 | £1519 | Gamma | 64.39 | 189.35 |
| Health state cost for HCC | £10,865 | £1910 | Gamma | 32.36 | 335.71 |
| Health state cost for LT1 (year of liver transplant) | £32,732 | £3296 | Gamma | 98.61 | 331.92 |
| Health state cost for LT2 (post-transplant) | £1852 | £355 | Gamma | 27.21 | 68.06 |
Appendix 10 Net benefit approach
Cost-effectiveness decision rules and the incremental cost-effectiveness ratio
In traditional cost-effectiveness analyses, standard decision rules are considered to establish the cost-effectiveness of an intervention compared with a given comparator. These are typically outlined using the cost-effectiveness plane. If the incremental cost is negative and the incremental effect is positive (south-east quadrant), the intervention is unequivocally cost-effective (it is dominant, achieving better outcomes at lower cost). If the incremental cost is positive and the incremental effect is negative (north-west quadrant), the intervention is unequivocally not cost-effective (it is dominated, achieving poorer outcomes at higher cost). Where both the incremental cost and the incremental effect are negative (south-west quadrant), or both the incremental cost and the incremental effect are positive (north-east quadrant), no such unequivocal statements can be made. Determining whether or not the intervention is cost-effective depends on a threshold value, defined as the maximum amount society is willing to pay for an incremental health gain or, equivalently, the minimum amount society is willing to accept for forgoing an incremental health gain.
One of the drawbacks of the ICER is that the location of negative ICERs [whether they are in the south-east (dominant) or north-west (dominated) quadrant] cannot be determined without reference to other contextual information. The INB provides an unambiguous decision rule, although this implies knowledge of the threshold value.
For further explanation see appendix 8 of the previous HTA report. 41
List of abbreviations
- AG
- Assessment Group
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- BNF
- British National Formulary
- BRIEF
- Behaviour Rating Inventory of Executive Function
- BSA
- body surface area
- BSC
- best supportive care
- CBCL
- Child Behaviour Checklist
- CDI
- Children’s Depression Inventory
- CEAC
- cost-effectiveness acceptability curve
- CHQ
- Child Health Questionnaire
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- DSA
- deterministic sensitivity analysis
- EOT
- end-of-treatment virological response
- EQ-5D
- European Quality of Life-5 Dimensions
- EVR
- early virological response
- FDA
- Food and Drug Administration
- GP
- general practitioner
- HAI
- histological activity index
- HCC
- hepatocellular carcinoma
- HCHS
- Hospital and Community Health Services
- HCV
- hepatitis C virus
- HIV
- human immunodeficiency virus
- HPA
- Health Protection Agency
- HRQoL
- health-related quality of life
- HTA
- health technology assessment
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- INB
- incremental net benefit
- IU
- international unit
- MS
- manufacturer’s submission
- MSD
- Merck Sharp & Dohme
- NICE
- National Institute for Health and Care Excellence
- PCR
- polymerase chain reaction
- PSA
- probabilistic sensitivity analysis
- PSS
- personal and social services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RBV
- ribavirin
- RCT
- randomised controlled trial
- RNA
- ribonucleic acid
- RVR
- rapid virological response
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SG
- standard gamble
- SHTAC
- Southampton Health Technology Assessments Centre
- SVR
- sustained virological response
- TA
- technology appraisal
- TTO
- time trade-off
- VAS
- visual analogue scale
- VT
- vertical transmission
- WTP
- willingness to pay