Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 05/515/01. The contractual start date was in May 2009. The draft report began editorial review in August 2013 and was accepted for publication in April 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Wolf et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Seriously ill children admitted to paediatric intensive care (PIC) for treatment and supportive therapy require both analgesia and sedation as part of their management to maintain comfort and provide pain relief associated with invasive procedures, mechanical ventilation and the need to lie relatively still. Sedation is also needed to prevent distress from the presence of unfamiliar personnel and from the high level of background noise, which can disturb sleeping patterns. 1 Undersedation and oversedation are both harmful. Inadequate sedation is unacceptable in a vulnerable child: the child may ‘fight’ the ventilator, leading to ineffective gas exchange, adverse haemodynamic/stress responses, accidental extubation or the loss of invasive access or monitors. In intensive care, agitation and inadequate sedation has been correlated with adverse short- and longer-term outcomes. 2 In contrast, oversedation delays recovery, promotes tolerance to the drugs and leads to distressing symptoms on withdrawal of the drugs: agitation, seizures, hallucinations, psychosis, fever and tachycardia. 3,4
Physician focus in the critically ill child is primarily directed at diagnosis and treatment of the primary disease and often minimal attention is given to the attendant sedation, particularly once the patient has been paralysed with neuromuscular blocking agents. This is reflected in the limited available studies of sedation in the paediatric intensive care unit (PICU), despite common understanding of its problematic nature. This is compounded by the difficulty of undertaking such studies, which require cumbersome observations and recordings of sedation levels, and close observation and manipulation of dose administration to remain within chosen sedation parameters. The limitation of available published data with a large cohort makes the need for a larger-scale trial important but, at the same time, makes planning of such a trial difficult.
Benzodiazepines
Currently in the UK, midazolam is the most popular sedative used in critically ill children, usually given in combination with an opioid by intravenous (i.v.) infusion at doses between 50 and 300 µg/kg/hour. 5 Alternative agents to midazolam include diazepam, clonidine, chloral hydrate and promethazine. The limited data on midazolam suggest a high incidence of side effects: in two studies6,7 designed to observe adverse reactions (ARs) to sedative agents the reported incidence was as high as 35% for midazolam, and this was related to duration of the infusion and cumulative dose. The duration of abnormal behaviour after drug withdrawal was as long as 1 week. Limiting the benzodiazepine dose may delay the onset of tolerance but is often unobtainable because of the need to maintain adequate sedation. The frequency and severity of symptoms are related to the cumulative amount of drug given and the duration of the infusion and are commonly identified as agitation, prolonged crying, abnormal movements, vomiting and cardiovascular disturbance. 5 Of concern is that in a study on neonatal sedation and neurological outcome the use of midazolam appeared to have an adverse effect on outcome compared with morphine or placebo. 8 This has led to a significant reduction of midazolam use in the neonatal intensive care. Moreover, recent studies on neurodevelopment have raised serious concerns with regard to the effects of even relatively brief exposure of the young child to gamma-aminobutyric acid (GABA) agonists, including midazolam, in terms of long-term behavioural and intellectual development. 9,10 The intrinsic effects of midazolam and morphine on outcome have never been compared with other regimens.
Clonidine
In recent years, considerable interest has been shown in the use of α2-agonist drugs as an alternative to benzodiazepines in intensive care sedation both in adults and children. 11 Clonidine is a lipid-soluble, partial α2-agonist with antihypertensive, analgesic and sedative effects. Its primary antihypertensive action is attributed to its central α2 effect on the sympathetic outflow, resulting in reduced heart rate, vasodilatation and lowered blood pressure (BP). 12,13 More recently it has gained recognition for its sedative and analgesic properties. The mechanism for the sedative and analgesic actions is not clear but is thought to be a combination of central effects that modulate descending inhibitory nociceptive mechanisms and spinal analgesia, acting on the dorsal horn of the spinal cord. 14 Elimination is through both hepatic metabolism to inactive metabolites and direct renal excretion. 12 It is a drug that may have a protective effect on the developing brain in that, unlike benzodiazepines, it is not associated with apoptotic changes on exposure to the drug. 15,16
Caudal epidural and spinal clonidine have been evaluated in paediatric anaesthesia. It has been shown to augment pain relief and increase the duration of postoperative analgesia with minimal side effects,17–19 and is now used routinely in paediatric practice. Given as an oral premedicant, clonidine has similar anxiolytic and improved sedative properties compared to preoperative benzodiazepines20 but, in addition, it can attenuate haemodynamic responses to nociception and provide postoperative analgesia. These effects on central sympathetic outflow and centrally based analgesia mechanisms reduces intraoperative anaesthetic requirements and metabolic responses to surgery. 21 In anaesthesia of the critically ill neonatal cardiac patient, this has shown to have improved outcome in terms of survival. 22
In the last 10 years, following experience with clonidine in paediatric anaesthesia and its use in adults withdrawing from alcohol and opioids, it has become increasingly used for sedation and analgesia in the critically ill child in the PICU. 5 However, despite its widespread use there are few data on effectiveness, dose requirement and safety. A limited dose-finding study in the PICU has demonstrated that it can provide dose-dependent sedation in place of morphine using an i.v. infusion rate of 1–2 µg/kg/hour without haemodynamic compromise in terms of heart rate, BP or cardiac output. 23 A small prospective study of critically ill children demonstrated that concomitant administration of oral clonidine significantly reduces morphine and lorazepam requirements without additional side effects. 24 Clonidine has a good safety profile in the general population, even in extreme overdose,25,26 although it can be associated with significant side effects that include bradycardia, hypotension and rebound hypertension. There remains an unmet need for improved sedation in the PICU, and although clonidine is being increasingly used in the clinical situation in the PICU, a formal objective evaluation of i.v. clonidine as an alternative to i.v. midazolam needs to be undertaken.
Possible beneficial effects
The reduction of sympathetic outflow associated with clonidine may have specific benefits to critically ill children in the PICU. Studies in animals suggest that α2-agonists can improve neurological outcome that is associated with ischaemic cerebral injury. 27–31 These beneficial effects are α2-specific and reversed with selective α2-antagonists. 29 The protective mechanism of action is unclear but may be due to suppression of extracellular glutamate and aspartate release during energy failure. 32 Recent data have also demonstrated that preconditioning before the insult can both reduce infarct size and improve neurological outcome after insult. 33
Trauma surgery and critical illness are associated with a variety of neurohumoral responses (the stress response), which can result in organ dysfunction. 34 More specifically, renal function deteriorates after both adult and paediatric cardiac surgery, and this effect is due, in part, to the increase in sympathetic outflow and the rise in circulating vasoconstrictors such as noradrenaline, vasopressin and angiotensin. 34,35 Clonidine has been demonstrated to suppress these responses and prevent the associated decline in renal function after adult cardiac surgery. 36 In addition, clonidine has independent local effects on tubular function which promote both diuresis and natriuresis. 37 In terms of cardiovascular responses, reduction in stress responses by α2-agonists have been shown to reduce perioperative myocardial ischaemia in adults who are undergoing both cardiac and non-cardiac surgery. 38
Toxicity
Clonidine can cause significant side effects after accidental overdose in children: pallor, bradycardia, hypotension, miosis, unconsciousness, hypotonia and hypothermia,39,40 although in healthy children the tolerance to extreme overdose (up to 1000 times the therapeutic dose) appears to be reassuring. 25,26 In adults the peripheral α1 effects can cause hypertension and vasoconstriction in overdose, but this appears to be far less common in children. The only deaths in the literature have been associated with multiple drug ingestion and were not thought to be related to clonidine. 41
Rationale
Although there are few data on the use of clonidine in PIC, this drug has been adopted widely in PIC as a mainstream sedative agent and as a treatment for drug withdrawal in children after prolonged exposure to sedatives. 5 Clonidine has specific attributes that make it potentially a better choice than midazolam as an adjunct to morphine: co-analgesia through a different mechanism than opioids, reduction in sympathetic tone, improved cardiac and renal function, protection from ischaemic/reperfusion injury and reduced tolerance/withdrawal. The combination of morphine and clonidine seems to be a rational alternative to the current use of morphine and midazolam. This is particularly pertinent given the high frequency of adverse responses to midazolam on withdrawal of the drug, the risks of longer-term central nervous system damage in the developing brain associated at least in a primate model with benzodiazepines, and the potential advantages documented above with use of clonidine. Given these theoretical advantages and the limited clinical information on the use of α2-agonists for sedation in the PICU, there is a need to evaluate this drug objectively and to determine if it has outcome benefits compared with the routine use of midazolam.
A previous pilot data set defined dose effectiveness of i.v. clonidine, which allows assumptions of dose equivalence of midazolam with clonidine. 23 For clonidine, an effective dose (ED) 95% (ED95%) for the COMFORT score in the effective range was provided by an infusion rate of 2 µg/kg/hour. This compares with an effective range of 50–200 µg/kg/hour for midazolam,6,7 with an ED95% of 150 µg/kg/hour. As clonidine continues to be used with increasing frequency in PIC without any benchmark data, there is an urgent need to define safety and efficacy in a larger group of patients in a more rigorous fashion than the previous pilot studies. Apart from the issue of quality of sedation, and potential cost savings by avoiding complications associated with sedation and analgesia in PIC, the use of i.v. clonidine provides modest cost savings over i.v. midazolam. The cost of midazolam at 150 µg/kg/hour is currently £1.60 per day for the drug, compared with 90p per day for 2 µg/kg/hour of clonidine.
Chapter 2 Methods
Objectives
Primary objective
To determine whether or not i.v. clonidine can provide equivalent control of sedation in the critically ill child when compared with i.v. midazolam.
Secondary objectives
To determine whether or not clonidine reduces side effects associated with sedation practice in intensive care compared with midazolam at clinically appropriate dosing regimens. To determine if there are any benefits on clinical outcomes using clonidine compared with midazolam.
Design
A prospective, controlled, double-blind, multicentre, randomised equivalence trial42 comparing clonidine and midazolam as i.v. sedative agents in critically ill children.
This trial was designed as an equivalence trial owing to the current variation in practices and use of both clonidine and midazolam. The equivalence margin was originally determined by discussion with a limited number of clinicians by considering a range that excluded values that would influence their choice of sedative. This equivalence range (± 0.10) was later widened to ± 0.15, based on wider feedback across principal investigators (PIs) at each site involved in the trial.
A validated scoring system to make objective observations in guiding infusion rates is the COMFORT score43,44 (see Appendix 2). This scoring system uses a variety of behavioural and physiological measurements to give a numeric value of between 8 and 40, with a value of < 17 being regarded as oversedated, and value of > 26 regarded as undersedated. The aim of the bedside carers was to maintain sedation within the 17–26 range during the study by adjustment of morphine and trial drugs according to a defined regimen.
Participants
To be eligible for the study the child had to meet the following inclusion and exclusion criteria.
Inclusion criteria
-
Children aged 30 days (≥ 37 weeks’ gestation) to 15 years inclusive. Children born before 37 weeks’ gestation are eligible if they are a minimum of 30 days post delivery and their corrected gestation is ≥ 37 weeks.
-
Admitted to PICU, ventilated and likely to require ventilation for > 12 hours.*
-
Recruitment within 120 hours of arrival in the PICU/intensive care unit (ICU).*
-
Child is ≤ 50 kg in weight.
-
Able to perform a COMFORT score on the child.
-
Adequately sedated: COMFORT score within the range of ≥ 17 and ≤ 26.
-
Fully informed written proxy consent.
*Eligibility criteria amended during trial and summarised (see Table 3); details provided in Appendix 5.
Exclusion criteria
-
Those patients with open chests following cardiac surgery.
-
Those patients chronically treated for raised BP.
-
Current treatment with beta-blockers (if patients have not received beta-blockers for 24 hours prior to entry into the trial then they are eligible to participate).
-
Acute traumatic brain injury.
-
Status epilepticus or active fitting (two or more seizures regularly on a daily basis).
-
Those patients requiring haemodialysis or haemofiltration.
-
Those patients requiring extracorporeal membrane oxygenation (ECMO) treatment.
-
Those patients with severe neuromuscular problems/impairment on whom you cannot perform a COMFORT score.
-
Known allergy to either of the trial medications (clonidine, midazolam or morphine).
-
Current treatment with continuous or intermittent muscle relaxants.
-
Those patients known to be pregnant.
-
Currently participating in a conflicting clinical study or participation in a clinical study involving a medicinal product in the last month.
-
Previously participated in Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation (SLEEPS) trial.
The use of midazolam or clonidine to establish sedation did not preclude entry into the trial.
Interventions
Study treatments were manufactured and supplied by SCM Pharma. Treatment packs contained a number of ampoules providing sufficient treatment for a patient for 7 days. Ampoules of clonidine were 5 ml in volume and contained a concentration of 150 µg/ml of clonidine. Ampoules of midazolam were 5 ml in volume and contained a concentration of 10 mg/ml of midazolam. Ampoules of midazolam and clonidine were identical in appearance, and the volumes of infusions delivered per hour for either drug were similar, such that the maximum hourly dose of midazolam (200 µg/kg/hour) was delivered at an infusion rate that also corresponded to the maximum hourly dose of clonidine (3 µg/kg/hour).
Table 1 illustrates the preparation of trial treatment for infusion, rate range of infusion, the loading dose, the maintenance rate and incremental steps to be applied for each weight group for two trial treatments, and the dosage administered based upon using these ampoules. Additional details can be found in Appendix 6.
| Regimen | Child’s weight (kg) | ||
|---|---|---|---|
| < 10 | 10–25 | > 25–50 | |
| Preparation for infusion | 5-ml trial treatment in 50 ml of 5% dextrose | 6.25-ml trial treatment in 50 ml of 5% dextrose | 25-ml trial treatment in 50 ml of 5% dextrose |
| Providing: | Providing: | Providing: | |
| Clonidine: 15 µg/ml | Clonidine: 18.75 µg/ml | Clonidine: 75 µg/ml | |
| Midazolam: 1 mg/ml | Midazolam: 1.25 mg/ml | Midazolam: 5 mg/ml | |
| Rate range of infusion | 0.05–0.20 ml/kg/hour | 0.04–0.16 ml/kg/hour | 0.01–0.04 ml/kg/hour |
| Providing: | Providing: | Providing: | |
| Clonidine: 0.75–3 µg/kg/hour | Clonidine: 0.75–3 µg/kg/hour | Clonidine: 0.75–3 µg/kg/hour | |
| Midazolam: 50–200 µg/kg/hour | Midazolam: 50–200 µg/kg/hour | Midazolam: 50–200 µg/kg/hour | |
| Loading dose (first hour of trial treatment) | 0.2 ml/kg over 1 hour | 0.16 ml/kg over 1 hour | 0.04 ml/kg over 1 hour |
| Providing: | Providing: | Providing: | |
| Clonidine: 3 µg/kg/hour | Clonidine: 3 µg/kg/hour | Clonidine: 3 µg/kg/hour | |
| Midazolam: 200 µg/kg/hour | Midazolam: 200 µg/kg/hour | Midazolam: 200 µg/kg/hour | |
| Maintenance rate (second hour of trial treatment) | 0.1 ml/kg/hour | 0.08 ml/kg/hour | 0.02 ml/kg/hour |
| Providing: | Providing: | Providing: | |
| Clonidine: 1.5 µg/kg/hour | Clonidine: 1.5 µg/kg/hour | Clonidine: 1.5 µg/kg/hour | |
| Midazolam: 100 µg/kg/hour | Midazolam: 100 µg/kg/hour | Midazolam: 100 µg/kg/hour | |
| Incremental steps (from third hour; reviewed hourly and adjusted according to COMFORT score) | Increase in steps of 0.05 ml/kg/hour | Increase in steps of 0.04 ml/kg/hour | Increase in steps of 0.01 ml/kg/hour |
Loading dose of trial intervention and morphine
After consent and randomisation, and before starting the trial drugs, sedation with the pre-existing drugs were adjusted to ensure that the COMFORT score was within the desired range (17–26). This necessitated that the child was not on muscle relaxants and did not have suppressed motor function and, therefore, was evaluable by the COMFORT score. At this point the trial morphine and the study drug (either midazolam or clonidine) were then given in a standardised loading fashion, irrespective of the pre-existing drugs that had been used prior to study. Loading with i.v. morphine consisted of a dose of 100 µg/kg over 15 minutes. The trial drug was then administered, over 1 hour, from the syringe that had been made up in blinded fashion. For midazolam this corresponded to 200 µg/kg over 1 hour followed by an initial maintenance infusion rate of 100 µg/kg/hour. For clonidine this corresponded to a loading dose of 3 µg/kg over the first hour followed by an initial maintenance infusion rate of 1.5 µg/kg/hour. After this first hour, the pre-existing sedative/analgesic drugs were discontinued.
Maintenance rates of trial interventions
From this point onwards the study drug and, if necessary, morphine infusion rates were changed, in a formalised fashion, either upwards or downwards according to the objective measure of the COMFORT score. The incremental changes allowed five delivery options: for clonidine, 0.00, 0.75, 1.50, 2.25 and 3.00 µg/kg/hour; for midazolam 0, 50, 100, 150 and 200 µg/kg/hour.
Morphine usage
After the initial loading dose of morphine of 100 µg/kg over a 15-minute period, the initial maintenance infusion rate of morphine was set at 20 µg/kg/hour, with an option to increase the morphine infusion if the maximum treatment dose of the study drugs had been reached. In addition, provision was made for the bedside nurses to increase morphine infusion rates if they considered that the COMFORT score had risen through pain rather than sedation, allowing a morphine infusion of up to 60 µg/kg/hour. This was particularly relevant in the case of children who were in the PICU immediately after surgery. Multiple changes of the infusion rates were allowable within a 1-hour period, provided that there was an accompanying COMFORT record that supported a change in infusion rate.
The flow diagram used to direct infusion rates of morphine and trial drug in response to the COMFORT score are shown in Figure 1.
FIGURE 1.
Trial interventions and morphine. a, If a COMFORT score of < 17 is recorded for 2 consecutive hours then reduce morphine or trial sedation as clinically indicated incrementally down to 20 µg/kg/hour of morphine and to the minimum trial infusion rate for the weight group. If minimum infusion rate is administered and the morphine infusion rate is 20 µg/kg/hour and the child still has a COMFORT score below 17, then if there are no analgesic requirements, the morphine can be further decreased by an increment to 10 µg/kg/hour. If the COMFORT score is still below 17, the morphine can be stopped (providing there are no analgesic requirements). If the COMFORT score is still below 17, the trial sedation can be temporarily stopped until the COMFORT score rises to 17 or more.
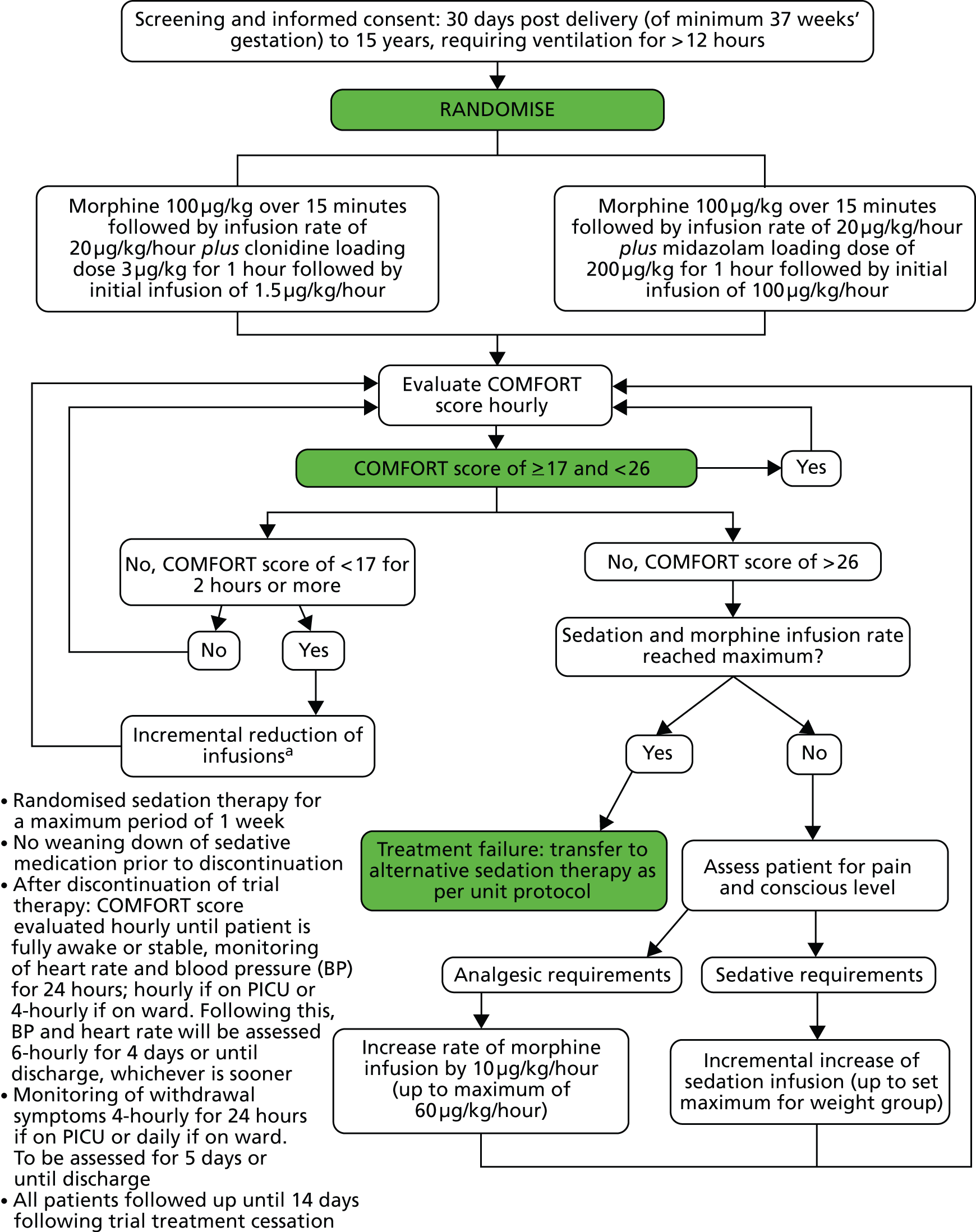
Interventions
In addition to recording hourly COMFORT scores and modulation of the infusion rates required to maintain children within the desired 17–26 score range, events either related to PIC or additional sedative/analgesia control were documented during the study.
Children who became unsettled and were outside the ideal COMFORT score within each hourly period were brought back into the acceptable range with increase in trial drug or morphine according to the treatment protocol. If the change was deemed to be urgent or there was clinical need then sedation, anaesthesia and, if necessary, rescue muscle relaxant drugs could be given at any point. However, If this occurred three times in a 12-hour period, the trial treatments were deemed to have failed and the study drugs replaced with conventional medication according to individual PICU practice.
Children who required invasive procedures or investigations, such as magnetic resonance imaging (MRI) scan or computed tomography scan were allowed to remain in the study provided that the study drugs could be continued throughout. In this situation, anaesthetic, analgesic or muscle relaxant drugs could be administered to ensure appropriate unconsciousness, pain relief and, if necessary, immobilisation. If muscle relaxants were administered, this temporarily caused some of the behavioural measures of the COMFORT score to be invalid, and therefore the BP and heart rate became the sole measures of the COMFORT score until muscle function returned.
The study drugs were continued until the patient had recovered sufficiently to allow extubation, or had completed 7 days of the study drug, or had failed treatment due to reaching the maximum allowed dose of study drug and morphine and still inadequate after an hour, or had required more than two rescue treatments in any 12-hour period. In addition, patients requiring advanced organ support, such as haemofiltration or extracorporeal life support, did not continue to receive study drugs, although they continued to be monitored as part of the study.
Study procedures
In each of the participating PICUs, patients were reviewed by the consultant staff or designated research nurse each morning to identify potentially eligible patients. Screening of a patient’s possible eligibility for the study was documented on the ‘screening log’. If a patient was assessed to be eligible for the study, the parent or legally acceptable representative of the patient was provided with the patient information and consent forms (Table 2).
| Procedures | Enrolment and baselinea | T+ (DAYS) | Premature discontinuation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum no. of treatment days | Follow-up days (F) | ||||||||||||||||
| T0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | F1 | F2 | F3 | F4 | F5 | F14 | ||||
| Signed informed consenta | ✗ | ||||||||||||||||
| Randomisationa | ✗ | ||||||||||||||||
| Verify consent/assent (as appropriate when sedation ceases) | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ||||||||||
| Assessment of eligibility criteria | ✗ | ||||||||||||||||
| Review of medical history | ✗ | ||||||||||||||||
| Review of concomitant medications | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Study interventionb | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||
| COMFORT scorec | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| BP and heart rated | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | (✗) | |||
| Fluid balancee | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | (✗) | |||||||||
| Withdrawal symptomsf | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ✗ | ✗ | ✗ | ✗ | ✗ | (✗) | ||||
| Assessment of AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | (✗) | |||
| Clinical laboratoryg | Chemistry | ✗ | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ✗ | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ||
| Urinalysis | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ✗ | (✗) | (✗) | (✗) | (✗) | (✗) | (✗) | ||||
| PK/PD and phthalate study (limited no. of centres participating in blood and urine sampling for PK/PD and phthalate substudy, but only Bristol taking samples for urinary VMA and cardiac function for PK/PD study) | Blood samplingh | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||
| Urine samplingi | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Urinary VMAi | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Cardiac functionj | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
To be eligible for the study the child was required to meet the inclusion and exclusion criteria (see Chapter 2, Inclusion criteria and Exclusion criteria). Eligible patients for whom informed consent was obtained were allocated the next available sequentially numbered treatment pack within their weight strata. If it was not possible to weigh the child then the weight was estimated using the formula/method routinely used on the Unit.
Public and patient involvement
A layperson was involved at the start of the trial during protocol development and trial design. During the development of the Patient Information Sheets (PISs), the Medicines for Children Research Network (MCRN) Young Persons Advisory Group and the MCRN Parents Group reviewed the documents and suggested changes. When it became apparent that there was a high decline rate for the trial, advice was sought again from the MCRN Parents Group in order to further revise the PIS and to review the proposed poster for the parents’ room prior to application for ethical approval.
Data collection
During trial treatment administration, data were prospectively collected for BP, heart rate, the COMFORT score, trial treatment rate, morphine dose, any additional analgesia, sedation and muscle relaxants, and reasons for administration of these medications. The case report form (CRF) designed for use during trial treatment administration was carefully designed to be as similar as possible to the PICU charts used to collect data clinically to try to make this as straightforward as possible for PICU bedside staff.
Data for blood biochemistry, urinalysis, fluid balance, incidence of hypotension requiring intervention, and information regarding whether or not feeds had been tolerated, whether or not bowels had opened and presence of bowel sounds were collected retrospectively. Forms were designed to collect these data retrospectively to alleviate some of the burden on the PICU bedside staff.
Following trial treatment, data were prospectively collected for BP, heart rate, the COMFORT score (until fully awake), any use of sedatives and analgesics, and withdrawal symptoms, and any treatment required for these. Blood biochemistry, urinalysis, whether or not feeds had been tolerated, whether or not bowels had opened and presence of bowel sounds were collected retrospectively post-trial treatment.
The final follow-up at 14 days post-trial treatment collected data on whether or not the patient had completed the study, the number of days spent on each type of ward, date of discharge, general practitioner (GP) attendances, and hospital attendances/admissions.
Adverse reactions and serious adverse events (SAEs) were collected prospectively from the time of consent and up until 14 days following trial treatment cessation. Any ARs were submitted to the Clinical Trials Unit by post and SAEs were notified by fax and telephone to ensure timely processing and completion.
Outcomes
Primary outcome
Adequate sedation defined as at least 80% of total evaluated time spent sedated within a COMFORT score range of 17 to 26.
Secondary outcomes
During study treatment phase
-
Percentage of time spent adequately sedated.
-
Time to reach the maximum permitted dose of sedation.
-
Time to reach the maximum permitted dose of morphine.
-
Profile in rise of daily cumulative sedative infusion.
-
Profile in rise of daily cumulative morphine infusion.
-
Maximum permitted dose of sedative reached.
-
Maximum permitted dose of morphine reached.
-
Fall in BP judged by clinician to require intervention.
-
Increased inotropic support required in first 12 hours after randomisation.
-
Supplementary analgesia required during sedation.
-
Daily urine output.
-
Treatment failure defined as inadequate sedation after 1 hour of maximum doses of sedative and morphine infusions (determined by a COMFORT score above 26) or treatment failure defined as three ‘events’* where rescue medications are needed to re-establish sedation or pain control occurring within any one 12-hour period during trial treatment.
-
Blood biochemistry and urinalysis.
-
Urinary concentration of gamma-glutamyl transpeptidase (Bristol only).**
-
Urinary concentration of alkaline phosphatase (Bristol only).**
*An ‘event’ is described as a point when control of sedation is deemed to be acutely lost requiring immediate intervention. The intervention can involve more than one drug given over a short period of time to establish rapid control (within approximately a 30-minute window to allow safe titration if necessary).
**The pharmacokinetic/pharmacodynamic substudy at the Bristol site did not go ahead as planned so these data were not collected.
Following study treatment phase
-
Time from stopping all sedation to being fully awake (determined by a sustained* score of 4 on the alertness category of the COMFORT score).
-
Rebound hypertension.
-
Signs of withdrawal measured using an 11-point assessment for abnormal behaviour (to be recorded until 5 days after treatment cessation or until discharge, whichever is soonest).
-
Withdrawal symptoms requiring clinical intervention (to be recorded until 5 days after treatment cessation or until discharge, whichever is soonest).
*Sustained for ≥ 2 hours.
Throughout the duration of study
-
Adverse reactions and SAEs (to be recorded until 14 days post-trial treatment cessation).
Health economics
-
Cost per additional case of adequate sedation [see also separate Statistical Analysis Plan (SAP) in Appendix 4 for health economics].
Sample size calculations
Sample size calculations were undertaken using NQuery Advisor software version 4.0 (Statistical Solutions, Saugus, MA, USA).
The trial was originally designed with an equivalence margin of ± 0.10. During teleconferences with PIs at sites it was suggested that this margin was too narrow. Recruitment into the trial was challenging, and consideration was given to widening the margin of equivalence as suggested by site PIs. The revision to the sample size calculation was submitted to ethics and the Medicines and Healthcare products Regulatory Agency (MHRA) on 18 April 2011 and accepted by the MHRA on 26 May 2011 and ethics on 15 June 2011. The original and revised sample size calculations are presented in full in the next two sections below.
Original sample size calculations
The proportion of children adequately sedated on midazolam is reported to be 0.65,45 with an expected proportion of 0.66 on clonidine. A two-group, large-sample normal approximation test of proportions with a two-sided 5% significance level to have 80% power to reject the null hypothesis that midazolam and clonidine are not equivalent (with margin of equivalence ± 0.10) would require 440 children in each group. The trial would therefore aim to recruit a total of 1000 children across both treatment groups to allow for approximately 10% loss to follow-up.
Sample size calculation revision
The revised sample size calculation uses a 15% margin, as agreed by the PIs and Trial Steering Committee (TSC) members, and indicates the statistical power that could be achieved with expected recruitment rate. Owing to observed completeness of the data collected at the time of the sample size revision, the 10% loss to follow-up adjustment was removed.
The proportion of children adequately sedated on midazolam is reported to be 0.65,45 with an expected proportion of 0.66 on clonidine. When the sample size in each group is 125, a two-group large-sample normal approximation test of proportions with a one-sided 0.025 significance level will have 64% power to reject the null hypothesis that the test and the standard are not equivalent (the difference in proportions, pT – pS, is 0.150 or farther from zero in the same direction) in favour of the alternative hypothesis that the proportions in the two groups are equivalent.
Randomisation and blinding
Randomisation was stratified by centre and weight in a 1 : 1 ratio between the two groups. Weight was not considered to be a prognostic indicator but randomisation was stratified by this factor to reduce wastage and costs associated with preparing all treatment packs to contain sufficient medicinal product to allow for higher-weight participants.
Separate randomisation lists were generated for each stratum in Stata version 9.0 (StataCorp LP, College Station, TX, USA) by the SLEEPS randomising statistician (independent of the SLEEPS trial statistics team) using simple block randomisation with random variable block length:
-
weight group A (< 10 kg) – block sizes of 4 and 6
-
weight group B (10 –25 kg) – block sizes of 4 and 6
-
weight group C (> 25–50 kg) – block sizes of 2 and 4.
Randomisation lists were supplied to SCM Pharma (Prudhoe, UK) who prepared treatment packs. Treatment packs within strata were identical in appearance. Each treatment pack contained sufficient ampoules to allow 7 days of treatment at the highest weight range of the strata. The ampoules within treatment packs were identical in appearance.
Batches of treatment packs were sent to pharmacies at each site, and they issued a number of treatment packs for secure storage on PICU so that patients could be recruited into the trial at any time. The trial treatment packs were sequentially numbered such that upon randomisation the next pack in the sequence for the appropriate weight group was selected. The randomisation log was completed and the start date, patient’s initials and the patient’s weight were completed on the treatment pack by the member of the research team randomising the patient.
Statistical methods
Interim monitoring
Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation was monitored by an Independent Data and Safety Monitoring Committee (IDSMC), having agreed procedures based on a Charter. 46 The IDSMC was responsible for reviewing and assessing recruitment, interim monitoring of safety and effectiveness, trial conduct and external data. The extent and type of missing data were monitored, and strategies were developed to minimise its occurrence.
All interim analysis results were confidential to the IDSMC members. The IDSMC considered patient safety alongside treatment efficacy when making recommendations regarding continuation, amendment or discontinuation of the trial. In order to estimate the effect of clonidine and midazolam for the primary outcome it was planned that the Haybittle–Peto approach would be used for requested interim analyses considering superiority, with 99.9% confidence intervals (CIs) calculated for the effect estimate. This method was chosen to ensure that interim efficacy results would have to be extreme before recommending early termination in order to be convincing to the clinical community.
Analysis plan
All analyses were conducted according to the SAP (see Appendix 3), which provides a detailed and comprehensive description of the main, preplanned analyses for the study. Analyses were performed with standard statistical software (SAS, version 9.3; SAS Institute Inc., Cary, NC, USA), apart from those in the health-economic analyses that were undertaken using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) (see Appendix 4 for details).
The main features of the SAP are summarised below.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is used to summarise representativeness of the study sample and patient throughput. Baseline characteristics are presented by treatment group and overall. Continuous variables presented with means and standard deviations (SDs) if normally distributed [median and interquartile range (IQR) if skewed], along with the minimum and maximum values. Categorical variables are presented with numbers and percentages.
The intention-to-treat (ITT) principle is used as far as practically possible. Equivalence for the primary outcome will be determined with margin of equivalence ± 0.15, as defined within the revised sample size calculation. As the ITT principle may not be conservative for equivalence trials, a per-protocol analysis was planned. Protocol deviations were defined prior to analysis and classified as major or minor, the intention being that participants with major protocol deviations would not be included within the per-protocol analysis. The number (and percentage) of patients with major and minor protocol deviations are summarised by treatment group, with details of type of deviation provided. The patients included in the ITT analysis data set, as defined in section 13 of the SLEEPS SAP (see Appendix 3), are used as the denominator to calculate the percentages.
For the secondary outcomes, statistical significance will be determined by a p-value of ≤ 0.05. Dichotomous outcomes will be analysed using the chi-squared test (or Fisher’s exact test if any of the cells in the 2 × 2 contingency table have expected counts of < 5), relative risks will be calculated and reported with 95% CIs. Two sample t-tests will be used for normally distributed continuous outcomes, with difference in means reported with 95% CIs. The difference in medians will be calculated for skewed data using the Hodges–Lehman estimate with the corresponding Moses distribution-free 95% CIs. The p-value for non-parametric two-sample Mann–Whitney U-test for a difference in medians will be presented.
The log-rank test is used for time to event outcomes that have no competing risks and reported with Kaplan–Meier curves and hazard ratios with 95% CIs. Medians from the Kaplan–Meier plots with 95% CIs will be presented, along with 25% and 75% quartiles with 95% CIs. Cumulative incidence curves are used for time to event outcomes with competing risks. The CIs are approximate, as they are calculated using interpolation of estimated subdistribution function and corresponding variances. Longitudinal data analysis using mixed models will be used to examine sedative and morphine doses over time between the groups. Mean profile plots by treatment groups will be presented with one standard error bars displayed for each hour.
Health economic methods
Health economic methods
The economic evaluation aimed to assess the cost-effectiveness of clonidine compared with midazolam in the treatment of critically ill children using clinical data from the SLEEPS trial. The cost-effectiveness analysis focused on the short-term costs and consequences of the two trial drug interventions. The primary analysis (base case) used cost data from the point of randomisation until 14 days post-treatment cessation, and was carried out from a UK NHS hospital services perspective. We used a cut-off of 14 days post-treatment cessation as the time horizon of interest for the base case analysis. This decision was based on discussions with clinical experts and published evidence of clinical effectiveness describing the use of sedative agents in a PIC context. 12,23 The measure of benefit used in the economic evaluation mirrored that adopted for SLEEPS as a whole, namely an additional case of adequate sedation defined within the primary outcome as ‘at least 80% of total time spent sedated within a COMFORT score of 17 to 26’. Given the methodological limitations surrounding preference-based outcomes measurement in young children, outcomes were not expressed in terms of preference-based metrics, such as the quality-adjusted life-year (QALY).
A number of sensitivity analyses were planned to test the robustness of the base case economic evaluation results. In addition, a scenario analysis was planned, adopting a wider perspective and including additional direct NHS economic costs [e.g. those costs attributable to GP visits and accident and emergency (A&E) attendances] utilising data from the point of randomisation to 14 days post-treatment cessation.
Collection of resource-use data
The SLEEPS study CRFs captured all resource use related to the child’s primary hospital admission, including trial drug treatments as well any transfers between wards and hospitals. Wider NHS resource use (e.g. GP visits, A&E visits, and readmissions to hospital) that took place within 14 days of treatment cessation were also recorded.
Specifically, individualised hospital services resource use was estimated for trial drug interventions, including pharmaceuticals and consumables [e.g. clonidine, midazolam, morphine, needle, syringe, line extension kit, line filter), duration of primary hospital admission in PICUs, ward transfers, length of stay (LoS) in any ward post PICU [e.g. stays in high-dependency units (HDUs) and/or general medical paediatric wards (GMs)], hospital–hospital transfers, and theatre time incurred during the treatment of SAEs.
All resource-use data were entered directly from the SLEEPS CRFs into the MACRO (InferMed Ltd, London, UK) trial database, with in-built safeguards against inconsistent entries.
Valuation of resource-use cost data
Unit costs for resources used by children who participated in the study were obtained from a variety of secondary sources. All unit costs utilised followed recent guidelines on costing health-care services as part of an economic evaluation [National Institute for Health and Care Excellence (NICE47)]. Where necessary, secondary information was obtained from ad hoc studies reported in the literature. Unit costs of hospital and community health-care costs were largely derived from national sources and took account of the cost of the health professionals’ qualifications (Curtis48). All PICU and HDU costs were valued using the NHS Reference Costs,49 a catalogue of costs compiled by the Department of Health in England (Department of Health49).
The main cost driver in the economic evaluation was the cost of critical care. The 24-hour critical care (per diem) NHS reference cost was calculated on a full absorption costing basis and included hotel services, nursing/medical and other clinical staff, therapy services and staff, ward consumables, blood and blood products, drugs, diagnostics, and medical and surgical equipment. Stays in critical care were valued using a half-a-day PICU cost for periods of < 12 hours and a full-day PICU cost for periods of between 12 and 24 hours.
Drug costs were obtained from the British National Formulary (BNF 201250) and Monthly Index of Medical Specialities (MIMS 201351). Consumables were costed using data from NHS Supply Chain catalogue. 52 All costs were expressed in pound sterling and valued at 2011–12 prices (with the exception of a small number of drug costs; see Appendix 10). None of the costs were inflated or deflated for use in the economic evaluation. For the base case analysis, unit costs were combined with resource volumes to obtain a net cost per child covering all categories of hospital costs. A range of sensitivity analyses also explored the implications of uncertainty surrounding the values of key cost parameters (described below). Further details on the methods used to value resource use are provided in Appendix 10.
Cost-effectiveness analytic models
As described above, the primary measure was an additional case of adequate sedation. The results of the economic evaluation were restricted to the patients for whom the primary outcome in the SLEEPS trial was available. In the cost-effectiveness analysis, the incremental cost-effectiveness ratio (ICER) was calculated as the difference in average costs (ΔC) divided by the difference in average effects (ΔE) between the clonidine and midazolam groups and expressed as the incremental cost per case of adequate sedation. No discounting of costs or benefits to present values was necessary as the time horizon of the economic evaluation (period of follow-up) was < 12 months.
Independent-sample t-tests were used to test for differences in resource use, costs and primary clinical outcomes between treatment groups. All statistical tests were two-tailed. Differences in resource use, costs and effects between the comparator groups were considered significant if two-tailed p-values were ≤ 0.05.
In common with many trial-based economic evaluations, the distributions for costs were skewed. Consequently, non-parametric bootstrap estimation was used to derive 95% CIs for mean cost differences between the comparator groups. 53 Each of these CIs was calculated using 1000 bias-corrected bootstrap replications. Non-parametric bootstrap simulation of the cost–effect pairs was also performed to generate 1000 replications of the ICER; these were subsequently represented graphically on a four-quadrant cost-effectiveness plane as described by Black et al. 54 As illustrated in the paper by Stinnett and Mullahy,55 mean net benefits, defined as Rc.ΔE – ΔC, were estimated for alternative values of Rc, the maximum acceptable ICER or cost-effectiveness threshold for the primary outcome, namely each additional case of adequate sedation. Although both stated and revealed that preference techniques have been used to estimate maximum acceptable ICERs or cost-effectiveness thresholds for generic measures of health outcome, such as the QALY (Gray et al. 56), no comparative data are available for the primary health outcome for this study. Consequently, the cost-effectiveness threshold was varied in our analyses between hypothetical values of £0 and £5000 per additional case of adequate sedation. A value of £1000 per additional case of adequate sedation was selected as the primary cost-effectiveness threshold for statements about cost-effectiveness and mean net benefits.
Cost-effectiveness acceptability curves (CEACs) showing the probability that clonidine is cost-effective relative to midazolam across a range of cost-effectiveness thresholds were also generated based on the proportion of bootstrap replicates with positive incremental net benefits. 55,57 The probability that clonidine is less costly or more effective than midazolam was based on the proportion of bootstrap replicates that had negative incremental costs or positive incremental health benefits, respectively.
Sensitivity analyses
Several sensitivity analyses were undertaken to assess the impact on cost-effectiveness results of areas of uncertainty surrounding components of the base case economic evaluation. All of these sensitivity analyses comprised complete data for 120 children (mirroring the strategy adopted for the primary efficacy assessments):
-
Sensitivity analysis (1) We varied the cost of higher-level inpatient care (stay in the PICU or HDU) by applying upper-quartile NHS Reference Costs49 across trusts.
-
Sensitivity analysis (2) We varied the cost of higher-level inpatient care (stay in the PICU or HDU) by applying lower quartile NHS Reference Costs49 across trusts.
-
Sensitivity analysis (3) We used exact proportions of 24-hour periods to value total lengths of stay rather than apply either one half day or full day per diems to 0–12 hour or 12–24 periods for costing purposes.
-
Sensitivity analysis (4) We extended the time horizon of the economic evaluation to cover the period between randomisation and 14 days postventilation cessation.
-
Sensitivity analysis (5) We widened the primary outcome definition to ‘. . . at least 75% of total time spent sedated within a COMFORT score of 17 to 26’.
-
Sensitivity analysis (6) We narrowed the primary outcome definition to ‘at least 85% of total time spent sedated within a COMFORT score of 17 to 26’.
In addition, a scenario analysis was performed, which comprised 106 children for whom complete data were available, and included wider NHS costs incurred within 14 days post-treatment cessation. These wider costs included costs associated with GP visits, A&E attendances and hospital readmissions. These additional costs are unlikely to be attributable to choice of sedative agents used in PICUs; hence their relegation to a scenario analysis.
Protocol amendments
Key protocol amendments are summarised within Table 3, and details are provided within Appendix 5.
| Area of protocol amendment | Version containing amendment | Details |
|---|---|---|
| Eligibility criteria | 5.0 | Reduction from 24 hours to 12 hours for number of hours for which a child is likely to require intubation and ventilation |
| 5.0 | Increase from 48 hours to 120 hours for the period children can be entered into the trial following admission to PICU. Addition of ‘ICU’, as child may have been admitted to ICU initially rather than PICU | |
| 4.0 | Amendment from ‘likely to require intubation and ventilation for more than 48 hours’ to ‘likely to require intubation and ventilation for more than 24 hours’ | |
| Allocated treatment regimen and morphine administration | 5.0 | Adjustment to trial treatment and morphine administration to allow bedside nurse to evaluate child for pain and conscious level to decide whether trial treatment or morphine should be adjusted |
| 5.0 | Addition of text to say that when a COMFORT score of < 17 is recorded, the score must remain below 17 for two consecutive hours before the morphine is reduced | |
| 4.0 | Addition of text to state that if a child is receiving the minimum infusions of trial sedation and morphine and the child is oversedated, the morphine can be further reduced by an increment of 10 µg/kg/hour to 10 µg/kg/hour, providing that there are no requirements for analgesia. If the child is still oversedated, the morphine can be stopped (as long as there are no analgesic requirements), although the trial sedation should continue | |
| Outcome definitions/recording | 5.0 | Addition of text to indicate that following trial treatment cessation, the only COMFORT score category that needs to be completed is ‘Alertness’, and that if sedation is still required following trial treatment cessation then the COMFORT score should continue to be measured hourly until the child is stable on the new sedative |
| 5.0 | Definition of treatment failure changed from the administration of three rescue boluses within any one 12-hour period to three ‘events’ where rescue medication(s) are needed to re-establish sedation or pain control occurring within any one 12-hour period during trial treatment |
Chapter 3 Results
Recruitment
Recruitment rate targets
The initial target sample size of the trial (1000 participants) was expected to be achieved within a 2-year recruitment period. This was based on average accrual of one patient per week at each of 12 sites. The proposal was presented to the Paediatric Intensive Care Society Study Group (PICSSG) and each site agreed with the target recruitment rates and considered them achievable at the outset.
Screening
A total of 10,023 participants were screened and assessed for eligibility to be randomised, of whom 9196 did not meet the inclusion criteria, leaving 827 who were eligible. A summary of the screening results by site is provided in Table 4, with the reasons for ineligibility provided in Table 5 and 6. The most common reasons for ineligibility were that the patient was aged < 30 days; did not require sedation; was not intubated; was not expected to be ventilated for sufficient time; or was on muscle relaxants.
| Centre code | Hospital | Screened | Not eligible, n (%) | Eligible and not randomised,a n (%) | Eligible and randomised, n (%) |
|---|---|---|---|---|---|
| 30 | Leeds General Infirmary | 160 | 147 (91.9) | 12 (7.5) | 1 (0.6) |
| 116 | Bristol Royal Children’s Hospital | 1713 | 1510 (88.1) | 187 (10.9) | 16 (0.9) |
| 133 | Birmingham Children’s Hospital | 2372 | 2281 (96.2) | 65 (2.7) | 26 (1.1) |
| 213 | Queen’s Medical Centre, Nottingham | 688 | 596 (86.6) | 48 (7.0) | 44 (6.4) |
| 243 | Royal Liverpool Children’s Hospital | 2527 | 2310 (91.4) | 207 (8.2) | 10 (0.4) |
| 246 | Royal Manchester Children’s Hospital | 944 | 916 (97.0) | 26 (2.8) | 2 (0.2) |
| 371 | The Royal Hospital for Sick Children, Glasgow | 727 | 655 (90.1) | 70 (9.6) | 2 (0.3) |
| 499 | Leicester Royal Infirmary | 325 | 265 (81.5) | 41 (12.6) | 19 (5.8) |
| 522 | University Hospital North Staffordshire | 324 | 294 (90.7) | 22 (6.8) | 8 (2.5) |
| 540 | Royal Belfast Hospital for Sick Children | 243 | 222 (91.4) | 20 (8.2) | 1 (0.4) |
| Total | 10,023 | 9196 (91.7) | 698 (7.0) | 129 (1.3) |
| Centre code | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | R13 | R14 | R15 | R16 | R17 | R18 | R19 | R20 | R21 | Not eligible other | Not eligible, missing reason |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 6 | – | 3 | 11 | 1 | – | 1 | – | – | 2 | 5 | 6 | – | – | 6 | – | 21 | – | 54 | 29 | – | 20 | – |
| 116 | 270 | 14 | 482 | 29 | 46 | 81 | – | 7 | 4 | 12 | 30 | 74 | 15 | 1 | 51 | 7 | 177 | 13 | 326 | 114 | 2 | 59 | 19 |
| 133 | 459 | 25 | 103 | 65 | 18 | 67 | 61 | 144 | – | 4 | 46 | 78 | 21 | 15 | 19 | 3 | 219 | 2 | 920 | 513 | 7 | 18 | 5 |
| 213 | 38 | 19 | 141 | 14 | 16 | 5 | 9 | – | 6 | 2 | 38 | 50 | 14 | – | 21 | – | 53 | 1 | 229 | 29 | 7 | 14 | 5 |
| 243 | 597 | 54 | 379 | 20 | 30 | 60 | 133 | 47 | 2 | 2 | 42 | 50 | 19 | 5 | 110 | 2 | 153 | 3 | 782 | 150 | – | 35 | 10 |
| 246 | 83 | 18 | 60 | 7 | 7 | 9 | 18 | – | 2 | 3 | 55 | 62 | 24 | 1 | 85 | – | 28 | – | 316 | 137 | 1 | 18 | 3 |
| 371 | 82 | 6 | 107 | 18 | 6 | – | 1 | 3 | – | 4 | 1 | 1 | 5 | 13 | 20 | – | 98 | – | 227 | 72 | – | 13 | 7 |
| 499 | 18 | 3 | 28 | 17 | 1 | 2 | 13 | – | 1 | – | 4 | 15 | – | – | 8 | – | 51 | – | 60 | 37 | 3 | 19 | 2 |
| 522 | 20 | 13 | 36 | 3 | 3 | 1 | 1 | – | 2 | – | 5 | 13 | 2 | 2 | 26 | 2 | 34 | – | 125 | 43 | 2 | 4 | 6 |
| 540 | 38 | – | 16 | 2 | 5 | – | – | – | – | 1 | 5 | 13 | 3 | 1 | 24 | – | 9 | – | 111 | – | – | 4 | 2 |
| Total | 1611 | 152 | 1355 | 186 | 133 | 225 | 237 | 201 | 17 | 30 | 231 | 362 | 103 | 38 | 370 | 14 | 843 | 19 | 3150 | 1124 | 22 | 204 | 59 |
| Code | Reason for exclusion |
|---|---|
| R1 | < 30 days of age (< 37 weeks’ gestation) (children born before 37 weeks’ gestation are eligible if they are a minimum of 30 days post delivery and their corrected gestation is ≥ 37 weeks) |
| R2 | ≥ 16 years of age |
| R3 | Not likely to require ventilation for > 12 hours |
| R4 | On PICU for > 120 hours (please ensure patient has not already been included on a screening log during this PICU stay) |
| R5 | > 50 kg in weight |
| R6 | Currently participating in conflicting clinical study (i.e. CHiP or StePS) or participation in clinical study involving a medicinal product in the last month |
| R7 | Not adequately sedated (COMFORT score of < 17 or > 26) |
| R8 | Open chest following cardiac surgery |
| R9 | Chronically treated for raised BP |
| R10 | Current treatment with beta-blockers |
| R11 | Acute traumatic brain injury |
| R12 | Status epilepticus or active fitting |
| R13 | Haemodialysis/haemofiltration required |
| R14 | ECMO required |
| R15 | Severe neuromuscular problems/impairment (not possible to perform a COMFORT score) |
| R16 | Known allergy to clonidine, midazolam or morphine |
| R17 | Treatment with continuous or intermittent muscle relaxants (not possible to perform a COMFORT score) |
| R18 | Known to be pregnant |
| R19 | Not intubated |
| R20 | Sedation not required |
| R21 | Previously participated in the SLEEPS trial |
Screening logs were monitored throughout the trial. Teleconferences and a face-to-face meeting were held with PIs and research nurses to learn from the processes and experiences at sites with the highest recruitment rates, and to identify and resolve barriers to recruitment across sites. This led to changes to the inclusion criteria to increase the time allowed for the child to be on PICU prior to randomisation; decrease the time for which the child was expected to be ventilated (key amendments summarised within Table 3 and details provided within Appendix 5); and increase the flexibility in the administration of the interventions and concomitant medications, including morphine.
Eligible patients
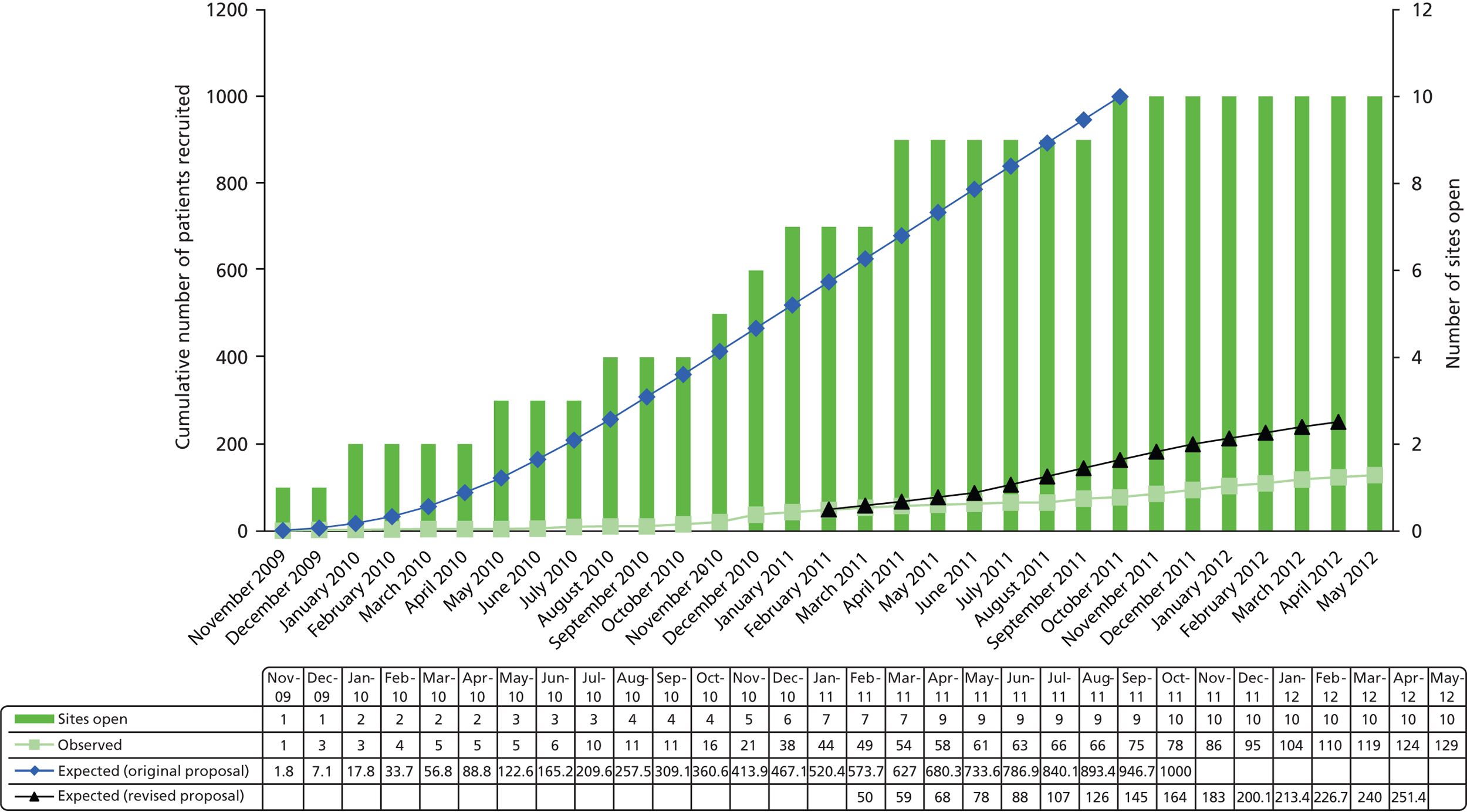
The number of eligible participants was lower than anticipated and the percentage converted to randomised participants much lower with considerable variation across sites. Figure 2 displays predicted recruitment compared with actual recruitment across sites during the trial recruitment period. The recruitment period was extended, and the original and revised recruitment curves are displayed. Of the 827 eligible participants, 698 (84%) participants were eligible and not randomised. Key reasons for eligible patients not being randomised are provided in Table 7 and show that 14% patients were missed, consent was not obtained for 23% (194/827) and various other reasons for 41%. A breakdown of ‘other reasons’ provided are given in Appendix 8, Table 40.
FIGURE 2.
Expected versus actual recruitment rates.
| Centre code | Hospital | Patient missed | Lack of GCP staff | Too busy | No consentb | Eligible other | Eligible missing reason | Total reasons | Total patients |
|---|---|---|---|---|---|---|---|---|---|
| 30 | Leeds General Infirmary | – | – | – | 4 | 9 | – | 13 | 12 |
| 116 | Bristol Royal Children’s Hospital | 75 | 2 | 5 | 37 | 76 | 6 | 201 | 187 |
| 133 | Birmingham Children’s Hospital | 5 | 1 | – | 28 | 28 | 8 | 70 | 65 |
| 213 | Queen’s Medical Centre, Nottingham | 4 | 3 | – | 24 | 10 | 10 | 51 | 48 |
| 243 | Royal Liverpool Children’s Hospital | 7 | 1 | 7 | 63 | 136 | 7 | 221 | 207 |
| 246 | Royal Manchester Children’s Hospital | 6 | 5 | – | 7 | 9 | – | 27 | 26 |
| 371 | The Royal Hospital for Sick Children, Glasgow | 4 | 18 | 2 | 5 | 45 | 1 | 75 | 70 |
| 499 | Leicester Royal Infirmary | 15 | 1 | 1 | 14 | 13 | 3 | 47 | 41 |
| 522 | University Hospital North Staffordshire | 3 | 1 | 1 | 7 | 8 | 3 | 23 | 22 |
| 540 | Royal Belfast Hospital for Sick Children | – | 8 | 2 | 5 | 6 | 2 | 23 | 20 |
| Total | 119 | 40 | 18 | 194 | 340 | 40 | 751 | 698 |
Discussions and monitoring across sites also led to suggestions that consent rates (Table 8) were higher the earlier that parents were first approached to discuss the trial. This was supported by data recording times of first contact and consent. The importance of an early approach to inform parents that the hospital was participating in the trial and that they maybe approached at a later time for consent was stressed to sites. However, all sites recognised that parents were more reluctant to enter the trial than expected when the trial was initially planned. Parents entering into PICU with a critically ill child were reluctant to give consent once the child had stabilised on the standard sedation medication, even although the agents used were often the same drugs as used in the study. This reflects both the fear of changing from something that was perceived as being one stable aspect of their care to an unknown, and the societal change to be less willing to participate in paediatric research studies (consent rates for drug based/intervention paediatric studies have fallen in recent years). In addition, pressures on PICU beds, the need for reduced patient day occupancy on PICU, and techniques in non-invasive ventilation have driven forward clinical techniques of early extubation. Although this may have been beneficial for patient care, a side effect of this has been the reduction in available ventilated clinical cases that could be entered into the study. Specifically, children undergoing cardiac surgery that would have been ventilated for several days 5 years ago are now being extubated the same day or even in the operating theatre (fast-track and ultra-fast-track surgery). 58 At the planning stage of the study it had been envisaged that a significant number of the patient recruitment would come from the postoperative cardiac patients, but owing to the above issue the best recruitment came from non-cardiac intensive care patients and only four patients were entered into the trial post cardiac surgery.
| Reason | Total patients |
|---|---|
| Parents declined (no reason given) | 128 |
| Declined all research | 20 |
| Child settled, so did not want to change sedation | 16 |
| Parental stress | 12 |
| Previously declined to SLEEPS | 6 |
| No reason given | 2 |
| Child fostered, so unable to give consent | 1 |
| Child has been through enough already | 1 |
| Do not want clonidine owing to its side effects | 1 |
| Mum declined but was initially keen – child was going for further surgery | 1 |
| Mum not willing to alter child’s regime | 1 |
| Mum said ‘no’ to blinded aspect of trial | 1 |
| Other – parents did not want to introduce any new treatments owing to uncertainty of child’s condition | 1 |
| Parents did not want their child to be used as a guinea pig | 1 |
| Strict protocol does not suit her | 1 |
| Too many decisions/treatment plans at present | 1 |
| Total | 194 |
Additional feedback from the sites was that the inclusion criteria requiring the child to be adequately sedated at the time of randomisation were a barrier to participation. This appeared within the ‘other reasons’ provided across sites and was discussed extensively among the trial management team, PIs and research nurses, but the eligibility criteria could not be amended. It was felt by participating centres that commencing the study when baseline control of sedation had not been obtained would be unacceptable for clinical management, even although it was acknowledged that loading of sedative and analgesia drugs with the commencement of the study would improve the COMFORT scores towards the target range.
The reasons why the eligible 698 patients were not randomised; more than one reason per patient could be recorded are summarised above (see Table 7).
Flow of randomised participants
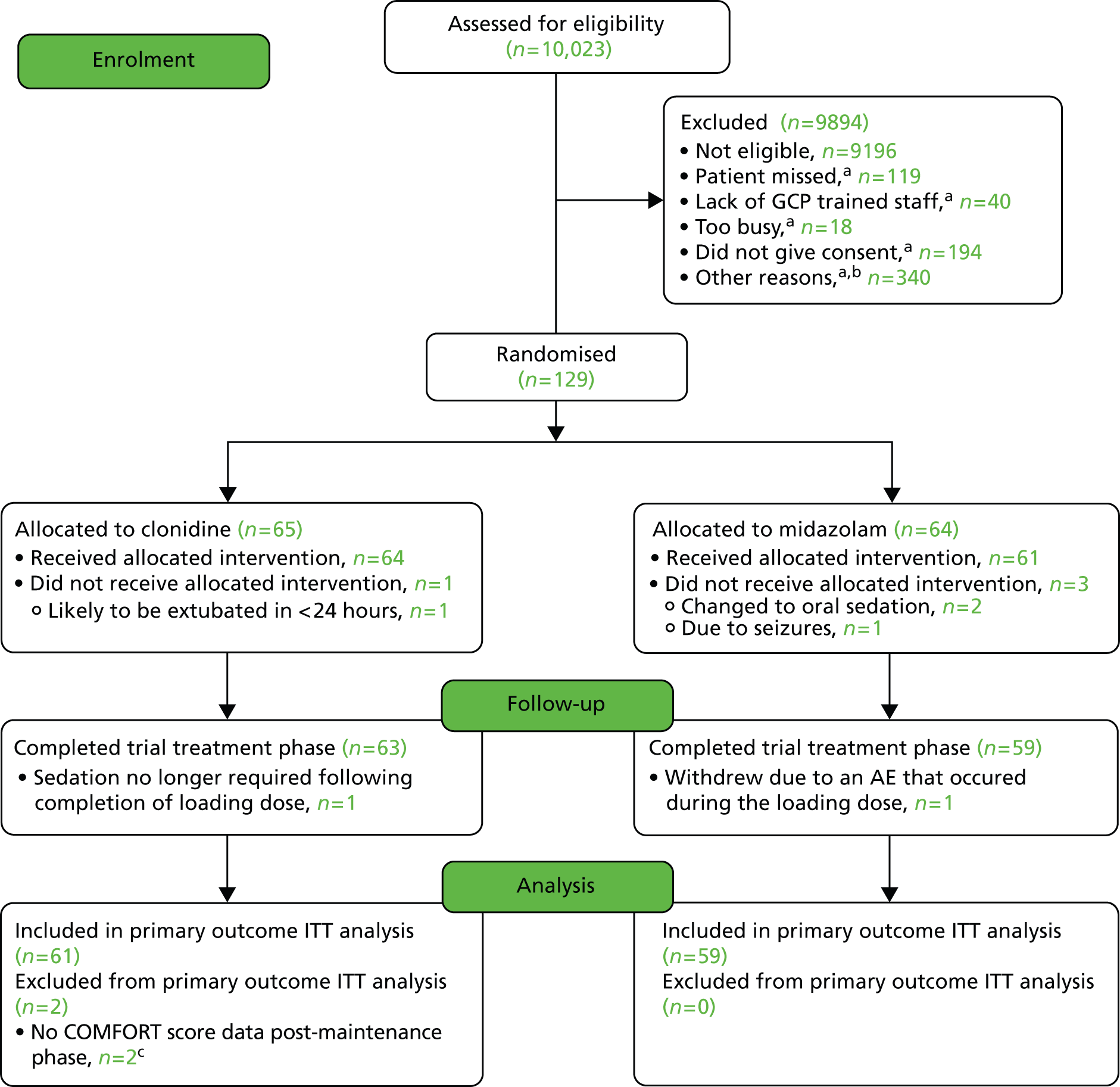
Figure 2 shows the actual rates of recruitment compared with the predicted rates of recruitment. Table 9 shows the dates the site was opened/closed to recruitment, the dates of first/last randomisations and the number of participants randomised for each of the 10 recruiting sites. The first patient was randomised into the trial on 18 November 2009 and the last patient randomised was on 19 May 2012. The participant flow diagram is provided in Figure 3. Of the 129 randomised participants, four did not receive their allocated treatment: one (2%) in the clonidine group because he/she was likely to be extubated within 24 hours; and three (5%) in the midazolam group – two of these because they were changed to oral sedation and one because of seizures. The study continued until its funding was exhausted.
FIGURE 3.
Participant flow diagram. a, Else otherwise eligible patients (more than one reason could be selected); b, breakdown of other reasons is listed in Appendix 8, Table 40; c, sedation stopped after 2 hours 15 minutes and 2 hours 35 minutes post randomisation.
| Centre code | Hospital | Date site: | Date of: | No. randomised | ||
|---|---|---|---|---|---|---|
| Opened to recruitment | Closed to recruitment | First randomisation | Last randomisation | |||
| 213 | Queen’s Medical Centre, Nottingham | 13 July 2010 | 31 May 2012 | 18 July 2010 | 18 May 2012 | 44 |
| 133 | Birmingham Children’s Hospital | 26 April 2010 | 31 May 2012 | 2 August 2010 | 19 May 2012 | 26 |
| 499 | Leicester Royal Infirmary | 24 November 2010 | 31 May 2012 | 24 November 2010 | 3 May 2012 | 19 |
| 116 | Bristol Royal Children’s Hospital | 13 October 2009 | 31 May 2012 | 18 November 2009 | 22 December 2011 | 16 |
| 243 | Royal Liverpool Children’s Hospital | 11 January 2010 | 31 May 2012 | 17 June 2010 | 14 May 2012 | 10 |
| 522 | University Hospital North Staffordshire | 16 March 2011 | 31 May 2012 | 4 April 2011 | 5 April 2012 | 8 |
| 246 | Royal Manchester Children’s Hospital | 17 January 2011 | 31 May 2012 | 6 January 2012 | 6 January 2012 | 2 |
| 371 | The Royal Hospital for Sick Children, Glasgow | 25 October 2010 | 31 May 2012 | 3 December 2010 | 7 March 2011 | 2 |
| 030 | Leeds General Infirmary | 6 April 2011 | 31 May 2012 | 16 September 2011 | 16 September 2011 | 1 |
| 540 | Royal Belfast Hospital for Sick Children | 27 October 2011 | 31 May 2012 | 14 December 2011 | 14 December 2011 | 1 |
| Total | 129 | |||||
Three participants who received at least one dose of their allocated treatment did not complete the trial treatment phase; one participant (2%) in the clonidine group because sedation was no longer required following completion of the loading dose and two (3%) participants in the midazolam group, who both withdrew because of an adverse event (AE) that occurred during the loading dose.
Two (3%) participants in the clonidine group that completed the trial treatment phase did not have any COMFORT score data post maintenance phase (on treatment for 2 hours 15 minutes and 2 hours 35 minutes, respectively) so could not contribute data to the primary outcome. The primary outcome analysis includes data for 61 of 65 (93.8%) participants allocated to the clonidine group, and 59 of 64 (92.2%) participants in the midazolam group.
Baseline comparability of randomised groups
Table 10 shows that the demographic characteristics of the 129 randomised participants were similar. Table 11 provides a summary of disease characteristics at baseline. Overall, the groups were similar at baseline; however, there were a higher percentage of children randomised in the midazolam group with chest disease. It was intended that the Paediatric Logistic Organ Dysfunction (PELOD) score be calculated for participants; however, only 15 patients (4 clonidine, 11 midazolam) out of 129 randomised had complete data for all of the categories of the PELOD scoring system. The majority of the patients (112, 86.8%) had an incomplete ‘Hepatic’ section because international normalised ratio (INR) and prothrombin time were not measured routinely with blood samples. Table 12 shows the level of sedation as measured by the COMFORT score, sedatives and inotropic support received at trial entry. There was a higher proportion of children receiving inotropic support prior to consent in the clonidine group; however, numbers were small (10 vs. 4). Patients undergoing cardiac surgery (3 vs. 1; see Table 11) would be on inotropes on entering the study, leaving the difference of inotropes at trial entry as 7 for clonidine and 3 for midazolam.
| Baseline characteristic | Clonidine (N = 65) | Midazolam (N = 64) | Total (N = 129) |
|---|---|---|---|
| Patients randomised, n | 65 | 64 | 129 |
| Gender, n (%) | |||
| Male | 43 (66.2) | 38 (59.4) | 81 (62.8) |
| Female | 22 (33.8) | 26 (40.6) | 48 (37.2) |
| Missing | 0 | 0 | 0 |
| Age at consent (years) | |||
| Median | 0.60 | 0.53 | 0.60 |
| IQR | 0.18–1.84 | 0.27–1.30 | 0.24–1.40 |
| Minimum | 0.08 | 0.09 | 0.08 |
| Maximum | 13.85 | 9.53 | 13.85 |
| Missing | 0 | 0 | 0 |
| Weight of child (kg) | |||
| Median | 6.60 | 7.00 | 6.80 |
| IQR | 4.20–12.00 | 4.00–10.00 | 4.00–10.60 |
| Minimum | 2.60 | 2.20 | 2.20 |
| Maximum | 50.00 | 30.00 | 50.00 |
| Missing | 0 | 0 | 0 |
| Weight group, n (%) | |||
| N = 65 | N = 64 | N = 129 | |
| < 10 kg | 43 (66.2) | 47 (73.4) | 90 (69.8) |
| 10–25 kg | 18 (27.7) | 15 (23.4) | 33 (25.6) |
| > 25–50 kg | 4 (6.1) | 2 (3.2) | 6 (4.6) |
| Category | Baseline characteristic | Clonidine (N = 65) | Midazolam (N = 64) | Total (N = 129) |
|---|---|---|---|---|
| Patients randomised, n | 65 | 64 | 129 | |
| General | Reasons for admission to PICU, n (%): | |||
| Patients with one reason | 64 (98.5) | 58 (90.6) | 122 (94.6) | |
| Patients with two reasonsa | 1 (1.5) | 6 (9.4) | 7 (5.4) | |
| Sepsis | 9 (13.9) | 8 (12.5) | 17 (13.2) | |
| Chest disease | 37 (56.9) | 51 (79.7) | 88 (68.2) | |
| Cardiac disease | 1 (1.5) | 1 (1.6) | 2 (1.6) | |
| Post cardiac surgery | 3 (4.6) | 1 (1.6) | 4 (3.1) | |
| Neurological disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Otherb | 16 (24.6) | 9 (14.1) | 25 (19.4) | |
| Missing | 0 | 0 | 0 | |
| (Note: above categories are not mutually exclusive) | ||||
| Glasgow Coma Scale score total, n: | 48 | 55 | 103 | |
| Mean | 9.31 | 8.85 | 9.07 | |
| SD | 3.37 | 3.20 | 3.27 | |
| Minimum | 3.00 | 3.00 | 3.00 | |
| Maximum | 15.00 | 15.00 | 15.00 | |
| Missing: | 17 | 9 | 26 | |
| – Missing ‘Verbal’ section | 12 | 8 | 20 | |
| – Missing ‘Eyes open’ section | 1 | 0 | 1 | |
| – Missing all sections | 4 | 1 | 5 | |
| Pacing system, n (%): | ||||
| Yes | 3 (4.6) | 0 (0.0) | 3 (2.3) | |
| No | 62 (95.4) | 64 (100.0) | 126 (97.7) | |
| Missing | 0 | 0 | 0 | |
| Cardiovascular | BP, systolic (mmHg) | |||
| Mean | 88.38 | 86.52 | 87.46 | |
| SD | 13.65 | 14.33 | 13.97 | |
| Minimum | 59.00 | 55.00 | 55.00 | |
| Maximum | 124.00 | 125.00 | 125.00 | |
| Missing | 0 | 0 | 0 | |
| BP, diastolic (mmHg) | ||||
| Mean | 45.23 | 45.04 | 45.14 | |
| SD | 9.54 | 9.85 | 9.66 | |
| Minimum | 26.00 | 14.00 | 14.00 | |
| Maximum | 70.00 | 68.00 | 70.00 | |
| Missing | 0 | 0 | 0 | |
| Heart rate (bpm): | ||||
| Mean | 131.52 | 132.16 | 131.84 | |
| SD | 24.46 | 20.27 | 22.40 | |
| Minimum | 70.00 | 84.00 | 70.00 | |
| Maximum | 195.00 | 170.00 | 195.00 | |
| Missing | 0 | 0 | 0 | |
| Average BP MAP over 4 hours previous to trial entry (mmHg) | ||||
| Median | 60.00 | 60.00 | 60.00 | |
| IQR | 55.00–65.00 | 52.50–66.50 | 55.00–66.00 | |
| Minimum | 40.00 | 40.00 | 40.00 | |
| Maximum | 80.00 | 134.00 | 134.00 | |
| Missing | 0 | 0 | 0 | |
| Average heart rate over 4 hours previous to trial entry (bpm) | ||||
| Mean | 130.31 | 132.14 | 131.22 | |
| SD | 22.52 | 18.23 | 20.44 | |
| Minimum | 75.00 | 98.00 | 75.00 | |
| Maximum | 191.00 | 172.00 | 191.00 | |
| Missing | 0 | 0 | 0 | |
| Pulmonary | PaO2 (KPa) | |||
| Median | 9.75 | 9.15 | 9.60 | |
| IQR | 6.40–12.60 | 6.90–12.00 | 6.60–12.40 | |
| Minimum | 3.50 | 4.20 | 3.50 | |
| Maximum | 100.50 | 87.00 | 100.50 | |
| Missing | 7 | 0 | 7 | |
| FiO2 (%) | ||||
| Median | 38.00 | 40.00 | 40.00 | |
| IQR | (30.00, 50.00) | (30.00, 50.00) | (30.00, 50.00) | |
| Minimum | 20.00 | 4.00 | 4.00 | |
| Maximum | 100.00 | 87.00 | 100.00 | |
| Missing | 1 | 0 | 1 | |
| PaCO2 (KPa) | ||||
| Median | 6.25 | 5.85 | 6.00 | |
| IQR | 5.30–7.05 | 5.05–6.80 | 5.10–6.90 | |
| Minimum | 3.70 | 3.80 | 3.70 | |
| Maximum | 10.90 | 12.80 | 12.80 | |
| Missing | 5 | 0 | 5 | |
| Neurological | Pupillary reaction, n (%) | |||
| Both reactive | 64 (100.0) | 63 (98.4) | 127 (99.2) | |
| Both fixed | 0 (0.0) | 1 (1.6) | 1 (0.8) | |
| Missing | 1 | 0 | 1 | |
| Clinical laboratoryc | Prothrombin time (seconds)c | |||
| Mean | 13.07 | 13.96 | 13.55 | |
| SD | 1.97 | 4.07 | 3.28 | |
| Minimum | 10.40 | 1.00 | 1.00 | |
| Maximum | 18.20 | 26.20 | 26.20 | |
| Missingc | 36 | 30 | 66 | |
| INRc | ||||
| Median | 1.10 | 1.30 | 1.2 | |
| IQR | 1.10–1.30 | 1.10–1.30 | 1.10–1.30 | |
| Minimum | 1.00 | 0.90 | 0.90 | |
| Maximum | 1.80 | 2.60 | 2.60 | |
| Missingc | 50 | 33 | 103 | |
| WBC (109/l)c | ||||
| Median | 8.30 | 8.25 | 8.30 | |
| IQR | 6.30–13.70 | 5.60–13.00 | 5.80–13.10 | |
| Minimum | 1.60 | 1.20 | 1.20 | |
| Maximum | 31.10 | 43.50 | 43.50 | |
| Missingc | 4 | 6 | 10 | |
| Platelets (109/l)c | ||||
| Median | 279.00 | 260.00 | 270.50 | |
| IQR | 143.00–352.00 | 143.00–358.00 | 172.0–354.50 | |
| Minimum | 44.00 | 29.00 | 29.00 | |
| Maximum | 587.00 | 685.00 | 685.00 | |
| Missingc | 4 | 5 | 9 |
| Category | Baseline characteristic | Clonidine (N = 65) | Midazolam (N = 64) | Total (N = 129) |
|---|---|---|---|---|
| Patients randomised, n | 65 | 64 | 129 | |
| Inotropic support | Children receiving inotropic support prior to consent, n (%): | |||
| Yes | 10 (30.3) | 4 (14.8) | 14 (23.3) | |
| No | 23 (69.7) | 23 (85.2) | 46 (76.7) | |
| Missinga | 32 | 37 | 69 | |
| Analgesia received prior to consent | Children receiving ‘any analgesia’ prior to consent, n (%): | 63 (100.0) | 64 (100.0) | 127 (100.0) |
| No information on analgesia prior to consent | 2 | 0 | 2 | |
| Numbers who received each analgesia prior to consent, n (%): | ||||
| Clonidine | 3 (4.8) | 2 (3.1) | 5 (3.9) | |
| Fentanyl | 3 (4.8) | 6 (9.4) | 9 (7.1) | |
| Ketamine | 2 (3.2) | 2 (3.1) | 4 (3.1) | |
| Morphine | 62 (98.4) | 60 (93.8) | 122 (96.1) | |
| Paracetamol | 6 (9.5) | 4 (6.3) | 10 (7.9) | |
| Sedation received prior to consent | Time from ‘any sedation’ to consent (hours), n | 20 | 25 | 45 |
| Median | 34.54 | 20.50 | 24.50 | |
| IQR | 11.26–42.29 | 17.50–29.83 | 16.83–39.17 | |
| Minimum | 2.25 | 2.00 | 2.00 | |
| Maximum | 89.33 | 109.33 | 109.33 | |
| Missing/incorrect start date | 4 | 1 | 5 | |
| No information on sedation prior to consent | 2 | 0 | 2 | |
| Numbers who received each sedative prior to consent, n (%): | ||||
| Alimemazine | 1 (1.6) | 1 (1.6) | 2 (15.7) | |
| Chloral hydrate | 5 (7.9) | 8 (12.5) | 13 (10.2) | |
| Clonidine | 3 (4.8) | 2 (3.1) | 5 (3.9) | |
| Ketamine | 2 (3.2) | 2 (3.1) | 4 (3.1) | |
| Lorazepam | 1 (1.6) | 1 (1.6) | 2 (15.7) | |
| Midazolam | 18 (28.6) | 16 (25.0) | 34 (26.8) | |
| Trimeprazine | 0 (0.0) | 1 (1.6) | 1 (0.8) | |
| COMFORT score total at trial entry: | ||||
| Median | 18.00 | 19.00 | 19.00 | |
| IQR | 17.00–20.00 | 18.00–21.00 | 18.00–20.00 | |
| Minimum | 17.00 | 14.00b | 14.00b | |
| Maximum | 32.00b | 25.00 | 32.00b | |
| Missing | 0 | 0 | 0 | |
| Start of treatment | Time from consent to commencing trial treatment (hours): | |||
| Median | 2.37 | 2.33 | 2.33 | |
| IQR | 1.63–5.12 | 1.25–6.62 | 1.3–5.25 | |
| Minimum | 0.50 | 0.47 | 0.47 | |
| Maximum | 21.92 | 27.50 | 27.50 | |
| Missingc | 1 | 3 | 4 |
Unblinding of randomised treatments
During the trial, treatment allocations for two participants were unblinded. Both participants had received clonidine and in each case the participants were unblinded to facilitate treatment of a SAE. Details are provided below:
-
Patient 1 Trial intervention stopped because of SAE:
-
SAE description: bradycardia requiring intervention
-
Severity: moderate
-
Time on treatment (hours): 2.6
-
-
Patient 2 SAE following cessation of trial treatment:
-
SAE description: failed extubation requiring reintubation
-
Severity: severe
-
Time on treatment (hours): 42.3
-
Time from treatment cessation to SAE onset (hours): 1.8.
-
Protocol deviations
The full list of protocol deviations can be found in Appendix 7, Table 39.
There was a total of 658 protocol deviations from 113 (90.4%) participants: 271 protocol deviations in the clonidine group, with 58 (90.6%) participants having at least one, and 387 protocol deviations in the midazolam group, with 55 (90.2%) participants having at least one.
Of the protocol deviations, 557 of the 658 were major: 227 major protocol deviations in the clonidine group, with 56 (87.5%) participants having at least one, and 330 major protocol deviations in the midazolam group, with 53 (86.9%) participants having at least one.
However, 101 of the 658 protocol deviations were minor protocol deviations: 44 minor protocol deviations in the clonidine group, with 26 (40.6%) participants having at least one, and 57 minor protocol deviations in the midazolam group, with 28 (45.9%) participants having at least one.
Primary outcome
The primary objective for SLEEPS is to determine whether or not clonidine and midazolam are equivalent in terms of efficacy. A two-group, large-sample normal approximation test of proportions using two one-sided tests (TOST) for equivalence analysis59 was used with the Wald method, which was used to calculate the asymptotic confidence limits. The TOST approach includes a right-sided test for the lower margin δL and a left-sided test for the upper margin δU using one-sided 0.025 significance levels. The overall p-value is taken to be the larger of the two p-values from the lower and upper tests. The null hypothesis for the equivalence test of the difference between two proportions is H0:p1−p2≤−δL or p1−p2≥δU compared with the alternative Ha:δL<p1−p2<δU, where δL is the lower margin and δU is the upper margin. Rejection of the null hypothesis would indicate that the two binomial proportions are equivalent. The revised sample size calculation for SLEEPS uses a ± 15% (δL = –15%, δU = 15%) equivalence margin.
The results for the primary outcome (proportion of participants adequately sedated for ≥ 80% of the time) are presented in Table 13. To be included in the analysis, participants had to have been on treatment for > 2 hours (such that the loading dose is complete followed by the first hour of maintenance dose) and have had at least one COMFORT score assessed at the end of the second hour. A total of 120 participants (61 clonidine, 59 midazolam) were included in the analysis.
| Primary outcome | Clonidine (N = 61) | Midazolam (N = 59) | Total (N = 120) | Difference in proportions | 95% CIa | p-valuea |
|---|---|---|---|---|---|---|
| Adequately sedated ≥ 80%, n (%) | 21 (34.4%) | 18 (30.5%) | 39 (32.5%) | 0.04 | –0.13 to 0.21 | p = 0.10a |
The difference in proportions (clonidine – midazolam) was 0.04 with 95% CI (–0.13 to 0.21). The margin of equivalence was predefined as (–0.15 to 0.15). This is displayed graphically in Figure 4. For equivalence to be declared the two-sided 95% CI of the differences between the two groups should lie entirely within the interval (–0.15 to 0.15) labelled within the graph as region B. Values falling within region A would indicate superiority of midazolam, and those in region C indicating superiority of clonidine. The lower limit of the 95% CI (–0.13) does not extend beyond the lower limit of the equivalence margin (–0.15), thereby excluding values that would suggest midazolam is clinically superior to clonidine. The upper limit of the 95% CI (0.21) extends beyond the upper limit of the margin of equivalence (0.15), thereby including values that would suggest that clonidine is clinically superior to midazolam. As the 95% CI includes values outside the margin of equivalence, the null hypothesis that the two interventions do not provide equivalent sedation, as defined by the primary outcome, cannot be rejected. Non-inferiority of clonidine to midazolam is demonstrated but the study was underpowered to detect equivalence.
FIGURE 4.
Primary outcome result with 95% CI and equivalence margin. Note: adequately sedated ≥ 80% of the time: difference in proportions (clonidine–midazolam) with 95% CIs.
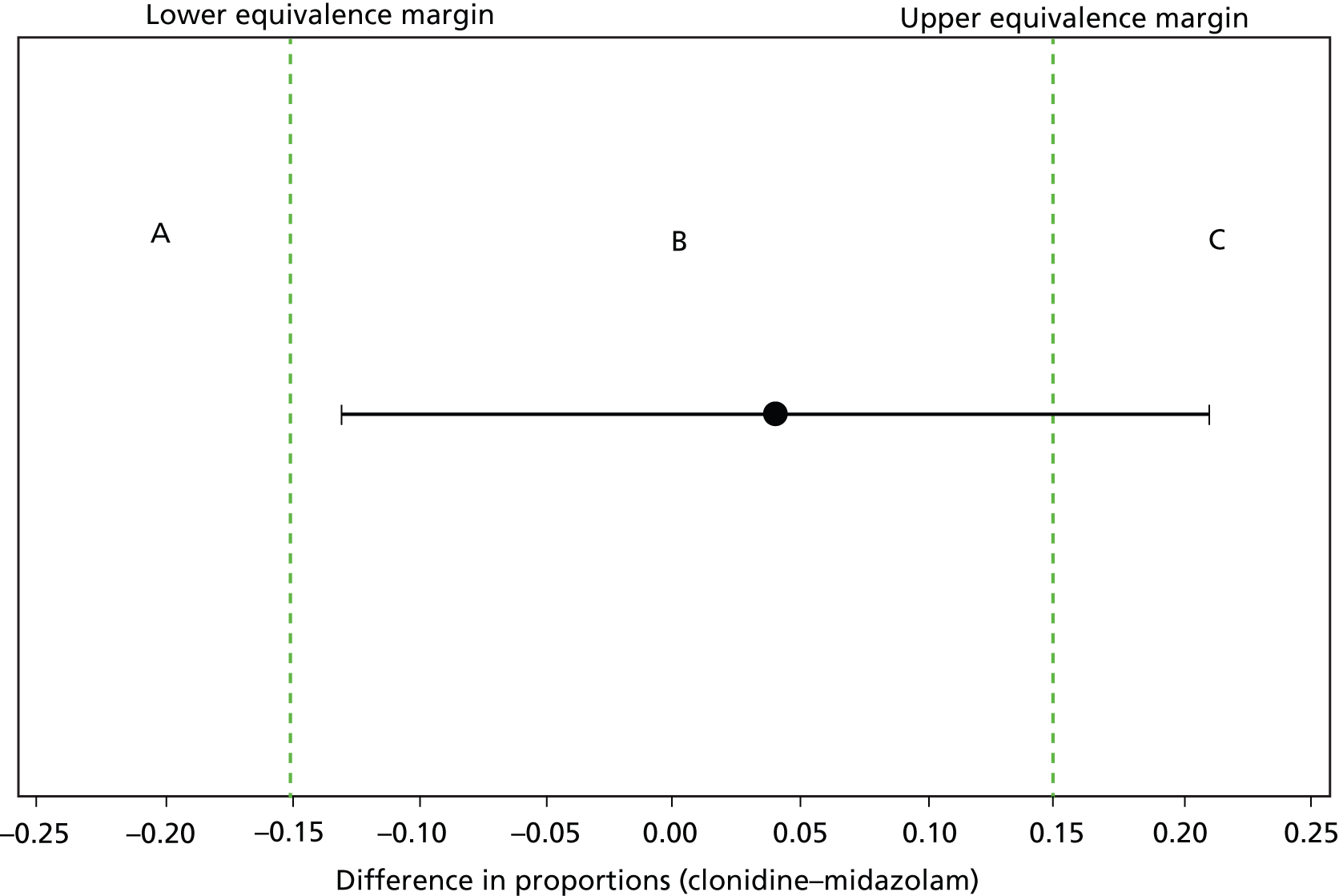
The numbers of participants with at least one missing COMFORT score during trial treatment were similar [35/61 (57.4%) clonidine, 39/59 (66.1%) midazolam] and the breakdown of the numbers of missing COMFORT scores was again similar (Table 14). Of the 54 patients (27 clonidine, 27 midazolam) that have only one missing COMFORT score hour, 48 of them [27/27 (100%) clonidine, 21/27 (77.8%) midazolam] have their final score missing with 41 of these [24/27 (88.9%) clonidine, 17/21 (81.0%) midazolam] being for an incomplete final hour. Participants in the midazolam group [median 38.25 with IQR (20.45–61.50)] were on treatment for longer than the clonidine group [median 22.83 with IQR (15.83–43.67)]. The proportions of time spent inadequately sedated, oversedated and undersedated are similar. When participants were inadequately sedated (outside the COMFORT score range of 17 to 26) they were more likely to be oversedated than undersedated.
| Category | Clonidine | Midazolam | Total |
|---|---|---|---|
| Included in primary outcome analysis | 61 | 59 | 120 |
| Missing COMFORT scores during trial treatment, n (%) | |||
| One or more missing | 35 (57.4) | 39 (66.1) | 74 (61.7) |
| 0 | 26 (42.6) | 20 (33.9) | 46 (38.3) |
| 1 | 27 (44.3) | 27 (45.8) | 54 (45.0) |
| 2 | 5 (8.2) | 8 (13.6) | 13 (10.8) |
| 3 | 2 (3.3) | 2 (3.4) | 4 (3.3) |
| 4 | 1 (1.6) | 1 (1.7) | 2 (1.7) |
| 5 | – | – | – |
| 6 | – | 1 (1.7) | 1 (0.8) |
| Hours sedateda | |||
| Median | 22.83 | 38.25 | 32.79 |
| IQR | 15.83–43.67 | 20.45–61.50 | 16.54–46.79 |
| Minimum | 0.25 | 2.00 | 0.25 |
| Maximum | 114.25 | 165.58 | 165.58 |
| Missing | 0 | 0 | 0 |
| Proportion of time spent inadequately sedated | |||
| Median | 0.26 | 0.27 | 0.26 |
| IQR | 0.16–0.45 | 0.18–0.36 | 0.17–0.42 |
| Minimum | 0.00 | 0.00 | 0.00 |
| Maximum | 1.00 | 0.67 | 1.00 |
| Missing | 0 | 0 | 0 |
| Proportion of time spent oversedated | |||
| Median | 0.17 | 0.18 | 0.18 |
| IQR | 0.05–0.33 | 0.11–0.28 | 0.07–0.29 |
| Minimum | 0.00 | 0.00 | 0.00 |
| Maximum | 1.00 | 0.55 | 1.00 |
| Missing | 0 | 0 | 0 |
| Proportion of time spent undersedated | |||
| Median | 0.07 | 0.04 | 0.05 |
| IQR | 0.00–0.13 | 0.00–0.12 | 0.00–0.13 |
| Minimum | 0.00 | 0.00 | 0.00 |
| Maximum | 0.35 | 0.54 | 0.54 |
| Missing | 0 | 0 | 0 |
The reasons for end of sedation for those 120 participants who are included in the primary analysis are provided in Table 15.
| Reason for end of allocated sedative | Clonidine (N = 61), n (%) | Midazolam (N = 59), n (%) | Total (N = 120), n (%) |
|---|---|---|---|
| Sedation no longer required | 39 (63.9) | 45(76.3) | 84 (70.0) |
| Treatment failure | 12 (19.7) | 7 (11.9) | 19 (15.8) |
| AE occurred | 5 (8.2) | 2 (3.4) | 7 (5.8) |
| Continuous use of muscle relaxants required | 2 (3.3) | 2 (3.4) | 4 (3.3) |
| Completed 7 days’ treatment | 0 | 2 (3.4) | 2 (1.7) |
| Other reasons, na | 4 (6.6) | 4 (6.8) | 8 (6.7) |
| Multiple reasons, nb | 1 | 3 | 4 |
Secondary outcomes
During trial treatment
The percentages of time spent adequately sedated (Table 16) per group are similar (73.86% clonidine, 72.73% midazolam). The lower limits of the IQRs demonstrate the difficulty in maintaining adequate sedation, with one-quarter of participants being adequately sedated only ≤ 58% of the time across both groups.
| Percentage of time spent adequately sedated | Clonidine (n = 61) | Midazolam (n = 59) | Total (n = 120) | Difference in mediansa (95% CI); p-valueb |
|---|---|---|---|---|
| Median | 73.86 | 72.73 | 73.68 | 0.66 (–5.25 to 7.24); p = 0.81 |
| IQR | 54.68–83.66 | 64.29–82.19 | 58.46–82.50 | |
| Minimum | 0.00 | 32.58 | 0.00 | |
| Maximum | 100.00 | 100.00 | 100.00 | |
| Missing | 0 | 0 | 0 |
Competing risk analyses were conducted for time to maximum permitted dose of sedative and time to maximum permitted dose of morphine instead of the pre-planned Kaplan–Meier analyses. Kaplan–Meier analyses were not carried out because participants withdrew from treatment due to reasons such as experiencing an AE, receiving muscle relaxants, etc. (all reasons listed in Table 34) before the events of interest could be observed. The treatment withdrawals for other reasons constitutes a competing risk, and a cumulative incidence analysis for time to events is more appropriate.
Of the 125 participants (64 clonidine, 61 midazolam) that received at least one dose of trial treatment, a total of 19 participants (15.2%) were a treatment failure, with 12 (18.8%) in the clonidine group and seven (11.5%) in the midazolam group. As the midazolam participants tended to be on treatment for longer, a post hoc ‘time to treatment failure’ analysis was conducted.
Table 17 shows the categorisation of participants for these competing risks analyses. Those participants who withdrew from treatment due to sedation no longer being required or those who completed the full 7 days of trial treatment are censored. The cumulative incidence curves for competing reasons of reaching maximum permitted dose of sedative/morphine and treatment withdrawal for each treatment group are shown in Figures 5–7.
| Time to maximum permitted dose of sedative, n (%) | |||
|---|---|---|---|
| Category (censoring indicator) | Clonidine ( N = 62) | Midazolam ( N = 59) | Total ( N = 121) |
| 0 – sedation no longer required or completed 7 days of trial treatment | 29 (46.8) | 33 (56.0) | 62 (51.2) |
| 1 – reached maximum permitted dose of sedative | 20 (32.3) | 21 (35.6) | 41 (33.9) |
| 2 – treatment failure | 5 (8.1) | 1 (1.7) | 6 (5.0) |
| 3 – other reasonsa | 8 (12.9) | 4 (6.8) | 12 (9.9) |
| Time to maximum permitted dose of morphine, n (%) | |||
| Category (censoring indicator) | Clonidine ( N = 62) | Midazolam ( N = 59) | Total ( N = 121) |
| 0 – sedation no longer required or completed 7 days of trial treatment | 36 (58.1) | 41 (69.5) | 77 (63.6) |
| 1 – reached maximum permitted dose of morphine | 10 (16.1) | 10 (17.0) | 20 (16.5) |
| 2 – treatment failure | 6 (9.7) | 2 (3.4) | 8 (6.6) |
| 3 – other reasonsa | 10 (16.1) | 6 (10.2) | 16 (13.2) |
| Time to treatment failure, n (%) | |||
| Category (censoring indicator) | Clonidine ( N = 64) | Midazolam ( N = 61) | Total ( N = 125) |
| 0 – sedation no longer required or completed 7 days of trial treatment | 40 (62.5) | 46 (75.4) | 86 (68.8) |
| 1 – treatment failure | 12 (18.8) | 7 (11.5) | 19 (15.2) |
| 2 – other reasonsa | 12 (18.8) | 8 (13.1) | 20 (16.0) |
FIGURE 5.
Time to reach the maximum permitted dose of sedation: cumulative incidence plot.
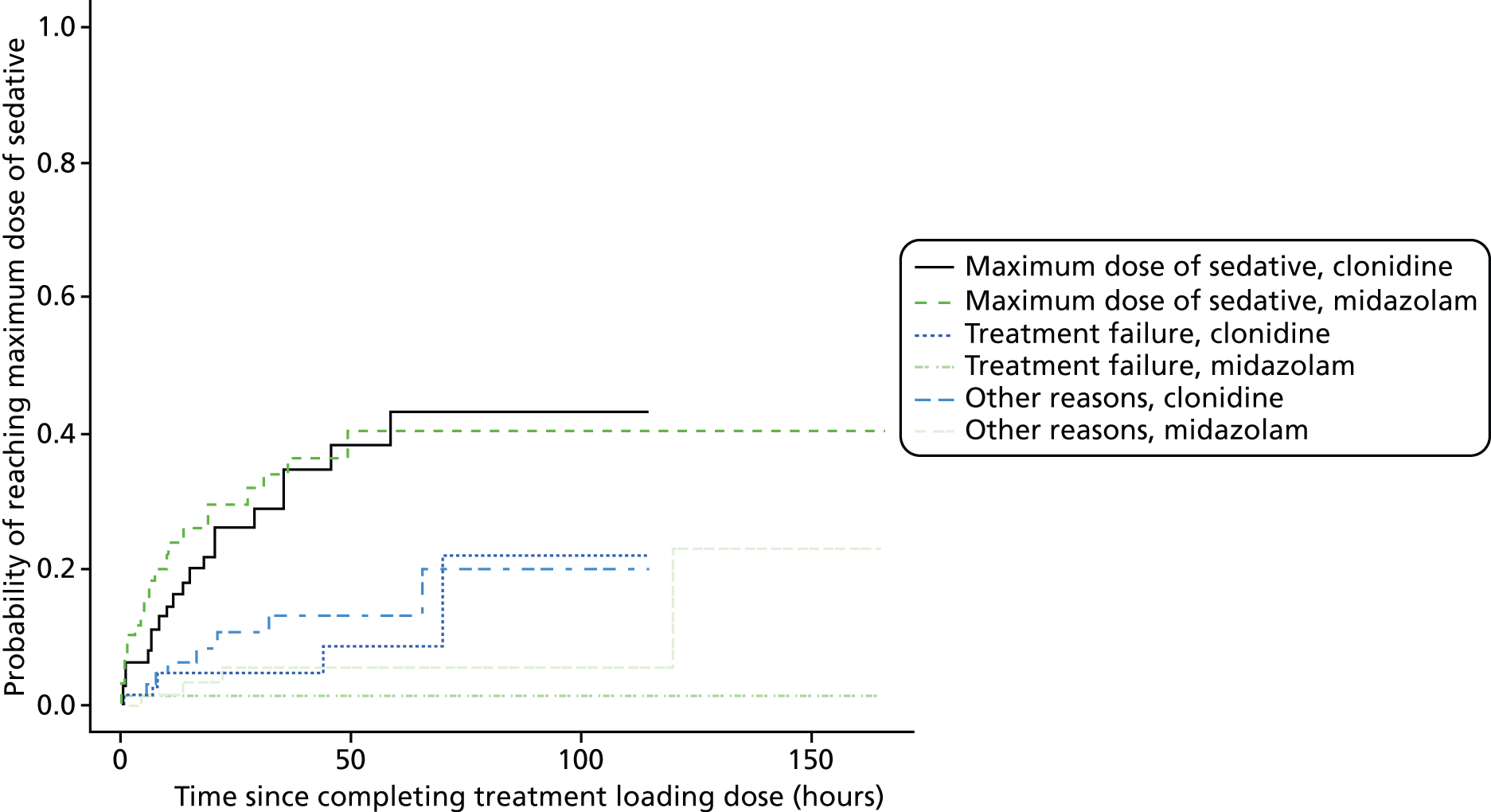
FIGURE 6.
Time to reach the maximum permitted dose of morphine: cumulative incidence plot.
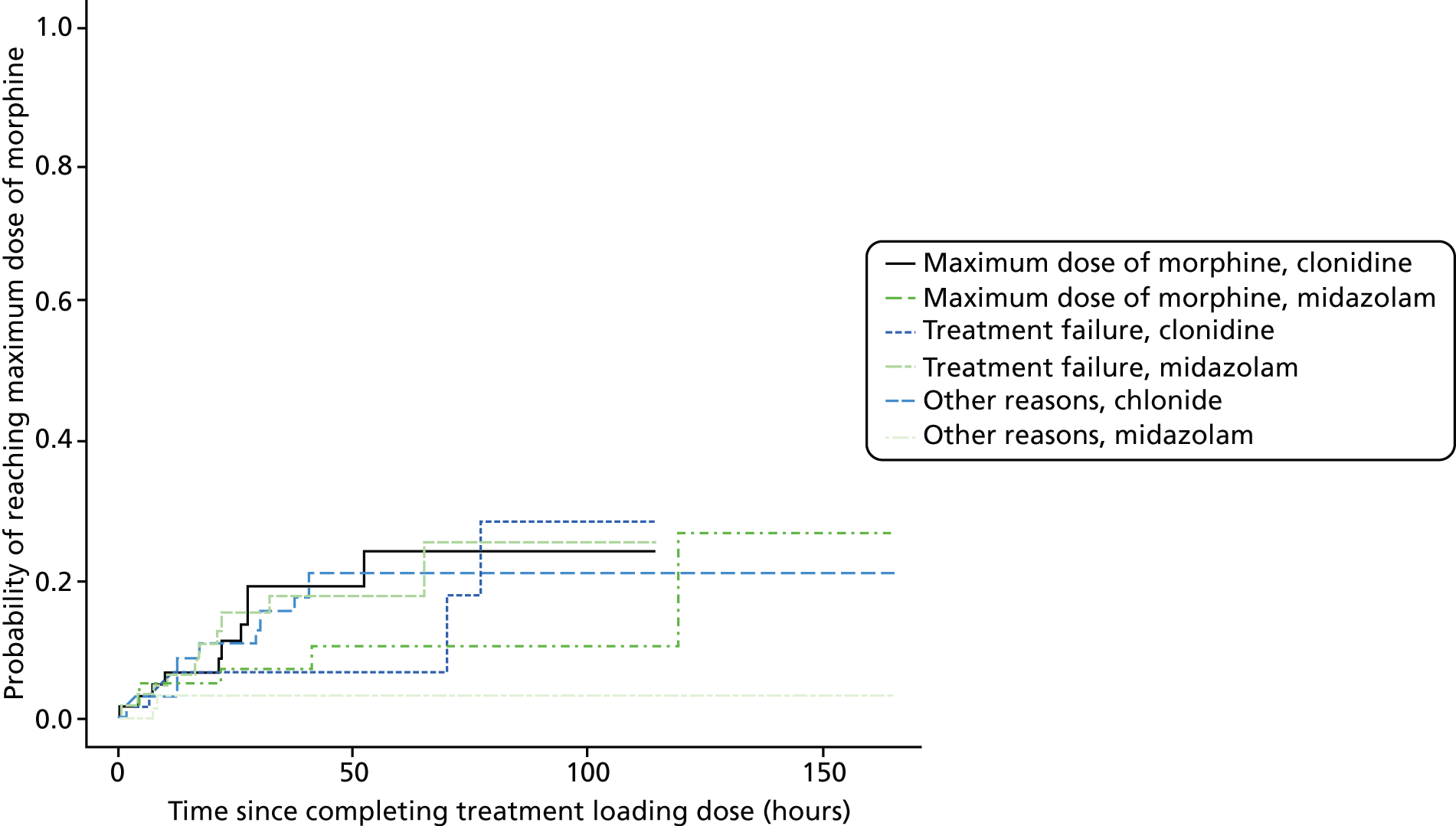
FIGURE 7.
Cumulative incidence curves for competing reasons of treatment withdrawal for clonidine and midazolam.
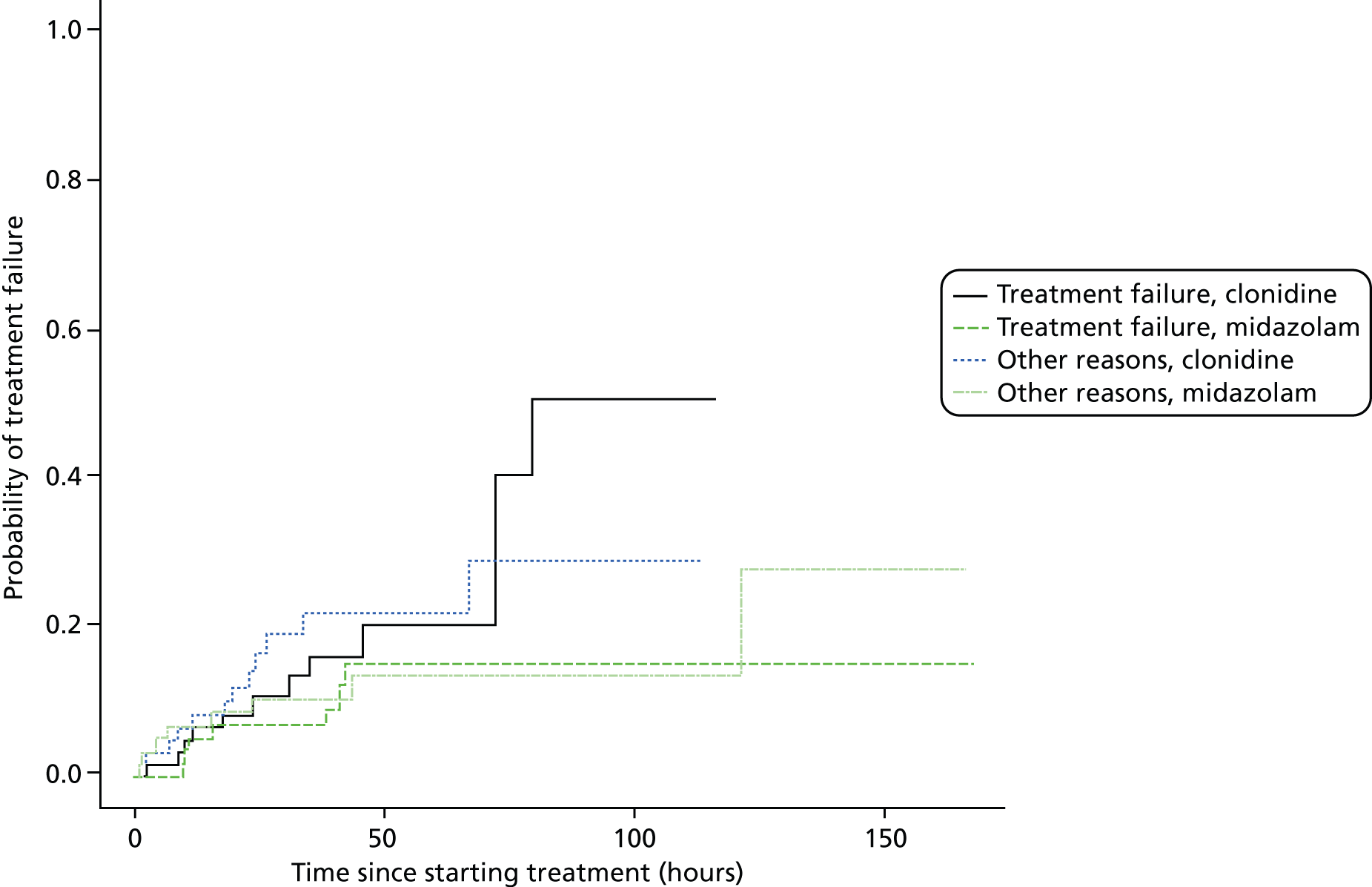
For time to maximum permitted dose of sedative (Table 18), the Gray’s test60 indicates no difference detected between the treatments for either competing risks [p-values of 0.75 (reaching maximum dose of sedative), 0.07 (treatment failure) and 0.11 (other reasons)].
| Outcome | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | Hazard ratio (95% CI); Gray’s test p-value |
|---|---|---|---|---|
| Time to reach the maximum permitted dose of sedation (hours) | ||||
| ( N = 62 a ) | ( N = 59 a ) | ( N = 121 a ) | ||
| 1. Reached maximum permitted dose of sedative | ||||
| 25% quartile (95% CI) | 21.00 (10.08 to 45.67) | 13.67 (1.42 to 19.25) | 19.30 (8.50 to 35.08) | 0.90 (0.49 to 1.65); p = 0.75 |
| Median | NR | NR | NR | |
| 75% quartile | NR | NR | NR | |
| 2. Treatment failure | ||||
| 25% quartile | NR | NR | NR | 5.34 (0.60 to 47.26); p = 0.07 |
| Median | ||||
| 75% quartile | ||||
| 3. Other reasons | ||||
| 25% quartile | NR | NR | NR | 2.21 (0.69 to 7.04); p = 0.11 |
| Median | ||||
| 75% quartile | ||||
| Time to reach the maximum permitted dose of morphine (hours) | ||||
| ( N = 62 a ) | ( N = 59 a ) | ( N = 121 a ) | ||
| 1. Reached maximum permitted dose of morphine | ||||
| 25% quartile (95% CI) | NR | NR | NR | 1.05 (0.44 to 2.52); p = 0.88 |
| Median (95% CI) | ||||
| 75% quartile (95% CI) | ||||
| 2. Treatment failure | ||||
| 25% quartile (95% CI) | 77.67 (0.00 to NR) | NR | NR | 3.30 (0.64 to 17.09); p = 0.10 |
| Median (95% CI) | NR | |||
| 75% quartile (95% CI) | NR | |||
| 3. Other reasons | ||||
| 25% quartile (95% CI) | 65.10 (16.58 to NR) | 119.53 (0.00 to NR) | 119.53 (10.25 to NR) | 1.89 (0.70 to 5.10); p = 0.15 |
| Median (95% CI) | NR | NR | NR | |
| 75% quartile (95% CI) | NR | NR | NR | |
| Time to treatment failure (hours) | ||||
| ( N = 64) | ( N = 61) | ( N = 125) | ||
| 1. Treatment failure | ||||
| 25% quartile (95% CI) | 72.08 (17.41 to 79.67) | NR | 79.67 (41.42 to NR) | 1.99 (0.77 to 5.17); p = 0.12 |
| Median (95% CI) | 79.67 (46.00 to NR) | NR | NR | |
| 75% quartile (95% CI) | NR | NR | NR | |
| 2. Other reasons | ||||
| 25% quartile (95% CI) | 67.10 (19.83 to NR) | 121.53 (0.00 to NR) | 121.53 (18.58 to NR) | 1.68 (0.69 to 4.10); p = 0.19 |
| Median (95% CI) | NR | NR | NR | |
| 75% quartile (95% CI) | NR | NR | NR | |
For time to maximum permitted dose of morphine, the Gray’s test60 indicates no difference detected between the treatments for either competing risks [p-values of 0.88 (reaching maximum dose of morphine), 0.10 (treatment failure) and 0.15 (other reasons)].
Therefore, overall there were no differences detected in the time taken to reach the maximum permitted doses of allocated sedative or morphine. Similarly, there were no differences in the proportions who reached the maximum dose (see Table 20).
For time to treatment failure, the Gray’s test60 indicates no difference detected between the treatments for either competing risks [p-values of 0.12 (treatment failure) and 0.19 (other reasons)]. Similarly, there were no differences in the proportion of patients experiencing treatment failure (see Table 20).
The mean profile rise of daily cumulative sedative infusion is given in Figure 8 and cumulative morphine infusions in Figure 9. The numbers contributing data at each time point for each analysis are given in Table 19. The figures show large overlapping regions of the standard error bars at each time point in the cumulative mean profile plots. The increasing widths of the standard error bars within the figure demonstrate increasing uncertainty as the number of participants contributing data decrease with increasing time according to Table 19. Note that post 80 hours there is only one patient in the clonidine group and hence the standard error bar is 0.
FIGURE 8.
Mean profile plots for cumulative sedative infusion. Note: the bars on the plot are one standard error bars. Where there are no standard error bars on the clonidine profile there is only one patient providing data.
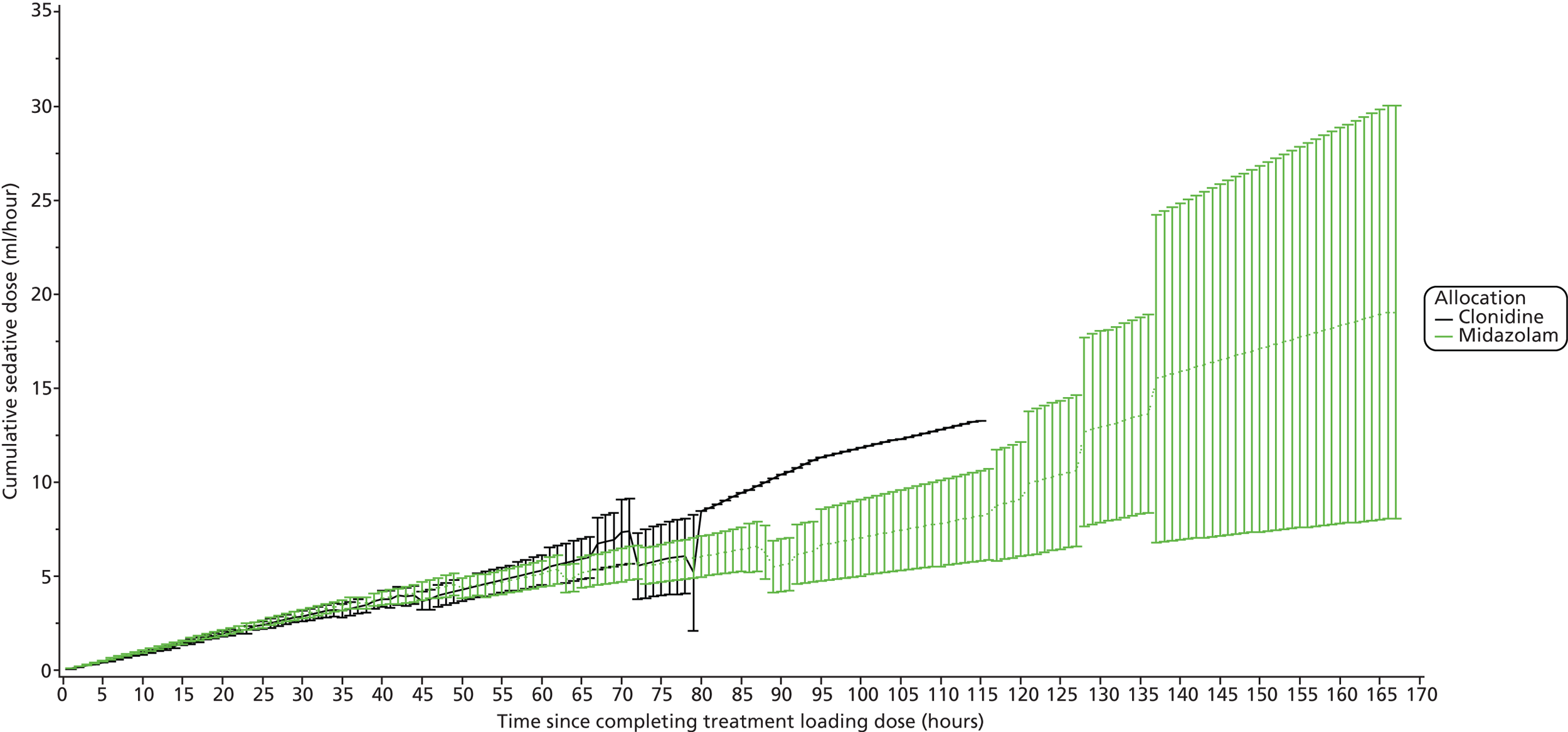
FIGURE 9.
Mean profile plots for cumulative morphine infusion. Note: the bars on the plot are one standard error bars. Where there are no standard error bars on the clonidine profile there is only one patient providing data.
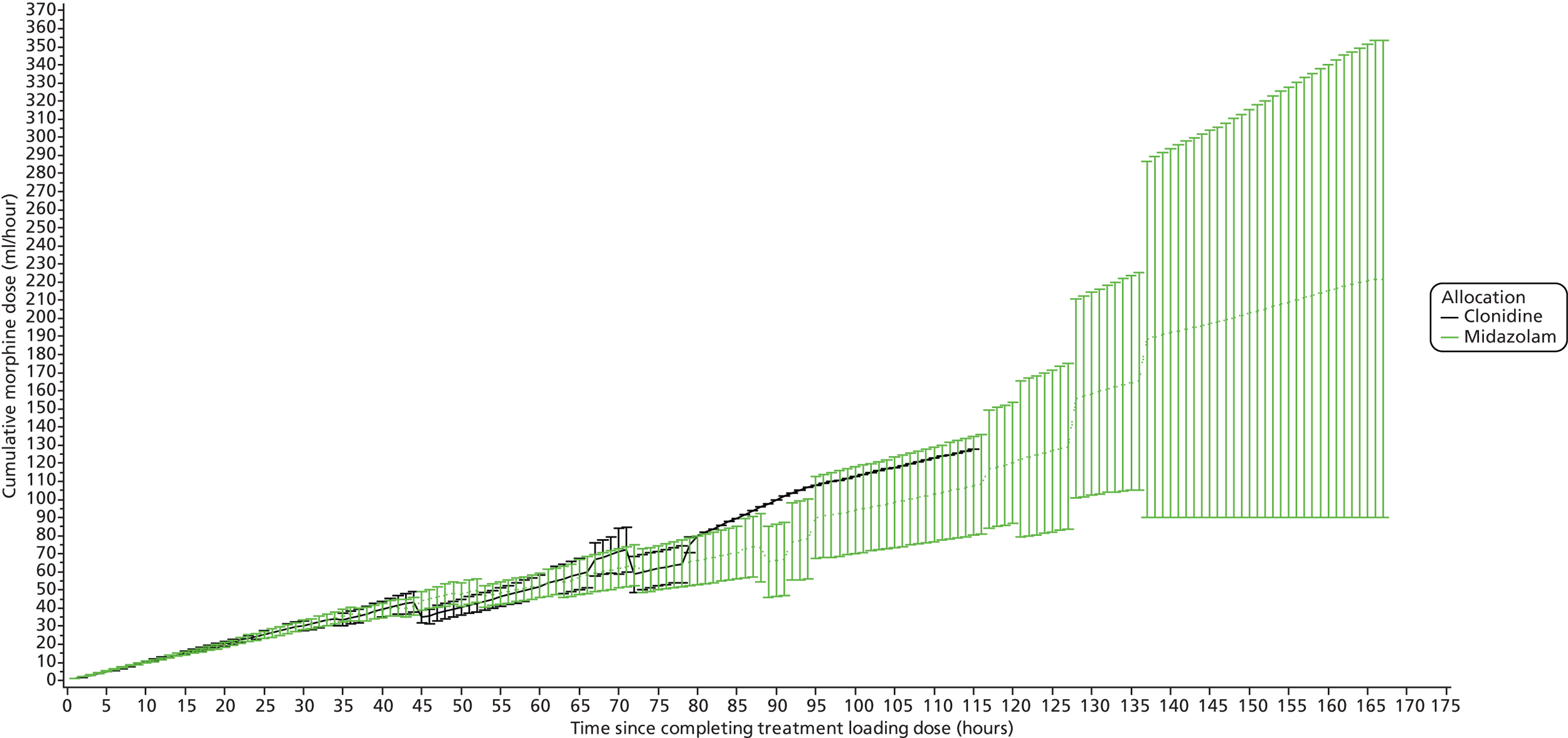
| Treatment | Time point (hours) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 | 105 | 110 | 115 | 120 | 125 | 130 | 135 | 140–165 | 170 | |
| Clonidine | 62 | 59 | 55 | 49 | 39 | 28 | 26 | 23 | 21 | 14 | 11 | 11 | 10 | 8 | 5 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Midazolam | 59 | 58 | 53 | 47 | 45 | 39 | 38 | 36 | 29 | 21 | 18 | 16 | 16 | 12 | 12 | 11 | 11 | 11 | 8 | 6 | 6 | 6 | 6 | 6 | 5 | 4 | 3 | 3 | 2 | 0 |
The results of the longitudinal mixed model for mean profile, rise in daily cumulative sedation gave least-squares mean estimates of 3.28 and 3.14, with standard errors of 0.17 and 0.17, respectively, for clonidine and midazolam. The difference in least-squares means and 95% CI is 0.14 (–0.33 to 0.61), with a p-value of 0.56.
From the longitudinal mixed model for mean profile, rise in daily cumulative morphine infusions gave least-squares mean estimates of 33.70 and 33.86, with standard errors of 1.80 and 1.80, respectively, for clonidine and midazolam. The difference in least-squares means and 95% CI is –0.16 (–5.20 to 4.88), with a p-value of 0.95.
A post hoc analysis of cumulative sedative/morphine infusion data has been summarised in 5-hour intervals with medians and IQRs split by patients who achieved the primary outcome of ≥ 80% adequately sedated in Appendix 11, Tables 46 and 48, and those patients who did not achieve the primary outcome of ≥ 80% adequately sedated (see Appendix 11, Tables 47 and 49). This post hoc summary aims to provide information for clinicians on dose response for those who were adequately sedated and those who were not.
There were no differences identified in the proportion of participants who had at least 1 day with a fall in BP requiring intervention (Table 20) or the number of days with a fall in BP judged by the clinician to require intervention.
| Outcomes | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | RR (95% CI); p-value |
|---|---|---|---|---|
| Maximum dose of sedative reached, n (%) | ||||
| Yes | 20 (32.3) | 21 (35.6) | 41 (33.9) | 0.91 (0.55 to 1.49); p = 0.70a |
| No | 42 (67.7) | 38 (64.4) | 80 (66.1) | |
| Missing | 2 | 2 | 4 | |
| Maximum dose of morphine reached, n (%) | ||||
| Yes | 10 (16.1) | 10 (17.0) | 20 (16.5) | 0.91 (0.43 to 2.12); p = 0.90a |
| No | 52 (83.9) | 49 (83.0) | 101 (83.5) | |
| Missing | 2 | 2 | 4 | |
| At least 1 day with a fall in BP requiring intervention, n (%) | ||||
| Yes | 5 (7.8) | 5 (8.3) | 10 (8.1) | 0.94 (0.29 to 3.08); p = 1.00b |
| No | 59 (92.2) | 55 (91.7) | 114 (91.9) | |
| Missing | 0 | 1 | 1 | |
| No. of days had a fall in BP, judged by clinician to require intervention, n (%) | ||||
| 0 | 59 (92.2) | 55 (91.7) | 114 (91.9) | p = 0.75c |
| 1 | 4 (6.3) | 4 (6.7) | 8 (6.5) | |
| 2 | 1 (1.6) | 0 (0.0) | 1 (0.8) | |
| 3 | 0 (0.0) | 1 (1.7) | 1 (0.8) | |
| Missing | 0 | 1 | 1 | |
| Increased inotropic support required in first 12 hours after randomisation, n (%) | ||||
| Yes | 5 (11.1) | 3 (5.8) | 8 (8.3) | 1.93 (0.49 to 7.61); p = 0.47b |
| No | 40 (88.9) | 49 (94.2) | 89 (91.7) | |
| Missingd | 19 | 9 | 28 | |
| Data not collected | 17 | 8 | 25 | |
| Data collected but missing | 2 | 1 | 3 | |
| At least one instance requiring supplementary analgesia during sedation, n (%) | ||||
| Yes | 53 (82.8) | 53 (86.9) | 106 (84.8) | 0.95 (0.82 to 1.11); p = 0.53a |
| No | 11 (17.2) | 8 (13.1) | 19 (15.2) | |
| Missing | 0 | 0 | 0 | |
| Treatment failure, n (%) | ||||
| Yes | 12 (18.8) | 7 (11.5) | 19 (15.2) | 1.63 (0.69 to 3.88); p = 0.26a |
| No | 52 (81.2) | 54 (88.5) | 106 (84.8) | |
| Missing | 0 | 0 | 0 | |
A higher proportion of participants on clonidine (5/64, 11.1%) than on midazolam (3/61, 5.8%) required increased inotropic support in the first 12 hours after randomisation (see Table 20); however, the number of events is small and the CI width is wide. This variable was added to data collection part of the way through the trial and is available for 77.6% of participants. In addition, at the time of consent a greater proportion of participants on clonidine were on inotropic support (30.3% clonidine vs. 14.8% midazolam). This baseline variable was collected throughout the trial but incompletely recorded, being available in 46.5% of participants. Of the five who required increased inotropic support in the first 12 hours on clonidine, only one participant was known to be receiving inotropic support at baseline. The status of the three participants on midazolam at baseline is unknown.
Similarly, there were no differences in the proportion of patients with at least one instance requiring supplementary analgesia during sedation (Table 20).
Table 21 shows the number of instances when participants required supplementary analgesia/sedation. Participants randomised to midazolam received supplementary analgesia with greater frequency than those receiving clonidine (p = 0.01). Table 21 also shows the median and IQR for time on treatment for each number of instances participants required supplementary analgesia/sedation. This shows that the number of instances increases with the length of time on sedation, with Table 13 showing those allocated to midazolam were sedated for longer. Details of the supplementary analgesia used are provided in Table 22 with reasons for use in Appendix 9.
| Instance | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | Cochran–Armitage trend test, p-value | |||
|---|---|---|---|---|---|---|---|
| n (%) | Median (IQR) (min., max.) | n (%) | Median (IQR) (min., max.) | n (%) | Median (IQR) (min., max.) | ||
| 0 | 11 (17.2) | 12.25 (4.08–18.50) (2.00, 61.77) | 8 (13.1) | 11.58 (4.08–15.95) (1.00, 20.00) | 19 (15.2) | 12.25 (4.08–17.83) (1.00, 61.77) | 0.01 |
| 1 | 18 (28.1) | 23.96 (19.62–45.17) (2.25, 116.25) | 10 (16.4) | 32.46 (22.45–40.25) (4.00, 96.00) | 28 (22.4) | 24.96 (19.81–44.26) (2.25, 116.25) | |
| 2 | 12 (18.8) | 23.91 (16.18–39.67) (11.60, 70.67) | 7 (11.5) | 45.00 (23.72–63.67) (16.00, 65.00) | 19 (15.2) | 25.25 (17.12–54.00) (11.60, 70.67) | |
| 3 | 5 (7.8) | 22.08 (19.83–57.83) (10.00, 72.17) | 7 (11.5) | 37.75 (23.37–43.50) (9.67, 53.33) | 12 (9.6) | 32.17 (20.96–48.42) (9.67, 72.17) | |
| 4 | 8 (12.5) | 30.20 (22.25–42.10) (17.42, 46.08) | 8 (13.1) | 43.21 (11.44–55.71) (10.17, 63.50) | 16 (12.8) | 34.83 (17.58–45.88) (10.17, 63.50) | |
| 5 | 2 (3.1) | 51.33 (23.00–79.67) (23.00, 79.67) | 7 (11.5) | 41.97 (37.25–48.08) (24.08, 88.00) | 9 (7.2) | 41.97 (37.25–48.08) (23.00, 88.00) | |
| 6 | 3 (4.7) | 45.45 (34.25–72.08) (34.25, 72.08) | 1 (1.6) | 86.50 (86.50–86.50) (86.50, 86.50) | 4 (3.2) | 58.77 (39.85–79.29) (34.25, 86.50) | |
| 7 | 0 | – | 4 (6.6) | 64.55 (27.60–103.58) (15.77, 117.50) | 4 (3.2) | 64.55 (27.60–103.58) (15.77, 117.50) | |
| 8 | 3 (4.7) | 42.28 (23.50–67.10) (23.50, 67.10) | 3 (4.9) | 43.25 (39.67–129.00) (39.67, 129.00) | 6 (4.8) | 42.77 (39.67–67.10) (23.50, 129.00) | |
| 9 | 0 | – | 1 (1.6) | 42.25 (42.25–42.25) (42.25, 42.25) | 1 (0.8) | 42.25 (42.25–42.25) (42.25, 42.25) | |
| ≥ 10 | 2 (3.1) | 63.29 (46.00–80.58) (46.00, 80.58) | 5 (8.2) | 137.65 (121.53–167.58) (92.32, 167.58) | 7 (5.6) | 121.53 (80.58–167.58) (46.00, 167.58) | |
| Specific supplementary analgesias required during sedation | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n patients | % | n events | n patients | % | n events | n patients | % | n events | |
| 01 = additional morphine | 9 | 14.1 | 17 | 16 | 26.2 | 22 | 25 | 20.0 | 39 |
| 02 = alfentanil | – | – | – | – | – | – | – | – | – |
| 03 = anaesthetic block | – | – | – | 1 | 1.6 | 3 | 1 | 0.8 | 3 |
| 04 = desflurane | – | – | – | – | – | – | – | – | – |
| 05 = diazepam | – | – | – | – | – | – | – | – | – |
| 06 = fentanyl | 3 | 4.7 | 4 | 6 | 9.8 | 13 | 9 | 7.2 | 17 |
| 07 = ibuprofen | 2 | 3.1 | 4 | 6 | 9.8 | 16 | 8 | 6.4 | 20 |
| 08 = isoflurane | 1 | 1.6 | 1 | 1 | 1.6 | 1 | 2 | 1.6 | 2 |
| 09 = ketamine | 16 | 25.0 | 25 | 17 | 27.9 | 37 | 33 | 26.4 | 62 |
| 10 = lorazepam | 3 | 4.7 | 3 | 1 | 1.6 | 4 | 4 | 3.2 | 7 |
| 11 = midazolam | 24 | 37.5 | 38 | 29 | 47.5 | 59 | 53 | 42.4 | 97 |
| 12 = muscle relaxant | 16 | 25.0 | 20 | 21 | 34.4 | 41 | 37 | 29.6 | 61 |
| 13 = paracetamol | 30 | 46.9 | 79 | 34 | 55.7 | 106 | 64 | 51.2 | 185 |
| 14 = propofol | – | – | – | 3 | 4.9 | 3 | 3 | 2.4 | 3 |
| 15 = remifentanyl | – | – | – | – | – | – | – | – | – |
| 16 = sevoflurane | – | – | – | 2 | 3.3 | 2 | 2 | 1.6 | 2 |
| 17 = thiopentone | – | – | – | – | – | – | – | – | – |
| NK = not known | 1 | 1.6 | 1 | 4 | 6.6 | 4 | 5 | 4.0 | 5 |
There were no differences between the groups for daily urine output (Table 23).
| Average daily urine output | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | Difference in mediansa (95% CI); p-valueb |
|---|---|---|---|---|
| ml/hour | ||||
| Median | 21.77 | 23.35 | 22.74 | c |
| IQR | 13.87–35.19 | 15.35–32.15 | 14.96–34.57 | |
| Minimum | 4.00 | 0.00 | 0.00 | |
| Maximum | 147.83 | 96.85 | 147.83 | |
| Missing | 0 | 1 | 1 | |
| ml/day | ||||
| Median | 522.40 | 560.43 | 545.70 | –19.99 (–127.57 to 101.55); p = 0.73 |
| IQR | 332.86–844.58 | 368.45–771.65 | 359.00–829.62 | |
| Minimum | 96.00 | 0.00 | 0.00 | |
| Maximum | 3547.87 | 2324.34 | 3547.87 | |
| Missing | 0 | 1 | 1 | |
There were insufficient data to be able to analyse the urinalysis outcomes, as this was not required to be routinely measured.
No discernible differences were noted for any of the blood biochemistry variables (Table 24). The numbers to have at least one abnormal result not expected for the patients’ condition were low (just 0–4 for each group). No patients in either of the two groups had any abnormal results that were not expected for the patient’s condition for chloride or creatinine.
| Measurement | At least one abnormal result not expected for the patient’s condition: | |||
|---|---|---|---|---|
| Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | RR (95% CI); Fisher’s exact p-value | |
| Sodium, n (%) | ||||
| Yes | 0 (0.0) | 1 (1.7) | 1 (0.8) | 0.31 (0.01 to 7.52); p = 0.48 |
| No | 63 (100.0) | 58 (98.3) | 121 (99.2) | |
| Missing | 1 | 2 | 3 | |
| Potassium, n (%) | ||||
| Yes | 0 (0.0) | 2 (3.4) | 2 (1.6) | 0.19 (0.01 to 3.83); p = 0.23 |
| No | 63 (100.0) | 57 (96.6) | 120 (98.4) | |
| Missing | 1 | 2 | 3 | |
| Chloride, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A (no events) |
| No | 34 (100.0) | 35 (100.0) | 69 (100.0) | |
| Missing | 30 | 26 | 56 | |
| Urea, n (%) | ||||
| Yes | 1 (1.6) | 1 (1.7) | 2 (1.7) | 0.95 (0.06 to 14.85); p = 1.00 |
| No | 60 (98.4) | 57 (98.3) | 117 (98.3) | |
| Missing | 3 | 3 | 6 | |
| Creatinine, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | N/A |
| No | 62 (100.0) | 57 (100.0) | 119 (100.0) | (no events) |
| Missing | 2 | 4 | 6 | N/A |
| Bilirubin, n (%) | ||||
| Yes | 1 (2.0) | 0 (0.0) | 1 (1.0) | 3.12 (0.13 to 74.76); p = 0.49 |
| No | 50 (98.0) | 53 (100.0) | 103 (99.0) | |
| Missing | 13 | 8 | 21 | |
| ALT, n (%) | ||||
| Yes | 3 (5.9) | 4 (7.6) | 7 (6.7) | 0.78 (0.18 to 3.31); p = 1.00 |
| No | 48 (94.1) | 49 (92.4) | 97 (93.3) | |
| Missing | 13 | 8 | 21 | |
| AST, n (%) | ||||
| Yes | 1 (9.1) | 0 (0.0) | 1 (3.9) | 4.00 (0.18 to 89.85); p = 0.42 |
| No | 10 (90.9) | 15 (100.0) | 25 (96.1) | |
| Missing | 53 | 46 | 99 | |
| Alkaline phosphate, n (%) | ||||
| Yes | 1 (2.0) | 2 (3.6) | 3 (2.9) | 0.55 (0.05 to 5.88); p = 1.00 |
| No | 49 (98.0) | 53 (96.4) | 102 (97.1) | |
| Missing | 14 | 6 | 20 | |
Secondary outcomes post-trial treatment phase
Results for the outcome time from stopping sedation to being fully awake are available in Table 25 and Figure 10. Being fully awake was determined by a score of 4 or 5 on the alertness category of the COMFORT score sustained for ≥ 2 hours or more. The Kaplan–Meier curve and the hazard ratio suggest that children who had been allocated to midazolam tended to take less time to be fully awake than those allocated to clonidine (see Table 25). This is also supported by the results for the outcome ‘fully awake within 24 hours’ provided in Table 26; however, results are not statistically significant.
| Time from stopping all sedation to being fully awake (hours)a | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | Hazard ratio (95% CI); log-rank p-value |
|---|---|---|---|---|
| n | 64 | 61 | 125 | |
| 25% quartile (95% CI) | 4.50 (2.50 to 8.00) | 2.00 (1.00 to 4.00) | 3.50 (2.00 to 5.00) | 0.64 (0.38 to 1.08); p = 0.09 |
| Median (95% CI) | 11.17 (6.17 to NR) | 6.22 (3.92 to 16.50) | 9.00 (6.00 to 17.17) | |
| 75% quartile (95% CI) | NR | NR | NR |
FIGURE 10.
Time from stopping all sedation to being fully awake: Kaplan–Meier plot. Note: ‘+’ = censored; log-rank p = 0.0898.
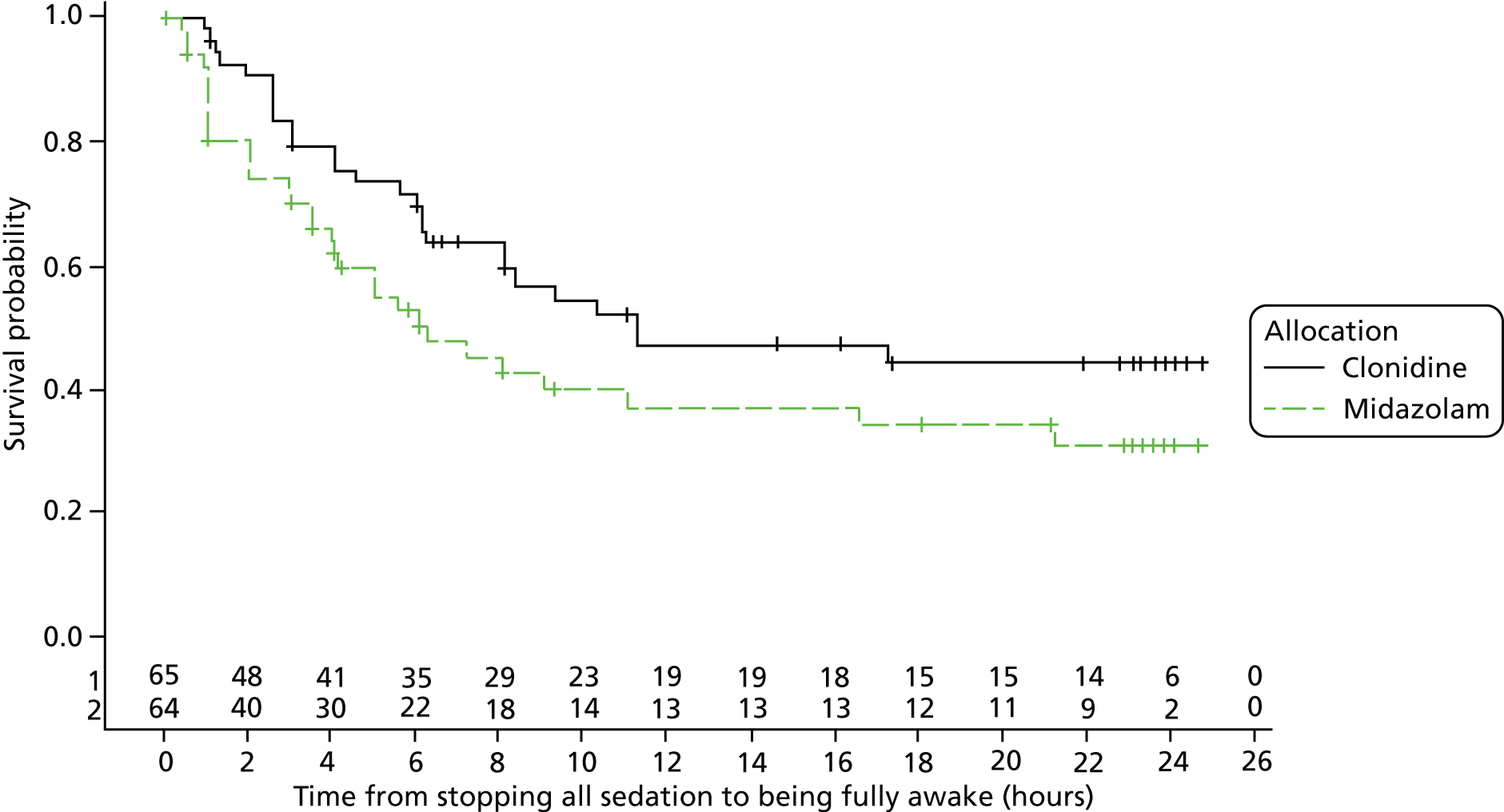
| Outcomes | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | RR (95% CI); p-value |
|---|---|---|---|---|
| Fully awake within 24 hours, n (%)a | ||||
| Yes | 27 (81.8) | 31 (93.9) | 58 (87.9) | 0.87 (0.73 to 1.05); p = 0.26b |
| No | 6 (18.2) | 2 (6.1) | 8 (12.1) | |
| Missing | 31 | 28 | 59 | |
| Moved from PICU to the ward before 24 hours | 27 | 20 | 47 | |
| Last recording a 4 or 5 but not two consecutive hours of 4 or 5 | 4 | 8 | 12 | |
| Fully awake within 24 hours, n (%) | ||||
| Sensitivity analysis 1a | 58 (90.6) | 59 (96.7) | 117 (93.6) | 0.94 (0.86 to 1.03); p = 0.27b |
| Sensitivity analysis 2a | 27 (42.2) | 31 (50.8) | 58 (46.4) | 0.83 (0.57 to1.21); p = 0.37b |
| One or more instance of rebound hypertension, n (%) | ||||
| Yes | 1 (1.6) | 0 (0.0) | 1 (0.8) | 2.86 (0.12 to 68.92); p = 1.00c |
| No | 63 (98.4) | 61 (100.0) | 124 (99.2) | |
| Missing | 0 | 0 | 0 | |
| Routine activities affected by withdrawal,d n (%) | ||||
| Yese | 28 (46.7) | 30 (52.6) | 58 (49.6) | 0.89 (0.62 to 1.28); p = 0.58 |
| No | 32 (53.3) | 27 (47.4) | 59 (50.4) | |
| Missing or no complete assessments | 4 | 4f | 8f | |
| Routine activities affected by withdrawal,d n (%) | ||||
| Sensitivity analysis 1: yes | 29 (48.3) | 30 (51.7) | 59 (50.0) | 0.93 (0.65 to1.34); p = 0.71b |
| Sensitivity analysis 2: yes | 47 (78.3) | 44 (75.9) | 91 (77.1) | 1.03 (0.85 to 1.26); p = 0.75b |
| Missinge | 4 | 3 | 7 | |
| Withdrawal symptoms requiring clinical intervention, n (%) | ||||
| Yes | 11 (18.3) | 16 (27.6) | 27 (22.9) | 0.66 (0.34 to 1.31); p = 0.23b |
| No | 49 (81.7) | 42 (72.4) | 91 (77.1) | |
| Missing | 4 | 3 | 7 | |
There were no differences in the proportions experiencing withdrawal symptoms or the ‘average total score per day’ (Table 27, not significant); however, a higher proportion of participants allocated to midazolam required clinical intervention for those symptoms (see Table 26).
| Average total score per daya | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | Difference in medians (95% CI); Mann–Whitney U-test p-value |
|---|---|---|---|---|
| Median | 1.13 | 1.13 | 1.13 | 0.08 (–0.36 to 0.50); p = 0.62 |
| IQR | 0.45–2.21 | 0.22–2.40 | 0.38–2.40 | |
| Minimum | 0.00 | 0.00 | 0.00 | |
| Maximum | 12.00 | 9.75 | 12.00 | |
| Missing | 4 | 4b | 8b | |
| Sensitivity analysis 1c | ||||
| Median | 0.84 | 0.65 | 0.80 | 0.10 (–0.23 to 0.45); p = 0.44 |
| IQR | 0.37–1.75 | 0.09–1.97 | 0.23–1.90 | |
| Minimum | 0.00 | 0.00 | 0.00 | |
| Maximum | 7.17 | 8.38 | 8.38 | |
| Missing | 4 | 3 | 7 | |
| Sensitivity analysis 2d | ||||
| Median | 5.82 | 3.42 | 4.12 | 0.72 (–0.63 to 2.54); p = 0.29 |
| IQR | 1.76–8.71 | 0.63–9.60 | 1.17–9.13 | |
| Minimum | 0.00 | 0.00 | 0.00 | |
| Maximum | 27.75 | 31.97 | 31.97 | |
| Missing | 4 | 3 | 7 | |
Signs of withdrawal were measured using an 11-point assessment for abnormal behaviour and were recorded until 5 days following trial treatment cessation or until discharge, whichever was soonest. The 11 descriptors that make up this assessment are scored 0 = none, 1 = mild (does not interfere with routine activities), 2 = moderate (interferes with routine activities) and 3 = severe (impossible to perform routine activities). Therefore, higher scores indicate worse withdrawal symptoms. Table 28 gives details of the text descriptors provided when the ‘Other’ category was selected.
| Treatment | Patient | Follow-up day no. | Reason |
|---|---|---|---|
| Clonidine | 1 | Day 1 | Throwing out of right arm |
| 2 | Day 1 | Mild – jittery | |
| Mild – sneezing | |||
| Mild – tachycardic | |||
| 3 | Day 3 | Mild – shell-shock quiet | |
| 4 | Day 1 | Occasionally startles when asleep | |
| 5 | Day 1 | Unsettled – crying and coughing | |
| Unsettled, coughing | |||
| 6 | Day 1 | Refusing feed/medicine | |
| 7 | Day 1 | Moderate | |
| Severe | |||
| 8 | Day 1 | Moderate | |
| Day 2 | Mild – loose stool | ||
| Day 3 | Mild – loose stool | ||
| Day 4 | Mild – fidgety | ||
| 9 | Day 1 | Reintubated at 16.00 | |
| 10 | Day 1 | Neuromuscular blockade atracurium | |
| Midazolam | 1 | Day 2 | Mild – not going into a deep sleep |
| Mild – only napping for a few minutes | |||
| Day 3 | Mild – every 4–5 hours | ||
| Mild – sleeping only 15–30 minutes | |||
| Day 4 | Mild – slept all night with help of pain relief | ||
| Day 5 | Mild – napping 10–15 minutes | ||
| Mild – slept for 2 hours | |||
| 2 | Day 3 | Mild – mum reports not sleeping | |
| Day 4 | Mild – mum reports still not sleeping | ||
| Day 5 | Mild – mum reports still not sleeping | ||
| 3 | Day 3 | Moderate – jittery | |
| 4 | Day 1 | Oramorph given | |
| 5 | Day 1 | Settled after feed | |
| Slept well | |||
| Very settled | |||
| Woke 02.10, rubbing eyes and irritable | |||
| 6 | Day 1 | Teeth grinding | |
| 7 | Day 2 | Mild – grip right hand | |
| Day 3 | Mild – decreased grip in the right hand | ||
| 8 | Day 1 | Moderate | |
| 9 | Day 3 | Severe – tachycardia | |
| 10 | Day 1 | Awake most of the night | |
| 11 | Day 1 | Moderate | |
| 12 | Day 1 | Itchy eyes | |
| Moaning (grunting) | |||
| 13 | Day 2 | Severe – diarrhoea | |
| Day 4 | Moderate – loose stool | ||
| Day 5 | Moderate – loose stool ×4 | ||
| 14 | Day 1 | Nasal flaring | |
| Pyrexial | |||
| 15 | Day 1 | Boluses required of fentanyl medication | |
| Continues on fentanyl and midazolam | |||
| 16 | Day 2 | Mild – slightly upset | |
| 17 | Day 2 | Moderate – no energy, lethargic |
There was just one case of rebound hypertension in the study. This was a mild case for a patient in the clonidine group.
Safety
All patients who received at least one dose of intervention are included in the safety analysis data set. There were no crossovers, so all patients who received one dose of intervention are included in their randomised groups. This was prespecified in section 13 of the SLEEPS SAP. There is a total of 125 patients in the safety analysis data set (64 on clonidine, 61 on midazolam). Safety data are provided in Tables 29–33.
Adverse reactions
| AR | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | |||
|---|---|---|---|---|---|---|
| Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | |
| Unexpected hypotension that requires intervention | 7 | 4 (6.3) | 3 | 3 (4.9) | 10 | 7 (5.6) |
| Bradycardia not requiring intervention | 6 | 2 (3.1) | – | – | 6 | 2 (1.6) |
| Bradycardia that requires intervention | 1 | 1 (1.6) | 3 | 2 (3.3) | 4 | 3 (2.4) |
| Hypertension not requiring intervention | 1 | 1 (1.6) | 1 | 1 (1.6) | 2 | 2 (1.6) |
| Constipation | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) |
| Hypertension following cessation of trial treatment | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Petechial rash | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) |
| Total | 16 | 9 | 9 | 8 | 25 | 17 |
Adverse reactions by severity
| AR | Severity | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | |||
|---|---|---|---|---|---|---|---|
| Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | ||
| Unexpected hypotension that requires intervention | Mild | 3 | 2 (3.1) | 1 | 1 (1.6) | 4 | 3 (2.4) |
| Moderate | 4 | 2 (3.1) | 1 | 1 (1.6) | 5 | 3 (2.4) | |
| Severe | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) | |
| Bradycardia not requiring intervention | Mild | 6 | 2 (3.1) | – | – | 6 | 2 (1.6) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Bradycardia that requires intervention | Mild | – | – | 2 | 1 (1.6) | 2 | 1 (0.8) |
| Moderate | 1 | 1 (1.6) | 1 | 1 (1.6) | 2 | 2 (1.6) | |
| Severe | – | – | – | – | – | – | |
| Hypertension not requiring intervention | Mild | 1 | 1 (1.6) | 1 | 1 (1.6) | 2 | 2 (1.6) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Constipation | Mild | – | – | – | – | – | – |
| Moderate | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) | |
| Severe | – | – | – | – | – | – | |
| Hypertension following cessation of trial treatment | Mild | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Petechial rash | Mild | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Total | Mild | 11 | 6 (9.4) | 5 | 4 (6.6) | 11 | 10 (8.0) |
| Moderate | 5 | 3 (4.7) | 3 | 3 (4.9) | 8 | 6 (4.8) | |
| Severe | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) | |
| Overall total | 16 | 9 | 9 | 8 | 25 | 17 | |
| Patient | Treatment | ARs |
|---|---|---|
| 1 | Clonidine | Moderate: unexpected hypotension that requires intervention (no. of times event occurred = 3) |
| 2 | Clonidine | Mild: unexpected hypotension that requires intervention (no. of times event occurred = 2) and Mild: hypertension following cessation of trial treatment (no. of times event occurred = 1) |
| 3 | Clonidine | Mild: bradycardia not requiring intervention (no. of times event occurred = 5) |
| 4 | Midazolam | Mild: bradycardia that requires intervention (no. of times event occurred = 2) |
| 5 | Midazolam | Moderate: constipation (no. of times event occurred = 1) and Mild: Petechial rash (no. of times event occurred = 1) |
Serious adverse events
Serious adverse events
| SAE | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | |||
|---|---|---|---|---|---|---|
| Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | |
| Accidental extubation | 1 | 1 (1.6) | 1 | 1 (1.6) | 2 | 2 (1.6) |
| Self-extubation not requiring reintubation | 1 | 1 (1.6) | 1 | 1 (1.6) | 2 | 2 (1.6) |
| Bradycardia requiring intervention | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Death from primary disease after active phase of trial complete | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Endotracheal tube migrated down right main bronchus due to wet retaining tapes | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) |
| Failed extubation requiring reintubation | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Infection requiring antibiotics | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Postextubation stridor | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Postoperative wound infection | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Recurrence of original disease after discharge from hospital | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Reintubation due to stridor | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Total | 10 | 10 | 3 | 3 | 13 | 13 |
Serious adverse events by severity
| SAE | Severity | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | |||
|---|---|---|---|---|---|---|---|
| Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | ||
| Accidental extubation | Mild | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Moderate | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) | |
| Severe | – | – | – | – | – | – | |
| Self-extubation not requiring reintubation | Mild | – | – | – | – | – | – |
| Moderate | 1 | 1 (1.6) | 1 | 1 (1.6) | 2 | 2 (1.6) | |
| Severe | – | – | – | – | – | – | |
| Bradycardia requiring intervention | Mild | – | – | – | – | – | – |
| Moderate | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) | |
| Severe | – | – | – | – | – | – | |
| Death from primary disease after active phase of trial complete | Mild | – | – | – | – | – | – |
| Moderate | – | – | – | – | – | – | |
| Severe | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) | |
| Endotracheal tube migrated down right main bronchus due to wet retaining tapes | Mild | – | – | – | – | – | – |
| Moderate | – | – | 1 | 1 (1.6) | 1 | 1 (0.8) | |
| Severe | – | – | – | – | – | – | |
| Failed extubation requiring reintubation | Mild | – | – | – | – | – | – |
| Moderate | – | – | – | – | – | – | |
| Severe | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) | |
| Infection requiring antibiotics | Mild | – | – | – | – | – | – |
| Moderate | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) | |
| Severe | – | – | – | – | – | – | |
| Postextubation stridor | Mild | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Postoperative wound infection | Mild | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Recurrence of original disease after discharge from hospital | Mild | – | – | – | – | – | – |
| Moderate | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) | |
| Severe | – | – | – | – | – | – | |
| Reintubation due to stridor | Mild | 1 | 1 (1.6) | – | – | 1 | 1 (0.8) |
| Moderate | – | – | – | – | – | – | |
| Severe | – | – | – | – | – | – | |
| Total | Mild | 4 | 4 | – | – | 4 | 4 (3.2) |
| Moderate | 4 | 4 | 3 | 3 | 7 | 7 (5.6) | |
| Severe | 2 | 2 | – | – | 2 | 2 (1.6) | |
| Overall total | 10 | 10 | 3 | 3 | 13 | 13 | |
There was one patient who had multiple SAEs, the first being ‘Failed extubation requiring reintubation’ with a severity of ‘severe’, and the other being ‘Postoperative wound infection’ with a ‘mild’ severity.
Line listings for SAE data are provided in Appendix 7, Table 50.
Two patients in the study had SAEs that were assessed as sudden unexpected serious adverse reactions (SUSARs). Both occurred in patients receiving clonidine. In one patient (as discussed previously), the heart rate fell to a low point of 64 beats per minute (bpm), 2 hours after completing the loading dose and during the maintenance phase. Although other patients receiving clonidine did have either significant heart rate reduction or BP that required intervention, this was the only event that resulted in withdrawal from study, prompted a discussion with the principal investigator (PI) at the local centre and was reported to the IDSMC. In normal clinical practice without blinding, clinicians would be aware that clonidine was being used and there would be more confidence in either treating the problem or reducing the dosage as it occurred. The second SUSAR involved a failed extubation after the use of clonidine. Although this was reported as a SUSAR, this was in a complex post cardiac patient and failure of extubation in these circumstances is not uncommon. However, the team in the local centre had not expected the child to fail extubation and the clinical features were of pulmonary oedema. It was therefore reported as a SUSAR and reviewed by the IDSMC.
Withdrawals
Three participants who received at least one dose of their allocated treatment did not complete the trial treatment phase: one (2%) in the clonidine group because sedation was no longer required following completion of the loading dose and two (3%) participants in the midazolam group, both because they withdrew because of an AE that occurred during the loading dose.
Completeness of follow-up
There were two phases to the trial: during treatment phase and post-treatment follow-up. All 125 participants who received at least one dose of trial treatment have a reason for the end of the treatment phase (Table 34; for ‘other’ reasons, see Table 35). Multiple reasons were indicated for cessation of treatment in four participants (one clonidine, three midazolam), with details provided in Table 36. Of note, the number of treatment failures and AEs were small but a higher proportion occurred in the clonidine group.
| Reasons | Clonidine | Midazolam | Total |
|---|---|---|---|
| No. who received treatment | 64 | 61 | 125 |
| Withdrawal from treatment reason: n (%) Sedation no longer required |
40 (62.5) | 45 (73.8) | 85 (68.0) |
| Treatment failure occurred | 12 (18.8) | 7 (11.5) | 19 (15.2) |
| An AE occurred | 6 (9.4) | 4 (6.6) | 10 (8.0) |
| Other | 4 (6.3) | 4 (6.6) | 8 (6.4) |
| Continuous use of muscle relaxants required | 3 (4.7) | 2 (3.3) | 5 (4.0) |
| 7 × 24 hours of trial treatment administered | – | 2 (3.3) | 2 (1.6) |
| Patient | Treatment | Reasons | Withdrawal from studya (yes/no) |
|---|---|---|---|
| 1 | Clonidine | Parents decided to withdraw as child not settled on sedation – risk of self-extubation, nearing treatment failure (consent withdrawn during follow-up) | No |
| 2 | Clonidine | Mum felt child needed more sedation than the trial permitted (consent withdrawn during follow-up) | No |
| 3 | Clonidine | Bypass surgery – general anaesthetic and chest open | No |
| 4 | Clonidine | Extubated 10.20, reintubated 11.25 but paralysed and sedated | Yes |
| 5 | Midazolam | Child not sedated adequately on maximum trial drug; clinically not needing an increase in morphine; child at risk of potential extubation and oedema of airway | No |
| 6 | Midazolam | Patient was pyrexial and required cooling, rocuronium was given | No |
| 7 | Midazolam | Withdrawn off study; request by parents (consent withdrawn at time of treatment cessation) | Yes |
| 8 | Midazolam | A medication was administered that was not permitted | No |
| Patient | Treatment | Reasons |
|---|---|---|
| 1 | Clonidine | Treatment failure occurred and mum felt that child needed more sedation than the trial permitted (consent withdrawn during follow-up) |
| 2 | Midazolam | Treatment failure occurred and continuous use of muscle relaxants required |
| 3 | Midazolam | Sedation no longer required and an AE occurred |
| 4 | Midazolam | Treatment failure occurred and a medication was administered that was not permitted |
Post-treatment follow-up consent was withdrawn for three participants (two clonidine, one midazolam). At least one post-treatment withdrawal assessment was carried out on the two clonidine participants prior to withdrawal of consent. The midazolam participant withdrew consent at the time of treatment cessation so had no post-treatment withdrawal assessments.
Six participants (four clonidine, two midazolam) withdrew from study at treatment cessation for reasons other than withdrawal of consent, so no post-treatment follow-up data were collected. The reasons why these seven participants came off treatment are as follows:
-
Three participants (two clonidine, one midazolam) required continuous muscle relaxation.
-
One clonidine participant was extubated but then needed reintubating, as he/she needed to be paralysed and sedated.
-
One clonidine participant was lost to follow-up.
-
One midazolam participant was a treatment failure and no post-treatment data were recorded.
In addition to the clonidine patient who was lost to follow-up with no post-treatment data, there were four more participants (three clonidine, one midazolam) who were lost to follow-up, who had some post-treatment follow-up data collected. They were all transferred without any further data collection. All four participants had a reason for treatment cessation of ‘sedation no longer required’.
Only two participants, both on midazolam, required sedating for > 7 days.
Health economic evaluation results
Resource use and costs
Table 37 provides a summary of the key resource-use values for each arm of the SLEEPS trial; results are presented separately for the clonidine and midazolam groups. There were no statistically significant differences between the trial arms in any category of resource use, with the exception of length of time on treatment; patients in the midazolam group had a statistically significant longer time on treatment than patients in the clonidine group. There were no deaths during the two time horizons considered in the economic evaluation.
| Resource-use variable (randomisation to 14 days post-treatment cessation) | Clonidine (n = 61) | Midazolam (n = 59) | p-value | Unit cost (£) |
|---|---|---|---|---|
| Initial LoS in the PICU (days) | 4.74 (3.63) | 4.89 (3.43) | 0.81 | NHS Reference Costs 2011–1249 |
| Post-PICU LoS (days) | 5.42 (3.63) | 6.56 (4.38) | 0.13 | NHS Reference Costs 2011–12,49 Alder Hey Finance Department 2012 (Alder Hey Hospital, Liverpool, 2012, personal communication) |
| Total LoS (days) | 10.17 (4.40) | 11.45 (4.94) | 0.14 | NHS Reference Costs 2011–1249 |
| Time on treatment (days) | 1.41 (0.95) | 2.05 (1.61) | 0.01 | BNF 2012,50 MIMS 2013,51 NHS Supply Chain catalogue 201252 |
| Transfers to different hospital (%) | 0.11 (0.32) | 0.11 (0.32) | 0.95 | NHS Reference Costs 2011–1249 |
| LoS in different hospital (days) | 6.35 (5.52) | 4.22 (3.83) | 0.34 | NHS Reference Costs 2011–1249 |
| SAEs (n) | 0.02 (0.128) | 0 (0) | 0.32 | Alder Hey Finance Department, NHS salary scales 2012 (Alder Hey Hospital, personal communication) |
Table 38 shows clearly that the most costly resource category was LoS in hospital. Three specific categories of LoS were estimated: LoS in admitting ward (PICU), LoS in any ward after PICU (this may have included stays in HDUs, GM wards or a return to PICUs) and LoS in GM wards in a different hospital. All other costs (drugs, consumables, SAEs and transfers) were relatively inexpensive compared with the per diem costs associated with hospital admissions.
| Cost category | Clonidine (N = 61) | Midazolam (N = 59) | Mean difference | p-valuea | Bootstrapped (95% CI)b |
|---|---|---|---|---|---|
| Initial stay in the PICU (£) | 8666.02 (6999.80 to 10,332.23) | 8944.31 (7346.13 to 10,542.48) | –278.29 | 0.814 | (–2602.77 to 1982.53) |
| Post-PICU hospital stay (£) | 2366.42 (1741.70 to 2991.14) | 3044.30 (2293.40 to 3795.19) | –677.88 | 0.176 | (–1738.90 to 273.88) |
| Total hospital stay (£) | 11,032.43 (9376.70 to 12,688.17) | 11,988.60 (10,261.73 to 13,715.48) | –956.17 | 0.435 | (–3316.94 to 1167.15) |
| Drug treatments (£) | 9.12 (5.95 to 12.30) | 17.69 (11.71 to 23.66) | –8.57 | 0.150 | (–9.22 to –5.00) |
| Consumables | 25.10 (22.09 to 28.11) | 32.49 (27.40 to 37.57) | –7.39 | 0.160 | (–9.22 to –5.00) |
| AEs | 12.80 (–12.28 to 37.88) | 0 (0 to 0) | 12.80 | 0.321 | (0.00 to 38.39) |
| Transfers to different hospitals | 26.39 (7.84 to 44.94) | 27.29 (8.15 to 46.43) | –0.89 | 0.948 | (–4.15 to 2.75) |
| Hospital stays in hospitals following transfer | 339.23 (78.03 to 600.43) | 210.20 (35.41 to 384.99) | 129.03 | 0.423 | (49.84 to 194.74) |
| Total cost of care from randomisation to 14 days post-treatment cessation (£) | 11,445.07 (9811.71 to 10,978.43) | 12,276.26 (10,554.40 to 13,998.13) | –831.19 | 0.494 | (–3148.65 to 1468.91) |
Statistical analysis revealed that there were no statistical differences, at the 5% level, between the two trial groups in any cost category when all 120 children considered in the primary efficacy assessments were included in the economic analyses and the perspective was restricted to NHS hospital costs only. The mean total NHS hospital service cost in the clonidine group (n = 61) was £11,445 and the mean total NHS hospital service cost in the midazolam group (n = 59) was £12,276, generating a mean difference in costs of –£831 (p = 0.494).
Results of the cost-effectiveness analysis
The economic evaluation assessed the cost-effectiveness of clonidine compared with midazolam in terms of natural units of health gain, expressed as the incremental cost per additional case of adequate sedation. The time horizon in the base case analysis covered the period from randomisation to 14 days post-treatment cessation. The incremental cost-effectiveness of clonidine is shown in Appendix 7, Table 51. For the 120 children receiving clonidine (n = 61) or receiving midazolam (n = 59), we had complete cost and outcomes data. Within the base case analysis, the average cost was £11,445 in the clonidine group compared with £12,276 in the midazolam group, generating a mean cost saving of £831 (p = 0.494). There was no statistically significant difference in total costs between the two groups, with 71% of bootstrap replicates suggesting that clonidine is, on average, less costly than midazolam in terms of hospital service costs. However, the results of the cost-effectiveness analysis should be interpreted with caution, as the SLEEPS trial did not have sufficient power to identify any difference in the primary outcome between children in the trial arms should there have been one.
In the base case analysis, the incremental cost-effectiveness was estimated at –£21,216 per additional case of adequate sedation. However, there was substantial uncertainty around this finding. The variability around the base case estimate of cost-effectiveness is evident in the cost-effectiveness plane shown in Figure 11.
FIGURE 11.
Cost-effectiveness plane for base case cost-effectiveness analysis.
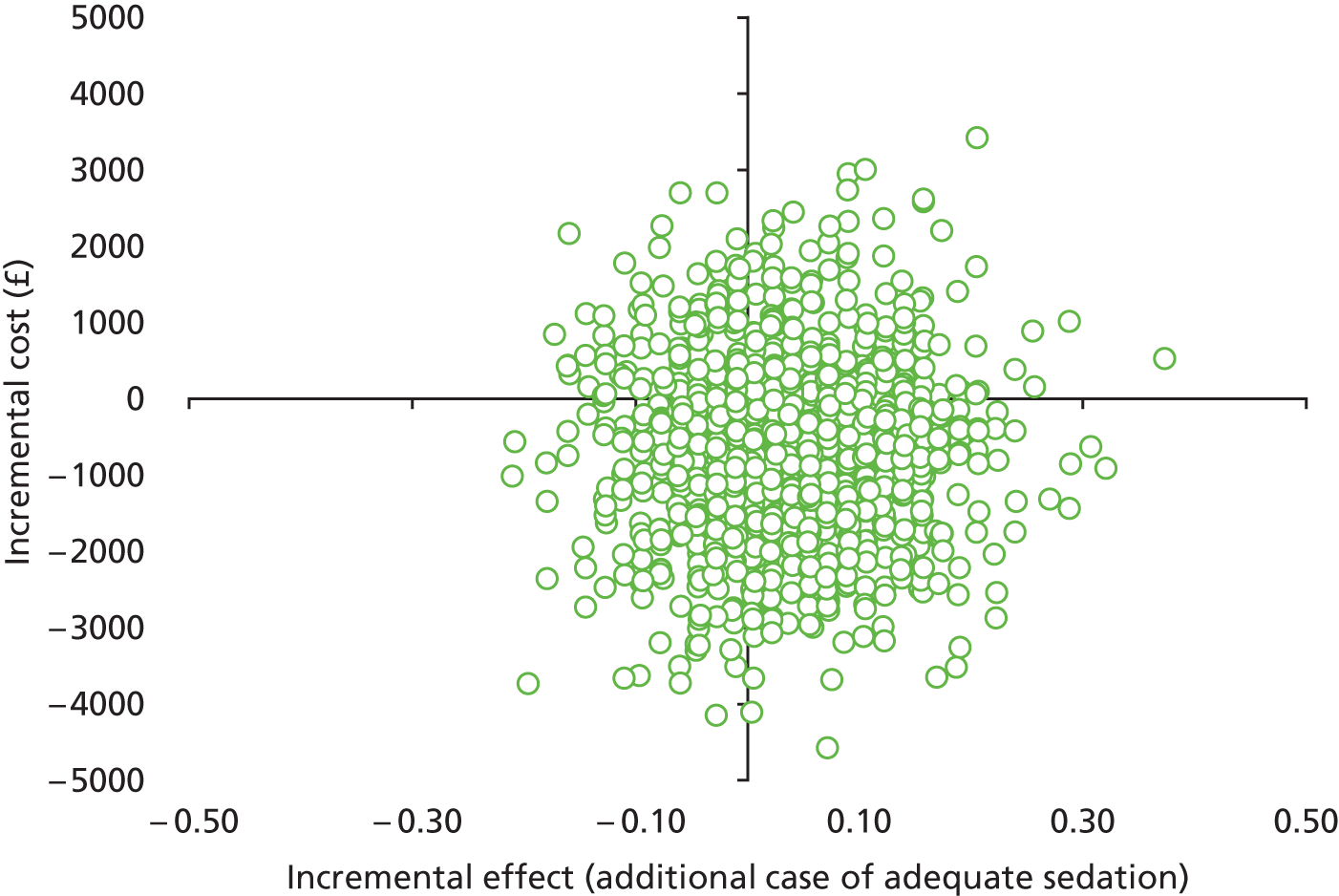
As the bootstrapped replications fall across all four quadrants of the cost-effectiveness plane, the CI around the mean ICER is difficult to interpret. For example, a negative ICER might represent lower costs and improved outcomes attributable to clonidine (south-east quadrant of cost-effectiveness plane) or higher costs and worse outcomes (north-west quadrant). Similarly, a positive ICER might represent higher costs and improved outcomes attributable to clonidine (north-east quadrant of cost-effectiveness plane) or lower costs and worse outcomes (south-west quadrant). As a result, a meaningful ordering of the bootstrapped replicates required to make the CI surrounding the mean ICER interpretable is very difficult. Under these circumstances, CEACs provide an appropriate approach to representing the uncertainty surrounding the mean ICER.
The CEAC for the primary clinical outcome measure is shown in Figure 12, and indicates that, despite being relatively flat, the higher the value that decision-makers place on an additional case of adequate sedation, the slightly higher the probability that clonidine will be cost-effective. At the notional cost-effectiveness threshold (or ceiling ratio) of £1000 per additional case of adequate sedation, the probability that clonidine is cost-effective compared with midazolam is 73%. Although no previous research has shown how much society or the NHS may or should be willing to pay for an additional case of adequate sedation for this group of children experiencing intensive care, the economic burden of not being able to adequately sedate a child is likely to be significant. Indeed, a recent NICE clinical guideline61 that focuses on sedation in children and young people states that ‘sedation failure is both distressing for the child and has major NHS cost implications’. If decision-makers are willing to pay as much as £5000 per additional case of adequate sedation then the probability that clonidine is cost-effective compared with midazolam increases to 76%.
FIGURE 12.
Cost-effectiveness acceptability curve for base case cost-effectiveness analysis.
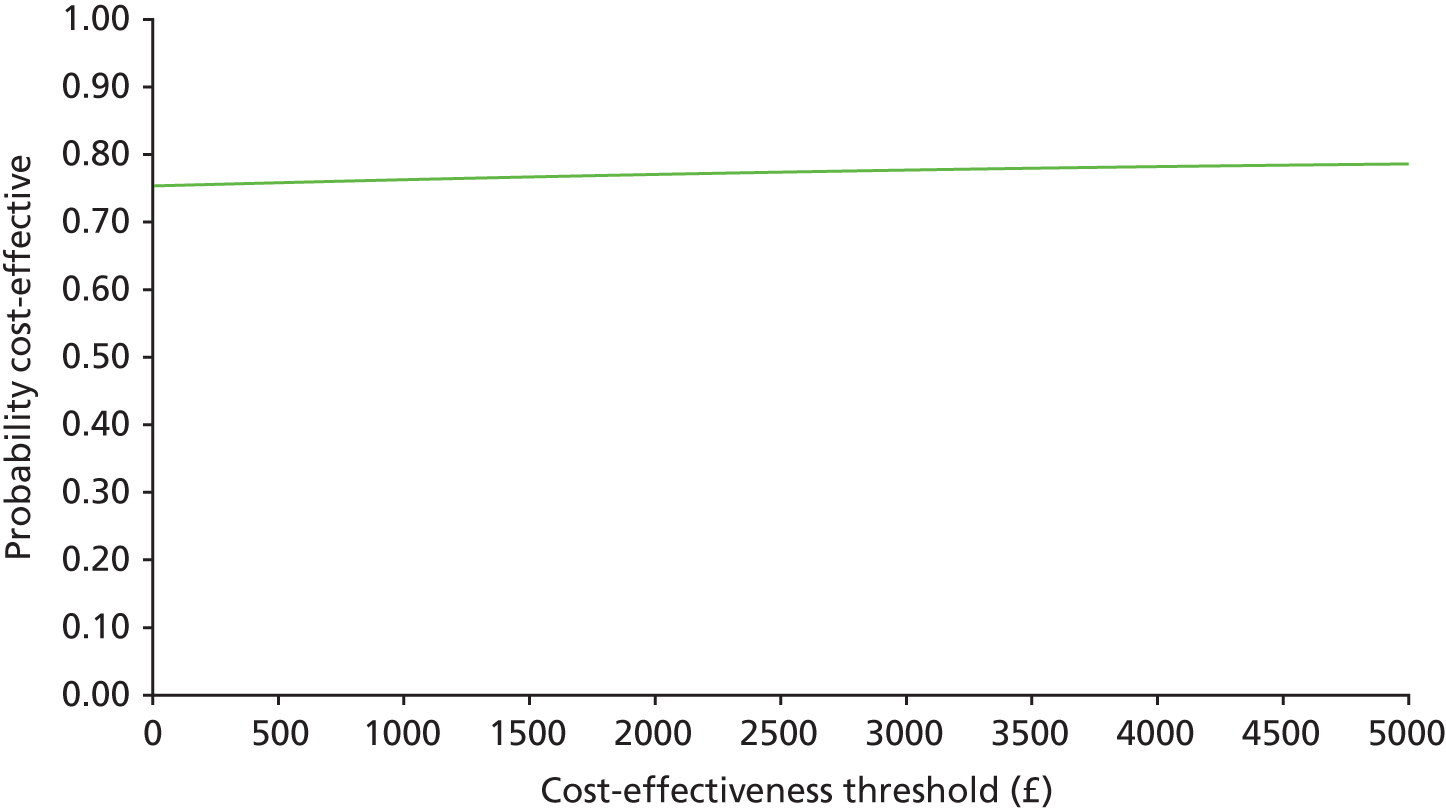
Mean net benefits were estimated for alternative cost-effectiveness thresholds per additional case of adequate sedation (see Appendix 7, Table 52). Assuming that the cost-effectiveness threshold equals £1000 per additional case of adequate sedation generates a mean net benefit to the health service attributable to each additional use of clonidine of £679 (i.e. on average, there is a net gain to the health service in monetary terms). This is analogous to stating that if the actual benefit of clonidine, in terms of additional cases of adequate sedation, is multiplied by a willingness to pay of £1000 per additional case of adequate sedation, and the net cost is subtracted, then the benefit to the NHS of adopting clonidine is, on average, positive in monetary terms. Note, however, that the 95% CI surrounding the mean net benefit (–1818 to 3086) includes negative values, i.e. there is a possibility of a net monetary loss associated with clonidine (see Appendix 7, Table 52). If the cost-effectiveness threshold is increased as high as £5000 per additional case of adequate sedation, the mean net benefit increases to £932 (95% CI –£1799 to £3212).
Sensitivity analyses were conducted to determine the impact of changing particular parameter values or assumptions on the magnitude of the mean ICER (see Appendix 7, Table 52). Assuming that higher-level inpatient care (e.g. stays in PICUs or HDUs) is valued using upper quartile NHS Reference Costs49 results in the mean cost difference between the trial arms increasing to –£997, with a corresponding ICER of –£24,933. Assuming that higher level inpatient care (e.g. stays in PICUs or HDUs) is valued using lower quartile NHS Reference Costs49 results in the mean cost difference between the trial arms falling to –£716 with a corresponding ICER of –£18,299. In both sensitivity analyses, the probability of clonidine being cost-effective compared with midazolam at a £1000 cost-effectiveness threshold increases from baseline. Assuming that part of a day spent by a child in an inpatient ward equates to a proportional period for costing purposes and, that, consequently, the vacated inpatient bed would be filled immediately, reduces the mean cost difference between the trial arms to –£753, with a corresponding ICER of –£19,224; under this assumption, there is no change from baseline in terms of probability of cost-effectiveness. Varying the costs of care associated with hospital admissions does not have a substantial effect on the magnitude of the base case ICER.
Extending the time horizon of the economic evaluation results in a mean cost difference of –£809, with a corresponding ICER of –£20,651. Even although extending the time horizon meant that for some children a slightly longer length of hospital stay was captured by the economic evaluation, and for one child the additional cost of a SAE was also captured, the size of the mean ICER does not vary substantially. The probability of clonidine being cost-effective in this sensitivity analysis is higher (76%) than the baseline value (73%).
The primary clinical outcome in the trial was framed around a case of adequate sedation; the definition of adequate sedation was ‘at least 80% of total time sedated within a COMFORT score range of 17 to 26’. In post hoc sensitivity analyses, we increased this proportion to 85% and also reduced this proportion to 75%. With a narrower definition of adequate sedation (85%), there was a reduction in the mean effect size in both groups and an increase in the mean difference in effect size (0.06); the corresponding ICER was –£13,979. With a broader definition of adequate sedation (75%), there was an increase in the mean effect size in both groups and an increase in the mean difference in effect size (0.07); the corresponding ICER was –£12,111.
In summary, all of the mean ICERs generated in the base case analysis and sensitivity analyses are negative, suggesting that clonidine is, on average, more effective and cheaper than midazolam. However, none of the differences in mean costs or consequences between the comparison groups was statistically significant, regardless of assumptions surrounding key parameters of the economic evaluation over which there was a degree of uncertainty. Under these circumstances, it is important to assess the likelihood that clonidine is cost-effective, primarily through the use of CEACs, rather than testing any particular hypothesis concerning its cost-effectiveness.
Cost-effectiveness acceptability curves generated following each sensitivity analysis are shown in Figure 13. Estimates of net monetary benefits for notional cost-effectiveness thresholds for an additional case of adequate sedation are shown in Appendix 7, Tables 52 and 53. For example, assuming that the cost-effectiveness threshold equals £1000 per additional case of adequate sedation, adopting a broad definition of adequate sedation (at least 75% of total time spent sedated within a COMFORT range of 17–26) generates a mean net benefit to the health service of £933, attributable to clonidine (i.e. there is a net gain to the health service in monetary terms). Note, however, that the 95% CI surrounding the mean net benefit (95% CI –£1414 to £3426) includes negative values, i.e. there is a possibility of a net monetary loss associated with clonidine (see Appendix 7, Table 53).
FIGURE 13.
Cost-effectiveness acceptability curves for base case analysis and sensitivity analyses.
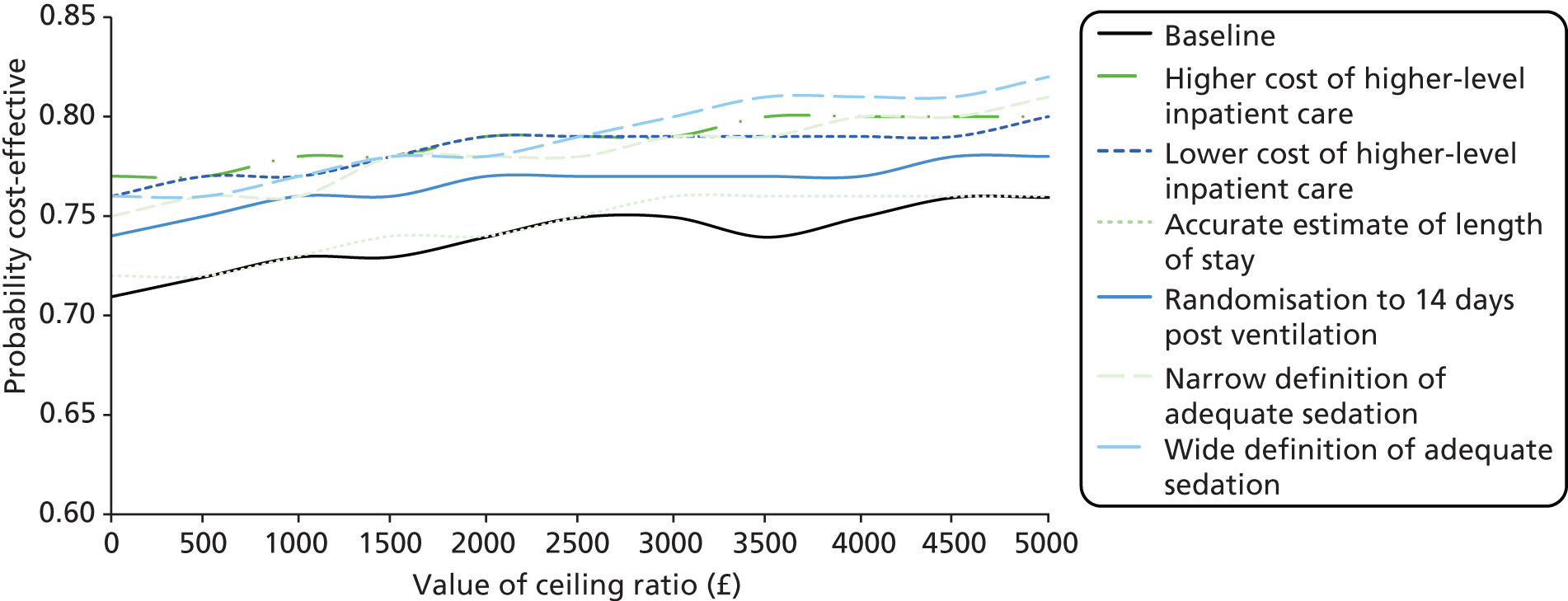
In addition to sensitivity analyses, we also conducted a scenario analysis using data from a sample of children (n = 106) for whom complete data were available on wider NHS resource use. We were able to collect data describing wider NHS resource use (e.g. GP visits, A&E visits and hospital readmissions) experienced by this group of children for 14 days after their treatment had ceased. At this time point, 29% (15/52) of patients in the clonidine arm were still in hospital and 31% (17/54) of patients in the midazolam arm were still in hospital. Clearly, not all of the patients included in this wider analysis incurred additional costs; this wider NHS resource use could have taken place only if the child had been discharged from hospital during this time period. In this analysis, the mean cost of care in the clonidine group is still cheaper than in the midazolam group, with a mean cost difference of –£552. However, midazolam is now slightly more effective than clonidine, with a mean effect difference of –0.006. With an ICER of £86,102 per additional case of adequate sedation there is a 48% probability of clonidine being more effective than midazolam, a 67% probability of clonidine being less costly than midazolam, and a 63% probability of clonidine being cost-effective compared with midazolam (see Appendix 7, Table 52). The variability around the base case estimate of cost-effectiveness is evident in the cost-effectiveness plane shown in Figure 14. The CEAC for the scenario analysis is shown in Figure 15, and indicates that the probability that clonidine is cost-effective declines slightly with increasing values that decision-makers place on an additional case of adequate sedation. Again, there were no statistically significant differences in costs or health consequences between the comparison groups. Estimates of net monetary benefits attributable to clonidine across alternative notional cost-effectiveness thresholds are shown in Appendix 7, Table 54. Assuming that the cost-effectiveness threshold equals £1000 per additional case of adequate sedation, including wider NHS costs generates a mean net benefit of £485 to the health service, attributable to clonidine (i.e. there is a net gain to the health service in monetary terms). Note, however, that the 95% CI surrounding the mean net benefit (–£2080 to £3174) includes negative values, i.e. there is again a possibility of a net monetary loss associated with clonidine (see Appendix 7, Table 54).
FIGURE 14.
Cost-effectiveness plane for cost-effectiveness scenario.
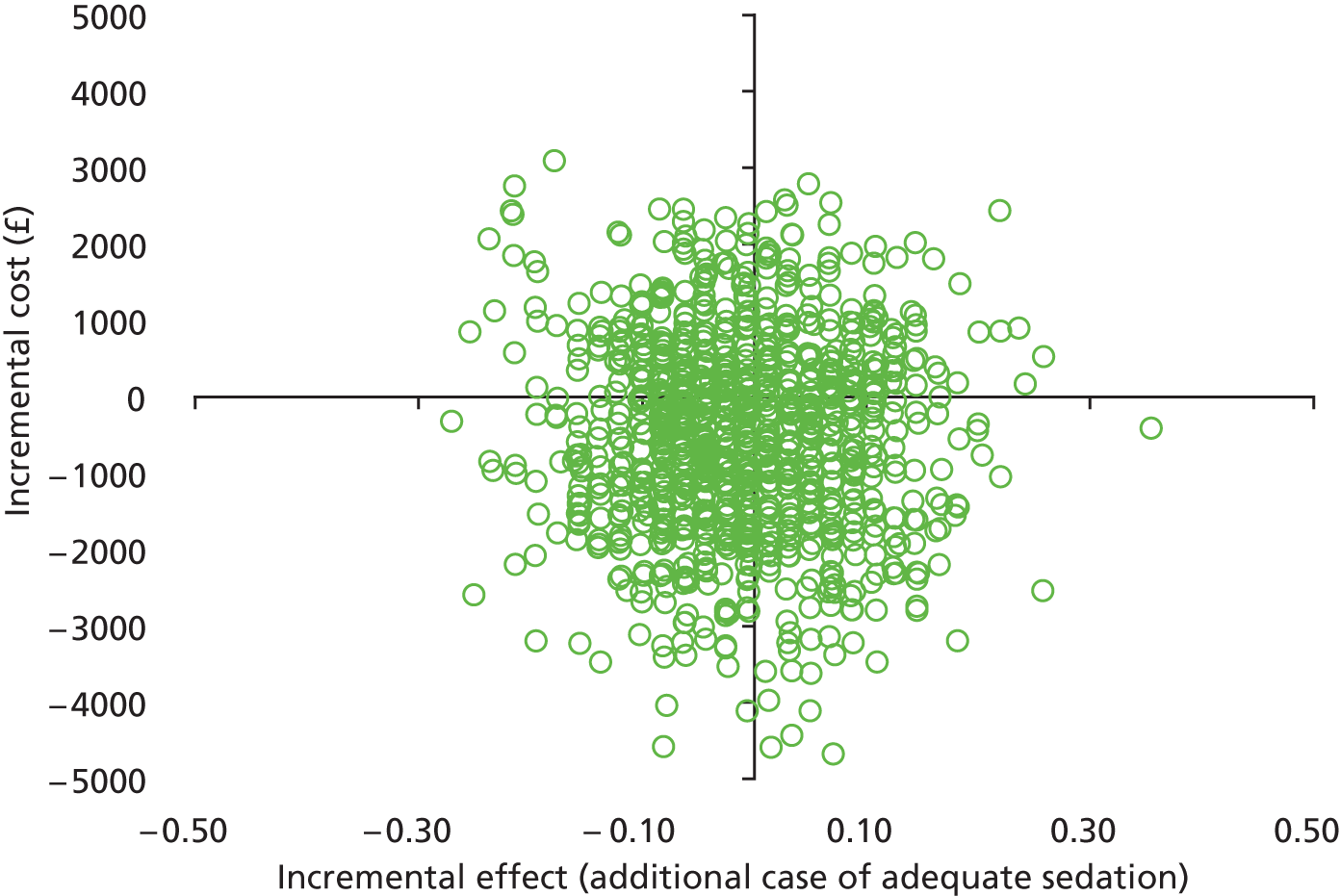
FIGURE 15.
Cost-effectiveness acceptability curve for cost-effectiveness scenario analysis.
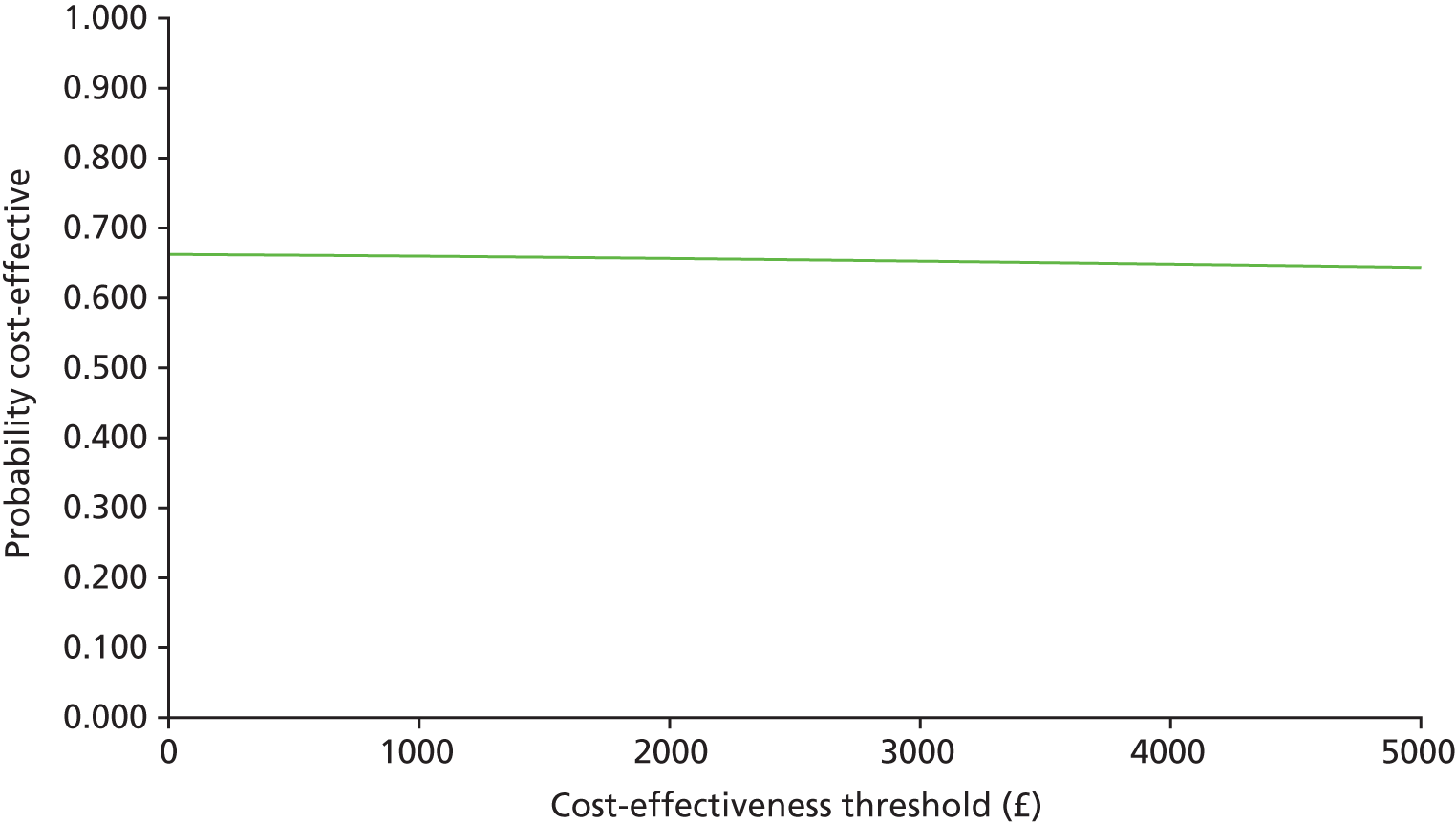
Chapter 4 Discussion
Main findings
Primary outcome
The trial did not recruit to target and was substantially underpowered in its objective to demonstrate equivalence. Equivalence between the treatment arms (± 0.15) for the proportion of children who were adequately sedated for ≥ 80% or more of the time was not demonstrated: 21 of 61 (34.4%) clonidine; 18 of 59 (30.5%) midazolam; difference in proportions 0.04 (95% CI –0.13 to 0.21). Non-inferiority of clonidine to midazolam was supported. However, this should be interpreted cautiously, as the wider CI that included values from outside the equivalence range favouring clonidine could have been due to the reduced numbers in the trial rather than the better performance of clonidine.
Other outcomes
Participants in the midazolam group were sedated for longer than those receiving clonidine (38.25 hours vs. 22.83 hours), but also took less time to become fully awake once sedation was stopped (medians 11.17 hours vs. 6.22 hours).
Fewer treatment failures were observed on midazolam [12/64 (18.8%) clonidine, 7/61 (11.5%) midazolam]. Only one case of rebound hypertension was observed (clonidine group). There were no discernible differences in the urine analysis or blood biochemistry results, and no differences in the proportions experiencing withdrawal symptoms; however, a higher proportion of participants allocated to midazolam required clinical intervention for those symptoms [11/60 (18.3%) clonidine, 16/58 (27.6%) midazolam].
Clonidine is an α2-agonist of a different pharmacological group to midazolam, which acts as a GABA agonist. Although clonidine affects sympathetic outflow from the brain (thereby reducing BP and heart rate), provides some analgesia and has a calming effect, the major actions of benzodiazepines are to provide both sleep/anaesthesia and amnesia. Although both drugs are used in the PICU, and can provide reasonable sedation in conjunction with morphine, it is clear that the drugs are different in their characteristics. Both agents provided reasonable sedation, and the amounts of time adequately sedated during the treatment phase were similar (73.8% clonidine, 72.8% midazolam). The data do also show that sedation in the PICU is far from perfect, in that 25% of patients are adequately sedated for only 58% of the time. This clearly indicates that the regimens currently used in the PICU with morphine and a second sedative drug (either clonidine or midazolam) remain suboptimal, regardless of choice of current agents, and strongly indicates that a third-line drug may be needed to approach the goal of adequate sedation for ≥ 80% of the time in the PICU.
The study has been able to confirm, but also quantifies, the risks associated with individual side effects of the two drugs and this will be helpful in informing the clinician on the selection of agent to use.
One of the barriers to increasing the use of clonidine has been concern about the potential cardiovascular side effects in the unstable and critically ill child. There have been very few data on incidence of side effects of hypotension, bradycardia or other dysrhythmias prior to the SLEEPS study other than anecdotal evidence or case reports. The study demonstrated that use of clonidine in comparison with midazolam was associated with increased inotrope delivery and/or fluid administration during the loading phase and in the first 12 hours. These effects were classified by the observers as mild and did not result in withdrawal from study. One subject did develop significant bradycardia without hypotension 4 hours after commencing clonidine, prompting the investigator to abandon the study and report a SUSAR. Recovery was spontaneous and required no intervention. The implication for clinical practice is that specific attention needs to be taken during the loading and early infusion phase when clonidine is used, and anticipation of fluid or drug intervention should be made. The other concern with clonidine has been the fear of rebound hypertension on abrupt withdrawal of the drug, which, although a known feature of adult sedation with clonidine, has not been observed in children. Nevertheless, the fear of this has led to a clinician practice that is not based on evidence of reducing clonidine dosage very gradually after even short exposure in the PICU. The SLEEPS study identified only a single case of increased BP after withdrawal of the sedative agents. It was categorised as mild and required no intervention. Although future use of clonidine must still be aware of the possibility of rebound hypertension, it would appear that it is not common and that the practice of tailoring the dosage of clonidine downwards over days and weeks for fear of this effect (which, in itself, has led to delayed discharge from hospital) should now be reviewed.
The key clinical concerns with midazolam prior to study were the rise in infusion requirements due to tolerance of the drug and the subsequent high incidence of withdrawal phenomena after the drug was stopped. These two phenomena are interrelated in that previous studies have shown that the incidence and severity of withdrawal phenomena with midazolam depend on the infusion rate of the drug, but also the duration and hence cumulative amount of drug received. The results of the SLEEPS study showed that there were no differences in times to achieve maximum sedation/analgesia in the groups, indicating similar tolerance/tachyphylaxis with midazolam compared to clonidine. There were fewer treatment failures in the midazolam group but this was associated with an increased requirement for supplementary analgesia compared with the clonidine group (p = 0.01), however patients were sedated for longer on midazolam. In terms of withdrawal associated with midazolam usage the SLEEPS study identified similar withdrawal symptoms for both groups. However, the proportion of patients requiring clinical intervention was higher in the midazolam group and this is in keeping with the known high instance of significant withdrawal side effects previously attributed to midazolam usage in the PICU. However, there may be some confounding with the greater length of time midazolam patients were sedated for and this is known to be a risk factor for increasing severity of withdrawal symptoms.
Strengths and weaknesses
Design
Despite the much smaller size of this study than that anticipated, SLEEPS is the largest trial comparing sedative agents in a PICU setting. SLEEPS has provided robust data targeting the concerns around the use of clonidine and cardiovascular instability and those around developing tolerance and withdrawal side effects of midazolam. We have demonstrated that the conventional approach of providing just midazolam and morphine in an unparalysed patient results in breakthrough sedation at a high frequency that is unacceptable. In conventional practice, many of the more sick children are given neuromuscular blocking agents with morphine midazolam combinations, which may mask the inadequacy of the sedation quality. This study should provoke solutions to this problem, which will involve the use of higher-efficacy opioids, such as fentanyl or alfentanil, and the more routine use of an additional sedative agent. Although clonidine performed no better than midazolam, it also was broadly similar in efficacy and had an acceptable safety profile. The trial therefore confirms that this is a viable alternative to midazolam. The rigour of the double-blind design and the thorough exploration of the data will expand the evidence base for these sedatives and provide useful data to inform clinical practice. Future study would require the results of the SLEEPS data to be taken into account. A new study would necessitate a more relaxed protocol, which would allow greater numbers of children with a greater variety of conditions to be recruited, more effective drug combinations that could include three drugs and a higher-efficacy opioid, and earlier enrolment to allow sicker children to be entered into the study regardless of their current sedation regimen or the use of muscle relaxants.
There is a tendency to oversedate children on PICU using multiple drugs at high doses and the SLEEPS trial protocol entwined the scoring system with the increases/decreases in sedative and analgesia. From the data presented it is clear that when children were outside the adequate sedation score range they were more likely to be oversedated than undersedated. However, both undersedation and oversedation are harmful. The scoring system used was systematic in its application; however, a consequence of the criteria used meant that it was not possible to use muscle relaxants. Although this impacted on numbers recruited, it may also impact on the generalisability of results.
The definition of the primary outcome, which used an 80% cut-off point for the proportion of time spent adequately sedated, as defined by the COMFORT score range, may be considered to be somewhat arbitrary. The ideal would have been an ED of 95%, as in many drug studies, but clearly this study was well short of this target.
A large number of protocol deviations were observed. It had been intended to conduct a per-protocol analysis alongside the ITT analysis, which may not be conservative within an equivalence trial. Further, the high degree of non-compliance may have increased the type I error rate in this study. The volume of protocol deviations shows the difficulty in applying a sedation protocol within a PICU.
Studies on sedation require intensive recorded monitoring, evaluation by an observer who has been trained and validated in the use of a sedation score, and rapid manipulation and documentation of changes in the infusion rates. This is in addition to the large administrative load in surveillance, recording, storing and processing of data. This has huge resource implications for any participating unit and, practically, this study was only possible by using the bedside nurses to run the evaluations and sedation changes. Individual training of large numbers of nursing staff to this level (180 staff on largest unit) and ensuring that their COMFORT scoring was standardised took considerable time, and the provision at each centre of just one part-time research nurse was inadequate for this trial. Future studies will need to address this requirement at the outset or relax the stringent inclusion criteria for nurse observers. The COMFORT score itself is relatively cumbersome but it is one of the few validated tools for evaluation of sedation in a PICU. The COMFORT scale, which eliminates the heart rate and BP observations, is simpler but has been only partially validated.
Patients in a PICU represent a heterogeneous mixture of ages and pathologies. This presents a difficult challenge in the study of these patient groups: on one hand there is a desire to have the conformity to achieve valid and reproducible results with a relatively small homogeneous group, but on the other hand there is a need to represent the entire PICU population. Despite considerable planning, training and meetings with the participating centres, protocol deviations were inevitable, representing the changing needs of the patients and demonstrating the difficulties experienced with adhering to differences from standard practice. During the set-up of the trial, one unit that had originally expressed an interest in participating then decided to decline. The unit had recently implemented a change in the sedation practices and reported the difficulties in adherence as the reason against participation, as SLEEPS would have necessitated further changes.
Examination of the protocol deviations indicate that these were most commonly related to failure to adjust sedation/analgesia to a change in COMFORT score, primary outcome data missing for ≥ 1 hour or delays in timing of action in response to COMFORT score-directed changes. Although these are not ideal, this represents what actually happens in the PICU in the management of the critically ill patient in an environment that is changing constantly and is difficult to control. The SLEEPS study represents the largest randomised sedation trial of PICU children and, as such, the lessons learnt from this are clear in terms of setting a more relaxed treatment protocol, which would allow increased participation and maintain more patients in the study. This is already helping to inform a following trial that has recently received funding and the senior author (ARW) is actively involved in an advisory capacity in this work. Relaxing the study protocol to accept more variability in the drug administration, and thereby obtaining more patient numbers without excessive protocol deviation, would appear to be a potential way forward in future work.
Considerations for dose and concomitant medications
Standard analgesic regimens used in the PICU and for postoperative analgesia describe doses of morphine infusions of between 0 and 60 µg/kg/hour. Correspondingly, the dose of midazolam used in the PICU is generally 0–200 µg/kg/hour. Previous studies have shown that midazolam side effects, including withdrawal phenomena, increase once the dose exceeds 100 µg/kg/hour, although some PICU continue to use doses of up to 300 µg/kg/hour. These doses were chosen for the SLEEPS study because they were applicable and relevant to clinical practice. Similarly, the limited data on i.v. clonidine suggested that a dose infusion in the order of 0–2.5 µg/kg/hour would provide reasonable analgesia. However, in clinical practice it is not uncommon to provide muscle relaxants for a limited period, particularly in the seriously sick infant, and also to prescribe pro re nata doses of additional drugs, whether oral or i.v. In addition to the obvious effects of muscle relaxant drugs, they may also have an intrinsic deafferenting effect, which can reduce sedation requirements in themselves. The use of muscle relaxants may be necessary in the sick infant with cardiac or airway disease but the consequence of their use is that COMFORT score cannot be assessed. The SLEEPS study needed to recognise this, and therefore it was a contraindication to entry and contributed to the low recruitment rates throughout the study. The requirement for additional as required i.v. sedation drugs on top of the two agent regimens was built into the protocol to allow for fluctuations in conscious level and sudden arousal. It was considered that if more than two doses of an additional drug were required in any 12-hour period then it would indicate that the two-dose regimen was insufficient. The data from the SLEEPS study indicated that neither midazolam plus morphine or clonidine plus morphine was able to provide the efficacy of sedation control alone with conventional doses, and that there remains a requirement for a re-evaluation of drug infusions or the need for a regular third drug.
Recruitment and retention
Recruitment into the trial was slower than expected, in part due to the number of eligible patients being lower than expected; however, retention of randomised participants was high.
The projected numbers for the study were 1000 patients, with 500 in each group. Initially, with 10 participating centres, this appeared feasible, requiring an average of one patient per week to be enrolled. Despite extension of the study, and a screening that totalled 10,023 children, only 129 children were randomised of whom 120 (93.0%) contributed data for the primary outcome of the study. The low recruitment rate, despite every effort – including several protocol amendments to try to improve the figures – has implications for other PICU studies of this type in the future. The specific issues identified are:
-
Conflict with other studies and elective cardiac cases:
-
Recruitment in a PICU is challenging. The potential competition with other ongoing studies [Control of Hyperglycaemia in Paediatric Intensive Care (CHiP) study; Steroids in Paediatric Sepsis (StePS) study] was recognised early on and discussed during planning of the SLEEPS study. The need for co-enrolment in PICU settings to support the research demands is important and has been discussed elsewhere. 62,63 The main trial that overlapped with recruitment of potential participants with SLEEPS was the CHiP trial, and coenrolment was clinically contraindicated for the two trials. The CHiP trial was more suitable for elective cardiac patients, and, in discussion with the PIs, it was decided that CHiP would concentrate on this, whereas SLEEPS would look to concentrate on non-cardiac patients until the CHiP trial ended.
-
In the last 5 years it has been recognised that early extubation of many of the postsurgical cardiac cases is beneficial to recovery. With this understanding, anaesthetic techniques have been developed to aid extubation and accelerated recovery. As a result, many infants and children have become ineligible for the SLEEPS trial and the initial entry criteria of expecting to be ventilated for 48 hours was changed to 12 hours. In addition, those children not undergoing early extubation are usually sufficiently unwell that they are paralysed, delaying entry into the study. Again, the protocol was amended to try to extend the time from initial ventilation to study entry from the initial value of ‘within 48 hours’ to ‘within 120 hours’. This allowed extra time for the sickest patient to become well enough to be sedated without additional paralysis. However, despite this the results show that recruitment of patients after cardiac surgery was poor, even after the CHiP trial ended.
-
-
Parental issues:
-
Parents of children admitted to PICU in an emergency are highly stressed. Consenting for research studies in children have reduced considerably in the last 10 years, even for simple elective observational studies. There was considerable refusal rate from parents [194/827 (23.5%) eligible patients]. Reasons were multifactorial but a common reason was that if the child was settled on the ventilator in intensive care at the time that consent was asked for parents could see no gain for their child in participating. In addition, clinicians may feel anxious64 about approaching parents for consent, and this may be exacerbated if they begin to expect a negative response.
-
-
Timing of consent:
-
Once parents had arrived and settled with their children in the PICU, and the seriousness of their condition had become apparent, parents were more reluctant to give consent to any procedure other than one that was life saving. One of the possible solutions to the above problem would be to have taken consent at the earliest clinical point of contact but still allowing parents to make an informed choice. An additional complication was that many of the patients come from referral hospitals before retrieval to the regional centres. This further delays the ability to access parents for consent. Discussions were held about removing the criteria for children to be adequately sedated before entry into the trial, which would have necessitated a deferred consent approach. However, owing to the retrieval nature of many of the cases and the potential concerns around cardiovascular instability with clonidine this was not considered appropriate. Future studies would be improved by achieving deferred consent, allowing children to be initiated into the study at the outset of critical care, even if muscle relaxants are being used. This would increase patient recruitment considerably, and generalisability of results to evaluate immediate sedative requirements.
-
-
Clinicians’ issues:
-
All of the PIs and the PICU teams were committed to the study but, despite this, the clinicians in charge felt, in some cases, that the child was too unstable to enter into the study. This was one of the first studies to institute a blinded randomised controlled medication trial in the PICU on sedation. Although the study itself has led to more confidence with the use of clonidine in the PICU, at the time there were concerns about cardiovascular instability or ineffectiveness. In situations when there was clinical concern this led to abandoning of consent for the study and, although frustrating, was understandable. The severity of illness in the children under study and the effects on clinician decision-making cannot be overestimated. When faced with a critically ill child, clinicians will naturally tend towards conservatism. For example, although many intensivists will use morphine liberally in the child with asthma, a few will avoid the drug because of the potential concern of exacerbation of bronchospasm. Similarly, although there is evidence that muscle relaxation increases complications, including the risk of nosocomial infection, a common failure to recruit was due to the use of these agents. These drugs were often used to simplify a complex situation so that the physician could concentrate on facilitating treatment of the primary disease while effectively removing the need to consider sedation in a generic fashion. The results of the SLEEPS study would raise some concerns about this practice, in that at the standard doses of midazolam–morphine or clonidine–morphine reliable sedation cannot be guaranteed.
-
-
Research nurse time:
-
The amount of adequate research nurse support in complex interventional trials should not be underestimated. It is required in terms of education of staff and thus avoidance of protocol violation. Paucity of research nurses cannot be underestimated as an impediment to recruitment.
-
-
Delay in study start:
-
From the time of obtaining funding for the study there were significant delays in opening to recruitment. The key delay was the production of the blinded drugs, which required feasibility work, bioburden and postfiltration validation and testing, analytical method development and validation, stability protocol development, and obtaining of stability data. The manufacturers experienced delays with receiving supplies of the midazolam active ingredient, which delayed the feasibility work and consequently all time points with regards to the Investigational Medicinal Product (IMP). We were also required to carry out a systematic review and to produce a Simplified Investigational Medicinal Product Dossier as part of the clinical trial application (CTA) approval process. This meant that the process took a year from the signing of the contract with the IMP manufacturers until the CTA was granted by the MHRA. Furthermore, there were significant delays of several months in opening sites to recruitment once the regulatory approvals were in place. This was due to the training needs associated with the study and the volume of staff on each PICU. With research nurse time equivalent to 1 day per week, it was very difficult for the research nurse to train the large number of staff required to run the trial, especially as it was necessary to find time within their clinical roles for this to take place. During the time between first applying for funding and opening to recruitment, shifts in practice became apparent, with moves towards oral sedation and reductions in the length of sedation.
-
-
Compliance with the protocol:
-
This study highlights the difficulties of adhering to a tight sedation protocol and attempting to apply this to a wide age group in children with different disease processes. This approach led to difficulty in recruitment and in those who were recruited, and difficulties in maintaining the patients within the tight sedation regimen. Future studies in sedation will need to approach this by having more relaxed entry criteria, by allowing the use of additional drugs and to accept periods when data acquisition is not possible. Such a study will require large patient numbers and careful stratification by age and disease in order to further our understanding. It will also require considerable resources and funding, with research staff independent of the clinical care so that there is tighter matching of drug delivery in response evaluations, and more rapid response to behavioural change that would include allowance for incremental dosing with study drugs or other alternatives. Alternatively, there should be a reversion to small tightly controlled single-centre studies focusing on one age group and one disease group.
-
Discussion of economic evaluation results
The economic evaluation undertaken alongside the SLEEPS trial compared the use of i.v. clonidine with i.v. midazolam in the sedation of critically ill children. It represents, to our knowledge, the first economic evaluation of i.v. clonidine in critically ill children from a NHS hospital services perspective. The economic evaluation was conducted according to nationally agreed design and reporting standards. 47,65 A key strength of the economic evaluation is that it is based on the prospective collection of cost and clinical effectiveness data from the SLEEPS trial, which recruited children from across representative clinical centres in the UK; this means that the source of the data is likely to be reliable and appropriate to inform health-care decision-making in the NHS. As resource-use data were collected via the trial CRFs, almost complete health economics data were available for analysis and we are therefore confident that we have been able to identify, measure and value resource use reliably for both groups of children.
The economic evaluation revealed no statistically significant differences between the clonidine patients and the midazolam patients for any of the cost categories. The results of the cost-effectiveness analysis demonstrate that use of clonidine compared with midazolam yields a relatively high probability (73%) of cost-effectiveness at a threshold of £1000 for an additional case of adequate sedation. Increasing the cost-effectiveness threshold resulted in the use of clonidine becoming increasingly cost-effective: at a cost-effectiveness threshold of £5000, the probability that clonidine is cost-effective increases to 76%. Clearly, how much society or the NHS may, or should be, willing to pay for a case of adequate sedation is unknown, and this is the challenge faced by health-care decision-makers. Future preference elicitations studies in this area should aid their decision-making. Indeed, a separate discrete choice experiment that we are currently conducting among a sample of 1000 members of the UK public, which aims to elicit preferences for attributes associated with sedative drugs to facilitate artificial ventilation in PIC, should inform decision-making in this context. It is noted that a recent Evidence Update Report from NICE supports the view that optimum methods for the sedation of children and young people should be a research priority and require further study. 66
The results of five of the six sensitivity analyses confirm that our probability estimates of the cost-effectiveness of clonidine are robust; probabilities ranged from 72% to 77% at a cost-effectiveness threshold of £1000 per additional case of adequate sedation. The exception was the scenario analysis that broadened the study perspective (to cover wider NHS resource use).
It is clear from the analyses performed that length of hospital stay was the key cost driver in the economic evaluation. By focusing attention on per diem hospital costs associated with general medical, PICU and HDU wards, any minor effects of clonidine or midazolam on activity within the critical care unit may have been missed. However, we are confident that any costs related to substantial changes in morbidity (with either clonidine or midazolam in the PICU, HDU or general ward) have been captured by our cost estimates of inpatient LoS. Regardless of the method of valuing this cost, the magnitude of the mean ICER remained largely unchanged. All of the mean ICERs in the base case analysis and sensitivity analyses were negative, as clonidine was, and, on average, cheaper and more effective than midazolam. However, the interpretation of negative ICERs is challenging and requires careful consideration. As noted above, a negative ICER might represent lower costs and improved outcomes attributable to clonidine (south-east quadrant of cost-effectiveness plane) or higher costs and worse outcomes (north-west quadrant). The scenario analysis is the only analysis that yielded a positive mean ICER. In this scenario, clonidine was, on average, cheaper (negative costs) and slightly less effective (negative effects) than midazolam. Nevertheless, the importance of uncertainty surrounding the magnitude of the estimated mean ICERs was evident in all the cost-effectiveness analyses.
Two key components of the economic evaluation merit further discussion. First, choosing a time frame for the analysis of costs in this economic evaluation was problematic. In the clinical trial, the definition of adequate sedation was prespecified to be ‘at least 80% of total time sedated within a COMFORT score range of 17 to 26’. However, to use ‘total time sedated’ as the time horizon would have underestimated the costs incurred, as children were not immediately discharged from hospital after being sedated, and sedation itself can be associated with longer-term sequelae. In contrast, to have adopted the period ‘from randomisation to final discharge’ as the time horizon would most likely have overestimated the costs, as some children stayed in hospital and received other interventions unrelated to mode of sedation and/or their underlying health condition. The results of the economic evaluation may therefore have limited applicability to these complex patients who have significantly long periods of sedation and ventilation that are consequences of the patients’ needs rather than the choice of sedation drug. The base case analysis was based on the period from ‘randomisation to 14 days post-treatment cessation’ and this was chosen in collaboration with clinical experts and in keeping with recommendations for PIC. In addition, we also used the period from ‘randomisation to 14 days post-ventilation cessation’ in a sensitivity analysis; this assumption resulted in a slightly longer time horizon for the economic evaluation but did not substantially change the results or conclusions of the economic evaluation. Second, there is no published or unpublished estimate of willingness to pay for an adequately sedated child (the value of the cost-effectiveness threshold). When estimating net benefits, we assumed that the economic value placed on an additional case of adequate sedation lies somewhere between £0 and £5000; however, the true value of this benefit is currently unknown. Separate research we are conducting, in the form of a discrete choice experiment, should generate monetary values placed on an additional case of adequate sedation in PIC and consequently inform decision-making in this area.
The main limitation of the economic evaluation is that it is based on the results of the SLEEPS trial, which may not have had sufficient power to identify any difference in the primary outcome between children in the trial arms should there have been one. If this were the case, then the results of the economic evaluation may have been different. Nevertheless, in keeping with broader methodological practices, we have concentrated on estimating cost and effect differences, and assessing the likelihood that clonidine is cost-effective rather than testing any particular hypothesis concerning its cost-effectiveness. In addition, the authors recognise that, as in all economic evaluations, other valid approaches to costing could have been adopted.
In conclusion, the results of our analyses suggest that, from a NHS hospital services perspective, clonidine is likely to be a cost-effective sedative agent in PIC in comparison with midazolam. The results of the baseline and sensitivity analyses showed that the probability of clonidine being cost-effective is > 70% at a cost-effectiveness threshold of £1000 per additional case of adequate sedation. It is anticipated that data collected on the costs and consequences of children undergoing i.v. sedation as part of the SLEEPS trial will be used to inform future economic evaluations and other empirical research studies in this area.
Comparison with other studies
The SLEEPS study safety monitoring was informed by other relevant studies. 67 The most relevant systematic review69 was of midazolam in neonates, which included only three trials, and concluded that there was no evidence to support its use. Studies on neonates have demonstrated that midazolam has limited value in sedation in this age group, and that morphine alone can be sufficient. In addition, the concerns related to apoptosis in the developing brain, associated with exposure to benzodiazepines, gives concern regarding the use of these drugs in the very young. In contrast, infants and older children require sedative drugs, sometimes at high doses, to prevent discomfort even when opioids are used. The SLEEPS study demonstrates that infusions of midazolam at standard doses in combination with morphine cannot reliably achieve ideal sedation. Higher doses of midazolam can be used but are associated with increasing tolerance and withdrawal. Therefore, the inclusion of a third background sedative drug from a different pharmacological group may become necessary as a drug-sparing agent in those who cannot be managed by two agents alone. The results of SLEEPS have generally been consistent with those reported within Gamble et al. 67
Generalisability
Conclusions based on studies of sedation in children in PIC can be difficult to interpret by clinicians when they apply this to specific management of individual patients. Even entirely normal neonates, infants and children have large differences in both pharmacokinetic and pharmacodynamic responses to sedative drugs due to variation in maturity of excretion and elimination routes (kinetic variation) in addition to the changes in target receptors and responses with age (pharmacodynamics variation). Moreover, specific diseases greatly affect drug response. Children with sepsis or cardiac disease are identifiably different from each other due at least in part to altered pharmacokinetics. 69–71 Nevertheless, the current study comparing two commonly used sedative drugs in a clinical environment has outcomes that can provide broad guidelines for clinicians wishing to sedate patients in the PICU without paralysis. It clearly shows that for many patients the combinations of midazolam–morphine or clonidine–morphine are unable to provide sedation in the unparalysed patient within the ideal sedation zone as defined by the COMFORT score.
The current study appeared to preferentially recruit children who required sedation for relatively short periods (median sedation times of 22.83 hours for clonidine and 38.25 hours for midazolam). In general this occurred because the sickest children were either ineligible because of the need for neuromuscular blockade or concern from individual physicians to enter an unstable patient into the study. Moreover, children who require long stays in the PICU are often those with underlying neurological or neuromuscular pathology and most of these are ineligible for study. The SLEEPS study was focused on control of sedation over a maximum of 7 days and the side effects and the development of drug tolerance with two different drugs. Several groups were not well represented in the study and this included cardiac patients and long-stay patients.
The initial study envisaged that many more cardiac patients would be recruited. Unfortunately as discussed elsewhere the move to fast track cardiac surgery for a larger number of elective cases and the move towards early radical repair in the neonatal period resulted in fewer patients being recruited that expected from this group. Both midazolam and clonidine have effects on the cardiovascular system but in clinical practice muscle relaxants are used in the early phase after complex surgery and it is likely that this results in lower doses of sedation drugs being used. In the unparalysed patient the results of the study would suggest that two drugs alone in the doses commonly used are insufficient and, in general, we believe that the clinician should include a third agent as a background routine dose to moderate consciousness. This would then allow the i.v. opioid plus midazolam/clonidine to be used in a variable fashion within the usually prescribed limits. Further study would need to test this hypothesis and is being actioned in the current design of the current CloSed Consortium study.
This study demonstrates that combinations of midazolam–morphine or clonidine–morphine, at infusions that are generally accepted as normal dose ranges in clinical practice, fail to provide reliable sedation in a significant proportion of patients. This is reflected in the treatment failures recorded for both treatment groups. This has important implications; currently, the shortfall in sedation efficacy is compensated for by the addition of additional sedative agents (given orally, intravenously or per rectum) or by introducing neuromuscular blocking drugs. This last approach is of some concern in that evaluation of adequacy of sedation in the paralysed patient is limited. The implication for this is clear in that sedation regimens need either to routinely make a third sedative agent available or to use higher-efficacy drugs, such as fentanyl or alfentanil, in place of morphine. Data have already shown that increasing doses of midazolam are associated with greater side effects, such as withdrawal, and therefore allowing increased doses of midazolam may not be beneficial. The advent of alternatives to clonidine, such as dexmedetomidine, may open up the possibility of α2-agonist drugs with greater efficacy.
Despite using a relatively conventional treatment approach, the study results demonstrate the rapid swings from oversedation to undersedation with both drugs and the need for rapid intervention with bolus rescue drugs. Adding a third agent will improve this but the study underlines the need for frequent sedation measurement and the ability to respond to early arousal.
The SLEEPS study was underpowered to demonstrate equivalence due to failure to recruit to target; however, non-inferiority of clonidine to midazolam was shown. The SLEEPS study demonstrates that clonidine is a viable alternative to midazolam without substantial safety issues. Clonidine and midazolam have different pharmacological characteristics requiring the clinician to select them on individual needs and pathologies of the child. Specific attention needs to be taken during the loading and early infusion phase when clonidine is used due to its potential to reduce heart rate and BP. However, once the drug has been established it does not appear to be associated with major cardiovascular side effects. Relatively few patients were recruited who were receiving inotropic drugs and reflected the lack of inclusion of cardiac patients or sick patients with sepsis due to the requirement for neuromuscular blockade on arrival in the PICU and during the window of recruitment.
Before this study, concerns had been raised about rebound hypotension when clonidine was discontinued. Within the limitations of the study this did not seem to be a concern. The practice of tailoring the dosage of clonidine downwards after short-term sedation over fear of rebound hypertension should be reviewed. After long-term sedation it is likely that the drug will continue to be weaned gradually as an agent to protect from withdrawal of sedation. The data demonstrate that tolerance and withdrawal are reported features for both drugs, although possibly worse for midazolam. The SLEEPS study was not designed to look at more chronic sedation in the PICU and how longer-term tolerance develops. However, given that a significant number of patients are ventilated for > 7 days and require ongoing PICU sedation, further studies are needed to address this separate issue, which is acknowledged to be problematic.
Conclusions
Interpretation
This is the first study to document and compare the applicability of two commonly used sedation and analgesia regimens in critically ill ventilated children in a prospective blinded randomised fashion. The results have indicated that although both clonidine or midazolam can provide effective sedation some of the time, neither are able to achieve a target of 80% time in the targeted sedation zone. Additional supplementary medication can be used to maintain sedation but, as the trial did not allow more than two doses per 12-hour period, treatment failure occurred (clonidine in 18.8%, midazolam 11.5%). Although the drugs were not shown to be equivalent, this is not surprising, given the low statistical power to detect equivalence. However, these drugs do have very different pharmacology, with separate target sites, and so different profiles may be expected. Although their ability to provide controlled dose-dependent sedation is broadly similar, their characteristics and side effect profile are different. The study demonstrated the need to be aware of cardiovascular side effects, with clonidine in particular within the first 12 hours, and that patients who have been sedated with midazolam may require additional treatment for withdrawal phenomena afterwards.
Implications for health care
This study demonstrates that combinations of midazolam–morphine or clonidine–morphine, at infusions generally accepted as normal dose ranges in clinical practice, fail to provide reliable sedation in a significant proportion of patients. This is reflected in the proportion of time spent adequately sedated for both treatment groups. This has important implications: currently, the shortfall in sedation efficacy is compensated for by the addition of another sedative agent (given orally, intravenously or per rectum) or by introducing neuromuscular blocking drugs. This latter approach is of some concern in that evaluation of adequacy of sedation in the paralysed patient is limited. The implication for this is clear in that sedation regimens need either to routinely make a third sedative agent available or to use higher-efficacy drugs, such as fentanyl or alfentanil, in place of morphine. Data have already shown that increasing doses of midazolam are associated with greater side effects, such as withdrawal, and therefore allowing increased doses of midazolam may not be beneficial. The advent of alternatives to clonidine such as dexmedetomidine may open up the possibility of α2-agonist drugs with greater efficacy.
Despite using a relatively conventional treatment approach, the study results demonstrate the rapid swings from oversedation to undersedation with both drugs, and the need for rapid intervention with bolus rescue drugs. Adding a third agent will improve this, but the study underlines the need for frequent sedation measurement and the ability to respond to early arousal.
The SLEEPS study demonstrates that clonidine is a viable alternative to midazolam without substantial safety issues from this study. Clonidine and midazolam have different pharmacological characteristics, requiring the clinician to select them on individual needs and pathologies of the child. Specific attention needs to be taken during the loading and early infusion phase when clonidine is used because of its potential to reduce heart rate and BP. Once the drug has been established, the drug does not appear to be associated with major cardiovascular side effects. Before this study, concerns had been raised about rebound hypotension when clonidine was discontinued. Within the limitations of the study, this did not seem to be a concern, although after long-term sedation it is likely that the drug will continue to be weaned gradually as an agent to protect from withdrawal of sedation. Although future use of clonidine must still be aware of the possibility of rebound hypertension, it would appear that this is not common and that the practice of tailoring the dosage of clonidine downwards over days and weeks for fear of this effect (which, in itself, has led to delayed discharge from hospital) should now be reviewed.
The study will help to guide clinicians into making a rational choice between these drugs in the PICU: clonidine may be chosen for those patients with excessive sympathetic drive but avoided in children immediately after cardiac surgery. When the trial was designed, it was envisaged that many of those recruited would be children post cardiac surgery. Unfortunately, the development of fast-track cardiac surgery in the last 10 years with extubation within 12 hours after surgery has reduced the potential recruitment from this population and, as a result, few of the study patients were receiving inotropes while receiving the trial drugs. This is unfortunate in that as clonidine has interactions with the sympathetic nervous system it remains important to understand the relative effects compared with midazolam.
Implications for research
Sedation of the critically ill child with the current regimens is still far from perfect. Having shown that the current regimens of midazolam–morphine or clonidine–morphine does not on its own provide reliable sedation without supplementation, future study needs to focus on either improving clinical effectiveness without introducing further side effects either during or after sedation.
Of the 10,023 patients screened and reported in the screening log, only 698 (8.3%) were eligible according to the strict inclusion criteria. The common causes of failure (see Table 4) to reach entry criteria were patients were not intubated or were likely to be extubated within a short period of admission to the PICU (particularly those who had undergone cardiac surgery), those who required muscle relaxants throughout the potential recruitment period and those whom the physicians felt unable to allow recruitment on the grounds of clinical state. Interestingly, the units that were able to recruit the most patients (such as Nottingham) were those that had a good throughput of single organ dysfunction, such as chest infections. When the trial was conceived, it was hoped to have a large throughput of cardiac cases with periods of ventilation that would be well within the trial criteria. During the long process required to activate the study, the emphasis on early extubation of cardiac surgery became a significant clinical feature (fast-track and ultra-fast-track cardiac surgery). As a result, early extubation for common conditions such as Fallot’s tetralogy, ventriculoseptal defect and single ventricle palliation became common. This then left the neonatal and complex cardiac cases that clinically were either ineligible or unsuited to the institution of the SLEEPs protocol until after the allotted window of recruitment. Similarly, with the recent developments in management of infants with chest infections, such as respiratory syncytial virus bronchiolitis, many of these infants are now managed with non-invasive ventilation or converted on to a non-invasive (non-intubated strategy) rapidly after arrival. Only the sickest of these patients remain intubated for extended periods of time, and, of these, many are deemed too unwell for study by the local clinician or are managed with neuromuscular blocking drugs. A newly funded similar international multicentre sedation study with sedation in the PICU using clonidine (the current CloSed Consortium study) has used the knowledge of the SLEEPS study (and direct experience) to improve recruitment by allowing later entry of PICU patients into the study, with an understanding that several days may need to pass with other sedative regimens and neuromuscular paralysing drugs before the patients can be recruited.
This study demonstrates clearly that regimens using the conventional doses of midazolam–morphine or clonidine–morphine are not often going to provide acceptable sedation on their own. Although there is considerable variability, for the majority of patients a third sedative agent is necessary or substitution of morphine with a more potent opioid, such as fentanyl or alfentanil, is required. To better describe the effectiveness of sedative agents in the PICU, the results and experience of the SLEEPS trial need to be recognised and allowance made in future trials to incorporate third agents or more potent opioids into the trial protocol. This is, indeed, what is being proposed in the CloSed study. Although this may make this study, and future studies, less easy to interpret, it would at least allow a much increased recruitment practice, which would better reflect a true clinical population of critically ill children in the PICU. Increasing the patient numbers at the expense of the rigidity of the study would at least provide a more accurate reflection of the practices within PICU.
Research directions could include investigating the use of a third agent that could act as a ‘sparing drug’ to reduce side effects, replacing morphine with a higher-efficacy opioid, such as fentanyl, or encouraging development of a novel high-efficacy sedative agent. In addition, research into techniques that allow earlier extubation and reduce both duration and quantity of sedation, such as non-invasive ventilation and fast-track surgery, will hasten recovery and discharge from the PICU. This would have profound effects of reducing NHS costs for PICU stays and increasing PICU bed availability.
Sedation remains a key cause of delay in extubation and discharge from the PICU, and is associated with frequent morbidity. However, although the problem is acknowledged, research in this area is not popular, possibly because it is not directly connected with ‘curing’ disease and that the problems associated with sedation are perceived simply as iatrogenic. In clinical practice, difficulty with inadequate sedation is usually managed in the short term by adding additional agents until a child is acceptably asleep, even though this will further increase the likelihood of drug tolerance and delayed recovery in the longer term.
Fundamental and difficult questions need to be addressed even before considering the drug management and monitoring. In western culture, with the emphasis on child-centred and parent-directed management, there is a common perception that only a completely anaesthetised child is a comfortable child. In the neonatal nursery, where the sensitivity to central nervous system agents is increased and the newborn child has less mobility, it has been easier to move away from heavy sedation bordering on anaesthesia. The infant and young child is far more difficult to maintain in a comfortable and quiescent state: their conscious state varies in almost a binary fashion from asleep to awake and moving vigorously over minutes. Other cultures, such as Japanese culture, have accepted this situation without resorting to deep sedation, but it does require constant attention from parents and caregivers to reassure a child that will need to remain relatively still in a cot (Clinical Investigator, personal observation and communication). Children managed in this way, with the emphasis on intense human support and minimal drug delivery rather than high-dose pharmacological intervention, are much more labour intensive to manage but they avoid the effects of withdrawal and tolerance and are allowed accelerated recovery. Efforts to implement this practice in a rigorous fashion, perhaps using quality improvement methodology, might prove valuable in the future. However, it will be essential to ensure that this approach does not result in either short- or long-term distress to the child.
One of the difficulties in this research is that there is a degree of concern not only from parents, but also from the clinicians in undertaking a study that requires a change in general management away from the more comfortable ‘Unit Policy’, particularly when this does not pertain to the primary pathology and treatment. This was a serious problem in setting up the protocol for the trial, and, in order to achieve a collaborative multicentre trial, compromises were required to achieve a protocol that could be agreed by different units. Although the current emphasis in clinical trials is to have large numbers of patients enrolled, it is a difficult model for the PICU. The patient numbers are small and PICU practices are both conservative and varied, making multicentre trials in sedation research difficult, with experience demonstrating the struggle of overcoming the barriers. To guard against the need for more research and maximise the value of return for research investment we would promote consideration of the use of external pilots conducted in two to three centres within this setting. External pilots could have more exacting protocols, and consideration could be given to how they should be upscaled as an output. Evaluation of the upscaling of the clinical protocol across centres in a main trial should be a progression criterion that is evaluated within an internal pilot. The impact of this approach on the additional time required to answer the clinical question and move from an external pilot to a main trial should be considered.
Acknowledgements
The SLEEPS Trial Management Group (TMG) is very grateful to all of the PIs, research practitioners, site pharmacists, children, and their carers and families for their commitment, energy and patience. The SLEEPS TMG is also grateful to the following: the Local Research Networks in England, whose work and support was invaluable in the early stages of SLEEPS; the TSC (chaired by Professor Adam Finn, Dr Kerry Hood, Dr John Henderson) and IDSMC [Professor Diana Elbourne (chair), Dr Richard Howard, Dr Mike Sury] for their support and work throughout the study. We would like to thank Dr Girvan Burnside for his role in providing statistical quality assurance of the outcomes via independent programming. We would also like to thank the SLEEPS data managers. Clare Jackson and Lola Awoyale.
Contributions of authors
Andrew Wolf (chief investigator) led the clinical developmental of the protocol and on-going oversight and management of the study and prepared the report for publication.
Andrew McKay (trial statistician) performed the statistical analyses and prepared the report for publication.
Catherine Spowart and Heather Granville contributed to protocol development, trial co-ordination and commented on the draft of the report.
Angela Boland and Stavros Petrou conducted the health-economic evaluations and wrote the respective sections of the report.
Adam Sutherland provided pharmaceutical advice throughout the trial and commented on the draft of the report.
Carrol Gamble (Professor in Medical Statistics) led the statistical team and contributed to the design of the study, its conduct and analysis and wrote the report for publication.
The SLEEPS Study Group
| Site | PI name(s) | Research nurse name(s) |
|---|---|---|
| Bristol Royal Children’s Hospital | Professor Andy Wolf | Marian Allen, Nicky Robinson |
| Alder Hey Children’s Hospital | Dr Frank Potter, Dr Marie Horan | Sarah Siner, Clare Sellers, Helen Hill |
| Birmingham Children’s Hospital | Dr Kevin Morris | Joanne Faulkner |
| Royal Manchester Children’s Hospital | Dr Stephen Playfor | Maria Macdonald |
| Leeds General Infirmary | Dr Tim Haywood | Darren Hewett |
| Royal Hospital for Sick Children Glasgow | Dr David Ellis, Dr Richard Levin | Liz Waxman |
| Queen’s Medical Centre Nottingham | Dr Asrar Rashid, Dr Patrick Davies | Dan Walsh, Joseph Manning |
| Leicester Royal Infirmary | Dr Amish Vora | Rekha Patel, Claire Brunskill |
| Royal Belfast Hospital for Sick Children | Dr Anthony Chisakuta | Patricia McCreesh, Diane Moore, Katie Dowdie |
| University Hospital of North Staffordshire | Dr Pavanasam Ramesh | Sue Lownds, Hilary Shepley, Marie Phipps |
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Tobias JD, Rasmussen GE. Pain management and sedation in the pediatric intensive care unit. Pediatr Clin North Am 1994;41:1269-92.
- Tonner PH, Weiler N, Paris A, Sholz J. Sedation and analgesia in the intensive care unit. Curr Opin Anaesthesiol 2003;16:113-21. http://dx.doi.org/10.1097/00001503-200304000-00003.
- Tobias JD. Tolerance, withdrawal and physical dependency after long term sedation and analgesia of children in the intensive care unit. Crit Care Med 2000;28:2122-32. http://dx.doi.org/10.1097/00003246-200006000-00079.
- Wolf AR, Jackman LJ. Analgesia and sedation after pediatric cardiac surgery. Pediatr Anesth 2011;21:567-76. http://dx.doi.org/10.1111/j.1460-9592.2010.03460.x.
- Jenkins IA, Playfor SD, Bevan C, Davies G, Wolf A. Current United Kingdom sedation practice in pediatric intensive care. Pediatric Anesth 2007;17:675-83. http://dx.doi.org/10.1111/j.1460-9592.2006.02180.x.
- Parkinson L, Hughes J, Gill A, Billingham I, Ratcliffe J, Choonara I. A randomised controlled trial of sedation in the critically ill. Paediatr Anaesth 1997;7:405-10. http://dx.doi.org/10.1046/j.1460-9592.1997.d01-109.x.
- Hughes J, Gill A, Leach HJ, Nunn AJ, Billingham I, Ratcliffe J, et al. A prospective study of the adverse effects of midazolam on withdrawal in critically ill children. Acta Paediatr 1994;83:1194-9. http://dx.doi.org/10.1111/j.1651-2227.1994.tb18280.x.
- Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Arch Pediatr Adolescent Med 1999;153:331-8. http://dx.doi.org/10.1001/archpedi.153.4.331.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003;23:876-82.
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 2010;112:834-41. http://dx.doi.org/10.1097/ALN.0b013e3181d049cd.
- Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: from second-to first-line sedative agents in a critical care setting. J Intensive Care Med 2012;27:219-37. http://dx.doi.org/10.1177/0885066610396815.
- Aantaa R, Scheinin M. Alpha-2-adrenergic agents in anaesthesia. Acta Anaesthesiol Scand 1993;37:433-48. http://dx.doi.org/10.1111/j.1399-6576.1993.tb03743.x.
- Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth 2001;86:5-11. http://dx.doi.org/10.1093/bja/86.1.5.
- Wolff M, Heugel P, Hempelmann G, Scholz A, Muhling J, Olschewski A. Clonidine reduces the excitability of spinal dorsal horn neurones. Br J Anaesth 2007;98:353-61. http://dx.doi.org/10.1093/bja/ael379.
- Ponten E, Viberg H, Gordh T, Eriksson P, Fredriksson A. Clonidine abolishes the adverse effects on apoptosis and behaviour after neonatal ketamine exposure in mice. Acta Anaesthesiol Scand 2012;56:1058-65. http://dx.doi.org/10.1111/j.1399-6576.2012.02722.x.
- Walker SM, Grafe M, Yaksh TL. Intrathecal clonidine in the neonatal rat: dose-dependent analgesia and evaluation of spinal apoptosis and toxicity. Anesth Analg 2012;115:450-60. http://dx.doi.org/10.1213/ANE.0b013e3182501a09.
- Lee JJ, Rubin AP. Comparison of a bupivacaine-clonidine mixture with plain bupivacaine for caudal analgesia in children. Br J Anaesth 1994;72:258-62. http://dx.doi.org/10.1093/bja/72.3.258.
- De Negri P, Ivani G, Visconti C, De Vivo P, Lonnqvist PA. The dose-response relationship for clonidine added to a postoperative continuous epidural infusion of ropivacaine in children. Anesth Analg 2001;93:71-6. http://dx.doi.org/10.1097/00000539-200107000-00016.
- Rochette A, Raux O, Troncin R, Dadure C, Verdier R, Capdevila X, et al. Clonidine prolongs spinal anesthesia in newborns: a prospective dose ranging study. Anesth Analg. 2004;98:56-9. http://dx.doi.org/10.1213/01.ANE.0000093229.17729.6C.
- Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies. Acta Anaesthesiol Scand 2010;54:397-402. http://dx.doi.org/10.1111/j.1399-6576.2009.02207.x.
- Nishina K, Mikawa K, Maekawa N, Shiga M, Obara H. Effects of oral clonidine premedication on plasma glucose and lipid homeostasis associated with exogenous glucose infusion in children. Anaesthesiology 1998;88:922-7. http://dx.doi.org/10.1097/00000542-199804000-00011.
- Anand KJS, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med 1992;326:1-12. http://dx.doi.org/10.1056/NEJM199201023260101.
- Ambrose C, Sale S, Howells R, Bevan C, Jenkins I, Weir P, et al. Intravenous clonidine infusion in critically ill children: dose-dependent sedative effects and cardiovascular stability. Br J Anaesth 2000;84:794-6. http://dx.doi.org/10.1093/oxfordjournals.bja.a013594.
- Arenas-Lopez S, Riphagen S, Tibby SM, Durward A, Thomlin S, Davies G, et al. Use of oral clonidine for sedation in ventilated paediatric intensive care patients. Intensive Care Med 2004;30:1625-9. http://dx.doi.org/10.1007/s00134-004-2319-0.
- Romano MJ, Dinh A. A 1000-fold overdose of clonidine caused by a compounding error in a 5-year old child with attention-deficit/hyperactivity disorder disease. Pediatrics 2001;108:471-2. http://dx.doi.org/10.1542/peds.108.2.471.
- Meyer C, Cambray R. One hundred times the intended dose of caudal clonidine in three pediatric patients. Paediatric Anaesth 2008;18:888-90. http://dx.doi.org/10.1111/j.1460-9592.2008.02583.x.
- Hoffman WE, Kocks E, Werner C, Thomas C, Albrecht RF. Dexmedatomidine improves neurological injury from incomplete ischaemia in the rat: reversal by the [alpha] 2-antagonist atipamezole. Anesthesiology 1991;75:328-32. http://dx.doi.org/10.1097/00000542-199108000-00022.
- Kuhmonen J, Pokorny J, Miettinen R, Haapalinna A, Jolkkonnen J, Riekkinnen P, et al. Neuroprotective effects of dexmedatomidine in the gerbil hippocampus after transient global ischaemia. Anesthesiology 1997;87:371-7. http://dx.doi.org/10.1097/00000542-199708000-00025.
- Yuan SZ, Runold M, Hagberg H, Bona E, Lagercrantz H. Hypoxic-ischaemic brain damage in immature rats: effect of adrenoreceptor modulation. Eur J Paediatr Neurol 2001;5:29-35. http://dx.doi.org/10.1053/ejpn.2001.0401.
- Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P, et al. Effects of alpha-2-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedatomidine. Anesthesiology 2002;96:134-41. http://dx.doi.org/10.1097/00000542-200201000-00026.
- Zhang Y. Clonidine preconditioning decreases infarct size and improves neurological outcome from transient forebrain ischemia in the rat. Neuroscience 2004;125:625-31. http://dx.doi.org/10.1016/j.neuroscience.2004.02.011.
- Donello JE, Padillo EU, Webster ML, Wheeler LA, Gill JW. Alpha-2-adrenoreceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J Pharmacol Exp Ther 2001;296:216-23.
- Wolf AR, Bissonnette B, Dalens B. Paediatric Anaesthesia. New York, NY: McGraw-Hill; 2002.
- Burch M, Lum L, Elliott M, Carter N, Slater D, Smith A, et al. Influence of cardiopulmonary bypass on water balance hormones in children. Br Heart J 1992;68:309-12. http://dx.doi.org/10.1136/hrt.68.9.309.
- Dorman T, Clarkson K, Rosenfeld BA, Shanholtz C, Lipsett PA, Breslow MJ. Effects of clonidine on prolonged postoperative sympathetic response. Crit Care Med 1997;25:1147-52. http://dx.doi.org/10.1097/00003246-199707000-00015.
- Kulka PJ, Tryba M, Zenz M. Preoperative alpha2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomised, controlled trial. Crit Care Med 1996;24:947-52. http://dx.doi.org/10.1097/00003246-199606000-00012.
- Hohage H, Schlatter E, Greven J. Effects of moxonidine and clonidine on renal function and blood pressure in anaesthetised rats. Clin Nephrol 1997;47:316-24.
- Wijeysundera DN, Naik JS, Beattie WS. Alpha-2-adrenergic agonists to prevent perioperative cardiovascular complications: a meta-analysis. Am J Med 2003;114:742-52. http://dx.doi.org/10.1016/S0002-9343(03)00165-7.
- Fiser DH, Moss MM, Walker W. Care for clonidine poisoning in toddlers. Crit Care Med 1990;18:1124-8. http://dx.doi.org/10.1097/00003246-199010000-00014.
- Wiley JF, Wiley CC, Torrey SB, Henretig FM. Clonidine poisoning in young children. J Pediatr 1990;116:645-8. http://dx.doi.org/10.1016/S0022-3476(05)81621-X.
- Maloney MJ, Schwam JS. Clonidine and sudden death. Pediatrics 1995;96:1176-7.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG. for the CONSORT Group . Reporting of noninferiority and equivalence randomized trials. Extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. http://dx.doi.org/10.1001/jama.2012.87802.
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Pediatr Psychol 1992;17:95-109. http://dx.doi.org/10.1093/jpepsy/17.1.95.
- van Dijk M, de Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ, et al. The reliability and validity of the COMFORT scale as a post-operative pain instrument in 0- to 3-year-old infants. Pain 2000;84:367-77. http://dx.doi.org/10.1016/S0304-3959(99)00239-0.
- Haishima Y, Seshimo F, Higuchi T, Yamazaki H, Hasegawa C, Izumi S, et al. Development of a simple method for predicting the levels of di(2-ethylhexyl) phthalate migrated from PVC medical devices into pharmaceutical solutions. Int J Pharm 2005;298:126-42. http://dx.doi.org/10.1016/j.ijpharm.2005.04.009.
- Foresterhill A. The DAMOCLES Study Group . A proposed charter for clinical trial 2005 data monitoring committees: helping them do their job well. Lancet 2005;365:711-22. http://dx.doi.org/10.1016/S0140-6736(05)17965-3.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. http://publications.nice.org.uk/guide-to-the-methods-of-technology-appraisal-2013-pmg9 (accessed May 2013).
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2010.
- Department of Health . NHS Reference Costs 2011–12 n.d. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed December 2012).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary n.d. www.bnf.org/bnf/ (accessed August 2012).
- Haymarket Media Group . Monthly Index of Medical Specialities 2013. http://MIMS.co.uk (accessed April 2013).
- Department of Health . NHS Supply Chain n.d. www.supplychain.nhs.uk (accessed December 2012).
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. http://dx.doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Black WC. The cost-effectiveness plane: a graphical representation of cost-effectiveness. Med Decis Making 1990;10:212-15. http://dx.doi.org/10.1177/0272989X9001000308.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:S65-80. http://dx.doi.org/10.1177/0272989X9801800209.
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2010.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;2:377-92. http://dx.doi.org/10.1002/hec.766.
- Wolf AR. Fast track paediatric surgery: feasible or foolhardy. Ann Card Anaesth 2004;7:109-13.
- Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 1987;15:657-80. http://dx.doi.org/10.1007/BF01068419.
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141-54. http://dx.doi.org/10.1214/aos/1176350951.
- National Institute for Health and Care Excellence (NICE) . Sedation in Diagnostic and Therapeutic Procedures in Children and Young People 2010. http://guidance.nice.org.uk/CG/Wave18/52/Consultation/Latest.
- Krige A, Pattison N, Booth M, Walsh T. Co-enrolment to intensive care studies: a UK perspective. J Intens Care Soc 2013;14:103-6.
- Harron K, Lee T, Ball T, Mok Q, Gamble C, Macrae D, et al. Making co-enrolment feasible for randomised controlled trials in paediatric intensive care. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0041791.
- Shilling V, Williamson P, Hickey H, Sowden E, Beresford M, Smyth RL, et al. Communication about children’s clinical trials as observed and experienced: qualitative study of parents and practitioners. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0021604.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- National Institute for Health and Care Excellence (NICE) . Sedation in Children and Young People (May 2012). Evidence Update 19 2012. www.evidence.nhs.uk/evidence-update-19 (accessed May 2013).
- Gamble C, Wolf A, Sinha I, Spowart C, Williamson P. The role of systematic reviews in pharmacovigilance planning and clinical trials authorisation application: example from the SLEEPS Trial. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0051787.
- Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev 2012;6. http://dx.doi.org/10.1002/14651858.CD002052.pub2.
- Lynn A, Nespeca MK, Bratton SL, Strauss SG, Shen DD. Clearance of morphine in postoperative infants during intravenous infusion: the influence of age and surgery. Anesthes Analg 1998;86:958-63.
- Murray DM, Thorne GC, Rigby-Jones AE, Tonucci D, Grimes S, Tooley M, et al. Electroencephalograph variables, drug concentrations and sedation scores in children emerging from propofol infusion anaesthesia. Paediatr Anaesth 2004;14:143-51. http://dx.doi.org/10.1046/j.1460-9592.2003.01175.x.
- Rigby-Jones AE, Priston MJ, Sneyd JR, Wolf AR. Remifentanil-midazolam sedation for pediatric patients receiving mechanical ventilation after cardiac surgery. Br J Anaesth 2007;99:252-61. http://dx.doi.org/10.1093/bja/aem135.
Appendix 1 Scheme for drug delivery
Blinded syringe production and presentation
The ampoules of study treatment will be stored in the PICU drugs cupboard at room temperature.
The nurse will prepare the study drug for infusion according to which weight group the patient falls into:
-
< 10 kg (yellow pack)
-
10–25 kg (blue pack)
-
> 25–50 kg (pink pack).
Morphine will be prepared as per usual fashion (on the PICU by the nursing staff).
Preparations and strength
-
< 10 kg:
-
Midazolam Put 50 mg (5 ml) midazolam to a total of 50 ml of 5% dextrose (1 mg/ml).
-
Clonidine Put 750 µg (5 ml) clonidine to a total of 50 ml of 5% dextrose (15 µg/ml).
-
-
10–25 kg:
-
Midazolam Put 62.5 mg (6.25 ml) midazolam to a total of 50 ml of 5% dextrose (1.25 mg/ml).
-
Clonidine Put 937.5 µg (6.25 ml) clonidine to a total of 50 ml of 5% dextrose (18.75 µg/ml).
-
-
> 25–50 kg:
-
Midazolam Put 250 mg (25 ml) to a total of 50 ml of 5% dextrose (5 mg/ml).
-
Clonidine Put 3750 µg (25 ml) to a total of 50 ml of 5 % dextrose (75 µg/ml).
-
Morphine (a, b, c) Put 1 mg/kg in 50 ml of 5% dextrose.
-
Dose range
-
Strength:
-
Midazolam Dose range is 0.05 ml/kg/hour (50 µg/kg/hour) to 0.2 ml/kg/hour (200 µg/kg/hour).
-
Clonidine Dose range is 0.05 ml/kg/hour (0.75 µg/kg/hour) to 0.2 ml/kg/hour (3 µg/kg/hour).
-
-
Strength:
-
Midazolam Dose range is 0.04 ml/kg/hour (50 µg/kg/hour) to 0.16 ml/kg/hour (200 µg/kg/hour).
-
Clonidine Dose range is 0.04 ml/kg/hour (0.75 µg/kg/hour) to 0.16 ml/kg/hour (3 µg/kg/hour).
-
-
Strength:
-
Midazolam Dose range 0.01 ml/kg/hour (50 µg/kg/hour) to 0.04 ml/kg/hour (200 µg/kg/hour).
-
Clonidine Dose range 0.01 ml/kg/hour (0.75 µg/kg/hour) to 0.04 ml/kg/hour (3 µg/kg/hour).
-
Morphine (a–c) 0.5 ml/hour (10 µg/kg/hour) and 3 ml/hour (60 µg/kg/hour).
-
Loading
-
< 10 kg:
-
Midazolam Load for 1 hour at start of trial with 0.2 ml/kg over 1 hour (200 µg/kg/hour).
-
Clonidine Load for 1 hour at start of trial with 0.2 ml/kg over 1 hour (3 µg/kg/hour).
-
Morphine Load with 100 µg/kg over 15 minutes.
-
-
10–25 kg:
-
Midazolam Load for 1 hour at start of trial with 0.16 ml/kg over 1 hour (200 µg/kg/hour).
-
Clonidine Load for 1 hour at start of trial with 0.16 ml/kg over 1 hour (3 µg/kg/hour).
-
Morphine Load with 100 µg/kg over 15 minutes.
-
-
> 25–50 kg:
-
Midazolam Load for 1 hour at start of trial with 0.04 ml/kg over 1 hour (200 µg/kg/hour).
-
Clonidine Load for 1 hour at start of trial with 0.04 ml/kg over 1 hour (3 µg/kg/hour).
-
Morphine Load with 100 µg/kg over 15 minutes.
-
Maintenance and incremental change
-
< 10 kg:
-
Midazolam Start infusion at 0.1 ml/kg/hour (100 µg/kg/hour). Change in steps of 0.05 ml/kg/hour.
-
Clonidine Start infusion at 0.1 ml/kg/hour (1.5 µg/kg/hour). Change in steps of 0.05 ml/kg/hour.
-
Morphine Start at 20 µg/kg/hour (1 ml/hour).
-
-
10–25 kg:
-
Midazolam Start infusion at 0.08ml/kg/hour (100 µg/kg/hour). Change in steps of 0.04 ml/kg/hour.
-
Clonidine Start infusion at 0.08ml/hour/kg/hour (1.5 µg/kg/hour). Change in steps of 0.04 ml/kg/hour.
-
Morphine Start at 20 µg/kg/hour (1 ml/hour).
-
-
> 25–50 kg:
-
Midazolam Start infusion at 0.02 ml/kg/hour (100 µg/kg/hour). Change in steps of 0.01 ml//kg/hour.
-
Clonidine Start infusion at 0.02 ml/kg/hour (1.5 µg/kg/hour). Change in steps of 0.01 ml/kg/hour.
-
Morphine Start at 20 µg/kg/hour (1 ml/hour).
-
Scheme for adjustment of infusions
-
Load and start infusions.
-
COMFORT score hourly (target of ≤ 26, ≥ 17).
-
Increase or decrease study medication infusion based on hourly COMFORT score as per schematic diagram (Protocol, p. 10) and using the incremental changes described above. Protocol dictates that the decision to increase or decrease study medication or morphine should be made hourly according to the COMFORT score. If the patient has a COMFORT score of > 26 then nurses at the bedside will need to assess the patient for pain and their conscious level to determine whether morphine or trial sedation should be increased. If the patient is judged to be in pain then the morphine should be increased by 10 µg/kg/hour (up to a maximum of 60 µg/kg/hour). If the patient is judged to have a lack of sedation then the trial drug should be increased by the designated amount (Protocol, see table 1, section 7.3). Only one incremental change of either trial medication or morphine can occur per documented COMFORT score.
-
If the patient develops a COMFORT score of ≥ 27 in between hourly assessments, it is acceptable to formally score the patient and increase the sedative infusion delivery before the formal hourly assessment. This will need to be recorded.
-
The maximum dose of clonidine is 3 µg/kg/hour and the maximum dose of midazolam is 200 µg/kg/hour.
-
If sedation is re-established and COMFORT score falls to < 17 and a score of < 17 is sustained for 2 hours (two subsequent COMFORT scores), reduce morphine or trial sedation infusion incrementally as clinically indicated (according to subsequent COMFORT scores), down to a minimum of 20 µg/kg/hour for morphine or down to the minimum trial infusion rate for the appropriate weight group.
-
However, if sedation remains inadequate after an hour of maximum study drug and maximum morphine (60 µg/kg/hour), treatment failure will have been deemed to have occurred. Switch to alternative sedation as per unit policy. Continue with measurements of COMFORT and BP described above.
-
If the minimum trial infusion rate and a morphine infusion rate of 20 µg/kg/hour is administered and the COMFORT score of the child is still < 17 then, if there are no analgesic requirements, the morphine can be further decreased by an increment of 10 µg/kg/hour to 10 µg/kg/hour. If at the subsequent COMFORT score, the COMFORT score is still < 17, the morphine can be stopped (providing there are no analgesic requirements). If at the subsequent COMFORT score, the COMFORT score is still < 17, the trial sedation can be temporarily stopped.
-
If during study a painful procedure is required necessitating additional anaesthesia or analgesia this can be provided. Trial medication should remain at the same infusion rate throughout this period until the effects of other drugs have worn off. Drugs used may include propofol, volatile anaesthetic agents, thiopentone, ketamine, fentanyl morphine, midazolam or diazepam. Careful documentation of these concomitant medications will be made and evaluations as per the study will continue. Muscle relaxants are also permissible for procedures where this is deemed necessary by the independent clinician.
-
If during the infusion there is a sudden and extreme loss of sedation control (which may be associated with incidental manipulations, nursing cares or simply with sudden arousal), it is permissible to deliver a rescue dose of additional i.v. analgesia or sedation as deemed necessary. This will be recorded. The trial will then proceed as before, with upwards adjustment of trial medications as per protocol. However, if three such episodes requiring intervention occur within a 12-hour period then this will terminate the study for that patient, which will then be described as a treatment failure.
Appendix 2 COMFORT score
COMFORT Scale scoring
Alertness
Rates the patient’s response to ambient stimulation in the environment including responses to sound (noises from monitors, intercoms, people talking, pagers, etc.), movement, light, etc. To rate this category, no stimulus is introduced by the observer.
-
Deeply asleep The state of least responsiveness to the environment. The patient’s eyes are closed, breathing is deep and regular, and the patient shows minimal responses to changes in the environment.
-
Lightly asleep The patient has their eyes closed throughout most of the observation period, but still responds somewhat to the environment as evidenced by slight movements, facial movements, unsuccessful attempts at eye openings, etc.
-
Drowsy The patient closes their eyes frequently or makes laboured attempts to open eyes and is less responsive to the environment.
-
Alert and awake The patient is responsive and interactive with the environment, but without an exaggerated response to the environment. The patient’s eyes remain open most of the time or open readily in response to ambient stimuli.
-
Hyper-alert The patient is hyper-vigilant, may be wide-eyed, attends rapidly to subtle changes in the environmental stimuli and has exaggerated responses to environmental stimuli.
Guidelines: If two or more of the following items achieve a score of ≥ 2 then the child is classified as lightly asleep – respiratory response, physical movement, muscle tone.
Calmness/agitation
Rates the patient’s level of emotional arousal and anxiety.
-
Calm The patient appears serene and tranquil. There is no evidence of apprehension or emotional distress.
-
Slightly anxious The patient is not completely calm. The patient shows slight apprehension and emotional distress.
-
Anxious The patient appears somewhat apprehensive and emotionally distressed, but remains in control.
-
Very anxious The patient appears very apprehensive. Emotional distress is apparent but the patient remains somewhat in control.
-
Panicky The patient’s total demeanour conveys immediate and severe emotional distress with loss of behavioural control.
Respiratory response
Rates the patient’s oral and respiratory responses to an endotracheal tube and intermittent ventilation.
-
No coughing or no spontaneous respiration Only ventilator-generated breaths are apparent. No respiratory movement is apparent between ventilator breaths. No oral movement or chest wall movement occurs except as created by the ventilator.
-
Spontaneous respiration The patient breathes at a regular, normal respiratory rate in synchrony with the ventilator. No oral movement or chest wall movement occurs which is contrary to the ventilator movement.
-
Occasional cough/resists ventilator The patient has occasional oral or chest wall movement contrary to the ventilator pattern. The patient may occasionally breathe out of synchrony with the ventilator.
-
Actively breathes against ventilator The patient has frequent oral or chest wall movement contrary to the ventilator pattern, coughs regularly or frequently breathes out of synchrony with the ventilator.
-
Fights ventilator – coughs/chokes/gags The patient actively makes oral or chest wall movement contrary to the ventilator pattern, and coughs and/or gags in a manner which may interfere with ventilation.
Physical movement
Rates frequency and intensity of physical movement.
-
None The patient shows complete absence of independent movement.
-
Occasional, slight movements The patient shows three or fewer small amplitude movements of the fingers or feet, or very small head movement.
-
Frequent, slight movement The patient shows more than three small amplitude movements of the fingers or feet, or very small head movements.
-
Vigorous movements of extremities only The patient shows movements of greater amplitude, speed or vigour of hands, arms or legs. The head may move slightly. Movement is vigorous enough to potentially disrupt cannulas.
-
Vigorous movements of extremities, torso and head The patient shows movements of greater amplitude, speed or vigour of the head and torso, such as head-thrashing, back-arching or neck-arching. Extremities may also move. Movement is vigorous enough to potentially disrupt placement of an endotracheal tube.
Guidelines Occasional movement is defined as less than once per minute. Frequent movement is defined as more than once per minute.
Blood pressure
Mean arterial blood pressure (MAP) rates the frequency of elevations above (or below) a normal baseline. The baseline may need to be reset on a daily basis or occasionally more frequently, depending on changes in clinical conditions (e.g. change in temperature or the addition of inotropes, etc.). Each re-evaluation will set the cardiovascular baselines for each ‘rating period’.
At the beginning of the rating period, ‘baseline’, ‘15% below baseline’ and ‘above baseline’ values are recorded on the rating sheet in an easily observable location. The rater observes the monitor for MAP during the observational period of an hour and records, with a hash mark, each observation above or below the baseline. Ratings are made upon the number of readings above the baseline.
-
BP 15% below baseline
-
BP consistently at baseline
-
infrequent elevations of ≥ 15% (one to three during observation period)
-
frequent elevations of ≥ 15% (more than three during observation period)
-
sustained elevation of ≥ 15%.
Guidelines The baseline to use initially for BP will be an average of the measurements taken hourly over the 4 hours previous to trial entry. Following this, the baseline should be recalculated on a daily basis for each patient (or occasionally more frequently) if this is felt to be clinically appropriate for the individual.
Heart rate
Heart rate score is based on the frequency of elevations above (or below) a normal baseline. The baseline may need to be reset on a daily basis or occasionally more frequently, depending on changes in clinical conditions (e.g. change in temperature or the addition of inotropes, etc.). Each re-evaluation will set the cardiovascular baselines for each ‘rating period’. At the beginning of the rating period, baseline, 15% above baseline and 15% below baseline values are recorded on the rating sheet in an easily observable location. The observer observes the heart rate throughout the hour and records, with a hash mark, each episode of elevation above the baseline or episodes below the baseline. Ratings are made based upon the number of readings above the baseline.
-
heart rate 15% below baseline
-
heart rate consistently at baseline
-
infrequent elevations of ≥ 15% (one to three during observation period)
-
frequent elevations of ≥ 15% (more than three during observation period)
-
sustained elevation of ≥ 15%.
Guidelines The baseline to use initially for BP will be an average of the measurements taken hourly over the 4 hours previous to trial entry. Following this, the baseline should be recalculated on a daily basis for each patient (or occasionally more frequently), if this is felt to be clinically appropriate for the individual.
Guidance on interpretation of heart rate and blood pressure values
If heart rate and BP values are inappropriate to the rest of the COMFORT score owing to other clinical events, then the heart rate and BP should be scored as ‘2’, i.e. at baseline. For example, if a patient has a temperature of 39 °C and a heart rate of 190 bpm, which is not consistent with a child that otherwise has COMFORT score criteria that indicate adequate analgesia and sedation, then the heart rate will be scored as ‘2’.
Muscle tone
Muscle tone is assessed in relation to normal tone in a patient who is awake and alert. The rating is based upon patient response to rapid and slow flexion and extension on a non-instrumented extremity (i.e. elbow or knee without an i.v. line, tape, arterial line or physical restraint). A wrist or ankle may be used if no other joint is available. This rating is the only one that requires active intervention by the rater and is performed at the end of the observation period.
-
Relaxed/none Muscle tone is absent. There is no resistance to movement.
-
Reduced muscle tone The patient shows less resistance to movement than normal but muscle tone is not totally absent.
-
Normal muscle tone Resistance to movement is normal.
-
Increased tone/flexion – fingers/toes The patient shows resistance to movement that is clearly greater than normal but the joint is not rigid.
-
Extreme rigidity/flexion – fingers/toes Muscle rigidity is the patient’s predominant state throughout the observation period. This may be observed even without manipulating an extremity.
Facial tension
Facial tension assesses tone and tension of facial muscles. The standard of comparison is a patient who is awake and alert.
-
Relaxed The patient shows no facial muscle tone, with absence of normal mouth and eye closing. The mouth may look slack and the patient may drool. Brow smooth.
-
Normal tone The patient shows no facial muscle tension with mouth and eyes closing appropriately. Small movements of the lips, mouth or tongue. Brow smooth.
-
Some tension This does not include sustained tension of muscle groups – such as the brow, forehead or mouth – but you may see a frown or eye squeezing.
-
Full facial tension The patient shows notable, sustained tension of facial muscle groups, including the brow, forehead, mouth, chin or cheeks.
-
Hyper-alert The patient demonstrates facial grimacing with an expression that conveys an impression of crying, discomfort and distress. This generally includes extreme furrowing of brow.
Appendix 3 Statistical analysis plan
Appendix 4 Health economic analysis plan
Appendix 5 Details of protocol amendments
Version 5.0 (1 March 2011)
Substantial amendment version 4.0 (6 May 2010) to version 5.0 (1 March 2011)
| Page no. | Comment |
|---|---|
| Throughout | Updated version and date |
| 9 | Addition of ‘ICU = Intensive Care Unit’ to Glossary |
| 10 | 1000 removed Reduction from 24 hours to 12 hours for number of hours for which child is likely to require intubation and ventilation Increase from 48 hours to 120 hours for the period children can be entered into the trial following admission to PICU. Addition of ‘ICU’, as child may have been admitted to ICU initially rather than PICU ‘12’ changed to ‘10’ for number of participating sites |
| 11 | Updated flow chart replaced previous flow chart to explain change to protocol regarding administration of trial treatment and morphine |
| 16 | ‘The Specials Clinical Manufacturing Unit’ changed to ‘SCM Pharma’ |
| 23 | Definition of treatment failure for secondary end point no. 12 changed from the administration of three rescue boluses within any one 12-hour period to three ‘events’, for which rescue medication(s) are needed to re-establish sedation or pain control occurring within any one 12-hour period during trial treatment Description of an ‘event’ provided |
| 24 | Change to inclusion/exclusion criteria: |
|
|
| 25 | Addition of ‘A requirement for continuous infusion of muscle relaxants’ as a reason for patients to be withdrawn from the trial intervention |
| 26 | Addition of text to state that parents of eligible patients can be approached regarding the trial during transfer and a summary information sheet can be given to the parents at this point Removal of recording ‘inotropic administration’ as a baseline assessment Addition of recording of previous sedation and analgesic therapy |
| 28 | ‘The Specials Clinical Manufacturing Unit’ changed to ‘SCM Pharma’ |
| 33 | Addition of chlorpromazine, haloperidol and promethazine to allowed supplementary anaesthesia |
| 34 | Addition of guidance doses for allowed concomitant medications |
| 35 | Adjustment to trial treatment and morphine administration to allow bedside nurse to evaluate child for pain and conscious level to decide whether trial treatment or morphine should be adjusted Treatment failure changed from requiring three rescue doses within a 12-hour period to three ‘events’ for which rescue medication is needed to re-establish sedation or pain control occur within a 12-hour period. Description of an ‘event’ given Removal of guidance dose for fentanyl |
| 36 | Adjustment to trial treatment and morphine administration to allow bedside nurse to evaluate child for pain and conscious level to decide whether trial treatment or morphine should be adjusted Addition of text to say that when a COMFORT score of < 17 is recorded, the score must remain below 17 for 2 consecutive hours before the morphine is reduced |
| 37 | Addition of text to say that when a COMFORT score of < 17 is recorded, the score must remain at < 17 for two consecutive hours before the morphine is reduced. Clarification of adjustments to trial sedation and morphine provided Text added to say that the trial sedation can be temporarily stopped if the morphine has been stopped and the COMFORT score still remains < 17 |
| 38 | ‘24 hours’ changed to ‘12 hours’ Addition of text ‘and morphine’, as the COMFORT score will dictate whether increases or decreases in study medication and morphine occur |
| 39 | Addition of text to say that following trial treatment cessation, the only COMFORT score category that needs to be completed is ‘Alertness’ and that if sedation is still required following trial treatment cessation then the COMFORT score should continue to be measured hourly until the child is stable on the new sedative |
| 41 | Increase from 48 hours to 120 hours for the period children can be entered into the trial following admission to PICU. Addition of ‘ICU’, as child may have been admitted to ICU initially rather than PICU |
| 46 | Change to description of treatment failure to three ‘events’ for which rescue medication are needed to re-establish sedation or pain control occurring within any one 12-hour period during trial treatment Description of event provided |
| 48 | Revised sample size calculation provided Margin of equivalence altered to 0.15 |
Version 4.0 (6 May 2010)
Substantial amendment version 3.0 (5 October 2010) to version 4.0 (6 May 2010)
| Page | Comment |
|---|---|
| Throughout | Updated version and date |
| 3 | Amendment to contact details for Funder |
| 4 | Amendment to Chief Investigator’s telephone and fax number |
| 5 | Details for data manager added |
| 10 | Amendment from ‘likely to require intubation and ventilation for more than 48 hours’ to ‘likely to require intubation and ventilation for more than 24 hours’ Clarification that the trial will be conducted in 12 of the sites listed on the Participating Sites document |
| 11 | Text added to box at bottom of page to explain actions to be taken regarding morphine should a child be oversedated on minimum trial sedation and minimum morphine Text amended to show that all patients are followed up until 14 days following trial treatment cessation rather than until hospital discharge |
| 19 | Amendment to state that if an intervention is required to treat a withdrawal symptom then this will be recorded on the withdrawal symptom chart rather than the concomitant medications page |
| 24 | Text to explain that children who are born before 37 weeks’ gestation are eligible for the trial if they are a minimum of 30 days post delivery and their corrected gestation is ≥ 37 weeks 48 hours amended to 24 hours for Inclusion Criterion b Amendment from 3 months to 1 month to Exclusion Criteria |
| 25 | Clarification that a need to commence haemodialysis or haemofiltration will result in the child being withdrawn from the trial Amendment from withdrawal CRF to End of Study CRF |
| 26 | Amendment of ‘Screening and Enrolment Log’ to ‘Screening Log’ Addition of text to state that if a child is likely to be suitable for the trial following surgery then the parent or legally acceptable representative of the child can be approached prior to surgery The physical examination has been removed from the baseline assessments (physical examinations were removed in a previous amendment but this had been missed) ‘Time sedation therapy administered at trial entry stopped’ removed Text to state that baseline assessments can be completed retrospectively |
| 27 | Addition of lower storage temperature for trial medications of 2 °C |
| 28 | Addition of text to state which colour pack each weight group will be presented in |
| 32 | Addition of lower storage temperature for trial medications of 2 °C |
| 33 | Clarification of recording of concomitant medications required to treat withdrawal symptoms |
| 35 | Addition of text to state that if a child is receiving the minimum infusions of trial sedation and morphine, and the child is oversedated, the morphine can be further reduced by an increment of 10 µg/kg/hour to 10 µg/kg/hour, providing that there are no requirements for analgesia. If the child is still oversedated, the morphine can be stopped (as long as there are no analgesic requirements), although the trial sedation should continue Amendment from 3 months to 1 month for co-enrolment guidelines |
| 36 | Amendment from ‘48 hours’ to ‘24 hours’ Clarification that for the first 24 hours following treatment cessation, withdrawal symptoms will be recorded 4 hourly, whether on the ward or in PICU |
| 38 | Amendment to text to state that fluid balance will be recorded only during trial treatment |
| 39 | Addition of Day 7 to Treatment days Amendment to text to state that fluid balance will be recorded only during trial treatment |
| 43 | Removal of text saying that the child’s GP and/or district nurse will be asked to contact the family and provide follow-up information to the recruiting centre |
| 50 | Amendment from ‘below’ to ‘on the following page’ |
| 54 and 55 | Addition of text to state that the parent/legal representative can be approached prior to their child having surgery Clarification that the consent process can be carried out by a member of the research team identified in the trial signature and delegation log Removal of text ‘at this stage’ |
| 55 | Removal of text saying that if a child is unable to assent then this will be documented on the age and stage of development-specific Patient Information Sheet and Consent form |
| 59 | Amendment to presentation of missing data codes and addition of N/R (not received) and N/K (not known) codes Amendment to Monitoring at Clinical Trials Unit section detailing the assessment of data and how data queries will be processed |
| 63 | Removal of Dr Simon Nadel from the TMG and TSC Addition of Dr Frank Potter and Dr Marie Horan to the TMG and TSC |
| 77 | Muscle Tone and Alertness swapped order in COMFORT score Amendment from ‘Blood Pressure/Heart Rate below baseline’ to ‘Blood Pressure/Heart Rate 15% below baseline’ |
| 81 | Amendment from ‘Blood Pressure/Heart Rate below baseline’ to ‘Blood Pressure/Heart Rate 15% below baseline’ |
| 82 | Removal of ‘2 minute’ from Muscle Tone |
| 84 | Addition of text to state which colour pack each weight group will be presented in |
| 86 | Addition of text to state that if a child is receiving the minimum infusions of trial sedation and morphine, and the child is oversedated, the morphine can be further reduced by an increment of 10 µg/kg/hour to 10 µg/kg/hour, providing that there are no requirements for analgesia. If the child is still oversedated, the morphine can be stopped (as long as there are no analgesic requirements), although the trial sedation should continue |
Version 3.0 (5 October 2009)
Substantial amendment version 2.1 (14 September 2009) to version 3.0 (5 October 2009)
| Page | Comment |
|---|---|
| Throughout | Updated version and date |
| 22 | Addition of ‘Blood biochemistry and urinalysis’ to secondary end points Addition of ‘Percentage of time spent adequately sedated’ to secondary end points |
| 31 | Change of text from ‘PICU’ to ‘pharmacy’ |
| 43 | Addition of ‘Blood biochemistry and urinalysis’ to secondary end points |
| Addition of “Percentage of time spent adequately sedated” to secondary end points | |
| 57 | Removal of text marking source data sections of CRF with Ⓢ |
| 73 | Removal of Participating Sites from protocol (Change of PI from Dr Kate Parkins at Royal Liverpool Children’s Hospital to Dr Frank Potter. Change of Trust name from Royal Liverpool Children’s Hospital to Alder Hey Children’s NHS Foundation Trust.) Participating sites are now a supporting document |
| Throughout | Amendments to order of appendices and references to appendices following removal of participating sites from appendices |
Version 2.1 (14 September 2009)
Non-substantial amendment version 2.0 (5 May 2009) to version 2.1 (14 September 2009)
| Page | Comment |
|---|---|
| Throughout | Updated version and date |
| Throughout | Change of wording from ‘subject’ to ‘participant’ |
| 4 | Addition of Jake Harley being authorised to sign the protocol and protocol amendments on behalf of the Sponsor Change of name from ‘Fell’ to ‘Spowart’ Changes to telephone numbers for Andrew McKay |
| 5 | ‘Diane’ amended to ‘Diana’ |
| 10 | Amendment to clarify that the study is an equivalence trial |
| 20 | Change of spelling from ‘Principle’ to ‘Principal’ |
| 21 | Clarification of length of time for recording withdrawal symptoms and AEs |
| 25 | Amendment to clarify that the study is an equivalence trial |
| 34 | Amendment to clarify that the study is an equivalence trial |
| 36 | Clarification of length of time AEs are required to be reported |
| 43 | Clarification of length of time for recording withdrawal symptoms |
| 44 | Clarification of length of time for recording withdrawal symptoms and AEs |
| Removal of word efficacy to clarify that this is an equivalence trial | |
| 45 | Amendment to analysis plan to clarify that the study is an equivalence trial |
| 50 | Change of wording from ‘subject’ to ‘randomisation’ |
| 51 | Change of wording from ‘subjects’ to ‘study participants’ |
| Change of wording from ‘subject’ to ‘randomisation’ | |
| 52 | Change of wording from ‘subjects’ to ‘study participants’ |
| 57 | Change of wording from ‘subject’ to ‘randomisation’ |
| 62 | Change of name of ‘Fell’ to ‘Spowart’ Diane amended to ‘Diana’ |
| 63 | Addition of text to say that the protocol may be submitted for publication |
| 73 | Addition of Dr Margrid Schindler as Qualified Physician responsible for Trial-Site Related Medical Decisions at Above Site |
Version 2.0 (5 May 2009)
Substantial amendment version 1.0 (1 October 2008) to version 2.0 (5 May 2009)
| Page no. | Comment |
|---|---|
| Throughout | Updated version and date |
| 2 | Change to e-mail address from sleeps@mcrnctu.org.uk to helpdesk@mcrnctu.org.uk |
| 3 | Change to e-mail address from sleeps@mcrnctu.org.uk to helpdesk@mcrnctu.org.uk Addition of contact extension number of 0266 for Mary Perkins |
| 4 | Change to e-mail address from sleeps@mcrnctu.org.uk to helpdesk@mcrnctu.org.uk Change of fax number from 00 44 (0) 151 252 5456 to 00 44 (0) 151 282 4721 Addition of contact extension number of 0266 for Mary Perkins Andrew McKay has replaced Ashley Jones as statistician |
| 5 | Change of Data Monitoring Committee member from Professor Peter Collins to Dr Mike Sury |
| 18 and 19 | Change in reporting requirements of AEs. Text now states that only ARs and SAEs must be reported. Non-SAEs no longer need to be reported to alleviate the burden on PICU bedside nurses |
| 19 | Error in previous text. The substudy is not limited to the Bristol centre |
| 21 | Addition of site-specific assessment by local R&D Department Inclusion criteria for centres changed from able to recruit a minimum of ‘82 patients in 2 years’ to ‘84 patients in 2 years’ |
| 23 | Text altered to remove requirement to report non-SAEs |
| 25 | Change in recording requirements of concomitant medications to alleviate the burden on PICU nurses. Only administration of inotropes, sedation and analgesia will be recorded at baseline. Other concomitant medications no longer need to be recorded at baseline |
| 26 | Storage temperature of trial treatment has changed from < 25 °C to ≤ 30 °C. Following consultation with participating PICUs and measurement of maximum temperatures on PICU, there was a concern that temperatures were exceeding 25 °C on a regular basis. A review of the stability data for the trial treatments followed, which has resulted in us being able to assign a 12-month shelf life at ≤ 30 °C. A change has now been made to the stability protocol to include ‘real time’ at 6 months and 12 months time points for 30 °C/65% relative humidity |
| 26 | Text has now been altered to state that the shelf life of the trial treatment is now 12 months (all subsequent batches will have a shelf life of 12 months) |
| 27 | Clarification of the upper limit (50 kg) for the largest weight group |
| 27 | Addition of text to state that a 21-gauge needle (0.81 mm outer diameter) or smaller should be used to draw out the treatment from the vial to ensure that the extractable volume is adequate |
| 28 | Clarification that a dedicated line should be used for the administration of trial treatment and morphine has been provided Advice regarding administration of trial treatment provided |
| 31 | Storage temperature of trial treatment changed from < 25 °C to ≤ 30 °C |
| 31 and 32 | Text altered to reflect change in concomitant medications recordings required to alleviate the burden on PICU bedside nurses. Throughout the trial, any medications administered at the time of a SAE, SAR or SUSAR must be recorded Aside from this:
|
| 34 | Addition of upper weight limit for Weight Group C for clarification |
| 35 | Text altered to remove requirement to report non-SAEs |
| 36 | Text altered to remove requirement to report non-SAEs Clarification that only ARs and SAEs must be recorded |
| 37 | Clarification that fluid balance and clinical laboratory to be recorded only if these measurements are available Addition of recording the number of ventilated days for each patient |
| 38 | Physical examination removed from schedule of study procedures Addition of recording whether or not feeds are tolerated and whether or not bowels have opened |
| 44 | Addition of the upper limit (50 kg) for the largest weight group |
| 47–52 | Text altered to remove requirement to report non-SAEs. Clarification that only ARs and SAEs must be recorded. Clarification of reporting procedures and requirements. Diagram beneath 10.7.2 amended |
| 55 | Addition of site-specific assessment by local R&D Department. If it is after April 2009 then site-specific assessment will be carried out by the local R&D Department alone and not by the relevant Local Research Ethics Committee |
| 64 | Change of TMG member and TSC member from ‘Ashley Jones’ to ‘Andrew McKay’ |
| 64 | Change of Data Monitoring Committee member from ‘Professor Peter Collins’ to ‘Dr Mike Sury’ |
| 84 and 85 | Addition of upper weight limit for weight Group C for clarification |
Appendix 6 Protocol deviations
| Protocol deviations | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) |
|---|---|---|---|
| Any protocol deviation, n (%) | 58 (90.6) | 55 (90.2) | 113 (90.4) |
| Total protocol deviations, n | 271 | 387 | 658 |
| At least one major protocol deviation, n (%) | 56 (87.5) | 53 (86.9) | 109 (87.2) |
| Total major protocol deviations, n | 227 | 330 | 557 |
| n occurrences [n patients] (% of total patients) | |||
| Child aged < 7 days GA/CGA | – | – | – |
| Child aged ≥ 18 years | – | – | – |
| Not ventilated (identified by ‘No’ having been selected for this criterion) | – | – | – |
| Recruitment > 120 hours of arrival in PICU/ICU | – | – | – |
| Child weighed > 100 kg | – | – | – |
| Unable to perform a COMFORT score on the child | – | – | – |
| COMFORT score of < 17a | – | – | – |
| COMFORT score of > 26 | 1 [1] (1.6) | – | 1 [1] (0.8) |
| Fully informed written consent not provided or provided with inaccuracies | – | – | – |
| Patient with open chest following cardiac surgery | – | – | – |
| Patient chronically treated for raised blood pressure | – | – | – |
| Patient’s current treatment with beta-blockers 24 hours prior to entry | – | – | – |
| Patient had an acute traumatic brain injury | – | – | – |
| Patient in status epilepticus or active fitting | – | – | – |
| Patient required haemodialysis or haemofiltration | – | – | – |
| Patient required ECMO treatment | – | – | – |
| Patient with severe neuromuscular problems/impairment, on whom a COMFORT score cannot be performed | – | – | – |
| Patient had a known allergy to either of the trial medications | – | – | – |
| Patient’s current treatment with continuous or intermittent muscle relaxants | – | – | – |
| Patient was pregnant | – | – | – |
| Patient was currently participating in a conflicting clinical study or participation in a clinical study involving a medicinal product in the month prior | – | – | – |
| Previously participated in SLEEPS trial | – | – | – |
| Randomised to incorrect weight group (i.e. colour pack incorrect) | 1 [1] (1.6) | – | 1 [1] (0.8) |
| Patient randomised out of sequencea | 8 [8] (12.5) | 7 [7] (11.5) | 15 [15] (12) |
| COMFORT score of < 17 and no action taken instead of a morphine dose decrease | 52 [9] (14.1) | 34 [11] (18.0) | 86 [20] (16.0) |
| COMFORT score of < 17 and no action taken instead of a trial treatment dose decrease | 30 [12] (18.8) | 93 [9] (14.8) | 123 [21] (16.8) |
| COMFORT score of < 17 and no action taken instead of a morphine dose decrease/trial treatment dose decrease (depending on clinical assessment) | 16 [8] (12.5) | 33 [10] (16.4) | 49 [18] (14.4) |
| COMFORT score of < 17 and no action taken instead of stopping trial treatment | 2 [1] (1.6) | 17 [3] (4.9) | 19 [4] (3.2) |
| COMFORT score of > 26 and no action taken instead of a morphine dose increase | 12 [8] (12.5) | 18 [12] (19.7) | 30 [20] (16.0) |
| COMFORT score of > 26 and no action taken instead of a trial treatment dose increase | 7 [5] (7.8) | 2 [2] (3.3) | 9 [7] (5.6) |
| COMFORT score of > 26 and no action taken instead of a morphine dose increase/trial treatment dose increase (depending on analgesic or sedative requirements) | 1 [1] (1.6) | 3 [3] (4.9) | 4 [4] (3.2) |
| COMFORT score of < 17 and no action taken (no drug information available but confirmed protocol deviation) | 0 [0] (0.0) | 1 [1] (1.6) | 1 [1] (0.8) |
| COMFORT score of > 26 and no action taken (no drug information available but confirmed protocol deviation) | 1 [1] (1.6) | 1 [1] (1.6) | 2 [2] (1.6) |
| Treatment failure had occurred and trial treatment not stopped | 2 [2] (3.1) | 9 [2] (3.3) | 11 [4] (3.2) |
| COMFORT score between 17 and 26 and both trial treatment and morphine increased instead of no action taken | 1 [1] (1.6) | 2 [2] (3.3) | 3 [3] (2.4) |
| COMFORT score between 17 and 26 and morphine decreased instead of no action taken | 4 [4] (6.3) | 4 [4] (6.6) | 8 [8] (6.4) |
| COMFORT score between 17 and 26 and morphine increased instead of no action taken | 3 [3] (4.7) | 5 [5] (8.2) | 8 [8] (6.4) |
| COMFORT score between 17 and 26 and trial treatment decreased instead of no action taken | 2 [2] (3.1) | 4 [4] (6.6) | 6 [6] (4.8) |
| COMFORT score between 17 and 26 and trial treatment increased instead of no action taken | 9 [7] (10.9) | 13 [11] (18.0) | 22 [18] (14.4) |
| COMFORT score between 17 and 26 and dose decreased instead of no action taken (no drug information available but confirmed protocol deviation) | 0 [0] (0.0) | 1 [1] (1.6) | 1 [1] (0.8) |
| COMFORT score indicates trial treatment increase/decrease but dose was increased/decreased by two increments or more rather than one | 3 [3] (4.7) | 3 [3] (4.9) | 6 [6] (4.8) |
| COMFORT score indicates morphine dose increase/decrease but dose was increased/decreased by two increments or more rather than one | 4 [3] (4.7) | 3 [3] (4.9) | 7 [6] (4.8) |
| COMFORT score calculated incorrectly as being between 17 and 26 when a dose decrease should have occurred | 2 [2] (3.1) | 11 [3] (4.9) | 13 [5] (4.0) |
| COMFORT score calculated incorrectly as being between 17 and 26 when a dose increase should have occurred | 2 [2] (3.1) | 1 [1] (1.6) | 3 [3] (2.4) |
| COMFORT score calculated incorrectly and dose increase occurred when COMFORT score actually between 17 and 26 | 4 [3] (4.7) | 3 [3] (4.9) | 7 [6] (4.8) |
| Trial treatment maintenance rate calculated incorrectly therefore administered at the incorrect dose | 2 [2] (3.1) | 4 [4] (6.6) | 6 [6] (4.8) |
| Morphine maintenance rate calculated incorrectly therefore administered at the incorrect dose | 1 [1] (1.6) | 2 [2] (3.3) | 3 [3] (2.4) |
| Morphine decreased rather than trial treatment decreased | 6 [5] (7.8) | 5 [4] (6.6) | 11 [9] (7.2) |
| Trial treatment decreased rather than morphine decreased | 0 [0] (0.0) | 1 [1] (1.6) | 1 [1] (0.8) |
| Trial treatment increased rather than morphine increased | 5 [2] (3.1) | 3 [3] (4.9) | 8 [5] (4.0) |
| Both trial treatment and morphine increased instead of just morphine being increased | 2 [2] (3.1) | 4 [4] (6.6) | 6 [6] (4.8) |
| Both trial treatment and morphine increased instead of just trial treatment being increased | 3 [3] (4.7) | 1 [1] (1.6) | 4 [4] (3.2) |
| Both trial treatment and morphine decreased instead of just morphine or trial treatment being decreased (depending on analgesic or sedative requirements) | 0 [0] (0.0) | 2 [1] (1.6) | 2 [1] (0.8) |
| Both trial treatment and morphine decreased (no drug information available but confirmed protocol deviation) | 1 [1] (1.6) | 0 [0] (0.0) | 1 [1] (0.8) |
| Morphine increased instead of morphine being decreased | 1 [1] (1.6) | 0 [0] (0.0) | 1 [1] (0.8) |
| Trial treatment decreased instead of trial treatment being increased | 0 [0] (0.0) | 1 [1] (1.6) | 1 [1] (0.8) |
| Both trial treatment and morphine decreased instead of a morphine increase | 1 [1] (1.6) | – | 1 [1] (0.8) |
| Patient randomised following temperature deviation/unreliable temperature recording | 1 [1] (1.6) | – | 1 [1] (0.8) |
| Primary outcome data missing for ≥ 1 hourb | 37 [37] (57.8) | 39 [39] (63.9) | 76 [76] (60.8) |
| At least one minor, n (%) | 26 (40.6) | 28 (45.9) | 54 (43.2) |
| Total minor protocol deviations, n | 44 | 57 | 101 |
| n occurrences [n patients] (% of total patients) | |||
| Child aged ≥ 7 days but < 30 days GA/CGA | – | – | |
| Child aged > 15 years but < 18 years | – | – | – |
| Assumed patient would remain ventilated for > 12 hours but was actually ventilated for < 12 hours | – | – | – |
| Child weighed > 50 kg but < 100 kg | – | – | – |
| Dose increase/decrease has been recorded as the action taken, but the change in trial treatment/morphine is reflected in the following hour | 21 [17] (26.6) | 21 [16] (26.2) | 42 [33] (26.4) |
| Trial treatment increase/decrease dose increment is either between zero and one, or one and two, times the intended dose increment according to the trial protocol | 19 [13] (20.3) | 24 [9] (14.8) | 43 [22] (17.6) |
| Morphine increase/decrease dose increment is either between zero and one, or one and two, the intended dose increment according to the trial protocol | 1 [1] (1.6) | 2 [1] (1.6) | 3 [2] (1.6) |
| Morphine decreased instead of no action taken as need to sustain a COMFORT score of < 17 for 1 hour | 1 [1] (1.6) | 5 [4] (6.6) | 6 [5] (4.0) |
| Trial treatment decreased instead of no action taken as need to sustain a COMFORT score of < 17 for 1 hour | 1 [1] (1.6) | 2 [2] (3.3) | 3 [3] (2.4) |
| Both trial treatment decreased and morphine increased instead of a morphine increase trial treatment decreased instead of trial treatment being temporarily stopped | – | – | – |
| Patient commenced trial treatment after 24-hour window following consent | – | 3 [3] (4.9) | 3 [3] (2.4) |
| Patient started both trial treatment and morphine at the same time instead of morphine followed by trial treatment 15 minutes later | 1 [1] (1.6) | – | 1 [1] (0.8) |
Appendix 7 Reasons for ineligibility and eligible, but not randomised, participants
| Other reason | Not eligible (N = 204) | Eligible (N = 340) | Total (N = 544) |
|---|---|---|---|
| Consultant decisiona | 14 | 41 | 55 |
| Parents unavailable | 6 | 33 | 39 |
| Cardiacb | 3 | 29 | 32 |
| Language barrier | 9 | 22 | 31 |
| On fentanyl | 17 | 12 | 29 |
| Transferred | 18 | 11 | 29 |
| Sedation weaned | 11 | 16 | 27 |
| Social issues | 9 | 17 | 26 |
| No IMP | – | 23 | 23 |
| Ongoing sedation regime | 4 | 19 | 23 |
| Patient death | 16 | 7 | 23 |
| Treatment withdrawn | 12 | 10 | 22 |
| No decision within time frame by parents | 7 | 12 | 19 |
| Closed to recruitment | 2 | 14 | 16 |
| Discharged | 15 | 1 | 16 |
| Alternative combination of sedatives required | 5 | 5 | 10 |
| No dedicated i.v. line | 3 | 6 | 9 |
| Long-term ventilation | 6 | 2 | 8 |
| Oral sedation | 6 | 2 | 8 |
| Pulmonary hypertension | – | 5 | 5 |
| Already on morphine, midazolam and clonidine | 3 | 1 | 4 |
| Asthmatic | 2 | 2 | 4 |
| On ketamine | 3 | 1 | 4 |
| Unlikely to survive | 2 | 2 | 4 |
| Alternative sedation/analgesia required | 1 | 2 | 3 |
| Burns | 1 | 1 | 2 |
| Changed to enteral sedation | – | 2 | 2 |
| High analgesic requirement | – | 2 | 2 |
| No trial medications when was sedated | 2 | – | 2 |
| On B17c | 1 | 1 | 2 |
| Parental stress: not approached | – | 2 | 2 |
| Required exchange blood transfusion | 1 | 1 | 2 |
| Unknown neurological status | 2 | – | 2 |
| Unstable airway | 2 | – | 2 |
| Absent corpus callosum – poor prognosis | – | 1 | 1 |
| Additional sedatives routinely given at home (diazepam, chloral and melatonin) | – | 1 | 1 |
| Approached but then sedation stopped as oversedated | – | 1 | 1 |
| Approached for CHiP study as was going to have open chest following surgery the next day | – | 1 | 1 |
| Approached for CHiP trial | 1 | – | 1 |
| Behavioural issues, difficult to sedate | 1 | – | 1 |
| Being screened for flu study on 5 March, sedation off on 6 March | – | 1 | 1 |
| Bradycardic, < 80 bpm | 1 | – | 1 |
| Bronchospasm | 1 | – | 1 |
| Cannot receive any opioids | 1 | – | 1 |
| Child protection case | – | 1 | 1 |
| Childs condition deteriorated before could be entered into trial | – | 1 | 1 |
| Chronic BP | 1 | – | 1 |
| Consented but became ineligible (no details provided) | – | 1 | 1 |
| Daily returns to theatre | – | 1 | 1 |
| Decision made to extubate | 1 | – | 1 |
| Diagnosis NFR | 1 | – | 1 |
| Diagnosis of meningitis, abnormal movements | – | 1 | 1 |
| Dysrhythmias | 1 | – | 1 |
| Eligible overnight; plan to stop midazolam, restart beta-blockers | – | 1 | 1 |
| Extubated 15 hours post operation; muscle relaxant off on 19 January 2012 08.00, extubated 9 hours later | – | 1 | 1 |
| Fixed, dilated pupils | – | 1 | 1 |
| Going for cardiac surgery today | – | 1 | 1 |
| Guillain–Barré syndrome | 1 | – | 1 |
| Had bad experience when participated in a clinical study during previous admission | – | 1 | 1 |
| Just intubated at screening | 1 | 1 | |
| Lost i.v. access then only one peripheral cannula | – | 1 | 1 |
| Midazolam given to control spasms | – | 1 | 1 |
| Needs to have a new cannula | – | 1 | 1 |
| Neuromuscular condition | 1 | – | 1 |
| On CPAP | – | 1 | 1 |
| On melatonin | 1 | – | 1 |
| On regular anticonvulsants | 1 | – | 1 |
| On vecuronium | – | 1 | 1 |
| On to single sedation once paralysis stopped | 1 | – | 1 |
| Oversedated | – | 1 | 1 |
| Palliative | – | 1 | 1 |
| Paralysed until just after 48 hours | 1 | – | 1 |
| Parental stress, child’s diagnosis and stay on PICU uncertain; happy to have midazolam for 48 hours but wanted clonidine if intubated and ventilated for longer | 1 | – | 1 |
| Parents asked in anticipating paralysis coming off | 1 | – | 1 |
| Patient approached for CHiP trial – declined then > 48 hours admission deadline for SLEEPS | – | 1 | 1 |
| Patient consented but not randomised (no reason given) | 1 | – | 1 |
| Patient consented but not randomised as became ineligible (no reason given) | – | 1 | 1 |
| Patient is receiving diamorphine in his/her epidural | – | 1 | 1 |
| Patient referred to CHiP – consent pre operation | 1 | – | 1 |
| Patient too unstable | – | 1 | 1 |
| Patient too unwell | – | 1 | 1 |
| Plan for PEG insertion and then extubate but this was delayed | – | 1 | 1 |
| Plan to extubate when 12 hours but then removed on CPAP for further sedation offd | – | 1 | 1 |
| Poor i.v. access, difficult to obtain | – | 1 | 1 |
| Previously screened for SLEEPS | 1 | – | 1 |
| Problems with heart rate to continue on current sedation | – | 1 | 1 |
| Renal transplant epidural in | – | 1 | 1 |
| Research nurse on A&E and ward would be only second patient in SLEEPS so instructed not to recruit | – | 1 | 1 |
| Respiration status unstable | – | 1 | 1 |
| Terminal care | – | 1 | 1 |
| Ventilated for 2 weeks in another hospital and has received sedation for 2 weeks | – | 1 | 1 |
| Consultant decision reason | Not eligible (N = 14) | Eligible (N = 41) | Total (N = 55) |
|---|---|---|---|
| No reason given | 3 | 11 | 14 |
| Requires minimal sedation | 6 | 2 | 8 |
| Airway clinically unstable | – | 3 | 3 |
| Preference for fentanyl | – | 3 | 3 |
| Requires higher dose of morphine | 1 | 2 | 3 |
| Clinically unstable | 1 | 1 | 2 |
| Requires higher dose of sedative | 1 | 1 | 2 |
| Requires higher doses of sedative and morphine | – | 2 | 2 |
| Patient in complete heart block, internal pacemaker, not for clonidine | – | 1 | 1 |
| Awaiting surgery day 2 of admission – postoperative | – | 1 | 1 |
| BP low, did not want extra sedation | 1 | – | 1 |
| Did not want to give sedation as BP down and on inotropes | – | 1 | 1 |
| Did not want to sedate | – | 1 | 1 |
| Owing to clinical condition | – | 1 | 1 |
| Enteral sedation | – | 1 | 1 |
| Frequent bradycardia, changed from morphine to fentanyl | – | 1 | 1 |
| Neuroimpairment | 1 | – | 1 |
| Patient being paced | – | 1 | 1 |
| Patient is asthmatic – does not want patient on morphine | – | 1 | 1 |
| Preference as neurological monitoring required | – | 1 | 1 |
| Preference for oral sedation and morphine | – | 1 | 1 |
| Preference for propofol | – | 1 | 1 |
| Preference in complete heart block, not wanting clonidine | – | 1 | 1 |
| Previous sedation issues | – | 1 | 1 |
| Requires smaller dose of morphine | – | 1 | 1 |
| To stay on fentanyl, as severe pulmonary hypertension | – | 1 | 1 |
| Cardiac reason | Not eligible (N = 3) | Eligible (N = 29) | Total (N = 32) |
|---|---|---|---|
| No further information provided | 1 | 14 | 15 |
| Pulmonary hypertension | – | 6 | 6 |
| Unstable | 1 | 4 | 5 |
| To remain on fentanyl | – | 3 | 3 |
| Care | – | 1 | 1 |
| Complex | – | 1 | 1 |
| Surgery being carried out within 24 hours of admission | 1 | – | 1 |
Appendix 8 Supplementary analgesia required during sedation
| Specific supplementary analgesias required during sedation | Reason why analgesia was requireda | Clonidine (N = 64) | Midazolam (N = 61) | Total (N = 125) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n patients | (%) | n events | n patients | (%) | n events | n patients | (%) | n events | ||
| 01 = additional morphine | A = agitated/discomfort | 5 | 7.8 | 10 | 6 | 9.8 | 11 | 11 | 8.8 | 18 |
| B = limit movement | 1 | 1.6 | 1 | 1 | 1.6 | 2 | 2 | 1.6 | 2 | |
| C = painful/clinical procedure | 4 | 6.3 | 6 | 10 | 16.4 | 14 | 14 | 11.2 | 17 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | 2 | 3.1 | 3 | 3 | 4.9 | 5 | 5 | 4.0 | 6 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 02 = alfentanil | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | – | – | – | – | – | – | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 03 = anaesthetic block | A = agitated/discomfort | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 2 |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 04 = desflurane | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | – | – | – | – | – | – | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 05 = diazepam | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | – | – | – | – | – | – | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 06 = fentanyl | A = agitated/discomfort | 3 | 4.7 | 3 | 2 | 3.3 | 5 | 5 | 4.0 | 6 |
| B = limit movement | 2 | 3.1 | 3 | 1 | 1.6 | 3 | 3 | 2.4 | 4 | |
| C = painful/clinical procedure | – | – | – | 4 | 6.6 | 4 | 4 | 3.2 | 8 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | 2 | 3.3 | 2 | 2 | 1.6 | 2 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| NK = not known | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| 07 = ibuprofen | A = agitated/discomfort | 1 | 1.6 | 1 | 2 | 3.3 | 3 | 3 | 2.4 | 3 |
| B = limit movement | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| C = painful/clinical procedure | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 2 | |
| D = pyrexia | 2 | 3.1 | 4 | 3 | 4.9 | 5 | 5 | 4.0 | 9 | |
| E = other | – | – | – | 2 | 3.3 | 2 | 2 | 1.6 | 3 | |
| F = general care | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 2 | |
| NK = not known | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| 08 = isoflurane | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | 1 | 1.6 | 1 | 1 | 1.6 | 2 | 2 | 1.6 | 2 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 09 = ketamine | A = agitated/discomfort | 1 | 1.6 | 1 | 1 | 1.6 | 2 | 2 | 1.6 | 2 |
| B = limit movement | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| C = painful/clinical procedure | 14 | 21.9 | 23 | 17 | 27.9 | 31 | 31 | 24.8 | 56 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | 1 | 1.6 | 1 | 2 | 3.3 | 3 | 3 | 2.4 | 3 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 10 = lorazepam | A = agitated/discomfort | 1 | 1.6 | 1 | 1 | 1.6 | 2 | 2 | 1.6 | 4 |
| B = limit movement | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 3 | |
| C = painful/clinical procedure | 1 | 1.6 | 1 | 1 | 1.6 | 2 | 2 | 1.6 | 2 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | 1 | 1.6 | 1 | – | – | 1 | 1 | 0.8 | 1 | |
| 11 = midazolam | A = agitated/discomfort | 15 | 23.4 | 22 | 17 | 27.9 | 32 | 32 | 25.6 | 51 |
| B = limit movement | 8 | 12.5 | 11 | 4 | 6.6 | 12 | 12 | 9.6 | 15 | |
| C = painful/clinical procedure | 5 | 7.8 | 7 | 15 | 24.6 | 20 | 20 | 16.0 | 29 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | 4 | 6.3 | 6 | 7 | 11.5 | 11 | 11 | 8.8 | 14 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 12 = muscle relaxant | A = agitated/discomfort | 1 | 1.6 | 1 | 1 | 1 | 0.8 | 1 | ||
| B = limit movement | 3 | 4.7 | 3 | 1 | 1.6 | 4 | 4 | 3.2 | 4 | |
| C = painful/clinical procedure | 10 | 15.6 | 13 | 20 | 32.8 | 30 | 30 | 24.0 | 47 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | 4 | 6.3 | 5 | 5 | 8.2 | 9 | 9 | 7.2 | 11 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 13 = paracetamol | A = agitated/discomfort | 9 | 14.1 | 12 | 14 | 23.0 | 23 | 23 | 18.4 | 34 |
| B = limit movement | 1 | 1.6 | 1 | 2 | 3.3 | 3 | 3 | 2.4 | 3 | |
| C = painful/clinical procedure | 3 | 4.7 | 3 | 3 | 4.9 | 6 | 6 | 4.8 | 6 | |
| D = pyrexia | 15 | 23.4 | 38 | 24 | 39.3 | 39 | 39 | 31.2 | 103 | |
| E = other | 7 | 10.9 | 17 | 6 | 9.8 | 13 | 13 | 10.4 | 24 | |
| F = general care | 3 | 4.7 | 11 | 5 | 8.2 | 8 | 8 | 6.4 | 20 | |
| NK = not known | – | – | – | 2 | 3.3 | 2 | 2 | 1.6 | 2 | |
| 14 = propofol | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | 3 | 4.9 | 3 | 3 | 2.4 | 3 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 15 = remifentanyl | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | – | – | – | – | – | – | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 16 = sevoflurane | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| 17 = thiopentone | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | – | – | – | – | – | – | – | – | – | |
| D = pyrexia | – | – | – | – | – | – | – | – | – | |
| E = other | – | – | – | – | – | – | – | – | – | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| NK = not known | A = agitated/discomfort | – | – | – | – | – | – | – | – | – |
| B = limit movement | – | – | – | – | – | – | – | – | – | |
| C = painful/clinical procedure | 1 | 1.6 | 1 | 1 | 1.6 | 2 | 2 | 1.6 | 2 | |
| D = pyrexia | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| E = other | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| F = general care | – | – | – | – | – | – | – | – | – | |
| NK = not known | – | – | – | 1 | 1.6 | 1 | 1 | 0.8 | 1 | |
| Allocation | Patient | Day | Time | Specific analgesia required during sedation | Other reason why analgesia was required |
|---|---|---|---|---|---|
| Clonidine | 1 | Day 2 | 14:00:00 | 13 = paracetamol | Ongoing analgesia |
| 2 | Day 2 | 12:00:00 | 13 = paracetamol | In pain following physiotherapy | |
| Day 3 | 00:00:00 | 13 = paracetamol | Ongoing analgesia | ||
| 20:00:00 | 13 = paracetamol | Missing reason | |||
| 3 | Day 1 | 00:00:00 | 11 = midazolam | Undersedated | |
| 23:00:00 | 13 = paracetamol | Missing reason | |||
| 4 | Day 1 | 23:00:00 | 12 = muscle relaxant | Desaturation, fighting the ventilator | |
| 5 | Day 1 | 23:00:00 | 13 = paracetamol | In preparation of nursing cares/suction, etc. | |
| Day 3 | 01:00:00 | 11 = midazolam | Emergency bolus midazolam score of > 28 | ||
| 6 | Day 1 | 09:00:00 | 11 = midazolam | Bradycardic plus desaturation decrease 60%, splinting chest | |
| 10:00:00 | 11 = midazolam | Bradycardic plus desaturation decrease 30%, splinting chest | |||
| 22:00:00 | 11 = midazolam | Splinting chest, oxygen saturations decreased to 50%, bradycardia of 80 bpm | |||
| 7 | Day 2 | 08:00:00 | 13 = paracetamol | Not receiving analgesia despite previous surgery | |
| 8 | Day 1 | 04:00:00 | 13 = paracetamol | Postoperative cardiac patient; routine paracetamol. Pain relief | |
| 22:00:00 | 13 = paracetamol | Postoperative cardiac patient; routine paracetamol. Pain relief | |||
| Day 2 | 03:00:00 | 13 = paracetamol | Postoperative cardiac patient; regular paracetamol | ||
| 10:00:00 | 13 = paracetamol | Postoperative cardiac patient; routine paracetamol for pain relief | |||
| 16:00:00 | 13 = paracetamol | Postoperative cardiac patient procedure – chest drain removal | |||
| 22:00:00 | 13 = paracetamol | Postoperative cardiac patient; routine paracetamol | |||
| Day 3 | 10:00:00 | 13 = paracetamol | Postoperative cardiac patient; routine paracetamol | ||
| 9 | Day 1 | 00:00:00 | 13 = paracetamol | Routine postcardiac surgery analgesia | |
| 18:00:00 | 13 = paracetamol | Routine postcardiac surgery analgesia | |||
| Day 2 | 06:00:00 | 13 = paracetamol | Routine postoperative analgesia | ||
| 10 | Day 1 | 00:00:00 | 01 = additional morphine | Fighting ventilator | |
| 01:00:00 | 11 = midazolam | Fighting ventilator | |||
| 02:00:00 | 01 = additional morphine | Prior to physiotherapy | |||
| 05:00:00 | 12 = muscle relaxant | Fighting ventilator | |||
| 11 | Day 3 | 09:00:00 | 12 = muscle relaxant | ETT retaped | |
| 09 = ketamine | ETT retaped | ||||
| 12 | Day 2 | 11:00:00 | 01 = additional morphine | Decreased SaO2, splinting chest (vecuronium given) and morphine to aid comfort while vecuronium given | |
| 12 = muscle relaxant | Decreased SaO2, splinting chest (vecuronium given) | ||||
| Midazolam | 1 | Day 1 | 21:00:00 | 07 = ibuprofen | For pain relief, as no morphine |
| Day 2 | 03:00:00 | 13 = paracetamol | Routine analgesia, as no i.v. morphine | ||
| 06:00:00 | 07 = ibuprofen | Routine analgesia, as no i.v. morphine | |||
| 2 | Day 1 | 20:00:00 | 13 = paracetamol | Regular intravenous paracetamol prescribed post surgery pain relief and temperature control | |
| 3 | Day 2 | 14:00:00 | 11 = midazolam | To prevent swelling of airway | |
| 4 | Day 2 | 11:00:00 | NK = not known | Patient struggling and turning over in bed | |
| 5 | Day 2 | 06:00:00 | 13 = paracetamol | Ongoing analgesia care/requirements | |
| 22:00:00 | 13 = paracetamol | Ongoing general care (ongoing analgesia requirements) | |||
| 6 | Day 2 | 08:00:00 | 09 = ketamine | Ketamine was used at 08:30 due to an episode of desaturation and to relax the child chest as splinting so as to administer oxygen therapya | |
| 7 | Day 6 | 00:00:00 | 07 = ibuprofen | Missing reason | |
| 21:00:00 | 13 = paracetamol | Missing reason | |||
| 8 | Day 2 | 12:00:00 | 13 = paracetamol | Missing reason | |
| 9 | Day 2 | 11:00:00 | 12 = muscle relaxant | Required CT scan, rocuronium bolus given for transfer | |
| Day 3 | 16:00:00 | 01 = additional morphine | MRI scan | ||
| 11 = midazolam | |||||
| 12 = muscle relaxant | |||||
| 16 = sevoflurane | |||||
| 10 | Day 2 | 03:00:00 | 11 = midazolam | Desat + 49 bagged (oxygen saturation fell to 49% requiring hand bag ventilation)b | |
| 04:00:00 | 12 = muscle relaxant | Desat + 49 bagged (oxygen saturation fell to 49% requiring hand bag ventilation) | |||
| 11 | Day 1 | 21:00:00 | 11 = midazolam | Safe positioning for chest radiograph | |
| 12 | Day 3 | 14:00:00 | 11 = midazolam | Tachycardic | |
| 13 | Day 2 | 04:00:00 | 11 = midazolam | Wild ETT unstable, sedation score 32 increase trial increase morphine ×2c | |
| 05:00:00 | 11 = midazolam | Awake ETT unstable | |||
| 14 | Day 2 | 22:00:00 | 13 = paracetamol | Paracetamol was given to keep patient settled | |
| 15 | Day 4 | 11:00:00 | 11 = midazolam | PEG insertion | |
| 16 | Day 5 | 23:00:00 | 12 = muscle relaxant | Retaping of ETT | |
| 17 | Day 3 | 13:00:00 | 06 = fentanyl | For retaping ETT | |
| 14:00:00 | 12 = muscle relaxant | Retaping ETT | |||
| 18 | Day 1 | 05:00:00 | 01 = additional morphine | Central venous line leaking, patient desaturating and not receiving sedation | |
| 03 = anaesthetic block | |||||
| 19 | Day 2 | 03:00:00 | 06 = fentanyl | Breathing against ventilation, increase CO2 | |
| 20 | Day 1 | 05:00:00 | 01 = additional morphine | Facilitate ventilation and ventilation | |
| 12 = muscle relaxant | |||||
| 21 | Day 1 | 21:00:00 | 09 = ketamine | Turn, bed change and mouth care |
Appendix 9 Health economic appendix
| From randomisation to 14 days post-treatment cessation | ||
|---|---|---|
| Intervention | Estimation | Drug treatments were made up for each child every 24 hours. No matter how much of the drug was used, a new batch was made up every 24 hours. Time on treatment (from initial loading dose) was recorded by nursing staff for all children. All unused drugs were discarded. The consumables associated with daily drug treatments were estimated by nursing and clinical staff |
| Valuation | Price of clonidine was taken from MIMS (2013).51 There is no entry for clonidine in MIMS 2012. We have assumed that, as the price is very low, it is not unreasonable to assume the same price for 2012 Price of midazolam and morphine were taken from BNF (2012)50 Price of consumables and dextrose were taken from NHS Supply Chain catalogue (2012).52 Consumables include syringe, needle, extension line kit, line filter and line tap |
|
| Hospital stay | Hospital stays were divided into three categories: per diem, per diem GM ward and per diem HDU Critical care paediatric bed-days: The PICU cost (£1826) was taken from the NHS Reference Costs 2011–1249 (XB05Z). The HDU cost (£920) was taken from the NHS Reference Costs 2011–1249 (XB07Z). The per diem GM ward cost (£331) was provided by the Finance/Accounts Department of Alder Hey Hospital, Liverpool Hospital admissions are often made up of stays in different wards. All transfers between wards were recorded on the Patient Transfer form. Of the 108 children in the analysis, 13 did not have a completed Patient Transfer form. Data on LoS in PICU, GM and HDU were then obtained from the completed End of Study form. Only one child did not have this information recorded. For this child, an average of LoS in PICU was estimated using data from the 108 children with completed Patient Transfer forms. LoS in PICU was then subtracted from the total LoS to estimate days in the GM ward Duration and therefore cost of inpatient stay is a key driver in the economic evaluation, and required careful consideration in the sensitivity analyses, in which various approaches were used to test the robustness of the economic evaluation results to changes in the cost of a hospital inpatient admission. For the most part, LoS was recorded accurately in terms of hours and minutes. However, only discharge dates were recorded (no time). We therefore assumed that all children were discharged from hospital at 23:59 In the base case cost estimates of LoS, if a child had spent > 12 hours in a ward, a full per diem cost was applied. If a child had spent < 12 hours in a ward, a half day cost was applied. Full days incurred the full per diem cost In the sensitivity analysis, three different approaches to costing LoS were undertaken:
|
|
| Hospital transfer | All children who were transferred between hospitals during the initial hospital admission were costed using the NHS reference cost of £230 (ASS02). Where no further information was available on LoS, it was assumed that all children had a stay in hospital at least until 14 days post-treatment cessation | |
| Additional days in different hospital | Children were sometimes transferred to a different hospital for continuation of treatment. If the extended LoS was known then this estimate was used in the analysis. If the extended LoS was unknown then it was assumed that the child stayed in hospital at least until the time horizon used in the analysis (14 days post-treatment cessation or 14 days postventilation cessation) | |
| SAEs | Total length of hospital stay costs already include any additional days in hospital due to a SAE After careful examination of CRFs, only SAEs pertaining to two children required additional costing over and above the per diem cost. One child went from a GM ward to theatre on two separate occasions for a simple procedure that took 30 minutes. The cost of the SAE for this child was made up of (basic) theatre cost plus surgeon (average) cost per hour. This event was costed in the base case analysis and therefore subsequent sensitivity and scenario analyses. One child suffered a SAE while in the PICU and went to theatre for a cerebral drainage. The cost of the SAE for this child was made up of a (neurosurgery) theatre cost plus (high) surgeon cost per hour. This event was costed only in the sensitivity analysis with the extended time horizon (14 days postventilation cessation) Cost source: Alder Hey Finance Department (Alder Hey Hospital, personal communication)
|
|
| Death | It was to be assumed that any child who died during the trial within the time horizon of the economic evaluation incurred the cost of a post-mortem as a proxy for the costs associated with dying in hospital. However, none of the children died in the trial during the two time periods of interest Cost source: Alder Hey Finance Department 2012 (Alder Hey Hospital, personal communication)
|
|
| Scenario analysis: wider NHS costs (14 days post-treatment cessation) | ||
| GP attendance | Cost source: Personal Social Services Research Unit 2012 (Curtis48)
|
|
| A&E attendance | The cost estimate used in the analysis depended on whether or not the child was admitted to hospital as a result of attendance Cost source: Personal Social Services Research Unit 2012 (Curtis48)
|
|
| Hospital admission | The GM per diem cost used in the baseline analysis (£331) was used to estimate the cost of any additional day spent in hospital as part of a re-admission within 14 days post-treatment cessation | |
| Additional sensitivity analyses | ||
A further three sensitivity analyses were undertaken:
|
||
Appendix 10 Cumulative sedative–morphine infusion split by primary outcome ‘yes/no’
| Hour | Clonidine | Midazolam | ||||
|---|---|---|---|---|---|---|
| No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | |
| 0 | 21 | – | – | 18 | – | – |
| 5 | 20 | 0.50 (0.36–0.55) | 0.20, 0.97 | 17 | 0.42 (0.26–0.50) | 0.09, 0.84 |
| 10 | 20 | 1.00 (0.75–1.11) | 0.40, 1.98 | 17 | 0.83 (0.52–1.00) | 0.19, 1.82 |
| 15 | 18 | 1.48 (1.06–1.50) | 0.60, 3.00 | 16 | 1.29 (0.71–1.57) | 0.24, 2.79 |
| 20 | 15 | 1.76 (1.55–2.00) | 0.96, 4.02 | 15 | 1.85 (1.04–2.17) | 0.74, 3.77 |
| 25 | 11 | 2.20 (1.85–2.50) | 1.16, 5.03 | 13 | 2.08 (1.30–2.50) | 0.93, 4.69 |
| 30 | 11 | 2.76 (2.28–3.00) | 1.36, 6.05 | 12 | 2.75 (1.58–3.29) | 1.11, 5.69 |
| 35 | 11 | 3.40 (2.72–3.51) | 1.56, 7.07 | 12 | 3.24 (1.84–4.11) | 1.30, 6.69 |
| 40 | 9 | 4.00 (3.42–4.21) | 2.28, 8.08 | 11 | 4.13 (2.10–5.33) | 1.48, 7.69 |
| 45 | 6 | 4.50 (4.34–4.69) | 3.82, 5.05 | 8 | 4.75 (2.34–5.53) | 1.67, 8.10 |
| 50 | 5 | 4.88 (4.80–5.00) | 4.39, 5.18 | 7 | 5.41 (2.20–6.93) | 1.85, 9.10 |
| 55 | 5 | 5.43 (5.05–5.50) | 4.96, 5.66 | 7 | 5.98 (2.39–7.68) | 2.04, 10.10 |
| 60 | 5 | 6.00 (5.53–6.12) | 5.30, 6.15 | 7 | 6.70 (2.57–8.43) | 2.22, 11.10 |
| 65 | 4 | 6.21 (5.67–6.78) | 5.55, 6.94 | 4 | 5.70 (3.07–8.42) | 2.41, 9.18 |
| 70 | 2 | 7.43 (7.11–7.75) | 7.11, 7.75 | 4 | 6.21 (3.30–9.17) | 2.59, 9.93 |
| 75 | 1 | 7.79 (7.79–7.79) | 7.79, 7.79 | 3 | 4.29 (2.78–10.68) | 2.78, 10.68 |
| 80 | 1 | 8.44 (8.44–8.44) | 8.44, 8.44 | 3 | 4.57 (2.96–11.43) | 2.96, 11.43 |
| 85 | 1 | 9.41 (9.41–9.41) | 9.41, 9.41 | 3 | 4.85 (3.15–12.18) | 3.15, 12.18 |
| 90 | 1 | 10.38 (10.38–10.38) | 10.38, 10.38 | 2 | 4.23 (3.33–5.12) | 3.33, 5.12 |
| 95 | 1 | 11.30 (11.30–11.30) | 11.30, 11.30 | 2 | 4.46 (3.52–5.40) | 3.52, 5.40 |
| 100 | 1 | 11.83 (11.83–11.83) | 11.83, 11.83 | 2 | 4.69 (3.70–5.68) | 3.70, 5.68 |
| 105 | 1 | 12.31 (12.31–12.31) | 12.31, 12.31 | 2 | 4.92 (3.89–5.96) | 3.89, 5.96 |
| 110 | 1 | 12.80 (12.80–12.80) | 12.80, 12.80 | 2 | 5.16 (4.07–6.24) | 4.07, 6.24 |
| 115 | 1 | 13.28 (13.28–13.28) | 13.28, 13.28 | 2 | 5.39 (4.26–6.51) | 4.26, 6.51 |
| 120 | 0 | – | – | 1 | 4.59 (4.59–4.59) | 4.59, 4.59 |
| 125 | 0 | – | – | 1 | 4.78 (4.78–4.78) | 4.78, 4.78 |
| Hour | Clonidine | Midazolam | ||||
|---|---|---|---|---|---|---|
| No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | |
| 0 | 40 | – | – | 41 | – | – |
| 5 | 39 | 0.40 (0.25–0.48) | 0.05, 1.00 | 41 | 0.50 (0.39–0.66) | 0.10, 1.02 |
| 10 | 35 | 0.80 (0.49–1.05) | 0.10, 1.95 | 36 | 1.00 (0.76–1.33) | 0.16, 2.05 |
| 15 | 31 | 1.21 (0.75–1.90) | 0.18, 2.90 | 31 | 1.55 (1.05–2.07) | 0.21, 3.00 |
| 20 | 24 | 1.55 (1.01–2.58) | 0.33, 3.90 | 30 | 2.09 (1.30–2.61) | 0.26, 4.00 |
| 25 | 17 | 2.24 (1.30–3.55) | 0.48, 4.46 | 26 | 2.62 (1.42–3.27) | 0.66, 5.00 |
| 30 | 15 | 2.38 (1.50–4.40) | 0.65, 5.49 | 26 | 3.06 (1.67–4.07) | 0.89, 6.00 |
| 35 | 12 | 2.03 (1.62–5.03) | 0.85, 6.19 | 24 | 3.38 (1.89–4.73) | 1.12, 7.00 |
| 40 | 12 | 2.35 (1.84–5.81) | 1.06, 7.16 | 18 | 3.56 (2.13–4.48) | 1.36, 8.00 |
| 45 | 8 | 2.60 (1.91–3.19) | 1.26, 8.13 | 13 | 3.05 (2.37–6.10) | 1.59, 9.00 |
| 50 | 6 | 2.97 (1.97–4.33) | 1.46, 9.10 | 11 | 2.97 (2.64–6.59) | 1.83, 7.73 |
| 55 | 6 | 3.72 (2.17–4.93) | 1.67, 10.06 | 9 | 3.38 (2.88–4.23) | 2.06, 8.48 |
| 60 | 5 | 4.10 (2.36–4.85) | 1.86, 11.03 | 9 | 3.69 (3.12–4.43) | 2.30, 9.23 |
| 65 | 4 | 4.20 (2.26–8.92) | 1.96, 12.00 | 8 | 3.80 (3.24–6.39) | 2.48, 10.03 |
| 70 | 3 | 6.85 (2.01–12.97) | 2.01, 12.97 | 8 | 4.06 (3.47–6.79) | 2.48, 11.03 |
| 75 | 2 | 4.90 (2.06–7.75) | 2.06, 7.75 | 8 | 4.27 (3.70–7.23) | 2.48, 12.03 |
| 80 | 0 | – | – | 8 | 4.49 (3.93–7.60) | 2.48, 13.03 |
| 85 | 0 | – | – | 8 | 4.71 (4.16–8.13) | 2.48, 14.03 |
| 90 | 0 | – | – | 6 | 4.71 (4.19–5.10) | 2.48, 15.03 |
| 95 | 0 | – | – | 4 | 5.29 (4.74–10.76) | 4.41, 16.03 |
| 100 | 0 | – | – | 4 | 5.63 (4.92–11.54) | 4.62, 17.03 |
| 105 | 0 | – | – | 4 | 5.99 (5.14–12.28) | 4.84, 18.03 |
| 110 | 0 | – | – | 4 | 6.24 (5.37–12.92) | 5.05, 19.03 |
| 115 | 0 | – | – | 4 | 6.58 (5.65–13.63) | 5.38, 20.03 |
| 120 | 0 | – | – | 4 | 6.95 (6.01–14.39) | 5.88, 21.03 |
| 125 | 0 | – | – | 3 | 8.50 (6.29–22.03) | 6.29, 22.03 |
| 130 | 0 | – | – | 3 | 9.30 (6.51–23.03) | 6.51, 23.03 |
| 135 | 0 | – | – | 3 | 10.10 (6.72–23.83) | 6.72, 23.83 |
| 140 | 0 | – | – | 2 | 15.88 (6.94–24.83) | 6.94, 24.83 |
| 145 | 0 | – | – | 2 | 16.49 (7.15–25.83) | 7.15, 25.83 |
| 150 | 0 | – | – | 2 | 17.10 (7.36–26.83) | 7.36, 26.83 |
| 155 | 0 | – | – | 2 | 17.71 (7.58–27.83) | 7.58, 27.83 |
| 160 | 0 | – | – | 2 | 18.31 (7.79–28.83) | 7.79, 28.83 |
| 165 | 0 | – | – | 2 | 18.92 (8.01–29.83) | 8.01, 29.83 |
| Hour | Clonidine | Midazolam | ||||
|---|---|---|---|---|---|---|
| No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | |
| 0 | 21 | – | – | 18 | – | – |
| 5 | 20 | 5.00 (5.00–5.00) | 5.00, 11.50 | 17 | 5.00 (5.00–5.00) | 2.50, 8.00 |
| 10 | 20 | 10.00 (10.00–10.00) | 10.00, 24.50 | 17 | 10.00 (10.00–10.00) | 3.50, 18.00 |
| 15 | 18 | 15.00 (15.00–15.00) | 13.50, 35.50 | 16 | 15.00 (13.00–15.00) | 3.50, 28.00 |
| 20 | 15 | 20.00 (20.00–20.00) | 16.00, 45.50 | 15 | 20.00 (18.00–20.00) | 3.50, 38.00 |
| 25 | 11 | 25.00 (25.00–25.00) | 19.00, 56.50 | 13 | 25.00 (24.00–25.00) | 3.50, 40.25 |
| 30 | 11 | 30.00 (30.00–30.00) | 26.00, 70.50 | 12 | 30.00 (22.75–30.75) | 3.50, 51.50 |
| 35 | 11 | 35.00 (35.00–35.00) | 35.00, 85.50 | 12 | 35.00 (26.50–37.13) | 3.50, 65.50 |
| 40 | 9 | 40.00 (40.00–40.00) | 40.00, 100.50 | 11 | 40.00 (28.50–49.25) | 3.50, 78.00 |
| 45 | 6 | 45.00 (45.00–45.00) | 44.00, 45.00 | 8 | 45.00 (37.75–52.13) | 14.50, 64.25 |
| 50 | 5 | 50.00 (50.00–50.00) | 50.00, 50.00 | 7 | 50.00 (38.50–69.25) | 17.00, 71.75 |
| 55 | 5 | 55.00 (55.00–55.00) | 55.00, 55.00 | 7 | 55.00 (43.00–79.25) | 19.50, 79.25 |
| 60 | 5 | 60.00 (60.00–60.00) | 60.00, 60.00 | 7 | 60.00 (43.00–86.75) | 22.00, 89.25 |
| 65 | 4 | 65.00 (65.00–65.00) | 65.00, 65.00 | 4 | 72.13 (37.25–96.75) | 24.50, 99.25 |
| 70 | 2 | 71.25 (70.00–72.50) | 70.00, 72.50 | 4 | 77.13 (39.75–105.50) | 27.00, 109.25 |
| 75 | 1 | 75.00 (75.00–75.00) | 75.00, 75.00 | 3 | 55.00 (28.75–118.25) | 28.75, 118.25 |
| 80 | 1 | 80.00 (80.00–80.00) | 80.00, 80.00 | 3 | 57.50 (31.25–125.75) | 31.25, 125.75 |
| 85 | 1 | 89.75 (89.75–89.75) | 89.75, 89.75 | 3 | 60.00 (33.75–134.50) | 33.75, 134.50 |
| 90 | 1 | 99.75 (99.75–99.75) | 99.75, 99.75 | 2 | 49.38 (36.25–62.50) | 36.25, 62.50 |
| 95 | 1 | 107.75 (107.75–107.75) | 107.75, 107.75 | 2 | 51.13 (37.25–65.00) | 37.25, 65.00 |
| 100 | 1 | 112.75 (112.75–112.75) | 112.75, 112.75 | 2 | 53.63 (39.75–67.50) | 39.75, 67.50 |
| 105 | 1 | 117.75 (117.75–117.75) | 117.75, 117.75 | 2 | 56.63 (43.25–70.00) | 43.25, 70.00 |
| 110 | 1 | 122.75 (122.75–122.75) | 122.75, 122.75 | 2 | 59.13 (45.75–72.50) | 45.75, 72.50 |
| 115 | 1 | 127.75 (127.75–127.75) | 127.75, 127.75 | 2 | 61.38 (48.75–74.00) | 48.75, 74.00 |
| 120 | 0 | – | – | 1 | 53.25 (53.25–53.25) | 53.25, 53.25 |
| 125 | 0 | – | – | 1 | 54.25 (54.25–54.25) | 54.25, 54.25 |
| Hour | Clonidine | Midazolam | ||||
|---|---|---|---|---|---|---|
| No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | No. of participants remaining | Cumulative dose: median (IQR) | Cumulative dose: min., max. | |
| 0 | 40 | – | – | 41 | – | – |
| 5 | 39 | 5.00 (4.00–5.00) | 1.00, 14.75 | 41 | 5.00 (5.00–5.00) | 1.75, 13.75 |
| 10 | 35 | 10.00 (8.00–10.00) | 1.00, 22.00 | 36 | 10.00 (9.75–10.08) | 2.00, 28.25 |
| 15 | 31 | 15.00 (10.00–17.00) | 1.00, 29.00 | 31 | 15.00 (14.00–16.00) | 2.00, 26.00 |
| 20 | 24 | 20.00 (16.38–24.88) | 2.00, 34.25 | 30 | 20.00 (17.25–21.00) | 2.00, 38.50 |
| 25 | 17 | 25.00 (16.00–30.00) | 7.00, 48.00 | 26 | 25.00 (19.75–35.00) | 2.00, 53.50 |
| 30 | 15 | 29.50 (15.00–38.75) | 7.00, 62.50 | 26 | 30.00 (22.25–43.00) | 2.00, 68.50 |
| 35 | 12 | 30.13 (14.75–35.00) | 7.00, 68.25 | 24 | 35.00 (22.38–54.38) | 2.00, 80.00 |
| 40 | 12 | 33.38 (19.75–39.50) | 7.50, 83.25 | 18 | 36.13 (24.00–44.00) | 5.50, 88.00 |
| 45 | 8 | 29.97 (17.25–36.88) | 10.00, 46.50 | 13 | 43.00 (29.00–45.00) | 5.50, 102.50 |
| 50 | 6 | 32.75 (17.50–43.75) | 12.50, 56.50 | 11 | 41.50 (30.25–50.00) | 5.50, 117.50 |
| 55 | 6 | 38.54 (22.50–48.75) | 15.00, 70.00 | 9 | 42.75 (35.75–55.00) | 5.50, 99.80 |
| 60 | 5 | 36.00 (27.50–52.15) | 17.50, 85.00 | 9 | 45.50 (41.25–59.50) | 5.50, 107.30 |
| 65 | 4 | 44.85 (26.25–78.60) | 20.00, 100.00 | 8 | 55.63 (29.88–64.00) | 5.50, 115.30 |
| 70 | 3 | 62.24 (37.50–115.00) | 37.50, 115.00 | 8 | 59.25 (31.38–69.00) | 5.50, 127.80 |
| 75 | 2 | 55.25 (43.20–67.29) | 43.20, 67.29 | 8 | 63.00 (31.38–74.00) | 5.50, 140.30 |
| 80 | 0 | – | – | 8 | 66.75 (31.63–79.00) | 5.50, 154.30 |
| 85 | 0 | – | – | 8 | 69.25 (33.63–84.00) | 5.50, 169.30 |
| 90 | 0 | – | – | 6 | 71.75 (11.00–88.00) | 5.50, 184.30 |
| 95 | 0 | – | – | 4 | 86.00 (74.25–145.15) | 69.50, 197.30 |
| 100 | 0 | – | – | 4 | 90.00 (76.75–151.65) | 69.50, 207.30 |
| 105 | 0 | – | – | 4 | 95.00 (79.63–158.65) | 70.25, 216.30 |
| 110 | 0 | – | – | 4 | 100.00 (83.38–166.15) | 72.75, 226.30 |
| 115 | 0 | – | – | 4 | 105.00 (87.63–173.65) | 76.25, 236.30 |
| 120 | 0 | – | – | 4 | 110.00 (92.63–181.15) | 81.25, 246.30 |
| 125 | 0 | – | – | 3 | 110.00 (86.25–256.80) | 86.25, 256.80 |
| 130 | 0 | – | – | 3 | 116.50 (89.25–269.30) | 89.25, 269.30 |
| 135 | 0 | – | – | 3 | 121.50 (90.25–281.80) | 90.25, 281.80 |
| 140 | 0 | – | – | 2 | 192.03 (90.25–293.80) | 90.25, 293.80 |
| 145 | 0 | – | – | 2 | 197.03 (90.25–303.80) | 90.25, 303.80 |
| 150 | 0 | – | – | 2 | 202.78 (90.25–315.30) | 90.25, 315.30 |
| 155 | 0 | – | – | 2 | 209.03 (90.25–327.80) | 90.25, 327.80 |
| 160 | 0 | – | – | 2 | 215.28 (90.25–340.30) | 90.25, 340.30 |
| 165 | 0 | – | – | 2 | 220.78 (90.25–351.30) | 90.25, 351.30 |
Appendix 11 Serious adverse events line listings
| SAE no. | Treatment | Time from treatment start to SAE onset (hours) | Time from treatment cessation to SAE onset (if occurred post treatment) (hours) | SAE description | SAE group | Seriousness (PI/ChI) | Severity | Expectedness (PI/ChI) | Relationship (PI/ChI) | Cause | Outcome | Patient status | Unblinded | SUSAR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | Clonidine | 196.67 | 151.50 | Patient had two line insertions under general anaesthetic on two occasions Patient had 3 weeks of i.v. antibiotics. Temperature settled after this. Patient had one further week of oral antibiotics. Inflammatory markers returned to normal 29 March 2010 clinic appointment: chest radiograph; minimal change at left base For 1 week, antibiotics |
Infection requiring antibiotics | PI: Prolonged existing hospitalisation and infection requiring long-term antibiotics ChI: Prolonged existing hospitalisation and infection requiring long-term antibiotics |
Moderate | Unexpected/unexpected | Unlikely/unlikely | Disease under study | Resolved with sequelae | Completed trial | No | No |
| 002 | Clonidine | 44.12 | 1.83 | Study drug off at 13.20 Extubated at 15.00 approximately. Patient cries 2–3 minutes later, agitated, desaturated Output preserved on arterial line (BP systolic > 100 mmHg; HR > 100 bpm) Colour/perfusion very poor Reintubation – pink, blood-stained secretions Echocardiography – no tampenade, good function, forward flow through valve CT, brain normal |
Failed extubation requiring reintubation | PI: Immediately life-threatening, prolonged existing hospitalisation and patient required reintubation and reventilation, no long-term sequelae ChI: Immediately life-threatening |
Severe | Unexpected/unexpected | Possibly/unlikely | Disease under study | Resolved | Completed trial | Yes | Yes |
| 003 | Clonidine | 358.45 | 316.17 | Admitted from cardiac clinic to the cardiac ward due to a sternotomy wound infection Commenced on oral antibiotics Discharged home on 7 July 2010 |
Postoperative wound infection | PI: Required hospitalisation and patient required reintubation and reventilation, no long-term sequelae ChI: Required hospitalisation and medically significant/important |
Mild | Unexpected/unexpected | Unrelated/unrelated | Disease under study | Not resolved/ongoing | Continuing in trial | No | No |
| 004 | Clonidine | 34.25 | 0.00 | Patient woke, coughed and then self-extubated – his tube was coughed up – and did not use his hands to cause it to be dislodged | Accidental extubation | PI: Medically significant/important and self-extubation ChI: Medically significant/important and self-extubation |
Mild | Expected/expected | Almost certainly/almost certainly | Lack of efficacy | Resolved | Completed trial | No | No |
| 005 | Clonidine | 288.52 | 276.92 | Patient was taken by his mother to his local A&E at 18.00 on 24 October 2010 due to another asthma attack Oxygen and nebulisers were administered; he was transferred to the assessment unit and seen by paediatrician, and admitted to the paediatric ward He was discharged the following day at 18.30 |
Recurrence of original disease after discharge from hospital | PI: Required hospitalisation ChI: Required hospitalisation |
Moderate | Unexpected/unexpected | Unlikely/unlikely | Other illness | Resolved | Completed trial | No | No |
| 006 | Clonidine | 2.58 | 0.02 | Randomised at 15:00 on 22 December 2010 Loading dose given at 16:30–17:30 Heart rate remained stable 100–110 bpm during and for > 1 hour post infusion Bradycardia (? sinus) developed at 19:05 down to 64 bpm, no obvious cause/no hypoxia Trial drug and morphine stopped Heart rate recovered within 10 minutes to 100 bpm |
Bradycardia requiring intervention | PI: Medically significant/important and profound bradycardic episode following the loading doses ChI: Medically significant/important |
Moderate | Unexpected/unexpected | Possibly/possibly | Does not need to be completed if relationship to study drug is not unrelated or unlikely | Resolved | Withdrawn from trial | Yes | Yes |
| 007 | Midazolam | 27.92 | N/A – SAE occurred during trial treatment | Patient COMFORT score was 17 at the time of the event (21.15) On 28 July 2011 at 11.40 patient ETT was pulled back by 1 cm due to right upper lobe collapse The tapes were changed again at 17.15 as they had become wet and unsecure because the patient was dribbling a lot At 21.15 it was reported that patient coughed his ETT tube out and required to be reintubated. Tube was reported to be un secure because of being wet, but was documented in the notes as being pulled out |
ETT migrated down right main bronchus due to wet retaining tapes | PI: Immediately life-threatening and prolonged existing hospitalisation ChI: Medically significant/important |
Moderate | Unexpected/expected | Unrelated/unrelated | Disease under study | Resolved | Continuing in trial | No | No |
| 008 | Clonidine | 24.4 | 0.00 | Patient was awake and orientated, started gagging on his oral ETT and managed to self-extubate with his tongue He remained comfortable and did not require reintubating |
Self-extubation not requiring reintubation | PI: Medically significant/important ChI: Medically significant/important |
Moderate | Expected/expected | Unrelated/unrelated | Prior or concomitant treatment | Resolved | Continuing in trial | No | No |
| 009 | Midazolam | 15.07 | N/A – SAE occurred during trial treatment | Patient turned for sheet change – COMFORT score = 17 desaturated ETT suctioned Patient coughing and reaching for tube Reconnected to vent, realised tube out SLEEPS drug stopped |
Accidental extubation | PI: Medically significant/important and self-extubated, did not require reintubation ChI: Medically significant/important and required unplanned cessation of ventilation, which was successful |
Moderate | Expected/expected | Unrelated/unrelated | Lack of efficacy | Resolved | Continuing in trial | No | No |
| 010 | Clonidine | 48.85 | 5.50 | Trial medication discontinued at 12.30 on 19 February 2012 The child was extubated at 16.30 but required reintubation at 19.30 owing to stridor and the need to protect his airway. This is a known risk of extubation and not related to trial medication |
Reintubation owing to stridor | PI: Prolonged existing hospitalisation ChI: Prolonged existing hospitalisation |
Mild | Unobtainable/expected | Unrelated/unrelated | Other illness | Resolved | Completed trial | No | No |
| 011 | Clonidine | 26.83 | 2.00 | RSV bronchiolitis, extubated, postintubation stridor, reintubation | Postextubation stridor | PI: Medically significant/important and reintubation ChI: Medically significant/important and reintubation |
Mild | Unexpected/unexpected | Unrelated/unrelated | Other illness | Resolved | Completed trial | No | No |
| 012 | Clonidine | 338.4 | 312.85 | Child with acute leukaemia in relapse developed sepsis and admitted to PICU Drainage of cerebral subdural collection Mucorymycosis On SLEEPS drugs from 20 March 2012 to 21 March 2012 until extubated Later developed multiple organ problems, which led to death after |
Death from primary disease after active phase of trial complete | PI: Death ChI: Death |
Severe | Unexpected/unexpected | Unrelated/unrelated | Other illness | Fatal | Completed trial | No | No |
| 013 | Midazolam | 6.67 (0.00) | 6.67 (0.00) | Self-extubation Also pulled out i.v. cannula Some stridor Settled with non-invasive mask ventilation (non-invasive BIPAP) Able to progress to CPAP within 12 hours. No drugs required (no nebulised) |
Self-extubation not requiring reintubation | PI: Medically significant/important and self-extubation and needed non-invasive BIPAP for 6 hours ChI: Medically significant/important and required non-invasive support for 6 hours due to premature extubation |
Moderate | Expected/expected | Probably/probably | Lack of efficacy | Resolved | Withdrawn from trial | No | No |
Appendix 12 Supplementary health economics tables
| Analysis (n = 120) | Mean costs (95% CI), £ | Mean effects (95% CI) | ICER | Probability that clonidine is: | Cost-effective (%)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clonidine | Midazolam | Difference | Clonidine | Midazolam | Difference | More effective (%) | Less costly (%) | |||
| Base case | 11,445 (9811 to 13,078) | 12,276 (10,554 to 13,998) | –831 (–3204 to 1542) | 0.34 (0.22 to 0.46) | 0.30 (0.19 to 0.42) | 0.04 (–0.13 to 0.21) | –21,216 | 66 | 71 | 73 |
| Sensitivity analysis (n = 120) | ||||||||||
| Higher cost of higher-level inpatient care | 12,448 (10,640 to 14,256) | 13,425 (11,486 to 15,363) | –977 (–3627 to 1674) | 0.34 (0.22 to 0.46) | 0.30 (0.19 to 0.42) | 0.04 (–0.13 to 0.21) | –24,933 | 71 | 77 | 78 |
| Lower cost of higher-level inpatient care | 10,027 (8642 to 11,411) | 10,744 (9287 to 12,200) | –716 (–2726 to 1292) | 0.34 (0.22 to 0.46) | 0.30 (0.19 to 0.42) | 0.04 (–0.13 to 0.21) | –18,299 | 69 | 73 | 77 |
| Accurate LoS | 10,981 (9264 to 12,518) | 11,644 (9925 to 13,364) | –753 (–3119 to 1614) | 0.34 (0.22 to 0.46) | 0.30 (0.19 to 0.42) | 0.04 (–0.13 to 0.21) | –19,224 | 67 | 72 | 73 |
| 14 days postventilation cessation | 11,535 (9856 to 13,213) | 12,344 (10,583 to 14,105) | –809 (–3241 to 1623) | 0.34 (0.22 to 0.46) | 0.30 (0.19 to 0.42) | 0.04 (–0.13 to 0.21) | –20,651 | 70 | 74 | 76 |
| Narrow definition of adequate sedation | 11,445 (9811 to 13,078) | 12,276 (10,554 to 13,998) | –831 (–3204 to 1542) | 0.25 (0.14 to 0.35) | 0.19 (0.09 to 0.29) | 0.06 (–0.09 to 0.21) | –13,979 | 78 | 75 | 77 |
| Wider definition of adequate sedation | 11,445 (9811 to 13,078) | 12,276 (10,554 to 13,998) | –831 (–3204 to 1542) | 0.48 (0.35 to 0.60) | 0.41 (0.28 to 0.53) | 0.07 (–0.11 to 0.24) | –12,111 | 79 | 75 | 76 |
| Scenario analysis (n = 106) | ||||||||||
| Wider NHS perspective up to 14 days post-treatment cessation | 11,832 (9978 to 13,686) | 12,384 (10,586 to 14,182) | –552 (–3143 to 2031) | 0.33 (0.20 to 0.46) | 0.33 (0.21 to 0.46) | –0.0006 (–0.19 to 0.17) | 86,102 | 48 | 67 | 63 |
| Value of threshold (£) | Base case | Higher-level inpatient care | Accurate LoS | |||||
|---|---|---|---|---|---|---|---|---|
| Higher cost | Lower cost | |||||||
| % cost-effective | Mean net benefit (95% CI) | % cost-effective | Mean net benefit (95% CI) | % cost-effective | Mean net benefit (95% CI) | % cost-effective | Mean net benefit (95% CI) | |
| 0 | 71 | 640 (–1854 to 2993) | 77 | 967 (–1688 to 3279) | 76 | 730 (–1308 to 2860) | 72 | 732 (–1531 to 3038) |
| 500 | 72 | 659 (–1817 to 3022) | 77 | 990 (–1708 to 3336) | 77 | 751 (–1302 to 2881) | 72 | 751 (–1517 to 3084) |
| 1000 | 73 | 679 (–1818 to 3086) | 78 | 1035 (–1638 to 3397) | 77 | 772 (–1291 to 2885) | 73 | 769 (–1523 to 3084) |
| 1500 | 73 | 699 (–1799 to 3080) | 78 | 1013 (–1691 to 3345) | 78 | 793 (–1278 to 2885) | 74 | 787 (–1428 to 3124) |
| 2000 | 74 | 717 (–1797 to 3108) | 79 | 1058 (–1586 to 3402) | 79 | 814 (–1268 to 2899) | 74 | 806 (–1479 to 3173) |
| 2500 | 75 | 736 (–1777 to 3100) | 79 | 1081 (–1572 to 3440) | 79 | 834 (–1264 to 2911) | 75 | 824 (–1494 to 3193) |
| 3000 | 75 | 755 (–1783 to 3129) | 79 | 1103 (–1658 to 3429) | 79 | 855 (–1300 to 2917) | 76 | 843 (–1481 to 3236) |
| 3500 | 74 | 774 (–1789 to 3133) | 80 | 1126 (–1661 to 3457) | 79 | 876 (–1319 to 2986) | 76 | 861 (–1453 to 3289) |
| 4000 | 75 | 793 (–1799 to 3190) | 80 | 1149 (–1657 to 3501) | 79 | 897 (–1344 to 3055) | 76 | 879 (–1415 to 3308) |
| 4500 | 76 | 813 (–1799 to 3190) | 80 | 1171 (–1639 to 3580) | 79 | 918 (–1333 to 3102) | 76 | 898 (–1371 to 3328) |
| 5000 | 76 | 932 (–1799 to 3212) | 80 | 1194 (–1674 to 3630) | 80 | 939 (–1319 to 3128) | 76 | 916 (–1341 to 3380) |
| Value of threshold (£) | Base case | 14 days postventilation cessation | Adequate sedation | |||||
|---|---|---|---|---|---|---|---|---|
| Narrow definition | Wider definition | |||||||
| % cost-effective | Mean net benefit (95% CI) | % cost effective | Mean net benefit (95% CI) | % cost-effective | Mean net benefit (95% CI) | % cost-effective | Mean net benefit (95% CI) | |
| 0 | 71 | 640 (–1854 to 2993) | 74 | 807 (–1597 to 3153) | 75 | 871 (–1426 to 3182) | 76 | 867 (–1432 to 3300) |
| 500 | 72 | 659 (–1817 to 3022) | 75 | 828 (–1633 to 3191) | 76 | 902 (–1400 to 3213) | 76 | 900 (–1423 to 3335) |
| 1000 | 73 | 679 (–1818 to 3086) | 76 | 849 (–1636 to 3239) | 76 | 933 (–1395 to 3283) | 77 | 933 (–1414 to 3426) |
| 1500 | 73 | 699 (–1799 to 3080) | 76 | 870 (–1592 to 3284) | 78 | 964 (–1374 to 3359) | 78 | 966 (–1405 to 3472) |
| 2000 | 74 | 717 (–1797 to 3108) | 77 | 891 (–1549 to 3288) | 78 | 995 (–1342 to 3382) | 78 | 999 (–1397 to 3491) |
| 2500 | 75 | 736 (–1777 to 3100) | 77 | 911 (–1593 to 3301) | 78 | 1026 (–1313 to 3454) | 79 | 1032 (–1387 to 3545) |
| 3000 | 75 | 755 (–1783 to 3129) | 77 | 932 (–1600 to 3354) | 79 | 1056 (–1298 to 3531) | 80 | 1065 (–1386 to 3609) |
| 3500 | 74 | 774 (–1789 to 3133) | 77 | 953 (–1624 to 3407) | 79 | 1087 (–1256 to 3594) | 81 | 1098 (–1385 to 3668) |
| 4000 | 75 | 793 (–1799 to 3190) | 77 | 974 (–1616 to 3459) | 80 | 1118 (–1282 to 3664) | 81 | 1131 (–1441 to 3731) |
| 4500 | 76 | 813 (–1799 to 3190) | 78 | 995 (–1640 to 3512) | 80 | 1149 (–1312 to 3737) | 81 | 1164 (–1389 to 3815) |
| 5000 | 76 | 932 (–1799 to 3212) | 78 | 1015 (–1662 to 3587) | 81 | 1180 (–1287 to 3809) | 82 | 1197 (–1441 to 3887) |
| Value of threshold (£) | Base case | Wider NHS costs (n = 106): | ||
|---|---|---|---|---|
| % cost-effective | Mean net benefit (95% CI) | % cost-effective | Mean net benefit (95% CI) | |
| 0 | 71 | 640 (–1854 to 2993) | 64 | 496 (–2036 to 3191) |
| 500 | 72 | 659 (–1817 to 3022) | 63 | 490 (–2058 to 3177) |
| 1000 | 73 | 679 (–1818 to 3086) | 63 | 485 (–2080 to 3174) |
| 1500 | 73 | 699 (–1799 to 3080) | 63 | 479 (–2083 to 3134) |
| 2000 | 74 | 717 (–1797 to 3108) | 63 | 474 (–2096 to 3149) |
| 2500 | 75 | 736 (–1777 to 3100) | 63 | 468 (–2108 to 3165) |
| 3000 | 75 | 755 (–1783 to 3129) | 63 | 462 (–2127 to 3178) |
| 3500 | 74 | 774 (–1789 to 3133) | 63 | 457 (–2158 to 3170) |
| 4000 | 75 | 793 (–1799 to 3190) | 63 | 451 (–2166 to 3181) |
| 4500 | 76 | 813 (–1799 to 3190) | 63 | 446 (–2176 to 3180) |
| 5000 | 76 | 932 (–1799 to 3212) | 62 | 440 (–2204 to 3223) |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AR
- adverse reaction
- BNF
- British National Formulary
- BP
- blood pressure
- bpm
- beats per minute
- CEAC
- cost-effectiveness acceptability curve
- CHiP
- Control of Hyperglycaemia in Paediatric Intensive Care
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTA
- clinical trial application
- ECMO
- extracorporeal membrane oxygenation
- ED
- effective dose
- GABA
- gamma-aminobutyric acid
- GM
- general medical
- GP
- general practitioner
- HDU
- high-dependency unit
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IDSMC
- Independent Data and Safety Monitoring Committee
- IMP
- Investigational Medicinal Product
- INR
- international normalised ratio
- IQR
- interquartile range
- ITT
- intention to treat
- i.v.
- intravenous
- LoS
- length of stay
- MAP
- mean arterial pressure
- MCRN
- Medicines for Children Research Network
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MIMS
- Monthly Index of Medical Specialities
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- PELOD
- Paediatric Logistic Organ Dysfunction
- PI
- principal investigator
- PIC
- paediatric intensive care
- PICSSG
- Paediatric Intensive Care Society Study Group
- PICU
- Paediatric intensive care unit
- PIS
- Patient Information Sheet
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- Statistical Analysis Plan
- SD
- standard deviation
- SLEEPS
- Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TOST
- two one-sided tests
- TSC
- Trial Steering Committee