Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/104/16. The contractual start date was in September 2011. The draft report began editorial review in September 2014 and was accepted for publication in April 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
P Ronan O’Connell reports a contract research agreement with Medtronic to study neuromodulation in an animal model of faecal incontinence. Sandra Eldridge reports grants from Queen Mary University of London during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Horrocks et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Faecal incontinence (FI) poses a significant UK public health problem. Its prevalence is difficult to accurately assess, although the best studies estimate this at 11–15% in the adult population1 and as high as 50% in care homes. 2,3 As prevalence and severity increase with age,4 FI is expected to become a greater problem in an increasingly aged population. It is known to be an under-reported problem, with many symptomatic patients suffering in silence. 5–8 FI has a significant impact on quality of life, causing social and psychological disability,9,10 and often leads to people suffering from stigmatisation and social exclusion. 5,11,12 The attendant socioeconomic burden of FI is high not only because of the cost of health-care utilisation, but also because of job absenteeism. 13,14
Management of faecal incontinence
Management of FI is challenging because of a widespread lack of expertise, high prevalence and multiple aetiologies. Initial management involves a tailored stepwise approach, beginning with more conservative strategies (diet, toilet training and medications) and moving on to appropriate nurse-led bowel retraining programmes and psychosocial support. Combinations of these treatments often prove effective;15,16 however, many patients suffer refractory symptoms, for which the National Institute for Health and Care Excellence recommends moving to more invasive measures. 17 Depending on local expertise, surgery – for example sphincter repair, artificial sphincter, dynamic graciloplasty or a permanent stoma – may be the only option for these patients. Surgical procedures are invasive and have, at best, variable success rates with significant risk of morbidity. 18–21
Neuromodulation is a relatively new treatment modality for FI which is bridging the gap between conservative strategies and invasive surgery in centres where expertise exists. It is based on recruitment of residual anorectal neuromuscular function pertinent to continence by electrical stimulation of the peripheral nerve supply, without the need for surgery to the anus itself.
Sacral nerve stimulation (SNS) employs direct electrical stimulation to the sacral nerve roots (mainly the S3 nerve root) and is a safe, effective treatment for FI, with short-, medium- and long-term median success rates reported as 63% (range 33–66%), 58% (range 52–81%) and 54% (range 50–58%) respectively. 22–28 SNS has become the first-line surgical treatment option for FI. 17 Despite largely favourable data, SNS requires two operations and is not without risk of morbidity. 29 Although it is cost-effective compared with other surgical options,30 SNS does have high associated costs, recently estimated as £20,484 for the first 10 years of a patient’s treatment,31,32 because of the combination of equipment, hospital admission and ongoing care.
Tibial nerve stimulation in faecal incontinence
Tibial nerve stimulation is a minimally invasive neuromodulatory modality. The tibial nerve contains afferent and efferent fibres originating from the fourth and fifth lumbar and first, second and third sacral nerves. Thus, stimulation of the tibial nerve is thought to lead to similar changes in anorectal neuromuscular function as observed with SNS (owing to shared sacral root effects) but without the need for a permanent surgically implanted device. Tibial nerve stimulation (TNS) is an outpatient treatment, which can be delivered by any trained health-care professional, and is consequently much cheaper than SNS. Initially described for urinary incontinence,33 TNS was first used for FI in 2003. 34 Since then, there have been several publications regarding the use of TNS to treat FI. Two main delivery methods are described:
-
Percutaneous tibial nerve stimulation (PTNS) involves electrical stimulation via a needle placed adjacent to the tibial nerve just above the ankle. This is delivered via the Urgent® PC neuromodulation system (Uroplasty Limited, Manchester, UK). Treatment is typically delivered as 12 30-minute treatments, given either weekly for 12 weeks or twice-weekly for 6 weeks.
-
Transcutaneous tibial nerve stimulation (TTNS) involves electrical stimulation which is delivered via two-pad electrodes placed over the tibial nerve just above the ankle. This is usually delivered via a transcutaneous electrical nerve stimulation (TENS) machine. Treatment regimens vary considerably, although administration is usually in 20- to 30-minute sessions over a period of weeks or months.
The main advantage of PTNS over TTNS is the proximity of the needle to the tibial nerve, enabling higher treatment amplitude to be delivered while avoiding the painful skin sensations associated with transcutaneous treatment.
Evidence for percutaneous tibial nerve stimulation in faecal incontinence
Published studies of PTNS include nine case series34–42 (one study34 included a ‘control’ group for comparison), one small single-centre randomised single-blind trial (PTNS vs. TTNS vs. sham),43 one comparative case-matched study (PTNS vs. SNS)44 and one prospective clinical audit with a ‘pseudo’ case–control model (PTNS vs. SNS). 31 A recent review by the authors summarises the results. 45 Five publications are from the same institution and report results from an accumulating database. Interpretation and comparison of these studies is hampered by a lack of standardised and universally accepted outcome measures, observer and patient blinding, performance and interpretation bias and attrition bias.
When considering data from bowel diaries to assess treatment success (the most universally accepted method in the SNS literature), two studies39,43 reported that 63% and 82% of patients had a ≥ 50% reduction in the weekly number of faecal incontinence episodes (FIEs) immediately after treatment. Two studies reported longer-term follow-up, with 59% of patients experiencing treatment success after 1 year39 and 53% at a median of 22 months,42 based on the same outcome measure. When considering FIEs as a count, six studies1–3,6,8,9 reported this outcome, with a median reduction from five episodes to one per week immediately following treatment (a statistically significant reduction in three of these studies36,38,42) and a median reduction from six episodes to one in the two studies that reported this outcome in the longer term (at 1 year39 and at a median of 29 months42), which led to a statistically significant reduction in both.
The randomised single-centre study of PTNS versus TTNS versus sham treatment in 30 patients43 reported that 82% of patients in the PTNS group, 45% of patients in the TTNS group and 13% of those in the sham group had ≥ 50% reduction in the weekly number of FIEs immediately after treatment. This was statistically significant across all groups (p = 0.035).
In summary, the observational studies of PTNS showed improvements in most outcome measures (bowel diary, Cleveland Clinic Incontinence Score and quality-of-life measures) after treatment compared with baseline. A small three-arm RCT of PTNS versus TTNS versus sham showed effects of both treatments over sham, with PTNS appearing superior. 45
It seems that PTNS may offer a low-cost (estimated at £5916 for the first 10 years based on 6-monthly ‘top-up’ sessions)31 minimally invasive outpatient technique with almost no associated morbidity. 46 If this is true, and PTNS offers similar efficacy to SNS, PTNS may be considered as a genuinely new option in the pathway between conservative management and the more invasive surgical procedure of SNS.
Limitations of the current published evidence for percutaneous tibial nerve stimulation in faecal incontinence
To our knowledge, no double-blind placebo-controlled trial of PTNS in patients with FI has been performed. Thus, notably, the effect of PTNS in FI, over and above that of meetings with a nurse specialist alone, remains unknown. This is important because:
-
PTNS is available in several centres in the UK and, although much cheaper than SNS, there is still a cost associated with its use. Increasing numbers of centres are using it, but many are doing so with speculation that it is little more than an expensive form of acupuncture.
-
The possible placebo effect of PTNS should not be underestimated:
-
High placebo responses are almost universally observed in trials of therapy for functional47,48 and organic48,49 colorectal diseases.
-
Therapeutic responses have been achieved by acupuncture alone in FI,50 noting that the medial ankle is an established acupuncture site for the viscera (‘sanyinjiao’ or ‘spleen 6’).
-
Regular meetings with a specialist nurse may confer some benefit even without formal bowel retraining. 51
-
-
The influence of unblinded observers, especially when interpreting bowel diary data, is also a potential source of bias.
Study aims
We aimed to assess the clinical effect of PTNS, compared with sham electrical stimulation, in the treatment of patients with significant FI in whom conservative management strategies have already failed.
We also planned to test the effect of PTNS versus sham electrical stimulation on:
-
improvements in validated incontinence scores
-
patient-centred FI-related symptoms
-
disease-specific and generic quality-of-life measures.
Hypothesis
A 12-week course of PTNS results in a clinical response rate of 55% compared with a sham response rate of 35%, with clinical response defined as a reduction in the weekly number of FIEs of ≥ 50%.
Chapter 2 Methods
Overview: study design
The CONFIDeNT (CONtrol of Faecal Incontinence using Distal NeuromodulaTion) study was a UK-based multicentre, pragmatic, parallel-arm, double-blind, randomised controlled trial comparing PTNS with sham electrical stimulation, with equal allocation, stratified by sex and centre, in the treatment of FI, and assessing outcomes following a standard 12-week treatment schedule. The detailed trial protocol is available to view online (www.nets.nihr.ac.uk). The study method is summarised in a flow diagram (see Appendix 1). Events occurring at each visit are detailed in Appendix 2. All case report forms used can be seen in Appendix 3.
Study outcomes
Clinical outcomes
These were assessed at baseline (prior to therapy) and 2 weeks following completion of a 12-week course of treatment. Clinical outcomes were derived from 2-week bowel diaries and a series of validated, investigator-administered questionnaires.
Primary
Responder versus non-responder: responder defined as a patient achieving ≥ 50% reduction in total FIEs per week, as recorded on a 2-week self-completed bowel diary.
Secondary
-
Percentage change in FIEs per week (i.e. patients achieving ≥ 25%, ≥ 75% or 100% reduction in weekly FIEs).
-
Change in FIEs per week as a continuous measure.
-
Change in symptom severity score: St Mark’s Continence Score (SMCS). A score from 0 (best) to 24 (worst) with > 5 indicating significant symptoms. 52
-
Change in disease-specific quality-of-life scores:
-
Change in general quality-of-life measures: Short Form Questionnaire-36 items (SF-36). 55 A score with eight domains with scores given as percentages.
-
Change in patients’ health status and overall health using European Quality of Life-5 Dimensions (EQ-5D)56 questionnaire.
-
Change in patient-centred outcomes questionnaire. A derivative of the International Consultation on Incontinence Modular Questionnaire – Bowel57 with a score from 1 (best) to 80 (worst).
-
Likert scale of patients’ global impression of success (scale of 0–10).
-
Qualitative data:
-
patient-perceived impression of change in use of incontinence pads and constipating medications
-
patient-perceived impression of change in urinary symptoms
-
patient impression of the treatment in general
-
patient-perceived allocation (PTNS or sham).
-
Other outcomes recorded at each visit:
-
stimulation parameters
-
adverse events and concomitant medications.
In addition to this, patients completed a bowel diary after six treatments and this formed a further secondary outcome.
Clinical centres
Centres with specialist expertise in FI, including nurse-led (or equivalent) incontinence services, were invited to participate in the study. Centres had to demonstrate experience with PTNS, having previously completed a full set of 12 treatments in a minimum of three patients. Each centre also required a minimum of two staff members to run the trial and ensure satisfactory blinding.
Study population
All adult patients attending the specialist continence or pelvic floor clinics at each of the centres were considered for participation in the study. This included patients with FI symptoms sufficiently severe to warrant intervention and in whom appropriate conservative therapies, such as diet, pelvic floor exercises, biofeedback and loperamide, had failed. Specialist investigations including structural and functional anorectal assessment were not mandatory, and anal sphincter injury was not a contraindication.
Inclusion criteria
-
Faecal incontinence sufficiently severe to warrant intervention (as recommended by the principal investigator at each site).
-
Failure of appropriate conservative therapies.
-
Age ≥ 18 years.
Exclusion criteria
-
Inability to provide informed consent for the research study.
-
Inability to fill in the detailed bowel diaries required for outcome assessments (this will exclude participants who do not speak/read English).
-
Neurological diseases, such as diabetic neuropathy, multiple sclerosis and Parkinson’s disease (including any participant with painful peripheral neuropathy).
-
Anatomical limitations that would prevent successful placement of needle electrode.
-
Other medical conditions precluding stimulation, for example bleeding disorders, certain cardiac pacemakers, peripheral vascular disease or ulcer, lower leg cellulitis.
-
Congenital anorectal anomalies or absence of native rectum as a result of surgery.
-
A cloacal defect.
-
Present evidence of external full-thickness rectal prolapse.
-
Previous rectal surgery (rectopexy/resection) done < 12 months prior to the study (24 months for cancer).
-
Stoma in situ.
-
Chronic bowel diseases such as inflammatory bowel disease leading to chronic uncontrolled diarrhoea.
-
Pregnancy or intention to become pregnant.
-
Previous experience of SNS or PTNS.
Following in-depth discussion with the research ethics committee, it was decided that, as some of the outcome questionnaires had not been validated in languages other than English, we should exclude people who do not understand written or spoken English from the study.
Data collection
We planned that each patient should attend for 14 visits and the events that occurred at each visit were as follows.
Visit 1: interest – eligibility
At this appointment, or over the telephone, a local researcher trained in good clinical practice determined eligibility by interview on the basis of defined inclusion and exclusion criteria listed on case report form (CRF) 1. The participants’ details were recorded on the screening log, and each participant was allocated a unique participant identifier number (see below). These data were used to complete the Consolidated Standard of Reporting Trials (CONSORT) flow chart and to generate reports on non-recruited patients for discussion at management group meetings.
Eligible subjects were provided with adequate explanation of the aims, methods, expected benefits and risks of participating in the study and given a patient information sheet containing this information. Participants were allowed 1 week to consider their participation (in accordance with good clinical practice). Participants who remained interested were provided with a bowel diary to complete over the next 2 weeks. Each participant was counselled on how to fill this diary in. Appointments were then booked for visits 2–14, with visit 2 being at least 2 weeks later to allow time for diary completion.
Unique participant identifier codes
Once a participant was registered on the screening log, he or she was allocated a unique participant identifier code. This consisted of six characters: three letters followed by three numbers. The letters denoted the study centre code, and the number was allocated on a consecutive basis, for example 001 for the first participant, and so on.
Visit 2: consent – confirm eligibility – baseline assessment – randomisation – first intervention
At this appointment, a member of the local team (trained in informed consent) answered any further questions and then asked the participant to sign the study consent form (also countersigned by the local researcher). All prospective participants were reminded of the need to be logistically able to complete the full protocol of 12 sessions at weekly intervals. Once the consent form was signed, the local investigator confirmed eligibility by recording data on CRF 1. If the participant was a female of childbearing potential, a urine pregnancy test was performed at this point.
The researcher then recorded all baseline data of FI history, past medical history and medication usage (using CRF 2 – initial assessment). The participant was asked to fill in the questionnaires (CRF 3) and to hand in the completed bowel diary, which was checked for completeness. Prior to randomisation the consent form, eligibility criteria (CRF 1) and initial assessment (CRF 2) were verified by another member of the research team.
Participants who failed to complete the bowel diary properly were given another 2-week bowel diary to complete and returned 2 weeks later for the trial to commence. If they failed a second attempt, they became a screen failure, and were withdrawn. Another participant was recruited in their place.
The researcher performed the randomisation, recorded this information on CRF 4 and (now unblinded) delivered the first 30-minute intervention (real PTNS or sham). Parameters of stimulation were recorded (CRF 5). The participant’s details were entered on the enrolment log, and a general practitioner (GP) letter, informing the GP of the participant’s involvement in the trial, was sent out.
Visits 3–13: intervention – interim information
At appointments 3–13, an unblinded researcher (who might be the same person as in visit 2) delivered the 30-minute intervention, having checked CRF 4 to confirm randomisation allocation. They enquired about adverse events, concomitant medication usage and pad usage, and recorded these on CRF 5.
At visit 7, participants were given a 1-week interim bowel diary to complete between visits 7 and 8. This bowel diary was collected and checked at visit 8.
At visit 13, participants were given a 2-week bowel diary to complete prior to attending visit 14, 2 weeks later.
Visit 14: final study visit
The final study visit was performed by a blinded member of the research team (i.e. somebody who was not present at visits 2–13). At this appointment, the bowel diary was collected and checked for completeness. The participant was then asked to complete the questionnaire document (CRF 3) and the post-treatment questionnaire (CRF 6).
The researcher then ensured that all documents were present and filled in correctly, prior to the principal investigator completing and signing off CRF 7. The participant was then unblinded as to treatment allocation and further follow-up was arranged as necessary.
Participants who failed to complete the interim bowel diary between visits 7 and 8 attempted this again the following week, and this was recorded as a protocol deviation. Participants who failed to complete the final bowel diary were again asked to complete this after visit 14, and they returned for another final study visit 2 weeks later. This was also a protocol deviation.
After completion of trial
After visit 14, participants who received ‘sham’ stimulation were offered PTNS on an open-label basis. Participants who received real PTNS and who derived significant benefit were offered ‘top-up’ sessions as per local departmental protocols. Participants who received real PTNS but derived no significant benefit were offered further treatments on an ‘open-label’ basis, following local departmental protocols.
Study procedures: delivery of percutaneous tibial nerve stimulation or sham
Participants received PTNS or sham using the recommended standard of 12 weekly 30-minute outpatient stimulations. Appendix 4 details exactly how PTNS and sham electrical stimulation were administered. Treatments were tailored to participants’ needs but protocol tolerance stipulated a minimum of 10 treatments, no fewer than 5 days or greater than 10 days apart, to be completed in 13 weeks. Treatments given outside these windows were classed as a protocol deviation.
Percutaneous tibial nerve stimulation was delivered via the Urgent® PC neuromodulation system, a reusable external pulse generator that provides visual and auditory feedback. It has an adjustable current setting from 0 to 9 mA in pre-set 0.5-mA increments, a fixed-pulse frequency of 20 Hz and a pulse width of 200 microseconds.
Transcutaneous electrical nerve stimulation was used for the delivery of sham electrical stimulation (Biostim M7 TENS unit, Biomedical Life Systems, Vista, CA, USA). The sham treatment was a modification of that used and validated in the pivotal level I trial of Peters et al. 58 in overactive bladder (OAB) syndrome. 59 However, this was improved upon by inserting (at the same site) the Urgent® PC needle in all subjects. In the Peters study, the Urgent PC® needle was used in the PTNS arm, but the sham arm employed an acupuncture technique using a Streitberger needle, which does not puncture the skin.
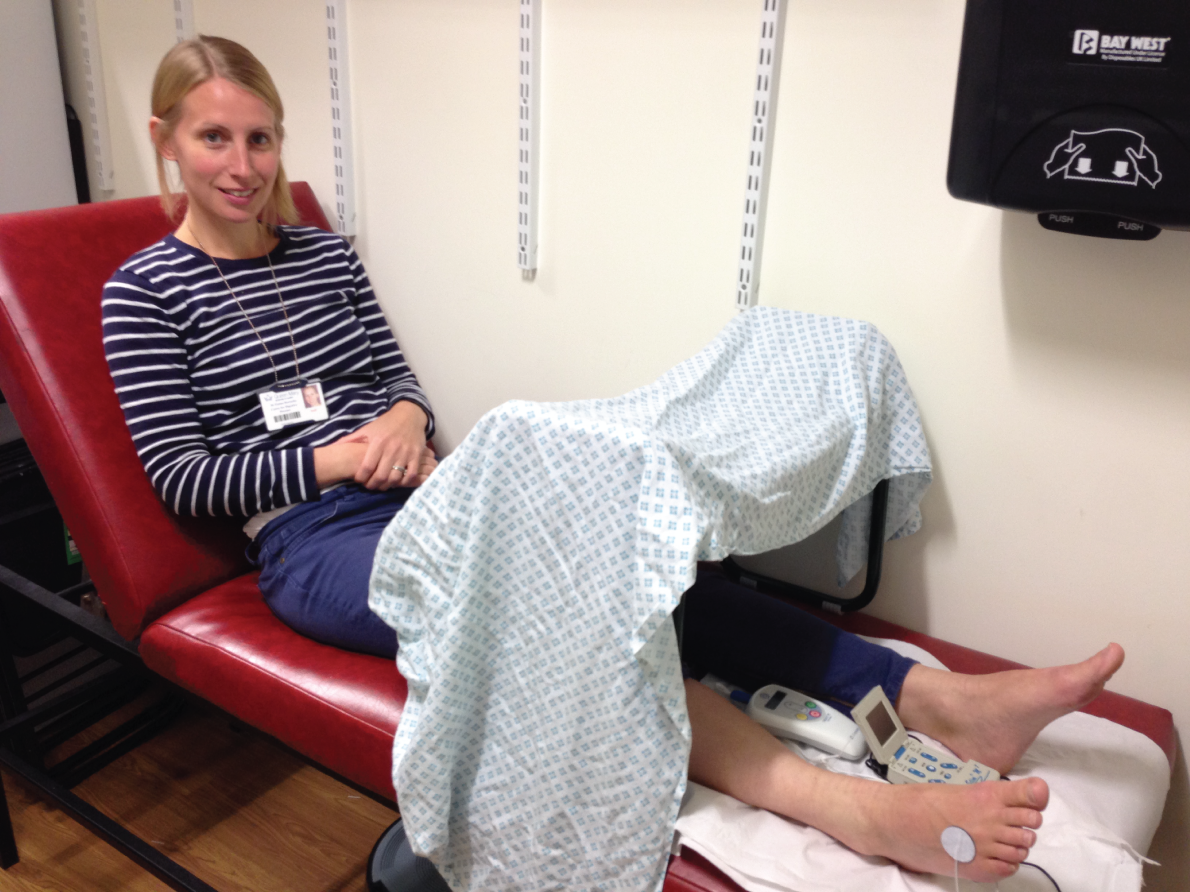
Treatments were always given in individual treatment rooms, with participants lying supine on a clinical couch. They were asked to remove clothing and shoes so as to bare legs from the knees downwards. A ‘gardener’s kneeling stool’ was placed over both legs, just below the knees, and a sheet draped over this to hide the participant’s feet from their view (Figure 1). Once the participant was comfortable, but prior to equipment set-up, each researcher read a standardised paragraph to the participant, informing them of what to expect. This read:
I am now going to start the nerve stimulation treatment. I will be inserting a small electrode needle, like an acupuncture needle, into your leg and putting sticky electrodes onto your foot. When I turn the machine on you will be asked when you can first feel an electrical sensation in your ankle or foot. I will carry on increasing the intensity of this until it is slightly uncomfortable, then I will turn it down a little if necessary. Occasionally you may also feel numbness or slight movement of your toes. This is normal. I will set the machine up and leave it running for 30 minutes. You may or may not continue to feel the stimulation during this time – this is normal also. After 30 minutes have elapsed I will remove the needle and sticky electrodes (the machine automatically turns off at this time). If the treatment becomes uncomfortable at any point please let me know and I will turn it down or stop the machine.
FIGURE 1.
Photographs of equipment set-up.
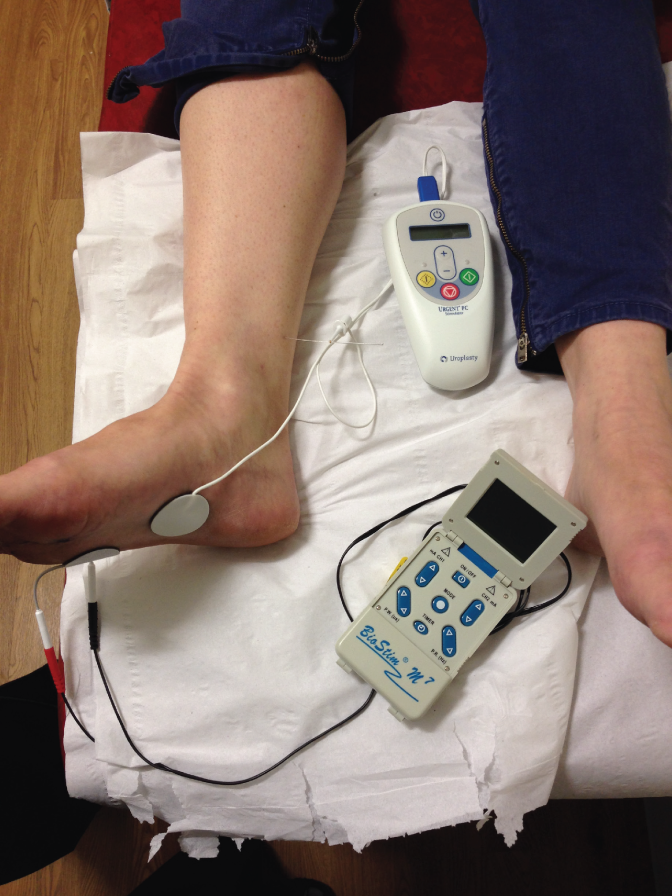
All participants then had an Urgent® PC machine and a TENS machine set up on their right foot, unless there was a reason why the right foot could not be used, under which circumstances the left foot was used. In the true PTNS arm, the Urgent® PC was used as normal, and the TENS machine left turned off. In the sham arm, the TENS machine was used to provide the electrical stimulation and the Urgent® PC was turned on only to provide the auditory stimulus. Following satisfactory treatment commencement, the sheet was draped fully over the participant’s feet, ensuring that accidental unblinding could not take place. The researcher then filled in the paperwork for this visit and left the room, returning after 30 minutes to remove the equipment.
Treatment arm
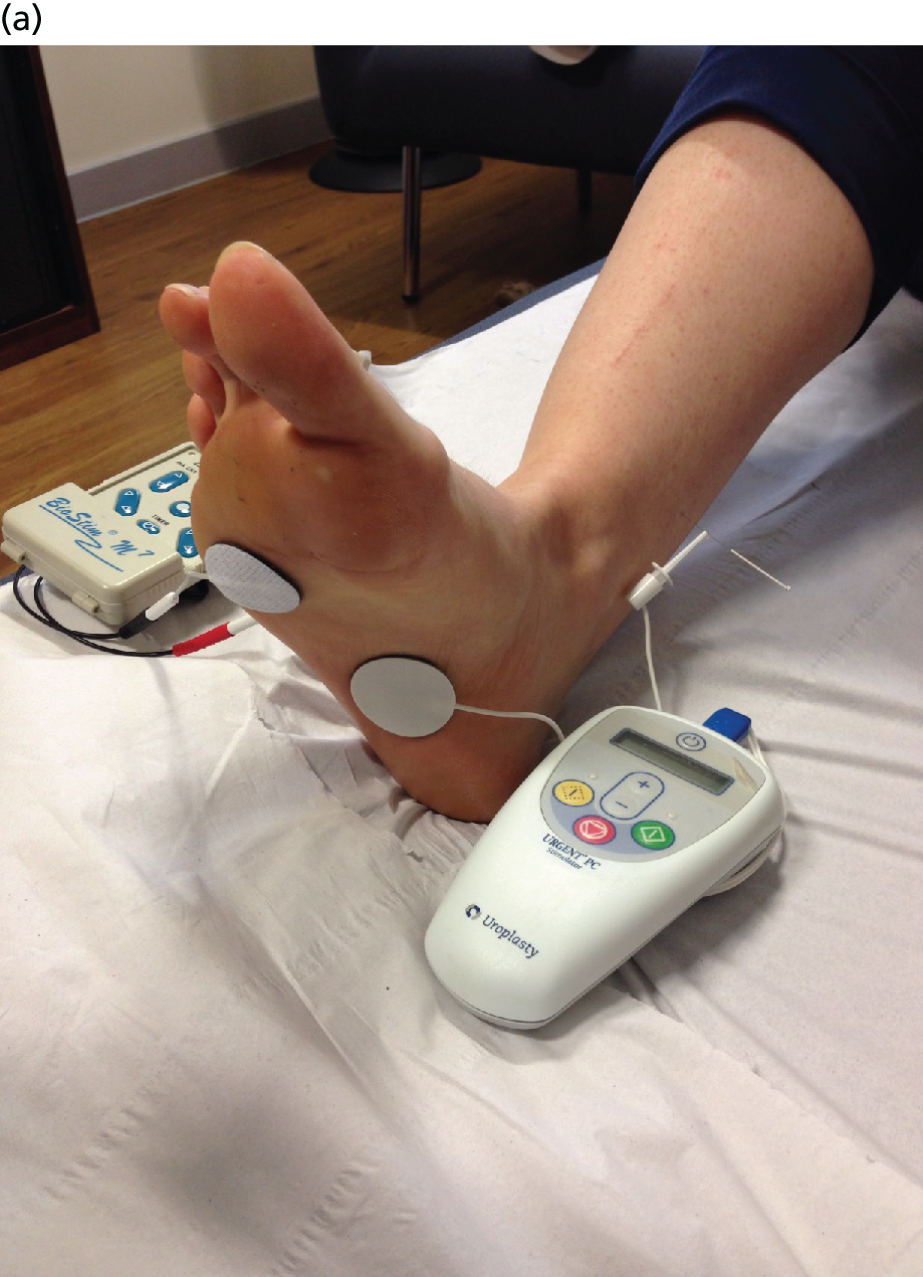
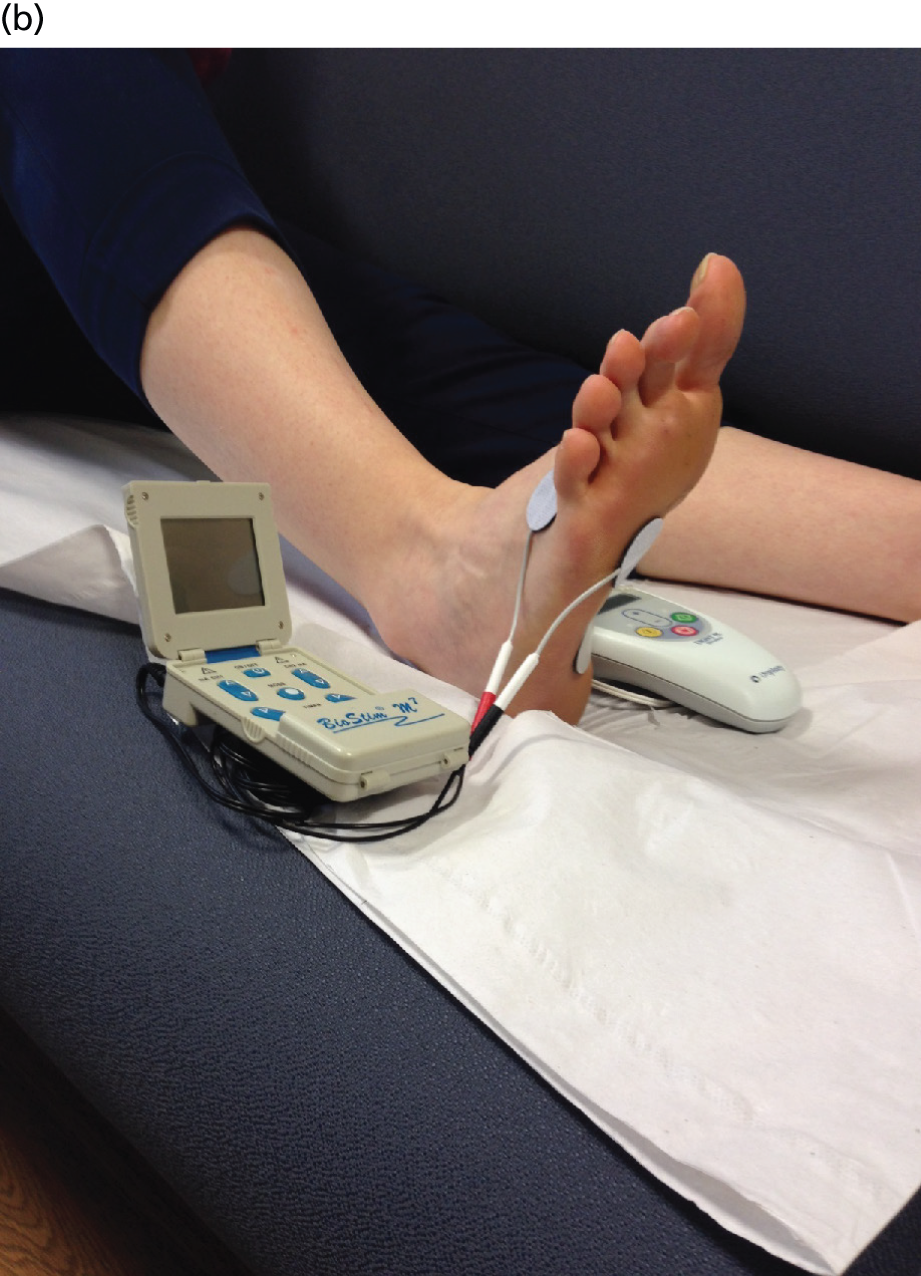
After checking equipment, which should have included the Urgent® PC machine, lead wire, alcohol wipe and electrode needle with tube assembly, the site of needle insertion was identified on the lower inner aspect of the right leg approximately three finger breadths (5 cm) cephalad to the medial malleolus and approximately one finger breadth (2 cm) posterior to the tibia. The area was cleaned with ethanol and the needle electrode–guide tube assembly placed over the identified insertion site at a 60° angle between electrode and ankle. The 34-gauge needle electrode was gently tapped to pierce the skin and thence advanced using a rotating motion approximately 2 cm. The lead wire was then connected to the stimulator and to the ipsilateral calcaneal reference electrode (Figure 2a). The lead wire was then taped to the participant’s leg so that the PTNS participant experienced the same sensations as the sham participant. The TENS machine was connected to two electrodes, one placed under the little toe and one on top of the foot (Figure 2b). The TENS machine was not turned on. The setting for PTNS therapy was determined by increasing the current slowly while observing the participant’s sensory response (appropriate response being in great toe or sole of foot) or motor response (plantar flexion of foot or great toe). Current was then reduced by one level for therapy, and continued for 30 minutes, at which point the electrode was removed.
FIGURE 2.
Equipment set-up. (a) PTNS needle and calcaneal electrode and (b) TENS surface electrode placements.
Sham arm
The same protocol was followed as for the treatment arm. The only difference was that the needle was inserted only 2 mm into the skin and subcutaneous tissue, that is just in far enough not to fall out and not deep enough to be close to the tibial nerve. The lead was then taped to the participant’s leg near to, but not touching, the needle. The purpose of this was to prevent unblinding in the event of the participant inadvertently seeing the equipment. The PTNS surface electrode (see Figure 2a) on the calcaneus was also attached. The two active TENS surface electrodes were employed as shown in Figure 2b, with one placed under the little toe and one on top of the foot. Once all equipment was set up, the practitioner picked up both the Urgent® PC machine and the TENS machine, one in each hand. Both machines were turned on. The TENS machine was set to a pulse frequency of 10 Hz and a pulse width of 200 microseconds. Then, after pressing buttons simultaneously on the Urgent® PC machine and the TENS machine, the practitioner increased the adjustable current setting (which ranged from 0 to 10 mA in pre-set 1-mA increments on the TENS machine). The setting for therapy was determined in the usual way by observing the participant’s sensory reactions or their foot for toe/ankle extensor motor responses, and if necessary the current was reduced by one level for therapy. The reason both machines were used together was so that the audible sounds produced by the Urgent® PC stimulator were the same in both the PTNS and the sham arms, to decrease auditory variation between the study arms.
This sham treatment was shown in a departmental pilot to be both more acceptable and more realistic than that described by Peters et al. ,58 which involved the placement of a Streitberger needle. We also confirmed that this sham, using TENS to deliver the electrical stimulation, does not stimulate the posterior tibial nerve (proven in a neurophysiological pilot by the consultant neurophysiologist).
Treatment quality control
The importance of quality control and standardisation of technique between individuals and centres was recognised. In order to keep the quality high, each researcher was taught and certified to give PTNS by a uroplasty-approved trainer. Each researcher also underwent a personal training session at the site initiation visit by the trial research fellow (EH) on how to deliver PTNS and sham according to the CONFIDeNT protocol. Each researcher was then observed delivering both treatments. Six-monthly site visits throughout the duration of the trial involved assessment of technique. Retraining was undertaken where necessary.
Withdrawal criteria
Participants were withdrawn from the treatment or the trial if they fulfilled any of the criteria below at any point following delivery of the first treatment.
Withdrawn from treatment only (follow-up data still collected)
-
Participant no longer wished to be involved in trial treatments.
-
Participant developed a medical condition listed in the exclusion criteria.
-
Participant became pregnant or intended to become pregnant.
-
Unblinding occurred.
-
Participant had an intercurrent illness.
Withdrawn from the trial (no follow-up data collected)
-
Participant was lost to follow-up (could not be contacted by telephone or other means).
-
Participant no longer wished to be involved in the trial.
-
Death.
Early withdrawal was documented carefully and all participants were followed up in the NHS in the usual way. In the case of each participant who withdrew, permission was sought to use the data that had already been collected.
Randomisation
Participants were randomised, with allocation concealment, using a bespoke web-based computer program held at Nottingham Clinical Trials Unit. Each centre randomised its own participants to receive either PTNS or sham following baseline data collection and immediately prior to delivery of the first treatment. The computer program required the researcher to input the unique participant identifier code, sex and date of birth, and immediate on-screen randomisation occurred. Allocation was on an equal basis with initial stratification by sex and then stratification of females by centre. Stratification by sex was used to reduce the potential confounding effects of variation in outcomes between male and female participants. As males represent only 10% of patients and only one or two male participants were expected from each centre (owing to differing pathophysiologies60), randomisation stratified on centre would increase the probability that all the males were allocated to PTNS or sham by chance. To avoid this situation, only females were stratified by centre, achieving a near balance of PTNS and sham arms and allowing comparability by centre.
Blinding
Blinding of participants
Participants were blinded to allocation, but had knowledge of the 50% chance of receiving sham treatment. For both PTNS and sham interventions (1) a standardised description of the technique was read from a card prior to each treatment, which described what the patient should expect – an electrical sensation variably in the ankle or foot with or without motor responses in the foot (note: there is significant variability in conscious sensation and motor responses even between participants undergoing only PTNS); (2) the lower extremity was draped from view, ensuring participants had no knowledge of equipment set-up; and (3) the audible sounds present during PTNS and sham treatments were identical.
Performance bias considerations
In order to avoid either arm receiving more advice or reassurance, the interaction of the administering researcher was standardised and limited to a general welcome, addressing any concerns (while recording adverse events) and answering questions regarding loperamide dosages and incontinence pad use (both recorded in outcome variables). The standardised description of the technique (as stated above) was read to the participant, the equipment set up and fully covered and then participant left to receive the 30-minute treatment.
Blinding of trial staff
At least two researchers were available at each site to run the study, one of whom performed the randomisation and all treatments, and was necessarily unblinded, while the other remained blinded and carried out the final data collection. Blinding and unblinding procedures are detailed in Appendix 5.
Sample size calculation
Data published at the time of sample size calculation35,39,46,61 and our own data36 on 50 patients suggested a 60% success rate for PTNS based on our chosen primary outcome measure. There were no RCT data for PTNS in FI; however, the pivotal level I SumiT trial of PTNS in OAB symptoms,58 which used a similar global response assessment of urinary incontinence and intention-to-treat analysis, observed a moderate or marked improvement in symptoms in 55% in the PTNS arm and only 21% in the sham arm. On the basis that placebo responses are frequently higher for bowel than for bladder symptoms,47–49 we selected a sham response rate of 35% while keeping the more conservative estimate of treatment response of 55% (the difference of 20% we believe remains clinically important in relation to other therapies such as SNS). In total, 212 participants were required to detect this difference with 80% power at the 5% significance level. We expected to screen 235 participants at baseline to allow for a 10% failure to attend for randomisation, baseline data collection and first treatment.
Statistical methods
Statistical methods are detailed in the statistical analysis plan (see Appendix 6). This document was drawn up by the trial statisticians and reviewed by the Trial Steering Committee (TSC) and Data and Safety Monitoring Committee (DSMC), and received formal sign-off from both committees prior to unblinding and analysis.
The analysis was carried out using Stata version 12.1 (StataCorp LP, College Station, TX, USA), interfacing with Realcom Impute (2007, Centre for Multilevel Modelling, University of Bristol, Bristol, UK), which was used to multiply impute missing outcome and baseline covariate data. 62
All patients randomised who received the first treatment were included in the intention-to-treat analysis of the primary end point. Those in whom post-treatment data were unavailable at 14 weeks for any reason (loss to follow-up or failure to complete treatment) had their outcome multiply imputed under the assumption of data missing at random using variables prognostic of outcome, such as measure of outcome made at baseline, and others predictive of ‘missingness’ (i.e. the reason it is missing) such as mean number of FIEs per week at baseline, age, sex and, where available, mid-study bowel diary data. The numbers of variables included in each imputation model were limited by the relatively small number of study centres. Multilevel multiple imputation was performed using the multivariate normal distribution in Realcom Impute, using treatment allocation, patient sex and allocation as auxiliary variables. After a burn-in of 1000 runs of the Monte Carlo Markov chain sampler, missing values were filled every 500th run to create a total of 10 completed data sets for analysis. The data were analysed in Stata and the results pooled by Rubin’s rules. 62
The final analysis was adjusted for variables that were selected prior to data extraction. A decision was made to fit fixed effects for sex, randomisation and baseline level of outcome and to fit a random effect for study centre. In order to handle potential clustering effects of patients within centre, the intracluster correlation coefficients (ICCs) and their 95% confidence intervals (CIs) for the outcomes by centre were estimated using the user-contributed Stata command sea_obi, which allows the ICC to be negative. 63 Random-effects models were fitted by restricted maximum likelihood estimation (e.g. xtmixed. . ., reml). For outcomes with a negative ICC, linear regression models were fitted (without clustering) using the regress command.
For binary outcomes, logistic mixed-effects models were used, adjusting for baseline mean number of FIEs per week and sex and with a random effect for study centre. Estimates from these models are presented as adjusted odds ratios with 95% CIs. For continuous outcomes, linear mixed-effects models were used, adjusting for baseline measure of outcome and sex, and with a random effect for study centre. Estimates from these models are presented as adjusted difference in means.
Per-protocol analysis was carried out for all outcome measures to include those patients who received a full course of treatment as per the protocol, that is at least 10 treatments in 13 weeks that were no fewer than 5 and no more than 10 days apart. Sensitivity analyses were performed for all outcome measures, excluding any patients who had reported no episodes of FI in their 14-day baseline bowel diary, and excluding those centres that had randomised fewer than five patients.
Subgroup analyses for the primary outcome, fitting an interaction term between the categorical variable defining the subgroups and the randomisation variable, were performed, as follows:
-
males versus females
-
severity of FI (those with ≥ 7 weekly FIEs vs. those with < 7 weekly FIEs)
-
age (< 40 years, 40–60 years and > 60 years)
-
type of FI (both urge and passive, urge only or passive only).
Ethical arrangements and research governance
This trial was granted ethical approval in June 2010 (Research Ethics Committee reference 10/H0703/25).
The trial was conducted in compliance with the principles of the Declaration of Helsinki (1996),64 and in accordance with all applicable regulatory requirements including but not limited to the Research Governance Framework for Health and Social Care,65 trust and research office policies and procedures, and any subsequent amendments. The trial was compliant with the approved protocol and research ethics committee conditions of approval, and in line with good clinical practice guidelines. 66
Information regarding study participants was kept confidential and managed by each study site in accordance with the Data Protection Act,67 NHS Caldicott Guardian Agreements,68 The Research Governance Framework for Health and Social Care65 and research ethics committee approval.
Important changes to protocol after study commencement
Following study commencement, two amendments were made to the protocol, one major and one minor, but with no change to the study intervention. The following were amended:
-
The post-treatment information questionnaire (CRF 6) was amended following recommendation by the TSC. It suggested that the recording of week-by-week incontinence pad and loperamide usage was neither satisfactory nor accurate, and that this information would be better captured by questionnaire at the end. Thus, two extra questions were added to the final questionnaire.
-
Cleveland Clinic Incontinence Score was updated to the SMCS. This was used throughout but misnamed in the original protocol.
-
Clarification was added to the protocol to include details of the per-protocol analysis criteria.
-
Statistical analysis section was updated:
-
Multiple imputation method for handling missing outcome data rather than the last value carried forward method was included on recommendation from the Health Technology Assessment, as this is the widely accepted standard.
-
Regression models fitted to estimate treatment effect were changed from fixed centre effects to random centre effects. 69
-
-
Centre eligibility criteria were updated to remove the absolute requirement of a minimum of five participants recruited, following discussion with the TSC that this was an arbitrary and unnecessary requirement.
Trial oversight
The trial was under the auspices of the chief investigator and the pragmatic clinical trials unit at Barts and The London School of Medicine and Dentistry. The project was overseen by a TSC.
The TSC had an independent chairperson, and met every 6 months throughout to provide overall supervision and ensure the trial was conducted to the rigorous standards set out in the Medical Research Council’s guidelines for good clinical practice. 66 Specifically, the TSC’s role was to ensure:
-
that the views of users and carers were always taken into consideration
-
the scientific rigour of the study and adherence to protocol
-
that project milestones were met
-
that expertise/advice was provided to the Trial Management Group (TMG).
Membership of the TSC was:
-
senior statistician – Sandra Eldridge
-
independent chairperson – Professor Christine Norton, Professor of Nursing (King’s College London)
-
independent external member – Professor Ronan O’Connell, clinical and research expertise in lower gastrointestinal neuromodulation (University College Dublin)
-
patient and public involvement representative – Deborah Gilbert, chief executive (Bowel & Cancer Research charity).
The TMG was responsible for day-to-day project delivery in each participating centre. It met monthly and was answerable to the TSC. The group was responsible for overseeing and managing:
-
trial recruitment and retention rates
-
site initiation, training, monitoring, compliance and correction/preventative actions
-
data management (collection, quality control, entry and query management)
-
adverse and serious adverse event (SAE) reporting
-
study milestones
-
study reporting
-
budget expenditure and accruals.
The TMG comprised:
-
chief investigator – Charles Knowles
-
academic clinical fellow – Emma Horrocks
-
trial manager – Natasha Stevens
-
trial statistician – Stephen Bremner.
A DSMC was appointed to monitor unblinded comparative data and make recommendations to the TSC. The DSMC initially met together with the TSC, and subsequently 2 weeks prior to the TSC to enable any findings/recommendations to be submitted to the TSC. DMSC meeting timings and conclusions can be seen in Appendix 7. A DAMOCLES DSMC charter70 was adopted (see Appendix 8), and an independent pragmatic clinical trials unit statistician provided the DSMC with an unblinded comprehensive report prior to each meeting.
The DSMC comprised:
-
independent lead – Professor Dion Morton, Professor of Surgery, University of Birmingham
-
independent member – Professor Elaine Denny, Professor of Health Sociology, University of Birmingham
-
independent statistician – Dr Daniel Altmann, Senior Lecturer in Medical Statistics, London School of Hygiene & Tropical Medicine, University of London.
Patient and public involvement
Patient and public involvement was considered at all stages of this trial from conception to dissemination. This is described in detail in Appendix 9.
Chapter 3 Results
Participant flow
The CONSORT diagram shows the flow of participants through the trial (Figure 3). Non-completing participants either withdrew from treatment (and remained in the trial) or withdrew from the trial (in which case no further data were collected from them). Permission was, however, sought to use the data that had already been collected.
FIGURE 3.
Flow of patients through the study. a, See eligibility criteria box; b, withdrawal from treatment only.
Trial recruitment
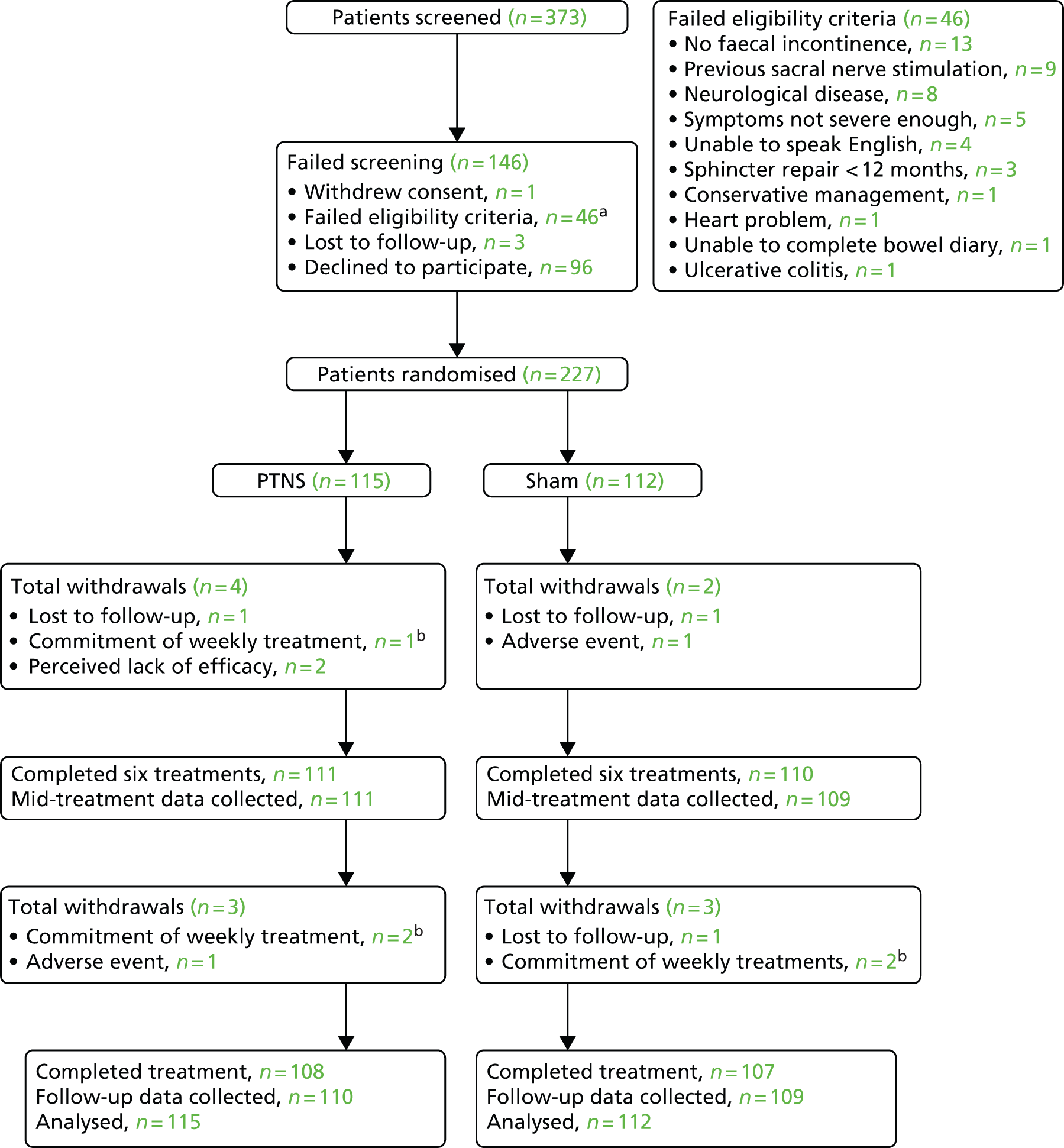
Seventeen of the 18 UK centres recruited participants for the trial between 23 January 2012 and 31 October 2013. The remaining centre was unable to participate because of staff shortages. Trial centres were Barts Health NHS Trust, London; Aintree University Hospitals NHS Foundation Trust, Liverpool; University Hospital Southampton NHS Foundation Trust, Southampton; Sandwell and West Birmingham NHS Trust, Birmingham; Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield; The Community Specialist Colorectal Clinic, Ching Way Medical Centre, London; Leicester General Infirmary, Leicester; Queen’s Medical Centre, Nottingham; Castle Hill Hospital, Hull; University College Hospital, London; Bristol Royal Infirmary, Bristol; St Mark’s Hospital, London; Guy’s and St Thomas’ Hospital, London; Poole Hospital NHS Foundation Trust, Poole; Leeds Royal Infirmary, Leeds; Pilgrim Hospital, Boston, Lincolnshire; and University Hospital of South Manchester, Wythenshawe. Centre recruitment rate is shown in Figure 4.
FIGURE 4.
Recruitment of sites.
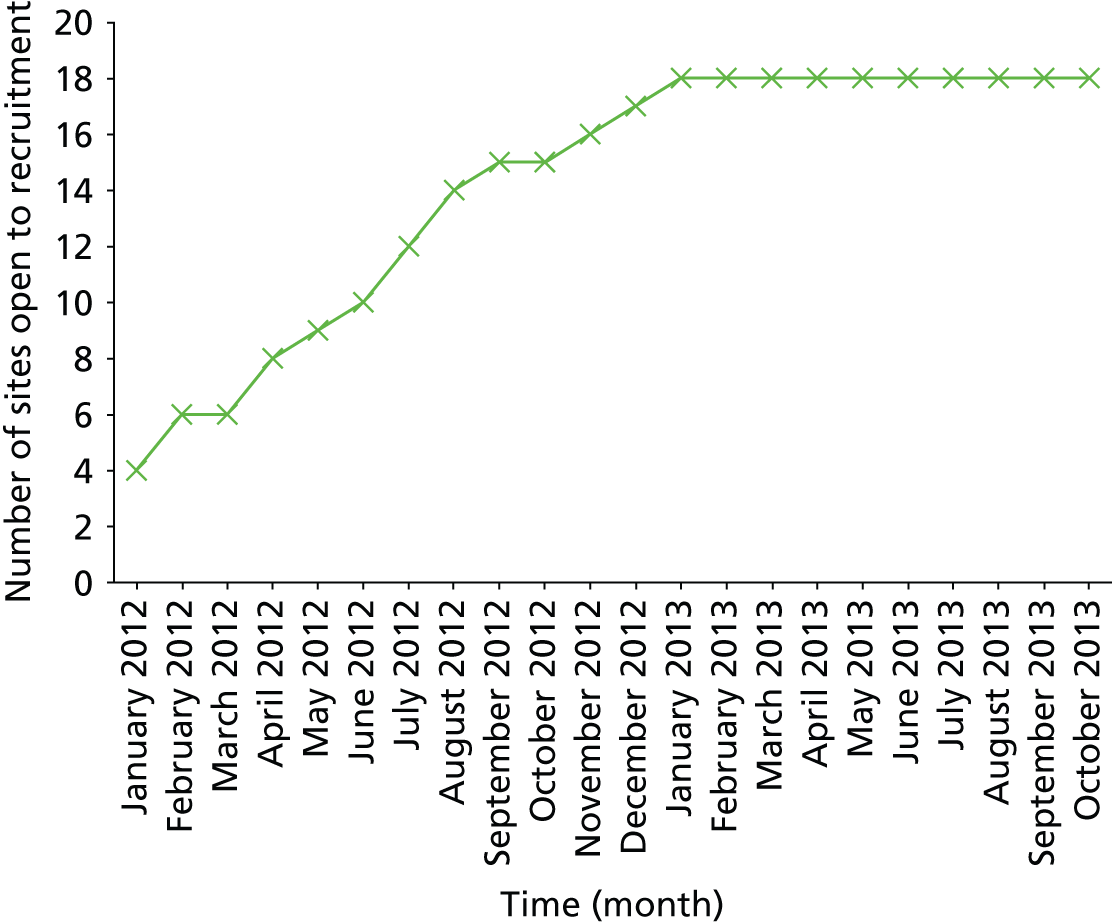
In total, 373 participants were screened and, of these, 227 (61%) were randomised. The overall recruitment rate is shown in Figure 5. The number of participants per site ranged from 1 to 45. There were 12 participant withdrawals: seven from the trial and five from treatment (see Figure 3).
FIGURE 5.
Participant recruitment.
Data quality
Data return was generally very high and quality was very good. Data from bowel diaries were 97.7% complete. This was probably a consequence of bowel diary training for each patient prior to completing the diary and the vigilant checking of bowel diaries on return. Questionnaire completion was also very good (mean 90.4%, range 77.6–100%). For all data, percentages were calculated from the corrected denominator; however, as data return was so high, individual values for number of patients for each outcome have not been recorded in the tables. These are available in Appendix 10.
Baseline data
In total, 227 participants were randomised: 115 to receive PTNS and 112 to receive sham electrical stimulation.
Baseline findings
Ninety per cent of participants were female, with a mean age of 57 years (range 20–85 years). Mean symptom duration was 8 years (range 5 months to 50 years). Baseline demographics and clinical data are summarised in Table 1. Previous treatments and relevant past medical history are summarised in Tables 2 and 3 respectively. Complete lists of past medical history and regular medications are presented in Appendices 11 and 12. Demographics of the two arms were evenly matched for age and sex as per stratification. Of note, approximately 40% of participants appeared to have concomitant symptoms of FI and evacuatory difficulties (39% in PTNS arm and 44% in sham arm), and approximately 60% had concomitant urinary symptoms (61% in PTNS arm and 64% in sham arm).
| Outcome | PTNS | Sham |
|---|---|---|
| Sex (female), n (%) | 104 (90) | 101 (90) |
| Age (years), median (IQR) | 58 (50–67) | 58 (48–65) |
| Duration of symptoms (months), median (IQR) | 60 (24–168) | 48 (24–108) |
| Obstetric history,a n (%) | 95 (91) | 96 (95) |
| Vaginal deliveries only,a n (%) | 90 (95) | 96 (100) |
| C-sections only,a n (%) | 5 (5) | 0 (0) |
| Episiotomies or tears,a n (%) | 78 (87) | 82 (85) |
| Passive FI, n (%) | 88 (77) | 86 (77) |
| Urge FI, n (%) | 94 (82) | 93 (83) |
| Flatus incontinence, n (%) | 74 (64) | 83 (74) |
| Evacuatory difficulties, n (%) | 44 (39) | 49 (44) |
| Straining, n (%) | 34 (30) | 37 (33) |
| Digitation, n (%) | 12 (10) | 15 (13) |
| Urinary symptoms, n (%) | 70 (61) | 72 (64) |
| Urinary urgency, n (%) | 50 (43) | 49 (44) |
| Urinary urge incontinence, n (%) | 39 (34) | 42 (38) |
| Treatment | PTNS | Sham |
|---|---|---|
| Antidiarrhoeal medications, n (%) | 77 (67) | 67 (60) |
| Biofeedback, n (%) | 56 (49) | 59 (53) |
| Pelvic floor exercises, n (%) | 37 (32) | 36 (32) |
| Fibre supplementation, n (%) | 18 (16) | 30 (27) |
| Laxatives/suppositories/irrigation, n (%) | 20 (17) | 16 (14) |
| Anal sphincter repair, n (%) | 4 (3) | 4 (4) |
| Other anal surgery, n (%) | 11 (10) | 8 (7) |
| Defecatory advice, n (%) | 9 (8) | 7 (6) |
| Other, n (%) | 5 (4) | 8 (7) |
| Outcome | PTNS | Sham |
|---|---|---|
| Hysterectomy,a n (%) | 30 (29) | 24 (24) |
| Vaginal operation,a n (%) | 3 (3) | 2 (2) |
| Pelvic operation,a n (%) | 19 (18) | 16 (16) |
| Abdominal operation, n (%) | 28 (24) | 30 (27) |
| Anal operation, n (%) | 6 (5) | 9 (8) |
| Neck or back pain, n (%) | 15 (13) | 21 (19) |
| OAB, n (%) | 15 (13) | 7 (6) |
| Diverticular disease, n (%) | 4 (3) | 6 (5) |
| Irritable bowel syndrome, n (%) | 1 (1) | 4 (4) |
Bowel diary data at baseline
Baseline bowel diaries demonstrated a median of 6.0 FIEs per week in PTNS patients, comprising a median of 3.0 urge faecal incontinent episodes and a median of 2.0 passive episodes. In the sham arm there was a median of 6.9 FIEs per week, but with a slightly higher rate of passive FI (median 3.0 episodes) than urge episodes (median 2.5 episodes) (Table 4).
| Outcome | PTNS | Sham |
|---|---|---|
| FIEs per week | ||
| Median (IQR) | 6.0 (2.0–14.0) | 6.9 (2.5–16.0) |
| Mean (SD) | 9.9 (11.2) | 10.4 (10.9) |
| Urge FIEs per week | ||
| Median (IQR) | 3.0 (0.9–8.0) | 2.5 (0.5–7.0) |
| Mean (SD) | 5.3 (7.2) | 4.8 (5.9) |
| Passive FIEs per week | ||
| Median (IQR) | 2.0 (0.0–7.5) | 3.0 (0.0–8.0) |
| Mean (SD) | 4.6 (6.0) | 5.7 (7.6) |
Other baseline outcome measures
Baseline SMCSs were similar between the arms, with a mean score of 14.4 (standard deviation 3.7) in the PTNS arm and of 15.4 (standard deviation 4.1) in the sham arm. All 211 patients who completed their SMCS had significant FI on the basis of their score being > 5 (Table 5).
| Outcome | PTNS | Sham |
|---|---|---|
| SMCSa | ||
| Median (IQR) | 14.0 (12.0–17.0) | 16.0 (13.0–18.0) |
| Mean (SD) | 14.4 (3.7) | 15.4 (4.1) |
| SMCS > 5, n (%) | 110 (100) | 101 (100) |
| FIQoL scores | ||
| Lifestyleb | ||
| Median (IQR) | 2.7 (1.8–3.4) | 2.5 (1.7–3.6) |
| Mean (SD) | 2.6 (0.9) | 2.6 (1.0) |
| Coping and behaviourb | ||
| Median (IQR) | 1.7 (1.2–2.3) | 1.6 (1.1–2.6) |
| Mean (SD) | 1.9 (0.7) | 1.9 (0.9) |
| Depression and self-perceptionb | ||
| Median (IQR) | 3.1 (2.0–3.4) | 2.6 (2.0–3.7) |
| Mean (SD) | 2.8 (0.9) | 2.7 (0.9) |
| Embarrassmentc | ||
| Median (IQR) | 2.0 (1.7–2.7) | 2.0 (1.3–2.7) |
| Mean (SD) | 2.2 (0.8) | 2.1 (0.8) |
| Patient-centred outcomesd | ||
| Median (IQR) | 8.9 (7.8–9.8) | 9.2 (8.3–10.0) |
| Mean (SD) | 8.5 (1.6) | 8.7 (1.7) |
| GIQoLe | ||
| Median (IQR) | 130.0 (113.0–141.0) | 126.5 (109.0–139.0) |
| Mean (SD) | 126.7 (18.8) | 123.8 (20.2) |
| SF-36 scores (%) | ||
| Physical functioning | ||
| Median (IQR) | 70.0 (45.0–90.0) | 65.0 (40.0–85.0) |
| Mean (SD) | 65.7 (27.4) | 61.4 (28.4) |
| Role-physical | ||
| Median (IQR) | 50.0 (0.0–100.0) | 25.0 (0.0–75.0) |
| Mean (SD) | 46.4 (42.1) | 36.4 (41.4) |
| Bodily pain | ||
| Median (IQR) | 60.0 (40.0–90.0) | 57.5 (32.5–90.0) |
| Mean (SD) | 61.3 (30.0) | 58.2 (31.5) |
| General health | ||
| Median (IQR) | 50.0 (35.0–70.0) | 50.0 (30.0–70.0) |
| Mean (SD) | 51.2 (23.4) | 50.3 (23.8) |
| Vitality | ||
| Median (IQR) | 45.0 (30.0–57.5) | 50.0 (30.0–60.0) |
| Mean (SD) | 43.9 (22.1) | 42.7 (22.8) |
| Social functioning | ||
| Median (IQR) | 62.5 (37.5–75.0) | 62.5 (37.5–87.5) |
| Mean (SD) | 58.4 (28.8) | 59.3 (31.6) |
| Role-emotional function | ||
| Median (IQR) | 66.7 (0.0–100.0) | 33.3 (0.0–100.0) |
| Mean (SD) | 58.4 (28.8) | 59.3 (31.6) |
| Mental health | ||
| Median (IQR) | 60.0 (44.0–76.0) | 64.0 (48.0–76.0) |
| Mean (SD) | 60.3 (21.0) | 60.8 (21.6) |
| EQ-5D index scoref | ||
| Median (IQR) | 0.73 (0.62–0.85) | 0.73 (0.62–0.85) |
| Mean (SD) | 0.69 (0.27) | 0.63 (0.34) |
Primary outcome
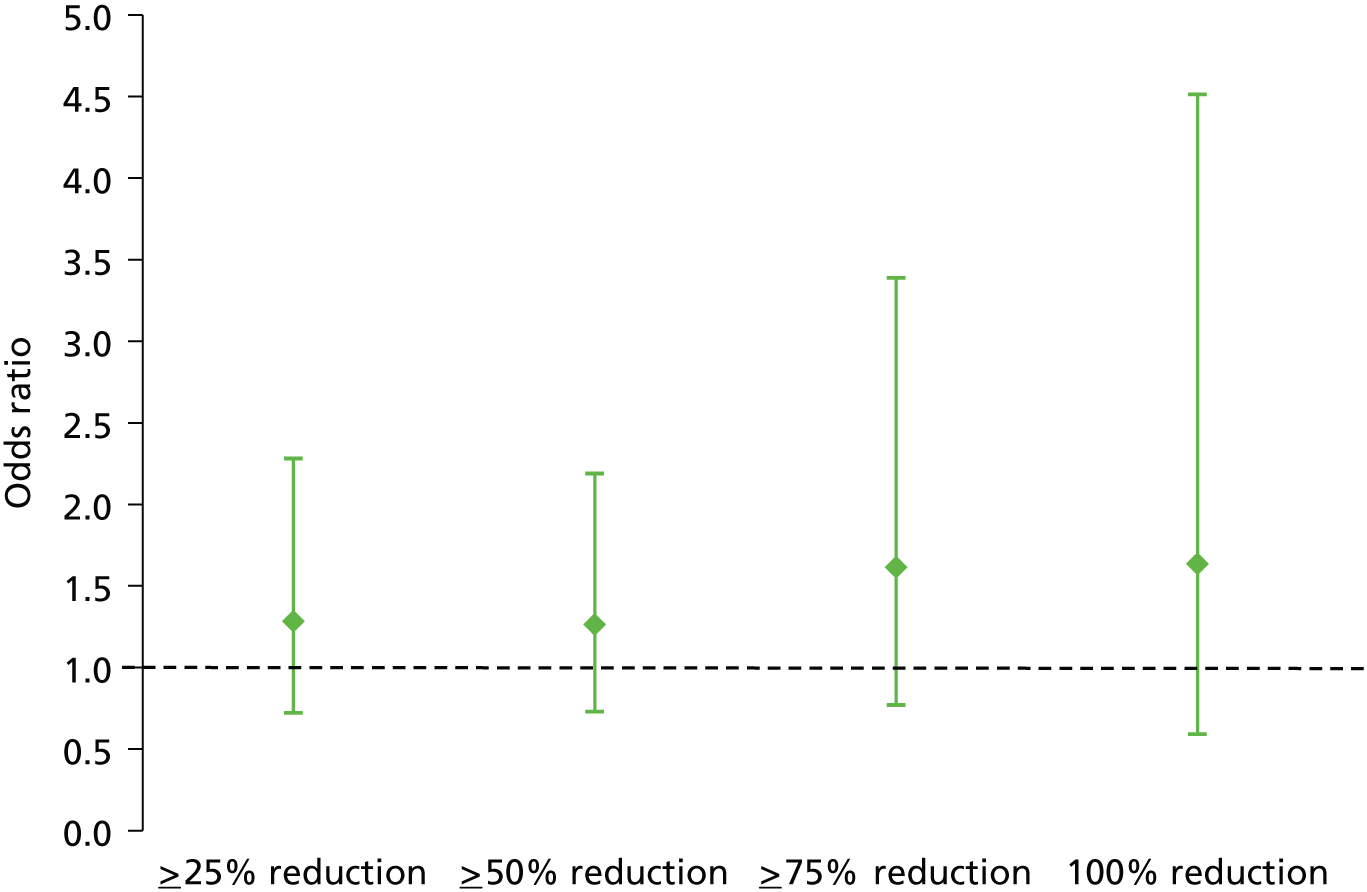
The percentage of patients achieving a ≥ 50% reduction in weekly FIEs was similar in both arms at 38% (39 out of 103) for PTNS and 31% (32 out of 102) for sham treatment (unadjusted odds ratio 1.333; adjusted odds ratio 1.283, 95% CI 0.722 to 2.281; p = 0.396) (Tables 6 and 7).
| Outcome | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| ≥ 50% reduction FIEs (primary outcome) | 1.283 | 0.722 to 2.281 | 0.396 |
| ≥ 25% reduction in FIEs | 1.264 | 0.730 to 2.190 | 0.404 |
| ≥ 75% reduction in FIEs | 1.615 | 0.770 to 3.388 | 0.205 |
| 100% reduction in FIEs | 1.635 | 0.592 to 4.514 | 0.344 |
| Difference in means | |||
| Change in FIEs | –2.262 | –4.185 to –0.339 | 0.021 |
| Change in urge FIEs | –1.456 | –2.693 to –0.219 | 0.021 |
| Change in passive FIEs | –0.635 | –1.668 to 0.397 | 0.228 |
| FIQoL embarrassment | 0.036 | –0.151 to 0.223 | 0.706 |
| FIQoL coping | 0.013 | –0.171 to 0.197 | 0.889 |
| FIQoL lifestyle | 0.086 | –0.075 to 0.248 | 0.290 |
| FIQoL depression | 0.014 | –0.297 to 0.324 | 0.927 |
| SF-36 physical functioning | –1.854 | –6.992 to 3.284 | 0.479 |
| SF-36 role-physical | 1.113 | –8.866 to 11.092 | 0.826 |
| SF-36 bodily pain | –1.026 | –6.815 to 4.764 | 0.728 |
| SF-36 general health | –0.158 | –4.749 to 4.433 | 0.946 |
| SF-36 vitality | –3.142 | –8.129 to 1.845 | 0.215 |
| SF-36 social functioning | 5.209 | –0.740 to 11.157 | 0.087 |
| SF-36 role emotional | –4.815 | 14.802 to 5.171 | 0.343 |
| SF-36 mental health | –0.509 | –4.831 to 3.814 | 0.817 |
| SMCS | –0.047 | –1.033 to 0.939 | 0.925 |
| Patient-centred outcomes | –0.545 | –1.081 to –0.008 | 0.047 |
| EQ-5D index score | –0.017 | –0.078 to 0.044 | 0.583 |
| GIQoL | –1.300 | –5.168 to 2.568 | 0.506 |
| Likert scale of success | 0.808 | –0.055 to 1.672 | 0.068 |
| Outcome | Baseline | End of treatment | ||
|---|---|---|---|---|
| PTNS | Sham | PTNS | Sham | |
| FIEs per week | ||||
| Median (IQR) | 6.0 (2.0–14.0) | 6.9 (2.5–16.0) | 3.5 (1.0–10.0) | 4.8 (1.5–12.8) |
| Mean (SD) | 9.9 (11.2) | 10.4 (10.9) | 6.4 (7.6) | 9.1 (10.7) |
| Urge FIEs per week | ||||
| Median (IQR) | 3.0 (0.9–8.0) | 2.5 (0.5–7.0) | 1.5 (0.0–4.5) | 1.5 (0.5–5.5) |
| Mean (SD) | 5.3 (7.2) | 4.8 (5.9) | 3.0 (4.2) | 4.4 (6.5) |
| Passive FIEs per week | ||||
| Median (IQR) | 2.0 (0.0–7.5) | 3.0 (0.0–8.0) | 1.5 (0.0–5.0) | 1.5 (0.0–6.5) |
| Mean (SD) | 4.6 (6.0) | 5.7 (7.6) | 3.4 (4.6) | 4.7 (6.6) |
Secondary outcomes
Percentage change in faecal incontinence episodes
No significant difference was observed between the PTNS and sham arms in the number of participants achieving > 25%, > 75% and 100% reductions in weekly FIEs (see Table 6 and Figure 6).
FIGURE 6.
Adjusted odds ratios and 95% CIs of percentage reduction in FIEs: PTNS vs. sham.
Change in faecal incontinence episodes as a continuous measure
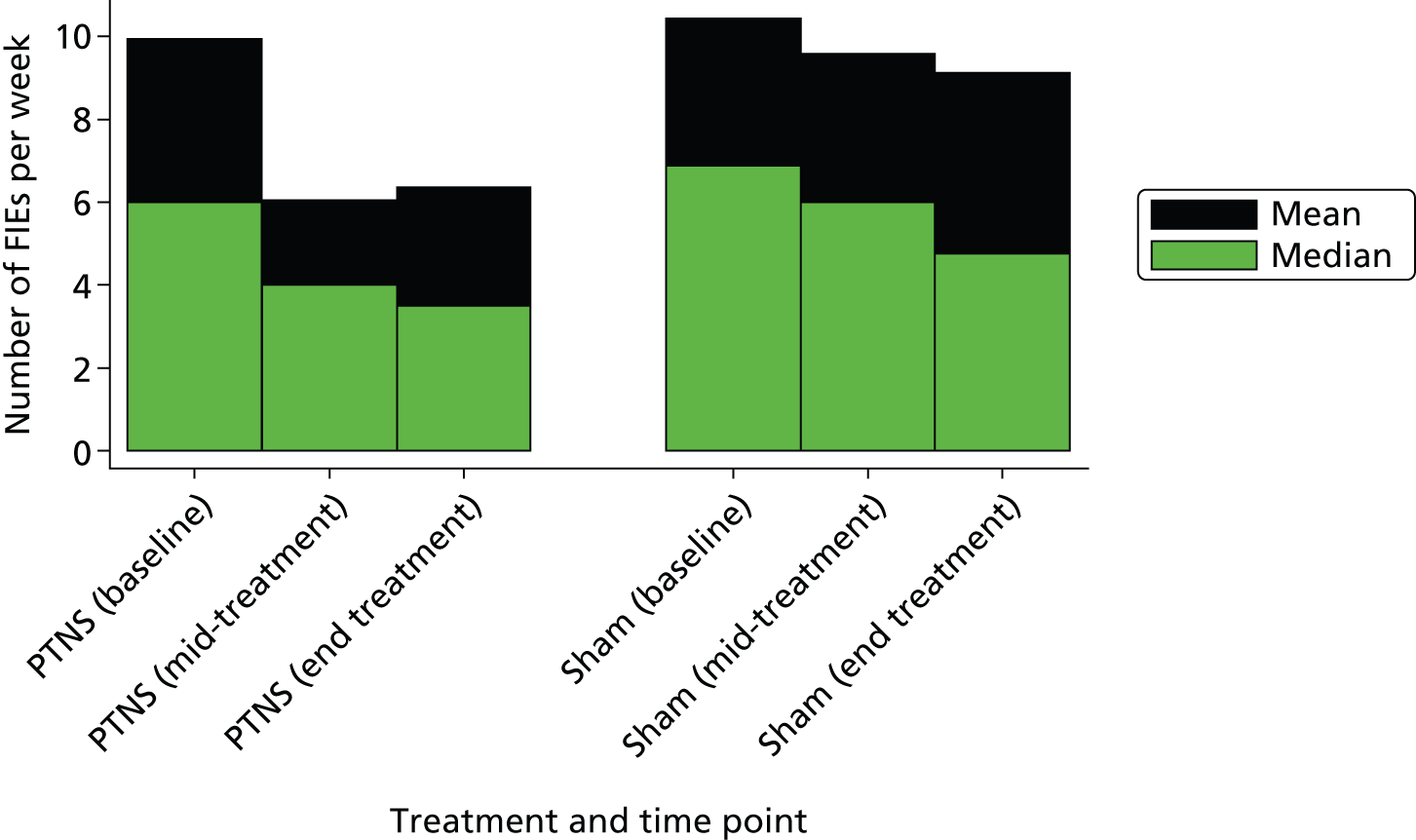
There was a greater decrease in total number of FIEs per week in the PTNS than the sham arm (difference in means –2.3, 95% CI –4.2 to –0.3) episodes per week, and this difference was significant (p = 0.02). This comprised a reduction in urge FIEs (–1.5, 95% CI –2.7 to –0.2; p = 0.02) but not in passive FIEs (–0.64, 95% CI –1.67 to 0.40; p = 0.23) per week (see Table 6 and Figures 7 and 8). There was very little continued improvement from mid-treatment to end of treatment (indicating that those who are likely to respond to treatment will have done this by week 6).
FIGURE 7.
Adjusted difference in mean (95% CI) number of FIEs per week: PTNS vs. sham.
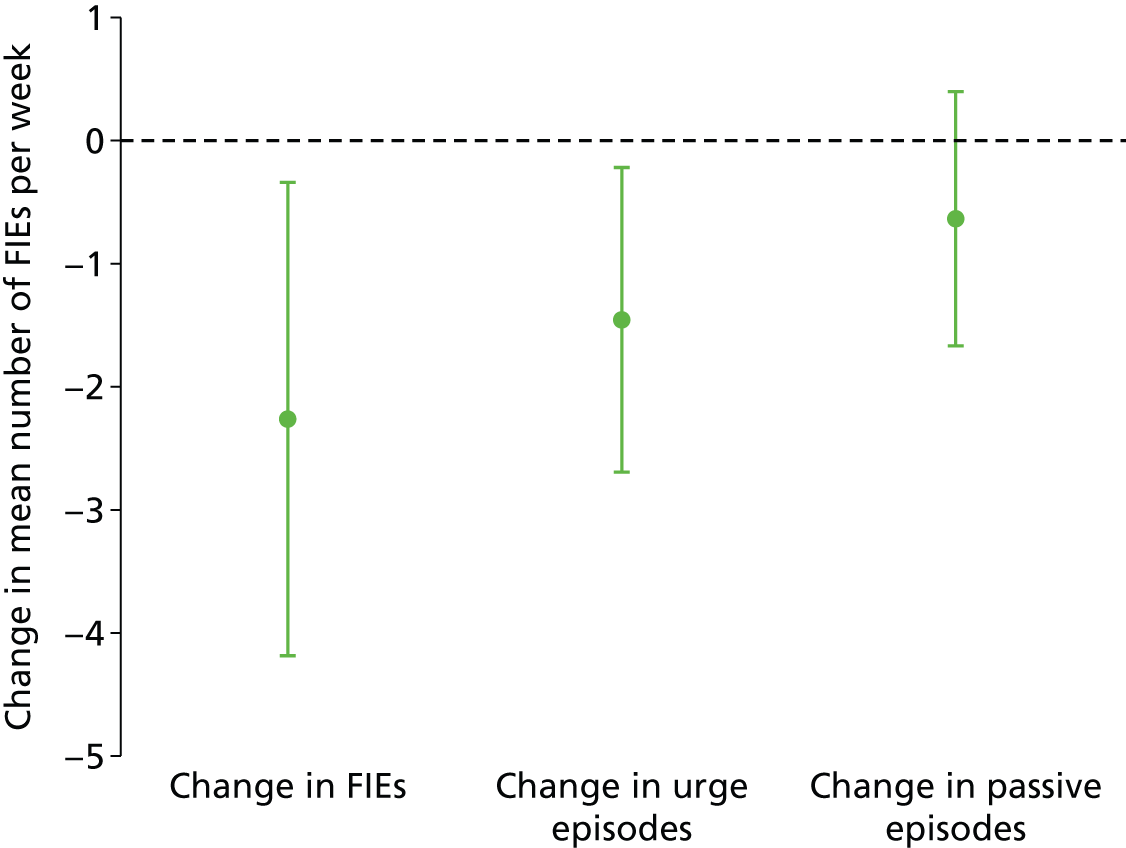
FIGURE 8.
Faecal incontinence episodes per week by treatment arm and time point.
Change in symptom severity score: St Mark’s Continence Score
No significant difference in SMCS was observed between the PTNS and sham arms following treatment (difference in means –0.047, 95% CI –1.033 to 0.939; p = 0.93) (Table 8 and see Table 6).
| Outcome | Baseline | End of treatment | ||
|---|---|---|---|---|
| PTNS | Sham | PTNS | Sham | |
| SMCS | ||||
| Median (IQR) | 14.0 (12.0–17.0) | 16.0 (13.0–18.0) | 14.0 (11.0–17.0) | 15.0 (11.0–18.0) |
| Mean (SD) | 14.4 (3.7) | 15.4 (4.1) | 13.9 (4.3) | 14.6 (4.6) |
| SMCS > 5 | ||||
| n (%) | 110 (100) | 101 (100) | 104 (100) | 101 (100) |
Change in quality-of-life measures
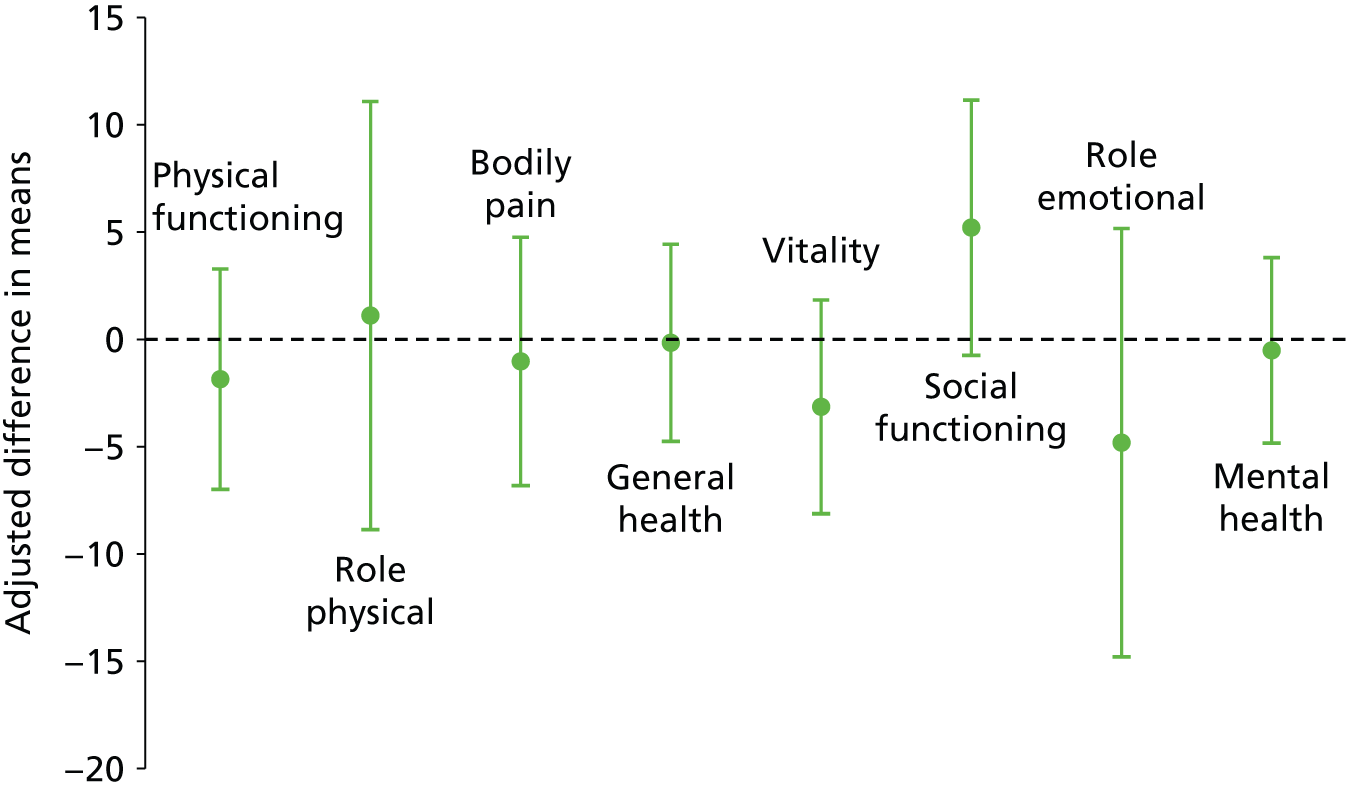
No significant differences were seen in the disease-specific (FIQoL and GIQoL) or generic (SF-36) quality-of-life measures between the PTNS and sham arms following treatment (Table 9 and Figures 9 and 10; see also Table 6).
| Outcome | Baseline | End of treatment | ||
|---|---|---|---|---|
| PTNS | Sham | PTNS | Sham | |
| FIQoL scores | ||||
| Lifestylea | ||||
| Median (IQR) | 2.7 (1.8–3.4) | 2.5 (1.7–3.6) | 3.0 (2.2–3.7) | 2.9 (1.9–3.7) |
| Mean (SD) | 2.6 (0.9) | 2.6 (1.0) | 2.8 (0.9) | 2.8 (1.0) |
| Coping and behavioura | ||||
| Median (IQR) | 1.7 (1.2–2.3) | 1.6 (1.1–2.6) | 1.9 (1.3–2.6) | 1.7 (1.2–2.9) |
| Mean (SD) | 1.9 (0.7) | 1.9 (0.9) | 2.0 (0.8) | 2.0 (1.0) |
| Depression and self-perceptiona | ||||
| Median (IQR) | 3.1 (2.0–3.4) | 2.6 (2.0–3.7) | 3.1 (2.2–3.7) | 2.6 (2.0–3.9) |
| Mean (SD) | 2.8 (0.9) | 2.7 (0.9) | 2.9 (1.0) | 2.8 (1.0) |
| Embarrassmentb | ||||
| Median (IQR) | 2.0 (1.7–2.7) | 2.0 (1.3–2.7) | 2.7 (1.7–3.0) | 2.3 (1.7–3.0) |
| Mean (SD) | 2.2 (0.8) | 2.1 (0.8) | 2.4 (0.8) | 2.3 (0.9) |
| GIQoL scoresc | ||||
| Median (IQR) | 130.0 (113.0–141.0) | 126.5 (109.0–139.0) | 135.0 (115.0–148.0) | 134.0 (120.0–146.0) |
| Mean (SD) | 126.7 (18.8) | 123.8 (20.2) | 132.0 (20.6) | 131.6 (20.5) |
| SF-36 scores (%) | ||||
| Physical functioning | ||||
| Median (IQR) | 70.0 (45.0–90.0) | 65.0 (40.0–85.0) | 75.0 (47.5–90.0) | 70.0 (45.0–90.0) |
| Mean (SD) | 65.7 (27.4) | 61.4 (28.4) | 67.1 (27.7) | 63.8 (29.0) |
| Role-physical | ||||
| Median (IQR) | 50.0 (0.0–100.0) | 25.0 (0.0–75.0) | 62.5 (0.0–100.0) | 25.0 (0.0–100.0) |
| Mean (SD) | 46.4 (42.1) | 36.4 (41.4) | 54.4 (44.1) | 46.2 (44.8) |
| Bodily pain | ||||
| Median (IQR) | 60.0 (40.0–90.0) | 57.5 (32.5–90.0) | 67.5 (45.0–90.0) | 67.5 (35.0–90.0) |
| Mean (SD) | 61.3 (30.0) | 58.2 (31.5) | 64.3 (28.3) | 62.1 (31.0) |
| General health | ||||
| Median (IQR) | 50.0 (35.0–70.0) | 50.0 (30.0–70.0) | 55.0 (30.0–75.0) | 50.0 (35.0–70.0) |
| Mean (SD) | 51.2 (23.4) | 50.3 (23.8) | 52.8 (24.6) | 50.6 (23.9) |
| Vitality | ||||
| Median (IQR) | 45.0 (30.0–57.5) | 50.0 (30.0–60.0) | 50.0 (25.0–60.0) | 50.0 (35.0–65.0) |
| Mean (SD) | 43.9 (22.1) | 42.7 (22.8) | 45.6 (22.2) | 46.7 (23.1) |
| Social functioning | ||||
| Median (IQR) | 62.5 (37.5–75.0) | 62.5 (37.5–87.5) | 75.0 (50.0–87.5) | 62.5 (37.5–87.5) |
| Mean (SD) | 58.4 (28.8) | 59.3 (31.6) | 66.4 (28.6) | 60.6 (31.7) |
| Role-emotional function | ||||
| Median (IQR) | 66.7 (0.0–100.0) | 33.3 (0.0–100.0) | 100.0 (0.0–100.0) | 83.3 (0.0–100.0) |
| Mean (SD) | 58.4 (28.8) | 59.3 (31.6) | 61.7 (45.3) | 60.2 (44.1) |
| Mental health | ||||
| Median (IQR) | 60.0 (44.0–76.0) | 64.0 (48.0–76.0) | 64.0 (48.0–84.0) | 64.0 (52.0–76.0) |
| Mean (SD) | 60.3 (21.0) | 60.8 (21.6) | 62.7 (25.1) | 63.0 (21.4) |
FIGURE 9.
Adjusted difference in means (95% CI) for FIQoL: PTNS vs. sham.
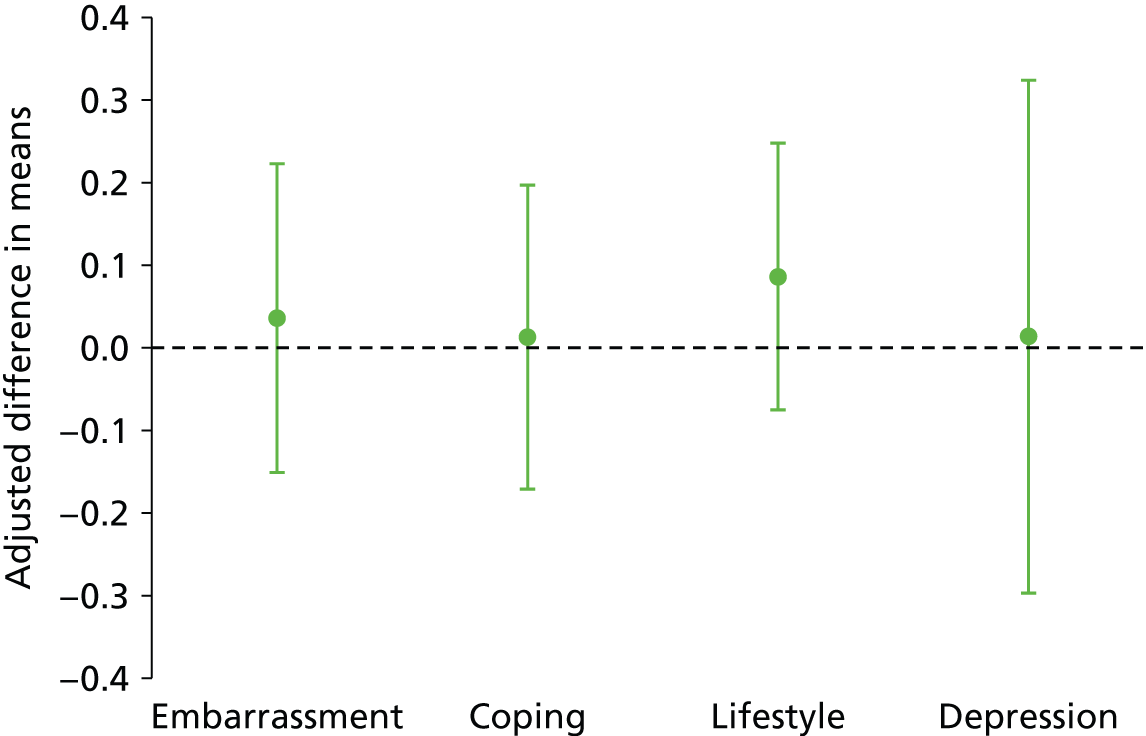
FIGURE 10.
Adjusted difference in means (95% CI) for SF-36: PTNS vs. sham.
Change in patient-centred outcomes score
Improvement in patient-centred outcomes (i.e. a reduction in score) was significantly greater in the PTNS arm than in the sham arm (difference in means –0.545, 95% CI –1.081 to –0.008; p = 0.047) (Table 10; see also Table 6).
| Outcome | Baseline | End of treatment | ||
|---|---|---|---|---|
| PTNS | Sham | PTNS | Sham | |
| Median (IQR) | 8.9 (7.8–9.8) | 9.2 (8.3–10.0) | 8.4 (6.9–9.4) | 9.3 (7.6–10.0) |
| Mean (SD) | 8.5 (1.6) | 8.7 (1.7) | 7.8 (2.0) | 8.4 (2.1) |
Likert scale of patients’ global impression of success (scale 0–10)
No significant difference existed in patients’ global impression of success between the PTNS and sham arms (difference in means 0.808, 95% CI –0.055 to 1.672; p = 0.068) (Table 11; see also Table 6).
| Outcome | PTNS | Sham |
|---|---|---|
| Median (IQR) | 4.8 (0.0–6.8) | 2.1 (0.0–4.9) |
| Mean (SD) | 4.0 (3.3) | 3.2 (3.1) |
European Quality of Life-5 Dimensions analysis
There were virtually no differences between the two arms either at baseline or after treatment in respect of EQ-5D index and visual analogue scale (VAS) scores, with scores on both scales remaining unchanged over time (Table 12 and see Table 6). The full report can be viewed in Appendix 13.
| Outcome | Baseline | End of treatment | ||
|---|---|---|---|---|
| PTNS | Sham | PTNS | Sham | |
| EQ-5D index, mean (SD) | 0.69 (0.27) | 0.63 (0.34) | 0.68 (0.28) | 0.65 (0.34) |
| EQ-5D VAS, mean (SD) | 64.50 (21.72) | 64.04 (21.24) | 64.25 (22.32) | 63.69 (23.66) |
Other outcomes
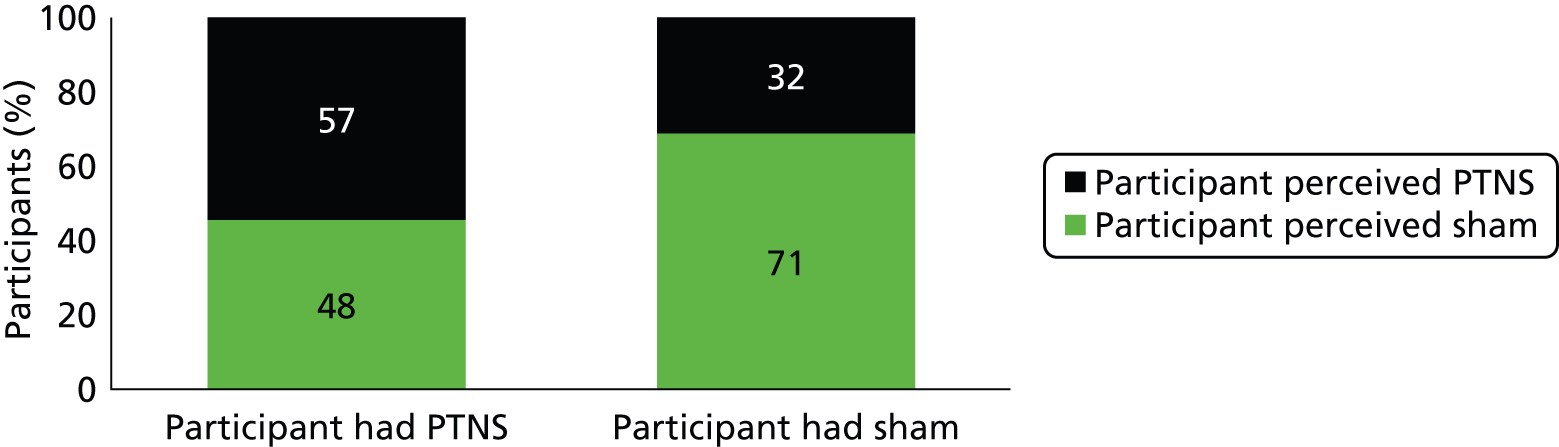
In the PTNS arm, 57 out of 107 (54%) participants thought that they had received PTNS and 48 out of 107 (46%) thought that they had received sham treatment (Figure 11). In the sham arm, 32 out of 103 (31%) participants thought that they had received PTNS and 71 out of 103 (69%) participants thought that they had received sham treatment. Overall, 208 patients answered this question, of whom 62% perceived correctly and 38% perceived incorrectly.
FIGURE 11.
Participants’ perception of treatment.
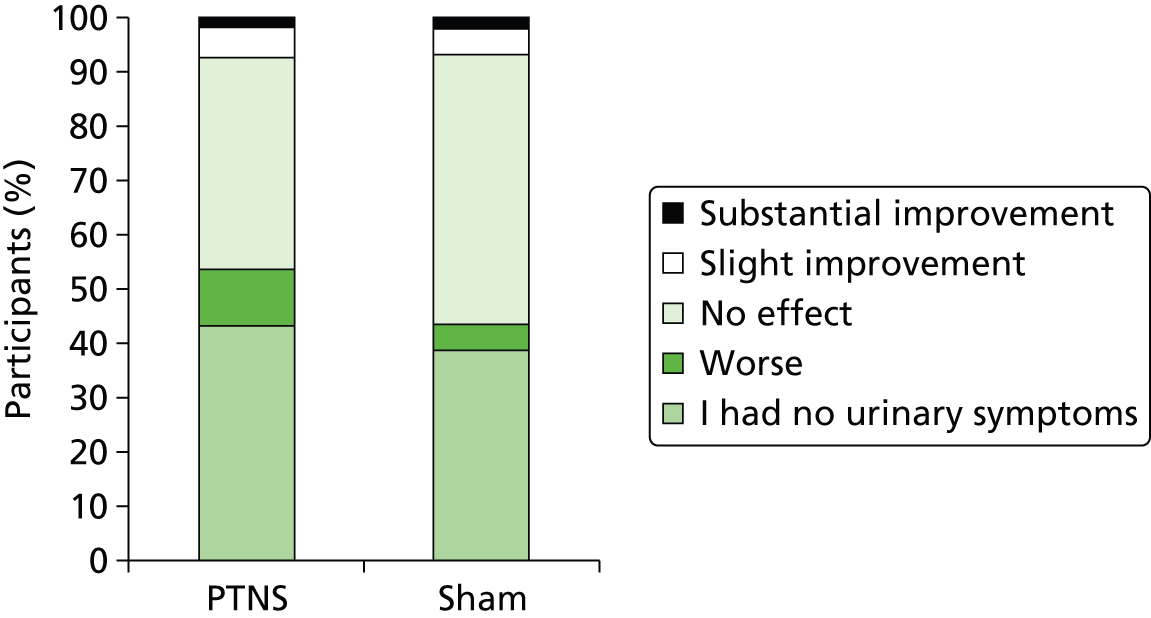
Only 13% (8 out of 61) of patients in the PTNS arm experienced slight or substantial improvement in urinary symptoms and the figure in the sham arm was similar, at 11% (7 out of 64). Most symptomatic patients reported no effect: 39% in the PTNS arm and 50% in the sham arm. Indeed, more patients in the PTNS arm than in the sham arm reported a worsening of urinary symptoms (10% vs. 5%) (Figure 12).
FIGURE 12.
Effect of treatment on urinary symptoms.
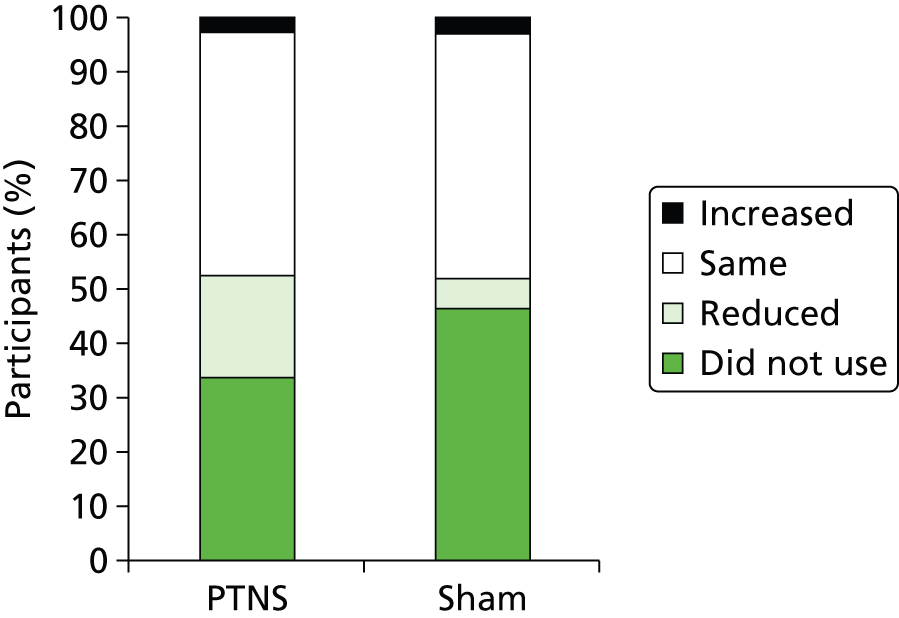
Of participants who used loperamide at baseline, the majority in both the PTNS (33 out of 49 = 67%) and sham (32 out of 38 = 84%) arms reported no change in use throughout the trial. Similar percentages in each arm (4% in PTNS vs. 5% in sham) reported increasing loperamide use. A higher percentage of patients in the PTNS arm than in the sham arm reduced their loperamide use (29% vs. 11%) (Figure 13).
FIGURE 13.
Effect of treatment on loperamide use.
Other potentially relevant concomitant medication usage can be seen in Appendix 14. There has been minimal concomitant medication usage and this has not been considered significant.
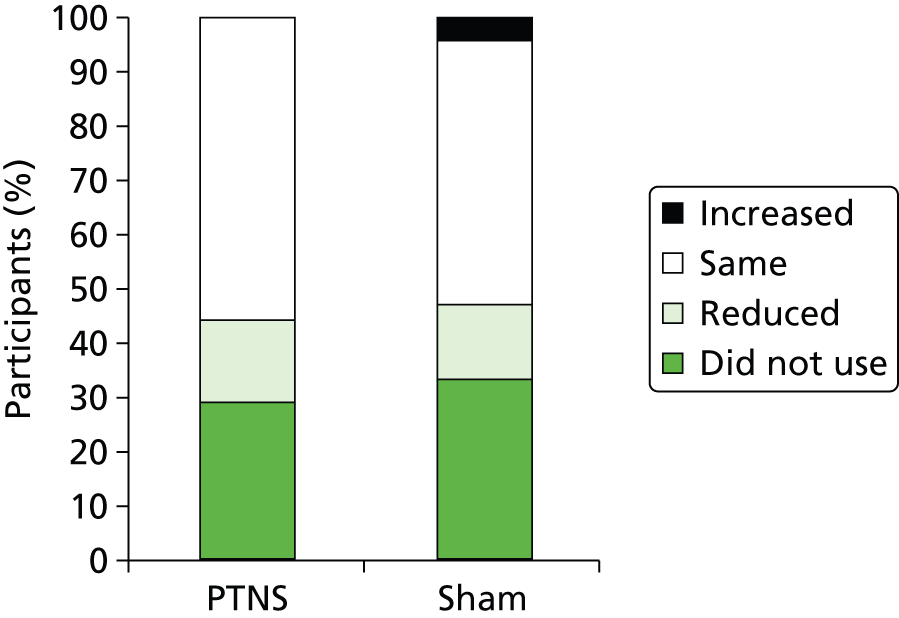
Of the participants who used incontinence pads, the majority [56% (44 out of 79) in PTNS arm and 49% (35 out of 72) in sham arm] reported no change in use over the period of the trial. Similar percentages of participants reduced their pad usage through the course of the trial (15% in the PTNS arm and 14% in the sham arm), while 4% participants in the sham arm had to increase their pad usage compared with none in the PTNS arm (Figure 14).
FIGURE 14.
Effect of treatment on incontinence pad usage.
Per-protocol analysis
Per-protocol analysis was carried out subsequent to the intention-to-treat analysis. To be included in these analyses, patients were required to have at least 10 treatments within 13 weeks, with 10 treatments no fewer than 5 days and no more than 10 days apart. This was to ensure that patients attended for treatments regularly and in a time frame spread evenly throughout the treatment duration.
In total, 197 of the 227 patients completed the treatment per protocol. Table 13 presents the results of the analysis of the primary outcome. The conclusion from this analysis remains unchanged and other important outcomes remain unchanged apart from the Likert scale of success, which shows that those in the PTNS arm were more likely than those in the sham arm to perceive that the treatment was successful; this difference was statistically significant.
| Outcome | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| ≥ 50% reduction FIEs (primary outcome) | 1.269 | 0.688 to 2.341 | 0.446 |
| ≥ 25% reduction in FIEs | 1.247 | 0.698 to 2.228 | 0.456 |
| ≥ 75% reduction in FIEs | 1.631 | 0.781 to 3.409 | 0.194 |
| 100% reduction in FIEs | 1.658 | 0.590 to 4.655 | 0.338 |
| Difference in means | |||
| Change in FIEs | –2.233 | –4.275 to –0.191 | 0.032 |
| Change in urge FIEs | –1.486 | –2.778 to –0.194 | 0.024 |
| Change in passive FIEs | –0.600 | –1.663 to 0.463 | 0.268 |
| SMCS | 0.202 | –0.855 to 1.258 | 0.708 |
| GIQoL | –1.750 | –5.864 to 2.364 | 0.401 |
| FIQoL embarrassment | 0.059 | –0.141 to 0.260 | 0.563 |
| FIQoL coping | –0.007 | –0.211 to 0.196 | 0.944 |
| FIQoL lifestyle | 0.093 | –0.079 to 0.266 | 0.286 |
| FIQoL depression | 0.030 | –0.302 to 0.361 | 0.853 |
| SF-36 physical functioning | –0.601 | –5.964 to 4.761 | 0.826 |
| SF-36 role-physical | 1.562 | –9.062 to 12.186 | 0.772 |
| SF-36 bodily pain | –2.933 | –8.975 to 3.108 | 0.341 |
| SF-36 general health | 0.612 | –3.989 to 5.213 | 0.794 |
| SF-36 vitality | –2.872 | –7.967 to 2.224 | 0.268 |
| SF-36 social functioning | 5.665 | –0.518 to 11.848 | 0.074 |
| SF-36 role emotional | –6.562 | –16.988 to 3.863 | 0.216 |
| SF-36 mental health | –0.300 | –4.633 to 4.033 | 0.892 |
| Patient-centred outcomes | –0.593 | –1.141 to –0.044 | 0.034 |
| EQ-5D index score | –0.020 | –0.082 to 0.042 | 0.524 |
| Likert scale of success | 0.934 | 0.037 to 1.831 | 0.042 |
Subgroup analyses
Preplanned subgroup analyses were performed for the primary outcome only. The following subgroups were selected:
-
sex (male vs. female)
-
FI severity (> 7 episodes per week vs. < 7 episodes per week on initial bowel diary)
-
age (< 40 years, 40–60 years, > 60 years)
-
both urge and passive incontinence, only urge, only passive.
The primary outcome was negative for each of these subgroup analyses (see Appendix 15).
Sensitivity analysis
Sensitivity analysis was carried out, removing the patients who scored ‘zero’ on their initial bowel diaries (see Appendix 16). This excluded 16 patients, nine from the PTNS arm and seven from the sham arm. The primary outcome was negative for this analysis (odds ratio 1.325, 95% CI 0.736 to 2.385; p = 0.348).
Further sensitivity analysis was carried out excluding patients who were recruited from poorly recruiting centres (defined as centres recruiting fewer than five patients) (see Appendix 17). This excluded four patients from two centres, two from each arm. The primary outcome was negative for this analysis (odds ratio 1.234, 95% CI 0.693 to 2.196; p = 0.476).
Centre effect
Data were analysed to allow for a centre effect, that is, outcomes among patients being treated by the same study centre may be correlated, indicating that treatment at some centres may be more effective. The ICC was very small (< 0.001 for most outcomes), indicating no significant centre effect. The only outcomes for which the ICC was substantial were ≥ 75% reduction (ICC = 0.222) in FIEs, 100% reduction in FIEs (ICC = 0.012), change in passive FIEs (ICC = 0.106), FIQoL coping (ICC = 0.104), SF-36 social functioning (ICC = 0.012), SF-36 mental health (ICC = 0.038), EQ-5D (ICC = 0.019) and the Likert scale of success (ICC = 0.02).
Serious adverse events
There were four SAEs during the trial (Table 14). None was related to the trial treatment and all were resolved.
| SAE | Allocation | Grade | Duration (days) | Action | Relatedness | Outcome |
|---|---|---|---|---|---|---|
| Flexible cystoscopy for botulinum toxin type A (Botox®, Allergan) | PTNS | Moderate | 3 | H | U | R |
| Sleeve gastrectomy | Sham | Severe | 1 | H | U | R |
| Pilonidal abscess | Sham | Moderate | 26 | H | U | R |
| Shoulder manipulation | PTNS | Severe | 1 | H | U | R |
Adverse events
A total of 204 adverse events were noted in the trial, 107 in the PTNS arm and 97 in the sham arm. Table 15 reports severity by relatedness in each arm. There were seven mild related adverse events in each arm.
| Outcome | PTNS | Sham | ||||||
|---|---|---|---|---|---|---|---|---|
| Related | Possibly related | Unrelated | Total | Related | Possibly related | Unrelated | Total | |
| Mild | 7 | 25 | 40 | 72 | 7 | 18 | 33 | 58 |
| Moderate | 0 | 13 | 17 | 30 | 0 | 14 | 21 | 35 |
| Severe | 0 | 4 | 1 | 5 | 0 | 1 | 3 | 4 |
Related and possibly related adverse events can be seen in Table 16. A full list of all adverse events can be seen in Appendix 18.
| Outcome | Adverse event | PTNS | Sham |
|---|---|---|---|
| Related | Pain at needle site | 4 | 3 |
| Bruising at needle site | 2 | 1 | |
| Altered sensation at needle site | 1 | 0 | |
| Bleeding at needle site | 0 | 2 | |
| Altered sensation in toe | 0 | 1 | |
| Possibly related | Pain in abdomen | 4 | 2 |
| Pain in back | 1 | 0 | |
| Pain in leg or foot | 13 | 10 | |
| Pain in perineum | 0 | 1 | |
| Altered sensation in leg or foot | 4 | 0 | |
| Altered sensation in perineum | 0 | 1 | |
| Weakness in leg | 0 | 1 | |
| Constipation | 0 | 1 | |
| Diarrhoea | 8 | 3 | |
| FI | 0 | 1 | |
| Urinary symptoms | 0 | 4 | |
| Headache/migraine | 6 | 7 | |
| Dizziness | 5 | 0 | |
| Nausea/vomiting | 0 | 1 | |
| Anxiety/depression | 1 | 1 | |
| Skin disorder | 1 | 0 |
Chapter 4 Discussion
Although PTNS and sham electrical stimulation offer some improvement in FI symptoms by reducing weekly episodes, no clinically significant benefit of PTNS over sham was demonstrated. This was demonstrated by the primary outcome, with 38% of participants in the PTNS arm and 31% in the sham arm achieving at least a 50% reduction in FIEs.
Some of the secondary outcome variables, namely reduction in mean total weekly FIEs, reduction in mean urge FIEs and improvement on the patient-centred outcomes form (a derivative of the validated International Consultation on Incontinence Modular Questionnaire – Bowel), demonstrated a significant benefit of PTNS over sham treatment. However, the margin of clinical benefit must be considered small even though statistical significance was achieved, as this improvement was based on a reduction in weekly FIEs from a median of 6.0 (IQR 2.0–14.0) to a median of 3.5 (IQR 1.0–10.0), meaning that many participants still have significant FI. It is interesting to note that, if a treatment effect was going to occur, it would have done so by six treatments.
There was no significant improvement in the SMCS in the PTNS arm compared with the sham arm. Patients in the PTNS arm of the trial showed no significant improvement in any of the quality-of-life measures, compared with patients in the sham arm.
The results of this study may seem surprising when considered in the context of other published studies of PTNS from FI. The 12 published studies on PTNS, including 10 case series, one small randomised study and one comparative case-matched study of PTNS and SNS, allude to a 63–82% response rate using the same primary outcome, which is considerably higher than the 38% reported here. The results of this study are, however, closer to a recently conducted randomised study of PTNS compared with SNS, which found that 40% of patients in the PTNS arm reported treatment success at 3 months, again significantly lower than any other previously reported data. 71 Interestingly, a double-blind placebo-controlled randomised controlled trial of TTNS for FI in the literature shows no discernible benefit of TTNS over sham treatment in the treatment of FI. 72
These findings highlight the necessity of conducting well-designed randomised controlled trials to answer clinical questions. The other previously published non-randomised studies are prone to significant bias, which may account for the difference in results.
Case series provide poor evidence and are open to significant bias. First and foremost, there is no control arm for comparison, leading to performance bias. There is no way of unpicking the effect of natural change in disease status over time or the well-recognised placebo effect of this nurse-led face-to-face intervention. Both of these issues can be ameliorated only by including a control arm. Selection bias in case series is a large problem unless subjects are truly selected consecutively. In addition to this, case series are often subject to attrition bias, as patients may be lost to follow-up or researchers may selectively report only subjects with positive findings. In case series, both the patient and the observer are often unblinded. This can introduce bias from both perspectives: patients may experience a high level of expectation, which may influence reporting; and, in addition to this, bias may be introduced from the observers’ perspective, as clinicians often have a vested interest in treatment and publication. This problem is particularly important in trials of FI that involve bowel diary data, as diaries are notoriously poorly completed and open to interpretation. 73
The only other RCTs of PTNS in the literature are those on OAB. Of these studies, two double-blinded RCTs that compare PTNS with sham electrical stimulation showed a statistically significant improvement in urinary frequency and urge urinary incontinence in the active PTNS arm compared with the sham arm (71% vs. 0% responders in the smaller study; p < 0.001;74 and 54.5% vs. 24.9% in the larger pivotal trial;58 p < 0.001). One had a significantly smaller sample size than the other (n = 35 and n = 174 respectively).
Both of these studies reported a higher treatment effect of PTNS than seen in the CONFIDeNT trial, and one that is significantly beneficial compared with sham. There could be a number of reasons for this. It could simply be a result of PTNS having efficacy in OAB but not FI. Alternatively, it could be a result of these studies selecting purely patients who had OAB, that is patients who experience bladder urgency (more akin to faecal urgency or urge FI), which may account for the CONFIDeNT trial showing no overall benefit in patients, but significant reductions in urge FIEs.
The disparity could also be a factor of primary outcome measure selection. Peters et al. 58 used a subjective primary end point involving number of patients who graded their overall bladder symptoms as moderately or markedly improved on a global response assessment. It is unclear whether or not this assessment tool has been validated. The smaller study chose an objective primary end point, equivalent to that used in the CONFIDeNT trial, of those patients who experienced a > 50% reduction in number of urge urinary incontinence episodes.
Both urological studies also reported a significantly lower treatment effect of the sham, or placebo. The placebo effect in trials of functional bowel disease is well acknowledged to be high; indeed, meta-analyses of 45 published trials estimate the placebo response rate in functional dyspepsia to be between 6% and 72%75,76 and in 50 placebo-controlled irritable bowel syndrome trials it is estimated to be between 3% and 84%. 77–79 This may indicate that the 0% placebo effect in the Finazzi-Agro et al. 74 trial is a product of a small sample size, inadequate blinding or both. The apparently lower sham response in both studies may be a result of a less effective sham.
The sham stimulation used was different in both urological studies and also different from that used in the CONFIDeNT trial. In one study, the needle was placed in the medial head of gastrocnemius muscle and electrical stimulation activated for only 30 seconds prior to the stimulator being turned off;74 in the other study, a Streitberger needle was used, which does not pierce the skin, and electrical stimulation was delivered via TENS. The sham chosen in the CONFIDeNT trial was designed to give a very similar feeling to that produced by the active treatment, by giving the sensation of the skin being pierced by the needle and by providing a constant electrical sensation.
The sham treatment in the CONFIDeNT trial was well conducted and blinding was maintained in each treatment session with no information revealed to patients. The trial team feel that this was an improvement on the sham used in the Peters et al. 58 study of OAB, as the sham treatment was carried out using the same needle as in the PTNS arm, and it did pierce the skin to give the same sensation as the PTNS arm, the only difference being that the needle was not advanced as far. The sham was shown not to stimulate the tibial nerve during neurophysiological testing.
Limitations
This study was generally conducted to a high standard and there were no major methodological flaws or protocol violations. The effect of sham stimulation was correctly predicted at 35%. It would have been difficult to predict such a marginal treatment effect based on the previous literature; however, the effect of treatment was still less than our conservative estimate of 55%. The data do not indicate that the sample size was inadequate, as the 95% CI of the primary outcome analysis precludes a clinically significant reduction in the odds of success.
The study did, however, have some limitations. As is the nature of FI, there was significant heterogeneity in the population of patients selected to take part in the trial. This may have affected the results, especially given that there may have been a treatment effect among those suffering with urge FI (akin to OAB).
Patients suffering with frequent loose stools, or those with significant constipation, may indicate different disease pathophysiologies, thus complicating the results with regard to efficacy of PTNS. As seen from the demographics, approximately 40% of patients in this study suffer with concomitant rectal evacuatory problems. Similarly, it could be argued that, based on the paper by Hoturas et al. ,80 which suggested that those with urge FI benefit more from PTNS, which would go along with a similar concept that PTNS works for OAB, the CONFIDeNT trial could have selected patients with pure urge FI. These stipulations would have added further complexity to the trial, requiring all patients to undergo anorectal physiology testing, and would have adversely affected recruitment by significantly reducing the number of patients eligible for the trial. Moreover, this was a pragmatic trial testing a treatment aimed as a first-line treatment for FI, for example in GP surgeries or nursing homes, where such patient selection would not be feasible or possible, and results are required to be generalisable.
Another limitation of this study, and one that widely affects studies of FI, is that there is no perfect or universally accepted outcome measure. 73 Weekly FIEs, as a count, has an overdispersed Poisson distribution, that is greater variability than expected. Therefore, attempting to define a clinically significant mean reduction in FIE per week in a population of patients with widely dispersed starting FI frequencies is very difficult. This study, along with many in the SNS literature, chose to counter this problem by adopting a categorical measure of percentage reductions, that is the proportion of patients who have a ≥ 50% reduction in FIE per week, which is likely to be a much more realistic indication of success. Although subject to criticism, this was chosen as the primary outcome for the study not least because it has most often been used to assess SNS, thus allowing comparisons to be drawn between the two treatment modalities. Further, the 50% criterion has been applied as the primary end point in both of the pivotal trials of contemporary treatments in FI,27,81 with these treatments subsequently reviewed favourably by the US Food and Drug Administration. It could be argued that a 50% reduction in FIEs is not life-changing because this may still signify significant FI; however, until another outcome measure is introduced into the literature this is the most widely accepted outcome for treatment comparison. It is also important to remember that bowel diary data were collected for only a 2-week period at the beginning and end of treatment, and a score of ‘zero’ on a bowel diary does not necessarily signify that a patient’s incontinence is cured (a point ignored in previous literature, in which terms such as ‘complete continence’ are used to denote this eventuality).
Consideration has been given to the fact that, because the use of regular medications was not prohibited, a change in antidiarrhoeal medication (e.g. loperamide) may have reduced the effect size of PTNS. The decision was made not to limit the use of such medications, as it is likely that people would have continued to use the medications anyway. Instead, the decision was made to record patients’ loperamide use throughout the trial. Patients were asked each week about loperamide use; however, following the first meeting of the TSC and DSMC, it was felt that this was not accurately recalled by patients and a decision was made that the best way to collect this information would be to ask at the end of the trial whether their usage had remained the same, increased or decreased. As this decision was made partway into the trial, this information was not collected from all patients. We did, however, feel that it important to attempt to quantify this because it is a potential confounder. The question on loperamide usage was answered by 144 out of 227 patients (64%), and this was in the main because 55 patients completed the trial prior to implementation of the new CRF. Those who did answer the question were evenly balanced between treatment arms. As can be seen from the results, of those who were taking loperamide, almost three times as many patients in the PTNS arm reduced this as in the sham arm. Simple chi-squared testing, however, found this difference to be non-significant (p = 0.06). This calculation should be interpreted with caution, as the numbers using loperamide at baseline were comparatively small and, because they are not the full randomised samples, may differ systematically (i.e. there may be confounding).
Another criticism of this study could be that patients were not excluded on the basis of having ‘zero’ FIEs reported on their baseline bowel diary, so long as the principal investigator was convinced that the participant had FI significant enough to warrant intervention. This decision was taken because FI is often a problem which happens in bouts and it is not impossible for a patient to experience two symptom-free weeks by chance. The alternatives would have been either to exclude these patients, which might have seemed unfair given that they do have significant FI and should be entitled to try this treatment, or to give the patients another chance to complete the bowel diary. It was felt that these patients might well then fabricate the bowel diary if zero FIEs were to occur again, thus potentially confounding the results. Sensitivity analysis was done removing the 16 patients to whom this applied, who happened to be spread evenly across the two arms, and this made no difference to the overall results.
A further limitation of this study is the short follow-up period, as outcomes were assessed 2 weeks after the end of treatment. Many trials of this nature assess outcomes at 6 months; however, there are moves to extend this to 12 months following treatment [as per ROME IV (Rome Diagnostic Criteria for Functional Gastrointestinal Disorders); Professor Charles Knowles, unpublished data].
Finally, this trial could be criticised for lack of formal health economic analysis. This was not performed because of the lack of clinical effectiveness, and failure to demonstrate any changes in the EQ-5D questionnaire. The authors acknowledge that since then a potentially more sensitive five-level questionnaire has been developed, which might have yielded a different result.
Chapter 5 Conclusions
The CONFIDeNT study was a well-conducted, definitive trial, carried out to a high standard with an absence of any methodological flaws or serious breaches.
Percutaneous tibial nerve stimulation did not show significant clinical benefit over sham electrical stimulation in the treatment of FI based on the proportions of patients who reported at least a 50% reduction in weekly FIE. There was, however, a significant improvement in those patients who had PTNS in mean reduction in total weekly FIE, urge weekly FIE and patient-centred outcomes compared with those who had sham treatment.
Based on the evidence presented, it would be hard to justify recommending this therapy for the patient population in the trial.
In view of the relatively low costs associated with this treatment and its high acceptability, there may be a justification in continuing to treat a subgroup of patients with troublesome urge FI symptoms in whom directed therapy may cause symptomatic improvement. Further studies of PTNS should be directed at those with urge FI to determine whether or not this approach has value. Long-term follow-up of participants in the CONFIDeNT study will also be useful to further gauge response to treatment.
Acknowledgements
We would like to acknowledge the following in relation to the CONFIDeNT study.
The CONFIDeNT Group
CONFIDeNT Clinical Advisor: Professor Norman Williams.
Principal investigators: Dr Anton Emmanuel, Miss Carolynne Vaizey, Mr Paul Durdey, Mr Charles Maxwell-Armstrong, Miss Katherine Gill, Mr Pasquale Giordano, Miss Karen Nugent, Mr Paul Skaife, Mr Steven Brown, Mr Alexis Schizas, Mr Justin Yeung, Mr Graeme Duthie, Mr Dermot Burke, Mr Pradeep Agarwal, Ms Karen Telford, Mr Andrew Clarke and Dr Yan Yannikou.
Dr Adam Smith, PhD, Project Director of Outcomes Research, York Health Economics Consortium Ltd, for his EQ-5D analysis.
Nottingham Clinical Trials Unit for their randomisation support.
All members of the DSMC for their support throughout the trial.
Data Management and Quality Assurance Team: Ms Sandy Smith, Mr Mike Waring, Mrs Lara Edwards, Ms Anitha Manivannan, Mr Glenn Poon and Mr Syed Arafath.
Staff at all 18 UK centres for their hard work throughout the trial, including Barts Health NHS Trust, London; University College Hospital, London; St Mark’s Hospital, London; Bristol Royal Infirmary, Bristol; Queen’s Medical Centre, Nottingham; Sandwell and West Birmingham NHS Trust, Birmingham; The Community Specialist Colorectal Clinic, Ching Way Medical Centre, London; University Hospital Southampton NHS Foundation Trust, Southampton; Aintree University Hospitals NHS Foundation Trust, Liverpool; Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield; Guy’s and St Thomas’ Hospital, London; Leicester General Infirmary, Leicester; Castle Hill Hospital, Hull; Leeds Royal Infirmary, Leeds; Pilgrim Hospital, Boston (Lincolnshire); University Hospital of South Manchester, Wythenshawe; Poole Hospital NHS Foundation Trust, Poole; and University Hospital of North Durham, Durham.
Contributions of authors
Miss Emma J Horrocks (academic clinical fellow) contributed to the trial design, data collection, analysis and interpretation of the data, and drafting and submitting the final report.
Dr Stephen A Bremner (trial statistician) contributed to the trial design, data analysis and interpretation, and revising the final report.
Ms Natasha Stevens (trial manager) contributed to the trial design, data synthesis and revising the final report.
Professor Christine Norton (professor of nursing and chairperson of the TSC) contributed to modification of the study design, interpretation of the data and revising the final report.
Ms Deborah Gilbert (chief executive of Bowel & Cancer Research charity and patient and public involvement representative) contributed to the trial design, data interpretation and revising the final report.
Professor P Ronan O’Connell (professor of surgery and member of the TSC) contributed to the trial design, interpretation of the data and revision of the final report.
Professor Sandra Eldridge (professor of medical statistics and senior trial statistician) contributed to the acquisition of funding, trial design, supervision of the data analysis, data interpretation and revising the final report.
Professor Charles H Knowles (clinical professor of surgical research and chief investigator) contributed to the trial conception and design, the acquisition of funding, oversight of data collection, data interpretation, and revising and final approval of the final report.
Publication
Knowles CH, Horrocks EJ, Bremner SA, Stevens N, Norton C, O’Connell PR, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic, parallel-group randomised controlled trial [published online ahead of print 17 August 2015]. Lancet 2015.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum 2004;47:1341-9. http://dx.doi.org/10.1007/s10350-004-0593-0.
- Nelson RL. Epidemiology of fecal incontinence. Gastroenterology 2004;126:S3-7. http://dx.doi.org/10.1053/j.gastro.2003.10.010.
- Perry S, Shaw C, McGrother C, Matthews RJ, Assassa RP, Dallosso H, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002;50:480-4. http://dx.doi.org/10.1136/gut.50.4.480.
- Pretlove SJ, Radley S, Toozs-Hobson PM, Thompson PJ, Coomarasamy A, Khan KS. Prevalence of anal incontinence according to age and gender: a systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct 2006;17:407-17. http://dx.doi.org/10.1007/s00192-005-0014-5.
- Damon H, Guye O, Seigneurin A, Long F, Sonko A, Faucheron JL, et al. Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol 2006;30:37-43. http://dx.doi.org/10.1016/S0399-8320(06)73076-7.
- Hughes BT, Chepyala P, Hendon S, Crowell MD, Olden KW. Fecal incontinence in an inpatient population: a not uncommon finding. Dig Dis Sci 2009;54:2215-19. http://dx.doi.org/10.1007/s10620-008-0592-4.
- Norderval S, Nsubuga D, Bjelke C, Frasunek J, Myklebust I, Vonen B. Anal incontinence after obstetric sphincter tears: incidence in a Norwegian county. Acta Obstet Gynecol Scand 2004;83:989-94. http://dx.doi.org/10.1111/j.0001-6349.2004.00647.x.
- Whitehead WE. Diagnosing and managing fecal incontinence: if you don’t ask, they won’t tell. Gastroenterology 2005;129. http://dx.doi.org/10.1053/j.gastro.2005.05.043.
- Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol 2006;4:1004-9. http://dx.doi.org/10.1016/j.cgh.2006.01.003.
- Kamm MA. Faecal incontinence. BMJ 1998;316:528-32. http://dx.doi.org/10.1136/bmj.316.7130.528.
- Collings S, Norton C. Women’s experiences of faecal incontinence: a study. Br J Community Nurs 2004;9:520-3. http://dx.doi.org/10.12968/bjcn.2004.9.12.17239.
- Cotterill N, Norton C, Avery KN, Abrams P, Donovan JL. A patient-centered approach to developing a comprehensive symptom and quality of life assessment of anal incontinence. Dis Colon Rectum 2008;51:82-7. http://dx.doi.org/10.1007/s10350-007-9069-3.
- Finne-Soveri H, Sorbye LW, Jonsson PV, Carpenter GI, Bernabei R. Increased work-load associated with faecal incontinence among home care patients in 11 European countries. Eur J Public Health 2008;18:323-8. http://dx.doi.org/10.1093/eurpub/ckm085.
- Miner PB. Economic and personal impact of fecal and urinary incontinence. Gastroenterology 2004;126:S8-13. http://dx.doi.org/10.1053/j.gastro.2003.10.056.
- Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev 2012;7. http://dx.doi.org/10.1002/14651858.cd002111.pub3.
- Sun WM, Read NW, Verlinden M. Effects of loperamide oxide on gastrointestinal transit time and anorectal function in patients with chronic diarrhoea and faecal incontinence. Scand J Gastroenterol 1997;32:34-8. http://dx.doi.org/10.3109/00365529709025060.
- Faecal Incontinence: The Management of Faecal Incontinence in Adults. London: NICE; 2007.
- Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet 2000;355:260-5. http://dx.doi.org/10.1016/S0140-6736(99)05218-6.
- Tillin T, Gannon K, Feldman RA, Williams NS. Third-party prospective evaluation of patient outcomes after dynamic graciloplasty. Br J Surg 2006;93:1402-10. http://dx.doi.org/10.1002/bjs.5393.
- Guidelines for Faecal Incontinence. London: NICE; 2011.
- Brown SR, Wadhawan H, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database Syst Rev 2013;7. http://dx.doi.org/10.1002/14651858.cd001757.pub4.
- Mowatt G, Glazener C, Jarrett M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev 2007;3. http://dx.doi.org/10.1002/14651858.cd004464.pub2.
- Leroi AM, Parc Y, Lehur PA, Mion F, Barth X, Rullier E, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662-9. http://dx.doi.org/10.1097/01.sla.0000186281.09475.db.
- Tjandra JJ, Chan MKY, Yeh CH, Murray-Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum 2008;51:494-502. http://dx.doi.org/10.1007/s10350-007-9103-5.
- Jarrett ME, Mowatt G, Glazener CM, Fraser C, Nicholls RJ, Grant AM, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg 2004;91:1559-69. http://dx.doi.org/10.1002/bjs.4796.
- Matzel KE, Kamm MA, Stosser M, Baeten CG, Christiansen J, Madoff R, et al. Sacral spinal nerve stimulation for faecal incontinence: multicentre study. Lancet 2004;363:1270-6. http://dx.doi.org/10.1016/S0140-6736(04)15999-0.
- Wexner SD, Coller JA, Devroede G, Hull T, McCallum R, Chan M, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg 2010;251:441-9. http://dx.doi.org/10.1097/SLA.0b013e3181cf8ed0.
- Thin NN, Horrocks EJ, Hotouras A, Palit S, Thaha MA, Chan CL, et al. A systematic review of the clinical effectiveness of neuromodulation in the treatment of faecal incontinence. Br J Surg 2013;100:1430-47. http://dx.doi.org/10.1002/bjs.9226.
- Dudding TC, Meng Lee E, Faiz O, Pares D, Vaizey CJ, McGuire A, et al. Economic evaluation of sacral nerve stimulation for faecal incontinence. Br J Surg 2008;95:1155-63. http://dx.doi.org/10.1002/bjs.6237.
- Gladman MA, Knowles CH. Surgical treatment of patients with constipation and fecal incontinence. Gastroenterol Clin North Am 2008;37:605-25. http://dx.doi.org/10.1016/j.gtc.2008.06.009.
- Hotouras A, Murphy J, Allison M, Curry A, Williams NS, Knowles CH, et al. Prospective clinical audit of two neuromodulatory treatments for fecal incontinence: sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS). Surg Today 2014;44:2124-30. http://dx.doi.org/10.1007/s00595-014-0898-0.
- Sprange K, Clift M, Burke M, Whitehead SR, Hutton J. Evidence Review: Sacral Nerve Stimulation for Faecal Incontinence. Derby: Healthcare Innovation and Technology Evaluation Centre (HITEC); 2009.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol 1983;129:78-9.
- Shafik A, Ahmed I, El-Sibai O, Mostafa RM. Percutaneous peripheral neuromodulation in the treatment of fecal incontinence. Eur Surg Res 2003;35:103-7. http://dx.doi.org/10.1159/000069399.
- de la Portilla F, Rada R, Vega J, Gonzalez CA, Cisneros N, Maldonado VH. Evaluation of the use of posterior tibial nerve stimulation for the treatment of fecal incontinence: preliminary results of a prospective study. Dis Colon Rectum 2009;52:1427-33. http://dx.doi.org/10.1007/DCR.0b013e3181a7476a.
- Boyle DJ, Prosser K, Allison ME, Williams NS, Chan CL. Percutaneous tibial nerve stimulation for the treatment of urge fecal incontinence. Dis Colon Rectum 2010;53:432-7. http://dx.doi.org/10.1007/DCR.0b013e3181c75274.
- Findlay JM, Yeung JM, Robinson R, Greaves H, Maxwell-Armstrong C. Peripheral neuromodulation via posterior tibial nerve stimulation: a potential treatment for faecal incontinence?. Ann R Coll Surg Engl 2010;92:385-90. http://dx.doi.org/10.1308/003588410X12628812459652.
- Hotouras A, Thaha MA, Allison ME, Currie A, Scott SM, Chan CL. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis 2012;27:927-30. http://dx.doi.org/10.1007/s00384-011-1405-3.
- Govaert B, Pares D, Delgado-Aros S, La Torre F, Van Gemert WG, Baeten CG. A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence. Colorectal Dis 2010;12:1236-41. http://dx.doi.org/10.1111/j.1463-1318.2009.02020.x.
- de la Portilla F, Laporte M, Maestre MV, Diaz-Pavon JM, Gollonet JL, Palacios C, et al. Percutaneous neuromodulation of the posterior tibial nerve for the treatment of faecal incontinence: mid-term results – is retreatment required?. Colorectal Dis 2014;16:304-10. http://dx.doi.org/10.1111/codi.12539.
- Arroyo A, Parra P, Lopez A, Pena E, Ruiz-Tovar J, Benavides J, et al. Percutaneous posterior tibial nerve stimulation (PPTNS) in faecal incontinence associated with an anal sphincter lesion: results of a prospective study. Int J Surg 2014;12:146-9. http://dx.doi.org/10.1016/j.ijsu.2013.11.020.
- Hotouras A, Murphy J, Walsh U, Allison M, Curry A, Williams NS, et al. Outcome of percutaneous tibial nerve stimulation (PTNS) for fecal incontinence: a prospective cohort study. Ann Surg 2014;259:939-43. http://dx.doi.org/10.1097/SLA.0b013e3182a6266c.
- George AT, Kalmar K, Sala S, Kopanakis K, Panarese A, Dudding TC, et al. Randomized controlled trial of percutaneous versus transcutaneous posterior tibial nerve stimulation in faecal incontinence. Br J Surg 2013;100:330-8. http://dx.doi.org/10.1002/bjs.9000.
- Asari SA, Meurette G, Mantoo S, Kubis C, Wyart V, Lehur PA. Percutaneous tibial nerve versus sacral nerve stimulation for faecal incontinence: a comparative case-matched study. Colorectal Dis 2014;16:O393-9. http://dx.doi.org/10.1111/codi.12680.
- Horrocks EJ, Thin N, Thaha MA, Taylor SJ, Norton C, Knowles CH. Systematic review of tibial nerve stimulation to treat faecal incontinence. Br J Surg 2014;101:457-68. http://dx.doi.org/10.1002/bjs.9391.
- Allison M. Percutaneous tibial nerve stimulation: a new treatment for faecal incontinence. Gastrointest Nurs 2009;7:22-9. http://dx.doi.org/10.12968/gasn.2009.7.1.39370.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med 1999;107:S91-7. http://dx.doi.org/10.1016/S0002-9343(99)00086-8.
- Musial F, Klosterhalfen S, Enck P. Placebo responses in patients with gastrointestinal disorders. World J Gastroenterol 2007;13:3425-9. http://dx.doi.org/10.3748/wjg.v13.i25.3425.
- Ilnyckyj A, Shanahan F, Anton PA, Cheang M, Bernstein CN. Quantification of the placebo response in ulcerative colitis. Gastroenterology 1997;112:1854-8. http://dx.doi.org/10.1053/gast.1997.v112.pm9178676.
- Scaglia M, Delaini G, Destefano I, Hulten L. Fecal incontinence treated with acupuncture: a pilot study. Auton Neurosci 2009;145:89-92. http://dx.doi.org/10.1016/j.autneu.2008.10.014.
- Enck P, Van der Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterol Motil 2009;21:1133-41. http://dx.doi.org/10.1111/j.1365-2982.2009.01345.x.
- Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut 1999;44:77-80. http://dx.doi.org/10.1136/gut.44.1.77.
- Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmulling C, Neugebauer E, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 1995;82:216-22. http://dx.doi.org/10.1002/bjs.1800820229.
- Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 2000;43:9-16. http://dx.doi.org/10.1007/BF02237236.
- Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey: reliability and validity in a patient population. Med Care 1988;26:724-35. http://dx.doi.org/10.1097/00005650-198807000-00007.
- The EuroQoL Group . EuroQoL: a new facility for the measurement of health-realted quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Cotterill N, Norton C, Avery KN, Abrams P, Donovan JL. Psychometric evaluation of a new patient-completed questionnaire for evaluating anal incontinence symptoms and impact on quality of life: the ICIQ-B. Dis Colon Rectum 2011;54:1235-50. http://dx.doi.org/10.1097/DCR.0b013e3182272128.
- Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183:1438-43. http://dx.doi.org/10.1016/j.juro.2009.12.036.
- Peters K, Carrico D, Burks F. Validation of a sham for percutaneous tibial nerve stimulation (PTNS). Neurourol Urodyn 2009;28:58-61. http://dx.doi.org/10.1002/nau.20585.
- Lunniss PJ, Gladman MA, Hetzer FH, Williams NS, Scott SM. Risk factors in acquired faecal incontinence. J R Soc Med 2004;97:111-16. http://dx.doi.org/10.1258/jrsm.97.3.111.
- Queralto M, Portier G, Cabarrot PH, Bonnaud G, Chotard JP, Nadrigny M, et al. Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic fecal incontinence. Int J Colorectal Dis 2006;21:670-2. http://dx.doi.org/10.1007/s00384-005-0068-3.
- Goldstein H. REAL COM-IMPUTE software for multilevel multiple imputation with mized response types. J Stat Softw 2011;45:1-12.
- Ukoumunne OC. A comparison of confidence interval methods for the intraclass correlation coefficient in cluster randomized trials. Stat Med 2002;21:3757-74. http://dx.doi.org/10.1002/sim.1330.
- World Medical Association . Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects 1996. www.wma.net/en/30publications/10policies/b3/ (accessed 1 September 2014).
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- MRC Guidelines for Good Clinical Practice in Clinical Trials. London: MRC; 1998.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Health and Social Care Information Centre . Caldicott Guardians n.d. http://systems.hscic.gov.uk/infogov/caldicott (accessed 1 September 2014).
- Kahan BC, Morris TP. Analysis of multicentre trials with continuous outcomes: when and how should we account for centre effects?. Stat Med 2013;32:1136-49. http://dx.doi.org/10.1002/sim.5667.
- Sydes M, Neal D. National Data Monitoring Committees in Clinical Trials: Guidance for Research Ethics Committees. Bristol: National Research Ethics Services; 2010.
- Thin NN. Gastroenterology 2014;146. http://dx.doi.org/10.1016/S0016-5085(14)60549-7.
- Leroi AM, Siproudhis L, Etienney I, Damon H, Zerbib F, Amarenco G, et al. Transcutaneous electrical tibial nerve stimulation in the treatment of fecal incontinence: a randomized trial (CONSORT 1a). Am J Gastroenterol 2012;107:1888-96. http://dx.doi.org/10.1038/ajg.2012.330.
- Vaizey CJ. Faecal incontinence: standardizing outcome measures. Colorectal Dis 2014;16:156-8. http://dx.doi.org/10.1111/codi.12566.
- Finazzi-Agro E, Petta F, Sciobica F, Pasqualetti P, Musco S, Bove P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol 2010;184:2001-6. http://dx.doi.org/10.1016/j.juro.2010.06.113.
- Mearin F, Balboa A, Zarate N, Cucala M, Malagelada JR. Placebo in functional dyspepsia: symptomatic, gastrointestinal motor, and gastric sensorial responses. Am J Gastroenterol 1999;94:116-25. http://dx.doi.org/10.1111/j.1572-0241.1999.00781.x.
- Allescher HD, Bockenhoff A, Knapp G, Wienbeck M, Hartung J. Treatment of non-ulcer dyspepsia: a meta-analysis of placebo-controlled prospective studies. Scand J Gastroenterol 2001;36:934-41. http://dx.doi.org/10.1080/003655201750305440.
- Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2001;15:355-61. http://dx.doi.org/10.1046/j.1365-2036.2001.00937.x.
- Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2003;15:79-86. http://dx.doi.org/10.1046/j.1365-2982.2003.00389.x.
- Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med 2003;163:265-74. http://dx.doi.org/10.1001/archinte.163.3.265.
- Hotouras A, Thaha MA, Boyle D, Allison ME, Currie A, Knowles CH, et al. Short-term outcome following percutaneous tibial nerve stimulation (PTNS) for faecal incontinence: a single-centre prospective study. Colorectal Dis 2012;14:1101-5. http://dx.doi.org/10.1111/j.1463-1318.2011.02906.x.
- Graf W, Mellgren A, Matzel KE, Hull T, Johansson C, Bernstein M, et al. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, sham-controlled trial. Lancet 2011;377:997-1003. http://dx.doi.org/10.1016/S0140-6736(10)62297-0.
- Centre of the Cell . Centre of the Cell™ n.d. www.centreofthecell.org (accessed 1 September 2014).
Appendix 1 Flow diagram of study
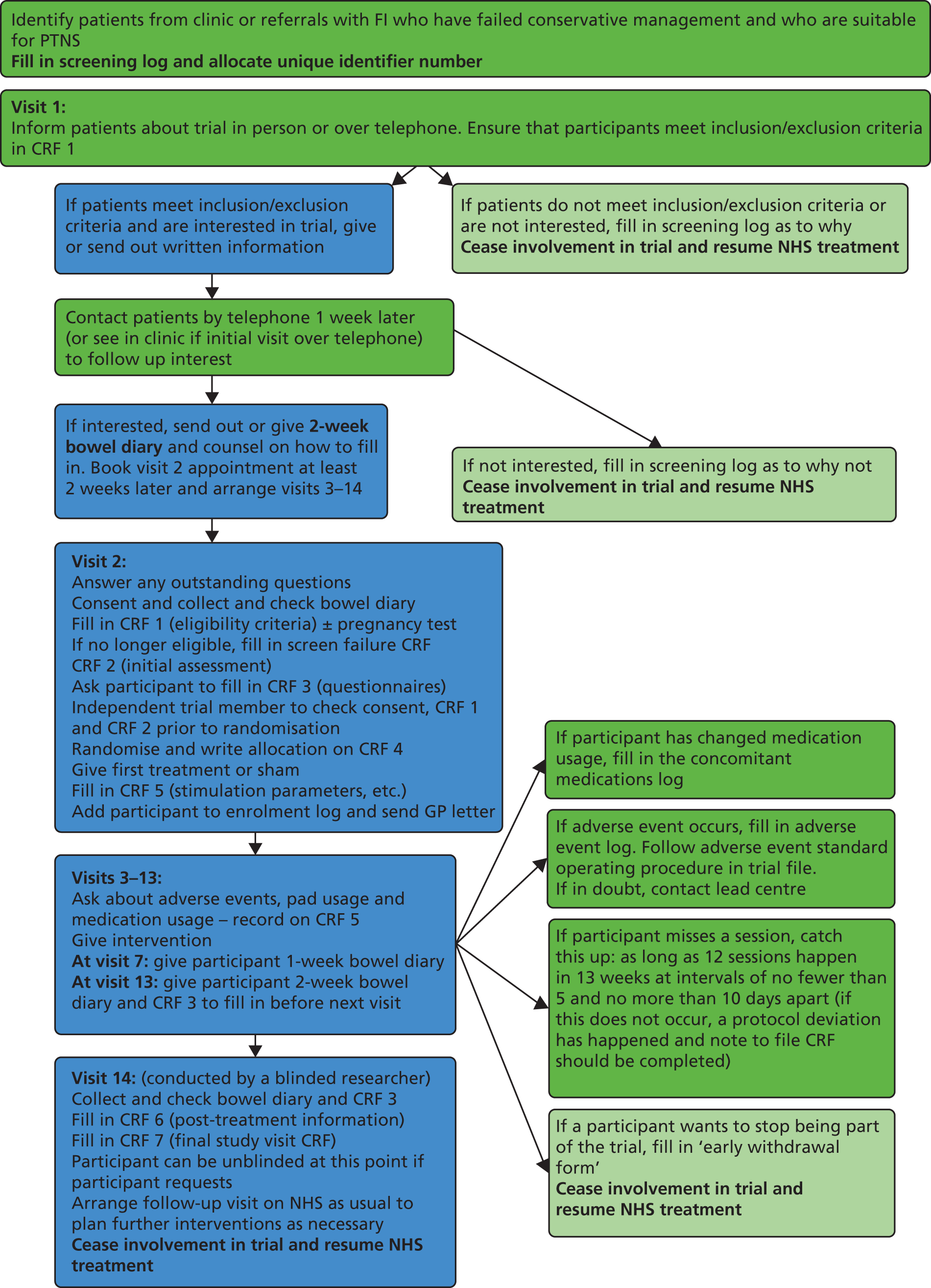
Appendix 2 Events at each visit
| Event | Visit 1 | Telephone conversation | Visit 2 | Visits 3–13 | Visit 14 |
|---|---|---|---|---|---|
| Eligibility assessment | ✗ | ||||
| Bowel diary | ✗ | Visit 7–8 | ✗ | ||
| Consent | ✗ | ||||
| Participant contact information sheet | ✗ | ||||
| Eligibility assessment (CRF 1) | ✗ | ||||
| Initial assessment (CRF 2) | ✗ | ||||
| Questionnaires (CRF 3) | ✗ | ✗ | |||
| Randomisation | ✗ | ||||
| Randomisation information (CRF 4) | ✗ | ||||
| Intervention | ✗ | ✗ | |||
| Record stimulation parameters adverse events and medication/pad usage (CRF 5) | ✗ | ✗ | |||
| Adverse events log | ✗ | ✗ | ✗ | ||
| Concomitant medications log | ✗ | ✗ | ✗ | ||
| Post-treatment information (CRF 6) | ✗ | ||||
| Final study visit information (CRF 7) | ✗ |
Appendix 3 Case report forms
Appendix 4 Standardised percutaneous tibial nerve stimulation and sham
Appendix 5 Standard operating procedures
Appendix 6 Statistical analysis plan
Appendix 7 Data and Safety Monitoring Committee input
Data Safety and Monitoring Committee meeting timings and conclusions
Meeting 1: 6 February 2012
Conclusions
The DSMC agreed to meet at least 3 weeks prior to each TSC to enable sufficient time for analysis and interpretation of data prior to reporting to the TSC. It was also recognised that safety was not really an issue (owing to the nature of the treatment) and therefore the DSMC would concentrate on the ethical basis of the trial, assessing whether or not it was being conducted according to the protocol and whether or not it was able to answer the clinical question being asked. The timelines for future meetings were agreed, with the next scheduled for 5 months later to enable sufficient data to be collected. The remaining meetings would be at 4-monthly intervals. The author and format of the DSMC charter was agreed and the method of providing unblinded data by an independent statistician was confirmed. The trial statistician also agreed to draft the statistical analysis plan in time for the next DSMC meeting.
At subsequent meetings, the trial manager and trial statistician presented reports on the trial progress to date and reviewed the open data report, with the DSMC making clarifications or further elaborating as required. The DSMC then conducted their review of the unblinded report at a closed meeting and reported their recommendations and conclusions to the TSC in writing.
Meeting 2: 24 October 2012
Recommendations
The covariates for analysis were discussed and the per protocol analysis definitions agreed. The DSMC recommended that concomitant medications usage be presented by treatment arm but also by relevance and irrelevance to the treatment of FI. The DSMC was concerned there might be a significant difference in the drop-out rate between arms and agreed to monitor this and centre effects closely. The format of the report was discussed, with recommendations made for presenting the data. It was requested that the stratification factors for randomisation be included in the next report so that the DSMC could assess balance in the randomisation. It was also requested that AEs and SAEs by allocation be included in the next report.
Conclusions
The DSMC concluded that its remit should be to assure patient safety (although not a major concern in this trial), to ensure that the treatment is not futile, to assess whether or not the outcomes are sensitive enough to measure change and to ensure that the statistical analysis plan is fair and appropriate. The committee was delighted with the general progress of the trial, noting that centres were opening rapidly and recruitment increasing appropriately. For a challenging invasive functional bowel disease trial, the CRF returns also seemed satisfactory. Review of the unblinded data revealed no specific concerns and the DSMC was happy for the trial to progress.
Meeting 3: 18 March 2013
Recommendations
The DSMC made several recommendations for the formatting and presentation of data, particularly with regard to outcome data, AEs and withdrawals. The committee was interested in the data provided for the use of constipating agents and the use of bowel medication. The DSMC recommended providing an additional two tables: the first table to show ‘use of bowel medication’ at baseline and at end of treatment; the second table to show the numbers of people who increased their intake of constipating agents or decreased their intake of constipating agents, and the same for bowel medication, for each of the two groups. Finally, the DSMC suggested meeting again in 6 months before database lock, so that a final review of the data set could be performed and so the committee could contribute to the final report.
Conclusions
The DSMC was delighted with the conduct and the progress in the trial. Recruitment was running close to target and the trial was likely to close in the summer of 2013. The DSMC was pleased to see that the completeness of the data collection was good and no concerns were raised at the open meeting.
Meeting 4: 25 November 2013
Recommendations
The DSMC recommended that SAEs needed to be categorised into probably related and unrelated. The committee suggested analysing men separately as well as together with women, owing to the small number of men in the study. It was also noted that urinary symptoms in men and women were very different and these should also be analysed separately because of different physiological processes. The committee also recommended including data from centres where fewer than five patients had been recruited and analysing with and without these data, conducting sensitivity analysis if required. The committee expected that there was unlikely to be any centre effect.
Conclusions
The committee reviewed the confidential report and had no concerns over the data. The committee congratulated the team on a very successful trial. The committee did not consider another meeting to be necessary; however, the chairperson offered to look over the final data set.
Appendix 8 Data and Safety Monitoring Committee Charter
CHARTER FOR DATA MONITORING COMMITTEE FOR THE CONFIdeNT STUDY
DRAFT FOR APPROVAL
Introduction
Study name
CONFIDeNT: CONtrol of Faecal Incontinence using Distal NeuromodulaTion.
Study registration
ISRCTN88559475.
Objectives of the study
To determine the effectiveness of PTNS versus sham electrical stimulation, based on (primary outcome) reductions in weekly FIEs and on (secondary outcomes) improvements in validated incontinence scores and other symptoms and quality-of-life measures.
Purpose of charter
The purpose of this document is to describe the roles and responsibilities of the independent Data and Monitoring Ethics Committee (DMEC) for the CONFIDeNT study, including the timing of meetings, methods of providing information to and from the TSC, frequency and format of meetings and statistical issues.
Roles and responsibilities of the Data Monitoring and Ethics Committee
Broad statement of aim of committee
To protect and serve study participants (especially regarding safety) and to assist and advise the chief investigator so as to protect the validity and credibility of the trial.
Terms of reference
The DMEC will receive and review interim results of the study.
The DMEC is responsible to the TSC of the study. Although it may choose to communicate interim results or make recommendations, the final decisions about the study rest with the TSC.
During the period of recruitment to the study, data will be supplied to the Chair of the DMEC as frequently as is requested. Meetings of the committee, either in person or by phone, will be arranged periodically, as considered appropriate by the Chair.
In the light of the interim data, the DMEC will inform the TSC if, in their view, there is proof beyond reasonable doubt that the data indicate that the treatment under investigation is either clearly indicated or clearly contraindicated.
If the DMEC does choose to inform the TSC that in their view the situation described above pertains, the TSC will consider modifying or stopping intake into the study. Unless modification or cessation is recommended by the DMEC, however, the TSC, collaborators and administrative staff (except staff who produce the confidential analyses) will remain ignorant of the interim results.
Collaborators, and all others associated with the study, may write directly to the Chair of the DMEC, to draw attention to any concern they may have about the possibility of harm arising from the use of the treatment under study, or about any other matters that may be relevant.
Specific roles of Data Monitoring and Ethics Committee
These will include interim reviews of the trial’s progress including:
-
updated figures on recruitment and losses to follow-up
-
data quality, including completeness and adherence to protocol by participants and investigators
-
evidence for treatment harm, for example safety data and adverse events
-
advice on any protocol modifications suggested by investigators or sponsors.
Before or early in the trial
Before the start of recruitment, the TSC met with the DMEC to discuss various aspects of the trial and protocol. All DMEC members had the opportunity to see the study protocol, and will have the opportunity to comment on the contents of this charter.
Among the points raised, it was suggested that a draft statistical analysis plan be drafted for the next DMEC meeting. Other points raised, relevant to the DMEC, are mentioned later in this document.
Composition of Data Monitoring and Ethics Committee and relationship to trial steering committee
Membership and size
The members are independent of the trial, and include a clinician and a statistician.
The members of the CONFIDeNT DMEC are:
Professor Dion Morton (chair)
Professor of Surgery
Academic Department of Surgery
University of Birmingham
Queen Elizabeth Hospital
Mindelsohn Way
Edgebaston
Birmingham, B15 2WB
E-mail: XX
Professor Elaine Denny (independent member)
Professor of Health Sociology
Birmingham City University
City North Campus
Birmingham, B42 2SU
E-mail: XX
Dr Dan Altmann (independent statistician)
Senior Lecturer in Medical Statistics
Department of Medical Statistics
London School of Hygiene & Tropical Medicine
Keppel Street
London, WC1E 7HT
E-mail: XX
The responsibilities of the trial statistician
The trial statistician, Dr Stephen Bremner, in consultation with the senior trialstatistician Professor Sandra Eldridge, will organise the interim reports to the DMEC. These reports will contain unblinded data, although the trial statistician will not see the unblinded version. It is not expected that the trial statistician will need to participate in DMEC meetings, but he should be available to respond to any queries arising from the interim reports.
Payment to Data Monitoring and Ethics Committee members
Members will be reimbursed for travel to and from DMEC meetings if required.
Competing interests
Data Monitoring and Ethics Committee members should disclose to the TSC any competing interests, not restricting to financial matters. DMEC members will respect the confidentiality of the interim reports, and will not use interim results to influence or inform financial trading. The format for a short competing interests form is given in Annex 1, and should be completed by DMEC members and returned to the chief investigator [CI].
Organisation of Data Monitoring and Ethics Committee meetings
Timing of data monitoring and ethics committee meetings
The first DMEC meeting should be at 5 months, and will be held in Birmingham; subsequently meetings will be held 3 to 4 weeks prior to planned TSC meetings. The format of subsequent DMEC meetings will be decided by DMEC.
Trial documentation and procedures to ensure confidentiality and proper communication
It is anticipated that the first interim report for DMEC, organised by the trial statistician, should contain data presented, where possible, by unblinded trial allocation group, to include (1) recruitment flow chart (showing attendance at scheduled visits and any reported non-adherence and withdrawal from the trial); (2) primary and secondary outcomes; and (3) adverse events and any other safety-related data. A dummy report before the first DMEC meeting, although helpful, is not necessary. Changes to format or content may be requested by DMEC for subsequent DMEC meetings.
Access to the accumulating data and interim results
Data and Safety Monitoring Committee members do not have the right to share confidential information with anyone outside the DMEC, including the CI and named applicants.
Responsibility for identifying and circulating external evidence
Identification and circulation of external evidence (e.g. from other trials/systematic reviews) is not the responsibility of the DMEC members. The TSC will usually collate any such information if appropriate.
To whom the data monitoring and ethics committee will communicate the decisions/recommendations that are reached
The DMEC will report its recommendations in writing to the TSC, copying to the trial statistician, in time for consideration at the next TSC meeting. It is hoped and anticipated that, routinely, if the trial is to continue largely unchanged, then a note from the DMEC Chair back to TSC along the lines given in Annex 2 will be sufficient response.
Ensuring safety of confidential papers
The DMEC members should store interim report papers safely after each meeting so they may check the next report against them. After the trial is reported, the DMEC members should destroy all interim reports.
Decision-making
What decisions/recommendations will be open to the Data Monitoring and Ethics Committee?
Possible recommendations could include:
-
no action needed, trial continues as planned
-
early stopping as a result, for example, of clear benefit or harm of a treatment, futility or external evidence
-
extending recruitment (based on actual control arm response rates being different to predicted) or extending follow-up
-
sanctioning and/or proposing protocol changes.
Statistical methods
Any planned interim analyses should be tabled by the trial statistician before the first DMEC meeting for discussion and agreement.
How decisions or recommendations will be reached within the Data Monitoring and Ethics Committee
Data Monitoring and Ethics Committee members must agree on a process of decision-making, including whether or not there will be voting or other formal or informal methods of achieving consensus.
Every effort should be made by the DMEC to reach a unanimous decision. If the DMEC cannot achieve this, a vote may be taken, although details of the vote should not be routinely included in the report to the TSC, as these may inappropriately convey information about the state of the trial data. It is important that the implications (e.g. ethical, statistical, practical, financial) for the trial be considered before any recommendation is made.
When the Data Monitoring and Ethics Committee is quorate for decision-making
Owing to the relatively small size of the DMEC, decisions should involve all three members, but not necessarily at a face-to-face meeting: teleconference or e-mail contact may be sufficient. DMEC members who will not be able to attend a planned face-to-face meeting may pass comments to the DMEC Chair for consideration during any discussions.
Reporting
Minutes of meetings
Separate minutes of DMEC meetings will be taken by the Chair of the DMEC. The DMEC Chair must sign off all minutes.
What will be done if there is disagreement between the Data Monitoring and Ethics Committee and the trial steering committee?
If the DMEC has serious problems or concerns with the TSC decision, a meeting of these groups should be held. The information to be shown would depend upon the action proposed and the DMEC’s concerns. Depending on the reason for the disagreement confidential data may have to be revealed to all those attending such a meeting. The meeting should be chaired by a senior member of the TSC or an external expert who is not directly involved with the trial. The funder may be invited to such meetings.
After the trial
Publication of results
At the end of the trial there will be a meeting to allow the DMEC to discuss the final data with the chief investigator and give advice about data interpretation.
The information about the Data Monitoring and Ethics Committee that will be included in published trial reports
Data Monitoring and Ethics Committee members will be named and their affiliations listed in the main trial report, unless they explicitly request otherwise. A brief summary of the timings and conclusions of DMEC meetings should be included in the body of this paper.
Will the Data Monitoring and Ethics Committee have the opportunity to comment on publications before submission?
The DMEC will be given an opportunity to read and comment on any publication before it is submitted.
Constraints on Data and Safety Monitoring Committee members divulging information about their deliberations after the trial has been published
The DMEC may discuss issues from their involvement in the trial 12 months after the primary trial results have been published.
Annex 1 Competing interests form
Potential competing interests of DMEC members for CONFIDeNT, ISRCTN88559475.
The avoidance of any perception that members of a DMEC may be biased in some fashion is important for the credibility of the decisions made by the DMEC and for the integrity of the CONFIDeNT study. Possible competing interests should be disclosed: in many cases simple disclosure should be sufficient. Otherwise, the DMEC member should remove the conflict or stop participating in the DMEC. Table 18 lists potential competing interests.
| Stock ownership in any commercial companies involved |
| Stock transaction in any commercial company involved (if previously holding stock) |
| Consulting arrangements with the sponsor |
| Frequent speaking engagements on behalf of the intervention |
| Career tied up in a product or technique assessed by trial |
| Hands-on participation in the trial |
| Involvement in the running of the trial |
| Emotional involvement in the trial |
| Intellectual conflict, for example strong prior belief in the trial’s experimental arm |
| Involvement in regulatory issues relevant to the trial procedures |
| Investment (financial or intellectual) in competing products |
| Involvement in the publication |
Please complete the following section and return to the Chief Investigator.
______ No, I have no competing interests to declare
______ Yes, I have competing interests to declare (please detail below)
Please provide details of any competing interests:
_____________________________________________________________
Name: _______________________________
Signed: ______________________________ Date: ________________
Annex 2 Suggested report from the Data Monitoring and Ethics Committee to the Trial Steering Committee where no recommendations are being made
[Insert date]
To: Chair of Trial Steering Committee
Dear [Chair of Trial Steering Committee]
The Data Monitoring and Ethics Committee (DMEC) for the [insert trial name] trial met on [meeting date] to review its progress and interim accumulating data. [List members] attended the meeting and reviewed the report.
The trial question remains important and, on the basis of the data reviewed at this stage, we recommend continuation of the trial according to the current version of the protocol [specify protocol version number and date] with no changes.
We shall next review the progress and data [provide approximate timing]
Yours sincerely,
[Name of meeting Chair]
Chair of Data Monitoring and Ethics Committee
On behalf of the DMEC (all members listed below)
DMEC members:
(1) [Insert name and role]
(2) [Insert name and role]
(3) [Insert name and role]
Appendix 9 Patient and public involvement
The CONFIDeNT trial has had active collaboration with the Bowel & Cancer Research charity from the early stages of the trial and throughout trial implementation and dissemination of results. The chief executive of this charity has been a lay representative on the TSC and has been influential in portraying the lay understanding and interpretation of trial materials, reviewing the trial protocol and patient information material, and aiding in the production of a lay summary of trial results for dissemination to patients and user groups, a copy of which will be published on the Bowel & Cancer Research charity website.
The researchers recognise that there was limited patient and public involvement in the development of the research question, in the grant application and in the review of patient materials because of the lack of an established patient and public involvement network at the time of trial design. Through linking with the Bowel & Cancer Research charity, and in collaboration with the National Centre for Bowel Research and Surgical Innovation, we have strengthened our patient and public involvement position for future trials by establishing a patient and public involvement group and now have an established patient and public involvement group with over 70 members. This group is specifically interested in areas of bowel research being conducted in the centre, including FI and constipation, inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis, bowel cancer and stomas. Now that this database of patient and public involvement members has been established, we are able to consult members for feedback on surveys and questionnaires, on trial design and acceptability and on acceptability of documentation developed for patients, including advertising materials, patient information sheets, diaries and questionnaires, as well as able to invite members for inclusion in TSCs and attendance at meetings and workshops.
This collaboration held a highly successful event at Barts and the London School of Medicine and Dentistry in December 2012 to showcase the research being undertaken within the centre. Members of the PPI group were invited to attend and patient representatives were also invited to speak about experience participating in research. A similar event was run in April 2015, which involved more interactive workshops to aid understanding of what it means to be involved in a patient and public involvement group and deliver training on the various tasks that may be undertaken as a patient and public involvement member.
Finally, should HTA funding be agreed, we plan to develop a ‘pod’ and live science show at the Centre of the Cell. This is a centre for public engagement which ‘combines an award-winning interactive science centre plus live science shows, workshops, debates, science talks and a widening participation for East London and beyond’. 82 We have been in initial discussions with the Centre of the Cell, and have come up with some fantastic ideas on how to disseminate the results of this trial while raising awareness of bowel problems such as FI and try to improve education about the healthy bowel. As part of this, we plan to develop a high-tech, multimedia, interactive pod show for children. This will provide longevity in disseminating the message of preventing bowel disease from an early age and also educate children on the type of medical research undertaken to help prevent and treat bowel disease.
The following text is a lay summary of the CONFIDeNT trial results, which has been edited by our patient and public involvement group and will be sent out to all trial participants:
Thank you very much for participating in this important research project.
The aim of the study was to assess the effectiveness of PTNS (percutaneous tibial nerve stimulation) compared to placebo or sham (pretend) electrical stimulation treatment in reducing FIEs.
In total, 227 patients from 17 NHS hospitals across the UK were involved in the study. Half of the patients had PTNS treatment and half had sham treatment, each undergoing a course of 12 treatments over 12 weeks. We recorded your faecal incontinence episodes before treatment commencement, after 6 treatments and at the end of 12 treatments. Your treatment was deemed successful if you experienced a 50% or more reduction in faecal incontinence episodes as recorded on bowel diaries. We also measured changes in your quality of life from questionnaires.
This study is now complete and we write to you to share the results. There was no significant difference between the number of patients in each group who experienced a 50% or more reduction in faecal incontinence episodes. In the PTNS group, 38% of patients had successful treatment and in the sham group 31% of patients had successful treatment. This does not amount to a significant difference between the two groups. There was also no difference in improvement in quality of life between the group that received real PTNS and those that received sham treatment.
Further analysis did however show that patients experienced a significant reduction in the overall number of faecal incontinence episodes in the PTNS group, and this did not happen in the sham group. This seemed to be a reduction in the ‘rush’ type episodes rather than the ‘no warning’ type episodes. This could be important for tailoring future treatment and further research into this area is required.
The results of this study will be shared with doctors and other medical practitioners through national and international conferences in a detailed scientific report.
If you would like to read more about these findings, please refer to the lay summary of results on the Bowel & Cancer Research Charity website www.bowelcancerresearch.org. A full detailed report will be available on the funder’s website, National Institute of Health Research www.nihr.ac.uk.
Once again thank you for your participation, without which we could not have completed the study. We enclose with this letter an invitation for your continued involvement in our patient and public involvement group (PPI group) and do hope you may be interested in working with us in the future to promote public awareness and engagement with research, particularly in the area of bowel diseases.
Appendix 10 Raw data
| Outcome | PTNS | Sham | Total |
|---|---|---|---|
| Total weekly FIEs at baseline | |||
| n | 111 | 108 | 219 |
| Mean (SD) | 9.9 (11.2) | 10.4 (10.9) | 10.2 (11.0) |
| Median (IQR) | 6.0 (2.0–14.0) | 6.9 (2.5–16.0) | 6.5 (2.0–14.6) |
| Minimum to maximum | 0.0 to 57.0 | 0.0 to 71.0 | 0.0 to 71.0 |
| Urge FIEs per week at baseline | |||
| n | 114 | 109 | 223 |
| Mean (SD) | 5.3 (7.2) | 4.8 (5.9) | 5.1 (6.6) |
| Median (IQR) | 3.0 (0.9–8.0) | 2.5 (0.5–7.0) | 3.0 (0.5–7.0) |
| Minimum to maximum | 0.0 to 42.5 | 0.0 to 41.0 | 0.0 to 42.5 |
| Passive FIEs per week at baseline | |||
| n | 112 | 108 | 220 |
| Mean (SD) | 4.6 (6.0) | 5.7 (7.6) | 5.2 (6.8) |
| Median (IQR) | 2.0 (0.0–7.5) | 3.0 (0.0–8.0) | 2.5 (0.0–7.5) |
| Minimum to maximum | 0.0 to 27.0 | 0.0 to 43.0 | 0.0 to 43.0 |
| Total weekly FIEs after treatment | |||
| n | 105 | 104 | 209 |
| Mean (SD) | 6.4 (7.6) | 9.1 (10.7) | 7.7 (9.3) |
| Median (IQR) | 3.5 (1.0–10.0) | 4.8 (1.5–12.8) | 4.0 (1.0–11.0) |
| Minimum to maximum | 0.0 to 44.5 | 0.0 to 43.5 | 0.0 to 44.5 |
| Urge FIEs per week after treatment | |||
| n | 106 | 105 | 211 |
| Mean (SD) | 3.0 (4.2) | 4.4 (6.5) | 3.7 (5.5) |
| Median (IQR) | 1.5 (0.0–4.5) | 1.5 (0.5–5.5) | 1.5 (0.0–4.5) |
| Minimum to maximum | 0.0 to 23.5 | 0.0 to 35.5 | 0.0 to 35.5 |
| Passive FIEs per week after treatment | |||
| n | 105 | 105 | 210 |
| Mean (SD) | 3.4 (4.6) | 4.7 (6.6) | 4.0 (5.8) |
| Median (IQR) | 1.5 (0.0–5.0) | 1.5 (0.0–6.5) | 1.5 (0.0–5.5) |
| Minimum to maximum | 0.0 to 21.0 | 0.0 to 33.5 | 0.0 to 33.5 |
| Outcome | PTNS | Sham | Total |
|---|---|---|---|
| SMCS at baseline | |||
| n | 110 | 101 | 211 |
| Mean (SD) | 14.4 (3.7) | 15.4 (4.1) | 14.9 (3.9) |
| Median (IQR) | 14.0 (12.0–17.0) | 16.0 (13.0–18.0) | 15.0 (13.0–18.0) |
| Minimum to maximum | 5.0 to 22.0 | 5.0 to 24.0 | 5.0 to 24.0 |
| SMCS after treatment | |||
| n | 104 | 101 | 205 |
| Mean (SD) | 13.9 (4.3) | 14.6 (4.6) | 14.3 (4.4) |
| Median (IQR) | 14.0 (11.0–17.0) | 15.0 (11.0–18.0) | 14.0 (11.0–18.0) |
| Minimum to maximum | 6.0 to 23.0 | 5.0 to 24.0 | 5.0 to 24.0 |
| Outcome | PTNS (N = 115) | Sham (N = 112) | Total (N = 227) |
|---|---|---|---|
| Sex | |||
| Male | 11 (10%) | 11 (10%) | 22 (10%) |
| Female | 104 (90%) | 101 (90%) | 205 (90%) |
| Age (years) | |||
| n | 115 | 112 | 227 |
| Mean (SD) | 57.8 (12.4) | 56.5 (12.1) | 57.2 (12.2) |
| Median (IQR) | 58.0 (50.0–67.0) | 58.0 (48.0–65.0) | 58.0 (49.0–66.0) |
| Minimum to maximum | 20.0 to 85.0 | 23.0 to 79.0 | 20.0 to 85.0 |
| Duration of symptoms (months) | |||
| n | 112 | 110 | 222 |
| Mean (SD) | 112.6 (117.5) | 79.7 (88.4) | 96.3 (105.2) |
| Median (IQR) | 60.0 (24.0–168.0) | 48.0 (24.0–108.0) | 60.0 (24.0–120.0) |
| Minimum to maximum | 5.0 to 600.0 | 6.0 to 540.0 | 5.0 to 600.0 |
| Number of vaginal deliveries | |||
| n | 90 | 96 | 186 |
| Mean (SD) | 2.3 (1.2) | 2.4 (1.1) | 2.4 (1.2) |
| Median (IQR) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| Minimum to maximum | 1.0 to 7.0 | 1.0 to 7.0 | 1.0 to 7.0 |
| Caesarean deliveries | |||
| No, n (%) | 90 (95) | 96 (100) | 186 (97) |
| Yes, n (%) | 5 (5) | 0 (0) | 5 (3) |
| Obstetric history | |||
| No, n (%) | 9 (9) | 5 (5) | 14 (7) |
| Yes, n (%) | 95 (91) | 96 (95) | 191 (93) |
| Episiotomy/tear | |||
| No, n (%) | 12 (13) | 14 (15) | 26 (14) |
| Yes, n (%) | 78 (87) | 82 (85) | 160 (86) |
| Previous biofeedback | |||
| No, n (%) | 56 (50) | 48 (45) | 104 (47) |
| Yes, n (%) | 56 (50) | 59 (55) | 115 (53) |
| Previous sphincter repair | |||
| No, n (%) | 101 (91) | 101 (94) | 202 (92) |
| Yes, n (%) | 10 (9) | 7 (6) | 17 (8) |
| Previous treatment (other), n (%) | 18 (17) | 21 (21) | 39 (19) |
| Increased stool frequency, n (%) | 69 (61) | 69 (62) | 138 (62) |
| Urgency to pass stool, n (%) | 99 (90) | 99 (88) | 198 (89) |
| Passive FI, n (%) | 88 (77) | 86 (77) | 174 (77) |
| Urge FI, n (%) | 94 (82) | 93 (83) | 187 (82) |
| Flatus incontinence, n (%) | 74 (64) | 83 (74) | 157 (69) |
| Evacuatory difficulties, n (%) | 44 (39) | 49 (44) | 93 (41) |
| Straining, n (%) | 34 (30) | 37 (33) | 71 (31) |
| Prolapse, n (%) | 4 (4) | 8 (7) | 12 (5) |
| Soils underwear, n (%) | 104 (91) | 103 (92) | 207 (92) |
| Use pads, n (%) | 77 (67) | 73 (65) | 150 (66) |
| Unable to defer defecation, n (%) | 52 (47) | 43 (41) | 95 (44) |
| Unable to distinguish faeces from flatus, n (%) | 44 (38) | 38 (35) | 82 (36) |
| Sense of rectal blockage or bulge, n (%) | 21 (18) | 22 (20) | 43 (19) |
| Digitation required, n (%) | 12 (10) | 15 (13) | 27 (12) |
| Anxiety/panic, n (%) | 75 (65) | 84 (75) | 159 (70) |
| Urinary symptom history, n (%) | 70 (61) | 72 (64) | 142 (63) |
| Increased frequency of urine, n (%) | 44 (38) | 43 (38) | 87 (38) |
| Urinary urgency, n (%) | 50 (43) | 49 (44) | 99 (44) |
| Urinary stress incontinence, n (%) | 43 (37) | 42 (38) | 85 (37) |
| Urinary urge incontinence, n (%) | 39 (34) | 42 (38) | 81 (36) |
| Other urinary symptoms, n (%) | 15 (14) | 12 (12) | 27 (13) |
| Outcome | PTNS (N = 115) | Sham (N = 111) | Total (N = 226) |
|---|---|---|---|
| Total weekly FIEs at baseline | |||
| n | 111 | 108 | 219 |
| Mean (SD) | 9.9 (11.2) | 10.4 (10.9) | 10.2 (11.0) |
| Median (IQR) | 6.0 (2.0–14.0) | 6.9 (2.5–16.0) | 6.5 (2.0–14.6) |
| Minimum to maximum | 0.0 to 57.0 | 0.0 to 71.0 | 0.0 to 71.0 |
| Urge FIEs per week at baseline | |||
| n | 114 | 109 | 223 |
| Mean (SD) | 5.3 (7.2) | 4.8 (5.9) | 5.1 (6.6) |
| Median (IQR) | 3.0 (0.9–8.0) | 2.5 (0.5–7.0) | 3.0 (0.5–7.0) |
| Minimum to maximum | 0.0 to 42.5 | 0.0 to 41.0 | 0.0 to 42.5 |
| Passive FIEs per week at baseline | |||
| n | 112 | 108 | 220 |
| Mean (SD) | 4.6 (6.0) | 5.7 (7.6) | 5.2 (6.8) |
| Median (IQR) | 2.0 (0.0–7.5) | 3.0 (0.0–8.0) | 2.5 (0.0–7.5) |
| Minimum to maximum | 0.0 to 27.0 | 0.0 to 43.0 | 0.0 to 43.0 |
| Controlled defecations per week at baseline | |||
| n | 115 | 111 | 226 |
| Mean (SD) | 13.2 (12.2) | 12.4 (9.5) | 12.8 (11.0) |
| Median (IQR) | 11.0 (4.7–17.5) | 10.5 (6.5–17.0) | 11.0 (6.0–17.0) |
| Minimum to maximum | 0.0 to 83.5 | 0.0 to 51.5 | 0.0 to 83.5 |
| Days of underwear staining per week at baseline | |||
| n | 114 | 110 | 224 |
| Mean (SD) | 3.8 (2.2) | 4.0 (2.4) | 3.9 (2.3) |
| Median (IQR) | 3.7 (2.0–6.0) | 4.5 (2.0–6.0) | 4.0 (2.0–6.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days using pad per week at baseline | |||
| n | 112 | 106 | 218 |
| Mean (SD) | 3.6 (3.2) | 3.7 (3.3) | 3.6 (3.3) |
| Median (IQR) | 4.5 (0.0–7.0) | 4.3 (0.0–7.0) | 4.5 (0.0–7.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days with an enema per week at baseline | |||
| n | 112 | 107 | 219 |
| Mean (SD) | 0.3 (1.1) | 0.2 (0.7) | 0.2 (0.9) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly solid at baseline | |||
| n | 108 | 109 | 217 |
| Mean (SD) | 2.6 (2.5) | 2.6 (2.2) | 2.6 (2.3) |
| Median (IQR) | 2.0 (0.0–4.8) | 2.3 (0.5–3.8) | 2.0 (0.5–4.2) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly mushy at baseline | |||
| n | 108 | 109 | 217 |
| Mean (SD) | 0.8 (1.4) | 1.0 (1.4) | 0.9 (1.4) |
| Median (IQR) | 0.0 (0.0–1.2) | 0.0 (0.0–1.5) | 0.0 (0.0–1.4) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly liquid at baseline | |||
| n | 108 | 109 | 217 |
| Mean (SD) | 3.5 (2.3) | 3.4 (2.1) | 3.5 (2.2) |
| Median (IQR) | 3.5 (1.8–5.5) | 3.5 (2.0–5.0) | 3.5 (1.9–5.3) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Outcome | PTNS (N = 115) | Sham (N = 112) | Total (N = 227) |
|---|---|---|---|
| GIQoL at baseline | |||
| n | 98 | 98 | 196 |
| Mean (SD) | 126.7 (18.8) | 123.8 (20.2) | 125.3 (19.5) |
| Median (IQR) | 130.0 (113.0–41.0) | 126.5 (109.0–139.0) | 128.0 (112.0–140.0) |
| Minimum to maximum | 78.0 to 162.0 | 68.0 to 160.0 | 68.0 to 162.0 |
| EQ-5D at baseline | |||
| n | 115 | 109 | 224 |
| Mean (SD) | 0.69 (0.27) | 0.63 (0.34) | 0.66 (0.31) |
| Median (IQR) | 0.73 (0.62–0.85) | 0.73 (0.62–0.85) | 0.73 (0.62–0.85) |
| Minimum to maximum | –0.18 to 1.00 | –0.24 to 1.00 | –0.24 to 1.00 |
| SMCS at baseline | |||
| n | 110 | 101 | 211 |
| Mean (SD) | 14.4 (3.7) | 15.4 (4.1) | 14.9 (3.9) |
| Median (IQR) | 14.0 (12.0–17.0) | 16.0 (13.0–18.0) | 15.0 (13.0–18.0) |
| Minimum to maximum | 5.0 to 22.0 | 5.0 to 24.0 | 5.0 to 24.0 |
| Patient-centred outcomes at baseline | |||
| n | 100 | 92 | 192 |
| Mean (SD) | 8.5 (1.6) | 8.7 (1.7) | 8.6 (1.7) |
| Median (IQR) | 8.9 (7.8–9.8) | 9.2 (8.3–10.0) | 9.0 (8.0–9.9) |
| Minimum to maximum | 1.9 to 10.0 | 1.6 to 10.0 | 1.6 to 10.0 |
| SF-36 physical functioning at baseline | |||
| n | 108 | 107 | 215 |
| Mean (SD) | 65.7 (27.4) | 61.4 (28.4) | 63.6 (27.9) |
| Median (IQR) | 70.0 (45.0–90.0) | 65.0 (40.0–85.0) | 70.0 (40.0–85.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 role-physical at baseline | |||
| n | 111 | 107 | 218 |
| Mean (SD) | 46.4 (42.1) | 36.4 (41.4) | 41.5 (41.9) |
| Median (IQR) | 50.0 (0.0–100.0) | 25.0 (0.0–75.0) | 25.0 (0.0–100.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 bodily pain at baseline | |||
| n | 113 | 112 | 225 |
| Mean (SD) | 61.3 (30.0) | 58.2 (31.5) | 59.8 (30.7) |
| Median (IQR) | 60.0 (40.0–90.0) | 57.5 (32.5–90.0) | 57.5 (32.5–90.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 general health at baseline | |||
| n | 114 | 108 | 222 |
| Mean (SD) | 51.2 (23.4) | 50.3 (23.8) | 50.8 (23.6) |
| Median (IQR) | 50.0 (35.0–70.0) | 50.0 (30.0–70.0) | 50.0 (35.0–70.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 95.0 | 0.0 to 100.0 |
| SF-36 vitality at baseline | |||
| n | 108 | 104 | 212 |
| Mean (SD) | 43.9 (22.1) | 42.7 (22.8) | 43.3 (22.4) |
| Median (IQR) | 45.0 (30.0–57.5) | 50.0 (30.0–60.0) | 50.0 (30.0–60.0) |
| Minimum to maximum | 0.0 to 85.0 | 0.0 to 95.0 | 0.0 to 95.0 |
| SF-36 social functioning at baseline | |||
| n | 115 | 112 | 227 |
| Mean (SD) | 58.4 (28.8) | 59.3 (31.6) | 58.8 (30.1) |
| Median (IQR) | 62.5 (37.5–75.0) | 62.5 (37.5–87.5) | 62.5 (37.5–87.5) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 role-emotional function at baseline | |||
| n | 113 | 111 | 224 |
| Mean (SD) | 56.3 (43.0) | 48.9 (44.2) | 52.7 (43.7) |
| Median (IQR) | 66.7 (0.0–100.0) | 33.3 (0.0–100.0) | 66.7 (0.0–100.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 mental health at baseline | |||
| n | 109 | 106 | 215 |
| Mean (SD) | 60.3 (21.0) | 60.8 (21.6) | 60.6 (21.3) |
| Median (IQR) | 60.0 (44.0–76.0) | 64.0 (48.0–76.0) | 64.0 (44.0–76.0) |
| Minimum to maximum | 12.0 to 100.0 | 0.0 to 96.0 | 0.0 to 100.0 |
| FIQoL lifestyle at baseline | |||
| n | 93 | 92 | 185 |
| Mean (SD) | 2.6 (0.9) | 2.6 (1.0) | 2.6 (1.0) |
| Median (IQR) | 2.7 (1.8–3.4) | 2.5 (1.7–3.6) | 2.7 (1.7–3.5) |
| Minimum to maximum | 1.0 to 4.0 | 1.0 to 4.0 | 1.0 to 4.0 |
| FIQoL coping at baseline | |||
| n | 79 | 77 | 156 |
| Mean (SD) | 1.9 (0.7) | 1.9 (0.9) | 1.9 (0.8) |
| Median (IQR) | 1.7 (1.2–2.3) | 1.6 (1.1–2.6) | 1.7 (1.2–2.4) |
| Minimum to maximum | 1.0 to 4.0 | 1.0 to 4.0 | 1.0 to 4.0 |
| FIQoL depression at baseline | |||
| n | 75 | 81 | 156 |
| Mean (SD) | 2.8 (0.9) | 2.7 (0.9) | 2.8 (0.9) |
| Median (IQR) | 3.1 (2.0–3.4) | 2.6 (2.0–3.7) | 2.9 (2.0–3.6) |
| Minimum to maximum | 1.1 to 4.1 | 1.0 to 4.1 | 1.0 to 4.1 |
| FIQoL embarrassment at baseline | |||
| n | 110 | 106 | 216 |
| Mean (SD) | 2.2 (0.8) | 2.1 (0.8) | 2.1 (0.8) |
| Median (IQR) | 2.0 (1.7–2.7) | 2.0 (1.3–2.7) | 2.0 (1.3–2.7) |
| Minimum to maximum | 1.0 to 4.0 | 1.0 to 3.7 | 1.0 to 4.0 |
| Outcome | PTNS (N = 110) | Sham (N = 110) | Total (N = 220) |
|---|---|---|---|
| Total weekly FIEs mid-treatment | |||
| n | 97 | 99 | 196 |
| Mean (SD) | 6.1 (7.6) | 9.6 (11.5) | 7.8 (9.9) |
| Median (IQR) | 4.0 (1.0–9.0) | 6.0 (1.0–14.0) | 4.0 (1.0–10.0) |
| Minimum to maximum | 0.0 to 46.0 | 0.0 to 51.0 | 0.0 to 51.0 |
| Urge FIEs per week mid-treatment | |||
| n | 102 | 102 | 204 |
| Mean (SD) | 3.3 (4.3) | 4.4 (6.3) | 3.9 (5.4) |
| Median (IQR) | 2.0 (0.0–5.0) | 2.0 (0.0–7.0) | 2.0 (0.0–6.0) |
| Minimum to maximum | 0.0 to 25.0 | 0.0 to 29.0 | 0.0 to 29.0 |
| Passive FIEs per week mid-treatment | |||
| n | 100 | 102 | 202 |
| Mean (SD) | 3.0 (4.6) | 5.0 (7.3) | 4.0 (6.2) |
| Median (IQR) | 1.0 (0.0–4.5) | 1.0 (0.0–8.0) | 1.0 (0.0–6.0) |
| Minimum to maximum | 0.0 to 24.0 | 0.0 to 37.0 | 0.0 to 37.0 |
| Controlled defecations per week mid-treatment | |||
| n | 104 | 102 | 206 |
| Mean (SD) | 13.0 (11.5) | 11.4 (7.6) | 12.2 (9.8) |
| Median (IQR) | 11.0 (6.0–15.5) | 10.0 (7.0–15.0) | 10.0 (7.0–15.0) |
| Minimum to maximum | 0.0 to 84.0 | 0.0 to 44.0 | 0.0 to 84.0 |
| Days of underwear staining per week mid-treatment | |||
| n | 99 | 97 | 196 |
| Mean (SD) | 3.3 (2.3) | 3.4 (2.6) | 3.4 (2.5) |
| Median (IQR) | 3.0 (1.0–5.0) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days using pad per week mid-treatment | |||
| n | 99 | 99 | 198 |
| Mean (SD) | 3.7 (3.2) | 3.8 (3.4) | 3.8 (3.3) |
| Median (IQR) | 4.0 (0.0–7.0) | 7.0 (0.0–7.0) | 5.5 (0.0–7.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days using an enema per week mid-treatment | |||
| n | 95 | 97 | 192 |
| Mean (SD) | 0.2 (0.7) | 0.2 (0.8) | 0.2 (0.8) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Minimum to maximum | 0.0 to 4.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly solid mid-treatment | |||
| n | 65 | 70 | 135 |
| Mean (SD) | 3.2 (2.3) | 3.2 (2.4) | 3.2 (2.3) |
| Median (IQR) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly mushy mid-treatment | |||
| n | 65 | 70 | 135 |
| Mean (SD) | 0.5 (1.0) | 1.0 (1.7) | 0.8 (1.4) |
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) |
| Minimum to maximum | 0.0 to 4.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly liquid mid-treatment | |||
| n | 65 | 70 | 135 |
| Mean (SD) | 3.3 (2.4) | 2.8 (2.6) | 3.1 (2.5) |
| Median (IQR) | 3.0 (2.0–5.0) | 2.5 (0.0–5.0) | 3.0 (0.0–5.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Outcome | PTNS (N = 109) | Sham (N = 108) | Total (N = 217) |
|---|---|---|---|
| Primary outcome | |||
| ≥ 50% reduction in FIEs, n (%) | 39 (38) | 32 (31) | 71 (35) |
| ≥ 25% reduction in FIEs, n (%) | 51 (50) | 46 (45) | 97 (47) |
| ≥ 75% reduction in FIEs, n (%) | 26 (25) | 17 (17) | 43 (21) |
| 100% reduction in FIEs, n (%) | 11 (11) | 7 (7) | 18 (9) |
| Total weekly FIEs after treatment | |||
| n | 105 | 104 | 209 |
| Mean (SD) | 6.4 (7.6) | 9.1 (10.7) | 7.7 (9.3) |
| Median (IQR) | 3.5 (1.0–10.0) | 4.8 (1.5–12.8) | 4.0 (1.0–11.0) |
| Minimum to maximum | 0.0 to 44.5 | 0.0 to 43.5 | 0.0 to 44.5 |
| Urge FIEs per week after treatment | |||
| n | 106 | 105 | 211 |
| Mean (SD) | 3.0 (4.2) | 4.4 (6.5) | 3.7 (5.5) |
| Median (IQR) | 1.5 (0.0–4.5) | 1.5 (0.5–5.5) | 1.5 (0.0–4.5) |
| Minimum to maximum | 0.0 to 23.5 | 0.0 to 35.5 | 0.0 to 35.5 |
| Passive FIEs per week after treatment | |||
| n | 105 | 105 | 210 |
| Mean (SD) | 3.4 (4.6) | 4.7 (6.6) | 4.0 (5.8) |
| Median (IQR) | 1.5 (0.0–5.0) | 1.5 (0.0–6.5) | 1.5 (0.0–5.5) |
| Minimum to maximum | 0.0 to 21.0 | 0.0 to 33.5 | 0.0 to 33.5 |
| Controlled defecations per week after treatment | |||
| n | 104 | 107 | 211 |
| Mean (SD) | 13.0 (9.9) | 11.6 (9.2) | 12.3 (9.6) |
| Median (IQR) | 11.0 (7.0–16.3) | 10.5 (5.5–15.5) | 11.0 (6.5–16.0) |
| Minimum to maximum | 0.0 to 67.0 | 0.0 to 68.5 | 0.0 to 68.5 |
| Days of underwear staining per week after treatment | |||
| n | 106 | 106 | 212 |
| Mean (SD) | 3.1 (2.2) | 3.3 (2.5) | 3.2 (2.4) |
| Median (IQR) | 2.5 (1.0–4.5) | 3.0 (1.0–6.0) | 2.8 (1.0–5.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days using pad per week after treatment | |||
| n | 103 | 103 | 206 |
| Mean (SD) | 3.6 (3.2) | 3.6 (3.3) | 3.6 (3.3) |
| Median (IQR) | 4.0 (0.0–7.0) | 4.5 (0.0–7.0) | 4.3 (0.0–7.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days using an enema per week after treatment | |||
| n | 103 | 102 | 205 |
| Mean (SD) | 0.4 (1.3) | 0.2 (0.9) | 0.3 (1.1) |
| Median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly solid after treatment | |||
| n | 100 | 106 | 206 |
| Mean (SD) | 3.6 (2.4) | 3.0 (2.4) | 3.3 (2.4) |
| Median (IQR) | 3.5 (1.7–5.5) | 3.0 (0.5–5.1) | 3.2 (1.0–5.4) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly mushy after treatment | |||
| n | 100 | 106 | 206 |
| Mean (SD) | 0.7 (1.3) | 0.9 (1.5) | 0.8 (1.4) |
| Median (IQR) | 0.0 (0.0–0.8) | 0.0 (0.0–1.1) | 0.0 (0.0–1.0) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Days per week stool was mostly liquid after treatment | |||
| n | 100 | 106 | 206 |
| Mean (SD) | 2.7 (2.1) | 3.1 (2.1) | 2.9 (2.1) |
| Median (IQR) | 2.5 (1.1–4.0) | 3.0 (1.5–4.5) | 2.9 (1.4–4.5) |
| Minimum to maximum | 0.0 to 7.0 | 0.0 to 7.0 | 0.0 to 7.0 |
| Outcome | PTNS (N = 107) | Sham (N = 107) | Total (N = 214) |
|---|---|---|---|
| GIQoL after treatment | |||
| n | 95 | 91 | 186 |
| Mean (SD) | 132.0 (20.6) | 131.6 (20.5) | 131.8 (20.5) |
| Median (IQR) | 135.0 (115.0–48.0) | 134.0 (120.0–146.0) | 134.5 (118.0–148.0) |
| Minimum to maximum | 86.0 to 167.0 | 74.0 to 171.0 | 74.0 to 171.0 |
| EQ-5D after treatment | |||
| n | 108 | 106 | 214 |
| Mean (SD) | 0.68 (0.28) | 0.65 (0.34) | 0.67 (0.31) |
| Median (IQR) | 0.76 (0.62–0.85) | 0.73 (0.56–0.85) | 0.73 (0.62–0.85) |
| Minimum to maximum | –0.02 to 1.00 | –0.24 to 1.00 | –0.24 to 1.00 |
| Patient-centred outcomes after treatment | |||
| n | 87 | 85 | 172 |
| Mean (SD) | 7.8 (2.0) | 8.4 (2.1) | 8.1 (2.1) |
| Median (IQR) | 8.4 (6.9–9.4) | 9.3 (7.6–10.0) | 8.8 (7.0–9.8) |
| Minimum to maximum | 1.0 to 10.0 | 1.0 to 10.0 | 1.0 to 10.0 |
| SMCS after treatment | |||
| n | 104 | 101 | 205 |
| Mean (SD) | 13.9 (4.3) | 14.6 (4.6) | 14.3 (4.4) |
| Median (IQR) | 14.0 (11.0–17.0) | 15.0 (11.0–18.0) | 14.0 (11.0–18.0) |
| Minimum to maximum | 6.0 to 23.0 | 5.0 to 24.0 | 5.0 to 24.0 |
| SF-36 physical functioning after treatment | |||
| n | 96 | 105 | 201 |
| Mean (SD) | 67.1 (27.7) | 63.8 (29.0) | 65.4 (28.4) |
| Median (IQR) | 75.0 (47.5–90.0) | 70.0 (45.0–90.0) | 70.0 (45.0–90.0) |
| Minimum to maximum | 5.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 role-physical after treatment | |||
| n | 108 | 106 | 214 |
| Mean (SD) | 54.4 (44.1) | 46.2 (44.8) | 50.4 (44.6) |
| Median (IQR) | 62.5 (0.0–100.0) | 25.0 (0.0–100.0) | 50.0 (0.0–100.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 bodily pain after treatment | |||
| n | 109 | 109 | 218 |
| Mean (SD) | 64.3 (28.3) | 62.1 (31.0) | 63.2 (29.6) |
| Median (IQR) | 67.5 (45.0–90.0) | 67.5 (35.0–90.0) | 67.5 (37.5–90.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 general health after treatment | |||
| n | 107 | 105 | 212 |
| Mean (SD) | 52.8 (24.6) | 50.6 (23.9) | 51.7 (24.2) |
| Median (IQR) | 55.0 (30.0–75.0) | 50.0 (35.0–70.0) | 50.0 (30.0–70.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 95.0 | 0.0 to 100.0 |
| SF-36 vitality after treatment | |||
| n | 105 | 104 | 209 |
| Mean (SD) | 45.6 (22.2) | 46.7 (23.1) | 46.1 (22.6) |
| Median (IQR) | 50.0 (25.0–60.0) | 50.0 (35.0–65.0) | 50.0 (30.0–60.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 95.0 | 0.0 to 100.0 |
| SF-36 social functioning after treatment | |||
| n | 109 | 109 | 218 |
| Mean (SD) | 66.4 (28.6) | 60.6 (31.7) | 63.5 (30.3) |
| Median (IQR) | 75.0 (50.0–87.5) | 62.5 (37.5–87.5) | 62.5 (37.5–87.5) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 role-emotional after treatment | |||
| n | 108 | 108 | 216 |
| Mean (SD) | 61.7 (45.3) | 60.2 (44.1) | 61.0 (44.6) |
| Median (IQR) | 100.0 (0.0–100.0) | 83.3 (0.0–100.0) | 100.0 (0.0–100.0) |
| Minimum to maximum | 0.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| SF-36 mental health after treatment | |||
| n | 101 | 107 | 208 |
| Mean (SD) | 62.7 (25.1) | 63.0 (21.4) | 62.8 (23.2) |
| Median (IQR) | 64.0 (48.0–84.0) | 64.0 (52.0–76.0) | 64.0 (52.0–84.0) |
| Minimum to maximum | 4.0 to 100.0 | 0.0 to 100.0 | 0.0 to 100.0 |
| FIQoL lifestyle after treatment | |||
| n | 90 | 88 | 178 |
| Mean (SD) | 2.8 (0.9) | 2.8 (1.0) | 2.8 (1.0) |
| Median (IQR) | 3.0 (2.2–3.7) | 2.9 (1.9–3.7) | 2.9 (2.0–3.7) |
| Minimum to maximum | 1.0 to 4.0 | 1.0 to 4.0 | 1.0 to 4.0 |
| FIQoL coping after treatment | |||
| n | 68 | 71 | 139 |
| Mean (SD) | 2.0 (0.8) | 2.0 (1.0) | 2.0 (0.9) |
| Median (IQR) | 1.9 (1.3–2.6) | 1.7 (1.2–2.9) | 1.8 (1.2–2.9) |
| Minimum to maximum | 1.0 to 4.0 | 1.0 to 4.0 | 1.0 to 4.0 |
| FIQoL depression after treatment | |||
| n | 64 | 64 | 128 |
| Mean (SD) | 2.9 (1.0) | 2.8 (1.0) | 2.9 (1.0) |
| Median (IQR) | 3.1 (2.2–3.7) | 2.6 (2.0–3.9) | 2.9 (2.1–3.9) |
| Minimum to maximum | 1.0 to 4.4 | 1.1 to 4.4 | 1.0 to 4.4 |
| FIQoL embarrassment after treatment | |||
| n | 102 | 102 | 204 |
| Mean (SD) | 2.4 (0.8) | 2.3 (0.9) | 2.3 (0.9) |
| Median (IQR) | 2.7 (1.7–3.0) | 2.3 (1.7–3.0) | 2.3 (1.7–3.0) |
| Minimum to maximum | 1.0 to 4.0 | 1.0 to 4.0 | 1.0 to 4.0 |
| Likert scale of success after treatment | |||
| n | 110 | 107 | 217 |
| Mean (SD) | 4.0 (3.3) | 3.2 (3.1) | 3.6 (3.2) |
| Median (IQR) | 4.8 (0.0–6.8) | 2.1 (0.0–4.9) | 3.4 (0.0–6.0) |
| Minimum to maximum | 0.0 to 10.0 | 0.0 to 10.0 | 0.0 to 10.0 |
| Do you think you had PTNS or sham? | |||
| Sham, n (%) | 48 (46) | 71 (69) | 119 (57) |
| PTNS, n (%) | 57 (54) | 32 (31) | 89 (43) |
| Did you have any adverse events? | |||
| No, n (%) | 95 (87) | 88 (81) | 183 (84) |
| Yes, n (%) | 14 (13) | 20 (19) | 34 (16) |
| Was there any effect on urinary symptoms? | |||
| No urinary incontinence, n (%) | 47 (44) | 41 (39) | 88 (41) |
| Made it worse, n (%) | 11 (10) | 5 (5) | 16 (8) |
| No effect, n (%) | 42 (39) | 52 (50) | 94 (44) |
| Slight improvement, n (%) | 6 (6) | 5 (5) | 11 (5) |
| Substantial improvement, n (%) | 2 (2) | 2 (2) | 4 (2) |
| What was your loperamide use? | |||
| Did not use, n (%) | 25 (34) | 33 (46) | 58 (40) |
| Decreased, n (%) | 14 (19) | 4 (6) | 18 (12) |
| Same, n (%) | 33 (45) | 32 (45) | 65 (45) |
| Increased, n (%) | 2 (3) | 2 (3) | 4 (3) |
| What was your pad use? | |||
| Did not use, n (%) | 23 (29) | 24 (33) | 47 (31) |
| Decreased, n (%) | 12 (15) | 10 (14) | 22 (15) |
| Same, n (%) | 44 (56) | 35 (49) | 79 (52) |
| Increased, n (%) | 0 (0) | 3 (4) | 3 (2) |
Appendix 11 Past medical history
| Outcome | PTNS | Sham |
|---|---|---|
| Hysterectomy | 30 | 24 |
| Vaginal operation | 3 | 2 |
| Pelvic operation | 19 | 16 |
| Abdominal operation | 28 | 30 |
| Anal operation | 6 | 9 |
| Sphincter repair | 4 | 4 |
| Neck or back pain or back problem | 15 | 21 |
| OAB or bladder incontinence | 15 | 7 |
| Constipation | 1 | 0 |
| Diarrhoea | 1 | 0 |
| Diverticular disease | 4 | 6 |
| FI | 8 | 11 |
| Irritable bowel syndrome | 1 | 4 |
| Crohn’s disease/proctitis | 1 | 1 |
| Abdominal problem | 3 | 2 |
| Perianal or anal problem | 4 | 3 |
| Anxiety/depression/psychiatric illness | 22 | 24 |
| Breast problem or cancer | 8 | 2 |
| Cardiovascular problem | 34 | 27 |
| ENT problem | 7 | 5 |
| Eye problem or operation | 11 | 7 |
| Fatigue/fibromyalgia/myalgic encephalopathy | 6 | 4 |
| Gynaecological problem | 6 | 9 |
| Haematological disorder | 6 | 3 |
| Headache/migraine | 4 | 6 |
| HIV infection or infectious disease | 1 | 2 |
| Metabolic disorder | 0 | 4 |
| Minor operation | 1 | 5 |
| Neurological problem | 5 | 4 |
| Orthopaedic problem | 23 | 16 |
| Respiratory problem | 14 | 22 |
| Rheumatological problem (other than back or neck pain) | 22 | 16 |
| Rhinitis or hay fever | 3 | 7 |
| Skin disorder | 7 | 9 |
| Thoracic operation | 0 | 1 |
| Upper GI reflux | 20 | 18 |
Appendix 12 Regular medications
| Medication | PTNS | Sham |
|---|---|---|
| Codeine phosphate | 5 | 4 |
| Laxative | 5 | 11 |
| Loperamide | 30 | 30 |
| Analgesia | 20 | 26 |
| Antimalarial | 0 | 1 |
| Antibiotic/antiviral/antifungal | 3 | 3 |
| Antidepressant/antianxiety/psychological medication | 34 | 24 |
| Antihistamine | 5 | 8 |
| Autoimmune condition medication | 2 | 3 |
| Cardiovascular medication | 31 | 24 |
| Chemotherapy | 2 | 0 |
| Cold or flu remedy | 0 | 0 |
| Eye medication | 8 | 4 |
| Gynaecological medication | 2 | 0 |
| Metabolic medication | 0 | 0 |
| Neurological condition medication | 4 | 6 |
| Other GI medications | 0 | 0 |
| Respiratory medication | 13 | 22 |
| Skin condition medication | 4 | 5 |
| Urinary incontinence/OAB medication | 7 | 7 |
| Vaccine | 0 | 0 |
Appendix 13 European Quality of Life-5 Dimensions summary
ANALYSIS OF EUROPEAN QUALITY OF LIFE-5 DIMENSIONS DATA FROM THE CONFIDeNT TRIAL
Background
European Quality of Life-5 Dimensions data have been collected as part of the CONFIDeNT trial comparing PTNS against a sham treatment (TENS) alongside other patient-reported outcome (PRO) measures, symptom diaries and clinical outcomes.
York Health Economics Consortium has been requested by Queen Mary, University of London to conduct a basic statistical analysis of the EQ-5D (Option 1).
Methods and results
Methods
A Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) data file containing the data was provided by Queen Mary, University of London. The data were anonymised and comprised a unique study code for each patient, the group (PTNS or sham) to which the patients had been allocated, the EQ-5D data for two time points (week 2 baseline and week 14 end of study), that is the raw scores for mobility, self-care, usual activities, pain/discomfort, anxiety/depression, the EQ-5D VAS score and the EQ-5D index score. The data were analysed using Stata 12.1 software. The descriptive statistics were derived for the EQ-5D VAS score and EQ-5D index score, that is the average VAS scores and index scores for each arm of the trial at the two time points and the differences between the two arms, as well as the standard deviations, 95% CIs and the average change and standard deviation of change. Descriptive statistics were also derived for the categorical (ordinal) data (EQ-5D raw scores), consisting of the category frequencies at the two time points, as well as a cross-tabulation to highlight changes over time in EQ-5D scores.
Results
Data were available from 227 patients: 115 in the PTNS arm and 112 in the sham arm. The mean scores by group (arm) over time are shown in Table 29.
| Variable | Group | n | Mean | SD | Minimum | Maximum | LCL | UCL |
|---|---|---|---|---|---|---|---|---|
| VAS week 2 | PTNS | 114 | 64.50 | 21.72 | 5.00 | 100 | 60.51 | 68.49 |
| VAS week 2 | Sham | 112 | 64.04 | 21.24 | 20.00 | 100 | 60.10 | 67.97 |
| VAS week 14 | PTNS | 109 | 64.25 | 22.32 | 8.00 | 100 | 60.06 | 68.44 |
| VAS week 14 | Sham | 109 | 63.69 | 23.66 | 10.00 | 100 | 59.25 | 68.13 |
| Index week 2 | PTNS | 115 | 0.69 | 0.27 | –0.18 | 1 | 0.64 | 0.74 |
| Index week 2 | Sham | 109 | 0.63 | 0.34 | –0.24 | 1 | 0.57 | 0.70 |
| Index week 14 | PTNS | 108 | 0.68 | 0.28 | –0.02 | 1 | 0.63 | 0.74 |
| Index week 14 | Sham | 106 | 0.65 | 0.34 | –0.24 | 1 | 0.59 | 0.72 |
It may be seen that there were virtually no differences between the two arms either at week 2 (baseline) or at week 14 in respect of both the EQ-5D VAS scores and the EQ-5D index scores, with scores on both scales remaining unchanged over time.
The mean change from baseline (difference between week 14 and week 2) is shown in Table 30, for the EQ-5D VAS and the EQ-5D index scores. It may be seen that there was a slight decrease in EQ-5D VAS and EQ-5D index scores for the PTNS from baseline. For the sham treatment, VAS score increased slightly from baseline, whereas the index score remained almost unchanged.
| Variable | Group | n | Mean | SD |
|---|---|---|---|---|
| VAS | PTNS | 108 | –0.67 | 18.88 |
| VAS | Sham | 109 | 0.32 | 23.74 |
| Index | PTNS | 108 | –0.01 | 0.23 |
| Index | Sham | 105 | 0.02 | 0.25 |
The mean differences between the two groups are shown in Table 31. As may be seen from Table 31, although the PTNS were marginally higher at each point, the differences in scores between the two groups were minimal.
| Variable | Mean difference | SE | LCL | UCL |
|---|---|---|---|---|
| VAS week 2 | 0.46 | 2.86 | –5.17 | 6.10 |
| VAS week 14 | 0.56 | 3.12 | –5.58 | 6.70 |
| Index week 2 | 0.05 | 0.04 | –0.03 | 0.13 |
| Index week 14 | 0.03 | 0.04 | –0.05 | 0.12 |
The cross-tabulations for the individual EQ-5D domains are shown in Table 32. Few changes were observed for the mobility and self-care domains both over time and between the two groups, that is the vast majority of patients did not record any change for these two domains. These domains were also the only two for which patients did not record the more severe scores.
| Mobility week 2 | Mobility week 14 | |||||||
|---|---|---|---|---|---|---|---|---|
| PTNS | Sham | |||||||
| 1 | 2 | 3 | Total | 1 | 2 | 3 | Total | |
| 1 | 69 | 4 | 0 | 73 | 63 | 9 | 0 | 72 |
| 2 | 7 | 29 | 0 | 36 | 6 | 29 | 0 | 35 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 76 | 33 | 0 | 109 | 69 | 38 | 0 | 107 |
| Self-care week 2 | Self-care week 14 | |||||||
| PTNS | Sham | |||||||
| 1 | 2 | 3 | Total | 1 | 2 | 3 | Total | |
| 1 | 92 | 3 | 0 | 95 | 90 | 5 | 0 | 95 |
| 2 | 7 | 7 | 0 | 14 | 0 | 10 | 0 | 10 |
| 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total | 99 | 10 | 0 | 109 | 90 | 16 | 0 | 106 |
| Usual activities week 2 | Usual activities week 14 | |||||||
| PTNS | Sham | |||||||
| 1 | 2 | 3 | Total | 1 | 2 | 3 | Total | |
| 1 | 48 | 7 | 0 | 55 | 42 | 15 | 0 | 57 |
| 2 | 16 | 31 | 0 | 47 | 13 | 27 | 1 | 41 |
| 3 | 0 | 5 | 2 | 7 | 3 | 3 | 3 | 9 |
| Total | 64 | 43 | 2 | 109 | 58 | 45 | 4 | 107 |
| Pain/discomfort week 2 | Pain/discomfort week 14 | |||||||
| PTNS | Sham | |||||||
| 1 | 2 | 3 | Total | 1 | 2 | 3 | Total | |
| 1 | 28 | 13 | 2 | 43 | 26 | 8 | 0 | 34 |
| 2 | 12 | 43 | 3 | 58 | 14 | 38 | 4 | 56 |
| 3 | 0 | 3 | 4 | 7 | 1 | 5 | 9 | 15 |
| Total | 40 | 59 | 9 | 108 | 41 | 51 | 13 | 105 |
| Anxiety/depression week 2 | Anxiety/depression week 14 | |||||||
| PTNS | Sham | |||||||
| 1 | 2 | 3 | Total | 1 | 2 | 3 | Total | |
| 1 | 34 | 13 | 0 | 47 | 37 | 12 | 0 | 49 |
| 2 | 14 | 33 | 8 | 55 | 11 | 25 | 7 | 43 |
| 3 | 0 | 5 | 2 | 7 | 1 | 7 | 7 | 15 |
| Total | 48 | 51 | 10 | 109 | 49 | 44 | 14 | 107 |
A greater number of changes were recorded for the other three domains. For usual activities, 26% and 32% of scores changed from week 2 to week 14 for the PTNS and sham arms respectively; for pain/discomfort the figures were 31% for both arms; and for anxiety/depression they were 28% and 35% respectively.
For anxiety/depression there was an almost equal percentage change observed for improvement (e.g. a change from 3 to 2, or 2 to 1) and deterioration in scores (e.g. 1 to 2, or 2 to 3) between the PTNS and sham arms. For improvement in scores, the percentage changes were 17% and 18% respectively (PTNS and sham), and 19% and 18% for deterioration in scores. For usual activities, the percentage change in improvement was virtually the same between groups: 19% for PTNS and 18% for sham. However, there was a greater percentage of deterioration in usual activities for the sham group: 6% for PTNS and 15% for sham. This pattern was reversed for pain/discomfort: 14% (PTNS) and 19% (sham) for improvement and 17% and 11% for deterioration.
Conclusions
The results demonstrated that there were no differences between the two arms, PTNS and sham, in terms of patients’ overall health (EQ-5D VAS) or their health status (EQ-5D index score). Furthermore, there were no changes in the overall scores on these two measures over time. Differences were observed for the individual domains of the EQ-5D, particularly for usual activities, pain/discomfort and anxiety/depression. Patients in the sham arm experienced a greater degree of deterioration in their usual activities than those in the PTNS arm. However, in contrast to this, those patients in the PTNS arm reported a greater degree of pain/discomfort, whereas those in the sham arm noted an improvement in their levels of pain/discomfort.
These results should be interpreted in the context of the other outcome measures derived in the study, and with the caveat that the EQ-5D may not be sensitive to change in this patient population; however, the results suggest that the PTNS treatment – although it may not have an impact on overall quality of life – may improve patients’ usual activities, but perhaps at the cost of an increased degree of pain or discomfort.
Appendix 14 Concomitant medications
| Allocation | Medication | Indication | Duration (days) | Ongoing |
|---|---|---|---|---|
| PTNS | Cocodamol | Arm pain | 4 | No |
| PTNS | Cocodamol | Headache | 1 | No |
| PTNS | Codydramol | Leg pain | 1 | No |
| PTNS | Coproxamol | Pain | n/a | Yes |
| PTNS | Coproxamol | Neck pain | 2 | No |
| Sham | Coproxamol | Dental pain | n/a | Yes |
| Sham | Cocodamol | Leg pain | 3 | No |
| Sham | Morphine | Osteoarthritis and fibromyalgia | n/a | Yes |
| Sham | Tramadol | Fractured coccyx | 14 | No |
| Sham | Tramadol | Abdominal pain | n/a | Yes |
| Sham | Tramadol | Knee pain | n/a | Yes |
| Sham | Tramadol | Chest pain | n/a | Yes |
| PTNS | Ispaghula husk (Fybogel®, Reckitt Benckiser) | Unknown | Unknown | Unknown |
| PTNS | Ispaghula husk (Fybogel®, Reckitt Benckiser) | Diarrhoea | 2 | Yes |
| PTNS | Klean-Prep® (Norgine, Helsinn-Birex Pharmaceuticals Ltd) | Preparation for colonoscopy | 1 | No |
| PTNS | Klean-Prep® (Norgine, Helsinn-Birex Pharmaceuticals Ltd) | Preparation for colonoscopy | 1 | No |
| PTNS | Sodium picosulfate with magnesium citrate (Picolax®, Ferring) | Preparation for colonoscopy | 1 | No |
| Sham | Ispaghula husk (Fybogel®, Reckitt Benckiser) | Constipation | 7 | No |
| Sham | Lactulose | Post surgery | 8 | No |
| Sham | Lactulose | Constipation | Unknown | No |
Appendix 15 Subgroup analyses
| Outcome | Main treatment effect | Interaction term(s) | Interaction term global p-value | |||||
|---|---|---|---|---|---|---|---|---|
| PTNS vs. sham, OR | 95% CI | p-value | Interaction term 1, OR | 95% CI | Interaction term 2, OR | 95% CI | ||
| Moderator | Male vs. female | |||||||
| Sex (male vs. female) | 1.143 | 0.629 to 2.078 | 0.661 | 5.418 | 0.451 to 65.065 | n/a | n/a | 0.183 |
| ≥ 7 FIEs per week base vs. < 7 | 1.177 | 0.535 to 2.590 | 0.686 | 1.212 | 0.389 to 3.776 | n/a | n/a | 0.740 |
| Age groups (years) | 40–60 vs. < 40 | > 60 vs. < 40 | ||||||
| < 40, 40–60, > 60 | 1.575 | 0.205 to 12.106 | 0.662 | 0.745 | 0.075 to 7.371 | 0.857 | 0.088 to 8.385 | 0.957 |
| Type of incontinence | Urge only vs. urge and passive | Passive only vs. urge and passive | ||||||
| Urge or passive or both | 1.589 | 0.730 to 3.456 | 0.243 | 0.824 | 0.185 to 3.675 | 0.424 | 0.090 to 1.995 | 0.554 |
Appendix 16 Sensitivity analysis 1
| Outcome | Type | n | Estimate | LCL | UCL | p-value | Model-based ICC |
|---|---|---|---|---|---|---|---|
| ≥ 50% reduction in FIEs (primary outcome) | OR | 211 | 1.325 | 0.736 | 2.385 | 0.348 | < 0.001 |
| ≥ 25% reduction in FIEs | OR | 211 | 1.314 | 0.747 | 2.311 | 0.344 | < 0.001 |
| ≥ 75% reduction in FIEs | OR | 211 | 1.643 | 0.775 | 3.484 | 0.195 | 0.212 |
| 100% reduction in FIEs | OR | 211 | 1.670 | 0.596 | 4.674 | 0.330 | 0.008 |
| Change in FIEs | Beta | 211 | –2.468 | –4.533 | –0.403 | 0.019 | < 0.001 |
| Change in rush FIEs | Beta | 211 | –1.557 | –2.881 | –0.232 | 0.021 | < 0.001 |
| Change in passive leakage FIEs | Beta | 211 | –0.736 | –1.850 | 0.378 | 0.195 | 0.1 |
| FIQoL embarrassment | Beta | 211 | 0.049 | –0.149 | 0.247 | 0.630 | < 0.001 |
| FIQoL coping | Beta | 211 | 0.021 | –0.172 | 0.214 | 0.831 | 0.116 |
| FIQoL lifestyle | Beta | 211 | 0.105 | –0.070 | 0.280 | 0.236 | < 0.001 |
| FIQoL depression | Beta | 211 | 0.025 | –0.294 | 0.344 | 0.873 | < 0.001 |
| SF-36 physical functioning | Beta | 211 | –2.025 | –7.515 | 3.464 | 0.469 | < 0.001 |
| SF-36 role-physical | Beta | 211 | 1.646 | –8.860 | 12.152 | 0.758 | n/a |
| SF-36 bodily pain | Beta | 211 | –1.039 | –7.114 | 5.036 | 0.737 | < 0.001 |
| SF-36 general health | Beta | 211 | 0.159 | –4.759 | 5.076 | 0.949 | < 0.001 |
| SF-36 vitality | Beta | 211 | –2.930 | –8.194 | 2.334 | 0.273 | n/a |
| SF-36 social functioning | Beta | 211 | 6.343 | 0.010 | 12.676 | 0.051 | 0.017 |
| SF-36 role emotional | Beta | 211 | –5.461 | –15.881 | 4.959 | 0.302 | n/a |
| SF-36 mental health | Beta | 211 | 0.031 | –4.477 | 4.540 | 0.989 | 0.065 |
| SMCS | Beta | 211 | –0.139 | –1.163 | 0.885 | 0.790 | < 0.001 |
| Patient-centred outcomes | Beta | 211 | –0.562 | –1.123 | –0.001 | 0.050 | < 0.001 |
| EQ-5D index score | Beta | 211 | –0.017 | –0.081 | 0.048 | 0.610 | 0.019 |
| GIQoL | Beta | 211 | –1.558 | –5.566 | 2.449 | 0.442 | n/a |
| Likert scale of success | Beta | 211 | 0.786 | –0.123 | 1.694 | 0.091 | 0.009 |
Appendix 17 Sensitivity analysis 2
| Outcome | Type | n | Estimate | LCL | UCL | p-value | Model-based ICC |
|---|---|---|---|---|---|---|---|
| ≥ 50% reduction in FIEs (primary outcome) | OR | 223 | 1.234 | 0.693 | 2.196 | 0.476 | < 0.001 |
| ≥ 25% reduction in FIEs | OR | 223 | 1.220 | 0.698 | 2.132 | 0.485 | < 0.001 |
| ≥ 75% reduction in FIEs | OR | 223 | 1.634 | 0.776 | 3.438 | 0.196 | 0.214 |
| 100% reduction in FIEs | OR | 223 | 1.690 | 0.609 | 4.693 | 0.315 | 0.006 |
| Change in FIEs | Beta | 223 | –2.158 | –4.034 | –0.283 | 0.024 | < 0.001 |
| Change in rush FIEs | Beta | 223 | –1.501 | –2.752 | –0.25 | 0.019 | < 0.001 |
| Change in passive leakage FIEs | Beta | 223 | –0.592 | –1.637 | 0.453 | 0.267 | 0.094 |
| FIQoL embarrassment | Beta | 223 | 0.020 | –0.166 | 0.206 | 0.83 | < 0.001 |
| FIQoL coping | Beta | 223 | 0.017 | –0.168 | 0.203 | 0.855 | 0.1 |
| FIQoL lifestyle | Beta | 223 | 0.092 | –0.072 | 0.257 | 0.27 | < 0.001 |
| FIQoL depression | Beta | 223 | 0.010 | –0.301 | 0.321 | 0.945 | < 0.001 |
| SF-36 physical functioning | Beta | 223 | –1.479 | –6.674 | 3.717 | 0.576 | < 0.001 |
| SF-36 role-physical | Beta | 223 | 1.462 | –8.684 | 11.608 | 0.777 | n/a |
| SF-36 bodily pain | Beta | 223 | –0.844 | –6.712 | 5.024 | 0.778 | < 0.001 |
| SF-36 general health | Beta | 223 | –0.021 | –4.676 | 4.634 | 0.993 | < 0.001 |
| SF-36 vitality | Beta | 223 | –3.857 | –8.875 | 1.161 | 0.131 | n/a |
| SF-36 social functioning | Beta | 223 | 5.419 | –0.585 | 11.423 | 0.078 | 0.012 |
| SF-36 role emotional | Beta | 223 | –5.297 | –15.405 | 4.811 | 0.303 | n/a |
| SF-36 mental health | Beta | 223 | –0.877 | –5.237 | 3.483 | 0.693 | 0.031 |
| SMCS | Beta | 223 | 0.052 | –0.928 | 1.032 | 0.917 | < 0.001 |
| Patient-centred outcomes | Beta | 223 | –0.575 | –1.121 | –0.029 | 0.039 | < 0.001 |
| EQ-5D index score | Beta | 223 | –0.020 | –0.082 | 0.042 | 0.524 | 0.017 |
| GIQoL | Beta | 223 | –1.269 | –5.182 | 2.643 | 0.521 | n/a |
| Likert scale of success | Beta | 223 | 0.856 | –0.016 | 1.728 | 0.055 | 0.02 |
Appendix 18 Adverse events
| Personal information number | Adverse event | Site | Related | Duration (days) | Grade | Action taken | Outcome |
|---|---|---|---|---|---|---|---|
| ANT002 | Orthopaedic injury | – | Unrelated | 73 | Moderate | Other | Resolved |
| ANT007 | Bleeding | Rectal | Unrelated | 1 | Mild | Other | Resolved |
| ANT007 | Cough/cold/flu | – | Unrelated | – | Mild | Concomitant medication given | Unresolved |
| ANT011 | Fall | – | Unrelated | 1 | Mild | No action taken | Resolved |
| ANT011 | Fall | – | Unrelated | 1 | Mild | No action taken | Resolved |
| ANT019 | Breast lump | – | Unrelated | – | Mild | No action taken | Unknown |
| ANT019 | Vomiting/nausea | – | Unrelated | 2 | Mild | No action taken | Resolved |
| ANT028 | Cough/cold/flu | – | Unrelated | 4 | Mild | No action taken | Resolved |
| ANT040 | Pain | Back | Unrelated | 8 | Moderate | No action taken | Resolved |
| BLT002 | Headache/migraine | – | Possible | 8 | Moderate | Concomitant medication given | Resolved |
| BLT002 | Infection | Chest | Unrelated | 8 | Moderate | Concomitant medication given | Resolved |
| BLT002 | Pain | Arm | Unrelated | 3 | Mild | No action taken | Resolved |
| BLT002 | Pain | Back | Unrelated | 2 | Moderate | Concomitant medication given | Resolved |
| BLT003 | Allergic reaction | – | Unrelated | 25 | Mild | Concomitant medication given | Resolved |
| BLT003 | Dizziness | – | Possible | 1 | Mild | No action taken | Resolved |
| BLT003 | Infection | Tooth/gum | Unrelated | 6 | Mild | Concomitant medication given | Resolved |
| BLT005 | Diarrhoea | – | Possible | 3 | Mild | No action taken | Resolved |
| BLT005 | Infection | Skin | Unrelated | 14 | Mild | No action taken | Resolved |
| BLT005 | Infection | Urinary tract | Unrelated | 9 | Mild | Concomitant medication given | Resolved |
| BLT005 | Pain | Leg | Unrelated | 14 | Mild | No action taken | Resolved |
| BLT018 | Dizziness | – | Possible | 23 | Mild | No action taken | Resolved |
| BLT018 | Infection | Tooth/gum | Unrelated | 32 | Mild | Concomitant medication given | Resolved |
| BLT041 | Bruising | Needle site | Related | 14 | Mild | No action taken | Resolved |
| BLT041 | Cough/cold/flu | – | Unrelated | 7 | Mild | No action taken | Resolved |
| BLT041 | Cough/cold/flu | – | Unrelated | 12 | Mild | No action taken | Resolved |
| BLT041 | Infection | Ear | Unrelated | 19 | Mild | Concomitant medication given | Resolved |
| BLT042 | Cough/cold/flu | – | Unrelated | 5 | Mild | Concomitant medication given | Unresolved |
| BLT042 | Cough/cold/flu | – | Unrelated | 17 | Mild | Concomitant medication given | Resolved |
| BLT053 | Cough/cold/flu | – | Unrelated | 8 | Mild | Concomitant medication given | Resolved |
| BLT053 | Pain | Leg | Possible | 1 | Severe | No action taken | Resolved |
| BLT053 | Pain | Leg | Possible | – | Moderate | Concomitant medication given | Unresolved |
| BLT066 | Orthopaedic injury | – | Unrelated | 48 | Moderate | No action taken | Resolved |
| BLT068 | Pain | Back | Unrelated | 17 | Moderate | No action taken | Resolved |
| BLT074 | Headache/migraine | – | Possible | 2 | Moderate | Concomitant medication given | Resolved |
| BLT074 | Infection | Urinary tract | Unrelated | 15 | Moderate | No action taken | Resolved |
| BLT074 | Pain | Leg | Possible | 3 | Moderate | No action taken | Resolved |
| BLT074 | Pain | Foot | Possible | 21 | Severe | No action taken | Resolved |
| BLT074 | Pain | Leg | Possible | 1 | Moderate | No action taken | Resolved |
| BLT074 | Skin disorder | – | Possible | 9 | Mild | No action taken | Resolved |
| CHH006 | Pain | Arm | Unrelated | 5 | Mild | Concomitant medication given | Resolving |
| GST003 | Pain | Leg | Possible | 10 | Mild | No action taken | Resolved |
| LGI003 | Dizziness | – | Possible | – | Mild | Other | Unresolved |
| PHT003 | Altered sensation | Leg | Possible | – | Mild | No action taken | Unresolved |
| PHT003 | Altered sensation | Leg | Possible | – | Mild | No action taken | Unresolved |
| PHT003 | Fall | – | Unrelated | – | Mild | No action taken | Unresolved |
| PHT004 | Cough/cold/flu | – | Unrelated | 21 | Moderate | No action taken | Resolved |
| PHT004 | Rheumatoid arthritis | – | Unrelated | – | Moderate | Concomitant medication given | Unresolved |
| PHT008 | Anxiety/depression/psychiatric illness | – | Possible | – | Mild | Concomitant medication given | Resolving |
| PHT009 | Altered sensation | Foot | Possible | 1 | Mild | No action taken | Resolved |
| PHT009 | Altered sensation | Foot | Possible | 1 | Mild | No action taken | Resolved |
| PHT009 | Dizziness | – | Possible | 1 | Mild | No action taken | Resolved |
| PHT009 | Pain | Back | Possible | Mild | No action taken | Unresolved | |
| QMC002 | Pain | Needle site | Related | 16 | Mild | Other | Resolved |
| QMC003 | Diarrhoea | – | Possible | 5 | Moderate | No action taken | Resolved |
| QMC003 | Pain | Needle site | Related | 22 | Mild | Other | Resolved |
| SMH001 | Cough/cold/flu | – | Unrelated | 26 | Mild | Concomitant medication given | Resolved |
| SMH001 | Headache/migraine | – | Possible | 3 | Mild | Concomitant medication given | Resolved |
| SMH001 | Pain | Neck | Unrelated | 6 | Mild | Concomitant medication given | Resolved |
| SMH010 | Bruising | Needle site | Related | 8 | Mild | No action taken | Resolved |
| SMH010 | Dizziness | – | Possible | 1 | Mild | No action taken | Resolved |
| SMH010 | Pain | Abdomen | Possible | 9 | Moderate | No action taken | Resolved |
| SOT005 | Diarrhoea | – | Possible | 3 | Mild | Concomitant medication given | Resolved |
| SOT009 | Vomiting/nausea | – | Unrelated | – | Mild | Concomitant medication given | Resolving |
| STH002 | Headache/migraine | – | Possible | – | Severe | Concomitant medication given | Resolved |
| STH006 | Altered sensation | Needle site | Related | 1 | Mild | No action taken | Resolved |
| STH007 | Cough/cold/flu | – | Unrelated | 8 | Mild | Concomitant medication given | Resolved |
| STH007 | Cough/cold/flu | – | Unrelated | 8 | Mild | Concomitant medication given | Resolved |
| STH013 | Pain | Needle site | Related | – | Mild | No action taken | Unknown |
| SWB002 | Vitamin D deficiency | – | Unrelated | – | Mild | Concomitant medication given | Unresolved |
| SWB010 | Renal problem | – | Unrelated | – | Mild | Concomitant medication given | Unresolved |
| SWB021 | Diarrhoea | – | Possible | 6 | Mild | No action taken | Resolved |
| SWB022 | Pain | Toe | Possible | – | Mild | No action taken | Unknown |
| SWB022 | Vomiting/nausea | – | Unrelated | 3 | Moderate | No action taken | Resolved |
| SWB023 | Pain | Heel | Possible | 2 | Mild | No action taken | Resolved |
| UCL004 | Infection | Chest | Unrelated | – | Mild | No action taken | Resolving |
| UCL008 | Headache/migraine | – | Possible | 2 | Moderate | No action taken | Resolved |
| UCL013 | Pain | Leg | Possible | 2 | Mild | No action taken | Resolved |
| UCL016 | Pain | Leg | Possible | 2 | Moderate | Concomitant medication given | Resolved |
| UCL016 | Pain | Needle site | Related | 2 | Mild | No action taken | Resolved |
| UCL016 | Vomiting/nausea | – | Unrelated | 1 | Mild | No action taken | Resolved |
| ULH003 | Pain | Breast | Unrelated | – | Mild | Concomitant medication given | Unresolved |
| ULH003 | Upper GI reflux | – | Unrelated | 64 | Moderate | Concomitant medication given | Resolved |
| ULH011 | Bleeding | Rectal | Unrelated | 1 | Moderate | No action taken | Resolved |
| ULH014 | Operative procedure/hospital procedure | – | Unrelated | 3 | Moderate | Hospitalisation | Resolved |
| USM005 | Diarrhoea | – | Possible | 4 | Mild | No action taken | Resolved |
| USM023 | Skin disorder | – | Unrelated | 9 | Mild | Other | Resolved |
| USM025 | Infection | Tooth/gum | Unrelated | – | Mild | Concomitant medication given | Unknown |
| USM026 | Fall | – | Unrelated | 1 | Mild | No action taken | Resolving |
| USM030 | Pain | Heel | Possible | – | Moderate | No action taken | Unresolved |
| WCU001 | Pain | Abdomen | Possible | 3 | Mild | No action taken | Resolved |
| WCU004 | Altered sensation | Arm | Unrelated | 1 | Moderate | No action taken | Resolved |
| WCU004 | Infection | Urinary tract | Unrelated | 6 | Mild | Concomitant medication given | Resolved |
| WCU004 | Pain | Toe | Possible | 1 | Moderate | No action taken | Resolved |
| WCU004 | Pain | Leg | Possible | 1 | Moderate | No action taken | Resolved |
| WCU010 | Diarrhoea | – | Possible | 2 | Mild | No action taken | Resolved |
| WCU011 | Bleeding | Rectal | Unrelated | 1 | Moderate | Concomitant medication given | Unresolved |
| WCU011 | Pain | Abdomen | Possible | 4 | Mild | No action taken | Resolved |
| WCU012 | Pain | Abdomen | Possible | – | Mild | No action taken | Unresolved |
| WCU014 | Infection | Chest | Unrelated | 6 | Mild | Concomitant medication given | Resolved |
| WCU021 | Infection | Urinary tract | Unrelated | 11 | Mild | Concomitant medication given | Unknown |
| WCU021 | Vomiting/nausea | – | Unrelated | 2 | Mild | No action taken | Resolved |
| WCU033 | Diarrhoea | – | Possible | 1 | Severe | Concomitant medication given | Unknown |
| WCU033 | Operative procedure/hospital procedure | – | Unrelated | 1 | Severe | Hospitalisation | Resolved |
| WCU038 | Headache/migraine | – | Possible | 2 | Moderate | Concomitant medication given | Resolved |
| WCU038 | Skin disorder | – | Unrelated | 4 | Mild | No action taken | Resolved |
| WCU043 | Pain | Groin | Unrelated | 1 | Moderate | No action taken | Resolved |
| WCU043 | Pain | Ear | Unrelated | 3 | Moderate | No action taken | Resolved |
| WCU049 | Diarrhoea | – | Possible | – | Mild | No action taken | Resolved |
| Personal information number | Adverse event | Site | Related | Duration (days) | Grade | Action taken | Outcome |
|---|---|---|---|---|---|---|---|
| ANT001 | Skin disorder | – | Unrelated | – | Moderate | No action taken | Unknown |
| ANT008 | Pain | Leg | Possible | 70 | Mild | No action taken | Resolved |
| ANT014 | Operative procedure/hospital procedure | – | Unrelated | 1 | Mild | Concomitant medication given | Resolved |
| ANT018 | Altered sensation | Toe | Related | 1 | Mild | No action taken | Resolved |
| ANT038 | Operative procedure/hospital procedure | – | Unrelated | 8 | Moderate | No action taken | Resolved |
| ANT041 | Infection | Chest | Unrelated | 8 | Mild | Concomitant medication given | Resolved |
| ANT041 | Orthopaedic injury | – | Unrelated | 15 | Moderate | Concomitant medication given | Resolved |
| ANT044 | Upper GI reflux | – | Unrelated | – | Moderate | Concomitant medication given | Resolving |
| BLT001 | Cough/cold/flu | – | Unrelated | 3 | Mild | Concomitant medication given | Resolved |
| BLT001 | Diarrhoea | – | Possible | 2 | Moderate | No action taken | Resolved |
| BLT013 | Bruising | Needle site | Related | 5 | Mild | No action taken | Resolved |
| BLT013 | Infection | Chest | Unrelated | 19 | Moderate | Concomitant medication given | Resolved |
| BLT013 | Pain | Ankle | Possible | 8 | Mild | No action taken | Resolved |
| BLT019 | Altered sensation | Perineum | Possible | 1 | Mild | No action taken | Resolved |
| BLT019 | Altered sensation | Perineum | Possible | 1 | Mild | No action taken | Resolved |
| BLT019 | Operative procedure/hospital procedure | – | Unrelated | 1 | Moderate | No action taken | Resolved |
| BLT019 | Orthopaedic injury | – | Unrelated | 3 | Mild | No action taken | Resolved |
| BLT019 | Pain | Shoulder | Unrelated | 2 | Mild | Concomitant medication given | Resolved |
| BLT026 | Cough/cold/flu | – | Unrelated | 14 | Moderate | Concomitant medication given | Resolved |
| BLT026 | Cough/cold/flu | – | Unrelated | 4 | Mild | Concomitant medication given | Resolved |
| BLT026 | Urinary symptoms | – | Possible | 1 | Mild | No action taken | Resolved |
| BLT031 | Headache/migraine | – | Possible | 3 | Mild | No action taken | Resolved |
| BLT031 | Pain | Back | Unrelated | – | Moderate | Concomitant medication given | Unresolved |
| BLT046 | Cough/cold/flu | – | Unrelated | 7 | Mild | No action taken | Resolved |
| BLT046 | Diarrhoea | – | Possible | 2 | Mild | No action taken | Resolved |
| BLT046 | Pain | Abdomen | Possible | 13 | Mild | No action taken | Resolved |
| BLT046 | Pain | Abdomen | Possible | 22 | Mild | No action taken | Resolved |
| BLT046 | Urinary symptoms | – | Possible | – | Moderate | No action taken | Resolving |
| BLT051 | Cough/cold/flu | – | Unrelated | 5 | Mild | Concomitant medication given | Resolved |
| BLT051 | Headache/migraine | – | Possible | 1 | Moderate | No action taken | Resolved |
| BLT058 | Altered sensation | Lip | Unrelated | 8 | Mild | No action taken | Resolved |
| CHH002 | Diarrhoea | – | Possible | 1 | Mild | No action taken | Resolved |
| CHH004 | Infection | Tooth/gum | Unrelated | 8 | Mild | Concomitant medication given | Resolving |
| LRI002 | Pain | Leg | Possible | 3 | Mild | Concomitant medication given | Resolved |
| LRI002 | Skin disorder | – | Unrelated | 3 | Moderate | Concomitant medication given | Resolved |
| LRI006 | Anxiety/depression/psychiatric illness | – | Possible | – | Moderate | Other | Unresolved |
| LRI007 | Headache/migraine | – | Possible | 5 | Moderate | Concomitant medication given | Resolved |
| PHT005 | Upper GI reflux | – | Unrelated | – | Moderate | Concomitant medication given | Unresolved |
| PHT006 | Bleeding | Rectal | Unrelated | 2 | Mild | No action taken | Resolved |
| PHT010 | Pain | Back | Unrelated | 1 | Mild | No action taken | Resolving |
| QMC001 | Infection | Skin | Unrelated | 8 | Mild | No action taken | Resolved |
| QMC001 | Pain | Needle site | Related | – | Mild | No action taken | Resolved |
| QMC001 | Pain | Needle site | Related | 30 | Mild | No action taken | Resolved |
| QMC004 | Infection | Chest | Unrelated | 9 | Mild | Concomitant medication given | Resolved |
| SMH007 | Cough/cold/flu | – | Unrelated | 156 | Mild | Concomitant medication given | Resolved |
| SMH007 | Urinary symptoms | – | Possible | – | Mild | No action taken | Unknown |
| SMH013 | Pain | Leg | Possible | 11 | Moderate | Concomitant medication given | Unresolved |
| SOT002 | Infection | Chest | Unrelated | 6 | Moderate | Concomitant medication given | Resolved |
| STH012 | Pain | Leg | Possible | – | Moderate | No action taken | Unknown |
| SWB016 | Infection | Chest | Unrelated | 7 | Mild | Concomitant medication given | Resolved |
| SWB020 | Headache/migraine | – | Possible | 6 | Severe | Concomitant medication given | Resolved |
| SWB020 | Headache/migraine | – | Possible | – | Mild | No action taken | Unknown |
| UCL002 | Infection | Chest | Unrelated | 8 | Mild | Concomitant medication given | Resolving |
| UCL006 | Infection | Chest | Unrelated | 4 | Moderate | Concomitant medication given | Resolving |
| UCL006 | Infection | Cold sores | Unrelated | 6 | Mild | Concomitant medication given | Resolving |
| UCL010 | Cough/cold/flu | – | Unrelated | 9 | Mild | Concomitant medication given | Resolved |
| UCL012 | Skin disorder | – | Unrelated | 8 | Mild | No action taken | Unresolved |
| ULH001 | Infection | Urinary tract | Unrelated | 6 | Moderate | Concomitant medication given | Resolved |
| ULH001 | Infection | Chest | Unrelated | 7 | Moderate | Concomitant medication given | Resolved |
| ULH006 | Infection | Urinary tract | Unrelated | 8 | Moderate | Concomitant medication given | Resolved |
| ULH007 | Infection | Chest | Unrelated | 8 | Moderate | Concomitant medication given | Resolved |
| ULH009 | Bleeding | Rectal | Unrelated | 3 | Mild | No action taken | Resolved |
| ULH013 | Water retention | – | Unrelated | – | Mild | No action taken | Resolved |
| ULH018 | Operative procedure/hospital procedure | – | Unrelated | 1 | Mild | No action taken | Unknown |
| ULH018 | Operative procedure/hospital procedure | – | Unrelated | 1 | Moderate | No action taken | Unknown |
| ULH018 | Operative procedure/hospital procedure | – | Unrelated | 3 | Mild | No action taken | Unknown |
| ULH022 | Bleeding | Needle site | Related | 4 | Mild | No action taken | Resolved |
| USM019 | Upper GI reflux | – | Unrelated | – | Mild | Concomitant medication given | Resolving |
| USM022 | Infection | Urinary tract | Unrelated | 8 | Mild | Concomitant medication given | Resolved |
| USM022 | Upper GI reflux | – | Unrelated | – | Moderate | Concomitant medication given | Unknown |
| USM028 | Fall | – | Unrelated | 1 | Moderate | No action taken | Resolved |
| USM028 | Pain | Leg | Possible | 11 | Moderate | No action taken | Resolved |
| USM028 | Vomiting/nausea | – | Possible | 6 | Moderate | No action taken | Resolved |
| USM032 | Infection | Diverticulitis | Unrelated | 14 | Moderate | Concomitant medication given | Resolved |
| WCU003 | Weakness | Leg | Possible | 1 | Moderate | No action taken | Resolved |
| WCU006 | FI | – | Possible | 4 | Mild | No action taken | Resolved |
| WCU006 | Infection | Skin | Unrelated | Mild | Other | Unknown | |
| WCU006 | Skin disorder | – | Unrelated | 1 | Mild | No action taken | Resolved |
| WCU008 | Infection | Skin | Unrelated | 26 | Moderate | Hospitalisation | Resolved |
| WCU008 | Operative procedure/hospital procedure | – | Unrelated | 1 | Severe | Hospitalisation | Resolved |
| WCU008 | Vomiting/nausea | – | Unrelated | 2 | Mild | No action taken | Resolved |
| WCU017 | Pain | Rib | Unrelated | 1 | Severe | Concomitant medication given | Resolving |
| WCU017 | Pain | Rib | Unrelated | – | Severe | Concomitant medication given | Resolving |
| WCU018 | Constipation | – | Possible | – | Mild | Concomitant medication given | Unresolved |
| WCU018 | Headache/migraine | – | Possible | 2 | Mild | Concomitant medication given | Resolved |
| WCU018 | Pain | Perineum | Possible | 2 | Moderate | No action taken | Resolved |
| WCU018 | Pain | Leg | Possible | 1 | Moderate | No action taken | Resolved |
| WCU018 | Pain | Leg | Possible | 1 | Moderate | No action taken | Resolved |
| WCU029 | Infection | Urinary tract | Unrelated | – | Mild | Concomitant medication given | Unresolved |
| WCU029 | Infection | Urinary tract | Unrelated | Mild | No action taken | Unresolved | |
| WCU029 | Pain | Ankle | Possible | 1 | Moderate | No action taken | Resolved |
| WCU029 | Pain | Needle site | Related | 1 | Mild | No action taken | Resolved |
| WCU035 | Pain | Foot | Possible | 1 | Moderate | No action taken | Resolved |
| WCU041 | Bleeding | Needle site | Related | 1 | Mild | No action taken | Resolved |
| WCU041 | Cough/cold/flu | – | Unrelated | 9 | Mild | Concomitant medication given | Resolved |
| WCU041 | Headache/migraine | – | Possible | 67 | Mild | Concomitant medication given | Resolved |
| WCU047 | Infection | Urinary tract | Unrelated | 6 | Mild | Concomitant medication given | Resolved |
| WCU047 | Urinary symptoms | – | Possible | – | Mild | No action taken | Unresolved |
List of abbreviations
- CI
- confidence interval
- CONFIDeNT
- CONtrol of Faecal Incontinence using Distal NeuromodulaTion
- CONSORT
- Consolidated Standard of Reporting Trials
- CRF
- case report form
- DSMC
- Data and Safety Monitoring Committee
- EQ-5D
- European Quality of Life-5 Dimensions
- FI
- faecal incontinence
- FIE
- faecal incontinence episode
- FIQoL
- Faecal Incontinence Quality of Life Index
- GIQoL
- Gastrointestinal Quality of Life Index
- GP
- general practitioner
- ICC
- intracluster correlation coefficient
- OAB
- overactive bladder
- PTNS
- percutaneous tibial nerve stimulation
- SAE
- serious adverse event
- SF-36
- Short Form Questionnaire-36 items
- SMCS
- St Mark’s Continence Score
- SNS
- sacral nerve stimulation
- TENS
- transcutaneous electrical nerve stimulation
- TMG
- Trial Management Group
- TNS
- tibial nerve stimulation
- TSC
- Trial Steering Committee
- TTNS
- transcutaneous tibial nerve stimulation
- VAS
- visual analogue scale