Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/69/17. The protocol was agreed in June 2016. The assessment report began editorial review in January 2017 and was accepted for publication in June 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Duarte et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Epidemiology
Psoriasis is a chronic but non-contagious inflammatory disease of the skin and joints. 1 The disease predominantly affects body parts such as the scalp, elbows, knees and lower back and results in typical red, scaly and flaky skin, also known as plaque psoriasis. 2 Plaque psoriasis is the most common type of psoriasis, although there are also other types of psoriasis such as guttate psoriasis (mostly in the trunk area), flexural psoriasis (affecting the flexures), palmoplantar pustulosis psoriasis (affecting the palms) and psoriatic nail diseases. 2 In children, plaque lesions appear most frequently on the scalp, followed by the extensor surfaces of the extremities and the trunk. 3
Psoriasis can appear at any age although it predominantly starts during adulthood. 1,2,4 The prevalence of psoriasis varies across the world, ranging from 0% to 2.1% in children and from 0.91% to 8.5% in the adult population. 5 The prevalence of psoriasis in the UK is estimated to be around 0.4% in children (including adolescents) and 2.2% in adults, with both sexes affected equally. 6
Aetiology, pathology and prognosis
The aetiology of psoriasis remains largely unknown; however, a genetic predisposition and environmental factors are believed to be the key players. 7,8 It is estimated that the heritability of psoriasis is 60–90%; however, a worldwide positive family history of psoriasis ranges between 4.5% and 88%. 9 Among environmental factors, alcohol consumption, infection, emotional stress, medications, obesity and smoking may be risk factors for psoriasis. 1,9
The natural history of psoriasis varies by clinical subtype, that is, it may present as chronic, stable plaques with intermittent remissions and exacerbations or acutely with a rapid progression and widespread involvement. 1 Plaque psoriasis usually manifests as a chronic disease, with intermittent remissions and, in some cases, the joints and eyes can be involved. 1 In contrast to adults, plaque psoriasis in children is less scaly and the lesions are often smaller and thinner. This can result in delayed diagnosis of the disease. 3 In addition, in children, plaques appear most frequently on the scalp and may lead to hair loss (psoriatic alopecia) if severe. 3
Significance in terms of ill health
The impact of psoriasis encompasses functional, psychological and social dimensions. 10 Factors that contribute to this include symptoms specifically related to the skin (e.g. chronic itch, bleeding, scaling and nail involvement), problems related to treatments (mess, odour, inconvenience and time), psoriatic arthritis and the effect of living with a highly visible, disfiguring skin disease (difficulties with relationships, difficulties with securing employment and poor self-esteem). Even people with minimal involvement (less than the equivalent of three palm areas) state that psoriasis has a major effect on their life. The combined long-term therapy costs and social costs of the disease have a major impact on health-care systems and on society in general. 11
Mortality primarily as a result of psoriasis is not common; however, the chronic and incurable nature of psoriasis means that associated morbidity is significant. 11 Studies show that a significant proportion of children with psoriasis (12–37%) do not grow out of it,12 which implies that childhood psoriasis has a substantial long-term social and economic impact on individuals and the community. 13
Some reports also suggest that adult psoriasis patients who were diagnosed during childhood have worse lifetime quality of life than those diagnosed during adulthood,14,15 although this claim is not supported by other studies. 16
Assessment and management of psoriasis in children
Currently, there is no treatment pathway specific to psoriasis for children in the UK. Treatment depends to some extent on the extent and severity of an individual’s disease and local customs and practice. Existing psoriasis guidance for all age groups [National Institute for Health and Care Excellence (NICE) clinical guideline CG153 in England11) states that traditional topical therapies (such as corticosteroids, vitamin D and analogues, dithranol and tar preparations) can be prescribed as first-line therapy. Second-line therapies can include phototherapy, broad- or narrow-band ultraviolet B (NBUVB) light, with or without the supervised application of complex topical therapies such as dithranol in Lassar’s paste or crude coal tar and photochemotherapy, psoralen plus UVA light (PUVA) and non-biological systemic agents such as ciclosporin, methotrexate and acitretin. Third-line therapies include systemic biological therapies that use molecules designed to block specific molecular steps important in the development of psoriasis, such as the tumour necrosis factor (TNF) antagonists, and anti-interleukin (IL)-12/IL-23 monoclonal antibodies. However, this guideline highlights special considerations for children (e.g. avoidance of very potent corticosteroids, PUVA and acitretin) and recommends that children and young people with any type of psoriasis should be referred to a specialist at presentation.
Assessment of treatment response and quality of life
In children, a variety of clinical scales are used to assess treatment response in psoriasis, including the Physician Global Assessment (PGA), the Psoriasis Area and Severity Index (PASI),17 the Children’s Dermatology Life Quality Index (CDLQI),18 the Pediatric Quality of Life Inventory (PedsQL™)19 and the Teenager’s Quality of Life Index (T-QoL). 20
Physician Global Assessment
The PGA is an instrument that provides a subjective overall evaluation of plaque psoriasis severity using a scale of seven categories (‘clear’, ‘almost clear’, ‘mild’, ‘mild to moderate’, ‘moderate’, ‘moderate to severe’, ‘severe’). 21 There are two primary forms: a static form [Static Physician Global Assessment (sPGA)], which measures the physician’s impression of the disease at a single point, and a dynamic form [Dynamic Physician Global Assessment (dPGA)], in which the physician assesses the global improvement from baseline. 17
The sPGA uses seven scaled scores to describe the severity of disease: 0 = ‘clear’, 1 = ‘almost clear’, 2 = ‘mild’, 3 = ‘mild to moderate’, 4 = ‘moderate’, 5 = ‘moderate to severe’ and 6 = ‘severe’. 17,22 The dPGA, on the other hand, uses six scaled scores to describe either improvement or deterioration of disease. For disease improvement the scores are +1 = ‘mild’, +2 = ‘moderate’, +3 = ‘moderate to large’, +4 = ‘large’ and +5 = ‘very large’. For disease deterioration the scores are –1 = ‘mild’, –2 = ‘moderate’, –3 = ‘moderate to large’, –4 = ‘large’ and –5 = ‘very large’. A score of zero indicates no or minimal change.
As the sPGA scoring system is simpler to use than the dPGA scoring system, because, with the dPGA, physicians have to record the severity of psoriasis at baseline to evaluate the change in disease status after a follow-up period, the sPGA has become a widely used treatment response assessment tool in practice. 17 However, the sPGA does not discriminate small changes and the score ranges are not robust. 17
Psoriasis Area and Severity Index
In clinical trials of patients with psoriasis, assessment of the response to treatment is usually based on the PASI. 17 Although it is widely used, the PASI also has a number of deficiencies: its constituent parameters have never been properly defined; it is insensitive to change in mild-to-moderate psoriasis; estimation of disease extent is notoriously inaccurate; and the complexity of the formula required to calculate the final score further increases the risk of errors. It combines an extent and a severity score for each of the four body areas (head, trunk, upper extremities and lower extremities). The extent score of 0–6 is allocated according to the percentage of skin involvement (e.g. 0 and 6 represent no psoriasis and 90–100% involvement respectively). The severity score of 0–12 is derived by adding scores of 0–4 for each of the qualities of erythema (redness), induration and desquamation, representative of the psoriasis within the affected area. It is probable, but usually not specified in trial reports, that most investigators take induration to mean plaque thickness without adherent scale and desquamation to mean thickness of scale rather than severity of scale shedding. The severity score for each area is multiplied by the extent score and the resultant body area scores, weighted according to the percentage of total body surface area (BSA) that the body area represents (10% for head, 30% for trunk, 20% for upper extremities and 40% for lower extremities), are added together to give the PASI score. Although the PASI score can theoretically reach 72, scores in the upper half of the range (> 36) are not common, even in severe psoriasis. Furthermore, the PASI score fails to capture the disability that commonly arises from involvement of functionally or psychosocially important areas (hands, feet, face, scalp and genitalia), which together represent only a small proportion of total BSA. 23 However, PASI-based measures have discriminatory capability and are generally accepted for the assessment of treatment effects. However, clinical expert opinion is that the PASI is not widely used in clinical practice.
Despite the fact that it has not been validated in children and young people as a measure of disease severity, the PASI was chosen as the primary outcome variable for psoriasis in the economic evaluation because it is used in the majority of randomised controlled trials (RCTs). Typically, the PASI is reported as a dichotomous measure indicating a 50%, 75% or 90% reduction in PASI score from baseline (PASI 50, PASI 75 and PASI 90 respectively).
Children’s Dermatology Life Quality Index
The CDLQI is a 10-item questionnaire that aims to measure the quality of life of children (aged 4–16 years) based on how much they have been affected by a skin problem over the week preceding the date of questioning. 18 The 10 items cover six areas of daily activities: symptoms and feelings, leisure, school or holidays, personal relationships, sleep and treatment. 24,25 Usually, children, either alone or with the help of their parents, choose one of the four possible replies (scored from 0 to 3), with a maximum overall score of 30 and with a high score corresponding to low quality of life and vice versa. 25
Children’s Dermatology Life Quality Index scores can be divided into scoring bands – band 0 (score of 0–1), band 1 (score of 2–6), band 2 (score of 7–12), band 3 (score of 13–18) and band 4 (score of 19–30) – that respectively correspond to no, small, moderate, very large or extremely large effects on the child’s quality of life. 25 However, the CDLQI is not considered appropriate for use as a health-related quality-of-life (HRQoL) assessment tool beyond the age of 16 years.
Pediatric Quality of Life Inventory
The PedsQL is a modular instrument for measuring HRQoL in children and adolescents (age 2–18 years). It consists of 23 items in four domains: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items) and school functioning (5 items). Each item receives a score of 0–4 (0 = ‘never a problem’, 1 = ‘almost never a problem’, 2 = ‘sometimes a problem’, 3 = ‘often a problem’, 4 = ‘almost always a problem’) and are reverse scored and linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0), so that higher scores indicate better HRQoL. 26 Paediatric self-report is measured in children and adolescents aged 5–18 years and parent proxy report of child HRQoL is measured for children and adolescents aged 2–18 years.
Teenager’s Quality of Life Index
Built on qualitative data from patients, the Teenager’s Quality of Life Index (T-QoL) is a validated tool to quantify the impact of skin disease on adolescents’ quality of life. 20 The index consists of 18 items categorised into three domains: self-image, physical well-being and the future, and psychological impact and relationships. The authors have proposed the T-QoL as an outcome measure in both clinical practice and clinical research. 20
General issues with quality-of-life measurement in childhood psoriasis
Quality-of-life measurements may not be particularly meaningful in younger children with psoriasis, who are less good at articulating how much the disease is bothering them. In the case of younger children, proxy measurements may more accurately reflect parental perception or concern. There is only moderate correlation between PASI/PGA response measures and the CDLQI;27 some children with relatively mild disease can have very poor HRQoL scores, whereas others with more severe disease can have acceptable HRQoL. As well as disease symptoms and consequences, the frequency of injections can be an important quality-of-life consideration in children.
Description of the technology under assessment
Biological therapies, or biologics, are agents that are extracted or semi-synthesised from biological sources and which are used for treating specific medical conditions, including autoimmune diseases. They are frequently produced using recombinant deoxyribonucleic acid (DNA) technology and are designed to act on specific parts of the human immune system. For example, biologics such as certolizumab, etanercept (Enbrel®, Pfizer, New York, NY, USA), adalimumab (HUMIRA®, AbbVie, Maidenhead, UK), infliximab (Remicade®, Janssen Biotech, Inc., Horsham, PA, USA) and golimumab (Simponi®, Janssen Biotech, Inc., Horsham, PA, USA) block tumour necrosis factor alpha (TNF-α), and ustekinumab (STELARA®, Janssen Biotech, Inc., Titusville, NJ, USA) and secukinumab (Cosentyx®, Novartis, Basel, Switzerland) inhibit IL-12/IL-23 and IL-17-A respectively. Such biologics are indicated for a range of conditions, including psoriasis, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis and inflammatory bowel disease.
Three biologics (adalimumab, etanercept and ustekinumab) have regulatory approval for the treatment of plaque psoriasis in children and young people (Table 1).
| Treatment | Age range | Disease status | Mechanism of action | Dose/frequency | Treatment pathway |
|---|---|---|---|---|---|
| Adalimumab | ≥ 4 years | Severe chronic plaque psoriasis | TNF-α inhibitor | 0.8 mg/kg up to a maximum of 40 mg at weeks 0 and 1, then every 2 weeks thereafter | When topical therapy and phototherapies are inadequate or inappropriate |
| Etanercept | ≥ 6 years | Severe chronic plaque psoriasis | TNF-α inhibitor | 0.8 mg/kg up to a maximum of 50 mg weekly for up to 24 weeks | When systemic therapies or phototherapies are inadequate or not tolerated |
| Ustekinumab | ≥ 12 years | Moderate to severe plaque psoriasis | IL-12/IL-23 inhibitor | 0.75 mg/kg for body weight of < 60 kg, 45 mg for body weight of 60–100 kg and 90 mg for body weight of > 100 kg at weeks 0 and 4, then every 12 weeks thereafter | When systemic therapies or phototherapies are inadequate or not tolerated |
Adalimumab is a fully human immunoglobulin G1 monoclonal antibody that inhibits the activity of TNF-α. It has a marketing authorisation in the UK for treating severe chronic plaque psoriasis in children and adolescents from 4 years of age who have an inadequate response to, or who are inappropriate candidates for, topical therapy and phototherapies.
Etanercept is a recombinant human TNF-α receptor fusion protein that inhibits the activity of TNF-α. It has a marketing authorisation in the UK for treating chronic severe plaque psoriasis in children and adolescents from the age of 6 years who are inadequately controlled by, or who are intolerant to, other systemic therapies or phototherapies.
Ustekinumab is a fully human monoclonal antibody that acts as a cytokine inhibitor by targeting IL-12 and IL-23. It has a marketing authorisation for treating moderate to severe plaque psoriasis in adolescent patients from the age of 12 years who are inadequately controlled by, or who are intolerant to, other systemic therapies or phototherapies.
More recently, versions of biological drugs have become available that have been manufactured after the expiry of an original innovator agent’s patent. These ‘biosimilars’ are developed to be highly similar to the existing biological agents in physicochemical and biological terms and are typically cheaper than the original agents. Biosimilar medicines are usually licensed for all indications specified in the licence of the originator biological medicine, but this requires appropriate scientific justification on the basis of demonstrated or extrapolated equivalence. Benepali® (Biogen Idec Ltd, Maidenhead, UK), a biosimilar of etanercept, has been approved in Europe for use in adults with moderate to severe rheumatoid arthritis, psoriatic arthritis, severe ankylosing spondylitis, severe non-radiographic axial spondyloarthritis and moderate to severe plaque psoriasis. Currently, three biosimilars of infliximab (Inflectra®, Pfizer; Remsima, Pfizer; and Flixabi®, Biogen, Cambridge, MA, USA) are approved for use in ankylosing spondylitis, Crohn’s disease, psoriatic arthritis, psoriasis, rheumatoid arthritis and ulcerative colitis.
Chapter 2 Definition of the decision problem
According to NICE guideline CG153 in England,11 psoriasis patients are treated in three stages. First-line therapy includes traditional topical therapies (such as corticosteroids, vitamin D and vitamin D analogues, dithranol and tar preparations). Second-line therapies include the phototherapies NBUVB light and PUVA and systemic non-biological agents such as ciclosporin, methotrexate and acitretin. Systemic biological therapies such as the TNF antagonists adalimumab, etanercept and infliximab and the monoclonal antibody ustekinumab, which targets IL-12 and IL-23, can be provided as third-line therapy.
The three biologics that have regulatory approval for the treatment of plaque psoriasis in children and young people (adalimumab, etanercept and ustekinumab) have not yet been appraised by NICE and no NICE technology appraisal (TA) guidance is available for treating children and adolescents in the UK with these treatments for this indication.
Objective
The aim of this study was to determine the clinical effectiveness and cost-effectiveness of adalimumab, etanercept and ustekinumab within their respective licensed indications for the treatment of plaque psoriasis in children and young people.
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 3 Assessment of clinical effectiveness
Methods for the synthesis of evidence of clinical effectiveness
A systematic review of the clinical effectiveness of adalimumab, etanercept and ustekinumab within their respective licensed indications for the treatment of plaque psoriasis in children and young people was performed following the general principles recommended in the Centre for Reviews and Dissemination (CRD)’s guidance28 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 29 A protocol was registered with PROSPERO.
Literature searching: adalimumab, etanercept and ustekinumab
The literature search for the clinical effectiveness review aimed to systematically identify relevant RCTs of adalimumab, etanercept and ustekinumab used to treat children and young people with plaque psoriasis.
The search strategy was developed in MEDLINE (via Ovid) and included search terms for:
-
psoriasis
-
adalimumab, etanercept, ustekinumab or biosimilars
-
children or young people.
The three sets of terms were combined using the Boolean operator AND. Search terms were developed through discussion with the review team and use of database thesauri and online drug information resources. No language, date, geographical or study design limits were applied. The MEDLINE strategy was adapted for use in the other resources searched.
The searches were carried out on 24/25 May 2016 and updated during September 2016. The following databases were searched: MEDLINE (including MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, Database of Abstracts of Reviews of Effects (DARE), EMBASE, Health Technology Assessment (HTA) database, NHS Economic Evaluation Database (NHS EED), PubMed and Science Citation Index.
In addition, the following resources were searched for ongoing, unpublished or grey literature: ClinicalTrials.gov, Conference Proceedings Citation Index – Science, EU Clinical Trials Register, PROSPERO and World Health Organization (WHO) International Clinical Trials Registry Platform portal.
A search for guidelines on psoriasis in children or young people was carried out through the following guideline websites: National Guideline Clearinghouse (www.guideline.gov; accessed 14 June 2017), NICE Clinical Knowledge Summaries (https://cks.nice.org.uk/; accessed 14 June 2017), NHS Evidence (www.evidence.nhs.uk; accessed 14 June 2017), NICE evidence summaries: new medicines (www.evidence.nhs.uk/Search?q=Evidence+summary+new+medicine; accessed 14 June 2017) and the NICE website (www.nice.org.uk/; accessed 14 June 2017).
In addition to utilising these published and unpublished data resources, requests for clinical study reports (CSRs) relating to adalimumab, etanercept and ustekinumab were made to AbbVie, Pfizer and Janssen respectively.
The search results were imported into EndNote X7 (Thomson Reuters, CA, USA) and deduplicated. Full search strategies can be found in Appendix 1.
Literature searching: network meta-analysis
Alternative treatments in children and young people
To inform the network meta-analysis (NMA), searches were undertaken to identify relevant RCTs of systemic non-biological (acitretin, methotrexate and ciclosporin) and other biological (infliximab, secukinumab) therapies used in children and young people with plaque psoriasis. No language, date, geographical or study design limits were applied to the searches.
The searches were carried out on 31 May 2016 in the following databases: MEDLINE (including MEDLINE Epub Ahead of Print, MEDLIINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE), CENTRAL, CDSR, CINAHL Plus, DARE, EMBASE, HTA database, PubMed and Science Citation Index.
In addition, the following resources were searched for ongoing, unpublished or grey literature: ClinicalTrials.gov, Conference Proceedings Citation Index – Science, EU Clinical Trials Register, PROSPERO and WHO International Clinical Trials Registry Platform portal.
The search results were imported into EndNote X7 and deduplicated. The search was updated in September 2016 to capture more recent studies. Full search strategies can be found in Appendix 1.
Registry data
To identify longer-term follow-up evidence, a literature search was conducted within the MEDLINE database for the search terms ‘psoriasis AND regist*’. The results of this search were screened for publications from psoriasis registries, secondary analyses of registry data and systematic reviews of broader dermatological and psoriasis registry data. The list of registries generated through these searches was compared against those in three relevant systematic reviews30–32 to verify the studies included and to identify any that had been overlooked. Twenty patient registries for psoriasis treatment were identified in this way; 14 were located in European countries, three were international in scope, two were based in the USA and one was based in Malaysia. Each registry name was then separately used as a search term in MEDLINE and any publications referencing these that had not been found in the initial searches were retrieved.
In addition, representatives of the 14 psoriasis registries from European countries (Austria, Australia, Czech Republic, Denmark, France, Germany, Italy, Netherlands, Portugal, Slovenia, Spain, Sweden, Switzerland and the UK) were contacted and asked to provide any relevant information on the use of the biologics adalimumab, etanercept and ustekinumab for the treatment of psoriasis in children and young people.
Inclusion and exclusion criteria
Two reviewers independently screened all titles and abstracts. Full manuscripts for any potentially relevant titles/abstracts were obtained when possible and the relevance of each study was assessed by two reviewers according to the following criteria. Any discrepancies were resolved by consensus and, if necessary, a third reviewer was consulted. Studies available only as abstracts were included and attempts were made to contact the authors for further details.
Study design
Randomised controlled trials (including any open-label extensions of RCTs) were eligible for the review of clinical efficacy.
Information on adverse events (AEs) was also sought from regulatory sources when appropriate. Registries and observational studies were included when relevant outcome data were available.
To address longer-term measures of efficacy and drug survival, published analyses based on large and long-term data sets (including studies of registry data) were also considered.
Participants
Studies of children and/or young people with moderate to severe plaque psoriasis were included. Severity could be defined using the PASI, PGA, BSA or other measures, alone or in combination, although there is no universal definition of severity for this population. Studies of guttate, erythrodermic and pustular psoriasis were excluded, as were studies of psoriatic arthritis.
Studies in children or young people with psoriasis in whom topical therapies, systemic therapies or phototherapies were inadequate, inappropriate or not tolerated were eligible for inclusion. Participants aged < 12 years were considered to be children whereas those aged 12–17 years were considered to be young people.
Interventions
The relevant interventions were adalimumab, etanercept and ustekinumab.
Comparators
The relevant comparators were:
-
alternative biological therapies with relevant marketing authorisation (adalimumab, etanercept or ustekinumab)
-
non-biological systemic therapy (including, but not limited to, ciclosporin and methotrexate)
-
topical therapy (for people in whom non-biological systemic therapy is not suitable), that is, best supportive care (BSC)
-
biological treatments used outside their marketing authorisation (such as infliximab, adalimumab, etanercept or ustekinumab if used outside the constraints of the relevant marketing authorisation in children and young people)
-
biosimilars of etanercept, adalimumab or ustekinumab
-
placebo.
Outcomes
Data on effectiveness, adverse effects, patient-centred outcome measures, costs to the health service and cost-effectiveness were eligible for inclusion, including the following outcomes:
-
severity of psoriasis (e.g. BSA, PGA score)
-
response and remission rates (e.g. PASI 50/75/90 response)
-
relapse rate
-
rates of treatment discontinuation and withdrawal
-
short- and long-term adverse effects of treatment (e.g. injection site and allergic reactions, serious infections, reactivation of infections including tuberculosis, malignancy)
-
HRQoL [e.g. CDLQI, PedsQL and EuroQol-5 Dimensions (EQ-5D)33 scores].
Data extraction
Data relating to both study design and study quality were extracted by one reviewer using a standardised data extraction form and independently checked for accuracy by a second reviewer. Disagreements were resolved through consensus and, if necessary, a third reviewer was consulted. Data from studies with multiple publications were extracted and reported as a single study.
Quality assessment
The quality of RCTs was assessed using the Cochrane risk-of-bias tool,34 with additional assessments made for baseline imbalance of important prognostic indicators. 35 Relevant prognostic and treatment response indicators were identified from both published research and clinical advice. The risk-of-bias assessment was performed by one reviewer and independently checked by a second. Disagreements were resolved through consensus and, if necessary, a third reviewer was consulted.
The quality of non-randomised studies was assessed using a checklist based on CRD guidance28 and used in previous technology assessments for NICE. 36 This assesses study eligibility criteria and recruitment methods, the baseline similarity of comparison groups, the blinding of allocation, the completeness of follow-up and outcome reporting.
Methods of data synthesis
The analysis and synthesis of clinical data in this review was conducted in distinct sections. In the absence of sufficient trials to conduct pairwise meta-analysis, the results of included studies are presented in a series of structured tables and summarised narratively and subjected to detailed critical appraisal.
To assess the relative clinical effectiveness of the three biologics (i.e. adalimumab, etanercept and ustekinumab), syntheses of both pairwise (head-to-head) and indirect comparative data were planned. When possible, treatment response (PASI) outcomes were to be synthesised using Bayesian NMA methods. Bayesian statistical methods provide information on the benefits of the active treatments relative to the appropriate comparators and each other. 37 Meta-analysis using mixed-treatment comparisons enables the estimation of different parameters from several studies with similar comparisons to be combined when direct evidence on comparisons of interest is absent or sparse. 38 For example, should active treatments being evaluated have a common comparator of placebo, this would allow a network to be established between them, providing information on the benefits of these treatments relative to placebo and to each other.
However, the available trials conducted in children precluded the construction of the necessary network. To inform the economic evaluation, trials conducted in adults were included in a NMA. Full details of the methods and results are presented in Chapter 4 (see Framework of analysis for informing the relative efficacy of the interventions).
Results
Quantity of identified evidence
A total of 2386 non-duplicate records were identified from the clinical effectiveness database searches. Of these, 2284 records were excluded after title or abstract screening. In addition, eight relevant regulatory documents were retrieved. Thus, a total of 111 records were read in full, resulting in 63 records being excluded and a total of 48 records being included in the review,39–86 relating to nine studies (three RCTs and six open-label or observational follow-up studies) (Figure 1). The included records are summarised in Appendix 2. Appendix 3 lists the excluded studies and reasons for exclusion.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of studies in children and young people.
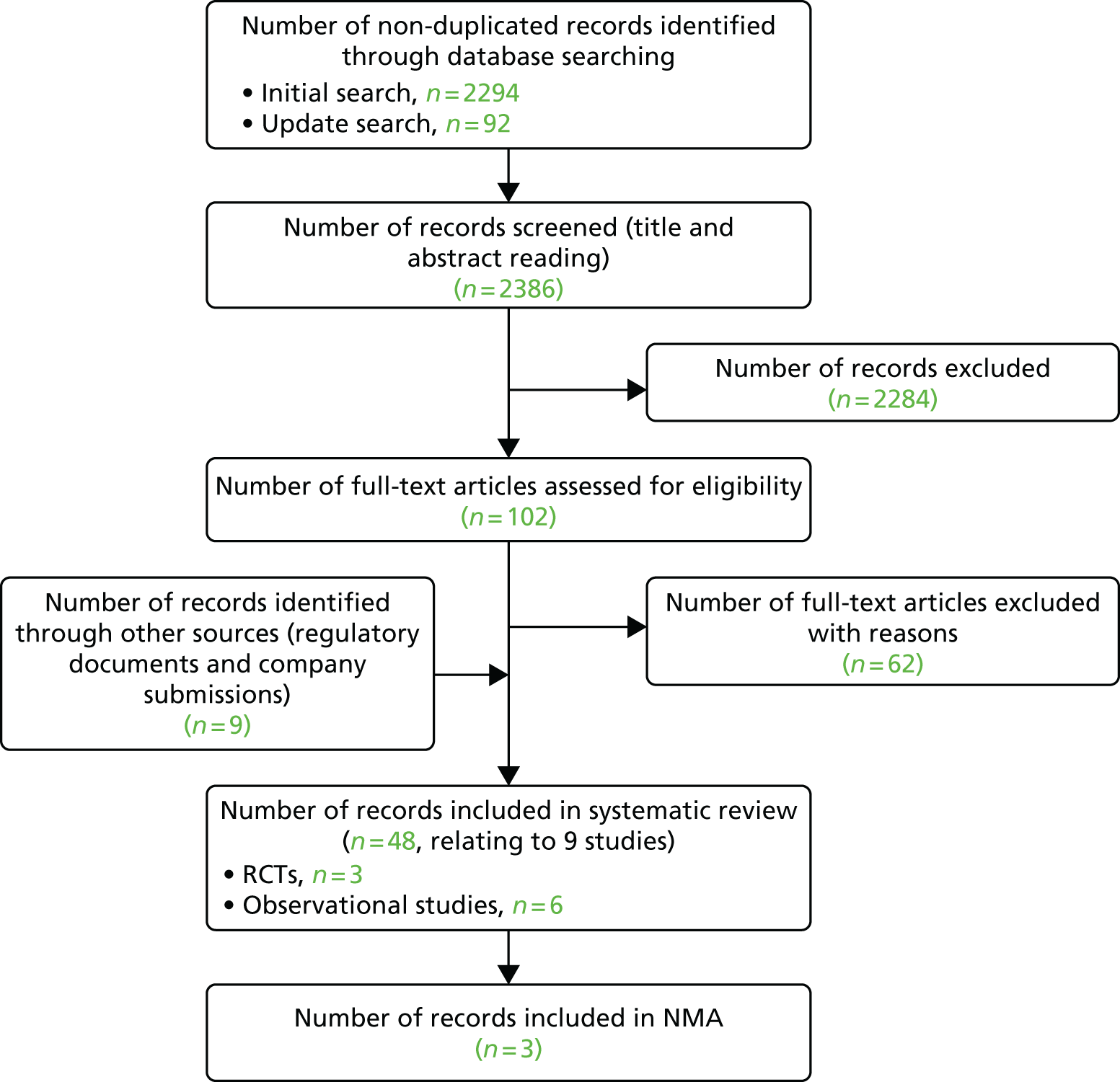
Searches for relevant registry data identified 685 publications. Three publications from two registries were found to include children with psoriasis who were treated with biologics. Of the 14 national psoriasis registry representatives contacted, seven responded but no relevant additional data were available.
Characteristics of the included studies
Three RCTs were retrieved, one for each of the biologics of interest (i.e. adalimumab,39–47,79,80,87 etanercept48–69 and ustekinumab72–75,81,82,88). The RCTs investigated short-term clinical efficacy and AEs. The etanercept and ustekinumab trials included 12 weeks of follow-up and used placebo as a comparator whereas the adalimumab trial was of 16 weeks’ duration and included oral methotrexate, a non-biological systemic treatment, as the comparator. Participant selection criteria for these trials are reported in Table 2. Each RCT also incorporated an open-label phase (Table 3). These open-label or observational periods investigated longer-term efficacy and AEs, incorporating withdrawal and/or retreatment phases. The adalimumab, etanercept and ustekinumab trials had 52, 312 and 60 weeks of follow-up data available respectively.
| Criteria | Study | ||
|---|---|---|---|
| Adalimumab (M04-717) | Etanercept (20030211) | Ustekinumab (CADMUS) | |
| Inclusion criteria |
|
|
|
| Exclusion criteria |
|
|
|
| Study | Relevant dosing and regimens used | Duration of randomised and blinded phase | Post-randomised period design details | Latest time point with available results | Anticipated time to response: information from Summary of Product Characteristics |
|---|---|---|---|---|---|
| Adalimumab (M04-717) | Adalimumab: standard dose (initial 0.8 mg/kg up to a maximum of 40 mg, followed by 0.8 mg/kg every other week) or half-dose. Methotrexate: initial dose 0.1 mg/kg, up to a maximum of 7.5 mg per week, followed by a dose of up to 0.4 mg/kg from week 1 onwards, up to a maximum dose of 25 mg per week | 16 weeks | After the primary treatment phase (period A – blinded period), responders from period A were withdrawn from active treatment for up to 36 weeks and monitored for loss of disease control (withdrawal phase or period B). Participants from period B who had experienced loss of disease control were treated with adalimumab for up to 16 weeks (retreatment phase or period C). Participants from periods A, B and C who met entry criteria to the long-term follow-up phase or period D received adalimumab or were observed off treatment (if disease remained under control during period B) | 52 weeks | Continued therapy beyond 16 weeks should be carefully considered in a patient not responding within this time period |
| Etanercept (20030211) | Etanercept: dose of 0.8 mg/kg of body weight up to a maximum intended dose of 50 mg | 12 weeks | A 24-week, open-label treatment period (weeks 13–36) to assess the efficacy of etanercept therapy in all patients and a 12-week, randomised, double-blind, withdrawal–retreatment period (weeks 37–48) to examine the effects of withdrawal of the study drug and subsequent retreatment | 312 weeks | The recommended dose is 0.8 mg/kg (up to a maximum of 50 mg per dose) once weekly for up to 24 weeks. Treatment should be discontinued in patients who show no response after 12 weeks. If retreatment with etanercept is indicated, the above guidance on treatment duration should be followed |
| Ustekinumab (CADMUS) | Ustekinumab: standard dose (0.75 mg/kg for those weighing up to 60 kg, fixed 45 mg for those weighing 60–100 kg, fixed 90 mg for those weighing > 100 kg) or half-dose at 0 and 4 weeks and every 12 weeks subsequently | 12 weeks | After the double-blinded period (12 weeks), those in the placebo group were allowed to cross over to receive either standard or half-dose ustekinumab at weeks 12 and 16 and then every 12 weeks. Participants were followed for efficacy and safety through weeks 52 and 60 respectively | 60 weeks | Consideration should be given to discontinuing treatment in patients who have shown no response up to 28 weeks of treatment |
Baseline characteristics of participants
The baseline characteristics of the participants in the included RCTs are presented in Table 4. Although only older children and adolescents (aged 12–17 years) were included in the ustekinumab trial, the median age of children across the three trials did not differ greatly, as it appears that relatively few younger children were included in the adalimumab and etanercept trials.
| Characteristic | Study | |||||||
|---|---|---|---|---|---|---|---|---|
| Adalimumab (M04-717) | Etanercept (20030211) | Ustekinumab (CADMUS) | ||||||
| Adalimumab 0.8 mg/kg | Adalimumab 0.4 mg/kg | Methotrexate | Etanercept 0.8 mg/kg | Placebo | Ustekinumab 0.75 mg/kg | Ustekinumab 0.375 mg/kg | Placebo | |
| Study duration (weeks) | 16 | 16 | 16 | 12 | 12 | 12 | 12 | 12 |
| Number of participants | 38 | 39 | 37 | 106 | 105 | 36 | 37 | 37 |
| Age (years), median (range) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 14 (4–17) | 13 (4–17) | 15.0 (12–17) | 15.0 (12–17) | 16 (12–17) |
| Age (years), mean (SD) | 13.0 (3.3) | 12.6 (4.4) | 13.4 (3.5) | – | – | 14.8 (1.7) | 15.1 (1.7) | 15.6 (1.5) |
| Male (%) | 44.7 | 53.8 | 29.7 | 52 | 50 | 44.4 | 48.6 | 54.1 |
| Duration of psoriasis (years), mean (SD) | 5.0 (3.8) | 4.8 (3.3) | 5.1 (3.8) | – | – | 5.6 (3.8) | 5.9 (4.0) | 6.2 (5.0) |
| Duration of psoriasis (years), median (range) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 6.8 (0.3–17.9) | 5.8 (0.3–15.8) | 5.5 (0.6–13.6) | 5.7 (0.5–15.0) | 5.1 (0.4–17.8) |
| Weight (kg), mean (SD) | – | – | – | – | – | 62 (17.1) | 68.2 (24.5) | 64.7 (14.7) |
| Weight (kg), median (range) | 48.5 (17–95) | 53 (15–108) | 52 (20–87) | 59.6 (17.7–168.3) | 59.8 (17.2–131.5) | 61.7 (33.8–109.5) | 62.0 (32.0–173.5) | 60.3 (43.8–107.0) |
| Height (cm), mean (SD) | – | – | – | – | – | 163.9 (9.2) | 168 (11.0) | 169.7 (11.3) |
| Height (cm), median (range) | 156.5 (104–185) | 157 (121–182) | 157 (121–182) | 159 (104–188) | 158 (104–191) | 163.0 (145.0–181.0) | 168.0 (142.0–188.0) | 171.3 (147.0–188.0) |
| BSA % affected, mean (SD) | 27.7 (20.4) | 26.0 (16.2) | 30.3 (21.2) | – | – | 31.9 (23.2) | 33.6 (21.4) | 27.4 (16.4) |
| BSA % affected, median (range) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 21 (10–90) | 20 (10–95) | – | – | – |
| PASI score, median (range) | 15.3 (10.2–50.4) | 15.6 (6.1–29.4) | 17.5 (5.0–51.4) | 16.7 (12.0–51.6) | 16.4 (12.0–56.7) | 16.8 | 19.5 | 19.6 |
| PASI score, mean (SD) | 18.9 (10) | 16.9 (5.8) | 19.2 (10) | 18.5 (6.7) | 18.6 (6.8) | 21.7 (10.4) | 21.0 (8.5) | 20.8 (8.0) |
| PGA score of ≥ 3 (%) | 92 | 90 | 97 | 99 | 99 | 100 | 100 | 100 |
| Psoriatic arthritis (%) | 0 | 2.6 | 0 | 5 | 13 | 5.6 | 5.4 | 5.4 |
| Previous use of topical therapy (%) | 100 | 100 | 100 | – | – | 91.7 | 83.8 | 91.9 |
| Previous use of phototherapy (%) | 44.7 | 59 | 51.4 | – | – | 38.9 | 48.6 | 29.7 |
| Previous use of systemic therapy (%) | 36.8 | 28.2 | 24.3 | 55a | 59a | 47.2 | 37.8 | 43.2 |
| Previous use of biological therapy (%) | 10.5 | 10.3 | 8.1 | 0 | 0 | 8.3 | 10.8 | 13.5 |
| CDLQI score, mean (SD) | 10.9 (6.6) | 11.6 (7.9) | 11.4 (5.6) | 8.7 (6.0) | 10 (6.4) | 10.3 (6.6) | 9.4 (6.5) | 9.1 (6.4) |
| CDLQI score, median (range) | 10 (1–23) | 10.5 (0–27) | 12 (1–23) | 7.0 (0–26) | 9.5 (0–29) | 9.0 (1.0–26.0) | 10.5 (0.0–24.0) | 10.0 (1.0–26.0) |
| PedsQL score, mean (SD) | 70.4 (14.2) | 70.4 (21.3) | 78.8 (14.9) | 74.8 (17.8) | 76.1 (16.9) | 76.4 (15.3) | 75.2 (16.2) | 73.3 (17.5) |
| PedsQL score, median (range) | 72.3 (41.3–93.5) | 75 (5.4–100) | 84.8 (38.98.9) | 77.2 (5.4–100) | 79.9 (79.9–100) | 79.4 (42.4–100.0) | 77.7 (34.8–97.8) | 77.2 (26.1–98.9) |
All three trials used a composite measure of disease severity incorporating baseline PASI, PGA and BSA measurements. When used in isolation, a PASI score between 10 and 20 is considered to indicate moderate to severe psoriasis, whereas severe psoriasis has a score of > 20. Across the included studies, the average PASI score ranged from 18.3 to 21.2, with 93–100% of participants having a PGA score of > 3 (mild/moderate disease). Although adalimumab and etanercept are licensed for severe chronic plaque psoriasis and ustekinumab is licensed for moderate to severe plaque psoriasis, on average, measures of disease duration and the component measures of severity did not appear to differ markedly between the three trials. The degree of psoriasis affecting high-impact and difficult-to-treat sites (e.g. face, scalp, palms, soles, flexures and genitals) across the three studies was less clear.
A key difference between the licences for the three agents is the availability of adalimumab for patients for whom topical therapy and phototherapy are inadequate or inappropriate. Unlike the licences for etanercept and ustekinumab, there is no mention in the licence for adalimumab of previous non-biological systemic treatment. However, a substantial minority of participants in the adalimumab trial (29.8%) had received prior systemic therapy, compared with 42.7% of participants in the ustekinumab trial; 56.8% of participants in the etanercept trial had received either prior systemic therapy or prior phototherapy (separate data were not reported).
A similar proportion of participants in the adalimumab and ustekinumab trials had received some form of biological treatment prior to enrolment (9.6% and 10.8% respectively). As etanercept was the first TNF-α inhibitor to be approved for psoriasis, none of the participants recruited to the etanercept trial had previously been treated with biological therapy.
Although there were noticeable differences in participant characteristics between the trials, these were not as clear as the respective licences for the three treatments might suggest. Notwithstanding methodological differences, there appears to be sufficient overlap in the trial populations to discuss these three trials together.
Length of follow-up and early escape
The initial randomised treatment period was 12 weeks in the etanercept and ustekinumab trials and 16 weeks in the adalimumab trial. Twelve-week outcome data were not available for the adalimumab trial, although clinical advice (Dr Ruth Murphy, Consultant Dermatologist, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, 25 October 2016, personal communication) suggested that the difference in length of follow-up between treatments was acceptable.
All three trials allowed participants to ‘escape’ from the randomised treatment period before the 12-/16-week follow-up. The criteria for early escape and statistical handling of early escape data are discussed separately for each trial in the relevant sections.
Post-randomised treatment periods are briefly summarised in Table 3.
Outcomes
The adalimumab and etanercept trials considered the PASI 75 response to be the primary outcome measure, whereas the ustekinumab trial used a primary outcome measure of a PGA score of 0 or 1 (‘clear’ or ‘almost clear’). However, all three trials reported PASI and PGA scores and some measure of HRQoL (CDLQI and/or PedsQL), which are presented in the following sections.
Efficacy and safety of adalimumab
One multicentre RCT (M04-717) comparing two doses of adalimumab against methotrexate met the selection criteria for the review. Although this trial has not been published in a peer-reviewed journal, data were available from regulatory documentation,46,47 conference proceedings39–44,87 and a CSR provided by the manufacturer. 45
The M04-717 trial was separated into four periods:
-
period A – double-blind RCT of initial treatment (16 weeks)
-
period B – observational study of treatment withdrawal (up to 36 weeks)
-
period C – double-blind retreatment study based on original randomisation in period A (16 weeks)
-
period D – long-term follow-up (up to 52 weeks).
The double-blind RCT (period A) recruited paediatric patients (aged 4–17 years, weighing ≥ 13 kg) with severe chronic psoriasis from 42 centres across 13 countries. Severe chronic psoriasis was defined as failure to respond to topical therapy, requiring systemic treatment to control disease, and one of the following: (1) sPGA of ≥ 4, (2) BSA involvement of > 20%, (3) very thick lesions with BSA involvement of > 10%, (4) PASI score of > 20, or (5) PASI score of > 10 plus one of the following: (i) active psoriatic arthritis unresponsive to non-steroidal anti-inflammatory drugs, (ii) clinically relevant facial involvement, (iii) clinically relevant genital involvement, (iv) clinically relevant hand and/or foot involvement, or (v) CDLQI score of > 10.
In total, 114 participants were randomised: 38 to standard-dose adalimumab (subcutaneous; initial dose of 0.8 mg/kg up to a maximum of 40 mg, followed by 0.8 mg/kg every other week); 39 to low-dose adalimumab (subcutaneous; initial dose of 0.4 mg/kg up to a maximum of 20 mg, followed by 0.4 mg/kg every other week); and 37 to methotrexate (orally; initial dose of 0.1 mg/kg up to a maximum of 7.5 mg, followed by a weekly dose of up to 0.4 mg/kg, up to a maximum dose of 25 mg/week). To maintain blinding, participants allocated to adalimumab received placebo tablets and participants allocated to methotrexate received a placebo injection according to the adalimumab schedule. As methotrexate is a folic acid antagonist, all participants received folic acid (0.8–1.0 mg/day) as a dietary supplement (to maintain study blinding).
Previous therapy received by trial participants included topical therapy (100%), phototherapy (52%), non-biological systemic therapy (30%) and biological therapy (10%; all etanercept).
Risk-of-bias assessment
The risk of bias for the trial was low for most domains, with appropriate methods used for the allocation of participants, blinding, handling of missing data and reporting of outcomes (on the basis of information reported in the CSR;45 Table 5). Baseline characteristics were mostly balanced across treatment groups, with the exception of percentage of males, which appeared to be lower in the methotrexate arm. It should be noted that only six of the 114 children randomised were aged < 7 years at recruitment, all of whom were randomised to the low-dose adalimumab group. This means that, despite adalimumab having a marketing authorisation in children aged ≥ 4 years, this particular trial does not provide any comparative efficacy data on the licensed standard dose of adalimumab in children aged 4–6 years.
| Assessment criterion | Risk-of-bias judgement | Support for judgement |
|---|---|---|
| Sequence generation | Low | ‘Participants were randomised by interactive voice/web response system to receive adalimumab 0.8 mg/kg, adalimumab 0.4 mg/kg, or MTX in a 1 : 1 : 1 ratio, respectively. Randomisation was stratified by prior treatment with etanercept’ (p. 15)45 |
| Allocation concealment | Low | Participants were randomised using an interactive voice/web response (IVR/IWR) system |
| Baseline comparability | Moderate | There was a higher proportion of female participants in the methotrexate group than in the adalimumab groups. Only six children aged < 7 years were included in the trial, all of whom were in the 0.4 mg/kg adalimumab group. There was a higher baseline PedsQL score in the methotrexate group |
| Blinding of participants, personnel and outcome assessors | Low | ‘All AbbVie personnel with direct oversight of the conduct and management of the trial, (with the exception of the AbbVie Drug Supply Management Team), the PI, study site personnel, and the participant were to remain blinded to each participant’s treatment throughout the blinded period of the study. The IVR/IWR system was to provide access to blinded participant treatment information in the case of medical emergency’ (p. 15).45 There was one participant for whom the blind was broken because of a serious adverse event (proctocolitis) that occurred on day 195 of period B and who was thus non-treatment emergent |
| Incomplete outcome data | Low | Eight participants ‘early escaped’ by week 8 of period A: five initially randomised to methotrexate, two randomised to low-dose adalimumab and one randomised to standard-dose adalimumab |
| (Confidential information has been removed) | ||
| Selective reporting | Low | All outcomes from the trial protocol were reported in the CSR45 |
In total, 16 of the 114 participants received the wrong medication. Regulatory documents indicate that the incidence of the error ‘wrong medication’ occurred at single time points and was unlikely to have affected the results of the study. 45 A small number of patients (n < 5) across the three treatment arms received topical therapies during the randomised period, despite it being prohibited under the trial protocol, although this is unlikely to have had a substantial impact on the efficacy estimates.
Primary efficacy end points for the randomised controlled period were a ≥ PASI 75 response at week 16 and a sPGA rating of ‘cleared’ or ‘minimal’ (0 or 1) at week 16. Secondary outcomes included PASI 50, 90 and 100 responses, a PGA score of 0 and CDLQI and PedsQL scores.
Participants were evaluated at all visits for worsening of psoriasis. Up to and including the week 8 visit, participants were eligible for ‘early escape’ if they met one of the following criteria: (1) PASI scores increased by 50% at week 4 relative to baseline or (2) PASI scores increased by 25% relative to baseline and by ≥ 4 points at each of two consecutive study visits (prior to or at week 8). After week 8, participants were to continue in the trial until the week 16 visit.
Participants entering ‘early escape’ were permitted to enter a longer-term observational study period (period D; see Period D: long-term follow-up) in which they received open-label adalimumab at a dose of 0.8 mg/kg every other week (up to a maximum of 40 mg).
Primary efficacy analyses were conducted in the intention-to-treat (ITT) population (i.e. all randomised participants). Participants with missing or incomplete data at week 16 (including those entering ‘early escape’) were imputed to be non-responders for categorical variables (non-responder imputation method) and had their last observation carried forward for continuous variables. Analyses using per-protocol and ‘as observed’ data were also reported in the CSR. 45 The safety analysis was conducted in the safety population (i.e. all participants who received at least one dose of the study medication).
Efficacy of adalimumab at 16 weeks
The absolute and relative results for PASI, sPGA, CDLQI and PedsQL outcomes at week 16 are shown in Tables 6 and 7.
| Treatment | Participants who achieved the outcome, n/N (%) | Mean (SD) change from baseline | ||||
|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 or 1 | CDLQI | PedsQL | |
| ADA 0.8 mg/kg | Confidential information has been removed | 22/38 (57.9) | 11/38 (28.9) | 23/38 (60.5) | 6.6 (6.2) n = 38 | 10.8 (15.4) n = 38 |
| ADA 0.4 mg/kg | Confidential information has been removed | 17/39 (43.6) | 12/39 (30.7) | 16/39 (41.0) | 4.9 (6.2) n = 38 | 9.5 (12.3) n = 38 |
| MTX 0.1 mg/kg | Confidential information has been removed | 12/37 (32.4) | 8/37 (21.6) | 15/37 (40.5) | 5 (7.1) n = 36 | 1.9 (10.4) n = 36 |
| Treatment | Relative risk (95% CI) | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 or 1 | CLDQI | PedsQL | |
| ADA 0.8 mg/kg | Confidential information has been removed | 1.79 (1.04 to 3.06) | 1.34 (0.61 to 2.95) | 1.49 (0.94 to 2.38) | 1.6 (–1.44 to 4.64) | 8.9 (2.94 to 14.86) |
| ADA 0.4 mg/kg | Confidential information has been removed | 1.34 (0.75 to 2.42 | 1.42 (0.65 to 3.08) | 0.81 (0.46 to 1.41) | –0.1 (–3.14 to 2.94) | 7.6 (2.42 to 12.78) |
| MTX 0.1 mg/kg (reference) | Confidential information has been removed | 1.00 | 1.00 | 1.00 | 0.00 | 0.00 |
Psoriasis Area and Severity Index response
(Confidential information has been removed) and PASI 75 response rates at 16 weeks were significantly greater for standard-dose adalimumab (0.8 mg/kg) than for methotrexate [(confidential information has been removed) 58% vs. 32% respectively)]. Low-dose adalimumab (0.4 mg/kg) did not show a statistically significant improvement over methotrexate for these outcomes [(confidential information has been removed) and 44% vs. 32% respectively]. PASI 90 response rates did not differ significantly between the three treatment arms (Table 8).
| Subgroup (years) | Treatment | Participants who achieved the outcome, n/N (%) | |||
|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 or 1 | ||
| 4–6 | ADA 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ADA 0.4 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| MTX | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| > 6 to 9 | ADA 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ADA 0.4 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| MTX | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| > 9 to 12 | ADA 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ADA 0.4 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| MTX | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| > 12 to 15 | ADA 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ADA 0.4 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| MTX | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| > 15 | ADA 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ADA 0.4 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| MTX | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
(Confidential information has been removed.)
Physician Global Assessment
The proportion of participants achieving a sPGA score of 0 or 1 (‘clear’ or ‘minimal’) at 16 weeks was greater for standard-dose adalimumab than for low-dose adalimumab or methotrexate (61% vs. 41% vs. 41% respectively), although this difference was not statistically significant.
(Confidential information has been removed.)
Quality of life
Two HRQoL measures, CDLQI and PedsQL, were reported at 16 weeks. All three treatment groups showed improvements from baseline in the dermatology-specific quality-of-life measure (CDLQI), exceeding the published minimal clinically important difference (MCID) of a 2.5-point change from baseline. 48 However, these improvements were similar across the three treatment groups, with no significant difference between either dose of adalimumab or methotrexate (6.6 for adalimumab 0.8 mg/kg vs. 4.9 for adalimumab 0.4 mg/kg vs. 5.0 for methotrexate respectively).
Unlike the CDLQI, improvements on the generic HRQoL measure (PedsQL) significantly favoured both doses of adalimumab over methotrexate (mean changes of 10.8 and 9.5 for standard- and low-dose adalimumab, respectively, vs. 1.9 for methotrexate). The mean changes in the adalimumab groups both exceeded the published MCID of 4.4 for the PedsQL. 26
It is unclear why PedsQL scores would increase in the absence of dermatology-related quality-of-life benefits as measured by the CDLQI. However, both mean and median PedsQL scores at baseline were noticeably higher in the methotrexate arm than in the adalimumab treatment arms (see Table 4) and so the observed PedsQL change scores in the adalimumab arms may be overestimates because of regression to the mean. 89
Longer-term efficacy of adalimumab
Period B: withdrawal
(Confidential information has been removed.)
Period C: retreatment
(Confidential information has been removed.)
| Disease status at the end of period B and retreatment patterns in period C | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
|---|---|---|---|---|
| Participants from period B who experienced loss of disease control, retreated with the originally randomised dose of adalimumab of 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Participants from period B who experienced loss of disease control, retreated with the originally randomised dose of adalimumab of 0.4 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Participants from period B who experienced loss of disease control who were initially randomised to methotrexate, retreated with adalimumab at a dose of 0.8 mg/kg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
(Confidential information has been removed.)
Period D: long-term follow-up
(Confidential information has been removed.)
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
|---|---|---|---|---|
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
Safety of adalimumab
Adverse events at 16 weeks
Adverse event rates were comparable among the three treatment groups (Table 11). Three serious adverse events (SAEs) considered unrelated to treatment (hand fracture, gastrointestinal infection from food poisoning and agitation as a result of alcohol consumption) were reported, all of which occurred in participants receiving 0.4 mg/kg of adalimumab. One participant in the same treatment arm withdrew because of an AE (moderate psoriasis flare).
| Treatment | Participants with safety reports, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Withdrawals because of AEs | |
| ADA 0.8 mg/kg | 26/38 (68.4) | 0/38 (0.0) | 18/38 (47.4) | 0/38 (0.0) | 4/38 (10.5) | 0/38 (0.0) | 0/36 (0.0) |
| ADA 0.4 mg/kg | 30/39 (76.9) | 3/39 (7.7)a | 22/39 (56.4) | 1/39 (2.6) | 3/39 (7.7) | 0/39 (0.0) | 1/39 (2.6)b |
| MTX | 28/37 (75.7) | 0/37 (0.0) | 20/37 (54.1) | 0/37 (0.0) | 3/37 (8.1) | 0/37 (0.0) | 0/37 (0.0) |
Longer-term safety of adalimumab
Table 12 shows that the overall numbers of AEs during patient follow-up across all four study periods were similar across treatment arms. A total of nine SAEs were reported in six participants. In terms of episodes per 100 patient-years, the total rate of SAEs was 5.9 for all participants ever treated with 0.8 mg/kg of adalimumab from the first dose of 0.8 mg/kg adalimumab and 7.4 for all participants treated with adalimumab (0.4 mg/kg and 0.8 mg/kg) from the first dose of 0.8 mg/kg adalimumab.
| Follow-up period | Participants with safety reports, n | |||||||
|---|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Tuberculosis | Withdrawals because of AEs | |
| Period B | ||||||||
| ADA 0.8 mg/kg (n = 23) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0 | 0 | 0 | 0 | 0 |
| ADA 0.4 mg/kg (n = 18) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0 | 0 | 0 | 0 | 0 |
| MTX (n = 13) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0 | 0 | 0 | 0 | 0 |
| Period C | ||||||||
| ADA 0.8 mg/kg (n = 19) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0 | 0 | 2 | 0 | 0 |
| ADA 0.4 mg/kg (n = 11) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0 | 0 | 0 | 0 | 0 |
| MTX (n = 8) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0 | 0 | 0 | 0 | 1 |
| Period D | ||||||||
| ADA 0.8 mg/kg (n = 36) | Confidential information has been removed | 3 | 25 | 0 | 2 | 0 | 1 | 0 |
| ADA 0.4 mg/kg (n = 36) | Confidential information has been removed | 1 | 15 | 0 | 1 | 0 | 1 | 0 |
| MTX (n = 36) | Confidential information has been removed | 1 | 22 | 0 | 1 | 0 | 0 | 1a |
One SAE (haemorrhagic ovarian cyst) occurred in period B in a participant who had been initially randomised to 0.8 mg/kg of adalimumab.
Five SAEs occurred during period D, including one death from an accidental fall, one tendon injury in a participant receiving 0.4 mg/kg of adalimumab, one maculopapular rash in a participant receiving 0.8 mg/kg of adalimumab, one case of chest pain in a participant randomised to methotrexate but receiving 0.8 mg/kg of adalimumab and one case of eye naevus in a participant receiving 0.8 mg/kg of adalimumab. All SAEs were considered by investigators to be unrelated or probably unrelated to the study drug with the exception of the case of eye naevus, which was assessed as being possibly related.
In addition to the participant who discontinued treatment because of a moderate psoriasis flare in period A, one participant initially randomised to methotrexate but receiving 0.8 mg/kg of adalimumab during period D discontinued treatment because of severe urticaria.
The rate of all infections reported by participants receiving 0.8 mg/kg of adalimumab was 170.4 episodes per 100 patient-years. Only two events of tuberculosis occurred, both during period D.
Summary of the efficacy and safety of adalimumab
-
There was evidence from one 16-week RCT comparing adalimumab with methotrexate in children and young people with severe chronic psoriasis.
-
This trial did not provide comparative evidence for children aged 4–6 years.
-
Adalimumab at the licensed dose of 0.8 mg/kg (up to 40 mg) leads to significantly greater responses than methotrexate for the outcomes of PASI 50 and PASI 75, but not for the outcome of PASI 90.
-
PGA 0/1 response rates were higher for 0.8 mg/kg of adalimumab than for methotrexate, although the difference was not statistically significant.
-
The benefits of half-dose adalimumab were not statistically greater than those observed for methotrexate.
-
Evidence on quality of life was inconsistent across different measures, possibly because of baseline imbalances on the PedsQL.
-
(Confidential information has been removed.)
-
In children and young people, adalimumab does not appear to be associated with an increase in adverse effects relative to methotrexate over 16 weeks, (confidential information has been removed).
-
However, because of the small numbers of observed participants, the possibility of rare AEs cannot be entirely excluded.
Efficacy and safety of etanercept
One multicentre RCT (20030211) comparing etanercept with placebo met the selection criteria for the review. Data on short-term safety and efficacy (blinded period) were available from published peer-reviewed journal papers,48–53 conference proceedings54–60 and regulatory documents. 61–71
This double-blind RCT recruited children aged between 4 and 17 years from 42 sites in the USA and Canada who had stable, moderate-to-severe plaque psoriasis at screening. Moderate to severe plaque psoriasis was defined as a PASI score of ≥ 12 (PASI scores range from 0 to 72, with higher scores indicating a worse condition); a sPGA of ≥ 3 (in which 0 indicates clear and 5 indicates severe psoriasis) and psoriasis involvement of ≥ 10% of the BSA; a history of psoriasis for ≥ 6 months; and previous or current treatment with phototherapy or systemic psoriasis therapy (e.g. methotrexate, ciclosporin or retinoids) or psoriasis considered by the investigator as poorly controlled with topical therapy.
Within each age stratum, participants were randomised in a 1 : 1 ratio to either 0.8 mg/kg of etanercept once weekly up to a maximum dose of 50 mg or placebo.
The primary outcome measure used in the RCT was the PASI 75 response at week 12. The secondary outcome measures were PASI 50 response, PASI 90 response, clear or almost clear status on the sPGA and percentage improvement from baseline in the CDLQI at week 12.
A total of 264 participants were screened and 211 children were randomised to etanercept (n = 106) or placebo (n = 105). At baseline, both groups were similar in terms of age and sex, BSA and PASI and PGA scores, although the placebo group had a slightly higher proportion of patients with psoriatic arthritis (13% vs. 5%). There was no previous use of biological therapy in either group (see Table 4). It should be noted that only 19 children included in the study (9.0%) were aged < 8 years and only nine (4.3%) were aged < 6 years.
At or after week 4, participants with a > 50% increase or an absolute increase of ≥ 4 points in the PASI score from baseline were allowed to enter an ‘escape’ arm to receive open-label etanercept every week up to week 12. During this initial 12-week comparative period, a higher number of participants from the placebo group (n = 27/105) than the etanercept group (n = 5/106) entered the early escape arm. Participants who entered the escape arm were recorded as non-responders at the time that they entered the escape arm. Data for those participants from before they entered the escape arm were not changed. For participants who had missing data, their missing data were imputed as non-responses but their existing data were included as observed.
Risk-of-bias assessment
The trial had a low overall risk of bias for most domains, with appropriate methods used for randomisation, handling of missing data and reporting of outcomes (Table 13). The study was described as ‘double-blinded’, although the methods used to achieve blinding were not described.
| Assessment criterion | Risk-of-bias judgement | Support for judgement |
|---|---|---|
| Sequence generation | Low | Interactive voice or web response system was used |
| Allocation concealment | Low | Interactive voice or web response system was used during randomisation |
| Baseline comparability | Low | No obvious baseline imbalances although slightly higher psoriatic arthritis rate (13% vs. 5%) in the placebo group |
| Blinding of participants and personnel | Unclear | Although double blinded initially, patients could enter an escape arm and receive open-label etanercept. In total, 27/105 placebo-allocated patients entered the escape arm vs. 5/106 etanercept-allocated patients. For binary end points, efficacy measures taken after entering the escape group were imputed as non-responses. Blinding methods not described |
| Blinding of outcome assessment | Unclear | Participants, caregivers, investigators and outcomes assessors were blinded, although the method of blinding was not described |
| Incomplete outcome data | Low | For binary measures, missing post-baseline data were imputed as non-responses. Continuous measures were imputed to have baseline values |
| Selective reporting | Low | The reported treatment response and HRQoL outcomes match those described in the study protocol |
Efficacy of etanercept at week 12
Data on the treatment response outcomes were available from publications and regulatory documents. 49–70,87,89 PASI and PGA scores are reported in Tables 14 and 15.
| Treatment | Participants who achieved the outcome, n/N (%) | Mean (SD) change from baseline | ||||
|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 or 1 | CDLQI | PedsQL | |
| ETA | 79/106 (74.5) | 60/106 (56.6) | 29/106 (27.4) | 56/106 (52.8) | 5.4 (5.6) | 6.8 (17.6) |
| PLB | 24/105 (22.9) | 12/105 (11.4) | 7/105 (6.7) | 14/105 (13.3) | 3.1 (5.1) | 3.8 (10.1) |
| Treatment | Relative risk (95% CI) | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 or 1 | CDLQI | PedsQL | |
| ETA | 3.26 (2.26 to 4.71) | 4.95 (2.84 to 8.65) | 4.10 (1.88 to 8.95) | 3.96 (2.36 to 6.66) | 2.3 (0.85 to 3.74) | 3.0 (–0.87 to 6.87) |
| PLB | 1.00 | 1.00 | 1.00 | 1.00 | 0.00 | 0.00 |
Psoriasis Area and Severity Index response
Psoriasis Area and Severity Index 50, 75 and 90 responses in the etanercept group were 74.5%, 56.6% and 27.4% respectively. Response rates for the placebo group were 22.9%, 11.4% and 6.7%. When translated into relative risk (RR) values, the etanercept group had a significantly higher probability of achieving PASI 50, 75 and 90 responses, with RRs of 3.26 [95% confidence interval (CI) 2.26 to 4.71], 4.95 (95% CI 2.84 to 8.65) and 4.10 (95% CI 1.88 to 8.95) respectively.
Physician Global Assessment
The proportion of participants achieving a PGA score of 0 or 1 (‘clear’ or ‘minimal’) at 12 weeks was significantly greater in the etanercept group than in the placebo group (52.8% vs. 13.3%), equating to a RR of 3.96 (95% CI 2.36 to 6.66).
Quality of life
Data for two HRQoL measures, CDLQI and PedsQL, were available at 12 weeks (see Table 14). Both the etanercept group and the placebo group showed improvements from baseline in CDLQI scores, exceeding the published MCID of a 2.5-point change from baseline,48 although the improvement in the etanercept group was statistically significantly greater than that in the placebo group (mean difference 2.3, 95% CI 0.85 to 3.74).
Both treatment groups also showed improvements on the PedsQL, although for the placebo group this fell below the published MCID of 4.4. The mean change in PedsQL score from baseline, although favouring etanercept, was not statistically significantly different between the treatment groups (mean difference 3.0, 95% CI –0.87 to 6.87).
Subgroup outcomes
Age-based subgroup analysis results of PASI responses for the etanercept trial (20030211) were available (Table 16). A higher proportion of the etanercept treatment group than the placebo group achieved PASI 50, 75 and 90 responses in all age categories. Imputation of treatment failure for participants entering the early escape arm reduced the magnitude of the difference between treatments, although these differences remained formally statistically significant for all comparisons, with the exception of PASI 90, which was of borderline statistical significance (p = 0.054).
| Age (years) | Treatment | Participants who achieved the outcome, n/N (%) | ||
|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | ||
| ≥ 8 | ETA | 70/95a (73.7) | 52/95a (54.7) | 26/95a (27.4) |
| PLB | 23/97a (23.7) | 11/97a (11.3) | 6/97a (6.2) | |
| 4–11 | ETA | 29/38 (76.3), 30/38 (78.9)b | 22/38 (57.9), 22/38 (57.9)b | NA, 12/38 (31.6)b |
| PLB | 8/38 (21.1), 16/38 (42.1)b | 4/38 (10.5), 10/38 (26.3)b | NA, 5/38 (13.2)b | |
| 12–17 | ETA | 50/68 (73.5), 51/68 (75.0)b | 38/68 (55.9), 38/68 (55.9)b | NA, 17/68 (25.0)b |
| PLB | 16/67 (23.9), 21/67 (31.3)b | 8/67 (11.9), 11/67 (16.4)b | NA, 4/67 (5.6)b | |
Longer-term efficacy of etanercept
Weeks 12–36: open-label etanercept treatment
At the end of the 12-week double-blind period a total of 208 participants (105 and 103 of the original etanercept and placebo groups respectively) entered an open-label treatment phase (i.e. all were treated with etanercept) and were followed up until week 36.
Patients who did not achieve a PASI 50 response at week 24 were given the option to discontinue the study or enter the incomplete responder arm. Participants in the incomplete responder arm had the option to receive topical psoriasis therapy according to the standard of care in addition to receiving open-label etanercept (Figure 2).
FIGURE 2.
Short-term and long-term participant follow-up flow chart for the etanercept trial.
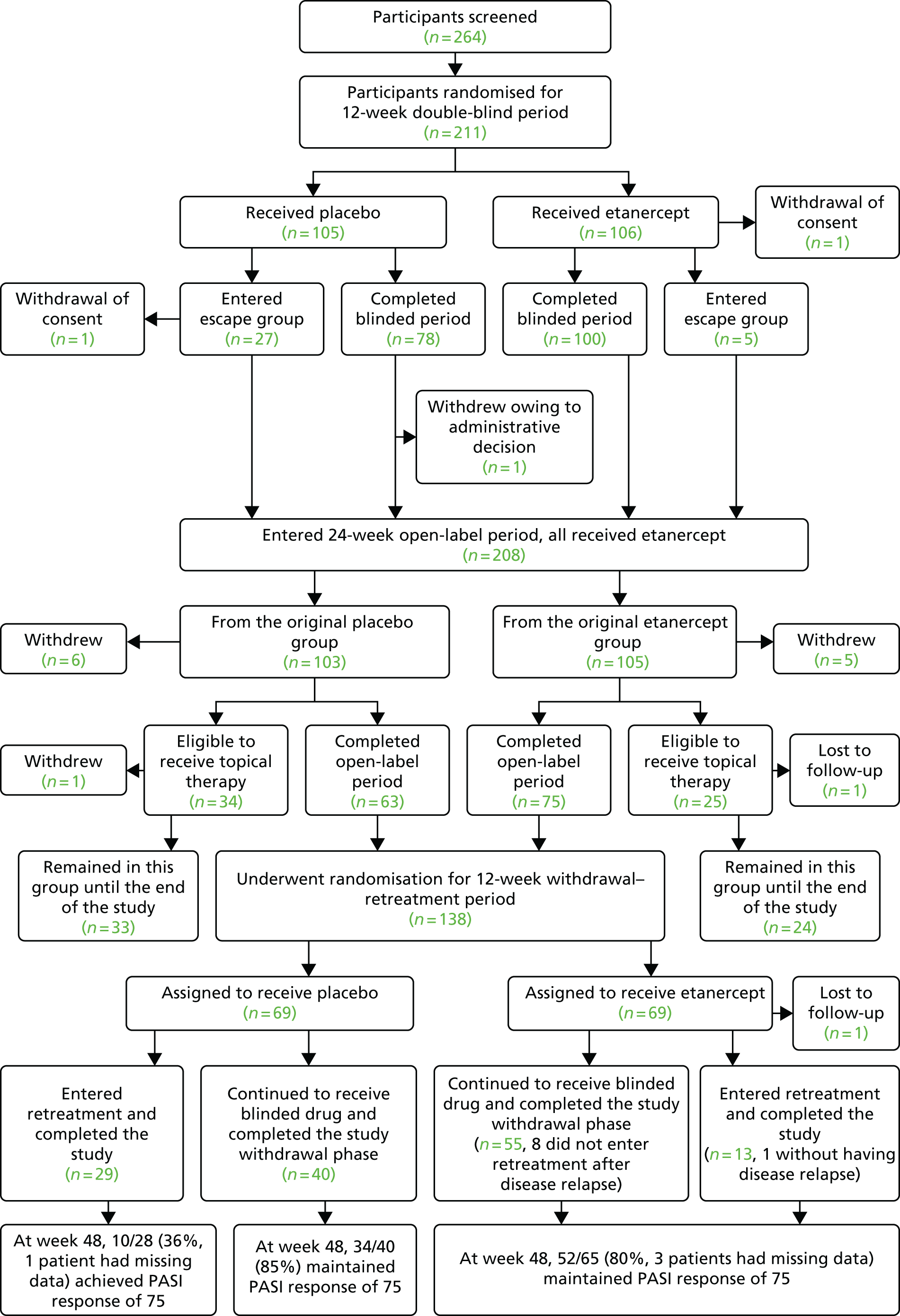
By weeks 24 and 36 (i.e. after 12 and 24 weeks of open-label etanercept respectively), participants who were originally randomised to placebo during the double-blind period achieved similar PASI and PGA responses as participants receiving etanercept throughout (Table 17).
| Follow-up | Participants who achieved the outcome, n/N (%) | ||
|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | |
| Week 24 | |||
| ETA/ETA | 92/105 (88) | 72/105 (69) | 39/105 (37) |
| PLB/ETA | 80/103 (78) | 64/103 (62) | 37/103 (36) |
| Week 36 | |||
| ETA/ETA | 91/105 (87) | 71/105 (68) | 43/105 (41) |
| PLB/ETA | 89/103 (86) | 67/103 (65) | 39/103 (38) |
Weeks 36–48: re-randomised ‘withdrawal–retreatment’ period
At week 36, 138 patients who had achieved a PASI 50 response at week 24 or a PASI 75 response at week 36 were randomised in a 1 : 1 ratio to receive either etanercept or placebo in a double-blinded fashion. Patients were followed up for a further 12 weeks until week 48.
During the follow-up, 42 participants from the ITT population (29/69 and 13/69 from the placebo and etanercept arms respectively) lost their PASI 75 response and so were allocated to receive etanercept in an open-label fashion until week 48.
Overall, 52 out of 65 participants (80%) who received etanercept throughout the withdrawal–retreatment period maintained a PASI 75 response and 85% of those re-randomised to placebo and who did not lose a PASI 75 response during follow-up retained their response at week 48. Only 36% of those who were retreated with open-label etanercept after losing a PASI 75 response on placebo had regained a response by week 48 (Table 18). The use of PASI 75 response as both a retreatment rule and as an outcome makes these results difficult to interpret; however, a relatively high rate of late crossover from placebo to etanercept could partly explain a lack of response on PASI and PGA measures among these participants.
| Re-randomisation status at week 36 and treatment course until week 48 | Participants who achieved the outcome, n/N (%) | |
|---|---|---|
| PASI 75 | sPGA 0 or 1 | |
| Re-randomised to etanercept and received blinded etanercept (no loss of PASI 75 response) or open-label etanercept (after loss of PASI 75 response) | 52/65 (80) | 38/65 (58) |
| Re-randomised to placebo and stayed on blinded placebo until week 48 (no loss of PASI 75 response) | 34/40 (85) | 27/40 (68) |
| Re-randomised to placebo but received open-label etanercept after loss of PASI 75 response | 10/28 (36) | 8/28 (29) |
Weeks 48–312 (20050111)
In total, 194 participants completed 48 weeks of follow-up in the etanercept trial (20030211) (57 participants who received etanercept and topical therapy starting from the open-label treatment phase, 95 participants who were randomised to the etanercept and placebo arms and who continued to receive the blinded drug and 42 participants who were randomised to either etanercept or placebo but who did not achieve a PASI 75 response and who were retreated with etanercept until the end of the study).
Of the 194 participants who completed the etanercept trial (20030211), 182 were enrolled in an open-label extension study (20050111)66 to establish the long-term safety of etanercept. Participants received 0.8 mg/kg of etanercept (up to a maximum dose of 50 mg) subcutaneously once weekly for a further 264 weeks. In total, 63 participants (34.6%) completed 264 weeks of follow-up.
During the 264 weeks of further follow-up, the probability of achieving a PASI 50, 75 and 90 response was similar across all of the outcome recording points (Table 19). However, it should be noted that, by week 264, 63.6% of the participants (115/181) had withdrawn from the study and the reasons for withdrawal were unavailable.
| Week | Participants who achieved the outcome, n/N (%) | ||||
|---|---|---|---|---|---|
| ≥ PASI 50 | ≥ PASI 75 | ≥ PASI 90 | sPGA 0 | sPGA 0 or 1 | |
| 12 | 162/181 (89.5) | 122/181 (67.4) | 64/181 (35.4) | 24/181 (13.3) | 97/181 (53.6) |
| 48 | 150/168 (89.3) | 113/168 (67.3) | 55/168 (32.7) | 18/168 (10.7) | 82/168 (48.8) |
| 96 | 123/138 (89.1) | 84/138 (60.9) | 41/138 (29.7) | 16/139 (11.5) | 66/139 (47.5) |
| 144 | 101/114 (88.6) | 71/114 (62.3) | 32/114 (28.1) | 9/114 (7.9) | 52/114 (45.6) |
| 192 | 80/92 (87.0) | 64/92 (69.6) | 33/92 (35.9) | 19/92 (20.7) | 44/92 (47.8) |
| 240 | 68/74 (91.9) | 48/74 (64.9) | 27/74 (36.5) | 13/74 (17.6) | 37/74 (50.0) |
| 264 | 58/66 (87.9) | 42/66 (63.6) | 19/66 (28.8) | 8/66 (12.1) | 25/66 (37.9) |
Safety of etanercept
Adverse events at 12 weeks
The number of AEs reported during the 12-week randomised phase was similar for etanercept and placebo (68 vs. 62). There were 50 infections and seven injection site reactions in the etanercept group, compared with 33 infections and five injection site reactions in the placebo group. Although the difference in rate of infections fell short of formal statistical significance, there were noticeably more infections in the etanercept group (47.2% vs. 31.4%; p = 0.0683). One participant in the etanercept group withdrew because of an adverse effect (no further details available). No SAEs were observed during the 12-week randomised phase (Table 20).
| Treatment | Participants with safety reports, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Withdrawals because of AEs | |
| ETA | 68/106 (64.2) | NR | 50/106 (47.2) | 0/106 (0.0) | 7/106 (6.6) | NR | 1/106 (0.9) |
| PLB | 62/105 (59.0) | NR | 33/105 (31.4) | 0/105 (0.0) | 5/105 (4.8) | NR | 0/105 (0.0) |
Adverse events at weeks 12–36
Up to week 36, 282 infections were reported during treatment with etanercept (238.18 events per 100 person-years). The most common infections were upper respiratory tract infection, nasopharyngitis, influenza, pharyngitis, streptococcal pharyngitis and viral upper respiratory tract infection (59.97, 33.79, 16.05, 15.20, 8.45 and 8.45 events per 100 participant-years respectively). During the same period, a single serious non-infectious AE (benign ovarian mass) and one serious case of gastroenteritis with dehydration were observed (Table 21).
| Treatment | Participants with safety reports, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Withdrawals because of AEs | Deaths | |
| ETA | 584.48 events per 100 person-years | 1/208 (0.5) | 238.18 events per 100 person-years | 1/208 (0.5) | 26/208 (12.5) | 0 | 5/208 (2.4)a | 0/208 (0.0) |
Over the same time period, 2.4% of participants (5/208) withdrew because of AEs: three participants withdrew because of non-infectious AEs (psoriasis, atopic dermatitis and muscle cramps) and two participants withdrew because of infections (pneumonia and skin infection).
Adverse events at weeks 48–312
A total of 161 participants (89.0%) reported at least one AE up to week 264 of the follow-up study (20050111). Seven participants (3.9%) reported a SAE, with each participant reporting a single event: anxiety, cellulitis, infectious mononucleosis, postoperative intestinal obstruction, osteonecrosis and a thyroid cyst, with the seventh participant undergoing an elective abortion (Table 22). Of the seven SAEs, only the cellulitis infection was considered by the investigator to be related to etanercept treatment.
| Treatment | Participants with safety reports, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Withdrawals because of AEs | |
| ETA | 161/181 (89.0) | 7/181 (3.9) | 140/181 (77.3) | 2/181 (1.1) | 16/181 (8.8) | NR | 6/181 (3.3)a |
Six participants (3.3%) withdrew from the study because of either an infectious or a non-infectious AE. Two participants withdrew because of Crohn’s disease and one participant each withdrew because of glomerulonephritis (secondary to infection), psoriasis, sinusitis and nerve paralysis. The case of glomerulonephritis and one of the cases of Crohn’s disease were considered to be related to treatment.
No SAE led to study withdrawal. No opportunistic infections or deaths occurred during the study and no malignancies were reported.
Summary of the efficacy and safety of etanercept
-
One multicentre RCT (20030211) compared etanercept with placebo in children aged 4–17 years with moderate to severe plaque psoriasis.
-
Relatively few young children (9% aged < 8 years; 4.3% aged < 6 years) were included in the study.
-
At 12 weeks etanercept was significantly more effective than placebo in improving the severity of plaque psoriasis based on PASI 50, 75 and 90 and PGA cleared or minimal scores.
-
Improvements in HRQoL were larger for etanercept than for placebo, but reached statistical significance only for the CDLQI.
-
Adverse events rates were similar between the etanercept group and the placebo group at 12 weeks, with no SAEs observed for either treatment. However, the observed higher rate of infections among participants receiving etanercept was of borderline statistical significance.
-
A subsequent open-label extension study followed up participants for up to 6 years from entry into the original RCT. The proportions of PASI and PGA responders were stable over time, although only 36% of participants were available at the last follow-up point. The proportion of participants withdrawing because of lack of efficacy is unknown. These longer-term uncontrolled observational response data may therefore overestimate the efficacy of etanercept.
-
Withdrawals because of AEs were infrequent and no deaths or malignancies were observed up to 264 weeks of additional follow-up.
Efficacy and safety of ustekinumab
One multicentre RCT (1275PSO3006; CADMUS) comparing standard and half-standard dosages of ustekinumab with placebo met the selection criteria for the review. Data on safety and efficacy (blinded period) were available from one peer-reviewed journal paper,72 a conference abstract,73 regulatory documentation74 and a CSR provided by the manufacturer. 88
The CADMUS RCT was a double-blind, placebo-controlled study in adolescent participants (aged 12–17 years) who had had a diagnosis of moderate to severe plaque psoriasis for ≥ 6 months, which was conducted at multiple sites in Europe (Belgium, France, Germany, Hungary, Portugal, Russian Federation, Sweden, Ukraine and the UK) and Canada. Moderate-to-severe disease was defined as a PASI score of ≥ 12, a PGA score of ≥ 3 and BSA involvement of ≥ 10%.
A total of 157 participants were screened, of whom 110 were eligible and randomised (37 participants to placebo, 37 participants to the half-standard dosage of ustekinumab and 36 participants to the standard dosage of ustekinumab). The standard dosage of ustekinumab was 0.75 mg/kg for participants weighing ≤ 60 kg, 45 mg for participants weighing 60–100 kg and 90 mg for participants weighing > 100 kg. The half-standard dosage of ustekinumab was 0.375 mg/kg for participants weighing 60–100 kg and 45 mg for participants weighing > 100 kg. Randomisation was stratified by investigational site and baseline weight (≤ 60 kg or > 60 kg).
The study had three periods:
-
Controlled period (0–12 weeks): participants received either ustekinumab (full dose or half dose) or placebo. In the ustekinumab groups, participants were allowed to escape early at week 8 and receive moderate- to high-potency topical steroid preparations up to week 12 if their PASI scores increased by ≥ 50% from baseline. However no participants entered the escape route during this period.
-
Placebo crossover and active treatment period (12–52 weeks): participants randomised to placebo during the controlled period were allowed to cross over to the full or half-standard dose of ustekinumab at week 12.
-
Follow-up period (52–60 weeks): participants continued to be followed for safety analysis.
To preserve blinding, participants in the half-standard-dosage and standard-dosage groups received ustekinumab at week 0 and week 4 followed by doses every 12 weeks until week 40. Participants in the placebo group also received placebo at week 0 and week 4 and crossed over to receive either the half-standard dosage or standard dosage of ustekinumab at week 12 or week 16, followed by 12 weekly doses of either a half-standard dosage or standard dosage of ustekinumab, with the last dose at week 40. All participants were followed up for efficacy up to week 52 and for safety up to week 60.
The primary outcome measure was the proportion of participants who achieved a sPGA score of ‘cleared’ or ‘minimal’ at week 12. Data from all randomised participants were analysed according to their assigned treatment group. Participants who met treatment failure criteria prior to week 12 or who entered the early escape arm were considered non-responders at week 12. In addition, participants who had a missing PGA score at week 12 were considered as not achieving the primary end point at week 12.
The secondary outcome measures were PASI 50, 75 and 90 response at week 12 based on all randomised participants and changes from baseline in CDLQI score at week 12 based on efficacy-evaluable participants.
Risk-of-bias assessment
Based on the Cochrane risk-of-bias assessment tool,34 the CADMUS trial double-blind period had a low risk of bias: appropriate randomisation and blinding techniques were implemented, no obvious difference in baseline characteristics between treatment arms was apparent, missing data were handled appropriately and all protocol-stated outcome measures were reported (Table 23).
| Assessment criterion | Risk-of-bias judgement | Support for judgement |
|---|---|---|
| Sequence generation | Low | Dynamic central randomization was implemented in conducting this study. Participants were randomly assigned to 1 of 4 treatment groups based on an algorithm implemented in the Interactive Voice/Web Response System (IVRS or IWRS) before the studypp. 26–7742010National Library of Medicine |
| Allocation concealment | Low | Based on the algorithm, the IVRS/IWRS assigned a unique treatment code, which dictated the treatment assignment |
| Baseline comparability | Low | No obvious differences in baseline characteristics |
| Blinding of participants, personnel and outcome assessors | Low | The Sponsor, investigative study sites, and participants remained blinded to treatment assignment until the last participant enrolled completed the Week 60 evaluations and the database was lockedpp. 26–7742010National Library of Medicine |
| Incomplete outcome data | Low | Participants who discontinued study treatment due to lack of efficacy, an adverse event (AE) of worsening of psoriasis, or who started a protocol-prohibited medication/therapy during the study that could affect their psoriasis were considered as treatment failures. A participant who met 1 or more treatment failure criteria was considered as a treatment failure from that point onward. The baseline values were used for all directly measured endpoints regardless of the actual measurements. Zeros were assigned to improvements and percent improvements, and nonresponder status was assigned to binary response variablesp. 27742010National Library of Medicine |
| Participants who used a moderate to high potency topical steroid as a result of being eligible to early escape were considered as nonresponders at Week 12 for binary endpoints and their continuous outcomes at Week 12 were imputed by the last value at or prior to week 8. The analysis at Week 16 was the observed data without imputation. After Week 16, if participants continued to use a moderate to high potency topical steroid, treatment failure rules were applied to those participantsp. 27742010National Library of Medicine | ||
| Selective reporting | Low | Primary and secondary outcomes reported match the study protocol |
Efficacy of ustekinumab
Efficacy at week 12
Data on treatment response (PASI and sPGA) and HRQoL (CDLQI and PedsQL) outcomes for the CADMUS RCT are presented in Tables 24 and 25.
| Treatment | Participants who achieved the outcome, n/N (%) | Mean (SD) change from baseline | |||||
|---|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 or 1 | sPGA 0 | CDLQI | PedsQL | |
| UST 0.75 mg/kg | 32/36 (88.9) | 29/36 (80.6) | 22/36 (61.1) | 25/36 (69.4) | 17/36 (47.2) | –6.7 (5.6) n = 32 | 8.03 (10.4) n = 32 |
| UST 0.375 mg/kg | 30/37 (81.2) | 29/37 (78.4) | 20/37 (54.1) | 25/37 (67.6) | 12/37 (32.4) | –5.6 (6.4) n = 35 | 10.81 (12.9) n = 35 |
| PLB | 11/37 (29.7) | 4/37 (10.8) | 2/37 (5.4) | 2/37 (5.4) | 1/37 (2.7) | –1.5 (3.2) n = 32 | 3.35 (10.0) n = 32 |
| Treatment | Outcome, RR (95% CI) | Mean difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0/1 | sPGA 0 | CDLQI | PedsQL | |
| UST 0.75 mg/kg | 2.99 (1.79 to 4.97) | 7.5 (2.9 to 19.1) | 11.0 (2.8 to 43.5) | 12.9 (3.3 to 50.3) | 17.5 (2.5 to 124.5) | 5.2 (2.96 to 7.44) | 8.9 (2.46 to 15.34) |
| UST 0.375 mg/kg | 2.72 (1.62 to 4.48) | 7.3 (2.8 to 18.6) | 10.0 (2.5 to 39.8) | 12.5 (3.2 to 49) | 12.0 (1.6 to 87.7) | 4.1 (1.7 to 6.5) | 7.6 (2.16 to 13.04) |
| PLB | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.00 | 0.00 |
Physician Global Assessment
Significantly greater proportions of participants in the standard-dosage and the half-standard-dosage groups (69.4% and 67.6% respectively) than in the placebo group (5.4%) achieved a PGA score of cleared (0) or minimal (1) at week 12. The proportions of participants who achieved a PGA score of cleared (0) were also higher in the standard-dosage and half-standard-dosage groups (47.2% and 32.4% respectively) than in the placebo group (2.7%). The RRs for these outcomes are shown in Table 25.
Psoriasis Area and Severity Index response
Higher proportions of participants in the standard-dosage and the half-standard-dosage groups than in the placebo group achieved PASI 50, 75 and 90 responses. For example, 80.6% and 78.4% of the standard-dosage and half-standard-dosage groups, respectively, achieved a PASI 75 response at week 12 whereas only 10.8% of the placebo group achieved the same PASI 75 response (see Table 24). The RR values also show that both ustekinumab dosage groups had significantly higher probabilities of achieving the PASI 50, 75 and 90 responses than the placebo group (see Table 25).
Health-related quality of life
Changes from baseline in CDLQI score were significantly greater in both the standard-dosage and half-standard-dosage groups (mean of –6.7 and –5.6 respectively) than in the placebo group (–1.5). Although both ustekinumab treatment groups showed improvements from baseline in the CDLQI that exceed the published MCID of a 2.5-point change from baseline, this was not the case for the placebo group. The mean difference values indicate that CDLQI changes were significantly greater for both ustekinumab dosage groups than for the placebo group (mean difference: standard dosage 5.2, 95% CI 2.96 to 7.44; half-standard dosage 4.1, 95% CI 1.7 to 6.5; see Table 25).
Participants in both the standard-dosage group and the half-standard-dosage group showed significantly larger improvements in the PedsQL total scale scores from baseline (mean 8.03 and 10.81 respectively) than participants in the placebo group (mean 3.35). The mean changes for the half-standard dosage and standard dosage were above the published MCID of 4.4 whereas the mean change in the placebo group was below the MCID. Mean differences at 12 weeks indicate that the standard-dosage and half-standard-dosage groups had a significantly higher improvement in PedsQL score than the placebo group (mean difference: standard dosage 8.9, 95% CI 2.46 to 15.34; half-standard dosage 7.6, 95% CI 2.16 to 13.04).
Subgroup efficacy outcomes
Subgroup efficacy results for the PASI 75 and PGA 0 or 1 outcomes were available from the CSR88 (Table 26). (Confidential information has been removed.)
| Subgroup | Participants who achieved the outcome, n/N | |
|---|---|---|
| PASI 75a | sPGA 0 or 1a | |
| Age | ||
| ≤ 15 years | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
| > 15 years | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
| Sex | ||
| Male | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
| Female | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
| Weight | ||
| ≤ 60 kg | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
| > 60–≤ 100 kg | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
| > 100 kg | ||
| UST 0.75 mg/kg | Confidential information has been removed | Confidential information has been removed |
| UST 0.375 mg/kg | Confidential information has been removed | Confidential information has been removed |
| PLB | Confidential information has been removed | Confidential information has been removed |
Longer-term efficacy of ustekinumab
Psoriasis Area and Severity Index response
Among participants continuing ustekinumab treatment, PASI responses observed in week 12 appeared to be sustained at week 52, with few participants lost to follow-up (one participant was lost from the standard-dose arm and two from the half-standard-dose arm) (Table 27).
| Treatment | Participants who achieved the outcome, n/N (%) | ||||
|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | sPGA 0 | sPGA 0 or 1 | |
| UST 0.75 mg/kg | Confidential information has been removed | 28/35 (80.0) | 23/35 (65.7) | 18/36 (50) | 26/36 (72) |
| UST 0.375 mg/kg | Confidential information has been removed | 23/34 (67.6) | 17/34 (50.0) | 13/37 (35) | 23/37 (62) |
| PLB → UST 0.75 mg/kg | Confidential information has been removed | 17/17 (100.0) | 16/17 (94.1) | 11/17 (65) | 16/17 (94) |
| PLB → UST 0.375 mg/kg | Confidential information has been removed | 12/17 (70.6) | 9/17 (52.9) | 9/19 (47) | 13/19 (68) |
Participants who were randomised to placebo and who crossed over to the standard ustekinumab dosage (0.75 mg/kg) achieved better PASI responses than those who were randomised to placebo and crossed over to the half-standard dosage of ustekinumab (0.375 mg/kg) (see Table 27).
Physician Global Assessment
A similar pattern of responses was seen for the sPGA, with similar response rates at week 52 as at week 12 among participants continuing ustekinumab treatment and a large improvement between weeks 12 and 52 for participants crossing over to active treatment from placebo (see Table 27).
Safety of ustekinumab
Adverse events at week 12
The percentage of participants reporting AEs did not differ significantly between the ustekinumab groups (44.4% standard-dosage group and 51.4% half-standard-dosage group) and the placebo group (56.8%) (Table 28). No SAEs were reported in the standard-dosage group or the placebo group, whereas one participant in the half-standard-dosage group was hospitalised for worsening of psoriasis (see Table 28).
| Treatment | Participants with safety reports, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Withdrawals because of AEs | |
| UST 0.75 mg/kg | 16/36 (44.4) | 0/36 (0.0) | 8/36 (22.2) | 0/36 (0.0) | 1/36 (2.8) | 0/36 (0.0) | 0/36 (0.0) |
| UST 0.375 mg/kg | 19/37 (51.4) | 1/37 (2.7)a | 12/37 (32.4) | 0/37 (0.0) | 0/37 (0.0) | 0/37 (0.0) | 0/37 (0.0) |
| PLB | 21/37 (56.8) | 0/37 (0.0) | 14/37 (37.8) | 0/37 (0.0) | 0/37 (0.0) | 0/37 (0.0) | 0/37 (0.0) |
One participant in the standard dosage group had a mild injection site reaction. There were no incidences of serious infection, tuberculosis, malignancy or withdrawals because of AEs during the initial 12-week treatment period (see Table 28).
Discontinuation up to week 40
Up to week 40, 8.2% of the participants (9/110) discontinued the trial. The most common reasons for discontinuation were a lack of efficacy and AEs (Table 29). Five participants (13.5%) who were originally randomised to the half-standard-dosage group discontinued the trial compared with two participants (5.6%) in the standard-dosage group. Two patients who crossed over from placebo to the half-standard dosage of ustekinumab withdrew because of AEs.
| Treatment | Participants with safety reports, n/N (%) | |||
|---|---|---|---|---|
| Total discontinued | Because of AEs | Because of death | Because of lack of efficacy | |
| UST 0.75 mg/kg | 2/36 (5.6) | 0/36 (0.0) | 0/36 (0.0) | 2/36 (5.6) |
| UST 0.375 mg/kg | 5/37 (13.5) | 1/37 (2.7) | 1/37 (2.7) | 3/37 (8.1) |
| PLB → UST 0.75 mg/kg | 0/18 (0.0) | 0/18 (0.0) | 0/18 (0.0) | 0/18 (0.0) |
| PLB → UST 0.375 mg/kg | 2/19 (10.5) | 2/19 (10.5) | 0/19 (0.0) | 0/19 (0.0) |
Adverse events up to week 60
Up to week 60, 81.8% of participants (90/110) in the ustekinumab combined group reported one or more AEs (Table 30). Of the 74 participant-recorded infections, 18 (24%) were considered reasonably related to ustekinumab treatment.
| Treatment | Participants with safety reports, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|
| AEs | SAEs | Infections | Serious infections | Injection site reactions | Malignancies | Withdrawals because of AEs | |
| UST 0.75 mg/kg | 29/36 (80.6) | 1/36 (2.8)b | 24/36 (66.7) | 1/36 (2.8)b | 1/36 (2.8) | 0/36 (0.0) | 0/36 (0.0) |
| UST 0.375 mg/kg | 33/37 (89.2) | 5/37c,d (13.5) | 26/37 (70.3) | 1/37d (2.7) | 0/37 (0.0) | 0/37 (0.0) | 2/37 (5.4) |
| PLB → UST 0.75 mg/kg | 13/18 (72.2) | 0/18 (0.0) | 11/18 (61.1) | 0/18 (0.0) | 0/18 (0.0) | 0/18 (0.0) | 0/18 (0.0) |
| PLB → UST 0.375 mg/kg | 15/19 (79.0) | 0/19 (0.0) | 13/19 (68.4) | 0/19 (0.0) | 0/19 (0.0) | 0/19 (0.0) | 2/19 (10.5) |
In total, 5.5% of participants (6/110) in the ustekinumab combined group (five participants in the half-standard-dosage group and one participant in the standard-dosage group) reported SAEs (see Table 30). Four (one because of worsening of psoriasis) of the 110 participants in the ustekinumab combined group discontinued the trial because of an AE by week 60.
Summary of the efficacy and safety of ustekinumab
-
Both the standard dosage and the half-standard dosage of ustekinumab were significantly more effective than placebo in improving the severity of plaque psoriasis based on PASI 50, 75 and 90 responses, sPGA cleared or minimal response and sPGA cleared response at 12 weeks.
-
Both ustekinumab dosages also led to significantly greater improvements in HRQoL (CDLQI and PedsQL) at 12 weeks than placebo.
-
Among participants originally allocated to ustekinumab, PASI and PGA effects observed at 12 weeks appeared to be largely sustained at 52 weeks, with few withdrawals because of lack of efficacy.
-
Participants originally allocated to placebo showed substantial improvements in PASI and PGA responses at 52 weeks after crossing over to ustekinumab treatment at week 12. There was some indication that the gains were greater among those who received the standard dosage of ustekinumab than among those who received the half-standard dosage.
-
There were no notable differences between the ustekinumab group and the placebo group in terms of short-term and longer-term AEs, although the number of observations was small and the longest follow-up time was only 60 weeks. Few participants withdrew because of adverse effects.
Additional observational evidence
Retrospective case series
Two retrospective case series reported the use of adalimumab, etanercept and ustekinumab from 4 to 165 weeks in children with moderate to severe psoriasis. 76,77 The key characteristics and results from these studies are reported in Table 31.
| Study, country | Treatment (dose) | Number of patients | Treatment duration (weeks) | Age (years), median (range) | Mean PGA score at baseline | Reported outcomes/key AEs |
|---|---|---|---|---|---|---|
| Garber et al., 2015,76 USA | Adalimumab (40 mg every other week) | 7 | 146 | – | – | Achieved clearance, n = 4/6; secondary failure, n = 3; injection site reaction, n = 1; minor infection, n = 3a |
| Etanercept (50 mg weekly) | 13 | 87 | – | – | Achieved clearance, n = 6/9; injection site reaction, n = 1; secondary failure, n = 6; lack of response, n = 3; minor infection, n = 4b | |
| Ustekinumab (45 mg at weeks 0 and 4, then every 12 weeks) | 3 | 165 | – | – | Achieved clearance, n = 1/3 | |
| Adalimumab + methotrexate | 2 | 11 | – | – | Achieved clearance, n = 1/2 | |
| Etanercept + methotrexate | 2 | 121 | – | – | Achieved clearance, n = 2/2 | |
| Etanercept + ciclosporin | 1 | 20 | – | – | – | |
| Klufas et al., 2016,77 USA | Adalimumab (40 mg every other week) | 11 | 3–134 | 16.5 (7.0–18.0) | 2.4 | Mean PGA scorec 0.7 |
| Injection site reaction, n = 1 | ||||||
| Etanercept (25 or 50 mg once or twice weekly) | 23 | 8–135 | 14.0 (8.0–18.0) | 3.0 | Mean PGA scorec 1.5 | |
| Injection site reaction, n = 2 | ||||||
| Ustekinumab (45 or 90 mg at weeks 0 and 4, then every 12 weeks) | 6 | 4–72 | 16.5 (7.0–18.0) | 2.6 | Mean PGA scorec 1.5 | |
| Adalimumab (40 mg eow) + methotrexate (7.5–15 mg weekly) | 9 | 8–118 | 15.0 (11.0–17.0) | 2.4 | Mean PGA scorec 1.0 | |
| Injection site reaction, n = 1 | ||||||
| Etanercept (50 mg once or twice weekly + methotrexate (7.5–15 mg weekly) | 5 | 4–30 | 15.0 (13.0–17.0) | 3.1 | Mean PGA scorec 1.8 | |
| Ustekinumab (45 mg at weeks 0 and 4, then every 12 weeks) + methotrexate (12.5 mg weekly) | 2 | NR | 16.5 (16.0–17.0) | 3.8 | Mean PGA scorec 1.3 |
Garber et al. 76 reported a retrospective chart review of 27 participants (19 males, 8 females) attending a single US general dermatology clinic from 2008 to 2014. Insufficient details were reported to establish how many patients received more than one biologic over this period. Clearance rates [defined as 99% reduction on the Simple Measure for Assessing Psoriasis Severity or (S-MAPA)] were reported (see Table 31). No SAEs were reported. Although the authors concluded that the use of adalimumab, etanercept and ustekinumab is safe in paediatric psoriasis and that these treatments are efficacious, this study provides insufficient data on the efficacy or safety profiles of these agents in practice.
Klufas et al. 77 similarly reported a retrospective case series evaluating 51 children with moderate to severe psoriasis treated with systemic therapies for AE occurrence and PGA-measured disease response. For all biologics (alone or in combination with methotrexate), mean PGA values fell at 5–7 months’ follow-up. In total, 29 AEs were reported in relation to 80 treatment data points (some patients received more than one biologic); most were minor subjective side effects, with no infections or SAEs reported. Again, limitations in the sample size and study design preclude strong inferences being drawn from these data.
Registry data
Published findings or papers detailing study design were identified for 16 registries. Information on biological drug safety in their psoriasis cohorts was published by nine registries, with 11 articles on biological efficacy and nine articles including drug survival data. This does not necessarily mean that the other registries did not record these outcomes, but they were not covered in the identified literature output.
Registry data for children
Further screening was carried out to find registry publications making explicit reference to children with psoriasis and specifically providing information on the survival of biological treatment. Two registries (Child-CAPTURE and DERMBIO) were found to include children with psoriasis who were treated with biologics. Child-CAPTURE (the Netherlands) contained seven children treated with etanercept,90 but did not differentiate between biological and non-biological therapies in drug survival analyses. We identified from the 2014 annual report91 by the DERMBIO (Denmark) registry that there are 37 children enrolled who are undergoing treatment with adalimumab, etanercept or ustekinumab, although data for this group were not reported separately. Cox regression modelling of covariates in two studies found no significant predictive relationship between patient age and drug survival, suggesting that treatment withdrawal rates among children were similar to those in adults. 92,93
Wider registry data
In one 2015 DERMBIO study following 1277 (predominantly adult) psoriasis patients for up to 10 years,92 median drug survival for etanercept was 30 months (95% CI 25.1 to 34.9 months), which was significantly lower than for adalimumab (59 months, 95% CI 45.6 to 72.4 months) and ustekinumab (median not reached). Year-on-year drug survival for etanercept (estimated from the Kaplan–Meier curve) was 0.70 at 1 year, 0.53 at year 2 and 0.30 at year 5, whereas year-on-year drug survival for ustekinumab was 0.85 after 1 year, 0.78 at year 2 and 0.65 at year 5 (Table 32). Loss of efficacy was the most likely reason for drug discontinuation, but this was of greater significance proportionally for etanercept than for the other biologics analysed.
| Biologic | Drug survival | ||
|---|---|---|---|
| 1 year | 2 years | 5 years | |
| Adalimumab (n = 567) | 0.77 | 0.67 | 0.48 |
| Etanercept (n = 364) | 0.70 | 0.53 | 0.30 |
| Infliximab (n = 176) | 0.75 | 0.62 | 0.43 |
| Ustekinumab (n = 170) | 0.85 | 0.78 | 0.65 |
Findings from the British Association of Dermatologists Biologic Interventions Register (BADBIR) were broadly similar to those seen in the Danish cohort. A study by Warren et al. 94 on drug survival over 3 years in 3523 biologic-naive patients found that 77% of patients remained on biologic treatment over the first year, falling to 53% by the third year. Again, there were significant differences in the treatment withdrawal rates between biologics. Ustekinumab exhibited the highest first-course survival rate at 0.89 at year 1 and 0.75 at year 3. Adalimumab showed the highest survival of the anti-TNF-α drugs, at 0.79 at year 1 and 0.59 at year 3. Disregarding the very small population of patients on infliximab, etanercept was consistently the worst-performing drug in terms of treatment withdrawal, with a 1-year survival rate of 0.70, dropping to 0.40 at 3 years (Table 33). Etanercept was also found to be a significant predictor of discontinuation of therapy because of loss of efficacy. Other significant predictors of treatment withdrawal were female sex, smoking status and a higher baseline Dermatology Life Quality Index (DLQI) score.
| Biologic | Drug survival | ||
|---|---|---|---|
| 1 year | 2 years | 3 years | |
| Adalimumab (n = 1879) | 0.79 | 0.67 | 0.59 |
| Etanercept (n = 1098) | 0.70 | 0.51 | 0.40 |
| Infliximab (n = 96) | 0.65 | 0.50 | 0.35 |
| Ustekinumab (n = 450) | 0.89 | 0.82 | 0.75 |
Overview of the randomised controlled trial results
Despite differences in inclusion criteria, the relative lack of younger children in the adalimumab and etanercept trials meant that the median age of children across the three trials did not differ greatly. Similarly, measures of disease duration and the component measures of severity did not appear to differ markedly between the three trials. Few participants in any trial had previous experience of biological treatment.
The biologics and their respective comparators in the relevant RCTs in children and young people are summarised in Table 34.
| Treatment | Class of therapy | Dosage | Comparator |
|---|---|---|---|
| Adalimumab | Anti-TNF-α | Standard (0.8 mg/kg) | Methotrexate |
| Half-standard (0.4 mg/kg) | |||
| Etanercept | Anti-TNF-α | Standard (0.8 mg/kg) | Placebo |
| Ustekinumab | Anti-IL-12/-IL-23 | Standard (0.75 mg/kg) | Placebo |
| Half-standard (0.375 mg/kg) |
There were no head-to-head comparative data available for the three biologics. In addition, although the etanercept and ustekinumab trials had a placebo as a common comparator, the adalimumab trial used methotrexate as a comparator.
Table 35 shows the relative effects for all three biologics from the three RCTs. However, an implicit comparison is not useful for the purposes of the decision-analytic modelling required for the economic evaluation. Chapter 4 therefore describes a formal evidence synthesis to inform the relative efficacy of these interventions.
| Trial | PASI outcome, RR (95% CI) | ||
|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | |
| Adalimumab vs. methotrexate (M04-717) (16 weeks) | |||
| Standard dosage (0.8 mg/kg) | Confidential information has been removed | 1.79 (1.04 to 3.06)a | 1.34 (0.61 to 2.95) |
| Half-standard dosage (0.4 mg/kg) | Confidential information has been removed | 1.34 (0.75 to 2.42)a | 1.42 (0.65 to 3.08) |
| Etanercept vs. placebo (20030211) (12 weeks) | |||
| Standard dosage (0.8 mg/kg) | 3.26 (2.26 to 4.71) | 4.95 (2.84 to 8.65)a | 4.10 (1.88 to 8.95) |
| Ustekinumab vs. placebo (CADMUS) (12 weeks) | |||
| Standard dosage (0.75 mg/kg) | 2.99 (1.79 to 4.97) | 7.5 (2.9 to 19.1) | 11.0 (2.8 to 43.5) |
| Half-standard dosage (0.375 mg/kg) | 2.72 (1.62 to 4.48) | 7.3 (2.8 to 18.6) | 10.0 (2.5 to 39.8) |
Chapter 4 Evidence synthesis to inform the relative efficacy of the interventions
Overview
Randomised controlled trials of the effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people have been discussed and summarised in Chapter 3. The efficacy end point consistently reported across the trials was PASI response rates, which is the key efficacy parameter used in the economic analysis. To determine the relative efficacy of the interventions, it would be ideal to have results from good-quality adequately powered RCTs comparing the active treatments with one another in the population of children and young people. However, the evidence base presents a number of challenges for informing the relative efficacy of the interventions in this population. First, the interventions of interest have not been directly compared in head-to-head RCTs. Second, no common comparator (e.g. placebo) exists across all of the RCTs. Third, the age of the populations included in the trials differs across the RCTs and the interventions of interest have marketing authorisation for different age groups. Fourth, the severity of plaque psoriasis is defined differently in the populations included in the RCTs and the interventions are licensed for different levels of psoriasis severity in children and young people. These challenges mean that a number of assumptions are required to inform the benefits of the active treatments relative to the appropriate comparators and each other.
Meta-analysis using mixed-treatment comparisons enables the estimation of different parameters from several studies with similar comparisons to be combined when direct evidence on comparisons of interest is absent or sparse. The statistical synthesis method of NMA enables the comparison of multiple treatment options using both direct comparisons of interventions from RCTs and indirect comparisons across trials based on a common comparator. 95,96 As suggested by the term, NMA needs a ‘network of evidence’ to be established between all of the interventions of interest. However, with neither direct comparisons nor a common comparator in the evidence base for children and young people from which to derive indirect comparisons of comparator treatments, the evidence base is structured as a ‘disconnected network’ (Figure 3).
FIGURE 3.
Network of evidence for children and young people. ADA, adalimumab 0.8 mg/kg, maximum 40 mg/week; ETA, etanercept 0.8 mg/kg, maximum 50 mg/week; MTX, methotrexate 0.1–0.4 mg/kg/week; PLB, placebo; UST 45, ustekinumab 0.75 mg/kg or 45 mg/week. Trial names are stated when trial evidence informs the network treatment link.
In the following sections we build on the challenges listed above by exploring treatment efficacy by age subgroup and by performing a naive indirect treatment comparison of adalimumab and etanercept, highlighting the limitations of such analysis. Furthermore, a framework of analysis is described that uses different levels of evidence from the adult population to specifically address the issue of having a disconnected network structure.
Efficacy differences by age subgroup
Adalimumab, etanercept and ustekinumab have marketing authorisation for different age groups in the population of children and young people (≥ 4 years for adalimumab, ≥ 6 years for etanercept and ≥ 12 years for ustekinumab). This is the result of variation in the ages of the patient populations included in the RCTs for these interventions. Furthermore, the trial population for etanercept also included patients who were younger than the licensed age group (i.e. the inclusion criteria for the etanercept trial was children and adolescents aged 4–17 years and nine children were included in the trial who were younger than the subsequent licensed age group of ≥ 6 years). To establish the relative efficacy of the interventions it was necessary to either (1) assume that the PASI response rates for the treatments are independent of age within the full population of children and young people or (2) consider outcomes in a population subgroup by age.
Chapter 3 presents the PASI response rates for each study by age subgroup. On inspection of the PASI response rates, there does not appear to be a pattern across the efficacy outcomes for the different age subgroups within the same study, which could explain any differences in efficacy as a result of age. This would seem to suggest that the PASI response rates for the study as a whole are reflective of the outcomes expected in a particular subpopulation by age. This was examined further by using standard parametric statistical tests to assess the equality of proportions (i.e. the probability of PASI 50/75/90 response rates) across different age subgroups within each study. Within each study there were no statistically significant differences identified across the age subgroups for each of the PASI response rates of 50, 75 or 90 (Table 36). Therefore, to compare the relative efficacy of the interventions, it was assumed that the PASI response rates for the treatments are independent of age within the full population of children and young people and that the studies are comparable for this population.
| Study/treatment arma | All | Age subgroup (years) | Hypothesis test of equality of proportions, p-value | ||||
|---|---|---|---|---|---|---|---|
| 4–6 | > 6–9 | > 9–12 | > 12–15 | > 15 | |||
| M04-717 | |||||||
| Adalimumab | n = 38 | n = 0 | n = 7 | n = 8 | n = 13 | n = 10 | |
| PASI 50 (%) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.72 |
| PASI 75 (%) | 57.9 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.84 |
| PASI 90 (%) | 28.9 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.47 |
| Methotrexate | n = 37 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| PASI 50 (%) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.91 |
| PASI 75 (%) | 32.4 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.44 |
| PASI 90 (%) | 21.6 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.77 |
| Study/treatment arma | All | Age subgroup (years) | Hypothesis test of equality of proportions, p-value | ||||
| ≤ 15 | > 15 | ||||||
| CADMUS | |||||||
| Placebo | n = 37 | n = 15 | n = 22 | ||||
| PASI 50 (%) | 29.7 | NA | NA | NA | |||
| PASI 75 (%) | 10.8 | Confidential information has been removed | Confidential information has been removed | 0.90 | |||
| PASI 90 (%) | 5.4 | NA | NA | NA | |||
| Ustekinumab | n = 36 | n = 20 | n = 16 | ||||
| PASI 50 (%) | 88.9 | NA | NA | NA | |||
| PASI 75 (%) | 80.6 | Confidential information has been removed | Confidential information has been removed | 0.60 | |||
| PASI 90 (%) | 61.1 | NA | NA | NA | |||
| Study/treatment arma | All | Age subgroup (years) | Hypothesis test of equality of proportions, p-value | ||||
| 4–11 | > 12–17 | ||||||
| 20030211 | |||||||
| Placebo | n = 105 | n = 38 | n = 67 | ||||
| PASI 50 (%) | 22.9 | 21.1 | 23.9 | 0.93 | |||
| PASI 75 (%) | 11.4 | 10.5 | 11.9 | 1.00 | |||
| PASI 90 (%) | 6.7 | NA | NA | NA | |||
| Etanercept | n = 106 | n = 38 | n = 68 | ||||
| PASI 50 (%) | 74.5 | 76.3 | 73.5 | 0.93 | |||
| PASI 75 (%) | 56.6 | 57.9 | 55.9 | 1.00 | |||
| PASI 90 (%) | 27.4 | NA | NA | NA | |||
Indirect treatment comparison
Figure 3 shows that there is no common comparator arm between the adalimumab trial (M04-717) and the trials of etanercept (20030211) and ustekinumab (CADMUS), with the adalimumab trial having a comparator of methotrexate and the other two trials having a comparator of placebo. Therefore, it is not possible to establish an indirect comparison between adalimumab and etanercept or ustekinumab without drawing on evidence from other sources (e.g. evidence on the relative efficacy of the interventions in adults) or by creating a common comparator (e.g. assuming that the methotrexate and placebo response rates are exchangeable between the trials). In this section, attention is focused on the indirect comparison that can be established between etanercept and ustekinumab.
An indirect treatment comparison of PASI response rates at 12 weeks was performed between the licensed doses of etanercept (0.8 mg/kg up to a maximum dose of 50 mg) and ustekinumab (standard dose) using placebo as a common comparator. A Bayesian indirect treatment comparison was undertaken using a probit model for ordered multinomial outcomes of PASI response rates (using a fixed-effect model with multinomial likelihood and a probit link (see Appendix 4). Table 37 presents the absolute probabilities of PASI 50, 75 and 90 responses for etanercept and ustekinumab and Table 38 presents the relative treatment effects expressed as RRs with 95% Bayesian credible intervals (CrIs).
| Treatment | PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) |
|---|---|---|---|
| Placebo | 0.265 (0.190 to 0.346) | 0.131 (0.082 to 0.191) | 0.042 (0.021 to 0.073) |
| Etanercepta | 0.744 (0.631 to 0.841) | 0.565 (0.437 to 0.688) | 0.330 (0.218 to 0.454) |
| Ustekinumabb | 0.896 (0.797 to 0.962) | 0.781 (0.632 to 0.898) | 0.571 (0.395 to 0.742) |
| PLB | 4.95 (2.84 to 8.65) | 7.50 (2.90 to 19.10) |
| 4.48 (2.99 to 6.64) | ETAa | – |
| 6.27 (3.80 to 10.00) | 1.41 (0.99 to 1.93) | USTb |
The results demonstrate that ustekinumab appears to be more effective than etanercept in this population. The PASI 75 absolute probability of response for ustekinumab at 12 weeks was estimated to be 78% (95% CrI 63% to 90%) whereas that for etanercept was estimated to be 57% (95% CrI 44% to 69%). The 95% CrIs were wide and overlap, which reflects the small sample size and limited number of data points used in this analysis. The pooled RR presented in Table 38 for ustekinumab compared with etanercept is 1.41, but this is not statistically significant as the 95% CrI includes 1. The indirect comparison results are in line with the direct evidence from the clinical trials.
The company submission for ustekinumab presented a similar indirect treatment comparison for ustekinumab compared with etanercept [Janssen. Adalimumab, Etanercept and Ustekinumab for Treating Plaque Psoriasisin Children and Young People – Company Evidence Submission. ID854. 2016 (unpubished)]. The company’s analysis produced results for the full population and for a subgroup aged 12–17 years. The results of the company’s full population NMA are broadly similar to the results from the Assessment Group’s (AG) analysis, for example the ustekinumab PASI 75 response was estimated to be 79.8%, compared with 78.1% in Table 37.
It is important to note that these analyses are limited for a number of reasons.
-
It draws conclusions only regarding the short-term use of ustekinumab and etanercept in the population of children and young people from the corresponding trials.
-
The placebo arms in the etanercept trial (20030211) and the ustekinumab trial (CADMUS) are assumed to be exchangeable between the trials.
-
Inclusion criteria for age were different between the trials.
-
There is uncertainty in both the within-trial and between-trial treatment effect estimates because of the small sample sizes in the trials.
-
There are differences between the trials in terms of baseline characteristics and trial design: these differences have been explored separately in Chapter 3.
-
The indirect treatment comparison does not provide sufficient information to inform the economic analysis as the use of adalimumab has been excluded from the analysis because of a lack of a common comparator.
As a consequence of the above limitations, in particular the exclusion of the adalimumab trial (M04-717) evidence, the results in Table 37 could not be used to assess the relative cost-effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people.
Framework of analysis for informing the relative efficacy of the interventions
Because of the lack of a common comparator arm between the adalimumab trial (M04–717) and the ustekinumab (CADMUS) and etanercept (20030211) trials, an analysis plan was developed that entailed exploring the possibility of using PASI response data from adults with moderate to severe plaque psoriasis to fill the evidence gap in the population of children and young people. The use of data from the adult population was supported by our clinical advisor (Dr Ruth Murphy, personal communication), who did not see any reason why the relative effectiveness of the interventions in adults could not be used to infer relative effectiveness in children and young people, especially in the absence of evidence in the latter population.
A framework of analysis was thus developed for the NMA approach that allowed all available and relevant evidence to be included. The framework explored two separate networks, which differed according to the extent of evidence utilised from the adult trials.
-
The network of trials in children and young people was connected by bringing the minimum amount of evidence required from the adult population to link the adalimumab trial with the other trials in the disconnected network in Figure 3.
-
The network of trials in children and young people was connected by bringing together all relevant evidence on the efficacy of all of the interventions in adults.
This approach allows treatment-specific estimates to be modelled in each population by drawing strength from the network of evidence available. The use of a NMA in preference to pairwise meta-analyses enables the inclusion of all relevant evidence, allowing for precise estimates of treatment effects to be calculated. In addition, the results from the NMA feed directly into the economic model to provide the relevant cost-effectiveness of adalimumab, etanercept and ustekinumab compared with relevant comparators and each other. This approach has been used in previous NICE TAs for the treatment of plaque psoriasis in adults (TA103,97 TA134,98 TA146,99 TA180,100 TA350,101 and TA368102).
In each of the NICE TAs in adults the evidence network was updated with new studies reported since the previous appraisal. Therefore, we took the most recent single TAs in adults (TA368102 and TA350101) as the starting point for developing a network of studies that could potentially connect the adalimumab trial in children and young people to the other interventions. The Evidence Review Groups (ERGs) for these appraisals generally rated the systematic reviews underpinning the identification of trials for inclusion in the NMA as appropriate and the evidence networks were subsequently used to inform NICE recommendations in these appraisals. Therefore, it was assumed that the vast majority of relevant evidence for the interventions in adults had been captured in the most recent appraisals in 2015. 101,102 Relevant adult trials were identified based on the indirect comparison and/or multiple treatment comparisons reported within these appraisals. Lists of excluded trials and reasons for exclusion were also reviewed and relevant trials identified. To supplement this review, the results of a recently published systematic review and NMA,103 which adjusted for cross-trial differences in the comparative efficacy of biological treatments for moderate to severe psoriasis in adults, were also examined to cross-check that the majority of relevant studies had been identified in the previous appraisals. Furthermore, we also considered studies reported in the original multiple TA in adults (TA10397), which included interventions such as methotrexate and ciclosporin. The key inclusion and exclusion criteria used to identify relevant trials for the NMA are shown in Table 39. A list of excluded trials (n = 18) and reasons for exclusion can be found in Appendix 5. Table 40 presents a summary of the trials in adults, including the comparator agents used in each trial, which was used to inform the NMA.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
| Study | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adalimumab (dose, mg/week) | Etanercept (dose, mg/week) | Ustekinumab (dose, mg/week) | Methotrexate | Apremilasta | Ciclosporin | Fumaric acid | Infliximab | Placebo | |
| Adalimumab (n = 5) | |||||||||
| Gordon et al., 2006104 | ✓ (40) | ✓ | |||||||
| Menter et al., 2008105 (REVEAL) | ✓ (40) | ✓ | |||||||
| Saurat et al., 2008106 (CHAMPION) | ✓ (40) | ✓ | ✓ | ||||||
| Asahina et al., 2010107 | ✓ (40) | ✓ | |||||||
| Bissonnette et al., 2013108 | ✓ (40) | ✓ | |||||||
| Etanercept (n = 6) | |||||||||
| Gottlieb et al., 2003109 | ✓ (50) | ✓ | |||||||
| Leonardi et al., 2003110 | ✓ (50) | ✓ | |||||||
| Elewski et al., 2004111 | ✓ (50) | ✓ | |||||||
| Papp et al., 2005112 | ✓ (50) | ✓ | |||||||
| van de Kerkhof et al., 2008113 | ✓ (50) | ✓ | |||||||
| Reich et al., 2016114 (LIBERATE) | ✓ (50) | ✓ | ✓ | ||||||
| Ustekinumab (n = 7) | |||||||||
| Tsai et al., 2011115 (PEARL) | ✓ (45) | ✓ | |||||||
| Zhu et al., 2013116 (LOTUS) | ✓ (45) | ✓ | |||||||
| Krueger et al., 2007117 | ✓ (45, 90) | ✓ | |||||||
| Leonardi et al., 2008118 (PHOENIX I) | ✓ (45, 90) | ✓ | |||||||
| Papp et al., 2008119 (PHOENIX II) | ✓ (45, 90) | ✓ | |||||||
| Griffiths et al., 2010120 (ACCEPT) | ✓ (45, 90) | ✓ | |||||||
| Igarashi et al., 2012121 | ✓ (45, 90) | ✓ | |||||||
| Methotrexate (n = 4) | |||||||||
| Heydendael et al., 2003122 | ✓ | ✓ | |||||||
| Flytström et al., 2008123 | ✓ | ✓ | |||||||
| Barker et al., 2011124 (RESTORE I) | ✓ | ✓ | |||||||
| Fallah et al., 2011125 | ✓ | ✓ | |||||||
| Apremilast (n = 3) | |||||||||
| Papp et al., 2012126 | ✓ | ✓ | |||||||
| Papp et al., 2015127 (ESTEEM I) | ✓ | ✓ | |||||||
| Paul et al., 2015128 (ESTEEM II) | ✓ | ✓ | |||||||
| Ciclosporin (n = 2) | |||||||||
| Guenther et al., 1991129 | ✓ | ✓ | |||||||
| Meffert et al., 1997130 | ✓ | ✓ | |||||||
| Fumaric acid (n = 1) | |||||||||
| Altmeyer et al., 1994131 | ✓ | ✓ | |||||||
| Infliximab (n = 6) | |||||||||
| Chaudhari et al., 2001132 | ✓ | ✓ | |||||||
| Gottlieb et al., 2004133 (SPIRIT) | ✓ | ✓ | |||||||
| Reich et al., 2005134 (EXPRESS I) | ✓ | ✓ | |||||||
| Menter et al., 2007135 (EXPRESS II) | ✓ | ✓ | |||||||
| Torii et al., 2010136 | ✓ | ✓ | |||||||
| Yang et al., 2012137 | ✓ | ✓ | |||||||
Thirty-four trials in adults with moderate to severe plaque psoriasis were found to be relevant for the NMA; 29 of these considered a placebo arm and six were three-arm trials. As described in the ERG reports for the previous TAs, selected studies were mostly comparable in terms of their inclusion criteria regarding previous and concomitant medication use. The majority of studies included patients who had failed or who had had an insufficient response to previous topical therapy and conventional systemic agents such as ciclosporin or methotrexate. Some studies included only biologic-naive individuals, whereas others allowed previous biological therapy use. Almost all of the studies did not allow concomitant treatment with systemic agents or phototherapy. A few studies did not mention their criteria regarding concomitant medication use.
The full set of interventions and comparators included adalimumab, etanercept, 45 mg of ustekinumab, 90 mg of ustekinumab, apremilast, methotrexate, ciclosporin, fumaric acid, infliximab and placebo. Response rates for PASI 50, 75 and 90 from the selected trials were identified and extracted, together with sample size and key baseline patient characteristics by treatment arm. Table 41 presents a summary of the data extracted together with the corresponding data from the three trials in children and young people.
| Study | Treatment/weekly dose | Population | Time point (weeks) | n | Age (years), mean | Male (%) | PASI 50 (n) | PASI 50 (%) | PASI 75 (n) | PASI 75 (%) | PASI 90 (n) | PASI 90 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003021148–71 | Placebo | Children and young people | 12 | 105 | – | 50 | 24 | 22.9 | 12 | 11.4 | 7 | 6.7 |
| Etanercept 0.8 mg/kg (maximum 50 mg) | 12 | 106 | – | 52 | 79 | 74.5 | 60 | 56.6 | 29 | 27.4 | ||
| CADMUS 201572–75 | Placebo | Children and young people | 12 | 37 | 16 | 54 | 11 | 29.7 | 4 | 10.8 | 2 | 5.4 |
| Ustekinumab SD (maximum 45 mg) | 12 | 36 | 15 | 44 | 32 | 88.9 | 29 | 80.6 | 22 | 61.1 | ||
| M04-71739–47,87 | Adalimumab 0.8 mg/kg (maximum 40 mg) | Children and young people | 16 | 37 | 13 | 45 | 30 | 78.9 | 22 | 57.9 | 11 | 28.9 |
| Methotrexate | 16 | 38 | 13 | 30 | 20 | 54.1 | 12 | 32.4 | 8 | 21.6 | ||
| Guenther et al., 1991129 | Placebo | Adults | 10 | 11 | – | – | 1 | 9.0 | – | – | – | – |
| Ciclosporin | 10 | 12 | – | – | 12 | 100.0 | – | – | – | – | ||
| Altmeyer et al., 1994131 | Placebo | Adults | 16 | 51 | – | – | – | – | 1 | 2.0 | – | – |
| Fumaric acid | 16 | 49 | – | – | – | – | 12 | 24.5 | – | – | ||
| Meffert et al., 1997130 | Placebo | Adults | 10 | 43 | – | – | – | – | 2 | 4.7 | – | – |
| Ciclosporin | 10 | 41 | – | – | – | – | 4 | 9.8 | – | – | ||
| Chaudhari et al., 2001132 | Placebo | Adults | 10 | 11 | 45 | 73 | – | – | 2 | 18.2 | – | – |
| Infliximab 5 mg/kg | 10 | 11 | 51 | 64 | – | – | 9 | 81.8 | – | – | ||
| Gottlieb et al., 2003109 | Placebo | Adults | 12 | 55 | 47 | 67 | 6 | 10.9 | 1 | 1.8 | 0 | 0.0 |
| Etanercept 50 mg | 12 | 57 | 48 | 58 | 40 | 70.2 | 17 | 29.8 | 6 | 10.5 | ||
| Heydendael et al., 2003122 | Methotrexate | Adults | 16 | 43 | 42 | 65 | – | – | 26 | 60.5 | – | – |
| Ciclosporin | 16 | 42 | 38 | 69 | – | – | 30 | 71.4 | – | – | ||
| Leonardi et al., 2003110 | Placebo | Adults | 12 | 166 | 46 | 63 | 24 | 14.5 | 6 | 3.6 | 1 | 0.6 |
| Etanercept 50 mg | 12 | 162 | 45 | 67 | 94 | 58.0 | 55 | 34.0 | 19 | 11.7 | ||
| Elewski et al., 2004111 | Placebo | Adults | 12 | 193 | 45 | 64 | 18 | 9.3 | 6 | 3.1 | 1 | 0.5 |
| Etanercept 50 mg | 12 | 196 | 45 | 65 | 126 | 64.3 | 67 | 34.2 | 2 | 1.0 | ||
| Gottlieb et al., 2004133 (SPIRIT) | Placebo | Adults | 10 | 51 | 45 | 61 | 11 | 21.6 | 3 | 5.9 | 1 | 2.0 |
| Infliximab 5 mg/kg | 10 | 99 | 44 | 74 | 96 | 97.0 | 87 | 87.9 | 57 | 57.6 | ||
| Papp et al., 2005112 | Placebo | Adults | 12 | 193 | 44 | 64 | 18 | 9.3 | 6 | 3.1 | 1 | 0.5 |
| Etanercept 50 mg | 12 | 196 | 46 | 65 | 126 | 64.3 | 67 | 34.2 | 21 | 10.7 | ||
| Reich et al., 2005134 (EXPRESS I) | Placebo | Adults | 10 | 77 | 44 | 79 | 6 | 7.8 | 2 | 2.6 | 1 | 1.3 |
| Infliximab 5 mg/kg | 10 | 301 | 43 | 69 | 274 | 91.0 | 242 | 80.4 | 172 | 57.1 | ||
| Gordon et al., 2006104 | Placebo | Adults | 12 | 52 | 43 | 65 | 7 | 13.5 | 2 | 3.8 | 0 | 0.0 |
| Adalimumab 40 mg | 12 | 45 | 46 | 71 | 34 | 75.6 | 24 | 53.3 | 11 | 24.4 | ||
| Krueger et al., 2007117 | Placebo | Adults | 12 | 64 | 44 | 72 | 7 | 10.9 | 1 | 1.6 | 1 | 1.6 |
| Ustekinumab 45 mg | 12 | 64 | 45 | 61 | 59 | 92.2 | 43 | 67.2 | 28 | 43.8 | ||
| Ustekinumab 90 mg | 12 | 64 | 44 | 81 | 59 | 92.2 | 52 | 81.3 | 33 | 51.6 | ||
| Menter et al., 2007135 (EXPRESS II) | Placebo | Adults | 10 | 208 | 44 | 69 | – | – | 4 | 1.9 | 1 | 0.5 |
| Infliximab 5 mg/kg | 10 | 314 | 45 | 65 | – | – | 237 | 75.5 | 142 | 45.2 | ||
| Flytström et al., 2008123 | Methotrexate | Adults | 12 | 37 | 48 | 76 | 24 | 64.9 | 9 | 24.3 | 4 | 10.8 |
| Ciclosporin | 12 | 31 | 45 | 87 | 27 | 87.1 | 18 | 48.6 | 9 | 24.3 | ||
| Leonardi et al., 2008118 (PHOENIX I) | Placebo | Adults | 12 | 255 | 45 | 72 | 26 | 10.2 | 8 | 3.1 | 5 | 2.0 |
| Ustekinumab 45 mg | 12 | 255 | 46 | 69 | 213 | 83.5 | 171 | 67.1 | 106 | 41.6 | ||
| Ustekinumab 90 mg | 12 | 256 | 45 | 68 | 220 | 85.9 | 170 | 66.4 | 94 | 36.7 | ||
| Menter et al., 2008105 (REVEAL) | Placebo | Adults | 16 | 398 | 45 | 65 | 60 | 15.1 | 28 | 7.0 | 8 | 2.0 |
| Adalimumab 40 mg | 16 | 614 | 44 | 67 | 667 | 81.9 | 578 | 71.0 | 366 | 45.0 | ||
| Papp et al., 2008119 (PHOENIX II) | Placebo | Adults | 12 | 410 | 47 | 69 | 41 | 10.0 | 15 | 3.7 | 3 | 0.7 |
| Ustekinumab 45 mg | 12 | 409 | 45 | 69 | 342 | 83.6 | 273 | 66.7 | 173 | 42.3 | ||
| Ustekinumab 90 mg | 12 | 411 | 47 | 67 | 367 | 89.3 | 311 | 75.7 | 209 | 50.9 | ||
| Saurat et al., 2008106 (CHAMPION) | Placebo | Adults | 16 | 53 | 41 | 66 | 16 | 30.2 | 10 | 18.9 | 6 | 11.3 |
| Adalimumab 40 mg | 16 | 108 | 43 | 65 | 95 | 88.0 | 86 | 79.6 | 55 | 50.9 | ||
| Methotrexate | 16 | 110 | 42 | 66 | 68 | 61.8 | 39 | 35.5 | 15 | 13.6 | ||
| van de Kerkhof et al., 2008113 | Placebo | Adults | 12 | 46 | 44 | 54 | 4 | 8.7 | 1 | 2.2 | 1 | 2.2 |
| Etanercept 50 mg | 12 | 96 | 46 | 62 | 66 | 68.8 | 36 | 37.5 | 13 | 13.5 | ||
| Asahina et al., 2010107 | Placebo | Adults | 16 | 46 | 44 | 89 | 9 | 19.6 | 2 | 4.3 | 0 | 0.0 |
| Adalimumab 40 mg | 16 | 43 | 44 | 83 | 35 | 81.4 | 27 | 62.8 | 17 | 39.5 | ||
| Griffiths et al., 2010120 (ACCEPT) | Ustekinumab 45 mg | Adults | 12 | 209 | 45 | 64 | 182 | 87.1 | 141 | 77.5 | 76 | 53.9 |
| Ustekinumab 90 mg | 12 | 347 | 45 | 67 | 319 | 91.9 | 256 | 80.3 | 155 | 60.5 | ||
| Torii et al., 2010136 | Placebo | Adults | 10 | 19 | 43 | 74 | 2 | 10.5 | 0 | 0.0 | 0 | 0.0 |
| Infliximab 5 mg/kg | 10 | 35 | 47 | 63 | 29 | 82.9 | 26 | 68.6 | 19 | 54.3 | ||
| Barker et al., 2011124 (RESTORE I) | Methotrexate | Adults | 16 | 215 | 42 | 69 | 130 | 60.5 | 90 | 41.9 | 41 | 19.1 |
| Infliximab 5 mg/kg | 16 | 653 | 44 | 67 | 567 | 86.8 | 508 | 77.8 | 356 | 54.5 | ||
| Fallah et al., 2011125 | Methotrexate | Adults | 12 | 27 | 41 | 59 | 15 | 55.6 | 6 | 22.2 | 2 | 7.4 |
| Fumaric acid | 12 | 27 | 43 | 74 | 11 | 40.7 | 5 | 18.5 | 1 | 3.7 | ||
| Tsai et al., 2011115 (PEARL) | Placebo | Adults | 12 | 60 | 40 | 88 | 8 | 13.3 | 3 | 5.0 | 1 | 1.7 |
| Ustekinumab 45 mg | 12 | 61 | 41 | 82 | 51 | 83.6 | 41 | 67.2 | 30 | 49.2 | ||
| Igarashi et al., 2012121 | Placebo | Adults | 12 | 31 | 49 | 84 | 4 | 12.9 | 2 | 6.5 | 1 | 3.2 |
| Ustekinumab 45 mg | 12 | 64 | 45 | 83 | 53 | 82.8 | 38 | 59.4 | 21 | 32.8 | ||
| Ustekinumab 90 mg | 12 | 62 | 44 | 76 | 52 | 83.9 | 42 | 67.7 | 27 | 43.5 | ||
| Papp et al., 2012126 | Placebo | Adults | 16 | 88 | 44 | 60 | 22 | 25.0 | 5 | 5.7 | 1 | 1.1 |
| Apremilast | 16 | 88 | 44 | 57 | 53 | 60.2 | 36 | 40.9 | 10 | 11.4 | ||
| Yang et al., 2012137 | Placebo | Adults | 10 | 45 | 40 | 78 | 6 | 13.2 | 1 | 2.2 | 0 | 0.0 |
| Infliximab 5 mg/kg | 10 | 84 | 39 | 71 | 79 | 94.0 | 68 | 81.0 | 48 | 57.1 | ||
| Bissonnette et al., 2013108 | Placebo | Adults | 16 | 10 | 57 | 60 | – | – | 2 | 20.0 | – | – |
| Adalimumab 40 mg | 16 | 20 | 56 | 85 | – | – | 14 | 70.0 | – | – | ||
| Zhu et al., 2013116 (LOTUS) | Placebo | Adults | 12 | 162 | 40 | 78 | 32 | 19.8 | 18 | 11.1 | 5 | 3.1 |
| Ustekinumab 45 mg | 12 | 160 | 49 | 84 | 146 | 91.3 | 132 | 82.5 | 107 | 66.9 | ||
| Papp et al., 2015127 (ESTEEM I) | Placebo | Adults | 16 | 282 | 47 | 69 | 48 | 17.0 | 15 | 5.3 | 1 | 0.4 |
| Apremilast | 16 | 562 | 46 | 67 | 330 | 58.7 | 186 | 33.1 | 55 | 9.8 | ||
| Paul et al., 2015128 (ESTEEM II) | Placebo | Adults | 16 | 137 | 46 | 73 | 27 | 19.7 | 8 | 5.8 | 1 | 0.7 |
| Apremilast | 16 | 274 | 45 | 64 | 152 | 55.5 | 79 | 28.8 | 24 | 8.8 | ||
| Reich et al., 2016114 (LIBERATE) | Placebo | Adults | 16 | 84 | – | – | 28 | 33.3 | 10 | 11.9 | – | – |
| Etanercept 50 mg | 16 | 83 | – | – | 69 | 83.1 | 40 | 48.2 | – | – | ||
| Apremilast | 16 | 83 | – | – | – | – | 33 | 39.8 | – | – |
Network meta-analysis using minimum evidence from the adult population
The disconnected network of evidence in children and young people was connected in the first instance by bringing together the minimum amount of evidence required from the adult population to link the adalimumab trial with the other trials (Figure 4). Among the studies presented in Table 40, there was only one trial in adults that could directly connect methotrexate with placebo and adalimumab with placebo (CHAMPION106). A number of trials compared adalimumab with placebo alone but inclusion of these trials would mean that methotrexate was connected only indirectly through adalimumab and placebo, potentially undermining the evidence from M04-717 on this agent. Therefore, the CHAMPION study represented the best way to connect adalimumab and methotrexate to etanercept and ustekinumab using the least amount of evidence drawn from the adult population.
FIGURE 4.
Network of evidence using minimum evidence from the adult population. ADA, adalimumab 0.8 mg/kg, maximum 40 mg/week; ETA, etanercept 0.8 mg/kg, maximum 50 mg/week; MTX, methotrexate 0.1–0.4 mg/kg/week; PLB, placebo; UST 45, ustekinumab 0.75 mg/kg or 45 mg/week. Trial names are stated when trial evidence informs the network treatment link.
In the CHAMPION trial,106 the primary efficacy end point was the proportion of individuals achieving a PASI 75 response at 16 weeks. Adalimumab was found to have significantly greater efficacy (79.6% achieving a PASI 75 response) than either methotrexate (35.5%) or placebo (18.9%). PASI outcome data and key baseline characteristics for the CHAMPION trial are provided in Table 41. The average age of patients recruited into the CHAMPION trial was approximately 42 years. The CHAMPION trial was a larger trial than the trials carried out in children and young people (n = 271 vs. n = 75 in the M04-717 trial), with an approximately 10–20% higher proportion of males.
The PASI 75 response rates for adalimumab and methotrexate in the CHAMPION trial were similar to those reported in the M04-717 trial in children and young people. An important difference between the CHAMPION trial and the trials in children and young people was the observed placebo effect on the primary end point of PASI 75. Whereas in the etanercept trial (20030211) and the ustekinumab trial (CADMUS) the proportion of individuals achieving a PASI 75 response in the placebo arm was approximately 11%, the proportion achieving a PASI 75 response in the placebo arm in the CHAMPION trial106 was approximately 19%. The authors of the CHAMPION trial identified two reasons for this anomalous placebo response: (1) placebo response rates are generally greater in European studies and (2) the observed placebo response may partly have resulted from the correction of an underlying folate deficiency following folate supplementation, which was mandatory for all study patients.
Given that the CHAMPION trial connects the adalimumab trial in children and young people (M04-717) to etanercept and ustekinumab through placebo, it is important to ensure that the differences in placebo response rates do not ‘artificially’ inflate or deflate the PASI response outcomes for the interventions of interest. Therefore, as well as using a baseline unconstrained prediction model, whereby baseline risk (placebo response rates) is predicted using evidence from all studies included in the network (analysis 1a), a baseline constrained prediction model was also considered, whereby placebo response rates are predicted based on the placebo arm trials in children and young people only [i.e. the etanercept (20030211) and ustekinumab (CADMUS) trials] (analysis 1b). As the number of trials to inform each treatment effect is small, a fixed-effect model was used. The results of this analysis are presented in Results.
Network meta-analysis using full evidence from the adult population
The second approach to the NMA involved connecting the evidence from the adalimumab trial in children and young people to the evidence from the other trials (20030211 and CADMUS) by drawing strength from the full network of evidence available in adults. The relative efficacy of adalimumab, etanercept and ustekinumab has been evaluated extensively in adults with moderate to severe plaque psoriasis. Given the limited evidence base in children and young people, and the expectation that the difference in response rates between the interventions is predominantly the result of the relative efficacy of the biologics rather than age or other patient characteristics, it would seem appropriate to combine the weight of evidence from all relevant trials and comparators, including those in adults. This wider network of evidence can be used to facilitate an indirect comparison of adalimumab with etanercept and ustekinumab by examining the relationships that exist between the different treatments and study populations and drawing strength from the full network of evidence.
Figure 5 presents the full network of evidence in both populations. This wider network considers nine active treatments and placebo, encompassing 37 RCTs in total (three in children and young people and 34 in adults), with six of these being three-arm trials. The majority of network links (‘head-to-head trial comparisons’) are populated by more than one study.
FIGURE 5.
Wider network of evidence in children and young people and adult populations. ADA, adalimumab 0.8 mg/kg, maximum 40 mg/week; APRE, apremilast; CIC, ciclosporin; ETA, etanercept 0.8 mg/kg, maximum 50 mg/week; FUM-A, fumaric acid; INF, infliximab 5 mg/kg; MTX, methotrexate 0.1–0.4 mg/kg/week; PLB, placebo; UST 45, ustekinumab 0.75 mg/kg or 45 mg/week; UST 90, ustekinumab 90 mg/week. Trial names are stated when trial evidence informs the network treatment link. Discontinued lines indicate where three-arm trials inform the evidence network, with the number of three-arm trials indicated in boxes.
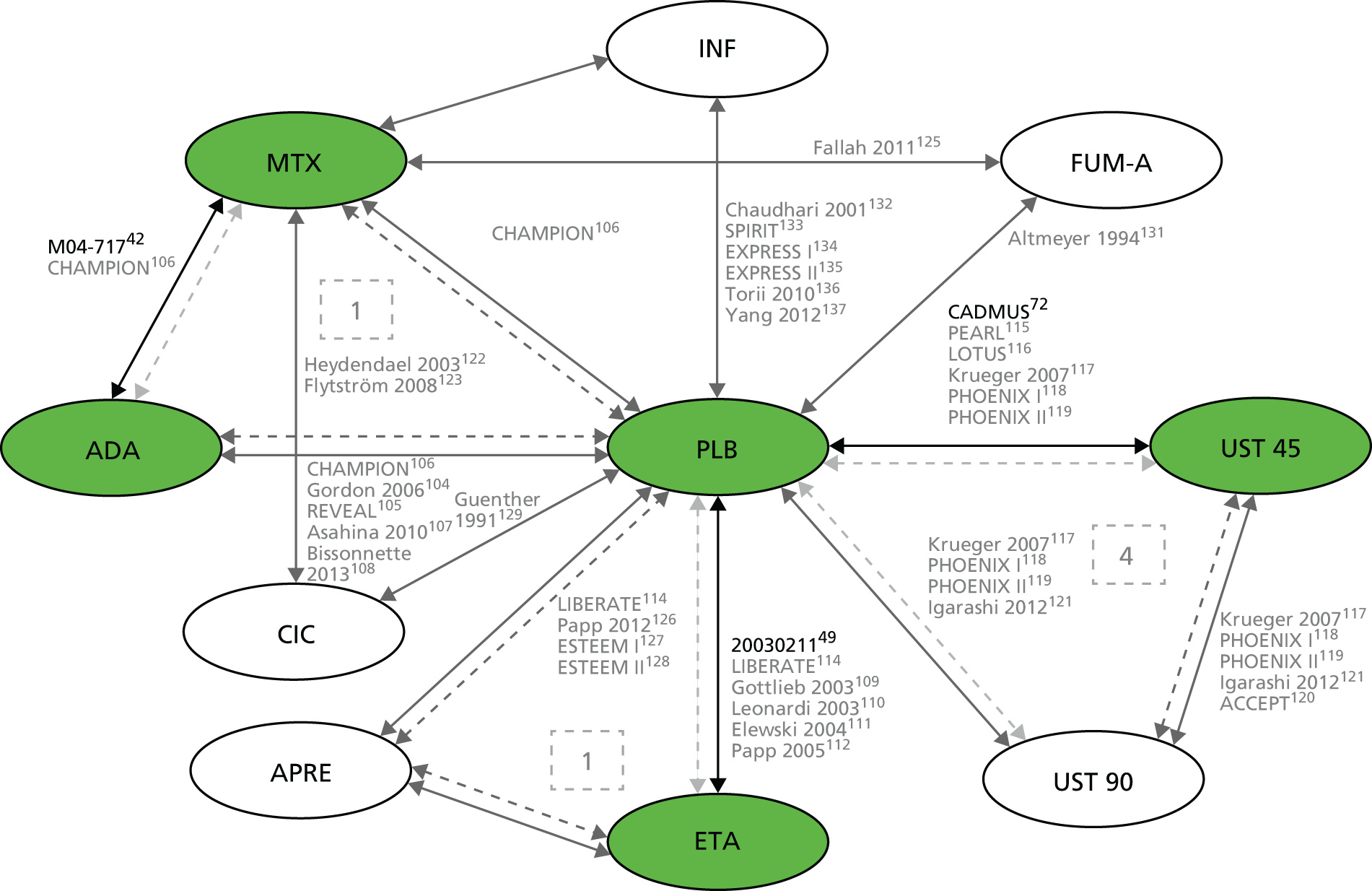
A Bayesian evidence synthesis approach was employed that draws on the relationships that exist between treatments and populations while also preserving differences that exist across populations by adjusting for age and placebo response rates. NMA meta-regression models on baseline risk (i.e. placebo response) were explored. 103 These models impose a common interaction effect between baseline risk and relative effectiveness that accounts for variation in reference arm response across trials. NMA meta-regression models that explore variability caused by age effects were also implemented. These models impose an age group interaction effect at the study level (binary variable: 1 if study is from a child or young adult population, 0 otherwise) that attempts to explain the heterogeneity between treatment effects when considering both adult treatment response data and data from children and young people. The age-adjusted meta-regression models provided pooled PASI response rates by treatment for both children and young people, and adults. A common treatment × age interaction effect was imposed. The common interaction assumption is the least data demanding (i.e. only one extra parameter needs to be estimated), but it also imposes the strongest assumption as it implies that the same age group effect exists regardless of treatment (excluding placebo). 138 For example, if the age interaction effect (of children and young people vs. adults) is estimated to be positive and of average magnitude 25% on the absolute PASI scale, PASI response rates in children and young people will be approximately 25% higher, on average, than those in adults, irrespective of treatment. Further details on the implemented synthesis models and their assumptions, including the WinBUGS code (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK), are provided in Appendix 5.
Fixed- and random-effects analyses were explored for two separate scenarios: (1) a meta-regression model with adjustment for baseline risk (i.e. placebo response rates) and (2) a meta-regression model with adjustment for baseline risk and age. Irrespective of scenario and according to deviance information criterion (DIC) and total residual deviance statistics, the random-effects approach provided a better fit to the data than the fixed-effect counterpart. Therefore, only results from the random-effects model are presented and discussed here. The results from the fixed-effect model are provided in Appendix 6.
Table 42 provides a summary of the models implemented together with the key modelling assumptions. As no evidence was found to support the existence of a class effect, all models considered treatments to be independent of each other. In models in analyses 2a and 2b it was assumed that treatments were independent of each other, but treatment effects were adjusted with the trial-specific baseline effects, assuming a common interaction term. In addition, models in analysis 2b were adjusted for trial-specific age effects, also assuming a common interaction term. This age adjustment enabled the estimation of treatment effects separately by age (adults and children and young people). All implemented synthesis models assumed fixed effects on PASI response cut-off points.
| Analysis | Study | Meta-regression | Baseline prediction |
|---|---|---|---|
| 1a | Fixed effects | No adjustment | Unconstrained |
| 1b | Fixed effects | No adjustment | Constrained to studies of children and young people |
| 2 | Random effects | No adjustment | Unconstrained |
| 2a | Random effects | Common interaction term for baseline effect | Unconstrained; baseline adjusted |
| 2b | Random effects | Common interaction term for baseline effect and for age effect | Unconstrained; baseline adjusted |
Results
Analysis 1: results using minimum evidence from the adult population
Table 43 summarises the results of the NMA in terms of absolute PASI response rates for the unconstrained (no explicit adjustment for differences in placebo response rates across the trials) and constrained (placebo response rates predicted based on the placebo arm trials in children and young people only) models. The results of both sets of analyses show that all active treatments are more effective than placebo. In terms of mean response rates (analysis 1b results), ustekinumab is estimated to have the highest probability of achieving a PASI 50 (90%, 95% CrI 81% to 96%), PASI 75 (79%, 95% CrI 64% to 90%) and PASI 90 (57%, 95% CrI 39% to 74%) response compared with any of the other treatments, suggesting that it is the most effective intervention. This is followed by adalimumab, etanercept and methotrexate in both sets of analyses, that is, the ranking of treatments based on mean response rates is unchanged in the different models.
| Treatment | Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| 1a | 1b | |||||||
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.371 (0.29 to 0.46) | 0.203 (0.14 to 0.27) | 0.071 (0.04 to 0.11) | 5 | 0.267 (0.19 to 0.35) | 0.131 (0.08 to 0.19) | 0.039 (0.02 to 0.07) | 5 |
| Etanercept | 0.830 (0.73 to 0.91) | 0.676 (0.54 to 0.79) | 0.431 (0.30 to 0.57) | 3 | 0.747 (0.63 to 0.84) | 0.566 (0.43 to 0.69) | 0.321 (0.21 to 0.44) | 3 |
| Ustekinumab 45 mg | 0.941 (0.87 to 0.99) | 0.859 (0.73 to 0.95) | 0.677 (0.49 to 0.85) | 1 | 0.901 (0.81 to 0.96) | 0.787 (0.64 to 0.90) | 0.569 (0.39 to 0.74) | 1 |
| Adalimumab | 0.832 (0.74 to 0.91) | 0.678 (0.56 to 0.79) | 0.433 (0.31 to 0.56) | 2 | 0.746 (0.60 to 0.87) | 0.567 (0.40 to 0.73) | 0.324 (0.19 to 0.49) | 2 |
| Methotrexate | 0.432 (0.33 to 0.54) | 0.251 (0.17 to 0.34) | 0.096 (0.06 to 0.15) | 4 | 0.323 (0.20 to 0.47) | 0.170 (0.09 to 0.28) | 0.057 (0.02 to 0.11) | 4 |
| Residual deviance, mean (95% CI) | 46.6a (39.7 to 57.6) | 46.6a (39.7 to 57.6) | ||||||
| DIC | 158.60 | 158.60 | ||||||
The unconstrained baseline model (analysis 1a), however, predicts a placebo effect for PASI 75 of 20.3% (95% CrI 14% to 27%) whereas the constrained baseline model (analysis 1b) predicts a placebo effect of 13.1% (95% CrI 8% to 19%). This difference is driven by the CHAMPION trial,106 which had a substantially higher placebo response rate of approximately 19% for PASI 75 compared with the placebo response rates observed in the trials of children and young people (approximately 11% in 20030211 and CADMUS). The constrained baseline model (analysis 1b) adjusts the baseline predictions to consider only placebo effect evidence from trials in the younger population. In this analysis, the mean PASI 75 response rate for placebo is reduced and closer to the observed response in the children and young people trials.
As shown by the CrIs around the mean response rates, which are wide and overlap, there is uncertainty around these response rates. This is also shown in terms of the RRs of each treatment compared with placebo and their CrIs for the best-fitting model 1b (Table 44).
| PLB | 4.95 (2.84 to 8.65) | 7.50 (2.90 to 19.10) | – | – |
| 4.37 (3.02 to 6.56) | ETA | – | – | – |
| 6.10 (3.84 to 10.01) | 1.39 (1.00 to 1.97) | UST 45 | – | – |
| 4.36 (3.10 to 6.31) | 1.00 (0.71 to 1.39) | 0.72 (0.48 to 1.01) | ADA | 0.49 (0.38 to 0.59) |
| 1.28 (0.78 to 1.98) | 0.29 (0.16 to 0.50) | 0.21 (0.11 to 0.38) | 0.29 (0.19 to 0.43) | MTX |
Analysis 2: results using all relevant evidence from the adult population
Table 45 summarises the absolute PASI response rates from the NMA that uses the full network of evidence in both populations for the unadjusted random-effects model (analysis 2). Relative treatment effects for analysis 2 for PASI 75 response are presented in Table 46. The random-effects approach outperformed the fixed-effect approach in terms of model fit, suggesting that accounting for between-study heterogeneity is an important factor (τ2 = 0.02).
| Treatment | Analysis 2 | |||
|---|---|---|---|---|
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.141 (0.12 to 0.16) | 0.049 (0.04 to 0.06) | 0.010 (0.01 to 0.01) | 5 |
| Etanercept | 0.633 (0.57 to 0.70) | 0.404 (0.34 to 0.47) | 0.172 (0.13 to 0.22) | 3 |
| Ustekinumab 45 mg | 0.885 (0.85 to 0.92) | 0.732 (0.67 to 0.79) | 0.466 (0.40 to 0.53) | 1 |
| Adalimumab | 0.818 (0.76 to 0.87) | 0.629 (0.55 to 0.70) | 0.354 (0.28 to 0.43) | 2 |
| Methotrexate | 0.562 (0.47 to 0.65) | 0.336 (0.25 to 0.42) | 0.130 (0.08 to 0.18) | 4 |
| Residual deviance, mean (95% CI) | 378.1a (355.6 to 404.0) | |||
| DIC | 1241.07 | |||
| PLB | 9.52 (7.46 to 12.35) | 14.49 (11.43 to 18.28) | 8.08 (6.18 to 10.53) | 1.88 (1.02 to 3.47) |
| 8.03 (6.61 to 9.64) | ETA | – | – | – |
| 14.24 (12.17 to 16.58) | 1.78 (1.50 to 2.12) | UST 45 | – | – |
| 12.34 (10.10 to 14.82) | 1.54 (1.25 to 1.88) | 0.87 (0.74 to 0.99) | ADA | 0.49 (0.38 to 0.59) |
| 6.72 (4.83 to 8.90) | 0.84 (0.60 to 1.11) | 0.47 (0.35 to 0.60) | 0.55 (0.42 to 0.68) | MTX |
The results of this analysis suggest that ustekinumab is the most effective intervention, with the highest mean probability of PASI response (PASI 75: 73%, 95% CrI 67% to 79%), followed by adalimumab (PASI 75: 63%, 95% CrI 55% to 70%), etanercept (PASI 75: 40%, 95% CrI 34% to 47%) and methotrexate (PASI 75: 34%, 95% CrI 25% to 42%). Ustekinumab was statistically significantly more effective than any other agent based on relative effect estimates for PASI 75 (vs. etanercept: RR 1.78, 95% CrI 1.50 to 2.12; vs. adalimumab: RR 1.15, 95% CrI 1.01 to 1.35) and adalimumab was statistically significantly more effective than etanercept (RR 1.54, 95% CrI 1.25 to 1.88). The estimated pooled placebo absolute effect is in line with that observed, on average, across all studies in all populations.
These unadjusted results, however, do not consider an explicit adjustment for differences in placebo response rates across trials or differences across the populations (i.e. children and young people compared with adults). In the following sections, the results from the adjusted analyses are presented.
Adjustment for differences in placebo response rates across the trials
The NMA in the full population compares treatment outcomes across a large number of separate clinical trials. The reliability of these comparisons depends on the cross-trial similarity of the patient populations included in the network. An important difference between the included trials is the observed PASI response rates in the placebo arms of the trials, which is a common reference treatment across the majority of the trials. Table 41 showed that the PASI response rates in the placebo arms of the trials ranged from 0%136 to 20%. 108 All of the trials varied by design, eligibility criteria, previous medication use, average age and other characteristics. All of these variations could contribute to differences in placebo response rates and, therefore, to differences in the relative efficacy of the intervention compared with placebo. However, there is no systematic way to identify the reasons for these differences. A ‘placebo creep’ phenomenon has been discussed in the literature, which identifies a relationship between placebo response rates and time since publication of the trial results. However, such a phenomenon has not been identified in the trials considered in the NMA (Figure 6). The average PASI 75 response rate in the placebo arm across all trials is 6.2%, whereas the average rate in studies of adult populations is 5.9% and in studies of children and young people is 11.1%. Three adult studies106,108,132 have substantially higher placebo response rates (approximately 18–20%) than the other studies. Four studies, including the two trials in children and young people72 and two in adults,114,116 have approximately double the average placebo rate.
FIGURE 6.
Probability of a PASI 75 response in the placebo arms of trials in the NMA by publication year.
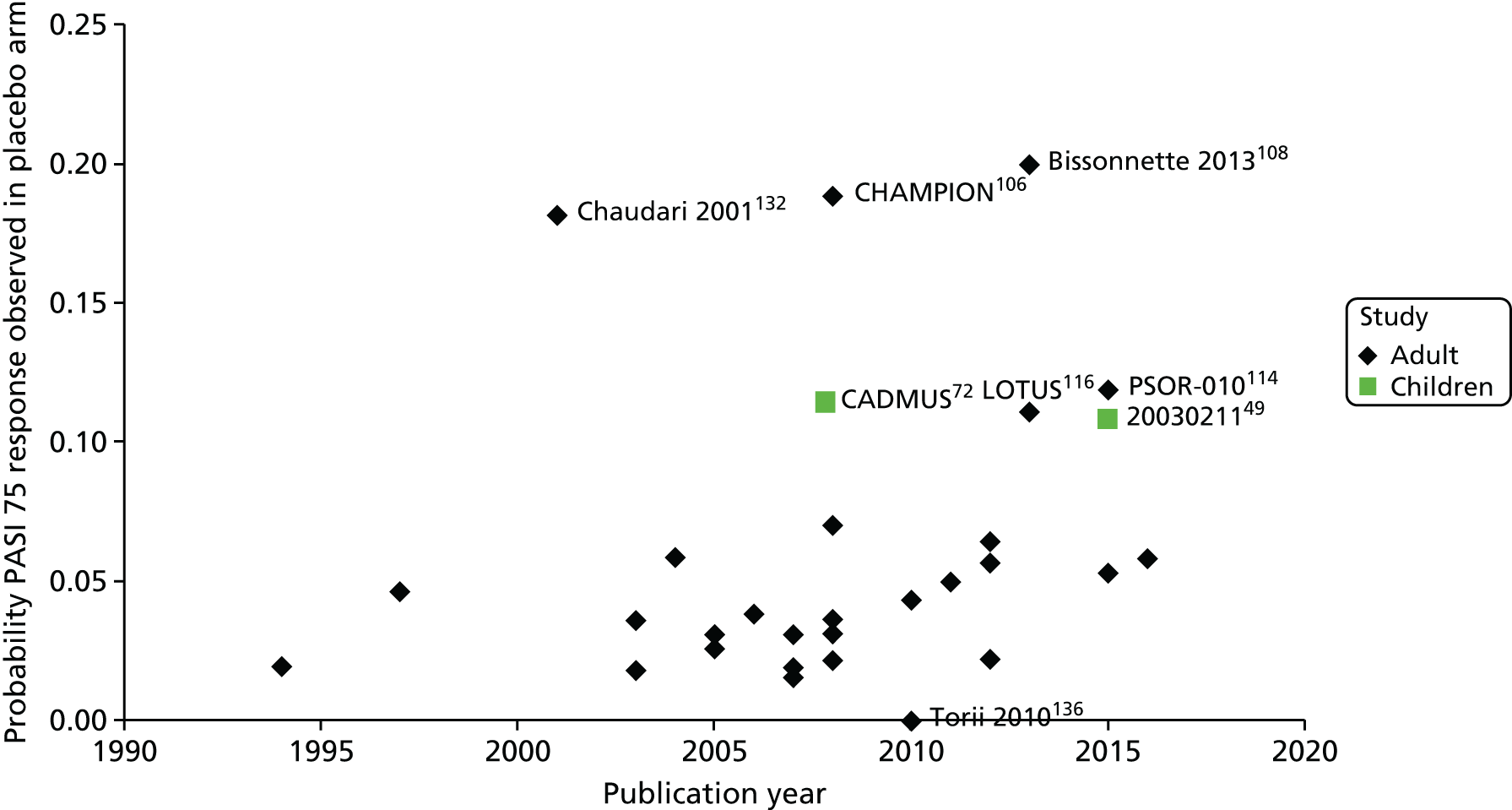
It is not clear exactly how these varying placebo rates affect treatment effects; however, it is clear that any differences will affect the relative efficacy of the interventions compared with placebo. Therefore, a potential relationship between baseline risk and relative treatment effect was explored103 in analysis 2a.
Tables 47 and 48 present the results of the model that adjusts for differences in placebo response rates. As for the unadjusted analysis (i.e. analysis 2), the baseline adjusted random-effects model was found to fit the data considerably better than the fixed-effect counterpart (DIC: 1303.7 fixed effects vs. 1177.6 random effects; total residual deviance: 473.5 fixed effects vs. 380.9 random effects). Furthermore, the 95% CRIs for the estimated mean baseline effect derived in the baseline-adjusted model do not include zero (–0.93, 95% CrI –0.97 to –0.88). This suggests that adjusting for baseline risk heterogeneity is important to explain existing between-study variation.
| Treatment | Analysis 2a | |||
|---|---|---|---|---|
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.151 (0.13 to 0.17) | 0.053 (0.04 to 0.06) | 0.010 (0.01 to 0.01) | 5 |
| Etanercept | 0.642 (0.57 to 0.71) | 0.414 (0.35 to 0.49) | 0.180 (0.12 to 0.25) | 3 |
| Ustekinumab 45 mg | 0.882 (0.84 to 0.92) | 0.727 (0.66 to 0.79) | 0.461 (0.37 to 0.56) | 1 |
| Adalimumab | 0.839 (0.78 to 0.89) | 0.660 (0.58 to 0.74) | 0.349 (0.25 to 0.45) | 2 |
| Methotrexate | 0.570 (0.46 to 0.67) | 0.344 (0.25 to 0.44) | 0.178 (0.10 to 0.28) | 4 |
| Residual deviance, mean (95% CI) | 381.7a (357.5 to 409.4) | |||
| DIC | 904.5 | |||
| PLB | 9.52 (7.46 to 12.35) | 14.49 (11.43 to 18.28) | 8.08 (6.18 to 10.53) | 1.88 (1.02 to 3.47) |
| 7.86 (6.46 to 9.44) | ETA | – | – | – |
| 13.82 (11.70 to 16.32) | 1.77 (1.48 to 2.11) | UST 45 | – | – |
| 12.53 (10.34 to 15.01) | 1.60 (1.31 to 1.95) | 0.91 (0.78 to 1.04) | ADA | 0.49 (0.38 to 0.59) |
| 6.52 (4.68 to 8.55) | 0.84 (0.58 to 1.12) | 0.47 (0.34 to 0.61) | 0.52 (0.38 to 0.67) | MTX |
The results of analysis 2a suggest that ustekinumab is the most effective intervention, with the highest mean probability of PASI response (PASI 75: 73%, 95% CrI 66% to 79%), followed by adalimumab (PASI 75: 66%, 95% CrI 58% to 74%), and etanercept (PASI 75: 41%, 95% CrI 35% to 49%) and methotrexate (PASI 75: 34%, 95% CrI 25% to 44%). Ustekinumab is statistically significantly more effective than etanercept based on relative effect estimates for PASI 75 (RR 1.77, 95% CrI 1.48 to 2.11), but not statistically significantly more effective than adalimumab (RR 1.10, 95% CrI 0.96 to 1.28). Adalimumab is also statistically significantly more effective than etanercept (RR 1.60, 95% CrI 1.31 to 1.95).
Adjusting for differences in population and placebo response rates
Although evidence from trials in both children and young people and adults contributed to the full network of evidence (effectively assuming independence between age and treatment effectiveness), it is important to recognise that the age of the population could contribute to differences in treatment efficacy. Therefore, in analysis 2b we adjusted for differences in the population and differences in placebo response rates (as the placebo response rates were considerably different in the trials of children and young people and the trials of adults). Table 49 summarises the results of this analysis in terms of PASI response outcomes for both populations. Table 50 presents the corresponding RRs for PASI 75 for children and young people.
| Treatment | Analysis 2b population | |||||||
|---|---|---|---|---|---|---|---|---|
| Children and young people | Adults | |||||||
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.265 (0.15 to 0.40) | 0.115 (0.05 to 0.20) | 0.029 (0.01 to 0.06) | 5 | 0.151 (0.13 to 0.17) | 0.053 (0.04 to 0.06) | 0.010 (0.01 to 0.01) | 5 |
| Etanercept | 0.752 (0.62 to 0.86) | 0.544 (0.39 to 0.69) | 0.279 (0.16 to 0.42) | 3 | 0.619 (0.54 to 0.69) | 0.390 (0.32 to 0.47) | 0.162 (0.12 to 0.22) | 3 |
| Ustekinumab 45 mg | 0.934 (0.87 to 0.97) | 0.824 (0.71 to 0.91) | 0.594 (0.43 to 0.74) | 1 | 0.872 (0.83 to 0.91) | 0.711 (0.64 to 0.78) | 0.441 (0.36 to 0.52) | 1 |
| Adalimumab | 0.915 (0.83 to 0.97) | 0.790 (0.64 to 0.90) | 0.546 (0.37 to 0.72) | 2 | 0.844 (0.78 to 0.90) | 0.667 (0.58 to 0.75) | 0.393 (0.30 to 0.48) | 2 |
| Methotrexate | 0.708 (0.53 to 0.85) | 0.492 (0.31 to 0.68) | 0.240 (0.11 to 0.40) | 4 | 0.567 (0.45 to 0.68) | 0.342 (0.24 to 0.45) | 0.134 (0.08 to 0.20) | 4 |
| Residual deviance, mean (95% CI) | 380.8a (356.2 to 408.6) | 380.8a (356.2 to 408.6) | ||||||
| DIC | 1229.5 | 1229.5 | ||||||
| PLB | 4.95 (2.84 to 8.65) | 7.50 (2.90 to 19.10) | – | – |
| 5.09 (3.30 to 8.05) | ETA | – | – | – |
| 7.91 (4.46 to 14.14) | 1.54 (1.28 to 1.92) | UST 45 | – | – |
| 7.53 (4.37 to 12.98) | 1.47 (1.23 to 1.79) | 0.96 (0.85 to 1.05) | ADA | 0.49 (0.38 to 0.59) |
| 4.55 (3.01 to 6.94) | 0.91 (0.66 to 1.15) | 0.59 (0.41 to 0.77) | 0.62 (0.44 to 0.78) | MTX |
The model from analysis 2b fits the data as well as model 2a, as both present similar average total residual deviance [380.8 (2b) vs. 381.7 (2a)]. However, the DIC is substantially higher for model 2b. This suggests that this model is being penalised because of issues of parsimony. The children and young people subgroup effect is estimated not to be statistically significantly different from the adult subgroup effect, implying that the PASI absolute effect distributions of these populations overlap. This is not unexpected because of the limited number of existing studies in the population of children and young people.
The adjustment for population resulted in similar treatment rankings for children and young people when compared with the whole population results (see Table 47). The pooled placebo response rate for children and young people was estimated to be higher than that for adults (PASI 75: 12%, 95% CrI 5% to 20% in children and young people vs. 5%, 95% CrI 4% to 6% in adults), reflecting the higher placebo response rates observed in the trials in children and young people. This affects the efficacy of treatments by substantially increasing the estimated absolute PASI response rates across all treatments, but affecting the relative effects to a smaller extent. On average, PASI 75 response rates were estimated to be 10–15% higher in children and young people than in adults. The treatment rankings, however, remained unchanged. This is consistent with clinical opinion, with efficacy rates expected to be generally higher in children and young people than in adults as the biological interventions tend to work better in individuals with a lower body weight. Also, children and young people tend to have fewer comorbidities and generally have a greater exposure to ultraviolet (UV) light from participating in outside activities. The CrIs for PASI 75 response for children and young people and adults overlap, as shown in Figure 7.
FIGURE 7.
Absolute PASI 75 probability of response for children and young people and adults from NMA model 2b. ADA, adalimumab 0.8 mg/kg, maximum 40 mg/week; ETA, etanercept 0.8 mg/kg, maximum 50 mg/week; MTX, methotrexate 0.1–0.4 mg/kg/week; PLB, placebo; UST 45, ustekinumab 0.75 mg/kg or 45 mg/week.
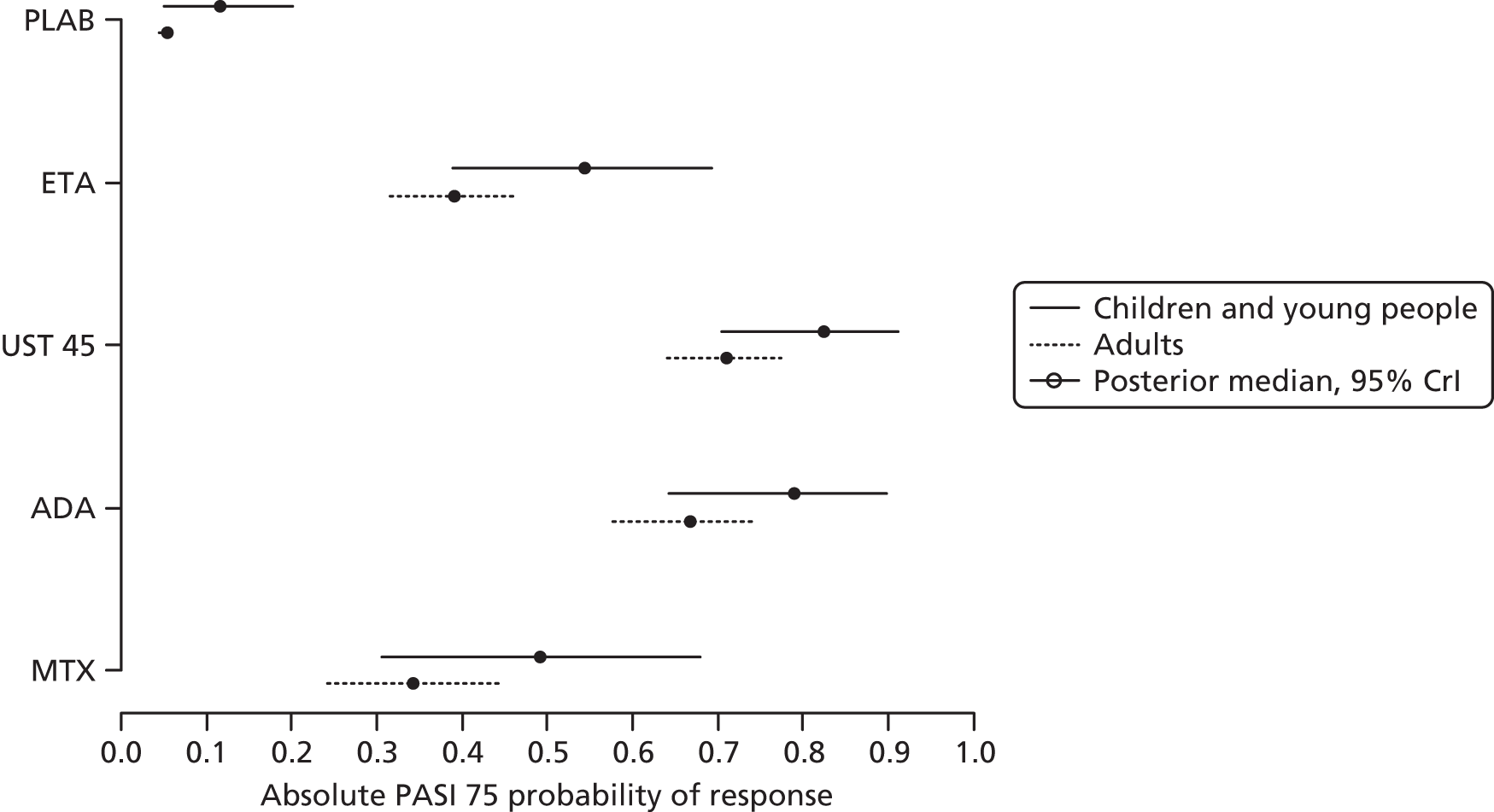
The results of analysis 2b in children and young people suggest that ustekinumab is the most effective intervention with the highest mean probability of PASI response (PASI 75: 82%, 95% CrI 71% to 90%), followed by adalimumab (PASI 75: 79%, 95% CrI 64% to 90%), etanercept (PASI 75: 54%, 95% CrI 39% to 69%) and methotrexate (PASI 75: 49%, 95% CrI 31% to 68%). The relative efficacy of ustekinumab and adalimumab is similar based on relative effectiveness estimates for PASI 75 response (ADA vs. UST 45: RR 0.96, 95% CrI 0.85 to 1.05). In children and young people, ustekinumab (RR 1.54, 95% CrI 1.28 to 1.92) and adalimumab (RR 1.47, 95% CrI 1.23 to 1.79) are statistically significantly more effective than etanercept.
A consistency assessment was undertaken that involved excluding the trials of children and young people from the evidence network. This assessment indicated that the results were consistent across populations (see Appendix 7 for further details).
Summary of the findings on relative efficacy from the network meta-analysis
There was no direct trial evidence that could be used to establish the relative effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people. Furthermore, there was no common comparator across the three included trials, which precluded establishing an indirect comparison between all of the interventions without drawing on evidence from other sources, namely from a different age population (i.e. adults).
Several NMA analyses were conducted to overcome the challenges involved in formally assessing the relative efficacy of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people.
First, statistical testing was performed on age subgroup efficacy data from the clinical trials in children and young people to establish whether or not it is reasonable to assume that the PASI response rates for the treatments are independent of age within the full population of children and young people, as the trials included participants of different age ranges. An indirect treatment comparison based solely on children and young people trial data for etanercept and ustekinumab was then performed and the results presented. However, this analysis was of limited use for the economic analysis as the network did not incorporate the full set of relevant interventions. Finally, a framework of analysis using different levels of evidence from the adult population was developed to address the issue of having a disconnected network structure. Previously appraised adult trial evidence was reviewed and extracted, and was assumed exchangeable with evidence from children and young adults, for inclusion in the evidence base. Two main approaches were considered, one in which the network of trials in children and young people was connected by bringing the minimum amount of evidence required from the adult population to link the three existing trials and the other in which all relevant efficacy evidence identified in adults was incorporated in the network. For each NMA model fixed- and random-effects model approaches were investigated. The latter approach was shown to be preferable, highlighting that it was important to account for variability across trials. The rate of placebo response was identified as a source of heterogeneity. Also, population-adjusted models allowed subpopulation-specific estimates to be obtained for (1) children and young people and (2) adults. The different model adjustments were explored and the age- and placebo-adjusted model was identified as the best-fitting model. For comparison and comprehensiveness, unadjusted and adjusted model results were presented.
The PASI response results were generally consistent across the different models, both adjusted and unadjusted. Overall, PASI responses were estimated to be higher for ustekinumab, followed by adalimumab and etanercept. However, there was no statistically significant difference (at the 5% significance level) between adalimumab and ustekinumab across the majority of models for PASI 75 response. Methotrexate was the least efficacious active agent, followed by placebo. The economic model in Chapter 6 uses the results for the children and young people subgroup of the placebo and population random-effects adjusted NMA (2b; see Table 49) to inform the effectiveness estimates. This NMA model was considered to provide the most appropriate set of efficacy estimates to inform the economic analysis because (1) it considers all relevant evidence, (2) it adjusts for placebo heterogeneity, (3) it adjusts for age effects and (4) it enables the estimation of age subgroup-specific effects. Scenario analyses were also conducted in which the results from the unadjusted baseline constrained model with minimum adult evidence (1b; see Table 43) are applied in the model. Partial comparisons with direct trial data and the indirect comparison reported in Indirect treatment comparison were also incorporated in a scenario analysis for completeness.
Chapter 5 Assessment of existing cost-effectiveness evidence
Introduction
This chapter aims to provide an overview of the existing evidence on the cost-effectiveness of adalimumab, etanercept, ustekinumab and relevant comparators for the treatment of plaque psoriasis in children and young people. The overview includes the company submissions from Janssen (ustekinumab) and AbbVie (adalimumab) [Pfizer (etanercept) did not provide a company submission]. An overview of the cost-effectiveness evidence from related NICE TAs of the treatment of plaque psoriasis in adults (TA103,97 TA134,98 TA146,99 TA180,100 TA350101 and TA368102) is also presented. The differences in the model structures and assumptions used across the studies are examined to identify any important differences in approaches and areas of uncertainty. The findings from the review provide the basis for the development of a new decision-analytic model in children and young people reported in Chapter 6.
Methods
The searches described in Chapter 3 (see Appendix 1 for details) were used to identify potentially relevant studies for inclusion in the assessment of the cost-effectiveness of the interventions compared with any comparator in children and young people. A broad range of studies was considered for inclusion in the assessment of cost-effectiveness, including economic evaluations conducted alongside clinical trials and modelling studies. Only full economic evaluations that compared two or more options and considered both costs and consequences in children and/or young people were considered. The inclusion criteria allowed for studies in adults to be included as long as data were reported separately for a subpopulation of children and/or young people. Only studies with ‘cost’ in the title or abstract were included. The searches were not restricted to level of disease severity, as a dearth of evidence was anticipated in the population of children and young people.
Titles and abstracts were assessed independently by two reviewers for inclusion and any discrepancies were resolved by consensus. Additional hand-searching of related TAs in adults was undertaken.
Results
Identified published studies
A total of 293 unique records were identified from the systematic literature review of existing cost-effectiveness evidence in children and young people, of which only one study subsequently met the inclusion criteria. 139 This study, on the use of etanercept for the treatment of chronic severe plaque psoriasis in children and adolescents from the age of 8 years in NHS Wales who are inadequately controlled by, or who are intolerant to, other systemic therapies or phototherapies, was by the All Wales Medicines Strategy Group (AWMSG).
One previous NICE multiple technology appraisal (MTA) (TA10397,140) and five single technology appraisals (STAs) (TA134,98 TA146,99 TA180,100 TA350,101 and TA368102) were identified in adults with chronic plaque psoriasis.
Review of existing published cost-effectiveness studies
This review starts with an overview of the AWMSG cost-effectiveness model for the assessment of etanercept in children and adolescents from the age of 8 years and then considers the cost-effectiveness evidence submitted by the companies for ustekinumab and adalimumab in children and young people. The final section provides an overview of the cost-effectiveness modelling used in the previous TAs in adults.
Etanercept All Wales Medicines Strategy Group cost-effectiveness model in children and young people
The only published economic model identified was that reported as part of AWMSG advice no. 138 for the use of etanercept within NHS Wales for the treatment of chronic severe plaque psoriasis in children and adolescents from the age of 8 years. 139 The cost-effectiveness evidence presented was deemed insufficient for AWMSG to recommend the use of etanercept in NHS Wales. The cost-effectiveness modelling was not reported in sufficient detail to be very informative. AWMSG considered that it was not possible to judge whether or not the analysis presented by the company (Pfizer) in its submission represented the most plausible estimate of the cost-effectiveness of etanercept compared with placebo in this population. This was because of a number of limitations in the economic evidence and decision-analytic model submitted by the company.
The economic model was a Markov model with a 28-day cycle length to represent intermittent treatment with etanercept compared with placebo/non-systemic therapy over a 10-year time horizon. The perspective of the evaluation was NHS Wales. For treatment with 0.8 mg/kg of etanercept weekly (up to a maximum of 50 mg), individuals were modelled to receive initial therapy for a ‘trial period’ of 12 weeks, after which their PASI 50 response was used to determine whether or not they were considered responders or non-responders to treatment. Those who achieved a PASI 50 response were considered responders and continued treatment with continuous etanercept until week 24. At week 24, those who did not achieve a PASI 50 response discontinued treatment whereas those with a PASI response between 50 and 75 remained on continuous etanercept. Of those with a PASI 75 response, 25% were assumed to remain on continuous etanercept and the remainder received intermittent etanercept (consisting of a treatment-free period, with treatment reinitiated in those who experienced a relapse). Non-responders at 12 and 24 weeks were assumed to discontinue treatment.
The effectiveness evidence was sourced from a placebo-controlled RCT of the use of etanercept in children and adolescents aged 4–17 years with moderate to severe psoriasis and with previous or current treatment with phototherapy or systemic therapies or psoriasis considered by the investigator to be poorly controlled with topical treatments. 57 The AWMSG estimated that only 57% of the trial population met the licensed indication for etanercept at the time of the submission.
The HRQoL estimates applied in the model were derived from adult studies of etanercept through the mapping of adult DLQI scores to EQ-5D utility values. The utility gains from baseline were assumed to be independent of treatment and varied according to the severity of disease based on PASI response rates. The AWMSG noted that there was no discussion of the uncertainty surrounding the use of utility values from adults to inform the population of children and adolescents.
The drug costs of etanercept were based on the doses used in the RCT in children and adolescents,57 at 0.8 mg/kg up to a maximum dose of 50 mg, delivered in prefilled syringes. However, the AWMSG noted discrepancies between what was reported and the doses used in the model, with a lower dose of 25 mg weekly used in the model for all patients instead of 44% of patients receiving the maximum dose of 50 mg per week and 56% of patients receiving a weekly dose of 0.8 mg/kg in the trial. The median weight in the trial was approximately 60 kg, which equates to a median dose closer to 50 mg per week than 25 mg per week. The AWMSG noted that the model was very sensitive to the assumed weekly cost of etanercept.
The number of clinic visits was informed by the British Association of Dermatologists (BAD) guidelines and length of hospital stay for patients who failed treatment was sourced from TA10397 in adults. A number of other model parameters were not discussed in the company’s submission but appeared to have been sourced from the original NICE MTA (TA10397) in adults. The use of adult data to populate the model and the implications of assuming transferability of adult data to inform the decision problem in children and adolescents did not appear to have been discussed by the company during the AWMSG appraisal.
The results of the company’s model showed that etanercept was both less expensive and more effective (i.e. etanercept was the dominant treatment strategy) than placebo in children and adolescents aged ≥ 8 years. Sensitivity analysis was poorly reported and it was uncertain whether or not probabilistic sensitivity analysis had been performed. The AWMSG considered it impossible to establish whether or not the base-case analysis represented the most plausible estimate of the cost-effectiveness of etanercept in this population based on the limited information provided in the submission. As a result, the AWMSG was unable to recommend etanercept for children and young people because of the uncertainties inherent in the economic model.
Janssen submission on the use of ustekinumab in children and young people
Within its submission supporting this appraisal, Janssen explored the possibility of constructing an economic model to assess the cost-effectiveness of ustekinumab for the treatment of moderate to severe psoriasis in children and young people. However, given the limited clinical evidence identified in its systematic review of effectiveness, Janssen decided not to pursue the development of an economic model. Janssen noted that the only previous economic evaluation in this population (i.e. for etanercept139) resulted in an adaptation of an adult model in psoriasis and relied on simplifying assumptions in the cost-effectiveness analysis. Therefore, because of the limitations of the evidence base, it concluded that any estimation of the cost-effectiveness of biologics in children and young people with psoriasis will be subject to a number of insuperable uncertainties and will largely be based on a number of assumptions taken from the adult population. Janssen’s submission did, however, provide an overview of the available evidence in children and adults to aid the development of an economic model by the AG. However, no cost-effectiveness results were presented in Janssen’s submission on children and young people.
AbbVie submission on the use of adalimumab in children and young people
AbbVie undertook a targeted review to identify publications and major health technology assessment bodies reporting cost-effectiveness analyses of the use of adalimumab in children and young people with plaque psoriasis. Its submission indicates that only one relevant study was identified. 141 This study estimated the number needed to treat to achieve a PASI 75 response based on a Bayesian NMA of efficacy outcomes for adalimumab, etanercept, infliximab and ustekinumab and evaluated the incremental cost per PASI 75 responder for the biological treatments during the first 10–16 weeks of treatment. Based on the results of this study, AbbVie indicated that adalimumab was found to be the most cost-effective treatment option in terms of incremental cost per PASI 75 responder compared with the other biologics. However, the AG notes that the study by Langley et al. 141 was not based on a population of children and young people and did not present the cost-effectiveness of the biologics in terms of costs and quality-adjusted life-years (QALYs) over a time horizon that was sufficiently long to capture differences between the interventions. Furthermore, the study has been published only in abstract form rather than as a full publication and therefore limited details are available to adequately critique the study. AbbVie’s submission did not include an economic model for the assessment of the use of adalimumab in children and young people.
Cost-effectiveness models in adults
Overview
Given that the literature review identified only one unpublished model assessing the cost-effectiveness of etanercept in children and young people and that this model was adapted from TA10397 in adults and largely populated with adult data, additional hand-searching of published documents associated with the previous NICE TAs of plaque psoriasis in adults was carried out. The aim was to examine existing decision-analytic models to identify important structural assumptions, highlight key areas of uncertainty and outline the potential issues associated with generalising evidence from the adult population to a population of children and young people.
The first NICE TA on biological therapies for the treatment of psoriasis was a MTA examining the cost-effectiveness of etanercept and efalizumab (Raptiva®, Serono Europe Ltd, London, UK) within their licensed indications in adults (TA103,97 published in July 2006). As part of this appraisal, the York Assessment Group developed a de novo cost-effectiveness model, which was subsequently referred to as ‘the York model’. Five subsequent STAs followed TA103:
-
TA134 – Infliximab for the Treatment of Adults with Psoriasis (published in January 2008)98
-
TA146 – Adalimumab for the Treatment of Adults with Psoriasis (published in June 2008)99
-
TA180 – Ustekinumab for the Treatment of Adults with Moderate to Severe Psoriasis (published in September 2009)100
-
TA350 – Secukinumab for Treating Moderate to Severe Plaque Psoriasis (published in July 2015)101
-
TA368 – Apremilast for Treating Moderate to Severe Plaque Psoriasis (published in November 2015). 102
All of these STAs employed a similar modelling approach to that used in the original York model in TA103. 97 The only study identified that deviated from the original model was the most recent STA of apremilast (TA368102), which included the modelling of sequences of treatment. Therefore, the main differences between the TAs lie in the evidence base, intervention and comparators rather than there being any major structural differences in the modelling approach used. A summary of the York model and the key differences between the assumptions and evidence base used in subsequent adaptations of the model are described in the following section. Table 51 provides an overview of the NICE TAs in adults.
| Characteristic | NICE TA | |||||
|---|---|---|---|---|---|---|
| ETA and EFA (TA10397) | INF (TA13498) | ADA (TA14699) | UST (TA180100) | SEC (TA350101) | APR (TA368102) | |
| Modelling approach | Markov model, which became known as the ‘York model’ | Based on the York model | Based on the York model | Based on the York model | Based on the York model but explicitly incorporates a decision tree for the trial period followed by a Markov model | Based on the York model but with treatment sequences |
| Intervention | EFA; ETA 25 mg b.i.w. continuous; ETA 50 mg b.i.w. intermittent | INF | ADA | UST 45 mg; UST 90 mg | SEC | Primary analysis: APR → ADA → ETA → BSC; subgroup analysis: APR → BSC; scenario analysis APR → ADA → ETA/UST → BSC |
| Comparators | Primary analysis: BSC; secondary analysis: CS, Fumaderm® (combination of fumaric acid esters), MTX, INF | EFA; ETA 25 mg b.i.w. continuous; ETA 25 mg b.i.w. intermittent; ETA 50 mg b.i.w. intermittent; BSC | INF; EFA; ETA 25 mg b.i.w. continuous; ETA 25 mg b.i.w. intermittent; ETA 50 mg b.i.w. intermittent; BSC | ADA; INF; EFA; ETA 25 mg b.i.w. continuous; ETA 25 mg b.i.w. intermittent; ETA 50 mg b.i.w. intermittent; BSC | ADA; UST; INF; ETA; BSC | Primary analysis: ADA → ETA → BSC; subgroup analysis: BSC; scenario analysis ADA → ETA/UST → BSC |
| Time horizon and justification | 10 years; justification NR | 10 years; sufficient time for all future costs and outcomes to be included | 10 years; based on the York model | 10 years; based on the York model | 10 years; time horizon reflective of the treatment duration of moderate to severe plaque psoriasis | 10 years; to maintain consistency with previous analyses and in the base case the majority of patients are on BSC by the end of 10 years |
| Cycle length | 12 months (not explicit) | 12 months | 12 months | 3 months | 12 months | 28 days |
| Discount rates | 6.0% costs, 1.5% QALYs | 3.5% | 3.5% | 3.5% | 3.5% | 3.5% |
| Mortality | Not considered | Not considered | Not considered | Not considered | Not considered | All-cause mortality incorporated |
| HRQoL instrument | DLQI scores mapped to EQ-5D utility values | Utilities from the York model | EQ-5D | DLQI scores mapped to EQ-5D utility values; SF-6D used in sensitivity analysis | EQ-5D | Utilities from the York model and EQ-5D; DLQI scores mapped to EQ-5D utility values |
| Link between utility and clinical efficacy | EQ-5D mapped from ΔDLQI by ΔPASI (coefficients not reported) | In the base case estimates from the York model were used, but only for those in the fourth quartile of the DLQI score (worst HRQoL). Additional analyses used utility values estimated by mapping SF-36 data collected in two trials to EQ-5D utility values using an unpublished mapping algorithm | EQ-5D association with DLQI and changes in PASI response rates estimated from trial data | EQ-5D mapped from ΔDLQI by ΔPASI (used a mapping algorithm based on the published scatterplot in the York model): EQ-5D = –0.0162 × DLQI + 0.8554 | Changes in EQ-5D from baseline at a given time point as a function of (1) PASI response at that time point, (2) baseline DLQI score difference from the pooled mean baseline DLQI score, (3) interaction between these terms | Changes in utility associated with changes from baseline PASI were taken from the York model for the DLQI score of > 10 population. For the DLQI score of ≤ 10 population, EQ-5D data collected in trials were used; direct link between %ΔPASI and ΔEQ-5D in patients with a DLQI score of ≤ 10. The same baseline utility score (0.7) from a published study was used for both populations |
| Total costs | Incremental vs. BSC: BSC £0; ETA 25 mg £7743; EFA £9382; ETA 25 mg continuous £9665; ETA 50 mg £14,860 | Incremental vs. BSC: ETA 25 mg continuous £1531; INF £4562 | Incremental vs. BSC: MTX –£3844; ciclosporin –£1987; BSC £0; ETA intermittent £4114; ETA high intermittent £4699; EFA £4942; ADA £4993; ETA £5058; INF £7736 | Incremental vs. BSC: BSC £0; EFA £5264; ETA 25 mg intermittent £3989; ETA 25 mg continuous £4829; ETA 50 mg continuous £5333; ADA £4660; UST £4615; INF £6327 | BSC £73,610; ETA 25 mg £75,788; SEC 300 mg £76,361; ADA 40 mg £76,981; UST 45 mg £79,544; UST 90 mg £79,732; INF 5 mg/kg £93,539 | DLQI > 10: apremilast sequence £89,374; comparator sequence £92,589 |
| Total QALYs | BSC: 0; ETA 25 mg 0.116; EFA 0.112; ETA 25 mg continuous 0.116; ETA 50 mg continuous 0.123 | ETA 25 mg continuous 0.089; INF 0.205 | Incremental vs. BSC: MTX 0.129; ciclosporin 0.079; ETA intermittent 0.11; ETA high intermittent 0.123; EFA 0.124; ADA 0.164; ETA 0.134; INF 0.182 | Incremental vs. BSC: EFA 0.1308; ETA 25 mg intermittent 0.1325; ETA 25 mg continuous 0.1409; ETA 50 mg continuous 0.1483; ADA 0.1502; UST 0.156; INF 0.1616 | BSC 0.97; ETA 25 mg 1.13; SEC 300 mg 1.36; ADA 40 mg 1.22; UST 45 mg 1.30; UST 90 mg 1.33; INF 5 mg/kg 1.36 | DLQI > 10: apremilast sequence 6.83; comparator sequence 6.69 |
Summary of the York model (TA103) and subsequent adaptations (TA134, TA146, TA180, TA350 and TA368)
The York model was a cohort Markov model that was developed to estimate the costs and QALYs of etanercept and efalizumab compared with BSC over a time horizon of 10 years (primary analysis). 97 A secondary analysis was also conducted to compare the interventions with additional systemic therapies of ciclosporin, Fumaderm® (combination of fumaric acid esters), methotrexate and infliximab. The model adopted the perspective of the UK NHS. The price year for costs was 2004–5 and an annual discount rate of 6% for costs and 1.5% for outcomes was applied (in line with NICE guidance142 at the time of the appraisal).
The model consisted of a two-part structure: a ‘trial’ period for initial response and a ‘treatment’ period for long-term response to treatment (Figure 8). The initial response period was used to determine initial response rates and the decision to continue treatment. The duration of the trial period was based on the period over which response was assessed in the efficacy trials for each treatment – this was 12 weeks for etanercept and efalizumab and between 10 and 16 weeks for the other systemic therapies. Individuals with a PASI 75 response were considered ‘responders’ and continued treatment after the trial period (i.e. they entered the treatment period), whereas individuals who were ‘non-responders’ discontinued treatment and received BSC. The treatment duration for responding individuals was based on an annual withdrawal rate of 20%. On withdrawal, individuals were assumed to receive BSC.
FIGURE 8.
Structure of the York model. Source: TA103. 97 © NICE 2016 Etanercept and Efalizumab for the Treatment of Adults with Psoriasis. Available from www.nice.org.uk/guidance/ta103. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
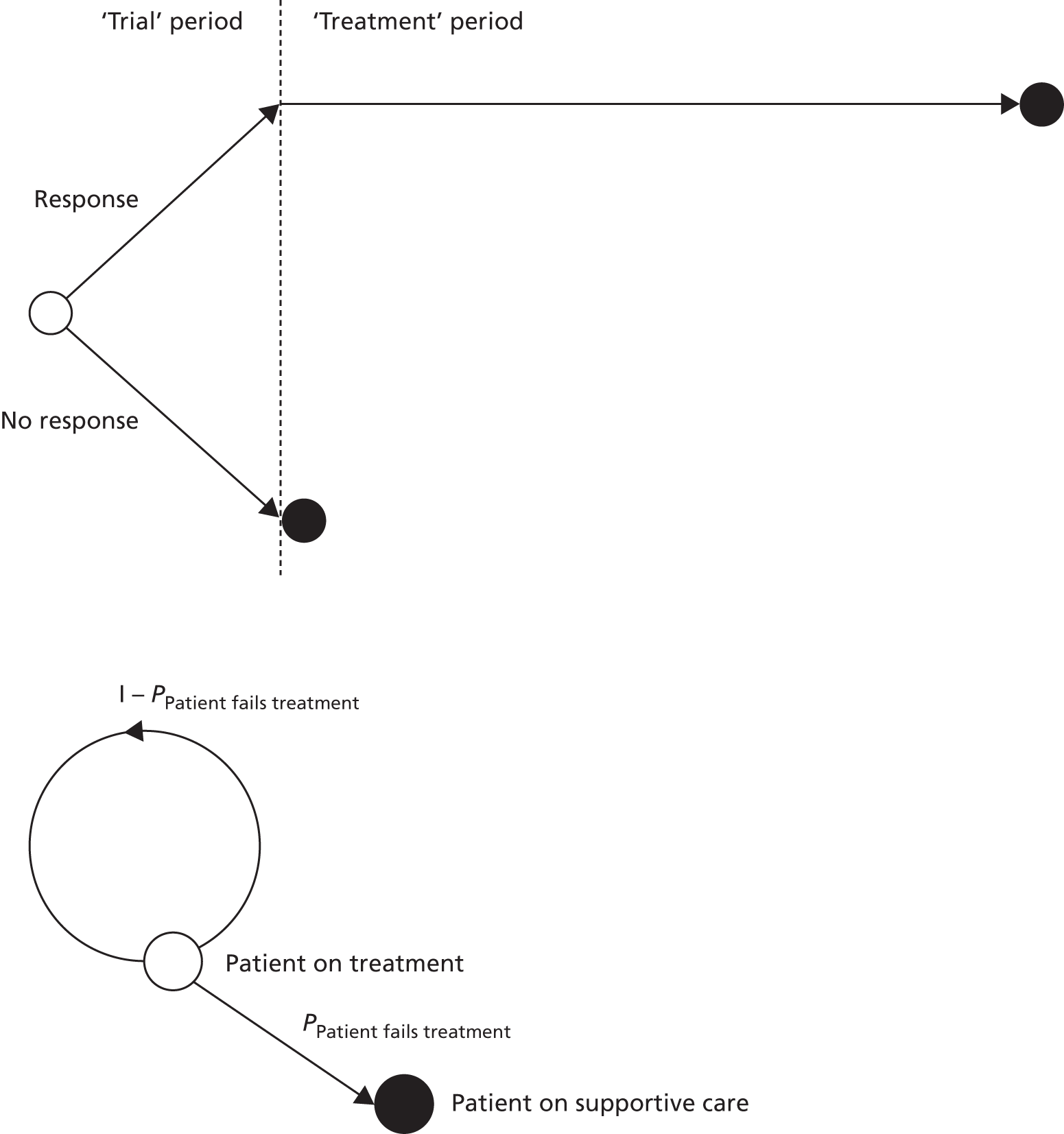
The base-case analysis considered a single line of therapy consisting of a biological treatment (etanercept or efalizumab) followed by BSC. Although specific sequences of treatments were not considered in the York model, an analysis showing the expected costs and QALYs associated with each treatment option compared with BSC was used to determine the most ‘cost-effective order’ in which to give the treatments, which varied according to the cost-effectiveness threshold.
The same model structure based on a single line of treatment was used in the subsequent TAs – TA134,98 TA146,99 TA180100 and TA350101 – and to a lesser extent in TA368,102 in which it was used only for the subpopulation of patients with a DLQI score of ≤ 10. The only difference in the modelling approach across the appraisals was a variation in the cycle length of the Markov model (12 months in TA134,98 TA14699 and TA350;101 3 months in TA180;100 and 28 days in TA368102), which was adapted to reflect the different length of the trial periods when treatment response was assessed. All appraisals used a time horizon of 10 years in their base-case analysis and used additional scenarios to show the implications of a change in the time horizon.
In TA368,102 the company adapted the model structure to allow a comparison of treatment sequences, with up to five sequential lines of treatment. The model structure followed the same approach as in the York model but, if the treatment response was considered inadequate at the end of the trial period, individuals moved into the trial period of the next line of treatment (or to BSC if the end of the treatment sequence had been reached). The company’s original economic model considered only apremilast as the first treatment in the sequence and compared different treatment sequences with apremilast as an additional line of therapy, rather than replacing an existing biological therapy in the sequence. However, following the ERG critique additional analyses were presented that compared the use of apremilast at different positions within a sequence.
Clinical effectiveness evidence in the York model and subsequent appraisals
The response rates used in the York model were based on a Bayesian NMA comparing the interventions with a broad range of comparators, including systemic therapies. An ordered probit model was used to predict PASI 50, PASI 75 and PASI 90 response rates, with PASI 75 used as the primary measure of response at the end of the trial period. If a trial reported only PGA 0/1 response (clear or almost clear) as the end point, it was assumed to be equivalent to the PASI 75 response. A similar Bayesian NMA was used in subsequent appraisals but was updated with additional evidence as more interventions and comparators became available. There were some differences across the appraisals in terms of how heterogeneity was accounted for in the meta-analysis and whether or not any adjustment had been made for differences in placebo response rates across the trials.
The effectiveness data were considered to be an area of uncertainty in the previous appraisals,97–102 mainly because of the lack of direct head-to-head comparisons between the biological treatments and the paucity of longer-term data. Although the evidence base expanded over time with many more RCTs included in the NMA, other concerns were raised relating to differences between the trial populations in the network (e.g. exposure to previous therapies and severity of disease). The definition of placebo or BSC across the different trials included in the NMA was also a contentious issue.
Health-related quality of life in the York model and subsequent appraisals
The utility values associated with treatment in the York model were based on the proportion of patients in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) and the change in utility from baseline associated with the PASI response category. Utility values were estimated based on a two-stage process:
-
Mean change in DLQI score between baseline and week 12 in the etanercept trials was estimated for patients with different levels of PASI response and different baseline DLQI scores. This analysis was facilitated by access to patient-level data from the trials and the placebo and treatment groups were pooled.
-
The DLQI data collected in the etanercept trials were then mapped onto EQ-5D values. This was achieved through access to data from the Health Outcomes Data Repository (HODaR), which included patients who had completed both the DLQI and EQ-5D. These data were used to map the change in DLQI score associated with PASI responses to changes in EQ-5D utility values.
The two-stage process was used to estimate average EQ-5D gains in utility from baseline for the different PASI response categories: 0.05 for PASI < 50, 0.17 for PASI 50–74, 0.19 for PASI 75–89 and 0.21 for PASI ≥ 90. Estimated gains in utility were also presented for individuals in the fourth quartile of the baseline DLQI score, that is, for patients with the worst baseline quality of life. The utility values from the York model were applied directly in TA368102 for the population with a DLQI score of > 10 and in TA13498 (values for the fourth quartile of the baseline DLQI score). For TA146,99 TA350101 and TA368102 (scenario analysis), the company had access to EQ-5D data collected in the trials, which were pooled across treatment groups and reported by PASI response category. For TA180,100 a similar modelling approach was used but the mapping algorithm used in the York model was applied to ustekinumab trial data to generate utility gains by PASI response category based on DLQI scores in the trial. The utility values applied in the TAs are summarised in Table 52.
| Key characteristics | Previous TA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TA10397 | aTA13498 | TA14699 | TA180100 | bTA350101 | TA368102 | ||||||||
| Populationc | All | Fourth-quartile DLQI score | Fourth-quartile DLQI score | All | DLQI > 10 | DLQI ≤ 10 | DLQI ≥ 10 | DLQI ≥ 10 | All | DLQI > 10 | DLQI ≤ 10 | ||
| Analysis | BC | SA | BC | SA | BC | SA | BC | SA | BC | BC | SA | BC | SA |
| BL PASI | – | – | – | – | – | – | – | – | 0.642 | 0.7 | 0.7 | 0.7 | 0.7 |
| Source | NR | NR | NR | NR | NR | NR | NR | NR | RCT | Revicki et al.143 | |||
| Incremental gain in utility from baseline | |||||||||||||
| PASI < 50 | 0.050 | 0.120 | 0.120 | 0.054 | 0.063 | 0.045 | 0.04 | 0.0016 | 0.109 | 0.05 | 0.0134 | 0 | –0.0024 |
| PASI 50–74 | 0.170 | 0.290 | 0.290 | 0.140 | 0.178 | 0.102 | 0.17 | 0.0424 | 0.193 | 0.17 | 0.0537 | 0.02 | 0.0275 |
| PASI 75–89 | 0.190 | 0.380 | 0.380 | 0.22 | 0.0970 | 0.226 | 0.19 | 0.1150 | 0.03 | 0.0256 | |||
| PASI ≥ 90 | 0.210 | 0.410 | 0.410 | 0.219 | 0.308 | 0.130 | 0.25 | 0.1276 | 0.264 | 0.21 | 0.1333 | 0.07 | 0.0704 |
| Source | Trial DLQI data by PASI category, mapped to EQ-5D utility values | TA10397 | Pooled trial EQ-5D data. Relationship with PASI established by mixed model | Trial DLQI data by PASI category, mapped to EQ-5D utility values using mapping algorithm from TA10397 | RCT sourced SF-6D data by PASI response category | Pooled trial EQ-5D data and a statistical model used to predict the change in HRQoL from BL by categories of PASI response | TA10397 | Pooled trial EQ-5D data by PASI response category | Pooled trial EQ-5D data by PASI response category | Pooled trial EQ-5D data by PASI response category. Included all available apremilast trial data available | |||
None of the TAs included a disutility associated with AEs from treatment. Only one TA considered a disutility from flare-ups associated with time off treatment (intermittent etanercept) (TA14699); however, the disutility value applied was not reported in the published documentation.
The modelling approach used in the previous TAs assumed that:
-
The PASI response is a perfect proxy for the change in utility arising from treatment. In other words, by conditioning on PASI response, utility is independent of treatment.
-
Similarly, if utility is conditioned on DLQI score change, then utility is independent of PASI response.
-
The relationship between DLQI score and utility is linear.
-
The impact of AEs on HRQoL is unimportant.
The main critique of the approach used in the York model relates to the uncertainty introduced by mapping from the DLQI to the EQ-5D, based on a small sample of 86 patients. The NICE Appraisal Committees favoured the use of EQ-5D data collected directly from the trials when available. Scenario analysis in TA10397 showed that the cost-effectiveness results were very sensitive to the selection of utility values, with greater QALY gains from treatment in the fourth quartile of the baseline DLQI score (subgroup with the worst baseline HRQoL) than in the overall trial population. Furthermore, as the utility values were conditioned on the PASI response at the end of the trial period, these values were extrapolated over the time horizon of the model, which is a key driver of differences between the treatments.
Resource use and costs in the York model and subsequent appraisals
Resource use and costs included in the York model and subsequent appraisals related to drug acquisition, administration and monitoring, outpatient visits and inpatient hospitalisation stays. The cost of tests to assess eligibility for biological treatment was excluded. With the exception of infliximab, all treatments were assumed to be self-administered. Drug costs were sourced from the most recent information in the British National Formulary (BNF). A range of monitoring and laboratory costs were considered, including for a full blood count, liver function tests and regular physician visits. No AE costs associated with treatment were included in the York model. TA350101 was the only appraisal in which the costs of AEs were considered. The rates of AEs applied were sourced from the secukinumab trials and published literature and included non-melanoma skin cancer (NMSC), other malignancies and severe infections.
Resource use and costs included in the earliest TAs (TA134,98 TA14699 and TA180100) mostly followed the assumptions of the York model and sourced their resource use and unit costs from TA103. 97 There were small differences in resource use for drug monitoring and administration between the TAs but these differences had only a minor impact on the cost-effectiveness results. Later TAs (TA350,101 TA368102) based their resource use and cost estimates on the NICE clinical guideline on psoriasis (CG15311) for the cost-effectiveness of second-line biologics and on the accompanying costing report. CG153 included the same categories of costs as the York model but expanded on them to better characterise the costs of BSC. The costs associated with BSC were identified as a key driver of the cost-effectiveness results in TA10397 and were considered to be an area of substantial uncertainty in subsequent TAs.
In all of the appraisals, non-responders to treatment were assumed to receive BSC (with the exception of TA368,102 in which the sequential use of treatments was considered and non-responders moved to BSC only when all other treatment options were exhausted). The costs associated with BSC differ across the appraisals as there was no clear guidance on what BSC consists of. Table 53 provides a summary of the resource use and costs relating to BSC included in each of the TAs, as well as those reported in CG153.
| Study | Treatments included as part of BSC | Outpatient visits (annual) | Day centre care (annual) | Hospitalisations (annual) | Reported total annual cost of BSC (£) |
|---|---|---|---|---|---|
| TA368102 | 45% of patients on MTX, 45% of patients on continuous CS, 16% of patients undergo 24 sessions of NBUVB per year | 10% of patients undergo five visits | All patients undergo five visits | 82% of high-need patients have one hospitalisation, with a mean LOS of 20.8 days; 18% of very high need patients have 2.55 hospitalisations, with a mean LOS of 53.04 days. The resulting weighted average yielded 26.6 days | 11,542.73 |
| TA350101 | 45% of patients on MTX (15 mg/week), 45% of patients on continuous CS (300 mg/day) for a maximum of 2 years, 3.84 sessions of NBUVB per year | 4 | 5 | 10.7 | 9015.00 |
| CG15311 | 45% of patients on MTX, 45% of patients on continuous CS for a maximum of 2 years, 16% of patients undergo 24 sessions of NBUVB per year | 10% of patients undergo five visits | All patients undergo five visits | 82% of high-need patients have one hospitalisation, with a mean LOS of 20.8 days; 18% of very high need patients have 2.55 hospitalisations, with a mean LOS of 53.04 days (average of 26.6 days) | 10,730.00 |
| TA180100 | No treatments | 2 | 0 | 21 | 6209.54 |
| TA14699 | No treatments | 2 | 0 | 21 | 5493.00 |
| TA13498 | No treatments | 18a | 0 | 21 | 7365.00 |
| TA10397 | No treatments | 2 | 0 | 0/21b | 113.20/5327.71 |
The cost of BSC for non-responders in the York model was limited to two annual outpatient visits in the base-case analysis, but included one annual hospitalisation with a 21-day length of stay (LOS) in a scenario analysis. The rate of hospitalisations for patients on BSC was based on expert opinion, with LOS sourced from Hospital Episode Statistics (HES) data and two local surveys. Subsequent TAs (TA134,98 TA14699 and TA18098) used the 21-day inpatient stay for BSC in their base-case analysis. In each of these appraisals it was assumed that no treatments were given as part of BSC.
National Institute for Health and Care Excellence CG15311 departed from this definition of BSC (see Appendix P of the guideline) because it was believed that it does not reflect what currently happens in clinical practice for patients who require a second-line biologic. In CG153 it was assumed that 45% of patients would receive treatment with methotrexate, 45% would receive continuous ciclosporin for a maximum of 2 years and 16% would undergo 24 sessions of NBUVB therapy per year while on BSC. Furthermore, it was reported in CG153 that patients meeting the eligibility criteria for biological therapy are generally high-need patients who use a sizeable number of health-care resources through inpatient admissions, lengthy hospital stays and frequent visits to day clinics for specialist-applied topical treatments and ultraviolet B (UVB) and who require monitoring for toxicity because of the use of systemic treatments. The NICE Guideline Development Group (GDG) sourced the resource use estimates for BSC from two published cohort studies of patients with a high level of need (i.e. those with severe psoriasis), which were conducted in tertiary dermatology units in the UK (n = 76)143 and the Netherlands (n = 67). 144 Both of these studies estimated the mean number of inpatient days in the year preceding initial treatment with biological therapy. In addition, estimates of LOS from a multicentre prospective service review based on four specialist dermatology centres in the UK145 were used in a scenario analysis. The GDG for CG153 emphasised that there is substantial variability in the long-term costs reported for patients with psoriasis. As a result, CG153 included extensive sensitivity analyses for the elements of cost associated with BSC. These included variations in the number of hospitalisations per year and the average LOS by level of need. The cost-effectiveness of second-line biological therapy compared with BSC in CG153 was highly sensitive to the assumptions about BSC.
The more recent TAs (TA368102 and TA350101) largely followed the resource use reported in CG153 for BSC (Table 54). The NICE Appraisal Committees for TA350 and TA368 noted that in both cases the costs of BSC were likely to have been overestimated. The committees considered that the patient population in CG153 and Fonia et al. 144 did not match that in the appraisals and reflected a sicker group of patients. In particular, Fonia et al. 144 described care in a tertiary care centre, which is known to be associated with treating the most severely affected cases of psoriasis. Furthermore, during the consultation process for TA368,102 the company provided NHS HES data that showed that the average LOS associated with BSC was 3.5 days. However, this was argued by the company to be an underestimate as it included patients with different disease severities and patients receiving concomitant medication. The duration of hospital stay for BSC in adults with moderate to severe psoriasis remains highly uncertain.
| Severity of psoriasis | Proportion of patients (%) | Number of admissions (annual) | Assumed average LOS (days) | Patient days in hospital | Number of bed-days per annum in the model | Base-case assumptions and variations in scenario analysis |
|---|---|---|---|---|---|---|
| High need | 82 | 1 | 20.8 | 20.8 | 26.6 | Base case: proportion of patients by level of need sourced from the study by Driessen et al.146 Average LOS taken from the study by Woods et al.145 for patients with a baseline PASI score of 10–20 (20.8 days). Number of hospitalisations calculated to match that in the study by Driessen et al.146 (average LOS for very high-need patients in the year prior to biological therapy 53 days) |
| Very high need | 18 | 2.55 | 20.8 | 53.0 | ||
| High need | 82 | 1 | 23.7 | 23.7 | 30.3 | Scenario 1: average LOS taken from Woods et al.145 for patients with a baseline PASI score of > 20 (23.7 days) |
| Very high need | 18 | 2.55 | 23.7 | 60.4 | ||
| High need | 70 | 1 | 20.8 | 20.8 | 30.5 | Scenario 2: 30% very high need |
| Very high need | 30 | 2.55 | 20.8 | 53.0 | ||
| High need | 95 | 1 | 20.8 | 20.8 | 22.4 | Scenario 3: 5% very high need |
| Very high need | 5 | 2.55 | 20.8 | 53.0 | ||
| High need | 82 | 0.25 | 20.8 | 5.2 | 13.8 | Scenario 4: aimed to match the estimates of average LOS in the study by Driessen et al.146 (53 days for patients with a LOS of ≥ 30 days and 14.9 days for the full study population) by changing the number of hospitalisations per year. However, the number of hospitalisations per year for the high-need patients would have to be 0.75 to yield an average LOS of 14.9 days, as reported in the study |
| Very high need | 18 | 2.55 | 20.8 | 53.0 | ||
| High need | 82 | 0.5 | 20.8 | 10.4 | 16.0 | Scenario 5: 0.5 hospitalisations for high-need patients and 2 hospitalisations for very-high-need patients |
| Very high need | 18 | 2 | 20.8 | 41.6 | ||
| High need | 82 | 1 | 20.8 | 20.8 | 20.8 | Scenario 6: 1 hospitalisation for all |
| Very high need | 18 | 1 | 20.8 | 20.8 | ||
| High need | 82 | 0.312 | 20.8 | 6.49 | 6.5 | Scenario 7: aimed to match the estimate of average LOS (6.49 days) in the study by Fonia et al.144 by changing the number of hospitalisations per year |
| Very high need | 18 | 0.312 | 20.8 | 6.49 |
Cost-effectiveness results from the York model and subsequent appraisals
The cost-effectiveness results for the base-case analyses in the previous NICE TAs in adults are summarised in Table 55, alongside the drivers of cost-effectiveness stated in the TA documentation. The results reported for TA134,98 TA146,99 TA180,100 TA350101 and TA368102 correspond to those in the company submissions.
| Appraisal | Previous adult TA | |||||
|---|---|---|---|---|---|---|
| ETA and EFA (TA10397) | INF (TA13498) | ADA (TA14699) | UST (TA180100) | SEC (TA350101) | APR (TA368102) | |
| Base-case analysis results [ICERs (per QALY)] | Incremental analysis:
|
Incremental analysis:
|
Incremental analysis (biologics only):
|
ICERs vs. BSC:
|
Incremental analysis:
|
APR sequence dominated the comparator sequence |
| Stated drivers of cost-effectiveness | Identified by scenario analysis in the AG report:
|
Identified by one-way sensitivity analysis in the MS:
|
Identified by scenario analysis in the MS:
|
Identified by one-way sensitivity analysis in the MS:
|
Identified by scenario analysis in the MS:
|
Differences in costs (mostly because of hospitalisation LOS for those on BSC) and outcomes for APR compared with BSC were the main drivers given the high assumed costs of BSC and the assumption of no PASI response for BSC |
In the base-case full incremental analysis for TA103,97 which compared etanercept in three dosing regimens (25 mg intermittent, 25 mg continuous and 50 mg intermittent), efalizumab and BSC, and assuming no hospitalisations for non-responders to biological treatment, BSC was the most cost-effective strategy at cost-effectiveness thresholds of < £66,703 per QALY gained. At a threshold of ≥ £66,703 per QALY gained, intermittent etanercept at 25 mg would be the cost-effective intervention, dominating (i.e. being less costly and more effective than) continuous etanercept at 25 mg and efalizumab. Intermittent etanercept at 50 mg had an incremental cost-effectiveness ratio (ICER) exceeding £1M per QALY gained compared with etanercept at a lower dose (25 mg intermittent).
Inclusion of 21 days of hospitalisation for non-responders to the biological drugs reduced the ICER for intermittent etanercept at 25 mg compared with BSC to £29,420 per additional QALY. When, in addition to the 21 days of hospitalisation, the estimates of utility gains per PASI response were sourced from the subgroup of patients in the highest (worst HRQoL) quartile of the baseline DLQI score (the group with the highest gain in utility from improvement in PASI score), the ICER for intermittent etanercept at 25 mg compared with BSC further reduced to £15,297 per QALY. In both of these scenario analyses, continuous etanercept at 25 mg and efalizumab remained dominated by intermittent etanercept at 25 mg, whereas the ICER for intermittent etanercept at 50 mg compared with intermittent etanercept at 25 mg decreased but not enough to make it cost-effective at commonly accepted cost-effectiveness threshold ranges. In the secondary analysis that compared the full range of systemic therapies (namely infliximab, methotrexate, ciclosporin and Fumaderm) and assumed 21 days of hospitalisation for non-responders, methotrexate dominated all interventions with the exception of infliximab, including BSC. Infliximab was more costly and more effective than methotrexate but the resulting ICER for this comparison exceeded £1M per QALY gained, with methotrexate emerging as the cost-effective intervention for this analysis.
The appraisals subsequent to TA103 (TA134,98 TA146,99 TA180,100 TA350101 and TA368102) all included a cost associated with hospitalisation for non-responders (LOS ranging from 10.7 to 26.6 days per annum). This generally resulted in more favourable cost-effectiveness estimates when biological therapies were compared with BSC. Consistent with the findings of the York model, the duration of hospitalisation for non-responders was identified as a key driver of cost-effectiveness for biological therapies across the appraisals. The base-case analysis in the majority of the appraisals98–100 used estimates of utility gains by PASI response in a subgroup with lower baseline HRQoL (TA134, TA146 and TA180), leading to higher QALY gains for the most effective drugs in terms of PASI response. This parameter can be considered the second driver of cost-effectiveness in the adult models.
The base-case cost-effectiveness results in the company submissions for infliximab, adalimumab and ustekinumab (TA134,98 TA14699 and TA180100 respectively) place the ICERs for these drugs at the upper end of the currently accepted NICE cost-effectiveness threshold range, as long as the assumptions about HRQoL and the costs of hospitalisation for non-responders referred to above hold. The estimates of cost-effectiveness for secukinumab and apremilast presented by the manufacturers in TA350101 and TA368102 were considered overly optimistic by the NICE Appraisal Committees and were largely driven by the costs of BSC in non-responders to biological therapy. These costs were considerably higher than in previous appraisals because of the assumption of a higher consumption of health-care resources by non-responders, in line with CG15311 (see Table 54).
The National Institute for Health and Care Excellence recommended the following biological treatments in adults with psoriasis: efalizumab,97 etanercept,97 infliximab,98 adalimumab,99 ustekinumab100 and secukinumab. 101 With the exception of infliximab the biological treatments were recommended for severe psoriasis, defined as a baseline PASI score of ≥ 10 and a DLQI score of > 10 in patients who had previously failed or who had a contraindication/intolerance to non-biological systemic therapy. The recommendation for efalizumab further required that patients had failed on etanercept or had a contraindication/intolerance to the drug. Efalizumab is no longer marketed in the UK.
Infliximab was recommended only for very severe psoriasis, defined as a baseline PASI score of ≥ 20 and a DLQI score of > 18 in patients who had previously failed or who had a contraindication/intolerance to non-biological systemic therapy. The recommendation for ustekinumab and secukinumab was conditional on the availability of Patient Access Schemes. The Patient Access Scheme for ustekinumab guarantees a flat price for 45 mg and 90 mg of ustekinumab so that the 90-mg dose is provided at the same price as the 45-mg dose for patients weighing > 100 kg, whereas the Patient Access Scheme for secukinumab consists of a confidential discount over the drug list price.
The NICE recommendations for all of these biological treatments require treatment termination if a response is not produced at the end of the ‘trial’ period (12 weeks for etanercept and secukinumab, 10 weeks for infliximab and 16 weeks for adalimumab and ustekinumab). Treatment response is defined as achieving a PASI 75 or PASI 50 response accompanied by a 5-point reduction in DLQI score from baseline.
Summary of the key areas of uncertainty in adult models and motivation for a de novo model in children and young people
There are no studies comparing the cost-effectiveness of biological therapies for plaque psoriasis in children and young people. Furthermore, none of the companies participating in this appraisal have submitted an economic evaluation in this population. Our review of previous NICE TAs of plaque psoriasis in adults was conducted to examine existing decision-analytic models and identify important structural assumptions and highlight key areas of uncertainty and the potential issues associated with generalising evidence from the adult population to a population of children and young people. In this section we summarise the key areas of uncertainty identified in adults in light of potential implications for the de novo model in children and young people.
Model structure
Although in clinical practice, treatment with biological therapy is expected to be sequential, that is, patients are switched to further lines of biological therapy on failure of the first-line biological therapy, the majority of the TAs did not consider treatment sequencing. Lack of evidence to inform treatment sequencing, especially on the efficacy of the treatments depending on the previous therapies received, appeared to be the main reason for not formally modelling treatment sequences in all but one appraisal (TA368102). Given that there is very limited evidence to support the cost-effectiveness of the sequential use of treatments in adults and that no evidence exists in children and young people (see Chapter 3), any attempt to model treatment sequences in the population of children and young people will be highly uncertain.
Clinical effectiveness evidence
Because of a lack of head-to-head trials comparing the biological treatments with each other, NMA was used to compare the treatments with each other indirectly. There was concern that not all trial populations matched those of the decision problem because of variation in the inclusion criteria, with some trials not explicitly excluding individuals who had not failed non-biological systemic therapy. Placebo or BSC was not defined consistently across the trials, which introduced heterogeneity in placebo response rates. Similar issues were identified in the clinical effectiveness evidence for children and young people (see Chapter 3), with the evidence base even more sparse, with only three RCTs and no common comparator across the trials. Chapter 4 describes how NMA was used to expand the evidence base in children and young people by drawing strength from the full network of evidence available for adults, while attempting to account for heterogeneity between trial populations (i.e. children and young people vs. adults) and placebo response rates.
Long-term response and withdrawal rates
To extrapolate data beyond the clinical trials, previous appraisals in adults have assumed that responders to treatment maintain their PASI response rate over time until treatment withdrawal. The same all-cause withdrawal probability of 20% per annum has been assumed for all biological therapies in the absence of any long-term withdrawal data. Given the paucity of long-term data in children and young people, this parameter will also be uncertain in this population.
Health-related quality of life
Most of the previous TAs in adults used utility values based on an estimate of the relationship between PASI response rates and changes in DLQI scores mapped onto EQ-5D utility values. Although some TAs applied EQ-5D data collected directly in RCTs, this was limited to data collected in the trials sponsored by the companies and no evidence synthesis methods were used to synthesise the utility estimates. The estimates of utility gains from treatment were variable across subgroups of patients defined by baseline DLQI score, with greater gains achieved for individuals with worse baseline HRQoL. The size of the utility gains in previous appraisals was considered to be largely uncertain and it represented a key driver of the cost-effectiveness results. It is expected that utility gains associated with treatment will also be highly uncertain in the population of children and young people because of an absence of EQ-5D data in this population. In Chapter 6, a review of HRQoL data in children and young people is reported. Scenario analyses are used to explore the impact of uncertainty on the cost-effectiveness results.
Resource use and costs
The resource use and costs associated with BSC has been one of the key drivers of cost-effectiveness in adult appraisals. In particular, the duration of and costs associated with inpatient hospitalisation stays for individuals who do not respond adequately to treatment have been highly uncertain. Until the publication of CG153,11 the resource use and costs associated with BSC in adult TAs were largely informed by assumptions and expert opinion. The two TAs that followed the guideline (TA350101 and TA368102) supplemented it with resource use data from cohort studies of patients treated for psoriasis with biological treatments. However, the patient population from whom the data were collected was likely to reflect a sicker population than that defined by the NICE scope for these appraisals and the uncertainty associated with the estimates was not sufficiently explored. The search described in Chapter 5 (see Methods) did not identify any evidence on the resource use and costs involved in BSC in children and young people. The use of evidence in adults supplemented by clinical expert opinion to inform the costs of BSC in children and young people is discussed in depth in Chapter 6. Scenario analyses are used to explore the implications for the cost-effectiveness results of uncertainty in the assumptions made about BSC, particularly in relation to hospitalisation LOS.
Each of these areas of uncertainty is considered in more detail in the following chapter as part of the decision-analytic model developed to evaluate the cost-effectiveness of adalimumab, etanercept and ustekinumab in children and young people.
Chapter 6 Independent economic assessment
Introduction
The review of cost-effectiveness evidence in the population of children and young people and the absence of company models highlights the challenges of developing an economic model in this population. The fundamental challenge is the limited clinical evidence base for both short- and long-term outcomes to inform a model. Therefore, any estimation of the cost-effectiveness of biological therapies in children and young people will be subject to a number of uncertainties. These uncertainties cannot be avoided but a clear and transparent approach, that highlights the assumptions entering the economic model, can be pursued to help the decision-maker assess the cost-effectiveness of biological therapies in this population.
Plaque psoriasis is a chronic non-progressive disease that manifests itself in children and young people in a similar manner to that in adults. The main difference between the younger population and adults is the presence of comorbidities in adults (such as high blood pressure, liver impairment and renal impairment), which tend to make adults less well with psoriasis than a younger population. Currently, there is no treatment pathway specific to psoriasis in children and young people in the UK. The management of treatment and approach to care seems to mirror that used in adults. Our clinical advisor, Dr Ruth Murphy, indicated that when there is an absence of evidence it would be reasonable to extrapolate data from the adult population to children and young people. The company submission for ustekinumab also supports this approach for the development of an economic model given that there are few significant differences in the posology or management of chronic plaque psoriasis in children, young people and adults.
The management and treatment of plaque psoriasis depends on the extent and severity of an individual’s disease, local custom and practice. If an individual patient does not respond to or tolerate a particular treatment option, an alternative one is usually tried. This means that treatments are usually ‘trialled’ on an individual basis until an effective option is found. If an effective treatment is not found, then a patient will receive some form of BSC. This approach to treatment appears to be the same for children and young people and adults, but usually more caution is exercised in the younger population because of the limited availability of licensed treatment options.
The trialling of treatments on treatment failure or intolerance suggests that sequences of treatments could be considered in an cost-effectiveness model, whereby after failure of a first treatment option patients are trialled on a second option and so on, until all options are exhausted. However, this would require additional clinical evidence on the efficacy of the treatments conditional on the previous therapies received. There is very limited evidence to support the cost-effectiveness of the sequential use of treatments in adults and no evidence exists in children and young people (see Chapter 3). Therefore, although the model should ideally explore the sequential use of treatments, any attempt to do so in the population of children and young people would be highly uncertain. Furthermore, the optimum treatment sequence may not be suitable for an individual patient with specific characteristics and when treatment in this population is usually tailored to the child or adolescent because of needle phobia or the presence of psoriatic arthritis. Therefore, an alternative approach may be better whereby the optimum ordering of treatments, in terms of their cost-effectiveness, is established. This can be achieved by comparing each of the alternative treatment options with BSC and then indicating the most cost-effective order in which to give the therapies based on total expected costs and QALYs associated with each treatment option.
The previous York model appears to be the most widely accepted model of chronic plaque psoriasis. 97 The five NICE TAs that followed TA103 for the treatment of moderate to severe psoriasis in adults98–102 followed the framework of the York model and these have been accepted by NICE as being relevant to plaque psoriasis. The main changes that have followed since the advent of the York model have been the availability of new evidence, the methodology for linking efficacy estimates to HRQoL utility values, the parameters used in the model to inform BSC, updated unit costs, time on treatment and the modelling of treatment sequences in the most recent appraisal of apremilast. 102 It would therefore seem appropriate that the same modelling framework is used for children and young people but with an evidence base informed by outcomes in the younger population. Hence, the structure of our model is very similar to that used in previous TAs in adults and, when evidence is lacking or limited in the population of children and young people, data have been extrapolated from the adult population and supplemented by expert opinion.
Decision problem and patient population
The decision problem addresses the cost-effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people. The population in the model reflects the marketing authorisations of the three interventions. However, the marketing authorisations differ in terms of the age of the population and the severity of psoriasis at baseline and also in terms of the positioning of the biologic in the pathway of care.
A stepwise approach to treatment for the management of plaque psoriasis is usually pursued in which topical therapies are offered as first-line treatment followed by phototherapies and/or systemic non-biological therapies such as methotrexate as second-line treatment and then biological treatments as third-line treatment when previous therapies have been found to be ineffective. However, adalimumab is licensed in a paediatric population for individuals who have an inadequate response to, or who are inappropriate candidates for, topical therapy and phototherapies, whereas etanercept and ustekinumab are licensed for individuals who are inadequately controlled by, or who are intolerant to, other systemic therapies or phototherapies. Therefore, adalimumab is the only biological treatment indicated in the population of children and young people who have not failed previous systemic therapies.
The biological interventions also differ in their marketing authorisations by age and severity of psoriasis (Figure 9). Both adalimumab (age ≥ 4 years) and etanercept (age ≥ 6 years) are indicated for younger ages and severe psoriasis, whereas ustekinumab is indicated for an adolescent population (age ≥ 12 years) and moderate to severe psoriasis. The definition of severity differs in the corresponding trials of the biologics in children and young people (Table 56). In adults, severe psoriasis is defined by a total PASI score of ≥ 10 and a DLQI score of > 10. However, there is not a clear consensus on the definition of moderate or severe psoriasis in children and young people. This is partly because the PASI has not been validated as a disease severity assessment tool for use in this population and no other tool is available. Mean PASI scores at baseline in the trials were 18.6 for etanercept, 18.3 for adalimumab and 21.1 for ustekinumab. Therefore, although the licence for ustekinumab includes those with moderate to severe psoriasis, patients in the ustekinumab trial (CADMUS) had a disease severity that was more comparable to that in patients with severe disease in the etanercept trial (20030211) and adalimumab trial (M04-717). Hence, the population in the model was chosen to reflect severe psoriasis as defined by the baseline characteristics of the populations in the trials of children and young people.
FIGURE 9.
Marketing authorisations of biological therapies in children and young people by age and severity.
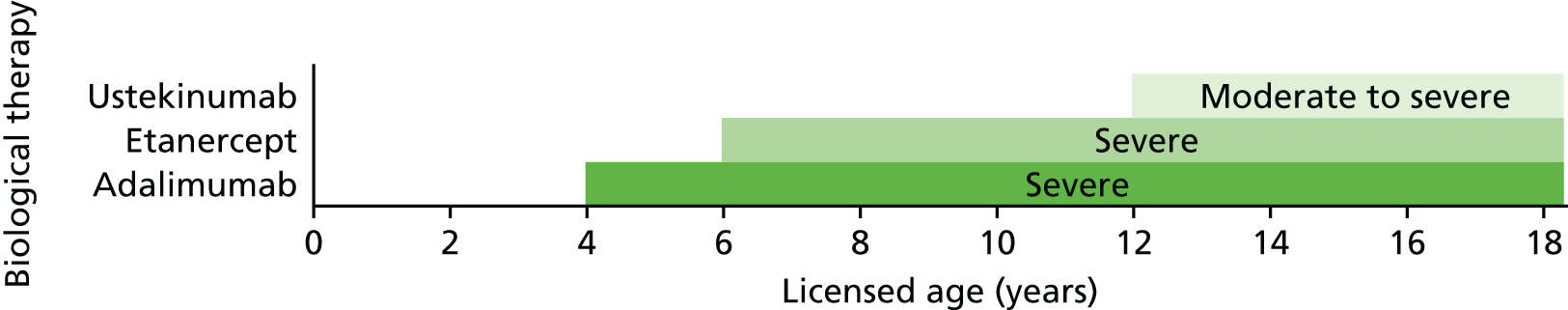
| Marketing authorisation | Etanercept | Ustekinumab | Adalimumab |
|---|---|---|---|
| Licence | Severe chronic plaque psoriasis | Moderate to severe plaque psoriasis | Severe chronic plaque psoriasis |
| Trial population | Moderate to severe plaque psoriasis with baseline PASI score of ≥ 12, PGA score of ≥ 3 and involvement of ≥ 10% of BSA | Moderate to severe plaque psoriasis with baseline PASI score of ≥ 12, PGA score of ≥ 3 and involvement of ≥ 10% of BSA for ≥ 6 months | Severe plaque psoriasis with baseline PASI score of ≥ 20, PGA score of ≥ 4 and involvement of ≥ 20% of BSA or very thick lesions and involvement of ≥ 10% of BSA or baseline PASI score of ≥ 10 and one of the following: (1) active psoriatic arthritis non-responsive to non-steroidal anti-inflammatory drugs, (2) clinically relevant facial involvement, (3) clinically relevant genital involvement, (4) clinically relevant hand or foot involvement or (5) CDLQI score of > 10 |
To reflect the differences in marketing authorisation by age and positioning of treatment in the pathway, three separate populations were considered in the base-case cost-effectiveness analysis:
-
Before systemic therapy – children and young people aged 4–17 years with adalimumab as the only licensed intervention for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, topical therapy and phototherapies, that is, as an alternative to systemic therapies.
-
After systemic therapy (1) – children and young people aged 6–11 years with adalimumab and etanercept for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies.
-
After systemic therapy (2) – children and young people aged 12–17 years with adalimumab, etanercept and ustekinumab for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies.
The population aged 4–5 years with adalimumab as the only licensed intervention for the treatment of severe plaque psoriasis after systemic therapy was not considered as a separate population because no children aged < 6 years were included in the adalimumab trial (M04-717); therefore, efficacy estimates for this age group were assumed to be the same as those for children aged 6–11 years, which results in similar cost-effectiveness estimates for adalimumab compared with BSC for ages 6–11 years.
The starting age used in the model was 4 years, 6 years and 12 years for the three populations described above respectively. The time horizon of the model extended until individuals reached 18 years of age. At this point, the population reaches adulthood and separate NICE recommendations for the use of the interventions in adults apply. The differences in the marketing authorisations of the interventions by age inevitably mean that the time horizon of the model differs according to the population. To explore the impact of the time horizon, a separate scenario analysis is presented that considers a common time horizon of 14 years for all populations. The time horizon of 14 years (which is greater than the time horizon of 10 years used in previous TAs in adults) is sufficient to capture differences in costs and effects between the interventions under comparison.
Intervention and comparators
The interventions considered in the cost-effectiveness analysis were adalimumab, etanercept and ustekinumab within their marketing authorisations. The following comparators were considered in the NICE scope:147
-
Non-biological systemic therapy (including, but not limited to, ciclosporin and methotrexate).
-
Topical therapy (for people in whom non-biological systemic therapy is not suitable), that is, BSC.
-
Biological treatments used outside their marketing authorisation (such as infliximab, adalimumab, etanercept or ustekinumab if used outside the constraints of the relevant marketing authorisation in children and young people).
-
When appropriate, adalimumab, etanercept and ustekinumab will be compared with each other.
Because of the positioning of adalimumab in the stepwise management of psoriasis, non-biological systemic therapy is only a relevant comparator for adalimumab as it is the only licensed intervention representing an alternative to systemic therapy; etanercept and ustekinumab are licensed for individuals who are inadequately controlled by, or who are intolerant to, previous systemic therapies. Standard systemic therapies such as methotrexate, ciclosporin and acitretin are not licensed for psoriasis in children and young people. However, it is evident from the UK audit of the assessment and management of psoriasis in children that 19% of children have received systemic drugs (9% methotrexate, 5% acitretin, 4% ciclosporin and 1% dapsone) outside their licensed indications. 12,148 The non-biological systemic therapy considered as a comparator in the cost-effectiveness analysis for adalimumab is methotrexate as it is the most widely used systemic therapy in the population of children and young people and was used as a comparator in the M04-717 trial.
If biological treatments are found not to be effective, individuals are usually offered some form of BSC rather than no treatment. Therefore, BSC is considered a relevant comparator for individuals who have exhausted all treatment options including conventional systemic therapy and phototherapy. BSC tends to include a mix of active non-biological systemic therapies such as methotrexate and ciclosporin and palliative care, including phototherapy, even though these treatments may have been proven to be largely ineffective.
The interventions of etanercept, adalimumab and ustekinumab were compared with each other as appropriate to the licensed population. The use of these interventions outside the age constraints of their licence (e.g. the use of etanercept in children aged < 6 years and ustekinumab in children aged < 12 years) was considered relevant in a scenario analysis. The use of other off-label biological treatments such as infliximab outside its licensed indication in adults was not considered. Advice from our clinical expert (Dr Ruth Murphy, personal communication) suggested that it is very unlikely that an unlicensed TNF inhibitor would be used as an alternative to a biological treatment that is licensed and available in this population. Furthermore, there are no RCTs comparing the use of infliximab with the use of any comparator (or placebo) in the population of children and young people. Infliximab also requires intravenous infusion in hospital and our clinical expert suggested that this is not a favourable option in this young population.
The biosimilar of etanercept, namely Benepali (50 mg), is not licensed for use in children and young people. Therefore, the biosimilar was not considered a relevant comparator in the base-case analysis. However, a scenario analysis was considered in which the drug cost of etanercept was reduced by approximately 10% to match the cost of Benepali in adults (£164.00 per prefilled syringe). 149
The drug doses for the interventions and comparators considered in the cost-effectiveness analysis are shown in Table 57. These are based on licensed doses for etanercept, adalimumab and ustekinumab and expected doses for methotrexate and BSC. Continuation of treatment was conditioned on response to treatment at the end of the trial period, corresponding to the time point specified in the Summary of Product Characteristics for children and young people. For etanercept and adalimumab, this was 12 and 16 weeks respectively. For ustekinumab, the Summary of Product Characteristics specifies that consideration should be given to discontinuation if there is no response up to 28 weeks. In the analysis, the time point for response to ustekinumab was taken to be 16 weeks, corresponding to its administration at 12 weeks after the dose given at 4 weeks. This is the same time point that was used to assess response to ustekinumab in adults (TA180100). It was assumed that all treatments are used continuously in responders to treatment until treatment withdrawal. Ciclosporin (used as part of BSC) was assumed to have a maximum treatment duration of 2 years.
| Treatment | Dose | Response assessment (weeks) |
|---|---|---|
| Etanercept | 0.8 mg/kg up to a maximum of 50 mg weekly for up to 24 weeks | 12 |
| Adalimumab | 0.8 mg/kg up to a maximum of 40 mg at weeks 0 and 1, then every 2 weeks thereafter | 16 |
| Ustekinumab | 0.75 mg/kg for those weighing < 60 kg, 45 mg for those weighing 60–100 kg and 90 mg for those weighing > 100 kg at weeks 0 and 4, then every 12 weeks thereafter | 16 |
| Methotrexate | 0.1–0.4 mg/kg weekly | 16 |
| Ciclosporin (as part of BSC) | 2–5 mg/kg daily for up to 2 years | Not applicable |
Methods
Overview
A de novo decision-analytic model was developed to estimate the cost-effectiveness of adalimumab, etanercept and ustekinumab for the treatment of plaque psoriasis in children and young people. The cost-effectiveness model consists of a Markov cohort transition model developed in Microsoft Excel® (2013; Microsoft Corporation, Redmond, WA, USA). The structure of the model is very similar to that used in previous TAs of moderate to severe plaque psoriasis in adults. The model was developed in accordance with the NICE reference case. 150 The time horizon of the model extends until individuals reach 18 years of age, when they then become adults and current NICE recommendations for the use of the interventions in adults apply. The length of the time horizon varies by the starting age of individuals in the model. As indicated previously, three starting ages were considered in the model to reflect the restrictions of the marketing authorisation of the interventions.
The outcomes of the model are expressed using QALYs. The QALY provides a summary measure combining estimates of the remaining length of life (life-years) with the associated quality of life. QALYs are derived by multiplying a utility value (quality of life) by the time spent with this utility (length of life). The utility values used in the model were generated from PedsQL trial data using a mapping algorithm to convert them to EQ-5D utility values. The utilities associated with treatment were based on the proportion of individuals in the different PASI response categories (see Health-related quality of life). All costs were considered from the perspective of the NHS and Personal Social Services (PSS). Health-care resource use and cost categories include the cost of treatment (acquisition, administration, monitoring and AE costs) and changes in health service resource use because of loss of response to treatment (see Best supportive care costs).
The parameters for the model were sourced from published literature, information reported in the company submissions and the results of the evidence synthesis described in Chapter 4. Both costs and QALYs were discounted at 3.5% per annum, in line with current NICE guidance. 150
Model structure and assumptions
The model consists of four health states: ‘trial period’, ‘continued use’, BSC and death (Figure 10). Individuals enter the model in the trial period and receive one of the three biological interventions or a relevant comparator. The length of the trial period is dependent on the intervention and can last from 12 weeks for etanercept to 16 weeks for adalimumab and ustekinumab, corresponding to the time point at which response to treatment is assessed. The cycle length in the model corresponds to 28 days (4 weeks), which takes account of the different lengths of time spent in the trial period.
FIGURE 10.
Schematic of the model structure.
At the end of the trial period, individuals are assessed as responders or non-responders to treatment based on PASI response rates. PASI response in the base-case analysis is taken to be PASI 75, that is, response is assessed based on whether or not an individual achieves a 75% reduction in baseline PASI score. Individuals who do not have an adequate response to treatment at the end of the trial period move to BSC. Individuals who are considered responders to treatment transition to the health state of continued use, remaining in this state until they withdraw from treatment and move to BSC. During the period of continued use, individuals continue to receive the active therapy and are assumed to maintain their level of PASI response until treatment discontinuation from any cause, such as lack of efficacy, the presence of AEs or non-compliance to treatment (modelled together as an overall risk of all-cause withdrawal).
On treatment discontinuation (in either the trial period or the continued use state), individuals transition to BSC. BSC consists of non-biological supportive therapies. The only transition out of the BSC state is to the ‘death’ state. Death is an all-cause mortality state to which transition is possible from any health state. Mortality is not conditioned on treatment or treatment response. Mortality rates by age were sourced from life tables in England and Wales for the years 2013–15151 and averaged across sexes.
Effectiveness data
The measure of treatment effectiveness used in the model was the proportion of individuals achieving a specific threshold of PASI response relative to baseline. Relative change in PASI response is the most widely reported outcome in clinical trials and has been used as the main outcome in previous models in adults. The PASI response rates used in the model were taken directly from the children and young people efficacy estimates from the NMA that incorporated all relevant adult evidence (see Chapter 4, Framework of analysis for informing the relative efficacy of the interventions). Scenario analyses were also conducted in which the results from the unadjusted baseline constrained model with minimum adult evidence (see Chapter 4, Framework of analysis for informing the relative efficacy of the interventions) were applied in the model; partial comparisons with direct trial data and the indirect comparison (see Chapter 4, Indirect treatment comparison) were also incorporated in scenario analyses for completeness. None of the three trials of biological therapies in children and young people with psoriasis required previous failure on non-biological systemic therapy as an inclusion criterion. Therefore, it was assumed in the model that treatment effectiveness is independent of failure on non-biological systemic therapy prior to starting biological therapy. It is unknown how the position on the care pathway is likely to affect treatment effectiveness. In the base-case analysis, PASI 75 response rates were taken as the measure of effectiveness for treatment continuation. Individuals who meet the threshold of PASI 75 are classified as responders at the end of the trial period and are assumed to maintain their response for as long as they are in the health state of continued use. In a separate scenario analysis, the threshold of PASI 50 was taken as the measure of effectiveness for treatment continuation.
The PASI response rates from the NMA were also used in the model to inform the HRQoL utility values. Gains in utility associated with treatment were conditioned on PASI response rates (see Chapter 5, Health-related quality of life in the York model and subsequent appraisals), an approach that has been taken in previous models for the treatment of psoriasis in adults. PASI response rates for BSC were assumed to be equivalent to those for placebo in the NMA.
In the absence of data to model time-varying transition probabilities, response rates were assumed to be constant per cycle in the model. The response rates used to inform the model are presented later in this chapter (see Table 70). The uncertainty in the predicted response rates from the NMA was reflected in the model by directly exporting the simulated posterior distributions from the Markov chain Monte Carlo analysis in WinBUGS to the cost-effectiveness analysis, preserving any correlations in the data.
Treatment withdrawal rates
Responders to treatment were assumed to maintain their response until treatment discontinuation. Discontinuation was modelled as an overall risk of withdrawal from any cause, such as lack of efficacy, the presence of AEs or non-compliance to treatment. Previous TAs in adults assumed a constant withdrawal rate of 20% per annum for all treatments.
A literature search, described in Chapter 3, was conducted with the aim of identifying registry data on long-term treatment response to biologics in children and young people with psoriasis. Two registries were identified: Child-CAPTURE90 (Netherlands) and DERMBIO92 (Denmark). However, none of the published studies from these registries allowed the estimation of long-term withdrawal rates in individuals who are responders to treatment; in addition, the DERMBIO registry included only a small number of children. The data indicated that there was no significant predictive relationship between age and treatment continuation, which may suggest that treatment withdrawal rates used in the adult population can be extrapolated to children and young people in the absence of any alternative source of data. Data from the DERMBIO registry suggest that the withdrawal rate on biological therapies is constant over the treatment period (with no obvious plateau),92 which supports the use of a constant withdrawal rate over time.
A recent study on the long-term drug survival rates of four biologics (adalimumab, etanercept, infliximab and ustekinumab) based on data from the UK BADBIR audit of 3523 biologic-naive adult patients indicated that loss of efficacy is a major reason for treatment discontinuation, with efficacy decreasing from 77% in the first year of use to 53% in the third year of use. 94 This is consistent with a withdrawal rate of 20% per annum, which has been used in previous TAs in adults. This study also suggested that there may be differences in the withdrawal rate by treatment, with ustekinumab having a significantly higher survival rate than adalimumab and etanercept. However, the study did not distinguish between discontinuation because of a lack of treatment response in the short term, that is, during the initial trial period, and discontinuation because of a lack of treatment response in the long-term for patients who are responders to treatment. Therefore, the differences in withdrawal rates by treatment may reflect the higher efficacy of ustekinumab than adalimumab and etanercept, rather than reflecting differences between the treatments conditional on response at the initial assessment point.
In the absence of sufficient evidence on the long-term withdrawal rates in children and young people, and given that observational data generally suggest that a constant 20% annual withdrawal rate is a reasonable assumption in adults, the same withdrawal rate was assumed in the model (this rate equates to a 28-day discontinuation rate of 1.70% per cycle).
All-cause mortality
All-cause mortality was incorporated in the model by applying a risk of death during each cycle. The mortality risk was assumed to be independent of response status or treatment received. A common mortality risk was thus assumed for all patients based on the general population mortality risk. The general population mortality risk was obtained from sex-specific life tables for England and Wales for the period between 2013 and 2015, with the risk averaged across males and females, assuming equal proportions. 151
Health-related quality of life
Review of utility data in children and young people with psoriasis
A systematic literature review was conducted to identify utility values for plaque psoriasis in children and young people. The aim of the search was to identify any studies that reported utility values or other measures of HRQoL that could be converted into utility values specifically for the population of children and young people.
The search strategy was developed in MEDLINE (via Ovid) by an information specialist with input from the project team. The strategy included terms for psoriasis combined, using the Boolean operator AND, with terms for quality of life/utilities or named instruments. No language, geographical or date limits were applied. A search filter to limit retrieval to quality-of-life studies was used when available. The search strategy was adapted for use in the other resources searched. Full search strategies can be found in Appendix 1.
The following databases were searched on 12 July 2016: MEDLINE [including MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)], the Cost-effectiveness Analysis (CEA) Registry [see https://research.tufts-nemc.org/cear4/ (accessed 10 August 2017)] EMBASE, PubMed and the School of Health and Related Research Health Utilities Database (ScHARRHUD) [see www.scharrhud.org/ (accessed 10 August 2017)].
The Health Economics Research Centre (HERC) database of mapping studies from the University of Oxford152 was also searched to identify any suitable mapping algorithms that would allow conversion of clinical measures routinely collected in studies of psoriasis in children and young people into utility values.
The results from the searches were imported into an EndNote X7 library and deduplicated. After deduplication, 286 records in total were identified. The titles and abstracts were assessed independently by two reviewers for inclusion and any discrepancies were resolved by consensus. None of the titles identified reported utility values collected in children and young people with psoriasis. The search of the HERC mapping algorithm database identified one study on the development of a mapping algorithm to estimate EQ-5D-Y (EuroQol-5 Dimensions for Youth) utility values from PedsQL general core scales. 153 The PedsQL is a generic instrument for measuring HRQoL in children and adolescents with acute and chronic health conditions. The PedsQL measures core dimensions of health as delineated by the WHO, as well as role (school) functioning. The four multidimensional scales are physical functioning (eight items), emotional functioning (five items), social functioning (five items) and school functioning (five items). 19 The EQ-5D-Y is the youth version of the EQ-5D, which has been specifically adapted in terms of language for children aged 8–11 years and adolescents aged 12–18 years. In the absence of a tariff set specifically for the EQ-5D-Y, the authors153 applied the UK national tariff for the valuation of the EQ-5D-3L (EuroQol-5 Dimensions three-level version) to generate utility values from the EQ-5D-Y instrument. 154
Khan et al. 153 assessed different mapping methods for estimating EQ-5D-Y health utilities from PedsQL response scores. The study used data collected in a cross-sectional survey conducted in four secondary schools in England among children aged 11–15 years. The sample on which the mapping models were estimated included 559 children and the validation sample included 337 children. Children in the full study sample (n = 896) were on average aged 13.3 years (standard deviation 1.3 years), 54% were male and approximately 40% were of non-white ethnicity. The authors explored both direct and response mapping approaches to predict EQ-5D-Y utility values, as well as a number of functional forms, including ordinary least squares (OLS) regression, generalised linear models, two-part logit–OLS regression, censored least absolute deviation and Tobit regression. Model performance was assessed on the validation sample and models were re-estimated on the full study sample. Table 58 presents the two best-fitting models for the mapping algorithm. These correspond to the models estimated using OLS regression with (1) age and sex terms included as regressors and (2) excluding age and sex terms as regressors.
| Variables | Mapping algorithm | |||
|---|---|---|---|---|
| OLS regression with age and sex terms (1) | OLS regression without age and sex terms (2) | |||
| Coefficient | SE | Coefficient | SE | |
| Age (years) | –0.006136 | 0.004741 | – | – |
| Sex | –0.009385 | 0.012292 | ||
| PedsQL domain scores | ||||
| Physical functioning (PF) | 0.009067 | 0.002571 | 0.009127 | 0.002568 |
| Emotional functioning (EF) | 0.006807 | 0.002533 | 0.006611 | 0.002530 |
| Social functioning (SF) | 0.00563 | 0.002831 | 0.005705 | 0.002829 |
| School functioning (SchF) | 0.005802 | 0.002371 | 0.006011 | 0.002367 |
| Quadratic terms | ||||
| PF squared | 0.00002 | 0.000025 | 0.00002 | 0.000025 |
| EF squared | –0.000049 | 0.000018 | –0.000048 | 0.000018 |
| SF squared | 0.000011 | 0.000016 | 0.000011 | 0.000016 |
| SchF squared | –0.000017 | 0.000015 | –0.000017 | 0.000015 |
| Interaction terms | ||||
| PF*EF | –0.000005 | 0.000027 | –0.000004 | 0.000027 |
| PF*SF | –0.000053 | 0.000029 | –0.000055 | 0.000029 |
| PF*SchF | –0.000066 | 0.000030 | –0.000066 | 0.000030 |
| EF*SF | –0.000011 | 0.000023 | –0.000009 | 0.000023 |
| EF*SchF | 0.000061 | 0.000021 | 0.000059 | 0.000021 |
| SF*SchF | –0.000026 | 0.000022 | –0.000027 | 0.000022 |
| Constant | –0.335861 | 0.118035 | –0.428496 | 0.094210 |
The two models were considered to have similar prediction accuracy for mean EQ-5D-Y values. Model 1, which included age and sex as regressors, had a better fit across a wider range of EQ-5D-Y values than model 2. Model 2 reported a better fit for the EQ-5D-Y utility score range of 0.8–1.0 category. There are a number of potential limitations to the use of this algorithm to predict EQ-5D-Y utilities. The sample on which the models were estimated consisted of healthy children aged 11–15 years, which may limit the predictive accuracy in sicker populations or populations outside this age range. The authors recognise that there is a need for further validation and testing of the algorithm but, in the absence of an alternative source, it remains a useful tool for estimating EQ-5D-Y utility values in situations in which only the PedsQL has been administered.
Utility data reported in company submissions
Health-related quality-of-life assessments were carried out in the etanercept trial (2003021149), the ustekinumab trial (CADMUS72) and the adalimumab trial (M04-71742) using the CDLQI and the PedsQL at selected time points. EQ-5D or EQ-5D-Y values were not collected in any of the trials. Therefore, the only way to include EQ-5D utility values in the model was by mapping from either CDLQI scores or PedsQL scores. The literature review described earlier did not identify any studies that estimated the relationship between CDLQI scores and EQ-5D values, whereas the study by Khan et al. 153 was the only study that estimated the relationship between PedsQL scores and EQ-5D values. The AG requested from the companies access to individual patient data (IPD) for PedsQL domain scores at baseline and follow-up by category of PASI response and PedsQL summary scores at the domain level by response category. The AG did not receive access to IPD; however, Janssen (ustekinumab) submitted aggregated summary data (mean and standard deviation) from the CADMUS trial for the PedsQL subscale and total scale scores by treatment arm (placebo and ustekinumab standard dose) and PASI response category at 12 weeks (< 50, 50–74, 75–89, ≥ 90) for baseline and 12, 28 and 52 weeks.
Utility estimates used in the model
The utility values associated with treatment in previous models in adults were based on the proportion of individuals in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) and the change in utility from baseline associated with the PASI response categories. Therefore, PASI response rates from the NMA were assumed to be a perfect proxy for change in utility arising from treatment.
The relationship between utility and PASI response was estimated in previous TAs in adults using either DLQI data mapped onto EQ-5D utility values or directly from EQ-5D data collected in the trials. In the population of children and young people, the only possibility of obtaining EQ-5D values was by mapping from PedsQL scores to EQ-5D-Y values, as described earlier. Without access to the IPD, which would allow full uncertainty to be reflected in the values, the mapping algorithm was applied to the summary scores at the domain level from the CADMUS trial.
Validation of the algorithm was performed by examining data reported in a study by Varni et al. ,155 which compared self-reported HRQoL (based on PedsQL scores) between paediatric patients with moderate to severe plaque psoriasis and a healthy population sample. The sample used to represent the psoriasis population corresponded to individuals in the main efficacy trial for etanercept (n = 208, age 4–17 years) and measurements of PedsQL scores at baseline were pooled across the two treatment arms (etanercept and placebo). The healthy population sample was taken from a US children’s health insurance programme evaluation (n = 5079) open to children and young people aged 2–16 years. Table 59 summarises the PedsQL subscale scores reported in Varni et al. 155 for the psoriasis and healthy populations, alongside the estimates obtained by applying the two best-fitting mapping algorithms to obtain EQ-5D-Y utility values (model 1 includes age and sex terms whereas model 2 excludes these variables).
| Covariates | Population | |||||
|---|---|---|---|---|---|---|
| Psoriasis (n = 208) | Healthy (n = 5079) | |||||
| Mean | EQ-5D-Y utility in model 1 | EQ-5D-Y utility in model 2 | Mean | EQ-5D-Y utility in model 1 | EQ-5D-Y utility in model 2 | |
| Age (years) | 12.71 | 0.869 | 0.864 | 9.72 | 0.936 | 0.913 |
| Sexa | 0.519 | 0.517 | ||||
| Physical functioning score | 82.5 | 87.8 | ||||
| Emotional functioning score | 67.1 | 79.2 | ||||
| Social functioning score | 80.7 | 85 | ||||
| School functioning score | 70.2 | 70.2 | ||||
| PedsQL total score | 75.5 | 83.9 | ||||
The EQ-5D utility estimates were higher in the healthy population than in the population with psoriasis, irrespective of the model used to map PedsQL scores to EQ-5D-Y values. The distinction between the models was minimal, especially in the psoriasis population: model 1 provided slightly higher utility values than model 2 (0.6% and 2.5% higher in the psoriasis and healthy populations respectively). Model 2 was subsequently used in the base-case analysis as the reference category for the variable sex was unclear in the study by Khan et al. 153
The mapping algorithm (model 2 in Table 58) was used to estimate change in EQ-5D-Y utility values from baseline based on PedsQL data from the ustekinumab trial (CADMUS) at baseline and 12 weeks’ follow-up (the time point at which response to treatment was assessed in the trial and blinding of randomised subjects in the trial was terminated; after this point crossover between treatment arms was possible). The mean change in EQ-5D-Y values between baseline and week 12 was estimated for individuals with different levels of PASI response. Table 60 reports the EQ-5D-Y utility values estimated for the base-case-analysis, with the placebo and treatment arms pooled.
| Baseline utilitya (n = 73) | Utility increment at the end of the trial period by PASI response category | |||
|---|---|---|---|---|
| < 50 (n = 30) | 50–74 (n = 10) | 75–89 (n = 9) | ≥ 90 (n = 24) | |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
The baseline utility estimate is similar to that derived from the etanercept trial (20030211) (0.864 in Table 59) and is lower than the general healthy population estimate of 0.913 based on the study by Varni et al. 155 The mean changes in EQ-5D utility from baseline by PASI response category are much smaller than the corresponding changes in EQ-5D utility observed in previous TAs in adults. For example, the EQ-5D changes in utility by PASI response category in the York model of adults were 0.05 for PASI < 50, 0.17 for PASI 50–74, 0.19 for PASI 75–89 and 0.21 for PASI ≥ 90.
To examine whether or not the changes in EQ-5D-Y utility values were accompanied by similar changes in other measures of HRQoL in the population of children and young people, the changes in EQ-5D-Y values were compared with reported CDLQI values by PASI response (Table 61). A comparison of EQ-5D and DLQI values by PASI response in adults is also shown in Table 61 (taken from TA180100 for ustekinumab, which was the only TA in adults that reported both outcomes). The mean changes in CDLQI score by PASI response in the CADMUS trial are much smaller than the mean changes in DLQI score in TA180,100 which is consistent with the smaller mean changes estimated for the EQ-5D-Y in the paediatric population than for the EQ-5D in adults. These differences, however, should be interpreted with caution as CDLQI and DLQI scores are not directly comparable and the number of observations was much smaller in the population of children and young people than in the adult population in TA180. 100
| PASI response category | CADMUS trial | TA180101 | ||||
|---|---|---|---|---|---|---|
| Sample size, n | Mean change in CDLQI score | Mean change in EQ-5D-Y utility | Sample size, n | Mean change in DLQI score | Mean change in EQ-5D utilitya | |
| < 50 | 30 | –1.2 | Confidential information has been removed | 430 | –2.5 | 0.04 |
| 50–74 | 10 | 2.0 | Confidential information has been removed | 160 | –10.3 | 0.17 |
| 75–89 | 9 | –5.6 | Confidential information has been removed | 207 | –13.4 | 0.22 |
| ≥ 90 | 24 | –8.1 | Confidential information has been removed | 318 | –15.3 | 0.25 |
The EQ-5D-Y/EQ-5D utility estimates suggest that improvements in HRQoL associated with reductions in PASI response rates are of a much smaller magnitude in children and young people than in adults; however, the evidence is highly uncertain because of the small sample size and the limited data available to validate the findings. In the absence of an alternative source to estimate EQ-5D values for the model, these values were used in the base-case analysis. It is important to highlight a number of limitations of this approach. First, the use of a mapping algorithm to estimate utilities introduces uncertainty compared with direct EQ-5D measurement. Second, the Khan et al. 153 mapping algorithm has not been validated in children aged < 11 years or in a population with psoriasis. Third, the CADMUS trial, from which the PedsQL data that were mapped to EQ-5D utilities were sourced, excluded children aged < 12 years; therefore, it remains uncertain whether or not the mapped utilities are reflective of this population. Fourth, in populations aged < 12 years, there may be issues with lack of agreement or consistency between self-reported and proxy (parent)-reported measurements. 156 Therefore, even if PedsQL data were available for younger children, the mapping algorithm might not consistently perform for self-reported and parent-reported measurements of the instrument. Finally, Khan et al. 153 used the EQ-5D-3L value set as a proxy for EQ-5D-Y in the absence of an alternative tariff set, but this approach is currently not recommended. 157 These limitations reduce the robustness of the utility estimates used in the model.
There might be other potential benefits of treatment that fall outside the QALY estimation. First, children and young people may miss schooldays to attend health-care appointments and may be absent for longer periods from school while experiencing symptoms. This can have a negative impact on their education/academic achievements and, in future, their ability to gain employment. It may also affect their social and psychological health through the reduced ability to participate in social and leisure activities and sport. Second, early treatment of children and young people with biological agents may prevent long-term multisystem morbidity (e.g. hypertension, cardiovascular disease, depression), which has a higher prevalence in adults with psoriasis than in the general population. 158 Finally, there may also be other aspects of HRQoL that are outside the perspective defined by NICE’s reference case,150 namely the potential impact on the HRQoL of carers of children and young people with psoriasis if treatment with biologics reduces the spillover disutility of illness by improving patients’ outcomes. The impact on carers may also extend to a reduced ability to participate in normal activities, both work- and non-work related. Because of an absence of quantitative estimates of the impact on the HRQoL of children and young people with psoriasis receiving any of the interventions and the potential benefits to their carers, it was not possible to incorporate them into the economic analysis. Any attempt to add arbitrary values to the utility estimates, which are already highly uncertain, would introduce further uncertainty.
Given the uncertainty surrounding the utility estimates for children and young people, scenario analyses were conducted using utility estimates from previous TAs in adults for etanercept, adalimumab and ustekinumab. Table 62 summarises the utility estimates considered in the scenario analyses.
| Analysis | Baseline utility | Utility gain by PASI response category | |||
|---|---|---|---|---|---|
| < 50 | 50–74 | 75–89 | ≥ 90 | ||
| Base-case analysis | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| TA10397 utility values | 0.7a | 0.050 | 0.170 | 0.190 | 0.210 |
| TA14699 utility valuesb | 0.692c | 0.063 | 0.178 | 0.178 | 0.308 |
| TA180100 utility valuesb | 0.7a | 0.04 | 0.17 | 0.22 | 0.25 |
Utility estimates by health state
The HRQoL utility values were applied in the model based on PASI response to treatment. The utility values in the trial period and period of continued use for each treatment were based on the proportions of individuals in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) and the change in utility from baseline associated with a PASI response. During the trial period, individuals are assigned utility values based on treatment response at the end of the trial period:
where u00 is the utility gain for individuals not achieving a PASI 50 response; u50 is the utility gain for individuals achieving a PASI 50 response but not a PASI 75 response; u75 is the utility gain for individuals achieving a PASI 75 response but not a PASI 90 response; u90 is the utility gain for individuals achieving a ≥ PASI 90 response; and ptrtPASIxx is the probability of a PASI XX response with treatment.
During the period of continued use, individuals are assigned utility values based on maintaining a treatment response at the end of the trial period, which is based on meeting the minimum of a PASI 75 response:
Individuals who discontinue treatment progress to BSC. The utility associated with BSC was based on the proportion of individuals in the different PASI response categories (< 50, 50–74, 75–89, ≥ 90) for BSC (assumed to be equal to the placebo response from the NMA):
A scenario analysis was considered in which the utility of individuals receiving BSC was set to be equal to baseline utility, that is, there are no health benefits from BSC.
On entering the death state, individuals are assigned a utility value of zero. Table 63 summarises the utility estimates applied in the base-case analysis by treatment and health state.
| Treatment | Health state in the model | ||
|---|---|---|---|
| Trial period | Continued use | BSC | |
| Adalimumab | 0.9156 | 0.9261 | 0.8713 |
| Etanercept | 0.8974 | 0.9177 | 0.8713 |
| Ustekinumab | 0.9186 | 0.9274 | 0.8713 |
| Methotrexate | 0.8994 | 0.9164 | 0.8713 |
| BSC | – | – | 0.8713 |
Given the paucity of evidence on AEs in children and young people receiving biological treatment for psoriasis, and similarly to the majority of previous TAs in adults, no disutility from treatment was applied in the model.
Resource utilisation and costs
Resource use and costs included in the model correspond to direct NHS costs and include treatment acquisition costs, administration costs, monitoring costs, costs associated with AEs and the costs of BSC. Costs were sourced from NHS reference costs 2014–15,159 the Monthly Index of Medical Specialities (MIMS),160 the BNF,161 Curtis and Burns and published literature. When costs were not available for 2015–16, they were inflated to 2014–15 prices based on the Hospital & Community Health Services Index published in Curtis and Burns. The systematic literature review described in Chapter 3 (see Methods for the synthesis of evidence of clinical effectiveness) considered broad search terms to capture resource utilisation and costs associated with the treatment of psoriasis in the population of children and young people. The search identified five studies162–166 that estimated resource use and the costs of biological therapies in psoriasis from insurance claim databases, but on further examination of the populations included in the studies it became clear that only adults were considered in the databases. In addition, the studies used data from US insurance databases, which are unlikely to reflect health-care resource use in the UK.
Given the lack of data on resource use and the costs of treatment for psoriasis in children and young people, previous NICE TAs for adults were hand-searched to identify relevant resource use categories and potential sources of resource use estimates and unit costs. These were tabulated and sent to our clinical advisor (see Chapter 5, Resource use and costs in the York model and subsequent appraisals), who then worked with us to help establish the transferability of the adult data and resource use assumptions to the population of children and young people.
According to our clinical advisor, the management of psoriasis in children and young people is very similar to that in adults. Therefore, it seems reasonable to assume that the resource use associated with the administration of the treatments and monitoring costs in children and young people would be similar to those used in previous TAs in adults. The assumptions used for resource use and costs for each of the cost categories are described in the following sections.
Drug acquisition costs
Table 64 details the dose and frequency of administration for each treatment and comparator, including ciclosporin, which forms part of BSC, and the unit costs associated with each treatment.
| Treatment | Administration route | Dose and frequency | Presentation and unit cost | Source |
|---|---|---|---|---|
| Adalimumab | SC | 0.8 mg/kg up to a maximum of 40 mg at weeks 0 and 1, then every 2 weeks thereafter | Prefilled syringe, 40 mg – £352.14 | MIMS160 |
| Etanercept | SC | 0.8 mg/kg up to a maximum of 50 mg weekly for up to 24 weeks | Prefilled syringe, 25-mg/vial – £89.38; prefilled syringe, 50 mg – £178.75 | MIMS160 |
| Ustekinumab | SC | 0.75 mg/kg for those weighing < 60 kg, 45 mg for those weighing 60–100 kg and 90 mg for those weighing > 100 kg at weeks 0 and 4, then every 12 weeks thereafter | Injectable solution, 45-mg vial – £2147.00 | MIMS160 |
| Methotrexate | Oral (72%), SC (24%), IM (4%) | 0.1–0.4 mg/kg weekly | Oral solution (2 mg/ml), 65 ml – £125.00; injectable solution, 50-mg vial – £2.62 | MIMS,160 BNF161 |
| Ciclosporin | Oral | 2–5 mg/kg daily for up to 2 years | Oral solution (100 mg/ml), 50 ml – £102.30 | BNF161 |
The dosages of the biological therapies were taken from the Summaries of Product Characteristics. 167–169 For methotrexate and ciclosporin, which are currently not licensed for paediatric use, the dosages were sourced from published literature78,170,171 and confirmed with our clinical advisor to ensure that they reflected UK clinical practice in this population. Methotrexate can be administered orally or injected subcutaneously or intramuscularly. In the model it was assumed that 72% of individuals are given methotrexate in oral solution and 28% in injectable solution, which reflects the distribution of administration identified in the UK psoriasis audit of the use of systemic treatments in children and young people. 148 Therefore, the unit cost per mg for methotrexate is a weighted average of the unit cost per mg of the oral and injectable solutions (i.e. £0.71/mg). Unit costs were sourced from MIMS160 and supplemented with data from the BNF. 161
Figure 11 illustrates the number of doses administered in the first five cycles of the model for each treatment based on the licensed dose. Adalimumab is administered at weeks 0 (baseline) and 1 and then every 2 weeks thereafter until response assessment at the end of week 16. If individuals are responders to treatment they continue to receive adalimumab every 2 weeks until treatment withdrawal (highlighted in grey). Ustekinumab is administered at weeks 0 and 4 and then every 12 weeks thereafter, with response assessment at week 16. Etanercept and methotrexate are administered weekly, with response assessment at weeks 12 and 16 respectively.
FIGURE 11.
Drug dose distribution during the first five cycles in the model. ADA, adalimumab; ETA, etanercept; MTX, methotrexate; UST, ustekinumab; •, ADA administration; ♦, ETA administration; ▪, UST administration; Δ, MTX administration.
The dosages of the biological treatments are dependent on patient weight. The median weight by age and sex in the population of children and young people was extracted from the Royal College of Paediatrics and Child Health’s school-age growth charts. 172 Table 65 shows the weights used in the model by age. These were based on an average of the weight of boys and girls (and when the weight estimate in the growth chart did not correspond to an integer, the next-highest integer was used).
| Age (years) | Median weight (kg) | ||
|---|---|---|---|
| Girls | Boys | Used in the model | |
| 4 | 17 | 18 | 17.5 |
| 5 | 19 | 19 | 19 |
| 6 | 21 | 21 | 21 |
| 7 | 23 | 23 | 23 |
| 8 | 26 | 26 | 26 |
| 9 | 29 | 29 | 29 |
| 10 | 33 | 32 | 32.5 |
| 11 | 36 | 35 | 35.5 |
| 12 | 41 | 39 | 40 |
| 13 | 46 | 44 | 45 |
| 14 | 50 | 50 | 50 |
| 15 | 54 | 56 | 55 |
| 16 | 56 | 61 | 58.5 |
| 17 | 57 | 66 | 61.5 |
| 18 | 58 | 67 | 62.5 |
The weight by age was used to estimate the correct dosage of each treatment and the corresponding cost. Table 66 summarises the dosages used in the model for each treatment by age and the corresponding costs per dose. Following clinical advice it was assumed that the vial with the lowest dose available would be used to allow administration of a single dose in the paediatric population. This inevitably results in wastage of the remainder of the vial. For example, for individuals who weigh < 60 kg the full cost of the 45-mg vial of ustekinumab is assumed as the remaining product in the vial cannot be stored. Vial splitting across individuals was considered unlikely because in most cases the majority of the vial is used for a single patient and treating patients together is less likely to occur in this population because of low patient numbers. Therefore, the cost per dose was fixed for adalimumab and ustekinumab (£352.14 and £2147.00 respectively). For etanercept, the 25-mg vial (£89.38) is used for children aged < 10 years whereas the 50-mg vial (£178.75) is used for those aged ≥ 10 years.
| Age | Weight (kg) | Drug | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adalimumab (0.8 mg/kg, maximum 40 mg) | Etanercept (0.8 mg/kg, maximum 50 mg) | Ustekinumab (0.75 mg/kg for weight < 60 kg; 45 mg for weight 60–100 kg) | Methotrexate (0.4 mg/kg) | Ciclosporin (5 mg/kg) | |||||||
| Dosage (mg) | Cost per dose (£) | Dosage (mg) | Cost per dose (£) | Dosage (mg) | Cost per dose (£) | Dosage (mg) | Cost per dose (£) | Dosage (mg) | Cost per dose (£) | ||
| 4 | 17.5 | 14 | 352.14 | – | – | – | – | 7 | 6.73 | 87.5 | 1.79 |
| 5 | 19 | 15.2 | 352.14 | – | – | – | – | 7.6 | 7.31 | 95 | 1.94 |
| 6 | 21 | 16.8 | 352.14 | 16.8 | 89.38 | – | – | 8.4 | 8.08 | 105 | 2.15 |
| 7 | 23 | 18.4 | 352.14 | 18.4 | 89.38 | – | – | 9.2 | 8.85 | 115 | 2.35 |
| 8 | 26 | 20.8 | 352.14 | 20.8 | 89.38 | – | – | 10.4 | 10.00 | 130 | 2.66 |
| 9 | 29 | 23.2 | 352.14 | 23.2 | 89.38 | – | – | 11.6 | 11.15 | 145 | 2.97 |
| 10 | 32.5 | 26 | 352.14 | 26 | 178.75 | – | – | 13 | 12.50 | 162.5 | 3.32 |
| 11 | 35.5 | 28.4 | 352.14 | 28.4 | 178.75 | – | – | 14.2 | 13.65 | 177.5 | 3.63 |
| 12 | 40 | 32 | 352.14 | 32 | 178.75 | 30.0 | 2147.00 | 16 | 15.38 | 200 | 4.09 |
| 13 | 45 | 36 | 352.14 | 36 | 178.75 | 33.8 | 2147.00 | 18 | 17.31 | 225 | 4.60 |
| 14 | 50 | 40 | 352.14 | 40 | 178.75 | 37.5 | 2147.00 | 20 | 19.23 | 250 | 5.12 |
| 15 | 55 | 40 | 352.14 | 44 | 178.75 | 41.3 | 2147.00 | 22 | 21.15 | 275 | 5.63 |
| 16 | 58.5 | 40 | 352.14 | 46.8 | 178.75 | 43.9 | 2147.00 | 23.4 | 22.50 | 292.5 | 5.98 |
| 17 | 61.5 | 40 | 352.14 | 49.2 | 178.75 | 45.0 | 2147.00 | 24.6 | 23.65 | 307.5 | 6.29 |
| 18 | 62.5 | 40 | 352.14 | 50 | 178.75 | 45.0 | 2147.00 | 25 | 24.04 | 312.5 | 6.39 |
Drug administration costs
Adalimumab, etanercept and ustekinumab were assumed to be self-administered. In the case of younger children it was assumed that a parent or carer would administer the subcutaneous injection. Subcutaneous injections were assumed to incur administration costs only for nurse training for self-administration (or parent/carer administration) in the induction phase. In line with previous TAs in adults, this was assumed to require 3 hours of nurse time, which was costed based on the cost per working hour of a band 5 hospital nurse with qualifications (£43 per hour). A cost of £129 was applied in the first cycle of the model for the administration of the biologics.
Monitoring costs
Table 67 summarises the resource use assumptions made in relation to monitoring and the corresponding unit costs applied in the model. In the absence of evidence specifically relating to the population of children and young people, resource use estimates associated with monitoring and routine laboratory tests for biological and non-biological systemic treatments were taken from NICE CG153,173 which used similar assumptions to those in the original York model (TA10397) and subsequent NICE appraisals of biological treatments. 98–102
| Item | Frequency of testing | Unit cost | |||||
|---|---|---|---|---|---|---|---|
| Biological therapy | Methotrexate | Ciclosporin | |||||
| Trial period | Continued use (annual) | Trial period | Continued use/BSC (annual) | BSC (annual) | Per item (£) | Source | |
| Liver function test | 2 | 4 | 2 | 4 | 4 | 0.77a | TA10397,140 |
| Full blood count | 2 | 4 | 2 | 4 | 4 | 3.05a | TA10397,140 |
| Glomerular filtration | 0 | 0 | 0 | 0 | 1 | 195.07b | NHS reference costs, 2014–15159 |
| Urea and electrolytes | 2 | 4 | 2 | 4 | 4 | 1.41a | TA10397,140 |
| Outpatient/physician visits | 2 | 4 | 2 | 4 | 4 | 119.99c | NHS reference costs, 2014–15159 |
Individuals on biological therapy were assumed to undertake a series of tests during the initial trial period, namely a full blood count, liver function test and urea and electrolytes test. During the trial period the tests were assumed to be carried out during two routine outpatient visits that occur at treatment initiation and at the end of the trial period (treatment response assessment visit). As methotrexate was not included as a comparator in CG153173 (only as part of BSC), it was assumed that the resource use for the monitoring of methotrexate in the trial period is the same as that for biological treatment. In the maintenance period (corresponding to the health state of ‘continued use’), individuals on systemic therapies were assumed to be monitored once every 3 months. 173 The unit costs for glomerular filtration rate and outpatient visits were taken from NHS reference costs 2014–15,159 whereas the costs of the remaining monitoring items were inflated to 2014–15 prices based on estimates presented in TA103. 97
The costs of tests undertaken solely to screen individuals for eligibility for treatment were excluded from the analysis, namely chest radiography, tests for tuberculosis or biopsies of lesions atypical of psoriasis. These costs were also excluded in previous appraisals in adults. The cost of folic acid used in conjunction with methotrexate to prevent side effects was also excluded from previous appraisals as the annual cost of this drug is very low (< £1). Our clinical advisor indicated that children and young people would be tested for herpes zoster before treatment initiation; however, as this test would be performed on every patient not immune to the virus regardless of treatment, it was excluded from the analysis. The costs of liver biopsy and type III procollagen peptide (PIIINP) monitoring for the purpose of assessing liver function in individuals treated with methotrexate were also excluded from the analysis based on clinical advice; liver biopsy is seldom conducted in children and young people given its invasiveness, whereas PIIINP is a marker of growth in this population rather than of hepatic toxicity.
Best supportive care costs
Best supportive care corresponds to the management of individuals after failure of conventional systemic therapies. BSC is also considered a relevant comparator to biological treatments. If biological treatments are found not to be effective, individuals are usually offered some form of BSC rather than no treatment. BSC tends to include a mix of active non-biological systemic therapies such as methotrexate and ciclosporin and palliative care, including phototherapy, as well as outpatient visits and hospitalisations to manage disease flare-ups.
The resource use and costs associated with BSC have represented a significant area of uncertainty in the analysis of the cost-effectiveness of biological treatments for moderate to severe psoriasis in adults. In TAs prior to CG153,97–100 the definition of BSC in terms of resource use and costs was restricted to outpatient visits and hospitalisations to manage the symptoms of psoriasis, with these largely informed by assumptions and clinical opinion. In CG153,173 the definition of BSC was expanded to also include non-biological systemic treatments, phototherapy and attendance at tertiary day centres. As discussed previously (see Chapter 5, Resource use and costs in the York model and subsequent appraisals), this guideline used estimates of resource use from observational studies in the UK143 and the Netherlands144 but also relied heavily on clinical opinion and assumptions. In the absence of evidence for children and young people, the definition of BSC from CG153173 was used in the model, with input from our clinical advisor on the appropriateness of the assumptions for a younger population.
Table 68 summarises the resource use assumptions for BSC by category of cost in CG153173 and those applied in the model, alongside the associated unit costs. Unit costs were sourced from the BNF,161 MIMS160 and NHS reference costs 2014–15. 159 The relative proportions of individuals on active treatment with methotrexate and ciclosporin were modified from those used in CG153173 based on clinical opinion that children and young people are less likely to be managed with ciclosporin than adults because of the renal toxicity of the drug. Data from a UK psoriasis audit on the use of systemic treatments in children and young people were used to inform the relative proportion of individuals on methotrexate and ciclosporin. 148 In CG153, it was assumed that 90% of individuals receiving BSC would be on active treatment with systemic drugs. However, in the audit, 53 patients were treated with non-biological systemic treatments, of whom 25 patients were treated with methotrexate and 12 with ciclosporin. Therefore, instead of assuming that individuals are equally distributed between methotrexate and ciclosporin, a ratio (25/37 and 12/37 for those on methotrexate and ciclosporin respectively) for each treatment was applied to the overall proportion of 90% to reflect the distribution of children and young people receiving these treatments in the audit. The corresponding proportions of individuals assumed to receive methotrexate and ciclosporin as part of BSC were 61% and 29%, respectively. As in CG153,173 treatment with ciclosporin was assumed to be discontinued after a maximum duration of 2 years (because of the increased risk of renal toxicity). Monitoring costs associated with the use of these non-biological systemic therapies were applied in the model as presented in Monitoring costs.
| Cost element | CG153173 | Current analysis | |||
|---|---|---|---|---|---|
| Resource use | Unit cost | Resource use | Unit cost | Source | |
| Drug acquisition costs | |||||
| Methotrexate | 45% of patients on methotrexate once weekly | £0.05 per mg | 61% of patients on methotrexate once weekly | £0.71 per mg | MIMS,160 BNF161 |
| Ciclosporin | 45% of patients on ciclosporin daily for a maximum of 2 years | £0.02 per mg | 29% of patients on ciclosporin daily for a maximum of 2 years | £0.02 per mg | BNF161 |
| Health-care utilisation costs | |||||
| NBUVB | 16% of patients have 24 sessions of NBUVB per year | £85.16 | 16% of patients have 24 sessions of NBUVB per year (same as CG153173) | £95.53a | NHS reference costs 2014–15159 |
| Monitoring | Four monitoring visits per year for all patients on systemic treatment (including at each one outpatient visit, one FBC, one LFT and one U&E test) plus 0.04 liver biopsies and four PIIINP tests per year for patients on methotrexate and one GFR test per year for patients on ciclosporin | £86.85 per monitoring visit, £553 per liver biopsy, £25.29 per PIIINP test, £233.00 per GFR test | Four monitoring visits per year for all patients on systemic treatment (including at each one outpatient visit, one FBC, one LFT and one U&E test) plus one GFR test per year for patients on ciclosporin (same as CG153173 but without liver biopsies and PIIINP for patients on methotrexate) | £125.22 per monitoring visit for all patients on systemic treatment, £195.07 per GFR testb | Calculated (see Monitoring costs). For GFR, NHS reference costs 2014–15159 |
| Day centre care | Five visits per year | £362.60 per visit | Five visits per year (same as CG153173) | £472.55 per visitc | NHS reference costs 2014–15159 |
| Outpatient visits | Five visits per year for the 10% of patients who are not on systemic treatment | £82 | Five visits per year for the 10% of patients who are not on systemic treatment (same as CG153173) | £119.99d | NHS reference costs 2014–15159 |
| Hospitalisations | 26.6 bed-days | £271.17 | 0 (base case). Alternative values explored in scenario analysis | £295.80e | NHS reference costs 2014–15159 |
In line with CG153,173 16% of the population were assumed to undergo 24 sessions of phototherapy per year (NBUVB) and five outpatient visits per annum were assumed for the 10% of individuals not managed with systemic therapies. All individuals were assumed to incur the costs of five visits per annum to a specialist dermatology day centre, in line with CG153. 173
The resource use associated with hospitalisations for individuals on BSC was identified as an area of high uncertainty and a key driver of cost-effectiveness in the previous TAs in adults. The number of bed-days assumed in CG153173 (26.6 days per year) was based on the average LOS for psoriasis patients with a baseline PASI of 10–20 points taken from a UK observational study145 combined with the average number of hospitalisations for individuals at high need (one hospitalisation per year) and very high need (2.55 hospitalisations per year) from a Dutch observational study. 144 The total of 26.6 days of hospitalisation per annum was considered by the NICE Appraisal Committees for TA350101 (secukinumab) and TA368102 (apremilast) in adults to be too high. Our clinical advisor suggested that hospitalisations in children and young people are very rare. This is largely because children and young people have not yet developed the comorbidities that often lead to hospitalisations in adults with psoriasis. Therefore, in the base-case analysis it was assumed that children and young people do not incur any inpatient stays. In separate scenario analyses, estimates of 26.6 days of hospitalisation per annum173 and 6.49 days of hospitalisation per annum144 were considered.
Adverse event costs
As discussed in Chapter 5 (see Resource use and costs in the York model and subsequent appraisals), only one previous TA in adults101 considered the costs of hospitalisations resulting from AEs in the cost-effectiveness analysis. The AEs that were assumed to lead to relevant resource use consumption (i.e. those leading to hospitalisations) in this evaluation were (1) NMSC, (2) malignancies other than NMSC and (3) severe infections. The rates of AEs as reported in the literature (for adalimumab, etanercept, ustekinumab and infliximab) and from trial data (for secukinumab) were applied to each treatment arm as per the rates of these events occurring.
The safety data from the clinical trials of biological drugs for the treatment of severe-to-moderate psoriasis (see Chapter 3, Safety of adalimumab, Safety of etanercept and Safety of ustekinumab) suggested that there was little difference in the short- and long-term rates of AEs between trial arms, with the potential exception of etanercept, for which a higher rate of infections (not statistically significant) was observed than for placebo. However, the trial data included a small number of observations for each treatment and a limited follow-up period (from 52 weeks for adalimumab46,47 to 312 weeks for etanercept). 49,52,80 Observational studies in children and young people with psoriasis76,77 (see Chapter 3, Additional observational evidence) did not report any increase in infections or SAEs associated with the use of biological therapies.
Given the paucity of robust evidence on the incidence of AEs in children and young people with moderate-to-severe psoriasis, the costs of these were not included in the base-case analysis. However, scenario analyses were conducted to explore the impact on the cost-effectiveness results of including the costs associated with hospitalisations resulting from serious infections and malignancies (both NMSC and other). The rates of AEs were sourced from TA350101 and supplemented with data from Dixon et al. 174 for methotrexate, whereas the unit costs were taken from NHS reference costs 2014–15. 159 Table 69 summarises the adverse event rates applied in the model, alongside the corresponding unit costs.
| AE | AE rate (per patient-year) | Unit cost (£) | |||
|---|---|---|---|---|---|
| Adalimumab | Etanercept | Ustekinumab | Methotrexate | ||
| NMSC | 0.0097 | 0.0354 | 0.0065 | – | 2160.37a |
| Non-NMSC malignancies | 0.006 | 0.00043 | 0.0016 | – | 4974.76b |
| Severe infections | 0.0519 | 0.0513 | 0.01 | 0.0414 | 2679.66c |
The costs of AEs associated with biological therapies and methotrexate were applied in the model to individuals while on treatment. Individuals treated with BSC were assumed not to develop AEs.
Analytical methods
Base-case analysis
The expected costs and QALYs of the interventions and comparators were determined for each population and the relative cost-effectiveness was established using standard decision rules and reported using ICERs as appropriate. The ICER examines the additional cost that one treatment option incurs over another and compares this with the additional health benefits to give the additional cost of the treatment for each additional QALY gained. When more than two treatment options are being compared, the ICERs are calculated using the following process:
-
The treatment options are ranked in terms of mean QALYs (from the least effective to the most effective).
-
If a treatment option is more costly and less effective than any other option, then this treatment is said to be dominated and is excluded from the calculation of the ICERs.
-
The ICERs are calculated for each successive alternative, from the least effective to the most effective. If the ICER for a given treatment option is higher than that of any more effective option, then this treatment option is ruled out on the basis of extended dominance.
-
Finally, the ICERs are recalculated, excluding any treatment options that are ruled out by principles of dominance or extended dominance.
The resulting ICERs provide the basis for establishing which treatment appears optimal based on cost-effectiveness considerations. Guidance from NICE150 suggests that an incremental cost per additional QALY of around £20,000–30,000 is considered to represent an appropriate threshold for the health opportunity costs to the NHS.
The ICER comparing all interventions and comparators relates to a situation in which the decision-maker can choose only one of the treatment options. However, in psoriasis, as indicated previously, if an individual patient does not respond to or tolerate one of the biological therapies, an alternative one is usually tried. This means that treatments are usually trialled on an individual basis until an effective option is found. The ICERs comparing each intervention with BSC (after systemic therapy) or methotrexate (before systemic therapy) are also presented, to indicate the optimum ordering of treatments in terms of their cost-effectiveness. The most cost-effective order in which to give the therapies based on total expected costs and QALYs associated with each treatment option is dependent on the cost-effectiveness threshold.
Probabilistic sensitivity analysis was used to represent uncertainty in the cost-effectiveness results. The effectiveness data were entered as simulated posterior distributions from the Markov chain Monte Carlo analysis to reflect uncertainty in the mean estimates. Monte Carlo simulation was used to propagate the uncertainty in the input parameters over 10,000 draws, from which mean costs and QALYs were then obtained by averaging over the 10,000 simulations. The probability that a treatment is first in the sequence was also estimated.
Differences in the marketing authorisations of the interventions by age and the positioning of adalimumab before non-biological systemic therapy means that the comparative cost-effectiveness of the interventions needs to be evaluated by age and before or after use of systemics. The relevant comparator also depends on the position of the particular intervention in the pathway. Before systemic therapy, methotrexate is the relevant comparator (as the current standard of care), whereas after systemic therapy BSC represents the most relevant comparator. Three base-case populations are presented:
-
children and young people aged 4–17 years with adalimumab compared with methotrexate, that is, as a second-line therapy in individuals who are inadequately controlled by, or who are intolerant to, topical therapy and phototherapies
-
children and young people aged 6–11 years with adalimumab and etanercept compared with BSC and with each other, that is, as third-line therapy in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies
-
children and young people aged 12–17 years with adalimumab, etanercept and ustekinumab compared with BSC and with each other, that is, as third-line therapy in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies.
Table 70 summarises the input parameters used in the base-case analysis.
| Parameter | Mean | SE | Source |
|---|---|---|---|
| Baseline age (years) | 4, 6 or 12 | – | According to the licence for each comparator |
| Discount rate (per year) | 3.5% | – | NICE methods guidance150 |
| Time horizon (years) | 14, 12 or 6 | – | Assumption: until individuals reach 18 years |
| Duration of treatment trial period (weeks) | |||
| Adalimumab | 16 | – | Licence definition of timing for treatment response assessment |
| Etanercept | 12 | – | |
| Ustekinumab | 16 | – | |
| Methotrexate | 16 | – | Response assessment in the adalimumab trial (M04-717) |
| Treatment response: adalimumab | |||
| Probability of PASI 50 | 91.5% | Simulated posterior distribution from the Bayesian NMA | NMA (see Chapter 4, Adjusting for differences in population and placebo response rates) |
| Probability of PASI 75 | 79.0% | ||
| Probability of PASI 90 | 54.6% | ||
| Treatment response: etanercept | |||
| Probability of PASI 50 | 75.2% | Simulated posterior distribution from the Bayesian NMA | NMA (see Chapter 4, Adjusting for differences in population and placebo response rates) |
| Probability of PASI 75 | 54.4% | ||
| Probability of PASI 90 | 27.9% | ||
| Treatment response: ustekinumab | |||
| Probability of PASI 50 | 93.4% | Simulated posterior distribution from the Bayesian NMA | NMA (see Chapter 4, Adjusting for differences in population and placebo response rates) |
| Probability of PASI 75 | 82.4% | ||
| Probability of PASI 90 | 59.4% | ||
| Treatment response: methotrexate | |||
| Probability of PASI 50 | 70.8% | Simulated posterior distribution from the Bayesian NMA | NMA (see Chapter 4, Adjusting for differences in population and placebo response rates) |
| Probability of PASI 75 | 49.2% | ||
| Probability of PASI 90 | 23.9% | ||
| Treatment response: BSC | |||
| Probability of PASI 50 | 26.5% | Simulated posterior distribution from the Bayesian NMA | NMA (see Chapter 4, Adjusting for differences in population and placebo response rates) |
| Probability of PASI 75 | 11.5% | ||
| Probability of PASI 90 | 2.9% | ||
| Withdrawal rate (annual) | 0.20 | Mean/4 | Assumption based on previous TAs in adults |
| Mortality rate | Age dependent | – | Life table data for England and Wales 2013–15151 |
| Baseline utility | (Confidential information has been removed) | – | PedsQL data from the CADMUS trial mapped onto EQ-5D utility values using the algorithm from Khan et al.153 |
| Utility increment for PASI < 50 | (Confidential information has been removed) | – | |
| Utility increment for PASI 50–74 | (Confidential information has been removed) | – | |
| Utility increment for PASI 75–89 | (Confidential information has been removed) | – | |
| Utility increment for PASI ≥ 90 | (Confidential information has been removed) | – | |
| Drug acquisition resource use and costs | |||
| Adalimumab administrations in the ‘trial period’ | 9 | – | According to licence |
| Etanercept administrations in the ‘trial period’ | 12 | – | |
| Ustekinumab administrations in the ‘trial period’ | 2 | – | |
| Methotrexate administrations in the ‘trial period’ | 16 | – | |
| Adalimumab administrations per cycle in ‘continued use’ | 2 | – | |
| Etanercept administrations per cycle in ‘continued use’ | 4 | – | |
| Ustekinumab administrations per cycle in ‘continued use’ | 0.33 | – | |
| Methotrexate administrations per cycle in ‘continued use’ and ‘BSC’ | 4 | – | |
| Ciclosporin administrations per cycle in ‘BSC’ | 28 | – | |
| Proportion of patients on methotrexate in ‘BSC’ | 61% | – | Assumption |
| Proportion of patients on ciclosporin in ‘BSC’ | 29% | – | |
| Dosage of methotrexate (per kg) | 0.4 mg | – | Same as in the adalimumab trial (M04-717); de Jager et al.78 |
| Dosage of ciclosporin (per kg) | 5 mg | – | Clinical opinion of Dr Ruth Murphy; Mahé et al.;170 Pereira et al.175 |
| Adalimumab cost per dose | £352.14 | – | MIMS160 |
| Etanercept cost per dose (< 10 years old) | £89.38 | – | MIMS160 |
| Etanercept cost per dose (≥ 10 years old) | £178.75 | – | MIMS160 |
| Ustekinumab cost per dose | £2147.00 | – | MIMS160 |
| Methotrexate cost per mg | £0.71 | – | BNF161 |
| Ciclosporin cost per mg | £0.02 | – | BNF161 |
| Drug administration costs | |||
| Self-administration instruction (hours) | 3 | – | Assumption |
| Cost of hospital nurse band 5 (per hour) | £43 | – | Curtis and Burns |
| Monitoring frequency | |||
| FBC, LFT, U&E and physician visits for adalimumab, etanercept, ustekinumab and methotrexate in the ‘trial period’ | 2 | – | Assumption based on adult data, as described in Chapter 5, Resource use and costs in the York model and subsequent appraisals |
| FBC, LFT, U&E and physician visits for adalimumab, etanercept, ustekinumab and methotrexate per annum in ‘continued use’ | 4 | – | |
| FBC, LFT, U&E and physician visits for ciclosporin and methotrexate per annum in ‘BSC’ | 4 | – | |
| GFR for ciclosporin per annum in ‘BSC’ | 1 | – | |
| Monitoring test costs | |||
| FBC | £3.05 | – | TA10397,140 |
| LFT | £0.77 | – | |
| U&E | £1.41 | – | |
| GFR | £195.07 | – | NHS reference costs 2014–15159 |
| Physician monitoring visit | £119.99 | – | |
| Palliative care resource use and costs in BSC | |||
| NBUVB sessions per cycle | 3.84 | – | Assumption based on CG153173 (adults) |
| Day centre care visits | 5 | – | |
| Outpatient visits | 0.5 | – | |
| NBUVB cost per sessions | £95.53 | – | NHS reference costs 2014–15159 |
| Day centre care cost per visit | £472.55 | – | |
| Outpatient cost per visit | £119.99 | – | |
Scenario analysis
A number of alternative scenarios were considered in which the assumptions used as part of the base-case analysis were varied. These analyses were undertaken to assess the robustness of the base-case results to variation in the assumptions and sources of the data used to populate the model. Table 71 summarises the alternative scenarios considered. For each element, the position in the base-case analysis is outlined, alongside the alternative assumptions applied. The cost-effectiveness of the interventions was considered under each of the scenarios for each of the licensed populations.
| Scenario | Element | Position in the base-case analysis | Variation in scenario analysis |
|---|---|---|---|
| Intervention and comparators | |||
| 1 | Off-label use of biologics outside age constraints | Adalimumab licensed for those aged ≥ 4 years | Adalimumab, etanercept and ustekinumab for those aged ≥ 4 years |
| Etanercept licensed for those aged ≥ 6 years | |||
| Ustekinumab licensed those for aged ≥ 12 years | |||
| Model time horizon | |||
| 2 | Time horizon of the model | 14 years, 12 years and 6 years for the adalimumab, etanercept and ustekinumab populations respectively (i.e. until individuals reach 18 years) | Common time horizon of 14 years |
| Treatment effectiveness estimates | |||
| 3a | Direct trial evidence for treatment effects in children and young people | NMA using full network of evidence in children, young people and adults | M04-717 trial44,46 used to inform adalimumab vs. methotrexate |
| 20030211 trial49 used to inform etanercept vs. BSC | |||
| CADMUS trial72 used to inform ustekinumab vs. BSC | |||
| 3b | Indirect treatment comparison in children and young people | Indirect treatment comparison used to inform etanercept vs. ustekinumab vs. BSC | |
| 3c | Treatment effects from the NMA using minimum evidence from the adult population | NMA using minimum evidence from the adult population (CHAMPION study106) to link the trials in children and young people | |
| 3d | PASI response assessment | PASI 75 | PASI 50 |
| HRQoL utility values | |||
| 4a | EQ-5D utility estimates from adults | PedsQL data mapped onto EQ-5D-Y utility values | EQ-5D values from TA10397 |
| TA14699 | |||
| TA180100 | |||
| 4b | Utility estimates for BSC | Utility gains for BSC weighted by PASI response associated with placebo from the NMA | Utility in BSC equal to the baseline value (i.e. no utility gain associated with BSC) |
| BSC costs | |||
| 5 | Hospitalisations for BSC | No inpatient stay included for children and young people | 6.49 days per annum based on data in adults from Fonia et al.144 |
| 26.6 days per annum based on data in adults from CG153173 | |||
| AE costs | |||
| 6 | Costs associated with AEs | Not included | Costs of severe infections included |
| Costs of severe infections and malignancies included | |||
| Treatment withdrawal rates | |||
| 7 | Withdrawal rates from treatment | 20% per annum | 10% per annum |
| 30% per annum | |||
| Biosimilars | |||
| 8 | Biosimilar for etanercept | Unit cost of etanercept | Unit cost of 50 mg of Benepali (biosimilar) |
Results
Results of the base-case cost-effectiveness analysis
Table 72 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy. Adalimumab is more costly (additional cost of £27,084) but also more effective than methotrexate (incremental gain in QALYs of 0.088). The resulting ICER is £308,329 per QALY gained. The small incremental gain in QALYs for adalimumab compared with methotrexate is a result of the modest utility increments in EQ-5D-Y for the different PASI response categories (< 50, 50–74, 75–89, ≥ 90). The average proportion of individuals achieving a PASI 75 response is 79% for adalimumab compared with 49% for methotrexate but the utility gains for individuals achieving a PASI 75–89 response and PASI ≥ 90 response are very small at 0.0340 and 0.0810 respectively. Therefore, the difference in effectiveness translates into a small utility gain while on treatment with adalimumab compared with methotrexate. The difference in total costs for adalimumab compared with methotrexate is driven by the difference in treatment costs: adalimumab has a cost of £704.28 per 4-week cycle in the model (i.e. £352.14 per dose every 2 weeks) whereas methotrexate has a cost of approximately £60 per 4-week cycle. The difference in treatment costs is partly offset by the greater efficacy associated with adalimumab, which results in lower costs associated with BSC (i.e. less time spent in BSC) for non-responders compared with higher costs on BSC with methotrexate, but this offset is not sufficient to outweigh the difference in treatment costs. The probability that adalimumab is cost-effective at a threshold of £30,000 per additional QALY is zero.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| MTX | 34,914 | 9.939 | – | – | – | MTX |
| ADA | 61,999 | 10.027 | 27,084 | 0.088 | 308,329 | |
Table 73 presents the cost-effectiveness results for the interventions after failed systemic therapy by age group. The difference by age group reflects the fact that ustekinumab does not have marketing authorisation for use in children and young people aged <12 years. For the younger age group of 6–11 years, adalimumab is the most effective treatment (8.890 QALYs), followed by etanercept (8.813 QALYs) and BSC (8.710 QALYs). In terms of costs, adalimumab is the most costly treatment (£57,251), followed by etanercept (£43,808) and BSC (£36,406). Based on a fully incremental analysis, the ICER of etanercept compared with BSC is £71,903 per additional QALY, whereas the ICER of adalimumab compared with etanercept is £174,519 per additional QALY. The individual pairwise ICERs for etanercept and adalimumab compared with BSC are £71,903 and £115,825 per additional QALY respectively.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.710 | – | – | – | – | BSC |
| ETA | 43,808 | 8.813 | 7402 | 0.103 | 71,903 | 71,903 | |
| ADA | 57,251 | 8.890 | 13,444 | 0.077 | 174,519 | 115,825 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.804 | – | – | – | – | BSC |
| ETA | 33,199 | 4.887 | 11,450 | 0.084 | ED ADA | 137,059 | |
| ADA | 37,852 | 4.950 | 16,103 | 0.146 | 110,430 | 110,430 | |
| UST | 39,975 | 4.960 | 2123 | 0.011 | 201,507 | 116,568 | |
For children and young people aged 12–17 years, ustekinumab is the most effective treatment (4.960 QALYs), followed by adalimumab (4.950 QALYs), etanercept (4.887 QALYs) and BSC (4.804 QALYs). In terms of costs, ustekinumab is the most costly treatment (£39,975), followed by adalimumab (£37,852), etanercept (£33,199) and BSC (£21,749). Based on a fully incremental analysis, etanercept is extendedly dominated by adalimumab (i.e. etanercept produces additional gains in effectiveness at incremental costs higher than those of the next most effective strategy of adalimumab, observed by a higher ICER for etanercept than for adalimumab), the ICER of adalimumab compared with BSC is £110,430 per additional QALY and the ICER of ustekinumab compared with adalimumab is £201,507 per additional QALY. The individual pairwise ICERs for etanercept, adalimumab and ustekinumab compared with BSC are £137,059, £110,430 and £116,568 per additional QALY respectively.
There are two important differences to note between the two age populations. First, the reduction in total costs and QALYs for the interventions in the older age group is an artefact of the difference in the model time horizon used in each analysis (i.e. 12 years for age group 6–11 years and 6 years for age group 12–17 years). The time horizon of the model extends until individuals reach 18 years of age, at which point it was assumed that separate NICE recommendations for the interventions in adults apply. A separate scenario analysis is presented below that considers a common time horizon of 14 years for both populations, which is sufficient to capture differences in costs and effects between the interventions. Second, the total costs of etanercept are proportionally greater in the older age group than in the younger age group. This is because of the higher drug acquisition costs of etanercept once individuals reach the age of 10 years, that is, etanercept costs £715 per 4-week cycle in the model (i.e. £178.75 per 50-mg dose each week) for those aged ≥ 10 years and £357.50 per 4-week cycle in the model (i.e. £89.38 per 25-mg dose each week) for those aged < 10 years.
For children and young people aged 6–11 years, adalimumab is the most effective treatment but the incremental gain in QALYs compared with etanercept is relatively small because the utility gains in EQ-5D-Y associated with higher PASI response rates are small. Therefore, the benefits of achieving a greater PASI response do not translate into a large improvement in health outcomes. The benefit of more individuals achieving a higher PASI response rate manifests itself in lower costs associated with less time spent in BSC. The average proportion of individuals achieving a PASI 75 response is 79% for adalimumab and 54% for etanercept. The higher efficacy associated with adalimumab compared with etanercept, which results in fewer individuals accumulating the costs associated with BSC (approximately £284 per 4-week cycle), is not sufficient to offset the additional treatment costs of adalimumab, which are £704.28 per 4-week cycle (i.e. £352.14 per dose every 2 weeks), compared with £357.50 per 4-week cycle for etanercept in children aged < 10 years and £715 per 4-week cycle for children aged ≥ 10 years (note that, although the costs for etanercept increase at age 10 years, there are fewer individuals receiving treatment at this point because the starting age in the model is 6 years and the treatment withdrawal rate is assumed to be 20% per annum).
For children and young people aged 12–17 years, ustekinumab is the most effective treatment but again the incremental gain in QALYs compared with the alternative interventions is relatively small because of the small magnitude of utility gains for the different PASI response categories in the population of children and young people compared with adults (see Utility estimates by health state). The drug acquisition costs of etanercept in young people aged ≥ 12 years are greater than those of adalimumab (£715 for etanercept vs. £704.28 for adalimumab per 4-week cycle) whereas the efficacy for adalimumab is greater than that for etanercept, which reduces the time spent on BSC for those treated with adalimumab. As a result, it might be expected that the total costs of adalimumab would be lower than those for etanercept; however, the improved efficacy of adalimumab also extends the time that individuals receive the intervention and therefore the overall costs of adalimumab increase. Despite this, the incremental costs of etanercept relative to BSC are greater for each additional gain in QALYs than the incremental costs of adalimumab relative to BSC for each QALY gain. As a result, adalimumab extendedly dominates etanercept, which rules out etanercept as a potential cost-effective treatment option.
Treatment with ustekinumab results in the highest average proportion of individuals achieving a PASI 75 response rate (82% vs. 79% for adalimumab and 54% for etanercept), but also has the highest total costs. The higher total costs for ustekinumab compared with adalimumab are the result of the marginally higher drug acquisition costs associated with ustekinumab [£715.67 per 4-week cycle (i.e. £2147.00 per dose with each dose given at 12 weekly intervals) vs. £704.28 per 4-week cycle (i.e. £352.14 per dose at fortnightly intervals) for adalimumab] and the greater cost of ustekinumab during the induction period (i.e. a cost of £2147.00 per dose given at baseline, 4 weeks and 16 weeks) than of adalimumab in the induction period (i.e. a cost of £352.14 per dose given at baseline, 1 week and then every 2 weeks up to week 16). The higher efficacy associated with ustekinumab compared with adalimumab, with an average of 3% more individuals achieving a PASI 75 response, results in a reduction in costs associated with individuals on ustekinumab remaining off BSC for longer, but this reduction is not sufficient to offset the additional treatment costs associated with ustekinumab.
The pairwise ICERs for each of the interventions compared with BSC indicate the ICER at which the particular therapy might enter a sequence. Under base-case assumptions, these ICERs are very high, ranging from £110,430 (adalimumab) to £137,059 (etanercept) per additional QALY in children and young people aged 12–17 years. The optimal treatment option is BSC up until the threshold reaches £111,000 per QALY gained, when adalimumab would then enter as the first treatment in the sequence. The fact that BSC is the only form of management available until the threshold reaches £111,000 per QALY suggests that, under base-case assumptions, none of the biological therapies are sufficiently cost-effective to enter the sequence until this threshold is used. The probability that any of the biologics are cost-effective at a threshold of £30,000 per additional QALY is zero.
Cost-effectiveness results for alternative scenarios
Intervention and comparators
Scenario 1: off-label use of biologics outside age constraints and position in the pathway
As discussed in Decision problem and patient population, the biological interventions differ in their marketing authorisation by age and positioning of treatment in the pathway. Adalimumab is licensed for the youngest age group from ≥ 4 years and is the only biological treatment positioned as a second-line therapy in individuals who are inadequately controlled by, or who are intolerant to, topical therapy and phototherapies, that is, as an alternative to systemic therapy. This makes the comparison of adalimumab with etanercept and ustekinumab more problematic as the latter interventions are licensed as third-line therapies in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies and who are aged ≥ 6 years in the case of etanercept and ≥ 12 years in the case of ustekinumab. In this scenario, the off-label use of the biologics outside their age constraints and positioning in the management pathway is considered.
In the absence of clinical effectiveness evidence in a systemic therapy-naive population, the same efficacy estimates as in the base-case analysis were used in this scenario. Therefore, the only difference between this scenario and the base-case assumptions is the comparator, which is methotrexate in the analysis that considers biologics as an alternative to systemic therapy, and the time horizon of the model, which extends to 14 years because the starting age in the model is now 4 years.
Table 74 presents the cost-effectiveness results for the use of the interventions as an alternative to systemic therapy for all ages (4–17 years). Ustekinumab is the most effective treatment, followed by adalimumab, etanercept and methotrexate, as the efficacy of the treatments follow in this order. In terms of costs, ustekinumab is the most costly treatment, followed by adalimumab, etanercept and methotrexate. The reason for this ordering is the same as in the base-case results, with ustekinumab costing £715.67 per 4-week cycle compared with a cost per cycle of £704.28 for adalimumab, £357.50 and £715 for etanercept for those aged < 10 years and ≥ 10 years, respectively, and approximately £60 for methotrexate, with the reduction in costs associated with improved efficacy (i.e. less time spent on BSC) not sufficient to offset the additional treatment costs. Based on a fully incremental analysis, the incremental costs of etanercept and adalimumab relative to methotrexate are greater for each additional gain in QALY than the incremental costs of ustekinumab relative to methotrexate for each QALY gain. Therefore, etanercept and adalimumab are extendedly dominated by ustekinumab. The ICER of ustekinumab compared with methotrexate is very high at £293,117 per QALY gained. As a result, the optimal treatment option in a systemic therapy-naive population is methotrexate.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | |||||||
| MTX | 34,914 | 9.939 | – | – | – | – | MTX |
| ETA | 46,767 | 9.948 | 11,853 | 0.009 | ED ADA | 1,319,539 | |
| ADA | 61,999 | 10.027 | 27,084 | 0.088 | ED UST | 308,329 | |
| UST | 64,426 | 10.040 | 29,512 | 0.101 | 293,117 | 293,117 | |
Table 75 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for all ages (4–17 years). The only difference between this scenario and the base-case analysis is the starting age of 4 years used in the model. The total absolute costs and QALYs are greater than in the base case because of the longer model time horizon of 14 years. The ordering of the treatments in terms of costs and QALYs follows that in the base case, with ustekinumab the most effective but most costly treatment, followed by adalimumab, etanercept and BSC. Based on a fully incremental analysis, adalimumab is extendedly dominated by ustekinumab. Compared with the base-case population aged 12–17 years, etanercept is no longer extendedly dominated because more individuals receive a lower dose of etanercept, at the cost of a 25-mg vial rather than a 50-mg vial. The ICERs are lower than in the base-case analysis but the optimal treatment option remains BSC. BSC is the optimal option until the threshold reaches £60,000 per QALY gained, when etanercept would then enter as the first treatment in the sequence.
| Intervention | Mean cost (£) | Mean QALYs | Incremental costs vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | |||||||
| BSC | 40,478 | 9.843 | – | – | – | – | BSC |
| ETA | 46,767 | 9.948 | 6289 | 0.105 | 59,924 | 59,924 | |
| ADA | 61,999 | 10.027 | 15,231 | 0.079 | ED UST | 117,080 | |
| UST | 64,426 | 10.040 | 17,659 | 0.092 | 193,573 | 121,779 | |
Model time horizon
Scenario 2: time horizon of the model
The time horizon of the model was chosen to reflect the fact that once individuals reach 18 years of age separate NICE recommendations for the use of the interventions in adults apply. To incorporate these recommendations, evidence on the efficacy of the treatments in biologic-experienced patients (i.e. effectiveness estimates conditional on previous biological therapy) would be required. This would involve modelling the sequential use of therapies, with every possible potential treatment sequence considered based on current recommendations in adults. As well as being outside the scope of this appraisal, this would represent a significant challenge for two reasons: first, there is very limited evidence on the efficacy of biologics when used in sequence, that is, in biologic-experienced patients, and, second, current NICE recommendations for the use of biologic therapies in moderate to severe psoriasis in adults have been informed by a series of STAs98–102 rather than a MTA that establishes the optimal sequence of treatments in adults.
Furthermore, the differences in the marketing authorisations of the interventions by age inevitably mean that the time horizon of the model will differ according to age group. In this scenario, the impact of the time horizon was assessed by considering a common time horizon of 14 years for all age groups, but with the same starting age for each group as used in the base-case analysis. The time horizon of 14 years is sufficient to capture differences in costs and effects between the interventions under comparison because all individuals on each treatment in the model have moved to BSC by 14 years. This time horizon is also greater than the 10 years used in previous TAs in adults.
The base case already considers a time horizon of 14 years for adalimumab as an alternative to systemic therapy because the starting age is 4 years. Therefore, Table 76 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for a common time horizon of 14 years. By extending the time horizon, the total costs and QALYs for the interventions are greater than in the base case, but the relative cost-effectiveness of the interventions remains the same, that is, the ICERs for each intervention relative to the next best treatment option or BSC are similar to those observed in the base case. Therefore, the model time horizon used in the base-case analysis is sufficient to capture the differences between the interventions in terms of costs and QALYs.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 41,413 | 9.842 | – | – | – | – | BSC |
| ETA | 49,109 | 9.948 | 7696 | 0.105 | 73,153 | 73,153 | |
| ADA | 62,723 | 10.027 | 13,614 | 0.079 | 172,000 | 115,592 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 44,010 | 9.836 | – | – | – | – | BSC |
| ETA | 58,286 | 9.942 | 14,275 | 0.105 | ED ADA | 135,354 | |
| ADA | 64,204 | 10.021 | 20,194 | 0.184 | 109,531 | 109,531 | |
| UST | 66,503 | 10.033 | 2299 | 0.012 | 188,715 | 114,439 | |
Treatment effectiveness estimates
Scenario 3a: direct trial evidence for treatment effects in children and young people
As discussed in Chapter 4, a NMA was used in the base-case analysis to connect the evidence from the adalimumab trial (M04-717) in children and young people to the evidence from the etanercept (20030211) and ustekinumab (CADMUS) trials by drawing strength from the wider network of evidence in adults. In this scenario, the relative cost-effectiveness of adalimumab compared with methotrexate and of etanercept and ustekinumab compared with BSC is considered using the direct efficacy estimates derived from their corresponding trials. The limitation of this approach is that it does not allow the relative cost-effectiveness of all three biologics to be assessed in the same analysis. However, it may give an indication of how much influence the wider network of evidence has on the individual pairwise comparisons.
Table 77 presents the cost-effectiveness results for use of adalimumab as an alternative to systemic therapy using the efficacy estimates from the M04-717 trial alone. The incremental cost (£20,256) and QALYs (0.037) for adalimumab compared with methotrexate are lower than the base-case incremental cost (£27,084) and QALYs (0.088). The PASI 75 response rate is 58% for adalimumab and 32% for methotrexate in the M04-717 trial compared with 79% and 49%, respectively, in the NMA. The NMA estimates higher absolute values for PASI 75 response but the incremental difference between adalimumab and methotrexate is of a similar magnitude in the NMA (30% difference in PASI 75 response) and the M04-717 trial (26% difference in PASI 75 response). This smaller difference in relative effectiveness between adalimumab and methotrexate in the M04-717 trial means that the incremental cost for each additional gain in QALYs is greater for adalimumab compared with methotrexate. The resulting ICER increases from £308,329 per additional QALY in the base-case analysis to £549,899 per additional QALY using the direct trial evidence.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| MTX | 36,601 | 9.919 | – | – | – | MTX |
| ADA | 56,857 | 9.956 | 20,256 | 0.037 | 549,899 | |
Table 78 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using the efficacy estimates from the etanercept trial (20030211) and the ustekinumab trial (CADMUS). The total costs and QALYs for etanercept and ustekinumab compared with BSC are very similar to those in the base case. This is because the PASI 75 response rates estimated from the NMA for etanercept (54%), ustekinumab (82%) and placebo (11.5%) are very similar to the corresponding response rates from the individual trials (CADMUS: 81% for ustekinumab vs. 11% for placebo; 20030211: 57% for etanercept vs. 11.4% for placebo). As a result, the pairwise ICERs for etanercept and ustekinumab compared with BSC are similar to those in the base-case analysis: the ICER for etanercept compared with BSC increases from £71,903 per QALY in the base-case analysis to £75,350 per QALY using the direct trial evidence and the ICER for ustekinumab compared with BSC increases marginally from £116,568 per QALY in the base-case analysis to £116,982 per QALY using the direct trial evidence.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. BSC (£) | Incremental QALYs vs. BSC | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | ||||||
| BSC | 36,406 | 8.720 | – | – | – | BSC |
| ETA | 44,108 | 8.822 | 7701 | 0.102 | 75,350 | |
| Children and young people aged 12–17 years | ||||||
| BSC | 21,749 | 4.814 | – | – | – | BSC |
| UST | 39,622 | 4.966 | 17,873 | 0.153 | 116,982 | |
Scenario 3b: indirect treatment comparison estimates in children and young people
In this scenario, the relative cost-effectiveness of etanercept and ustekinumab compared with BSC is considered using the indirect treatment comparison estimates from the 20030211 and CADMUS trials, with placebo used as a common comparator. The limitation of this approach is that it does not allow the relative cost-effectiveness of etanercept and ustekinumab compared with adalimumab to be determined because of the absence of a placebo arm in the M04-717 trial.
Table 79 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using efficacy estimates from an indirect treatment comparison of etanercept from the 20030211 trial and ustekinumab from the CADMUS trial. The total costs and QALYs for etanercept, ustekinumab and BSC are similar to those in the base-case analysis. This is expected as the efficacy estimates from the individual trials for these interventions are similar to those estimated in the NMA. Etanercept is extendedly dominated by ustekinumab as the incremental costs of etanercept relative to BSC are greater for each additional gain in QALYs than the incremental costs of ustekinumab relative to BSC for each QALY gain. This occurs because ustekinumab has a better efficacy (78% PASI 75 response) than etanercept (57% PASI 75 response), which results in improved health outcomes for ustekinumab. Interestingly, the total cost for ustekinumab is greater than that for etanercept despite the fact that the drug acquisition costs are similar between the two treatments in children and young people aged 12–17 years. This arises because, although the improved efficacy of ustekinumab reduces the time spent on BSC, it also means that a greater proportion of time is spent on a cost-ineffective treatment option. The ICER of ustekinumab compared with BSC is £119,092 per QALY gained. As a result, the optimal treatment option is BSC unless the cost-effectiveness threshold reaches £120,000 per additional QALY.
| Intervention | Mean cost (£) | Mean QALYs | Incremental costs vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.809 | – | – | – | – | BSC |
| ETA | 33,662 | 4.901 | 11,913 | 0.092 | ED UST | 128,903 | |
| UST | 39,105 | 4.955 | 17,356 | 0.146 | 119,092 | 119,092 | |
Scenario 3c: treatment effects from the network meta-analysis using minimum evidence from the adult population
In Chapter 4 the disconnected network of evidence in children and young people was connected in the first instance by bringing together the minimum amount of evidence required from the adult population to link the adalimumab trial with the other paediatric trials. The CHAMPION study in adults,106 which was a three-arm trial comparing adalimumab, methotrexate and placebo, represented the best way of connecting adalimumab to etanercept and ustekinumab using the least amount of evidence borrowed from the adult population. In this scenario, the relative cost-effectiveness of the interventions was considered in the base-case populations using the treatment effects estimated from the minimum network of evidence.
Table 80 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy using the NMA with minimum links to the adult evidence. The incremental cost of £18,422 for adalimumab compared with methotrexate is lower than the incremental cost of £27,084 in the base-case analysis. This is because of a larger difference in PASI 75 response rates between adalimumab and methotrexate in the minimum NMA (approximately 40% difference) than in the full network of evidence (approximately 30% difference). Although there is a higher efficacy difference between adalimumab and methotrexate in this scenario, the health outcomes also depend on the utility associated with BSC, which is based on the proportion of individuals in the different PASI response categories in the placebo arm in the NMA. The PASI response rates for placebo are greater in the minimum NMA than in the full network. Therefore, the gain in utility associated with better efficacy on adalimumab is offset by a higher gain in utility associated with BSC. As a result, the incremental QALYs for adalimumab compared with methotrexate are very similar to those in the base-case analysis. The corresponding ICER for adalimumab compared with methotrexate is reduced from £308,329 per additional QALY in the base case to £211,259 per additional QALY.
| Intervention | Mean cost (£) | Mean QALYs | Incremental costs vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| MTX | 38,177 | 9.879 | – | – | – | MTX |
| ADA | 56,599 | 9.966 | 18,422 | 0.087 | 211,259 | |
Table 81 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using the NMA with minimum links to the adult evidence. The incremental costs and QALYs for etanercept and ustekinumab compared with BSC are similar to those in the base-case analysis, but the incremental costs and QALYs for adalimumab are reduced in both age groups. This is because the differences in PASI 75 response rate between the interventions and BSC in the minimum NMA and the full NMA are similar for etanercept (44% vs. 43%) and ustekinumab (66% vs. 71%) but are much smaller for adalimumab (44% vs. 68%). As a result, adalimumab is less cost-effective in children and young people aged 6–11 years (ICER vs. BSC increases from £115,825 per additional QALY in the base case to £137,329 per additional QALY) and is extendedly dominated by ustekinumab in the 12–17 years age group. The ICER for etanercept is reduced by £3400 in children aged 6–11 years, but etanercept is also extendedly dominated by ustekinumab in children aged 12–17 years. The ICER for ustekinumab compared with BSC increases slightly from the base-case value of £116,568 per QALY gained to £118,515 per QALY gained using the minimum NMA.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.717 | – | – | – | – | BSC |
| ETA | 44,063 | 8.828 | 7657 | 0.112 | 68,485 | 68,485 | |
| ADA | 52,067 | 8.831 | 8004 | 0.002 | 3,587,196 | 137,329 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.807 | – | – | – | – | BSC |
| ETA | 33,598 | 4.898 | 11,849 | 0.091 | ED UST | 130,389 | |
| ADA | 33,977 | 4.899 | 380 | 0.001 | ED UST | 132,682 | |
| UST | 39,264 | 4.955 | 17,515 | 0.148 | 118,515 | 118,515 | |
Scenario 3d: Psoriasis Area and Severity Index response assessment
In this scenario, PASI 50 is considered as the primary efficacy end point for response assessment at the end of the trial period instead of PASI 75, as used in the base-case analysis.
Table 82 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy using PASI 50 as the primary efficacy end point. The incremental costs and QALYs for adalimumab compared with methotrexate increase compared with the base case because there is a smaller difference in PASI 50 response rates between the interventions (91.5% adalimumab vs. 71% methotrexate) than for PASI 75 response rates (79% adalimumab vs. 49% methotrexate). As a result, the ICER increases from £308,329 per QALY gained to £353,148 per QALY gained.
| Intervention | Mean cost (£) | Mean QALYs | Incremental costs vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| MTX | 32,765 | 9.932 | – | – | – | MTX |
| ADA | 65,008 | 10.023 | 32,243 | 0.091 | 353,148 | |
Table 83 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using PASI 50 as the primary efficacy end point. The incremental cost per additional QALY gained is greater for all interventions than in the base-case analysis. This is because the total costs have increased (a greater proportion of individuals continue treatment as responders) but the total QALYs have decreased across the interventions. The difference in PASI 50 response rate between the interventions and BSC is similar to the difference observed in PASI 75 response rates. The decrease in QALYs results from the proportionally smaller utility gain associated with the PASI 50–75 response category than with the PASI 75–90 and PASI ≥ 90 response categories. BSC remains the optimal treatment option and the probability that any of the biologics are cost-effective at a threshold of £30,000 per additional QALY is zero.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.710 | – | – | – | – | BSC |
| ETA | 46,396 | 8.807 | 9990 | 0.097 | 103,388 | 103,388 | |
| ADA | 60,091 | 8.886 | 13,695 | 0.079 | 172,967 | 134,724 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.804 | – | – | – | – | BSC |
| ETA | 36,930 | 4.882 | 15,180 | 0.078 | ED ADA | 193,536 | |
| ADA | 40,024 | 4.947 | 18,275 | 0.143 | 127,783 | 127,783 | |
| UST | 41,833 | 4.957 | 1809 | 0.010 | 131,128 | 131,128 | |
Health-related quality-of-life utility values
Scenario 4a: EuroQol-5 Dimensions utility estimates from adults
The HRQoL utility values in children and young people are subject to considerable uncertainty. EQ-5D-Y values mapped from PedsQL data from the CADMUS trial (ustekinumab) at baseline and 12 weeks’ follow-up were used to estimate utility gains from baseline associated with different PASI response categories (< 50, 50–74, 75–89, ≥ 90). The utility values associated with treatment were then based on the proportion of individuals in the different PASI response categories from the NMA and the associated utility gain for each PASI category. As discussed in Utility estimates used in the model, the estimated EQ-5D-Y utility gains mapped from the PedsQL data were of a much smaller magnitude than the EQ-5D values used in previous TAs in adults. 97–102 It was also noted that the gains in CDLQI score by PASI response category were of a smaller magnitude than the DLQI values reported in adults. It is not clear whether these smaller utility increments observed in children and young people are a reflection of a lower impact of severe psoriasis on quality of life in a paediatric population or a result of the small sample sizes and the limited data in this population.
In this scenario, EQ-5D utility values from the adult population were used to inform the gains in utility associated with PASI response in children and young people. Utility values from TA10397 (etanercept) were used; however, the implications of using alternative adult utility values from TA14699 (adalimumab) and TA180100 (ustekinumab) were also considered (see Table 62 for a comparison of utility values in children and young people and adults).
Table 84 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy using utility estimates from an adult population. The total QALYs for the interventions are lower than those in the base-case analysis, but this is because of the use of a lower baseline utility value in this scenario to prevent the utility values rising above 1.0. Note that changing the baseline utility value used in the model does not significantly affect the cost-effectiveness results because the model is driven by the incremental changes in utility from baseline. The incremental QALYs of 0.150 for adalimumab compared with methotrexate are significantly higher than the incremental QALYs of 0.088 in the base case. As a result, the ICER for adalimumab compared with methotrexate reduces from £308,329 to £180,773 per additional QALY. The implications of using adult utility values from TA180100 and TA14699 are even more pronounced, with an incremental gain in QALYs of 0.204 and 0.260, respectively, for adalimumab compared with methotrexate, resulting in corresponding ICERs of £132,616 and £104,010 per additional QALY (see Appendix 8 for results based on utility estimates from TA180100).
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Utility estimates sourced from TA103:97 children and young people aged 4–17 years | ||||||
| MTX | 34,931 | 9.116 | – | – | – | MTX |
| ADA | 62,043 | 9.266 | 27,112 | 0.150 | 180,773 | |
| Utility estimates sourced from TA146:99 children and young people aged 4–17 years | ||||||
| MTX | 34,919 | 9.229 | – | – | – | MTX |
| ADA | 62,000 | 9.489 | 27,081 | 0.260 | 104,010 | |
Table 85 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy using utility estimates from an adult population. The incremental QALYs for the interventions compared with BSC are substantially greater than those in the base case. Ustekinumab is the most effective intervention, followed by adalimumab, etanercept and BSC, and the incremental gain in QALYs from moving from one intervention to the next is greater than in the base case. As a result, all of the ICERs are substantially lower than in the base case, falling by 55–61%. Etanercept shows the largest reduction in the ICER and, at a threshold of £30,000 per additional QALY, etanercept becomes the optimal treatment in the 6–11 years age group. In the 12–17 years age group, etanercept is extendedly dominated by adalimumab because of the higher drug acquisition costs associated with this age group requiring more than a 25-mg dose. In those aged 12–17 years, the optimal treatment option remains BSC up until a threshold of £51,000 per QALY gained, when adalimumab would then enter as the first treatment in the sequence. At a threshold of £60,000 per QALY, adalimumab represents the only cost-effective treatment option based on a fully incremental analysis, whereas all of the biologics would be considered cost-effective based on a pairwise comparison with BSC.
| Intervention | Mean cost (£) | Mean QALYs | Incremental costs vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Utility estimates sourced from TA103:97 children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 7.844 | – | – | – | – | ETA |
| ETA | 43,798 | 8.102 | 7392 | 0.257 | 28,740 | 28,740 | |
| ADA | 57,257 | 8.237 | 13,459 | 0.135 | 99,419 | 53,112 | |
| Utility estimates sourced from TA146:99 children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 7.890 | – | – | – | – | ETA |
| ETA | 43,829 | 8.219 | 7423 | 0.329 | 22,578 | 22,578 | |
| ADA | 57,215 | 8.450 | 13,386 | 0.232 | 57,762 | 37,125 | |
| Utility estimates sourced from TA103:97 children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.326 | – | – | – | – | BSC |
| ETA | 33,181 | 4.535 | 11,432 | 0.209 | ED ADA | 54,717 | |
| ADA | 37,844 | 4.644 | 16,095 | 0.318 | 50,578 | 50,578 | |
| UST | 39,968 | 4.661 | 2124 | 0.016 | 131,702 | 54,491 | |
| Utility estimates sourced from TA146:99 children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.326 | – | – | – | – | BSC |
| ETA | 33,195 | 4.618 | 11,446 | 0.292 | ED ADA | 39,247 | |
| ADA | 37,873 | 4.807 | 16,124 | 0.481 | 33,517 | 33,517 | |
| UST | 39,928 | 4.837 | 2055 | 0.029 | 69,895 | 35,612 | |
The implications of using adult utility values from TA180100 and TA14699 are even more pronounced than when using utility gains from TA10397 because of the greater utility gains in the PASI 75–89 and ≥ 90 categories. The ICERs for children and young people aged 6–11 years are £22,578 (TA14699) and £21,546 (TA180100) for etanercept compared with BSC and £37,125 (TA14699) and £39,682 (TA180100) for adalimumab compared with BSC. The lowest ICERs for children and young people aged 12–17 years are £33,517 for adalimumab compared with BSC, £35,612 for ustekinumab compared with BSC and £39,247 for etanercept compared with BSC.
Scenario 4b: utility estimates for best supportive care
The base-case analysis assumes that the utility associated with BSC is based on the proportion of individuals in the different PASI response categories in the placebo arm of the NMA. In this scenario, the utility for BSC was set equal to the baseline value, that is, assuming that there is no utility gain associated with BSC.
Table 86 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy assuming that there is no utility benefit associated with BSC. For the comparison of adalimumab and methotrexate, the assumption of no utility benefit on BSC affects only the utility of non-responders. The total QALYs for both interventions are reduced and the incremental QALYs for adalimumab compared with methotrexate increase from 0.088 in the base case to 0.102 because of the higher efficacy of adalimumab, which reduces the time spent in BSC.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| MTX | 34,925 | 9.833 | – | – | – | MTX |
| ADA | 62,010 | 9.935 | 27,085 | 0.102 | 266,161 | |
Table 87 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy assuming that there is no utility benefit associated with BSC. This assumption reduces the total QALYs for the comparator of BSC and the utility of non-responders. As a result, the incremental QALYs for the interventions compared with BSC increase and the incremental gain in QALYs for the interventions relative to the next best option (e.g. ustekinumab is the most effective treatment, followed by adalimumab and etanercept) also increases as less time is spent on BSC. Consequently, the ICERs for the interventions are reduced compared with the base-case values.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.593 | – | – | – | – | BSC |
| ETA | 43,785 | 8.724 | 7378 | 0.131 | 56,430 | 56,430 | |
| ADA | 57,208 | 8.812 | 13,423 | 0.089 | 151,299 | 94,780 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.739 | – | – | – | – | BSC |
| ETA | 33,193 | 4.846 | 11,444 | 0.106 | ED ADA | 107,462 | |
| ADA | 37,844 | 4.917 | 16,095 | 0.178 | 90,292 | 90,292 | |
| UST | 39,969 | 4.929 | 2124 | 0.012 | 180,232 | 95,871 | |
Costs associated with best supportive care
Scenario 5a: number of hospitalisations per annum for best supportive care
The resource use associated with BSC, in particular the number of hospitalisations per annum, was identified as an area of high uncertainty and a key driver of cost-effectiveness in previous TAs in adults. 97–102 Two main sources have been referred to in previous appraisals: (1) NICE CG153,173 in which an average of 26.6 inpatient days per year was estimated for individuals whose psoriasis has not responded to treatment, and (2) Fonia et al. ,144 who estimated an average of 6.49 days of hospitalisation per annum. During previous NICE appraisals, the clinical experts considered that both sources are likely to overestimate the actual number of hospital days and resource use associated with BSC. This is in part because of the populations considered in CG153173 and the study by Fonia et al. ,144 with CG153173 considering a high-need population with very severe psoriasis and the study by Fonia et al. 144 describing care in a tertiary care centre known for treating the most severely affected individuals. The clinical experts in recent appraisals also noted that the number of individuals hospitalised for severe psoriasis has fallen over time and is continuing to fall. They also indicated that BSC is mostly given to individuals during their outpatient visits. As a result, the resource use associated with BSC is an area of considerable uncertainty and both sources of data have a number of shortcomings, even in the adult population.
In the base-case analysis in children and young people, it was assumed that there are no hospitalisations for psoriasis in this population. This was informed by clinical opinion, with our clinical advisor suggesting that hospitalisations in children and young people are very rare, partly because this population has not yet developed the comorbidities that often complicate more severe cases of psoriasis in adults. In this scenario, the implications of assuming no inpatient stays for children and young people were explored by using an estimate of 6.49 hospitalisations per annum based on the study by Fonia et al. 144 and 26.6 hospitalisations per annum based on CG153173 in adults.
Table 88 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy, assuming hospitalisations for BSC. For the comparison of adalimumab and methotrexate, the total costs for both interventions are increased, but the incremental cost of adalimumab compared with methotrexate decreases because of the higher efficacy associated with adalimumab, which reduces the time spent in BSC. The resulting ICER decreases from £308,329 per additional QALY in the base case to £281,029 per additional QALY for 6.49 inpatient days per annum and £202,571 per additional QALY for 26.6 inpatient days per annum.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| 6.49 hospitalisation days per annum for BSC:144 children and young people aged 4–17 years | ||||||
| MTX | 52,280 | 9.939 | – | – | – | MTX |
| ADA | 77,153 | 10.027 | 24,873 | 0.089 | 281,029 | |
| 26.6 hospitalisation days per annum for BSC:173 children and young people aged 4–17 years | ||||||
| MTX | 106,053 | 9.939 | – | – | – | MTX |
| ADA | 123,929 | 10.027 | 17,876 | 0.088 | 202,571 | |
Table 89 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy, assuming hospitalisations for BSC. Under this assumption, the costs of BSC increase by £147.67 and £605.25 per 4-week cycle for 6.49 and 26.6 inpatient days per annum respectively. As a result, the total costs associated with the comparator of BSC increase and the costs for non-responders increase. For children and young people aged 6–11 years, the reduction in the incremental cost of etanercept compared with BSC is sufficient to make etanercept the optimal treatment option at a threshold of £30,000 per QALY for a stay of 6.49 inpatient days per annum. When the hospitalisation LOS per annum is increased to 26.6 days in this age group, etanercept becomes the least costly treatment option and BSC becomes dominated by etanercept (i.e. BSC costs more than etanercept but produces fewer QALYs). Adalimumab enters as a cost-effective option only if the threshold increases to £70,000 per QALY gained.
| Intervention | Mean cost (£) | Mean QALYs | Incremental costs vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| 6.49 hospitalisation days per annum for BSC144 | |||||||
| Children and young people aged 6–11 years | |||||||
| BSC | 55,597 | 8.710 | – | – | – | – | ETA |
| ETA | 58,500 | 8.813 | 2903 | 0.103 | 28,286 | 28,286 | |
| ADA | 70,016 | 8.891 | 11,516 | 0.078 | 148,586 | 80,046 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 32,333 | 4.804 | – | – | – | – | BSC |
| ETA | 40,099 | 4.887 | 7766 | 0.083 | ED ADA | 93,102 | |
| ADA | 43,188 | 4.950 | 10,855 | 0.146 | 74,501 | 74,501 | |
| UST | 45,064 | 4.960 | 1875 | 0.010 | 186,634 | 81,735 | |
| 26.6 hospitalisation days per annum for BSC173 | |||||||
| Children and young people aged 6–11 years | |||||||
| BSC | 115,063 | 8.710 | 5550 | –0.180 | Dominated | – | ETA |
| ETA | 104,113 | 8.813 | – | – | – | Dominant | |
| ADA | 109,512 | 8.891 | 5399 | 0.077 | 69,797 | Dominant | |
| Children and young people aged 12–17 years | |||||||
| BSC | 65,129 | 4.804 | 4119 | –0.156 | Dominated | – | ADA |
| ETA | 61,537 | 4.887 | 1777 | –0.062 | Dominated | Dominant | |
| ADA | 59,760 | 4.950 | – | – | – | Dominant | |
| UST | 61,010 | 4.960 | 1250 | 0.011 | 118,665 | Dominant | |
For children and young people aged 12–17 years, etanercept is dominated by adalimumab. The ICER for adalimumab compared with BSC is £74,501 per QALY when 6.49 inpatient days per annum are assumed. When 26.6 inpatient days per annum are assumed, adalimumab becomes the least costly treatment option and the most cost-effective option at a threshold of £30,000 per QALY. The ICER for ustekinumab compared with adalimumab is £118,665 per QALY for a stay of 26.6 inpatient days per annum.
Costs associated with adverse events
Scenario 6: costs of severe infections and malignancies
In the absence of robust evidence on the incidence of AEs associated with treatment in children and young people, the base-case analysis assumed that there were no AEs associated with treatment. In this scenario, the costs associated with SAEs, including NMSC, malignancies other than NMSC and severe infections, are included. These events are expected to be very rare.
Table 90 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy with the costs of AEs included. The incremental cost of adalimumab increases by only £400. The resulting impact on the ICER is minor, with an increase from £308,329 per QALY gained in the base case to £311,067 per QALY gained.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| MTX | 35,176 | 9.939 | – | – | – | MTX |
| ADA | 62,694 | 10.027 | 27,518 | 0.088 | 311,067 | |
Table 91 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy with the costs of AEs included. As expected, the incremental costs for the interventions relative to BSC increase, but the resulting impact on the ICERs for all interventions is very minor.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.710 | – | – | – | – | BSC |
| ETA | 44,310 | 8.813 | 7904 | 0.103 | 76,810 | 76,810 | |
| ADA | 57,911 | 8.891 | 13,601 | 0.077 | 176,012 | 119,357 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.804 | – | – | – | – | BSC |
| ETA | 33,584 | 4.887 | 11,835 | 0.083 | ED ADA | 142,041 | |
| ADA | 38,382 | 4.950 | 16,633 | 0.146 | 113,974 | 113,974 | |
| UST | 40,063 | 4.960 | 1682 | 0.010 | 169,254 | 117,497 | |
Treatment withdrawal rates
Scenario 7: withdrawal rates from treatment
In the base-case analysis discontinuation from treatment is modelled as an all-cause withdrawal probability of 20% per annum, which is applied to all interventions. This withdrawal rate has been used in all previous TAs in adults97–102 and is consistent with long-term survival rates of biologics from the BADBIR audit. 94 In the absence of alternative data in children and young people, this scenario considered two separate withdrawal rates of 10% and 30% per annum.
Table 92 presents the cost-effectiveness results for adalimumab as an alternative to systemic therapy for treatment withdrawal rates of 10% and 30% per annum. The lower withdrawal rate implies that individuals spend longer on treatment before moving to BSC, whereas the higher withdrawal rate means that individuals spend less time on treatment and more time on BSC. The total costs for adalimumab increase for the 10% rate and decrease for the 30% rate, whereas the total costs for methotrexate decrease for the 10% rate and increase for the 30% rate. This opposite effect between the treatments arises because the drug acquisition cost of adalimumab (£704.28 per 4-week cycle) is proportionally greater than the cost of BSC (approximately £284 per 4-week cycle) compared with the drug acquisition cost of methotrexate (approximately £60 per 4-week cycle) relative to the cost of BSC. As a result, the withdrawal rate has less impact on the total cost of methotrexate than on the total cost of adalimumab. The incremental costs of adalimumab compared with methotrexate are £40,781 and £19,692 for the 10% and 30% annual withdrawal rates, respectively, compared with the base-case incremental cost of £27,084. In terms of health outcomes, the more time spent on treatment the higher the utility gains; therefore, the QALYs increase for the lower withdrawal rate and decrease for the higher withdrawal rate. The resulting ICERs for adalimumab compared with methotrexate are £298,846 and £318,188 per additional QALY for the 10% and 30% annual withdrawal rates, respectively, compared with the base-case value of £308,329 per additional QALY.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| Withdrawal rate of 10% per annum | ||||||
| MTX | 32,274 | 9.990 | – | – | – | MTX |
| ADA | 73,055 | 10.126 | 40,781 | 0.136 | 298,846 | |
| Withdrawal rate of 30% per annum | ||||||
| MTX | 36,364 | 9.912 | – | – | – | MTX |
| ADA | 56,057 | 9.974 | 19,692 | 0.062 | 318,188 | |
Table 93 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for treatment withdrawal rates of 10% and 30% per annum. The total costs for all of the interventions increase for the 10% rate and decrease for the 30% rate because of the accumulation of higher drug acquisition costs while on treatment for longer. This increase in costs is counterbalanced by an increase in utility gains while on treatment. The resulting impact on the ICERs is minimal. BSC remains the optimal treatment option at a threshold of £30,000 per QALY gained.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Withdrawal rate of 10% per annum | |||||||
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.710 | – | – | – | – | BSC |
| ETA | 49,361 | 8.864 | 12,955 | 0.154 | 84,138 | 84,138 | |
| ADA | 66,830 | 8.977 | 17,469 | 0.113 | 154,817 | 114,029 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.804 | – | – | – | – | BSC |
| ETA | 36,194 | 4.911 | 14,445 | 0.107 | ED ADA | 135,131 | |
| ADA | 42,002 | 4.989 | 20,253 | 0.185 | 109,399 | 109,399 | |
| UST | 44,400 | 5.002 | 2398 | 0.013 | 182,511 | 114,244 | |
| Withdrawal rate of 30% per annum | |||||||
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.710 | – | – | – | – | BSC |
| ETA | 40,978 | 8.784 | 4572 | 0.074 | 61,924 | 61,924 | |
| ADA | 51,745 | 8.841 | 10,766 | 0.056 | 190,888 | 117,774 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.804 | – | – | – | – | BSC |
| ETA | 30,952 | 4.870 | 9203 | 0.066 | ED ADA | 139,568 | |
| ADA | 34,732 | 4.920 | 3780 | 0.050 | 75,289 | 111,784 | |
| UST | 36,599 | 4.928 | 1867 | 0.008 | 230,608 | 119,527 | |
Biosimilars
Scenario 8: reduction in the cost of etanercept
The biosimilar of etanercept, Benepali (50 mg), does not have marketing authorisation for use in children and young people. In this scenario, the drug acquisition cost of etanercept was reduced by approximately 10% to match the cost of Benepali in adults.
Table 94 presents the cost-effectiveness results for treatment with the interventions after failed systemic therapy for a 10% reduction in the acquisition cost of etanercept. For children and young people aged 6–11 years, the incremental cost of etanercept relative to BSC is reduced by £580, which reduces the ICER from £71,903 to £66,240 per additional QALY. For children and young people aged 12–17 years, the incremental cost of etanercept relative to BSC is reduced by £1480, which is a greater reduction than that observed in the younger age group because it is assumed that the 10% reduction in the drug acquisition cost of etanercept applies only to children aged ≥ 10 years who require 50 mg of etanercept. The cost reduction has a very minor impact on the cost-effectiveness results.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 8.710 | – | – | – | – | BSC |
| ETA | 43,225 | 8.813 | 6819 | 0.103 | 66,240 | 66,240 | |
| ADA | 57,272 | 8.891 | 14,047 | 0.077 | 181,897 | 115,815 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.804 | – | – | – | – | BSC |
| ETA | 31,719 | 4.887 | 9970 | 0.083 | ED ADA | 119,501 | |
| ADA | 37,826 | 4.949 | 16,077 | 0.146 | 110,437 | 110,437 | |
| UST | 39,908 | 4.960 | 2082 | 0.010 | 205,422 | 116,619 | |
Discussion of the cost-effectiveness results and alternative scenarios
The results of the base-case analysis suggest that adalimumab is not a cost-effective treatment option when positioned in the pathway as an alternative to systemic therapy, with an ICER of £308,329 per QALY gained compared with methotrexate. When positioned after systemic therapy, the ICER for adalimumab compared with BSC is £115,825 per QALY gained for ages 6–11 years and £110,430 per QALY gained for ages 12–17 years. At a threshold of £30,000 per QALY gained, etanercept is not a cost-effective option for the treatment of severe plaque psoriasis in individuals who are inadequately controlled by, or who are intolerant to, systemic therapies or phototherapies. The ICER for etanercept compared with BSC is £71,903 per QALY gained for ages 6–11 years and etanercept is extendedly dominated by adalimumab for those aged 12–17 years. Ustekinumab is the most effective treatment in children and young people aged 12–17 years but it is also the most costly treatment. Based on a fully incremental analysis, the ICER for ustekinumab compared with adalimumab is £201,507 per QALY gained, whereas the ICER for ustekinumab compared with BSC is £116,568 per QALY gained. The base-case results suggest that BSC is the only cost-effective form of management for the treatment of severe plaque psoriasis unless the threshold reaches ≥ £111,000 per additional QALY. The probability that any of the biologics are cost-effective at a threshold of £30,000 per QALY is zero.
The lack of cost-effectiveness appears to result from the very modest QALY gains associated with treatment. The small incremental difference in health benefits between the treatments is a result of the relatively small EQ-5D-Y utility gains associated with higher PASI response rates. As a consequence, the benefits of achieving a greater PASI response do not translate into large improvements in health outcomes. The acquisition costs of the treatments are also not substantially different: ustekinumab costs £715.67 per 4-week cycle (i.e. £2147.00 per dose with each dose given at 12 weekly intervals) compared with £704.28 per 4-week cycle (i.e. £352.14 per dose given every 2 weeks) for adalimumab and £715.00/£357.50 per 4-week cycle (i.e. £178.75 per 50-mg/£89.38 per 25-mg dose given each week) for etanercept, depending on patient weight.
A number of scenarios were used to explore the impact of alternative assumptions on the cost-effectiveness of the biological treatments. Tables 95 and 96 summarise the cost-effectiveness results for the scenario analyses for adalimumab as an alternative to systemic therapy and the use of the interventions after failed systemic therapy respectively.
| Analysis | ICER: ADA vs. MTX (£/QALY) |
|---|---|
| Children and young people aged 4–17 years | |
| Base case | 308,329 |
| Scenario 1: off-label use of biologics outside age constraints | – |
| Scenario 2: common time horizon of 14 years | – |
| Scenario 3a: direct trial evidence estimates of effect | 549,899 |
| Scenario 3b: indirect treatment comparison estimates | – |
| Scenario 3c: treatment effects from the NMA using minimum evidence from the adult population | 211,259 |
| Scenario 3d: PASI 50 response assessment | 353,148 |
| Scenario 4a | |
| EQ-5D utility estimates from TA10397 in adults | 180,773 |
| EQ-5D utility estimates from TA14699 in adults | 104,010 |
| Scenario 4b: utility on BSC equal to baseline value | 266,161 |
| Scenario 5 | |
| Hospitalisations of 6.49 days per annum | 281,029 |
| Hospitalisations of 26.6 days per annum | 202,571 |
| Scenario 6: costs associated with AEs | 311,067 |
| Scenario 7 | |
| Treatment withdrawal rate of 10% per annum | 298,846 |
| Treatment withdrawal rate of 30% per annum | 318,188 |
| Scenario 8: unit cost of biosimilar for etanercept | – |
| Analysis | ICER (£/QALY) | ||
|---|---|---|---|
| ADA vs. BSC | ETA vs. BSC | UST vs. BSC | |
| Base case | 117,080 | – | – |
| Scenario 1: off-label use of biologics outside age constraints | 117,080 | 59,924 | 121,779 |
| Children and young people aged 6–11 years | |||
| Base case | 115,825 | 71,903 | – |
| Scenario 1: off-label use of biologics outside age constraints | – | – | – |
| Scenario 2: common time horizon of 14 years | 115,592 | 73,153 | – |
| Scenario 3a: direct trial evidence estimates of effect | – | 75,350 | – |
| Scenario 3b: indirect treatment comparison estimates | – | – | – |
| Scenario 3c: treatment effects from the NMA using minimum evidence from the adult population | 137,329 | 68,485 | – |
| Scenario 3d: PASI 50 response assessment | 134,724 | 103,388 | – |
| Scenario 4a | |||
| EQ-5D utility estimates from TA10397 in adults | 53,112 | 28,740 | – |
| EQ-5D utility estimates from TA14699 in adults | 37,125 | 22,578 | – |
| Scenario 4b: utility in BSC equal to baseline value | 94,780 | 56,430 | – |
| Scenario 5 | |||
| Hospitalisations of 6.49 days per annum | 80,046 | 28,286 | – |
| Hospitalisations of 26.6 days per annum | Dominant | Dominant | – |
| Scenario 6: costs associated with AEs | 119,357 | 76,810 | – |
| Scenario 7 | |||
| Treatment withdrawal rate of 10% per annum | 114,029 | 84,138 | – |
| Treatment withdrawal rate of 30% per annum | 117,774 | 61,924 | – |
| Scenario 8: unit cost of biosimilar for etanercept | 115,815 | 66,240 | – |
| Children and young people aged 12–17 years | |||
| Base-case | 110,430 | 137,059 | 116,568 |
| Scenario 1: off-label use of biologics outside age constraints | – | – | – |
| Scenario 2: common time horizon of 14 years | 109,531 | 135,354 | 114,439 |
| Scenario 3a: direct trial evidence estimates of effect | – | – | 116,982 |
| Scenario 3b: indirect treatment comparison estimates | – | 128,903 | 119,092 |
| Scenario 3c: treatment effects from the NMA using minimum evidence from the adult population | 132,682 | 130,389 | 118,515 |
| Scenario 3d: PASI 50 response assessment | 127,783 | 193,536 | 131,128 |
| Scenario 4a | |||
| EQ-5D utility estimates from TA10397 in adults | 50,578 | 54,717 | 54,491 |
| EQ-5D utility estimates from TA14699 in adults | 33,517 | 39,247 | 35,612 |
| Scenario 4b: utility in BSC equal to baseline value | 90,292 | 107,462 | 95,871 |
| Scenario 5 | |||
| Hospitalisations of 6.49 days per annum | 74,501 | 93,102 | 81,735 |
| Hospitalisations of 26.6 days per annum | Dominant | Dominant | Dominant |
| Scenario 6: costs associated with AEs | 113,974 | 142,041 | 117,497 |
| Scenario 7 | |||
| Treatment withdrawal rate of 10% per annum | 109,399 | 135,131 | 114,244 |
| Treatment withdrawal rate of 30% per annum | 111,784 | 139,568 | 119,527 |
| Scenario 8: unit cost of biosimilar for etanercept | 110,437 | 119,501 | 116,619 |
The scenarios that have the most impact on the cost-effectiveness results are (1) use of utility estimates from an adult population (scenario 4a), (2) assuming that no health benefits are associated with BSC (scenario 4b) and (3) assuming that hospitalisations are associated with BSC (scenario 5).
The gains in utility in the adult population for the different PASI response categories are up to 6.6 times greater than the utility gains estimated in children and young people. It is unclear whether this difference reflects a lower impact of severe psoriasis on HRQoL in children and young people or the limited data available in this population and the significant uncertainty surrounding quality-of-life estimates in paediatric psoriasis populations. The use of utility values from an adult population brings the ICER for etanercept compared with BSC under the threshold of £30,000 per QALY gained in children and young people aged 6–11 years. The ICERs for ustekinumab and adalimumab using adult utility data are reduced significantly but remain above the £30,000 per QALY threshold, even using the most favourable estimates from TA146. 99 Under the assumption of no health benefits associated with BSC, the ICERs are reduced by up to £30,000 from the base-case values but remain quite high, with the lowest ICER of £56,430 per QALY gained for etanercept compared with BSC.
The number of hospitalisations associated with BSC is a key driver of the cost-effectiveness of the biological interventions. This was also identified as a key consideration in previous TAs in adults. 97–102 Based on clinical opinion, in the base-case analysis it was assumed that hospitalisations for severe psoriasis are very rare in children and young people. If the average hospitalisation LOS per annum is increased to 6.49 days based on the study by Fonia et al. ,144 the ICERs for the interventions reduce significantly; however, the only ICER that falls below the threshold of £30,000 is for the use of etanercept compared with BSC in children and young people aged 6–11 years. If the average number of hospitalisations per annum is increased significantly to 26.6 days per annum, based on the very high-need population described in CG153,173 the biological treatments compared with BSC are all considered cost-effective in individuals who have failed systemic therapy. However, recent appraisals in adults have considered the estimate of 26.6 days per annum to be too high.
The combined impact of the most optimistic utility estimates in adults (TA14699), 6.49 inpatient days per annum and no health benefits for BSC are presented in Tables 97 and 98 for the use of the interventions before and after systemic therapy. The combined impact of the utility gains from an adult population and an assumption of 6.49 hospitalisations per annum is sufficient to reduce the pairwise ICERs for the interventions compared with BSC to below a threshold of £30,000 per additional QALY, whereas the additional assumption of no health benefits for BSC reduces the ICERs further to below a threshold of £20,000 per additional QALY. Based on a fully incremental analysis, etanercept is the optimal treatment for children and young people aged 6–11 years, whereas adalimumab is the optimal treatment for children and young people aged 12–17 years.
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children and young people aged 4–17 years | ||||||
| Adult utility data (TA146) + 6.49 hospitalisations per annum | ||||||
| MTX | 52,273 | 9.229 | – | – | – | MTX |
| ADA | 77,106 | 9.489 | 24,834 | 0.260 | 95,527 | |
| Adult utility data (TA146) + 6.49 hospitalisations per annum + no health benefits for BSC | ||||||
| MTX | 52,291 | 8.351 | – | – | – | MTX |
| ADA | 77,136 | 8.727 | 24,845 | 0.376 | 66,126 | |
| Intervention | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| Adult utility data (TA146) + 6.49 hospitalisations per annum | |||||||
| BSC | 55,597 | 7.890 | – | – | – | – | ETA |
| ETA | 58,515 | 8.218 | 2917 | 0.328 | 8897 | 8897 | |
| ADA | 69,982 | 8.451 | 11,467 | 0.233 | 49,274 | 25,657 | |
| Adult utility data (TA146) + 6.49 hospitalisations per annum + no health benefits for BSC | |||||||
| BSC | 55,597 | 6.918 | – | – | – | – | ETA |
| ETA | 58,506 | 7.474 | 2909 | 0.557 | 5227 | 5227 | |
| ADA | 70,021 | 7.809 | 11,515 | 0.334 | 34,438 | 16,190 | |
| Children and young people aged 12–17 years | |||||||
| Adult utility data (TA146) + 6.49 hospitalisations per annum | |||||||
| BSC | 32,333 | 4.351 | – | – | – | – | ADA |
| ETA | 40,102 | 4.618 | 7769 | 0.266 | ED ADA | 29,177 | |
| ADA | 43,193 | 4.807 | 10,860 | 0.455 | 23,861 | 23,861 | |
| UST | 45,087 | 4.837 | 1894 | 0.031 | 61,722 | 26,253 | |
| Adult utility data (TA146) + 6.49 hospitalisations per annum + no health benefits for BSC | |||||||
| BSC | 32,333 | 3.815 | – | – | – | – | ADA |
| ETA | 40,100 | 4.269 | 7767 | 0.454 | ED ADA | 17,108 | |
| ADA | 43,194 | 4.537 | 10,861 | 0.722 | 15,040 | 15,040 | |
| UST | 45,099 | 4.579 | 1905 | 0.042 | 45,818 | 16,716 | |
Chapter 7 Assessment of factors relevant to the NHS and other parties
The potential extra cost to the NHS of providing adalimumab, etanercept or ustekinumab to children and young people with moderate to severe plaque psoriasis is largely uncertain, given the paucity of evidence on the health-care resource use specific to this population and the uncertainties in the effectiveness evidence base. The resource use associated with BSC in terms of the expected number of hospitalisations per annum was identified as a key area of uncertainty, as in previous TAs of psoriasis in adults. Reducing uncertainty at this level would allow a more accurate assessment of the potential impact on the consumption of NHS resources of providing biological treatment to children and young people with moderate to severe plaque psoriasis.
Chapter 8 Discussion
Statement of principal findings
One multicentre RCT (M04-717) found that adalimumab at the licensed dose of 0.8 mg/kg (up to 40 mg) led to a significantly greater response than methotrexate for the outcomes of PASI 50 and PASI 75, but not PASI 90, at 16 weeks. PGA 0/1 response rates were non-significantly higher for 0.8 mg/kg of adalimumab than for methotrexate. The benefits of half-dose adalimumab were not statistically greater than those for methotrexate. Evidence on quality of life was inconsistent across different measures, possibly because of baseline imbalances on the PedsQL. In children and young people, adalimumab did not appear to be associated with an increase in adverse effects relative to methotrexate over 16 weeks, although the possibility of rare AEs cannot be entirely excluded. The trial did not provide any comparative evidence for children aged 4–6 years. (Confidential information has been removed.)
One multicentre RCT (20030211) found etanercept to be significantly more effective than placebo in improving the severity of plaque psoriasis based on PASI 50, 75 and 90 and PGA 0/1 response rates at 12 weeks. Improvements in HRQoL were larger for etanercept than for placebo, but reached statistical significance only when measured by the CDLQI. AE rates were mostly similar in the etanercept and placebo groups at 12 weeks, with no serious AEs observed for either treatment. However, a higher observed rate of infections among participants receiving etanercept was of borderline statistical significance. Relatively few young children (9% aged < 8 years; 4.3% aged < 6 years) were included in the study. Up to 6 years of open-label follow-up (20050111) found that the proportions of PASI and PGA responders were stable over time, although only 36% of participants were available at the latest follow-up point. The proportion of participants withdrawing because of lack of efficacy is unknown. Through 264 weeks of follow-up, withdrawals because of AEs were infrequent and no deaths or malignancies were observed.
One multicentre trial (CADMUS) in children aged 12–17 years found that both the standard dosage and the half-standard dosage of ustekinumab were significantly more effective than placebo in improving the severity of plaque psoriasis based on PASI 50, 75 and 90 and PGA 0/1 responses at 12 weeks. Both ustekinumab dosages also led to significantly greater improvements in HRQoL (measured using the CDLQI and PedsQL). Among participants originally allocated to ustekinumab, PASI and PGA effects observed at 12 weeks appeared to be largely sustained at 52 weeks, with few withdrawals because of lack of efficacy. There were no notable adverse effects associated with ustekinumab, although the number of observations was small and the longest follow-up time was just 60 weeks. Few participants withdrew because of adverse effects.
No statistically significant differences were identified in PASI response outcomes across different age groups within the trials. Therefore, to establish the relative efficacy of the interventions the analyses assumed that treatment effects were exchangeable across ages in the population of children and young people. Based on an indirect treatment comparison of PASI response outcomes at 12 weeks from the 20030211 trial and the CADMUS trial, using the placebo arms of the trials as a common comparator, ustekinumab is a more effective treatment option than etanercept. The lack of a common comparator arm between the adalimumab trial (M04-717) and etanercept trial (20030211) and the CADMUS trial meant that it was not possible to draw conclusions about the relative efficacy of adalimumab, etanercept and ustekinumab based on the three trials in children and young people alone. To fill this evidence gap for the economic analysis it was necessary to draw strength from a wider evidence base of trials examining the efficacy of the interventions in adults. This wider network of evidence was used to facilitate an indirect comparison of adalimumab with etanercept and ustekinumab by examining the relationships that exist between the different interventions and study populations (i.e. children and young people and adults) and drawing conclusions for each population based on the full network of evidence. Adjustments were also made for differences in placebo response rates across the trials. The NMA results – adjusted for differences in population and placebo response rates – demonstrated that ustekinumab is the most effective intervention in children and young people, followed by adalimumab, etanercept and methotrexate. These rankings also matched those in the adult population. The absolute PASI response outcomes were estimated to be higher in children and young people than in adults because of higher placebo response rates in the etanercept trial (20030211) and the ustekinumab trial (CADMUS), but the relative effectiveness of the interventions was similar across the two populations.
The cost-effectiveness of adalimumab, etanercept and ustekinumab was evaluated by comparing the additional costs of the interventions relative to each other and to either methotrexate or BSC, depending on the position of the interventions in the pathway, with the additional health benefits over a time horizon that was sufficient to capture differences in costs and effects. Health outcomes were expressed in QALYs and all costs were considered from the perspective of the NHS and PSS. Because of differences in the marketing authorisations of the interventions by age and the positioning of adalimumab before non-biological systemic therapy, cost-effectiveness estimates were presented for three base-case populations: (1) children and young people aged 4–17 years, with adalimumab compared with methotrexate; (2) children and young people aged 6–11 years, with adalimumab and etanercept compared with BSC; and (3) children and young people aged 12–17 years, with adalimumab, etanercept and ustekinumab compared with BSC.
The paucity of clinical and economic evidence to inform the evaluation of cost-effectiveness in children and young people resulted in there being a number of strong assumptions and uncertainties in the analysis. These assumptions arose from the need to extrapolate data from the adult population to inform the population of children and young people. A number of alternative scenarios were considered to examine the impact of these assumptions on the cost-effectiveness results. The base-case cost-effectiveness results indicated that adalimumab was not a cost-effective treatment option when positioned in the treatment pathway as an alternative to systemic therapy. When positioned after systemic therapy, the ICER for adalimumab compared with BSC was more favourable, but it still remained well above conventional NICE thresholds of cost-effectiveness. Etanercept was also not considered a cost-effective option after systemic therapy for ages 6–11 years and was extendedly dominated by adalimumab for ages 12–17 years. Ustekinumab was the most effective treatment in children and young people aged 12–17 years but it was also the most costly treatment. The ICERs for ustekinumab compared with adalimumab and BSC were > £100,000 per QALY gained. Based on the base-case assumptions, the probability that any of the biological treatments would be considered cost-effective at the higher end of the NICE threshold of £30,000 per QALY was zero.
The lack of cost-effectiveness of the biologics compared with BSC was the result of the very modest QALY gains associated with improvements in PASI response outcomes. The difference in total costs between the interventions was driven by the time spent on BSC (non-responders). The acquisition costs of the biologics were not significantly different. The key drivers of cost-effectiveness were the utility estimates, the health benefits associated with BSC and the number of hospitalisations on BSC. Extrapolating utility estimates from the adult population to the population of children and young people reduced the ICERs by > 50% because the gains in utility associated with different PASI response outcomes were up to 6.6 times greater than the estimated utility gains from mapping PedsQL data from the CADMUS trial onto EQ-5D-Y utility values. The choice of EQ-5D utility values from other previous TAs in adults also had a significant impact. The base-case analysis included the possibility that psoriasis can improve with BSC and used the response rates for placebo from the NMA to inform this. When this assumption was altered to assume that there were no health benefits from BSC, the ICERs were reduced by £20,000–30,000 per additional QALY. The resource use associated with BSC in terms of the expected number of hospitalisations per annum also had a major impact on the cost-effectiveness results, reducing the ICERs compared with BSC considerably the more days of hospitalisation were assumed. This was also identified as a key area of uncertainty in previous TAs of psoriasis in adults.
Strengths and limitations of the assessment
Strengths
The reviews of clinical effectiveness and cost-effectiveness were based on comprehensive searches of the literature, which were supplemented by data from multiple additional sources, including European Medicines Agency and US Food and Drugs Agency documents and CSRs, allowing the inclusion of unpublished studies and data.
The clinical effectiveness review focused directly on evidence relating to children and young people with plaque psoriasis, resulting in the identification of only four relevant studies for the three biologics of interest. Consequently, the total number of included participants and the average length of follow-up (for adalimumab and ustekinumab) were limited. However, this provides the best evidence of the efficacy and short- to medium-term safety of adalimumab, etanercept and ustekinumab directly relevant to the decision problem.
A key strength of this evaluation was the fact that it went beyond the scope of the appraisal by bringing together evidence from the adult population to support an economic evaluation in children and young people. The review of cost-effectiveness evidence in this population, and the absence of economic models from the companies, highlighted the challenges involved in evaluating the cost-effectiveness of biological interventions in children and young people with plaque psoriasis. The fundamental challenge was the limited clinical evidence base for short- and long-term outcomes. Therefore, any estimation was going to be subject to a number of uncertainties. Clinical opinion suggests that the management and approach to care of treatment appears to mirror that used in adults. Therefore, in the absence of evidence, it seemed reasonable to extrapolate data from the adult population to inform the economic model in children and young people. This approach was also supported by the companies.
A major strength of the NMA was the fact that it brought together clinical evidence from the adult population to allow the evidence from the M04-717 trial to be connected with evidence from the other paediatric trials, while making an adjustment for any differences in PASI outcomes by population. This enabled the relative effectiveness of adalimumab, etanercept and ustekinumab to be estimated in children and young people by using what is already known about the relative effectiveness of the interventions in adults.
The economic model represents the first attempt to evaluate the cost-effectiveness of biological treatments in children and young people. This model used the same approach as the most widely accepted York model, which has been used in previous TAs in adults. This ensures consistency in the approaches undertaken for both populations. The main changes have been the availability of new evidence, including evidence in a paediatric population, HRQoL outcomes specific to a paediatric population and resource use and cost estimates. The analysis also attempted to reflect differences between the interventions in terms of their marketing authorisation by age and positioning of treatment before and after systemic therapy.
Limitations
The flow of participants through the etanercept studies was complex, with data spread across a number of publications and regulatory data sources. No CSR data were available to investigate this in further detail. Similarly, the lack of a CSR meant that some details about study conduct required for a complete risk-of-bias assessment were unavailable. Whenever possible, we avoided making assumptions and presented the most complete data as reported. The open-label design of the included follow-up studies may also have introduced additional biases.
In the absence of sufficient clinical evidence and economic data in children and young people, a simplified modelling approach was undertaken. This simplified approach involved modelling a single line of therapy before receiving BSC. However, plaque psoriasis is a lifelong chronic condition and, if the condition no longer responds to a biological treatment, individuals are usually offered another biological treatment, a pattern that is likely to be repeated over their lifetime. This means that treatments are usually trialled on an individual basis until an effective option is found. If individuals do not respond to multiple biological interventions, then the only remaining option is BSC. This approach to treatment is expected to be similar for children and young people and adults. However, much more caution is usually exercised in the younger population because of the limited availability of licensed treatment options. Therefore, the modelling approach undertaken is likely to be a simplification of reality. This simplification was necessary because of an absence of evidence on the sequential use of biological treatments in children and young people, in which treatment response would need to be conditioned on the previous biological treatments received. The modelling of sequential treatments also requires every potential treatment permutation to be considered. This has already presented a significant issue in the most recent TAs in adults that did consider more than one line of therapy (TA368102 for apremilast and the appraisal of ixekizumab,176 which is currently out for consultation), in which the modelled sequences did not reflect current clinical practice. Any attempt at treatment sequencing in the population of children and young people would be highly uncertain; this is not only because of the lack of data but also because of the further complication of the differences between the biological treatments in terms of marketing authorisation by age, severity and positioning in the treatment pathway. Furthermore, if a cost-effectiveness analysis identifies the optimal treatment sequence in children and young people, this is less likely to be helpful to clinical practice as that particular sequence may not be suitable for all (or any) individuals. For example, treatment in this population is usually tailored to the child or young person because of needle phobia or the presence of psoriatic arthritis.
Uncertainties
Evidence on the efficacy and safety of adalimumab, etanercept and ustekinumab in younger children is mostly absent from the included RCTs. The ustekinumab trial (CADMUS) restricted inclusion to participants aged > 12 years. Only one subject aged < 6 years received the licensed dose of adalimumab (0.8 mg/kg), with the majority of participants in the adalimumab trial being aged 9–18 years. Similarly, only 19 children (9%) included in the trial evaluating etanercept were aged < 8 years.
It has not been possible to define moderate or severe psoriasis in children and young people. The definition varied across the three trials in this population, with ustekinumab licensed for moderate to severe psoriasis and etanercept and adalimumab licensed for severe psoriasis. Previous TAs in adults defined severe psoriasis as a PASI score of ≥ 10 and a DLQI score of > 10. The trial populations for etanercept and ustekinumab included children or young people with a baseline PASI score of ≥ 12 (the mean score was around 18–21), with ≥ 10% of BSA affected (for ≥ 6 months in the case of ustekinumab) and a PGA score of ≥ 3. The trial population for adalimumab included a baseline PASI score of > 20, involvement of > 20% of BSA or very thick lesions and involvement of > 10% of BSA, a sPGA score of ≥ 4 or a baseline PASI score of > 10 (the mean score was 18.3 in the trial) and a number of other characteristics such as active psoriatic arthritis or a CDLQI score of > 10. There does not appear to be a standard routine assessment because the PASI and CDLQI tools have not been validated for the purpose of disease severity measurement in this population. There were also differences in the inclusion criteria in the trials in children and young people with regard to the previous use of topical therapies, with only one trial requiring a previous failure to respond to topical therapies (adalimumab, M04-717 trial). Another area of uncertainty pertains to the lack of exact correspondence between the populations in the trials of biological agents for psoriasis in children and young people and the marketing authorisations for these drugs in terms of the requirement for previous failure on non-biological systemic therapy. None of the three trials of biological therapies in children and young people with psoriasis required previous failure on non-biological systemic therapy as an inclusion criterion. Therefore, the model assumes that treatment effectiveness is independent of failure on non-biological systemic therapy prior to starting biological therapy. It is unknown how the position in the care pathway is likely to affect treatment effectiveness.
The complex flow of participants and the use of open-label designs limit inferences being made about the longer-term efficacy and safety of adalimumab, etanercept and ustekinumab and their withdrawal rates.
The paucity of clinical and economic evidence to inform the cost-effectiveness of biological treatments in a population of children and young people has led to a number of uncertainties. The most significant of these is the HRQoL gains associated with treatment. The incremental health benefits between the biological treatments are very sensitive to the utility gains associated with PASI response outcomes. In the base-case analysis these gains were estimated based on mapping PedsQL data from the ustekinumab trial onto EQ-5D-Y utility values. The PedsQL data were based on a very small sample size. In the absence of any other data, this represented the only method available to estimate EQ-5D values in this population. However, the PedsQL or CDLQI instruments have not been validated for the assessment of disease severity in a population of children and young people with psoriasis and the PedsQL instrument does not appear to be used routinely in clinical practice. Furthermore, these instruments are not specific to psoriasis and therefore may not capture all of the important impacts of the condition, such as on anxiety, depression, schooling and social interactions with friends. The PedsQL data from the ustekinumab trial are also based on a population aged 12–17 years; therefore, these data are unlikely to reflect the same quality-of-life outcomes in younger children. For example, very young children may not have developed a certain level of self-awareness, which means that any quality-of-life instrument in young children is unlikely to be accurate. Quality-of-life outcomes in children and young people can be lower because of the fear of subcutaneous injections rather than because of the severity of the condition itself. There are other potential benefits of the treatment of psoriasis in children and young people for which no quantitative estimates are available for the interventions in this MTA. These potential benefits include educational benefits from improved school attendance and a reduction in the prevalence of comorbidities later in life for children and young people with psoriasis, as well as reduced spillover disutility from the illness to carers. In the absence of quantitative estimates of these potential benefits, we were unable to incorporate these into the economic analysis. Any attempt to add arbitrary values to the utility estimates that are already highly uncertain will introduce further uncertainty.
The cost-effectiveness results are also very sensitive to the benefits and resource use associated with BSC. The number of hospitalisations per annum in children and young people is an area of considerable uncertainty. Extrapolating the data on hospitalisations from adults to this population is also subject to uncertainty because of a number of shortcomings that exist among all sources of data on resource use for BSC. Given these uncertainties the results from the base-case cost-effectiveness analysis should be considered alongside the results of the separate scenarios.
Chapter 9 Conclusions
Etanercept and ustekinumab, within their licensed indications, lead to significantly greater improvements in psoriasis symptoms than placebo at 12 weeks’ follow-up. Quality-of-life benefits were also observed. Although these effects appear to persist beyond 12 weeks, their magnitude and persistence is less certain.
Adalimumab at the licensed dose of 0.8 mg/kg (up to 40 mg) leads to significantly greater improvements in psoriasis symptoms than methotrexate for some, but not all, measures at 16 weeks. Observed quality-of-life benefits were inconsistent across different measures.
There was a lack of comparative evidence for these biologics in very young children.
With the exception of a non-significantly higher observed rate of infections among participants receiving etanercept, there was little evidence of short-term AEs. However, the relatively small number of observations and the limited length of follow-up across trials cannot exclude the possibility of rare events being undetected.
The absence of head-to-head comparisons of the three drugs meant that these treatments would have to be compared indirectly. In addition, the lack of a common comparator meant that a wider network of data from adults with psoriasis needed to be used to connect the network. This further increased the uncertainty about the relative effects of these treatments and further diminished the relative contribution of data from children to the analysis.
Based on the economic assessment, the majority of ICERs for the use of biologics in children and young people were in excess of NICE’s usual cost-effectiveness threshold and were reduced significantly only when combined assumptions that align with those in the management of psoriasis in adults were adopted.
Implications for service provision
Although two biologics are licensed for younger children with plaque psoriasis (adalimumab from age 4 years, etanercept from age 6 years), the existing randomised trials include very few young children. Consequently, evidence on the effectiveness and safety of these treatments in younger children has been generalised from observations in older children and young people.
Suggested research priorities
-
With the introduction of biological treatments in the population of children and young people, continued collection of data through biological therapy registries for children and young people aged < 18 years is warranted to investigate safety, patterns of treatment switching and long-term withdrawal rates. Although randomised clinical trials are the gold standard for evidence collection, the small number of children and young people eligible for biological therapy in psoriasis hinders the feasibility of carrying out large studies in this area. Registry data would constitute a potentially more viable source of evidence to reduce the uncertainty around safety, patterns of treatment switching and long-term withdrawal rates and inform clinical practice. The collection of observational data via these registries may also contribute to quantifying the impact of biological therapy for psoriasis early in life on the prevalence of comorbidities (e.g. hypertension, obesity and depression).
-
Adequately powered RCTs could substantially reduce the uncertainty surrounding the effectiveness of biological treatments in biologic-experienced populations of children and young people, that is, treatment response rates conditional on previous treatment are required. In the absence of head-to-head trials, placebo-controlled trials could facilitate NMAs.
-
In particular, further evidence is needed to inform the clinical effectiveness and safety of adalimumab and etanercept in younger children.
-
Further research is needed to establish the impact of biological therapies on improving the HRQoL of children and young people. Future trials should consider collecting direct estimates of EQ-5D-Y utility values. Research in this area also needs to consider whether or not the HRQoL measures capture the potential impact of any educational benefits from improved school attendance and a reduction in the prevalence of comorbidities later in life for children and young people with psoriasis (e.g. obesity, cardiovascular disease, metabolic syndrome and mental health disorders), as well as reduced spillover disutility from the illness to carers.
-
There is a need for the PASI instrument and/or other tools to be validated for disease severity assessment in a population of children and young people.
-
Further research is needed into the resource use and costs associated with BSC.
Acknowledgements
We thank Matthew Walton (Research Training Fellow in Systematic Review) for his contribution to the section on registry data. We also thank the manufacturers for responding to our requests for additional data.
Contributions of authors
Ana Duarte (Research Fellow, Health Economics) contributed to the protocol, the development of the economic model, the review of the economic analyses, the interpretation of the results and the writing of the report.
Teumzghi Mebrahtu (Research Fellow, Evidence Synthesis) contributed to the protocol, study selection, data extraction, the validity assessments and synthesis of the included studies, the interpretation of the results and the writing of the report.
Pedro Saramago Goncalves (Research Fellow, Health Economics) contributed to the protocol and conducted the network meta-analyses. He also contributed to the interpretation of the results and the writing of the report.
Melissa Harden (Information Specialist) developed the search strategies, conducted a range of searches to locate studies and wrote the sections of the report relating to the literature searches.
Ruth Murphy (Consultant Dermatologist, Paediatric Dermatology) provided expert clinical advice, contributed to the protocol and the interpretation of the results and commented on drafts of the report.
Stephen Palmer (Professor of Health Economics) contributed to the protocol, advised on the economic analysis and the interpretation of the results and commented on drafts of the report.
Nerys Woolacott (Reader in Health Technology Assessment) contributed to the protocol, advised on the clinical evidence review and the interpretation of the results and commented on drafts of the report.
Mark Rodgers (Research Fellow, Evidence Synthesis) contributed to the protocol, study selection, data extraction and the validity assessments and synthesis of the included studies. He also contributed to the interpretation of the results and the writing of the report. He took overall responsibility for the clinical effectiveness section.
Claire Rothery (Senior Research Fellow, Health Economics) had overall responsibility for the cost-effectiveness sections. She contributed to the development of the protocol, the network meta-analyses, the economic model and the economic analyses. She also contributed to the interpretation of the results and the writing of the report.
Data sharing statement
This report uses published primary research and other publicly available data, supplemented by evidence provided by the manufacturers of adalimumab (AbbVie) and ustekinumab (Janssen Biotech, Inc.). The full list of data sources is provided in the appendices. Further information can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Basko-Plluska JL, Petronic-Rosic V. Psoriasis: epidemiology, natural history, and differential diagnosis. Psoriasis 2012;2:67-76.
- Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005;64:ii18-23. https://doi.org/10.1136/ard.2004.033217.
- Benoit S, Hamm H. Childhood psoriasis. Clin Dermatol 2007;25:555-62. https://doi.org/10.1016/j.clindermatol.2007.08.009.
- Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum 2009;61:1373-8. https://doi.org/10.1002/art.24608.
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85. https://doi.org/10.1038/jid.2012.339.
- Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011;164:602-9. https://doi.org/10.1111/j.1365–2133.2010.10134.x.
- Elder JT, Nair RP, Henseler T, Jenisch S, Stuart P, Chia N, et al. The genetics of psoriasis 2001: the odyssey continues. Arch Dermatol 2001;137:1447-54. https://doi.org/10.1001/archderm.137.11.1447.
- Richardson SK, Gelfand JM. Update on the natural history and systemic treatment of psoriasis. Adv Dermatol 2008;24:171-96. https://doi.org/10.1016/j.yadr.2008.09.006.
- Burden-Teh E, Thomas KS, Ratib S, Grindlay D, Adaji E, Murphy R. The epidemiology of childhood psoriasis: a scoping review. Br J Dermatol 2016;174:1242-57. https://doi.org/10.1111/bjd.14507.
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010;146:891-5. https://doi.org/10.1001/archdermatol.2010.186.
- Psoriasis: Assessment and Management. London: NICE; 2012.
- Burden-Teh E, Lam M, Taibjee S, Taylor A, Webster S, Jury C, et al. Real-life experiences of managing childhood psoriasis: a UK multicentre audit of the assessment and management of psoriasis in children. Br J Dermatol 2015;173:152-3. https://doi.org/10.1111/bjd.13819.
- Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol 2011;164:1-14. https://doi.org/10.1111/j.1365–2133.2011.10280.x.
- Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis. Pediatr Dermatol 2015;32:656-62. https://doi.org/10.1111/pde.12416.
- Jankovic S, Raznatovic M, Marinkovic J, Jankovic J, Kocev N, Tomic-Spiric V, et al. Health-related quality of life in patients with psoriasis. J Cutan Med Surg 2011;15:29-36. https://doi.org/10.2310/7750.2010.10009.
- de Jager ME, de Jong EM, van de Kerkhof PC, Evers AW, Seyger MM. An intrapatient comparison of quality of life in psoriasis in childhood and adulthood. J Eur Acad Dermatol Venereol 2011;25:828-31. https://doi.org/10.1111/j.1468–3083.2010.03872.x.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005;64:ii65-8. https://doi.org/10.1136/ard.2004.031237.
- Salek MS, Jung S, Brincat-Ruffini LA, MacFarlane L, Lewis-Jones MS, Basra MK, et al. Clinical experience and psychometric properties of the Children’s Dermatology Life Quality Index (CDLQI), 1995–2012. Br J Dermatol 2013;169:734-59. https://doi.org/10.1111/bjd.12437.
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126-39. https://doi.org/10.1097/00005650-199902000-00003.
- Basra MKA, Finlay AY, Salek M-S. Development and validation of teenagers’ Quality of Life (T-QoL (C)) index: a dermatology-specific measure for adolescents. J Invest Dermatol 2013;133.
- Berth-Jones J, Grotzinger K, Rainville C, Pham B, Huang J, Daly S, et al. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment. Br J Dermatol 2006;155:707-13. https://doi.org/10.1111/j.1365–2133.2006.07389.x.
- Farhi D, Falissard B, Dupuy A. Global assessment of psoriasis severity and change from photographs: a valid and consistent method. J Invest Dermatol 2008;128:2198-203. https://doi.org/10.1038/jid.2008.68.
- Rodgers M, Epstein D, Bojke L, Yang H, Craig D. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15100.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995;132:942-9. https://doi.org/10.1111/j.1365-2133.1995.tb16953.x.
- Holme SA, Man I, Sharpe JL, Dykes PJ, Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol 2003;148:285-90. https://doi.org/10.1046/j.1365-2133.2003.05157.x.
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329-41. https://doi.org/10.1367/1539–4409(2003)003<0329:TPAAPP>2.0.CO;2.
- De Jager MEA, Van De Kerkhof PCM, De Jong EMGJ, Seyger MMB. A cross-sectional study using the Children’s Dermatology Life Quality Index (CDLQI) in childhood psoriasis: negative effect on quality of life and moderate correlation of CDLQI with severity scores. Br J Dermatol 2010;163:1099-101. https://doi.org/10.1111/j.1365–2133.2010.09993.x.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. https://doi.org/10.1016/j.jclinepi.2009.06.005.
- Eissing L, Rustenbach SJ, Krensel M, Zander N, Spehr C, Radtke MA, et al. Psoriasis registries worldwide: systematic overview on registry publications. J Eur Acad Dermatol Venereol 2016;30:1100-6. https://doi.org/10.1111/jdv.13634.
- DiMarco G, Hill D, Feldman SR. Review of patient registries in dermatology. J Am Acad Dermatol 2016;75:824-9. https://doi.org/10.1016/j.jaad.2016.03.020.
- Rencz F, Kemény L, Gajdácsi JZ, Owczarek W, Arenberger P, Tiplica GS, et al. Use of biologics for psoriasis in Central and Eastern European countries. J Eur Acad Dermatol Venereol 2015;29:2222-30. https://doi.org/10.1111/jdv.13222.
- EuroQol Research Foundation . EQ-5D n.d. https://euroqol.org/ (accessed 15 September 2017).
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods 2014;5:79-85. https://doi.org/10.1002/jrsm.1090.
- Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17520.
- Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. PharmacoEconomics 2008;26:753-67. https://doi.org/10.2165/00019053-200826090-00006.
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med 1996;15:2733-49. https://doi.org/10.1002/(SICI)1097-0258(19961230)15:24<2733::AID-SIM562>3.0.CO;2-0.
- Papp K, Thaci D, Landells I, Unnebrink K, Amer F. Baseline characteristics in pediatric patients with chronic plaque psoriasis from a Phase 3, randomized, double-blind study of adalimumab versus methotrexate treatment. J Dtsch Dermatol Ges 2014;12:37-8. https://doi.org/10.1111/j.1610–0387.2014.12387.
- Papp K, Thaci D, Landells I, Unnebrink K, Williams DA. Study design and baseline characteristics from a Phase 3, randomized, double-blind study of adalimumab versus methotrexate treatment in pediatric patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol 2013;27:27-8. https://doi.org/10.1111/jdv.12186.
- Papp K, Marcoux D, Landells I, Weilbel L, Ghislain PD, Unnebrink K, et al. Adalimumab long-term safety/efficacy results for pediatric patients with chronic plaque psoriasis from a Phase 3, randomized study. J Am Acad Dermatol 2016;74. https://doi.org/10.1016/j.jaad.2016.02.822.
- Papp K, Thaci D, Marcoux D, Weibel L, Unnebrink K, Williams DA. Efficacy and safety of adalimumab versus methotrexate treatment in pediatric patients with severe chronic plaque psoriasis: results from the 16-week randomized, double-blind period of a Phase 3 study. J Invest Dermatol 2015;135.
- Phillipp S, Pierre-Dominique PD, Landells I, Unnebrink K, Williams DA. Efficacy, safety of adalimumab versus methotrexate in pediatric patients with severe chronic plaque psoriasis: results from the treatment withdrawal and double-blind retreatment periods of a Phase 3 study. J Invest Dermatol 2015;135.
- Papp K, Williams D, Thaci D, Landells I, Unnebrink K. Study design and baseline characteristics from a Phase 3, randomized, double-blind study of adalimumab versus methotrexate treatment in pediatric patients with chronic plaque psoriasis. J Am Acad Dermatol 2014;70. https://doi.org/10.1016/j.jaad.2014.01.788.
- A Double Blind Study in Pediatric Subjects with Chronic Plaque Psoriasis, Studying Adalimumab vs. Methotrexate. Bethesda, MD: National Library of Medicine; 2010.
- Extension of Indication Variation Assessment Report: Humira (Adalimumab). Procedure No. EMEA/H/C/000481/II/0134. London: European Medicines Agency; 2015.
- Assessment Report for Paediatric Studies Submitted According to Article 46 of the Regulation (EC) No. 19012006: Humira (Adalimumab). London: European Medicines Agency; 2015.
- Langley RG, Paller AS, Hebert AA, Creamer K, Weng HH, Jahreis A, et al. Patient-reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12-week results from a Phase III randomized controlled trial. J Am Acad Dermatol 2011;64:64-70. https://doi.org/10.1016/j.jaad.2010.02.060.
- Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med 2008;358:241-51. https://doi.org/10.1056/NEJMoa066886.
- Paller AS, Eichenfield LF, Langley RG, Leonardi CL, Siegfried EC, Creamer K, et al. Subgroup analyses of etanercept in pediatric patients with psoriasis. J Am Acad Dermatol 2010;63:e38-41. https://doi.org/10.1016/j.jaad.2009.11.001.
- Landells I, Paller AS, Pariser D, Kricorian G, Foehl J, Molta C, et al. Efficacy and safety of etanercept in children and adolescents aged ≥ 8 years with severe plaque psoriasis. Eur J Dermatol 2010;20:323-8. https://doi.org/10.1684/ejd.2010.0911.
- Paller AS, Siegfried EC, Eichenfield LF, Pariser D, Langley RG, Creamer K, et al. Long-term etanercept in pediatric patients with plaque psoriasis. J Am Acad Dermatol 2010;63:762-8. https://doi.org/10.1016/j.jaad.2010.04.004.
- Paller AS, Siegfried EC, Pariser DM, Rice KC, Trivedi M, Iles J, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol 2016;74:280-7.e1. https://doi.org/10.1016/j.jaad.2015.09.056.
- Levy ML, Jahreis A, Paller AS, Walker S, Molta C, Freundlich B. Etanercept in children and adolescents with psoriasis. J Eur Acad Dermatol Venereol 2005;19.
- Siegfried E, Levy M, Jahreis A, Paller A. Etanercept in children and adolescents with psoriasis. J Am Acad Dermatol 2006;54.
- Paller A, Siegfried E, Langley R, Gottlieb A. A 12-week Phase 3 study of efficacy and safety of etanercept therapy in children and adolescents with moderate to severe plaque psoriasis. J Am Acad Dermatol 2007;56.
- Paller A, Langley R, Siegfried E, Gottlieb A. Etanercept treatment in children and adolescents with plaque psoriasis. J Am Acad Dermatol 2008;58. https://doi.org/10.1016/j.jaad.2007.10.055.
- Paller AS, Pariser D, Foehl J, Pedersen R, Molta C. Interim results of a long-term safety and tolerability study of etanercept treatment in children and adolescents age 8 to 17 years with plaque psoriasis. Eur J Pediatr Dermatol 2010;20:52-3.
- Paller A, Pariser D, Siegfried E, Kricorian G. Safety and efficacy of etanercept treatment in children and adolescents with plaque psoriasis: 96-week results of open-label extension study. J Am Acad Dermatol 2010;62. https://doi.org/10.1016/j.jaad.2009.11.080.
- Paller AS, Siegfried EC, Pariser DM, Rice KC, Trivedi M, Iles J, et al. Five-year open-label extension study of safety and efficacy of etanercept in children and adolescents with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016;74. https://doi.org/10.1016/j.jaad.2016.02.977.
- Enbrel (Etanercept) for the Treatment of Pediatric Plaque Psoriasis. Silver Spring, MD: Department of Health and Human Services, US Food and Drug Administration; 2008.
- Background Information for the Dermatologic and Ophthalmologic Drugs Advisory Committee (DODAC) Meeting, 18 June 2008. Silver Spring, MD: Department of Health and Human Services, US Food and Drug Administration; 2008.
- Assessment Report for Enbrel. International Nonproprietary Name: INN – Etanercept. Procedure No. EMEA/H/C/262/II/94. London: European Medicines Agency; 2008.
- Assessment Report for Enbrel. International Nonproprietary Name: Etanercept. Procedure No. Type II Variation EMEA/H/C/262/II/134. London: European Medicines Agency; 2011.
- An Open-Label Extension Study to Evaluate the Safety of Etanercept in Pediatric Subjects with Plaque Psoriasis. European Medicines Agency; 2012.
- Pediatric Open-Label Extension Study. Bethesda, MD: National Library of Medicine; 2005.
- Enbrel: Etanercept. Procedure No. EMEA/H/C/000262/A46/134. CHMP Assessment Report for Paediatric Use Studies Submitted According to Article 46 of the Regulation (EC) No. 19012006. London: European Medicines Agency; 2013.
- Study Evaluating the Safety and Effectiveness of Etanercept for the Treatment of Pediatric Psoriasis. Bethesda, MD: National Library of Medicine; 2010.
- Post Marketing Surveillance to Observe Safety and Efficacy of Enbrel in Pediatric Patients with Psoriasis. Bethesda, MD: National Library of Medicine; 2011.
- Pediatric Study in Children and Adolescents with Severe Plaque Psoriasis. Bethesda, MD: National Library of Medicine; 2015.
- A Randomized, Double-blind, Placebo- and Active Controlled Multicenter Trial to Demonstrate Efficacy of Subcutaneous Secukinumab Compared to Placebo and Etanercept (in a Single Blinded Arm) after Twelve Weeks of Treatment, and to Assess the Safety, Tolerability, and Long-term Efficacy in Subjects from 6 to Less than 18 Years of Age with Severe Chronic Plaque Psoriasis. European Medicines Agency; 2014.
- Landells I, Marano C, Hsu MC, Li S, Zhu Y, Eichenfield LF, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized Phase 3 CADMUS study. J Am Acad Dermatol 2015;73:594-603. https://doi.org/10.1016/j.jaad.2015.07.002.
- Landells I, Marano C, Hsu MC, Li S, Eichenfield L, Hoeger P, et al. Safety and efficacy of ustekinumab in adolescent patients with moderate to severe plaque psoriasis: results through 1 year of the Phase 3 CADMUS trial. J Am Acad Dermatol 2015;72. https://doi.org/10.1016/j.jaad.2015.02.820.
- A Study of the Safety and Efficacy of Ustekinumab in Adolescent Patients with Psoriasis (CADMUS). Bethesda, MD: National Library of Medicine; 2010.
- Assessment Report: Stelara. International Non-proprietary Name: Ustekinumab. London: European Medicines Agency; 2015.
- Garber C, Creighton-Smith M, Sorensen EP, Dumont N, Gottlieb AB. Systemic treatment of recalcitrant pediatric psoriasis: a case series and literature review. J Drugs Dermatol 2015;14:881-6.
- Klufas DM, Wald JM, Strober BE. Treatment of moderate to severe pediatric psoriasis: a retrospective case series. Pediatr Dermatol 2016;33:142-9. https://doi.org/10.1111/pde.12782.
- de Jager ME, de Jong EM, van de Kerkhof PC, Seyger MM. Efficacy and safety of treatments for childhood psoriasis: a systematic literature review. J Am Acad Dermatol 2010;62:1013-30. https://doi.org/10.1016/j.jaad.2009.06.048.
- Etanercept (Enbrel®) in Psoriasis – Pediatrics. Bethesda, MD: National Library of Medicine; 2004.
- Siegfried EC, Eichenfield LF, Paller AS, Pariser D, Creamer K, Kricorian G. Intermittent etanercept therapy in pediatric patients with psoriasis. J Am Acad Dermatol 2010;63:769-74. https://doi.org/10.1016/j.jaad.2009.10.046.
- An Efficacy, Safety, and Pharmacokinetics Study of Subcutaneously Administered Ustekinumab in the Treatment of Moderate to Severe Chronic Plaque Psoriasis in Pediatric Participants Greater than 6 to Less than 12 Years of Age. Bethesda, MD: National Library of Medicine; 2016.
- A Phase 3 Open-Label Study to Assess the Efficacy, Safety, and Pharmacokinetics of Subcutaneously Administered Ustekinumab in the Treatment of Moderate to Severe Chronic Plaque Psoriasis in Pediatric Subjects Greater than 6 to Less than 12 years of age. European Medicines Agency; 2016.
- Chingcuanco F. TNF-Inhibitors: Comparing the Safety, Efficacy and Physicochemical Profiles of Biosimilars and Innovators 2015. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025262 (accessed 24 May 2016).
- Smith C, Jabbar-Lopez Z, Yiu Z, Samarasekera E, Mustapa MFM, Exton L. In People With Psoriasis (All Types), What Are the Clinical Effectiveness Efficacy, Safety and Tolerability of Systemic Biologics (Adalimumab, Etanercept, Infliximab, Secukinumab or Ustekinumab) Compared With Each Other, With Methotrexate or With Placebo? 2015. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015017538 (accessed 24 May 2016).
- Sanclemente G, Murphy R, Contreras J, García H, Bonfill Cosp X. Anti-TNF agents for paediatric psoriasis. Cochrane Database Syst Rev 2015;11. https://doi.org/10.1002/14651858.CD010017.pub2.
- Seyger MMB, De Jager MEA, De Jong EMG, van De Kerkhof PCM. Efficacy and safety of treatments in childhood psoriasis: a systematic literature review. Eur J Pediatr Dermatol 2010;20.
- Thaci D, Seyger M, Philipp S, Unnebrink K, Williams D. Safety and Efficacy for Pediatric Patients With Chronic Plaque Psoriasis Who Did Not Respond to 16 Weeks of Double-Blind Methotrexate Treatment and Switched to Adalimumab n.d.
- Clinical Study Report: A Phase 3 Multicenter, Randomized, Double-blind, Placebo-controlled Study Evaluating the Efficacy and Safety of Ustekinumab in the Treatment of Adolescent Subjects with Moderate to Severe Plaque-type Psoriasis – CADMUS. Janssen Research & Development, LLC; 2014.
- Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123-4. https://doi.org/10.1136/bmj.323.7321.1123.
- Oostveen AM, De Jager MEA, van De Kerkhof PCM, Donders ART, De Jong EMGJ, Seyger MMB. The influence of treatments in daily clinical practice on the Children’s Dermatology Life Quality Index in juvenile psoriasis: a longitudinal study from the Child-CAPTURE patient registry. Br J Dermatol 2012;167:145-9. https://doi.org/10.1111/j.1365–2133.2012.10996.x.
- Ibfelt EH, Jensen DV, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clinical Epidemiology 2016;8:737-42. https://10.2147/CLEP.S99490.
- Gniadecki R, Bang B, Bryld LE, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015;172:244-52. https://doi.org/10.1111/bjd.13343.
- Gniadecki R, Kragballe K, Dam TN, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011;164:1091-6. https://doi.org/10.1111/j.1365–2133.2011.10213.x.
- Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker J, Burden AD, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015;135:2632-40. https://doi.org/10.1038/jid.2015.208.
- Dias S, Welton NJ, Sutton AJ, Ades AE. A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. London: NICE Decision Support Unit; 2014.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. https://doi.org/10.1002/sim.1875.
- Etanercept and Efalizumab for the Treatment of Adults with Psoriasis. London: NICE; 2006.
- Infliximab for the Treatment of Adults with Psoriasis. London: NICE; 2008.
- Adalimumab for the Treatment of Adults with Psoriasis. London: NICE; 2008.
- Ustekinumab for the Treatment of Adults with Moderate to Severe Psoriasis. London: NICE; 2009.
- Secukinumab for Treating Moderate to Severe Plaque Psoriasis. London: NICE; 2015.
- Apremilast for Treating Moderate to Severe Plaque Psoriasis. London: NICE; 2015.
- Signorovitch JE, Betts KA, Yan YS, LeReun C, Sundaram M, Wu EQ, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. Br J Dermatol 2015;172:504-12. https://doi.org/10.1111/bjd.13437.
- Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol 2006;55:598-606. https://doi.org/10.1016/j.jaad.2006.05.027.
- Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled Phase III trial. J Am Acad Dermatol 2008;58:106-15. https://doi.org/10.1016/j.jaad.2007.09.010.
- Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008;158:558-66. https://doi.org/10.1111/j.1365–2133.2007.08315.x.
- Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010;37:299-310. https://doi.org/10.1111/j.1346–8138.2009.00748.x.
- Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-alpha antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging 2013;6:83-90. https://doi.org/10.1161/CIRCIMAGING.112.975730.
- Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003;139:1627-32. https://doi.org/10.1001/archderm.139.12.1627.
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept Psoriasis Study Group . Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349:2014-22. https://doi.org/10.1056/NEJMoa030409.
- Elewski BE, Boh E, Papp K, Zitnik R. Efficacy and safety of etanercept in patients with psoriasis: results of a global Phase III study. J Am Acad Dermatol 2004;50. https://doi.org/10.1016/j.jaad.2003.10.559.
- Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global Phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005;152:1304-12. https://doi.org/10.1111/j.1365-2133.2005.06688.x.
- van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol 2008;159:1177-85. https://doi.org/10.1111/j.1365–2133.2008.08771.x.
- Reich K, Papp K, van de Kerkhof P, Zhang Z, Nograles K, Soung J. 52-week efficacy in patients with moderate to severe psoriasis continued on apremilast or switched from etanercept: the LIBERATE study. Australas J Dermatol 2016;57:69-70. https://doi.org/10.1111/ajd.12480.
- Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a Phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci 2011;63:154-63. https://doi.org/10.1016/j.jdermsci.2011.05.005.
- Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a Phase 3 clinical trial (LOTUS). J Drugs Dermatol 2013;12:166-74.
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007;356:580-92. https://doi.org/10.1056/NEJMoa062382.
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665-74. https://doi.org/10.1016/s0140–6736(08)60725–4.
- Papp K, Bissonnette R, Rosoph L, Wasel N, Lynde CW, Searles G, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled Phase III study. Lancet 2008;371:1337-42. https://doi.org/10.1016/S0140–6736(08)60593–0.
- Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010;362:118-28. https://doi.org/10.1056/NEJMoa0810652.
- Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Japanese Ustekinumab Study Group . Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a Phase 2/3 clinical trial. J Dermatol 2012;39:242-52. https://doi.org/10.1111/j.1346–8138.2011.01347.x.
- Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med 2003;349:658-65. https://doi.org/10.1056/NEJMoa021359.
- Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol 2008;158:116-21.
- Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, van Hoogstraten H, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol 2011;165:1109-17. https://doi.org/10.1111/j.1365–2133.2011.10615.x.
- Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. Br J Dermatol 2011;164:855-61. https://doi.org/10.1111/j.1365–2133.2010.10195.x.
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet 2012;380:738-46. https://doi.org/10.1016/S0140–6736(12)60642–4.
- Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a Phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol 2015;73:37-49. https://doi.org/10.1016/j.jaad.2015.03.049.
- Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a Phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol 2015;173:1387-99. https://doi.org/10.1111/bjd.14164.
- Guenther L, Wexler DM. Inducing remission of severe psoriasis with low dose cyclosporin A. Can J Dermatol 1991;3:163-7.
- Meffert H, Bräutigam M, Färber L, Weidinger G. Low-dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Derm Venereol 1997;77:137-41.
- Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol 1994;30:977-81. https://doi.org/10.1016/S0190-9622(94)70121-0.
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001;357:1842-7. https://doi.org/10.1016/S0140-6736(00)04954-0.
- Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2004;51:534-42. https://doi.org/10.1016/j.jaad.2004.02.021.
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a Phase III, multicentre, double-blind trial. Lancet 2005;366:1367-74. https://doi.org/10.1016/S0140-6736(05)67566-6.
- Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007;56:31.e1-15. https://doi.org/10.1016/j.jaad.2006.07.017.
- Torii H, Nakagawa H. Japanese Infliximab Study Investigators . Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010;59:40-9. https://doi.org/10.1016/j.jdermsci.2010.04.014.
- Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J 2012;125:1845-51.
- Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med 2009;28:1861-81. https://doi.org/10.1002/sim.3594.
- Final Appraisal Report Etanercept (Enbrel). Penarth: All Wales Medicines Strategy Group; 2010.
- Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Vergel YB, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10460.
- Langley RG, Signorovitch J, Wang K, Betts KA, Sundaram M, Mulani P, et al. Number needed to treat and cost per responder for biologic therapies for the treatment of moderate to severe psoriasis. J Am Acad Dermatol 2014;70. https://doi.org/10.1016/j.jaad.2014.01.008.
- Guide to the Methods of Technology Appraisal. London: NICE; 2004.
- Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol 2008;158:549-57. https://doi.org/10.1111/j.1365–2133.2007.08236.x.
- Fonia A, Jackson K, LeReun C, Grant DM, Barker JNWN, Smith CH. A retrospective cohort study of the impact of biologic therapy initiation on medical resource use and costs in patients with moderate to severe psoriasis. Br J Dermatol 2010;163:807-16. https://doi.org/10.1111/j.1365–2133.2010.09944.x.
- Woods AL, Rutter KJ, Gardner LS, Lewis VJ, Saxena S, George SA, et al. Inpatient management of psoriasis: a multicentre service review to establish national admission standards. Br J Dermatol 2008;158:266-72. https://doi.org/10.1111/j.1365–2133.2007.08338.x.
- Driessen RJ, Bisschops LA, Adang EM, Evers AW, van De Kerkhof PC, De Jong EM. The economic impact of high-need psoriasis in daily clinical practice before and after the introduction of biologics. Br J Dermatol 2010;162:1324-9. https://doi.org/10.1111/j.1365-2133.2010.09693.x.
- National Institute for Health and Care Excellence . Multiple Technology Appraisal. Adalimumab, Etanercept and Ustekinumab for Treating Plaque Psoriasis in Children and Young People. Final Scope n.d. www.nice.org.uk/guidance/ta455/documents/final-scope (accessed 15 September 2017).
- Burden-Teh E, Lam ML, Taibjee SM, Taylor A, Webster S, Dolman S, et al. How are we using systemic drugs to treat psoriasis in children? An insight into current clinical UK practice. Br J Dermatol 2015;173:614-18. https://doi.org/10.1111/bjd.13671.
- E Etanercept biosimilar (Benepali®) for the Treatment of the Following Diseases: Rheumatoid Arthritis (RA), Axial Spondylitis (AS), Psoriatic Arthritis and Plaque Psoriasis. n.d.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Office for National Statistics . National Life Tables: England &Amp; Wales, Based on Data for the Years 2013–2015 2016. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables (accessed 10 October 2016).
- Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477–7525–11–151.
- Khan KA, Petrou S, Rivero-Arias O, Walters SJ, Boyle SE. Mapping EQ-5D utility scores from the PedsQL™ generic core scales. PharmacoEconomics 2014;32:693-706. https://doi.org/10.1007/s40273–014–0153-y.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Varni JW, Globe DR, Gandra SR, Harrison DJ, Hooper M, Baumgartner S. Health-related quality of life of pediatric patients with moderate to severe plaque psoriasis: comparisons to four common chronic diseases. Eur J Pediatr 2012;171:485-92. https://doi.org/10.1007/s00431–011–1587–2.
- Upton P, Lawford J, Eiser C. Parent–child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res 2008;17:895-913. https://doi.org/10.1007/s11136–008–9350–5.
- van Reenen M, Janssen B, Oppe M, Kreimeier S, Greiner W. EQ-5D-Y User Guide: Basic Information on How to Use the EQ-5D-Y Instrument 2014.
- Strohal R, Kirby B, Puig L. on behalf of the Psoriasis Expert Panel . Psoriasis beyond the skin: an expert group consensus on the management of psoriatic arthritis and common co-morbidities in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol 2014;28:1661-9. https://doi.org/10.1111/jdv.12350.
- National Schedule of Reference Costs: the Main Schedule. London: Department of Health; 2015.
- MIMS . MIMS Online 2016. www.mims.co.uk/ (accessed September 2016).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Bonafede MM, Gandra SR, Watson C, Princic N, Fox KM. Cost per treated patient for etanercept, adalimumab, and infliximab across adult indications: a claims analysis. Adv Ther 2012;29:234-48. https://doi.org/10.1007/s12325–012–0007-y.
- Howe A, Eyck LT, Dufour R, Shah N, Harrison DJ. Treatment patterns and annual drug costs of biologic therapies across indications from the Humana commercial database. J Manag Care Spec Pharm 2014;20:1236-44. https://doi.org/10.18553/jmcp.2014.20.12.1236.
- Schabert VF, Watson C, Gandra SR, Goodman S, Fox KM, Harrison DJ. Annual costs of tumor necrosis factor inhibitors using real-world data in a commercially insured population in the United States. J Med Econ 2012;15:264-75. https://doi.org/10.3111/13696998.2011.644645.
- Shah N, Harrison D, Wu N, Lee YC. Cost per treated patient for etanercept and ustekinumab in patients treated for moderate to severe plaque psoriasis. J Am Acad Dermatol 2014;70. https://doi.org/10.1016/j.jaad.2014.01.690.
- Wu N, Lee YC, Shah N, Harrison DJ. Cost of biologics per treated patient across immune-mediated inflammatory disease indications in a pharmacy benefit management setting: a retrospective cohort study. Clin Ther 2014;36:1231-41. https://doi.org/10.1016/j.clinthera.2014.06.014.
- European Medicines Agency . Product Information: Annex I, Summary of Product Characteristics n.d. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000481/human_med_000822.jsp&mid=WC0b01ac058001d124 (accessed 8 September 2017).
- European Medicines Agency . Product Information: Annex I, Summary of Product Characteristics n.d. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000262/WC500027361.pdf (accessed 8 September 2017).
- European Medicines Agency . Product Information: Annex I, Summary of Product Characteristics n.d. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000958/WC500058513.pdf (accessed 8 September 2017).
- Mahé E, Bodemer C, Pruszkowski A, Teillac-Hamel D, de Prost Y. Cyclosporine in childhood psoriasis. Arch Dermatol 2001;137:1532-3.
- Leman J, Burden D. Psoriasis in children: a guide to its diagnosis and management. Paediatr Drugs 2001;3:673-80. https://doi.org/10.2165/00128072-200103090-00005.
- Royal College of Paediatrics and Child Health . Royal College of Paediatrics and Child Health’s School Age Growth Charts 2013. www.rcpch.ac.uk/child-health/research-projects/uk-who-growth-charts/uk-growth-chart-resources-2–18-years/school-age (accessed September 2016).
- Psoriasis: Assessment and Management. London: NICE; 2012.
- Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DPM. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti–tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:2368-76. https://doi.org/10.1002/art.21978.
- Pereira TM, Vieira AP, Fernande JC, Sousa-Basto A. Cyclosporin A treatment in severe childhood psoriasis. J Eur Acad Dermatol Venereol 2006;20:651-6. https://doi.org/10.1111/j.1468-3083.2006.01562.x.
- National Institute for Health and Care Excellence . Single Technology Appraisal. Ixekizumab for Treating Moderate to Severe Plaque Psoriasis (ID904). Committee Papers 2016. www.nice.org.uk/guidance/ta442/documents/committee-papers-3 (accessed 15 September 2017).
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). 2011.
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of clinical trials published world-wide – the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerg Themes Epidemiol 2008;5. https://doi.org/10.1186/1742-7622-5-13.
- Vencovsky J, Sylwestrzak A, Leszczynski P, Porawska W, Baranauskaite A, Tseluyko V, et al. A Phase III randomised, double-blind clinical study comparing SB4, an etanercept biosimilar, with etanercept reference product (Enbrel) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy (24-week results). Ann Rheum Dis 2015;74:467-8. https://doi.org/10.1136/annrheumdis-2015-eular.1220.
- Tarp S, Bryld LE, Iversen L, Skov L, Broesby-Olsen S, Brock B, et al. Comparative Effectiveness Associated With the Use of Biologics and Small-Molecules for Psoriasis: Protocol for a Systematic Review and Meta-Analysis 2015. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015029122 (accessed 24 May 2016).
- Soliman A, Nofal E, Nofal A, El desouky F, Asal M. Combination therapy of methotrexate plus NBUVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat 2015;26:528-34. https://doi.org/10.3109/09546634.2015.1034069.
- Ruano J, Epstein D, Isla-Tejera B, Gomez-Garcia F, Lorente A, Vélez García-Nieto A. Short-Term Effectiveness and Safety of New Biologic Agents Targeting IL-23Th17 Pathway for Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis 2015. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015025472 (accessed 24 May 2016).
- Puig L, Ruiz-Salas V. Long-term efficacy, safety and drug survival of ustekinumab in a Spanish cohort of patients with moderate to severe plaque psoriasis. Dermatology 2015;230:46-54. https://doi.org/10.1159/000366499.
- Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015;373:1318-28. https://doi.org/10.1056/NEJMoa1503824.
- Langley RG, Lebwohl M, Krueger GG, Szapary PO, Wasfi Y, Chan D, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol 2015;172:1371-83. https://doi.org/10.1111/bjd.13469.
- Kimball AB, Rothman KJ, Kricorian G, Pariser D, Yamauchi PS, Menter A, et al. OBSERVE-5: observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol 2015;72:115-22. https://doi.org/10.1016/j.jaad.2014.08.050.
- Brănişteanu DE, Voicu CM, Creţu A, Dimitriu A, Luca MC, Sălăvăstru CM. Adverse reactions of biological therapy for psoriasis. Rev Med Chir Soc Med Nat Iasi 2015;119:38-44.
- Ali F, Cueva A, Atwan A, Salek S, Finlay A, Piguet V, et al. A Systematic Review of the Impact on Quality of Life of Topical, Systemic and Biologic Therapies for Psoriasis 2015. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015009193 (accessed 24 May 2016).
- Adalimumab (Humira) for Severe Chronic Plaque Psoriasis in Children and Adolescents – Second Line. Birmingham: NIHR Horizon Scanning Centre, University of Birmingham; 2014.
- Umezawa Y, Nobeyama Y, Hayashi M, Fukuchi O, Ito T, Saeki H, et al. Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol 2013;40:1008-13. https://doi.org/10.1111/1346–8138.12353.
- Strohal R, Chimenti S, Vena GA, Girolomoni G. Etanercept provides an effective, safe and flexible short- and long-term treatment regimen for moderate-to-severe psoriasis: a systematic review of current evidence. J Dermatol Treat 2013;24:199-208. https://doi.org/10.3109/09546634.2012.713462.
- Park KK, Wu JJ, Koo J. A randomized, ‘head-to-head’ pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB-UVB) phototherapy in obese psoriasis patients. J Eur Acad Dermatol Venereol 2013;27:899-906. https://10.1111/j.1468-3083.2012.04611.x.
- Ustekinumab (Stelara) for Plaque Psoriasis in Adolescents. Birmingham: NIHR Horizon Scanning Centre, University of Birmingham; 2013.
- López-Ferrer A, Vilarrasa E, Gich IJ, Puig L. Adalimumab for the treatment of psoriasis in real life: a retrospective cohort of 119 patients at a single Spanish centre. Br J Dermatol 2013;169:1141-7. https://doi.org/10.1111/bjd.12543.
- Lebwohl MG, Kircik L, Callis Duffin K, Pariser D, Hooper M, Wenkert D, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2013;69:385-92. https://doi.org/10.1016/j.jaad.2013.03.031.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol 2013;27:e384-9. https://doi.org/10.1111/jdv.12004.
- Gisondi P, Cazzaniga S, Chimenti S, Giannetti A, Maccarone M, Picardo M, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol 2013;27:e30-41. https://doi.org/10.1111/j.1468–3083.2012.04450.x.
- da Silva CAP, Von Kossel K, Leszczynski M, Melnik T, Riera R. Methotrexate for psoriasis. Cochrane Database Syst Rev 2013;4. https://doi.org/10.1002/14651858.cd010498.
- Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD009481.pub2.
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23,458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis 2013;72:517-24. https://doi.org/10.1136/annrheumdis-2011-201244.
- Balzola F, Cullen G, Hoentjen F, Ho GT, Russell R. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Inflamm Bowel Dis Monit 2013;13.
- All Wales Therapeutics and Toxicology Centre . AWMSG Secretariat Assessment Report. Etanercept (Enbrel®) 2013. www.awmsg.org/awmsgonline/app/appraisalinfo/1437 (accessed 6 June 2016).
- Strand V, Sharp V, Koenig AS, Park G, Shi Y, Wang B, et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann Rheum Dis 2012;71:1143-50. https://doi.org/10.1136/annrheumdis-2011–200387.
- Lynde CW, Gupta AK, Guenther L, Poulin Y, Levesque A, Bissonnette R. A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept. J Dermatol Treat 2012;23:261-7. https://doi.org/10.3109/09546634.2011.607795.
- Kim IH, West CE, Kwatra SG, Feldman SR, O’Neill JL. Comparative efficacy of biologics in psoriasis: a review. Am J Clin Dermatol 2012;13:365-74. https://doi.org/10.2165/11633110–000000000–00000.
- Famenini S, Wu JJ. The safety of ustekinumab in psoriasis. J Drugs Dermatol 2012;11:907-10.
- Chiu HY, Wang TS, Chang CY, Tsai TF. The effectiveness and safety of adalimumab in the treatment of non-reimbursed patients with mild-to-moderate psoriasis. J Eur Acad Dermatol Venereol 2012;26:991-8. https://doi.org/10.1111/j.1468–3083.2011.04199.x.
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda A. Long-term safety of adalimumab in patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, and Crohn’s disease. Ann Rheum Dis 2012;71:514-15. https://doi.org/10.1136/annrheumdis-2012-eular.3077.
- Young MS, Horn EJ, Cather JC. The ACCEPT study: ustekinumab versus etanercept in moderate-to-severe psoriasis patients. Expert Rev Clin Immunol 2011;7:9-13. https://doi.org/10.1586/eci.10.92.
- Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA 2011;306:864-71. https://doi.org/10.1001/jama.2011.1211.
- Lara-Corrales I, Xi N, Pope E. Childhood psoriasis treatment: evidence published over the last 5 years. Rev Recent Clin Trials 2011;6:36-43. https://doi.org/10.2174/157488711793980174.
- Brunasso AM, Puntoni M, Salvini C, Delfino C, Curcic P, Gulia A, et al. Tolerability and safety of biological therapies for psoriasis in daily clinical practice: a study of 103 Italian patients. Acta Derm Venereol 2011;91:44-9. https://doi.org/10.2340/00015555–0959.
- Menter A, Gordon KB, Leonardi CL, Gu Y, Goldblum OM. Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis. J Am Acad Dermatol 2010;63:448-56. https://doi.org/10.1016/j.jaad.2009.09.040.
- Esposito M, Giunta A, Mazzotta A, Babino G, Talamonti M, Chimenti MS, et al. Continuous treatment of plaque-type psoriasis with etanercept: an observational long-term experience. Int J Immunopathol Pharmacol 2010;23:503-9. https://doi.org/10.1177/039463201002300212.
- Etanercept (Enbrel) for Moderate-to-Severe Plaque Psoriasis in Children and Adolescents. Birmingham: National Horizon Scanning Centre; 2008.
- Romero-Maté A, García-Donoso C, Córdoba-Guijarro S. Efficacy and safety of etanercept in psoriasis/psoriatic arthritis: an updated review. Am J Clin Dermatol 2007;8:143-55. https://doi.org/10.2165/00128071-200708030-00002.
- Ranjan N, Sharma NL, Shanker V, Mahajan VK, Tegta GR. Methotrexate versus hydroxycarbamide (hydroxyurea) as a weekly dose to treat moderate-to-severe chronic plaque psoriasis: a comparative study. J Dermatol Treat 2007;18:295-300. https://doi.org/10.1080/09546630701499291.
- Krueger GG, Elewski B, Papp K, Wang A, Zitnik R, Jahreis A. Patients with psoriasis respond to continuous open-label etanercept treatment after initial incomplete response in a randomized, placebo-controlled trial. J Am Acad Dermatol 2006;54:S112-19. https://doi.org/10.1016/j.jaad.2005.10.054.
- Gordon K, Korman N, Frankel E, Wang H, Jahreis A, Zitnik R, et al. Efficacy of etanercept in an integrated multistudy database of patients with psoriasis. J Am Acad Dermatol 2006;54:101-11. https://doi.org/10.1016/j.jaad.2005.11.1088.
- Amornpinyokeit N, Asawanonda P. 8-Methoxypsoralen cream plus targeted narrowband ultraviolet B for psoriasis. Photodermatol Photoimmunol Photomed 2006;22:285-9. https://doi.org/10.1111/j.1600–0781.2006.00249.x.
- Bigby M. A randomized controlled trial of methotrexate and cyclosporine in the treatment of psoriasis. Arch Dermatol 2004;140:347-8. https://doi.org/10.1001/archderm.140.3.347.
- Heydendael VMR, Spuls PI, Ten Berge IJM, Opmeer BC, Bos JD, De Rie MA. Cyclosporin trough levels: is monitoring necessary during short-term treatment in psoriasis? A systematic review and clinical data on trough levels. Br J Dermatol 2002;147:122-9. https://doi.org/10.1046/j.1365–2133.2002.04836.x.
- Faerber L, Braeutigam M, Weidinger G, Mrowietz U, Christophers E, Schulze HJ, et al. Cyclosporine in severe psoriasis. Results of a meta-analysis in 579 patients. Am J Clin Dermatol 2001;2:41-7. https://doi.org/10.2165/00128071-200102010-00007.
- Ho VC, Griffiths CE, Albrecht G, Vanaclocha F, León-Dorantes G, Atakan N, et al. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. The PISCES Study Group. Br J Dermatol 1999;141:283-91. https://doi.org/10.1046/j.1365-2133.1999.02977.x.
- Zachariae H, Abrams B, Bleehen SS, Bräutigam M, Burrows D, Ettelt MJ, et al. Conversion of psoriasis patients from the conventional formulation of cyclosporin A to a new microemulsion formulation: a randomized, open, multicentre assessment of safety and tolerability. Dermatology 1998;196:231-6. https://doi.org/10.1159/000017880.
- Koo J. A randomized, double-blind study comparing the efficacy, safety and optimal dose of two formulations of cyclosporin, Neoral and Sandimmun, in patients with severe psoriasis. OLP302 Study Group. Br J Dermatol 1998;139:88-95. https://doi.org/10.1046/j.1365-2133.1998.02319.x.
- Laburte C, Grossman R, Abi-Rached J, Abeywickrama KH, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic severe plaque psoriasis. Br J Dermatol 1994;130:366-75. https://doi.org/10.1111/j.1365-2133.1994.tb02935.x.
- Italian Multicenter Study Group on Cyclosporin in Psoriasis . Cyclosporin versus etretinate: Italian multicenter comparative trial in severe plaque-form psoriasis. Dermatology 1993;187:8-18. https://doi.org/10.1159/000247286.
- Christophers E, Mrowietz U, Henneicke HH, Färber L, Welzel D. Cyclosporine in psoriasis: a multicenter dose-finding study in severe plaque psoriasis. The German Multicenter Study. J Am Acad Dermatol 1992;26:86-90. https://doi.org/10.1016/0190-9622(92)70012-5.
- Tanew A, Guggenbichler A, Hönigsmann H, Geiger JM, Fritsch P. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol 1991;25:682-4. https://doi.org/10.1016/0190-9622(91)70253-X.
- Ruzicka T, Sommerburg C, Braun-Falco O, Koster W, Lengen W, Lensing W, et al. Efficiency of acitretin in combination with UV-B in the treatment of severe psoriasis. Arch Dermatol 1990;126:482-6. https://doi.org/10.1001/archderm.1990.01670280066012.
- Kragballe K, Jansen CT, Geiger JM, Bjerke JR, Falk ES, Gip L, et al. A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study. Acta Derm Venereol 1989;69:35-40.
- Takashima A, Sunohara A, Matsunami E, Mizuno N. Comparison of therapeutic efficacy of topical PUVA, oral etretinate, and combined PUVA and etretinate for the treatment of psoriasis and development of PUVA lentigines and antinuclear antibodies. J Dermatol 1988;15:473-9. https://doi.org/10.1111/j.1346-8138.1988.tb01194.x.
- Geiger JM, Czarnetzki BM. Acitretin (Ro 10-1670, etretin): overall evaluation of clinical studies. Dermatologica 1988;176:182-90. https://doi.org/10.1159/000248701.
- Melis M. Treatment of plaque psoriasis with an aromatic retinoid (etretinate). Rev Med Chil 1984;112:20-5.
- Christiansen JV, Holm P, Møller R, Reymann F, Schmidt H. Etretinate (Tigason) and betamethasone valerate (Celeston valerate) in the treatment of psoriasis. A double-blind, randomized, multicenter trial. Dermatologica 1982;165:204-7. https://doi.org/10.1159/000249942.
- Ustekinumab Safety and Surveillance Program using the Ingenix NHI Database. Bethesda, MD: National Library of Medicine; 2010.
- Study Evaluating the Safety of Enbrel (Etanercept). Bethesda, MD: National Library of Medicine; 2008.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. https://doi.org/10.1177/0272989x12458724.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641-56. https://doi.org/10.1177/0272989X12455847.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. Sheffield: NICE Decision Support Unit; 2011.
Appendix 1 Search strategies
The following searches were carried out to identify:
-
RCTs of adalimumab, etanercept and ustekinumab for the treatment of children and young people with plaque psoriasis
-
cost-effectiveness studies of adalimumab, etanercept and ustekinumab for the treatment of children and young people with plaque psoriasis
-
quality-of-life values for children and young people with plaque psoriasis that could be incorporated in the decision model.
Clinical and cost-effectiveness review
Database search strategies
MEDLINE [MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)]
Via Ovid (http://ovidsp.ovid.com/)
Date range searched: 1946 to present.
Date searched: 24 May 2016.
Records retrieved: 334.
The search was updated on 30 September 2016 and retrieved 347 records.
Search strategy
-
Psoriasis/ (29,080)
-
(psorias$ or psoriat$).ti,ab. (36,767)
-
parapsoriasis.ti,ab. (525)
-
pustul$ adj2 palm$).ti,ab. (785)
-
or 2 or 3 or 4 (42,607)
-
dalimumab/ (3349)
-
adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (5219)
-
adfrar or exemptia or MSB11022 or MSB 11022 or GP2017 or GP 2017 or GP2015 or GP 2015 or M923 or “M 923” or ABP501 or ABP 501).af. (19)
-
Etanercept/ (4651)
-
(etanercept or enbrel or 185243-69-0).af. (6612)
-
(benepali or brenzys or SB4 or CHS-0214 or CHS0214).af. (107)
-
Ustekinumab/ (414)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (796)
-
or/6-13 (10,289)
-
5 and 14 (2651)
-
exp Child/ (1,665,584)
-
exp Infant/ (1,007,205)
-
Adolescent/ (1,731,066)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenage$ or toddler$ or youth or youths or young people or young person$).ti,ab. (1,671,736)
-
16 or 17 or 18 or 19 (3,484,944)
-
15 and 20 (334)
Key
/ = indexing term [medical subject heading (MeSH heading)].
exp = exploded indexing term (MeSH heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = terms in any field.
adj = terms next to each other (order specified).
adj2 = terms within two words of each other (any order).
Cochrane Central Register of Controlled Trials (CENTRAL)
Via Wiley Online Library (http://onlinelibrary.wiley.com/).
Date range searched: issue 4 of 12, April 2016.
Date searched: 24 May 2016.
Records retrieved: 32
The strategy below was used to search CENTRAL and CDSR. The search was updated on 30 September 2016 and retrieved 39 records from CENTRAL.
Search strategy
#1 MeSH descriptor: [Psoriasis] this term only (1891)
#2 (psorias* or psoriat*):ti,ab,kw (4321)
#3 parapsoriasis:ti,ab,kw (3)
#4 (pustul* near/2 palm*):ti,ab,kw (72)
#5 #1 or #2 or #3 or #4 (4353)
#6 MeSH descriptor: [Adalimumab] this term only (239)
#7 (adalimumab or humira or D2E7 or (D2 next E7) or “331731-18-1”) (1088)
#8 (adfrar or exemptia or MSB11022 or “MSB 11022” or GP2017 or “GP 2017” or GP2015 or “GP 2015” or M923 or “M 923” or ABP501 or “ABP 501”) (2)
#9 MeSH descriptor: [Etanercept] this term only (383)
#10 (etanercept or enbrel or “185243-69-0”) (1162)
#11 (benepali or brenzys or SB4 or CHS-0214 or CHS0214) (2)
#12 MeSH descriptor: [Ustekinumab] this term only (49)
#13 (ustekinumab or stelara or “CNTO1275” or “CNTO-1275” or “815610-63-0”) (194)
#14 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 (2054)
#15 #5 and #14 (614)
#16 MeSH descriptor: [Child] explode all trees (173)
#17 MeSH descriptor: [Infant] explode all trees (14,329)
#18 MeSH descriptor: [Adolescent] this term only (85,135)
#19 (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) (193,089)
#20 #16 or #17 or #18 or #19 (193,089)
#21 #15 and #20 (50)
Note that results at line #21 are the total results for all databases within The Cochrane Library.
Key
MeSH descriptor = indexing term (MeSH heading).
* = truncation.
ti,ab,kw = terms in either title or abstract or keyword fields.
near/2 = terms within two words of each other (any order).
next = terms are next to each other.
“ “ = phrase search.
Cochrane Database of Systematic Reviews (CDSR)
Via Wiley Online Library (http://onlinelibrary.wiley.com/).
Date range searched: issue 5 of 12, May 2016.
Date searched: 24 May 2016.
Records retrieved: 10.
See above under CENTRAL for search strategy used. The search was updated on 30 September 2016 and retrieved 10 records.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus
Via EBSCOhost (www.ebscohost.com/)
Date range searched: from inception to 23 May 2016.
Date searched: 24 May 2016.
Records retrieved: 69.
The search was updated on 30 September 2016 and retrieved 77 records.
Search strategy
S19 S14 AND S18 (69)
S18 S15 OR S16 OR S17 (815,757)
S17 TX adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons” (815,757)
S16 (MH “Adolescence+”) (355,038)
S15 (MH “Child+”) (459,154)
S14 S5 AND S13 (532)
S13 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 (2252)
S12 TX ustekinumab or stelara or CNTO1275 or “CNTO-1275” or “815610-63-0” (157)
S11 TX benepali or brenzys or SB4 or “CHS-0214” or CHS0214 (3)
S10 TX etanercept or enbrel or “185243-69-0” (1528)
S9 (MH “Etanercept”) (701)
S8 TX adfrar or exemptia or MSB11022 or “MSB 11022” or GP2017 or “GP 2017” or GP2015 or “GP 2015” or M923 or “M 923” or ABP501 or “ABP 501” (4)
S7 TX (adalimumab or humira or D2E7 or “D2-E7” or “D2 E7” or “331731-18-1”) (933)
S6 (MH “Adalimumab”) (124)
S5 S1 OR S2 OR S3 OR S4 (5573)
S4 TI (pustul* N2 palm*) OR AB (pustul* N2 palm*) (50)
S3 TI parapsoriasis OR AB parapsoriasis (11)
S2 TI ( psorias* or psoriat* ) OR AB ( psorias* or psoriat* ) (4364)
S1 (MH “Psoriasis”) (3589)
Key
MH = indexing term (CINAHL heading).
* = truncation.
TI = terms in the title.
AB = terms in the abstract.
TX = all text – search of all of the database’s searchable fields.
“ “ = phrase search.
N2 = terms within two words of each other (any order).
Database of Abstracts of Reviews of Effects (DARE)
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 31 March 2015.
Date searched: 24 May 2016.
Records retrieved: 4
Search strategy
This search strategy was not updated as DARE closed at the end of March 2015.
-
MeSH DESCRIPTOR Psoriasis (202)
-
(psorias* or psoriat*) (311)
-
(parapsoriasis) (1)
-
(pustul* NEAR2 palm*) (2)
-
(palm* NEAR2 pustul*) (3)
-
#1 OR #2 OR #3 OR #4 OR #5 (311)
-
MeSH DESCRIPTOR Adalimumab (112)
-
(adalimumab or humira or D2E7 or D2-E7 or “D2 E7” or “331731-18-1”) (240)
-
MeSH DESCRIPTOR Etanercept (99)
-
(etanercept or enbrel or “185243-69-0”) (246)
-
MeSH DESCRIPTOR Ustekinumab (16)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or “815610-63-0”) (32)
-
(adfrar or exemptia or MSB11022 or “MSB 11022” or GP2017 or “MSB-11022” or “GP 2017” or “GP-2017” or GP2015 or “GP 2015” or “GP-2015” or M923 or “M 923” or “M-923” OR ABP501 or “ABP 501” or “ABP-501”) (0)
-
(benepali or brenzys or SB4 or CHS-0214 or CHS0214) (0)
-
#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 (355)
-
MeSH DESCRIPTOR child EXPLODE ALL TREES (4890)
-
MeSH DESCRIPTOR infant EXPLODE ALL TREES (2947)
-
MeSH DESCRIPTOR adolescent (4584)
-
(adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) (13,284)
-
#16 OR #17 OR #18 OR #19 (13,284)
-
#6 AND #15 AND #20 (11)
-
(#6 AND #15 AND #20) IN DARE (4)
-
(#6 AND #15 AND #20) IN HTA (7)
-
(#6 AND #15 AND #20) IN NHSEED (0)
Key
MeSH DESCRIPTOR = indexing term (MeSH heading).
* = truncation.
“ “ = phrase search.
NEAR2 = terms within two words of each other (order specified).
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 20 May 2016.
Date searched: 23 May 2016.
Records retrieved: 771.
The search was updated on 30 September 2016 and retrieved 826 records.
Search strategy
-
exp psoriasis/ (57,775)
-
(psorias$ or psoriat$).ti,ab. (53,835)
-
parapsoriasis.ti,ab. (571)
-
(pustul$ adj2 palm$).ti,ab. (1042)
-
1 or 2 or 3 or 4 (70,014)
-
adalimumab/ (20,228)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (20,663)
-
(adfrar or exemptia or MSB11022 or MSB 11022 or GP2017 or GP 2017 or GP2015 or GP 2015 or M923 or “M 923” or ABP501 or ABP 501).af. (49)
-
etanercept/ (22718)
-
(etanercept or enbrel or 185243-69-0).af. (23,579)
-
(benepali or brenzys or SB4 or CHS-0214 or CHS0214).af. (85)
-
ustekinumab/ (2696)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (2813)
-
or/6-13 (34,307)
-
5 and 14 (8172)
-
exp child/ (2,315,907)
-
exp adolescent/ (1,350,949)
-
juvenile/ (26,103)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenage$ or toddler$ or youth or youths or young people or young person$).ti,ab. (2,067,003)
-
16 or 17 or 18 or 19 (3,558,532)
-
15 and 20 (771)
Key
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = all fields.
adj2 = terms within two words of each other (any order).
Health Technology Assessment (HTA) database
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 24 May 2016.
Date searched: 24 May 2016.
Records retrieved: 7.
See above under DARE for search strategy used. The search was updated on 30 September 2016 and retrieved seven records.
NHS Economic Evaluation Database (NHS EED)
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 24 May 2016.
Date searched: 24 May 2016.
Records retrieved: 0.
See above under DARE for search strategy used. This search strategy was not updated as NHS EED closed at the end of March 2015.
PubMed
Date searched: 24 May 2016.
Records retrieved: 333.
The search was updated on 30 September 2016 and retrieved 347 records.
Search strategy
(((((((((((((((((“Adalimumab”[Mesh:noexp]) OR ((adalimumab OR humira OR D2E7 OR “D2 E7” OR “D2-E7” OR “331731-18-1”))) OR ((adfrar OR exemptia))) OR ((“MSB11022” OR “MSB 11022” OR “MSB 11022”))) OR “Etanercept”[Mesh:noexp]) OR ((etanercept OR enbrel OR “185243-69-0”))) OR ((benepali OR brenzys))) OR ((“SB4” OR “CHS-0214” OR “CHS0214”))) OR “Ustekinumab”[Mesh:noexp]) OR ((ustekinumab OR stelara OR CNTO1275 OR “CNTO-1275” OR “815610-63-0”))) OR (“M923”[All Fields] OR “M 923”[All Fields] OR “M-923”[All Fields])) OR (((“ABP501”) OR “ABP 501”) OR “ABP-501”)) OR (((“GP2017”) OR “GP-2017”) OR “GP 2017”)) OR ((“GP2015”[All Fields] OR “GP-2015”[All Fields] OR “GP 2015”[All Fields])))) AND ((((“Psoriasis”[Mesh:noexp]) OR ((psorias*[Title/Abstract] OR psoriat*[Title/Abstract]))) OR parapsoriasis[Title/Abstract]) OR ((pustul*[Title/Abstract] AND palm*[Title/Abstract]))))) AND ((((“Child”[Mesh]) OR “Infant”[Mesh]) OR “Adolescent”[Mesh]) OR ((adolescen*[Title/Abstract] OR baby[Title/Abstract] OR babies[Title/Abstract] OR child[Title/Abstract] OR children[Title/Abstract] OR boy[Title/Abstract] OR boys[Title/Abstract] OR girl[Title/Abstract] OR girls[Title/Abstract] OR infant*[Title/Abstract] OR infanc*[Title/Abstract] OR juvenile*[Title/Abstract] OR paediatric[Title/Abstract] OR pediatric[Title/Abstract] OR preschooler*[Title/Abstract] OR schoolboy*[Title/Abstract] OR schoolgirl*[Title/Abstract] OR schoolchild*[Title/Abstract] OR teens[Title/Abstract] OR teenage*[Title/Abstract] OR toddler*[Title/Abstract] OR youth[Title/Abstract] OR youths[Title/Abstract] OR “young people”[Title/Abstract] OR “young person”[Title/Abstract] OR "young persons”[Title/Abstract])))
Key
[Mesh] = exploded indexing term (MeSH heading).
[Mesh:noexp] = indexing term (MeSH heading) not exploded.
* = truncation.
“ “ = phrase search.
[Title/Abstract]) = terms in either title or abstract fields.
Science Citation Index
Via Web of Science, Thomson Reuters (http://thomsonreuters.com/thomson-reuters-web-of-science/).
Date range searched: 1900 to 20 May 2016.
Searched on: 23 May 2016.
Records retrieved: 256.
The search was updated on 30 September 2016 and retrieved 272 records.
Search strategy
| #13 | 256 |
#12 AND #11 Indexes=SCI-EXPANDED Timespan=All years |
| #12 | 1,516,336 |
TS = (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) Indexes = SCI-EXPANDED Timespan = All years |
| #11 | 3490 |
#10 AND #4 Indexes = SCI-EXPANDED Timespan = All years |
| #10 | 13,549 |
#9 OR #8 OR #7 OR #6 OR #5 Indexes = SCI-EXPANDED Timespan = All years |
| #9 | 1006 |
TS = (ustekinumab or stelara or CNTO1275 or CNTO-1275 or “815610-63-0”) Indexes = SCI-EXPANDED Timespan = All years |
| #8 | 145 |
TS = (benepali or brenzys or SB4 or CHS-0214 or CHS0214) Indexes = SCI-EXPANDED Timespan = All years |
| #7 | 8405 |
TS = (etanercept or enbrel or “185243-69-0”) Indexes = SCI-EXPANDED Timespan = All years |
| #6 | 12 |
TS = (adfrar or exemptia or MSB11022 or “MSB 11022” or GP2017 or “GP 2017” or GP2015 or “GP 2015” or M923 or “M 923” or ABP501 or “ABP 501”) Indexes = SCI-EXPANDED Timespan = All years |
| #5 | 6376 |
TS = (adalimumab or humira or D2E7 or D2-E7 or “D2 E7” or “331731-18-1”) Indexes = SCI-EXPANDED Timespan = All years |
| #4 | 46,577 |
#3 OR #2 OR #1 Indexes = SCI-EXPANDED Timespan = All years |
| #3 | 806 |
TS = (pustul* NEAR/2 palm*) Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 480 |
TS = parapsoriasis Indexes = SCI-EXPANDED Timespan = All years |
| #1 | 45,734 |
TS = (psorias* or psoriat*) Indexes = SCI-EXPANDED Timespan = All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
“ “ = phrase search.
NEAR/2 = terms within two words of each other (any order).
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
Date searched: 24 May 2016.
Records retrieved: 23.
Searches were carried out as per the search strings below. In total, 28 studies were found, which was reduced to 23 studies after deduplication of the results.
The search was updated on 15 September 2016 (see Network meta-analysis searches, Search 2). Update searches for the biosimilar drugs (lines 2, 3, 4 and 6) were carried out on 30 September 2016 but did not identify any further studies.
-
Six studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1)
-
No studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (adfrar OR exemptia)
-
No studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (MSB11022 OR “MSB 11022” OR MSB-11022 OR GP2017 OR “GP 2017” OR GP-2017 OR GP2015 OR “GP 2015” OR GP-2015)
-
No studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (M923 OR “M 923” OR M-923 OR ABP501 OR “ABP 501” OR ABP-501)
-
Fifteen studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (etanercept OR enbrel OR 185243-69-0)
-
No studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (benepali OR brenzys OR SB4 OR CHS-0214 OR CHS0214)
-
Seven studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0)
Conference Proceedings Citation Index – Science
Via Web of Science, Thomson Reuters (http://thomsonreuters.com/thomson-reuters-web-of-science/).
Date range searched: 1990 to 20 May 2016.
Date searched: 23 May 2016.
Records retrieved: 21.
The search was updated on 30 September 2016 and retrieved 21 records.
Search strategy
| #13 | 21 |
#12 AND #11 Indexes = CPCI-S Timespan = All years |
| #12 | 148,800 |
TS = (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) Indexes = CPCI-S Timespan = All years |
| #11 | 633 |
#10 AND #4 Indexes = CPCI-S Timespan = All years |
| #10 | 2726 |
#9 OR #8 OR #7 OR #6 OR #5 Indexes = CPCI-S Timespan = All years |
| #9 | 179 |
TS = (ustekinumab or stelara or CNTO1275 or CNTO-1275 or “815610-63-0”) Indexes = CPCI-S Timespan = All years |
| #8 | 30 |
TS = (benepali or brenzys or SB4 or CHS-0214 or CHS0214) Indexes = CPCI-S Timespan = All years |
| #7 | 1341 |
TS = (etanercept or enbrel or “185243-69-0”) Indexes = CPCI-S Timespan = All years |
| #6 | 6 |
TS = (adfrar or exemptia or MSB11022 or “MSB 11022” or GP2017 or “GP 2017” or GP2015 or “GP 2015” or M923 or “M 923” or ABP501 or “ABP 501”) Indexes = CPCI-S Timespan = All years |
| #5 | 1351 |
TS = (adalimumab or humira or D2E7 or D2-E7 or “D2 E7” or “331731-18-1”) Indexes = CPCI-S Timespan = All years |
| #4 | 6375 |
#3 OR #2 OR #1 Indexes = CPCI-S Timespan = All years |
| #3 | 68 |
TS = (pustul* NEAR/2 palm*) Indexes = CPCI-S Timespan = All years |
| #2 | 18 |
TS = parapsoriasis Indexes = CPCI-S Timespan = All years |
| #1 | 6317 |
TS = (psorias* or psoriat*) Indexes = CPCI-S Timespan = All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
“ “ = phrase search.
NEAR/2 = terms within two words of each other (any order).
EU Clinical Trials Register
www.clinicaltrialsregister.eu/ctr-search/search
Date searched: 24 May 2016.
Records retrieved: 10.
The search was updated on 19 September 2016 (see Network meta-analysis searches, Search 2). Update searches for the biosimilar drugs (lines 2, 3, 4 and 6) were carried out on 30 September 2016 but did not identify any further studies.
-
Two result(s) found for (Psoriasis OR psoriatic) AND (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1). Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
No result(s) found for (Psoriasis OR psoriatic) AND (adfrar OR exemptia). Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
One result(s) found for (Psoriasis OR psoriatic) AND (MSB11022 OR “MSB 11022” OR MSB-11022 OR GP2017 OR “GP 2017” OR GP-2017 OR GP2015 OR “GP 2015” OR GP-2015) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
No result(s) found for (Psoriasis OR psoriatic) AND (M923 OR “M 923” OR M-923 OR ABP501 OR “ABP 501” OR ABP-501). Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
Five result(s) found for (Psoriasis OR psoriatic) AND (etanercept OR enbrel OR 185243-69-0) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
No result(s) found for (Psoriasis OR psoriatic) AND (benepali OR brenzys OR SB4 OR CHS-0214 OR CHS0214). Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
Two result(s) found for (Psoriasis OR psoriatic) AND (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
PROSPERO
Date searched: 24 May 2016.
Records retrieved: 32.
Search: psoriasis in all fields.
The search was updated on 30 September 2016 and retrieved 13 new records, added since the previous search on 24 May 2016.
World Health Organization (WHO)’s International Clinical Trials Registry Platform
Date searched: 24 May 2016.
Records retrieved: 32.
The search was updated on 19 September 2016 (see Network meta-analysis searches, Search 2). Update searches for the biosimilar drugs (lines 2, 3, 4 and 6) were carried out on 30 September 2016 but did not identify any further studies.
-
Eight trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1) limited to clinical trials in children (birth to 18 years)
-
No trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (adfrar OR exemptia) limited to clinical trials in children (birth to 18 years)
-
No trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (MSB11022 OR “MSB 11022” OR MSB-11022 OR GP2017 OR “GP 2017” OR GP-2017 OR GP2015 OR “GP 2015” OR GP-2015) limited to clinical trials in children (birth to 18 years)
-
No trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (M923 OR “M 923” OR M-923 OR ABP501 OR “ABP 501” OR ABP-501) limited to clinical trials in children (birth to 18 years)
-
Fifteen trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (etanercept OR enbrel OR 185243-69-0) limited to clinical trials in children (birth to 18 years)
-
Two trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (benepali OR brenzys OR SB4 OR CHS-0214 OR CHS0214) limited to clinical trials in children (birth to 18 years)
-
Seven trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0) limited to clinical trials in children (birth to 18 years)
Guideline searches
The following resources were searched for relevant guidelines on 25 May 2016. The same searches were repeated on 30 September 2016, with one further guideline identified from NHS Evidence.
National Guideline Clearinghouse
Date searched: 25 May 2016 (update search on 30 September 2016).
Records retrieved: 4
Keyword: psorias* or psoriat*
Age of target population: Infant, Newborn (to 1 month), Infant (1 to 23 months), Child (2 to 12 years), Adolescent (13 to 18 years)
Ten results were browsed and four were identified as being potentially relevant.
National Institute for Health and Care Excellence Clinical Knowledge Summaries
Date searched: 25 May 2016 (update search on 30 September 2016).
Records retrieved: 1
The topics section was browsed and one Clinical Knowledge Summary for psoriasis was identified.
NHS Evidence
Date searched: 25 May 2016 (update search on 30 September 2016).
Records retrieved: 22
((intitle:psorias* OR intags: psorias* OR inurl:psorias*) AND (child* or infant* or adolescen*))
Results filtered by type of information = guidance. The results were scanned for relevance and 22 documents were identified.
National Institute for Health and Care Excellence Evidence summaries: new medicines
www.nice.org.uk/about/what-we-do/our-programmes/nice-advice/evidence-summaries-new-medicines
Date searched: 24 May 2016 (update search on 30 September 2016).
Records retrieved: 0
Browsed 63 titles of published evidence summaries – no relevant summaries found.
National Institute for Health and Care Excellence website
Date searched: 25 May 2016 (update search on 30 September 2016).
Records retrieved: 4.
Browsed psoriasis topic page (www.nice.org.uk/guidance/conditions-and-diseases/skin-conditions/psoriasis) – 4 relevant documents identified.
Network meta-analysis searches
The following searches were carried out to identify:
-
RCTs of systemic non-biological (acitretin, methotrexate, ciclosporin) and biological (infliximab, secukinumab) therapies in children and young people with plaque psoriasis
-
RCTs of adalimumab, etanercept, ustekinumab, acitretin, methotrexate, ciclosporin, infliximab or secukinumab in adults with plaque psoriasis.
Search 1
Randomised controlled trials of systemic non-biological (acitretin, methotrexate, ciclosporin) and biological therapies (infliximab, secukinumab) in children and young people with plaque psoriasis.
Database search strategies
MEDLINE [MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)]
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to present.
Date searched: 31 May 2016.
Records retrieved: 760
The search was updated on 30 September 2016 and retrieved 784 records.
Search strategy
-
Psoriasis/ (29,106)
-
(psorias$ or psoriat$).ti,ab. (36,848)
-
parapsoriasis.ti,ab. (525)
-
(pustul$ adj2 palm$).ti,ab. (787)
-
or/1-4 (42,692)
-
Acitretin/ (922)
-
(acitretin$ or etretin or neotigason or soriatane or 55079-83-9).af. (2571)
-
Methotrexate/ (33,972)
-
(methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or 15475-56-6 or 59-05-2 or 7413-34-5).af. (48,192)
-
exp Cyclosporins/ (37,382)
-
(cyclosporin$ or ciclosporin$ or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or 59865-13-3 or 63798-73-2).af. (55,065)
-
Infliximab/ (7830)
-
(infliximab or remicade or 170277-31-3).af. (11,015)
-
(inflectra or remsima or CT-P13).af. (63)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (193)
-
or/6-15 (111,288)
-
5 and 16 (5655)
-
exp Child/ (1,666,387)
-
exp Infant/ (1,007,661)
-
Adolescent/ (1,732,216)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenage$ or toddler$ or youth or youths or young people or young person$).ti,ab. (1,674,732)
-
or/18-21 (3,488,828)
-
17 and 22 (761)
-
exp animals/ not humans/ (4,247,320)
-
23 not 24 (760)
Key
/ = indexing term (MeSH heading)
exp = exploded indexing term (MeSH heading)
$ = truncation
ti,ab = terms in either title or abstract fields
af = terms in any field
adj2 = terms within two words of each other (any order)
Cochrane Central Register of Controlled Trials (CENTRAL)
Via Wiley Online Library (http://onlinelibrary.wiley.com/).
Date range searched: issue 5 of 12, May 2016.
Date searched: 31 May 2016.
Records retrieved: 70.
The strategy below was used to search CENTRAL and CDSR. The search was updated on 30 September 2016 and retrieved 79 records from CENTRAL.
Search strategy
#1 MeSH descriptor: [Psoriasis] this term only (1891)
#2 (psorias* or psoriat*):ti,ab,kw (4328)
#3 parapsoriasis:ti,ab,kw (3)
#4 (pustul* near/2 palm*):ti,ab,kw (73)
#5 #1 or #2 or #3 or #4 (4360)
#6 MeSH descriptor: [Acitretin] this term only (66)
#7 (acitretin* or etretin or neotigason or soriatane or “55079-83-9”) (162)
#8 MeSH descriptor: [Methotrexate] this term only (3050)
#9 (methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) (7671)
#10 MeSH descriptor: [Cyclosporins] explode all trees (2699)
#11 (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) (6059)
#12 MeSH descriptor: [Infliximab] this term only (433)
#13 (infliximab or remicade or “170277-31-3”) (1347)
#14 (inflectra or remsima or “CT-P13”) (17)
#15 (secukinumab or Cosentyx or “AIN457” or “AIN-457” or “1229022-83-6”) (153)
#16 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 (14,174)
#17 #5 and #16 (822)
#18 MeSH descriptor: [Child] explode all trees (178)
#19 MeSH descriptor: [Infant] explode all trees (14,343)
#20 MeSH descriptor: [Adolescent] this term only (85,203)
#21 (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) (193,591)
#22 #18 or #19 or #20 or #21 (193,591)
#23 #17 and #22 (89)
#24 #17 and #22 in Cochrane Reviews (Reviews and Protocols) (15)
#25 #17 and #22 in Trials (70)
Key
MeSH descriptor = indexing term (MeSH heading)
* = truncation
ti,ab,kw = terms in either title or abstract or keyword fields
near/2 = terms within two words of each other (any order)
“ “ = phrase search
Cochrane Database of Systematic Reviews (CDSR)
Via Wiley Online Library (http://onlinelibrary.wiley.com/).
Date range searched: issue 5 of 12, May 2016.
Date searched: 31 May 2016.
Records retrieved: 15.
See above under CENTRAL for search strategy used. The search was updated on 30 September 2016 and retrieved 15 records from CDSR.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus
Via EBSCOhost (www.ebscohost.com/).
Date range searched: from inception to 30 May 2016.
Date searched: 31 May 2016.
Records retrieved: 68.
The search was updated on 30 September 2016 and retrieved 74 records.
Search strategy
S22 S17 AND S21 (68)
S21 S18 OR S19 OR S20 (816,943)
S20 TX adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons” (816,943)
S19 (MH “Adolescence+”) (355,343)
S18 (MH “Child+”) (459,529)
S17 S5 AND S16 (718)
S16 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 (9936)
S15 TX (secukinumab or Cosentyx or “AIN457” or “AIN-457” or “1229022-83-6”) (73)
S14 TX (inflectra or remsima or “CT-P13”) (34)
S13 TX (infliximab or remicade or “170277-31-3”) (2106)
S12 (MH “Infliximab”) (1001)
S11 TX (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) (2710)
S10 (MH “Cyclosporins”) OR (MH “Cyclosporine”) (1875)
S9 TX methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) (5106)
S8 (MH “Methotrexate”) (3692)
S7 TX acitretin* or etretin or neotigason or soriatane or “55079-83-9” (90)
S6 (MH “Retinoids”) (662)
S5 S1 OR S2 OR S3 OR S4 (5,585)
S4 TI (pustul* N2 palm*) OR AB (pustul* N2 palm*) (50)
S3 TI parapsoriasis OR AB parapsoriasis (11)
S2 TI ( psorias* or psoriat* ) OR AB ( psorias* or psoriat* ) (4375)
S1 (MH “Psoriasis”) (3599)
Key
MH = indexing term (CINAHL heading).
* = truncation.
TI = terms in the title.
AB = terms in the abstract.
TX = all text – search of all of the database’s searchable fields.
“ “ = phrase search.
N2 = terms within two words of each other (any order).
Database of Abstracts of Reviews of Effects (DARE)
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 31 March 2015.
Date searched: 31 May 2016.
Records retrieved: 6.
This search strategy was not updated as DARE closed at the end of March 2015.
Search strategy
-
MeSH DESCRIPTOR Psoriasis (202)
-
(psorias* or psoriat*) (311)
-
(parapsoriasis) (1)
-
(pustul* NEAR2 palm*) (2)
-
(palm* NEAR2 pustul*) (3)
-
#1 OR #2 OR #3 OR #4 OR #5 (311)
-
MeSH DESCRIPTOR Acitretin (7)
-
(acitretin* or etretin or neotigason or soriatane or “55079-83-9”) (25)
-
MeSH DESCRIPTOR Methotrexate (176)
-
(methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) (452)
-
MeSH DESCRIPTOR Cyclosporins EXPLODE ALL TREES (109)
-
(cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) (279)
-
MeSH DESCRIPTOR Infliximab (163)
-
(infliximab or remicade or “170277-31-3”) (349)
-
(inflectra or remsima or “CT-P13”) (5)
-
(secukinumab or Cosentyx or “AIN457” or “AIN-457” or “1229022-83-6”) (11)
-
#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 (921)
-
#6 AND #17 (118)
-
MeSH DESCRIPTOR child EXPLODE ALL TREES (4890)
-
MeSH DESCRIPTOR infant EXPLODE ALL TREES (2947)
-
MeSH DESCRIPTOR adolescent (4585)
-
(adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler*) (13,225)
-
(schoolboy* or schoolgirl* or schoolchild* or teens or teenage*) (148)
-
(toddler* or youth or youths or “young people” or “young person” or “young persons”) (614)
-
#19 OR #20 OR #21 OR #22 OR #23 OR #24 (13,340)
-
#18 AND #25 (7)
-
(#18 AND #25) IN DARE (6)
-
(#18 AND #25) IN HTA (1)
-
(#18 AND #25) IN NHSEED (0)
Key
MeSH DESCRIPTOR = indexing term (MeSH heading).
* = truncation.
“ “ = phrase search.
NEAR2 = terms within two words of each other (order specified).
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 27 May 2016.
Date searched: 31 May 2016.
Records retrieved: 1467.
The search was updated on 30 September 2016 and retrieved 1564 records.
Search strategy
-
exp psoriasis/ (57,814)
-
(psorias$ or psoriat$).ti,ab. (53,874)
-
parapsoriasis.ti,ab. (571)
-
(pustul$ adj2 palm$).ti,ab. (1043)
-
1 or 2 or 3 or 4 (70,062)
-
etretin/ (4892)
-
(acitretin$ or etretin or neotigason or soriatane or 55079-83-9).af. (5033)
-
methotrexate/ (146,811)
-
(methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or 15475-56-6 or 59-05-2 or 7413-34-5).af. (152,425)
-
cyclosporin derivative/ (1950)
-
cyclosporin/ (70,557)
-
cyclosporin A/ (65,595)
-
(cyclosporin$ or ciclosporin$ or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or 59865-13-3 or 63798-73-2).af. (139,555)
-
infliximab/ (35,519)
-
(infliximab or remicade or 170277-31-3).af. (36,260)
-
(inflectra or remsima or CT-P13).af. (157)
-
secukinumab/ (768)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (849)
-
or/6-18 (289,812)
-
5 and 19 (15,485)
-
exp child/ (2,317,206)
-
exp adolescent/ (1,351,704)
-
juvenile/ (26,120)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenage$ or toddler$ or youth or youths or young people or young person$).ti,ab. (2,068,480)
-
or/21-24 (3,560,620)
-
20 and 25 (1468)
-
(animal/ or nonhuman/) not exp human/ (5,037,476)
-
26 not 27 (1467)
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = all fields.
adj2 = terms within two words of each other (any order).
Health Technology Assessment (HTA) database
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 31 May 2016.
Date searched: 31 May 2016.
Records retrieved: 1.
See above under DARE for search strategy used. The search was updated on 30 September 2016 and retrieved one record.
PubMed
Date searched: 31 May 2016.
Records retrieved: 698.
The search was updated on 30 September 2016 and retrieved 715 records.
Search strategy
(((((((((((((“Acitretin”[Mesh:NoExp]) OR ((acitretin* OR etretin OR neotigason OR soriatane OR “55079-83-9”))) OR “Methotrexate”[Mesh:NoExp]) OR ((methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal OR trexall OR otrexup OR rasuvo OR “15475-56-6” OR “59-05-2” OR “7413-34-5”))) OR “Cyclosporins”[Mesh]) OR ((cyclosporin$ OR ciclosporin$ OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin OR sandimmun OR syncloral OR vanquoral OR gengraf OR “59865-13-3” OR “63798-73-2”))) OR “Infliximab”[Mesh:NoExp]) OR ((infliximab OR remicade OR “170277-31-3”))) OR ((inflectra OR remsima OR CT-P13))) OR ((secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR “1229022-83-6”)))) AND ((((“Psoriasis”[Mesh:NoExp]) OR ((psorias*[Title/Abstract] OR psoriat*[Title/Abstract]))) OR parapsoriasis[Title/Abstract]) OR ((pustul*[Title/Abstract] AND palm*[Title/Abstract]))))) AND ((((“Child”[Mesh]) OR “Infant”[Mesh]) OR “Adolescent”[Mesh:NoExp]) OR ((adolescen*[Title/Abstract] OR baby[Title/Abstract] OR babies[Title/Abstract] OR child[Title/Abstract] OR children[Title/Abstract] OR boy[Title/Abstract] OR boys[Title/Abstract] OR girl[Title/Abstract] OR girls[Title/Abstract] OR infant*[Title/Abstract] OR infanc*[Title/Abstract] OR juvenile*[Title/Abstract] OR paediatric[Title/Abstract] OR pediatric[Title/Abstract] OR preschooler*[Title/Abstract] OR schoolboy*[Title/Abstract] OR schoolgirl*[Title/Abstract] OR schoolchild*[Title/Abstract] OR teens[Title/Abstract] OR teenage*[Title/Abstract] OR toddler*[Title/Abstract] OR youth[Title/Abstract] OR youths[Title/Abstract] OR “young people”[Title/Abstract] OR “young person”[Title/Abstract] OR “young persons”[Title/Abstract])))
Key
[Mesh] = exploded indexing term (MeSH heading).
[Mesh:NoExp] = indexing term (MeSH heading) not exploded.
* = truncation.
“ “ = phrase search.
[Title/Abstract]) = terms in either title or abstract fields.
Science Citation Index
Via Web of Science, Thomson Reuters (http://thomsonreuters.com/thomson-reuters-web-of-science/).
Date range searched: 1900 to 30 May 2016.
Date searched: 31 May 2016.
Records retrieved: 402.
The search was updated on 30 September 2016 and retrieved 420 records.
Search strategy
| #14 | 402 |
#13 AND #12 Indexes = SCI-EXPANDED Timespan = All years |
| #13 | 1,529,440 |
TS = (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) Indexes = SCI-EXPANDED Timespan = All years |
| #12 | 6098 |
#11 AND #4 Indexes = SCI-EXPANDED Timespan = All years |
| #11 | 122,506 |
#10 OR #9 OR #8 OR #7 OR #6 OR #5 Indexes = SCI-EXPANDED Timespan = All years |
| #10 | 329 |
TS = (secukinumab or Cosentyx or AIN457 or AIN-457 or “1229022-83-6”) Indexes = SCI-EXPANDED Timespan = All years |
| #9 | 78 |
TS = (inflectra or remsima or CT-P13) Indexes = SCI-EXPANDED Timespan = All years |
| #8 | 16,040 |
TS = (infliximab or remicade or “170277-31-3”) Indexes = SCI-EXPANDED Timespan = All years |
| #7 | 67,052 |
TS = (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) Indexes = SCI-EXPANDED Timespan = All years |
| #6 | 44,388 |
TS = (methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) Indexes = SCI-EXPANDED Timespan = All years |
| #5 | 1259 |
TS = (acitretin* or etretin or neotigason or soriatane or “55079-83-9”) Indexes = SCI-EXPANDED Timespan = All years |
| #4 | 46,956 |
#3 OR #2 OR #1 Indexes = SCI-EXPANDED Timespan = All years |
| #3 | 808 |
TS = (pustul* NEAR/2 palm*) Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 480 |
TS = parapsoriasis Indexes = SCI-EXPANDED Timespan = All years |
| #1 | 46,113 |
TS = (psorias* or psoriat*) Indexes = SCI-EXPANDED Timespan = All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
“ “ = phrase search.
NEAR/2 = terms within two words of each other (any order).
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
Date searched: 31 May 2016.
Records retrieved: 20.
Searches were carried out as per the search strings below. In total, 47 studies were found, which was reduced to 20 studies after deduplication of the results.
The search was updated on 15 September 2016 (see Network meta-analysis searches, Search 2).
-
Three studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (acitretin OR etretin OR neotigason OR soriatane OR 55079-83-9)
-
Nine studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal)
-
Nine studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (trexall OR otrexup OR rasuvo OR 15475-56-6 OR 59-05-2 OR 7413-34-5)
-
Seven studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (cyclosporin OR ciclosporin OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin)
-
Seven studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (sandimmun OR syncloral OR vanquoral OR gengraf OR 59865-13-3 OR 63798-73-2)
-
Nine studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (infliximab OR remicade OR 170277-31-3)
-
No studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (inflectra OR remsima OR CT-P13)
-
Three studies found for (Psoriasis OR psoriatic) AND (adolescent OR adolescence OR adolescents OR child OR children OR infant OR infancy OR infants) AND (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6)
Conference Proceedings Citation Index – Science
Via Web of Science, Thomson Reuters (http://thomsonreuters.com/thomson-reuters-web-of-science/).
Date range searched: 1990 to 30 May 2016.
Date searched: 31 May 2016.
Records retrieved: 16.
The search was updated on 30 September 2016 and retrieved 16 records.
Search strategy
| #14 | 16 |
#13 AND #12 Indexes = CPCI-S Timespan = All years |
| #13 | 149,897 |
TS = (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenage* or toddler* or youth or youths or “young people” or “young person” or “young persons”) Indexes = CPCI-S Timespan = All years |
| #12 | 592 |
#11 AND #4 Indexes = CPCI-S Timespan = All years |
| #11 | 15,410 |
#10 OR #9 OR #8 OR #7 OR #6 OR #5 Indexes = CPCI-S Timespan = All years |
| #10 | 76 |
TS = (secukinumab or Cosentyx or AIN457 or AIN-457 or “1229022-83-6”) Indexes = CPCI-S Timespan = All years |
| #9 | 8 |
TS = (inflectra or remsima or CT-P13) Indexes = CPCI-S Timespan = All years |
| #8 | 2781 |
TS = (infliximab or remicade or “170277-31-3”) Indexes = CPCI-S Timespan = All years |
| #7 | 8929 |
TS = (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) Indexes = CPCI-S Timespan = All years |
| #6 | 4027 |
TS = (methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) Indexes = CPCI-S Timespan = All years |
| #5 | 129 |
TS = (acitretin* or etretin or neotigason or soriatane or “55079-83-9”) Indexes = CPCI-S Timespan = All years |
| #4 | 6417 |
#3 OR #2 OR #1 Indexes = CPCI-S Timespan = All years |
| #3 | 68 |
TS = (pustul* NEAR/2 palm*) Indexes = CPCI-S Timespan = All years |
| #2 | 18 |
TS = parapsoriasis Indexes = CPCI-S Timespan = All years |
| #1 | 6359 |
TS = (psorias* or psoriat*) Indexes = CPCI-S Timespan = All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
“ “ = phrase search.
NEAR/2 = terms within two words of each other (any order).
EU Clinical Trials Register
www.clinicaltrialsregister.eu/ctr-search/search
Date searched: 31 May 2016.
Records retrieved: 7.
The search was updated on 19 September 2016 (see Network meta-analysis searches, Search 2).
-
No result(s) found for (Psoriasis OR psoriatic) AND (acitretin* OR etretin OR neotigason OR soriatane OR 55079-83-9). Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
Five result(s) found for (Psoriasis OR psoriatic) AND (methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal OR trexall OR otrexup OR rasuvo OR 15475-56-6 OR 59-05-2 OR 7413-34-5) ). Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
No results found for (Psoriasis OR psoriatic) AND (cyclosporin* OR ciclosporin* OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin OR sandimmun OR syncloral OR vanquoral OR gengraf OR 59865-13-3 OR 63798-73-2) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
One result(s) found for (Psoriasis OR psoriatic) AND (infliximab OR remicade OR 170277-31-3) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
No results(s) found for (Psoriasis OR psoriatic) AND (inflectra OR remsima OR CT-P13) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
-
One result(s) found for (Psoriasis OR psoriatic) AND (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6) Limited by age range to adolescent, children, infant and toddler, newborn, preterm, under 18
Key
* = truncation.
World Health Organization (WHO)’s International Clinical Trials Registry Platform
Date searched: 30 May 2016.
Records retrieved: 25.
The search was updated on 19 September 2016 (see Network meta-analysis searches, Search 2).
-
One trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (acitretin* OR etretin OR neotigason OR soriatane OR 55079-83-9) limited to clinical trials in children (birth to 18 years)
-
Five trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal OR trexall OR otrexup OR rasuvo OR 15475-56-6 OR 59-05-2 OR 7413-34-5) limited to clinical trials in children (birth to 18 years)
-
Two trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (cyclosporin* OR ciclosporin* OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin OR sandimmun OR syncloral OR vanquoral OR gengraf OR 59865-13-3 OR 63798-73-2) limited to clinical trials in children (birth to 18 years)
-
Five trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (infliximab OR remicade OR 170277-31-3) limited to clinical trials in children (birth to 18 years)
-
One trial found for Condition: (psoriasis OR psoriatic) AND Intervention: (inflectra OR remsima OR CT-P13) limited to clinical trials in children (birth to 18 years)
-
Twelve trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6) limited to clinical trials in children (birth to 18 years)
Search 2
Randomised controlled trials of adalimumab, etanercept, ustekinumab, acitretin, methotrexate, ciclosporin, infliximab or secukinumab in adults with plaque psoriasis.
Database search strategies
MEDLINE [MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)]
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to present.
Date searched: 14 September 2016.
Records retrieved: 274.
The Cochrane Highly Sensitive Search Strategy (sensitivity-maximising version) for identifying randomised trials in Ovid MEDLINE was used to limit retrieval to RCTs (lines 26–35). 177
Search strategy
-
Psoriasis/ (29,604)
-
(psorias$ or psoriat$).ti,ab. (37,897)
-
parapsoriasis.ti,ab. (529)
-
(pustul$ adj2 palm$).ti,ab. (804)
-
1 or 2 or 3 or 4 (43,797)
-
Adalimumab/ (3560)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (5547)
-
Etanercept/ (4829)
-
(etanercept or enbrel or 185243-69-0).af. (6906)
-
Ustekinumab/ (449)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (859)
-
or/6-11 (10,712)
-
Acitretin/ (944)
-
(acitretin$ or etretin or neotigason or soriatane or 55079-83-9).af. (2610)
-
Methotrexate/ (34,625)
-
(methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or 15475-56-6 or 59-05-2 or 7413-34-5).af. (49,306)
-
exp Cyclosporins/ (37,792)
-
(cyclosporin$ or ciclosporin$ or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or 59865-13-3 or 63798-73-2).af. (55,843)
-
Infliximab/ (8112)
-
(infliximab or remicade or 170277-31-3).af. (11,419)
-
(inflectra or remsima or CT-P13).af. (78)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (236)
-
or/13-22 (113,486)
-
5 and 12 (2777)
-
5 and 23 (5831)
-
randomized controlled trial.pt. (430,970)
-
controlled clinical trial.pt. (91,709)
-
randomized.ab. (370,512)
-
placebo.ab. (179,018)
-
clinical trials as topic.sh. (179,518)
-
randomly.ab. (263,747)
-
trial.ti. (162,078)
-
26 or 27 or 28 or 29 or 30 or 31 or 32 (1,067,990)
-
exp animals/ not humans.sh. (4,316,367)
-
33 not 34 (984,770)
-
24 and 35 (611)
-
25 and 35 (851)
-
36 or 37 (1163)
-
(2014$ or 2015$ or 2016$).ed,dc. (3,860,024)
-
38 and 39 (274)
Key
/ = indexing term (MeSH heading).
exp = exploded indexing term (MeSH heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
adj2 = terms within two words of each other (any order).
af = terms in any field.
sh = subject heading.
ed = entry date field.
dc = date record created field.
pt = publication type.
Cochrane Central Register of Controlled Trials (CENTRAL)
Via Wiley Online Library (http://onlinelibrary.wiley.com/).
Date range searched: issue 8 of 12, August 2016.
Date searched: 14 September 2016.
Records retrieved: 280.
Search strategy
The strategy below was used to search CENTRAL and CDSR.
#1 MeSH descriptor: [Psoriasis] this term only (1903)
#2 (psorias* or psoriat*):ti,ab,kw (4457)
#3 parapsoriasis:ti,ab,kw (3)
#4 (pustul* near/2 palm*):ti,ab,kw (75)
#5 #1 or #2 or #3 or #4 (4489)
#6 MeSH descriptor: [Adalimumab] this term only (253)
#7 (adalimumab or humira or D2E7 or (D2 next E7) or “331731-18-1”) (1167)
#8 MeSH descriptor: [Etanercept] this term only (391)
#9 (etanercept or enbrel or “185243-69-0”) (1216)
#10 MeSH descriptor: [Ustekinumab] this term only (50)
#11 (ustekinumab or stelara or “CNTO1275” or “CNTO-1275” or “815610-63-0”) (207)
#12 #6 or #7 or #8 or #9 or #10 or #11 (2186)
#13 MeSH descriptor: [Acitretin] this term only (66)
#14 acitretin* or etretin or neotigason or soriatane or “55079-83-9” (167)
#15 MeSH descriptor: [Methotrexate] this term only (3087)
#16 methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5” (7891)
#17 MeSH descriptor: [Cyclosporins] explode all trees (2708)
#18 cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2” (6124)
#19 MeSH descriptor: [Infliximab] this term only (445)
#20 infliximab or remicade or “170277-31-3” (1404)
#21 inflectra or remsima or “CT-P13” (19)
#22 secukinumab or Cosentyx or “AIN457” or “AIN-457” or “1229022-83-6” (187)
#23 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 (14,517)
#24 #5 and #12 (651)
#25 #5 and #23 (869)
#26 #24 or #25 (1271)
#27 #24 or #25 Publication Year from 2014 to 2016 (305)
Note that results at line #27 are the total results for this search including all databases within The Cochrane Library.
Key
MeSH descriptor = indexing term (MeSH heading).
* = truncation.
ti,ab,kw = terms in either title or abstract or keyword fields.
near/2 = terms within two words of each other (any order).
“ “ = phrase search.
Cochrane Database of Systematic Reviews (CDSR)
Via Wiley Online Library (http://onlinelibrary.wiley.com/).
Issue 9 of 12, September 2016.
Date searched: 14 September 2016.
Records retrieved: 10.
See above under CENTRAL for search strategy used.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus
Via EBSCOhost (www.ebscohost.com/).
Date range searched: from inception to 14 September 2016.
Date searched: 14 September 2016.
Records retrieved: 108.
Search strategy
S43 S40 OR S42 (108)
S42 S38 AND S41 (28)
S41 (ZD “in process”) (225,905)
S40 S38 AND S39 (80)
S39 EM 2014- (914,196)
S38 S25 AND S37 (378)
S37 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 1,073,032
S36 TX allocat* random* (5244)
S35 (MH “Quantitative Studies”) (14,844)
S34 (MH “Placebos”) (9797)
S33 TX placebo* (39,440)
S32 TX random* allocat* (5244)
S31 (MH “Random Assignment”) (41,555)
S30 TX randomi* control* trial* (109,672)
S29 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) (850,155)
S28 TX clinic* n1 trial* (189,284)
S27 PT Clinical trial (79,715)
S26 (MH “Clinical Trials+”) (202,495)
S25 S12 OR S24 (1104)
S24 S5 AND S23 (746)
S23 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 (10,196)
S22 TX (secukinumab or Cosentyx or “AIN457” or “AIN-457” or “1229022-83-6”) (83)
S21 TX (inflectra or remsima or “CT-P13”) (37)
S20 TX (infliximab or remicade or “170277-31-3”) (2163)
S19 (MH “Infliximab”) (1013)
S18 TX (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) (2766)
S17 (MH “Cyclosporins”) OR (MH “Cyclosporine”) (1909)
S16 TX methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) (5246)
S15 (MH “Methotrexate”) (3761)
S14 TX acitretin* or etretin or neotigason or soriatane or “55079-83-9” (95)
S13 (MH “Retinoids”) (672)
S12 S5 AND S11 (567)
S11 S6 OR S7 OR S8 OR S9 OR S10 (2369)
S10 TX ustekinumab or stelara or CNTO1275 or “CNTO-1275” or “815610-63-0” (176)
S9 TX etanercept or enbrel or “185243-69-0” (1590)
S8 (MH “Etanercept”) (708)
S7 TX ( adalimumab or humira or D2E7 or “D2-E7” or “D2 E7” or “331731-18-1” ) (1003)
S6 (MH “Adalimumab”) (135)
S5 S1 OR S2 OR S3 OR S4 (5815)
S4 TI (pustul* N2 palm*) OR AB (pustul* N2 palm*) (52)
S3 TI parapsoriasis OR AB parapsoriasis (11)
S2 TI ( psorias* or psoriat* ) OR AB ( psorias* or psoriat* ) (4590)
S1 (MH “Psoriasis”) (3656)
Key
MH = indexing term (CINAHL heading).
* = truncation.
TI = terms in the title.
AB = terms in the abstract.
TX = all text – search of all of the database’s searchable fields.
“ “ = phrase search.
N2 = terms within two words of each other (any order).
Database of Abstracts of Reviews of Effects (DARE)
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 31 March 2015.
Date searched: 14 September 2016.
Records retrieved: 15.
Search strategy
-
MeSH DESCRIPTOR Psoriasis (203)
-
(psorias* or psoriat*) (312)
-
(parapsoriasis) (1)
-
(pustul* NEAR2 palm*) (2)
-
(palm* NEAR2 pustul*) (3)
-
#1 OR #2 OR #3 OR #4 OR #5 (312)
-
MeSH DESCRIPTOR Adalimumab (113)
-
(adalimumab or humira or D2E7 or D2-E7 or “D2 E7” or “331731-18-1”) (241)
-
MeSH DESCRIPTOR Etanercept (99)
-
(etanercept or enbrel or “185243-69-0”) (246)
-
MeSH DESCRIPTOR Ustekinumab (17)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or “815610-63-0”) (33)
-
#7 OR #8 OR #9 OR #10 OR #11 OR #12 (357)
-
#6 AND #13 (111)
-
MeSH DESCRIPTOR Acitretin (7)
-
(acitretin* or etretin or neotigason or soriatane or “55079-83-9”) (25)
-
MeSH DESCRIPTOR Methotrexate (176)
-
(methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) (453)
-
MeSH DESCRIPTOR Cyclosporins EXPLODE ALL TREES (109)
-
(cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) (279)
-
MeSH DESCRIPTOR Infliximab (164)
-
(infliximab or remicade or “170277-31-3”) (350)
-
(inflectra or remsima or “CT-P13”) (5)
-
(secukinumab or Cosentyx or “AIN457” or “AIN-457” or “1229022-83-6”) (11)
-
#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 (923)
-
#6 AND #25 (119)
-
#14 OR #26 (155)
-
(#14 OR #26) IN DARE, HTA FROM 2014 TO 2016 (26)
Key
MeSH DESCRIPTOR = indexing term (MeSH heading).
* = truncation.
“ “ = phrase search.
NEAR2 = terms within two words of each other (order specified).
EMBASE
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1974 to 13 September 2016.
Date searched: 14 September 2016.
Records retrieved: 832.
Search strategy
A search strategy developed by Lefebvre et al. to limit retrieval of studies to RCTs was used (see lines 29–45). 178
-
exp psoriasis/ (59,685)
-
(psorias$ or psoriat$).ti,ab. (55,841)
-
parapsoriasis.ti,ab. (576)
-
(pustul$ adj2 palm$).ti,ab. (1072)
-
1 or 2 or 3 or 4 (72,314)
-
adalimumab/ (21,367)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (21,859)
-
etanercept/ (23,491)
-
(etanercept or enbrel or 185243-69-0).af. (24,393)
-
ustekinumab/ (2986)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (3119)
-
or/6-11 (35,874)
-
etretin/ (5060)
-
(acitretin$ or etretin or neotigason or soriatane or 55079-83-9).af. (5207)
-
methotrexate/ (149,388)
-
(methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or 15475-56-6 or 59-05-2 or 7413-34-5).af. (155,196)
-
cyclosporin derivative/ (1951)
-
cyclosporin/ (71,661)
-
cyclosporin A/ (66,255)
-
(cyclosporin$ or ciclosporin$ or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or 59865-13-3 or 63798-73-2).af. (141,395)
-
infliximab/ (36,969)
-
(infliximab or remicade or 170277-31-3).af. (37,773)
-
(inflectra or remsima or CT-P13).af. (227)
-
secukinumab/ (909)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (994)
-
or/13-25 (295,191)
-
5 and 12 (8698)
-
5 and 26 (16,051)
-
random$.ti,ab. (1,123,629)
-
factorial$.ti,ab. (28,599)
-
crossover$.ti,ab. (59,028)
-
cross-over$.ti,ab. (26,272)
-
placebo$.ti,ab. (244,396)
-
(doubl$ adj blind$).ti,ab. (172,319)
-
(singl$ adj blind$).ti,ab. (18,249)
-
assign$.ti,ab. (296,256)
-
allocat$.ti,ab. (107,971)
-
volunteer$.ti,ab. (211,580)
-
Crossover Procedure/ (48,681)
-
double blind procedure/ (134,149)
-
Randomized Controlled Trial/ (420,204)
-
single blind procedure/ (23,202)
-
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 (1,760,154)
-
(animal/ or nonhuman/) not exp human/ (5,111,869)
-
43 not 44 (1,564,985)
-
27 and 45 (1443)
-
28 and 45 (1756)
-
46 or 47 (2351)
-
(2014$ or 2015$ or 2016$).em. (4,971,904)
-
48 and 49 (832)
Key
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = all fields.
adj2 = terms within two words of each other (any order).
em = entry date.
Health Technology Assessment (HTA) database
Via www.crd.york.ac.uk/CRDWeb/
Date range searched: from inception to 14 September 2016.
Date searched: 14 September 2016.
Records retrieved: 11.
See above under DARE for search strategy used.
PubMed
Date searched: 14 September 2016.
Records retrieved: 225.
Search strategy
The Cochrane Highly Sensitive Search Strategy (sensitivity-maximising version) for identifying randomised trials in PubMed was used to limit retrieval to clinical trials. 177
((((((“Psoriasis”[Mesh:NoExp]) OR ((psorias*[Title/Abstract] OR psoriat*[Title/Abstract] OR parapsoriasis[Title/Abstract]))) OR ((pustul*[Title/Abstract] AND palm*[Title/Abstract])))) AND ((((((((“Adalimumab”[Mesh:NoExp]) OR ((adalimumab OR humira OR D2E7 OR “D2 E7” OR “331731-18-1”))) OR “Etanercept”[Mesh:NoExp]) OR ((etanercept OR enbrel OR “185243-69-0”))) OR “Ustekinumab”[Mesh:NoExp]) OR ((ustekinumab OR stelara OR CNTO1275 OR “CNTO-1275” OR “815610-63-0”)))) OR ((((((((((((acitretin* OR etretin OR neotigason OR soriatane OR “55079-83-9”))) OR “Acitretin”[Mesh:NoExp]) OR “Methotrexate”[Mesh:NoExp]) OR ((methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal OR trexall OR otrexup OR rasuvo OR “15475-56-6” OR “59-05-2” OR “7413-34-5”))) OR “Cyclosporins”[Mesh]) OR ((cyclosporin$ OR ciclosporin$ OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin OR sandimmun OR syncloral OR vanquoral OR gengraf OR “59865-13-3” OR “63798-73-2”))) OR “Infliximab”[Mesh:NoExp]) OR ((infliximab OR remicade OR “170277-31-3”))) OR ((inflectra OR remsima OR CT-P13))) OR ((secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR “1229022-83-6”)))))) AND (((((((((randomized controlled trial [pt]) OR controlled clinical trial [pt]) OR randomized [tiab]) OR placebo [tiab]) OR clinical trials as topic [mesh: noexp]) OR randomly [tiab]) OR trial [ti])) NOT ((animals [mh] NOT humans [mh]))) Filters: Publication date from 2014/01/01 to 2016/12/31
Key
[Mesh] = exploded indexing term (MeSH heading).
[Mesh:NoExp] = indexing term (MeSH heading) not exploded.
* = truncation.
“ “ = phrase search.
[Title/Abstract]) = terms in either title or abstract fields.
[tiab] = terms in either title or abstract fields.
[pt] = publication type.
[mh] = exploded indexing term (MeSH heading).
Science Citation Index
Via Web of Science, Thomson Reuters (http://thomsonreuters.com/thomson-reuters-web-of-science/).
Date range searched: 1900 to 13 September 2016.
Date searched: 14 September 2016.
Records retrieved: 820.
Search strategy
| #28 | 820 |
#26 not #27 Indexes = SCI-EXPANDED Timespan = 2014-2016 |
| #27 | 3,866,779 |
TS = (animal or animals or dog or dogs or hamster* or mice or mouse or rat or rats or bovine or sheep or guinea*) Indexes = SCI-EXPANDED Timespan = All years |
| #26 | 3,492 |
#24 AND #18 Indexes = SCI-EXPANDED Timespan = All years |
| #25 | 3492 |
#24 AND #18 Indexes = SCI-EXPANDED Timespan = All years |
| #24 | 5,910,291 |
#23 OR #22 OR #21 OR #20 OR #19 Indexes = SCI-EXPANDED Timespan = All years |
| #23 | 5,038,629 |
TS = (placebo* or random* or control* or prospectiv* or volunteer*) Indexes = SCI-EXPANDED Timespan = All years |
| #22 | 518,826 |
TS = (clinic* SAME trial*) Indexes = SCI-EXPANDED Timespan = All years |
| #21 | 15,215 |
TS = (singl* SAME mask*) or TS = (doubl* SAME mask*) or TS = (trebl* SAME mask*) or TS = (tripl* SAME mask*) Indexes = SCI-EXPANDED Timespan = All years |
| #20 | 218,910 |
TS = (singl* SAME blind*) or TS = (doubl* SAME blind*) or TS = (trebl* SAME blind*) or TS = (tripl* SAME blind*) Indexes = SCI-EXPANDED Timespan = All years |
| #19 | 1,076,694 |
TS = (stud* SAME design*) Indexes = SCI-EXPANDED Timespan = All years |
| #18 | 8309 |
#17 OR #9 Indexes = SCI-EXPANDED Timespan = All years |
| #17 | 6234 |
#16 AND #4 Indexes = SCI-EXPANDED Timespan = All years |
| #16 | 123,797 |
#15 OR #14 OR #13 OR #12 OR #11 OR #10 Indexes = SCI-EXPANDED Timespan = All years |
| #15 | 374 |
TS = (secukinumab or Cosentyx or AIN457 or AIN-457 or “1229022-83-6”) Indexes = SCI-EXPANDED Timespan = All years |
| #14 | 99 |
TS = (inflectra or remsima or CT-P13) Indexes = SCI-EXPANDED Timespan = All years |
| #13 | 16,441 |
TS = (infliximab or remicade or “170277-31-3”) Indexes = SCI-EXPANDED Timespan = All years |
| #12 | 67,409 |
TS = (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) Indexes = SCI-EXPANDED Timespan = All years |
| #11 | 44,932 |
TS = (methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) Indexes = SCI-EXPANDED Timespan = All years |
| #10 | 1288 |
TS = (acitretin* or etretin or neotigason or soriatane or “55079-83-9”) Indexes = SCI-EXPANDED Timespan = All years |
| #9 | 3641 |
#8 AND #4 Indexes = SCI-EXPANDED Timespan = All years |
| #8 | 13,940 |
#7 OR #6 OR #5 Indexes = SCI-EXPANDED Timespan = All years |
| #7 | 1098 |
TS = (ustekinumab or stelara or CNTO1275 or CNTO-1275 or “815610-63-0”) Indexes = SCI-EXPANDED Timespan = All years |
| #6 | 8642 |
TS = (etanercept or enbrel or “185243-69-0”) Indexes = SCI-EXPANDED Timespan = All years |
| #5 | 6703 |
TS = (adalimumab or humira or D2E7 or D2-E7 or “D2 E7” or “331731-18-1”) Indexes = SCI-EXPANDED Timespan = All years |
| #4 | 47,963 |
#3 OR #2 OR #1 Indexes = SCI-EXPANDED Timespan = All years |
| #3 | 819 |
TS = (pustul* NEAR/2 palm*) Indexes = SCI-EXPANDED Timespan = All years |
| #2 | 482 |
TS = parapsoriasis Indexes = SCI-EXPANDED Timespan = All years |
| #1 | 47,116 |
TS = (psorias* or psoriat*) Indexes = SCI-EXPANDED Timespan = All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
“ “ = phrase search.
NEAR/2 = terms within two words of each other (any order).
SAME = terms in the same record.
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
Date searched: 15 September 2016.
Records retrieved: 105.
Searches were carried out as per the search strings below. In total, 171 studies were found, which was reduced to 105 studies after deduplication of the results.
-
Twenty-six studies found for (Psoriasis OR psoriatic) AND (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1) | Studies received from 1 January 2014 to 15 September 2016
-
Twenty-two studies found for (Psoriasis OR psoriatic) AND (etanercept OR enbrel OR 185243-69-0) | Studies received from 1 January 2014 to 15 September 2016
-
Twenty-three studies found for (Psoriasis OR psoriatic) AND (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0) | Studies received from 1 January 2014 to 15 September 2016
-
Five studies found for (Psoriasis OR psoriatic) AND (acitretin OR etretin OR neotigason OR soriatane OR 55079-83-9) | Studies received from 1 January 2014 to 15 September 2016
-
Twenty-four studies found for (Psoriasis OR psoriatic) AND (methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal) | Studies received from 1 January 2014 to 15 September 2016
-
Twenty-four studies found for (Psoriasis OR psoriatic) AND (trexall OR otrexup OR rasuvo OR 15475-56-6 OR 59-05-2 OR 7413-34-5) | Studies received from 1 January 2014 to 15 September 2016
-
Four studies found for (Psoriasis OR psoriatic) AND (cyclosporin OR ciclosporin OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin) | Studies received from 1 January 2014 to 15 September 2016
-
Four studies found for (Psoriasis OR psoriatic) AND (sandimmun OR syncloral OR vanquoral OR gengraf OR 59865-13-3 OR 63798-73-2) | Studies received from 1 January 2014 to 15 September 2016
-
Six studies found for (Psoriasis OR psoriatic) AND (infliximab OR remicade OR 170277-31-3) | Studies received from 1 January 2014 to 15 September 2016
-
Three studies found for (Psoriasis OR psoriatic) AND (inflectra OR remsima OR CT-P13) | Studies received from 1 January 2014 to 15 September 2016
-
Thirty studies found for (Psoriasis OR psoriatic) AND (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6) | Studies received from 1 January 2014 to 15 September 2016
Conference Proceedings Citation Index – Science
Via Web of Science, Thomson Reuters (http://thomsonreuters.com/thomson-reuters-web-of-science/).
Date range searched: 1990 to 13 September 2016.
Daten searched: 14 September 2016.
Records retrieved: 33.
Search strategy
| #28 | 33 |
#26 not #27 Indexes = CPCI-S Timespan = 2014-2016 |
| #27 | 306,516 |
TS = (animal or animals or dog or dogs or hamster* or mice or mouse or rat or rats or bovine or sheep or guinea*) Indexes = CPCI-S Timespan = All years |
| #26 | 235 |
#24 AND #18 Indexes = CPCI-S Timespan = All years |
| #25 | 235 |
#24 AND #18 Indexes = CPCI-S Timespan = All years |
| #24 | 1,276,541 |
#23 OR #22 OR #21 OR #20 OR #19 Indexes = CPCI-S Timespan = All years |
| #23 | 1,060,361 |
TS = (placebo* or random* or control* or prospectiv* or volunteer*) Indexes = CPCI-S Timespan = All years |
| #22 | 41,861 |
TS = (clinic* SAME trial*) Indexes = CPCI-S Timespan = All years |
| #21 | 5279 |
TS = (singl* SAME mask*) or TS = (doubl* SAME mask*) or TS = (trebl* SAME mask*) or TS = (tripl* SAME mask*) Indexes = CPCI-S Timespan = All years |
| #20 | 19,759 |
TS = (singl* SAME blind*) or TS = (doubl* SAME blind*) or TS = (trebl* SAME blind*) or TS = (tripl* SAME blind*) Indexes = CPCI-S Timespan = All years |
| #19 | 257,226 |
TS = (stud* SAME design*) Indexes = CPCI-S Timespan = All years |
| #18 | 1159 |
#17 OR #9 Indexes = CPCI-S Timespan = All years |
| #17 | 599 |
#16 AND #4 Indexes = CPCI-S Timespan = All years |
| #16 | 15,543 |
#15 OR #14 OR #13 OR #12 OR #11 OR #10 Indexes = CPCI-S Timespan = All years |
| #15 | 83 |
TS = (secukinumab or Cosentyx or AIN457 or AIN-457 or “1229022-83-6”) Indexes = CPCI-S Timespan = All years |
| #14 | 10 |
TS = (inflectra or remsima or CT-P13) Indexes = CPCI-S Timespan = All years |
| #13 | 2799 |
TS = (infliximab or remicade or “170277-31-3”) Indexes = CPCI-S Timespan = All years |
| #12 | 8983 |
TS = (cyclosporin* or ciclosporin* or capimune or capsorin or deximune or emulsoforal or neoral or neciclopin or sandimmun or syncloral or vanquoral or gengraf or “59865-13-3” or “63798-73-2”) Indexes = CPCI-S Timespan = All years |
| #11 | 4083 |
TS = (methotrexate or amethopterin or MTX or ebetrex or maxtrex or metoject or methofill or namaxir or zlatal or trexall or otrexup or rasuvo or “15475-56-6” or “59-05-2” or “7413-34-5”) Indexes = CPCI-S Timespan = All years |
| #10 | 133 |
TS = (acitretin* or etretin or neotigason or soriatane or “55079-83-9”) Indexes = CPCI-S Timespan = All years |
| #9 | 645 |
#8 AND #4 Indexes = CPCI-S Timespan = All years |
| #8 | 2720 |
#7 OR #6 OR #5 Indexes = CPCI-S Timespan = All years |
| #7 | 185 |
TS = (ustekinumab or stelara or CNTO1275 or CNTO-1275 or “815610-63-0”) Indexes = CPCI-S Timespan = All years |
| #6 | 1356 |
TS = (etanercept or enbrel or “185243-69-0”) Indexes = CPCI-S Timespan = All years |
| #5 | 1361 |
TS = (adalimumab or humira or D2E7 or D2-E7 or “D2 E7” or “331731-18-1”) Indexes = CPCI-S Timespan = All years |
| #4 | 6587 |
#3 OR #2 OR #1 Indexes = CPCI-S Timespan = All years |
| #3 | 69 |
TS = (pustul* NEAR/2 palm*) Indexes = CPCI-S Timespan = All years |
| #2 | 18 |
TS = parapsoriasis Indexes = CPCI-S Timespan = All years |
| #1 | 6529 |
TS = (psorias* or psoriat*) Indexes = CPCI-S Timespan = All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
“ “ = phrase search.
NEAR/2 = terms within two words of each other (any order).
SAME = terms in the same record.
EU Clinical Trials Register
www.clinicaltrialsregister.eu/ctr-search/search
Date searched: 19 September 2016.
Records retrieved: 85.
Date range searched: 1 January 2014 to 19 September 2016.
-
Seventeen result(s) found for (Psoriasis OR psoriatic) AND (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1
-
Nine result(s) found for (Psoriasis OR psoriatic) AND (etanercept OR enbrel OR 185243-69-0)
-
Eleven result(s) found for (Psoriasis OR psoriatic) AND (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0)
-
One result(s) found for (Psoriasis OR psoriatic) AND (acitretin* OR etretin OR neotigason OR soriatane OR 55079-83-9)
-
Twenty result(s) found for (Psoriasis OR psoriatic) AND (methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal OR trexall OR otrexup OR rasuvo OR 15475-56-6 OR 59-05-2 OR 7413-34-5)
-
Five result(s) found for (Psoriasis OR psoriatic) AND (cyclosporin* OR ciclosporin* OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin OR sandimmun OR syncloral OR vanquoral OR gengraf OR 59865-13-3 OR 63798-73-2)
-
Three result(s) found for (Psoriasis OR psoriatic) AND (infliximab OR remicade OR 170277-31-3)
-
One result(s) found for (Psoriasis OR psoriatic) AND (inflectra OR remsima OR CT-P13)
-
Eighteen result(s) found for (Psoriasis OR psoriatic) AND (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6)
Key
* = truncation.
World Health Organization (WHO)’s International Clinical Trials Registry Platform
Date searched: 19 September 2016.
Records retrieved: 188.
Date range searched: 1 January 2014 to 19 September 2016.
-
Forty-one trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1)
-
Twenty-six trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (etanercept OR enbrel OR 185243-69-0)
-
Twenty-five trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0)
-
Six trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (acitretin* OR etretin OR neotigason OR soriatane OR 55079-83-9)
-
Thirty trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (methotrexate OR amethopterin OR MTX OR ebetrex OR maxtrex OR metoject OR methofill OR namaxir OR zlatal OR trexall OR otrexup OR rasuvo OR 15475-56-6 OR 59-05-2 OR 7413-34-5)
-
Four trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (cyclosporin* OR ciclosporin* OR capimune OR capsorin OR deximune OR emulsoforal OR neoral OR neciclopin OR sandimmun OR syncloral OR vanquoral OR gengraf OR 59865-13-3 OR 63798-73-2)
-
Seven trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (infliximab OR remicade OR 170277-31-3)
-
Two trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (inflectra OR remsima OR CT-P13)
-
Forty-seven trials found for Condition: (psoriasis OR psoriatic) AND Intervention: (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6)
Quality-of-life review
MEDLINE & MEDLINE In-Process & Other Non-Indexed Citations
Via Ovid (http://ovidsp.ovid.com/).
Date range searched: 1946 to present.
Date searched: 12 July 2016.
Records retrieved: 140.
Search strategy
A search filter was used to limit retrieval to quality-of-life studies – see lines 1–28 below.
-
quality-adjusted life years/ (8623)
-
“Value of Life”/ (5514)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. (7149)
-
(quality adjusted or adjusted life year$).ti,ab,kf. (11,017)
-
disability adjusted life.ti,ab,kf. (1947)
-
daly$1.ti,ab,kf. (1798)
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).ti,ab,kf. (482)
-
(multiattribute$ or multi attribute$).ti,ab,kf. (639)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kf. (24,175)
-
utility.ab. /freq=2 (12,201)
-
utilities.ti,ab,kf. (5156)
-
disutili$.ti,ab,kf. (305)
-
(HSUV or HSUVs).ti,ab,kf. (28)
-
health$1 year$1 equivalent$1.ti,ab,kf. (40)
-
(hye or hyes).ti,ab,kf. (58)
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. (1129)
-
(illness state$1 or health state$1).ti,ab,kf. (4781)
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).ti,ab,kf. (6257)
-
(eq-sdq or eqsdq).ti,ab,kf. (0)
-
(short form$ or shortform$).ti,ab,kf. (23,633)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. (17,244)
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).ti,ab,kf. (2480)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kf. (3232)
-
(sf16 or sf 16 or sf sixteen or sfsixteen).ti,ab,kf. (20)
-
(sf20 or sf 20 or sf twenty or sftwenty).ti,ab,kf. (315)
-
(15D or 15-D or 15 dimension).ti,ab,kf. (4251)
-
(standard gamble$ or sg).ti,ab,kf. (7698)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. (1478)
-
or/1-28 (104,742)
-
Child$ Dermatolog$ Life Quality Index$.ti,ab,kf. (121)
-
CDLQI.ti,ab,kf. (114)
-
Dermatolog$ Life Quality Index$.ti,ab,kf. (1067)
-
DLQI.ti,ab,kf. (779)
-
P?ediatric Quality of Life Inventor$.ti,ab,kf. (792)
-
PedsQL$.ti,ab,kf. (998)
-
Teen$ quality of life questionnaire$.ti,ab,kf. (0)
-
T-QoL.ti,ab,kf. (2)
-
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 (2459)
-
29 or 38 (106,897)
-
Psoriasis/ (29,336)
-
(psorias$ or psoriat$).ti,ab. (37,350)
-
parapsoriasis.ti,ab. (527)
-
(pustul$ adj2 palm$).ti,ab. (797)
-
40 or 41 or 42 or 43 (43,220)
-
exp Child/ (1,694,336)
-
exp Infant/ (1,023,912)
-
Adolescent/ (1,758,843)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenage$ or toddler$ or youth or youths or young people or young person$).ti,ab. (1,712,033)
-
45 or 46 or 47 or 48 (3,547,384)
-
39 and 44 (758)
-
49 and 50 (140)
Key
/ = indexing term (MeSH heading).
exp = exploded indexing term (MeSH heading).
$ = truncation.
$1 = limited truncation – 1 or 0 characters after word.
? = wild card – 1 or 0 characters within a word.
ti,ab,kf = terms in either title or abstract or author keyword fields.
ab. /freq=2 = terms appear at least twice in the abstract.
adj3 = terms within three words of each other (any order).
Cost-effectiveness Analysis (CEA) Registry
https://research.tufts-nemc.org/cear4/
Date searched: 12 July 2016.
Records retrieved: 23.
-
psoriasis – 22 results
-
psoriatic – 0 results
-
parapsoriasis – 0 results
-
pustulosis – 0 results
-
pustular – 0 results
-
Child Dermatology Life Quality Index – 0 results
-
CDLQI – 0 results
-
Dermatology Life Quality Index – 4 results
-
DLQI – 3 results
-
Pediatric Quality of Life Inventory – 0 results
-
Paediatric Quality of Life Inventory – 0 results
-
PedsQL – 0 results
-
Teenagers quality of life questionnaire – 0 results
-
T-QoL – 0 results
EMBASE
Via Ovid http://ovidsp.ovid.com/
Date range searched: 1974 to 11 July 2016.
Date searched: 12 July 2016.
Records retrieved: 169.
Search strategy
-
quality adjusted life year/ (16,494)
-
“quality of life index”/ (2102)
-
“quality of life assessment”/ (1928)
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kw. (12,590)
-
(quality adjusted or adjusted life year$).ti,ab,kw. (15,220)
-
disability adjusted life.ti,ab,kw. (2301)
-
daly$1.ti,ab,kw. (2364)
-
((index adj3 wellbeing) or (quality adj3 wellbeing) or qwb).ti,ab,kw. (725)
-
(multiattribute$ or multi attribute$).ti,ab,kw. (788)
-
(utility adj3 (score$1 or scoring or valu$ or measur$ or evaluat$ or scale$1 or instrument$1 or weight or weights or weighting or information or data or unit or units or health$ or life or estimat$ or elicit$ or disease$ or mean or cost$ or expenditure$1 or gain or gains or loss or losses or lost or analysis or index$ or indices or overall or reported or calculat$ or range$ or increment$ or state or states or status)).ti,ab,kw. (34,463)
-
utility.ab. /freq=2 (17,405)
-
utilities.ti,ab,kw. (7886)
-
disutili$.ti,ab,kw. (539)
-
(HSUV or HSUVs).ti,ab,kw. (41)
-
health$1 year$1 equivalent$1.ti,ab,kw. (43)
-
(hye or hyes).ti,ab,kw. (106)
-
(hui or hui1 or hui2 or hui3).ti,ab,kw. (1578)
-
(illness state$1 or health state$1).ti,ab,kw. (7514)
-
(euro qual or euro qual5d or euro qol5d or eq-5d or eq5-d or eq5d or euroqual or euroqol or euroqual5d or euroqol5d).ti,ab,kw. (10,726)
-
(eq-sdq or eqsdq).ti,ab,kw. (0)
-
(short form$ or shortform$).ti,ab,kw. (29,716)
-
exp short form 36/ (18,971)
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kw. (26,999)
-
(sf6 or sf 6 or sf6d or sf 6d or sf six or sfsix or sf8 or sf 8 or sf eight or sfeight).ti,ab,kw. (3155)
-
(sf12 or sf 12 or sf twelve or sftwelve).ti,ab,kw. (5241)
-
(sf16 or sf 16 or sf sixteen or sfsixteen).ti,ab,kw. (35)
-
(sf20 or sf 20 or sf twenty or sftwenty).ti,ab,kw. (303)
-
(15D or 15-D or 15 dimension).ti,ab,kw. (5069)
-
(standard gamble$ or sg).ti,ab,kw. (10,415)
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kw. (1985)
-
or/1-30 (148,427)
-
Child$ Dermatolog$ Life Quality Index$.ti,ab,kw. (173)
-
CDLQI.ti,ab,kw. (172)
-
dermatology life quality index/ (1292)
-
Dermatolog$ Life Quality Index$.ti,ab,kw. (1864)
-
DLQI.ti,ab,kw. (1660)
-
P?ediatric Quality of Life Inventor$.ti,ab,kw. (1084)
-
PedsQL$.ti,ab,kw. (1779)
-
Teen$ quality of life questionnaire$.ti,ab,kw. (2)
-
T-QoL.ti,ab,kw. (8)
-
or/32-40 (4669)
-
31 or 41 (152,183)
-
exp psoriasis/ (58,850)
-
(psorias$ or psoriat$).ti,ab. (54,945)
-
parapsoriasis.ti,ab. (573)
-
(pustul$ adj2 palm$).ti,ab. (1062)
-
43 or 44 or 45 or 46 (71,249)
-
42 and 47 (2034)
-
exp child/ (2,336,115)
-
exp adolescent/ (1,362,547)
-
juvenile/ (26,527)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenage$ or toddler$ or youth or youths or young people or young person$).ti,ab. (2,090,417)
-
49 or 50 or 51 or 52 (3,590,832)
-
48 and 53 (169)
Key
/ = indexing term (MeSH heading).
exp = exploded indexing term (MeSH heading).
$ = truncation.
$1 = limited truncation – 1 or 0 characters after word.
? = wild card –1 or 0 characters within a word.
ti,ab,kf = terms in either title or abstract or author keyword fields.
ab. /freq=2 = terms appear at least twice in the abstract.
adj3 = terms within three words of each other (any order).
PubMed
Date searched: 12 July 2016.
Records retrieved: 154.
Search strategy
((((((((((((((((((((((((((((((((“Quality-Adjusted Life Years”[Mesh:noexp]) OR “Value of Life”[Mesh:noexp]) OR ((qaly*[Text Word] OR qald*[Text Word] OR qale*[Text Word] OR qtime*[Text Word]))) OR ((“quality adjusted”[Text Word] OR “adjusted life year”[Text Word] OR “adjusted life years”[Text Word]))) OR “disability adjusted life”[Text Word]) OR daly*[Text Word]) OR ((((index[Text Word] AND wellbeing[Text Word])) OR (quality[Text Word] AND wellbeing[Text Word])) OR qwb[Text Word])) OR ((multiattribute*[Text Word] OR “multi attribute”[Text Word] OR “multi attributes”[Text Word]))) OR ((utility[Text Word]) AND (score*[Text Word] OR scoring[Text Word] OR valu*[Text Word] OR measur*[Text Word] OR evaluat*[Text Word] OR scale*[Text Word] OR instrument*[Text Word] OR weight[Text Word] OR weights[Text Word] OR weighting[Text Word] OR information[Text Word] OR data[Text Word] OR unit[Text Word] OR units[Text Word] OR health*[Text Word] OR life[Text Word] OR estimat*[Text Word] OR elicit*[Text Word] OR disease*[Text Word] OR mean[Text Word] OR cost*[Text Word] OR expenditure*[Text Word] OR gain[Text Word] OR gains[Text Word] OR loss[Text Word] OR losses[Text Word] OR lost[Text Word] OR analysis[Text Word] OR index*[Text Word] OR indices[Text Word] OR overall[Text Word] OR reported[Text Word] OR calculat*[Text Word] OR range*[Text Word] OR increment*[Text Word] OR state[Text Word] OR states[Text Word] OR status[Text Word]))) OR utility[Title/Abstract]) OR utilities[Text Word]) OR disutili*[Text Word]) OR ((HSUV[Text Word] OR HSUVs[Text Word]))) OR ((((“healthy year equivalent”[Text Word] OR “healthy years equivalent”[Text Word]) OR “healthy years equivalents”[Text Word]) OR “healthy year equivalents”[Text Word]))) OR ((hye[Text Word] OR hyes[Text Word]))) OR ((hui[Text Word] OR hui1[Text Word] OR hui2[Text Word] OR hui3[Text Word]))) OR ((“illness state”[Text Word] OR “illness states”[Text Word] OR “health state”[Text Word] OR “health states”[Text Word]))) OR ((“euro qual”[Text Word] OR “euro qual5d”[Text Word] OR “euro qol5d”[Text Word] OR eq-5d[Text Word] OR eq5-d[Text Word] OR eq5d[Text Word] OR euroqual[Text Word] OR euroqol[Text Word] OR euroqual5d[Text Word] OR euroqol5d[Text Word]))) OR ((eq-sdq[Text Word] OR eqsdq[Text Word]))) OR ((“short form”[Text Word] OR “short forms”[Text Word] OR shortform*[Text Word]))) OR ((sf36*[Text Word] OR sf-36*[Text Word] OR “sf thirtysix”[Text Word] OR “sf thirty six”[Text Word]))) OR ((sf6[Text Word] OR “sf 6”[Text Word] OR sf6d[Text Word] OR “sf 6d”[Text Word] OR “sf six”[Text Word] OR sfsix[Text Word] OR sf8[Text Word] OR “sf 8”[Text Word] OR “sf eight”[Text Word] OR sfeight[Text Word]))) OR ((sf12[Text Word] OR “sf 12”[Text Word] OR “sf twelve”[Text Word] OR sftwelve[Text Word]))) OR ((sf16[Text Word] OR “sf 16”[Text Word] OR “sf sixteen”[Text Word] OR sfsixteen[Text Word]))) OR ((sf20[Text Word] OR “sf 20”[Text Word] OR “sf twenty”[Text Word] OR sftwenty[Text Word]))) OR ((15D[Text Word] OR 15-D[Text Word] OR “15 dimension”[Text Word]))) OR ((“standard gamble”[Text Word] OR “standard gambles”[Text Word] OR sg[Text Word]))) OR ((“time trade off”[Text Word] OR “time trade offs”[Text Word] OR “time tradeoff”[Text Word] OR “time tradeoffs”[Text Word] OR tto[Text Word] OR timetradeoff*[Text Word]))) OR ((Child* AND Dermatolog* AND Life Quality Index*[Text Word]) OR (CDLQI[Text Word]) OR (Dermatolog* AND Life Quality Index*[Text Word]) OR (DLQI[Text Word]) OR (Pediatric Quality of Life Inventor*[Text Word] OR Paediatric Quality of Life Inventor*[Text Word]) OR (PedsQL*[Text Word]) OR (Teen* AND quality of life questionnaire*[Text Word]) OR (T-QoL[Text Word])))) AND ((((“Psoriasis”[Mesh:noexp]) OR ((psorias*[Title/Abstract] OR psoriat*[Title/Abstract]))) OR parapsoriasis[Title/Abstract]) OR ((pustul*[Title/Abstract] AND palm*[Title/Abstract]))))) AND ((((“Child”[Mesh]) OR “Infant”[Mesh]) OR “Adolescent”[Mesh]) OR ((adolescen*[Title/Abstract] OR baby[Title/Abstract] OR babies[Title/Abstract] OR child[Title/Abstract] OR children[Title/Abstract] OR boy[Title/Abstract] OR boys[Title/Abstract] OR girl[Title/Abstract] OR girls[Title/Abstract] OR infant*[Title/Abstract] OR infanc*[Title/Abstract] OR juvenile*[Title/Abstract] OR paediatric[Title/Abstract] OR pediatric[Title/Abstract] OR preschooler*[Title/Abstract] OR schoolboy*[Title/Abstract] OR schoolgirl*[Title/Abstract] OR schoolchild*[Title/Abstract] OR teens[Title/Abstract] OR teenage*[Title/Abstract] OR toddler*[Title/Abstract] OR youth[Title/Abstract] OR youths[Title/Abstract] OR “young people”[Title/Abstract] OR “young person”[Title/Abstract] OR “young persons”[Title/Abstract])))
Key
[Mesh] = exploded indexing term (MeSH heading).
[Mesh:noexp] = indexing term (MeSH heading) not exploded.
* = truncation.
[Text Word] = searches in the title, abstract, MeSH headings and subheadings, other terms field, chemical names of substances.
[Title/Abstract] = terms in either title or abstract fields.
“ “ = phrase search.
School of Health and Related Research Health Utilities Database (ScHARRHUD)
Date searched: 12 July 2016.
Records retrieved: 20.
Search strategy
psorias* OR psoriat* OR parapsoriasis
Pustulosis OR Pustular
Child* Dermatolog* Life Quality Index*
CDLQI
Dermatolog* Life Quality Index*
DLQI
Pediatric Quality of Life Inventor* OR Paediatric Quality of Life Inventor*
PedsQL*
Teen* quality of life questionnaire*
T-QoL
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10)
Key
* = truncation.
Appendix 2 Summary of included records
| Study ID (ClinicalTrials.gov ID) | Design | First author and year | Title | Record type |
|---|---|---|---|---|
| Adalimumab | ||||
| M04-717 (NCT01251614) | RCT/open-label extension | AbbVie 201045 | A double blind study in pediatric participants with chronic plaque psoriasis, studying adalimumab vs. methotrexate | Protocol |
| Papp 201340 | Study design and baseline characteristics from a Phase 3, randomized, double-blind study of adalimumab versus methotrexate treatment in pediatric patients with chronic plaque psoriasis | Meeting abstract | ||
| Papp 201439 | Baseline characteristics in pediatric patients with chronic plaque psoriasis from a Phase 3, randomized, double-blind study of adalimumab versus methotrexate treatment | Meeting abstract | ||
| Papp 201444 | Study design and baseline characteristics from a Phase 3, randomised, double-blind study of adalimumab versus methotrexate treatment in paediatric patients with chronic plaque psoriasis | Meeting abstract | ||
| Papp 201542 | Efficacy and safety of adalimumab versus methotrexate treatment in pediatric patients with severe chronic plaque psoriasis: results from the 16-week randomized, double-blind period of a Phase 3 study | Meeting abstract | ||
| Phillip 201543 | Efficacy, safety of adalimumab versus methotrexate in pediatric patients with severe chronic plaque psoriasis: results from the treatment withdrawal and double-blind retreatment periods of a Phase 3 study | Meeting abstract | ||
| European Medicines Agency 201546 | Extension of indication variation assessment report: Humira (adalimumab). Procedure no. EMEA/H/C/000481/II/0134 | Regulatory documentation | ||
| European Medicines Agency 201547 | Assessment report for paediatric studies submitted according to Article 46 of the Regulation (EC) no. 1901/2006: Humira (adalimumab) | Regulatory documentation | ||
| Papp 201641 | Adalimumab long-term safety/efficacy results for pediatric patients with chronic plaque psoriasis from a Phase 3, randomized study | Meeting abstract | ||
| Amgen 200479 | Etanercept (Enbrel®) in Psoriasis – Pediatrics | |||
| Siegfried 201080 | Intermittent etanercept therapy in pediatric patients with psoriasis | |||
| Thaci 201587 | Safety and efficacy for pediatric patients with chronic plaque psoriasis who did not respond to 16 weeks of double-blind methotrexate treatment and switched to adalimumab | |||
| Etanercept | ||||
| 20030211 (NCT00078819) | RCT/open label | Amgen 200479 | Etanercept (Enbrel®) in psoriasis – pediatrics | Protocol |
| Levy 200554 | Etanercept in children and adolescents with psoriasis | Meeting abstract | ||
| Siegfried 200655 | Etanercept in children and adolescents with psoriasis | Meeting abstract | ||
| Paller 200756 | A 12-week Phase 3 study of efficacy and safety of etanercept therapy in children and adolescents with moderate to severe plaque psoriasis | Meeting abstract | ||
| Paller 200849 | Etanercept treatment for children and adolescents with plaque psoriasis | Journal article | ||
| Paller 200857 | Etanercept treatment in children and adolescents with plaque psoriasis | Meeting abstract | ||
| Langley 201148 | Patient-reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12-week results from a Phase III randomized controlled trial | Journal article | ||
| Siegfried 201080 | Intermittent etanercept therapy in pediatric patients with psoriasis | Journal article | ||
| Paller 201050 | Subgroup analyses of etanercept in pediatric patients with psoriasis | Research letter | ||
| Landells 201051 | Efficacy and safety of etanercept in children and adolescents aged ≥ 8 years with severe plaque psoriasis | Journal article | ||
| Paller 201058 | Interim results of a long-term safety and tolerability study of etanercept treatment in children and adolescents age 8 to 17 years with plaque psoriasis | Meeting abstract | ||
| US Food and Drug Administration 200861 | Enbrel (etanercept) for the treatment of pediatric plaque psoriasis | Regulatory documentation | ||
| Amgen (via US Food and Drug Administration) 200862 | Background information for the Dermatologic and Ophthalmologic Drugs Advisory Committee (DODAC) meeting, 18 June 2008 | Regulatory documentation | ||
| European Medicines Agency 200863 | Assessment report for Enbrel. International nonproprietary name: INN – etanercept. Procedure no. EMEA/H/C/262/II/94 | Regulatory documentation | ||
| European Medicines Agency 201164 | Assessment report for Enbrel. International nonproprietary name: etanercept. Procedure no. type II variation EMEA/H/C/262/II/134 | Regulatory documentation | ||
| 20050111 (NCT00141921) | Observational study (long-term extension of study 20030211) | Amgen 200566 | Pediatric open-label extension study | Protocol |
| Amgen 201265 | An open-label extension study to evaluate the safety of etanercept in pediatric participants with plaque psoriasis | Protocol | ||
| Paller 201052 | Long-term etanercept in pediatric patients with plaque psoriasis | Journal article | ||
| Paller 201059 | Safety and efficacy of etanercept treatment in children and adolescents with plaque psoriasis: 96-week results of open-label extension study | Meeting abstract | ||
| Paller 201553 | Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis | Journal article | ||
| Paller 201660 | Five-year open-label extension study of safety and efficacy of etanercept in children and adolescents with moderate to severe plaque psoriasis | Meeting abstract | ||
| European Medicines Agency 201367 | Enbrel: etanercept. Procedure no. EMEA/H/C/000262/A46/134. CHMP assessment report for paediatric use studies submitted according to Article 46 of the Regulation (EC) no. 1901/2006 | Regulatory documentation | ||
| NCT01100034 | Observational study | Pfizer 201068 | Study evaluating the safety and effectiveness of etanercept for the treatment of pediatric psoriasis | Protocol |
| NCT01432249 | Observational study | Pfizer 201169 | Post marketing surveillance to observe safety and efficacy of Enbrel In pediatric patients with psoriasis | Protocol |
| CAIN457A2310 (NCT02471144) | RCT | Novartis Pharmaceuticals, 201570 | Pediatric study in children and adolescents with severe plaque psoriasis | Protocol |
| Novartis Pharmaceuticals 201571 | A randomized, double-blind, placebo- and active controlled multicenter trial to demonstrate efficacy of subcutaneous secukinumab compared to placebo and etanercept (in a single blinded arm) after twelve weeks of treatment, and to assess the safety, tolerability, and long-term efficacy in participants from 6 to less than 18 years of age with severe chronic plaque psoriasis | Protocol | ||
| Ustekinumab | ||||
| CNTO1275PSO3006/CADMUS (NCT01090427) | RCT/open-label extension | Janssen Research & Development, LLC 201074 | A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS) | Protocol |
| Landells 201572 | Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized Phase 3 CADMUS study | Journal article | ||
| Landells 201573 | Safety and efficacy of ustekinumab in adolescent patients with moderate to severe plaque psoriasis: results through 1 year of the Phase 3 CADMUS trial | Meeting abstract | ||
| European Medicines Agency 201575 | Assessment report: Stelara. International non-proprietary name: ustekinumab. Procedure no. EMEA/H/C/000958/II/0042 | Regulatory documentation | ||
| CR108129/CNTO1275PSO3013/CADMUS Jr (NCT02698475) | Observational study | Janssen Research & Development, LLC 201681 | An efficacy, safety, and pharmacokinetics study of subcutaneously administered ustekinumab in the treatment of moderate to severe chronic plaque psoriasis in pediatric participants greater than 6 to less than 12 years of age | Protocol |
| Observational study | Janssen-Cilag International 201682 | A Phase 3 open-label study to assess the efficacy, safety, and pharmacokinetics of subcutaneously administered ustekinumab in the treatment of moderate to severe chronic plaque psoriasis in pediatric participants greater than 6 to less than 12 years of age | Protocol | |
| Multiple biological/systemic treatments | ||||
| Garber 2015 | Observational study | Garber 201576 | Systemic treatment of recalcitrant pediatric psoriasis: a case series and literature review | Journal article |
| Klufas 2016 | Observational study | Klufas 201677 | Treatment of moderate to severe pediatric psoriasis: a retrospective case series | Journal article |
| Systematic reviews | ||||
| PROSPERO2015:CRD42015025262 | Systematic review | Chingcuanco 201583 | TNF-inhibitors: comparing the safety, efficacy and physicochemical profiles of biosimilars and innovators | Systematic review protocol |
| PROSPERO2015:CRD42015017538 | Systematic review | Smith 201584 | In people with psoriasis (all types), what are the clinical effectiveness/efficacy, safety and tolerability of systemic biologics (adalimumab, etanercept, infliximab, secukinumab or ustekinumab) compared with each other, with methotrexate or with placebo? | Systematic review protocol |
| Sanclemente 2015 | Systematic review | Sanclemente 201585 | Anti-TNF agents for paediatric psoriasis | Systematic review |
| de Jager 2010 | Systematic review | de Jager 201078,86 | Efficacy and safety of treatments for childhood psoriasis: a systematic literature review | Systematic review |
Appendix 3 List of excluded studies with reasons for exclusion
| Study | Record title | Reason for exclusiona |
|---|---|---|
| Vencovsky 2015179 | A Phase III randomised, double-blind clinical study comparing SB4, an etanercept biosimilar, with etanercept reference product (Enbrel) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy (24-week results) | 1 |
| Tarp 2015180 | Comparative effectiveness associated with the use of biologics and small-molecules for psoriasis: protocol for a systematic review and meta-analysis | 1 |
| Soliman 2015181 | Combination therapy of methotrexate plus NBUVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis | 2 |
| Ruano 2015182 | Short-term effectiveness and safety of new biologic agents targeting IL-23/Th17 pathway for moderate to severe plaque psoriasis: a systematic review and network meta-analysis | 1 |
| Puig 2015183 | Long-term efficacy, safety and drug survival of ustekinumab in a Spanish cohort of patients with moderate to severe plaque psoriasis | 1 |
| Lebwohl 2015184 | Phase 3 studies comparing brodalumab with ustekinumab in psoriasis | 1 |
| Langley 2015185 | Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up | 1 |
| Kimball 2015186 | OBSERVE-5: observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results | 1 |
| Brănişteanu 2015187 | Adverse reactions of biological therapy for psoriasis | 2 |
| Ali 2015188 | A systematic review of the impact on quality of life of topical, systemic and biologic therapies for psoriasis | 1 |
| NIHR Horizon Scanning Centre 2014189 | Adalimumab (Humira) for severe chronic plaque psoriasis in children and adolescents – second line | 4 |
| Umezawa 2013190 | Drug survival rates in patients with psoriasis after treatment with biologics | 2 |
| Strohal 2013191 | Etanercept provides an effective, safe and flexible short- and long-term treatment regimen for moderate-to-severe psoriasis: a systematic review of current evidence | 1 |
| Park 2013192 | A randomized, ‘head-to-head’ pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB-UVB) phototherapy in obese psoriasis patients | 1 |
| NIHR Horizon Scanning Centre 2013193 | Ustekinumab (Stelara) for plaque psoriasis in adolescents | 4 |
| López-Ferrer 2013194 | Adalimumab for the treatment of psoriasis in real life: a retrospective cohort of 119 patients at a single Spanish centre | 1 |
| Lebwohl 2013195 | A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis | 1 |
| Janagond 2013196 | Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study | 2 |
| Gisondi 2013197 | Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry | 1 |
| da Silva 2013198 | Methotrexate for psoriasis | 5 |
| Chen 2013199 | Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis | 3 |
| Burmester 2013200 | Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease | 1 |
| Balzola 2013201 | Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease | 1 |
| All Wales Medicines Strategy Group 2013202 | AWMSG Secretariat Assessment Report. Etanercept (Enbrel®) | 4 |
| Strand 2012203 | Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment | 1 |
| Lynde 2012204 | A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept | 1 |
| Kim 2012205 | Comparative efficacy of biologics in psoriasis: a review | 1 |
| Famenini 2012206 | The safety of ustekinumab in psoriasis | 1 |
| Chiu 2012207 | The effectiveness and safety of adalimumab in the treatment of non-reimbursed patients with mild-to-moderate psoriasis | 1 |
| Burmester 2012208 | Long-term safety of adalimumab in patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, and Crohn’s disease | 1 |
| Young 2011209 | The ACCEPT study: ustekinumab versus etanercept in moderate-to-severe psoriasis patients | 1 |
| Ryan 2011210 | Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials | 1 |
| Lara-Corrales 2011211 | Childhood psoriasis treatment: evidence published over the last 5 years | 5 |
| Brunasso 2011212 | Tolerability and safety of biological therapies for psoriasis in daily clinical practice: a study of 103 Italian patients | 1 |
| Menter 2010213 | Efficacy and safety of adalimumab across subgroups of patients with moderate to severe psoriasis | 1 |
| Esposito 2010214 | Continuous treatment of plaque-type psoriasis with etanercept: an observational long-term experience | 1 |
| All Wales Medicines Strategy Group 2010139 | Final appraisal report etanercept (Enbrel) | 5 |
| National Horizon Scanning Centre 2008215 | Etanercept (Enbrel) for moderate-to-severe plaque psoriasis in children and adolescents | 4 |
| Flytström 2008123 | Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial | 1 |
| Romero-Maté 2007216 | Efficacy and safety of etanercept in psoriasis/psoriatic arthritis: an updated review | 1 |
| Ranjan 2007217 | Methotrexate versus hydroxycarbamide (hydroxyurea) as a weekly dose to treat moderate-to-severe chronic plaque psoriasis: a comparative study | 1 |
| Krueger 2006218 | Patients with psoriasis respond to continuous open-label etanercept treatment after initial incomplete response in a randomized, placebo-controlled trial | 1 |
| Gordon 2006219 | Efficacy of etanercept in an integrated multistudy database of patients with psoriasis | 1 |
| Amornpinyokeit 2006220 | 8-Methoxypsoralen cream plus targeted narrowband ultraviolet B for psoriasis | 3 |
| Bigby 2004221 | A randomized controlled trial of methotrexate and cyclosporine in the treatment of psoriasis | 1 |
| Heydendael 2002222 | Cyclosporin trough levels: Is monitoring necessary during short-term treatment in psoriasis? A systematic review and clinical data on trough levels | 1 |
| Faerber 2001223 | Cyclosporine in severe psoriasis. Results of a meta-analysis in 579 patients | 1 |
| Ho 1999224 | Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. The PISCES Study Group | 2 |
| Zachariae 1998225 | Conversion of psoriasis patients from the conventional formulation of cyclosporin A to a new microemulsion formulation: a randomized, open, multicentre assessment of safety and tolerability | 1 |
| Koo 1998226 | A randomized, double-blind study comparing the efficacy, safety and optimal dose of two formulations of cyclosporin, Neoral and Sandimmun, in patients with severe psoriasis. OLP302 Study Group | 1 |
| Laburte 1994227 | Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic severe plaque psoriasis | 1 |
| Italian Multicenter Study Group on Cyclosporin in Psoriasis 1993228 | Cyclosporin versus etretinate: Italian multicenter comparative trial in severe plaque-form psoriasis | 1 |
| Christophers 1992229 | Cyclosporine in psoriasis: a multicenter dose-finding study in severe plaque psoriasis. The German Multicenter Study | 1 |
| Tanew 1991230 | Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study | 1 |
| Ruzicka 1990231 | Efficiency of acitretin in combination with UV-B in the treatment of severe psoriasis | 1 |
| Kragballe 1989232 | A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicentre study | 1 |
| Takashima 1988233 | Comparison of therapeutic efficacy of topical PUVA, oral etretinate, and combined PUVA and etretinate for the treatment of psoriasis and development of PUVA lentigines and antinuclear antibodies | 1 |
| Geiger 1988234 | Acitretin (Ro 10-1670, etretin): overall evaluation of clinical studies | 1 |
| Melis 1984235 | [Treatment of plaque psoriasis with an aromatic retinoid (etretinate)] | 2 |
| Christiansen 1982236 | Etretinate (Tigason) and betamethasone valerate (Celeston valerate) in the treatment of psoriasis. A double-blind, randomized, multicenter trial | 2 |
| Janssen Biotech, Inc.237 | Ustekinumab safety and surveillance program using the Ingenix NHI database | 1 |
| Wyeth 2008238 | Study evaluating the safety of Enbrel (etanercept) | 1 |
Appendix 4 Evidence synthesis modelling, software and WinBUGS code
Bayesian NMA was conducted to pool trial results. NMA models were programmed in WinBUGS software (version 1.4.3) using a Bayesian statistical framework. WinBUGS is a Bayesian analysis software tool that, through the use of Gibbs sampling (a Markov chain Monte Carlo method), evaluates posterior distributions for the parameters of interest given likelihood functions derived from data and prior probabilities. Fixed- and random-effects models were evaluated. Model selection was determined by model fit statistics (i.e. DIC and total residual deviance) to identify the best model choice. Treatment effects were expressed in relation to placebo. Uninformative priors were used throughout. The Bayesian NMA for PASI utilised a framework of analysis that evaluated the probability of PASI responses in different categories of PASI thresholds (50, 75 and 90) within a single model. The analyses followed the principles outlined in the NICE Decision Support Unit (DSU). 95 The single synthesis multinomial model with a probit link is recommended by the NICE DSU and assumes that there is an underlying continuous variable that has been categorised by specifying the cut-off points. It assumes also that the treatment effect is the same regardless of the different cut-off points in each trial. All PASI response models were run for 10,000 iterations after a burn-in of 20,000 on two chains. Synthesis model results provide pooled probabilities of achieving PASI 50, 75 and 90 responses for each treatment of interest, alongside a measure of uncertainty, that is, the 95% CrI. In brief, trials report rikj, the number of patients in arm k of trial i belonging to different, mutually exclusive categories j = 1, 2, 3, where these categories represent the different thresholds of PASI score (e.g. 50%, 75% or 90% improvement). The responses for each arm k of trial i in category j follow a multinomial distribution:
which has been parameterised as a series of conditional binomial distributions, with parameters of interest the probabilities, pikj, that a patient in arm k (k = 1, 2, 3) of trial i (i = 1,. . . . .,I) belongs to category j (j = 1, 2, 3). A probit link function was used, the inverse of the normal cumulative distribution function Φ, to define pikj as a function of a set of threshold values, zj. The threshold values (estimated within the model) are such that the probability that the standard normal (the probit score) will take a value less than or equal to z1 will reflect the probability of obtaining a PASI response lower than 50%, that is, 1 – PASI50. The probability that the standard normal will take a value less than or equal to z2 will reflect the probability of obtaining a PASI response lower than 75%, that is, 1 – PASI75 and, analogously, evaluating Φ at z3 will approximate 1 – PASI90. Placebo and treatments are assumed to shift the mean of the distribution. This means that the pooled effect of taking the experimental treatment instead of the control is to change the probit score (or z-score) of the control arm by di,1 standard deviations. Therefore, the model is written as:(5)pikj = Φ(µi+zj+δi,1kI{k ≠ 1}). The terms zj are the differences on the standard normal scale between the response to category j and the response to category j – 1 in all of the arms of trial i. The correlation structure induced by three-arm trials was accounted for as a substantial proportion of the studies forming the evidence base had such characteristics.
We assumed that the baselines, µi, were trial specific (i.e. unconstrained – except for model 1b) and were given a non-informative prior. A non-informative prior was assigned to the treatment effects parameter (δt). A uniform prior was assigned to the parameter zj.
Alternative assumptions were tested in two analyses. The first assumed a meta-regression for placebo effects (model 2a). In a second analysis we explored the impact on treatment effects of adjusting for age, that is, explicitly modelling children and young people and adult subgroups (model 2b). The key assumptions implemented for PASI responses in the models and detailed coding of the models are presented in Table 99. The preferred model was used to evaluate the estimated probability of achieving PASI 50, 75 and 90 responses on treatment t, using:(6)Tajt = 1−Φ(A+δt+zj),for adults and:(7)Tcjt = 1−Φ(A+δt+zj+B),for children and young people, where A is the pooled baseline effect. The baseline effect, A, was estimated as:
where µi1 is the baseline effects, i is the studies and 1 = placebo, NS is the number of studies and B is the common regression (slope) coefficient relating to the treatment*age interaction, which is assumed to be identical for all treatments. This is a strong assumption but, because of only increasing the number of parameters in the model by 1, is the least data demanding. Other interaction assumptions were tested (i.e. independent and exchangeable)138 but the model was unable to appropriately estimate all parameters.
| Model 2 | Model 2a | Model 2b |
|---|---|---|
| Likelihoodrikj∼Binomial(pikj,nikj)Modelqikj=1−(pikCl,j=1/pikCl,j)θikj=µi+δti,k−δti,1+zjpikCi,j=1−ADikjADikj=ϕ(θik,j=1)δt∼dnorm(dt,σ2)Priorsσ∼dunif(0,2)µi∼dnorm(0,0.000001)zj∼dunif(0,5) | Likelihoodrikj∼Binomial(pikjnikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zj+β(µi−µ¯)pikCij=1−ADikjADikj=ϕ(θik,j−1)δt∼dnorm(dt,σ2)Priorsσ∼dunif(0,2)µi∼dnorm(0.0.0001)β∼dnorm(0,0.0001)zj∼dunif(0,5) | Likelihoodrikj∼Binomial(pikjnikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zj+β(µi−µ¯)+γ.xipikCij=1−ADikjADikj=ϕ(θik,j−1)δi∼dnorm(di,σ2)Priorsσ∼dunif(0,2)µi∼dnorm(0,0.0001)β∼dnorm(0,0.0001)γ∼dnorm(0,0.0001)zj∼dunif(0,5) |
Assumptions:
|
Assumptions:
|
Assumptions:
|
We adopted the WinBUGS code presented in DSU2239 for the analysis, although we identified that the model was not specifying the z-score correctly in the linear predictor specification when the first category of the response data (in this case PASI 50) was missing. A correction was made to incorporate the correct specification for the z-score in the linear predictor specification.
WinBUGS code for the preferred model
Appendix 5 Studies excluded from the network meta-analyses
No treatment arm of interest (12 studies)
Lebwohl 2003
Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med 2003;349:2004–13.
Gordon 2003
Gordon KB, Papp KA, Hamilton TK, Walicke PA, Dummer W, Li N, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 2003;290:3073–80.
ACD2058g
ACD2058g. Phase III, randomised double blind placebo-controlled study evaluating 12 weeks of therapy with XOMA1 efalizumab administered subcutaneously (SC), followed by either continued treatment for an additional 12 weeks or re-treatment for 12 weeks following relapse. In Clinical and Cost-effectiveness of Efalizumab (Raptiva) for Moderate to Severe Psoriasis. Industry submission. Feltham: Serono Ltd; 2004.
ACD2600g
ACD2600g. Phase IIIb, randomised, double-blind, parallel group, placebocontrolled, multicentre study evaluating 12 weeks therapy with subcutaneously administered Genentech efalizumab in adults with moderate to severe psoriasis who are candidates for systemic therapy. In Clinical and Cost-effectiveness of Efalizumab (Raptiva) for Moderate to Severe Psoriasis. Industry submission. Feltham: Serono Ltd; 2004.
IMP24011
IMP24011. Phase III, randomised, double blind, placebo-controlled, multicentre study evaluating 12 weeks subcutaneous therapy with Genentech efalizumab in patients with moderate to severe psoriasis who are candidates for systemic therapy. In Clinical and Cost-effectiveness of Efalizumab (Raptiva) for Moderate to Severe Psoriasis. Industry submission. Feltham: Serono Ltd; 2004.
Rich 2013
Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, Phase II regimen-finding study. Br J Dermatol 2013;168:402–11.
Papp 2013
Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo controlled Phase II dose-ranging study. Br J Dermatol 2013;168:412–21.
SCULPTURE 2013
Novartis. Study Comparing secukinumab Use in Long-term Psoriasis maintenance therapy: fixed regimens versus re-Treatment Upon start of Relapse (SCULPTURE). 2013.
Mroweitz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015;73:27–36.e1.
ERASURE 2014
Rich P, Karpov A, Papavassilis C, Marmur E, Klingo K, et al. Secukinumab efficacy stratified by body weight: a subanalysis from the ERASURE study. J Am Acad Dermatol 2014;70:AB186.
Papp K, Karpov A, Papavassilis C, Melendez E, Nakagawa H, et al. Secukinumab efficacy in relationship with response to previous biologic psoriasis therapy: a subanalysis from the ERASURE Study. J Am Acad Dermatol 2014;70:AB186.
Lebwohl M, Vender R, Menter A, Karpov A, Papavassilis C. ERASURE: Secukinumab Shows Sustained Efficacy in Subjects Regardless of Previous Biologic Exposure. European Association of Dermatology and Venereology (EADV) Congress, Amsterdam, the Netherlands, October 2014.
Gottlieb A, Gnanasakthy A, Strober B, Zhang JJ, Tran MH. Secukinumab’s time to posriasis response on patient-reported symptoms (ERASURE study). J Am Acad Dermatol 2014;70:AB189.
Novartis. Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis (ERASURE). 2013.
FEATURE 2014
Blauvelt A, Gottlieb A, Prinz J, Pathan R, Cooper S. Secukinumab efficacy and safety in subjects with moderate to severe plaque psoriasis: results from the Judging the efficacy of secUkinumab in patients with psoriasis using autoiNjector: a Clinical Trial evalUating treatment REsults trial (JUNCTURE). J Am Acad Dermatol 2014;70:AB185.
Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab adminstration by pre-filled syringe: efficacy, safety and usability results from a randomised controlled trial in psoriasis (FEATURE). Br J Dermatol 2015;172:484–93.
Novartis. First study of secukinumab in pre-filled syringes in subjects with chronic plaque-type psoriasis: response at 12 weeks (FEATURE). 2014. URL: https://clinicaltrials.gov/ct2/show/NCT01555125 (accessed 15 September 2017).
JUNCTURE 2015
Blauvelt A, Gottlieb A, Prinz J, Pathan R, Cooper S. Secukinumab efficacy and safety in subjects with moderate to severe plaque psoriasis: results from the Judging the efficacy of secUkinumab in patients with psoriasis using autoiNjector: a Clinical Trial evalUating treatment REsults trial (JUNCTURE). J Am Acad Dermatol 2014;70(Suppl. 1):AB185.
Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015;29:1082–90.
Novartis. Judging the Efficacy of SecUkinumab in Patients with Psoriasis using Autoinjector: a Clinical Trial EvalUating Treatment Results (JUNCTURE). 2014. URL: https://clinicaltrials.gov/ct2/show/NCT01636687 (accessed 15 September 2017).
Rivas E, Griffiths C, Rich P, Gong Y, Papavassilis C. FIXTURE: Secukinumab Shows Sustained Efficacy in Subjects Regardless of Previous Biologic Exposure. European Association of Dermatology and Venereology (EADV) Congress, Amsterdam, the Netherlands, October 2014.
FIXTURE 2014
Novartis. FIXTURE (Full year Investigative eXamination of secukinumab vs. eTanercept Using 2 dosing Regimens to determine Efficacy in psoriasis). 2013.
Reich K, et al. , editors. Sustainability of response with secukinumab to 52 weeks in moderate-to-severe plaque psoriasis: data from the full year investigative examination of secukinumab vs. etanercept using 2 dosing regimens to determine efficacy in psoriasis (FIXTURE) study. 2014.
Psoriasis Area and Severity Index outcomes reported at irrelevant time points (two studies)
van Joost 1988
van Joost T, Bos JD, Heule F, Meinardi MM. Low-dose cyclosporin A in severe psoriasis. A double-blind study. Br J Dermatol 1988;118:183–90.
Ellis 1991
Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med 1991;324:277–84.
Treatment arm of interest but not the recommended dose (four studies)
Tyring 2007
Tyring S, Gordon KB, Poulin Y, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Arch Dermatol 2007;143:719–26.
Bagel 2012
Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol 2012;67:86–92.
Tyring S, Bagel J, Lynde C, Klekotka P, Thompson EH, Gandra SR, et al. Patient-reported outcomes in moderate-to-severe plaque psoriasis with scalp involvement: results from a randomized, double-blind, placebo-controlled study of etanercept. J Eur Acad Dermatol Venereol 2013;27:125–8.
Gottlieb 2011
Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 2011;165:652–60.
Strober 2011
Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a Phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 2011;165:661–8.
Appendix 6 Evidence synthesis: fixed-effect model results
| Treatment | Analysis 2: fixed-effects approach | |||
|---|---|---|---|---|
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.212 (0.20 to 0.23) | 0.087 (0.08 to 0.10) | 0.020 (0.02 to 0.02) | 5 |
| Etanercept | 0.726 (0.68 to 0.77) | 0.517 (0.47 to 0.57) | 0.259 (0.22 to 0.30) | 3 |
| Ustekinumab | 0.863 (0.84 to 0.89) | 0.704 (0.67 to 0.74) | 0.439 (0.40 to 0.48) | 2 |
| Adalimumab | 0.868 (0.84 to 0.90) | 0.711 (0.67 to 0.76) | 0.447 (0.40 to 0.50) | 1 |
| Methotrexate | 0.369 (0.28 to 0.47) | 0.187 (0.13 to 0.26) | 0.099 (0.03 to 0.09) | 4 |
| Residual deviance, mean (95% CI) | 938.5a (921.2 to 959.8) | |||
| DIC | 1790.3 | |||
| Treatment | Analysis 2a: fixed-effects approach | |||
|---|---|---|---|---|
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.147 (0.13 to 0.17) | 0.051 (0.04 to 0.06) | 0.010 (0.01 to 0.01) | 5 |
| Etanercept | 0.595 (0.55 to 0.65) | 0.367 (0.32 to 0.42) | 0.148 (0.12 to 0.18) | 3 |
| Ustekinumab | 0.862 (0.83 to 0.89) | 0.695 (0.65 to 0.74) | 0.422 (0.37 to 0.47) | 1 |
| Adalimumab | 0.821 (0.79 to 0.85) | 0.632 (0.59 to 0.68) | 0.356 (0.31 to 0.40) | 2 |
| Methotrexate | 0.552 (0.49 to 0.62) | 0.326 (0.27 to 0.39) | 0.124 (0.09 to 0.16) | 4 |
| Residual deviance, mean (95% CI) | 406.3a (385.7 to 431.4) | |||
| DIC | 1259.5 | |||
Appendix 7 Consistency assessment
The validity of a NMA depends on an assumption of homogeneity/exchangeability between all of the trials included in the network, that is, that there are no essential differences between the methods, populations and interventions being studied and that any differences are due to chance (as in a standard meta-analysis). The lack of homogeneity/exchangeability between studies involving one of the treatments of interest and studies involving the other treatments of interest may generate inconsistency. The main potential threat to consistency of the evidence network is the pooling of evidence across trials in children and young people with evidence from adult populations.
The main network evidence loops that require close examination are placebo compared with methotrexate compared with adalimumab, placebo compared with etanercept compared with apremilast and placebo compared with 45 mg of ustekinumab compared with 90 mg of ustekinumab (see Figure 5), as these involve the main agents of interest. As illustrated in Figure 5, these evidence loops contain one, one and four three-arm trials respectively (discontinued line boxes). Within a three-arm trial no inconsistency exists, and no inconsistency is brought to the evidence network from these multiarm trials, potentially only between-trial heterogeneity. 240 These three evidence loops of interest include a mixture of two- and three-arm trial evidence. In these circumstances defining and assessing inconsistency creates inherent technical difficulties. Solutions to this problem are labelled by the NICE DSU Technical Support Document 4240,241 document on inconsistency of evidence as ‘not entirely satisfactory’ and are ‘predicated on the assumption that the majority of trials are two-arm trials and there is unlikely to be any material impact on detection of inconsistency’.
To overcome these potential inconsistency assessment issues, a scenario analysis was performed that consisted of excluding from the analysis the evidence from trials in children and young people and synthesising only the evidence from adult populations. Therefore, analysis 2a (i.e. a baseline risk-adjusted random-effects model) was replicated using only the evidence from the 34 adult trials and the results of this scenario analysis were compared with the results obtained using the full evidence base.
Table 102 presents the PASI response outcomes for the trials in adult populations only. Overall, the results were similar to those observed in analysis 2a (see Table 101). This provides some reassurance that consistency exists between the two subpopulations.
| Treatment | Consistency assessment: analysis 2a restricted to adult subpopulation | |||
|---|---|---|---|---|
| PASI 50, mean (95% CrI) | PASI 75, mean (95% CrI) | PASI 90, mean (95% CrI) | r | |
| Placebo | 0.144 (0.12 to 0.17) | 0.050 (0.04 to 0.06) | 0.010 (0.01 to 0.01) | 5 |
| Etanercept | 0.619 (0.55 to 0.69) | 0.389 (0.32 to 0.46) | 0.161 (0.12 to 0.21) | 3 |
| Ustekinumab 45 mg | 0.875 (0.83 to 0.91) | 0.714 (0.65 to 0.78) | 0.442 (0.37 to 0.52) | 1 |
| Adalimumab | 0.84 (0.78 to 0.89) | 0.654 (0.57 to 0.73) | 0.377 (0.30 to 0.46) | 2 |
| Methotrexate | 0.548 (0.44 to 0.65) | 0.322 (0.23 to 0.42) | 0.121 (0.07 to 0.18) | 4 |
| Residual deviance, mean (95% CI) | 360.3a (337.2 to 387.0) | |||
| DIC | 45.02 | |||
Appendix 8 Additional cost-effectiveness results
| Treatment | Mean cost (£) | Mean QALYs | Incremental cost vs. MTX (£) | Incremental QALYs vs. MTX | ICER vs. MTX (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|
| Children aged 4–17 years | ||||||
| MTX | 34,910 | 9.156 | – | – | – | MTX |
| ADA | 62,019 | 9.361 | 27,109 | 0.204 | 132,616 | |
| Treatment | Mean cost (£) | Mean QALYs | Incremental cost vs. next best option (£) | Incremental QALYs vs. next best option | ICER vs. next best option (£/QALY) | ICER vs. BSC (£/QALY) | Optimal treatment (£30,000 threshold) |
|---|---|---|---|---|---|---|---|
| Children and young people aged 6–11 years | |||||||
| BSC | 36,406 | 7.808 | – | – | – | – | |
| ETA | 43,779 | 8.150 | 7373 | 0.342 | 21,546 | 21,546 | ETA |
| ADA | 57,230 | 8.333 | 13,451 | 0.183 | 73,670 | 39,682 | |
| Children and young people aged 12–17 years | |||||||
| BSC | 21,749 | 4.306 | – | – | – | – | |
| ETA | 33,186 | 4.585 | 11,437 | 0.278 | ED ADA | 41,085 | BSC |
| ADA | 37,848 | 4.732 | 16,099 | 0.426 | 37,802 | 37,802 | |
| UST | 39,924 | 4.753 | 2075 | 0.021 | 100,423 | 40,700 | |
Appendix 9 Adalimumab risk-of-bias assessment for trial extension periods
| Item | Period | |||||
|---|---|---|---|---|---|---|
| B | C | D | ||||
| Judgement | Justification | Judgement | Justification | Judgement | Justification | |
| Is the population based on a representative sample selected from a relevant population? | Unclear | Only responders in period A (RCT) entered this stage of the study | No | Not representative of participants receiving first biological treatment or switching biologic treatments: all participants had received adalimumab or methotrexate and experienced loss of disease control before being retreated | Yes | Participants from periods A, B and C were included |
| Are the criteria for inclusion explicit? | Yes | Participants with a PASI 75 and PGA 0/1 response at the end of period A | Yes | Participants from period B who had 16 weeks of adalimumab or methotrexate and experienced loss of disease control were included | Yes | Participants from periods A, B and C who met entry criteria in period A were included |
| Were groups similar at baseline in terms of important confounding variables? If not, was the analysis adjusted to account for the imbalance? | NA | Unclear | Insufficient demographic information. PASI 50 rates differ (p = 0.06) | NA | Non-comparative observational period | |
| Was knowledge of the allocated intervention by outcome assessors adequately prevented during the study? | Unclear | No information provided | Yes | Blinded retreatment | NA | Non-comparative observational period. Adalimumab dose was blinded for some participants and open label for others |
| Were losses to follow-up < 20%? | Yes | 53 of 54 participants entering group B completed follow-up | Yes | 34 of 38 participants completed follow-up | Yes | 90 of 108 participants completed follow-up |
| Were all patients accounted for at the end of study follow-up? | Yes | Yes | Yes | |||
| Were reliable methods used to measure outcomes? | Yes | PGA worsening by at least two grades | Yes | Outcomes and methods were reported | Yes | Outcomes and methods were reported |
| Was the study sufficiently powered to detect a treatment effect? | Unclear | No information was provided | Unclear | No information was provided | Unclear | No information was provided |
| Was the study follow-up duration sufficient to detect a long-term treatment effect? | Yes | Participants were followed up for 36 weeks | Yes | As in period A, participants were followed up for 16 weeks | Yes | Participants were followed up for 52 weeks in period D |
Appendix 10 Etanercept risk-of-bias assessment for trial extension periods
| Item | Open-label treatment (24 weeks) | Withdrawal–retreatment period (12 weeks) | Long-term follow-up (264 weeks) | |||
|---|---|---|---|---|---|---|
| Judgement | Justification | Judgement | Justification | Judgement | Justification | |
| Is the population based on a representative sample selected from a relevant population? | Yes | All patients who entered the randomisation stage were included | No | Only those who achieved a PASI 50 response at week 24 or a PASI 75 response at week 36 entered the study | Yes | 182 of the 211 participants from the blinded period and completing the withdrawal–retreatment period entered this study |
| Are the criteria for inclusion explicit? | Yes | With the exception of three withdrawals, all patients who entered the randomisation stage were included | Yes | Those who achieved a PASI 50 response at week 24 or a PASI 75 response at week 36 entered the study | Yes | All participants who completed the withdrawal–retreatment period were included |
| Were groups similar at baseline in terms of important confounding variables? If not, was the analysis adjusted to account for the imbalance? | NA | All patients received the same treatment (etanercept), that is, no comparative efficacy analyses were planned | Yes | Participants were similar in terms of age, sex, weight, height, PASI scores and % BSA affected | NA | The follow-up study did not aim to carry out comparative analyses |
| Was knowledge of the allocated intervention by outcome assessors adequately prevented during the study? | Unclear | Every patient was receiving etanercept | Unclear | Although the participants, caregivers, investigators and outcome assessors were blinded during the blinding period, no information was available for this phase of the study | Unclear | Although the participants, caregivers, investigators and outcome assessors were blinded during the blinding period, no information was available for this phase of the study |
| Were losses to follow-up < 20%? | Yes | 94.7% (197/208) of participants entering this stage were present at the end of the stage | Yes | 137 out of 138 participants completed the study | No | 115 out of 182 participants withdrew by the end of the study (week 264) |
| Were all patients accounted for at the end of study follow-up? | Yes | 11 participants withdrew and 197 participants were present at the end of the study | Yes | 137 participants completed the study and one was lost to follow-up | Yes | Withdrawals and reasons (e.g. AEs, lost to follow-up, withdrawal consent and protocol deviations) reported |
| Were reliable methods used to measure outcomes? | Yes | PASI scores and AEs were recorded | Yes | PASI scores were reported | Yes | PASI scores and AEs reported |
| Was the study sufficiently powered to detect a treatment effect? | NA | Power analysis was not carried out or needed for the study | Unclear | No power calculation was carried out or reported for this phase of the study | Unclear | No power calculations were carried out or evidenced in the study reports |
| Was the study follow-up duration sufficient to detect a long-term treatment effect? | Yes | Participants were followed up for 24 weeks | Unclear | The follow-up period was 12 weeks | Yes | The follow-up period was 5 years |
Appendix 11 Ustekinumab risk-of-bias assessment for trial extension periods
| Item | Period | |||
|---|---|---|---|---|
| Placebo crossover and active treatment (12–52 weeks) | Follow-up (52–60 weeks) | |||
| Judgement | Justification | Judgement | Justification | |
| Is the population based on a representative sample selected from a relevant population? | Yes | All participants from the initial blinded period were eligible to enter the crossover phase of the study | Yes | All participants from previous phases were eligible for follow-up |
| Are the criteria for inclusion explicit? | Yes | All participants from the initial blinded period were eligible to enter the crossover phase of the study | Yes | All participants from previous phases were eligible for follow-up |
| Were groups similar at baseline in terms of important confounding variables? If not, was the analysis adjusted to account for the imbalance? | NA | This phase of the study did not aim for comparative analysis | NA | This phase of the study did not aim for comparative analysis |
| Was knowledge of the allocated intervention by outcome assessors adequately prevented during the study? | Yes | The sponsor, investigative study sites and participants remained blinded to treatment assignment until the last participant enrolled had completed the study | Yes | The sponsor, investigative study sites and participants remained blinded to treatment assignment until the last participant enrolled had completed the week 60 evaluations and the database was locked |
| Were losses to follow-up < 20%? | Yes | Only 7 out of 110 participants withdrew by the end of this phase | Unclear | The total loss to follow-up or withdrawals at this phase of the study were not reported |
| Were all patients accounted for at the end of study follow-up? | Yes | Withdrawals and reasons (e.g. AEs, death and lack of efficacy) were reported | Unclear | The total loss to follow-up for this phase of the study was not reported |
| Were reliable methods used to measure outcomes? | Yes | PASI scores were reported | NA | The follow-up was aimed only at safety reports |
| Was the study sufficiently powered to detect a treatment effect? | NA | The follow-up period did not aim for comparative analyses | NA | The follow-up period did not aim for comparative analyses |
| Was the study follow-up duration sufficient to detect a long-term treatment effect? | Yes | Participants were followed up for 40 weeks | No | Participants were followed up for only 8 weeks |
Appendix 12 Risk-of-bias assessment for observational multiple biological therapy studies
| Item | Study | |||
|---|---|---|---|---|
| Garber 201576 | Klufas 201677 | |||
| Judgement | Justification | Judgement | Justification | |
| Is the population based on a representative sample selected from a relevant population? | Yes | All patients with the disease code ICD-9-CM 696.1 were considered in the study | Yes | All patients with the disease code ICD-9-CM 696.1 were considered in the study |
| Are the criteria for inclusion explicit? | Yes | Patients with a S-MAPA score of ≥ 15 or otherwise documented moderate to severe psoriasis were included | Yes | Patients with moderate to severe psoriasis were included |
| Were groups similar at baseline in terms of important confounding variables? If not, was the analysis adjusted to account for the imbalance? | NA | Not a comparative study | NA | Not a comparative study |
| Was knowledge of the allocated intervention by outcome assessors adequately prevented during the study? | Unclear | No information provided | Unclear | No information provided |
| Were losses to follow-up < 20%? | NA | Retrospective analysis of health records | NA | Retrospective analysis of health records |
| Were all patients accounted for at the end of study follow-up? | NA | Retrospective analysis of health records | NA | Retrospective analysis of health records |
| Were reliable methods used to measure outcomes? | Unclear | No information was provided | Unclear | No information was provided |
| Was the study sufficiently powered to detect a treatment effect? | NA | Not a comparative analysis | NA | Not a comparative analysis |
| Was the study follow-up duration sufficient to detect a long-term treatment effect? | No | Mean duration of treatment was as short as 11 weeks in the etanercept + methotrexate group | No | Mean duration of treatment was as short as 3 weeks in the adalimumab + methotrexate group |
Glossary
- Adverse effect
- An abnormal or harmful effect, caused by and attributable to exposure to a chemical (e.g. a drug), that is indicated by some result such as death, a physical symptom or a visible illness. An effect may be classed as adverse if it causes functional or anatomical damage, causes irreversible change in the homeostasis of the organism or increases the susceptibility of the organism to other chemical or biological stress.
- Between-study variance
- A measure of statistical heterogeneity that depends on the scale of the outcome measured. It represents the variation in reported study effects over and above the variation expected given the within-study variation.
- Biological therapy (biologic)
- Any pharmaceutical product derived from biological sources. In psoriasis treatment, biological therapies are generally monoclonal antibodies that bind to and inactivate immune cell signalling molecules (e.g. tumour necrosis factor and interleukins), thereby dampening the inflammatory response.
- Biosimilar
- An imitation biological medical product (such as anti-tumour necrosis factor) usually marketed by a different manufacturer from the manufacturer of the original biological product, once a patent has expired. The biosimilar should be similar to the original licensed product in terms of safety and efficacy.
- Ciclosporin
- A medication originally developed to prevent the immune system from rejecting transplanted organs but which has also proved helpful in treating psoriasis.
- Confidence interval
- The typical (‘classical’ or ‘frequentist’) definition is the range within which the ‘true’ value (e.g. the size of effect of an intervention) would be expected to lie (e.g. 95% or 99%) if sampling could be repeated a large number of times.
- Cost-effectiveness analysis
- An economic analysis that expresses the effects or consequences of interventions on a single dimension. This would normally be expressed in ‘natural’ units (e.g. cases cured, life-years gained). The difference between interventions in terms of costs and effects is typically expressed as an incremental cost-effectiveness ratio (e.g. the incremental cost per life-year gained).
- Credible interval
- In Bayesian statistics, a credible interval is a posterior probability interval estimation that incorporates problem-specific contextual information from the prior distribution. Credible intervals are used for purposes similar to those of confidence intervals in frequentist statistics.
- Crohn’s disease
- An inflammatory condition of the digestive tract; rheumatic diseases are often associated with Crohn’s disease and ulcerative colitis is related to it.
- Deviance information criterion
- A model fit statistic and used for Bayesian model comparison. The model with the smallest deviance information criterion is estimated to be the model that would best predict a replicate data set that has the same structure as that currently observed.
- Disease-modifying antirheumatic drug
- A drug that is capable of modifying the progression of rheumatic disease. The term is applied to what are now considered to be traditional (or conventional) disease-modifying drugs, in particular sulfasalazine, methotrexate and ciclosporin, as well as azathioprine, cyclophosphamide, antimalarials, penicillamine and gold. The newer agent leflunomide is also a disease-modifying antirheumatic drug. Biologics are not generally referred to as disease-modifying antirheumatic drugs, although occasionally the term ‘bDMARD’ may be used.
- EuroQol-5 Dimensions
- A standardised instrument for measuring generic health-related quality of life, used in computation of quality-adjusted life-years.
- Fixed-effect model
- A statistical model that stipulates that the units under analysis (e.g. people in a trial or studies in a meta-analysis) are the ones of interest and thus constitute the entire population of units. Only within-study variation is taken to influence the uncertainty of the results (as reflected in the confidence interval) of a meta-analysis using a fixed-effect model.
- Health Assessment Questionnaire
- A self-administered questionnaire measuring an individual’s physical disability and pain. The ability to perform various activities is scored between 0 (without any difficulty) and 3 (unable to do). The final score is reported as an average of all activity scores.
- Heterogeneity
- In systematic reviews, heterogeneity refers to variability or differences between studies in the estimates of effects. A distinction is sometimes made between ‘statistical heterogeneity’ (differences in the reported effects), ‘methodological heterogeneity’ (differences in study design) and ‘clinical heterogeneity’ (differences between studies in terms of the key characteristics of the participants, interventions or outcome measures).
- Intention to treat
- An intention-to-treat analysis is one in which all of the participants in a trial are analysed according to the intervention to which they were allocated, regardless of whether they received it or not.
- Methotrexate
- One of the oldest chemotherapy drugs used in the treatment of cancer and autoimmune diseases such as rheumatoid and psoriatic arthritis.
- Network meta-analysis (synonyms: mixed-treatment comparison, indirect treatment comparison)
- Used when there is insufficient direct evidence linking two interventions, this is a meta-analysis comparing three or more different treatments using both direct comparison within randomised controlled trials and indirect comparison between trials based on a common comparator (such as placebo).
- Placebo
- An inactive substance or procedure administered to a patient, usually to compare its effects with those of a real drug or other intervention, but sometimes for the psychological benefit to the patient through a belief that he or she is receiving treatment.
- Plaque psoriasis
- The most common form of psoriasis, also known as psoriasis vulgaris, recognised by red, raised lesions covered by silvery scales. About 80% of patients with psoriasis have this type of disease.
- Psoriasis
- A chronic skin disease characterised by inflammation and scaling. Scaling occurs when cells in the outer layer of the skin are produced faster than normal and build up on the skin’s surface. It is thought to be caused by a disorder of the immune system.
- Psoriasis Area and Severity Index score
- A number representing the extent of skin coverage, redness, scaliness and thickness of a person’s psoriasis. The Psoriasis Area and Severity Index response is presented as PASI 50, PASI 75 or PASI 90. This represents the reduction of the individual’s Psoriasis Area and Severity Index score from baseline as a percentage.
- Psoriatic arthritis
- A disease characterised by stiffness, pain and swelling in the joints, especially of the hands and feet. It affects about 30% of people with psoriasis. Early diagnosis and treatment can help inhibit the progression of joint deterioration.
- Quality-adjusted life-year
- An index of health gain in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period. Quality-adjusted life-years have the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- Quality of life
- A concept incorporating all of the factors that might affect an individual’s life, such as the absence of disease or infirmity, as well as other factors that might affect their physical, mental and social well-being.
- Random-effects model
- A statistical model sometimes used in meta-analysis in which both within-study sampling error (variance) and between-study variation are included in the assessment of the uncertainty (confidence interval) of the results of a meta-analysis.
- Randomised controlled trial (synonym: randomised clinical trial)
- An experiment in which investigators randomly allocate eligible people into intervention groups to receive or not receive one or more of the interventions that are being compared.
- Relative risk (synonym: risk ratio)
- The ratio of risk in the intervention group to risk in the control group. The risk (proportion, probability or rate) is the ratio of people with an event in a group to the total number in the group. A relative risk of 1 indicates no difference between comparison groups. For undesirable outcomes, a relative risk of < 1 indicates that the intervention was effective in reducing the risk of that outcome.
- Residual deviance
- An analysis used for model comparison and goodness of fit. The residual deviance is equal to the deviance for a given model minus the deviance for a saturated model. A saturated model is one in which all of the predictions from the model are equal to the observed data values. Total residual deviance should approximate the number of data points for a good fit.
- Rheumatoid arthritis
- A chronic autoimmune disease characterised by pain, stiffness, inflammation, swelling and, sometimes, destruction of joints.
- Sensitivity analysis
- An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was carried out. Sensitivity analyses are used to assess how robust the results are to uncertain decisions or assumptions about the data and the methods that were used.
- Statistical significance
- An estimate of the probability of an association (effect) as large as or larger than that observed in a study occurring by chance, usually expressed as a p-value.
- Tumour necrosis factor alpha
- A cell signalling molecule (cytokine) involved in the inflammatory response pathway, known to be fundamental to the pathological processes causing psoriasis and psoriatic arthritis. It plays a key role in the onset and persistence of joint and skin inflammation.
List of abbreviations
- AE
- adverse event
- AG
- Assessment Group
- AWMSG
- All Wales Medicines Strategy Group
- BADBIR
- British Association of Dermatologists Biologic Interventions Register
- BNF
- British National Formulary
- BSA
- body surface area
- BSC
- best supportive care
- CDLQI
- Children’s Dermatology Life Quality Index
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CG
- clinical guideline
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- CSR
- clinical study report
- DARE
- Database of Abstracts of Reviews of Effects
- DIC
- deviance information criterion
- DLQI
- Dermatology Life Quality Index
- dPGA
- Dynamic Physician Global Assessment
- DSU
- Decision Support Unit
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions three-level version
- EQ-5D-Y
- EuroQol-5 Dimensions for Youth
- ERG
- Evidence Review Group
- GDG
- Guideline Development Group
- HERC
- Health Economics Research Centre
- HES
- Hospital Episode Statistics
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IL
- interleukin
- IPD
- individual patient data
- ITT
- intention to treat
- LOS
- length of stay
- MCID
- minimal clinically important difference
- MeSH
- medical subject heading
- MIMS
- Monthly Index of Medical Specialities
- MTA
- multiple technology appraisal
- NBUVB
- narrow-band ultraviolet B
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NMSC
- non-melanoma skin cancer
- OLS
- ordinary least squares
- PASI
- Psoriasis Area and Severity Index
- PedsQL™
- Pediatric Quality of Life Inventory
- PGA
- Physician Global Assessment
- PSS
- Personal Social Services
- PUVA
- psoralen plus UVA light
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- S-MAPA
- Simple Measure for Assessing Psoriasis Severity
- sPGA
- Static Physician Global Assessment
- STA
- single technology appraisal
- TA
- technology appraisal
- TNF
- tumour necrosis factor
- TNF-α
- tumour necrosis factor alpha
- T-QoL
- Teenager’s Quality of Life Index
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
