Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/31/02. The contractual start date was in July 2015. The draft report began editorial review in May 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rod S Taylor is the chairperson of the National Institute for Health Research (NIHR) Health Services and Delivery Research programme researcher-led panel and is a current member of the NIHR Priority Research Advisory Methodology Group (PRAMG). He was a member of the NIHR Health Technology Assessment General Board between 2014 and June 2017. Funding from BioQ Pharma has been awarded to the Swansea Centre for Health Economics to conduct a pilot study of the use of OneDose ReadyfusOR in the postoperative delivery of pain relief. Deborah Fitzsimmons is not involved in this study but is the Academic Director of the research centre that has received the grant. Stephanie Poulton reports personal fees from the Neuro Orthopaedic Institute (NOI) and personal fees from Pain and Performance, outside the submitted work. Pain and Performance is a private organisation that teaches and runs seminars about pain for professionals and treats people in pain (see www.painandperformance.com)
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Snidvongs et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Funding history
The FACET (Feasibility of Assessing the Clinical- and cost-Effectiveness of Therapeutic lumbar facet-joint injections) feasibility study was a commissioned proposal funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (reference number HTA 11/31/02); the funding contract was agreed in July 2013. A favourable ethical opinion was given on 21 May 2015 and NHS permission was granted on 20 January 2016, with the first participant recruited to the study on 22 January 2016. Following an extension agreement with the funder, recruitment ended on 30 September 2016 and the study closed on 31 March 2017.
Structure of this report
In this chapter, the existing evidence for therapeutic intra-articular lumbar facet-joint injections (FJIs) for non-specific low back pain (LBP) is reviewed and the need for a pilot trial is presented. In Chapter 2 the feasibility study procedure and associated methodological work are described, with the results of this work being presented in Chapter 3. The implications of our findings are discussed in Chapter 4 and the conclusions drawn with regard to a full trial are presented in Chapter 5.
Background
Low back pain causes more global disability than any other condition, and has a lifetime prevalence of up to 85%. 1 Non-specific LBP, in which symptoms are experienced without any recognisable pathology,2 is thought to affect around 90% of all LBP sufferers, with between 1% and 5% of patients presenting with LBP having a serious spinal pathology such as vertebral fracture, malignancy, infection and inflammatory disease. 3
The economic costs of LBP have been reported to be £12.3B per annum in the UK alone. 4 Chronic LBP, with a prevalence of 3–10%,5 is associated with depression, anxiety, deactivation, inability to work and substantial societal costs. 1,6
Common contributors to LBP in adults are thought to include lumbar facet-joints. 7 These are paired synovial joints between the superior and inferior articular processes of consecutive lumbar vertebrae and between the fifth lumbar vertebra and the sacrum. Encapsulated nerve endings have been demonstrated in these facet-joints, supplied by medial branches of the dorsal rami nerves (‘medial branch nerves’). Facet-joint contributions to LBP may arise from any structure that is part of the facet-joints, including the fibrous capsule, synovial membrane, hyaline cartilage and bone.
Low back pain with a likely facet-joint component can be treated with interventions targeting the facet-joints, including intra-articular (within the joint itself) FJIs, periarticular medial branch nerve blocks or radiofrequency denervation of the medial branch nerves innervating the joints. The technique of FJI is not standardised and may be carried out with or without radiological guidance to confirm needle placement. 8 Lumbar FJIs involve the injection of an active substance, typically steroids with or without a local anaesthetic, intra-articularly or next to the joint (periarticular injections). They are commonly carried out under radiological or fluoroscopic guidance, although they can be performed under ultrasound or computerised tomography scanning guidance. 9
The National Institute for Health and Care Excellence (NICE) guidelines for managing LBP were recently updated [NICE guideline (NG59)10]. These guidelines propose a care pathway in which all those aged ≥ 16 years with LBP with or without sciatica should be provided with advice and information, tailored to their needs and capabilities, to help them self-manage at all steps of the treatment pathway, including education on the nature of LBP and sciatica and encouragement to return to work and pursue normal activities of daily living. 11 Those with a specific episode or flare-up of LBP should consider a group exercise programme, manual treatment or a psychological therapy package. If these therapies fail, pharmacological options and combined physical and psychological (CPP) programmes should be offered, followed by radiofrequency denervation or surgical approaches such as fusion. The guidelines make specific ‘do not use’ recommendations for a range of groups of treatments including acupuncture and electrotherapy; traction, braces and corsets; disc replacement; and spinal injections (including FJIs). The NICE guidelines omit intra-articular FJIs on the grounds of there being insufficient high-quality evidence to support their use and recommend instead targeting the facet-joints’ nerve supply (medial branch nerves) as the predominant pain generator source. However, intra-articular FJIs remain in common use. 12
Review of the literature: a review of systematic reviews and meta-analyses
We undertook a literature search to identify systematic reviews and meta-analyses of randomised controlled trials of intra-articular lumbar FJIs for chronic LBP. We searched MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials from 1966 to February 2017; the search strategies used are detailed in Appendix 1. Two reviewers (Saowarat Snidvongs and Fausto Morell-Ducos) independently screened and assessed full-text articles for eligibility. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is illustrated in Figure 1. Eleven systematic reviews14–24 met the inclusion criteria; the sources of the reviews are summarised in Appendix 2. The Assessment of Multiple Systematic Reviews (AMSTAR) checklist,25 applied by two independent reviewers to assess the methodological quality of the systematic reviews, demonstrated significant variations between the reviews, with AMSTAR scores ranging between 2 and 10 out of a maximum of 11 points, as shown in Appendix 3 (a low score is associated with a higher risk of bias).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review search. Adapted from Moher et al. 13 © 2009 Moher et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
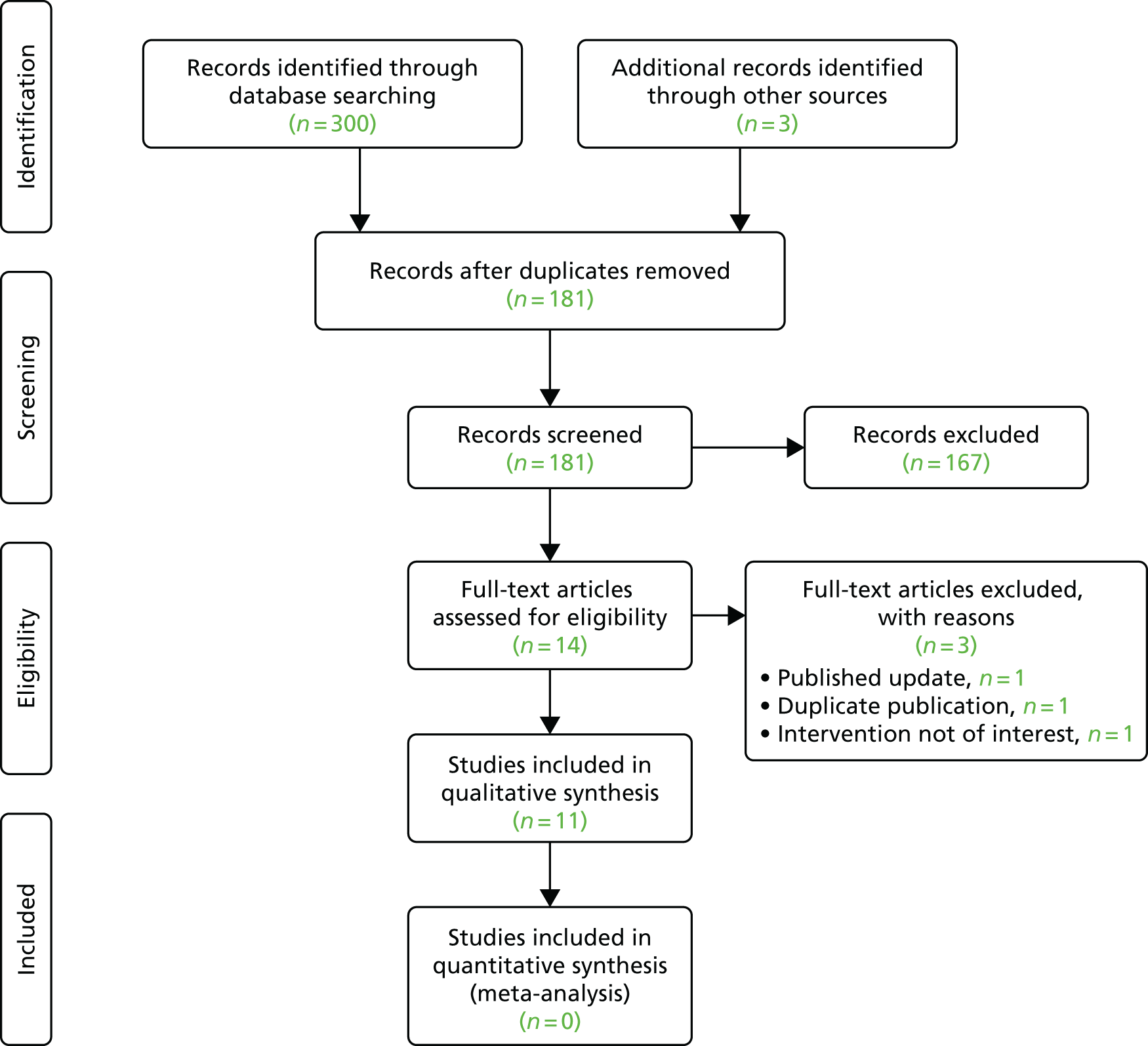
In total, 14 randomised controlled trials were identified across these 11 reviews; 13 were obtained as full-text articles and their findings are summarised in Table 1. Appendix 4 illustrates that, given the variation in dates when the reviews were undertaken and their precise inclusion/exclusion criteria, no one review included all of these trials. The authors of these systematic reviews concluded that the level of clinical heterogeneity across the included randomised controlled trials – different injection procedures, substances and comparators – precluded any meta-analyses.
| First author, year (country, n randomised) | Population | Intervention | Comparator | Primary outcome | Follow-up (months) | Key findings |
|---|---|---|---|---|---|---|
| Lilius, 198926 (Finland, n = 109) | Unilateral LBP for > 3 months, failed analgesics and physiotherapy, no diagnostic blocks | (1) Radiologically guided intra-articular lumbar FJIs with bupivacaine and methylprednisolone; (2) radiologically guided pericapsular injections with bupivacaine and methylprednisolone | Sham (radiologically guided intra-articular lumbar FJIs with physiological saline) | Not stated – assessed pain, disability and return to work | 3 months | No difference in outcomes at follow-up between the two active groups and the sham group. Improvement in pain, disability and work attendance in all groups |
| Carette, 199127 (Canada, n = 101) | LBP for > 6 months, normal neurological examination, > 50% pain reduction after single intra-articular diagnostic injection with lidocaine | Fluoroscopy-guided intra-articular lumbar FJIs with methylprednisolone and isotonic saline | Sham (fluoroscopy-guided intra-articular lumbar FJIs with isotonic saline) | Not stated – assessed pain severity, back mobility and limitation of function | 6 months | No differences in outcomes at 1 and 3 months between the two groups. At 6 months, patients in the intervention group reported greater self-rated improvement, lower pain intensity and less physical disability than patients in the sham group |
| Marks, 199228 (Scotland, UK, n = 86) | LBP for > 6 months, failed non-narcotic analgesics and physiotherapy, no diagnostic blocks | Radiologically guided intra-articular lumbar FJIs with lidocaine and methylprednisolone | Radiologically guided lumbar facet-joint medial branch nerve blocks with lidocaine and methylprednisolone | Pain intensity | 3 months | Marginally longer duration of response in the intervention group after 1 month, otherwise no difference in outcomes at other time points between the two groups. Some short-term pain relief seen in both groups |
| Revel, 199829 (France, n = 80) | LBP for > 3 months, failed analgesics and physical therapy, no diagnostic blocks | Fluoroscopy-guided intra-articular lumbar FJIs with lidocaine plus periarticular corticosteroid steroid injection (not evaluated) | Fluoroscopy-guided intra-articular lumbar FJIs with saline plus periarticular corticosteroid steroid injection (not evaluated) | Pain intensity using VAS | 30 minutes after injections | Significantly reduced pain scores in the intervention group compared with the comparator group |
| Manchikanti, 200130 (USA, n = 84) | LBP for > 6 months, failed conservative management, positive response following controlled comparative diagnostic blocks with lidocaine and bupivacaine | Lumbar facet medial branch nerve blocks with lidocaine or bupivacaine, Sarapin® (High Chemical Company, Levittown, PA, USA) and methylprednisolone | Lumbar facet medial branch nerve blocks with lidocaine or bupivacaine and Sarapin | Not stated – assessed pain characteristics, physical health, mental health, functional status, return to work and narcotic intake | Up to 2.5 years | No difference in outcomes at follow-up between the groups; improvement in pain and functional outcomes in groups I (IA and IB) and II (IIA and IIB) |
| Mayer, 200431 (USA, n = 70) | ‘Chronic disabling work related lumbar spinal disorder’ for > 6–12 months, ‘lumbar segmental rigidity’ on clinical examination, no diagnostic blocks | Fluoroscopy-guided bilateral intra-articular lumbar FJIs with lidocaine, bupivacaine and depot corticosteroid and home stretching exercise programme | Home stretching exercise programme only | Range of motion, pain and disability | Not specified – after completing the home stretching exercise programme | No difference in pain and disability reported at follow-up between the two groups, but greater improvement in range of motion in the intervention group |
| Fuchs, 200532 (Germany, n = 60) | LBP for > 3 months, facet-joint osteoarthritis on imaging | Computerised tomography-guided intra-articular lumbar FJIs with triamcinolone | Computerised tomography-guided intra-articular FJIs with sodium hyaluronate | Pain intensity, functioning and quality of life | 180 days | No difference in outcomes at follow-up between the two active groups; improvement in pain and functional outcomes in both groups |
| Manchikanti, 200833 (USA, n = 120) | LBP for > 6 months, failed conservative management, 80% pain relief following controlled comparative diagnostic blocks with lidocaine and bupivacaine | IA: lumbar facet-joint medial branch nerve blocks with bupivacaine; IB: lumbar facet-joint medial branch nerve blocks with bupivacaine and Sarapin | IIA: lumbar facet-joint medial branch nerve blocks with bupivacaine and steroid; and IIB: lumbar facet-joint medial branch nerve blocks with bupivacaine, steroid and Sarapin | Not stated – assessed pain relief, work status, opioid intake and functional status | 1 year | No difference in outcomes at follow-up between the groups; improvement in pain and functional outcomes in both groups |
| Kawu, 201134 (Nigeria, n = 18) | LBP for > 3 months, failed analgesics, MRI features of facet-joint arthropathy, no diagnostic blocks | Radiologically guided lumbar FJIs with bupivacaine and methylprednisolone | Physiotherapy (McKenzie regimen) | Not stated – assessed pain relief and satisfaction with treatment | 6 months | Greater decreases in pain and higher levels of satisfaction in the intervention group than in the comparator group |
| Celik, 201135 (Turkey, n = 80) | LBP for < 4 months, no diagnostic blocks | Fluoroscopy-guided lumbar FJIs with bupivacaine and methylprednisolone | Diclofenac, thiocolchicoside and bed rest for 4 days | Not stated – assessed LBP disability and pain intensity | 6 months | Greater decreases in pain and disability in the intervention group than in the comparator group; improvement in pain and functional outcomes in both groups |
| Yun, 201236 (Korea, n = 57) | LBP (no duration specified), clinical indicators of facet syndrome, no diagnostic blocks | Fluoroscopy-guided lumbar FJIs with lidocaine and triamcinolone | Ultrasound-guided lumbar FJIs with lidocaine and triamcinolone | Pain and activities of daily living | 3 months | No difference in outcomes at follow-up between the two active groups; improvement in pain and functional outcomes in both groups |
| Ribeiro, 201337 (Brazil, n = 60) | LBP for > 3 months, clinical diagnosis of lumbar facet-joint syndrome, no diagnostic blocks | Fluoroscopy-guided intra-articular lumbar FJIs with lidocaine and triamcinolone | Intramuscular paravertebral injections with lidocaine and triamcinolone | Not stated – assessed quality of life, functional capacity, pain on back extension, percentage improvement scale, analgesic usage | 24 weeks | ‘Slightly superior’ results in the intervention group than in the comparator group; improvement in pain and functional outcomes in both groups |
| Lakemeier, 201338 (Germany, n = 56) | LBP for > 24 months, > 50% pain reduction after single intra-articular diagnostic injection with bupivacaine | Fluoroscopy-guided intra-articular lumbar FJIs with bupivacaine and betamethasone | Radiofrequency denervation of the lumbar facet-joint medial branch nerves | LBP-related disability using the Rowland Morris Disability Questionnaire | 6 months | No difference in outcomes at follow-up between the two active groups; improvement in pain and functional outcomes in both groups |
The conclusions drawn from the systematic reviews were generally equivocal. Although there was some trial evidence demonstrating the effectiveness of therapeutic lumbar FJIs in the management of chronic LBP, the limited to moderate quality of this evidence was insufficient to support their use in practice. The current evidence base for FJI is neatly summed up by a recent and high-quality systematic review by Vekaria et al. ,23 which concluded that ‘The studies found here were clinically diverse and precluded any meta-analysis. A number of methodological issues were identified. The positive results, whilst interpreted with caution, do suggest that there is a need for further high-quality work in this area.’23
Rationale for a feasibility study
To provide further high-quality research in this area, the NIHR HTA programme issued a commissioning brief in 2011 to answer the research question, ‘Is a definitive study to assess the effectiveness and cost-effectiveness of facet-joint injections compared with best non-invasive care for people with persistent non-specific low back pain feasible?’ [see www.journalslibrary.nihr.ac.uk/programmes/hta/113102/#/ (accessed 31 October 2017)].
Two research teams were funded by the NIHR in response to this commissioned call: (1) the Facet Feasibility study (the addition of intra-articular facet-joint injections to best usual non-invasive care) (reference HTA 11/31/01) led by Professor Martin Underwood, University of Warwick [see www.journalslibrary.nihr.ac.uk/programmes/hta/113101/#/ (accessed 31 October 2017)] and (2) this project, the FACET feasibility study (a multicentre double-blind randomised controlled trial comparing intra-articular lumbar facet-joint injections with a sham procedure, followed by a combined physical and psychological programme) (reference HTA 11/31/02) [see www.journalslibrary.nihr.ac.uk/programmes/hta/113102/#/ (accessed 31 October 2017)] led by Professor Richard Langford, Barts Health NHS Trust.
Study aims and research questions
The aim of the FACET feasibility study was to assess the feasibility of conducting a definitive study to evaluate the clinical effectiveness and cost-effectiveness of FJIs compared with a sham procedure in patients with non-specific LBP of > 3 months’ duration.
To inform a full trial, a number of questions first need to be assessed by a feasibility study:
-
Given the multiple sites with the potential to generate back pain, can patient selection criteria be optimised, using clinical and investigative diagnostic methods?
-
Can the method of injection be standardised and an appropriate sham procedure be established?
-
Can justification for further studies to evaluate treatment methods to target and attenuate the source of chronic LBP of facet-joint origin be delivered?
-
Is a sham-controlled trial design acceptable to patients and clinicians?
-
Can a sufficient number of patients be recruited and retained?
Study objectives
-
To assess the eligibility criteria and recruitment and retention of patients in the two treatment arms (FJIs vs. sham procedure) by assessing the feasibility of recruitment in the three centres, reviewing the number of completed patient data sets, auditing the quality of data entry at the centres and assessing and analysing any protocol violations (such as failure to deliver the CPP programme – recommended therapy at the time of the establishing this study39), side effects and other adverse outcomes.
-
To assess the feasibility and acceptability of the two treatment arms from the point of view of patients and their pain teams.
-
To assess the feasibility of the proposed definitive study design, including:
-
testing of the randomisation and blinding procedures
-
development of appropriate active and sham procedures for FJIs
-
assessment of the consistency of the trial sites in terms of delivering the CPP programme
-
assessment of the ability to collect the outcomes proposed for the main trial (pain, functioning, health-related quality of life, anxiety and depression, health-care resource utilisation, complications and adverse events).
-
-
To estimate outcome standard deviations (SDs) to inform the power calculation for a definitive study.
-
To finalise the protocol design, statistical plan, number of centres required and study duration for the definitive study.
Chapter 2 Methods
Study design
This feasibility study utilised a blinded parallel two-arm pilot randomised controlled trial design. Following a positive diagnostic medial branch nerve block, participants with non-specific LBP were individually randomised in a 1 : 1 ratio to receive either the FJI (intervention group) or a sham (placebo injection) procedure (control group). Both the intervention group and the control group received a CPP programme after their active or sham injections.
Participants
The study sought patients with non-specific LBP based on the following inclusion and exclusion criteria.
Inclusion criteria
-
Patients aged 18–70 years attending pain clinics identified during routine clinical assessment of non-specific LBP. Clinical indicators of pain of facet-joint origin included tenderness over the facet-joints, referred leg pain above the knees and worsening pain on extension, flexion and rotation of the lumbar spine.
-
Low back pain of ≥ 3 months’ duration.
-
An average pain intensity score of ≥ 4 out of 10 in the 7 days preceding recruitment despite NICE-recommended treatment. NICE clinical guideline CG887 recommended providing patients with advice and information to promote self-management of their LBP and offering one of the following treatments, taking into account patient preference: an exercise programme, a course of manual therapy or a course of acupuncture.
-
Dominantly paraspinal (not midline) tenderness at two bilateral lumbar levels.
-
At least two components of NICE-recommended best non-invasive care completed, including education and one of a physical exercise programme, acupuncture or manual therapy. 7
-
Patients are suitable for the FJIs.
Exclusion criteria
-
Patient refusal to consent.
-
More than four painful lumbar facet-joints. No more than four facet-joints were to be injected to limit the total dose of intra-articular steroids.
-
Patient has not completed at least two components of NICE-recommended best non-invasive care, including education and one of a physical exercise programme, acupuncture or manual therapy. 7
-
‘Red flag’ signs. These are possible indicators of serious spinal pathology and include thoracic pain, fever, unexplained weight loss, bladder or bowel dysfunction, progressive neurological deficit and saddle anaesthesia. 40
-
Known hypersensitivity to study medications.
-
Dominantly midline tenderness over the lumbar spine, any other dominant pain or radicular pain.
-
Any major systemic disease or mental health illness that may affect the patient’s pain, disability and/or their ability to exercise and rehabilitate, as judged by the Principal Investigators.
-
Any active neoplastic disease, including primary or secondary neoplasm.
-
Pregnant or breastfeeding patients (verbal confirmation obtained at screening; prior to each interventional procedure involving radiography, local hospital procedures will be followed to confirm that female participants are not pregnant).
-
Any evidence of previous lumbar FJIs, previous lumbar spinal surgery or any major trauma or infection to the lumbar spine.
-
Patients with morbid obesity (body mass index of ≥ 35 kg/m2).
-
Participation in another clinical trial of an investigational medicinal product or disease-related intervention in the past 30 days.
-
Patients unable to commit to the 6-month study duration.
-
Patients involved in legal actions or employment or benefit tribunals related to their LBP.
-
Patients with a known history of substance abuse.
Recruitment procedures
It was originally planned to recruit patients from pain clinics at the three participating NHS centres and their associated community based pain clinics. However, because of the early termination of the study by the funder, recruitment was undertaken at only one centre, Barts Health NHS Trust. Recruitment took place over 9 months. The first participant was recruited in January 2016 and the last participant was recruited in September 2016.
Patients were referred by their general practitioner (GP) as a standard clinical referral with LBP requiring further specialist assessment, for reasons such as uncertain diagnosis, failure of conservative treatment and expectation of therapeutic interventions. Potentially eligible patients were identified by a pain clinician based on the inclusion and exclusion criteria.
Participants were free to withdraw from the study at any time without giving a reason; the participant information sheet stated that ‘a decision to withdraw from the study at any time will not affect the standard of care that you receive now or in the future’ (see Appendix 5). Participants who withdrew from the study received a routine follow-up appointment in their pain clinic for continuing assessment and management by their pain physician.
Informed consent
Potential participants were given a copy of the information sheet (see Appendix 5) and a verbal explanation of its contents, including information on the nature of the study, the implications and constraints of the study protocol and any known side effects and risks involved in taking part in the study. A medically qualified investigator on the delegation log obtained written informed consent.
Sample size calculation
At the outset of the study it was expected that a total of 60 patients would be recruited, to be able to estimate the precision of an assumed 20% attrition rate with an error of ±5% at the 95% confidence level. Assuming that 24 full data sets per arm were completed at the end of the study, this would give a reasonable estimate of variance of the outcomes. 41
Diagnostic test
Following screening and consent, all participants underwent a diagnostic medial branch nerve block. Those who achieved a positive response were randomised to the intervention group or the control (sham) group.
The diagnostic medial branch nerve injections were carried out at each painful lumbar level under radiological guidance. With the patient lying in the prone position on a radiolucent table, the investigator examined the patient’s back to elicit paraspinal tenderness and confirmed appropriate landmarks and facet-joints to be injected using radiological image intensification. The C-arm of the image intensifier was obliquely rotated as required to facilitate visualisation of the target for injection. The spinal needle was used to inject 0.5 ml of 1% lidocaine per level, with six levels injected. A positive response was defined as a ≥ 50% pain reduction lasting for > 30 minutes, that is, the duration of action of lidocaine, measured using a pain intensity numerical rating scale (NRS) and assessed in the standing position.
Interventions
The FJIs, sham procedure and diagnostic tests were carried out by the Principal Investigator at Barts Health NHS Trust, a Fellow of the Faculty of Pain Medicine of the Royal College of Anaesthetists in the UK. During all of the interventional procedures strict aseptic conditions were adhered to and local theatre protocols were followed with regard to admission and discharge criteria, including the use of the World Health Organization (WHO) Surgical Safety Checklist42 to identify the correct patient prior to starting the procedure. The investigator carrying out the injections was not blinded to randomisation group.
Active injection
Before undertaking this feasibility study, a web-based survey of 250 UK pain specialists was carried out utilising the Delphi method to agree on the choice of needle, injectate and volume of injection, as well as the choice of steroid, dose and volume and maximum dose of steroid (see Appendix 6).
In the intervention group, each participant received four FJIs at two bilateral lumbar levels, with 0.5 ml of 0.5% bupivacaine (Marcain Polyamp Steripack 0.5%, Aspen Pharma Trading Limited, Dublin, Ireland) and 20 mg of methylprednisolone (Depo-Medrone 40 mg/ml, Pfizer, Kent, UK) injected per joint. No more than four facet-joints were to be injected to avoid any potential confounding effect attributable to the systematic action of exceeding 80 mg of methylprednisolone. The volume of injectate did not exceed 1 ml per joint as it would be possible to rupture the intra-articular capsule with greater volumes, spreading the local anaesthetic and steroid to other potentially pain-generating structures.
Paraspinal tenderness was elicited as described previously. The skin was anaesthetised with 1% lidocaine (Lidocaine Hydrochloride Injection BP 1% w/v, Hameln Pharmaceuticals Ltd, Gloucester, UK) and a 22G 90-mm Quincke spinal needle was advanced through the skin, subcutaneous tissue and paraspinal muscle towards the facet-joint under radiological guidance. Entry of the needle was confirmed by visualisation of the needle position within the joint space and local anaesthetic and steroid were injected into the joint.
Sham procedure
The control group received four injections of 0.5 ml of normal saline (0.9% sodium chloride) at two bilateral lumbar levels. A low volume was chosen to avoid irritation of any structure that is part of the facet-joints, including the fibrous capsule, synovial membrane, hyaline cartilage and bone. The sham group would not receive systematic steroid administration as it has been shown that the addition of parenteral steroid does not contribute to the pain relief achieved by targeted injections. 43
Paraspinal tenderness was elicited as described previously. The skin was anaesthetised with 1% lidocaine and a 22G 90-mm Quincke spinal needle was advanced through the skin, subcutaneous tissue and paraspinal muscle towards the periarticular space under radiological guidance. Placement of the needle in the periarticular space was confirmed by visualisation of the needle position next to the joint space and normal saline was injected at this site.
Combined physical and psychological programme
Both the intervention group and the control group underwent a CPP programme delivered by trained physiotherapists. Research on CPP management of LBP has demonstrated that equally effective management can be achieved with far fewer than the 100 hours prescribed in the 2009 NICE guidance. 7 The study therefore proposed to deliver a programme drawing on the methods and evidence from the Back Skills Training (BeST) trial. 44
Physiotherapists on the delegation log were trained to deliver the Back Skills Training programme by the lead physiotherapist. Individual physiotherapists undertook approximately 10 hours of online training at www.backskillstraining.co.uk (accessed 11 November 2017) and received a certificate of completion and a trainer manual to support CPP programme delivery. The lead physiotherapist organised face-to-face meetings with each physiotherapist to ensure competency and standardised delivery.
Each participant attended an initial one-to-one hour-long assessment with a trained physiotherapist at which information was gathered, including the impact of pain on their activity and their thoughts and beliefs regarding LBP. Individualised goals were identified with one specific to physical activity. Participants then selected and practised an individualised exercise programme.
Six weekly 1.5-hour sessions of a group-based CPP programme were scheduled for each participant. Completion of the CPP programme was defined as having completed a minimum of four out of six sessions. The session contents are detailed on the website www.backskillstraining.co.uk and address the following:
-
understanding pain
-
pain fluctuations
-
unhelpful thoughts and feelings
-
restarting activities or hobbies
-
when pain worries us
-
coping with flare-ups.
One session per programme was observed by the lead physiotherapist to assess consistency of delivery and to provide feedback and support for the physiotherapists running the course. Two research physiotherapists in total, including the lead physiotherapist, delivered the programme to the study participants at Barts Health NHS Trust.
Each participant received a Back Skills Training Patient Workbook, which provided a summary of each week’s content for their reference at home. It was expected that participants would be in groups of fewer than 10 people; four to five groups of four to five participants per site were anticipated.
Regulatory approvals
The study was conducted in compliance with the principles of the Declaration of Helsinki (1996)45 and the principles of Good Clinical Practice46 and in accord with all applicable regulatory requirements including but not limited to the Research Governance Framework47 and the Medicines for Human Use (Clinical Trials) Regulations 2004,48 as amended in 2006 and 2008, the sponsor’s policies and procedures and any subsequent amendments.
The required regulatory approvals were obtained in the UK. The study received ethics approval from the National Research Ethics Service (NRES) Committee London – City & East (reference 15/LO/0500) and clinical trial authorisation from the Medicines and Healthcare products Regulatory Agency (MHRA) (reference 14620/0046/001–0001). The trial protocol was reviewed by the MHRA’s clinical trials team and the trial was considered to be a type A Clinical Trial of an Investigational Medicinal Product (CTIMP), that is, the risks are no higher than those of standard medical care. The Summary of Product Characteristics (SmPCs) for each investigational medical product (bupivacaine and methylprednisolone acetate) are available to view on the Electronic Medicines Compendium. 49,50 Health Research Authority (HRA) approval was obtained and the study was given permission by the sponsor’s Joint Research Management Office (JRMO) to recruit patients at Barts Health NHS Trust.
Imaging authorisation was given by the sponsor’s clinical radiation expert and medical physics expert, as all of the participants would receive ionising radiation in the form of X-rays for the diagnostic injections, FJIs and sham procedure.
Randomisation and blinding
Participants were allocated to either the intervention group or the control group in a 1 : 1 ratio, with stratification by centre and minimisation on baseline pain scores (categories). To ensure concealment, the allocation sequence was computer generated and provided through a password-protected web-based portal developed and maintained by the Peninsula Clinical Trials Unit (PenCTU).
It was not possible to blind the operator (Principal Investigator) as the injections were intentionally given at different sites (intra-articular vs. periarticular) and the injections looked different (methylprednisolone is provided as a cloudy suspension, whereas the sham injection was clear). However, study participants and the remainder of the research team, including the Chief Investigator, research nurses conducting the outcome assessments and data analysts, were blinded for the duration of the study. Unblinding took place at the end of the study once data analysis had been completed. A standard operating procedure was in place for emergency unblinding, in accordance with the sponsor guidelines.
Outcomes
The outcome questionnaire visits took place in research nurse-led clinics at baseline (pre randomisation) and at 6 weeks, 3 months and 6 months post randomisation. A sample case report form is shown in Appendix 7 and the schedule of outcome assessment is shown in Table 2. The outcome questionnaire covered a range of pain- and disability-related issues and was in accord with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)-recommended core outcome measures for chronic pain trials. 51 The following assessment tools were used in the study.
-
Pain intensity and characteristics – Brief Pain Inventory (BPI) (Short Form) Modified,52 with its 11-point NRS, and short-form McGill Pain Questionnaire version 2 (SF-MPQ-2). 53 As movement could potentially influence the intervention (lumbar FJIs or sham procedure), all numerical rating scores were assessed in the standing position.
-
Use of co-analgesics in the previous week – participant self-report.
-
Lack of efficacy of pain relief, or, for side effects, early withdrawal from the study.
-
Expectation of benefit (asked at baseline only) – measured on a scale from 0 to 6, ranging from ‘expect no improvement’ to ‘expect total improvement’.
-
Health-related quality of life – EuroQol-5 Dimensions five-level version (EQ-5D-5L)54 and Short Form questionnaire-12 items (SF-12). 55
-
Functional impairment – Oswestry Low Back Pain Disability Questionnaire56 and Pain Self-Efficacy Questionnaire (PSEQ). 57
-
Satisfaction with treatment (after treatment given) – NRS from 0 to 10, with 0 = ‘extremely dissatisfied’ and 10 = ‘extremely satisfied’.
-
Complications and adverse events – these were the subject of enquiry at visits and following procedures, as well as being spontaneously reported at any time. They were acted on as necessary and for the patients’ benefit and were fully documented on case report forms and medical notes.
-
Co-psychological well-being – Hospital Anxiety and Depression Scale (HADS),58 Pain Catastrophizing Scale (PCS),59 SF-12 and BPI.
-
Health-care utilisation and costs and impact on productivity –Stanford Presenteeism Scale (SPS) 6,60 self-reported measures of sickness absence over the previous 3 months and health-care utilisation in the form of hospital visits, treatments and medications. These data were collected at each outcome visit on the case report form.
| Assessment | Prescreening | Visit | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 (6 weeks after injections ± 2 weeks) | 5 (3 months after injections ± 2 weeks) | 6 (6 months after injections ± 2 weeks) | ||
| Informed consent | ✗ | ||||||
| Targeted physical examination | ✗ | ✗ | |||||
| Inclusion/exclusion criteria fulfilled | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Medical history recorded | ✗ | ||||||
| Demographic data recorded | ✗ | ||||||
| Drug history recorded | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Breakthrough analgesia recorded | ✗ | ✗ | ✗ | ✗ | |||
| Adverse events | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Outcome questionnaires | ✗ | ✗ | ✗ | ✗ | |||
| Expectation of benefit scale | ✗ | ||||||
| BPI (Short Form) | ✗ | ✗ | ✗ | ✗ | |||
| SF-MPQ-2 | ✗ | ✗ | ✗ | ✗ | |||
| EQ-5D-5L | ✗ | ✗ | ✗ | ✗ | |||
| SF-12 | ✗ | ✗ | ✗ | ✗ | |||
| Oswestry Low Back Pain Disability Questionnaire | ✗ | ✗ | ✗ | ✗ | |||
| PSEQ | ✗ | ✗ | ✗ | ✗ | |||
| HADS | ✗ | ✗ | ✗ | ✗ | |||
| Pain Catastrophizing Scale | ✗ | ✗ | ✗ | ✗ | |||
| SPS 6 | ✗ | ✗ | ✗ | ✗ | |||
| Satisfaction with treatment scale | ✗ | ✗ | ✗ | ||||
Adverse events
Adverse events were assessed by a blinded subinvestigator at each visit and an adverse event form was completed as necessary (see Appendix 8). Adverse events are defined as any untoward medical occurrence in a subject to whom a medicinal product has been administered; an adverse reaction is an untoward and unintended response in a subject to an investigational medicinal product (IMP) that is related to any dose administered to that subject. A serious adverse event or reaction results in death, is life-threatening, requires inpatient hospitalisation or prolongation of an existing hospitalisation, results in persistent or significant disability or incapacity or is a congenital anomaly or birth defect. A suspected unexpected serious adverse reaction is any serious adverse event that is both suspected to be related to the IMP and unexpected.
Adverse events not already identified locally were recorded at each trial visit and managed in accordance with the sponsor’s requirements. Serious adverse events were reported to the JRMO by the investigators within 24 hours of the research team becoming aware of them and causality and expectedness were confirmed by the Chief Investigator, as the sponsor’s medical representative.
Study management and committees
The Trial Management Group (TMG) was responsible for the overall management of the project and included all co-applicants and members of the study research team. A Trial Steering Committee (TSC) provided independent advice and support to the study and aimed to report to the funder on study progress. It was chaired by an independent clinician with experience of pain trials. A Data Monitoring Committee (DMC) had access to unblinded data and made recommendations to the TSC on whether there were any ethical or safety reasons why the trial should not continue. It included independent members who were all experts in pain medicine.
Patient and public involvement
Patients with personal experience of LBP collaborated in the early stages of study design, for example advising on the acceptability of study visits and the outcome questionnaires. The questionnaires were tested on patients presenting to the multidisciplinary pain clinics and were deemed to be acceptable. Patient representatives were invited to attend the TSC meetings; however, there was no patient or public involvement in the management or running of the trial beyond the initial set-up stage.
Statistical analysis
Analyses were conducted in accordance with the International Conference on Harmonisation (ICH) statistical guidelines for clinical trials61 and the Consolidated Standards for Reporting Trials (CONSORT) reporting checklist for trials. 62 As this was a feasibility study, it was not planned to formally inferentially test differences in outcomes or costs between or within the groups. Mean recruitment and attrition rates were calculated with 95% confidence intervals (CIs). Means and SDs for all outcomes for the two groups at baseline and at the follow-up visits were reported. A detailed statistical analysis plan was prepared by the study statistician (RST) prior to any data analysis. Analyses were performed blinded to group allocation using Stata® 14.2 (StataCorp LP, College Station, TX, USA).
Health economics analysis
A health economics analysis plan was developed in collaboration with the study’s health economist and statistician. A formal economic analysis was not proposed (as this was a feasibility study). Any outcomes from this feasibility study would be used in the design of the definitive study. In particular, the health economics analysis looked at the ability to collect the outcomes proposed for the main trial and to inform a robust framework to assess the cost-effectiveness of lumbar FJIs for persistent non-specific LBP for a future definitive randomised controlled trial.
The resources used in the delivery of the intervention and sham procedure were calculated from the case report forms and in consultation with the trial team. Health-care resource use was captured through administration of specific questions to trial participants at each assessment, with responses recorded on case report forms, with a focus on collecting data on the most relevant and important drivers of health resource use and costs. Published national costs were used to calculate the costs of delivering each treatment arm. A summary of the costing methods employed are presented in Appendix 9. In addition, a literature review was conducted to review the scope and quality of the current economic evidence base for the use of FJIs in patients with non-specific LBP (see Appendix 9).
Descriptive analyses of the outcomes were used to report utilities based on the relevant tariff for each of the health-related quality-of-life outcomes. Economic outcomes were measured using the EQ-5D-5L (the preferred approach of the study team and for NICE decision-making) and the Short Form questionnaire-6 Dimensions (SF-6D63), used as a means of calculating utilities from the SF-12. A descriptive analysis of the health-related quality of life outcomes was summarised and was to provide estimates of quality-adjusted life-years (QALYs) gained as a result of receiving the intervention within the study period.
Summary of changes to the study protocol
The minor and major amendments made to the protocol over the duration of the study are detailed in Appendix 10.
Chapter 3 Results
Screening and recruitment
It was originally planned to screen and recruit participants across three centres; however, given the delays in study set-up, the funder directed that screening and recruitment take place at one centre only. The timelines of the study and the reasons for the delays are presented at the end of this chapter.
Recruitment began at Barts Health NHS Trust on 20 January 2016 and was terminated on 30 September 2016. As shown in Figure 2, during this time 628 patients referred to the recruiting clinics by their GP with non-specific LBP were screened for eligibility to enter the study. Of the 50 patients who met the inclusion criteria, 16 agreed to take part in the study and 11 received the diagnostic test for facet-joint disease. Nine participants had a positive response and were randomised to receive either lumbar FJIs with steroid or a sham procedure. The target sample size in each centre was 20 participants. The actual participant screening-to-recruitment ratio was 70 : 1 (628 : 9), which contrasts with an expected prestudy ratio of 17 : 1 (1000 : 60). The recruitment rate varied between zero and four patients per month (median two participants per month (Table 3).
FIGURE 2.
Consolidated Standards for Reporting Trials (CONSORT) flow diagram showing the flow of participants through the trial.
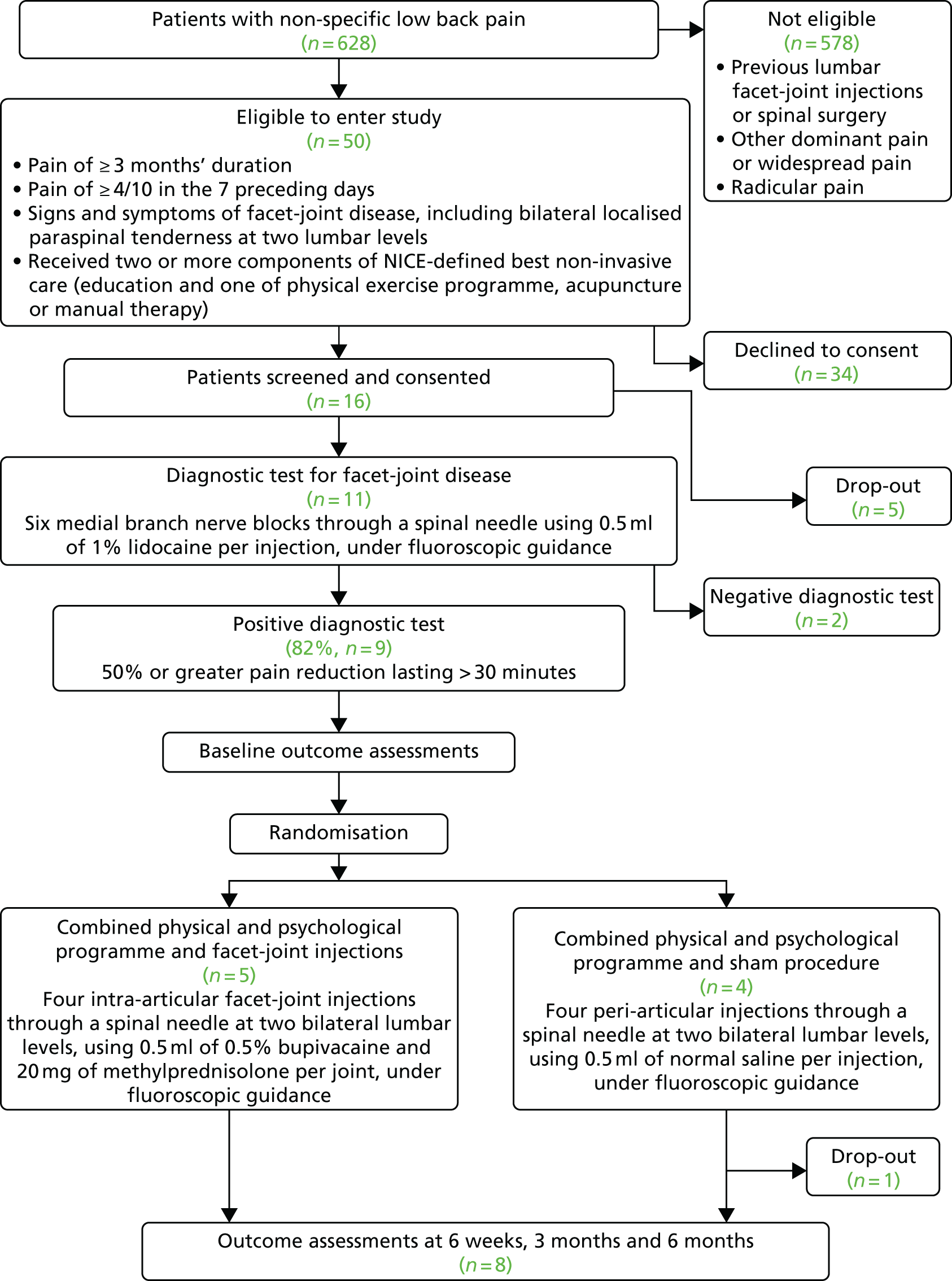
| Outcome | Recruiting month | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Recruited | 1 | 4 | 2 | 0 | 2 | 2 | 0 | 2 | 3 | 16 |
| Screened | 8 | 26 | 6 | 37 | 18 | 46 | 78 | 209 | 200 | 628 |
The reasons for screening failure are detailed in Table 4. The greatest proportion of patients was screened from the pain clinics at St Bartholomew’s Hospital, with a screened/recruited fraction of 1.69% (Table 5). Although only 16 patients were screened from the community pain clinic at the Essex Lodge GP surgery in Plaistow, East London, the screened/recruited fraction was higher at 12.5%. No participants were recruited from the spinal orthopaedic clinic at The Royal London Hospital or from the pain clinics at Whipps Cross University Hospital and Mile End Hospital.
| Reasons | Number of patients |
|---|---|
| Previous lumbar FJIs | 192 |
| Previous lumbar FJIs | 163 |
| Previous lumbar FJIs and no previous physiotherapy | 3 |
| Previous lumbar FJIs and radiofrequency denervation | 18 |
| Previous lumbar FJIs or radiofrequency denervation and aged > 70 years | 8 |
| Other dominant pain or widespread pain | 92 |
| Radicular pain | 64 |
| Aged > 70 yearsa | 42 |
| Aged > 70 years | 29 |
| Previous lumbar FJIs or radiofrequency denervation and aged > 70 years | 8 |
| Aged > 70 years and has radicular pain | 12 |
| Aged > 70 years and has widespread pain | 1 |
| Other reasons for not meeting inclusion/exclusion criteria | 36 |
| Did not wish to take part | 34 |
| Previous major trauma to the lumbar spine | 29 |
| ‘Red flag’ signs | 29 |
| Previous lumbar spinal surgery | 25 |
| Study team unable to contact | 17 |
| Already taking part in another study | 12 |
| Limited or no English language | 11 |
| Active neoplastic disease | 7 |
| No previous physiotherapy | 7 |
| Morbid obesity (body mass index of ≥ 35 kg/m2) | 7 |
| Learning difficulties or known mental health illness | 5 |
| Known history of substance abuse | 2 |
| Aged < 18 years | 1 |
| Clinic location | Number of patients | Screened/recruited fraction (%) | |
|---|---|---|---|
| Screened for eligibility | Randomised | ||
| Pain clinic, St Bartholomew’s Hospital | 413 | 7 | 1.69 |
| Spinal orthopaedic (‘fracture’) clinic, The Royal London Hospital | 180 | 0 | 0 |
| Community pain clinic, Essex Lodge GP surgery | 16 | 2 | 12.5 |
| Pain clinic, Whipps Cross Hospital | 12 | 0 | 0 |
| Tower Hamlets Persistent Pain Services, Mile End Hospital | 7 | 0 | 0 |
Nine out of the 11 enrolled participants had a positive response to the diagnostic lumbar facet medial branch nerve block (82%, 95% CI 48% to 98%).
Adherence to allocated treatment
Facet-joint active and placebo injection
All the participants received their randomised procedure as planned and no problems with the injections were reported. None of the participants received any additional interventional pain procedures during their time in the study.
Combined physical and psychological programme
All nine participants were invited to attend a CPP programme after they had received their randomised procedure. Three physiotherapy-led CPP programmes took place between study months 12 and 20, with four participants in the first group, three in the second group and two in the final group. Six of the nine (67%) participants successfully completed the CPP programme, defined in the protocol as having attended at least four out of the six sessions. The median number of CPP programme sessions attended was four. One participant attended the initial CPP programme assessment but did not attend the programme because of illness during that period and another participant was unable to attend the CPP programme for personal reasons. A third participant did not attend the CPP programme because of unplanned overseas leave.
Study dropout and attrition
Of the 16 patients recruited and who consented to take part in the study, five withdrew before they received their diagnostic injections (although all completed the baseline assessment questionnaires). One patient already had lumbar FJIs booked for a future date and two changed their mind about taking part (one for personal reasons and one for family reasons). Two were unable to attend for the injections as the study dates were not suitable for them.
Of the nine participants who were randomised, eight completed the study (defined as having completed the final set of questionnaires 6 months after the randomised procedure), resulting in an 11% (95% CI 0.2% to 48%) attrition rate. The expected attrition rate was 20%. Six study deviations were recorded: three participants did not complete the CPP programme and three participants did not complete all of the questionnaire sets (Figure 3). A ‘study deviation’ was defined as a participant who did not attend a study visit or CPP programme session but who did not drop out of the study completely.
FIGURE 3.
Flow diagram showing the study deviations and dropouts during the study. (a) Combined physical and psychological programme and facet-joint injections; and (b) combined physical and psychological programme and sham procedure.
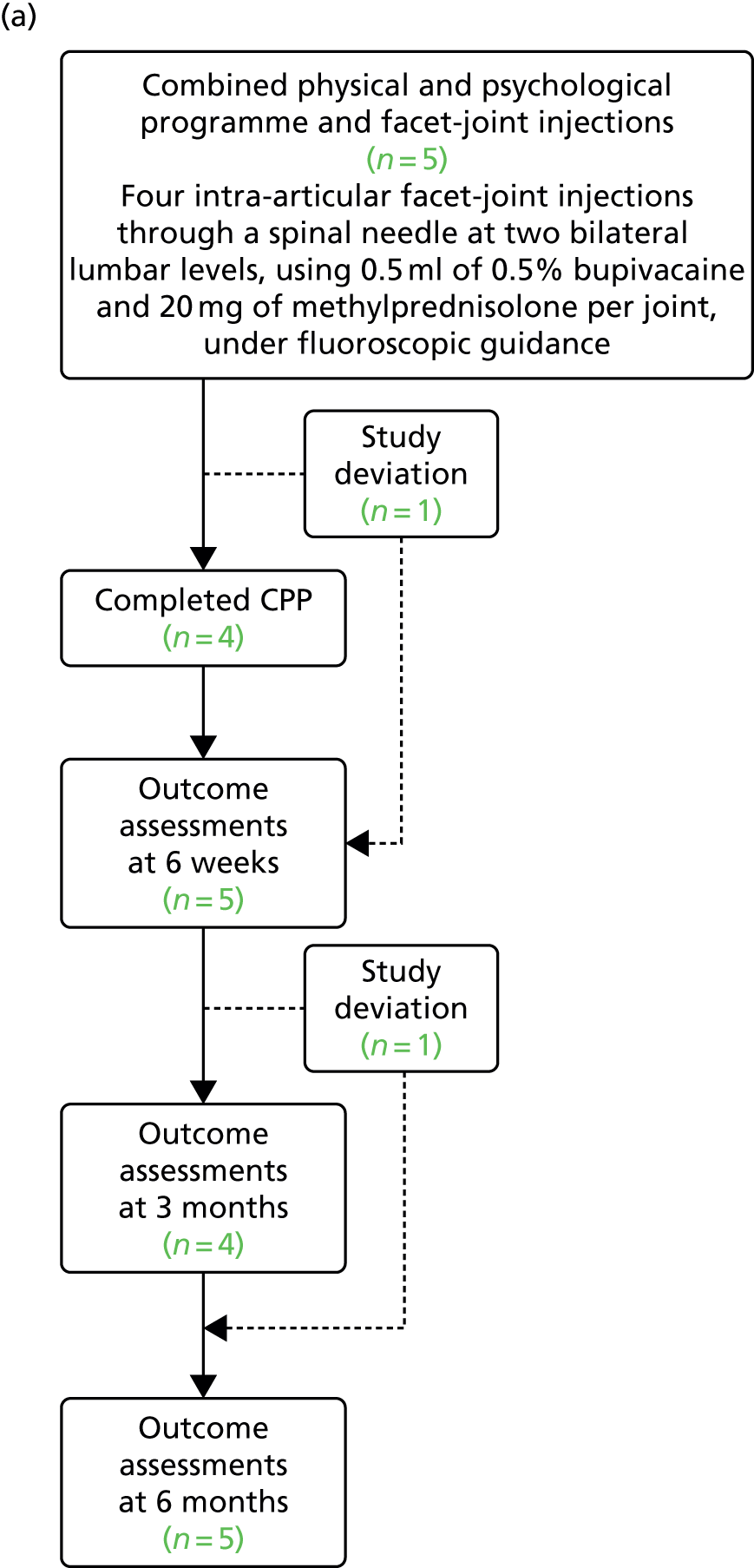
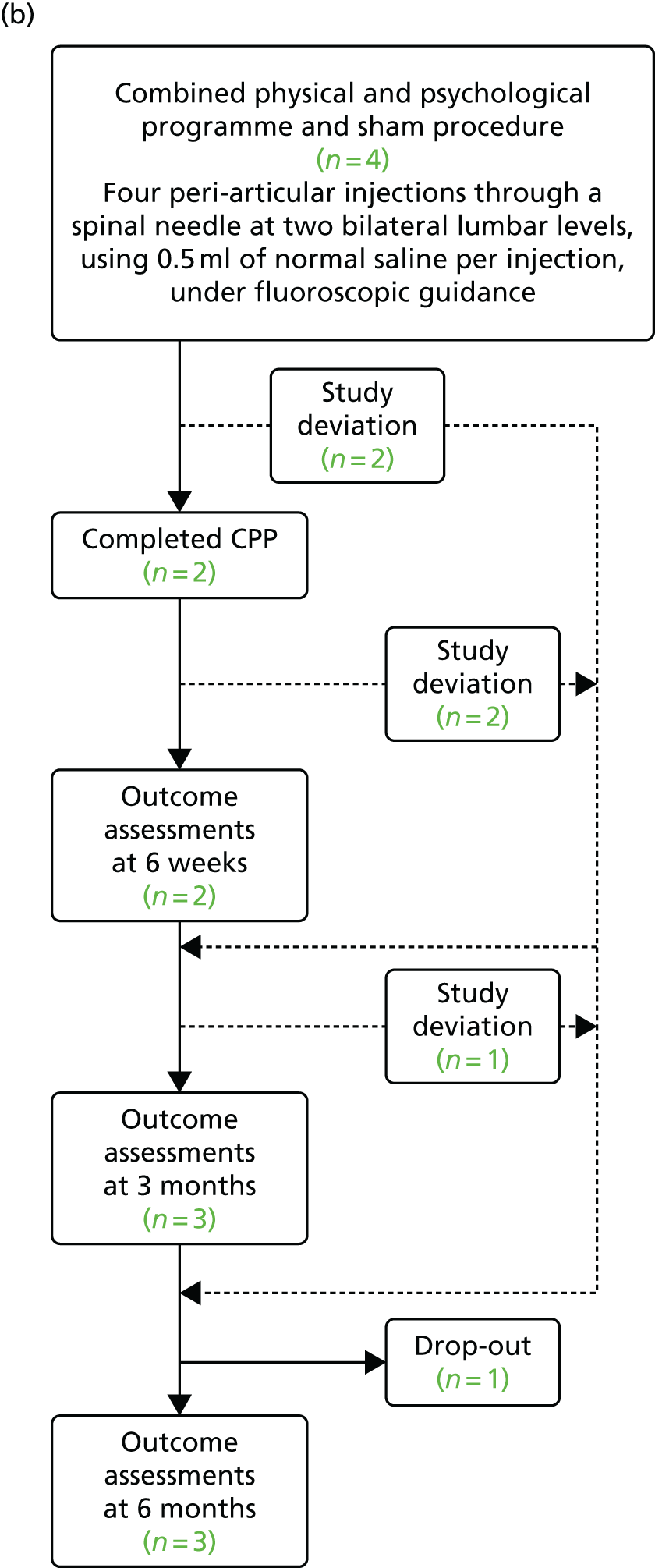
There were low levels of missingness of within-questionnaire data, as shown in Appendix 11. This is summarised in Table 6.
| Participant number | Questionnaires completed | Number of CPP programme sessions attended | CPP programme group | Randomisation group | |||
|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | 3 months | 6 months | ||||
| 1 | Y | Y | Y | Y | 5 | 1 | Intervention |
| 2 | Y | N | N | Y | 0 | 1 | Sham |
| 3 | Y | Y | Y | Y | 6 | 1 | Sham |
| 4 | Y | Y | Y | Y | 4 | 1 | Intervention |
| 5 | Y | Y | Y | Y | 6 | 2 | Sham |
| 6 | Y | Y | N | Y | 0 | 2 | Intervention |
| 7 | Y | Y | Y | Y | 5 | 2 | Intervention |
| 8 | Y | Y | Y | N | 0 | 3 | Sham |
| 9 | Y | Y | Y | Y | 4 | 3 | Intervention |
Baseline characteristics and outcomes
The mean age of eligible participants was 45 years, with a similar proportion of males and females. Six out of 14 participants (43%) were not working at baseline (Table 7).
| Characteristic | All eligible (N = 16a) | Not randomised (N = 7) | Randomisation group | |
|---|---|---|---|---|
| Sham (N = 4) | Intervention (N = 5) | |||
| Age (years), mean (SD) | 44.8 (13.2) | 44.4 (14.3) | 50.5 (14.4) | 40.8 (11.5) |
| Sex (male), n (%) | 9 (56) | 2 (29) | 2 (50) | 3 (60) |
| BMI (kg/m2), mean (SD) | 27.0 (5.1) | 29.9 (5.1) | 29.6 (4.7) | 27.7 (5.6) |
| Baseline pain (0–10 VAS), mean (SD) | 8.5 (1.5) | 8.0 (1.7) | 9.5 (1.0) | 8.4 (1.5) |
| Duration of pain (months), mean (SD) | 71.9 (88.7) | 46.0 (53.6) | 51.0 (46.3) | 124.8 (135.9) |
| Location of pain, n (%) | ||||
| Bilateral | 12 (75) | 5 (71) | 3 (75) | 4 (80) |
| Unilateral | 4 (25) | 2 (29) | 1 (25) | 1 (20) |
| Aware of pain (years), mean (SD) | 6.8 (7.6) | 5.2 (4.6) | 4.2 (3.9) | 10.4 (11.3) |
| Description of health, n (%) | ||||
| Excellent | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Very good | 1 (7) | 0 (0) | 0 (0) | 0 (0) |
| Good | 9 (64) | 3 (60) | 3 (75) | 1 (20) |
| Fair | 1 (7) | 1 (20) | 0 (0) | 3 (60) |
| Poor | 3 (21) | 1 (20) | 1 (25) | 1 (20) |
| Work status, n (%) | ||||
| Full time | 7 (50) | 1 (17) | 2 (50) | 4 (80) |
| Part time | 1 (7) | 0 (0) | 1 (25) | 0 (0) |
| Not working | 4 (29) | 3 (50) | 0 (0) | 1 (20) |
| Other | 2 (14) | 2 (33) | 1 (25) | 0 (0) |
| Illness caused participant to stop working, n (%) | ||||
| Yes | 10 (71) | 1 (20) | 3 (75) | 3 (60) |
| No | 4 (29) | 4 (80) | 1 (25) | 2 (40) |
| Missed work days, mean (SD) | 13.5 (31.1) | 0 (0) | 30.0 (52.0) | 4.5 (3.7) |
| Level of activity prior to procedure, n (%) | ||||
| Hard manual | 2 (29) | 0 (0) | 1 (33) | 1 (33) |
| Lifting | 1 (14) | 1 (100) | 0 (0) | 0 (0) |
| Walking | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sedentary | 4 (57) | 0 (0) | 2 (66) | 2 (66) |
| Current smoker, n (%) | ||||
| Yes | 11 (79) | 2 (40) | 0 (0) | 1 (20) |
| No | 3 (21) | 3 (60) | 4 (100) | 4 (80) |
| Alcohol (units per week), mean (SD) | 0.7 (1.1) | 0.6 (1.3) | 0.5 (1.0) | 1.0 (1.2) |
| Exercise per week, n (%) | ||||
| > 5 days | 2 (14) | 0 (0) | 1 (25) | 1 (20) |
| 3–5 days | 1 (7) | 1 (20) | 0 (0) | 0 (0) |
| 1–2 days | 5 (36) | 1 (20) | 1 (25) | 3 (60) |
| < 1 day | 6 (43) | 3 (60) | 2 (50) | 1 (20) |
Baseline patient-reported outcomes indicated a population with substantial levels of pain [mean 8.5 on a 0–10 visual analogue scale (VAS)] that was predominantly bilateral (12/16, 75%) and which had a mean duration of 72 months (see Table 7). In terms of the primary and secondary outcomes, high baseline levels of disability and mental ill health and poor overall health-related quality of life were seen (Tables 7 and 8).
| Outcome | All eligible (n = 16a) | Not randomised (n = 7) | Randomisation group, mean score (SD) | |
|---|---|---|---|---|
| Sham (n = 4) | Intervention (n = 5) | |||
| BPI (0–10) | ||||
| Worst pain | 8.5 (1.7) | 9.0 (0.9) | 9.3 (1.5) | 7.2 (2.2) |
| Least pain | 6.0 (2.7) | 6.2 (2.3) | 6.0 (2.7) | 6.0 (3.5) |
| Average pain | 7.4 (1.5) | 7.7 (1.5) | 7.5 (1.9) | 7.0 (1.6) |
| Pain now | 6.5 (2.8) | 5.2 (2.9) | 8.0 (2.3) | 6.8 (2.8) |
| Pain severity | 7.1 (1.6) | 7.0 (0.8) | 7.7 (1.7) | 6.8 (2.4) |
| General activity | 7.7 (2.5) | 7.7 (2.6) | 9.3 (1.5) | 6.6 (1.2) |
| Mood | 6.9 (2.2) | 6.7 (2.4) | 7.0 (2.4) | 7.0 (4.8) |
| Walking ability | 6.3 (2.8) | 6.2 (2.3) | 5.5 (2.0) | 7.0 (2.7) |
| Normal work | 8.1 (2.2) | 8.5 (2.1) | 8.5 (1.7) | 7.2 (2.9) |
| Relations | 6.2 (2.5) | 5.3 (3.3) | 6.5 (1.9) | 7.0 (4.8) |
| Sleep | 7.1 (3.0) | 5.8 (3.5) | 8.8 (1.9) | 7.4 (3.0) |
| Enjoyment | 7.4 (3.0) | 6.8 (3.9) | 8.3 (1.7) | 7.4 (3.0) |
| Interference | 7.1 (3.9) | 6.7 (4.7) | 7.7 (1.8) | 7.1 (2.4) |
| SF-MPQ-2 | ||||
| Continuous pain | 5.2 (2.0) | 5.6 (0.9) | 4.5 (3.0) | 5.3 (2.2) |
| Intermittent pain | 4.4 (2.5) | 4.7 (2.4) | 4.2 (2.6) | 4.3 (3.1) |
| Neuropathic pain | 2.7 (1.9) | 2.7 (2.2) | 2.1 (1.8) | 3.2 (1.7) |
| Affective descriptors | 4.0 (2.6) | 3.9 (2.8) | 2.0 (1.5) | 5.6 (2.4) |
| Total | 4.1 (1.7) | 4.2 (1.5) | 3.3 (2.0) | 4.5 (2.0) |
| Oswestry Low Back Pain Disability Questionnaire | ||||
| Total | 49.2 (17.6) | 55.8 (19.4) | 48.8 (19.9) | 43.0 (15.0) |
| PSEQ | ||||
| Total | 21.3 (12.8) | 16.5 (15.8) | 27.0 (7.7) | 22.6 (12.2) |
| SF-12 | ||||
| PCS | 33.5 (5.8) | 34.5 (5.8) | 32.7 (6.0) | 33.1 (6.7) |
| MCS | 35.7 (11.2) | 34.7 (14.7) | 43.4 (10.0) | 30.4 (4.6) |
| HADS | ||||
| Anxiety | 10.1 (4.0) | 10.3 (5.2) | 7.5 (3.4) | 12.0 (1.2) |
| Depression | 9.7 (4.1) | 11.0 (4.8) | 6.8 (3.9) | 10.4 (3.4) |
| Pain Catastrophizing Scale | ||||
| Rumination | 12.5 (3.9) | 11.7 (4.9) | 11.5 (4.0) | 14.2 (2.5) |
| Magnification | 7.1 (3.3) | 6.8 (3.3) | 6.3 (4.3) | 8.0 (3.2) |
| Helplessness | 15.7 (4.4) | 16.3 (4.8) | 11.7 (4.4) | 18.0 (4.8) |
| Total | 35.2 (11.1) | 34.8 (11.7) | 29.5 (12.3) | 40.2 (9.0) |
| EQ-5D-5L index | 0.41 (0.30) | 0.43 (0.29) | 0.40 (0.21) | 0.39 (0.35) |
| Expectation of benefit | 3.3 (1.7) | 2.7 (2.4) | 3.5 (0.6) | 3.8 (1.1) |
Given the small number of participants randomised, not unexpectedly there was evidence of imbalance in participant characteristics and patient-reported outcomes between the two groups at baseline (see Table 8).
Primary and secondary outcomes at follow-up
Given the feasibility nature of the study and the small number of participants randomised, the primary and secondary outcomes at 6 weeks’ and 3 and 6 months’ follow-up are presented descriptively, with no inferential between or within comparisons undertaken or reported (Table 9).
| Outcome | Follow-up time point, mean score (SD) | |||||
|---|---|---|---|---|---|---|
| 6 weeks | 3 months | 6 months | ||||
| Sham group (n = 2) | Intervention group (n = 5) | Sham group (n = 3) | Intervention group (n = 4) | Sham group (n = 3) | Intervention group (n = 5) | |
| BPI (0–10) | ||||||
| Worst pain | 5.0 (2.8) | 7.8 (1.9) | 7.3 (3.0) | 7.8 (1.7) | 6.3 (4.7) | 6.0 (3.5) |
| Least pain | 4.0 (1.4) | 5.2 (2.5) | 7.0 (3.6) | 5.3 (2.2) | 5.3 (4.5) | 5.0 (2.8) |
| Average pain | 4.5 (2.1) | 6.2 (2.5) | 6.0 (2.6) | 6.3 (2.5) | 5.3 (4.5) | 5.6 (2.6) |
| Pain now | 5.0 (2.8) | 6.6 (2.1) | 6.3 (3.0) | 5.8 (2.1) | 6.0 (4.6) | 5.2 (3.7) |
| Pain severity | 4.6 (2.3) | 6.5 (2.1) | 6.7 (3.0) | 6.3 (2.1) | 5.8 (4.5) | 5.5 (3.1) |
| General activity | 5.0 (4.2) | 6.2 (2.4) | 6.7 (2.3) | 7.3 (1.9) | 6.0 (5.3) | 5.6 (3.6) |
| Mood | 4.5 (2.1) | 7.2 (1.9) | 7.0 (3.6) | 7.8 (2.1) | 5.3 (5.0) | 5.6 (3.6) |
| Walking ability | 4.0 (1.4) | 7.2 (2.6) | 5.3 (1.5) | 6.0 (2.9) | 4.3 (4.9) | 5.0 (4.6) |
| Normal work | 5.5 (0.7) | 7.2 (2.6) | 6.0 (2.0) | 6.8 (2.8) | 5.0 (5.0) | 6.0 (4.7) |
| Relations | 3.5 (2.1) | 6.2 (3.4) | 5.7 (4.0) | 7.5 (1.9) | 4.0 (5.3) | 5.2 (3.2) |
| Sleep | 6.5 (4.9) | 8.0 (1.9) | 7.0 (2.6) | 6.3 (2.9) | 4.3 (5.1) | 6.0 (4.7) |
| Enjoyment | 4.5 (2.1) | 7.6 (2.3) | 7.7 (2.5) | 7.0 (2.2) | 4.7 (4.7) | 6.2 (3.3) |
| Interference | 4.8 (2.1) | 7.1 (1.9) | 6.5 (2.4) | 6.9 (2.2) | 4.8 (4.9) | 5.7 (3.8) |
| SF-MPQ-2 | ||||||
| Continuous pain | 3.8 (3.2) | 4.9 (3.1) | 6.3 (3.4) | 3.9 (1.4) | 4.1 (3.7) | 3.3 (2.6) |
| Intermittent pain | 4.4 (3.7) | 3.7 (2.5) | 4.9 (3.0) | 3.7 (3.5) | 3.5 (2.9) | 3.6 (3.8) |
| Neuropathic pain | 1.7 (1.8) | 4.0 (3.2) | 2.5 (1.5) | 2.0 (0.9) | 3.6 (3.4) | 3.0 (2.9) |
| Affective descriptors | 4.4 (4.8) | 4.6 (3.0) | 5.6 (4.0) | 5.4 (1.2) | 2.5 (2.5) | 3.7 (2.8) |
| Total | 3.5 (3.3) | 4.2 (2.3) | 4.8 (2.6) | 3.6 (1.5) | 3.5 (2.9) | 3.4 (2.8) |
| Oswestry Low Back Pain Disability Questionnaire | ||||||
| Total | 36.0 (17.0) | 48.4 (20.2) | 56.0 (14.4) | 39.0 (9. 9) | 42.6 (34.0) | 39.9 (26.0) |
| PSEQ | ||||||
| Total | 33.5 (10.6) | 21.2 (15.3) | 27.7 (9.6) | 31.8 (14.1) | 28.3 (21.7) | 33.2 (19.4) |
| SF-12 | ||||||
| PCS | 38.8 (10.3) | 33.7 (8.6) | 38.5 (6.8) | 40.8 (11.0) | 34.4 (12.5) | 39.5 (13.7) |
| MCS | 43.6 (15.4) | 31.3 (7.9) | 35.7 (7.8) | 37.8 (2.6) | 47.2 (22.1) | 38.1 (13.5) |
| HADS | ||||||
| Anxiety | 7.0 (1.4) | 12.8 (4.4) | 8.3 (3.8) | 11.5 (4.6) | 6.7 (5.7) | 10.0 (3.9) |
| Depression | 4.0 (4.3) | 10.8 (6.7) | 8.0 (3.5) | 9.5 (5.5) | 7.7 (8.1) | 8.4 (7.1) |
| Pain Catastrophizing Scale | ||||||
| Rumination | 11.0 (1.4) | 12.4 (4.9) | 15.0 (1.0) | 11.8 (4.8) | 15.8 (3.8) | 14.4 (5.5) |
| Magnification | 8.0 (1.4) | 6.6 (2.9) | 10.3 (0.6) | 5.0 (3.5) | 19.5 (3.5) | 13.8 (5.9) |
| Helplessness | 14.0 (2.8) | 15.0 (7.0) | 13.7 (6.5) | 15.0 (8.9) | 16.7 (6.1) | 15.5 (9.0) |
| Total | 33.0 (5.6) | 34.0 (14.0) | 19.0 (6.6) | 32.7 (17.2) | 16.7 (7.6) | 16.0 (7.4) |
| EQ-5D-5L index | 0.67 (0.30) | 0.43 (0.33) | 0.42 (0.10) | 0.62 (0.28) | 0.60 (0.50) | 0.51 (0.41) |
| Satisfaction | 6.3 (3.8) | 6.6 (0.9) | 7.3 (1.2) | 7.5 (0.6) | 9.7 (0.6) | 6.0 (2.1) |
Adverse events
Three study participants reported adverse events, with two serious events reported by one participant. All three participants reported a flare-up of their LBP, which resolved (Table 10).
| Participant number | Description of adverse event | Relationship to IMP, as judged by the Principal Investigator | Seriousness of the adverse event, as judged by the Principal Investigator | Randomisation group |
|---|---|---|---|---|
| 1 | Flare-up of LBP after the randomised procedure | Expected reaction, related to the IMP | Not serious | Sham |
| 4 | Flare-up of LBP 5 months after the randomised procedure | Expected reaction, related to the IMP | Not serious | Intervention |
| 7 | Urinary incontinence | Unexpected reaction, not related to the IMP | Serious adverse event (required overnight stay in hospital) | Intervention |
| Swelling at site of injections | Expected reaction, related to the procedure but not to the IMP | Serious adverse reaction (required overnight stay in hospital) | ||
| Flare-up of LBP after the randomised procedure | Expected reaction, related to the IMP | Not serious | ||
| Flare-up of LBP 5 months after the randomised procedure | Expected reaction, related to the IMP | Not serious |
Blinding to treatment allocation
To test the fidelity of blinding, participants were asked to guess which allocation group they had been randomised to, prior to being unblinded at the end of the study. Only one out of eight participants who completed the study correctly guessed their allocation group. The blinded outcome assessor correctly guessed the allocation group for four of the nine participants.
Health economics analysis
Given the importance of health economics evidence in informing decision-making, the incorporation of a health economics work package as part of a feasibility study can inform the delivery of a robust trial in the future, evaluating clinical effectiveness and cost-effectiveness. The main objective of the health economics analysis was to assess the feasibility of collecting data and produce an appropriate framework for a future full economic evaluation. This included a description of the main resource and cost drivers during the patient pathway in delivering the intervention and an assessment of collecting resource use information based on a resource use questionnaire devised for the feasibility study. An assessment of the performance of two preference-based health-related quality of life measures – the SF-12 (SF-6D)55 and EQ-5D-5L54 – within the feasibility study was also proposed in the original health economics analysis plan.
We intended to present both of the scores from the full EQ-5D (EuroQol-5 Dimensions) questionnaire, that is, the EQ-5D-5L descriptive system and the EQ-5D VAS, to present a comprehensive picture of patient-reported outcomes. Patients are asked to assess their health state on five dimensions – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – using five levels ranging from ‘no problems’ to ‘severe problems’. A single summary index is obtained by applying a formula that attaches weights to each of the levels in each dimension. This formula is based on the valuation of EQ-5D health states from a general population sample in the UK. 64 The EQ-5D VAS is a thermometer-type vertical 20-cm scale with the end points of best imaginable health state (at the top) and worst imaginable health state (at the bottom) having numerical values of 100 and 0 respectively. This is a self-rated valuation and represents the respondents’ views of their health state and how it has affected their life on the day.
The utilities derived from the EQ-5D-5L and SF-6D were converted into QALYs to present a profile of QALY gains/losses over time.
As this was not a full trial, no inferential statistical comparisons of health-related quality of life or service use between the intervention group and the control group were planned. Instead, the focus was on reporting descriptive findings for the two groups at baseline and at the follow-up points to inform a suitable framework for a future economic evaluation if the findings from the feasibility study could be used to inform a future definitive study.
For the feasibility study, the perspective adopted was that of the UK NHS, focusing on primary and secondary resource use. However, given the impact of back pain on the patient and wider society (e.g. because of reduced/lost work productivity), a preliminary assessment of employment-related outcomes using the SPS 660 was reported as part of the trial outcomes. The economic analysis was conducted using IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA).
Literature review
As part of the assessment of the feasibility of undertaking a future full economic analysis, a structured, rapid review of the literature was undertaken (see Appendix 9). This rapid review identified two partial economic evaluations (costs of FJIs)65,66 and one study that evaluated health-related quality of life. 37 We found limited economic evidence, with no cost-effectiveness studies identified of diagnostic or therapeutic FJIs. In undertaking any future trial, the feasibility of considering longer-term horizons should be fully assessed, for example whether a model-based analysis can be conducted to robustly extrapolate beyond the trial horizon, based on the suitability and quality of external literature sources.
Identification of the main resource and cost drivers associated with the delivery of facet-joint injections
A descriptive profile of resource use and costs was developed in consultation with the trial team to understand the opportunity costs associated with the delivery of the intervention. These were valued in Great British pounds in 2016 prices based on published unit costs or other literature sources. 10,67–69 The resource use reported at each of the trial visits was assessed with regard to whether it represented (1) research costs (which would be identified and excluded from any intervention costs), (2) costs attributed to routine usual NHS care or (3) opportunity costs (e.g. associated with additional staff time in managing a patient following FJIs) as a result of the intervention.
The intervention costs were considered in the following stages:
-
identification and screening of patients suitable for treatment (entry into the trial)
-
delivery of the intervention or sham procedure
-
delivery of the CPP programme
-
follow-up assessments.
The identification and screening of patients occurred during a routine consultant-led outpatient pain clinic appointment. The associated unit cost per patient was recorded as £148.03 for both groups. 67 Delivery of the intervention or sham procedure occurred at a routine day surgery unit at a cost of £691 per patient. The procedure was identified from the NHS national schedule of reference costs for 2015–1668 as an Injection of Therapeutic Substance into Joint for Pain Management (currency code AB19Z).
Delivery of the CPP programme had a mean cost of £2500 per patient. 70 However, as this programme was delivered to both groups, we excluded the CPP programme costs from the final cost analysis.
There were three follow-up assessments. Two assessments/data collection sessions were research-based, nurse-led appointments and were not included in the costs. The 6-month follow-up appointment was conducted by a consultant at an outpatient pain clinic as part of routine aftercare post procedure at a cost of £148.03 per patient for both groups. 69
Health resource use
An assessment of the feasibility of gathering appropriate resource use data from a patient sample was a major component of this evaluation. The resource use measure was developed by the clinical team based on standard clinical practice for the management of LBP and included questions on primary and secondary care resource use (e.g. hospital admissions, outpatient visits, GP surgery visits and medication use).
Feasibility of collecting resource use data
The resource use questionnaire was completed by the trial research assistant during scheduled visits to a nurse-led outpatient pain management clinic and took approximately 60 minutes per visit to complete. Although these are ‘research’ costs, the cumulative impact of collecting data will add to the costing of the time/resources for any future trial.
The focus was on collecting information on consultations with the NHS health-care professionals who would most likely be involved in the management of LBP. All costs were valued in Great British pounds using a price year of 2016. Table 23 (see Appendix 9) summarises this resource use. As the trial period was < 12 months, no discounting was applied to costs or outcomes. An additional question allowed patients to report any other resources used outside of the main categories; however, no other reported resource items were recorded.
Data on hospital contacts collected via the resource use questionnaire was compared with the data collected on adverse events and serious adverse events to ensure that there was no overlapping of data collected and, therefore, no double-counting of resource use. Prior to the analysis, appropriate decision rules were put in place for the costing of hospital admissions. If a patient was reported as having been admitted to hospital during the past 4 weeks, as a result of a serious adverse event, this was classified as an emergency hospital admission (non-elective inpatient stay). If (in an expected minority of patient cases) a hospital admission was for a condition unrelated to LBP, the reason for the unrelated hospital admission was recorded but the hospital admission was not included in the analysis.
The findings from the trial identified neither serious adverse events resulting in a hospital admission nor any other hospital admissions during the trial period. When documented adverse events were examined (n = 3 participants accounting for six health-care contacts), no additional impact on resource use was identified as one of the following: (1) already documented within the resource use questionnaire; (2) no further action or impact on resource use was documented; or (3) the adverse event was unrelated to the intervention. In two cases an adverse event was reported by the patient but no further information was available.
The resource use questionnaire collected information on current prescribed analgesics and any other medications at baseline. At the three follow-up time points, information on current analgesics and other medications was also collected along with any changes in medication during the follow-up period. The specific dates that prescriptions were issued, including the dates that any changes in medication occurred, were unknown because medications were prescribed by the patients’ GPs rather than by the trial team. Medication reported in the ‘other’ medication category that was not related to the treatment of LBP was excluded following examination of the data by the trial Chief Investigator. The costs of medication were then compiled for each time point and summated to give a total medication cost per group.
Resource use and costs
Table 11 summarises the NHS resource use for participants from baseline to 6 months’ follow-up. Overall, the total number of primary care visits was the same across the groups over the time period of the trial (seven GP visits recorded with a total cost per group of £252). 67 No emergency department admissions or hospital inpatient admissions (either elective or emergency) were recorded in either group. One outpatient appointment was recorded in the intervention group (received FJIs) at a cost of £148. 68
| Consultations for LBP | Visits | Total costs (aggregate of all follow-up time points) (£) | ||
|---|---|---|---|---|
| Sham group (n = 4) | Intervention group (n = 5) | Sham group (n = 4) | Intervention group (n = 5) | |
| Total GP visits | ||||
| Number of visits/cost | 7 | 7 | 252.00 | 252.00 |
| Mean (SD) | 1.75 (1.71) | 1.4 (2.19) | 63.00 (61.48) | 50.40 (78.87) |
| Min., max. | 0, 4 | 0, 5 | 0, 44.00 | 0, 180.00 |
| Total outpatient pain clinic appointments | ||||
| Number of visits/cost | 0 | 1 | 0 | 148.03 |
| Mean (SD) | ||||
| Min., max. | ||||
| Total hospital emergency inpatient admissions | ||||
| Number of visits/cost | 0 | 0 | 0 | 0 |
| Mean (SD) | ||||
| Min., max. | ||||
| Total emergency department admissions | ||||
| Number of visits/cost | 0 | 0 | 0 | 0 |
| Mean (SD) | ||||
| Min., max. | ||||
| Total medication costs | ||||
| Total cost | 48.83 | 562.51 | ||
| Mean (SD) | 12.21 (11.31) | 112.50 (81.25) | ||
| Min., max. | 1.21, 29.63 | 29.63, 231.76 | ||
The main numerical differences between the two groups occurred for medication use, with a total cost of £48.83 (mean cost £12.21 per participant; SD £11.31) in the sham group and £562.51 (mean cost £112.50 per participant; SD £81.25) in the intervention group, a mean difference of £100 per participant. Further details of the medication use over each time point are provided in Table 12.
| Follow-up time period | Randomisation group | |
|---|---|---|
| Sham (n = 4) (£) | Intervention (n = 5) (£) | |
| From baseline to 6 weeks’ follow-up (visit 4) | ||
| Total | 32.05 | 35.75 |
| Mean (SD) | 8.01 (12.04) | 7.15 (12.68) |
| Min., max. | 0, 25.78 | 0, 29.27 |
| From 6 weeks’ follow-up to 3 months’ follow-up (visit 5) | ||
| Total | 4.25 | 129.63 |
| Mean (SD) | 1.06 (2.13) | 25.93 (30.94) |
| Min., max. | 0, 4.25 | 0, 77.95 |
| From 3 months’ follow-up to 6 months’ follow-up (visit 6) | ||
| Total | 12.53 | 397.13 |
| Mean (SD) | 3.12 (3.82) | 79.43 (58.95) |
| Min., max. | 0, 7.75 | 23.25, 156.12 |
| Total medication costs after treatment | ||
| Total | 48.83 | 562.51 |
| Mean (SD) | 12.21 (11.31) | 112.50 (81.25) |
| Min., max. | 1.21, 29.63 | 29.63, 231.76 |
| Difference between groups | ||
| Total cost difference | 513.68 | |
| Mean cost difference | 100.29 | |
Although there are limitations of the analysis associated with the highly skewed costs and small sample size, this suggests that a potential downstream effect of FJI is a subsequent increase in medication use and associated costs within primary care.
Table 13 provides an estimation of the total mean costs of the FJI intervention and the sham procedure. This shows that the FJI intervention was assessed as costing £118 more per patient than the sham procedure.
| Mean cost | Randomisation group, mean (SD) | Mean cost difference | |
|---|---|---|---|
| Sham (n = 4) | Intervention (n = 5) | ||
| Total cost (£) | 75 (73) | 193 (219) | The intervention is £118 more expensive per patient than the sham procedure |
Outcomes
At baseline all nine patients completed the EQ-5D assessment. However, at the follow-up data collection points only six participants (two in the sham group and four in the intervention group) returned complete assessments across all three time points.
The baseline mean EQ-5D-5L and SF-6D utilities and EQ-5D VAS scores are presented in Tables 14 and 15, respectively, which compare the values for those patients who completed the assessments (complete cases) with the values for those who withdrew after completing the baseline assessments. As a point of reference, utilities from a UK population with LBP are presented. 71
| Outcome | Randomisation group | Group lost to follow-up | Utilities by disease (lower back pain)a | |
|---|---|---|---|---|
| Sham | Intervention | |||
| EQ-5D-5L utility score | ||||
| n | 4 | 5 | 5 | 265 |
| Mean (SD) | 0.402 (0.209) | 0.387 (0.355) | 0.429 (0.362) | 0.635 (0.266) |
| Min. | 0.147 | –0.068 | –0.0004 | –0.181 |
| Max. | 0.619 | 0.816 | 0.802 | 1.000 |
| SF-6D utility score | ||||
| n | 4 | 4 | 5 | 263 |
| Mean (SD) | 0.509 (0.068) | 0.532 (0.073) | 0.523 (0.064) | 0.658 (0.144) |
| Min. | 0.450 | 0.450 | 0.410 | 0.370 |
| Max. | 0.590 | 0.620 | 0.570 | 1.000 |
| EQ-5D VAS scorea | Randomisation group | Group lost to follow-up (n = 6) | |
|---|---|---|---|
| Sham (n = 4) | Intervention (n = 5) | ||
| Mean (SD) | 58.75 (23.94) | 45.60 (9.32) | 47.67 (22.46) |
| Min. | 25.0 | 38.0 | 20.0 |
| Max. | 80.0 | 60.0 | 80.0 |
At baseline, there were numerical differences in baseline EQ-5D-5L utilities, with those lost to follow-up having very slightly numerically higher utilities than those in the sham and intervention groups (0.43 vs. 0.40 vs. 0.39). The minimum and maximum values indicate that there is potentially a wide range of scores and the potential for heterogeneity within the patient sample. Although appropriate caution must be exercised in the interpretation of these baseline scores, further examination of baseline differences in utilities should be examined in any future trial. Brazier et al. 71 reported that, on average, the SF-6D generates utility values that are higher than those generated using the EQ-5D-5L and this is also reflected in this trial sample. The SF-6D results show that those in the intervention group have a marginally higher utility score than those who withdrew after the baseline assessment and those who received the sham procedure (0.53 vs. 0.52 vs. 0.51).
The EQ-5D VAS results showed a different picture, with VAS scores ranging from 45.60 in the intervention group to 47.67 in the group who withdrew after completing the baseline assessment and 58.75 in the sham group, again showing the potential for heterogeneity in the patient sample.
Stanford Presenteeism Scale 6 scores at baseline
Scores on the SPS 6 range from 6 to 30, with lower scores indicating lower presenteeism and higher scores indicating higher presenteeism. 60 Higher presenteeism scores are associated with decreased productivity and work quality. At baseline the sham group and the group lost to follow-up reported slightly higher scores of 15.8 and 15.2, respectively, with the intervention group having the lowest mean score of 14.4, as shown in Table 16.
| SPS 6 score | Randomisation group | Group lost to follow-up (n = 5)a | |
|---|---|---|---|
| Sham (n = 4) | Intervention (n = 5) | ||
| Mean (SD) | 15.8 (3.8) | 14.4 (5.5) | 15.2 (3.1) |
| Min. | 11.0 | 6.0 | 11.0 |
| Max. | 20.0 | 20.0 | 18.0 |
Stanford Presenteeism Scale 6 scores at the follow-up assessments
After treatment, at the follow-up assessments, the sham group still had slightly higher presenteeism scores than those who received the intervention (Table 17). Although the intervention group showed a reduction in presenteeism score at the 6-week time point, both groups tended to report higher presenteeism scores after treatment than at baseline.
| SPS 6 score | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 weeks (visit 4) | 3 months (visit 5) | 6 months (visit 6) | ||||
| Sham group (n = 2) | Intervention group (n = 5) | Sham group (n = 3) | Intervention group (n = 4) | Sham group (n = 3) | Intervention group (n = 5) | |
| Mean (SD) | 19.5 (3.5) | 13.8 (5.9) | 16.7 (6.1) | 15.5 (9.0) | 16.7 (7.6) | 16.0 (7.4) |
| Min. | 17.0 | 7.0 | 10.0 | 7.0 | 8.0 | 6.0 |
| Max. | 22.0 | 22.0 | 22.0 | 26.0 | 22 | 24.0 |
Comparison of utilities across assessment points
Table 18 summarises the utilities across the groups at the three follow-up points to 6 months using the EQ-5D-5L and SF-6D. It is important to acknowledge the impact of missing data, with two participants in the sham group missing data at the 6-week assessment, one participant from each group missing data at the 3-month assessment and one participant from the sham group missing data at the 6-month assessment. Although the small sample size precluded any formal assessment of missing data, this highlights the importance of ensuring that outcomes are as complete as possible over the follow-up period and, when necessary, that there are appropriate decision rules to handle missing data (e.g. suitable imputation methods) for formal economic analysis in any future trial.
| Outcome | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 weeks (visit 4) | 3 months (visit 5) | 6 months (visit 6) | ||||
| Sham group (n = 2) | Intervention group (n = 5) | Sham group (n = 3) | Intervention group (n = 4) | Sham group (n = 3) | Intervention group (n = 5) | |
| EQ-5D-5L utility score | ||||||
| Mean (SD) | 0.675 (0.118) | 0.433 (0.335) | 0.418 (0.098) | 0.617 (0.279) | 0.597 (0.499) | 0.520 (0.413) |
| Min. | 0.592 | 0.013 | 0.352 | 0.204 | 0.021 | 0.018 |
| Max. | 0.758 | 0.762 | 0.531 | 0.802 | 0.893 | 0.841 |
| SF-6D utility score | ||||||
| Mean (SD) | 0.708 (0.244) | 0.452 (0.080) | 0.557 (0.096) | 0.561 (0.160) | 0.622 (0.249) | 0.615 (0.149) |
| Min. | 0.540 | 0.380 | 0.470 | 0.410 | 0.370 | 0.450 |
| Max. | 0.880 | 0.570 | 0.660 | 0.730 | 0.860 | 0.820 |
For the EQ-5D-5L, the sham group showed an increase in utility from 0.40 at baseline to 0.68 at 6 weeks. This declined at 3 months to 0.42 before increasing to 0.60 at 6 months, indicating small changes during the treatment and follow-up period. In the intervention group, the baseline score of 0.39 increased slightly to 0.43 at 6 weeks and 0.62 at 3 months and then declined to 0.52 at 6 months. Although no quantitative assessment (given the limitations of the data) can produce a reliable numerical magnitude of effect, the direction of travel suggests that, overall, there are likely to be small effects on health-related quality of life. The SF-6D scores were, in the main, higher than the EQ-5D-5L scores. At 6 weeks’ follow-up the sham group reported a considerably higher score than the intervention group (0.71 vs. 0.45). However, at 3 months’ and 6 months’ follow-up both groups reported the same utility scores: 0.56 at 3 months increasing to 0.62 by the end of the trial.
The mean EQ-5D VAS scores at follow-up are presented in Table 19. A similar pattern of missing data was seen. Overall, there were numerical differences between each time point in both groups, with the sham group showing a very slight improvement in VAS score from baseline to 6 weeks (58.75 vs. 60.00), a reduction in VAS score between 6 weeks and 3 months (60.0 vs. 43.33) and an improvement in VAS score from 3 months to 6 months (43.33 vs. 53.33). In contrast, the intervention group showed a decline from baseline to 6 weeks (45.60 vs. 38.33), an improvement between 6 weeks and 3 months (38.33 vs. 53.33) and a decline from 3 months to 6 months (53.33 vs. 45.00).
| EQ-5D VAS score | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 weeks (visit 4) | 3 months (visit 5) | 6 months (visit 6) | ||||
| Sham group (n = 2) | Intervention group (n = 3) | Sham group (n = 3) | Intervention group (n = 3) | Sham group (n = 3) | Intervention group (n = 5) | |
| Mean (SD) | 60.00 (28.28) | 38.33 (10.41) | 43.33 (25.17) | 53.33 (25.17) | 53.33 (47.26) | 45.00 (29.15) |
| Min. | 40 | 30 | 20 | 30 | 0 | 10 |
| Max. | 80 | 50 | 70 | 80 | 90 | 80 |
Changes in quality-adjusted life-years
Given the potential inclusion of a cost–utility analysis within a future economic analysis, the utilities derived from the EQ-5D-5L and SF-6D were translated into QALYs using the methodology outlined in Appendix 9. Table 20 summarises the QALYs at each time point by group. With respect to the EQ-5D-5L, from baseline to 6 months the mean (SD) QALY gain was 0.31 (0.32) in the sham group and 0.28 (0.15) in the intervention group. The SF-6D produced slightly higher QALY gains at 6 months, with the sham group reporting a mean (SD) gain of 0.32 (0.076) and the intervention group reporting a mean (SD) gain of 0.30 (0.044).
| Outcome | Time point | Total QALY gain | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 weeks (baseline to visit 4) | 3 months (visit 4 to visit 5) | 6 months (visit 5 to visit 6) | ||||||
| Sham group | Intervention group | Sham group | Intervention group | Sham group | Intervention group | Sham group | Intervention group | |
| EQ-5D-5L-derived QALY gain | ||||||||
| n | 2 | 4 | 2 | 4 | 2 | 4 | 2 | 4 |
| Mean (SD) | 0.075 (0.003) | 0.059 (0.038) | 0.068 (0.014) | 0.069 (0.032) | 0.167 (0.015) | 0.155 (0.085) | 0.309 (0.320) | 0.283 (0.152) |
| Min. | 0.073 | 0.007 | 0.058 | 0.023 | 0.156 | 0.028 | 0.290 | 0.060 |
| Max. | 0.076 | 0.094 | 0.077 | 0.094 | 0.178 | 0.200 | 0.330 | 0.370 |
| SF-6D-derived QALY gain | ||||||||
| n | 2 | 3a | 2 | 3a | 2 | 3a | 2 | 3a |
| Mean (SD) | 0.076 (0.017) | 0.062 (0.007) | 0.077 (0.023) | 0.064 (0.014) | 0.165 (0.036) | 0.151 (0.037) | 0.318 (0.076) | 0.300 (0.044) |
| Min. | 0.064 | 0.055 | 0.060 | 0.047 | 0.139 | 0.107 | 0.260 | 0.250 |
| Max. | 0.089 | 0.071 | 0.093 | 0.078 | 0.191 | 0.185 | 0.370 | 0.330 |
In the health economics analysis plan, the original intention was to compare the psychometric properties of the EQ-5D-5L with those of the SF-6D using a similar set of analyses as reported by Mulhern et al. ,72 to inform the potential choice of preference-based measure of health-related quality of life in any future trial. However, given the limitations presented by the small sample size (and within this the further impact of missing data), this analysis could not be undertaken given that it did not meet any of the accepted criteria in terms of a sufficient sample size to conduct appropriate tests associated with assessing the reliability and validity of patient-reported outcomes. 73
Health economics summary
The health economics results are purely descriptive and with such a small sample size we are appropriately cautious about drawing any conclusions. The incorporation of an early-stage assessment of the requirements and parameters for an economic analysis in a future trial has allowed a provisional assessment of these, with evidence from the feasibility study providing a useful basis for the development of a suitable framework. Because of the constraints presented, in-depth examination was not undertaken and the provisional assessments (e.g. early assessment of the intervention costs associated with FJIs) must be interpreted with caution. A key learning point was the need for health economics analysis to be embedded into the feasibility study to ensure that important insights and lessons can be used to develop the framework for an economic analysis within a future trial. There are still questions to address but, even with the basic assessment carried out during this feasibility study, we have a good basis on which to take this development forward.
It seems clear that the study population are ‘users’ of health services, although there was little impact of the intervention on secondary care, with the main drivers of subsequent health resource use being associated with medications within primary care. Although the current resource use measure appears to capture relevant cost drivers, further work on developing a more precise measure (such as utilising routine secondary care data) to capture all relevant costs should be undertaken. Another possible area for consideration is the potential for imbalances between the groups at baseline. In a full randomised controlled trial, adjustment for baseline outcomes and other factors may be required. Although the feasibility study was designed to capture a UK NHS perspective only, the potential impact on patients, families and the wider society should be carefully considered in any future trial to capture the full picture.
The EQ-5D-5L and SF-6D data reflect the impact on health-related quality of life that the study participants experienced. Although formal conclusions cannot be drawn on the merits of either measure, the consensus from the team in light of this provisional investigation is that the EQ-5D-5L is likely to be the preferred measure, given its use in previous studies of back pain and its recommendation by UK decision-making bodies such as NICE74,75 as the primary method of deriving QALYs. Further exploration of the findings from the feasibility study compared with the findings of other studies using these measure should be undertaken to fully rationalise the selection of appropriate outcomes to inform a future economic evaluation.
Study timelines
The start date of the study was delayed by 18 months (546 days), with 244 days taken to obtain NHS permission to recruit after obtaining a favourable research ethics opinion (Figures 4 and 5). A no-cost negotiated 1-month extension period was granted by the funder to allow for collection of follow-up data and delivery of the final report 45 days after collection of the final outcome data.
FIGURE 4.
Study timelines and milestones. REC, Research Ethics Committee.
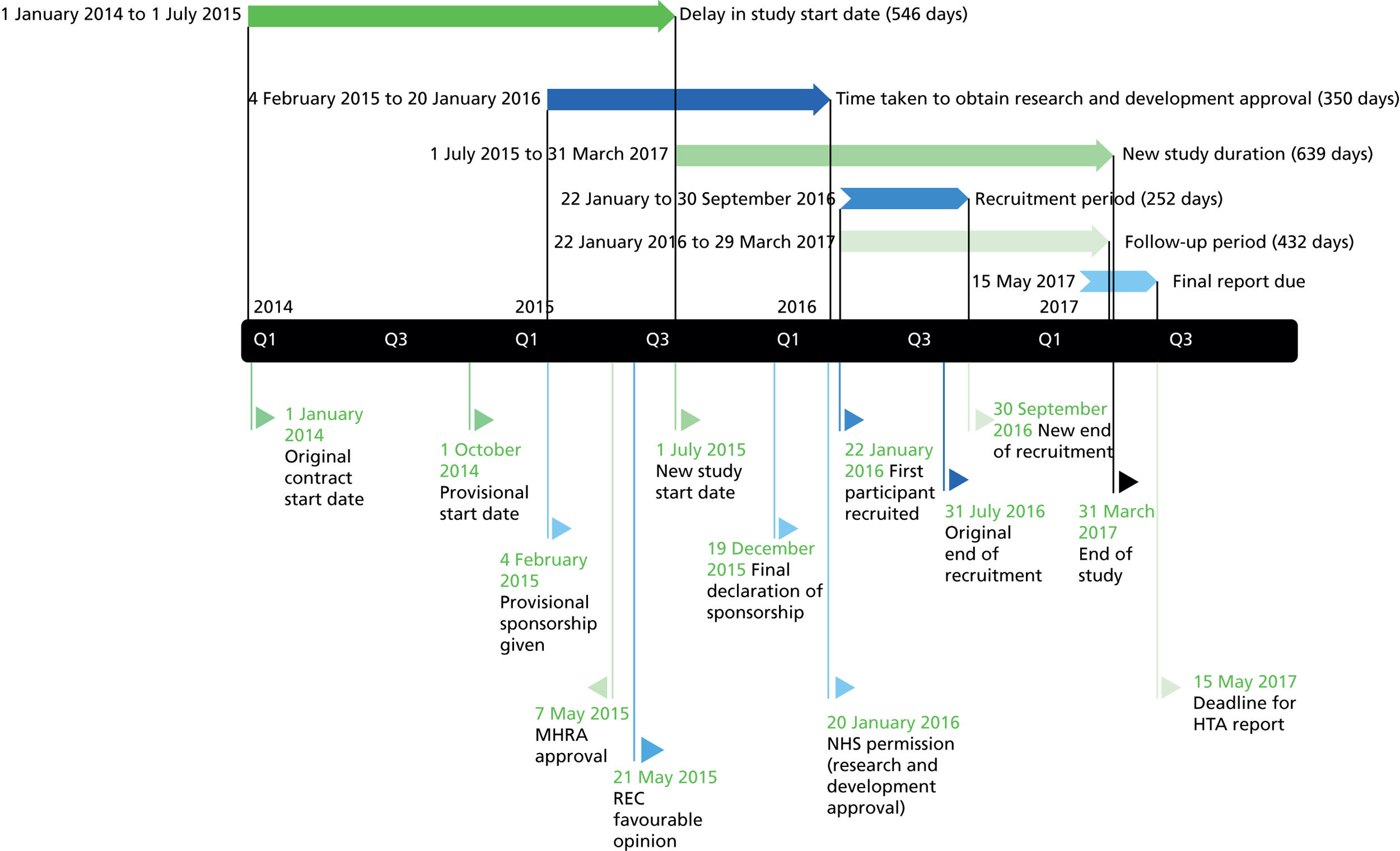
FIGURE 5.
Study Gantt chart.
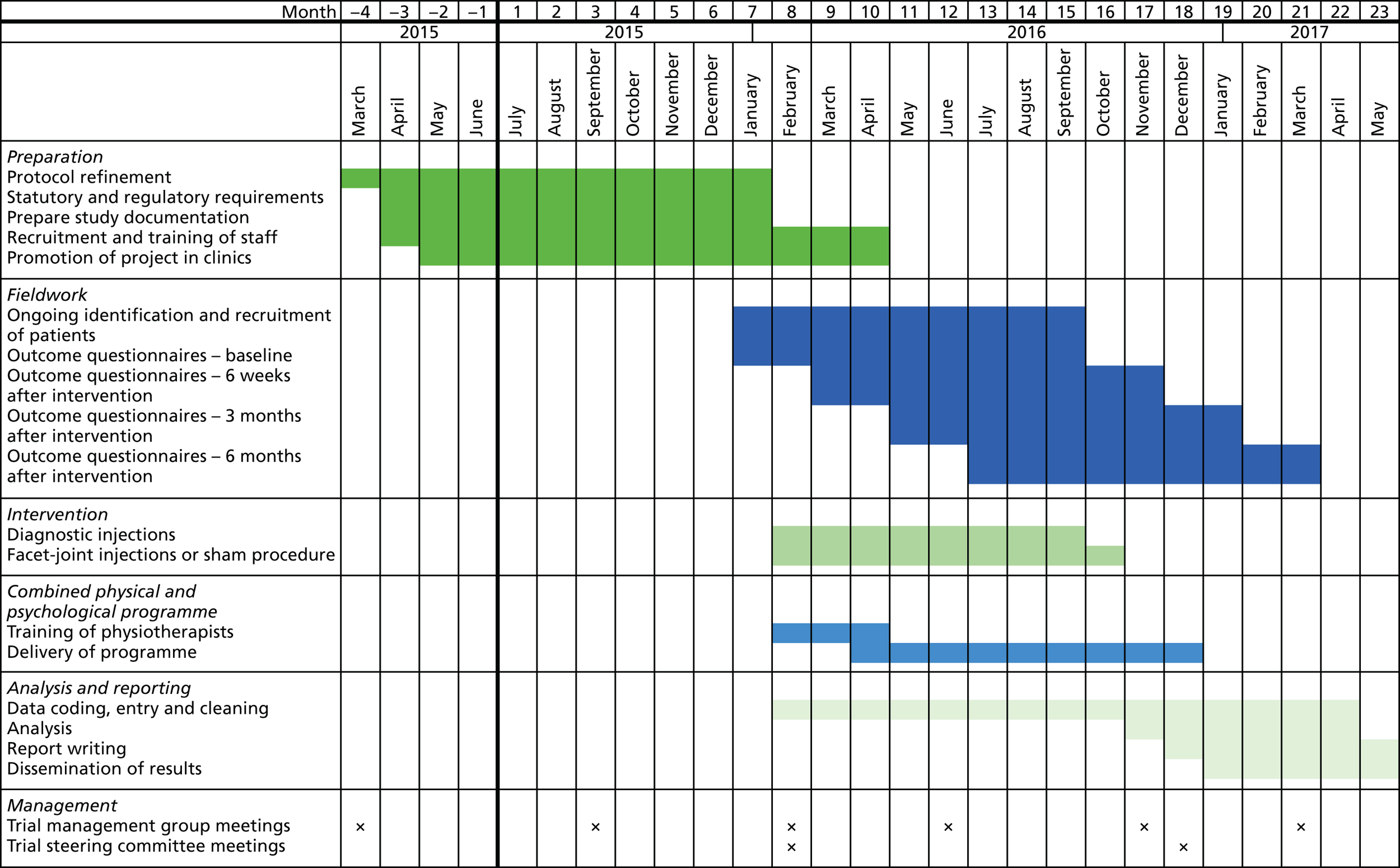
Chapter 4 Discussion
Summary of findings
The overarching aim of the FACET feasibility study was to assess the feasibility of conducting a definitive study to evaluate the clinical effectiveness and cost-effectiveness of FJIs compared with a sham procedure in patients with non-specific LBP of > 3 months’ duration.
We were unable to meet our feasibility objective of a recruitment target of 60 randomised LBP patients across three investigative sites. Instead, we were able to randomise only nine patients in one investigative site. However, we believe that we met our other pre-stated feasibility objectives within the constraints of this being a single-centre study with a small sample size: (1) we were able to select patients into the study using a joint clinical and diagnostic approach; (2) we have demonstrated successful standardisation of the methods of FJI and the sham procedure, using the Delphi method to generate consensus among interventional pain specialists in the UK; (3) we were able to carry out the sham procedure (periarticular injections with saline) in participants randomised to the control group; (4) the sham control study design was accepted by the clinicians involved in the study and was also deemed acceptable by patients; and (5) we were able to retain patients over the 6 months of follow-up.
The small number of participants recruited to the study and the feasibility design preclude us from drawing any conclusions on the clinical effectiveness or cost-effectiveness of intra-articular lumbar FJIs in the management of non-specific LBP. Additionally, although eight out of the nine patients randomised completed the study, the small sample size precludes any definitive conclusions being made about patient retention. However, the clinical procedures appeared to be safe and well tolerated, with no significant adverse events related to the steroid injection. Furthermore, we were able to successfully collect clinical and economic outcomes from the majority of the patients over the duration of the study.
Numerical differences between the groups were identified in the feasibility study (particularly with regard to increased medication costs in the intervention group) that warrant further attention, particularly given the inherent skewness in the data and the very small sample size.
The remainder of this chapter considers in more detail the findings of the feasibility study in terms of the detailed objectives, reviews the practical problems that were experienced and identifies the key learning outcomes for any definitive future trial in this area.
To assess the eligibility criteria and recruitment and retention of patients in the two treatment arms
Recruitment took place at only a single centre, largely because of the delay in study set-up and the decision by the funder to terminate the study early because of the lack of time available to open the other two sites. A number of reasons for this delay in set-up and time to first patient recruitment have been identified, including regulatory issues, staffing problems, specific recruitment challenges, factors affecting clinician and patient participation and the study population itself.
An unexpectedly high number of patients needed to be screened to identify eligible patients. The actual participant screening-to-recruitment ratio was 70 : 1 (628 : 9), which contrasts with the expected prestudy ratio of 17 : 1 (1000 : 60). The screening criteria and recruitment rates were discussed at each TMG meeting and it was perceived by some members of the group that the inclusion and exclusion criteria may have excluded potentially eligible patients, such as those aged > 70 years who would otherwise have been suitable.
Over the period of the trial, five participants (31%) dropped out of the study after giving consent but before receiving the diagnostic test (see Figure 3). This compared with our expected level of attrition. We were able to collect study data from participants at baseline and at the various follow-up points. The level of outcome completion varied across outcomes, with 11 out of 16 participants who completed the baseline questionnaire returning incomplete data sets, ranging from entire questionnaire visits being omitted to one section of a single questionnaire being missing or spoiled (illegible). Missing and incomplete outcome data are detailed further in Appendix 11. Manual double data entry, considered to be the gold standard for data transfer,76 was used by two independent researchers and ensured the high quality of data entry onto the electronic database.
An apparently high level of participant retention was seen in this trial, with an average attrition rate of 11%, that is, only one patient out of nine randomised failed to complete the final set of questionnaires at 6 months. This compares favourably with the expected attrition rate of 20% defined in the protocol. However, given the small sample size this estimate of attrition was very imprecise, with a 95% CI of 0.2% to 48%.
To assess the feasibility and acceptability of the two treatment arms from the point of view of patients and their pain teams
A Delphi exercise was undertaken with interventional pain physicians in the UK to agree on the methods for FJI and the sham procedure (see Appendix 6). The two treatments arms (FJIs and the sham procedure) can therefore be considered to be feasible and acceptable by pain clinicians. Furthermore, all six pain consultants at Barts Health NHS Trust screened and recruited participants to the study. Strong support was also given from the surgeons and research physiotherapists involved in screening patients from the spinal orthopaedic clinics at the centres involved in this study.
Of the 34 patients who met the eligibility criteria but who declined to take part in the study, none cited the study design as the reason for not wishing to take part.
To assess the feasibility of the proposed definitive study design
We believe that this study did allow us to demonstrate the feasibility of the study design to inform a definitive study. We were able to develop an appropriate active injection technique (intra-articular lumbar FJI) and sham procedure. No patients refused participation on the grounds of randomisation to an active or a sham procedure. We were able to maintain blinding of patients as only one of the eight participants who completed the study correctly guessed their allocation group. The outcome assessors correctly guessed four out of nine allocation groups over the period of the trial.
A key uncertainty going into this study was the feasibility of delivering a usual care CPP programme to both study arms. The 2009 NICE guidelines for the early management of persistent non-specific LBP (CG88)7 prescribed around 100 hours of a CPP programme over a maximum of 8 weeks for those who have already received at least one less-intensive treatment such as an exercise programme, manual therapy or acupuncture and who have ‘high disability and/or significant psychological distress’. 7 However, following careful discussion by the TMG and a review of the current evidence we instead decided to base delivery of our CPP programme in this trial on the BeST trial,44 which recommended six sessions of group-based therapy (up to 10 participants) of 90 minutes each. The study’s lead physiotherapist (SP) trained all of the study physiotherapists across the three sites to deliver the CPP programme and all received a certificate of training.
Three CPP programmes were consistently delivered at Barts Health NHS Trust, albeit to small groups of participants (one to three participants per group); the BeST trial found that a group size of six to 10 participants was clinically effective and cost-effective and popular in clinical practice in that it allows for group discussion and problem-solving. 44 Six out of nine (67%) participants successfully completed the CPP programme, defined in the protocol as having attended at least four out of the six sessions. The other three participants failed to meet this protocol definition of CPP programme attendance because of extenuating circumstances (illness, undisclosed personal reasons and travel abroad).
Since our original trial proposal, the NICE guidelines (CG88)7 on the management of LBP have been updated. The current 2016 NG5910 draws on evidence from the BeST trial44 and no longer specifies the length or duration of the CPP programme, instead stating:
Consider a combined physical and psychological programme, incorporating a cognitive behavioural approach (preferably in a group context that takes into account a person’s specific needs and capabilities), for people with persistent LBP or sciatica:
when they have significant psychosocial obstacles to recovery (for example, avoiding normal activities based on inappropriate beliefs about their condition) or
when previous treatments have not been effective.
Thus, we believe that CPP programme delivery in our trial was well aligned with the latest NICE guidance.
To estimate outcome standard deviations to inform the power calculation for a definitive study
We have reported the SDs for all proposed clinical and economic outcomes at baseline and follow-up. However, given the small final sample size realised we express caution in using these SDs to inform the sample size calculation for a future definitive study. Following their simulation analysis, Teare et al. 77 recommended that an external pilot study has at least 70 measured subjects (35 per group) when estimating the SD for a continuous outcome. Similarly, the sample size in this study was also inadequate to provide a sufficiently precise estimate of attrition. Probably the only parameter for a future definitive study that this study was able to estimate with precision was the screening/recruitment rate.
To finalise the protocol design, statistical plan, number of centres required and study duration of the definitive study
Given the failure to meet the study recruitment target and the small number of patients recruited from one centre, the study team deemed it inappropriate to present a finalised protocol for a definitive study on the basis of this feasibility study.
Issues encountered and how they were resolved
This feasibility study encountered system-level barriers but no significant delays in obtaining research ethics or MHRA approval.
Regulatory issues
The projected recruitment period for this feasibility study occurred during a time of change within research governance, which in turn led to significant delays in regulatory approvals being granted (see Figure 4). Delays were experienced in obtaining HRA approval and system errors led to further delays in obtaining NIHR portfolio adoption and NHS permission. The large numbers of collaborating centres involved led to delays in contracts being signed. The study team also noted the length of time taken for any issues to be addressed at a local level.
Staffing issues
The changes in the study timeline meant that key members of the co-applicant team were unable to commit to the study: the Chief Investigator (Richard Langford) and lead physiotherapist (Paul Watson) retired from clinical practice, the trial co-ordinator was replaced and others had conflicting interests and priorities. A high staff turnover rate within the local research governance team, including several changes of trial pharmacist and a change of trial monitor, resulted in some duplication of work, in part because of inadequate handovers. Substantial amendments to the protocol were made to reflect staff turnover and also on the request of the new trial monitor, resulting in further recruitment delays.
Recruitment challenges
The low recruitment rate from the pain clinics alone led to protocol amendments to allow for screening at and recruitment from physiotherapy-based musculoskeletal clinics and spinal orthopaedic clinics (see Appendix 10). A recruitment drive was put into place within the last 2 months of the recruitment phase, led by a newly appointed trial mentor, using strategies to increase the number of patients eligible for screening within the limitations of the study protocol. A no-cost recruitment extension period was also granted by the funder.
However, it can be seen in Appendix 12 that, although the number of patients screened increased as a result, the number of participants recruited did not. A future multicentre trial may wish to consider the early addition of a recruitment co-ordinator to the core study team, to closely monitor the recruitment rate at each site and implement a priori contingency plans with the sites’ Principal Investigators should the recruitment rate fall below the predicted rate. Based on our experience, we would advise the appointment of a study mentor (who is an experienced triallist) to review the recruitment rate at each site and to implement strategies to improve this. This may include the appointment of additional research assistants to screen for suitable patients from a wider range of clinics (e.g. associated community pain clinics and spinal orthopaedic clinics) and from GP referral letters. Protocol amendments were made to reflect these changes (see Appendix 10). McDonald et al. 78 demonstrated that recruitment strategies can change as a result of pilot or feasibility phases of a study.
We conclude, however, that the target population for the study was not always being referred to the hospital pain and spinal orthopaedic clinics. We were unable to explore this further in the feasibility study but would propose this as an area for future exploration in terms of the definitive trial design. The recruitment estimates used for the feasibility study were based on the clinical experience of the investigators and a retrospective audit of procedures carried out at each of the three centres. It was initially believed that five patients would be recruited from the pain clinics at Barts Health NHS Trust each week, with three patients recruited each from Basildon and Thurrock University Hospitals NHS Foundation Trust in Basildon and the Walton Centre NHS Foundation Trust in Liverpool. A comparison of the actual with the expected flow of participants through the study is illustrated in Appendix 13. Although the actual number of participants recruited in the study was lower than expected, it is hoped that this work will guide future research in this area by providing more realistic and up-to-date data and by highlighting the areas in the recruitment pathway that pose the greatest challenges to recruitment.
Clinician and patient participation
In their systematic review, Prescott et al. 79 identified a number of factors that may affect clinician participation in a study. These include time constraints and a lack of staff and training. Clinicians may also have competing priorities during the study recruitment period, for example from involvement in other trials. This feasibility study recruited from a busy NHS trust in which clinicians have time pressures from usual clinical practice in the pain clinics as well as ongoing management duties. All of the study interventions and pain consultant clinic appointments were scheduled alongside routine NHS appointments.
At the time of writing, the pain clinics at St Bartholomew’s Hospital at Barts Health NHS Trust accept referrals via the NHS e-Referral Service (formerly Choose and Book) from a wide catchment area across the country in addition to its local area in East London. Travel and travel costs can therefore be substantial for some patients, some of whom have limited mobility because of their pain condition and may even require hospital transport. Travel costs and the time taken to attend for appointments may therefore be a factor contributing towards non-participation. Study participants may also have other commitments that preclude their attendance at study visits, such as full- or part-time employment or caring for young children.
Five out of sixteen patients who consented to take part in the study dropped out before treatment for reasons that remain speculative. Of the nine patients who took part, six completed at least four sessions of the CPP programme. It may be possible to improve participant retention in a definitive trial by including more detailed information on the CPP programme in the participant information sheet and by involving the public in its preparation to make the information easier to understand.
Study population
The study population presenting to pain clinics at Barts Health NHS Trust was noted to have complex pain needs, often with co-existing radicular pain or chronic widespread pain conditions, in addition to suffering from psychological distress. Although approximately 1050 new patients are referred and seen annually in the pain clinics at St Bartholomew’s Hospital, it was clear that many of these ‘new’ patients were not new to the pain services, having had previous spinal injections or surgery, for example. None of the patients screened in the spinal orthopaedic clinics were eligible to take part in the study for these reasons and many of the referrals also had radicular pain, indicative of a lumbar disc pathology.
No patients were recruited from the Tower Hamlets Persistent Pain Services at Mile End Hospital. The London Borough of Tower Hamlets has a diverse ethnic population and experiences widespread deprivation and low average health deprivation scores, with a high rate of population mobility. 80 Although an inability to speak English was not an exclusion criterion for the study, translation and patient advocacy services for non-NHS study-related visits were not funded for. Language barriers have been highlighted by previous researchers as being a negative influence on recruitment, with altruism noted to be an important factor in deciding to take part. 81 We believe that this could be an area for further investigation in a future trial, to make research more accessible to diverse local populations.
The highest participant screening-to-recruitment ratio was seen in the community pain clinic at Essex Lodge GP surgery, which has strong, established links to primary care and is located at the same site as the GP surgery. Patients were thought to be referred to this pain service by the local GPs earlier in their pain trajectory than in other pain clinics in this study. This feasibility study has demonstrated the need for more primary care involvement at all stages of the study, from protocol design and patient screening and recruitment to dissemination of the results. A need for improved referral pathways between the pain services and some primary care providers has been identified.
Strengths and limitations
Many of the key uncertainties involved in undertaking a definitive study were addressed in this feasibility study.
Major strengths of the study were the Delphi exercise, which was undertaken to obtain a consensus on the procedural techniques, and that the interventional procedures themselves were shown to be safe and reproducible. Furthermore, the positive response in nine out of 11 participants (82%, 95% CI 48% to 98%) following the single diagnostic lumbar facet medial branch nerve block indicates some accuracy of the assessment performed by the clinical team in determining pain of lumbar facet-joint origin based on history and clinical examination alone. However, the results are imprecise because of the small sample size. We have shown that the CPP programme is deliverable at a single recruiting centre and that it was possible to train the study physiotherapists to deliver the programme at each of the three planned recruitment centres. We were able to retain most of the recruited participants for the duration of the study.
Embedding a health economics assessment into the feasibility study allowed us to obtain important insights that can be used to develop the framework for an economic analysis within a future trial. Although the feasibility study was not primarily designed to act as a formal microcosting exercise, we have endeavoured to capture all salient aspects of the patient pathway relevant to the intervention using a triangulation of data sources, including trial documentation and structured questions to the clinical experts and discussion with the trial team, with validation of any assumptions made throughout the costing procedure. We have also been transparent in the costing methods employed, as detailed in Appendix 9. A recognised limitation of our analysis is that the resource use questionnaire was not sufficiently comprehensive to capture all health-care resource use nor did we investigate the potential for other approaches (including capturing routine data in a systematic manner). In future feasibility studies we suggest that a worthwhile endeavour would be to undertake a more ‘bottom-up’ exercise of costing, subject to trial resources being available to do so. We therefore recommend that, in a future trial, the current measure is tested and challenged against existing measures [e.g. search of the DIRUM (Database of Instruments for Resource Use Measurement);82 see Table 29 in Appendix 9], including investigating ways that data can be collated [such as using routine sources such as Hospital Episode Statistics (HES) and/or utilising patient diaries to collect data, e.g. related to primary care contacts and medication use].
The main weakness of the study was the failure to achieve our expected recruitment targets and the consequent early closure by the funder. There were substantial system-level barriers that the study team were unable to control (such as delays in obtaining HRA approval and changes in key study personnel), which led to long delays in obtaining research governance approvals, and permission to recruit was granted at only a single site.
It became clear early in the recruitment phase that many patients presenting to the pain clinics with LBP of > 3 months’ duration were not suitable for the study and that patients screened in the spinal orthopaedic clinics also did not meet the eligibility criteria. Despite employing additional strategies in the final stages of recruitment, we can conclude that the patient population presenting to these hospital-based specialist clinics was generally not suitable because of the complexity of their pain problems and that we may have had better success in recruiting from primary care-based services.
We would advise caution in extrapolating the results of this study as the study took place at a single centre in a secondary care setting. Patients presenting to primary care may additionally have lower levels of functional disability, despite high levels of pain intensity.
The feasibility study resulted in a number of suggestions for refinements to data collection, including resource use. One of the key balances has to be the development of a measure (method) of capturing resource use that is sufficiently comprehensive and accurate enough to capture important and relevant changes while not overburdening the trial in capturing extensive and irrelevant drivers of costs. The derived measure was driven primarily by clinical expertise; however, this must of course be balanced with the needs of the health economist in terms of having data of sufficient quality to undertake a comprehensive and transparent costing exercise. Appendix 14 presents recommendations from reflecting on the current measure of resource capture.
Because of the trial limitations, we were able to present only a descriptive profile using the EQ-5D-5L and SF-6D to determine utilities; thus, any interpretation of our findings should be appropriately tentative. The choice of which measure to use should also be considered further, given the potential for different numbers of QALYs to be generated by different measures and the subsequent impact on calculations of cost-effectiveness. 73
Implications for a future definitive study
Neither of the research teams funded by the HTA programme to investigate the feasibility of carrying out a definitive study to assess the use of lumbar FJIs for persistent non-specific LBP met the target recruitment rate. Professor Underwood’s team concluded that a definitive study is indeed feasible, but that recruitment from the pain clinics alone was insufficient. 83 Both teams experienced significant delays in study set-up.
Any future definitive trial must consider patient and public involvement at all stages of the research cycle, including in the design and running of the trial and representation on the TMG.
Low back pain and sciatica were selected by the Trauma Programme of Care Board as their Pathfinder Project; these projects were established by NHS England in 2013 to set up clear ‘end-to-end’ generic pathways from primary care into specialised services as required, enabling collaborative commissioning and incorporation of the latest evidence into the pathways. The National Low Back and Radicular Pain Pathway 201784 was updated following the publication of NG5910 and includes all of NICE’s recommendations. Improved co-ordination and communication with GP practices is necessary for any future collaborative work and we envisage working alongside the Pathfinder Project (in which spinal injections are not recommended) to recruit patients from primary care with high levels of functional disability who may benefit from such an intervention and rehabilitation at an earlier stage. One finding from the feasibility study was the long duration of pain awareness among the study participants, which warrants further investigation as this may reflect the time taken for patients to be referred to specialist clinics.
Based on our findings, we propose three research recommendations. First, we would agree with the Underwood team that a definitive study is potentially feasible, with adjustments made to the target population and increased primary care involvement, to enable patients to be screened earlier in their pain trajectory. Second, a definitive study will need to draw on lessons learned from both research teams (such as the recruitment enhancement strategies) and could involve a future collaboration between the two research groups, combining the successful elements of the feasibility studies. The updated NICE guidelines10 recommended radiofrequency denervation of the lumbar facet-joint medial branch nerves in patients with LBP that have not responded to less invasive treatments, following a positive diagnostic block; this is in contrast to a recent Cochrane review that has not recommended its use. 85 There is, however, new evidence from a large multicentre Dutch trial that radiofrequency denervation offers no clinical benefit compared with a sham procedure. 86 Third, as both intra-articular injections and radiofrequency denervation are widely used in the UK, we contend that there remains a need for future definitive studies to assess the clinical effectiveness and cost-effectiveness of both of these procedures in the management of persistent LBP.
Chapter 5 Conclusions
A successful trial can be defined as one that achieves success in recruitment and is able to answer the research questions. Although we have successfully demonstrated our ability to develop a robust study protocol and deliver the intended interventions, and addressed many of the feasibility objectives, the failure to achieve the target recruitment rate remains a key finding of this study. However, there are lessons to be learned here that can be used to inform and improve patient recruitment for a future definitive study. To optimise recruitment for a definitive study we would contend that any future studies in this area should involve stronger collaborations with primary care physicians and musculoskeletal physiotherapy teams to identify and recruit potential participants earlier in their pain trajectory, with the aim of making these procedures more accessible to these patients who would not otherwise have been referred on for specialist services.
Acknowledgements
Original co-applicant team
Professor Richard Langford (former Chief Investigator).
Dr Vivek Mehta (Chief Investigator, former Principal Investigator at Barts Health NHS Trust).
Professor Rod S Taylor (Study Statistician).
Dr Simon Thomson (Principal Investigator at Basildon and Thurrock University Hospitals NHS Foundation Trust).
Dr Manohar Sharma (Principal Investigator at the Walton Centre NHS Foundation Trust).
Professor Ceri Phillips (former Study Health Economist).
Professor Andrew Moore (Study Methodologist).
Professor Paul Watson (former Lead Physiotherapist).
Dr Amanda Williams (Study Health Psychologist).
Mr Neil Buxton (Consultant Neurosurgeon at the Walton Centre NHS Foundation Trust).
Dr Stephanie Stokes (Patient Liaison Representative).
Trial Management Group
Dr Vivek Mehta (Chief Investigator).
Professor Rod S Taylor (Study Statistician and Trial Mentor).
Dr Simon Thomson (Principal Investigator at Basildon and Thurrock University Hospitals NHS Foundation Trust).
Dr Manohar Sharma (Principal Investigator at the Walton Centre NHS Foundation Trust).
Professor Deborah Fitzsimmons (Study Health Economist).
Dr Saowarat Snidvongs (Principal Investigator at Barts Health NHS Trust).
Ms Alia Ahmad (Trial Co-ordinator).
Trial Steering Committee
Dr Sanjeeva Gupta (Bradford Teaching Hospitals NHS Foundation Trust) (chairperson).
Dr Julius Bourke (Queen Mary University of London).
Dr Theresa Wodehouse (Queen Mary University of London).
Dr Thomas Gilkes (Homerton University Hospital NHS Foundation Trust).
Dr Vivek Mehta (Barts Health NHS Trust).
Data Monitoring Committee
Dr Ajoy Pandit (West Hertfordshire Hospitals NHS Trust) (chairperson).
Dr David Pang (Guy’s and St Thomas’ NHS Foundation Trust).
Dr Michael Husband (West Sussex Hospitals NHS Foundation Trust).
Trial management team (Pain and Anaesthesia Research Centre at Barts Health NHS Trust)
Ms Alia Ahmad (Trial Co-ordinator).
Ms Athina Karavasopoulou (Research Nurse).
Ms Georgina Wilson (Research Assistant).
Pain consultants at Barts Health NHS Trust
Dr Jayne Gallagher (Lead Clinician).
Dr Vivek Mehta.
Dr Serge Nikolic.
Dr Kristin Ullrich.
Dr Shankar Ramaswamy.
Dr Saowarat Snidvongs.
Spinal orthopaedic research team at Barts Health NHS Trust
Mr Alexander Montgomery (Consultant Spinal Surgeon).
Ms Jamila Kassam (Lead Research Physiotherapist).
Ms Catherine Hilton (Orthopaedic Research Physiotherapist).
Ms Shahnaz Ahmad (Orthopaedic Research Physiotherapist).
Study physiotherapists
Ms Stephanie Poulton (Lead Study Physiotherapist).
Ms Kasia Gabriel.
Ms Roisin Ormond.
Peninsula Clinical Trials Unit
Laura Cocking.
Brian Wainman.
Elliot Carter.
Dr Hardip Nandra (GP at Essex Lodge GP surgery, London).
Dr Fausto Morell-Ducos (Specialty Registrar in Anaesthesia and Pain Medicine) – second reviewer for the systematic reviews.
Ms Isatou N’jie (Clinical Support Librarian at Barts Health NHS Trust) – provided assistance in developing search terms for the literature searches.
Contributions of authors
Saowarat Snidvongs (Consultant in Anaesthesia and Pain Medicine) was the Principal Investigator at Barts Health NHS Trust, drafted the manuscript and carried out the diagnostic injections, facet-joint injections and sham procedure.
Rod S Taylor (Professor of Health Services Research, Director of the Exeter Clinical Trials Unit and NIHR Senior Investigator) was the study statistician and a co-applicant, contributed to the protocol design and development, drafted the statistical analysis plan, undertook statistical analyses for the study and contributed to writing of the manuscript.
Alia Ahmad (Research Assistant) was the trial co-ordinator and was responsible for facilitating and co-ordinating the study by maintaining trial activities and paperwork according to good clinical practice.
Simon Thomson (Consultant in Anaesthesia and Pain Medicine) was the Principal Investigator at Basildon and Thurrock University Hospitals NHS Foundation Trust and a co-applicant, contributed to the protocol design and development and reviewed the final manuscript.
Manohar Sharma (Consultant in Pain Medicine) was the Principal Investigator at the Walton Centre NHS Foundation Trust and a co-applicant, contributed to the protocol design and development and reviewed the final manuscript.
Angela Farr (Health Economist and Data Analyst) contributed to the writing of the health economics analysis plan, undertook the health economics analysis, drafted the health economics results and detailed costing methodology and provided feedback on all aspects of the health economics sections and the final draft of the manuscript.
Deborah Fitzsimmons (Professor of Health Outcomes Research and Academic Director of the Swansea Centre for Health Economics) was the lead study health economist, wrote the health economics analysis plan, had senior oversight of the health economics analysis, drafted the health economics sections of the manuscript and provided feedback on the final draft of the manuscript.
Stephanie Poulton (Clinical Specialist Physiotherapist in Pain Management) was the lead study physiotherapist co-ordinating the training of the study physiotherapists for delivery of the CPP programme.
Vivek Mehta (Consultant in Pain Medicine and Director of the Pain and Anaesthesia Research Centre at Barts Health NHS Trust) was the Chief Investigator and former Principal Investigator and was a grant holder and co-applicant, contributed to the protocol design and development, carried out the final outcome assessments and reviewed the final manuscript.
Richard Langford (Consultant in Anaesthesia and Pain Medicine and former Director of the Pain and Anaesthesia Research Centre at Barts Health NHS Trust) was the former Chief Investigator and was a co-applicant, contributed to the protocol design and development and reviewed the final manuscript.
Conferences
The study protocol was presented as a poster at the International Association for the Study of Pain’s 16th World Congress on Pain in Yokohama, Japan, in September 2016. The preliminary results were presented as a poster at the British Pain Society’s 50th Anniversary Annual Scientific Meeting in Birmingham, UK, in May 2017. The full study was presented as a poster at the European Pain Federation’s 10th Congress in Copenhagen, Denmark, in September 2017; the critical appraisal of systematic reviews was also presented as a poster at the same congress. The study has been accepted as a poster presentation at the Society for Back Pain Research in Northampton, UK, in November 2017.
Thesis in preparation
The FACET feasibility study will form the basis of Dr Snidvongs’s Doctor of Medicine thesis entitled ‘Lumbar facet-joint injections for the management of chronic low back pain’. This will be submitted to the University of Exeter in 2017.
Data sharing statement
We shall make data available to the scientific community with as few restrictions as feasible while retaining exclusive use until the publication of major outputs. Requests for the data should be made to the corresponding author (Dr Saowarat Snidvongs).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet 1999;354:581-5. https://doi.org/10.1016/S0140-6736(99)01312-4.
- Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet 2012;379:482-91. https://doi.org/10.1016/S0140–6736(11)60610–7.
- Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ 2006;332:1430-4. https://doi.org/10.1136/bmj.332.7555.1430.
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000;84:95-103. https://doi.org/10.1016/S0304-3959(99)00187-6.
- Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med 2009;169:251-8. https://doi.org/10.1001/archinternmed.2008.543.
- Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012;37:E668-77. https://doi.org/10.1097/BRS.0b013e318241e5de.
- Savigny P, Watson P, Underwood M. Guideline Development Group . Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ 2009;338. https://doi.org/10.1136/bmj.b1805.
- Nath S, Nath CA, Pettersson K. Percutaneous lumbar zygapophysial (Facet) joint neurotomy using radiofrequency current, in the management of chronic low back pain: a randomized double-blind trial. Spine 2008;33:1291-7. https://doi.org/10.1097/BRS.0b013e31817329f0.
- Wu T, Zhao WH, Dong Y, Song HX, Li JH. Effectiveness of ultrasound-guided versus fluoroscopy or computed tomography scanning guidance in lumbar facet joint injections in adults with facet joint syndrome: a meta-analysis of controlled trials. Arch Phys Med Rehabil 2016;97:1558-63. https://doi.org/10.1016/j.apmr.2015.11.013.
- Low Back Pain and Sciatica in Over 16s: Assessment and Management. London: NICE; 2016.
- National Institute for Health and Care Excellence . Managing Low Back Pain and Sciatica 2017. http://pathways.nice.org.uk/pathways/low-back-pain-and-sciatica (accessed 6 May 2017).
- Mars T, Ellard DR, Antrobus JH, Cairns M, Underwood M, Haywood K, et al. Intraarticular facet injections for low back pain: design considerations, consensus methodology to develop the protocol for a randomized controlled trial. Pain Physician 2015;18:473-93.
- Moher D, Liberati A, Tetzlaff J, Altman DG. the PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Slipman CW, Bhat AL, Gilchrist RV, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J 2003;3:310-16. https://doi.org/10.1016/S1529-9430(03)00025-1.
- Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician 2007;10:229-53.
- Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD001824.pub3.
- Datta S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician 2009;12:437-60.
- Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine 2009;34:1078-93. https://doi.org/10.1097/BRS.0b013e3181a103b1.
- Henschke N, Kuijpers T, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, et al. Injection therapy and denervation procedures for chronic low-back pain: a systematic review. Eur Spine J 2010;19:1425-49. https://doi.org/10.1007/s00586-010-1411-0.
- Falco FJ, Manchikanti L, Datta S, Sehgal N, Geffert S, Onyewu O, et al. An update of the effectiveness of therapeutic lumbar facet joint interventions. Pain Physician 2012;15:E909-53.
- Manchikanti L, Nampiaparampil DE, Manchikanti KN, Falco FJ, Singh V, Benyamin RM, et al. Comparison of the efficacy of saline, local anesthetics, and steroids in epidural and facet joint injections for the management of spinal pain: a systematic review of randomized controlled trials. Surg Neurol Int 2015;6:S194-235. https://doi.org/10.4103/2152-7806.156598.
- Manchikanti L, Kaye AD, Boswell MV, Bakshi S, Gharibo CG, Grami V, et al. A systematic review and best evidence synthesis of the effectiveness of therapeutic facet joint interventions in managing chronic spinal pain. Pain Physician 2015;18:E535-82.
- Vekaria R, Bhatt R, Ellard DR, Henschke N, Underwood M, Sandhu H. Intra-articular facet joint injections for low back pain: a systematic review. Eur Spine J 2016;25:1266-81. https://doi.org/10.1007/s00586–016–4455-y.
- Manchikanti L, Hirsch JA, Falco FJ, Boswell MV. Management of lumbar zygapophysial (facet) joint pain. World J Orthop 2016;7:315-37. https://doi.org/10.5312/wjo.v7.i5.315.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-10.
- Lilius G, Laasonen EM, Myllynen P, Harilainen A, Grönlund G. Lumbar facet joint syndrome. A randomised clinical trial. J Bone Joint Surg Br 1989;71:681-4.
- Carette S, Marcoux S, Truchon R, Grondin C, Gagnon J, Allard Y, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med 1991;325:1002-7. https://doi.org/10.1056/NEJM199110033251405.
- Marks RC, Houston T, Thulbourne T. Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain 1992;49:325-8. https://doi.org/10.1016/0304-3959(92)90239-8.
- Revel M, Poiraudeau S, Auleley GR, Payan C, Denke A, Nguyen M, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine 1998;23:1972-6.
- Manchikanti L, Pampati V, Bakhit CE, Rivera JJ, Beyer CD, Damron KS, et al. Effectiveness of lumbar facet joint nerve blocks in chronic low back pain: a randomized clinical trial. Pain Physician 2001;4:101-17.
- Mayer TG, Gatchel RJ, Keeley J, McGeary D, Dersh J, Anagnostis C. A randomized clinical trial of treatment for lumbar segmental rigidity. Spine 2004;29:2199-205. https://doi.org/10.1097/01.brs.0000142009.73869.8d.
- Fuchs S, Erbe T, Fischer HL, Tibesku CO. Intraarticular hyaluronic acid versus glucocorticoid injections for nonradicular pain in the lumbar spine. J Vasc Interv Radiol 2005;16:1493-8. https://doi.org/10.1097/01.RVI.0000175334.60638.3F.
- Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of a randomized, double-blind controlled trial: Clinical Trial NCT00355914. Pain Physician 2008;11:121-32.
- Kawu AA, Olawepo A, Salami AO. Facet joints infiltration: a viable alternative treatment to physiotherapy in patients with low back pain due to facet joint arthropathy. Niger J Clin Pract 2011;14:219-22. https://doi.org/10.4103/1119-3077.84021.
- Celik B, Er U, Simsek S, Altug T, Bavbek M. Effectiveness of lumbar zygapophysial joint blockage for low back pain. Turk Neurosurg 2011;21:467-70. https://doi.org/10.5137/1019-5149.JTN.4057-10.1.
- Yun DH, Kim HS, Yoo SD, Kim DH, Chon JM, Choi SH, et al. Efficacy of ultrasonography-guided injections in patients with facet syndrome of the low lumbar spine. Ann Rehabil Med 2012;36:66-71. https://doi.org/10.5535/arm.2012.36.1.66.
- Ribeiro LH, Furtado RN, Konai MS, Andreo AB, Rosenfeld A, Natour J. Effect of facet joint injection versus systemic steroids in low back pain: a randomized controlled trial. Spine 2013;38:1995-2002. https://doi.org/10.1097/BRS.0b013e3182a76df1.
- Lakemeier S, Lind M, Schultz W, Fuchs-Winkelmann S, Timmesfeld N, Foelsch C, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain: a randomized, controlled, double-blind trial. Anesth Analg 2013;117:228-35. https://doi.org/10.1213/ANE.0b013e3182910c4d.
- Low Back Pain in Adults: Early Management. London: NICE; 2009.
- Greenhalgh S, Selfe J. Red Flags II: a Guide to Solving Serious Pathology of the Spine. London: Churchill Livingstone; 2010.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40. https://doi.org/10.1002/sim.4780141709.
- World Health Organization . WHO Surgical Safety Checklist and Implementation Manual 2008. www.who.int/patientsafety/safesurgery/ss_checklist/en/ (accessed 1 November 2017).
- Bogduk N. A narrative review of intra-articular corticosteroid injections for low back pain. Pain Med 2005;6:287-96. https://doi.org/10.1111/j.1526-4637.2005.00048.x.
- Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14410.
- World Medical Association . World Medical Association Declaration of Helsinki 1996. http://www.chcuk.co.uk/pdf/Declaration_of_Helsinki_1996_version.pdf (accessed 2 November 2017).
- International Conference of Harmonisation Steering Committee . Guideline for Good Clinical Practice E6 (R1) 1996. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed 14 November 2017).
- Research Governance Framework for Health and Social Care, Second Edition. London: Department of Health; 2005.
- The Medicines for Human Use (Clinical Trials) Regulations 2004. London: The Stationery Office; 2004.
- Electronic Medicines Compendium . Summary of Product Characteristics for Marcain Polyamp Steripack 0.5%; Updated 7th February 2017 n.d. www.medicines.org.uk/emc/medicine/6013 (accessed 11 September 2017).
- Electronic Medicines Compendium . Summary of Product Characeteristics for Depo-Medrone 40mg Ml; Updated 1st February 2017 n.d. www.medicines.org.uk/emc/medicine/3549 (accessed 11 September 2017).
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. https://doi.org/10.1016/j.pain.2004.09.012.
- Brief Pain Inventory (Short Form). Houston, TX: The University of Texas, MD Anderson Cancer Center; 1991.
- Mapi Research Trust . Short-Form McGill Pain Questionnaire (SF-MPQ-2) 2009. https://eprovide.mapi-trust.org/instruments/short-form-mcgill-pain-questionnaire (accessed 14 November 2017).
- EuroQol Group . EuroQol-5 Dimensions Five-Level Version (EQ-5D-5L) 2009. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 14 November 2017).
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Mapi Research Trust . Oswestry Disability Index 2000. https://eprovide.mapi-trust.org/instruments/oswestry-disability-index (accessed 14 November 2017).
- Nicholas MK. Self-Efficacy and Chronic Pain n.d.
- GL Assessment . Hospital Anxiety and Depression Scale (HADS) 2017. www.gl-assessment.co.uk/products/hospital-anxiety-and-depression-scale-hads/ (accessed 14 November 2017).
- Mapi Research Trust . Pain Catastrophizing Scale 1995. https://eprovide.mapi-trust.org/instruments/pain-catastrophizing-scale (accessed 14 November 2017).
- Koopman C, Pelletier KR, Murray JF, Sharda CE, Berger ML, Turpin RS, et al. Stanford presenteeism scale: health status and employee productivity. J Occup Environ Med 2002;44:14-20. https://doi.org/10.1097/00043764-200201000-00004.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . Statistical Principles for Clinical Trials 1998. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf (accessed 27 September 2017).
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Short Form Six-Dimension (SF-6D). Sheffield: ScHARR, University of Sheffield; n.d.
- Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Office of Health Economics Research paper 16/01 . Valuing Health-Related Quality of Life: An Eq-5d-5l Value Set for England 2016. www.ohe.org/publications/valuing-health-related-quality-life-eq-5d-5l-value-set-england (accessed 17 November 2017).
- Cohen SP, Williams KA, Kurihara C, Nguyen C, Shields C, Kim P, et al. Multicenter, randomized, comparative cost-effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology 2010;113:395-40. https://doi.org/10.1097/ALN.0b013e3181e33ae5.
- Burnham RS, Holitski S, Dinu I. A prospective outcome study on the effects of facet joint radiofrequency denervation on pain, analgesic intake, disability, satisfaction, cost, and employment. Arch Phys Med Rehabil 2009;90:201-5. https://doi.org/10.1016/j.apmr.2008.07.021.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Department of Health . NHS Reference Costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 6 May 2017).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2017.
- National Institute for Health and Care Excellence (NICE) . Low Back Pain: Full Guidance Draft – Appendix E Health Economics Model 2008.
- Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ 2004;13:873-84. https://doi.org/10.1002/hec.866.
- Mulhern B, Mukuria C, Barkham M, Knapp M, Byford S, Soeteman D, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205:236-43. https://doi.org/10.1192/bjp.bp.112.122283.
- Streiner DL, Norman GR, Cairney J. Health Measurement Scales: a Practical Guide to their Development and Use. Oxford: Oxford University Press; 2014.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 6 May 2017).
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual n.d. www.nice.org.uk/process/pmg20/chapter/introduction-and-overview (accessed 6 May 2017).
- Paulsen A, Overgaard S, Lauritsen JM. Quality of data entry using single entry, double entry and automated forms processing – an example based on a study of patient-reported outcomes. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0035087.
- Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-264.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3.
- Tower Hamlets Council . Tower Hamlets Borough Statistics 2015. www.towerhamlets.gov.uk/lgnl/community_and_living/borough_statistics/borough_statistics.aspx (accessed 6 May 2017).
- Newington L, Metcalfe A. Factors influencing recruitment to research: qualitative study of the experiences and perceptions of research teams. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-10.
- Database of Instruments for Resource Use Measurement . Database of Instruments for Resource Use Measurement (DIRUM) n.d. www.dirum.org/ (accessed 6 May 2017).
- Ellard DR, Underwood M, Achana F, Antrobus JH, Balasubramanian S, Brown S, et al. Facet joint injections for people with persistent non-specific low back pain (Facet Injection Study): a feasibility study for a randomised controlled trial. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21300.
- NHS England Trauma Programme of Care . National Low Back and Radicular Pain Pathway 2017 n.d. www.ukssb.com/assets/PDFs/2017/February/National-Low-Back-and-Radicular-Pain-Pathway-2017_final.pdf (accessed 8 July 2017).
- Maas ET, Ostelo RW, Niemisto L, Jousimaa J, Hurri H, Malmivaara A, et al. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst Rev 2015;10. https://doi.org/10.1002/14651858.CD008572.pub2.
- Juch JNS, Maas ET, Ostelo RWJG, Groeneweg JG, Kallewaard JW, Koes BW, et al. Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain: the Mint randomized clinical trials. JAMA 2017;318:68-81. https://doi.org/10.1001/jama.2017.7918.
- Boswell MV, Colson JD, Spillane WF. Therapeutic facet joint interventions in chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician 2005;8:101-14.
- Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine 2009;34:49-5. https://doi.org/10.1097/BRS.0b013e3181909558.
- Boswell MV, Manchikanti L, Kaye AD, Bakshi S, Gharibo CG, Gupta S, et al. A best-evidence systematic appraisal of the diagnostic accuracy and utility of facet (zygapophysial) joint injections in chronic spinal pain. Pain Physician 2015;18:E497-533.
- Nash TP. Facet joints – intra-articular steroids or nerve block?. Pain Clin 1989;3:77-82.
- Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J. Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?. Health Technol Assess 2000;4.
- Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al. Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9320.
- Gilbert FJ, Grant AM, Gillan MG, Vale L, Scott NW, Campbell MK, et al. Does early imaging influence management and improve outcome in patients with low back pain? A pragmatic randomised controlled trial. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8170.
Appendix 1 Search strategies for the literature search of systematic reviews and meta-analyses of randomised controlled trials of intra-articular lumbar facet-joint injections for chronic low back pain
MEDLINE
Date range searched: 1966 to 6 February 2017.
| # | Search term | Results |
|---|---|---|
| 1 | exp *LOW BACK PAIN/ | 13,733 |
| 2 | (“low back pain”).ti,ab | 21,120 |
| 3 | exp *ZYGAPOPHYSEAL JOINT/ | 961 |
| 4 | (“facet joint”).ti,ab | 2227 |
| 5 | exp *CHRONIC PAIN/ OR exp *PAIN/ | 218,391 |
| 6 | (lumbar OR paravertebral).ti,ab | 91,494 |
| 7 | exp *LUMBAR VERTEBRAE/ | 26,956 |
| 8 | 1 OR 2 | 24,954 |
| 9 | 3 OR 4 | 2683 |
| 10 | 6 OR 7 | 98,948 |
| 11 | 5 AND 10 | 9560 |
| 12 | 8 OR 9 OR 10 OR 11 | 117,090 |
| 13 | exp *INJECTIONS, INTRA-ARTICULAR/ | 809 |
| 14 | (“intra articular*”).ti,ab | 12,196 |
| 15 | (facet ADJ2 injection).ti,ab | 143 |
| 16 | (facet ADJ2 joint).ti,ab | 2299 |
| 17 | exp *FLUOROSCOPY/ | 5060 |
| 18 | (fluoroscop*).ti,ab | 21,046 |
| 19 | exp *THERAPEUTICS/ | 2,895,415 |
| 20 | (therap*).ti,ab | 2,169,176 |
| 21 | (“percutaneous spinal”).ti,ab | 94 |
| 22 | 13 OR 14 | 12,630 |
| 23 | 15 AND 16 | 120 |
| 24 | 17 OR 18 | 23,098 |
| 25 | 19 OR 20 | 4,590,592 |
| 26 | exp *INJECTIONS/ | 20,026 |
| 27 | (injection*).ti,ab | 496,795 |
| 28 | 26 OR 27 | 505,646 |
| 29 | 21 AND 28 | 7 |
| 30 | 22 OR 23 OR 24 OR 25 OR 29 | 4,610,334 |
| 31 | 12 AND 30 | 49,755 |
| 32 | (“systematic review*”).ti,ab | 94,141 |
| 33 | 31 AND 32 | 1138 |
| 34 | (“meta analysis”).ti,ab | 88,442 |
| 35 | 31 AND 34 | 521 |
| 36 | (“control* trial*” OR RCT).ti,ab | 13,597 |
| 37 | 31 AND 36 | 179 |
| 38 | 33 OR 35 OR 37 | 1504 |
| 39 | (facet).ti,ab | 10,869 |
| 40 | 32 AND 39 | 123 |
EMBASE
Date range searched: 1966 to 6 February 2017.
| # | Search term | Results |
|---|---|---|
| 1 | exp *LOW BACK PAIN/ | 23,697 |
| 2 | (“low back pain”).ti,ab | 28,245 |
| 3 | exp *ZYGAPOPHYSEAL JOINT/ | 509 |
| 4 | (“facet joint”).ti,ab | 2999 |
| 5 | exp *CHRONIC PAIN/ OR exp *PAIN/ | 386,200 |
| 6 | (lumbar OR paravertebral).ti,ab | 123,427 |
| 7 | exp *LUMBAR VERTEBRAE/ | 7855 |
| 8 | 1 OR 2 | 35,444 |
| 9 | 3 OR 4 | 3251 |
| 10 | 6 OR 7 | 125,759 |
| 11 | 5 AND 10 | 16,010 |
| 12 | 8 OR 9 OR 10 OR 11 | 151,862 |
| 13 | exp *INJECTIONS, INTRA-ARTICULAR/ | 1884 |
| 14 | (“intra articular*”).ti,ab | 15,071 |
| 15 | (facet ADJ2 injection).ti,ab | 193 |
| 16 | (facet ADJ2 joint).ti,ab | 3056 |
| 17 | exp *FLUOROSCOPY/ | 6811 |
| 18 | (fluoroscop*).ti,ab | 34,353 |
| 19 | exp *THERAPEUTICS/ | 3,002,010 |
| 20 | (therap*).ti,ab | 3,111,511 |
| 21 | (“percutaneous spinal”).ti,ab | 138 |
| 22 | 13 OR 14 | 16,232 |
| 23 | 15 AND 16 | 165 |
| 24 | 17 OR 18 | 35,845 |
| 25 | 19 OR 20 | 5,235,530 |
| 26 | exp *INJECTIONS/ | 33,580 |
| 27 | (injection*).ti,ab | 635,413 |
| 28 | 26 OR 27 | 639,110 |
| 29 | 21 AND 28 | 11 |
| 30 | 22 OR 23 OR 24 OR 25 OR 29 | 5,270,243 |
| 31 | 12 AND 30 | 38,627 |
| 32 | (“systematic review*”).ti,ab | 118,882 |
| 33 | 31 AND 32 | 867 |
| 34 | (“meta analysis”).ti,ab | 117,968 |
| 35 | 31 AND 34 | 428 |
| 36 | (“control* trial*” OR RCT).ti,ab | 231,428 |
| 37 | 31 AND 36 | 2362 |
| 38 | 33 OR 35 OR 37 | 2906 |
| 39 | (facet).ti,ab | 12,624 |
| 40 | 32 AND 39 | 149 |
Cochrane Central Register of Controlled Trials
Date range searched: 1966 to 6 February 2017.
Search strategy
#1 MeSH descriptor: [Back Pain] explode all trees
#2 back near pain
#3 dorsalgia
#4 back disorder*
#5 backache
#6 MeSH descriptor: [Low Back Pain] explode all trees
#7 (lumbar next pain)
#8 (#1 or #2 or #3 or #4 or #5 or #6 or #7)
#9 MeSH descriptor: [Zygapophyseal Joint] explode all trees
#10 facet near joints
#11 zygapophysial*
#12 (#9 or #10 or #11)
#13 (#8 and #12)
Appendix 2 Systematic review articles on therapeutic lumbar facet-joint injections identified from the database searches
| Source | First author, year of publication | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slipman et al., 200314 | Boswell et al., 200587 | Boswell et al., 200715 | Staal et al., 200816 | Staal et al., 200988 | Datta et al., 200917 | Chou et al., 200918 | Henschke et al., 201019 | Falco et al., 201220 | Manchikanti et al., 201521 | Boswell et al., 201589 | Manchikanti et al., 201522 | Vekaria et al., 201623 | Manchikanti et al., 201624 | |
| MEDLINE | ||||||||||||||
| EMBASE | ||||||||||||||
| CENTRAL | ||||||||||||||
| Other sources | ||||||||||||||
| Reasons for exclusion if applicable | Published update | Duplicate publication | Intervention not of interest | |||||||||||
Appendix 3 Assessment of Multiple Systematic Reviews (AMSTAR) checklist scores for the included systematic reviews
| First author, year | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | AMSTAR scorea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slipman, 200314 | CA | CA | N | N | Y | Y | Y | Y | CA | N | CA | 4 |
| Boswell, 200715 | CA | CA | Y | CA | Y | Y | Y | Y | CA | N | CA | 5 |
| Staal, 200816 | Y | Y | Y | N | Y | Y | Y | Y | Y | N | CA | 8 |
| Datta, 200917 | CA | CA | Y | CA | Y | NA | NA | NA | NA | N | CA | 2 |
| Chou, 200918 | CA | Y | Y | Y | Y | N | Y | Y | N | N | CA | 6 |
| Henschke, 201019 | CA | CA | N | N | Y | Y | Y | Y | Y | Y | N | 6 |
| Falco, 201220 | CA | Y | Y | CA | Y | Y | Y | Y | Y | N | CA | 7 |
| Manchikanti, 201521 | CA | Y | Y | CA | Y | Y | Y | Y | Y | N | N | 7 |
| Manchikanti, 201522 | CA | Y | Y | CA | N | Y | Y | Y | Y | N | CA | 6 |
| Vekaria, 201623 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | CA | 10 |
| Manchikanti, 201624 | CA | Y | Y | CA | N | Y | Y | Y | N | N | CA | 5 |
Appendix 4 Venn diagram to illustrate the randomised controlled trials included in each systematic review
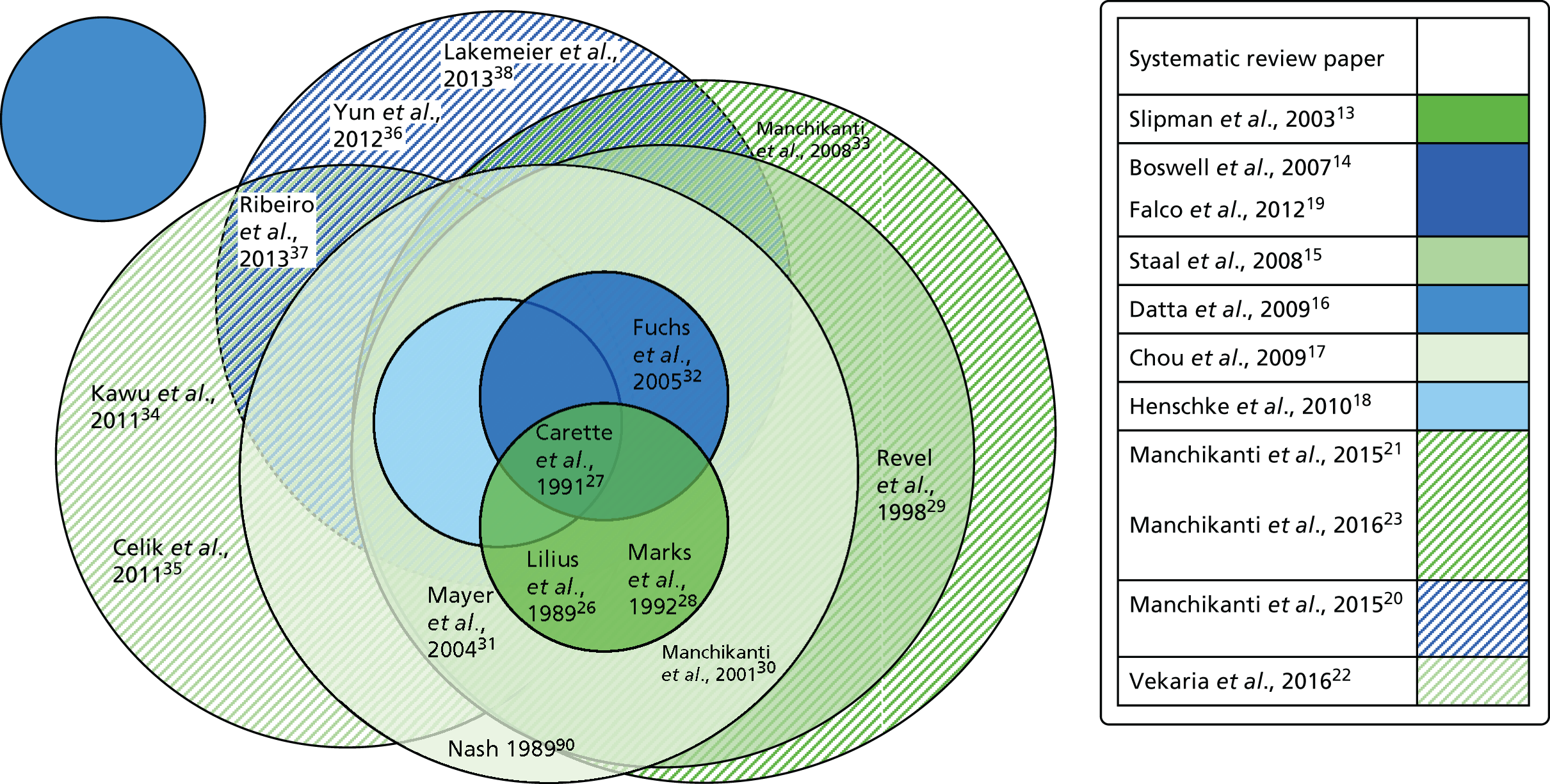
Appendix 5 Participant information sheet
Appendix 6 Delphi exercise
A web-based survey of pain specialists in the UK utilised the Delphi method to determine the choice of needle, injectate and volume of injection, as well as the choice of steroid, dose and volume and the maximum dose of steroid. Of approximately 250 pain specialists consulted, 42 took part in the survey.
| Question | Response (%) | Response count | ||||
|---|---|---|---|---|---|---|
| 1. At what maximum steroid dose, do you think, reviewers and general sceptics could claim that a positive result for a facet-joint injection was due to systematic action, rather than local benefit? | ||||||
| 60 mg of methylprednisolone | 25.0 | 10 | ||||
| 80 mg of methylprednisolone | 22.5 | 9 | ||||
| 100 mg of methylprednisolone | 20.0 | 8 | ||||
| 120 mg of methylprednisolone | 32.5 | 13 | ||||
| Response (%) | Response count | |||||
| 2. Which volume is closest to your choice in each facet-joint? | ||||||
| < 1 ml | 28.6 | 12 | ||||
| 1 ml | 38.1 | 16 | ||||
| 1.5 ml | 21.4 | 9 | ||||
| Response (%) | Response count | |||||
| 3. Assuming that we keep to a maximum of four joints, what steroid dose should we use in each joint? | ||||||
| 10 mg of methylprednisolone per joint | 40.5 | 17 | ||||
| 20 mg of methylprednisolone per joint | 57.1 | 24 | ||||
| 30 mg of methylprednisolone per joint | 2.4 | 1 | ||||
| Most likely, % (n) | Likely, % (n) | Not likely, % (n) | Does not affect the outcome, % (n) | Rating average | Response count | |
| 4. If we were not to use methylprednisolone, which of these two steroids would you prefer? | ||||||
| Triamcinolone | 85.7 (36) | 9.5 (4) | 2.4 (1) | 2.4 (1) | 1.21 | 42 |
| Dexamethasone | 12.5 (4) | 34.4 (11) | 50.0 (16) | 3.1 (1) | 2.44 | 32 |
| Response (%) | Response count | |||||
| 5. The sham group should have a fluoroscopic guided needle placed | ||||||
| Next to the periarticular surface with no injection | 38.1 | 16 | ||||
| Next to the periarticular surface with saline injected (same volume as the active group) | 28.6 | 12 | ||||
| Intra-articular placement with only contrast injected | 21.4 | 9 | ||||
| Intra-articular placement with contrast and placebo (saline) injection (same volume as the active group) | 11.9 | 5 | ||||
Appendix 7 Sample case report form
Appendix 8 Sample adverse event log
Appendix 9 Literature review to inform the health economics component and costing methodology
Literature review
Objectives
A rapid, systematically based review of the health economics literature was conducted to inform the development of the framework for an economic model to assess the longer-term cost-effectiveness of FJIs for persistent non-specific LBP. The review identified, and assessed the scope and quality of, the current evidence in relation to:
-
the cost-effectiveness of FJIs for persistent non-specific LBP
-
specific resources and associated costs associated with FJIs
-
relevant outcomes to inform a health economic analysis, for example health-related quality of life/utilities – this considered possible candidate measures used elsewhere, for example the EQ-5D or SF-6D
-
possible frameworks, for example economic models, that have been reported in the literature.
Methods
A PICO (problem/population, intervention, control, outcomes) approach was used to generate appropriate search terms (Table 22). PubMed and the NHS Economic Evaluation Database (NHS EED) were systematically searched to identify relevant studies (systematic reviews, randomised controlled trials and, when necessary, observational studies), following key PRISMA principles. 13
| Number | Problem/population | Intervention | Control | Outcomes |
|---|---|---|---|---|
| 1. | facet-joint | injection | sham | cost |
| 2. | cervical | analgesia | placebo | effectiveness |
| 3. | thoracic | denervation | standard of care | economic |
| 4. | lumbar | quality of life | ||
| 5. | utilities |
Cost of illness studies, costing studies or other partial economic evaluations were identified but not formally reviewed. In addition, searches to identify literature to inform health-related quality of life/utility estimates were undertaken.
Results
The search strategy identified only two studies65,66 evaluating the cost of FJIs, although these were partial economic evaluations rather than formal cost-effectiveness studies, and one study37 evaluating health-related quality of life.
Cohen et al. 65 evaluated lumbar z-joint denervation costs and outcomes using three paradigms: (1) radiofrequency denervation without the use of a screening block; (2) radiofrequency denervation if the patient obtained significant relief after a single diagnostic block; and (3) radiofrequency denervation only if an appropriate patient had a positive response to two confirmatory medial branch (facet-joint nerve) blocks to determine which treatment paradigm was associated with the highest overall and radiofrequency denervation success rates, and to evaluate the relative costs per successful treatment for each of the three groups. The primary end point as a measure of ‘clinical success’ was 3 months of significant pain relief. The cost of a diagnostic facet-joint block averaged US$350 for the first level and US$170 for each subsequent level; the cost of radiofrequency denervation averaged US$650 for the first joint and US$325 for each additional joint; the overall group success rate ranged between 17% and 32%; and the mean cost of successful treatment in the single-block group was US$5172 (SD US$860). The study found that proceeding straight to radiofrequency denervation without any diagnostic blocks was associated with both the lowest cost per successful procedure and the highest number of total successful procedures.
Burnham et al. 66 conducted an evaluation of the effectiveness of radiofrequency denervation of the lumbar facet-joints for pain, analgesic intake, disability, patient satisfaction, back pain-related costs and employment status in patients with chronic LBP of facet-joint origin in the context of a clinical audit of a new radiofrequency denervation programme. Both direct and indirect costs relating to LBP care that were or could potentially be borne directly by the subject were estimated. Direct costs were calculated by asking the subject to describe the type and number of any of the following treatments/services received for his or her LBP in the previous month: physician office visits, chiropractic treatments, physiotherapy treatments and treatments from other allied health practitioners (massage, acupuncture, psychology and so forth).
Post radiofrequency denervation, significant improvements were observed in pain, analgesic requirements, satisfaction, disability and direct costs occurred. These outcomes peaked at 3–6 months and gradually diminished thereafter. Satisfaction with medical care and living with current symptoms improved similarly. Overall, satisfaction with the radiofrequency denervation procedure was high and no complications were reported.
Based on these outcomes, it was deemed that radiofrequency denervation provides safe and significant short-term improvements in pain, analgesic requirements, function, satisfaction and direct costs in patients with chronic LBP of facet origin.
Ribeiro et al. 37 studied 60 subjects with a diagnosis of facet-joint syndrome who were randomised into an experimental group (intra-articular injection of six lumbar facet-joints with triamcinolone hexacetonide) or a control group (intramuscular injection of six lumbar paravertebral points with triamcinolone acetonide). No details were provided by the authors on trade names or manufacturer details for the drugs used. Outcome measures including a pain VAS, a pain VAS during extension of the spine, a Likert scale, an improvement percentage scale, the Roland Morris Disability Questionnaire and the SF-36 were administered at baseline and at 1, 4, 12 and 24 weeks after the interventions. The data revealed an improvement in the experimental group in terms of health-related quality of life, in the ‘role physical’ profile, assessed by the SF-36.
Conclusions
The literature search identified a very limited set of evidence on the cost-effectiveness of diagnostic or therapeutic FJIs and the studies identified were simple costing studies. As such, a knowledge gap remains for the simulation of longer-term outcomes to inform cost-effectiveness models with long-term horizons.
Resource use associated with facet-joint injections
Resource use: methodology
The patient questionnaire was completed by a research assistant during visits to a nurse-led outpatient pain management clinic and took approximately 1 hour to complete. The visits took place at 6 weeks (visit 4, research study-specific visit), 3 months (visit 5, research study-specific visit) and 6 months (visit 6, standard routine consultant-led outpatient check-up).
The questions regarding NHS resource use specifically asked about the most likely health-care professionals who would be engaged with during treatment for LBP; the results are detailed in Table 23.
| Coded variable | Variable type | Description of resource use and basis for the unit cost | Unit cost | Unit cost source |
|---|---|---|---|---|
| V01 _ EmergencyDepartmentVisits | Number, long integer | Emergency department visit (standard A&E attendance) | £137.74 | Department of Health68 |
| V01 _ LengthOfStay | Number, long integer | Length of stay in hospital (emergency admission as a result of serious adverse event) | Unit cost method to be decided – see data. National average unit cost: Non-Elective Inpatients – Long Stay £3058.14, Non-Elective – Short Stay £615.83 | Department of Health68 |
| V01 _ GPAppointments | Number, long integer | GP appointment (assumed to be a standard GP consultation with an average contact of 9.22 minutes) at a GP surgery | £36 | Curtis and Burns67 |
| V01 _ PainClinic | Number, long integer | Pain clinic (standard routine outpatient appointment at a pain management clinic) | Assumed to be consultant led unit cost – £148.03 (non-consultant-led unit cost £111.15) | Department of Health68 |
Medication
The patient questionnaire contained within the case report form collected information on current prescribed analgesics and any other medications at baseline. At the three follow-up time points information on current analgesics and other medications was also collected along with any changes in medication during the follow-up period. Information on medication use is reported in Table 24.
| Coded variable | Variable type | Description of resource use and basis for the unit cost | Unit cost source |
|---|---|---|---|
| V01 _ CurrentAnalgesics | Text | Current analgesics (name of medication, dosage and frequency) | BNF69 |
| V01 _ OtherMedications | Text | Other medications (name of medication, dosage and frequency) | BNF69 |
The specific dates that prescriptions were issued, including the dates that any changes in medication occurred, are unknown as medications were prescribed by the patients’ GPs rather than by the trial team. Medication reported in the ‘other’ medication category that was not related to the treatment of LBP was excluded.
Medication costs were calculated at baseline (visit 1) to evaluate the pain medication being taken before the intervention commenced. Costs were then calculated between baseline and the 6-week follow-up (visit 4), between visit 4 and the 3-month follow-up (visit 5) and between visit 5 and the 6-month follow-up (visit 6). As some patients had their pain medication prescriptions changed in the community by their GP between follow-up visits to the hospital, any change in prescription was assumed to be at the mid-point between follow-up visits for costing purposes. Table 25 shows how the time periods were classified in days to calculate the duration of prescribed medication.
| Time perioda | Duration in days |
|---|---|
| Duration of baseline prescribed medication | A period of 21 days before the baseline assessment (assumption as pretrial duration of prescribed medication is unknown) |
| When the same medication was reported at successive follow-up points the whole period between time points was considered as a continuous prescription | Baseline (visit 1) to 6-week follow-up (visit 4) = 42 days; 6-week follow-up (visit 4) to 3-month follow-up (visit 5) = 51 days; 3-month follow-up (visit 5) to 6-month follow-up (visit 6) = 93 days |
| When a change in prescribed medication was reported an approximate mid-point between follow-up visits was used to estimate prescription duration – the previous medication was allocated the first half of the number of days in that period and the new medication was allocated the second half of the number of days during that time period | At visit 4: old medication = first 21 days after baseline assessment, new medication = the 21 days before visit 4; at visit 5: old medication = first 25.5 days after visit 4, new medication = the 25.5 days before visit 5; at visit 6: old medication = first 46.5 days after visit 5, new medication = 46.5 days before visit 6 |
The cost of medication per day was calculated and this was then multiplied by the number of days the medication was prescribed.
Dealing with missing medication data
Table 26 contains the substitute costing rules applied when reported medication prescriptions were vague or information was missing. Table 27 lists the analgesic medication reported by trial participants and the accompanying unit costs.
| Medication dose and frequency of dose is reported within a possible range depending on need | The lower dose and frequency is used as a conservative estimate or a maintenance dose is used |
|---|---|
| Ibuprofen (200 mg) | The daily standard maintenance dose is used = two tablets, three times per day = six tablets = 1.2 g per day |
| Paracetamol (500 mg) | The daily standard dose of 500 mg every 4 hours = six tablets per day = 3 g per day |
| Co-codamol (30/500 mg) | A standard dose of two tablets four times a day = eight tablets per day |
| Naproxen (250 mg) | A standard maintenance dose of one tablet every 6 hours = four tablets per day = 1 g per day |
| Name of medication | Dose | Frequency of dose | Unit cost per pack (£) | Number of tablets per pack | Cost per tablet (£) |
|---|---|---|---|---|---|
| Amitriptyline | 10 mg | 3 × (at night) | 0.96 | 28 | 0.034286 |
| Amitriptyline | 25 mg | 1 × daily | 0.99 | 28 | 0.035357 |
| Amlodipine | 5 mg | 1 × daily | 0.91 | 28 | 0.0325 |
| Co-codamol | 30 mg | Two tablets each 4 × daily | 6.73 | 100 | 0.0673 |
| Co-codamol | 8/500 mg | 4 × daily | 1.16 | 30 | 0.038667 |
| Co-codamol | 30/500 mg | Daily as required | 6.73 | 100 | 0.0673 |
| Codeine | 30–60 mg | BD (2*)/TDS (3*), depending on severity | 1.44 | 28 | 0.051429 |
| Codeine phosphate | 30/500 mg | 6 × daily | 6.73 | 100 | 0.0673 |
| Diazepam | 5 mg | 1 × nightly | 1.06 | 28 | 0.037857 |
| Duloxetine | 60 mg | 1 × nightly | 27.72 | 28 | 0.99 |
| Duloxetine | 60 mg | 1 × daily | 27.72 | 28 | 0.99 |
| Gabapentin | 200 mg | 2 × daily | 3.17 | 100 | 0.0317 |
| Ibuprofen | 5% gel | 3 × daily | 1.28 | 1 | 1.28 |
| Ibuprofen | 400 mg | 3–4 × daily | 3.50 | 84 | 0.041667 |
| Lyrica | 300 mg | Max. 300 mg per day for neuropathic pain | 64.40 | 56 | 1.15 |
| Naproxen | As needed | Assumed standard dose | 1.12 | 28 | 0.04 |
| Nortriptyline | 10 mg | 1 × daily | 12.06 | 100 | 0.1206 |
| Palexia | (50 mg) | 2 × daily | 12.46 | 28 | 0.445 |
| Paracetamol | 500 mg as required | Assumed standard dose | 0.92 | 32 | 0.02875 |
| Pregabalin | 75 mg standard | 3 × daily, standard dose 75 mg twice daily | 64.40 | 56 | 1.15 |
| Solpadol | Assumed standard dose | Assumed standard dose | 6.74 | 100 | 0.0674 |
| Tremadol (assumed tramadol) | 50 mg | 2 × every 4–6 hours | 1.20 | 30 | 0.04 |
Calculating quality-adjusted life-years
For both groups in the study the number of QALYs gained was calculated for the complete cases for the 6 months between baseline (before treatment) and the final 6-month follow-up visit, as shown in Table 28.
| Time period | Calculation method |
|---|---|
| 6 weeks between baseline (visit 1) and 6-week follow-up (visit 4) | [(EQ-5D mean utility at visit 1 + EQ-5D mean utility at visit 4)/2] × (6 weeks) = QALYs gained at visit 4 |
| 6 weeks between 6-week follow-up (visit 4) and 3-month follow-up (visit 5) | [(EQ-5D mean utility at visit 4 + EQ-5D mean utility at visit 5)/2] × (6 weeks) = QALYs gained at visit 5 |
| 12 weeks between 3-month follow-up (visit 5) and 6-month follow-up (visit 6) | [(EQ-5D mean utility at visit 5 + EQ-5D mean utility at visit 6)/2] × (12 weeks) = QALYs gained at visit 6 |
| Total QALYs gained at the end of treatment | QALYs gained at visit 4 +QALYs gained at visit 5 +QALYs gained at visit 6 |
Existing measures used to collect resource use data in lower back pain studies
A search of the DIRUM82 to compare existing resource use measures specifically related to LBP was conducted. The results of this search are shown in Table 29.
| Disease category | Reference | Items of resource use being measured |
|---|---|---|
| Anaesthesia and pain control | Back Pain Questionnaire91 | GP visits, physiotherapy visits, other NHS (osteopath and chiropractor visits) |
| Anaesthesia and pain control | BeST trial follow-up questionnaire44 | GP visits, inpatient admissions, practice nurse visits, outpatient attendance, medication, A&E attendance, physiotherapy visits, psychologist visits, other NHS (scans, radiography, blood tests), other non-NHS (private visits: chiropractor, osteopath, physiotherapist, alternative therapy, etc.) |
| Anaesthesia and pain control | Lower Back Pain Resource Use Questionnaire92 | Inpatient admissions, outpatient attendance, A&E attendance, employer,a physiotherapy visits, health-care aids, other non-NHS (chiropractor, acupuncturist and osteopath visits) |
| Anaesthesia and pain control, orthopaedics and trauma | Scottish Back Trial Questionnaire93 | GP visits, inpatient admissions, outpatient attendance, medication, physiotherapy visits |
Appendix 10 Summary of changes to the protocol
| Amendment | Protocol version, date | Summary of changes |
|---|---|---|
| Minor amendment | 4, 4 February 2015 | Update to schedule of assessment table |
| Substantial amendment 1 | 5, 2 September 2015 | Update to patient safety information |
| Revised details for the TSC and DMC | ||
| Substantial amendment 2 | 6, 7 May 2016 | Change of Chief Investigator and Principal Investigator at Barts Health NHS Trust |
| Lidocaine renamed as a non-IMP | ||
| Additional recruitment from spinal orthopaedic and musculoskeletal clinics |
Appendix 11 Missing or incomplete questionnaire data
Eleven participants did not complete, or completed incorrectly, the different components of each questionnaire; this is detailed in Table 30.
| Participant number | Missing data | Details | |||
|---|---|---|---|---|---|
| Baseline | 6 weeks | 3 months | 6 months | ||
| 1002 | All | All | |||
| 1003 | BPI | ‘What treatments or medications are you receiving for your pain?’ – missing | |||
| 1004 | BPI | ‘Please mark on the diagram the area of your pain’ – spoiled | |||
| 1005 | SF-12 | ‘Climbing several flights of stairs’ – missing | |||
| 1006 | All | ||||
| 1007 | Oswestry | ‘Social life’ – spoiled | |||
| 1009 | All | ||||
| 1009 | SF-12 | ‘Physical health, limited to work, emotional problems, did work less carefully’ – missing | |||
| 1010 | SF-MPQ-2 | ‘Hot burning pain, splitting pain’ – missing | |||
| SF-12 | ‘Did work less carefully’ – missing | ||||
| Oswestry | ‘Personal care, sleeping’ – spoiled; ‘sex life’ – missing | ||||
| 1011 | SPS 6 | ‘I felt hopeless about finishing certain work tasks, due to my health problems’, ‘At work, I was able to focus on achieving my goals despite my health problem’ and ‘Despite having my health problem, I felt energetic enough to complete all my work’ – missing | |||
| 1014 | EQ-5D-5L | ‘Pain/discomfort’ – missing | |||
| Oswestry | ‘Sex life’ – missing | ||||
Appendix 12 Graph showing the number of patients screened compared with the recruitment rate by recruitment month
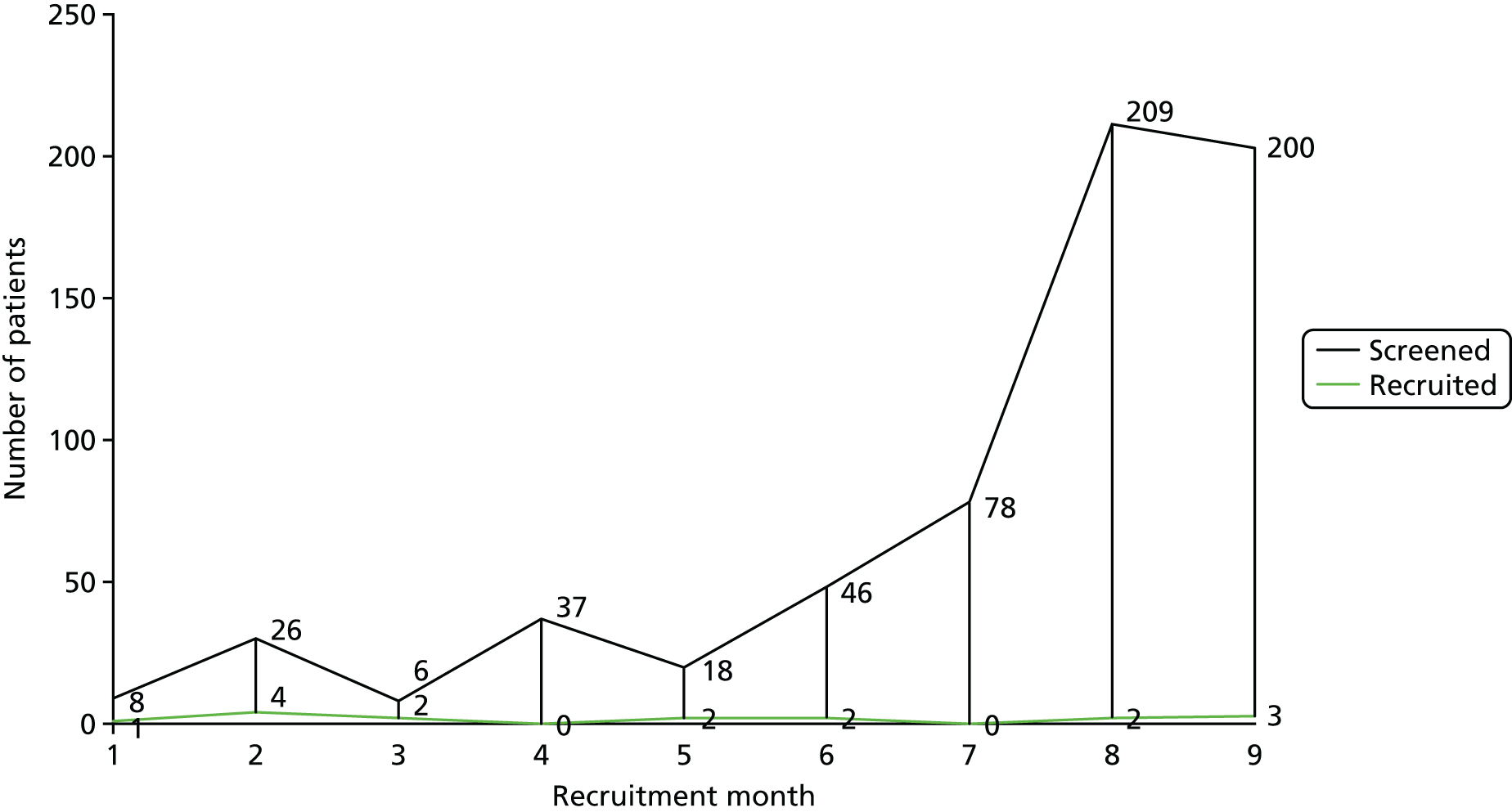
Appendix 13 Flow diagram showing the actual compared with the estimated flow of participants through the study
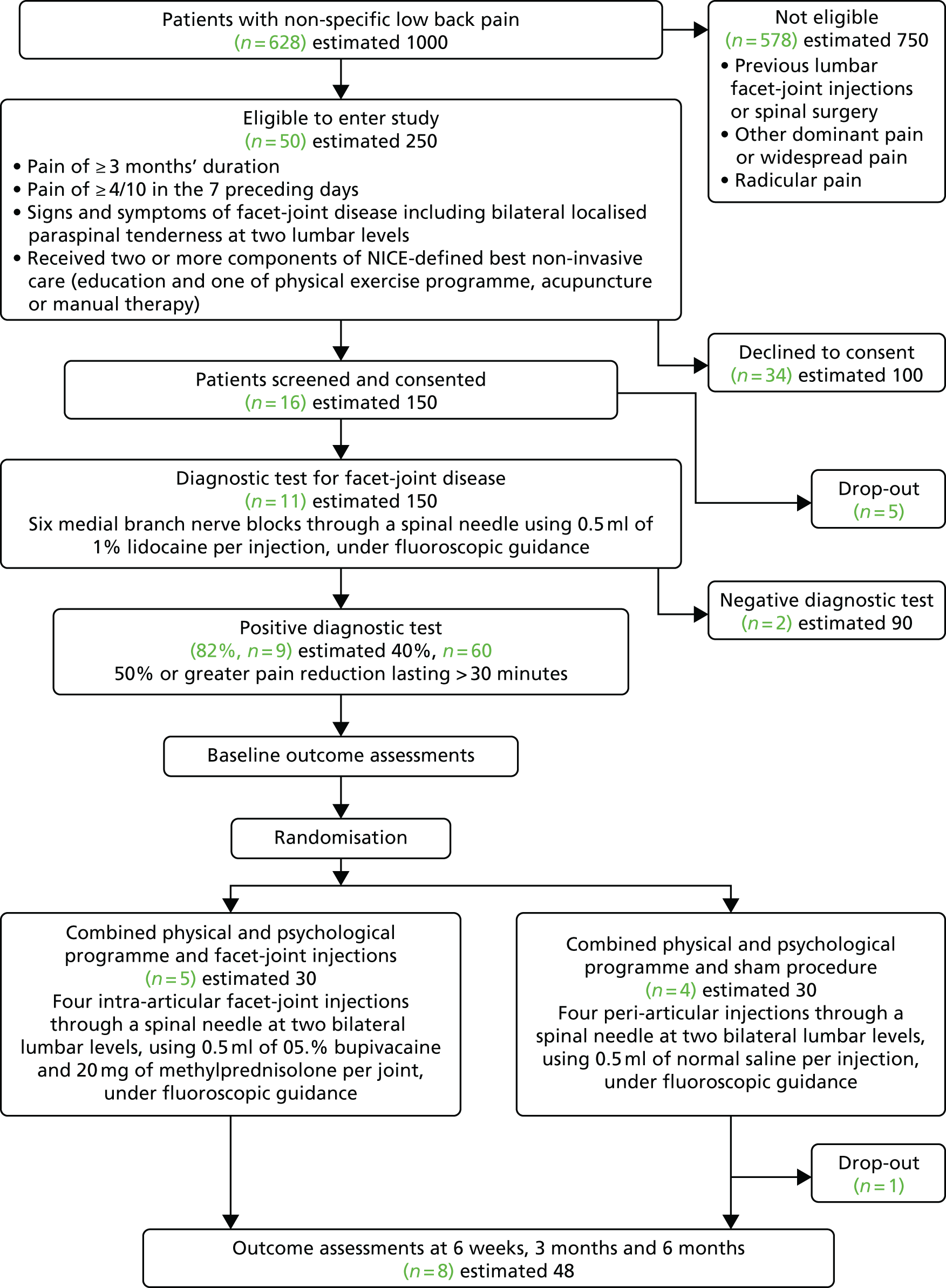
Appendix 14 Limitations of current resource use measures and recommendations for improving the accuracy of capturing future NHS service provision
| Resource use | Limitations and recommendations |
|---|---|
| Medication | Limitations:
|
Recommendations:
|
|
| Visits to health-care professionals | |
| Time scale of data capture at follow-up | Limitation:
|
Recommendation:
|
|
| Hospital inpatient stay | Limitation: no information on mode of admission or reason for admission |
Recommendation:
|
|
| Emergency/A&E attendances | Consider the type of service that trial participants are likely to require. Would they be admitted or treated and discharged? What is the level of investigation and treatment? Treatment and discharge is less costly than treatment and admittance. The construction of appropriate questions to capture information on attendance should be considered if this area of health-care resource use is an important driver of costs for the trial population. If not considered an important cost driver an average cost of £137.74 per attendance can be applied for simplicity |
| Ambulance service | Consider if trial participants would be potential users of the ambulance service and the implications of service type and associated costs:
|
| Outpatient clinics | Note in advance the type of service-led clinic if relevant. For example, a consultant-led outpatient clinic is more expensive than a non-consultant-led clinic |
| GP consultations | Limitation:
|
Recommendation:
|
|
Glossary
- Best usual care
- As described in National Institute for Health and Care Excellence clinical guideline 88, Low Back Pain: Early Management of Persistent Non-specific Low Back Pain, best usual care includes providing patients with advice and information to promote self-management of their low back pain and offering one of the following treatments, taking into account patient preference: an exercise programme, a course of manual therapy or a course of acupuncture.
- Combined physical and psychological programme
- A group-based programme focused around education and targeted training of self-management skills, utilising a psychological approach to improve physical activity and functioning.
- Definitive study
- An adequately powered randomised controlled trial to provide unequivocal evidence that supports or rejects the test hypothesis, for example whether or not a treatment shows a benefit to patients.
- Feasibility study
- A study that asks whether or not something can be done, whether or not we should proceed with it and, if so, how.
- Lumbar facet-joint injections
- The active intervention in which a needle is inserted into the facet-joint and a therapeutic substance, such as a steroid, is injected.
- Sham procedure
- A dummy procedure in which a needle is inserted near the facet-joint but no therapeutic substance is injected.
List of abbreviations
- AMSTAR
- Assessment of Multiple Systematic Reviews
- BeST
- Back Skills Training
- BPI
- Brief Pain Inventory
- CG
- clinical guideline
- CI
- confidence interval
- CPP
- combined physical and psychological (programme)
- DIRUM
- Database of Instruments for Resource Use Measurement
- DMC
- Data Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions five-level version
- FACET
- Feasibility of Assessing the Clinical- and cost-Effectiveness of Therapeutic lumbar facet-joint injections
- FJI
- facet-joint injection
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HRA
- Health Research Authority
- HTA
- Health Technology Assessment
- IMP
- investigational medicinal product
- JRMO
- Joint Research Management Office
- LBP
- low back pain
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NG
- NICE guideline
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRS
- numerical rating scale
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSEQ
- Pain Self-Efficacy Questionnaire
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-MPQ-2
- short-form McGill Pain Questionnaire version 2
- SPS
- Stanford Presenteeism Scale
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
