Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/141/04. The contractual start date was in December 2012. The draft report began editorial review in October 2016 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael J Sweeting reports grants from the National Institute for Health Research during the conduct of the study. Jessica K Barrett reports grants from the Medical Research Council during the conduct of the study. Roger M Greenhalgh serves as a salaried director of BIBA Medical and has an equity interest in the company, and also serves as an expert witness on behalf of patients with vascular disease.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Patel et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Abdominal aortic aneurysm
Abdominal aortic aneurysm (AAA) is defined as a localised enlargement of the abdominal aorta, such that the diameter is > 3 cm or > 50% larger than the normal diameter. 1–3 It was recognised if an aneurysm is found in one site, there is an increased chance of one being found elsewhere in the arterial tree. The commonest by far is AAA and patients with it have increased chance of thoracic aortic aneurysm and also popliteal aneurysm. It was soon recognised that all of the arteries of patients with aneurysms are wider and the term ‘dilating’ arterial disease was used. 4 In addition, it was described as ‘arteriomegaly’. 5 To support the concept that there were other causative factors of dilatation, diabetes mellitus was found in only 1% of ‘dilating disease patients’. 4
Epidemiology and risk factors
Abdominal aortic aneurysm is a common condition affecting particularly men aged > 60 years. In the second half of the twentieth century, there was a steady increase in mortality from aortic aneurysm in England and Wales. 6–8 According to population screening studies using ultrasonography, the prevalence of aortic aneurysm among 65-year-old men is 4%. 9 In the 65–74 years age group, the prevalence increases to 5% in men and to about one-third in women. 10 Following these reports, aneurysm screening programmes, usually of men aged 65 years, were implemented in several countries. These programmes have now reported a much lower prevalence of 1.9% in England and 1.7% in Sweden, respectively. 11,12 As well as the decline in incidence of clinically relevant aneurysms in men in England, Wales and Scotland, the age at which these aneurysms present has increased by 5–10 years. 13 Reports from other countries also show that aneurysm-related mortality is no longer increasing. 14–16
Increasing age and male gender are known to be the strongest associated risk factors. Other risk factors associated with AAAs include smoking and ethnicity. The wall weakens as a result of loss of elastin and collagen17 or fibrillin18 and the dilating process associated with more inflammation. 19
Age, sex and ethnicity
The risk of aneurysm increases dramatically after 60 years of age. Clinically relevant aneurysms (> 4 cm in diameter) are present in approximately 1% of men aged between 55 and 64 years, and the prevalence increases by 2–4% per decade thereafter. 20,21 In the UK, the rate of aneurysm in Caucasian men aged > 65 years is about 4.7%. Rupture of the aneurysm occurs in 1–3% of men aged ≥ 65 years, with a mortality rate of 70–95%. This equates to around 3000 deaths from rupture each year in men aged ≥ 65 years in England and Wales. Aneurysms are much less common in individuals of Asian and African descent. However, when adjusted for age, the rate of aneurysm-related mortality is higher among African Americans than in Caucasians. 22 Other studies have shown that the Asian population (men and women), who tend to be of smaller stature, are disadvantaged when being considered for endovascular aneurysm repair (EVAR), as the presence of smaller vessels is not conducive to easy deployment or long-term durability of the grafts. 23,24
Abdominal aortic aneurysms are four to six times more common in men than in women. 25 In addition, aneurysms develop in women approximately a decade later than in men. However, there is evidence that for women the risk of rupture, growth rates and operative mortality are all higher. 25–30
Smoking
Smoking has been found to be the principal risk factor for aneurysm formation, growth and rupture. 31,32 It is a much stronger risk factor than for coronary artery disease. The duration of exposure rather than the level of exposure appears to determine the risk of the development of an aneurysm in men aged > 50 years. The slow decline of risk after the cessation of smoking and the higher relative risk for small compared with large aneurysms suggests that smoking is a direct causative factor. 32
Other risk factors
Patients with aneurysms frequently have atherosclerosis, and numerous studies show an association with coronary heart disease and peripheral atherosclerosis. 33,34 A host of other risk factors, such as poor lung function,35 hypertension and high cholesterol levels,36,37 have been suggested to increase the risk of aneurysmal development, although there are conflicting reports in the current literature. A positive family history, especially in male first-degree relatives, is also associated with increased risk of aneurysm. 38,39 Studies suggest lower prevalence of diabetes for dilating (aneurysmal) disease, stenosing arterial disease and a protective role for diabetes on the development of aneurysms. 4 Diabetes has also been associated with slower aneurysm growth rates. 37,40,41
To obtain insight into the pathological processes associated with the vascular remodelling that accompanies aortic dilatation, early work in our group compared the histological features and the activity of matrix metalloproteinases (MMPs) in aneurysm biopsies. 19 The histological feature most clearly associated with enlarging aneurysm diameter was a higher density of inflammatory cells in the adventitia. This inflammation was non-specific. We also identified gelatinase A (MMP-2) as the principal metallogelatinase in small aneurysms and an increasing activity of gelatinase B (MMP-9) in large aneurysms. This early work identified that the recruitment of inflammatory cells into the adventitia, with subsequent elaboration of metalloproteinases, including gelatinase B, may contribute to the rapid growth and rupture of larger aneurysms.
We also found that a strong interaction occurs between fibrillin genotype and blood pressure and that this contributes to the development of aneurysmal disease. 18 Marfan syndrome is a congenital disorder of connective tissue that is associated with various systemic complications, and the defective gene has been mapped to the fibrillin-1 (FBN1) gene on chromosome 15. 42
Our group was also involved with early work exploring genetic variants of collagen III and AAA. Variations in collagen structure were recognised in Ehlers–Danlos syndrome type IV and could also be associated with a predisposition to aortic aneurysm. We reported, in 1991, that genetic variants of type III collagen may influence the extensile properties of the aortic wall and that mutations in the type III collagen gene may be associated with aortic aneurysms. 17 More specifically, variation at the haptoglobin locus could have a direct effect on the degradation of elastin in atherosclerotic aorta, whereas variation at the cholesteryl ester transfer protein locus could affect lipid metabolism and promote atherosclerosis. These results indicated that a gene on chromosome 16 was associated with aortic aneurysm.
Around the same time, Irizarry et al. 43 demonstrated the presence of interstitial collagenase [now known as matrix metalloproteinase 1 (MMP-1)] in specimens of AAAs and Curci et al. 44 reported that, because elastin represents a critical component of aortic wall structure and a matrix substrate for metalloelastases, human macrophage elastase may have a direct and singular role in the pathogenesis of aortic aneurysms.
Aneurysms result from chronic weakening of the arterial wall, and approximately 80% occur between the renal arteries and aortic bifurcation. Aneurysmal expansion can also be found in the suprarenal segment and can sometimes extend upwards into the thoracic segment of the aorta above the diaphragm or downwards beyond the aortic bifurcation into the common iliac arteries. When the aneurysm becomes large, it may be diagnosed by examining the abdomen of the supine patient and feeling for a large pulsatile mass. Large aneurysms in thin people are easy to detect, but the accuracy of the clinical examination is much reduced by obese body habitus and small aneurysm size. 45 There is also large variability in the interobserver sensitivity for detection of aneurysms, and even an experienced clinician may miss palpating an aneurysm in the presence of central obesity or abdominal distension. 46
The majority of aneurysms are asymptomatic and, if undetected, the aortic dilatation can continue for many years. It can lead to catastrophic rupture, which is fatal in > 80% of cases. If left untreated, aneurysms have been known to grow to very large sizes, up to ≥ 15 cm, and, in rare cases, have ruptured at more modest diameters as small as 3–4 cm. Large and life-threatening aneurysms are preceded by a long period of subclinical growth in the diameter of the aneurysm (about 1.6 mm/year on average). Although diagnosis is usually incidental, expansion may become painful and lead to pulsating sensations in the abdomen or pain in the chest, lower back or groin.
The treatment options for asymptomatic aneurysms are conservative management, surveillance with a view to eventual repair and immediate repair. Repair is indicated if the aneurysm is > 5.5 cm, grows > 1 cm per year or is symptomatic (tender). 47,48 The successful management of the condition depends on the clinician finding the correct balance between careful surveillance of the aneurysm diameter until it enlarges to a point at which the risk of rupture is deemed to exceed the risk of death from elective surgery.
Detection and treatment of abdominal aortic aneurysm
Detection and screening
Owing to the asymptomatic nature of the disease, a considerable number of cases present as an emergency following a rupture, which has only a 10–20% survival rate. 49 The in-hospital survival rate for treating a ruptured aneurysm is just above 50%, but many patients never have the opportunity to undergo surgical intervention. 21
As the condition is not always found early, population screening programmes have been set up. The basis for such programmes is the known increased risk of rupture for aneurysms > 5.5 cm, whereas neither open surgery50,51 nor endovascular repair52 is of benefit for aneurysms of 4.0–5.4 cm. Both of these aneurysms being more common in men, the number of women affected is much lower and, therefore, the real threshold of intervention could be lower in women, though trial data do not show this. 53
The method of population screening is based on ultrasound. Duplex ultrasound scanning of the abdomen is safe, inexpensive and non-invasive. Ultrasound-based assessments quantify the maximal anterior–posterior and transverse diameter of the aorta. Among asymptomatic patients, ultrasound detects the presence of aneurysms accurately and reproducibly. Sensitivity and specificity are both close to 100% when compared with operative findings, making ultrasound an ideal test for mass screening. 54,55
Ellis et al. 56 showed that variability can be 8 mm if a different operator or system is used, but 4 mm for the same operator and ultrasound system. In the UK Small Aneurysm Trial,57 trained nurses had a reproducibility of 2 mm. The UK Small Aneurysm Trial57 and Aneurysm Detection And Management (ADAM)51 trial criterion of 5.5 cm was based on an outer-to-outer front-to-back ultrasound measurement. Scott et al. ,58 for UK screening, chose inner to inner.
However, when assessing aneurysm size, ultrasound is less precise than computed tomography (CT). Reproducible measurement of aneurysm size is important because it is the single component of prognosis and also because it provides a baseline to determine the aneurysm growth rate. An aneurysm growth rate of > 1 cm/year has been suggested as a threshold for surgery irrespective of size,59 even though there is no evidence to suggest that the growth rate influences rupture risk independently of aneurysm size.
The efficiency of ultrasound-based screening in men aged ≥ 65 years has been very well demonstrated in population-based randomised controlled trials (RCTs) and subsequent meta-analyses. 60–67 The aneurysm-associated mortality rate, the number of ruptured aneurysms and the number of emergency operations can be reduced significantly by a single ultrasound examination in men over the age of 65 years. At the same time, there is a two- to threefold increase in the number of elective aneurysm operations. In the UK, The NHS Abdominal Aortic Aneurysm Screening Programme68 was introduced after research and analysis of data from a number of randomised trials and existing local screening programmes in England that showed a reduction in aneurysm-related mortality when men aged ≥ 65 years were offered ultrasound screening. The evidence was assessed by the UK National Screening Committee against a set of internationally recognised criteria that confirmed that screening all men aged ≥ 65 years saves lives. The programme is aimed at reducing deaths from ruptured aneurysms through early detection, appropriate monitoring and treatment. The introduction of aneurysm screening to men aged 65 years is estimated to reduce premature death from ruptured aneurysms by up to 50% over 10 years. The prevalence of aneurysms in men is three times higher than in women and, currently, there is no good evidence to support aneurysm screening in older women.
Investigation of abdominal aortic aneurysm for possible intervention
In the EVAR trials, CT was used to investigate the suitability of aneurysms for intervention. Initially, three-dimensional scanning was not well advanced, but the technique greatly improved with time, and with it the interpretation of the findings and sizing of the infrarenal aorta.
Follow-up after aneurysm repair
At the beginning of the EVAR trials, routine follow-up of AAAs, even annually, was not usual and patients with open repair (OR) were frequently discharged to primary care. Ultrasound gathered favour with relation to population screening and detection.
Intervention for aortic aneurysm
Intervention for aortic aneurysm depends on the size or diameter of the aneurysm and is a balance between the risk of rupture and the operative mortality for aneurysm repair. Appropriate patient selection and timing of repair for the aneurysm is based on identifying individuals at the greatest risk of aneurysm rupture. Patients undergoing surgical intervention have immediate perioperative risks that must be weighed against the low likelihood of rupture before death from other causes. 69
Frequency of screening
Most people with aneurysms in the diameter range 3.0–5.5 cm are kept under review in surveillance programmes. There is consensus that very small aneurysms (3.0–3.9 cm) have a very small risk of rupture and, therefore, do not require surgical intervention. Most aneurysms initially measuring < 40 mm are very unlikely to expand to a size necessitating repair within 5 years,70 and patients with aneurysms of this size should receive ultrasound surveillance at regular intervals. Over the last two decades longitudinal studies of patients with smaller aneurysms have provided insights into the ideal timing of repair and the need for, and frequency of, ultrasound surveillance if an expectant management strategy is followed. A Cochrane review summarising the results from four trials to date demonstrated no advantage to early repair (via open or endovascular surgery) for small aneurysms in the range 4.0–5.5 cm. A policy of ultrasound surveillance is also advised as the ‘best care’ for patients with asymptomatic aneurysms of this size. 48 Once the aneurysm reaches 5.5 cm (measured by duplex ultrasound in males) or the aneurysm growth rate exceeds 1 cm per year, it is recommended that a vascular surgeon review the patient immediately (or within 2 weeks) to prevent interval rupture. If fit enough for surgery the patients should be considered for elective surgical repair at this juncture. For females, a maximum aortic diameter of 5.0 cm, as measured by duplex ultrasound, is considered to be the point at which a referral to a vascular surgeon is mandated.
Treatment of abdominal aortic aneurysm once detected
Medical therapy
Patients diagnosed with AAA who do not meet the criteria for intervention at the time of the initial diagnosis should be managed conservatively based on the results of randomised trials. The criteria for intervention are an aneurysm diameter of < 5.5 cm, a growth rate of > 1 cm per year or a tender aneurysm. 50 However, the natural history of aneurysms is one of progressive expansion necessitating regular clinical evaluation and surveillance of aneurysm diameter to identify aneurysms that satisfy the criteria for justified intervention.
Medical therapies focus on the management of modifiable risk factors for aneurysm and cardiovascular disease with the goals of reducing the need for intervention as a result of aneurysm expansion or rupture, reducing morbidity and mortality associated with repair, and reducing cardiovascular morbidity and mortality. 71 Although many pharmacological therapies aimed at limiting aneurysm expansion and preventing rupture have been investigated, currently there is no proven focused therapy that reduces aneurysm growth. A systematic review and meta-analysis investigating the impact of a range of pharmaceutical agents (including beta-blockers, other antihypertensive therapies, antibiotics and anti-inflammatory agents including statins) demonstrated little evidence of reduction in aneurysm growth rates. 72 Among the factors associated with aneurysm expansion and rupture, smoking is the most important modifiable risk factor and smoking cessation is recommended for all patients with aneurysms. 73 Although reduced aneurysm expansion and rupture risk have not been clearly demonstrated among those who have stopped smoking, smoking cessation has other benefits. In addition, because aneurysm is regarded as a coronary risk equivalent, most guidelines recommend aspirin, statins and antiplatelet therapy for patients with aneurysms to reduce the risk of a future cardiovascular event. Other medical conditions, such as hypertension, should be treated as appropriate. In general, there is surprisingly little high-quality evidence on medical treatment for small aneurysms, especially in relation to the use of newer beta-blockers, angiotensin-converting enzyme inhibitors and statins. 74
Open surgical repair
For patients whose AAA satisfies criteria for intervention, OR was regarded as the gold standard before these UK trials. It involves an incision of the abdomen to directly visualise the aortic aneurysm. Dubost et al. described how the technique involves access either through a transabdominal route or via a retroperitoneal approach. 75 During OR the aneurysmal portion of the aorta is replaced with a graft, usually made of DACRON® (Invista™, Wichita, KA, USA) or polytetrafluoroethylene. 76 The graft is sutured within the aorta. The DACRON is anastomosed to the aortic neck and either to the aortic bifurcation below (tube graft) or to the two iliac arteries (bifurcation device).
Recovery after OR is substantial and, in the UK, operative mortality has been reported to be 5.6%. 47 Immediately following surgery, patients can expect to spend 1–3 days in the intensive care unit (ICU), followed by 4–10 days on the hospital ward. After discharge, patients will take 3–6 months to fully recover their energy and return to their preoperative daily activities.
Advent of endovascular aortic repair
Before the advent of EVAR, OR was the only surgical treatment available for aortic aneurysms. In the spring of 1990, word came of a novel endovascular technique which was later reported in 1991 by Parodi et al. in Argentina. 77 However, it later transpired that Volodos et al. in the Ukraine (at the time of the Soviet Union), a student of Dotter, had already done such a procedure, unknown to the Western world. 78
By 1996 it was evident that commercial EVAR devices would become available shortly. The urge was to learn the new device method, but it would require comparison with OR for those patients medically fit for OR, although Parodi et al. 77 had championed the method for those patients not fit enough for OR.
The EVAR trials [EVAR trial 1 (EVAR-1) and EVAR trial 2 (EVAR-2)] were set up to meet the above need and funded by the Health Technology Assessment (HTA) programme under the then director, Sir Miles Irving.
Even though a registry of devices was also introduced at about the same time,79 this lacked the RCT discipline of comparison with the then alternative. Sir Miles Irving called for, and delivered, device funding by NHS subventions such that the only way to achieve funding was as part of the EVAR trials. The shorthand for the procedure was taken from these world-first trials: ‘EVAR’.
A feature of the trials was for complete endorsement by the Vascular Surgical Society of Great Britain and Ireland and the British Society of Interventional Radiology. Together these societies recommended collaboration of both disciplines for the trial and together the societies founded the EVAR trial centres.
Endovascular aneurysm repair was seen as a minimally invasive option which involves the insertion of a stent graft into the lumen, effectively excluding the aneurysm from blood flow and minimising the risk of rupture. The concept of endograft is to deploy inside the aneurysm through femoral access to exclude the aneurysm sac from circulation. EVAR requires adequate aortic and iliac fixation sites for effective sealing and fixation. The first devices were assembled in the sterile operating room and deployed. There was inevitable delay before commercial higher-quality devices became available. The earliest systems used handmade stent grafts that allowed a repair to lie entirely within the abdominal aorta (aortoaortic graft). Subsequently, it was shown that the aortoaortic endovascular grafts were suitable for < 10% of patients and bifurcation systems were developed; these allowed a greater proportion of aneurysms to be managed by an endovascular method. 80 In-house systems were introduced in the UK that employed an aorto-uniiliac stent graft system made with the second side being occluded using a DACRON sac and stent and the procedure completed with a femoro–femoral crossover graft. 81,82 This left patients with two small incisions in the groin and minimum pain. These in-house systems were soon superseded as commerical higher-quality devices became available.
The imagined advantages of EVAR over OR included reduced operative time, avoidance of general anaesthesia, less trauma and postoperative pain, reduced hospital length of stay and use of intensive care facilities, reduced blood loss and reduced immediate postoperative mortality.
Post-surgical surveillance
The European Society for Vascular Surgery, in agreement with the EVAR protocol, recommends that all EVAR patients should undergo CT 30 days post procedure. If there is any endoleak, then further CT at 6 and 12 months is recommended. In patients with no early endoleak and stable or shrinking aneurysm, annual duplex ultrasound scanning is recommended. Any subsequent new endoleak or increase in aneurysm sac size should prompt more detailed imaging with CT. 83 Many clinicians now follow patients almost exclusively by ultrasound to measure maximum aneurysm diameter and obtain further imaging only if the sac fails to shrink or expands > 5 mm.
Currently, the European Society for Vascular Surgery recommends colour duplex ultrasound imaging at least once every 5 years following OR. 83 Imaging protocols following EVAR are controversial and currently the topic of much debate.
Endoleaks
The inability to obtain or maintain a secure seal between a vessel wall and a transluminally implanted intra-aneurysmal graft was a complication unique to the evolving technique of endovascular aneurysm exclusion. As the term ‘leak’ had long been associated with aneurysm rupture, the term ‘endoleak’ was proposed as a more definitive description of this phenomenon. White et al. 84 first proposed standardisation of the terminology describing this important sequela to endovascular aneurysm exclusion. This was important to facilitate uniform reporting of clinical trial data vital to the evaluation of this emerging technique.
Endoleaks represent the most common complication of EVAR and occur in 20–25% of patients. 85,86 They are frequently identified in late imaging follow-up and used to justify lifelong follow-up of these patients. If left untreated, endoleaks can lead to fatal ruptures. 87 There are five different types of endoleaks, all of which are characterised by persistent blood flow within the aneurysm sac but outside the stent graft. 88 The classification is determined by the source of vessels that causes the inflow into the sac. Type I endoleaks are leaks at the proximal aorta and stent graft (type IA) or leaks at the distal iliac and stent graft limb (type IB). Type II endoleaks are caused by retrograde flow through collateral vessels into the aneurysm sac. Type III endoleaks are holes, defects or separations in the stent graft material. Type IV endoleaks represent porous graft walls. Type V endoleaks have been described as being as result of endotension with an enlarging aneurysm sac without a visible endoleak. 89 Other complications that can occur after EVAR include device migration90–92 and postoperative stent graft kinking. 93 CT angiography has been the most commonly used modality for follow-up after EVAR and is currently the best method for detecting endoleaks.
Clinical trials comparing endovascular aneurysm repair and open repair
We searched MEDLINE and EMBASE for all articles published from 1 January 2000 to 31 May 2016 using search terms ‘15 year follow-up of EVAR for intact abdominal aortic aneurysm’, ‘long-term elective repair’, ‘abdominal aortic aneurysm’, ‘minimally invasive surgery’, ‘vascular surgical procedures’, ‘endovascular surgery’ and ‘open surgery’.
EVAR trial 1, the first and largest randomised trial of endovascular aortic repair compared with OR of aortic aneurysms, began in 1999, alongside its sister trial EVAR-2, to evaluate the efficacy of endovascular repair in patients too frail to be considered for OR and compared best medical therapy with endovascular aortic repair. 94–96 A number of trials have now been undertaken to compare endovascular with OR, but EVAR-2 remains unique in addressing the question of whether or not endovascular repair is justified in patients in whom OR is not an option, usually on the grounds of poor anaesthetic fitness. Other randomised clinical trials with protocols like that of EVAR-1, comparing open and endovascular repair, followed in the Netherlands [the Dutch Randomised Endovascular Aneurysm Management (DREAM) trial],97 USA [Open Versus Endovascular Repair (OVER) trial]98 and France (Anévrysme de l’aorte abdominale, Chirurgie versus Endoprothése; ACE). 99
EVAR trial 1
Before the current report of late follow-up to 15 years, EVAR-1 offered the longest follow-up of any of the RCTs comparing EVAR with OR up to 10 years. Early 30-day results were reported in 2004,100 mid-term results in 2005101 and long-term follow-up over 8 years (median 6 years) in 2010. 94 Baseline characteristics were not significantly different between treatment groups. The perioperative (30-day) mortality was significantly lower for EVAR (1.8% vs. 4.3%). During 6904 person-years of follow-up, 524 deaths occurred, 76 of which were aneurysm related. Kaplan–Meier survival curves for all-cause mortality among the two cohorts converged at 2 years, demonstrating that the survival advantage favouring EVAR is lost. With regard to aneurysm-related mortality, Kaplan–Meier curves converged at 6 years because of endograft failure leading to late rupture-related mortality, increasing substantially in the EVAR group. Secondary outcomes including graft complications, reinterventions and costs favoured OR over the long term. Although there was a health-related quality of life (HRQoL) benefit with EVAR at 3 months, this benefit was lost at 1 year. Based on these findings, the EVAR-1 investigators concluded that, although EVAR offered lower operative mortality, there was no difference in total or aneurysm mortality. Furthermore, EVAR was associated with increased rates of complications, reinterventions and costs.
The Dutch Randomised Endovascular Aneurysm Management trial
One year after the start of EVAR-1, the DREAM trial, which used a similar protocol to EVAR-1, began enrolling patients from 26 centres in the Netherlands and four centres in Belgium. Three hundred and fifty-one patients whose maximum aneurysm diameter was > 5.0 cm and who were deemed to have acceptable risk for both procedures were randomised to undergo either open or endovascular repair. Operative mortality was reported in 2004, 2-year follow-up in 2005 and long-term follow-up in 2010. 97,102–104 The primary study outcomes were rates of death from any cause and reinterventions. The 30-day postoperative mortality was 1.2% after EVAR and 4.6% after OR. At 2 years post procedure, the cumulative rates of aneurysm-related death remained lower for EVAR than for OR (2.1% vs. 5.7%), a difference that was entirely attributable to the initial perioperative outcomes. 104 Survival curves for all-cause mortality converged at 1 year and, by the end of follow-up, all-cause mortality was equivalent between groups. As with the EVAR-1, the rates of graft-related complications and reinterventions were higher after EVAR.
The Open Versus Endovascular Repair trial
The OVER trial was the first RCT in the USA and enrolled patients between 2002 and 2008 from 42 Veterans Affairs Medical Centres. Again, trial design was based on that of EVAR-1 but only men were recruited. Eight hundred and eighty-one patients with an aneurysm of maximum diameter > 5.0 cm, an iliac aneurysm of diameter > 3.0 cm or an aneurysm of diameter > 4.5 cm and rapid enlargement (> 0.5 mm in 6 months), were randomised to undergo either open or endovascular repair. Interim data analysis was reported in 2009 and long-term results in 2012. 98,105 The primary study outcomes were procedure failure, secondary therapeutic procedures, length of stay, quality of life, erectile dysfunction, major morbidity and death. The 30-day mortality was 0.5% after EVAR and 3.0% after OR. Survival curves converged at 2 years and over 9 years of follow-up, mortality rates were not significantly different between groups (7.0% EVAR vs. 9.8% OR). There were no differences between the two groups in major morbidity, procedure failure, secondary therapeutic procedures, aneurysm-related hospitalisations, HRQoL or erectile dysfunction. In this trial, incisional hernia was included as an OR-related complication. As a result, the rates of secondary intervention were found to be similar between groups (13.7% EVAR vs. 12.5% OR), with the majority of secondary procedures in the EVAR group as a result of endovascular revisions compared with repair of incisional hernia in the OR group.
The Anévrysme de l’aorte abdominale, Chirurgie versus Endoprothése trial
The fourth RCT, with a similar design to EVAR-1, was the ACE trial, which randomly assigned 316 patients with an aneurysm > 5.0 cm to EVAR or OR surgery. 99 As with the trials above, there were no significant differences in the cumulative survival or major adverse events rates at the 1 year’ follow-up and at 3 years’ follow-up. In contrast to the other three trials, operative mortality was also not significantly different (0.6% EVAR vs. 1.3% OR). The need for reintervention was higher in the EVAR group than in the OR group (16% vs. 2.4%).
EVAR trial 2
Unlike EVAR-1, EVAR-2 remains unique and its publication altered global practice. Endovascular repair of AAA was originally developed for patients who were considered to be physically ineligible for open surgical repair. Whereas the four previously described major RCTs enrolled patients who were physically fit enough to undergo either EVAR or OR, EVAR-2 enrolled patients who were too high risk for open surgical repair, randomising such unfit patients to EVAR or observation. The enrolment protocol and data collection methods were identical to those of EVAR-1. 106 Three hundred and thirty-eight patients were enrolled and randomised between September 1999 and December 2003. 107 After mid-term analysis on this cohort, an additional 66 patients were recruited for long-term follow-up, giving a total of 404 patients. 95 A total of 197 patients were randomised to EVAR while 207 were assigned to have no intervention. However, of the 207 patients in the observation arm, 70 eventually underwent some form of aneurysm repair attributable to rupture, rapid growth, symptomatic aneurysm or patient preference. Patients were followed for rates of death, graft-related complications and reinterventions, and costs up to the end of 2009. The 30-day operative mortality was 7.3% in the EVAR group. The overall rate of aneurysm rupture in the no-intervention group was 12.4 per 100 person-years. Aneurysm-related mortality was lower in the EVAR group, but this advantage did not result in any benefit in terms of total mortality. A total of 48% of patients who survived endovascular repair had graft-related complications, and 27% required reintervention within the first 6 years. During 8 years of follow-up, endovascular repair was considerably more expensive than no repair (cost difference £9826).
After the 10-year results of EVAR-2, the funding and enthusiasm for EVAR use in situation dropped as these HTA-funded trials had shown that no operation was not only giving false hope, unnecessary cost and no change in the expected date of death. The per-protocol analysis hinted at some benefit in patients who actually underwent EVAR.
Evidence outside the trials
Outside the evidence from these trials, Schermerhorn et al. 108 recently assessed perioperative and long-term survival, reinterventions and complications after EVAR compared with OR of AAA in propensity score-matched cohorts of Medicare beneficiaries who underwent repair during the period 2001–8 and were followed until 2009. The authors found that endovascular repair, compared with OR, was associated with early survival advantage that gradually decreased over time, with catch-up of mortality after 3 years. The rate of rupture after aneurysm repair was significantly higher under EVAR than OR.
A recent observational study from a single institution in Queensland, Australia,109 reported no differences in 5-, 10- and 15-year survival rates between OR (n = 982, median follow-up 6.5 years) and EVAR (n = 358, median follow-up 4.0 years), but suffered from incomplete patient reporting. The EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair (EUROSTAR) database has reported that rate of secondary sac rupture after EVAR is low for the first 4 years, but after this time the rate appears to increase, particularly in those with known sac expansion. 110 A previous report from the EVAR trials defined a ‘cluster’ of findings (type I endoleak, type III endoleak, type II endoleak with sac expansion, kinking and migration), which was associated with secondary sac rupture with 67% risk of death. 111
There has been no previous comprehensive report of very long-term follow-up of EVAR or OR beyond 10 years. EVAR-1 reported follow-up for aneurysm-related and total mortality for a period of 8 years,94 at which point there was no difference between endovascular and open abdominal aneurysm repair and the problem of secondary sac rupture after EVAR, associated with 67% mortality, was just becoming apparent. 111 The original trial protocol stated that if concerns became apparent about the durability of EVAR, the trial should be extended to address this issue. In this report we present long-term results up to 15 years, of EVAR-1 in terms of aneurysm-related and total mortality, cause of death, aneurysm-related reinterventions and cost-effectiveness. We also report results up to 15 years for EVAR-2 in terms of overall mortality.
Rationale for follow-up from 10 years up to 15 years
In July 1999, the UK EVAR-1 and EVAR-2 trials were commissioned by the NHS research and development (R&D) HTA programme [now known as the National Institute for Health Research (NIHR) HTA programme]. The trials were funded for 4 years (from July 1999 to 2003), followed by a 2-year extension, which was granted to ensure that recruitment targets were met. Subsequently, long-term follow-up support was awarded for a further 5 years of follow-up until July 2010 (up to 10 years of follow-up). The trial objectives were to assess the safety and efficacy of EVAR against the established open surgical treatment in the management of large aneurysms of at least 5.5 cm diameter as measured using CT. Two trials were instigated: EVAR-1 would compare EVAR against OR in patients who were considered fit and suitable for both procedures and EVAR-2 would compare EVAR against no intervention for patients who were considered suitable for EVAR but unfit for OR. The primary outcome for both trials was mortality, with secondary outcomes of complications, reinterventions, HRQoL, adverse events, costs and cost-effectiveness.
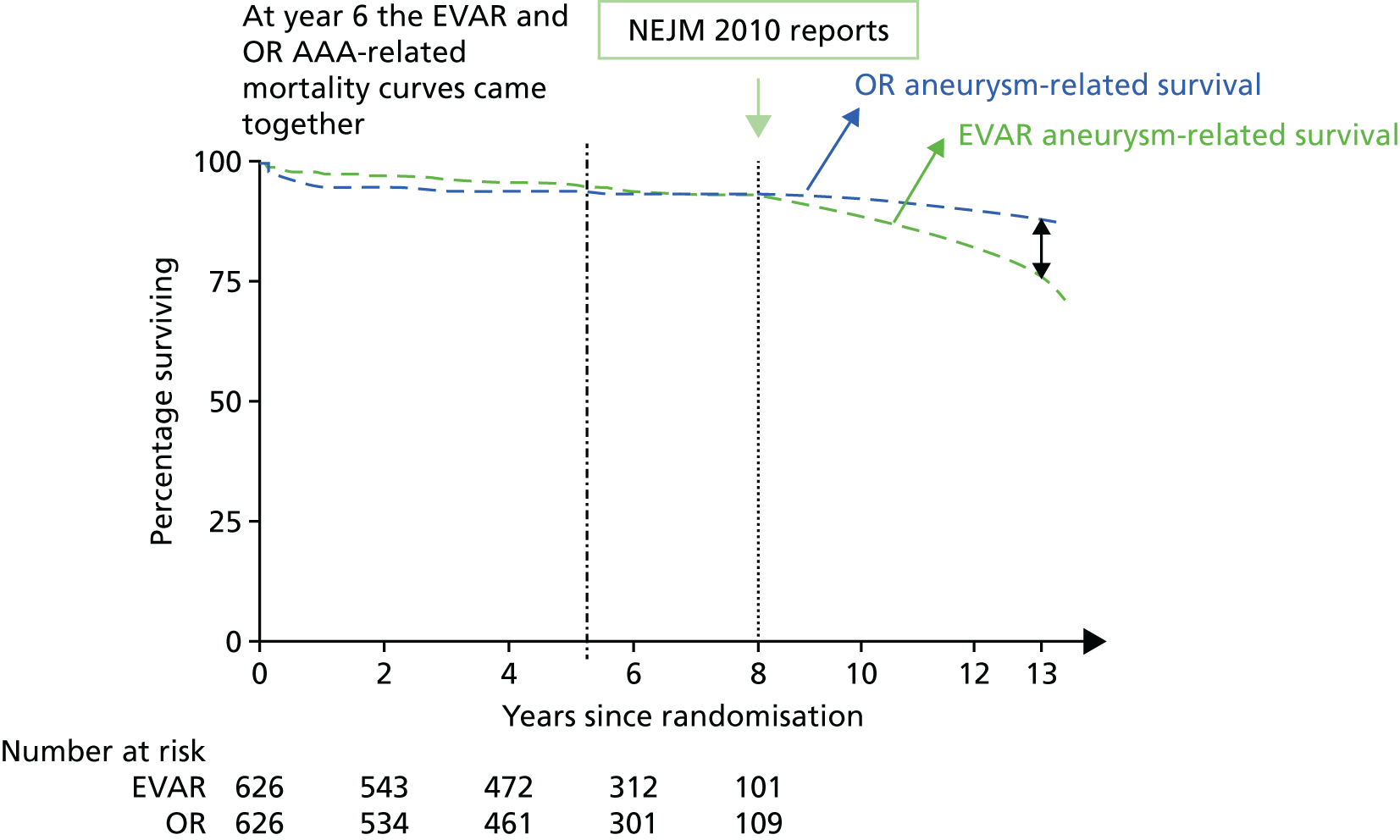
In follow-up until December 2009, 27 sac ruptures had occurred after EVAR, carrying a 67% mortality rate. Type I endoleak, type III and type II with sac expansion, kinking and migration were identified as the ‘cluster’ of complications associated with these late EVAR secondary sac ruptures. 111 It is possible that the early aneurysm-related mortality benefit of EVAR is lost as a result of these late sac ruptures and death. If this trend were to continue, after 15 years, OR would have a lower aneurysm-related mortality than endovascular repair, even with every attempt made to intervene to correct known causes of sac expansion to reduce the risk of secondary rupture in EVAR patients. The possible future projection is shown graphically for EVAR-1 in Figure 1. The rationale for continuing follow-up to 15 years in EVAR-1 was to test this hypothesis. The primary outcome measure for the 15-year follow-up was aneurysm-related mortality and total mortality.
FIGURE 1.
Projected aneurysm-related survival after 8 years of follow-up in EVAR-1 based on observed trends. NEJM, New England Journal of Medicine.
Our secondary outcomes included reinterventions (time to first reintervention, to first reintervention for a life-threatening problem and to first serious reintervention).
Other secondary outcomes included complications, cost and cost-effectiveness. The evaluation of cost-effectiveness of OR and EVAR after a 15-year time period is particularly pertinent because EVAR-related complications continue to occur many years after the original procedure. This objective required knowledge of continuing graft-related complications and use of hospital resources to the end of 2014.
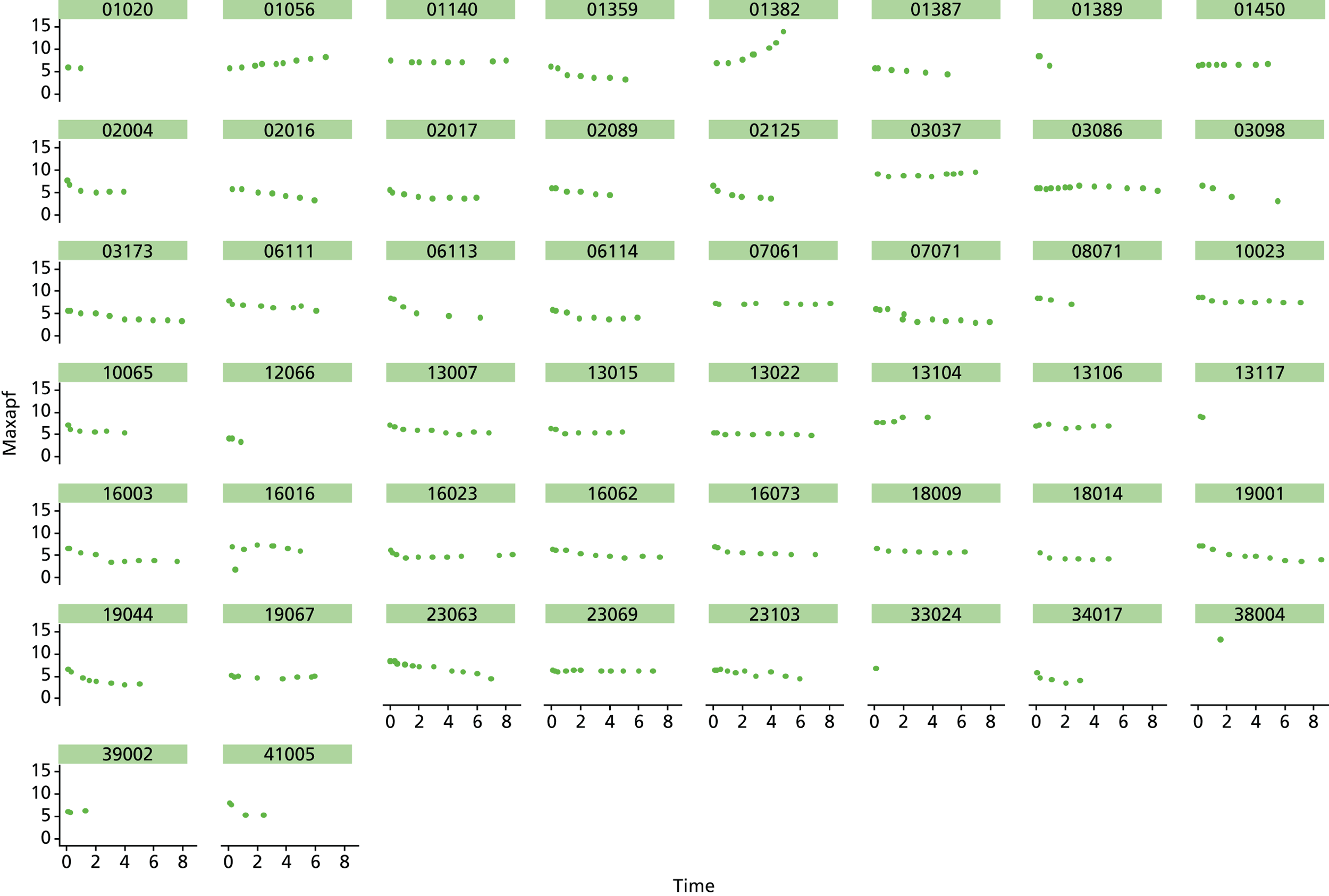
Based on the 27 sac ruptures reported by Wyss et al. ,111 in 2010, it was hypothesised that sac growth after EVAR is associated with an increased risk of rupture and the presence of a significant complication or endoleak. It was therefore assumed that patterns of sac growth might predict the need for reintervention, even in the absence of a defined endoleak. A further outcome was therefore to investigate any association between sac growth (Figure 2) and secondary rupture, complication, endoleak and reintervention correction.
FIGURE 2.
Change in sac diameter of a random selection of 50 patients (post-EVAR measurements only, preoperative measurements excluded). Graphs by ID.
In 2012, there was a call with vignette (NIHR Evaluation, Trials and Studies Coordinating Centre – HTA) for addressing the question ‘What is the optimal management strategy for type II endoleaks?’ The then Director of the HTA programme, Professor Tom Walley, questioned whether or not the HTA-funded EVAR trials would produce data that could answer this question. The authors of the EVAR trials considered the Director’s question and considered that an individual patient data (IPD) meta-analysis of EVAR-1, the DREAM trial, the OVER trial and ACE would produce much useful information and among it some on type II endoleaks. We informed the HTA programme that we would be able to address the question ‘What is the optimal management strategy for type II endoleaks?’ without requiring further data collection.
An IPD meta-analysis of four multicentre randomised trials of EVAR compared with OR was conducted to a prespecified analysis plan, reporting on mortality, aneurysm-related mortality and reinterventions.
Data from the IPD meta-analysis have been used to address the question of the management of type II endoleaks following EVAR.
Chapter 2 Methods
Detailed descriptions of the trial design, methodology and results after 10 years of follow-up have previously been reported, including an earlier HTA programme report which was published in February 2012. 96
Participants
Men and women aged ≥ 60 years were enrolled into EVAR-1 between 1 September 1999 and 31 August 2004 from 37 hospitals in the UK. Twelve hundred and fifty-two patients whose maximum aortic aneurysm diameter was ≥ 5.5 cm, with aortic morphology compatible with endograft placement within the manufacturer’s instructions for use (IFU) and who were deemed to have an acceptable risk of postoperative death for both procedures were randomised to undergo either OR or EVAR.
EVAR trial 2 was designed to address the question of whether or not endovascular repair reduces the rate of death among patients who were considered to be physically ineligible for OR. During the same time period, patients of both sexes who were at least 60 years of age with large aneurysms (≥ 5.5 cm in diameter) and who were deemed unfit for OR, were randomly assigned to undergo either EVAR or no repair.
Interventions
In EVAR-1, patients were randomised to undergo either OR or EVAR. In EVAR-2, patients were randomly assigned to undergo either EVAR or no repair.
Objectives
The objective was to follow up the world’s first RCT of EVAR compared with OR with up to 15 years of follow-up; to reconvene the EVAR trial centres and brief them on the uncertainty of outcome versus OR in the very long term and to inform them of the risk of secondary rupture;111 to re-establish a link via the new EVAR trial manager and identify and brief the current lead vascular surgeon and radiologist at each of the 37 trial centres; with funding for clinical trial data collection and results of CT scanning or other imaging, to identify the contacts for these purposes; and then to be in a position to achieve the 10–15 years of additional clinical follow-up for all alive patients from 1 September 2009.
Outcomes (measures)
Our primary outcome was aneurysm-related mortality and total mortality. Aneurysm-related mortality was defined as all deaths from aneurysm rupture before repair, within 30 days of the primary procedure, within 30 days of any reintervention attributable to the aneurysm, from other aneurysm-related causes (including graft infection or fistula), or from secondary aneurysm rupture after repair. Our secondary outcomes included reintervention (time to first reintervention, first reintervention for a life-threatening problem and first serious reintervention). Further secondary outcomes included complications, sac growth and risk of late complications, costs and cost-effectiveness.
For the primary mortality outcome, patients were followed up from 1 September 1999 to 30 June 2015 [using record linkage from the Office for National Statistics (ONS), with death classification based on death certificates and clinical information provided to the Endpoint Committee]. Patients were followed up for death (total and aneurysm related), dates as above, and for graft-related complications and reinterventions from 1 September 2009 to 31 March 2015. For graft-related reinterventions between 1 September 2009 and 31 March 2015, follow-up was predominantly using record linkage to administrative data for hospital readmissions and reinterventions via Hospital Episode Statistics (HES).
Reinterventions, including incisional hernia repair throughout the trial and other operative procedures preceding death, were subsequently checked with the trial centres, with 89% concordance between administrative and clinical site data. Graft-related complications and reinterventions were also directly obtained from the trial centres with a new case record form for our follow-up between 1 September 2009 and 31 March 2015. The primary analysis compared rates of total mortality and aneurysm-related mortality to 30 June 2015; aneurysm-related mortality was adjudicated and confirmed by the Endpoint Committee.
Sample size
As of 1 September 2009, there were 711 patients with an average age of 80 years reported alive and under follow-up in EVAR-1 (357 and 354 in the EVAR and OR groups, respectively), and 96 patients with an average age of 82 years reported alive and under follow-up in EVAR-2 (50 and 46 in the EVAR and no-intervention groups, respectively). This gives 80% power at the 5% significance level to detect hazard ratios (HRs) of 1.25 and 1.83 during the extended follow-up periods in EVAR-1 and EVAR-2, respectively, assuming 10% to be still alive at the end of June 2015.
Randomisation and blinding
Randomisation was conducted using computer-generated sequences of randomly permuted blocks stratified by centre at the trial hub (Charing Cross Hospital). Patients in EVAR-1 were randomly allocated (1 : 1) to undergo either OR or endovascular repair. In EVAR-2, patients were randomly allocated (1 : 1) to undergo either EVAR or to have no intervention. There was no blinding in either trial.
Statistical methods
All analyses were performed according to a predefined statistical analysis plan and were based on the intention-to-treat (ITT) principle, with outcomes assessed from the time of randomisation. Cox regression modelling was used to compare total mortality, aneurysm-related mortality and time to first graft-related reintervention. Crude regression estimates were presented as well as ones adjusted for two sets of baseline covariates: primary adjustment for age, sex, aneurysm diameter, forced expiratory volume in 1 second, log-creatinine level and statin use; secondary adjustment for the primary covariates as well as body mass index (BMI), smoking status (current, past and never), systolic blood pressure and serum cholesterol level. For the graft-related reintervention analysis, additional secondary adjustment was made for top neck aortic diameter at the level of the lowest renal artery, neck length (distance between the lowest renal artery and the start of the aneurysm) and common iliac diameter (largest of both legs). The primary and secondary adjustment results were very similar and the latter are reported. Baseline data were almost complete, with 94% and 92% of patients in EVAR-1 and EVAR-2, respectively, having a complete set of covariates for the adjusted analyses. HRs were presented as the EVAR group relative to the OR group for EVAR-1 and the EVAR group relative to the no-intervention group for EVAR-2. Owing to non-proportional hazards during the first 8 years of follow-up,94,95 data were analysed by splitting follow-up into four time periods: 0–6 months, 6 months–4 years, 4–8 years and > 8 years. HRs presented in these time periods help to explain the non-proportionality, but must be interpreted conditional on surviving up to the time point in question and are therefore dependent on how the randomised groups fared in the follow-up prior to the time period. Deviations from the proportional hazards assumption were assessed overall and within these periods by regressing scaled Schoenfeld residuals against log of time. Regression estimates are presented both unadjusted and adjusted for baseline covariates. Kaplan–Meier estimates were used to show survival probabilities up to 15 years in each group. Tests of interaction were performed for total mortality and aneurysm-related mortality between randomised group and sex, age and aneurysm diameter (the last two as continuous variables).
In EVAR-1, two sensitivity analyses were performed to allow inclusion of patients with missing covariates in the adjusted models: first, the missing indicator method112 and, second, multiple imputation using chained equations that included terms for the event outcome and the estimate of the cumulative baseline hazard. 113–117
In addition, a per-protocol analysis was performed in EVAR-1 on data from patients who had undergone their randomly assigned treatment. This analysis excluded patients who did not undergo aneurysm repair, those who underwent emergency repair, those in whom the repair was abandoned during surgery (i.e. the aorta was left unrepaired) and those who did not undergo the randomly assigned procedure.
In the EVAR-1 analysis, time to first reintervention analyses were conducted separately for any graft-related reintervention, any serious reintervention (see two or three stars in Table 1) and any life-threatening condition (see four or five stars in Table 1). The criteria used to censor individuals are provided in Severity score for reinterventions. Further analyses were also undertaken in patients without any reintervention between randomisation and 2 years and without any reintervention between randomisation and 5 years.
All analyses were carried out in Stata version 13 (StataCorp LP, College Station, TX, USA). The Data Monitoring and Ethics Committee (DMEC) approved the statistical analysis plan.
Methods used for very long-term follow-up and changes to protocol
Patients in both trials were followed up comprehensively for rates of perioperative and late death, graft-related complications, reinterventions and resource use until September 2009. 94
By the end of the 10-year follow-up in September 2009, three of the participating centres had merged with other local participating centres into single NHS trusts (Leeds, Belfast and Glasgow). All the trial participants at four further centres that had recruited very small numbers of patients had died and so were also not included in late follow-up. A total of 31 centres therefore participated in late follow-up to 15 years. Ethics approval for extended patient follow-up was obtained on 16 February 2011 from the UK North West Multicentre Research Ethics Committee, which did not require patients to be reconsented for ongoing follow-up of the EVAR trials up to 15 years (reference number 98/8/26 for EVAR-1 and reference number 98/8/27 for EVAR-2). Patient consent for the ongoing audit of medical notes for follow-up was given by each patient before randomisation. The EVAR trials’ extended follow-up for both trial 1 and trial 2 was to be based on a review of hospital notes and continued flagging with the Medical Research Information Service for date and cause of death. As with the earlier follow-up, a lead clinician and a local co-ordinator were identified at each of the centres. The co-ordinator was to be remunerated for annual data extraction of clinical information from patient hospital notes relating to aneurysm-related complications and reinterventions from routine hospital visits for surveillance of the aneurysm repair for 2010–14.
Very long-term follow-up for reinterventions: October 2009 to March 2015
For the current phase of very long-term follow-up, funding was not achieved until 26 April 2012 and the trial manager was appointed on 1 December 2012. There was a delay in the signing of contracts between Imperial College London and the NIHR. The collaboration agreement was a four-way agreement that included the University of Cambridge (statistics) and the University of York (cost-effectiveness). Final contracts were not signed until 28 August 2013. In addition, local R&D approval was required from the 32 NHS trusts (30 in England, one in Scotland and one in Northern Ireland). First contact with each of the NHS trust R&D offices was made by the trial manager in December 2012. This process was not completed until 28 January 2014. A new case record form (see Appendix 1) was designed with the expectation of collecting data on complications and reinterventions retrospectively for 2010–12 from clinical notes review and then prospectively for a further 3 years. Imaging scans and reports were also gathered as well as information on adverse events such as strokes, myocardial infarctions (MIs), chronic renal failure and amputation.
Owing to the delays described above, prospective data collection did not start until late in 2013. After 1 year of data collection, it became clear that for EVAR-1 the majority of OR patients (72%) and some EVAR patients (32%) were no longer being followed up at the original trial centres and some hospital mergers had taken place leading to patients being followed up at non-trial hospitals. For EVAR-2, nearly all of the patients had died. At a meeting of the Data Management Subcommittee of the Trial Management Committee (TMC) on 4 November 2014, it was decided to obtain permission for record linkage follow-up for all English trial patients alive or lost to follow-up in September 2009, by obtaining administrative data for hospital readmissions and procedures for patients from EVAR-1 only. There were so few EVAR-2 patients surviving, that given the need to provide robust justification to obtain ethics approval for using administrative data, this option was not pursued for EVAR-2 patients.
Obtaining permission to do this was a two-stage procedure. As patients had not given consent for access to their routine hospital administrative data on enrolment during 1999–2004, we had to obtain ethics approval (section 251) from the Confidentiality Advisory Group (CAG) to enable disclosure of confidential patient information. This required a lengthy process to demonstrate our institution met the stringent information governance requirements. The application to the CAG was submitted on 23 September 2014 and approval was granted on 18 February 2015. The second part of the process involved an application for HES data, which was submitted on 19 February 2015. HES is a government data warehouse containing records of all patients, tracked by their unique NHS number, admitted to NHS hospitals in England and contains details of inpatient care, outpatient appointments and accident and emergency attendance records. HES data allowed us to obtain patient data on all hospital readmissions and procedures (with procedure coding) until 31 March 2015 for 655 of the 724 (90%) patients alive in September 2009, including 13 patients previously lost to follow-up. Only seven English patients evaded HES record linkage. All procedures identified via HES which were potentially related to the original aneurysm repair, including abdominal hernia repairs, were validated with the relevant trial centre or where possible with other non-trial hospitals. There was 89% concordance between administrative data and clinical site data.
Sixty-nine patients, including 62 in either Scotland or Northern Ireland, could not be followed up via HES, of whom 48 (70%) had local follow-up at the trial centre. These 48 patients included 8 out of 15 patients in Scotland, 38 out of 47 patients in Northern Ireland and two out of seven English patients who were unmatched by HES. There were no Welsh centres. This process of using complementary sources of information from record linkage with local validation increased the completeness of patient follow-up in EVAR-1 from 51% to 97%, with only 25 patients being lost to follow-up for reinterventions by 31 March 2015.
Given the time taken for this process, the NIHR agreed to the project end date being extended until 31 August 2016.
Severity score for reinterventions
There is no recognised reporting system for reintervention or reintervention severity, to facilitate allocation of resource use. Therefore, the grading of the severity of aneurysm-related reinterventions and the associated use of high dependency or intensive care (high-dependency unit; HDU/ICU) were obtained by a questionnaire that was sent to each of the principal investigators at the 32 trial centres. Thus the EVAR trials severity score obtained is shown in Table 1.
| Reintervention category | Severity | Mean estimated days in HDU | Mean estimated days in ICU |
|---|---|---|---|
| Conversion to OR | ***** | 2.1 | 1.9 |
| Reintervention for graft infection: OR | ***** | 2.9 | 2.9 |
| Reintervention for graft infection: EVAR | ***** | 2.3 | 0.7 |
| Reoperation of OR: OR | ***** | 2.5 | 2.7 |
| Known aneurysmal extension above or below original graft: OR | ***** | 2.2 | 1.6 |
| Known aneurysmal extension above or below original graft: EVAR | ***** | 1.3 | 0.5 |
| Replacement stent graft | ***** | 1.5 | 0.6 |
| Fenestrated EVAR | ***** | 1.7 | 0.3 |
| Axillobifemoral bypass graft | ***** | 1.2 | 0.0 |
| Added stent; proximal (type Ia endoleak) or distal (type Ib endoleak) | **** | 0.4 | 0.1 |
| Staple or ligation: EVAR | *** | 0.5 | 0.0 |
| Embolisation (of endoleak) | *** | 0.1 | 0.0 |
| Sclerosis of endoleaks | *** | 0.1 | 0.0 |
| Femorofemoral crossover graft | *** | 0.2 | 0.0 |
| Distal limb procedure/revascularisation: OR | *** | 0.6 | 0.0 |
| Distal limb procedure/revascularisation: EVAR | *** | 0.1 | 0.0 |
| Amputation | *** | 0.3 | 0.0 |
| Reintervention for thrombosis of graft limb: EVAR | ** | 0.6 | 0.2 |
| Reintervention for thrombosis of graft limb: OR | ** | 0.6 | 0.2 |
| Incisional hernia: OR | ** | 0.5 | 0.0 |
| False femoral aneurysm: OR | ** | 0.1 | 0.0 |
| Minor reintervention | * | 0.0 | 0.0 |
Reinterventions recorded during follow-up included those that took place during primary admission and subsequent admissions. Also included are previously ignored reinterventions for ORs [e.g. incisional hernias (see Table 1 for full list of reinterventions recorded)]. The date of any reintervention during the primary admission was taken to be the mid-point between the date of admission and the date of discharge or in-hospital death. The censoring time for reinterventions was determined as follows:
-
For all patients from Scotland and Northern Ireland and those from England unmatched in HES with a known follow-up in 2014/15, the date of their latest follow-up was used for censoring.
-
For alive patients in Scotland and Northern Ireland and those from England unmatched in HES without a follow-up in 2014/15, the date of last follow-up or the date of audit of their notes was used for censoring.
-
For all patients in England tracked via HES, censoring was taken as 31 March 2015, unless the date of death was earlier.
-
For dead patients without a follow-up in 2014/15, the date of death was used for censoring, providing it occurred within the year after their last follow-up or date their notes were audited, otherwise these latter dates were used for censoring.
Very long-term follow-up for complications: October 2009 to March 2015
As administrative data do not report complications, only reinterventions, this meant that it would no longer be possible to conduct a comparison of complication rates by randomised group. Complication data were obtained in satisfactory numbers only for analysis of complication rate by time period in the group randomised to EVAR.
Complications were only obtained from the trial centres using the new case record form for late follow-up (see Appendix 1). The new case record form was designed to capture all information about the cluster of complications identified by Wyss et al. 111 as preceding rupture as well as all reinterventions over the whole 5-year follow-up period. Trial centres were reminded that all patients should continue in regular follow-up (the protocol specified annual follow-up including clinical, imaging and serum creatinine level assessment) and all patients, including lapsed follow-ups, should be recalled for a final clinical and imaging follow-up in 2014. As previously, the management of aneurysm-related complications was left to the discretion of the trial centre.
Very long-term follow-up for mortality: October 2009 to June 2015
Follow-up for mortality was unchanged throughout the trial and data for the date, place and cause of death from the ONS were obtained from September 2009 to June 2015. These data, together with information about reinterventions, were assessed by the Endpoint Committee, who were blinded to randomised group and trial in which the patient participated. The committee considered all events from both EVAR-1 and EVAR-2.
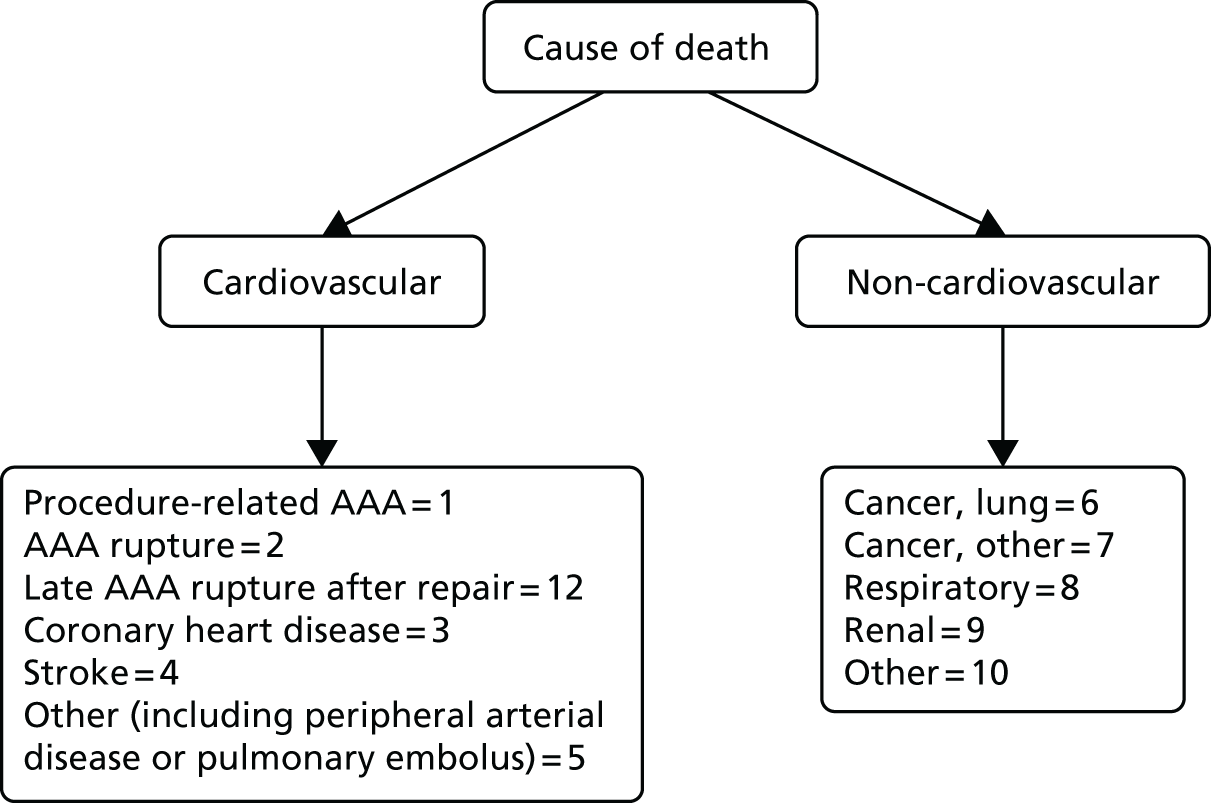
All death certificates were reviewed by an Endpoint Committee to agree cause of death (Figure 3). Aneurysm-related mortality was defined as all deaths (1) from aneurysm rupture before repair, (2) within 30 days of the primary procedure, (3) within 30 days of any reintervention attributable to the aneurysm, and (4) other aneurysm-related causes (including graft infection or fistula) or from secondary aneurysm rupture after repair. Deaths for which the underlying cause was attributed to International Classification of Diseases, Tenth Edition codes I713–719 were also classified as aneurysm related.
FIGURE 3.
Classification of death codes assigned by the Endpoint Committee.
Imaging follow-up
The original EVAR trials protocol included annual imaging via a CT scanning to record the aneurysm sac diameter in EVAR patients and anastomosis diameter in OR patients. For the late follow-up, wherever CT scanning was performed as part of routine follow-up, images were copied for transfer to the trial co-ordinating centre. After 2010, many of the centres had reverted to using duplex ultrasound and the number of CT scans available was largely curtailed. Often with duplex scans the infrarenal maximum outer-to-outer anteroposterior diameter is recorded. For EVAR, crucially, maximum sac diameter is an essential finding. Endoleaks and other complications were noted from the imaging reports reflecting the opinion of the local NHS radiologist. All CT scans that were available were examined in the trial centre core laboratory by a skilled and trained vascular fellow to note known factors associated with eventual secondary sac rupture.
Additional 231 patients not included in earlier reports
After publication of the 30-day mortality results (not the primary outcome of the trial), 26 of the 37 centres remained in equipoise and wished to continue randomising patients until the primary outcome results (mortality after minimum 1-year follow-up from randomisation) were available. The chairperson of the DMEC approved this further recruitment, provided it was undertaken as a separate study, which could report on these later cases, with more experienced clinicians responsible particularly for EVAR. Two hundred and thirty-one additional patients were therefore randomised to a separate study between 1 September 2004 and the publication of mid-term results on 15 June 2005 and are being reported for the first time. One hundred and seventy-five patients were randomised to either OR or EVAR in EVAR-1 and 56 patients to either EVAR or no intervention in EVAR-2 (total 231 patients). Patient follow-up for mortality was the same as for the main EVAR-1 and EVAR-2 patients. In EVAR-1 this included a further 175 patients who have now been included in sensitivity analyses for mortality. Of these 175 patients, 88 patients were randomised to the EVAR arm of the study, of whom 70 were alive in September 2009. Eighty-seven patients were randomised to the OR arm of the study, of whom 66 were still alive in September 2009. For EVAR-2, 28 patients were randomised to EVAR, of whom 11 were alive in September 2009 and 28 patients were assigned to the no-intervention arm, of whom eight were alive in September 2009. The delays described above made it very challenging to obtain retrospective follow-up data for these 231 patients. Evaluation of data collection for these patients by the Data Management Subcommittee of the TMC on 30 June 2014 showed follow-up information on only one-third. Where available, we therefore focused on obtaining details of the primary operation for these additional patients. This information included (1) the preoperative diameter of the aneurysm; (2) the date of operation; (3) whether the patient had an EVAR or OR; and (4) if EVAR, which device was used. All of these additional 231 patients were registered with the Medical Research Information Service for mortality reporting. This allowed the 175 EVAR-1 patients to be included in sensitivity analysis for all-cause mortality, but little else, including the impact of later generation endografts on complication and reintervention rates.
Data management
All data transferred to the co-ordinating centre at Imperial College London were entered into the trial database by the trial manager based at Charing Cross Hospital. Data were handled in a secure way according to good clinical trial practice with due consideration for patient data confidentiality. The pre-existing database and new database for late follow-up were both stored securely on a dedicated, stand-alone (non-networked) computer system that was maintained in a locked office. The system was protected by strong passwords and advanced encryption standard (14 rounds and a 256-bit key). It was accessible only to the trial manager and the data manager. The trial manager was the only person responsible for data entry. Data entry errors were assessed by carrying out double data entry on a randomly selected 10% of patients. The case record form used for late follow-up is included in Appendix 1.
The approval for obtaining HES data was granted on the condition that all data received should be stored on the Imperial College Healthcare NHS Trust system accessible to only the trial manager. The reason for this stipulation was the lack of a satisfactory information governance toolkit assessment at Imperial College London which the NHS trust had in place. The information governance toolkit is a performance tool produced by the Health and Social Care Information Centre for the Department of Health. It draws together legal rules and central guidance and presents them in one place as a set of information governance requirements. Organisations are required to carry out self-assessments of their compliance against these requirements, which include management structures and responsibilities, confidentiality and data protection and information security. A realistic time scale for completion of a satisfactory toolkit assessment is therefore in the order of several months, and in 2015 very few universities had a satisfactory assessment in place. This requirement was the reason for the delay in obtaining HES data and was satisfactorily resolved as a result of our institutions partnership with the NHS trust.
Computed tomography images that were transferred anonymously to the trial co-ordinating centre were also treated with the same level of security and confidentiality. These were subsequently transferred to the dedicated research workstation in the core laboratory at Charing Cross Hospital.
Patient and public involvement
Patient and public involvement has been a prominent aspect in the EVAR-1 and EVAR-2, and never more so than when explaining to patients the difference of the methods offered at randomisation. During follow-up we were increasingly concerned that failure to carry out clinical and imaging annual assessments could be detrimental to patient outcomes. Thus, the trial manager visited every trial centre co-ordinator who was given a copy of the Wyss paper (2010)111 to stress the need for ongoing follow-up and for this to be explained by them to patients.
In February 2015, we were granted approval to obtain confidential data on hospital admissions and procedures via HES. In June 2015, data were obtained for patients who were participants in EVAR-1 at hospitals in England and who had been lost to aneurysm-related follow-up. In order to obtain approval from the CAG we interviewed (face to face), trial patients at routine hospital visits. We explained to the patients that confidential records were held in a government database which stores information about all patient hospital visits in England. We asked them their view on access to confidential data from NHS and government records. All interviewed patients stated that they would be happy for us to access information in this way.
Organisational structure and committees
The late follow-up of the EVAR trials was managed centrally by the principal investigator (Professor Roger Greenhalgh), the trial manager (Dr Rajesh Patel) and Professor Janet Powell (co-applicant), who are based at the Charing Cross Hospital site of Imperial College London. Statistical expertise was provided by Dr Michael Sweeting (University of Cambridge) and costs and cost-effectiveness expertise were provided by Dr David Epstein who related to Professor Mark Sculpher (University of York).
Trial Management Committee
This committee was concerned with the day-to-day running of the trials and related to both the DMEC and the Trial Steering Committee. It was chaired by Professor Roger Greenhalgh and members included Professor Janet Powell (vascular biology), Dr Michael Sweeting (statistics), who related to Professor Simon Thompson, Dr David Epstein (health economics), Mr Colin Bicknell (vascular surgeon), Miss Regula Von-Allen (vascular surgeon), Mr Thomas Wyss (imaging core laboratory) and Dr Nick Burfitt (interventional radiologist). Dr Rajesh Patel (trial manager) attended all meetings to update committee members on trial progress and highlight any problematic issues.
Data Monitoring and Ethics Committee
This committee was regularly updated on trial progress and the DMEC communicated with the Trial Steering Committee. The committee was chaired by Professor Gerry Fowkes and included Dr Robert Morgan from the British Society of Interventional Radiology and Professor Bruce Campbell from the National Institute for Health and Care Excellence (NICE).
Trial Steering Committee
This committee was chaired by Professor Richard Lilford (University of Birmingham) and included Roger Greenhalgh for the applicants and TMC, as well as Michael Wyatt (Newcastle), Simon Thompson (statistics) and Mark Sculpher (health economics). The role of the committee was to liaise between the DMEC and TMC and oversee any issues relating to the progress of the trials or needs for additional funding.
Endpoint Committee
This committee was chaired by Professor Janet Powell and included an independent vascular surgeon (Professor Alison Halliday) and a consultant cardiologist (Dr Simon Gibbs). All death certificates were centrally coded at the ONS and were reviewed by this committee in relation to any aneurysm-related procedures. All available data relating to the death and a primary underlying cause of death were classified according to the groupings presented in Figure 3, where death codes 1, 2 and 12 were classified as aneurysm related. Aneurysm-related deaths were defined as all deaths occurring within 30 days of the primary aneurysm repair or any reintervention for a graft-related complication unless over-ruled by post-mortem findings or a separate procedure (unrelated to the aneurysm) that took place between the aneurysm intervention and death (code 1); all deaths from rupture of an unrepaired aneurysm (code 2); and all deaths from rupture of a repaired aneurysm, usually endograft rupture (code 12). In addition, late complications of aneurysm repair, such as aortoduodenal fistula or bowel obstruction, were recorded as procedure-related deaths (code 1).
Chapter 3 Results for EVAR trial 1
Patients
From 1 September 1999 until 31 August 2004, we recruited 1252 patients to participate in this trial, who were equally, and randomly, assigned to the two treatment groups. There were no differences in baseline characteristics between the groups, mean age 74 years, 1135 men (Table 2). 101
| Baseline characteristics by randomised group for EVAR trial 1 | ||
|---|---|---|
| Baseline characteristica | EVAR (n = 626) | OR (n = 626) |
| Age (years) | 74.1 (6.1) [0] | 74.0 (6.1) [0] |
| Number of males (%) | 565 (90) [0] | 570 (91) [0] |
| AAA diameter (cm) | 6.4 (0.9) [0] | 6.5 (1.0) [1] |
| BMI (kg/m2) | 26.5 (4.6) [1] | 26.5 (4.3) [6] |
| Diabetes (%) | 61 (10) [2] | 68 (11) [6] |
| Smoking status (%) | [1] | [1] |
| Current | 134 (21) | 136 (22) |
| Past | 419 (67) | 444 (71) |
| Never | 72 (12) | 45 (7) |
| History of cardiac diseaseb (%) | 269 (43) [0] | 261 (42) [0] |
| Systolic blood pressure (mmHg) | 148 (22) [5] | 147 (21) [2] |
| Diastolic blood pressure (mmHg) | 82 (12) [7] | 82 (13) [3] |
| ABPI (mean of both legs) | 1.01 (0.18) [13] | 1.03 (0.18) [27] |
| FEV1 (l) | 2.1 (0.7) [8] | 2.2 (0.7) [4] |
| Serum creatinine level (µmol/l)a | 102 (91–118) [1] | 102 (90–120) [4] |
| Serum cholesterol level (mmol/l) | 5.1 (1.2) [18] | 5.1 (1.1) [25] |
| Statin use (%) | 216 (35) [7] | 224 (36) [3] |
| Aspirin use (%) | 338 (54) [0] | 325 (52) [0] |
Baseline characteristics were compared between randomised groups and no differences were observed.
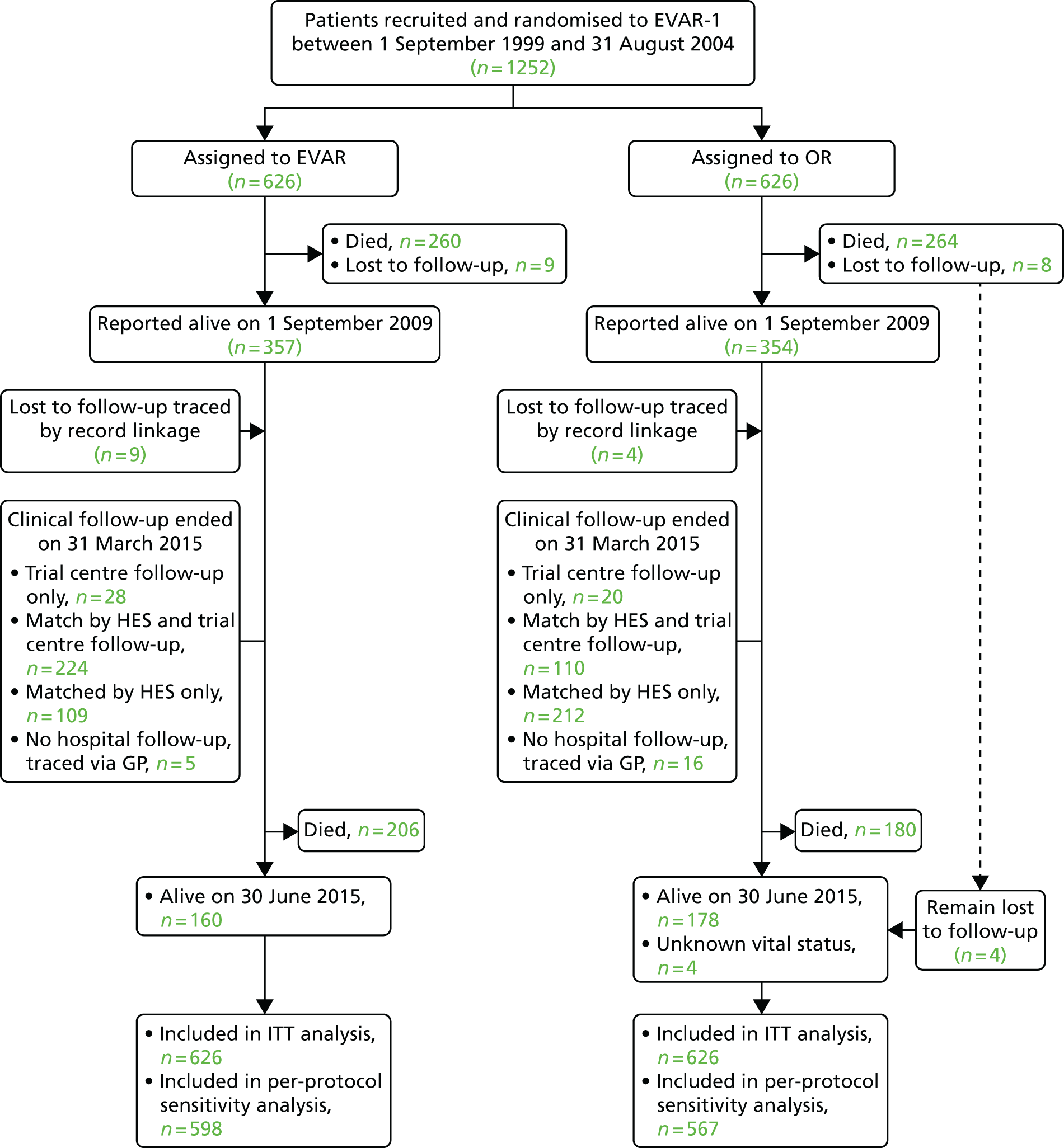
Patients were followed until 30 June 2015 (mean 12.7 years; median 12.4 years; minimum 1.8 years; maximum, 15.8 years); mean person-years observation to either death or end of the study was 8.0 years. By 30 June 2015 only four patients were lost to follow-up for mortality and 25 for reinterventions (this includes five patients in the EVAR group and 20 patients in the OR group), with data now available from record linkage for 13 of the 17 patients previously lost to mortality follow-up (Figure 4).
FIGURE 4.
CONSORT diagram for mortality and reinterventions. GP, general practitioner.
For 13 individuals, a cause of death was established based only on a death certificate. Annual clinical follow-up with CT scanning or duplex imaging reduced steadily over the period of the trial and was consistently lower in the OR group (Table 3).
| Years since randomisation | EVAR, n/N (%) | OR, n/N (%) | ||
|---|---|---|---|---|
| CT | Duplexa | CT | Duplexa | |
| < 1 | 532/581 (92) | 55/566 (10)b | ||
| 1–2 | 448/543 (83) | 414/534 (78) | ||
| 2–3 | 419/503 (83) | 363/500 (73) | ||
| 3–4 | 354/474 (75) | 306/464 (66) | ||
| 4–5 | 291/443 (66) | 262/439 (60) | ||
| 5–6 | 271/409 (66) | 221/399 (55) | ||
| 6–7 | 183/370 (49) | 24/56 (43) | 144/370 (39) | 6/52 (12) |
| 7–8 | 139/339 (41) | 59/147 (40) | 97/333 (29) | 6/139 (4) |
| 8–9 | 111/297 (37) | 70/189 (37) | 58/284 (20) | 9/172 (5) |
| 9–10 | 72/263 (27) | 73/208 (35) | 26/257 (10) | 11/212 (5) |
| 10–11 | 47/220 (21) | 69/214 (32) | 8/224 (4) | 15/219 (7) |
| 11–12 | 24/135 (18) | 42/135 (31) | 6/143 (4) | 13/143 (9) |
| 12–13 | 12/74 (16) | 20/74 (27) | 1/83 (1) | 13/83 (16) |
| 13–14 | 3/41 (7) | 9/41 (22) | 2/50 (4) | 6/50 (12) |
| 14–15 | 0/9 (0) | 2/9 (22) | 0/12 (0) | 0/12 (0) |
Over the course of follow-up, CT was carried out a median of six (interquartile range 3–8) times per patient in the EVAR group and three (interquartile range 1–6) times in the OR group. Of the patients who had not had death reported by 1 September 2009, 655 of 728 (90%) patients were tracked with HES, including 13 patients previously lost to follow-up, with local follow-up reported in 48 (70%) of the remaining patients (see Figure 4). After publication of the 30-day mortality results,100 26 of the 37 trial centres remained in equipoise and continued recruitment into a separate study from 1 September 2004 until 15 June 2005, when primary outcome results were published,101 with a further 175 patients not reported previously but now used in sensitivity analyses for mortality only. Operative characteristics are shown for the 1252 patients in the EVAR-1 in Table 4a and the additional 175 patients in Table 4b.
| Baseline characteristica | EVAR (n = 626) | OR (n = 626) |
|---|---|---|
| Age (years) | 74.1 (6.1) | 74.0 (6.1) |
| Number of males (%) | 565 (90) | 570 (91) |
| Mean AAA diameter (cm) | 6.4 (0.9) | 6.5 (1.0) |
| Procedure received | ||
| EVAR | 598 | 31 |
| OR | 16 | 571 |
| None | 12 (all died before operation) | 24 (including five who refused) |
| Unknown | 0 | 0 |
| Baseline characteristica | EVAR (n = 88) | OR (n = 87) |
|---|---|---|
| Age (years) | 75.1 (5.9) | 75.1 (5.9) |
| Number of males (%) | 83 (94) | 81 (93) |
| Mean AAA diameter (cm) | 6.5 (1.1) | 6.4 (0.8) |
| Procedure received | ||
| EVAR | 73 | 5 |
| OR | 3 | 61 |
| None | 3 | |
| Unknown | 12 | 18 |
Aneurysm-related and total mortality
During 9968 person-years of follow-up, 910 deaths occurred, 101 of which were aneurysm related (Table 5). Overall aneurysm-related mortality was 1.1 deaths per 100 person-years in the EVAR group and 0.9 deaths per 100 person-years in the OR group [adjusted HR 1.31, 95% confidence interval (CI) 0.86 to 1.99; p = 0.21]. For total mortality, there were 9.3 deaths per 100 person-years in the EVAR group and 8.9 deaths per 100 person-years in the OR group (adjusted HR 1.11, 95% CI 0.97 to 1.27; p = 0.14). The results for the sensitivity analyses that included patients with missing baseline covariates were similar for aneurysm-related and total mortality (Table 6).
| EVAR (n = 626) n/N (rate/100 person-years) | OR (n = 626) n/N (rate/100 person-years) | HR (95% CI) | p-valueb | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Total mortality | |||||
| All patients | 466/626 (9.3) | 444/626 (8.9) | 1.05 (0.92 to 1.19) | 1.11 (0.97 to 1.27) | 0.14 |
| Time since randomisation | |||||
| 0–6 months | 26/626 (8.5) | 45/626 (15.0) | 0.57 (0.35 to 0.92) | 0.61 (0.37 to 1.02) | 0.06 |
| 6 months–4 years | 126/600 (6.7) | 116/581 (6.3) | 1.07 (0.83 to 1.38) | 1.13 (0.87 to 1.47) | 0.35 |
| 4–8 years | 135/474 (8.3) | 129/464 (8.0) | 1.03 (0.81 to 1.31) | 1.07 (0.83 to 1.37) | 0.62 |
| > 8 years | 179/339 (14.9) | 154/333 (12.7) | 1.18 (0.95 to 1.47) | 1.25 (1.00 to 1.56) | 0.0484 |
| Aneurysm-related death | |||||
| All patients | 56/626 (1.1) | 45/626 (0.9) | 1.24 (0.84 to 1.83) | 1.31 (0.86 to 1.99) | 0.21 |
| Time since randomisation | |||||
| 0–6 months | 14/626 (4.6) | 30/626 (10.0) | 0.46 (0.24 to 0.87) | 0.47 (0.23 to 0.93) | 0.0307 |
| 6 months–4 years | 12/599 (0.6) | 8/581 (0.4) | 1.48 (0.60 to 3.62) | 1.46 (0.56 to 3.83) | 0.44 |
| 4–8 years | 14/474 (0.9) | 4/464 (0.2) | 3.46 (1.14 to 10.52) | 3.11 (0.99 to 9.72) | 0.05 |
| > 8 years | 16/339 (1.3) | 3/333 (0.2) | 5.50 (1.60 to 18.89) | 5.82 (1.64 to 20.65) | 0.0064 |
| HR (95% CI) | |||
|---|---|---|---|
| Complete-case analysis (secondary adjustment) | Missing indicator method (secondary adjustment) | Multiple imputation (secondary adjustment) | |
| Any death | |||
| All patients | 1.11 (0.97 to 1.27) | 1.08 (0.95 to 1.24) | 1.09 (0.95 to 1.24) |
| Time since randomisation | |||
| 0–6 months | 0.61 (0.37 to 1.02) | 0.57 (0.35 to 0.93) | 0.58 (0.36 to 0.95) |
| 6 months–4 years | 1.13 (0.87 to 1.47) | 1.11 (0.86 to 1.43) | 1.10 (0.85 to 1.41) |
| 4–8 years | 1.07 (0.83 to 1.37) | 1.03 (0.81 to 1.31) | 1.04 (0.81 to 1.32) |
| > 8 years | 1.25 (1.00 to 1.56) | 1.28 (1.03 to 1.59) | 1.27 (1.02 to 1.58) |
| Aneurysm-related death | |||
| All patients | 1.31 (0.86 to 1.99) | 1.28 (0.85 to 1.91) | 1.29 (0.87 to 1.91) |
| Time since randomisation | |||
| 0–6 months | 0.47 (0.23 to 0.93) | 0.44 (0.23 to 0.84) | 0.47 (0.25 to 0.89) |
| 6 months–4 years | 1.46 (0.56 to 3.83) | 1.44 (0.56 to 3.68) | 1.38 (0.56 to 3.42) |
| 4–8 years | 3.11 (0.99 to 9.72) | 3.44 (1.12 to 10.53) | 3.58 (1.17 to 10.94) |
| > 8 years | 5.82 (1.64 to 20.65) | 6.17 (1.75 to 21.74) | 6.14 (1.75 to 21.57) |
There was evidence of deviation from the proportional hazards assumption for aneurysm-related mortality (p < 0.0001), with an early benefit of EVAR during the first 6 months, counteracted by an increase in aneurysm-related mortality beyond 4 years, the difference being most marked after 8 years (adjusted HR 5.82, 95% CI 1.64 to 20.65; p = 0.0064) (see Table 5). There was also evidence of deviation from the proportional hazards assumption for total mortality (p = 0.0232), with an early benefit of EVAR during the first 6 months, similar mortality between the groups from 6 months to 8 years, but thereafter an increase in mortality in the EVAR group (adjusted HR 1.25, 95% CI 1.00 to 1.56; p = 0.0484) (see Table 5). Kaplan–Meier curves for aneurysm-related and total mortality are shown in Figure 5. Aneurysm-related mortality curves cross over between 6 and 8 years and total mortality curves diverge after 10 years. Median survival was 8.7 years and 8.3 years in the EVAR and OR groups, respectively (log-rank p-value 0.49). Sensitivity analyses, including the additional 175 patients from the separate 2004–5 study, yielded very similar results (Figure 6).
FIGURE 5.
Kaplan–Meier estimates for total and aneurysm-related survival over a maximum of 15 years’ follow-up.
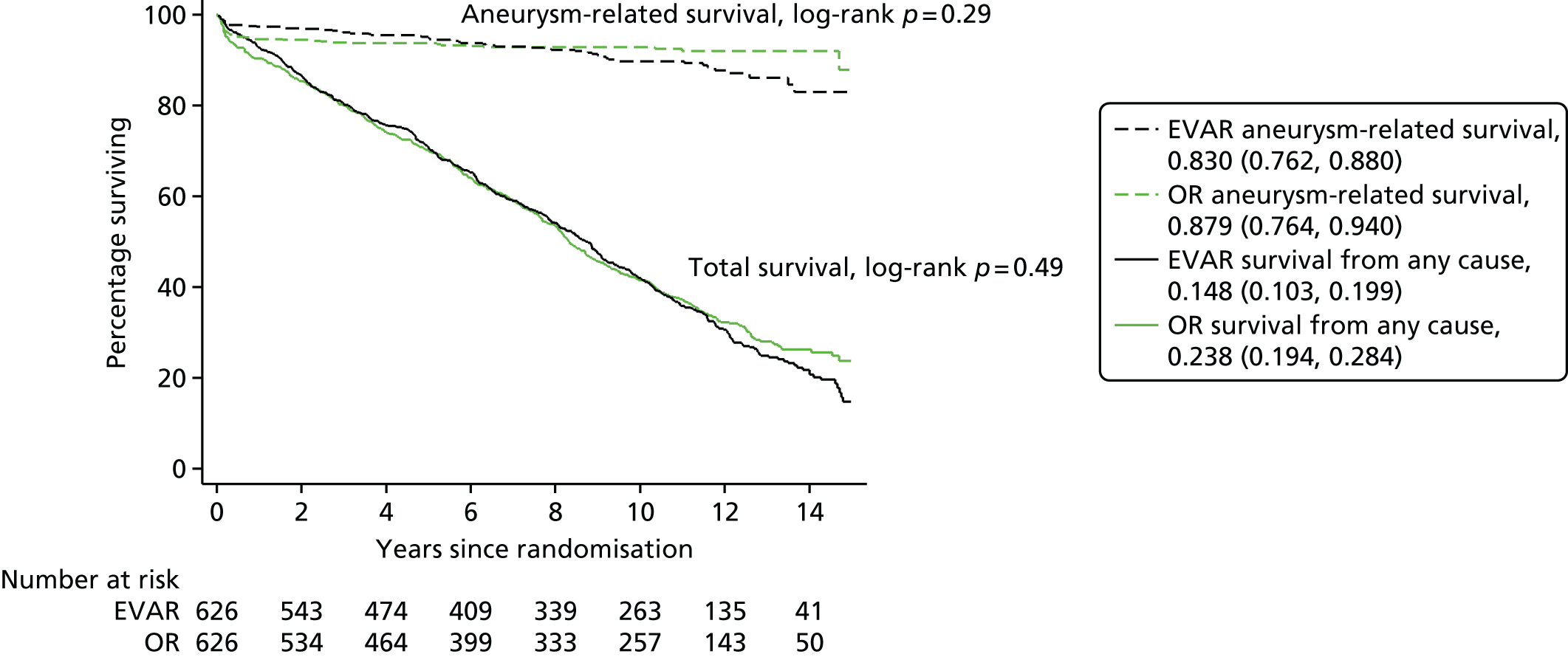
FIGURE 6.
Kaplan–Meier estimates for total and aneurysm-related survival including the 175 additional patients randomised in a separate study from 1 September 2004 to 15 June 2005 (EVAR, n = 88; OR, n = 87) for sensitivity analysis.
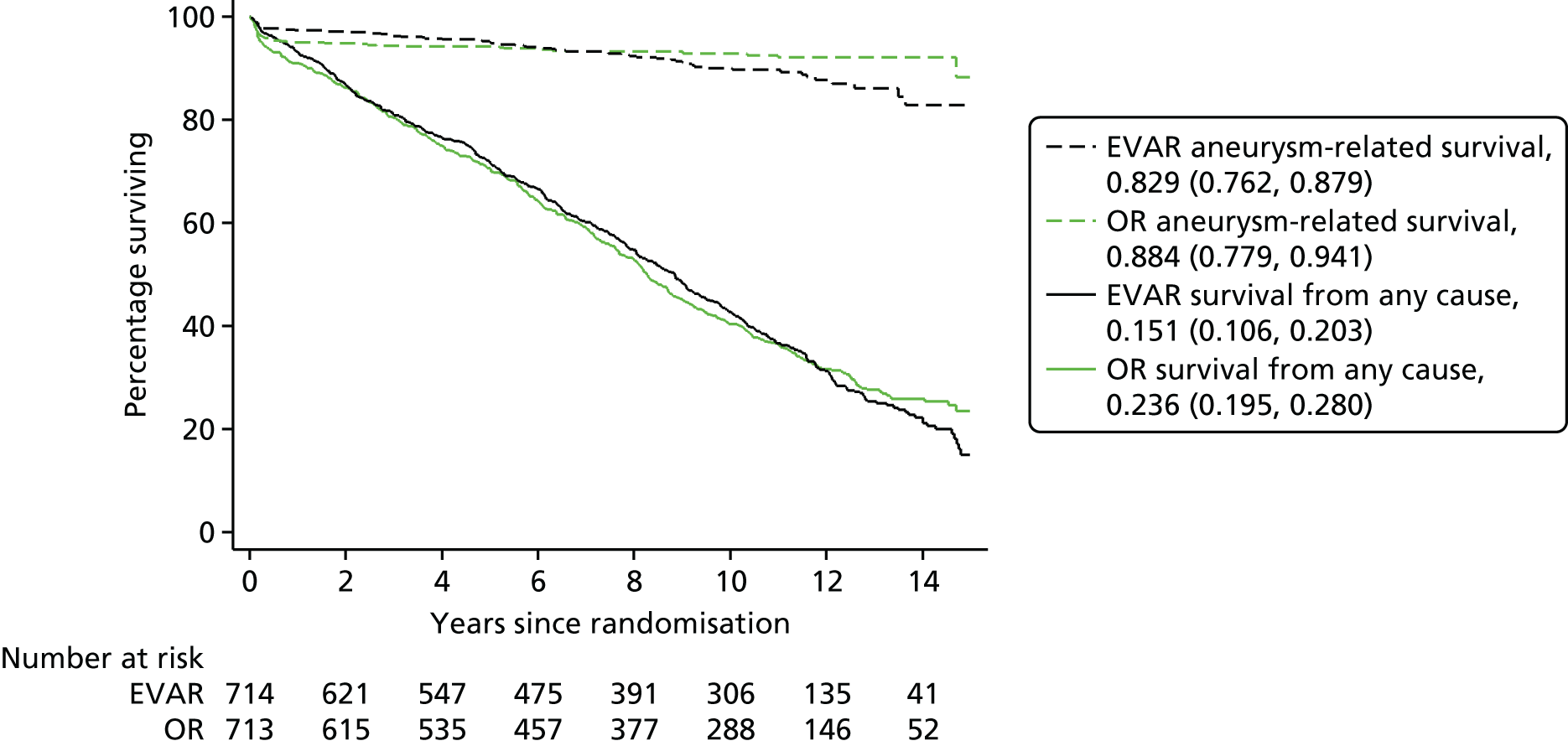
The full list of causes of death by time period are given in Table 7. Overall, there were 31 deaths from rupture after aneurysm repair in the EVAR group and five in the OR group. Two patients in the OR group had ruptures in 2010 and 2012 having refused operation. Overall, there was no evidence of a difference in cancer-related mortality between the groups (adjusted HR 1.09, 95% CI 0.84 to 1.40; p = 0.53), although there was some evidence of an increase in the EVAR group after 8 years (adjusted HR 1.87, 95% CI 1.19 to 2.96; p = 0.007) (Figure 7 and Table 8).
| Cause of death | EVAR, n (%) | OR, n (%) |
|---|---|---|
| Randomisation to 6 months | n = 26 | n = 45 |
| Aneurysm rupture before repair (primary) | 5 (19) | 5 (11) |
| Aneurysm related after repair | 7 (27) | 24 (53) |
| Aneurysm rupture after repair (secondary) | 2 (8) | 1 (2) |
| Coronary heart disease | 4 (15) | 4 (9) |
| Stroke | 0 (0) | 1 (2) |
| Other vascular disease | 2 (8) | 2 (4) |
| Cancer, lung | 1 (4) | 0 (0) |
| Cancer, other | 2 (8) | 0 (0) |
| Respiratory | 0 (0) | 5 (11) |
| Renal | 2 (8) | 0 (0) |
| Other | 1 (4) | 3 (7) |
| Unknown | 0 (0) | 0 (0) |
| 6 months–4 years | n = 126 | n = 116 |
| Aneurysm rupture before repair (primary) | 2 (2) | 5 (4) |
| Aneurysm related after repair | 2 (2) | 2 (2) |
| Aneurysm rupture after repair (secondary) | 8 (6) | 1 (1) |
| Coronary heart disease | 27 (22) | 25 (22) |
| Stroke | 11 (9) | 6 (5) |
| Other vascular disease | 6 (5) | 5 (4) |
| Cancer, lung | 19 (15) | 20 (17) |
| Cancer, other | 20 (16) | 29 (25) |
| Respiratory | 10 (8) | 16 (14) |
| Renal | 4 (3) | 1 (1) |
| Other | 15 (12) | 6 (5) |
| Unknown | 2 (2) | 0 (0) |
| 4–8 years | n = 135 | n = 129 |
| Aneurysm rupture before repair (primary) | 0 (0) | 1 (1) |
| Aneurysm related after repair | 6 (4) | 2 (2) |
| Aneurysm rupture after repair (secondary) | 8 (6) | 1 (1) |
| Coronary heart disease | 31 (23) | 28 (22) |
| Stroke | 16 (12) | 12 (9) |
| Other vascular disease | 7 (5) | 7 (5) |
| Cancer, lung | 12 (9) | 16 (12) |
| Cancer, other | 22 (16) | 27 (21) |
| Respiratory | 16 (12) | 22 (17) |
| Renal | 4 (3) | 3 (2) |
| Other | 13 (10) | 10 (8) |
| Unknown | 0 (0) | 0 (0) |
| > 8 years | n = 179 | n = 154 |
| Aneurysm rupture before repair (primary) | 0 (0) | 1 (1) |
| Aneurysm related after repair | 3 (2) | 0 (0) |
| Aneurysm rupture after repair (secondary) | 13 (7) | 2 (1) |
| Coronary heart disease | 33 (18) | 35 (23) |
| Stroke | 10 (6) | 15 (10) |
| Other vascular disease | 4 (2) | 12 (8) |
| Cancer, lung | 13 (7) | 10 (6) |
| Cancer, other | 37 (21) | 21 (14) |
| Respiratory | 29 (16) | 30 (19) |
| Renal | 5 (3) | 4 (3) |
| Other | 31 (17) | 24 (16) |
| Unknown | 1 (1) | 0 (0) |
FIGURE 7.
Kaplan–Meier estimates for cancer-related survival over a maximum of 15 years’ follow-up.
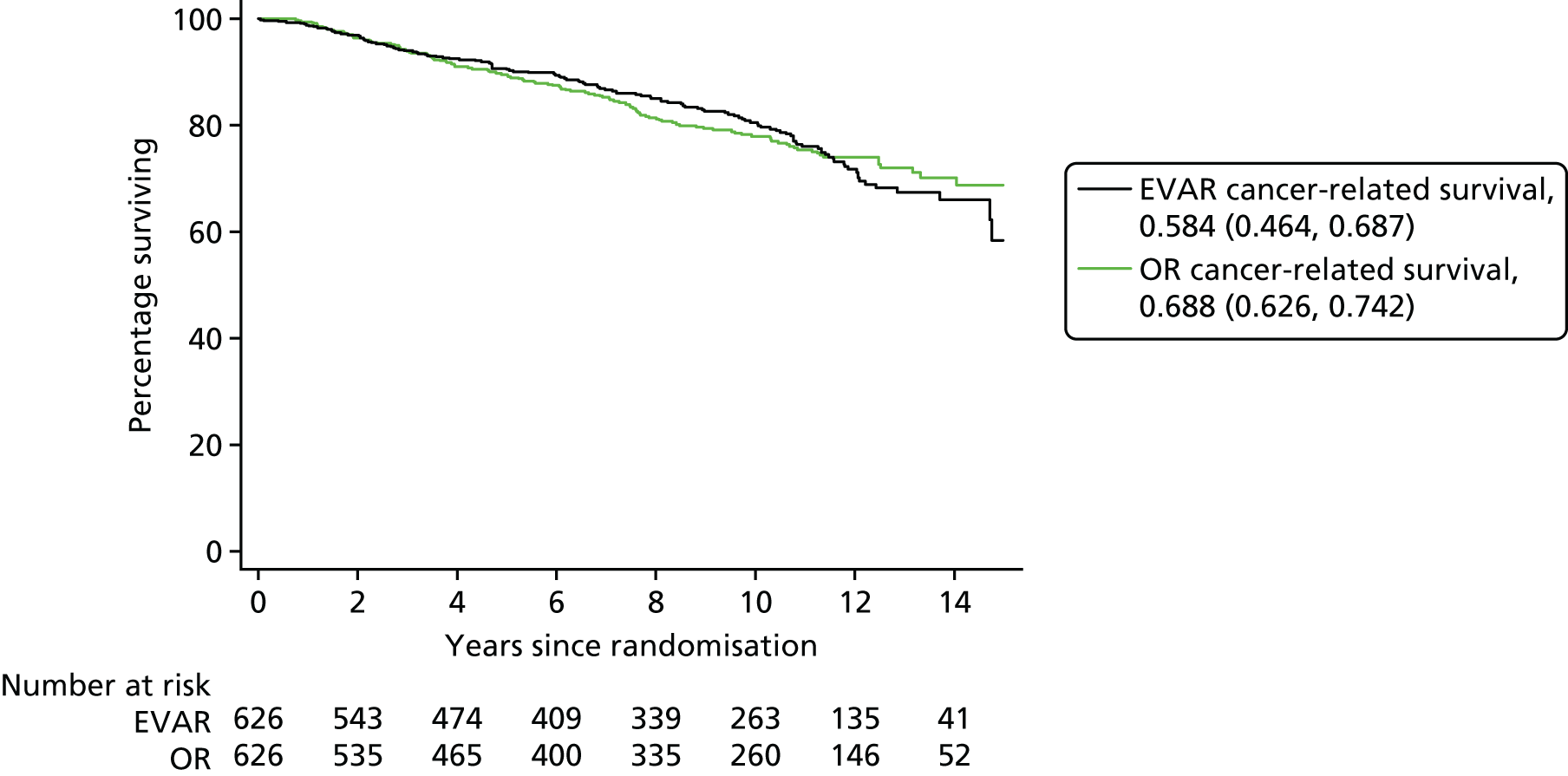
| EVAR (n = 626), n/N (rate/100 person-years) | OR (n = 626), n/N (rate/100 person-years) | HR (95% CI) | p-valueb | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Cancer-related death | |||||
| All patients | 126/626 (2.5) | 123/626 (2.5) | 1.02 (0.80 to 1.31) | 1.09 (0.84 to 1.40) | 0.53 |
| Time since randomisation | |||||
| 0–4 years | 42/626 (1.9) | 49/626 (2.3) | 0.85 (0.56 to 1.28) | 0.90 (0.59 to 1.37) | 0.63 |
| 4–8 years | 34/474 (2.1) | 43/464 (2.7) | 0.78 (0.50 to 1.22) | 0.76 (0.47 to 1.21) | 0.24 |
| > 8 years | 50/339 (4.2) | 31/333 (2.5) | 1.65 (1.05 to 2.58) | 1.87 (1.19 to 2.96) | 0.007 |
Details of the primary cancer sites underlying mortality after 8 years are shown in Table 9.
| Cancer site | EVAR, n | OR, n |
|---|---|---|
| Lung or pleura | 13 | 11 |
| Gastrointestinal or peritoneum | 11 | 6 |
| Genitourinary | 14 | 11 |
| Blood | 6 | 2 |
| Brain | 1 | 0 |
| Unspecified | 5 | 1 |
| Total | 50 | 31 |
There was no evidence of significant interactions between the randomly assigned treatment and age, sex or aneurysm diameter for either aneurysm-related or total mortality (p > 0.10 for all comparisons).
Per-protocol analysis including 598 patients in the EVAR group who received elective EVAR and 567 patients in the OR group who received elective OR again showed strongly the benefit of EVAR during the first 6 months, counteracted by an increase in aneurysm-related mortality at all subsequent time periods (Table 10); the increase being proportionately greater than for the analysis by randomised group. Overall, aneurysm-related mortality was significantly higher in the EVAR group (1.0/100 person-years) than in the OR group (0.6/100 person-years) (adjusted HR 1.76, 95% CI 1.07 to 2.89; p = 0.0263). There was weak evidence that total mortality was higher in the EVAR group, at 9.1 per 100 person-years, than in the OR group, at 8.4 per 100 person-years (adjusted HR 1.14, 95% CI 0.99 to 1.31; p = 0.07).
| EVAR (n = 598), n/N (rate/100 person-years) | OR (n = 567), n/N (rate/100 person-years) | HR (95% CI) | p-valueb | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Any death | |||||
| All patients | 443/598 (9.1) | 391/567 (8.4) | 1.10 (0.96 to 1.26) | 1.14 (0.99 to 1.31) | 0.07 |
| Time since randomisation | |||||
| 0–6 months | 17/598 (5.8) | 34/567 (12.4) | 0.47 (0.26 to 0.83) | 0.48 (0.26 to 0.90) | 0.0224 |
| 6 months–4 years | 120/581 (6.6) | 95/533 (5.6) | 1.19 (0.91 to 1.56) | 1.21 (0.92 to 1.60) | 0.17 |
| 4–8 years | 130/461 (8.2) | 121/437 (8.0) | 1.02 (0.80 to 1.31) | 1.05 (0.81 to 1.36) | 0.71 |
| > 8 years | 176/331 (15.0) | 141/314 (12.0) | 1.26 (1.01 to 1.57) | 1.30 (1.03 to 1.63) | 0.0254 |
| Aneurysm-related death | |||||
| All patients | 49/598 (1.0) | 29/567 (0.6) | 1.62 (1.03 to 2.57) | 1.76 (1.07 to 2.89) | 0.0263 |
| Time since randomisation | |||||
| 0–6 months | 9/598 (3.1) | 23/567 (8.4) | 0.37 (0.17 to 0.79) | 0.36 (0.15 to 0.85) | 0.0208 |
| 6 months–4 years | 10/580 (0.6) | 2/533 (0.1) | 4.70 (1.03 to 21.46) | 4.36 (0.92 to 20.67) | 0.06 |
| 4–8 years | 14/461 (0.9) | 2/437 (0.1) | 6.68 (1.52 to 29.40) | 5.80 (1.29 to 26.08) | 0.0220 |
| > 8 years | 16/331 (1.4) | 2/314 (0.2) | 8.27 (1.90 to 36.00) | 9.43 (2.09 to 42.59) | 0.0035 |
Aneurysm-related reinterventions
During 9715 person-years of follow-up, there were 258 graft-related reinterventions performed in 165 patients in the EVAR group and 105 graft-related reinterventions performed in 74 patients in the OR group, with rates to first reintervention of 4.1 and 1.7 per 100 person-years, respectively (adjusted HR 2.42, 95% CI 1.82 to 3.21; p < 0.0001) (Table 11). The reintervention rate was significantly higher in the EVAR group for any reintervention and serious reinterventions in the first 4 years (Figure 8a, see Table 11) and for life-threatening reinterventions (including conversion to OR, repeat EVAR and treatment of graft infection), in the periods 6 months to 4 years and beyond 8 years (Figure 8b, see Table 11). Even after 2 years (Figure 8c) or 5 years (Figure 8d) without any life-threatening reintervention, new life-threatening reinterventions occurred at any time to 15 years of follow-up. The relative difference in reintervention rate between the groups was highest in the period 6 months to 4 years after randomisation, particularly for the most serious reinterventions (see Table 11). A similar pattern, by time period, was observed for second and subsequent reinterventions (Table 12).
| EVAR (n = 626), n/N (rate/100 person-years) | OR (n = 626), n/N (rate/100 person-years) | HR (95% CI) | p-valueb | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Any reintervention | |||||
| All patients | 164/626 (4.1)c | 74/626 (1.7) | 2.37 (1.80 to 3.12) | 2.42 (1.82 to 3.21) | < 0.0001 |
| Time since randomisation | |||||
| 0–6 months | 67/626 (23.7) | 36/626 (12.5) | 1.89 (1.26 to 2.83) | 1.95 (1.28 to 2.98) | 0.0020 |
| 6 months–4 years | 56/536 (3.5) | 9/559 (0.5) | 6.81 (3.37 to 13.77) | 6.29 (3.09 to 12.78) | < 0.0001 |
| 4–8 years | 21/381 (1.6) | 16/436 (1.1) | 1.48 (0.77 to 2.84) | 1.60 (0.81 to 3.15) | 0.17 |
| > 8 years | 20/264 (2.3) | 13/282 (1.3) | 1.76 (0.88 to 3.54) | 1.51 (0.71 to 3.19) | 0.29 |
| Any serious reintervention | |||||
| All patients | 140/626 (3.3) | 57/626 (1.3) | 2.60 (1.91 to 3.54) | 2.62 (1.90 to 3.61) | < 0.0001 |
| Time since randomisation | |||||
| 0–6 months | 45/626 (15.5) | 19/626 (6.5) | 2.38 (1.39 to 4.06) | 2.46 (1.39 to 4.33) | 0.0019 |
| 6 months–4 years | 52/557 (3.1) | 8/570 (0.5) | 6.93 (3.29 to 14.58) | 6.45 (3.04 to 13.68) | < 0.0001 |
| 4–8 years | 21/403 (1.5) | 16/444 (1.1) | 1.43 (0.75 to 2.74) | 1.45 (0.73 to 2.88) | 0.29 |
| > 8 years | 22/277 (2.5) | 14/289 (1.4) | 1.76 (0.90 to 3.44) | 1.59 (0.78 to 3.26) | 0.20 |
| Life-threatening reintervention | |||||
| All patients | 85/626 (1.9) | 41/626 (0.9) | 2.12 (1.46 to 3.08) | 2.09 (1.42 to 3.08) | < 0.0002 |
| Time since randomisation | |||||
| 0–6 months | 22/626 (7.4) | 19/626 (6.5) | 1.14 (0.62 to 2.11) | 1.08 (0.57 to 2.08) | 0.81 |
| 6 months–4 years | 27/576 (1.5) | 2/570 (0.1) | 13.77 (3.27 to 57.92) | 12.78 (3.01 to 54.23) | 0.0006 |
| 4–8 years | 15/434 (1.0) | 11/450 (0.7) | 1.41 (0.65 to 3.06) | 1.41 (0.63 to 3.14) | 0.40 |
| > 8 years | 21/302 (2.1) | 9/300 (0.8) | 2.50 (1.14 to 5.45) | 2.44 (1.05 to 5.68) | 0.0385 |
FIGURE 8.
Kaplan–Meier estimates over a maximum of 15 years’ follow-up for (a) the time to first reintervention; (b) the time to first life-threatening reintervention; (c) the time to first life-threatening reintervention for individuals who have survived 2 years free of a life-threatening reintervention; and (d) the time to first life-threatening reintervention for individuals who have survived 5 years free of a life-threatening reintervention.
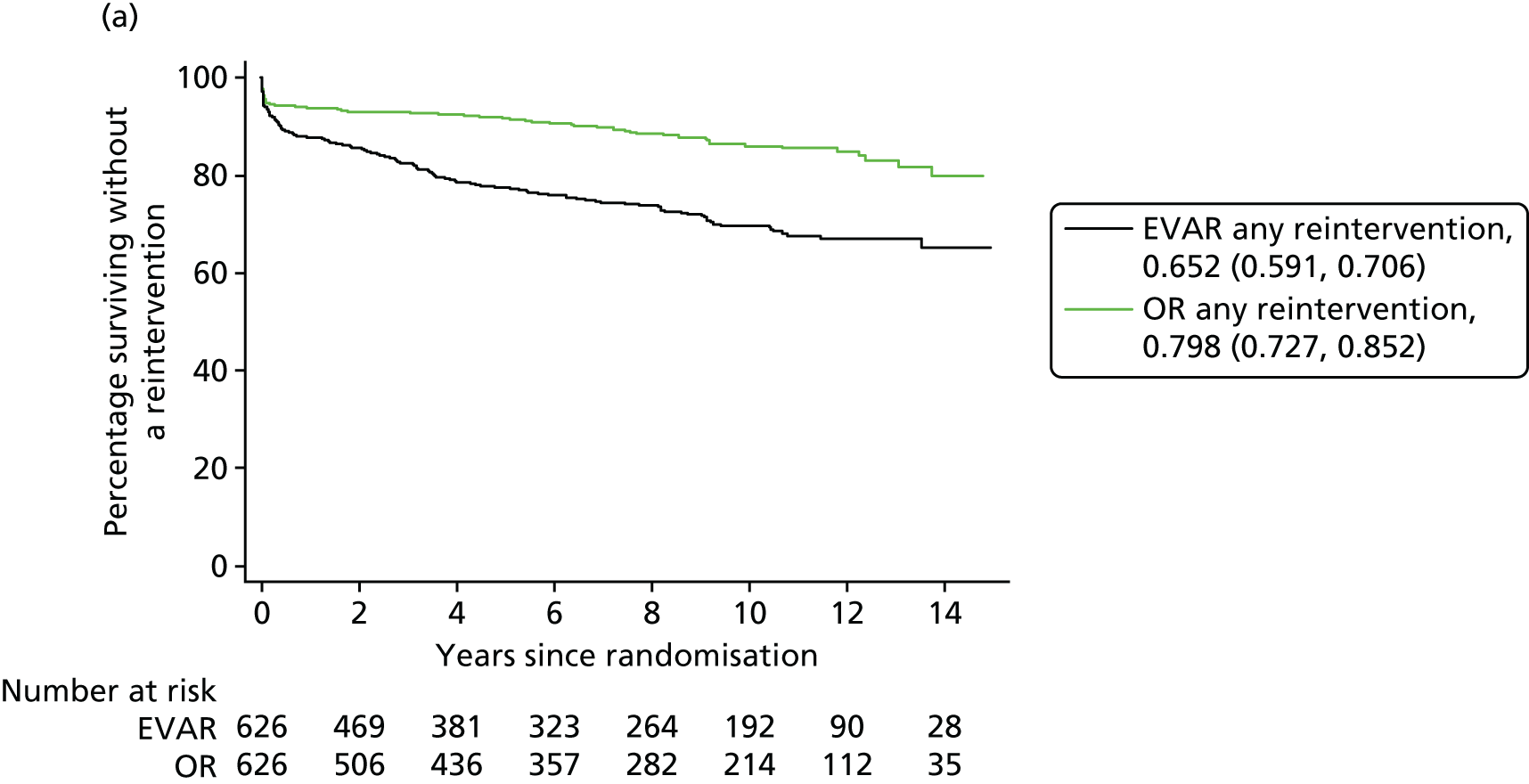
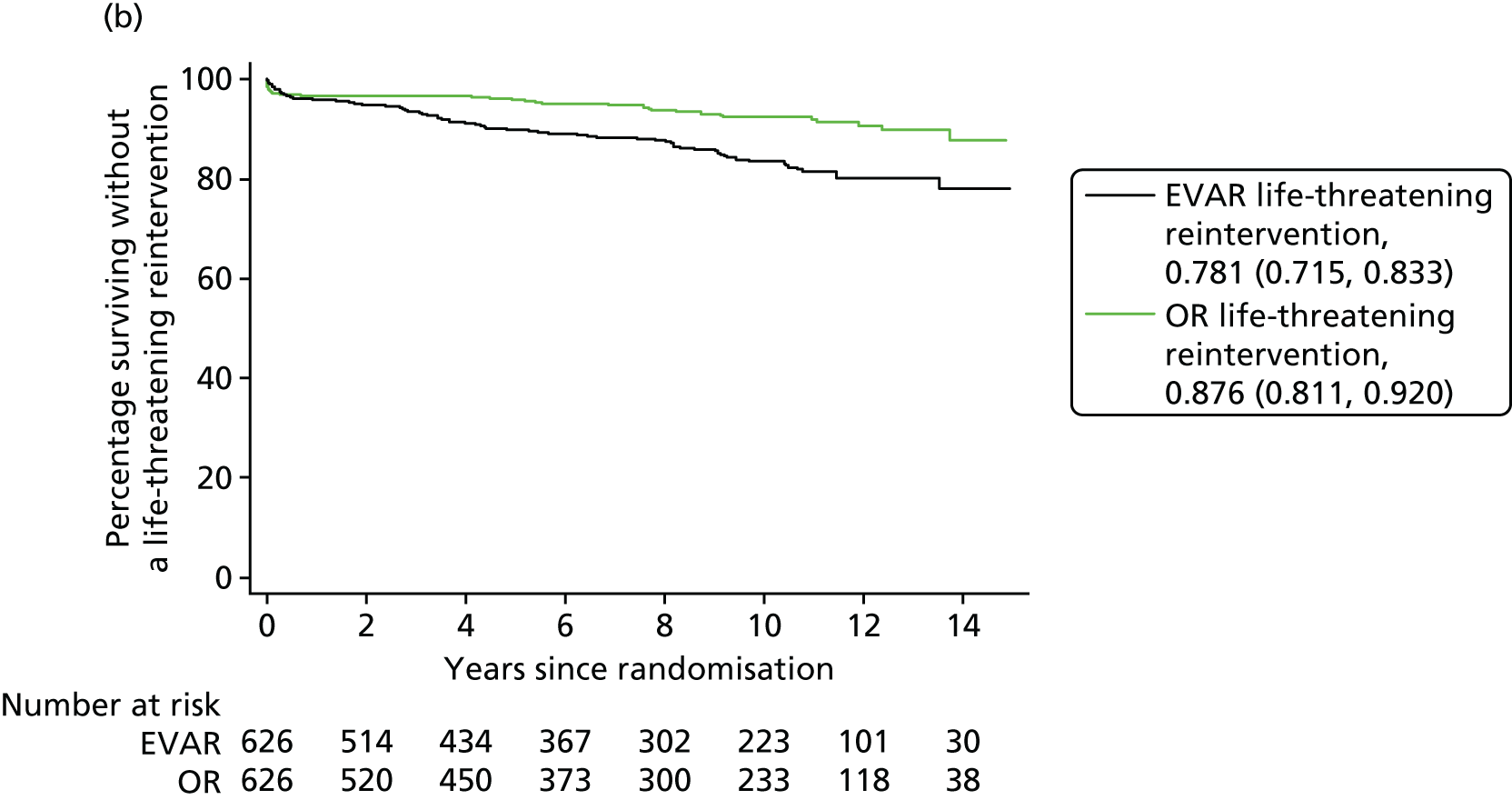
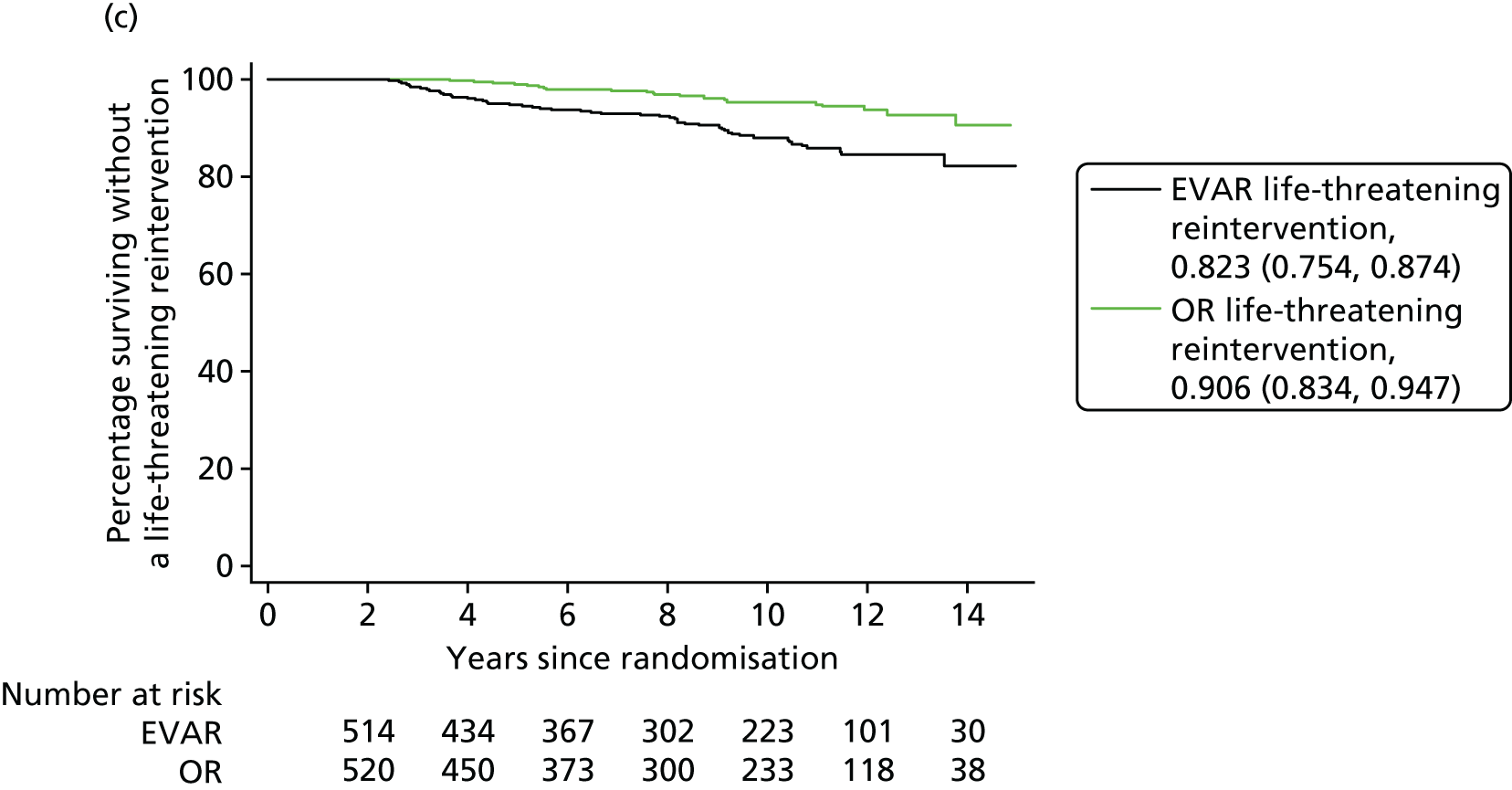
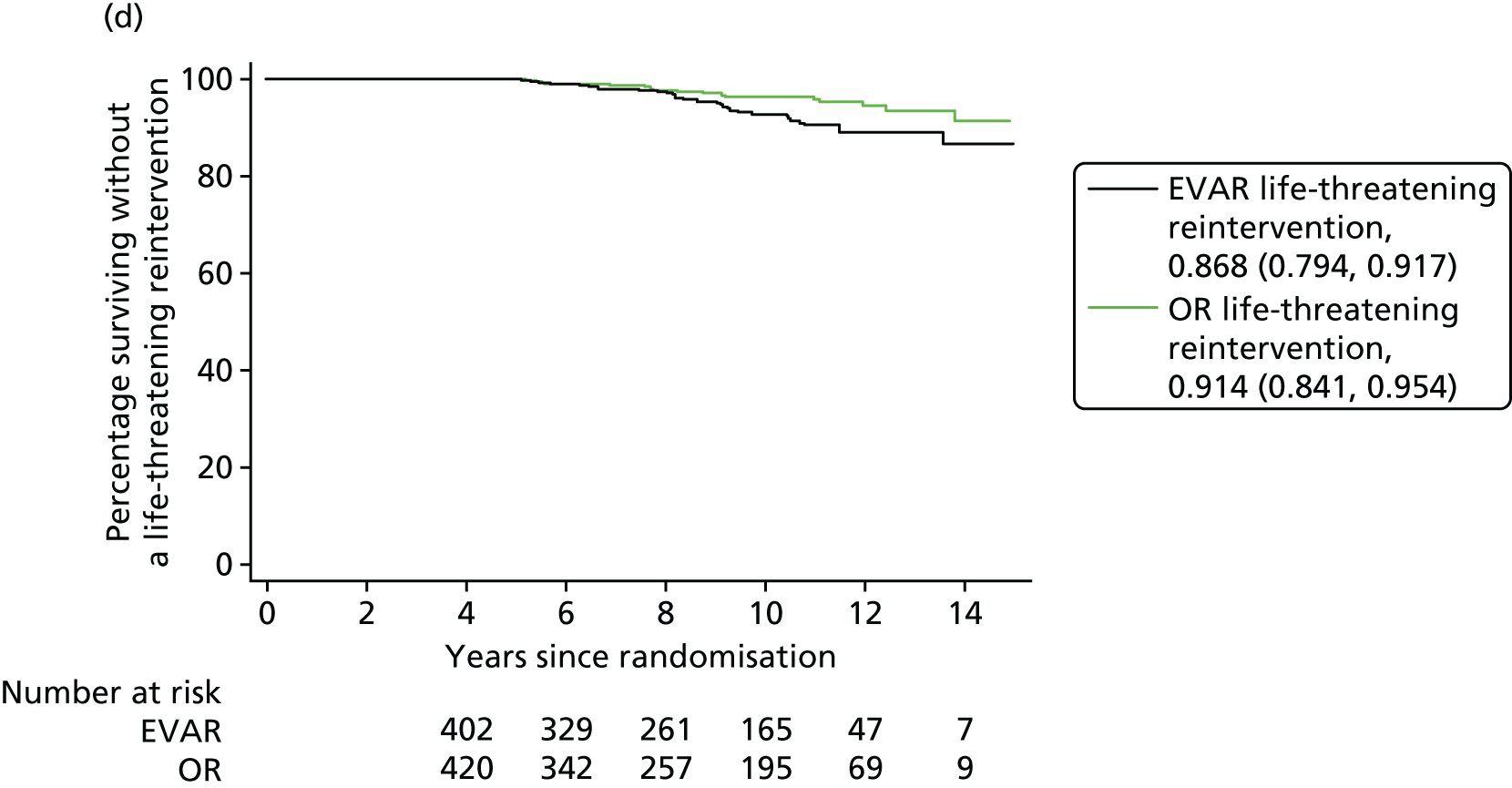
| Reintervention | EVAR, n | OR, n |
|---|---|---|
| 0–6 months | n = 81 | n = 36 |
| First | 67 | 36 |
| Second | 11 | 0 |
| Third | 2 | 0 |
| Fourth | 1 | 0 |
| 6 months–4 years | n = 73 | n = 10 |
| First | 56 | 9 |
| Second | 12 | 1 |
| Third | 4 | 0 |
| Fourth | 1 | 0 |
| 4–8 years | n = 46 | n = 35 |
| First | 21 | 16 |
| Second | 13 | 13 |
| Third | 9 | 4 |
| Fourth | 2 | 2 |
| Fifth | 1 | 0 |
| > 8 years | n = 57 | n = 24 |
| First | 20 | 13 |
| Second | 14 | 7 |
| Third | 10 | 3 |
| Fourth | 7 | 1 |
| Fifth | 4 | 0 |
| Sixth | 2 | 0 |
Discussion of EVAR trial 1
The most striking finding of these very long-term results is that both aneurysm-related and total mortality rates are greater in late follow-up for patients who were randomised to EVAR than those randomised to OR, although over the whole follow-up period the average total and aneurysm-related mortality rates were not significantly different between groups. The significant late divergence of the survival curves in favour of OR (see Figure 5) can be partly explained through greater contribution to late mortality from aneurysm-related and cancer deaths in the EVAR group, and the fact that these were elderly patients and the survival curves will inevitably start to converge in older ages.
Total and aneurysm-related mortality were lower in patients who received EVAR in the first 6 months, but increased after 6 months’ follow-up, leading to a significantly higher rate after 8 years’ follow-up in EVAR than in those who received OR. After the first 6 months, the increased aneurysm-related deaths in the EVAR group were predominantly from secondary sac rupture. Over the whole duration of follow-up, two aneurysm-related deaths followed reintervention, but 31 of the others were from secondary sac rupture, partly attributable to a failure to have corrected underlying causes of sac expansion from endoleak. 111 In those patients allocated to OR, there were five secondary ruptures, of which four were in patients who received EVAR and the remaining secondary rupture occurred more than 8 years after OR.
Reinterventions occurred in both groups throughout follow-up, including those who were free from reintervention after 2 or even 5 years, but the rate of reintervention was higher in the EVAR group at all time periods. These late reinterventions included those with high severity score, indicating that there was never a safe period to abandon follow-up for patients with EVAR. However, in this trial, some patients were discharged from surveillance and, therefore, lost the option of planned reintervention. With an average age of 74 years at randomisation, there could have been pressing clinical reasons not to reintervene for some patients with long-term follow-up because of age and frailty. A criticism in earlier reports from this trial, that not all incision-related reinterventions following OR were reported, was remedied in this long-term follow-up.
Limitations of EVAR trial 1
Limitations of this trial include that devices used were implanted between 1999 and 2004 and newer devices may be expected to have better results118 and imaging for sizing and placement of endografts has improved. The original trial protocol was for annual follow-up by CT, which was used in the early stages of the trial. However, in the later stages, many of the EVAR patients were followed up using ultrasonography. This change from CT to ultrasonography was influenced by increasing concern about radiation exposure. 119 Moreover, imaging follow-up declined over time, particularly for the OR patients. Consequently, reinterventions became less likely once surveillance ceased. Nor can we assume that follow-up practice is the same in the rest of the world as it is in the UK, where many patients were discharged from surveillance after several years. Since the EVAR group patients had more diligent follow-up than the OR patients, aneurysm-related mortality may have been underestimated in the OR group, although this does not affect our findings for total mortality. A further limitation is that, as a result of decreasing clinical follow-up at the original trial hospitals, the methodology to identify reinterventions changed after 2009 to predominantly using record linkage through the HES administrative data set, these reinterventions subsequently being validated at the trial hospitals. However, these data also captured data for patients whose care had moved to non-trial hospitals and recovered some patients previously lost to follow-up.
Patients prefer EVAR,120 and today it is the method of choice for repair of AAA. EVAR devices improve constantly, and sizing and imaging for deployment is better than between 1999 and 2004: a corollary is that experience in OR is declining. However, aortas with aneurysmal disease continue to dilate, and in time a good device can leak or migrate and even an OR can rupture. Challenges in the future to maintain the initially superior results of being in the EVAR group include the need to halt the dilating disease process as well as devices which allow for this inevitable dilating process over the years. It is fortunate that the long-term results of this study can act as a benchmark against which new endovascular technologies for aneurysm repair can be compared at each time point. In the meantime, surveillance must be addressed in clinical guidelines: it should be diligent, regular, easy and avoid CT scanning where possible, perhaps concentrating on the sac diameter after EVAR either by ultrasonography or novel implantable sensor devices. 121–125
Chapter 4 Costs: endovascular aneurysm repair compared with open repair over 15 years
Introduction
This chapter estimates the total cost per patient of each procedure over 15 years in patients who were suitable for EVAR and fit for OR. The costs for each patient include the index procedure, aneurysm-related reinterventions and surveillance, as received in EVAR-1. Other potentially related categories of acute and chronic health-care costs, such as MI, stroke, amputation, renal failure and cancer, are not included in this analysis, as data on health-care use for these reasons were not collected after 2009.
As this chapter presents a description of the data, rather than an economic evaluation, costs were not discounted. A full economic evaluation showing discounted costs and quality-adjusted life-years (QALYs) is presented in Chapter 5.
Methods
All resources in the current analysis were costed at 2014/15 prices. The follow-up is divided into two periods: (1) 1 September 1999–31 August 2009; and (2) 1 September 2009–31 March 2015. The method of costing is different for the two periods. During the first period (1999–2009), detailed hospital resource use data were available for the index procedure and aneurysm-related reinterventions for each patient from the case record forms. The same methodology is used here as in the previous publication,94 the only difference being that, in the previous publication, these resources were costed at 2008/9 prices. In the current analysis, these same resources have been recosted using largely new unit costs at 2014/15 prices (Table 13).
| Resource | Unit cost (£) | Source | Reference |
|---|---|---|---|
| EVAR device | 6558 | Average selling price of the EVAR device (across all manufacturers) in the UK | Marianna Stellino, W.L. Gore and Associates, 14 October 2015, personal communication |
| Other consumables and accessories: EVAR | 387 (at 2004 prices) | Survey of participating centres undertaken by the EVAR trials, May 2004 | Brown et al., 201296 |
| Graft: OR | 240 (at 2004 prices) | Survey of participating centres undertaken by the EVAR trials, May 2004 | Brown et al., 201296 |
| Other consumables: OR | 189 (at 2004 prices) | Survey of participating centres undertaken by the EVAR trials, May 2004 | Brown et al., 201296 |
| Blood products | 140.02 | National Blood Service | www.blood.co.uk (accessed November 2015) |
| Ultrasound | 88 | Ultrasound scanning | NHS Reference Costs 2014 to 2015 126 |
| CT | 103 | CT scanning | NHS Reference Costs 2014 to 2015 126 |
| Theatre time (per hour) | 1139 | NHS Scotland | Cost book 2014/15 R142X theatre services |
| Hospital stay (per day) | 342 | Day in ward, YR04Z endovascular repair, excess bed-day | NHS Reference Costs 2014 to 2015 126 |
| Critical care (per day) | 1142 | Activity-weighted average, adult critical care 0–6 organs supported | NHS Reference Costs 2014 to 2015 126 |
Detailed hospital resource use data were not widely available from case record forms after 1 September 2009 because of loss to follow-up, especially in patients in the OR group. To supplement the trial data and information from hospital notes, data on hospital episodes for each patient from 1 September 2009 to 31 March 2015 were obtained from national NHS HES (see Chapter 2 for the definition of the censoring date for reinterventions). Each episode was assigned a Hospital Resource Group code127 and matched with the published national average cost of that episode. 126 A Hospital Resource Group is similar to a Diagnostic Resource Group. HES data did not include time in critical care. The TMC estimated the likely time in ICU and HDU for each type of procedure during the follow-up via a survey of all the centre principal investigators (see Table 1). These estimates were used to calculate the costs of critical care for each episode in the follow-up after 1 September 2009 using published national average costs. 126
Surveillance was undertaken through attendance at vascular outpatient consultations, CT scanning and ultrasonography. These data were available for each patient in EVAR-1 over the entire period of follow-up (1999–2015) and these were costed at 2014/15 prices using national average costs. 126
Hospital notes were searched over the full trial period for incisional hernia repair procedures. These are almost exclusively carried out after OR. These reinterventions were not included in previous publications,94,101 but are included in this analysis and were costed by Hospital Resource Group at 2014/15 prices using national average costs. 126 Analysis is by ITT. Costs were converted to US dollars at purchasing power parities (£0.70 = US$1.00). 128 All data management and analysis was carried out in Stata version 13.
Handling missing data
Because of staggered recruitment to the trial between 1 September 1999 and 31 August 2004, follow-up data were available at the censoring date for reinterventions on 31 March 2015 from between 11 to 15.5 years. The aim of this chapter is to estimate mean costs per patient over the full time horizon of the trial (i.e. 15 years). As not all patients have the full 15 years of follow-up, we need to try to understand the reasons for the missing data and handle the missing data accordingly. 129 In this chapter we assume that the missing data have a monotone pattern. This means in practice that all patients are assumed to have complete follow-up for costs (no missing data on surveillance or reinterventions) until their censoring date for reinterventions. This administrative censoring was handled in two ways: (1) using inverse probability weighting (IPW); and (2) multiple imputation.
Inverse probability weighting
In the first method, the administrative censoring was handled using IPW. 130 It was assumed that data were missing monotonically and missing completely at random. The following steps were taken in data management prior to implementation of the IPW programme:
-
The cost, costit, for patient i for each year t from date of randomisation (t = 1 . . . 14) was calculated as the sum of the costs of interventions, reinterventions and surveillance during that year for that patient.
-
Patients who died before the index operation were assigned a cost of zero for all years up to the censoring date (if no other information is available).
-
If no interventions or surveillance were recorded in the trial or the HES data for a patient i in any year t before the censoring date (t < Xi), then costit was assigned a value of zero (assumption of monotone missing cost data).
-
Any surveillance or reintervention apparently incurred on a date after the designated censoring date for that patient was assumed to be an error and reassigned to the last period before censoring for that patient (assumption of monotone missing cost data).
-
If a patient died, then costit after the time of death (t > Xit) was assumed to be zero; this assumption ensures that the weighted cost includes both alive and dead individuals.
-
There were insufficient reinterventions in the final year to estimate reliable CIs; hence costs over 14 years are presented in this chapter.
-
Data were organised in the long format.
The IPW method sets up an indicator variable, d*it, which takes value 1 if the patient is dead or still in follow-up at time t, and zero otherwise. A Kaplan–Meier survival function is then calculated and used to estimate G*it, defined as the probability that patient i is still in follow-up or dead by time t. The probability G* is estimated without explanatory covariates and hence assumes that data are missing completely at random. The weight wit is then defined as d*it/G*it. This ensures that observations at the end of the trial (when there are fewer patients in follow-up) receive a greater weight in the analysis.
Weighted linear regression is carried out for each period t separately, where costit is the dependent variable and treati the independent variable. The coefficient βt on the treatit variable is then interpreted as the difference in mean cost per patient at time t between the treatment groups. The difference in the total mean cost per patient over the full 14 years (taking account of administrative censoring) is the sum of the coefficients for each year, ∑T = 14t = 1βt. CIs were estimated by bootstrap. 131
Multiple imputation
In the second method, the administrative censoring was handled using multiple imputation. 129 It was assumed that data were missing monotonically and missing at random.
The following steps were taken in data management prior to imputation:
-
The cost, costit, for patient i for each year t from date of randomisation (t = 1 . . . 14) was calculated as the sum of the costs of interventions, reinterventions and surveillance during that year for that patient.
-
Patients who died before the index operation were assigned a cost of zero for all years up to the censoring date (if no other information is available).
-
If no interventions or surveillance were recorded in the trial or the HES data for a patient i in any year t before the censoring date (t < Xi), then costit was assigned a value of zero (assumption of monotone missing cost data).
-
Any surveillance or reintervention apparently incurred on a date after the designated censoring date for that patient was assumed to be an error and reassigned to the last period before censoring for that patient (assumption of monotone missing cost data).
-
Data were organised in the wide format.
Multiple imputation was undertaken using predictive mean matching, drawing on the five closest neighbours. Imputation was carried out separately for each randomised treatment group (by treati). Fifteen imputed data sets were created (missing values; M = 15). For each dependent variable with missing data (costit), a customised conditional prediction equation was built with the following independent variables, diedt + 1, agei, sexi, eq5dsi0, costi1 if diedt = = 0, where diedt + 1 is a binary indicator variable which takes value 1 if the patient died in the next period. This takes account that health-care costs are often higher in the months leading up to death. The variables agei, sexi, eq5dsi0 are the age, gender and EuroQol-5 Dimensions (EQ-5D) index at baseline. The variable costi1 is the cost incurred in the first year. This takes account of the severity and complications incurred in the index procedure, which may predict subsequent need for health care. The conditional expression (if diedt = = 0) restricts estimation of costs and imputation to the patients known to be alive at time t.
After imputation, the total cost per patient (total_costi) was calculated passively as the sum of the costit in each of the 14 years. If a patient died, then costit after the time of death (t > Xit) was assumed to be zero.
Analysis was carried out using linear regression, which was used to estimate the difference in mean costs and standard errors (SEs) between the treatment groups, with total_costi as the dependent variable and treati as the independent variable. Rubin’s rule was applied to aggregate coefficients across the M data sets.
Results
Mean costs over 14 years estimated using inverse probability weighting
The cost results for the IPW method are shown in Table 14 and Figure 9. In the EVAR group there were 626 randomised patients, of whom 160 were alive on 30 June 2015. In the OR group, there were 626 randomised patients, of whom 181 were alive on 30 June 2015. Eleven patients in the EVAR group and 20 patients in the OR group did not receive any aneurysm repair and were assigned zero cost for the initial procedure. The overall difference in mean cost between the groups at 1 year is £2194. This difference increases gradually over 14 years to £3798 (95% CI £2338 to £5258). Most of this difference is as a result of the initial procedure cost and subsequent reinterventions.
| Year | Procedures and reinterventions (£) | Surveillance and imaging (£) | Total mean cost (£) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | EVAR | Difference | OR | EVAR | Difference | OR | EVAR | Difference | |
| 1 | 13,725 | 15,660 | 1935 | 218 | 405 | 187 | 13,857 | 16,051 | 2194 |
| 2 | 168 | 153 | –15 | 188 | 200 | 12 | 334 | 340 | 5 |
| 3 | 0 | 281 | 281 | 168 | 177 | 9 | 152 | 445 | 293 |
| 4 | 45 | 382 | 337 | 142 | 154 | 12 | 173 | 525 | 352 |
| 5 | 106 | 214 | 108 | 113 | 128 | 15 | 205 | 332 | 127 |
| 6 | 92 | 160 | 68 | 100 | 113 | 13 | 179 | 265 | 87 |
| 7 | 69 | 123 | 53 | 66 | 88 | 22 | 129 | 206 | 77 |
| 8 | 241 | 112 | –129 | 49 | 81 | 32 | 280 | 188 | –92 |
| 9 | 101 | 288 | 187 | 35 | 77 | 43 | 130 | 357 | 226 |
| 10 | 125 | 199 | 73 | 17 | 62 | 45 | 140 | 255 | 115 |
| 11 | 56 | 320 | 264 | 12 | 55 | 43 | 66 | 362 | 296 |
| 12 | 43 | 166 | 123 | 12 | 41 | 29 | 53 | 198 | 144 |
| 13 | 78 | 0 | –78 | 14 | 26 | 12 | 88 | 25 | –63 |
| 14 | 81 | 114 | 32 | 12 | 14 | 3 | 89 | 126 | 37 |
| Total mean | 14,931 | 18,171 | 3240 | 1147 | 1622 | 475 | 15,876 | 19,674 | 3798 |
| SE | 666 | 23 | 745 | ||||||
| 95% CI | 1934 to 4546 | 430 to 521 | 2338 to 5258 | ||||||
FIGURE 9.
Evolution of mean cost (£) per patient in each treatment group over time. Mean cost over 14 years estimated using multiple imputation.
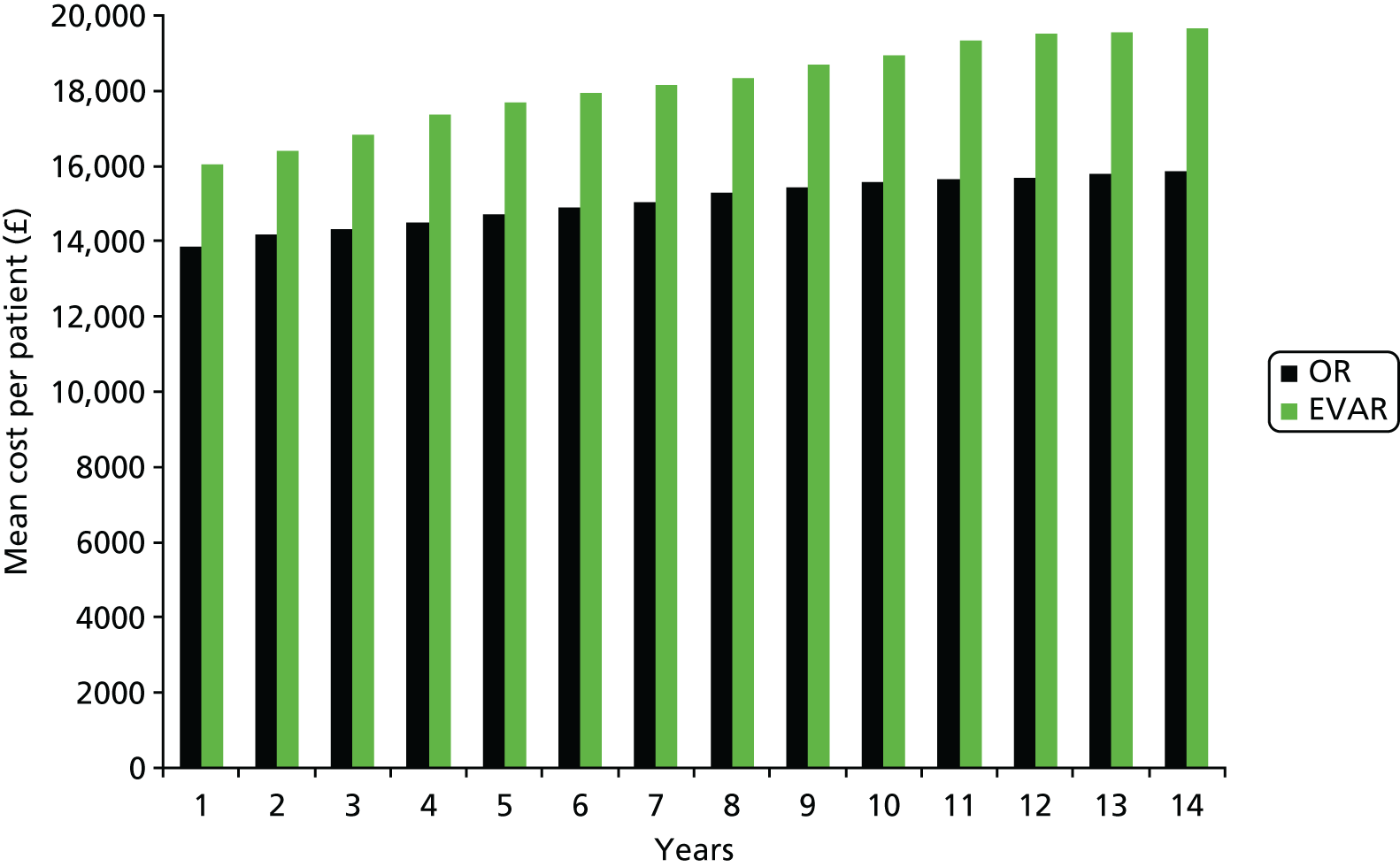
Table 15 shows the estimates from the regression of total cost on treatment group, using Rubin’s rules to aggregate coefficients over the M = 15 data sets. The estimated difference in mean cost per patient between the groups is £3757 (95% CI £2332 to £5182).
| Mean | SE | t | p-value | 95% CI | |
|---|---|---|---|---|---|
| Treat | 3757 | 726 | 5 | 0.000 | 2332 to 5182 |
| Intercept | 15,891 | 513 | 31 | 0.000 | 14,884 to 16,897 |
Discussion
This chapter has estimated mean costs per patient for EVAR and OR over 14 years follow-up. The estimate of the difference in mean costs between the treatment groups is robust to the method used for handling missing data. The difference in cost is £3798 (95% CI £2338 to £5258) under IPW and £3757 (95% CI £2332 to £5182) under multiple imputation.
The methods make different assumptions about the missing data mechanism. The IPW method assumes data are missing completely at random, that is, the probability of an observation being missing does not depend on observed or unobserved measurements. This may be reasonable if the data are administratively censored, where the censoring arises because of the (random) date of recruitment into the trial, rather than unplanned loss to follow-up. The multiple imputation method assumes that data are missing at random, that is, given the observed data, the probability of an observation being missing does not depend on the unobserved data. The high degree of similarity between the two estimates suggests that the missing completely at random assumption is reasonable in this case.
It is perhaps unsurprising that the two methods give similar results, because the number of missing data is modest in this study. This is because there is complete follow-up for reinterventions for 11.5 years (the end of randomisation until the closure of the trial) and data for reinterventions, were obtained from HES (and verified from hospital notes and trial documents).
Chapter 5 Cost-effectiveness of endovascular and open repair
Introduction
This chapter presents a cost-effectiveness model of EVAR compared with OR for aneurysm repair, in patients fit for OR and suitable for EVAR. Parameters are mainly based on the outcomes of EVAR-1. Nevertheless, 24% of the OR group are alive at 15 years; hence some assumptions about the rate of mortality beyond follow-up are needed.
Methods
Overview
A Markov model was constructed to compare costs and QALYs over the lifetime of aneurysm patients undergoing either OR or EVAR. The perspective is the UK NHS and the price year is 2014–15. The discount rate was 3.5% per annum. The structure of the model was the same as used in a previous publication,132 with some minor changes to reflect the new data and analyses presented in this report.
There are three ‘states’: alive, dead from AAA-related causes, or dead from other causes. The cycle length is 6 months. Aneurysm repair (EVAR or OR) takes place during the first model cycle, incurring costs and reduced HRQoL compared with that in the general population.
Patients may die during the first 6 months from an AAA-related cause (mainly operative deaths) or other causes. During each subsequent 6-month cycle, the patient may die of aneurysm causes or other causes, whereas survivors undergo surveillance and may require surgical or endovascular reintervention. Reintervention incurs a cost and decrement in HRQoL during that 6-month cycle.
Patients have one surveillance visit (outpatient attendance and ultrasound) per year after EVAR and one every 5 years under OR. The parameters used in the base-case model are shown in Table 16.
| Parameter | Variable | Parameter value | Mean | SE | LL | UL | Events | Patient-years at risk |
|---|---|---|---|---|---|---|---|---|
| Age at baseline | Age | 74 | ||||||
| Years | Cyclelength | 0.5 | ||||||
| One male | Gender | 1 | ||||||
| Trial OCM compared with population OCM | SMR | 1.175 | ||||||
| HR AAA deaths 0–6 months | HR_aaa_1 | 0.47 | 0.47 | 0.23 | 0.93 | |||
| HR 6 months–4 years | HR_aaa_2 | 1.46 | 1.46 | 0.56 | 3.83 | |||
| HR 4–8 years | HR_aaa_3 | 3.11 | 3.11 | 0.99 | 9.72 | |||
| HR > 8 years | HR_aaa_4 | 5.82 | 5.82 | 1.64 | 20.65 | |||
| HR after the end of the RCT | HR_aaa_5 | 1 | 1 | 0.36 | 2.62 | |||
| Absolute rate open AAA deaths 0–6 months | open_aaa_1 | 0.10 | 30 | 300 | ||||
| Absolute rate open AAA deaths 6 months–4 years | open_aaa_2 | 0.004 | 8 | 2000 | ||||
| Absolute rate open AAA deaths 4–8 years | open_aaa_3 | 0.002 | 4 | 2000 | ||||
| Absolute rate open AAA deaths > 8 years | open_aaa_4 | 0.002 | 3 | 1500 | ||||
| Costprocedure EVAR | evar_c_proc | 15104 | 15104 | 345 | ||||
| Costprocedure open | open_c_proc | 14127 | 14127 | 449 | ||||
| Costreintervention/episode | c_reint | 8670 | 8670 | 831 | ||||
| Costsurveillance/attendance | c_surv | 227 | ||||||
| Number of surveillance/year (OR) | s_open | 0.2 | ||||||
| Number of surveillance/year (EVAR) | s_evar | 1 | ||||||
| Difference utility EVAR – OR 0–3 months | Diff_u_1a | 0.0599 | 0.0599 | 0.0166 | ||||
| Difference utility EVAR – OR 3–6 months | Diff_u_1b | 0 | ||||||
| Difference utility EVAR – OR 6 months–4 years | Diff_u_2 | 0 | ||||||
| Difference utility EVAR – OR 4–8 years | Diff_u_3 | 0 | ||||||
| Difference utility EVAR – OR > 8 years | Diff_u_4 | 0 | ||||||
| Utility at baseline | u_0 | 0.804 | 0.804 | 0.021 | ||||
| Decrement in utility after OR 0–3 months | open_u_1a | –0.0802 | –0.0802 | 0.0119 | ||||
| Decrement in utility after OR 3–6 months | open_u_1b | –0.0802 | –0.0802 | 0.0119 | ||||
| Decrement in utility 6 months–4 years | open_u_2 | 0 | ||||||
| Decrement in utility 4–8 years | open_u_3 | 0 | ||||||
| Decrement in utility > 8 years | open_u_4 | –0.01 | ||||||
| Decrement in utility 0–6 months after reintervention | reint_u | –0.0604 | –0.0604 | 0.0258 | ||||
| Decrement in utility 0–6 months before death | death_u | –0.149 | –0.149 | 0.0166 | ||||
| HR reinterventions 0–6 months | HR_reint_1 | 1.95 | 1.95 | 1.28 | 2.98 | |||
| HR 6 months–4 years | HR_reint_2 | 6.29 | 6.29 | 3.09 | 12.78 | |||
| HR 4–8 years | HR_reint_3 | 1.6 | 1.6 | 0.81 | 3.15 | |||
| HR > 8 years | HR_reint_4 | 1.51 | 1.51 | 0.71 | 3.13 | |||
| Absolute rate OR reinterventions 0–6 months | open_reint_1 | 0.125 | 36 | 288 | ||||
| Absolute rate OR reinterventions 6 months–4 years | open_reint_2 | 0.005 | 9 | 1800 | ||||
| Absolute rate OR reinterventions 4–8 years | open_reint_3 | 0.011 | 16 | 1454 | ||||
| Absolute rate OR reinterventions > 8 years | open_reint_4 | 0.013 | 13 | 1000 | ||||
| EVAR OCM compared with OR OCM | evar_excess | 1.072 | ||||||
| Discountrate, year | discount | 0.035 |
Aneurysm-related mortality
The rates of aneurysm mortality during the trial follow-up period are taken from EVAR-1. The base-case model adheres, as far as possible, to the results of EVAR-1. The base case uses the ITT estimates from the RCT (see Table 5) and assumes no difference in aneurysm mortality after the RCT ends. Three sensitivity analyses are conducted using different assumptions about the relative rate of aneurysm mortality between the treatment groups. The first sensitivity analysis, scenario 1, assumes that the HR for aneurysm mortality observed at the end of the trial continues beyond the RCT. The second sensitivity analysis, scenario 2, assumes that the HR for aneurysm mortality after 4 years is equal to the lower limit of the 95% CI for the mean, rather than the central value, and no difference in aneurysm mortality after the RCT ends. A third sensitivity analysis, scenario 3, uses per-protocol estimates from the RCT (see Table 10) and assumes no difference in AAA after the RCT ends.
Deaths from other causes
The age-specific annual rates of non-aneurysm mortality (i.e. deaths from other causes) in the model are based on UK life tables for all-cause mortality in the general male population. 133 Approximately 90% of patients in EVAR-1 were male. The rates of non-aneurysm mortality are calibrated so that overall mortality after OR at 15 years estimated by the model is the same as that observed in EVAR-1, that is 23.8% survived up to 15 years. This is implemented in the model by a parameter representing the relative risk of EVAR-1 patients, relative to the general population. In this case, the relative risk is 1.175 (see Table 16). The higher rate of mortality in the EVAR-1 population, relative to the general population of the same age, probably arises from the comorbidities and risk factors that usually are associated with development of an AAA.
The differences in all-cause survival between endovascular and OR observed in the RCT over the whole 15-year follow-up can mostly be explained by differences in aneurysm-caused deaths, although it has been suggested that there may have been more deaths from other causes in the EVAR group, in particular mid-term cardiovascular deaths134 and cancer deaths after 8 years (see Chapter 3). The survival curves in the EVAR-1 trial were observed to converge at around 2–4 years (see Figure 5). To reproduce this catch-up in this model, the rates of non-aneurysm mortality after EVAR are set to be slightly greater for the first 2 years (HR 1.072). After 2 years these rates are assumed to be the same in both treatment groups.
Health-related quality of life
EVAR trial 1 collected HRQoL data in both groups using the EuroQol-5 Dimensions, three-level version, instrument up to 10 years. Extensive analyses of these data were undertaken as part of this study and are presented as a working paper. 135 Analysis of EQ-5D in EVAR-1 shows that HRQoL declines in the first 6 months relative to baseline in all patients and that EVAR patients have, on average, better HRQoL during this period than OR patients. 135 After 6 months from the initial operation there is no difference in mean EQ-5D score from baseline between the groups. However, HRQoL is impaired in the 6 months after a reintervention and in the 6 months prior to death (see Table 16).
Reinterventions
Survivors are at risk of reinterventions, which incur a hospital procedure cost and a decrement in HRQoL for 6 months. Reinterventions are those that were identified in EVAR-1 and include incisional hernia repair (mainly after OR) and aneurysm-related reinterventions such as stent graft replacement. The rates of reinterventions up to 15 years are taken from Table 11 of this report. It is assumed in the model that there is no difference in the rate of reinterventions after the 15-year follow-up of the RCT.
Costs
Hospital costs of the procedures for EVAR and OR were estimated using the methods described in Chapter 4. The costs of surveillance using ultrasound were estimated from NHS unit costs. 126 The cost of a reintervention was estimated as the mean hospital reintervention cost from EVAR-1 using the methods described in Chapter 4.
Sensitivity analyses
In addition to the three sensitivity analyses described earlier, a further sensitivity analysis (scenario 4) was conducted using the parameters of the model used in the guidance on the use of endovascular stent grafts issued by NICE in 2009 (technology appraisal number 167). 136 The following parameters were assumed to apply for EVAR by this guidance: a HR for late aneurysm deaths of 1.5; a HR for reinterventions of 1.5; an annual cost of surveillance after EVAR of £56; and no difference in the initial procedure cost. Other input parameters are kept the same as the base case.
Probabilistic sensitivity analysis
All results are presented as the mean of 1000 Monte Carlo simulations of the model. Results were unaltered when tested using 5000 simulations. HRs were assigned a log-normal distribution, rates were assigned gamma distributions, the costs of the procedures were assigned normal distributions and the decrements in utility were assigned normal distributions. The normal distribution for modelling costs and utility is acceptable here as the sample size in EVAR-1 was large and the SEs of the means for these parameters are small. Therefore, the central limit theorem can be assumed to apply.
Results
Table 17 shows the results of the model. The base case assumes that there is no difference in aneurysm mortality beyond the end of the trial and no difference in reinterventions beyond the end of the trial. Figure 10 shows the predicted survival curves under these assumptions. The curves cross at about 4 years for any-cause mortality. EVAR is not effective or cost-effective over the lifetime under the base-case assumptions, with greater overall health benefit, measured in QALYs, but also greater cost. The incremental cost-effectiveness ratio (ICER) is £202,776 per QALY.
| OR | EVAR | Difference | ICER | Probability that EVAR is cost-effective at a threshold of | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALY | Cost (£) | QALY | Cost (£) | QALY | £20,000/QALY | £30,000/QALY | ||
| Base case | 15,536 | 6.415 | 19,152 | 6.433 | 3616 | 0.018 | 202,776 | 0.063 | 0.191 |
| Scenario 1 | 15,512 | 6.421 | 19,150 | 6.432 | 3638 | 0.011 | 330,555 | 0.051 | 0.65 |
| Scenario 2 | 15,599 | 6.435 | 19,212 | 6.542 | 3653 | 0.107 | 34,026 | 0.223 | 0.480 |
| Scenario 3 | 15,521 | 6.421 | 19,057 | 6.199 | 3554 | –0.223 | D– | 0.003 | 0.015 |
| Scenario 4 | 15,271 | 6.431 | 16,315 | 6.526 | 1044 | 0.095 | 10,929 | 0.702 | 0.763 |
FIGURE 10.
Predicted survival curves from the base-case model (deterministic implementation).
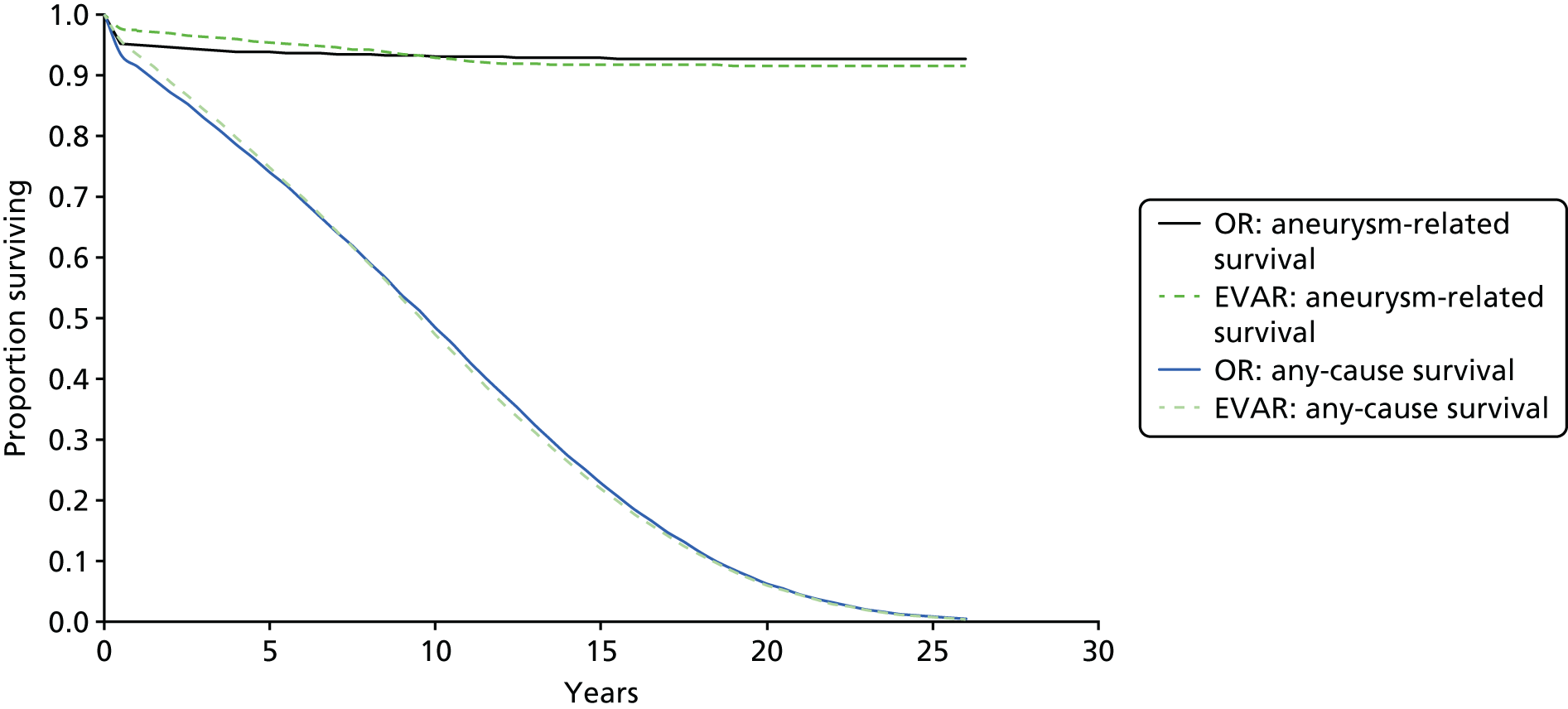
Figure 11 shows the distribution of simulations from the probabilistic implementation of the base-case model. The difference in QALYs is highly skewed, with a long tail. This arises from the wide CIs around the HRs for aneurysm-related deaths. The probability that EVAR is cost-effective is 0.06 at a threshold of £20,000 per QALY and 0.19 at a threshold of £30,000 per QALY.
FIGURE 11.
Cost-effectiveness plane (base-case model, probabilistic implementation).
The first sensitivity analysis, scenario 1, assumes that the HR for aneurysm mortality observed at the end of the trial continues beyond the RCT. This makes EVAR somewhat less effective than the base case, but the results are not substantially different. The second sensitivity analysis, scenario 2, assumes that the HR for aneurysm mortality after 4 years is equal to the lower limit of the 95% CI for the mean (rather than the central value). Under this scenario, the ICER reduces to £34,026 per QALY. Under the per-protocol analysis, scenario 3, EVAR is more effective in the short term than in the base case, but there are more aneurysm-related deaths in the long term, and EVAR becomes less effective and more costly than OR over the lifetime. Scenario 4 applies the model input values used in the NICE final appraisal document. EVAR is considerably more effective than OR in this scenario and the ICER reduces to £11,088 per QALY. The probability that EVAR is cost-effective is 0.70 at £20,000 per QALY and 0.76 at £30,000 per QALY. Figure 12 shows the cost-effectiveness acceptability curve for the base-case and sensitivity analyses.
FIGURE 12.
Cost-effectiveness acceptability curves for the base-case and sensitivity analyses. Note that the cost-effectiveness acceptability curve for scenario 1 is the same as the base case.
Discussion
The base-case model uses EVAR-1 data to inform outcomes within the trial follow-up and assumes that the rates of aneurysm mortality and reinterventions are the same as OR after the end of the trial. Under this model, EVAR showed greater overall health benefit but higher costs than OR. The initial benefit after EVAR was attenuated by late mortality and reinterventions, which are associated with a decrement in HRQoL. Over the lifetime of the patient, these two factors outweigh the initial gain in operative mortality and faster recovery from surgery. Lifetime costs are greater for EVAR because of the price of the stent graft, the need for surveillance and the need for reinterventions. The ICER in the base case is > £200,000 per QALY, considerably above the threshold of £20,000–30,000 per QALY that conventionally applies in the UK.
The base-case model is based closely on EVAR-1 during the first 15 years, which found that the initial gains in operative mortality were gradually lost over the longer term and that EVAR was associated with greater costs. EVAR might be considered cost-effective in certain scenarios. Scenario 2 assumes that late AAA mortality is lower than the base case. This might be plausible, but it is selective of the evidence. Given the trial data, it is equally likely that the ‘true’ HR for aneurysm mortality could be at the upper limit of the 95% CI, rather than at the lower limit.
The results shown here are similar to earlier estimates based on the 8-year data from EVAR-1. 132 The DREAM trial, based in the Netherlands, concluded that EVAR was not cost-effective even after 1 year. 137 The OVER trial, based in the USA, found that EVAR was effective and cost-effective at 2 years,138 but this was in a lower-risk group and did not take account of late mortality or reinterventions.
The NICE appraisal of endovascular stent grafts (technology appraisal number 167) was conducted in 2009 when the 4-year results of EVAR-1 were known. The committee acknowledged the high internal validity of the EVAR trial but were concerned that the study did not properly reflect the performance of the latest generation of endovascular stents, which were thought to have improved since the trial recruitment period 1999–2004. Hence, NICE assumed more favourable outcomes for the later generation of EVAR devices than were reported in the trial. In particular, it assumed lower aneurysm-related mortality, fewer reinterventions, fewer resources used in the hospital stay and less cost for surveillance than observed in EVAR-1. Using these values in the current model, this scenario produced an ICER of £10,929 per QALY, which would be highly cost-effective at conventional thresholds used in the UK. These assumptions, as yet, are not supported by evidence from RCTs. Nevertheless, this modelling does show how EVAR would need to improve in order to be considered cost-effective. For example, EVAR might be cost-effective if ways could be found to monitor sac expansion to trigger more timely and better targeted reintervention and reduce late aneurysm deaths.
Chapter 6 Individual patient data meta-analysis of EVAR trial 1, the Dutch Randomised Endovascular Aneurysm Management trial, the Open versus Endovascular Repair trial and Anévrysme de l’aorte abdominale, Chirurgie versus Endoprothése trial
Introduction
Open repair of AAA was first introduced by Dubost et al. in 1951. 75 In the 1990s the less invasive EVAR was introduced and the first multicentre randomised trial of EVAR compared with OR was started in 1999 (EVAR-1), in the UK. 100 This was soon followed by other multicentre trials in Europe (the DREAM and ACE trials) and the OVER trial in the USA. 98,99,104
Each of the randomised trials of EVAR compared with OR recruited patients (suitable for either OR or EVAR) with slightly different entry characteristics with respect to age, sex, aneurysm morphology and other demographics. The EVAR-1, DREAM and OVER trials all showed an early survival benefit for EVAR, whereas the ACE trial did not. This early survival benefit for EVAR was lost within 1–3 years for EVAR-1 and the DREAM trial, but not until later for the OVER trial. 94,98,104 This ‘catch-up’ in mortality has been noted in many other studies, including a Cochrane review and analyses of the Medicare database,108,139 but no satisfactory explanation for this phenomenon has emerged (the Cochrane review was limited by not being able to report aneurysm-related mortality or subgroup analyses). 139 Each trial individually has been too small to investigate the reasons for this ‘catch-up’ mortality in the EVAR groups or to answer the much-discussed question of whether or not younger and fitter patients (or other subgroups) should be offered OR, which is considered more durable than EVAR. 140,141 This ‘catch-up’ in mortality needs to be avoided if EVAR is to outperform OR in the longer term. To try to address some of these issues, the four randomised trials agreed to pool their data for an IPD meta-analysis.
Methods
In July 2013, MEDLINE, EMBASE and clinical trial databases were searched for randomised trials comparing OR and EVAR of AAAs. The search terms used were AAA, aneurysm, endovascular, stent, open repair and randomised trial. From 275 reports, we identified four eligible trials reporting mid-term follow-up. The methods for these four multicentre trials included in this meta-analysis have been published previously. 94,102,105,106 EVAR-1 randomised 1252 patients (91% male), with an aneurysm diameter of > 5.5 cm, between September 1999 and August 2004 in the UK. The DREAM trial randomised 351 patients (92% male), with an aneurysm diameter of ≥ 5 cm, between November 2000 and December 2003 in the Netherlands and Belgium. The OVER trial randomised 881 patients (99% male), with an aneurysm diameter of ≥ 5.0 cm, between October 2002 and April 2008 at Veteran Affairs hospitals in the USA. The ACE trial randomised 306 patients (99% male), with an aneurysm diameter of > 5.0 cm, between March 2003 and March 2008 in France (seven patients withdrew consent before discharge). All patients were considered fit for open surgery under general anaesthesia and all trials used approved devices for EVAR, predominantly within the manufacturers’ IFU, and followed up patients for a minimum of 3 years. Summary baseline characteristics for patients by trial are shown in Table 18.
| Baseline variable | EVAR-1 (n = 1252) | DREAM (n = 351) | OVER (n = 881) | ACE (n = 299) |
|---|---|---|---|---|
| Age (years), mean (SD) | 74 (6.1) | 70 (6.7) | 70 (7.8) | 69 (7.4) |
| Male sex, n (%) | 1135 (91) | 332 (92) | 876 (99) | 296 (99) |
| BMI (kg/m2), mean (SD) | 26.5 (4.5) | 26.7 (4.7) | 28.6 (5.4) | 27.2 (3.5) |
| Smoking status,a n (%) | ||||
| Current | 270 (22) | 130 (37) | 363 (41) | 72 (24) |
| Ex | 863 (69) | 78 (22) | 481 (55) | 75 (25) |
| Diabetes, n (%) | 128 (10) | 35 (10) | 200 (23) | 49 (16) |
| Previous angina/MI, n (%) | 492 (39) | 153 (44) | 268 (30) | 115 (38) |
| ABPI, mean (SD)b | 1.0 (0.18) | 1.0 (0.16) | 0.98 (0.18) | NA |
| Creatinine level (μmol/l), median (IQR) | 102 (90–119) | 95 (84–109) | 97 (80–110) | 93 (82–110) |
| EQ-5D score, mean (SD) | 0.82 (0.12) | 0.84 (0.11) | 0.85 (0.09) | NA |
| AAA diameter (cm), mean (SD) | 6.5 (0.9) | 6.0 (0.9) | 5.7 (0.9) | 5.6 (0.7) |
| AAA neck length (cm), mean (SD) | 2.8 (1.2) | 2.5 (1.2) | 2.6 (1.2) | 2.8 (1.0) |
| AAA neck diameter (cm), mean (SD) | 2.35 (0.30) | 2.39 (0.33) | 2.26 (0.35) | 2.36 (0.33) |
| Post-randomisation parameters | ||||
| Time (days) from randomisation to repair,c median (IQR) | 40 (1–576) | 39 (3–209) | 17 (0–290) | 27 (1–203) |
| Repair in compliance with randomisation, % | 93 | 96 | 96 | 93 |
| Follow-up for mortality (years), median (IQR) | 6.0 (3.9–7.3) | 6.0 (5.0–6.8) | 5.4 (4.1–6.8) | 3.1 (2.1–3.4) |
| 30-day operative mortality, n/N (%) | ||||
| EVAR | 11/614 (1.8) | 2/170 (1.2) | 1/439 (0.2) | 2/150 (1.3) |
| OR | 26/602 (4.3) | 5/173 (2.9) | 8/429 (1.9) | 1/147 (0.7) |
| Reintervention rate, n/person-years (rate per 100 person-years) | ||||
| EVAR | 174/3381 (5.1) | 77/906 (8.5) | 155/2334 (6.6) | 32/419 (7.6) |
| OR | 64/3309 (1.9) | 41/932 (4.4) | 104/2276 (4.6) | 10/408 (2.5) |
The four data sets were merged based on fields available in the case record forms of the largest trial (EVAR-1), range checks were conducted and queries resolved with the individual trial co-ordinating centres. The common baseline variables across the trials were age, sex, history of smoking, diabetes, coronary artery disease (defined as previous stable or unstable angina or MI), BMI, maximum aneurysm diameter, proximal aortic neck length and diameter, ankle–brachial pressure index (ABPI) and creatinine level, used for estimated glomerular filtration rate (eGFR),142 but without information on ethnicity. Each trial also contained some data about hypertension, which was included in a modified Wilkins cardiovascular survival risk score143 (Table 19). The reporting of both drug use (including antiplatelet and lipid-lowering agents) and reinterventions was very different in the four trials (particularly intestinal and wound-related reinterventions following OR). The postoperative surveillance protocol was identical for both randomised groups in all trials, except for the DREAM trial, in which, after 2 years, surveillance was relaxed for the OR group. However, for complications, only endoleaks after EVAR were reported similarly across the trials; laparotomy-related complications were not.
| Blood pressure | Previous MI | Diabetes | Smoking (never/ex/current) | EVAR-1, n | DREAM, n | OVER, n | ACE, n | |
|---|---|---|---|---|---|---|---|---|
| All optimal |
SBP < 120 mmHg and DBP < 80 mmHg (ACE trial only: no hypertension) AND |
No AND |
No AND |
Never | 4 (0) | 3 (1) | 7 (1) | 31 (10) |
| One or more not optimal |
SBP 120–139 mmHg or DBP 80–89 mmHg (ACE trial only: SBP controlled by one drug or DBP ≤ 90 mmHg) OR |
No AND |
No AND |
Never/ex | 173 (14) | 40 (11) | 182 (21) | 15 (5) |
| One or more elevated |
SBP 140–159 mmHg or DBP 90–99 mmHg (ACE trial only: one hypertensive drug for both SBP and DBP) OR |
No AND |
No AND |
Never/ex | 232 (19) | 44 (13) | 75 (9) | 49 (16) |
| One major |
SBP ≥ 160 mmHg or DBP ≥ 100 mmHg (ACE trial only: two or more hypertensive drugs for both SBP and DBP or uncontrollable) OR |
Yes OR |
Yes OR |
Current | 579 (46) | 149 (43) | 397 (45) | 109 (36) |
| Two or more majors |
SBP ≥ 160 mmHg or DBP ≥ 100 mmHg (ACE trial only: two or more hypertensive drugs or uncontrollable) AND/OR |
Yes AND/OR |
Yes AND/OR |
Current | 264 (21) | 112 (32) | 219 (25) | 95 (32) |
Statistical analysis
The primary analyses considered the groups ‘as randomised’ within each trial. Mortality after randomisation was assessed at 30 days, in hospital and then at three defined time periods: 0–6 months, 6 months–4 years and > 4 years after randomisation. Aneurysm-related mortality included death from (1) primary aneurysm rupture, (2) within 30 days of aneurysm repair or any reintervention; and (3) rupture after repair. Given the different times between randomisation and aneurysm repair in the four trials (Table 20), aneurysm-related mortality was also assessed at 30 days, 31 days–3 years and > 3 years after aneurysm repair. Kaplan–Meier survival curves by randomised group were generated from the combined data from all four trials and the restricted mean life-years up to a certain time estimated by the area under the curve up to that time. Logistic regression was used to compare operative (30-day) and in-hospital mortality among patients who underwent repair, and Cox proportional hazards regression was used to compare total and aneurysm-related mortality and time to reintervention. A two-stage IPD meta-analysis was performed. First analyses were conducted separately within each trial and then pooled time-period-specific estimates were calculated using random-effects meta-analysis with between-study heterogeneity estimated using the method of DerSimonian and Laird. 144 The proportion of between-trial variability beyond that expected by chance was quantified using the I2-statistic. 145 All analyses were then repeated adjusting for the following baseline covariates: age, sex, maximum aneurysm diameter and log-creatinine level.
| All-cause mortality | EVAR-1 (n = 1252), n deaths/N (rate/100 person-years) | DREAM (n = 351), n deaths/N (rate/100 person-years) | OVER (n = 881), n deaths/N (rate/100 person-years) | ACE (n = 299), n deaths/N (rate/100 person-years) | Pooled (n = 2783), n deaths/N (rate/100 person-years) |
|---|---|---|---|---|---|
| All patients | |||||
| EVAR | 260/626 (7.5) | 58/173 (6.2) | 146/444 (6.3) | 17/150 (4.1) | 481/1393 (6.7) |
| OR | 264/626 (7.7) | 60/178 (6.2) | 146/437 (6.4) | 12/149 (2.9) | 482/1390 (6.8) |
| Time since randomisation | |||||
| 0–6 months | |||||
| EVAR | 26/626 (8.5) | 6/173 (7.1) | 11/444 (5.0) | 3/150 (4.6) | 46/1393 (6.7) |
| OR | 45/626 (15.0) | 10/178 (11.6) | 17/437 (8.0) | 1/149 (1.4) | 73/1390 (10.9) |
| 6 months–4 years | |||||
| EVAR | 125/599 (6.7) | 33/167 (6.2) | 73/433 (5.2) | 13/146 (3.8) | 244/1345 (5.9) |
| OR | 116/581 (6.3) | 25/168 (4.6) | 78/420 (5.9) | 10/146 (3.0) | 229/1315 (5.7) |
| > 4 years | |||||
| EVAR | 109/472 (8.4) | 19/134 (6.0) | 62/348 (8.6) | 1/33 (17.7) | 191/987 (8.2) |
| OR | 103/461 (7.9) | 25/143 (7.4) | 51/331 (7.0) | 1/23 (17.5) | 180/958 (7.6) |
| Unadjusted HR (95% CI) | |||||
| All patients | 0.98 (0.82 to 1.16) | 1.01 (0.70 to 1.44) | 0.98 (0.78 to 1.23) | 1.52 (0.71 to 3.24) | 0.99 (0.87 to 1.13) |
| Time since randomisation | |||||
| 0–6 months | 0.57 (0.35 to 0.92) | 0.60 (0.22 to 1.66) | 0.63 (0.29 to 1.34) | 2.95 (0.31 to 28.40) | 0.61 (0.42 to 0.89)* |
| 6 months–4 years | 1.06 (0.82 to 1.37) | 1.36 (0.81 to 2.29) | 0.89 (0.65 to 1.23) | 1.25 (0.55 to 2.83) | 1.04 (0.87 to 1.25) |
| > 4 years | 1.06 (0.81 to 1.39) | 0.81 (0.45 to 1.47) | 1.23 (0.85 to 1.78) | 1.07 (0.88 to 1.32) | |
| EVAR-1 (n = 1246) | DREAM (n = 339) | OVER (n = 881) | ACE (n = 281) | Pooled (n = 2747) | |
| Adjusted HR (95% CI)a | |||||
| All patients | 1.00 (0.84 to 1.19) | 0.88 (0.61 to 1.27) | 1.04 (0.82 to 1.31) | 1.43 (0.63 to 3.22) | 1.01 (0.89 to 1.14) |
| Time since randomisation | |||||
| 0–6 months | 0.58 (0.36 to 0.95) | 0.42 (0.14 to 1.25) | 0.62 (0.29 to 1.33) | b | 0.57 (0.39 to 0.84)** |
| 6 months–4 years | 1.08 (0.84 to 1.40) | 1.15 (0.68 to 1.95) | 0.94 (0.69 to 1.30) | 1.14 (0.49 to 2.68) | 1.05 (0.87 to 1.26) |
| > 4 years | 1.11 (0.85 to 1.46) | 0.79 (0.43 to 1.44) | 1.30 (0.89 to 1.90) | b | 1.12 (0.91 to 1.38) |
The subgroups age, sex, eGFR, coronary artery disease, ABPI, the modified Wilkins cardiovascular survival risk score, maximum aneurysm diameter, proximal aneurysm neck diameter and neck length were assessed for differences in the effect of the EVAR and open strategies by including an interaction term between the subgroup and randomised group in a Cox regression model. Except for sex and coronary artery disease, all measures were entered as continuous variables to assess effect modification. Each interaction term was pooled across the trials using random-effects meta-analysis and its statistical significance assessed using a Wald test, taking the 5% level as significant. For presentation purposes only (and not for assessing significance), HRs are shown by dichotomising continuous measures at chosen cut-off points: age (72 years), eGFR (68.4), ABPI (0.9), cardiovascular risk score (2 major), maximum aneurysm diameter (5.9 cm), neck diameter (2.3 cm), neck length (2.5 cm), estimating the HRs within each subgroup and pooling these across studies.
The hazard of reintervention following aneurysm repair was analysed using an Anderson–Gill multiple failure time model. 146
All analyses were performed using Stata statistical software, version 13.
Results
A total of 2783 patients, with 14,245 person-years of follow-up, were included in this meta-analysis: their baseline characteristics are shown in Table 18, with significant intertrial differences in all variables. Patients in EVAR-1 were older and had larger aneurysms than patients in the other trials. Nearly all patients in the OVER and EVAR-1 trials had a history of smoking, compared with about half the patients in the DREAM and ACE trials. Nearly all patients in the OVER and EVAR-1 trials had a history of smoking, compared with about half the patients in the DREAM and ACE trials, and patients in the OVER trial had the highest BMI and highest proportion of patients with diabetes. Summary post-randomisation characteristics of the trials also are given in Table 18. The median follow-up was 6.0, 6.0, 5.4 and 3.1 years, for EVAR-1, the DREAM, OVER and ACE trials, respectively, 5.5 years for the pooled data and compliance with randomised allocation was 93% or higher in all trials.
Total mortality
Over the follow-up of all four trials there were 481 deaths in the EVAR groups and 482 in the OR groups. Kaplan–Meier curves by randomised group for total mortality across all four trials are shown in Figure 13. Overall, there was no difference in total mortality over the follow-up period of the trial (pooled HR 0.99, 95% CI 0.87 to 1.13) (Figure 14a and see Table 20). Between 0 and 6 months mortality was lower for the EVAR groups, with 46 deaths, than for OR, with 73 deaths (HR 0.61, 95% CI 0.42 to 0.89), with no evidence of heterogeneity between the trials. The early survival advantage of EVAR in the first 6 months was largely attributable to the lower 30-day operative mortality for EVAR than with OR (unadjusted pooled odds ratio 0.40, 95% CI 0.22 to 0.73) (Figure 14b). After this, the early advantage of the EVAR group was lost and the HRs moved (non-significantly) in the direction of OR. Adjusted HRs were similar. By 5 years the estimated survival was 73.6% (95% CI 71.1% to 75.9%) in both the EVAR and OR groups with an expected 0.06 additional life-years in the EVAR group, corresponding to 23 days (95% CI –16 to 61 days; p = 0.246). The causes of death by each time period are shown in Table 21.
FIGURE 13.
Kaplan–Meier survival curves for overall total mortality, by randomised group, for all four trials combined.
FIGURE 14.
(a) Total mortality, overall and at 0–6 months, 6 months–4 years and > 4 years since randomisation, unadjusted HRs; and (b) mortality within 30 days of operation, showing odds ratio.
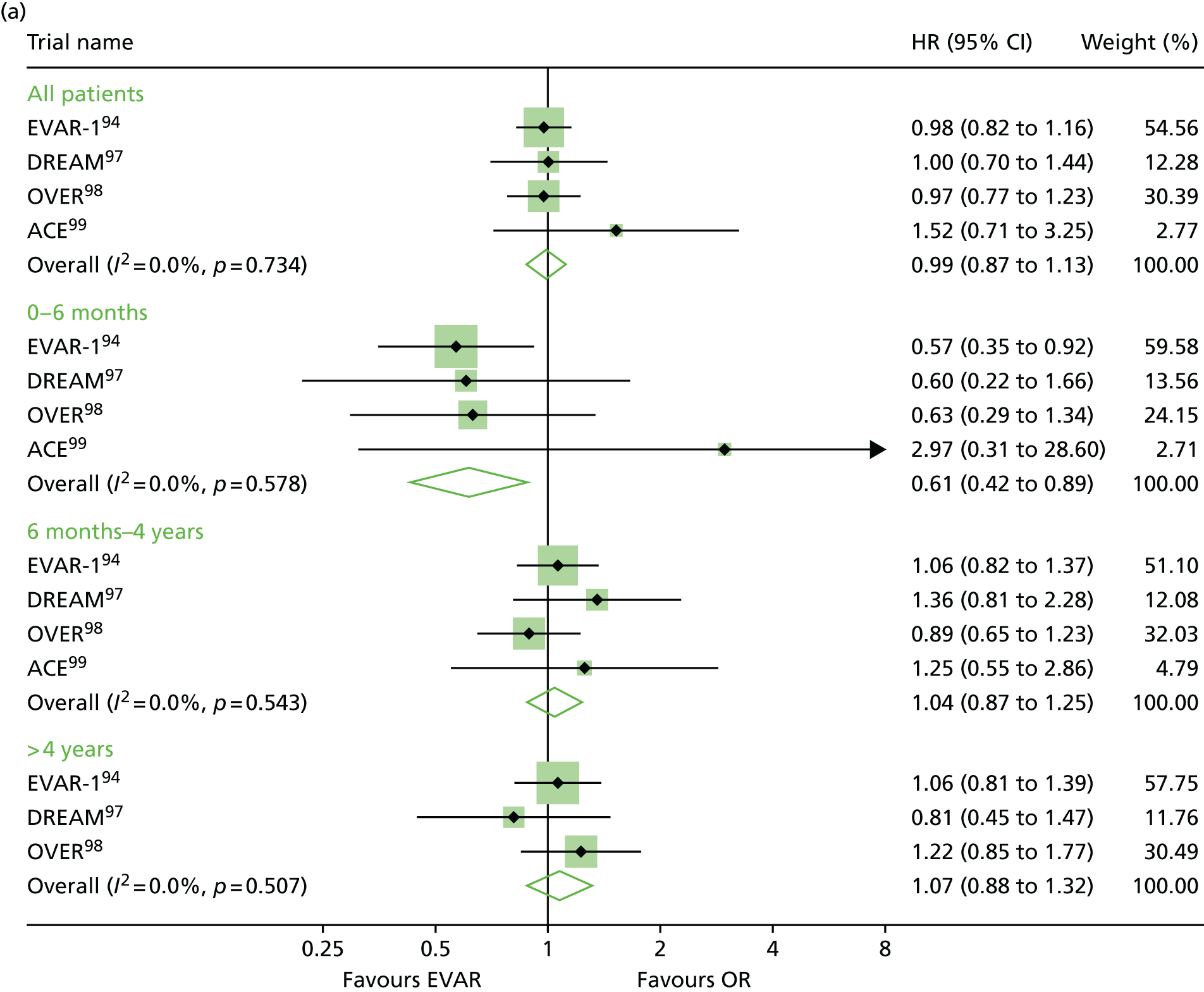
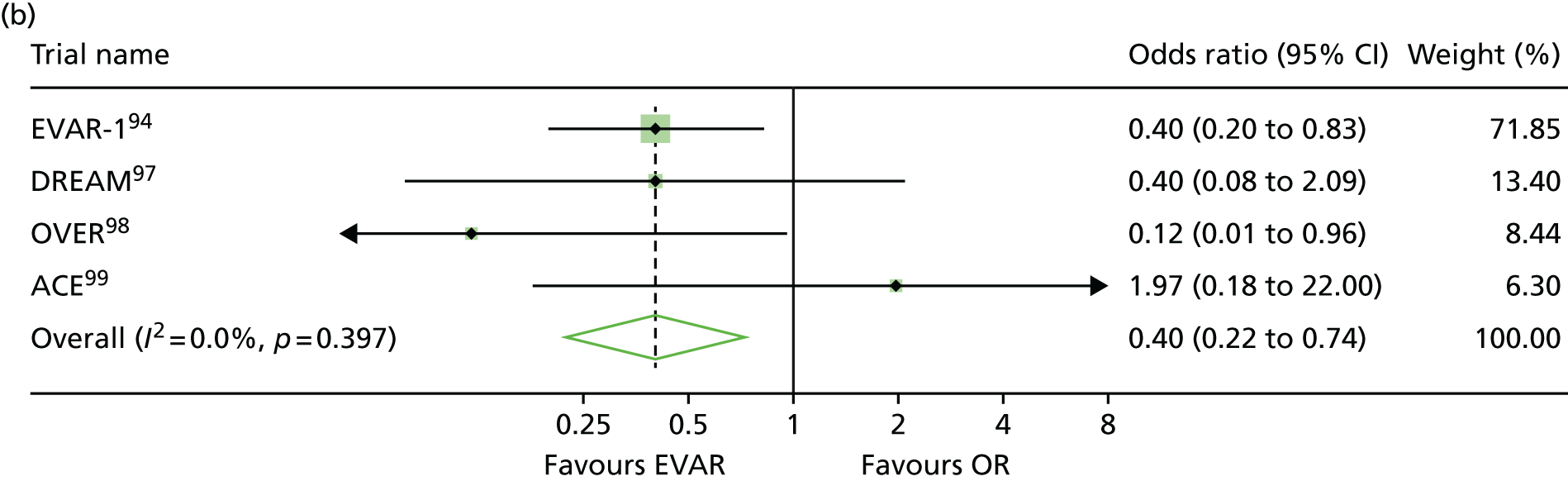
| Cause of death | EVAR-1, n (%) | DREAM, n (%) | OVER, n (%) | ACE, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| EVAR (n = 26) | OR (n = 45) | EVAR (n = 6) | OR (n = 10) | EVAR (n = 11) | OR (n = 17) | EVAR (n = 3) | OR (n = 1) | |
| 0–6 months | ||||||||
| AAA related | 14 (54) | 30 (67) | 3 (50) | 10 (100) | 5 (45) | 14 (82) | 3 (100) | 1 (100) |
| MI/other cardiac | 4 (15) | 4 (9) | 0 (0) | 0 (0) | 2 (18) | 0 (0) | 0 (0) | 0 (0) |
| Stroke | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 0 (0) | 0 (0) |
| Other vascular | 2 (8) | 2 (4) | 1 (17) | 0 (0) | 3 (27) | 0 (0) | 0 (0) | 0 (0) |
| Cancer, lung | 1 (4) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cancer, other | 2 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (18) | 0 (0) | 0 (0) |
| Pulmonary | 0 (0) | 5 (11) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Renal | 2 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 1 (4) | 3 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| EVAR (n = 125) | OR (n = 116) | EVAR (n = 33) | OR (n = 25) | EVAR (n = 73) | OR (n = 78) | EVAR (n = 13) | OR (n = 10) | |
| 6 months–4 years | ||||||||
| AAA related | 12 (10) | 8 (7) | 1 (3) | 1 (4) | 2 (3) | 0 (0) | 4 (31) | 0 (0) |
| MI/other cardiac | 27 (22) | 25 (22) | 8 (24) | 6 (24) | 16 (22) | 15 (19) | 2 (15) | 4 (40) |
| Stroke | 11 (9) | 6 (5) | 1 (3) | 2 (8) | 3 (4) | 1 (1) | 1 (8) | 0 (0) |
| Other vascular | 7 (6) | 6 (5) | 0 (0) | 3 (12) | 3 (4) | 4 (5) | 0 (0) | 0 (0) |
| Cancer, lung | 19 (15) | 20 (17) | 1 (3) | 7 (28) | 7 (10) | 12 (15) | 2 (15) | 2 (20) |
| Cancer, other | 20 (16) | 29 (25) | 12 (36) | 2 (8) | 18 (25) | 15 (19) | 2 (15) | 1 (10) |
| Pulmonary | 9 (7) | 15 (13) | 2 (6) | 2 (8) | 4 (5) | 8 (10) | 1 (8) | 1 (10) |
| Renal | 4 (3) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (8) | 1 (10) |
| Other | 15 (12) | 6 (5) | 4 (12) | 2 (8) | 12 (16) | 9 (12) | 0 (0) | 0 (0) |
| Unknown | 1 (1) | 0 (0) | 4 (12) | 2 (8) | 8 (11) | 13 (17) | 0 (0) | 1 (10) |
| EVAR (n = 109) | OR (n = 103) | EVAR (n = 19) | OR (n = 25) | EVAR (n = 62) | OR (n = 51) | EVAR (n = 1) | OR (n = 1) | |
| > 4 years | ||||||||
| AAA related | 10 (9) | 2 (2) | 2 (11) | 0 (0) | 3 (5) | 3 (6) | 0 (0) | 0 (0) |
| MI/other cardiac | 28 (26) | 26 (25) | 6 (32) | 9 (36) | 15 (24) | 10 (20) | 0 (0) | 0 (0) |
| Stroke | 11 (10) | 11 (11) | 1 (5) | 2 (8) | 4 (6) | 2 (4) | 0 (0) | 0 (0) |
| Other vascular | 8 (7) | 6 (6) | 0 (0) | 0 (0) | 5 (8) | 2 (4) | 0 (0) | 0 (0) |
| Cancer, lung | 9 (8) | 9 (9) | 0 (0) | 0 (0) | 7 (11) | 7 (14) | 0 (0) | 0 (0) |
| Cancer, other | 14 (13) | 20 (19) | 4 (21) | 7 (28) | 7 (11) | 11 (22) | 0 (0) | 0 (0) |
| Pulmonary | 14 (13) | 17 (17) | 3 (16) | 2 (8) | 9 (15) | 11 (22) | 0 (0) | 0 (0) |
| Renal | 4 (4) | 2 (2) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 11 (10) | 10 (10) | 3 (16) | 1 (4) | 5 (8) | 1 (2) | 0 (0) | 0 (0) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 3 (12) | 7 (11) | 4 (8) | 1 (100) | 1 (100) |
Aneurysm-related mortality
The findings for aneurysm-related mortality were similar in direction, with relative benefit for the EVAR groups 0–6 months after randomisation (25 aneurysm-related deaths vs. 55 in the OR groups; pooled unadjusted HR 0.44, 95% CI 0.26 to 0.76). In later time periods the results move in the direction of OR, 6 months–4 years and > 4 years, with pooled HRs of 1.43 (95% CI 0.61 to 3.34) and 2.29 (95% CI 0.49 to 10.85), respectively (Figure 15). For those who received aneurysm repair, analysis by time from repair showed a strong relative advantage for the EVAR group in the first 30 days, between 30 days and 3 years there was no difference between the groups, but after 3 years there was a significant relative advantage for the OR group, with three aneurysm-related deaths compared with 19 deaths in the EVAR groups (HR 5.16, 95% CI 1.49 to 17.89; p = 0.010) (Table 22).
FIGURE 15.
Aneurysm-related mortality, overall and at 0–6 months, 6 months–4 years and > 4 years since randomisation. Unadjusted HRs.
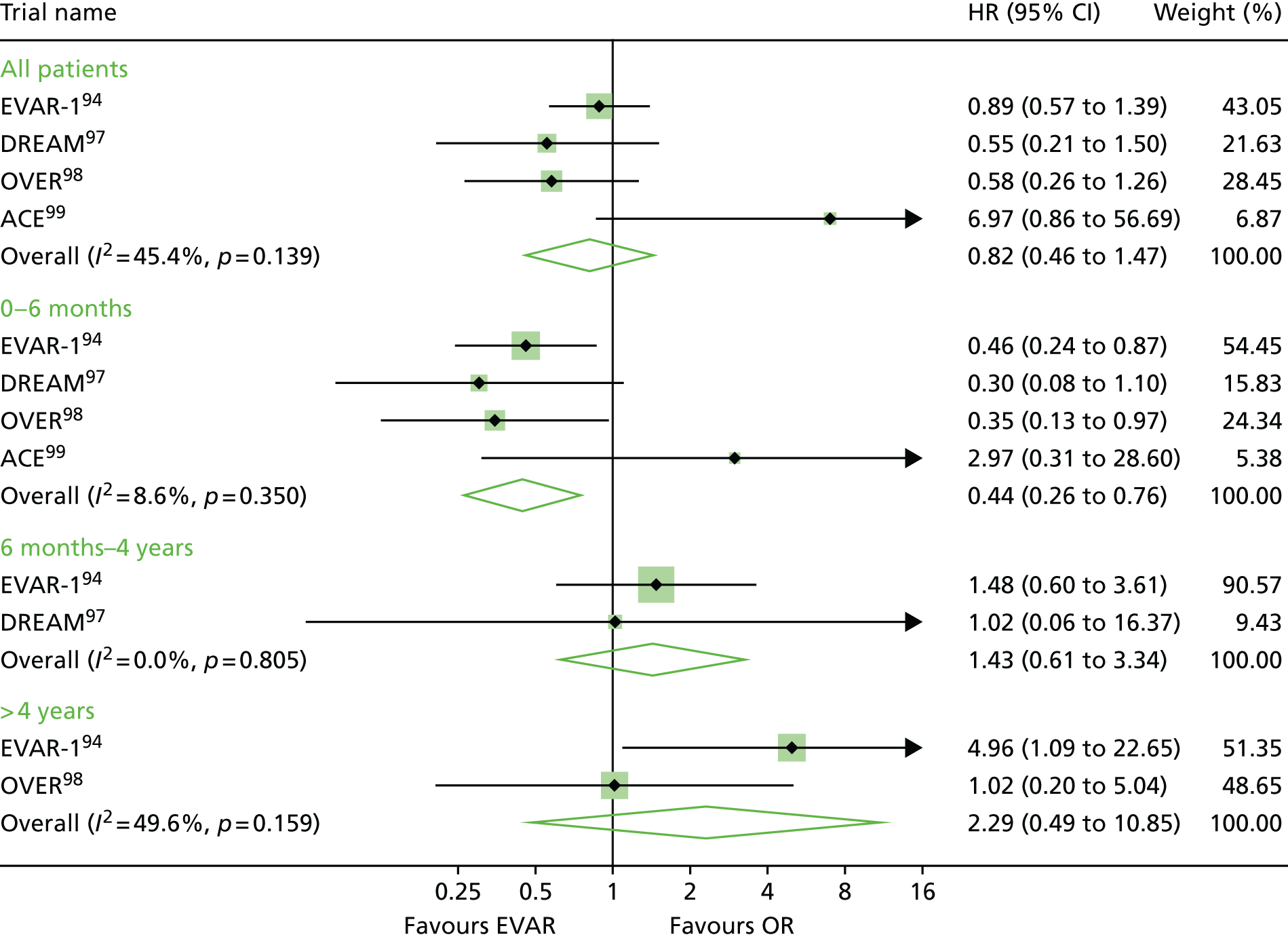
| Aneurysm-related mortality | EVAR-1 (n = 1216) n deaths/N (rate/100 person-years) | DREAM (n = 343) n deaths/N (rate/100 person-years) | OVER (n = 868) n deaths/N (rate/100 person-years) | ACE (n = 297) n deaths/N (rate/100 person-years) | Pooled (n = 2724) n deaths/N (rate/100 person-years) |
|---|---|---|---|---|---|
| All patients | |||||
| EVAR | 31/614 (0.9) | 6/170 (0.7) | 9/439 (0.4) | 7/150 (1.7) | 53/1373 (0.8) |
| OR | 32/602 (1.0) | 10/173 (1.1) | 13/429 (0.6) | 1/147 (0.3) | 56/1351 (0.8) |
| Time since operation | |||||
| 0–30 days | |||||
| EVAR | 11/614 (22.0) | 2/170 (14.3) | 1/439 (2.8) | 2/150 (16.4) | 16/1373 (14.2) |
| OR | 26/602 (53.7) | 5/173 (35.5) | 8/429 (22.9) | 1/147 (8.3) | 40/1351 (36.5) |
| 31 days–3 years | |||||
| EVAR | 7/603 (0.4) | 2/168 (0.4) | 5/438 (0.4) | 4/148 (1.1) | 18/1357 (0.5) |
| OR | 4/576 (0.3) | 5/168 (1.1) | 4/421 (0.3) | 0/146 (0.0) | 13/1311 (0.4) |
| > 3 years | |||||
| EVAR | 13/498 (0.8) | 2/140 (0.5) | 3/380 (0.3) | 1/78 (2.3) | 19/1096 (0.6) |
| OR | 2/484 (0.1) | 0/146 (0.0) | 1/352 (0.1) | 0/72 (0.0) | 3/1054 (0.1) |
| Unadjusted HR | |||||
| All patients | 0.94 (0.57 to 1.54) | 0.61 (0.22 to 1.68) | 0.68 (0.29 to 1.59) | 6.86 (0.84 to 55.78) | 0.89 (0.51 to 1.56) |
| Time since operation | |||||
| 0–30 days | 0.41 (0.20 to 0.83) | 0.40 (0.08 to 2.08) | 0.12 (0.02 to 0.97) | 1.97 (0.18 to 21.76) | 0.41 (0.22 to 0.74)** |
| 31 days–3 years | 1.69 (0.50 to 5.77) | 0.40 (0.08 to 2.08) | 1.20 (0.32 to 4.47) | a– | 1.07 (0.49 to 2.36) |
| > 3 years | 6.35 (1.43 to 28.15) | a– | 3.18 (0.33 to 30.64) | a– | 5.16 (1.49 to 17.89)* |
| EVAR-1 (n = 1211) | DREAM (n = 331) | OVER (n = 868) | ACE (n = 280) | Pooled (n = 2690) | |
| Adjusted HRb | |||||
| All patients | 0.97 (0.59 to 1.59) | 0.44 (0.15 to 1.30) | 0.71 (0.30 to 1.67) | a– | 0.81 (0.55 to 1.21) |
| Time since operation | |||||
| 0–30 days | 0.42 (0.21 to 0.86) | 0.21 (0.02 to 1.85) | 0.13 (0.02 to 1.05) | a– | 0.36 (0.19 to 0.67)** |
| 31 days–3 years | 1.82 (0.52 to 6.36) | 0.32 (0.06 to 1.65) | 1.13 (0.29 to 4.39) | a– | 0.98 (0.38 to 2.55) |
| > 3 years | 6.58 (1.48 to 29.21) | a– | 3.19 (0.33 to 31.26) | a– | 5.30 (1.52 to 18.46)** |
Total mortality by subgroups
There was no significant effect of age or sex on the relative effectiveness of EVAR in preventing deaths in any time period, including the first 6 months following randomisation (Figure 16). There were two subgroups of patients who appeared to have no early benefit (to 6 months) under EVAR compared with OR: patients with moderate renal dysfunction and those with coronary artery disease. For those with above-median eGFR, the pooled HR of 0.42 (95% CI 0.21 to 0.84) was significantly in favour of EVAR compared with the less favourable and non-significant pooled HR of 0.68 (95% CI 0.43 to 1.08) for those with worse renal function; therefore, the interaction between eGFR measure and treatment group was significant (interaction p = 0.024) (see Figure 16).
FIGURE 16.
Unadjusted HRs for total mortality by subgroups of age, sex and eGFR: overall and at 0–6 months, 6 months–4 years and > 4 years since randomisation. Interaction p-value for age and eGFR calculated using the continuous measures (median eGFR 68.4). Not all trials contribute to the subgroup analyses or every time point.
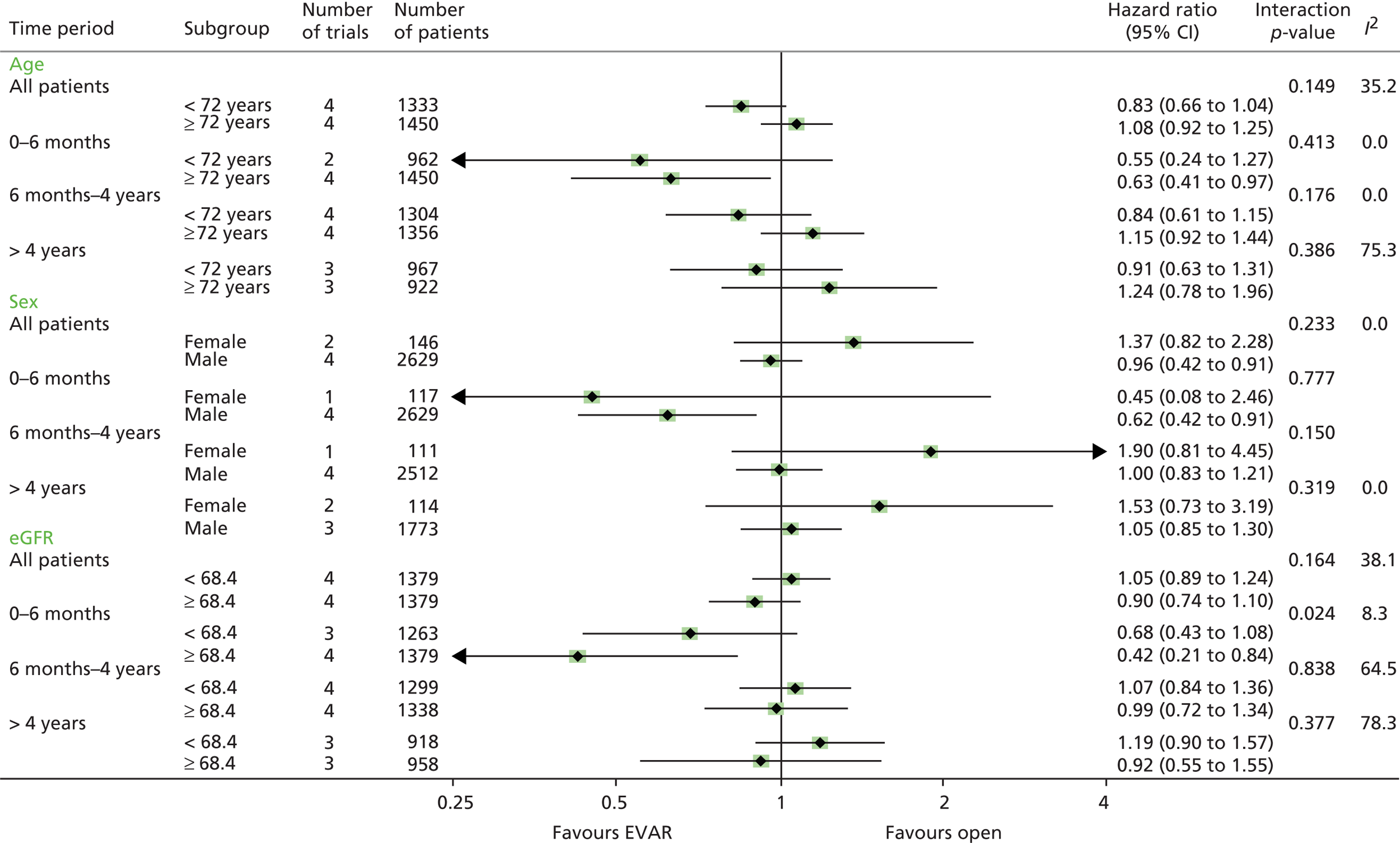
Similarly, patients with coronary artery disease gained no early advantage of being in the EVAR group, in comparison with patients without prior coronary artery disease (interaction p = 0.047) (Figure 17). None of the morphological aneurysm characteristics, smoking, diabetes or BMI was associated with mortality (Figures 18 and 19).
FIGURE 17.
Unadjusted HRs for total mortality by subgroups of history of angina or MI, ABPI and cardiovascular risk score: overall and at 0–6 months, 6 months–4 years and > 4 years since randomisation. Interaction p-values for ABPI and cardiovascular risk score calculated using the continuous measures. Not all trials contribute to the subgroup analyses or every time point.
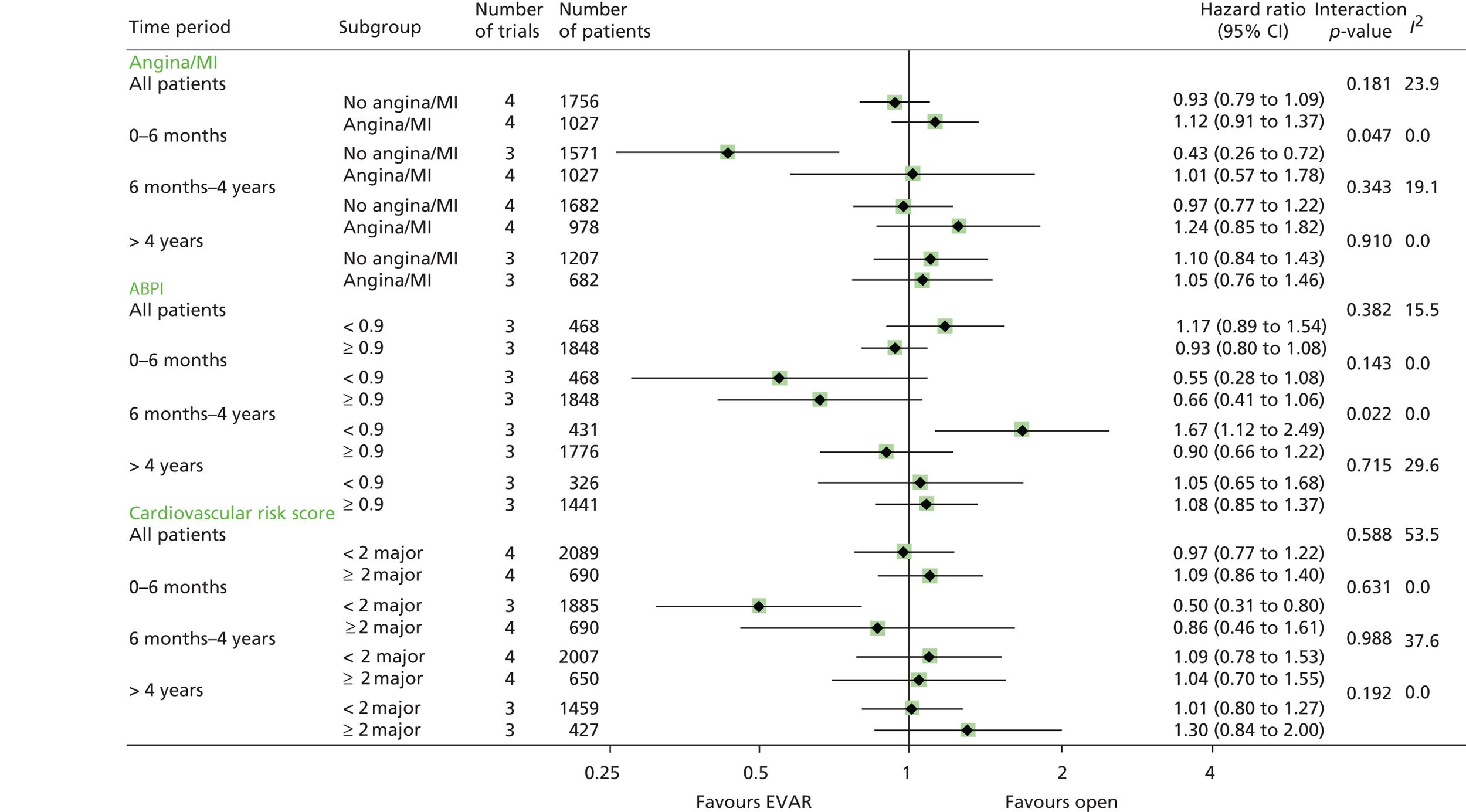
FIGURE 18.
Unadjusted HRs for total mortality by subgroups of maximum AAA diameter, neck diameter and neck length: overall and at 0–6 months, 6 months–4 years and > 4 years since randomisation. Interaction p-values for maximum AAA diameter, neck diameter and neck length calculated using the continuous measures. The number of trials contributing to each analysis is shown.
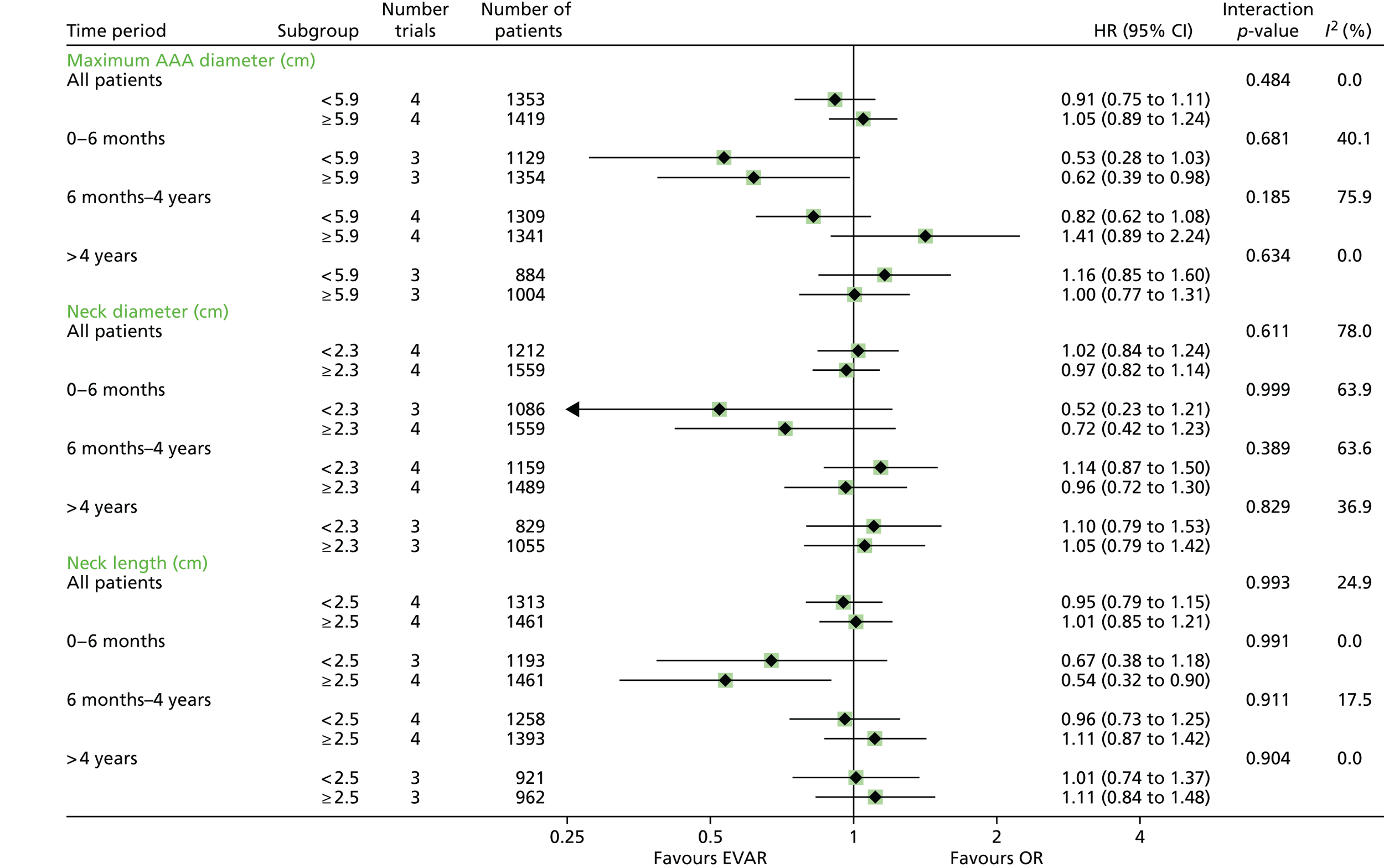
FIGURE 19.
Unadjusted HRs for total mortality by subgroups of history of diabetes, BMI and smoking status: overall and at 0–6 months, 6 months–4 years and > 4 years since randomisation. Interaction p-values for BMI calculated using the continuous measures. The number of trials contributing to each analysis is shown.
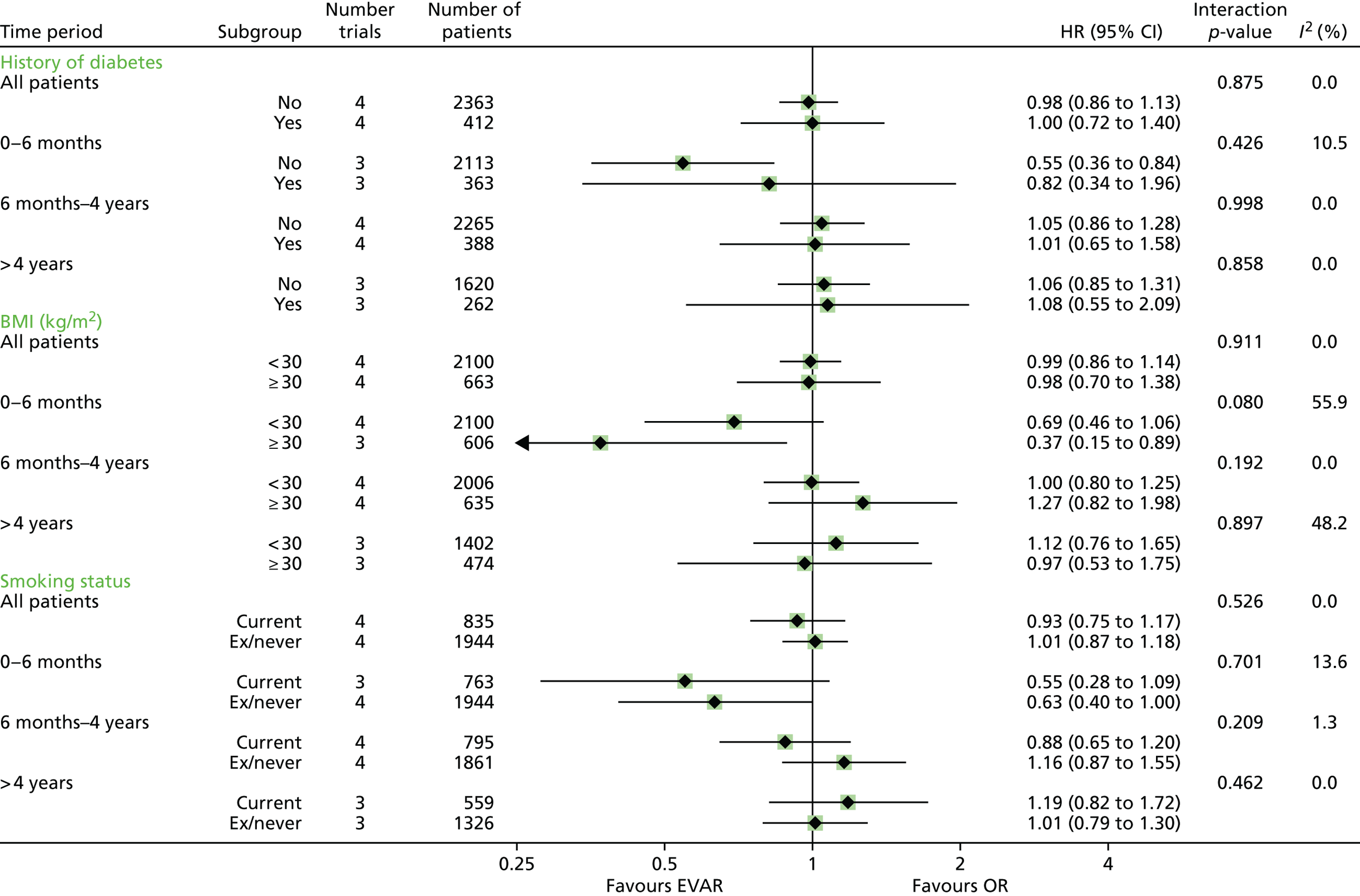
Baseline ABPI was not available for the ACE trial. In the other trials, patients with peripheral arterial disease (low ABPI of < 0.9) had a similar early survival advantage from being in the EVAR group as those with a ABPI of ≥ 0.9. However, in the 6 months to 4 years time period, for those with a ABPI of < 0.9, the OR group had the survival advantage (HR 1.67, 95% CI 1.12 to 2.49), in comparison with patients with a ABPI of > 0.9 (interaction p = 0.022). During the 6 months to 4 years time period, for those with a ABPI of < 0.9, total mortality was 9.6 and 5.7 per 100 person-years in the EVAR and OR groups, respectively, compared with 5.1 and 5.8 per 100 person-years, respectively, in the higher ABPI group. The cause of death in the two ABPI groups by time period is shown in Table 23. The operative mortality by subgroup shows that the highest mortality was in those with low ABPI (Table 24) and this group has higher aneurysm-related mortality throughout. Finally, a cardiovascular risk score was not discriminatory at any time period.
| Cause of death | ABPI < 0.9, n | ABPI ≥ 0.9, n | ||
|---|---|---|---|---|
| EVAR (n = 13) | OR (n = 23) | EVAR (n = 29) | OR (n = 43) | |
| 0–6 months | ||||
| AAA related | 7 (54) | 18 (78) | 15 (52) | 30 (70) |
| MI/other cardiac | 0 (0) | 1 (4) | 6 (21) | 3 (7) |
| Stroke | 0 (0) | 0 (0) | 1 (3) | 1 (2) |
| Other vascular | 4 (31) | 2 (9) | 2 (7) | 0 (0) |
| Cancer, lung | 1 (8) | 0 (0) | 1 (3) | 0 (0) |
| Cancer, other | 0 (0) | 0 (0) | 2 (7) | 3 (7) |
| Pulmonary | 0 (0) | 2 (9) | 0 (0) | 0 (0) |
| Renal | 1 (8) | 0 (0) | 1 (3) | 0 (0) |
| Other | 0 (0) | 0 (0) | 1 (3) | 3 (7) |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| EVAR (n = 62) | OR (n = 39) | EVAR (n = 149) | OR (n = 161) | |
| 6 months–4 years | ||||
| AAA related | 6 (10) | 3 (8) | 7 (5) | 6 (4) |
| MI/other cardiac | 15 (24) | 10 (26) | 33 (22) | 31 (19) |
| Stroke | 3 (5) | 1 (3) | 12 (8) | 7 (4) |
| Other vascular | 5 (8) | 3 (8) | 5 (3) | 5 (3) |
| Cancer, lung | 6 (10) | 5 (13) | 20 (13) | 30 (19) |
| Cancer, other | 8 (13) | 10 (26) | 40 (27) | 34 (21) |
| Pulmonary | 6 (10) | 3 (8) | 7 (5) | 20 (12) |
| Renal | 1 (2) | 0 (0) | 3 (2) | 2 (1) |
| Other | 7 (11) | 2 (5) | 17 (11) | 14 (9) |
| Unknown | 5 (8) | 2 (5) | 5 (3) | 12 (7) |
| EVAR (n = 35) | OR (n = 36) | EVAR (n = 144) | OR (n = 131) | |
| > 4 years | ||||
| AAA related | 3 (9) | 4 (11) | 12 (8) | 1 (1) |
| MI/other cardiac | 9 (26) | 14 (39) | 34 (24) | 25 (19) |
| Stroke | 2 (6) | 4 (11) | 13 (9) | 10 (8) |
| Other vascular | 4 (11) | 3 (8) | 8 (6) | 4 (3) |
| Cancer, lung | 0 (0) | 0 (0) | 14 (10) | 16 (12) |
| Cancer, other | 3 (9) | 3 (8) | 22 (15) | 35 (27) |
| Pulmonary | 7 (20) | 3 (8) | 19 (13) | 23 (18) |
| Renal | 0 (0) | 2 (6) | 4 (3) | 1 (1) |
| Other | 3 (9) | 0 (0) | 15 (10) | 12 (9) |
| Unknown | 4 (11) | 3 (8) | 3 (2) | 4 (3) |
| Subgroup | Operative mortality, n/N (%) |
|---|---|
| Age (years) | |
| < 72 | 12/1311 (0.9) |
| ≥ 72 | 44/1413 (3.1) |
| Sex | |
| Female | 6/150 (4.0) |
| Male | 50/2574 (1.9) |
| eGFR | |
| < 68.4 | 35/1341 (2.6) |
| ≥ 68.4 | 20/1360 (1.5) |
| Angina/MI | |
| No angina/MI | 35/1721 (2.0) |
| Angina/MI | 21/1003 (2.1) |
| ABPI | |
| < 0.9 | 19/455 (4.2) |
| ≥ 0.9 | 29/1813 (1.6) |
| Cardiovascular risk score | |
| < 2 major | 43/2052 (2.1) |
| ≥ 2 major | 13/668 (2.0) |
| Maximum AAA diameter (cm) | |
| < 5.9 | 18/1330 (1.4) |
| ≥ 5.9 | 37/1383 (2.7) |
| Neck diameter (cm) | |
| < 2.3 | 23/1191 (1.9) |
| ≥ 2.3 | 33/1521 (2.2) |
| Neck length (cm) | |
| < 2.5 | 29/1284 (2.3) |
| ≥ 2.5 | 27/1431 (1.9) |
| History of diabetes | |
| No | 47/2317 (2.0) |
| Yes | 9/399 (2.3) |
| BMI (km/m2) | |
| < 30 | 44/2057 (2.1) |
| ≥ 30 | 11/649 (1.7) |
| Smoking status | |
| Current | 14/814 (1.7) |
| Ex/never | 42/1906 (2.2) |
Complications and reinterventions
Complications (apart from endoleaks after EVAR) and reinterventions were reported heterogeneously across the four trials. The overall rates of reinterventions reported were higher in the EVAR group than in the OR group for all trials (see Table 18). The risk of reintervention by time period following aneurysm repair is shown in Figure 20, with substantial heterogeneity between trials for reinterventions recorded between 31 days and 3 years.
FIGURE 20.
Hazard ratio for any reintervention by time period following aneurysm repair for 2718 subjects undergoing aneurysm repair (seven subjects missing follow-up for reinterventions after aneurysm repair). Note that the number of reinterventions reported here may differ slightly from those reported by the individual trial publications, particularly for the OVER trial where more reinterventions after EVAR-1 were included in their trial report. This difference is mainly attributable to the different ways in which reinterventions were categorised across the trials. For instance, amputations were included in reinterventions for the OVER trial, but placed in a separate category for all the other trials and this meta-analysis. Similarly, below knee reconstructions were included as OVER trial reinterventions, but were not included for the meta-analysis unless there was clear evidence that these procedures were aneurysm related.
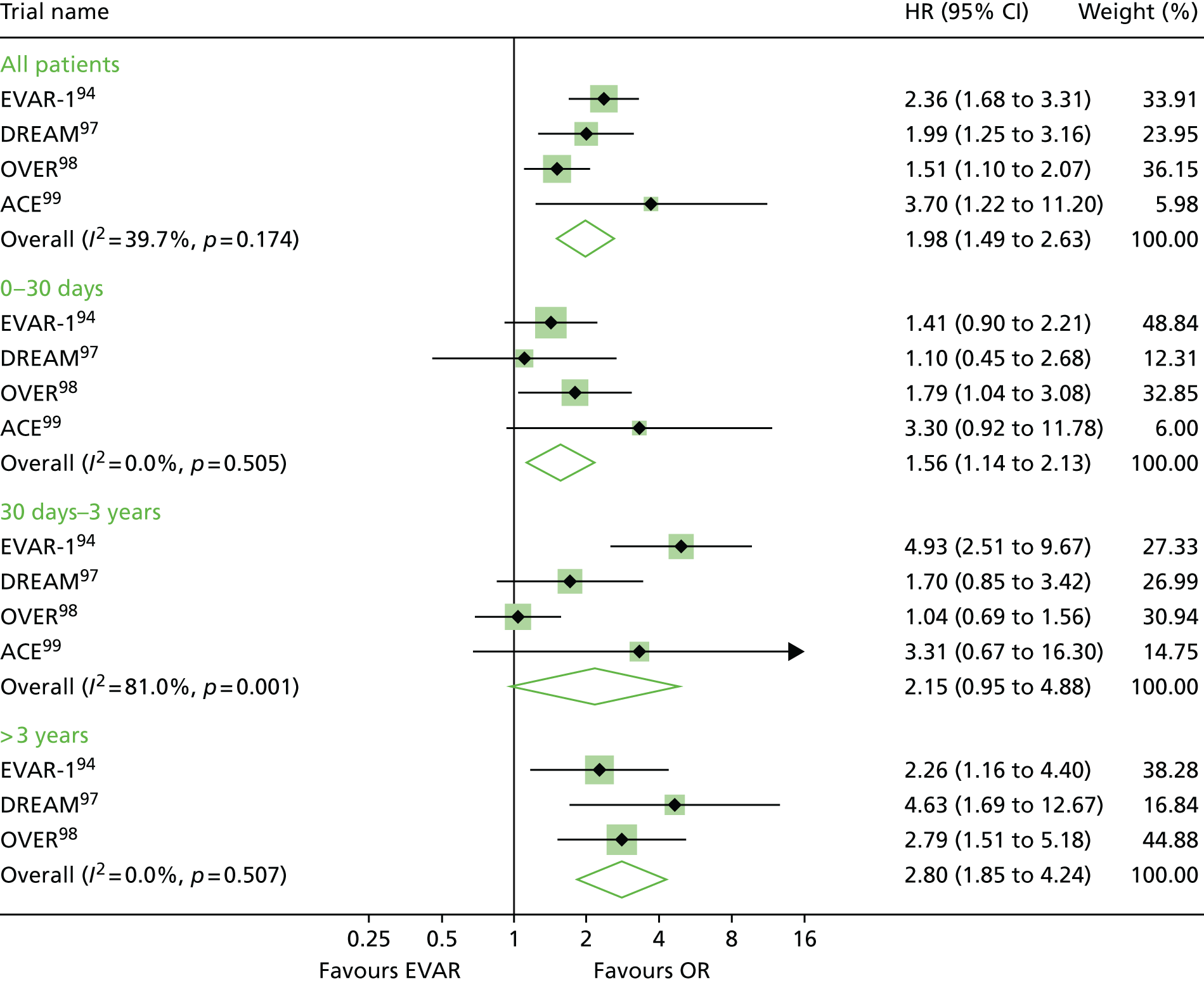
There was no indication that complications following EVAR decreased with the year in which the trial commenced: these rates, together with types and numbers of complications, are reported in Table 25. The most common reported complication after EVAR was type II endoleak, which overall was reported 435 times in 325 of 2783 (12%) patients, with corrective reintervention being performed in 99 of 435 (23%) detected type II endoleaks. The second most common type of complication was type I endoleak, for which 79 of 120 (66%) patients received an early reintervention. Similarly, early correction of other serious EVAR-related complications was attempted only in less than two-thirds of cases. Secondary sac rupture was reported in 37 patients – 33 in the EVAR randomised groups (2.4% of patients) and four in the OR groups (0.3% of patients); although all four of these patients were treated with EVAR, for those with secondary rupture following treatment with EVAR, the median time to rupture was 3.5 years (Figure 21). Of these patients, 11 (30%) had a type I endoleak, of which seven were treated, seven (19%) had a type II endoleak, of which three were treated, two (5%) had a type III endoleak, of which one was treated, and nine had known graft migration. Nineteen (51%) patients had no endoleaks detected before secondary sac rupture and one further patient had a thoracic endograft for proximal aortic dissection 3 days earlier. The mean time between detection of the first endoleak and rupture was 1.8 years. The 30-day mortality rate following rupture was 62% (n = 23).
| EVAR-1 (n = 1252) | DREAMa (n = 351) | OVER (n = 881) | ACE (n = 299) | Total (n = 2783) | |
|---|---|---|---|---|---|
| Follow-up for complications, years mean (SD) | 5.3 (2.5) | 5.2 (2.2) | 5.2 (2.1) | 2.8 (1.1) | 5.0 (2.4) |
| Rate of complications, n/person-years (rate per 100 person-years) | |||||
| EVAR | 315/3381 (9.3) | 125/906 (13.8) | 209/2334 (9.0) | 103/419 (24.6) | 752/7040 (10.7) |
| OR | 27/3309 (0.8) | 7/932 (0.8) | 13/2276 (0.6) | 8/408 (2.0) | 55/6925 (0.8) |
| Type of complication,b n (n treated) | |||||
| Type I endoleak | 56 (35) | 24 (17) | 25 (19) | 15 (8) | 121 (79) |
| Type II endoleak | 146 (39) | 73 (12) | 139 (38) | 77 (10) | 435 (99) |
| Type III endoleak | 25 (15) | 5 (1) | 7 (7) | 0 (0) | 37 (23) |
| Type IV endoleak | 0 (0) | 0 (0) | 5 (2) | 0 (0) | 5 (2) |
| Type V endoleak | 0 (0) | 0 (0) | 11 (3) | 5 (0) | 16 (3) |
| Secondary rupture | 27 (10) | 2 (2) | 5 (4) | 3 (3) | 37 (18) |
| Rate of secondary rupture after EVAR per 100 person-years | 0.7 | 0.1 | 0.2 | 0.7 | 0.5 |
FIGURE 21.
Time from repair to secondary rupture, in those who were treated with EVAR.
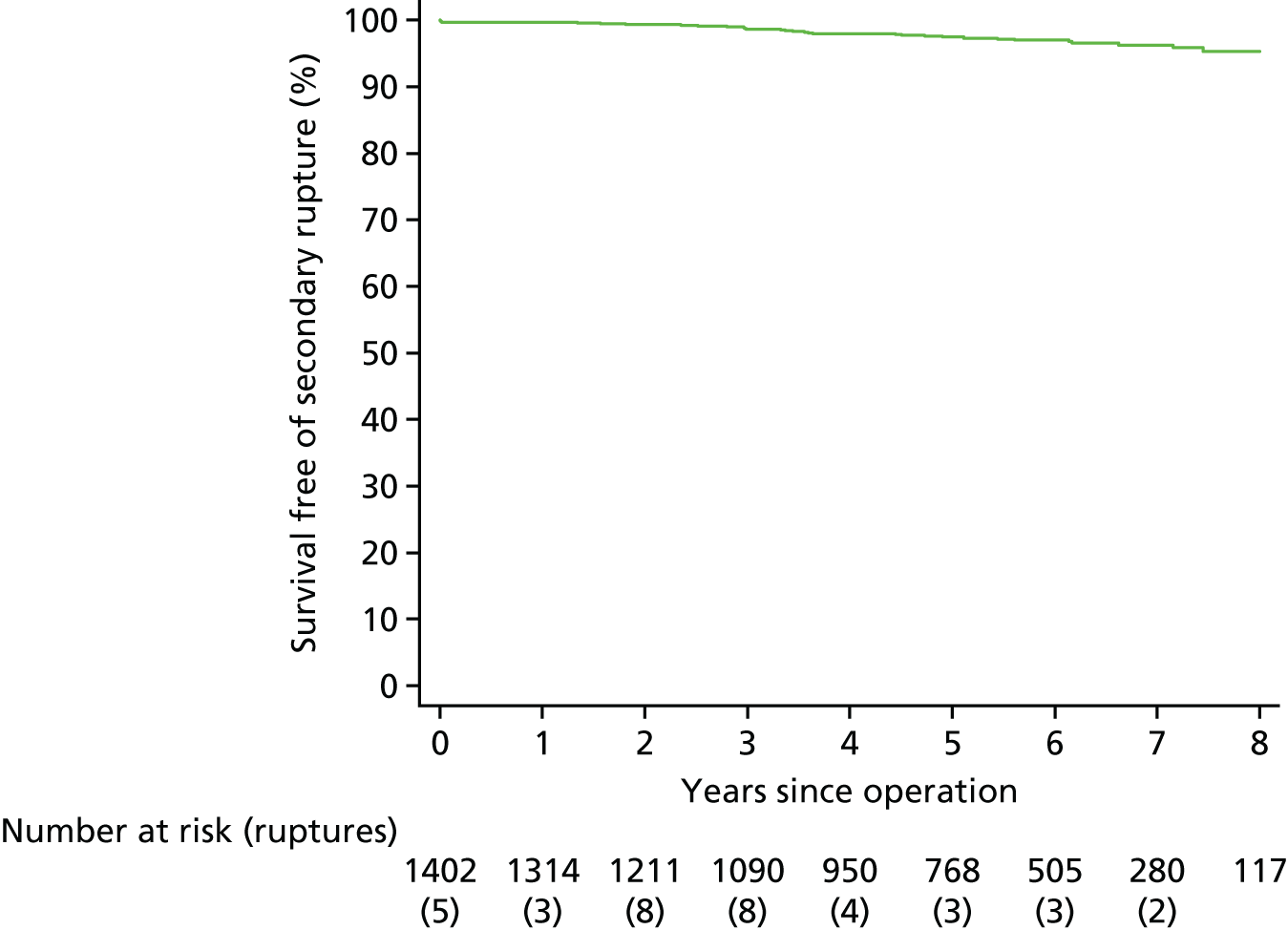
Discussion
These four randomised trials, in Europe and the USA, provide the best evidence for the early survival advantage offered by EVAR rather than OR. Patients prefer the less invasive method of AAA repair, EVAR, which has been widely adopted, and the majority of elective repairs are now performed using EVAR. 108,120,147 This meta-analysis, over a 5-year time horizon, confirms that overall there is an early survival advantage under EVAR, which is lost within 3 years of randomisation, so that the life-years saved from EVAR over a 5-year time period are minimal. Between 0 and 6 months after randomisation, total and aneurysm-related mortality were lower for the EVAR group, mainly because of the 2.5-fold lower operative mortality in this group. However, after this time period, the early EVAR group advantage was eroded progressively. By 3 years after aneurysm repair, aneurysm-related mortality was five times higher in the EVAR group (at this stage mainly because of secondary rupture or reinterventions), and this is likely to contribute to the ‘catch-up’ in mortality.
The next investigations focused on whether the early survival advantage was either maintained or lost in subgroups of patients categorised by their preoperative characteristics. Over a 5-year time horizon, there was no convincing evidence that being randomised to EVAR or OR resulted in differential survival between any subgroups of the population. This does not support the suggestion that younger and fitter patients with aortic morphology suitable for EVAR are likely to benefit from OR over 5 years. 140 However, differential effect modification was suggested in some subgroups (renal dysfunction, coronary artery disease) in the first 6 months and for those with peripheral arterial disease in the later 6 months–4 years time period, possibly caused by frailty effects.
Low ABPI was introduced as a measure of peripheral atherosclerosis148 and is a marker of generalised atherosclerosis. 149 This subgroup had the highest pooled operative mortality, although the relative early advantage of EVAR was maintained. However, between 6 months and 4 years, fortunes reversed in favour of a survival advantage in the OR group, a pattern which indicates that those with low ABPI are another contributor to the ‘catch-up’ in mortality phenomenon.
All trials enrolled patients with evidence of moderate renal dysfunction, 35% of the overall enrolment having eGFR < 60 (chronic kidney disease stage 3 or above). For these patients, there was no evidence of a benefit from being randomised to EVAR (vs. OR), even in the first 6 months. These data are in agreement with an earlier observational study showing a high early postoperative event rate for patients with a low eGFR. 150 Whether or not renal function deteriorates more rapidly after either elective EVAR or OR of an AAA has been debated fiercely and less attention has been focused on improving the perioperative care. 151 Similarly, patients with known coronary artery disease had no evidence of an early survival advantage from being randomised to EVAR. Given that EVAR is less invasive than OR, these findings for the moderate renal dysfunction and coronary artery disease are surprising. Perhaps the stress of EVAR in these subgroups has been underestimated. It also is possible that those randomised to EVAR received less stringent preoperative evaluations, resulting in better perioperative care for the OR group with these comorbidities.
This study has several limitations that restrict its scope. First, all endografts were implanted between 1999 and 2008 using general anaesthesia, and today newer endografts are used, often implanted under local anaesthesia. Second, reporting standards for baseline characteristics (e.g. smoking, drugs) differed between trials. Third, the reporting of complications and reinterventions (aneurysm related and other cardiovascular) was very heterogeneous across the trials. Moreover, today it is recognised that type II endoleaks with sac enlargement can be dangerous111 and that a type II endoleak might even hide a type I or type III endoleak. Fourth, at the time these trials recruited (1999–2008), no trial had a clear policy for reintervention in the presence of endoleaks after EVAR and even serious complications such as type I endoleak were not always corrected quickly, which may have contributed to the increasing aneurysm-related mortality rate in the EVAR group at ≥ 3 years after aneurysm repair.
The reintervention rate was consistently higher in the EVAR groups (see Table 18), although the data are heterogeneous and the largest trial did not report incision-related complications after OR. It would be reassuring to learn that by using more recent EVAR devices within IFU, coupled with more rigorous surveillance, the continuing aneurysm-related mortality in the EVAR group could be attenuated to minimise the ‘catch-up’ in mortality. To rely entirely on the introduction of new devices to prevent aneurysm-related mortality in the EVAR group, especially without adequate surveillance, may be unwise. Recent analyses of the Medicare database support this caution. 108
In summary, this meta-analysis confirms the advantage of lower mortality in the EVAR group in the first 6 months and provides some new insight of how this early advantage of the EVAR group is eroded (aneurysm-related mortality and inclusion of those with peripheral arterial disease). Additionally, two subgroups were identified who do not have lower mortality under EVAR at any time, to suggest that these groups (moderate renal dysfunction and established coronary artery disease) may benefit from improved perioperative care, especially for EVAR. Surveillance must focus on reducing aneurysm-related deaths in the mid-term and longer term, particularly deaths resulting from reinterventions and secondary ruptures after EVAR.
Chapter 7 The significance of type II endoleaks following endovascular aneurysm repair: results from the individual patient data meta-analysis
There was a call with vignette (NIHR Evaluation, Trials and Studies Coordinating Centre – HTA 2012 – Interventional Procedures Panel) for possible funding on the management of endoleaks following EVAR.
In the vignette, the incidence of type II endoleak is shown to range from 0% to 17% 30 days post EVAR (decreasing to 1–8% at 6 months’ follow-up). 152 It is also noted that current evidence on type II endoleak is insufficient to define criteria for intervention in isolated type II endoleak after EVAR. 153 In addition, the question of static sac size is raised. 154 Attempts have been made to classify diagnoses of management following endovascular thoracic and abdominal aortic repair. 155 Additionally, management of type II endoleak (preoperative vs. postoperative vs. expectant management) provided a summary of the known position in 2009156 and a retrospective study with CT angiography evaluating the potential outcome of predictors in type II endoleak was also available in 2011. 157
Type II endoleaks are a common complication of endovascular repair of AAA (EVAR). The sensitivity and specificity of the detection depends on the imaging modalities used for postoperative EVAR surveillance, with magnetic resonance imaging being described as the most sensitive method. Less sensitive but more commonly used is CT or duplex scanning, with or without contrast.
The natural history of type II endoleaks remains poorly understood and, as a result, the optimal management and long-term success of interventions are currently unknown. Studies regarding the significance of type II endoleak have shown mixed results. Some studies have shown that type II endoleaks are not associated with adverse outcomes, whereas other studies have shown that persistent type II endoleak is associated with aneurysm sac growth, increased risk of rupture, reintervention and need for conversion to OR. 158 However, most studies are in agreement that uncorrected type II endoleak may result in persistently elevated pressure in the sac without sac shrinkage. 159 There is a long-standing debate whether or not, in the absence of sac expansion, intervention is required for type II endoleaks and many are thought to resolve spontaneously. There is also a debate whether or not persistent type II endoleaks are in fact hidden type I endoleaks (with due required correction).
Individual patient data meta-analysis
This is described in Chapter 6. In the merging of the data from the four RCTs, for complications only endoleaks after EVAR were similar across the trials.
The hazard of reintervention following aneurysm repair was analysed using a multiple failure time model. In addition, for patients who received EVAR, mortality HRs were investigated in individuals without and with a detected/treated type II endoleak.
Results on type II endoleak from the individual patient data meta-analyses
For patients who received EVAR (including patients who were randomised to OR but crossed over), mortality HRs were investigated in individuals without and with a detected/treated type II endoleak. These analyses are no longer by randomised groups and are thus observational in nature. To investigate this, a three-level time-dependent categorical variable was created for each individual based on information from their first detected type II endoleak. Specifically, follow-up for each individual was split into periods in which (1) no type II endoleak had been detected since randomisation; (2) the first type II endoleak had been detected but as yet was untreated; and (3) the first type II endoleak had been detected and treated. A Cox regression model was fitted from randomisation with this time-dependent exposure to assess the HRs of detected untreated and detected treated type II endoleaks in relation to no detected type II endoleaks.
Table 26 shows data from EVAR-1, DREAM, OVER and ACE, in terms of number of deaths and number of aneurysm-related deaths, and also pooled data. As can be seen in Table 26, there is no difference between patients with no detected type II endoleak and either untreated or treated type II endoleaks, unadjusted or adjusted for age, sex, aneurysm diameter and log-creatinine.
| Analysis | EVAR-1 | DREAM | OVER | ACE | Pooled |
|---|---|---|---|---|---|
| Number contributing to analysis (n deaths/n AAA deaths) | n = 629 (255/32) | n = 171 (59/7) | n = 440 (143/8) | n = 163 (18/7) | n = 1403 (475/54) |
| Unadjusted | |||||
| No type II endoleak (reference) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Untreated type II endoleak | 0.83 (0.57 to 1.21) | 1.19 (0.61 to 2.30) | 1.56 (1.05 to 2.32) | a | 1.14 (0.75 to 1.76) |
| p = 0.538; I2 = 61% | |||||
| Treated type II endoleak | 1.87 (1.14 to 3.08) | 2.11 (0.65 to 6.84) | 0.50 (0.18 to 1.37) | a | 1.30 (0.56 to 3.03) |
| p = 0.546; I2 = 65% | |||||
| Any type II endoleak | 1.03 (0.75 to 1.41) | 1.31 (0.72 to 2.39) | 1.26 (0.87 to 1.84) | a | 1.14 (0.91 to 1.43) |
| p = 0.241; I2 = 0% | |||||
| Number contributing to analysis (n deaths/n AAA deaths) | n = 628 (255/32) | n = 165 (56/6) | n = 440 (143/8) | n = 154 (16/6) | n = 1387 (470/52) |
| Adjustedb | |||||
| No type II endoleak (reference) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Untreated type II endoleak | 0.86 (0.59 to 1.26) | 1.22 (0.63 to 2.38) | 1.46 (0.97 to 2.19) | a | 1.14 (0.79 to 1.62) |
| p = 0.488; I2 = 44% | |||||
| Treated type II endoleak | 1.71 (1.04 to 2.84) | 2.68 (0.81 to 8.88) | 0.48 (0.18 to 1.32) | a | 1.31 (0.54 to 3.17) |
| p = 0.549; I2 = 67% | |||||
| Any type II endoleak | 1.04 (0.76 to 1.44) | 1.38 (0.75 to 2.54) | 1.19 (0.81 to 1.74) | a | 1.14 (0.91 to 1.43) |
| p = 0.276; I2 = 0% | |||||
Overall, there was no evidence that a detected type II endoleak (either treated or untreated) was associated with worse overall survival, although results differed by trial (see Table 26). In EVAR-1, patients who had been treated for their type II endoleak had a higher hazard of mortality than patients with no detected type II endoleak, whereas patients with untreated type II endoleaks had a lower hazard. The converse was seen in the OVER trial, with the untreated type II endoleak patients having a higher mortality rate. These differences may be attributable to different decisions regarding who to treat in the two trial populations.
Further analyses from the individual patient data meta-analysis restricting to endoleaks detected within the first 3 years
Further analyses were conducted to assess whether type II endoleaks detected within the first 3 years of randomisation were associated with a higher rate of mortality. The following results are based on the EVAR-treated patients only, therefore are subject to potential confounding (non-randomised) comparisons. Data on treated and untreated type II endoleaks from the four RCTs, restricted to endoleaks detected within the first 3 years, were used to define a time-dependent variable in a Cox regression model.
Figure 22 shows survival beyond 3 years for each trial, separated by those with and without detected type II endoleak. There were no significant differences between the two groups in terms of overall survival in any of the trials. The HRs for a detected type II endoleak (within the first 3 years) for both all-cause and aneurysm-related mortality are shown in Table 27. As with the individual trial results, the pooled estimates show no evidence of a substantial increase in either all-cause or aneurysm-related mortality following a detected type II endoleak within the first 3 years, for individuals who survive beyond 3 years. However, because of the small numbers aneurysm-related mortality could be assessed only within EVAR-1.
FIGURE 22.
All-cause mortality for EVAR-operated patients surviving beyond 3 years, by whether or not a type-II endoleak is detected within the first 3 years. (a) ACE; (b) DREAM; (c) EVAR-1; and (d) OVER.
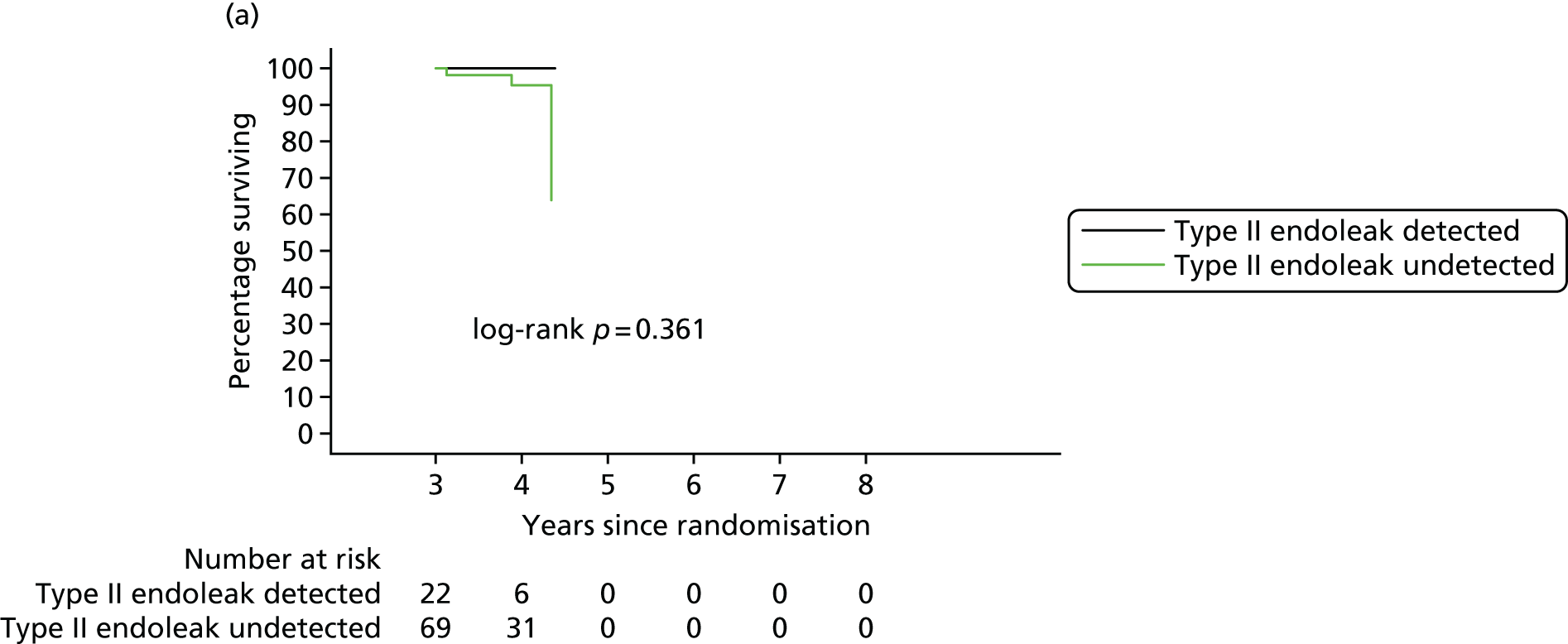
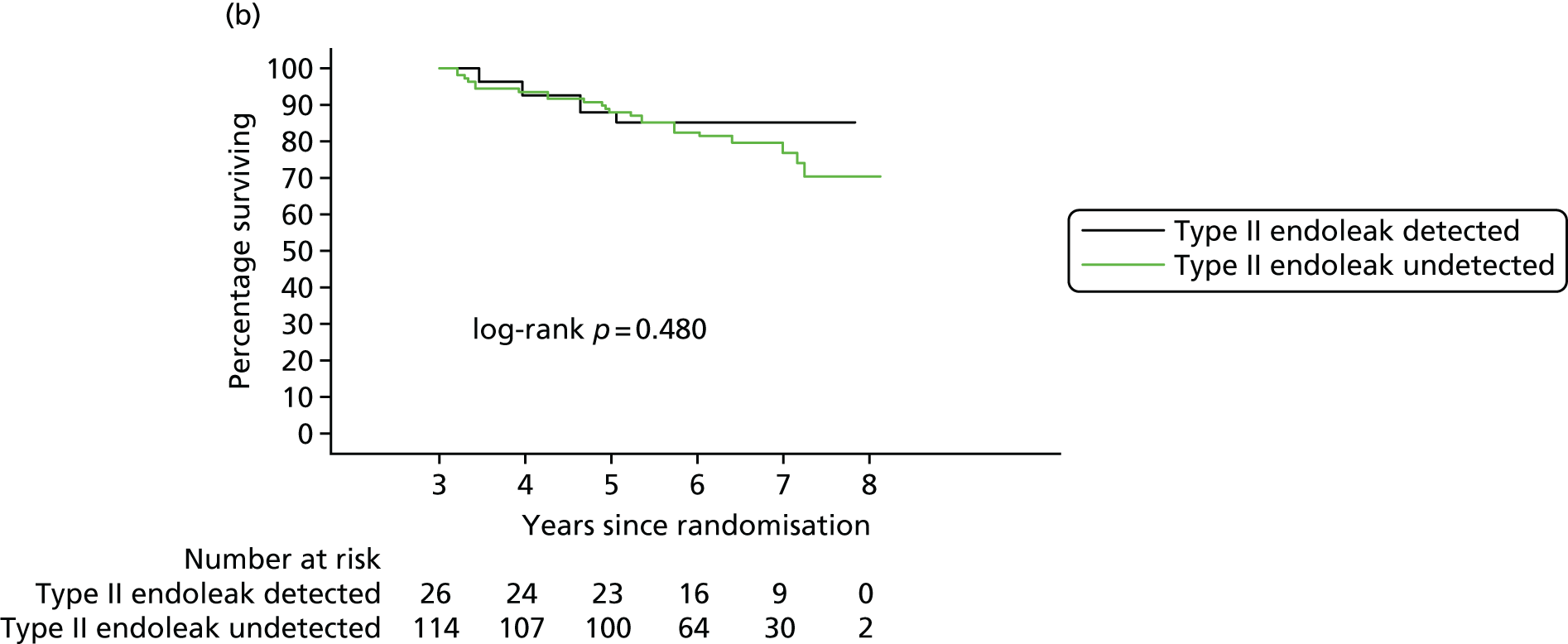
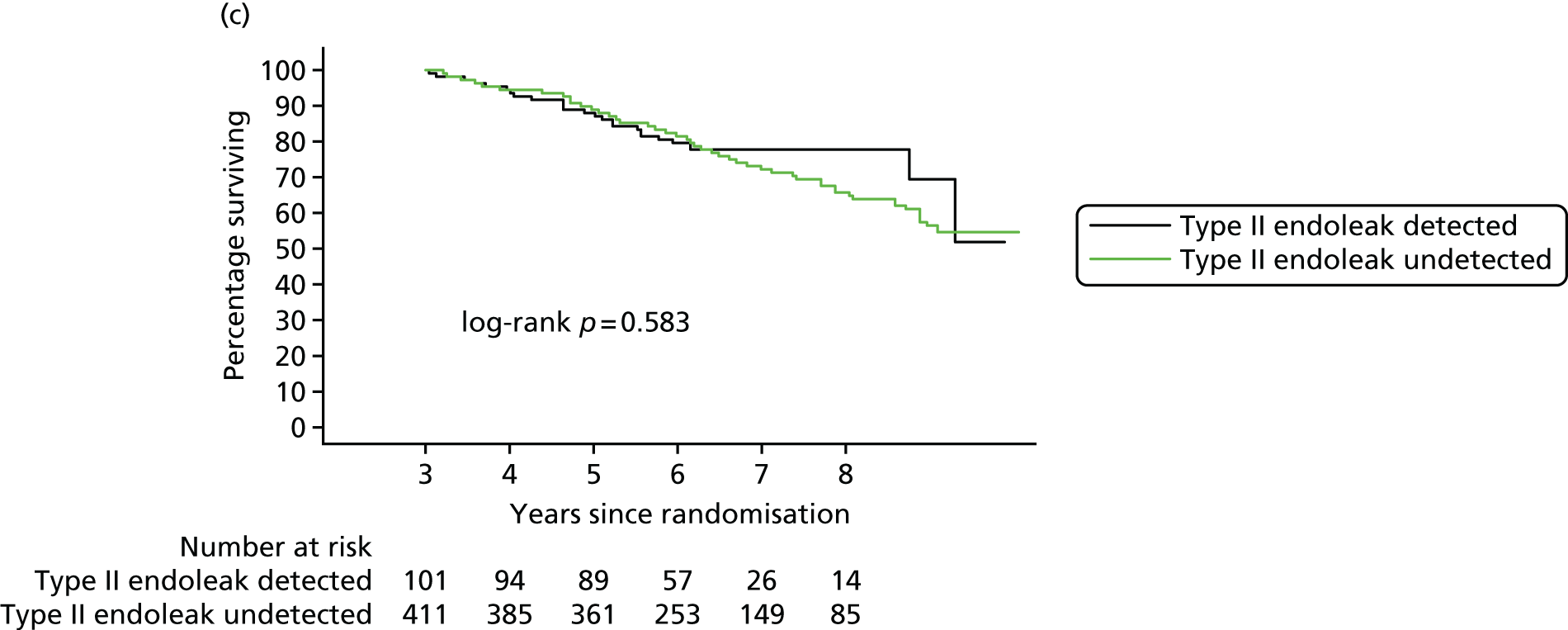
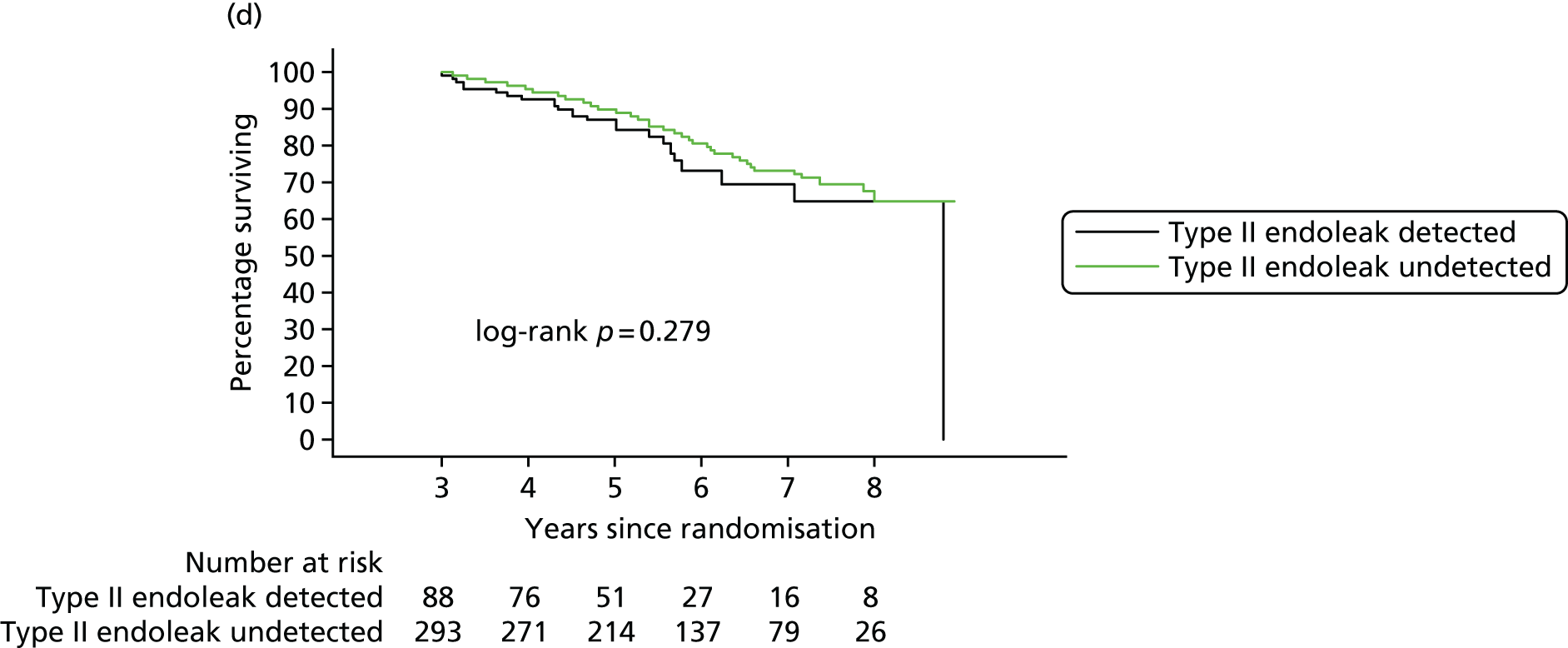
| ACE (n = 163) | DREAM (n = 171) | EVAR-1 (n = 629) | OVER (n = 440) | Total (n = 1403) | |
|---|---|---|---|---|---|
| Known type II endoleak before 3 years: yes, n (%) | 35 (21) | 36 (21) | 120 (19) | 102 (23) | 293 (21) |
| Unadjusted | |||||
| Number surviving beyond 3 years and not censored (n deaths/n AAA deaths) | n = 91 (3/1) | n = 140 (28/2) | n = 512 (140/14) | n = 381 (84/3) | n = 1124 (255/20) |
| All-cause mortality: HR (95% CI) of type II endoleak on post 3-year survival | 0.68 (0.24 to 1.97) | 0.88 (0.56 to 1.38) | 1.32 (0.80 to 2.18) | 1.01 (0.74 to 1.39) | |
| p = 0.935; I2 = 0% | |||||
| Aneurysm-related mortality: HR (95% CI) of type II endoleak on post 3-year survival | 1.70 (0.53 to 5.41) | 1.70 (0.53 to 5.41) | |||
| p = 0.373 | |||||
| Adjusted | |||||
| Number surviving beyond 3 years and not censored (n deaths/n AAA deaths) | n = 88 (3/1) | n = 135 (26/2) | n = 511 (140/14) | n = 381 (84/3) | n = 1115 (253/20) |
| All-cause mortality: HR (95% CI) of type II endoleak on post 3-year survival | 0.77 (0.26 to 2.28) | 0.88 (0.56 to 1.39) | 1.28 (0.77 to 2.13) | 1.01 (0.73 to 1.40) | |
| p = 0.936; I2 = 0% | |||||
| Aneurysm-related mortality: HR (95% CI) of type II endoleak on post 3-year survival | 1.67 (0.50 to 5.60) | 1.67 (0.50 to 5.60) | |||
| p = 0.405 | |||||
There was no overall evidence that type II endoleak (either treated or untreated) had a dramatic effect on subsequent survival.
Discussion
The pooled estimates show no evidence of a substantial increase in either all-cause or aneurysm-related mortality following a detected type II endoleak within the first 3 years, for individuals who survive beyond 3 years. However, as a result of small numbers, aneurysm-related mortality could be assessed only within EVAR-1.
As previously shown, type II endoleak as part of the ‘cluster’ of complications is associated with secondary rupture. However, this suggests that it is other complications that are listed in the cluster that are important and not type II endoleaks on their own. The ‘cluster’ did include type II endoleak with sac expansion in its definition and it seems that sac expansion is the important measure here.
Additionally, it is often difficult to be sure exactly where an endoleak is occurring. With improved experience and imaging quality, the certainty that a type II endoleak actually is one and nothing more serious should be improving. The big fear is that a type II endoleak is ‘a type I endoleak in disguise’. This is perhaps why the increase of sac diameter is such an important indicator of functionally important endoleak of one form or another.
Chapter 8 Modelling sac growth following elective endovascular aneurysm repair and associations with complications and secondary rupture
Introduction
Following elective EVAR surgery, the aneurysm sac should gradually shrink over time as the endograft diverts blood flow away from the aneurysm. It is important, however, to continue monitoring the sac over time, through repeat imaging, and the position of the endograft to allow early detection of serious complications such as endoleaks, which can lead to secondary rupture. In the EVAR trials, the follow-up protocol suggested CT imaging of the sac and EVAR device location initially at 1, 3 and 12 months postoperatively and then annually thereafter. In this chapter we investigate whether or not variables that are easily measured on a CT scan or ultrasound, such as the diameter of the sac, are (1) associated with the onset of a complication or secondary rupture and (2) can be used to accurately predict the occurrence of the event so that an appropriate imaging protocol can be set up or a reintervention can be planned. To achieve this it is first important to model and better understand the trajectory profile of sac diameters postoperatively. A suitable model of the sac trajectory over time can then be utilised to provide predictors of outcomes, such as the current predicted sac diameter or the current rate of growth (see Figure 23 for an illustration).
FIGURE 23.
Illustration of a modelled sac trajectory (solid curve) using CT measurements taken over time (crosses). The model can be used to relate important aspects of the sac trajectory at the landmark time of prediction, such as (1) the current predicted diameter (green cross) and (2) the current rate of sac growth (green line).
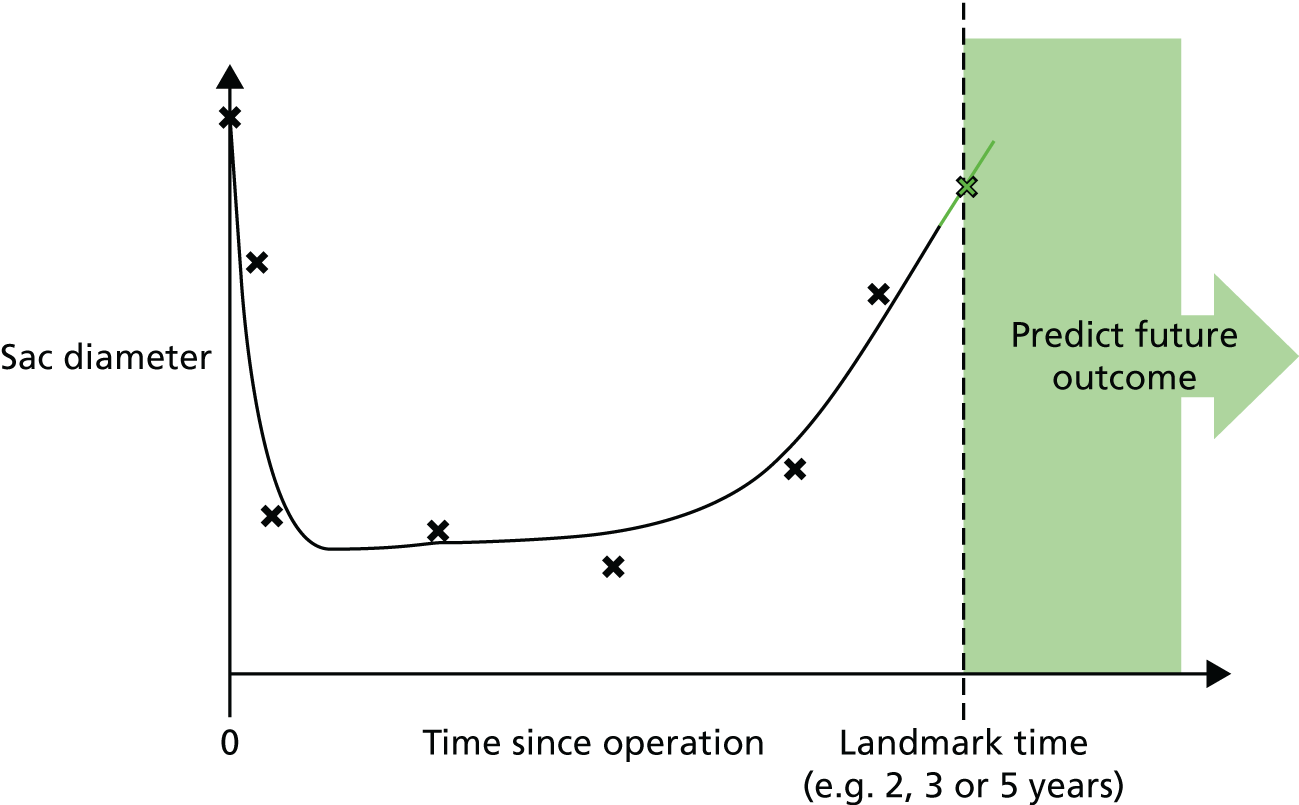
Data
A total of 1656 patients were randomised in the EVAR trials prior to September 2004 (EVAR-1, n = 1252; EVAR-2, n = 404) (Figure 24). Of these patients, 847 underwent elective EVAR without conversion to OR (EVAR-1, n = 623; EVAR-2, n = 224). After excluding individuals who underwent no postoperative CT (one individual), whose sac diameter measurements were all missing (58 individuals) or who received a straight graft (four individuals), the final analysis data set consisted of 784 individuals who underwent elective EVAR surgery, irrespective of their randomisation group.
FIGURE 24.
Flow diagram showing EVAR-1 and EVAR-2 patients used in the sac growth analysis.
Of these 784 individuals, 591 (75.4%) were from EVAR-1 and 193 (24.6%) were from EVAR-2. Six hundred and ninety-nine (89.2%) patients were male and the mean age was 75 years (range 54–94 years). Further patient characteristics are presented in Table 28.
| n = 785 | Number missing (%) | |
|---|---|---|
| Age, years | 74.5 (6.4) | 0 (0) |
| Sex (male), n (%) | 699 (89.2) | 0 (0) |
| Smoking status, n (%) | ||
| Current | 159 (20.3) | 1 (0.1) |
| Past | 548 (70.0) | |
| Never | 76 (9.7) | |
| Trial, n (%) | ||
| EVAR-1 | 591 (75.4) | 0 (0) |
| EVAR-2 | 193 (24.6) | |
| Preoperative AAA size (cm) | 6.5 (0.9) | 0 (0) |
| Graft shape, n (%) | ||
| Uni-iliac | 57 (7.5) | 20 (2.5) |
| Bi-iliac | 707 (92.5) | |
| Graft manufacturer, n (%) | ||
| Cook®/Zenith® | 434 (55.9) | 7 (0.9) |
| Medtronic/Talent® | 231 (29.7) | |
| GORE®/EXCLUDER® | 48 (6.2) | |
| Other | 64 (8.2) | |
| Number of postoperative sac measurements | 5.4 (2.7) | |
Methods
Modelling sac diameter following elective endovascular aneurysm repair operation
Each individual provided a set of repeated sac diameter measurements. To model the sac trajectories over time and account for between-patient variation in the sac trajectories we use a mixed-effects model. First, we tried to capture the shape of the trajectory by finding a model from the class of fractional polynomial models that best fitted the relationship between sac diameter and follow-up time. 160 As is traditional with such a class of models, we allowed polynomial powers of time up to cubic terms (i.e. x, x2 and x3), a square root transformation (x0.5), a log-transformation and the inverse polynomial and square root terms (e.g. powers –2, –1 and –0.5). Each power of time was assessed as both a fixed- and a random-effect term in a mixed-effects model and an independent variance covariance matrix was used for the random effects. A variable selection procedure was undertaken with the best fitting model (according to Akaike information criterion) chosen as the final model.
In addition to the chosen functional form of the sac trajectory over time, we also investigated including baseline covariates and linear time interactions in the mixed model in order to explain any heterogeneity in sac diameters and growth over time.
The final mixed-effects model was fitted once using all individuals and sac diameter measurements over follow-up to get accurate estimates of the fixed effects and variance components and in particular the long-term trajectory of sac diameter. Using these estimates, we then obtained predicted sac diameter measurements and rates of growth for each individual by calculating their empirical Bayes predicted random effects. This was done three times for each individual, corresponding to three landmark times at 2, 3 and 5 years in which predictions of future events are to be assessed, each time using only data available up to the landmark time in the calculation of the random effects.
Modelling future complications and reinterventions
We investigated modelling three possible future events:
-
any aneurysm-related complication except for sac expansion (see Table 29 for a full list of complications)
-
any cluster complication[2], defined here to be any of the following: a type I endoleak, a type II endoleak (with sac expansion), a type III endoleak, migration, or kinking
-
secondary rupture.
| Complication type | Cluster complication | Individuals reporting complication at least once, n (%) | Times complication reported during follow-up, n |
|---|---|---|---|
| Endoleak type I | Yes | 78 (10) | 93 |
| Endoleak type II | Yesa | 189 (24) | 240 |
| Endoleak type III | Yes | 32 (4) | 33 |
| Migration | Yes | 55 (7) | 63 |
| Kinking | Yes | 29 (4) | 33 |
| Other | No | 21 (3) | 21 |
| Thrombosis | No | 45 (6) | 48 |
| Graft infection | No | 4 (1) | 4 |
| False femoral aneurysm | No | 3 (0) | 3 |
| Neck expansion | No | 7 (1) | 7 |
| Renal failure | No | 1 (0) | 1 |
| Anastomotic aneurysm | No | 9 (1) | 9 |
| Rupture | No | 13 (2) | 14 |
| Stent fracture | No | 3 (0) | 3 |
| Renal infection | No | 6 (1) | 6 |
| Unknown | No | 1 (0) | 1 |
For each event we considered three potential times at which a prediction was to be made (the landmark times) (see Table 24).
These times were chosen to be at 2, 3 and 5 years postoperatively. We fitted Cox proportional hazards regression models to individuals still at risk at each of the landmark times, taking the landmark time as the entry time into the model. The time until the first occurrence of the event following the landmark time was of primary interest. Individuals were censored at their last CT/US date that assessed the complications of interest (the full set of complications were assessed up to September 2009, whereas cluster complications were assessed up until March 2015). Non-fatal rupture events were obtained via clinical follow-up and from HES, whereas fatal rupture events were obtained from the ONS. Therefore, the censoring date for the analysis of rupture events occurred at the earliest of the last data extraction for HES or ONS.
Along with a set of baseline covariates, we also investigated two aspects of the sac trajectory as predictors of the outcome: (1) the current predicted sac diameter; and (2) the current predicted rate of growth. A naive, albeit simple, estimate of the current sac diameter is to take the last observed sac diameter measurement, an approach labelled ‘last observation carried forward’, whereas an ‘observed’ sac growth can be calculated by dividing the difference in an individual’s last two measurements by the time elapsed. Alternatively, predictions of current sac diameter and current sac growth can be obtained from the fitted longitudinal model, which has the advantage of using all previous sac diameter measurements to derive a more precise estimate. The disadvantage of using predictions from a model is that they require some non-trivial calculations to be performed (e.g. using a computer program). Such an approach could, however, be easily facilitated through the development of a purpose-built web interface.
The predictive performance of the fitted Cox models at each landmark time was assessed in terms of predicting events occurring within 1, 2 or 5 years of the landmark time for any complication and cluster complication (the time horizon). As fewer rupture events were observed, ruptures were predicted within 5 or 10 years of the landmark time. A C-index was calculated and interpreted as the proportion of pairs of individuals in whom the ordering of the event times within the time horizon was correctly predicted. A C-index of 1.0 can be interpreted as perfect discrimination, whereas a value of 0.5 indicates that the model is predicting events no better than chance. To account for overoptimism, C-indices were calculated on a 10-fold cross-validated data set using nine-tenths of the data to fit the model and the remaining one-tenth to validate. Changes in C-index from a prediction model excluding any aspects of sac diameter were also calculated.
Results: pattern of sac growth over time
Table 30 shows the average number of sac diameter measurements per individual up until the landmark time of interest, and the number of individuals and complication events that occur after the landmark time contributing to the survival analysis.
| Landmark time | |||
|---|---|---|---|
| 2 years | 3 years | 5 years | |
| Number of patients contributing to mixed model | 784 | 784 | 784 |
| Mean number of sac measurements prior to landmark time | 2.8 | 3.5 | 4.6 |
| Number at risk for any complication at landmark time | 666 | 604 | 479 |
| Number experiencing any complication after landmark time | 136 | 100 | 50 |
| Number at risk for cluster complications at landmark time | 666 | 607 | 482 |
| Number experiencing any cluster complication after landmark time | 144 | 114 | 80 |
| Number at risk for secondary rupture at landmark time | 665 | 608 | 501 |
| Number experiencing a fatal or non-fatal secondary rupture after landmark time | 36 | 32 | 21 |
A plot of the repeated sac diameter measurements over time for each individual is shown in Figure 25, stratified by those who did not or did experience a rupture during follow-up. The time trend appears to be non-linear, with an initial decrease in sac diameter followed by a flattening out for those who did not experience a rupture. The time trend in the group who ruptured is similar, but with an increase in sac diameter prior to rupture for some individuals. (Note that the time of rupture may not necessarily be immediately after the last sac diameter measurement as a result of some patients ceasing regular clinical imaging.)
FIGURE 25.
Repeated sac diameter measurements against time since operation for each individual. (a) Individuals who did not experience a rupture during follow-up; and (b) individuals who had a rupture event.
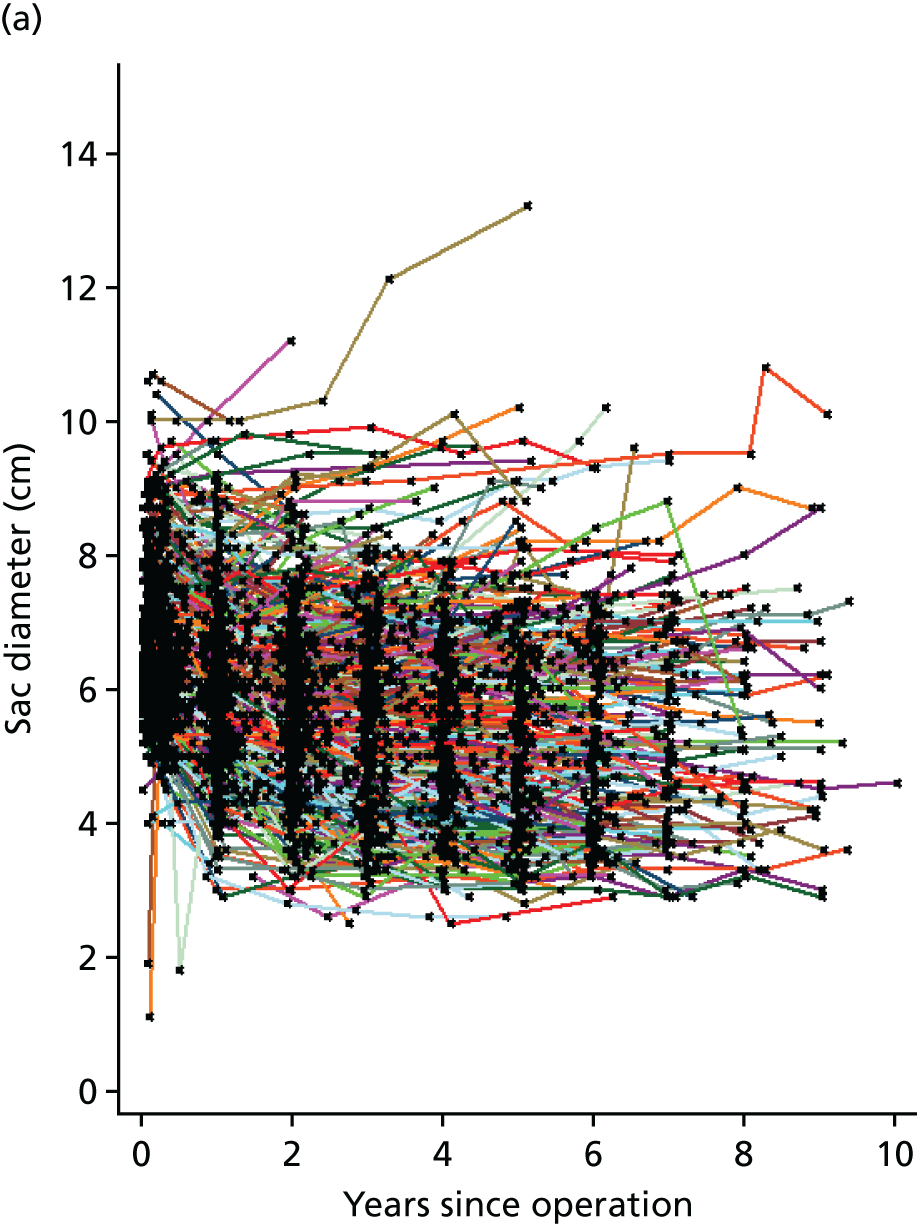
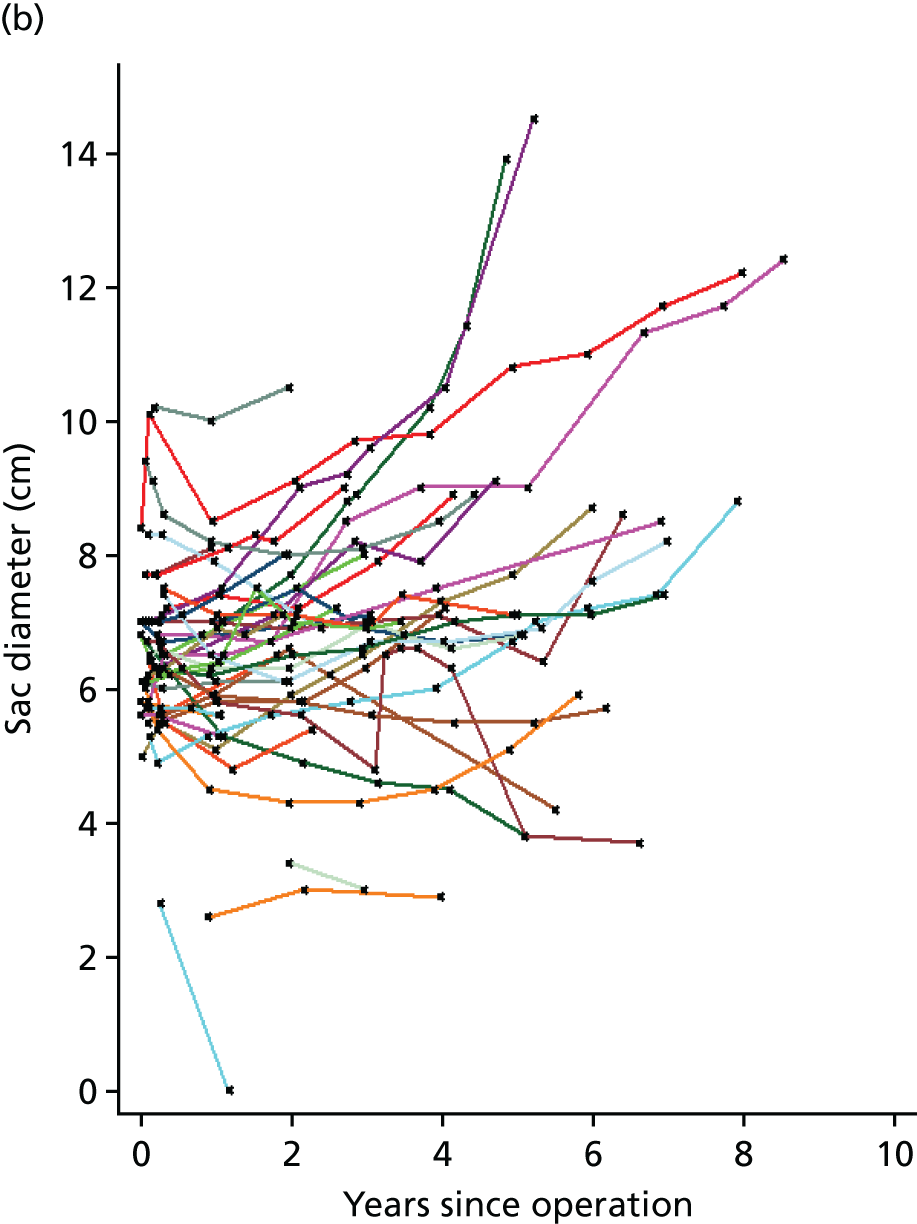
After comparing all models with a linear time trend and up to two fractional polynomial terms, our final mixed-effects model contained linear, quadratic and square root powers of time with both fixed- and random-effects terms for each time component. Figure 26 shows the population-averaged time trajectory for those with average or reference baseline covariates (see Table 31 for specification of reference baseline covariates). Sac diameter initially tends to decrease sharply postoperatively, followed by a period of flattening out and then a gradual growth.
FIGURE 26.
Population-averaged trajectory of postoperative sac diameter for an EVAR-1 individual with average preoperative AAA size, fitted with a uni-iliac, Cook/Zenith graft.
Table 31 shows the estimates for predictors of sac diameter and rate of growth from the final mixed-effects model (coefficients for the function of time and variance components are given in Table 40). Baseline age and sex were not included in the final model because they were not significantly associated with postoperative sac diameter after adjusting for preoperative AAA size. Larger preoperative AAA size was associated with larger postoperative sac diameters. Different rates of sac growth were observed for different graft shapes and graft manufacturers, with higher growth rates associated with the use of uni-iliac grafts and Medtronic/Talent® (Medtronic CardioVascular, Santa Rosa, CA, USA) and GORE®/EXCLUDER® (W.L. Gore and Associates, Flagstaff, AZ, USA) grafts relative to Cook®/Zenith® (Cook Medical, Bloomington, IN, USA) grafts.
| Change in mean sac diameter (cm) (95% CI) | p-value | |
|---|---|---|
| Preoperative AAA size, per cm increase | 0.937 (0.891 to 0.984) | < 0.001 |
| Graft shape | ||
| Uni-iliac | Reference | 0.479 |
| Bi-iliac/bifem | 0.059 (–0.104 to 0.221) | |
| Graft manufacturer | ||
| Cook/Zenith | Reference | 0.799 |
| Medtronic/Talent | –0.016 (–0.110 to 0.079) | |
| GORE/EXCLUDER | –0.047 (–0.231 to 0.137) | |
| Other | –0.074 (–0.231 to 0.084) | |
| Trial | ||
| EVAR-1 | Reference | < 0.001 |
| EVAR-2 | 0.194 (0.095 to 0.293) | |
| Change in rate of sac growth (cm/year) (95% CI) | p-value | |
| Graft shape | ||
| Uni-iliac | Reference | 0.015 |
| Bi-iliac/bifem | –0.116 (–0.210 to –0.023) | |
| Graft manufacturer | ||
| Cook/Zenith | Reference | < 0.001 |
| Medtronic/Talent | 0.191 (0.135 to 0.247) | |
| GORE/EXCLUDER | 0.245 (0.139 to 0.351) | |
| Other | 0.016 (–0.075 to 0.107) | |
Figure 27 shows fitted sac diameter trajectories at the landmark times of 2, 3 and 5 years post operation for six patients with differing outcomes. Each trajectory has been fitted using only the sac diameter measurements available up to the landmark time. Also plotted are all observed sac diameter measurements, up to September 2009, for each patient. The patient who experiences a rupture after 12 years (see Figure 27a) has initially stable, then increasing, sac diameter measurements, which is reflected in the fitted trajectories at 2, 3 and 5 years. At 5 years the fitted curve detects the increase in sac diameter and upward trajectory in the observed measurements. Patients who experience stable or decreasing measurements tend to have similar fitted trajectories at the different landmark times.
FIGURE 27.
Postoperative sac diameter measurements for six individuals and their fitted trajectories using only past measurements at 2, 3 and 5 years. (a) Rupture at 12 years post operation; (b) type II endoleak at 6 years post operation; (c) type II endoleak at 0.1 years post operation; and (d–f) no post-operation complications.
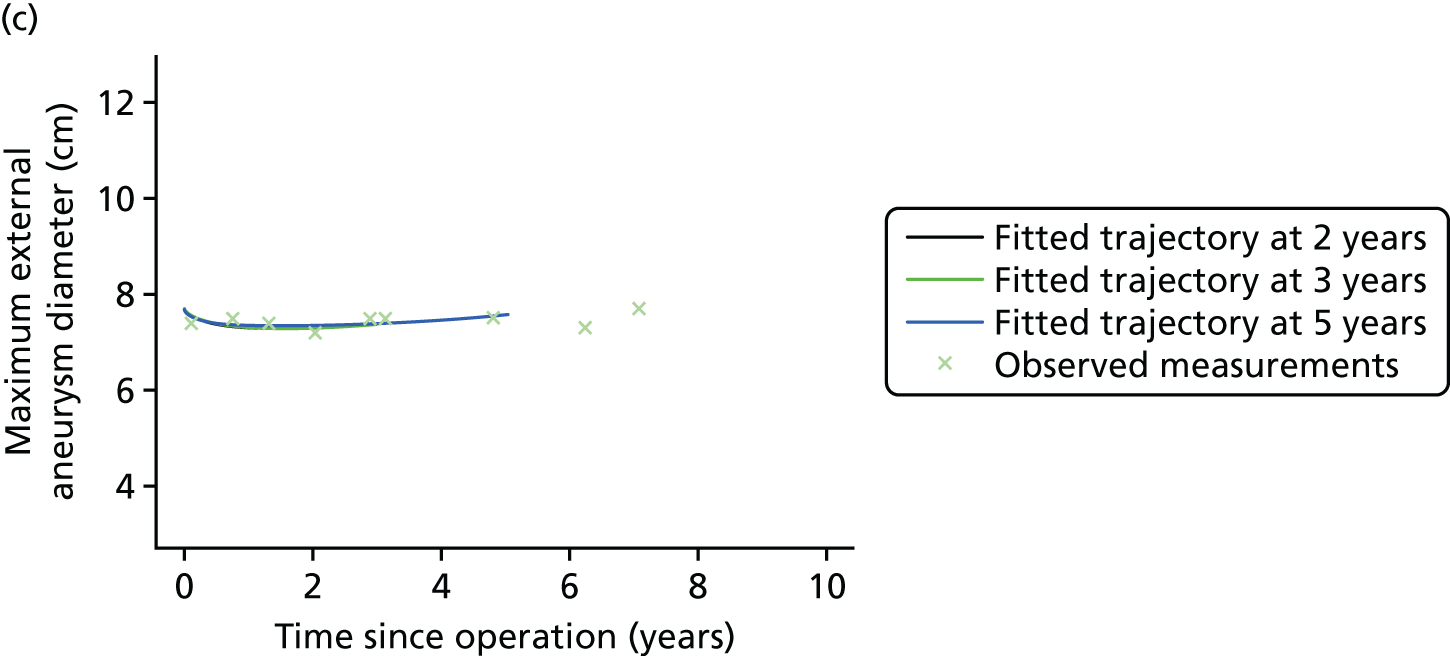
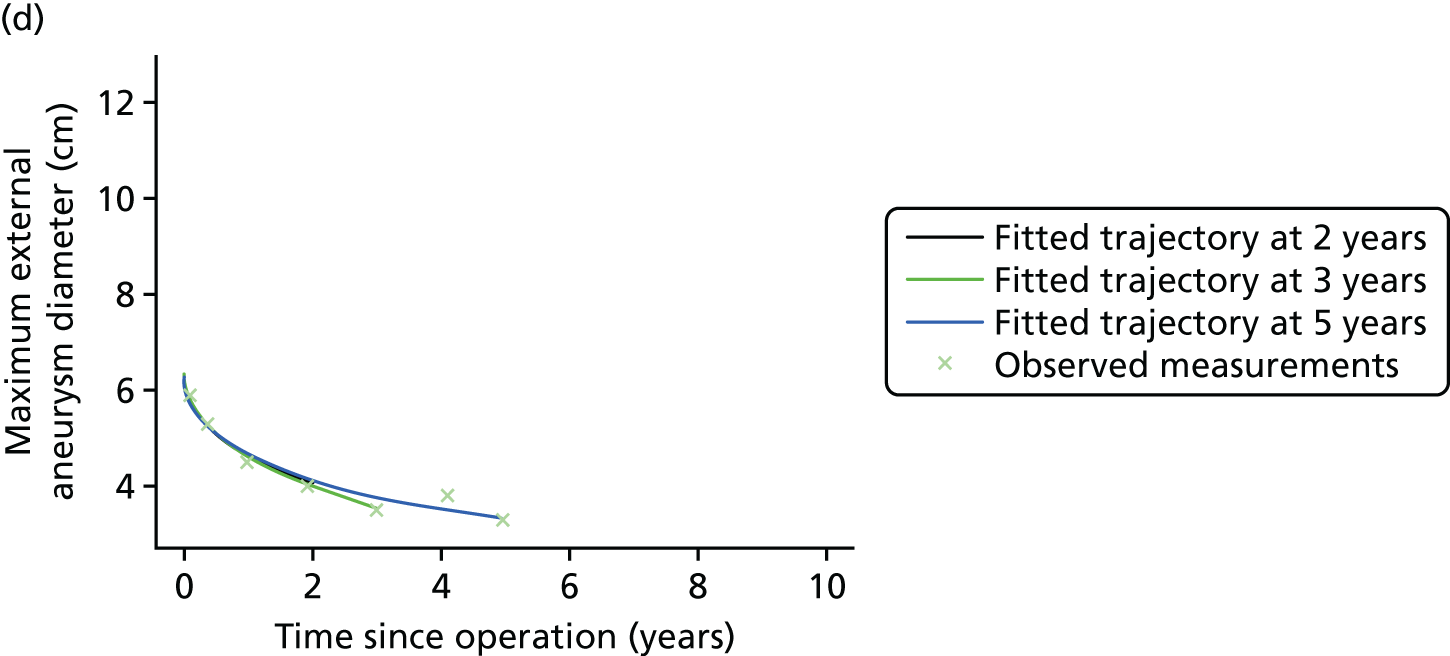
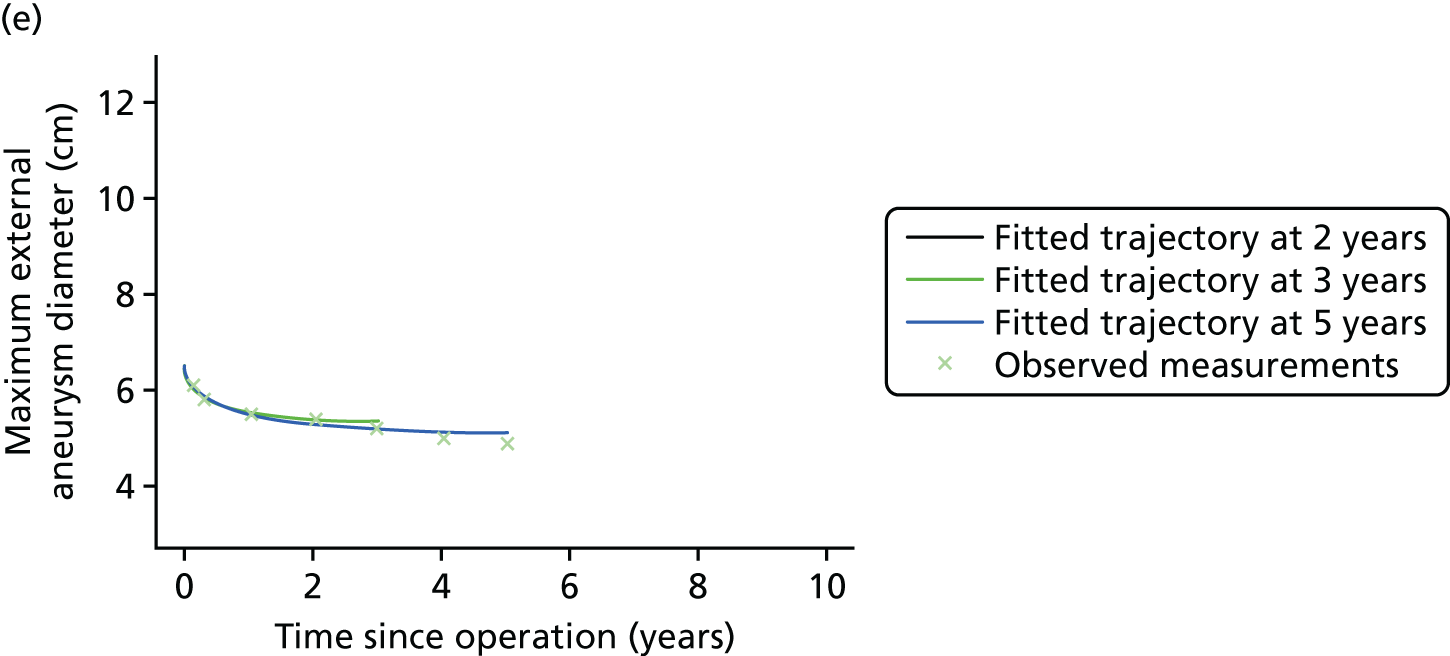
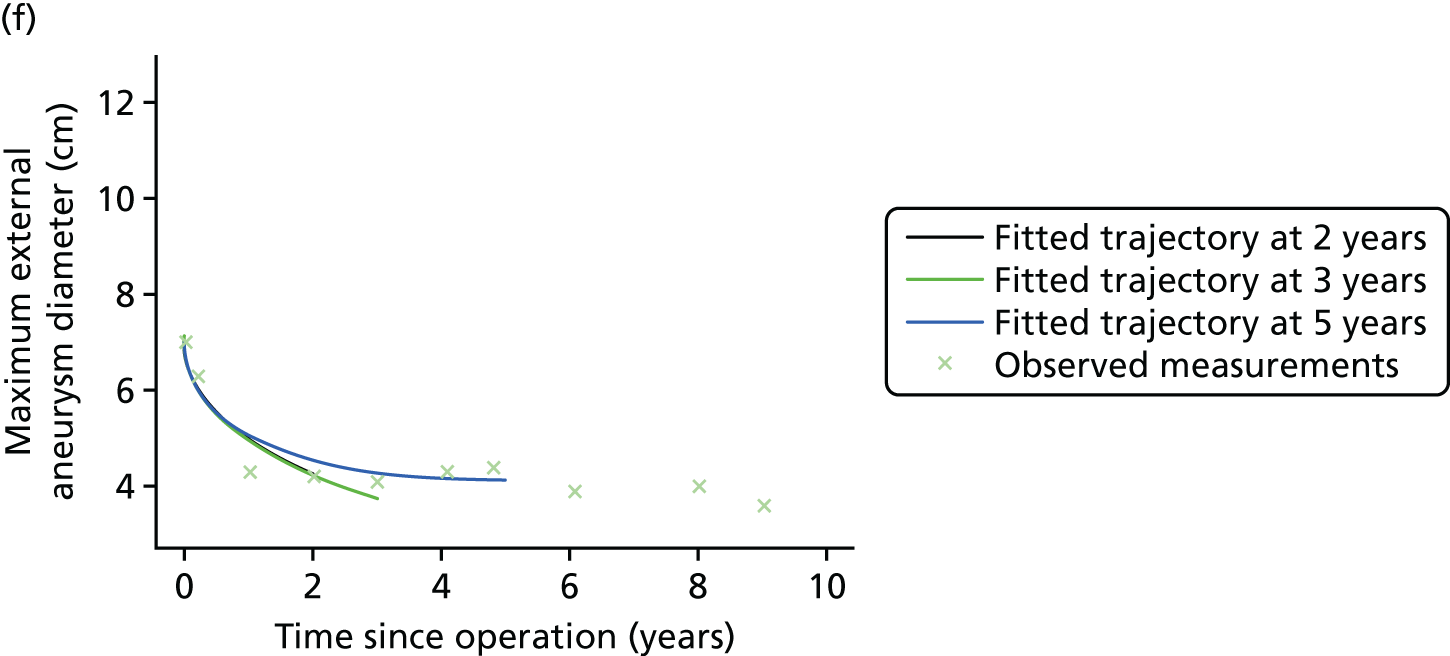
Results: associations between aspects of sac trajectory and risk of future events
Any future complication
There were 315 first-time complications that occurred in 2846 person-years of observation (crude risk of 11.1 per 100 person-years, 95% CI 9.9 to 12.4). There were 666, 604 and 479 individuals still under follow-up at the landmark forecasting times of 2, 3 and 5 years post operation, respectively. In univariate survival analyses, at 2 and 3 years following operation, there was found to be an association between the following factors and a heightened risk of developing any future complication: larger preoperative AAA size; a Medtronic/Talent or other graft; larger current sac diameter; and a larger current rate of sac growth (both predicted from the linear mixed model) (Table 32). However, after adjustment, only graft manufacturer remained statistically significant. At 5 years post operation, after adjustment, there were no variables that were convincingly associated with the risk of a future complication taking place.
| Landmark time | Univariate model,a HR (95% CI) | Multivariate model, HR (95% CI) |
|---|---|---|
| 2 years post operation (n = 638; number of events = 133) | ||
| Age (years) | 1.024 (0.996 to 1.052) | 1.016 (0.988 to 1.045) |
| p-value | 0.093 | 0.265 |
| Preoperative AAA size (cm) | 1.188 (1.003 to 1.407) | 1.401 (0.837 to 2.345) |
| p-value | 0.046 | 0.199 |
| Graft shape | ||
| Uni-iliac | Reference | Reference |
| Bi-iliac/bifem | 0.719 (0.397 to 1.302) | 0.900 (0.483 to 1.677) |
| p-value | 0.276 | 0.740 |
| Graft manufacturer | ||
| Cook/Zenith | Reference | Reference |
| Medtronic/Talent | 1.437 (0.982 to 2.102) | 1.126 (0.719 to 1.764) |
| GORE/EXCLUDER | 0.814 (0.352 to 1.883) | 0.540 (0.220 to 1.329) |
| Other | 2.420 (1.445 to 4.052) | 2.158 (1.284 to 3.627) |
| p-value | 0.004 | 0.008 |
| Current sac diameter (cm) | 1.226 (1.073 to 1.401) | 0.834 (0.494 to 1.410) |
| p-value | 0.003 | 0.498 |
| Current rate of growthb | 1.229 (1.054 to 1.433) | 1.422 (0.915 to 2.211) |
| p-value | 0.008 | 0.118 |
| 3 years post operation (n = 583; number of events = 98) | ||
| Age (years) | 1.035 (1.002 to 1.069) | 1.024 (0.990 to 1.059) |
| p-value | 0.037 | 0.163 |
| Preoperative AAA size (cm) | 1.234 (1.022 to 1.490) | 1.107 (0.679 to 1.807) |
| p-value | 0.029 | 0.683 |
| Graft shape | ||
| Uni-iliac | Reference | Reference |
| Bi-iliac/bifem | 1.450 (0.589 to 3.567) | 1.824 (0.720 to 4.620) |
| p-value | 0.419 | 0.205 |
| Graft manufacturer | ||
| Cook/Zenith | Reference | Reference |
| Medtronic/Talent | 1.448 (0.934 to 2.245) | 1.149 (0.694 to 1.904) |
| GORE/EXCLUDER | 0.502 (0.156 to 1.616) | 0.325 (0.096 to 1.101) |
| Other | 2.124 (1.165 to 3.871) | 1.828 (0.992 to 3.368) |
| p-value | 0.024 | 0.038 |
| Current sac diameter (cm) | 1.295 (1.126 to 1.490) | 1.123 (0.682 to 1.851) |
| p-value | < 0.001 | 0.648 |
| Current rate of growthb | 1.262 (1.060 to 1.502) | 1.192 (0.707 to 2.011) |
| p-value | 0.009 | 0.510 |
| 5 years post operation (n = 467; number of events = 49) | ||
| Age (years) | 1.004 (0.959 to 1.052) | 0.993 (0.946 to 1.043) |
| p-value | 0.850 | 0.783 |
| Preoperative AAA size (cm) | 1.240 (0.957 to 1.608) | 1.371 (0.906 to 2.074) |
| p-value | 0.104 | 0.135 |
| Graft shape | ||
| Uni-iliac | Reference | Reference |
| Bi-iliac/bifem | 0.990 (0.308 to 3.188) | 1.169 (0.353 to 3.867) |
| p-value | 0.987 | 0.799 |
| Graft manufacturer | ||
| Cook/Zenith | Reference | Reference |
| Medtronic/Talent | 1.291 (0.700 to 2.380) | 0.924 (0.486 to 1.757) |
| GORE/EXCLUDER | 0.296 (0.040 to 2.192) | 0.209 (0.028 to 1.566) |
| Other | 1.394 (0.569 to 3.412) | 1.287 (0.512 to 3.232) |
| p-value | 0.436 | 0.420 |
| Current sac diameter (cm) | 1.239 (1.036 to 1.481) | 0.939 (0.633 to 1.393) |
| p-value | 0.019 | 0.756 |
| Current rate of growthb | 1.317 (1.024 to 1.695) | 1.504 (0.919 to 2.463) |
| p-value | 0.032 | 0.105 |
Cluster complications
There were 288 first-time cluster complications that occurred in 3457 person-years of observation (crude risk of 8.3 per 100 person years, 95% CI 7.4 to 9.4). Stronger associations were observed with cluster complications (Table 33). At 2 and 3 years post operation, after adjustment for other potential confounders, graft manufacturer remained significantly associated with future complications, with Gore/Excluder grafts showing a fourfold lower risk than Cook/Zenith grafts (HR at 3 years = 0.26, 95% CI 0.08 to 0.86) and ‘other’ manufacturers estimated to have nearly double the risk as the Cook/Zenith grafts (HR at 3 years = 1.93, 95% CI 1.08 to 3.46). No other variables significantly predicted cluster complications at these time periods.
| Landmark time | Univariate model,a HR (95% CI) | Multivariate model, HR (95% CI) |
|---|---|---|
| 2 years post operation (n = 639; number of events = 137) | ||
| Age (years) | 1.023 (0.996 to 1.051) | 1.013 (0.986 to 1.042) |
| p-value | 0.096 | 0.342 |
| Preoperative AAA size (cm) | 1.285 (1.090 to 1.516) | 1.416 (0.854 to 2.347) |
| p-value | 0.003 | 0.178 |
| Graft shape | ||
| Uni-iliac | Reference | Reference |
| Bi-iliac/bifem | 0.912 (0.479 to 1.736) | 1.161 (0.595 to 2.266) |
| p-value | 0.778 | 0.661 |
| Graft manufacturer | ||
| Cook/Zenith | Reference | Reference |
| Medtronic/Talent | 1.454 (0.999 to 2.117) | 1.085 (0.698 to 1.686) |
| GORE/EXCLUDER | 0.910 (0.394 to 2.103) | 0.549 (0.224 to 1.349) |
| Other | 2.711 (1.637 to 4.489) | 2.450 (1.474 to 4.072) |
| p-value | 0.001 | 0.001 |
| Current sac diameter (cm) | 1.350 (1.184 to 1.540) | 0.916 (0.549 to 1.529) |
| p-value | < 0.001 | 0.737 |
| Current rate of growthb | 1.313 (1.126 to 1.532) | 1.451 (0.938 to 2.245) |
| p-value | 0.001 | 0.094 |
| 3 years post operation (n = 587; number of events = 108) | ||
| Age (years) | 1.025 (0.994 to 1.058) | 1.008 (0.976 to 1.041) |
| p-value | 0.117 | 0.648 |
| Preoperative AAA size (cm) | 1.315 (1.098 to 1.575) | 1.232 (0.789 to 1.922) |
| p-value | 0.003 | 0.359 |
| Graft shape | ||
| Uni-iliac | Reference | Reference |
| Bi-iliac/bifem | 1.610 (0.656 to 3.953) | 2.216 (0.879 to 5.585) |
| p-value | 0.298 | 0.091 |
| Graft manufacturer | ||
| Cook/Zenith | Reference | Reference |
| Medtronic/Talent | 1.325 (0.872 to 2.014) | 0.852 (0.532 to 1.365) |
| GORE/EXCLUDER | 0.519 (0.162 to 1.663) | 0.256 (0.077 to 0.855) |
| Other | 2.270 (1.276 to 4.035) | 1.929 (1.076 to 3.459) |
| p-value | 0.016 | 0.008 |
| Current sac diameter (cm) | 1.452 (1.268 to 1.663) | 1.102 (0.699 to 1.738) |
| p-value | < 0.001 | 0.676 |
| Current rate of growthb | 1.439 (1.213 to 1.709) | 1.493 (0.932 to 2.392) |
| p-value | < 0.001 | < 0.001 |
| 5 years post operation (n = 470; number of events = 75) | ||
| Age (years) | 1.014 (0.976 to 1.053) | 0.987 (0.949 to 1.027) |
| p-value | 0.481 | 0.523 |
| Preoperative AAA size (cm) | 1.399 (1.132 to 1.729) | 1.012 (0.710 to 1.443) |
| p-value | 0.002 | 0.948 |
| Graft shape | ||
| Uni-iliac | Reference | Reference |
| Bi-iliac/bifem | 1.210 (0.442 to 3.315) | 1.482 (0.531 to 4.137) |
| p-value | 0.711 | 0.453 |
| Graft manufacturer | ||
| Cook/Zenith | Reference | Reference |
| Medtronic/Talent | 1.183 (0.720 to 1.944) | 0.709 (0.426 to 1.181) |
| Gore/Excluder | 0.252 (0.035 to 1.839) | 0.133 (0.018 to 0.977) |
| Other | 1.583 (0.765 to 3.273) | 1.334 (0.637 to 2.792) |
| p-value | 0.279 | 0.083 |
| Current sac diameter (cm) | 1.629 (1.405 to 1.888) | 1.586 (1.116 to 2.252) |
| p-value | < 0.001 | 0.010 |
| Current rate of growthb | 1.697 (1.389 to 2.072) | 1.151 (0.774 to 1.712) |
| p-value | < 0.001 | 0.488 |
At 5 years post operation, the graft manufacturer was no longer associated with the onset of future cluster complications, whereas there was some evidence that a larger sac diameter at 5 years was an important predictor. After adjusting for current sac diameter, the rate of sac growth was no longer found to be an independent risk factor, as sac diameter and rate of growth were highly correlated by 5 years (ρ = 0.76) (Figure 28).
FIGURE 28.
Predicted current rate of change against predicted current sac diameter using past measurements at 2, 3 and 5 years. Prediction at (a) 2 years; (b) 3 years; and (c) 5 years.
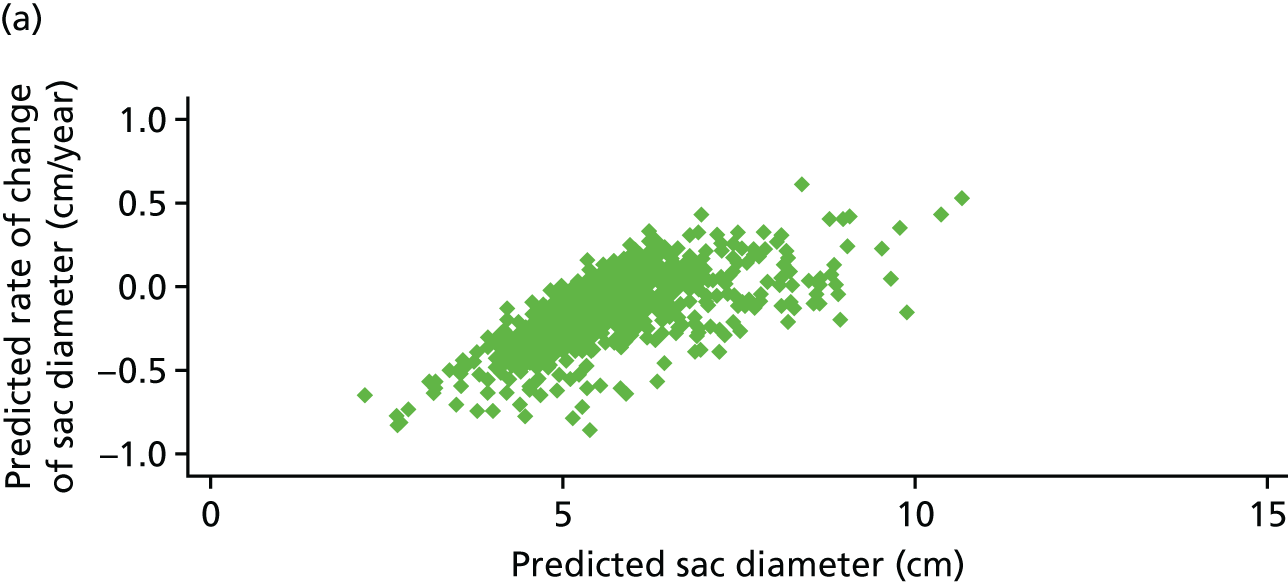
Secondary rupture
There were 41 secondary rupture events that occurred in 5666 person-years of observation [crude rupture risk of 0.72 per 100-person years, 95% CI 0.53 to 0.98). Survival free of secondary rupture is shown in Figure 29. Owing to the small number of ruptures, only current sac diameter and rate of growth were investigated as potential predictors of rupture. Both were strongly associated with rupture events at all landmark times in univariate analyses, but only (modelled) rate of sac growth remained an independent risk factor when adjusting for each other (Table 34). For a 0.2 cm per year increase in growth rate the risk of secondary rupture increases substantially (HR at 5 years = 2.61, 95% CI 1.49 to 4.57), a finding that is consistent across follow-up.
FIGURE 29.
Survival free of death from secondary rupture for 785 EVAR-operated patients in EVAR-1 and EVAR-2.
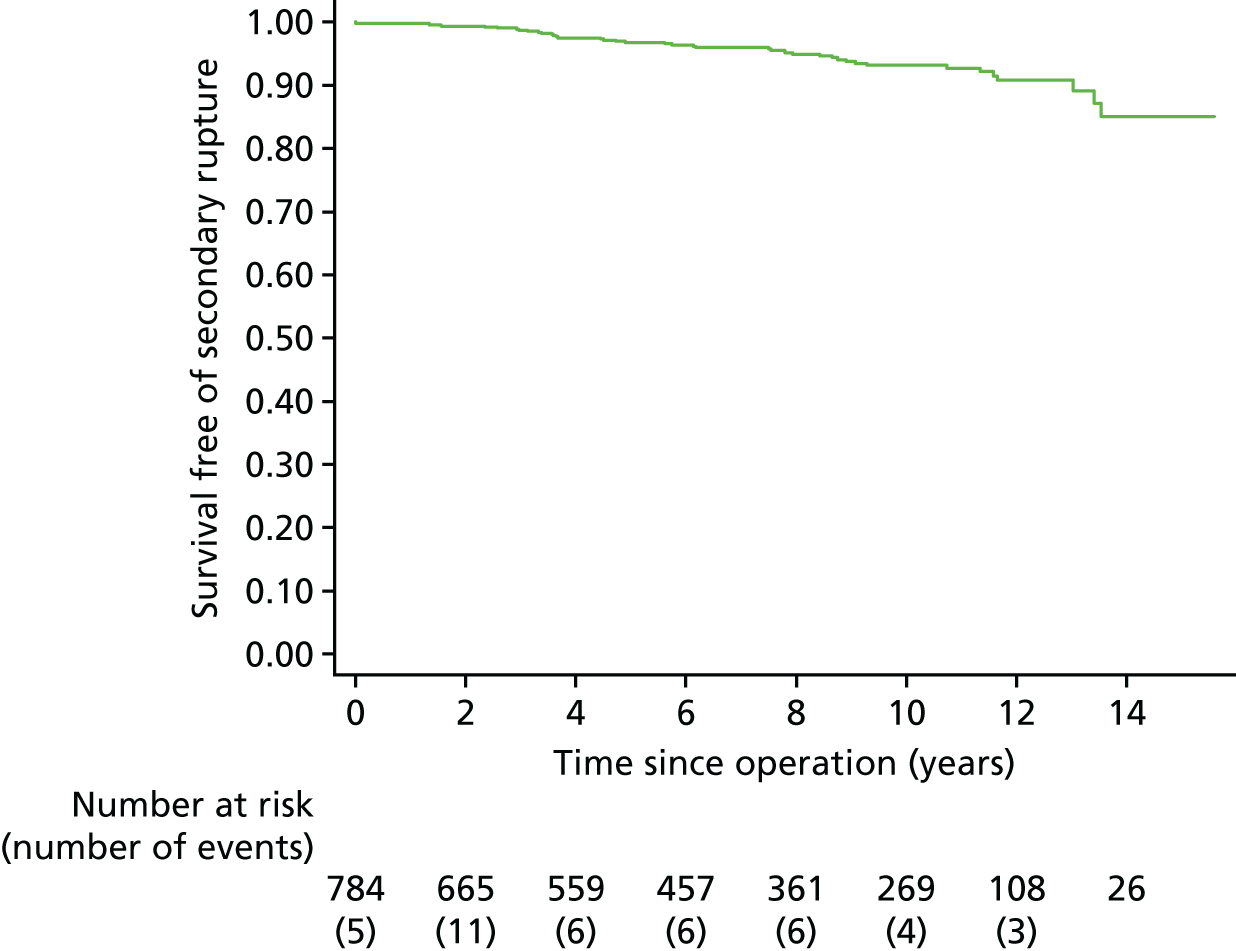
| Landmark time | Univariate model, HR (95% CI) | Multivariate model, HR (95% CI) | |
|---|---|---|---|
| 2 years post operation (n = 636; number of events = 36) | Current sac diameter (cm) | 1.673 (1.303 to 2.148) | 1.160 (0.820 to 1.640) |
| p-value | < 0.001 | 0.402 | |
| Current rate of growtha | 2.622 (1.847 to 3.723) | 2.372 (1.559 to 3.611) | |
| p-value | < 0.001 | < 0.001 | |
| 3 years post operation (n = 586; number of events = 32) | Current sac diameter (cm) | 2.033 (1.597 to 2.588) | 1.270 (0.883 to 1.828) |
| p-value | < 0.001 | 0.197 | |
| Current rate of growtha | 3.628 (2.550 to 5.163) | 2.993 (1.897 to 4.722) | |
| p-value | < 0.001 | < 0.001 | |
| 5 years post operation (n = 486; number of events = 21) | Current sac diameter (cm) | 2.407 (1.821 to 3.181) | 1.516 (0.989 to 2.324) |
| p-value | < 0.001 | 0.056 | |
| Current rate of growtha | 3.888 (2.625 to 5.758) | 2.609 (1.488 to 4.574) | |
| p-value | < 0.001 | 0.001 |
Results: predictive capabilities of a risk score
Predictive accuracy was assessed for any complication and any cluster complication in three models: model 1C included only baseline covariates (age, preoperative AAA size, graft shape and graft manufacturer); model 2C additionally included current sac diameter; and model 3C additionally included current sac diameter and rate of sac growth. The predictive accuracy measures, as estimated by the cross-validated C-index, are shown in Tables 35 and 36 for any complication and any cluster complication, respectively. Very low C-indices of magnitude between 0.53 and 0.63 are observed, indicating that the models are unable to accurately identify individuals who will develop complications in the time horizons studied. For example, a C-index of 0.5 shows that the model is no more likely than chance (i.e. probability of 0.5) to identify out of a randomly chosen pair of individuals which one will suffer a complication first. Slight improvements are observed when sac diameter or rate of growth is included in the prediction model though the overall predictive accuracy remains low.
| Landmark time | Time horizon (number of events) | C-index (95% CI) | ||
|---|---|---|---|---|
| Model 1C | Model 2C | Model 3C | ||
| 2 years | 1 year (n = 50) | 0.543 (0.482 to 0.604) | 0.567 (0.505 to 0.629) | 0.565 (0.503 to 0.626) |
| 2 years (n = 79) | 0.562 (0.515 to 0.610) | 0.587 (0.539 to 0.635) | 0.575 (0.527 to 0.623) | |
| 5 years (n = 125) | 0.576 (0.537 to 0.615) | 0.598 (0.559 to 0.637) | 0.585 (0.546 to 0.625) | |
| 3 years | 1 year (n = 35) | 0.585 (0.518 to 0.652) | 0.598 (0.532 to 0.665) | 0.593 (0.526 to 0.660) |
| 2 years (n = 56) | 0.578 (0.526 to 0.631) | 0.612 (0.561 to 0.663) | 0.607 (0.556 to 0.659) | |
| 5 years (n = 95) | 0.587 (0.542 to 0.631) | 0.613 (0.569 to 0.657) | 0.610 (0.566 to 0.654) | |
| 5 years | 1 year (n = 20) | 0.537 (0.455 to 0.620) | 0.561 (0.474 to 0.620) | 0.571 (0.485 to 0.657) |
| 2 years (n = 36) | 0.563 (0.498 to 0.628) | 0.566 (0.499 to 0.632) | 0.567 (0.500 to 0.633) | |
| 5 years (n = 50) | 0.533 (0.471 to 0.595) | 0.548 (0.482 to 0.613) | 0.554 (0.490 to 0.619) | |
| Landmark time | Time horizon (number of events) | C-index (95% CI) | ||
|---|---|---|---|---|
| Model 1C | Model 2C | Model 3C | ||
| 2 years | 1 year (n = 41) | 0.534 (0.470 to 0.599) | 0.566 (0.502 to 0.630) | 0.568 (0.504 to 0.631) |
| 2 years (n = 68) | 0.550 (0.499 to 0.601) | 0.589 (0.540 to 0.639) | 0.582 (0.533 to 0.632) | |
| 5 years (n = 114) | 0.578 (0.539 to 0.618) | 0.608 (0.569 to 0.647) | 0.599 (0.559 to 0.638) | |
| 3 years | 1 year (n = 32) | 0.602 (0.537 to 0.667) | 0.616 (0.550 to 0.683) | 0.604 (0.535 to 0.673) |
| 2 years (n = 47) | 0.580 (0.527 to 0.633) | 0.625 (0.574 to 0.677) | 0.617 (0.565 to 0.669) | |
| 5 years (n = 95) | 0.584 (0.541 to 0.628) | 0.623 (0.581 to 0.666) | 0.619 (0.577 to 0.662) | |
| 5 years | 1 year (n = 22) | 0.554 (0.468 to 0.640) | 0.613 (0.523 to 0.704) | 0.613 (0.523 to 0.704) |
| 2 years (n = 40) | 0.573 (0.508 to 0.639) | 0.611 (0.545 to 0.676) | 0.610 (0.544 to 0.675) | |
| 5 years (n = 72) | 0.553 (0.496 to 0.668) | 0.608 (0.552 to 0.665) | 0.611 (0.554 to 0.668) | |
Five models were considered for predicting secondary rupture: 1R, using the last observed sac diameter measurement; 2R, using ‘observed’ sac growth, calculated by dividing the difference in an individual’s last two measurements by the time elapsed; 3R, using model predicted current sac diameter only; 4R, using model predicted sac growth only; and 5R, using model predicted sac diameter and sac growth together. The predictive accuracy of each model at different prediction times and prediction intervals is shown in Table 37. The C-indices of the model with just model predicted sac diameter (model 3R) range from 0.71 to 0.78 and are similar to those of the model using the last observed measurement (model 1R). These increase to between 0.76 and 0.85 when predicted sac growth alone is included in the risk prediction model, which indicates relatively good predictive accuracy based on this measure alone. The model with ‘observed’ sac growth (model 2R) has the worst predictive performance in general, with C-indices ranging from 0.59 to 0.73, indicating that precise measures of sac growth cannot be obtained from just two repeat measurements of sac diameter. The predictive capability of the model is not significantly enhanced when both predicted sac diameter and growth are included together (model 5R).
| Landmark time | Time horizon (number of events) | C-index (95% CI) | ||||
|---|---|---|---|---|---|---|
| Model 1R | Model 2R | Model 3R | Model 4R | Model 5R | ||
| 2 years | 5 years (n = 14) | 0.732 (0.625 to 0.838) | 0.606 (0.483 to 0.728) | 0.733 (0.624 to 0.842) | 0.796 (0.665 to 0.927) | 0.798 (0.672 to 0.924) |
| 10 years (n = 27) | 0.699 (0.610 to 0.787) | 0.588 (0.488 to 0.688) | 0.706 (0.616 to 0.796) | 0.755 (0.649 to 0.861) | 0.754 (0.651 to 0.856) | |
| 3 years | 5 years (n = 13) | 0.784 (0.686 to 0.881) | 0.726 (0.604 to 0.848) | 0.783 (0.681 to 0.884) | 0.828 (0.718 to 0.938) | 0.818 (0.709 to 0.927) |
| 10 years (n = 25) | 0.744 (0.652 to 0.837) | 0.709 (0.610 to 0.809) | 0.743 (0.646 to 0.839) | 0.776 (0.671 to 0.881) | 0.768 (0.664 to 0.873) | |
| 5 years | 5 years (n = 12) | 0.768 (0.623 to 0.913) | 0.611 (0.434 to 0.788) | 0.772 (0.628 to 0.916) | 0.846 (0.750 to 0.942) | 0.817 (0.692 to 0.942) |
| 10 years (n = 20) | 0.778 (0.654 to 0.901) | 0.623 (0.470 to 0.775) | 0.783 (0.660 to 0.907) | 0.844 (0.757 to 0.930) | 0.822 (0.715 to 0.928) | |
The relationship between sac growth and risk of rupture is further highlighted in Table 38, in which the proportion of rupture events that occur in a 5-year time period after the landmark time of interest is subdivided by categories of sac growth (< 0 mm/year, 0–2 mm/year, 2–4 mm/year and ≥ 4 mm/year). Individuals who are not censored in this 5-year time period are classified either as a case (a rupture event occurs within 5 years) or as a control (no rupture event occurs within the 5 years). For an individual, sac growth can be calculated using either a prediction from a fitted linear mixed model or an ‘observed’ growth, as described in the previous paragraph. The advantage of the first approach is that it should give a more accurate prediction of current sac growth as it is based on all past sac diameter measurements, whereas the latter approach will be more sensitive to measurement error of the sac, resulting in a wider variability in the estimated rates of growth. The advantage of the second approach, however, is that it can be easily calculated without the need for a computer algorithm. From Table 38 it can be clearly seen that predicted sac growth is a relatively good predictor of risk of rupture, with a 15% or greater observed risk of rupture in individuals with predicted sac growth ≥ 4 mm per year compared with < 1% risk in individuals with predicted growth < 0 mm per year. Using an ‘observed’ growth rate, it is more difficult to discriminate between the high- and low-risk populations.
| Landmark time | Predicted or naive (observed) | Sac growth, n/N (%) | |||
|---|---|---|---|---|---|
| < 0 mm/year | 0–2 mm/year | 2–4 mm/year | ≥ 4 mm/year | ||
| 2 years | Predicted (n = 14) | 4/529 (0.8) | 4/177 (2.3) | 9/39 (23.1) | 2/6 (33.3) |
| Observed (n = 13) | 6/456 (1.3) | 4/110 (3.6) | 1/46 (2.2) | 7/90 (7.8) | |
| 3 years | Predicted (n = 13) | 3/433 (0.7) | 4/232 (1.7) | 7/79 (8.9) | 5/11 (45.5) |
| Observed (n = 13) | 4/425 (0.9) | 5/145 (3.5) | 1/58 (1.7) | 9/99 (9.1) | |
| 5 years | Predicted (n = 12) | 1/341 (0.3) | 4/267 (1.5) | 4/115 (3.5) | 5/34 (14.7) |
| Observed (n = 12) | 4/370 (1.1) | 2/182 (1.1) | 1/63 (1.6) | 7/115 (6.1) | |
Finally, it may be useful to consider a sac growth cut-off point at which a clinician may choose to instigate further investigations or more detailed clinical imaging. The sensitivity (the proportion of future rupture cases that are correctly identified for further investigation) and the specificity (the proportion of cases that do not rupture that are not chosen for further investigation) of different growth rate cut-off points are shown in Table 39, based on rupture events (cases) and non-rupture events (controls) that occur within 5 years (excluding individuals who are censored during this time). Cut-off points that yield both sensitivity and specificity > 70% are highlighted. At 2 years, identifying individuals with any positive growth (> 0 mm/year) would result in 79% of the future rupture cases being identified, but at a cost of 28% of non-rupture cases also undergoing further investigation. If only individuals with > 1 mm per year predicted growth are chosen for further investigation, then only 68% of future ruptures (within 5 years) would be identified, although only 14% of non-rupture cases would be identified for further investigation. At 3 years post operation, sac growth can be more readily detected and the sensitivity to predict future ruptures is higher, 84% using a cut-off point of 0 mm per year and 79% using a cut-off point of 1 mm per year. But a cut-off point of 0 mm per year gives quite a low specificity, suggesting that any observed growth at this time point is not necessarily related to potential future rupture. Specificities at 5 years post operation are generally lower.
| Landmark time | Predicted or naive (observed) | Sensitivity (%)/specificity (%) cut-off point for further investigations: sac growth (mm/year) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| 2 years | Predicted (n = 14) | 79/72 | 68/86 | 58/95 | 26/99 | 11/99 |
| Observed (n = 13) | 67/66 | 50/77 | 44/81 | 39/85 | 39/88 | |
| 3 years | Predicted (n = 13) | 84/58 | 79/76 | 63/89 | 42/97 | 26/99 |
| Observed (n = 13) | 79/59 | 63/74 | 53/79 | 53/84 | 47/87 | |
| 5 years | Predicted (n = 12) | 93/46 | 86/62 | 64/81 | 43/92 | 36/96 |
| Observed (n = 12) | 71/51 | 64/68 | 57/76 | 57/81 | 50/85 | |
Conclusions/implications for imaging protocols following elective endovascular aneurysm repair
We have shown in this chapter that patterns of sac growth, if modelled correctly, can be useful predictors for future serious rupture events. Estimates of sac expansion should be derived from longitudinal modelling of the sac diameter trajectory, which accounts for all previous measurements and thus produces more accurate estimates of current sac growth. Naive estimates of growth based on the last two observed diameter measurements are too crude to be of practical use in a prediction model. The current sac diameter was not found to be an independent risk factor of rupture after accounting for the growth rate of the sac and the predictive accuracy of a model just using sac diameter was lower than a model that used sac growth.
Further work is planned to recommend clinical cut-off points for which high-risk individuals could be flagged for more comprehensive surveillance and imaging. This would require a careful consideration of an acceptable trade-off between ensuring a high sensitivity (correctly identifying those who will truly go on and rupture), while keeping 1 – specificity relatively low (i.e. not flagging too many individuals who will not rupture). For such cut-off points to be used in clinical practice will require (1) stringent validation on external data sources; (2) acceptance by the clinical community; and (3) software implementation to allow real-time decisions to be made. This could be coupled with developments in potential real-time monitoring of sac expansion.
We have shown in these analyses that a decision threshold based on sac growth may not be constant over time because of the non-linear nature of the sac trajectory. At 2 years post operation any sac expansion is highly indicative of a possible future rupture event, whereas at 5 years post operation the majority of patients who were not observed to have a rupture also had non-negative growth rates (albeit sometimes close to zero). This finding suggests two things: first, that the cut-off point for further investigations could be raised slightly at 3 or 5 years post operation; and, second, that surveillance cannot stop at 2 years post operation in the face of a shrinking sac, as some sacs do not start to expand until after this time.
Developing a model to predict future complications or cluster complication was less successful, with none of the developed models providing high predictive accuracy. This could partly be due to the non-specific nature of the outcomes under consideration, particularly with respect to ‘any complication’, which was a composite outcome of 16 different aneurysm-related complications (see Table 29).
Limitations of this work are that there were relatively few rupture events (36 occurred beyond 2 years post operation). Therefore, the results may be overly optimistic and require validation using external data sources. C-indices were calculated using cross-validation in an attempt to mitigate this issue, but the sensitivities and specificities shown in Table 39 could be overly optimistic. A further limitation of this work is that reinterventions that occurred before secondary rupture are ignored in the modelling. Patients with high sac expansion may therefore have undergone corrective procedures, resulting in a lower risk of subsequent rupture. This could have led us to underestimate the association between sac growth and rupture. Future analyses could therefore consider alternative end points such as aneurysm-related mortality or a composite end point of reintervention or secondary rupture.
In developing a model for describing sac trajectories over time, we found that grafts produced by different manufacturers behaved differently with respect to the rate of subsequent sac expansion. It should, however, be noted that these grafts were all first-generation grafts and the results are in no way indicative of today’s newer-generation endovascular devices. Furthermore, some grafts may have been more widely used on more difficult aneurysms, which were then more likely to expand post operation (Table 40).
| Coefficient | p-value | |
|---|---|---|
| Fixed effects | ||
| Time (years) | 0.300 (0.167 to 0.432) | < 0.001 |
| Quadratic time | 0.000 (–0.007 to 0.008) | 0.905 |
| Square root time | –1.116 (–1.285 to 0.947) | < 0.001 |
| Between-person standard deviation | ||
| Time | 0.130 (0.088 to 0.191) | |
| Quadratic time | 0.022 (0.019 to 0.027) | |
| Square root time | 0.644 (0.594 to 0.698) | |
| Within-person (residual) standard deviation | 0.383 (0.371 to 0.395) | |
Chapter 9 Results from EVAR trial 2
The EVAR trial 2 patients and their follow-up
Between 1 September 1999 and 31 August 2004, 404 patients were recruited to participate in EVAR-2. A total of 197 patients were randomly assigned to the endovascular group and 207 were assigned to the no-intervention group. There were no significant differences between the two study groups with respect to baseline characteristics, mean age was 76.8 years and 347 (86%) were men (Table 41), but these patients were older and more physically frail than patients randomised to EVAR-1. 95
| Baseline characteristics by randomised group for EVAR trial 2 | ||
|---|---|---|
| Baseline characteristica | EVAR (n = 197) | No intervention (n = 207) |
| Age (years) | 77.2 (6.3) [0] | 76.4 (6.7) [0] |
| Number of males (%) | 168 (85) [0] | 179 (86) [0] |
| AAA diameter (cm) | 6.8 (1.0) [0] | 6.7 (1.0) [0] |
| BMI (kg/m2) | 26.4 (5.0) [1] | 26.5 (4.4) [1] |
| Diabetes (%) | 30 (15) [2] | 29 (14) [2] |
| Smoking status (%) | [0] | [0] |
| Current | 33 (17) | 37 (18) |
| Past | 152 (77) | 156 (75) |
| Never | 12 (6) | 14 (7) |
| History of cardiac diseaseb (%) | 132 (67) [0] | 153 (74) [0] |
| Systolic blood pressure (mmHg) | 140 (20) [0] | 139 (23) [0] |
| Diastolic blood pressure (mmHg) | 79 (12) [0] | 79 (12) [3] |
| ABPI (mean of both legs) | 0.99 (0.20) [10] | 0.98 (0.19) [8] |
| Forced expiration volume in 1 second (l) | 1.6 (0.6) [7] | 1.7 (0.7) [4] |
| Serum creatinine (µmol/l)a | 107 (90–134) [0] | 112 (94–140) [2] |
| Serum cholesterol (mmol/l) | 4.8 (1.2) [13] | 4.8 (1.1) [7] |
| Statin use (%) | 82 (42) [1] | 86 (42) [0] |
| Aspirin use (%) | 114 (58) [1] | 114 (55) [0] |
Patients were followed for mortality until 30 June 2015 (mean 12.3 years, median 12.2 years); mean follow-up to either death or end of the study was 4.2 years. Mortality was from regular reports from NHS Digital, which were assessed by the Endpoint Committee in a consistent manner for all deaths reported between September 2009 and June 2015. Out of 98 patients still under follow-up in September 2009, 45 (46%) had local follow-up at the trial centre. In contrast to follow-up of EVAR-1 patients, the EVAR-2 patients were not tracked using the administrative database of HES from NHS Digital. The Consolidated Standards of Reporting Trials diagram is shown in Figure 30.
FIGURE 30.
Consolidated Standards of Reporting Trials diagram: EVAR-2.
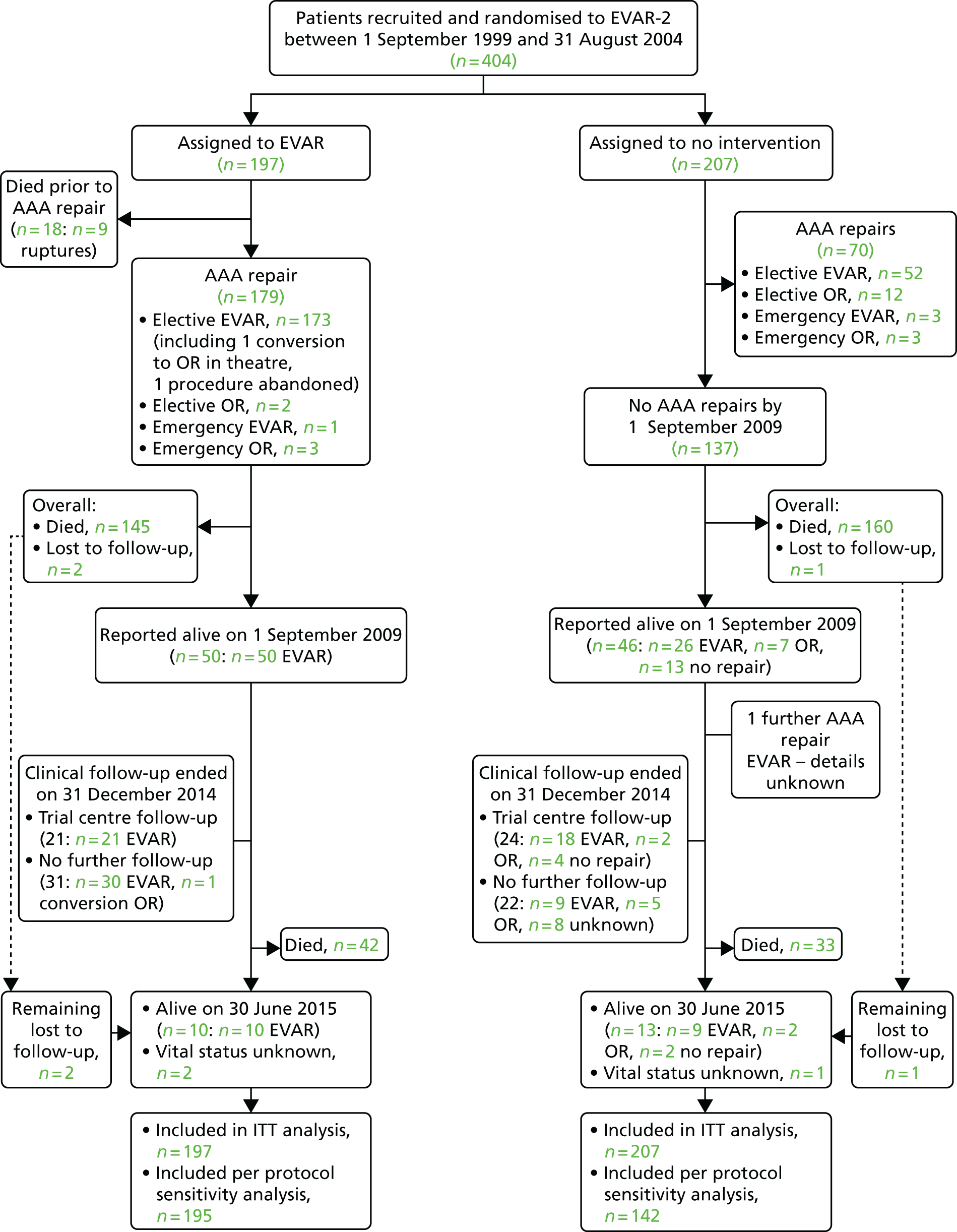
Long-term survival of patients randomised in the EVAR trial 2
After the 5- and 10-year results of EVAR-2 were known,95,107 it has become increasingly recognised that there is a cohort of patients who have such a limited life expectancy or such extensive comorbidities that repair of an AAA should not be considered. 161,162 The difficulty is in defining carefully this group of patients. In EVAR-2, the participating clinicians appeared to have had good judgement in selecting patients for this trial: by 4 years < 40% of patients remained alive and by 8 years this had reduced to < 20%. 95 Nevertheless, the approximately 20% of patients who remain alive after 8 years were perhaps physically the fittest of all the patients enrolled in EVAR-2 and it is of interest to compare their very long-term survival both by ITT and per-protocol analysis. This starts to enable description of the patients who, although physically frail and ineligible for OR, may have many life-years ahead and might benefit from EVAR, particularly if conducted under local anaesthesia. Such information could be important for updating the guidelines for the management of physically frail patients with large aneurysms.
Aneurysm-related and total mortality
During 1713 person-years of follow-up, 381 deaths occurred and 84 of which were aneurysm related. Overall aneurysm-related mortality was 3.2 deaths per 100 person-years in the EVAR group and 6.5 deaths per 100 person-years in the no-intervention group (adjusted HR 0.55, 95% CI 0.34 to 0.90; p = 0.018). Beyond 8 years of follow-up, the rate of aneurysm-related mortality was very low, with two aneurysm-related deaths in the EVAR group (at 8.0 and 13.2 years after randomisation) and one aneurysm-related death in the no-intervention group (at 9.3 years after randomisation), with survival curves remaining parallel (Figure 31). However, at 8 years only 10 of 38 patients in the no-intervention group remained with an intact unrepaired aneurysm compared with 0 of 32 patients in the EVAR group.
FIGURE 31.
Kaplan–Meier estimates for total survival and aneurysm-related survival up to 12 years of follow-up. Twelve-year survival probabilities are reported in the key.
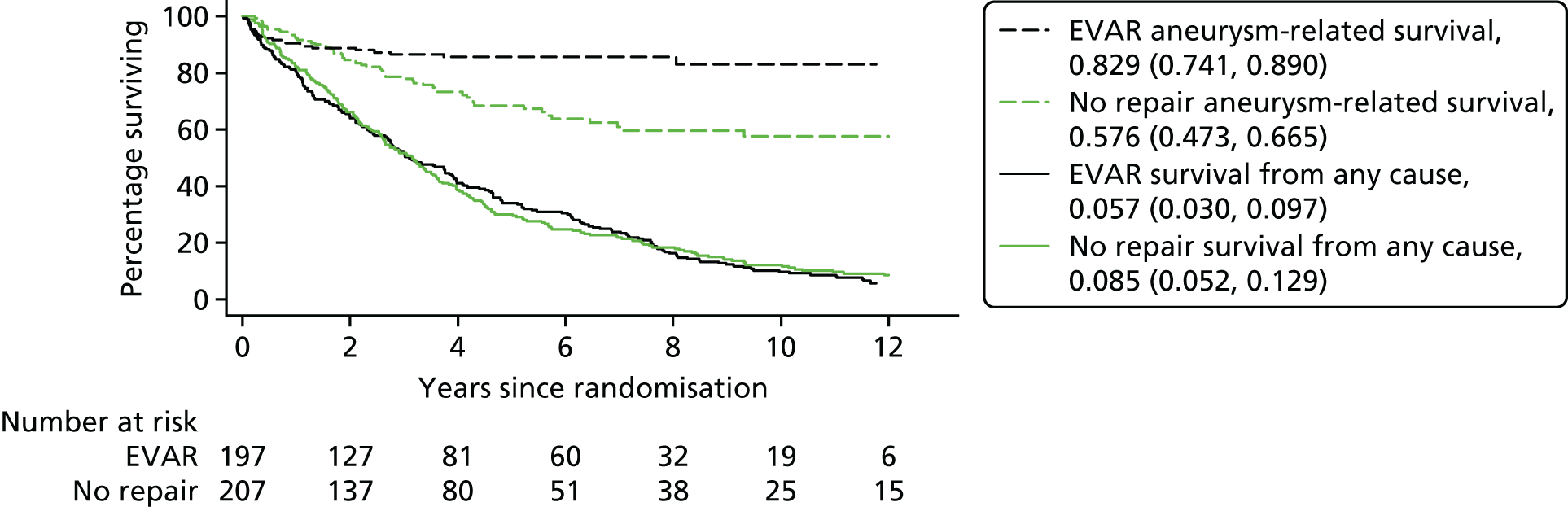
For total mortality, there were 22.4 deaths per 100 person-years in the EVAR group and 22.1 deaths per 100 person-years in the no-intervention group. Beyond 8 years the number of deaths and mortality rate were similar in the two randomised groups. By 12 years of follow-up the estimated survival was 5.7% (95% CI 3.05 to 9.7%) in the EVAR group and 8.5% (95% CI% 5.2 to 12.9%) in the no-intervention group (see Figure 31). There was no significant difference in life expectancy (restricted to 12 years of follow-up), between the groups (4.2 years in both the EVAR and the no-intervention groups; p = 0.99).
Deaths according to time since randomisation are given in Table 42; deaths from any cause and aneurysm-related causes according to time since randomisation are given in Table 43; and the full causes of death by time period are given in Table 44. Overall, there were 16 deaths from aneurysm rupture in the EVAR group (13 procedure related and three secondary ruptures) and 53 in the no-intervention group (52 primary and one secondary rupture).
| EVAR (n = 197) | No repair (n = 207) | |
|---|---|---|
| Any death, n (%) | ||
| All patients | 187 (94.9) | 194 (93.7) |
| Time of death, n (%) | ||
| 0–6 months | 24 (13) | 19 (10) |
| 6 months–4 years | 92 (49) | 108 (56) |
| 4–8 years | 49 (26) | 42 (22) |
| > 8 years | 22 (12) | 25 (13) |
| Cause of death, n (%) | ||
| Procedure-related AAA | 11 (6) | 3 (2) |
| Procedure-related AAA rupture | 13 (7) | 53 (27) |
| Coronary heart disease | 45 (24) | 60 (31) |
| Stroke | 4 (2) | 5 (3) |
| Other (including PAD) | 3 (2) | 3 (2) |
| Cancer, lung | 13 (7) | 8 (4) |
| Cancer, other | 27 (14) | 20 (10) |
| Respiratory | 47 (25) | 18 (9) |
| Renal | 3 (2) | 4 (2) |
| Other | 18 (10) | 15 (8) |
| Secondary AAA rupture | 3 (2) | 1 (1) |
| Unknown | 0 (0) | 4 (2) |
| Treatment group, n/N | HR (95% CI) | p-value | |||
|---|---|---|---|---|---|
| EVAR (n = 197), n/N (rate/100 person-years) | No repair (n = 207), n/N (rate/100 person-years) | Unadjusted | Adjusted (primary, secondary) | ||
| Any death | |||||
| All patients | 187/197 (22.4) | 194/207 (22.1) | 1.02 (0.84 to 1.25) | 1.05 (0.85 to 1.30) | 0.55 |
| 1.07 (0.86 to 1.33) | |||||
| Time since randomisation | |||||
| 0–6 months | 24/197 (26.0) | 19/207 (19.0) | 1.38 (0.76 to 2.52) | 1.48 (0.80 to 2.71) | 0.41 |
| 1.32 (0.68 to 2.54) | |||||
| 6 months–4 years | 92/173 (21.4) | 108/188 (23.6) | 0.90 (0.69 to 1.20) | 0.96 (0.72 to 1.29) | 0.92 |
| 1.02 (0.75 to 1.37) | |||||
| 4–8 years | 49/81 (21.4) | 42/80 (19.7) | 1.09 (0.72 to 1.65) | 1.01 (0.66 to 1.56) | 0.82 |
| 0.95 (0.60 to 1.50) | |||||
| > 8 years | 22/32 (26.3) | 25/38 (23.2) | 1.15 (0.65 to 2.04) | 1.24 (0.66 to 2.36) | 0.70 |
| 1.14 (0.59 to 2.20) | |||||
| Aneurysm-related death | |||||
| All patients | 27/197 (3.2) | 57/207 (6.5) | 0.50 (0.32 to 0.80) | 0.55 (0.34 to 0.88) | 0.018 |
| 0.55 (0.34 to 0.90) | |||||
| Time since randomisation | |||||
| 0–6 months | 15/197 (16.3) | 9/207 (9.0) | 1.82 (0.80 to 4.16) | 1.93 (0.84 to 4.46) | 0.19 |
| 1.78 (0.75 to 4.21) | |||||
| 6 months–4 years | 10/173 (2.3) | 35/188 (7.6) | 0.31 (0.15 to 0.62) | 0.33 (0.16 to 0.68) | 0.0050 |
| 0.34 (0.16 to 0.72) | |||||
| 4–8 years | 0/81 (0.0) | 12/80 (5.6) | 0 | NC | NC |
| > 8 years | 2/32 (2.4) | 1/38 (0.9) | 2.57 (0.23 to 28.45) | 3.90 (0.14 to 108.15) | NC |
| NC | |||||
| > 4 years | 2/81 (0.6) | 13/80 (4.0) | 0.15 (0.03 to 0.68) | 0.18 (0.04 to 0.80) | 0.029 |
| 0.17 (0.04 to 0.84) | |||||
| Cause of death | EVAR | No repair |
|---|---|---|
| Randomisation to 6 months | n = 24 | n = 19 |
| Primary-related AAA | 8 | 1 |
| Procedure-related AAA rupture | 7 | 8 |
| Coronary heart disease | 2 | 5 |
| Stroke | 0 | 2 |
| Other (including PAD) | 0 | 0 |
| Cancer, lung | 1 | 0 |
| Cancer, other | 0 | 0 |
| Respiratory | 5 | 1 |
| Renal | 0 | 1 |
| Other | 1 | 1 |
| Secondary AAA rupture | 0 | 0 |
| Unknown | 0 | 0 |
| 6 months–4 years | n = 92 | n = 108 |
| Primary-related AAA | 3 | 0 |
| Procedure-related AAA rupture | 6 | 35 |
| Coronary heart disease | 30 | 32 |
| Stroke | 3 | 2 |
| Other (including PAD) | 0 | 3 |
| Cancer, lung | 7 | 3 |
| Cancer, other | 14 | 11 |
| Respiratory | 19 | 9 |
| Renal | 2 | 3 |
| Other | 7 | 8 |
| Secondary AAA rupture | 1 | 0 |
| Unknown | 0 | 2 |
| 4–8 years | n = 49 | n = 41 |
| Primary-related AAA | 0 | 3 |
| Procedure-related AAA rupture | 0 | 9 |
| Coronary heart disease | 11 | 12 |
| Stroke | 1 | 0 |
| Other (including PAD) | 0 | 0 |
| Cancer, lung | 4 | 4 |
| Cancer, other | 8 | 5 |
| Respiratory | 15 | 5 |
| Renal | 1 | 0 |
| Other | 9 | 3 |
| Secondary AAA rupture | 0 | 0 |
| Unknown | 0 | 0 |
| > 8 years | n = 22 | n = 25 |
| Primary-related AAA | 0 | 0 |
| Procedure-related AAA rupture | 0 | 0 |
| Coronary heart disease | 2 | 11 |
| Stroke | 0 | 1 |
| Other (including PAD) | 3 | 0 |
| Cancer | ||
| Cancer, lung | 1 | 1 |
| Cancer, other | 5 | 4 |
| Respiratory | 8 | 3 |
| Renal | 0 | 0 |
| Other | 1 | 3 |
| Secondary AAA rupture | 2 | 1 |
| Unknown | 0 | 1 |
Aneurysm-related reinterventions
During 1663 person-years of follow-up, 54 graft-related reinterventions were performed in 38 patients in the EVAR group and 21 graft-related reinterventions were performed in 15 patients in the no-intervention group (Table 45). HRs for risk of a reintervention are shown in Table 46 by time epoch. There was a significantly higher rate of reinterventions in the EVAR group in the 6 months following randomisation. Beyond 4 years the rate of reintervention was similar between the two groups, as a result of a decreasing rate in the EVAR group and a slightly increasing rate in the no-intervention group as surviving individuals among those who underwent aneurysm repair.
| EVAR (n = 197) | No repair (n = 207) | |
|---|---|---|
| Any reintervention | ||
| All patients, n patients (n reinterventions) | 38 (54) | 15 (21) |
| Time of reintervention | ||
| Randomisation to 6 months | 26 | 6 |
| 6 months–4 years | 20 | 8 |
| 4–8 years | 8 | 4 |
| > 8 years | 0 | 3 |
| Type of reintervention | ||
| Added stent | 18 | 4 |
| Staple or ligation | 0 | 0 |
| Embolisation (of endoleak) | 9 | 2 |
| Sclerosis of endoleaks | 0 | 0 |
| Conversion to OR | 1 | 2 |
| Other | 6 | 2 |
| Known aneurysmal extension above or below original graft | 0 | 1 |
| Reintervention for thrombosis of graft limb | 0 | 0 |
| Reintervention for graft infection | 0 | 1 |
| Incisional hernia | 0 | 0 |
| False femoral aneurysm | 0 | 1 |
| Minor reintervention | 2 | 1 |
| Femorofemoral crossover graft | 3 | 1 |
| FEVAR | 0 | 1 |
| Axillobifemoral graft | 0 | 0 |
| Distal limb procedure/revascularisation | 11 | 2 |
| Reoperation of OR | 1 | 0 |
| Replacement stent graft | 0 | 2 |
| Further open abdominal surgery in primary admission | 3 | 0 |
| Amputation | 0 | 0 |
| Unknown | 0 | 1 |
| EVAR (n = 197) n unique reintervention episodesa/total N person-years (rate/100 person-years) | No intervention (n = 207) n unique reintervention episodesa/total N person-years (rate/100 person-years) | HR (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusted (primary,a secondary) | ||||
| Any reintervention | |||||
| All patients | 47/809 (5.8) | 21/854 (2.5) | 2.36 (1.28 to 4.37) | 2.42 (1.31 to 4.48) | 0.005 |
| 2.54 (1.32 to 4.87) | |||||
| Time since randomisation | |||||
| 0–6 months | 23/91 (25.3) | 6/100 (6.0) | 4.10 (1.50 to 11.22) | 4.31 (1.53 to 12.10) | 0.004 |
| 4.94 (1.65 to 14.81) | |||||
| 6 months–4 years | 16/418 (3.8) | 8/451 (1.8) | 2.17 (0.85 to 5.56) | 2.15 (0.80 to 5.77) | 0.077 |
| 2.56 (0.90 to 7.29) | |||||
| > 4 years | 8/300 (2.7) | 7/303 (2.3) | 1.12 (0.39 to 3.25) | 1.15 (0.43 to 3.08) | 0.99 |
| 0.99 (0.29 to 3.36) | |||||
Further analysis was carried out to compare baseline characteristics between those who did not survive > 8 years and the 70 (17%) patients who did (Table 47). The patients who survived ≥ 8 years were younger, with higher BMI, lower creatinine levels and better lung function than patients who survived < 8 years. There was also a small difference in aneurysm diameter, with slightly smaller aneurysms in the long-term survivors.
| Baseline characteristica | Did not survive > 8 years (n = 334) | Survived ≥ 8 years (n = 70) | p-valueb |
|---|---|---|---|
| Age (years) | 77.2 (6.6) [0] | 74.9 (6.0) [0] | 0.0048 |
| Number of males (%) | 287 (86) [0] | 60 (86) [0] | 0.96 |
| AAA diameter (cm) | 6.7 (1.0) [0] | 6.6 (1.1) [0] | 0.021 |
| BMI (kg/m2) | 26.2 (4.7) [2] | 27.9 (4.7) [0] | 0.0078 |
| Diabetes (%) | 47 (14) [3] | 12 (17) [1] | 0.50 |
| Smoking status (%) | [0] | [0] | 0.97 |
| Current | 58 (17) | 12 (17) | |
| Past | 255 (76) | 53 (76) | |
| Never | 21 (6) | 5 (7) | |
| History of cardiac diseasec (%) | 233 (70) [0] | 52 (74) [0] | 0.45 |
| Systolic blood pressure (mmHg) | 139 (21) [0] | 139 (24) [0] | 0.93 |
| Diastolic blood pressure (mmHg) | 79 (12) [3] | 80 (12) [0] | 0.41 |
| ABPI (mean of both legs) | 0.98 (0.20) [24] | 1.00 (0.21) [1] | 0.59 |
| Forced expiration volume in 1 second (l) | 1.6 (0.7) [10] | 1.9 (0.7) [1] | 0.0047 |
| Serum creatinine level (µmol/l)a | 110 (94–141) [1] | 105 (87–119) [1] | 0.014 |
| Serum cholesterol level (mmol/l) | 4.8 (1.1) [19] | 4.8 (1.3) [1] | 0.97 |
| Statin use (%) | 138 (41) [1] | 30 (43) [0] | 0.83 |
| Aspirin use (%) | 186 (56) [1] | 42 (60) [0] | 0.53 |
Discussion of EVAR trial 2
Findings from these EVAR-2 results have shown that the benefit of EVAR in terms of aneurysm-related mortality persists throughout follow-up. However, although some patients (< 10%) survive to 12 years, either with or without aneurysm repair, the majority of EVAR-2 patients had a limited life expectancy, and hence at no time does aneurysm repair confer an overall survival benefit. Given the rather limited clinical and imaging follow-up of these patients beyond 8 years, aneurysm-related mortality could have been underestimated during the final few years of follow-up and, therefore, is a less reliable outcome for EVAR-2 than for EVAR-1 and the magnitude of the difference in aneurysm-related mortality appears to be unchanged between 8 and 12 years after randomisation. Our earlier interpretation, that it is advisable to take time to optimise patient fitness before considering EVAR, continues to hold up. By the end of patient follow-up at least 71 of 207 (34%) patients assigned to no intervention had undergone aneurysm repair. Some patients had their aneurysm repair many years after randomisation and over 12 years of follow-up there was no difference in either survival or average life-years between the randomised groups.
Inspection of the baseline characteristics of the long-term survivors suggested ‘the survival of the fittest’ phenomenon. The long-term survivors had notably better baseline lung function than the remainder, which might have impacted on the ability of the long-term survivors to cope well with general anaesthesia. During the randomisation period, many of the EVAR procedures are likely to have been conducted under general anaesthesia, although today there is more widespread use of local anaesthesia in physically frail patients. The differences in creatinine levels may be of less significance, as with their younger age and higher BMI, the glomerular filtration rates might be quite similar for the long-term survivors and the remainder. Aneurysm diameter is an accepted indicator of the risk of cardiovascular mortality, so that the patients with lower baseline aneurysm diameter are likely to have been at lower risk of cardiovascular death. 163–165
Although longer follow-up shows benefits in terms of aneurysm-related mortality, primarily through prevention of late aneurysm rupture, patients in this trial had a limited life expectancy, regardless of whether the aneurysm was repaired or no intervention was performed. Life expectancy was estimated to be 4.2 years in both the EVAR and no-intervention groups. By 1 September 2009, only 98 patients had survived, and by 30 June 2015 only 23 patients were still alive. The clinical factors leading to the assessment that these patients were physically ineligible for OR seem likely to have contributed to a high subsequent rate of death from any cause. This rate was not influenced by assignment to EVAR.
Beyond 8 years of follow-up, there were no reinterventions recorded in the EVAR group and just three reinterventions in the group randomised to no repair. By September 2009 there were so few EVAR-2 patients surviving that, given the need to provide robust justification to obtain ethics approval for using administrative data, HES data were not pursued for EVAR-2 patients. In hindsight, this might have been a mistake. HES data may have allowed us to capture further primary aneurysm repairs as well as more reinterventions, including those carried out at non-trial hospitals, and better define the cause of late deaths. However, the main reason for the low number of reinterventions is the attrition as a result of high mortality in EVAR-2, leaving less time for graft-related complications to develop. Throughout the trial, one might have expected a lower reintervention rate than in EVAR-1, as EVAR-2 patients were frailer and less fit. However, up to September 2009 patient fitness did not seem to have influenced the decision to intervene, with a similar reintervention rate in both EVAR groups of EVAR-1 and EVAR-2. In late follow-up from September 2009, in those patients that underwent endovascular repair (including a considerable number of patients in the no-intervention group that had aneurysm repair), it is likely that reinterventions, even when deemed necessary, may not have been performed because of physical frailty.
Like EVAR-1, EVAR-2 used principally early-generation endografts; later iterations of grafts would now be the commonly used. The long-term durability of these later iterations of endografts has not been evaluated, but it is hoped that they would be associated with lower rates of complications and reinterventions.
It is perhaps unsurprising that long-term survival is associated with younger age, smaller aneurysm and better lung function at baseline. It is thus likely that future selection for EVAR in the relatively frail might likely be influenced by age, size of aneurysm and respiratory status.
Chapter 10 Conclusions and recommendations
Suggestions of possible implications on practice and local health-care delivery
EVAR trial 1 was performed in the UK as a collaborative venture between the two disciplines of vascular surgery and interventional radiology, which worked well for elective repair of the abdominal aorta by endovascular methods. Recruitment was ahead of schedule both because of this high-level national support for the EVAR trials and also because Sir Miles Irving linked NIHR funding of EVAR to subventions which were triggered only by randomisation with local freedom of choice of EVAR manufacturer device.
The results have been segmented into four periods: 0–6 months, 6 months–years, 4–8 years and > 8 years. From a benefit in mortality in favour of the EVAR group up to 6 months, beyond 4 years this was counterbalanced by an increase in aneurysm-related mortality, the difference being most marked after 8 years. In terms of total mortality, there was benefit in favour of EVAR during the first 6 months. Groups were similar between 6 months and 8 years, but after 8 years total mortality was significantly worse in the EVAR group than in the OR group.
Reinterventions were greater in the EVAR group throughout EVAR-1 in all periods, and there was never a time when there was evidence that the interventions were expected to be so low that follow-up surveillance could stop.
Despite warnings to the trial centres to recommend ongoing annual clinical follow-up and imaging, this fell away. Even though many centres switched from CT to ultrasound, this did not stop the reduction of enthusiasm to follow patients at this late stage.
In EVAR-2 after 10 years there was poor clinical follow-up and we did not use HES to track reinterventions. This was because the number of patients alive at that stage was very small. However, we had robust data on mortality and our evidence shows superior aneurysm-related mortality in favour of EVAR increasing with time, but there was no difference in the groups in terms of all-cause mortality.
The IPD meta-analysis confirms the advantage of lower mortality in the EVAR group in the first 6 months and provides some new insight of how this early advantage of the EVAR group is eroded (aneurysm-related mortality and inclusion of those with peripheral arterial disease). Additionally, we identified two subgroups in which mortality was not lower at any time, suggesting that these groups (patients with moderate renal dysfunction and established coronary artery disease) may benefit from improved perioperative care, especially if undergoing EVAR. Surveillance must focus on reducing aneurysm-related deaths in the mid-term and longer term, particularly deaths resulting from reinterventions and secondary ruptures after EVAR.
In the IPD meta-analysis there was no overall evidence that type II endoleak in itself is associated with a higher rate of mortality, although, as previously shown, type II endoleak as part of the ‘cluster’ of complications is associated with secondary rupture. However, this suggests that it is other complications that are listed in the ‘cluster’ that are sinister and not type II endoleaks alone. The ‘cluster’ included definition of the type II endoleak with sac expansion and it seems that sac expansion is the important factor here. The findings here on type II endoleak might suggest that the enthusiasm to correct type II endoleak when on its own is misplaced. On the other hand, when type II endoleak is associated the so-called ‘cluster’ it is far from a benign condition. In addition, type II endoleak can be wrongly diagnosed and a more severe endoleak, such as types IA or IB, can be at the root of the problem.
The mean difference in costs over 14 years was £3798 (95% CI of £2338 to £5258). Decision modelling based on EVAR-1 showed that the lifetime difference in cost was £3616 and the difference in QALYs was 0.018, with a cost per QALY of £202,776. The cost per QALY exceeds conventional thresholds used in the UK. The DREAM and ACE trials (conducted in the Netherlands and France, respectively) found similar results. If EVAR is to be considered a cost-effective use of NHS resources, it needs to demonstrate fewer reinterventions and fewer late aneurysm deaths than were observed in the EVAR trial.
Patterns of sac growth, if modelled correctly, can be useful predictors for future serious rupture events. Estimates of sac expansion should be derived from longitudinal modelling with a sac diameter trajectory, which accounts for all previous measurements and thus produces more accurate estimates of current sac growth. Naive estimates of growth based on the last two observed diameter measurements are too crude to be of critical use in a prediction model. The current sac diameter was not found to be an independent risk factor of rupture after accounting for the growth rate of the sac and the predictive accuracy of the model just using sac diameter was lower than a model that used sac growth.
Recommendations for future research
To pursue annual follow-up with appropriate imaging is the first priority, although a simple switch from annual CT scanning (with associated radiation exposure) to ultrasound is in itself not enough for follow-up to be continued adequately.
As EVAR is more costly over the patient’s lifetime, an earlier discharge from secondary care to simple follow-up in primary care might be a worthwhile aim in the future. In order for EVAR to be considered effective and cost-effective, an area of further research is to find better ways to target reintervention of patients who are at risk of secondary rupture and avoid reintervention in patients at very low risk. Our early findings suggest that an algorithm could be developed based on annual measurements of aortic sac diameter only. This might have excellent predictive value for future rupture. If effective, it would need substantial validation on a separate cohort of patients.
The long-term findings of EVAR-2 present an ethical problem for consideration. Our evidence is that endovascular repair does not alter mortality from all causes in any way, but EVAR can lower aneurysm-related mortality. It is entirely possible that greater enthusiasm to perform endovascular repair may re-emerge and a view on the attitudes to this will become important.
The determination of which frail patients merit aortic repair needs further determination. We see early encouragement that such a cohort could be defined. It would need to be clinically tested. This may be the only realistic way that EVAR could become effective and cost-effective.
Acknowledgements
We thank Sir Miles Irving, Director of the NIHR HTA programme in 1993–9, for championing the funding of devices solely within the context of a RCT; and Louise Brown, who was the EVAR-1 and EVAR-2 trial manager in 1999–2001, and Philip Poole Wilson, chairperson of the DMEC 1999–2009, in helping to achieve ongoing funding. We would like to thank the principal investigators of the RCTs who were involved in the IPD meta-analysis: Professor Jan Blankensteijn (DREAM trial), Professor Frank Lederle (OVER trial) and Professor Jean-Pierre Becquemin (ACE trial). We are grateful to Dr Pinar Ulug who meticulously combined the data from the four trials.
These trials are a collaborative effort and their success is a result of the enormous enthusiasm and support from all of the committee members and participating centres. A full list of EVAR trial participants is given in Appendix 2.
Contributions of authors
Rajesh Patel drafted and compiled the report as trial manager, referring always to the other authors.
Janet T Powell critically reviewed the manuscript and added value at every stage.
Michael J Sweeting drafted all statistical methods as he performed all statistical analyses. He also drafted Chapter 8 with Jessica K Barrett and critically reviewed the manuscript.
David M Epstein performed cost and cost-effectiveness analyses, and drafted Chapters 4 and 5.
Jessica K Barrett performed analysis for Chapter 8 and co-drafted this chapter with Michael J Sweeting.
Roger M Greenhalgh had overall responsibility, co-ordination and supervision, with special responsibility for clinical issues, and he edited the report.
Publications
Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004;27:372–81.
EVAR Trial Participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843–8.
EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179–86.
EVAR Trial Participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187–92.
Greenhalgh RM, Brown LC, Powell JT. High risk and unfit for open repair are not the same. Eur J Vasc Endovasc Surg 2007;34:154–5.
Brown LC, Greenhalgh RM, Howell S, Powell JT, Thompson SG. Patient fitness and survival after abdominal aortic aneurysm repair in patients from the UK EVAR trials. Br J Surg 2007;94:709–16.
Brown LC, Greenhalgh RM, Kwong GP, Powell JT, Thompson SG, Wyatt MG. Secondary interventions and mortality following endovascular aortic aneurysm repair: device-specific results from the UK EVAR trials. Eur J Vasc Endovasc Surg 2007;34:281–90.
Powell JT, Brown LC, Greenhalgh RM, Thompson SG. The rupture rate of large abdominal aortic aneurysms: is this modified by anatomical suitability for endovascular repair? Ann Surg 2008;247:173–9.
Epstein DM, Sculpher MJ, Manca A, Michaels J, Thompson SG, Brown LC, et al. Modelling the long-term cost-effectiveness of endovascular or open repair for abdominal aortic aneurysm. Br J Surg 2008;95:183–90.
Rodway AD, Powell JT, Brown LC, Greenhalgh RM. Do abdominal aortic aneurysm necks increase in size faster after endovascular than open repair? Eur J Vasc Endovasc Surg 2008;35:685–93.
Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG, on behalf of the UK EVAR Trial Participants. Renal function and abdominal aortic aneurysm: the impact of different management strategies on long-term renal function in the UK EndoVascular Aneurysm Repair (EVAR) Trials. Ann Surg 2010;251:966–75.
Brown LC, Greenhalgh RM, Thompson SG, Powell JT. Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair? Results from the randomised EVAR Trial 2. Eur J Vasc Endovasc Surg 2010;39:396–402.
Brown LC, Greenhalgh RM, Powell JT, Thompson SG. Use of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2010;97:1207–17.
The UK EVAR Trial Investigators. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010;362:1872–80.
The UK EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863–71.
Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR Trials. Ann Surg 2010;252:805–12.
Brown LC, Thompson SG, Greenhalgh RM, Powell JT, on behalf of the EVAR Trial participants. Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm: results from the randomised EVAR trial 1. Br J Surg 2011;98:935–42.
Epstein D, Sculpher MJ, Powell JT, Thompson SG, Brown LC, Greenhalgh RM. Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysm based on four randomized clinical trials. Br J Surg 2014;101:623–31.
Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16(9).
Wyss TR, Dick F, Brown LC, Greenhalgh RM. The influence of thrombus, calcification, angulation, and tortuosity of attachment sites on the time to the first graft-related complication after endovascular aneurysm repair. J Vasc Surg 2011;54:965–71.
Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, for the EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366–74.
Powell JT, Sweeting MJ, Ulug P, Blankensteijn JD, Lederle FA, Becquemin JP, Greenhalgh RM. Endovascular or open repair for abdominal aortic aneurysm over 5 years: Individual patient data meta-analysis from EVAR 1 DREAM, OVER and ACE trials. Br J Surg 2017;104:166–78.
Sweeting MJ, Patel R, Powell JT, Greenhalgh RM. Endovascular repair of abdominal aortic aneurysm in patients physically ineligible for open repair: very long-term follow-up in the EVAR-2 randomized controlled trial. Ann Surg 2017;266:713–19.
IMPROVE Trial Investigators. Comparative clinical effectiveness and cost effectiveness of endovascular strategy vs. open repair for ruptured abdominal aortic aneurysm: three year results of the IMPROVE randomised trial. BMJ 2017;359:4859.
Data sharing statement
Any requests for data should be sent to the corresponding author, Professor Roger Greenhalgh.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Collin J, Araujo L, Walton J, Lindsell D. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet 1988;2:613-15. https://doi.org/10.1016/S0140-6736(88)90649-6.
- Sonesson B, Länne T, Hansen F, Sandgren T. Infrarenal aortic diameter in the healthy person. Eur J Vasc Surg 1994;8:89-95. https://doi.org/10.1016/S0950-821X(05)80127-6.
- Wanhainen A, Themudo R, Ahlstrom H, Lind L, Johansson L. Thoracic and abdominal aortic dimension in 70-year-old men and women – a population-based whole-body magnetic resonance imaging (MRI) study. J Vasc Surg 2008;47:504-12. https://doi.org/10.1016/j.jvs.2007.10.043.
- Greenhalgh RM, Rosengarten DS, Mervart I, Lewis B, Calnan JS, Martin P. Serum lipids and lipoproteins in peripheral vascular disease. Lancet 1971;2:947-50. https://doi.org/10.1016/S0140-6736(71)90269-8.
- Thomas ML. Arteriomegaly. Br J Surg 1971;58:690-4. https://doi.org/10.1002/bjs.1800580915.
- Fowkes FG, Macintyre CC, Ruckley CV. Increasing incidence of aortic aneurysms in England and Wales. BMJ 1989;298:33-5. https://doi.org/10.1136/bmj.298.6665.33.
- Filipovic M, Goldacre MJ, Roberts SE, Yeates D, Duncan ME, Cook-Mozaffari P. Trends in mortality and hospital admission rates for abdominal aortic aneurysm in England and Wales, 1979-1999. Br J Surg 2005;92:968-75. https://doi.org/10.1002/bjs.5118.
- Choke E, Vijaynagar B, Thompson J, Nasim A, Bown MJ, Sayers RD. Changing epidemiology of abdominal aortic aneurysms in England and Wales: older and more benign?. Circulation 2012;125:1617-25. https://doi.org/10.1161/CIRCULATIONAHA.111.077503.
- Heather BP, Poskitt KR, Earnshaw JJ, Whyman M, Shaw E. Population screening reduces mortality rate from aortic aneurysm in men. Br J Surg 2000;87:750-3. https://doi.org/10.1046/j.1365-2168.2000.01476.x.
- Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg 1995;82:1066-70. https://doi.org/10.1002/bjs.1800820821.
- Earnshaw JJ. Doubts and dilemmas over abdominal aortic aneurysm. Br J Surg 2011;98:607-8. https://doi.org/10.1002/bjs.7495.
- Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011;124:1118-23. https://doi.org/10.1161/CIRCULATIONAHA.111.030379.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012;43:161-6. https://doi.org/10.1016/j.ejvs.2011.11.014.
- Schwarze ML, Shen Y, Hemmerich J, Dale W. Age-related trends in utilization and outcome of open and endovascular repair for abdominal aortic aneurysm in the United States, 2001-2006. J Vasc Surg 2009;50:722-9.e2. https://doi.org/10.1016/j.jvs.2009.05.010.
- Norman PE, Spilsbury K, Semmens JB. Falling rates of hospitalization and mortality from abdominal aortic aneurysms in Australia. J Vasc Surg 2011;53:274-7. https://doi.org/10.1016/j.jvs.2010.08.087.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011;98:645-51. https://doi.org/10.1002/bjs.7461.
- Powell JT, Adamson J, MacSweeney ST, Greenhalgh RM, Humphries SE, Henney A. Genetic variants of collagen III and abdominal aortic aneurysm. Eur J Vasc Surg 1991;5:145-8. https://doi.org/10.1016/S0950-821X(05)80679-6.
- Powell JT, MacSweeney ST, Greenhalgh RM, Turner RJ, Henney AM. Interaction between fibrillin genotype and blood pressure and the development of aneurysmal disease. Ann N Y Acad Sci 1996;800:198-207. https://doi.org/10.1111/j.1749-6632.1996.tb33310.x.
- Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic-aneurysm. Arterioscl Throm Vasc Biol 1995;15:1145-51. https://doi.org/10.1161/01.ATV.15.8.1145.
- Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø study. Am J Epidemiol 2001;154:236-44. https://doi.org/10.1093/aje/154.3.236.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003;348:1895-901. https://doi.org/10.1056/NEJMcp012641.
- Gillum RF. Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol 1995;48:1289-98. https://doi.org/10.1016/0895-4356(95)00045-3.
- Masuda EM, Caps MT, Singh N, Yorita K, Schneider PA, Sato DT, et al. Effect of ethnicity on access and device complications during endovascular aneurysm repair. J Vasc Surg 2004;40:24-9. https://doi.org/10.1016/j.jvs.2004.02.035.
- Cheng SW, Ting AC, Ho P, Poon JT. Aortic aneurysm morphology in Asians: features affecting stent-graft application and design. J Endovasc Ther 2004;11:605-12. https://doi.org/10.1583/04-1268R.1.
- Lederle FA, Johnson GR, Wilson SE. Aneurysm Detection and Management Veterans Affairs Cooperative Study . Abdominal aortic aneurysm in women. J Vasc Surg 2001;34:122-6. https://doi.org/10.1067/mva.2001.115275.
- Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873-U2893. https://doi.org/10.1093/eurheartj/ehu281.
- Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation 2007;115:2865-9. https://doi.org/10.1161/CIRCULATIONAHA.106.671859.
- Stenbaek J, Granath F, Swedenborg J. Outcome after abdominal aortic aneurysm repair. Difference between men and women. Eur J Vasc Endovasc Surg 2004;28:47-51. https://doi.org/10.1016/j.ejvs.2004.02.013.
- Mofidi R, Goldie VJ, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg 2007;94:310-14. https://doi.org/10.1002/bjs.5573.
- Hultgren R, Granath F, Swedenborg J. Different disease profiles for women and men with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2007;33:556-60. https://doi.org/10.1016/j.ejvs.2006.11.030.
- Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg 1999;30:1099-105. https://doi.org/10.1016/S0741-5214(99)70049-2.
- Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg 2003;38:329-34. https://doi.org/10.1016/S0741-5214(03)00136-8.
- Golledge J, Norman PE. Atherosclerosis and abdominal aortic aneurysm: cause, response, or common risk factors?. Arterioscl Throm Vasc Biol 2010;30:1075-7. https://doi.org/10.1161/ATVBAHA.110.206573.
- Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health 2004;14:343-9. https://doi.org/10.1093/eurpub/14.4.343.
- Fowkes FG, Anandan CL, Lee AJ, Smith FB, Tzoulaki I, Rumley A. Reduced lung function in patients with abdominal aortic aneurysm is associated with activation of inflammation and hemostasis, not smoking or cardiovascular disease. J Vasc Surg 2006;43:474-80. https://doi.org/10.1016/j.jvs.2005.11.018.
- Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg 2000;87:195-200. https://doi.org/10.1046/j.1365-2168.2000.01353.x.
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997;126:441-9. https://doi.org/10.7326/0003-4819-126-6-199703150-00004.
- Powell JT, Greenhalgh RM. Multifactorial inheritance of abdominal aortic aneurysm. Eur J Vasc Surg 1987;1:29-31. https://doi.org/10.1016/S0950-821X(87)80020-8.
- Larsson E, Granath F, Swedenborg J, Hultgren R. A population-based case–control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg 2009;49:47-50. https://doi.org/10.1016/j.jvs.2008.08.012.
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. UK Small Aneurysm Trial Participants . Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004;110:16-21. https://doi.org/10.1161/01.CIR.0000133279.07468.9F.
- Mattes E, Davis TM, Yang D, Ridley D, Lund H, Norman PE. Prevalence of abdominal aortic aneurysms in men with diabetes. Med J Aust 1997;166:630-3.
- Lee B, Godfrey M, Vitale E, Hori H, Mattei MG, Sarfarazi M, et al. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature 1991;352:330-4. https://doi.org/10.1038/352330a0.
- Irizarry E, Newman KM, Gandhi RH, Nackman GB, Halpern V, Wishner S, et al. Demonstration of interstitial collagenase in abdominal aortic aneurysm disease. J Surg Res 1993;54:571-4. https://doi.org/10.1006/jsre.1993.1087.
- Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest 1998;102:1900-10. https://doi.org/10.1172/JCI2182.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000;160:833-6. https://doi.org/10.1001/archinte.160.6.833.
- Guide to Clinical Preventive Services. Baltimore, MD: Williams & Wilkins; 1996.
- The UK Small Aneurysm Trial Participants . Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998;352:1649-55. https://doi.org/10.1016/S0140-6736(98)10137-X.
- Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD001835.pub4.
- Ingoldby CJ, Wujanto R, Mitchell JE. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. Br J Surg 1986;73:551-3. https://doi.org/10.1002/bjs.1800730711.
- The UK Small Aneurysm Trial Participants . Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. New Engl J Med 2002;346:1445-52. https://doi.org/10.1056/NEJMoa013527.
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 2000;160:1425-30. https://doi.org/10.1001/archinte.160.10.1425.
- Ouriel K, Clair DG, Kent KC, Zarins CK. Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators . Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010;51:1081-7. https://doi.org/10.1016/j.jvs.2009.10.113.
- Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG. SWAN Collaborative Group . Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg 2016;103:1097-104. https://doi.org/10.1002/bjs.10225.
- Wilmink AB, Forshaw M, Quick CR, Hubbard CS, Day NE. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen 2002;9:125-7. https://doi.org/10.1136/jms.9.3.125.
- Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999;17:472-5. https://doi.org/10.1053/ejvs.1999.0835.
- Ellis M, Powell JT, Greenhalgh RM. Limitations of ultrasonography in surveillance of small abdominal aortic aneurysms. Br J Surg 1991;78:614-16. https://doi.org/10.1002/bjs.1800780529.
- The UK. Small Aneurysm Trial Participants . The U.K. Small Aneurysm Trial: design, methods and progress. Eur J Vasc Endovasc Surg 1995;9:42-8. https://doi.org/10.1016/S1078-5884(05)80223-0.
- Scott RA, Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA. The long-term benefits of a single scan for abdominal aortic aneurysm (AAA) at age 65. Eur J Vasc Endovasc Surg 2001;21:535-40. https://doi.org/10.1053/ejvs.2001.1368.
- Lederle FA, Wilson SE, Johnson GR, Littooy FN, Acher C, Messina LM, et al. Design of the abdominal aortic Aneurysm Detection and Management Study. ADAM VA Cooperative Study Group. J Vasc Surg 1994;20:296-303. https://doi.org/10.1016/0741-5214(94)90019-1.
- Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg 2007;94:696-701. https://doi.org/10.1002/bjs.5780.
- Lindholt JS, Juul S, Henneberg EW. High-risk and low-risk screening for abdominal aortic aneurysm both reduce aneurysm-related mortality. A stratified analysis from a single-centre randomised screening trial. Eur J Vasc Endovasc Surg 2007;34:53-8. https://doi.org/10.1016/j.ejvs.2006.12.031.
- Lindholt JS, Norman P. Screening for abdominal aortic aneurysm reduces overall mortality in men. A meta-analysis of the mid- and long-term effects of screening for abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2008;36:167-71. https://doi.org/10.1016/j.ejvs.2008.03.006.
- Lindholt JS, Sorensen J, Sogaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010;97:826-34. https://doi.org/10.1002/bjs.7001.
- Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004;329. https://doi.org/10.1136/bmj.38272.478438.55.
- Thompson SG, Ashton HA, Gao L, Scott RA. Multicentre Aneurysm Screening Study Group . Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009;338. https://doi.org/10.1136/bmj.b2307.
- Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA. Multicentre Aneurysm Screening Study Group . Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 2012;99:1649-56. https://doi.org/10.1002/bjs.8897.
- Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD002945.pub2.
- Davis M, Harris M, Earnshaw JJ. Implementation of the National Health Service Abdominal Aortic Aneurysm Screening Program in England. J Vasc Surg 2013;57:1440-5. https://doi.org/10.1016/j.jvs.2012.10.114.
- Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med 1996;124:577-84. https://doi.org/10.7326/0003-4819-124-6-199603150-00007.
- Vega de Ceniga M, Gomez R, Estallo L, Rodriguez L, Baquer M, Barba A. Growth rate and associated factors in small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2006;31:231-6. https://doi.org/10.1016/j.ejvs.2005.10.007.
- Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2007;34:267-73. https://doi.org/10.1016/j.ejvs.2007.03.006.
- Guessous I, Periard D, Lorenzetti D, Cornuz J, Ghali WA. The efficacy of pharmacotherapy for decreasing the expansion rate of abdominal aortic aneurysms: a systematic review and meta-analysis. PLOS ONE 2008;3. https://doi.org/10.1371/journal.pone.0001895.
- Greenhalgh RM, Fowkes FG, Powell JT, Forbes JF, Ruckely CV. Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg 2000;19:636-42. https://doi.org/10.1053/ejvs.2000.1066.
- Rughani G, Robertson L, Clarke M. Medical treatment for small abdominal aortic aneurysms. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD009536.pub2.
- Dubost C, Allary M, Oeconomos N. Treatment of aortic aneurysms; removal of the aneurysm; re-establishment of continuity by grafts of preserved human aorta. Mem Acad Chir 1951;77:381-3.
- Prager MR, Hoblaj T, Nanobashvili J, Sporn E, Polterauer P, Wagner O, et al. Collagen- versus gelatine-coated Dacron versus stretch PTFE bifurcation grafts for aortoiliac occlusive disease: long-term results of a prospective, randomized multicenter trial. Surgery 2003;134:80-5. https://doi.org/10.1067/msy.2003.179.
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491-9. https://doi.org/10.1007/BF02015271.
- Volodos NL, Karpovich IP, Troyan VI, Kalashnikova Yu V, Shekhanin VE, Ternyuk NE, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. VASA Suppl 1991;33:93-5.
- Harris PL, Buth J, Mialhe C, Myhre HO, Norgren L. The need for clinical trials of endovascular abdominal aortic aneurysm stent-graft repair: The EUROSTAR Project. EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair. J Endovasc Surg 1997;4:72-9. https://doi.org/10.1583/1074-6218(1997)004<0072:TNFCTO>2.0.CO;2.
- Andrews SM, Cuming R, Macsweeney ST, Barrett NK, Greenhalgh RM, Nott DM. Assessment of feasibility for endovascular prosthetic tube correction of aortic aneurysm. Br J Surg 1995;82:917-19. https://doi.org/10.1002/bjs.1800820720.
- Yusuf SW, Baker DM, Hind RE, Chuter TA, Whitaker SC, Wenham PW, et al. Endoluminal transfemoral abdominal aortic aneurysm repair with aorto-uni-iliac graft and femorofemoral bypass. Br J Surg 1995;82. https://doi.org/10.1002/bjs.1800820719.
- Nasim A, Thompson MM, Sayers RD, Bolia A, Bell PR. Endovascular repair of abdominal aortic aneurysm: an initial experience. Br J Surg 1996;83:516-19. https://doi.org/10.1002/bjs.1800830428.
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41:S1-58. https://doi.org/10.1016/j.ejvs.2010.09.011.
- White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Ther 1997;4:152-68. https://doi.org/10.1177/152660289700400207.
- Veith FJ, Baum RA, Ohki T, Amor M, Adiseshiah M, Blankensteijn JD, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002;35:1029-35. https://doi.org/10.1067/mva.2002.123095.
- Hobo R, Buth J. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg 2006;43:896-902. https://doi.org/10.1016/j.jvs.2006.01.010.
- Schlosser FJ, Gusberg RJ, Dardik A, Lin PH, Verhagen HJ, Moll FL, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted?. Eur J Vasc Endovasc Surg 2009;37:15-22. https://doi.org/10.1016/j.ejvs.2008.10.011.
- White GH, May J, Waugh RC, Chaufour X, Yu W. Type III and type IV endoleak: toward a complete definition of blood flow in the sac after endoluminal AAA repair. J Endovasc Surg 1998;5:305-9. https://doi.org/10.1583/1074-6218(1998)005<0305:TIATIE>2.0.CO;2.
- Rosen RJ, Green RM. Endoleak management following endovascular aneurysm repair. J Vasc Interv Radiol 2008;19:S37-43. https://doi.org/10.1016/j.jvir.2008.01.017.
- Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048-60. https://doi.org/10.1067/mva.2002.123763.
- Tonnessen BH, Sternbergh WC, Money SR. Late problems at the proximal aortic neck: migration and dilation. Semin Vasc Surg 2004;17:288-93. https://doi.org/10.1053/j.semvascsurg.2004.09.005.
- Tonnessen BH, Sternbergh WC, Money SR. Mid- and long-term device migration after endovascular abdominal aortic aneurysm repair: a comparison of AneuRx and Zenith endografts. J Vasc Surg 2005;42:392-400. https://doi.org/10.1016/j.jvs.2005.05.040.
- Fransen GA, Desgranges P, Laheij RJ, Harris PL, Becquemin JP. EUROSTAR Collaborators . Frequency, predictive factors, and consequences of stent-graft kink following endovascular AAA repair. J Endovasc Ther 2003;10:913-18. https://doi.org/10.1177/152660280301000511.
- The UK EVAR Trial Investigators . Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71. https://doi.org/10.1056/NEJMoa0909305.
- The UK EVAR Trial Investigators . Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010;362:1872-80. https://doi.org/10.1056/NEJMoa0911056.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16090.
- De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1881-9. https://doi.org/10.1056/NEJMoa0909499.
- Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Kohler TR, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med 2012;367:1988-97. https://doi.org/10.1056/NEJMoa1207481.
- Becquemin JP, Pillet JC, Lescalie F, Sapoval M, Goueffic Y, Lermusiaux P, et al. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low- to moderate-risk patients. J Vasc Surg 2011;53:1167-73. https://doi.org/10.1016/j.jvs.2010.10.124.
- Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR Trial Participants . Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843-8. https://doi.org/10.1016/S0140-6736(04)16979-1.
- EVAR Trial Participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. https://doi.org/10.1016/S0140-6736(05)66627-5.
- Prinssen M, Buskens E, Blankensteijn JD. The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial. Background, design and methods. J Cardiovasc Surg 2002;43:379-84.
- Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607-18. https://doi.org/10.1056/NEJMoa042002.
- Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 2005;352:2398-405. https://doi.org/10.1056/NEJMoa051255.
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Matsumura JS, Kohler TR. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 2009;302:1535-42. https://doi.org/10.1001/jama.2009.1426.
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004;27:372-81. https://doi.org/10.1016/j.ejvs.2003.12.019.
- EVAR Trial Participants . Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187-92. https://doi.org/10.1016/S0140-6736(05)66628-7.
- Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med 2015;373:328-38. https://doi.org/10.1056/NEJMoa1405778.
- Khashram M, Jenkins JS, Jenkins J, Kruger AJ, Boyne NS, Foster WJ, et al. Long-term outcomes and factors influencing late survival following elective abdominal aortic aneurysm repair: a 24-year experience. Vascular 2016;24:115-25. https://doi.org/10.1177/1708538115586682.
- Koole D, Moll FL, Buth J, Hobo R, Zandvoort HJ, Bots ML, et al. Annual rupture risk of abdominal aortic aneurysm enlargement without detectable endoleak after endovascular abdominal aortic repair. J Vasc Surg 2011;54:1614-22. https://doi.org/10.1016/j.jvs.2011.06.095.
- Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR Trials. Ann Surg 2010;252:805-12. https://doi.org/10.1097/SLA.0b013e3181fcb44a.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Little R, Rubin D. Statistical Analysis With Missing Data. Hoboken, NJ: John Wiley & Sons; 2002.
- Clark TG, Altman DG. Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol 2003;56:28-37. https://doi.org/10.1016/S0895-4356(02)00539-5.
- Royston P. Multiple imputation of missing values: update of ice. Stata J 2005;5:527-36.
- Moons KG, Donders RA, Stijnen T, Harrell FE. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006;59:1092-101. https://doi.org/10.1016/j.jclinepi.2006.01.009.
- White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982-98. https://doi.org/10.1002/sim.3618.
- Verzini F, Isernia G, De Rango P, Simonte G, Parlani G, Loschi D, et al. Abdominal aortic endografting beyond the trials: a 15-year single-center experience comparing newer to older generation stent-grafts. J Endovasc Ther 2014;21:439-47. https://doi.org/10.1583/13-4599MR.1.
- Motaganahalli R, Martin A, Feliciano B, Murphy MP, Slaven J, Dalsing MC. Estimating the risk of solid organ malignancy in patients undergoing routine computed tomography scans after endovascular aneurysm repair. J Vasc Surg 2012;56:929-37. https://doi.org/10.1016/j.jvs.2012.02.061.
- Reise JA, Sheldon H, Earnshaw J, Naylor AR, Dick F, Powell JT, et al. Patient preference for surgical method of abdominal aortic aneurysm repair: postal survey. Eur J Vasc Endovasc Surg 2010;39:55-61. https://doi.org/10.1016/j.ejvs.2009.08.008.
- Ellozy SH, Carroccio A, Lookstein RA, Minor ME, Sheahan CM, Juta J, et al. First experience in human beings with a permanently implantable intrasac pressure transducer for monitoring endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2004;40:405-12. https://doi.org/10.1016/j.jvs.2004.06.027.
- Ellozy SH, Carroccio A, Lookstein RA, Jacobs TS, Addis MD, Teodorescu VJ, et al. Abdominal aortic aneurysm sac shrinkage after endovascular aneurysm repair: correlation with chronic sac pressure measurement. J Vasc Surg 2006;43:2-7. https://doi.org/10.1016/j.jvs.2005.09.039.
- Milner R, Kasirajan K, Chaikof EL. Future of endograft surveillance. Semin Vasc Surg 2006;19:75-82. https://doi.org/10.1053/j.semvascsurg.2006.03.002.
- Ohki T, Ouriel K, Silveira PG, Katzen B, White R, Criado F, et al. Initial results of wireless pressure sensing for endovascular aneurysm repair: the APEX Trial – Acute Pressure Measurement to Confirm Aneurysm Sac Exclusion. J Vasc Surg 2007;45:236-42. https://doi.org/10.1016/j.jvs.2006.09.060.
- Kurosawa K, Ohta H, Sumi M, Ohki T. Physiologic monitoring data: will it change our follow-up paradigms?. Semin Vasc Surg 2007;20:115-20. https://doi.org/10.1053/j.semvascsurg.2007.04.002.
- NHS Reference Costs 2014 to 2015. London: Department of Health; 2015.
- HRG4+ 201415 Reference Costs Grouper User Manual. London: NHS Digital; 2015.
- Organisation for Economic Co-operation and Development . Purchasing Power Parities for GDP and Related Indicators 2015 2015.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45. https://doi.org/10.1002/sim.1794.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Epstein D, Sculpher MJ, Powell JT, Thompson SG, Brown LC, Greenhalgh RM. Long-term cost-effectiveness analysis of endovascular versus open repair for abdominal aortic aneurysm based on four randomized clinical trials. Br J Surg 2014;101:623-31. https://doi.org/10.1002/bjs.9464.
- Office for National Statistics . National Life Tables, United Kingdom: 2012–2014 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2015-09-23.
- Brown LC, Thompson SG, Greenhalgh RM, Powell JT. Endovascular Aneurysm Repair Trial Participants . Incidence of cardiovascular events and death after open or endovascular repair of abdominal aortic aneurysm in the randomized EVAR trial 1. Br J Surg 2011;98:935-42. https://doi.org/10.1002/bjs.7485.
- Epstein D. Health Related Quality of Life After Surgical Repair of Asymptomatic Abdominal Aortic Aneurysms. Granada: University of Granada; 2016.
- Endovascular Stent–Grafts for the Treatment of Abdominal Aortic Aneurysms. London: National Institute for Health and Care Excellence; 2009.
- Prinssen M, Buskens E, de Jong SE, Buth J, Mackaay AJ, van Sambeek MR, et al. Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2007;46:883-90. https://doi.org/10.1016/j.jvs.2007.07.033.
- Stroupe KT, Lederle FA, Matsumura JS, Kyriakides TC, Jonk YC, Ge L, et al. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg 2012;56:901-9. https://doi.org/10.1016/j.jvs.2012.01.086.
- Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev 2014;1. https://doi.org/10.1002/14651858.CD004178.pub2.
- Cronenwett JL. Endovascular aneurysm repair: important mid-term results. Lancet 2005;365:2156-8. https://doi.org/10.1016/S0140-6736(05)66629-9.
- Kontopodis N, Antoniou SA, Georgakarakos E, Ioannou CV. Endovascular vs open aneurysm repair in the young: systematic review and meta-analysis. J Endovasc Ther 2015;22:897-904. https://doi.org/10.1177/1526602815606937.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461-70. https://doi.org/10.7326/0003-4819-130-6-199903160-00002.
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012;308:1795-801. https://doi.org/10.1001/jama.2012.14312.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982;10:1100-20. https://doi.org/10.1214/aos/1176345976.
- Mani K, Björck M, Wanhainen A. Changes in the management of infrarenal abdominal aortic aneurysm disease in Sweden. Br J Surg 2013;100:638-44. https://doi.org/10.1002/bjs.9046.
- Sumner DS, Strandness DE. The relationship between calf blood flow and ankle blood pressure in patients with intermittent claudication. Surgery 1969;65:763-71.
- Heald C, Fowkes F, Murray G, Price J. Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle/brachial index: systematic review. Atherosclerosis 2006;189:61-9. https://doi.org/10.1016/j.atherosclerosis.2006.03.011.
- Saratzis A, Sarafidis P, Melas N, Saratzis N, Kitas G. Impaired renal function is associated with mortality and morbidity after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2013;58:879-85. https://doi.org/10.1016/j.jvs.2013.03.036.
- Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg 2009;50:880-96. https://doi.org/10.1016/j.jvs.2009.07.001.
- Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg 2006;20:69-74. https://doi.org/10.1007/s10016-005-9382-z.
- Karthikesalingam A, Thrumurthy SG, Jackson D, Choke E, Sayers, RD, Loftus IM, et al. Current evidence is insufficient to define an optimal threshold for intervention in isolated type II endoleak after endovascular aneurysm repair. J Endovasc Ther 2012;19:200-8. https://doi.org/10.1583/11-3762R.1.
- Patatas K, Ling L, Dunning J, Shrivastava V. Static sac size with a type II endoleak post-endovascular abdominal aortic aneurysm repair: surveillance or embolization?. Interact Cardiovasc Thorac Surg 2012;15:462-6. https://doi.org/10.1093/icvts/ivs201.
- Cao P, De Rango P, Verzini F, Parlani G. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg 2010;51:53-69.
- Jonker FH, Aruny J, Muhs BE. Management of type II endoleaks: preoperative versus postoperative versus expectant management. Semin Vasc Surg 2009;22:165-71. https://doi.org/10.1053/j.semvascsurg.2009.07.008.
- Keedy AW, Yeh BM, Kohr JR, Hiramoto JS, Schneider DB, Breiman RS. Evaluation of potential outcome predictors in type II endoleak: a retrospective study with CT angiography feature analysis. AJR Am J Roentgenol 2011;197:234-40. https://doi.org/10.2214/AJR.10.4566.
- Brown A, Saggu GK, Bown MJ, Sayers RD, Sidloff DA. Type II endoleaks: challenges and solutions. Vasc Health Risk Manag 2016;12:53-6. https://doi.org/10.2147/VHRM.S81275.
- Gandhi RT, Bryce Y, Ganguli S, McWilliams J, Vatakencherry G. Management of type II endoleaks. Endovascular Today 2016;15:42-8.
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999;28:964-74. https://doi.org/10.1093/ije/28.5.964.
- Egorova N, Giacovelli JK, Gelijns A, Greco G, Moskowitz A, McKinsey J, et al. Defining high-risk patients for endovascular aneurysm repair. J Vasc Surg 2009;50:1271-9. https://doi.org/10.1016/j.jvs.2009.06.061.
- De Martino RR, Goodney PP, Nolan BW, Robinson WP, Farber A, Patel VI, et al. Optimal selection of patients for elective abdominal aortic aneurysm repair based on life expectancy. J Vasc Surg 2013;58:589-95. https://doi.org/10.1016/j.jvs.2013.03.010.
- Brady AR, Fowkes FG, Thompson SG, Powell JT. Aortic aneurysm diameter and risk of cardiovascular mortality. Arterioscler Thromb Vasc Biol 2001;21:1203-7. https://doi.org/10.1161/hq0701.091999.
- Freiberg MS, Arnold AM, Newman AB, Edwards MS, Kraemer KL, Kuller LH. Abdominal aortic aneurysms, increasing infrarenal aortic diameter, and risk of total mortality and incident cardiovascular disease events: 10-year follow-up data from the Cardiovascular Health Study. Circulation 2008;117:1010-17. https://doi.org/10.1161/CIRCULATIONAHA.107.720219.
- Norman PE, Muller J, Golledge J. The cardiovascular and prognostic significance of the infrarenal aortic diameter. J Vasc Surg 2011;54:1817-20. https://doi.org/10.1016/j.jvs.2011.07.048.
Appendix 1 Case record form
Appendix 2 The UK EndoVascular Aneurysm Repair trial participants
Trial investigators
Grant applicants
RM Greenhalgh (principal investigator), JT Powell, MJ Sweeting, M Sculpher, D Epstein and CD Bicknell.
Trial Management Committee
RM Greenhalgh, MD (chairperson); JT Powell, MD; MJ Sweeting, PhD; D Epstein, PhD; CD Bicknell, MD; R Von-Allmen, MD; TR Wyss, MD; and N Burfitt, FRCR.
Trial Steering Committee
RJ Lilford (chairperson), RM Greenhalgh, M Wyatt, SG Thompson and M Sculpher.
Data Monitoring and Ethics Committee
G Fowkes (chairperson), R Morgan and B Campbell.
Endpoint Committee
JT Powell (chairperson), A Halliday and S Gibbs.
Data and Trial Management
R Patel (trial manager) and M Kaderbhai (data manager).
Trial Manager (1999–2010)
LC Brown.
Statistical and costs analyses
MJ Sweeting and D Epstein.
Regional Trial Investigators Committee
(Numbers in parentheses indicate the number of patients entered into both EVAR-1 and EVAR-2.)
K Varty and C Cousins, Addenbrookes Hospital, Cambridge (10); D Harkin, RJ Hannon and L Johnston, Belfast City Hospital, Belfast (53); AW Bradbury and MJ Henderson, Birmingham Heartlands Hospital, Birmingham (8); D Ritoo, SD Parvin and DFC Shepherd, Bournemouth General Hospital, Bournemouth (68); C Bicknell, RM Greenhalgh and AW Mitchell, Charing Cross Hospital, London (27); S Dimitri, PR Edwards and GT Abbott, Countess of Chester Hospital, Chester (15); DJ Higman and A Vohra, University Hospital Coventry, Coventry (8); S Ashley and C Robottom, Derriford Hospital, Plymouth (2); MG Wyatt and JDG Rose, Freeman Hospital, Newcastle (121); K Daly, D Byrne and R Edwards, Gartnavel General Hospital, Glasgow (12); K Daly, DP Leiberman and DH McCarter, Glasgow Royal Infirmary, Glasgow (19); B Modarai, PR Taylor and JF Reidy, Guy’s and St. Thomas’ Hospital, London (124); P McCollum, AR Wilkinson and DF Ettles, Hull Royal Infirmary, Hull (29); I Nichol, AE Clason, GLS Leen and J Cook, University Hospital, Middlesborough (19); NV Wilson and M Downes, Kent and Canterbury Hospital, Canterbury (1); J Abraham, SR Walker and JM Lavelle, Lancaster General Infirmary, Lancaster (12); MJ Gough and S McPherson, Leeds General Infirmary, Leeds (38); DJA Scott and DO Kessell, Leeds St James’s Hospital, Leeds (11); R Naylor, R Sayers and NG Fishwick, Leicester Royal Infirmary, Leicester (148); S Vallabhaneni, PL Harris and DA Gould, Liverpool Royal Hospital, Liverpool (143); JV Smyth, MG Walker and NC Chalmers, Manchester Royal Infirmary, Manchester (96); A Garnham and MA Collins, New Cross Hospital, Wolverhampton (1); S Thomas, JD Beard and PA Gaines, Northern General Hospital, Sheffield (77); MY Ashour and R Uberoi, Queen Elizabeth Hospital, Gateshead (18); S Macsweeney, B Braithwaite and SC Whitaker, Queen’s Medical Centre, Nottingham (116); JN Davies and S Travis, Royal Cornwall Hospital, Truro (26); G Hamilton, A Platts and M Davis, Royal Free Hospital, London (42); A Shandall and BA Sullivan, Royal Gwent Hospital, Newport (1); S Sarkar, M Sobeh and M Matson, Royal London Hospital, London (7); AD Fox and R Orme, Royal Shrewsbury Hospital, Shrewsbury (7); W Yusef and T Doyle, Royal Sussex County Hospital, Brighton (6); J Budd, M Horrocks and J Hardman, Royal United Hospital, Bath (34); D Harkin, PHB Blair and PK Ellis, Royal Victoria Hospital, Belfast (46); G Morris and A Odurny, Southampton General Hospital, Southampton (39); A Tiwari, R Vohra and M Duddy, Selly Oak Hospital, Birmingham (22); M Thompson, TML Loosemore, AM Belli and R Morgan, St. George’s Hospital, London (54); O Agu, M Adiseshiah and JAS Brookes, University College Hospital, London (69); and, CN McCollum and R Ashleigh, University Hospital of South Manchester, Manchester (127).
Trial co-ordinators
M Aukett, C Bailey, S Baker, E Barbe, G Bate, N Batson, J Bell, J Blundell, D Boardley, S Boyes, H Brooks, O Brown, J Bryce, J Burrough, M Carmichael, T Chance, S Clarke, J Coleman, C Cosgrove, G Curran, L Dabee, L Dali-Kemmery, A Datson, M Davis, T Dennison, C Devine, N Dewhirst, B Errington, H Fairey, H Farrell, C Fisher, P Fulford, M Gough, C Graham, C Harrison, R Hooper, G Horne, L Horrocks, B Hughes, T Hutchings, M Ireland, C Judge, L Kelly, J Kemp, A Kite, M Kivela, M Lapworth, C Lee, L Linekar, A Mahmood, L March, J Martin, N Matharu, K McGuigen, P Morris-Vincent, S Murray, A Murtagh, J Myerscough, G Owen, V Ramoutar, C Rippin, J Rowley, L Sequeira, J Sinclair, S Spencer, V Taylor, H Thompson, C Tomlinson, S Ward, V Wealleans, J West, K White, J Williams, L Wilson and A Wye.
List of abbreviations
- AAA
- abdominal aortic aneurysm
- ABPI
- ankle–brachial pressure index
- ACE
- Anévrysme de l’aorte abdominale, Chirurgie versus Endoprothése
- BMI
- body mass index
- CAG
- Confidentiality Advisory Group
- CI
- confidence interval
- CT
- computed tomography
- DMEC
- Data Monitoring and Ethics Committee
- DREAM
- Dutch Randomised Endovascular Aneurysm Management
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- EuroQol-5 Dimensions
- EVAR
- endovascular aneurysm repair
- EVAR-1
- EVAR trial 1
- EVAR-2
- EVAR trial 2
- HDU
- high-dependency unit
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IFU
- instructions for use
- IPD
- individual patient data
- IPW
- inverse probability weighting
- ITT
- intention to treat
- M
- missing values
- MD
- Doctor of Medicine
- MI
- myocardial infarction
- MMP
- matrix metalloproteinase
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OR
- open repair
- OVER
- Open Versus Endovascular Repair
- PhD
- Doctor of Philosophy
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- SE
- standard error
- TMC
- Trial Management Committee
