Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/38/01. The contractual start date was in September 2009. The draft report began editorial review in September 2016 and was accepted for publication in June 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Keith Greene is the founder and shareholder of K2 Medical Systems (Plymouth, UK) and Clinical Director for the development of the INFANT system. Christopher Mabey is employed by, and is a shareholder of, K2 Medical Systems, the technology provider for the study. Edmund Juszczak reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) and Efficacy and Mechanism Evaluation programmes during the conduct of the study, and is a member of the NIHR HTA Commissioning Board. Peter Brocklehurst reports grants and personal fees from the Medical Research Council and grants from the National Institute for Health and Care Excellence, NIHR Health Services and Delivery Research, NIHR HTA and Wellcome Trust, outside the submitted work, and is chairperson of the NIHR HTA Women and Children’s Health panel and is a member of the HTA Prioritisation Group. Sara Kenyon is a member of the NIHR HTA Women and Children’s Health panel and received NIHR funding to undertake the HOLDS (High Or Low Dose Syntocinon® for delay in labour) trial, and was part funded by the NIHR Collaboration for Leadership in Applied Health Research and Care West Midlands.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Brocklehurst et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The justification for the trial, the supporting literature and the methods of the trial were published as a trial protocol in BMC Pregnancy and Childbirth. Sections of this chapter are reproduced from Brocklehurst. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The text below includes minor additions and formatting changes to the original text.
Continuous electronic fetal monitoring (EFM) in labour is widely used throughout the developed world. However, its potential for improving fetal and neonatal outcomes has not been realised. The reasons for this are probably complex, but are likely to include difficulty of interpreting the fetal heart rate trace correctly during labour, when the birth attendant has many competing tasks. For intrapartum monitoring to improve fetal and neonatal outcomes, the interpretation of the fetal heart rate has to be substantially and consistently improved. This standard has to be sustained and be independent of any health professional’s individual ability. Computerised interpretation of the fetal heart rate and intelligent decision support has the potential to deliver this improvement in care. The aim of EFM is to detect abnormalities of the fetal heart rate pattern during labour that are associated with asphyxia so that action can be taken to expedite delivery and prevent stillbirth and the development of neonatal encephalopathy (NNE). Therefore, the potential benefits of EFM are immense. Prevention of even a modest proportion of perinatal asphyxia will improve the health and well-being of thousands of children and their families throughout the world each year. In addition, the cost to the NHS Litigation Authority (NHSLA) for obstetrics is very large and rising. EFM could contribute to a substantial reduction. Furthermore, if this technology can work in the complex process of labour, it also has the potential to improve patient safety in a wide range of health-care settings.
The problem of perinatal asphyxia
Perinatal asphyxia, if severe, can result in intrapartum stillbirth. If less severe, it results in the development of an encephalopathic state in the newborn. This is characterised by a decreased level of consciousness, altered reflexes, abnormal tone and ultimately permanent damage to the brain. Moderate or severe NNE occurs in approximately 2 out of 1000 births. 2 With more severe asphyxial encephalopathy there is an increased risk of death or neurodevelopmental abnormalities: 25% of infants who have moderate asphyxial encephalopathy will develop cerebral palsy and around 80% of infants who have severe encephalopathy and survive will develop cerebral palsy. 3 Perinatal asphyxia may account for up to 30% of all cases of cerebral palsy4 and it is a very significant health-care and financial burden on the NHS. A reduction in the number of babies born with perinatal asphyxia would reduce the associated mortality and, among survivors, the burden of ill health and incapacity. It could also result in substantial savings in litigation costs in the UK.
Efficacy of continuous electronic fetal monitoring
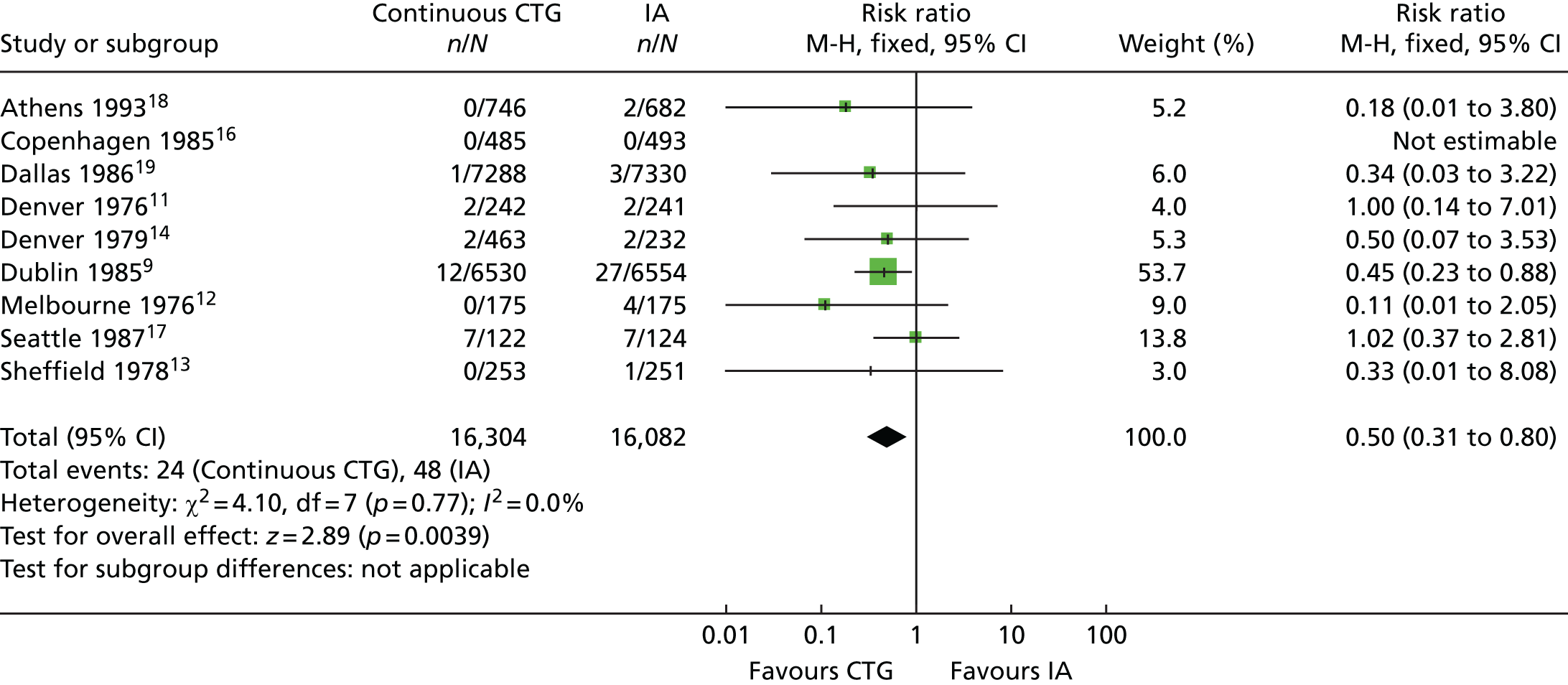
Continuous electronic fetal monitoring was invented in the 1960s. 5,6 The recorder displays the fetal heart rate and maternal uterine activity on a continuous line graph, called the cardiotocograph (CTG) tracing. EFM was widely introduced in the 1970s7 and it became controversial in the 1980s when it was shown to poorly predict Apgar scores and fetal acid–base status at delivery. 8 The largest randomised controlled trial (RCT) (the Dublin trial) showed no reduction in perinatal mortality or in cerebral palsy using EFM. 9 However, systematic reviews and meta-analyses of all trials indicated some benefits of EFM: for example, a 58% reduction in odds of deaths attributable to intrapartum hypoxia [95% confidence interval (CI) 2% to 83%]10 (Table 1) and a 50% reduction in risk of neonatal seizures (95% CI 20% to 69%) (Figure 1). 20 EFM is widely used on many women during labour in the UK. National Institute for Health and Care Excellence (NICE) guidelines for fetal monitoring in the NHS detail explicit criteria for implementing EFM; as a result, EFM is carried out in capproximately 60% of all women in labour. 21 EFM has been shown to be associated with an increase in caesarean sections and instrumental vaginal births. In a review of 11 trials involving a total of 18,961 women, there was a 63% increase in the odds of a caesarean section (95% CI 1.29 to 2.07), and in 10 trials involving a total of 18,615 women there was a 15% increase in the odds of an instrumental vaginal birth (95% CI 1.01 to 1.33). 20
| Study and year of publication | Patients in the (n) | Perinatal deaths (n) | Perinatal deaths as a result of fetal hypoxia (n) | |||
|---|---|---|---|---|---|---|
| EFM group | IA group | EFM | IA | EFM | IA | |
| Haverkamp et al., 197611 | 242 | 241 | 2 (FD 0, ND 2) | 1 (FD 0, ND 1) | 0 | 0 |
| Renou et al., 197612 | 175 | 175 | 1 (FD 0, ND 1) | 1 (FD 1, ND 0) | 0 | 1 (FD) |
| Kelso et al.,197813 | 253 | 251 | 0 | 1 (FD 0, ND 1) | 0 | 1 (ND) |
| Haverkamp et al., 197914 |
230 229 |
231 | 3 (FD 0, ND 3) | 0 | 0 | 0 |
| Wood et al., 198115 | 445 | 482 | 1 (FD 0, ND 1) | 0 | 0 | 0 |
| MacDonald et al., 19859 | 6474 | 6490 | 14 (FD 3, ND 11) | 14 (FD 2, ND 12) | 7 (FD 3, ND 4) | 7 (FD 2, ND 5) |
| Neldam et al., 198616 | 482 | 487 | 0 | 1 (FD 1, ND 0) | 0 | 1 (FD) |
| Luthy et al., 198717 | 122 | 124 | 17 (FD 1, ND 16) | 18 (FD 1, ND 17) | 0 | 1 (FD) |
| Vintzileos et al., 199318 | 746 | 682 | 2 (FD 0, ND 2) | 9 (FD 2, ND 7) | 0 | 6 (FD 2, ND 4) |
| Total | 9398 | 9163 | 40 (4.2/1000) | 45 (4.9/1000) | 7 (0.7/1000)a | 17 (1.8/1000)a |
FIGURE 1.
The effect of EFM vs. intermittent auscultation on the incidence of neonatal seizures. Review: continuous cardiotocography as a form of EFM for fetal assessment during labour; comparison: continuous cardiotocography vs. intermittent auscultation; outcome: 26 neonatal seizures. CTG, cardiotocography; df, degrees of freedom; FBS, fetal blood sample; IA, intermittent auscultation; M-H, Mantel–Haenszel.
Human error and systems failure
In the late 1980s it became apparent that a human element might be a factor in the failure of EFM to deliver improved outcomes. In one case–control study, the intrapartum management of 38 babies severely asphyxiated at birth was compared with that of 120 controls. 22 Cardiotography was abnormal in 29% of babies in the control group, but in only 9% was the abnormality severe. In contrast, 87% of the babies asphyxiated at birth had an abnormal CTG and in 61% of cases the abnormality was severe. However, the most striking finding was the length of time required for the staff to recognise the CTG abnormality. With moderate abnormalities, the mean time until recognition was 91 minutes [standard deviation (SD) 61 minutes]; paradoxically, with severe abnormalities it was 128 minutes (SD 100 minutes). The authors could give no plausible reason for the standard of CTG interpretation being so poor. However, it was clear from this study that, if the quality of interpretation of the intrapartum CTG had been higher, the benefits from EFM would almost certainly have been significantly and substantially enhanced.
In 1990, Ennis and Vincent published the results of their study of 64 cases of poor perinatal outcome from the archives of the Medical Protection Society. 23 In 11 cases, EFM was not performed, despite being indicated, and in six cases the technical quality of the tracing was inadequate. In 19 cases the CTG trace was missing, and in 14 cases a significant abnormality in the CTG trace either was unnoticed or did not result in any action being taken; in only 14 cases was appropriate monitoring performed and action taken. In only 16 cases was a consultant involved to aid in the interpretation of the CTG. In a further case–control study based in Oxford, published in 1994, intrapartum care was assessed in 141 cases of cerebral palsy and in 62 perinatal deaths with a probable intrapartum cause. 24 The authors found that, compared with control babies, abnormal fetal heart rate patterns were 2.3 times as common in babies who went on to develop cerebral palsy and 6.7 times as common in fetuses that died in the perinatal period. In addition, the authors found that clinicians failed to respond to these clear signs of abnormality in 26% of cerebral palsy cases and 50% of perinatal deaths, compared with 7% of control cases. On the basis of these figures, it can be estimated that approximately one case of cerebral palsy and one perinatal death can possibly be prevented in every 4500 deliveries. If one assumes 700,000 births per annum in the UK, 174 cases of cerebral palsy and 158 perinatal deaths could be prevented each year. Stewart et al. 25 reported that perinatal mortality in the UK is twice as high at night as during the day, and twice as high in July and August as in the rest of the year. They suggested that excess deaths may be because of over-reliance on inexperienced staff at night and a shortage of staff during the peak summer holiday months; they also suggested that the excess might be related to physical and mental fatigue of the caregivers. In 1999, the Confidential Enquiry into Stillbirths and Deaths in Infancy (CESDI) studied the proportion of 567 cases for which there was evidence of suboptimal care in labour. CESDI then looked at whether or not improved care could possibly or probably have prevented the adverse outcome. 26 Suboptimal care was identified in 71% of cases; a better outcome could possibly (in 28% of cases) or probably (in 22% of cases) have been anticipated, if care had been adequate. The report authors noted that interpretation of the CTG remained the most frequent problem identified as a cause of suboptimal care.
Does improving training solve the problem?
In a study of the efficacy of intrapartum intervention, Young et al. 27 found evidence of substandard care in labour in 74% of babies with low Apgar scores. Following the introduction of regular audit of low Apgar scores, with intensive feedback to clinical staff, this proportion fell to 23%, but then increased to 32% over the following year. However, following the introduction of compulsory training in CTG interpretation for all staff, the proportion of low Apgar score cases associated with substandard care fell back once again to only 9%. It is clear from this study that improved interpretation of CTGs during labour can bring about a striking increase in the quality of care, with measurable impacts on neonatal condition. However, intensive education is not sustainable in most clinical settings. With recent changes in the training of junior medical and midwifery staff, it is clear that there is a need to develop other systems that are less reliant on individual motivation and training. These systems need to work equally well, regardless of the time of day, day of the week, month of the year, and the level of staffing on the labour ward.
Litigation and the costs to families and society
Maternity services are associated with far higher litigation costs than other services. This is reflected in the various arrangements for the development of risk management standards across the UK (Clinical Negligence Scheme for Trusts in England; Welsh Risk Pool; Clinical Negligence and Other Risks Indemnity Schemes and NHS Quality Improvement Scotland in Scotland).
The total cost of claims reported to the NHSLA over the period 1996–2006 was £3.8B [Great British pounds (GBP)]. The annual figures for the value of maternity claims paid out (Table 2) demonstrate an increase of almost sixfold over the last 13 years and the rate of increase shows no signs of slowing. In response to a parliamentary question on 29 January 2007, it was stated that the total NHS compensation payout in 2006 was £593M, with £68M resulting from just 10 cases, all of which were related to pregnancy and childbirth. By 2015/16, the total payout had risen to £1.488B, with £578M being attributable to maternity cases alone. 28 In 2007, the BBC reported a settlement of £6M for a child with cerebral palsy after doctors were alleged to have mismanaged the birth. 29 By 2015, the cost of a single case of cerebral palsy had risen to over £10M. 30 Even successful defence can cost up to £0.5M. In 2016, the NHSLA reported that the annual value of submitted claims related to pregnancy-related cerebral palsy had risen from £354M in 2004/5 to £989.7M in 2015/16. 28 In 2000, the British Medical Journal highlighted the importance of ‘system errors’ in medical disasters31 and analogies were drawn with errors in aviation. It suggested that some techniques used in this industry could be applied effectively to medical care, such as safety drills, revalidation, ‘nearmiss’ reporting and a ‘no blame’ culture. The role of expert systems and ‘intelligent alarms’ was highlighted.
| Year maternity claims paid out | Total cost in millions (GBP) |
|---|---|
| 2003/4 | 96 |
| 2004/5 | 121 |
| 2005/6 | 144 |
| 2006/7 | 171 |
| 2007/8 | 162 |
| 2008/9 | 222 |
| 2009/10 | 197 |
| 2010/11 | 234 |
| 2011/12 | 422 |
| 2012/13 | 508 |
| 2013/14 | 458 |
| 2014/15 | 501 |
| 2015/16 | 578 |
The potential solution: development of the intelligent decision support software
A group in Plymouth has been working on the problem of resolving human error in the management of labour for many years [Medical Research Council (MRC) funded for 10 years] and has developed intelligent computer systems as decision aids to support clinicians. The group was funded by the MRC for development and clinical validation of a decision support tool for the management of labour using the CTG. It comprises feature extraction of all relevant data from the CTG and clinical history which have been found to influence clinicians’ decision-making, and then an analysis of these within a rule-based expert system. The specific piece of decision support software to be evaluated in INFANT has been designed by K2 Medical Systems (Plymouth, UK) (a spin-off company from the University of Plymouth) to run on the K2 Medical Systems data collection system (Guardian®). Guardian is a system for managing information from labour monitoring.
The data collection system (Guardian)
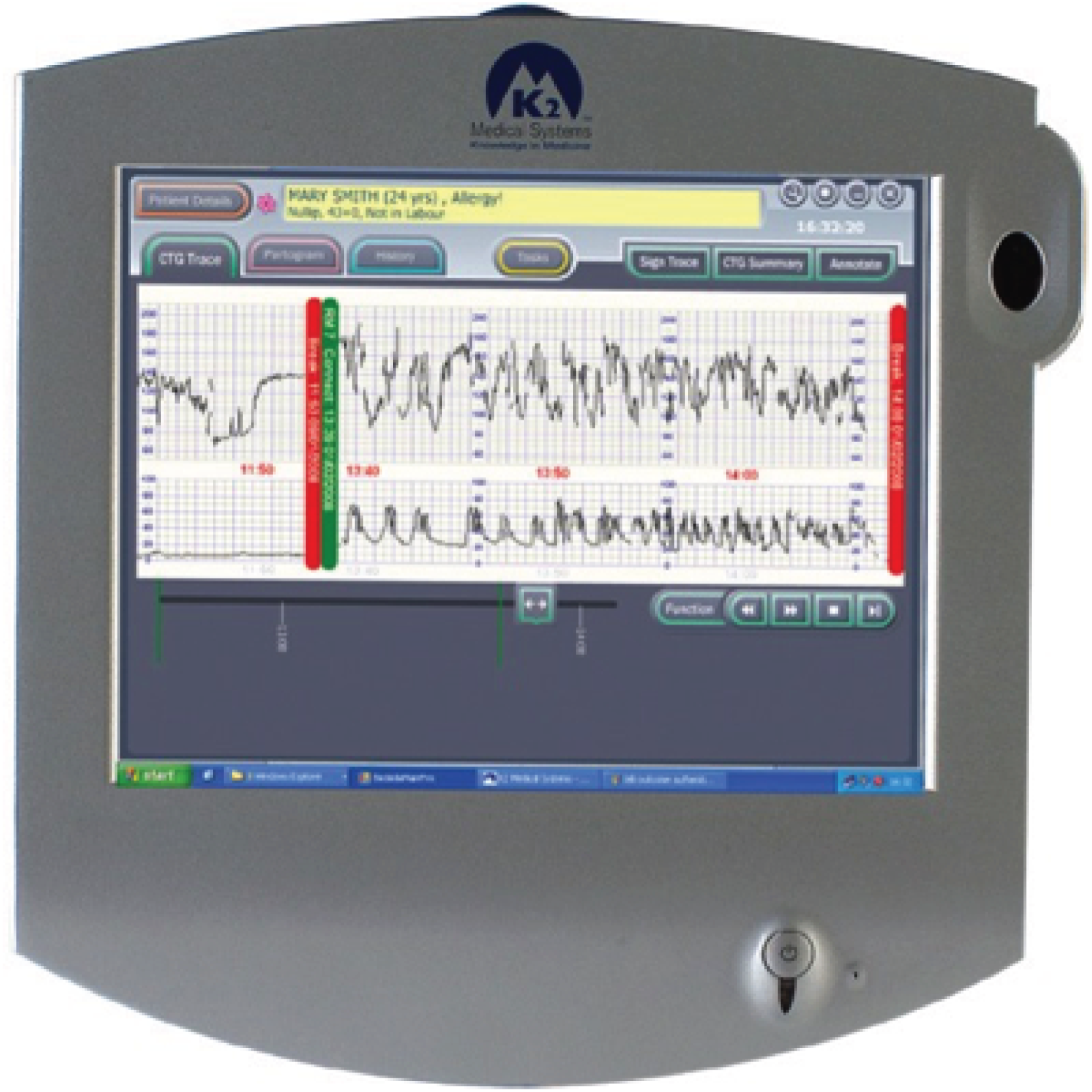
The Guardian system consists of a medical-grade personal computer (PC) platform (Figure 2) that meets the Medicines and Healthcare products Regulatory Agency standards for a class IIa device. The design has been informed by user preference studies and ethnographic and audio-visual observations of clinical care and decision-making. 32,33 It has a touch-screen user interface (Figure 3) and is connected to a conventional CTG recorder at the woman’s bedside.
FIGURE 2.
Guardian system: example of hardware.
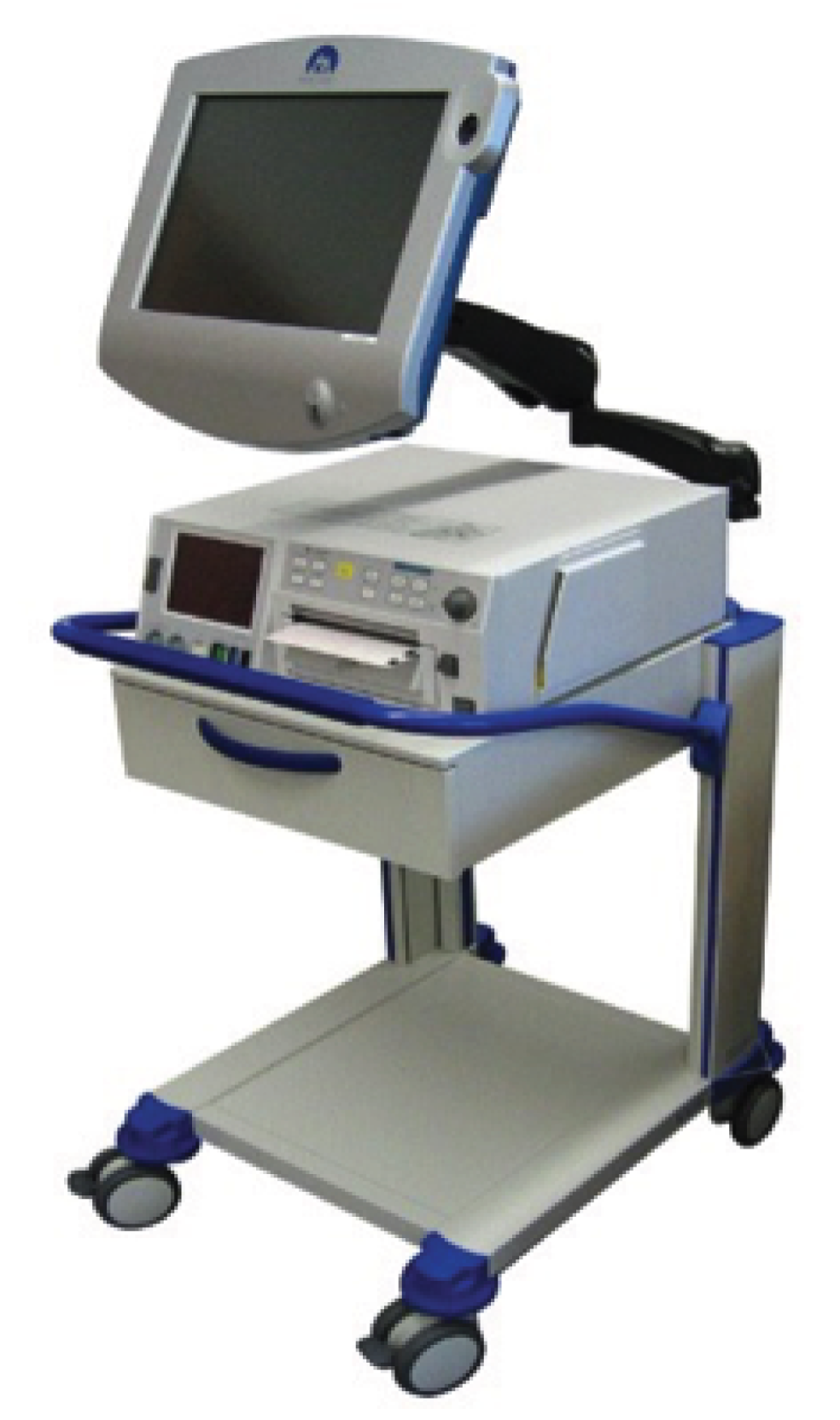
FIGURE 3.
Guardian system: example of screen.
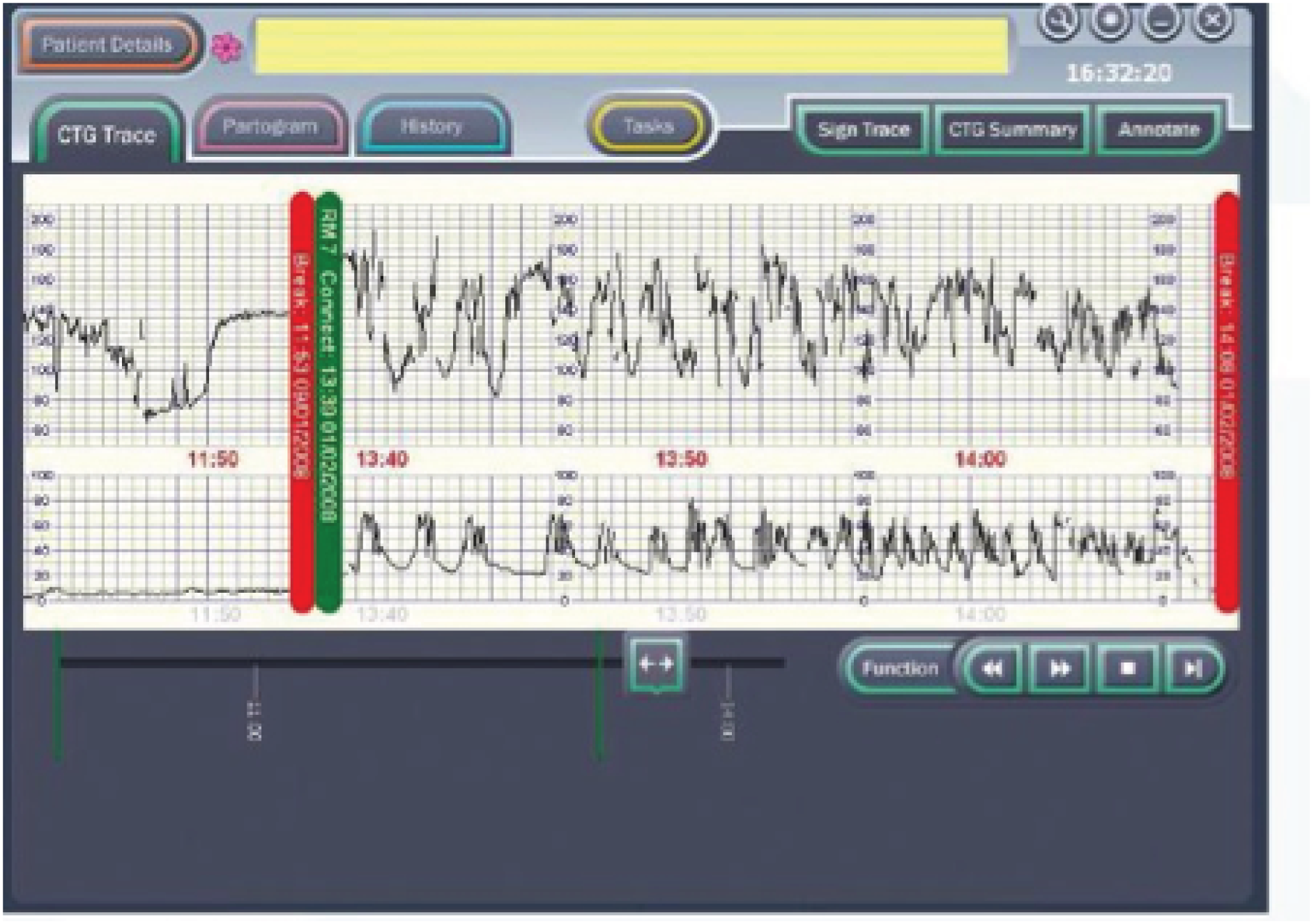
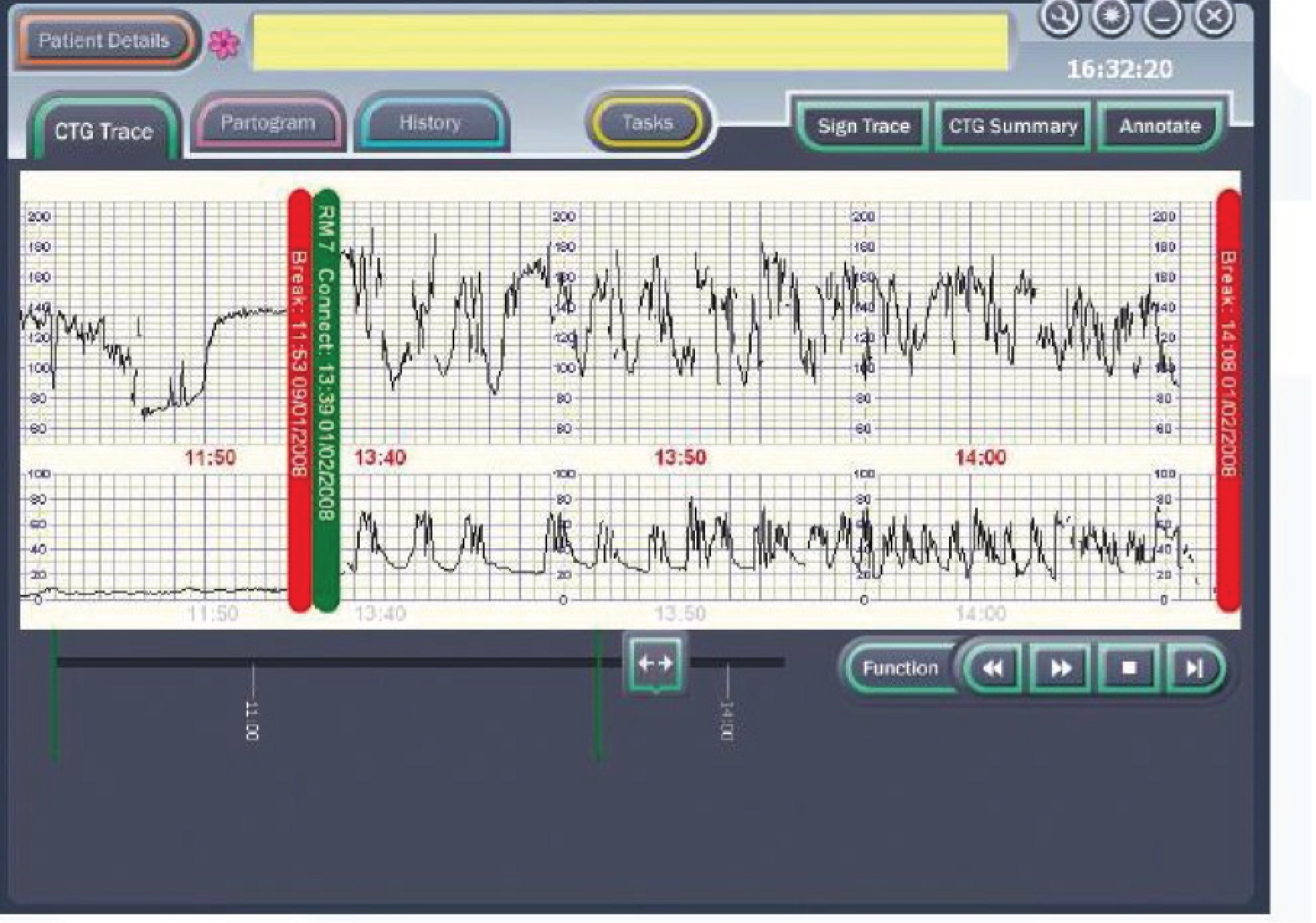
The PC uses the Microsoft Windows® (Microsoft Corporation, Redmond, WA, USA) operating system and runs the decision support software developed by the Plymouth group. The clinician enters clinical information (antenatal risk factors, vaginal examination data, fetal blood sample results, etc.) via the touch-screen. This information is displayed as a partogram (Figure 4). It displays the CTG on a computer screen alongside other clinical data [e.g. the partogram, maternal vital signs (including Modified Early Warnings Systems charts) and details of maternal anaesthesia and analgesia] that are collected as part of routine clinical care. Guardian does not interpret any of the data being collected, but acts as an interface to collect and display data at the bedside, centrally on the labour ward, in consultants’ offices or remotely. The system requires little or no training to use and has been used for routine clinical care by a number of hospitals throughout the UK. 34 If CTG is performed by ultrasound or electrocardiography (ECG) clip, the PC system automatically collects these data from the RS 232 digital data port of any CTG recorder. The system displays the CTG data on the screen (Figure 5).
FIGURE 4.
Guardian system: example of partogram on screen.
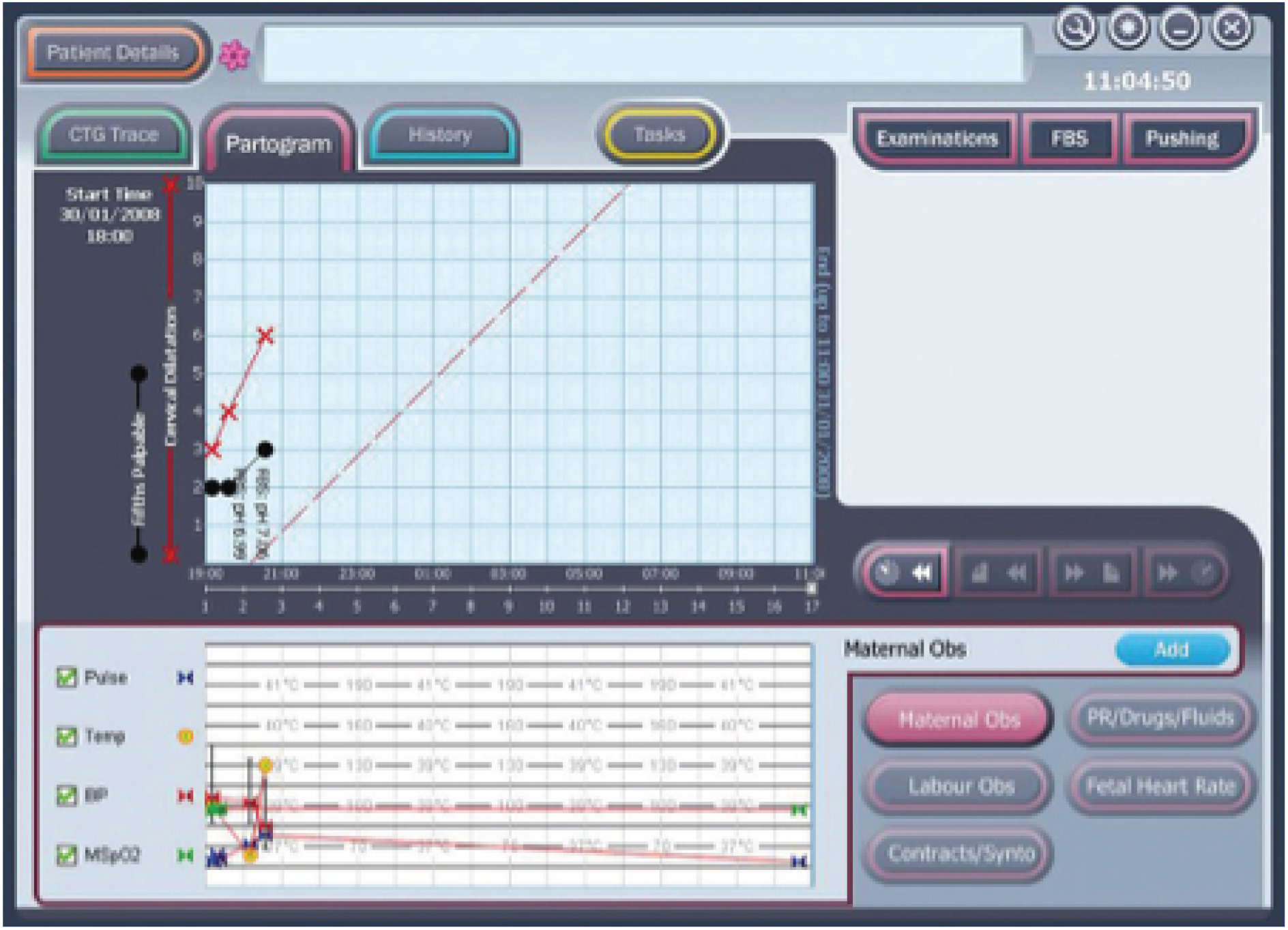
FIGURE 5.
Guardian system: example of decision support on screen.
The decision support software
The decision support software is a specific piece of software that has been developed to run on the Guardian system. It extracts the important features of baseline heart rate, heart rate variability, accelerations, type and timing of decelerations, the quality of the signal and the contraction pattern from the CTG. The decision support software then analyses these data along with the quality of the signals. The system’s assessment of the CTG is presented as a series of colour-coded alerts depending on the severity of the abnormality detected (Figures 6–8). The system can therefore be viewed as an intelligent prompt, but by recording the chronology of events it also offers the opportunity to later audit the actual clinical decision-making process in a similar way to an aircraft’s black box.
FIGURE 6.
Example of a ‘blue level of concern’.
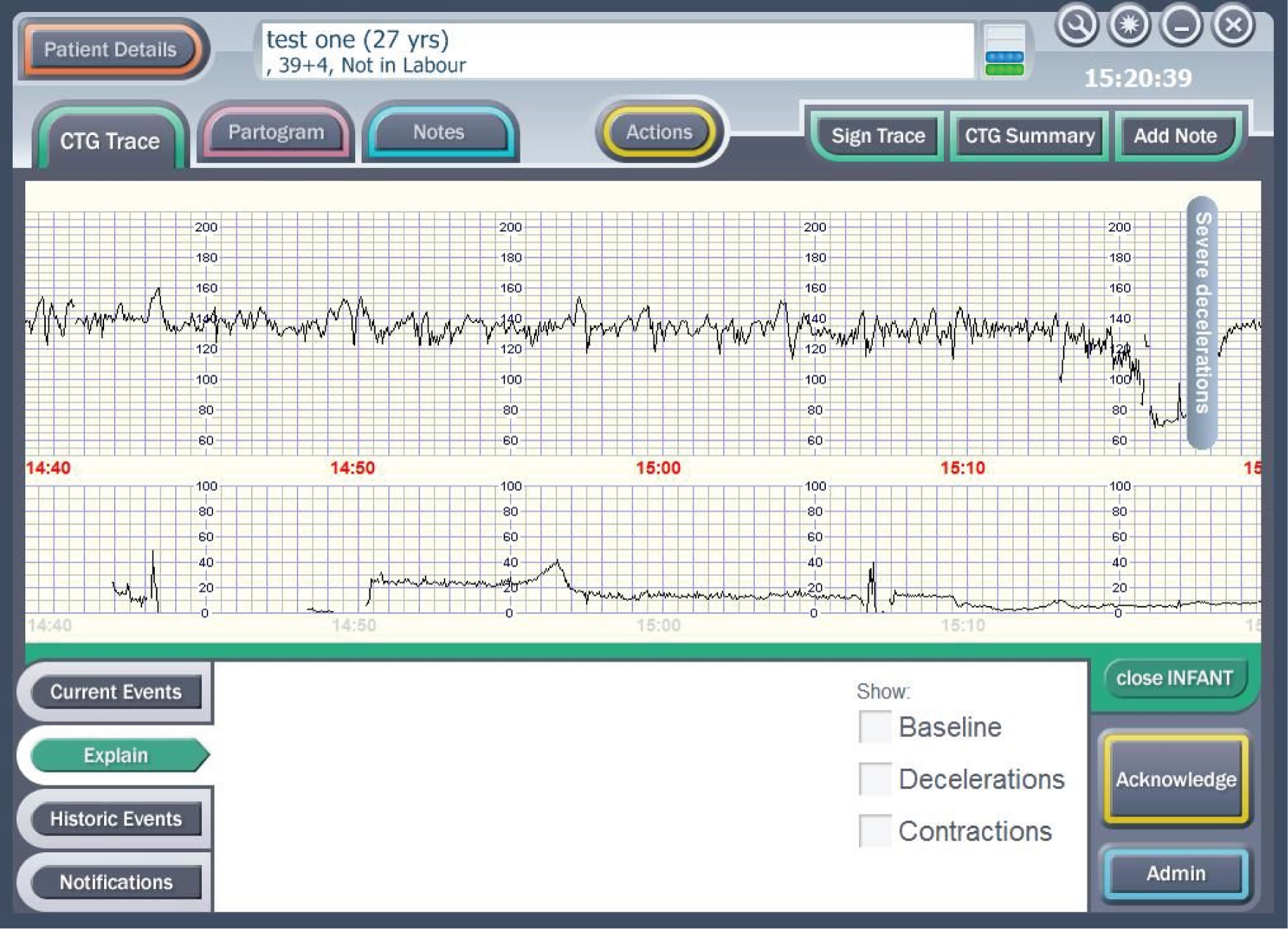
FIGURE 7.
Example of a ‘yellow level of concern’.
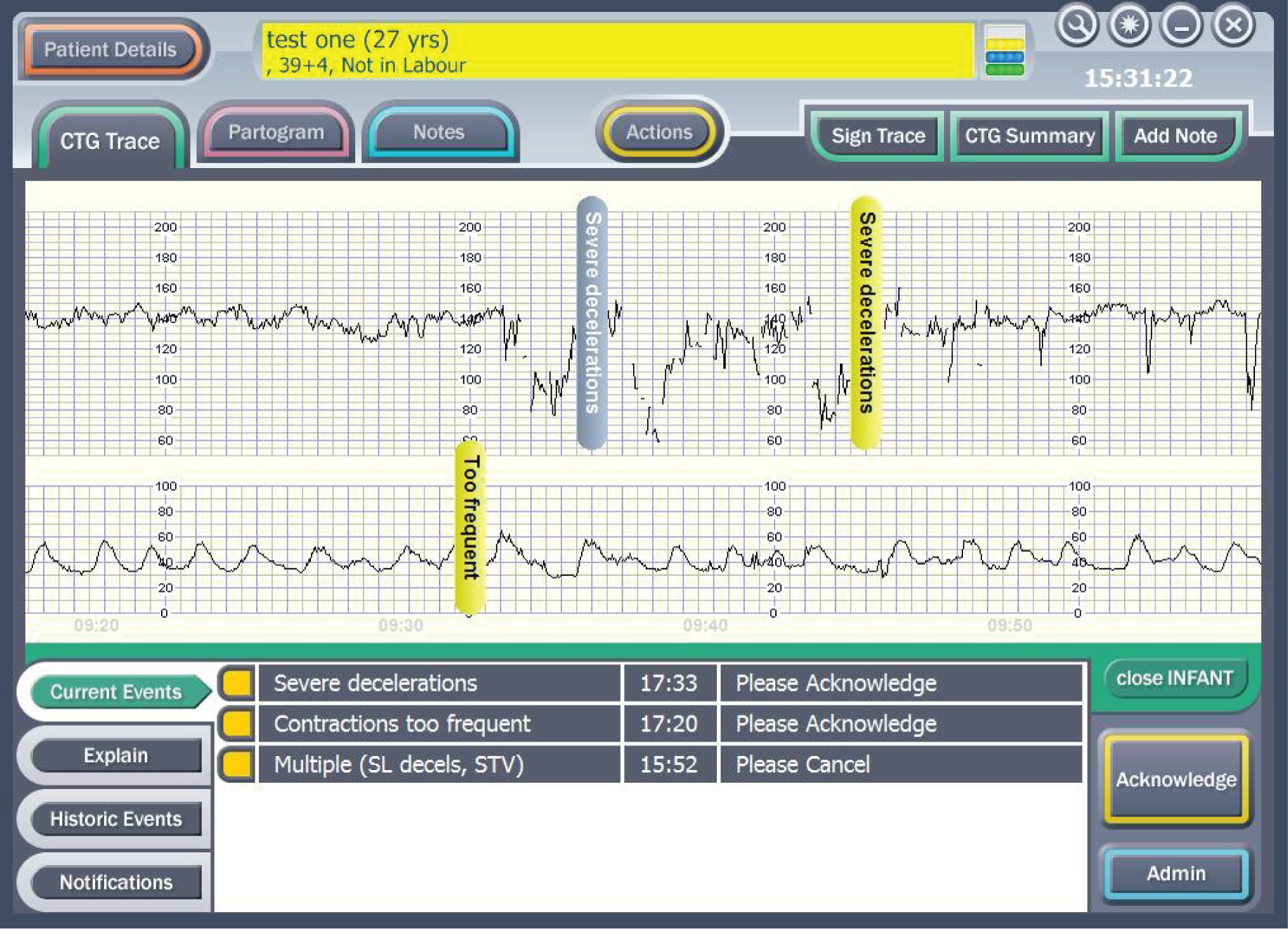
FIGURE 8.
Example of a ‘red level of concern’.
Studies using the intelligent support software
Three studies conducted by the Plymouth group35–37 demonstrated that the software, when used ‘offline’, performed as well as expert obstetricians in interpreting the CTG and managing labour subsequently, and that the system performed better than routine clinical practice. The system identified more cases that went on to have a poor outcome and anticipated clinical decision-making. In one of these studies involving labours that had resulted in a stillbirth, the system ‘intervened’ (i.e. recognised the abnormality which would have prompted delivery) more than 6 hours earlier than happened in actual clinical practice and more than 2 hours before the experts. If this translated into clinical practice, it would be reasonable to expect that a number of such deaths might have been prevented if the software had been in use at the time. In all other poor-outcome groups the system ‘intervened’ much earlier than had happened in routine clinical practice and at a similar time as the experts. The system failed to predict one perinatal death, whereas the experts in the ‘offline’ study, and those functioning in routine clinical practice, failed to predict several deaths. These extensive ‘offline’ validation studies have shown that the system matches the performance of an expert obstetrician in interpreting the CTG and performed considerably better than routine clinical practice. Furthermore, the system is not overinterventional. From these data it seems reasonable to hypothesise that the clinical use of this computer-based decision support software would decrease the incidence of perinatal mortality and morbidity.
Current practice
Continuous electronic fetal monitoring is widely used for the majority of women during labour and birth in the UK. NICE guidelines for fetal monitoring detail explicit criteria indicating which women should be offered EFM during labour; approximately 60% of all women in labour meet these criteria. 21 This study did not aim to influence the number of women who received EFM.
Research objectives
Our hypotheses were that:
-
a substantial proportion of substandard care results from a failure to correctly identify abnormal fetal heart rate patterns
-
improved recognition of abnormality would reduce substandard care and poor outcomes
-
improved recognition of normality would reduce unnecessary intervention.
These led to the objectives of the study, to:
-
determine whether or not intelligent decision support can improve interpretation of the intrapartum CTG and, therefore, improve the management of labour for women who are judged to require EFM. Specifically, will the system, compared with current clinical practice:
-
identify more clinically significant heart rate abnormalities?
-
result in more prompts and timely action on clinically significant heart rate abnormalities?
-
result in fewer poor neonatal outcomes?
-
change the incidence of operative interventions?
-
-
assess whether or not the use of intelligent decision support improved the quality of routine care received by women undergoing EFM during labour. This information was important for evaluating whether or not the decision support software decreases the risk of suboptimal care in labour; it was also useful to explore the effect that such an intervention may have on litigation costs for obstetrics
-
determine whether or not the use of the decision support software was cost-effective in terms of the incremental cost per poor perinatal outcome prevented
-
determine whether or not use of the decision support software had any effect on the longer-term neurodevelopment of children born to women participating in the INFANT study.
Chapter 2 Methods
The INFANT study was an individually randomised controlled trial of 46,000 women who were judged to require EFM in labour. Follow-up was completed at 2 years for a sample of 7000 surviving children born to women participating in the INFANT study.
Trial eligibility and randomisation
Inclusion criteria
Women admitted to a participating labour ward who fulfilled all of the following criteria were eligible to be recruited and randomised.
-
Women were judged to require EFM by the local clinical team based on existing guidelines, and the woman consented to have EFM, and EFM was possible.
-
Note that EFM is defined as the active decision of the health-care professional and the woman to initiate EFM for the purpose of fetal monitoring, usually because of perceived risk factor(s) that increase the likelihood of fetal compromise occurring in labour.
-
The decision to initiate EFM can occur at any time during labour. Some women with known factors that place them at higher risk of fetal compromise during labour would already know that EFM throughout labour was planned. Others started labour with intermittent monitoring and then were judged to require EFM at some point during the labour. Women were eligible to participate at any stage of labour.
-
-
Women were pregnant with a single fetus or twins.
-
Gestational stage was ≥ 35 weeks (≥ 245 days).
-
There was no known gross fetal abnormality, including any known fetal heart arrhythmia such as heart block.
-
Women were aged ≥ 16 years.
-
Women were able to give consent to participate in the trial as judged by the attending clinicians.
Exclusion criteria
-
Triplet or higher-order pregnancy.
-
Criteria for EFM not met, including elective caesarean section prior to the onset of labour.
Information for women and obtaining informed consent
Information about the trial was provided to women during the antenatal period (see Appendix 1), after their booking appointment. This process was individualised for each participating centre depending on their routine practices. For example, in some centres, women were provided with information about the trial at their routine ultrasound scan appointment (18–22 weeks). All women had the opportunity to ask questions.
When a woman presented in early labour to the labour ward in a participating centre, she was given a copy of the participant information leaflet (see Appendix 2) and a verbal explanation of the INFANT trial. She was then asked whether or not she would like to participate in the study and, if she agreed, she was asked to sign an INFANT trial consent form. Then, if at any point EFM was commenced during labour, the midwife responsible for the woman’s care checked her eligibility to participate in the trial and that she was still happy to take part. This was documented, and then the woman was randomised by the Guardian system to either the decision support (intervention) arm or the no decision support (control) arm.
All women in labour admitted to the participating centres were expected to have their labour information recorded in the Guardian system, in accordance with the current practice in each centre. This did not change the way health professionals managed labour; it merely changed the way they managed the information generated by the process of monitoring labour and how they recorded this information. It was clearly stated that women were free to withdraw from the study at any time for any reason without prejudice to future care, and with no obligation to give the reason for withdrawal.
Written informed consent was obtained by means of a dated signature from the woman and the signature of the person who obtained informed consent (see Appendix 3); this would be the principal investigator (or a qualified health-care professional with delegated authority). A copy of the signed informed consent document was given to the woman. A further copy was retained in the woman’s medical notes, a copy was retained by the principal investigator and a final copy was sent to the trial co-ordinating centre.
A senior investigator was available at all times to discuss concerns raised by women or clinicians during the course of the trial.
Randomisation
The Guardian system prompted the health professional providing care to consider whether or not the woman was eligible for the INFANT trial when EFM had been used for > 5 minutes. Intermittent use of EFM for durations of up to 5 minutes could be employed for intermittent monitoring, but when used for longer periods of time this would indicate that a decision had been made to initiate EFM, in which case the woman may have been eligible to participate in the trial. If the health-care professional indicated that the woman was not yet eligible because an active decision had not been made to initiate EFM, then the Guardian system prompted the health-care professional again, if the CTG continued to be recorded for longer than 5 minutes in that or any subsequent episode of monitoring.
When the health-care professional indicated that a woman was eligible to participate, the Guardian system clarified that the necessary eligibility criteria for trial entry had been met (i.e. that the health professional gave the required answers to a number of questions posed by the Guardian system, and then the Guardian system randomly allocated the women in the ratio 1 : 1 to either ‘CTG with no decision support’ or ‘CTG with decision support’) (Figure 9). The allocations were computer generated in Stata® version 10 (StataCorp LP, College Station, TX, USA) using stratified block randomisation employing variable block sizes to balance between the two trial arms by singleton or twin pregnancy, and within each participating centre. The procedures for randomisation were fully documented, reviewed and signed off prior to the start of the trial and monitored by the co-ordinating centre during the trial.
FIGURE 9.
Flow diagram.
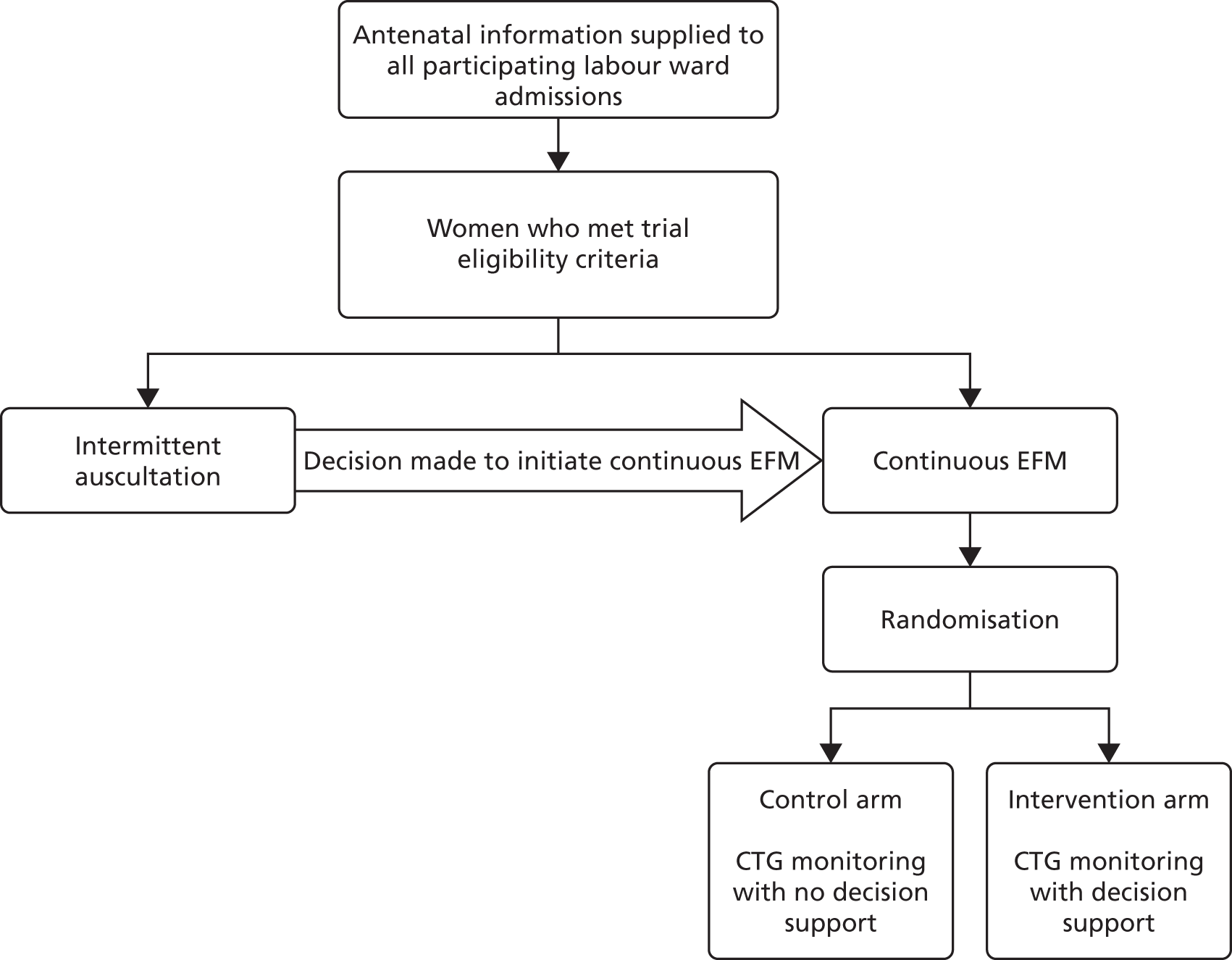
Planned interventions
The intervention was the use of decision support software. In order to accurately reflect any potential impact of the decision support software in contemporary NHS practice, such as changes in midwifery presence during labour consequent upon knowledge of the allocation, it was desirable that clinicians were not masked to allocation.
Clinical management
The Guardian system was developed to be used with all women in labour in the participating centres. It was only the decision support software that runs on this system that was being tested in this trial.
Clinicians in all participating centres were initially trained in the use of the decision support software by staff from the trial co-ordinating centre (see Appendix 4). This process included developing a ‘training team’ at each site which was responsible for cascading training among the local clinicians. The clinical management of women in the trial was not altered by their participation; however, staff caring for women in the decision support arm received a series of graded alerts or alarms when abnormalities of the CTG were detected by the system, which increased in urgency with the severity of the abnormality as judged by the system. No additional training was provided to clinical staff in how to respond to fetal heart rate abnormalities as part of their participation in the trial because, in the UK and Republic of Ireland, staff supervising labour and delivery are expected to have had such training, for example by completing computer-based training packages every 6–12 months, attending annual lectures and attending regular CTG review meetings.
Primary outcome measures
Primary short-term outcome
The primary short-term outcome was a composite of poor neonatal outcome including deaths [intrapartum stillbirths plus neonatal deaths (i.e. deaths up to 28 days after birth) except deaths as a result of congenital anomalies], significant morbidity (moderate or severe NNE, defined as the use of whole-body cooling) and admissions to a neonatal unit within 48 hours of birth for ≥ 48 hours (with evidence of feeding difficulties or respiratory illness and when there was evidence of compromise at birth suggesting that the condition was the result of mild asphyxia and/or mild encephalopathy).
Note: we recognised that the signs of mild encephalopathy can be subtle and hence a number of babies identified as having this condition were likely to have a range of non-specific signs such as respiratory difficulty and poor feeding rather than features more specifically associated with encephalopathy. 38 Therefore, we included ‘admission to the neonatal unit within 48 hours of birth for ≥ 48 hours where there is evidence of compromise at birth’. Since this was a mature group of babies (born ≥ 35 weeks), any difference in the incidence of these admissions was felt likely to result from differences in perinatal asphyxia.
Primary long-term outcome
The primary long-term outcome was the Parent Report of Children’s Abilities-Revised (PARCA-R) composite score39,40 at the age of 2 years for a subset of 7000 infants.
Note: neurodevelopmental delay and cerebral palsy are the most important long-term adverse outcomes associated with perinatal asphyxia. However, the incidence of moderate or severe cerebral palsy is around 1.5–2.5 per 1000 live births, depending on the definition and the method of ascertainment. There is also uncertainty about the proportion of these cases that results from intrapartum asphyxia in mature infants; however, 30% appears to be a reasonable estimate. 4 Therefore, given the rarity of this outcome, it was unlikely that a clear difference could be demonstrated between the two groups in a trial of 46,000 births. In order to have reassurance that any benefits of the intervention, with respect to short-term outcomes, had not occurred at the expense of later neurodevelopmental delay, we measured neurodevelopment in a proportion of the surviving children at the age of 2 years.
Secondary outcome measures
Secondary short-term outcomes
Neonatal
-
Intrapartum stillbirth (excluding deaths as a result of congenital anomalies).
-
Neonatal deaths up to 28 days after birth (excluding deaths as a result of congenital anomalies).
-
Moderate or severe encephalopathy.
-
Admission to neonatal unit within 48 hours of birth for ≥ 48 hours with evidence of feeding difficulties, respiratory illness or encephalopathy (when there was evidence of compromise at birth).
-
Admission to a higher level of care.
-
An Apgar score of < 4 at 5 minutes after birth.
-
The distribution of cord blood gas data for cord artery pH.
-
Metabolic acidosis (defined as a cord artery pH of < 7.05 and a base deficit in extracellular fluid of ≥ 12 mmol/l).
-
Resuscitation interventions.
-
Seizures.
-
Destination immediately after birth.
-
Length of hospital stay.
Maternal
-
Mode of delivery.
-
Operative intervention (caesarean section or instrumental delivery) for:
-
fetal indication or
-
failure to progress or
-
a combination of fetal distress and failure to progress or
-
other reason.
-
-
Grade of caesarean section.
-
Episiotomy.
-
Any episode of fetal blood sampling.
-
Length of:
-
first stage of labour from trial entry
-
second stage of labour from trial entry
-
labour from trial entry (total).
-
-
Destination immediately after birth.
-
Admission to a higher level of care.
Secondary long-term outcomes (infant)
Health and development outcomes at 24 months
-
Non-verbal cognition scale (PARCA-R).
-
Vocabulary subscale (PARCA-R).
-
Sentence complexity subscale (PARCA-R).
-
Late deaths up to 24 months (after the neonatal period).
-
Diagnosed with cerebral palsy.
-
Non-major disability.
-
Major disability.
-
Breastfeeding (collected at 12 and 24 months).
Quality-of-care outcomes
-
Adverse outcome (trial composite primary outcome plus metabolic acidosis) when it is judged that suboptimal care has occurred in labour: levels 1 to 3 separately and combined.
-
Level 1: suboptimal care, but different management would have made no difference to outcome.
-
Level 2: suboptimal care, and different management might have made a difference to outcome.
-
Level 3: suboptimal care, and different management would reasonably be expected to have made a difference to the outcome.
Note: in all cases of adverse outcome (trial primary outcome plus a cord artery pH of < 7.05 with a base deficit of ≥ 12 mmol/l) and all neonatal deaths and intrapartum stillbirths care in labour was assessed, to determine if it was suboptimal, by panel review similar to that undertaken by the Confidential Enquiry into Maternal and Child Health (CEMACH). 26 Intrapartum notes were copied and anonymised, and all references to trial allocation removed. The notes were then examined by a panel of an experienced obstetrician, midwife and neonatologist to identify if there was suboptimal care, particularly in relation to interpretation of the CTG and actions that flowed from any identification of CTG abnormalities.
Process outcomes (after trial entry)
-
Total number of CTG abnormalities (blue, yellow and red levels of concern) detected by the decision support software.
-
Number of blue levels of concern on the decision support software, indicating a mild abnormality on the CTG.
-
Number of yellow levels of concern on the decision support software, indicating a moderate abnormality on the CTG.
-
Number of red levels of concern on the decision support software, indicating a severe abnormality on the CTG.
-
Number of women with at least one yellow level of concern on the decision support software, indicating an abnormality on the CTG.
-
Number of women with at least one red level of concern on the decision support software, indicating a severe abnormality on the CTG.
-
Time from first red level of concern to birth.
Note that it was important to collect and analyse process outcomes in the trial, as a failure to detect differences in clinical or quality-of-care outcomes between the two randomised groups may be because of poor adherence with the alerts of the system, rather than the system not correctly identifying abnormalities with the CTG. In addition, as the trial allocation was not masked, it was important to measure any change that resulted from clinicians being aware of whether or not the decision support system was in operation.
-
Number of thumb entries per hour from time of trial entry to first yellow level of concern or until fully dilated (10 cm) if no abnormality detected or first yellow level of concern occurred prior to trial entry.
-
Number of vaginal examinations.
-
Epidural analgesia.
-
Labour augmentation.
-
Presence of meconium.
Note: some of these later process outcomes (e.g. the number of thumb entries per hour) were proxy measures to determine the presence of a health professional in the delivery room during the labour, which allowed us to quantify any differences between the groups with respect to support offered to women during labour. Although unlikely, knowledge of the trial allocation could have resulted in less frequent contact with the woman allocated decision support in labour. Less frequent contact would have resulted in a lower number of these process measures.
Data collection
For all participating women and babies, labour variables and outcomes were stored automatically and contemporaneously on the Guardian system. Data collected via the system were sent electronically to the trial co-ordinating centre. Data were extracted from the notes of babies admitted to the neonatal unit and for all neonatal deaths (see Appendices 5–7), as well as for mothers admitted for a higher level of care (see Appendix 8). Not all data fields were collected at every centre. However, when an item was collected, these data were sent to the clinical trials unit. The trial did not collect the reason why EFM was being used, as this was not recorded. All children surviving to be discharged home from hospital following their birth were ‘flagged’ at the NHS Information Centre for those born in England, and for those born in Scotland comparable systems were used. All deaths occurring after discharge home from hospital were notified to the trial co-ordinating centre. At 2 years after trial entry a sample of 7000 surviving children (3500 in each group) were followed up. The family was sent a two-part parent-completed questionnaire to assess the child’s health, development and well-being (see Appendix 9). The first part of the questionnaire comprised the PARCA-R, which had been previously validated as a means of assessing neurodevelopment in a trial setting. 39,40 The second part focused on general health issues, and had also been used previously.
Calculation of proposed sample size
The proposed total sample size was 46,000 births.
The following data sources and assumptions were used in the calculation of the trial sample size.
Incidence of intrapartum stillbirth
This was estimated as 0.35 per 1000 births. This estimate was derived from the following incidence data: 0.51 per 1000 births for women of all gestation periods (England, Wales and Northern Ireland, 2004)41 and 0.27 per 1000 births for women at ≥ 37 weeks’ gestation (Trent Region, 2005). 42 This trial restricted eligibility to women at ≥ 35 weeks’ gestation; therefore, the incidence was expected to be lower than in women at all gestational ages, which includes those who deliver preterm. However, it was also expected to be higher than for all women at term. As the women being recruited were all judged to require EFM and it was assumed that women in this ‘risk group’ would be at increased risk of adverse outcomes, the incidence was likely to be higher. In addition, these estimates used a denominator of all modes of births and so they included women having elective caesarean sections, who are not at risk of intrapartum stillbirth as there is no intrapartum period. Approximately 7% of women have elective caesarean sections, and removal of these women would increase the incidence further. Therefore, an estimate of an incidence of 0.35 per 1000 births appeared reasonable.
Incidence of neonatal death
This was estimated as 0.7 per 1000 births. This estimate was derived from the following data: 3.4 per 1000 births for women of all gestation periods (England, Wales and Northern Ireland, 2004)41 and 0.89 per 1000 births in those women at ≥ 37 weeks’ gestation (Trent Region, 2005). 42 This trial restricted eligibility to women at ≥ 35 weeks’ gestation; therefore, the incidence would be expected to be lower than among women of all gestational ages, which includes those who deliver preterm. However, it would be higher than for all women at term. A reasonable estimate of neonatal death for babies at 35 weeks’ gestation or more was therefore considered to be 1.0 per 1000 births. Using data from the Trent Survey 200542, 30% of neonatal deaths were as a result of congenital anomalies. Therefore, this rate was reduced to 0.7 per 1000 births. As the women being recruited were all judged to require EFM and it was assumed that women in this ‘risk group’ would be at increased risk of adverse outcomes, the incidence may have been higher. Therefore, an estimate of an incidence of 0.7 per 1000 births appeared reasonable.
Incidence of severe and moderate neonatal encephalopathy
The most appropriate estimate of the incidence of NNE in babies born at ≥ 35 weeks’ gestation was 1.3 per 1000 births (Trent & Northern Region, 2002). 43 However, as above, women being recruited to this trial were all judged to require EFM, which means that they may have been at increased risk of adverse outcomes; therefore, the incidence may have been higher.
Combined outcomes
Data were available on some combined outcomes. For example, the incidence of intrapartum stillbirths plus deaths on the labour ward assumed to be as a result of intrapartum asphyxia (the incidence of which is much lower than neonatal mortality) plus severe and moderate NNE was 1.7 per 1000 births (95% CI 1.5 to 1.9; range 0.8–2.3) (18 hospitals, Trent, 2003–4) and 1.9 per 1000 births (95% CI 1.6 to 2.3; range 0.6–2.3) (12 hospitals, Yorkshire Neonatal Network, 2004–5). 43 These data are for babies born at ≥ 35 weeks’ gestation, with the incidence of these outcomes being higher in the larger hospitals, which attract women with more complicated pregnancies.
Incidence of primary outcome for INFANT
We assumed an incidence of the primary outcome of 3 per 1000 births. This was calculated by summing the rate of intrapartum stillbirth, neonatal death, and moderate and severe encephalopathy, which gave an incidence of 2.35 per 1000 births. However, added to this figure was mild encephalopathy, which was reported to occur in 1.25 per 1000 births, and other significant morbidity [other admissions to the neonatal unit within 48 hours of birth for ≥ 48 hours (e.g. with feeding difficulties, respiratory symptoms or seizures)], for which there were no good estimates of incidence. The estimate of 3 per 1000 births erred on the side of caution and an increased incidence of this outcome in the trial would either (a) increase the power of the trial to demonstrate the same effect size or (b) allow detection of a smaller effect size with the same trial size or (c) necessitate a smaller trial if the postulated effect size (or larger) was detected.
Effect size
The effect size that can be detected with 46,000 women (23,000 in each group), assuming a 5% level of significance and 90% power, was a 50% reduction in poor neonatal outcome rate from 3 to 1.5 per 1000 births. We approximated the number of women recruited with the number of infants born, even though women with a twin pregnancy were eligible to join the trial. Approximately 1 in 80 pregnancies are twin pregnancies; however, a proportion of these births will occur at < 35 weeks’ gestation and a large proportion of the term births would be by elective caesarean section. Therefore, we estimated that < 1% of all births in the study would be twins. In a study of 164 preterm infants,40 the mean PARCA-R composite score at 2 years was 80 points (SD 33 points) and the mean Mental Development Index (Bayley Scales of Infant Development II) score was approximately half a SD below the standardised mean of 100. We assumed that a normal group of term-born infants would have a PARCA-R composite score half a SD above this sample of preterm infants, so we estimated a mean 2-year score of 96 points (SD 33 points). Based on this estimate, a follow-up sample of 7000 children (3500 per arm) in the INFANT study would have over 90% power to detect a difference of 3 points in the PARCA-R composite score with a two-sided 5% significance level. The incidence of severe metabolic acidosis (a cord artery pH of < 7.05) has been reported as 10 per 1000 births. 44–47 The proposed sample size was therefore able to detect a 28% relative risk reduction in this incidence with > 80% power in those babies who had their cord artery pH measured.
Assumptions
Variations in some of the assumptions of incidence produced marked variations in the required sample size as the anticipated overall incidence was so low. For example, Table 3 illustrates the impact on the required sample size of varying the incidence of the primary outcome, assuming a 5% level of significance and 90% power for the same effect size (a 50% relative risk reduction).
| Incidence of primary outcome in (per 1000 births) | Relative risk | Total sample size required | |
|---|---|---|---|
| No decision support group | Decision support group | ||
| 3.0 | 1.5 | 0.5 | 46,000 |
| 4.0 | 2.0 | 0.5 | 34,000 |
| 5.0 | 2.5 | 0.5 | 27,000 |
| 6.0 | 3.0 | 0.5 | 22,000 |
Table 4 illustrates the variation on the effect size that could be detected with a sample size of approximately 46,000 women with variations in incidence.
| Total sample size | Relative risk | Incidence of primary outcome in (per 1000 births) | |
|---|---|---|---|
| No decision support group | Decision support group | ||
| 46,000 | 0.50 | 3.00 | 1.50 |
| 46,000 | 0.56 | 4.00 | 2.25 |
| 46,000 | 0.61 | 5.00 | 3.05 |
| 46,000 | 0.64 | 6.00 | 3.85 |
Loss to follow-up
It was assumed that loss to follow-up for the short-term primary outcome would be negligible, as most of this information would be collected before the woman left the delivery room in which she had been recruited. For neonatal outcomes, a small number of babies were admitted to a neonatal unit separate from where they were born or planned to be born, in which case data were collected from these sites through the research midwives employed by the study in the participating centres. At the time of entry to the study all women were asked for permission for their contact details to be downloaded to the trial co-ordinating centre along with their clinical details from the Guardian system. The families selected for follow-up at 2 years were contacted by post 8 weeks after birth and informed that they had been selected for the follow-up study. Contact with families who agreed to take part was maintained during the period between birth and the follow-up assessment by sending a birthday card each year along with a FREEPOST change-of-address card to facilitate communication with University College London (UCL) Comprehensive Clinical Trials Unit about updated contact details.
Trial management
Research governance
The sponsor of the trial was initially the University of Oxford (January 2009 to May 2012), but changed to UCL when the trial moved (May 2012 to June 2016). The trial was run on a day-to-day basis by the project management group. This group reported to the Trial Steering Committee (TSC), which was responsible to the research sponsor. At each participating centre, local principal investigators reported to the project management group via the project-funded staff based at the UCL Comprehensive Clinical Trials Unit.
Insurance
NHS indemnity operated in respect of the clinical treatment being provided. In addition, the sponsor had appropriate insurance-related arrangements in place.
Trial Steering Committee
The trial was supervised by an independent TSC. The precise terms of reference for the TSC were agreed at its first meeting. A TSC charter similar to that used by the Data Monitoring Committee (DMC) (see Data Monitoring Committee) was completed.
Data Monitoring Committee
An independent DMC was established for the trial. This was independent of the trial organisers. The terms of reference for the DMC were agreed at the first meeting. A DMC charter was completed following the recommendations of the DAMOCLES (DAta MOnitoring Committees: Lessons, Ethics, Statistics) study. 48
During the period of recruitment to the trial, interim analyses were supplied, in strict confidence, to the DMC, together with any other analyses the DMC requested. In the light of interim data, and other evidence from relevant studies, the DMC would inform the TSC if, in its view, there was proof beyond reasonable doubt that the data indicated that any part of the protocol under investigation was either clearly indicated or contraindicated, either for all women or for a particular subgroup of trial participants. A decision to inform the TSC would in part be based on statistical considerations. Appropriate criteria for proof beyond reasonable doubt could not be specified precisely. A difference of at least three standard errors (SEs) in the interim analysis of a major endpoint may have been needed to justify halting, or modifying, the study prematurely. This criterion had the practical advantage that the exact number of interim analyses would be of little importance, and so no fixed schedule was proposed. 49 Unless modification or cessation of the protocol was recommended by the DMC, the TSC, collaborators and administrative staff (except those who supply the confidential information) would remain masked to the results of the interim analysis. Collaborators and all others associated with the study were able to write, through the trial office, to the DMC, to draw attention to any concerns they may have about the possibility of harm arising from the treatment under study, or about any other matters that may have been relevant.
Publication policy
The chief investigator was responsible for co-ordinating the dissemination of data from this study. All publications using data from the study to undertake original analyses were submitted to the TSC for review before release. To safeguard the scientific integrity of the trial, it was agreed that data from the study would not be presented in public before the main results were published, without the prior consent of the TSC. The success of the trial depended on a large number of midwives and obstetricians. For this reason, chief credit for the results would be given not to the committees or central organisers, but to all who collaborated and participated in the study. It was agreed that authorship at the head of the primary results paper would take the form ‘The INFANT Collaborative Group’, to avoid giving undue prominence to any individual.
Chapter 3 Substudy of maternal anxiety in labour during recruitment to the pilot phase of the INFANT trial
During the process of application to the Research Ethics Committee (REC) for approval to undertake the INFANT trial, the committee was concerned that the use of the decision support technology during labour may increase anxiety among the women taking part. The committee asked the trial team to provide some reassurance that participating in the trial would not result in unacceptable anxiety for the women taking part.
We developed the study described below to address this. Sections of this chapter have been reproduced with permission from Barber et al. 50 Copyright © 2013, MA Healthcare Limited. Quotations from participants in this qualitative study have been reproduced verbatim from this publication with permission from the journal.
Introduction
Anxiety is common in pregnancy51 and EFM can lead to increased anxiety. 52 A study in Australia reported interviews with 100 women shortly after a straightforward birth and found that only 15% reported no anxiety during labour and birth. 53
Confidential enquiries into perinatal deaths have repeatedly demonstrated that most poor infant outcomes arise during labour. EFM was introduced into clinical practice to try to prevent these poor outcomes; however, we know that poor interpretation of fetal heart rate patterns occurs. Improvements in CTG interpretation have to be sustainable and ideally be independent of clinicians’ abilities. Computerised interpretation and decision support have the potential to improve care.
If women are aware of an effective method of interpreting their baby’s heart rate during labour, and understand that this is safer than an individual clinician’s interpretation, this may result in reduced anxiety.
The INFANT decision support software assesses the CTG and provides a colour-coded ‘ladder of concern’, which appears on the CTG screen (see Chapter 1 for more detail).
The aim of this study was to explore whether or not the use of EFM during labour increases or reduces anxiety levels among women and whether or not the addition of the INFANT decision support software has a positive or negative effect on these anxiety levels. We initially used a survey to measure anxiety in women randomised to each arm of the INFANT trial. We then used qualitative interviews in a smaller number of women to explore their feelings of anxiety associated with the use of monitoring in labour and the decision support system.
Methods
Survey
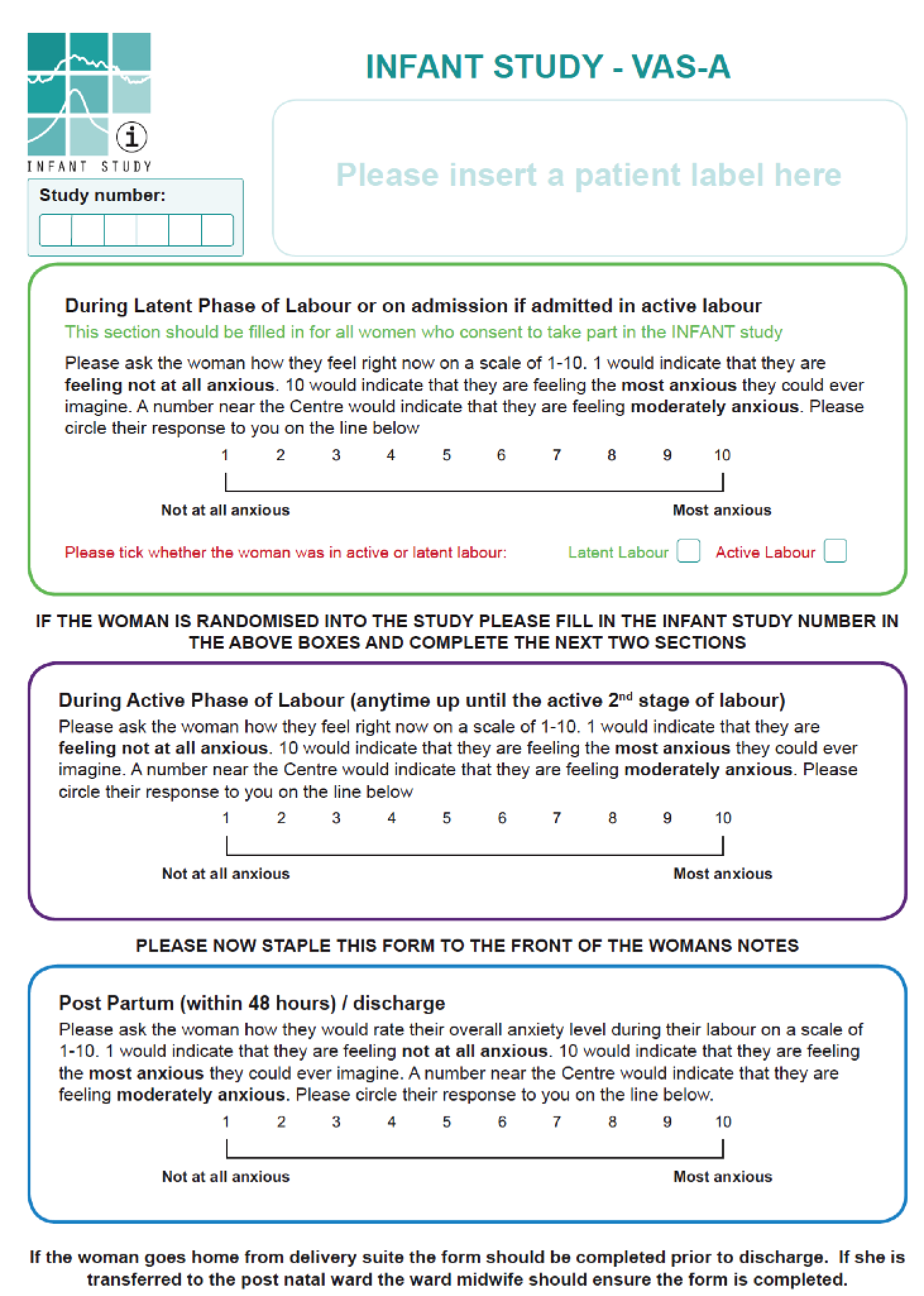
From 5 January 2010 to 18 July 2010, 469 women were recruited to the pilot phase of the INFANT trial from the Royal Blackburn Hospital. A total of 234 women were recruited to the CTG monitoring only (control) group and 235 were recruited to the CTG monitoring plus decision support (intervention) group. The eligibility criteria and process of recruitment are described in Chapter 2. In a subset of women approached to participate in the INFANT trial, we measured anxiety in the latent phase of labour when cervical dilatation was ≤ 3 cm with an effaced cervix. If the woman was recruited into the trial, we measured anxiety at a further two time points: during the active phase of labour when the cervical dilatation was 4–7 cm, and within 48 hours post partum. At each time point, the midwife asked women to rate their anxiety using a visual analogue scale – anxiety (VAS-A) from 1 (not at all) to 10 (very much so). Their responses were recorded on the VAS-A study form (Figure 10).
FIGURE 10.
The INFANT study visual analogue scale form.
Statistical analysis
The change in VAS-A scores in the two groups (control and intervention) (1) from the latent to the active phase of labour and (2) from the latent phase to the postpartum period was analysed using repeated measures analysis of covariance (ANCOVA). This enabled us to include all women in the analysis, even those with data at only one time point. The correlation between scores between phases was calculated using the Pearson correlation coefficient. Two-sided significance tests were used, taking a p-value of 0.05 as significant. The analysis was conducted using statistical software Stata version 11.1.
Qualitative study
Women from two of the study sites in the INFANT trial (Warrington Hospital and University Hospital of North Staffordshire) were approached to be interviewed about their experiences of birth and fetal monitoring by a single trained qualitative researcher. Women were approached after giving birth and before hospital discharge. A purposive sampling approach was used to ensure that equal numbers of women from the two arms of the trial were recruited, and that the number and severity of alerts was wide-ranging and well balanced between the two groups. The trial’s statistician identified potential women to be included and informed the qualitative researcher of their study number. The interviewer was masked to the women’s trial allocation and pattern of alerts until after the interviews were complete.
The interviews collected views of the whole birth experience but included semistructured prompting to explore women’s feelings about monitoring and their understanding of the INFANT trial. The interviewer and another senior qualitative researcher undertook the analysis jointly. An initial thematic analysis was undertaken using NVivo (QSR International, Warrington, UK) software to support the coding process. This was followed by a framework analysis, summarising the experiences and attitudes of each woman and mapping these against the woman’s characteristics (hospital of birth, study allocation, maternal age, gestation at trial entry and number of yellow and red alerts).
Results
Quantitative study
The VAS-A score sheets were completed by 275 out of 469 (59%) women [CTG monitoring only: 142 out of 234 (61%); CTG monitoring plus decision support: 133 out of 235 (57%)]. In the control group, data were available for 128 (55%) women from the latent phase, 104 (44%) for the active phase and 81 (35%) for the postpartum period. In the intervention group, data were available for 124 (53%) women for the latent phase, 106 (45%) for the active phase and 81 (34%) for the postpartum period.
The VAS-A scores are shown in Table 5. The scores were approximately normally distributed. In each group, anxiety levels increased from a score of around 5 points in the latent phase to around 6 points in the active phase, then dropped below 5 points in the postpartum period. There was no difference between groups in the change in anxiety from the latent to the active phase (p = 0.84) or from the latent phase to the postpartum period (p = 0.88). The scores were positively correlated: 0.48 between latent and active phase, 0.41 between the latent phase and the postpartum period, and 0.44 between the active phase and the postpartum period.
| Phase | VAS-A scores | Between-group difference in mean change (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| CTG monitoring only | CTG monitoring plus decision support | |||||
| n | Mean (SD) | n | Mean (SD) | |||
| Latent | 128 | 5.0 (2.7) | 124 | 4.8 (2.6) | ||
| Active | 104 | 5.7 (2.7) | 106 | 5.5 (2.9) | –0.08 (–0.93 to 0.76) | 0.84 |
| Postpartum | 81 | 4.5 (2.4) | 81 | 4.4 (2.4) | –0.06 (–0.84 to 0.71) | 0.88 |
Qualitative study
A total of 18 women were interviewed, including six with their birthing partner, who was either their partner or mother. Table 6 provides a list of the women interviewed with details of their hospital of birth, trial group, age, gestation at trial entry and number of alerts. Four women had no alerts, seven had at least one yellow but no red alerts and seven had at least one red alert. The number of red and yellow alerts were evenly distributed across the trial groups.
| Interview IDa | Hospital | Trial arm | Maternal age (years) | Gestation at randomisation (weeks) | Number of yellow alerts | Number of red alerts |
|---|---|---|---|---|---|---|
| 01 | Warrington | Control | 30 | 41 | 2 | 2 |
| 02 | Warrington | Control | 23 | 39 | 3 | 0 |
| 08 | Warrington | Control | 30 | 40 | 1 | 0 |
| 14 | Warrington | Control | 23 | 26 | 4 | 1 |
| 21 | Warrington | Control | 29 | 40 | 5 | 1 |
| 22 | Warrington | Control | 36 | 38 twins | 13 | 2 |
| 07 | Warrington | Decision support | 49 | 41 | 5 | 0 |
| 15 | Warrington | Decision support | 28 | 39 | 0 | 0 |
| 18 | Warrington | Decision support | 26 | 41 | 1 | 0 |
| 19 | Warrington | Decision support | 38 | 37 | 4 | 2 |
| 20 | Warrington | Decision support | 36 | 38 | 3 | 0 |
| 04 | North Staffs | Control | 37 | 39 | 0 | 0 |
| 05 | North Staffs | Control | 31 | 37 | 0 | 0 |
| 11 | North Staffs | Control | 39 | 42 | 3 | 0 |
| 03 | North Staffs | Decision support | 27 | 40 | 0 | 0 |
| 09 | North Staffs | Decision support | 28 | 41 | 8 | 2 |
| 10 | North Staffs | Decision support | 21 | 41 | 6 | 1 |
| 16 | North Staffs | Decision support | 31 | 38 | 4 | 0 |
Levels of understanding
Among participants, there was a patchy understanding of what the trial was about, beyond a general view that it was seeking to understand people’s experiences in labour and improve care for other women in the future. This is a finding that is widely reflected in the literature. 54–56 Women were frequently unclear about what is ‘normal’ monitoring of the baby in labour and what is part of the trial, and whether or not they would have been monitored anyway:
It was nothing, you know, that was intervening with anything, so I hadn’t minded at all. I just hadn’t been totally aware that I’d be hooked up to a machine the whole time. I just thought it was if you needed to be on the machine, they’d then monitor it through the computer . . . I wasn’t sure whether I had to be on the machine for the sake, you know, for the baby’s sake and whatnot or whether it was because I was, because of the study. I wasn’t too sure what it was that I had to actually be on it for.
Participant 02, control arm
My understanding at the time of it was really just that it would be somebody maybe 1 or 2 years after the birth would be following up with a questionnaire and maybe a phone call or something just to see what my experience was . . . I don’t recall the monitoring being part of the study but a huge deal wasn’t made of it anyway, but as I say it wouldn’t have been a problem if it was, but I was going to be monitored anyway with being induced.
Participant 05, control arm
Participant 05 (control arm) also said, ‘I didn’t remember them saying which group I was in. Is there different groups?’. Similarly, participant 14 (control group) said, ‘I don’t remember anything to do with groups’. Furthermore, she did not clearly recall any monitoring at all. This participant had a vague recollection of ‘bands’ being used for monitoring her baby’s heart, and that the baby’s heartbeat had dropped at one stage, but said, ‘I definitely don’t remember anything being put round me’ other than her transcutaneous electrical nerve stimulation machine. Similarly, a woman in the intervention arm (participant 18) said, ‘Groups? They might have mentioned it but I don’t remember it’.
One couple (04, control arm) knew they had been allocated to a group but were unaware of how and why they had been selected for participation. The male partner had spent quite a lot of time trying to work out for himself what the monitor readings meant:
It would be nice to know.
You know, why your specific case was chosen. Was it just it was randomly chosen or was it because they wanted to, to look at it for a certain reason?
Or is it just that we were placed in this particular bracket, you know?
Well, was it just random that our, you know, results were picked out? Or was it because, like we think, [female] had a rapid delivery? Or, you know, or something come up on the results why they decide?
However, other participants in both groups were aware that there were different arms and demonstrated an awareness of which arm they were in as well as how they were allocated to a group:
Basically what they told us, well it says in the leaflet as well, that you might not be selected for the trial on a computer. Basically she tapped in some information on the screen and then it come up whether you’d be selected for it. And she typed it in and she said we weren’t selected for it.
Participant 01, control arm
It was frequently suggested by participants that being given information about the trial towards the end of pregnancy rather than during the labour episode may improve understanding, as it was difficult for people to absorb the information or give it any priority during labour. This approach of providing information during pregnancy should have been happening in the centres, and a few women did recall discussing it at antenatal appointments or seeing posters about it in previous visits, but others did not. Women also mentioned feeling ‘vague’ or ‘confused’ about the information provided during labour because of the pain relief medication they had taken.
There was little evidence that feeling underinformed had led women to regret taking part. This was because EFM was commonly seen as routine care and not particularly invasive. Even women who disliked not being able to mobilise because of the monitoring failed to express major concerns about the information and consent process.
One exception to this was a woman in the decision support arm who said that she felt ‘mithered’ by having to answer all the questions needed to take part in the trial:
I didn’t want to be asked; I just wanted to be left alone to get on with going through the labour . . . I just wanted to be left alone and that took like 15, 20 minutes to do all that so like she was asking me questions and I was contracting as well . . . I signed it because I thought – and I said this to my mum this morning – the only reason that I signed it was because I thought if the midwife thinks that we’re co-operating with this then she’ll give us some drugs [laughter]. She’ll give me some more drugs, that’s what I thought . . . It was something that was so shoved in my face and I didn’t really have a choice basically . . . because I wanted to keep my midwife nice and sweet.
Participant 15, decision support arm
She complained that she ‘hated’ being monitored because it restricted her movements. It did not make her feel more anxious, but she regretted taking part because she felt that it spoilt her otherwise good experience of a spontaneous vaginal birth. She was so bothered by the restrictions on movement that she requested that the monitoring be stopped. It is clear from her interview that she did not understand that she would have had monitoring anyway and that it was not a consequence of taking part in the trial. She said that monitoring should be used ‘only if it’s an emergency for the baby’ and did not understand that the purpose of monitoring was to detect concerns before they become emergencies. When this was discussed during the interview she commented:
So in that case it does change my views differently then, then yes, if I would have known it was something to do with protecting the baby then yes I would have had it on in labour.
Participant 15, decision support arm
Monitoring and reassurance
Women in both groups of the trial reported finding monitoring reassuring. There was no difference in the pattern of responses between the two groups, or between women with few alerts and those with many.
For example, one woman said:
That showed what the heartbeat was doing, you know, ranging from sort of whatever it was, 100 to 150. And there was a guide next to it to say what’s acceptable and what’s, you know, risky. [um] So that was quite reassuring, wasn’t it?
Participant 04, control arm, no alerts
Oh, I thought it was brill, to be honest, because as I say a lot of the time I felt a little bit out of the loop. From where I was sitting I could see all the screens and what was going on so I found that, you know, sort of quite comforting.
Participant 03, decision support arm, no alerts
Being able to monitor what was happening with [baby 1] and sort of midway through what was happening with [baby 2] with their heart rates and things made me very reassured.
Participant 22, control arm, 13 yellow and two red alerts
I did quite enjoy having the monitors on actually. . . . you can just see, see sort of their heartbeat and how strong your contractions were, whereas normally you couldn’t, you haven’t got that.
Participant 09, decision support arm, eight yellow and two red alerts
Several women (or their partners) said that the monitor also helped reassure the partner. This generated a sense of involvement because they could observe a contraction and support their partner appropriately. Participant 19 (decision support arm) said that her partner ‘kept checking the paper. I think he was fascinated by it’.
Monitoring and restriction of movement
On those occasions when objections were raised about monitoring, this was most usually because of the restrictions it placed on movement. Some women said that they did not find the monitor restrictive or uncomfortable, including participant 19 (decision support arm). When asked if she could move around she said:
Yes, I was on my side for most of it, they said that’s the most comfortable position for me so I just stayed on my side.
Did you mind being monitored at all?
No, no anything that helps really.
When you say helps, in what way?
I mean, well like just in case there’s any complications, I’d rather be monitored and have them spot them, so.
You found it sort of reassuring?
Yes, yes extra reassurance.
However, other women talked about wanting to alleviate pain by moving around or to be able to go to the toilet. The monitor restricted this movement and some women felt that the monitor straps were uncomfortable. Some women expressed a preference for a wireless monitor. One woman had heard that this was available in other hospitals. Women who disliked being monitored expressed varying degrees of resignation, assuming that they would have their movement restricted anyway (e.g. because of an epidural) or that the disadvantages of limited movement had to be balanced against the benefits for the baby and their own peace of mind:
The fact that it restricted me, that was a bit of a pain, but I don’t know whether I would have moved round that much or, you know. Because I mean I could have sat on the ball, you know, the blow up ball . . . rather than the chair, which is one of the things they recommend. But I didn’t want it. I just wanted to sit on the chair . . . No, it’s reassuring, I think because you know you don’t want your baby’s heart rate to go down. And it was quite good as well in that my husband could see when the contractions were coming on . . . I think we, it made no difference to us taking part, you know, it wasn’t detrimental, it wasn’t, I was going to be monitored anyway whether I took part in the trial or not.
Participant 20, decision support arm, three yellow alerts
And the reason I agreed, why I thought it was brilliant, was because it’s extra checks, it’s extra checks for him. And I think well he’s going to be monitored closely from now on which is amazing but, you know, because I’d do anything for him, you know, healthy baby, and if it picks up on something, fantastic, and it’s doing research for everything else, so yes . . . So it turned out that I was on this monitor for this and my heart and everything so I couldn’t move off the bed for all them hours, they wouldn’t let me move. So I couldn’t walk round, so my plan had gone way out the window. I wasn’t walking round, I’d not had my bath at all, I’m stuck on this bed, I’ve been induced. . . . The birth plan was just sit on the ball, stay upright and move as much as you can. That was basically it. And nothing happened like that and I’m saying ‘Can I get off this bed?’ ‘No, you’re being monitored for this and you’re being monitored for your heart, you’re being monitored, baby’s being monitored because of the poo because that’s dangerous, no you can’t move off this bed’. I was like ‘Ohhhh’, so I just lay down the whole day. Which was really nice and very boring.
Participant 11, control arm, three yellow alerts
Monitoring and anxiety
Women did describe anxious moments during their labour; however, these did not seem to be associated with monitoring, but were more related to the urgent comments and behaviour of staff as they responded to the monitoring results or to other clinical concerns.
There was only one clear exception to this:
So it was quite good because obviously people could see what was going on . . . because the doctor come in and said I’ve been watching you on the screen . . . Sometimes, I kept hearing her say when the heartbeat, because they kept saying she’s being naughty, they just kept saying that, and it was flashing messages up to them and I heard her say a couple of times ‘I think that heartbeat’s okay for the minute’ but that was telling her it was, so . . . They did talk about it quite a bit, they used it as a guide but said they would use their own judgement to make any decisions . . . In a way I don’t know if it’s a good thing or a bad thing, though, because right towards the end because her heartbeat had been playing up all day, we were all focused on it . . . Everyone was just focused on this monitor and the heartbeat, so I think that got a little bit stressful, because I did end up telling him [partner] ‘Stop telling me what’s happening or talking about it’ because it was making me panic . . . He says to me afterwards that he was, it was the most scared he’d ever been in his life but at the time he seemed really quite cool and took it all in his stride but obviously he was just putting a show on for me.
Participant 16, decision support arm, four yellow alerts
Nonetheless, participant 16 concluded: ‘The study didn’t really affect the birthing experience at all so that, you know, I’d do it again, that’s fine’.
This woman’s interpretation was that the staff used the decision support to justify postponing any intervention. She was glad not to have a caesarean section, but she felt that she could have had an instrumental delivery earlier: ‘I do think that because her heartbeat had been playing up so long throughout the day and things weren’t moving on for me that they should have looked at me a bit earlier and made a decision a bit earlier’. The woman’s mother, who was present during the labour and birth, and was interviewed with her, was even more convinced of the need for an early delivery:
It was about half nine and I can remember the midwife saying, ‘We’re going to leave you till eleven and if nothing’s happened by eleven we’ll get the doctor and we’ll see about taking you to theatre. But then when 11 o’clock came they had a look at you and you’d dilated quite a bit by then so they said we’ll leave you another hour, that took you to 12.00 and then again we’ll leave you another 2 hours, and that annoyed me because that’s what they kept saying. Although, you know, she was well into labour I think they should have took her for a C-section at about 11 o’clock, I do.
Mother of participant 16, decision support arm
This example suggests that CTG with decision support could lead to some cases of raised anxiety levels. There were two examples in the control group in which monitoring without decision support also caused some anxiety. Participant 08 reported that her mother had been worried at one point, although she herself was relaxed and asleep:
My mum was watching where they was monitoring me and I think I fell asleep so as I had diamorphine. And at one point, because they had one on me and one obviously for the baby’s heartbeat, and the baby must have moved so it went to zero and my mum thought it was me. She said she couldn’t see my chest rising or anything and thought I wasn’t breathing and she looked at this monitor and seen zero and she was like, ‘She’s not breathing, she’s not breathing’ and I woke up and I was like ‘What?’ [laughter].
Participant 08, control arm
The partner of participant 21 (five yellow alerts and one red alert) recalled his own fears:
Yes it was helpful, but it was also, I think, in some respects that she couldn’t see it was as well because there was quite a few scary moments.
You see, I couldn’t see that because it was . . .
Yes, I mean his heart, I could see his heartbeat going down and she couldn’t see it, so I was a bit worried there.
But as soon as his heartbeat started going down they all, them sitting on their desk came in to have a look.
They were both surprised at the amount of paperwork the monitoring generated for the midwife, and felt that this detracted staff from providing reassurance and support:
You need somebody that’s talking to you, explaining things and actually maintaining the relationship with you as opposed to just doing, writing down and checking the monitors and things like that because otherwise you end up panicked and it’s not good.
Participant 21, control arm, 5 yellow alerts and 1 red alert
Discussion
We found no evidence from the quantitative analysis of anxiety, using VAS-A scores, that CTG monitoring plus decision support is associated with a change in women’s anxiety levels compared with CTG monitoring alone. Labour appears to be an anxious time for most women, and uncertainty about whether or not their baby will be all right contributes to this anxiety. Alerts indicating abnormalities of the fetal heart rate are very likely to lead to anxiety for parents and clinicians, but this anxiety is appropriate if the alert also prompts a response that ensures that the baby is kept safe. If the decision support software results in the number of false ‘abnormal’ heart rate patterns being reduced, and the true-positive rate is optimised (i.e. abnormal CTGs are not overlooked), then any anxiety engendered by monitoring would be minimised and would probably be acceptable to women.
Findings from the qualitative interview illustrate that EFM per se did lead to significant anxiety for the women interviewed. Concerns about monitoring were commonly to do with discomfort and restriction of movement. Some women reported finding the activity of monitoring reassuring. There is a possibility that the use of monitoring itself leads to a belief that birth is a risky process, which therefore needs careful monitoring. This in turn may lead people to report that they find monitoring reassuring. It has been argued that women in labour ‘became very susceptible to the reassurance of the cultural props around them. The symbolic messages of the hospital setting gave them something to cling onto’. 57 Women’s feelings of control during labour are an important predictor of a positive birth experience, and may help in decreasing anxiety. 58 This paper also argued that information giving during labour and participation in decision-making were crucial in helping women to achieve feelings of control. It is possible that the trial information, given out in the booking appointment in early pregnancy, at the time of the 22-week anomaly scan and at the routine 34-week appointment, contributed to women achieving these feelings of control. In a trial examining different approaches to the presentation of information about prenatal screening,59 the information provided did not increase anxiety. Very detailed information has been shown to have the potential to reduce anxiety. 59
This small qualitative sample cannot be generalised to all of the women who participated in the trial. However, when considered alongside the results of the survey, we feel confident that raised anxiety levels resulting directly from EFM with decision support is uncommon. When women are anxious in labour, it appears to be more to do with general feelings of anxiety about the baby’s health, which may be exacerbated by staff behaviours. However, there was one woman in our sample whose anxiety seemed to be directly linked to the use of decision support. This means that we need to be aware of the possibility of this happening with other women and consider ways to provide reassurance and explanation. We also found that CTG monitoring without decision support caused some anxiety and we need to consider how further explanation and reassurance can help these women.
Details of ethics approval
The Northern and Yorkshire REC gave approval to the study. The reference number of the ethics approval is 09/H0903/31.
Chapter 4 Analysis plan for the trial
Primary analysis
An outline analysis plan was developed and agreed by the TSC and the DMC before any data were collected, and a detailed plan was agreed before data were unmasked and analysed. Demographic factors and clinical characteristics were summarised with counts (percentages) for categorical variables, means (SDs) for normally distributed continuous variables or medians [interquartile ranges (IQRs) or entire ranges] for other continuous variables. The primary analysis was a comparison of the management approaches assigned at randomisation. Participants were analysed in the groups to which they were randomly assigned regardless of deviation from the protocol or treatment received. Comparative statistical analysis entailed calculating the risk ratio (RR) plus 95% CI for the primary outcome (99% CIs for all other dichotomous outcomes), the mean difference (plus 99% CI) for normally distributed continuous outcomes or the median difference (plus 99% CI) for skewed continuous variables. Analyses were adjusted for the stratification factors used in the randomisation procedure: centre and singleton/twin pregnancy. Analysis of secondary outcomes was clearly delineated from the primary analysis in the statistical reports produced. For secondary outcomes, a 1% level of statistical significance was employed to take account of the number of comparisons. The trial is reported according to the principles of the Consolidated Standards of Reporting Trials (CONSORT) statement.
Prespecified subgroup analysis
The consistency of the effect of decision support on the babies of groups of women recruited to the trial was explored to see whether or not decision support is of particular help to the babies of specific subgroups of women using the statistical test of interaction. Therefore, the categories of prespecified subgroup analysis were:
-
singletons versus twins
-
suspected growth restriction at labour onset versus no growth restriction
-
centre
-
body mass index (BMI) group [underweight (12–18.4 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and/or unrecorded obese (30–70 kg/m2)].
Data collection schedule
Information was to be collected at the following times:
-
electronically during labour via the Guardian system
-
all women
-
-
Post Birth Data Collection (PBDC) Form M (Mother) (see Appendix 8)
-
all women receiving a higher level of care, surgery or a procedure in theatre following delivery
-
-
PBDC Form B (Baby) (see Appendix 5)
-
all babies receiving a higher level of care or surgery following birth
-
-
PBDC Chart B (Baby) Neonatal Encephalopathy Data (see Appendix 6)
-
all babies receiving a higher level of care and classified as having NNE
-
-
Death of a baby in the INFANT study form (see Appendix 7)
-
all intrapartum stillbirths and neonatal deaths up to 28 days after birth
-
-
Parent’s Questionnaire at 24 months (health and development outcomes)
-
a subset of 7000 participants consenting to follow-up
-
-
Parent’s Questionnaire at 12 and 24 months (health economic items)
-
a subset of 700 participants within the follow-up sample of 7000.
-
Derivation of variables
Primary outcomes
-
Composite of poor neonatal outcomes, defined as any of:
-
intrapartum stillbirths, except deaths as a result of congenital anomalies (Table 7)
-
neonatal deaths up to 28 days after birth, except deaths as a result of congenital anomalies (Table 8)
-
moderate or severe NNE (Table 9)
-
admission to neonatal unit within 48 hours of birth for ≥ 48 hours with evidence of feeding difficulties, respiratory illness or NNE (Table 10).
-
-
PARCA-R composite score at 24 months:
-
Total sum of scores on the PARCA-R in section A, B and C of the Parent Questionnaire at 24 months (Appendix 9).
-
| Baby outcome = ‘Stillbirth’ in Guardian | |
| MINUS | Any deaths as a result of congenital anomalies recorded on the death of a baby in the INFANT study form |
| Baby outcome = ‘Early neonatal death’ in Guardian and died within 28 days after birth | |
| OR | A ‘Yes’ response to Q14 on the PBDC Form B (Baby) and died within 28 days after birth |
| OR | A completed death of a baby in the INFANT study form and died within 28 days after birth |
| OR | Deaths notified by the NHS Information Centre (England) and the NHS Greater Glasgow & Clyde Safe Haven (Scotland) up to 28 days after birth |
| MINUS | Any deaths as a result of congenital anomalies recorded on the death of a baby in the INFANT study form |
| A ‘Yes’ response to therapeutic hypothermia on the PBDC Chart B (Baby) Neonatal Encephalopathy Data | |
| AND | Confirmed by a masked review committee |
| A ‘Yes’ response to Q2 on the PBDC Form B (Baby) | |
| AND | A ‘Yes’ response to Q5 or Q6 on the PBDC Form B (Baby) |
| AND | A ‘Yes’ response to Q8 on the PBDC Form B (Baby) |
| AND | A ‘Yes’ response to Q9 on the PBDC Form B (Baby) |
| AND | Confirmed as compromised at birth by a masked review committee (score of ≥ 3 on Primary Outcome Review Scoring Sheet) |
Neonatal secondary outcomes
-
Intrapartum stillbirth except deaths as a result of congenital anomalies.
-
Neonatal deaths up to 28 days after birth except deaths as a result of congenital anomalies.
-
Moderate or severe NNE.
-
Admission to neonatal unit within 48 hours of birth for ≥ 48 hours with evidence of feeding difficulties, respiratory illness or NNE.
-
(For the above, see definition in Primary outcomes.)
-
-
Admission to a higher level of care within 48 hours of birth for ≥ 48 hours [A ‘Yes’ response to Q2 on the PBDC Form B (Baby)].
-
An Apgar score of < 4 (from the Guardian variable ‘Apgars 5 mins’).
-
Distribution of cord blood gas data for cord artery pH (summarise the variable cord artery results in Guardian).
-
Metabolic acidosis.
-
A cord artery pH of < 7.05 and a base excess of ≥ 12 mmol/l for the variable cord artery results in Guardian.
-
-
Resuscitation interventions.
-
Count of the number of interventions (listed under ‘Resuscitation Type’ in Guardian).
-
-
Seizures.
-
A ‘Yes’ response to Q7 on the PBDC Form B (Baby).
-
-
Destination immediately after birth.
-
Summarise the Guardian variable ‘infant transfer dest’.
-
-
Length of stay (Table 11).
| ‘Infant Discharge Date’ in Guardian | |
| OR | Date of discharge recorded in Q12 of the PBDC Form B (Baby) |
| MINUS | ‘Delivery Date Time’ in Guardian |
Maternal secondary outcomes
-
Mode of delivery.
-
Recorded in Guardian in field ‘Mode of Delivery’.
-
-
Operative intervention (caesarean section and instrumental delivery) for:
-
fetal distress or
-
failure to progress or
-
combination of fetal distress and failure to progress or
-
other reason.
-
Indications in Guardian recorded in the fields: ‘Forceps Indicators’, ‘Ventouse Indicators’ and ‘CS Indicators’ were coded into these four categories according the rules listed in Table 12. When a woman had more than one indication recorded and at least one was fetal distress and at least one was failure to progress, she was classified as category iii.
-
-
-
Grade of caesarean section.
-
Recorded in Guardian in field ‘CS Priority’.
-
-
Episiotomy.
-
Recorded in Guardian in field ‘Episiotomy’.
-
-
Any episode of fetal blood sampling.
-
Recorded in Guardian in field ‘No of FBS’.
-
-
Length of first stage, second stage and total length of labour from trial entry.
-
Recorded in Guardian, was calculated from fields ‘Labour start time’, ‘Second stage/fully dilated’ and ‘Delivery Date Time’.
-
-
Destination immediately after birth.
-
Recorded in Guardian in field ‘Mother transfer dest’.
-
| Indications for instrumental vaginal delivery and caesarean section | Fetal distress | Failure to progress | Other |
|---|---|---|---|
| Abnormal CTG | Yes | ||
| Abnormal FBS pH | Yes | ||
| Abnormal presentation or lie | Yes | ||
| APH | Yes | ||
| Intrapartum haemorrhage | Yes | ||
| Breech presentation | Yes | ||
| Cephalopelvic disproportion | Yes | ||
| Chorioamnionitis | Yes | ||
| Cord prolapse or presentation | Yes | ||
| Delay in first stage | Yes | ||
| Delay in second stage | Yes | ||
| Eclampsia | Yes | ||
| Failed induction | Yes | ||
| Failed forceps/ventouse | Yes | ||
| Failed trial of forceps | Yes | ||
| Failed trial of ventouse | Yes | ||
| Failure to progress | Yes | ||
| Fetal compromise – abnormal CTG | Yes | ||
| Fetal compromise – meconium stained liquor | Yes | ||
| Fetal compromise suspected or indicated | Yes | ||
| Fetal reason | Yes | ||
| HELLP | Yes | ||
| Intrapartum haemorrhage | Yes | ||
| IUGR | Yes | ||
| Low scalp pH | Yes | ||
| Malpresentation | Yes | ||
| Malpresentation/unstable lie | Yes | ||
| Maternal condition | Yes | ||
| Maternal distress/exhaustion | Yes | ||
| Maternal effort contraindicated | Yes | ||
| Maternal hypertension | Yes | ||
| Maternal medical condition | Yes | ||
| Maternal medical disease | Yes | ||
| Maternal request | Yes | ||
| Multiple pregnancy | Yes | ||
| Non-reassuring CTG | Yes | ||
| Non-reassuring FBS | Yes | ||
| Obstructed twin/triplet | Yes | ||
| Other | Yes | ||
| Other (fetal) | Yes | ||
| Other (maternal) | Yes | ||
| Other maternal medical history | Yes | ||
| Placenta abruption | Yes | ||
| Placenta praevia | Yes | ||
| Planned as elective | Yes | ||
| Pre-eclampsia | Yes | ||
| Presumed fetal compromise | Yes | ||
| Previous caesarean section | Yes | ||
| Previous infertility | Yes | ||
| Previous lower-segment caesarean section | Yes | ||
| Previous poor obstetric outcome | Yes | ||
| Previous obstetric history | Yes | ||
| Previous traumatic vaginal delivery | Yes | ||
| Previous uterine surgery | Yes | ||
| Prolonged second stage | |||
| Pyrexia in labour | Yes | ||
| Ruptured uterus | Yes | ||
| Slow progress in first stage | Yes | ||
| Slow progress in second stage | Yes | ||
| Suspected fetal distress | Yes | ||
| Unstable lie | Yes |
Quality-of-care outcome
Adverse outcome and suboptimal care (Table 13).
| Composite primary outcome (see definition in Primary outcomes) | |
| AND | Metabolic acidosis (see definition in Neonatal secondary outcomes) |
| AND | Judged to have experienced suboptimal care in labour by a panel of experienced clinicians masked to allocation |
| OR | Stillbirth or neonatal death not as a result of a congenital anomaly |
Process outcomes
Process outcomes were derived for the control group in the same way as the intervention group; the decision support software was running in the background even though it was not displayed, so it was possible to review the pattern of alerts retrospectively across the trace.
-
Number of CTG abnormalities (blue, yellow and red levels of concern) detected by the decision support software, after trial entry.
-
Recorded in Guardian in fields ‘Number of Blue after randomisation’, ‘Number of Yellow after randomisation’ and ‘Number of Red after randomisation’.
-
-
Number of blue levels of concern on the decision support software, indicating a mild abnormality on the CTG, after trial entry.
-
Recorded in Guardian in field ‘Number of Blue after randomisation’.
-
-
Number of yellow levels of concern on the decision support software, indicating a moderate abnormality on the CTG, after trial entry.
-
Recorded in Guardian in field ‘Number of Yellow after randomisation’.
-
-
Number of red levels of concern on the decision support software, indicating a severe abnormality on the CTG, after trial entry.
-
Recorded in Guardian in field ‘Number of Red after randomisation’.
-
-
Number of women with at least one yellow level of concern on the decision support software, indicating an abnormality on the CTG, after trial entry.
-
Recorded in Guardian in field ‘Number of Yellow after randomisation’.
-
-
Number of women with at least one red level of concern on the decision support software, indicating a severe abnormality on the CTG, after trial entry.
-
Recorded in Guardian in field ‘Number of Red after randomisation’.
-
-
Time from first red level of concern after trial entry to birth.
-
Recorded in Guardian, calculated from fields ‘Date Time 1st Red after randomisation’ and ‘Delivery Date Time’.
-
-
Number of thumb entries per hour from time of trial entry to first yellow level of concern or until fully dilated (10 cm) if no abnormality detected or first yellow level of concern occurred prior to trial entry.
-
Recorded in Guardian in fields ‘Count of thumbprints between randomisation and first yellow’ and ‘Count of thumbprints between randomisation and fully dilated’. The rate will be calculated using the fields ‘Date time 1st yellow after randomisation’, ‘Second stage/fully dilated’ and ‘Delivery Date Time’.
-
-
Number of vaginal examinations after trial entry.
-
Recorded in Guardian in field ‘No of VEs after randomisation’.
-
-
Epidural analgesia after trial entry.
-
Recorded in Guardian in fields ‘PD did woman have an epidural’ and ‘PD did woman have an epidural time’. Only count if first recorded after the time of trial entry.
-
-
Labour augmentation after trial entry.
-
Recorded in Guardian in fields, ‘Syntocinon in 1st or 2nd stage’ and ‘Syntocinon in 1st or 2nd stage time’. Only counted if the first recording was after the time of trial entry.
-
-
Presence of meconium after trial entry.
-
Recorded in Guardian in field ‘PD any meconium recorded during labour’ and ‘PD any meconium recorded during labour time’. Only counted if the first recording was after the time of trial entry.
-
Health and development outcomes at 24 months
-
Components of the PARCA-R:
-
Non-verbal cognition scale. (Sum of scores in Section A of the Parent Questionnaire at 24 months: ‘Your child at play’ Q1–33.)
-
Vocabulary subscale. (Sum of scores of the 100 items in Section B of the Parent Questionnaire at 24 months: ‘What your child can say’.)
-
Sentence complexity subscale. (Sum of scores in Section C of the Parent Questionnaire at 24 months: ‘Your child’s understanding’ Q1–18.)
-
-
Infant deaths at 24 months (Table 14).
-
Disability status at age 2 years (Table 15).
-
Cerebral palsy diagnosis.
-
A ‘Yes’ response to Q9 in Section G of the Parent Questionnaire at 24 months.
-
-
Health economic outcomes.
-
Ever been given breast milk or put to the breast.
-
A ‘Yes’ response in Section 1 of the Health Economic Parent Questionnaire at 24 months.
-
-
Age when last breastfed or put to the breast (days).
-
Convert response categories to days in Section 1 of the Health Economic Parent Questionnaire at 24 months.
-
-
| Baby outcome = ‘Early neonatal death’ in Guardian | |
| OR | A ‘Yes’ response to Q14 on the PBDC Form B (Baby) |
| OR | A completed Death of a baby in the INFANT study form |
| MINUS | Any deaths as a result of congenital anomalies recorded on the death of a baby in the INFANT study form |
| AND | Deaths notified by the NHS Information Centre (England) and NHS Greater Glasgow & Clyde Safe Haven (Scotland) up to 2 years after birth |
| Sections A–C: cognitive function |
|
| Sections B–C: communication |
|
| Section D: physical ability | Walking:
|
Sitting:
|
|
Hand use:
|
|
Head control:
|
|
| Section E: vision |
|
| Section F: hearing |
|
| Section G: growth |
|
| Section G: seizures |
|
| Section G: feeding |
|
| Section G: respiratory |
|
| Section G: other disability |
|
Missing data for any components of the disability classification questions meant that the overall classification may result in several categories:
-
Major.
-
At least one component in sections A–G is classified as major.
-
-
At least non-major.
-
One component in sections A–G is classified as non-major, but one item is missing and therefore it is not possible to conclude that the overall classification is not major.
-
-
Non-major.
-
The most severe item in sections A–G is classified as non-major and there are no other missing data.
-
-
At most non-major.
-
Any missing items in sections A–G could only be classified as non-major or no disability and all other items are classified as no disability.
-
-
None.
-
No data re missing and all items in sections A–G are classified as no disability.
-
-
Not known.
-
No disability reported but when some data items in sections A–G are missing.
-
In order to derive the classification ‘Major’ or ‘Non-major’ in the final report, all infants with ‘At least non-major’ disability were classified as ‘Non-major’ and all infants with ‘At most non-major’ disability were classified as ‘None’.
Protocol violation
A protocol violation is the intended failure to comply with the final study protocol as approved by the REC and research and development departments, an example being serious non-compliance with the protocol resulting from fraud or misconduct that affects participant rights, safety and/or the integrity of the resultant data. Any violations were reported to the sponsor and REC as soon as possible.
Protocol deviation
A protocol deviation is an unintended failure to adhere to the final study protocol.
In this trial, the following were defined as protocol deviations.
Participants randomised in error
These include women:
-
who did not receive EFM
-
who had triplets or a higher-order pregnancy
-
who were at < 35 weeks’ gestation
-
whose infant had a known gross fetal abnormality
-
who were aged < 16 years
-
who were not able to give consent to participate as judged by the attending clinicians
-
who received an elective caesarean section prior to the onset of labour.
Participants who do not receive the allocated intervention
These included women in the following arms:
-
‘CTG with no decision support’ arm who received partial or full decision support
-
‘CTG with decision support’ arm who did not receive decision support.
Follow-up completed outside set time window
This included infants who were aged 2 years ± 3 months when the 2-year follow-up questionnaire was completed.
Primary analysis strategy
For the primary analysis, participants were analysed in the groups into which they were randomly allocated (i.e. comparing the outcomes of all women and babies allocated ‘CTG with no decision support’ with ‘CTG with decision support’), regardless of the allocation received.
The two groups were compared by calculating the treatment difference adjusted for the stratification factors used in the randomisation (centre and singleton/twin pregnancy). The adjusted analysis took account of the correlation between treatment groups introduced by stratifying the randomisation (which forces outcomes between treatment arms to be similar apart from any treatment effect). 62 Both adjusted and unadjusted estimates were presented for all outcomes, but the primary inference was based on the adjusted analysis.
The unit of randomisation was birth episode, which raises the issue of non-independence of observations. Some women had more than one delivery over the study period and may have been randomised into the trial more than once if they were eligible, but this was estimated to be very unlikely. Based on national statistics and average interpregnancy intervals in the UK, we anticipated that around 10% of women in this cohort would have a subsequent delivery within the study period, but a smaller proportion would have two consecutive births monitored by CTG. 63,64 In addition, around 1.5% of women would be expected to have twin deliveries,65 but this proportion was likely to be lower in this cohort as some twin births will occur before 35 weeks’ gestation and a large proportion of twin term births are by elective caesarean section.
We anticipated the proportion of non-independent observations within and between pregnancies to be much lower than 10%; however, some outcomes can have a large intracluster correlation coefficient – in particular the 2-year outcomes collected via the parent questionnaire – so clustering was taken into account in the analysis. 66,67
Descriptive analysis population
Baseline demographic and clinical characteristics were reported for each delivery for all women randomised for whom we had data available, excluding protocol violations and women randomised in error who did not give consent or who were aged < 16 years.
Comparative analysis population
-
Maternal outcomes.
-
All women randomised for whom data were available, excluding protocol violations and women randomised in error who did not give consent or who were aged < 16 years. For women with more than one birth episode during the study period, baseline characteristics and maternal outcomes were reported on each occasion. For twin births the mode of delivery of the first twin delivered was reported.
-
-
Short-term neonatal outcomes.
-
All babies including both twins, excluding protocol violations and women randomised in error who did not give consent or who were aged < 16 years.
-
-
The 24-month health and development outcomes.
-
A sample of 7000 infants recruited during the first 2 years of the trial, excluding protocol violations, women randomised in error who did not give consent or who were aged < 16 years and adopted children.
-
Interim analysis population
Different denominators were used in the annual interim analysis.
-
The total number of trial participants, excluding protocol violations and women randomised in error who did not give consent or who were aged < 16 years.
-
The number of women with post-birth data.
-
The number of babies with post-birth and/or 24-month follow-up data.
Representativeness of trial population and participant throughput
The flow of participants through each stage of the trial was summarised using a CONSORT diagram. 68 We reported the numbers of women who:
-
were randomly assigned
-
received the intended intervention
-
withdrew before or during CTG monitoring
-
were included in the primary analysis at discharge
-
were lost to follow-up
-
were included in the analysis at 24 months.
Baseline comparability of randomised groups
Participants in the original two randomised groups were described separately with respect to:
-
maternal age
-
ethnic group
-
singleton or twin pregnancy
-
gestational age at trial entry
-
BMI at booking visit (if recorded)
-
smoking history at booking visit (if recorded)
-
parity
-
obstetric history
-
cervical dilatation at trial entry (if recorded)
-
intrauterine growth restriction (IUGR) suspected at labour onset
-
labour induction.
Numbers (with percentages) for binary and categorical variables, and means (and SDs) or medians (with lower and upper quartiles) for continuous variables were presented; no tests of statistical significance were performed nor were CIs calculated for differences between randomised groups on any baseline variable.
Losses to follow-up
The numbers (with percentages) of losses to follow-up among women selected for the 24-month assessment were reported and compared between the two trial arms, and the reasons recorded. Any deaths (and their causes) were reported separately.
Description of available data
The pattern of missing data for primary and secondary outcomes, from baseline to the end of follow-up, was summarised for the two treatment groups, with differentiation between fully and partially completed forms/Guardian fields and those that were missing completely.
Not all data are routinely collected by all hospitals, for example BMI, smoking history, cervical dilatation, cord artery pH and base deficit. These data were reported separately for the subset of hospitals that do collect them routinely.
Description of compliance with intervention
A summary of the intervention received was provided, which included intermittent use of the decision support software and/or withdrawal of consent during labour.
Unmasking of randomised treatments
In order to accurately reflect any potential impact of the decision support software in contemporary NHS practice, such as changes in midwifery presence during labour consequent on knowledge of the allocation, the clinicians were not masked to allocation. The local community midwives and participants may also have been aware of the allocation. All other persons involved in the trial (except for the trial statistician and trial programmer), including the UCL trial co-ordinating centre, did not have access to the aggregate list of randomisation codes. K2 Medical Systems remained masked until the data were frozen at the end of the trial.
Statistical methods used for analysis of primary outcomes
The numbers (percentages) of babies with poor neonatal outcomes were presented for each group and the RR plus 95% CI calculated. RRs were estimated using generalised estimating equations (GEEs), or a similar method, adjusting for the stratification factors used in the randomisation (centre and singleton/twin pregnancy). This method of analysis accounted for the correlation in outcomes between twins and siblings delivered in a subsequent pregnancy during the trial period. A log binomial model was planned to be used in the first instance but, if convergence was not achieved, then a log-Poisson model would be used with a robust variance estimator. 69 The mean (SD) PARCA-R composite score was presented for each group and the mean difference between groups plus 95% CI was calculated and compared using GEE (Gaussian model with identity link), adjusting for stratification factors.
Significance levels
For the analysis of the primary outcomes, a p-value of 0.05 (5% significance level) was used to indicate statistical significance. Comparisons of all other outcomes including the components of the primary outcome were reported in full for completeness and transparency. For all other analyses, a p-value of 0.01 (1% significance level) was used to indicate statistical significance, to take into account the number of comparisons. Two-sided statistical tests and corresponding p-values were presented throughout.
Missing data
Missing data for the short-term primary outcome were likely to be negligible, as most of the data were collected electronically on the Guardian system before the woman left the delivery room. If any data items were missing on the PBDC forms, completed for babies and women admitted to a higher level of care, every effort was made to extract these data from the hospital involved.
For any partially completed PARCA-R scales in the 24-month parent questionnaires, the following strategies for estimation of the total and subscale scores were employed when items were missing:
-
Non-verbal cognition scale (PARCA-R: ‘Your child at play’ Q1–33)
-
pro rata estimation if < 10% of (up to three) items are missing
-
if Q6 is ‘No’, and Q6a is missing, code ‘No’ for Q6a
-
if Q6 is ‘Don’t know’, and Q6a is missing, code ‘Don’t know’ for Q6a.
-
-
Vocabulary subscale (PARCA-R: ‘What your child can say’)
-
non-ticked items were coded to zero.
-
-
Sentence complexity subscale (PARCA-R: ‘Your child’s understanding Q1–18)
-
if Q1–6 were missing, coded to zero and analysed as ‘Not Yet’
-
if Q6 is ‘Often’ or ‘Sometimes’ and any of Q7–18 were not completed, coded to zero.
-
Prespecified subgroup analysis
To examine whether or not the effect of decision support was consistent across specific subgroups of women and babies, the following subgroup analyses were planned and carried out:
-
singletons versus twins
-
suspected IUGR at labour onset compared with no growth restriction
-
BMI group [underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (≥ 30 kg/m2) or unrecorded]
-
centre.
For the trial composite primary outcome, results were presented in forest plots showing the RR plus 95% CI for each subgroup, by treatment group, with the p-value for the statistical test of interaction. 70 For the PARCA-R composite score, the difference between the mean treatment effects was reported, within each subgroup, with a 95% CI and the corresponding p-value. 71
Using these statistical methods, we performed subgroup analyses for all prespecified neonatal outcomes and instrumental vaginal deliveries. In addition, we analysed process outcomes by centre.
Prespecified sensitivity analysis
Following early DMC meetings, it was reported that the number of babies admitted to a neonatal unit within 48 hours for ≥ 48 hours exceeded the number anticipated in the sample size calculation by an order of magnitude. Following a review of cases by the masked review panel at the end of the trial, the number still exceeded that anticipated in the sample size calculation, although to a lesser degree. Hence this component far outweighed the other rarer components, such as stillbirth, neonatal death and moderate or severe NNE, and strongly influenced the composite primary outcome. To explore the impact of this on the main findings, a sensitivity analysis of the primary composite outcome was performed, including only the most severely affected babies admitted to a neonatal unit and allocated a score of ≥ 7 points on the Primary Outcome Review Scoring Sheet by the masked review panel.
Statistical software employed
Stata/SE version 13 for Microsoft Windows was used for this analysis.
Statistical methods used for analysis of secondary outcomes
Generalised estimating equations, or a similar method, was used for the analysis of secondary outcomes, adjusting for the stratification factors used in the randomisation procedure (centre and singleton/twin pregnancy). For normally distributed continuous outcomes, we presented the mean and SD for each group, calculating the mean difference plus 99% CI using a Gaussian model with the identity link. For the length of labour, we presented the geometric mean ratio (GMR). For binary and categorical outcomes, we presented counts and percentages for each group and calculated the RR with corresponding 99% CI using a binomial or Poisson model with the log-link. For the number of thumb entries per hour from trial entry to the first yellow level of concern or full dilatation, the rate ratio plus 99% CI was calculated using a Poisson model with the log-link. For skewed continuous outcomes, we presented the median and IQR (or entire range, whichever was appropriate) for each group and compared the difference in medians between groups using quantile regression. We were unable to adjust for the correlation among twins and siblings using this method.
Deviation from analysis described in protocol
For the count variables, median differences were all zero with zero CIs, although some were statistically significant and adjusted quantile regression models were not performing well (lack of convergence), so rate ratios were presented as the effect measure instead of median differences. Medians (IQR) were still presented for each variable as planned. In addition, the hazard ratio was presented for the time from the first red alert to birth instead of the median difference, in keeping with the comparison of rates for the rest of the process outcomes. This was agreed at the INFANT project management group meeting on 23 October 2014.
The outcome time from the first red level of concern to delivery was changed to time from the last red level of concern to delivery. This was agreed at the INFANT coinvestigator group meeting on 15 July 2015 following a review by Professor Philip Steer. It was found that the first red level of concern was frequently an artefact and, therefore, did not accurately capture information relating to prompt action at or around delivery following a red alert.
Chapter 5 Trial conduct
Training
An extensive programme of training in the trial processes was delivered by the trial team at each participating site. This included training about the use of the decision support software, how to complete the electronic data input in preparation for download to the trial co-ordinating centre and how to complete the necessary paper data collection forms for babies of women admitted to higher-level care (see Appendix 4).
Recruitment
The aim was to randomise 46,000 women in the trial over 36 months. Therefore, approximately 320 women per week needed to be recruited. A conservative estimate of the proportion of women who receive EFM in labour, and were therefore eligible for trial entry, was 60%. The eligibility rate was expected to be higher in some centres; for example, in one study, 80% of primigravid women who gave birth in Liverpool Women’s Hospital were monitored continuously. In total, there were around 42,500 deliveries per annum in the centres originally planned to participate in INFANT (the average for each centre was 4250 deliveries per annum). This was approximately 817 deliveries per week, among which an estimated 490 women per week (60%) would receive EFM.
Pilot work carried out in Plymouth to investigate recruitment rates found that only 2 out of 105 eligible women declined to take part. Although this indicated that uptake was likely to be high, strategies to promote and support recruitment were implemented in all participating centres.
During the course of the trial, recruitment graphs (e.g. Figure 11) were sent to all recruiting sites each month. This showed the percentage of target recruitment over the years of the trial. In the example below, this centre exceeded its recruitment target throughout the duration of the trial, achieving a very high recruitment rate of approximately 230% of its target in 2011.
FIGURE 11.
Example of monthly recruitment data sent to the centres.
Overall, despite most centres exceeding their recruitment target, there was a delay in reaching the final recruitment target (Figure 12). This was caused by longer than expected delays in initiating new centres into the trial.
FIGURE 12.
Actual vs. target recruitment.
Review of primary outcome
Following the TSC meeting on 2 October 2013, the INFANT project management group agreed to undertake further work to clarify the classification of the primary outcome for the trial. This issue had arisen because the incidence of one of the components of the composite primary outcome (admission to the neonatal unit within 48 hours of birth for ≥ 48 hours with evidence of respiratory or feeding problems or NNE) was substantially higher than had been anticipated, and an initial review of these cases (by PB and DF, masked to allocation) suggested that a number of cases were included in this outcome that were unrelated to perinatal hypoxia (Table 16). It was also recognised that, for many of these uncertain cases, the detail provided by the INFANT data collection forms was insufficient (Figure 13). All cases were included in this outcome if question 2 was answered ‘Yes’ and ‘Yes’ was also answered for one or more of questions 5, 6, 8 and/or 9 (Figure 13).
| Component of primary outcome | Expected | Observed | ||
|---|---|---|---|---|
| Event rate (n/1000) | Number (n = 29,233a) | Event rate (n/1000) | Number (n = 29,233a) | |
| Intrapartum stillbirth | 0.35 | 10 | 0.07 | 2 |
| Neonatal death up to 28 days after birth | 0.70 | 20 | 0.48 | 14 |
| Moderate or severe NNE | 1.30 | 38 | 0.92 | 27 |
| Admission to NICU within 48 hours of birth for ≥ 48 hours with evidence of respiratory or feeding problems or NNE | 1.25 | 37 | 18.10 | 529 |
| Overall (total) | 3.60b | 105 | 19.57 | 572 |
FIGURE 13.
Definition of ‘Admission to neonatal intensive care unit within 48 hours of birth for ≥ 48 hours with evidence of respiratory or feeding problems or NNE’. See Appendix 5.
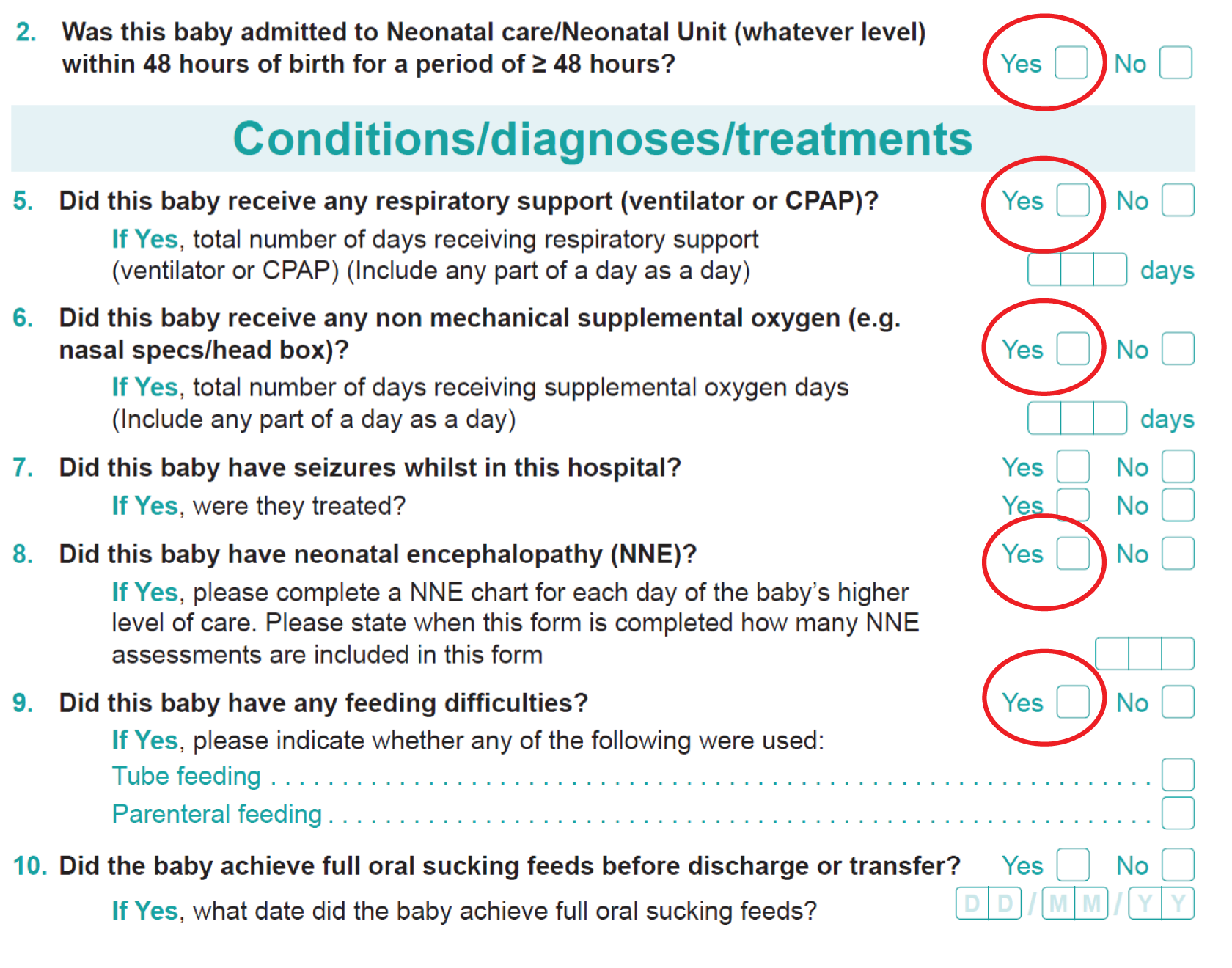
It was agreed that neonatal unit discharge summaries would be collected for all babies who were admitted to the neonatal unit within 48 hours of birth for ≥ 48 hours. A more formal process of review of a proportion of these cases would then be undertaken by Peter Brocklehurst, David Field, Keith Greene and Nikki Robertson (a neonatologist independent of the trial who is an expert on perinatal hypoxia in term babies) to devise a more structured way of assessing the classification of these babies. This system would then be implemented by David Field and Keith Greene for the purposes of data monitoring. There was also agreement that this process would be repeated by an independent panel of neonatologists at the end of the trial.
Peter Brocklehurst, David Field, Keith Greene and Nikki Robertson met on 16 January 2013 and reviewed approximately 40 sets of discharge summaries (masked to trial allocation) and devised a data extraction form, which included the key elements of the neonatal course which are most likely to be related to intrapartum hypoxia. This form was not an established or validated list of criteria, but the numeric scoring was devised to try and give some quantification of the severity of elements of the clinical course. A score of ≥ 3 points was agreed to be evidence that the condition of the baby was likely to be associated with intrapartum hypoxia, acknowledging that there remained uncertainty about this, as there is no absolute measure of intrapartum hypoxia. However, having reviewed the discharge summaries with the data extraction form, the criteria were felt to be a good reflection of the baby’s clinical condition.
Process
David Field and Keith Greene then reviewed all the discharge forms independently to score the cases. A teleconference on 22 February 2012 resolved, by consensus with Peter Brocklehurst, the uncertain cases so that a decision could be made as to whether or not to include each baby as having evidence of the primary outcome (Table 17).
| Component of primary outcome | Expected | Observed | ||
|---|---|---|---|---|
| Event rate (n/1000) | Number (n = 29,233a) | Event rate (n/1000) | Number (n = 29,233a) | |
| Intrapartum stillbirth | 0.35 | 10 | 0.07 | 2 |
| Neonatal death up to 28 days after birth | 0.70 | 20 | 0.48 | 14 |
| Moderate or severe NNE | 1.30 | 38 | 0.92 | 27 |
| Admission to NICU within 48 hours of birth for ≥ 48 hours with evidence of respiratory or feeding problems or NNE | 1.25 | 37 | 3.18 | 93 |
| Overall (total) of those reviewed | 3.60 | 105 | 4.65 | 136 |
This produced a slightly revised definition of this component of the composite primary outcome:
-
Admission to the neonatal unit within 48 hours of birth for ≥ 48 hours with evidence of respiratory or feeding problems or NNE (when there is evidence of compromise at birth).
This process and revised definition were reviewed and accepted by the TSC on 18 March 2013 and by the DMC on 21 March 2013.
Cases continued to be reviewed by David Field and Keith Greene on a monthly basis for the purposes of data monitoring. These data were entered into the INFANT database accordingly.
Independent review panel
An independent panel of neonatologists, to review all cases of the primary outcome (all stillbirths, early neonatal deaths, cases of NNE and babies admitted to the neonatal intensive care unit within 48 hours for ≥ 48 hours with evidence of respiratory or feeding difficulties), was assembled. An advertisement was placed in the British Association of Perinatal Medicine newsletter for prospective panel members. In order to ensure the independence of the panel, members could not be practising at any of the INFANT trial recruiting centres. Brief information of what would be expected as part of the review, how often they would be required to meet and details of payments to be made were included in the advertisement. The role was remunerated at £500 per day. Applicants were selected by the INFANT coinvestigators group.
After an initial face-to-face meeting to establish methods of working, panel members were sent a proportion of the cases once trial recruitment had finished. There was an overlap of cases so that every case was reviewed by at least two reviewers. When there was lack of agreement by two members of the panel, consensus was reached by discussion with the whole panel, at either a face-to-face meeting or teleconference.
Follow-up of deaths to age 2 years for entire trial cohort
After the initial analysis of the results of the trial, there was an unusual finding noted with the data from the 2-year follow-up.
In the original INFANT protocol, 46,000 women would be recruited and their babies would be followed up until hospital discharge, with a sample of 7000 of these babies being followed up at 2 years to assess longer-term outcomes, including neurodevelopmental outcomes. These 7000 babies were planned to be selected from the early phase of trial recruitment so that follow-up would not prolong the duration of the trial and the time to publication. We also initiated a system of ‘flagging’ with the NHS that notified the trial team of later deaths (after initial hospital discharge) to report deaths among all of the 46,000 babies by the time follow-up was completed. The duration of this mortality follow-up was intended to be dependent on when the baby was born and, for babies whose mothers were recruited later in the trial, would be < 2 years.
Once the planned follow-up was complete, the preliminary results from the INFANT trial were analysed and presented to the coinvestigators. There was an apparent difference in the number of longer-term deaths between groups. Given the lack of any difference in the primary outcome (early mortality and major morbidity), this result was surprising. At a joint meeting of the TSC and DMC in December 2014, it was suggested that the trial team should undertake a structured review of all the deaths, including obtaining as complete information about the later deaths as possible and, masked to allocation, ascribe cause-of-death classifications so that we could get some insight into whether this difference in deaths was likely to be real or spurious.
The distribution of all deaths between trial entry and follow-up at this time was 20 in one arm compared with 40 in the other arm. Masked to allocation, the cause of death was classified for babies for whom we had known cause-of-death data, and cause-of-death data were sought from the relevant national bodies (in England and Scotland) so that the later deaths could also be classified. Some cause-of-death data were still missing at this time. If an infant death is a coroner’s case, there is often a delay in the cause-of-death data becoming available.
Once the coding was undertaken, the allocations were revealed and these data were presented to the coinvestigator group at its meeting on 4 February 2015. We compared the causes of death, including identifying deaths for congenital anomalies and lethal genetic conditions in which the decision support system could not be expected to make a difference. There were no differences in the numbers of deaths as a result of lethal congenital anomalies or lethal genetic conditions between the two groups. There appeared to be a possible difference between the two groups in terms of sudden unexplained death in infancy and other conditions, such as sepsis, in which there might potentially be a link between condition at birth and these later health outcomes. Although conditions might not be sufficient or severe enough to register as an event in the primary outcome, they might predispose the child to later morbidity and mortality.
The coinvestigator group decided that they could not conclude that this was a spurious result and agreed with the recommendations of the TSC/DMC that we should continue to follow up the remaining children until they reached age 2 years to determine the complete number of deaths by 2 years for the entire cohort.
The last baby was born in August 2013, resulting in a continuation of follow-up until after August 2015, allowing approximately 4 months for the majority of coroner’s case cause-of-death data to be entered into the national systems.
During the further follow-up, it became apparent that an error had been made by the national authority in England when searching for cases of death. Despite the denominator of the trial growing each time the data were submitted for flagging, the search for deaths continued to be carried out for the initial submitted cohort, which was less than half of the entire trial population.
Once this error was recognised, a greater number of deaths were identified and, by the time the final child had reached age 2 years, the total numbers of deaths in each arm (excluding lethal congenital anomalies) were similar, with 29 in the decision support arm and 35 in the no decision support arm, adjusted risk ratio (aRR) 0.83 (99% CI 0.44 to 1.59).
Chapter 6 Results
Between 6 January 2010 and 31 August 2013, 47,062 women were randomised to the INFANT trial from the participating centres (Table 18). A total of 1020 women (2.2%) were excluded from the analysis of the primary outcome (Figure 14). The majority of these exclusions were because of missing or incomplete consent forms. Data at the time of birth were available for 100% of women and babies eligible to be analysed. Follow-up data at 2 years were available for 56% (7066/12,704) of those contacted, although data were sufficiently complete for the analysis for 6707 children.
| Centre | Trial arm, n (%) | |
|---|---|---|
| Decision support (N = 22,987) | No decision support (N = 23,055) | |
| Birmingham Women’s Hospital | 1131 (4.9) | 1131 (4.9) |
| Burnley General Hospital | 2058 (9.0) | 2062 (8.9) |
| Chelsea and Westminster Hospital, London | 441 (1.9) | 449 (2.0) |
| Derriford Hospital, Plymouth | 1641 (7.1) | 1626 (7.1) |
| Homerton University Hospital, London | 944 (4.1) | 940 (4.1) |
| Liverpool Women’s Hospital | 1524 (6.6) | 1559 (6.8) |
| Northwick Park Hospital, London | 926 (4.0) | 936 (4.1) |
| Nottingham City Hospital and Queens Medical Centre | 902 (3.9) | 903 (3.9) |
| Princess Anne Hospital, Southampton | 585 (2.5) | 581 (2.5) |
| Princess Royal Hospital and Southern General Hospital, Glasgow | 2041 (8.9) | 2033 (8.8) |
| Queen Alexandra Hospital, Portsmouth | 1370 (6.0) | 1372 (6.0) |
| Rotunda Hospital, Dublin | 1735 (7.6) | 1728 (7.5) |
| Royal Bolton Hospital | 499 (2.2) | 510 (2.2) |
| Royal Derby Hospital | 421 (1.8) | 425 (1.8) |
| St Mary’s Hospital, Manchester | 935 (4.1) | 945 (4.1) |
| Stoke Mandeville Hospital | 1214 (5.3) | 1216 (5.3) |
| University College Hospital, London | 196 (0.9) | 192 (0.8) |
| University Hospital Coventry | 600 (2.6) | 607 (2.6) |
| University Hospital of North Staffordshire | 1670 (7.3) | 1679 (7.3) |
| Warrington Hospital | 1730 (7.5) | 1732 (7.5) |
| Warwick Hospital | 424 (1.8) | 429 (1.9) |
FIGURE 14.
Consolidated Standards of Reporting Trials (CONSORT) flow of participants. a, One woman who withdrew (with consent to use of data) was also randomised in error; b, the 46,042 women included in the analysis includes 448 women (1%) with two singleton birth episodes and six women with one singleton and one twin birth episode in the study period and the allocation received for the subsequent delivery was independent of the first allocation received; and c, data from the Republic of Ireland not included in the numerator (n = 1 decision support) or denominator (n = 1754 in decision support and n = 1752 in no decision support) as data on deaths after discharge were not available. Deaths in the flow chart include stillbirths (n = 1 in decision support and n = 2 in no decision support).
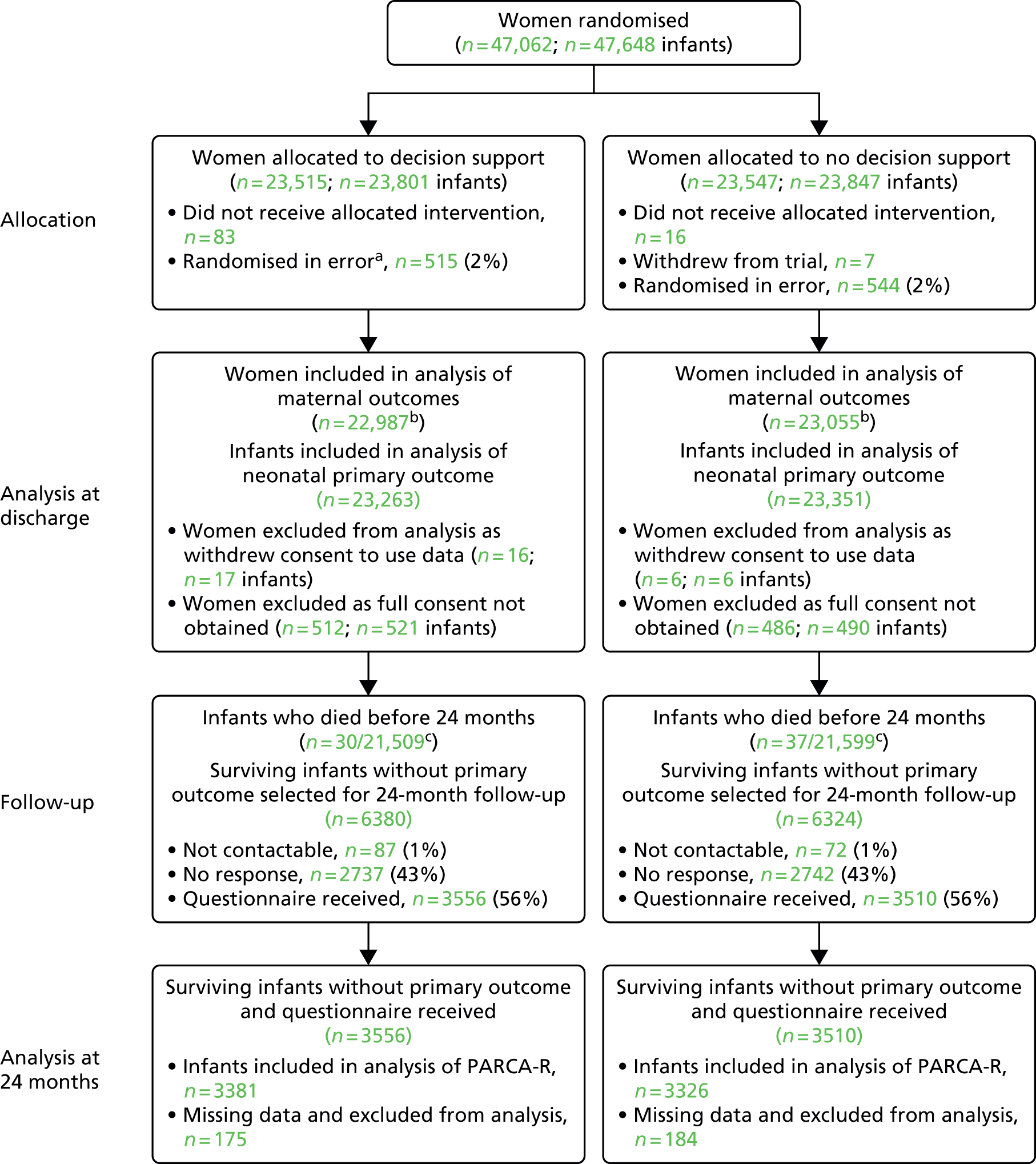
A total of 30 women withdrew from the trial, 23 in the decision support group and seven in the no decision support group. The reasons given are in Table 19. A total of 1059 women were randomised in error, and the reasons for this are given in Table 20.
| Reason | Trial arm, number of participants | |
|---|---|---|
| Decision support (N = 23) | No decision support (N = 7) | |
| No longer wishes data to be collected/used | 2 | 2 |
| Did not like alerts | 4 | 0 |
| Twin baby died | 1 | 0 |
| No longer wishes to be followed up | 1 | 0 |
| Not happy with care/approach | 1 | 2 |
| Too distressed | 1 | 0 |
| Not happy with allocation | 4 | 0 |
| Not known/other | 9 | 3 |
| Reason | Trial arm, number of participants | |
|---|---|---|
| Decision support (N = 515) | No decision support (N = 544) | |
| No labour/elective caesarean section | 378 | 371 |
| Date of randomisation after date of delivery | 92 | 120 |
| Fetal abnormality | 18 | 19 |
| Gestational age at entry of < 35 weeks or not known | 10 | 15 |
| Not CTG monitored | 9 | 9 |
| Not known/other | 8 | 10 |
Baseline characteristics were similar between the two groups in the trial (Table 21). Mean maternal age was 29 years. The majority of women participating in the trial were of white ethnic origin and the median BMI at booking was 25 kg/m2. Nearly 60% of women were having their first baby, and the majority of women in both groups had a gestational age of between 38 and 41 completed weeks, although 11% were < 38 weeks’ gestation and 6.3% were 42 weeks or later. Very few women had experienced a previous stillbirth (1%) and approximately 6% had undergone a caesarean section in a previous birth. In almost 60% of women, labour was induced.
| Maternal characteristic | Trial arm | |
|---|---|---|
| Decision support (N = 22,987)a | No decision support (N = 23,055)a | |
| Maternal age at trial entry (years) | ||
| Median (IQR) | 29 (25–33) | 29 (25–33) |
| Ethnic group, n (%)b | ||
| White | 17,234 (83.3) | 17,213 (83.0) |
| Indian | 743 (3.6) | 724 (3.5) |
| Pakistani | 736 (3.6) | 802 (3.9) |
| Bangladeshi | 98 (0.5) | 113 (0.5) |
| Black Caribbean | 116 (0.7) | 135 (0.6) |
| Black African | 461 (2.2) | 505 (2.4) |
| Any other ethnic group | 1296 (6.3) | 1249 (6.0) |
| Unknown | 2303 | 2314 |
| Twin pregnancy, n (%) | 276 (1.2) | 296 (1.3) |
| Gestational age at entry (completed weeks) | ||
| Median (IQR) | 40 (39–41) | 40 (39–41) |
| < 35+0, n (%) | 4 (–d) | 6 (–d) |
| 35+0 to 37+6, n (%) | 2529 (11.0) | 2522 (10.9) |
| 38+0 to 39+6, n (%) | 7322 (31.9) | 7266 (31.5) |
| 40+0 to 41+6, n (%) | 11,688 (50.9) | 11,795 (51.2) |
| ≥ 42+0, n (%) | 1437 (6.3) | 1457 (6.3) |
| BMI (kg/m2), at booking visit | ||
| Median (IQR) | 25 (22–30) | 25 (22–30) |
| < 18.5, n (%) | 379 (2.5) | 384 (2.6) |
| 18.5 to 24.9, n (%) | 6302 (42.1) | 6225 (41.6) |
| 25 to 29.9, n (%) | 4531 (30.3) | 4560 (30.5) |
| 30 to 34.9, n (%) | 2178 (14.5) | 2237 (14.9) |
| 35 to 39.9, n (%) | 1024 (6.8) | 1025 (6.8) |
| ≥ 40, n (%) | 565 (3.8) | 544 (3.6) |
| Unknown, n | 8008 | 8080 |
| Smoking, at booking visit, n (%) | ||
| Yes | 2448 (14.3) | 2536 (14.7) |
| No | 14,724 (85.7) | 14,722 (85.3) |
| Unknown | 5815 | 5797 |
| Parity, n (%) | ||
| Nulliparous | 13,736 (59.8) | 13,650 (59.2) |
| Parous | 9247 (40.2) | 9390 (40.8) |
| Obstetric history, n (%) | ||
| Previous stillbirth | 273 (1.2) | 223 (1.0) |
| Previous elective caesarean section | 208 (0.9) | 253 (1.1) |
| Previous emergency caesarean section | 1240 (5.4) | 1224 (5.3) |
| Previous neonatal death | 80 (0.4) | 95 (0.4) |
| Cervical dilatation at time of trial entry (cm) | ||
| Median (IQR) | 4 (2–6) | 4 (2–5) |
| Unknown, n | 16,184 | 16,339 |
| Fetal growth restriction suspected at labour onset, n (%) | 859 (3.7) | 914 (4.0) |
| Labour induction, n (%) | ||
| Induced | 13,516 (59.2) | 13,568 (59.2) |
| Spontaneous | 8955 (39.2) | 8967 (39.2) |
| No labour | 376 (1.7) | 367 (1.6) |
| Epidural analgesia, n (%) | ||
| Yes | 2682 (26.0) | 2766 (26.8) |
| No | 7628 (74.0) | 7549 (73.2) |
| Unknowna | 12,677 | 12,740 |
| Presence of meconium, n (%) | ||
| Yes | 449 (4.5) | 454 (4.5) |
| No | 9454 (95.5) | 9535 (95.5) |
| Unknownc | 13,084 | 13,066 |
There was no evidence of a difference in the incidence of the primary outcome of the composite of poor neonatal outcome between the groups, with 172 babies (0.7%) having a poor outcome in the decision support group compared with 171 babies (0.7%) in the no decision support group (aRR 1.01, 95% CI 0.82 to 1.25) (Table 22). Similarly, there was no evidence of a difference in any individual component of the composite primary outcome between the groups.
| Outcome | Trial arm | Adjusteda RR (CI) unless otherwise indicated | |
|---|---|---|---|
| Decision support (N = 23,263) | No decision support (N = 23,351) | ||
| Composite primary outcome (95% CI) | |||
| Composite primary outcome 1–4, n (%)b | 172 (0.7) | 171 (0.7) | 1.01 (0.82 to 1.25) |
| (1) Intrapartum stillbirths, n (%)c | 1 (–) | 2 (–) | 0.50 (0.05 to 5.53) |
| (2) Neonatal deaths up to 28 days after birth, n (%)d | 6 (–) | 4 (–) | 1.51 (0.42 to 5.33) |
| (3) Moderate or severe NNE (requiring cooling), n (%) | 18 (0.1) | 21 (0.1) | 0.86 (0.46 to 1.61) |
| (4) Admission to neonatal unit within 48 hours of birth for ≥ 48 hours because of feeding difficulties, respiratory illness/symptoms or NNE and evidence of compromise at birth, n (%) | 147 (0.6) | 144 (0.6) | 1.02 (0.81 to 1.29) |
| Other neonatal outcomes (99% CI) | |||
| Admission to a higher level of care, n (%) | 1389 (6.0) | 1429 (6.1) | 0.98 (0.89 to 1.08) |
| An Apgar score of < 4 at 5 minutes, n (%) | 43 (0.2) | 65 (0.3) | 0.67 (0.40 to 1.11) |
| Cord artery pH, n (%) | |||
| < 7.15 | 1625 (11.3) | 1695 (11.8) | 0.96 (0.88 to 1.04) |
| < 7.05 | 268 (1.9) | 278 (1.9) | 0.95 (0.77 to 1.19) |
| Mean (SD) | 7.24 (0.08) | 7.24 (0.08) | |
| Unknown | 8829 | 8981 | |
| Metabolic acidosis, n (%)e | |||
| Yes | 148 (1.1) | 131 (1.0) | 1.12 (0.82 to 1.52) |
| No | 13,538 (98.9) | 13,533 (99.0) | |
| Unknown | 9577 | 9687 | |
| Resuscitation, n (%) | |||
| None | 18,457 (87.3) | 18,605 (87.6) | |
| One intervention | 2139 (10.1) | 2116 (10.0) | 1.03f (0.96 to 1.09) |
| Two or more interventions | 554 (2.6) | 524 (2.5) | |
| Unknown | 2113 | 2106 | |
| Seizures while in hospital, n (%) | 39 (0.2) | 41 (0.2) | 0.95 (0.54 to 1.70) |
| Destination of baby immediately after birth, n (%) | |||
| Postnatal ward | 21,571 (93.6) | 21,664 (93.6) | |
| Home | 467 (2.0) | 485 (2.1) | 1.00g (0.99 to 1.00) |
| Transitional care unit | 277 (1.2) | 235 (1.0) | |
| Neonatal unit | 653 (2.8) | 690 (3.0) | |
| Transferred hospital | 4 (–) | 7 (–) | |
| Stillbirth | 1 (–) | 2 (–) | |
| Other | 69 (0.3) | 53 (0.2) | |
| Length of hospital stay to discharge (days) | |||
| Median (IQR) | 2 (1–3) | 2 (1–3) | Hazard ratio: 0.99 (99% CI 0.97 to 1.01) |
In a prespecified sensitivity analysis that used a different cut-off point for defining compromise at birth (a score of ≥ 7 points indicating a more severe compromise than a score of ≥ 3 points) this made no difference to the interpretation of the measure of effect for the primary outcome (aRR 0.97, 95% CI 0.58 to 1.63; Table 23).
| Outcome | Decision support (N = 23,263) | No decision support (N = 23,351) | Adjusteda relative risk (95% CI) |
|---|---|---|---|
| Composite primary outcome 1–4, n (%)b | 28 (0.1) | 29 (0.1) | 0.97 (0.58 to 1.63) |
| Unknown | 1 | 2 | |
| (1) Intrapartum stillbirths, n (%)c | 1 (–d) | 2 (–d) | 0.50 (0.05 to 5.53) |
| (2) Neonatal deaths up to 28 days after birth, n (%)e | 6 (–d) | 4 (–d) | 1.51 (0.42 to 5.33) |
| (3) NNE (requiring cooling), n (%) | 18 (0.1) | 21 (0.1) | 0.86 (0.46 to 1.61) |
| (4) Admission to neonatal unit within 48 hours of birth for ≥ 48 hours as a result of feeding difficulties, respiratory illness/symptoms or NNE and evidence of compromise at birth with panel review score of ≥ 7, n (%) | 3 (–d) | 2 (–d) | 1.51 (0.25 to 9.01) |
There was no evidence of any difference in any of the trial’s secondary outcomes for the baby (see Table 22), including Apgar score, all admissions to the neonatal unit, metabolic acidosis of cord blood sample, the need for neonatal resuscitation and duration of hospital stay.
Just over half of all births were spontaneous vaginal births and there was no statistically significant difference between the two groups [11,823 women (50.8%) in the decision support group vs. 11,959 women (51.2%) in the no decision support group; aRR 0.99, 99% CI 0.97 to 1.01]. Of the operative births, half were by caesarean section and half were instrumental (Table 24). More women in the decision support group underwent fetal blood sampling: 2366 (10.3%) in the decision support group versus 2187 (9.5%) in the no decision support group (aRR 1.08, 99% CI 1.01 to 1.16). No other statistically significant differences were found between the two groups from trial entry to birth in clinical outcomes (see Table 24).
| Outcome | Trial arm | Adjusted RR (99% CI)a | |
|---|---|---|---|
| Decision support | No decision support | ||
| Number of infants in denominator | 23,263 | 23,351 | |
| Mode of delivery, n (%) | |||
| Spontaneous cephalic vaginal | 11,823 (50.8) | 11,959 (51.2) | 0.99 (0.97 to 1.01) |
| Caesarean section | 5669 (24.4) | 5555 (23.8) | |
| Instrumental | 5698 (24.5) | 5765 (24.7) | |
| Vaginal breech | 73 (0.3) | 72 (0.3) | |
| Indications for any operative intervention (caesarean section and instrumental delivery), n (%) | |||
| Fetal distress | 4278 (18.4) | 4262 (18.3) | 1.04b (1.00 to 1.08) |
| Failure to progress | 5059 (21.8) | 5175 (22.2) | 1.01b (0.97 to 1.05) |
| Fetal distress and failure to progress | 1774 (7.6) | 1599 (6.9) | |
| Other reason | 229 (1.0) | 247 (1.1) | |
| Indication for instrumental vaginal deliveries, n (%) | |||
| Fetal distress | 2608 (11.2) | 2559 (11.0) | 1.03b (0.97 to 1.09) |
| Failure to progress | 2262 (9.7) | 2396 (10.3) | 0.97b (0.91 to 1.03) |
| Fetal distress and failure to progress | 700 (3.0) | 660 (2.8) | |
| Other reason | 117 (0.5) | 134 (0.6) | |
| Caesarean section, n (%) | |||
| Grade 1 (immediate threat to life) | 1138 (4.9) | 1121 (4.8) | 1.02c (0.92 to 1.13) |
| Grade 2 (some threat of compromise) | 3754 (16.2) | 3605 (15.5) | 1.04c (0.99 to 1.09) |
| Grade 3 (no threat of compromise) | 645 (2.8) | 689 (3.0) | 1.02c (0.98 to 1.07) |
| Grade 4 (elective – timing to suit) | 12 (0.1) | 12 (0.1) | |
| Number of women in denominator | 22,987 | 23,055 | |
| Episiotomy, n (%) | 6396 (28.9) | 6498 (29.3) | 0.99 (0.95 to 1.03) |
| Unknown | 826 | 840 | |
| Any episode of fetal blood sampling, n (%) | 2366 (10.3) | 2187 (9.5) | 1.08 (1.01 to 1.16) |
| Destination of mother immediately after birth, n (%) | |||
| Ward | 21,554 (94.6) | 21,614 (94.5) | |
| Home | 429 (1.9) | 462 (2.0) | 1.00d (0.99 to 1.00) |
| ICU | 15 (0.1) | 19 (0.1) | |
| High-dependency unit | 793 (3.5) | 768 (3.4) | |
| Theatre | 0 (–) | 0 (–) | |
| Other hospital | 0 (–) | 8 (–) | |
| Admission to a higher level of care, n (%) | 1245 (5.4) | 1193 (5.2) | 1.05 (0.95 to 1.16) |
| Length of labour from trial entry (minutes)e | |||
| Geometric mean and GMR | 379 | 381 | 0.99 (0.98 to 1.01) |
| Median (IQR) | 404 (234–638) | 408 (236–640) | |
| Unknown | 871 | 924 | |
| Length of first stage from trial entry (minutes)e | |||
| Geometric mean and GMR | 169 | 168 | 1.01 (0.98 to 1.04) |
| Median (IQR) | 200 (100–351) | 201 (96–354) | |
| Unknown | 6422 | 6292 | |
| Length of second stage from trial entry (minutes)e | |||
| Geometric mean and GMR | 39 | 39 | 0.99 (0.96 to 1.03) |
| Median (IQR) | 49 (15–113) | 50 (16–114) | |
| Unknown | 6036 | 5934 | |
Quality of care was assessed by an expert panel for all neonatal deaths and intrapartum stillbirths as well as for all babies with an adverse outcome (trial primary outcome plus a cord artery pH of < 7.05 with a base deficit of ≥ 12 mmol/l) (Tables 25 and 26). The addition of cord artery metabolic acidosis as a criterion for review substantially reduced the number of babies considered by the panel. The overall proportion of cases with poor outcome in which babies were judged to have suboptimal care likely to have affected the outcome was 38% (27/71), which is similar to that reported previously. 72 This included all instances of suboptimal care, regardless of whether this was related to CTG interpretation or subsequent management decisions. We could not investigate whether or not in all cases appropriate action was taken in response to recognised abnormality, although that aspect has been examined in cases with a composite adverse outcome and biochemical evidence of asphyxia (the analysis of suboptimal care will be reported separately).
| Levels of carea | Trial arm | Adjustedb RR (99% CI) | |
|---|---|---|---|
| Decision support | No decision support | ||
| Babies with an adverse outcomec | N = 35 | N = 36 | |
| Babies with an adverse outcome for whom care has been judged not to be suboptimal (level 0), n (%) | 11 (31.4) | 15 (41.7) | |
| Babies with an adverse outcome for whom care has been judged to be suboptimal (levels 1, 2 and 3), n (%) | 24 (68.6) | 21 (58.3) | 1.18 (0.82 to 1.68) |
| Level 1 | 7 (20.0) | 2 (5.6) | |
| Level 2 | 3 (8.6) | 6 (16.7) | |
| Level 3 | 14 (40.0) | 13 (36.1) | |
| Levels of carea | Trial arm | Adjustedb relative risk (99% CI) | |
|---|---|---|---|
| Decision support | No decision support | ||
| All babies | N = 23,263 | N = 23,351 | |
| Babies with an adverse outcomec for whom care has been judged not to be suboptimal (level 0), n (%) | 11 (0.05) | 15 (0.06) | |
| Babies with an adverse outcome for whom care has been judged to be suboptimal (levels 1, 2 and 3), n (%) | 24 (0.10) | 21 (0.09) | 1.15 (0.64 to 2.06) |
| Level 1 | 7 (0.03) | 2 (0.01) | |
| Level 2 | 3 (0.01) | 6 (0.03) | |
| Level 3 | 14 (0.06) | 13 (0.06) | |
The process outcomes collected in the trial are shown in Table 27. For women in the no decision support group, the presence of alerts was calculated by the software during labour but not revealed to the woman or her caregivers. Using women with any level of concern as the denominator, blue levels of concern (the least severe alert) occurred most frequently, with a median of nine such alerts during these labours (a rate of just below 1.4 per hour). The next more severe alert – a yellow level of concern – occurred a median of twice per labour for both women in the decision support group and those in the no decision support group. There was evidence of a lower rate of yellow levels of concern in the decision support group (adjusted rate ratio 0.87, 99% CI 0.84 to 0.89; Table 27). The most severe alert, the red level of concern, occurred infrequently (a median of once per labour, with a rate of 0.14 per hour) and there was no evidence of a difference between the two groups (aRR 0.98, 99% CI 0.92 to 1.04).
| Outcome | Trial arm | Adjustedb effect measure (99% CI) | |
|---|---|---|---|
| Decision support (N = 22,987) | No decision supporta (N = 23,055) | ||
| No labour, n | 378 | 371 | |
| Date and time of randomisation after date and time of delivery, n | 92 | 120 | |
| Number of remaining participants | 22,517 | 22,564 | |
| Epidural analgesia, n (%) | |||
| Yes | 2770 (27.3) | 2689 (26.5) | RR 1.03 (0.97 to 1.09) |
| No | 7383 (72.7) | 7453 (73.5) | |
| Unknownc | 12,364 | 12,422 | |
| Labour augmentation, n (%) | |||
| Yes | 2705 (30.9) | 2750 (31.3) | RR 0.99 (0.93 to 1.04) |
| No | 6047 (69.1) | 6042 (68.7) | |
| Unknownc | 13,765 | 13,772 | |
| Presence of meconium, n (%) | |||
| Yes | 440 (4.5) | 469 (4.8) | RR 0.94 (0.80 to 1.11) |
| No | 9316 (95.5) | 9346 (95.2) | |
| Unknownc | 12,761 | 12,749 | |
| Number of women with at least one blue, yellow or red level of concern, n (%) | 21,950 (97.5) | 22,021 (97.6) | RR 1.00 (1.00 to 1.00) |
| Number of women with at least one blue level of concern (mild abnormality), n (%) | 21,863 (97.1) | 21,913 (97.1) | RR 1.00 (1.00 to 1.00) |
| Number of women with at least one yellow level of concern (moderate abnormality), n (%) | 16,765 (74.5) | 16,722 (74.1) | RR 1.00 (0.99 to 1.02) |
| Number of women with at least one red level of concern (severe abnormality), n (%) | 2335 (10.8) | 2413 (11.1) | RR 0.97 (0.90 to 1.04) |
| Unknownd | 822 | 833 | |
| Number of blue, yellow or red levels of concern in women with at least one level of concern | |||
| Median (IQR) | 9 (5–15) | 9 (5–15) | Rate ratio 0.98 (0.96 to 1.00) |
| Rate per hour | 1.37 | 1.40 | |
| Unknowne | 765 | 824 | |
| Number of blue levels of concern in women with a blue level | |||
| Median (IQR) | 7 (4–11) | 7 (4–11) | Rate ratio 1.01 (0.99 to 1.03) |
| Rate per hour | 1.06 | 1.05 | |
| Unknowne | 740 | 800 | |
| Number of yellow levels of concern in women with a yellow level | |||
| Median (IQR) | 2 (1–4) | 2 (1–5) | Rate ratio 0.87 (0.84 to 0.89) |
| Rate per hour | 0.35 | 0.40 | |
| Unknowne | 354 | 421 | |
| Number of red levels of concern in women with a red level | |||
| Median (IQR) | 1 (1–1) | 1 (1–1) | Rate ratio 0.98 (0.92 to 1.04) |
| Rate per hour | 0.14 | 0.14 | |
| Unknownd,e | 41 | 55 | |
| Interaction with Guardian system via number of thumbprint entries from time of trial entry to first yellow level of concern, or until cervix fully dilated if no abnormality detected | |||
| Median (IQR) | 5 (0–16) | 4 (0–15) | Rate ratio 0.99 (0.95 to 1.03) |
| Rate per hour | 4.22 | 4.21 | |
| Unknown | 1723 | 1603 | |
| Number of vaginal examinations | |||
| Median (IQR) | 2 (1–3) | 2 (1–3) | Rate ratio 1.03 (1.00 to 1.05) |
| Rate per hour | 0.28 | 0.27 | |
| Unknown | 877 | 929 | |
| Time from last red level of concern to delivery (minutes) | |||
| Median (IQR) | 58 (13–279) | 58 (13–264) | HR 0.99 (0.92 to 1.06) |
| Unknownd | 822 | 823 | |
Although there was a worry within the trial term that women in the decision support group would be left alone in labour more frequently because the decision support software was running, there was no evidence to suggest that caregivers interacted with the Guardian system less frequently in this group. The rate of thumbprint entries on the Guardian system was 4.22 per hour in the decision support group and 4.21 per hour in the no decision support group (aRR 0.99, 99% CI 0.95 to 1.03; see Table 27).
The time from the last red level of concern to birth was similar in both groups, with a median of 58 minutes. Although this appears lengthy, there were red levels of concern that did not prompt immediate delivery, for example when the CTG monitor was picking up the maternal heart rate. In a subgroup of 500 traces (with a similar number of consecutive cases from each contributing centre) containing at least one red level of concern, the last red level of concern was judged (by an expert coinvestigator, PS) to be a valid fetal concern for 55%. For the remainder of these traces, the maternal heart rate triggered the red level of concern in 70%, and it was triggered for other reasons in 30%.
Follow-up at 2 years
Families were contacted when the surviving child(ren) born in the INFANT trial reached age 2 years. A total of 7066 families returned a questionnaire. There were statistically significant differences in the characteristics of the mothers who responded and those of the entire trial cohort, as well as between mothers who did and did not respond to an invitation to complete the questionnaire (Tables 28 and 29). Many of these differences are very small, but, given the large number of participants in the trial, are statistically significant. In general, the questionnaire responders, when compared with the entire trial cohort, were more likely to be slightly older, to be of white ethnic origin, to have given birth at a later gestational age and to have been having their first baby and were less likely to smoke.
| Characteristic | Follow-up status | p-valueb | |
|---|---|---|---|
| Non-responders or not followed up at 2 years (N = 38,669)a | Responders at 2 years (N = 6986)a | ||
| Maternal age (years) | |||
| Median (IQR) | 29 (24–33) | 30 (26–34) | < 0.001 |
| Ethnic group, n (%)c | |||
| White | 28,714 (81.9) | 5461 (90.9) | < 0.001 |
| Indian | 1318 (3.8) | 130 (2.2) | |
| Pakistani | 1356 (3.9) | 166 (2.8) | |
| Bangladeshi | 190 (0.5) | 19 (0.3) | |
| Black Caribbean | 231 (0.7) | 19 (0.3) | |
| Black African | 917 (2.6) | 37 (0.6) | |
| Any other ethnic group | 2354 (6.7) | 176 (2.9) | |
| Unknown | 3589 | 978 | |
| Twin pregnancy, n (%) | 486 (1.3) | 80 (1.2) | 0.44 |
| Gestational age at entry (completed weeks) | |||
| Median (IQR) | 40 (38–41) | 40 (39–41) | < 0.001 |
| < 35+0, n (%) | 9 (–) | 1 (–) | |
| 35+0 to 37+6, n (%) | 4314 (11.2) | 682 (9.8) | |
| 38+0 to 39+6, n (%) | 12,467 (32.3) | 2035 (29.1) | |
| 40+0 to 41+6, n (%) | 19,584 (50.7) | 3702 (53.0) | |
| ≥ 42+0, n (%) | 2282 (5.9) | 566 (8.1) | |
| BMI (kg/m2), at booking visit | |||
| Median (IQR) | 25 (22–30) | 25 (22–29) | 0.64 |
| < 18.5, n (%) | 644 (2.6) | 110 (2.2) | |
| 18.5–24.9, n (%) | 10,321 (41.8) | 2125 (42.3) | |
| 25–29.9, n (%) | 7467 (30.2) | 1563 (31.1) | |
| 30–34.9, n (%) | 3638 (14.7) | 735 (14.6) | |
| 35–39.9, n (%) | 1703 (6.9) | 319 (6.4) | |
| ≥ 40, n (%) | 936 (3.8) | 169 (3.4) | |
| Unknown | 13,960 | 1965 | |
| Smoking (at booking visit), n (%) | |||
| Yes | 4198 (15.0) | 747 (11.9) | < 0.001 |
| No | 23,681 (85.0) | 5518 (88.1) | |
| Unknown | 10,795 | 721 | |
| Parity, n (%) | |||
| Nulliparous | 22,792 (59.0) | 4317 (61.8) | < 0.001 |
| Parous | 15,858 (41.0) | 2669 (38.2) | |
| Obstetric history, n (%) | |||
| Previous stillbirth | 425 (1.1) | 70 (1.0) | 0.47 |
| Previous elective caesarean section | 359 (0.9) | 100 (1.4) | < 0.001 |
| Previous emergency caesarean section | 2028 (5.2) | 413 (5.9) | 0.02 |
| Previous neonatal death | 153 (0.4) | 19 (0.3) | 0.12 |
| Cervical dilatation at time of trial entry (cm) | |||
| Median (IQR) | 4 (2–6) | 4 (2–5) | 0.09 |
| Unknown | 27,528 | 4750 | |
| Fetal growth restriction suspected at labour onset, n (%) | 1506 (3.9) | 247 (3.5) | 0.08 |
| Labour induction, n (%): | |||
| Induced | 22,848 (59.5) | 4022 (57.9) | 0.01 |
| Spontaneous | 14,932 (38.9) | 2829 (40.7) | |
| No labour | 632 (1.7) | 101 (1.5) | |
| Epidural analgesia, n (%) | |||
| Yes | 4966 (28.0) | 425 (15.9) | < 0.001 |
| No | 12,798 (72.0) | 2257 (84.2) | |
| Unknownd | 20,905 | 4304 | |
| Presence of meconium, n (%) | |||
| Yes | 771 (4.5) | 113 (4.1) | 0.30 |
| No | 16,202 (95.5) | 2642 (95.9) | |
| Unknownd | 21,696 | 4231 | |
| Characteristic | Follow-up status | p-valueb | |
|---|---|---|---|
| Non-responders at 2 years (N = 5560)a | Responders at 2 years (N = 6986)a | ||
| Maternal age (years) | |||
| Median (IQR) | 27 (23–31) | 30 (26–34) | < 0.001 |
| Ethnic group, n (%)c | |||
| White | 4094 (86.8) | 5461 (90.9) | < 0.001 |
| Indian | 140 (3.0) | 130 (2.2) | |
| Pakistani | 230 (4.9) | 166 (2.8) | |
| Bangladeshi | 18 (0.4) | 19 (0.3) | |
| Black Caribbean | 15 (0.3) | 19 (0.3) | |
| Black African | 54 (1.1) | 37 (0.6) | |
| Any other ethnic group | 167 (3.5) | 176 (2.9) | |
| Unknown | 842 | 978 | |
| Twin pregnancy, n (%) | 78 (1.4) | 80 (1.2) | 0.20 |
| Gestational age at entry (completed weeks) | |||
| Median (IQR) | 40 (38–41) | 40 (39–41) | < 0.001 |
| < 35+0, n (%) | 2 (–) | 1 (–) | |
| 35+0 to 37+6, n (%) | 665 (12.0) | 682 (9.8) | |
| 38+0 to 39+6, n (%) | 1728 (31.1) | 2035 (29.1) | |
| 40+0 to 41+6, n (%) | 2787 (50.1) | 3702 (53.0) | |
| ≥ 42 0, n (%) | 377 (6.8) | 566 (8.1) | |
| BMI (kg/m2), at booking visit | |||
| Median (IQR) | 26 (22–30) | 25 (22–29) | 0.01 |
| < 18.5 | 130 (3.3) | 110 (2.2) | |
| 18.5–24.9 | 1559 (39.1) | 2125 (42.3) | |
| 25–29.9 | 1187 (29.8) | 1563 (31.1) | |
| 30–34.9 | 607 (15.2) | 735 (14.6) | |
| 35–39.9 | 313 (7.9) | 319 (6.4) | |
| ≥ 40 | 192 (4.8) | 169 (3.4) | |
| Unknown | 1572 | 1965 | |
| Smoking (at booking visit), n (%) | |||
| Yes | 994 (20.7) | 747 (11.9) | < 0.001 |
| No | 3807 (79.3) | 5518 (88.1) | |
| Unknown | 759 | 721 | |
| Parity, n (%) | |||
| Nulliparous | 3043 (54.7) | 4317 (61.8) | < 0.001 |
| Parous | 2517 (45.3) | 2669 (38.2) | |
| Obstetric history, n (%) | |||
| Previous stillbirth | 77 (1.4) | 70 (1.0) | 0.05 |
| Previous elective caesarean section | 61 (1.1) | 100 (1.4) | 0.10 |
| Previous emergency caesarean section | 345 (6.2) | 413 (5.9) | 0.49 |
| Previous neonatal death | 22 (0.4) | 19 (0.3) | 0.23 |
| Cervical dilatation at time of trial entry (cm) | |||
| Median (IQR) | 4 (2–5) | 4 (2–5) | 0.53 |
| Unknown | 3730 | 4750 | |
| Fetal growth restriction suspected at labour onset, n (%) | 266 (4.8) | 247 (3.5) | < 0.001 |
| Labour induction, n (%) | |||
| Induced | 3108 (56.2) | 4022 (57.9) | 0.18 |
| Spontaneous | 2336 (42.2) | 2829 (40.7) | |
| No labour | 86 (1.6) | 101 (1.5) | |
| Epidural analgesia, n (%) | |||
| Yes | 266 (13.8) | 425 (15.9) | 0.05 |
| No | 1665 (86.2) | 2257 (84.2) | |
| Unknownd | 3629 | 4304 | |
| Presence of meconium, n (%) | |||
| Yes | 66 (3.1) | 113 (4.1) | 0.06 |
| No | 2085 (96.9) | 2642 (95.9) | |
| Unknownd | 3409 | 4231 | |
Data could be analysed for 6707 of the 7066 infants for whom a questionnaire was returned (95%). There was no evidence of a difference between the two groups in any of the 2-year outcomes, including the long-term primary outcome of the PARCA-R, with a mean composite score of 98.0 points (SD 33.8 points) in the decision support group and 97.2 points (SD 33.4 points) in the no decision support group (mean difference 0.63 95% CI –0.98 to 2.25) (Table 30).
| Outcome | Trial arm | Adjusteda RR (99% CI) unless otherwise indicatedb | |
|---|---|---|---|
| Decision support | No decision support | ||
| Infant deaths at 2 years, n/N (%)c | 29/21,508 (0.13) | 35/21,597 (0.16) | 0.83 (0.44 to 1.59) |
| Number of surviving infants without the primary outcome | 3556 | 3510 | |
| PARCA-R composite scoreb | |||
| Mean score (points) (SD) | 98.0 (33.8) | 97.2 (33.4) | |
| Median score (points) (IQR) | 98 (73–126) | 97 (72–125) | Mean difference (95% CI): 0.63 (–0.98 to 2.25) |
| Unknown | 175 | 184 | |
| Components of the PARCA-R | |||
| Non-verbal cognition scale | |||
|
27.7 (3.7) | 28.0 (3.6) | |
|
28 (26–30) | 28 (26–31) | Mean difference (99% CI): –0.22 (–0.44 to 0.01) |
| Vocabulary subscale | |||
|
57.4 (27.8) | 56.5 (27.7) | |
|
58 (36–81) | 56 (35–80) | Mean difference (99% CI): 0.82 (–0.91 to 2.54) |
| Sentence complexity subscale | |||
|
12.4 (5.4) | 12.3 (5.3) | |
|
12 (9–6) | 12 (9–16) | Mean difference (99% CI): 0.07 (–0.26 to 0.41) |
| Cerebral palsy, n (%) | 4 (0.12) | 4 (0.12) | 0.99 (0.16 to 6.1) |
| Unknown | 111 | 114 | |
| Non-major or major disability, n (%)d | 942 (40.4) | 840 (37.4) | 1.08 (0.98 to 1.18) |
| Unknown | 1225 | 1266 | |
| Major disability, n (%)d | 134 (5.8) | 135 (6.0) | 0.95 (0.70 to 1.29) |
| Unknown | 1225 | 1266 | |
Nearly 6% of children were classified as having major disability. The classification of disability used resulted in relatively large numbers of children being assigned a major disability as a consequence of poor growth (between 2.8% and 3% of all children) and cognitive difficulties (between 1.2 and 1.5% of all children). 60,61 Other major disabilities, such as physical disability, blindness and deafness, were all very uncommon (Table 31).
| Disability component | Trial arm | |
|---|---|---|
| Decision support (N = 3556) | No decision support (N = 3510) | |
| Cognition, n (%) | ||
| None | 3159 (93.4) | 3134 (94.2) |
| Non-major | 180 (5.3) | 143 (4.3) |
| Major | 42 (1.2) | 49 (1.5) |
| Unknown | 175 | 184 |
| Communication, n (%) | ||
| None | 3132 (88.6) | 3096 (88.7) |
| Non-major | 394 (11.2) | 392 (11.2) |
| Major | 8 (0.2) | 4 (0.1) |
| Unknown | 22 | 18 |
| Physical ability, n (%) | ||
| None | 3161 (92.3) | 3166 (93.7) |
| Non-major | 245 (7.2) | 199 (5.9) |
| Major | 19 (0.6) | 15 (0.4) |
| Unknown | 131 | 130 |
| Vision, n (%) | ||
| None | 3409 (99.6) | 3373 (99.7) |
| Non-major | 14 (0.4) | 8 (0.2) |
| Major | 1 (–) | 1 (–) |
| Unknown | 132 | 128 |
| Hearing, n (%) | ||
| None | 3307 (99.0) | 3293 (99.2) |
| Non-major | 30 (0.9) | 25 (0.8) |
| Major | 5 (0.2) | 2 (0.1) |
| Unknown | 214 | 190 |
| Growth, n (%) | ||
| None | 1899 (90.5) | 1882 (92.2) |
| Non-major | 137 (6.5) | 102 (5.0) |
| Major | 63 (3.0) | 58 (2.8) |
| Unknown | 1457 | 1468 |
| Seizures, n (%) | ||
| None | 3302 (99.0) | 3259 (98.9) |
| Non-major | 31 (0.9) | 30 (0.9) |
| Major | 4 (0.1) | 5 (0.2) |
| Unknown | 219 | 216 |
| Feeding, n (%) | ||
| None | 3385 (99.9) | 3326 (99.9) |
| Non-major | 0 (0.0) | 0 (0.0) |
| Major | 3 (0.1) | 5 (0.2) |
| Unknown | 168 | 179 |
| Respiratory, n (%) | ||
| None | 3284 (97.0) | 3266 (97.4) |
| Non-major | 100 (3.0) | 86 (2.6) |
| Major | 0 (0.0) | 0 (0.0) |
| Unknown | 172 | 158 |
| Other disability, n (%) | ||
| None | 3550 (99.9) | 3501 (99.8) |
| Non-major | 0 (0.0) | 0 (0.0) |
| Major | 3 (0.1) | 6 (0.2) |
| Unknown | 3 | 3 |
A number of subgroup analyses were prespecified (Figures 15–40 and Tables 32–35). There was no evidence that the decision support software produced different outcomes in any of the subgroups (e.g. multiple pregnancy, suspected fetal growth restriction, BMI of the mother) for either the primary outcome or a limited range of prespecified secondary outcomes. There were also no differences in the distribution of cord blood pH measurements (Figure 41). The number of alerts in the analysis differed by centre (Figures 33, 35–39). The reasons for this are unclear, particularly as there were no statistically significant differences in the other outcomes by centre.
FIGURE 15.
Maternal and neonatal outcomes, by twin pregnancy. a, Hazard ratios reported.
FIGURE 16.
Maternal and neonatal outcomes, by suspected fetal growth restriction. a, Hazard ratios reported.
FIGURE 17.
Maternal and neonatal outcomes, by BMI at booking visit. a, Hazard ratios reported.
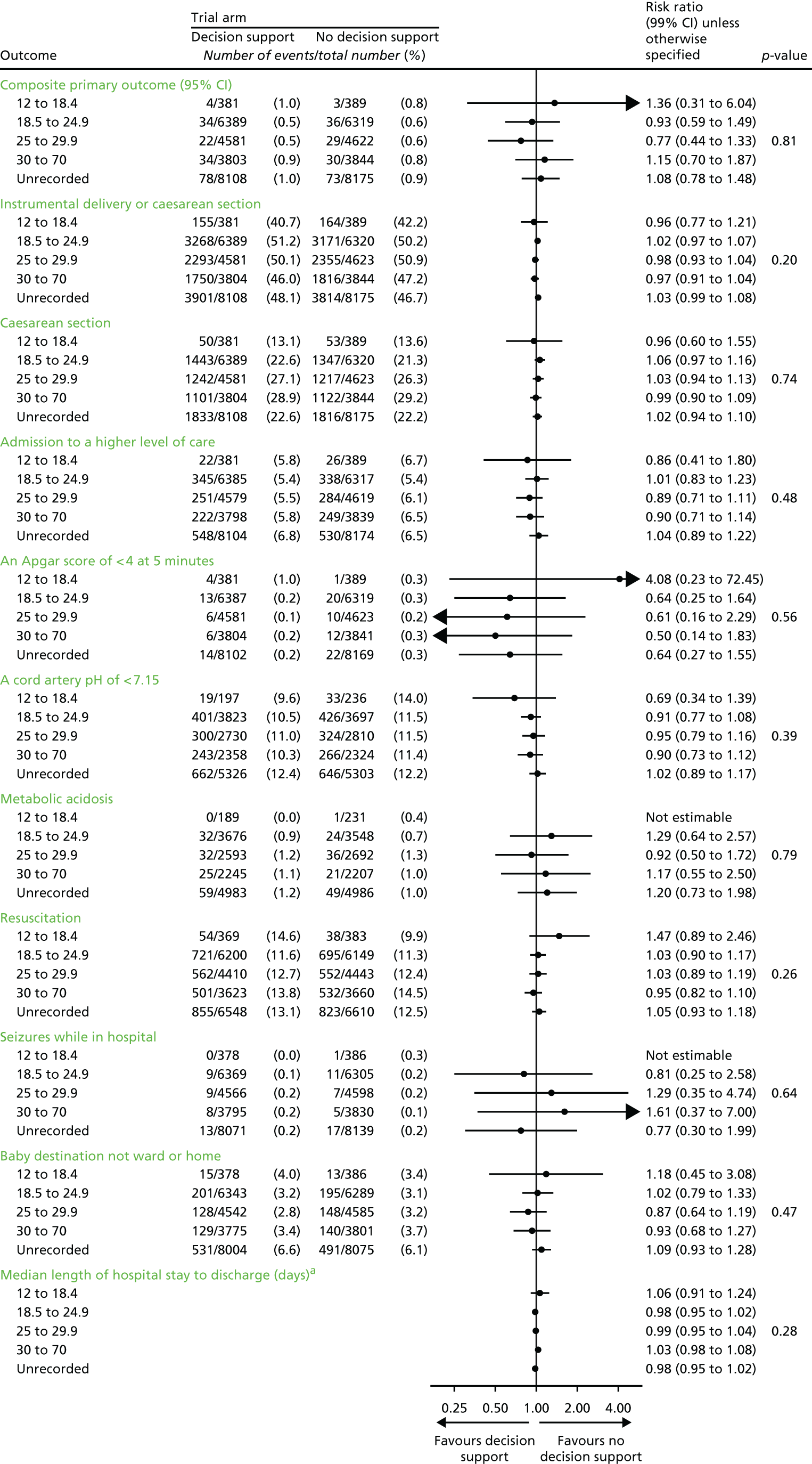
FIGURE 18.
Composite primary outcome by centre.
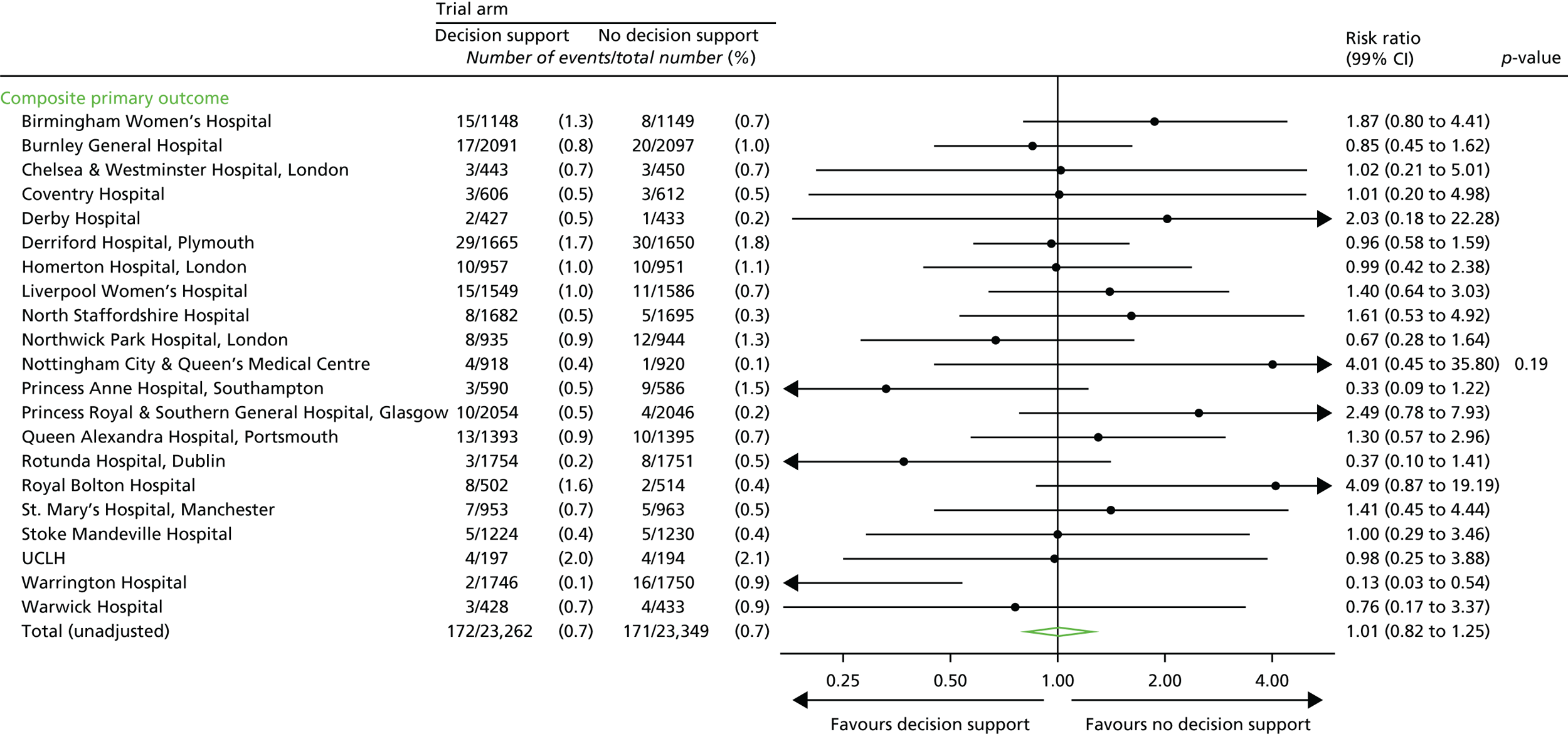
FIGURE 19.
Instrumental delivery or caesarean section, by centre.
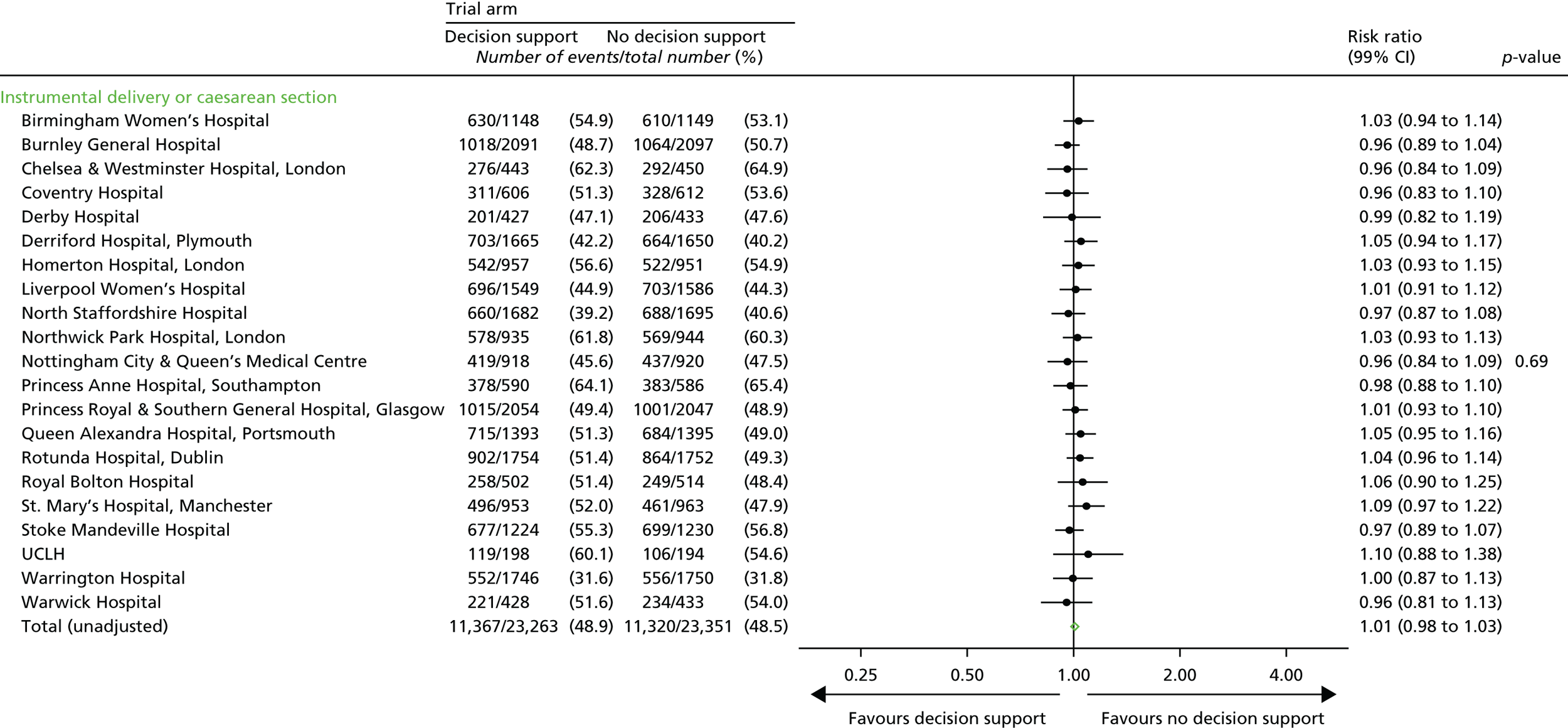
FIGURE 20.
Caesarean section by centre.
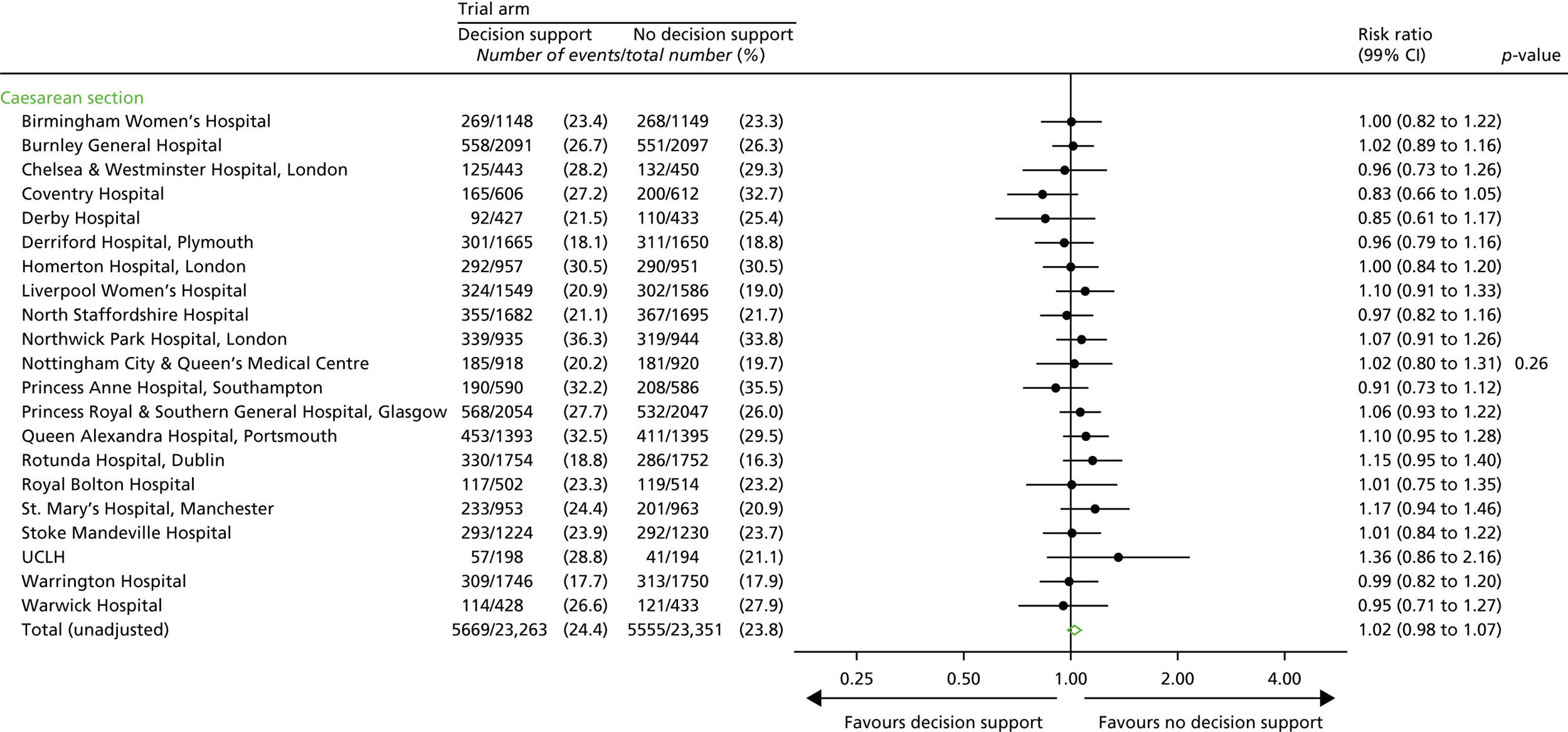
FIGURE 21.
Admission of infant to a higher level of care, by centre.
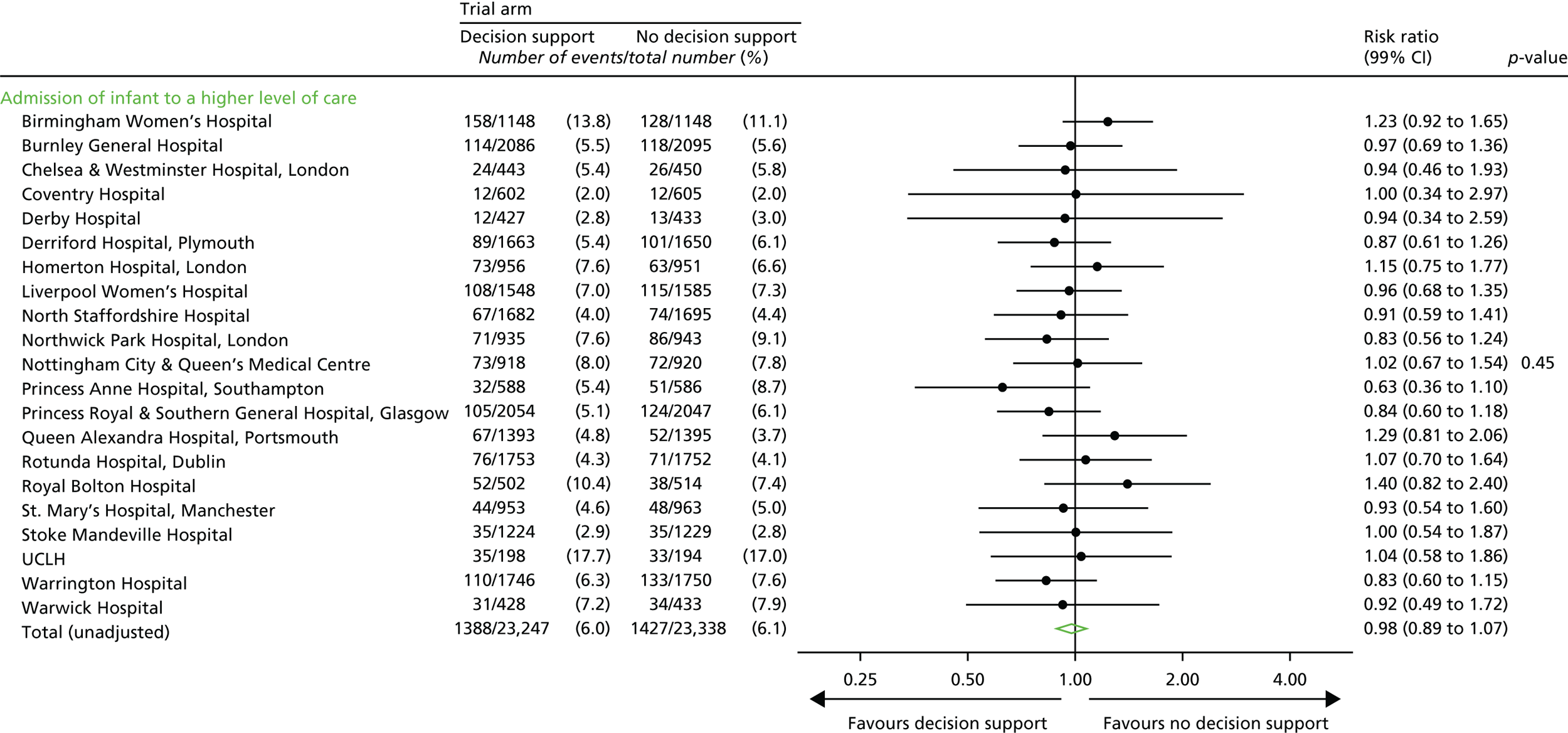
FIGURE 22.
Apgar score by centre. a, p-value calculated using centres with estimable RRs.
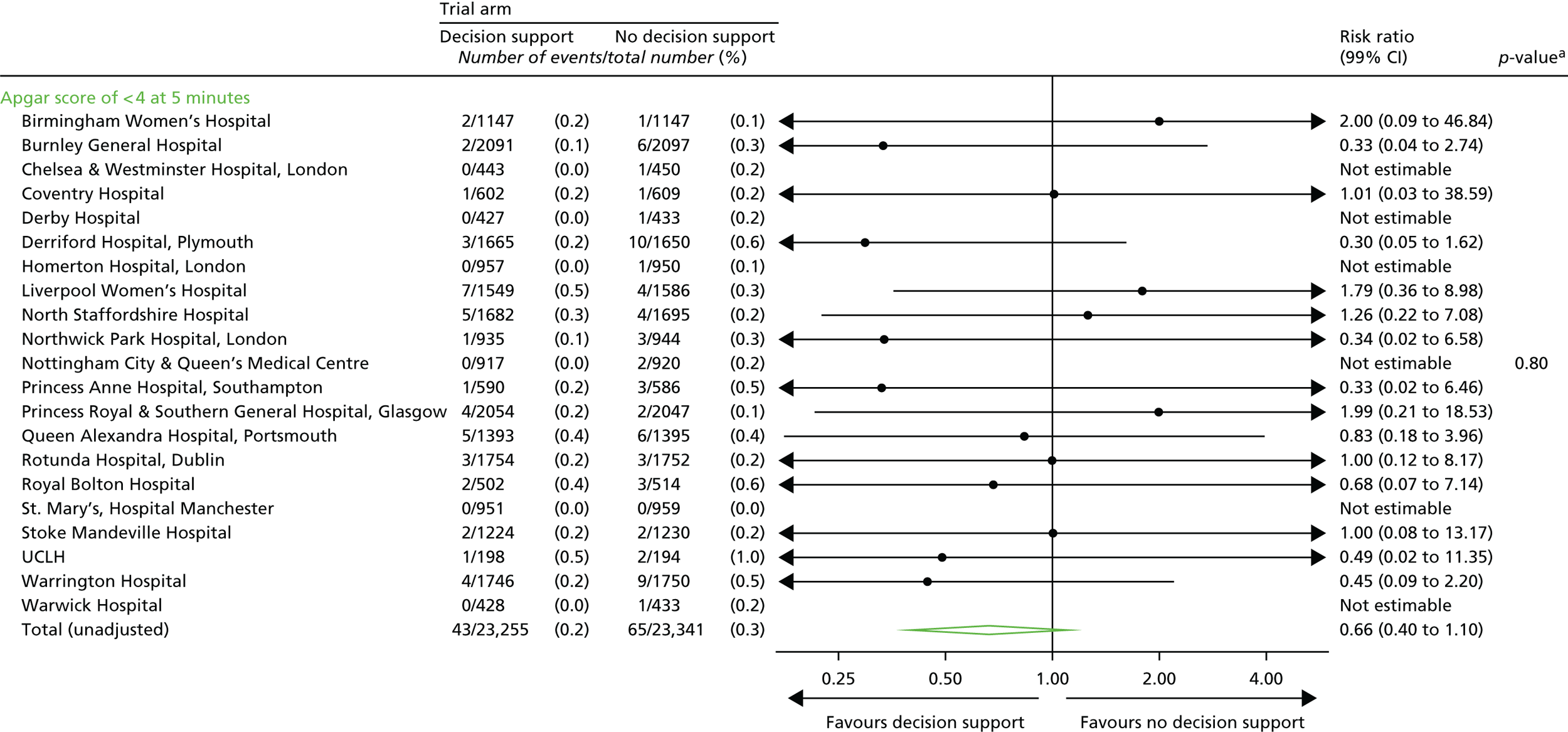
FIGURE 23.
Cord artery pH by centre.
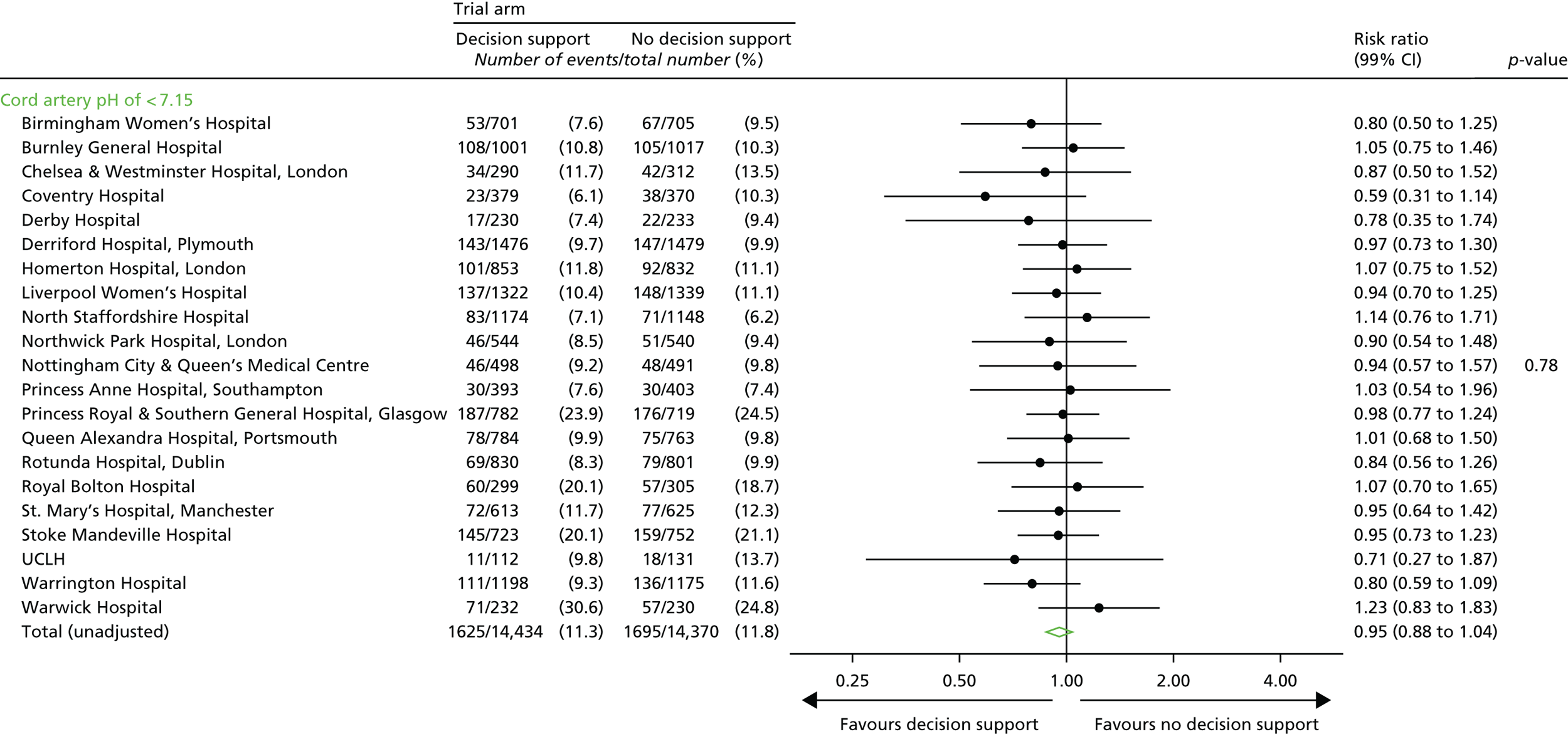
FIGURE 24.
Metabolic acidosis by centre. a, p-value calculated using centres with estimable RRs.
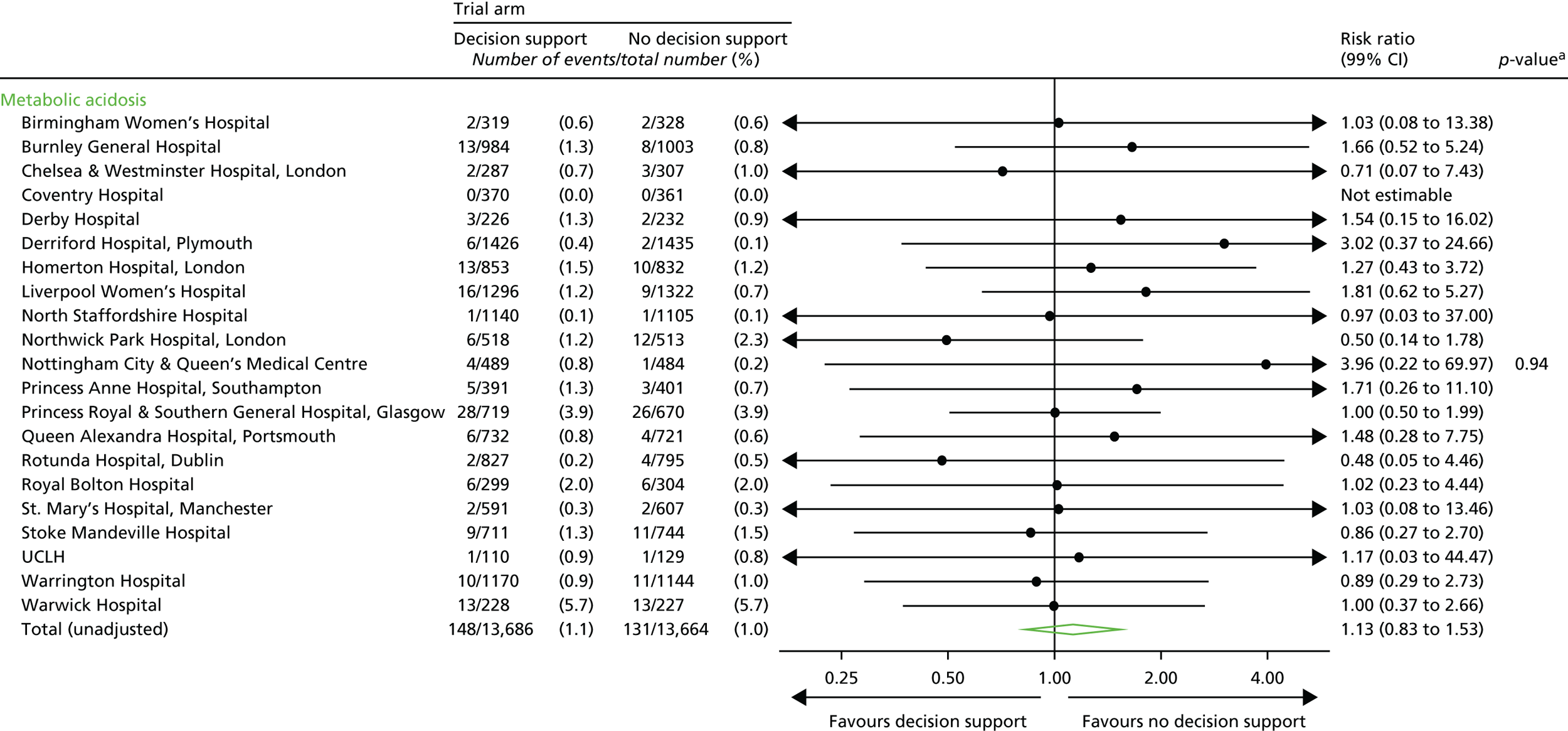
FIGURE 25.
Resuscitation by centre. a, p-value calculated using centres with estimable RRs. b, Resuscitation data were not recorded in the Guardian system at Birmingham Women’s Hospital and Homerton University Hospital.
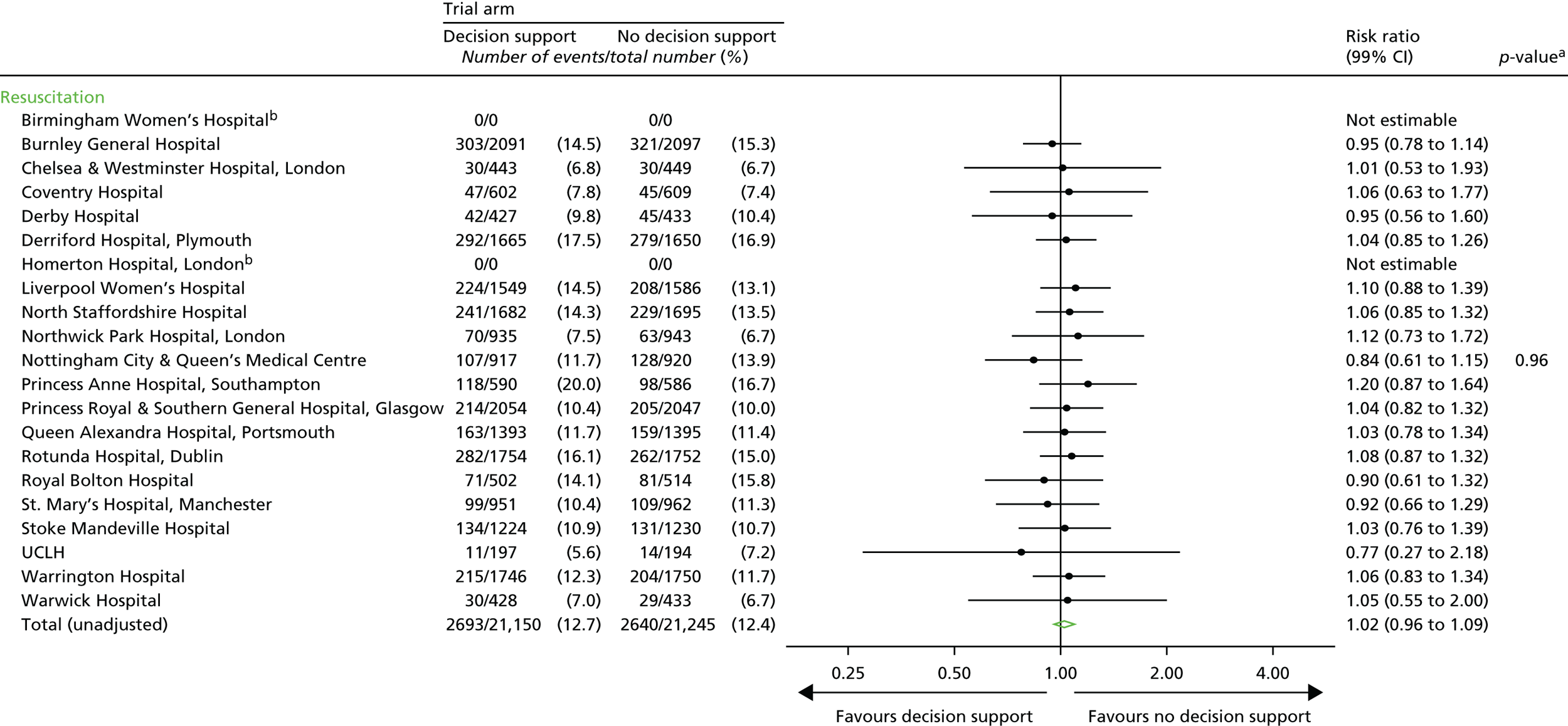
FIGURE 26.
Seizures by centre. a, p-value calculated using centres with estimable RRs.
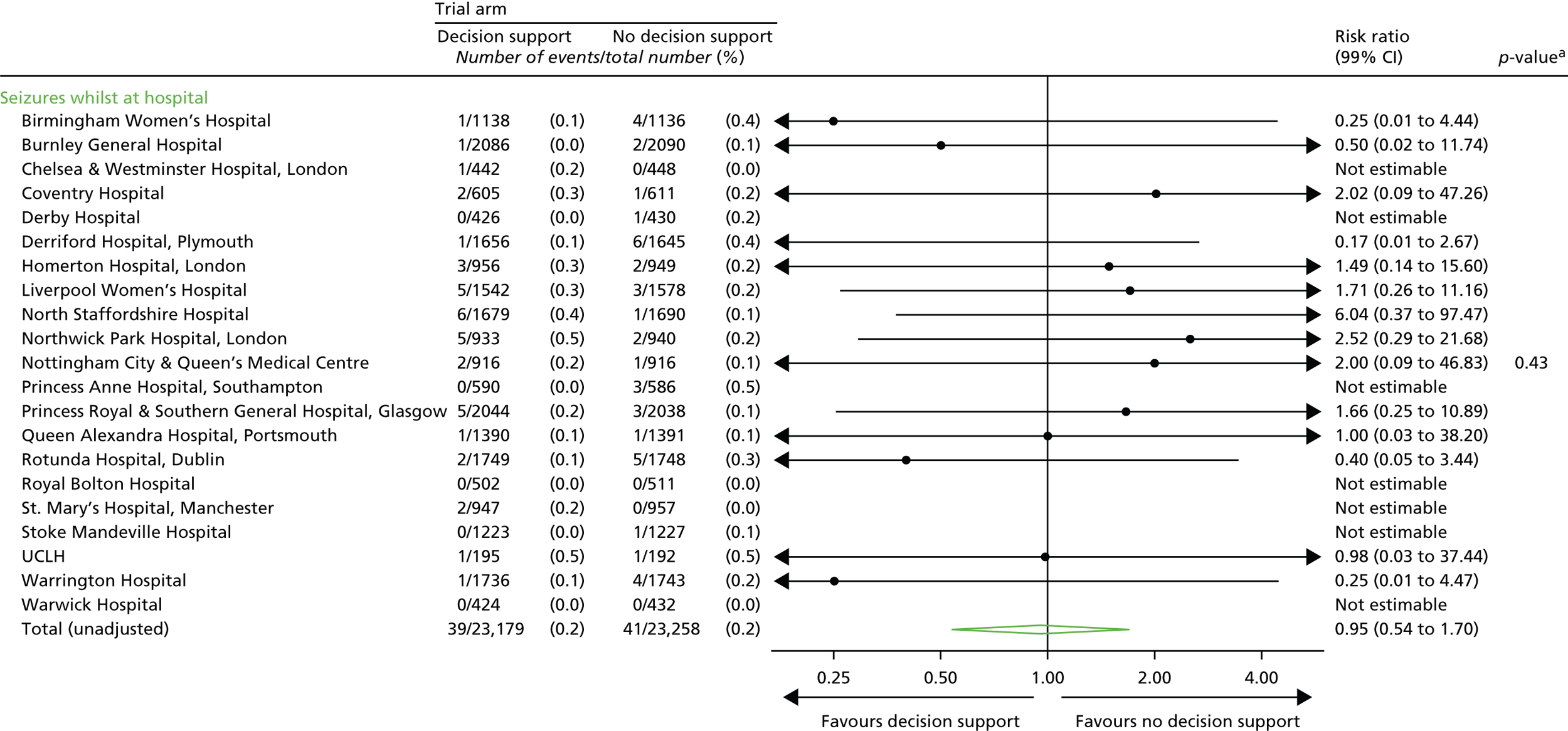
FIGURE 27.
Infant destination immediately after birth, by centre.
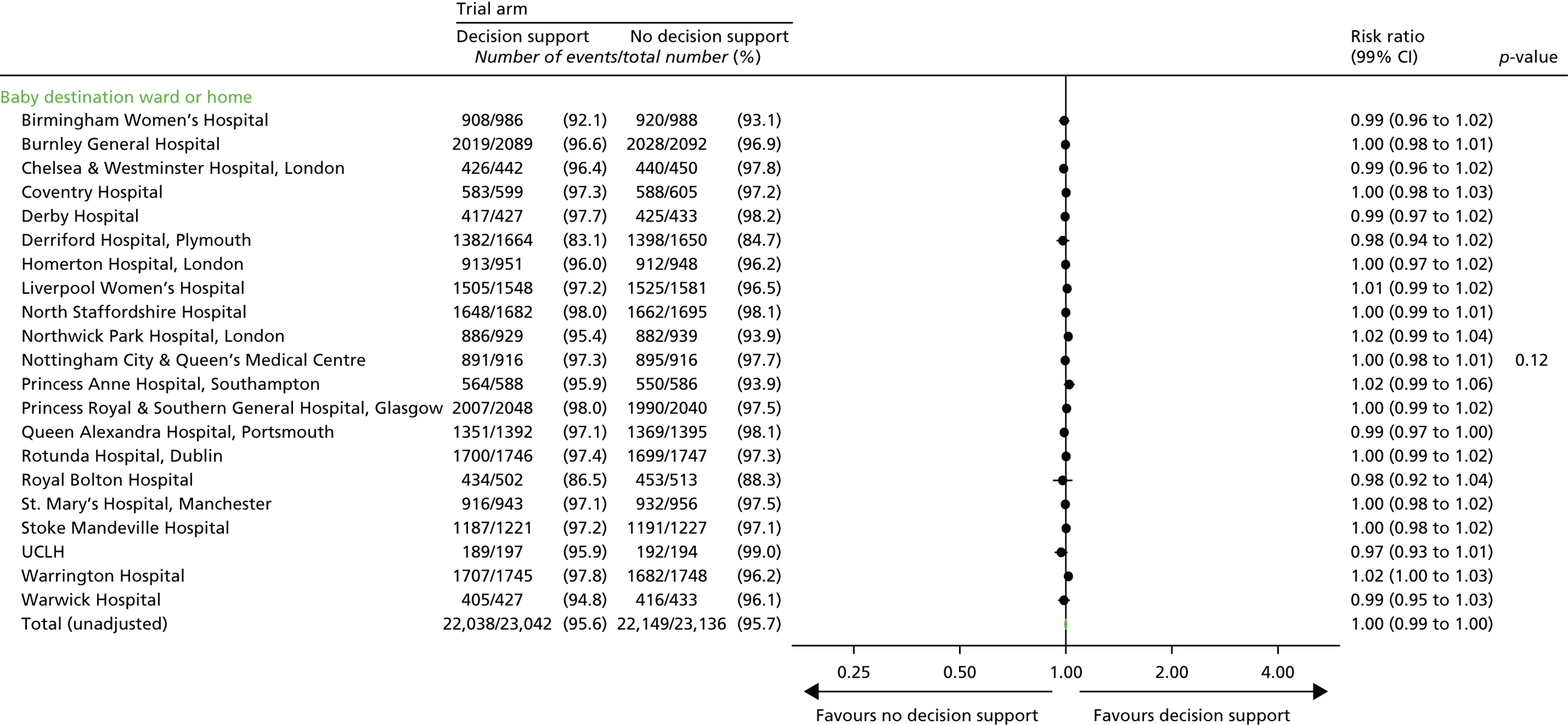
FIGURE 28.
Length of hospital stay to discharge, by centre.
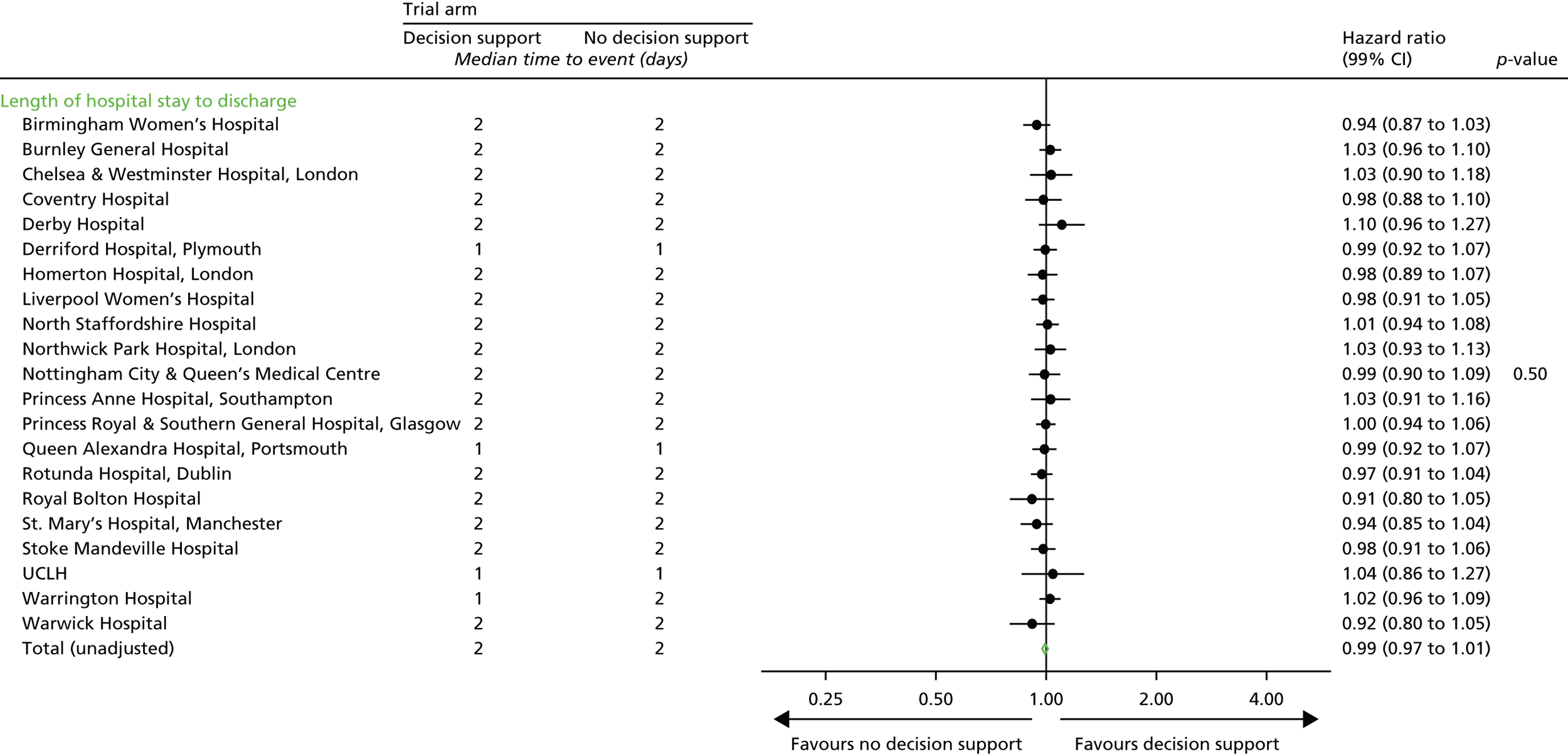
FIGURE 29.
Epidural analgesia after trial entry, by centre. a, p-value calculated using centres with estimable RRs. b, Timing of epidural in relation to trial entry only collected from 2013 onwards for each centre.
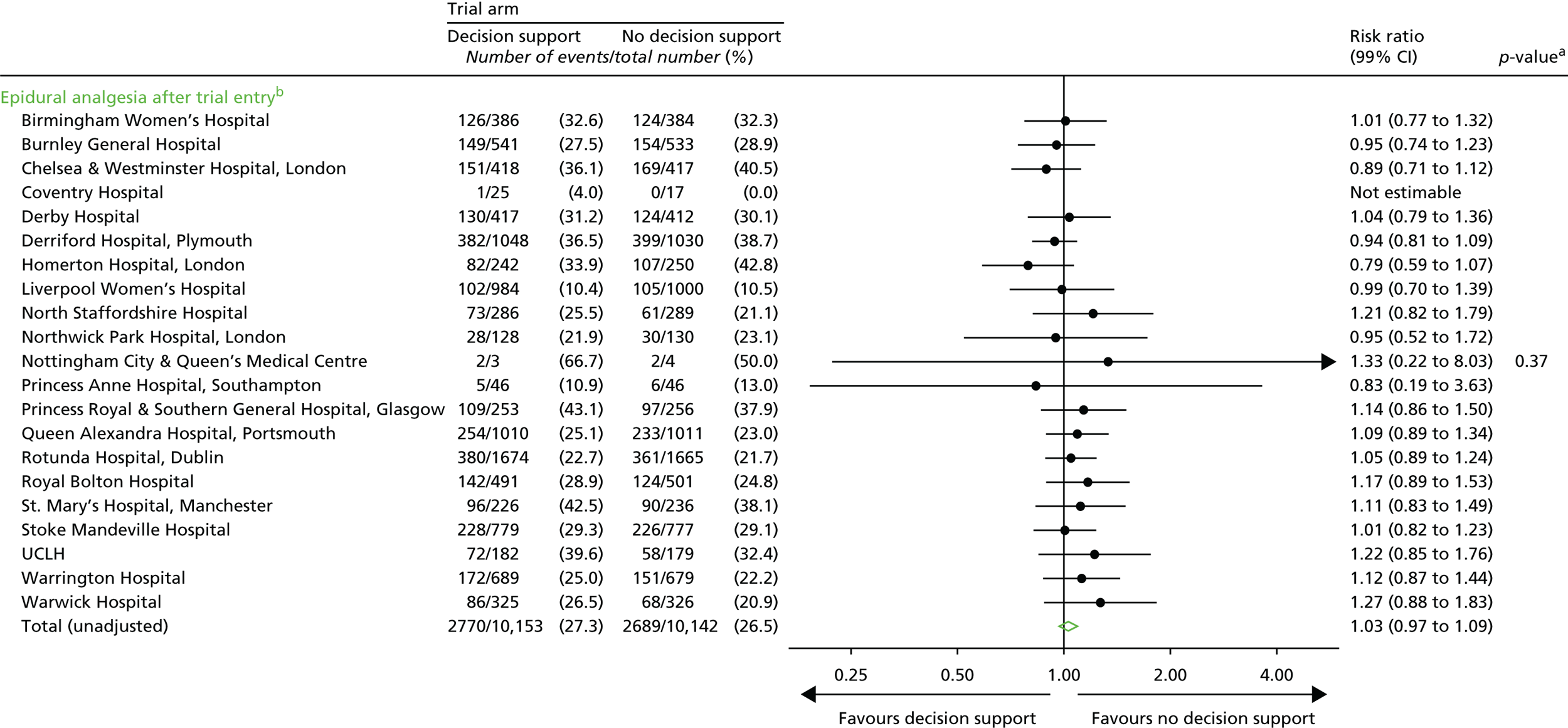
FIGURE 30.
Labour augmentation after trial entry, by centre. a, p-value calculated using centres with estimable RRs. b, Timing of labour augmentation in relation to trial entry only collected from 2013 onwards for each centre.
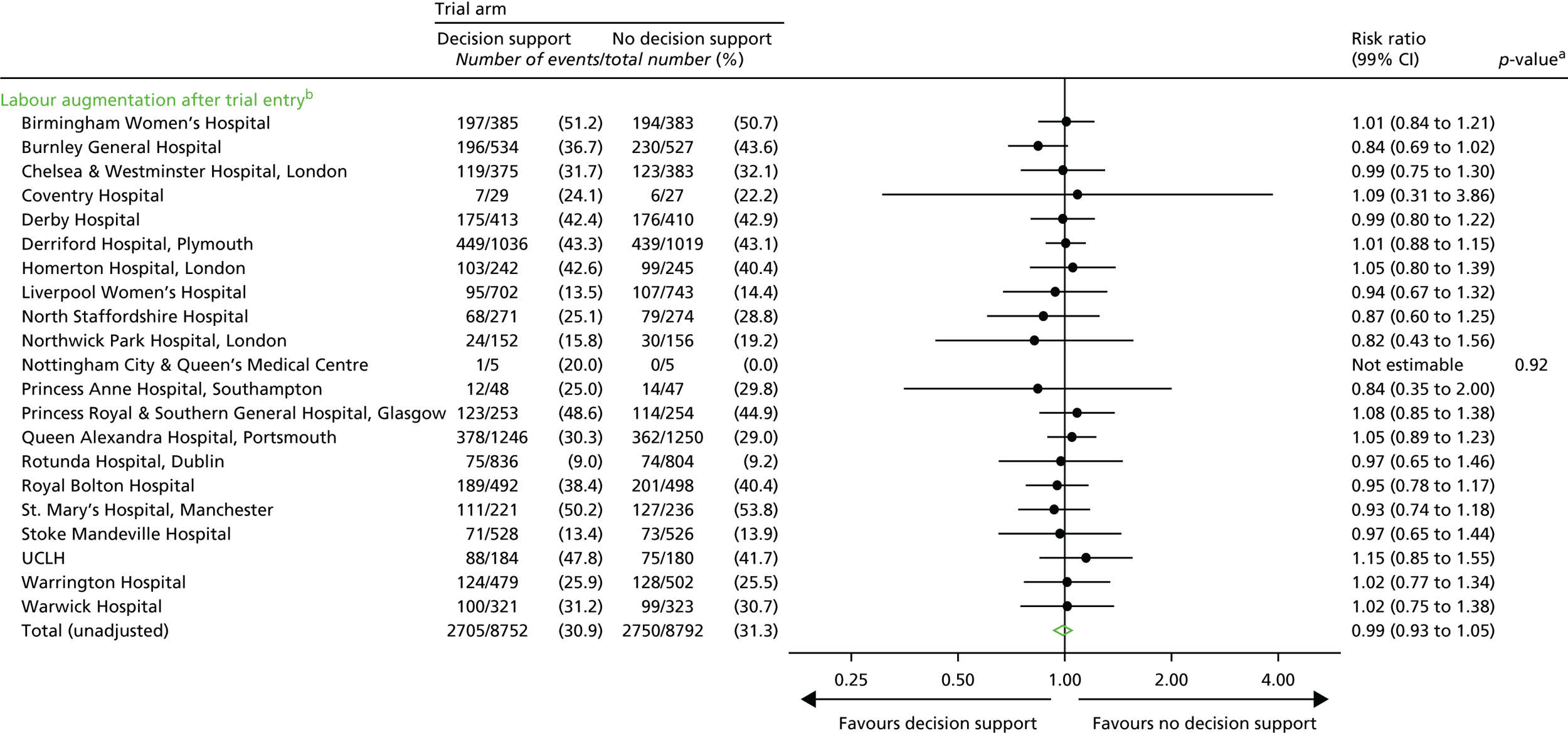
FIGURE 31.
Presence of meconium after trial entry, by centre. a, p-value calculated using centres with estimable RRs. b, Timing of meconium in relation to trial entry only collected from 2013 onwards for each centre.
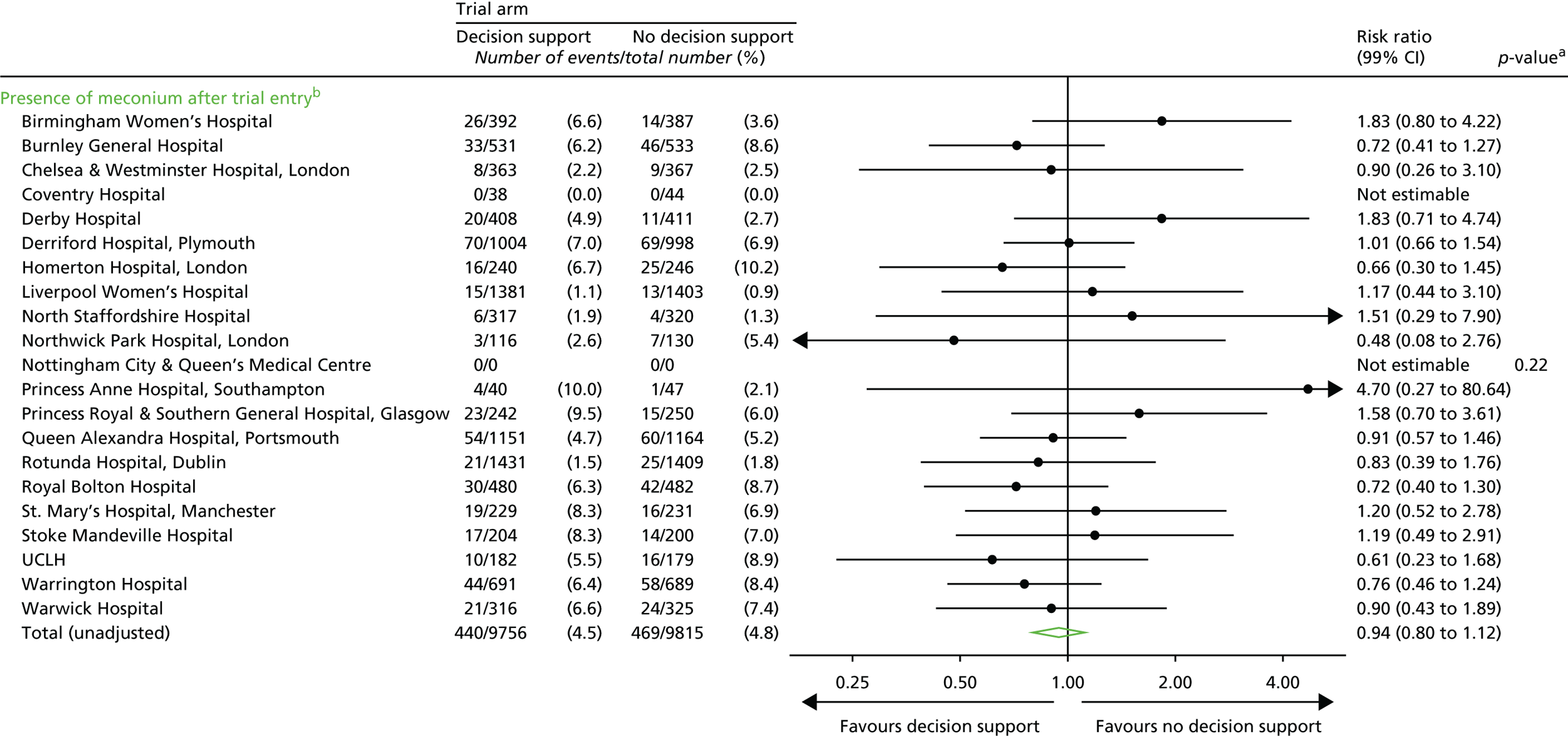
FIGURE 32.
Number of women with at least one blue, yellow or red level of concern, by centre.
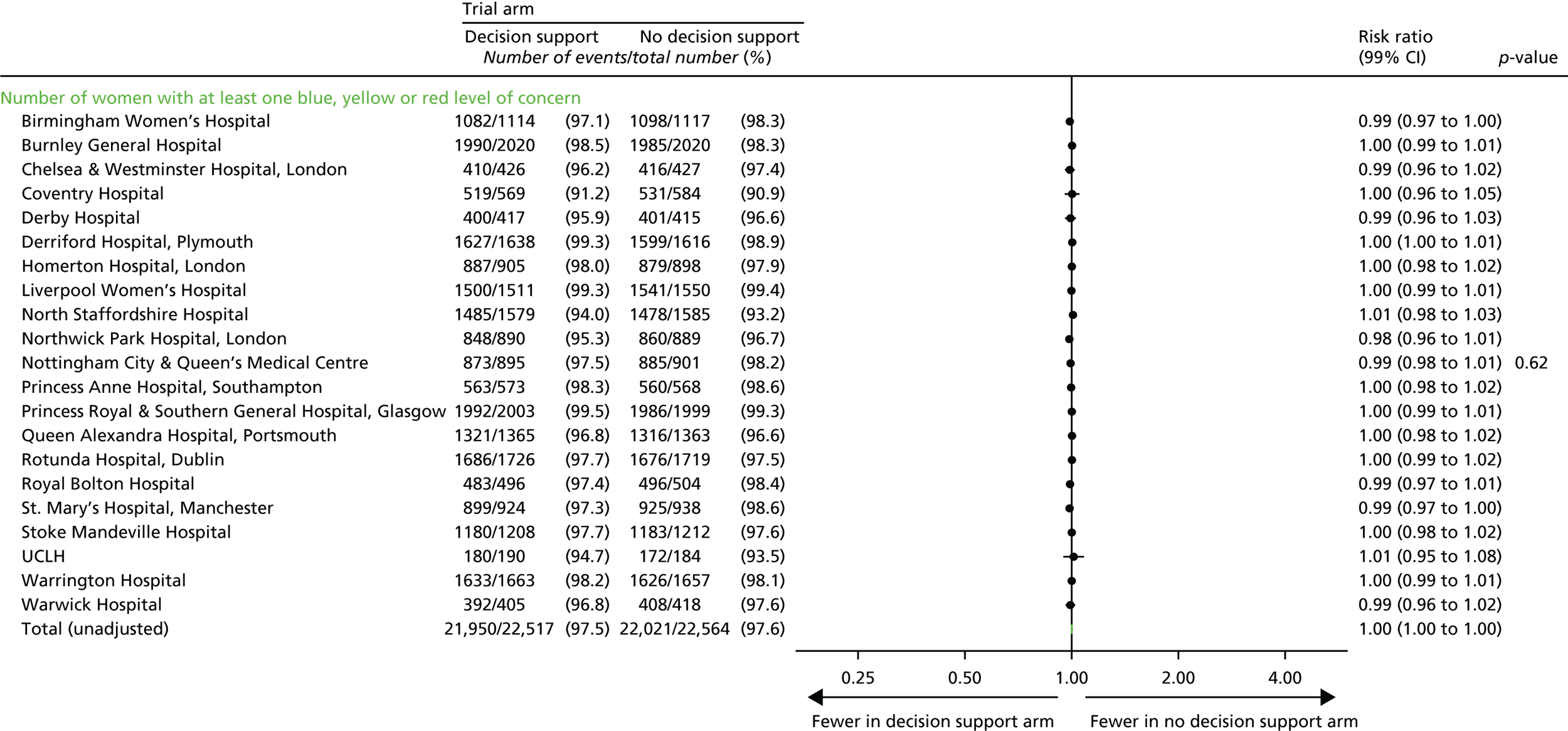
FIGURE 33.
Number of women with at least one blue level of concern, by centre.
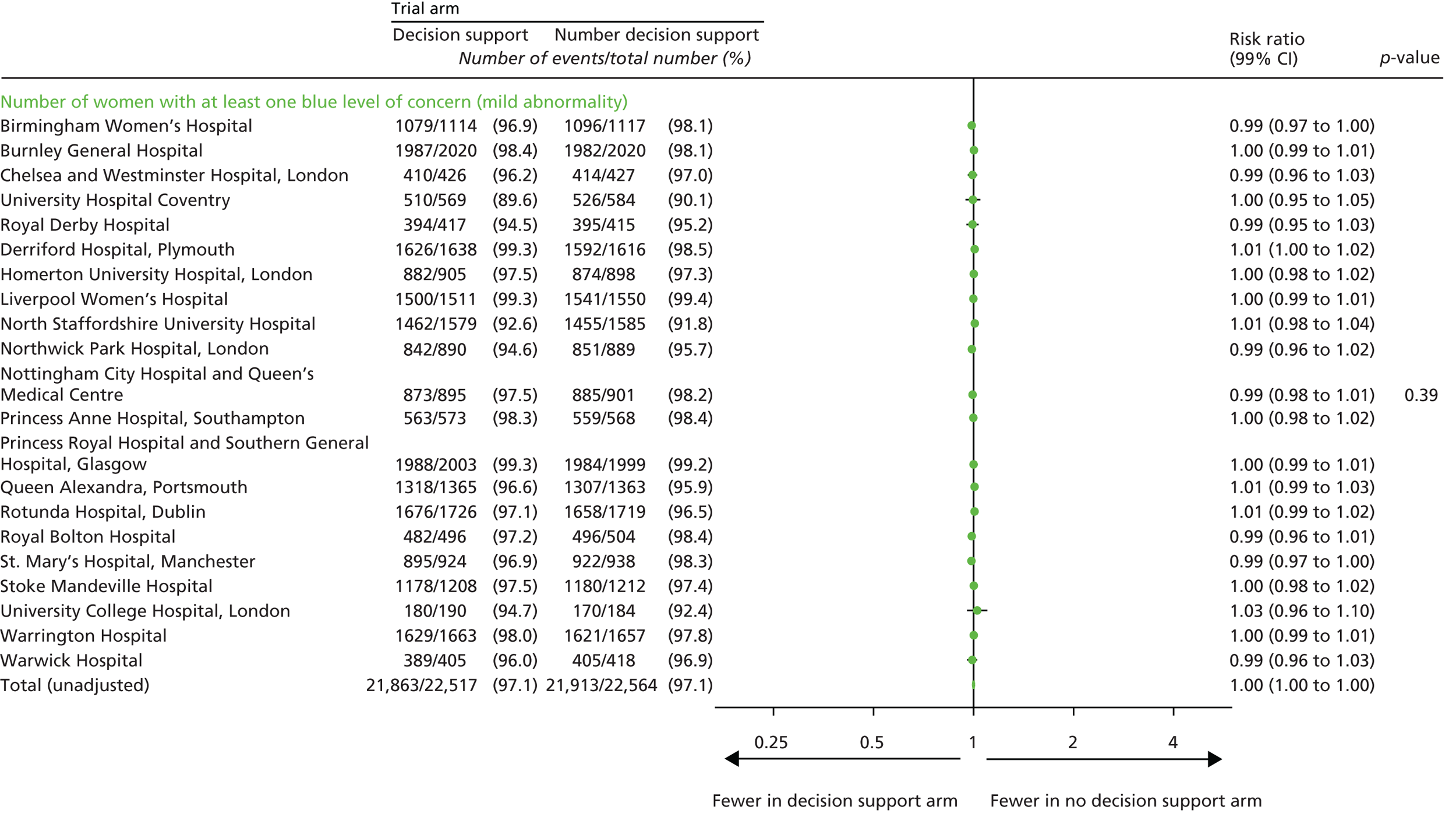
FIGURE 34.
Number of women with at least one yellow level of concern, by centre.
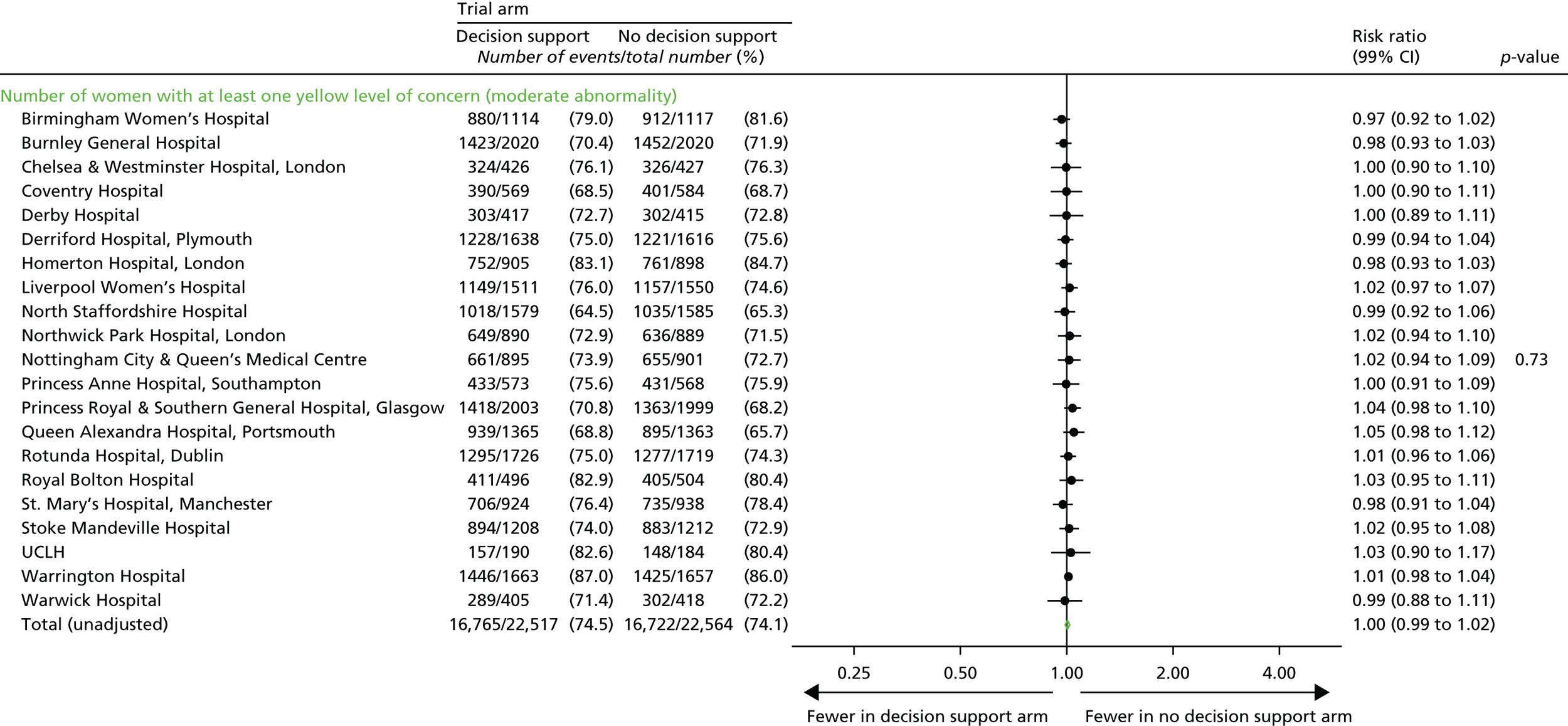
FIGURE 35.
Number of women with at least one red level of concern, by centre. a, Data on timing of red level of concern are not available for two centres: Warwick Hospital (n = 823) and Derby Hospital (n = 832).
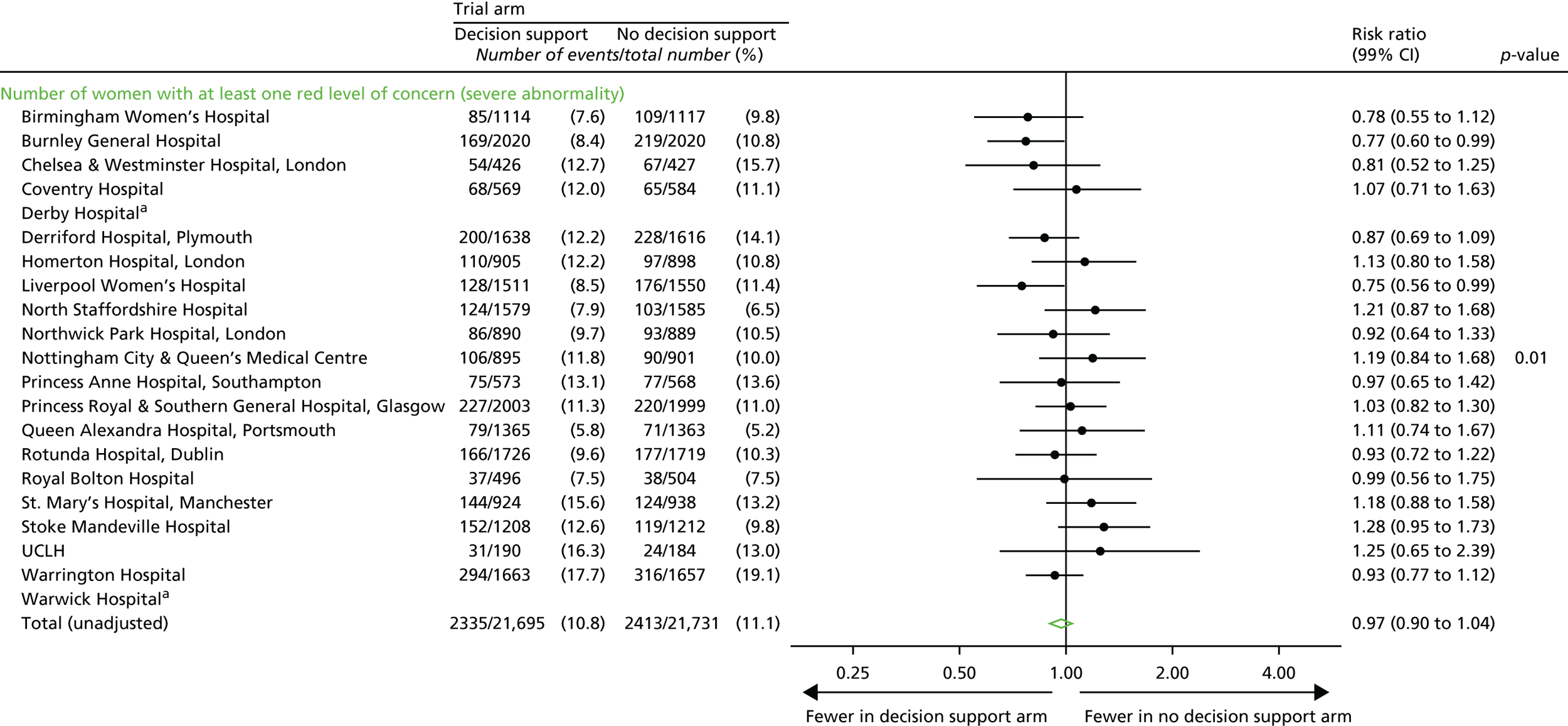
FIGURE 36.
Number of blue, yellow and red levels of concern, by centre.
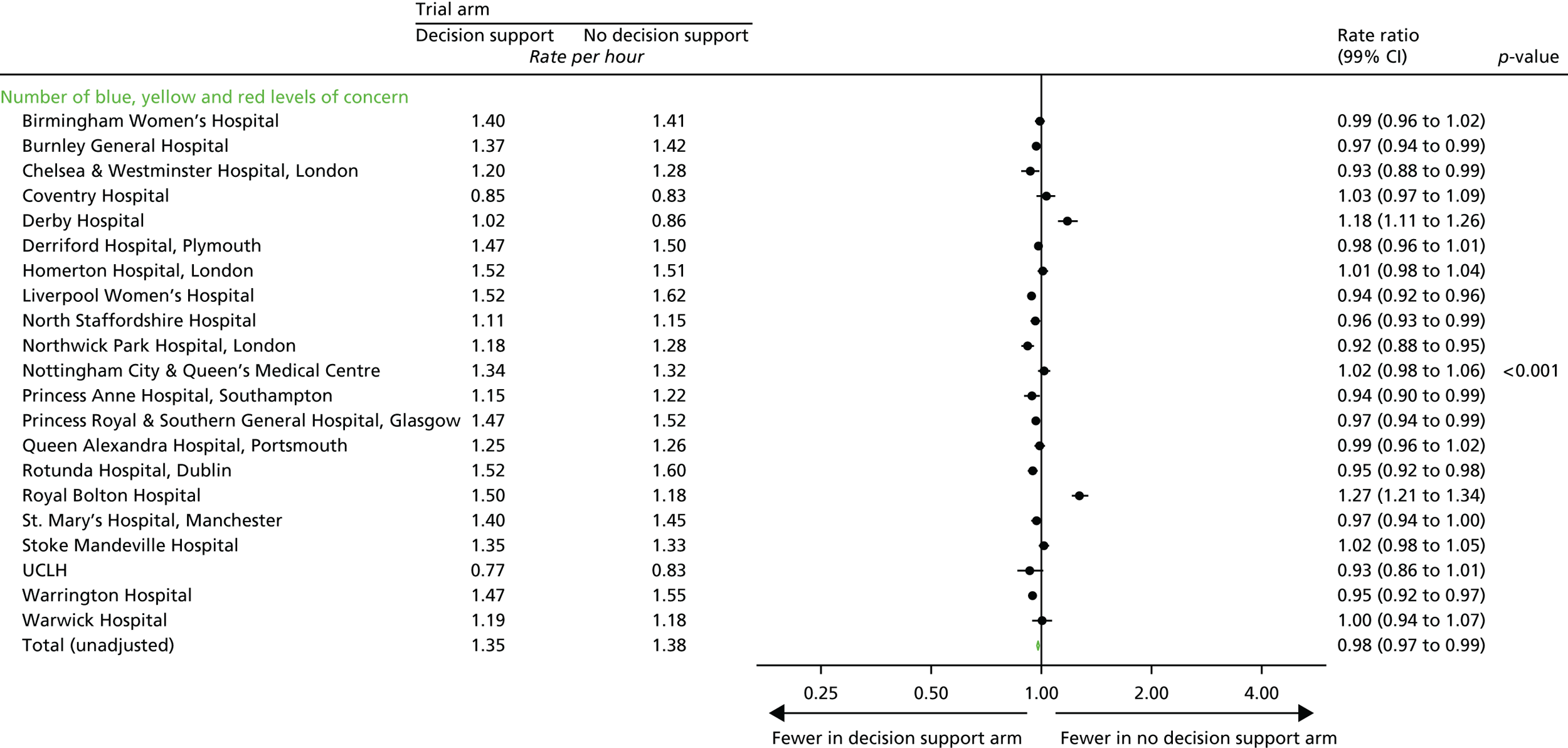
FIGURE 37.
Number of blue levels of concern, by centre.
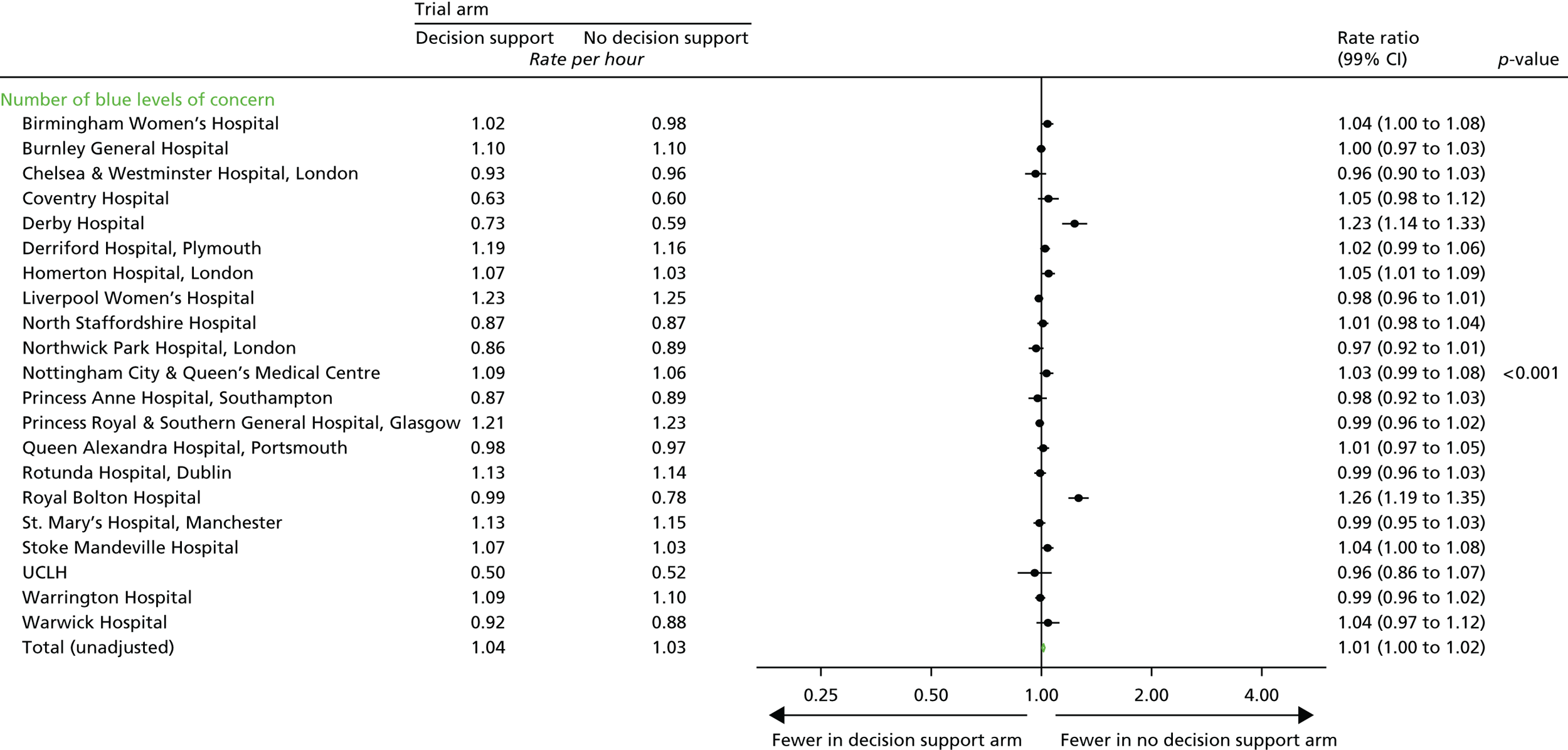
FIGURE 38.
Number of yellow levels of concern, by centre.
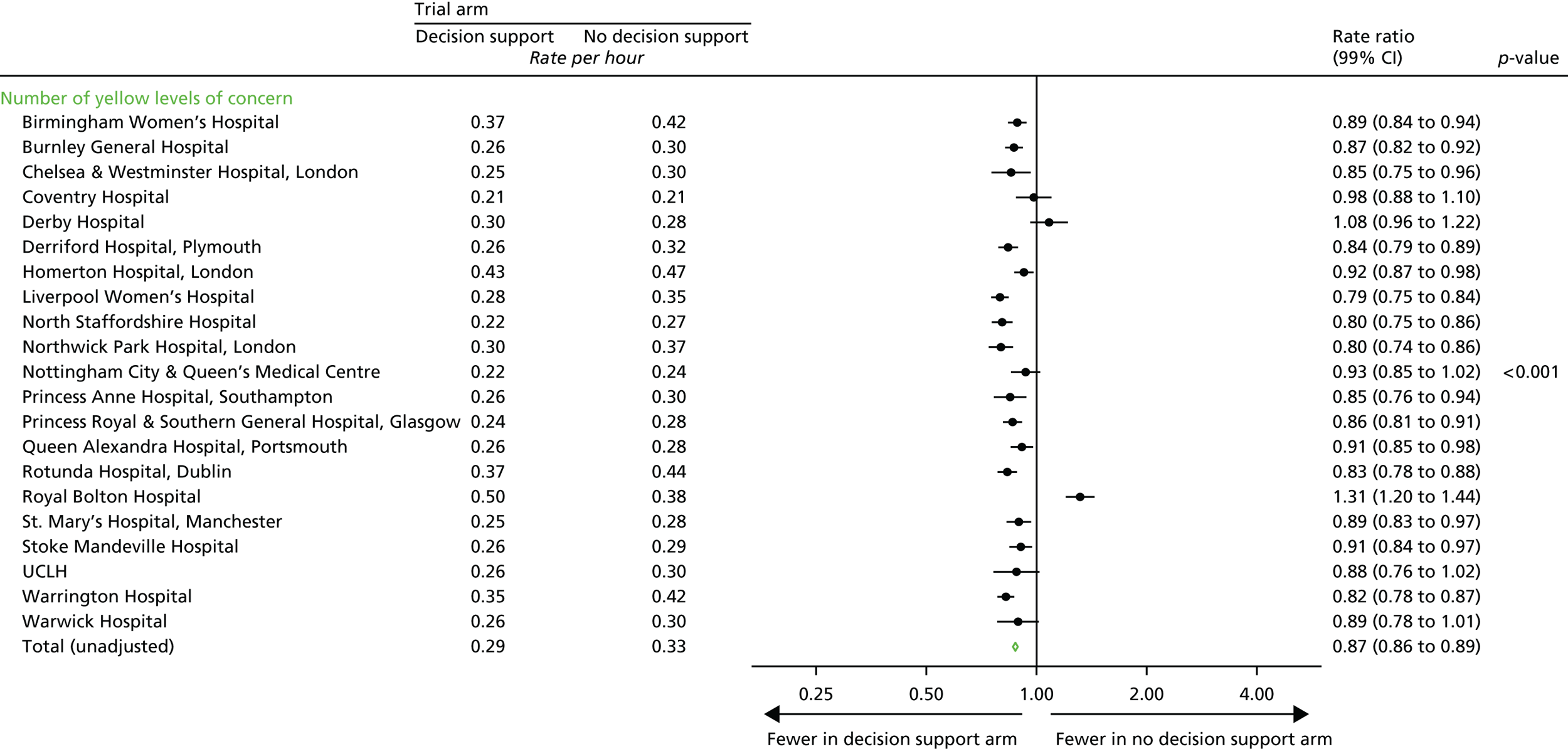
FIGURE 39.
Number of red levels of concern, by centre. a, Data on timing of red level of concern are not available for two centres: Warwick Hospital (n = 823) and Derby Hospital (n = 832).
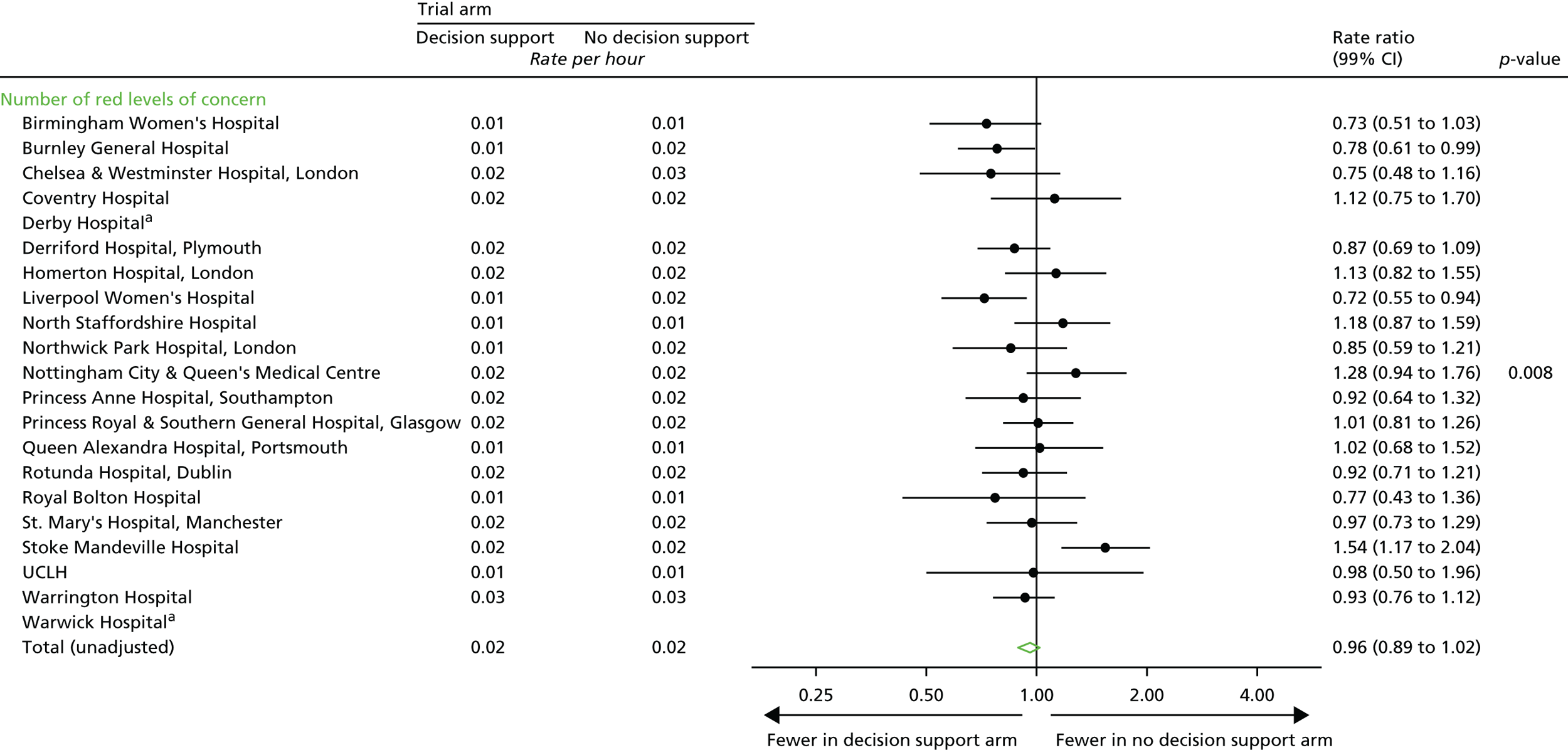
FIGURE 40.
Time from last red level of concern to delivery. a, Hazard ratio of > 1 favours decision support as this implies that time to delivery (from last red level of concern) was reached more quickly than with no decision support. b, Data on timing of red level of concern are not available for two centres: Warwick Hospital (n = 823) and Derby Hospital (n = 832).
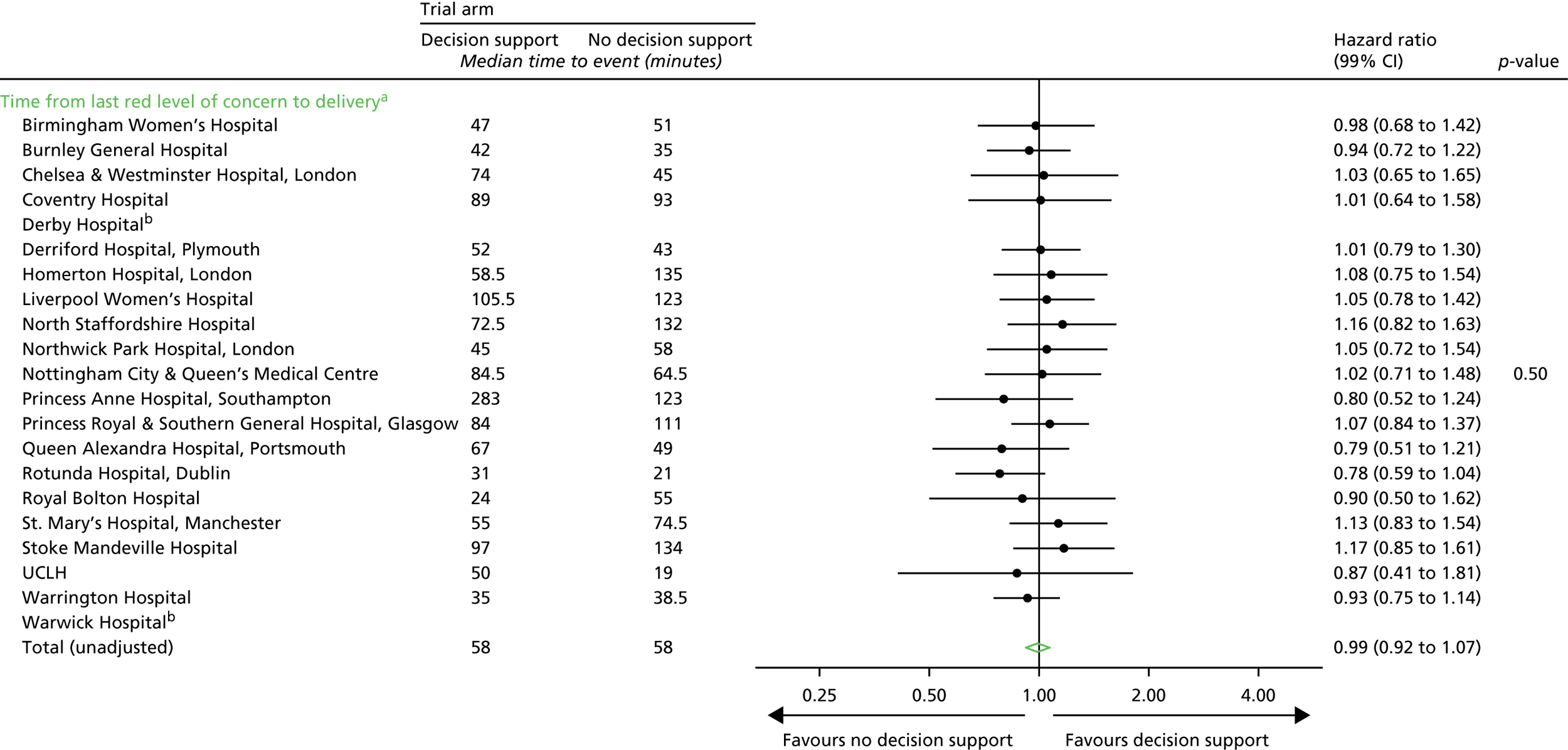
Predefined subgroup analyses of short-term outcomes
Note: results are presented on forest plots showing the RR plus 95% CI for each subgroup, by treatment group, with the p-value for the statistical test of interaction. Subgroup analyses are not adjusted for the stratification factors used at randomisation because of the small number of events in some subgroup categories.
Predefined subgroup analyses of long-term outcomes
| Singleton vs. twins | n (%) | Trial arm, mean score (points) (SD) | Mean difference (95% CI) | |
|---|---|---|---|---|
| Decision support | No decision support | |||
| Singleton | 6555 (98) | 98.2 (33.7) | 97.4 (33.4) | 0.82 (–0.81 to 2.44) |
| Twin | 152 (2) | 85.7 (37.3) | 89.7 (32.4) | –3.95 (–19.4 to 11.5) |
| Total (unadjusted) | 6707 (100) | 98.0 (33.8) | 97.2 (33.4) | 0.73 (–0.88 to 2.34) |
| Unknown | 359 | |||
| FGR | n (%) | Trial arm, mean score (points) (SD) | Mean difference (95% CI) | |
|---|---|---|---|---|
| Decision support | No decision support | |||
| No FGR | 6472 (96) | 98.3 (33.7) | 97.5 (33.4) | 0.77 (–0.89 to 2.42) |
| FGR | 235 (4) | 89.4 (35.0) | 90.9 (34.1) | –1.50 (–10.5 to 7.54) |
| Total (unadjusted) | 6707 (100) | 98.0 (33.8) | 97.2 (33.4) | 0.73 (–0.88 to 2.34) |
| Unknown | 359 | |||
| BMI (kg/m2) | n (%) | Trial arm, mean score (points) (SD) | Mean difference (95% CI) | |
|---|---|---|---|---|
| Decision support | No decision support | |||
| 12 to 18.4 | 104 (2) | 94.6 (37.5) | 99.9 (37.9) | –5.27 (–20.0 to 9.41) |
| 18.5 to 24.9 | 2039 (30) | 98.2 (33.7) | 97.4 (32.8) | 0.86 (–2.07 to 3.78) |
| 25 to 29.9 | 1508 (23) | 98.3 (34.8) | 98.6 (33.3) | –0.34 (–3.82 to 3.14) |
| 30 to 70 | 1157 (17) | 96.7 (34.8) | 95.3 (33.1) | 1.43 (–2.53 to 5.39) |
| Unrecorded | 1899 (28) | 98.3 (32.4) | 97.1 (34.0) | 1.27 (–1.76 to 4.30) |
| Total (unadjusted) | 6707 (100) | 98.0 (33.8) | 97.2 (33.4) | 0.73 (–0.88 to 2.34) |
| Unknown | 359 | |||
| Centrea | n (%) | Trial arm, mean score (points) (SD) | Mean difference (95% CI) | |
|---|---|---|---|---|
| Decision support | No decision support | |||
| Birmingham Women’s Hospital | 262 (4) | 95.1 (32.0) | 96.8 (31.0) | –1.74 (–9.54 to 6.06) |
| Burnley General Hospital | 832 (12) | 94.0 (35.7) | 94.0 (33.4) | 0.02 (–4.73 to 4.78) |
| Derriford Hospital, Plymouth | 814 (12) | 95.6 (33.0) | 94.7 (34.3) | 0.84 (–3.85 to 5.53) |
| Liverpool Women’s Hospital | 725 (11) | 102.4 (33.4) | 97.6 (34.6) | 4.79 (–0.28 to 9.85) |
| Northwick Park Hospital, London | 146 (2) | 86.3 (34.8) | 88.4 (31.4) | –2.19 (–13.3 to 8.87) |
| Nottingham City Hospital and Queens Medical Centre | 135 (2) | 98.1 (33.2) | 104.4 (30.0) | –6.30 (–17.1 to 4.50) |
| Princess Anne Hospital, Southampton | 205 (3) | 99.1 (32.0) | 100.9 (32.0) | –1.77 (–10.7 to 7.15) |
| Princess Royal Hospital and Southern General Hospital, Glasgow | 333 (5) | 106.1 (31.9) | 105.4 (32.4) | 0.69 (–6.27 to 7.65) |
| Queen Alexandra Hospital, Portsmouth | 693 (10) | 98.2 (33.7) | 97.7 (32.4) | 0.56 (–4.44 to 5.56) |
| Rotunda Hospital, Dublin | 161 (2) | 109.8 (32.2) | 110.0 (34.9) | –0.19 (–10.7 to 10.3) |
| St Mary’s Hospital, Manchester | 208 (3) | 100.0 (34.8) | 96.2 (32.4) | 3.73 (–5.73 to 13.2) |
| Stoke Mandeville Hospital | 462 (7) | 98.3 (32.4) | 96.4 (32.0) | 1.86 (–4.08 to 7.80) |
| University Hospital of North Staffordshire | 826 (12) | 97.1 (34.3) | 97.9 (33.3) | –0.75 (–5.37 to 3.88) |
| Warwick Hospital | 905 (14) | 97.4 (33.6) | 96.8 (34.4) | 0.61 (–3.88 to 5.10) |
| Total (unadjusted) | 6707 (100) | 98.0 (33.8) | 97.2 (33.4) | 0.73 (–0.88 to 2.34) |
| Unknown | 359 | |||
Chapter 7 Economic evaluation
In previous chapters we showed that the use of decision support software for the management of labour did not translate into statistically significant differences in the two primary outcomes in this trial. In addition, we reported that other neonatal secondary outcomes at hospital discharge and at 2 years’ follow-up were similar between the two groups.
This chapter adds an additional layer of relevant information to the clinical outcomes and includes a cost–consequences analysis conducted alongside INFANT. It was originally intended to be presented as a cost-effectiveness analysis and a health economics analysis plan had been developed to support this (see Appendix 10). Given the trial findings, a cost–consequences approach was deemed more appropriate and helpful, as is discussed in detail later in this chapter.
The cost–consequences analysis was conducted from a NHS perspective and included direct costs to mothers and their babies. A detailed overview of the analysis conducted from trial entry to hospital discharge and up to 2 years’ follow-up is provided in this chapter. In addition to the composite primary outcome at discharge, PARCA-R composite scores for a subset of surviving infants without the composite primary outcome at 2 years and maternal health-related quality of life (HRQoL) at 12 and 24 months post birth are presented.
Methods
NHS health-care resource use
A comprehensive list of health-care resource use information for mothers and their babies was collected for the study. Data collection began when women arrived in hospital in labour and ended when postnatal care for both mother and baby was complete. For most women, this was following hospital discharge. A subset of women who consented to be followed up post birth were sent a questionnaire regarding health-care resource use and maternal HRQoL information at 12 and 24 months’ follow-up.
Labour-related resource use data included procedures undertaken for mothers and infants before discharge and were collected using the Guardian system. Additional information, such as maternal transfers after birth, whether or not babies or mothers had been admitted to a higher level of care unit and neonatal deaths, was collected using bespoke data collection forms. These were overseen by the trial’s co-ordinating research midwife, who ensured that all care was documented. The forms were then posted to the INFANT trial administrative centre for data entry.
Health-care utilisation at 12- and 24-month follow-up was identified using a postal questionnaire that collected information about acute and community care, secondary care and maternal HRQoL. It included outpatient appointments and inpatient stays (e.g. for operations), follow-up care and numbers of visits to relevant health-care professionals. The questionnaires were sent to a subsample of mothers of surviving infants who consented to follow-up and who gave birth in the first year of the trial. The 12-month follow-up questionnaire collected resource use data between post-birth discharge and 1 year, and the 24-month questionnaire asked about resources consumed in the previous 12 months. Two postal reminders were sent by the trial management team for questionnaires not returned. All data collected using the postal questionnaire were double data entered and cleaned prior to analysis. Table 36 presents categories of NHS health-care resource use collected during the study.
| Resource use item | Unit cost (GBP) | Source | Notes |
|---|---|---|---|
| Maternal | |||
| Birth related | |||
| Induction | 173 | Schroeder et al., 201273 | |
| Episiotomy | 27 | Schroeder et al., 201273 | |
| Perineal tear | |||
| First- and second-degree tears | 23 | Schroeder et al., 201273 | |
| Third- and fourth-degree tears | 649 | Schroeder et al., 201273 | |
| Manual removal of the placenta | 752 | Schroeder et al., 201273 | |
| Blood transfusion | 158 | Schroeder et al., 201273 | Per blood pack |
| Higher level of care admissions | |||
| Level of care, per day | |||
| Special care | 898 | NHS Reference Costs 2014 to 2015, 201574 | |
| High-dependency care | 1278 | NHS Reference Costs 2014 to 2015, 201574 | |
| Intensive care | 1432 | NHS Reference Costs 2014 to 2015, 201574 | |
| Surgery/post-birth procedures | |||
| Management of post-partum haemorrhage using the Bakri technique | 987 | NHS Reference Costs 2014 to 2015, 201574 | |
| Management of post-partum haemorrhage using EUA | 1201 | NHS Reference Costs 2014 to 2015, 201574 | |
| Hysterectomy | 1388 | NHS Reference Costs 2014 to 2015, 201574 | |
| Perineal haematoma | 987 | NHS Reference Costs 2014 to 2015, 201574 | |
| Transfer to another hospital | 439 | Schroeder et al., 201273 | |
| Follow-up | |||
| Secondary care | |||
| Hospital inpatient (per day) | 492 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by data submissions of regular day or night admissions |
| Postnatal ward stay (per day) | 104 | Schroeder et al., 201273 | |
| A&E department (per visit) | 169 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by data submissions of non-admitted to emergency medicine |
| Outpatient clinic | 111 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by activity of non-paediatric outpatient attendances |
| Day case | 1078 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by data submissions of non-paediatric day cases |
| Community care | |||
| GP appointment | 45 | Curtis and Burns, 201575 | Per patient contact lasting 11.7 minutes |
| Practice nurse appointment | 15 | Curtis and Burns, 201575 | Per surgery consultation lasting 15.5 minutes |
| Community nurse appointment | 67 | Curtis and Burns, 201575 | Per visit lasting 60 minutes |
| Physiotherapy appointment | 38 | Curtis and Burns, 201575 | Per visit lasting 60 minutes |
| Hospital community counselling | 51 | Curtis and Burns, 201575 | Per visit lasting 60 minutes |
| Other | 43 | Average of community care visits | |
| Infant | |||
| Birth related: mode of birth | |||
| Vaginal delivery | 1724 | NHS Reference Costs 2014 to 2015, 201574 | Normal delivery, cc = 0 (HRG data) |
| Breech delivery | 2311 | NHS Reference Costs 2014 to 2015, 201574 | Normal delivery, cc = 1 (HRG data) |
| Assisted delivery | 2046 | NHS Reference Costs 2014 to 2015, 201574 | Assisted delivery, cc = 0 (HRG data) |
| Caesarean section delivery | 3895 | NHS Reference Costs 2014 to 2015, 201574 | Emergency C-section |
| Resuscitation | 177 | Schroeder et al., 201273 | |
| Higher level of care admissions | |||
| Level of care, per day | |||
| Special care | 486 | NHS Reference Costs 2014 to 2015, 201574 | |
| High-dependency care | 847 | NHS Reference Costs 2014 to 2015, 201574 | |
| Intensive care | 1176 | NHS Reference Costs 2014 to 2015, 201574 | |
| Transfer to another hospital | 1101 | NHS Reference Costs 2014 to 2015, 201574 | Neonatal critical care, transportation |
| Neonatal death | 703 | Schroeder et al., 201273 | |
| Consultations | |||
| General | 167 | NHS Reference Costs 2014 to 2015, 201574 | |
| Orthopaedic | 279 | NHS Reference Costs 2014 to 2015, 201574 | |
| Surgery | |||
| Paediatric cardiology | 3895 | NHS Reference Costs 2014 to 2015, 201574 | |
| Plastic surgery | 1828 | NHS Reference Costs 2014 to 2015, 201574 | |
| Gastrointestinal surgery | 3706 | NHS Reference Costs 2014 to 2015, 201574 | |
| Paediatric neurosurgery | 1105 | NHS Reference Costs 2014 to 2015, 201574 | |
| Total body cooling | 6424 | Regier et al., 200976 | |
| Follow-up | |||
| Secondary care | |||
| Hospital inpatient (per day) | 757 | NHS Reference Costs 2014 to 2015, 201574 | Paediatric high-dependency ward |
| Postnatal ward stay (per day) | 104 | Schroeder et al., 201273 | |
| A&E department (per visit) | 169 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by data submissions of non-admitted to emergency medicine |
| Outpatient clinic | 180 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by activity of paediatric outpatient attendances |
| Day case | 661 | NHS Reference Costs 2014 to 2015, 201574 | Weighted average by data submissions of paediatric day cases |
| Community care | |||
| GP appointment | 45 | Curtis and Burns, 201575 | Per patient contact lasting 11.7 minutes |
| Practice nurse appointment | 15 | Curtis and Burns, 201575 | Per surgery consultation lasting 15.5 minutes |
| Health visitor | 76 | Curtis and Burns, 201575 | Per visit lasting 60 minutes |
| Community nurse appointment | 67 | Curtis and Burns, 201575 | Per visit lasting 60 minutes |
| Community paediatrician | 274 | NHS Reference Costs 2014 to 2015, 201574 | Non-admitted face-to-face attendance |
| Physiotherapy appointment | 80 | NHS Reference Costs 2014 to 2015, 201574 | Per one-to-one child session |
| Other | 93 | Average of community care visits | |
To estimate whether or not the use of decision support software in INFANT would incur additional NHS resources beyond the implementation of the Guardian system, the health economics team met with a representative from K2 Medical Systems to identify a base-case cost for the software. All sites (and hence all women) participating in the trial used the Guardian system, but we identified three additional aspects to cost for the decision support tool.
The first was the price to be paid by the NHS for the new software. A price for the decision support software had not been determined at the time the study was concluded. We understand that this price would be determined using a particular commercial strategy by K2 Medical Systems and would probably be selected in view of the trial results. Therefore, for the base-case scenario it was initially assumed that the software would be made available free of charge to the NHS. Scenarios to address this were identified for a multiway sensitivity analysis, to include any cost shifting in actual practice.
Second, an annual maintenance fee would be needed for software updates and other related information technology issues. A maintenance fee for the whole Guardian system had already been paid by the trial sites so it was assumed that further maintenance needed for the software would be included. Although the fee could potentially increase as a result of the installation of the decision support software, in the base case it was assumed that the fee would not change.
Finally, training of NHS staff members to develop familiarity and technical competence with the software was reviewed. All training received by staff during the preparation of the trial was delivered during working hours and was fitted into regular working patterns. Therefore, staff did not have to take additional time off work to learn how to use the decision support tool. It was assumed that a similar model would be used across the NHS and that no separate training costs were required. Consequently, in our base case, none of the three identified elements would incur additional NHS resource use, so no specific costs for the use of decision support software in INFANT were assigned. Scenarios to address this were identified for a multiway sensitivity analysis, to include any cost shifting in actual practice.
Unit costs
Sources and associated estimates of unit costs for the different categories of health-care resource use are presented in Table 36. Information was primarily extracted from secondary national sources including the Personal Social Services Research Unit75 and NHS Reference Costs. 74 The unit costs associated with induction, episiotomy, perineal tear, manual removal of the placenta, blood transfusion and neonatal death were not available in any of the secondary data sources consulted. Therefore, we replicated the ‘bottom-up’ costing survey conducted in a recent cost-effectiveness analysis of the Birthplace in England programme to estimate the unit costs for these items. 73 A bottom-up costing proforma was circulated to all trial midwives to complete. These were then followed up with face-to-face interviews. The proformas represented a detailed approach to capturing all possible NHS resources used in the care of the mother and baby during the period between admission and discharge. A working document was generated to capture the generalisability and variability of the procedures. For each scenario, the trial midwife was asked to describe in detail the ‘standard procedures’ that would be undertaken for labour and birth events and, when possible, the typical ratios of ‘staff-to-woman’ care. Scenarios were then varied between the least and the most complex, and included a description of the associated change in activity, staffing level and related resource use. Each of the interviews included approximately 1.5 hours of structured time. The data were then compiled into comparative resource use spreadsheets and were cross-referenced. The original unit costs (calculated for the Birthplace in England study) were then revised to be trial specific. The cost associated with total body cooling was not available in the Personal Social Services Research Unit or NHS reference costs data and was extracted from a study investigating the cost-effectiveness of total-body hypothermia plus intensive care versus intensive care alone to treat NNE. 76
All costs were expressed in 2014–15 GBP inflated to this base using the most up-to-date Hospital and Community Health Service inflation index. 77 Costs incurred between 12 and 24 months’ follow-up were discounted at an annual rate of 3.5%, as recommended by current guidance. 78
Cost analysis
Categories of resource use and associated costs are presented separately for mothers and their babies. Quantities of resource use in each category were multiplied by the corresponding unit cost to estimate the cost in a particular category. This was then averaged across each trial arm to obtain a mean cost per mother or baby. Costs from post-birth discharge to 12 months, and then from 12 to 24 months’ follow-up, were combined to estimate the overall costs at 2 years post birth. The total costs for the following categories are presented separately for the mother–baby dyad: trial entry to hospital discharge, community professional visits and secondary care. Given that we present the economic evaluation as a cost–consequences analysis, and that the numbers of participants used to estimate costs to hospital discharge and to 2 years’ follow-up were different, an overall total cost over the trial period was not calculated. Analyses were by ‘intention to treat’, indicating that the costs incurred were attributed to the original trial arm.
A conditional two-stage top-down costing approach was designed to cost prescribed medications for mothers or their babies. In the first stage, the proportion of mothers or babies with prescribed medications and the number of courses received were compared between trial arms. Were statistically significant differences to be observed between the groups, a microcosting method would be followed to cost the type of medication and the number of courses received on an individual basis. No statistically significant differences in the medications or the number of courses prescribed were observed between the groups and the cost of medication was excluded from the analysis.
Health outcome measures
The two primary outcomes of the trial were a composite of poor neonatal outcome and the PARCA-R composite score at age 2 years. These were used for the cost–consequences analysis. PARCA-R information was collected on a subset of infants selected from those without the composite primary outcome at birth. The parents of all babies born without the primary outcome in the first and second years of the trial were sent follow-up questionnaires. This was to maximise the effort to reach the sample size of approximately 7000 at 24 months’ follow-up by the end of the recruitment period for the larger trial. The subset of participants completing PARCA-R questionnaires and those completing the questionnaires collecting health-care resource use and maternal HRQoL data overlapped, with some women receiving both questionnaires.
The maternal HRQoL information was collected using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), at 12 and 24 months’ follow-up. 79 The EQ-5D-3L is a multiattribute generic instrument widely used in the conduct of cost–utility analysis of competing technologies and is recommended by reimbursement organisations such as NICE. 78 It has two components: a descriptive system and a ‘feeling thermometer’ using a visual analogue scale. The descriptive system covers five dimensions (mobility, self-care, usual activity, pain/discomfort and anxiety/depression), with each dimension including three levels (no problem, some problems and extreme problems). The EQ-5D-3L identifies 243 different health states that can be converted into a preference-based score using a value set obtained from a representative sample of the UK general population. 80
Statistical analysis
Health-care resource use between treatment arms was compared using RRs for binomial variables and mean differences for continuous covariates. Costs and EQ-5D-3L scores were compared using mean differences between treatment arms. Parametric and non-parametric methods accurately estimate the true SEs of means when large sample sizes for continuous variables are used even when the data are highly skewed. 81 Hence, mean resource use, cost and EQ-5D-3L score differences, and their associated uncertainty between the INFANT decision support and no decision support groups were estimated using parametric methods. In line with the statistical analysis of the primary outcomes, differences between treatment arms were adjusted using a random intercept binomial (for RRs) or linear (for mean differences) model adjusting for the stratification factors at randomisation (centre and twin birth) and clustering because of twins and multiple-birth episodes. A 95% significance level was used in all the comparisons made.
In all categories except for two health-care resource categories (manual removal of the placenta and infant resuscitation), the level of missing data was < 5%. Therefore, the cost analysis to the point of hospital discharge was conducted using a complete case analysis. Nevertheless, the 12- and 24-month follow-ups suffered from a larger number of missing data (specifically for resource use and EQ-5D-3L) and a multiple imputation framework with a chained equation was implemented. 82 This was developed using recent guidance for handling missing data in cost-effectiveness analysis. 83 We constructed an imputation model that included covariates with complete data on trial entry characteristics (maternal age at trial entry, twin pregnancy, gestational age at trial entry, whether the mother was nulliparous or multiparous, the baby’s birth weight and mode of delivery), EQ-5D-3L scores and all individual categories of resource use variables at 12 and 24 months’ follow-up. We used prediction mean matching, estimated 50 different imputations and the imputation model was implemented separately by trial allocation. Mean estimates and estimates of SEs were combined between imputed data sets using Rubin’s rule84 and were also adjusted using a random intercept binomial (for RRs) or linear (for mean differences) model adjusting for the stratification factors at randomisation (centre and twin birth) and clustering because of twins and multiple-birth episodes.
Results
Figure 41 presents the flow of participants and the sources of information used in each component of the cost–consequences analysis. For completeness, the numbers of participants for the two primary outcomes already reported in the CONSORT diagram in Figure 14 are also included in this flow chart. The totals of 46,042 women and 46,614 infants are those included in the analysis (i.e. excluding women with missing consent forms and those who withdrew consent to use their data). Estimating the cost analysis to the point of hospital discharge used all women and infants participating in the trial although there were more missing data given the number of categories used for the calculation of costs up to this point. A subset of 12,704 women with surviving babies without the composite primary outcome were sent a questionnaire that included the PARCA-R at the 24-month follow-up. A total of 7066 questionnaires (56%) were received, resulting in 6707 infants with complete PARCA-R information. The health-care resource use and maternal HRQoL follow-up questionnaire at 12- and 24-month follow-ups was circulated to a subset of 3875 women with surviving babies in the trial. A total of 2389 questionnaires (62%) were received and multiple imputation was used to handle missing data and to present the cost analysis over the 24-month follow-up period on 3798 women and 3875 babies.
FIGURE 41.
Flow of participants and data availability included in each component of the cost–consequences analysis.
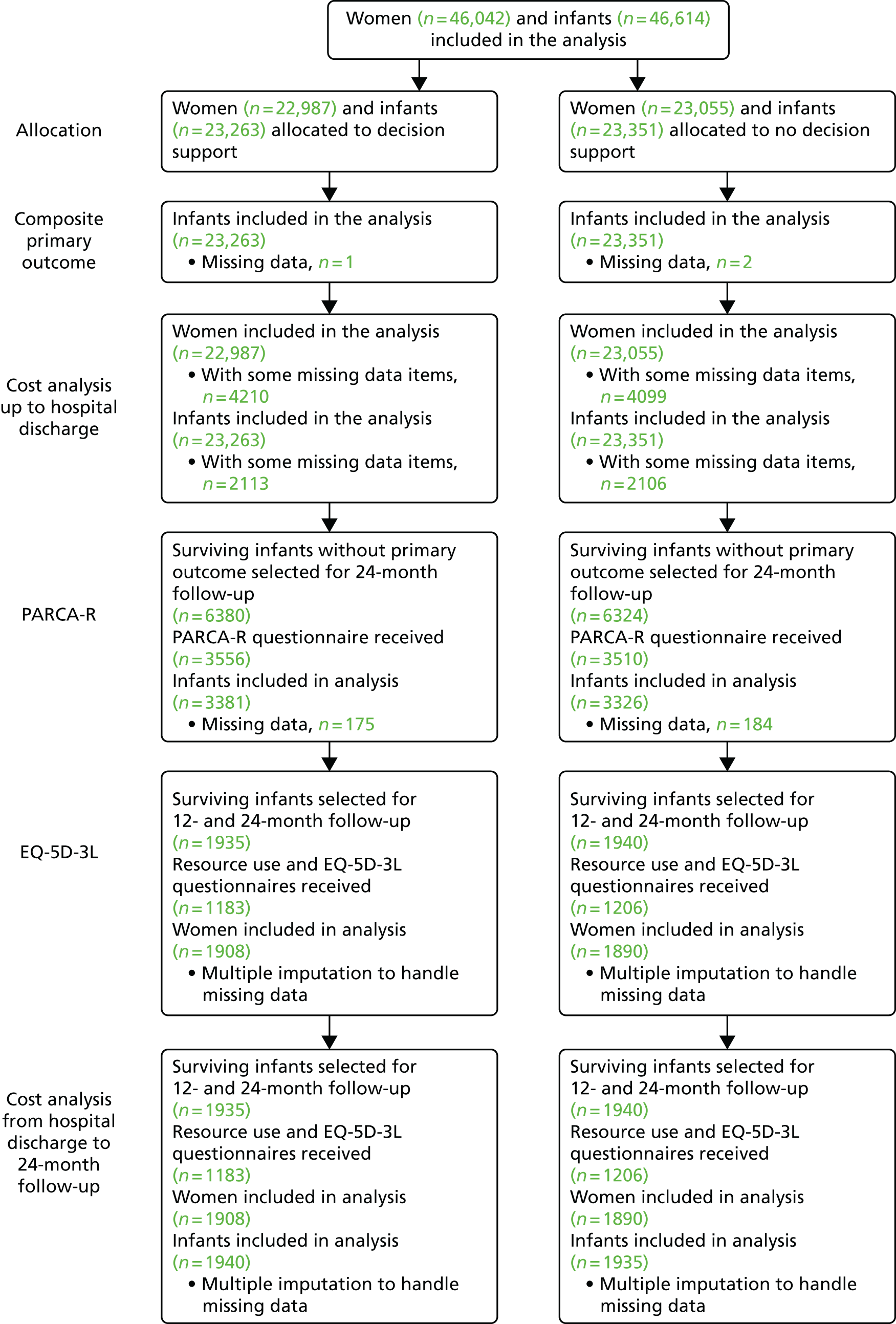
Tables 37 and 38 present the maternal and infant health-care resource use from trial entry to postnatal hospital discharge. No statistically significant differences were detected in any category of resource use assessed.
| Resource | Trial arm | RR/mean difference (95% CI)a | |
|---|---|---|---|
| Decision support (N = 22,987) | No decision support (N = 23,055) | ||
| Induction, n (%) | 13,516 (59.16) | 13,568 (59.24) | 1.00 (0.99 to 1.02) |
| Unknown | 140 | 153 | |
| Episiotomy, n (%) | 6396 (28.86) | 6498 (29.25) | 0.99 (0.96 to 1.02) |
| Unknown | 826 | 840 | |
| Perineal tear, n (%) | |||
| First- and second-degree tear | 8105 (36.26) | 8226 (37.13) | 0.98 (0.95 to 1.00) |
| Third- and fourth-degree tear | 652 (2.95) | 697 (3.15) | 0.94 (0.84 to 1.04) |
| Unknown | 881 | 902 | |
| Manual removal of the placenta, n (%) | 396 (1.72) | 421 (1.83) | 0.93 (0.81 to 1.06) |
| Unknown | 3703 | 3626 | |
| Medical and surgical management post birth, n (%) | |||
| Management of postpartum haemorrhage using the Bakri technique | 18 (< 0.1) | 23 (< 0.1) | 0.78 (0.42 to 1.45) |
| Management of postpartum haemorrhage using EUA | 44 (< 0.1) | 49 (< 0.1) | 0.90 (0.60 to 1.36) |
| Hysterectomy | 8 (< 0.1) | 5 (< 0.1) | 1.61 (0.53 to 4.91) |
| Perineal haematoma | 19 (< 0.1) | 10 (< 0.1) | 1.90 (0.88 to 4.09) |
| PPH with blood transfusion | 1 (< 0.1) | 2 (< 0.1) | 0.50 (0.045 to 5.50) |
| Hospital length of stay, n (%) | |||
| Length of stay (days), mean (SD) | 2 (8.87) | 2 (8.33) | –0.01 (–0.17 to 0.14) |
| Unknown | 42 | 32 | |
| Higher level of care admissions (days), mean (SD) | |||
| High-dependency care | 0.04 (0.32) | 0.04 (0.24) | 0.003 (–0.002 to 0.008) |
| Intensive care | 0.00 (0.29) | 0.00 (0.07) | 0.001 (–0.002 to 0.005) |
| Resource | Trial arm | RR/mean difference (95% CI)a | |
|---|---|---|---|
| Decision support (N = 23,263) | No decision support (N = 23,351) | ||
| Mode of birth, n (%) | |||
| Spontaneous vaginal birth | 11,823 (50.82) | 11,959 (51.21) | 0.99 (0.97 to 1.01) |
| Breech birth | 73 (0.31) | 72 (0.31) | 1.06 (0.78 to 1.43) |
| Ventouse vaginal birth | 2522 (10.84) | 2509 (10.74) | 1.01 (0.96 to 1.06) |
| Forceps vaginal birth | 3176 (13.65) | 3256 (13.94) | 0.98 (0.93 to 1.02) |
| Caesarean section | 5669 (24.37) | 5555 (23.79) | 1.02 (0.99 to 1.06) |
| Resuscitation, n (%) | |||
| Initial | 2139 (10.11) | 2116 (9.96) | 1.02 (0.96 to 1.07) |
| Intensive | 554 (2.62) | 524 (2.47) | 1.07 (0.94 to 1.20) |
| Unknown | 2113 | 2106 | |
| Higher level of care admissions (days), mean (SD) | |||
| Special care | 0.21 (1.61) | 0.23 (2.86) | –0.029 (–0.07 to 0.013) |
| High-dependency care | 0.04 (0.75) | 0.04 (0.91) | –0.006 (–0.021 to 0.009) |
| Intensive care | 0.05 (0.85) | 0.04 (0.64) | 0.007 (–0.007 to 0.021) |
| Neonatal surgery, n (%) | 23 (0.10) | 26 (0.11) | |
| Paediatric cardiology | 7 (0.03) | 5 (0.02) | 1.41 (0.45 to 4.43) |
| Plastic surgery | 5 (0.02) | 10 (0.04) | 0.50 (0.17 to 1.47) |
| Gastrointestinal surgery | 10 (0.04) | 11 (0.05) | 0.85 (0.34 to 2.11) |
| Paediatric neurosurgery | 2 (0.01) | 0 | – |
| Total body cooling | 19 (0.1) | 21 (0.1) | 0.90 (0.48 to 1.69) |
| Intrapartum stillbirth, n (%) | 1 (–) | 2 (–) | 0.46 (0.04 to 6.05) |
| Neonatal death, n (%) | 6 (–) | 4 (–) | 1.51 (0.42 to 5.33) |
Tables 39 and 40 show the results of the maternal and infant cost analysis from trial entry to hospital discharge. The total mean maternal costs from trial entry to hospital discharge were estimated to be £431 (SE £7) and £433 (SE £6) in the decision support and no decision support groups, respectively; the non-significant mean-adjusted cost difference was –£2 (95% CI –£28 to £24). The total mean infant costs from trial entry to hospital discharge were estimated to be £2539 (SE £12) and £2541 (SE £15) in the decision support and no decision support groups, respectively; the non-significant mean-adjusted cost difference was £1 (95% CI –£37 to £39).
| Resource | Trial arm | Mean difference (95% CI)b | |||
|---|---|---|---|---|---|
| Decision support (N = 22,987) | No decision support (N = 23,055) | ||||
| n a | Mean (SE) | n a | Mean (SE) | ||
| Induction | 22,847 | 102 (0.56) | 22,902 | 102 (0.56) | |
| Episiotomy | 22,161 | 8 (0.08) | 22,215 | 8 (0.08) | |
| Perineal tear | 22,106 | 27 (0.71) | 22,153 | 28 (0.73) | |
| Manual removal of the placenta | 19,284 | 12 (0.65) | 19,429 | 14 (0.66) | |
| Medical and surgical management | 22,987 | 4 (0.46) | 23,055 | 4 (0.46) | |
| Blood transfusion | 22,987 | 2 (1.3) | 23,055 | 3 (1.3) | |
| Maternal transfer | 22,987 | 0.22 (0.06) | 23,055 | 0.13 (0.05) | |
| Hospital length of stay | 22,945 | 226 (6.07) | 23,023 | 227 (5.69) | |
| Higher level of care admissions | |||||
| High-dependency care | 22,987 | 49 (2.68) | 23,055 | 45 (2.01) | |
| Intensive care | 22,987 | 4 (2.72) | 23,055 | 2 (0.62) | |
| Total maternal cost from trial entry to hospital discharge | 18,777 | 431.3 (6.8) | 18,956 | 432.6 (6.4) | –2 (–28 to 24) |
| Resource | Trial arm | Mean difference (95% CI)b | |||
|---|---|---|---|---|---|
| Decision support (N = 23,263) | No decision support (N = 23,351) | ||||
| n a | Mean (SE) | n a | Mean (SE) | ||
| Mode of birth | 23,263 | 2334 (5.88) | 23,351 | 2322 (5.82) | |
| Resuscitation | 21,150 | 8 (0.19) | 21,245 | 8 (0.19) | |
| Neonatal surgery | |||||
| Paediatric cardiology | 23,263 | 1 (0.44) | 23,351 | 0.83 (0.37) | |
| Plastic surgery | 23,263 | 0.39 (0.18) | 23,351 | 0.78 (0.25) | |
| Gastrointestinal surgery | 23,263 | 1.6 (0.50) | 23,351 | 2 (0.50) | |
| Paediatric neurosurgery | 23,263 | 0.09 (0.07) | 23,351 | 0 | |
| Total body cooling | 23,263 | 5 (1.20) | 23,351 | 6 (1.26) | |
| Neonatal death | 23,263 | 0.18 (0.07) | 23,351 | 0.12 (0.06) | |
| Higher level of care admissions | |||||
| Special care | 23,263 | 99 (5.13) | 23,351 | 114 (9.11) | |
| High-dependency care | 23,263 | 36 (4.21) | 23,351 | 41 (5.05) | |
| Intensive care | 23,263 | 54 (6.61) | 23,351 | 46 (5.00) | |
| Total infant cost from trial entry to hospital discharge | 21,150 | 2539.3 (12.4) | 21,245 | 2540.9 (14.9) | 1 (–37 to 39) |
Table 41 presents the results of the maternal health-care resource use and associated costs over 24 months since hospital discharge. A significant adjusted mean cost difference of –£166 (95% CI –£319 to –£12) was observed in secondary care admissions for mothers, favouring the decision support group. Such difference was driven by slightly more admissions to all units in the no decision support group since hospital discharge. However, when adding up all categories of costs (community plus secondary care), the total mean (SE) maternal follow-up costs were estimated to be £753 (SE £35) and £904 (SE £77) in the decision support and no decision support groups, respectively; the non-significant mean-adjusted cost difference was –£149 (95% CI –£314 to £16). Table 42 reports similar information for the infants. None of the categories of costs or overall costs resulted in any statistically significant difference between the groups of infants.
| Resource | Trial arm | Mean cost difference (95% CI)a | |||
|---|---|---|---|---|---|
| Decision support (n = 1908) | No decision support (n = 1890) | ||||
| Mean resource use (SE) | Mean cost (SE) | Mean resource use (SE) | Mean cost (SE) | ||
| Community professional visits | |||||
| General practice | 5.30 (0.16) | 234 (7) | 5.01 (0.12) | 222 (5) | 12 (–6 to 30) |
| Practice nurse | 1.48 (0.08) | 21 (1) | 1.23 (0.06) | 18 (1) | 4 (1 to 6)* |
| Community nurse | 0.16 (0.04) | 11 (2) | 0.25 (0.06) | 17 (4) | –5 (–14 to 3) |
| Physiotherapy | 0.61 (0.07) | 23 (2) | 0.67 (0.08) | 25 (3) | –2 (–9 to 6) |
| Hospital community counselling | 0.12 (0.03) | 6 (2) | 0.12 (0.03) | 6 (1) | –0.09 (–5 to 4) |
| Other community professionals | 1.09 (0.10) | 46 (4) | 0.90 (0.10) | 38 (4) | 8 (–3 to 20) |
| Total community professional visits | 341 (11) | 325 (10) | 171 (–14 to 48) | ||
| Secondary care | |||||
| Outpatient visits | 0.98 (0.06) | 107 (7) | 0.88 (0.07) | 96 (8) | 11 (–9 to 31) |
| A&E visits | 0.35 (0.03) | 58 (5) | 0.35 (0.03) | 57 (5) | 1 (–13 to 14) |
| Intensive care unit (nights) | 0.00 (0.00) | 0 (0) | 0.04 (0.02) | 59 (34) | –60 (–128 to 9) |
| High-dependency ward/unit (nights) | 0.05 (0.02) | 58 (19) | 0.11 (0.04) | 120 (41) | –61 (–147 to 25) |
| General ward (nights) | 0.42 (0.05) | 43 (5) | 0.47 (0.05) | 48 (5) | –4 (–20 to 11) |
| Other admissions | 0.18 (0.03) | 87 (14) | 0.29 (0.06) | 140 (28) | –52 (–114 to 10) |
| Day case | 0.06 (0.01) | 59 (8) | 0.06 (0.01) | 59 (8) | 0 (–23 to 23) |
| Total secondary care | 412 (30) | 580 (73) | –166 (–319 to –12)* | ||
| Total maternal follow-up | 753 (35) | 904 (77) | –149 (–314 to 16) | ||
| Resource use | Trial arm | Mean cost difference (95% CI)a | |||
|---|---|---|---|---|---|
| Decision support (n = 1940) | No decision support (n = 1935) | ||||
| Mean resource use (SE) | Mean cost (SE) | Mean resource use (SE) | Mean cost (SE) | ||
| Community professional visits | |||||
| General practice | 6.72 (0.16) | 297 (7) | 6.86 (0.16) | 303 (7) | –6 (–25 to 14) |
| Practice nurse | 0.88 (0.06) | 12 (0.81) | 0.81 (0.04) | 12 (0.63) | 0.99 (–1 to 3) |
| Health visitor | 1.44 (0.11) | 107 (8) | 1.48 (0.10) | 110 (7) | –2 (–23 to 19) |
| Community nurse | 0.20 (0.03) | 13 (2) | 0.18 (0.04) | 12 (2) | 2 (–4 to 8) |
| Community paediatrician | 0.31 (0.03) | 84 (9) | 0.31 (0.03) | 84 (9) | 3 (–23 to 28) |
| Physiotherapy | 0.14 (0.03) | 11 (2) | 0.32 (0.07) | 25 (5) | –14 (–26 to –2) |
| Other community professionals | 0.54 (0.06) | 49 (5) | 0.63 (0.08) | 58 (7) | –8 (–24 to 9) |
| Total community professional visits | 575 (20) | 603 (21) | –24 (–82 to 34) | ||
| Secondary care | |||||
| Outpatient visits | 1.36 (0.08) | 242 (15) | 1.25 (0.07) | 223 (12) | 24 (–14 to 62) |
| A&E visits | 1.14 (0.05) | 188 (9) | 1.01 (0.04) | 167 (7) | 21 (–2 to 44) |
| Intensive care unit (nights) | 0.17 (0.05) | 201 (58) | 0.18 (0.06) | 205 (69) | –3 (–187 to 181) |
| High-dependency ward/unit (nights) | 0.20 (0.05) | 131 (35) | 0.12 (0.03) | 78 (21) | 53 (–29 to 134) |
| General ward (nights) | 0.61 (0.06) | 63 (6) | 0.56 (0.05) | 58 (6) | 4 (–12 to 21) |
| Other admissions | 0.12 (0.03) | 88 (21) | 0.07 (0.02) | 51 (14) | 37 (–14 to 87) |
| Day case | 0.05 (0.01) | 32 (5) | 0.06 (0.01) | 40 (6) | –8 (–23 to 7) |
| Total secondary care | 945 (90) | 822 (80) | 128 (–120 to 375) | ||
| Total infant follow-up | 1520 (100) | 1425 (92) | 104 (–174 to 382) | ||
Maternal HRQoL EQ-5D-3L scores at 12 and 24 months’ follow-up are presented in Table 43. Mothers reported scores similar to the population norm for English females aged 25–34 years (currently a mean of 0.925 using the EuroQoL EQ-5D-3L index85) in both groups and, therefore, no statistically significant mean differences in scores were observed at any follow-up point.
| EQ-5D-3L follow-up point | Trial arm | Mean difference (95% CI)a | |||
|---|---|---|---|---|---|
| Decision support (n = 1908) | No decision support (n = 1890) | ||||
| Mean score | SE | Mean score | SE | ||
| 12 months | 0.908 | 0.005 | 0.913 | 0.005 | –0.005 (–0.020 to 0.012) |
| 24 months | 0.909 | 0.006 | 0.919 | 0.005 | –0.008 (–0.023 to 0.007) |
A summary of the different components included in the cost–consequences analysis is reported in Table 44. For each of the maternal and infant total mean cost components from trial entry to hospital discharge and over 24 months’ follow-up since hospital discharge, no statistically significant differences were observed between the groups. Similarly, no statistically significant mean differences were observed between the groups in any of the consequences evaluated; the composite primary outcome, PARCA-R and maternal quality-of-life scores.
| Component | Trial arm, mean estimate | Mean difference (unless otherwise stated) (95% CI)a | |
|---|---|---|---|
| Decision support | No decision support | ||
| Costs (2014–15 GBP) | |||
| Total maternal from trial entry to hospital discharge | 431 | 432 | –2 (–28 to 24) |
| Total infant from trial entry to hospital discharge | 2539 | 2540 | 1 (–37 to 39) |
| Total maternal follow-up | 753 | 904 | –149 (–314 to 16) |
| Total infant follow-up | 1520 | 1425 | 104 (–174 to 382) |
| Consequences: infant | |||
| Composite primary outcome, n (%) | 172 (0.7) | 171 (0.7) | RR: 1.01 (0.82 to 1.25) |
| PARCA-R score | 98.0 | 97.2 | 0.63 (–0.98 to 2.25) |
| Consequences: maternal | |||
| EQ-5D-3L 12 months | 0.908 | 0.913 | –0.005 (–0.020 to 0.012) |
| EQ-5D-3L 24 months | 0.909 | 0.919 | –0.008 (–0.023 to 0.007) |
Discussion
Presentation of the results
The findings of the clinical study in Chapter 6 showed no differences in the composite primary outcomes at birth, or in PARCA-R composite scores at 2 years. Given these findings, we chose to present the results of our economic evaluation (using the clinical outcomes reported previously) with a cost–consequences analysis. Our statistical analysis plan refers to the estimation of a cost-effectiveness analysis, but with no differences in costs or effects between the groups, there was no evidence of differences between the groups to generate this. A cost–utility analysis could not be conducted, owing to the lack of HRQoL data at baseline and postnatally, to derive quality-adjusted life-years. Given the overall trial results, we considered that presenting the data in a disaggregated manner to show the costs and benefits (consequences) for different time periods provides the most useful information to complement the clinical findings.
The study was conducted at multiple labour ward sites across England, Scotland and the Republic of Ireland, with differing configurations of care and economies of scale strengthening the generalisability of our results. A useful ancillary benefit of this study is that it provides detailed costs of intrapartum care, which contributes to the broader field of evidence available for health economic evaluations in the perinatal research arena.
Findings
Both the clinical and cost–consequences analyses did not identify statistically significant differences between the trial arms. There were also no differences in the direction of costs, namely a consistent pattern of cost increase in one trial arm compared with the other. Within the cost categories there were small differences, such as secondary care for mothers post birth, but, overall, the differences between groups did not reflect a direction. We had anticipated that a decision support tool to prevent poor perinatal outcomes might have lifelong effects, that these could be modelled using decision-analytic or Markov models, and that ‘obstetric litigation costs averted’ would have been a key cost driver. Nevertheless, there were no differences in cost or effectiveness to model between the arms of the trial to 24 months post birth and for the longer term. Research was undertaken during the study to identify a composite ‘quality of care’ variable that would identify substandard (and potentially negligent) clinical care during birth. The consistent and methodical documentation of birth information captured in the Guardian system and decision support software (INFANT) might assist an interrogation of the ‘quality-of-care’ for clinical staff and may be useful in future studies. The software did not assist research for a longer-term cost-effectiveness study here but it has the potential to shorten an obstetric litigation process and even potentially to alter the outcome of some cases. This hypothesis is currently untested and may be relevant for future research.
The trial was not designed to assess the cost and benefit of the Guardian system and the value it provides for labour ward staff. No research was undertaken to explicitly cost Guardian separately from the decision support tool (INFANT). However, we did probe whether or not the combined information display (at the patient’s bedside, centrally or remotely) and the interaction with the decision support tool caused a cost shifting or change to practice for staff for efficiency purposes. We conducted informal interviews at 11 out of 23 sites, which were all sites where research midwives agreed to discuss the health economics component including whether or not the sites had:
-
installed Guardian for the study and had changed to be wholly paper-free, using it for all births since the start of the trial
-
installed Guardian for the study, but did not use it consistently in the ward
-
used Guardian prior to and after the trial for all births.
In summary, feedback from the interviews was consistent across the sites. Interactions with Guardian and the decision support (INFANT) system produced negligible changes and affected clinical staff differently. Midwives generally reported no change to practice but stated that the alarms during the second stage of labour sometimes distressed patients and might require additional checks or review, or even the option to switch off the software. There were a few reports of increased visits to the ward by clinical staff, querying yellow and red alerts. Most midwifery co-ordinators reported that the convenience and accessibility of the display platforms increased time efficiency for staff co-ordination and allocation. Clinicians reported that, although the duration of their staff shift did not change, they had greater flexibility around location (with an increased freedom to be desk based, enabling work time to be more productive). Central hospital information technology staff identified an increase in their staff time dedicated to the labour ward, providing technical support to the midwifery and medical staff. It was difficult to separate out the impact of Guardian and decision support (INFANT) for this particular activity, as both collect and display information. However, there did not seem to be a striking change to the workload models for clinical staff, for cost shifting or efficiency. Having documented the findings of this research question, we did not attribute a cost impact.
Limitations
As discussed, the economic evaluation presented here was originally intended to be a cost-effectiveness analysis. The incremental cost-effectiveness ratio (ICER) was to be expressed as an incremental cost per poor perinatal outcome prevented (at hospital discharge following birth). Two longer-term cost-effectiveness analyses were planned: (1) to estimate the cost-effectiveness of the decision support software (INFANT) when surviving children reached 2 years of age and (2) to incorporate lifetime cost and health consequences of the decision support software (INFANT) for the mother and child within an economic modelling framework. Given that there were no statistically significant differences between costs and outcomes at post-birth discharge or at 24 months, we would not add value to this research with a longer-term framework. A cost-effectiveness analysis in the short term to include the cost of the software would only make the INFANT software more expensive and, thus, less cost-effective. Our original plan to perform multiway sensitivity analyses, to estimate and value the initial and annual maintenance fees for the decision support tool (INFANT), would make the decision support arm more expensive. As stated earlier, several strategies had been developed to estimate the additional software costs (apportioned per birth) via estimates of installation and maintenance fees or via a commercial strategy by K2 Medical Systems, but given the findings in the trial, we did not pursue a final cost estimate for the INFANT software.
The study is limited by different components of missing data. The trial suffered a large loss to follow-up; 46% of women contacted at 12-month follow-up and 38% of women contacted at 24-month follow-up returned questionnaires. Although numerous methods and substantial effort was invested to increase the response rate, it was an unsurprising outcome. Labour is a life-changing but acute event, and the majority of the women in this study had uncomplicated pregnancies and a fairly straightforward labour (albeit requiring EFM during the event). Most also had healthy babies so we surmise that they would not have felt an innate need to personally ‘invest’ in the study, in a manner equivalent to suffering a severe long-term illness. We also identified women pregnant with their second baby while completing questionnaires about their first, reflecting a progression in their life stage from the original birth event. Our only contact with the women was via postal address and, with limited resources, once this connection was lost as a result of residential change, we were not able to trace them further. Finally, conversations with research midwives revealed that, although recruitment into the trial was explicitly and clearly communicated to women and their partners, and consent was required for participation, many would not have identified the use of the decision support software (INFANT) separately from their overall birth experience. It was primarily used by midwives and would have been associated with other clinical activity, such as documenting patient information into the system.
As described throughout this chapter, we resolved the issue of missing data in different ways. We ensured that missing data are noted in detail throughout, in the flow chart (see Figure 41), in each table of resource use and in the final cost analyses (see Tables 37 and 38). For the 12- and 24-month follow-ups, we account for missing data using multiple imputation techniques.
Value of this research
This cost–consequences analysis fills an evidence gap regarding the use of computerised interpretation of the CTG in women receiving EFM during labour. It identifies and presents all the key resource use associated with the intervention. It also adds evidence to a growing body of unit cost information collected through primary sources for perinatal care.
Economic evaluation adds value to research by providing decision-makers with information that systematically considers all the evidence, balances trade-offs to ensure that health care provides value for money and explores uncertainty around key cost drivers and health outcomes. We have captured and combined the key clinical and cost impacts of the decision support software (INFANT). Based on the results presented in this chapter and previous chapters, there is no evidence to support the use of computerised interpretation of the CTG in women in the UK and the Republic of Ireland.
Chapter 8 Discussion and conclusion
In this trial of 46,000 women, there is no evidence of a difference in the risk of a poor neonatal outcome using CTG interpretation software to support decision-making. Another randomised trial (of 7730 women), which evaluated the use of decision support in women who were monitored during labour using fetal ECG monitoring, also found no evidence that CTG interpretation decision support improved the primary outcome of cord blood metabolic acidosis. 86
Using a composite primary outcome is not always helpful if different components of the outcome respond differently to the intervention. If one component of the composite dominates the others, then effectively the trial results reflect any differences detected within this dominant component. 87 We initially hypothesised that the indicence of components of the composite outcome (extended perinatal mortality, NNE and prolonged admission to a neonatal unit following birth in a poor condition) would be similar, with each component likely to contribute approximately one-third to the composite.
Estimates of the incidence of the components of the primary outcome for the eligible study population were difficult to find before the trial commenced. 1 The observed perinatal mortality in the study (stillbirth and neonatal death, excluding lethal congenital anomalies) was lower than the prior estimate (13/46,614 births or 0.3 per 1000 births vs. 1.05 per 1000 births), and the incidence of NNE requiring cooling was also lower (39/46,614 births or 0.8 births per 1000 births vs. 1.3 births per 1000 births see Chapter 2). However, prolonged neonatal unit admission with evidence of compromise at birth, for which we had no good data at the time the trial was planned, occurred more frequently than expected (291/46,614 births or 6 per 1000 births), contributing substantially to the higher than anticipated overall primary event rate of 7 per 1000 births, compared with our estimated 3 per 1000 births. This allowed us to have the power to detect more modest differences in the composite outcome than we had originally planned.
The very low numbers of perinatal deaths and longer-term adverse outcomes, such as cerebral palsy, mean that this trial is unable to rule out even large differences in these individual outcomes. However, given this very low event rate, the numbers needed to treat to prevent one perinatal death would be very substantial even if the intervention was effective.
The strength of this study lies in its contemporaneous data collection and its size, the latter being designed to detect differences in substantive perinatal outcomes, as well as in more frequent outcomes such as cord metabolic acidosis and operative delivery. Potential weaknesses include the potential for staff to learn from exposure to the decision support arm of the trial, resulting in improved outcomes in the control arm. This potential weakness was identified when the trial was being planned. We acknowledged that passive learning from the decision support system was possible and the only way to completely rule out this effect would be to conduct a cluster randomised trial. Such a design was unfeasible given the limited number of centres with the Guardian system in the UK and Republic of Ireland and the very low incidence of the primary outcome measure. Moreover, part of our prior hypothesis was that, although some poor CTG interpretation is as a result of a lack of training, some clinicians have a poor intrinsic pattern recognition ability that is not susceptible to improvement by training. Such an intrinsic disability would, by definition, not be affected by training, and the performance of such clinicians would be particularly improved by assistance from automatic interpretation. Therefore, we collected a range of process outcomes to measure the impact on clinician behaviour during the trial. There was some evidence that clinical behaviour was changed in the decision support arm of the trial: the incidence of fetal blood sampling was higher in the decision support group (10.3% vs. 9.5% in the no decision support group) and the incidence of repeated yellow alerts was lower (0.35 per hour vs. 0.40 per hour in the no decision support group). It may be that different action was taken in response to the alerts in the decision support arm of the trial, for example the clinicians might have reduced the dose of an oxytocin infusion in women having their labour augmented if this was leading to very frequent contractions, or changed maternal position if the CTG abnormality resulted from vena caval compression. Such actions could have prevented further yellow alerts, leading to a decrease in this group, but we do not have any direct evidence showing that this was the case. Even if it was, it did not result in any significant change in clinical outcomes. Another potential weakness is that the UK and Republic of Ireland setting, where EFM is not routine,21 makes generalisability of the findings to settings where EFM is routine more uncertain.
Detecting abnormalities in the fetal heart rate can only improve outcome if caregivers respond appropriately to the alerts. An expert panel reviewed all severe adverse outcomes in the trial and found no evidence that there were differences in suboptimal care between the two groups. Therefore, we conclude that our hypothesis, that substandard care is largely related to failure to identify pathological fetal heart rate patterns, is not supported. It appears that most adverse outcomes associated with preventable substandard care involved a failure to take appropriate management decisions once the CTG abnormality had been recognised. This aspect will be reported on in detail in a follow-up paper. Our hypothesis that unnecessary intervention would be reduced was also not supported.
The decision support software used in this trial clearly identifies most fetal heart rate abnormalities. 35–37 However, the alerts do not take into account other information about the labour, such as the duration of labour, the rate of labour progress, presence of meconium, whether or not the woman has an elevated temperature and whether or not there is suspected fetal growth restriction, all of which may modify the way a clinician interprets the fetal heart rate and acts on this information. Further development of decision support software to include these variables may improve the quality of the feedback the system provides to clinicians and therefore may make a positive difference to outcomes. Given the importance of the consequences of intrapartum hypoxia for parents, clinicians and health services, there continues to be an urgent need to improve knowledge and training about the appropriate response to CTG abnormalities, including timely intervention.
Currently, there is no evidence to support the use of computerised interpretation of the CTG in women who have EFM in labour to improve clinical outcomes for mothers or babies.
Acknowledgements
Contributions of others
We gratefully acknowledge K2 Medical Systems and its Director, Dr Robert Keith, for providing support for running of the trial, trial design and oversight, advice on technical aspects of the INFANT software, analysis and interpretation of the results.
Recruiting centres
Birmingham Women’s Hospital
-
Nina Johns, Principal Investigator
-
Tracey Johnston, Principal Investigator
-
Gemma Barnfield, Recruiting Midwife
-
Karen Davies, Recruiting Midwife.
Chelsea and Westminster Hospital, London
-
Mark Johnson, Principal Investigator
-
Holly Patterson, Recruiting Midwife.
Derriford Hospital, Plymouth
-
Imogen Montague, Principal Investigator
-
Sally Watmore, Recruiting Midwife
-
Alison Stolton, Recruiting Midwife.
Homerton University Hospital, London
-
Maryam Parisaei, Principal Investigator
-
Natasha McGhee, Recruiting Midwife
-
Silvia Segovia, Recruiting Midwife.
Lancashire Women and Newborn Centre
-
Elizabeth Martindale, Principal Investigator
-
Hilary Jackson, Recruiting Midwife
-
Josephine Holleran, Recruiting Midwife.
Liverpool Women’s Hospital
-
Devender Roberts, Principal Investigator
-
Siobhan Holt, Recruiting Midwife.
Northwick Park Hospital, London
-
Bosko Dragovic, Principal Investigator
-
Miriam Willmott-Powell, Recruiting Midwife
-
Laura Hutchinson, Recruiting Midwife
-
Benedek Toth, Recruiting Midwife
-
Gemma Chandler, Recruiting Midwife
-
Suzanne Ridley, Recruiting Midwife.
Nottingham City Hospital
-
George Bugg, Principal Investigator
-
Anna Molnar, Recruiting Midwife
-
Denise Lochrie, Recruiting Midwife.
Princess Anne Hospital, Southampton
-
Jillian Connor, Principal Investigator
-
David Howe, Principal Investigator
-
Katie Head, Recruiting Midwife
-
Sue Wellstead, Recruiting Midwife.
Princess Royal Hospital, Glasgow
-
Alan Mathers, Principal Investigator
-
Laura Walker, Recruiting Midwife
-
Isobel Crawford, Recruiting Midwife.
Queen Alexandra Hospital, Portsmouth
-
David Davies, Principal Investigator
-
Zoe Garner, Recruiting Midwife
-
Lucy Galloway, Recruiting Midwife.
Queen’s Medical Centre
-
George Bugg, Principal Investigator
-
Yvette Davies, Recruiting Midwife
-
Carys Smith, Recruiting Midwife
-
Gill Perkins, Recruiting Midwife.
Rotunda Hospital, Dublin
-
Mike Geary, Principal Investigator
-
Fiona Walsh, Recruiting Midwife
-
Ursula Nagle, Recruiting Midwife.
Royal Blackburn Hospital
-
Elizabeth Martindale, Principal Investigator
-
Hilary Jackson, Recruiting Midwife
-
Louise O’Malley, Recruiting Midwife.
Royal Bolton Hospital
-
Narmada Katakam, Principal Investigator
-
Heather White, Recruiting Midwife
-
Emma Tanton, Recruiting Midwife.
Royal Derby Hospital
-
Rosie Hamilton, Principal Investigator
-
Hilary Glanowski, Recruiting Midwife
-
Ethel Forde, Recruiting Midwife.
Southern General Hospital, Glasgow
-
Alan Mathers, Principal Investigator
-
Christina MacDonald, Recruiting Midwife
-
Lorna McKay, Recruiting Midwife.
St. Mary’s Hospital, Manchester
-
Leroy Edoziern, Principal Investigator
-
Paula Doran, Recruiting Midwife
-
Julie Dillon, Recruiting Midwife
-
Cara Taylor, Recruiting Midwife
-
Paula Evans, Recruiting Midwife.
Stoke Mandeville Hospital
-
Veronica Miller, Principal Investigator
-
Christopher Wayne, Principal Investigator
-
Julie Tebbutt, Recruiting Midwife
-
Ellie Hendy, Recruiting Midwife.
University College Hospital, London
-
Patrick O’Brien, Principal Investigator
-
Seni Subair, Principal Investigator
-
Helen Dent, Recruiting Midwife
-
Camille Mallet, Recruiting Midwife.
University Hospital Coventry
-
Siobhan Quenby, Principal Investigator
-
Jane Hillen, Recruiting Midwife.
University Hospital of North Staffordshire
-
Peter Young, Principal Investigator
-
Tracey Harrison, Recruiting Midwife
-
Louise Wood, Recruiting Midwife.
Warrington Hospital
-
Rita Arya, Principal Investigator
-
Lindsay Roughley, Recruiting Midwife.
Warwick Hospital
-
Olanrewaju Sorinola, Principal Investigator
-
Carole Rogers, Recruiting Midwife
-
Janet Phipps, Recruiting Midwife.
Trial Steering Committee
-
Bob Arndtz, Audit Manager, NHS Litigation Authority, London, UK.
-
Denis Azzopardi, Professor in Paediatrics and Neonatal Medicine, Hammersmith Hospital, London, UK.
-
Zoe Chivers, Head of Services, Bliss, London, UK. (from May 2014).
-
Andy Cole, Chief Executive, Bliss, London, UK. (until May 2014).
-
Professor Max Parmar (Chairperson until November 2015), Professor of Medical Statistics and Epidemiology, Director of MRC Clinical Trials Unit and the Institute of Clinical Trials and Methodology, UCL, London, UK.
-
Tracy Roberts, Professor in Health Economics, University of Birmingham, Birmingham, UK.
-
Dr Julia Sanders, Consultant Midwife, University Hospital of Wales, Cardiff, UK.
-
Professor Derek Tuffnell (Chairperson from November 2015), Professor of Obstetrics and Gynaecology, Bradford Royal Infirmary, Bradford, UK.
Data Monitoring Committee
-
Professor Deborah Ashby (Chairperson), Professor of Medical Statistics and Clinical Trials, Imperial College London, UK.
-
Professor Jane Norman, Professor of Maternal and Fetal Health, University of Edinburgh, Edinburgh, UK (previous member of the National Institute for Health Research NIHR Health Technology Assessment HTA and Efficacy and Mechanism Evaluation Editorial Board).
-
Professor Andy Shennan, Professor of Obstetrics, St Thomas’ Hospital, London, UK.
-
Professor Helen Spiby, Professor of Midwifery, Faculty of Medicine and Health Sciences, University of Nottingham, Nottingham, UK.
-
Dr Win Tin, Consultant Neonatologist, The James Cook University Hospital, Middlesbrough, UK.
Clinical Trials Research Unit staff
National Perinatal Epidemiology Unit, University of Oxford (from January 2009 to May 2012)
-
Vicki Barber, Trial Director
-
Emma Haines, Data and Administrative Co-ordinator
-
Andy Kirk, Webmaster and Design Co-ordinator
-
Louise Linsell, Senior Medical Statistician
-
Katie Lean, Recruiting Midwife Co-ordinator
-
Linda Mottram, Trial Co-ordinator
-
Liz Schroeder, Perinatal Economist
-
Clare Shakeshaft, Study Co-ordinator.
Comprehensive Clinical Trials Unit, University College London (from 1 May 2012 to 1 June 2016)
-
Julie Bakobaki, Clinical Project Manager
-
Philip Bakobaki, IT Developer
-
James Blackstone, Data Manager
-
Mary Yip Braidley, Trial Manager
-
Jackie Coleman, Trial Co-ordinator
-
Jade Dyer, Data Manager
-
Abigail Howarth, Data Co-ordinator
-
Dawn Letchford, Data Entry Assistant
-
Victoria McCudden, Clinical Project Manager
-
Guy Schroeter, Clinical Project Manager
-
Heather Short, Trial Manager
-
Irene Simmonds, Trial Manager.
Contributions of authors
Peter Brocklehurst (Professor of Women’s Health, Director of the Birmingham Clinical Trials Unit) was responsible for the trial design, oversight, analysis and interpretation of the findings. He was also responsible for the first draft and co-ordination of the production of this report and is its guarantor.
David Field (Professor of neonatology, Department of Health Sciences, University of Leicester) was responsible for the trial design and oversight; advice on measures of neonatal and longer-term child outcome, congenital anomalies, primary outcome review panel for interim analyses, and final analysis; and interpretation of the results.
Keith Greene (Retired in 2011 from Plymouth Hospitals NHS Trust and University of Plymouth) was responsible for the trial design and oversight, advice on labour and maternal outcomes, primary outcome review panel for interim analyses and analysis and interpretation of the results.
Edmund Juszczak (Director, National Perinatal Epidemiology Unit, Clinical Trials Unit, Nuffield Department of Population Health, University of Oxford) was responsible for the trial design and oversight, statistical advice and interpretation of the results.
Sara Kenyon (Reader in Evidence Based Maternity Care, Institute of Applied Health Research, University of Birmingham) was responsible for the trial design and oversight, advice on recruitment and follow-up questionnaire processes, maternal measures, analysis and interpretation of the results.
Louise Linsell (Senior Medical Statistician, National Perinatal Epidemiology Unit, Clinical Trials Unit, Nuffield Department of Population Health, University of Oxford) was responsible for the trial design, oversight of production of statistical analysis plan, analysis and interpretation of the results.
Chris Mabey (Senior Engineer, K2 Medical Systems, Plymouth) was responsible for the trial oversight, advice on technical aspects of INFANT software, analysis and interpretation of the results.
Mary Newburn [Consultant, Health Researcher/Public and Parent Involvement Lead (maternity theme), Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South London, Kings College London] was responsible for the trial design and oversight, advice on participant information material, analysis and interpretation of the findings.
Rachel Plachcinski (Research Engagement Officer, National Childbirth Trust) was responsible for the trial design and oversight, advice on participant information material, analysis and interpretation of the findings.
Maria Quigley (Professor of Statistical Epidemiology, National Perinatal Epidemiology Unit, Clinical Trials Unit, Nuffield Department of Population Health, University of Oxford) was responsible for the trial design and oversight, analysis and interpretation of the results.
Philip Steer (Emeritus Professor, Imperial College London) was responsible for the trial design and oversight, advice on labour and maternal outcomes, was the chairperson of adverse outcome review panel and was responsible for the analysis and interpretation of the results.
Liz Schroeder (Senior Lecturer, Department of Health Systems and Populations, Faculty of Medicine and Health Sciences, Macquarie University, Sydney, Australia) was responsible for the oversight of production of health economics analysis plan, health economic analysis and interpretation of the results.
Oliver Rivero-Arias (Associate Professor, Senior Health Economist, National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford) was responsible for the production of the health economic analysis and interpretation of the results.
Publications
Barber VS, Lean KA, Shakeshaft CE. Computers & CTGs: where are we at? Br J Midwifery 2010;18:644–9
Barber V, Linsell L, Locock L, Powell L, Shakeshaft C, Lean K, et al. Electronic fetal monitoring during labour and anxiety levels in women taking part in a RCT. Br J Midwifery 2013;21:394–403.
The INFANT Collaborative Group. Computerised interpretation of the fetal heart rate during labour (INFANT): a randomised controlled trial. Lancet 2017;389:1719–29.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Brocklehurst P. INFANT Collaborative Group . A study of an intelligent system to support decision making in the management of labour using the cardiotocograph – the INFANT study protocol. BMC Pregnancy Childbirth 2016;16. https://doi.org/10.1186/s12884-015-0780-0.
- Levene MI, Levene MI, Lilford RJ, Bennett MJ, Punt J. Fetal and Neonatal Neurology and Neurosurgery. London: Churchill Livingstone; 1995.
- Volpe JJ, Volpe JJ. Neurology of the Newborn. Philadelphia, PA: W.B. Saunders; 1994.
- Macfarlane A, Johnson A, Mugford M, Rennie JM, Roberton NRC. Textbook of Neonatology. London: Churchill Livingstone; 1999.
- Hon EH. The classification of fetal heart rate. I. A working classification. Obstet Gynecol 1963;22:137-46.
- Hon EH. Instrumentation of fetal heart rate and fetal electrocardiography. II. A vaginal electrode. Am J Obstet Gynecol 1963;86:772-84. https://doi.org/10.1016/S0002-9378(16)35194-8.
- Gillmer MD, Combe D. Intrapartum fetal monitoring practice in the United Kingdom. Br J Obstet Gynaecol 1979;86:753-8. https://doi.org/10.1111/j.1471-0528.1979.tb10689.x.
- Steer PJ, Eigbe F, Lissauer TJ, Beard RW. Interrelationships among abnormal cardiotocograms in labor, meconium staining of the amniotic fluid, arterial cord blood pH, and Apgar scores. Obstet Gynecol 1989;74:715-21.
- MacDonald D, Grant A, Sheridan-Pereira M, Boylan P, Chalmers I. The Dublin randomized controlled trial of intrapartum fetal heart rate monitoring. Am J Obstet Gynecol 1985;152:524-39. https://doi.org/10.1016/0002-9378(85)90619-2.
- Vintzileos AM, Nochimson DJ, Guzman ER, Knuppel RA, Lake M, Schifrin BS. Intrapartum electronic fetal heart rate monitoring versus intermittent auscultation: a meta-analysis. Obstet Gynecol 1995;85:149-55. https://doi.org/10.1016/0029-7844(94)00320-D.
- Haverkamp AD, Thompson HE, McFee JG, Cetrulo C. The evaluation of continuous fetal heart rate monitoring in high-risk pregnancy. Am J Obstet Gynecol 1976;125:310-17. https://doi.org/10.1016/0002-9378(76)90565-2.
- Renou P, Chang A, Anderson I, Wood C. Controlled trial of fetal intensive care. Am J Obstet Gynecol 1976;126:470-6. https://doi.org/10.1016/0002-9378(76)90641-4.
- Kelso IM, Parsons RJ, Lawrence GF, Arora SS, Edmonds DK, Cooke CD. An assessment of continuous fetal heart rate monitoring in labor: a randomized trial. Am J Obstet Gynecol 1978;131:526-31. https://doi.org/10.1016/0002-9378(78)90114-X.
- Haverkamp AD, Orleans M, Langendoerfer S, McFee J, Murphy J, Thompson HE. A controlled trial of the differential effects of intrapartum fetal monitoring. Am J Obstet Gynecol 1979;134:399-412. https://doi.org/10.1016/S0002-9378(16)33082-4.
- Wood C, Renou P, Oats J, Farrell E, Beischer N, Anderson I. A controlled trial of fetal heart rate monitoring in a low-risk obstetric population. Am J Obstet Gynecol 1981;141:527-34. https://doi.org/10.1016/S0002-9378(15)33273-7.
- Neldam S, Osler M, Hansen PK, Nim J, Smith SF, Hertel J. Intrapartum fetal heart rate monitoring in a combined low- and high-risk population: a controlled clinical trial. Eur J Obstet Gynecol Reprod Biol 1986;23:1-11. https://doi.org/10.1016/0028-2243(86)90099-7.
- Luthy DA, Shy KK, van Belle G, Larson EB, Hughes JP, Benedetti TJ, et al. A randomized trial of electronic fetal monitoring in preterm labor. Obstet Gynecol 1987;69:687-95. https://doi.org/10.1097/00006254-198710000-00002.
- Vintzileos AM, Antsaklis A, Varvarigos I, Papas C, Sofatzis I, Montgomery JT. A randomized trial of intrapartum electronic fetal heart rate monitoring versus intermittent auscultation. Obstet Gynecol 1993;81:899-907.
- Leveno KJ, Cunningham FG, Nelson S, Roark ML, Williams ML, Guzick DS, et al. A prospective comparison of selective and universal electronic fetal monitoring in 34,995 pregnancies. N Engl J Med 1986;315:615-19. https://doi.org/10.1056/NEJM198609043151004.
- Alfirevic Z, Devane D, Gyte GML. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database of Syst Rev 2013;5. https://doi.org/10.1002/14651858.CD006066.pub2.
- Intrapartum Care: Care of Healthy Women and their Babies During Childbirth. London: NICE; 2007.
- Murphy KW, Johnson P, Moorcraft J, Pattinson R, Russell V, Turnbull A. Birth asphyxia and the intrapartum cardiotocograph. Br J Obstet Gynaecol 1990;97:470-9. https://doi.org/10.1111/j.1471-0528.1990.tb02515.x.
- Ennis M, Vincent CA. Obstetric accidents: a review of 64 cases. BMJ 1990;300:1365-7. https://doi.org/10.1136/bmj.300.6736.1365.
- Gaffney G, Sellers S, Flavell V, Squier M, Johnson A. Case–control study of intrapartum care, cerebral palsy, and perinatal death. BMJ 1994;308:743-50. https://doi.org/10.1136/bmj.308.6931.743.
- Stewart JH, Andrews J, Cartlidge PH. Numbers of deaths related to intrapartum asphyxia and timing of birth in all Wales perinatal survey, 1993–5. BMJ 1998;316:657-60. https://doi.org/10.1136/bmj.316.7132.657.
- Confidential Enquiry into Stillbirths and Deaths in Infancy: 6th Annual Report. London: Maternal and Child Health Research Consortium; 1999.
- Young P, Hamilton R, Hodgett S, Moss M, Rigby C, Jones P, et al. Reducing risk by improving standards of intrapartum fetal care. J R Soc Med 2001;94:226-31.
- Annual Report and Accounts 2015/16. London: NHS Litigation Authority; 2016.
- BBC News . Girl, 12, Wins £6m Compensation n.d. http://news.bbc.co.uk/1/hi/england/beds/bucks/herts/6310805.stm (accessed 16 September 2016).
- BBC News . Girl, 7, Awarded £10m in Damages from King’s College Hospital Trust 2015. www.bbc.co.uk/news/uk-england-london-30943477 (accessed 3 October 2017).
- Sexton JB, Thomas EJ, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ 2000;320:745-9. https://doi.org/10.1136/bmj.320.7237.745.
- Harris M. An Investigation of Labour Ward Care to Inform the Design of a Computerised Decision Support System for the Management of Childbirth. Plymouth: University of Plymouth; 2000.
- Harris M, Jagodzinski AP, Greene KR. Roles for knowledge-based computer systems: case studies in maternity care. AI &Amp; Society 2001;15:386-95. https://doi.org/10.1007/BF01206117.
- Feinmann J. Clinical IT. Ticker taped. Health Serv J 2003;113:28-9.
- Keith RDF, Beckley S, Garibaldi JM, Westgate JA, Ifeachor EC, Greene KR. A multicentre comparative study of 17 experts and an intelligent computer system for managing labour using the cardiotocogram. Br J Obstet Gynaecol 1995;102:688-700. https://doi.org/10.1111/j.1471-0528.1995.tb11425.x.
- Skinner JF, Harris M, Greene KR. Computerised Decision Support for Managing Labour Using the Cardiotocogram: 500 Cases With the Range of Abnormality n.d.
- Keith RDF, Greene KR. Development, evaluation and validation of an intelligent decision support tool for the management of labour. Baillière’s Clinical Obstetrics and Gynaecology 1994;8:583-605. https://doi.org/10.1016/S0950-3552(05)80200-7.
- Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev 1985;11:21-6. https://doi.org/10.1016/0378-3782(85)90115-X.
- Johnson S, Marlow N, Wolke D, Davidson L, Marston L, O’Hare A, et al. Validation of a parent report measure of cognitive development in very preterm infants. Dev Med Child Neurol 2004;46:389-97. https://doi.org/10.1017/S0012162204000635.
- Johnson S, Wolke D, Marlow N. Preterm Infant Parenting Study Group . Developmental assessment of preterm infants at 2 years: validity of parent reports. Dev Med Child Neurol 2008;50:58-62. https://doi.org/10.1111/j.1469-8749.2007.02010.x.
- Perinatal Mortality Surveillance Report 2004: England, Wales and Northern Ireland. London: CEMACH; 2006.
- Kurinczuk JJ, Barralet JH, Redshaw M, Brocklehurst P. Report to the Patient Safety Research Programme (Policy Research Programme of the Department of Health): Monitoring the Incidence of Neonatal Encephalopathy – What Next?. Oxford: NPEU; 2005.
- Field DJ, Manktelow BM, Gill B, Draper ED. The Neonatal Survey and Yorkshire Neonatal Network Report 2005.
- Westgate J, Harris M, Curnow JS, Greene KR. Plymouth randomized trial of cardiotocogram only versus ST waveform plus cardiotocogram for intrapartum monitoring in 2400 cases. Am J Obstet Gynecol 1993;169:1151-60. https://doi.org/10.1016/0002-9378(93)90273-L.
- Westgate J, Garibaldi JM, Greene KR. Umbilical cord blood gas analysis at delivery: a time for quality data. Br J Obstet Gynaecol 1994;101:1054-63. https://doi.org/10.1111/j.1471-0528.1994.tb13581.x.
- Goldaber KG, Gilstrap LC, Leveno KJ, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol 1991;78:1103-7.
- Eskes TK, Jongsma HW, Houx PC. Percentiles for gas values in human umbilical cord blood. Eur J Obstet Gynecol Reprod Biol 1983;14:341-6. https://doi.org/10.1016/0028-2243(83)90010-2.
- The Damocles Study Group . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)70939-9.
- Peto R, Pike M, Armitage P, Breslow N, Cox D, Howard S, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. 1. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- Barber V, Linsell L, Locock L, Powell L, Shakeshaft C, Lean K, et al. Electronic fetal monitoring during labour and anxiety levels in women taking part in a RCT. Br J Midwifery 2013;21:394-403. https://doi.org/10.12968/bjom.2013.21.6.394.
- Bewley S, Cockburn J. Responding to fear of childbirth. Lancet 2002;359:2128-9. https://doi.org/10.1016/S0140-6736(02)09113-4.
- Mancuso A, De Vivo A, Fanara G, Denaro A, Laganà D, Accardo FM. Effects of antepartum electronic fetal monitoring on maternal emotional state. Acta Obstet Gynecol Scand 2008;87:184-9. https://doi.org/10.1080/00016340701823892.
- McLachlan H, Waldenström U. Childbirth experiences in Australia of women born in Turkey, Vietnam, and Australia. Birth 2005;32:272-82. https://doi.org/10.1111/j.0730-7659.2005.00370.x.
- Edwards SJ, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J. Ethical issues in the design and conduct of randomised controlled trials. Health Technol Assess 1998;2.
- Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA 2004;292:1593-601. https://doi.org/10.1001/jama.292.13.1593.
- Cox AC, Fallowfield LJ, Jenkins VA. Communication and informed consent in phase 1 trials: a review of the literature. Support Care Cancer 2006;14:303-9. https://doi.org/10.1007/s00520-005-0916-2.
- Machin D, Scamell M. The experience of labour: using ethnography to explore the irresistible nature of the bio-medical metaphor during labour. Midwifery 1997;13:78-84. https://doi.org/10.1016/S0266-6138(97)90060-7.
- Gibbins J, Thomson AM. Women’s expectations and experiences of childbirth. Midwifery 2001;17:302-13. https://doi.org/10.1054/midw.2001.0263.
- Graham W, Smith P, Kamal A, Fitzmaurice A, Smith N, Hamilton N. Randomised controlled trial comparing effectiveness of touch screen system with leaflet for providing women with information on prenatal tests. BMJ 2000;320:155-60. https://doi.org/10.1136/bmj.320.7228.155.
- Johnson A. Follow up studies: a case for a standard minimum data set. Arch Dis Child Fetal Neonatal Ed 1997;76:F61-3. https://doi.org/10.1136/fn.76.1.F61.
- Disability and Perinatal Care: A Report of Two Working Groups Convened by the National Perinatal Epidemiology Unit and the Former Oxford Regional Health Authority. Oxford: NPEU and ORHA; 1994.
- Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e5840.
- Office for National Statistics . Birth Statistics Statistical Bulletin 2008 2009.
- Smith GCS, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. BMJ 2003;327:313-9. https://doi.org/10.1136/bmj.327.7410.313.
- Birth Statistics: Review of the National Statistician on Births and Patterns of Family Building in England and Wales, 2008. London: ONS; 2009.
- Yelland LN, Salter AB, Ryan P, Makrides M. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account?. Paediatr Perinat Epidemiol 2011;25:283-97. https://doi.org/10.1111/j.1365-3016.2011.01196.x.
- Marston L, Peacock JL, Yu K, Brocklehurst P, Calvert SA, Greenough A, et al. Comparing methods of analysing datasets with small clusters: case studies using four paediatric datasets. Paediatr Perinat Epidemiol 2009;23:380-92. https://doi.org/10.1111/j.1365-3016.2009.01046.x.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:698-702. https://doi.org/10.1136/bmj.c332.
- Yelland LN, Salter AB, Ryan P. Relative risk estimation in randomized controlled trials: a comparison of methods for independent observations. Int J Biostat 2011;7. https://doi.org/10.2202/1557-4679.1278.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7382.219.
- Matthews JN, Altman DG. Interaction 3: How to examine heterogeneity. BMJ 1996;313. https://doi.org/10.1136/bmj.313.7061.862.
- Berglund S, Grunewald C, Pettersson H, Cnattingius S. Severe asphyxia due to delivery-related malpractice in Sweden 1990–2005. BJOG 2008;115:316-23. https://doi.org/10.1111/j.1471-0528.2007.01602.x.
- Schroeder E, Petrou S, Patel N, Hollowell J, Puddicombe D, Redshaw M, et al. Cost effectiveness of alternative planned places of birth in woman at low risk of complications: evidence from the Birthplace in England national prospective cohort study. BMJ 2012;344. https://doi.org/10.1136/bmj.e2292.
- Department of Health . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 2 June 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Regier DA, Petrou S, Henderson J, Eddama O, Patel N, Strohm B, et al. Cost-effectiveness of therapeutic hypothermia to treat neonatal encephalopathy. Value Health 2010;13:695-702. https://doi.org/10.1111/j.1524-4733.2010.00731.x.
- Financial Matters; NewsLetters. London: NHS Finance Manual; 2016.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Nixon RM, Wonderling D, Grieve RD. Non-parametric methods for cost-effectiveness analysis: the central limit theorem and the bootstrap compared. Health Econ 2010;19:316-33. https://doi.org/10.1002/hec.1477.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Little RJ, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: Wiley InterScience; 2002.
- Szende A, Janssen BM, Cabases JM. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht: Springer Open; 2014.
- Nunes I, Ayres-de-Campos D, Ugwumadu A, Amin P, Banfield P, Nicoll A, et al. FM-ALERT: A Randomised Clinical Trial of Intrapartum Fetal Monitoring With Computer Analysis Alerts Versus Previously Available Monitoring n.d. www.omniview.eu/Cache/binImagens/2015_BritishIsles_7730patient_RCT-647.pdf (accessed 5 April 2016).
- Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty?. JAMA 2003;289:2554-9. https://doi.org/10.1001/jama.289.19.2554.
Appendix 1 Information for women during the antenatal period
Appendix 2 Participant information leaflet
Appendix 3 Consent form
Appendix 4 Training summary
Appendix 5 Data collection form for a baby admitted to a neonatal unit
Appendix 6 Data collection form for a baby with neonatal encephalopathy
Appendix 7 Data collection form for a neonatal death
Appendix 8 Data collection form for a mother admitted to a higher level of care
Appendix 9 Two-year follow-up questionnaire
Appendix 10 Economic evaluation analysis plan
INFANT economic evaluation analyses plan
Liz Schroeder
February 2014
Glossary
- CEA
- cost-effectiveness analysis
- CUA
- cost–utility analysis
- ICER
- incremental cost-effectiveness ratio
- QALY
- quality-adjusted life-year
Aims of the INFANT Health Economic Evaluation
As shown above, determining whether or not the use of the decision support software is cost-effective for the management of labour and birth is an objective of the trial. This objective will be met through component studies that address specific research questions (aims).
Firstly to consider the incremental cost of an adverse perinatal outcome prevented at hospital discharge.
However, this outcome is likely to have longer-term consequences in terms of health status and health service utilisation over the infant’s lifetime. Two longer-term evaluations are therefore planned.
In the first instance to estimate the cost-effectiveness of the decision support when surviving children reach age 2 years (aim 2), estimated as the incremental cost per disability free life-years gained at 2 years. These estimates will be informed with individual patient level data collected from parents participating in the INFANT trial.
Secondly, to extrapolate long-term outcomes and costs over a lifetime expressed as an incremental cost per quality-adjusted life-year (QALY) gained using decision–analytic modelling techniques (aim 3).
Finally, to explore the potential effect of the intervention on litigation claims for obstetrics (aim 4) in a ‘stand-alone’ study.
These aims are shown in the following table (Table 45).
| Aim | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Outcome measure | Poor perinatal outcome averted | Disability-free life-years | ‘derived’ QALYs (using clinical diagnosis of mild, moderate or severe outcomes at 2 years) | Predicted changes in obstetric claims (modelled scenarios) |
| Timeframe | Initial hospital discharge | 2 years | Long term (e.g. lifetime/18-year time horizon) | Long term (e.g. lifetime/18-year time horizon) |
| Data | 47,112 women | 5–7000 + all primary outcome cases (for which we have 2-year outcome data) | As in (1) & (2) + secondary information derived from literature reviews – mapping economic data between health states obtained from literature | Use of INFANT data for possible changes to baby outcomes, modelled scenarios using litigation information from the NHS LA |
| 1500 for resource use data | ||||
| Data collection | Guardian system, hospital information system + observational data | As in (1) + parent questionnaires at 1 and 2 years | As in (1&2) + literature reviews | As in 3, + NHS LA 10-year litigation data |
| Analysis | Intention-to-treat analysis, ICERs and net benefit statistics presented using non-parametric statistical methods with 95% confidence ellipses | As in (1) | Extrapolation model for long-term outcomes (+ decision-analytic modelling + sensitivity analysis) | Modelled scenarios of changes to clinical practice; baby outcomes; modelled longer-term outcomes and litigation payouts |
| PARCA-R defined predictors of mild, moderate and severe disability mapped to disability-adjusted life-years | ||||
Methods
Data collection
A prospective economic evaluation is being conducted alongside the trial, with the aim of estimating the cost-effectiveness of the intelligent decision support software. Information on resource utilisation will be collected through the Guardian data collection system, hospital-patient administration and maternity information systems. Observational research methods will be used to collect additional costs in intrapartum, postpartum or neonatal care for the first analysis (study aim 1).
Postal questionnaires sent to parents at 1 year and 2 years post discharge collect resource use data for the health service over that period (study aim 2).
Decision-analytic modelling methods (or Markov methods if required) synthesising primary INFANT and secondary cost and epidemiological data will be used to estimate the cost-effectiveness of longer-term outcomes (study aim 3).
A stand-alone study exploring litigation for obstetrics in the NHS and the potential effect that this intervention may have, using current primary data sources provided by the NHS LA and outcomes from the trial will also be undertaken (study aim 4).
The recruitment of study participants and data collection for the health economics can be viewed in the following flow chart (Figure 42).
FIGURE 42.
Flow chart depicting recruitment and data collection for INFANT health. a, +/– represents with or without additional data collected by the midwife as necessary. b, Only mothers recruited during the first year of the trial so that follow-up of this group can be completed around the time that the trial stops recruiting. c, x represents the actual numbers that would be collected.
Brief overview of cost-effectiveness analysis and cost–utility analysis
Cost-effectiveness analysis (CEA) is a form of economic evaluation that compares the relative costs and outcomes (effects) of two or more courses of action, using a common outcome measure. In cost-effectiveness analyses, the costs are expressed in monetary units, while benefits are expressed in natural or physical units, such as survival, physical abilities and health-related quality of life. CEA involves calculating the difference in costs and difference in outcomes between the health-care interventions being compared, and then expressing these as a ratio. Typically the ratio is measured as a value where the denominator is a gain in health from a measure (years of life, premature births averted) and the numerator is the cost associated with the health gain. The ICER represents the additional cost of one unit of outcome gained by a healthcare intervention or strategy, when compared with the next best alternative, mutually exclusive intervention or strategy. Three incremental cost-effectiveness ratios, or ICERs, will represent the additional cost of one unit of outcome gained by the incorporation of the intelligent decision support system for the management of women who are judged to require continuous electronic fetal monitoring (EFM) compared with current clinical practice. These are the incremental cost of poor perinatal outcome prevented at hospital discharge, the incremental cost per disability free life-years gained at 2 years and the incremental cost per quality-adjusted life-year (QALY) gained at 18 years. A threshold value is often set by policy makers, who may decide that only interventions with an ICER below the threshold are cost-effective (and therefore should be funded). Various thresholds around £20,000/QALY and £40,000/QALY gained will be explored in the analysis.
Economic evaluation design issues for INFANT study aims 1 and 2 (within-trial economic evaluation)
Trial design
INFANT is a multicentre individually randomised controlled trial of 47,112 women who are judged to require continuous electronic fetal monitoring in labour. It is a two-arm parallel trial with one arm allocated to CTG monitoring with decision support and one arm allocated to CTG monitoring only. At 2 years after trial entry, a random sample of 5000–7000 children (2500–3500 in each group) are followed up at 2 years. This sample is taken from within the sample recruited during the second year of the trial so that follow-up of this group can be completed around the time that the trial stops recruiting.
For the economic evaluation, a subsample of approximately 750 infants from each trial arm randomly selected within the second year, as well as all babies with the primary outcome who survive to hospital discharge, are followed up at 1 and 2 years for resource use data in order to estimate the incremental cost per disability free life-years gained at 2 years. Decision-analytic modelling techniques will be used to extrapolate cost-effectiveness over a lifetime.
Time horizon and perspective of the INFANT economic evaluation
The economic evaluation will be conducted from a health service perspective and therefore only direct costs to NHS hospital providers will be included. Societal costs, such as travel time and lost productivity to families will not be captured. (Estimates of the longer-term costs of care including litigation costs are discussed in Economic evaluation design issues for INFANT study aim 3 (modelling longer-term outcomes) and Economic evaluation design issues for INFANT study aim 4 (modelling clinical scenarios that will impact on obstetric litigation claims).
Sample size and power
The sample size of the INFANT economic evaluation is based on the primary clinical outcomes of the INFANT trial, which is currently a pragmatic approach to the determination of sample size and power calculation. Different techniques have been proposed for the estimation of statistical power and sample size for economic evaluation in randomised trials, but current practice tends to prefer power calculations based on primary clinical outcomes. This is partly because of the complexity of trying to predict the main outcome of interest to economists, which is the joint distribution of the difference in costs and benefits between treatment arms. 6
Study end points
In the baseline study (study aim 1), we are using an intermediate outcome (poor perinatal outcome prevented at hospital discharge) and hence additional resource use and unit cost data are needed to capture longer-term costs and outcomes. This is the purpose of the study at 2 years where the outcome measure is quantified in terms of disability free life-years gained (study aim 2) and for the extrapolation of longer-term outcomes expressed as QALYs over a lifetime or to 18 years (study aim 3).
Database design and management
Collection and management of the economic data are fully integrated with the management of the clinical data and, as such, there will be no distinction between the data sets for study aim 1. Ongoing data quality monitoring occurs timeously to address missing and poor-quality data issues. Data queries are consistently managed to maximise data completeness and quality. The data formatting procedures needed for the economic analysis were prespecified such that the transfer of all necessary data for the economics study is timely, and the design and piloting of the data capture system is complete. Double data entry for the 12- and 24-month parental questionnaires is in process.
Collection and measurement of trial costs
Trial costs/resource use
The economic evaluation focuses on main cost drivers (such as days spent in intensive care) as well as resources that are expected to differ between the trial arms. All resource use including intervention related resources will be included in the cost analysis. Table 46 documents the resource use data identified for the health economics component.
| Resource use variable | Trial arm, Mean resource use (SE) | Unit cost (£) | |
|---|---|---|---|
| Intervention group | Standard care group | ||
| Inpatient stay | |||
| Intensive care (level 1) | |||
| High-dependency care (level 2) | |||
| Special care (level 3) | |||
| Ordinary care (level 4) | |||
| Cooling (additional to IC care) | |||
| Readmission after initial discharge | |||
| Associated with transfer (number of transfers) | |||
| Transfer | |||
| Community resource use in first year (visits) | |||
| General practitioner | |||
| Health visitor | |||
| Practice nurse | |||
| Community nurse | |||
| Community paediatrician | |||
| Physiotherapist | |||
| Community resource use in second year (visits) | |||
| General practitioner | |||
| Health visitor | |||
| Practice nurse | |||
| Community nurse | |||
| Community paediatrician | |||
| Physiotherapist | |||
| Resource use of mother | |||
| Inpatient stay | |||
| Inpatient stay ward | |||
| Inpatient stay IC | |||
| Additional investigations | |||
| Radiography | |||
| Ultrasound scans | |||
| Surgery | |||
| Other procedures (number of) | |||
| Associated with transfer (number of transfers) | |||
| Transfer | |||
For each resource, the level of aggregation will be prospectively determined. As an example, inpatient hospitalizations might be considered in disaggregated units, such as staff time, or in highly aggregated units, such as numbers of hospitalizations or days in the hospital. A mixed case approach to costing will be used, dependent on resource use patterns expected, and availability of national standardised cost data (from Department of Health reference costs, typically used in economic evaluations). Items will be captured in disaggregated units where relevant, and such costing is typically labelled ‘bottom up’. This will be achieved by asking midwives/clinicians to document relevant staffing, medications and equipment. They will then be sent a micro-costing sheet to correct with their own resource components (Table 47) which will be very detailed, capturing all resource components that might be used. Further information will also be captured during formal interviews to include cost of the intervention itself, including the potential impact on staff working patterns.
| Staffing | Medications | Equipment | Total Cost | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Staff title and grade | Length of contact time/procedure | Staffing cost (£) | Drug | Dose | Mode of administration | No of treatments per day | Cost (£) | Piece | Llifespan | Annuitised cost (£) | ∑ of costs |
Total cost will be measured by multiplying unit costs to resource use data. Unit costs are the cost per standard unit. Unit costs will be consistent with measured resource use, the study’s perspective, and its time horizon (for instance valued at 2012 prices). In some cases, unit costs will be estimated from trial data collection sites, but more commonly they will be derived from national data sources (Department of Health reference costs).
Statistical tests
The purpose of clinical trial cost analysis is to estimate costs, cost differences associated with treatment, the variability of differences, and whether or not the differences occurred by chance.
Once resources have been identified and valued, differences between groups must be summarised. Arithmetic mean cost differences are generally considered the most appropriate and robust measure, however, cost data often do not conform to the assumptions for standard statistical tests for comparing differences in arithmetic means. Nonparametric methods of estimating incremental net benefit will be used to compute the cost-effectiveness acceptability curves and the confidence ellipses.
Missing data
Missing data are inevitable in economic analyses conducted alongside trials. Eliminating cases with missing data is not recommended because it may introduce bias or severely reduce power to test hypotheses. Nevertheless, ignoring small amounts of missing data is acceptable if a reasonable case can be made that doing so is unlikely to bias treatment group comparisons. Imputation refers to replacing missing fields with estimates. A strict quality control is currently in place for the INFANT data collection to minimise missing data. However, it is expected that some missing data will occur and we will be using appropriate methods such as multiple imputation if necessary to impute missing resource use and health-related quality of life data.
Uncertainty
Results of economic assessments in trials are subject to a number of sources of uncertainty, including sampling uncertainty and uncertainty in parameters such as unit costs. The revised point estimate and revised 95% CIs that result from the sensitivity analysis will be reported.
Reporting the methods and results
Cost-effectiveness acceptability curves and incremental net benefit statistics with 95% CIs will be presented. The differences in resource use and costs tested using t tests and differences in effects will be presented using relative risks. Net benefits are defined as Rc.ΔE – ΔC, (where Rc is the threshold cost ratio, ΔE is the change in effects between the trial arms, and ΔC the change in costs for the trial arms). These will be estimated for alternative values of Rc, together with their 95% confidence ellipses.
A series of sensitivity analyses will be undertaken to explore the implications of uncertainty on the base-case incremental cost-effectiveness ratios. This will include varying variables found to be the key cost-drivers in early analyses for cost.
The health economist will receive a ‘cleaned’ database of resource use and effectiveness data from the main INFANT statistical team conducting the primary analysis. All analyses will be performed with a microcomputer using Stata version 13, and Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) software.
Economic evaluation design issues for INFANT study aim 3 (modelling longer-term outcomes)
Long-term cost-effectiveness of intelligent system to support decision-making in the management of labour using cardiotocogram
An analysis of the cost-effectiveness of the intelligent decision support software during labour will take into account the potential long-term outcomes to mother and child. Cost-effectiveness will be calculated in terms of the incremental cost per QALY gained. The analysis will be intended to account for the expected lifetime of the children, but depending on data availability we will consider shorter life horizons that can be populated with good evidenced data such as 18 years. Tables 48 and 49 show the primary data inputs that will be derived from the INFANT trial to populate the model.
| Parameter | Trial arm, mean (95% CI) | |
|---|---|---|
| Intervention group | Standard care group | |
| First 12 months: outcomes, and costs | ||
| Survival with NNE | ||
| Death of child | ||
| Composite: poor neonatal outcomes | ||
| EQ-5D of mother | ||
| Cost of ECG decision software | ||
| Hospital costs to discharge for surviving children | ||
| Hospital costs to discharge for women | ||
| Hospital costs for non-surviving children | ||
| Inpatient costs of children post discharge | ||
| Community care costs of children post discharge | ||
| 12 to 24 months: outcomes and costs | ||
| Age assessed for DQ | ||
| Survival without neurological abnormality (using Development Quotient) | ||
| Survival with neurological abnormality (using Development Quotient) | ||
| EQ-5D child (from mapping study) | ||
| EQ-5D of mother | ||
| Death of child | ||
| Inpatient costs, children without neurological abnormality | ||
| Inpatient costs, children with neurological abnormality | ||
| Community care costs, children without neurological abnormality | ||
| Community care costs, children with neurological abnormality | ||
| Parameter | Trial arm, mean (95% CI) | Mean difference† (95% CI) | |
|---|---|---|---|
| Intervention group | Standard care group | ||
| Analysis 1 (incremental cost per poor neonatal event averted) | |||
| Cost | |||
| Effectiveness | |||
| ICER | |||
| λ = 20,000 | |||
| λ = 30,000 | |||
| Analysis 2 (incremental cost per disability free life-year gained; using the DQ obtained at 2 years) | |||
| Cost | |||
| Effectiveness | |||
| ICER | |||
| λ = 20,000 | |||
| λ = 30,000 | |||
| Analysis 3 (incremental cost per quality-adjusted life-year gained; long-term analysis) | |||
| Cost | |||
| Effectiveness | |||
| ICER | |||
| λ = 20,000 | |||
| λ = 30,000 | |||
| Sensitivity Analysis 1 (incremental cost per quality-adjusted life-year gained including medical legal claims) | |||
| Cost | |||
| Effectiveness | |||
| ICER | |||
| λ = 20,000 | |||
| λ = 30,000 | |||
| Sensitivity Analyses 2 to 4 will vary the inputs to each of the main analysis | |||
| Cost | |||
| Effectiveness | |||
| ICER | |||
| λ = 20,000 | |||
| λ = 30,000 | |||
The long-term economic evaluation will require extrapolating and identifying future health-care costs and the health status of mothers and infants from literature as well as the application of decision-analytic methods to synthesise information from different sources.
A brief review of the NIHR HTA website (www.hta.ac.uk/project/htapubs.asp), the NHS Economic Evaluation Database (NHS-EED, www.crd.york.ac.uk/) and PubMed (www.pubmed.gov) reveals only one previous study of the long-term cost-effectiveness of cardiotocography methods in fetal monitoring during labour. 7 We will build on the results of this literature, but we will develop a de novo cost-effectiveness model that will be populated based on available evidence, including the data collected during the trial. At this stage, the proposed design is to use a Markov model described and simplified in Figure 43. A structured review of the published literature available for cost-effectiveness and cost-utility modelled from patient level data is currently in progress. The data, including an inventory of health state utility weights is being searched through the Paediatric Economic Database Evaluation (PEDE) made available by the Research Institute at The Hospital for Sick Children, Toronto.
FIGURE 43.
Markov model for long-term cost-effectiveness model.
Decisions about the model structure might be revised after systematic searches of the literature are undertaken and expert clinical input is considered. Following decisions about model structure, a list of parameter estimates required for the model will be developed. These are likely to include the series of parameters reported in Table 50. Data collected in this trial will provide information to populate the model which will be supplemented with available evidence in the literature following systematic searches. Parameters may require additional modelling to capture the long-term and time-dependent nature of the estimate values.
| Transition probability parameters | |
|---|---|
| Caesarean delivery | Mortality first 2 years after moderate encephalopathy |
| Instrumental vaginal delivery | Mortality first 2 years after severe encephalopathy |
| Spontaneous vaginal delivery | Remain without disability |
| No encephalopathy after caesarean section | From no disability to mild disability |
| No encephalopathy after instrumental delivery | From no disability to moderate disability |
| No encephalopathy after spontaneous delivery | From no disability to severe disability |
| Moderate encephalopathy after caesarean section | From no disability to death |
| Moderate encephalopathy after instrumental delivery | Remain with mild disability |
| Moderate encephalopathy after spontaneous delivery | From mild disability to no disability |
| Severe encephalopathy after caesarean section | From mild disability to moderate disability |
| Severe encephalopathy after instrumental delivery | From mild disability to severe disability |
| Severe encephalopathy after spontaneous delivery | From mild disability to death |
| Perinatal death after caesarean section | Remain with moderate disability |
| Perinatal death after instrumental delivery | From moderate disability to no disability |
| Perinatal death after spontaneous delivery | From moderate disability to mild disability |
| No disability after no encephalopathy | From moderate disability to severe disability |
| No disability after moderate encephalopathy | From moderate disability to death |
| No disability after severe encephalopathy | Remain with severe disability |
| Mild disability after no encephalopathy | From severe disability to no disability |
| Mild disability after moderate encephalopathy | From severe disability to mild disability |
| Mild disability after severe encephalopathy | From severe disability to moderate disability |
| Moderate disability after no encephalopathy | From severe disability to death |
| Moderate disability after moderate encephalopathy | Litigation if mild disability |
| Moderate disability after severe encephalopathy | Litigation if moderate disability |
| Severe disability after no encephalopathy | Litigation if severe disability |
| Severe disability after moderate encephalopathy | Litigation if perinatal death |
| Severe disability after severe encephalopathy | Litigation if death within first 2 years |
| Mortality first 2 years after no encephalopathy | Litigation if death after first 2 years |
| Resource cost | |
| Cost of caesarean section in CG | Cost moderate encephalopathy in first 2 years |
| Cost of caesarean section in IG | Cost severe encephalopathy in first 2 years |
| Cost of instrumental delivery in CG | Costs no disability |
| Cost of instrumental delivery in TG | Costs mild disability |
| Cost of spontaneous delivery in CG | Cost moderate disability |
| Cost of spontaneous delivery in TG | Cost severe disability |
| Cost of decision support software | Cost of litigation if mild disability |
| Cost no encephalopathy until discharge | Cost of litigation if moderate disability |
| Cost moderate encephalopathy until discharged | Cost of litigation if severe disability |
| Cost severe encephalopathy until discharged | Cost of litigation if perinatal death |
| Cost of perinatal death | Cost of litigation if death within first 2 years |
| Cost no encephalopathy in first 2 years | Cost of litigation if death after first 2 years |
| Outcomes | |
| Utility no encephalopathy – child | Utility no encephalopathy – parent |
| Utility moderate encephalopathy – child | Utility moderate encephalopathy – parent |
| Utility severe encephalopathy – child | Utility severe encephalopathy – parent |
| Utility no disability – child | Utility no disability – parent |
| Utility mild disability – child | Utility mild disability – parent |
| Utility moderate disability – child | Utility moderate disability – parent |
| Utility severe disability – child | Utility severe disability – parent |
We will undertake deterministic and probabilistic sensitivity analysis. For the latter input parameters will be assigned probability distributions to reflect their imprecision and Monte Carlo techniques will be used to reflect this uncertainty in the results. We will construct cost-effectiveness acceptability curves and cost-effectiveness confidence ellipses.
In addition to this analysis, we will also consider the effect on potential medico-legal claims that result from adverse events during the intrapartum and neonatal periods. We will model the probability of these claims and estimate the litigation costs related to them. We will explore different scenarios in our analyses as can be seen in Economic evaluation design issues for INFANT study aims 4 (Modelling clinical scenarios that will impact on obstetric litigation claims).
Economic evaluation design issues for INFANT study aim 4 (modelling clinical scenarios that will impact on obstetric litigation claims)
The prevention of a modest proportion of perinatal asphyxia will improve the health and well-being of thousands of children and their families throughout the world each year. A reduction in the number of babies born with perinatal asphyxia will reduce the associated mortality and, among survivors, the burden of ill health and incapacity over their lifetime. The implications of this cost burden to society is that maternity services are associated with far higher litigation costs than other services and a single ‘successful’ litigation case may result in a settlement worth millions of pounds.
Medical negligence data is one method of estimating the longer-term costs of perinatal asphyxia because the financial projections should be indicative of the cost implications for the value of life for the rest of life. However, evaluations for litigation purposes are often not reached until 5–6 years after the birth event, when neuro-paediatricians can identify patterns of brain damage reflecting birth asphyxia and when the baby is likely to have cerebral palsy. It is possible that some claims are processed in 2–3 years and others over 10–15 years.
The prevention of a modest proportion of perinatal asphyxia could thus result in substantial savings in litigation costs in the UK. However, litigation claims and pay outs are only slightly associated with negligence. All cases of litigation which are paid are likely to be associated with negligence. For various reasons outcomes as a result of negligence may not lead to litigation, and other cases [which incur economic (lawyers) costs] are brought where no settlement is made because there was no negligence.
Given that the NHSLA is the recipient of the Clinical Negligence Scheme for Trusts risk pooling schemes and has a unique database of all claims for births, we have proposed a stand-alone study with the NHSLA to understand the proportion of cases of perinatal asphyxia that have resulted in both successful and unsuccessful litigation (to generate baseline estimates of all successful litigation cases), the consequent pay-outs in the cases of successful claims and how these estimates have been derived over the past 10 years.
This would use a database of time series data that shows the costs involved in settled claims, including the initial capital compensation and then the ‘periodicals’ (annual payment for life), or any payments processed for deaths would satisfy this evidence gap. We will then develop a model estimating the longer-term cost of perinatal asphyxia. We understand that financial projections should be indicative of the cost implications for the value of life for the rest of life. Furthermore, it may encompass health-care and family costs, though may not include other therapies and state funded or local authority costs, so we would estimate these separately.
Together, these estimates will also assist us to identify potential savings in litigation costs in the UK from the decision support.
List of abbreviations
- aRR
- adjusted risk ratio
- BMI
- body mass index
- CESDI
- Confidential Enquiry into Stillbirths and Deaths in Infancy
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTG
- cardiotocograph
- DMC
- Data Monitoring Committee
- ECG
- electrocardiography
- EFM
- continuous electronic fetal monitoring
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GBP
- Great British pounds
- GEE
- generalised estimating equation
- GMR
- geometric mean ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IQR
- interquartile range
- IUGR
- intrauterine growth restriction
- MRC
- Medical Research Council
- NHSLA
- NHS Litigation Authority
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NNE
- neonatal encephalopathy
- PARCA-R
- Parent Report of Children’s Abilities-Revised
- PBDC
- Post Birth Data Collection
- PC
- personal computer
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SD
- standard deviation
- SE
- standard error
- TSC
- Trial Steering Committee
- UCL
- University College London
- VAS-A
- Visual Analogue Scale – Anxiety
