Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/104/16. The contractual start date was in August 2013. The draft report began editorial review in March 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
During the preparation of this report Howard Ring was the chairperson of the Health Technology Assessment Mental, Psychological and Occupational Health Panel and he was a member of the Psychological and Community Therapies Panel during the project. Cam Donaldson was a member of the Medical Research Council Methodology Research Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Ring et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Text in this chapter has been reproduced from Ring et al. 1 © Ring et al. 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
A note on terminology
A number of terms are in use to describe the intellectual functioning of individuals with a significant impairment of cognitive and other abilities arising from congenital, including genetic, disturbances of development or from pathological processes arising during the developmental period. In this trial the description ‘intellectual disability’ (ID) will be used. The other term still in common use to describe the same population is ‘learning disability’ (LD). In both instances the heterogeneous group of conditions being referred to are characterised by the presence of significant limitations in intellectual functioning, usually considered to be equivalent to a measured intelligence quotient (IQ) of < 70, together with deficits in social and adaptive behaviours, with onset during the developmental period (< 18 years of age).
Similarly, several terms are used to describe the training of nurses working with people with an ID. These nurses will generally have undertaken a nursing course specifically developed for this field and may describe themselves as either LD nurses or ID nurses.
With respect to experience and training in the field of epilepsy, again several terms are in use in the UK. Epilepsy specialist nurses are individuals with a primary training in general and/or ID nursing who have undertaken additional formal specialist training in epilepsy and its management. The great majority of nurses working with people who have an ID and epilepsy have not undertaken the additional formal specialist training that would lead to the title of epilepsy nurse specialist (ENS), but they will, in the course of their training and practice, have gained some level of experience in epilepsy. This is quantified in the Epilepsy And Intellectual Disability (EpAID) clinical trial by use of the competency framework that underpins the experimental intervention. Thus, the majority of nurses delivering the intervention in the EpAID trial were ID nurses with a level of expertise in epilepsy described by the framework. A small proportion, however, met the criteria for ENSs.
Epilepsy and intellectual disability
Nearly one million adults in England have an ID; epilepsy is the most common medical illness in this group, affecting around 26%, with higher rates in those with more severe IDs. 2 Individuals with an ID and epilepsy have a worse outcome than those in the general population with epilepsy, with an increased seizure frequency, higher frequencies of multiple antiepileptic drug (AED) use and side effects, higher treatment costs, higher rates of mortality and a greater incidence of behavioural problems. 1,3–5
Reflecting these observations, it has been reported that, between 2005 and 2009, the most common cause of avoidable acute hospital admission for people with an ID was seizures associated with poorly controlled epilepsy. 6 A survey by the Improving Health and Lives: Learning Disabilities Observatory reported that the second most frequent potentially preventable cause of death in those with an ID was epilepsy or convulsions (occurring in 13% of those with an ID). 7 These observations highlight the need to improve outcomes for people with epilepsy and an ID.
Currently, in the UK, secondary care of epilepsy and IDs is generally provided by community ID services or hospital-based neurological services. 8 It is common for people with an ID to be on multiple therapies and they are likely to have tried several AEDs to reduce seizure severity and frequency. 3 Only around 30% of this population will achieve seizure freedom, compared with 70% of the general population. 1,4 Therefore, in most people with epilepsy and an ID, the aim of treatment with AED is to reduce seizure severity and frequency while keeping associated side effects to a minimum. 9 Achieving this balance is often difficult in adults with an ID because of the severity of the epilepsy and the frequent presence of complex associated morbidities.
Associated morbidities of epilepsy in adults with an ID include motor and mobility problems, communication difficulties, attentional deficits and a range of emotional, cognitive and behavioural problems. 1 Therefore, identifying and differentiating presenting symptoms from an individual’s ID, epilepsy and medication side effects is often challenging.
Additional challenges are faced by treating clinicians in communicating effectively with people who have an ID and comorbid communication difficulties and psychobehavioural conditions such as affective disorders, autism or a range of challenging behaviours that limit their ability to co-operate with the demands of treatment. 3 Individuals with epilepsy and an ID also often lack the capacity to understand and make decisions regarding their treatment, meaning that ‘best interest’ decisions may be required, often involving tripartite discussion between the individual, his or her family members or paid carer and the treating clinician. 10 These challenges and the need for innovative approaches to overcome them were recognised by Public Health England in their 2014 paper, Making Reasonable Adjustments to Epilepsy Services for People with Learning Disabilities. 11 These observations all indicate that a multidisciplinary, holistic approach, supported by judicious use of specialist nursing input, may improve clinical management for people with an ID and epilepsy, who historically have frequently been excluded from clinical research and overlooked by health services.
The role of nurses with expertise in the management of epilepsy
Nurses with enhanced expertise in the management of epilepsy, often described as ENSs, offer a broad spectrum of services to patients with epilepsy. Depending on their level of training and expertise they contribute to activities that may include patient assessment, medication management and ordering and interpreting investigations. 12 They also provide education, support and counselling to patients and families, which are often overlooked by other clinicians. 13–15 ENSs may also have more time to speak to patients16 and may improve the continuity of, and accessibility to, care, with the potential to improve communication between people with epilepsy and their primary health-care services. 17,18 Thus, it might be predicted that ENSs would be ideally placed to champion and enhance the unpredictable, complex and long-term needs of people with epilepsy. 19
However, a Cochrane review20 of five trials of the use of epilepsy specialist nursing found no convincing evidence across the general population that ENSs improved overall outcomes for people with epilepsy. Nevertheless, an open prospective survey of the effects of introducing paediatric ENSs, published after the Cochrane review, suggested that paediatric ENSs might reduce emergency admissions by as much as 50%. 21
In terms of financial costs, a trial22 in patients recruited from a hospital-based epilepsy service, of whom just under 10% had an ID, noted that the use of an epilepsy nurse cost less than standard care, with reduced numbers of outpatient clinic hospital attendances with doctors and a potential decrease in general practitioner (GP) consultations after 6 months.
The great majority of previous research into the use of ENSs has been carried out in the general population, in studies that have largely or completely excluded adults with IDs, perpetuating the health inequalities that people with IDs have been subject to. Advocacy organisations, especially Mencap,23 have highlighted examples of such inequalities in health care, noting that clinicians regularly fail people with IDs by failing to consult with or involve parents and family members in care and treatment decisions. In addition, and importantly, a survey of epilepsy services for a community sample of adults with an ID and epilepsy24 found that only 34% of respondents had seen an ENS. This low exposure rate was found despite indications that ENSs may enhance the care of people with an ID and epilepsy. 25–27 For instance, an open study28 demonstrated that implementing the National Institute for Health and Care Excellence (NICE) epilepsy guidelines for people with an ID, including having a central role for an ENS, led to identifiable improvements in patient care. However, that conclusion was based on an audit of just 23 patients.
Rationale for the trial
Some anecdotal evidence and data from open studies suggests that ENS-led management may improve outcomes and reduce the costs of care for adults with epilepsy and an ID. However, this has not been tested in a definitive clinical trial and the results from previous studies in the general population cannot necessarily be generalised to adults with an ID, given the often greater severity and complexity of epilepsy and associated morbidities in those with ID. In addition, the majority of previous research has examined the role and effects of specific ENS interventions. However, as noted above, ENSs are a relatively rare resource not accessed by the majority of adults with an ID and epilepsy. It is the case, however, that nurses trained to work with people who have an ID also have experience and often some additional training in the management of epilepsy. In the absence of data to clearly support or refute the value of nurse-led epilepsy services for adults, either in the general population or among those with an ID, currently this skilled, potentially clinically effective resource is utilised variably and inefficiently. 29,30 The EpAID trial thus aimed to determine whether or not the use of a nurse-led intervention was associated with clinically effective and cost-effective benefits in the management of epilepsy in adults with an ID. Specifically, the trial aimed to test the effectiveness of the recently developed Learning Disability Epilepsy Specialist Nurse Competency Framework31 to improve outcomes for adults with an ID and epilepsy.
A key aspect of this framework is that it was designed to be applicable to the practice and professional development of all ID nurses, not just the small number of ENSs. Thus, if the use of the framework by nurses representative of those working in more or less all community intellectual disability team (CIDTs) was found to be effective, it could be readily implemented across the NHS.
Chapter 2 Methods
Text in this chapter has been reproduced from Ring et al. 1 © Ring et al. 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Aim and objectives
The aim of this trial was to test the hypothesis that an ENS-led intervention would cost-effectively reduce seizure severity and improve overall quality of life (QoL) for patients and those who provide care for them in the community.
Primary objective
The primary objective of the trial was to establish whether or not nurses with a range of previous experience in epilepsy and ID, reflecting the skill mix currently existing in the NHS, working in accord with the Learning Disability Epilepsy Specialist Nurse Competency Framework,31 could improve epilepsy-related clinical and QoL outcomes in the management of epilepsy in adults with an ID compared with treatment as usual (TAU), as measured using the Epilepsy and Learning Disabilities Quality of Life seizure severity scale (ELDQoL-SSS). 32
Secondary objectives
The main secondary outcome was to establish whether or not any perceived benefits represent good value for money after consideration of the costs associated with the intervention from the perspective of health and social services. Additional secondary outcomes consisted of a measure of carer strain, the Epilepsy and Learning Disabilities Quality of Life (ELDQoL) subscales for AED side effects, mood and behaviour and semistructured interviews of samples of clinicians, family members and paid carers to examine how the competency framework, compared with TAU, had an impact on relationships that are critical in delivering ongoing care for adults with an ID and epilepsy.
Trial design
The study was a cluster randomised controlled trial (RCT) with two arms: a TAU control arm and an experimental arm involving the use of the ID epilepsy nurse competency framework. 31 The trial complied with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for cluster randomised trials. 33 The study also contained a nested qualitative component. A cluster randomised design was selected because the intervention, a change in how nurses worked within the community team setting, needed to be implemented at the level of the clinical team as a whole. This was because, first, pilot work had previously indicated that within a community team the nurses would share duties from time to time and therefore all would need to follow the same treatment approach and, second, as the active intervention also involved training of the nurses administering it, those nurses could no longer be considered to be able to continue to deliver TAU reliably. The trial was therefore developed such that a community team constituted a single cluster.
The design of the trial is shown in Figure 1. A flow diagram is provided in Figure 2, which describes the order in which cluster and participant recruitment, cluster randomisation, data collection and intervention processes at each research cluster took place.
FIGURE 1.
Design of the EpAID trial. CSRI, Client Service Receipt Inventory; EQ-5D-5L, EuroQol-5 Dimensions, five-level version; KCTU, King’s Clinical Trial Unit; MCSI, Modified Caregiver Strain Index; WTP, willingness to pay. This figure has been reproduced from Ring et al. 1 © Ring et al. 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
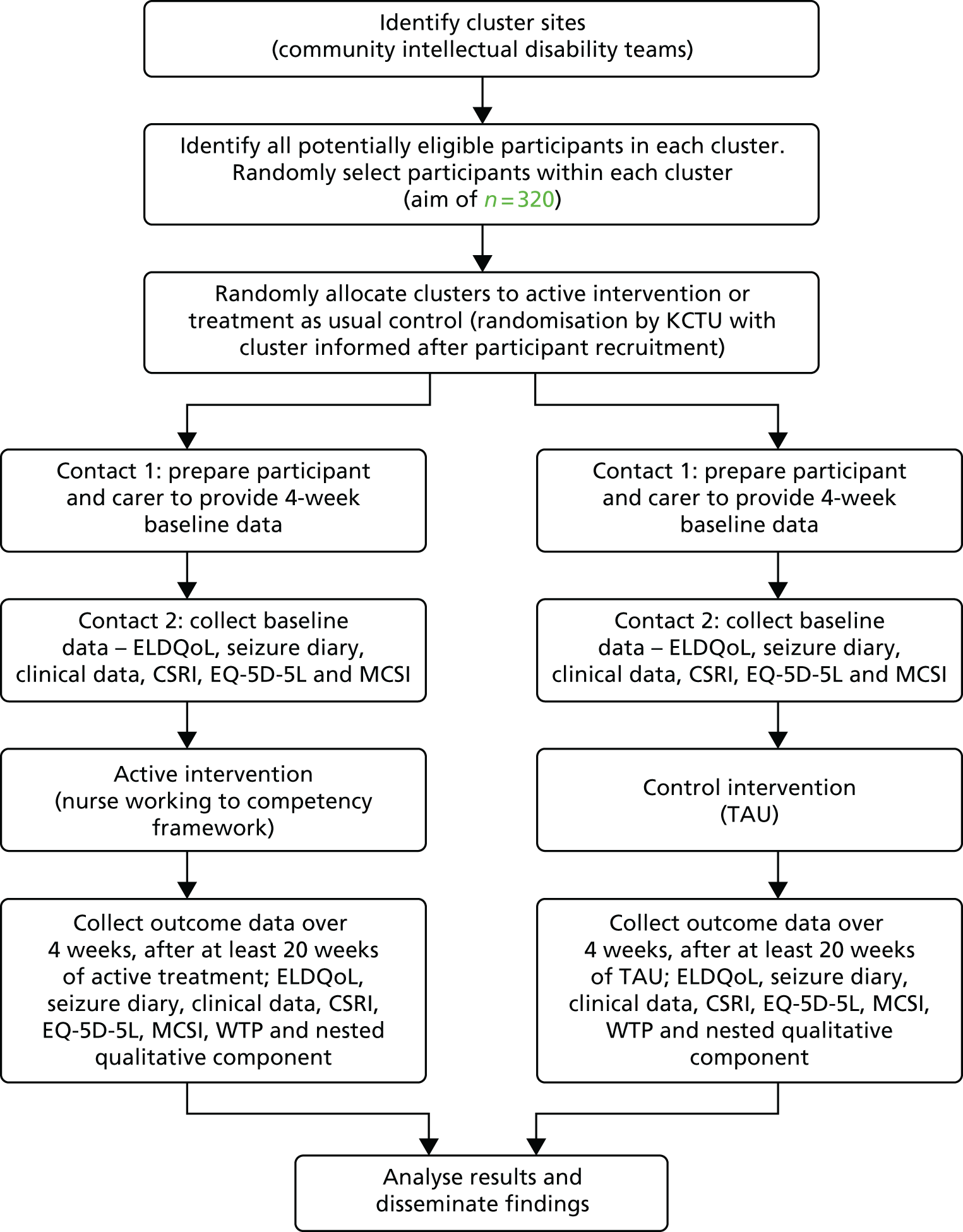
FIGURE 2.
Flow diagram depicting the EpAID trial processes. This figure has been reproduced from Ring et al. 1 © Ring et al. 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
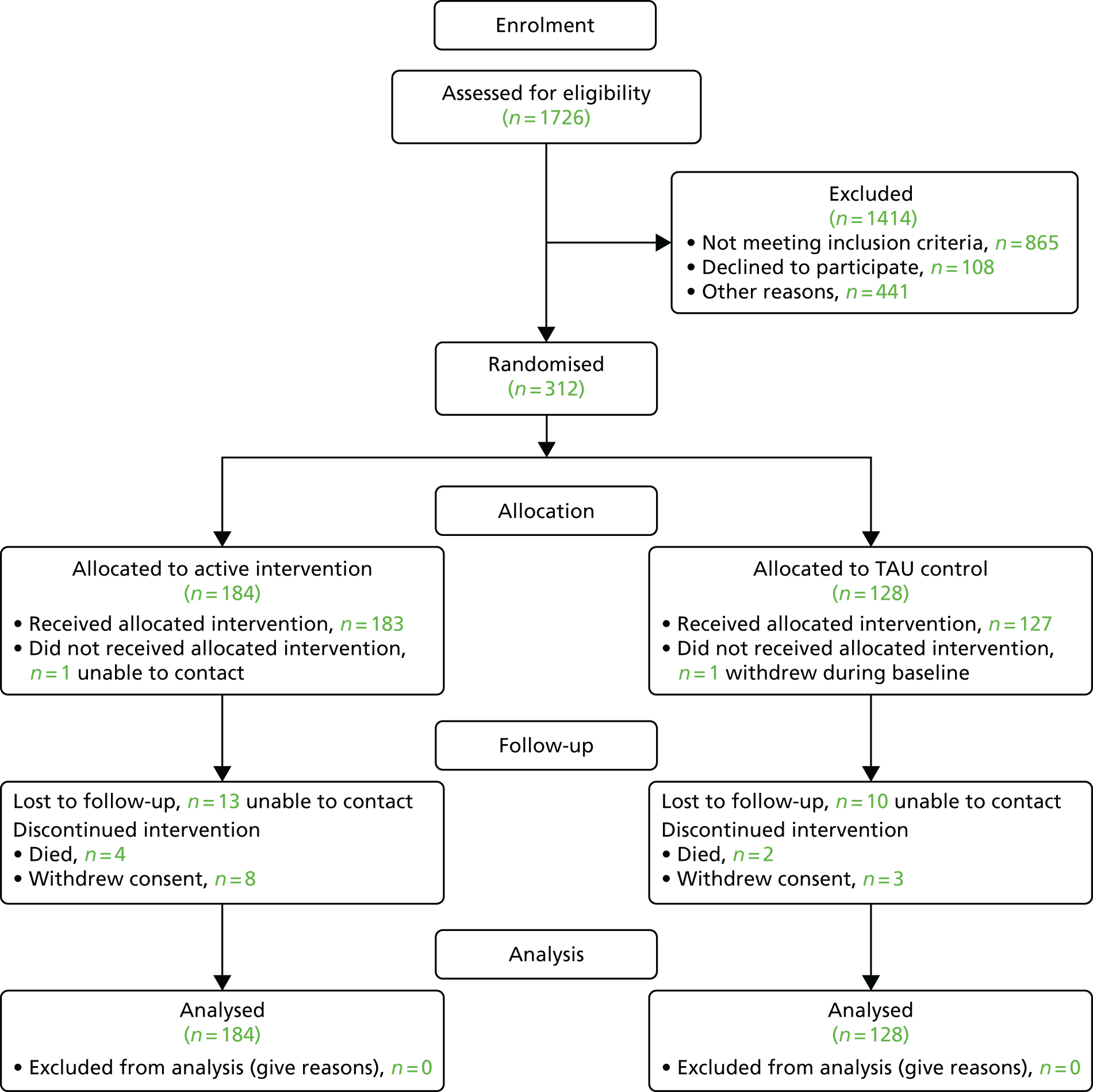
It is important to note that participant recruitment at a site was completed before the King’s Clinical Trials Unit (KCTU) communicated which arm of the trial that site would be randomised to. The involvement of participants in the trial commenced with a 4-week period of baseline observation; this was followed by a minimum of 24 weeks of trial intervention, during which they received either TAU or care according to the competency framework, after which they underwent a 4-week period of follow-up observations. The trial intervention also continued during the follow-up phase.
Assignment of interventions
Randomisation of the clusters was undertaken independently by KCTU using block randomisation with fixed block sizes. A minimum of two sites were randomised at a time to preserve allocation concealment. Site details were submitted to KCTU after potential participants within the centre had been identified. Cluster randomisation to treatment arm took place close to the start of the intervention phase to minimise the risk of clusters withdrawing between randomisation and the start of the trial.
Concealment mechanism
The nature of the active intervention – a change in working protocols for the nurses delivering the intervention – was such that the study could not be fully blind. However, the following measures were taken to minimise the risk of bias being introduced.
-
There was random selection of participants into the trial in each cluster and random allocation of each cluster to treatment arm.
-
To minimise expectations of the participants, their carers and families and the clinical staff at each cluster, they were not informed that there were two arms in the trial (an ‘active’ arm and a TAU arm), nor were they informed which arm of the study they had been randomised to. They were told instead about the range of interventions included in the trial and that patient treatment may or may not change.
-
The individual cluster sites were not informed what the intervention that they were to deliver should look like until the month prior to the intervention phase and they were informed only after participant recruitment at that site had been completed. As indicated above, the information provided to participants and clusters included a degree of obfuscation around the design of the trial.
-
As in the normal course of events, there are some changes from time to time in how epilepsy is managed in an individual with continuing seizures; it was expected that this would blur, to some extent, any perceived variations in management associated with the two arms of the study.
-
The only people working on the trial who were informed which arm a cluster had been randomised to were staff at KCTU undertaking the randomisation process and two senior nurse trainers who provided training to the nurses delivering the trial interventions. The chairperson of the trial Data Monitoring and Ethics Committee (DMEC) could also receive this information but only following a specific request. Such a request was not in fact made during the trial. All other members of the research team remained blind to which arm a cluster had been randomised to.
-
The nurses delivering the treatment interventions were not present while members of the research team collected baseline or follow-up data.
-
The primary outcome measure and the majority of the secondary outcome measures took the form of structured questionnaires and respondents were asked to consider their responses to these questions before reporting them to the researchers collecting the data.
Participants
All participants were adults recruited from adult CIDTs. To be eligible to receive treatment from a CIDT an individual needs to meet administrative criteria defining them as somebody with an ID. The criteria used in the UK are described in Chapter 1, A note on terminology.
Eligibility criteria
Inclusion criteria
-
Aged 18–65 years.
-
The presence of a developmental ID with an IQ of ≤ 70.
-
A diagnosis of epilepsy with a history of at least one seizure in the 6 months preceding recruitment into the trial (not considered by those managing the epilepsy to have been a non-epileptic seizure).
-
Nurse in the CIDT with a current role in delivering some aspects of epilepsy management at the time of both screening and consent.
-
Family carers, paid support workers and nurses were eligible for the semistructured interviews comprising the qualitative element of the trial if they had participated in the trial and had indicated a willingness to be approached for an interview.
Exclusion criteria
-
The presence of a rapidly progressive physical or neurological illness.
-
Alcohol or drug dependence.
The intervention
The experimental intervention was the Learning Disability Epilepsy Specialist Nurse Competency Framework. 31 This provides guidelines describing a structure and goals to support the delivery of epilepsy care and management by LD-trained nurses. The guidelines were developed by the UK EpilepSy Nurses Association (ESNA) in association with the UK Royal College of Nursing.
The competency framework provides a collection of competencies considered by the authors of the framework to be central to effective clinical performance. It describes a series of interventions that can be taken in clinical, educational and professional domains relevant to the optimal delivery of epilepsy management in adults with an ID and epilepsy, tailored to the competency level of the nurse delivering the interventions. The definitions and assessment of competency level, which are described in the competency framework,31 are derived from Benner’s five-level model of nursing competence34 and enable all nurses working in the field to be allocated to one of three levels of practice: ‘novice’, ‘competent’ and ‘expert’. The framework itself, as described in the ESNA document,31 addresses the nine domains listed in Box 1.
A. Clinical diagnosis and management of epilepsy.
I. Diagnosis of epilepsy.
II. Assessing and managing seizures.
(i) Assessing, planning, implementing and evaluating care.
III. Assessing and managing medicines.
(i) Antiepileptic drugs.
(ii) Emergency medication.
IV. Assessing and managing linked health conditions.
B. Assessing and managing risk.
C. Impact of epilepsy.
I. Assessing and managing the impact of epilepsy.
II. People with ID, families and carers.
D. Capacity and consent to treatment.
E. Personal planning and organisation.
I. Autonomy, accountability and management.
II. Telephone management relationships.
III. Time management.
F. Multidisciplinary team working.
G. Personal and professional development.
H. Evidence-based practice.
I. Development of educational programmes.
Adapted with permission from Doherty et al. 31 (pp. 16–17). © 2013 ESNA. All rights reserved.
For each of the nine domains, the specific actions that nurses should take at the appropriate level of competence are split into performance criteria and the knowledge and understanding that would enable these levels of performance. For example, under the domain of ‘assessing and managing linked health conditions’, the following performance criteria are listed.
-
For a novice nurse to:
-
identify any common links between LD diagnosis and epilepsy prognosis
-
carry out a basic health assessment, including developing health action plans
-
discuss recognised LD conditions and syndromes linked to epilepsy
-
demonstrate a basic understanding of the relationship between aetiology, diagnosis and prognosis
-
complete health assessments
-
direct the patient to their GP for an annual health check within an individual’s epilepsy management plan
-
-
For a competent nurse to:
-
demonstrate an understanding of the risk factors of developing epilepsy depending on the patient’s LD syndromic classification, for example, Down Syndrome
-
discuss the link between seizure control and physical ill-health
-
take a written history which includes aetiology of LD, epilepsy syndrome, seizure diagnosis and treatment neurological conditions
-
discuss recognised epilepsy syndromes and potential impact on learning and development
-
confidently discuss with others the evidence-based relationship between aetiology and diagnosis with seizure presentation and treatment prognosis
-
understand the potential link between physical ill health, seizure frequency and AEDs.
-
-
For an expert specialist nurse:
-
to interpret and influence practice
-
to assess the relationship between epilepsy and concomitant conditions, in particular chest infection, dysphagia, sleep disorder, diabetes
-
to assess the impact of epilepsy on the individual’s mental health status and/or behaviour
-
to assess and record the impact of syndrome-specific features
-
to assess and respond to the relationship between epilepsy and concomitant conditions
-
to consider the overall impact of epilepsy in relation to specific conditions, which may be exacerbated by seizures and or treatment and vice versa, and the health and well-being of the individual
-
to manage potential ill-health implications of long-term medication administration.
-
General guidance on how an individual nurse is placed at the appropriate level of competence is provided in the competency framework (pp. 14–15). 31 Each nurse’s competence was based on their own self-rating of their level of competence on the following domains: clinical diagnosis and management of epilepsy, impact of epilepsy, capacity and consent to treatment and multidisciplinary team working. Competency was determined for each nurse delivering management at every site participating in the trial. This determination took place during the trial-specific training received by each nurse shortly before the end of the follow-up period at each site.
The activities undertaken by nurses delivering the intervention were recorded by them in a daily activity diary for subsequent analysis (a copy of the diary template is provided in Appendix 1). The diaries provided a record of the reasons for carrying out each intervention through the duration of the trial and the care delivered during the interventions.
No potential treatments were precluded by being in the trial. A key element of the competency framework is that it is not a manualised treatment guideline for epilepsy but rather a list of what management a nurse should be able to deliver at their given level of competence. However, what management is actually delivered will also depend on the way in which individual clinical services are arranged. Within these constraints, nurses delivered their interventions at a frequency determined by clinical need, through home visits, telephone clinics and visits to the local primary care or ID team base as appropriate. When nurses considered that it was appropriate, and as described in the competency framework, they also delivered epilepsy education to patients and carers. In addition, interactions with other clinicians, for example participants’ primary care health service, local community ID health team and/or local neurology service, were facilitated by the nurses as and when they considered clinically appropriate.
The control condition
The control condition was TAU. This was the existing management approach for each participant. As was the case for the participants randomised to the competency framework arm, no potential treatments were precluded by being in the trial. The activities undertaken by each nurse in the control arm when interacting with any of the participants recruited into the trial in the control clusters were also recorded in a daily activity diary for subsequent analysis.
Training of nurses delivering the active or control interventions
All of the nurses involved in delivering management to participants in the competency framework and TAU arms received trial-specific training. This was led by two senior specialist nurses, experienced in nurse education, research and epilepsy. Nurses from both arms received training on the general principles associated with undertaking a clinical trial, the eligibility criteria for participants in the EpAID trial and how to complete the daily activity diary. In addition, each nurse had their competency level established according to the three levels described in the competency framework, by discussing with the trainer their level of expertise under each of the headings described in Box 1. The training was delivered at the nurses’ clinical base and staff from each cluster were trained separately.
For nurses in the TAU arm the training lasted for approximately 3 hours. Those nurses working in sites allocated to the active intervention also received 3 hours of additional training specifically focusing on the competency framework. This consisted of a workshop in which each of the competencies was considered in detail, making use of guided discussion and reflections by each nurse on his or her own working practices. The learning objectives for the competency-specific training were for each nurse to understand the concept of nursing competencies, identify his or her individual level of expertise based on Benner’s nursing competency framework,34 understand how the competency framework should be used in practice, explain how competencies relate to the NHS Knowledge and Skills Framework35 and identify the competencies that they should attain based on their level of expertise in relation to the diagnosis of epilepsy, classification of epileptic seizures, assessing and managing seizures, assessing, planning, implementing and evaluating care, collecting clinical information (seizure frequency, side effects, behavioural symptoms and effects of seizures on daily life from patient and carers), assessing and managing the impact of epilepsy and QoL, working with people with an ID, their families and carers, offering education, support and advice and directing to additional services.
The training was provided by a nurse familiar with the competency framework and experienced in the management of epilepsy in adults with IDs. The framework itself was used as the core text for the training. A copy was given to each nurse and training was provided in how to make use of the framework document for individual ongoing clinical practice and development.
For each cluster it was planned that the training would take place at the end of the baseline period. At some sites, some nurses were trained earlier in the baseline period; when that happened for nurses in the competency framework arm, they were asked by the trainer not to make use of the framework until the intervention period started.
Trial setting
The trial took place within secondary care services in the community. Participants were recruited from CIDTs and the trial interventions were delivered by telephone or in face-to face contact in participants’ homes or community day centres or in clinics held in CIDT bases by nurses working with the teams.
End points: definitions and acquisition
Primary end point
The primary end point was the ELDQoL-SSS32 (see Appendix 2). This was completed by carers and provided a detailed measure of the carers’ views of the physical severity of seizures experienced by participants in the preceding 4 weeks, including any associated injuries and the level of distress manifested by participants after a seizure. Data were collected for 4 weeks at baseline, prior to the start of the intervention (time point B2; Table 1) and again for 4 weeks after at least 24 weeks of intervention (time point F, see Table 1). Possible scores ranged from 10 to 56, with higher scores indicating greater concerns of informants regarding participants’ seizure severity. If a participant had no seizure during the preceding 4 weeks then this was noted. In the statistical analysis of the seizure severity scale (SSS) scores, when a participant had not had a seizure during the 4 weeks covered by the SSS, the lowest possible SSS score was imputed.
| Form | Content | Time point | Ongoing | Withdrawal | |||
|---|---|---|---|---|---|---|---|
| Screening (B1) | Baseline (B2) | Intervention | Follow-up (F) | ||||
| Registration form | Year of birth, sex | ✗ | |||||
| Demographics | Epilepsy management, ethnicity | ✗ | |||||
| Eligibility form | Inclusion/exclusion criteria | ✗ | |||||
| ELDQoL | Individual items of each ELDQoL subscale | ✗ | ✗ | ✗ | |||
| Epilepsy and ID history form | Epilepsy history (diagnosis, seizure type, triggers), level of ID | ✗ | |||||
| CSRI | Accommodation, care received | ✗ | ✗ | ||||
| MCSI | Individual items of the MCSI | ✗ | ✗ | ||||
| EQ-5D-5L | Individual items of the EQ-5D-5L | ✗ | ✗ | ||||
| Seizure diary (for baseline and follow-up) | Number of each type of seizure (collected daily for 4 weeks) | ✗ | ✗ | ||||
| Enrolment form | Date of enrolment | ✗ | |||||
| Seizure diary (clinical) | Number of each seizure type | ✗ | |||||
| WTP | Individual items of WTP | ✗ | |||||
| Visit information | Information about visit (change of accommodation, carer present) | ✗ | ✗ | ||||
| Medication list | Types of medication, dose, frequency | ✗ | ✗ | ||||
| Serious adverse event form | Date, seriousness, effects, causality (related to EpAID trial or not) | ✗ | |||||
| Withdrawal form | Date of and reason for withdrawal from the study | ✗ | |||||
| Nurse self-completion activity diary | Date and time of visit, location, reasons for intervention, care given | ✗ | |||||
| Clinical information at follow-up | ✗ | ||||||
| Nurse registration form | Nurse details (year of birth, sex, cluster, competence level) | ✗ | |||||
Secondary end points
Economic evaluation
This consisted of three separate but related evaluations. First, a cost-effectiveness analysis was undertaken on the primary outcome measure of the trial, the ELDQoL-SSS score. Second, a cost–utility analysis was undertaken after calculating changes in quality-adjusted life expectancy associated with the intervention, derived from measurements of QoL captured using the EuroQoL-5 Dimensions, five-level version (EQ-5D-5L)36 (described in more detail in Economic analysis methodology). The EQ-5D-5L was collected at baseline and after at least 24 weeks of intervention (time point F; see Table 1), to be used in conjunction with population survey data to calculate quality-adjusted life-years (QALYs). Third, a cost–benefit analysis was undertaken for the subset of patients living with a friend or relative in which the benefit of the intervention was assessed in monetary terms. Resource use was captured using data recorded on a modified version of the Client Service Receipt Inventory (CSRI)37 over a 4-week period at baseline (time point B2; see Table 1) and again for 4 weeks after at least 24 weeks of intervention (time point F; see Table 1).
Carer preferences
Carer preferences were identified using willingness-to-pay (WTP) methods. Preferences were collected from an adult with primary responsibility for the care of a participant using a questionnaire at follow-up designed to elicit a monetary valuation of the intervention. Data were collected at least 24 weeks after commencement of the intervention (time point F; see Table 1).
Number of seizures per month
The number of seizures per month was calculated from daily entries by participants’ carers in a seizure diary. Seizure frequency data were collected to record the total number of tonic–clonic seizures and the total number of all other seizures experienced by participants during a 4-week period at baseline (time point B2; see Table 1) and again for a 4-week period after at least 24 weeks of intervention (time point F; see Table 1).
Epilepsy and Learning Disabilities Quality of Life subscales for antiepileptic drug side effects, mood and behaviour
These data were collected for 4 weeks at baseline, prior to the start of the intervention (time point B2; see Table 1) and again for 4 weeks after at least 24 weeks of intervention (time point F; see Table 1). The total possible scores ranged from 19 to 76 for the AED side effects profile, from 9 to 36 for the behaviour scale and from 16 to 64 for the mood scale. For each of the subscales, higher scores indicate poorer QoL/functioning.
Modified Caregiver Strain Index38
This instrument was employed to measure the possible effects of the intervention on emotional, financial and practical stresses experienced by carers. The Modified Caregiver Strain Index (MCSI)38 was collected in the baseline period and again after at least 24 weeks of intervention. The MCSI was collected only from family respondents identified as a family member in response to question V6 of the relevant visit information questionnaire [i.e. at baseline (time point B2; see Table 1) and follow-up (time point F; see Table 1)]. Possible MCSI scores range from 0 to 26, with higher scores indicating a higher strain on the carer.
A series of semistructured interviews with clinicians, family and paid carers
These interviews were conducted to examine how the competency framework, compared with TAU, affected relationships between the nurses and family carers/paid support workers with respect to (1) reported perceptions of patient health and QoL, (2) the involvement of patients in treatment decisions and (3) the active engagement of carers with clinical epilepsy services. The interviews were conducted by telephone. They lasted between 20 and 30 minutes and were audio recorded and transcribed.
The nurse self-completion daily activity diary
The nurse self-completion daily activity diary was completed throughout the trial by all of the nurses delivering any intervention to any participant in either arm. These self-completion daily activity diaries aimed to provide a reliable account of epilepsy-related nursing activity on a daily basis and at relatively low cost. 39 They have been used successfully as a data collection method in a number of studies,30,40,41 in which no difficulties were encountered in relation to attrition, missing data or failure to complete. The diaries were prefaced with instructions for completion and a model example of how entries should appear. Diary entry was expected to take each nurse approximately 15 minutes a day. For each visit, the diaries were used to record the start and end time of the visit, where the visit took place (home, clinic, general practice, by telephone, other), the reasons for intervention (1. assessment; 2. counselling; 3. education; 4. health facilitation; 5. management planning; 6. monitoring epilepsy; 7. monitoring health/behaviour; 8. monitoring treatment; 9. responding to urgent health or behavioural concerns; 10. other) and details of the care given (1. education of family carer; 2. education of paid staff; 3. education of patient; 4. health facilitation; 5. investigation request; 6. management planning; 7. medication issues; 8. prescribing; 9. review and monitoring of medication; 10. other). Multiple reasons for intervention and details of care given at any given visit could be recorded.
Descriptive measures
The community intellectual disability team epilepsy service availability questionnaire
This was completed by psychiatric and nursing staff employed in each cluster. It was used to describe the resources for epilepsy treatment available to the CIDTs locally at each cluster and the approach employed in making use of these resources.
Demographic and clinical data
Demographic and clinical descriptors of participants were collected from participants’ clinical notes at baseline. The descriptors included the level of ID (mild, moderate, severe, profound), the nature of their accommodation (living alone, living with family, living in a group home or other community residential care setting) (also collected at follow-up), sex, age, current antiepilepsy treatment (also collected at follow-up) and any additional ID syndrome, psychiatric and neurological diagnoses.
Safety procedures and adverse events
The approach to defining, identifying and recording safety procedures and adverse events is described in Appendix 3.
Data collection methods
The consenting of participants and the collection of baseline and follow-up data were undertaken either by research assistants employed by the University of Cambridge or by Clinical Research Network (CRN) nurses employed at the cluster sites. Consent was always obtained face to face. The majority of the data were collected over the telephone by research assistants from family carers or paid support workers who knew the participants well. The informants were sent the questionnaires in advance and their answers were noted by a research assistant during a prearranged telephone conversation. Qualitative interviews were conducted by telephone and audio recorded for later transcription. A WTP health economic questionnaire was collected over the telephone during the follow-up phase.
The trial employed an electronic case report form (eCRF) created by KCTU in collaboration with the trial statistician and the chief investigator using the MACRO Electronic Data Capture system (version 4; InferMed, London, UK) and maintained by KCTU. It was hosted on a dedicated secure server within KCTU’s host academic institution. The system is regulatory compliant42–44 with a full audit trail, data discrepancy functionality and database lock functionality and supports real-time data cleaning and reporting.
Clinical outcome analyses
Analyses were undertaken to assess the efficacy of the use of the competency framework in comparison to TAU in reducing the severity of seizures and improving QoL outcomes and to provide a measure of how cost-effective this intervention was.
Primary efficacy analysis
The primary analysis assessed the efficacy of ENS-led care in comparison to TAU in reducing the severity of seizures. The primary analysis was an intention-to-treat (ITT) analysis and the primary outcome was the difference in ELDQoL-SSS score between baseline and follow-up. Because the trial was cluster randomised and a clustering effect was expected, a linear mixed-effects model was fitted. Random effects to model the effect of cluster were included. Baseline patient-level covariates included were ELDQoL-SSS score, age, level of ID (mild, moderate, severe or profound), number of tonic–clonic seizures per month, living circumstances (living independently, living with family members or living in a group home) and deprivation index of cluster area. Cluster-level covariates included were mean seniority level across the cluster of the nurses working in the cluster, to investigate possible therapist effects, and mean caseload of the nurses working in the cluster. Multiple imputation (MI) was performed when > 3% of the covariates were missing.
The null hypothesis was that the treatment effect is zero. The alternative hypothesis was that there is a difference in the change in ELDQoL-SSS score from baseline to follow-up between the competency framework arm and the TAU arm. A Wald test for the effect of the intervention was used in the primary analysis.
The following planned exploratory subgroup analyses were performed:
-
interaction between treatment and number of seizures
-
interaction between treatment and number of seizure types (one seizure type, more than one seizure type)
-
interaction between treatment and level of ID (mild/moderate, severe/profound)
-
interaction between treatment and living circumstances (group home, with family members, independently, other)
-
interaction between treatment and baseline ELDQoL-SSS score (above the median, below the median)
-
interaction between change in MCSI score and baseline number of tonic–clonic seizures.
Secondary outcomes were analysed using a suitable mixed-effects model (linear mixed-effects model for continuous outcomes and a Poisson generalised linear mixed-effects model for count data) or by accounting for the clustering using robust Huber–White standard errors. Secondary clinical end points included the change in MCSI score, the number of seizures and the change in other subscales of the ELDQoL (AED side effects, mood and behaviour). The covariates used for the analysis of the secondary outcomes were the same as those included for the primary outcome measure. Each secondary end point was analysed including its baseline value as a covariate.
Derived variables
The methods used to convert the individual items on each ELDQoL subscale into a total subscale score are provided in Appendix 4. The 13 individual items on the MCSI can be scored as 0, 1 or 2. To obtain the total MCSI score the sum of the individual items is calculated. When half or fewer than half of the MCSI items were missing, the total MCSI score was calculated as the mean of the present items multiplied by the total number of items in the instrument. If more than half of the items were missing the score was set as ‘missing’.
The primary outcome of the trial – the difference in ELDQoL-SSS score – was calculated as the follow-up ELDQoL-SSS score minus the baseline ELDQoL-SSS score. The secondary outcomes of MCSI score, number of seizures per month and ELDQoL subscale scores for AED side effects, mood and behaviour were also calculated by subtracting baseline values from follow-up values. The outcome of number of seizures per month included the number of tonic–clonic seizures rather than the total number of seizures. This figure was used as earlier pilot work had demonstrated that the recorded frequency of tonic–clonic seizures is relatively reliable but that counts of other seizure types, such as focal seizures, absence seizures or atonic seizures, are less so, as the phenomena in question may be either missed (on account of a very brief duration or subtle manifestation) or misdiagnosed.
The variable age was derived using the date of the visit and the year of birth. The exact date of birth was not recorded and so date of birth was assumed to be 30 June in the year of birth.
The competence level of the nurses was scored as follows: novice = 1, competent = 2 and expert = 3. An overall nurse competence score for each cluster was calculated by taking the mean competence score for all of the nurses who worked with the trial participants in that cluster. For example, if a cluster had three novice, six competent and five expert nurses, the nurse competence score for the cluster was 2.14.
The caseload of the nurses in each cluster was calculated as the mean number of patients that each nurse working in a cluster was treating.
The deprivation index involved ranking all of the postcodes, with the most deprived postcode being given a ranking of 1. Postcodes were ranked within England, Scotland and Wales and so the ranks are not comparable. The deprivation index was therefore converted into a categorical variable ranging from 1 to 5 based on the quintiles of the ranks, with the most deprived 20% of areas given a score of 1 and the least deprived 20% given a score of 5. It should be noted that, although quintiles are more directly comparable than ranks, they may still differ across countries because of differences in poverty between countries.
Assessment of treatment delivered (as recorded in the nurse activity diaries) was analysed as follows. Summary statistics for the duration of the visits and where the visits took place were produced by arm. The absolute number of visits by arm was calculated for each reason for intervention and details of the care given are provided. The proportion of visits by arm was also calculated for each reason for intervention and details of the care given are provided.
Sample size calculation
As this was a cluster randomised trial, the true value of the intraclass correlation coefficient (ICC) would have a large impact on the power of the trial. Data from an earlier observational study of epilepsy management in adults with an ID45 were used to estimate the ICC for the ELDQoL-SSS. The estimated ICC was close to 0, but with a wide confidence interval (CI). We chose to power the study for the change in ELDQoL-SSS score between baseline and follow-up at 24 weeks assuming an ICC of 0.05, which was above the estimated value in the earlier observational study. The estimated standard deviation (SD) for the change in ELDQoL-SSS score from this observational study was 6.55.
When planning the trial we originally aimed to recruit 12 clusters (six in each arm) of 30 patients to provide 90% power at a one-sided 0.025 significance level to detect a true mean intervention effect of 3.6. However, because of a lower than expected number of eligible patients per cluster, we modified the trial’s sample size to assume 15 patients per cluster. In this case, 16 clusters (eight in each arm) would provide 90% power at a one-sided 0.025 significance level using the same SD, target effect size and ICC value.
Timing of the analyses
The final analyses were performed after the collection of follow-up data from all of the clusters and after data cleaning and locking had taken place.
Analysis population
The primary and all secondary end points were analysed using all of the participants in each cluster who were randomised to the competency framework intervention or to TAU, who were not lost to follow-up.
Safety population
Safety data were analysed using all of the participants who received either the competency framework intervention or TAU for any length of time. Participants who dropped out before receiving either treatment were excluded.
Missing data
Covariate data
The primary analysis included participants in whom the outcome was observed. As a linear mixed-effects model was used, missing outcome data were dealt with using a missing at random (MAR) assumption. When > 3% of data for covariates were missing, MI was used for the primary analysis. Five imputations were used unless the percentage missingness was greater than this. In this case, the number of imputations was equal to the percentage missingness (e.g. if 10% of participants were missing baseline data, we would use 10 imputations).
Outcome data
A further analysis was performed in which missing primary outcome data were imputed. Because of the way that the ELDQoL-SSS was scored (see Appendix 4), there were a number of ways that the outcome measure could be missing. These were if the participant:
-
was lost to follow-up
-
attended the visit but did not answer any questions on the ELDQoL-SSS
-
answered < 50% of the questions and therefore was defined as missing.
If a participant had no seizures in the previous month, he or she was unable to answer any of the questions on the ELDQoL-SSS questionnaire and therefore the outcome was missing. In this case, the missingness was informative of the outcome. When this was the reason for a missing baseline or follow-up ELDQoL-SSS score the best possible score on the ELDQoL-SSS scale was imputed as it was assumed that no seizures equates to a low seizure severity score. For this reason, the numbers of participants for whom the outcome was missing (baseline and follow-up) are summarised by arm.
Secondary analyses
Multiple imputation was also performed on missing covariate and outcome data for all secondary clinical analyses in a similar way to that performed for the primary analysis.
Economic analysis methodology
The analyses applied a health and social care perspective as the primary perspective, as recommended by NICE. 46 A societal perspective, which included additional costs falling on patients’ families, was applied in sensitivity analyses.
Data collection for economic analyses
Resource use
We sought to collect data on all resource use relevant to the intervention, but to exclude resource use unconnected to participants’ epilepsy or ID and hence unlikely to be influenced by the intervention. In practice, it was impractical to assess all health or social care resource use for relevance to epilepsy/ID; we did so only for hospitalisations when the risk was greatest for high-cost unrelated episodes to influence the analysis. We used a modified version of the CSRI,47 which we had applied in a previous study of care for adults with epilepsy and ID. 8 The modified CSRI measured resource use relating to accommodation, respite including holidays, primary health and social care, day care, secondary health care including tests and investigations, mode of transport to health-care appointments and informal care. It asked predominantly about the previous month, with the exception of respite care and holidays, in which the relevant recall period was 6 months. In this, we sought to strike a balance between the accuracy of data recall and the potential for seasonal differences to influence the results. In addition, data on medications were collected separately.
Accommodation was recorded using one of four categories: group home, family home, independently or other. The number of residents with special needs in the home was recorded. The number of nights of respite care received and the provider of that respite care were recorded, along with the patient/family contribution to the cost of respite care when applicable. The total number of nights spent on holiday over the last 6 months was recorded, along with the patient/family contribution to the cost of holidays. The numbers of contacts with doctors, nurses, allied health professionals and social workers in the last month were recorded; contacts were categorised as home or office visits and the average duration of contact was recorded. Data were collected on the provision of care assistance in the home, cleaning and laundry services and Meals on Wheels, along with the duration of support when relevant and whether or not the patient or family contributed to the cost. The numbers of visits to day centres, social clubs and drop-in centres, attendance at adult education classes and miscellaneous activities were recorded. The miscellaneous activities were further categorised as one-to-one or group activities. Hospital admissions were categorised as accident and emergency (A&E) department visits, inpatient admissions, outpatient attendances or day hospital attendances and respondents were asked to distinguish between care related to epilepsy and care unrelated to epilepsy. Respondents were also asked to report the mode of transport that participants used to travel to and from hospital. The numbers of radiography scans, electroencephalograms (EEGs), computed tomography (CT) scans and magnetic resonance imaging (MRI) scans received by participants were recorded. Finally, the CSRI included a section that quantified the hours of informal care provided along with the occupation (and hence the potential market wage) of the primary carer (when applicable). This section included further questions on the mode of transport that participants used to attend routine engagements.
Quality of life
Participant QoL was measured at baseline and follow-up using a condition-specific measure and a generic measure. The condition-specific measure was the ELDQoL scale,32 described in End points: definitions and acquisition. The generic measure was the EQ-5D-5L. 48 This measure assesses health functioning across five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The five-level version elicits responses to each dimension at one of five levels, from no problems to severe problems. The resulting responses are not simply summed; a ‘tariff’ or health state value for each of the 3125 combinations of responses has been generated from a survey of the UK population. 49
Questionnaires on QoL were completed by participants when feasible, with help from their primary carer, or by the primary carer when necessary (proxy valuations). We did not attempt to distinguish between patient and proxy valuations for two reasons. First, we considered that the dichotomisation of responses would disguise a continuum of carer involvement in completion of the questionnaires, from modest guidance to full, proxy completion. Second, the analysis considers the change in QoL rather than the absolute value for each participant between baseline and follow-up. As a consequence, the impact of proxy involvement is likely to be attenuated, assuming the same degree of involvement at baseline and follow-up. Proxy valuations have been shown to be accurate for the physical aspects of health functioning, but less so for the mental aspects. 50
A cost–utility analysis was used to evaluate effectiveness. The cost–utility analysis enabled comparison of cost-effectiveness against commonly accepted thresholds of acceptability in terms of the cost per QALY gained. 51 However, given the potential for a lack of sensitivity of the EQ-5D-5L to benefits of the intervention for carers and for participants, we also undertook a cost–benefit analysis. The time horizon of each evaluation was 6 months and the primary perspective was health and social services. A broader societal perspective was considered in sensitivity analyses.
Cost differences across the two arms of the trial were estimated using questionnaires assessing the provision of health and social care at baseline and after 6 months and from time diaries completed by ENSs. QALY gains were estimated as 0.5 × health-related QoL at 6 months after controlling for QoL at baseline, ELDQoL-SSS score, age, level of ID, mean number of seizures per month (calculated as described earlier) and living circumstances (as described earlier).
Willingness-to-pay data were collected from family carers but not paid support workers. They were collected as open-ended responses with the use of prompt cards showing a range of values. Bootstrapping was used to estimate uncertainty in mean costs and outcomes and to facilitate construction of cost-effectiveness acceptability curves (CEACs) for the cost–utility analysis. Bootstrapping refers to a statistical technique used to estimate uncertainty around a parameter without assuming a parametric distribution for the population distribution of that parameter.
Monetary valuation of outcomes
When we could identify an informal carer with primary responsibility for a participant’s welfare, we sought to elicit a monetary valuation of the benefit that he or she perceived from any changes in epilepsy-related support from the nurse(s) working with that participant. At follow-up, carers in both trial arms were asked if they thought that the support that they and the participant had received from their nurse had stayed the same, improved or worsened. If they perceived that there had been a change, we sought a monetary valuation of their preferred service in the form of the maximum monthly payment that they would be prepared to make to maintain that service. We first presented respondents with a series of 13 payment cards containing amounts varying from £1 to £1000 in random order and asked them to sort the cards into amounts that they would pay, amounts that they would not pay and amounts for which they were unsure whether or not they would pay. We then asked for the maximum that they would pay, with prompting regarding the acceptable range indicated by the ‘card sort’.
We took reasonable steps to explain the purpose of the survey and emphasise that the exercise was purely to value the care that participants had received and was not part of a process to determine an appropriate charge for care. However, when respondents perceived a change in care but were unwilling to state a payment value we sought to distinguish responses that indicated a value of zero from those that indicated an unwillingness to provide a positive value (so called protest responses). 52 Respondents who were unwilling to state a value for the care that they had received were asked to select the reason why from a menu of preset responses. The response ‘I do value the support I received but I believe the government should pay for it’ was deemed to indicate a protest response (and hence a missing valuation). All other responses were deemed to indicate a value of zero.
Data preparation for the economic analysis
Valuation of resource use
Unit costs for resource use were taken from three nationally relevant and recognised sources when possible: NHS Reference Costs,53 Unit Costs of Health and Social Care (UCHSC)54 and the British National Formulary. 55 Unit costs for the financial year 2014/15 were applied. When necessary, costs were inflated to 2014/15 prices using the Hospital and Community Health Services inflation index. 54 Costs for primary health and social care contacts were derived from the unit costs per patient contact hour reported in the UCHSC combined with the relevant mean contact time reported by respondents. Only a cost per hour was available for psychiatrists, psychologists and chiropodists. The unit cost was multiplied by 1.35 to estimate the cost per patient contact hour. This figure was derived from the ratio of the cost per hour to the cost per patient contact hour for consultant surgeons reported in an earlier version of the UCHSC. 56 Costs per contact hour for home visits were available in the UCHSC only for occupational therapists. We calculated the ratio of the cost per patient contact hour between home visits and clinic visits for occupational therapists (1.158) and applied this to the relevant clinic visit costs to estimate the unit cost per patient contact hour of home visits for all of the other health-care professionals.
A unit cost of care assistance at home of £24 per hour was taken from the UCHSC. A cost of £5.08 (after inflation) for each meal on wheels was derived from a Health Technology Assessment article. 57 The costs of a cleaner and laundry services were derived from online suppliers. The costs of day care including one-to-one and group activities were taken from the UCHSC. Hospital A&E and outpatient visit and day unit costs were taken from the UCHSC. An inpatient cost of £400 per bed-day was derived from Department of Health estimates published in response to a freedom of information request. 58 Unit costs for tests and investigations were derived from NHS Reference Costs. 53 Emergency ambulance and passenger transport costs were derived from the UCHSC. Hospital car costs were estimated from a BBC report on hospital expenditure on taxis. 59
Informal care was valued at the gross market wage rate for carers when these data were available. In the absence of data, or when carers were retired, informal care was valued at the legal minimum wage rate for 2014/15 (£6.50 per hour from October 2014). Median gross market wage rates by category of employment for 2015 were drawn from data published by the Office for National Statistics60 and converted to an hourly rate, assuming a working week of 32.2 hours. 61 Estimation of car travel costs assumed an 8-mile round trip for a visit by a nurse to a service user, costing 40p per mile.
Quantification of intervention benefits
Responses to the ELDQoL questionnaire at baseline and follow-up were scored according to the recommended algorithms, which included imputing missing responses using the mean score for completed questions for domains in which at least half of the questions had been completed (see Appendix 4).
Responses on the EQ-5D-5L at baseline and follow-up were scored using an algorithm published by the EuroQoL group62 and based on a valuation exercise undertaken with a sample of the UK general population. 49 A missing score was recorded when responses to any of the five dimensions were missing. We assumed that changes in QoL from baseline occurred quickly and that any differences in baseline and follow-up QoL applied to the entire intervening period. QALYs were calculated as the product of time in years and change in QoL. For example, an increase in QoL of 0.1 measured at 6 months from baseline would equate to a QALY gain of 0.05 over that period.
A planned comparison of mean WTP values elicited at 6 months for participants in the intervention and control arms was not undertaken because of the low number of responses.
Economic analysis
Regression analysis was used to determine the impact of treatment on costs and outcomes while controlling for a prespecified list of baseline characteristics at participant and ENS level: baseline QoL (EQ-5D-5L tariff), baseline SSS score (ELDQoL), baseline mood score (ELDQoL), baseline AED side effects profile score (ELDQoL), baseline behaviour score (ELDQoL), costs in month prior to baseline, sex, age, number of tonic–clonic seizures, ID level, neighbourhood deprivation score, nurse workload and nurse competence. ID level entered the regression models as a categorical variable with four levels: mild, moderate, severe and profound. A treatment*ID level interaction term was included to allow exploration of differential impacts on the two subgroups of mild/moderate ID and severe/profound ID. The neighbourhood deprivation score was determined as the Index of Multiple Deprivation derived from participants’ postcodes. 63 Scores were assigned to quintiles according to the relevant national ranking for England, Scotland or Wales. Nurse competence was self-assessed at three levels: novice, competent and expert. The side effect profile score was log-transformed to achieve a distribution that was closer to normal.
Generalised linear modelling was used to examine a range of potential distributions in modelling both costs and EQ-5D-5L tariffs. The Park test64 was used to assess the suitability of alternative distributions and the Hosmer–Lemeshow,65 Pregibon66 and Pearson67 tests were used to test the suitability of different link functions.
Missing data were addressed using MI. 68 Such an approach assumes that any data are MAR, that is, missingness is random contingent on the observed data. Imputation was undertaken using chained equations. Predictive mean matching was used for each variable with missing data to allow for non-normal distributions. 69 Regression models were fitted across 20 imputations and the results were combined using Rubin’s rules to estimate the incremental cost and incremental effectiveness attributable to treatment. The regression-adjusted estimates of the treatment impact on costs and outcomes were used to express cost-effectiveness as the incremental cost-effectiveness ratios (ICERs) for both QALYs and ELDQoL-SSS scores. ICERs were calculated as the cost difference attributable to treatment, ΔC, divided by the difference in outcome, ΔE, attributable to treatment:
Sampling uncertainty in the data was captured by bootstrapping. This ensured that any correlation in costs and outcomes would be reflected in the distribution of cost-effectiveness estimates. A total of 1000 bootstrap replicates were created. Missing data were imputed using MI, with 20 imputations undertaken on each bootstrap replicate. Regression models were fitted across the 20 imputations and the results were combined using Rubin’s rules to estimate the incremental cost and incremental effectiveness attributable to treatment. Thus, a single estimate for the impact of treatment on costs, SSS score and QoL (EQ-5D-5L tariff) was derived for each bootstrap replicate after adjusting for baseline differences and imputing missing data.
The bootstrap replicates were used to plot CEACs. For each bootstrap replicate the net monetary benefit was calculated by multiplying the difference in outcomes attributable to treatment by a threshold value and subtracting the difference in costs attributable to treatment. Calculations were undertaken for a range of threshold values starting at £0. For each threshold value the proportion of the bootstrap replicates in which the net monetary benefit was greater than £0 (treatment is cost-effective at that threshold) was calculated. The CEAC could then be plotted as the proportion of bootstrap replicates with a positive net monetary benefit (cost-effective) as a function of the threshold value. The threshold values were varied from £0 to £50,000 per QALY and from £0 to £2000 per point on the SSS.
Sensitivity analyses
The primary economic analysis took a health and social care perspective as recommended by NICE. 46 However, a considerable part of the care and support of adults with epilepsy and ID is likely to fall on their family members. In the sensitivity analysis we considered a broader perspective, including costs falling on participants or their family members and the impact of informal care. Transport, respite and holiday costs falling on families were included. Informal care, valued as described in Valuation of resource use, was also included. Cost-effectiveness was reported in the form of ICERs and CEACs generated from the bootstrap replicates after inclusion of the additional costs.
Subgroup analysis according to ID level was undertaken by inclusion of a treatment*ID level interaction term in the imputation and regression models. ID level was dichotomised into mild/moderate and severe/profound. Missing data in the interaction term were imputed using the ‘just another variable’ approach as recommended. 70
Accommodation costs were large and subject to greater variation between baseline and follow-up in the TAU arm than in the treatment arm. There was a possibility that inaccurate costing or changes unrelated to the intervention might have driven the relative differences between the intervention arm and the TAU arm. A sensitivity analysis was undertaken to examine health and social care costs and EQ-5D-5L tariff values after excluding accommodation costs.
Finally, the analytical approach used, in which the data were bootstrapped and then imputed, did not allow for explicit recognition of clustering by site in the bootstrap routine. A sensitivity analysis was undertaken in which the data were first imputed (20 imputations) and then the differences in costs and outcomes between the intervention arm and the TAU arm were estimated on each imputation using a two-stage bootstrap routine. 71
Qualitative analysis methodology
The semistructured interviews were analysed using a systematic process of indexing and interpretation. Answers to individual questions were summarised and then examined for content, with emergent themes identified and coded. 72 We examined how the competency framework, compared with TAU, affected relationships between the ENSs and family members/paid carers with respect to (1) reported perceptions of patient health and QoL, (2) the involvement of patients in treatment decisions and (3) the active engagement of carers with clinical epilepsy services.
Trial governance
The trial was overseen by a Trial Steering Committee and a DMEC, both of which had an independent chairperson. These committees were constituted and worked according to the research governance guidelines issued by the National Institute for Health Research (NIHR)73 and the Medical Research Council. 44
In addition, a Trial Advisory Group (TAG) was developed to provide ongoing public and patient observation of the trial and advice. The TAG members included the mother of a young woman with a severe ID and complex epilepsy, the manager of a community residential home for adults with an ID and epilepsy and a representative from the charity Epilepsy Action who was also able to consult through that charity’s volunteer and adviser contacts. The group considered and advised on issues relevant to recruitment and the retention of participants, the outcome measures employed in the trial and the dissemination of findings.
The trial safety procedures are described in Appendix 3.
Research ethics approvals
The trial received ethics approval for England and Wales from the National Research Ethics Service (London Queen Square Committee) and for Scotland from the Scotland A Research Ethics Committee. Amendments were reported to all study sites and the trial oversight committees. To enable inclusion of adults lacking the capacity to decide whether or not to participate in research, appropriate approvals were sought from family or care providers in line with sections 30–34 of the Mental Capacity Act 200574 (England and Wales) or section 51 of the Adults with Incapacity (Scotland) Act 2000. 75
Chapter 3 Results
Recruitment and attrition of participants
Figure 3 presents the CONSORT diagram describing the flow of potential participants through the trial. In total, 312 individuals were recruited into the study. Of the 17 research sites included in the trial, eight were randomised to the framework intervention and nine to TAU. A total of 128 participants were recruited in sites randomised to TAU and 184 were recruited in sites randomised to the competency framework. The numbers entering the trial at each site are provided in Table 2. Recruitment of research sites into the trial was staggered, with the first site recruited in September 2014 and the final site recruited in September 2015. Follow-up data collection was completed in October 2016.
FIGURE 3.
The EpAID trial CONSORT diagram. This figure has been reproduced from Ring et al. 1 © Ring et al. 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
| Site | Number of participants |
|---|---|
| 1 | 28 |
| 2 | 24 |
| 3 | 10 |
| 4 | 16 |
| 5 | 22 |
| 6 | 20 |
| 7 | 20 |
| 8 | 20 |
| 9 | 23 |
| 10 | 17 |
| 11 | 10 |
| 12 | 18 |
| 13 | 5 |
| 14 | 34 |
| 15 | 17 |
| 16 | 12 |
| 17 | 16 |
The greatest attrition of potential participants from the total of those initially screened for eligibility arose as a consequence of 50.1% of those screened not meeting the trial eligibility criteria. This is not surprising as the screening populations used by the recruitment sites were all patients with an ID and epilepsy, whereas the trial criteria required there to have been at least one seizure in the 6 months prior to the screening date, thereby ruling out, by design, those whose seizures were well controlled. Of those who were considered on screening to be eligible for the trial, there were three main causes of attrition: an inability to contact potential participants during the recruiting window, accounting for 30% of those screened as eligible but who did not proceed to enter the trial; no agreement from potential participants for their contact details to be passed to the research team, accounting for 29% of those screened as eligible who did not proceed to enter the trial; and refusal by the participant (or their consultee) to agree to participate in the trial, accounting for 20% of those screened as eligible who did not proceed to enter the trial.
Of those entered into the trial, 41 (13%) either withdrew from the trial (n = 35) or died (n = 6) between the start of the baseline period and the end of the follow-up period. In addition, a further two participants died after their follow-up data had been collected but before the trial had been closed at the sites in which they were being treated.
There was no significant difference in the rates of withdrawal between the two arms of the trial. Between the start of baseline recording and the end of the follow-up period, 24 participants (13%) (including four who had died) withdrew from sites at which the competency framework was being trialled and 17 participants (13%) (including two who had died) withdrew from sites at which TAU was being delivered. Excluding death, withdrawals occurred for one of three reasons. The most frequent reason was the failure of the research team to be able to make contact with the participant or their informant (23 cases), with withdrawal being at the request of the family member or paid carer acting as the informant in nine cases and at the request of the participant in three cases.
The numbers of missing data sets for the baseline and outcome measures are reported in Appendix 5. These numbers include data missing both as a result of a participant being withdrawn and as a result of that particular outcome measure not being completed. The data presented in the results tables include the number of relevant data sets included in each instance.
Characteristics of the clinical teams taking part in the trial
The relevant staff skill mix and working practices of the contributing community teams were assessed using the CIDT epilepsy service availability questionnaire (see Appendix 6). These data are reported in Box 2. In addition, to help provide an indication of how representative the services participating in the trial were of the wider population of CIDTs, Box 2 also provides comparative data from a previous survey of 53 CIDTs that took place 3 years before the EpAID trial commenced (four of the CIDTs who contributed to the first survey also took part in the EpAID trial) (unpublished data held by Dr Howard Ring). There were a range of differences between the teams in which the EpAID trial took place, in terms of both the way that the teams delivered epilepsy management and the staff and resources that they had access to to do this. However, these variations generally resembled the variations observed in the earlier survey, suggesting that the services hosting the EpAID trial were relatively representative of CIDTs in the UK more generally. The range of differences in the CIDT teams engaged in the EpAID trial reflects the impression gained by members of the research team when visiting the research sites that the way in which epilepsy treatment was delivered by CIDTs varied between teams.
England (n = 13), Scotland (n = 2) and Wales (n = 2).
England (n = 47), Scotland, (n = 2), Wales (n = 1), Northern Ireland (n = 1), Channel Islands (n = 1) and the Republic of Ireland (n = 1).
How many patients in total are looked after by the service?16 responses (94%); mean = 925, median = 638, range = 120–3500.
38 responses (72%); mean = 3437.79 (with one outlier reporting a total catchment population of 110,000), median = 400, range = 60–110,000.
How many adults looked after by the service have a diagnosis of epilepsy?8 responses (47%); mean = 299, median = 207, range = 50–600.
34 responses (36%); mean = 123, median = 78, range = 0–550.
Does the service maintain an updated epilepsy register?17 responses (100%); no = 12 (71%), yes = 5 (29%).
52 responses (98%); no = 45 (86.5%), yes = 7 (13.50%).
Does the service run an epilepsy clinic?17 responses (100%); no = 10 (59%), yes = 7 (41%).
52 responses (98%); no = 31 (60%), yes = 21 (40%).
Who delivers the epilepsy clinic?7 responses (100% of eligible responses); ID psychiatrist + ID nurse (n = 5), ENS (n = 1); visiting neurologist (n = 1).
15 responses (71% of eligible responses); ID psychiatrist + ID nurse (n = 4), ID psychiatrist + ENS (n = 4), ENS (n = 2), visiting neurologist (n = 1), ID consultant (n = 2), ID consultant + neurology consultant (n = 2).
Does the community ID service include a consultant psychiatrist with a particular interest in epilepsy?17 responses (100%); no = 8 (47%), yes = 9 (53%).
53 responses (100%); no = 9 (17%), yes = 44 (83%).
CIDT members15 responses (88%).
-
Consultant psychiatrist:
100% of responding teams have at least one; median = 2, range = 1–7
92% of responding teams have at least one; median = 1, range = 0–8.
-
Non-consultant doctors:
63% of responding teams have at least one; median number = 1.5, range = 1–6
71% of responding teams have at least one; median = 1, range = 0–8.
-
Community ID/LD nurses:
94% of responding teams have at least one; median = 6, range = 0–19
86% of responding teams have at least one; median = 4, range = 0–35.
-
ENSs:
44% of responding teams have at least one; median = 0, range = 0–3
21% of responding teams have at least one; median = 0, range = 0–2.
-
ID/LD nurses with a special interest in epilepsy:
81% of responding teams have at least one; median = 1.5, range = 0–9
61% of responding teams have at least one; median = 1, range = 1–6.
What role(s) does the epilepsy nurse specialist/intellectual disability nurse with a special interest in epilepsy have in epilepsy management delivered by your service?17 responses (100%).
46 responses (87%).
-
Initial assessment of epilepsy:
undertaken in 14 (82%) services
undertaken in 30 (65%) services.
-
Ongoing follow-up of epilepsy:
undertaken in 14 (82%) services
undertaken in 37 (80%) services.
-
Training/care plan writing for emergency medication for prolonged seizures:
undertaken in 17 (100%) services
undertaken in 42 (91%) services.
-
Telephone contact to support and advise family:
undertaken in 16 (94%) services
undertaken in 40 (87%) services.
-
Visits to support and advise families:
undertaken in 16 (94%) services
undertaken in 43 (94%) services.
-
Liaison with other services:
undertaken in 17 (100%) services
undertaken in 38 (83%) services.
Do intellectual disability services without an epilepsy nurse specialist have access to epilepsy nurse specialists in other clinical services?11 responses (100% of eligible responses); yes = 10 (91%).
52 responses (98% of eligiable responses); yes = 31 (60%).
Can the community intellectual disability service directly access relevant investigations for epilepsy (e.g. electroencephalography, brain imaging)?17 responses (100%); yes = 10 (59%).
51 responses (96%); yes = 45 (88%).
Does the service provide training to carers about emergency treatment of seizures?17 responses (100%); yes = 16 (94%).
53 responses (100%); yes = 52 (98%).
Is the intellectual disability service the main provider of epilepsy management to any of its patients?17 responses (100%); no = 8 (47%), yes = 9 (53%).
53 responses (100%); no = 8 (15%); yes = 45 (85%).
Among those services for which this is the case, for what proportion of its patients is it the main provider of their epilepsy management?12 responses; mean = 45%, range = 0–98%.
41 responses; mean = 74%, range = 5–100%.
Is a local neurology service the main provider of epilepsy management to any of the community intellectual disability team’s patients?16 responses (94%); no = 1 (6%), yes = 15 (94%).
52 responses: no = 11 (21%); yes = 41 (79%).
Among those intellectual disability services for which this is the case, for what proportion of its patients is a local neurology service the main provider of their epilepsy management?14 responses; mean = 43%, range = 2–100%.
33 responses; mean = 23%; range = 1–100%.
For those services referring patients to a local neurology service for their epilepsy management, what referral criteria are employed?15 responses; referral of all patients – in 2 (13%) services providing an answer, referral of those with the most difficult to treat epilepsy – in 10 (67%) services providing an answer.
39 responses; referral of all patients – in 7 (18%) services providing an answer, referral of those with the most difficult to treat epilepsy – in 28 (68%) services providing an answer.
Is there a joint management approach between the community intellectual disability service and a local neurology service for any patients?16 responses (94%); no = 7 (44%), yes = 9 (56%).
52 responses (98%); no = 27 (52%), yes = 25 (48%).
If so, for what proportion of community intellectual disability patients with epilepsy is this the case?11 responses; mean = 12%, range = 0–40%.
23 responses; mean = 18%, range = 1–1000%.
Does the community intellectual disability service have a role in diagnosing new cases of epilepsy?16 responses (94%); no = 6 (37.5%), yes = 10 (62.5%).
40 responses (76%); no = 6 (15%), yes = 34 (85%).
Does the community intellectual disability service liaise with other organisations for the epilepsy management of its patients?17 responses (100%); 14 services (82%) do liaise with other organisations.
35 responses (66%); 30 services (86%) do liaise with other organisations.
Does the community intellectual disability service follow an epilepsy care pathway that makes explicit reference to people with intellectual disability?17 responses (100%); yes = 9 (53%).
41 responses (78%); yes = 16 (39%).
In 2 services (22% of services with an epilepsy care plan) it is used just for the ongoing management of epilepsy.
In 4 services (25% of services with an epilepsy care plan) it is used just for the ongoing management of epilepsy.
In 7 services (78% of services with an epilepsy care plan) it is used for both the diagnosis and the management of epilepsy.
In 12 services (75% of services with an epilepsy care plan) it is used for both the diagnosis and the management of epilepsy.
In 7 services (78%) the epilepsy care pathway was designed by the ID service itself.
In 10 services (63%) the epilepsy care pathway was designed by the ID service itself.
Are there currently any plans to change or restructure the epilepsy management offered by the community intellectual disability service?17 responses (100%); yes = 4 (23.5% of services).
51 responses (96%); yes = 32 (63% of services).
Comparative data from a previous survey of 53 CIDTs are shown in green. This first survey took place 3 years before the EpAID trial commenced. Four of the CIDTs that contributed to the first survey also took part in the EpAID trial.
As described in Chapter 2 (see The intervention), the nurses delivering the intervention were allocated to one of three levels of competence in terms of their experience at managing epilepsy in adults with an ID. The ratings of these levels of competence are reported in Table 3. It should be noted that, although only 2% of the nurses delivering the intervention in the TAU arm were nurse prescribers, 29% of the nurses (corresponding to five nurses) in the competency framework arm were nurse prescribers. It should also be noted that there were more than twice the number of nurses involved in delivering treatment in the TAU arm than there were in the competency framework arm.
| Variable | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n/N | % | n/N | % | n/N | % | |
| Competence level | ||||||
| Novice | 0/18 | 0.0 | 8/50 | 16.0 | 8/68 | 11.8 |
| Competent | 11/18 | 61.1 | 32/50 | 64.0 | 43/68 | 63.2 |
| Expert | 7/18 | 38.9 | 10/50 | 20.0 | 17/68 | 25.0 |
| Prescriber | ||||||
| No | 12/17 | 70.6 | 44/45 | 97.8 | 56/62 | 90.3 |
| Yes | 5/17 | 29.4 | 1/45 | 2.2 | 6/62 | 9.7 |
| Full-/part-time | ||||||
| Full-time | 8/14 | 57.1 | 17/24 | 70.8 | 25/38 | 65.8 |
| Part-time | 6/14 | 42.9 | 7/24 | 29.2 | 13/38 | 34.2 |
| Nurse salary band | ||||||
| Band 5 | 2/15 | 13.3 | 11/46 | 23.9 | 13/61 | 21.3 |
| Band 6 | 7/15 | 46.7 | 23/46 | 50.0 | 30/61 | 49.2 |
| Band 7 | 3/15 | 20.0 | 11/46 | 23.9 | 14/61 | 23.0 |
| Band 8a | 3/15 | 20.0 | 0/46 | 0.0 | 3/61 | 4.9 |
| Band 8b | 0/15 | 0.0 | 1/46 | 2.2 | 1/61 | 1.6 |
Baseline clinical characteristics of the participants
The baseline clinical and demographic characteristics of the participants are reported in Table 4.
| Variable | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n/N | % | n/N | % | n/N | % | |
| Age (years) | 177 | Mean 39.6 (SD 13.3); minimum, maximum 18.1, 65.5 | 126 | Mean 37.01 (SD 12.5); minimum, maximum 18.4, 63.5 | ||
| Sex | ||||||
| Male | 99/184 | 53.8 | 61/128 | 47.7 | 160/312 | 51 |
| Female | 85/184 | 46.2 | 67/128 | 52.3 | 152/312 | 48.7 |
| Ethnicity | ||||||
| Asian | 8/179 | 4.5 | 10/125 | 8.0 | 18/304 | 5.9 |
| Black | 4/179 | 2.2 | 6/125 | 4.8 | 10/304 | 3.3 |
| White | 164/179 | 91.6 | 100/125 | 80.0 | 264/304 | 86.8 |
| Mixed | 2/179 | 1.1 | 3/125 | 2.4 | 5/304 | 1.6 |
| Level of ID | ||||||
| Mild | 19/173 | 11.0 | 21/107 | 19.6 | 40/280 | 14.3 |
| Moderate | 31/173 | 17.9 | 24/107 | 22.4 | 55/280 | 19.6 |
| Severe | 101/173 | 58.4 | 53/107 | 49.5 | 154/280 | 55.0 |
| Profound | 22/173 | 12.7 | 9/107 | 8.4 | 31/280 | 11.1 |
| Accommodation arrangements | ||||||
| In a group home | 78/177 | 44.1 | 40/122 | 32.8 | 118/299 | 39.5 |
| With family members | 57/177 | 32.2 | 57/122 | 46.7 | 114/299 | 38.1 |
| Independently | 13/177 | 7.3 | 9/122 | 7.4 | 22/299 | 7.4 |
| Other | 29/177 | 16.4 | 16/122 | 13.1 | 45/299 | 15.1 |
| Deprivation indexa | ||||||
| Most deprived (0–20%) | 33/179 | 18.4 | 27/126 | 21.4 | 60/305 | 19.7 |
| Deprived (20–40%) | 34/179 | 19.0 | 27/126 | 21.4 | 61/305 | 20.0 |
| Middle (40–60%) | 31/179 | 17.3 | 30/126 | 23.8 | 61/305 | 20.0 |
| Not deprived (60–80%) | 42/179 | 23.5 | 20/126 | 15.9 | 62/305 | 20.3 |
| Least deprived (80–100%) | 39/179 | 21.8 | 22/126 | 17.5 | 61/305 | 20.0 |
The characteristics of the participants’ epilepsy at baseline are described in Tables 5 and 6.
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Age (years) at epilepsy diagnosis | 138 | 5.6 (7.6) | 0, 43 | 75 | 7.3 (9.9) | 0, 59 |
| Age (years) at onset of recurrent seizures | 144 | 4.8 (7.2) | 0, 43 | 87 | 5.7 (9.4) | 0, 59 |
| Number of episodes of status in past 6 months | 168 | 0.4 (2.7) | 0, 32 | 101 | 1.6 (6.3) | 0, 56 |
| Number of prolonged seizures in past 6 months | 154 | 2.5 (11.3) | 0, 100 | 91 | 4.6 (16.3) | 0, 100 |
| Use of rescue medication in past 6 months | 100 | 1.3 (3.0) | 0, 18 | 63 | 6.6 (15.7) | 0, 100 |
| Variable | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Epilepsy syndrome identified | ||||
| No | 128/178 | 71.9 | 33/95 | 34.7 |
| Yes | 50/178 | 28.1 | 62/95 | 65.3 |
| Epilepsy syndrome | ||||
| Focal | 15/46 | 32.6 | 15/54 | 27.8 |
| Generalised | 20/46 | 43.5 | 28/54 | 51.9 |
| Undetermined | 1/46 | 2.2 | 7/54 | 13.0 |
| Special syndromes | 10/46 | 21.7 | 4/54 | 7.4 |
| Seizure type | ||||
| Focal | 11/173 | 6.4 | 7/85 | 8.2 |
| Generalised | 69/173 | 39.9 | 33/85 | 38.8 |
| Focal and generalised | 93/173 | 53.8 | 45/85 | 52.9 |
Causes of epilepsy, when known, and the seizure types experienced in the 2 years preceding entry into the EpAID trial are described in Tables 7 and 8. The most common type of seizure experienced by participants, occurring at similar rates in both arms of the trial, was tonic–clonic seizures.
| Variable | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Genetic cause of epilepsy | ||||
| Fragile X syndrome | 1/171 | 0.6 | 0/109 | 0.0 |
| Tuberous sclerosis | 5/171 | 2.9 | 3/109 | 2.8 |
| Rett syndrome | 1/171 | 0.6 | 6/109 | 5.5 |
| Angelman syndrome | 2/171 | 1.2 | 1/109 | 0.9 |
| Acquired causes of epilepsy | ||||
| Birth injury | 11/171 | 6.4 | 2/109 | 1.8 |
| Cerebral palsy | 25/171 | 14.6 | 4/109 | 3.7 |
| Infantile brain injury | 3/171 | 1.8 | 2/109 | 1.8 |
| Brain infection | 10/171 | 5.8 | 1/109 | 0.9 |
| Prolonged febrile convulsion | 2/171 | 1.2 | 3/109 | 2.8 |
| Intracranial haemorrhage | 1/171 | 0.6 | 1/109 | 0.9 |
| Other causes of epilepsy | 34/171 | 19.9 | 19/109 | 17.4 |
| Unknown reason | 76/171 | 44.4 | 67/109 | 61.5 |
| Non-epileptic seizure(s) reported in the past 6 months in those known to have them | ||||
| No | 3/13 | 23.1 | 4/8 | 50.0 |
| Yes | 10/13 | 76.9 | 4/8 | 50.0 |
| No knowledge of non-epileptic seizure diagnostic status | 36/175 | 20.6 | 37/107 | 34 |
| Seizure type | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Absence | 56/174 | 32.2 | 26/105 | 24.8 |
| Atonic | 24/174 | 13.8 | 9/105 | 8.6 |
| Tonic | 36/174 | 20.7 | 20/105 | 19.0 |
| Clonic | 5/174 | 2.9 | 4/105 | 3.8 |
| Tonic–clonic | 153/174 | 87.9 | 86/105 | 81.9 |
| Simple partial (focal) | 27/174 | 15.5 | 5/104 | 4.8 |
| Complex partial (focal) | 80/174 | 46.0 | 32/104 | 30.8 |
| Myoclonic | 23/174 | 13.2 | 26/105 | 24.8 |
| Drop attacks | 11/174 | 6.3 | 11/105 | 10.5 |
Additional comorbid pathologies are relatively common in adults with ID and epilepsy. In Table 9 the frequencies of a range of the more common current physical, psychiatric and behavioural comorbidities known by CIDTs to be experienced by participants are listed. It is noted that, in general, the rates of comorbidities were somewhat higher among the participants in the TAU arm than among those in the competency framework arm.
| Comorbid condition | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Cardiovascular | 13/178 | 7.3 | 5/111 | 4.5 |
| Respiratory | 20/178 | 11.2 | 23/111 | 20.7 |
| Gastrointestinal | 25/177 | 14.1 | 37/111 | 33.3 |
| Genitourinary | 11/178 | 6.2 | 17/111 | 15.3 |
| Endocrine | 4/177 | 2.3 | 12/111 | 10.8 |
| Neurological | 49/177 | 27.7 | 44/110 | 40.0 |
| Musculoskeletal | 47/178 | 26.4 | 33/110 | 30.0 |
| Dermatological | 13/178 | 7.3 | 16/111 | 14.4 |
| Sight | 22/177 | 12.4 | 32/111 | 28.8 |
| Hearing | 8/178 | 4.5 | 9/111 | 8.1 |
| Autism | 49/178 | 27.5 | 25/110 | 22.7 |
| Challenging behaviour | 54/178 | 30.3 | 46/111 | 41.4 |
| Psychiatric | 19/178 | 10.7 | 19/111 | 17.1 |
| Other | 14/176 | 8.0 | 31/107 | 29.0 |
The AEDs taken by the participants at baseline are described in Table 10. The same five AEDs were the most frequently prescribed agents in both arms of the trial, albeit with the first and second most commonly prescribed and the third and fourth most commonly prescribed agents reversed in order between the two arms. However, in neither arm was there a change in the relative frequency of prescription of these AEDS between baseline and follow-up.
| AED | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active, n | TAU, n | |||||
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | |
| Carbamazepine | 55 | 51 | –4 | 35 | 34 | –1 |
| Clobazam | 35 | 38 | 3 | 27 | 19 | –8 |
| Clonazepam | 4 | 6 | 2 | 9 | 12 | 3 |
| Eslicarbazepine | 1 | 1 | 0 | 0 | 0 | 0 |
| Ethosuximide | 1 | 1 | 0 | 0 | 0 | 0 |
| Gabapentin | 2 | 1 | –1 | 3 | 3 | 0 |
| Lacosamide | 16 | 19 | 3 | 10 | 11 | 1 |
| Lamotrigine | 77 | 71 | –6 | 51 | 41 | –10 |
| Levetiracetam | 68 | 64 | –4 | 33 | 30 | –3 |
| Oxcarbazepine | 4 | 3 | –1 | 5 | 5 | 0 |
| Perampanel | 3 | 0 | –3 | 1 | 1 | 0 |
| Phenobarbitone | 8 | 5 | –3 | 3 | 3 | 0 |
| Phenytoin | 11 | 7 | –4 | 14 | 14 | 0 |
| Pregabalin | 5 | 6 | 1 | 3 | 1 | –2 |
| Primidone | 1 | 1 | 0 | 0 | 0 | 0 |
| Rufinamide | 0 | 2 | 2 | 3 | 1 | –2 |
| Sodium valproate | 76 | 68 | –8 | 61 | 54 | –7 |
| Topiramate | 18 | 17 | –1 | 11 | 11 | 0 |
| Vigabatrin | 2 | 1 | –1 | 2 | 2 | 0 |
| Zonisamide | 7 | 7 | 0 | 6 | 4 | –2 |
Clinical outcomes
Baseline and follow-up end point data
The primary outcome measure employed to assess the effect of introducing the competency framework was the ELDQoL-SSS. This is a 14-item questionnaire that measures the views of an informant as to how severe the physical and behavioural manifestations and consequences of a participant’s seizures have been, overall, in the preceding 4 weeks. The ELDQoL inventory also provides three further subscales that rate carer concerns regarding AED side effects experienced by participants, participants’ behaviour and participants’ mood. The mean values obtained from the trial participants on the ELDQoL subscales at baseline and at the follow-up assessment are provided in Tables 11 and 12.
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SSS | 176 | 22.48 (9.55) | 9.0, 45.0 | 123 | 23.07 (9.70) | 9.0, 46.0 |
| AED side effects scale | 154 | 28.18 (10.76) | 18.0, 62.0 | 117 | 30.73 (11.68) | 18.0, 69.0 |
| Behaviour scale | 176 | 16.21 (6.50) | 8.0, 32.0 | 125 | 17.38 (6.45) | 8.0, 33.0 |
| Mood scale | 175 | 27.30 (8.69) | 15.0, 48.0 | 125 | 29.13 (9.64) | 15.0, 52.0 |
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SSS | 161 | 21.83 (10.36) | 9.0, 43.0 | 111 | 21.14 (9.98) | 9.0, 45.0 |
| AED side effects scale | 147 | 27.48 (11.06) | 18.0, 61.0 | 105 | 28.67 (10.69) | 18.0, 62.0 |
| Behaviour scale | 161 | 15.65 (6.51) | 8.0, 33.0 | 110 | 16.28 (6.77) | 8.0, 33.0 |
| Mood scale | 157 | 26.01 (8.74) | 15.0, 43.0 | 111 | 26.64 (8.81) | 15.0, 46.0 |
The average magnitude of change from baseline to follow-up on each of these subscales is summarised in Table 13. Before controlling for cluster effects and baseline individual- and cluster-level covariates, all average ELDQoL subscale scores in both arms of the trial reduced by small amounts from baseline to follow-up.
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| SSS | 160 | –0.75 (9.83) | –33.0, 31.0 | 109 | –1.21 (8.62) | –30.0, 25.0 |
| AED side effects scale | 131 | –0.07 (9.16) | –30.0, 28.0 | 100 | –1.76 (11.72) | –39.0, 27.0 |
| Behaviour scale | 160 | –0.56 (6.29) | –22.0, 19.0 | 109 | –0.94 (5.78) | –17.0, 21.0 |
| Mood scale | 156 | –1.51 (8.95) | –33.0, 25.0 | 110 | –2.11 (10.48) | –37.0, 23.0 |
The number of tonic–clonic seizures recorded by family carers or paid support workers was also collected as end point data. Although many participants also had other seizure types, including focal or myoclonic seizures, and a minority (see Table 8) did not have any tonic–clonic seizures, the number of tonic–clonic seizures was selected as one of the end-point data sets as their occurrence is generally associated with greater health risks for people with epilepsy than other seizure types and they can be more reliably detected and counted. The numbers of tonic–clonic seizures recorded for participants during the baseline and follow-up periods are reported in Table 14.
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Baseline | 173 | 4.4 (10.5) | 0, 83 | 118 | 4.3 (14.9) | 0, 145 |
| Follow-up | 156 | 6.3 (19.9) | 0, 160 | 100 | 3.2 (7.3) | 0, 36 |
| Change | 153 | 2.0 (16.8) | –29, 151 | 98 | –0.2 (8.4) | –47, 36 |
Modified Carer Strain Index scores were also collected from family carers, but not from paid carers, as a measure of emotional, financial and practical stresses experienced by carers. Family carers’ scores on the MCSI are reported in Table 15.
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Mean (SD) | Minimum, maximum | n | Mean (SD) | Minimum, maximum | |
| Baseline | 54 | 13.42 (6.67) | 0, 25 | 54 | 13.07 (5.89) | 2, 26 |
| Follow-up | 41 | 13.93 (6.80) | 0, 25 | 34 | 13.13 (5.09) | 3, 26 |
| Change | 36 | –0.37 (5.21) | –13, 12 | 28 | –1.06 (4.38) | –13, 5 |
Primary outcome analysis
Using an ITT analysis and controlling for baseline individual- and cluster-level variables, as reported in Tables 16 and 17 (which respectively report the analyses of the collected data set and the full data set with MI), there was no significant difference in ELDQoL-SSS score between the two arms of the trial (–0.326, 95% CI –4.382 to 3.731; p = 0.875) (possible scores range from 10 to 56, with a higher score indicating greater severity).
| Variable | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | –0.326 (2.070) | 0.875 | –4.382 to 3.731 |
| Sex | |||
| Male | Baseline | ||
| Female | 0.679 (1.078) | 0.529 | –1.433 to 2.791 |
| Baseline ELDQoL-SSS score | –0.444 (0.062) | < 0.001 | –0.566 to –0.322 |
| Baseline number of tonic–clonic seizures | 0.007 (0.043) | 0.872 | –0.078 to 0.092 |
| Age | 0.037 (0.047) | 0.433 | –0.055 to 0.129 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | 2.515 (1.467) | 0.086 | –0.360 to 5.390 |
| Independently | 3.176 (2.207) | 0.150 | –1.150 to 7.502 |
| Other | 0.147 (1.700) | 0.931 | –3.185 to 3.478 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | 0.287 (1.924) | 0.881 | –3.484 to 4.058 |
| Severe | 0.075 (1.679) | 0.964 | –3.215 to 3.366 |
| Profound | 1.975 (2.372) | 0.405 | –2.673 to 6.623 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –0.915 (1.831) | 0.618 | –4.504 to 2.675 |
| Middle (40–60%) | –0.687 (1.833) | 0.708 | –4.279 to 2.905 |
| Not deprived (60–80%) | 1.078 (1.829) | 0.555 | –2.506 to 4.663 |
| Least deprived (80–100%) | –1.836 (1.816) | 0.312 | –5.394 to 1.722 |
| Nurse competency level | –0.732 (2.620) | 0.780 | –5.867 to 4.403 |
| Overall nurse workload | 0.039 (0.107) | 0.718 | –0.171 to 0.249 |
| Constant | 6.800 (6.707) | 0.311 | –6.345 to 19.946 |
| Variable | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | –0.213 (1.959) | 0.913 | –4.053 to 3.626 |
| Sex | |||
| Male | Baseline | ||
| Female | 0.664 (0.999) | 0.507 | –1.295 to 2.622 |
| Baseline ELDQoL-SSS score | –0.461 (0.058) | < 0.001 | –0.574 to –0.347 |
| Baseline number of tonic–clonic seizures | 0.025 (0.043) | 0.552 | –0.059 to 0.109 |
| Age | 0.021 (0.044) | 0.629 | –0.065 to 0.108 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | 1.438 (1.328) | 0.279 | –1.164 to 4.041 |
| Independently | 2.283 (2.100) | 0.277 | –1.832 to 6.399 |
| Other | 0.217 (1.580) | 0.891 | –2.879 to 3.313 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | 0.390 (1.859) | 0.834 | –3.253 to 4.033 |
| Severe | –0.276 (1.711) | 0.872 | –3.630 to 3.079 |
| Profound | 1.789 (2.350) | 0.446 | –2.817 to 6.395 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –1.587 (1.656) | 0.338 | –4.833 to 1.659 |
| Middle (40–60%) | –0.905 (1.696) | 0.594 | –4.229 to 2.420 |
| Not deprived (60–80%) | 0.916 (1.735) | 0.598 | –2.485 to 4.317 |
| Least deprived (80–100%) | –2.494 (1.676) | 0.137 | –5.779 to 0.791 |
| Nurse competency level | 0.241 (2.443) | 0.922 | –4.547 to 5.029 |
| Overall nurse workload | 0.052 (0.099) | 0.597 | –0.142 to 0.246 |
| Constant | 6.029 (6.150) | 0.327 | –6.025 to 18.083 |
In determining the sample size for the trial, we chose to power it for the change between baseline and follow-up in the ELDQoL-SSS score, assuming an ICC of 0.05. However, analysis of the scores collected in the trial revealed an ICC of 0.139 [standard error (SE) 0.072, 95% CI 0.047 to 0.344]. Thus, the power of the trial to detect a significant difference between the arms in ELDQoL-SSS score between baseline and follow-up will have been smaller than that originally anticipated.
The analyses reported in Tables 16 and 17 indicate that, across the trial arms, there was a significant effect of baseline ELDQoL-SSS score on follow-up score, with higher scores at baseline falling further at follow-up.
Secondary outcome analysis
Analyses of the secondary outcomes, controlling for baseline individual- and cluster-level variables, are reported in Tables 18–22, using full data sets with MI when appropriate.
| Category | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | 0.194 (1.620) | 0.905 | –2.981 to 3.369 |
| Baseline ELDQoL-SSS score | –0.499 (0.060) | < 0.001 | –0.617 to –0.382 |
| Baseline number of tonic–clonic seizures | 0.003 (0.056) | 0.964 | –0.107 to 0.112 |
| Sex | |||
| Male | Baseline | ||
| Female | 0.509 (1.163) | 0.662 | –1.771 to 2.789 |
| Age | 0.035 (0.053) | 0.499 | –0.067 to 0.138 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | 1.900 (1.578) | 0.228 | –1.192 to 4.993 |
| Independently | 2.652 (2.622) | 0.312 | –2.488 to 7.792 |
| Other | 1.472 (1.967) | 0.454 | –2.384 to 5.327 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | –2.541 (2.181) | 0.244 | –6.815 to 1.734 |
| Severe | –1.159 (1.871) | 0.536 | –4.826 to 2.508 |
| Profound | –1.364 (2.846) | 0.632 | –6.942 to 4.214 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –1.879 (2.000) | 0.348 | –5.798 to 2.041 |
| Middle (40–60%) | –2.591 (1.927) | 0.179 | –6.368 to 1.186 |
| Not deprived (60–80%) | –1.236 (1.945) | 0.525 | –5.047 to 2.576 |
| Least deprived (80–100%) | –4.357 (1.961) | 0.026 | –8.201 to –0.514 |
| Nurse competency level | 1.646 (1.902) | 0.387 | –2.081 to 5.373 |
| Overall nurse workload | –0.044 (0.077) | 0.564 | –0.196 to 0.107 |
| Constant | 11.192 (5.464) | 0.041 | 0.483 to 21.902 |
| Category | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | 0.661 (0.998) | 0.508 | –1.295 to 2.617 |
| Baseline ELDQoL-SSS score | –0.507 (0.059) | < 0.001 | –0.622 to –0.391 |
| Baseline number of tonic–clonic seizures | 0.023 (0.028) | 0.414 | –0.032 to 0.077 |
| Sex | |||
| Male | Baseline | ||
| Female | 0.023 (0.659) | 0.972 | –1.268 to 1.315 |
| Age | 0.017 (0.029) | 0.568 | –0.040 to 0.073 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | 1.160 (0.862) | 0.179 | –0.531 to 2.850 |
| Independently | 0.624 (1.444) | 0.666 | –2.206 to 3.454 |
| Other | 0.229 (1.061) | 0.830 | –1.852 to 2.309 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | 0.125 (1.188) | 0.916 | –2.454 to 2.203 |
| Severe | 1.319 (1.060) | 0.213 | –0.759 to 3.398 |
| Profound | 3.250 (1.575) | 0.039 | 0.162 to 6.338 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –0.470 (1.115) | 0.673 | –2.655 to 1.715 |
| Middle (40–60%) | –0.772 (1.157) | 0.504 | –3.040 to 1.495 |
| Not deprived (60–80%) | 0.265 (1.181) | 0.823 | –2.050 to 2.581 |
| Least deprived (80–100%) | –1.070 (1.138) | 0.347 | –3.299 to 1.160 |
| Nurse competency level | 0.295 (1.232) | 0.811 | –2.120 to 2.709 |
| Overall nurse workload | 0.054 (0.050) | 0.283 | –0.045 to 0.153 |
| Constant | 3.346 (3.333) | 0.315 | –3.187 to 9.879 |
| Category | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | 0.854 (1.541) | 0.580 | –2.167 to 3.874 |
| Baseline ELDQoL-SSS score | –0.645 (0.058) | < 0.001 | –0.760 to –0.531 |
| Baseline number of tonic–clonic seizures | 0.049 (0.041) | 0.231 | –0.031 to 0.129 |
| Sex | |||
| Male | Baseline | ||
| Female | 0.425 (0.973) | 0.662 | –1.482 to 2.332 |
| Age | 0.012 (0.043) | 0.778 | –0.072 to 0.096 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | 1.968 (1.274) | 0.122 | –0.529 to 4.465 |
| Independently | 2.999 (2.255) | 0.184 | –1.421 to 7.418 |
| Other | 1.578 (1.562) | 0.313 | –1.485 to 4.640 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | 0.436 (1.853) | 0.814 | –3.195 to 4.067 |
| Severe | 0.940 (1.616) | 0.561 | –2.227 to 4.107 |
| Profound | 1.220 (2.337) | 0.601 | –3.359 to 5.800 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –1.486 (1.603) | 0.354 | –4.628 to 1.656 |
| Middle (40–60%) | –1.549 (1.624) | 0.340 | –4.732 to 1.634 |
| Not deprived (60–80%) | 1.610 (1.673) | 0.336 | –1.670 to 4.889 |
| Least deprived (80–100%) | –1.955 (1.631) | 0.231 | –5.151 to 1.242 |
| Nurse competency level | 0.281 (1.913) | 0.883 | –3.468 to 4.030 |
| Overall nurse workload | 0.066 (0.078) | 0.399 | –0.087 to 0.219 |
| Constant | 11.326 (5.382) | 0.035 | 0.778 to 21.874 |
| Category | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | –0.569 (1.631) | 0.727 | –3.766 to 2.629 |
| Baseline ELDQoL-SSS score | –0.386 (0.105) | < 0.001 | –0.592 to –0.180 |
| Baseline number of tonic–clonic seizures | –0.005 (0.030) | 0.876 | –0.064 to 0.055 |
| Sex | |||
| Male | Baseline | ||
| Female | 1.148 (1.358) | 0.398 | –1.514 to 3.810 |
| Age | –0.022 (0.071) | 0.763 | –0.161 to 0.118 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | –3.084 (3.697) | 0.404 | –10.330 to 4.162 |
| Independently | 0.324 (4.173) | 0.938 | –7.855 to 8.504 |
| Other | –2.694 (4.491) | 0.549 | –11.496 to 6.109 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | 1.668 (2.321) | 0.472 | –2.882 to 6.217 |
| Severe | 0.464 (2.215) | 0.834 | –3.878 to 4.806 |
| Profound | 0.635 (2.711) | 0.815 | –4.678 to 5.949 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –0.046 (1.922) | 0.981 | –3.814 to 3.722 |
| Middle (40–60%) | –0.206 (2.097) | 0.922 | –4.316 to 3.904 |
| Not deprived (60–80%) | –0.937 (2.198) | 0.670 | –5.246 to 3.372 |
| Least deprived (80–100%) | –0.279 (1.985) | 0.888 | –4.169 to 3.610 |
| Nurse competency level | 0.041 (1.869) | 0.982 | –3.621 to 3.704 |
| Overall nurse workload | –0.052 (0.072) | 0.472 | –0.194 to 0.090 |
| Constant | 8.964 (6.093) | 0.141 | –2.978 to 20.906 |
| Category | β (SE) | p-value | 95% CI |
|---|---|---|---|
| Treatment arm | |||
| Active | Baseline | ||
| TAU | –3.143 (2.898) | 0.278 | –8.823 to 2.537 |
| Baseline number of tonic–clonic seizures | –0.405 (0.140) | 0.004 | –0.680 to –0.130 |
| Sex | |||
| Male | Baseline | ||
| Female | 0.025 (1.669) | 0.988 | –3.246 to 3.296 |
| Age | –0.034 (0.071) | 0.635 | –0.173 to 0.105 |
| Accommodation | |||
| In a group home | Baseline | ||
| With family members | 2.761 (2.123) | 0.193 | –1.400 to 6.923 |
| Independently | 1.199 (3.444) | 0.728 | –5.551 to 7.948 |
| Other | 3.566 (2.739) | 0.193 | –1.802 to 8.934 |
| Level of ID | |||
| Mild | Baseline | ||
| Moderate | 0.046 (2.977) | 0.988 | –5.789 to 5.880 |
| Severe | 1.410 (2.580) | 0.585 | –3.647 to 6.467 |
| Profound | –0.314 (3.812) | 0.934 | –7.786 to 7.158 |
| Deprivation index | |||
| Most deprived (0–20%) | Baseline | ||
| Deprived (20–40%) | –4.072 (2.695) | 0.131 | –9.354 to 1.210 |
| Middle (40–60%) | –3.147 (2.770) | 0.256 | –8.577 to 2.284 |
| Not deprived (60–80%) | –3.122 (2.953) | 0.290 | –8.909 to 2.665 |
| Least deprived (80–100%) | –4.659 (2.894) | 0.107 | –10.331 to 1.014 |
| Nurse competency level | 2.384 (3.543) | 0.501 | –4.560 to 9.328 |
| Overall nurse workload | –0.131 (0.144) | 0.362 | –0.413 to 0.151 |
| Constant | 3.471 (8.877) | 0.696 | –13.927 to 20.869 |
There were no significant differences between the arms in ELDQoL AED side effects scale score (p = 0.905) (see Table 18), ELDQoL behaviour scale score (p = 0.508) (see Table 19), ELDQoL mood scale score (p = 0.580) (see Table 20), MCSI score (p = 0.727) (see Table 21) or number of tonic–clonic seizures (p = 0.278) (see Table 22).
As was the case for the primary outcome, for each of these secondary outcomes there was a significant effect of baseline level on the follow-up result, with participants who achieved more pathological scores at baseline demonstrating more of a decrease towards less pathological scores at follow-up.
Subgroup analyses
The planned subgroup analyses, as specified in the trial statistical analysis plan before data analysis commenced (see Chapter 2, Clinical outcome analyses), are reported in Table 23. There was a significant interaction between ELDQoL-SSS score and treatment arm and level of ID.
| Outcome | Variable | Category | n | β (SE) | p-value | 95% CI |
|---|---|---|---|---|---|---|
| Change in ELDQoL-SSS score | Baseline number of tonic–clonic seizures | 312 | –0.055 (0.084) | 0.513 | –0.221 to 0.110 | |
| Change in ELDQoL-SSS score | Baseline SSS | Below median | 312 | Baseline | ||
| Above median | 1.932 (1.915) | 0.313 | –1.820 to 5.684 | |||
| Change in ELDQoL-SSS score | Level of ID | Mild/moderate | 312 | Baseline | ||
| Severe/profound | –5.280 (2.229) | 0.018 | –9.649 to –0.910 | |||
| Change in ELDQoL-SSS score | Accommodation | In a group home | 312 | Baseline | ||
| With family | 1.581 (2.522) | 0.531 | –3.361 to 6.524 | |||
| Independently | 1.036 (4.363) | 0.812 | –7.515 to 9.586 | |||
| Other | 3.737 (3.289) | 0.256 | –2.711 to 10.184 | |||
| Change in ELDQoL-SSS score | Baseline number of seizure types | 0/1 seizure types | 279 | Baseline | ||
| > 1 seizure type | 0.900 (1.884) | 0.633 | –2.793 to 4.593 | |||
| Change in MCSI score | Baseline number of tonic–clonic seizures | 96 | –0.022 (0.067) | 0.738 | –0.154 to 0.109 |
Further analysis of this interaction revealed that, for those with a mild/moderate ID (n = 95), the follow-up SSS score minus the baseline SSS score was, on average, 3.376 higher (i.e. worse) in the TAU arm than in the competency framework arm (a non-significant trend: SE 2.005, 95% CI –0.554 to 7.307; p = 0.092). However, among participants with a severe/profound group ID (n = 185), the follow-up SSS score minus the baseline SSS score was 1.968 lower in the TAU arm than in the competency framework arm (a non-significant change: SE = 2.557, 95% CI –6.981 to 3.044; p = 0.442). However, the treatment effect was not significant in either arm.
The other planned subgroup analyses did not indicate any other significant interactions between the primary end point and baseline variables (see Table 23).
Trial-related clinical activities carried out by the treating nurses
Using the nurse self-completion daily activity diary, as described in End points: definitions and acquisition, data were collected on the duration and nature of all clinical work, pertaining to the participants, undertaken by the nurses during the intervention period. The aim of collecting these data was to generate profiles of the clinical needs addressed and the interventions given to participants in the two study arms to provide an indication of the extent to which the competency framework altered clinical practice. Overall, it was found that the reasons for intervention (χ2 = 54.50, degrees of freedom = 10; p < 0.001) and the details of the care given (χ2 = 94.69, degrees of freedom = 10; p < 0.001) were both significantly different between the two arms of the trial.
Table 24 demonstrates that, on average, the nurses working in the active intervention arm engaged in fewer episodes of care with participants than those working in the TAU arm (mean difference = 1.961 fewer contacts in the active intervention arm, SE 0.586, 95% CI –3.644 to –0.277; p = 0.023). Although within each arm there was a wide variation in the duration of individual contacts, the clinical contacts in both arms had a similar average duration of around 40 minutes.
| Variable | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n | Median | Minimum, maximum | n | Median | Minimum, maximum | |
| Number of contactsa | 184 | 2 | 0, 36 | 128 | 5 | 0, 40 |
| Duration of contact (minutes) | 900 | 30 | 0, 603 | 890 | 30 | 0, 420 |
Tables 25–27 provide, respectively, details of the location of each episode of care, the reason or reasons for it and the nature of the care provided or intervention delivered.
| Location | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Home | 292/940 | 31.1 | 336/904 | 37.2 |
| Clinic | 105/940 | 11.2 | 72/904 | 8.0 |
| GP surgery | 6/940 | 0.6 | 9/904 | 1.0 |
| Telephone | 200/940 | 21.3 | 285/904 | 31.5 |
| Other | 337/940 | 35.9 | 202/904 | 22.3 |
| Reasona | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Assessment | 48/943 | 5.1 | 93/907 | 10.3 |
| Counselling | 10/943 | 1.1 | 11/907 | 1.2 |
| Education | 43/943 | 4.6 | 51/907 | 5.6 |
| Health facilitation | 188/943 | 19.9 | 155/907 | 17.1 |
| Management planning | 159/943 | 16.9 | 173/907 | 19.1 |
| Monitoring epilepsy | 444/943 | 47.1 | 445/907 | 49.1 |
| Monitoring health/behaviour | 243/943 | 25.8 | 321/907 | 35.4 |
| Monitoring treatment | 278/943 | 29.5 | 240/907 | 26.5 |
| Responding to urgent health or behavioural concern | 116/943 | 12.3 | 81/907 | 8.9 |
| Other | 163/943 | 17.3 | 129/907 | 14.2 |
| Interventiona | Treatment arm | |||
|---|---|---|---|---|
| Active | TAU | |||
| n/N | % | n/N | % | |
| Education of family carer | 39/943 | 4.1 | 61/907 | 6.7 |
| Education of paid staff | 131/943 | 13.9 | 111/907 | 12.2 |
| Education of patient | 25/943 | 2.7 | 50/907 | 5.5 |
| Health facilitation | 273/943 | 29.0 | 284/907 | 31.3 |
| Investigation request | 39/943 | 4.1 | 53/907 | 5.8 |
| Management planning | 288/943 | 30.5 | 302/907 | 33.3 |
| Medication issues | 206/943 | 21.8 | 140/907 | 15.4 |
| Prescribing | 76/943 | 8.1 | 21/907 | 2.3 |
| Review and monitoring of medication | 319/943 | 33.8 | 206/907 | 22.7 |
| Other | 255/943 | 27.0 | 262/907 | 28.9 |
Nurses could make use of as many of the available responses as they considered appropriate to describe the reasons for and nature of each episode of care. Examination of these tables and Figures 4 and 5 provides an impression of the activities that the treating nurses in the two arms engaged in and why.
FIGURE 4.
Reasons for each intervention as a proportion of all reasons cited for an intervention.
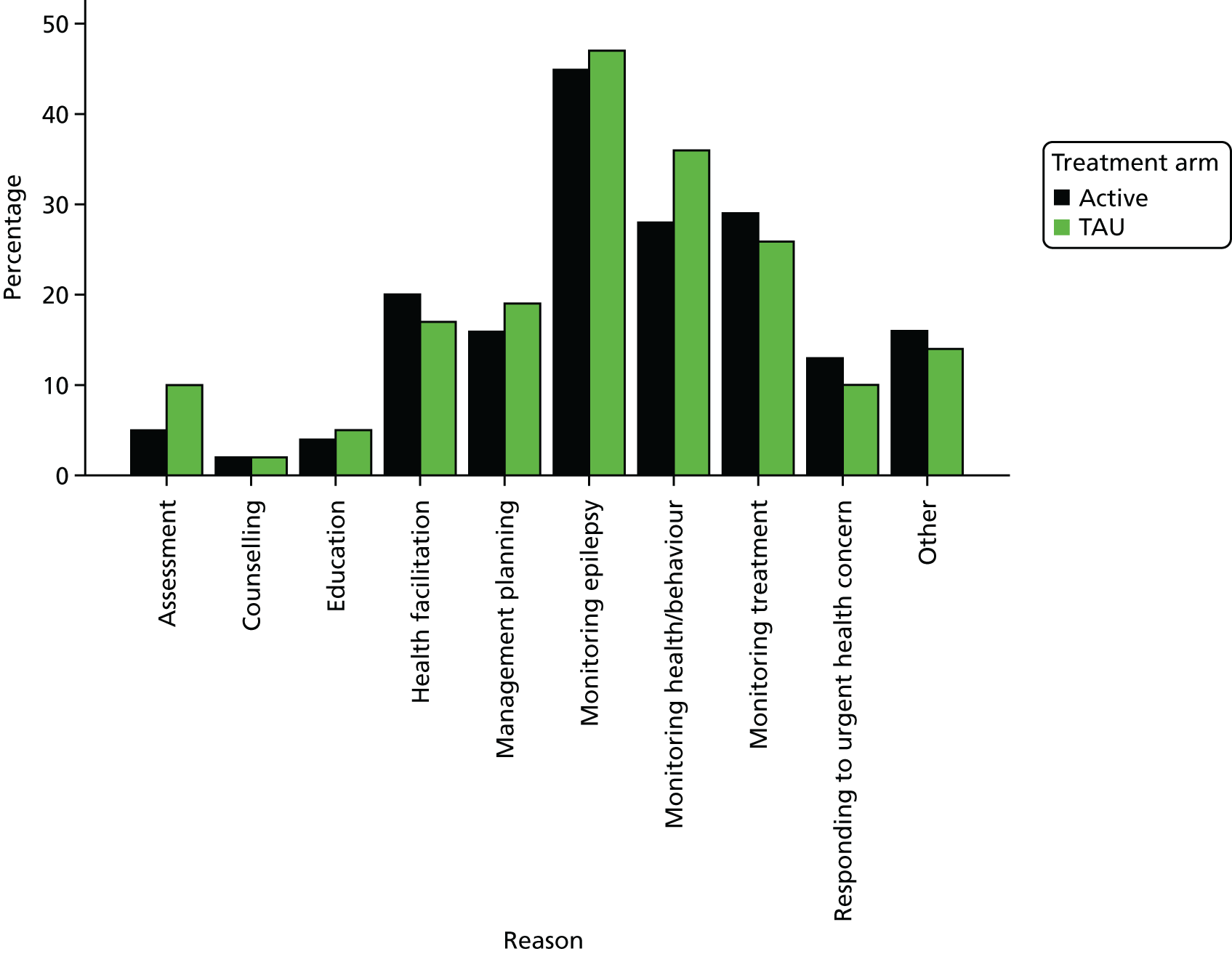
FIGURE 5.
Care delivered during an intervention as a proportion of all interventions delivered.
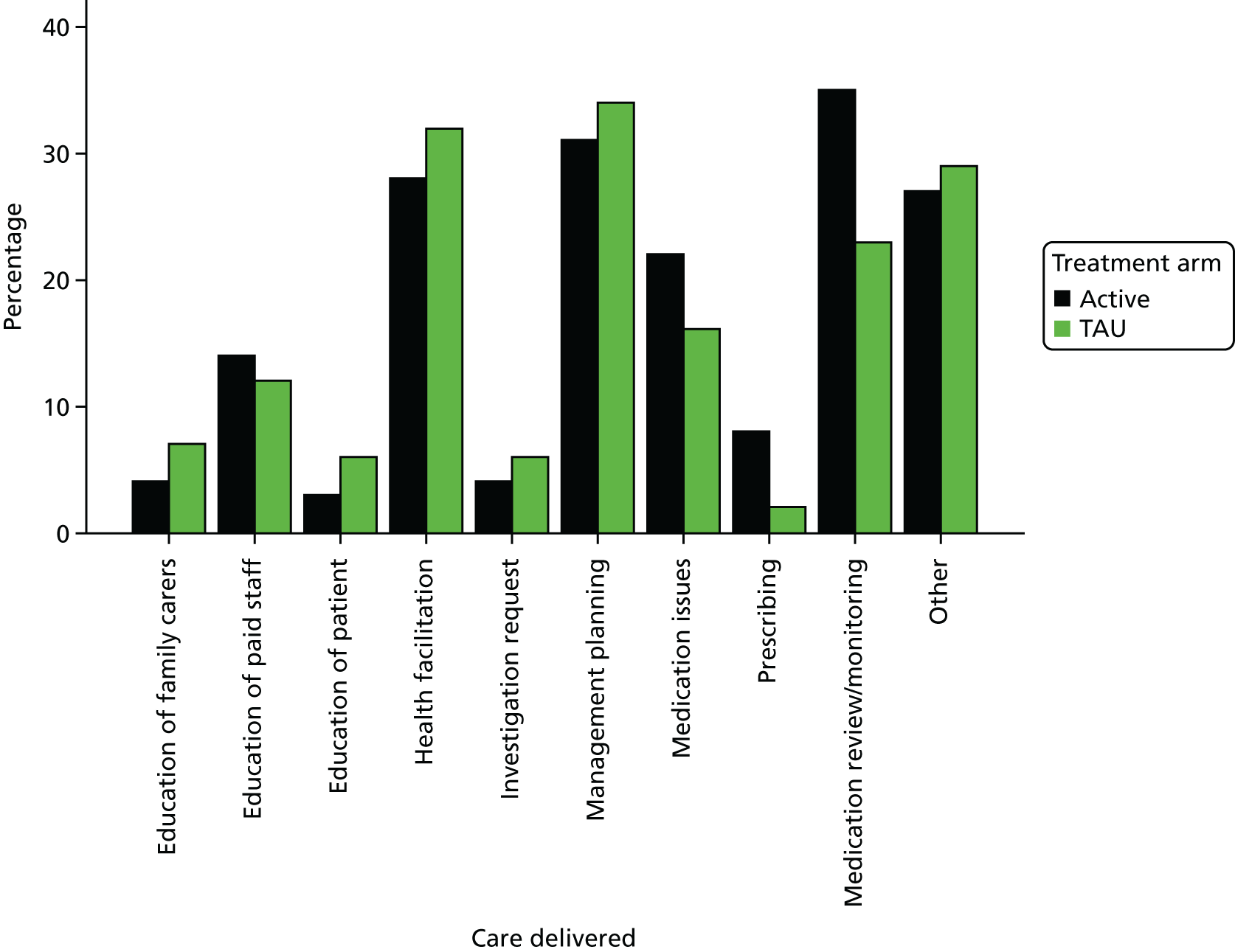
In addition, planned comparisons were carried out to examine a priori hypotheses that the nature of the competency framework guidelines would result in a higher proportion of contacts for health facilitation and a lower proportion of contacts in response to urgent health or behavioural concerns in the competency framework arm. As reported in Table 26, there was no significant difference between the arms with respect to the proportion of contacts for health facilitation, whereas, against expectations, the proportion of contacts relating to urgent health or behavioural concerns was greater in the active arm.
Safety findings
The frequency and proportions of serious adverse events (SAEs), including deaths, that occurred during the trial in the two arms are reported in Table 28, summarised as the consequence of the SAE, the view of the local principal investigator (PI) as to whether or not the SAE was likely to have been caused by participation in the EpAID trial and the outcome of the event. Overall, there was no significant difference between the two arms of the trial in the proportion of participants who experienced a SAE. The proportion of participants in the competency framework arm who experienced a SAE was 0.136 and in the TAU arm was 0.094 (estimated difference 0.042, 95% CI –0.029 to 0.113; p = 0.258).
| Variable | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n/N | % | n/N | % | n/N | % | |
| Consequences | ||||||
| Death | 4/184 | 2.2 | 2/128 | 1.6 | 8/312 | 2.6 |
| Hospitalisation | 30/184 | 16.3 | 20/128 | 15.6 | 50/312 | 16.0 |
| Other | 3/184 | 1.6 | 0/128 | 0.0 | 3/312 | 1.0 |
| Caused by participation in the EpAID trial | ||||||
| No | 33/184 | 17.9 | 21/128 | 16.4 | 54/312 | 17.3 |
| Yes | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| Outcome | ||||||
| Resolved | 17/184 | 9.2 | 12/128 | 9.4 | 29/312 | 9.3 |
| Ongoing | 6/184 | 3.3 | 2/128 | 1.6 | 8/312 | 2.6 |
| Death | 6/184 | 3.3 | 2/128 | 1.6 | 8/312 | 2.6 |
A full list of the SAEs reported is provided in Appendix 3.
Of the 184 participants entered into the active arm of the trial, four died at some point between being consented into the trial and completing the period of follow-up observation. Of the 128 participants randomised to the TAU arm, two people died over the same period. Of these six deaths, five were attributed to respiratory pathology and one to cardiac disease. None were directly associated with epilepsy and in no instance did the local PI consider that the death was associated with participation in the EpAID trial.
Economic analyses: results
Raw resource use and outcome data
All 312 participants included in the primary statistical analysis were included in the economic analysis. Table 29 provides mean values and the proportion of missing data for cost and outcome data at baseline and follow-up in the intervention and control arms. The number of missing data for costs was small at baseline and modest at follow-up. The number of missing data for outcomes was greater, but in all cases at least 70% of data were non-missing. Over half of the costs falling on health and social care were accommodation costs. Social care and day care costs were also high. The costs falling on participants/families for the provision of respite care, holidays, social care and transport constitute a modest share of the total monthly costs, but at around £200 per month may represent a significant burden. Informal care costs were much higher and constituted the main difference between costs falling on health and social care and societal costs. Drug costs were generally low but included some high-cost items such as rescue medication and fortified drinks. The costs of holidays, hospitalisations and contact with the treating nurse were low. There was a sizeable increase in accommodation costs in the control arm at follow-up compared with baseline, which was not replicated in the intervention arm.
| Variable | Treatment arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Active (n = 184) | TAU (n = 128) | |||||||
| Baseline | Follow-up | Baseline | Follow-up | |||||
| Mean | Proportion missing (%) | Mean | Proportion missing (%) | Mean | Proportion missing (%) | Mean | Proportion missing (%) | |
| Costs by category (£) | ||||||||
| Drugs | 160 | 0 | 174 | 0 | 200 | 0 | 160 | 0 |
| Accommodation | 3853 | 4 | 3938 | 14 | 2949 | 5 | 3351 | 17 |
| Respite care | 176 | 4 | 153 | 14 | 129 | 5 | 165 | 19 |
| Holidays | 71 | 4 | 54 | 14 | 70 | 5 | 70 | 19 |
| Primary health | 181 | 4 | 162 | 14 | 221 | 5 | 244 | 19 |
| Social care | 795 | 4 | 559 | 14 | 859 | 5 | 775 | 19 |
| Day care | 996 | 4 | 1062 | 14 | 980 | 5 | 1259 | 19 |
| Hospital visits | 25 | 4 | 54 | 14 | 50 | 5 | 93 | 19 |
| Patient costs | 208 | 0 | 224 | 0 | 156 | 0 | 165 | 0 |
| Informal care | 1745 | 4 | 1783 | 14 | 2537 | 4 | 2652 | 19 |
| Treating nurse | NA | 63 | 0 | NA | 58 | 0 | ||
| Total health and social care | 6276 | 4 | 6253 | 14 | 5470 | 5 | 6288 | 19 |
| Total societal | 8237 | 4 | 8295 | 14 | 8191 | 5 | 9142 | 20 |
| Outcomes | ||||||||
| EQ-5D-5L tariff | 0.63 | 17 | 0.60 | 22 | 0.62 | 16 | 0.62 | 28 |
| EQ-5D-5L VAS | 76 | 5 | 76 | 14 | 74 | 7 | 77 | 20 |
| ELDQoL-SSS | 26.6 | 16 | 27.0 | 21 | 26.1 | 13 | 25.6 | 23 |
| ELDQoL mood | 31.2 | 5 | 30.9 | 15 | 31.6 | 2 | 30.9 | 13 |
| ELDQoL behaviour | 18.5 | 5 | 18.5 | 13 | 18.8 | 2 | 18.5 | 14 |
| ELDQoL AED SEP | 31.3 | 20 | 31.1 | 25 | 32.5 | 9 | 31.9 | 20 |
Figure 6 illustrates the changes in overall mean costs (after excluding missing data) by category between baseline and follow-up. The largest absolute change was seen for accommodation costs and the largest relative change was seen for hospital costs. However, the data for hospital costs along with holiday costs contained a large number of zero observations, increasing sensitivity to outliers.
FIGURE 6.
Costs by category at baseline and follow-up for all participants.
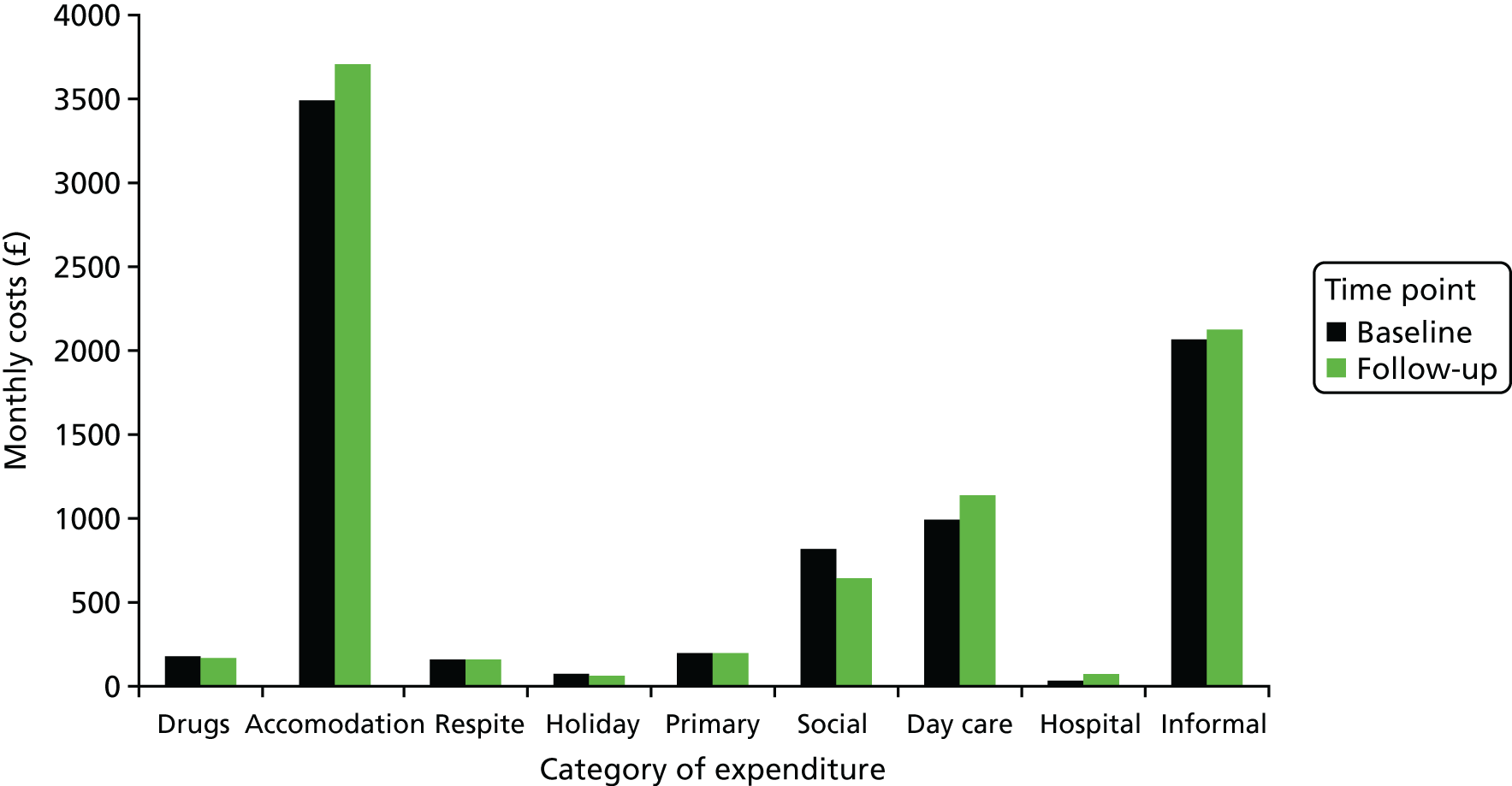
The distribution of overall health and social care costs was moderately skewed. Figure 7 shows the distribution for all patients at follow-up. After removing accommodation costs, the distribution of the remaining costs exhibited greater skew. Using generalised linear modelling, alternative distributions and link functions were examined to see if they would provide a better fit with the data than the assumption of normally distributed residuals underpinning linear regression. However, tests of the variance and link functions did not reliably identify superior alternatives to linear regression. The distribution of EQ-5D-5L tariffs was also moderately skewed. Figure 8 shows the distribution of EQ-5D-5L tariff values at follow-up.
FIGURE 7.
Distribution of monthly health and social care costs at follow-up.
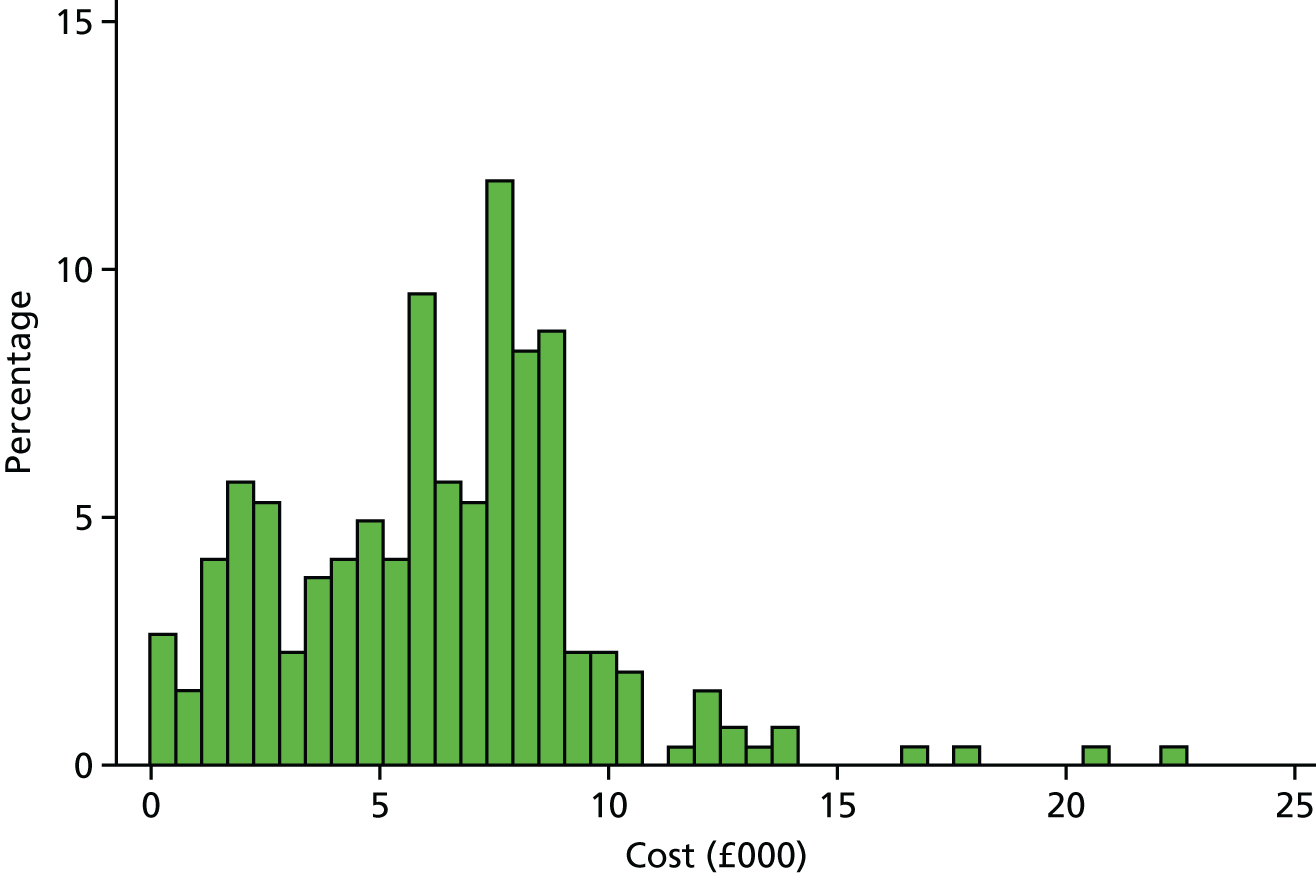
FIGURE 8.
Distribution of EQ-5D-5L tariff values at follow-up.
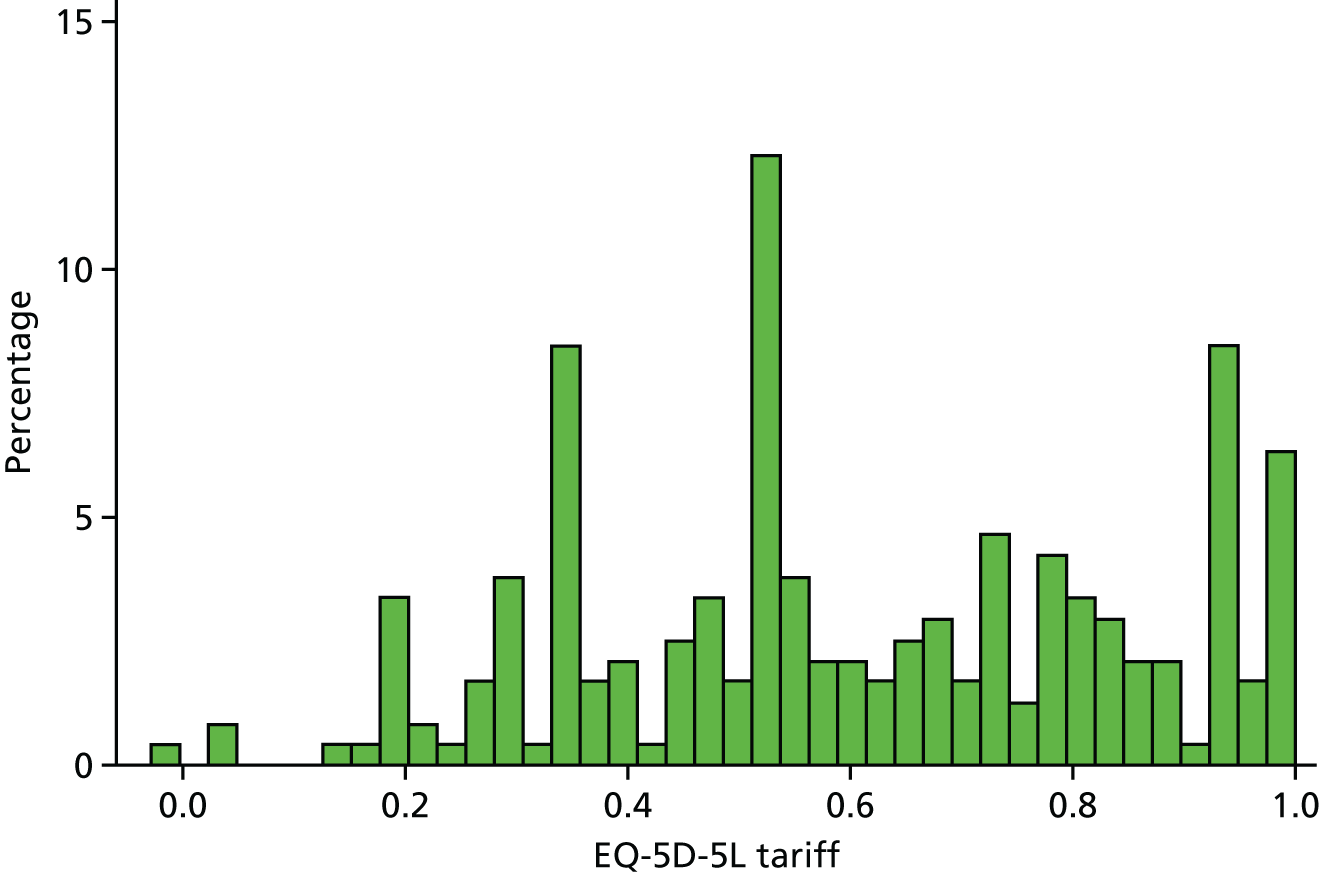
Table 30 reports the mean treatment effects and the non-parametric 95% CIs derived following MI of missing data and adjustment for differences at baseline using linear regression.
| Costs and outcomes | Mean | p-value | 95% CI |
|---|---|---|---|
| Total annual health and social care costs (£) | –358 | 0.36 | –1119 to 294 |
| Total annual societal costs (£) | –612 | 0.19 | –1523 to 299 |
| QoL: EQ-5D-5L tariff | –0.020 | 0.45 | –0.071 to 0.032 |
| QoL: ELDQoL-SSS | –0.50 | 0.66 | –2.76 to 1.76 |
The data suggest that the intervention is associated with a reduction in monthly costs compared with TAU. The data also indicate that the treatment is associated with a reduction in QoL. In all cases the effect associated with treatment is not significant at the 95% confidence level. The data suggest that the competency framework might reduce the cost of supporting people with epilepsy and an ID but provide worse outcomes than usual care.
Table 30 reports differences in costs over the previous month at follow-up between the treatment group and the control group after adjusting for baseline differences. Assumptions are required regarding the impact of treatment on costs in the intervening 5 months. Likewise, the EQ-5D-5L captures patients’ health status on the day that the questionnaire is completed and assumptions are needed regarding QoL measures between baseline and follow-up. The most common assumption is a linear interpolation between baseline and follow-up. With respect to the ICER, this is mathematically equivalent to assuming an immediate change in QoL and costs following commencement of the intervention (the proportionate effect on the numerator and the denominator is the same). Using Equation 1 for the ICER (see Economic analysis), either assumption generates ICERs of £220,000 per QALY from a health and social care perspective and £376,000 per QALY from a societal perspective. Usual care is more effective but far more expensive and, hence, is not cost-effective. Interpreted a little differently, the intervention results in a reduction in QoL but generates considerable cost savings, which would justify its introduction at currently accepted thresholds. An increase in ELDQoL-SSS score represents a worsening of severity. The ICERs for a point reduction in SSS score are £8600 and £14,700 from a health and social care and a societal perspective respectively. It should be noted at this point that, although it is not incorrect to interpret the mean ICERs generated from the bootstrap replicates, the considerable uncertainty surrounding the results requires explicit consideration.
Uncertainty and correlation between costs and outcomes
The cost-effectiveness plane derived from the difference in health and social care costs and QoL estimated using EQ-5D-5L tariff values is plotted in Figure 9. In the cost-effectiveness plane, each pair of cost and QoL treatment effect estimates from the 1000 bootstrap replicates are plotted. The majority of points lie in the south-west quadrant representing a reduction in both costs and QoL associated with treatment. Any correlation between costs and outcomes appears to be small. This reflects the underlying data in which only a modest correlation between changes in costs and changes in EQ-5D-5L tariffs at the patient level was observed (r = 0.12).
FIGURE 9.
Cost-effectiveness plane plotted using health and social care costs and QoL measured with the EQ-5D-5L.
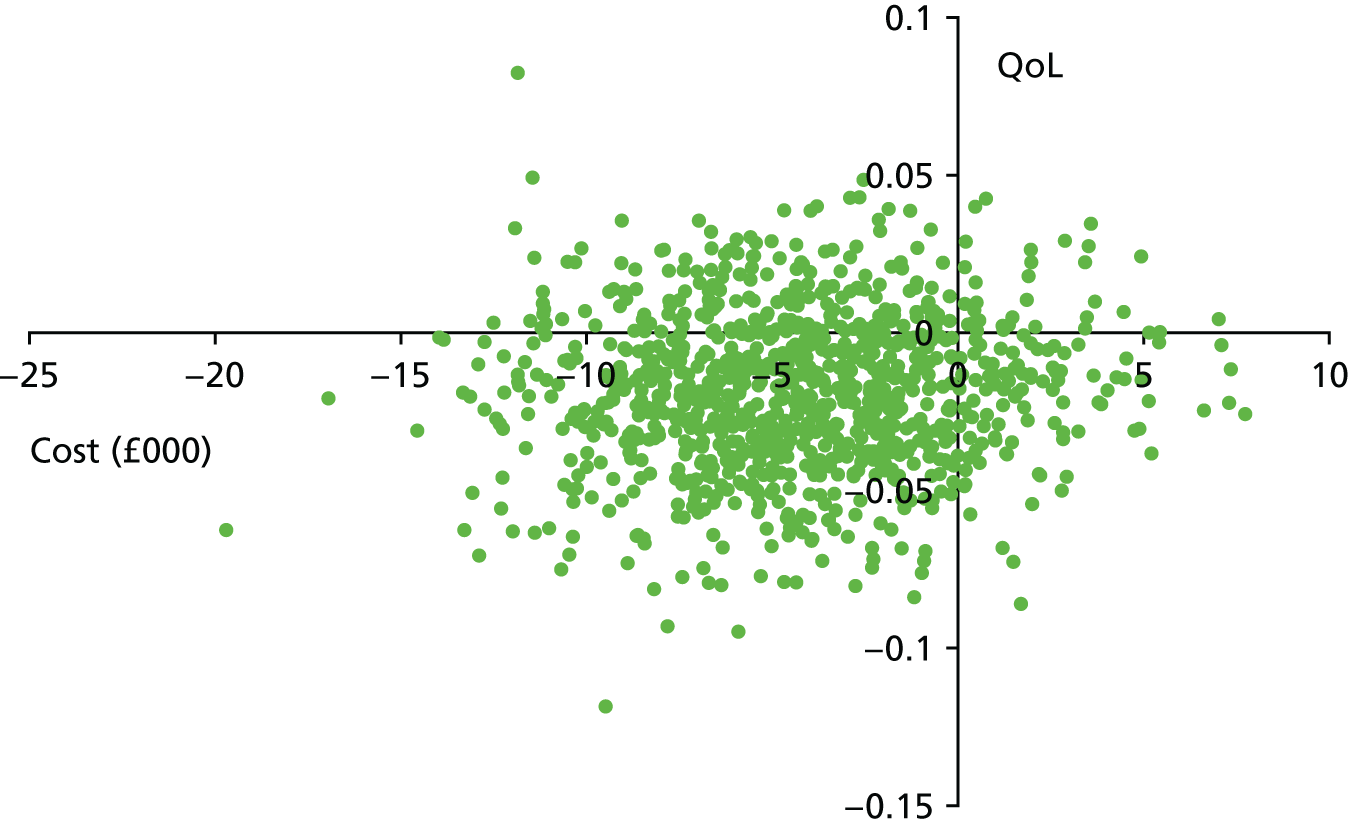
The pairs of treatment costs and effects from the bootstrap replicates plotted in the cost-effectiveness plane can also be used to generate the CEAC. The resulting plot is shown in Figure 10. The CEAC suggests that there is a roughly 85% likelihood, given sampling uncertainty, that the intervention is cost-effective across a range of values placed on a QALY from £0 to £50,000.
FIGURE 10.
Cost-effectiveness acceptability curve plotted using health and social care costs and QoL measured with the EQ-5D-5L.
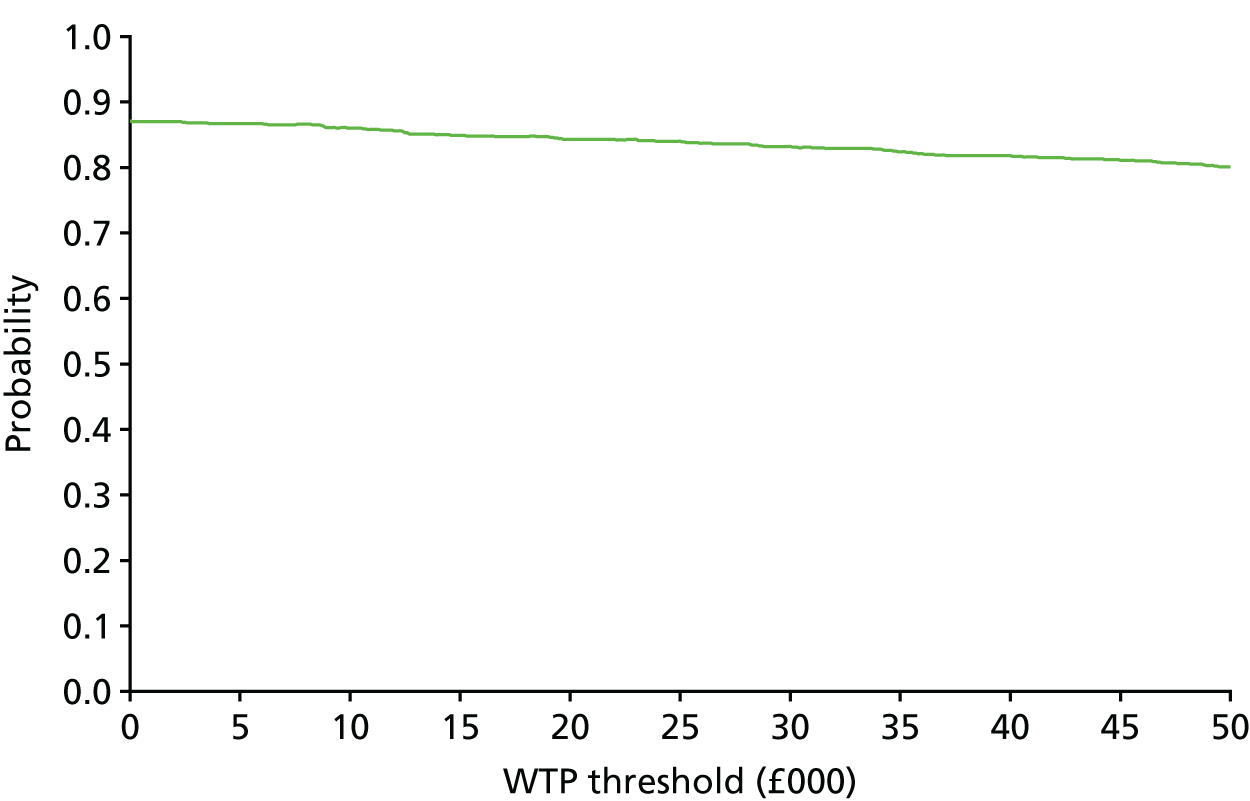
The intervention is less effective than the control, and so, as the value placed on a QALY increases, the likelihood that the intervention is cost-effective falls – but not by much because the differential impact on QoL is small compared with costs. Over the range £0–50,000 the likelihood that the intervention is cost-effective falls from 87% to 80%. The inference is the same as that derived from the mean ICER – the defined epilepsy nurse role is cost-effective. However, the CEAC conveys the uncertainty around that conclusion, which is considerable.
When societal costs are included, the treatment effect on costs tends to more negative values. This is best seen in the corresponding cost-effectiveness plane (Figure 11).
FIGURE 11.
Cost-effectiveness plane plotted using societal costs and QoL measured with the EQ-5D-5L.
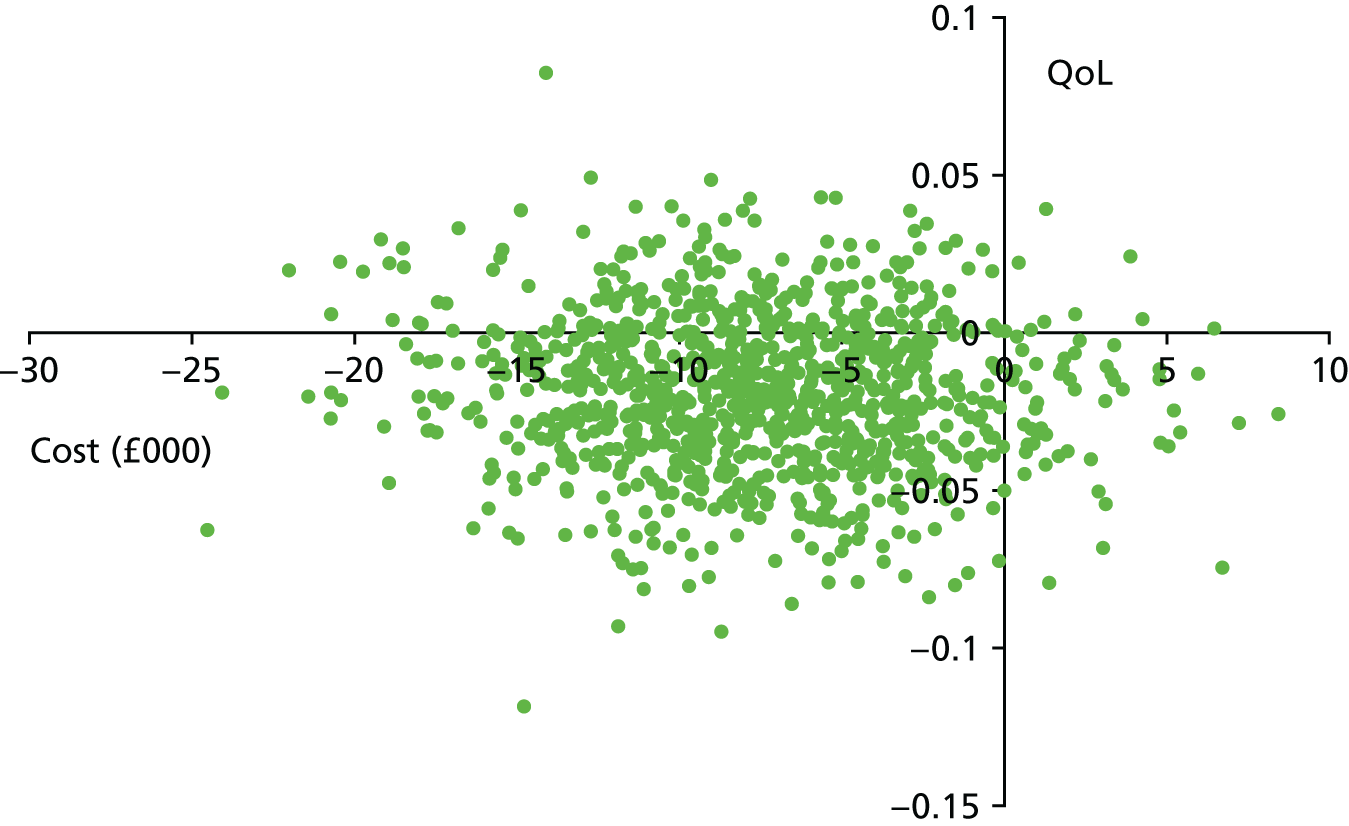
Comparing this figure with the cost-effectiveness plane for health and social care costs (see Figure 9) it can be seen that the cloud of cost and effect pairs is shifted to the left. The effect appears to be modest but it is sufficient to influence the resulting CEAC (Figure 12).
FIGURE 12.
Cost-effectiveness acceptability curve plotted using societal costs and QoL measured with the EQ-5D-5L.
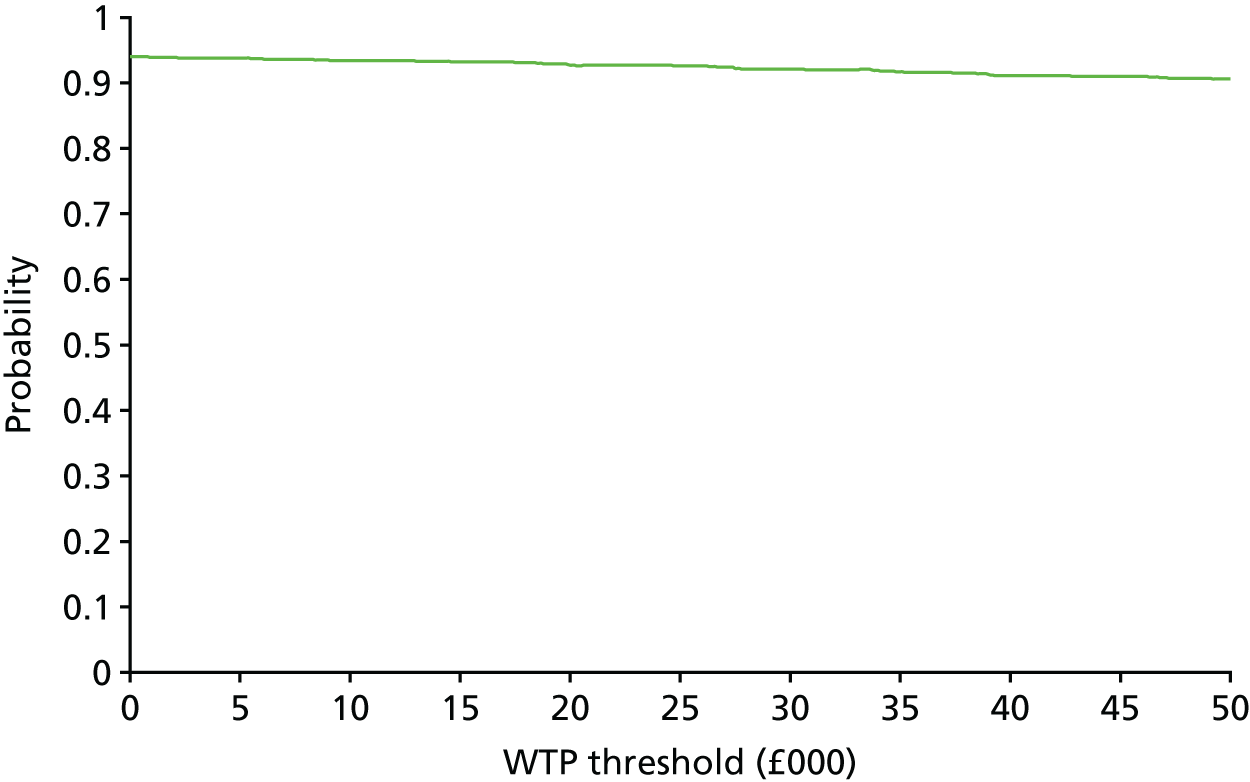
Again, the threshold value placed on a QALY has a very modest impact on uncertainty. Now, however, the likelihood that the intervention is cost-effective increases to a little over 90%. This reflects additional cost savings attributable to the intervention that are not included in the health and social care perspective (costs falling on patients/families and the cost of informal care).
To generate a CEAC for the primary clinical outcome measure, a range of values capturing the threshold value that a decision-maker might place on the outcome (a unit reduction in SSS score) needs to be specified. The score range for the SSS score is 10–56. We chose a range of values from £0 to £2000 per point. At the maximum value of £2000, movement from the worst possible to the best possible SSS score would be valued at £90,000. The resulting CEACs from the health and social care (Figure 13) and societal (Figure 14) perspectives are very similar to those generated when QoL is assessed using EQ-5D-5L tariffs (utility). Again, the treatment protocol appears to be cost-effective.
FIGURE 13.
Cost-effectiveness acceptability curve plotted using health and social care costs and SSS scores.
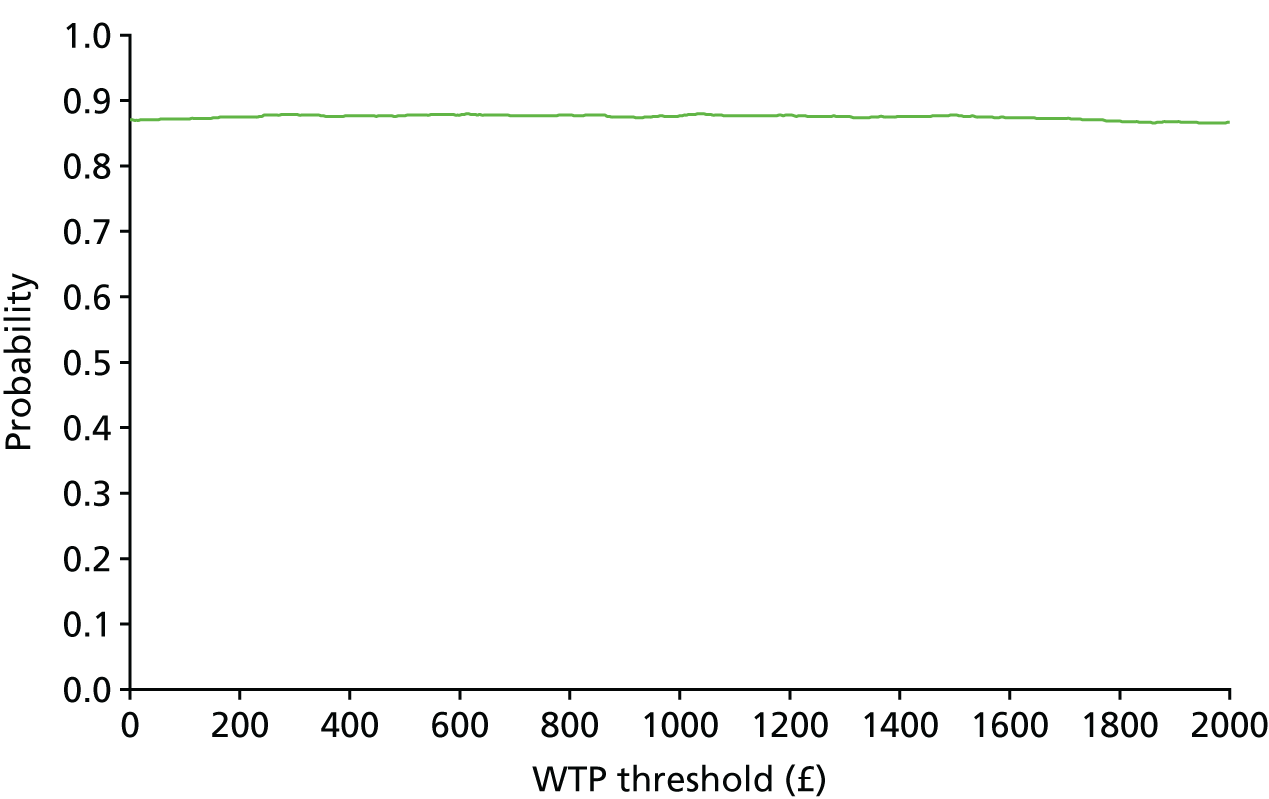
FIGURE 14.
Cost-effectiveness acceptability curve plotted using societal costs and SSS scores.
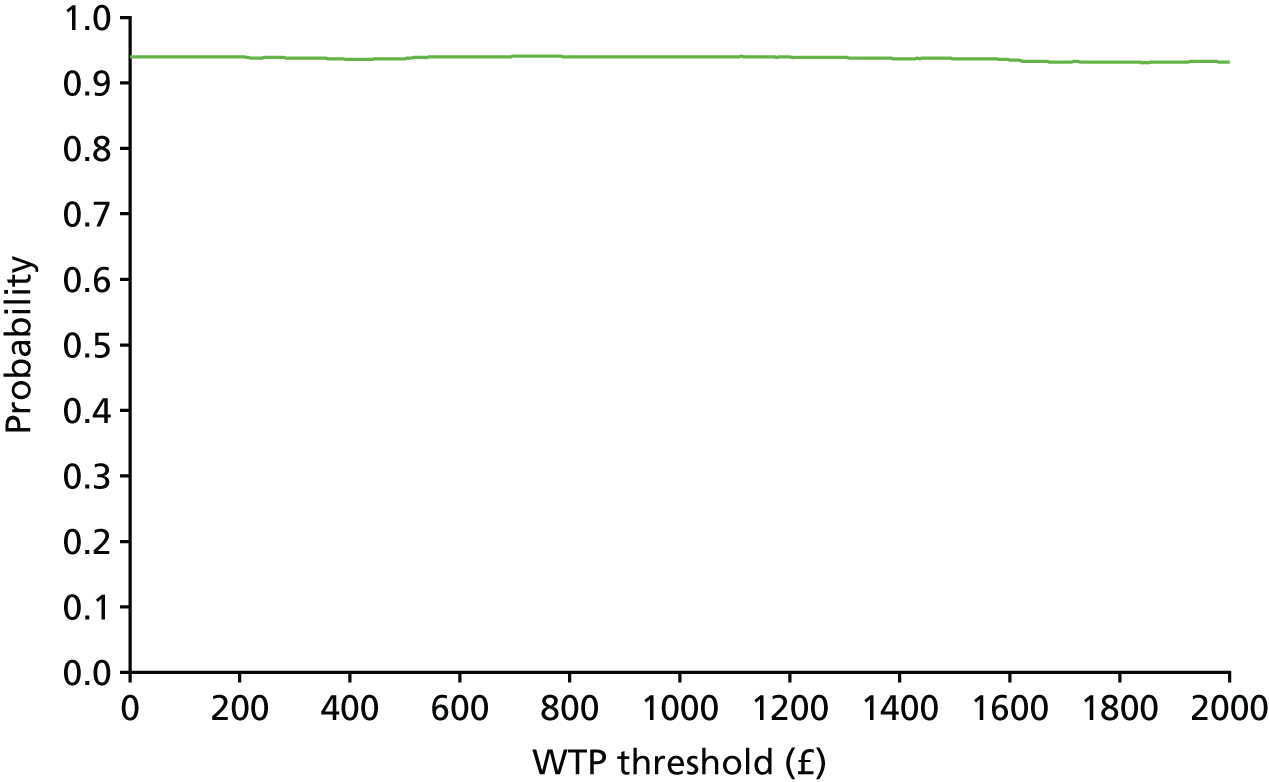
Sensitivity analysis
Subgroup analysis according to intellectual disability
The impact of the extent of ID was investigated in subgroup analysis. The resulting CEACs for the mild/moderate and severe/profound subgroups are shown in Figures 15–18. In the mild/moderate group there is considerable uncertainty regarding the cost-effectiveness of the intervention when the outcome measure is the EQ-5D-5L score, which increases as the threshold value placed on a QALY is increased (see Figures 15 and 16). Indeed, at £50,000 there is a < 50% chance that the intervention is cost-effective. In contrast, for those with severe/profound ID there is a greater chance that the intervention is cost-effective and the value placed on outcomes has no influence on cost-effectiveness at all. These results are consistent with the intervention effects, as measured by changes in QoL on the EQ-5D-5L, being concentrated among patients with mild or moderate ID. The impact of the intervention on costs is more consistent across ID subgroups but is more likely to be cost saving in patients with severe or profound ID.
FIGURE 15.
Cost-effectiveness acceptability curve plotted using health and social care costs and QoL measured with the EQ-5D-5L for patients with mild/moderate ID.
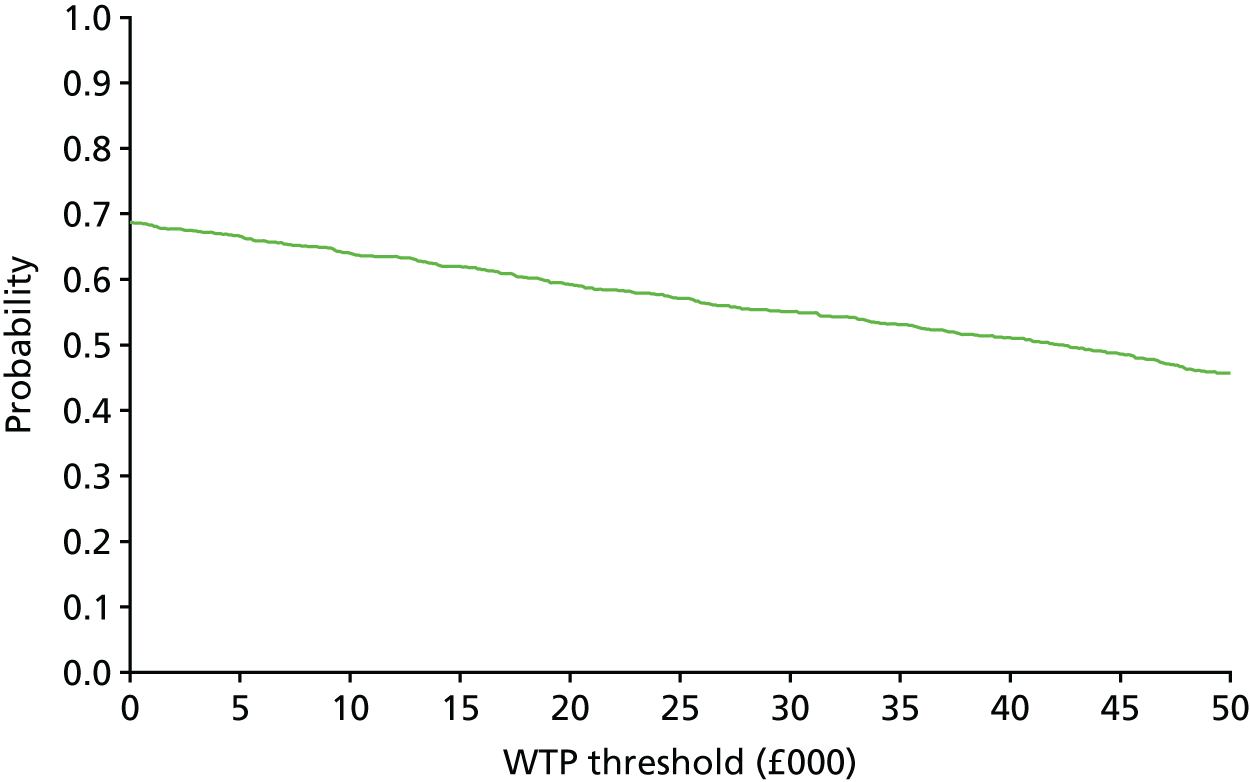
FIGURE 16.
Cost-effectiveness acceptability curve plotted using health and social care costs and QoL measured with the EQ-5D-5L for patients with severe/profound ID.
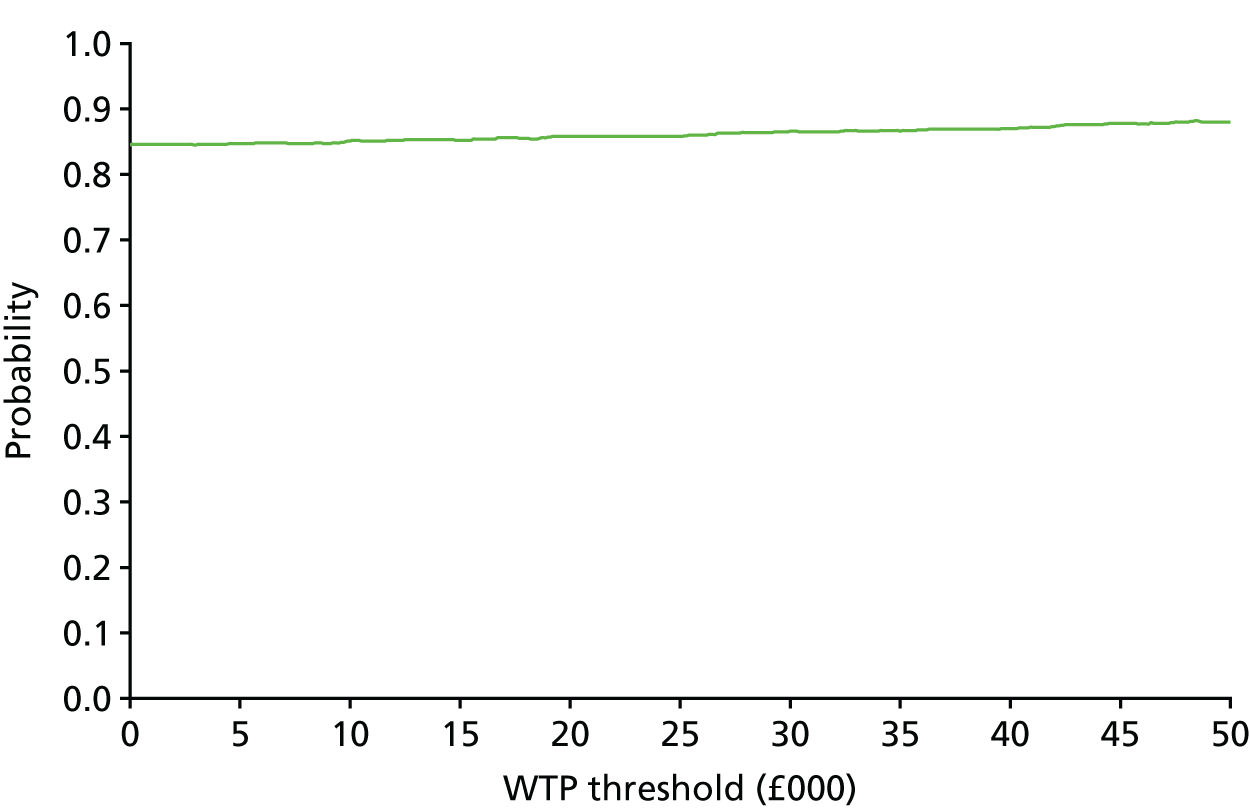
FIGURE 17.
Cost-effectiveness acceptability curve plotted using health and social care costs and QoL measured with the ELDQoL-SSS for patients with mild/moderate ID.
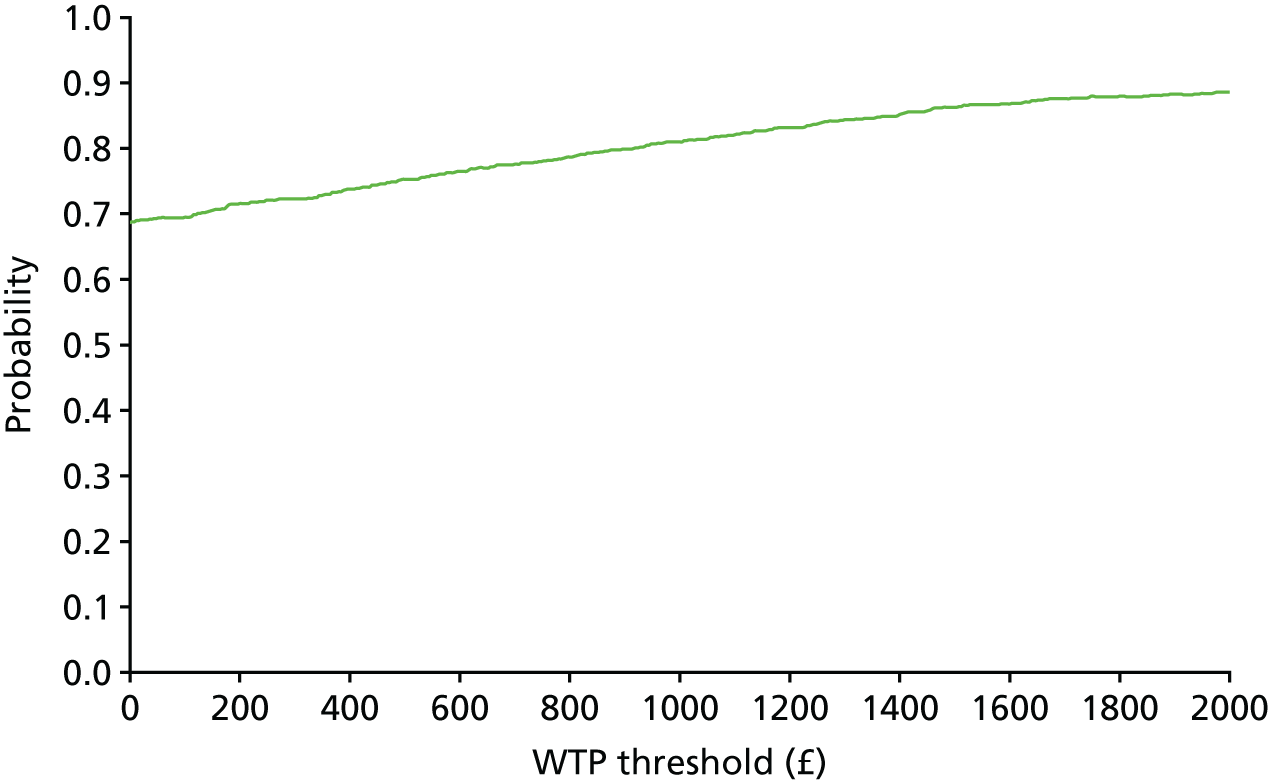
FIGURE 18.
Cost-effectiveness acceptability curve plotted using health and social care costs and QoL measured with the ELDQoL-SSS for patients with severe/profound ID.
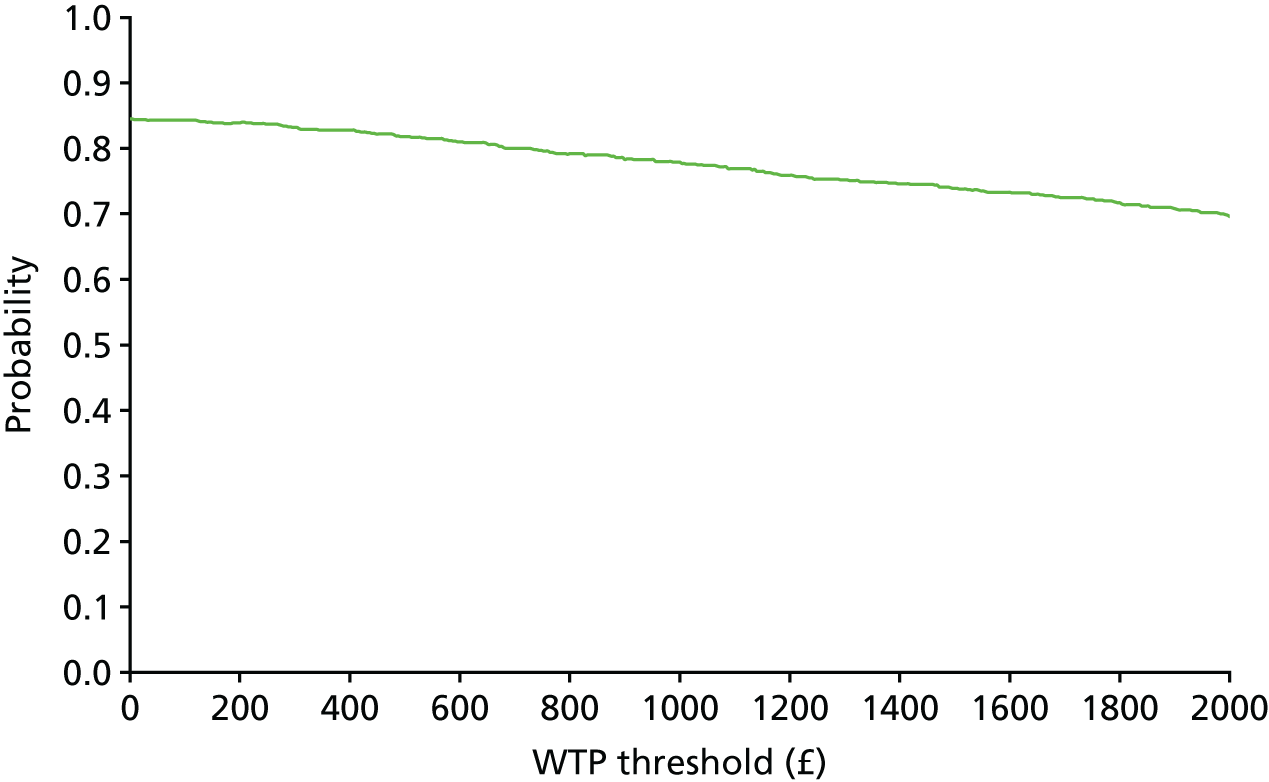
A slightly different picture emerges when the outcome measure is the SSS score. Here, the intervention has a modestly positive impact in patients with mild or moderate ID. This is evident in the CEAC (see Figure 17), which rises as the value placed on a point improvement in the SSS score increases. This is paired with a smaller chance of cost savings in this subgroup compared with those with severe/profound ID so that, across the range of values examined, the cost-effectiveness increases from 68% to 88%. For patients with severe/profound ID the impact of the intervention on SSS score is negative. In the absence of consideration of outcome (threshold value of £0) the intervention has an 84% chance of being cost-effective in this group (see Figure 18). However, as the value placed on a unit improvement in SSS score increases, the likelihood of cost-effectiveness falls, to around 70% at a threshold value of £2000 per unit gain in SSS score.
Exclusion of accommodation costs
The impact of excluding accommodation costs is to increase the likelihood that the intervention is cost-effective (Figure 19). This is somewhat counterintuitive when one considers the raw trial data, which indicate a much larger rise in accommodation costs in the control arm from baseline to follow-up than in the intervention arm. The overall cost savings from the intervention will be smaller in the absence of those accruing from changes in accommodation. However, accommodation costs for those in the formal care sector are high and are likely to dwarf other costs. Excluding these costs may reduce the overall variability in the treatment effect on costs, with the result that a higher proportion of bootstrap replications generate cost savings for the intervention arm, albeit of more modest size.
FIGURE 19.
Cost-effectiveness acceptability curve plotted using health and social care costs, excluding accommodation costs, and QoL measured with EQ-5D-5L.
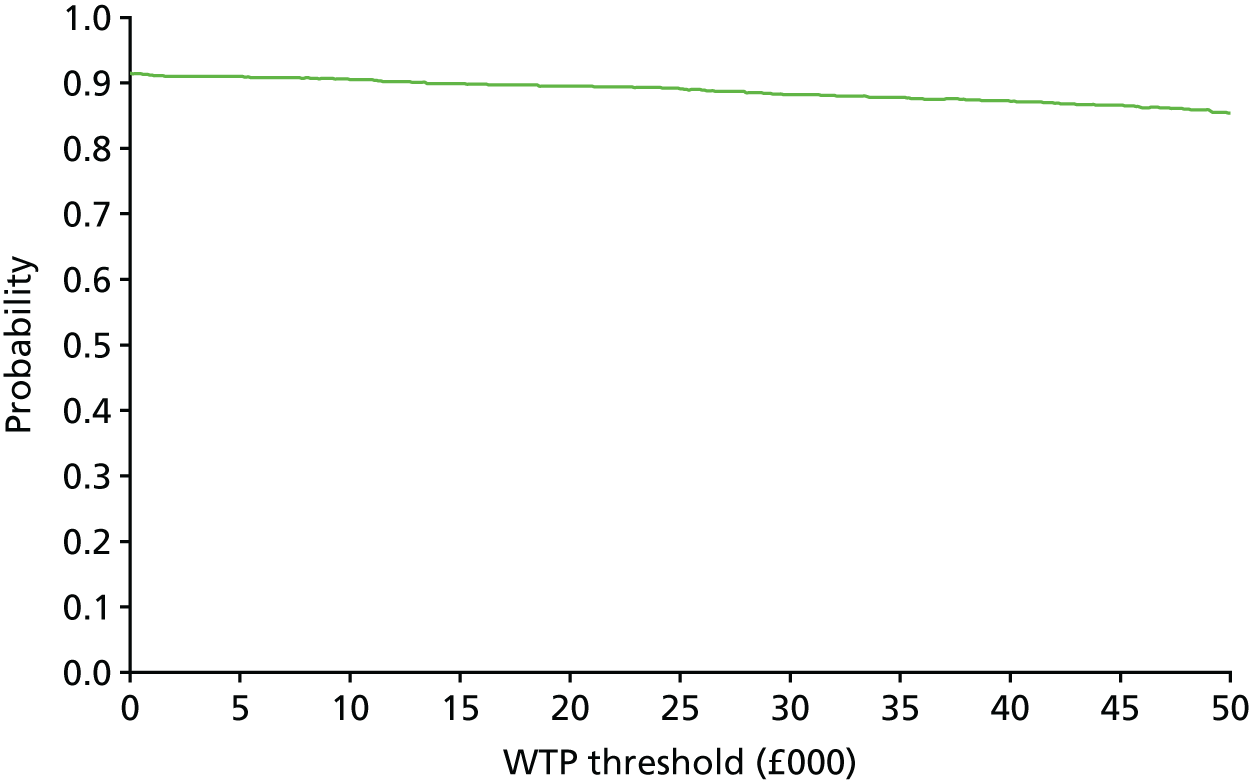
Application of two-stage bootstrapping
When the data were first imputed and then bootstrapped using a two-stage bootstrap routine, the treatment effect on monthly health and social care costs and on EQ-5D-5L tariff values was –£646 and –0.029 respectively. Applying the previously stated assumptions regarding the interpolation of costs and outcomes between baseline and follow-up results in an ICER for usual care of £264,000 per QALY.
Preferences of carers
The responses of participants’ primary carers when asked to value the care that the participants had received are reported in Table 31. Of the 129 participants with a friend or relative as a primary carer, we received a questionnaire response from 75 (58%). Table 31 reports the proportion of respondents who perceived either an improvement or a worsening of care at follow-up compared with baseline. The vast majority of respondents did not report any change in the quality of care, with no difference between the intervention arm and the control arm. Carers who perceived a change in care were asked to value the care that they perceived as superior. Nine of the 13 carers stated a maximum monthly payment that they would be prepared to pay for their preferred level of support. Values ranged from £5 to £1000. No further analysis of these data was undertaken.
| Treatment arm | Judgement on support over the last 6 months | ||
|---|---|---|---|
| Improved | No change | Worsened | |
| Framework | 4 | 40 | 3 |
| TAU | 3 | 22 | 3 |
Qualitative outcomes
The interviewees consisted of 11 family carers, of whom six were in the active arm; eight nurses, of whom four were in the active arm; and 10 paid support workers, of whom six were in the active arm.
Interviews with family carers
Family carers’ experiences of epilepsy nurses varied considerably. In part, this was because of differences in the severity and frequency of the seizures affecting their children, but also because their adult children received care and treatment from nurses with various levels of training and expertise. Some patients saw nurse prescribers, whereas other patients’ epilepsy was managed by a psychiatrist or a neurologist and they saw nurses with little in the way of specialist epilepsy training. Family carers in this latter group described nurses who had little direct involvement in epilepsy management, although these nurses might visit them at home and respond to other concerns about the health and/or social care of their son or daughter. Irrespective of the qualifications of the nurse (and the trial arm in which participants had been treated), what these family carers appreciated was a nurse who was approachable and who could be contacted quickly when there was a problem. Moreover, family carers particularly valued nurses who they saw as being able to communicate effectively with other health and social care professionals, especially with respect to writing care plans, providing or arranging epilepsy training for paid support staff and securing social care funding for specialist equipment. All of the family carers who we interviewed were appreciative of the nursing service that they were receiving but it was the personal characteristics and manner of the nurses that were valued rather than their level of training and expertise in epilepsy. That said, family carers did expect nurses to be aware of the side effects of AEDs and how these might impede opportunities for social inclusion, as well as the impact of comorbid physical health problems. Nonetheless, it was also apparent that, apart from the personal qualities of the nurses, these family carers were generally poorly equipped to evaluate the service received by their son or daughter – they were largely unaware of the possibility that epilepsy management could be differently organised. They were also unable to comment on the effectiveness of the care and treatment received, except to note that their son or daughter’s medication had not been changed for some time.
Interviews with nurses
Interestingly, nurses in both arms struggled to recall any ‘training’ that they had received as part of their participation in the EpAID trial. Whether in the competency framework or the TAU arm, the nurses who we interviewed were alert to the fact that epilepsy management was complicated by the treatment of physical illnesses; they were sensitive to the potential side effects of AEDs and the impact that this could have on people’s lives. All respondents were aware of their duties under the Mental Capacity Act 200574 (England and Wales) to involve patients in decisions about their care and treatment, even if they lacked the capacity to make treatment decisions. Whether a patient was supported by family members or by paid support workers, all of the nurses who we spoke to were aware of the important role that these carers played in managing and reporting seizure activity. All of the nurses described working in multidisciplinary clinical teams and saw this as beneficial. A minority of the nurses did report, however, that there was less integration with social care than there had been in the past.
Paid support staff
Like family carers, the paid support staff who we spoke to had different experiences of nursing involvement in the care and treatment of a person’s epilepsy, which related to the different levels of professional expertise of the nurses and differences in the arrangements of the services in which they worked. Nonetheless, all of the paid support staff spoke highly of the nurses who they saw and again this was related to the personal qualities of the nurses, in particular their ability to communicate and share information. As with the family carers, paid support workers reported that the nurses who they saw were alert to the side effects of medications, appreciated the significance of comorbid physical health problems and understood that epilepsy and the medication used to treat it could affect opportunities for social inclusion.
Overall conclusions from the qualitative interviews
There was nothing in the interview data to suggest that there were any clearly recognisable differences between the two arms of the study. In both arms it appeared to be the personal characteristics and manner of the nurses that were important in determining how family carers and paid support workers felt about the nursing input received by the participants. It was observed that services in which the nurses worked, and in which participants – supported by their family or paid workers – accessed epilepsy care and treatment, varied significantly, both within and between treatment arms. Although we did not specifically ask respondents about how epilepsy services were organised, their accounts nevertheless revealed considerable differences: epilepsy treatments were accessed through psychiatrics, neurologists or nurse prescribers; services had different set-ups with respect to the holding of regular clinics and the making of home visits; there were variations in the degree to which nurses saw patients themselves or referred them on to other clinicians; and there were variations in how health services related to members of social care teams. However, there was no evidence that how these different aspects of service delivery were perceived by the respondents was particularly determined by which arm of the trial they were associated with.
Chapter 4 Discussion
The intended goals of the EpAID trial
Anecdotal evidence and data from open studies have suggested that epilepsy nurse-led management may improve some clinical outcomes and reduce treatment costs in adults with epilepsy and ID. 12,13,15–21,30 However, a recently updated Cochrane review of non-pharmacological management of epilepsy76 did not identify any RCTs of nurse-led interventions for epilepsy and indeed found only one study, of a neurosurgical intervention, that met the requirements for inclusion in the whole review. Against this background, the aim of the EpAID trial was to test the hypothesis that a nurse-led intervention, the Learning Disability Epilepsy Specialist Nurse Competency Framework, would lead to cost-effective reductions in seizure severity and improvements in other measures of QoL for patients with ID and epilepsy and those who provide care for them in the community compared with TAU.
Interpretation of findings from the primary and secondary end points
The primary objective of the trial was to determine whether or not the participants in the active arm of the trial experienced a reduction in their seizure severity, as measured by the ELDQoL-SSS, compared with the participants who had continued to receive their usual treatment. Using an ITT analysis controlling for key baseline individual and cluster-level variables, no significant difference between the experimental arm and the TAU arm in this measure of seizure severity was observed.
Considering the secondary end points – scores on the ELDQoL AED side effects scale, the ELDQoL behaviour scale, the ELDQoL mood scale, the MCSI (a measure of the strains and burdens of providing care as perceived by family carers) and the number of tonic–clonic seizures – during the follow-up compared with the baseline observation period, again, there were no significant differences between the experimental arm and the TAU arm.
There is thus no evidence in adults with an ID and active epilepsy, as a group, that the introduction of the competency framework led to a reduction in overall seizure severity or to improvements in any of a range of other consequences of epilepsy and its treatment for the participants or their family carers. The absence of any change on the MCSI is in line with the finding that, when carers who were family members were asked during follow-up, the great majority did not report any change in the quality of care received or the perceived value of it. Likewise, the qualitative analysis of semistructured interviews with family carers and paid support workers demonstrated that, although these respondents were alert to various aspects of the perceived nature and quality of their interactions with nurses delivering treatment, their perceptions were not shaped in line with which arm of the study they had been associated with but with the personal qualities, as the respondents perceived them, of the nurses.
It is noted that for all ELDQoL and MCSI outcomes there was a significant effect of baseline scores on follow-up outcomes, with higher scores at baseline being associated with greater decreases in pathological scores at follow-up. This pattern of results is most likely to reflect simple ‘regression to the mean’. The Hawthorne effect – a non-specific change in behaviour as a motivational response to the interest, care or attention received through observation and assessment77 – could also have resulted in improved outcomes over the course of the trial but is less likely to have led to those with more pathological states at baseline gaining relatively larger benefits at follow-up. With respect to changes in SSS scores in particular, in several of the services, entry into the team in which the trial took place may have followed a recent deterioration in a participant’s epilepsy. It is possible that this deterioration may have been self-limiting, irrespective of any epilepsy treatment received, for instance, if it occurred as a result of an infection that was subsequently treated. Although this could also have contributed to the observation that higher baseline scores were associated with greater falls at baseline, this referral model was used in only a minority of the teams and we do not consider that it is likely to have played a significant role.
With respect to the economic analyses, however, the results indicate that nurses asked to work in line with the competency framework are likely to be cost-effective compared with nurses working in the TAU arm, although there is considerable uncertainty in this finding. Thus, the use of the competency framework appeared to result in a modest decrement in QoL assessed using the EQ-5D-5L and the SSS of the ELDQoL, but a sizeable reduction in the costs associated with caring for participants. However, the 95% confidence limits estimated from the bootstrap replicates include zero for both societal and health and social care costs and both QoL measures. Likewise, the likelihood that the intervention is cost-effective is always below 95% across the range of thresholds considered in the base case and all sensitivity analyses for both QoL outcome measures.
Overall, the economic analyses indicated that there appears to be only a very modest chance that nurses working according to the competency framework will increase costs and a far greater chance that this will reduce treatment costs compared with TAU. The data also suggest that such a role was detrimental to patient care, albeit the impact is small. Such findings might be consistent if the framework role reduced duplication and unnecessary services, which occasionally left patients less well supported overall.
The origin of the apparent cost-effectiveness also needs to be considered. The raw data reported in Table 29 showed that there was very little change in total health and social service and societal costs from baseline to follow-up in the framework arm but a larger increase in these costs in the usual care arm. This pattern of results is reflected in the relative cost savings in the framework arm reported in Table 30 following bootstrapping and adjustment for baseline variables. Thus, over the course of the trial the costs rose more for those in the TAU arm than they did for those in the framework arm. The reasons for this are not clear. One speculative interpretation is that, as a result of being involved in the trial, nurses in both arms changed their behaviours but that, although the competency framework led nurses in the active arm to engage in actions that resulted in cost-effective interventions, nurses in the TAU arm, without that set of guiding principles, made changes that resulted in increased costs. Against this, however, it is noted that in both arms the, albeit, small number of responses from family carers revealed that most had not perceived any difference in the treatment received by participants during the intervention period. In addition, it is hard to see how such an explanation could explain the larger increase in societal costs during follow-up for those in the TAU arm.
Overall, however, at conventionally accepted WTP thresholds, the competency framework appears to be cost-effective. Although less clinically effective than usual care, the role appears to be associated with a reduction in the cost of supporting patients compared with TAU as delivered in this trial, which would justify its application. Differences in both costs and outcomes were not significant at the 5% level; at a threshold of £20,000–30,000 per QALY the likelihood that the intervention is cost-effective is 75–80%. It should be noted that, although our analysis indicates a potential for the competency framework to reduce costs, it is possible that there are additional costs associated with the implementation of the competency framework that were not captured within our trial.
Interpretation of the planned subgroup analyses
Although no benefits of the competency framework were observed across the participants randomised to the active arm as a whole, there was a significant interaction between ELDQoL-SSS score and treatment arm and level of ID, suggesting a differential response to the intervention according to whether a participant had a mild to moderate or a severe to profound ID. Thus, for those with a severe to profound ID, there was no evidence of either treatment being superior, whereas among those with a mild to moderate ID there was a trend towards a beneficial effect in the active treatment arm. This possible effect should, however, be considered with caution as the treatment effect was not significant in either arm.
The EpAID trial is, by some distance, the largest reported study of a nursing intervention related to epilepsy in adults with an ID and, as far as the authors are aware, no previous study has examined the question of whether or not there is any relationship between the severity of a person’s ID and the extent to which they may benefit from more structured nursing input. It is possible that, as people with a severe-to-profound ID generally have more severe and pervasive brain dysfunction than those with a less severe ID, the capacity for their epilepsy to improve in response to the use of the competency framework is more limited, especially as the framework tends to focus on optimising current treatment approaches. Certainly, in any future research on, or application of, the competency framework, our results suggest that consideration should be given to the severity of the ID present.
Issues to consider regarding delivery of the interventions
Examination of how the nurses involved in the trial delivered management to the participants suggests another possible benefit of employing the competency framework. It was observed that, on average, the nurses in the competency framework arm engaged in five clinical contacts with their patients over the course of the intervention, compared with an average of seven contacts reported by the nurses delivering TAU. Given that the outcomes in the two arms of the trial were relatively similar it may be that the use of the competency framework facilitates more efficient working.
However, when considering this possibility it should also be noted that, although community teams were randomly allocated to the competency framework and the TAU arms, subsequent examination of the nurse competencies recorded at baseline indicates that in the control arm there were more ‘novice’ nurses, with limited previous experience of managing epilepsy in adults with ID, whereas in the experimental arm there were more nurse prescribers with high levels of relevant prior clinical experience. These differences in therapist expertise between the arms were taken into account in the analysis of the primary and secondary end points. However, when examining the pattern of activities engaged in by the nurses during the trial, it is important to consider the effect that this imbalance could have exerted. For instance, the greater proportion in the competency framework arm of clinical contacts involving a response to urgent health or behavioural concerns may have arisen because the nurses in that arm were in general more experienced and therefore perhaps more likely to deliver such interventions; in teams in which the nurses were less experienced, such interventions may have been delivered by other members of the multidisciplinary team. Similarly, the greater proportion in the competency framework arm of contacts involving the prescribing of AED is likely to relate to the greater number of nurse prescribers working in that arm.
Safety issues
Six participants died between their entry into the trial and the end of the collection of their follow-up data. In addition, a further two participants died after their involvement in the trial had finished but before their research sites had been closed. As noted in Chapter 3 (see Safety findings), there was no significant difference between the arms in the proportion of participants who died.
As stated in Chapter 1 (see Epilepsy and intellectual disability), people with ID and epilepsy have particularly high rates of death. 5 Robertson et al. ,78 in a systematic review of 16 studies drawn from eight high-income countries, observed that mortality rates were higher in those with an ID and epilepsy than in comparable populations with just one of these conditions. They also noted, as others have done,79 that increased mortality rates in people who have both an ID and active epilepsy may in part be explained by the observations that those with a more severe ID are also more likely to have epilepsy than those with milder levels of intellectual impairment and that people with a more severe ID in general have higher mortality rates.
For six of the eight participants who died, their death was attributed to a respiratory problem. This is in line with data from the analysis by Glover and Ayub7 of causes of death in those with an ID, as described on death certificates for all deaths in England between 2004 and 2008, which found that the most common cause of death for people with an ID was respiratory disease, reported in 52% of the sample. Although there are significant limitations of this analysis, in that the authors estimated that only around half the number of death certificates that they would have expected to mention an ID actually mentioned one, based on the prevalence of ID in England, it is the largest study of its kind to have analysed these figures.
Overall, no differences were observed between the study arms in the frequency of SAEs. Although in both arms 16% of the participants were admitted to hospital on at least one occasion, it is already known that the clinical population under investigation has, as summarised in Chapter 1 (see Epilepsy and intellectual disability), high rates of morbidity. Hence, in summary, there is no evidence that the use of the competency framework was associated with morbidity or mortality rates that differed from those seen for TAU.
Qualitative outcomes
As noted earlier, although family carers and paid support workers formed various views about the nurses with whom they had contact during the trial, there was no evidence that these views were shaped by which arm of the trial they had been exposed to. However, it is apparent from the data that we collected that family carers and paid support workers had very few grounds on which to evaluate the service that they received from nurses, beyond how they felt about an individual nurse; in this trial, in both arms, it was observed that these respondents were likely to be appreciative of the nurses working with them. Therefore, although some respondents spoke negatively about past experiences or reported that the nurse who they saw was not particularly involved in the practicalities of epilepsy management, because the person concerned also saw a psychiatrist, they lacked sufficient awareness of variations between services to evaluate the service that they used. Given the uncertainties of epilepsy management, they were also unable to comment on the effectiveness of a person’s treatment beyond noting – in some cases – that his or her medication had not been changed for some time.
The nurses interviewed were not asked which arm of the trial they thought that they had been allocated to. Rather, in this part of the analysis we explored whether or not there were implicit differences in the nature of the nurses’ responses that could be related to which arm of the trial they had been allocated to. As noted in Chapter 3, none was found. The impression gained from the nurses’ interviews, supported by the comments made by family carers and paid support workers, was that, across both arms of the study, nurses considered that they were able to conscientiously discharge their responsibilities. It is reassuring that the nurses had this perception. However, from the point of view of the trial we cannot conclude whether or not those nurses allocated to the competency framework arm thought that their clinical practice had been changed by the use of the framework. However, given that the trial design involved obscuring from nurses which arm they were in, and that, in general, they did not, when asked, actually recall any formal training related to the trial, this is not surprising and it is likely, at least in the small sample of nurses interviewed, that they did not have expectations that could have biased their performance in the trial according to which arm they were actually allocated to.
The generalisability of the trial findings
Approach to data analysis
Given the challenges of collecting cost and outcome data in this group of patients, the levels of missing data were relatively modest. However, the analysis of cost-effectiveness in cluster randomised trials, with missing data and the potential for baseline differences to influence the results, is challenging. 80 We prioritised the need to account for missing data in a principled manner along with adjustment for any differences in baseline covariates. In the economic analysis we used bootstrapping to quantify the uncertainty in costs and outcomes. Such an approach makes no parametric assumptions (although our adjustment for baseline imbalances did) and captures any correlation between costs and outcomes. However, this approach did not explicitly allow for the clustering of data in the sampling routine. We addressed this in the economic analysis with a sensitivity analysis in which we undertook the imputation of missing data first and then applied a two-stage bootstrap routine to each of the imputed data sets. This latter approach has the weakness that it did not support adjustment for baseline imbalances in patient covariates. However, the similarity in the results of the two approaches is encouraging. Hospital costs were limited to costs associated with epilepsy.
In the course of the analysis, a number of secondary hypotheses were tested. No corrections for multiple comparisons were made and therefore the results of these tests of the secondary hypotheses should be considered with caution.
Study blinding
Clinicians in both arms were aware that they were part of a treatment trial and all had received direct training from a senior nurse. Although those in the TAU arm were not explicitly taught about the competency framework, they were aware that the focus of the research was on how to manage epilepsy in people with an ID and epilepsy. As a consequence they may have tried to improve the management that they provided for patients involved in the study,81 potentially overshadowing the effects of the intervention. However, the authors are not aware of any clusters in which the nurses delivering treatment were explicitly aware of what approaches were being compared and which arm they were in.
Variability in clinical services
It has been noted previously that undertaking RCTs of complex interventions in ID services may be difficult and, in particular, that regional variations in practice contribute to difficulties in evaluating such interventions. 82 In the case of epilepsy management in adults with an ID, variations in practice exist internationally, as reflected in an earlier exercise to develop international expert consensus-based guidelines,9 as well as locally. 83 However, variability in how epilepsy is managed in this clinical group has persisted and was one of the key rationales for undertaking the EpAID trial. Nonetheless, as a consequence of this, although within the competency framework arm the expectation was that this variability would be reduced, in the TAU arm it is likely that any existing variability between services persisted. It has been pointed out in other contexts that, when there is such variability, to support generalisability to other clinical settings, management in the control group should not deviate too much from existing local practices. 81 When there are major baseline differences in practice between the participating centres, having limited numbers of clusters in the arms may also limit generalisability.
In defence of the generalisability of the trial in the face of appreciable baseline variability in service delivery, the following points may be made. The competency framework acknowledges that there is large variability in how community ID services operate and in how they are set up to manage epilepsy. The EpAID trial was designed to test the effects of making use of the competency framework in this real-world environment. Hence, no structural or personnel changes were made in any of the clinical services in which the research clusters were located. The research did not examine what happened when a service delivered management exactly as optimally described in the framework. Rather, it tested the hypothesis that nurses, working as far as they could towards the goals described by the framework and within the constraints of their existing service, would improve epilepsy-related outcomes for adults with an ID compared with nurses working without this set of explicit competency aspirations. The variable nature of pre-existing epilepsy services in the various sites comprising each arm was in theory taken account of by the random allocation of services to the treatment arms. This approach had the benefit that the potential value of using the competency framework could be more readily generalised to the full range of community ID services in England, Scotland and Wales and that the treatment being trialled did not require the more unrealistic and potentially expensive process of remodelling CIDTs specifically to enable full delivery of all of the competency aspirations. However, it also meant that, within the active intervention arm, the extent to which the competency framework was delivered varied from service to service. This may have reduced the scope for finding significant differences between the arms. However, in support of being able to generalise to other ID services, the EpAID trial made use of cluster randomisation to protect against contamination between arms84 and within each cluster the nurses managed participants using just one approach. In addition, the recruitment of 17 individual cluster sites from across England, Scotland and Wales, with each site managed by a different health-care delivery organisation (NHS trust), optimised the external validity of the trial.
Specific limitations associated with the undertaking of a treatment trial in adults with an intellectual disability living in the community
Recruitment to the trial proved relatively challenging. This was partly explained by the fact that, not only did the participants themselves need to be willing – if they had capacity – to consent to participate but, for the majority who lacked capacity, an appropriate individual also needed to be identified who was able and willing to act as a consultee (in England and Wales) or guardian, welfare attorney or nearest relative (in Scotland) and who would then also agree to the trial. To overcome these challenges we worked closely with the community teams delivering care to this patient group and, through them, aimed to constructively involve carers, particularly family carers.
The importance of this approach was illustrated in a previous qualitative study of parents of adults with an ID and epilepsy, which observed that, in their role as gatekeepers to access by health services to their grown-up children, the extent to which parents facilitated their offspring’s participation in therapeutic activities depended on the parents’ views, as opposed to the clinicians’ views, of those activities. 10
A related and necessary consequence of the ID of all of the trial participants was the key role in the trial of family carers and/or paid support workers in terms of providing the outcome data. Thus, the outcome measures depended on the perceptions of these others of the state and progress of the participants and the outcome measures were selected to be appropriate for completion by these informants.
As the research team were aware of these potential challenges to recruitment, the following steps to minimise attrition were designed into the trial: (1) participation in the trial resulted in very few active demands being made on the participants, (2) participants were eligible because they had ongoing seizures, meaning that they and those supporting them were able to readily identify the potential value of the trial, (3) participation in the trial did not preclude receipt of any other clinically indicated treatment and (4) the research team followed a flexible informant-led approach to gathering baseline and follow-up data, with contacts taking place at times and locations suggested by participants and respondents and with, as far as possible, contacts being made face-to-face, by telephone or by post, as preferred by respondents.
There are, however, issues that may arise when using telephone contact to complete questionnaires. Questionnaire interviews conducted over the telephone may have been more readily misunderstood than face-to-face interviews, even if informants had a copy of the relevant questionnaire in front of them (which was not always the case). On the other hand, telephone calls may have been more convenient as they were scheduled around the needs of the respondents, including being able to receive calls during the evening and at weekends, which is likely to have reduced the risk of a delay in obtaining, or failure to obtain, baseline or follow-up measures.
Further issues that may have affected the trial relate to the different accommodation and care arrangements that existed for the participants. In both arms, the great majority of participants lived either in a group home or with members of their family. However, in the competency framework arm, a slightly higher proportion of participants lived in a group home than with family, with the opposite pattern observed in the TAU arm. The potential consequences of these different living arrangements may relate to issues around consent, data gathering and how paid or family carers might differ in delivering recommended treatments. Informed consent can be more difficult to gauge when the participant is reliant on others. Participants’ choices may be limited: they may be used to saying ‘yes’ or ‘no’ to every option, which clouds their ability to make a judgement related to informed consent. Furthermore, the researcher may have been seen as an authority figure, leading to acquiescence by the potential participant. 85 With respect to the gathering of baseline and follow-up data for participants living with family carers, in general a relatively small number of carers were involved in delivering care, meaning that the informant completing the baseline and follow-up assessments may have been more likely to be in possession of all of the required information. For the participants living in a group home, however, where care duties may be split and a larger number of paid support workers are often involved in delivering care, the quality of information transfer relevant to providing the outcome measures will have been an important factor. In terms of how paid or family carers might differ in actually delivering recommended treatments, it is noted in a study from the USA that the adherence to prescribed AED regimens was greater for those living in a group home than for those living with their family. 86
In summary, there are a variety of ways in which living the life of a person with an ID may introduce challenges into the implementation and interpretation of a clinical trial of a complex intervention.
Conclusions
Differences in outcomes between the competency framework arm and the TAU arm were limited and associated with various degrees of uncertainty, as quantified in Chapter 3. As noted in Chapter 1, there is currently a very limited database from which to draw firm conclusions about how best to manage AED-refractory epilepsy in adults with an ID and, as discussed in earlier sections of this chapter, there are a range of challenges in undertaking a trial of a complex intervention in this population. The EpAID trial did not identify any significant clinical differences between the outcomes in the two arms to warrant changing clinical practice to incorporate the competency framework as it was employed in the trial. However, although noting both the small effects of the intervention and the limitations of the trial, the results suggest that the competency framework is likely to be cost-effective when employed in the CIDT context to inform the management of epilepsy in adults with an ID, although, for the population of adults with an ID and epilepsy as a whole, there was no clinical benefit compared with TAU.
The observation of a trend towards the use of the competency framework in those with a mild-to-moderate ID being associated with a small amount of benefit in terms of reducing concerns over seizure severity suggests that this could usefully be explored in future research. It should also be noted that the EpAID trial tested the use of the framework in the short term, meaning that the elements of continuing professional development that are built into the framework were not tested. Nurses with experience in ID and epilepsy could be well placed to deliver or facilitate the epilepsy management recommended for adults with an ID by the relevant clinical guidelines. 87 Future research will be able to explore the specific value of the competency framework for those with a mild to moderate ID and the potential for greater long-term benefits arising from application of the continuing professional development element of the framework.
Acknowledgements
This research was supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) East of England at Cambridgeshire and Peterborough NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR or Department of Health and Social Care.
This study was further supported by the UK Clinical Research Collaboration (UKCRC)-registered KCTU, which is part funded by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC).
The research team are very grateful to all of the participants and their families and paid carers for supporting the trial. We are also grateful to the nurses who delivered the competency framework and the TAU interventions, to the local PIs at the research sites and to the members of several CRNs who supported the trial by collecting consent and data from some of the participants.
Change to the protocol
At the start of the trial the recruitment strategy aimed to recruit up to 34 participants in each of 12 clusters. However, recruitment progress in the early clusters was limited and the recruitment strategy was altered to the aim of recruiting 20 participants from each of 16 clusters.
Contributions of authors
Howard Ring (Psychiatrist) was the chief investigator. He was responsible for protocol development and trial management and contributed to drafting of the report.
James Howlett (Statistician) contributed to statistical analyses and drafting of the report.
Mark Pennington (Health Economist) contributed to protocol development, and drafting of the report and was responsible for the economic methodology and analyses.
Christopher Smith (Trial Co-ordinator) was responsible for the day-to-day running of the trial and data collection and contributed to drafting of the report.
Marcus Redley (Sociologist) contributed to protocol development, development and analysis of the qualitative components of the trial and drafting of the report.
Caroline Murphy (Manager of KCTU) contributed to protocol and drafting of the report and was responsible for database development.
Roxanne Hook (Research Assistant) contributed to data collection and drafting of the report.
Adam Platt (Research Assistant) contributed to data collection and drafting of the report.
Nakita Gilbert (Research Assistant) contributed to data collection and drafting of the report.
Elizabeth Jones (Learning Disability Nurse) contributed to protocol development, and training of the nurses delivering the interventions, coordinated service user involvement and contributed to drafting of the report.
Joanna Kelly (Strategic Data Management Lead and Data Analyst) contributed to database development and maintenance, and drafting of the report.
Angela Pullen (Epilepsy Services Manager, Epilepsy Action) contributed to protocol development, collection or patient and public views on the study development and drafting of the report.
Adrian Mander (Statistician) contributed to development of the statistical methodology employed in the trial, and drafting of the report.
Cam Donaldson (Health Economist) contributed to health economic protocol development and drafting of the report.
Simon Rowe (Senior NHS Commissioning Manager) contributed to protocol development and drafting of the report.
James Wason (Statistician) contributed to protocol development, oversaw the development of the statistical methodology employed in the trial and the statistical analysis of the results, apart from the analyses of the economic data, and drafting of the report.
Fiona Irvine (Senior Academic Nurse) provided expertise in the development of training and training guidelines and the assessment of nurse activity. She also contributed to protocol development, training of the nurses delivering the intervention and drafting of the report.
Publication
Ring H, Gilbert N, Hook R, Platt A, Smith C, Irvine F, et al. Improving outcomes in adults with epilepsy and intellectual disability (EpAID) using a nurse-led intervention: study protocol for a cluster randomised controlled trial. Trials 2016;17:297.
Data sharing statement
All available data may be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ring H, Gilbert N, Hook R, Platt A, Smith C, Irvine F, et al. Improving outcomes in adults with epilepsy and intellectual disability (EpAID) using a nurse-led intervention: study protocol for a cluster randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1429-7.
- McGrother CW, Bhaumik S, Thorp CF, Hauck A, Branford D, Watson JM. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure 2006;15:376-86. https://doi.org/10.1016/j.seizure.2006.04.002.
- Kerr M, Rugg-Gunn FJ, Sander JW, Smalls JE. Epilepsy 2011: From Science to Society. Chalfont: International League Against Epilepsy and Epilepsy Society; 2011.
- Bell GS, Sander JW. The epidemiology of epilepsy: the size of the problem. Seizure 2001;10:306-14. https://doi.org/10.1053/seiz.2001.0584.
- Forsgren L, Edvinsson SO, Nyström L, Blomquist HK. Influence of epilepsy on mortality in mental retardation: an epidemiologic study. Epilepsia 1996;37:956-63. https://doi.org/10.1111/j.1528-1157.1996.tb00533.x.
- Glover G, Evison F. Hospital Admissions That Should Not Happen: Admissions for Ambulatory Care Sensitive Conditions for People With Learning Disabilities in England 2013. www.ndti.org.uk/uploads/files/IHAL-2013-02_Hospital_admissions_that_should_not_happen_ii.pdf (accessed 16 November 2016).
- Glover G, Ayub M. How People With Learning Disabilities Die 2010. www.improvinghealthandlives.org.uk/uploads/doc/vid_9033_IHAL2010-06%20Mortality.pdf (accessed 16 November 2016).
- Pennington M, Prince E, Bateman N, Gray J, Croudace TJ, Redley M, et al. Factors influencing the costs of epilepsy in adults with an intellectual disability. Seizure 2012;21:205-10. https://doi.org/10.1016/j.seizure.2011.12.012.
- Kerr M, Scheepers M, Arvio M, Beavis J, Brandt C, Brown S, et al. Consensus guidelines into the management of epilepsy in adults with an intellectual disability. J Intellect Disabil Res 2009;53:687-94. https://doi.org/10.1111/j.1365-2788.2009.01182.x.
- Redley M, Prince E, Bateman N, Pennington M, Wood N, Croudace T, et al. The involvement of parents in healthcare decisions where adult children are at risk of lacking decision-making capacity: a qualitative study of treatment decisions in epilepsy. J Intellect Disabil Res 2013;57:531-8. https://doi.org/10.1111/j.1365-2788.2012.01556.x.
- Making Reasonable Adjustments to Epilepsy Services for People with Learning Disabilities. London: Public Health England; 2014.
- All Party Parliamentary Group on Epilepsy . The Human and Economic Cost of Epilepsy in England 2007. www.epilepsy.org.uk/sites/epilepsy/files/images/campaigns/arygrouponepilepsy_wasted_money_wasted_lives.pdf (accessed 16 November 2016).
- Hopkins J, Irvine F. Qualitative insights into the role and practice of epilepsy specialist nurses in England: a focus group study. J Adv Nurs 2012;68:2443-53. https://doi.org/10.1111/j.1365-2648.2012.05941.x.
- Hopkins J, Irvine F. Identifying and Monitoring the Cost-Effectiveness of the Epilepsy Specialist Nurse 2010. www.epilepsy.org.uk/sites/epilepsy/files/images/campaigns/ss-of-epilepsy-specialist-nurse-final-report.pdf (accessed 16 November 2016).
- Yedidia MJ. Transforming doctor–patient relationships to promote patient-centered care: lessons from palliative care. J Pain Symptom Manage 2007;33:40-57. https://doi.org/10.1016/j.jpainsymman.2006.06.007.
- Ridsdale L, Morgan M, O’Connor C. Promoting self-care in epilepsy: the views of patients on the advice they had received from specialists, family doctors and an epilepsy nurse. Patient Educ Couns 1999;37:43-7. https://doi.org/10.1016/S0738-3991(98)00094-9.
- Mills N, Bachmann MO, Harvey I, Hine I, McGowan M. Effect of a primary-care-based epilepsy specialist nurse service on quality of care from the patients’ perspective: quasi-experimental evaluation. Seizure 1999;8:1-7. https://doi.org/10.1053/seiz.1998.0232.
- Mills N, Campbell R, Bachmann MO. What do patients want and get from a primary care epilepsy specialist nurse service?. Seizure 2002;11:176-83. https://doi.org/10.1053/seiz.2001.0615.
- Beagan B, Ells C. Values that matter, barriers that interfere: the struggle of Canadian nurses to enact their values. Can J Nurs Res 2009;41:86-107.
- Bradley PM, Lindsay B. Care delivery and self-management strategies for adults with epilepsy. Cochrane Database Syst Rev 2008;1:1-28. https://doi.org/10.1002/14651858.CD006244.pub2.
- Johnson K, McGowan T, Dunkley C. A Review of Epilepsy Specialist Nurse’s Clinical Activity and Impact on Paediatric Admissions 2010. www.cewt.org.uk/CEWT/Research_files/ESN%20BPNA%202010.pdf (accessed 16 November 2016).
- Meads C, Bradley P, Burls A. The Effectiveness of Specific Epilepsy Services. A West Midlands Health Technology Assessment Group Report 2001. www.birmingham.ac.uk/Documents/college-mds/haps/projects/WMHTAC/REPreports/2001/Epilepsyservices.pdf (accessed 16 November 2016).
- Mencap . Death by Indifference: Following up the Treat Me Right! Report 2007. www.mencap.org.uk/sites/default/files/2016-06/DBIreport.pdf (accessed 16 November 2016).
- Reuber M, Gore J, Wolstenhome J, Jonas P, Frankson C, Murray C, et al. Examining a community model of epilepsy care for people with learning disabilities. Seizure 2008;17:84-91. https://doi.org/10.1016/j.seizure.2007.07.004.
- Christodoulou M. Neurological nurse specialists: a vital resource under threat. Lancet Neurol 2012;11:210-11. https://doi.org/10.1016/S1474-4422(12)70030-3.
- Graydon M. Short communication: do learning disability services need epilepsy specialist nurses?. Seizure 2000;9:294-6. https://doi.org/10.1053/seiz.2000.0410.
- Hannah JA, Brodie MJ. Epilepsy and learning disabilities – a challenge for the next millennium?. Seizure 1998;7:3-13. https://doi.org/10.1016/S1059-1311(98)90002-4.
- Whitten E, Griffiths A. Implementing epilepsy guidelines within a learning disability service. Seizure 2007;16:471-8. https://doi.org/10.1016/j.seizure.2007.03.008.
- Goodwin M, Higgins S, Lanfear JH, Lewis S, Winterbottom J. The role of the clinical nurse specialist in epilepsy. A national survey. Seizure 2004;13:87-94. https://doi.org/10.1016/S1059-1311(03)00149-3.
- Irvine F, Hopkins J. The true cost of care. Epilepsy Professional 2010;16:32-5.
- Doherty C, Hughes D, Stafford B, Whitten E, Irvine F. The Learning Disability Epilepsy Specialist Nurse Competency Framework 2013. www.epilepsy.org.uk/sites/epilepsy/files/professionals/LD%20ESN%20Competency%20Framework_v0%203_20Feb13.pdf (accessed 16 November 2016).
- Buck D, Smith M, Appleton R, Baker GA, Jacoby A. The development and validation of the Epilepsy and Learning Disabilities Quality of Life (ELDQOL) scale. Epilepsy Behav 2007;10:38-43. https://doi.org/10.1016/j.yebeh.2006.10.010.
- Campbell MK, Elbourne DR, Altman DG. CONSORT group . CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. https://doi.org/10.1136/bmj.328.7441.702.
- Benner P. From Novice to Expert: Excellence and Power in Clinical Nursing Practice. Menlo Park, CA: Addison Wesley; 1984.
- The NHS Knowledge and Skills Framework (NHS KSF) and the Development Review Process. London: Department of Health; 2004.
- EuroQol Group . EuroQoL – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Beecham J, Snell T, Perkins M, Knapp M. Health and social care costs for young adults with epilepsy in the UK. Health Soc Care Community 2010;18:465-73. https://doi.org/10.1111/j.1365-2524.2010.00919.x.
- Robinson BC. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- Corti L. Using Diaries in Social Research. Social Research Update 1993. http://sru.soc.surrey.ac.uk/SRU2.html (accessed 16 November 2016).
- Higgins S, Lanfear J, Goodwin M. Quantifying the role of nurse specialists in epilepsy: data from diaries and interviews. Br J Neurosci Nurs 2006;2:239-46. https://doi.org/10.12968/bjnn.2006.2.5.21526.
- Higgins S. Outlining and defining the role of the epilepsy specialist nurse. Br J Nurs 2008;17:154-7. https://doi.org/10.12968/bjon.2008.17.3.28403.
- US Food and Drug Administration . CFR – Code of Federal Regulations Title 21 n.d. www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11&showFR=1 (accessed 29 January 2018).
- Great Britain . The Medicines for Human Use (Clinical Trials) Regulations 2004 n.d. www.legislation.gov.uk/uksi/2004/1031/contents/made (accessed 29 January 2018).
- Medical Research Council . MRC Guidelines for Good Clinical Practice in Clinical Trials 1998. www.mrc.ac.uk/documents/pdf/good-clinical-practice-in-clinical-trials/ (accessed 16 November 2016).
- Wagner AP, Croudace TJ, Bateman N, Pennington MW, Prince E, Redley M, et al. Clinical services for adults with an intellectual disability and epilepsy: a comparison of management alternatives. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0180266.
- The Social Care Guidance Manual. London: NICE; 2013.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Devlin N, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2017;27:7-22. https://doi.org/10.1002/hec.3564.
- Pickard AS, Knight SJ. Proxy evaluation of health-related quality of life: a conceptual framework for understanding multiple proxy perspectives. Med Care 2005;43:493-9. https://doi.org/10.1097/01.mlr.0000160419.27642.a8.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. https://doi.org/10.1136/bmj.329.7459.224.
- Jorgensen BS, Syme GJ, Bishop BJ, Nancarrow BE. Protest responses in contingent valuation. Environ Resour Econ 1999;14:131-50. https://doi.org/10.1023/A:1008372522243.
- Department of Health . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 3 January 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, et al. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial – a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17070.
- Department of Health . Freedom of Information Request 2015. https://data.gov.uk/data-request/nhs-hospital-stay (accessed 19 January 2017).
- Triggle N. Patients’ Taxis Cost NHS Millions 2011. www.bbc.co.uk/news/health-15115652 (accessed 19 January 2017).
- Office for National Statistics . Annual Survey of Hours and Earnings (ASHE) 2016. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2016provisionalresults#earnings-by-occupation (accessed 19 January 2017).
- Organisation for Economic Cooperation and Development . Average Annual Hours Actually Worked Per Worker 2016. http://stats.oecd.org/index.aspx?DataSetCode=ANHRS (accessed 19 January 2017).
- Dolan P. Modelling valuarius for EuroQoL health states. Med Care 1997;35:1095-108.
- Noble M, Wright G, Smith G, Dibben C. Measuring multiple deprivation at the small-area level. Environ Plan A 2006;38:169-85. https://doi.org/10.1068/a37168.
- Park RE. Estimation with heteroscedastic error terms. Econometrica 1966;34. https://doi.org/10.2307/1910108.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: Wiley; 1995.
- Pregibon D. Goodness of link tests for generalized linear models. J R Stat Soc Ser C (Appl Stat) 1980;29:15-24. https://doi.org/10.2307/2346405.
- Pearson ES. The test of significance for the correlation coefficient. J Am Stat Assoc 1931;26:128-34. https://doi.org/10.1080/01621459.1931.10503208.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Vink G, Frank LE, Pannekoek J, Buuren S. Predictive mean matching imputation of semicontinuous variables. Stat Neerl 2014;68:61-90. https://doi.org/10.1111/stan.12023.
- Seaman SR, Bartlett JW, White IR. Multiple imputation of missing covariates with non-linear effects and interactions: an evaluation of statistical methods. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-46.
- Ng ES, Grieve R, Carpenter J. Two-stage non-parametric bootstrap sampling with shrinkage correction for clustered data. Stata J 2013;13:141-64.
- Cirourel A. Method and Measurement in Sociology. New York: Free Press; 1964.
- National Institute for Health Research . Research Governance Guidelines n.d. www.nihr.ac.uk/funding-and-support/documents/current-funding-opportunities/hsdr/NETSCC_TSC_SSC-Guidance_April-2016.pdf (accessed 29 January 2018).
- Department of Health . Mental Capacity Act 2005 n.d. www.legislation.gov.uk/ukpga/2005/9/contents (accessed 16 November 2016).
- Scottish Government . Adults With Incapacity (Scotland) Act 2000 n.d. www.legislation.gov.uk/asp/2000/4/section/51 (accessed 16 November 2016).
- Jackson CF, Makin SM, Marson AG, Kerr M. Non-pharmacological interventions for people with epilepsy and intellectual disabilities. Cochrane Database Syst Rev 2015;9. https://doi.org/10.1002/14651858.CD005502.pub3.
- Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ 2015;351. https://doi.org/10.1136/bmj.h4672.
- Robertson J, Hatton C, Emerson E, Baines S. Mortality in people with intellectual disabilities and epilepsy: a systematic review. Seizure 2015;29:123-33. https://doi.org/10.1016/j.seizure.2015.04.004.
- Tyrer F, Smith LK, McGrother CW, Taub NA. The impact of physical, intellectual and social impairments on survival in adults with intellectual disability: a population-based register study. J Intellect Disabil Res 2007;20:360-7. https://doi.org/10.1111/j.1468-3148.2006.00343.x.
- Gomes M, Ng ES, Grieve R, Nixon R, Carpenter J, Thompson SG. Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med Decis Making 2012;32:350-61. https://doi.org/10.1177/0272989X11418372.
- Montenij L, de Waal E, Frank M, van Beest P, de Wit A, Kruitwagen C, et al. Influence of early goal-directed therapy using arterial waveform analysis on major complications after high-risk abdominal surgery: study protocol for a multicenter randomized controlled superiority trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-360.
- Oliver PC, Piachaud J, Done J, Regan A, Cooray S, Tyrer P. Difficulties in conducting a randomized controlled trial of health service interventions in intellectual disability: implications for evidence-based practice. J Intellect Disabil Res 2002;46:340-5. https://doi.org/10.1046/j.1365-2788.2002.00408.x.
- Ring H, Zia A, Bateman N, Williams E, Lindeman S, Himlok K. How is epilepsy treated in people with a learning disability? A retrospective observational study of 183 individuals. Seizure 2009;18:264-8. https://doi.org/10.1016/j.seizure.2008.10.009.
- Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract 2000;17:192-6. https://doi.org/10.1093/fampra/17.2.192.
- Goldsmith L, Skirton H, Webb C. Informed consent to healthcare interventions in people with learning disabilities – an integrative review. J Adv Nurs 2008;64:549-63. https://doi.org/10.1111/j.1365-2648.2008.04829.x.
- Hom CL, Touchette P, Nguyen V, Fernandez G, Tournay A, Plon L, et al. The relationship between living arrangement and adherence to antiepileptic medications among individuals with developmental disabilities. J Intellect Disabil Res 2015;59:48-54. https://doi.org/10.1111/jir.12123.
- Epilepsies: Diagnosis and Management. London: NICE; 2012.
Appendix 1 The EpAID trial nurse self-completion diary version 1.4
Appendix 2 The EpAID trial Epilepsy and Learning Disabilities Quality of Life questionnaire version 1.6
Appendix 3 Safety procedures and adverse events
Reporting processes
It was not expected that there would be any major negative effects on participants resulting from participating in the EpAID trial. No clinically indicated treatments were withheld in either arm of the trial. However, SAEs that could have been expected in association with participation in the trial included an increase in seizure frequency or severity, occurrence of uncontrolled seizures requiring paramedic support or hospital admission and emergence of AED-related adverse effects.
The PI at each cluster site was responsible for recording all SAEs and reporting them to the chief investigator, via the trial co-ordinator, on trial SAE forms. The chief investigator then reported all SAEs to the sponsor. Any SAEs classified as ‘related’ and ‘unexpected’ were to be reported to the main research ethics committee within 15 days of the chief investigator becoming aware of them.
Adverse events
An adverse event was defined as any untoward medical occurrence in a participant involved in the trial. Adverse events occurring from time to time in patients participating in this trial in both the TAU arm and the intervention arm were generally not serious in nature and were not considered significant in terms of the safety assessment. They were not recorded unless considered serious, as defined in the following section.
Serious adverse events
A SAE was defined as any untoward medical occurrence that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a genetic abnormality or birth defect
-
was otherwise considered medically significant by the investigator.
Serious adverse events that might have been expected in the EpAID trial participants were:
-
increased seizure frequency or severity
-
the occurrence of uncontrolled seizures requiring paramedic support or hospital admission
-
the emergence of AED-related adverse effects
-
any event that the treating clinician would expect in a patient with an ID and epilepsy.
For each SAE identified the likely cause, in the view of the local PI (either EpAID trial related or not EpAID trial related), and the outcome (recovered, recovered with sequelae, ongoing, unknown and death) were recorded along with the start date, the criteria for designation, expectedness and causality.
Hospital admissions
In adults with an ID, regardless of whether or not they have epilepsy, there are several causes of hospital admission that are relatively common and would be expected and unrelated to participation in the trial. Admissions to hospital for these reasons were not reported as SAEs unless a participant’s local clinical team considered that an admission primarily for one of the reasons on the list was nevertheless directly or indirectly associated with the participant’s involvement in the trial. These causes of hospital admission are:
-
constipation
-
complications of diabetes mellitus
-
respiratory infections
-
asthma
-
cellulitis
-
dental infections
-
kidney disease.
Serious adverse events reported
The complete list of adverse events reported is provided in Table 32.
| Site | Person identification number | SAE | Consequence | Caused by EpAID trial | Outcome |
|---|---|---|---|---|---|
| 01 | 1 | Admitted for prolonged seizures | Hospitalisation | No | Unknown |
| 01 | 4 | Admitted for prolonged seizures | Hospitalisation | No | Unknown |
| 01 | 12 | Hospitalised for management of deep-vein thrombosis | Hospitalisation | No | Resolved |
| 01 | 12 | Admitted following 2–3 weeks of poor eating and drinking | Hospitalisation | No | Unknown |
| 01 | 19 | Admitted for uncontrolled seizures secondary to poor food and fluid intake and medication compliance | Hospitalisation | No | Unknown |
| 02 | 2 | Admitted for back pain and laboured breathing | Hospitalisation | No | Resolved |
| 02 | 3 | Admitted because of coughing and general lethargy alongside associated foul-smelling urine | Hospitalisation | No | Resolved |
| 02 | 3 | Admitted following five seizures overnight | Hospitalisation | No | Resolved |
| 02 | 4 | Admitted because of increased seizure activity | Hospitalisation | No | Resolved |
| 02 | 6 | Percutaneous endoscopic gastrostomy tube had fallen out overnight. Admitted for re-siting. Discharged same day | Hospitalisation | No | Resolved |
| 02 | 10 | Admitted to hospital with community-acquired pneumonia | Hospitalisation | No | Resolved |
| 02 | 10 | Admitted to hospital following repeated seizures despite rescue medication | Hospitalisation | No | Resolved |
| 02 | 14 | Admitted for mental health breakdown | Hospitalisation | No | Ongoing |
| 02 | 21 | Pneumonia and multiple organ failure | Death | No | Death |
| 02 | 21 | Constipation | Hospitalisation | No | Resolved |
| 04 | 1 | Participant admitted as had been difficult to rouse and was not taking food or fluids | Hospitalisation | No | Unknown |
| 04 | 2 | Seizures at respite care, rescue medication administered, further seizures and raised temperature | Hospitalisation | No | Resolved |
| 04 | 2 | Cluster of seizures | Hospitalisation | No | Resolved |
| 04 | 3 | Unwitnessed seizure/fall | Hospitalisation | No | Resolved |
| 05 | 5 | Previously diagnosed with malignant tumour. Epilepsy relapsed as could not tolerate medication | Hospitalisation | No | Unknown |
| 05 | 5 | Inoperable abdominal tumour | Other | No | Unknown |
| 05 | 5 | Patient had a terminal illness | Death | No | Death |
| 05 | 22 | Prolonged seizures that required hospitalisation to stabilise | Hospitalisation | No | Resolved |
| 06 | 5 | Routine change of baclofen, no complications | Hospitalisation | No | Resolved |
| 06 | 5 | High temperature with possible underlying renal infection | Hospitalisation | No | Resolved |
| 06 | 11 | Admitted to hospital because of breathing difficulties arising from chest infection | Hospitalisation | No | Resolved |
| 07 | 19 | Mini stroke | Hospitalisation | No | Resolved |
| 07 | 19 | Participant fell and hit head. Admitted but no cause found | Hospitalisation | No | Unknown |
| 07 | 19 | Participant fell and hit head. Admitted but no cause found | Hospitalisation | No | Unknown |
| 07 | 19 | Participant fell downstairs while carrying a bag of rubbish and sustained small fracture to the skull | Hospitalisation | No | Unknown |
| 08 | 13 | Death from natural causes: aspiration of blood following bleeding oesophagitis | Death | Yes | Death |
| 08 | 14 | Seizures continued after administration of rescue medication | Hospitalisation | No | Resolved |
| 08 | 14 | Seizures continued after administration of rescue medication | Hospitalisation | No | Resolved |
| 08 | 16 | Admitted for low pulse rate and hypertension; treated for infection | Hospitalisation | No | Unknown |
| 08 | 16 | Treated for infection | Hospitalisation | No | Resolved |
| 08 | 16 | Hospitalisation for re-siting of percutaneous endoscopic gastrostomy tube | Hospitalisation | No | Resolved |
| 08 | 16 | Admitted for treatment of chest infection | Hospitalisation | No | Resolved |
| 08 | 17 | Admitted for further administration of rescue medication | Hospitalisation | No | Unknown |
| 08 | 17 | Admitted for administration of further rescue medication to control seizures | Hospitalisation | No | Resolved |
| 09 | 1 | Admitted to hospital following blood test. Patient aspirated, resulting in pneumonia and collapsed lung | Death | No | Death |
| 09 | 2 | Fell during seizure, sustained a broken leg in two places | Hospitalisation | No | Unknown |
| 09 | 5 | In hospital since November 2015, death from respiratory causes | Death | No | Death |
| 09 | 5 | Feeding tube dislodged, pneumonia delayed surgical re-implantation | Other | No | Ongoing |
| 09 | 9 | Admitted to hospital because of prolonged clonic seizures | Hospitalisation | No | Unknown |
| 09 | 9 | Long clonic seizure, GP thought patient was dehydrated | Hospitalisation | No | Resolved |
| 09 | 14 | Hospitalisation after seizure during which the participant fell to the floor and broke an ankle | Hospitalisation | No | Unknown |
| 09 | 20 | Lump in breast | Other | No | Unknown |
| 11 | 5 | Multiple infections, acute bronchiolitis, enterocolitis (Clostridium difficile), urinary tract infection | Hospitalisation | No | Ongoing |
| 11 | 5 | Attended A&E for low oxygen saturation. Suffered respiratory arrest | Death | No | Death |
| 11 | 10 | Head injury following a fall after a seizure | Hospitalisation | No | Resolved |
| 13 | 13 | Admitted because of epilepsy episode but diagnosed with a chest infection | Hospitalisation | No | Resolved |
| 15 | 29 | 18 seizures in 24 hours, which did not respond to rescue medication | Hospitalisation | No | Resolved |
| 15 | 29 | Assumed post-ictal state | Hospitalisation | No | Resolved |
| 16 | 5 | Multiple seizures, chest infection, constipation | Hospitalisation | Response not provided by respondent | Ongoing |
| 16 | 5 | Continuation of condition | Hospitalisation | Response not provided by respondent | Ongoing |
| 16 | 5 | Increase in seizures, bowel perforation | Hospitalisation | Response not provided by respondent | Ongoing |
| 16 | 12 | Participant died in her sleep of a heart attack | Death | Response not provided by respondent | Death |
| 16 | 16 | Diagnosis of community-acquired pneumonia – CURB-65 pneumonia severity score of 2, chronic type 2 respiratory failure | Hospitalisation | No | Resolved |
| 16 | 16 | Admitted to hospital because of a cough. Diagnosis was community-acquired pneumonia | Hospitalisation | Response not provided by respondent | Ongoing |
| 16 | 16 | Admitted to hospital with a chest infection. Died of pneumonia | Death | Response not provided by respondent | Death |
| 17 | 12 | Participant attended A&E because of uncontrollable pain. Changes made to insulin medication | Hospitalisation | No | Ongoing |
Appendix 4 Scoring instructions for the Epilepsy and Learning Disabilities Quality of Life questionnaire
Appendix 5 Missing data
| Baseline | Follow-up | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Active | TAU | ||||||
| n/N | % | n/N | % | n/N | % | ||
| Present | Present | 100/184 | 54.3 | 72/128 | 56.3 | 172/312 | 55.1 |
| Present | No seizures (4 weeks) | 26/184 | 14.1 | 16/128 | 12.5 | 42/312 | 13.5 |
| Present | Missing | 10/184 | 5.4 | 14/128 | 10.9 | 24/312 | 7.7 |
| < 50% answered | No seizures (4 weeks) | 1/184 | 0.5 | 1/128 | 0.8 | 2/312 | 0.6 |
| < 50% answered | Missing | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| No seizures (4 weeks) | Present | 13/184 | 7.1 | 10/128 | 7.8 | 23/312 | 7.4 |
| No seizures (4 weeks) | No seizures (4 weeks) | 21/184 | 11.4 | 11/128 | 8.6 | 32/312 | 10.3 |
| No seizures (4 weeks) | Missing | 6/184 | 3.3 | 0/128 | 0.0 | 6/312 | 1.9 |
| Missing | Present | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| Missing | Missing | 7/184 | 3.8 | 2/128 | 1.6 | 9/312 | 2.9 |
| Baseline | Follow-up | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Active | TAU | ||||||
| n/N | % | n/N | % | n/N | % | ||
| Present | Present | 82/184 | 44.6 | 62/128 | 48.4 | 144/312 | 46.2 |
| Present | < 50% answered | 5/184 | 2.7 | 3/128 | 2.3 | 8/312 | 2.6 |
| Present | No seizures (4 weeks) | 18/184 | 9.8 | 17/128 | 13.3 | 35/312 | 11.2 |
| Present | Missing | 9/184 | 4.9 | 14/128 | 10.9 | 23/312 | 7.4 |
| < 50% answered | Present | 7/184 | 3.8 | 4/128 | 3.1 | 11/312 | 3.5 |
| < 50% answered | < 50% answered | 6/184 | 3.3 | 3/128 | 2.3 | 9/312 | 2.9 |
| < 50% answered | No seizures (4 weeks) | 9/184 | 4.9 | 0/128 | 0.0 | 9/312 | 2.9 |
| < 50% answered | Missing | 1/184 | 0.5 | 1/128 | 0.8 | 2/312 | 0.6 |
| No seizures (4 weeks) | Present | 10/184 | 5.4 | 10/128 | 7.8 | 20/312 | 6.4 |
| No seizures (4 weeks) | < 50% answered | 3/184 | 1.6 | 0/128 | 0.0 | 3/312 | 1.0 |
| No seizures (4 weeks) | No seizures (4 weeks) | 21/184 | 11.4 | 11/128 | 8.6 | 32/312 | 10.3 |
| No seizures (4 weeks) | Missing | 6/184 | 3.3 | 0/128 | 0.0 | 6/312 | 1.9 |
| Missing | Present | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| Missing | Missing | 7/184 | 3.8 | 2/128 | 1.6 | 9/312 | 2.9 |
| Baseline | Follow-up | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Active | TAU | ||||||
| n/N | % | n/N | % | n/N | % | ||
| Present | Present | 99/184 | 53.8 | 71/128 | 55.5 | 170/312 | 54.5 |
| Present | < 50% answered | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| Present | No seizures (4 weeks) | 27/184 | 14.7 | 17/128 | 13.3 | 44/312 | 14.1 |
| Present | Missing | 10/184 | 5.4 | 15/128 | 11.7 | 25/312 | 8.0 |
| < 50% answered | Present | 1/184 | 0.5 | 0/128 | 0.0 | 1/312 | 0.3 |
| No seizures (4 weeks) | Present | 13/184 | 7.1 | 10/128 | 7.8 | 23/312 | 7.4 |
| No seizures (4 weeks) | No seizures (4 weeks) | 21/184 | 11.4 | 11/128 | 8.6 | 32/312 | 10.3 |
| No seizures (4 weeks) | Missing | 6/184 | 3.3 | 0/128 | 0.0 | 6/312 | 1.9 |
| Missing | Present | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| Missing | Missing | 7/184 | 3.8 | 2/128 | 1.6 | 9/312 | 2.9 |
| Baseline | Follow-up | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Active | TAU | ||||||
| n/N | % | n/N | % | n/N | % | ||
| Present | Present | 97/184 | 52.7 | 72/128 | 56.3 | 169/312 | 54.2 |
| Present | < 50% answered | 1/184 | 0.5 | 0/128 | 0.0 | 1/312 | 0.3 |
| Present | No seizures (4 weeks) | 27/184 | 14.7 | 17/128 | 13.3 | 44/312 | 14.1 |
| Present | Missing | 10/184 | 5.4 | 15/128 | 11.7 | 25/312 | 8.0 |
| < 50% answered | Present | 1/184 | 0.5 | 0/128 | 0.0 | 1/312 | 0.3 |
| < 50% answered | < 50% answered | 1/184 | 0.5 | 0/128 | 0.0 | 1/312 | 0.3 |
| No seizures (4 weeks) | Present | 11/184 | 6.0 | 10/128 | 7.8 | 21/312 | 6.7 |
| No seizures (4 weeks) | < 50% answered | 2/184 | 1.1 | 0/128 | 0.0 | 2/312 | 0.6 |
| No seizures (4 weeks) | No seizures (4 weeks) | 21/184 | 11.4 | 11/128 | 8.6 | 32/312 | 10.3 |
| No seizures (4 weeks) | Missing | 6/184 | 3.3 | 0/128 | 0.0 | 6/312 | 1.9 |
| Missing | Present | 0/184 | 0.0 | 1/128 | 0.8 | 1/312 | 0.3 |
| Missing | Missing | 7/184 | 3.8 | 2/128 | 1.6 | 9/312 | 2.9 |
| Baseline | Follow-up | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Active | TAU | ||||||
| n/N | % | n/N | % | n/N | % | ||
| Present | Present | 153/184 | 83.2 | 98/128 | 76.6 | 251/312 | 80.4 |
| Present | Missing | 20/184 | 10.9 | 20/128 | 15.6 | 40/312 | 12.8 |
| Missing | Present | 3/184 | 1.6 | 2/128 | 1.6 | 5/312 | 1.6 |
| Missing | Missing | 8/184 | 4.3 | 8/128 | 6.3 | 16/312 | 5.1 |
| Baseline | Follow-up | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|---|
| Active | TAU | ||||||
| n/N | % | n/N | % | n/N | % | ||
| Present | Present | 37/165 | 22.4 | 30/118 | 25.4 | 67/283 | 23.7 |
| Present | Missing | 12/165 | 7.3 | 19/118 | 16.1 | 31/283 | 11.0 |
| Missing | Present | 0/165 | 0.0 | 2/118 | 1.7 | 2/283 | 0.7 |
| Missing | Missing | 116/165 | 70.3 | 67/118 | 56.8 | 183/283 | 64.7 |
| Variable | Treatment arm | Overall | ||||
|---|---|---|---|---|---|---|
| Active | TAU | |||||
| n/N | % | n/N | % | n/N | % | |
| Age | 7/184 | 3.8 | 2/128 | 1.6 | 9/312 | 2.9 |
| Accomodation | 7/184 | 3.8 | 6/128 | 4.7 | 13/312 | 4.2 |
| Level of ID | 11/184 | 6.0 | 21/128 | 16.4 | 32/312 | 10.3 |
| Deprivation Index | 5/184 | 2.7 | 2/128 | 1.6 | 7/312 | 2.2 |
| Number of tonic–clonic seizures | 11/184 | 6.0 | 10/128 | 7.8 | 21/312 | 6.7 |
Appendix 6 Community intellectual disability team epilepsy service availability questionnaire version 1
List of abbreviations
- A&E
- accident and emergency
- AED
- antiepileptic drug
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIDT
- community intellectual disability team
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- Clinical Research Network
- CSRI
- Client Service Receipt Inventory
- DMEC
- Data Monitoring and Ethics Committee
- ELDQoL
- Epilepsy and Learning Disabilities Quality of Life
- ELDQoL-SSS
- Epilepsy and Learning Disabilities Quality of Life seizure severity scale
- ENS
- epilepsy nurse specialist
- EpAID
- Epilepsy And Intellectual Disability
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- ESNA
- EpilepSy Nurses Association
- GP
- general practitioner
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ID
- intellectual disability
- IQ
- intelligence quotient
- ITT
- intention to treat
- KCTU
- King’s Clinical Trials Unit
- LD
- learning disability
- MAR
- missing at random
- MCSI
- Modified Caregiver Strain Index
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PI
- principal investigator
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SSS
- seizure severity scale
- TAG
- Trial Advisory Group
- TAU
- treatment as usual
- UCHSC
- Unit Costs of Health and Social Care
- WTP
- willingness to pay
