Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/09/10. The contractual start date was in July 2016. The draft report began editorial review in August 2017 and was accepted for publication in January 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Waugh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction to age-related macular degeneration
Age-related macular degeneration (AMD) is a progressive degenerative disease of the retina in which the macula is most affected. 1 It is the commonest cause of blindness in the UK and it affects mainly older people.
Age-related macular degeneration goes through various stages, called early, intermediate and advanced. The first signs are yellowish deposits in the retina called drusen. Then abnormalities in the colour of the retina develop: paler areas called hypopigmentation, and darker areas with hyperpigmentation. Advanced AMD takes two forms, wet and dry, both of which lead to visual loss. Advanced dry AMD is characterised by atrophy of the retina – it wastes away and patches of retina and vision are lost. Because the patches were thought to resemble countries on a map, it became called ‘geographic atrophy’ (GA). The central most detailed vision is lost, making it difficult to drive, read or recognise faces.
Wet AMD, also called exudative AMD, is characterised by the development of abnormal new vessels [choroidal neovascularisation (CNV) and retinal angiomatous proliferation (RAP)]. It is now treated with drugs that inhibit a compound called vascular endothelial growth factor (VEGF), so they are called ‘anti-VEGF drugs’. They include bevacizumab (Avastin, Roche), ranibizumab (Lucentis, Novartis, Basel, Switzerland) and aflibercept (Eylea, Bayer). The AMD sections of this report are concerned with treatments for only dry AMD, at all stages, from prevention of early changes progressing to advanced AMD, both dry and wet, and treatment of advanced dry AMD. As part of the background, we also look at some epidemiological studies of risk factors for AMD.
Prevalence
The prevalence of AMD increases with age. 2 Owen and colleagues3 reported an overall prevalence of advanced AMD in 2007–9 of 2.4% in the over 50s rising to 12.2% in the over 80s. The estimated number of people with advanced AMD in the UK was 513,000, about 2.4% of the population aged ≥ 50 years, with just over half (1.3%) having dry AMD. In the UK, there are about 2.6 million people with early AMD.
The Bridlington Eye Assessment Project (BEAP) showed that 38% of those aged > 65 years have no sign of AMD, 54% have early AMD, 2.8% have intermediate AMD and 4.5% have advanced AMD. The prevalence of advanced AMD rises with age, from 2.1% in those aged 65–70 years, to 7.5% in those aged 80–85 years, and 16% in those aged > 85 years. 4 Visual acuity (VA) is often maintained at 6/9 or better in most eyes before the development of GA. AMD is by far the commonest cause of blind and partial sight certifications in the UK, accounting for about 59%. 5
We have an ageing population with more people living longer; therefore, more people will live to develop AMD. They may otherwise be fit with a good quality of life, and so visual loss may have a dramatic effect in their remaining years.
We need to distinguish rates and numbers. The most recent meta-analysis of the prevalence of AMD in Europe, by Colijn and colleagues6 from the EYE-RISK consortium and the European Eye Epidemiology (E3) consortium, concludes that the prevalence of advanced AMD is now declining, perhaps because of healthier lifestyles. However, the number of people with any AMD will almost double.
Impact
Age-related macular degeneration causes central visual loss leading to gaps on items on which the eye naturally focuses, such as words on pages, bus numbers, faces and television. Vision becomes distorted, colours can fade and adaptation to dark can be impaired. Driving may become impossible. Visual impairment increases the risk of falls and injuries and can lead to depression and social isolation. Getting out and about safely, for example to go shopping, may become difficult. Independent living may become impossible. Sight loss is a leading cause of suicide among older people. 7
Age-related macular degeneration reduces quality of life. Brown and colleagues8 assessed the quality of life among patients with mild [VA of 20/20 to 20/40 in the better-seeing eye (BSE)], moderate (VA 20/50 to 20/100 in BSE), severe (≤ 20/200) and very severe AMD (≤ 20/800). They used the time trade-off method, which asks how much of remaining life would be given up in return for perfect vision.
Patients scored their quality of life as:
-
0.83 with mild AMD (similar to having moderate angina)
-
0.68 with moderate AMD (similar to life following a moderate stroke, or having AIDS)
-
0.47 with severe AMD (similar to end-stage renal failure on dialysis)
-
0.40 with very severe AMD – a 60% loss of quality of life (similar to being bedridden after a major stroke or advanced prostate cancer with intractable pain).
Aetiology
The causes of AMD are not known. Risk factors include age, genetic predisposition, exposure to light, race, smoking, overweight and obesity, and diet. 9–11 High fat diets and obesity increase the risk, whereas antioxidant nutrients protect. In the Danish Inter99 study, Munch et al. 12 found that among people aged 30–60 years, macular drusen of > 63 µm was associated with physical inactivity, higher waist measurements (in men) and higher serum triglycerides (in women).
Chakravarthy and colleagues13 carried out a systematic review of risk factors for AMD, drawing on 18 cohort and six case–control studies. They found that cigarette smoking and a family history of AMD showed strong associations, and that there were moderate but consistent associations with risk factors for cardiovascular disease such as higher BMI, hypertension and higher plasma fibrinogen.
Smoking greatly increases the risk of AMD. The European Eye Study14 reported that current smokers had 2.6 times the risk of wet AMD and 4.8 times the risk of advanced dry AMD (GA) as opposed to non-smokers. The Melbourne Collaborative Cohort Study15 looked at patterns of diet, and found that diets rich in fruits, vegetables, chicken and nuts and low in red meat were associated with a lower prevalence of advanced AMD. Interestingly, they divided foods by method of cooking and noted that steamed fish conferred a lower risk than fried fish, probably reflecting broader dietary patterns. An earlier paper from the same study16 had reported that high red meat and processed red meat intake increased the risk of AMD, but that higher chicken intake reduced it. A third paper17 reported that higher trans-unsaturated fat intake was associated with increased prevalence of late AMD. Higher olive oil intake (> 100 ml/week) was associated with an odds ratio (OR) of 0.48 [95% confidence interval (CI) 0.22 to 1.04] compared with an intake of < 1 ml/week.
In a recent review, Zhu and colleagues18 provide a high-quality review of fish consumption and the incidence of AMD, with a meta-analysis of eight prospective cohort studies from the USA (n = 4), Australia (n = 2), Ireland (n = 1) and the Netherlands (n = 1). Some of the studies adjusted for a wide range of confounding variables, others for only a few. The incidence was reduced by 24% overall (OR 0.76, 95% CI 0.65 to 0.90, I2 = 50%; but heterogeneity in effect size not direction). Fish consumption is not clearly defined in the review but a diagram of the dose–response relationship shows that the reduction in OR increases with frequency, with once a week consumption reducing the risk by only 11% (RR 0.89, 95% CI 0.83 to 0.96). However, after an increase to three times a week, the relative risk (RR) plateaus at the 0.76 level.
High intake of dietary salt has also been suggested as a contributory cause. 19 This could be mediated through its effect on blood pressure. The Complications of Age-related Macular Degeneration Prevention Trial (CAPT) research group reported that, compared with people who had normal blood pressure, those with definite hypertension (defined as a systolic blood pressure of ≥ 160, diastolic blood pressure of ≥ 95, or on treatment) had 1.55 times the risk of wet AMD and 1.86 times the risk of GA. 20
Low-dose aspirin (100 mg on alternate days) taken for 10 years had no significant effect compared with placebo, with the hazard ratio (HR) for developing new AMD of 0.82 (95% CI 0.64 to 1.06). 21 Heavy alcohol consumption (more than three standard drinks per day) was reported by Chong et al. 22 to increase the risk of early AMD.
The prevalence varies among ethnicities, with the frequency of late AMD highest in white people, and lowest in Africans. 10 This is partly due to varying genetic susceptibilities.
The presence of some genes increases susceptibility, particularly the complement factor H (CFH) gene, which is linked to the complement pathway, part of the immune system, and the Age-Related Maculopathy Susceptibility Gene 2 (ARMS2). These two genes are involved in > 60% of cases of advanced AMD. 10 Conversely, some genes such as some variants of the apolipoprotein E gene, which regulates lipid and cholesterol transport in the central nervous system, appear to be protective. The mechanism underlying the protection may be via better transport of cholesterol and other metabolites out of the cells in the retinal pigment epithelium (RPE).
The structure of the eye
The sclera
The outer layer of the eyeball is the sclera, which forms part of the supporting wall of the eye. It is the ‘white of the eye’ and it surrounds most of the eye. However at the front of the eye, it is replaced by the cornea, which is transparent and allows light through.
The choroid
Inside the sclera, the next layer at the back of the eye is the choroid, which is the vascular layer of the eye, composed of blood vessels and connective tissue. There are sublayers within the choroid, including the choriocapillaris and Bruch’s membrane. The choriocapillaris consists of the capillaries that provide oxygen and nutrients to the retina.
Bruch’s membrane
Bruch’s membrane is the innermost part of the choroid, in contact with the retina. The innermost part of Bruch’s membrane is formed by the basement membrane of the RPE, which transmits waste products of metabolism from the photoreceptors (PRs) in the retina into the blood vessels in the choroid. The RPE and the choroid provide nourishment to the retinal PR cells.
Bruch’s membrane gets thicker with age, and this slows the transport of metabolites. With ageing, lipids accumulate in Bruch’s membrane. The conduction of fluids (hydraulic conductivity – the ability to let fluids pass) through the membrane is reduced. 23,24 It is thought that oxidative change in the lipids may trigger an inflammatory process, including activation of complement. Reduced transport of nutrients into the retina and reduced transport of waste products of metabolism out of it may trigger a release of VEGF in an attempt to provide more blood supply, and this may lead to the development of abnormal new blood vessels in wet AMD. The RPE has a symbiotic relationship with the choriocapillaris; if the RPE is lost, then the choriocapillaris closes down. This is believed to be the result of reduced production of VEGF by the RPE.
There is a large variation in thickening of Bruch’s membrane with age. Lommatzsch et al. 23 suggest that half of the thickening is due to natural ageing and half is due to other factors, such as genetic susceptibility and environmental factors. In early AMD, there is thickening of Bruch’s membrane due to lipid and protein deposits (drusen). 25
The retinal pigment epithelium
The RPE lies between Bruch’s membrane and the PRs. Boulton and Dayhaw-Barker26 provide a good review of its functions, which include transport of ions, fluid and metabolites; support for the visual cycle; clearance of debris; protection against light and free radicals; and production of growth factors. The RPE changes with age. In AMD, accumulation of lipofuscin in the RPE can damage it.
The retina
The retina contains the PR cells, rods and cones, and it is only 0.5 mm thick. Retinal pigmentation is partly due to the presence of melanin in the RPE. There is more melanin in the macula so it appears darker. 26 Melanin is protective but the amount of melanin falls with age, and one effect is to reduce antioxidant potential. 26
The macula
The macula is an oval area near the centre of the retina, only 5.5 mm across. It is the most sensitive part of the retina. At the centre of the macula is the fovea. The macula is responsible for high acuity and colour vision. The macula is yellowish in colour due to the macular pigments.
Macular pigments
These consist of lutein, zeaxanthin and meso-zeaxanthin, which are found in high concentration in the macula and are known as macular pigments. The first two are obtained from the diet, and meso-zeaxanthin is formed in the macula from lutein. The levels are measured as macular pigment optical density (MPOD). Their distributions in the retina vary, with meso-zeaxanthin dominating in the centre of the macula and lutein at the periphery. Carpentier et al. 27 provide an overview, with points including:
-
Adipose tissue may compete with the retina for uptake of lutein and zeaxanthin so obesity may lower MPOD.
-
Macular pigments protect the retina from the effects of blue light.
-
In the USA, the combined intake of lutein and zeaxanthin is about 2 mg per day.
-
Higher intakes appear to reduce the risk of AMD.
-
Taking supplements increases MPOD when that is low and some studies report improvements in visual function.
Lutein and zeaxanthin are members of the xanthophyll family. Most lutein and zeaxanthin comes from vegetables with highest concentrations, dark leafy vegetables such as spinach and kale, and egg yolk and maize. These carotenoids have antioxidant effects, protecting the RPE from oxidative stress. Increasing dietary intake leads to an increase in macular levels. A Dutch trial28 showed increases in VA and improvement in dark adaptation.
However, Stevens et al. 29 from Aston University reported that among 158 patients recruited via the Macular Society helpline, those with AMD consumed a daily average 3.3 mg of lutein and zeaxanthin (text – table says under 2 mg/day), which is well below the 10 mg recommended after the AREDS 2 study. 30 Many patients were not eating vegetables such as spinach and kale, but a control group of people without AMD had better intake.
Pathology
Drusen are small yellow or white accumulations of extracellular material that build up between Bruch’s membrane and the RPE. Drusen come in two main forms: hard and soft. Most people > 40 years have a few small hard drusen, but if they are more numerous or if they are larger, they may be the start of macular degeneration.
Small drusen (< 63 µm) are considered by Holz et al. 11 not to be associated with progression to AMD, but to be a non-specific change due to ageing. However, drusen volume31 or size32 are strong predictors of progression to GA or wet AMD.
Reticular pseudodrusen
Reticular pseudodrusen (RPD) are a specific phenotype of early AMD first described by Mimoun et al. 33 as a yellowish interlacing network in the outer macula of AMD patients, best visualised under blue light. Other terms that have been used for RPD are subretinal drusenoid deposits, reticular macular disease and reticular drusen.
Arnold et al. 34 reported RPDs typical predominant location between the upper edge of the fovea and the supero-temporal arcade. The fundus autofluorescence (FAF) findings of RPD and its common association with RAP were first noted by McBain et al. 35 and Lois et al. 36 and subsequently supported by others. 37
The prevalence of RPD was initially reported to be 0.7% in the Beaver Dam Eye study38 and 1.95% in the Blue Mountains Eye study. 39 The 15-year incidences were 3% and 4%, respectively. Later studies utilising multimodal imaging have reported a higher prevalence of the condition [4.9% in the Rotterdam study40 and 13.4% in the Alienor (Antioxydants, Lipides Essentiels, Nutrition et Maladies OculaiRes) study41]. This could be attributed to the fact that the former studies only utilised colour fundus photography (CFP) to detect RPD but the latter used newer imaging technologies. Known risk factors for RPD include age and female gender. 38–41
Reticular pseudodrusen are associated with all stages of AMD as well as being more prevalent in late AMD. 42–46 The prevalence of RPD in early AMD was reported to be 8.4% in the AREDS study,47 36–54% in neovascular age-related macular degeneration (nAMD) and ranging from 29% to 92% in GA. 42 The Beaver Dam Eye study reported that eyes with RPD are at a sixfold higher risk of progressing to late AMD within 5 years than eyes with indistinct soft drusen but no RPD. 38 The Blue Mountains Eye study reported a fourfold increased risk. 39 Gil and colleagues48 studied the fellow eyes of patients with unilateral wet AMD and found that 58% had RPD, and that RPD increased the risk of progression compared with patients without RPD.
Fellow eyes of patients with unilateral wet AMD are known to have a higher risk of progression to late AMD. 47,49 Studies in this group have shown that presence of RPD is an independent and additional risk factor (when combined with drusen and pigmentary changes) for progression to late AMD in the fellow eye. 46,48,50,51 Eyes with RPD tend to progress to GA50,51 but some studies have also reported a higher risk of wet AMD. 34,44 In patients with established GA, Marsiglia et al. 52 reported that eyes with RPD have a higher rate of progression than eyes without RPD.
Reticular pseudodrusen have been reported to cause significant deterioration in rod function,42 although central VA is preserved, as reported by Hogg et al. 50 using the Smith-Kettlewell low luminance acuity test and by Steinberg et al. 53 using microperimetry. Compared with areas with no pathologic morphology, areas with RPD demonstrated a large and sharp decrease of scotopic sensitivity while there was only a mild decrease in photopic sensitivity. 53 Other studies have shown that RPD are associated with reduction in photopic sensitivity when compared with healthy controls or people with typical drusen. 54–56 Ooto et al. 57 suggested that in order to truly reflect a patient’s visual function, other parameters, such as contrast sensitivity and mesopic sensitivity, should be measured along with VA, as RPD are associated with deterioration in both.
Corvi et al. 58 compared MPOD in patients with RPD and people without AMD, and reported lower levels. They also reported reduced best corrected visual acuity (BCVA) and retinal sensitivity. After 3 months supplementation with lutein 10 mg/day and zeaxanthin 2 mg/day, the mean MPOD in the RPD group improved to the same levels as in the control group. However, no significant improvements were seen in BCVA or retinal sensitivity. This may be because changes in function take longer to accrue. In the CREST study, Nolan and colleagues59 found that MPOD increased by 3 months but that changes in contrast sensitivity took 12 months to reach statistical significance.
Pigmentation
Melanin in the choroid is protective against oxidative damage, and a reduction in pigment in the eye may increase the risk of developing AMD.
Retinal hypopigmentation results in paler areas and is usually associated with loss of the RPE cells. Conversely, hyperpigmentation can occur in early AMD. Neither change is specific to AMD. Another pigment, lipofuscin, appears harmful. It is composed of lipids and protein. A major component is a retinoid A2E (N-retinyl-N-retinylidene ethanolamine) which is a by-product of the visual cycle.
The ‘visual cycle’
Light reaching the photoreceptors in the retina triggers the conversion of the light-sensitive retinoid 11-cis-retinal into a different form, 11-cis-retinol, thereby generating an electrical signal to the brain. The trans form is then converted back to the cis form in the RPE and then returns to the photoreceptors, completing the visual cycle. If the two molecules of the trans form combines with one of the lipids (phosphatidilethanolomine) in the RPE, A2E is formed, and this can impair RPE function. Because the edges of patches of GA are thought to have A2E accumulation [as reflected in increased autofluorescence (AF)], reducing that accumulation may be one target of drug treatment.
Oxidative stress
Oxidative stress is defined by Betteridge60 as a disturbance in the balance between the production of reactive oxygen species (free radicals) and antioxidant defences. The retina has a very high metabolic rate, reflected in high oxygen consumption, and has a high concentration of polyunsaturated fatty acids and exposure to light, which, if coupled with inadequate levels of antioxidants, can make it very susceptible to oxidative stress. Yehoshua and Rosenfeld61 report that the evidence for cumulative oxidative damage being the cause of AMD has been growing, but that a mechanism for it is not yet known.
Barnett and Handa62 suggest that oxidative stress can affect the immune system, turning it from a protective to a pathological response, and can also lead to chronic inflammation.
The immune system
Ambati and colleagues63 have reviewed the immunology of AMD. In brief, they consider that overactivity in the alternative pathway of the complement system is involved in the development of AMD. This is associated with the genetic susceptibility via a variant of the CFH gene, known as CFH (402His), which causes a greater than normal complement response to retinal injury. Ambati and colleagues63 suggest that in individuals with ‘a complement hyperinflammatory phenotype’ there is an over-reaction to cellular damage in the retina.
Anderson, Hageman and colleagues64 first described the role of inflammation in AMD, and put forward the hypothesis that drusen were the result of local immune-mediated processes and the junction of the RPE and Bruch’s membrane.
The pathological role of the complement system has led to trials of drugs to inhibit that system.
Age-related macular degeneration
Classification (Macular Research Classification Committee 2013):65
-
Normal ageing – people with small drusen (< 63 µm), also termed drupelets, should be considered to have normal ageing changes with no clinically relevant increased risk of late AMD developing.
-
Early AMD – medium drusen (≥ 63 to < 125 µm), but without pigmentary abnormalities thought to be related to AMD.
-
Intermediate AMD – large drusen or with pigmentary abnormalities associated with at least medium drusen.
-
Late AMD – neovascular (wet) AMD or GA (advanced dry AMD).
In this report, we use the term dry AMD to cover all stages from early AMD to GA.
Early and intermediate AMD is characterised by drusen, and/or by changes in pigmentation. 25 However, most people with drusen will not progress to severe visual loss and drusen may cause only mild or no visual symptoms. Up to 80% of people > 60 years have some drusen. Hard drusen are well-defined yellowish deposits with little risk of progression.
Soft drusen are larger, not well demarcated and are associated with a high risk of progression to late AMD. They may become larger and merge over time and can lead to RPE detachments, called drusenoid RPE detachments. They may disappear, but this is usually associated with atrophy of the outer retina. Drusen are associated with thinning of the overlying RPE. Fleckenstein and colleagues66 consider that GA is the natural end stage of soft drusen. A key component of drusen is amyloid beta,67 which is a waste product.
The underlying processes include locally intensive metabolism, oxidative stress, chronic inflammation, a pathological immune response and lipofuscin accumulation. 9 Lipofuscin is considered toxic.
In atrophic AMD, there may be a single patch of atrophy or several. Over time, the patches may get bigger and may merge. The foveal centre (the area responsible for central vision) is lost last as atrophy occurs around the centre of the macula first before expanding into the fovea, which is the very centre of the macula. This potentially gives time for treatment before the central vision is lost.
Vision is lost from atrophic patches and the gaps in vision are called scotomas.
The atrophy is due to loss of the RPE, outer layers of the retina and the underlying choriocapillaris. 11,66 On optical coherence tomography (OCT), GA appears as a flat patch where the retinal has withered away. A total of 20% of people with legal blindness have lost central vision due to GA. It tends to be of similar extent in both eyes66 but patients can have GA in one eye and wet AMD in the other, and can also have both GA and wet AMD in the same eye, if late dry AMD turns to wet AMD.
Geographic atrophy is also seen in patients treated with anti-VEGF drugs for wet AMD. 35,68 In both the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT)69 and Inhibition of VEGF in Age-related choroidal Neovascularisation (IVAN)70 trials (of ranibizumab vs. bevacizumab in wet AMD) it was observed that about one-fifth of patients developed GA after 2 years of anti-VEGF treatment. 71 This GA appeared to be clinically similar to the GA that is seen in dry AMD and may occur because VEGF is required for the maintenance of the choriocapillaris by the RPE.
Progression and natural history
Data on natural history studies are important because natural history may be the only comparator for some interventions reported in observational studies.
Wet AMD will develop in 10–15% of people with intermediate AMD. 72 In the AREDS trial, the average time to atrophy was 5–6 years in people with large drusen and hyperpigmentation, but 2.5 years in those with hypopigmentation.
Most people with AMD are at the early stage,2 as shown in Table 1.
| Age (years) | Drusen | Advanced dry AMD |
|---|---|---|
| 65–69 | M 9.7%, F 9.8% | M 0.5%, F 0.1% |
| 70–74 | M 12.5%, F 17.3% | M 0.6%, F 1.0% |
| 75–79 | M 18.7%, F 18.1% | M 1.9%, F 1.2% |
| ≥ 80 | M 23.3%, F 28.9% | M 1.4%, F 5.8% |
| All ages > 65 | 15.4% | 1.2% |
The KORA (Cooperative Health Research in the Region of Augburg) study from South Germany reported features of AMD in people < 50 years. 73
The Geographic Atrophy Study by Sunness et al. 74,75 reported that GA enlarged at 2.6 mm2 per year over a median follow-up of 4.3 years, in 212 eyes in 131 patients, mean age 78 years. However, there was a very wide range of progression rates from none to almost 14 mm2 per year. They noted a high concordance in rates of enlargement between eyes.
The Geographic Atrophy Progression Study,76 in patients with a mean age of 77 years, found that the GA enlarged by an average of 1.85 mm2 over 12 months, based on AF, and this was slightly higher based on CFP.
The Beaver Dam Study77 found a progression rate of 1.3 mm2 per year in 53 eyes of 32 patients (mean age about 81 years) over 5 years.
The AREDS trial group reported progression of 1.7 mm2 per year in 251 eyes of 181 patients (mean age 70 years) over a median follow-up of 6 years.
The FAM (Fundus Autofluorescence in age-related Macular Degeneration) study78 reported a similar progression rate of 1.75 mm2 per year (mean) or 1.52 mm2 per year (median) in 195 eyes of 129 patients (mean age 74 years), but over a median follow-up of only 1.8 years. They also reported a wide range of progression rates. They used FAF to determine areas of GA.
Decision problem
The questions for this review include:
-
Can treatment of early AMD prevent or slow progression to advanced forms (wet or dry)?
-
Can any treatments improve, or slow deterioration in, GA?
-
Can any treatments prevent GA progressing to wet AMD?
As our aim is to identify interventions that might have reached a stage where they could be assessed by the NIHR programmes [mainly Efficacy Mechanism and Evaluation (EME) and Health Technology Assessment (HTA)], we are not interested in –
-
rehabilitation methods such as external low visual aids
-
diagnostics
-
research still at basic science stage, such as in vitro, including cell work, or methods of carriage of gene therapies into cells using viral carriers
-
treatments with some evidence of efficacy in animal studies but not yet tested in humans. Such research might fall within the remit of the Medical Research Council (MRC) Translational Research Programme.
Potential treatments might be divided into the following groups:
-
Treatments where proof of concept in humans has already been achieved but where research is needed to evaluate clinical efficacy, and which might be suitable for the EME programme.
-
Treatments where there is evidence that shows they can be effective, but where further research is needed to establish the clinical effectiveness and cost-effectiveness for the NHS in comparison with the current best alternative. Such research falls within the remit of the HTA programme.
-
Interventions where there is sufficient evidence of lack of benefit, so that no further research is justified.
-
Interventions where there is no good evidence of any benefit and on which no money should be spent. Identifying these may help people who see unjustified claims or adverts.
Outcomes
The most important outcomes are those that matter to patients: VA, contrast sensitivity, adverse effects of treatment, reading speed, ability to drive, health-related quality of life, progression of disease and patient preference.
However, VA loss is a late manifestation of AMD and not a good primary outcome in most trials, especially when the treatment is aiming at prevention of visual loss before it occurs. Early AMD may cause minimal or no symptoms. VA depends only on the centre of the fovea but this tends to go last in atrophic AMD and many patients have large areas of atrophy and experience considerable problems before the fovea goes. Reading and seeing faces of people can be extremely difficult and the ability to drive may be lost.
Progression of dry AMD is slow, and so it could be years before a trial could show a decline in vision. Therefore, predictors or biomarkers of future decline can be accepted if there is good evidence that they are strong predictors of subsequent visual outcomes. These will include changes detectable by investigation, but not necessarily by people with AMD, including:
-
Rod function, which may not correlate with VA as central VA acuity (as measured using VA charts) depends on foveal function, and the fovea is cone rich. But rod function is one of the earliest abnormalities detected in people who will later develop GA in AMD.
-
Macular pigment density, because it appears to be protective.
-
Integrity of the RPE layer, as determined by FAF and OCT.
-
Drusen volume and number. Disappearance of drusen may be a sign of developing GA.
-
Macular sensitivity, which can be measured by microperimetry.
-
Dark adaptation.
Both photopic and scotopic vision need to be considered. Scotopic vision refers to low levels of light such as in near darkness.
One issue is the clinical significance of changes in VA. In past evaluations, for example of the anti-VEGF drugs, a clinically significant difference in VA has usually been considered as a change of ≥ 10 letters. Changes of < 5 letters are not regarded as clinically relevant as may indicate normal variability. Changes of 5–9 letters are not regarded as clinically useful but might be regarded a valuable outcome to investigate if seen in a short-term study, suggesting that a larger or longer trial is justified.
In dry AMD, no change (which could be lack of deterioration) could be regarded as clinically meaningful if observed over a long enough period.
Microperimetry
Microperimetry can detect changes in macular sensitivity in patients with early AMD and normal VA. 79
Macular sensitivity measured using microperimetry focuses on the central macula instead of the entire visual field. 80–83
Testing is performed either with a modified Humphrey Field Analyser or with a microperimeter. 83
There is limited evidence of the reproducibility of microperimetry in patients with AMD, but current studies have suggested that it provides consistent and reproducible readings. 84–86
Early AMD patients have rod sensitivity loss87 and impaired rod-mediated parameters of dark adaptation,88 which worsen as AMD progresses. The association between early AMD changes and macular sensitivity was further established by the observation that a correlation existed between altered AF signal and reduced macular sensitivity. 79,89 In GA, macular sensitivity was reduced in areas of increased fundus AF signal at the junctional zone of areas of atrophy. 90 However, this observation has not yet been proven to be a predictor of GA enlargement over time. 90
Current evidence suggests that macular sensitivity is a valuable biomarker for early AMD and microperimetry has proven to be an easy and reliable test to measure it. It is not widely used in clinical practice, but has been used in clinical trials to evaluate the effects of treatments on macular sensitivity. 91–96
Review methods
For reasons of space, we summarise methods here. Further details are provided in Appendix 1, including the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
Search strategies
MEDLINE, EMBASE, Web of Science and The Cochrane Library were searched from 2005 to 13 July 2017 for reviews, journal articles and meeting abstracts. Searches were limited to English language.
Initial searches of all databases were undertaken in June 2016 and updated searches were run in June 2017 to check for any articles added in the previous year. The Association for Research in Vision and Ophthalmology (ARVO) website was also searched for meeting abstracts.
References of reviews were checked for relevant studies and clinical experts were also consulted for any other relevant literature.
Studies were selected for inclusion through a two-stage process using predefined and explicit criteria. Titles and abstracts of 7948 articles from the full literature search results were screened independently by two reviewers to identify all citations that appeared likely to have met the inclusion criteria, and checked by a third. The full texts of 398 articles were obtained for further screening and checking of references and 112 articles were included in the final report.
ClinicalTrials.gov, the WHO search portal and UK Clinical Trials gateway were searched for ongoing and recently completed clinical trials.
Full details of the search strategies are in Appendix 1.
Inclusion and exclusion criteria
Participants
People with a confirmed diagnosis of dry AMD or Stargardt disease (STGD).
Interventions
Any interventions that aim to preserve or restore vision in dry AMD or STGD.
Exclusions
To avoid overlap, we excluded studies on some interventions being reviewed in the NICE guideline process (e.g. smoking cessation, diagnostic technologies, monitoring and review, and rehabilitation support).
Outcomes
These are as above.
Design
We placed no restriction on study design so included randomised controlled trials (RCTs), controlled clinical trials (CCTs) with a concurrent control group, and observational studies. This was partly so that we could assess the evidence base for treatments that might be advocated without a sufficient evidence base.
Systematic reviews were assessed for quality and summarised if they met quality criteria. Reviews were also used as a source for identifying primary studies, and for identifying studies published before 2005 that seemed relevant, such as earlier studies of included treatments.
Study selection and data extraction
Studies published as abstracts or conference presentations were only data extracted and included if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken. If such details were not available, key points from abstracts were summarised in the text.
Data were extracted by one reviewer using a standard data extraction form and checked by a second reviewer. At each stage, any disagreements between reviewers were resolved by consensus or, if necessary, by arbitration by a third reviewer.
Quality assessment strategy
The methodological quality of primary research studies was assessed using criteria based on those recommended by the Cochrane Collaboration and National Institutes of Health (NIH), National Heart, Lung and Blood Institute (NHLBI) (for further details, see Appendix 1). Quality criteria were applied by one reviewer and checked by a second reviewer, with any differences in opinion resolved by consensus or by arbitration by a third reviewer.
The quality of systematic reviews was assessed using the Centre for Reviews and Dissemination (CRD) checklist, with reviews assessed as good if four or more criteria were met.
Method of data synthesis
Studies were synthesised through a narrative review with tabulation of results of included studies. Formal synthesis through meta-analysis was not possible because studies were not of sufficient quality and were heterogeneous in terms of participant characteristics, outcomes and study design.
References
Trials and other studies listed by National Clinical Trial (NCT) numbers are available on the ClinicalTrials.gov website (https://clinicaltrials.gov/) by searching using NCT number. This website is a service of the US NIH.
Changes to the protocol
An outline protocol was registered on PROSPERO at an early stage. (This is mandatory for reviews commissioned by the HTA programme). However, during the systematic review, the protocol evolved over time, as agreed by the funder. The main change was to include additional outcomes, or predictors of outcomes, because of the awareness that many studies were relying on VA, which is a late outcome.
Quantity of evidence
We included 108 primary studies reported in 112 articles (see Figure 2). Of 104 dry AMD studies, there were 26 of pharmacological treatments, 30 in physical therapies, 3 of cell transplants and 45 of nutritional supplements. There were four studies in Stargardt’s, two of physical therapies and two of nutritional supplements. Two studies had subgroups of people with dry AMD and Stargardt’s97,98 making a total of six studies in STGD.
An overview of the study characteristics can be seen in Report Supplementary Material 6. There was a range of study designs, with 60 RCTs and CCTs, 24 cohort studies and cross-sectional studies, 13 single-arm before-and-after studies, 5 case–control studies, and 6 case series. Many studies had small sample sizes, the durations of intervention and follow-up were often short, and there were differences in the outcomes reported. We reported all outcomes of relevance if they were reported by the authors of each study. Further details are provided in Chapters 2–6. Baseline characteristics of participants are summarised in Report Supplementary Material 6. There was generally poor reporting of baseline characteristics across the studies. The risks of bias of RCTs and CCTs and quality of non-randomised studies are summarised in Report Supplementary Material 6. The overall quality of each study is reported within the results chapters of this report.
Details of methods and quality assessments are in Appendix 1. Report Supplementary Material 1–5 contain data extraction and quality assessment tables and can be downloaded as separate files from the HTA programme website (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/150910/#/documentation). Report Supplementary Material 6 has a list of excluded studies, most of which were excluded because they were on wet AMD or basic science, or were superseded by later studies.
Chapter 2 Stargardt disease
Background
Stargardt disease is caused by inheritance of a faulty gene: the ABCA4 gene. Genes are in pairs, one inherited from the father and one from the mother. If abnormal ABCA4 genes are inherited from both parents, then the disease will occur. Because of these abnormal genes, a build-up of waste material from metabolism called lipofuscin occurs in the retina. This material should be cleared away but because of the disease it is not and damages the retina, and some of the retinal cells die. This causes loss of vision, but the amount of loss varies. There are many different forms of the faulty gene and, as a result, some people have more severe disease than others.
The German ophthalmologist Karl Stargardt described this condition in 1909 (see Fishman99 for a historical review). It has also been called fundus flavimaculatus and, luckily, it is quite rare. The prevalence is often quoted to be about 1 in 10,000,100 which is compatible with a recent UK study of incidence101 carried out under the auspices of the British Ophthalmological Surveillance Unit (BOSU). The BOSU study reported an incidence of 1.1 to 1.3 per million population (all ages), based on 81 new cases reported to BOSU over a 12-month period. The median age at onset was 27 years, range 5–64 years, with a female majority of 61%. The BOSU authors note that not all patients with STGD have only macular disease, and that a minority have wider retinal involvement, with both central and peripheral visual loss. They therefore suggest that it be referred to as a retinal disease rather than a macular one.
Stargardt disease leads to atrophy of the RPE but it appears that once atrophy is established and the RPE is lost, the choriocapillaris may be lost to a greater degree than is observed in GA in AMD. Giani et al. 102 found that areas of atrophy in STGD demonstrated hypofluorescence on indocyanine green angiography, whereas in AMD they were hyperfluorescent, suggesting a loss of the choriocapillaris in STGD. Pellegrini and colleagues103 used a new imaging technique, OCT angiography, in addition to indocyanine green angiography, to study the choriocapillaris in patients with AMD and STGD. They found areas of ‘dark atrophy’ in 65% of patients with STGD but in none of the patients with AMD and GA.
If this is the case, then RPE transplantation at this late stage may not be able to improve function. Transplanted RPE would be unlikely to survive because of lack of nourishment if there is no choriocapillaris.
The defective ABCA4 gene in STGD encodes a protein involved in the visual cycle, which is found in PR cells but nowhere else in the body. Many mutations in this gene have been identified. Some cause retinopathies other than STGD. There is a similar disease (referred to as STGD-like disease) caused by a dominant gene, the ELOV1 gene.
As reported in the BOSU study,101 STGD often affects people in their 20s but the onset can occur at any age. The loss of vision is usually slowly progressive but can be more rapid when the condition develops in younger people.
Lambertus et al. 104 report a cohort of early-onset STGD with mean age at onset of 7 years (range 1–10 years). VA declined rapidly. This early-onset group represents the more severe end of the STGD spectrum.
A very large study from Gerald Fishman’s group in Chicago105 reported data from 361 patients. At presentation:
-
22% were aged ≤ 20 years, of whom 13% had VA 20/40 or better
-
41% were aged 21–40 years, with 22% having VA 20/40 or better
-
31% were aged 41–40 years, with 28% having VA 20/40 or better
-
6% were > 60 years.
This study reported that the younger the patient at presentation, the shorter the time to go from VA ≥ 20/40 to ≤ 20/200. Patients aged < 20 years at diagnosis with VA ≥ 20/40 took a median of 7 years to reach ≤ 20/200, whereas the median times for such progress were 22 years and 29 years for those initially seen who were aged 21–40 years or 41–60 years, respectively. The authors note an implication for research studies in that, given that progress often takes decades, intervention studies need a long follow-up before there can be certainty about prevention of progression.
The ProgStar group also reported an older age at onset group in which progression is slower. 106 Lambertus et al. 104 from Nijmegen, the Netherlands, defined late-onset STGD as age at onset of > 45 years, plus at least one ABCA4 mutation. They report a case series of 47 patients followed for a median of almost 5 years. The time to mild visual impairment averaged 2.7 years, the time to moderate impairment was 10.2 years and the time to severe impairment was 11.4 years.
Lambertus et al. 104 also report that there is a higher frequency of asymmetric progression in late-onset STGD. 107 In 29% of patients, atrophic areas encircled the fovea but did not involve it. There was considerable variation in effects on VA. Some patients had eventual foveal involvement without a preceding foveal-sparing stage.
van Huet and colleagues108 also report a group of patients (13 out of 198 with STGD) who had foveal sparing in at least one eye. All were confirmed to have an ABCA4 mutation. As in the study by Lambertus et al. ,104 they were later onset (mean age 52 years, range 32–67 years), with only three developing symptoms before the age of 45 years. Progression was slower than in younger onset patients. van Huet et al. 108 suggest that people with foveal sparing may therefore be particularly suitable for trials because therapies may have more time to take effect, but results of such trials might only be applicable to this subgroup.
Lois et al. 109 classified patients with STGD into three groups based on functional loss. People can have loss of macular function, loss of peripheral cone function in addition to macular function, or loss of both peripheral cone and rod function in addition to macular function. This last form is extremely severe. The earlier the age at onset, the more likely the disease is to be more severe.
-
Group 1: dysfunction confined to the macula
-
Group 2: macular and generalised cone electroretinography (ERG) abnormalities
-
Group 3: macular and both generalised cone and rod ERG abnormalities
These groups may represent distinct phenotypes of STGD with different prognoses. In a study of 59 patients, all with central visual loss at baseline, with mean follow-up of 10.5 years,110 progression varied as shown in Table 2.
| Group | Proportion at baseline | Median age (years) at onset | ERG deterioration |
|---|---|---|---|
| 1 | 46% | 25 | 22% |
| 2 | 29% | 20 | 65% |
| 3 | 25% | 14 | 100% |
In those with normal full-field ERG at baseline, only 20% showed clinically significant progression. However, all those with abnormal full-field rod dysfunction on ERG progressed.
This study showed that the groups were not just at different stages but had different forms of STGD. Another study, with only 12 patients, showed that the different groups had different progression rates, with areas of atrophy at the macula enlarging at a faster pace and new areas of atrophy at the macula developing more frequently in group 3. 111
Childhood onset has a poorer prognosis. Fujinami et al. 110 from Moorfields Hospital reported that in a series of 42 patients diagnosed at median age of 8.5 years,110 childhood-onset STGD was associated with severe visual loss, probably associated with more severe variants in the ABCA4 gene.
A characteristic of STGD is deposition of A2E (a major component of lipofuscin) in the RPE. The accumulation of A2E can be reduced by isotretinoin, which is an inhibitor of retinal dehydrogenase and rhodopsin regeneration. Fenretinide (ReVision Therapeutics, San Diego, CA, USA) also reduced the accumulation of A2E and lipofuscin in RPE cells in the mouse model of STGD, the ABCA4 knockout mouse (ABCA4–/–) (reviewed by Lu et al. 112). The ABCA4–/– mice model of STGD has been used to validate hypotheses about how ABCR, the protein codified by the ABCA4 gene, functions in the retina. The mice have also been used to demonstrate the impact of light on the accumulation of lipofuscin in the retina – when the ABCA4 knockout mice were raised in the dark, the accumulation of lipofuscin was prevented. 113
Aleman and colleagues114 noted that in macular degenerations associated with ABCA4 mutations, the fovea is often spared until late. They hypothesised that this might be due to the macular pigment concentration, which may reduce the build up of lipofuscin and that, if so, lutein and zeaxanthin supplements might slow progression. Their intervention study is described later. 104
Fundus autofluorescence has also been proposed as a good way to determine progression and monitor response to treatment111,115 and a recent study from the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) study group116 supports this.
There are three main ways of trying to treat STGD:
-
prevent the harmful accumulation of lipofuscin
-
gene therapy (to give the retina a new ABCA4 gene to replace the faulty ones)
-
cell transplantation to replace the dead cells with new ones.
Quantity and quality of research
Reviews
One recent review of treatments for STGD has been published by Lu et al. 112 It claims to be systematic but no details of methods are given, and not all therapies were covered. Three other non-systematic reviews were identified. 100,117,118 A very thorough report from the National Horizon Scanning Centre (NHSC)119 was found, covering all inherited retinal conditions, based on extensive searches and discussions with experts. All these reviews were used to check for references, including to ongoing research. The NHSC report119 was particularly useful.
Lu et al. 112 conclude that the most promising drug treatments for STGD are drugs that reduce lipofuscin accumulation, such as deuterated vitamin A (ALK-001; Alkeus Pharmaceuticals, Boston, MA, USA), fenretinide (Sirion Therapeutics, Tampa, FL, USA) and A1120, a non-retinoid RB4 antagonist.
A more recent review by Tanna and colleagues120 provides a detailed review of diagnostic methods but adds little on treatments. Lambertus et al. 104 have also reported ways of monitoring progression of STGD, focusing on late-onset STGD.
Microcurrent stimulation
In this treatment, very small electrical currents (800 µA) are applied to the eyelids, eyebrow or cornea.
Two studies including a total of 15 people (11 treated, 4 placebo) assessed the effects of electrical stimulation. One of these was a RCT by Röck and colleagues. 121 The RCT was a small three-arm comparison of two doses of transcorneal electrical stimulation and a sham comparator applied weekly for 6 weeks; the duration of follow-up was unclear. There were four participants in each group and the mean age of participants was 40 years. The study was undertaken in Germany and received commercial funding. The RCT had an unclear risk of selection bias. 121
Röck and colleagues121 included patients with logarithm of minimum angle of resolution (logMAR) VA 0.02–0.9. The eye with the worse VA was selected as the study eye. Although there was a small improvement in BCVA in the group given stimulation at 150% of the electrically stimulated evoked phosphene potential, and a deterioration in the 66% stimulation group and placebo group, the mean change in BCVA at follow-up was not significantly different among groups (Table 3). Mean intraindividual changes were also not significantly different between the three groups. No adverse events (AEs) were reported.
|
Röck 2013121 Röck 2011126 RCT; unclear ROB |
Sham, n = 4 | Stimulation 66%, n = 4 | Stimulation 150%, n = 4 | p-value |
|---|---|---|---|---|
| BCVA change, logMAR, mean (SE) | +0.03 (0.01) | +0.03 (0.01) | –0.02 (0.01) | 0.07 |
| Kondrot97 (B + A study; PQ) | Mixed treatment including MCS, n = 3 (6 eyes) | |||
| Acuity improvement, ETDRS chart, mean | 6.6 letters (range 2–13) | |||
| Contrast sensitivity improvement, mean | 3.67 letters (range 0–10) | |||
The other study was of a subgroup of three patients with STGD from a study by Kondrot,97 which also included people with dry AMD and other eye conditions (see Chapter 3). This study involved 3 days of a mixture of ‘alternative’ treatments, which means that it would not be possible to say which, if any, was effective. There was no external funding but participants paid US$3000 to have the treatment. The timing of assessment of results is not clear but may have been at the end of the course. The study scores poorly on quality assessment (see Report Supplementary Material 1). 97 The mean age was not reported.
Kondrot97 reported that in the three people with STGD, there were improvements in acuity of 6.6 letters and in contrast sensitivity of 3.67 letters after 3 days of mixed treatment. No statistical analyses were presented. There was also reported visual field ‘expansion’ in all six eyes. The author does not claim that the benefits were due only to microcurrent, but simply states that ‘In this article, I demonstrated that certain natural interventions given in a short period can reverse eye disease and improve vision’.
He suggested a trial with a control group.
Adverse events were not reported.
An observational study (NCT01790958) in 50 patients with various eye conditions, including STGD, was reported to be under way in Hawaii with an end date December 2012, but no results have been posted.
Summary of microstimulation
The small RCT by Röck and colleagues,121 with an unclear risk of bias, did not find any statistically significant benefit from MCS. We found no good evidence that MCS worked.
Light protection
Molecules can exist in different forms, called cis- and trans-isomers. In the visual cycle, light converts 11-cis-rhodopsin to the all trans form. This is then recycled back to the cis-form through a number of stages in which the ABCR protein is involved. The ABCR protein is encoded by the ABCA4 gene, which is defective in STGD. This results in the harmful compound, A2E, being produced and accumulating as the main component of lipofuscin in the RPE.
Exposure of the retina to light therefore stimulates production of A2E and the rationale of light therapy is to reduce the light reaching the retina in order to reduce the formation of A2E.
Light protection has been tried. The theory is that in people with STGD, light exposure may lead to more lipofuscin accumulation. So reducing incoming light might help to reduce the amount of lipofuscin and the damage caused. There is evidence from mice with STGD that darkness protects the eye.
One study of five participants by Teussink et al. 122 assessed the effects of light exposure protection using a black contact lens covering > 90% of light in the visible spectrum, worn on the better eye during waking hours for a year. 122 The other eye acted as a control. The study was undertaken in the Netherlands. Follow-up assessments were undertaken at a mean of 17.8 months. The study was assessed as poor quality (see Report Supplementary Material 1). Funding was from a non-commercial source. The mean age of participants was 22.6 years, and three were male. The BCVA at baseline was provided only for individual participants. Further details are provided in Report Supplementary Material 1.
Teussink and colleagues122 included people with typical clinical symptoms associated with STGD and at least one ABCA4 mutation. BCVA in the treated eye was reported only for individual participants and no mean value was provided. In four out of the five patients, progression was less in the light-protected eye. The study reported that BCVA was stable in all but one patient during the study period. No participants developed atrophy and there were no AEs reported.
Summary of light protection
One very small study reported reduced progression in the light-protected eye in four out of five participants. So although the evidence base is very weak, and we need a proper trial, we could recommend that people with STGD should wear sunglasses or dark contact lenses to protect their retinas from light exposure.
Retinal pigment epithelium transplant and stem cells
Two publications from a small before-and-after study in STGD conducted in the USA were identified. 98,123 Schwartz and colleagues98,123 recruited nine participants with STGD, and the eye with the worst vision was treated. A single treatment of subretinal transplantation of human embryonic stem cells (hESCs) derived RPE with 12 weeks of immune suppression was assessed. Median follow-up was 22 months. The study was assessed fair quality. Funding was from both commercial and non-commercial sources. Mean age was 50 years and 44% of participants were male. Baseline VA ranged from 20/200 (severe vision loss) to hand motion (near blindness). The STGD study, now completed, was registered as NCT01345006 and sponsored by the Astellas Institute for Regenerative Medicine (Malborough, MA, USA).
Schwartz and colleagues98,123 included people with end-stage STGD, peripheral visual field constriction and BCVA 20/400 or worse in the study eye. Unfortunately, three patients with STGD developed cataracts in the treated eyes, and one suffered from a post-surgical endophthalmitis. Of the other five patients, VA had improved by a median of 12 letters at 12 months, whereas the improvement in the untreated control eyes was only 2 letters. In two of the patients who developed cataracts, vision improved by 6 or 7 letters during cataract progression, and further after cataract removal. Quality of life, assessed by the National Eye Institute Visual Function Questionnaire-25 (NEIVFQ-25) improved for general vision, peripheral vision, near activities, distance activities and mental health (Table 4). Other outcomes are reported in Report Supplementary Material 1.
| Schwartz et al.98,123 (before and after; FQ) | RPE transplant (7 patients with 12-month follow-up) |
|---|---|
| VA (ETDRS) (12 months) improved by | |
| ≥ 15 letters | 3 eyes |
| 11–14 eyes | 0 eyes |
| ≤ 10 letters (stable) | 3 eyes |
| Worsened | |
| 10 letters | 1 eye |
| NEIVFQ-25 change from baseline | |
| General vision, median | Change at 12–52 weeks: +20.0 |
| Peripheral vision, median | Change at 12–52 weeks: +12.5 |
| Near activities, median | Change at 12–52 weeks: +8.3 |
| Distance activities, median | Change at 12–52 weeks: +12.5 |
| Mental health, median | Change at 12–52 weeks: +9.4 |
An update was presented at the 2017 American Academy of Ophthalmology meeting when 10 patients had a mean follow-up of 3 years with no serious side effects, with some gaining > 10 letters. 127
Adverse events
There were no AEs specifically from the cellular therapy (e.g. acute transplant rejection or abnormalities in retinal vascular or choroidal circulations) but one patient developed an endophthalmitis following surgery, which was a potentially devastating complication.
Summary of retinal pigment epithelium transplantation
One small before-and-after study98,123 found improvements in VA in most of the nine eyes after 12 months, whereas there was little change in the untreated fellow eyes. Improvements in quality of life were also noted.
Discussion
The NHSC report119 discussed the prospects for RPE cell transplants, quoting expert opinion doubts about the effectiveness of hESC-derived RPE cells. This was on the grounds that most people with STGD may need replacement of both RPE cells and PRs, if the treatment is undertaken at a late stage of disease, when PR cells may be already lost and permanent damage to the choriocapillaries may have taken place. Indeed, the early clinical trials referred to above selected patients with very advanced STGD. The treatment could potentially be more promising if the disease were to be treated at an earlier stage and this could be done provided that the risks of the treatment were small.
Research in progress
Research in collaboration with Ocata Therapeutics (formerly Advanced Cell Technology, now part of Astellas) has been under way at Moorfields since 2012.
NCT02445612 is the long-term (15 years, to 2029) follow-up of the NCT01345006 study by Schwartz et al. described above.
NCT02941991 is a 5-year follow-up study in Moorfields and Newcastle of people treated with hESC RPE cells, due to complete data collection in December 2019. The sponsor is the Astellas Institute for Regenerative Medicine.
NCT01469832 was called ‘Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With STGD Macular Dystrophy (SMD)’. It was being carried out in Moorfields, Newcastle and Edinburgh, aiming to end by 2015. It was sponsored by Astellas Institute for Regenerative Medicine. NCT02941991 looks to be a continuing follow-up, which is due to end in 2019. The entry for NCT01469837 has disappeared from Clinical trials.Gov.
NCT01625559 is a small study in Korea aiming to recruit three patients. No details have been added recently.
NCT02749734 aims to recruit 15 patients in China (Southwest Hospital) for a 12-month study.
Two stem cell studies are reported to be under way, called Stem Cell Ophthalmology Treatment Study (SCOTS) I and II, which are sponsored by MD Stem Cells. These are being carried out in nine different eye diseases, and involve injecting bone marrow cells (from the hip) into the eye by different routes. There is no control group. SCOTS I (NCT01920867) is due to end in August 2017. The start date for SCOTS II (NCT03011541) was January 2016 and end date will be 2020. The sites are Florida (Retinal Associates of South Florida) and Dubai (Al Zahra Hospital). A couple of single case reports have been published, but not in STGD, except for a case report from another centre of a retinal detachment following stem cell transplantation in a SCOTS I patient (Leung et al. 128). Media reports are that patients are being charged $20,000 for the treatment. 129
NCT02903576 is under way in São Paulo, Brazil, recruiting 18 patients with STGD and AMD, and due to end in 2018.
Many of the studies registered on ClinicalTrials.gov are not trials, and patients should be aware that registration does not mean approval by scientific authorities. Several who saw an entry for NCT02024269 had adipose tissue cells injected into their eyes. Kuriyan and colleagues130 report on the resulting visual loss. The patients were charged for the treatment and the ‘trial’ registration has been withdrawn.
Nutritional supplements
Two studies assessed the effects of nutritional supplements in people with STGD. 114,125
In a before-and-after study, Aleman and colleagues114 included nine people with STGD with foveal fixation and known or suspected disease-causing mutations in the ABCA4 gene. The study received non-commercial funding. The study was of fair quality and the mean age was 32 years, range 14–56 years. Follow-up assessments were undertaken immediately after the intervention period.
Baseline VA ranged from 20/20 to 20/50. The selected patients had relatively spared foveal function in at least one eye. Patients had reduced MPOD compared with normal eyes, and foveal thickness was reduced in patients compared with controls. The mean increase from baseline in foveal MPOD after 6 months of treatment with lutein 20 mg daily was reported to be statistically significant (p < 0.001) at 2 degrees and 5 degrees. MPOD correlated with serum lutein and nearly all patients had increases in serum lutein, but only 63% had increases in MPOD. There were no significant differences in foveal sensitivity between MPOD responders and non-responders, and there were no differences in logMAR VA.
Other outcomes are reported in Report Supplementary Material 1, including subgroups for those classified as responders and those as non-responders. AEs were not reported in the study by Aleman and colleagues. 114
The retina has a high concentration of omega-3 fatty acids and, in particular, of docosahexaenoic acid (DHA). Querques and colleagues125 hypothesised that DHA supplements might protect the retina. In their case series, 20 participants with late-onset STGD (defined as > 18 years, but mean age was 45 years, range 27–72 years) received DHA supplementation for 6 months. VA improved in only four patients, and only slightly (e.g. 20/25 to 20/20). The study quality was fair. The funding source was not reported. BCVA at baseline was only reported for individual patients. Other results are reported in Report Supplementary Material 1.
No AEs were recorded by Querques and colleagues. 125
A trial (NCT00420602) appears to be under way in Utah, in STGD3, using over-the-counter DHA/eicosapentaenoic acid (EPA) dietary supplementation with 1000 mg/day DHA/EPA. There is also a trial registered of DHA as having been done in Maryland (NCT00060749) but no results have been posted.
NCT03927515 (details first posted September 2017) will be a trial of omega-3 fatty acids versus placebo (sunflower oil) called MADEOS – MAcular DEgeneration Omega-3 study. The primary outcome is given as VA after 24 weeks, which seems very short.
NCT01278277 is a trial of saffron supplements (20 mg once a day) versus placebo, being carried out in 30 people by Falsini and colleagues in Rome, due to end December 2017. The primary outcome is ERG after 6 months.
Too high a vitamin A intake may be harmful. Sofi et al. 131 found that in 24 patients with STGD, those with low vitamin A intake (< 600 µg RAE per day) had better visual function, but there were only four such patients. There is support from the work of Radu et al. 132 who showed that in the STGD mice model (ABCA4–/–), vitamin A supplementation led to increased accumulation of lipofuscin in the RPE.
Summary of nutritional supplementation
One small short-term study of lutein supplementation failed to find a beneficial effect on VA and dark-adapted sensitivity. 114 A trial of DHA supplementation found only slight improvement in VA in 4 out of 20 people after 6 months of treatment. 125 It should be noted that treatments may be effective without improving vision if they prevent further deterioration, but this could only be shown by longer-term RCTs.
We suggest an exploratory EME study of lutein and zeaxanthin in STGD. There is theoretical support for them being of value in protecting the centre of the macula, through protection of visual cycle products from photo-oxidation, and hence reducing accumulation of the toxic A2E. Aleman and colleagues114 found that the eyes of those with STGD have lower MPOD than normal eyes and about half the serum levels of the carotenoids, compared with a control group, despite similar dietary intakes. That study was published in 2007. No trials of carotenoid supplements are registered on ClinicalTrials.gov.
We suggest an EME ‘proof of concept’ trial using sensitive measures of retinal function rather than changes in VA. MPOD and VA would be measured, but the aim would be to recruit people before vision was significantly impaired to determine whether functional loss could be delayed or prevented. So the study should use macular microperimetry or multifocal ERG to test macular function, reading vision and reading speed, visual-related quality of life and AF. The power of the study would be increased by recruiting faster progressors in STGD, which would probably allow some outcomes to be determined after shorter follow-up, perhaps 1 year. Because treatment would be systemic, both eyes will be treated (so no possibility of using one eye as treated and one eye as control) and, therefore, adequate controls would be required. It would be essential that all patients (treated and controls) are similar with regard to characteristics known to affect speed of progression of disease as well as genotype, which could be achieved by a large enough trial, and/or by stratified randomisation. Although genotyping may pose problems if mutations on the ABCA4 gene are not found in both alleles. A matched placebo would be required to reduce the risk of the control group self-treating with supplements.
Gene therapy
The status of gene therapy in STGD was reviewed in 2015 by Aurichio et al. 133 and in 2016 by Dalkara et al. 134 Aurichio et al. 133 note that the major problem is the size of the ABCA4 coding sequence (6.8 kb) and expression of the ABCA4 gene/protein exclusively in PR cells; therefore, the vectors need large carrying capacity and ability to transduce PR cells. Vectors with efficient PR transduction, such as adenoviruses, cannot carry more than 4.7 kb. However, the gene can be split in two halves and loaded on two vectors. Adeno associated virus appears safe and has been trialled in Leber congenital amaurosis (with some increase in vision). This approach seems promising but unproven. Aurichio et al. 133 report some success in a mouse model of STGD with ABCA4 transgene expression for up to 8 months with improved dark adaptation and reduced lipofuscin accumulation.
Dalkara and colleagues134 report proof-of-concept studies in mice, wherein subretinal injection of a lentivirus vector carrying the human ABCA4 gene was followed by reduced A2E and lipofuscin levels. Lentivirus can carry larger genes (8–10 kb).
A Phase I/II trial (NCT01367444) started in 2011 in Oregon and Paris with lentivirus carriage, with the equine infectious anaemia virus (StarGen, Oxford Biomedica) in humans, sponsored by Sanofi, and will test a range of doses. It aims to report results in 46 patients in 2018. No interim results have been published but a press release in 2013 reported that the first dose caused no safety problems. 135
A second trial (NCT01736592) began in 2012 and aims to follow up patients for 20 years. Results at 48 weeks were presented at ARVO 2017, but so far show no difference in VA between treated and untreated eyes. 136
A third trial (NCT01367444) is registered as being under way in three US centres and Paris. The Sanofi product is known as SAR422459.
Drug treatments
Our review found no evidence of any drug yet having been shown to be effective in STGD.
Soraprazan
Soraprazan is a proton potassium-competitive acid-blocker developed for use in dyspepsia, but discontinued for that use. It was granted orphan drug status by European Medicines Agency (EMA) for use in STGD in Germany in November 2013. 137 An orphan designation is not a marketing authorisation, but only approval for investigation. The EMA noted that no trials had been started.
The EMA document provides an accessible explanation of how soraprazan might work:138
Soraprazan is expected to be able to enter the cells of the retina, where it attaches to the abnormal deposits that damage the retina cells. Soraprazan is thought to cause the deposits to break up and partly dissolve. The broken-down deposits can then be expelled by the cell’s own natural mechanisms, reducing their build-up and the damage to the cell.
Reproduced with permission from © European Medicines Agency, 2013138
Isotretinoin
This was reported in a review by Battaglia Parodi et al. 139 to delay visual loss in STGD but to have adverse effects including liver toxicity. The evidence comes from the mouse model (ABCA4–/–), in which it reduced A2E and lipofuscin granules, but at a dose too high for human use.
Dobesilate
Dobesilate has been reported in a single case history95,140 to improve VA 4 weeks after a single injection, but with no changes in fundus photography, fluorescein angiography or foveal thickness. Dobesilate is an inhibitor of fibroblast growth factor.
4-methylpyrazole
A placebo controlled trial (NCT00346853) of 4-methylpyrazole was conducted in Utah in healthy adults to see if it would improve dark adaptation. Jurgensmeier et al. 141 reported that there were no significant differences after six intravenous (i.v.) injections and they concluded that further trials in humans were not justified.
ALK-001
This compound is C20 deuterated vitamin A. In deuterated compounds, hydrogen is replaced by deuterium (deuterium is an isotope of hydrogen which has a neutron in the nucleus, as in ‘heavy water’).
In the retina, vitamin A has a tendency to ‘dimerise’, which means that two vitamin A molecules join together. The combined compound is A2E, which is a major component of lipofuscin. Deuterated vitamin A is much less likely to dimerise, and so the deposition of A2E in lipofuscin is reduced by about 80% in a study in mice by Charbel Issa and colleagues. 142 For a recent review of rationale and of animal studies, see Saad and Washington 2016. 143
The rationale for use is that ALK-001 interferes with the vitamin A processes that lead to the formation of A2E and lipofuscin accumulation. A Phase I trial assessed safety in healthy volunteers (NCT02230228) and a Phase II trial is under way, called TEASE – Tolerability and Effects of AKL-001 on Stargardt disease (NCT02402660), which aims to recruit 50 patients followed for 24 months. The sponsor is Alkeus Pharmaceuticals, Boston, MA, USA.
Fenretinide
Fenretinide is a synthetic form of vitamin A. Administration leads to reduced levels of retinol binding protein (RBP) which, in turn, leads to decreased levels of vitamin A in the eye and reduced accumulation of the toxic A2E. Safety concerns (angiosarcomas) have been raised following research in mice (see Lu et al. 112 for review) but it has been used in a large study144 of 246 patients with dry AMD in two doses: 100 mg and 300 mg (see Chapter 5). Adverse reactions were common in a group with a median age of 79 years, and 17% of the lower-dose group and 20% of the high-dose group stopped the drug, compared with 6% on placebo. It may be worth a trial in STGD, in a much younger and fitter population. We have not found any such trial on Clinicaltrials.gov. It has been trialled in many cancers and other diseases so safety data are available in younger age groups.
LBS-500
This drug (www.linbioscience.com/Pipeline/LBS008)145 from Lin BioSciences (Taipei City, Taiwan) is expected to work in a similar way to fenretinide by reducing RBP4. It was granted orphan drug status by the Food and Drug Administration (FDA) for STGD, and a Phase I trial will be carried out in the USA in collaboration with Columbia University, supported by NIH. 146
A1120
This was originally developed for type 2 diabetes (to improve insulin sensitivity) but was ineffective. However, it competes with vitamin A for binding on to RBP and reduces accumulation of A2E and lipofuscin in a mouse model of STGD. 147 We have not found any human studies in STGD.
Emixustat
A Phase II pharmacodynamic study (NCT03033108) started in January 2017, which will look at safety and retinal responses to flashes of light (measured by electroretinogram), and was sponsored by Acucela (Seattle, WA, USA). There will be three different doses but no placebo group. Data collection is due to end in December 2017.
Fenofibrate
A very recent conference abstract by Moiseyev and colleagues148 has reported that fenofibrate may be a visual cycle inhibitor, and, if so, may have potential to decrease the accumulation of A2E in STGD.
Fenofibrate has been used for many years in hyperlipidaemia, but is currently of interest in diabetic retinopathy. An unexpected finding from the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) trial149 was a reduction in progression of diabetic retinopathy. The LENS (Lowering Events in Non-proliferative retinopathy in Scotland) trial150 of fenofibrate to reduce progression of diabetic retinopathy is under way, funded by the HTA programme.
Full publication of the study by Moiseyev and colleagues148 is awaited, and further pre-clinical research is probably required before clinical trials can start. However, fenofibrate is an old, cheap and safe drug so if it is proven to be a visual cycle inhibitor, then a trial in STGD (and dry AMD) would be justified.
Avacincaptad pegol
Avacincaptad (Zimura, Ophthotech, New York, NY, USA) is a C5 complement inhibitor or anti-C5 aptamer. A trial (NCT03364153) in STGD is due to start in December 2017, aiming to recruit 120 patients. An earlier smaller trial, NCT00950638, does not seem to have been published yet.
Another trial (NCT02686658) is under way in the USA and Hungary. The aim is to recruit 300 people to three arms: two doses of the drug and sham. It is due to end in December 2018.
There is another Zimura registration on the EU Clinical Trials Register, EUCTR2015-003991-56-HU, for a trial with a target of 900 patients, but without any sites mentioned, although the ‘HU’ implies Hungary.
Prevention of Stargardt
Sohrab and colleagues151 report a single case in which a man with STGD and his wife, who was a carrier but unaffected (i.e. heterozygous, carrying only one copy of the responsible gene), had IVF and pre-implantation diagnosis to identify an embryo with a normal maternal allele before implantation. The child was born healthy and will be an unaffected carrier.
Summary and conclusions
The evidence on treatment of STGD is sparse. We found only one RCT and it had only 12 patients. Most studies did not have a control group, were far too short term (did not last long enough to determine the effect and potential side effects of the treatment), and the quality of some studies was poor. There has been very little research into the treatment of STGD compared with that of AMD.
In prevention of lipofuscin accumulation, several drugs may have potential. Early trials of ALK-001 and emixustat (Acucela, Seattle, WA, USA) are under way. Fenretinide has shown promise in dry AMD and we think that a trial in STGD may be justified. We have not found any. We have not found any trials of A1120 in humans with STGD.
Gene therapy is at an early stage, but a study (StarGen NCT01736592) is under way in Oregon and Paris.
Cell transplantation to replace the RPE has been tried in one study in only nine people with STGD, but looks promising. RPE transplants may not be suitable for all people with STGD because other parts of the eye (the choriocapillaris, which provides the blood supply) can be affected and RPE cells will not replace those. There is also an issue of timing – if the cells are given at a very late stage then they may not be of benefit if other cells that are needed, such as those in the choriocapillaris or even PR cells, are already lost.
There is a plausible rationale for the benefits of lutein and zeaxanthin supplementation but no evidence.
Nutritional supplements have been used in one short study wherein people with STGD were found to have lower levels of macular pigments than people without the condition. 114 There were no changes in vision. Although this could be seen as negative result (no improvement), it may suggest a beneficial effect (no progression) but the study was really too small and too short to determine whether lutein supplements help. There are plausible theories that lutein (and zeaxanthin, another type of macular pigment) could slow progression, and we think that a longer and larger trial should be considered.
One supplement that should not be taken is vitamin A. There are some data from basic laboratory research suggesting it would cause harm in people with STGD by increasing lipofuscin in the retina.
Two key aspects will be the stage at which intervention is undertaken and case selection. Some treatments may be potentially suitable for an earlier stage and others for a later stage.
It is very important that outcome measures of trials are carefully selected. If function is lost, it may not be recoverable and thus vision may not improve. In inherited retinal diseases, main outcomes should include prevention of functional and structural deterioration (rather than improvement) as measured by best corrected vision, or ERG, or prevention of enlargement of atrophy on AF or prevention of loss of structure by OCT.
It is important that a clear definition of STGD is used for inclusion in the studies. Unfortunately, in many patients, mutations in the ABCA4 gene are identified in only one allele, so a confirmed genetic diagnosis may not be made in all cases. Some studies included patients in whom only one mutation was identified.
Chapter 3 Physical treatments for age-related macular degeneration
In this chapter we examine the evidence for seven interventions. There are considerable problems with some of the evidence that we have, or that we do not have, with one concern being publication bias. For example, we have one trial of acupuncture that reports benefit. 152 We do not know how many negative unpublished acupuncture trials there may be. This problem also applies to other interventions, and we note elsewhere that some registered trials have never been reported.
Acupuncture
Quantity and quality of research
Reviews
The American Academy of Ophthalmology153 reviewed the evidence published from January 1970 up to March 2007 on the safety and effectiveness of acupuncture, and concluded that there was insufficient evidence to justify its use. They found no relevant articles in MEDLINE, but found two articles from an internet search, from a journal not indexed in MEDLINE. Both articles were non-randomised case series. We therefore focused on publications in later years.
Studies
One before-and-after study conducted in Austria with 328 participants was identified. 152 Acupuncture was delivered twice a day, 5 days per week for 2 weeks. The study was assessed as poor quality and the funding source was not reported (Table 5). Mean age was 77.4 years [standard deviation (SD) 8.6 years]. VA was reported as the percentages of lines correctly read from 3 m [22%, interquartile range (IQR) 0–5%] and from 40 cm (45%, IQR 20–67%).
| Krenn et al.152 (before and after; PQ) | Acupuncture, n = 328 |
|---|---|
| Median (IQR) VA reading from 3-m distance (% lines correctly read) |
Baseline 22 (0–55)a 2 weeks 33 (0–66)a |
| Median (IQR) VA reading from 40-cm distance (% lines correctly read) |
Baseline 45 (20–67)a 2 weeks 66 (50–82)a |
| Vision at 3 m (%) | |
| Improved | 44.2 |
| Stable | 51.5 |
| Worsened | 4.3 |
| Vision at 40 cm (%) | |
| Improved | 88.4 |
| Stable | 8.8 |
| Worsened | 2.7 |
Results
Consecutive patients with dry AMD (details of stage not given), as diagnosed by their ophthalmologist, were included. After 2 weeks of acupuncture, the median VA at 3-m distance and at 40-cm distance improved in 44.2% and 88.4% of participants, respectively. Vision at 3 m and at 40 cm worsened in 4.3% and 2.7% of participants, respectively. No statistical analyses were presented.
Summary
One large before-and-after study found improvement in vision after 2 weeks of acupuncture; however, no statistical analyses were presented and the study was assessed as low quality.
Registered studies
The study NCT02255981 aims to use acupuncture to treat 33 patients in Columbia with unspecified macular diseases. With no control group, it is unlikely to be helpful.
Blue-light-filtering intraocular lenses
Mechanism of action
The outer layer of the retina has an abundant supply of oxygen from the choriocapillaris and oxidative damage, triggered by the effects of light, is a risk. Several studies have shown that people most exposed to sunlight, like fishermen, have a higher risk of AMD (see Cuthbertson review154). The risk may be higher in people who are both exposed to sunlight and have low antioxidant levels. 155
The structure of the human eye reduces penetration of harmful light. Protective mechanisms include the structure in front of the retina (especially the lens), various enzymes and the macular pigments lutein and zeaxanthin, which absorb blue light.
Cataract surgery is common in older people and the natural lens is replaced by an artificial one – an artificial intraocular lens (IOL). These can be protective only against ultraviolet radiation (UVR) or against both UVR and blue light. The argument against additional blue-light-filtering appears to be based on the effects on vision in poor light (scotopic vision) and circadian rhythms and sleep. Scotopic vision declines with age and is reduced by blue-light-filtering IOLs.
People with artificial lenses are described as ‘pseudophakic’. People who still have their own lens are called phakic.
There are several uncertainties:
-
Are people more at risk of AMD after cataract removal?
-
If so, do blue-light-filtering IOLs prevent AMD?
-
In people with existing AMD, do blue-light-filtering IOLs reduce or prevent progression?
Quantity and quality of research
Reviews
Four good reviews of blue-light-filtering IOLs were found (Cuthbertson et al. ,154 Davison et al. ,156 Henderson and Grimes157 Lai et al. 158) but they could not be described as systematic.
The reviews came to different conclusions. Cuthbertson and colleagues154 consider that evidence is lacking on whether blue-light-filtering IOLs are beneficial, and that only large long-term studies could provide evidence. They made no recommendation. Davison and colleagues156 conclude that blue-light-filtering IOLs should be used even without definite proof of protection against AMD. They conclude that such IOLs have no significant proven harms, can reduce glare and could be protective against the development and progression of AMD in pseudophakic eyes. They admit that definite proof of protection against AMD is lacking.
Henderson and Grimes157 conclude that there is no evidence that definitively demonstrates that blue-light-blocking IOLs have any effect on AMD. However, the evidence that they have disadvantages compared with UVR-only blocking IOLs is weak. Henderson and Grimes157 argue that the likelihood of any further RCTs being done to assess the effect on AMD is very low and that blue-light filters should be used because it is very likely that they would protect the eye.
Writing early in 2014, Lai and colleagues158 provide another non-systematic but useful review. They note that yellow-tinted, blue-light-filtering IOLs reduce blue-light irradiance of the retina by 62–82%, whereas clear UV-blocking IOLs reduce it by 43–64%; these ranges reflect the variation across studies. They also note that epidemiological studies of the association between light exposure and the development of AMD are conflicting. They suggest that there are at least two reasons for this. First, it is difficult to retrospectively quantify lifetime light exposure in people with and without AMD. Second, the development of AMD depends on many factors, including genes, smoking and diet. For example, Fletcher and colleagues155 found that the risk of AMD was increased only in those with low antioxidant levels. Lai and colleagues158 conclude that uncertainties remain over both the association between cataract surgery and the risk of AMD, and the value of blue-light-filtering IOLs after cataract surgery.
Chew and colleagues,159 from the AREDS study, found no definite effect of cataract surgery on the risk of progression of AMD. Their patients received clear UV-blocking IOLs. In a later study, available only as an ARVO abstract160 with data from AREDS 2, they again report no increase in progression of AMD after cataract extraction. Indeed, fewer eyes developed AMD after cataract surgery than eyes that did not have cataracts removed.
In a recent update, Downes,161 who was one of the authors of the Cuthbertson review,154 noted that there was little new research on whether the risk of AMD or of progression among those who had it was increased after cataract surgery.
However, since the previous reviews were written, several new studies on choice of IOL have been published.
Studies
Four studies were included.
Pipis and colleagues162 from Germany assessed progression in 66 eyes, 27 with a blue filter and 39 with a non-blue filter at 1 year, non-randomised, in people with pseudophakia and GA. Funding sources were not reported. GA progression was measured using spectral domain OCT and automated software analysis. At 1 year, progression was statistically significantly less in eyes with a blue-light filter than eyes with a no-colour filter [0.72 mm2 (SD 0.39 mm2) vs. 1.48 mm2 (SD 0.88 mm2); p = 0.002]. AEs were not reported.
The Pipis study162 was assessed as poor quality because on the NIH risk-of-bias checklist it was unclear how participants were selected, the sample size was not justified, the outcome assessors were not blinded to the intervention, and potential confounding variables were not adjusted for.
Nagai and colleagues163 reported a similar study in which they compared 52 eyes with blue-light-filtering (yellow-tinted) IOLs and 79 eyes with standard UV-filtering (colourless) IOLS. The groups were not randomised. They recorded FAF, GA and any wet AMD at baseline (cataract removal) and 2 years later. No abnormal AF was observed in the blue-light-filtering group but it developed or increased in 15% of the colourless IOL group (p = 0.0016) (Table 6).
| Study and outcome | Intervention | Comparator | p-value |
|---|---|---|---|
| Pipis et al.162 | Blue-light filter, n = 39 eyes | No blue filter, n = 27 eyes | |
| Retrospective cohort; PQ | |||
| GA progression in 1 year (mm2), mean (SD) | 0.72 (0.39) | 1.48 (0.88) | p = 0.0002 |
| Nagai et al.163 | Blue-light filter, n = 52 eyes | Colourless lens, n = 79 eyes | |
| Cohort study; FQ | |||
| Abnormal FAF development or increase in size or density, n (%) over 2 years | 0 | 12 (15.2) | 0.0016 |
| Abnormal FAF decrease, n (%) | 3 (5.8) | 2 (2.5) | NR |
| Wet AMD or GA development | 1 (1.9) | 9 (11.4) | 0.042 |
| Lavric and Pompe164 | Blue-light-filter IOL, n = 30 eyes | UV-filter IOL, n = 30 eyes | |
| Cohort study; PQ | |||
| Signs of early DRAMD (e.g. drusen or RPE changes), n (%) | 5 (17%) over 32 months (SD 8) | 5 (17%) over 34 months (SD 8) | |
Chong and colleagues,165 in a study available only as an abstract, carried out a study in 128 people who had cataracts removed from both eyes. The study could not be assessed as good quality because of lack of detail. They implanted a standard clear UV-light-filtering IOL in one eye and a blue-light-blocking IOL in the other. They found no significant difference in the progression of AMD after a mean follow-up of 26 months, but no detailed results were presented. The authors recommend longer follow-up but it is not clear whether they intend further publications.
Lavric and Pompe164 report the results of a study in 30 people having bilateral cataracts removed, with a UV-only light-filtering IOL placed in one eye and a blue-light-filtering IOL in the other. They report that after at least 2 years, there was no significant difference in proportions developing early signs of AMD.
Other studies
A study reported by Łak and colleagues166 is in Polish and so only the English abstract is available to us. Forty eyes of 20 patients had cataracts extracted, with a standard IOL in one eye and a blue-light-filtering IOL in the other. After 18 months, they concluded that there was no protection from blue-light-filtering IOLs.
Brockmann and colleagues167 have noted that available blue-light-filtering IOls may vary in the protective characteristics, but that most mimic the protection of the natural lens.
Summary
The evidence is mixed. The Davison156 and Henderson157 reviews make a good case for routine use of blue-light-filtering IOLs. The Pipis trial162 provides some support for a reduction in progression but scores poorly on quality. The Chong,165 Lavric164 and Łak166 studies, which have a stronger design with patients having standard IOLs in one eye versus blue-light-filtering ones in the other, report no differences, but two (Chong165 and Łak166) are available only as abstracts. The adverse effects on scotopic vision and sleep disturbance do not seem to be clinically significant.
However, a very large trial is under way, called the CLOCK-IOL colour study. Nishi and colleagues168 from Nara in Japan report that they plan to randomise 1000 patients after cataract surgery to a standard IOL or a blue-light-blocking IOL, with AMD one of the primary outcomes, assessed first after 1 year but with follow-up planned for up to 20 years. The protocol has been published. 168 The trial is registered on the University Hospital Medical Information Network as UMIN000014680. 169
Rheopheresis
Rheopheresis, also called haemopheresis, is a procedure in which blood is removed from the body, passed through a filter to remove the larger molecules, and then returned into the bloodstream. It is similar to renal haemodialysis. The patient’s entire blood volume is filtered over a 2- to 4-hour session. In the largest trial, eight treatments were given over 10 weeks. Blood is taken from one arm, filtered, and reinfused into the other arm. The filtration removes only larger molecules, including immunoglobulins such as immunoglobulin M (IgM), fibrinogen, von Willebrand factor and low-density lipoprotein (LDL) cholesterol. About half of these are removed. Smaller molecules, such as albumin and high-density lipoprotein (HDL) cholesterol, are not extracted.
Mechanism of action
Brunner and colleagues170 suggest that a key feature in AMD is impaired choroidal perfusion, and that reduction of plasma viscosity can improve this. However, reduced viscosity may be more important in the retinal circulation where vessels are smaller (3–10 µm compared with 20–40 µm in the choroidal capillaries). Perfusion pressure may be more important than viscosity as choroidal vessels are wider, fenestrated and have lower pressure than retinal vessels. 171,172
Quantity and quality of research
Reviews
One non-systematic review by Pulido and colleagues173 was identified (see Report Supplementary Material 2). It concluded that:
Rheopheresis treatment shows strong promise as a viable clinical option for patients suffering from the dry form of AMD in terms of minimizing vision loss, vision restoration, and overall quality of life factors.
Pulido and colleagues173
However, this was based largely on the interim results of the Multicenter Investigation of Rheopheresis for Age-related macular degeneration (MIRA) trial,174 of which Dr Pulido was an investigator, and the final results showed no overall benefit.
Studies
Eight studies of haemopheresis were identified. Five studies were RCTs: Koss 2009,175 Pulido 2006,176 Brunner 2000,170 Rencova 2015177 and Swartz. 178 The RCTs randomised 168 patients to haemopheresis and 132 as controls, but the largest174 was bigger than all the rest put together, with 104 randomised to haemopheresis. Two were CCTs (non-randomised) (Blaha 2013179 and Studnicka 2013180) and one was a large retrospective cohort study from the Rheonet Registry. 181
Further details are given in Report Supplementary Material 2.
The studies were conducted in the Czech Republic (n = 3),116,179,180 Germany (n = 3)170,175,181 and the USA (n = 2). 174,178 In all but one study170 participants received between 8 and 10 treatments. Duration of follow-up was between 20 weeks and 3.5 years (some participants in the Brunner study continued treatment and were followed up to 4 years). One RCT175 had a low risk of bias, with four of the Cochrane risk of bias criteria graded low and three high. The remaining RCTs were assessed as having uncertain risk of selection bias;170,176–178 and the CCTs were assessed as at high risk of selection bias. 179,180 The retrospective cohort study was assessed as poor quality. 181 Five studies had small sample sizes (< 22 participants per arm). Where reported, mean age ranged between 64 and 76 years and studies typically had a greater proportion of women than men. BCVAs at baseline were reported in six studies. The funding source was not reported by two of the studies,163,164 three received commercial funding158,162,168 and three received non-commercial funding. 165–167
Two studies182,183 used rheophoresis in combination with plasma exchange and selective adsorption.
The earliest studies (not included above) came from a group in Cologne. In 1995, Brunner and colleagues184 reported that in a series of 10 patients with ‘various macular diseases’ (no details given so not an inclusion), haemophoresis (which they called membrane differential filtration) reduced plasma viscosity by 15% by removing the large molecules. The largest reductions (measured the day after treatment) were in IgM (down to 33% of pre-treatment level), alpha-2-macroglobulin (30%), LDL (33%) and cholesterol (33%) but there were also reductions in HDL cholesterol (62% of pre-treatment level) and IgG (59%). VA was reported to improve by at least one Early Treatment Diabetic Retinopathy Study (ETDRS) line in 7 out of the 10 patients the day after treatment, but no details of baseline or final VA are given.
A second case series from 1996182 reported that a combination of plasma exchange, selective adsorption and membrane differential filtration improved VA by at least one ETDRS line 24 hours after treatment in 11 out of 17 patients (15 out of 31 eyes) with AMD (about half with wet AMD and results not given separately). One ETDRS line is not usually considered a clinically important change.
Brunner and colleagues 2000170 randomised 40 people with AMD in at least one eye, of whom 22 had dry AMD (based on the presence of drusen, pigment clumping, areolar atrophy) and VA 20/160 to 20/32, to membrane differential filtration with five sessions over 21 weeks. Mean age was 71 years. At follow-up (11 months for treated group, 12 for controls) there was deterioration in VA in both groups. The change was not significantly smaller in the haemopheresis group than for the controls (Table 7).
| Koss 2009175 | Rheopheresis, n = 22 | Control, n = 21 | p-value | |
|---|---|---|---|---|
| Change in BCVA, 7.5 months, ETDRS lines, mean (95% CI) | 0.63 (0.28 to 0.99) | –0.31 (–0.64 to 0.02) | Difference 0.9 (0.2, 1.7), p = 0.014 | |
|
Pulido 2006,174 Pulido 2005176 RCT; unclear ROB |
Rheopheresis, n = 104 | Placebo, n = 69 | p-value | |
| Mean logMAR ETDRS VA at 12 months (SD) | 0.02 (0.213) | 0.02 (0.20) | p = 0.977 | |
|
Brunner 2000170 RCT; unclear ROB |
Membrane differential filtration, n = 20 | Control, n = 20 | p-value | |
| Change in VA at follow-up, ETDRS lines, mean (SD), approximately 11 months | –0.21 (2.4) | –1.83 (2.9) | Difference 1.6, p = 0.06 | |
|
Swartz and Rabetoy 1999178 RCT; unclear ROB |
Apheresis, n = 10 | No filtration, n = 10 | No treatment, n = 10 | p-value |
| BCVA mean change (logMAR) ETDRS chart lines | 1.9 | 1.3 | 0.6 | Not reported |
|
Rencova 2015177 RCT; unclear ROB |
Rheopheresis, n = 12 | Control, n = 12 | p-value | |
| BCVA, ETDRS letters, median (95% CI), 2.5 years | 79.0 (57.3 to 83.4) | 72.5 (23.4 to 83.1) | p = 0.021 | |
| Drusenoid pigment epithelial detachment, mm2, mean (SD) | 0.71 (1.27) | 9.19 (9.51) | p < 0.001 | |
|
Blaha 2013179 CCT; high ROB |
Rheohaemopheresis, n = 37 | Control, n = 34 | p-value | |
| BCVA (95% CI) at 2.5 years, Snellen lines | 0.68 (0.35 to 1.00) | 0.52 (0.25 to 0.80) | p = 0.09 | |
| Studnička 2013180 | Rheohaemopheresis, n = 19 | Control, n = 18 | p-value | |
|
Mean BCVA (95% CI) at 3.5 years, Snellen lines CCT; high ROB |
0.79 (0.41 to 1.0) | 0.7 (0.32 to 0.87) | p = 0.125 | |
| Mean (SD) DPED, mm2 | 4.13 (3.84) | 6.69 (4.2) | p = 0.015 | |
|
Klingel 2010181 Retrospective cohort; PQ |
AMD, eyes, n = 428 | Controls, eyes, n = 85 | p-value | |
| % of eyes with improvement in VA (difference of ≥ 0.1 log Mar) | 42 | 26 | p < 0.01 | |
| % of eyes with loss in VA (difference of ≥ 0.1 log Mar) | 17 | 40 | p < 0.01 | |
In a longer-term case series with 20 participants with dry AMD (available only as an abstract),183 Brunner and colleagues reported significant improvements in BCVA 1.9 lines at 2 years and 1.2 at 3 years but not at 4 years follow-up, after combined plasma exchange, selective adsorption and filtration.
Koss and colleagues175 included 43 people with advanced AMD (wet or dry) in the non-study eye but not in the study eye. For inclusion, patients had to be between 45 and 85 years old, with AMD (no details, but confirmed by fluorescein angiography and fundus photography) in both eyes, and to have had dry AMD in the study eye. Study eyes had to have BCVA 0.1–0.8 determined with the use of the ETDRS charts. Treatment was 10 sessions over 17 weeks. At 7.5 months there was a statistically significant difference in the change in BCVA between the two groups favouring haemopheresis (see Table 7). Although not statistically analysed, there were higher proportions demonstrating improvement in VA and lower proportions demonstrating deterioration in the haemopheresis groups than the control group. At 25 months, about half of the patients were retested (others did not return after the end of the trial). The rheophoresis patients had lost an average of 1.7 lines and the controls had lost an average of 3.1 lines. By 25 months, four control patients and two haemopheresis patients had developed wet AMD.
A pilot study in Utah does not appear to have been published in full, but only as a conference abstract. Swartz and Rabetoy178 included 30 people with non-exudative AMD characterised by large soft drusen and VA 20/40 – 20/100 in one eye. They were randomised to three groups: rheophoresis, sham rheophoresis (blood moved from arm to arm through tubing but not filtered) and controls. No mean baseline values for BCVA by group were reported but the improvement at 20 weeks was reported to be greater (+1.9 ETDRS lines in active group and +1.3 lines in controls) in those treated with rheopheresis than the two control groups, although no statistical testing was undertaken. The sham group appeared to do better than the control group and it was suggested that this might be because large molecules adhered to the tubing.
Lane 2004185 wrote an article for Eurotimes (www.eurotimes.org, a journal of ESCRS – European Society for Cataract and Refractive Surgery) in which he interviewed experts in the field. He describes the Utah pilot by Swartz and Rabetoy178 as ‘the FDA pilot study’, and states that the unexpected sham result was the reason why FDA did not wish to include it in what was expected to be the definitive study of rheophoresis, the MIRA trial, approved by FDA.
The MIRA trial by Pulido et al. 174 was a RCT with 216 people with dry AMD in the study eye with ≥ 10 drusen and BCVA 20/32 to 20/125. GA was allowed as long as it covered less than three disk areas. Wet AMD was excluded. The mean groups were well matched at baseline. Quality assessment was mixed, with risks of selective reporting, incomplete outcome data, and allocation concealment unclear. Baseline VA was quite well matched. The interim results based on the first 43 patients (28 rheopheresis and 15 controls) were promising. 186 At 12 months, 16% of the rheopheresis group, but none of the controls, had improved VA by ≥ 3 lines. A total of 58% of the rheopheresis group achieved VA 20/40 or better compared with 14% of the controls. However, it should be noted that numbers are small as 14% of the controls is two patients. A total of 29% of the controls (four patients) lost 3 lines of vision compared with 5% of the rheopheresis group.
However in the final analysis, Pulido and colleagues174,176 found no statistically significant difference in VA between groups at 12 months (see Table 7). Many patients entered into the trial did not meet the inclusion criteria, and 37% of the treated patients had to be excluded. Reanalysis after removing them did show a statistically significant difference between arms but only of 0.09 lines, which is not clinically relevant. The authors concluded that ‘At best this was a flawed study in that 37% of the treated cases did not meet inclusion criteria, and at worst there was no evidence of effect. 174
A case series (not an inclusion because it is not published) with a similar intervention to MIRA was carried out in Canada, called Prospective Evaluation of visual functioning with Rheopheresis treatment for age-related macular degeneration in Canada (PERC). The only reports we have found so far are a conference abstract by Wong and colleagues187 from the 2006 ARVO meeting and presentations by members of OccuLogix (San Diego, CA, USA) reported in Primary Care Optometry News (www.healio.com/optometry) and in business media news. The abstract provided little detail but an Occulogix press release reports that the PERC results were presented at the American Society of Retinal Specialist 2005 annual meeting, and that 12 out of the 30 PERC patients gained ≥ 1 line of vision, with 16 being unchanged and two losing 1 line of vision or more. 188
The Rencova 2015 RCT177 included 38 people with dry AMD. Recruits had high-risk, dry AMD with soft drusen, confluent soft drusen and drusenoid pigment epithelium detachment (DPED). Baseline BCVAs were 74 letters in both groups. Median BCVA was higher in the haemopheresis group than the control group at 2.5 years (79 vs. 72.5 letters) and the DPED area as measured by fundus photography was significantly smaller in the haemopheresis group (see Table 7). A higher proportion of participants in the haemopheresis group demonstrated improvement in DPED area and fewer demonstrated deterioration compared with the control group (Report Supplementary Material 2). This study has a possible overlap of participants from Blaha179 and Studnička,180 which are reported next.
Blaha and colleagues 2013179 included 38 people with AMD in both eyes, confirmed in one or both eyes by fluorescein angiography and fundus photography, with soft drusen, confluent soft drusen and DPED. There were 34 control patients. At 2.5 years there were no statistically significant differences between groups in BCVA (see Table 3). Higher proportions of participants had improvement and lower proportions had deterioration in VA in the haemopheresis groups than the control group, although it is unclear whether or not this was analysed on the whole sample (Report Supplementary Material 6). No participants progressed to wet AMD.
In a non-randomised (but with some controls) study from the same centre, Studnička and colleagues 2013180 included 19 patients with bilateral soft drusen and, in 17 patients, DPED. No GA is mentioned. Baseline BCVA was 0.74 (95% CI 0.34 to 1.0). At 2 years’ follow-up, there was a significant difference between groups in BCVA, but at 3.5 years, no significant difference was seen (see Table 7). DPED area was smaller in the haemopheresis group than the control group. Two eyes in the haemopheresis group and six eyes in the control group developed CNV.
Klingel and colleagues 2010181 included in their large retrospective cohort study from the Rheonet Registry people with dry AMD, soft drusen, pigmentary abnormalities and VA between 0.1 – 0.63. Dry AMD was not defined, but did not include people with just soft drusen. Mean BCVA was not reported. They compared results in 428 eyes of 279 patients treated with rheophoresis, with those in 85 eyes of 55 patients who were deemed suitable for rheophoresis but not treated for various reasons (poor vascular access, no reimbursement, unwilling to be treated), but no comparison of the baseline data from treated and untreated groups is given, so we cannot say whether like was compared with like. The study was more about safety and adverse effects. Significantly more eyes improved in the haemopheresis group and significantly more eyes deteriorated in the control group (see Table 7).
Combinations of rheophoresis and other interventions
Two studies182,183 used rheophoresis in combination with plasma exchange and selective adsorption.
Brunner et al. 182 reported in a case series in 1996 that a combination of plasma exchange, selective adsorption and membrane differential filtration improved VA by at least 1 line, 24 hours after treatment in 11 out of 17 patients (15 out of 31 eyes) with AMD (about half with wet AMD and results not given separately). A single ETDRS line is not usually considered clinically important.
In a longer-term case series with 20 participants with dry AMD (available only as an abstract), Widder and colleagues183 reported significant improvements in BCVA 1.9 lines at 2 years and 1.2 at 3 years but not at 4 years follow-up, after combined plasma exchange, selective adsorption and filtration.
A small trial by Kamami-Levy and colleagues189 is as yet available only as an abstract from ARVO 2014. Patients were randomised to rheopheresis (n = 10) or controls (n = 11). The control intervention, if any, is not specified. After 24 months there were no statistically significant differences in VA or atrophic area.
Adverse events
Adverse event rates were low in three studies (0%, Studnička 2013;180 2%, Koss 2009;175 5%, Blaha 2013;179) (for specific events see Report Supplementary Material 2). However, Pulido 2006174 reported that during the period of follow-up, 34% of haemopheresis participants and 28% of control participants experienced an AE, with serious AEs in 24% of treated participants. Few details are given of what these serious AEs were. No serious AEs recorded by Brunner 2000. 170 In the study by Klingel 2010,181 0.24% of people with dry AMD experienced retinal bleeding (Table 8). AEs were not reported in the Rencova177 or Swartz178 studies.
| Koss 2009175 | Rheopheresis, n = 25 (%) | Control, n = 22 (%) |
|---|---|---|
| Any AE | 2.1 | |
| AE requiring treatment | 0.8 | 4.5 (not treatment related) |
| Serious AE | 0 | |
| Pulido 2006,174 Pulido 2005176 | Rheopheresis, n = 129 (%) | Placebo, n = 69 (%) |
| AE requiring intervention during day of treatment | 24.0 | 5.8 |
| AE during follow-up (after treatment phase) | 34.4 | 27.5 |
| AE requiring intervention during follow-up (after treatment phase) | 30.3 | 27.5 |
| Blaha 2013179 | Rheohaemapheresis, n = 37 (%) | Control, n = 34 (%) |
| Any AE | 5.4 | |
| Any AE requiring intervention | 1.0 | |
| AE resulting in treatment termination | 0 | |
| Klingel 2010181 | AMD, n = 833 (%) | |
| Retinal bleeding | 0.24 |
Summary
Seven intervention studies, with unclear risk of bias in four170,174,176–178 and poor quality in two,190,191 were included. Only one study (Koss192) was thought to have a low risk of selection bias. Results were mixed, with statistically, but not really clinically, significant effects of haemopheresis on VA reported in only three studies, all of which included very small numbers of patients. The studies that did not show differences between groups in BCVA included Pulido,174 with by far the largest numbers, and Studnicka 2013180 and Blaha 2013179 with longer periods of follow-up.
Overall, the results do not provide a strong case for the value of haemopheresis on VA. The two studies from Germany by Koss et al. 192 and Brunner et al. 170 reported statistically significant differences, and Lane179 reports that the largest German medical insurance company, Deutsche Kranken-Versicherung AG, agreed to fund rheopheresis. The largest study, the MIRA trial by Pulido et al.,174 was deemed to be negative and we could not find any FDA approval for the treatment on the FDA website. One interesting feature, reported by Brunner and colleagues,170 was that the effect of rheophoresis persisted for much longer than the reduction in viscosity, implying that a relatively short period of reduced viscosity could have longer-lasting effects.
The practicality and acceptability of having older people attend for 2- to 4-hour sessions, about 10 times a year, would have to be considered.
A related technology is HELP (heparin-induced extracorporeal lipoprotein precipitation). A study, NCT01840683, was registered in 2013 to be carried out at the Canadian Centre for Advanced Eye Therapeutics, a private company in Mississauga, Ontario, Canada. Some results were published by Ali and Armogan in Retina Today (Sept/October 2008, 72–75). After eight HELP sessions, VA was reported to have improved by at least 1 line in just over half of 33 eyes of 19 patients, and by ≥ 3 lines in 15% (about 5 patients). Drusenoid macular thickness was reduced in over half the eyes. No full publications were found, but there is a conference abstract. 193
Conclusion: given that most positive studies were small, that they mostly had uncertain risks of bias, that effect sizes were modest, that the largest one was negative and that treatment would be inconvenient to older people patients, we do not see rheopheresis as a research priority.
Microcurrent stimulation
Electrical current stimulation with transcutaneous electrical nerve stimulation (TENS) is commonly used in medicine to relieve musculoskeletal pain. 194 Recently there has been interest in using minute amounts of electrical stimulation, termed MCS, in an attempt to improve or restore vision in dry AMD.
The MCS uses electrical stimulation to nerve fibres via cutaneous electrodes at a much lower current that TENS. 194 These are attached to a controller (microcurrent stimulator), which delivers a fixed current, commonly of between 50 and 500 µA, with biphasic waveform at various frequencies set by the therapist. 194 MCS was first used for evoking phosphenes (visual perceptions induced by stimuli other than light) in early visual prostheses for blind patients. 195 A minimum of 150 µA seems to be needed to elicit phosphenes in patients with AMD so that is the minimum current used.
One study described below, by Anastassiou and colleagues,196 used 30–50 µA above the minimum. Sehic and colleagues197 suggest that the optimum level may vary among retinal diseases. Clinically, MCS can be applied via various routes, namely transpalpebral, transorbital, transcorneal, subretinal, epiretinal, transchoroidal and direct stimulation of the optic nerve or brain. 197 The most common MCS delivery method currently used in AMD studies is the transpalpebral approach. 196,198,199
The mechanism of action is not understood and many suggestions have been made. Current thinking on the mechanism of action of MCS comes from studies on experimental animal models, summarised in a narrative review by Sehic et al. 197 The main mechanisms proposed are neuroprotective, increasing cellular activity, improving cellular permeability, increasing nerve conduction velocity, reducing inflammation and increasing blood flow to the retina. 194,197
Electrical stimulation may have a neuroprotective effect on retinal ganglion cells by increasing expression of brain-derived neurotrophic factor, ciliary nerve trophic factor (CNTF) and of Bcl-2 (family of proteins involved in the regulation of apoptosis) by Müller cells. 197 An anti-inflammatory action may be achieved through inhibition of microglial secretion of interleukin-1β and tumour necrosis factor α. 197 Electrical stimulation can also increase intracellular calcium influx, thus causing neuronal depolarisation and increased cyclic adenosine monophosphate levels. 197 It was also demonstrated that retinal proteins associated with cellular signalling and neuronal transmission are up regulated with MCS. 197 In a study involving 10 healthy volunteers, Kurimoto et al. 200 found that transcorneal MCS increased choroidal blood flow. Although it is not clear whether or not the investigators were masked to the treatment allocation, if this finding were to be reproduced in other studies it could offer further support to the potential beneficial effect of MCS in AMD196,198,201 as inadequate choroidal perfusion appears to play a role on the pathogenesis of this disease. 201,202 Another potential mechanism by which MCS could affect the retina is by its potential effect in the RPE, for which there is evidence from in vitro research on cells alone. 203
In brief, if it works, the mechanism(s) are not known but there are possibilities.
Quantity and quality of research
Reviews
The American Academy of Ophthalmology Taskforce194 on complementary therapy assessment published a systematic review in 2004. The review concluded that there was no strong evidence to demonstrate the effectiveness of MCS for AMD. It only included three studies: Michael and Allen 1993,204 Allen 1998205 and Wallace 1997. 206 The review did not identify any serious adverse effects on health but did comment that there might be a significant cost associated.
The Task Force advised patients considering the treatments to ask the following questions of the providers:
-
Is the treatment being provided as part of a FDA-authorised study?
-
What are the results and benefits compared with a control group?
-
What other treatment options are available and how do they compare?
-
Is lifelong treatment with MCS necessary to maintain benefits?
These seem useful questions.
Studies
Six studies including 213 participants were identified (203 treated, 10 given placebo). One study207 and one subgroup97 were in STGD207 and are reported in Chapter 2. A number of studies included eyes with other retinal conditions. Data on these were not extracted. One study was a RCT by Anastassiou et al. 2013. 196 Four studies were single-arm before-and-after studies: Shinoda 2008,199 Chaikin 2015,198 Kondrot 2002208 and Kondrot 2015. 97 One case series reported two substudies. 204,205 The studies were conducted in Japan (n = 1), Germany (n = 1) and the USA (n = 4). The duration of treatments varied between 3 days and 3 months, where reported. In two studies, participants were also given nutritional supplements. 97,204 Follow-up was between 3 days and 24 months. The RCT196 was assessed as having unclear risk of selection bias and a risk of outcome detection bias. One of the before-and-after studies199 was of fair quality. The remaining studies were of poor quality10,97,198,204,208 (see Report Supplementary Material 2).
In the RCT and prospective before-and-after studies, the sample sizes were small as each had < 30 participants. The retrospective study included 70 participants with dry AMD. 97 Mean ages ranged from 76 to 83 years. The funding sources were not reported. In one study, participants paid US$3000 to have the treatment.
Results
Anastassiou and colleagues 2013196 included 22 people with dry AMD, no neovascular disease and VA between 25 and 45 letters. Details of the stage of AMD were not given. At 6 months, there was no statistically significant difference in the change in VA, contrast sensitivity or macular sensitivity (Table 9). However, they did report an improvement in VA after 1 week, an improvement of > 10 letters in three patients at 4 weeks, and an increase in contrast sensitivity at 4 weeks. Anastassiou and colleagues 2013196 concluded that transpalpebral electrostimulation led to a temporary increase in visual function in some patients, but that further research was needed.
|
Anastassiou et al. 2013196 RCT; unclear ROB |
Microstimulation, n = 12 | Placebo, n = 10 | p-value |
|---|---|---|---|
| VA, change letters at 6 months | 4.1 | –1.0a | p = 0.3 |
| Contrast sensitivity change, number of optotypes at 6 months | 1.5 | 0a | p = 0.9 |
| Macular sensitivity, change dB at 6 months | 0.1b | –0.8a | p = 0.4 |
|
Shinoda 2008199 Prospective B + A; FQ |
Dry AMD, eyes n = 7 | ||
|
Mean (SE) ETDRS p-value change from baseline at 4 weeks |
42.9 (4.9) p = 0.0401 |
||
|
Kondrot 2002208 Prospective B + A; PQ |
Microstimulation, n = 28 | ||
| Per cent of eyes with improvement of acuity at possible 1-year follow-up | 66 | ||
| Range of improvement, lines of VA | 0 to 2.5 lines | ||
|
Chaikin 2015198 Prospective B + A; PQ |
Frequency specific MCS, dry AMD eyes = 25 | ||
| VA, logMAR, change from baseline (95% CI) at 3 months | (n = 7) –0.1 (–0.2 to –0.01)c | ||
|
Kondrot 201597 Retrospective B + A; PQ |
Dry AMD, n = 70 (140 eyes) | ||
| Acuity improvement, ETDRS chart, mean letters, at possible 3 days’ follow-up | 5.5 | ||
| Contrast improvement mean letters | 3.8 | ||
|
Michael 1993,204 Allen 1998205 Case series; PQ |
Study 1, n = 25 | Study 2, cohort 1, n = 12 | Study 2, cohort 2, n = 34 |
| Mean numbers of letter change in VA, both eyes | –0.3 | NR | NR |
| Loss of letters of VA, mean by eye | NR | 3 | NR |
| Gain of letters of VA, mean by eye | NR | NR | 8.5 |
Shinoda and colleagues 2008199 included people with wet or dry AMD (wet not reported here). The inclusion criteria for dry AMD imply that GA was present. The study was graded as fair on the NIH checklist but it was very small, containing only seven patients with dry AMD. They reported a statistically significant improvement in the number of ETDRS letters in the dry AMD group from 39.8 to 42.9 (p = 0.04) after 4 weeks (see Table 9). However, they conclude that the limitations of the study (small numbers, short follow-up and the absence of a control group) means that it cannot be used to justify the use of MCS. They recommend a larger RCT with longer follow-up.
The Kondrot 2002208 study was graded as very poor quality, achieving only one positive answer out of 12 items on the NIH checklist. Kondrot 2002208 treated people with dry AMD (no details are given) with a combination of MCS, vitamins and nutritional supplements (details of those not given), starting with a pilot of 10 patients. There was no loss of vision, change in Amsler grid or change in intraocular pressure (IOP) in the first group of 10 patients, so the study was then expanded to treat 56 eyes of 28 patients with no control group. Before treatment, the range of VA was 20/25 to 1/400. After treatment, up to 1-year follow-up, the range of VA was reported to be between 20/20 and 3/800. The proportion of participants with improvement of VA was 66% and the range of improvement was mean 0.48 lines, range 0–2.5 lines. No other outcomes were reported. Dr Kondrot suggested that a double-blind study was desirable to address the possible placebo effect.
Chaikin and colleagues 2015198 included six people with wet and 25 with dry AMD (details not provided). MCS was given by the palpebral route, for 35 minutes once a week, for an average of 4.8 sessions (range 2–10). We report only results in dry AMD. There was no control group. The study was graded poor, achieving only four out of 12 items on the checklist. At 3 months, treated eyes showed an improvement in logMAR VA (see Table 9). There was a sharp improvement in the first week, continuing in the first month, followed by a levelling off. The authors stated that the mean letter change from baseline was statistically significant (p = 0.012), although a figure in the publication suggests this was not significant (p = 0.059). There was improvement in VA (defined as a gain of ≥ 1 letter) in 52% of eyes and deterioration in 28%. The authors cautiously conclude that the short follow-up and small numbers, the training effect of repeated VA testing and the lack of masking of the VA testers mean that long-term efficacy is unproven. They encourage further studies including a control arm with sham treatment. They conclude that if further studies supported the positive results from their study, that the treatment would be an easy one to administer in offices or at home.
In a large retrospective before-and-after study, graded poor on quality assessment, of a package of alternative treatments (i.v. nutrition, oxidative therapy, MCS and ‘syntonic light therapy’) combined with a stress reduction programme and a detoxification programme, Kondrot 201597 reported a 5.5-letter improvement in VA at follow-up (likely immediately following 3 days of treatment) (see Table 9). Improvement of < 1 line was apparent in 35.7%, 1–2 lines in 37.9% and > 2 lines in 15.7%. There was also an improvement in contrast sensitivity of 3.8 letters (Report Supplementary Material 2). However, given the combination of therapies, no conclusions on MCS stimulation can be reached.
In one publication, two linked studies of MCS in people with dry AMD (no further inclusion criteria) were reported, one of which had two separate cohorts of 12 and 34 patients, all patients treated, with no controls. 204,205 In the first study there was a decrease in VA after 2–7 years’ follow-up (see Table 9). In the second study, few results were reported; there was a mean loss of 3 letters of VA in cohort 1, and a mean gain of 8.5 letters in cohort 2. Few other results were reported (see Report Supplementary Material 2). In addition to electrotherapy, a nutrition extract of taurine, rutin and bilberry was administered in the second study by Allen et al. 205 There was no control group.
Adverse events
Anastassiou et al. 196 reported that there were no adverse effects of treatment. Shinoda et al. 199 reported that there were no ocular or systemic complications except in one participant who developed contact dermatitis on eye lids, which was treated as a serious AE. It is not clear from the publication if this was in a dry AMD patient. Three studies did not report AEs. 97,198,208
A pilot study (not included above) with 17 patients from Mumbai by Natarajan et al. 209,210 reported improvements in VA after MCS treatment with the ScyFIX device (Chanhassen, MN, USA; http://www.scyfix.org/.) Natarajan et al. 210 recommend a RCT with long-term follow-up.
Summary
The American Academy of Opthamology Taskforce concluded there was no strong evidence on the effectiveness of MCS stimulation, but it preceded many of the studies listed above. The studies reported mixed results of MCS. Follow-up was generally short and sample sizes small. In the RCT there were no significant benefits of treatment. In the before-and-after studies and the case series, results were difficult to interpret owing to differences in measures, time points, small samples, study quality and a lack of statistical analysis. In addition, in the case series, participants were given long-term nutritional supplements. In the immediate to short term following treatment, there may be some improvement in VA, but most authors expressed caution and suggested RCTs. Few studies reported other outcomes.
Overall, the evidence base for MCS is weak. Despite this, we note that Nova Oculus Partners (Indian Wells, CA, USA), manufacturers of an ‘electrotherapeutic device’ to treat dry AMD (unspecified), are hoping to get International Organization for Standardization (ISO) certification worldwide. 211
A device is being marketed by ScyFIX for ‘microcurrent neuromodulation’. It has a Conformité Européene (CE) mark in Europe. The manufacturer claims that microcurrent neuromodulation has been shown to be effective. 212 In their submission to the FDA, ScyFIX cite a study by Wallace 1997206 who reported treating 43 patients with dry AMD with microcurrent (200 µA for 20 minutes for 36 sessions) after which 54% improved by 1–4 Snellen lines. 212
ScyFIX212 also cite a paper by Paul (undated) which claims a 72% success rate in AMD after microcurrent and nutritional treatment. They also cite a presentation by Halloran and Reader213 (Fourth Annual Symposium on Biologically Closed Electrical Circuits 1997) in which improvement in visual field function was reported to be 4.61 decibels, and in VA 0.98 lines. The treatment was part of a package of treatments, including some described as applied kinesiology, neurolymphatic deep stimulation, deep tissue acupressure, colour-shape identification therapy and nutritional supplements.
Current research
We have identified five trials in ClinicalTrials.gov (accessed 16 April 2017).
NCT01600300 aimed to do a feasibility trial of the Tesmac device versus sham Tesmac (Acuity Medical International, Minneapolis, MN, USA). The completion date was 2004 but no results have been posted. The Tesmac device is now owned by The Eye Machine (Indian Wells, CA, USA). 214
NCT00804102: the study details mention a range of eye diseases including dry AMD but the only publication posted was on retinitis pigmentosa. However, the research group includes Röck from Tubingen, so this may have been the Röck 2013121 study described in Chapter 2.
NCT0170958 was an observational study in AMD, STGD and retinitis pigmentosa, led by Papastergiou in the Retinal Institute in Hawaii. No results have been posted. Completion date was 2012.
NCT02699216 is listed as currently recruiting in the Du Bois Vision Clinic, PA, USA, but expected completion date was December 2016. It will assess with the Novo Oculus device (The Eye Machine).
NCT02540148 was to be another trial of the Novo Oculus device against sham treatment, but is listed as not yet recruiting. The details were provided by The Eye Machine Canada (Indian Wells, CA, USA) but have not been updated since 2015.
We are aware of proposals for a further study of MCS being planned by Oxford Bioelectronics. A leaflet in February 2017 said they would be embarking on a pilot study to establish safety and clinical acceptability of the device as a treatment for dry AMD, and that if successful, a clinical trial for regulatory approval of a bioelectronic treatment would follow.
It is not clear if the pilot will have any control patients. If it does not, it will contribute little new. However, the proposal for a full clinical trial, if that is a RCT, is welcome. No further details were on the company website (www.oxfordbioelectronics.com/amd/; accessed 31 July 2017).
Lasers
Laser photocoagulation treatment of early age-related macular degeneration
Prophylactic laser photocoagulation has been proposed to prevent progression of early AMD.
Mechanism of action
Gass215 first reported drusen regression with application of laser photocoagulation remote to the area of drusen in 1971. A review by Cukras and Fine noted several studies that suggested that the laser need not be applied directly to the drusen. 216 Subsequent smaller RCTs217–220 have showed positive results favouring laser photocoagulation in terms of drusen regression, improvement in VA and similar or fewer incidences of CNV than with the fellow untreated eyes.
In order to maintain photoreceptor health, healthy RPE and Bruch’s membrane are required to aid diffusion of nutrients from the choroidal circulation. In old age, the hydraulic conductivity between the RPE, Bruch’s membrane and the choroid decreases due to accumulation of hydrophobic debris in the RPE and Bruch’s membrane. 221 In addition, matrix metalloproteinase (MMP), an enzyme responsible for degradation of extracellular matrix in the Bruch’s membrane and thus keeping the Bruch’s membrane thin, decreases in ageing. 221 These processes seem to occur earlier in eyes with AMD.
The mechanism(s) by which laser photocoagulation may help to remove drusen deposition is still poorly understood. It has been proposed that laser irradiation rejuvenates Bruch’s membrane by leading to a transient increase of MMP-2 and MMP-9 and by stimulating RPE migration 4–7 days after laser exposure. 221,222 It has been observed that laser photocoagulation enhances clearance of debris by choroidal phagocytic cells that have been seen protruding from the choroidal endothelium extending its processes towards Bruch’s membrane. 222 These subsequently leads to a thinner Bruch’s membrane, increasing hydraulic conductivity and egress of water and debris. 221 Utilising Fourier OCT, Huang et al. 223 demonstrated reduction in perifoveal RPE elevation compared with controls after applying barely visible laser photocoagulation in a horseshoe-shaped area temporal to the fovea. In a more recent review,224 it was stated that thermal induced stress of the RPE results in production of heat shock proteins that aid in repairing damaged tissues and increased apoptotic threshold of RPE cells to thermal, inflammatory, oxidative or hypoxic injury.
Laser photocoagulation as a therapeutic modality to clear drusen and increase the hydraulic conductivity of Bruch’s membrane has been performed either at threshold (visible burns) or at subthreshold (invisible/barely visible burns) levels. One of the earliest RCTs, the Choroidal Neovascularisation Prevention Trial (CNVPT)225,226 utilised threshold argon laser photocoagulation by applying either three rows of fundoscopically visible burns in a horseshoe-shaped manner or two concentric rings with identical settings, both of which were applied no closer than 750 µm from the fovea. Although significant drusen reduction was detected, there was a significant increase in incidence of CNV. 225,226 This result was mirrored in the Drusen Laser Study,227 which also applied laser at threshold levels.
From the CNVPT, Kaiser et al. 228 showed that the increased incidence of CNV appears to be related to the laser intensity used. It was postulated that with thermal lasers, excessive heat is spread to the overlying photoreceptors after being absorbed by the RPE, causing thermal damage. 221,224 The intense heat could also rupture Bruch’s membrane and promote CNV formation. 224,228 In addition, lateral spread to adjacent RPE occurs and causes damage beyond the specified laser spot diameter, giving an enlarged scar. 224 As a result, this could potentially lead to a visually significant GA if the fovea is involved. 229
In order to minimise damage to the adjacent structures while stimulating the RPE, subsequent studies used laser at subthreshold levels by reducing the duration of laser exposure. As a result, no burns are caused. Although two studies230,231 utilising this laser modality showed an increase in incidence of CNV, a recent Cochrane review222 showed that there was no significant difference between threshold and subthreshold laser treatment in terms of CNV or GA progression.
Although most RCTs have shown significant drusen reduction in treated eyes when compared with controls, this did not improve functional outcomes for patients. In fact, Virgili et al. 222 pointed out in their Cochrane review that prophylactic laser treatment (both threshold/subthreshold) did not affect VA, contrast sensitivity and reading ability when compared with untreated eyes. However, this review did not include the newer studies using micropulse or nanosecond (2RT) lasers. It was postulated that despite utilising subthreshold laser treatment, structures adjacent to the RPE (the intended target) still suffered collateral thermal damage from heat conduction due to the use of conventional continuous wave lasers. 224 Hence, micropulse and nanosecond lasers, which allow further reduction of duration of exposure, may provide improved outcomes.
In subthreshold diode micropulse lasers, multiple micropulses of laser energy (100–300 microseconds of exposure) are delivered; the laser is only ‘on’ for microseconds and then there is an ‘off’ time that prevents the increased heat that takes place using conventional lasers. ‘On’ and ‘off’ episodes are repeated while the treatment is being applied. To apply micropulse laser spots, users typically start with a test spot in continuous wave mode to determine the power required to create a barely visible threshold burn. When this is achieved, the laser is switched to the micropulse mode to create a subthreshold burn. 224 Brader and Young224 concluded in their review that SDM laser treats the RPE selectively without neurosensory retinal damage while better preserving the electrophysiological function than conventional lasers.
Nanosecond laser is delivered in a single pulse with a pulse duration of 3 nanoseconds and a spot size of 400 µm. Again, test shots are applied to get a threshold burn before reducing the power by 20% to achieve a subthreshold treatment end point. 232 A subsequent study233 showed that nanosecond laser was very specific to the RPE, with no damage seen in the adjacent neurosensory retinal or Bruch’s membrane. At the laser sites, it was observed that adjacent RPE cells enlarged and extend to reform the disrupted RPE monolayers in both the human and animal models. From the animal model, RPE cells around the borders of laser treatment sites were observed to be undergoing active cell proliferation. In addition, the immune response around the subthreshold laser sites was milder than threshold laser, reducing the risk of PR damage. The authors also observed that nanosecond laser induced thinning of the Bruch’s membrane by remodelling the extracellular matrix through increased expression of the MMP-2 and MMP-3 genes in the RPE. These observations have further reinforced the proposed mechanisms by which laser photocoagulation induce drusen regression as outlined above.
Quantity and quality of research
Reviews
We identified one good-quality systematic review from the Cochrane Eyes and Vision Group222 and four non-systematic reviews by Brader and Young,224 Geneva,234 Cukras and Fine 2007216 and Hsu et al. 2005. 235 The Cochrane review concluded that, although drusen area can be reduced, laser treatment is not associated with improved outcomes for patients. The RCTs included in the Cochrane review had a low risk of bias. Studies of laser treatment appear to include people with drusen, before GA develops.
The other reviews came to similar conclusions, but covered different topics. The earliest by Hsu et al. 235 noted that laser treatment had sometimes been reported to increase the risk of dry AMD progressing to wet AMD. Brader and Young224 reviewed subthreshold diode laser studies and concluded that there was only low evidence of efficacy but that the treatments given varied and were mostly not micropulsed. They also noted that one study by Friberg et al. 2006230 had been stopped prematurely because of an increased risk of wet AMD. Cukras and Fine216 concluded that the benefits of laser therapy were unproven.
Most prophylactic laser studies are in two high risk groups:
-
Bilateral intermediate AMD where one eye is treated and the other is the control.
-
Unilateral wet AMD where the non-wet eye receives laser.
Studies
The Cochrane review included only RCTs. We identified seven non-RCT studies, including 439 patients undergoing laser therapy. The Huang223 and Prahs236 studies were CCTs, and the Guymer232 and Ivandic237 studies were prospective cohort studies. There was one retrospective cohort study by Luttrull et al. ,96 one before-and-after study by Merry et al. 238 and one case series with a randomised element by Figueroa et al. 239 The studies were conducted in China (n = 1), Germany (n = 2), the USA (n = 1), Australia, Canada and Spain (n = 1). Between one and nine treatments were applied across the studies, with average follow-up ranging from 1 month to 8 years. The Huang223 and Prahs236 CCTs were assessed as having a high risk of bias. Three of the other studies were assessed as poor quality. 96,237,239 In the Figueroa et al. 239 trial section, most items on the Cochrane risk of bias checklist could not be completed because of lack of detail. The Ivandic237 study had positive answers for only 5 out of the 14 items on the NIH checklist. The Guymer232 study was assessed as fair to good, getting positive answers on 9 out of the 14 NIH checklist items and three negatives. The Merry238 study were assessed as poor quality, partly on some weak criteria (unclear if all eligible patients met criteria, small sample, no blinding, unclear loss to follow-up) and partly because details were not given for several items. Funding source was not reported by five of the studies,96,223,237–239 one received non-commercial funding236 and one received both non-commercial and commercial funding232 (see Report Supplementary Material 2). The Prahs236 and Merry238 studies had small numbers of recruits receiving laser treatment. Where reported, mean age ranged from 62 to 78 years across the studies and 30–45% of participants were men.
Results
Key results from these studies can be seen in Table 10.
|
Figueroa et al. , 1997239 Case series and RCT; PQ |
Cohort 1, n = 30 Intervention, 30 eyes |
Cohort 1, n = 30 Control, 30 eyes |
Cohort 2, n = 16 32 eyes |
|---|---|---|---|
| Choroidal neovascular membrane developed, n/N (%) | 0/30 eyes |
1/30 (3.3) eyes p = 0.5 vs. intervention cohort 1 |
3/16 (18) patients |
| Improvement in Snellen VA ≥ 1 line, after subfoveal drusen disappearance | 10/30 (33.2) patients | 5/16 (31.25) patients | |
| Snellen VA, 3 years | |||
| Improved ≥ 1 line | 5/30 (16.6) eyes | 0/30 eyes | 5/16 (31.25) patients |
| No change | 10 (33.3) eyes | 15 (50) eyes | – |
| Deterioration ≥ 1 line (due to cataract progression) | 15 (50) eyes | 15 (50) eyes | – |
|
Guymer et al. , 2014232 Prospective cohort; FQ |
Laser, n = 50 eyes | No laser, n = 50 eyes | p-value |
| Mean change from baseline in BCVA, ETDRS letters | –0.1 | 0.8 | Not reported |
| Improved by ≥ 5 letters, n (%) | 8 (16) | 4 (8) | |
| Lost ≥ 5 letters, n (%) | 7 (14) | 4 (8) | |
| Reduction in drusen area (%) | 44 | 22 | |
| Increase in drusen area (%) | 24 | 18 | |
|
Ivandic et al. , 2008237 Prospective cohort; PQ |
Laser, n = 193 eyes | ||
| People without cataracts | |||
| VA (%) | |||
| Improved overall | 97.3a | ||
| By one row Snellen | 19.8 | ||
| By two rows | 37.0 | ||
| By three rows | 19.2 | ||
| By four or five rows | 8.2 | ||
| By six rows | 4.1 | ||
| By seven rows | 0.7 | ||
| Unchanged | 2.7 | ||
| People without cataracts | |||
| VA (%) | |||
| Improved overall | 94.5a | ||
| By one row Snellen | 24.7 | ||
| By two rows | 41.2 | ||
| By three rows | 13.7 | ||
| By four or five rows | 8.8 | ||
| By six rows | 3.8 | ||
| By seven rows | 1.6 | ||
| Unchanged | 0.5 | ||
| People without cataracts | 5.5 | ||
| VA (%) | |||
|
Prahs 2010236 CCT; high ROB |
Treated eyes, n = 6 | Untreated eyes, n = 6 | p-value |
| Mean atrophic area, mm2 (range) at baseline | 6.3 (1.5–14.9) | 6.4 (0.9–15.4) | NR |
| Mean atrophic area, mm2 (range) after 12 months | 9.2 (3.1–16.4) | 8.3 (1.4–16.8) | Not reported |
| Mean (SD) progression rate, mm2 per year | 3.0 (2.8) | 1.9 (1.6) | Not reported |
|
Merry 2016238 Before and after; FQ |
Photobiomodulation, n = 24, 42 eyes | ||
| Change in BCVA letter score at 3 months |
+ 5.14 p < 0.001 |
||
| Change in CS 1.5 cycles per degree (log-CS) at 3 months |
+ 0.080 p = 0.056 |
||
| Change in CS 3.0 cycles per degree (log-CS) at 3 months |
+ 0.166 p = 0.016 |
||
| Change in CS 6.0 cycles per degree (log-CS) at 3 months |
+ 0.10 p = 0.036 |
||
| Change in drusen volume (mm3) at 3 months |
–0.029 p = 0.021 |
||
Figueroa and colleagues 1997239 included two cohorts of people: (1) bilateral confluent soft drusen and pigmentary changes (eyes were randomised to treatment or no treatment) and (2) high-risk drusen in one eye and choroidal neovascular membrane in fellow eye (where the eye with dry AMD was lasered). A green argon laser was used, and 0.1 seconds and 160-µm spot sizes. Light grey-white take was considered the end point for the treatments. Drusen disappeared in 45 out of 46 patients during an average of 3.5 months and untreated drusen (located far from laser scars) disappeared in 43 out of 46 patients during an average of 8.6 months (cohort not stated). Initial improvement in VA was seen in 33% of patients in cohort 1, but this was lost after 3 years’ follow-up in half of these. After an average of 3 years’ follow-up, 17% of eyes in cohort 1 and 31% of participants in cohort 2 had at least 1-line improvement in VA, compared with none of the untreated eyes. No statistical analyses were presented.
Guymer and colleagues 2014232 included 50 people with bilateral intermediate AMD, excluding those with evidence of GA or presence of CNV. They describe their study as a pilot. Only one eye was treated. They used an ultra-low energy nanosecond laser aiming at ‘retinal rejuvenation therapy’: 12 spots around the macula, 400-µm diameter spots, 3-nanosecond pulse length, 532-nm wavelength. This was estimated to give 1000 times less radiant exposure than conventional macula thermal lasers. At 12 months’ follow-up, a treatment effect was seen in both treated and untreated eyes; BCVA improved by at least 5 letters in 16% and 8% of eyes, and drusen area was reduced in 44% of treated and 22% of untreated eyes, but p-values were not reported.
Guymer’s proposed explanation for the improvement in untreated eyes is that laser to one eye exposes the drusen to the immune system. She suggests that the exposure starts the immune system cleaning up of drusen in both eyes. 240 Another possible explanation is that laser treatment breaks up the drusen and triggers an inflammatory response.
Ivandic and colleagues 2008237 included all stages of AMD (dry and wet forms with or without cataracts) and a VA ≤ 20/20. They applied low-level laser treatment: four sessions semi-conductor laser diode, continuous emission at 780 nm, spot diameter of 3 µm. They reported a statistically significant increase in VA by study end (not defined, assume 4 weeks, no data presented; p < 0.00001) for patients undergoing laser treatment, whereas there was no change in a small control group with no treatment.
In a retrospective pilot study, Luttrull and colleagues96 recruited 108 people (158 eyes) with high-risk AMD (presence of multiple large, diffuse, or bilateral macular drusen; macular pigment disturbance; extrafoveal or subfoveal GA; and/or CNV in the fellow eye). The laser used was diode micropulse laser at subthreshold levels. Follow-up was 1 month after one course of panmacular laser treatment. Snellen VA was unchanged (data not reported, p = 0.75, includes eight participants with inherited PR degeneration) but several secondary outcomes, pattern ERG (an early indicator of retinal dysfunction) and visual function (measured using central vision analyser and automated microperimetry) improved.
Huang and colleagues 2011223 allocated one eye to laser treatment and one eye to control in 10 people with bilateral soft drusen. Treatment was with argon green laser: 514 nm, 100 spots, 200-µm spot size, 0.1-second duration, at 55 m to 100mW, to give barely visible spots. After at least 8 years’ follow-up, soft drusen in the treated eyes were reduced (although new drusen had appeared), whereas soft drusen had increased ‘significantly’ in the untreated eyes (presented in figures only). BCVA did not reduce ‘significantly’ in either eye, and at 2-year follow-up there was no difference in retinal contrast sensitivity. No CNV occurred during the study.
Prahs et al. 236 treated one eye of each of six patients with bilateral GA with laser therapy (described as ‘selective retinal therapy’) while the other eye served as control. The laser used was neodymium-doped yttrium lithium fluoride (Nd:YLF), 527 nm, 200-ns pulse duration, 30 pulses at 100 Hz. After 1 year, the mean GA area and progression rate (mm2 per year) were slightly higher in the treated eyes, but statistical analyses were not reported.
Merry and colleagues 2016238 included 42 eyes of 24 people with dry AMD, with AREDS grades 2 (21%), 3 (48%) or 4 (with GA but no CNV, 31%) and a BCVA letter score of 50 (logMAR 1.0, Snellen 20/200) or better. They used low-level laser therapy, with three wavelengths: yellow 590 nm, red 670 nm and near-infrared 790 nm. They describe their approach as ‘photobiomodulation’, thought to act by biostimulation of PRs, increasing blood flow and stimulating cellular functions. It differs from earlier forms of laser therapy that created thermal effects. NCT00940407
Three months after laser treatment in all affected eyes, there was an increase in mean BCVA letter score (5.14 letters; p < 0.001) and an increase in contrast sensitivity that was statistically significant at 3.0 and 6.0 (but not 1.5) cycles per degree (see Table 10). A reduction in drusen volume was also found (p = 0.021), but there was no difference in other outcomes. No new wet AMD or GA developed during the study.
An abstract from the RANZCO 2011 conference by Beaumont and colleagues241 reported a study of subthreshold laser photocoagulation of large drusen in AMD in 121 patients in which one eye was treated and the other acted as control. After a mean of 5.5 years’ follow-up, wet AMD had developed in 4% of treated eyes and 8% of untreated eyes. GA developed in 10% of treated eyes and 7% of control eyes. In eyes which developed neither wet AMD nor GA, drusen had resolved in 57% of treated eyes and partially resolved in 34%. In control eyes, most drusen were unchanged. However, the overall results did not reach statistical significance.
One small study by Scalinci and colleagues242 from Bologna, Milan and Rome reported that photobiomodulation with a device called mnemosline (Mnemosline, Vicenza, Italy), which provided pulsed light at 650 nm for 10 minutes twice a day, 5 days a week for 3 months, was associated with modest improvements in BCVA, pattern ERG and retinal sensitivity. Only an abstract is available and no statistical significance analysis is provided. The authors call for a larger trial.
Adverse events
Guymer and colleagues 2014232 reported dot haemorrhage in one patient. Three studies reported no AEs occurred. 96,236,237
Summary
The Cochrane review of RCTs with a low risk of bias concluded that although laser treatment can reduce drusen area, it is not associated with improved outcomes for patients. Seven additional primary studies were identified: two CCTs with a high risk of bias, two studies assessed as fair quality and three assessed as poor quality. They used different forms of laser treatment. Outcome measures and length of follow-up varied. There may be some improvement in VA but it is unclear if this is maintained. Drusen appear to be reduced. Overall, the effect of laser treatment is inconclusive based on the review and primary studies.
The results in the study by Guymer et al. 232 were intriguing. A similar phenomenon was reported by Figueroa239 – most un-lasered drusen in the treated eye, distant from laser scars, regressed.
Registered research studies
NCT01799564 has the title Micropulse Laser for Geographic Atrophy and is being carried out by Mones and colleagues in Barcelona. In 15 patients, one eye will be randomised to laser to receive subthreshold micropulse spots in healthy RPE close to areas of GA. Estimated completion date is June 2017.
NCT02725762 is the Study of Photobiomodulation to Treat Dry AMD (LIGHTSITE 1) in Toronto. A total of 30 patients will be randomised to laser therapy or sham therapy. The laser is the LumiThera LT 300 (LumiThera, Poulsbro, WA, USA). Patients will be treated three times a week for 3 weeks, repeated at 6 months.
NCT02800356 is the Subthreshold Laser Treatment for Reticular Pseudodrusen and Geographic Atrophy Secondary to AMD. This is a non-randomised pilot study by Querques and colleagues, using a 577 nm yellow Pascal synthesis laser from Topcon (Tokyo, Japan). It aims to recruit 20 patients and is due to finish at end of 2017.
NCT01790802: Laser Intervention in Early Age-Related Macular Degeneration Study (LEAD). The purpose of this RCT is to determine whether or not 2RT nanosecond laser therapy slows the progression to advanced age-related macular degeneration. It aims to recruit 240 patients with early AMD (no GA). The principal investigator is Robyn Guymer. Final data collection should be in June 2018.
NCT00000167 is a trial of low-intensity laser treatment supported by the National Eye Institute in the USA. It aimed to recruit 1052 patients, and was due to end in 2007.
NCT02569892 is a trial of subthreshold Pascal laser versus sham laser, being carried out in Stanford University and the Bascom Palmer Eye Institute, in 56 patients with large, high-risk drusen. The aim is to see whether or not laser treatment will reduce progression to GA or wet AMD. The trial is due to end in 2018.
Ozone
In this procedure, around 200 ml of the patient’s own blood is withdrawn, treated with ozone, and replaced into the bloodstream (‘autohaemotherapy’ or AHT). This is done twice a week for 9–10 weeks, and then every 10 days, for 12–24 months.
Quantity and quality of research
All the publications come from the same group in Sienna, Italy.
Reviews
Five non-systematic reviews were identified, all from the same group, and mostly reviewing their own studies in AMD. First authors were Bocci 2007,243 Bocci 2011,244 Borelli 2013,245 Bocci 2015246 and Zanardi 2016. 247 (see Report Supplementary Material 2). However, some reviews covered other uses of ozone.
Studies
One RCT, by Borrelli et al. ,248 and one CCT, by Bocci,244 were identified, with a total of 217 participants (124 allocated to ozone therapy). Those in the RCT had 12 months of treatment and follow-up, whereas participants in the CCT had around 7 weeks of treatment and follow-up was at 18 months. The risk of selection bias was high in the CCT, in which patients had their blood treated with ozone or with oxygen. In the RCT, recruits were randomised to ozone therapy or to the AREDS nutritional supplement. The investigator and patients were aware of which treatment was used. Funding source was not reported by either study. Mean age was 71 years248 (range 63–81 years). 244 Baseline VA was reported by both studies (Table 11).
|
Borrelli 2012248 RCT; low ROB |
O3-AHT, n = 70 | AREDS Control, n = 70 | p-value |
|---|---|---|---|
| LogMAR change from baseline at 12 months, mean (SD) | –0.2 (0.01) | 0.3 (0.01) | p > 0.05a |
| BCVA, change from baseline at 12 months (%) | |||
| Loss of > 2 lines | 0 | 40 | |
| Loss of > 3 lines | 0 | 38 | p < 0.05b |
| Gain of > 1 line | 25 | 0 | p < 0.05b |
|
Bocci 2011244 CCT; high ROB |
Ozonated AHT, n = 54 | Oxygenated AHT, n = 23 | p-value |
| VA logMAR, change from baseline at 18 months | 0.15c | –0.2c | NR |
| VA (%) with: | |||
| improvement (> 2 ETDRS lines) | 66.6 | 30.4 | Statistically significant (p-value NR) |
| equal (≤ 2 ETDRS lines) | 33.3 | 68.5 | |
Results
Borrelli and colleagues 2012248 included people with bilateral AMD, with > 10 large semi-soft and/or confluent drusen within 3 mm of centre of fovea in the study eye and BCVA between 20/32 and 20/125. After 12 months, no statistically significant difference in logMAR was reported between the treatment and control groups, but the proportion of people gaining 1 line of vision was reported to be 25% in the ozone group and zero in the control group (p < 0.05). It is not clear what change happened in the other 75% but it is reported that none of the ozone group lost ≥ 2 lines, so we could presume that they had no change or a loss of not more than 1 line. The control group had lost 0.3 ETDRS lines by 12 months whereas the ozone group gained 0.3 lines, giving a difference of 0.6 lines.
Participants in the study by Bocci 2011244 presented with dry AMD, most commonly with soft confluent drusen followed by GA. At 18 months’ follow-up, there was a slight improvement in VA in the treatment group and a slight deterioration in the control group, but a statistical comparison was not reported. Two-thirds of the treatment group had an improvement of > 2 ETDRS lines compared with less than one-third of the control group; this was described as statistically significant (p-value not reported).
Adverse events
Temporary face redness was experienced by 3%. 248 No AEs were reported in the study by Bocci. 244
Summary
Five non-systematic reviews, one RCT (low risk bias) and one CCT (high risk of bias) were identified. Both studies reported that higher proportions of people with ozone therapy gained letters of VA. All the publications came from the same group, so they reviewed their own studies.
There is a lack of convincing evidence for ozone therapy.
Intraocular telescopes or lenses
Quantity and quality of research
These studies were done in people with late advanced AMD, in contrast with most of the patients in the previous studies who had mainly early disease.
Reviews
One non-systematic review by Hau and colleagues249 was identified (see Report Supplementary Material 2). Hau and colleagues249 concluded that:
We believe the IMT is a viable option for people who otherwise have limited options to improve QoL [quality of life] due to end-stage AMD.
Studies
One CCT from the implantable miniature telescope (IMT)-002 group250–253 in the USA and one case series by Qureshi et al. 254 in the UK were identified, with a total of 218 participants (total 452 eyes, 235 eyes allocated to intervention). Follow-up was 24 months in the CCT and 4 months in the case series. The CCT had a high risk of selection bias, and the case series was assessed as fair quality but the sample size was small (n = 12). Both studies received commercial funding.
The Qureshi254 paper authors assessed their own device in patients with bilateral intermediate or advanced AMD with central scotomas.
Mean age was 76–77 years and about half of the CCT and one-third of the case series participants were men. VA at baseline was reported by the case series only, but both reported categories of visual impairment (more details in Report Supplementary Material 2).
A number of other studies were found but were excluded because they had very small numbers, including:
-
Agarwal 2008 with four patients255
-
Hengerer 2015 with two patients256
-
Primo 2010 (Optometry) with two patients257
-
Scharioth 2015 with eight patients (using the Scharioth device). 258
The article by Taberno et al. 2015259 was just a description of their device.
Older reviews were not used.
Results
The IMT-002 group (Hudson, 2006250 Boyer, 2015251 Hudson 2008252 and Lane 2006253) included people with bilateral, stable, central VA loss by untreatable end-stage AMD (GA, disciform scar or both); phakic with evidence of cataract in the study eye; a distance BCVA 20/80 to 20/800 (ETDRS); and at least a 5-letter improvement with an external telescope used for 3 days. The patients’ own lenses were removed (which would alone would be expected to improve vision) and replaced with the IMT-002 device (VisionCare Opthalmic Technologies, Saratoga, CA, USA). Compared with untreated fellow eyes, there was a statistically significant improvement in both near and distance mean BCVA at 12 months, with two-thirds of treated eyes gaining ≥ 3 lines of distance vision versus 12.5% of untreated eyes (p < 0.0001), and two-thirds of treated eyes gaining ≥ 3 lines of near vision versus one-third of untreated eyes (p < 0.0001) (Table 12). At 24 months, 0.6% and 7.5% of implanted and untreated eyes, respectively, had lost ≥ 3 lines of vision (p = 0.013), and all categories of gains and losses except gain of ≥ 6 lines for BCVA were statistically significant between eyes in favour of the study eye (presented in figure only). Statistically significant improvements from baseline were found on the NEIVFQ-25 and activities of daily living scale.
|
Hudson et al. 2006250 CCT, high ROB |
Implanted eye, n = 192 | Fellow eyes, n = 192 | p-value |
|---|---|---|---|
| BCVA (distance) mean lines improvement at 12 months, logMAR | 3.47 | 0.76 | p < 0.0001 |
| BCVA (near) mean lines improvement at 12 months, logMAR | 3.18 | 1.78 | p < 0.0001 |
| BCVA (distance) gain of ≥ 3 lines at 12 months (%) | 66.7 | 12.5 | p < 0.0001 |
| BCVA (near) gain of ≥ 3 lines at 12 months (%) | 67.7 | 33.3 | p < 0.0001 |
| BCVA (distance) loss of ≥ 2 lines at 12 months (%) | 2.1 | 8.9 | p = 0.005 |
| BCVA gain of ≥ 3 lines at 24 months (%) |
N = 173 59.5 |
N = 174 10.35 |
p < 0.0001 |
| BCVA loss of ≥ 3 lines at 24 months (%) |
N = 173 0.6 |
N = 174 7.5 |
p = 0.013 |
| Mean BCVA line change from baseline at 24 monthsa |
N = 173 3.2 |
N = 174 0.4 |
p < 0.0001 |
| NEIVFQ-25 (mean SD) | |||
| Baseline | 43.9 (13.3), N = 206 | ||
| Change at 12 months |
+ 6.1 (14.4), N = 192 p < 0.0001 |
||
| ADL, mean (SD) | |||
| Baseline | 41.4 (15.7), N = 206 | ||
| Change at 12 months |
+ 14.1, N = 192 p < 0.0001 |
||
|
Qureshi et al. 2015254 Case series, FQ |
Telescope n = 18 eyes | ||
| Mean CDVA (assume SE) | 0.20 (0.13) | ||
| Mean CDVA % improvement | 67 | ||
| Mean BCVA (assume SE) | 0.21 (0.11) | ||
| Mean BCVA % improvement | 50 |
Qureshi and colleagues 2015254 implanted telescopes in 18 eyes of 12 participants. Inclusion criteria were bilateral, intermediate or advanced dry AMD with central scotomata, minimal cataract or pseudophakia, Snellen BCVA < 0.25 and improvement with an external telescope. A 67% improvement in VA and a 50% improvement in corrected near VA was found after 4 months, with improvement of visual impairment classification in 11 (61%) eyes (see Table 12). One eye deteriorated from severe to profound, and six eyes were unchanged in terms of classification.
Adverse events
Adverse events are summarised in Table 13 and Report Supplementary Material 2. Hudson and colleagues250–253 stated that no retinal detachments, CNV or visually significant cases of posterior capsule opacification occurred during the 2-year follow-up. However, ocular AEs and complications occurred, including inflammatory deposits, increased IOP and corneal oedema. Qureshi and colleagues254 reported no cases of clinical corneal decompensation, signs of cystoid macular oedema or active CNV.
| Hudson et al. 2006250 | n = 206 eyes |
|---|---|
| Ocular AEs in ≥ 5% at 12 months (%) | |
| Inflammatory deposits | 21 |
| Pigment deposits | 10 |
| Guttae | 8 |
| Posterior synechiae | 6 |
| Ocular complications in > 5% at 12 months (%) | |
| Increased IOP (7 days) | 28 |
| Corneal oedema (30 days) | 7 |
| Iris prolapse | 6 |
| Corneal abrasion | 5 |
| Corneal decompensation at 12 months (%) | 1 |
| Intraoperative iris prolapse | 0.5 |
| Ocular AEs in ≥ 5% at 24 months (%) | |
| Inflammatory deposits | 25 |
| Pigment deposits | 11 |
| Guttae | 8 |
| Posterior synechiae | 7 |
| Iris transillumination (> 21 days) | 5 |
| Iritis (> 30 days) | 6 |
| Qureshi et al. 2015254 | n = 18 eyes |
| Replacement IOL | 1 |
| Raised IOP | 1 |
A good-quality cost-effectiveness analysis by Brown et al. 260 concluded that when using US costs (not necessarily applicable to other countries), the IMT was cost-effective, improving quality of life by 12.5%.
The CentraSight telescope implant261 (VisionCare Opthalmic Technologies, Saratoga, CA, USA) is now being used in Manchester Royal Eye Hospital and appears to be the one used in the IMT-200 trial.
Summary
One large CCT and one small case series were included. The CCT found improvement in VA in implanted eyes compared with untreated eyes, although some AEs occurred. Compared with baseline, improvements in quality of life were found. Improvements in VA were also found by the case series but no statistical analyses were presented. In both cases, some improvement could have been due to cataract removal.
A trial is under way in the UK [the Efficacy of the telescopic mirror implant for age-related macular degeneration: the MIRROR Trial, Efficacy and Mechanism Evaluation (EME) project 13/160/03]. 262,263
A trial of the CentraSight telescope, NCT03011554, is being run in the USA in people with advanced AMD who have had cataracts removed and replaced with an IOL. The IOL will be exchanged for the telescope lens.
Qureshi and colleagues264 have reported a case series of 244 eyes into which the Eyemax IOL had been implanted, but the patients had a mix of dry and wet AMD, and their results are not reported separately, making the study an exclusion for our purposes.
Night-time light
The rationale behind a trial by McKeague and colleagues265 is that, paradoxically, the retina is most metabolically active in darkness. So the trial will test the effect of wearing a mask that produces a dim green light, during the night. Recruits will have wet AMD in one eye and early in the other. They will wear the mask for 12 months. The protocol has been published (ISRCTN82148651). 265
Summary and conclusions
We think that there is insufficient good-quality evidence to recommend the use of acupuncture, MCS or ozone.
There is some evidence on rheopheresis (a treatment similar in some ways to renal dialysis), but the largest trial showed no benefit. Given that most positive studies were small and mostly with uncertain risks of bias, that most effect sizes were modest, that the largest trial was negative, and that treatment would be inconvenient to older people, we do not see rheopheresis as a research priority. Even if stronger evidence emerged, there might be problems implementing it into routine care in the UK, creating a capacity problem for the NHS, although that would depend on the number of sessions required per patient per year.
We think the evidence for the use of blue-light-filtering IOLs after cataract extraction is currently insufficient to justify their routine use, but further research is under way.
The evidence on laser treatment is mixed, but newer forms look promising enough for further research. However, some is under way, with a large trial, the LEAD trial from a world centre of excellence in Melbourne, so we should perhaps wait for their results. 266
Telescopes or lenses also look promising, but there are different forms. Some articles are written by their inventors, so bias may be a problem. A trial funded by the NIHR EME programme, the MIRROR RCT, is under way. 262
Research priorities
If the HTA programme were to consider research into physical treatments for dry AMD, we think that the top priorities would be independent research into implanted lenses and a trial of newer forms of laser treatment, both of which are under way.
Chapter 4 Age-related macular degeneration treatment: cell therapy
In this report, we cover only dry AMD. Techniques such as autologous transplantation of patches of RPE and macular translocation have been used mostly in wet AMD, so are not included.
Background
In AMD, the accumulation of waste products in the RPE, such as lipofuscin, is believed to lead to the dysfunction and death of RPE cells, and the development of areas of GA. One approach to treatment is to try to replace these cells with cells grown from stem cells.
Human embryonic stem cells have been used. Schwartz and colleagues267 reported that these cells could be differentiated into RPE cells > 99% purity.
Research is also under way to convert skin cells from patients back into a form of stem cells, called induced pluripotent stem cells (IPSCs), which can then be persuaded to develop into RPE cells in vitro. The IPSCs, being from the same patient, have the advantage of not causing an immune reaction, whereas the allogenic HESCs derived RPE cells require, at present, a short course of immunosuppressant drugs.
Cells can be implanted as suspensions of cells or as sheets of cells on a biodegradable scaffold.
Quantity and quality of research
Reviews
We found many reviews: > 30 in the last 3 years alone. None appeared to be true systematic reviews but given the very small number of actual primary studies, it is not difficult to be comprehensive. There appear to be rather more reviews than treated patients.
A review by Ramsden et al. 268 from London, identified three teams around the world doing cell transplants: Ocata from California, the Riken Centre in Japan in collaboration with Kobe City General Hospital (one patient at time of article, but now at least two, with five candidates enrolled), and the London Project to Cure Blindness (one patient at time of article, although with wet AMD, but another since then, also with wet AMD). 269
Ramsden et al. 268 summarise some of the problems that are being encountered, including:
-
having to deal with multiple regulatory bodies
-
the need to find ways to scale up production of cells
-
limitation of cell transplant to advanced disease until the balance of risks from cell therapy are known
-
in this advanced group, problems of assessing benefit when there is already visual loss
-
determining the optimal immunosuppression regimens.
They identify three issues when assessing success:
-
safety
-
cell survival
-
visual outcome.
Dalkara and colleagues134 regard human IPSCs as an unlimited source of cells for transplantation, and report that at least 15 trials were under way in late 2015, of which 10 are in dry AMD or in mixed groups including dry AMD.
Zarbin270 gives a good account of ‘challenges to clinical translation’, which include:
-
Cell production.
-
Cell delivery, in suspensions or sheets on scaffolds, but with many different materials being studied as potential scaffolds.
-
Cell survival and differentiation. Survival may be impaired in areas of GA because of changes in supporting structures such as Bruch’s membrane.
-
Immune reactions. Although the subretinal space is less accessible to the immune system, this privilege is not complete and immune reactions can occur. Not all patients (most of whom are elderly) can tolerate immunosuppressive drugs.
-
A theoretical risk of mutation to cancerous cells.
Studies
Two linked publications reporting two small before-and-after studies conducted in the USA were identified. 98,123 One of the studies was in STGD, reported in Chapter 2. A total of nine patients with atrophic AMD were included, and the eye with the worst vision was treated. After pars plana vitrectomy, a single subretinal injection of hESC-derived RPE with 12 weeks of immune suppression was assessed. Tacrolimus and mycophenolate were given for 6 weeks, then mycophenolate alone for another 6 weeks. Median follow-up was 22 months. The study was assessed as fair quality and funding was from both commercial and non-commercial sources. Mean age was 77 (range 70–88) years and one-third of participants were men. Baseline VA ranged from 20/200 (severe vision loss) to hand motion (near blindness). These trials were sponsored by Advanced Cell Technology, now known as Ocata Therapeutics (Santa Monica, CA, USA).
A small study by Song et al. 271 from Korea had only four male patients, two of these had dry AMD (aged 65 and 79 years) and two STGD (aged 40 and 45 years). The eye with the worst VA was treated with a subretinal injection of hESC-derived RPE. Participants were followed up at 1 year. The study was funded via commercial and non-commercial organisations and as a small case series study was assessed as having fair quality.
Results
The Schwartz study (Table 14) included people with advanced atrophic AMD with > 250 µm of GA involving the central fovea. 98,123 After 12 months, VA had improved in 3 of 7 eyes and was stable in 3 of 7 eyes. Quality of life, assessed by the NEIVFQ-25 improved for general vision, peripheral vision, near activities, distance activities and mental health. The possibility of a placebo effect on the VFQ-25 scores cannot be excluded.
|
Schwartz et al. , 2015,123 201698 Before and after, FQ |
RPE transplant (7 eyes) |
|---|---|
| VA (ETDRS) (12 months) improved by | |
| ≥ 15 letters | 3 eyes |
| 11–14 eyes | 1 eye |
| ≤ 10 letters (stable) | 3 eyes |
| Worsened, 10 letters | 0 eyes |
| NEIVFQ-25 change from baseline | |
| General vision, median |
Baseline: 40.0 12–52 weeks: 20.0 |
| Peripheral vision, median |
Baseline: 50.0 12–52 weeks: 25.0 |
| Near activities, median |
Baseline: 20.8 12–52 weeks: 25.0 |
| Distance activities, median |
Baseline: 37.5 12–52 weeks: 16.7 |
| Mental health, median |
Baseline: 37.5 12–52 weeks: 18.8 |
In the study by Song et al. 271 improvements were seen in BCVA at 12 months in all patients in the study eyes, ranging from 1 to 19 letters improvement. Fellow eyes deteriorated in the two dry AMD patients but improved in the two patients with STGD. This may be chance because of the very small sample size.
Adverse events
There were no AEs from cellular therapy (e.g. acute transplant rejection or abnormalities in retinal or choroidal circulations). 98,123 AEs from the surgical procedure included one case of endophthalmitis and one eye developing worsening cataracts requiring surgery. Adverse effects thought to be possibly related to the systemic immunosuppression were one urinary tract infection, two people with gastrointestinal symptoms and two cases of non-melanoma skin cancer. Without a control group, it is not possible to say whether or not these were due to the immunosuppression.
No patients had ocular or systemic serious AEs in the study by Song et al. 271
An update of NCT01344993 was presented at the 2017 meeting of the American Academy of Ophthalmology by Gregori and colleagues, with mean follow-up of 3 years in 10 patients, with reassuring safety data and gains in VA > 10 letters in some patients.
At the same meeting, Banin and colleagues from Jerusalem presented data from a Phase1/2a study (NCT02286089) of hESC-derived RPE cells (OpRegen, Cell Cure Neurosciences, Jerusalem, Israel) in patients with GA. After up to 15 months of follow-up, there were no serious adverse effects and BCVA remained stable. 272
Summary
Three very small studies were identified. Two were assessed as fair quality. The other272 was available only as an abstract. Improvements in VA were found in over half of treated eyes, sustained for at least 1 year. Improvements in quality of life were also noted in one study, although AEs occurred. The evidence base is still very sparse, but this seems a promising development. Given the very small number of studies and the lack of RCTs this remains a treatment that should be performed under the context of research.
Case selection would be essential because if PRs have degenerated, adding just RPE will not be beneficial. However, if the technique is rapid, low cost and safe, then potentially it could be done at an early stage, when RPE atrophy is just started (as detected by say AF) in order to prevent progression.
Stem cells grow well in the laboratory and are thought to be capable of providing an unlimited supply of cells for transplantation. Progress is slow, but many more trials are starting. If these are successful, the problem may be how to increase production and implantation for the very large number of people who may be able to benefit. The costs and cost-effectiveness also need to be considered.
NT-501
The NT-051 implant (Neurotech, Cumberland, RI, USA) contains genetically modified human cells that secrete CNTF. The cells are contained in a semi-permeable capsule that allows CNTF to diffuse out, but presumably protects against an immune response. The capsule can be extracted. 273
Quantity and quality of research
Studies
Zhang et al. 273 carried out a Phase II three-arm pilot RCT at eight sites in the USA with 51 participants (NCT00277134). The arms were sham surgery (not explained), low-dose implants and high-dose implants. The low dose was predicted to have little effect – almost a placebo – and for some analyses the sham and low-dose groups were combined. The aim was to assess safety, evaluate the effects of CNTF in GA, and to determine the dose and end points for future studies. Treatment and follow-up were of 12 months’ duration. A high dose (n = 27) and a low dose of NT-501 (n = 12) were assessed. A sham arm (n = 12) was also included. The trial had an unclear risk of selection bias and received some commercial funding. Mean age was 75–78 years across groups and 37–58% were men. Baseline VA was reported as mean 53.5 (SD 9.0), 49.9 (SD 10.2) and 55.3 (SD 7.3) across the three groups, respectively.
Results
In people with BCVA 20/50 to 20/200 and category 3 or 4 AMD GA, Zhang and colleagues273 (Table 15) found a higher proportion of patients with stable VA after the implant, but this was not statistically significant. A subgroup analysis in patients with baseline BCVA 20/63 or better did show a statistically significant difference with none of the 10 in the high-dose group losing > 15 letters, compared with five out of nine in a combined sham and low-dose group. There was no significant difference in area of GA, but numbers were small and duration short. Retinal thickness increased in the high-dose group. Other outcomes can be seen in Report Supplementary Material 3.
|
Zhang et al. , 2011273 RCT; unclear ROB |
High dose NT-501, n = 27 | Low dose NT-501, n = 12 | Sham 2, n = 12 | p-value |
|---|---|---|---|---|
| VA stabilisation, % losing < 3 lines (15 letters) of VA | 96.3 | 83a | 75 | 0.078 high vs sham |
| Change in area of GA, mm2, mean (SD) | 2.03 (1.04) | 2.19 (1.87) | 2.42 (1.95) | 0.788 |
Adverse events
Adverse events were few and none were higher in the treatment groups.
Summary
This small RCT with an unclear risk of bias found no statistically significant overall differences in VA stabilisation or area of GA between people treated with NT-501 and those not treated. However, it was a small proof of concept trial. No further trials of NT-501 are registered with ClinicalTrials.gov (accessed 18 June 2017).
Current research
There are two main strands of cell therapy research: hESCs from donors and using the patient’s own cells (autologous cells) using IPSCs. Research on the latter is under way in Newcastle. Producing RPE cells from iSPCs used to take many months but this is changing.
Research in collaboration with Ocata Therapeutics (formerly Advanced Cell Technology) has been under way at Moorfields since 2012, as part of the London Project to Cure Blindness. Ten patients will take part in a trial of stem cell treatment and the first two patients have had the procedure. 274,275
NCT01632527 was a small Phase I/II study aiming to recruit 15 people with GA, who would have injections of human central nervous system stem cells into their eyes. The primary purpose was to establish safety. Clinical sites were in California, New York and Texas, USA. The sponsor was StemCells Inc. (Microbot Medical, Hingham, MA, USA). It was due to end June 2015 but no results have yet been posted. However, a long-term safety follow-up study, NCT02137915, from the same sponsor has been terminated, with a comment that termination was a business decision not due to any safety concern. Another stem cells trial, NCT02467634, has been terminated.
NCT03046407 is being carried out in Henan, China, in the First Affiliated Hospital of Zhengzhou University, sponsored by the Chinese Academy of Sciences. The plan is to recruit 10 patients with dry AMD and inject hESCs into the subretinal space. The main aim is to establish safety and tolerance. The study is due to end in December 2020.
The Chinese Academy of Sciences is also sponsoring NCT02755428, subretinal transplantation of RPE, in 10 patients in Beijing, which is due to end 2020.
NCT02749734 is called Clinical Study of Subretinal Transplantation of Human Embryo Stem Cell Derived Retinal Pigment Epithelium in Treatment of Macular Degeneration Diseases. It is being carried out in Southwest Hospital, Chongqing, China. It aims to recruit 15 patients with either dry AMD or STGD.
NCT02755248 is called Subretinal Transplantation of Retinal Pigment Epitheliums in Treatment of Age-related Macular Degeneration Diseases. It is being carried out in Beijing Tongren Hospital, sponsored by the Chinese Academy of Sciences. Recruitment target is 10. The primary aim is safety and tolerance over 1 year, and completion date is December 2020.
NCT02590692 is called A Phase I/IIa Safety Study of Subretinal Implantation of CPCB-RPE1 (Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Seeded on a Polymeric Substrate) in Subjects with Advanced, Dry Age-related Macular Degeneration. It aims to recruit 20 people in California, in two cohorts of 10. The first will have advanced GA with BCVA 20/200 or worse. If safety is shown, a second cohort with BCVA 20/80 to 20/400 will be added. The study is sponsored by Regenerative Patch Technologies LLC (Glendale, CA, USA), and completion date is 2022.
NCT02286089 is called Safety and Efficacy Study of OpRegen for Treatment of Advanced Dry-Form Age-Related Macular Degeneration. It is a Phase I/IIa study of dose escalation, safety and efficacy, being run in Israel in stages, first recruiting nine legally blind people then, if safety is shown, recruiting another six people with BCVA 20/100 or less. This is due to end in 2018. The manufacturer is BioTime, Alameda, CA, USA.
Janssen Pharmaceutical have a product derived from adult umbilical tissue cells called palucorcel (formerly CNTO 2476), which is injected subretinally. It was assessed from safety and for dose decision in NCT01226628, with gains of ≥ 10 letters in 35% and of ≥ 15 in 24% reported by Ho et al. 276 However, complications of surgery including retinal perforations (6 out of 35) and detachments (13 out of 35) were of concern and Ho et al. 276 suggest that a different surgical approach will be needed in further studies. A large multicentre trial in the USA (13 centres) and Canada (two centres) – NCT02659086 – will aim to recruit 285 people, followed up for 5 years. There will be a sham arm. The trial is called PRELUDE; it is not clear how this abbreviation was derived.
Chapter 5 Drug treatment in dry age-related macular degeneration
Statins
As noted earlier, AMD and cardiovascular diseases, such as stroke and heart disease, share some risk factors. Given the success of statins in reducing heart disease, and the presence of lipids in drusen, there was therefore interest in whether they could prevent, reverse or delay progression of dry AMD.
The case for statins in AMD could be based on several types of evidence:
-
pathophysiological – hypotheses based on changes in the retina in AMD, such as lipid-containing drusen
-
trials of statins in AMD
-
epidemiological studies of the risk of AMD in people using statins for cardiovascular disease
-
basic science studies, such as studies of the effect of statins on retinal cells in the laboratory.
The second group would be the most useful, but as reported below, there are few data, so we have examined a wider range of evidence.
The epidemiological studies compare people taking statins with those not taking them, but as allocation is not random, there are likely to be problems with other factors being different.
Guymer and colleagues277 reviewed the evidence for the effect of statins in 2005. They noted that there could be several possible mechanisms, including lipid-lowering, anti-inflammatory and anti-angiogenic effects. One problem with the literature was that some of the large epidemiological studies of AMD had started before statin use became common. Guymer and colleagues278 also reviewed the evidence on dietary factors and noted that high cholesterol and high dietary fat were associated with an increased risk of AMD, whereas intakes of omega 3 fatty acids and fish were associated with a lower risk.
Gehlbach and colleagues278 suggested four mechanisms by which statins could affect AMD:
-
serum-lipid lowering, which might reduce deposition of lipids in Bruch’s membrane
-
preserving vascular supply
-
an anti-inflammatory effect
-
an antioxidant effect.
Quantity and quality of research
Reviews of effectiveness of statins
Two systematic reviews by Gehlbach et al. 278 and Ma et al.,279 one non-systematic ‘mini-review’ (authors’ term) by Peponis et al. 280 and one narrative review based on systematic searches (Tsao and Fong281) of the effectiveness of statins were identified. One of the systematic reviews278 was rated as of good quality and the other of fair quality. 279
The Cochrane review278 was last updated in March 2016. It included only two trials. One, Martini and colleagues,282 used a very low dose of simvastatin (20 mg) for only 3 months in 15 people and was graded as having unclear risk of bias. The other trial was a good-quality trial from Guymer and colleagues283 in Melbourne, which is described in detail below.
Gelbach et al. 278 concluded that evidence from currently available RCTs is insufficient to conclude that statins have a role in preventing or delaying the onset or progression of AMD.
The systematic review by Ma and colleagues279 was of observational studies, seven cohort, five case–control and two cross-sectional. The seven cohort studies mostly reported that statins were protective in early AMD, but none had statistically significant effects. However, once pooled, the RR was 0.83 (95% CI 0.66 to 0.99; I2 13.5%). Statin use was associated with a reduced risk of soft indistinct drusen (RR 0.55, 95% CI 0.20 to 0.81) and a slightly reduced risk of large drusen (RR 0.84, 95% CI 0.68 to 0.99). In all AMD there was no significant effect overall but with such high heterogeneity (I2 91.7%) that a meta-analysis might be thought inappropriate. The RRs in the studies ranged from 0.30 (95% CI 0.21 to 0.44) in McGwin 2003284 to 1.30 (95% CI 1.17 to 1.44) in Etiman 2008. 285 Wet AMD was slightly reduced (RR 0.90, 95% CI 0.80 to 0.99) but there was no effect on GA (RR 1.16, 95% CI 0.77 to 1.56). No analysis by individual statin or dose was provided.
Ma and colleagues279 noted an age difference, with statins not protective in people > 65 years, but with a RR of 0.75 (95% CI 0.53 to 0.98) in the those < 65 years. However, there were only four studies reporting results in those < 65 years.
Ma and colleagues279 concluded that statin use was protective for early and exudative AMD, but that additional large studies are required to determine the potential effect of statins on AMD prevention. They suggest that the effects of statins may vary by stage, with lipid-lowering in early AMD, perhaps reducing the development of drusen, and an anti-inflammatory effect in late AMD.
Peponis and colleagues280 carried out systematic searches for a narrative review and found 23 studies. They stated that no conclusion could safely be reached on whether statins protected against AMD. They did not analyse by which statin or dose.
Tsao and Fong281 also carried out systematic searches for a narrative review of observational studies of the role of statins in reducing the risk of AMD. They note some evidence of benefit from cross-sectional and case–control studies, for example in reducing the risk of developing drusen, but because of weaknesses in study design, their overall conclusion is that there was insufficient evidence that statins could prevent AMD.
Primary studies
Two intervention studies were included. One was a RCT by Guymer and colleagues283 and the other was a before-and-after study by Vavvas and colleagues. 286 We excluded the Martini study282 because of dose and duration, as noted above.
The remaining studies below were observational in nature, identifying people taking statins and compared results with those who were not, including some narrative reviews of observational studies.
Results
Intervention studies
The RCT [Age-Related Maculopathy Statin Study (ARMSS)] by Guymer and colleagues283 included 114 people with intermediate AMD at high risk of progression, VA at least 20/60 in one eye and either high-risk drusen in both eyes, or late AMD (CNV, central GA) in one eye and any drusen or pigment change in the study eye. Randomisation was to 40 mg of simvastatin or placebo for 3 years. It was assessed as being at low risk of bias. However, it did have problems with 24% of recruits failing to attend the 3-year follow-up visit (recorded by Gehlbach et al. 278 based on communication with the authors), and 40 mg of simvastatin would not be regarded as a potent dose, compared with, for example, atorvastatin 80 mg daily.
Late AMD was seen in 42% at baseline. Recruits had to have normal plasma lipid levels (defined as not meeting National Heart Foundation of Australia criteria for treatment). Baseline levels were total cholesterol 5.71 and 5.63 mmol/l in placebo and statin groups, respectively. LDL-C levels were 3.34 and 3.27 mmol/l and HDL-C levels were 1.86 and 1.78 mmol/l. The main outcome measure was progression of AMD, assessed by four masked observers, independently, using a six-level severity scale. VA was not reported at the end of study but was reported to show no statistically significant differences in the 12-month preliminary results. At 3 years’ follow-up (after 3-years of treatment with 40 mg simvastatin daily or placebo) 70% of participants in the placebo group had had progression compared with 54% of the simvastatin group. The OR was not statistically significant in univariate analysis (OR 0.51, 95% CI 0.23 to 1.09; p = 0.08) but was in multivariate analyses adjusting for baseline age, sex, smoking, and unilateral advanced AMD status (OR 0.43, 95% CI 0.18, 0.99; p = 0.047). The effect of simvastatin was greater in the intermediate group with non-advanced AMD, in which 49% of the placebo group and 32% of the statin group progressed: OR 0.23 (95% CI 0.07 to 0.75), with no benefit in the advanced groups, in which 21% progressed in each arm. The effect varied by genotype, with a much greater protective effect in people with the CFH genotype CC(Y402H). Guymer and colleagues283 describe the study as a ‘proof of concept’ one.
Simvastatin 40 mg daily is not a very potent dose. In a small before-and-after study, Vavvas and colleagues286 in Boston, USA, and Heraklion, Crete, treated 24 patients with intermediate AMD (many large drusen and DPEDs but no CNV or GA in either eye) with high-dose (80 mg daily) atorvastatin. Baseline BCVA was 77.6 and the authors found that 10 (43.5%) participants had significant regression of drusen after an average of 1.5 years’ follow-up. Near complete resolution of drusen was reported in 34.8%. The responders gained 3.3 letters in VA whereas the non-responders lost 2.3 letters (not quite statistically significant).
In a small pilot study, reported only as an abstract, Tzotzas and colleagues287 treated seven women with simvastatin 20–40 mg for 12 months, and reported a reduction in drusen score (based on number, size and area covered).
Observational studies
Observational studies are always susceptible to confounding variables. If AMD is associated with cardiovascular disease, then it is likely to appear associated with statin use, because people with cardiovascular disease are likely to be on statins.
In one of the earlier studies from the UK (Sheffield), in people 66–75 years, Hall and colleagues288 reported an OR of AMD in statin users of 0.14 compared with non-users, but the confidence interval was wide (95% CI 0.02 to 0.83). They provide a useful example of possible confounding, in that AMD was strongly associated with coronary revascularisation, which was in turn associated with statin use. However, after logistic regression adjusting for various factors including revascularisation, use of statins remained protective.
A case–control study, from the Atherosclerosis Risk in Communities study284 also found an association between statins and a reduced risk of AMD, but the OR was only modestly reduced (0.79, 95% CI 0.63 to 0.99) and statistical significance was only reached after adjusting for age, gender and race. No data are provided on which statin or dose.
Conversely, the Beaver Dam study289 found no association between statin use and the incidence or progression of AMD. Again, no data on statin or dose are provided.
Tan and colleagues from the Blue Mountain study290 reported that statin use was associated with a reduced risk of indistinct soft drusen – HR 0.33, 95% CI 0.13 to 0.84. Interestingly, they also show that statin use was ineffective in reducing total cholesterol, because the mean in those prescribed statins was 5.69 mmol/l. This was statistically significantly lower than in people not on statins (mean 6.00 mmol/l) but not clinically significantly so.
In a prospective cohort study, Al-Holou and colleagues291 assessed the risk of progression to late AMD in those taking part in a RCT of nutritional supplements (AREDS2) who reported taking any statin. A total of 44% of AREDS 2 patients were statin users. Details of which and dose were not provided. Participants had bilateral large drusen or unilateral late AMD in one eye and large drusen in the fellow eye. Al-Holou and colleagues291 used propensity scoring to create two subgroups of the AREDS 2 patients who differed, as far as was known, only in statin use.
After a median follow-up of 5 years, 43.5% of participants progressed to late AMD (any). This was not reduced among statin users with HRs of 1.08 (95% CI 0.83 to 1.41; p = 0.56) before adjusting for competing risk of death and 0.94 (95% CI 0.72 to 1.22) after adjustment (Table 16). Similarly, there were no statistically significant associations between statins and progression to GA, nAMD or central GA. Unfortunately, the AREDS group did not have data on which statins were used or the doses.
|
Guymer et al. , 2008292 2013283 RCT; low ROB |
Simvastin, N = 57 | Placebo, N = 57 |
|---|---|---|
| Total progression of AMD from baseline, by person, n (%) at 3 years | 31/57 (54) | 40/57 (70) |
| Progressed to advanced AMD, n (%) | 12 (21.1) | 12 (21.1) |
| Progressed, but not to advanced AMD, n (%) | 18 (31.6) | 28 (49.1) |
|
Vavvas et al. , 2016286 B + A study; PQ |
Atorvastatin, N = 23 | |
| Significant regression of drusen, n (%) | 10 (43.5) | |
| Near complete regression of drusen, n (%) | 8 (34.8) | |
| VA, mean (SDa) | 77.7 (8.4) | |
Al-Holou and colleagues291 also reviewed 14 previous studies. Nine reported that statins were protective, but only four of these had an upper CI that did not overlap with no difference. Two reported ORs of 1.0. Three studies showed an increase in progression of AMD associated with statin use but all overlapped with no difference. None showed a statistically significant increase in AMD progression on statins.
Maguire and colleagues293 examined the effect of statins in a cohort study nested in the CAPT laser trial. CAPT included people with at least 10 drusen, no evidence of CNV and a VA at least 20/40. Statins were used by 40% of participants, but most started statins after entry to CAPT. After 5–6 years’ follow-up, statin use had no significant overall effect on progression to advanced AMD (see Table 16). There was an increase in the risk of CNV (RR 1.35, 95% CI 0.99 to 1.83) and a decrease in the risk of GA (0.80, 95% CI 0.46 to 1.39), but neither was statistically significant. No data provided on which statin or dose. Maguire and colleagues293 did find a strong association with hypertension.
It may be worth noting that statins belong to two subgroups – hydrophilic (e.g. pravastatin) and lipophilic (e.g. simvastatin).
Barbosa and colleagues294 used data from the cross-sectional National Health and Nutrition Examination Survey (NHANES) 2005–8 survey, from people with complete ophthalmological examinations with retinal photographs. Only 22% of the population used a statin, but of those diagnosed with AMD, 28.6% were taking a statin. They found a statistically significantly lower rate of AMD diagnosis in those aged ≥ 68 years who were long-term users of statins than in those not using statins – unadjusted OR 0.64, 95% CI 0.49 to 0.84 (see Table 16). However, in the age range 40–67 years, there was no reduction, but an increase for early AMD (OR 1.61, 95% CI 0.85 to 3.03). For any AMD, the unadjusted OR was 2.15 (95% CI 1.26 to 3.66). They provide no details of which statins were used.
The overall association between statin use and risk of any AMD diagnosis was only statistically significant in analyses unadjusted for age, gender, demographic characteristics, health-related behaviours, comorbidities, and general health condition. However, the risk reduction in those aged ≥ 68 years remained statistically significant, although a confounding variable not included in the adjusted analysis was duration of statin use, which was longer (61 vs. 44 months) in those > 68 years.
In a case–control study, McGwin and colleagues295 included men aged > 50 years who had at least one visit to a Veterans Affairs Medical Center in Alabama during the study period, and were newly diagnosed with AMD. Ten matched controls without a diagnosis of AMD were selected for each case. The study reported prescriptions for any statin use, but no details on which or doses. Men with AMD were statistically significantly less likely to have filled a statin prescription prior to the study start (see Table 16): OR 0.30 (95% CI 0.21 to 0.45). Similar associations were seen for current or past statin used, and according to various data cuts of duration of use. ORs for other outcomes were reported without the proportions by cases and controls, see Report Supplementary Material 4 for details.
VanderBeek and colleagues296 included 486,124 people aged ≥ 60 years who had been enrolled for at least two years with a national insurance claims database, and who had had at least one visit to an eye care provider. Statins were used by 46% but details of which and doses were not provided. The development of dry AMD [identified by the recording of ICD (International Classification of Diseases)-9 code 362–50, 362.51 or 362–57] was observed in 4.3% of eligible participants, 52.9% of whom used statins. There was no association between using statins and the development of dry AMD. Wet AMD developed in 7% of participants, of which 57.5% used statins. Those on statins for longer than 12 months had a higher risk of progressing to wet AMD than those on statins for shorter periods (see Table 16). Progression from dry AMD to wet AMD (defined by recording of ICD-9 code 362.52) was seen in 3.8% of people with dry AMD at baseline, of which 55% used statins. The risk was highest in people who used statins for longer. Use of statins for 19–24 months was associated with 1.6 times the risk than use of under 6 months. All analyses were controlled for various characteristics, including age, sex, race, region of the country (see Report Supplementary Material 4 for more details). However, there were associations between risks, statin use and lipid levels. Looking only at those on statins for > 1 year, the risks of progression from dry to wet AMD were 0.54 (95% CI 0.40 to 0.73) in those with normalised LDL, but 2.1 (95% CI 1.29 to 3.36) in those with high LDL. Therefore, statins appeared protective only if LDL was reduced to normal. Curiously, a normal HDL was associated with a higher risk of progression (HR 1.96, 95% CI 1.58 to 2.42) than lower HDLs. Normal HDL is usually regarded as good to have.
Kaiserman and colleagues297 studied people aged > 50 years who were members of a health maintenance organisation and who had received photodynamic therapy for nAMD, and used statins for 2 years. The proportion who had AMD was higher in those who had used statins (0.27%) than had not used statins (0.16%). The RR (unadjusted) was 1.66 (95% CI 1.29 to 2.19) but this was not statistically significant (p = 0.07) after adjustment for age, gender, socioeconomic status, place of residence, hypertension, hyperlipidemia, place of birth, ischaemic heart disease (IHD), diabetes and congestive heart failure. They also carried out a case–control study (reported in the same publication) with five matched controls for each AMD case. The proportions using statins were similar in those with (37.7%) and those without (37.6%) AMD (see Table 16). The OR was 1 (95% CI 0.8 to 1.3). Neither study showed any association between statin use and the risk of nAMD. Kaiserman and her colleagues therefore concluded that statins did not reduce the risk of wet AMD.
Fong and colleagues298 included all people from the Kaiser Permanente health plan in Southern California who had had an eye examination in the preceding year (86,635 people). They compared 719 people with a new diagnosis of exudative AMD with 78,650 people who did not have a diagnosis of AMD. As seen in Table 16, the study found no association with wet AMD and statin use (all statins combined), with an OR of 0.89 (95% CI 0.77 to 1.03). This was also the case in analyses of a subset of participants with longer term use of statins (OR 0.89, 95% CI 0.77 to 1.04; p = 0.14) and in those just using statins in the year of study (OR 0.83, 95% CI 0.59, 1.14; p = 0.64).
In a case–control study, Etminan and colleagues285 included people aged > 65 years in Quebec who had received a coronary revascularisation procedure, either angioplasty or bypass. Cases were those (2867) with a diagnosis of wet AMD (based on a recording of ICD-9 codes 362.5 and 362.52) and for each case four controls were chosen randomly from the cohort and matched by age. They reported a small increased risk of AMD in current users of statins compared with controls (RR 1.30, 95% CI 1.17 to 1.44; p-value not reported; see Table 16). Use of statins in the past year was similarly associated. The analyses were adjusted for gender, age, comorbidity, prior history of diabetic medications, myocardial infarction, stroke, IHD and congestive heart disease.
Lipoproteins and age-related macular degeneration
High-density lipoprotein cholesterol is usually regarded as ‘good cholesterol’ because of an association with reduced cardiovascular risk. The picture in AMD is mixed. Dashti and colleagues299 identified 20 studies of AMD risk and HDL cholesterol: seven reported higher risk with higher HDL, four showed reduced risk, eight showed no effect and one showed a mixed pattern.
In a more recent review, Wang and colleagues300 included 19 studies. They carried out meta-analysis and concluded that higher HDL cholesterol was associated with an increased risk of early AMD, but not of wet AMD or GA. High LDL-C was associated with a lower risk, but only just (RR 0.93, 95% CI 0.88 to 0.99).
In a case–control study with 82 patients with AMD (early or late, with late including both wet AMD or GA), Colak and colleagues,301 from Belgrade, found no association between HDL cholesterol and AMD, but did find higher total and LDL cholesterols in the AMD group (total 6.25 mmol/l vs. 5.57 mmol/l, p < 0.001; LDL 3.99 mmol/l vs. 3.60 mmol/l, p = 0.018). However, when they examined the subfractions of HDL cholesterol, they found differences in the HLD3 subfraction in men but not in women. The HLD3 sub fraction is not regarded as ‘good HDL’.
Therefore, looking just at HDL and LDL may be too broad in AMD, and we may need to explore gender differences in risks. To complicate things further, it has been suggested that the lipid deposition in Bruch’s membrane and drusen may not all be derived from plasma sources such as LDL cholesterol – some may be produced in the RPE itself. 302
Problems with statin studies
An almost universal problem with the observational studies is that they record use of any statin, but do not analyse by individual statin or dose, thereby lumping low-potency and high-potency statins together. The older studies probably reflect the use of older, less potent statins, rather than the more recent high-potency ones, such as atorvastatin 80 mg or rosuvastatin. There are other problems. One is that ‘use’ may mean prescription, not consumption. Another is that statin use may only have an effect if lipid levels are reduced and we do not know how great a reduction is required to affect AMD, if there is an effect.
One study by Shalev et al. 303 did subdivide statins into:
-
low efficacy – fluvastatin 40 mg or less, pravastatin 40 mg or less, simvastatin 10 mg or less, cerivastatin 0.2 mg, lovastatin 40 mg or less
-
moderate efficacy – fluvastatin 80 mg, rosuvastatin < 10 mg, simvastatin 20 or 40 mg, atorvastatin 10 mg
-
high efficacy – atorvastatin 20 mg or more, simvastatin 80 mg, lovostatin 80 mg, rosuvastatin > 10 mg, pravastatin 80 mg.
These bands were based on LDL lowering. Note that high-dose statins are now regarded as doses such as atorvastatin 80 mg daily.
Shalev et al. 303 noted that only about one-fifth of patients took statins on ≥ 90% of days. Only 19% were prescribed a high-efficacy dose.
One problem with some older negative studies is that they may not have enough patients on statins. Smeeth and colleagues304 used UK data from General Practice Research Database from the years 1987 to 2002, but only 2.1% of people on the database had ever been prescribed a statin. In a later study305 using The Health Improvement Network (THIN) data, 1996–2006, they found much larger numbers on statins and were able to match 129,288 statins users with > 600,000 controls who had not been prescribed statins. They also provide data on which statins were used, but not dosages. Simvastatin was used by 39% of people on the database, atorvastatin by 21% patients and more than one statin by 33% patients. They reported that statin use was associated, after adjustment, with HR of 1.17 (95% CI 1.00 to 1.38) for any AMD (as recorded on the THIN database). 302 Another problem is that people treated with statins are at high risk of cardiovascular disease and may have other confounding factors that cannot be entirely adjusted for.
Adverse events
Adverse events were recorded in the two intervention studies (Table 17). Simvastatin was well tolerated in the RCT by Guymer and colleagues283 with major illnesses reported in seven participants in the statin group and 15 in the control group. Muscle aches were reported by two recruits on placebo and five on simvastatin (see Report Supplementary Material 4 for full details.) In the before-and-after study by Vavvas and colleagues,286 three participants withdrew from study due to AEs.
|
Al-Holou et al. , 2015291 Prospective cohort; FQ |
All, N = 3791 | HR (95% CI); p-value | |
|---|---|---|---|
| Progression to late AMD (any) | 1650 (43.5%) |
a1.08 (0.83 to 1.41); p = 0.56 b0.94 (0.72 to 1.22) |
|
| Progression to GA (any) | 869 (22.9%) |
a1.21 (0.85 to 1.73) b1.06 (0.74 to 1.51) |
|
| Progression to nAMD | 998 (26.3%) |
a1.24 (0.89 to 1.73) b1.07 (0.80 to 1.50) |
|
| Progression to central GA | 479 (12.6%) |
a1.08 (0.67 to 1.74) b0.92 (0.57 to 1.48) |
|
|
Maguire et al. , 2009293 Cross-sectional; FQ |
All patients, N = 744 | Adjusted risk ratios (95% CI) associated with statin usec | |
| End-point GA, n/N (%) | 80/743d (10.8) | 0.75 (0.43 to 1.30) | |
| States that analyses are adjusted for age, per cent of retinal area covered by drusen, level of focal hyperpigmentation, and RPE depigmentation. Also reports unadjusted risk ratios (not data extracted) | |||
| CNV, n/N (%) | 176/744 (23.7) | 1.32 (0.95 to 1.84) | |
| Analyses adjusted for age, cigarette smoking status, hypertension, and level of focal hyperpigmentation. Also reports unadjusted risk ratios (not data extracted) | |||
| Advanced AMD, n/N (%) | 242/744 (32.5) | 1.19 (0.89 to 1.60) | |
| Analyses adjusted for risk factors for either CNV or GA. Also reports unadjusted risk ratios (not data extracted) | |||
|
Barbosa et al. , 2014294 Cross-sectional; FQ |
Statin use, N = 1231 | No statin use, N = 4374 | p-value |
| AMD diagnosis | 9.9 | 5.8 | p = 0.0003 |
| Statin users with AMD, n = 126, OR (95% CI) | p-value | ||
| Risk of Any AMD diagnosis | 1.77 (1.32 to 2.38) | Unadjusted | p < 0.0001 |
| Risk of early AMD | 0.95 (0.67 to 1.33) | Adjustede | p = 0.745 |
| Risk of late AMD | 0.78 (0.34 to 1.80) | p = 0.556 | |
|
McGwin et al. , 2015295 Case–control study; FQ |
Cases of ARM, N = 550 | Controls, N = 5500 | OR (95% CI)f |
| Proportion of patients with a statin prescription filled before the index date (%) | 6.7 | 13.6 | 0.30 (0.21 to 0.45) |
| Current statin use (%) | 4.4 | 8.0 | 0.34 (0.21 to 0.53) |
| Past statin use (%) | 2.4 | 5.6 | 0.26 (0.14 to 0.47) |
| Duration of use (%) | |||
| < 12 months | 2.0 | 4.3 | 0.32 (0.20 to 0.52) |
| 12–23 months | 2.0 | 2.9 | 0.29 (0.12 to 0.67) |
| > 23 months | 2.7 | 6.3 | 0.29 (0.15 to 0.56) |
|
VanderBeek et al. , 2013296 Case–control; FQ |
|||
| Statin use | |||
| 0–6 months | 1.0 (reference) | ||
| 7–12 months | 0.99 (95% CI 0.69 to 1.41); p = 0.952 | ||
| 13–18 months | 1.57 (95% CI 1.16 to 2.13); p = 0.003 | ||
| 19–24 months | 1.48 (95% CI 1.17 to 1.88); p = 0.001 | ||
| Progression From non-exudative to exudative AMD, HR (95% CI) | |||
| Statin use | |||
| 0–6 months | 1.0 (reference) | ||
| 7–12 months | 1.04 (95% CI 0.62 to 1.75); p = 0.870 | ||
| 13–18 months | 1.27 (95% CI 0.78 to 2.06); p = 0.337 | ||
| 19–24 months | 1.63 (95% CI 1.16 to 2.29); p = 0.005 | ||
|
Kaiserman et al., 2009297 Case control (two studies); FQ |
|||
| Study 1 | Statins, n = 107 | No statins, n = 176 | p-value |
| Proportion with AMD (had PDT) | 0.27% (95% CI 0.20 to 0.34) | 0.16% (95% CI 0.14 to 0.18) | p = 0.002 |
| Study 2 | AMD, n = 334 | Matched controls, n = 1670 | p-value |
| Proportions using statins, any | 126 (37.7%) | 628 (37.6%) | 0.97 |
|
Fong et al. , 2010298 Case–control; FQ |
Statin use, N = 43,026 | No statin use, N = 36,343 | |
| Wet AMD (%) | 51.5 | 48.5 | |
| No wet AMD (%) | 54.2 | 45.8 | |
| OR 0.89 (95% CI 0.77 to 1.03); p = 0.14 | |||
| Recent longer-term use of statins (3 years to 2007) | Statin use, n = 32,743 | No statin use, n = 46,626 | |
| Wet AMD (%) | 38.5 | 61.5 | |
| No wet AMD (%) | 41.3 | 58.7 | |
| OR 0.89 (95% CI 0.77 to 1.04); p = 0.14 | |||
| Lipid-lowering agent use in 2006 | Statin use, n = 5016 | No statin use, n = 74,353 | |
| Wet AMD (%) | 5.3 | 94.7 | |
| No wet AMD (%) | 6.3 | 93.7 | |
| OR 0.83 (95% CI 0.59 to 1.14); p = 0.64 | |||
|
Etminan et al. , 2008285 Nested case–control; FQ |
Cases, N = 2867 | Controls, N = 11,468 | Adjusted RR (95% CI) |
| Current users: statins, n | 642 | 2042 | 1.30 (1.17 to 1.44) |
| Use in past year: statins, n | 1268 | 4268 | 1.31 (1.20 to 1.43) |
Summary
The evidence from observational studies is rather mixed and probably best regarded as inconclusive overall. Some studies have attempted to adjust for confounding variables, for example by propensity scoring. Most studies gave no details of which statins were used or dosages. Patients on statins are more likely to have had cardiovascular disease, which is associated with an increased risk of AMD.
The trial by Guymer and colleagues,283 using 40 mg of simvastatin, did show benefit in terms of reduced progression in patients with non-advanced AMD. The results in the study of high-dose atorvastatin by Vavvas and colleagues286 were more impressive and visual benefits were reported, but there were no controls.
Conclusion
We recommend a trial of high efficacy statins in early and intermediate AMD.
A trial may be getting under way in Spain, called Statins4 drusen. 306
Visual cycle inhibitors
Mechanism of action
Detection of light in the PRs (rods and cones) begins with the transformation of 11-cis-retinal (a photosensitive molecule derived from vitamin A, attached to an opsin protein) into all-trans-retinal. This molecule is not light sensitive so in order to replenish the 11-cis-retinal stocks, a recycling process is required. This process is called the visual cycle and is illustrated in Figure 1.
FIGURE 1.
Visual cycle.
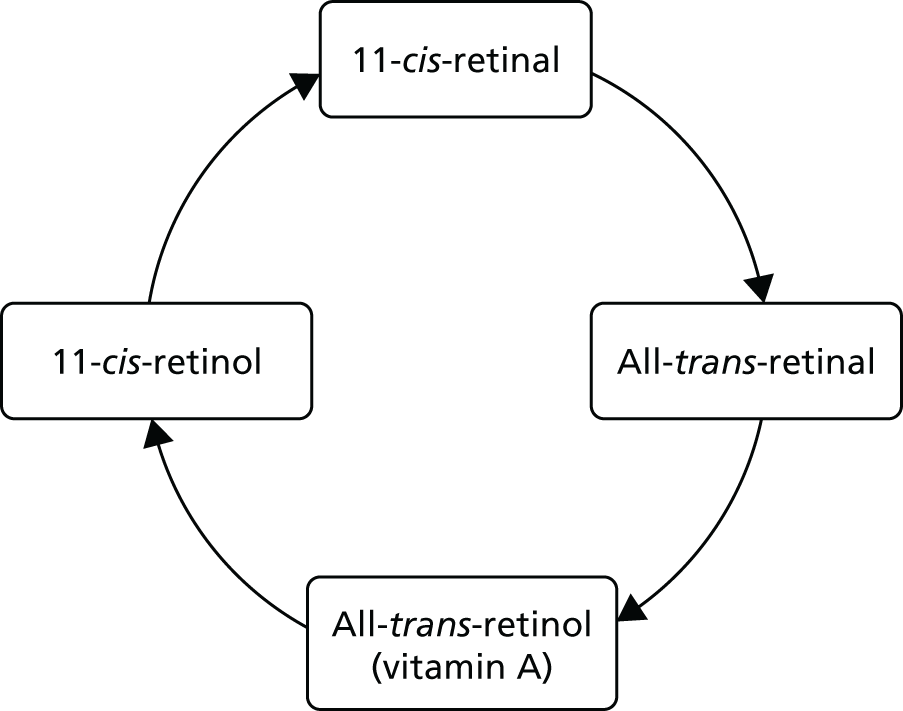
In AMD, this cycle malfunctions, and some all-trans-retinal goes down a different pathway into the A2E dimer, which is a major component of the toxic lipofuscin deposition. A2E is formed of two molecules of all-trans vitamin A (bisretinoid) and one molecule of ethanoloamine. 307 The aim of visual cycle inhibitors (also called modulators) is to reduce the accumulation of A2E.
Emixustat
Emixustat is an oral drug that inhibits RPE65, which is found only in the eye. So adverse effects elsewhere are unlikely.
One RCT was found. Dugel and colleagues308 included adults with a clinical diagnosis of GA, and BCVA 20/400 or better in the study eye, in a dose-ranging, Phase II RCT (NCT01002950) in 15 centres in the USA. The trial had an unclear risk of selection bias, although random generation was adequate. A total of 72 participants were randomised to one of five doses of oral emixustat or placebo for 90 days. The 7-mg and 10-mg groups were discontinued by the sponsor at an early stage owing to the frequency of adverse effects. Change in GA area is summarised in Table 18, but no statistical comparisons were presented. Two participants in the emixustat cohorts (one in the 7 mg dose arm, one in the 5 mg dose one) had a decrease in VA ≥ 15 letters, compared with none of the placebo group.
|
Dugel and colleagues 2015308 RCT, unclear ROB |
2 mg qAM (N = 12) | 5 mg qAM (N = 12) | 5 mg qPM (N = 12) | 7 mg qAMa (N = 12) | 10 mg qAM (N = 6) | Placebo (N = 18) |
|---|---|---|---|---|---|---|
| GA lesion size change from baseline at Day 90, Total area, mm2 by | ||||||
| Colour photography, mean (SD) (n) | 0.2 (0.5) 11 | 0.3 (0.5) 10 | 0.1 (0.5) 8 | 0.4 (0.7) 9 | ||
| FAF photography mean (SD) (n) | –0.1 (1.4) 11 | 0.0 (0.2) 4 | 0.0 (1.0) 8 | 0.2 (0.4) 8 | ||
| Fluorescein angiography, mean (SD) (n) | 0.2 (0.6) 12 | 0.5 (0.5) 10 | 0.2 (0.6) 9 | 0.4 (0.5) 12 | ||
| VA (decrease of ≥ 15 letters) | 0 | 0 | 1 | 1 | 0 | 0 |
Another trial has been registered, testing three doses of emixustat (2.5 mg, 5 mg and 10 mg) and placebo, but no results have yet been posted (NCT01802866). The primary outcome was area of GA. This is also registered as EUCTR2012-004952-12-DE.
Adverse events
Overall, there were 29 discontinuations due to ocular AEs (emixustat, n = 23; placebo, n = 6) in the study by Dugel and colleagues. 308 Serious AEs were reported by three participants in the emixustat groups (one exacerbation of chronic obstructive pulmonary disease and two with chromatopsia, a disturbance of colour vision, sufficient to interfere with driving) and none of the placebo group. At least one ocular event was experienced by 93% of the combined emixustat groups and 28% of the placebo group. The adverse ocular events included chromatopsia (57% emixustat vs. 17% placebo), delayed dark adaptation (48% vs. 6%), visual impairment (26% vs. 6%), reduced VA (11% vs. 0%) and blurred vision (15% vs. 6%).
Dugel and colleagues308 considered that the adverse effects were not sufficient to prevent the drug being trialled in a longer RCT with progression of GA and VA as the outcomes.
Another study of emixustat has been reported in a EURORETINA 2016 conference abstract to have shown no difference in GA growth rates between drug and placebo. 309
Fenretinide
Fenretinide inhibits bisretinoid accumulation by binding with retinol binding protein 4 (RBP4).
Quantity and quality of research
Reviews
Fenretinide was mentioned in three non-systematic reviews of multiple interventions. 9,310,311 However, all three reviews include only one trial, by Mata et al. ,144 described below.
Studies
Mata et al. 144 from the USA carried out a RCT (NCT00429936) in 246 participants. The aim was to see if reductions in RBP retinol would slow GA growth rates. The RCT was a three-arm comparison of oral fenretinide 100 mg (n = 80), oral fenretinide 300 mg (n = 84) and placebo (n = 82), taken daily for 24 months (details from trial registration). The study had an unclear risk of selection bias (see Report Supplementary Material 4). The median age of participants was around 79–80 years, and between 35% and 46% were male. Baseline mean BCVA was between 67 and 69. Mata is based in ReVision Therapeutics (La Jolla, VA, USA), which supported the trial jointly with Sirion Therapeutics (San Diego, CA, USA). The authors included people from both companies. The trial was carried out in 30 sites in the USA but no details of the locations or investigators are given. One author was from Retina Associates of Cleveland, OH, USA.
The aim was to slow growth of GA, which is logical as the aim is to slow down deterioration.
Mata et al. 144 included people with GA secondary to dry AMD and a BCVA between 20/20 and 20/100. GA was measured by FAF and recruits had total atrophic areas of one to eight disk areas. There were no significant differences in GA growth rates overall, but a trend towards a slower growth rate was seen in patients whose RBP fell < 2 mg/dl (Table 19). There were too few of these to show any statistical significance. At 25 months, VA had reduced by 10–11 letters in all groups (see Table 19). The time to first CNV event showed a reduced incidence of CNV in both fenretinide groups, with incidence 18.3% on placebo, 8.8% on fenretinide 100 mg and 9.6% on 300 mg (OR for placebo group 2.2; p = 0.06).
|
Mata et al. , 2013144 RCT; unclear ROB |
Fenretinide 100, n = 80 | Fenretinide 300, n = 84 | Placebo, n = 82 | p-value |
|---|---|---|---|---|
| VA change from baseline (mean letters lost) at 25 monthsa | –11.0 | –10.0 | –8.0 | NR |
| Mean % change in delayed dark adaptation grade | 28 | 38 | 16 | NR |
| Incidence of CNV onset in study or fellow eye (%) | ||||
| No CNV event | 91.3 | 90.4 | 81.7 | Reporting unclear |
| ≥ 1 CNV event | 8.8 | 9.6 | 18.3 | |
| Growth rate | 2.14 | 1.95 | 2.03 | – |
| Growth rate in patients with RBP ≤ 2m/dl | 2.53 | 1.70 | – | – |
Adverse events
The study reported higher rates of AEs leading to withdrawal in the two fenretinide groups than the placebo group (17.5% fenretinide 100; 20.2% fenretinide 300; 6.1% placebo). The most commonly reported AEs leading to withdrawal were eye disorders such as visual disturbance and night blindness in the fenretinide 300 mg group (for specific events see Report Supplementary Material 4). Other commonly reported AEs that did not lead to withdrawal were also reported.
Fenretinide has been very widely used in cancer trials, both of treatment and prevention, with thousands treated. De Palo and colleagues312 report three trials with 1422 women with breast cancer on fenretinide 200 mg daily and 363 with basal cell cancer. Veronesi and colleagues313 randomised 2972 women to fenretinide 200 mg daily or no treatment for 5 years in a trial of prevention of second breast cancer. We cite these studies to show that fenretinide can be well tolerated in younger patients; however, it causes fetal abnormalities so could not be used in women likely to become pregnant.
In a trial by Camerini et al. 314 of 5 years of fenretinide in 2867 women to prevent a second breast cancer, the commonest adverse effects were diminished dark adaptation in 19% and skin problems in 19% (placebo rates 3% for both).
It has been trialled for prevention of oral cancers in Italy. Chiesa and colleagues from Milan315 reported that only seven of 137 patients had to stop because of adverse effects.
In a breast cancer prevention trial, Costa et al. 316 tried three doses (100 mg, 200 mg and 300 mg for 6 months and then 200 mg for another 6 months). Impaired night vision was seen only with the 300 mg doses.
There was a lag between reduction in RBP retinol levels (maximum reached by 3 months) and reduced GA growth rates (not seen until after 12 months). Mata and colleagues144 argue that VA is unlikely to be correlated with reduced GA growth rates because the GA is outside the fovea, and that slowing of GA growth rate over 24 months would not be expected to affect VA. We agree.
The aim of slowing of GA growth rates is primarily to preserve vision, which was not shown in this trial. However, restriction of the size of GA may be also helpful as if one has a small area of GA it would be more likely that extrafoveally fixating patients may be able to still read with visual aids whereas if the area of GA is very large, extrafoveal fixation is very difficult or impossible so people may not do so well even with visual aids. The drop-out rate was quite high in the fenretinide groups (17.5% and 20.2%) compared with 6% in the placebo groups. Despite this, Mata et al. 144 say that the drug was well tolerated.
Summary
The RCT by Mata et al. 144 had an unclear risk of bias. It reported slowing of growth of areas of GA, but reported no difference in VA. The study found that fewer people in the treated groups progressed to wet AMD over 2 years of follow-up, which was a reduction of about half. AEs were more common in the fenretinide groups and, in particular, the higher-dose group, but no serious AEs due to the drug were reported.
The authors conclude that the results justify further trials.
Complement inhibitors
Mechanism of action
The complement system is part of the body’s immune system. There are three main complement pathways: classic, alternative and mannose-binding pathway. Complement components have been found in drusen and in AMD, and it is thought that the complement system is inappropriately activated. The aim of complement inhibitors is to reduce the activity.
One drug, lampalizumab, has been shown to have some effect. For completeness, a brief account is given here, but as trials are already under way, it is not anticipated that the HTA programme would commission any further trials.
Lampalizumab is an antigen-binding fragment targeting complement factor D, which is a key component of the alternative complement pathway. The MAHALO Phase II trial317 recruited 129 patients randomised to sham injections, or to two lampalizumab regimens, 10 mg once a month or twice a month, by intravitreal injection. The primary outcome measure was spread of GA, measured by AF, and follow-up was for 18 months. Almost one-quarter of patients dropped out. There was a 20% reduction in GA area on lampalizumab compared with sham. In a subgroup with the biomarker complement factor 1, the reduction was 44%.
Two very large trials are under way, CHROMA and SPECTRI.
SPECTRI is NCT02247531 and will compare lampalizumab 10 mg given every 4 weeks and every 6 weeks with sham injections. It aims to recruit 936 people in 160 sites worldwide (so only about six per site, which suggests a marketing element), although most are in the USA. There are 14 UK sites. Data collection is due to end in November 2017.
CHROMA (NCT02247479) aims to recruit 936 patients in 144 sites, again mainly USA but four in the UK. The design is the same as SPECTRI. Data collection is due to end in September 2018.
OMASPECT (NCT024745119) will recruit patients coming out of these two RCTs.
There is also another smaller trial, NCT02288559, that will compare two doses, 10 mg every 2 weeks versus 10 mg every 4 weeks, with sham injections, in 96 patients with GA in 37 sites in the USA. Final data collection is due in 2017.
All these trials are sponsored by Genentech-Roche (South San Francisco, CA, USA).
A trial without a NCT number is registered on the WHO trials portal. It looks very similar to NCT0268658 but has a slightly different start date and a recruitment target of 900. 318
NCT02515942 is a trial of the combination of CLG561, an antiproperdin drug, with tesidolumab (formerly LFG316), a C5 blocker, in GA. CLG561 was not effective on its own (NCT01527500). The trial is due to end in November 2017.
NCT02503333 is the FILLY trial of the complement inhibitor, APL-2 from Apellis Pharmaceuticals (Crestwood, KY, USA). Early results have been posted on the Apellis website (www.apellis.com; accessed 26 December 2017). In this Phase 2 study, APL-2 slowed the growth of GA by 29% compared with sham injections, but with a greater reduction becoming apparent in later months. However, in a subgroup with wet AMD in one eye, there was a higher incidence of wet AMD.
Quantity and quality of research
Reviews
One Cochrane review by Williams et al. 319 in 2014 and two non-systematic reviews320,321 were identified (see Report Supplementary Material 4). The Cochrane reviews found no trials – it preceded the MAHALO study. So no evidence based recommendations could then be made on the potential safety and efficacy of complement inhibitors for prevention or treatment of AMD. Complement inhibitors were also considered in four non-systematic reviews of multiple interventions. 9,310,311,322
Studies
Eculizumab
Eculizumab (Soliris®, Alexion Pharmaceuticals, New Haven, CT, USA) is a C5 inhibitor. One RCT of eculizumab has been reported, the COMPLETE (Complement Inhibition with Eculizumab for the Treatment of Nonexudative Macular Degeneration) study (NCT00935883).
This study was conducted in the USA, had an unclear risk of selection bias (although random sequence generation was adequate), and received commercial and non-commercial funding. Mean age was 79 years. Baseline VA was reported (Table 20).
|
Yehoshua et al. , 2014323 RCT, unclear ROB |
Eculizumab, n = 20 eyes | Placebo, n = 10 eyes | p-value |
|---|---|---|---|
| Mean change in GA at 52 weeks, mm (SD) | 0.37 (0.21) | 0.37 (0.22) | p = 0.93 |
| Change in ETDRS VA at 52 weeks | 0.7 (7.2) | 2.9 (7.0) | p = 0.43 |
| Change in ETDRS letters (%) | |||
| ≤ –15 | 5 | 10 | |
| –6 to –14 | 10 | 0 | |
| Within ± 5 | 70 | 90 | |
| 5 to 14 | 10 | 0 | |
| ≥ 15 | 5 | 0 | |
Results
In the Phase II COMPLETE Study, Yehoshua and colleagues323 included people with total GA area of 1.25–18 mm2 and VA 20/63 or better. A total of 30 participants were randomised to treatment with i.v. eculizimab, (high or low dose) or placebo for 24 weeks, with one eye selected as the study eye. There was no difference in change from baseline for GA or VA between eculizumab and placebo at 12 months’ follow-up (see Table 20). Yehoshua and colleagues323 suggest several possible reasons for the lack of effect. First, it may just be that the drug has no effect. Second, it may have been that the effect was smaller than detectable given the power of the trial, which could detect a reduction in GA growth rate down to 55%. It was powered to detect a 75% reduction. Third, the drug might have had more effect if given intravitreally.
Adverse events
Yehoshua et al. 323 reported no AEs.
Summary
The Cochrane review319 (last updated January 2014) found no published trials on the use of complement inhibitors in AMD.
One small RCT323 with an unclear risk of bias found no significant improvement with eculizumab. It seems unlikely that further research on eculizumab would be a priority.
Lampalizumab looks more promising but a considerable amount of research is under way.
L-dopa
Mechanism of action
Brilliant and colleagues324 report their discovery of a protein receptor, GPR143, that is activated by levodopa (L-dopa). This receptor is found in the RPE and their hypothesis is that GPR143 signalling might protect people from AMD, possibly by altering the balance between pigment epithelium derived factor and VEGF.
Quantity and quality of research
One study was identified. Brilliant and colleagues324 carried out a large retrospective cohort study of three registries with a total of 15,252,958 people in the USA. Data on exposure were according to L-dopa prescriptions, but the duration of follow-up was not reported. The study was funded by non-commercial grants, including two from NIH. Mean age was reported only according to L-dopa treatment and AMD diagnosis with and without L-dopa treatment, for each of the registries separately (Table 21). No baseline characteristics of the participants were reported.
|
Brilliant et al. , 2016324 Retrospective cohort, PQ |
Marshfield Clinic, n = 20,000 | Marshfield Epidemiology study, n = 17,500 | TruvenMarket Scan (Truven Health Analytics, Ann Arbor, MI, USA), n = 15,215,458 |
|---|---|---|---|
| Age, years mean (SD) |
L-dopa treatment 67.1 AMD diagnosis without L-dopa 71.2 AMD with L-dopa 79.3 |
L-dopa treatment 67.2 AMD diagnosis without L-dopa 71.3 |
L-dopa treatment 68 AMD diagnosis without L-dopa 71.4 AMD with L-dopa 79.3 |
| Clinic Personalised Medicine Research Project, n = 20,000: | |||
| AMD present | 1142/20,000 (5.7%) | ||
| AMD present and prescribed L-dopa | 39/20,000 (0.2%) | ||
| Marshfield Epidemiologic study area, n = 17,500 | |||
| AMD present and prescribed L-dopa | 20/17,500 (0.1%) | ||
Brilliant and colleagues324 found that age of AMD onset was significantly later in patients who used L-dopa than in non-users (79.3 years vs. 71.2–71.3 years; p < 0.01). L-dopa users were significantly less likely to have a diagnosis of AMD (OR 0.78, 95% CI 0.76 to 0.80; p < 0.001) after controlling for age and gender.
Adverse events were not reported.
The authors consider that the results justify clinical trials.
Summary
This huge study found that people who used L-dopa were less likely to have an AMD diagnosis and that the age of AMD onset was delayed, suggesting that L-dopa protects against AMD.
The study does not tell us whether L- DOPA would be useful in treating dry AMD. It is a very cheap drug with few adverse effects.
Alprostadil
Alprostadil is prostaglandin E1 and one proposed mechanism of action is to improve blood flow by vasodilation to improve choroidal blood flow, which is reduced in people with dry AMD compared with age-matched patients without it. However, prostaglandin E has other effects, including an anti-inflammatory one. It has been used in peripheral vascular disease, and a chance finding in a study for that, was an improvement in VA. 325 This led to a pilot study by Ladewig et al.,326 which was followed by a trial by Augustin et al. 325 The studies were conducted in Germany and Austria, respectively.
Studies
The pilot study by Ladewig et al. 326 recruited a series of 11 patients and compared them with 10 untreated patients, all with dry AMD (≥ 10 soft and/or hard drusen, early GA and pigment epithelial detachment without indications of CNV and BCVA ≥ 0.2 and ≤ 0.8). The distributions of stages of AMD are reported to be similar but details are not given. The study was graded as poor quality (see Report Supplementary Material 4). Participants were treated with daily i.v. infusion of 60 µg alprostadil for 3 weeks. The duration of follow-up was 6 months. Funding was from an independent source.
Visual acuity at 6 months improved in the alprostadil-treated group and deteriorated in the non-treated group; however, no analysis of the difference between groups was reported (Table 22). Other outcomes were reported only for the treated group.
|
Augustin et al. 2013325 RCT: uncertain ROB |
Alprostadil, n = 16 | Placebo, n = 17 | p-value |
|---|---|---|---|
| Change in BCVA ETDRS lines, 6 months mean (SD) [95% CI] | 1.47 (0.569) [0.30 to 2.64] | –0.04 (0.613) [–1.30 to 1.22] | NR |
| Progression of dry AMD, recorded at least once | 11/16 (68.8%) | 12/17 (70.6%) | NR |
| Stabilisation or amelioration of dry AMD | 5/16 (31.3%) | 5/17 (29.4%) | NR |
| Contrast sensitivity study eye (Pelli Robson), mean (SD) [95% CI] at 6 months | 1.81 (0.299) [1.02 to 1.34] | 1.094 (0.224) [0.98 to 1.21] | NR |
| Colour vision, change from baseline at 6 months, n | |||
| Normal – pathological | 1 | 0 | |
| Unchanged | 12 | 15 | |
| Pathological – normal | 3 | 2 | 0.47 |
|
Ladewig et al. , 2005326 Prospective cohort study: PQ |
Prostaglandin E1 (Alprostadil) n = 11 | No treatment, n = 10 | p-value |
| Change in VA at 6 months,% of patients | |||
| Improvement of 3 lines | 9 | NR | |
| Improvement of 1 line | 27 | NR | |
| No change | 45 | NR | |
| Decline by 1 line | 18 | NR | |
| Mean change from baseline in VA, ETDRS lines | 0.4a | –0.8 | |
| Change in contrast vision at 6 months, % of patients | |||
| Improvement of 1 line | 18 | NR | |
| Impairment of 1 line | 18 | NR | |
The RCT by Augustin et al. 325 was carried out at six sites in Germany and Austria. The trial (NCT00619229) was sponsored by UCB Pharma SA (Brussels, Belgium) and two authors were from the company. Augustin et al. 325 recruited 36 people with dry AMD with hard drusen and possible early GA limited to the perifoveal area in one eye and VA between 0.2 and 0.7 logMAR. They received daily i.v. infusion of 60 µg of alprostadil for 3 weeks. The controls received a placebo infusion. Follow-up was for 6 months. There was an unclear risk of selection bias. Mean age was 74 years and half of the recruits were male.
The study was stopped prematurely after the first interim analysis, which led to a need to recalculate the sample size, which showed a need for greater numbers if power was to be achieved. In addition, recruitment was slow. So results were based on interim data because the study was stopped early. Augustin et al. 325 state that only exploratory results can be presented.
The BCVA (using ETDRS charts) improved in the alprostadil-treated group, with benefit seen at end of treatment and at 3 and 6 months, but with greatest improvement at 6 months (see Table 22). BCVA deteriorated in the placebo group, but no analysis of the difference between groups was reported (see Table 22). Progression of dry AMD, stabilisation of dry AMD, contrast sensitivity and colour vision appeared to be similar in each group. No participants in either group developed nAMD.
Adverse events
There were no drug-related AEs in the pilot study. 327 In the RCT325 there were fewer AEs in the alprostadil group (11.1%) than the placebo group (33.3%). No serious AEs were experienced.
Summary
The trial did suggest some benefit in BCVA but this was not statistically significant with the limited numbers recruited (no p-value reported but the CIs overlap), and there was no improvement in progression, although the duration may have been too short. Whether daily infusion for 3 weeks would be acceptable in wider populations is not known, and nor do we know how long benefit would last. The value of prostaglandin infusion is therefore unproven.
Dorzolomide
Mechanism of action
Dorzolomide (Trusopt, Chibret, Munich, Germany) is a carbonic acid or carboanhydrase inhibitor. Such agents have been used in glaucoma and the rationale of testing in AMD is that in addition to lowering IOP, they also improve choroidal perfusion.
Quantity and quality of research
Only one study was identified. A placebo-controlled RCT by Remky and colleagues328 from Germany included 20 participants in the dorzolamide group and 20 in the placebo group. Treatment with a three-times-daily eye drop (dorzolamide 0.2% eye drop, placebo an artificial tear drop) lasted for 12 weeks with follow-up immediately afterwards. The study had an unclear risk of selection bias (see Report Supplementary Material 4). Participants were aged 70 years and 60–70% were male. Baseline BCVA was 0.12 to 0.13 logMAR. The funding source for the study was not reported.
Results
Remky and colleagues328 included people with AMD with VA > 0.4 (20/50). The eye with better VA was selected. At 12 weeks, VA was the same in each groups (Table 23). Pericentral visual field analysis showed an increased retinal sensitivity (1.55 dB, comparison from baseline p = 0.04) in the dorzolomide group but no significant change in the placebo group (+0.58 dB; p = 0.1). Other outcomes were also similar between groups (see Report Supplementary Material 4). The study was small and short, so power was limited.
|
Remky et al. , 2005328 RCT; unclear ROB |
Dorzolamide, n = 20 | Placebo, n = 20 | p-value |
|---|---|---|---|
| VA, mean LogMAR (SD) | 0.14 (0.12) | 0.14 (0.12) | NR |
Adverse events
There were no severe AEs in the study. Minor AEs were reported (see Report Supplementary Material 4).
Summary
This pilot RCT with an unclear risk of bias showed no significant differences in VA or other outcomes between those treated with dorzolamide and those treated with placebo, but the duration of treatment was very short and the power of the study was low. Few AEs were reported. This group of drugs have been used for many years with no serious adverse effects.
Glatiramer acetate (copaxone)
The proposed mechanism of action extrapolates from a study in a mouse model of Alzheimer’s disease in which glatiramer reduced plaque size.
Quantity and quality of research
Two publications from Landa et al. 329,330 The first study was a pilot CCT that included 14 participants treated with glatiramer acetate (seven participants) or placebo (seven participants). The second study was a pilot RCT that included six participants (four participants treated with glatiramer acetate and two participants treated with a placebo). The studies were conducted in the USA. Participants were treated for 12 weeks in both studies and follow-up was immediate. The intervention was by subcutaneous injection (for dosing details see Report Supplementary Material 4). The studies had an unclear risk of selection bias. Ages, male sex and baseline BCVA were not reported. The funding source was not reported.
Results
Landa and colleagues330 included people with dry AMD (no further inclusion criteria reported). At 12 weeks the CCT reported a statistically significant difference in the proportion of convex drusen disappeared or shrank (Table 24), but no difference in the proportion of total drusen or concave drusen that disappeared or shrank. In the pilot RCT the change in drusen area was reported. This appeared to be lower in the glatiramer acetate treated people than the placebo controls but no statistical analysis was undertaken.
|
Landa et al. , 2011330 CCT; unclear ROB |
Glatiramer acetate, n = 7 | Placebo, n = 7 | p-value |
|---|---|---|---|
| % drusen disappeared or shrank at 12 weeks | 19.2 | 6.5 | 0.13 |
| % convex drusen disappeared or shrank at 12 weeks | 27.8 | 6.8 | 0.008 |
| % concave drusen disappeared or shrank at 12 weeks | 4.7 | 5.6 | 0.89 |
|
Pilot study RCT; unclear ROB |
Glatiramer acetate, n = 4 | Placebo, n = 2 | |
| Change in drusen area, arbitrary units |
Baseline: 48,130 12 weeks: 16,205 |
Baseline: 32,294 12 weeks: 32,781 |
Adverse events
The study did not report AEs. 330
Summary
Two linked studies with unclear risk of bias were included. The studies were small and of short duration, and the outcome measure used was drusen area, which was reported to have reduced. The main interest of the authors seems to have been to test an outcome measure, using high-resolution spectral domain OCT, that could predict response. The evidence is too sparse to come to any conclusions on efficacy of glatarimer.
OT 551
OT 551 (Othera Pharmaceuticals, Exton, PA, USA) is a drug with antioxidant properties, that forms a compound called Tempol-H, which has been reported to protect RPE cells in the laboratory from oxidative damage. It is given as eye drops, three times daily.
Quantity and quality of research
One pilot RCT92 conducted in the USA with 10 participants with bilateral GA was included. Treatment and follow-up were 2 years duration and one eye of each participant was randomised to treatment or observation. The trial had unclear risk of selection bias. The funding source was non-commercial. The mean age of participants was 77 years and 40% were men. Mean baseline BCVA was 46.1–55.7 letters.
Results
Wong and colleagues92 included participants with bilateral GA. A statistically significant difference between treated eyes and untreated eyes was found for change in BCVA at 2 years (p = 0.026), but no significant difference was found for contrast sensitivity, GA area or drusen area (Table 25). No eyes progressed to nAMD.
|
Wong et al. 201092 RCT; unclear ROB |
OT-551, n = 10 (eyes) | No treatment, n = 10 (eyes) | p-value |
|---|---|---|---|
| BCVA letters change at 104 weeks, mean (SD) | 0.2 (13.3) | –11.3 (7.6) | 0.0259 |
| Loss of BCVA, 104 weeks (%) | |||
| ≥ 5 letters | 30 | 90 | |
| ≥ 10 lettersa | 30 | 60 | |
| ≥ 15 lettersa | 10 | 30 | |
| CS, change at 104 weeks, mean (SD) | –0.075 (0.33) | –0.15 (0.27) | 0.6059 |
| Increase in GA area at 104 weeks (mm2) fundus photos mean (SD) | 2.46 (1.25) | 2.47 (0.73) | 0.9502 |
| Total drusen area at 104 weeks, by fundus photosa | 0.32 | 0.39 | 0.5391 |
Adverse events
No serious AEs occurred. There were 32 grade 1 and four grade 2 events (see Report Supplementary Material 4).
Summary
One small RCT with an unclear risk of bias found a statistically significant improvement in BCVA in eyes treated with OT-551 for 2 years compared with untreated eyes. There was no significant difference in other outcomes. The authors conclude that OT 551 has limited or no benefit in GA. A larger trial was carried out with 137 subjects, using the eye drops on one eye four times a day for up to 2 years, but we found no publications other than a conference abstract331 reporting no benefit.
Sirolimus
Sirolimus is an immunosuppressant drug.
Quantity and quality of research
Two small RCTs, Wong 2013332 and Petrou 201594 conducted by the same group in the USA were included, with a total of 17 participants (total 34 eyes, 17 eyes treated). Randomisation was by eye, with the other eye acting as control. The first study used subconjunctival sirolimus, the second intravitreal. The studies had 12 months’ and 24 months’ follow-up, respectively. Both studies had an unclear risk of selection bias, although in one332 the random sequence was generated by a computer algorithm. Both studies received non-commercial funding, although the drug was donated by a commercial entity. Mean age was 74–78 years and the majority of participants were men. Visual baseline acuity was reported by both studies (Table 26) (NCT00766649 and NCT01445548).
|
Wong et al. , 2013332 RCT; unclear ROB |
Sirolimus, n = 8 (eyes) | No treatment, n = 8 (eyes) | p-value |
|---|---|---|---|
| Change in GA area (mm2) mean (SD), by fundus photography at 24 monthsa | 2.46 (1.18) | 2.08 (0.83) | 0.17 |
| Change in drusen area (mm2) mean (SD), by fundus photography at 24 monthsa | 0.04 (0.58) | 0.08 (0.36) | 0.81 |
| Change in BCVA letters at 24 months, mean (SD) | –21.0 (21.5) | –3.0 (8.1) | p = 0.03 difference 18 letters (95% CI 0.9 to 25 letters) |
| Proportion of eyes with ≥ 10 letters vision loss at 24 monthsb | 50 | 12.5 | |
|
Petrou et al. , 201594 RCT; unclear ROB |
Sirolimus, n = 5 (eyes) | No treatment, n = 5 (eyes) | p-value |
| Change in GA area (mm2) mean (SD), by fundus photography at 12 monthsc | 2.26 (0.94)d | 1.53 (0.75)d | 0.15 |
| Change in BCVA at 12 months, mean (SD) | –15.6 (7.23)d | 0 (13.47)d | 0.013 |
| Proportion of eyes with ≥ 10 letters vision loss at 12 months | 80c | 20c | NR |
Results
Both studies had similar eligibility criteria: bilateral GA with area of a half or more of a disc area, one or more large drusen (≥ 125 µm) in each eye, and BCVA 20/20 to 20/400 in each eye. Eyes from each participant were randomised to receive sirolimus injection or no treatment. Wong and colleagues333 administered sirolimus by subconjunctival injection every 3 months for 2 years. Petrou and colleagues94 administered sirolimus every 2 months for 1 year.
Both studies found that treated eyes had a statistically significantly greater decrease in BCVA compared with untreated eyes (p = 0.03,333 p = 0.01394) and that a higher proportion of treated eyes lost ≥ 10 lines of vision (50% vs. 12.5%,332 80% vs. 20%;94 p-value not reported). A non-significant greater increase in GA area in treated eyes was also found in both studies. No eyes developed CNV during either study.
Adverse events
Wong and colleagues332 reported mild to moderate ocular AEs, including small haemorrhages, raised IOP, and one death unrelated to the study medication. Petrou and colleagues94 reported accelerated retinal thinning in two treated eyes and treatment was suspended.
A third trial, NCT01675947, was terminated in 2014.
Summary
Two small RCTs with an unclear risk of bias found no benefits, but rather a worsening of vision in eyes treated with sirolimus injection. Serious AEs occurred in some participants. There seems to be no place for sirolimus in AMD.
Prednisolone
Quantity and quality of research
One prospective cohort study conducted on 475 participants (prednisolone, n = 400; control, n = 75) was identified. 334 Participants from Croatia were given either peribulbar injections of prednisolone 5 mg for 5 days, or received multivitamins. Allocation was temporal – the first 400 patients received the steroid. Follow-up was at 6 months. The study was of poor quality (see Report Supplementary Material 4). Participants were aged between 39–80 years, no further baseline characteristics were provided. The funding source was not reported. The study was published in the journal of the Croatian Anthropological Society.
Results
Vojniković and colleagues334 included people with dry AMD but no other inclusion criteria were reported. At 6 months, peripheral visual field had improved by 10% to 25% in participants in the prednisolone group but there was no improvement in the control participants (Table 27). Central visual field improved by 5% to 20% in the prednisolone group (only a subgroup of controls were reported, assume no improvement was seen in the remaining participants). No statistical analyses were reported. AEs were not reported.
|
Vojniković et al. , 2008334 Prospective cohort; PQ |
Prednisolone, n = 400 | Control, n = 75 | p-value |
|---|---|---|---|
| Peripheral visual field | Improvement of 10 to 25% | No significant improvement | NR |
| Central visual field | Improvement of 5–20% | Improvement of 0.5–1% in 43 patients | NR |
Summary
One study of poor quality provided sparse evidence on the effects of prednisolone on dry AMD.
Tandospirone
Tandospirone is a 5-HT1A agonist, referred to also as AL-8309B. 5-hydroxytriptamine (5-HT) is also known as serotonin. The 5-HT1A agonists are used in depression but have also been thought to be ‘neuroprotective’ and in a rat model exposed to severe photo-oxidative stress, they protected the PR and RPE cells.
Quantity and quality of research
One three-arm RCT was identified, the Geographic Atrophy Treatment Evaluation (GATE) trial. 190 The study was undertaken in 48 sites in a number of countries including the USA, Germany, Canada and the UK (see Report Supplementary Material 4). Participants were allocated to eye drops of 1% tandospirone (n = 252), 1.75% tandospirone (n = 259) or placebo (n = 261). Treatment was given twice daily for 24 months. Participant follow-up was at 30 months. The RCT had an unclear risk of selection bias. The mean age of participants was around 78 years and between 37% and 48% of participants were male. Baseline BCVA was not reported. The study was sponsored by Alcon Research (NCT00890097 and EUCTR2008-007705–37-DE).
Results
Jaffe and colleagues190 (Table 28) included people with GA secondary to dry AMD and no evidence of CNV. BCVA was required to be at least 35 letters. At 30 months VA was reduced in all groups, but the difference between groups was not statistically significant (see Table 28). The primary outcome for the study was the annualised lesion growth rate, this was not statistically significantly different between the active groups and the placebo.
|
Jaffe and colleagues 2015190 RCT; unclear ROB |
Tandospirone 1.0% n = 250 | Tandospirone 1.75% n = 258 | Placebo n = 260 |
|---|---|---|---|
| Annualised lesion growth rate, mean (95% CI)a | 1.725 (1.595 to 1.855) | 1.758 (1.626 to 1.890) | 1.707 (1.585 to 1.830) |
| BCVA change (ETDRS) estimated from figure | –0.8 | –0.6 | –0.7 |
Adverse events
The study reported similar rates of any ocular AEs across the three treatment groups. There were slightly higher rates of serious ocular AEs in the placebo group (Table 29 and see Report Supplementary Material 4).
| Jaffe and colleagues 2015190 | Tandospirone 1.0% n = 250 | Tandospirone 1.75% n = 258 | Placebo n = 260 |
|---|---|---|---|
| Any ocular AEs in study eye (%) | 66 | 67 | 60 |
| Serious ocular AEs in study eye (%) | 0 | 1 | 2 |
Summary
This RCT found no benefit on BCVA or GA growth from tandospirone eye drops in in people with GA. AEs were similar in the tandospirone groups and the placebo group.
Trimetazidine
Trimetazidine is a drug used in France as a prophylactic for angina, and also for vertigo and tinnitus, and for visual field loss presumed to be due to vascular causes.
Quantity and quality of research
One placebo-controlled RCT by Cohen et al. 335 from France (324 centres), Belgium (6 centres) and Spain (11 centres) compared 594 participants given trimetazidine 35 mg with 598 given a matched placebo. Treatment was one tablet twice daily for a mean of 37.6 months. Follow-up was a minimum of 3 years. The study had a low risk of selection bias (see Report Supplementary Material 4). Drop-out rates were 23% in the trimetazidine group and 27% in the placebo arm. Participants had a mean age of 73.5 years and 38% were male. Baseline distance VA was at least 0.5 (20/40) in 91.5% of the trimetazidine treated participants and 93% in the placebo group. The study was sponsored by Servier Laboratories, Suresnes, France.
Results
Cohen and colleagues335 included people with AMD and unilateral CNV, and the unaffected eye was the study eye. At follow-up, CNV incidence was similar between groups (p = 0.78) (Table 30). Five-year incidence of CNV, atrophy of greater than one-third disc diameters and atrophy of greater than one-third disc diameters were similar between groups. In atrophy greater than one-third disc diameters, the trimetazidine group did slightly better – incidences per 100 patient-years 5.11 on drug versus 6.45 on placebo; HR 0.76, 95% CI 0.56 to 1.02. In some subgroups (prespecified) the drug group did better, such as the under 75s where HR for atrophy was 0.57 (95% CI 0.38 to 0.88) based on 11% and 17% developing atrophy greater than one-third disc diameters. VA was not reported.
|
Cohen et al. , 2012335 RCT; low ROB |
Trimetazidine, n = 546 | Placebo, n = 540 | p-value |
|---|---|---|---|
| CNV Incidence per 100 patient-years | 10.86 | 11.13 | HR = 0.97 (95% CI 0.79 to 1.19); p = 0.78 |
| CNV 5-year cumulative incidence, mean (SD) (%) | 45.35 (3.27) | 48.50 (3.59) | NR |
Adverse events
Adverse events including eye disorders were similar between groups with any AEs experienced in 75% of the trimetazidine group and 79% of the placebo group.
Summary
This RCT was essentially negative overall, with no differences in the incidence of CNV and atrophy between those treated with trimetazidine and those treated with placebo.
Further research does not seem to be indicated.
Visaline
Visaline is a drug and nutrient mixture registered in Switzerland for AMD. The drug is buphenine, a beta-agonist to increase perfusion. The other components are beta-carotene, vitamin C and tocopherol.
Quantity and quality of research
One study was identified. Kaiser and colleagues from Switzerland336 carried out a placebo-controlled pilot RCT with nine participants in the visaline group and 11 in the placebo group. Treatment was with two tablets twice daily, 5 days per week (buphenine HCI 1.5 mg, beta-carotine 10 mg, tocopherol acetate 10 mg and ascorbic acid 50 mg or placebo) for 6 months with follow-up immediately afterwards. The study had an unclear risk of selection bias (see Report Supplementary Material 4). Participants were aged 73 years and 55% of treated participants and 9% (1 of 9) of placebo participants were male. Baseline near VA was 0.45 – 0.57. The funding source for the study was not reported.
Results
Kaiser and colleagues 1995336 included people with early AMD with BCVA between 20/100 and 20/25. The eye with better VA was selected if both eyes were affected. At 6 months, near and far VA were similar in each group as shown in Table 31. The proportions with improved visual function (not defined) was greater in the visaline group than the placebo group (44% vs. 27%); however, there appears to be some missing data (see Report Supplementary Material 4 for unchanged and worsened categories). Other outcomes were similar between groups.
|
Kaiser et al. , 1995336 RCT; unclear ROB |
Visaline, n = 9 | Placebo, n = 11 | p-value |
|---|---|---|---|
| Far VA at 6 months, mean (SD) | 0.67 (0.2) | 0.6 (0.22) | NS |
| Near VA at 6 months, mean (SD) | 0.62 (0.14) | 0.55 (0.23) | NS |
Adverse events
There were no AEs.
Summary
This RCT showed no difference in VA or other outcomes between those treated with visaline and those treated with placebo.
Antihypertensive drugs and risk of developing wet age-related macular degeneration
Beta-blockers, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers are very commonly used for controlling high blood pressure. It has been reported that they can induce regression of CNV in rodent models of AMD and so the question was raised whether or not they could reduce the risk of wet AMD. However, Thomas and colleagues337 found no such protection. They compared 250 patients with dry AMD and 250 with wet AMD and found no difference in the frequency with which these drugs were used. However, they did note that 65% of people with AMD had hypertension, with no difference between wet and dry AMD.
The case–control study by Etminan and colleagues285 from Vancouver in people who had had coronary angioplasty or bypass found that those with AMD (based on recording of ICD-9 codes) had a slightly higher rate of using ACE inhibitors (RR 1.19, 95% CI 1.07 to 1.33).
Ranibizumab
Ranibizumab is an anti-VEGF drug used in wet AMD and other eye conditions.
Quantity and quality of research
One small before-and-after study of ranibizumab by Gallego-Pinazo and colleagues338 was identified. The study was described as a pilot. It was conducted in Spain. Six participants received intravitreal ranibizumab 0.5 mg/0.05 ml at least once. Retreatment was permitted if there was persistence or recurrence of elevation of the RPE contour on OCT, or if retinal fluid was seen on OCT, or if ≥ 5 letters were lost. The mean number of injections was two and the range was one to five. Participants were then followed up at 12 months. The study was of fair quality apart from the very small size. The mean age of participants was 69 years and 33% were male. Baseline VA was 0.40 (decimal ETDRS equivalent). The study funder was not reported.
Results
Gallego-Pinazo and colleagues338 included people with BCVA < 20/30 and DPED from AMD. At 12 months the mean BCVA had improved (p = 0.046, Table 32) and the study found that 33.3% of patients gained between 19 and 21 letters, with no participants experiencing loss of BCVA. There was a decrease in central macular thickness but this was not statistically significant.
|
Gallego-Pinazo et al. , 2013338 Prospective B + A; FQ |
Ranibizumab, n = 6 | p-value |
|---|---|---|
| BCVA, mean (decimal ETDRS equivalent) | ||
| Baseline | 0.40 (0.15) | 0.046 |
| End of study | 0.58 (0.3) | |
Adverse events
The study did not report AEs.
Summary
In a very small before-and-after study of fair quality, a significant improvement on BCVA was seen at 12 months in people with DPEDs. AEs were not reported. The authors recommend a long-term randomised trial. If there is any prospect of benefit in dry AMD, the manufacturers will no doubt pursue it vigorously.
Brimonidine
Brominidine is an alpha-adrenergic agonist used in glaucoma, but there is some evidence from animal and in vitro cell studies that it may protect RPE. In glaucoma it is used as eye drops, but when given thus, little of the drug reaches the retina. It is made by Allergan (Dublin, Ireland), who also produce the dexamethasone implant (Ozurdex®, Allergan, Dublin, Ireland). Allergan have used the same technology to produce implants of brimonidine.
A Phase IIA trial (NCT00658619) comparing implants with two doses of brimonidine in a slow-release matrix versus sham injections was reported at ARVO 2017. 339 Recruits had AMD with bilateral GA. A total of 113 patients were randomised to brimonidine 132 or 264 µg or sham, given at baseline and 6 months. The primary end point was change from baseline in GA area. At 12 months, growth of GA was lower in the brimonidine groups (p = 0.032) by 19% for the lower dose and 28% for the higher. After 24 months, the difference was less, and GA growth was reported to be reduced only in the higher dose group (p = 0.059). This might imply that treatment would need to be given every 6 months (or even every 4 months, based on the timescale of release of dexamethasone from the Ozurdex implant). A Phase IIB trial, the Beacon Trial (NCT02087085), is now under way with brimonidine 400 µg given every 3 months. The matrix carrier slowly dissolves, leaving no foreign bodies floating in the eye.
Integrin inhibitors: luminate
Luminate is a new drug currently being trialled in wet AMD and DMO, but results from a case series of five patients with intermediate dry AMD (‘intact PR and RPE layers with no subretinal fluid’) were reported at ARVO 2017. 340 Four out of five patients were reported to show improved VA after 3 months.
Tetracyclines
NCT01782989 is using the Oracea brand of doxycycline (Galderma Laboratories, Fort Worth, TX, USA) in a trial to see if it slows progression of GA. The trial is called TOGA (Treatment with ORACEA for Geographic Atrophy). It is due to complete data collection by end of 2018.
A trial of oral minocycline, NCT02564978, is under way in GA, sponsored by the National Eye Institute, and involving centres in Maryland, USA, and Bristol and Moorfields. It aims to recruit 40 people and to end in 2021.
Other drugs
A drug called MC-1101 (MacuCLEAR®, MacuCLEAR Inc., Richardson, TX, USA) was being trialled in dry AMD. It could be given topically by spray on to the ocular surface and was said to improve choroidal blood flow. No data have been added to the registration for NCT02127463, a Phase II/III trial aiming to recruit 60 patients since 2014. Data collection was expected to be complete by 2016. NCT01601483 has been terminated, and NCT01922128 was completed in 2013.
NCT02684578 is a trial of metformin, an old, very cheap drug used in type 2 diabetes, versus placebo, in California. The drug will be given to people without diabetes for 18 months. The trial will end in 2019.
NCT00332657 and NCT00307398 were trials of anecortave in people with wet AMD in one eye and dry in the other, with the aim of preventing the development of wet AMD in the dry eye. It recruited 2500 people but was terminated because of lack of effectiveness.
NCT01603043 was also terminated was a trial of Alcon’s AL-788898A, a compstatin analogue, in GA.
NCT01342926 was a trial of GSK933776 (GlaxoSmithKline, Middlesex, UK), an antibody targeting amyloid beta, but it had no effect on growth of GA or on VA.
Another antibody against amyloid beta was (or is) RN6G from Pfizer (New York, NY, USA). Three trials were registered as NCT00877032 with 57 patients, NCT01577381 with 10, and NCT01003691 with 24. The second was terminated early after only the 10 recruits. No results are yet published.
Summary
We have reviewed the evidence on 23 drugs or groups of drugs. In 10 cases, there was some evidence showing no or very little benefit, or even harm, so when it comes to reporting back to the NIHR programmes, mainly HTA, we are minded to exclude these from further consideration. They are alprostadil, eculizumab, dorzolomide, OT 551 eye drops, prednisolone, sirolimus, tandospirone, trimetazidine, visaline and emixustat. In the case of prednisolone, we note that a trial of an implantable steroid is under way.
The current evidence on lampalizumab suggests benefit, but very large trials are under way, sponsored by the manufacturer, so no new research is indicated meantime.
There is a little evidence of benefit from glatarimer, but only shrinkage of drusen. This is too sparse to prompt NIHR research and we think future trials can be left to the manufacturer in the meantime. It is used in multiple sclerosis and the manufacturer, Teva Pharmaceutical Industries (headquartered in Petah Tikva, Israel), is one of the fifteenth largest Pharma companies in the world [according to their website (www.tevapharm.com; accessed 19 June 2107)]. So if glatarimer (also known as copaxone) works, there is a major incentive to do further research. However, no new research has been published since 2008, from which we conclude that Teva do not think the drug is worth pursuing (they may have further unpublished data).
There is some evidence of benefit from ranibizumab in pigment epithelial detachment (PED) in apparently dry AMD, but we think that more sensitive assessment of patients with PED may disclose evidence of wet AMD, so we are excluding this.
L-dopa is a drug very widely used in Parkinson’s disease. An impressively large study324 from the USA found that people taking L-dopa were less likely to develop AMD and that if they did develop it, they did so about 7 years later than people not taking L-dopa. Epidemiological studies such as this can be difficult to interpret due to confounding variables (i.e. the people taking L-dopa may be different in other ways from people not taking it, so the L-dopa may be a correlate not a cause). There have been past occasions where a large observational study suggests benefit but a subsequent trial has not shown any. Nevertheless, we think L-dopa merits further study, perhaps using the very large UK general practice databases such as Clinical Practice Research Datalink (CPRD) or THIN. This epidemiological research does not fit with NIHR remits. It might appear to fit with the MRC Population Systems and Medicine theme, but that appears to exclude vision and other central nervous system conditions. The MRC Neurosciences and Mental Health Board seems focused on basic science research. So it is not clear who would fund a UK study into whether or not L-dopa protects against AMD.
That leaves two topics that seem to justify NIHR research. The first is high-potency statins, such as atorvastatin 80 mg daily. The evidence from observational studies (mainly comparing the frequency of AMD in people taking statins and those not taking statins) is mixed and contradictory. The evidence from intervention studies comes mainly from a RCT by Guymer et al. ,283 using simvastatin 40 mg, which showed some benefit. But simvastatin 40 mg is not a potent dose. Vavvas et al. 286 found more impressive benefits from atorvastatin 80 mg but in a case series not a RCT. Atorvastatin is now out of patent and cheap, so no drug company would fund a trial. We recommend that the HTA programme do so. Any such study should use genetic subtyping, because Guymer and colleagues283 found that simvastatin worked much better in some people than others. There is also an issue about stage of AMD, with statins appearing more effective if used early. So selection of people for a statin trial would be important.
The second is fenretinide, which is a visual cycle inhibitor which may reduce the deposition of abnormal and toxic lipofuscin. There is one trial, run and written up by the manufacturer’s staff, which had mixed results. Progression of GA was little different overall, but there were better results in the subgroup that achieved the most plasma RBP lowering. However, progression to wet AMD was halved by fenretinide. We think, based on the understanding of the physiopathology of AMD and the visual cycle, and on the results from Mata et al. ,144 that an independent trial in a selected subgroup may be justified.
Chapter 6 Nutritional interventions in dry age-related macular degeneration
Introduction
There are many studies of nutrition and nutritional interventions in AMD but a number of issues need to be considered in their interpretation, including:
-
Are the interventions for primary prevention of AMD or to slow progression, or reverse changes?
-
Studies can be epidemiological, looking at associations between diet and AMD, or interventional. In epidemiological studies, it is important to think about correlation, causation and confounding. For example, people who eat a lot of fish may differ in other ways from people who do not eat fish. They may not eat much red meat. So any difference could be either due to eating fish or to not eating red meat. In the best studies, the authors try to adjust for known confounding variables, but there may be unknown ones. In intervention studies, exposures are much more under the control of the investigators, but trials may be much smaller and shorter than large epidemiological studies.
-
Are studies of dietary intake or of supplements? If the latter, what doses are used?
-
If supplements, what form is used? For example, zinc comes up a lot in AMD studies, but can be used as zinc oxide, zinc sulphate, zinc gluconate and perhaps others, and bioavailability varies.
-
If supplements are given in combinations, do some ingredients affect others? For example, if beta-carotene and lutein are given together, is absorption of lutein reduced?
-
How long are supplements given for? For example, after supplementation with lutein and zeaxanthin, serum levels rise quickly, but macular pigment concentration increases over several months, and visual function may take a year or two to reach statistically significant changes. 341
-
Responses to supplements may vary according to baseline levels. Those with the lowest baseline level may have more to gain. Conversely, if a trial (such as AREDS, see below) recruits people who are better nourished than the average member of the population, the recruits may have less to gain, and the results may under-estimate the benefits to the wider population.
-
Effects may vary across different manifestations of AMD, for example having more effect on the development of wet AMD than on GA. So an outcome of ‘advanced AMD’ may need to be refined.
The Age-Related Eye Disease Study trials
The first Age-Related Eye Disease Study (AREDS) trial started in 1992. The background was that some, but not all, epidemiological studies had found an association between the intake of antioxidants and zinc intake, and one small placebo-controlled trial of zinc342 had reported a reduction in VA loss in the zinc arm. Marketing of supplements to preserve vision had become common but had outstripped the evidence base. This led the National Eye Institute (part of the US NIH) to support a trial, by the AREDS research group.
The first AREDS study recruited 4757 participants. 32 They were divided by extent of AMD, as shown in Table 33.
| AMD category | Drusen size | Drusen area | Pigment abnormalities | Fellow eye |
|---|---|---|---|---|
| 1 | None or small | 5–15 small drusen | None | Same as first |
| 2 | Small or intermediate, or none if pigment abnormalities present | About 1/150 disk area, at least one drusen | Absent or present but no GA | Same as first or category 1 |
| 3a | Intermediate or large or non-central GA | About 1/16 disc area if soft indistinct drusen; about 1/5 if no soft drusen | Absent or present but no central GA | Same as or better than first eye |
| 3b | As for 3a | VA < 20/32 | ||
| 4 | As above | Advanced AMD or VA < 20/32 due to AMD |
The generalisability of predictive capacity of the AREDS trial categorisation was tested by Liew and colleagues343 from the Blue Mountains Eye Study (BMES) Group, which found very similar rates of progression when the simplified AREDS severity scale categories 1 and 2 were applied the Blue Mountains population. A 10-year progression to late AMD from category 2 was 7.3% in BMES and 8.4% in AREDS.
The outcome measures chosen were progression to advanced AMD (wet or GA), based on colour photography, and VA loss of ≥ 15 letters, equivalent of progress from, for example, 20/20 to 20/40 or worse.
These broad inclusion criteria lead to some problems. In category 1, only 5 out of 1117 recruits developed advanced AMD over the mean follow-up of 6.3 years. Participants in category 2 had only a 1.3% probability of progression to advanced AMD by year 5, whereas progression was expected in 18% of category 3 patients and in 43% of category 4.
So the analysis used mainly participants in categories 3 and 4. Randomisation was to one of four arms:
-
placebo
-
zinc (80 mg zinc oxide)
-
antioxidants (vitamin C 500 g, vitamin E 400 IU, beta-carotene 15 mg, daily)
-
zinc + antioxidants.
Compared with placebo for progression to advanced AMD from categories 3 and 4, risk reductions were (note the use of 99% CIs):
-
antioxidants OR 0.83 (99% CI 0.66 to 1.06)
-
zinc OR 0.79 (99% CI 0.62 to 0.99)
-
antioxidants + zinc OR 0.66 (99% CI 0.47 to 0.93) (p = 0.001).
For VA loss, the OR was 0.73 (99% CI 0.54 to 0.99) with the combination, but the reductions with zinc alone or antioxidants alone were not statistically significant.
Compared with placebo, antioxidant and zinc combination significantly reduced progression to wet AMD (OR 0.62, 99% CI 0.43 to 0.90). Progression to wet AMD was significantly reduced by zinc (OR 0.76, 99% CI 0.58 to 0.98) but not by antioxidants (OR 0.79, 99% CVI 0.56 to 1.13). None of the reductions in OR for development of central GA were statistically significant, possibly because the number of GA events was much lower than the number of wet AMD events (257 vs. 592), so that the AREDS study had only 40% power to show a significant 25% reduction in risk.
The AREDS 1 trial showed benefit which persisted for 7 years, with a modest but useful slowing of progression, which could mean that 30% of people expected to progress to advanced AMD over a 5-year period, would not. 344 The trial did not have enough power to confirm, or not, effects in categories 1 and 2.
An extension of the AREDS 1 trial207 showed that at 10 years, despite all arms taking the AREDS 1 formula (when available – there was a 2-year gap), fewer of the category 3 and 4 recruits who had been randomised to antioxidants + zinc progressed to advanced AMD (34% vs. 44% randomised to placebo).
An analysis of dietary data obtained at recruitment345 found that participants reporting the highest intake of the macular pigments, lutein and zeaxanthin, were less likely to have advanced GA. Lutein and zeaxanthin protect the retina against photochemical damage and oxidative stress by filtering harmful short wavelength light.
No serious AEs were reported. A protocol amendment was made in 1996 to allow participants who were current smokers to discontinue masked medication following evidence of the potential harmful effects of beta-carotene in smokers. This led to 72 participants stopping medication and 84 being reassigned, but analyses remained by intention to treat.
The quality of the AREDs trial32,346 was good as shown in Table 34.
| Quality criterion | Risk of bias (high, unclear, low) | Support for statement |
|---|---|---|
| Random sequence generation (selection bias) | Low | Computer randomisation |
| Allocation concealment (selection bias) | Low | Performed by co-ordinating centre |
| Blinding participants and personnel (performance bias), objective outcomes | Low | Described as double masked (including participants and investigators). Tablets identical in appearance and taste |
| Blinding participants and personnel (performance bias), subjective outcomes | N/A | N/A |
| Blinding outcome assessors (detection bias), objective outcomes | Low | Double masked (participants, investigators and Reading Centre personnel) |
| Blinding outcome assessors (detection bias), subjective outcomes | N/A | N/A |
| Incomplete outcome data (attrition bias), objective outcomes | Low | 2.4% lost to follow-up, states balanced across groups, only stated reason was missed at least 2 appointments. Some participants did not have photographic or VA assessment, also balanced across groups |
| Incomplete outcome data (attrition bias), subjective outcomes | N/A | N/A |
| Selective reporting (reporting bias) | Low | No evidence of selective reporting |
| Other biases | Low | No other bias |
Concerns about the generalisability of the AREDS 1 results has been raised, including in the NICE guideline on AMD. 347 The AREDS recruits are described as well-nourished and better educated than the general population. However, the key point is that if AREDS 1 recruited people who were more health conscious and were eating a healthier diet than the average person, the AREDS 1 results would underestimate the benefits in the general population. The AREDS 2 investigators noted that their recruits tended to have higher intakes of lutein and zeaxanthin than the general population (about 20% higher than the NHANES population), which reduced the power of the study to show benefit from supplementation. Some of those in the highest decile of dietary lutein and zeaxanthin intake were already taking more than was in the supplement. A daily intake of 10 mg of lutein may be rather more than is required.
Mortality among the AREDS recruits was about half that in the general population, confirming the healthier habits.
At the time when AREDS 1 was being carried out (1992–8), neither lutein nor zeaxanthin were available as supplements for research purposes. Observational studies suggested that higher intake of these carotenoids protected against AMD. One such study345 came from the AREDS 1 study, where participants aged ≥ 60 years (4519 people) completed a food frequency questionnaire at recruitment. Recruits reporting the highest intake of lutein and zeaxanthin were less likely to have advanced AMD, with ORs versus the lowest quintile of intake of 0.65 (95% CI 0.45 to 0.93) for wet AMD, 0.45 (95% CI 0.24 to 0.86) for GA, and 0.73 (95% CI 0.56 to 0.96) for large or extensive drusen.
AREDS 2
The AREDS 2 trial30 assessed the value of adding lutein and zeaxanthin supplements to the original AREDS formula. It recruited 4203 people aged 50 to 85 years, at high risk of progression to advanced AMD, with either bilateral large drusen or non-foveal GA, or large drusen or non-foveal GA in one eye and advanced AMD in the other eye, in 82 clinical centres. The primary outcome was the development of advanced AMD. Secondary outcomes included three or more lines of VA loss or treatment for wet AMD.
The primary randomisation included arms in which supplements of the long-chain omega-3 polyunsaturated fatty acids, DHA and EPA were given. This results in four arms:
-
placebo
-
lutein 10 mg and zeaxanthin 2 mg
-
DHA 350 mg and EPA 650 mg
-
lutein, zeaxanthin, DHA and EPA.
In the second randomisation, recruits (27% declined) were allocated to the above with:
-
the original AREDS 1 supplement (vitamins C and E, beta-carotene and zinc)
-
AREDS 1 without beta-carotene
-
AREDS 1 with reduced zinc (25 mg instead of 80 mg)
-
AREDS 1 with no beta-carotene and reduced zinc.
The quality of the AREDS 2 trial30,348 was good (Table 35).
| Quality criterion | Risk of bias (high, unclear, low) | Support for statement |
|---|---|---|
| Random sequence generation (selection bias) | Low | Random design using AREDS2 Advantage Electronic Data Capture system |
| Allocation concealment (selection bias) | Low | Undertaken by co-ordinating centre |
| Blinding participants and personnel (performance bias), objective outcomes | Low | Participants, investigators, study coordinators, and all other study personnel masked to treatment assignment |
| Blinding participants and personnel (performance bias), subjective outcomes | N/A | N/A |
| Blinding outcome assessors (detection bias), objective outcomes | Low | States all study personnel masked to treatment assignment, also masked graders at a central reading centre |
| Blinding outcome assessors (detection bias), subjective outcomes | N/A | N/A |
| Incomplete outcome data (attrition bias), objective outcomes | Low | Attrition reasonably balanced between groups |
| Incomplete outcome data (attrition bias), subjective outcomes | N/A | N/A |
| Selective reporting (reporting bias) | Low | No evidence of selective reporting |
| Other biases | Low | No other bias |
The median follow-up was 4.9 years. The mean age at recruitment was 73 years and 9% died during follow-up. Other loss to follow-up was good at only 3%. The recruits were a somewhat select group of highly educated and well-nourished people. Over 65% were educated beyond high-school level, and only 6.7% were smokers. 30
The main results were that:
-
There were no differences among the primary trial arms in progression to advanced AMD. For lutein and zeaxanthin compared with placebo, HR was 0.90 (99% CI 0.76 to 1.07).
-
Neither lowering the zinc dose nor omitting beta-carotene affected progression to advanced AMD.
-
Lutein and zeaxanthin supplements were more effective than beta-carotene – HR 0.82 (95% CI 0.69 to 0.96) for progression to advanced AMD, HR 0.78 (95% CI 0.64 to 0.94) for development of wet AMD and 0.94 (95% CI 0.70 to 1.26) for central GA.
-
Analysis by quintiles of baseline lutein and zeaxanthin, versus no lutein and zeaxanthin, showed a significant reduction in progression to advanced AMD only in the lowest quintile; HR 0.74 (95% CI 0.59 to 0.94).
-
A post hoc analysis across all arms that compared taking supplements with lutein and zeaxanthin versus those without, showed HR of 0.90 (95% CI 0.82 to 0.99; p = 0.04). The effect was seen more on wet AMD (HR 0.89, 95% CI 0.79 to 1.00) than GA (HR 0.92, 95% CI 0.78 to 1.07).
-
Adding DHA and EPA to the AREDS 1 formula conferred no benefit.
-
The reduction in zinc dose made no difference.
-
The VA outcome of ≥ 15 letters lost did not differ among arms.
One finding was that serum levels of lutein and zeaxanthin were lower when recruits also took beta-carotene, probably because beta-carotene competes with lutein and zeaxanthin for absorption.
One weakness of the study was that recruits had a much higher baseline intake of lutein and zeaxanthin than the general population (at least as reflected by NHANES participants).
The conclusion of AREDS 2 was that lutein and zeaxanthin should replace beta-carotene in the AREDS formula, and that the zinc could be reduced to 25 mg.
One problem with taking supplements is that they are bought over the counter, not prescribed. After the AREDS 1 study, Arora and colleagues349 from Wolverhampton identified 22 eye nutrient products but only two matched the dosages used in the trial.
There was some controversy over the role of genetic testing, with a theory put forward by Awh and colleagues350 that only some genetic subgroups benefited from antioxidants and zinc, and that some were actually harmed. This theory was refuted by Chew et al. 122 However, there may be different progression rates according to genetic factors. Seddon et al. 351 reported higher rates of progression in genotypes CFH Y402H (CC) and ARMS2 (TT).
Summary
The first AREDS trial showed that the supplement reduced the risk of progression by about 25%. At 10 years’ follow-up progression to advanced AMD was reduced from 44% to 34%. The AREDS 2 trial showed that beta-carotene should be replaced by lutein and zeaxanthin.
Other studies of lutein and zeaxanthin supplements
Quantity and quality of research
Reviews
Four systematic reviews of lutein alone or with zeaxanthin were identified. One by Ma and colleagues352 was about dietary intake rather than supplements. It was a high-quality review. Ma and colleagues352 concluded that dietary lutein and zeaxanthin did not reduce the incidence of early AMD, with a meta-analysis of six studies giving a RR between highest and lowest quintile of intake of 0.96. However, there was an association with a reduced progress to late AMD (wet AMD and GA) with a meta-analysis of four studies giving a RR of 0.74 (95% CI 0.57 to 0.97, I2 for heterogeneity 0%).
A systematic review of RCTs of lutein and zeaxanthin supplementation by Liu et al. 353 found that it improved both VA and contrast sensitivity. Although there is an error in the Liu review in that it uses a figure of 433 patients for the Beatty 2013 RCT at 36 months, whereas only 58 people were followed for that long. 354
Another high-quality review by Ma and colleagues in 2016355 examined the evidence for the effect of lutein, zeaxanthin and meso-zeaxanthin on macular pigment density. Ma and colleagues355 summarised some key points in the background to this review, including:
-
macular pigment is composed mainly of lutein, zeaxanthin and meso-zeaxanthin
-
the concentration of these carotenoids in the macular region is around 1000 times more than in the blood
-
it is thought that they protect the retina and the RPE from oxidative stress triggered by light
-
their previous trial of supplements had shown a trend towards improvement in BCVA after 4 weeks, in people with early AMD.
The Ma 2016 review355 included 20 RCTs of supplementation, in both people with AMD and healthy individuals. There were significant benefits of lutein, zeaxanthin and meso-zeaxanthin supplementation on MPOD augmentation both in AMD patients and healthy subjects with a dose–response relationship. In both cases, heterogeneity was high, but in effect size rather than direction.
Another systematic review and meta-analysis from China, by Wang and colleagues356 looked only at trials with only lutein supplementation. All these trials were included in the Ma 2016 review. 355 Wang and colleagues356 concluded that lutein supplements improved MPOD significantly but that the improvement in VA seen in five trials was modest and not statistically significant. Trial duration ranged from 4 to 12 months. They did conclude that there was nothing to be gained by using doses of > 10 mg daily.
In addition, one systematic review of vitamins and minerals by Evans et al. 357 was identified that included lutein and zeaxanthin among other supplements including minerals. This review concluded that there was no evidence that any of the supplements reduced the onset of AMD but that there was evidence supporting a reduction in progression. This analysis was dominated by the first AREDS trial (see Report Supplementary Material 5).
Eleven non-systematic reviews were also identified (see Report Supplementary Material 6). Some of these pre-dated AREDS 2, but most of the later ones supported use of the AREDS 2 supplement (Andreatta and El-Sherbiny 2014,358 Broadhead 2015,359 Gregori and Goldhardt 2015,360 Querques and Souied 2014361 and Scripsema 2015341).
Studies
Twenty-two studies were identified that compared the use of lutein and/or zeaxanthin either separately or in combinations with placebo or other active comparators. First authors and years were: Berrow 2013;362 Murray 2013;363 Weigert 2011;364 Wolf-Schnurrbusch 2015;365 Ma 2012;366 Richer 2011;367 Akuffo 2015;368 Huang 2015;192 Peng 2016;369 Wu 2015;370 Arnold 2013;371 Kelly 2014;372 Trieschmann 2007;373 Robman 2007;374 Olk 2015;375 Vishwanathan 2009;376 Bartlett 2007;377 Kelly 2017;378 Richer 2004;379 Dawczynski 2013;380 García-Layana 2013;381 Piermarocchi 2012. 382
There were 15 RCTs (1403 participants), two CCTs (186 participants), three prospective cohort studies (102,724 participants) and two before-and-after studies (112 participants). The studies were undertaken in the USA (5 studies), UK (3 studies, one of which was also undertaken in the Netherlands), China (2 studies), Germany (3 studies), the Netherlands (2 studies), Austria (1 study), Australia (1 study), Ireland (2 studies), Switzerland (1 study), Spain (1 study), Italy (1 study) and Taiwan (1 study). Duration of treatment ranged from 90 days to 2 years for RCTs, 8 to 24 weeks for the CCTs and 10 weeks to 2 years for the cohort studies and before-and-after studies. The duration of follow-up differed between the studies, ranging from 90 days to 3 years for RCTs, 8 weeks to 9 months for the CCTs and 18 weeks to 24 to 26 years for the cohort and before-and-after studies. The majority of studies assessed the effect of the nutrients on intermediate outcomes, such as MPOD, in people with early or intermediate AMD (15 studies: Berrow 2013;362 Murray 2013;363 Wolf-Schnurrbusch 2015;365 Weigert 2011;364 Bartlett 2007;377 Ma 2012;366 Richer 2011;367 Akuffo 2015;368 Huang 2015;192 Peng 2016;369 Arnold 2013;371 Piermarocchi 2012;382 Richer 2004;379 Dawczynski 2013;380 García-Layana 2013381), in a general population (3 studies: Vishwanathan 2009,376 Kelly 2014372 and Kelly 2017378) and in a mixed AMD/healthy population (Trieschmann 2007373).
The cohort study by Robman et al. 374 assessed the effects on progression of early AMD and another cohort study by Olk et al. 375 investigated whether the intervention reduced the risk of CNV in the fellow eye. In the remaining prospective cohort study by Wu et al. 370 the association between the nutrients and the occurrence of AMD was assessed in a general population.
Lutein was compared with:
-
placebo or no supplement (control) (7 RCTs: Berrow 2013,362 Murray 2013,363 Weigert 2011,364 Ma 2012,366 Huang 2015,192 Kelly 2014,372 Richer 2004379)
-
with other doses of lutein (2 RCTs: Ma 2012366 and Huang 2015192)
-
with lutein plus omega fatty acid (1 RCT: Wolf-Schnurrbusch 2015365)
-
with lutein plus vitamins and minerals (Richer 2004379)
-
and with lutein plus zeaxanthin or zeaxanthin (4 RCTs: Ma 2012,366 Huang 2015,192 Richer 2011367 and Kelly 2014372).
Lutein in combination with zeaxanthin or meso-zeaxanthin was compared with no supplements or placebo in two CCTs by Trieschmann373 and Kelly 2017,378 and with meso-zeaxanthin plus lutein plus zeaxanthin by Akuffo et al. 2015. 368 Lutein plus vitamins/minerals was compared with placebo in one RCT by Bartlett et al. 377 and lutein plus zeaxanthin plus ω–3 long-chain polyunsaturated fatty acids (LCPUFAs) were compared at different doses and with placebo in the RCT by Arnold et al. 371 The effects of the combination of lutein plus zeaxanthin were assessed in the before-and-after studies by Peng et al. 369 and Vishwanathan et al. ,376 and with fats in the cohort study by Robman et al. 374 Three RCTs compared lutein, zeaxanthin and other supplements with placebo or no supplement – Piermarocchi 2012,382 Dawczynski 2013380 and García-Layana 2013. 381 Wu et al. 370 looked at a combination of lutein and other carotenoids in the diet and the Olk et al. RCT375 compared zeaxanthin plus triple therapy (including intravitreal bevacizumab, intravitreal dexamethasone, photodynamic therapy with verteporfin) with triple therapy alone. Doses of lutein varied from 6 mg to 20 mg and zeaxanthin 0.6 mg to 17 mg whether provided separately or in combination treatments.
Selection bias was found to be low in two RCTs371,382 and high in one RCT381 and two CCTs. 373,378 In 12 RCTs the risk of selection bias was unclear. 192,362–368,372,377,379,380 The Peng et al. 369 before-and-after study was judged as being of good quality, but the Vishwanathan376 study to be of poor quality. Of the three cohort studies, Wu 2015370 was judged to be of good quality and the others fair quality374 or poor quality. 375
Sample sizes varied. Most studies had < 40 participants per arm but Wu,370 Trieschmann,373 Olk370 and Piermarocchi382 had arms with > 100 participants. The majority of studies had mean ages of 60–70 years. The proportion of male participants varied between 30% and 50% in most studies, although 96% of participants were male in two studies. 367,379 BCVA was reported at baseline in most studies. Most studies received some funding from commercial organisations, but often by provision of the supplement only.
Results
Lutein versus placebo or no active comparison
Seven studies included a lutein-only group and a placebo group or no active comparison. Huang and colleagues192 and Ma and colleagues366 used two different doses of lutein (Table 36) and also had a lutein + zeaxanthin arm. Richer and colleagues379 had a third arm with lutein plus vitamins and minerals.
|
Murray et al. , 2013363 RCT; unclear ROB |
Lutein 10 mg, n = 36 | Placebo, n = 36 | p-value | |
|---|---|---|---|---|
| Change in VA (ETDRS, logMAR), mean (12 months) | 0.01 | 0.04 | < 0.05 | |
|
Weigert et al. , 2011364 RCT; unclear ROB |
Lutein (20 mg to 10 mg), n = 84 | Placebo, n = 42 | p-value | |
| Change in VA, ETDRS letters, at 6 months mean (SD) | 2.1 (0.4) | 1a | 0.07 | |
|
RCT; unclear ROB |
Lutein 10 mg, n = 26 | Lutein 20 mg, n = 27 | Placebo, n = 28 | p-value |
| BCVA, logMAR, at 2 years, mean (SD) | 0.26 (0.15) | 0.28 (0.16) | 0.30 (0.25) | NR |
| Contrast sensitivity at 2 years, log, mean (SD) | ||||
| 3 cycles/degree | 1.47 (0.34) | 1.32 (0.25)b | 1.25 (0.32) | See below |
| 6 cycles/degree | 1.50 (0.33) | 1.54 (0.36)b | 1.25 (0.30) | |
| 12 cycles/degree | 1.10 (0.35) | 1.05 (0.36) | 0.87 (0.33) | |
| 18 cycles/degree | 0.59 (0.45) | 0.65 (0.39) | 0.40 (0.34) | |
|
RCT; unclear ROB |
Lutein 10 mg, n = 27 | Lutein 20 mg, n = 27 | Placebo, n = 27 | p-value |
| Change in BCVA, logMAR, mean (95% CI), 48 weeks | –0.04 (–0.11 to 0.03) | –0.02 (–0.11 to 0.06) | –0.00 (–0.06 to 0.05) | NR |
| Change in CS, log, mean (95% CI), 48 weeks | ||||
| 3 cycles/degree | 0.13 (0.03 to 0.29) | 0.18 (0.07 to 0.28)b | –0.03 (–0.19 to 0.13) | |
| 6 cycles/degree | 0.18 (0.03 to 0.34) | 0.21 (0.10 to 0.32)b | –0.01 (–0.17 to 0.16) | |
| 12 cycles/degree | 0.14 (0.02 to 0.27) | 0.15 (0.02 to 0.28) | 0.02 (–0.15 to 0.19) | |
| 18 cycles/degree | –0.01 (–0.18 to 0.15) | 0.10 (–0.06 to 0.26) | –0.02 (–0.18 to 0.13) | |
|
Richer et al. 2004379 RCT; unclear ROB |
Lutein, n = 29 | Lutein + other, n = 30 | Placebo, n = 30 | p-value |
| Near VA change, letters (95% CI) | 5.4 (2.5 to 8.2) | 3.5 (1.2 to 5.8) | –0.2 (–3.0 to 2.7) | 0.013 |
| Distance VA change, logMAR, right eye/left eye (95% CI) | –0.10 (–0.19 to –0.01)/–0.03 (–0.09 to 0.03) | –0.03 (–0.12 to 0.07)/–0.06 (–0.14 to 0.03) | –0.14 (–0.30 to 0.03)/0.05 (–0.14 to 0.23) | 0.01/NS |
Six studies assessed changes in VA. Murray 2013363 included people with AMD grade 0–4 in one eye and BCVA at least 0.5 logMAR; Weigert 2011364 included those with AMD, grade 2–4, no CNV and VA above 0.4; and Richer 2004379 included people with atrophic AMD and at least one vision-degrading abnormality. These studies reported a benefit in VA for those receiving lutein compared with placebo at 6 months (Weigert364) and 12 months (Murray363 and Richer379) with statistically significant benefit shown by the Murray and Richer studies. In contrast, Berrow 2013362 [in those with age-related maculopathy (ARM) and BCVA at least 0.2 logMAR], Huang 2015192 and Ma 201366 (both in those defined as having early AMD) found no or limited difference in VA for participants receiving either lutein or placebo at 40 weeks, 2 years and 48 weeks, respectively (statistical significance not reported for any of these studies, no data presented in the Berrow and colleagues362 study).
Changes in contrast sensitivity were examined by four RCTs. Compared with placebo, both Huang 2015192 and Ma 2012366 found significantly greater improvement for participants receiving lutein 20 mg (but not 10 mg) at 3 cycles/degree and 6 cycles/degree (see Table 36). Richer 2004379 reported significant within-group improvements from baseline at 3, 6 and 12 cycles/degree in the right eyes and at 6 and 12 cycles/degree in the left eyes of both lutein groups (data presented in a figure only, see Report Supplementary Material 5). Berrow 2013362 found no significant difference between lutein and placebo at 40 weeks (no data or p-value reported, therefore, not tabulated below). 362
Six RCTs192,363,364,366,372,379 assessed the effects of lutein supplementation on MPOD outcomes. Four reported an increase in MPOD. Ma366 reported no change and Kelly 2014372 found a slight decrease after 90 days of lutein supplementation in healthy volunteers (this study also had a zeaxanthin arm, reported below).
Adverse events
Four studies reported AEs. 192,364,366,379 Of these studies, only two (Weigert364 and Richer379) reported any actual events, reporting similar rates in the groups receiving lutein or placebo.
Overall, the picture is mixed. Supplementation with lutein alone has beneficial but modest effects on BCVA and contrast sensitivity in some of the studies, but the significance is doubtful. Lutein does increase MPOD.
A small trial of supplementation with lutein 20 mg daily is starting in Japan but with only 40 patients it is unlikely to make a major contribution. 385
Lutein plus zeaxanthin
Eleven studies are discussed in this section.
Four studies assessed the effect of combined lutein and zeaxanthin supplementation on VA in people with early to moderate AMD, including three RCTs (Huang et al. 2015,192 Ma et al. 2012366 and Richer et al. 367) and the before-and-after study by Peng et al. 369 (which confusingly, has ‘randomised controlled trial’ in the title). Huang and colleagues192 and Ma and colleagues366 found small improvements on BVCA logMAR for lutein (10 mg) plus zeaxanthin (10 mg) and for placebo at 2 years and 48 weeks follow-up, respectively, but little difference between groups (Table 37).
|
RCT; unclear ROB |
Lutein + zeaxanthin, n = 27 | Placebo, n = 28 | p-value | |
|---|---|---|---|---|
| BCVA, logMAR, at 2 years, mean (SD) | 0.27 (0.24) | 0.30 (0.25) | NR | |
| Contrast sensitivity at 2 years, log, mean (SD) | ||||
| 3 cycles/degree | 1.39 (0.39) | 1.25 (0.32) | See below | |
| 6 cycles/degree | 1.50 (0.36) | 1.25 (0.30) | ||
| 12 cycles/degree | 1.09 (0.35) | 0.87 (0.33) | ||
| 18 cycles/degree | 0.74 (0.33)a | 0.40 (0.34) | ||
|
RCT; unclear ROB |
Lutein 20 mg + zeaxanthin 10 mg, n = 27 | Placebo, n = 27 | p -value | |
| Change in BCVA, logMAR, mean (95% CI), 48 weeks | –0.04 (–0.10 to 0.01) | –0.00 (–0.06 to 0.05) | NS | |
| Change in CS, log, mean (95% CI), 48 weeks | ||||
| 3 cycles/degree | 0.18 (0.05 to 0.32) | –0.03 (–0.19 to 0.13) | NS | |
| 6 cycles/degree | 0.15 (0.04 to 0.31) | –0.01 (–0.17 to 0.16) | ||
| 12 cycles/degree | 0.12 (–0.04 to 0.28) | 0.02 (–0.15 to 0.19) | ||
| 18 cycles/degree | 0.09 (–0.11 to 0.29) | –0.02 (–0.18 to 0.13) | ||
|
Richer et al. , 2011 367 RCT; unclear ROB |
Zeaxanthin 8 mg + lutein 9 mg n = 25 | Lutein, 9 mg (faux placebo) n = 10 | p- value | |
| ETDSR Colenbrander average eye near high-contrast VA (SE) at 12 months | 92.8 (5.9) | 98.9 (5.7) | NR | |
| Colenbrander average eye low-contrast near VA, 12 months | 81.5 | 88.2 | NR | |
| Contrast sensitivity function, area under the curve at 5 special frequencies (SE), 12 months | 247.1 (35) | 310.5 (33.8) | NR | |
|
Kelly et al. , 2017 378 CCT; High risk of bias |
Carotenoid-enriched eggs, n = 25 | Placebo eggs, n = 25 | p- value | |
| BCVA mean (SD) final visit | 107.7 (4.45) | 105.4 (4.78) | p = 0.035 | |
|
Peng et al. , 2016 369 B + A; GQ |
Lutein complex (lutein 12 g + zeaxanthin 2 mg) n = 56 | p- value | ||
| BCVA (LogMAR), mean (SD) 5.5 months | 12.38 (3.41) | NR | ||
|
Akuffo et al. , 2015 368 RCT; unclear ROB |
Lutein 20 mg + zeaxanthin 2 mg n = 13 | Meso-zeaxanthin 10 mg + Lutein 10 mg + zeaxanthin 2 mg n = 16 | Meso-zeaxanthin 17 mg + Lutein 3 mg + zeaxanthin 2 mg n = 12 | p -value |
| Letter contrast sensitivity at 36 months, mean (SD),% change from baseline | ||||
| 1.2 cpd | 1.89 (0.16), 1 | 1.86 (0.18), 9 | 1.82 (0.20), 4 | NR |
| 2.4 cpd | 1.87 (0.17), 6 | 1.81 (0.21), 8 | 1.78 (0.21), 9 | NR |
| 6.0 cpd | 1.60 (0.15), 13 | 1.52 (0.25), 11 | 1.52 (0.27), 24 | NR |
| 9.6 cpd | 1.35 (0.16), 18 | 1.27 (0.34), 20 | 1.30 (0.22), 38 | NR |
| 15.15 cpd | 1.02 (0.23), 36 | 0.91 (0.38), 30 | 0.97 (0.25), 59 | NR |
Richer and colleagues,367 in their study of people with early and moderate AMD, identified limited differences on the ETDRS Colenbrander high-contrast VA score at 12 months’ follow-up, comparing zeaxanthin (8 mg) plus lutein (9 mg) and lutein alone (9 mg). On low-contrast VA, significance testing was not reported. The study also had a zeaxanthin only arm, see Supplementation with zeaxanthin alone below. The before-and-after study by Peng and colleagues369 reported that lutein (12 mg) plus zeaxanthin (2 mg) in people with early stage AMD and soft drusen, led to a statistically significant improvement in BCVA logMAR at 5.5 months’ follow-up (p < 0.05).
Kelly and colleagues378 compared eggs enriched with lutein and meso-zeaxanthin with standard eggs in healthy volunteers. After 8 weeks, a significant difference in BCVA was found in favour of the enriched eggs (NCT00527553).
Four RCTs (Huang 2015,192 Ma 2012,366 Richer 2011367 and Akuffo 2015368) assessed the effects of lutein and zeaxanthin on measures of contrast sensitivity in people with early mild to moderate AMD (mostly defined as soft drusen and/or pigmentary abnormalities with no GA or CNV). In people with early AMD, Akuffo and colleagues,368 with three active treatments and no controls, reported changes from baseline to 36 months in contrast sensitivity that did not differ among the treatments (i.e. lutein 20 mg plus zeaxanthin 2 mg, meso-zeaxanthin 10 mg plus lutein 10 mg plus zeaxanthin 2 mg, meso-zeaxanthin 17 mg plus lutein 3 mg plus zeaxanthin 2 mg). In Huang 2015192 and Ma 2012366 comparisons between lutein (10 mg) plus zeaxanthin (10 mg) and placebo did not differ significantly except in 18 cycles/degree in Huang192 (see Table 37). Richer and colleagues367 found that contrast sensitivity, assessed using area under the curve on five spatial frequencies, for people receiving zeaxanthin (8 mg) plus lutein (9 mg) and lutein alone (9 mg), appeared to show those receiving lutein appearing to benefit most (but statistical significance was not reported). In healthy volunteers, Kelly and colleagues378 found an improvement in the enriched egg group at 15.15 cycles/degree (p = 0.046); other data were not reported.
The Akuffo 2015368 RCT assessed progression in participants all receiving active interventions: lutein 20 mg plus zeaxanthin 2 mg, meso-zeaxanthin 10 mg plus lutein 10 mg plus zeaxanthin 2 mg, and meso-zeaxanthin 17 mg plus lutein 3 mg plus zeaxanthin 2 mg on the 11-step AREDS scale. 368 They found no statistically significant differences when assessing people making a two-step increase on the AREDS scale (p = 0.29) or for those likely to have either a low or high risk of progression to advanced AMD.
Two prospective cohort studies (Table 38) examined factors that may influence the development and progression of AMD, including the intake of lutein and zeaxanthin. Robman and colleagues374 found that an intake of lutein and zeaxanthin was associated with an increased OR of AMD progressing in people with early AMD, whether considering the worse eye, either eye, an increase ≥ 2 steps in the grades of size, total number, area occupied by a lesion and spread, or a qualitative assessment (i.e. better, worse, same) from macular photographs. The association found with lutein and zeaxanthin and progression of the worse eye was statistically significant (p = 0.02), but this depended on the definition of the progression used.
|
Robman et al. , 2007374 Cohort study: FQ |
Dietary intake of lutein, zeaxanthin and fats, n = 252 All 7 years’ follow-up |
p-value |
|---|---|---|
| Progression of AMD – Definition 1 | 2.65 (95% CI 1.13 to 6.22) | 0.02 |
| Progression of AMD – Definition 2 | 1.72 (95% CI 0.78 to 3.78) | 0.18 |
| Progression of AMD – Definition 3 | 1.84 (95% CI 0.84 to 2.96) | 0.13 |
| Definition 1: increase in AMD severity 1 or more levels in worse affected eye | ||
| Definition 2: increase in AMD severity 1 or more levels in either eye; or an increase in ≥ 2 steps in grades of size, total number, area occupied by a lesion and spread | ||
| Definition 3: qualitative (better, worse, same) from macular photographs | ||
|
Wu et al. , 2015370 Cohort study: GQ |
Dietary intake of lutein and zeaxanthin, n = 102,046 | p-value |
| Advanced AMD – RR | 0.59 (95% CI 0.48 to 0.73) | < 0.001 |
| Intermediate AMD – RR | 0.93 (95% CI 0.78 to 1.12) | 0.42 |
| Comparison of quintile 1 with 5 of predicted plasma carotenoid scores | ||
In contrast, Wu and colleagues370 reported that lutein and zeaxanthin was associated with a statistically significantly reduced risk of developing advanced AMD among people from a general population (i.e. without a diagnosis of AMD at baseline) (p < 0.001) (see Table 37). Although the RR of intermediate AMD was decreased with lutein and zeaxanthin, its effects were not statistically significant (p = 0.42).
The Huang 2015,192 Ma 2012,366 Akuffo 2015368 and Vishwanathan 2009376 RCTs, the Arnold 2013371 and Trieschman373 CCTs and the Kelly 2017378 before-and-after study assessed changes to MPOD (Table 39). Akuffo 2015368 found no statistically significant difference in MPOD at follow-up for the different active treatments, whereas some benefit was shown when active treatments were compared with the placebo or no supplement control in the Arnold 2013371 RCT and the Trieschmann CCT. 373 Arnold and colleagues371 found that participants with non-exudative AMD receiving a supplement of lutein, zeaxanthin and LCPUFAs at either single or double dose experienced a statistically significant increase in MPOD when compared with those receiving placebo (p-value not reported). Comparison between the two doses of the active supplement showed no significant difference in MPOD (p-value not reported). Akuffo and colleagues368 reported that change from baseline to follow-up at 36 months did not differ between the three active interventions of lutein 20 mg plus zeaxanthin 2 mg, meso-zeaxanthin 10 mg plus lutein 10 mg plus zeaxanthin 2 mg, and meso-zeaxanthin 17 mg plus lutein 3 mg plus zeaxanthin 2 mg (p-value not reported). In a CCT of people with no or minimal lens opacity, Trieschmann and colleagues373 identified a statistically significant difference in MPOD at 9 months’ follow-up for participants receiving lutein (12 mg) plus zeaxanthin (1 mg) when compared with the control who received no supplements (p < 0.0008). In a before-and-after study assessing the effects of egg yolk consumption on MPOD in people without AMD, Vishwanathan and colleagues376 found no statistically significant difference between baseline and follow-up at 5 or 14 weeks (p-value not reported).
|
Trieschmann et al. , 2007373 CCT; high ROB |
Lutein 12 mg and zeaxanthin 1 mg supplement n = 108 | No supplement (control) n = 28 | p-value | |
|---|---|---|---|---|
| MPOD at 0.5° eccentricity mean (SEM) difference at | ||||
| Baseline | 0.504 (0.197) | 0.525 (0.189) | 0.6 | |
| 9 months’ follow-up | 0.1 (0.009) | 0.03 (0.02) | < 0.0008 | |
|
Arnold et al. , 2013371 RCT; low ROB |
Supplement of lutein 10 mg, zeaxanthin 1 mg and LCPUFAs n = 50 | Supplement of lutein 20 mg, zeaxanthin 2 mg and LCPUFAs, n = 54 | Placebo n = 40 | p-value |
| MPOD units (degrees) | 0.22 | 0.25 | –0.01 | NR |
| Vishwanathan et al., 2009376 | Egg yolk consumption (lutein and zeaxanthin) n = 37 | p-value | ||
| B + A; PQ | ||||
| MPOD, mean (SE) at week 5 (end of 2-egg period) | ||||
| 0.25° | 0.55 (0.04) | NR | ||
| 0.5° | 0.52 (0.04) | NR | ||
| 1° | 0.37 (0.03) | NR | ||
|
Akuffo et al. , 2015368 RCT; unclear ROB |
Lutein 20 mg + zeaxanthin 2 mg, n = 13 | Meso-zeaxanthin 10 mg + Lutein 10 mg + zeaxanthin 2 mg, n = 16 | Meso-zeaxanthin 17 mg + Lutein 3 mg + zeaxanthin 2 mg, n = 12 | p-value |
| MPOD at 36 months, mean (SD),% change from baseline | ||||
| 0.25° eccentricity | 0.72 (0.24), 41 | 0.76 (0.23), 52 | 0.85 (0.25), 67, 0.000 | NR |
| 0.5° eccentricity | 0.62 (0.26), 51 | 0.64 (0.20), 42 | 0.68 (0.20), 74, 0.000 | NR |
| 1.0° eccentricity | 0.45 (0.19), 50 | 0.46 (0.15), 59 | 0.52 (0.16), 100, 0.000 | NR |
| 1.75° eccentricity | 0.23 (0.19), 35 | 0.28 (0.11), 87 | 0.34 (0.14), 183, 0.000 | NR |
Adverse events
Four studies reported AEs,192,366,367,378 although only Richer and colleagues367 identified any: two deaths unrelated to any intervention and a case of pneumonia.
Summary
The lutein + zeaxanthin studies show a mixed picture, partly because some studies had no placebo arm, and showed little or no difference among active arms. Most studies had quite small numbers of recruits. The cohort studies by Robman374 and Wu370 gave contrasting results on progression of AMD.
Supplementation with zeaxanthin alone
Two studies by Richer 2011367,372 and Olk 2015375 investigated zeaxanthin alone (Table 40).
| Olk et al., 2015375 cohort study: PQ | Triple therapy, n = 160 | Triple therapy + zeaxanthin, n = 80 | p-value |
|---|---|---|---|
| Fellow eyes that developed CNV (%) 12–24 months | 12.5 | 6.25 | 0.03 |
|
Richer et al. , 2011367 RCT; unclear ROB |
Zeaxanthin 8 mg, n = 25 | Lutein 9 mg (faux placebo), n = 10 | p-value |
| ETDSR Colenbrander Mixed Constrast Reading Card (Precision Vision, IL, USA) average eye near high-contrast VA (SE) at 12 months | 96.8 (8.35) | 98.9 (5.7) | NR |
| Colenbrander average eye low-contrast near VA, 12 months | 81.5 | 88.2 | NR |
| Contrast sensitivity function, area under the curve at 5 special frequencies (SE), 12 months | 254.7 (35.2) | 310.5 (33.8) | NR |
The prospective cohort study by Olk and colleagues375 assessed the effects of zeaxanthin plus triple therapy versus triple therapy alone [bevacizumab (Avastin® Roche, Basel, Switzerland), dexamethasone, photo-dynamic therapy with verteporfin (Visudyne®, Roche, Basel, Switzerland)] on the development of CNV in the fellow eyes of people with unilateral CNV. A significantly lower proportion of people who received zeaxanthin and triple therapy developed CNV in the fellow eye than those receiving triple therapy (p = 0.03). The Olk study375 had its weaknesses. It compared two consecutive cohorts of people with unilateral wet AMD, with zeaxanthin 20 mg daily being added after a specific date, and added a control group from a study of six RCTs of anti-VEGF treatment. The people were already taking an AREDS 1 supplement that may, because of the beta-carotene content, have reduced the bioavailability of zeaxanthin. Olk et al. 375 suggested that a RCT of zeaxanthin supplementation should be done, but that seems unnecessary after the AREDS trials. The reduction in progression to wet AMD in the zeaxanthin cohort was statistically significant (p = 0.03).
In the RCT by Richer and colleagues367 zeaxanthin 8 mg compared with lutein 9 mg did not appear to lead to any differences in high-contrast VA or low-contrast near VA.
Kelly and colleagues372 found no difference between groups in MPOD after 90 days of zeaxanthin supplementation from enriched eggs in healthy volunteers (this study also had lutein arms, see above) but had only about 20 recruits per arm. Their trial was more about the feasibility of increasing lutein and zeaxanthin intake by eggs rich in those carotenoids, by feeding hence diets with enriched amounts, sourced from maize and marigold sources.
In summary, there is little evidence on supplementation with zeaxanthin alone, the most significant being the reduction in progress to wet AMD reported by Olk et al. 375
Lutein plus zeaxanthin plus other nutrients versus control
Four studies compared lutein plus zeaxanthin plus vitamins and minerals (Piermarocchi382 and the Dawczynski380) or lutein plus zeaxanthin plus DHA (García-Layana381) with a placebo or no supplement (see Report Supplementary Material 5 for dose details). In the CARMA (Carotenoids in Age-related Maculopathy) trial, Beatty and colleagues354,386 randomised people to supplementation with lutein, zeaxanthin, vitamins E and C, zinc 20 mg and copper 0.4 mg daily, using the Ocuvite preparation (Bausch and Lomb, Berlin, Germany).
Piermarocchi and colleagues382 included people with dry AMD in at least one eye, having extensive intermediate drusen and at least one large drusen or GA not involving the macula centre, and BCVA in trial eye of ≥ 20/32. They found significantly better VA in the supplement group (lutein, zeaxanthin, astaxanthin, zinc and copper and antioxidant vitamins) after 24 months’ follow-up compared with no supplementation (Table 41).
|
Piermarocchi et al. 2012382 RCT; low ROB |
Lutein + zeaxanthin + vitamins/minerals, n = 84 | Control, n = 26 | p-value | |
|---|---|---|---|---|
| Mean (SD) BCVA at 24 months, ETDRS letter score | 81.4 (7.2) | 76.8 (8.9) | p = 0.003 | |
| Mean (95% CI) change in BCVA at 24 months, ETDRS letter score | –0.02 (–1.42 to 1.36) | –4.18 (–7.34 to –1.01) | p = 0.008 | |
| Mean (95% CI) change in contrast sensitivity at 24 months | 2 (0.80 to 3.19) | –1.15 (–2.86 to 0.54) | p = 0.01 | |
| Development of CNV (%) |
(n = 103) 12.7 |
(n = 43) 9.3 |
p = 0.760 | |
| NEIVFQ-25 composite score, mean (SD) 24 months | 82.1a (15.9) | 74.2b | NR | |
| NEIVFQ-25 composite score, mean (95% CI) change, 24 months | 3.6 (0.50 to 6.81) | –8.7 (–16.54 to –0.97) | NR | |
|
Dawczynski et al. 2013380 RCT; unclear ROB |
Lutein + zeaxanthin + vitamins/minerals dose 1, n = 50 | Lutein + zeaxanthin + vitamins/minerals dose 2, n = 55 | Placebo, n = 40 | p-value |
| BCVA, logMAR at 12 months | 0.104 (0.18) | 0.064 (0.16) | 0.127 (0.16) | See belowc |
| BCVA change in reading letters at 12 months, mean (SD) | 1.46 (2.8) | 2.02 (3.1) | 0.08 (2.8) | See belowd |
|
García-Layana et al. 2013381 RCT; high ROB |
Lutein/zeaxanthin/DHA, n = 23 | Placebo, n = 21 | p-value | |
| ETDRS letters, mean (SEM) at 1 year | 74.3 (9.2) | 75.9 (5.8) | NS | |
| Contrast sensitivity letters, mean (SEM) at 1 year | 26 (5) | 26 (6) | NS | |
|
Beatty et al., 2013354 RCT; low ROB |
Lutein + zeaxanthin + vitamins/minerals, n = 216 | Placebo, n = 217 | ||
| BCVA at 12 months mean (SD) | 79.7 (8.9) | 80.4 (6.5) | ||
Dawczynski and colleagues380 carried out a three-arm trial comparing two different doses of lutein plus zeaxanthin plus vitamins and minerals in people with non-exudative AMD in at least in one eye. They found a statistically significant change in BCVA reading letters at 12 months with both doses of lutein compared with placebo. However, differences in BCVA by logMAR at 12 months did not reach statistical significance. García-Layana and colleagues381 included people with early AMD and found no significant difference in VA between lutein plus zeaxanthin plus DHA and placebo at 12 months’ follow-up.
A significantly different change in contrast sensitivity at 24 months was found by Piermarocchi and colleagues,382 but no difference was found by García-Layana and colleagues. 381 Dawczynski and colleagues380 did not report contrast sensitivity.
Piermarocchi and colleagues382 found no difference in the development of CNV between groups. However, an improvement in NEIVFQ-25 score at 24 months was found in the supplement group, compared with a deterioration in the control group.
Beatty and colleagues reported the 36 month secondary outcomes in a full paper354 but the primary outcome of BCVA at 12 months only in a letter386 which followed the paper. There was no difference in VA at 12 months, after which numbers dropped off markedly because 12 months was the minimum follow-up.
Two studies reported MPOD outcomes. Dawczynski and colleagues380 reported a ‘considerable increase’ in MPOD. García-Layana and colleagues381 reported that after 12 months, MPOD increased by 0.059 units on placebo and 0.162 units on lutein + DHA (p < 0.05).
Adverse events
Adverse events were reported by Peirmarochi et al.,382 which found no significant systemic or ocular AEs related to the nutritional supplementation or adverse reactions leading to study withdrawal or discontinuation.
Summary
The main problem with such studies is that the relative contributions of the components cannot be assessed – we cannot say whether all the effects are due to lutein and zeaxanthin or whether the additional supplements have any marginal value. The other problem is that durations of 12 months are too short for changes in VA.
Lutein plus vitamins and minerals versus placebo
Lutein plus vitamins and minerals (retinol, vitamins C and E, zinc and copper) was compared with placebo in people with no ocular pathology, other than ARM, in at least one eye by Bartlett and colleagues. 377 VA outcomes were not reported in this small study. Although mean contrast sensitivity score at 9 months improved for people in the lutein plus vitamins and minerals at 9 months and worsened for participants receiving placebo, the difference between the groups was not statistically significant (p = 0.366).
Richer et al. 379 had a three-arm RCT comparing results in groups receiving lutein 10 mg alone, lutein 10 mg plus a mixture of 36 other ingredients including minerals, vitamins and other compounds, including black pepper (OcuPower, from Nutraceutical Sciences Institute, Boynton Beach, FL, USA), and maltodextrin as placebo. They found no difference between the lutein and OcuPower arms.
Adverse events
Bartlett and colleagues377 reported that no AEs were experienced as a result of either intervention.
Summary
The one study that assessed the effect of adding a package of minerals and other compounds found no advantage over lutein alone. 379
Lutein versus lutein plus omega-3 fatty acid
Wolf-Schnurrbusch and colleagues365 compared the efficacy of lutein and lutein plus omega-3 fatty acid (DHA + EPA 160 mg daily) in people with early to intermediate AMD in a RCT with unclear risk of selection bias. There were no changes in BCVA letter score at 6- or 12-month follow-up for either group (p-value not reported). Despite improvements in contrast sensitivity for the lutein group at 6 months’ follow-up, the statistically significant benefit compared with lutein plus omega had disappeared by 12 months’ follow-up (6-month post-treatment cessation). The study reported that MPOD and contrast sensitivity improved on lutein alone but not when DHA and EPA were added. The authors suggest that a possible explanation is that adding the omega-3 acids might reduce the bioavailability of lutein.
Adverse events
None reported with either lutein nor lutein plus omega groups.
Summary
There appeared to be no added value, and possible disbenefit, from adding omega-3 fats.
Summary: other studies of lutein and zeaxanthin supplements
Twenty-two studies (15 RCTs, two CCTs, two before-and-after studies and three cohort studies) compared lutein and/or zeaxanthin either separately or in combinations of intervention with themselves or inactive controls (i.e. placebo).
Three out of five lutein-only studies showed some improvement in VA, contrast sensitivity and MPOD.
The trials of lutein + zeaxanthin showed few differences but several compared different active arms, such as lutein + zeaxanthin versus lutein alone, with no placebo groups. Several studies reported reduced progression.
The three studies of zeaxanthin alone gave mixed results. One compared zeaxanthin alone versus lutein alone and found no differences. One found that zeaxanthin halved progression to wet AMD.
Several studies of combinations of lutein plus zeaxanthin plus other supplements reported benefit in BCVA and MPOD, but this could have been due to the lutein plus zeaxanthin, with other components of unproven benefit.
Fatty acids and antioxidants
One of the papers from AREDS 1387 followed up 1929 participants at moderate to high risk of progression (AREDS category 3a), and reported incidence of advanced AMD by baseline quintiles of ‘long-chain omega-3 polyunsaturated fatty acids’ – in effect DHA and EPA. Those in the highest quintile of baseline DHA and EPA had the lowest risk of progression: OR 0.65 (95% CI 0.45 to 0.92) for GA and 0.68 (95% CI 0.49 to 0.94) for wet AMD.
Reviews
One Cochrane review of fatty acids (Lawrenson and Evans 2015388) and one non-systematic review of fatty acids (Souied 2016389) were identified. Fatty acids were also mentioned in five reviews of multiple interventions but these were generally inconclusive except for the AREDS trials. 9,358,390–392
The Cochrane review388 concluded that there is no evidence from RCTs to support increasing omega 3 intake for preventing or slowing the progression of AMD. The systematic review included two studies, the AREDS 2 trial, already described, and the Nutritional AMD Treatment 2 (NAT-2) study by Souied et al.,393 described below.
The review by Souied389 noted that there was quite strong epidemiological evidence that diets rich in omega 3 fatty acid were protective against AMD, but that there was lack of evidence from intervention studies, including AREDS. However, they make the point that perhaps the well-nourished AREDS recruits had little to gain, but that people with low omega-3 fatty acid intake might gain more.
A systematic review by Huang et al. 394 examined the relationship between serum homocysteine, folic acid and vitamin B12 levels, and the risk of AMD. The authors concluded that AMD is associated with elevated homocysteine levels and decreased vitamin B12 levels, and that plasma homocysteine may act as a modulator of the risk for AMD.
One systematic review by Evans 2008357 and six non-systematic reviews (Bartlett 2003,395 Broadhead 2015,359 Evans and Lawrenson 2014,390 Gregori and Goldhardt 2015,360 Johnson 2010,391 Schmidl 2015396 and Scripsema 2015341) of antioxidants and nutritional supplements were identified. The Bartlett 2003395 review has been superseded by more recent ones. The Evans and Lawrenson 2014390 review summarises and updates their two Cochrane reviews. Gregori and Goldhardt360 described mainly the AREDS trials. The Johnson review391 preceded AREDS 2.
The Broadhead review, while not a systematic review, provides a very good summary of key issues, including:
-
consumption of leafy green vegetables and fish is recommended as a way of reducing the risk of AMD
-
a lot of studies have shown an inverse relationship between fish consumption and AMD
-
this may be related to the presence of omega-3 fatty acids in fish and carotenoids in green vegetables
-
the beneficial effects of eating a diet rich in fish are not replicated in intervention trials of DHA and EPA supplements
-
the role of omega-3 fatty acids in the diet may be linked with reduced intake of omega-6 fatty acids (such as linoleic) from red meats and cheese
-
it may be the overall diet that matters [increased fish consumption (2–4 helpings a week) may mean reduced red meat consumption]
-
carbohydrates with a low glycaemic index (meaning a slow rise in blood glucose after consumption) instead of those with a high glycaemic index, may reduce the risk of AMD
-
if supplements are to be used, the AREDS 2 formula should be used
-
all patients with AMD should be advised to eat fish at least twice a week, eat more green leafy vegetables, and restrict high glycaemic index foods.
The Evans review357 concluded that people with AMD, or early signs of the disease, may experience some benefit from taking supplements as used in the AREDS trial, but that current evidence does not support the use of vitamin E or beta-carotene supplements to prevent AMD. However, it did support the use of the AREDS supplement.
The present systematic review includes three studies from the Evans systematic review357 (Teikara 1998,397 Christen 2007398 and Taylor 2002399) because these studies add to the totality of the evidence base for the research question of relevance here.
Five reviews of multiple interventions (Buschini 2015,310 Girmens 2013,67 Hanus 2012,9 Querques 2014361 and Sin 2013392) covered nutritional supplements and some epidemiological relationships. They came to no firm conclusions other than those by Hanus9 and Sin392 recommending the AREDs supplement.
Studies
The AREDS 2 trial, reported above, found no advantage in adding DAH and EPA to the original AREDS formula.
Five studies evaluated fatty acids including three RCTs by Feher et al. 2005,400 Souied et al. 2013393 and Tao et al. 2016401 and two cohort studies by Reynolds et al. 2013402 and Cougnard-Gregoire et al. 2016. 403 A total of 251 participants were randomised to a fatty acid intervention and 255 to placebo, while the cohort studies assessed dietary intake in a total of 3494 healthy participants. The studies were conducted in France (n = 2), the USA (n = 1), Hungary (n = 1) and China (n = 1). Follow-up ranged from 3 months to 3 years in the RCTs, and 7–12 years in the cohort studies. The RCTs had an unclear risk of selection bias, and the cohort studies were assessed as fair quality403 and poor quality. 402 Where reported, mean age was 63–74 years and 31–56% were men.
A recent study by Wu and colleagues404 combined cohorts from the Nurses’ Health Study (75,889 women) and the Health Professionals Follow-up Study (38,961 men), in which 1589 people developed intermediate AMD (defined as at least one of intermediate drusen, pigmentary abnormalities, large drusen or non-central GA) and 1356 developed advanced (curiously 96% wet) AMD. Comparison of extreme quintiles of DHA showed a lower incidence with higher intake in intermediate AMD (HR 0.78, 95% CI 0.66 to 0.92) but not in advanced AMD (HR 1.01). The HR for intermediate AMD in those consuming five or more helpings of fatty fish was 0.61 (95% CI 0.46 to 0.81) compared with those who rarely are fatty fish. 404
Results
Fatty acids
Feher and colleagues 2005400 randomised people with early bilateral AMD and BCVA between 0.8 and 0.4 (Snellen chart) in the most affected eye to Phototrop (Sigma-Tau Industrie, Pomezia, Italy), which contains acetyl-L-carnitine (an amino acid) 100 mg, EPA 230 mg, DHA 160 mg and co-enzyme Q10 10 mg, or to a soy oil placebo. After 12 months no difference in change in VA was found between groups (Table 42), but a significantly greater proportion had VA categorised as improved or unchanged in the phototrop group compared with placebo (OR 2.48; p = 0.027). In addition, the drusen area decreased by 15% in the phototrop group while it increased by 11% in the placebo group (p = 0.045). Other outcomes, including other measures of VA and secondary analyses on the fellow eye can be seen in Report Supplementary Material 5.
|
Feher et al. 2005400 RCT; unclear ROB |
Phototrop, N = 48 | Placebo, N = 53 | p-value |
|---|---|---|---|
| Mean (SD) change in VA at 12 months, logMAR (study eye) | –0.009 (0.23) | 0.14 (0.23) | p = ns |
| Change in VA, logMAR, at 12 months (study eye) | |||
| Improved or unchanged (%) | 75 | 55 | OR 2.48, 0.027 |
| Deteriorated | 25 | 45 | |
| Drusen-covered area [ratio of drusen area at 12 months to screening (SD)] |
n = 46 0.85 (0.39) |
n = 52 1.11 (0.65) |
0.045 |
|
Souied et al. 2013393 RCT; unclear ROB |
DHA, N = 134 | Placebo, N = 129 | p-value |
| Mean (SD) BCVA change, logMAR at 3 years | –0.155 (0.297) | –0.116 (0.258) | 0.311 |
| Proportion with a decrease of > 15 letters on ETDRS at 3 years | 17.8 | 14.3 | 0.469 |
| Mean time to occurrence of CNV (months) | 19.5 (10.9) | 18.7 (10.6) | 0.613a |
| Proportion in whom CNV developed over 3 years | 28.4 | 25.6 | |
|
Tao et al. 2016401 RCT; unclear ROB |
α -lipoic acid, N = 50 | Placebo, N = 50 | p-value |
| BCVA (LogMAR), mean (SD) at 3 months | 0.66 (0.41) | 0.63 (0.42) | NS |
| Contrast sensitivity, mean (SD) | |||
| 3 cycles/degree, log | 1.02 (0.28) | 0.87 (0.29) | < 0.05 |
| 6 cycles/degree, log | 1.26 (0.39) | 1.15 (0.36) | NS |
| 12 cycles/degree, log | 0.92 (0.30) | 0.88 (0.35) | NS |
| 18 cycles/degree, log | 0.51 (0.34) | 0.44 (0.31) | NS |
| CLVQOL, mean (SD) | 82.6 (19.36) | 72.81 (18.05) | < 0.05 |
|
Cougnard-Grégoire et al. 2016403 Cohort study; FQ |
Olive oil, N = 936 eyes | No olive oil, N = 333 eyes | |
| No AMD (n = 945 eyes), n eyes (%) | 712 (75.3) | 233 (24.7) | |
| Early AMD (n = 268 eyes), n eyes (%) |
191 (71.3) OR 0.84 (95% CI 0.59, 1.24), p = 0.36b |
77 (28.7) | |
| Late AMD (n = 56 eyes), n eyes (%) |
33 (58.9) OR 0.44 (95% CI 0.21, 0.91), p = 0.03b |
23 (41.1) | |
The Souied 2013393 RCT compared DHA with placebo in people with early ARM (defined as any drusen or RPD with or without pigmentary changes) and VA of ≥ +0.4 logMAR units in the study eye, and nAMD in the fellow eye. After 3 years of treatment, no significant differences were found between groups in the mean time to occurrence of CNV, the proportion developing CNV, change in BCVA, the proportion with a decrease of > 15 letters on ETDRS (see Table 42) or drusen area (see Report Supplementary Material 5). GA was not reported.
Tao and colleagues 2016401 randomised people with dry AMD to 3 months’ treatment with α-lipoic acid (a fatty acid) or a vitamin C placebo. No significant differences were found between groups immediately following the intervention in BCVA or contrast sensitivity at 6, 12 or 18 cycles/degrees (see Table 42). However, significantly higher contrast sensitivity at 3 cycles/degree and Chinese-Version Low Vision Quality of Life score was found in the α-lipoic acid group in favour of the intervention.
Reynolds and colleagues 2013402 assessed intake of dietary omega-3 fatty acids and other fats in 2531 previous participants of the AREDs RCT, with 525 eyes that progressed and 4165 that did not. People were assigned a grade of no AMD, early AMD, intermediate AMD, or two forms of advanced or late stage AMD (GA and neovascular). There were 16.9% of participants progressing to GA over 10 years. In multivariate analysis controlling for covariates and genetic variants (see Report Supplementary Material 5 for details), there was a significant trend for a reduction in risk of progression to GA with increasing intake of DHA [p-trend = 0.008, HR 0.68 for quintile 1 vs. quintile 5 (95% CI 0.48 to 0.94)] and intake of DHA and EPA (p = 0.02). Further multivariate analysis found differing effects in people with different risk genotypes.
The prevalence of early and late AMD was compared between regular users and non-users (or occasional users) of olive oil among community-dwelling people, aged at least 65 years, in Bordeaux in the Alienor study by Cougnard-Grégoire and colleagues 2016. 403 Regular consumption of olive oil was found to be significantly associated with a lower risk of late AMD (OR 0.44, 95% CI 0.21 to 0.91; p = 0.03) but not early AMD (OR 0.84, 95% CI 0.59 to 1.24; p = 0.36) in multivariate analyses (see Table 42). No significant associations were found between AMD and consumption of other dietary fats, and no interaction with genetic factors was found.
Chong et al. 17 looked at the effect of trans-fatty acids and found an increased risk of AMD with higher intake, with an OR of 1.76 (95% CI 0.92 to 3.37; p = 0.02) when comparing the highest quartile of trans-fat intake with the lowest.
Adverse events
Three studies did not report AEs. 401–403
Adverse events were reported by Feher and colleagues 2005400 but these were unrelated to treatment (see Report Supplementary Material 5). Souied and colleagues393 found no statistically significant differences between groups in treatment related AEs, ocular AEs or serious non-ocular events (Table 43). However, worsening of cataract occurred more frequently in the placebo group (p = 0.032). Deaths were reported in both groups but were considered unlikely to be related to the study treatment.
|
Souied et al. , 2013393 RCT; unclear ROB |
DHA, n = 134 | Placebo, n = 129 | |
|---|---|---|---|
| Ocular AE | 58.7 | 50 | NS |
| Worsening of cataract | 50 | 62.5 | 0.032 |
| Serious non ocular eventa | 23.1 | 23.6 | NS |
| Deaths | 2.2 | 4.7 |
Summary
Results are mixed, with little difference in mean BCVA, but some evidence of reduced progression with increasing DHA and EPA intake, and a reduction in prevalence of AMD associated with olive oil intake.
The Cochrane review388 of two RCTs393,405 concluded there is no evidence from RCTs to support increasing omega-3 intake for preventing, or slowing the progression of, AMD.
Five primary studies evaluating fatty acids were identified: three RCTs with an unclear risk of bias,393,400,401 one fair-quality cohort study403 and one poor-quality cohort study. 402 Interventions, length of follow-up and outcomes measures varied, and results were inconsistent between studies. Overall, the effects of fatty acids are inconclusive.
NCT01782352 is an AMD substudy of the Vitamin D and Omega-3 Trial (VITAL) being run in Boston, Massachusetts by Christen and colleagues. The main VITAL trial is concerned with cardiovascular disease cancer and stroke (www.vitalstudy.org/). It is due to end in 2017, after recruiting almost 26,000 people.
NCT02613572 is a trial of alpha lipoic acid in GA, being carried out in Pennsylvania by Kim and colleagues. There are doubts about tolerability in older people.
NCT02379169 is a 6-month trial in Finland of sea buckthorn oil (which has beta-carotene and zeaxanthin) combined with lutein.
Homocysteine, folic acid and vitamins
The Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS) by Christen and colleagues406 randomised 5205 women, who did not have a diagnosis of AMD at baseline, to a daily supplement containing folic acid, vitamin B6 and vitamin B12 or to placebo. After 7.3 years of follow-up there were fewer cases of AMD in those randomised to a combination of folic acid 2.5 mg/day, vitamin B6 50 mg/day, and vitamin B12 1 mg/day than among those given daily placebo (Table 44). The RR was significant 0.66 (95% CI 0.47 to 0.93). The study also reported that visually significant AMD was significantly less frequent in those taking the folic acid/B6/B12 supplements (RR 0.59, 95% CI 0.36 to 0.95).
|
Christen et al. , 2009406 RCT; unclear ROB |
Folic acid/B6/B12 (n = 2607) | Placebo (n = 2598) | RR (95% CI); p-value |
|---|---|---|---|
| Total AMD, n cases | 55 | 82 | 0.66 (0.47 to 0.93); 0.02 |
| Visually significant AMD, n cases | 26 | 44 | 0.59 (0.36 to 0.95); 0.03 |
|
Gopinath et al. , 2013408 Cohort study; GQ |
With AMD, n = 219 | Without AMD, n = 1171 | p-value |
| Serum homocysteine (µmol/l) | 13.0 (4.6) | 12.0 (4.2) | p = 0.002 |
| Serum folate (nmol/l) | 18.0 (9.6) | 18.0 (8.5) | p = 0.96 |
| Serum vitamin B12 (pmol/l) | 263.4 (116.6) | 284.3 (138.0) | p = 0.02 |
Three studies focused on homocysteine levels in healthy populations.
Cohort studies by Merle 2016407 and Gopinath 2013408 assessed dietary intake of folate and vitamin B in a total of 3915 participants, and Gopinath 2013408 also assessed serum homocysteine, folate, and vitamin B12 levels. The studies were conducted in the USA (n = 2) and Australia (n = 1). Follow-up was 7.3 years in the RCT and 5–13 years in the cohort studies. The cohort studies were assessed as good quality. The three studies received non-commercial funding, although the supplements were provided by the manufacturer in the RCT. Where reported, mean age was 63–72 years and 0% to about 45% were men. Baseline VA was not reported by the studies.
Gopinath and colleagues 2013408 from the BMES in Australia, included people invited to attend an eye examination following a door-to-door census in the study area. Cross-sectional data from BMES had shown that increased serum homocysteine and low vitamin B12 were associated with an increased risk of AMD. In this cohort study, after 10 years’ (the BMES 4 visit) follow-up, those with AMD at 10 years had had higher baseline (at the BMES 2 visit) serum levels of homocysteine and lower levels of serum vitamin B12 than those without AMD (see Table 44). Serum levels of folate were not different between those with and those without AMD. They also examined the association with fish consumption (none), consumption of folate (no association) or vitamin B12 (lower in those with AMD, p = 0.03) supplements and total intake of folate (no association) and vitamin B12 (lower in those with AMD, p = 0.004).
Merle and colleagues 2016407 included participants from the AREDs trial with at least one eye with a VA no worse than 20/32 and at least one eye free from any disease that could complicate the assessments of AMD. The progression to GA was reported to be 16% after a mean of 8.7 years follow-up. The study reported that, after adjustment for age, sex and total energy intake, those who progressed to GA had lower intakes of thiamine (p = 0.01), riboflavin (p = 0.03) and folate (p = 0.001) than those who did not progress. In multivariate analysis, the trend for lower risk of progression was statistically significant for folate only (p = 0.007), see Report Supplementary Material 5 for more details. Subgroups by 10 single-nucleotide polymorphisms were reported for those who progressed and those who did not, and the difference in risk between quintiles of dietary folate intake was seen in some groups (subjects with C3 R102G CC, HR in highest quintiles about 0.4) but not others. So there was an interaction between genes and diet.
Summary
Folate supplementation may reduce the risk of AMD.
Vitamins
The potential antioxidant effects of vitamins were examined in five RTCs in healthy populations by Christen et al. 2007,398 Christen et al. 2010,409 Christen et al. 2014410 Taylor et al. 2002399 and Teikari et al. 1998411 and one cohort study in people with dry AMD in at least one eye by Cangemi et al. 2007. 411 A total of 20,529 participants were randomised to vitamin E/alpha-tocopherol, 10,819 to beta-carotene, 7111 to a multivitamin, 257 to alpha-tocopherol plus beta-carotene and 38,214 to placebo. The cohort study included 34 people receiving an ‘oral antioxidant and omega-3 supplement’ (see below for details). Four of the studies were conducted in the USA, one in Australia and one in Finland. Follow-up was from ≤ 4 years to 12 years in the RCTs and was 6 months in the cohort study. The RCTs all had an unclear risk of selection bias and the cohort study was assessed as fair quality. One study received commercial funding, one received both commercial and non-commercial funding, and four had non-commercial funding but the supplements were provided by the manufacturer in two of these. Mean age ranged from 52–76 years and 42–100% were men, where reported. Baseline VA was reported by three of the studies.
Christen and colleagues 2007398 assessed the incidence of ARM in healthy male physicians receiving beta-carotene (50 mg on alternate days) or placebo for 12 years in a large RCT. There was no difference between groups in the risk of visually significant ARM (RR 0.96, 95% CI 0.78 to 1.20), ARM with or without vision loss (RR 1.01, 95% CI 0.86 to 1.20) or advanced ARM (RR 0.97, 95% CI 0.69 to 1.37) (Table 45). This remained the case after excluding cases diagnosed during the first 2 or 5 years of follow-up.
|
Christen et al. , 2007398 RCT; unclear ROB |
Beta-carotene, N = 10,585 | Placebo, N = 10,557 | RRa (95% CI) | ||
|---|---|---|---|---|---|
| Visually significant ARM, n | 162 | 170 | 0.96 (0.78 to 1.20) | ||
| ARM with or without vision loss, n | 275 | 274 | 1.01 (0.86 to 1.20) | ||
| Advanced ARM, n | 63 | 66 | 0.97 (0.69 to 1.37) | ||
|
Christen et al. , 2010412 RCT; unclear ROB |
Vitamin E, N = 19,697 | Placebo, N = 19,724 | p-value | ||
| Visually significant AMD, n cases | 117 | 128 | 0.54 | ||
| Advanced AMD, n cases | 29 | 26 | 0.65 | ||
| All AMD ± vision loss, n cases | 280 | 313 | 0.20 | ||
|
Taylor et al. , 2002399 RCT; unclear ROB |
Vitamin E, N = 587 | Placebo, N = 592 | RR (95% CI) | ||
| 4-year incidence of early AMD (%) | |||||
| Photographsb | 8.6 | 8.1 | 1.05 (0.69 to 1.61) | ||
| Clinical gradingc | 7 | 7 | 1.12 (0.66 to 1.9) | ||
| Incidence of late AMD (%) | |||||
| Photographs | 0.8 | 0.6 | 1.36 (0.67 to 2.77) | ||
| Clinical grading | 1 | 1 | 1.00 (NA) | ||
| Incidence of drusen at 4 years (%) | |||||
| Soft intermediate | 19 | 18 | 1.05 (0.80 to 1.39) | ||
| Soft distinct | 6 | 6 | 1.05 (0.60 to 1.82) | ||
| Soft indistinct | 2 | 2 | 1.03 (0.77 to 1.38) | ||
| Progression of AMD (%) at 4 years | |||||
| Photographs | 19 | 18 | 1.09 (0.84 to 1.42) | ||
| Clinical grading | 7.9 | 6.0 | 1.31 (0.83 to 2.07) | ||
|
Teikari et al. , 1998397 RCT; unclear ROB |
Alpha-tocopherol N = 237 | Beta-carotene N = 234 | Alpha-tocopherol + beta-carotene N = 257 | Placebo N = 213 | p-value |
| ARM overall incidence (%) | 31.6 | 29.1 | 28.4 | 24.9 | 0.468 |
| ARM classd, n | |||||
| No ARM | 162 | 166 | 184 | 160 | |
| I | 65 | 64 | 64 | 46 | |
| II | 2 | 2 | 6 | 6 | |
| III | 6 | 2 | 2 | 0 | |
| IV | 2 | – | 1 | 1 | |
|
Christen et al. , 2014410 RCT; unclear ROB |
Multivitamin, N = 7111 | Placebo, N = 7122 | p-value | ||
| Visually significant AMD, n cases | 152 | 129 | 0.15 | ||
| Total AMD ± vision loss, n cases | 294 | 244 | 0.02 | ||
| Advanced AMD, n cases | 79 | 65 | 0.23 | ||
Christen and colleagues 2010412 included women from the Women’s Health Study who did not have a diagnosis of AMD at recruitment. With 10 years of follow-up there were fewer cases of visually significant AMD in those randomised to vitamin E and aspirin on alternate days than those given placebo, but the difference was not statistically significant (RR 0.93, 95% CI 0.72 to 1.19) (see Table 45). The incidence of advanced AMD was not significantly different between groups (RR 1.13, 95% CI 0.67 to 1.92), nor was the incidence of all AMD with or without vision loss (RR 0.90, 95% CI 0.77 to 1.06).
Taylor and colleagues 2002399 included healthy volunteers aged 55–80 years. The 4-year incidences of early AMD were similar between those randomised to high-dose vitamin E (500 IU daily) and those treated with placebo, with RR 1.05 (95% CI 0.60 to 1.61) (see Table 45). Results for late AMD, incidence of drusen and progression of AMD were similar between groups RR 1.36 (95% CI 0.67 to 2.77) for progression. The study reported that there was a significantly lower incidence of hypopigmentation in those taking vitamin E, but the data reported show a non-significant effect. No difference in BCVA was found (data not presented by the study). Quality of life was assessed using the visual function (VF-14) scale and it was stated that there were no differences between groups, but no data were presented.
Teikari and colleagues 1998397 compared the incidence of ARM among a subgroup of participants in a Finnish RCT on lung cancer prevention. Men who were ≥ 65 years and smoked at least five cigarettes a day were randomised to receive alpha-tocopherol, beta-carotene, alpha-tocopherol + beta-carotene or placebo. After approximately 6 years, no differences in the incidence of AMD were found among groups, RR for alpha-tocopherol 1.13 (95% CI 0.8 to 1.59) and 1.04 (95% CI 0.74 to 1.47) for beta-carotene (see Table 45) and there was no association between the supplements and prevalence of ARM after controlling for potential risk factors.
Christen and colleagues 2014410 included healthy male physicians from a cancer and cardiovascular prevention study (the Physicians Health Study II) who were not diagnosed with AMD. After 11.2 years of follow-up there was no significant difference between groups in cases of visually significant AMD (HR 1.19, 95% CI 0.94 to 1.50) or cases of advanced AMD (HR 1.22, 95% CI 0.88 to 1.70). However, the number of cases with total AMD with or without vision loss was significantly higher in those given multivitamins (HR 1.22, 95% CI 1.03 to 1.44).
Cangemi 2007411 compared one arm of their RCT, which compared MCS with sham microstimulation (abandoned because of lack of effect), with the placebo arm of another study. 186 As both arms of the original study received nutritional supplements, it is not clear why only the patients in the sham arm were included in this study. It was really a before-and-after study with no local controls. Participants in Cangemi 2007411 had at least one eye with dry AMD (> 10 large soft drusen of 63 µm in diameter, within 3000 µm of the fovea centre) and BCVA in the study eyes of 20/32 to 20/125 inclusive (ETDRS). After 6 months of a nutritional supplement containing vitamins A, C and E, zinc, copper, taurine, EPA fatty acid, DHA fatty acid, lutein and zeaxanthin (with sham microstimulation), VA was reported to have improved from baseline by 0.5 ETDRS lines (p = 0.045; see Table 45) compared with a deterioration in the placebo arm of the other study (no statistical comparison). BCVA improved in 56.7% of participants. There was little change in other outcomes, including contrast sensitivity and NEIVFQ-25 (see Report Supplementary Material 5). Because of the mixture of vitamins, metals, fatty acids and lutein given, it is not possible to say which ingredient had the reported effect.
Adverse events
Four papers (Christen and colleagues 2010,412 Christen and colleagues 2007,398 Teikari and colleagues,397 Christen and colleagues 2014410) did not report AEs.
Taylor and colleagues 2002399 found no serious AEs. Ocular AEs occurred in 18% of participants in the vitamin E group and 15% in the placebo group, not a significant difference. Cangemi 2007411 reported that there were no significant systemic or ocular AEs related to the nutritional supplement.
Summary
The Huang et al. 394 review concluded that AMD is associated with elevated homocysteine levels and decreased vitamin B12 levels. Four additional studies (Christen 2015,406,413 Merle 2016407 and Gopinath 2013408) were identified that assessed homocysteine levels, folic acid and B vitamins: one RCT (Christen 2009406) and three good-quality cohort studies (Christen 2015,413 Merle 2016,407 Gopinath 2013408) were identified that assessed homocysteine levels, folic acid and B vitamins. The results from the primary studies suggest an association with AMD and lower intake of B vitamins or reduced serum B12 levels. Results for the effect of folic acid were inconsistent.
The Evans review357 concluded that people with AMD, or early signs of the disease, may experience some benefit from taking supplements as used in the AREDS trial, but that current evidence does not support the use of antioxidant vitamin supplements to prevent AMD. Four large RCTs found no beneficial effect of beta-carotene or vitamin E/alpha-tocopherol in healthy individuals. One large RCT found higher total AMD in people given multivitamins. It may be that, as in category 1 in the AREDS trial, the rates of progression over the durations observed are too low to show any effect.
Ginkgo biloba extract
Ginkgo biloba extract is a popular herbal medicine, claimed by its advocates to be of benefit in a very wide range of diseases.
A Cochrane review by Evans 2013414 found only two trials. One compared ginkgo extract with placebo but had only 10 patients per arm followed for 6 months. In both groups, vision was report to improve but the ginkgo group was reported to have improved more. The other trial compared two doses of ginkgo and found no significant differences.
There is insufficient evidence for the use of ginkgo biloba in AMD.
HESA-A
HESA is described as a ‘natural drug’ but is a mixture of ingredients. A study by Ahmadi and colleagues415 from Iran included 140 participants in the HESA-A group and 140 in the placebo group. Details were sparse and risk of bias could not be assessed. Treatment with a twice daily oral tablet (25 mg/kg) containing ‘herbal-marine’ elements (including calcium 43.79%, diphosphorus pentoxide 6.63% and 24 other ingredients including various trace elements) or placebo (no details) lasted for 4 weeks with follow-up at 6 months. The study had an unclear risk of selection bias (see Report Supplementary Material 5). Participants were aged 69 years, on average, and 42–45% were male. Baseline BCVA was 1.7 logMAR. The funding source for the study was not reported.
Ahmadi and colleagues415 included people with a clinical diagnosis of wet or dry AMD but no breakdown is given. At 1 month, BCVA was reported to have improved in the HESA-A group and a statistically significant difference was reported between groups. The study reported that VA improved in 100% of participants in the treatment group after 4 weeks, sustained to 6 months but that no effect on BCVA was seen in the control group. No other outcomes were reported. Improvement was not defined and no data were presented.
Saffron
Saffron is the dried red stigma of Crocus sativus and contains a large number of compounds, including the carotenoids crocetin and zeaxanthin.
Quantity and quality of research
Reviews
Two non-systematic reviews of saffron use were identified. Milajerdi et al. 2015416 concluded that saffron was effective in a very wide range of diseases. The studies they included for saffron in AMD were by Falsini 2010417 and Marangoni 2013,418 described below. The other review by Bisti and colleagues 2014419 reviewed their own studies (including Falsini 2010417 and Marangoni 2013,418 in both of which Bisti is an author).
Broadhead et al. 359 also reviewed saffron use as part of a wider review of nutritional interventions in AMD, but concluded its value was unproven. However they have a trial under way, reported below.
Studies
Six studies were included. Three were from the same group in Italy: one crossover RCT by Falsini et al. 2010,417 a before-and-after study by Piccardi et al. 420 and a cohort study by Marangoni et al. 418 A total of 87 participants were included in the studies, all of whom received saffron.
The other three studies were RCTs by Lashay et al. 421 2016 from Tehran, Riazi et al. 422 2017 from Tehran (different hospitals) and Broadhead et al. 423 2016 from Australia. The Broadhead study is available only as a conference abstract.
In the Falsini RCT, saffron 20 mg or placebo were given for 90 days and then after a 15-day wash out participants received the alternate treatment for 90 days. Follow-up was immediate. In the before-and-after study participants received saffron (dose not reported) for 14 months and were followed up 1 month later, and in the cohort study saffron 20 mg was given for an average of 11 months and follow-up was immediate. The Falsini RCT417 had an unclear risk of selection bias, the before-and-after study by Piccardi420 appeared to be of good quality, and the cohort study418 to be poor quality. Mean age was 65–69 years across study groups, and around 46–55% of participants were men. Baseline VA was reported by two of the studies (see Report Supplementary Material 5). 417,420
Falsini and colleagues417 included people with bilateral early AMD and a BCVA at least 0.3 in the study eye (typically the eye with the best VA was selected as the study eye). After 90 days the mean VA was significantly better with saffron than placebo (Table 46). It is unclear whether or not this analysis accounts for the crossover appropriately. The VA was reported to have increased by 1 line in 80% and was unchanged in 20% of people treated with saffron. In the placebo group 100% were unchanged (see Table 46). The main outcome measure was flicker sensitivity.
|
Falsini et al. , 2010417 RCT; unclear ROB |
Saffron, n = 25 | Placebo, n = 25 | p-value | |
|---|---|---|---|---|
| Mean Snellen VA after 90 days (SD) | 0.80 (SD 0.20) | 0.72 (SD 0.24) | p < 0.01 | |
| VA (%) | ||||
| Increase by 1 line | 80 | 0 | ||
| Unchanged | 20 | 100 | ||
|
Piccardi et al. , 2012420 B + A; GQ |
Saffron, n = 29 | p-value | ||
| Mean VA |
Baseline: 0.75 15 months: 0.9 |
p < 0.01 versus baseline | ||
|
Riazi 2017 RCT; unclear ROB |
Saffron, n = 29 | Controls, n = 25 | ||
| Mean BVCA baseline logMAR | 0.46 (SD 0.41) | 0.62 (SD 0.54) | ||
| Mean BCVA at 12 weeks | logMAR 0.41 (SD 0.41) | 0.65 (SD 054); p = 0.001 | ||
| Change | 0.05 | + 0.03 | ||
In a before-and-after study, Piccardi and colleagues420 included people with bilateral early AMD and BCVA at least 0.5 in the study eye. After 14 months of treatment, mean VA improved by 2 Snellen lines compared with baseline values (VA 0.75 to 0.9; p < 0.01) (see Table 46). The primary outcome of the study was focal electroretinogram (fERG), which did not change during the study.
Marangoni and colleagues180 included people with bilateral early AMD and BCVA at least 0.5 in the study eye. Although VA was assessed as a secondary outcome, no data were reported. The primary outcome of the study, fERG, was reported to have improved significantly during the study compared with baseline values (see Report Supplementary Material 5).
Adverse events
All three of the studies reported no AEs.
Lashay et al. 421 randomised patients with both wet and dry AMD, but we are interested only in the 30 with dry AMD. Only 16 completed the study. No definition or details of dry AMD are given. The trial appeared to be of fair quality using the Cochrane risk-of-bias scale (four criteria low risk, unclear for allocation concealment). The outcomes were retinal sensitivity by ERG and macular thickness by OCT. The paper reports that VA was not improved but no data are provided.
Riazi et al. 422 included only patients with dry AMD and assigned them to 50 mg saffron daily or placebo capsules. The risk of bias in unclear due to lack of detail. After 3 months, improvements in VA and contrast sensitivity were reported in the saffron group but not in the controls.
Details of the trial by Broadhead and colleagues423 are too sparse to assess quality. It is reported as a double-blinded crossover trial in 100 people with non-advanced AMD. Saffron supplement (20 mg/day) or placebo was given for 3 months, followed by crossover for another 3 months. The abstract reports that saffron improved mean BCVA by 0.69 letters, which is not of clinical significance. The authors suggest that longer-term studies may show greater effect.
Summary
Two non-systematic reviews, four small studies and one abstract for a larger but short-term trial, were identified. The Falsini crossover RCT417 of uncertain risk of selection bias reported that VA was better with saffron treatment after 90 days. After 14 months of treatment with saffron, the Piccardi420 before-and-after study of good quality reported a significant increase in VA. Two studies did not report VA outcomes. No adverse effects of saffron supplementation were recorded. Three studies and one of the reviews were from the same centre.
Overall, the evidence suggests a potential for benefit but further evidence is required. An important point made by Bisti and colleagues419 in their review is that the content of saffron may vary depending on source.
Curcumin
Curcumin is the main component of turmeric, the spice used in Indian cooking. A review by Pescosolido 2014424 stated ‘It has been demonstrated that curcumin has beneficial effects on several ocular diseases such as . . . age-related macular degeneration . . .’. This review produced no evidence at all that this was the case and almost all the studies reviewed were at a basic science level.
A few other studies have been published but all are at the basic science stage, for example using RPE cells425 or rat models of light-induced retinal degeneration. 426 There is no evidence at present to support the use of turmeric products in AMD.
Zinc
Reviews
One high-quality systematic review by Vishwanathan and colleagues 2013427 was identified so we did not go back to the primary studies. Vishwanathan and colleagues 2013427 included 10 studies: four RCTs, four prospective cohort studies and two retrospective cohort studies. Outcomes were incidence of any AMD, early AMD and late AMD, BCVA, progression to GA. The review concluded that the current evidence on zinc intake for the prevention of AMD is inconclusive. The authors state that zinc treatment may be effective in preventing progression to advanced AMD but zinc alone may not be sufficient to produce clinically meaningful changes in VA. Recommendations for research were that evaluations of different forms of zinc supplementation (e.g. zinc oxide vs. zinc sulphate vs. zinc monocysteine) are required because bioavailability varies. The AREDS 1 trial used 80 mg of zinc oxide, AREDS 2 used 25 mg, Newsome 1988342 used zinc sulphate 100 mg, Newsome 2008428 used zinc monocysteine 25 mg twice daily, and Stur 1996429 used 200 mg of zinc sulphate. The Vishwanathan review was written before the AREDS 2 results were available.
Zinc was also mentioned in several non-systematic reviews310,322 of multiple interventions for dry AMD but these referred to the Vishwanathan review or the AREDS trial papers so did not add anything.
Studies
No published studies not covered by the Vishwanathan and colleagues 2013427 review were identified.
Summary: zinc
One good-quality systematic review concluded that there is inconclusive evidence for the use of zinc to prevent AMD but that it may be effective in prevention of progression to advanced AMD. It is included for this purpose in the AREDS 2 formula.
Chapter summary
There are many studies of nutritional supplements but some are too small and of too short duration, or are of combinations of compounds, making it difficult to assess the relative contributions of each. Supplements used include lutein and zeaxanthin, in combination or individually, or combined with a variety of other minerals and/or vitamins, the omega-3 fatty acids DHA and EPA, olive oil, folate acid, various vitamins, ginkgo biloba, turmeric, saffron and zinc.
The strongest evidence is for the AREDS 2 supplement, which contains:
500 mg vitamin C, 400 IU vitamin E, 10 mg lutein, 2 mg zeaxanthin, 25 mg zinc, 2 mg copper.
There is currently insufficient evidence to recommend any other nutritional supplements, although saffron extracts show promise and might justify further research. The combination of folic acid and vitamins B6 and B12 may also be worth further research, perhaps in people with early AMD. Olive oil may be protective as part of a healthy diet – the evidence is not on use as a supplement.
Chapter 7 Discussion and research needs
There were two aims for this review. The first was to provide an up-to-date systematic review of treatments for dry AMD and STGD. The second and more important aim was to identify treatments that were sufficiently promising for the NIHR programmes (HTA and EME) to consider commissioning primary research.
Statement of principal findings: dry age-related macular degeneration
Physical treatments
-
Newer forms of laser treatment show promise but a large trial, the LEAD trial from a world centre of excellence in Melbourne is ongoing (anticipated completion date June 2018), so we suggest waiting for their results.
-
Implantable telescopic lenses also show promise, but a NIHR EME study is under way (MIRROR) in advanced AMD.
-
There is insufficient good-quality evidence to recommend use of, or further research in, acupuncture, MCS or ozone.
-
There is some evidence on rheopheresis, but the largest trial showed no benefit, most studies reporting positive results were small with only modest effect sizes and mostly uncertain risks of bias, and treatment would be inconvenient to elderly patients. So we do not see rheopheresis as a research priority.
-
The evidence for the use of blue-light-filtering IOLs after cataract extraction is currently insufficient to justify their routine use, but further research is currently under way.
Cells
-
One small before-and-after study and one very small case series of cell (RPE) transplantation was identified. Improvements in VA were found in over half of treated eyes.
-
The evidence base is still very sparse, but this seems a promising development, and further research is under way.
Drug treatments
-
We think that there is sufficient evidence to justify a trial of a potent statin, such as atorvastatin 80 mg daily.
-
Fenretinide is a visual cycle inhibitor, which may reduce the deposition of lipofuscin. We found one trial, with an unclear risk of bias and run and written up by the manufacturer’s staff, which had mixed results. Progression of GA was little different overall, but was a bit lower in the subgroup that achieved greater serum RBP levels. However progression to wet AMD was halved by fenretinide. There were higher rates of AEs with fenretinide. Overall, we think a trial in early dry AMD may be justified, but the roughly 20% drop-out rates in the active drug arms should be noted.
-
An impressively large retrospective study from the USA found that people taking L-dopa were less likely to develop AMD, and that if they did develop it, it was about 7 years later than among people not taking L-dopa. Further research is needed, perhaps using the large UK general practice-based databases, THIN and CPRD, in order to assess whether a trial assessing its use in treating AMD could be justified.
-
The current evidence on lampalizumab suggests benefit, but very large trials are under way (sponsored by the manufacturer), so no new research is indicated meantime.
-
There is a little evidence of benefit from glatarimer acetate, but with only some shrinkage of drusen in two studies which had unclear risks of bias. The evidence is too sparse to justify NIHR research at present. Future trials can be left to the manufacturer if deemed worthwhile.
-
One small study reported benefit from prednisolone but it scored poorly on quality assessment so there is insufficient evidence to justify its use. Systemic steroids have adverse effects so if steroids were to be used, a topical one would seem better. The results of a trial of an implanted steroid, fluocinolone (NCT00008515), are awaited.
-
For nine drugs, there was some evidence showing no or very little benefit, or even harm, so we do not recommend further consideration. They are alprostadil, eculizumab, dorzolomide, OT 551 eye drops, sirolimus, tandospirone, trimetazidine, visaline and emixustat.
Nutrients
-
There are many studies of nutritional supplements but some are too small, of poor quality, of too short duration, or are of combinations of compounds, making it difficult to assess the relative contributions of each. Supplements used include lutein and zeaxanthin, in combination or individually, or combined with a variety of other minerals and/or vitamins, the omega-3 fatty acids DHA and EPA, olive oil, folate acid, various vitamins, ginkgo biloba, curcumin (from turmeric), saffron and zinc.
-
The first AREDS trial showed benefit in category 3 and 4 patients, which persisted for 7 years, with a modest but useful slowing of progression, which could mean that 30% of people expected to progress to advanced AMD over a 5-year period, would not. The trial did not have enough power to confirm, or not, effects in categories 1 and 2.
-
The AREDS 2 trial showed that beta-carotene should be replaced by lutein and zeaxanthin, and that the dose of zinc could be reduced.
-
We therefore think that there is good evidence that the AREDS 2 supplement should be used for patients meeting the AREDS 3 and 4 categories.
-
Saffron extracts have been reported to show some benefits in VA and might justify further research.
-
There is currently insufficient evidence to recommend any other nutritional supplements.
Statement of principal findings: Stargardt disease
The evidence on treatments for STGD is sparse. We found only one randomised trial with a control group (microstimulation), and it had only 12 patients and an unclear risk of bias. Most studies tested interventions with no comparison group, most were far too short term, and the quality of some studies was poor. There has been very little research into the treatment of STGD compared with AMD.
At present, the most promising treatments for STGD appear to be;
-
Prevention of lipofuscin accumulation. Several drugs may have potential, including fenretinide, deuterated vitamin A (ALK-001) and emixustat. Early trials of ALK-001 and emixustat are under way. Fenretinide has shown promise in dry AMD and we think a trial in STGD may be justified. A vignette has been written for the HTA programme.
-
Gene therapy is at an early stage, but a study (StarGen NCT01736592) is under way in Oregon and Paris.
-
Cell transplantation to replace the RPE has been tried in one small study in only nine people with STGD, but looks promising. Further research is already under way.
There are three other possible interventions that seem worth further research. One is light reduction, as reported in the very small trial by Teussink et al. ,122 where progression in the light-protected eye was reported to be less in four of the five participants. Second, there is a plausible rationale for the benefits of lutein and zeaxanthin supplementation to protect the macula (perhaps especially the fovea) but insufficient evidence. The small Aleman study114 was too short term. Scripsema and colleagues341 have pointed out that after supplementation with lutein and zeaxanthin, serum levels rise quickly, but macular pigment concentration increases over several months, and visual function may take a year or two to reach statistically significant changes.
The evidence for the third comes so far only from animal work, where fenofibrate appears to have some activity as a visual cycle inhibitor. Fenofibrate is an old, cheap and safe drug used for lipid-lowering, but is currently being trialled in diabetic retinopathy where it has shown some benefit in past studies.
Strengths and limitations
The strengths of this review come from the thorough searches, updated with weekly auto-alerts (to December 2017, so including the period of editorial review and allowing late updating), the rigorous systematic review methods used, including quality assessment of included studies, data extraction using predefined forms, checking of data extractions and reporting using structured tables.
Expert ophthalmological input was available within the team. Our links with the Macular Society research team fostered awareness and our panel of patient advisers met three times to hear and comment on drafts of the review.
The limitations came from the poor quality of much of the evidence. Many studies were too small and of poor quality. Durations were often very short and too short to capture effects on vision. Many studies used VA as their main outcome despite not having sufficient duration to observe changes.
The AREDS trials have been criticised for recruiting a well-educated and well-nourished group, who might not be representative of the general population (of the USA). However, their diets would have tended to reduce their risk of progression, which means that the AREDS trial may have underestimated the benefits of the supplement. The AREDS 1 supplement has been assessed as cost-effective in both the USA430 and Singapore. 431
Two other limitations in the evidence need to be mentioned. First, many of the studies are case series with no controls. Spontaneous improvement in VA can occur, as patients learn to use remaining retina more effectively. Sunness432 reported that 5 out of 48 worse-seeing eyes improved in patients with bilateral GA.
Second, publication bias is a likely problem but we have been unable to assess this formally. Prenner and colleagues433 found that half of the interventional trials registered on ClinicalTrials.gov had not been published ≥ 2 years after completion.
Earlier detection for clinical trials
As argued in the Chapter 1, VA is a late manifestation of AMD, and future studies should aim to detect, and intervene in, AMD earlier. Many people with early and intermediate (large drusen) AMD have no symptoms, and others with symptoms may attribute them to ageing. This implies that they would have to be found by screening. One option suggested by Chew and Schachat434 would be to detect people with drusen using the digital photographs taken for screening for diabetic retinopathy. Unfortunately, that would capture only those with diabetes and their retinopathy might require treated, which would complicate any AMD trials. Another option, being provided by one optometry practice in Scotland, is screening during annual eye tests, using both fundus photographs (and measurement of MPOD – although photographs are sufficient to identify people with drusen). 435 A network of screening practices could identify sufficient people for dry AMD trials.
However, work from the USA suggests that AMD is being missed in primary eye care, by both optometrists and ophthalmologists. Neely and colleagues436 examined 644 people aged ≥ 60 years who had been recorded as having normal maculae at their most recent dilated eye examination. They obtained three-field colour fundus photographs and found that 25% of people had some evidence of AMD. Of these, 69% had early and 31% had intermediate (large drusen) AMD.
Outcome measures
If we recruit people with early AMD (i.e. with drusen + RPE changes but no GA) to clinical trials, most are going to be asymptomatic in terms of central visual loss and, in most, vision is going to be good. Therefore, VA will not, in short-term follow-up, be a suitable outcome measure. Nor will GA, because enlargement of areas of GA will occur slowly, although GA could be an outcome over longer periods, with the right imaging technology. As Schaal and colleagues437 state: ‘The major disadvantage of using GA is that significant irreversible disease progression has already occurred’.
It will be necessary to look for reducing or preventing functional deterioration, such as changes in drusen number or volume (although with the caveat that resolution of drusen may be due to developing GA), preventing or reducing the development of reduced signal on AF (conventional 488 nm AF, 787 nm near-infrared AF), or lack of reduction/disorganisation of the IS/OS layer on OCT, or more sensitive measures such as macular function as measured by microperimetry (including using dense grids on microperimetry to more precisely determine macular function). Rod function is one of the earliest abnormalities detected in people who will later develop GA in AMD. Both photopic and scotopic vision need to be considered.
There appear to be subtypes of drusen as described by OCT that may have different prognostic significance. Veerappan and colleagues438 describe subtypes of drusen that predict progression to GA but not to wet AMD.
The appearances of GA on FAF (autoflorescence and fundus photography) also reveal subtypes of GA with different prognoses. Schmitz-Valckenberg and colleagues76 from the Geographic Atrophy Progression Study found more rapid growth in areas of GA in patients with multifocal atrophic spots than in those with unifocal spots.
Dark adaptation may also be an early sign of developing AMD. Several studies have reported that dark adaptation may be impaired in AMD before BCVA is affected. Owsley et al. 439 assessed rod-mediated dark adaptation in 325 people with no AMD and followed them up 3 years later. Those who had abnormal dark adaptation at baseline were twice as likely to have developed AMD. Alvarez et al. 440 reported that dark adaptation time measured by rod intercept time, increased over 2 years in eyes with AMD (with bilateral large drusen or more advanced AMD), but not in control eyes or in eyes without large drusen at baseline. They used the AdaptDx device (Maculogix, Middletown, PA, USA), which is said to take only 5 minutes. 441 Diaz and colleagues442 also reported that rod function was different between patients with early AMD (grades 2–3) and normal eyes, whereas BCVA was not. Another study from the same group443 described a digital dark adaptometer as providing a rapid and easy way of assessing progression in dry AMD, with high sensitivity and specificity in a study of 20 patients (ARED 2–4) and 20 normal controls. Lastly, Planas and colleagues444 compared patients with drusen with healthy controls, and found worse dark adaptation in the patients, especially those with RPD. Therefore, dark adaptation appears to be a quick test for monitoring early AMD, which is feasible in routine clinics.
Research under way in Northern Ireland shows that measuring the ability of people with AMD to see in the dark could provide evidence of deteriorating vision earlier than the tests usually used in Ophthalmology clinics. (Beirne R, University of Ulster, 2014).
We also need outcome measures that matter to people with AMD, such as being able to drive, reading speed, VA for both close and distant vision, and contrast sensitivity.
Kimel and colleagues445 emphasise the importance of reading for people with GA, and note that some people may have good BCVA, but have problems with reading. They have developed the functional reading independence index, which will be used in the trials of lampalizumab, mentioned in Chapter 5, Complement inhibitors.
A recent systematic review of biomarkers by Kersten and colleagues446 considered a very large number of possible biomarkers but concluded that few were likely to be useful. They also noted the potential of ‘hypothesis-free’ studies (i.e. those that measure lots of possible compounds rather than looking at specific ones where there may be a reason to suspect a link with AMD). An example of these was reported by Lains et al. 447 who studied 878 compounds and found that 87 metabolites differed between people with AMD and those without.
Wittes and Downs448 provide a useful review of the types of outcome measures in AMD and their relative advantages and disadvantages. They classify outcomes as:
-
continuous measures, such as VA over time
-
success or failure, such as proportions losing < 10 letters, or having no change over time
-
proportions requiring retreatment or alternative treatments
-
characteristics of the disease such as drusen volume.
They make several points worth considering. One is that using a continuous variable, such as mean VA, requires a smaller sample size, but will conceal the distribution of results and individual success or failure. To provide estimates of proportions requires a much larger sample size, but is clinically more useful.
Similarly in STGD, AF, macular sensitivity determined by microperimetry and VA are useful measures of progression, but may not capture all the functional effects. 449 One useful outcome in STGD is reading speed. Murro and colleagues450 found that reading speed was a strong determinant of quality of life (measured by the NEIVFQ-25).
One area where further research would be useful is in strengthening the links between early intermediate changes and longer-term visual outcomes. We argued that intermediate outcomes can be accepted if there is good evidence that they are strong predictors of later visual outcomes. However, some evidence comes from cross-sectional studies showing correlations, but we need more longitudinal studies showing that early intermediate outcomes predict later visual ones. As noted in the STGD chapter, this may require studies of long duration. There may be a place for population-based registries here.
Research priorities for National Institute for Health Research programmes
We think that at present the interventions where the NIHR programmes might consider primary studies are:
-
Fenretinide for STGD, and possibly for dry AMD, because it appears to reduce the accumulation of lipofuscin.
-
High-potency statins for AMD. We have inconclusive evidence from the trial by Guymer et al. 283 of simvastatin 40 mg but more promising results from an uncontrolled case series by Vavvas et al. 286 of atorvastatin 80 mg.
-
Lutein and zeaxanthin supplements for STGD, based on theoretical reasoning rather than results from intervention studies.
-
Screening for early asymptomatic AMD in order to provide recruits for trials at earlier stages.
Cell transplantation and gene therapy both look promising. Research is under way but it would be good if it could be accelerated. However, such research might, at present, lie more within the remit of the MRC than NIHR.
As noted earlier, there is good evidence that the AREDS 2 supplement is worthwhile in AREDS categories 3 and 4, but not in categories 1 and 2. Absence of evidence in categories 1 and 2 is because the AREDS trial lacked power to observe changes, because progression was (luckily) too slow to detect significant changes in the timescale of the trial. That raises two options for people in categories 1 and 2:
-
extrapolate from later stages, assume the AREDS 2 supplement will be effective, and advocate routine use
-
mount a large trial comparing the AREDS 2 supplement with a normal diet.
The first option would do no harm, and probably much good, but we cannot claim it to be evidence-based at present. The second would mean a long delay before people got what would probably be an effective treatment, and there might be ethical and recruitment problems.
In STGD, filtering of light by contact lenses looked promising in the one small study122 but it seems unlikely that the HTA programme would regard that as a sufficient basis for commissioning a large trial. That raises the question of who would fund a pilot study to see if there was enough evidence of efficacy to warrant a HTA trial.
The same might apply to saffron supplements in dry AMD, perhaps trialled against lutein and zeaxanthin, using MPOD as the intermediate outcome.
In any research, the outcomes should include functional changes that are important to patients, as well as morphological changes.
Prevention of age-related macular degeneration
It would be better if we could prevent or at least reduce AMD. Consideration of that topic is outside the remit of this review, but there is good evidence that healthier lifestyles – avoiding smoking, physical activity, healthy diets – can reduce the incidence, with a combination of lifestyle choices best. 451
A recent systematic review by McGuinness and colleagues452 of physical activity and AMD found slight protection against early AMD (OR 0.92, 95% CI 0.86 to 0.98) but a greater effect for late AMD (OR 0.59, 95% CI 0.49 to 0.72). The amount of activity in some studies was low, with just a few hours of small to moderate level of activity per week.
A recent Cochrane review453 concluded that there is, as yet, no evidence that taking supplements of vitamin E, beta-carotene, vitamin C or the multivitamin product Centrum (Pfizer Consumer Healthcare, New York, NY, USA), will prevent or postpone AMD.
Sources of advice
We have noted a large number of internet sites that offer information on AMD. Some provide reliable information, but many do not. Some are seriously misleading, making extravagant claims for treatments without a good evidence base.
One potential source of advice could be optometrists. Two studies have tried to find out what advice optometrists and ophthalmologist give to people at risk of, or who have, AMD. The response rates by ophthalmologists were very poor and could provide no useful data. Lawrenson and Evans454 got response rates of 16% from optometrists and 6% from ophthalmologists. About 60% of respondents gave advice, such as to eat leafy green vegetables and oily fish, and in people with advanced AMD in one eye and early in the other, supplements were recommended, but only a minority recommended evidence-based supplements. There may be considerable selection bias with responders being more likely than non-responders to give advice.
A survey in Sweden by Martin455 got a better response from optometrists (40%) but an equally poor response from ophthalmologists (5%). Over half of the optometrists would recommend macular carotenoids but not the full AREDS formula.
Conclusions
There are some promising developments in dry AMD, but research studies are already under way in some of these, and we suggest waiting for their results. We have suggested some topics where the NIHR programmes might consider primary research.
In STGD, there are fewer developments. Research is under way in some. Research into visual cycle inhibitors is under way but we have found no trials of fenretinide and we think a HTA trial may be justified.
Acknowledgements
We thank Professor Andrew Clegg, University of Central Lancashire, for advice in the early stages and for the first draft of some nutrients sections.
We thank three patient members of the Macular Society, David Reynolds, Jennifer Phillips and Elaine Barrett, for attending meetings at which results were presented and for their comments.
We thank Christine Clar for quality assessment and data extraction of a study written in German.
We thank Cathy Yelf, Chief Executive, the Macular Society; Simon Smith and Erika Wright, Warwick Medical School, for support during the application process; and Nick Eaton, Research Manager, NETSCC for support and advice during the project.
Contributions of authors
Norman Waugh, Pamela Royle and Geraldine Hoad checked retrieved studies for inclusions and exclusions.
Emma Loveman and Jill Colquitt did data extraction and quality assessment of included studies.
Pamela Royle carried out literature searches and helped with formatting and editing.
Jian Lee Yeong and Noemi Lois provided clinical advice and drafted some sections.
Geraldine Hoad liaised with the patient representatives and convened the workshops with them.
All authors reviewed the final report.
Data sharing statement
Supplementary files with quality assessments and data extractions of studies are available to download as supplementary files. Enquiries and further data requests should be submitted to the corresponding author for consideration.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39:367-74. https://doi.org/10.1016/S0039-6257(05)80092-X.
- LOC Support Unit . National Eye Health Epidemiological Model (NEHEM) 2017. www.eyehealthmodel.org.uk/# (accessed 18 December 2017).
- Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012;96:752-6. https://doi.org/10.1136/bjophthalmol-2011-301109.
- Wilde C, Poostchi A, Mehta RL, MacNab HK, Hillman JG, Vernon SA, et al. Prevalence of age-related macular degeneration in an elderly UK Caucasian population-The Bridlington Eye Assessment Project: a cross-sectional study. Eye 2017;31:1042-50. https://doi.org/10.1038/eye.2017.30.
- Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007-March 2008. Eye 2010;24:1692-9. https://doi.org/10.1038/eye.2010.122.
- Colijn JM, Buitendijk GHS, Prokofyeva E, Alves D, Cachulo ML, Khawaja AP, et al. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology 2017;124:1753-63. https://doi.org/10.1016/j.ophtha.2017.05.035.
- Waern M, Rubenowitz E, Runeson B, Skoog I, Wilhelmson K, Allebeck P. Burden of illness and suicide in elderly people: case-control study. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7350.1355.
- Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, Campanella J, et al. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc 2005;103:173-84. https://doi.org/10.1016/S0008-4182(05)80070-5.
- Hanus J, Zhao F, Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br J Ophthalmol 2016;100:122-7. https://doi.org/10.1136/bjophthalmol-2015-306972.
- Ho L, van Leeuwen R, de Jong P, Vingerling JR, Klaver CCW, Holz FG, et al. Age-related Macular Degeneration. Berlin: Springer; 2013.
- Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 2014;121:1079-91. https://doi.org/10.1016/j.ophtha.2013.11.023.
- Munch IC, Linneberg A, Larsen M. Precursors of age-related macular degeneration: associations with physical activity, obesity, and serum lipids in the inter99 eye study. Invest Ophthalmol Vis Sci 2013;54:3932-40. https://doi.org/10.1167/iovs.12-10785.
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol 2010;10. https://doi.org/10.1186/1471-2415-10-31.
- Chakravarthy U, Augood C, Bentham GC, de Jong PT, Rahu M, Seland J, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology 2007;114:1157-63. https://doi.org/10.1016/j.ophtha.2006.09.022.
- Amirul Islam FM, Chong EW, Hodge AM, Guymer RH, Aung KZ, Makeyeva GA, et al. Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study. Ophthalmology 2014;121:1428-34.e2. https://doi.org/10.1016/j.ophtha.2014.01.002.
- Chong EW, Simpson JA, Robman LD, Hodge AM, Aung KZ, English DR, et al. Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol 2009;169:867-76. https://doi.org/10.1093/aje/kwn393.
- Chong EW, Robman LD, Simpson JA, Hodge AM, Aung KZ, Dolphin TK, et al. Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol 2009;127:674-80. https://doi.org/10.1001/archophthalmol.2009.60.
- Zhu W, Wu Y, Meng YF, Xing Q, Tao JJ, Lu J. Fish consumption and age-related macular degeneration incidence: a meta-analysis and systematic review of prospective cohort studies. Nutrients 2016;8. https://doi.org/10.3390/nu8110743.
- Bringmann A, Hollborn M, Kohen L, Wiedemann P. Intake of dietary salt and drinking water: implications for the development of age-related macular degeneration. Mol Vis 2016;22:1437-54.
- Ying GS, Maguire MG, Liu C, Antoszyk AN. Complications of Age-related Macular Degeneration Prevention Trial Research Group . Night vision symptoms and progression of age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology 2008;115:1876-82. https://doi.org/10.1016/j.ophtha.2008.05.023.
- Christen WG, Glynn RJ, Chew EY, Buring JE. Low-dose aspirin and medical record-confirmed age-related macular degeneration in a randomized trial of women. Ophthalmology 2009;116:2386-92. https://doi.org/10.1016/j.ophtha.2009.05.031.
- Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol 2008;145:707-15. https://doi.org/10.1016/j.ajo.2007.12.005.
- Lommatzsch A, Wasmuth S, Pauleikhoff D, Holz FG, Bird AC, Holz FG, et al. Age-related Macular Degeneration. Berlin: Springer; 2013.
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology 1990;97:171-8. https://doi.org/10.1016/S0161-6420(90)32619-2.
- Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci 2013;54:ORSF68-80. https://doi.org/10.1167/iovs.13-12757.
- Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 2001;15:384-9. https://doi.org/10.1038/eye.2001.141.
- Carpentier S, Knaus M, Suh M. Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr 2009;49:313-26. https://doi.org/10.1080/10408390802066979.
- van der Made SM, Kelly ER, Kijlstra A, Plat J, Berendschot TT. Increased macular pigment optical density and vsual acuity following consumption of a buttermilk drink containing lutein-enriched egg yolks: a randomized, double-blind, placebo-controlled trial. J Ophthalmol 2016;2016. https://doi.org/10.1155/2016/9035745.
- Stevens R, Bartlett H, Cooke R. Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clin Nutr ESPEN 2015;10:e112-e117. https://doi.org/10.1016/j.clnesp.2015.03.080.
- Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 Report No. 1). Ophthalmology 2012;119:2282-9. https://doi.org/10.1016/j.ophtha.2012.05.027.
- Abdelfattah NS, Zhang H, Boyer DS, Rosenfeld PJ, Feuer WJ, Gregori G, et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci 2016;57:1839-46. https://doi.org/10.1167/iovs.15-18572.
- Age-Related Eye Disease Study Research Group . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmol 2001;119:1417-36. https://doi.org/10.1001/archopht.119.10.1417.
- Mimoun G, Soubrane G, Coscas G. Macular drusen. J Fr Ophtalmol 1990;13:511-30.
- Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina 1995;15:183-91. https://doi.org/10.1097/00006982-199515030-00001.
- McBain VA, Kumari R, Townend J, Lois N. Geographic atrophy in retinal angiomatous proliferation. Retina 2011;31:1043-52. https://doi.org/10.1097/IAE.0b013e3181fe54c7.
- Lois N, Owens SL, Coco R, Hopkins J, Fitzke FW, Bird AC. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol 2002;133:341-9. https://doi.org/10.1016/S0002-9394(01)01404-0.
- Sawa M, Ueno C, Gomi F, Nishida K. Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina 2014;34:761-7. https://doi.org/10.1097/01.iae.0000434566.57189.37.
- Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol 2008;145:317-26. https://doi.org/10.1016/j.ajo.2007.09.008.
- Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology 2013;120:2042-50. https://doi.org/10.1016/j.ophtha.2013.03.029.
- Buitendijk GH, Hooghart AJ, Brussee C, de Jong PT, Hofman A, Vingerling JR, et al. Epidemiology of reticular pseudodrusen in age-related macular degeneration: The Rotterdam Study. Invest Ophthalmol Vis Sci 2016;57:5593-601. https://doi.org/10.1167/iovs.15-18816.
- Chan H, Cougnard-Grégoire A, Delyfer MN, Combillet F, Rougier MB, Schweitzer C, et al. Multimodal imaging of reticular pseudodrusen in a population-based setting: The Alienor Study. Invest Ophthalmol Vis Sci 2016;57:3058-65. https://doi.org/10.1167/iovs.16-19487.
- Sivaprasad S, Bird A, Nitiahpapand R, Nicholson L, Hykin P, Chatziralli I. Moorfields UCL AMD Consortium . Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol 2016;61:521-37. https://doi.org/10.1016/j.survophthal.2016.02.005.
- Saade C, Smith RT. Reticular macular lesions: a review of the phenotypic hallmarks and their clinical significance. Clin Experiment Ophthalmol 2014;42:865-74. https://doi.org/10.1111/ceo.12353.
- Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol 2011;95:979-85. https://doi.org/10.1136/bjo.2010.194977.
- Kovach JL, Schwartz SG, Agarwal A, Brantley MA, Pan SS, Haines JL, et al. The relationship between reticular pseudodrusen and severity of AMD. Ophthalmology 2016;123:921-3. https://doi.org/10.1016/j.ophtha.2015.10.036.
- Pumariega NM, Smith RT, Sohrab MA, Letien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology 2011;118:1619-25. https://doi.org/10.1016/j.ophtha.2011.01.029.
- Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol 2005;123:1570-4. https://doi.org/10.1001/archopht.123.11.1570.
- Gil JQ, Marques JP, Hogg R, Rosina C, Cachulo ML, Santos A, et al. Clinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohort. Eye 2017;31:364-71. https://doi.org/10.1038/eye.2016.207.
- Wong TY, Wong T, Chakravarthy U, Klein R, Mitchell P, Zlateva G, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008;115:116-26. https://doi.org/10.1016/j.ophtha.2007.03.008.
- Hogg RE, Silva R, Staurenghi G, Murphy G, Santos AR, Rosina C, et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology 2014;121:1748-55. https://doi.org/10.1016/j.ophtha.2014.03.015.
- Finger RP, Wu Z, Luu CD, Kearney F, Ayton LN, Lucci LM, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology 2014;121:1252-6. https://doi.org/10.1016/j.ophtha.2013.12.034.
- Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE, Freund KB, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7362-9. https://doi.org/10.1167/iovs.12-11073.
- Steinberg JS, Fitzke FW, Fimmers R, Fleckenstein M, Holz FG, Schmitz-Valckenberg S. Scotopic and photopic microperimetry in patients with reticular drusen and age-related macular degeneration. JAMA Ophthalmol 2015;133:690-7. https://doi.org/10.1001/jamaophthalmol.2015.0477.
- Ooto S, Ellabban AA, Ueda-Arakawa N, Oishi A, Tamura H, Yamashiro K, et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am J Ophthalmol 2013;156:1184-91.e2. https://doi.org/10.1016/j.ajo.2013.06.036.
- Forte R, Cennamo G, de Crecchio G, Cennamo G. Microperimetry of subretinal drusenoid deposits. Ophthalmic Res 2014;51:32-6. https://doi.org/10.1159/000354117.
- Querques G, Massamba N, Srour M, Boulanger E, Georges A, Souied EH. Impact of reticular pseudodrusen on macular function. Retina 2014;34:321-9. https://doi.org/10.1097/IAE.0b013e3182993df1.
- Ooto S, Suzuki M, Vongkulsiri S, Sato T, Spaide RF. Multimodal visual function testing in eyes with nonexudative age-related macular degeneration. Retina 2015;35:1726-34. https://doi.org/10.1097/IAE.0000000000000608.
- Corvi F, Souied EH, Falfoul Y, Georges A, Jung C, Querques L, et al. Pilot evaluation of short-term changes in macular pigment and retinal sensitivity in different phenotypes of early age-related macular degeneration after carotenoid supplementation. Br J Ophthalmol 2017;101:770-3. https://doi.org/10.1136/bjophthalmol-2016-309115.
- Nolan JM, Power R, Stringham J, Dennison J, Stack J, Kelly D, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central Retinal Enrichment Supplementation Trials - Report 1. Invest Ophthalmol Vis Sci 2016;57:3429-39. https://doi.org/10.1167/iovs.16-19520.
- Betteridge DJ. What is oxidative stress?. Metab Clin Exp 2000;49:3-8. https://doi.org/10.1016/S0026-0495(00)80077-3.
- Yehoshua Z, Rosenfeld PJ, Holz FG, Pauleikhoff D, Spaide RF, Bird AC. Age-related Macular Degeneration. Berlin: Springer; 2013.
- Barnett BP, Handa JT. Retinal microenvironment imbalance in dry age-related macular degeneration: a mini-review. Gerontology 2013;59:297-306. https://doi.org/10.1159/000346169.
- Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol 2013;13:438-51. https://doi.org/10.1038/nri3459.
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 2010;29:95-112. https://doi.org/10.1016/j.preteyeres.2009.11.003.
- Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844-51. https://doi.org/10.1016/j.ophtha.2012.10.036.
- Fleckenstein M, Schmitz-Valckenberg S, S. SJ, Holz FG, Holz FG, Pauleikhoff D, et al. Age-related Macular Degeneration. Berlin: Springer; 2013.
- Girmens JF, Sahel JA, Marazova K. Dry age-related macular degeneration: a currently unmet clinical need. Intractable Rare Dis Res 2012;1:103-14. https://doi.org/10.5582/irdr.2012.v1.3.103.
- Lois N, McBain V, Abdelkader E, Scott NW, Kumari R. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina 2013;33:13-22. https://doi.org/10.1097/IAE.0b013e3182657fff.
- The CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908. https://doi.org/10.1056/NEJMoa1102673.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258-67. https://doi.org/10.1016/S0140-6736(13)61501-9.
- Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150-61. https://doi.org/10.1016/j.ophtha.2013.08.015.
- Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest 2014;124:1430-8. https://doi.org/10.1172/JCI71029.
- Brandl C, Breinlich V, Stark KJ, Enzinger S, Aßenmacher M, Olden M, et al. Features of age-related macular degeneration in the general adults and their dependency on age, sex, and smoking: results from the German KORA study. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0167181.
- Sunness JS, Bressler NM, Tian Y, Alexander J, Applegate CA. Measuring geographic atrophy in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 1999;40:1761-9.
- Sunness JS, Margalit E, Srikumaran D, Applegate CA, Tian Y, Perry D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology 2007;114:271-7. https://doi.org/10.1016/j.ophtha.2006.09.016.
- Schmitz-Valckenberg S, Sahel JA, Danis R, Fleckenstein M, Jaffe GJ, Wolf S, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology 2016;123:361-8. https://doi.org/10.1016/j.ophtha.2015.09.036.
- Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol 2008;146:692-9. https://doi.org/10.1016/j.ajo.2008.05.050.
- Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S. FAM-Study Group . Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol 2007;143:463-72. https://doi.org/10.1016/j.ajo.2006.11.041.
- Midena E, Vujosevic S, Convento E, Manfre’ A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol 2007;91:1499-503. https://doi.org/10.1136/bjo.2007.119685.
- Kalloniatis M, Luu C, Kolb H, Fernandez E, Nelson R. Webvision : The Organization of the Retina and Visual System. Salt Lake City, UT: University of Utah Health Sciences Center; 1995.
- Crossland MD, Luong VA, Rubin GS, Fitzke FW. Retinal specific measurement of dark-adapted visual function: validation of a modified microperimeter. BMC Ophthalmol 2011;11. https://doi.org/10.1186/1471-2415-11-5.
- Simunovic MP, Moore AT, MacLaren RE. Selective automated perimetry under photopic, mesopic, and scotopic conditions: detection mechanisms and testing strategies. Transl Vis Sci Technol 2016;5. https://doi.org/10.1167/tvst.5.3.10.
- Midena E, Pilotto E. Microperimetry in age: related macular degeneration. Eye 2017;31:985-94. https://doi.org/10.1038/eye.2017.34.
- Midena E, Vujosevic S, Cavarzeran F. Microperimetry Study Group . Normal values for fundus perimetry with the microperimeter MP1. Ophthalmology 2010;117:1571-6. https://doi.org/10.1016/j.ophtha.2009.12.044.
- Weingessel B, Sacu S, Vécsei-Marlovits PV, Weingessel A, Richter-Mueksch S, Schmidt-Erfurth U. Interexaminer and intraexaminer reliability of the microperimeter MP-1. Eye 2009;23:1052-8. https://doi.org/10.1038/eye.2008.237.
- Wu Z, Ayton LN, Guymer RH, Luu CD. Intrasession test-retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7378-85. https://doi.org/10.1167/iovs.13-12617.
- Owsley C, Jackson GR, Cideciyan AV, Huang Y, Fine SL, Ho AC, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41:267-73.
- Owsley C, McGwin G, Jackson GR, Kallies K, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology 2007;114:1728-35. https://doi.org/10.1016/j.ophtha.2006.12.023.
- Parisi V, Perillo L, Tedeschi M, Scassa C, Gallinaro G, Capaldo N, et al. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina 2007;27:879-90. https://doi.org/10.1097/IAE.0b013e318042d6aa.
- Schmitz-Valckenberg S, Bültmann S, Dreyhaupt J, Bindewald A, Holz FG, Rohrschneider K. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45:4470-6. https://doi.org/10.1167/iovs.03-1311.
- Caramoy A, Liakopoulos S, Menrath E, Kirchhof B. Autologous translocation of choroid and retinal pigment epithelium in geographic atrophy: long-term functional and anatomical outcome. Br J Ophthalmol 2010;94:1040-4. https://doi.org/10.1136/bjo.2009.161299.
- Wong WT, Kam W, Cunningham D, Harrington M, Hammel K, Meyerle CB, et al. Treatment of geographic atrophy by the topical administration of OT-551: results of a phase II clinical trial. Invest Ophthalmol Vis Sci 2010;51:6131-9. https://doi.org/10.1167/iovs.10-5637.
- Park SS, Bauer G, Abedi M, Pontow S, Panorgias A, Jonnal R, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci 2014;56:81-9. https://doi.org/10.1167/iovs.14-15415.
- Petrou PA, Cunningham D, Shimel K, Harrington M, Hammel K, Cukras CA, et al. Intravitreal sirolimus for the treatment of geographic atrophy: results of a phase I/II clinical trial. Invest Ophthalmol Vis Sci 2014;56:330-8. https://doi.org/10.1167/iovs.14-15877.
- Cuevas P, Outeiriño LA, Angulo J, Giménez-Gallego G. Treatment of dry age-related macular degeneration with dobesilate. BMJ Case Rep 2012;2012. https://doi.org/10.1136/bcr.02.2012.5942.
- Luttrull JK, Margolis BW. Functionally guided retinal protective therapy for dry age-related macular and inherited retinal degenerations: a pilot study. Invest Ophthalmol Vis Sci 2016;57:265-75. https://doi.org/10.1167/iovs.15-18163.
- Kondrot EC. Improvement in vision parameters for participants treated with alternative therapies in a 3-day program. Altern Ther Health Med 2015;21:22-35.
- Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years. Invest Ophthalmol Vis Sci 2016;57:ORSFc1-9. https://doi.org/10.1167/iovs.15-18681.
- Fishman GA. Historical evolution in the understanding of Stargardt macular dystrophy. Ophthalmic Genet 2010;31:183-9. https://doi.org/10.3109/13816810.2010.499887.
- Haddley K. Stargardt disease: light at the end of the tunnel. Drugs of the Future 2011;36:527-33. https://doi.org/10.1358/dof.2011.036.07.1673558.
- Spiteri Cornish K, Ho J, Downes S, Scott NW, Bainbridge J, Lois N. The epidemiology of Stargardt disease in the United Kingdom. Ophthalmol Retina 2017;1:508-13. https://doi.org/10.1016/j.oret.2017.03.001.
- Giani A, Pellegrini M, Carini E, Peroglio Deiro A, Bottoni F, Staurenghi G. The dark atrophy with indocyanine green angiography in Stargardt disease. Invest Ophthalmol Vis Sci 2012;53:3999-4004. https://doi.org/10.1167/iovs.11-9258.
- Pellegrini M, Acquistapace A, Oldani M, Cereda MG, Giani A, Cozzi M, et al. Dark atrophy: an optical coherence tomography angiography study. Ophthalmology 2016;123:1879-86. https://doi.org/10.1016/j.ophtha.2016.05.041.
- Lambertus S, Lindner M, Bax NM, Mauschitz MM, Nadal J, Schmid M, et al. Progression of late-onset Stargardt disease. Invest Ophthalmol Vis Sci 2016;57:5186-91. https://doi.org/10.1167/iovs.16-19833.
- Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology 2003;110:1151-8. https://doi.org/10.1016/S0161-6420(03)00333-6.
- Kong X, Strauss RW, Michaelides M, Cideciyan AV, Sahel JA, Muñoz B, et al. Visual acuity loss and associated risk factors in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 2). Ophthalmology 2016;123:1887-97. https://doi.org/10.1016/j.ophtha.2016.05.027.
- Lambertus S, Bax NM, Groenewoud JM, Cremers FP, van der Wilt GJ, Klevering BJ, et al. Asymmetric inter-eye progression in Stargardt disease. Invest Ophthalmol Vis Sci 2016;57:6824-30. https://doi.org/10.1167/iovs.16-20963.
- van Huet RA, Bax NM, Westeneng-Van Haaften SC, Muhamad M, Zonneveld-Vrieling MN, Hoefsloot LH, et al. Foveal sparing in Stargardt disease. Invest Ophthalmol Vis Sci 2014;55:7467-78. https://doi.org/10.1167/iovs.13-13825.
- Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol 2001;119:359-69. https://doi.org/10.1001/archopht.119.3.359.
- Fujinami K, Lois N, Davidson AE, Mackay DS, Hogg CR, Stone EM, et al. A longitudinal study of stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correlations. Am J Ophthalmol 2013;155:1075-88.e13. https://doi.org/10.1016/j.ajo.2013.01.018.
- McBain VA, Townend J, Lois N. Progression of retinal pigment epithelial atrophy in stargardt disease. Am J Ophthalmol 2012;154:146-54. https://doi.org/10.1016/j.ajo.2012.01.019.
- Lu LJ, Liu J, Adelman RA. Novel therapeutics for Stargardt disease. Graefes Arch Clin Exp Ophthalmol 2017;255:1057-62. https://doi.org/10.1007/s00417-017-3619-8.
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA 2000;97:7154-9. https://doi.org/10.1073/pnas.130110497.
- Aleman TS, Cideciyan AV, Windsor EA, Schwartz SB, Swider M, Chico JD, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Invest Ophthalmol Vis Sci 2007;48:1319-29. https://doi.org/10.1167/iovs.06-0764.
- Lois N, Halfyard AS, Bird AC, Holder GE, Fitzke FW. Fundus autofluorescence in Stargardt macular dystrophy-fundus flavimaculatus. Am J Ophthalmol 2004;138:55-63. https://doi.org/10.1016/j.ajo.2004.02.056.
- Strauss RW, Muñoz B, Ho A, Jha A, Michaelides M, Mohand-Said S, et al. Incidence of atrophic lesions in Stargardt disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: Report No. 5. JAMA Ophthalmol 2017;135:687-95. https://doi.org/10.1001/jamaophthalmol.2017.1121.
- Grob SR, Finn A, Papakostas TD, Eliott D. Clinical trials in retinal dystrophies. Middle East Afr J Ophthalmol 2016;23:49-5. https://doi.org/10.4103/0974-9233.173135.
- Han Z, Conley SM, Naash MI. Gene therapy for Stargardt disease associated with ABCA4 gene. Adv Exp Med Biol 2014;801:719-24. https://doi.org/10.1007/978-1-4614-3209-8_90.
- Smith J, Ward D, Michaelides M, Moore AT, Simpson S. New and emerging technologies for the treatment of inherited retinal diseases: a horizon scanning review. Eye 2015;29:1131-40. https://doi.org/10.1038/eye.2015.115.
- Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol 2017;101:25-30. https://doi.org/10.1136/bjophthalmol-2016-308823.
- Röck T, Schatz A, Naycheva L, Gosheva M, Pach J, Wilhelm B, et al. Effects of transcorneal electrical stimulation in patients with Stargardt’s disease. Ophthalmologe 2013;110:68-73. https://doi.org/10.1007/s00347-012-2749-y.
- Teussink MM, Lee MD, Smith RT, van Huet RA, Klaver CC, Klevering BJ, et al. The effect of light deprivation in patients with Stargardt disease. Am J Ophthalmol 2015;159:964-72.e2. https://doi.org/10.1016/j.ajo.2015.02.004.
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015;385:509-16. https://doi.org/10.1016/S0140-6736(14)61376-3.
- Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr 2007;86:180-8. https://doi.org/10.1093/ajcn/86.1.180.
- Querques G, Benlian P, Chanu B, Leveziel N, Coscas G, Soubrane G, et al. DHA supplementation for late onset Stargardt disease: NAT-3 study. Clin Ophthalmol 2010;4:575-80. https://doi.org/10.2147/OPTH.S10049.
- Röck T, Schatz A, Naycheva L, Willmann G, Bartz-Schmidt K-U, Zrenner E, et al. Effects of transcorneal electrical stimulation in patients with Stargardt disease – a prospective, randomized, sham-controlled pilot study. Invest Ophthalmol Vis Sci 2011;52.
- Gregori N, Schwartz S, Regillo C, Lam B, Eliott D, Rosenfeld P, et al. PA096 Long-Term Outcomes of Human Embryonic Stem cell–derived Retinal Pigment Epithelial Cell Transplantation for Retinal Degeneration from 2 Phase 1 2 Trials n.d.
- Leung EH, Flynn HW, Albini TA, Medina CA. Retinal detachment after subretinal stem cell transplantation. Ophthalmic Surg Lasers Imaging Retina 2016;47:600-1. https://doi.org/10.3928/23258160-20160601-16.
- The Niche-Knoepfler lab stem cell blog. Study That Injects Marrow Cells into Eye, Charges $20K Raises Many Questions 2016. https://ipscell.com/2016/02/study-that-injects-marrow-cells-into-eye-charges-20k-raises-many-questions/ (accessed 18 December 2017).
- Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE, et al. Vision loss after intravitreal injection of autologous ‘stem cells’ for AMD. N Engl J Med 2017;376:1047-53. https://doi.org/10.1056/NEJMoa1609583.
- Sofi F, Sodi A, Franco F, Murro V, Biagini D, Miele A, et al. Dietary profile of patients with Stargardt’s disease and Retinitis Pigmentosa: is there a role for a nutritional approach?. BMC Ophthalmol 2016;16. https://doi.org/10.1186/s12886-016-0187-3.
- Radu RA, Yuan Q, Hu J, Peng JH, Lloyd M, Nusinowitz S, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following vitamin A supplementation. Invest Ophthalmol Vis Sci 2008;49:3821-9. https://doi.org/10.1167/iovs.07-1470.
- Auricchio A, Trapani I, Allikmets R. Gene therapy of ABCA4-associated diseases. Cold Spring Harb Perspect Med 2015;5. https://doi.org/10.1101/cshperspect.a017301.
- Dalkara D, Goureau O, Marazova K, Sahel JA. Let there be light: gene and cell therapy for blindness. Hum Gene Ther 2016;27:134-47. https://doi.org/10.1089/hum.2015.147.
- OxfordBioMedica . SAR 422459: A Gene-Based Therapy for the Treatment of Stargardt Disease 2013. www.oxfordbiomedica.co.uk/pipeline-items/sar-422459-sanofi (accessed 18 December 2017).
- Wilson DJ, Sahel JA, Weleber RG, Erker LR, Lauer AK, Stout T, et al. One year results of a phase I/IIa study of SAR422459 in patients with Stargardt macular degeneration (SMD). Invest Ophthalmol Vis Sci 2017;58.
- Antoniu S. Fresh from the designation pipeline: orphan drugs recently designated in the European Union (September-November 2013). Expert Opin Orphan Drugs 2014;2:311-5. https://doi.org/10.1517/21678707.2014.897942.
- European Medicines Agency . Soraprazan for the Treatment of Stargardt’s Disease 2013. www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2013/11/WC500156187.pdf (accessed 18 December 2017).
- Battaglia Parodi M, La Spina C, Corradetti G, Berchicci L, Petruzzi G, Bandello F. Retinal hereditary and degenerative/dystrophic diseases (non-age-related macular degeneration). Dev Ophthalmol 2016;55:205-11. https://doi.org/10.1159/000431125.
- Cuevas P, Outeiriño LA, Angulo J, Giménez-Gallego G. Treatment of Stargardt disease with dobesilate. BMJ Case Rep 2012;2012. https://doi.org/10.1136/bcr-2012-007128.
- Jurgensmeier C, Bhosale P, Bernstein PS. Evaluation of 4-methylpyrazole as a potential therapeutic dark adaptation inhibitor. Curr Eye Res 2007;32:911-15. https://doi.org/10.1080/02713680701616156.
- Charbel Issa P, Barnard AR, Herrmann P, Washington I, MacLaren RE. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Natl Acad Sci USA 2015;112:8415-20. https://doi.org/10.1073/pnas.1506960112.
- Saad L, Washington I. Can vitamin a be improved to prevent blindness due to age-related macular degeneration, Stargardt disease and other retinal dystrophies?. Adv Exp Med Biol 2016;854:355-61. https://doi.org/10.1007/978-3-319-17121-0_47.
- Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, Singerman LJ. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 2013;33:498-507. https://doi.org/10.1097/IAE.0b013e318265801d.
- Lin BioScience . LBS-008. n.d. www.linbioscience.com/Pipeline/LBS008 (accessed 24 December 2017).
- Business Wire . Lin BioScience Receives US FDA Orphan Drug Status for LBS-008 for the Treatment of Stargardt Disease n.d. www.businesswire.com/news/home/20171026006756/en/Lin-BioScience-Receives-FDA-Orphan-Drug-Status (accessed 26 December 2017).
- Dobri N, Qin Q, Kong J, Yamamoto K, Liu Z, Moiseyev G, et al. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Invest Ophthalmol Vis Sci 2013;54:85-9. https://doi.org/10.1167/iovs.12-10050.
- Moiseyev GP, Shin Y, Takahashi Y, Ma JX. Fenofibrate is a competitive inhibitor of the RPE65 isomerase. Invest Ophthalmol Visl Sci 2016;57.
- Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849-61. https://doi.org/10.1016/S0140-6736(05)67667-2.
- National Institute for Health Research . A Randomised Placebo-Controlled Clinical Trial of Fenofibrate to Prevent Progression of Non-Proliferative Retinopathy in Diabetes (LENS : Lowering Events in Non-Proliferative Retinopathy in Scotland): HTA - 14 49 84 2017. www.journalslibrary.nihr.ac.uk/programmes/hta/144984/#/ (accessed 18 December 2017).
- Sohrab MA, Allikmets R, Guarnaccia MM, Smith RT. Preimplantation genetic diagnosis for stargardt disease. Am J Ophthalmol 2010;149:651-5.e2. https://doi.org/10.1016/j.ajo.2009.11.029.
- Krenn H. Acupuncture may improve vision in patients with age-related macular degeneration (AMD): an observational study. Deutsche Zeitschrift Fur Akupunktur 2008;51:25-8. https://doi.org/10.1016/j.dza.2008.08.001.
- AAO Complementary Therapy Task Force . Acupuncture for Age-Related Macular Degeneration CTA 2007. www.aao.org/complimentary-therapy-assessment/acupuncture-agerelated-macular-degeneration-cta--m-4 (accessed 18 December 2017).
- Cuthbertson FM, Peirson SN, Wulff K, Foster RG, Downes SM. Blue light-filtering intraocular lenses: review of potential benefits and side effects. J Cataract Refract Surg 2009;35:1281-97. https://doi.org/10.1016/j.jcrs.2009.04.017.
- Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol 2008;126:1396-403. https://doi.org/10.1001/archopht.126.10.1396.
- Davison JA, Patel AS, Cunha JP, Schwiegerling J, Muftuoglu O. Recent studies provide an updated clinical perspective on blue light-filtering IOLs. Graefes Arch Clin Exp Ophthalmol 2011;249:957-68. https://doi.org/10.1007/s00417-011-1697-6.
- Henderson BA, Grimes KJ. Blue-blocking IOLs: a complete review of the literature. Surv Ophthalmol 2010;55:284-9. https://doi.org/10.1016/j.survophthal.2009.07.007.
- Lai E, Levine B, Ciralsky J. Ultraviolet-blocking intraocular lenses: fact or fiction. Curr Opin Ophthalmol 2014;25:35-9. https://doi.org/10.1097/ICU.0000000000000016.
- Chew EY, Sperduto RD, Milton RC, Clemons TE, Gensler GR, Bressler SB, et al. Risk of advanced age-related macular degeneration after cataract surgery in the Age-Related Eye Disease Study: AREDS Report No. 25. Ophthalmology 2009;116:297-303. https://doi.org/10.1016/j.ophtha.2008.09.019.
- Chew EY, Clemons TE, Agron E, Martin DF, Bressler SB, Gensler G, et al. The lack of progression of age-related macular degeneration following cataract surgery in the age-related eye disease study 2 (AREDS2). Invest Ophthalmol Vis Sci 2015;56.
- Downes SM. Ultraviolet or blue-filtering intraocular lenses: what is the evidence?. Eye 2016;30:215-21. https://doi.org/10.1038/eye.2015.267.
- Pipis A, Touliou E, Pillunat LE, Augustin AJ. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur J Ophthalmol 2015;25:128-33. https://doi.org/10.5301/ejo.5000520.
- Nagai H, Hirano Y, Yasukawa T, Morita H, Nozaki M, Wolf-Schnurrbusch U, et al. Prevention of increased abnormal fundus autofluorescence with blue light-filtering intraocular lenses. Paper presented at the 12th Congress of the European Society of Retina Specialists, Milan, September 2012. J Cataract Refract Surg 2015;41:1855-9. https://doi.org/10.1016/j.jcrs.2015.01.017.
- Lavric A, Pompe MT. Do blue-light filtering intraocular lenses affect visual function?. Optom Vis Sci 2014;91:1348-54. https://doi.org/10.1097/OPX.0000000000000390.
- Chong CF, Pham T, Chew J, Lee KL, Chang A, Liu H. Progression of age-related macular degeneration after cataract surgery in patients with a blue blocking intraocular lens in one eye and a clear intraocular lens in the fellow eye. Clin Exp Ophthalmol 2011;39.
- Łak D, Lubiński W, Sylwestrzak Z, Szych Z, Karczewicz D. Comparative assessment of the course of age-related macular degeneration in patients after phacoemulsification cataract surgery with implantation of AcrySof Natural SN 60 at and AcrySof SA 60 at lenses. Ann Acad Med Stetin 2007;53:43-7.
- Brockmann C, Schulz M, Laube T. Transmittance characteristics of ultraviolet and blue-light-filtering intraocular lenses. J Cataract Refract Surg 2008;34:1161-6. https://doi.org/10.1016/j.jcrs.2008.03.039.
- Nishi T, Saeki K, Obayashi K, Miyata K, Tone N, Tsujinaka H, et al. The effect of blue-blocking intraocular lenses on circadian biological rhythm: protocol for a randomised controlled trial (CLOCK-IOL colour study). BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007930.
- Nishi T. A Randomized Controlled Trial to Determine the Long-Term Influence of Clear Intra Ocular Lens (IOL) and Yellow IOL With Cataract Surgery on Circadian Biological Rhythm and Related Health Outcomes 2014. https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000017070 (accessed 29 December 2017).
- Brunner R, Widder RA, Walter P, Lüke C, Godehardt E, Bartz-Schmidt KU, et al. Influence of membrane differential filtration on the natural course of age-related macular degeneration: a randomized trial. Retina 2000;20:483-91. https://doi.org/10.1097/00006982-200009000-00009.
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res 2010;29:144-68. https://doi.org/10.1016/j.preteyeres.2009.12.002.
- Wang Q, Kocaoglu OP, Cense B, Bruestle J, Jonnal RS, Gao W, et al. Imaging retinal capillaries using ultrahigh-resolution optical coherence tomography and adaptive optics. Invest Ophthalmol Vis Sci 2011;52:6292-9. https://doi.org/10.1167/iovs.10-6424.
- Pulido J, Sanders D, Winters JL, Klingel R. Clinical outcomes and mechanism of action for rheopheresis treatment of age-related macular degeneration (AMD). J Clin Apher 2005;20:185-94. https://doi.org/10.1002/jca.20047.
- Pulido JS, Winters JL, Boyer D. Preliminary analysis of the final multicenter investigation of rheopheresis for age related macular degeneration (AMD) trial (MIRA-1) results. Trans Am Ophthalmol Soc 2006;104:221-31.
- Koss MJ, Kurz P, Tsobanelis T, Lehmacher W, Fassbender C, Klingel R, et al. Prospective, randomized, controlled clinical study evaluating the efficacy of Rheopheresis for dry age-related macular degeneration. Dry AMD treatment with Rheopheresis Trial-ART. Graefes Arch Clin Exp Ophthalmol 2009;247:1297-306. https://doi.org/10.1007/s00417-009-1113-7.
- Pulido JS, Sanders D, Klingel R. Rheopheresis for age-related macular degeneration: clinical results and putative mechanism of action. Can J Ophthalmol 2005;40:332-40. https://doi.org/10.1016/S0008-4182(05)80076-6.
- Rencová E, Bláha M, Studnička J, Bláha V, Lánská M, Renc O, et al. Preservation of the photoreceptor inner/outer segment junction in dry age-related macular degeneration treated by rheohemapheresis. J Ophthalmol 2015;2015. https://doi.org/10.1155/2015/359747.
- Swartz M, Rabetoy G. Treatment of non-exudative agerelated macular degeneration using membrane differential filtration apheresis [meeting abstract from the Association for Research in Vision and Ophthalmology annual meeting. Fort Lauderdale, Florida, USA. May 9–14, 1999]. Invest Ophthalmol Vis Sci 1999;40.
- Blaha M, Rencova E, Langrova H, Studnicka J, Blaha V, Rozsival P, et al. Rheohaemapheresis in the treatment of nonvascular age-related macular degeneration. Atheroscler Suppl 2013;14:179-84. https://doi.org/10.1016/j.atherosclerosissup.2012.10.023.
- Studnička J, Rencová E, Bláha M, Rozsíval P, Lánská M, Bláha V, et al. Long-term outcomes of rheohaemapheresis in the treatment of dry form of age-related macular degeneration. J Ophthalmol 2013;2013. https://doi.org/10.1155/2013/135798.
- Klingel R, Fassbender C, Heibges A, Koch F, Nasemann J, Engelmann K, et al. RheoNet registry analysis of rheopheresis for microcirculatory disorders with a focus on age-related macular degeneration. Ther Apher Dial 2010;14:276-86. https://doi.org/10.1111/j.1744-9987.2010.00807.x.
- Brunner R, Widder RA, Fischer RA, Walter P, Bartz-Schmidt KU, Heimann K, et al. Clinical efficacy of haemorheological treatment using plasma exchange, selective adsorption and membrane differential filtration in maculopathy, retinal vein occlusion and uveal effusion syndrome. Transfus Sci 1996;17:493-8. https://doi.org/10.1016/S0955-3886(96)90083-1.
- Widder RA, Farvili E, Reis RJ, Luke C, Walter P, Kirchhof B, et al. The treatment of age-related macular degeneration (ARMD) with Etracorporeal treatment procedures. A follow-up of four years. Invest Ophthalmol Vis Sci 2002;43.
- Brunner R, Widder RA, Walter P, Borberg H, Oette K. Change in hemorrheological and biochemical parameters following membrane differential filtration. Int J Artif Organs 1995;18:794-8. https://doi.org/10.1177/039139889501801208.
- Lane N. Rheopheresis – Ready for Prime Time? 2004. http://nick-lane.net/publications/rheopheresis-ready-prime-time/ (accessed 18 December 2017).
- Pulido JS. Multicenter Investigation of Rheopheresis for AMD (MIRA-1) Study Group . Multicenter prospective, randomized, double-masked, placebo-controlled study of Rheopheresis to treat nonexudative age-related macular degeneration: interim analysis. Trans Am Ophthalmol Soc 2002;100:85-106.
- Wong DT, Siegel I, Jain S, Wong K. PERC Study: An open–label trial for rheopheresis in dry AMD patients. Invest Ophthalmol Vis Sci 2006;47.
- Business Wire . OccuLogix, Inc.: Positive PERC Data Presented at ASRS 2005 Annual Meeting 2005. www.businesswire.com/news/home/20050718005582/en/OccuLogix-Positive-PERC-Data-Presented-ASRS-2005 (accessed 18 December 2017).
- Kamami-Levy CJ, Glacet-Bernard A, Querques G, Dumont MEA, Saheb S, Soubrane G, et al. Rheopheresis in the treatment of nonexudative AMD. Invest Ophthalmol Vis Sci 2014;55.
- Jaffe GJ, Schmitz-Valckenberg S, Boyer D, Heier J, Wolf-Schnurrbusch U, Staurenghi G, et al. Randomized trial to evaluate tandospirone in geographic atrophy secondary to age-related macular degeneration: the GATE study. Am J Ophthalmol 2015;160:1226-34. https://doi.org/10.1016/j.ajo.2015.08.024.
- Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The incidence and progression of age-related macular degeneration over 15 years: the Blue Mountains Eye study. Ophthalmology 2015;122:2482-9. https://doi.org/10.1016/j.ophtha.2015.08.002.
- Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lin XM. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/564738.
- Ali F, Armogan N. Dry Age-Related Macular Degeneration Therapy Utilizing Heparin-Induced Extracorporeal Lipoprotein Precipitation (HELP) Apheresis n.d. www.euretina.org/barcelona2017/programme/posters-details.asp?id=13549 (accessed 24 December 2017).
- AAO Complementary Therapy Task Force . Microcurrent Stimulation for Macular Degeneration 2004. www.aao.org/Assets/f77155a3-802a-4f6c-9891-9e65e38d84af/634965436430000000/microcurrent-stimulation-for-macular-degeneration-pdf (accessed 18 December 2017).
- Naycheva L, Schatz A, Röck T, Willmann G, Messias A, Bartz-Schmidt KU, et al. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci 2012;53:7440-8. https://doi.org/10.1167/iovs.12-9612.
- Anastassiou G, Schneegans AL, Selbach M, Kremmer S. Transpalpebral electrotherapy for dry age-related macular degeneration (AMD): an exploratory trial. Restor Neurol Neurosci 2013;31:571-8. https://doi.org/10.3233/RNN-130322.
- Sehic A, Guo S, Cho KS, Corraya RM, Chen DF, Utheim TP. Electrical stimulation as a means for improving vision. Am J Pathol 2016;186:2783-97. https://doi.org/10.1016/j.ajpath.2016.07.017.
- Chaikin L, Kashiwa K, Bennet M, Papastergiou G, Gregory W. Microcurrent stimulation in the treatment of dry and wet macular degeneration. Clin Ophthalmol 2015;9:2345-53. https://doi.org/10.2147/OPTH.S92296.
- Shinoda K, Imamura Y, Matsuda S, Seki M, Uchida A, Grossman T, et al. Transcutaneous electrical retinal stimulation therapy for age-related macular degeneration. Open Ophthalmol J 2008;2:132-6. https://doi.org/10.2174/1874364100802010132.
- Kurimoto T, Oono S, Oku H, Tagami Y, Kashimoto R, Takata M, et al. Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin Ophthalmol 2010;4:1441-6. https://doi.org/10.2147/OPTH.S14573.
- Coleman DJ, Silverman RH, Rondeau MJ, Lloyd HO, Khanifar AA, Chan RV. Age-related macular degeneration: choroidal ischaemia?. Br J Ophthalmol 2013;97:1020-3. https://doi.org/10.1136/bjophthalmol-2013-303143.
- Gorovits R, Avidan N, Avisar N, Shaked I, Vardimon L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc Natl Acad Sci USA 1997;94:7024-9. https://doi.org/10.1073/pnas.94.13.7024.
- Gamboa OL, Pu J, Townend J, Forrester JV, Zhao M, McCaig C, et al. Electrical estimulation of retinal pigment epithelial cells. Exp Eye Res 2010;91:195-204. https://doi.org/10.1016/j.exer.2010.04.018.
- Michael DL, Allen MJ. Nutritional supplementation, electrical stimulation and age-related macular degeneration. J Orthomol Med 1993;8:161-71.
- Allen MJ, Jarding JB, Zehner R. Macular degeneration treatment with nutrients and micro current electricity. J Orthomol Med 1998;13:211-4.
- Wallace l. The treatment of macular degeneration and other retinal diseases using bioelectromagnetics therapy. J of Optometric Phototherapy 1997;4-5.
- Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Kurinij N, et al. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS Report No. 35. Ophthalmology 2013;120:1604-11.e4. https://doi.org/10.1016/j.ophtha.2013.01.021.
- Kondrot EC. Initial results of microcurrent stimulation in the treatment of age related macular degeneration. Townsend Letter for Doctors and Patients 2002;231:65-7.
- Natarajan S, Kar D, Uparkar M, Doctor P, Hussain A, Mhatre A, et al. Micro current neuromodulation in the management of dry age-related macular degeneration - a pilot study. Invest Ophthalmol Vis Sci 2009;50.
- Natarajan S, Uparkar M, Khulsange A, Gadgil D. Microcurrent neuromodulation in the management of dry age-related macular degeneration. Doc Ophthalmol 2008;117:24-5. https://doi.org/DOI 10.1007/s10633-008-9140-y.
- PRWeb . Nova Oculus Enlists Intertek to Audit ISO Certification Process 2017. www.prweb.com/releases/2017/04/prweb14202095.htm (accessed 18 December 2017).
- ScyFIX . Clinical Studies. n.d. www.scyfix.org/clinical_studies.htm (accessed 24 December 2017).
- Halloran G. Bioelectrical Stimulation in An Integrated Treatment for Macular Degeneration, Retinitis Pigmentosa, Glaucoma,CMV-Retinitis, &Amp; Diabetic Retinopathy n.d. https://organicmd.com/1997-presentation-study-on-the-better-eye-health-visual-healing-program (accessed 24 December 2017).
- PRWeb . The Eye Machine Acquires Acuity Medical and Two U.S. Patents 2015. www.prweb.com/releases/2015/6/prweb12789788.htm (accessed 18 December 2017).
- Gass JD. Photocoagulation of macular lesions. Trans Am Acad Ophthalmol Otolaryngol 1971;75:580-608.
- Cukras C, Fine SL. Thermal laser treatment in AMD: therapeutic and prophylactic. Int Ophthalmol Clin 2007;47:75-93. https://doi.org/10.1097/IIO.0b013e31802bda65.
- Little HL, Showman JM, Brown BW. A pilot randomized controlled study on the effect of laser photocoagulation of confluent soft macular drusen. Ophthalmology 1997;104:623-31. https://doi.org/10.1016/S0161-6420(97)30261-9.
- Frennesson C, Nilsson SE. Prophylactic laser treatment in early age related maculopathy reduced the incidence of exudative complications. Br J Ophthalmol 1998;82:1169-74. https://doi.org/10.1136/bjo.82.10.1169.
- Olk RJ, Friberg TR, Stickney KL, Akduman L, Wong KL, Chen MC, et al. Therapeutic benefits of infrared (810-nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age-related macular degeneration: two-year results of a randomized pilot study. Ophthalmology 1999;106:2082-90. https://doi.org/10.1016/S0161-6420(99)90487-6.
- Scorolli L, Corazza D, Morara M, Vismara S, Lugaresi ML, Meduri RA. Argon laser vs. subthreshold infrared (810-nm) diode laser macular grid photocoagulation in nonexudative age-related macular degeneration. Can J Ophthalmol 2003;38:489-95. https://doi.org/10.1016/S0008-4182(03)80028-5.
- Marshall J. The 2014 Bowman Lecture–Bowman’s and Bruch’s: a tale of two membranes during the laser revolution. Eye 2015;29:46-64. https://doi.org/10.1038/eye.2014.240.
- Virgili G, Michelessi M, Parodi MB, Bacherini D, Evans JR. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev 2015;10. https://doi.org/10.1002/14651858.CD006537.pub3.
- Huang YX, Xiang LN, Wang YL, Li MM, Hu YX. Long-term effect of prophylactic laser treatment for bilateral soft drusen. Chin Med J 2011;124:541-5.
- Brader HS, Young LH. Subthreshold diode micropulse laser: a review. Semin Ophthalmol 2016;31:30-9. https://doi.org/10.3109/08820538.2015.1114837.
- Choroidal Neovascularisation Prevention Trial Research Group . Laser treatment in eyes with large drusen. Short-term effects seen in a pilot randomized clinical trial. Choroidal Neovascularization Prevention Trial Research Group. Ophthalmology 1998;105:11-23.
- Choroidal Neovascularization Prevention Trial Research Group . Laser treatment in fellow eyes with large drusen: updated findings from a pilot randomized clinical trial. Ophthalmology 2003;110:971-8. https://doi.org/10.1016/S0161-6420(03)00098-8.
- Owens SL, Bunce C, Brannon AJ, Xing W, Chisholm IH, Gross M, et al. Prophylactic laser treatment hastens choroidal neovascularization in unilateral age-related maculopathy: final results of the drusen laser study. Am J Ophthalmol 2006;141:276-81. https://doi.org/10.1016/j.ajo.2005.08.019.
- Kaiser RS, Berger JW, Maguire MG, Ho AC, Javornik NB. Choroidal Neovascularization Prevention Trial Study Group . Laser burn intensity and the risk for choroidal neovascularization in the CNVPT Fellow Eye Study. Arch Ophthalmol 2001;119:826-32. https://doi.org/10.1001/archopht.119.6.826.
- Ruiz-Moreno J, De la Vega C, Zarbin MA. Macular atrophy after photocoagulation of soft drusen. Retina 2003;23:315-21. https://doi.org/10.1097/00006982-200306000-00005.
- Friberg TR, Musch DC, Lim JI, Morse L, Freeman W, Sinclair S. PTAMD Study Group . Prophylactic treatment of age-related macular degeneration report number 1: 810-nanometer laser to eyes with drusen. Unilaterally eligible patients. Ophthalmology 2006;113. https://doi.org/10.1016/j.ophtha.2005.10.066.
- Frennesson CI, Bek T, Jaakkola A, Nilsson SE. Prophylactic Laser Treatment Study Group . Prophylactic laser treatment of soft drusen maculopathy: a prospective, randomized Nordic study. Acta Ophthalmol 2009;87:720-4. https://doi.org/10.1111/j.1755-3768.2008.01396.x.
- Guymer RH, Brassington KH, Dimitrov P, Makeyeva G, Plunkett M, Xia W, et al. Nanosecond-laser application in intermediate AMD: 12-month results of fundus appearance and macular function. Clin Experiment Ophthalmol 2014;42:466-79. https://doi.org/10.1111/ceo.12247.
- Jobling AI, Guymer RH, Vessey KA, Greferath U, Mills SA, Brassington KH, et al. Nanosecond laser therapy reverses pathologic and molecular changes in age-related macular degeneration without retinal damage. FASEB J 2015;29:696-710. https://doi.org/10.1096/fj.14-262444.
- Geneva II. Photobiomodulation for the treatment of retinal diseases: a review. Int J Ophthalmol 2016;9:145-52. https://doi.org/10.18240/ijo.2016.01.24.
- Hsu J, Maguire MG, Fine SL. Laser prophylaxis for age-related macular degeneration. Can J Ophthalmol 2005;40:320-31. https://doi.org/10.1016/S0008-4182(05)80075-4.
- Prahs P, Walter A, Regler R, Theisen-Kunde D, Birngruber R, Brinkmann R, et al. Selective retina therapy (SRT) in patients with geographic atrophy due to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2010;248:651-8. https://doi.org/10.1007/s00417-009-1208-1.
- Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg 2008;26:241-5. https://doi.org/10.1089/pho.2007.2132.
- Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol 2016;95:e270-e277. https://doi.org/10.1111/aos.13354.
- Figueroa MS, Regueras A, Bertrand J, Aparicio MJ, Manrique MG. Laser photocoagulation for macular soft drusen. Updated results. Retina 1997;17:378-84. https://doi.org/10.1097/00006982-199717050-00004.
- Slezak M. Low-power laser may keep blindness at bay. New Sci 2013;217:8-9.
- Beaumont P, Kang HK, Gorbatov M, Do H, Matthew R. Prophylactic laser photocoagulation of drusen in early age-related macular degeneration. Clin Exp Ophthalmol 2011;39.
- Scalinci SZ, Milone F, Magnifico M, Berti C, Limoli PG, Vingolo EM, et al. Photobiomodulation (phototherapy) of retinal tissue in Stargardt disease. Invest Ophthalmol Vis Sci 2015;56.
- Bocci V. The case for oxygen-ozonetherapy. Br J Biomed Sci 2007;64:44-9. https://doi.org/10.1080/09674845.2007.11732755.
- Bocci V, Bocci V. Ozone: A New Medical Drug. Berlin: Springer; 2011.
- Borrelli E, Bocci V. Visual improvement following ozonetherapy in dry age related macular degeneration; a review. Med Hypothesis Discov Innov Ophthalmol 2013;2:47-51.
- Bocci V, Zanardia I, Valacchi G, Borrelli E, Travagli V. Validity of oxygen-ozone therapy as integrated medication form in chronic inflammatory diseases. Cardiovasc Hematol Disord Drug Targets 2015;15:127-38. https://doi.org/10.2174/1871529X1502151209114642.
- Zanardi I, Borrelli E, Valacchi G, Travagli V, Bocci V. Ozone: A multifaceted molecule with unexpected therapeutic activity. Curr Med Chem 2016;23:304-14. https://doi.org/10.2174/0929867323666151221150420.
- Borrelli E, Diadori A, Zalaffi A, Bocci V. Effects of major ozonated autohemotherapy in the treatment of dry age related macular degeneration: a randomized controlled clinical study. Int J Ophthalmol 2012;5:708-13. https://doi.org/10.3980/j.issn.2222-3959.2012.06.11.
- Hau VS, London N, Dalton M. The treatment paradigm for the implantable miniature telescope. Ophthalmol Ther 2016;5:21-30. https://doi.org/10.1007/s40123-016-0047-5.
- Hudson HL, Lane SS, Heier JS, Stulting RD, Singerman L, Lichter PR, et al. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology 2006;113:1987-2001. https://doi.org/10.1016/j.ophtha.2006.07.010.
- Boyer D, Freund KB, Regillo C, Levy MH, Garg S. Long-term (60-month) results for the implantable miniature telescope: efficacy and safety outcomes stratified by age in patients with end-stage age-related macular degeneration. Clin Ophthalmol 2015;9:1099-107. https://doi.org/10.2147/OPTH.S86208.
- Hudson HL, Stulting RD, Heier JS, Lane SS, Chang DF, Singerman LJ, et al. Implantable telescope for end-stage age-related macular degeneration: long-term visual acuity and safety outcomes. Am J Ophthalmol 2008;146:664-73. https://doi.org/10.1016/j.ajo.2008.07.003.
- Lane SS, Kuppermann BD. The implantable miniature telescope for macular degeneration. Curr Opin Ophthalmol 2006;17:94-8. https://doi.org/10.1097/01.icu.0000193067.86627.a1.
- Qureshi MA, Robbie SJ, Tabernero J, Artal P. Injectable intraocular telescope: pilot study. J Cataract Refract Surg 2015;41:2125-35. https://doi.org/10.1016/j.jcrs.2015.03.021.
- Agarwal A, Lipshitz I, Jacob S, Lamba M, Tiwari R, Kumar DA, et al. Mirror telescopic intraocular lens for age-related macular degeneration: design and preliminary clinical results of the Lipshitz macular implant. J Cataract Refract Surg 2008;34:87-94. https://doi.org/10.1016/j.jcrs.2007.08.031.
- Hengerer FH, Artal P, Kohnen T, Conrad-Hengerer I. Initial clinical results of a new telescopic IOL implanted in patients with dry age-related macular degeneration. J Refract Surg 2015;31:158-62. https://doi.org/10.3928/1081597X-20150220-03.
- Guo X, Zhu D, Lian R, Han Y, Guo Y, Li Z, et al. Matrigel and Activin A promote cell-cell contact and anti-apoptotic activity in cultured human retinal pigment epithelium cells. Exp Eye Res 2016;147:37-49. https://doi.org/10.1016/j.exer.2016.04.021.
- Scharioth GB. New add-on intraocular lens for patients with age-related macular degeneration. J Cataract Refract Surg 2015;41:1559-63. https://doi.org/10.1016/j.jcrs.2015.07.018.
- Tabernero J, Qureshi MA, Robbie SJ, Artal P. An aspheric intraocular telescope for age-related macular degeneration patients. Biomed Opt Express 2015;6:1010-20. https://doi.org/10.1364/BOE.6.001010.
- Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS, Lane SS. Comparative effectiveness and cost-effectiveness of the implantable miniature telescope. Ophthalmology 2011;118:1834-43. https://doi.org/10.1016/j.ophtha.2011.02.012.
- VisionCare . CentraSight 2017. www.centrasight.com/ (accessed 18 December 2017).
- NHS Health Research Authority . Efficacy of the Telescopic Mirror Implant for Age-Related Macular Degeneration: The MIRROR Trial. A Multicentre Randomised Controlled Clinical Trial 2016. www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/mirror-trial-version-10/ (accessed 18 December 2017).
- Silvestri G, Agus A, Murphy L, Wilkins M, McKibbin M, Gardner E, et al. Efficacy of the Telescopic Mirror Implant for Age-Related Macular Degeneration: The MIRROR Trial n.d. www.journalslibrary.nihr.ac.uk/programmes/eme/1316003/#/ (accessed 24 December 2017).
- Qureshi MA, Robbie SJ, Hengerer FH, Auffarth GU, Conrad-Hengerer I, Artal P. Consecutive case series of 244 age-related macular degeneration patients undergoing implantation with an extended macular vision IOL [published online ahead of print October 5 2017]. Eur J Ophthalmol 2017. https://doi.org/10.S301/ejo.5001052.
- McKeague C, Margrain TH, Bailey C, Binns AM. Low-level night-time light therapy for age-related macular degeneration (ALight): study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-246.
- Lek JJ, Brassington KH, Luu CD, Chen FK, Arnold JJ, Heriot WJ, et al. Subthreshold nanosecond laser intervention in intermediate age-related macular degeneration: study design and baseline characteristics of the Laser in Early Stages of Age-Related Macular Degeneration study (Report Number 1). Ophthalmol Retina 2017;1:227-39. https://doi.org/10.1016/j.oret.2016.12.001.
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 2012;379:713-20. https://doi.org/10.1016/S0140-6736(12)60028-2.
- Ramsden CM, da Cruz L, Coffey PJ. Stemming the tide of age-related macular degeneration: new therapies for old retinas. Invest Ophthalmol Vis Sci 2016;57:ORSFb1-3. https://doi.org/10.1167/iovs.15-18643.
- Da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration [published online ahead of print March 19 2018]. Nat Biotechnol 2018. https://doi.org/10.1038/nbt.4114.
- Zarbin M. Cell-based therapy for degenerative retinal disease. Trends Mol Med 2016;22:115-34. https://doi.org/10.1016/j.molmed.2015.12.007.
- Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Reports 2015;4:860-72. https://doi.org/10.1016/j.stemcr.2015.04.005.
- Banin E, Jaouni T, Gurevich M, Irving C, Cuzzani OE. Phase 1 and 2a Study of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Transplanted Subretinally In Advanced Dry-Form AMD Patients n.d.
- Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci USA 2011;108:6241-5. https://doi.org/10.1073/pnas.1018987108.
- Nommiste B, Fynes K, Tovell VE, Ramsden C, da Cruz L, Coffey P. Stem cell-derived retinal pigment epithelium transplantation for treatment of retinal disease. Prog Brain Res 2017;231:225-44. https://doi.org/10.1016/bs.pbr.2017.03.003.
- London Project to Cure Blindness . First Patient Receives Potential New Treatment for Wet Age-Related Macular Degeneration in London Project to Cure Blindness 2017. www.thelondonproject.org/ (accessed 18 December 2017).
- Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol 2017;179:67-80. https://doi.org/10.1016/j.ajo.2017.04.006.
- Guymer RH, Chiu AW, Lim L, Baird PN. HMG CoA reductase inhibitors (statins): do they have a role in age-related macular degeneration?. Surv Ophthalmol 2005;50:194-206. https://doi.org/10.1016/j.survophthal.2004.12.002.
- Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD006927.pub4.
- Ma L, Wang Y, Du J, Wang M, Zhang R, Fu Y. The association between statin use and risk of age-related macular degeneration. Sci Rep 2015;5. https://doi.org/10.1038/srep18280.
- Peponis V, Chalkiadakis SE, Bonovas S, Sitaras NM. The controversy over the association between statins use and progression of age-related macular degeneration: a mini review. Clin Ophthalmol 2010;4:865-9.
- Tsao SW, Fong DS. Do statins have a role in the prevention of age-related macular degeneration?. Drugs Aging 2013;30:205-13. https://doi.org/10.1007/s40266-013-0061-4.
- Martini E, Scorolli L, Burgagni MS, Fessehaie S. Evaluation of the retinal effects of simvastatin in patients with age-related macular degeneration. Ann Ottalmol Clin Ocul 1991;117:1121-6.
- Guymer RH, Baird PN, Varsamidis M, Busija L, Dimitrov PN, Aung KZ, et al. Proof of concept, randomized, placebo-controlled study of the effect of simvastatin on the course of age-related macular degeneration. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0083759.
- McGwin G, Xie A, Owsley C. The use of cholesterol-lowering medications and age-related macular degeneration. Ophthalmology 2005;112:488-94. https://doi.org/10.1016/j.ophtha.2004.10.027.
- Etminan M, Brophy JM, Maberley D. Use of statins and angiotensin converting enzyme inhibitors (ACE-Is) and the risk of age-related macular degeneration: nested case-control study. Curr Drug Saf 2008;3:24-6. https://doi.org/10.2174/157488608783333952.
- Vavvas DG, Daniels AB, Kapsala ZG, Goldfarb JW, Ganotakis E, Loewenstein JI, et al. Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment. EBioMedicine 2016;5:198-203. https://doi.org/10.1016/j.ebiom.2016.01.033.
- Tzotzas T, Apostolopoulou D, Memi E, Efthymiou H, Krassas GE. Th-P16:341 Simvastatin reduces the progression of the early stages (Drusen) of age-related macular degeneration: a pilot study. Atheroscler Suppl 2006;7:568-9. https://doi.org/10.1016/S1567-5688(06)82299-3.
- Hall NF, Gale CR, Syddall H, Phillips DI, Martyn CN. Risk of macular degeneration in users of statins: cross sectional study. BMJ 2001;323:375-6. https://doi.org/10.1136/bmj.323.7309.375.
- Klein R, Knudtson MD, Klein BE. Statin use and the five-year incidence and progression of age-related macular degeneration. Am J Ophthalmol 2007;144:1-6. https://doi.org/10.1016/j.ajo.2007.02.047.
- Tan JS, Mitchell P, Rochtchina E, Wang JJ. Statins and the long-term risk of incident age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol 2007;143:685-7. https://doi.org/10.1016/j.ajo.2006.11.021.
- Al-Holou SN, Tucker WR, Agrón E, Clemons TE, Cukras C, Ferris FL, et al. The association of statin use with age-related macular degeneration progression: the Age-Related Eye Disease study 2 Report Number 9. Ophthalmology 2015;122:2490-6. https://doi.org/10.1016/j.ophtha.2015.08.028.
- Guymer RH, Dimitrov PN, Varsamidis M, Lim LL, Baird PN, Vingrys AJ, et al. Can HMG Co-A reductase inhibitors (‘statins’) slow the progression of age-related macular degeneration? The age-related maculopathy statin study (ARMSS). Clin Interv Aging 2008;3:581-93. https://doi.org/10.2147/CIA.S2748.
- Maguire MG, Ying GS, McCannel CA, Liu C, Dai Y. Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group . Statin use and the incidence of advanced age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology 2009;116:2381-5. https://doi.org/10.1016/j.ophtha.2009.06.055.
- Barbosa DT, Mendes TS, Cíntron-Colon HR, Wang SY, Bhisitkul RB, Singh K, et al. Age-related macular degeneration and protective effect of HMG Co-A reductase inhibitors (statins): results from the National Health and Nutrition Examination Survey 2005-2008. Eye 2014;28:472-80. https://doi.org/10.1038/eye.2014.8.
- McGwin G, Owsley C, Curcio CA, Crain RJ. The association between statin use and age related maculopathy. Br J Ophthalmol 2003;87:1121-5. https://doi.org/10.1136/bjo.87.9.1121.
- VanderBeek BL, Zacks DN, Talwar N, Nan B, Stein JD. Role of statins in the development and progression of age-related macular degeneration. Retina 2013;33:414-22. https://doi.org/10.1097/IAE.0b013e318276e0cf.
- Kaiserman N, Vinker S, Kaiserman I. Statins do not decrease the risk for wet age-related macular degeneration. Curr Eye Res 2009;34:304-10. https://doi.org/10.1080/02713680902741670.
- Fong DS, Contreras R. Recent statin use and 1-year incidence of exudative age-related macular degeneration. Am J Ophthalmol 2010;149:955-8.e1. https://doi.org/10.1016/j.ajo.2009.12.037.
- Dashti N, McGwin G, Owsley C, Curcio CA. Plasma apolipoproteins and risk for age related maculopathy. Br J Ophthalmol 2006;90:1028-33. https://doi.org/10.1136/bjo.2006.093856.
- Wang Y, Wang M, Zhang X, Zhang Q, Nie J, Zhang M, et al. The association between the lipids levels in blood and risk of age-related macular degeneration. Nutrients 2016;8. https://doi.org/10.3390/nu8100663.
- Colak ES, Majkic-Singh NT, Stankovic SS, Kosanovic-Jakovic NG, Zoric LD, Radosavljevic AP, et al. Gender associated lipid and apolipoprotein profile in patients with age-related macular degeneration. EJIFCC 2011;22:16-23.
- Ebrahimi KB, Handa JT. Lipids, lipoproteins, and age-related macular degeneration. J Lipids 2011;2011. https://doi.org/10.1155/2011/802059.
- Shalev V, Sror M, Goldshtein I, Kokia E, Chodick G. Statin use and the risk of age related macular degeneration in a large health organization in Israel. Ophthalmic Epidemiol 2011;18:83-90. https://doi.org/10.3109/09286586.2011.560746.
- Smeeth L, Cook C, Chakravarthy U, Hubbard R, Fletcher AE. A case control study of age related macular degeneration and use of statins. Br J Ophthalmol 2005;89:1171-5. https://doi.org/10.1136/bjo.2004.064477.
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009;67:99-109. https://doi.org/10.1111/j.1365-2125.2008.03308.x.
- Institut de la Màcula . Statins4Drusen n.d. www.institutmacula.com/en/investigacion/statins4drusen/ (accessed 26 December 2017).
- Petrukhin K. Pharmacological inhibition of lipofuscin accumulation in the retina as a therapeutic strategy for dry AMD treatment. Drug Discov Today Ther Strateg 2013;10:e11-e20. https://doi.org/10.1016/j.ddstr.2013.05.004.
- Dugel PU, Novack RL, Csaky KG, Richmond PP, Birch DG, Kubota R. Phase ii, randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration. Retina 2015;35:1173-83. https://doi.org/10.1097/IAE.0000000000000606.
- Holz FG. Negative Dry AMD Results : Emixustat Fails to Slow Geographic Atrophy in 24-Month Trial 2016. www.eurotimes.org/negative-dry-amd-results/ (accessed 18 December 2017).
- Buschini E, Fea AM, Lavia CA, Nassisi M, Pignata G, Zola M, et al. Recent developments in the management of dry age-related macular degeneration. Clin Ophthalmol 2015;9:563-74. https://doi.org/10.2147/OPTH.S59724.
- Danis RP, Lavine JA, Domalpally A. Geographic atrophy in patients with advanced dry age-related macular degeneration: current challenges and future prospects. Clin Ophthalmol 2015;9:2159-74. https://doi.org/10.2147/OPTH.S92359.
- De Palo G, Veronesi U, Marubini E, Camerini T, Chiesa F, Nava M, et al. Controlled clinical trials with fenretinide in breast cancer, basal cell carcinoma and oral leukoplakia. J Cell Biochem 1995;58:11-7. https://doi.org/10.1002/jcb.240590803.
- Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, Mariani L, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst 1999;91:1847-56. https://doi.org/10.1093/jnci/91.21.1847.
- Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, et al. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol 2001;19:1664-70. https://doi.org/10.1200/JCO.2001.19.6.1664.
- Chiesa F, Tradati N, Marazza M, Rossi N, Boracchi P, Mariani L, et al. Fenretinide (4-HPR) in chemoprevention of oral leukoplakia. J Cell Biochem 1993;52:255-61. https://doi.org/10.1002/jcb.240531038.
- Costa A, Malone W, Perloff M, Buranelli F, Campa T, Dossena G, et al. Tolerability of the synthetic retinoid fenretinide (HPR). Eur J Cancer Clin Oncol 1989;25:805-8. https://doi.org/10.1016/0277-5379(89)90124-7.
- Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med 2017;9.
- Corporation O. A Clinical Trial to Assess the Safety and Efficacy of Intravitreous Administration of Zimura® in Subjects With Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration 2016. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-003991-56-HU (accessed 18 December 2017).
- Williams MA, McKay GJ, Chakravarthy U. Complement inhibitors for age-related macular degeneration. Cochrane Database Syst Rev 2014;1. https://doi.org/10.1002/14651858.CD009300.pub2.
- Kefauver SC. Emixustat hydrochloride: retinoid isomerohydrolase (RPE65) inhibitor treatment of age-related macular degeneration. Drugs of the Future 2014;39:615-25. https://doi.org/10.1358/dof.2014.039.09.2207181.
- Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglarnia AR, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest 2015;45:423-40. https://doi.org/10.1111/eci.12419.
- Querques G, Rosenfeld PJ, Cavallero E, Borrelli E, Corvi F, Querques L, et al. Treatment of dry age-related macular degeneration. Ophthalmic Res 2014;52:107-15. https://doi.org/10.1159/000363187.
- Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology 2014;121:693-701. https://doi.org/10.1016/j.ophtha.2013.09.044.
- Brilliant MH, Vaziri K, Connor TB, Schwartz SG, Carroll JJ, McCarty CA, et al. Mining retrospective data for virtual prospective drug repurposing: L-DOPA and age-related macular degeneration. Am J Med 2016;129:292-8. https://doi.org/10.1016/j.amjmed.2015.10.015.
- Augustin AJ, Diehm C, Grieger F, Bentz J. Alprostadil infusion in patients with dry age related macular degeneration: a randomized controlled clinical trial. Expert Opin Investig Drugs 2013;22:803-12. https://doi.org/10.1517/13543784.2013.794782.
- Ladewig MS, Ladewig K, Güner M, Heidrich H. Prostaglandin E1 infusion therapy in dry age-related macular degeneration. Prostaglandins Leukot Essent Fatty Acids 2005;72:251-6. https://doi.org/10.1016/j.plefa.2004.11.006.
- Seddon JM, Gensler G, Klein ML, Milton RC. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am J Ophthalmol 2006;141:201-3. https://doi.org/10.1016/j.ajo.2005.07.059.
- Remky A, Weber A, Arend O, Sponsel WE. Topical dorzolamide increases pericentral visual function in age-related maculopathy: pilot study findings with short-wavelength automated perimetry. Acta Ophthalmol Scand 2005;83:154-60. https://doi.org/10.1111/j.1600-0420.2005.00406.x.
- Landa G, Butovsky O, Shoshani J, Schwartz M, Pollack A. Weekly vaccination with Copaxone (glatiramer acetate) as a potential therapy for dry age-related macular degeneration. Curr Eye Res 2008;33:1011-13. https://doi.org/10.1080/02713680802484637.
- Landa G, Rosen RB, Patel A, Lima VC, Tai KW, Perez VR, et al. Qualitative spectral OCT/SLO analysis of drusen change in dry age-related macular degeneration patients treated with Copaxone. J Ocul Pharmacol Ther 2011;27:77-82. https://doi.org/10.1089/jop.2010.0109.
- Sternberg P, Rosenfeld PJ, Slakter JS, Koester JM, Reaves A. Topical OT-551 for treating geographic atrophy: phase ii results. Invest Ophthalmol Vis Sci 2010;51.
- Wong WT, Dresner S, Forooghian F, Glaser T, Doss L, Zhou M, et al. Treatment of geographic atrophy with subconjunctival sirolimus: results of a phase I/II clinical trial. Invest Ophthalmol Vis Sci 2013;54:2941-50. https://doi.org/10.1167/iovs.13-11650.
- Huang YM, Yan SF, Ma L, Zou ZY, Xu XR, Dou HL, et al. Serum and macular responses to multiple xanthophyll supplements in patients with early age-related macular degeneration. Nutrition 2013;29:387-92. https://doi.org/10.1016/j.nut.2012.06.009.
- Vojniković B, Kovacević D, Njirić S, Coklo M. Long term results of age-related macular degeneration therapy with prednisolone acetate – special refer to peripheral visual field changes. Coll Antropol 2008;32:351-3.
- Cohen SY, Bourgeois H, Corbe C, Chaine G, Espinasse-Berrod MA, Garcia-Sanchez J, et al. Randomized clinical trial France DMLA2: effect of trimetazidine on exudative and nonexudative age-relatedmacular degeneration. Retina 2012;32:834-43. https://doi.org/10.1097/IAE.0b013e31822058a3.
- Kaiser HJ, Flammer J, Stümpfig D, Hendrickson P. Visaline in the treatment of age-related macular degeneration: a pilot study. Ophthalmologica 1995;209:302-5. https://doi.org/10.1159/000310646.
- Thomas AS, Redd T, Hwang T. Effect of systemic beta-blockers, ace inhibitors, and angiotensin receptor blockers on development of choroidal neovascularization in patients with age-related macular degeneration. Retina 2015;35:1964-8. https://doi.org/10.1097/IAE.0000000000000603.
- Gallego-Pinazo R, Marina A, Suelves C, Frances-Munoz E, Millan JM, Arevalo JF, et al. Intravitreal ranibizumab for symptomatic drusenoid pigment epithelial detachment without choroidal neovascularization in age-related macular degeneration. Clin Ophthalmol 2011;5:161-5.
- Kuppermann BD, Patel SS, Boyer DS, Augustin AJ, Freeman WR, Kim T, et al. Brimonidine Drug Delivery System (DDS) generation 1 in patients with geographic atrophy: post-hoc analysis of a phase 2 study. Invest Ophthalmol Vis Sci 2017;58.
- Quiroz-Mercado H, Guerrero-Naranjo JL, Gonzalez-Salinas R, Hernandez-Zimbron LF, Zamora R, Gulías-Cañizo R, et al. Integrin peptide inhibitor for the treatment of intermediate age related macular degeneration. Invest Ophthalmol Vis Sci 2017;58.
- Scripsema NK, Hu DN, Rosen RB. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J Ophthalmol 2015;2015. https://doi.org/10.1155/2015/865179.
- Newsome DA, Swartz M, Leone NC, Elston RC, Miller E. Oral zinc in macular degeneration. Arch Ophthalmol 1988;106:192-8. https://doi.org/10.1001/archopht.1988.01060130202026.
- Liew G, Joachim N, Mitchell P, Burlutsky G, Wang JJ. Validating the AREDS simplified severity scale of age-related macular degeneration with 5- and 10-year incident data in a population-based sample. Ophthalmology 2016;123:1874-8. https://doi.org/10.1016/j.ophtha.2016.05.043.
- Richer S, Stiles W, Ulanski L, Carroll D, Podella C. Observation of human retinal remodeling in octogenarians with a resveratrol based nutritional supplement. Nutrients 2013;5:1989-2005. https://doi.org/10.3390/nu5061989.
- SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, Gensler G, Lindblad AS, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007;125:1225-32. https://doi.org/10.1001/archopht.125.9.1225.
- Age-Related Eye Disease Study Research Group . The Age-Related Eye Disease Study (AREDS): design implications. AREDS Report No. 1. Control Clin Trials 1999;20:573-600. https://doi.org/10.1016/S0197-2456(99)00031-8.
- Age-Related Macular Degeneration (NG82). London: NICE; 2018.
- Age-Related Eye Disease Study 2 Research Group . Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005-15. https://doi.org/10.1001/jama.2013.4997.
- Arora S, Musadiq M, Mukherji S, Yang YC. Eye nutrient products for age-related macular degeneration: what do they contain?. Eye 2004;18:470-3. https://doi.org/10.1038/sj.eye.6700700.
- Awh CC, Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology 2015;122:162-9. https://doi.org/10.1016/j.ophtha.2014.07.049.
- Seddon JM, Silver RE, Rosner B. Response to AREDS supplements according to genetic factors: survival analysis approach using the eye as the unit of analysis. Br J Ophthalmol 2016;100:1731-7. https://doi.org/10.1136/bjophthalmol-2016-308624.
- Ma L, Dou HL, Wu YQ, Huang YM, Huang YB, Xu XR, et al. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr 2012;107:350-9. https://doi.org/10.1017/S0007114511004260.
- Liu R, Wang T, Zhang B, Qin L, Wu C, Li Q, et al. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;56:252-8. https://doi.org/10.1167/iovs.14-15553.
- Beatty S, Chakravarthy U, Nolan JM, Muldrew KA, Woodside JV, Denny F, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology 2013;120:600-6. https://doi.org/10.1016/j.ophtha.2012.08.040.
- Ma L, Liu R, Du JH, Liu T, Wu SS, Liu XH. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016;8. https://doi.org/10.3390/nu8070426.
- Wang X, Jiang C, Zhang Y, Gong Y, Chen X, Zhang M. Role of lutein supplementation in the management of age-related macular degeneration: meta-analysis of randomized controlled trials. Ophthalmic Res 2014;52:198-205. https://doi.org/10.1159/000363327.
- Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye 2008;22:751-60. https://doi.org/10.1038/eye.2008.100.
- Andreatta W, El-Sherbiny S. Evidence-based nutritional advice for patients affected by age-related macular degeneration. Ophthalmologica 2014;231:185-90. https://doi.org/10.1159/000357528.
- Broadhead GK, Grigg JR, Chang AA, McCluskey P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr Rev 2015;73:448-62. https://doi.org/10.1093/nutrit/nuv005.
- Gregori NZ, Goldhardt R. Nutritional supplements for age-related macular degeneration. Curr Ophthalmol Rep 2015;3:34-9. https://doi.org/10.1007/s40135-014-0059-z.
- Querques G, Souied EH. The role of omega-3 and micronutrients in age-related macular degeneration. Surv Ophthalmol 2014;59:532-9. https://doi.org/10.1016/j.survophthal.2014.01.001.
- Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy – a randomised controlled trial. Br J Nutr 2013;109:2008-14. https://doi.org/10.1017/S0007114512004187.
- Murray IJ, Makridaki M, van der Veen RL, Carden D, Parry NR, Berendschot TT. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Invest Ophthalmol Vis Sci 2013;54:1781-8. https://doi.org/10.1167/iovs.12-10715.
- Weigert G, Kaya S, Pemp B, Sacu S, Lasta M, Werkmeister RM, et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:8174-8. https://doi.org/10.1167/iovs.11-7522.
- Wolf-Schnurrbusch UE, Zinkernagel MS, Munk MR, Ebneter A, Wolf S. Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. Invest Ophthalmol Vis Sci 2015;56:8069-74. https://doi.org/10.1167/iovs.15-17586.
- Ma L, Yan SF, Huang YM, Lu XR, Qian F, Pang HL, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012;119:2290-7. https://doi.org/10.1016/j.ophtha.2012.06.014.
- Richer SP, Stiles W, Graham-Hoffman K, Levin M, Ruskin D, Wrobel J, et al. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: the Zeaxanthin and Visual Function study (ZVF) FDA IND #78, 973. Optometry- J Am Optom Assoc 2011;82:667-80.e6. https://doi.org/10.1016/j.optm.2011.08.008.
- Akuffo KO, Nolan JM, Howard AN, Moran R, Stack J, Klein R, et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye 2015;29:902-12. https://doi.org/10.1038/eye.2015.64.
- Peng ML, Chiu HF, Chou H, Liao HJ, Chen ST, Wong YC, et al. Influence/impact of lutein complex (marigold flower and wolfberry) on visual function with early age-related macular degeneration subjects: A randomized clinical trial. J Functional Foods 2016;24:122-30. https://doi.org/10.1016/j.jff.2016.04.006.
- Wu J, Cho E, Willett WC, Sastry SM, Schaumberg DA. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol 2015;133:1415-24. https://doi.org/10.1001/jamaophthalmol.2015.3590.
- Arnold C, Winter L, Fröhlich K, Jentsch S, Dawczynski J, Jahreis G, et al. Macular xanthophylls and ω-3 long-chain polyunsaturated fatty acids in age-related macular degeneration: a randomized trial. JAMA Ophthalmol 2013;131:564-72. https://doi.org/10.1001/jamaophthalmol.2013.2851.
- Kelly ER, Plat J, Haenen GR, Kijlstra A, Berendschot TT. The effect of modified eggs and an egg-yolk based beverage on serum lutein and zeaxanthin concentrations and macular pigment optical density: results from a randomized trial. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0092659.
- Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res 2007;84:718-28. https://doi.org/10.1016/j.exer.2006.12.010.
- Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, et al. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol 2007;42:720-6. https://doi.org/10.3129/i07-116.
- Olk RJ, Peralta E, Gierhart DL, Brown GC, Brown MM. Triple combination therapy and zeaxanthin for the treatment of neovascular age-related macular degeneration: an interventional comparative study and cost-effectiveness analysis. Int J Retina Vitreous 2015;1. https://doi.org/10.1186/s40942-015-0019-2.
- Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am J Clin Nutr 2009;90:1272-9. https://doi.org/10.3945/ajcn.2009.28013.
- Bartlett HE, Eperjesi F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. Eur J Clin Nutr 2007;61:1121-7. https://doi.org/10.1038/sj.ejcn.1602626.
- Kelly D, Nolan JM, Howard AN, Stack J, Akuffo KO, Moran R, et al. Serum and macular response to carotenoid-enriched egg supplementation in human subjects: the Egg Xanthophyll Intervention clinical Trial (EXIT). Br J Nutr 2017;117:108-23. https://doi.org/10.1017/S0007114516003895.
- Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216-30. https://doi.org/10.1016/S1529-1839(04)70049-4.
- Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefes Arch Clin Exp Ophthalmol 2013;251:2711-23. https://doi.org/10.1007/s00417-013-2376-6.
- García-Layana A, Recalde S, Alamán AS, Robredo PF. Effects of lutein and docosahexaenoic acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients 2013;5:543-51. https://doi.org/10.3390/nu5020543.
- Piermarocchi S, Saviano S, Parisi V, Tedeschi M, Panozzo G, Scarpa G, et al. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol 2012;22:216-25. https://doi.org/10.5301/ejo.5000069.
- Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lu XR, et al. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: a randomised, double-blind, placebo-controlled trial. Br J Ophthalmol 2015;99:371-5. https://doi.org/10.1136/bjophthalmol-2014-305503.
- Ma L, Dou HL, Huang YM, Lu XR, Xu XR, Qian F, et al. Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: a randomized, double-masked, placebo-controlled trial. Am J Ophthalmol 2012;154:625-34.e1. https://doi.org/10.1016/j.ajo.2012.04.014.
- Sakai City Medical Center . Randomized Parallel-Group Trial of Lutein Supplementation for Macular Pigment Optical Density and Visual Function in the Patients With Unilateral Age-Related Macular Degeneration 2016. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000027962 (accessed 18 December 2017).
- Beatty S, Nolan JM, Muldrew KA, Woodside J, Stevenson MR, Chakravarthy U. Visual outcome after antioxidant supplementation. Ophthalmology 2013;120. https://doi.org/10.1016/j.ophtha.2012.09.013.
- Sangiovanni JP, Agrón E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, et al. Age-Related Eye Disease Study Research Group . {omega}-3 long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS Report No. 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr 2009;90:1601-7. https://doi.org/10.3945/ajcn.2009.27594.
- Lawrenson JG, Evans JR. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 2015;4. https://doi.org/10.1002/14651858.CD010015.pub3.
- Souied EH, Aslam T, Garcia-Layana A, Holz FG, Leys A, Silva R, et al. Omega-3 fatty acids and age-related macular degeneration. Ophthalmic Res 2015;55:62-9. https://doi.org/10.1159/000441359.
- Evans JR, Lawrenson JG. A review of the evidence for dietary interventions in preventing or slowing the progression of age-related macular degeneration. Ophthalmic Physiol Opt 2014;34:390-6. https://doi.org/10.1111/opo.12142.
- Johnson EJ. Age-related macular degeneration and antioxidant vitamins: recent findings. Curr Opin Clin Nutr Metab Care 2010;13:28-33. https://doi.org/10.1097/MCO.0b013e32833308ff.
- Sin HP, Liu DT, Lam DS. Lifestyle modification, nutritional and vitamins supplements for age-related macular degeneration. Acta Ophthalmol 2013;91:6-11. https://doi.org/10.1111/j.1755-3768.2011.02357.x.
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, et al. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 2013;120:1619-31. https://doi.org/10.1016/j.ophtha.2013.01.005.
- Huang P, Wang F, Sah BK, Jiang J, Ni Z, Wang J, et al. Homocysteine and the risk of age-related macular degeneration: a systematic review and meta-analysis. Sci Rep 2015;5. https://doi.org/10.1038/srep10585.
- Bartlett H, Eperjesi F. Age-related macular degeneration and nutritional supplementation: a review of randomised controlled trials. Ophthalmic Physiol Opt 2003;23:383-99. https://doi.org/10.1046/j.1475-1313.2003.00130.x.
- Schmidl D, Garhöfer G, Schmetterer L. Nutritional supplements in age-related macular degeneration. Acta Ophthalmol 2015;93:105-21. https://doi.org/10.1111/aos.12650.
- Teikari JM, Laatikainen L, Virtamo J, Haukka J, Rautalahti M, Liesto K, et al. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand 1998;76:224-9. https://doi.org/10.1034/j.1600-0420.1998.760220.x.
- Christen WG, Manson JE, Glynn RJ, Gaziano JM, Chew EY, Buring JE, et al. Beta carotene supplementation and age-related maculopathy in a randomized trial of US physicians. Arch Ophthalmol 2007;125:333-9. https://doi.org/10.1001/archopht.125.3.333.
- Taylor HR, Tikellis G, Robman LD, McCarty CA, McNeil JJ. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7354.11.
- Feher J, Kovacs B, Kovacs I, Schveoller M, Papale A, Balacco Gabrieli C. Improvement of visual functions and fundus alterations in early age-related macular degeneration treated with a combination of acetyl-L-carnitine, n-3 fatty acids, and coenzyme Q10. Ophthalmologica 2005;219:154-66. https://doi.org/10.1159/000085248.
- Tao Y, Jiang P, Wei Y, Wang P, Sun X, Wang H. α-lipoic acid treatment improves vision-related quality of life in patients with dry age-related macular degeneration. Tohoku J Exp Med 2016;240:209-14. https://doi.org/10.1620/tjem.240.209.
- Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology 2013;120:1020-8. https://doi.org/10.1016/j.ophtha.2012.10.020.
- Cougnard-Grégoire A, Merle BM, Korobelnik JF, Rougier MB, Delyfer MN, Le Goff M, et al. Olive oil consumption and age-related macular degeneration: the Alienor Study. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0160240.
- Wu J, Cho E, Giovannucci EL, Rosner BA, Sastry SM, Willett WC, et al. Dietary intakes of eicosapentaenoic acid and docosahexaenoic acid and risk of age-related macular degeneration. Ophthalmology 2017;124:634-43. https://doi.org/10.1016/j.ophtha.2016.12.033.
- Chew EY, Clemons TE, SanGiovanni JP, Danis R, Ferris FL, Elman M, et al. Lutein plus Zeaxanthin and omega-3 fatty acids for age-related macular degeneration. The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005-15. https://doi.org/10.1001/jama.2013.4997.
- Christen WG, Glynn RJ, Chew EY, Albert CM, Manson JE. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med 2009;169:335-41. https://doi.org/10.1001/archinternmed.2008.574.
- Merle BM, Silver RE, Rosner B, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: a prospective cohort study. Am J Clin Nutr 2016;103:1135-44. https://doi.org/10.3945/ajcn.115.117606.
- Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr 2013;98:129-35. https://doi.org/10.3945/ajcn.112.057091.
- Khan JC, Shahid H, Thurlby DA, Bradley M, Clayton DG, Moore AT, et al. Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br J Ophthalmol 2006;90:29-32. https://doi.org/10.1136/bjo.2005.073825.
- Christen WG, Glynn RJ, Manson JE, MacFadyen J, Bubes V, Schvartz M, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology 2014;121:525-34. https://doi.org/10.1016/j.ophtha.2013.09.038.
- Cangemi FE. TOZAL Study: an open case control study of an oral antioxidant and omega-3 supplement for dry AMD. BMC Ophthalmol 2007;7. https://doi.org/10.1186/1471-2415-7-3.
- Christen WG, Glynn RJ, Chew EY, Buring JE. Vitamin E and age-related macular degeneration in a randomized trial of women. Ophthalmology 2010;117:1163-8. https://doi.org/10.1016/j.ophtha.2009.10.043.
- Christen WG, Cook NR, Ridker PM, Buring JE. Prospective study of plasma homocysteine level and risk of age-related macular degeneration in women. Ophthalmic Epidemiol 2015;22:85-93. https://doi.org/10.3109/09286586.2015.1012272.
- Evans JR. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD001775.pub2.
- Ahmadi A, Ghanbari H, Soheilian M, Naseri M. The EFFEct of HESA-A (natural drug) on visual acuity in age related macular degeneration: a randomized double blind controlled clinical trial. Afr J Tradit Complement Altern Med 2009;6:549-53.
- Milajerdi A, Bitarafan V, Mahmoudi M. A review on the effects of saffron extract and its constituents on factors related to neurologic, cardiovascular and gastrointestinal diseases. J Medi Plants 2015;14:9-28.
- Falsini B, Piccardi M, Minnella A, Savastano C, Capoluongo E, Fadda A, et al. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:6118-24. https://doi.org/10.1167/iovs.09-4995.
- Marangoni D, Falsini B, Piccardi M, Ambrosio L, Minnella AM, Savastano MC, et al. Functional effect of saffron supplementation and risk genotypes in early age-related macular degeneration: a preliminary report. J Transl Med 2013;11. https://doi.org/10.1186/1479-5876-11-228.
- Bisti S, Maccarone R, Falsini B. Saffron and retina: neuroprotection and pharmacokinetics. Vis Neurosci 2014;31:355-61. https://doi.org/10.1017/S0952523814000108.
- Piccardi M, Marangoni D, Minnella AM, Savastano MC, Valentini P, Ambrosio L, et al. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med 2012;2012. https://doi.org/10.1155/2012/429124.
- Lashay A, Sadough G, Ashrafi E, Lashay M, Movassat M, Akhondzadeh S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: a double-blind, placebo-controlled, randomized trial. Med Hypothesis Discov Innov Ophthalmol 2016;5:32-8.
- Riazi A, Panahi Y, Alishiri AA, Hosseini MA, Zarchi AAK, Sahebkar A. The impact of saffron (Crocus sativus) supplementation on visual function in patients with dry age-related macular degeneration. Ital J Med 2017;11:196-201.
- Broadhead G, McCluskey P, Grigg J, Hong T, Schlub T, Chang A. Saffron therapy for the treatment of age-related macular degeneration. Clin Exp Ophthalmol 2016;44:31-2.
- Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med 2014;80:249-54. https://doi.org/10.1055/s-0033-1351074.
- Huynh TP, Mann SN, Mandal NA. Botanical compounds: effects on major eye diseases. Evid Based Complement Alternat Med 2013;2013. https://doi.org/10.1155/2013/549174.
- Wang LL, Sun Y, Huang K, Zheng L. Curcumin, a potential therapeutic candidate for retinal diseases. Mol Nutr Food Res 2013;57:1557-68. https://doi.org/10.1002/mnfr.201200718.
- Vishwanathan R, Chung M, Johnson EJ. A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:3985-98. https://doi.org/10.1167/iovs.12-11552.
- Newsome DA. A randomized, prospective, placebo-controlled clinical trial of a novel zinc-monocysteine compound in age-related macular degeneration. Curr Eye Res 2008;33:591-8. https://doi.org/10.1080/02713680802178437.
- Stur M, Tittl M, Reitner A, Meisinger V. Oral zinc and the second eye in age-related macular degeneration. Invest Ophthalmol Vis Sci 1996;37:1225-35.
- Rein DB, Saaddine JB, Wittenborn JS, Wirth K, Hoerger TJ, Narayan KM, et al. Technical appendix: cost-effectiveness of vitamin therapy for age-related macular degeneration. Ophthalmology 2007;114:e13-20. https://doi.org/10.1016/j.ophtha.2006.10.064.
- Saxena N, George PP, Heng BH, Lim TH, Yong SO. Cost-effectiveness of anti-oxidant vitamins plus zinc treatment to prevent the progression of intermediate age-related macular degeneration. A Singapore perspective. Indian J Ophthalmol 2015;63:516-23. https://doi.org/10.4103/0301-4738.158533.
- Sunness JS. Spontaneous improvement in visual acuity in age-related geographic atrophy of the macula. JAMA Ophthalmol 2014;132:356-7. https://doi.org/10.1001/jamaophthalmol.2014.21.
- Prenner JL, Driscoll SJ, Fine HF, Salz DA, Roth DB. Publication rates of registered clinical trials in macular degeneration. Retina 2011;31:401-4. https://doi.org/10.1097/IAE.0b013e3181eef2ad.
- Chew EY, Schachat AP. Should we add screening of age-related macular degeneration to current screening programs for diabetic retinopathy?. Ophthalmology 2015;122:2155-6. https://doi.org/10.1016/j.ophtha.2015.08.007.
- Mackie Eyecare . AMD Screening 2016. www.mackieopticians.co.uk/screening/amd-screening/ (accessed 18 December 2017).
- Neely DC, Bray KJ, Huisingh CE, Clark ME, McGwin G, Owsley C. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol 2017;135:570-5. https://doi.org/10.1001/jamaophthalmol.2017.0830.
- Schaal KB, Rosenfeld PJ, Gregori G, Yehoshua Z, Feuer WJ. Anatomic clinical trial endpoints for nonexudative age-related macular degeneration. Ophthalmology 2016;123:1060-79. https://doi.org/10.1016/j.ophtha.2016.01.034.
- Veerappan M, El-Hage-Sleiman AM, Tai V, Chiu SJ, Winter KP, Stinnett SS, et al. Optical coherence tomography reflective drusen substructures predict progression to geographic atrophy in age-related macular degeneration. Ophthalmology 2016;123:2554-70. https://doi.org/10.1016/j.ophtha.2016.08.047.
- Owsley C, McGwin G, Clark ME, Jackson GR, Callahan MA, Kline LB, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology 2016;123:344-51. https://doi.org/10.1016/j.ophtha.2015.09.041.
- Alvarez JA, Yazdanie M, Wong WT, Thompson D, Lipson R, Wiley H, et al. Longitudinal study of dark adaptation as a functional outcome measure for age-related macular degeneration. Invest Ophthalmol Vis Sci 2016;57.
- Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Evaluation of the AdaptDx™ for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;55. https://doi.org/10.1167/iovs.13-13745.
- Diaz ER, Tahir H, Kelly JM, Parry NRA, Aslam T, Carden D, et al. Functional and structural progression in early amd; dark adaptation best predicts morphology. Invest Ophthalmol Vis Sci 2016;57.
- Murray I, Carden D, Kelly JM. New rapid digital dark adaptometer that shows high sensitivity and specificity for early AMD. Invest Ophthalmol Vis Sci 2016;57.
- Planas MG, Biarnes M, Mones J. Dark adaptation impairment in patients with drusen. Invest Ophthalmol Vis Sci 2016;57.
- Kimel M, Leidy NK, Tschosik E, Dolan C, Souied EH, Varma R, et al. Functional Reading Independence (FRI) index: a new patient-reported outcome measure for patients with geographic atrophy. Invest Ophthalmol Vis Sci 2016;57:6298-304. https://doi.org/10.1167/iovs.16-20361.
- Kersten E, Paun CC, Schellevis RL, Hoyng CB, Delcourt C, Lengyel I, et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv Ophthalmol 2018;63:9-39. https://doi.org/10.1016/j.survophthal.2017.05.003.
- Lains I, Kelly RS, Miller JB, Silva R, Vavvas DG, Kim IK, et al. Human plasma metabolomics study across all stages of age-related macular degeneration identifies potential lipid biomarkers. Ophthalmology 2017;125:245-54. https://doi.org/10.1016/j.ophtha.2017.08.008.
- Wittes J, Downs M. Outcome measures to assess efficacy of treatments for age-related macular degeneration. Ophthalmology 2009;116:8-14. https://doi.org/10.1016/j.ophtha.2009.06.050.
- Cukras C, Jeffrey BG. The importance of outcome measure research in Stargardt disease. JAMA Ophthalmol 2017;135:704-5. https://doi.org/10.1001/jamaophthalmol.2017.1544.
- Murro V, Sodi A, Giacomelli G, Mucciolo DP, Pennino M, Virgili G, et al. Reading ability and quality of life in Stargardt disease. Eur J Ophthalmol 2017. https://doi.org/10.5301/ejo.5000972.
- Gopinath B, Liew G, Flood VM, Joachim N, Burlutsky G, Mitchell P. Combined influence of poor health behaviours on the prevalence and 15-year incidence of age-related macular degeneration. Sci Rep 2017;7. https://doi.org/10.1038/s41598-017-04697-3.
- McGuinness MB, Le J, Mitchell P, Gopinath B, Cerin E, Saksens NTM, et al. Physical activity and age-related macular degeneration: a systematic literature review and meta-analysis. Am J Ophthalmol 2017;180:29-38. https://doi.org/10.1016/j.ajo.2017.05.016.
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD000253.pub4.
- Lawrenson JG, Evans JR. Advice about diet and smoking for people with or at risk of age-related macular degeneration: a cross-sectional survey of eye care professionals in the UK. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-564.
- Martin L. Targeting modifiable risk factors in age-related macular degeneration in optometric practice in Sweden. Clin Optom 2017;9:77-83. https://doi.org/10.2147/OPTO.S129942.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- NIH Heart Lung and Blood Institute . Study Quality Assessment Tools 2014. www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed 18 December 2017).
Appendix 1 Methods
Quality assessment approach
We assessed the methodological quality of the included studies using recommended criteria. For RCTs and CCTs we used the Cochrane Handbook risk-of-bias criteria. 456 The Cochrane risk-of-bias assessment criteria looks at the extent to which the design of a study and how that study is conducted is likely to prevent bias (error) in the results. The tool covers six possible biases (selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias) that have been shown to reflect the main mechanisms for bias in RCTs (Higgins 2011457). These six biases are assessed by at least seven questions relating to the generation of the allocation sequence, concealment of the allocation sequence, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting and other threats to validity. In this review the assessment of performance bias, detection bias and attrition bias was evaluated separately for outcomes that were considered to be objective (e.g. BCVA) and subjective (e.g. quality-of-life measures). Each criteria is assigned a judgement of ‘high,’ ‘low’ or ‘unclear’ risk of bias. Narrative descriptions are also used to provide reasons for a particular judgement. In the review we used the risk of selection bias (generation and concealment of the allocation sequence) to establish the overall risk of bias for each study.
For non-randomised studies we used tools developed by the NIH NHLBI for a systematic review of cardiovascular risk. 458 The tools were developed by methodologists using quality assessment methods and concepts, and using other tools developed by groups such as Cochrane and the UK CRD. The tools focus on concepts including biases (selection, performance, detection and attrition), confounding, power and strengths of associations between treatments and outcomes. There are different tools for each major type of study; in this review we used the tools for cohort studies (two groups), before-and-after studies (one group), case–control studies (two group studies looking at associations) and case series studies. Specific guidance notes are provided for each tool. Each question is assigned a response of ‘yes’, ‘no’, ‘cannot determine’, ‘not reported’ or ‘not applicable’. The study is then assessed for overall quality (good, fair, poor) based on the responses to the individual questions, where a good study has the least risk of bias, and results are considered to be valid; a fair study is susceptible to some bias but this is not deemed sufficient to invalidate the results; and a poor study indicates that the study is at a significant risk of bias.
Quality scoring involved an element of judgement, because some criteria may be more important than others, and because some criteria may be assessed as not applicable, not reported or cannot determine, but as a rough rule of thumb we used the number of ‘yes’ responses:
-
for before-and-after studies, with 10 questions, good 8–10, fair 5–7, poor < 5
-
for cohort studies with 14 questions, good 10–14, fair 7–9, poor < 7
-
for case control studies with 12 questions, good 10–12, fair 7–9, poor < 7
-
for case series with 9 questions, good 8–9, fair 5–7, poor < 5.
Quality criteria were applied by one reviewer and checked by a second reviewer, with disagreement resolved by discussion.
Quantity of evidence
We included 108 primary studies (Table 47). A total of 104 studies were predominantly on people with (or at risk of) dry AMD and four studies were on people with STGD. Of the 104 dry AMD studies, there were 26 of pharmacological treatments, 30 in physical therapies, three of cell transplants and 45 of nutritional supplements. In the four studies in STGD there were two physical therapies and two nutritional supplements. Two studies had subgroups of people with dry AMD and STGD,97,98 making a total of six studies in STGD (see Table 47).
| Primary reference | Intervention(s) |
|---|---|
| STGD | |
| Aleman et al., 2007114 | Lutein |
| Querques et al., 2010125 | Nutritional supplements |
| Röck et al. 2013121 | Electrotherapy |
| Teussink et al., 2015122 | Light protection |
| Dry AMD | |
| Physical therapies | |
| Krenn et al., 2008152 | Acupuncture |
| Pipis et al., 2015162 | Blue-light filters |
| Lavric & Pompe 2014164 | Blue-light filters |
| Nagai et al., 2015163 | Blue-light filters |
| Chong et al., 2011 (abstract)165 | Blue-light filters |
| Blaha et al., 2013179 | Haemopheresis |
| Studnička et al. 2013180 | Haemopheresis |
| Klingel et al., 2010181 | Haemopheresis |
| Koss et al., 2009175 | Haemopheresis |
| Pulido et al., 2006174 | Haemopheresis |
| Rencová et al., 2015177 | Haemopheresis |
| Brunner et al., 2000170 | Haemopheresis |
| Swartz and Rabetoy 1999178 | Haemopheresis |
| Figueroa et al., 1997239 | Laser photocoagulation |
| Guymer et al., 2014232 | Laser |
| Ivandic et al., 2008237 | Laser |
| Luttrull et al., 201696 | Laser |
| Huang et al., 2011223 | Laser |
| Prahs et al., 2010236 | Laser |
| Merry et al., 2016238 | Photobiomodulation |
| Shinoda et al., 2008199 | Microcurrent |
| Chaikin et al., 2015198 | Microcurrent |
| Kondrot et al., 201597 | Microcurrent |
| Kondrot et al., 2002208 | Microcurrent |
| Anastassiou et al., 2013196 | Microcurrent |
| Michael et al., 1993204 | Microcurrent |
| Borrelli et al. 2012248 | Oxygen ozone-therapy |
| Bocci et al., 2011244 | Ozone |
| Hudson et al., 2006250 | Telescopes |
| Qureshi et al., 2015254 | Telescopes |
| Cell transplant technologies | |
| Schwartz et al., 2015123 | RPE transplant |
| Song et al., 2015271 | Stem cell transplant |
| Ho et al., 2017276 | Cell transplants |
| Pharmacological therapies | |
| Augustin et al. 2013325 | Alprostadil |
| Ladewig et al., 2005326 | Alprostadil |
| Remky et al., 2005328 | Dorzolamide |
| Yehoshua et al., 2014323 | Eculizumab |
| Dugal et al., 2015308 | Emixustat |
| Mata et al., 2013144 | Fenretide |
| Landa et al., 2011330 | Glatiramer acetate |
| Brilliant et al., 2016324 | L-dopa |
| Zhang et al., 2011273 | NT-501 |
| Wong et al., 201092 | Topical OT-551 |
| Vojniković et al., 2008334 | Prednisolone |
| Gallego-Pinazo et al., 2011338 | Ranibizumab |
| Petrou et al., 201594 | Sirolimus |
| Wong et al., 2013332 | Sirolimus |
| Maguire et al., 2009293 | Statins |
| Al-Holou 2015291 | Statins |
| Barbosa et al., 2014294 | Statins |
| Vavvas et al., 2016286 | Statins |
| McGwin et al., 2003295 | Statins |
| VanderBeek et al., 2013296 | Statins |
| Kaiserman et al., 2009297 | Statins |
| Fong et al., 2010298 | Statins |
| Etminan et al., 2008285 | Statins + ACE inhibitor |
| Jaffe et al., 2015190 | Tandospirone |
| Cohen et al., 2012335 | Trimetazidine |
| Kaiser et al., 1995336 | Visaline |
| Nutritional supplements | |
| AREDS 132 | Antioxidants and zinc |
| AREDS 2348 | Antioxidants, carotenoids and fatty acids |
| Berrow et al., 2013362 | Lutein |
| Murray et al., 2013363 | Lutein |
| Weigert et al., 2011364 | Lutein, lutein + zeaxanthin |
| Ma et al., 2012a366 | Lutein, zeaxanthin |
| Huang et al., 2015a192 | Lutein, zeaxanthin |
| Kelly et al., 2014372 | Lutein, zeaxanthin |
| Kelly et al., 2017378 | Lutein eggs |
| Richer et al., 2011367 | Lutein, zeaxanthin |
| Akuffo et al., 2015368 | Lutein, zeaxanthin |
| Peng et al., 2016369 | Lutein, zeaxanthin |
| Wu et al., 2015370 | Lutein, zeaxanthin |
| Trieschmann et al., 2007373 | Lutein, zeaxanthin |
| Arnold et al., 2013371 | Lutein, zeaxanthin, long-chain PUFA |
| Robman et al., 2007374 | Lutein, zeaxanthin |
| Vishwanathan et al., 2009376 | Eggs (lutein, zeaxanthin) |
| Olk et al., 2015375 | Triple therapy, zeaxanthin |
| Beatty et al., 2013354 | Lutein, zeaxanthin + others |
| Bartlett et al., 2007377 | Lutein, antioxidants |
| Richer et al. 2004379 | Lutein + carotenoids, antioxidants, vitamins, minerals |
| Dawczynski et al., 2013380 | Lutein, zeaxanthin, omega-3 |
| García-Layana et al., 2013381 | Lutein and DHA |
| Wolf-Schnurrbusch et al., 2015365 | Lutein, omega |
| Piermarocchi et al., 2012382 | Lutein, zeaxanthin |
| Reynolds et al., 2013402 | Omega-3 |
| Feher et al., 2005400 | Antioxidant and fatty acid |
| Souied et al., 2013393 | DHA |
| Tao et al., 2016401 | Alpha lipoic acid |
| Cougnard-Grégoire et al., 2016403 | Olive oil |
| Christen et al., 2009406 | Vitamin B6, B12, folate |
| Merle et al., 2016407 | Folate and vitamin B12 |
| Gopinath et al., 2013408 | Serum homocysteine, folate and vitamin B12 |
| Christen et al., 2007398 | Beta-carotene |
| Christen et al., 2010412 | Vitamin E |
| Christen et al., 2014410 | Multivitamins |
| Cangemi et al., 2007411 | Antioxidant and omega 3 |
| Taylor et al., 2002399 | Vitamin E |
| Teikari et al., 1998397 | Antioxidants |
| Ahmadi et al., 2009415 | HESA-A |
| Riazi et al., 2017422 | Saffron |
| Lashay et al., 2016421 | Saffron |
| Piccardi et al., 2012420 | Saffron |
| Falsini et al., 2010417 | Saffron |
| Marangoni et al., 2013418 | Saffron |
Summary overview of the study characteristics can be seen in Report Supplementary Material 6. There was a range of study designs, with 60 RCTs and CCTs, 24 cohort studies and cross-sectional studies, 13 single-arm before-and-after studies, 5 case–control studies and 6 case series. Many studies had small sample sizes, the durations of intervention and follow-up were often short, and there were differences in the outcomes reported. Further details are provided in Chapters 2–7. Baseline characteristics of participants are summarised in Report Supplementary Material 6. There was generally poor reporting of baseline characteristics across the studies. The risk of bias of RCTs and CCTs and quality of non-randomised studies are summarised in Report Supplementary Material 6. The overall quality of each study are reported within the results chapters of this report.
Review methods
Inclusion and exclusion criteria
Participants
-
People with a confirmed diagnosis of dry AMD or STGD.
Interventions
-
Any interventions which aim to preserve or restore vision in dry AMD or STGD.
Clinical experts were asked to identify treatments in development to ensure that all potential treatments were included in the review.
Exclusions: to avoid overlap we excluded studies on some interventions being reviewed in the NICE guideline process (e.g. smoking cessation, diagnostic technologies, monitoring and review, and rehabilitation support).
Outcomes
Primary outcomes were those that matter to patients and included:
-
visual acuity
-
contrast sensitivity
-
macular sensitivity
-
adverse effects of treatment
-
adherence to treatment
-
reading speed
-
ability to drive
-
health-related quality of life
-
progression of disease.
Although visual outcomes were preferred, because progression of dry AMD is slow we also included secondary outcomes where there was good evidence that they are strong predictors of subsequent visual outcomes. Secondary outcomes that were potentially eligible included:
-
rod function (may not correlate with VA as central VA, as measured using VA charts, but depends on foveal function, and the fovea is cone rich. Rod function is one of the earliest abnormalities detected in people who will later develop GA in AMD)
-
macular pigment density
-
macular function as measured by microperimetry
-
RPE thickness
-
AF
-
drusen volume.
Design
-
Randomised controlled trials.
-
Controlled clinical trials with a concurrent control group.
-
Observational studies.
Exclusions: we excluded observational studies with < 10 participants (or eyes) with the exception in studies for STGD and studies in stem cell treatments.
Systematic reviews and literature reviews that were identified by the searches were assessed for quality and summarised if they met quality criteria. Where there was a good-quality systematic review we did not review the primary studies. Reviews were also used as a source for identifying primary studies.
Study selection and data extraction
Studies were selected for inclusion through a two-stage process using predefined and explicit criteria. Titles and abstracts from the full literature search results were screened independently by two reviewers to identify all citations that appeared likely to have met the inclusion criteria. Full manuscripts of relevant studies were then retrieved and assessed for eligibility by one reviewer and checked by a second reviewer. Studies published as abstracts or conference presentations were only included if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken. As far as possible, full papers or abstracts describing the same study were linked together, with the article reporting key outcomes designated as the primary publication.
Data were extracted by one reviewer using a standard data extraction form and checked by a second reviewer. At each stage, any disagreements between reviewers were resolved by consensus or if necessary by arbitration by a third reviewer.
Method of data synthesis
Studies were synthesised through a narrative review with tabulation of results of included studies. Formal synthesis through meta-analysis was not possible because studies were not of sufficient quality and were heterogeneous in terms of participant characteristics, outcomes and study design.
Search strategies
MEDLINE, EMBASE, Web of Science and The Cochrane Library were searched from 2005 to 13 July 2017 for reviews, journal articles and meeting abstracts. Searches were limited to English language only.
Initial searches of all databases were undertaken in June 2016 and updated searches were run in June 2017 to check for any articles added in the previous year. The ARVO website was also searched for meeting abstracts.
References of reviews were checked for relevant studies and clinical experts were also consulted for any other relevant literature.
After removal of duplicate articles and screening out obviously irrelevant articles on the basis of their titles, the titles and abstracts of 7948 articles were screened for inclusion by two reviewers (PR, GH) and checked by a third (NW). The full texts of 398 articles were obtained for further screening and checking of references and 112 articles were included in the final report.
ClinicalTrials.gov, the WHO search portal and UK Clinical Trials gateway were searched for ongoing and recently completed clinical trials.
MEDLINE
-
(age related macular degeneration or age related maculopathy or AMD or ARMD or stargardt* or geographic atrophy).tw.
-
(therap* or intervention* or treatment* or prevent* or delay* or restore or preserve or trial).tw.
-
(telescop* or retinal implant* or intra-ocular lens* or intraocular lens* or IOLs).tw.
-
2 or 3
-
1 and 4
-
(age and macular degeneration).m_titl.
-
(ARMD or AMD or stargardt* or geographic atrophy).m_titl.
-
6 or 7
-
(dry adj3 (age-related macular degeneration or ARMD or AMD)).tw.
-
((early or intermediate) adj3 (age-related macular degeneration or ARMD or AMD)).tw.
-
((nonexudative or non-exudative) adj3 (age-related macular degeneration or AMD or ARMD)).tw.
-
((non-neovascular or nonneovascular) adj3 (age-related macular degeneration or ARMD or AMD)).tw
-
((varying or various or different) adj3 (age-related macular degeneration or ARMD or AMD)).tw.
-
geographic atrophy.tw.
-
(atrophic adj3 (age related macular degeneration or AMD or ARMD)).tw.
-
stargardt*.mp.
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
-
5 or 8 or 17
-
limit 18 to yr=‘2005 -Current’
-
(comment or letter or editorial).pt.
-
19 not 20
-
limit 21 to english language
Years searched: Ovid MEDLINE(R) 1946 to June Week 4 2016, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations July 01, 2016.
Updated search: Ovid MEDLINE(R) 1946 to June Week 4 2017; Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations July 12, 2017
EMBASE
-
(age related macular degeneration or age related maculopathy or AMD or ARMD or stargardt* or geographic atrophy).tw.
-
(therap* or intervention* or treat* or prevent* or delay* or restore or preserve or trial).tw.
-
(telescop* or retinal implant* or intra-ocular lens* or intraocular lens* or IOLs).tw.
-
2 or 3
-
1 and 4
-
(age and macular degeneration).m_titl.
-
(ARMD or AMD or stargardt* or geographic atrophy).m_titl.
-
6 or 7
-
(dry adj2 (age-related macular degeneration or ARMD or AMD)).tw.
-
((early or intermediate) adj2 (age-related macular degeneration or ARMD or AMD)).tw.
-
((nonexudative or non-exudative) adj2 (age-related macular degeneration or AMD or ARMD)).tw.
-
((non-neovascular or nonneovascular) adj2 (age-related macular degeneration or ARMD or AMD)).tw.
-
((varying or various or different) adj2 (age-related macular degeneration or ARMD or AMD)).tw.
-
geographic atrophy.tw.
-
(atrophic adj3 (age related macular degeneration or AMD or ARMD)).tw.
-
stargardt*.tw.
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
-
5 or 8 or 17
-
exp geographic atrophy/
-
exp age related macular degeneration/
-
exp Stargardt disease/
-
19 or 20 or 21
-
2 and 22
-
18 or 23
-
limit 24 to yr=‘2005 -Current’
-
(comment or letter or editorial).pt.
-
(neovascular or neo-vascular or wet or exudative or ranibizumab or bevacizumab or aflibercept).m_titl.
-
26 or 27
-
25 not 28
Years searched: OVID EMBASE 1974 to 2016 Week 27; updated search from 2016 to 2017 Week 28.
Web of Science
TITLE: (age related macular degeneration or age related maculopathy or stargardt* or geographic atrophy) AND TOPIC: (therap* or intervention* or treat* or prevent* or delay* or restore or progression or telescop* or retinal implant* or intra-ocular lens* or intraocular lens* or IOLs)
Years searched: 2005 to 5 July 2016; updated search: 2016 to 13 July 2017.
The Cochrane Library
Searched the Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials.
Search terms:
age related macular degeneration or age related maculopathy or stargardt* or geographic atrophy in Record Title AND therap* or intervention* or treat* or prevent* or delay* or restore or progression or telescop* or retinal implant* or intra-ocular lens* or intraocular lens* or IOLs in Title, Abstract, Keywords
Dates searched: June 2016 and updated in July 2017.
Clinical trials and ongoing studies
Searched ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP) and UK Clinical Trials gateway.
Terms used: ‘age related macular degeneration’ or ‘geographic atrophy’ or AMD or ‘stargardt’ or ‘stargardt’s’.
Searched in June 2016 and updated searches in December 2016 and July 2017.
FIGURE 2.
The PRISMA flow diagram.
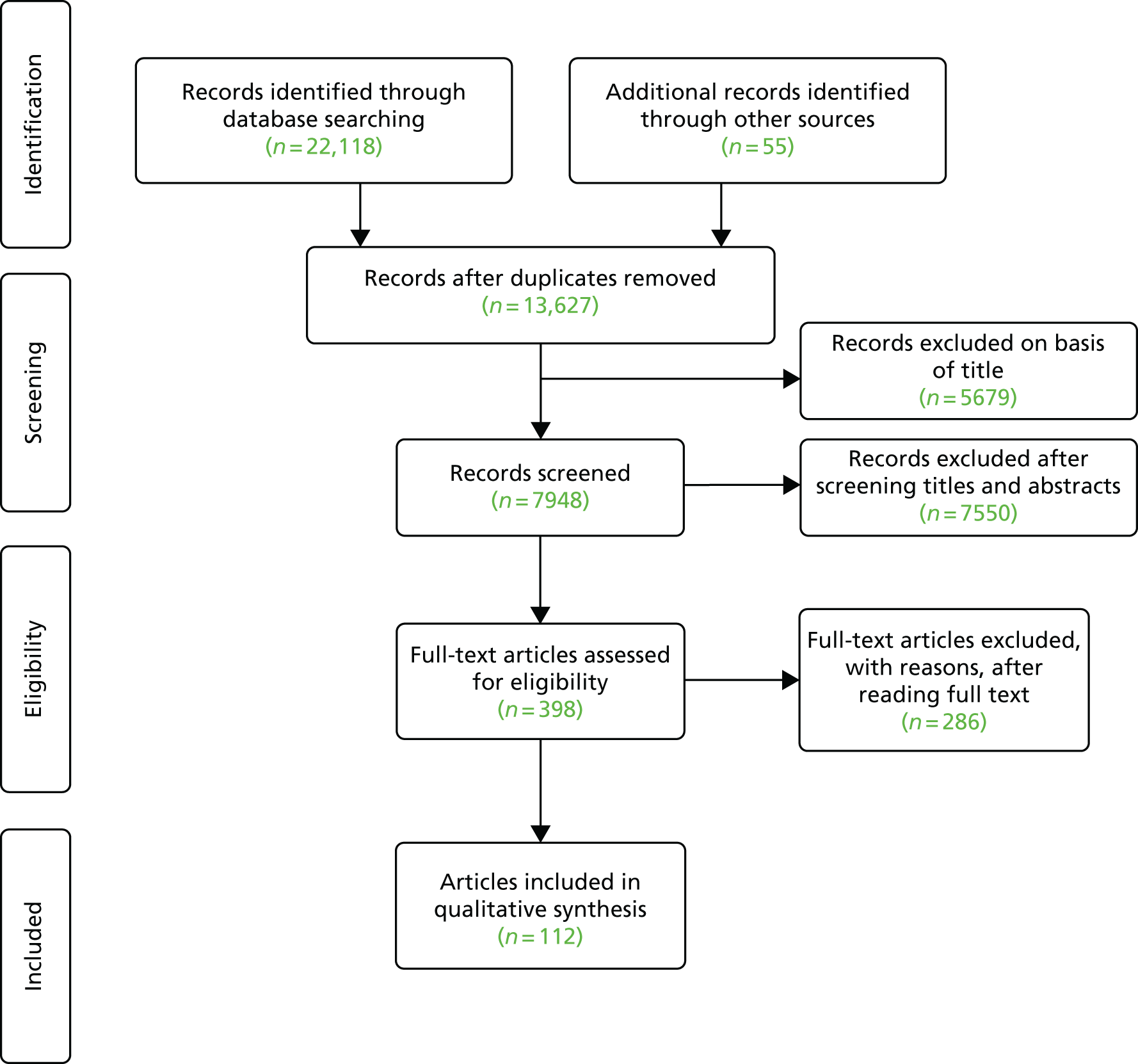
List of abbreviations
- 5-HT
- 5-hydroxytriptamine
- A2E
- N-retinyl-N-retinylidene ethanolamine
- ACE
- angiotensin-converting enzyme
- AE
- adverse event
- AF
- autofluorescence
- Alienor
- Antioxydants, Lipides Essentiels, Nutrition et maladies OculaiRes
- AMD
- age-related macular degeneration
- AREDS
- Age-Related Eye Disease Study
- ARM
- age-related maculopathy
- ARMS2
- Age-Related Maculopathy Susceptibility Gene 2
- ARMSS
- Age-Related Maculopathy Statin Study
- ARVO
- Association for Research in Vision and Ophthalmology
- BCVA
- best corrected visual acuity
- BMES
- Blue Mountains Eye Study
- BOSU
- British Ophthalmological Surveillance Unit
- BSE
- better-seeing eye
- CAPT
- Complications of Age-related Macular Degeneration Prevention Trial
- CCT
- controlled clinical trial
- CFH
- complement factor H
- CFP
- colour fundus photography
- CI
- confidence interval
- CNTF
- ciliary nerve trophic factor
- CNV
- choroidal neovascularisation
- CNVPT
- Choroidal Neovascularisation Prevention Trial
- COMPLETE
- Complement Inhibition with Eculizumab for the Treatment of Nonexudative Macular Degeneration
- CPRD
- Clinical Practice Research Datalink
- CRD
- Centre for Reviews and Dissemination
- DHA
- docosahexaenoic acid
- DPED
- drusenoid pigment epithelium detachment
- EMA
- European Medicines Agency
- EME
- Efficacy and Mechanism Evaluation
- EPA
- eicosapentaenoic acid
- ERG
- electroretinography
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- FAF
- fundus autofluorescence
- FDA
- Food and Drug Administration
- fERG
- focal electroretinogram
- GA
- geographic atrophy
- HDL
- high-density lipoprotein
- HELP
- heparin-induced extracorporeal lipoprotein precipitation
- hESC
- human embryonic stem cell
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICD
- International Classification of Diseases
- IgM
- immunoglobulin M
- IHD
- ischaemic heart disease
- IMT
- implantable miniature telescope
- IOL
- intraocular lens
- IOP
- intraocular pressure
- IPSC
- induced pluripotent stem cell
- IQR
- interquartile range
- i.v.
- intravenous
- LCPUFA
- long-chain polyunsaturated fatty acid
- LDL
- low-density lipoprotein
- L-dopa
- levodopa
- LEAD
- Laser Intervention in Early Age-Related Macular Degeneration Study
- logMAR
- logarithm of minimum angle of resolution
- MCS
- microcurrent stimulation
- MIRA
- Multicenter Investigation of Rheopheresis for Age-related macular degeneration
- MMP
- matrix metalloproteinase
- MPOD
- macular pigment optical density
- MRC
- Medical Research Council
- nAMD
- neovascular age-related macular degeneration
- NCT
- National Clinical Trial
- NEIVFQ-25
- National Eye Institute Visual Function Questionnaire-25
- NHANES
- National Health and Nutrition Examination Survey
- NHLBI
- National Heart, Lung and Blood Institute
- NHSC
- National Horizon Scanning Centre
- NIH
- National Institutes of Health
- NIHR
- National Institute for Health Research
- OCT
- optical coherence tomography
- OR
- odds ratio
- PED
- pigment epithelial detachment
- PERC
- Prospective Evaluation of Visual Functioning with Rheopheresis Treatment for Age-related Macular Degeneration in Canada
- PR
- photoreceptor
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RAP
- retinal angiomatous proliferation
- RBP
- retinol binding protein
- RCT
- randomised controlled trial
- RPD
- reticular pseudodrusen
- RPE
- retinal pigment epithelium
- RR
- relative risk
- SCOTS
- Stem Cell Ophthalmology Treatment Study
- SD
- standard deviation
- STGD
- Stargardt disease
- TENS
- transcutaneous electrical nerve stimulation
- THIN
- The Health Improvement Network
- UVR
- ultraviolet radiation
- VA
- visual acuity
- VEGF
- vascular endothelial growth factor
- VITAL
- Vitamin D and Omega-3 Trial
