Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/137/05. The contractual start date was in September 2015. The draft report began editorial review in April 2017 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Luke Vale is a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Clinical Evaluation and Trials Board. Noah M Ivers received grants from the Canadian Institutes of Health Research outside the submitted work. Jeremy M Grimshaw received grants from the Canadian Institutes of Health Research during the conduct of this study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Lawrensonet al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Globally, an estimated 380 million adults have diabetes mellitus. The majority have type 2 diabetes and the numbers are predicted to increase because of longer lifespans and rising levels of obesity. The majority of those affected live in low- or middle-income countries. 1Diabetes and its complications impose a considerable health and economic burden on individuals, families and health-care systems. 2
Diabetic retinopathy is the most common microvascular complication of diabetes and is one of the leading causes of blindness and visual impairment in the UK and throughout the world. 3,4Although effective treatments are available for sight-threatening retinopathy in the form of laser photocoagulation5and, more recently, the use of antivascular endothelial growth factor inhibitors,6the success of these interventions is dependent on early detection and timely referral for treatment. Screening for diabetic retinopathy fulfils the World Health Organization (WHO) criteria for a screening programme,7namely diabetes-associated visual impairment is an important public health problem; potentially sight-threatening retinopathy has a recognisable latent stage; a universally accepted and effective treatment is available; and screening is cost-effective compared with no screening in terms of sight-years preserved. 8,9Relatively few countries have introduced a national population-based diabetic retinopathy screening (DRS) programme. In most parts of the world screening remains opportunistic, although an annual or biennial retinal examination is recommended in diabetes clinical practice guidelines in many countries. 10–12
Given the value of screening for reducing the risk of sight loss among people with diabetes, it is essential that screening programmes provide consistent and equitable access for the target population. Furthermore, an appropriate infrastructure needs to be in place to manage those testing positive, with timely access to treatment for those who need it. To maximise screening coverage, those people with diabetes who would benefit from screening need to be identified and receive regular screening as part of their normal diabetic care.
UK diabetic retinopathy screening programme
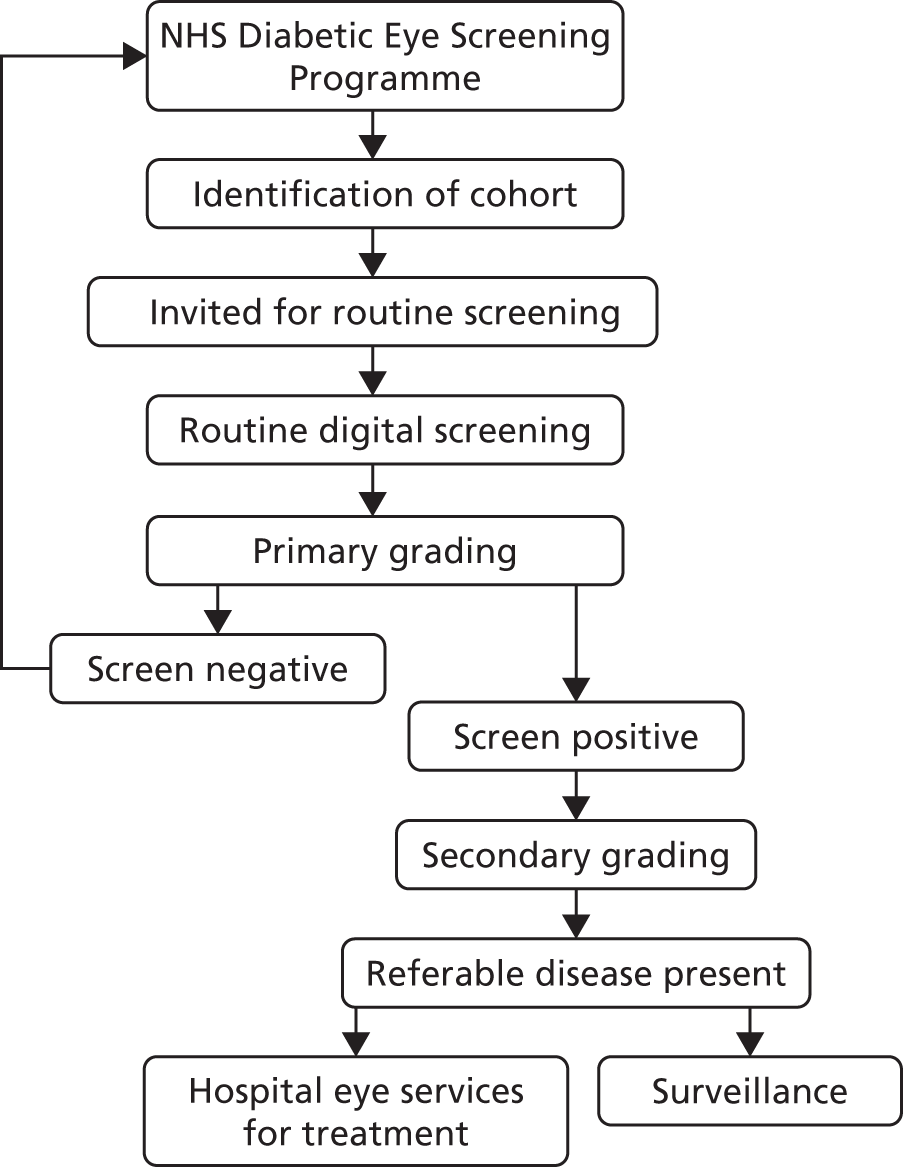
Recent developments in digital retinal photography have facilitated the rapid acquisition of high-quality fundus images that can be stored and subsequently graded. Digital imaging combined with trained graders has been shown to be an effective screening tool to identify sight-threatening diabetic retinopathy13and is increasingly gaining acceptance for population screening. 14–17The UK was the first country in the world to introduce a national population-based DRS scheme, which was based on annual digital fundus photography (Figure 1). This was initially introduced in England in 2003 as part of the National Service Framework for Diabetes18and by 2008 the scheme had become established throughout the UK. In England, DRS is overseen by the NHS Diabetic Eye Screening Programme, which offers screening to all people with diabetes aged > 12 years in approximately 70 local programmes. Screening services in Scotland, Wales and Northern Ireland are very similar but with slightly different operational procedures. If sight-threatening retinopathy is identified through the screening service, referral to a specialist eye unit is arranged within a specified time frame for further assessment and treatment. An early indicator of the success of the Diabetic Eye Screening Programme, combined with incentives to primary care practitioners to improve the quality of diabetes care,19comes from a recent longitudinal analysis of the national database of blindness certificates of vision impairment. 4Over 10 years leading up to 2009–10, the rate of working-age adults in England and Wales (aged 16–64 years) with blindness certifications attributable to diabetic retinopathy decreased by 3.7%, such that the condition is no longer the main cause of blindness in working adults.
FIGURE 1.
NHS Diabetic Eye Screening Programme.
Variation in attendance for diabetic retinopathy screening
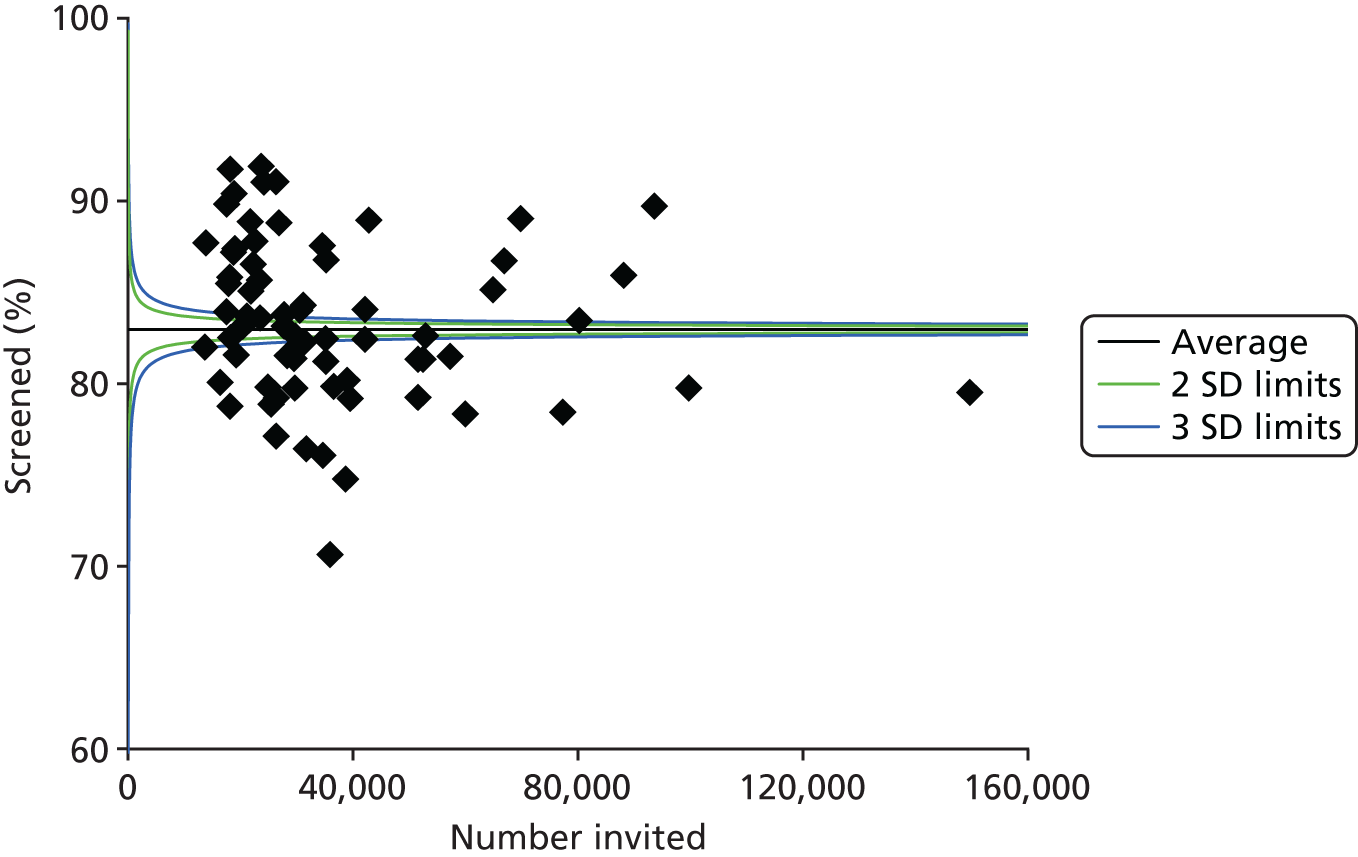
Despite evidence supporting the effectiveness of DRS in reducing the risk of sight loss in people with diabetes, screening coverage is consistently below recommended levels. 20–22Following a diagnosis of diabetes, not all of those who are eligible are referred into the retinopathy screening service and further barriers occur within the screening service itself. Those invited for screening have to receive the invitation, appreciate the importance of attending for screening and then attend for their appointment. Although a small proportion of the unscreened population is either excluded or suspended from the screening service, approximately 20% of those offered screening fail to attend, with wide geographical variation in screening attendance. Based on the most recently available information from screening programmes in England (from April 2015 to March 2016) (Figure 2), attendance varied from 71% to 92% of those referred into the screening service.
FIGURE 2.
Percentage of those offered a routine DRS appointment who attend and complete a routine digital screening event [based on DE1 (Diabetic Eye-Uptake of Digital Screening Encounter) key performance indicator data submissions from 1 April 2015 to 31 March 2016] across 72 screening programmes. SD, standard deviation.
Predictors of poor attendance for diabetic retinopathy screening and quality improvement interventions to increase screening attendance
Following the introduction of the UK NHS Diabetic Eye Screening Programme, published audits have reported significant inequity in DRS attendance and outcomes. Living in areas of high social deprivation, a younger age (< 40 years) and having a longer duration of diabetes have been found to be associated with lower rates of attendance. 8,20,23,24Ethnicity is also an important determinant of DRS attendance and outcomes. Black, Asian and minority ethnic groups with type 2 diabetes have a higher prevalence of diabetic retinopathy than white Europeans3and there is evidence to suggest that these groups are more likely to present with sight-threatening retinopathy and have higher rates of referral to ophthalmology following screening. 25,26Despite the associated risk, there is evidence that these ethnic groups are less likely to attend for screening. 20In addition to the obvious impact on eye health, the high rates of non-attendance have major financial consequences. For example, from April 2012 to April 2013, in the London borough of Tower Hamlets, the screening programme invited 13,894 people to participate in DRS. Of those invited, 4833 (34.7%) failed to attend for their appointment, without rebooking or cancelling. With each appointment costing £25, the total cost of non-attendance for that year can be crudely estimated at £120,825 (Tunde Peto, Queen’s University Belfast, 2016, personal communication).
The difference between health-care processes or outcomes observed in practice and those believed to be achievable has been referred to as the quality gap. 27DRS attendance can be regarded as a quality improvement (QI) target, which along with other processes of diabetes care can potentially be improved through the use of one or more QI interventions. A systematic review assessing the effectiveness of QI interventions to promote DRS was published in 2007. 22This review found evidence that a variety of intervention components targeting the patient, the health-care professional (HCP) or the health-care system can be effective in improving screening attendance. Patient-targeted QI components included (1) educational programmes to increase awareness of diabetic retinopathy and promote self-management and (2) the use of prompts/reminders. HCP-focused QI components included (1) clinician education and (2) audit and feedback. Interventions targeting the health-care system included (1) team changes, (2) establishing electronic registration and recall and (3) the use of telemedicine. In addition to QI interventions that specifically target DRS, general QI interventions for diabetes care may also be effective in improving screening coverage. Several systematic reviews of general QI interventions for improving the quality of diabetes care have included eye-screening outcomes (see Worswicket al. 28for an overview of these reviews). A systematic review by Triccoet al. 29explored the effectiveness of QI interventions in diabetes care and included eye screening as an outcome. QI interventions increased the likelihood that people with diabetes received screening for retinopathy [23 trials; relative risk (RR) 1.22, 95% confidence interval (CI) 1.13 to 1.32]. Although this review provided information on the relative effectiveness of different QI interventions, the optimal combination of intervention components remained unclear.
To develop and evaluate QI interventions for improving DRS attendance, it is important to understand the causal determinants of poor screening attendance. Moreover, it is unclear whether single or multicomponent interventions are needed, what the optimal combination of components in multicomponent interventions would be and whether or not interventions need to be adapted to particular patient groups (i.e. based on socioeconomic factors, comorbidities, reality, etc.). There is overwhelming evidence that behaviour change plays an important role in people’s health. 30Interventions to improve screening attendance are therefore likely to be more effective if they target the determinants of behaviour associated with DRS and tailor behaviour change strategies to specific patient groups. 31,32
Rationale for current evidence synthesis and methodological approach
Although there have been previous systematic reviews33,34on interventions to optimise adult screening programmes [including a National Institute for Health Research (NIHR)Health Technology Assessment(HTA) report35], it is likely that this evidence is not directly transferable to DRS. Screening for diabetic retinopathy differs from other forms of screening. It is technically a surveillance programme in that those in the target group have already been identified and have significant contact with the health-care system because of their underlying diabetes, with life-long monitoring (i.e. annual or biennial surveillance). However, the UK NHS Diabetic Eye Screening Programme is widely known as a screening programme and, for consistency, we will refer to screening rather than surveillance throughout this report.
Failure to attend for DRS is not only a UK problem but also a global public health problem. Multiple interventions have been studied in many countries in a variety of populations and contexts, including private and publicly-funded screening services. Given the likely complexity of the behavioural determinants of DRS attendance and the multicomponent nature of the interventions that have been used to increase screening attendance, it is important to consider evidence from settings outside the UK.
In the subsequent chapters we report an evidence synthesis of the published and grey literature to identify the effectiveness and cost-effectiveness of QI interventions for improving attendance for DRS globally and explore the barriers and enablers relating to participation in such activity. We use a validated taxonomy of behaviour change techniques (BCTs)36and theoretical frameworks37–39to specify the components of the interventions and theoretical determinants of screening behaviour. We assess the theoretical coherence between intervention components and determinants (i.e. barriers/enablers) associated with attendance for DRS. We use these data to assess whether or not components of interventions to improve attendance for DRS represent value for money for the UK NHS. We also report a formal process of knowledge exchange with stakeholders and end-users to discuss the interpretation and application of the project findings.
Chapter 2 Overview of methods
Aims and objectives
The aim of this project was to determine the most effective and cost-effective components of interventions that seek to increase attendance for DRS in people with type 1 or 2 diabetes mellitus and to identify likely determinants of poor uptake and ongoing attendance.
The specific objectives were as follows.
-
Systematically review the evidence from randomised controlled trials (RCTs) on the effectiveness and cost-effectiveness of QI interventions that seek to increase attendance for DRS.
-
Enrich the data set by contacting authors of included studies to obtain information on missing data relating to the content of the intervention and/or context.
-
Code descriptions of the interventions reported in the included RCTs in terms of the type of QI intervention components used in the studies and their constituent BCTs (with BCTs being the ‘active components’ of interventions).
-
Explore heterogeneity in effect size to identify factors associated with improved effectiveness (objectives 1–4 reported inChapter 3).
-
Systematically identify the published and grey literature reporting barriers and facilitators associated with DRS.
-
Code barriers and facilitators identified in objective 5 into domains from two theoretical frameworks (with domains being explanatory factors that are proposed to mediate change).
-
Assess whether or not the intervention components (from objective 3) target the proposed mediators (from objective 5) (objectives 5–7 reported inChapters 4and5).
-
Use data from objectives 1–7 to estimate the potential cost–consequences and cost–utility of interventions to increase attendance at DRS (seeChapter 6).
-
Integrate the findings (objectives 1–8), with input from stakeholders and end-users, to make recommendations for practice and future research aiming to improve the attendance for DRS in areas or population subgroups with low uptake of screening (seeChapter 7).
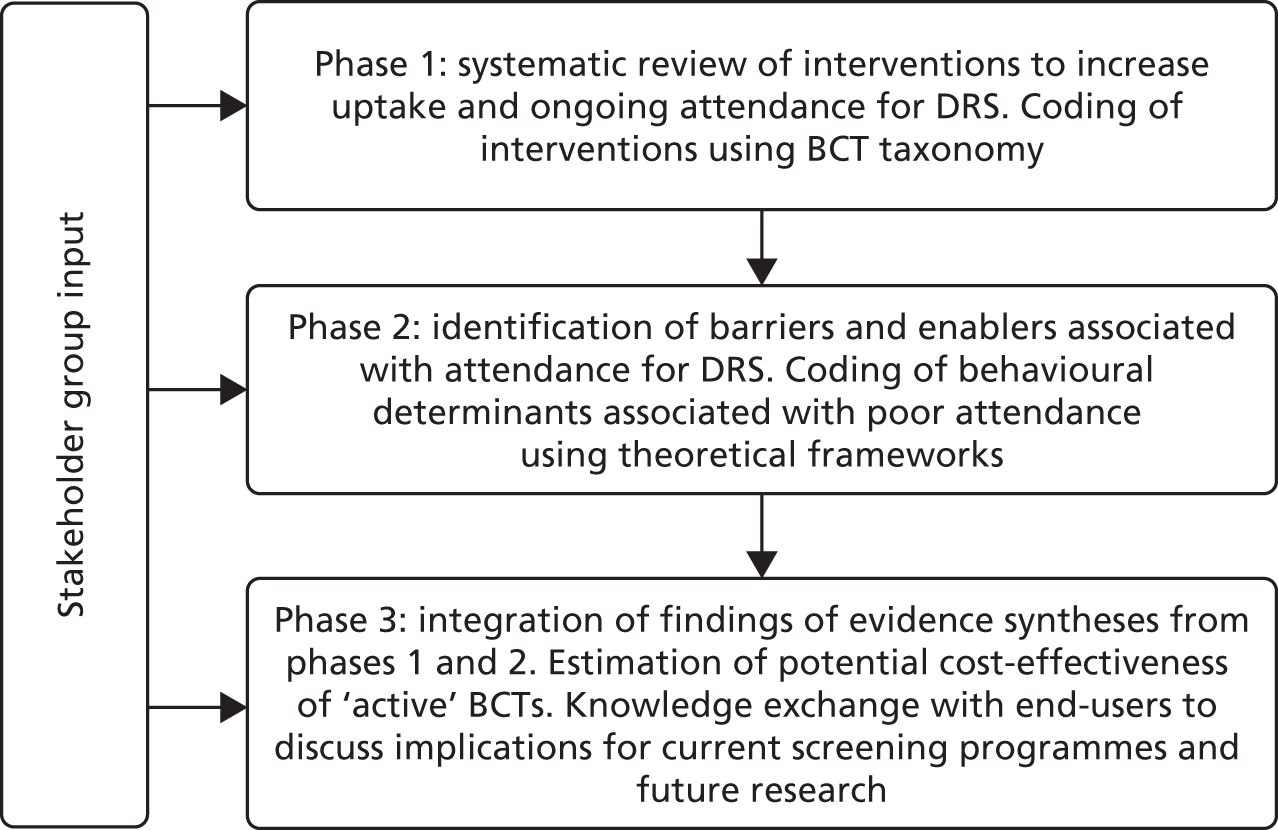
The study plan is detailed inFigure 3. The design used a validated taxonomy of BCTs36to code the components of QI interventions used in RCTs seeking to improve DRS attendance. We also applied a validated, theory-informed framework [Theoretical Domains Framework (TDF)]37,39to code the barriers to, and enablers of, screening behaviour in the studies identified from a systematic review of the published and grey literature. By employing established mapping tools, we assessed the extent to which BCTs used in existing interventions target the important theoretical determinants of DRS attendance. In parallel, a health economic evaluation provided data on the relative cost-effectiveness of the QI interventions, and their active components (BCTs), for increasing DRS attendance and determined the trade-off between the cost of the QI interventions and their effectiveness.
FIGURE 3.
Study plan.
Patient and public involvement
Patient and public involvement was integral to the project. Patient and public representatives were actively involved in the development of the protocols for the phase 1 and phase 2 systematic reviews through our stakeholder advisory group. The input of this group ensured that all important outcomes relevant to the study question were included in the evidence synthesis. The stakeholder advisory group (seeAppendix 1) included representatives of retinopathy screening services throughout the UK, the Public Health Development Manager and the Health Equalities Officer from the Royal National Institute of Blind People (RNIB) (who had been actively involved in community engagement projects to improve the uptake of DRS in minority ethnic groups); and the Director of Health Intelligence and Professional Liaison at Diabetes UK. The stakeholder advisory group also provided valuable intelligence on the problems of non-attendance for screening and directed the team towards relevant grey literature on barriers to attendance and local initiatives to improve attendance.
We conducted a formal 1-day knowledge exchange event with stakeholders and end-users to present the outputs from the evidence synthesis and health economic modelling and discussed their interpretations and service implications of the findings (phase 3). The event was attended by people with diabetes, representatives of leading charities (Diabetes UK, RNIB), screening providers from the screening programmes of the four nations and HCPs (representing ophthalmology, optometry and general medical practice). Before the event, all participants received a summary of the results of the project. On the day, members of the research team presented their research findings, which generated a lively debate. This was followed by three small discussion groups to reflect more deeply on the project results. These groups were facilitated by consultants who were independent of the research team. After the event, we prepared a formal report that included the themes that emerged across the three discussion groups and a discussion of the implications for UK screening programmes and for future research. A copy of the report was sent to participants for comments and amendments (seeAppendix 2). The outputs from the knowledge exchange event were used to inform the conclusions of, and recommendations originating from, this project.
Chapter 3 Interventions to increase attendance for diabetic retinopathy screening: systematic review and meta-analysis
Background
The majority of studies assessing the effectiveness of QI interventions to improve diabetes care (including those delivered specifically to improve DRS) involve multicomponent interventions that attempt to change the behaviour of HCPs (e.g. advising patients to attend DRS) or patients (e.g. actually attending) or both. As there is no consistent association between the number of intervention components and their effectiveness,40,41the ‘ideal’ number of components in such programmes is unknown. Furthermore, given the complexity of interventions tested to date, it is not always clear which specific components are the effective elements of these interventions (i.e. the ‘active ingredients’). Hence, the content of complex behaviour change interventions and the mechanisms through which they have their effect has been referred to as a ‘black box. 42Therefore, identification of effective interventions for increasing attendance for DRS first requires clarity about intervention content and the functional relationship between components of interventions and the intended outcomes. The Cochrane Effective Practice and Organisation of Care (EPOC) Group has developed a taxonomy that can be used to classify intervention content in systematic reviews. 43Although the EPOC Group taxonomy provides a common language and a useful summary description of interventions, the taxonomy may not be sufficiently detailed to specify intervention components in sufficient detail to facilitate replication. 44A complementary approach is to provide a comprehensive categorisation of the ingredients of interventions in terms of the BCTs used. BCTs are defined as the ‘observable, replicable and irreducible components of an intervention that are designed to alter or redirect causal processes regulating behaviour’. 36Recently, a taxonomy of 93 BCTs has been published (co-developed by team member JJF) to provide a common, consistent terminology [Behaviour Change Technique Taxonomy version 1 (BCTTv1)36by which the component BCTs in complex interventions may be identified and described. Examples of BCT labels in this taxonomy include ‘goal-setting (outcome)’, ‘self-monitoring of behaviour’, ‘feedback on behaviour’ and ‘problem-solving’. Review team members (JP, NMI and JMG) have demonstrated the feasibility of using the BCT taxonomy within trials of QI interventions for diabetes care. 44
Given the potential of screening for reducing the risk of sight loss among people with diabetes, it is essential that attendance for DRS is maximised as far as available resources allow. Wide geographical variation in screening coverage has been reported, with associated inequalities in outcomes. Furthermore, given the incremental costs (resource use) and benefits (effects) associated with interventions to improve attendance for DRS, it is important to consider whether or not such strategies are worthwhile. By identifying the active components of interventions that increase attendance for screening, this review contributes to the identification of implementation strategies for early detection of sight-threatening retinopathy. Furthermore, by exploring the differential effects of interventions in particular subgroups, the results may provide insight to help reduce inequalities in screening attendance and determine the impact of inequity on intervention effectiveness and efficiency.
Objectives
-
Systematically review the evidence from RCTs for the effectiveness and cost-effectiveness of QI interventions that seek to increase attendance for DRS.
-
Enrich the data set by contacting authors of included studies to obtain information on missing data relating to the content of the intervention and/or context.
-
Code descriptions of the interventions reported in the included RCTs in terms of the type of QI interventions used and their constituent BCTs.
-
Explore heterogeneity in effect size using conventional and innovative meta-analytic methods to identify factors (including BCTs) associated with greater effectiveness.
Methods
The protocol for this review was published as a Cochrane protocol in The Cochrane Library on 11 August 2016 (PROSPERO CRD42016044157). 45The economics aspects of the review were carried out according to the recently updated methodology for incorporating economic evidence into Cochrane intervention reviews.
Types of studies
We considered RCTs, both individually randomised and cluster RCTs, conducted in a primary or a secondary care setting. To investigate cost-effectiveness, we included full economic evaluations (cost-effectiveness analyses, cost–utility analyses and cost–benefit analyses), cost analyses and comparative resource utilisation studies conducted alongside or as part of an included RCT.
Types of participants
We included participants with type 1 and type 2 diabetes mellitus who were eligible for DRS.
Types of interventions
We included RCTs that used any planned strategy or combination of strategies to improve attendance for DRS targeted at individuals with diabetes, HCPs or the health-care system. Interventions included those specifically targeting DRS as well as those that were part of a general QI intervention for diabetes care. Comparator interventions were as specified in the included studies.
Types of outcome measures
Primary outcome
The primary outcome was one or more visits for DRS within a 2-year period following randomisation. This could be based on self-reports, medical insurance claims databases or health record audits.
Secondary outcomes
We considered the following secondary outcomes:
-
ongoing adherence to DRS based on attendance for screening following the initial screening post intervention
-
economic outcomes
-
resources (staff time, equipment, consumables) required to deliver interventions to increase attendance for screening
-
costs of staff used to provide interventions, costs of treatment and care, costs of primary care, lost wages and lost productivity (work output)
-
cost-effectiveness [incremental cost-effectiveness ratios (ICERs), incremental cost per quality-adjusted life-year (QALY), incremental cost per disability-adjusted life-year, incremental cost–benefit ratios and net benefits].
-
Search methods for identifying studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the NHS Economic Evaluation Database (NHS EED) on The Cochrane Library, Ovid MEDLINE, Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2017), EMBASE (January 1980 to February 2017), PsycINFO (1967 to February 2017), the Web of Science Conference Proceedings Citation Index – Science (CPCI-S) (January 1990 to February 2017) and Emerging Sources Citation Index (ESCI) (January 2015 to February 2017), ProQuest Family Health (January 1987 to February 2017) and OpenGrey (January 1980 to February 2017). We searched the following trials registers: International Standard Randomised Controlled Trial Number (ISRCTN) registry, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic searches. Appendix 3provides an example search strategy, with full details provided inReport Supplementary Material 1(seeSearch strategies for phase 1 systematic review).
Searching other resources
We searched the reference lists of included studies to identify additional relevant references. In particular, we used the reference list of a 2012 systematic review29co-authored by members of the current review team (NMI, JMG) that investigated the effectiveness of QI strategies for the management of diabetes. We also identified further studies from the review authors (NMI, JMG), who are currently updating this review. 46As the review of general QI interventions included only components directed at patients in conjunction with an intervention targeting HCPs,29we also screened the list of excluded studies, which was obtained from the review team.
Study selection and data extraction
Two review authors (JGL and JMB) independently screened the titles and abstracts of studies identified in the electronic searches. We obtained full copies of research papers in cases of uncertainty and resolved any differences of opinion between review authors by discussion. Two review authors (JGL and EGR) worked independently to extract data from the included studies using a modified version of the EPOC Group data collection form,47which incorporates information on study design, type and duration of interventions, participants, setting, methods, outcomes and results. For the extraction of data on the sociodemographic characteristics of participants that are known to be important from an equity perspective, we used the PROGRESS (place, race, occupation, gender, religion, education, socioeconomic status, social status) framework48and also recorded whether or not any interventions were aimed at disadvantaged or low- and middle-income country populations (defined using theWorld Bank Atlas Method – Detailed Methodology49).
Studies judged to potentially include economic data were identified and further assessed by an economics reviewer (PA). Data from included economic evaluations were extracted by one reviewer (PA) and checked by a second reviewer. Data collection was adapted from the format and guidelines used to produce the structured abstracts of full economic evaluations for inclusion in the NHS EED,50which were redesigned to accommodate specific data required for the review. Economic evaluations were classified based on their analytical framework and were coded appropriately.
Coding of intervention content
We coded intervention descriptions from all of the included studies using a validated taxonomy to characterise the constituent components of each intervention. The Cochrane EPOC Group has developed a comprehensive taxonomy to classify interventions for use in systematic reviews. 43We used a subset of the EPOC taxonomy that had been previously used by members of the review team in a review of the effectiveness of general QI implementation strategies for diabetes care29(seeAppendix 4). This adapted taxonomy incorporates 12 components that target health-care systems (case management, team changes, electronic patient registry, facilitated relay of information to clinicians, continuous QI), clinicians (audit and feedback, clinician education, clinician reminders, financial incentives) or patients (patient education, promotion of self-management and reminder systems). Two review authors (JGL and EGR) independently coded QI components as ‘present’ or ‘absent’ for all intervention and control arms. Any discrepancies were resolved by discussion.
To better characterise intervention content we also coded BCTs within interventions using the BCTTv1. 36Describing an intervention in terms of BCTs (i.e. ‘active ingredients’) provides a useful level of detail for synthesis and comparison44(seeAppendix 5for a description of the BCTs identified in the included studies). We coded BCTs as ‘present’ or ‘absent’ separately for patient and HCP recipients. 44We contacted all authors of included studies to ask for further information on the content of the intervention (e.g. a trial protocol, letters sent to patients, written or audio-visual materials) to clarify the BCT coding. We coded these materials using the BCT taxonomy in the same manner as for the corresponding published reports. Two review authors independently conducted BCT coding (EGR and FL), resolving discrepancies by discussion and, when necessary, by the involvement of a third reviewer (JJF).
Resource requirement needed to deliver interventions
We developed an ordered ranking scale to quantify the level of resource needed to deliver each intervention, based on the description of the intervention components in each included study (seeChapter 6for further details of the methodology). To determine the feasibility of this approach, we initially piloted the scale on a sample of 10 included studies using two members of the review team. Each intervention was initially graded between 1 (least resource intensive) and 5 (most resource intensive), or as 0 (unable to determine), with a record of how the reviewer graded each study also provided.
An algorithm was developed to derive the ordered rank. This mapped resource components and their intensity to the ordered rank. The following resource components were incorporated into the algorithm:
-
face-to-face minutes
-
telephone calls
-
patient home visits
-
printed materials/software
-
training.
We defined, a priori, a criterion for success of the ranking scale as reviewer scores from nine out of 10 studies being within one grade of each other following discussion. This criterion was achieved and the notes about how each study was graded were used to produce a reproducible description of the resource input associated with each grade on the ranking scale. The resource components and their intensity levels were then used to extract the resource use required to deliver the interventions in all included studies. This was conducted by two reviewers independently (JGL and EGR).
For the algorithm that mapped resource use onto the ordered ranking, the weights given to each resource component and their intensity were subsequently revised based on cost analyses (seeChapter 6). The revised grading system produced 18 ranks and these were recategorised into five ordered categories for the purposes of analysis.
Assessment of risk of bias
Two review authors (JGL and JMB) independently assessed study quality using the EPOC Group risk-of-bias tool,51which uses nine standard criteria.
-
Was the allocation sequence adequately generated?
-
Was the allocation adequately concealed?
-
Were baseline outcome measurements similar?
-
Were baseline characteristics similar?
-
Were incomplete outcome data adequately addressed?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Was the study adequately protected against contamination?
-
Was the study free from selective outcome reporting?
-
Was the study free from other risks of bias?
For cluster RCTs we considered particular biases, including (1) recruitment bias, (2) baseline imbalance, (3) loss of clusters and (4) unit of analysis errors. For each domain, two review authors performed the risk-of-bias assessment independently and assigned a judgement of low risk, high risk or unclear risk of bias. The review authors resolved any discrepancies between them by discussion.
The reliability of the data outputs from any full economic evaluation are in part predicated on the reliability of the estimates of the relative treatment effects (for benefits or harms) of the alternative courses of action [i.e. intervention(s) and comparator(s)) under investigation]. As the identified economic studies were a subset of the studies included in the review, the risk of bias was already assessed. However, assessment of the overall methodological quality of the economic component was still required and was carried out by one reviewer (PA) using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement52together with the Consensus on Health Economic Criteria (CHEC). 53In assessing the methodological quality of economic evaluations, the main objective is to assess the applicability of the scope of the analysis in terms of costs and outcomes. This helps to highlight the applicability and relevance of each economic evaluation. The checklists used differed from those that were originally included in our published Cochrane protocol because of the recent updates of the methods for the incorporation of economic evidence into Cochrane intervention reviews (seeReport Supplementary Material 1,Completed checklists for methodological quality assessment of economic evaluations, for the completed checklists for each included economic evaluation).
Data synthesis
Attendance at screening post intervention is a dichotomous outcome and our measure of intervention effect was the risk difference (RD), that is, the actual difference in the observed events between experimental and control interventions. For individual RCTs the unit of analysis was the individual participant. For cluster RCTs we analysed data after adjustment for clustering. In the case of cluster RCTs, when outcomes were presented at the patient level, we used an established method to adjust for clustering. 54This involved dividing the original sample size by the design effect, which was calculated from the average cluster size and the intraclass correlation coefficient (ICC). When the ICC was not reported, we imputed the most commonly reported value from studies in which it was reported. We contacted authors of included studies when important data were missing; we did not impute missing data if data were not available.
We conducted meta-analyses in Review Manager 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) using random-effects models to estimate the pooled RD across studies. Data from patient RCTs and cluster RCTs that were adjusted for clustering were included in the same meta-analyses. In the case of multiple intervention groups, as recommended in theCochrane Handbook for Systematic Reviews of Interventions,55we combined groups to create a single pairwise comparison.
A summary of the results of the included economic evaluations is available inReport Supplementary Material 1[seeFurther details of the review of economic evidence (phase 1 review)]; this is supplemented by a narrative description in the results and discussion sections. Costs for each study were adjusted to 2016 UK pounds using a web-based conversion tool based on implicit price deflators for gross domestic product (GDP), a measure of the wealth of a country, and GDP purchasing power parities. 56The tables inReport Supplementary Material 1[seeCharacteristics of included, ongoing and excluded studies (phase 1 review)] present the original currency and price year used in each included study. Users of this review who might want to adjust costs to another currency and price year suitable for their needs should use the costs for each study presented inReport Supplementary Material 1and not the adjusted costs presented in the main text of this report.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots and by formal statistical tests of heterogeneity (chi-squared test and theI2statistic). TheI2statistic is the proportion of variation between studies not due to chance and can take a value from 0% to 100%. Heterogeneity may be the result of variation in the ‘true’ effects underlying the studies but may also be the result of clinical diversity (participants, interventions, outcomes) and methodological diversity (varying degrees of bias).
We performed the following prespecified subgroup analyses to investigate whether or not the presence or absence of a particular covariant explained the variability in effect size:
-
QI intervention components/BCTs
-
resource requirements to deliver the intervention.
In our analyses, QI components (coded using the modified EPOC Group taxonomy) and BCTs of each intervention were assessed separately. When a study used multiple QI components and/or BCTs, the same effect size was applied to each component for the analysis. We compared effect estimates for subsets of studies that used a particular QI component/BCT or resource intensity and calculated a pooled effect size.
We further investigated associations between DRS attendance and effect size by meta-regression for a number of covariates including type of study design (individual/cluster RCT), baseline DRS attendance and QI component/BCT used in the intervention. For meta-regression we used a prespecified random-effects model and compared the RD of studies containing a particular explanatory variable with that of studies in which the variable was absent.
Subgroup analyses and meta-regression were conducted using Stata®14 (StataCorp LP, College Station, TX, USA) using the metan and metareg commands. We performed a sensitivity analysis to determine the impact on the pooled effect estimate of imputing the lower and upper range values for the ICC.
Methods used to assess the quality of the evidence for outcomes included in the summary of findings tables
We assessed the quality of the evidence using the evidence grading system developed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) collaboration57and described in section 12.2 of theCochrane Handbook for Systematic Reviews of Interventions. 55One review author (JGL) initially applied the GRADE evidence rating system and discussed the rating with other members of the review team. A final decision was reached by discussion and consensus.
We took the following into account when deciding whether or not to downgrade the quality of evidence for each outcome:
-
risk of bias
-
inconsistency of results
-
indirectness of evidence
-
imprecision of results
-
publication bias.
Results
Study selection
The database searches yielded 7244 titles and abstracts. We identified a further 33 studies from additional sources. After removing duplicates we screened 7277 studies and reviewed 130 full-text articles. We excluded 64 studies with reasons [seeReport Supplementary Material 1,Characteristics of included, ongoing and excluded studies (phase 1 review)] and included 66 studies that met our inclusion criteria [Figure 4provides a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram]. Of the included studies, 5058–107(75.8%) reported general QI interventions and evaluated the impact of the interventions across a range of outcomes, including DRS uptake; in 16 studies108–123(24.2%), the primary target of the intervention was to improve attendance for DRS. From the search of trials registers we identified nine ongoing studies [seeReport Supplementary Material 1,Characteristics of included, ongoing and excluded studies (phase 1 review)].
FIGURE 4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
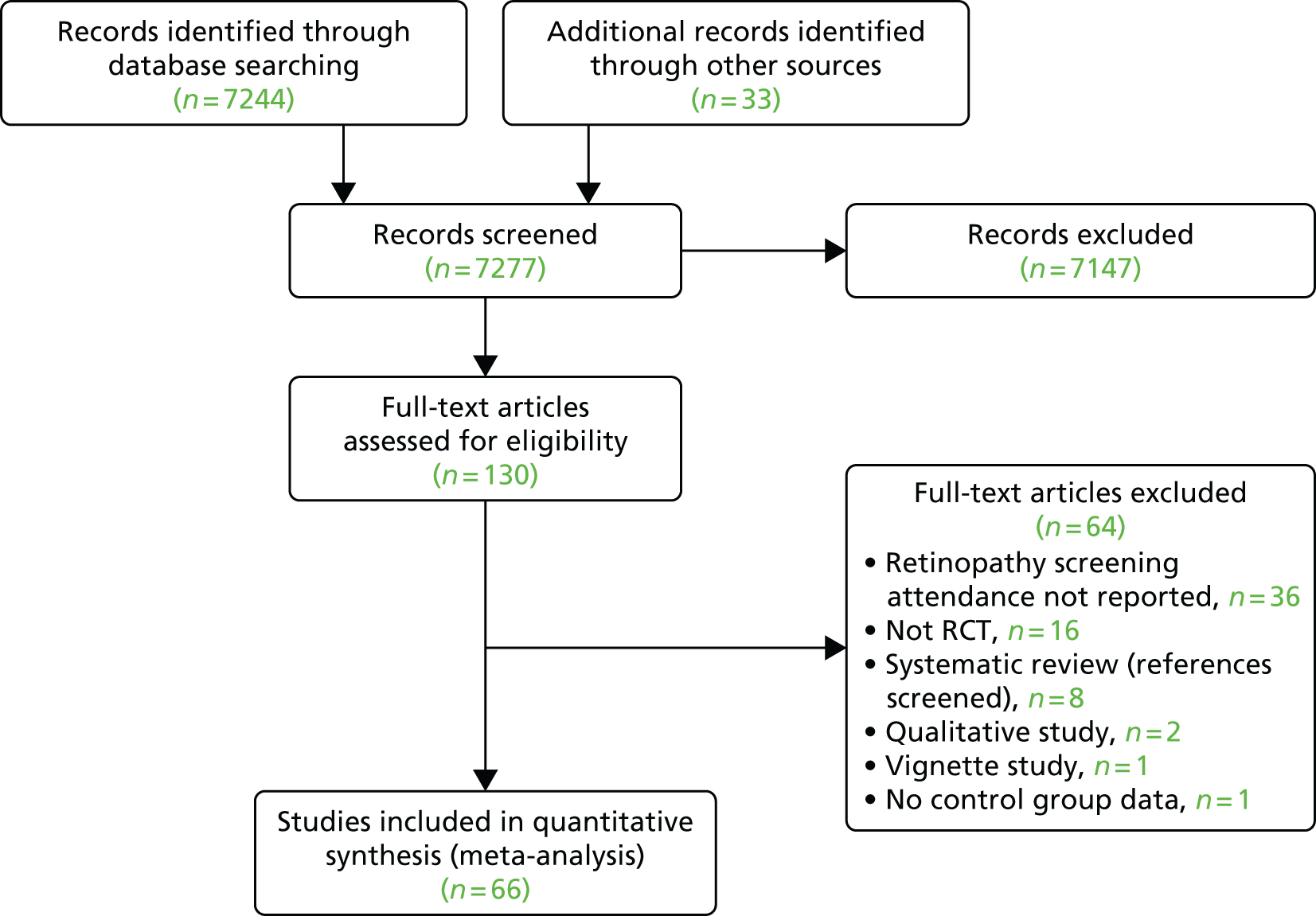
From the database searches we identified 22 references containing potential economic evaluations. Following full-text review, eight58,83,94,103,114,115,122,123of these references were excluded and 14 references59,62,67,69,70,85,87,89,95,105,117,124–126reporting economic outcomes were included in the review.
Characteristics of included studies
The included studies were conducted between 1988 and 2013. Thirty-five studies (53%) were parallel-group patient RCTs enrolling 237,025 patients59,61–63,71,72,75,80,82,85–89,95,96,98,99,102,103,107–109,111–122and 31 (47%) were cluster RCTs in which the HCP or the health-care setting was the unit of randomisation. 58,60,64–70,73,74,76–79,81,83,84,90–94,97,100,101,104–106,110,123These included 6126 clusters (range 6–4125 clusters). Fifty-nine studies (89%)58–63,65–78,80–93,95–105,107–116,118,120–122had two arms, six studies (9%)64,79,94,106,117,119had three arms and one study (2%)123had more than three arms. An overview of the included studies is provided inTable 1[for further details seeReport Supplementary Material 1,Characteristics of included, ongoing and excluded studies (phase 1 review)].
| Study characteristic | Target: DRS attendance (n = 16) | Target: general QI in diabetes care (n = 50) | Total (n = 66) |
|---|---|---|---|
| Study design | Individual RCT,n = 14 (87.5%); cluster RCT,n = 2 (12.5%) | Individual RCT,n = 21 (42.0%); cluster RCT,n = 29 (58.0%) | Individual RCT,n = 35 (53.0%); cluster RCT,n = 31 (47.0%) |
| Two arms,n = 13; three arms,n = 2; more than three arms,n = 1 | Two arms,n = 46; three arms,n = 4 | Two arms,n = 59 (89.4%); three arms,n = 6 (9.1%); more than three arms,n = 1 (1.5%) | |
| Location | USA,n = 12 (75.0%); Canada,n = 1 (6.3%); China,n = 1 (6.3%); Germany,n = 1 (6.3%); and the UK,n = 1 (6.3%). Conducted between 1995 and 2013 | USA,n = 29 (58.0%); Canada,n = 2 (4.0%); Netherlands,n = 4 (8.0%); Australia,:n = 3 (6.0%); the UK,n = 2 (4.0%); and other,n = 10 (20.0%). Conducted between 1988 and 2013 | USA,n = 41 (62.1%); Canada,n = 3 (4.6%); Netherlands,n = 4 (6.1%); Australia,n = 3 (4.6%); the UK,n = 3 (4.6%); and other,n = 12 (18.2%). Conducted between 1985 and 2013 |
| Setting | Primary care,n = 11 (68.8%); outpatient clinics,n = 4 (25.0%); unclear,n = 1 (6.3%) | Primary care,n = 40 (80.0%); outpatient clinics,n = 3 (6.0%); unclear,n = 7 (14.0%) | Primary care,n = 51 (77.3%); outpatient clinics,n = 7 (10.6%); unclear,n = 8 (12.1%) |
| Diabetes type | Type 2,n = 4 (25.0%); types 1 and 2,n = 3 (18.8%); not reported,n = 9 (56.3%) | Type 2,n = 34 (68.0%); types 1 and 2,n = 7 (14.0%); not reported,n = 9 (18.0%) | Type 2,n = 38 (57.6%); types 1 and 2,n = 10 (15.2%); not reported,n = 18 (27.3%) |
| Number of participants recruited | Individual RCT,n = 38,273; cluster RCT,n = 4135 clusters (182,513 patients); total,n = 220,786 (patients included) | Individual RCT,n = 198,752; cluster RCT,n = 1991 clusters (78,276 patients); total,n = 277,028 (patients included) | Individual RCT,n = 237,025; cluster RCT,n = 6126 clusters (260,789 patients); total,n = 497,814 (patients included) |
| Age (years), median (range) | 60.7 (51.1–72.7),n = 9 studies | 60.6 (46.8–74),n = 34 studies | 60.7 (46.8 to 74),n = 43 studies |
| Gender (% male), median (range) | 38.9 (25–98),n = 12 studies | 49.8 (25–97),n = 35 studies | 48 (25–98),n = 47 studies |
| Type of screening | Retinal examination,n = 12 (75.0%); grading of digital retinal images,n = 4 (25.0%) | Retinal examination,n = 49 (98.0%); grading of retinal images,n = 1 (2.0%) | Retinal examination,n = 61 (92.4%); grading of retinal images,n = 5 (7.6%) |
| Baseline screening attendance (in previous 12 or 24 months) (%), median (range) | 0 (0–48.4),n = 7 studies | 37.1 (0–88),n = 36 studies | 35.4 (0–87.8),n = 43 studies |
| Longest duration of follow-up (months), median (range)a | 6 (3–48),n = 14 studies | 12 (1–30),n = 49 studies | 12 (1–48),n = 63 studies |
Types of participants
Participant characteristics are reported inTable 1. We used PROGRESS elements to describe the characteristics of participants in the included studies that could have an impact on equity of access to health services. With the exception of gender (reported in 93.9% of studies) and ethnicity (reported in 56.1% of studies), the characteristics of participants were poorly described and the relative effectiveness of the interventions in subgroups in terms of PROGRESS elements was never reported. Seventeen studies (25.8%)60,62,63,68,72,75,91,96,107–110,112,113,116,120,121were conducted in disadvantaged populations and no studies were carried out in low- or middle-income countries.
Types of setting
Details of study location and setting are given inTable 1.
Intervention content in terms of quality improvement components (coded using the modified Effective Practice and Organisation of Care Group taxonomy)
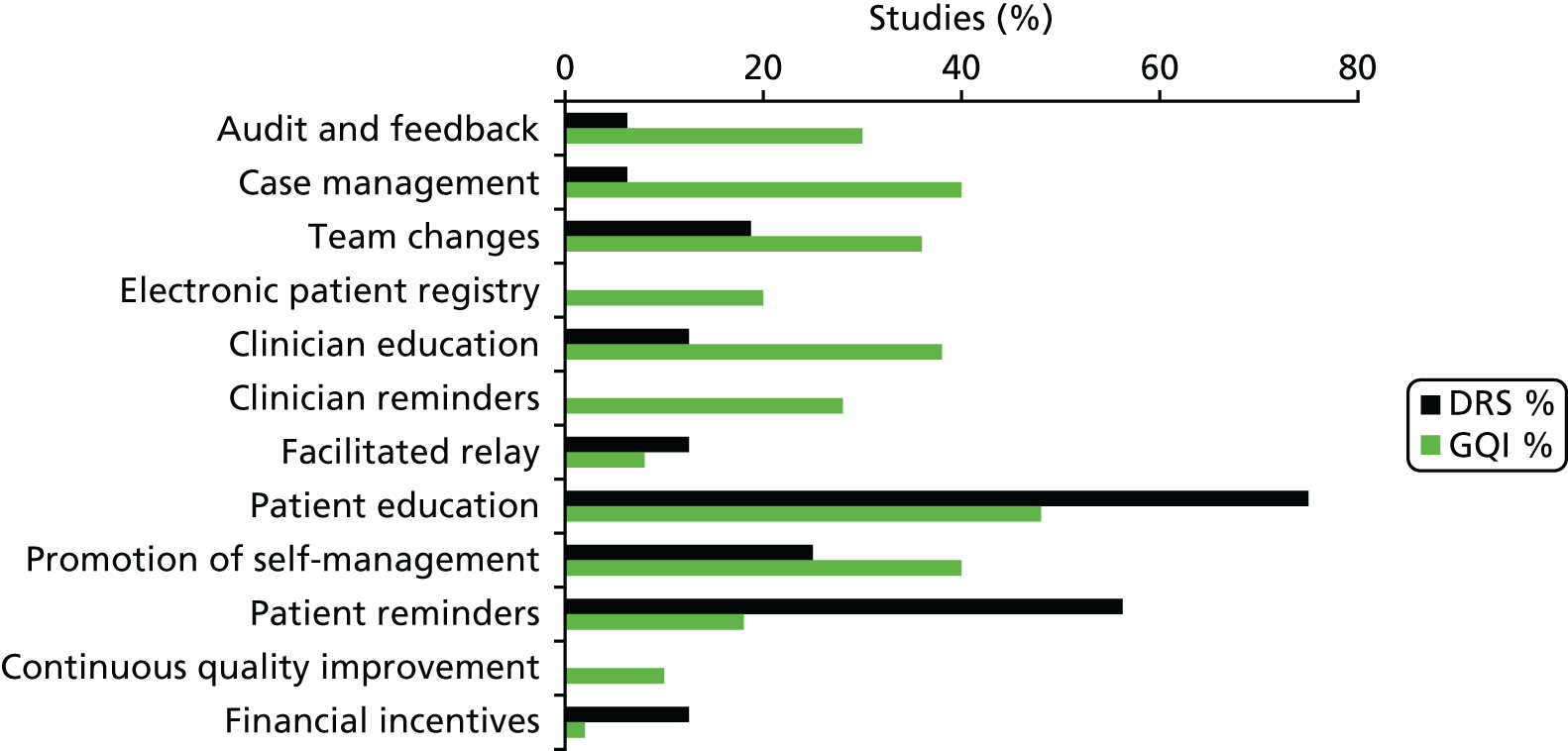
Interventions either were specifically targeted at improving attendance for DRS (n = 16) or were part of a general QI intervention to improve diabetes care (n = 50). For studies comparing any intervention with usual care, the majority of studies provided no description of usual care, which precluded coding of the comparator arm. All 12 QI intervention components, as defined by the modified EPOC taxonomy, were used in at least one study. Generally, interventions were multifaceted with several QI components per intervention arm (median 3, range 1–7). For interventions specifically targeting DRS attendance, the most commonly used QI components were ‘patient reminders’ (56% of studies) and ‘patient education’ (75%) (Figure 5). For general QI interventions, a greater number and range of strategies were used, including ‘patient education’ (48% of studies), ‘promotion of self-management’ (40%), ‘case management’ (40%), ‘clinician education’ (38%) and ‘team changes’ (36%).
FIGURE 5.
Quality improvement components used in the intervention arms of included studies. GQI, general quality improvement. Reproduced from Lawrensonet al.,127with permission from John Wiley & Sons, Ltd. Copyright © The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Intervention content in terms of behaviour change techniques (coded using the Behaviour Change Technique Taxonomy version 136)
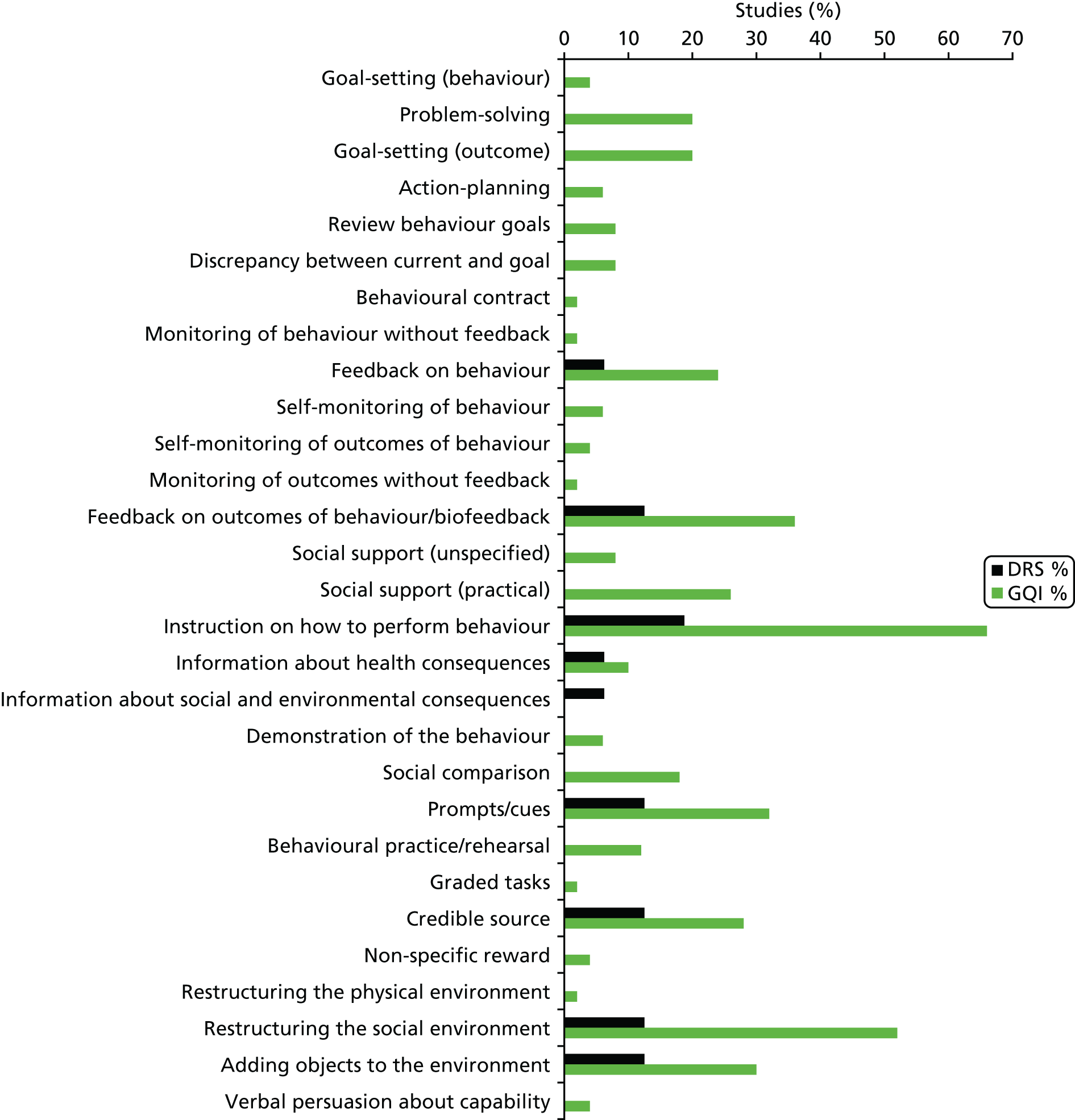
Overall, 39 out of the possible 93 BCTs (42%) were identified as targeting change in patient or HCP behaviour in at least one trial. Interventions specifically targeting DRS primarily used techniques aimed at patients, particularly ‘instruction on how to perform the behaviour’ (75% of studies), ‘prompts/cues’ (69%) and ‘information about consequences’ (56%) (Figure 6). Relatively few of these studies used BCTs that were aimed at HCPs (Figure 7). By contrast, the following HCP-directed strategies were more widely used in general QI interventions, in particular ‘instruction on how to perform the behaviour’ (66%), ‘restructuring the social environment’ (52%) and ‘feedback on outcomes of behaviour’ (36%). Table 2provides definitions and illustrative quotations for each BCT.
FIGURE 6.
Behaviour change techniques targeting patients used in the intervention arms of included studies. GQI, general quality improvement. Reproduced from Lawrensonet al.,127with permission from John Wiley & Sons, Ltd. Copyright © The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
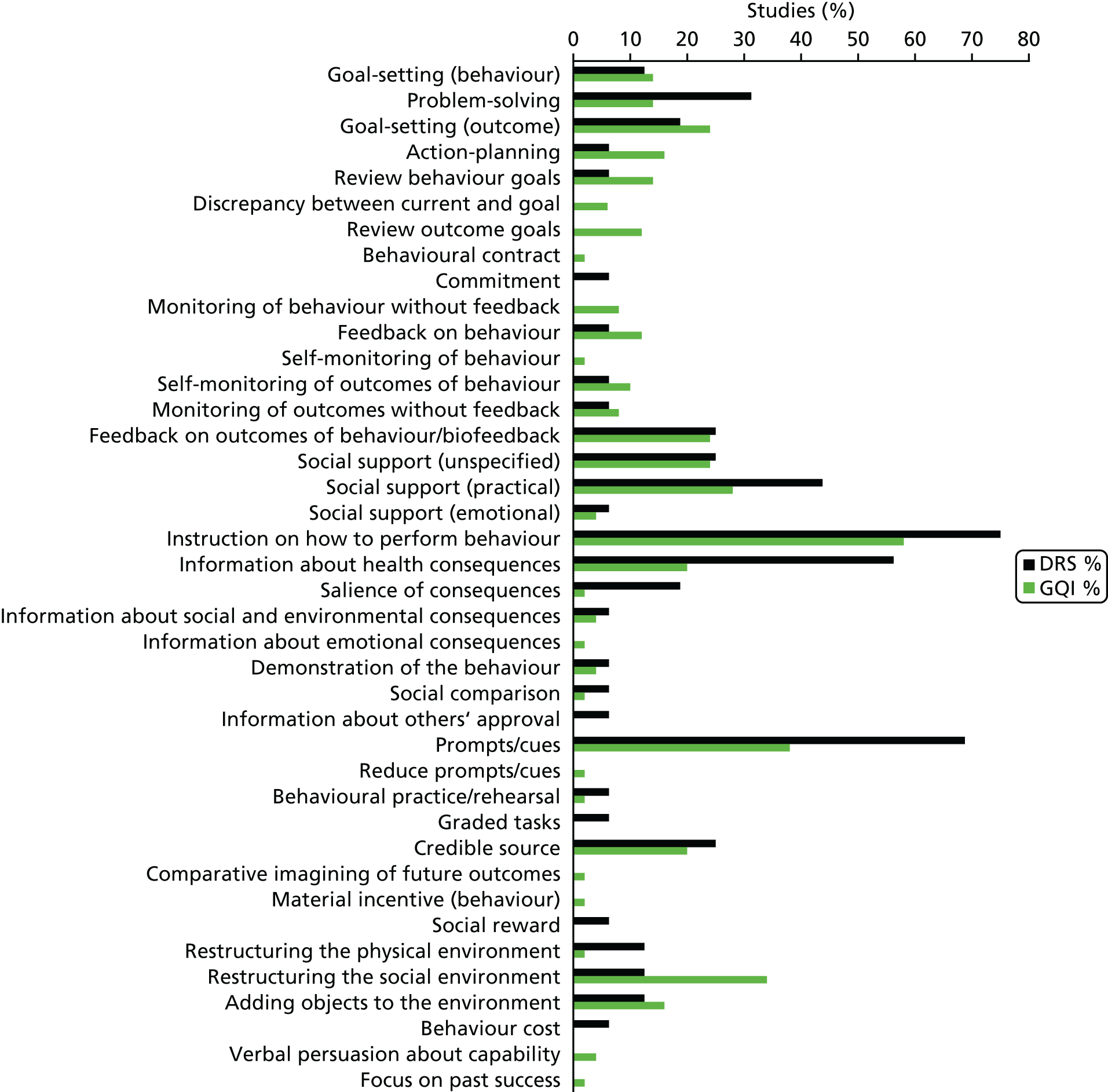
FIGURE 7.
Behaviour change techniques targeting HCPs used in the intervention arms of included studies. GQI, general quality improvement. Reproduced from Lawrensonet al.,127with permission from John Wiley & Sons, Ltd. Copyright © The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
| BCTs, abbreviated definitions and DRS-specific examples | Illustrative quotation from existing DRS interventions |
|---|---|
| Goals and planning | |
| Goal-setting (behaviour) | Practice nurses planned independent consultations with patients. The monitoring tool guided them through the consultations, and provided the opportunity to help the patient in selecting appropriate, concrete, behavioural goalsFreiet al.69(pp. 1040–1) |
| Set or agree a goal defined in terms of the behaviour to be achieved (e.g. set targets for how often patients should attend DRS, or general diabetes self-management, such as the frequency of blood glucose testing or the amount of carbohydrates to consume at each meal) | |
| Problem-solving | the health educator . . . offered one-on-one, interactive education and counselling. Having established rapport, she worked to identify and understand each subject’s reasons for and/or barriers to having a dilated retinal examination. Focused problem-solving then guided the subject toward making an informed choice about receiving an ophthalmic examinationBaschet al.109(p. 1879) |
| Analyse, or prompt the person to analyse, factors influencing the behaviour and generate or select strategies that include overcoming barriers and/or increasing facilitators (e.g. support patients to identify reasons for wanting or not wanting to attend DRS and help them select potential strategies for overcoming these barriers to screening attendance) | |
| Goal-setting (outcome) | During the case management sessions, patients and providers set management goals that were reasonable to achieveBarcelóet al.60(p. 147) |
| Set or agree a goal defined in terms of a positive outcome of wanted behaviour (e.g. agree with the patient target glycated haemoglobin level, blood pressure or cholesterol levels or target range for blood glucose levels) | |
| Action-planning | Behavioural activation for diabetic retinopathy prevention combined the principles of education about diabetes mellitus, behavioural therapy, and the health belief model to assist participants in identifying barriers to obtaining DFEs [dilated fundus examinations], problems-solving solutions to surmounting barriers, formulating action plans to facilitate DFEs, and gauging the success of action plansWeisset al.121(p. 1007) |
| Prompt detailed planning of performance of the behaviour (e.g. support the patient to develop a plan for how often they will attend DRS, where the DRS will occur and how they will get to their appointment) | |
| Review behaviour goals | Care managers were trained to use a patient-centred self-management approach that included review of the medical care needs and self-care goals that the patient identified and brainstorming additional strategies that patients could use to overcome barriers to their goalsGlasgowet al.73(p. 35) |
| Review behaviour goal(s) jointly with the person and consider modifying goal(s) or the behaviour change strategy in light of achievement [e.g. during scheduled diabetic review consultations, discuss with patients how they are progressing with their agreed self-management behavioural goals (e.g. frequency of blood glucose testing, attendance for DRS); when patients are not meeting agreed goals, either discuss how to adjust goals if needed to increase feasibility or engage in problem-solving to overcome any barriers to goal attainment] | |
| Discrepancy between current behaviour and goals | Physicians in the IG [intervention group] received a monthly report of their care quality with the top 10% quality of diabetes care score for all physicians being the achievable benchmarkHayashinoet al.77(p. 599) |
| Draw attention to discrepancies between a person’s current behaviour and the person’s previously set outcome goals, behaviour goals or action plans (e.g. provide feedback to HCPs on the proportion of patients who have received DRS in the previous 12 months and compare this against a gold standard for clinical practice based on clinical guidelines) | |
| Review outcome goal(s) | The telephone call was structured to first review the patient’s goals, followed by medication use, symptoms, glucose monitoring, blood pressure monitoring and self-management/care activitiesTayloret al.102(p. 1059) |
| Review outcome goal(s) jointly with the person and consider modifying goal(s) in light of achievement (e.g. review or alter target blood glucose levels towards a more feasible/achievable intermediate target) | |
| Behavioural contract | Care guides asked patients to sign a contract (which was scanned into the EHR [electronic health record]) agreeing to work toward their disease-specific goalsAdairet al.59(p. 176) |
| Create a written specification of the behaviour to be performed, agreed by the person and witnessed by another (e.g. ask the person with diabetes to sign a contract in their self-management plan or diary, undertaking to attend DRS once) | |
| Commitment | The initial goal was to elicit a verbal commitment to schedule an eye examinationBaschet al.109(p. 1879) |
| Ask the person to affirm or reaffirm the statement indicating commitment to change the behaviour (e.g. ask the person with diabetes to verbally affirm or reaffirm that they are committed to attending DRS at the agreed frequency and location) | |
| Feedback and monitoring | |
| Monitoring of behaviour by others without feedback | Foot examinations, blood pressure, and eye examinations were recorded on the reminder by clinic staff, collected after the patient visit and entered manuallyPetersonet al.58(p. 2239) |
| Observe or record behaviour with the person’s knowledge as part of a behaviour change strategy (e.g. record the proportion of patients who attend for a DRS examination as part of clinical audit, but do not feed back the results to the HCPs whose practice has been audited) | |
| Feedback on behaviour | In addition, diabetic members who did not have a record of a diabetic retinopathy exam received educational materials and a report of their current DRE [diabetic retinal examination] status directly from the HMO [health maintenance organisation] 2 weeks laterHalbertet al.114(p. 753) |
| Monitor and provide information or evaluative feedback on performance of the behaviour (e.g. form, frequency, duration, intensity) (e.g. provide a feedback report to HCPs, stating the proportion of their patients who have attended a DRS examination, had their blood pressure taken and had a foot examination) | |
| Self-monitoring of behaviour | We prepared feedback sheets for adherence to these eight indicators using data from the physicians’ self-report forms, as the physicians monitored and promoted these indicators to improve adherenceHayashinoet al.77(p. 601) |
| Establish a method for the person to monitor and record his or her behaviour(s) as part of a behaviour change strategy (e.g. a person with diabetes maintains a self-management diary in which they record their daily food intake and exercise and tick off on a checklist when they have attended the annual DRS examination) | |
| Self-monitoring of outcomes of behaviour | In general, case managers were directed to encourage patient self-management, including diet and exercise, provide reminders for recommended screening/tests, help with appointment scheduling; monitoring home glucose and blood pressure levelsKreinet al.85(p. 734) |
| Establish a method for the person to monitor and record the outcome(s) of their behaviour as part of a behaviour change strategy (e.g. a person with diabetes records in their self-management diary the results of their latest glycated haemoglobin test and DRS examination) | |
| Monitoring of outcomes of behaviour by others without feedback | The nurse case manager used behavioural goal setting, established individualized care plan, provide patient self-management education and surveillance of patients . . . ordered protocol-driven laboratory tests, tracked the outcomes using the computerized data registryGabbayet al.71(p. 30) |
| Observe or record outcomes of behaviour with the person’s knowledge as part of a behaviour change strategy (e.g. a person attends a DRS examination but is not provided with the results of the examination) | |
| Feedback on outcomes of behaviour | All persons who attended the screening clinics received a dilated eye exam by a volunteer community-based ophthalmologist. The eye exam included visual acuity . . . and a fundus examination through a dilated pupil . . . immediately after receiving the dilated eye exam, the patient was told the results by the examination ophthalmologistAndersonet al.108(p. 41) |
| Monitor and provide feedback on the outcome of performance of the behaviour [e.g. informing the person with diabetes of the results of the DRS examination (i.e. presence/absence of retinopathy)] | |
| Biofeedback | Immediately after receiving the dilated eye exam, the patient was told the results by the examination ophthalmologistAndersonet al.108(p. 41) |
| Provide feedback about the body (e.g. physiological or biochemical state)using an external monitoring device as part of a behaviour change strategy | |
| Social support | |
| Social support (unspecified) | Overall, the intervention included . . . and self-management support (provided by the practice nurse)Freiet al.69(p. 1041) |
| Advise on, arrange or provide social support (e.g. from friends, relatives, colleagues, ‘buddies’ or staff) or non-contingent praise or reward for performance of the behaviour – includes encouragement and counselling (e.g. provide general encouragement or reassurance to a person with diabetes to attend their DRS appointment) | |
| Social support (practical) | Referrals were facilitated to other clinicians when indicated, including ophthalmology, podiatry, nutrition and primary care for follow-up of acute or other chronic issues or when requested by patientsJacobset al.82(p. 616) |
| Advise on, arrange or provide practical help (e.g. from friends, relatives, colleagues, ‘buddies’ or staff) for performance of the behaviour (e.g. provide practical help for a patient with diabetes to attend DRS; this can include, for example, arranging a referral to DRS, arranging or providing transport to the clinic) | |
| Shaping knowledge | |
| Instruction on how to perform the behaviour | A direct mail reminder was sent to patients to reinforce the importance of annual eye examinations and included the following text:If you don’t have an eye doctor, ask your regular doctor to refer you to onePrelaet al.118(p. 258) |
| Advise or agree on how to perform the behaviour (includes ‘skills training’) (e.g. provide advice to a person with diabetes on how often guidelines recommend attending DRS, where they can obtain DRS and how to schedule an eye examination) | |
| Natural consequences | |
| Information about health consequences | A tailored telephone intervention was delivered by bilingual interventionists:Risk communications, such as the frequent lack of symptoms of retinopathy and that early treatment for retinopathy decreases the risk of blindness, were includedWalkeret al.120(p. 187) |
| Provide information (e.g. written, verbal, visual) about the health consequences of performing the behaviour (e.g. provide advice to the person with diabetes on the negative health consequences of retinopathy and the benefits of early detection.) | |
| Salience of consequences | The videotape used emotional appeals through storytelling to increase motivation to have a yearly dilated retinal examinationBaschet al.109(p. 1879) |
| Use methods specifically designed to emphasise the consequences of performing the behaviour with the aim of making them more memorable (e.g. give a person with diabetes a leaflet containing testimonials from other people with diabetes who suffer from retinopathy to emphasise the benefits of attending DRS and of early detection) | |
| Information about social and environmental consequences | A take-home reminder (aimed at patients, to remind them to make an appointment for an eye examination), to be given to patients by their family practitioner, included the following text:OHIP [Ontario Health Insurance Plan] covers annual eye checks for patients with diabetes so you will not have to payZwarensteinet al.123(p. 90) |
| Provide information (e.g. written, verbal, visual) about the social and environmental consequences of performing the behaviour (e.g. provide information on the costs of having a DRS examination) | |
| Information about emotional consequences | Group visit content, though patient-guided, was physician-directed to cover educational topics . . . and the emotional aspects of diabetesClancyet al.62(p. 621) |
| Provide information (e.g. written, verbal, visual) about the emotional consequences of performing the behaviour (e.g. provide a leaflet recognising the potential negative effects on emotional and mental health of managing a chronic illness such as diabetes) | |
| Comparison of behaviour | |
| Demonstration of the behaviour | The newsletter consisted of six sections, including a testimonial designed to model eye examination behaviourEllishet al.113(p. 1593) |
| Provide an observable sample of the performance of the behaviour, directly in person or indirectly (e.g. through film, pictures), for the person to aspire to or imitate (e.g. play a video demonstrating the DRS procedure) | |
| Social comparison | The system presented register data on their Type 2 diabetes population, giving them the option either to use the data during individual diabetes consultations or to gain an overview of the quality of their diabetes care and compare it with the corresponding quality in their colleagues’ practicesGuldberget al.74(p. 326) |
| Draw attention to others’ performance to allow comparison with the person’s own performance (e.g. provide HCPs with feedback on the proportion of their patients who have had a DRS examination and benchmark this in comparison to other hospitals or HCPs) | |
| Information about others’ approval | One of the messages in the targeted newsletter read:Even though you’ve been thinking about getting a dilated eye exam, we hope you’ll make the call nowEllishet al.113(additional information provided by the author) |
| Provide information about what other people think about their behaviour. The information clarifies whether others will like, approve of or disapprove of what the person is doing or will do (e.g. tell the person with diabetes that their family members would likely be keen for them to attend their DRS appointment) | |
| Associations | |
| Prompts/cues | For those who made an appointment, a reminder letter was mailed 3 weeks prior to the scheduled appointment. Additionally, there was an automated reminder call the day before the scheduled appointmentPizziet al.117(p. 255) |
| Introduce or define environmental or social stimulus with the purpose of prompting or cueing the behaviour (e.g. telephone the person with diabetes to remind them of their upcoming DRS appointment) | |
| Reduce prompts/cues | Recommendations for regular telephone follow-ups for diabetes patients, which will be monthly in the 1st half year and then will probably decreaseJansinket al.83(coded from protocol128) |
| Withdraw gradually prompts to perform the behaviour [e.g. decrease the frequency with which a person with diabetes is sent a reminder about their DRS attendance (i.e. from weekly to fortnightly, to monthly, to quarterly reminders)] | |
| Repetition and substitution | |
| Behavioural practice/rehearsal | During a 2-day training session, case managers received instruction on collaborative goal setting, with case examples and role-playing used to familiarize them with the treatment algorithmsKreinet al.85(p. 734) |
| Prompt practice or rehearsal of the performance of the behaviour one or more times in a context or at a time when the performance may not be necessary, to increase habit and skill (e.g. provide an opportunity for trainee HCPs to practise delivering a DRS examination to an actor role-playing a patient with diabetes) | |
| Graded tasks | Theoretically, this form of facilitation should be necessary for only a relatively short period of time, with the practice improvement team progressively assuming responsibility for the ongoing improvement efforts after the initial facilitationDickinsonet al.64(p. 10) |
| Set easy-to-perform tasks, making them increasingly difficult, but achievable, until the behaviour is performed (e.g. initially allocate a HCP responsibility for one component of the DRS examination and progressively increase their level of responsibility) | |
| Comparison of outcomes | |
| Credible source | Participants in the print-intervention group received a mailing of a colourful, 14-page booklet on preventing diabetes eye problems called Keep Your Eyes Healthy, in English or Spanish, developed by the National Institutes of HealthWalkeret al.120(p. 187) |
| Present verbal or visual communication from a credible source in favour of or against the behaviour (e.g. include the logos for national health institutes or cite published clinical guidelines to endorse information provided in leaflets regarding DRS) | |
| Reward and threat | |
| Material incentive (behaviour) | The automated system offered a live telephone call back to assist in scheduling test and also offered to send participants the following items: 1) a voucher that would allow the provider to waive the co-payment for a dilated eye examination . . .Simonet al.98(p. 1452) |
| Inform that money, vouchers or other valued objects will be delivered if and only if there has been effort and/or progress in performing the behaviour (e.g. advise the person with diabetes that they will receive a shopping voucher if they attend their upcoming DRS appointment) | |
| Social reward | When a subject reported having a dilated retinal examination a congratulatory letter was sentBaschet al.109(p. 1879) |
| Arrange a verbal or non-verbal reward if and only if there has been effort and/or progress in performing the behaviour (e.g. verbally praise the person with diabetes if they attend their DRS appointment) | |
| Non-specific reward | CME [continuing medical education] credits were given to the participating physicians in the workshopsVidal-Pardoet al.104(p. 752) |
| Inform that a reward will be delivered if and only if there has been effort and/or progress in performing the behaviour (e.g. inform the HCP that they will be rewarded for conducting a DRS examination with a target proportion of their patients) | |
| Antecedents | |
| Restructuring the physical environment | Care guide workstations were located in the clinic waiting rooms, to facilitate face-to-face interactions with patients, providers, and nursesAdairet al.59(p. 177) |
| Change, or advise changing, the physical environment to facilitate performance of the wanted behaviour or create barriers to performance of the unwanted behaviour (e.g. introduce mobile DRS vans in geographically remote areas to increase access to screening facilities) | |
| Restructuring the social environment | Three multi-lingual Link Workers already employed by Coventry Primary Care Trust (PCT) were trained in diabetes management and care and assigned to work with specific intervention GP [general practice] surgeriesBushet al.110(p. 295) |
| Change, or advise changing, the social environment to facilitate performance of the wanted behaviour or create barriers to performance of the unwanted behaviour (e.g. change a health-care team and team working, such as introducing a new specialist diabetes nurse role responsible for monitoring screening rates and telephoning people with diabetes to remind them to attend their DRS appointment) | |
| Adding objects to the environment | In addition 4500 diabetes passports were made available at the four hospitalsDijkstraet al.65(p. 128) |
| Add objects to the environment to facilitate performance of the behaviour (e.g. introduce new computerised software to a general practice to help monitor and remind HCPs about which patients need to be prompted to attend DRS) | |
| Scheduled consequences | |
| Behaviour cost | We were interested to find out whether a small copayment would be an important deterrent to the uptake of screening for diabetic retinopathy (DR) . . . We conducted a randomized trial in which one group was charged a small fee for DR screening and the other was provided with free accessLianet al.115(p. 1247) |
| Arrange for withdrawal of something valued if and only if an unwanted behaviour is performed (e.g. charging people with diabetes a fee for failing to attend a DRS examination) | |
| Self-belief | |
| Verbal persuasion about capability | Diabetes is a serious, lifelong condition, but there is so much that you can do to protect your health. Take charge of your health, not only for today, but also for the years to comeLafataet al.86(p. 523) |
| Tell the person that they can successfully perform the wanted behaviour, arguing against self-doubts and asserting that they can and will succeed (e.g. encourage or reassure the patient to attend a DRS examination, providing information as needed to address any concerns or self-doubts that they may have about attending for DRS) | |
| Focus on past success | A comprehensive programme that integrated lifestyle counselling based on motivational interviewing principles was integrated into structured diabetes careJansinket al.83(p. 119)In additional file 2, which is linked to the protocol for this study,128the authors describe the staff training programme. Within the text they explain that one guiding principle of motivational interviewing is to ‘support self-efficacy’. They state that ‘self-efficacy can be strengthened by affirming past success (i.e. reinforcement)’ |
| Advise thinking about or listing previous successes in performing the behaviour (or parts of it) (e.g. help the person with diabetes to remember the last time that they attended DRS and use this as an opportunity to reassure them of the benefits of attending) | |
For studies comparing any intervention with usual care, the majority of studies provided no description of usual care, which precluded coding of the comparator arm.
Outcome measures
In 12 (75%)96,108,109,111,113,114,117,119–123of the 16 studies in which the primary target of the intervention was to improve attendance for DRS, the outcome was a dilated fundus examination (DFE) conducted by an ophthalmologist or optometrist during the follow-up period post intervention (median follow-up 12 months). The DFE was confirmed by medical record audit, from a health claims database or by eye-care professional examination. In four studies (25%)110,112,115,116DRS consisted of screening of digital retinal images.
In the 50 studies in which DRS attendance was reported as part of a general QI intervention, DRS was usually listed as part of a number of processes of care based on diabetes guideline recommendations. DRS was variously described as a DFE/diabetic eye examination/retinal examination/eye examination in 49 studies (98%) and involved grading of retinal images in one study. 107DRS was confirmed by medical record audit, from health claims databases or from patient self-reports (both validated and unvalidated by an eye-care professional). The median duration of follow-up was 12 months (range 1–48 months).
In terms of economic outcomes, five studies reported a full economic evaluation. 67,117,124–126Three of these were cost-effectiveness analyses124–126and two were cost–consequence analyses. 67,117Nine studies reported partial economic evaluations: five were resource utilisation studies62,69,85,89,95and four were cost outcome descriptions. 59,70,87,105The full text of one of the cost-effectiveness studies could not be retrieved but the abstract124provided some information required for review alongside the clinical effectiveness report. 63
Excluded studies
SeeReport Supplementary Material 1,Characteristics of included, ongoing and excluded studies (phase 1 review).
Ongoing studies
SeeReport Supplementary Material 1,Characteristics of included, ongoing and excluded studies (phase 1 review).
Risk of bias in included studies
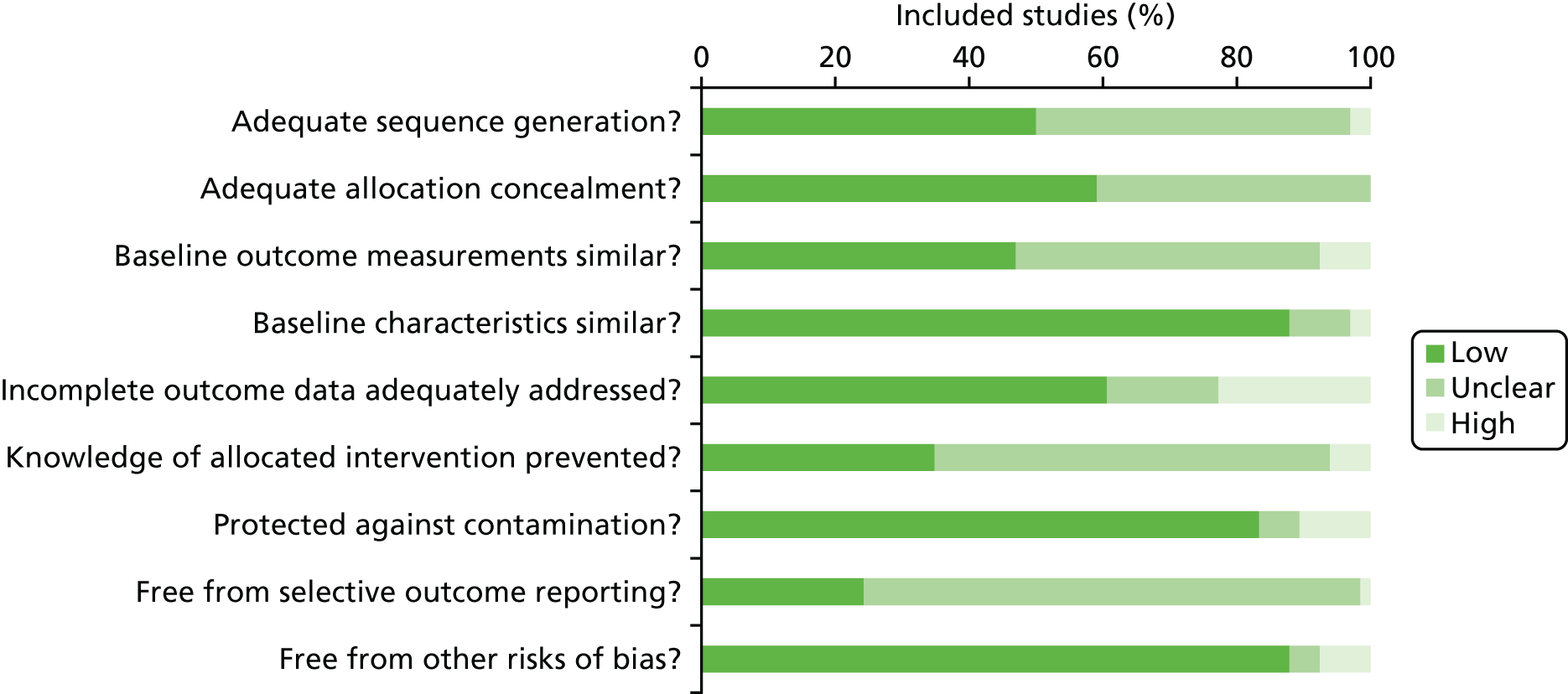
A risk-of-bias assessment was conducted using the Cochrane EPOC Group risk-of-bias tool (Figure 8). 51Overall, trials were judged to be at low or unclear risk of bias. However, 33 studies (48.5%)58–60,62,65,68,69,71,72,74,76,78,81–84,88,91–97,99,100,103–107,119,121were judged to be at high risk of bias in at least one domain. The domains most commonly at high risk of bias were ‘incomplete outcome data addressed’ [15 studies65,68,72,76,78,81–84,88,93,94,100,103,105(22.7%)], ‘protected against contamination’ [seven studies60,62,96,99,104,107,119(10.6%)] and ‘similar baseline outcome measurements’ [five studies58,69,92,95,106(7.6%)]. It was possible to judge if a study was free from selective outcome reporting for only 17 studies (25.7%)58,59,63,64,67,69,72,77,78,83,84,94,96,99,100,121,123because of a lack of availability of a prospectively published trial registration or protocol. Although studies were rarely judged to be at a high risk of bias for adequate sequence generation and adequate allocation concealment, the methods associated with these domains were frequently poorly reported.
FIGURE 8.
Risk-of-bias graph.
We explored publication bias using a funnel plot for the main comparison of any intervention with usual care (56 studies58–63,65–72,74–78,80–89,92–105,108–112,115–118,120–123). Although there were few data points for the less precise studies, those with greater precision were evenly distributed.
The studies that reported economic outcomes are a subset of the studies included in the systematic review and the risk of bias of these studies was very similar to the risk of bias of the main body of included studies. With respect to methodological quality, only five67,117,124–126of the 14 included economic studies reported full economic evaluations. One of these studies124was published as an abstract and lacked important methodological details. Only three96,117,125of the studies with full economic evaluations reported a sensitivity analysis to explore changes in the costs and outcomes under different scenarios. Discounting in economic evaluations is necessary to adjust future costs and outcomes of an intervention to their present values but was reported in only one96of the full economic outcomes. Its use would have been appropriate in those other studies with a stated follow-up of > 12 months. 67,70,85,89,105The methodological quality of the full economic evaluations was considered to be moderate. Full details of the methodological quality assessment for each of the included economic evaluations are available inReport Supplementary Material 1[seeFurther details of the review of economic evidence (phase 1 review)].
Effects of the interventions
Primary outcome
One or more visits for diabetic retinopathy screening within a 2-year period following implementation of the intervention
All 66 trials provided data for this outcome. There were two types of comparison: 56 (85%)58–63,65–72,74–78,80–89,92–105,108–112,115–118,120–123of the 66 studies compared an intervention against current usual care whereas 10 (15%) studies64,73,79,90,91,106,107,113,114,119compared a more intensive QI intervention or group of QI interventions against a less intensive intervention. As these addressed different questions, meta-analyses were conducted separately on the 56 and 10 studies.
Thirty-one (47%)58,60,64–70,73,74,76–79,81,83,84,90–94,97,100,101,104–106,110,123of the 66 trials were cluster RCTs. Only nine65,67,69,70,73,83,93,101,123of these reported an ICC and the ICC reported typically did not relate specifically to DRS outcomes. Of the nine cluster RCTs reporting an ICC, the most commonly reported value was 0.05 and so this was the value imputed for studies with no estimates of ICCs. The smallest value reported was 0.01 and the largest value was 0.2. A sensitivity analysis was conducted to investigate the impact on the computed effect estimates of using the lower and upper range values.
Of the 56 studies that compared any intervention against usual care, 13 (23%)108–112,115–118,120–123evaluated interventions specifically targeting DRS. The remaining 43 studies (77%)58–63,65–72,74–78,80–89,92–105evaluated interventions directed towards improving the general quality of diabetes care (including DRS attendance). Although there was substantial heterogeneity in the intervention effects (I2 > 90%), 48 out of the 56 studies showed an improvement in DRS attendance (Figure 9). As it may be argued that it is better to examine clinical differences in a meta-analysis rather than use them as a reason for not conducting one, we computed pooled estimates for each of these subgroups. A random-effects model was adopted, which can accommodate statistical heterogeneity between studies by assuming that different studies have different true effect sizes (Figure 9), but we acknowledge that use of the random-effects model does not in itself deal with heterogeneity. We assessed whether or not there was evidence of a subgroup effect and, as there was not (p = 0.15), subsequent analyses were conducted on the 56 studies. The pooled RD for comparisons between the intervention and usual care was 0.12 (95% CI 0.10 to 0.14). This corresponds to a 12% absolute increase in DRS attendance.
FIGURE 9.
Meta-analysis of any QI interventions compared with usual care. df, degrees of freedom; M-H, Mantel–Haenszel.
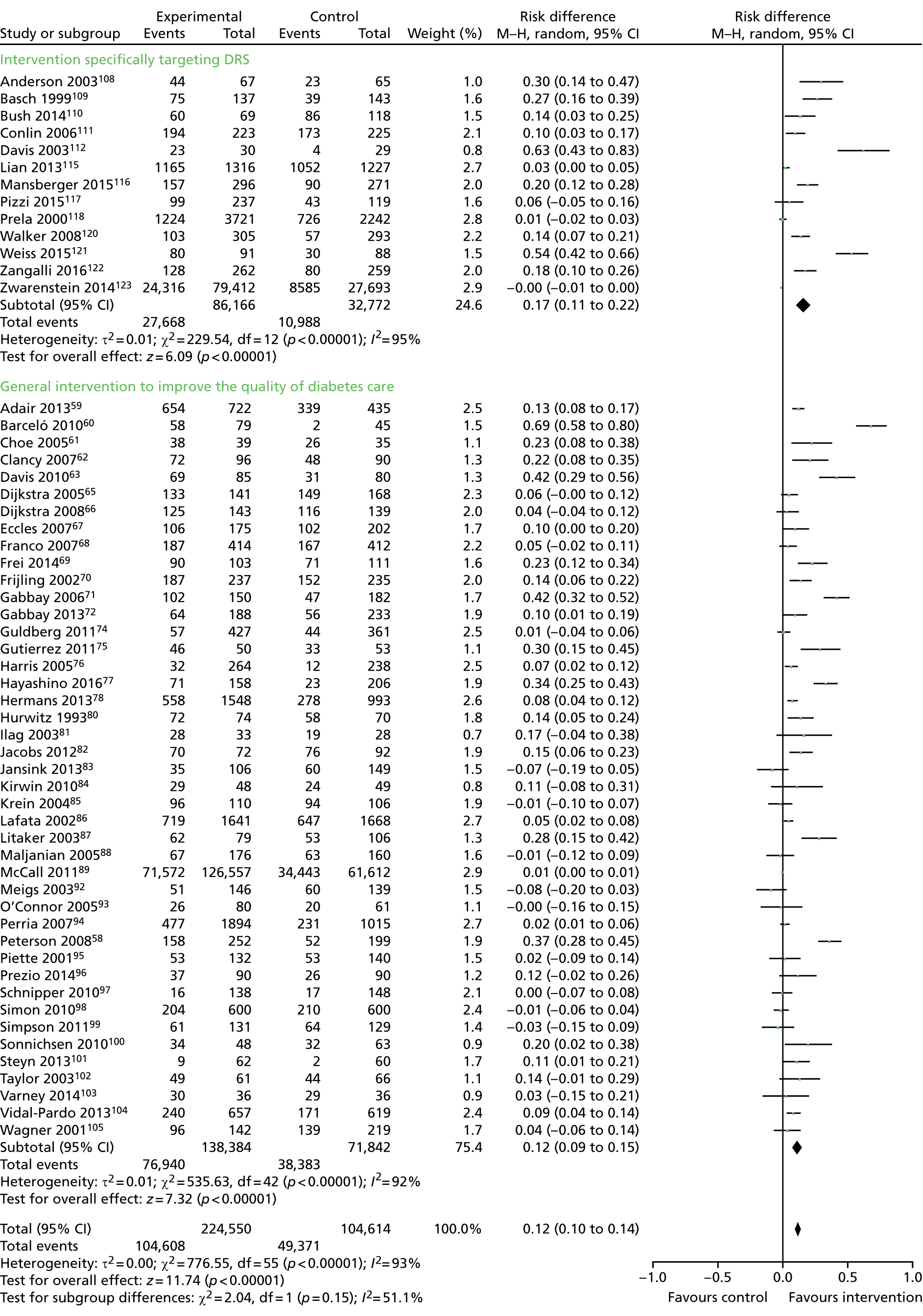
Examples of studies in this comparison included a tailored (individualised) newsletter compared with a generic patient education newsletter113and a comparison between audit and feedback to HCPs and audit and feedback combined with a diabetes outreach service. 91Ten studies64,73,79,90,91,106,107,113,114,119contributed to this analysis (Figure 10). Three (30%)113,114,119evaluated interventions specifically targeting DRS, whereas seven (70%)64,73,79,90,91,106,107evaluated interventions directed towards improving the general quality of diabetes care. The pooled RD for comparisons between a more intensive (stepped) intervention and a less intensive intervention was 0.05 (95% CI 0.02 to 0.09). This corresponds to a 5% absolute increase in DRS attendance for participants receiving the stepped intervention.
FIGURE 10.
Meta-analysis of stepped QI interventions compared with less intensive QI interventions. df, degrees of freedom; M-H, Mantel–Haenszel.
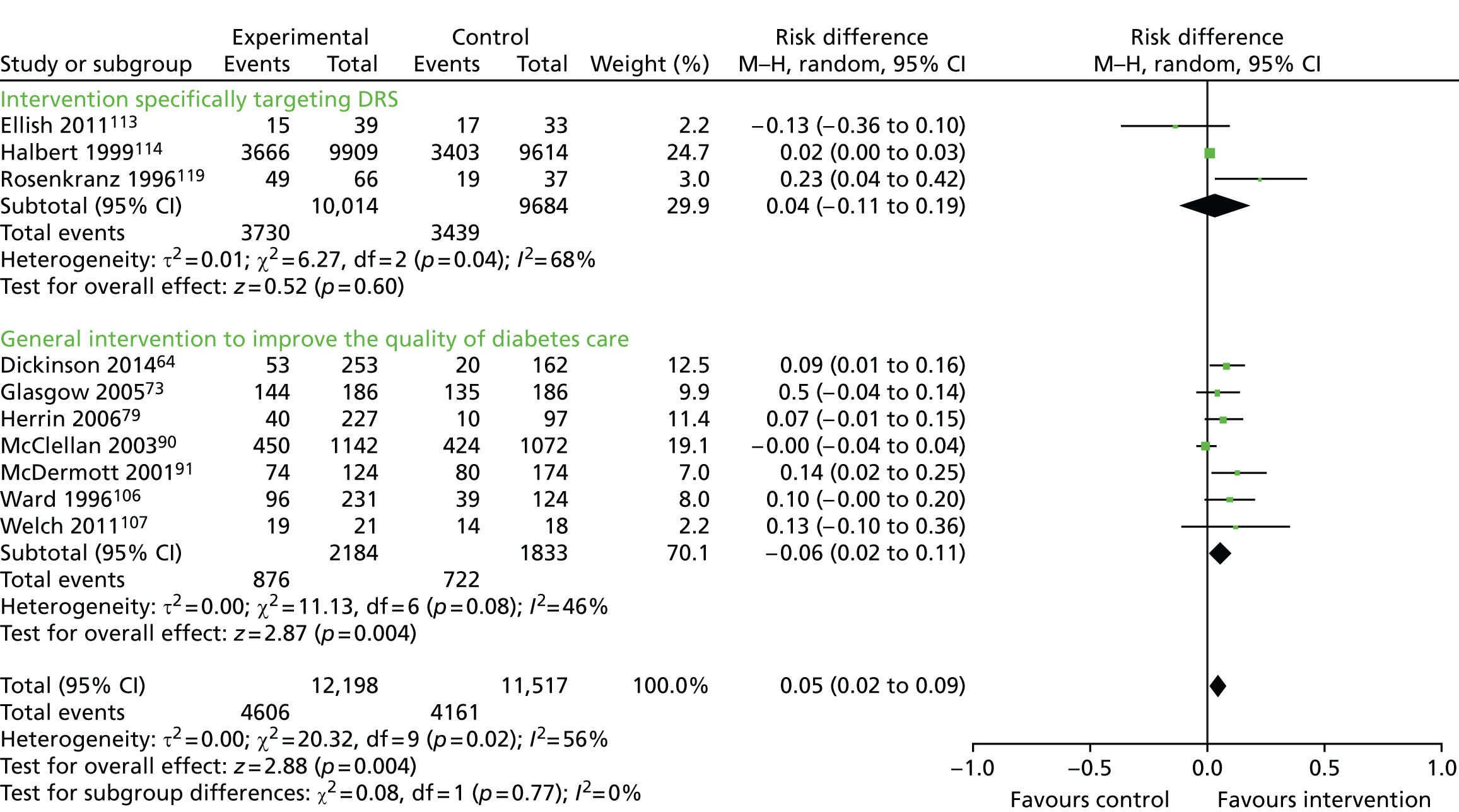
Secondary outcomes
Ongoing adherence to diabetic retinopathy screening based on attendance for screening following the initial screening post intervention
It was not possible to extract data on ongoing adherence to DRS (based on attendance for screening following the initial screening post intervention) because either it was not possible to identify unique screening episodes from pooled data reported at two time points or, in one study,116the intervention was offered to the comparator arm 18 months post randomisation.
Economic outcomes
Resources (staff time, equipment, consumables) required to deliver interventions to increase attendance for diabetic retinopathy screening
Resource utilisation was scored on a 1–5 ordered ranking scale, with 1 representing the least amount of resources used and 5 representing the most amount of resources used. Chapter 6provides a description of the ordered ranking scale and the justification for using an ordered ranking scale (seeThe ordered ranking scoreinReport Supplementary Material 4,Economic model). The percentage of studies in each resource grouping for the 56 studies comparing any intervention with usual care was as follows: 1 = 48.2%, 2 = 10.7%, 3 = 8.9%, 4 = 19.6% and 5 = 12.6%.
Costs of staff used to provide interventions, treatment and care, primary care, lost wages and lost productivity (work output)
All costs reported were converted to 2016 UK pounds and are summarised for each study inAppendix 6. Only two studies67,126reported both the direct and the indirect costs (productivity loss) of the interventions. In all other studies, the costs reported covered just the direct costs of providing the intervention. Five studies59,62,70,117,126reported the total direct costs of the intervention but the resources that they considered relevant and how they combined them to estimate total costs varied between studies. The components of the total cost for each intervention are reported inAppendix 6.
The types of resources included in the cost calculations for each study varied; hence, it is difficult to compare directly across the studies. The estimated training cost differed between the few studies that reported this information. In terms of the costs of treatment and care of diabetes, there was no obvious difference in between the intervention and the comparator in the studies that reported these data, primarily reflecting an absence of evidence.
Incremental cost-effectiveness ratio
Only three studies conducted in the USA124–126reported this outcome. Davis and Mayer-Davis124reported an incremental cost per QALY of £13,154 over 1 year for a diabetes telecare intervention compared with no intervention. However, it is unclear what tool was used to estimate QALYs. Prezioet al. 126used an established whole-disease model, the Archimedes model simulator, to estimate the incremental cost per QALY. Using a discount rate of 3% and programme effectiveness of 100%, the incremental cost per QALY was £73,683 over 5 years and £261 over 20 years for the intervention (a culturally tailored diabetes education programme delivered by a community health worker) compared with usual care. Schechteret al. 125also reported an ICER. In this study, the unit of effectiveness was the number of DFEs gained, which was associated with the number of diabetic retinopathies diagnosed. The incremental cost per DFE gained for a telephone intervention compared with a mailed/printed intervention was £333. Pizziet al. 117reported an ICER for a telephone intervention of £18.77 per additional patient attending a DFE compared with usual care. The ratio was not calculated for a mailed intervention because it was dominated by usual care.
Further details of these outcomes are reported inReport Supplementary Material 1[seeFurther details of the review of economic evidence (phase 1 review)].
Exploration of heterogeneity
Substantial heterogeneity was observed (I2 > 90%), which was investigated by subgroup analysis and meta-regression.
Subgroup analysis
Comparisons of studies grouped according to the QI component/BCTs present in the intervention arm were conducted as well as comparison of studies at high compared with low risk of bias and according to the resource intensity scale.
Although we attempted to code for the QI component/BCT in the control arm in all studies, we did not adjust for this in the analysis as, for the majority of these studies, usual care was not specified and therefore could not be coded.
A sufficient number of studies were available to investigate the effectiveness of nine out of the possible 12 QI components (seeAppendix 4for definitions of the QI components). Insufficient data were available to analyse ‘continuous quality improvement’, ‘financial incentives’ and ‘facilitated relay of information to clinicians’. Interventions incorporating all nine QI components evaluated in the subgroup analysis were associated with improvements in DRS attendance, with higher pooled effect estimates for interventions directed at patients (promotion of self-management and patient education) or the organisation of the health system (team changes or the establishment of an electronic patient registry) (Figure 11).
FIGURE 11.
Subgroup analysis of QI components.
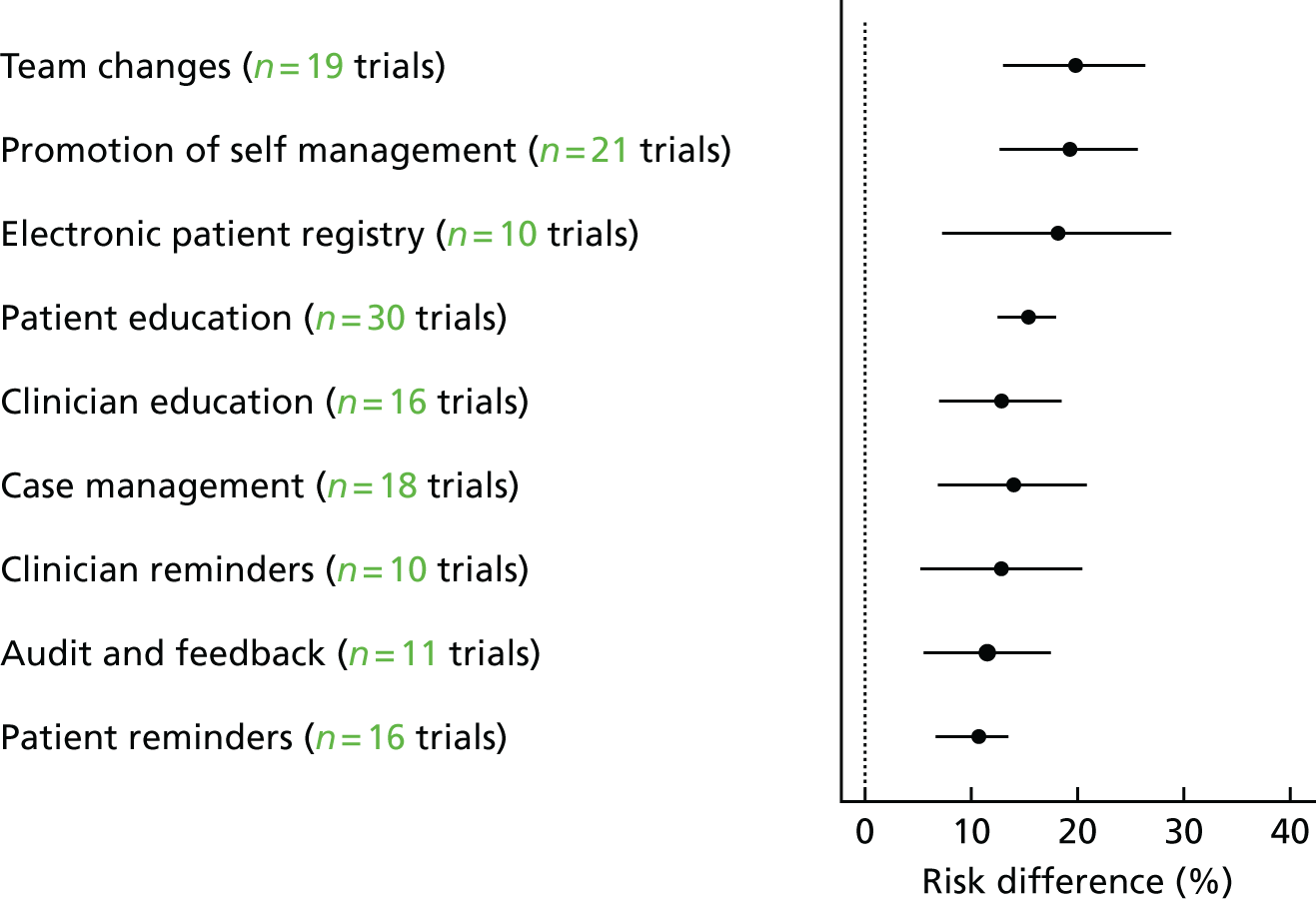
Sufficient studies were available to investigate the effectiveness of interventions containing particular BCTs (including 10 BCTs aimed at patients and seven aimed at HCPs) (Figures 12and13). Interventions incorporating all 17 BCTs included in the subgroup analysis were all shown to be effective at improving DRS attendance. For BCTs aimed at patients, higher pooled effect estimates were found for ‘goal-setting (outcome)’ and ‘credible source’; for BCTs aimed at HCPs, higher pooled effect estimates were found for ‘restructuring the social environment’ and ‘credible source’ (seeTable 2andAppendix 5for BCT descriptions).
FIGURE 12.
Subgroup analysis of BCTs aimed at patients.
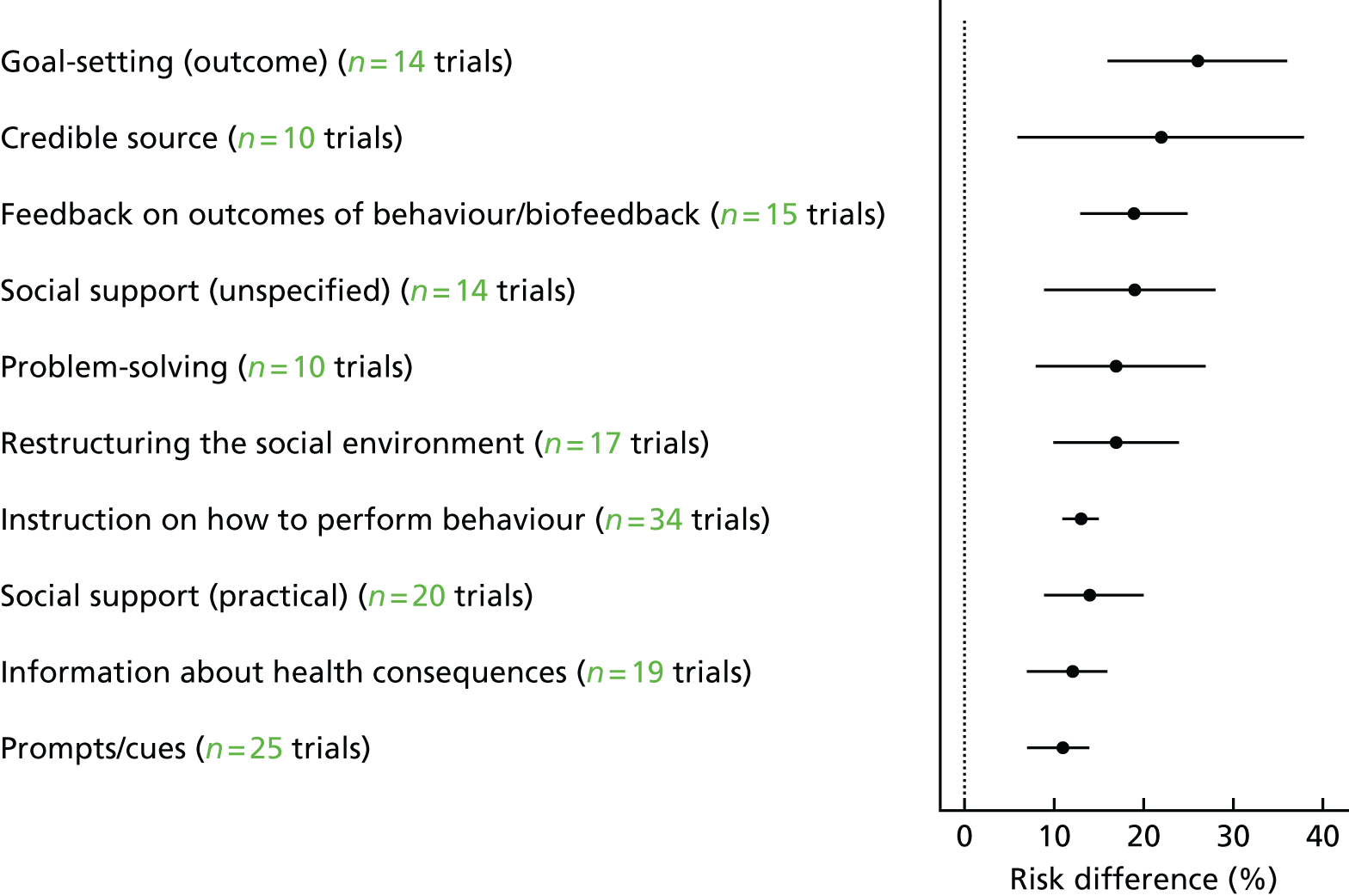
FIGURE 13.
Subgroup analysis of BCTs aimed at HCPs.
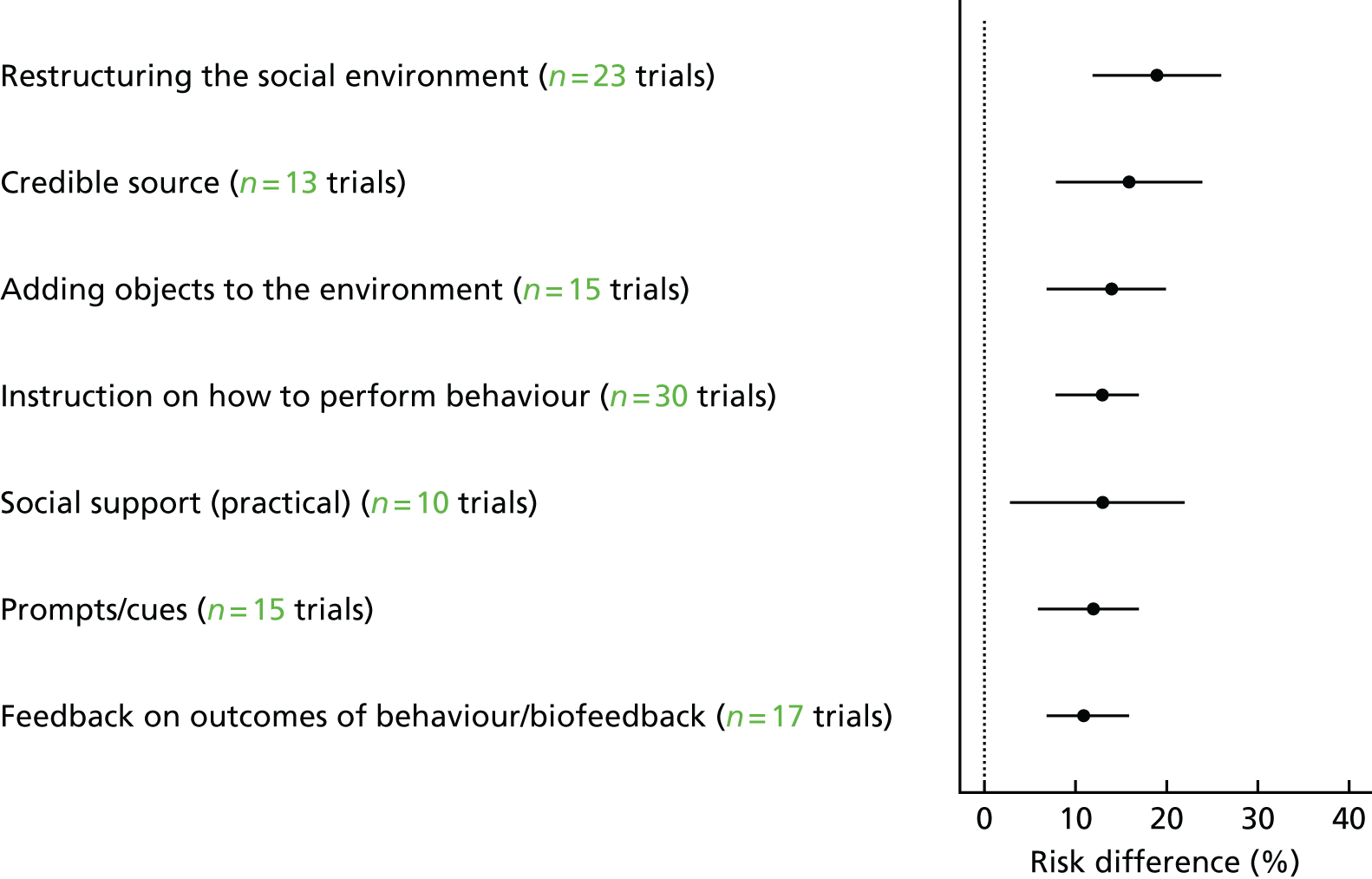
Analysis of subgroups according to resource intensity did not show a relationship between effect size and increasing resource intensity (Figure 14).
FIGURE 14.
Subgroup analysis based on resource utilisation.
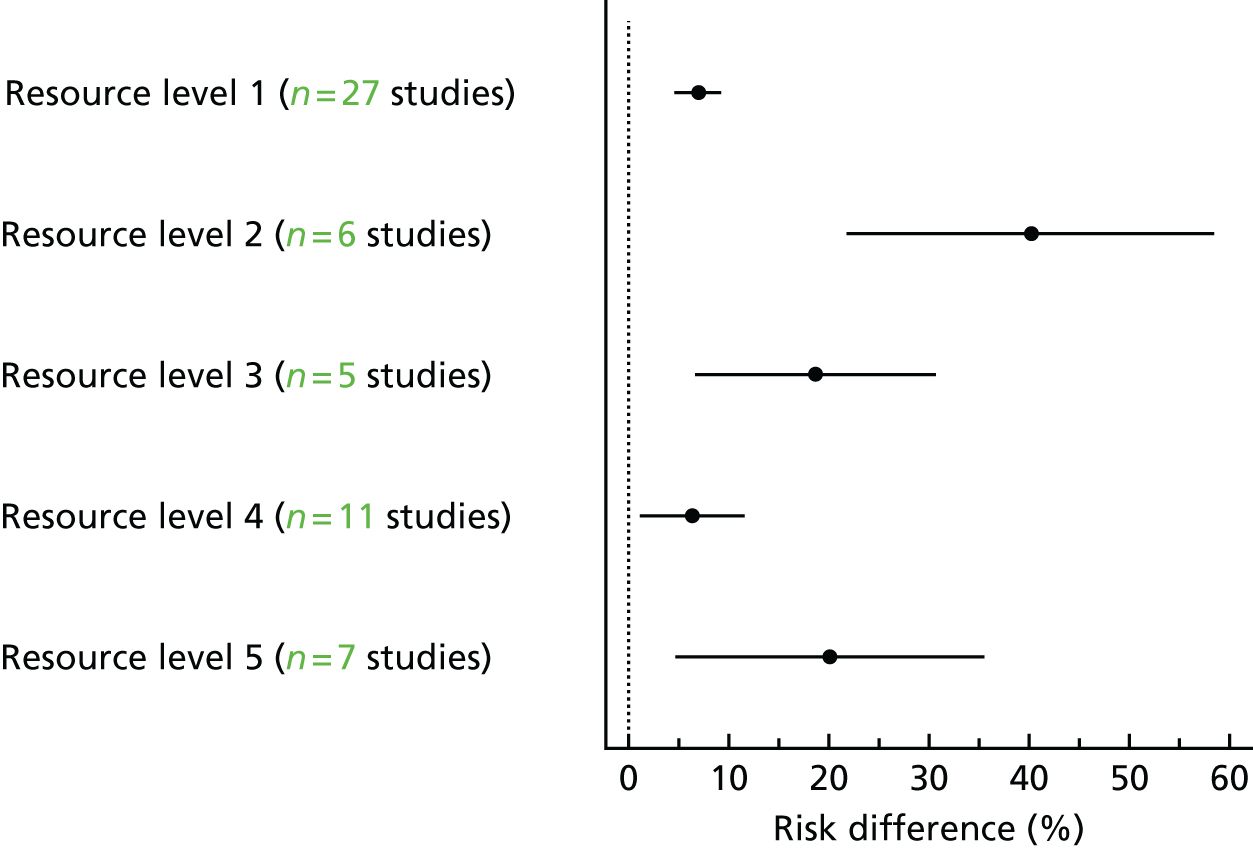
Meta-regression
The results of the meta-regression analysis are provided inTables 3–5. There was some evidence of an association between effect size and baseline risk, with larger effects in studies with poorer baseline DRS attendance (Figure 15). Because of regression to the mean, this association might be spurious, so a permutation test was conducted to allow for this (with 1000 permutations;p = 0.055).
| Explanatory variable | Regression coefficient (95% CI) | p-value | ResidualI2(%) |
|---|---|---|---|
| Baseline DRS attendance | –0.208 (–0.419 to 0.004) | 0.054 | 94 |
| High vs. low risk of bias | 0.008 (–0.136 to 0.094) | 0.079 | 92 |
| Cluster vs. individual RCT | –0.049 (–0.136 to 0.039) | 0.268 | 93 |
| QI component | |||
| Audit and feedback | –0.029 (–0.136 to 0.079) | 0.597 | 92 |
| Case management | 0.001 (–0.092 to 0.095) | 0.979 | 93 |
| Team changes | 0.089 (–0.001 to 0.178) | 0.052 | 91 |
| Electronic patient registry | 0.051 (–0.061 to 0.164) | 0.363 | 92 |
| Clinician education | –0.011 (–0.107 to 0.085) | 0.822 | 93 |
| Clinician reminders | –0.012 (–0.127 to 0.103) | 0.835 | 92 |
| Patient education | 0.075 (–0.009 to 0.160) | 0.081 | 92 |
| Promotion of self-management | 0.085 (–0.002 to 0.172) | 0.055 | 93 |
| Patient reminders | –0.033 (–0.128 to 0.063) | 0.493 | 93 |
| Resource requirement | 0.013 (–0.015 to 0.042) | 0.356 | 93 |
| Explanatory variable (HCP-BCTs) | Regression coefficient (95% CI) | p-value | ResidualI2(%) |
|---|---|---|---|
| Feedback on outcomes of behaviour/biofeedback | –0.035 (–0.129 to 0.060) | 0.465 | 92 |
| Social support (practical) | –0.013 (–0.128 to 0.102) | 0.821 | 93 |
| Instruction on how to perform the behaviour | –0.022 (–0.110 to 0.066) | 0.622 | 93 |
| Prompts/cues | –0.029 (–0.126 to 0.069) | 0.559 | 92 |
| Credible source | 0.028 (–0.075 to 0.132) | 0.586 | 93 |
| Restructuring the social environment | 0.085 (–0.001 to 0.172) | 0.053 | 91 |
| Adding objects to the environment | –0.003 (–0.102 to 0.095) | 0.946 | 92 |
| Explanatory variable (patient BCTs) | Regression coefficient | p-value | ResidualI2(%) |
|---|---|---|---|
| Problem-solving | 0.042 (–0.073 to 0.158) | 0.466 | 92 |
| Goal-setting (outcome) | 0.162 (0.070 to 0.254) | 0.001 | 90 |
| Feedback on outcomes of behaviour/biofeedback | 0.081 (–0.016 to 0.179) | 0.102 | 92 |
| Social support (unspecified) | 0.063 (–0.037 to 0.162) | 0.211 | 91 |
| Social support (practical) | 0.012 (–0.079 to 0.103) | 0.789 | 92 |
| Instruction on how to perform the behaviour | 0.031 (–0.059 to 0.120) | 0.497 | 92 |
| Information about health consequences | –0.023 (–0.114 to 0.069) | 0.624 | 93 |
| Prompts/cues | –0.050 (–0.137 to 0.037) | 0.251 | 93 |
| Credible source | 0.097 (–0.016 to 0.211) | 0.092 | 92 |
| Restructuring the social environment | 0.051 (–0.043 to 0.145) | 0.284 | 93 |
FIGURE 15.
Bubble plot showing the relationship between the RD and the baseline percentage screened. The RD represents the difference in DRS attendance between the intervention arm and the control arm. Each bubble corresponds to a different study and the size of the bubble reflects the number of participants. The regression line shows a trend towards reduced screening attendance with increasing baseline compliance. Reproduced from Lawrensonet al.,127with permission from John Wiley & Sons, Ltd. Copyright © The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
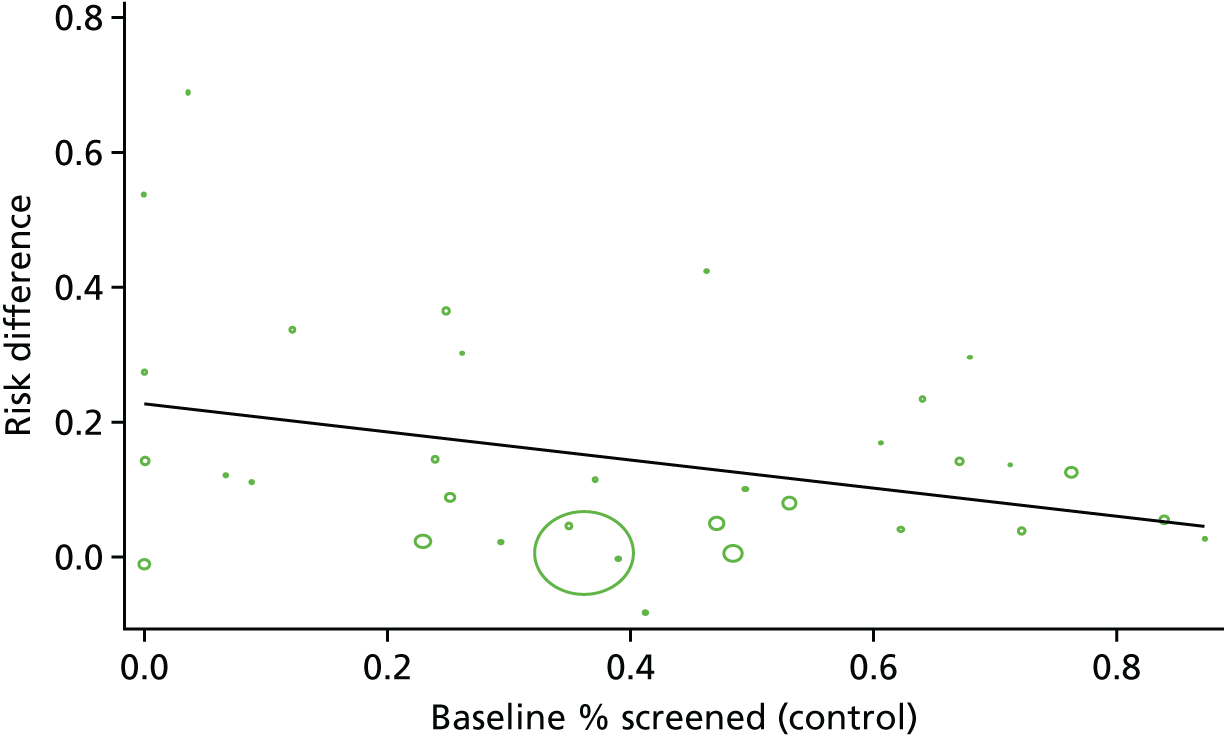
The effect sizes from studies at high risk of bias were slightly (but not statistically significantly) higher than those from studies at low risk of bias. Similarly, no statistically significant difference was found between individual RCTs and cluster RCTs (p = 0.268).
For the meta-regression analyses comparing studies containing particular QI components/BCTs with studies not including these components, no statistically significant differences were found, although there was evidence of an association for ‘team changes’ (p = 0.052) and ‘promotion of self-management’ (p = 0.055). The patient-targeted BCT ‘goal-setting (outcome)’ was associated with a significantly greater improvement in DRS attendance than in studies without this BCT (p = 0.001) and there was some evidence of an association for the HCP-targeted BCT ‘restructuring the social environment’ (p = 0.053).
Sensitivity analysis
The main meta-analysis for any intervention compared with usual care was repeated using the lower and upper range values for the ICCs to evaluate the impact on the computed effect estimates (Table 6).
| Model | ICC | ||
|---|---|---|---|
| 0.05 | 0.01 | 0.2 | |
| RD (95% CI) | RD (95% CI) | RD (95% CI) | |
| DRS | 0.17 (0.11 to 0.22) | 0.17 (0.11 to 0.22) | 0.17 (0.11 to 0.22) |
| GQI | 0.12 (0.09 to 0.15) | 0.12 (0.09 to 0.16) | 0.11 (0.08 to 0.15) |
| Combined | 0.12 (0.10 to 0.14) | 0.13 (0.11 to 0.15) | 0.12 (0.10 to 0.14) |
Overall quality of the evidence
The summary of findings for the primary outcome of this review is presented inTable 7. For the main comparison (any intervention vs. usual care), as most of the information came from studies at low or unclear risk of bias, we did not downgrade for risk of bias. We downgraded one step for inconsistency as there was substantial unexplained heterogeneity between the studies. We found no reasons to downgrade the evidence on the basis of indirectness, imprecision or publication bias. Figure 16provides a funnel plot for the main comparison.
| Outcomes | Anticipated absolute effectsa(95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Attendance with usual care | Attendance with any QI intervention | |||||
| Any QI intervention compared with usual care for DRS | ||||||
| Proportion of patients attending screening | 472 per 1000 | 580 per 1000 (557 to 604) | RR 1.23 (1.18 to 1.28) | 329,164 (56 RCTs) | ⊕⊕⊕○ moderate | There was substantial unexplained heterogeneity between studies (I2 = 93%;p< 0.00001) |
| Proportion of patients attending screening – strategy specifically targeting DRS | 335 per 1000 | 439 per 1000 (402 to 479) | RR 1.31 (1.20 to 1.43) | 118,938 (13 RCTs) | ⊕⊕⊕○ moderate | There was substantial unexplained heterogeneity between studies (I2 = 95%;p< 0.00001) |
| Proportion of patients attending screening – general strategy to improve the quality of diabetes care | 534 per 1000 | 662 per 1000 (625 to 700) | RR 1.24 (1.17 to 1.31) | 210,226 (43 RCTs) | ⊕⊕⊕○ moderate | There was substantial unexplained heterogeneity between studies (I2 = 92%;p< 0.00001) |
| Outcomes | Anticipated absolute effectsa(95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with intervention alone | Risk with stepped QI intervention compared with intervention alone | |||||
| Stepped QI intervention compared with intervention alone for DRS | ||||||
| Proportion of patients attending screening | 361 per 1000 | 405 per 1000 (372 to 437) | RR 1.12 (1.03 to 1.21) | 23,715 (10 RCTs) | ⊕⊕⊕○ moderate | There was substantial unexplained heterogeneity between studies (I2 = 56%;p = 0.02) |
| Proportion of patients attending screening – strategy specifically targeting DRS | 355 per 1000 | 384 per 1000 (295 to 497) | RR 1.08 (0.83 to 1.40) | 19,698 (3 RCTs) | ⊕⊕○○ low | There was substantial unexplained heterogeneity between studies (I2 = 68%;p = 0.04). The pooled estimate of effect includes both appreciable benefit and harm |
| Proportion of patients attending screening – general strategy to improve the quality of diabetes care | 394 per 1000 | 457 per 1000 (406 to 516) | RR 1.16 (1.03 to 1.31) | 4017 (7 RCTs) | ⊕⊕⊕○ moderate | There was moderate unexplained heterogeneity between studies (I2 = 46%;p = 0.08) |
FIGURE 16.
Funnel plot of comparison: any QI intervention compared with usual care. SE(RD), standard error of the risk difference. Reproduced from Lawrensonet al.,127with permission from John Wiley & Sons, Ltd. Copyright © The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
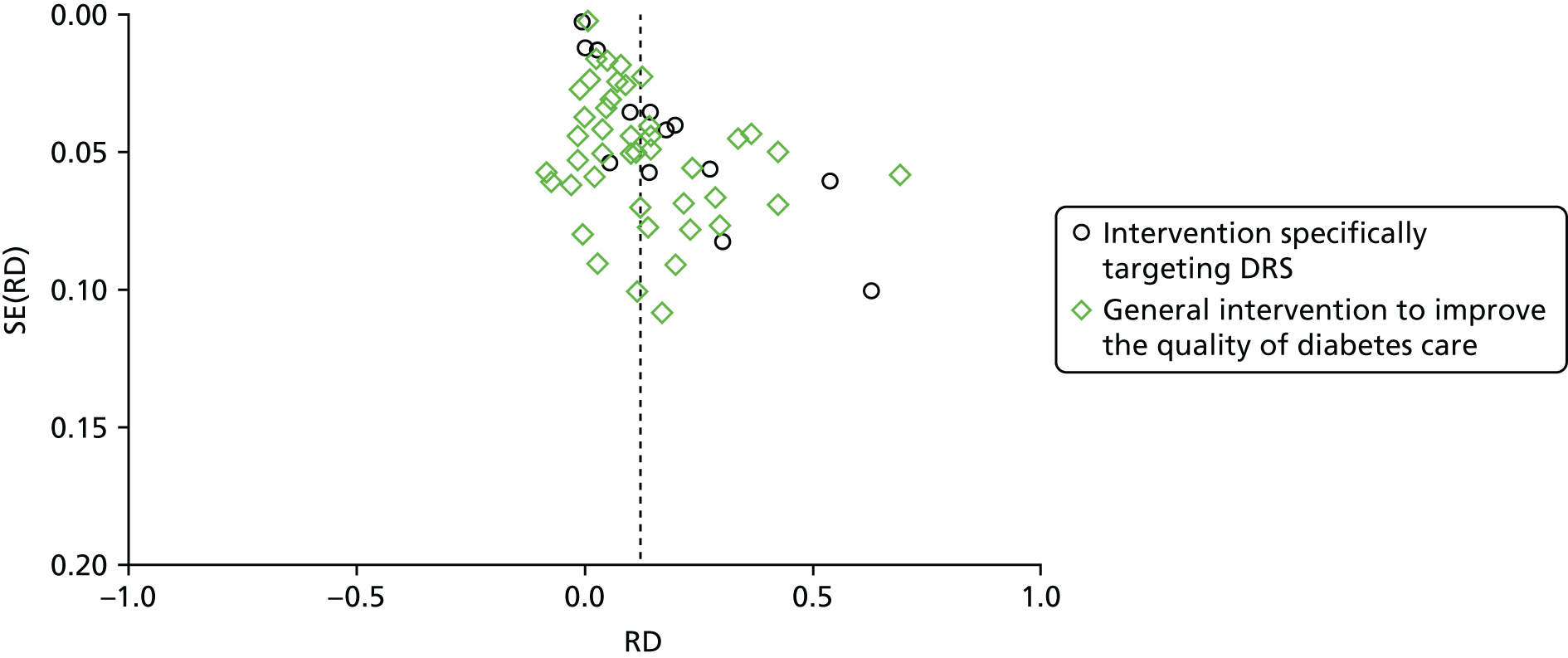
Discussion
Summary of the main results
Impact of quality improvement interventions on diabetic retinopathy screening
A comprehensive search of the literature identified 66 RCTs/cluster RCTs that investigated the effectiveness of interventions to improve attendance for DRS. Fifty-six studies (enrolling 446,078 patients) compared a variety of QI interventions with usual care. A meta-analysis of 56 of these studies found that QI intervention components that were aimed at patients, HCPs or the health-care system were associated with a 12% absolute increase in DRS attendance. In 13 of these studies, the QI intervention specifically targeted DRS and in 43 studies the intervention consisted of a general QI intervention to improve diabetes care. Although the pooled effect estimate was larger for DRS-targeted interventions than for non-targeted interventions (17% vs. 12% increase in DRS attendance), this difference was not statistically significant.
Ten studies (enrolling 51,736 patients) compared a less intensive intervention (‘active’ control) with a more intensive intervention. Three of these studies specifically targeted DRS and seven were general QI interventions. The aim of these studies was to determine whether stepping up the intensity of an intervention component, or introducing further components, would increase DRS attendance. The pooled effect estimate for these studies was smaller, with a 5% increase in DRS attendance in favour of the more intensive intervention, suggesting that it is possible to further enhance the effect size by using more intense interventions.
The main comparison in this review (any QI intervention vs. usual care) was associated with substantial heterogeneity. Heterogeneity was explored by subgroup analysis and meta-regression. There was some evidence for larger effect sizes in populations with lower baseline levels of DRS attendance; however, much of the observed heterogeneity was unexplained. Sufficient numbers of studies were available to investigate the impact of particular QI components or BCTs to identify the active ingredients of the interventions. All 12 QI components, as defined by the modified EPOC taxonomy, were used in at least one study and interventions were generally multifaceted with two to three QI components per intervention arm. QI components targeting patients, HCPs or the health-care system were all effective in subgroup analysis. A meta-regression comparing studies using particular QI components with those not using them showed no statistically significant difference between intervention components, although there was some evidence of an association for ‘team changes’ and ‘promotion of self-management’.
Behaviour change techniques that were particularly effective at improving DRS attendance when directed at patients included ‘goal-setting (outcome)’, that is, agreeing a goal defined in terms of a positive outcome resulting from the behaviour. There was a statistically significant improvement in DRS attendance in studies including this technique compared with those without. There was also some evidence of an association for ‘restructuring the social environment’ in interventions targeting HCPs. This technique involves changing the social environment to facilitate performance of the wanted behaviour, for example the assignment of multilingual link workers to work with specific intervention general practice surgeries in the study by Bushet al. 110The technique ‘credible source’ was effective for both patients and HCPs. This involved verbal or visual communication from a high-status professional or organisation in favour of the behaviour.
We found no studies reporting our secondary outcome measure of ongoing adherence to DRS following the initial screening appointment post intervention and no data on the relative effectiveness of interventions in particular population subgroups, for example according to socioeconomic characteristics.
Health economic outcomes
Fourteen studies reporting economic outcomes were included in the review; however, only five of these were full economic evaluations. Overall, we found that there is insufficient evidence to draw robust conclusions about the relative cost-effectiveness of the interventions compared with each other or with usual care. QI components aimed at patients directly appeared to be more resource intensive than those aimed at HCPs, with the exception of establishing an electronic patient registry, although there would be economies of scale in that there are high set-up costs but the ongoing running costs would be comparatively low.
The effectiveness of interventions that included specific QI or BCT components was calculated for levels of resource use as measured on an ordered ranking scale. The purpose was to explore whether or not more resource-intense interventions were more likely to be effective. The results indicate that there is no clear relationship between the two. Although it is possible that such a relationship may exist, there was no a priori reason to suppose that a more expensive intervention will also be more effective. Variation in resource use can usefully be categorised into variation in intensity of the same intervention and variation in the type of intervention. As an example of the first category, it may be supposed that spending more time face-to-face with a person with diabetes explaining the health consequences of behaviour may be more effective. Alternatively, designing, printing and distributing a 50-page document explaining health messages is likely be more expensive but possibly less effective than designing, printing and distributing a leaflet. As an example of the second category, providing text message prompts and cues is a completely different intervention from providing a leaflet on health consequences. The text messaging system is likely to be more expensive, but we cannot be sure it will be more effective. Furthermore, information about health consequences could also be delivered by telephone or in a leaflet. The telephone call is likely to be more expensive than the leaflet, but we cannot be sure that it will be more effective.
Quality of the evidence
Overall, we judged the quality of the evidence to be moderate using the GRADE evidence rating system. 57The level of evidence was downgraded one step because of inconsistency of findings in studies comparing QI interventions with usual care and one step for imprecision or inconsistency in studies comparing a less intensive with a more intensive intervention. Using the Cochrane EPOC risk-of-bias tool,51we found that nearly half of the included studies were at high risk of bias in at least one domain. This was most often because of incomplete outcome reporting. For many domains it was not possible to judge the risk of bias because of poor reporting. For example, because many of the RCTs did not have a prospectively published protocol, it was not possible to make a judgement whether or not outcomes were selectively reported. A subgroup analysis found that, although studies that were at a high risk of bias had slightly higher effect estimates than those at a low risk of bias, this difference was not statistically significant. The consensus of the review team was not to downgrade the quality of the evidence for risk of bias.
Of the 22 potential ‘economic’ studies identified by the review team, 14 studies were eligible for the review as partial or full economic evaluations. We identified a publication bias in two of the eight excluded studies. 94,123These studies failed to report the planned economic evaluations as no evidence of intervention effectiveness was identified. Such an approach could be considered as selective outcome reporting, such that potentially negative economic findings are not reported. This phenomenon of reporting bias has been recognised previously,129when studies with unfavourable effectiveness results are not published or are published later in low-impact journals. Furthermore, analytically, such an approach is substandard as these studies conflate absence of evidence with a finding of evidence of absence (of an effect).
Most of the economic evaluations had limitations in their reporting, with few providing a breakdown of the costs associated with delivering the different components of the intervention. There was also insufficient evidence to show whether or not part of the direct costs of the intervention and care may be offset by reduced productivity costs. However, it is important to note that the expected findings of an effective intervention would be gains in health and reductions in the costs of treating diabetes. The overall methodological quality of the included economic studies was mixed. The partial economic evaluations identified lack, by their nature, the methodological characteristics expected of an economic evaluation. The methodological quality of the full economic evaluations was considered to be moderate.
Agreements and disagreements with other studies or reviews
Only one systematic review22has previously investigated the effectiveness of interventions to increase the uptake of DRS. Although this review included 48 studies, only 12 of these were RCTs (10 of which were included in the current review). The authors similarly concluded that a variety of interventions can be effective in improving screening uptake, including increasing patient and provider awareness of diabetic retinopathy, introducing a computer-based registration/reminder programme and developing a community-based health-care system.
Compared with the paucity of systematic reviews of the impact of interventions to improve DRS outcomes, many reviews have evaluated the impact of general QI interventions to improve the overall quality of diabetes care. 28A systematic review published by members of the current team29included 48 cluster RCTs and 94 patient RCTs and found improvements in many important quality outcomes for patients with diabetes. A meta-analysis of a subset of 23 RCTs reporting the uptake of DRS found a similar pooled relative effect size to that in the current review (RR 1.22, 95% CI 1.13 to 1.32;I2 = 80.4%).
Limitations of the review
Our systematic review has some limitations. Coding of intervention content was challenging given the paucity of primary data sources, although in some cases this was offset by obtaining further information from researchers on intervention content, who also provided the materials used to deliver the interventions. We were not able to assess the impact of some QI intervention components because of too few trials being available for our subgroup and meta-regression analyses. Furthermore, we could not control for all potential confounding factors. Given the complexity of the interventions under investigation, which incorporate multiple QI components, it is likely that other covariates may have interacted synergistically or antagonistically with them. The short duration of the included RCTs (typically ≤ 12 months) also meant that we were unable to assess the effect of the QI interventions on ongoing DRS attendance or their impact on the progression of diabetic retinopathy.
Implications for practice
The results of this review provide evidence that QI interventions targeting patients, HCPs or the health-care system are likely to be associated with meaningful improvements in DRS attendance compared with usual care. There was no statistical difference between interventions specifically aimed at DRS and those that were part of a general QI strategy for improving diabetes care. This is a significant finding because of the additional benefits of general QI interventions in terms of improving glycaemic control, vascular risk management and screening for other microvascular complications. 28,29It is likely that further (but smaller) improvements in DRS attendance can also be achieved by increasing the intensity of a particular QI component or adding further components.
One of the main objectives of the review was to identify the ‘active’ components of successful interventions by using validated taxonomies to describe the content of the interventions. All of the QI components as defined by the modified EPOC taxonomy were associated with improvements in DRS attendance. To better characterise intervention content we coded the interventions in terms of patient and provider BCTs. For BCTs aimed at patients, higher effect estimates were found for interventions incorporating goal-setting; for BCTs aimed at HCPs, higher effect estimates were found for interventions involving environmental restructuring. However, only 42% of the 93 possible BCTs were reported in the included interventions and, although not all BCTs in the taxonomy might be appropriate for DRS, the findings of this review suggest that there may be opportunities to assess the potential of additional BCTs in future trials of novel interventions to improve screening attendance.
Although only three of the studies were conducted in the UK, we believe that the results of the review are applicable to a UK context. Two-thirds of the included studies were conducted in North America, where guideline recommendations include a DFE by an ophthalmologist or optometrist at least once every 2 years (or more frequently in high-risk individuals). Although the North American system for DRS is different from the national screening programme that operates in the UK, the two care pathways are similar in that DRS is generally conducted in a different health-care setting from that for other aspects of diabetes care.
Implications for research
The review highlighted a number of gaps within the evidence base. There was limited evidence on the relative effectiveness of QI interventions in particular population subgroups according to demographic characteristics that could have an impact on health equity, for example ethnicity, level of education or socioeconomic status. There is also insufficient evidence on the cost-effectiveness of QI interventions to improve DRS attendance.
Most of the included studies, whether targeting DRS or general QI, enrolled patients not achieving diabetes-relevant quality indicators. For example, five studies specifically targeting DRS exclusively recruited patients who were not meeting guideline recommendations for screening. It is not clear if the interventions would be as effective in populations with a higher level of screening attendance (> 80%). There was some evidence from our meta-regression analysis that the effectiveness of the interventions is negatively correlated with baseline level of DRS.
Although we have been able to show that interventions containing particular BCTs have a greater likelihood of success, given the multicomponent nature of interventions, it is likely that the presence of other BCTs or other effect modifiers in the intervention arm may also have an impact on effectiveness. The analysis conducted as part of this review did not attempt to isolate the impact of individual QI/BCT components, although exploratory analysis was conducted using multivariate meta-regression methods to determine cost-effectiveness (reported inChapter 6). Further research is needed to identify which components of interventions or combinations of components can optimally improve DRS attendance at an acceptable cost.
Chapter 4 Barriers to and enablers of diabetic retinopathy screening attendance: a systematic review of published and grey literature
Background
In addition to identifying the ‘active components’ in complex interventions (reported inChapter 3), evidence is also needed on how QI interventions might work. Understanding the theoretical determinants (e.g. barriers and enablers) of screening behaviour is an important step in intervention development and in interpretation of the results of evaluations of interventions. In phase 2 of this study, identification of barriers and enablers provides an evidence base to assess the extent to which existing QI interventions address the theoretical determinants of attendance behaviour, to offer guidance as to why some interventions might work better than others and to identify which theoretical determinants are likely to mediate the effects of interventions.
There is some evidence that theory-based interventions may be more effective than those that are not theory based. 31,32This suggests that interventions that target DRS behaviour are more likely to be effective if they target the theoretical determinants of screening behaviour. However, the explanatory factors (theoretical constructs) from different theories often overlap, making it challenging to identify the key determinants. 130
To address this, 128 explanatory constructs from 33 theories of behaviour change have been synthesised into an integrated framework, the TDF, which consists of 14 overarching ‘theoretical domains’. These domains are labelled (1) knowledge, (2) skills, (3) social/professional role and identity, (4) beliefs about capabilities, (5) optimism, (6) beliefs about consequences, (7) reinforcement, (8) intentions, (9) goals, (10) memory, attention and decision processes, (11) environmental context and resources, (12) social influences, (13) emotions and (14) behavioural regulation. 39Appendix 7presents the definitions of the TDF domains. Each domain represents a set of related constructs that may mediate behaviour change. For example, the ‘social influences’ domain includes constructs such as social support, group norms and social comparison. 130The TDF thus provides an accessible, comprehensive, theory-driven basis for investigating the potentially wide-ranging barriers to and enablers of behaviour change.
Barriers and enablers can operate at multiple levels in the health-care system. Ferlie and Shortell131propose four distinct levels of change that should be considered to maximise the likely effectiveness of QI interventions: individual, group or team, overall organisation and wider system or environment. The TDF can be applied at the level of the individual, team and/or health-care organisation to investigate barriers to and enablers of behaviour change. 130A related framework is the Consolidated Framework for Implementation Research (CFIR). The CFIR draws on a different theoretical literature from the TDF, primarily relating to organisational behaviour change. The CFIR provides an integrated framework of theory-based constructs to guide the identification of potential barriers to and enablers of health-care innovation across different organisational levels. It includes 39 constructs organised into five domains: (1) ‘intervention characteristics’, (2) ‘inner setting’, (3) ‘outer setting’, (4) ‘characteristics of the individuals involved’ and (5) the ‘process of implementation’. Appendix 8presents the constructs and definitions for each of the five CFIR domains. The CFIR can potentially be used concurrently with the TDF to offer an organisational focus to investigating barriers/enablers, which might identify further insights. 132
Both the TDF and the CFIR have been applied in numerous studies, primarily interview and survey studies, to systematically identify and characterise barriers to and enablers of implementation across a wide range of clinical contexts. More recently, the TDF has been applied in systematic reviews of barriers and enablers, as a coding framework for data synthesis. 133–136For example, Wilkinsonet al. 133undertook a review to identify barriers to and enablers of the translation of gestational diabetes guidelines into clinical practice. They used data including routinely collected hospital data, staff survey data, clinic observations and team discussions and evidence from relevant literature. Data representing barriers were then coded into the TDF domains and mapped onto potential implementation strategies. Identifying barriers and enablers in the literature, framing these in terms of theoretical domains and identifying their likely importance for screening attendance are steps that might explain why some interventions are more effective than others. This would enable intervention designers to optimise interventions by ensuring that they target the likely determinants of screening attendance.
Aim
In the evidence synthesis reported in this chapter we set out to complement the evidence on the effectiveness of QI interventions used in RCTs to improve attendance for DRS (seeChapter 3). We aimed to clarify how QI interventions might work and gain an understanding of DRS attendance behaviour by identifying the theoretical determinants of DRS attendance.
The specific objectives were to:
-
identify the published and grey literature reporting perceived barriers and enablers associated with DRS attendance
-
extract reported barriers/enablers and categorise them according to TDF/CFIR domains
-
identify key content themes within domains, regarding barriers to and enablers of DRS attendance
-
apply prespecified criteria to identify the likely importance of TDF/CFIR domains in influencing DRS attendance.
Methods
The protocol for this review was published inSystematic Reviewson 8 August 2016137(PROSPERO CRD42016032990).
Study eligibility criteria
Types of study
Studies were included if they reported primary data relating to barriers and enablers that might hinder or facilitate patient attendance at DRS. Such barriers or enablers included organisational, emotional, cognitive, behavioural and social factors; however, they were required to be potentially modifiable (i.e. not demographic or historical factors such as age, gender, ethnicity, socioeconomic status or duration of illness). By primary data we mean any data relating to participant perceptions of barriers and/or enablers or data that document specific modifiable factors associated with attendance.
Evaluations of QI interventions were excluded if they did not signal investigations of primary data relating to barriers and/or enablers in the title. This decision was based on the results of a scoping exercise performed on a subset of studies, returned by the search that reported evaluations of QI interventions. From this subset we identified that no information relevant to this review would be lost if we eliminated such studies based on screening at the abstract level. Reviews of studies reporting primary data were also excluded to avoid duplication of findings.
We included studies reported in English, conducted within the time period from January 1990 to March 2016, basing the lower date limiter on the publication of the St Vincent Declaration,138which set a target to reduce new cases of blindness in Europe by one-third or more, as this is arguably the catalyst for the development of screening programmes for diabetic retinopathy worldwide.
Participants
Those diagnosed with type 1 or type 2 diabetes and eligible for DRS were included in the review. There were no restrictions on participant demographics or characteristics. HCPs responsible for diabetes care were also included. In the context of this review a HCP was defined as anyone who provides information, guidance, screening or treatment to people with diabetes to assist with the management of their diabetes. Such professionals include, but are not limited to, general practitioners (GPs), diabetologists, diabetes specialist nurses, optometrists, ophthalmologists and retinal screeners. Samples from any country were included.
Context
There were no restrictions on population-based DRS programmes/models (e.g. fixed location screening services, mobile screening services, optometry-based services and mixed service delivery models).
Search strategy
Identifying published studies
We searched MEDLINE, EMBASE, PsycINFO, Web of Science, CENTRAL (The Cochrane Library) and ProQuest (from January 1990 to March 2016). The search strategy was adapted to suit each database. The reference list of any study that met the inclusion criteria was screened for any additional studies not identified in the database searches. The reference lists of any relevant reviews that were identified in the database search were also screened for any additional studies. An example of the search strategy for MEDLINE is provided inAppendix 9and the full search strategy for each database is provided inReport Supplementary Material 2(seeSearch strategies for phase 2 systematic review).
We undertook a scoping search to develop an appropriate search strategy and terms were agreed by the research team. Search terms addressed three distinct concepts: (1) diabetic retinopathy (e.g. diabetic retinopathy, proliferative retinopathy, diabetic eye disease), (2) screening (e.g. screening, vision tests, ophthalmoscopy, eye examination, fundus photography) and (3) potential barriers and enablers [e.g. (non)compliance, (non)responsive], including terms relating to background characteristics of the population for which there is evidence in the literature of disparities in health screening attendance (e.g. patient acceptance of health care, health promotion, health-care disparities, socioeconomic factors, education and ethnic groups).
In addition to the terms listed above, the search strategy included terms related to each of the TDF domains, to ensure that the potential range of barriers and enablers was represented. These were agreed by a consensus group exercise. Consensus group participants (seven research psychologists who were familiar with the TDF) were presented with the TDF domain labels and component constructs and asked to rank the constructs in terms of the extent to which they represent/summarise the domain. The highest ranking (i.e. the top two) constructs within each domain were selected and added to the list of keywords representing the TDF. For example, in the domain ‘emotions’ the top two constructs selected were ‘anxiety’ and ‘fear’.
Identifying grey literature
Established sources of grey literature were searched using two approaches. First, we used a modified version of the search strategy described above to search grey literature databases, for example OpenGrey, PsycEXTRA and the Healthcare Management Information Consortium (HMIC) database. Second, we carried out an internet search using the search engine Google (Google Inc., Mountain View, CA, USA) using the terms ‘diabetic retinopathy’ AND screening AND [barrier* OR ‘facilitate* OR enable]. We limited the Google search to the first 15 pages. We also consulted with the project representative stakeholder group to identify further sources of grey literature.
Study selection process
Following deduplication, one member of the research team (EGR) screened the titles and abstracts of all of the identified references against the inclusion/exclusion criteria to decide whether or not the full-text manuscripts should be retrieved. Each study was judged as either (1) not meeting the eligibility criteria for inclusion or (2) potentially meeting the eligibility criteria for inclusion. A second report author (FL) screened the titles of approximately 10% of the titles and abstracts. Inter-rater reliability statistics were calculated. The full-text screening was conducted using similar methods as used to screen titles and abstracts.
Data extraction and analysis materials/tools
We developed a data extraction form to extract study characteristics, including author/date, method of identification (e.g. database search, Google search), country where study was conducted, research objectives, topics of investigation (e.g. knowledge, attitudes, beliefs or perceptions of barriers/enablers), methodological/theoretical approach, data collection methods, data analysis methods, participant characteristics and sample size.
We prepared a codebook with the definitions of each of the 14 theoretical domains from the TDF and the 39 distinct constructs from the five domains of the CFIR, to facilitate coding consistency and reliability. Two report authors (EGR and FL) collaboratively coded three included studies, making reference to the codebook and amending it when appropriate.
Quality assessment
One review author (EGR) assessed the quality of the included studies using items selected from the Critical Appraisal Skills Programme (CASP) qualitative checklist139and the Mixed Methods Appraisal Tool (MMAT). 140Studies that reported mixed methods were appraised using both the quantitative and the qualitative appraisal tools. A quantitative survey that reported open-ended questions but which did not analyse the responses qualitatively was defined as a quantitative study. Studies presented as abstracts only were not subjected to quality appraisal. A second review author (JGL) assessed a random sample of studies (20%) and differences of opinion regarding quality were resolved by discussion.
Analysis
To the extent that it was possible in a secondary analysis context, we followed analysis methods that have been used in previous studies applying the TDF to interview transcripts from semistructured interviews. 141–143These methods typically follow a combined content and framework analysis approach and involved five steps: (1) data extraction, (2) deductive analysis (TDF coding), (3) inductive analysis (thematic synthesis), (4) CFIR coding and (5) identifying important domains. Figure 17presents a flow diagram of the steps in the analysis.
FIGURE 17.
Flow diagram of steps in the analysis.
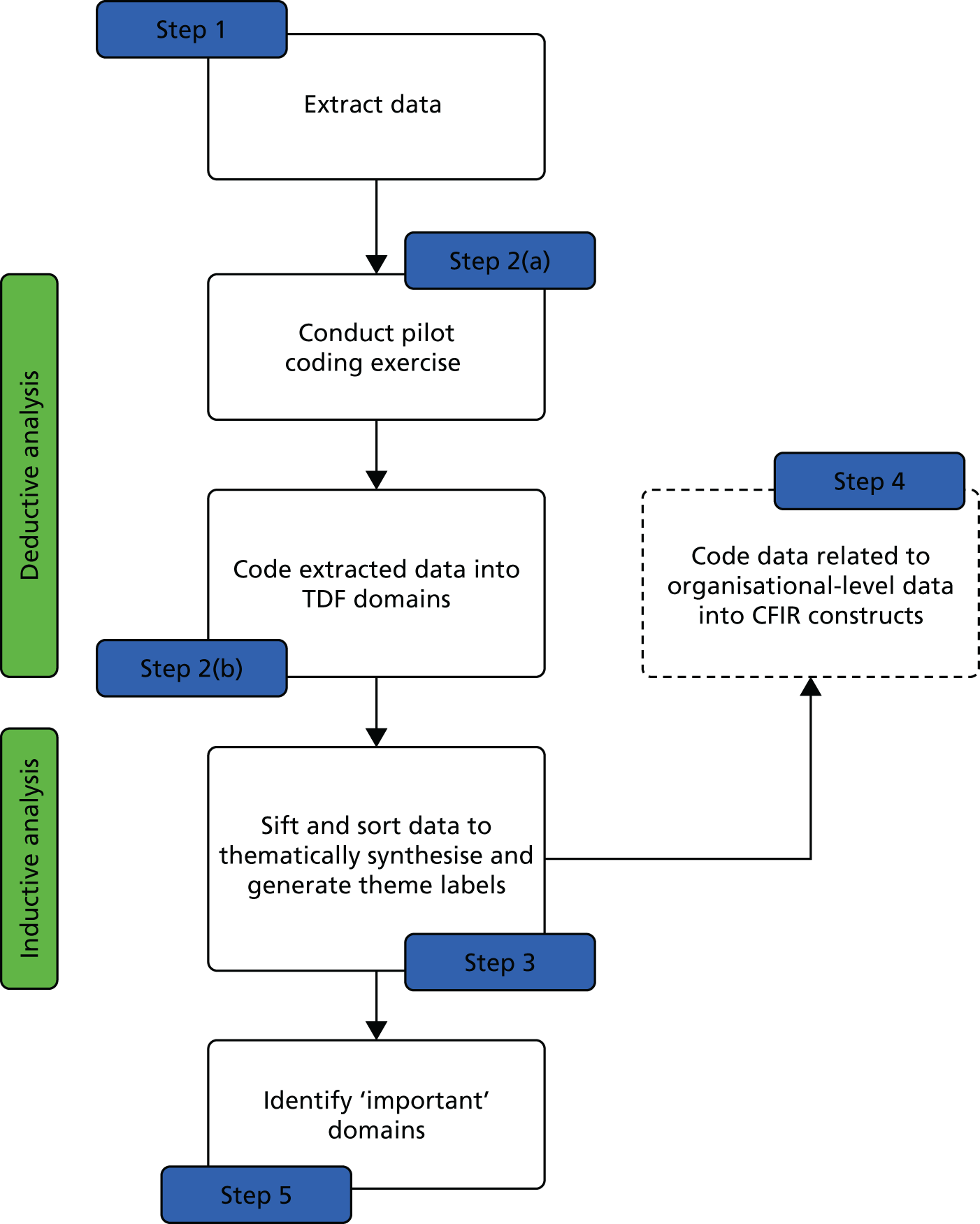
Data extraction
Step 1: data extraction
One report author (EGR) identified and extracted data reporting participants’ (e.g. people with diabetes and/or HCPs) perceptions of barriers to and enablers of DRS attendance that met our inclusion criteria, presented inTypes of studies. Extracted data included, for instance, participant quotations from qualitative studies, quantitative findings from questionnaire and survey studies and authors’ interpretive descriptions and summaries of results. Reported predictors of and associations with attendance/non-attendance reported in quantitative studies were also extracted.
Deductive analysis
Step 2a: pilot coding exercise
To practise coding extracted data into TDF domains, three pilot transcripts were collaboratively coded using the TDF as a coding framework. Emerging heuristics were documented (e.g. ‘knowledge’ may be clinically correct or incorrect but must relate/link to attendance). Any discrepancies were discussed until agreement was reached (seeAppendix 7for the TDF codebook).
Step 2b: Theoretical Domains Framework coding
One review author (EGR) coded the data extracted from all remaining studies. Extracted data were coded according to which TDF domain they were judged to represent, guided by the TDF codebook that was developed as a result of step 2a. For example, if a person with diabetes was quoted as saying, ‘I believe that having my eyes screened once a year will help preserve my vision’, this was coded into the domain ‘beliefs about consequences’. If a reported barrier/enabler was judged to concurrently represent more than one domain, it was coded into multiple domains. For example, if a person with diabetes was quoted as saying, ‘I am anxious about attending my screening appointment because I can’t drive home after receiving eye drops’, this was coded into two domains, ‘emotions’ and ‘beliefs about capabilities’.
Inductive analysis
Step 3: thematic synthesis
In line with a framework analysis approach, step 3 focused on sifting and sorting the data within each domain to thematically synthesise and identify key emerging content themes. 144,145One report author (EGR) grouped together similar data relating to perceived barriers to/enablers of DRS attendance for each of the 14 domains. Theme labels and, when appropriate, subtheme labels were then generated for each cluster of similar data to express these shared views. These initial grouping and theme labels were then reviewed in a consensus group exercise. Two additional report authors (FL, JJF) reviewed the initial groupings to assess (1) their agreement with the grouping of extracted data, (2) their agreement with the assigned theme labels and (3) if the themes were appropriately allocated to the given domains. Disagreements were discussed until consensus was reached. The theme groups/labels/allocation of domains were revised accordingly, based on consensus.
Additionally, EGR assigned the data within the themes as representing either barriers to or enablers of DRS attendance. The decision was made depending on the interpretation of the data from the study where the data originally came from. This was usually clear as data either were reported in a table entitled ‘barriers to DRS’ or ‘enablers to DRS’ attendance or were interpreted as one or the other by the study authors, who were familiar with the raw data. A theme/subtheme was then classified as (1) a barrier theme if the data points within it related to barriers only (e.g. receiving insufficient notice of appointments), (2) an enabler theme if the data points within it related to enablers only (e.g. support from local community groups/networks) and (3) both a barrier and an enabler theme if the data points related to both [e.g. (in)flexibility of choice of times/dates of appointments].
Step 4: Consolidated Framework for Implementation Research coding
To further explore potential organisational factors that might influence DRS attendance we recoded the themes identified in the inductive analysis (described above in step 3) into CFIR constructs. This exercise was carried out on the themes identified within the four TDF domains that are arguably related to the organisation: (1) ‘environmental context and resources’, (2) ‘social influences’, (3) ‘social/professional roles and identity’ and (4) ‘behavioural regulation’. 146
Identifying important domains
Step 5: applying importance criteria
Each domain identified in step 2 was reviewed against an established set of ‘importance criteria’146to determine which domains were likely to be important for influencing screening attendance. There were three importance criteria: (1) frequency (the number of studies that identified each theme), (2) level of elaboration (number of themes and subthemes) within each domain and (3) ‘expressed importance’ within each domain (either a statement from the authors’ interpretation of the study findings articulating specific beliefs as important influences or direct quotes from study participants expressing importance).
Results
Search results
Searches of MEDLINE, EMBASE, PsycINFO, Web of Science, CENTRAL and ProQuest generated 3251 records. Searches in OpenGrey, PsycEXTRA and the HMIC database generated a further 62 records. A further 144 records were identified through the other sources, totalling 3457 records. After deduplication, a total of 3010 records were screened based on title and abstract. In total, 2800 records were excluded, leaving 210 studies for full-text screening. When the full text of a record was not available but the abstract presented meaningful results, these were also screened against our inclusion/exclusion criteria. We identified 65 studies24,147–210that met our eligibility criteria. Figure 18presents the PRISMA flow diagram.
FIGURE 18.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
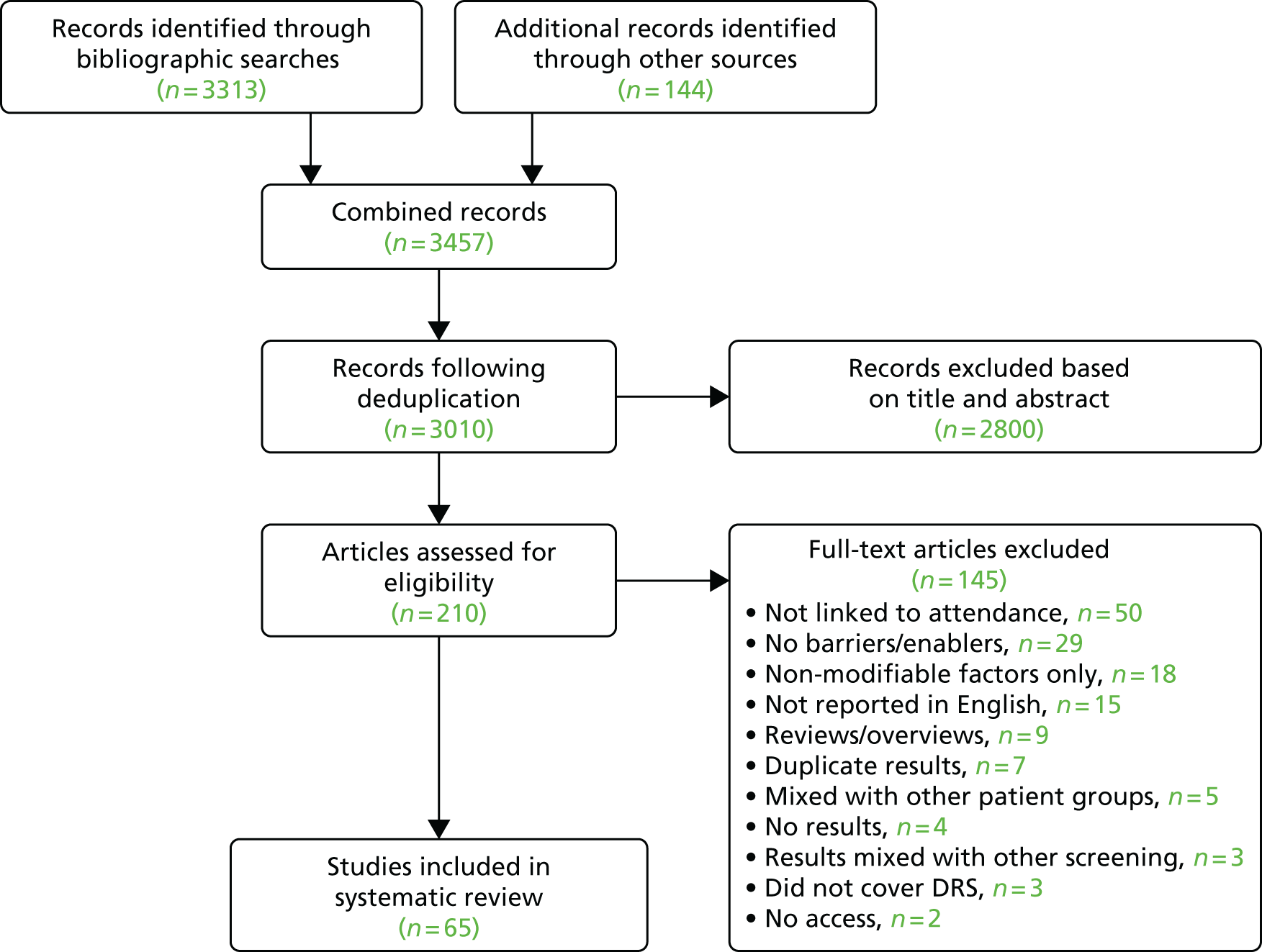
Characteristics of included studies
Report Supplementary Material 2[seeCharacteristics of the 65 included studies (phase 2 review)] shows the characteristics of the 65 included studies. Forty-one (63%)147,148,150,151,153,156,162–166,171,175–180,182,184–187,190,192,194–199,201–210used quantitative methods only (e.g. questionnaires, surveys), 18 (28%)149,152,154,155,157–160,168,169,172–174,181,188,189,191,200used qualitative (e.g. interviews/focus groups) methods only and six (9%)24,161,167,170,183,193used mixed methods. Forty-seven studies used quantitative methods in full or as part of a mixed-method design. Of these 47 studies, 37 (79%) used a cross-sectional survey design, nine (19%) used a follow-up design and one (2%) was a report of a questionnaire development study. Twenty-four studies used qualitative methods in full or as part of a mixed-method design. Of these 24 studies, 19 (79%) were descriptive studies that had no specific analytical or theoretical approach, one (4%) was part of an evaluation of a QI intervention, one (4%) used a theoretical framework (TDF), one (4%) used a grounded theory approach, one (4%) used a case-base approach and one (4%) was a service evaluation.
Twenty-nine (45%) of the studies were carried out in the USA, 12 (18%) in the UK, four (6%) in Australia, three (5%) in Canada, 10 (15%) in Asia, four (6%) in Africa, two (3%) in Europe and one (2%) in South America. Forty-eight studies (74%) were published as full texts in peer-reviewed journals, five (8%) were reported as unpublished full-text reports/dissertations and 12 (18%) were published in abstract/poster form only.
Fifty studies (77%) reported barriers/enablers from the perspective of the people with diabetes only, 14 (21%) reported barriers/enablers from the perspective of both people with diabetes and HCPs and one (2%) reported barriers/enablers from the perspective of HCPs who were diagnosed with diabetes. Twenty-nine studies (45%) reported barriers/enablers from a subset of patient samples. These included 10 studies (15%) that reported barriers/enablers from the perspective of specific ethnic groups (e.g. African Americans, American Indians, Aboriginal Canadians, people of South Asian or Hispanic origin), six studies (9%) that reported from the perspective of people who were classified as either non- or late attenders, four studies (6%) that reported from the perspective of older adults, two studies (3%) that reported from the perspective of younger adults, two studies (3%) that reported from the perspective of women only, two studies (3%) that reported from the perspective of people who had been diagnosed with diabetic retinopathy, one study (2%) that reported from the perspective of people with diabetes receiving treatment, one study (2%) that reported from the perspective of people with diabetes who had participated in a blindness prevention programme and one study (2%) that reported from the perspective of a Medicare population. Report Supplementary Material 2[seeExcluded studies (phase 2 review)] presents a description of articles that were assessed for inclusion at the full-text stage but which were subsequently excluded.
Quality of included studies
The quality appraisals of the included studies (n = 5324,147–161,163–170,172–175,177–180,182,184,185,187,190–192,194,195,197,199,201–210) are presented inReport Supplementary Material 2[seeQuality assessment (phase 2 review)]. Overall, studies were judged to be at low, medium or unclear risk of bias. However, studies were often poorly reported, which made the process of quality appraisal difficult. Limitations in the qualitative studies included (1) limited reporting or rigour of the data analysis and (2) limited reporting or consideration of the relationship between the authors and the participants. Generally, the strengths of these studies were (1) the research design (although there was often a lack of information justifying the approach), (2) the recruitment strategy and (3) the data collection procedures. Limitations in the quantitative studies related to (1) poor reporting of questionnaire validity and (2) low response rates.
Deductive analysis
In total, 612 units of data were extracted, of which 378 were qualitative (126 quotations from study participants and 252 from authors’ conclusions) and 234 were quantitative [e.g. percentages of participants agreeing with a questionnaire item or odds ratios (ORs)].
Reported barriers were identified in all but one of the TDF domains (‘skills’). Enablers were identified in all but two domains (‘beliefs about capabilities’ and ‘skills’). However, overall, there were almost three times as many themes/subthemes identified as barriers only than themes/subthemes identified as enablers only (60 vs. 22). Twenty-one themes/subthemes represented both barriers and enablers. Table 8reports the frequencies of barriers and enablers identified within each of the 15 TDF domains.
| TDF domain | Barriers only | Enablers only | Both barriers and enablers |
|---|---|---|---|
| Environmental context and resources | 18 | 3 | 6 |
| Social influences | 6 | 4 | 5 |
| Knowledge | 3 | 2 | 6 |
| Memory, attention and decision processes | 9 | 3 | – |
| Beliefs about consequences | 8 | 3 | – |
| Emotions | 6 | 2 | 1 |
| Social/professional role and identity | 5 | 1 | – |
| Goals | 2 | 1 | 1 |
| Beliefs about capabilities | 2 | – | – |
| Behavioural regulation | – | 2 | 1 |
| Intentions | 1 | 1 | – |
| Optimism | 1 | 1 | – |
| Reinforcement | – | – | 1 |
| Skills | – | – | – |
Inductive analysis
Report Supplementary Material 2(seeThemes/subthemes within each of the 14 domains from the Theoretical Domains Frameworks) presents all themes and subthemes identified within each domain, alongside frequencies, relevant studies and sample quotations. A narrative description of the themes, within domains, is presented later in this chapter, organised into two groups: (1) domains that were identified as being ‘high’ in importance (seeThematic synthesis for domains identified as having high importance) and (2) domains that were identified as being less important (seeDomains identified as being less important).
Consolidated Framework for Implementation Research: organisational analysis
Themes identified in the four TDF domains that arguably relate to the organisational level (as specified in step 4 of the methods) were recoded into CFIR constructs. The full results are presented inReport Supplementary Material 2(seeCoding of themes/subthemes into Consolidated Framework for Implementation Research constructs). The studies included in this review predominantly reported barriers and enablers for the person with diabetes, as reported by the patient, HCP or study author, rather than for the organisation or HCP, as conceptualised by the CFIR. Therefore, the majority of the themes were coded into the construct ‘needs and resources of those served by the organisation’ which, according to the CFIR, is defined as ‘the extent to which the needs of those served by the organisation (e.g. patient), as well as barriers and facilitators to meet those needs, are accurately known and prioritized by the organisation’ [reproduced from the CFIR. 211This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See:http://creativecommons.org/licenses/by/4.0/]. The focus of the CFIR on the HCP as the individual of interest and not the person with diabetes means that, in this study, it did not offer any additional insights over and above the TDF. As a consequence, no further analysis was conducted using the CFIR.
Importance of the Theoretical Domains Framework domains
This section reports the findings regarding the three importance criteria (frequency, elaboration and expressed importance).
Domain frequency
Most of the data units extracted from included studies were coded into the domains (1) environmental context and resources (50 studies24,147–151,153–170,172,174,175,177,179–191,194–196,199–202,206,207), (2) knowledge (33 studies24,151,152,155–161,165–169,172,174–178,181,188,190–195,200,203,205,206), (3) social influences (32 studies24,148–150,152,154,156–161,165–175,181,182,184,188,191,192,195,201,203), (4) memory, attention and decision processes (31 studies24,149,155–161,165,166,168–170,172,175,177,179–184,187,189,190,194,195,199,201,203), (5) beliefs about consequences (25 studies24,155–159,161,165–168,170,174,175,181–189,192,198) and (6) emotions (22 studies148,154,157–161,165,167,170,174,175,181,183,185,188–193,204). These six domains were identified by at least one-third of the included studies (Table 9).
| TDF domain | Frequency | Level of elaboration | |
|---|---|---|---|
| Number of studies identified | Number of themes | Number of subthemes | |
| 1. Environmental context and resources | 50 | 10 | 23 |
| 2. Social influences | 33 | 10 | 8 |
| 3. Knowledge | 33 | 5 | 9 |
| 4. Memory, attention and decision processes | 31 | 6 | 9 |
| 5. Beliefs about consequences | 25 | 7 | 8 |
| 6. Emotions | 22 | 6 | 4 |
| 7. Social/professional role and identity | 10 | 3 | 5 |
| 8. Goals | 11 | 2 | 3 |
| 9. Beliefs about capabilities | 9 | 1 | 2 |
| 10. Intentions | 7 | 2 | 0 |
| 11. Behavioural regulation | 7 | 2 | 0 |
| 12. Optimism | 3 | 2 | 0 |
| 13. Reinforcement | 1 | 1 | 0 |
| 14. Skills | 0 | 0 | 0 |
Level of elaboration
Approximately 83% of themes/subthemes relating to barriers and 80% relating to enablers were captured in the same six TDF domains. Table 9(columns 3 and 4) lists the numbers of themes and subthemes identified within each domain.
Rank order of domain importance
InTable 9, the fourteen TDF domains are presented in rank order. In general, there was good convergence between frequency (number of studies in which the domain was evident) and elaboration (number of themes and subthemes based on the inductive analysis). All three columns inTable 9identify environmental context and resources as the most important domain and, with one exception, the same six domains as more important than the remaining eight domains. One more subtheme was identified in the domain of social/professional role and identity than in the domain of emotions. However, the overall signal for importance was stronger for emotions, which was therefore ranked sixth for importance.
Expressed importance
Study authors’ interpretations of the study findings (e.g. in discussion sections) articulating specific beliefs as important influences were also considered to be evidence of the importance of barriers and enablers. Quotations expressing importance are presented inReport Supplementary Material 2(seeExpressed importance) alongside the TDF domain that they were judged to represent. For example, the quotation ‘Getting to and from screening appointment was important pragmatically for many patients, who had to overcome a range of issues’158represents expressed importance for the domain ‘environmental context and resources’ and the quotation ‘The main reason for refusal was the retinal photos taken might worsen sight’ represents expressed importance for the domain ‘beliefs about consequences’. 186After all quotations regarding expressed importance had been coded into the corresponding themes, the number of studies that identified each domain through expressed importance was counted, with the higher the count, the higher the expressed importance.
On the basis of expressed importance, domains judged to be important in the included studies were ‘environmental context and resources’ (19 studies148,152,154,155,158,160,161,166,170,179,185–187,189,191,194,199,201,207), ‘knowledge’ (19 studies152,158–161,165,167–169,173–175,181,190,191,193,195,200,205), ‘memory, attention and decision processes’ (11 studies156,166,170,179–182,187,189,193,201), ‘social influences’ (nine studies148,154,165,167,168,170,172,174,191), ‘beliefs about consequences’ (six studies158,165,183,186,189,204) and ‘emotions’ (four studies167,191,193,204). This list corresponds well with the list of six domains of high importance identified by the importance criteria frequency and elaboration. However, the domain ‘knowledge’ came out more strongly in expressed importance than it did in frequency or elaboration. No other domains were identified through expressed importance.
In summary, there was good convergence between all three criteria for identifying the importance of six theoretical domains, suggesting that these domains are likely to be key mediators of screening attendance behaviour.
Thematic synthesis for domains identified as having high importance
The content themes in the domains that were identified as being potentially important factors influencing screening attendance are described in further detail in the sections below.
Environmental context and resources
Theme: accessibility to the screening clinic
This was a theme identified in many studies in this review by both people with diabetes and HCPs. ‘Accessibility’ included issues with both transportation (e.g. lack/cost/poor quality) and distance to the DRS clinic. In one correlational study the author noted that those living ≥ 8 miles from the screening facility were significantly less likely to attend for screening than those living within an 8-mile radius. 147However, in another study the authors reported that half of the participants lived very close to the DRS clinic yet 86% still cited distance as a barrier. 148In several studies, the participants felt that having access to DRS services close to home was favourable and would improve attendance. 159,161,169
Theme: time (competing demands)
People with diabetes often cited time constraints as a barrier to attendance. Competing demands on their time from work commitments, family responsibilities (e.g. childcare) and clashes with other immoveable life events such as holidays and religious/cultural activities were all cited. For example, one HCP commented that ‘People go away . . . to the Caribbean, Africa, Asia, Pakistan, India . . . You find out in retrospect where they’ve been, and because they’re away they’re not going to get their screening done’. 149
Theme: financial concerns
Financial concerns, such as the cost of the eye examination/care and the cost or lack of insurance were prevalent, especially among those with diabetes participating in the US studies. Having private insurance was reported to be associated with higher attendance rates in two studies. 150,151However, in some cases people with diabetes were not aware that they were exempted from payment and this lack of awareness or ‘knowledge of benefits’ resulted in lower attendance rates. 152,153Self-employed people with diabetes had additional hidden financial costs as they lost income when they took time off work to attend their screening appointments. 154,155
Theme: scheduling appointment issues
Issues with scheduling appointments, including a long wait to receive an appointment and an inability to get an appointment, were understandably perceived to be barriers to attendance. Three studies, all from the UK, mentioned that people with diabetes had not received an invitation for screening or had been given insufficient notice, making it impossible to attend. 155–157In several studies people with diabetes expressed a preference for appointment flexibility and wanted to be able to choose a time that suited them. However, in one study older people with diabetes expressed a preference for fixed appointments. 24Centrally allocated appointments were perceived by some HCPs as a barrier as they counteracted their own attempts to bring patients to the clinic, especially if administrative systems were thought to be inflexible. 158,159However, when there was no alternative the centralised systems were viewed as beneficial. 149
Theme: time (service issues)
Long waiting times on the day of the appointment and lengthy appointments were identified as barriers to attendance by both people with diabetes and HCPs. Multiple appointments scheduled back to back could result in people with diabetes ‘waiting around’ all day. 160In one study this was identified as being especially problematic for people with diabetes, because of lengthy food abstinence. 158
Theme: referral issues
Issues with referrals were identified as a substantial problem for some. In one UK study the authors described how a person with diabetes who normally attends her screening appointments had attempted to access screening through her general practice but was refused as she was in temporary accommodation waiting to be rehoused. 158In some countries there was a lack of referral because of an absence of eye doctors where patients were registered. 161
Theme: specialist diabetes services and staff
The integration of specialist diabetes services or the use of ‘one-stop shops’ was viewed as beneficial in a number of studies; in one study a person with diabetes reasoned that ‘if the eye appointment was on the same day as the DM [diabetes mellitus] appointment I would definitely attend’. 161Inflexible or incompatible administration systems were perceived to cause problems. Furthermore, a practice employing a specialist nurse was associated with increased attendance in two studies. 162,163
Theme: availability of dedicated screening resources
Diabetic retinopathy screening clinics with dedicated resources, such as non-mydriatic cameras, were reported to improve attendance in one study. 164However, in another study such clinics were reported to be problematic when the practices did not have appropriate space for the equipment without causing staff overcrowding and communication problems between the screeners and practice staff. 149Integrating screening and routine care was perceived by HCPs in one study as being too resource intensive. 149
Social influences
Theme: doctor–patient communication
Doctor–patient communication was discussed in many of the studies included in this review. For example, a recommendation by a HCP to attend screening was voiced as an enabler to attend in some studies165,166and the absence of a recommendation from a HCP was viewed as a barrier in others. 161,167HCPs in one study suggested that the best time to advise a person with diabetes to attend screening is at the time of their diagnosis. 152However, elsewhere it was noted that when a person with diabetes is first diagnosed they are likely to have other more pressing diabetes-related complications that the HCP needs to address and providing recommendations or prompts to attend screening can often go unnoticed. 168Some people with diabetes reported a general lack of information provision, especially at the point of diagnosis. 169
Another barrier identified within the theme of doctor–patient communication was language and/or communication style, in particular for people with diabetes whose first language was not the same as that of the HCP. In some studies people with diabetes reported language difficulties as the primary reason for not attending at screening appointments (e.g. Sachdevaet al. 156). In one study a person with diabetes complained that she ‘didn’t understand her physician and was too intimidated to ask him to slow down when conversing’ and therefore was unaware of the recommendation to change her behaviour. 154Although language difficulties were viewed as a barrier by both people with diabetes and HCPs there was no consensus as to their significance. 159In some studies, participants felt that systems were in place to overcome this barrier, such as accompanying family members and the provision of interpreters. 149,159,169However, in other studies HCPs pointed out that in multicultural societies many different languages are spoken within any given practice area149and that accompanying relatives might not have the language skills needed to interpret correctly. 159
Theme: trust in doctors
Advice and recommendations from doctors were perceived to be an enabler in several studies. 150,161,165–167,169,170,188Conversely, in one study it was reported that a small number of people with diabetes did not trust doctors170and in another study it was found that low confidence in doctors was more common among non-attenders than attenders. 167At the system level, perceived discrimination in the health-care system was associated with longer time periods between screening visits. 171Conversely, one study reported that a culturally sensitive community-based clinic overcame such barriers in a small sample of Aboriginal people with diabetes in Canada. 154
Theme: presence or absence of support from family members
Family support, both on a practical level (e.g. providing transport to the clinic) and on an emotional level (e.g. providing encouragement and offering gentle reminders), was viewed as an enabler of attendance. 149,168–170,188Furthermore, an absence of such support was perceived to be a barrier. 157Family support appeared to be especially important to people with diabetes from communities that traditionally rely on their family members to look after them169or when the person with diabetes had a physical disability. 168
Theme: encouragement/support from local community groups/networks plus media attention and coverage
Encouragingly, community-based programmes were seen to foster trust and support among attendees in one study. 154Not having to rely exclusively on doctors to encourage, support and remind people with diabetes to attend screening was seen as an enabler when local community groups and support networks were also available sources of support and information. 169,172Furthermore, local media (television, newspapers, radio channels) were highlighted as potential forums to raise awareness and promote attendance at screening169,172and it was suggested by one HCP that such a lack of media attention could be a contributing factor to low attendance rates. 158
Theme: stigma
Some people with diabetes spoke of social stigma being attached to a diabetes diagnosis169,188and in one study women talked about feeling ashamed and not wanting people to know about their health issues. 169HCPs also spoke about the difficulties of being confronted by a patient’s perceptions of stigma. 173
Knowledge
Theme: (lack of) awareness of illness
Several studies reasoned that a patient’s lack of knowledge about diabetes, diabetic retinopathy and the link between the two was a barrier to attendance. People with diabetes reported that an understanding of how diabetes can affect vision was an essential and motivating factor associated with attendance:
If I had realised the possibility that it would suddenly go [sight], that I wouldn’t realise that it was coming on, I think I would have taken more care. It’s all great in hindsight but if I knew then what I know now, then this problem wouldn’t have happened. 174
In several studies HCPs argued that people with diabetes do not always have a clear idea about the link between diabetes and diabetic retinopathy. However, there was also some suggestion that HCPs were not always happy to make the link clear and were careful not to alarm their patients: ‘I would never say to someone that there is a possibility that you could go blind from diabetes’. 174
Theme: (lack of) awareness of screening and confusion between screening and routine eye tests
Similarly, but unsurprisingly, a lack of awareness of the need to screen (including the recommended frequency and an understanding of the importance of attendance) was seen as a barrier to attendance and awareness was seen as an enabler. Furthermore, several study authors asserted that some people with diabetes were not aware of the difference between DRS and routine eye examinations, resulting in some patients believing that they had attended screening when they had not. 155–159,169,175
Theme: education and training
This theme highlighted the association between attendance at education or training classes and attendance at screening. Some studies suggested that receiving diabetes self-management or blindness prevention classes significantly increased the likelihood of attendance at screening,151,176,177whereas people with diabetes who had not received such an education were screened significantly less often. 178
Memory, attention and decision processes
Theme: symptoms
The absence of symptoms often resulted in patients making the decision not to attend for screening. This barrier was evident across different countries and screening contexts (e.g. UK, USA, Africa, Asia and Australia). In one study > 50% of people with diabetes who had never had a dilated eye examination indicated that it was because they did not have problems with their eyes179and in another study this barrier appeared to be especially relevant for men. 161It was reported by both people with diabetes and HCPs that a small number of people were not motivated to attend screening until they had experienced symptoms. 149,155,159,168However, even when symptoms were experienced some people with diabetes did not always link these to diabetic retinopathy but rather to an inevitable consequence of getting older. 168
Theme: competing health problems
Many people with diabetes experience competing health problems that at times can overshadow any concern that they might have about their eyes. For some people with diabetes, missing a screening appointment might simply be the result of a temporary illness or health problem156–158but for others it might be as a consequence of comorbidities24,147,169,175,180or because of the burden of diabetes and its treatments. 160,181
Theme: forgetting
For some people with diabetes, the failure to attend screening was attributed to forgetting. This theme included forgetting to make an appointment,24forgetting to attend157,159,160,180,183,190and forgetting whether or not they had previously attended for screening. 159Several studies alluded to HCPs’ attempts to prompt or remind people with diabetes in advance of their upcoming appointment149,159and some reported that reminders prompted patients to maintain regular attendance. 172
Theme: patients’ perception that they have been checked elsewhere
Three studies stressed that people with diabetes did not always attend their screening appointments as they believed that they had been or were going to be checked elsewhere. In one study people with diabetes indicated that they had not received a dilated eye examination because they had been transferring their eye care to another specialist;180in two other studies some of the people with diabetes indicated that they had not had their eyes examined by the ophthalmologist or optometrist in the previous year as their eyes had been examined by a family physician or optician. 158,182
Beliefs about consequences
Theme: short-term effects of screening
Some people with diabetes reported that screening has negative short-term effects that can be off-putting and in some cases a barrier to attendance. For example, some studies reported that people with diabetes dislike mydriatic eye drops (given to temporarily dilate the pupils) so much that they do not attend. 165,170,182–184These drops were often described as uncomfortable or in some cases painful. 158,168,185In one case it was reported that a woman had developed a phobia of these eye drops. 157
Mydriatic drops were perceived as being negative not only because of the discomfort they cause but also because of the inconvenience of not being able to see well enough because of the temporary effects that they have on vision. This effect was described as inconvenient not only because people with diabetes were prohibited from driving until the effects of the drops had worn off but also because the effects on vision can make it difficult to navigate public transport. 158,165Furthermore, it was evident that some people with diabetes believed that the screening procedure could have long-term negative effects on vision, as a consequence of either the drops or the retinal photographs. 156,158,169,186
Theme: perceived necessity of screening
People with diabetes do not always perceive screening to be necessary. In many studies both patients and HCPs reported that people with diabetes do not attend for screening as they believe that there is ‘no need’ or that it is ‘unnecessary’. 158,166,175,187,192Specific reasons given for holding such a belief included ‘I was told that my eyes are fine at my last screening’,24,159,170‘my diabetes is under control’161,168,181and ‘screening is not useful at my age’. 167However, other people with diabetes reported that screening was necessary as it provided important information on the health status of their eyes. Some people reported that believing that screening will identify problems early was a motivating factor for them158,170,183,185,188and others reported that they were motivated to attend as screening can provide reassurance that all is well. 155,170,189In two studies people with diabetes explained that they attended screening as family members had experienced problems with diabetes or retinopathy in the past and this salience was a motivating factor to attend. 158,183
Emotions
Theme: fear or anxiety
For some people with diabetes, the fear of losing their vision acted as a strong incentive to attend screening (e.g. Applebee,159Hartnettet al. 160and Dervanet al. 165). However, fear of a diabetic retinopathy diagnosis was perceived as a barrier to attendance for others (e.g. Lewiset al. ,174Pasagian-Macaulayet al. 185and Njambi190). People with diabetes also expressed fear or anxiety about the screening procedure157,188,204and some feared a medical intervention if they were confronted with a diagnosis. 148,191Interestingly, one study reported that non-adherent people expressed less concern about losing their vision than adherent people. 192
Theme: defensive responses
In a small number of studies defensive responses were noted. In one study the authors explained that, despite the fact that the young adults who participated in their study wanted to attend screening, they actively engaged in strategies that prevented them from doing so. 189In other studies people with diabetes would offer no explanation for their refusal to attend. 157,161,181One HCP reported that ‘[the patient] is refusing to even discuss his condition, so all you can do is keep sending invites’. 157
Theme: emotional burden of diabetes
There was a sense that for some people with diabetes there was an emotional burden from having diabetes. Attending screening appointments sometimes reminded patients that they had not been able to control their diabetes. This could sometimes exacerbate negative emotions including a sense of failure, guilt, fear and anger. 159,160,174
Domains identified as being less important
The content themes in the domains that were not identified as being factors of high importance in influencing screening attendance are described in further detail in the sections below.
Social/professional role and identity
Theme: illness identity
Some people with diabetes communicated that they did not identify with having diabetes. Both people with diabetes and HCPs reasoned that some patients might be in denial and had not yet accepted that they have been diagnosed with diabetes. 157,158,180,193Other people with diabetes felt uncomfortable with identifying as a person with diabetes. One young person expressed how they found it difficult to attend diabetes-related groups as the other patients were so much older than they were and they did not feel that they fitted in. 188In another study a person explained that they just wanted to be treated as normal: ‘I can’t help it. I don’t like being diabetic . . . I am as normal as anybody else; I can do what anybody else can do’. 174
Beliefs about capabilities
Theme: physical capability to attend
Both people with diabetes and HCPs suggested that some people did not have the physical capability to attend screening because of a physical disability. 167,194,195Some had limited personal mobility because of poor overall health or were housebound. 157,180Additionally, if a person required physical assistance to attend but none was available this was also noted as being a barrier. 147,148,161,196In one study it was reported that requiring an accompanying person was more likely for non-attenders than for attenders. 167
Goals
Theme: goal priorities
Some regular attenders prioritised the health of their eyes and it was this prioritisation that motivated them to attend screening. 158,183,190One person explained: ‘I want my vision because I want to see my grandchildren’. 170Others attended screening from a desire to stay in control of their health. 170However, for others eye health was not always a priority. 166,192,194This lack of priority was linked to the burden of having diabetes and the expectation of attending multiple appointments. 159,169One HCP was quoted as saying, ‘Having diabetes is very hard work and the patient has to have a lot of incentives to actively comply with everything that is expected of them’. 159
Intentions
Themes: do not want to go or nothing would stop me
In a number of studies people with diabetes declared that the reason that they did not attend screening was because they just did not want to go or had no interest in attending. 167,183–185Often there was no explanation for why they did not want to attend. However, in one case a patient was quoted as saying, ‘one endocrinologist I went to just said “do this, do that” and I did not want to. I did not understand why I needed to’. 181For others, little would prevent them from attending: ‘I would go no matter what, except if insurance doesn’t cover it, but the Lord would provide a way’. 170
Behavioural regulation
Theme: (lack of) engagement with self-management
In one study the authors reported that for every 10-point increase in self-rated diabetes management the odds of receiving an eye examination increased by 16%. 197Both people with diabetes and HCPs noted that some people might not attend screening as they were disengaged with their diabetes care. 157One HCP argued that attendance relied on people with diabetes being proactive as appointment times were not always convenient and patients might need to call up the screening service and change their appointment. 158People setting their own prompts was seen by HCPs as a positive behaviour. 159,172
Optimism, reinforcement and skills
The domains ‘optimism’ and ‘reinforcement’ were sparsely populated in terms of thematic content. SeeReport Supplementary Material 2(Themes/subthemes within each of the 14 domains from the Theoretical Domains Frameworks) for themes, frequencies and accompanying quotes. None of the data extracted from the studies were coded into the domain ‘skills’ at step 2.
UK studies
Twelve24,149,155–159,162,169,174,183,189of the 65 studies (18%) were carried out in the UK, three162,183,189of which were reported in abstract form only. Most of the key issues (themes) identified from studies in other countries were also identified in at least one study carried out in the UK. Table 10lists the themes/subthemes that were identified in (1) UK studies only, (2) UK studies and studies from other countries and (3) non-UK studies only.
| Themes or subthemes identified in UK studies only | Themes or subthemes identified in UK and other country studies | Themes or subthemes not identified in UK studies |
|---|---|---|
| Environmental context and resources | ||
|
|
|
| Social influences | ||
|
|
|
| Memory, attention and decision processes | ||
|
|
|
| Knowledge | ||
|
|
|
| Beliefs about consequences | ||
|
|
|
| Emotions | ||
|
|
|
The themes and domains most frequently identified within the UK studies were (1) scheduling appointment issues (environmental context and resources), identified in eight24,149,155–159,169of the 12 UK studies (67%), (2) accessibility of the screening clinic (environmental context and resources), identified in eight149,156–159,162,169,183of the 12 UK studies (67%), (3) time – competing demands (environmental context and resources), identified in eight149,155–158,174,183,189of the UK studies (67%), (4) confusion between screening and routine eye tests (knowledge), identified in six155–159,169UK studies (50%), (5) symptoms (memory, attention and decision processes), identified in six149,155,158,159,169,189UK studies (50%), (6) competing health problems (memory, attention and decision processes), identified in six24,149,156–158,169UK studies (50%), (7) awareness of illness (knowledge), identified in five155,158,159,169,174UK studies (42%), (8) doctor–patient communication (social influence), identified in five24,149,156,159,169UK studies (42%), (9) fear and anxiety (emotions), identified in five157–159,174,183UK studies (42%) and (10) forgetting (memory, attention and decision processes), identified in five24,149,157,159,183UK studies (42%).
Patient and health-care professional perspectives
All studies included in this review either reported findings from the patient’s perspective or tested a hypothesis based on patient data. Only 14 studies (22%) reported barriers and enablers from the perspective of the HCP. Most of these studies did not distinguish between the two perspectives and integrated the findings from people with diabetes and HCPs. Only four studies noted any clear differences between the two perspectives. 152,160,169,174In one study the authors reported that HCPs had cited inadequate patient education as the major barrier, yet claimed that participating patients largely reported that diabetes education was adequate and instead felt that financial concerns were their biggest barrier. 160In another study the author reported that, for the most part, patients’ and HCPs’ perspectives mirrored each other. 169However, they did note that views on whether or not language was a barrier differed. Although people with diabetes reported to some degree that language was a barrier, most reported that there were systems in place to overcome these issues. In contrast, HCPs (in this case screeners) reported that language was an issue that they faced in everyday practice. In a third study, people with diabetes and HCPs agreed that regular attendance could be inconvenient but suggested that HCPs underestimated the difficulties that people could face when taking time off work. 174In the fourth study the author explained that HCPs and people with diabetes face different barriers to annual screening. For the person with diabetes the biggest barrier was a lack of understanding of the importance of screening and a lack of awareness of insurance coverage. The HCPs, on the other hand, identified the need for patient education on eye examinations and suggested that greater assistance was needed with tracking the patient through the system. 152
Discussion
The aim of this review was to investigate how QI interventions might work by identifying the theoretical determinants of DRS attendance. This was achieved by identifying studies reporting primary data on barriers to and enablers of DRS attendance from the perspective of people with diabetes and associated HCPs and framing them in terms of theoretical domains from the TDF and CFIR.
Summary of findings
The combined content and framework analysis identified six TDF domains [(1) ‘environmental context and resources’, (2) ‘social influences’, (3) ‘knowledge’, (4) ‘memory, attention and decision processes’, (5) ‘beliefs about consequences’ and (6) ‘emotions’] as representing the most important factors potentially influencing screening attendance. Hence, the hypothesis arising from this review is that interventions that target these domains are more likely to be effective at increasing screening attendance. This process also identified three TDF domains that potentially have the least influence on screening: (1) ‘optimism’, (2) ‘reinforcement’ and (3) ‘skills’. Hence, we propose that interventions targeting these three domains are less likely to be effective at increasing screening attendance (seeTables 8and9).
Inductive analysis within domains generated specific content themes that may help to identify potential targets for future QI interventions. The content themes were identified at multiple levels, including the person with diabetes (e.g. confusion between screening and routine eye tests), the HCP (e.g. absence or presence of a HCP recommendation), the health-care system (e.g. inaccurate register) and the wider community (e.g. lack of media coverage) (seeTable 10). Recoding the themes from the TDF domains into CFIR constructs did not offer any further insights and, as a consequence, we did not apply the importance criteria to the CFIR constructs. The large majority of barriers and enablers were identified in the UK and also in the context of health systems in other countries (seeTable 10).
Strengths, limitations and challenges
The combination of deductive coding (informed by theoretical frameworks to guide barrier identification) and inductive analysis (to allow more granular content themes, unanticipated findings and patient insights to emerge) is a strength of this review. Furthermore, this relatively novel approach of applying multilevel theoretical frameworks within the systematic review context acknowledges the potential influence of organisational and contextual factors on screening attendance. We were able to code all extracted data from the 65 studies into the TDF domains, thus demonstrating that the TDF framework provided a comprehensive coverage of barriers and enablers.
Another strength of this review is its inclusiveness. We included published and grey literature, qualitative and quantitative methodologies, patients’ and HCPs’ perspectives and any context and/or screening model. We reasoned that limiting the search to UK studies only or to one methodological perspective would risk overlooking potentially important and relevant findings. Although not all barriers and enablers will be relevant to all settings, this review gives a comprehensive overview of potential factors that may influence screening attendance.
The studies in this review predominantly identified barriers and enablers from the perspective of the person with diabetes rather than from the perspective of the organisation or HCP. Even the data that we had from the HCPs mostly focused on their views regarding patient barriers. Therefore, the CFIR did not offer additional insights over and above those obtained from the TDF. The CFIR could potentially contribute an interesting perspective but this would involve a slight reconceptualisation of the components of the framework. For example, it is plausible that, by conceptualising the patient as part of the ‘system’ (rather than the ‘outer context’) and describing DRS in terms of ‘innovation characteristics’ (seeAppendix 7), the CFIR could offer additional insights over and above those obtained from the TDF. This kind of reconceptualisation would need further investigation and methodological work, which is outside the scope of this study.
Although there were no major methodological concerns that we believe would have biased the outcomes of the included studies, it was unfortunate that a number of the studies were poorly or thinly described. This was problematic as it hampered our ability to differentiate between HCPs’ and patients’ perspectives or to distinguish between different patient subgroups. Furthermore, the data extracted and analysed in the present review were the data that were reported, analysed and interpreted by the study authors. It is possible that our data set may have been biased in that authors may have selectively reported findings on perceived barriers/enablers that were more prevalent or interesting or that had a better fit with the stated research question. There may have been additional views on barriers/enablers in the full, original data set that were not represented in the present findings. A final limitation of this review worth noting is that the theoretical frameworks used are limited in that they do not specify relationships between domains and hence the strength of the direct impact of barriers on behaviour is not known.
Implications for research
The findings of this review have important implications for the design of effective interventions to promote DRS attendance as they can be used to assess the extent to which the active components (BCTs) identified in existing QI interventions are actually targeting the theoretical domains that are important in determining attendance. This can be achieved by mapping the BCTs identified in the review reported inChapter 3against the theoretical domains identified in the current review using established mapping tools. The results of the mapping study are reported inChapter 5.
Implications for practice
The findings from this review can help to inform a set of recommendations on which theoretical domains to target in interventions (i.e. ‘environmental context and resources’, ‘social influences’, ‘knowledge’, ‘memory, attention and decision processes’, ‘beliefs about consequences’ and ‘emotions’) and which to avoid (i.e. ‘optimism’, ‘reinforcement’ and ‘skills’), thus enhancing the likely effectiveness of DRS programmes. Furthermore, within the TDF domains judged to be of high importance, the findings from the inductive analysis provide more detailed insight into which specific barriers and enablers may be associated with attendance behaviour. Findings from this level of analysis suggest potential targets for future QI interventions. Four key recommendations based on the findings from the thematic synthesis are presented in the following sections.
Reduce inconvenience to people with diabetes
Many of the barriers and enablers identified within the domains of ‘environmental context and resources’ and ‘memory, attention and decision processes’ relate to perceptions of convenience. Difficulties with transportation, distance to the screening clinic, competing health and time demands, lack of instrumental/pragmatic support and scheduling appointment issues were all reported to be important factors that may hinder attendance, whereas attempts to reduce inconvenience by improving accessibility, having flexible appointments and integrating services were reported to facilitate attendance. Therefore, providing local screening facilities and ‘one-stop shops’ (integrating screening with other diabetes appointments), offering flexible appointment systems and childcare facilities and providing transportation may be advantageous.
Increase awareness of the importance of screening
Within the ‘knowledge’ domain both people with diabetes and HCPs reported that a lack of awareness or understanding of diabetes and diabetic retinopathy and the link between the two was a barrier to attendance. Similarly, a lack of awareness of the importance of screening and the recommended screening frequency and a lack of targeted patient education were also reported to be patient barriers, whereas providing blindness prevention programmes and general diabetes self-management education was reported to be an enabler. Within the ‘social influences’ domain the perceived absence of a HCP recommendation to attend screening and/or a lack of information provision from the HCP were also perceived to be patient barriers. Using local media and local community networks to improve awareness and promote attendance was reported as a potential but often untapped strategy.
Increase patients’ sense of comfort and support
Within the ‘emotions’, ‘social influences’ and ‘beliefs about consequences’ domains, some people with diabetes reported barriers relating to difficulties with communicating with HCPs, a lack of trust in doctors, a lack of emotional support and negative emotions (e.g. fear, worry). Although there were limited reports of potential enablers to overcome such barriers, there was some mention that community-based clinics, social/cultural compatibility between the person with diabetes and the HCP and compassion from the HCP were enablers that might encourage patients’ feelings of comfort, support and trust.
Improve message content
Within the ‘memory, attention and decision processes’ domain we found that the absence of symptoms was a common barrier to attendance. Furthermore, within the ‘beliefs about consequences’ domain we found that some people with diabetes perceived that DRS was not necessary, especially if they felt that their diabetes was under control, they were not old or their previous test result was clear. Therefore, it would seem desirable to provide messages that highlight the asymptomatic nature of diabetic retinopathy and make salient the potential consequences if left unchecked. Likewise, providing messages that highlight the benefits of early detection, the safety of the procedure and the reassurance that a positive result can provide would all be desirable as they were identified within the ‘beliefs about consequences’ domain. In addition, a barrier identified within the ‘knowledge’ domain related to the confusion between DRS and routine eye tests. Messages that highlight the difference between the two and emphasise the importance of continuing to attend for DRS despite attendance at other eyes tests could be helpful. Furthermore, messages emphasising that DRS is a routine part of diabetes care could also be helpful as this belief was identified as an enabler within the ‘memory, attention and decision processes’ domain. The offer of a reminder to attend for DRS was also regarded as an enabler addressing this domain.
Recommendations for future research
In this review only 12 studies were conducted in the UK. Additional high-quality studies are therefore needed from the UK, from the perspective of both HCPs and people with diabetes. Ideally, such additional research would focus on sampling the subgroups that are potentially at the highest risk of blindness or that are least likely to attend for screening, as the pattern of important domains may differ between these subgroups depending on specific patient characteristics (e.g. location, age or socioeconomic status).
Future research could endeavour to identify which theoretical domains are most important for the subgroups that have been identified as being most at risk and/or least likely to attend for screening. For example, only two studies identified in this review explored factors that affect young adults. 188,189This patient group is not only under-researched but also at high risk of vision loss/blindness from diabetic retinopathy.
It is possible that the less frequently identified domains (e.g. ‘skills’, ‘intentions’, ‘optimism’) may simply not be as commonly assessed in quantitative work or are less likely to be spontaneously mentioned in response to open questions in interviews. Although such an occurrence may itself be an indication of low importance, it may be interesting to carry out a comparison of the results of this study against the results of more comprehensive assessments that identify not only omissions of these factors but provide evidence which shows that they have been assessed empirically and have been found to have no association with DRS attendance.
Concluding remarks
This review employed a systematic, theory-informed and replicable approach to identify barriers and enablers associated with DRS attendance. Six TDF domains were identified as having high importance and therefore are the theoretical domains most likely to be key mediators of DRS attendance behaviour. Thematic synthesis identified key content themes that offer further insights into which specific issues need to be addressed. Future research is needed to identify which domains are most important for subgroups of people with diabetes who have been identified as being most at risk.
Chapter 5 Do intervention components target theoretical determinants of diabetic retinopathy screening attendance? Mapping behaviour change techniques to barriers and enablers
Background
The benefits of designing interventions based on theory are widely recognised. 212Theory provides an explanatory, integrated summary of the proposed determinants of behaviour and the mechanisms involved in behaviour change. 213A wide range of BCTs can be applied to change the various theoretical determinants of behaviours. 214However, different BCTs are likely to be more, or less, effective at addressing different types of determinants. For example, the technique ‘behavioural rehearsal/practice’ is likely to be effective when the barrier is a lack of skill to perform the behaviour. This represents high theoretical coherence (i.e. match) between the intervention component and the theoretical determinant it targets. However, the same technique is unlikely to be effective where the determinant is a lack of motivation to perform the behaviour,214due to low theoretical coherence (i.e. mismatch) between the intervention component and the determinant it targets. It has been argued that interventions are more likely to be effective if they include components that specifically target the important theoretical determinants of behaviour and behaviour change. 214,215The research reported in this chapter addresses the question, ‘Do existing interventions to improve DRS attendance address the known barriers to attendance?’
The components of the existing interventions to increase DRS attendance that were included in the first systematic review (seeChapter 3) were identified and characterised using a validated taxonomy: the BCTTv1. 36The subgroup analyses reported inChapter 3identified BCTs that were associated with higher screening attendance. The theoretical determinants (e.g. barriers/enablers) of DRS attendance were identified in the second systematic review, reported inChapter 4. The TDF of behaviour37,39was used to classify reported barriers to and enablers of screening attendance from the perspectives of people with diabetes and HCPs. Important barriers to and enablers of screening attendance were identified (e.g. ‘emotions’ and ‘beliefs about consequences’ of screening) (seeChapter 4). In this chapter we explore the level of theoretical coherence between techniques in DRS interventions and theoretical domains in the barriers literature. We use the term ‘theoretical coherence’ to denote the extent to which a BCT included in existing DRS interventions targets an important theoretical domain. For example, high coherence would be demonstrated if the BCT ‘provide information on health consequences of the behaviour’ was identified as an intervention component in interventions included in the first systematic review and ‘knowledge’ was identified as an important domain in the second review. In this instance, interventions including this BCT would address the relevant theoretical determinant of the target behaviour. Conversely, low coherence would be displayed if the first systematic review identified the BCT ‘material reward’ as an intervention component, yet the second systematic review identified the theoretical domain ‘reinforcement’ to be of low importance to screening attendance.
This study therefore aimed to integrate the findings from the two reviews (seeChapters 3and4) to identify the extent to which frequently used BCTs address important barriers to screening attendance. To explore this in a systematic way, we used a structured tool to map BCTs against domains from the TDF. This tool was developed by integrating two published instruments that map BCTs against domains in the TDF, on the basis of expert consensus data,214,216and is presented inReport Supplementary Material 3(seeBehaviour change techniques theoretically coherent with important domains). Both matrices propose numerous theoretically coherent BCT and domain pairings, which achieved expert agreement that the BCTs are likely to be effective at addressing a given domain [e.g. the BCT ‘social support (practical)’ is theoretically coherent with the domain ‘social influences’].
This kind of tool is frequently used to design interventions by identifying BCTs to target important barriers to health-related behaviour change. For instance, Cadoganet al. 217conducted semistructured interviews based on the TDF with community pharmacists and GPs to examine barriers to/enablers of prescribing and dispensing of appropriate polypharmacy to older patients. Eight important domains were identified and the mapping tool216was used to select four BCTs for inclusion in an intervention for GPs or pharmacists (e.g. the use of the technique ‘prompts and cues’ to address the important barrier domain ‘memory, attention, decision processes’).
However, although BCT taxonomies and the TDF have both been recently applied in secondary analyses as part of systematic reviews,134,218to our knowledge no systematic review has reported the use of a mapping tool to identify the theoretical coherence in the manner described above. Evidence of coherence can facilitate interpretation of observed intervention effectiveness (or ineffectiveness). For example, if a BCT–domain pair has high coherence and coherence is associated with effectiveness, this can help to explain how interventions work to achieve target outcomes and why some interventions are more effective than others. Similarly, if coherence is associated with effectiveness, then BCT–domain pairs with low coherence would arguably be best avoided when designing DRS attendance interventions. Furthermore, such mapping between BCT and barriers or enablers may highlight potential ‘missed opportunities’ for intervention design, in the form of BCTs that are likely to be effective in targeting a given barrier or enabler, but which are not currently used in existing interventions. However, if coherence is not associated with effectiveness, this would suggest that there are limitations in the validity of the mapping tool approach. The objective of this research was thus to explain the findings from review 1 (seeChapter 3) and to propose possible enhancements to DRS attendance interventions.
Aims
This study aimed to integrate the findings fromChapters 3and4by applying a mapping tool to assess the theoretical coherence of existing DRS interventions, that is, the extent to which BCTs used in interventions (seeChapter 3) target the identified important theoretical determinants of screening attendance (seeChapter 4).
The specific research questions were:
-
What is the level of theoretical coherence of the intervention literature relating to DRS, that is, to what extent do BCTs frequently used in existing interventions to increase DRS attendance target the important barriers to attendance?
-
To what extent is observed theoretical coherence associated with intervention effectiveness, that is, higher screening attendance?
-
Are there any potential ‘missed opportunities’ for intervention design in terms of BCTs that are not frequently used in existing interventions but which are theoretically coherent with important barriers?
Methods
Sample
Data from the first systematic review (seeChapter 3) relevant to the work presented in this chapter included (1) the frequency with which each BCT was identified as present in intervention arms targeting people with diabetes and/or HCPs and, for those BCTs that were frequently identified (i.e. in ≥ 10 intervention arms), (2) the observed association between the BCT and intervention effectiveness (i.e. higher DRS rates in terms of RD, 95% CI). Findings from the review of perceived barriers to and enablers of screening attendance (seeChapter 4) relevant to the work presented in this chapter included the domains identified as important to screening attendance. Important domains (ranked in the top six) were ‘environmental context and resources’, ‘social influences’, ‘knowledge’, ‘memory, attention, and decision processes’, ‘beliefs about consequences’ and ‘emotions’ (seeTable 9).
Materials: mapping tool
A mapping tool was developed by integrating two previously published tools214,216(seeReport Supplementary Material 3). The integrated tool was used to identify the BCTs that are theoretically coherent with important domains (i.e. the domains that, arguably, should be used; presented inReport Supplementary Material 3,Behaviour change techniques theoretically coherent with important domains). The tool was also applied to the DRS literature to identify the domains that have been targeted by frequently used BCTs (i.e. the barriers that have been targeted in interventions; presented inReport Supplementary Material 3,Domains targeted by frequently used behaviour change techniques).
Procedure and analysis
Exploration of theoretical coherence between BCTs identified in the first review (seeChapter 3) and TDF domains identified in the second review (seeChapter 4) was conducted through two, bidirectional mapping processes, structured around the three research questions.
Research question 1: theoretical coherence of published diabetic retinopathy screening interventions
Frequently used BCTs were defined as those identified in a minimum of 10 patient and/or HCP intervention arms (as inChapter 3). The integrated tool was then used to identify which domains, according to expert consensus, the frequently used BCT would be potentially relevant to and effective at addressing. A BCT could be paired with more than one domain. Each pairing was classified as having high or low theoretical coherence. A pair was deemed to have high coherence if the frequently used BCT targeted an important domain (fromChapter 4). BCTs paired with more than one domain in the integrated mapping tool were classified as having high coherence if at least one domain was important, regardless of the importance classification of the remaining paired domains. Conversely, a BCT was deemed to have low coherence if it targeted only (i.e. was paired with only) domains of lower importance.
Research question 2: association between theoretical coherence and intervention effectiveness
Effect estimates for frequently used BCTs were obtained from the subgroup analyses reported inChapter 3(seeFigures 12and13). The relationship between BCT effectiveness and coherence was explored by comparing the numbers of reliably effective and ineffective BCTs that had high or low theoretical coherence.
Research question 3: ‘missed opportunities’ for intervention design
For the second mapping process, the integrated mapping tool was used to identify the BCTs paired with the six most important domains. ‘Missed opportunity’ was noted if the recommended BCT paired with the important domain was infrequently identified in published interventions (i.e. ≤ 10 patient and/or HCP intervention arms). Conversely, ‘opportunity seized’ was noted if the recommended BCT was frequently identified (i.e. ≥ 10 patient and/or HCP intervention arms).
Results
Interventions included a median of four BCTs targeting people with diabetes (range 0–16) and three targeting HCPs (range 0–14) (seeFigures 6and7). Ten BCTs were frequently identified in at least 10 interventions targeting people with diabetes and seven were frequently identified in at least 10 interventions targeting HCPs (Table 11and seeFigures 12and13).
| BCT | Intervention arms,n | RD (95% CI) | I 2 | Linked TDF domains according to integrated tool (maximumn = 14) | Domain importance rankinga | Coherence between BCT and domain |
|---|---|---|---|---|---|---|
| BCTs targeting people with diabetes | ||||||
| Goal-setting (outcome) | 14 | 0.26 (0.16 to 0.36) | 93 | Goals | 8 | |
| Skills | 14 | Low | ||||
| Behavioural regulation | 11 | |||||
| Feedback on outcomes of behaviour (including biofeedback) | 15 | 0.22 (0.15 to 0.29) | 94 | Beliefs about capabilities | 9 | |
| Beliefs about consequencesb | 5 | High | ||||
| Knowledgeb | 3 | |||||
| Credible source | 10 | 0.22 (0.06 to 0.38) | 95 | Beliefs about consequencesb | 5 | |
| Social influencesb | 2 | High | ||||
| Prompts and cues | 25 | 0.11 (0.07 to 0.14) | 92 | Memory, attention, decision makingb | 4 | High |
| Behavioural regulation | 11 | |||||
| Environmental context and resourcesb | 1 | |||||
| Social support (unspecified) | 14 | 0.19 (0.09 to 0.28) | 93 | Social influencesb | 2 | |
| Social professional role/identity | 7 | High | ||||
| Beliefs about capabilities | 9 | |||||
| Goals | 8 | |||||
| Problem-solving | 10 | 0.17 (0.08 to 0.27) | 89 | Skills | 14 | |
| Beliefs about capabilities | 9 | Low | ||||
| Goals | 8 | |||||
| Restructuring the social environment | 17 | 0.17 (0.10 to 0.24) | 85 | Environmental context and resourcesb | 1 | |
| Social influencesb | 2 | High | ||||
| Instruction on how to perform the behaviour | 35 | 0.13 (0.11 to 0.15) | 94 | Knowledgeb | 3 | |
| Skills | 14 | High | ||||
| Social support (practical) | 20 | 0.14 (0.09 to 0.20) | 90 | Social influencesb | 2 | High |
| Social/professional role and identity | 7 | |||||
| Beliefs about capabilities | 9 | |||||
| Goals | 8 | |||||
| Information about health consequences | 19 | 0.12 (0.07 to 0.16) | 92 | Knowledgeb | 3 | High |
| Beliefs about consequencesb | 5 | |||||
| Goals | 9 | |||||
| BCTs targeting HCPs | ||||||
| Restructuring the social environment | 23 | 0.19 (0.12 to 0.26) | 91 | Environmental context and resourcesb | 1 | |
| Social influencesb | 2 | High | ||||
| Credible source | 13 | 0.16 (0.08 to 0.24) | 95 | Beliefs about consequencesb | 5 | |
| Social influencesb | 2 | High | ||||
| Adding objects to the environment | 15 | 0.14 (0.07 to 0.20) | 88 | Environmental context and resourcesb | 1 | High |
| Social support (practical) | 10 | 0.13 (0.03 to 0.22) | 87 | Social influencesb | 2 | High |
| Social/professional role and identity | 7 | |||||
| Beliefs about capabilities | 9 | |||||
| Goals | 8 | |||||
| Instruction on how to perform the behaviour | 30 | 0.13 (0.08 to 0.17) | 93 | Knowledgeb | 3 | |
| Skills | 14 | High | ||||
| Prompts and cues | 15 | 0.12 (0.06 to 0.17) | 85 | Memory, attention, decision makingb | 4 | High |
| Behavioural regulation | 11 | |||||
| Environmental context and resourcesb | 1 | |||||
| Feedback on outcomes of behaviour (including biofeedback) | 17 | 0.11 (0.07 to 0.16) | 81 | Beliefs about capabilities | 9 | High |
| Beliefs about consequencesb | 5 | |||||
| Knowledgeb | 3 | |||||
Research question 1: theoretical coherence of existing diabetic retinopathy screening interventions
Overall, high levels of theoretical coherence between frequently used BCTs in existing interventions and important theoretical domains were observed. The majority (80%) of frequently used BCTs in interventions targeting people with diabetes and all (100%) frequently used BCTs in HCP interventions were paired with at least one important domain (fromChapter 4). Only two BCTs in interventions targeting people with diabetes were paired exclusively with domains of lesser importance and were thus deemed to have low theoretical coherence: ‘goal-setting (outcome)’ and ‘problem-solving’ (seeTable 11).
Research question 2: association between theoretical coherence and intervention effectiveness
The subgroup analyses reported inChapter 3(seeFigures 12and13) identified that all BCTs frequently used in patient and/or HCP interventions were associated with higher DRS attendance (seeTable 11). Higher effect estimates were observed for the BCTs ‘goal-setting (outcome)’ and ‘credible source’ in interventions targeting people with diabetes and for the BCTs ‘restructuring the social environment’ and ‘credible source’ in interventions targeting HCPs (seeFigures 12and13). However, with the exception of ‘goal-setting (outcome)’ (p = 0.001), differences in effect estimates between BCTs were not statistically significant (seeTable 5). All frequently used BCTs were associated with effectiveness, regardless of whether they had high or low theoretical coherence. However, the majority (n = 15/17) of statistically significant BCTs had high theoretical coherence according to the integrated mapping tool.
Research question 3: ‘missed opportunities’ for intervention design
Table 12presents the frequency with which BCTs paired with important TDF domains were identified in existing DRS interventions. Overall, the BCTs that paired with the six most important domains have not been used frequently in existing interventions targeting DRS attendance. This finding indicates numerous missed opportunities for intervention design. Opportunity seized was highest for the domains ‘memory, attention and decision processes’ (50% of the theoretically coherent BCTs were frequently used in interventions) and ‘knowledge’ (43% of the theoretically coherent BCTs were frequently used). The most missed opportunities were for the domain ‘emotions’ (no BCTs paired with this domain were frequently used in existing DRS interventions (range 0–3 intervention arms) (seeTable 11).
| BCTs paired with domain as per the merged tool | BCT frequency,nstudies | Mapping opportunity missed vs. opportunity seized for intervention design |
|---|---|---|
| Environmental context and resources | ||
| Restructuring the physical environment | 4 | Missed |
| Discriminative (learned) cue | 0 | Missed |
| Prompts/cues | 48 | Seized |
| Restructuring the social environment | 47 | Seized |
| Avoidance/changing exposure to cues for the behaviour | 0 | Missed |
| Social influences | ||
| Social comparison | 11 | Seized |
| Social support (unspecified) | 20 | Seized |
| Social support (emotional) | 3 | Missed |
| Social support (practical) | 34 | Seized |
| Information about others’ approval | 1 | Missed |
| Vicarious reinforcement | 0 | Missed |
| Restructuring the social environment | 47 | Seized |
| Identification of self as a role model | 0 | Missed |
| Social reward | 1 | Missed |
| Demonstration of the behaviour | 6 | Missed |
| Knowledge | ||
| Information on health consequences | 25 | Seized |
| Biofeedback | 36 | Seized |
| Information about antecedents | 0 | Missed |
| Feedback on behaviour | 20 | Seized |
| Information on social/environmental consequences | 4 | Missed |
| Information on emotional consequences | 1 | Missed |
| Salience of consequences | 4 | Missed |
| Memory, attention and decision processes | ||
| Self-monitoring of behaviour | 4 | Missed |
| Self-monitoring of outcome of behaviour | 8 | Missed |
| Action-planning | 12 | Seized |
| Prompts and cues | 35 | Seized |
| Beliefs about consequences | ||
| Information about emotional consequences | 1 | Missed |
| Salience of consequences | 4 | Missed |
| Covert sensitisation | 0 | Missed |
| Anticipated regret | 0 | Missed |
| Information about social/environmental consequences | 4 | Missed |
| Pros and cons | 0 | Missed |
| Vicarious reinforcement | 0 | Missed |
| Threat | 0 | Missed |
| Comparative imagining of future outcomes | 0 | Missed |
| Self-monitoring of behaviour | 4 | Missed |
| Self-monitoring of outcome of behaviour | 8 | Missed |
| Information on health consequences | 25 | Seized |
| Feedback on behaviour | 20 | Seized |
| Feedback on outcomes of behaviour | 36 | Seized |
| Credible source | 30 | Seized |
| Emotions | ||
| Reduce negative emotions | 0 | Missed |
| Information about emotional consequences | 1 | Missed |
| Self-assessment of affective consequences | 0 | Missed |
| Social support (emotional) | 3 | Missed |
| Conserving mental resources | 0 | Missed |
Discussion
This study applied a systematic approach to examine the extent to which components of interventions to increase DRS attendance target the important theoretical determinants of DRS attendance. This was achieved by integrating the findings on BCTs identified in existing interventions from the first systematic review (seeChapter 3) with the important theoretical domains identified from the studies of perceived barriers to/enablers of DRS attendance from the second review (seeChapter 4). An integrated tool merging two published BCT and domain mapping tools was applied to explore the level of theoretical coherence between currently used BCTs and important TDF domains. ‘High coherence’ was defined as the frequent use of BCTs that target important TDF domains. ‘Low coherence’ was defined as the frequent use of BCTs that target less important TDF domains. Missed opportunities for intervention design were noted as infrequent use of BCTs that are paired with, and which are potentially relevant to targeting, important domains. Findings on levels of coherence were used to address three research questions.
Research question 1: theoretical coherence of existing diabetic retinopathy screening interventions
Overall, there is a high degree of theoretical coherence between the components of existing DRS attendance interventions and the important theoretical determinants of screening attendance. Nearly all component BCTs frequently included in existing interventions were paired with at least one of the six most important theoretical domains representing the key barriers to or enablers of DRS attendance. This suggests that existing interventions may work as they are targeting important factors influencing whether or not people with diabetes attend DRS.
Research question 2: association between theoretical coherence and intervention effectiveness
It was not possible to formally evaluate the association between theoretical coherence and effectiveness as all frequently used BCTs were associated with improved screening attendance. This suggests that all BCTs are effective regardless of whether they have high or low theoretical coherence. However, the majority (88%) of frequently used BCTs with significant effect estimates were classified as having high theoretical coherence. It is possible that intervention designers more frequently select BCTs with high coherence because they are aware of theory and/or evidence or because they intuitively select BCTs that are more likely to address relevant barriers to and enablers of screening attendance.
Implications of mapping frequently used behaviour change techniques against theoretical domains
Complex interventions to change health-related behaviours often consist of multiple, interacting intervention components that are poorly specified. 219These interventions are also often developed without an explicit theoretical rationale for the selection of a particular intervention strategy or intervention component. 220This in turn renders it difficult to interpret intervention outcomes, identify the specific ‘active ingredients’ contributing to effectiveness or identify the pathways through which these active ingredients might have their effect. Identifying areas of high theoretical coherence for BCTs with demonstrated effectiveness can thus shed light on the mechanisms through which existing interventions might improve DRS attendance. Furthermore, the present findings may facilitate replication and inform the design of future interventions to increase DRS attendance or the enhancement of existing interventions. To maximise likely effectiveness, interventions may benefit from the inclusion of BCTs that are presently identified as being highly coherent with the important theoretical determinants of screening attendance.
The present findings may also inform intervention design in terms of how BCTs are operationalised and delivered. According to the integrated tool, the majority of BCTs with high theoretical coherence were paired with multiple domains, of which only one was typically classified as important. These BCTs were effective, despite also targeting theoretical domains that were less important to DRS attendance. It may be that these BCTs were effective because their specific content targeted the more important domain, rather than the less important domains. For example, the BCT ‘feedback on outcomes of behaviour’ maps onto both the domain ‘beliefs about consequences’ and the domain ‘beliefs about capabilities’. The BCT may have been associated with higher screening attendance because it contained information related to the consequences of screening attendance (domain ‘beliefs about consequences’) rather than providing motivational feedback aiming to boost self-efficacy (domain ‘beliefs about capabilities’), that is, the BCT may have targeted the important domain, ‘beliefs about consequences’. Indeed, examples of instances in which this BCT was coded in the first review suggest that this may have been the case: ‘immediately after receiving the dilated eye exam, the patient was told the results by the examining ophthalmologist’ (patient feedback)108and ‘the reviewers entered their findings into electronic forms within the telemedicine system, and the system automatically sent the evaluation reports to the clinics via email or fax’ (HCP feedback). 116Such findings may thus point to potential means of designing the delivery of specific BCTs to increase theoretical coherence and thus potential effectiveness.
Behaviour change techniques with low theoretical coherence
Two BCTs had low theoretical coherence for patient interventions: ‘problem-solving’ and ‘goal-setting (outcome)’. We had proposed that the inclusion of BCTs that target the less important domains of screening attendance may be less effective and dilute the impact of BCTs that do coherently map onto important theoretical determinants of DRS attendance. However, we found that the BCT ‘goal-setting (outcome)’ was significantly associated with the greatest effect estimates and also had low theoretical coherence. The observed low coherence may result from differences between how this BCT was operationalised in existing interventions included in the first review and how it was investigated in the second review. Goal-setting in existing interventions was typically related to general diabetes self-management or lifestyle behaviour change, for example ‘Participants developed a self-management action plan . . . They next selected a behaviour change goal in the area of smoking, diet, or exercise’ (patient goal-setting)73and ‘all practices were instructed to target the same values [glycated haemoglobin, systolic blood pressure, low-density lipoprotein]’ (HCP goal-setting). 58In contrast, the second review focused on understanding the role of the domain ‘goals’ specifically in the context of DRS attendance. Thus, the difference in focus and content of goal-setting between the data sets from both reviews may undermine the validity of the mapping process for this technique.
Despite low coherence, it is possible that ‘goal-setting (outcome)’ was effective when directed at people with diabetes for a number of other reasons. For example, attendance for DRS is only one of numerous, inter-related behaviours involved in general diabetes self-management. Thus, goal-setting around general diabetes self-management may have still been highly effective at increasing DRS attendance, despite not directly targeting it, because of spillover effects resulting from the setting of general self-management goals for other related behaviours. In addition, our method investigates BCTs in isolation rather than in combination. Interventions typically contain multiple BCTs. It is possible that goal-setting could interact or work synergistically with other BCTs, which could enhance its potency.
Research question 3: ‘missed opportunities’ for intervention design
Our second mapping process investigated the extent to which theoretically coherent BCT–domain pairs have been used in existing interventions to increase DRS attendance. We identified that the inclusion of BCTs that are potentially relevant to the important domains is limited and variable in existing interventions. All six important domains had at least one theoretically coherent BCT that was not frequently used in existing interventions. Such BCTs thus represent potential ‘missed opportunities’ for intervention design. Addressing these missed opportunities may thus increase intervention effects.
Specifically, all BCTs paired with the domain ‘emotions’ were used not at all, or only very infrequently, in existing interventions. There is thus a specific priority for the inclusion in future interventions and research of BCTs that target the emotional determinants of screening attendance. For instance, an important theme within the ‘emotions’ domain was fear/anxiety about vision loss. For some people with diabetes, the fear of losing their vision acted as a strong incentive to attend screening (e.g. Applebee,159Hartnettet al. 160and Dervanet al. 165). However, for others, fear of a diabetic retinopathy diagnosis and of the screening procedure were perceived barriers to attendance. 174,185,190This barrier to screening attendance could be addressed through the delivery of the BCTs ‘reduce negative emotions’, ‘information on health consequences of behaviour’ and ‘anticipated regret’, by providing information on treatment options for diabetic retinopathy. Such information could focus on providing reassurance by emphasising that effective treatments are available following early detection. The ‘missed opportunities’ identified through this second mapping process therefore represent a theoretically informed means of identifying BCTs that may be of greater relevance and effectiveness.
Strengths
The strengths of the current study include the transparent approach to systematically assessing the extent to which intervention components target theoretical determinants of behaviour. The use of available BCT × TDF domain mapping tools in a systematic review context represents a methodologically innovative aspect of this work and provides an example of how these recently developed tools may be further applied in secondary analyses of existing interventions. This systematic approach also demonstrates how these frameworks may be applied to identify limitations of, and missed opportunities in, the design of existing interventions,221and thus offers clear implications for future intervention refinement, design and research. In turn, this systematic approach has clear implications for future intervention design, refinement and research.
Additionally, to our knowledge, this is the first study to provide empirical findings that can be used to evaluate the validity of the proposed BCT and domain pairings in the mapping tools, which are based on expert opinion and consensus. Although based on a limited sample size, the frequently used BCTs that were effective and that map onto key TDF domains provide empirical support for the agreed BCT and domain pairings. Conversely, frequently used BCTs that were effective but that do not map onto key TDF domains with high coherence potentially refute the recommendations in the mapping tools. Given the high proportion of effective BCTs achieving high theoretical coherence, there is a trend towards a supportive pattern of findings for the proposed pairings in the available tools.
Limitations
An important caveat when interpreting these findings and a limitation to this methodological approach concerns the validity and applicability of the mapping tools. There are discrepancies across, and limitations of, the published tools132,216on which we based the integrated mapping tool. Hence, low observed coherence may not reflect the ‘true’ level of theoretical coherence and instead may be a product of limitations regarding the validity of the materials used to identify coherence. For instance, one may query if the BCT ‘goals’ is conceptually applicable to the domain ‘skills’ or if the BCT ‘feedback on outcomes of the behaviour’ is conceptually applicable to the domains ‘beliefs about consequences’ and ‘beliefs about capabilities’ (seeReport Supplementary Material 3,Behaviour change techniques theoretically coherent with important domains). It is also surprising that the BCTs involving ‘feedback’ are not paired with the domain ‘behavioural regulation’, given that feedback is regarded as the archetypal self-regulation strategy. 218,222,223Further research is thus needed to empirically evaluate and refine these mapping tools.
Furthermore, current BCT–domain mapping tools do not distinguish between different contexts, populations and types of behaviour, whereas theoretical coherence may depend on the nature of the behaviour and the population being investigated.
Conclusions
The first aim of this study was to assess the theoretical coherence of existing DRS interventions, that is, the extent to which BCTs used in interventions (seeChapter 3) target the identified important theoretical determinants of screening attendance (seeChapter 4). We identified high levels of theoretical coherence between frequently used BCTs in interventions to increase DRS attendance and important barriers to and enablers of screening attendance. The second aim was to identify whether high theoretical coherence was associated with intervention effectiveness (i.e. higher screening attendance). Although the large majority (88%) of frequently used BCTs with significant effect estimates had high theoretical coherence, it was not possible to formally evaluate the association between theoretical coherence and effectiveness, as all frequently used BCTs were associated with improved screening attendance. The third aim was to identify potential ‘missed opportunities’ for intervention design, in terms of infrequently used BCTs that are theoretically coherent with important barriers. A number of additional BCTs may be relevant to addressing important theoretical determinants of screening attendance that are not included in existing interventions. There are particular gaps around the inclusion of intervention components to address the emotional barriers to and enablers of screening attendance. Inclusion of such components in the design of future interventions, or the enhancement of existing interventions, may help to further improve DRS attendance and in turn ensure the timely referral and treatment of patients who test positive for diabetic retinopathy. There is also a need for further empirical research to evaluate the validity of mapping tools to facilitate the selection of coherent BCTs for future research and intervention design.
Chapter 6 Economic model
Introduction
The first objective of the economic analysis was to identify which QI and BCT components of interventions used to encourage attendance for DRS are most likely to be cost-effective and therefore which BCT or QI components should be prioritised for further research into interventions that encourage DRS attendance. The QI and BCT taxonomies used to code intervention components are described inAppendices 4and5respectively.
A further objective was to estimate the cost threshold below which an intervention designed to increase DRS attendance would be cost-effective at different levels of effectiveness. The systematic review reported inChapter 3identified which BCT and QI components were included in the main interventions in the RCTs. The QI components were included in one economic analysis and the BCT components were included in another economic analysis.
To address these objectives in a timely manner an existing economic model was used as the basis of the model reported here. This model was used to estimate the cost–utility of BCT and QI components of interventions that aimed to increase DRS attendance. Single BCT and QI components were compared in the model in terms of their cost-effectiveness. BCT or QI combinations were not evaluated. Although this is unrealistic in terms of an intervention, because of the way that the effectiveness of these components has been derived (as described inIntervention effect estimates), it does serve to illustrate the relative economic merits of each component and indicate which components could be prioritised in future research to develop interventions to increase DRS uptake and provide a benchmark against which local services could be judged with regard to whether or not they represent value for money. The BCTs and QI components evaluated were those that met the inclusion criteria for the meta-analyses reported inChapter 3; thus, only BCT and QI components that were recorded as intervention components in at least 10 studies were included in the economic analysis.
The model included a front end, which modelled the probability of DRS based on the intervention used to encourage uptake, and a back end, which modelled the long-term outcomes of DRS attendance and non-attendance. The long-term outcomes were based on data identified from a published economic model adapted for this analysis. 224
A literature search identified the published economic evaluations that could potentially provide the costs and benefits associated with DRS. The cost-effectiveness of DRS has been assessed in numerous studies. 61,225–227A published economic model of screening intervals for diabetic retinopathy, recently developed for the English and Welsh setting, with detailed published data to enable the reproduction of the model for the purpose of this analysis, was selected. This was a NIHR HTA report on the optimisation of DRS intervals by Scanlonet al. 224This study did not report cost and utility estimates for DRS strategies across a range of baseline probabilities of DRS attendance, but it provided sufficient information to model the long-term outcomes of DRS attendance and non-attendance and this enabled the cost-effectiveness of BCT and QI components to be estimated at different baseline probabilities of DRS attendance. A simplified model was produced from the data reported by Scanlonet al. 224and the probabilities of DRS attendance and the cost of BCT and QI components were modelled using the results of the effectiveness and cost analyses described in this chapter.
A full description of the economic methods is provided inReport Supplementary Material 4(seeEconomic model). This includes a full description of the model structure of the screening programmes and the progression of diabetic retinopathy disease, the costs and utilities associated with the model states and the effectiveness and cost estimates of the interventions. A summary of the key elements of the methods is reported here.
The economic model
It was not necessary to perfectly reproduce the analysis reported in Scanlonet al. 224as our objective was to evaluate the cost-effectiveness of interventions to improve DRS attendance. However, the essential features of the models are the same, as are the key assumptions, and the interested reader is referred back to the report by Scanlonet al. 224for a detailed description of these. For the same reason we have not reviewed all of the assumptions that underpin the Scanlonet al. 224model. We rely on sensitivity analyses to explore the significance of substantial changes to the model. The model compares the different BCT and QI components and used evidence from meta-analyses (and associated estimates of imprecision) to estimate their relative effectiveness and costs. The Scanlonet al. 224model modelled a cohort of people with diabetes based on a Gloucestershire Diabetic Eye Screening Service cohort of patients. The cohort represented all people within the screening programme. For simplicity, this analysis modelled a cohort of patients with the median values of the cohort modelled in the Scanlonet al. 224model (median age was 64 years). Although it is acknowledged that a lot of people will be eligible for a DRS programme at a much earlier age, a simple approach was required given the objectives and scope of this study, which was to identify the QI components and BCTs that are most likely to be cost-effective. As this age was not reflective of people offered DRS, it was varied in sensitivity analysis. Diabetic retinopathy is considered to consist of different stages affecting one or both eyes and people with diabetic retinopathy may progress or regress between them.
A pictorial representation of the front end of the model is provided inFigure 19. If an individual attends DRS there will be an opportunity to receive treatment, which will result in different costs and quality of life from those for an individual who did not attend DRS but who would have required treatment. An intervention to increase DRS attendance increases the probability that an individual will attend for screening and hence changes the health service costs and quality of life. Within the model the health outcomes of attending DRS were measured in QALYs, as this is the standard metric for informing the allocation of resources in the NHS in the UK.
FIGURE 19.
Pictorial representation of the initial stages of the economic model.
Diabetic retinopathy screening occurs at regular intervals; currently in the UK, annual screening is recommended. The results of the Scanlonet al. 224cost–utility analysis identified DRS every 3 years as the most cost-effective frequency for patients with diabetes and no preproliferative diabetic retinopathy or proliferative diabetic retinopathy or diabetic maculopathy. However, the UK National Screening Committee228has recommended that the screening interval for diabetic retinopathy should change from 1 year to 2 years for those at low risk (based on two screening episodes with no detected diabetic retinopathy). One-year intervals are recommended for those having any diabetic retinopathy in either of two previous screening episodes. The base-case model in our analysis assumed annual DRS screening, with 2-yearly and 3-yearly DRS used in sensitivity analyses. A positive screening episode was classified as screening positive for preproliferative diabetic retinopathy, proliferative diabetic retinopathy or diabetic maculopathy.
The outcomes of attending DRS and non-attendance are presented inFigure 20. Once an individual attends DRS he or she receives a positive or a negative test result. Individuals receiving a negative result from the initial screen or those not attending any of their appointments in a given screening period are invited for DRS a year later. Individuals with a positive result are referred to Hospital Eye Services (HES), where appropriate ophthalmic assessment takes place. The HES tests are assumed to have perfect sensitivity and specificity in the model. Once referred to HES, patients might or might not attend.
FIGURE 20.
Screening pathway for diabetic patients offered DRS. HES, Hospital Eye Services. Reproduced from Figure 15 of Scanlonet al. 224Contains information licensed under the Non-Commercial Government Licence v1.0.
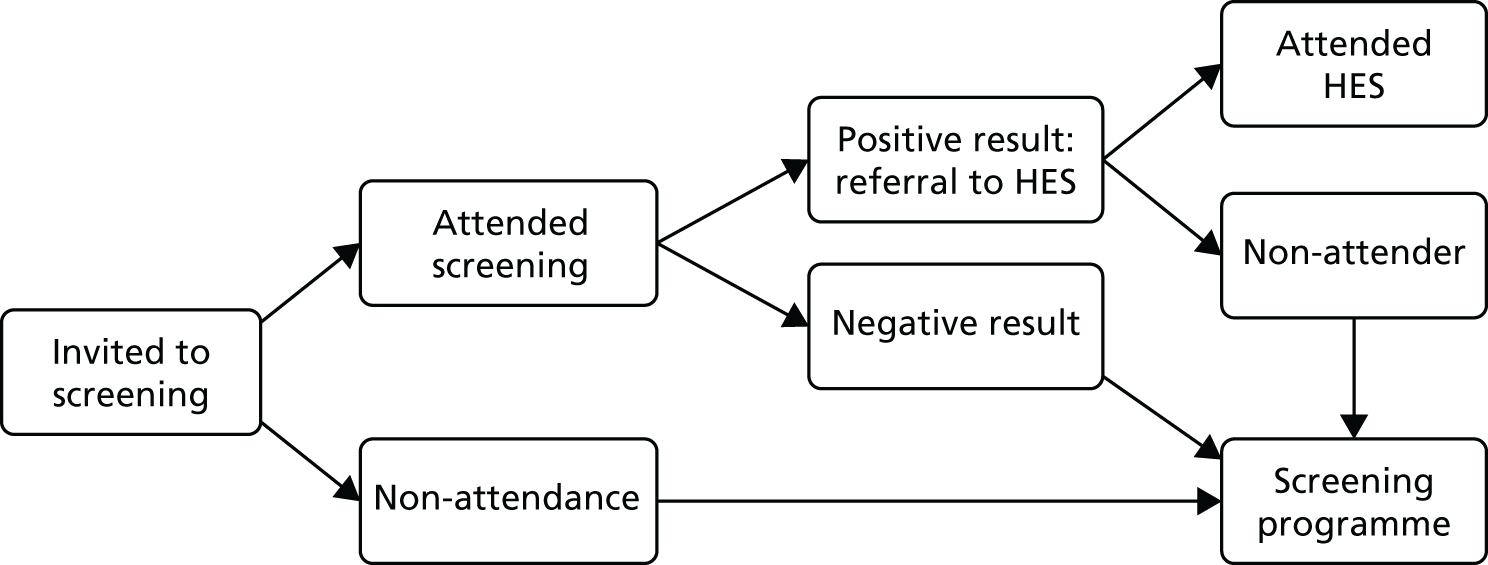
Following diagnosis, if an individual attends the HES and is diagnosed with preproliferative diabetic retinopathy, proliferative diabetic retinopathy or diabetic maculopathy, they may or may not be offered treatment. An individual who is offered treatment then re-enters the DRS programme and has the opportunity to be screened at the next screening interval. An individual who does not receive treatment is either monitored every 6 months in the HES or re-enters the DRS programme and has the opportunity to be screened at the next screening interval. The costs and utilities for the model states were derived from econometric analyses of the data set obtained from the Gloucestershire Diabetic Eye Screening Service and other sources, as described inReport Supplementary Material 4.
Intervention effect estimates
The effectiveness results reported in the included clinical studies are the effect estimates for the complex interventions studied. This economic analysis investigated the cost-effectiveness of individual components of these complex interventions and therefore effect estimates for individual QI components and BCTs were estimated by adjusting for other components. This is in contrast to the analysis inChapter 3, which sought to estimate mean effects for interventions that included a specific component (compared with interventions that did not include it).
A meta-regression analysis with multiple explanatory variables representing the different QI components was conducted for the effects of the QI components on the log OR of screening attendance. A separate analysis was conducted for the BCTs. Both the patient- and HCP-targeted techniques were included in the same regression analyses for both the resource use and the effect analyses. Although this is a large number of explanatory variables, there is a predefined set of competing interventions and there is no reason to exclude one over the other. In addition, no statistical tests were conducted as part of this analysis. These results in terms of the effect of QI and BCT components should be interpreted as additive, whereas the results inChapter 3are not additive.
The studies varied in their populations and other characteristics. Most studies were conducted outside the UK. Furthermore, there were insufficient data to model interaction effects between the BCTs and between the QI components. Consequently, the effect estimates are not precise estimates for a specific UK population. The purpose was to provide the parameter estimates for the cost-effectiveness model, which evaluates the probability that each QI/BCT is cost-effective accounting for each QI/BCT simultaneously. This can help prioritise further research by identifying the most promising intervention components and developing interventions utilising them.
Behaviour change techniques or QI components were excluded from the analysis if there were insufficient data to estimate a coefficient, if there was collinearity (overlap of studies between indicator variables) or if there were perfect predictions. As with the meta-analyses reported inChapter 3, only BCTs and QI components that occurred in at least 10 studies were included in the economic model and only these results are reported here. These were considered to be less prone to a spurious result. It is likely that there is variation between interventions that are coded as a particular QI or BCT. It is also likely that different approaches to implementing a QI or BCT intervention are present when QI components and BCTs are coded in different studies. Random-effects meta-regressions were performed in Stata®15 (StataCorp LP, College Station, TX, USA). The dependent variable was the log OR. Fifty-six studies58–63,65–72,74–78,80–89,92–105,108–112,115–118,120–123with a usual care comparator were included in the analyses.
The full set of regression results is presented inReport Supplementary Material 4. The results transformed into RRs with 95% CIs for the QI components that occur in at least 10 studies and the BCTs that occur in at least 10 studies are reported inTables 13and14respectively. The ordered resource ranking analysis that appears inTables 13and14is explained inResource use and cost estimates.
| QI components | Ordered resource ranking: proportional RR (95% CI) | Effect meta-regression: RR (95% CI) |
|---|---|---|
| Audit and feedback | 1.22 (0.79 to 1.38) | 0.99 (0.78 to 1.16) |
| Case management | 1.40 (1.28 to 1.42) | 0.87 (0.67 to 1.05) |
| Team changes | 1.26 (0.97 to 1.38) | 1.14 (1.00 to 1.24) |
| Electronic patient registry | 0.69 (0.17 to 1.23) | 1.01 (0.74 to 1.21) |
| Clinician education | 0.89 (0.42 to 1.24) | 1.06 (0.89 to 1.19) |
| Clinician reminders | 1.26 (0.73 to 1.40) | 1.08 (0.83 to 1.25) |
| Patient education | 0.80 (0.38 to 1.18) | 1.09 (0.92 to 1.22) |
| Promotion of self-management | 1.28 (0.85 to 1.40) | 1.12 (0.93 to 1.26) |
| Patient reminders | 0.64 (0.24 to 1.09) | 1.02 (0.84 to 1.16) |
| BCT components | Likert resource ranking: proportional RR (95% CI) | Effect meta-regression: RR (95% CI) |
|---|---|---|
| Patient-targeted BCTs | ||
| Problem-solving | 1.37 (1.03 to 1.42) | 0.95 (0.73 to 1.13) |
| Goal-setting (outcome) | 1.27 (0.57 to 1.41) | 1.24 (1.10 to 1.32) |
| Feedback on outcomes of behaviour/biofeedback | 0.59 (0.13 to 1.19) | 1.17 (1.02 to 1.27) |
| Social support (unspecified) | 0.15 (0.01 to 0.81) | 1.07 (0.87 to 1.22) |
| Social support (practical) | 1.29 (0.75 to 1.41) | 0.95 (0.76 to 1.11) |
| Instruction on how to perform the behaviour | 1.38 (1.09 to 1.42) | 0.89 (0.70 to 1.06) |
| Information about health consequences | 0.17 (0.02 to 0.76) | 1.15 (1.00 to 1.25) |
| Prompts/cues | 0.17 (0.02 to 0.81) | 0.91 (0.73 to 1.07) |
| Credible source | 0.38 (0.01 to 1.33) | 0.85 (0.56 to 1.10) |
| Restructuring the social environment | 0.80 (0.05 to 1.40) | 0.82 (0.58 to 1.03) |
| HCP-targeted BCTs | ||
| Feedback on outcomes of behaviour/biofeedback | 0.51 (0.12 to 1.11) | 0.85 (0.68 to 1.01) |
| Social support (practical) | 1.42 (1.30 to 1.43) | 1.27 (1.10 to 1.35) |
| Instruction on how to perform the behaviour | 0.90 (0.28 to 1.32) | 0.81 (0.61 to 0.99) |
| Prompts/cues | 1.34 (0.67 to 1.42) | 0.96 (0.67 to 1.18) |
| Credible source | 0.89 (0.22 to 1.34) | 0.95 (0.73 to 1.13) |
| Restructuring the social environment | 1.42 (1.33 to 1.43) | 1.13 (0.94 to 1.25) |
| Adding objects to the environment | 0.19 (0.02 to 0.93) | 0.98 (0.76 to 1.15) |
In the model, the baseline probability of DRS attendance was based on a minimum standard set by the UK National Screening Committee,229not on the probabilities in the usual care arms in the RCTs. The probability of DRS attendance varied in a sensitivity analysis. Details of how the effect estimates were utilised within the economic model are provided inReport Supplementary Material 4.
Resource use and cost estimates
The level of the cost of resource use in each included study was of interest for two purposes in this study. First, in the economic analysis, we were interested in estimating the cost of the individual components of the complex interventions included in each study rather than the cost of the complex interventions. This requires a form of multivariable regression with the QI and BCT components as explanatory variables, as used in the intervention effectiveness analysis. Second, inChapter 3the association between resource use and intervention effectiveness was investigated. InChapter 3the average effectiveness was calculated at different levels of resource use and resource use intensity was added as a covariate in a meta-regression.
A measure of resource use intensity was needed for each study. As there were 56 studies with a usual care comparator, an efficient method was required to derive this measure. The approach taken was to design a data abstraction method for recording predefined key categories of resource use and the levels of each category. The details are provided inReport Supplementary Material 4. The process of determining the resource categories and levels involved agreement between reviewers on an ordered ranking of resource use for each study. The cost of each level of each resource category was then estimated through random sampling of at least three studies with each category and level of resource use, costing the resource use associated with the intervention description using national cost estimates and calculating the average. The total cost of a complex intervention in each study is the product of the resource categories and estimated costs for each category. The cost variable was therefore categorical and the applicable regression method to estimate the incremental cost of each QI/BCT component was an ordinal logistic regression, with the ordered resource use ranking as the dependent variable.
The costs of the interventions from the 56 included studies were distributed across a total of 16 ordered ranks, which were utilised in the economic model. InChapter 3these 16 ranks were put into five groups to facilitate the analysis of an association between resource use and intervention effectiveness. The grouping was as follows: ranks 1–3 became rank 1; ranks 4–6 became rank 2; ranks 7–9 became rank 3; ranks 10–12 became rank 4; and ranks 13–16 became rank 5.
The cost analysis for the economic model differs from the work conducted in the review of economic outcomes reported inChapter 3in that the economic model utilises unit cost estimates for England and Wales to value the described interventions rather than converting the costs reported in the studies from one currency to another. Arguably, such data would be more transferable as the resources required to provide an intervention may not vary between settings.
It was assumed that all people with diabetes would be eligible for DRS and that BCT/QI interventions would target all eligible people. It is noted that some of the evidence on the effectiveness of BCT/QI interventions came from studies targeting those most likely not to attend DRS. It was not possible to control for this factor in the meta-regression data and we have assumed a constant effect. However, we have explored the impact of different baseline uptake rates of DRS to illustrate the relative impact of BCTs/QI components when the baseline uptake is lower (seeBase-case and sensitivity analyses).
Resource use may be incurred at different organisational levels. It may differ between individuals or at the GP or practice level or even be determined at the level of the NHS. It was assumed that all software development and the design of educational pamphlets would be financed at a higher organisational level than, for example, a general practice, for simplicity taken as the national level. This assumes that a country takes advantage of economies of scale. The total numbers of people with diabetes (n = 2,913,538),230general practices (n = 8151) and GPs (n = 40,265)231in England were used to derive a cost per patient for each intervention. The price year was 2016.
Details of how the ordered resource use and cost estimates were incorporated in the model are provided inReport Supplementary Material 4(seeEconomic model).
Analyses and interpretation
Point estimates of incremental cost-effectiveness
To identify the interventions that are most likely to be cost-effective at a specific cost-effectiveness threshold, £20,000 per QALY in the main analyses, the estimated costs and effectiveness of each intervention were incorporated into the model and the total costs (including the long-term cost of health service use) and benefits (measured in QALYs) were calculated for intervention and no intervention. The results at a threshold of £30,000 per QALY were also calculated and are reported inAppendix 10. Interventions with an ICER of < £20,000 per QALY are commonly considered to be cost-effective according to National Institute for Health and Care Excellence guidelines. 232A cost of £30,000 per QALY is quoted as the upper value for the plausible range of willingness-to-pay thresholds.
Probabilistic analysis
The cost-effectiveness results depend on all of the model parameters listed inResource use and cost estimates. As explained above, only uncertainty in the effect and cost parameters of the BCTs and QI components was included in the model. The imprecision in estimates of the effect and cost of the BCTs and QI components was accounted for by conducting probabilistic sensitivity analyses. This involved simultaneously sampling from probability distributions assigned to each model parameter. For example, the normal distribution for the log OR effect of each intervention was derived from the point estimate and standard error of the relative effectiveness and constant reported in the meta-regression analyses. Uncertainty associated with regression model specification could not be accounted for. For example, there were insufficient data to model interactions between BCTs and QI components in the multiple ordered logit regression for resource use and the multivariable meta-regression for effectiveness.
Although the Scanlonet al. 224model accounted for uncertainty in all of the model parameters, in this model no uncertainty was accounted for in the parameters related to the outcomes of being screened or not being screened.
Two sets of results were produced from the probabilistic sensitivity analyses. The first is the probability that each intervention is cost-effective compared with no intervention at a specific cost-effectiveness threshold of £20,000 per QALY. The results at a threshold of £30,000 per QALY were also calculated and are reported inAppendix 10.
The model estimates a long-term benefitBifor interventioniand a long-term benefitBnifor no interventionni. The model also estimates a total costCifor intervention, which includes the long-term cost of health service use and the cost of intervention. The total cost of long-term health service use for no intervention wasCni.
For a specific cost-effectiveness thresholdγ(£20,000 per QALY in the main analyses), the incremental net benefit of the intervention compared with no intervention was calculated:
Given that the purpose of this analysis was to identify the BCT and QI components that are most likely to be cost-effective and therefore most worthy of further consideration when developing an intervention, the absolute costs, benefits and net benefits are not reported in the results section. What is of most interest is the probability that each BCT/QI is cost-effective at a specific cost-effectiveness threshold given the available evidence.
The probability that the incremental net benefit is positive was derived from the probabilistic sensitivity analysis. For example, consider the comparison of an intervention and no intervention. A probability of 0.5 indicates that the intervention and no intervention are equally likely to be considered cost-effective. A probability of 0.95 indicates that the intervention is highly likely to be the cost-effective option. A probability of 0.05 indicates that the no-intervention option is highly likely to be the cost-effective option.
The second set of results is the probabilityxthat each intervention is the most cost-effective, accounting for all of the interventions and no intervention simultaneously. First, the net benefit is calculated for each intervention, as described in the following equation:
Second, the proportion of times that the net benefit of interventionXhas the highest net benefit is the probability that interventionXis cost-effective relative to the other interventions. The probabilityxis a measure of certainty and the complement (1 – x) is the error probability. The highest probability does not necessarily indicate that the corresponding intervention is expected to be the most cost-effective; it means that it is the most likely to be the most cost-effective of all of the interventions compared.
It should also be noted that the more comparators there are, the lower the probabilities are likely to be for any given intervention. For example, if 10 interventions are each equally likely to be cost-effective then for each intervention the probability of being cost-effective would be 0.1. As the objective of this analysis is to identify which of the BCT/QI components is the ‘best bet’ in economic terms, to consider further when developing an intervention, it is the relative size of the probabilities that is important.
Threshold analysis
This analysis identified the maximum cost that an intervention must have for any given level of effectiveness of DRS such that society’s maximum willingness to pay for a QALY is not exceeded. Thus, this provides us with a maximum amount that we should devote to increasing the uptake of DRS for any expected uptake rate of DRS that we would expect to achieve.
A hypothetical intervention was assumed to have a hypothetical clinical effectivenessφ, which was associated with a long-term benefitBi. The long-term benefit of a no-intervention alternative wasBni. The hypothetical intervention was assumed to have a hypothetical intervention costω, which was associated with a total costCi, which includes the long-term cost of health service use. The total cost of long-term health service use of no intervention wasCni. For a specific cost-effectiveness thresholdγ, £20,000 per QALY in the main analyses, the incremental net benefit of the intervention compared with no intervention was calculated:
For each level of assumed intervention effectivenessφ, the value of the intervention costωwas varied in a threshold analysis. The highest value ofωwas identified that resulted in a positive incremental net benefit for each value ofφ. The highest cost for each level of effectiveness then creates a maximum cost curve. The maximum cost curve was then utilised to obtain the maximum cost associated with the mean estimate of effect for each BCT/QI and with the lower and upper limits of the 95% CI. The results of this analysis are reported inAppendix 11.
Base-case and sensitivity analyses
The base-case model assumed that:
-
screening would be conducted annually
-
the baseline probability of screening uptake would be 0.7
-
a cohort of 64-year-olds is modelled
-
the probability of a patient being monitored is 0.78 following HES referral and a positive diagnosis
-
there are no social care cost implications.
Sensitivity analyses to evaluate the impact of different scenarios on the results were conducted for the probabilistic analyses.
Annual DRS is currently the most common screening frequency in the UK. The results of the Scanlonet al. 224cost–utility analysis identified DRS every 3 years as the most cost-effective screening frequency for patients with diabetes and no preproliferative diabetic retinopathy or proliferative diabetic retinopathy or diabetic maculopathy. However, the UK National Screening Committee228has recommended that the screening interval for diabetic retinopathy should change from 1 year to 2 years for those at low risk (based on two screening episodes with no detected diabetic retinopathy). One-year intervals are recommended for those having any diabetic retinopathy in either of two previous screening episodes. Consequently, sensitivity analyses were conducted assuming that screening was carried out every 2 years and every 3 years, with the latter analysis conducted to help benchmark the results with those from Scanlonet al. 224
The diabetic eye screening (DRS) uptake rate was 83.1% in England in 2015 based on data from the 81.9% of diabetic eye screening programmes that reported data against the new DRS pathway for 2015. 233The minimum standard set by the UK National Screening Committee234is at least 70%, with an achievable objective of at least 80%. The base-case analysis used a baseline uptake rate of 70% based on the minimum standard. Sensitivity analyses were conducted assuming screening uptake rates of 50%, 60%, 80% and 90%. These rates were chosen to represent rates that may apply to the UK and specific subgroups eligible for screening in the UK. For example, and as noted earlier, the lower rates may be more applicable to subgroups less likely to engage with the DRS service. The economic model used in this analysis was a simplified version of the Scanlonet al. 224model, which utilised the data reported in the HTA report. An interquartile range was reported for the baseline age of the screening cohort in the Scanlonet al. 224model. This indicates that a heterogeneous population may have been modelled. For simplicity, in this base-case analysis a cohort of 64-year-olds enters the model at the start of the analysis (64 years was the median age in the baseline population in the Scanlonet al. 224model). To understand the effect on the results of modelling baseline populations with different ages, sensitivity analyses were conducted with cohorts of 56-year-olds and 70-year-olds.
In the Scanlonet al. 224model, in the base-case analysis it was assumed that, of the people who are not treated following a positive diagnosis of preproliferative diabetic retinopathy, proliferative diabetic retinopathy or diabetic maculopathy, the probability that a patient would be monitored was 0.78. This assumption was also made in this base-case analysis. In sensitivity analysis the probability was reduced to 0.4.
As described earlier, the cost of social care was excluded in the base-case model. In sensitivity analysis it was assumed that 10% of people with diabetic maculopathy in both eyes lived in nursing/residential homes.
Sensitivity analyses for the threshold analysis to derive the maximum cost curve were informed by the sensitivity analyses conducted for the probabilistic analyses.
Results
Quality improvement results
The base-case analysis includes a baseline probability of DRS attendance of 0.7. The probability that each QI component is more cost-effective than no QI intervention at improving attendance at DRS is presented inTable 15. All of these results are based on effectiveness and cost analyses that adjust for the other QI components. They provide an indication of which QI components are worth prioritising over which others when designing a DRS attendance intervention, which is likely to consist of a combination of QI components. The results of the sensitivity analyses including baseline DRS attendance probabilities of 0.5, 0.6, 0.8 and 0.9 are also reported inTable 15. These results assume a societal willingness-to-pay threshold for a QALY of £20,000. The results assuming a threshold of £30,000 per QALY are reported inAppendix 10.
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| Audit and feedback | 0.329 | 0.229 | 0.109 | 0.031 | 0.001 |
| Case management | 0.012 | 0.005 | 0.000 | 0.000 | 0.000 |
| Team changes | 0.764 | 0.625 | 0.360 | 0.041 | 0.001 |
| Electronic patient registry | 0.536 | 0.479 | 0.418 | 0.319 | 0.084 |
| Clinician education | 0.652 | 0.587 | 0.491 | 0.307 | 0.038 |
| Clinician reminders | 0.530 | 0.421 | 0.246 | 0.069 | 0.010 |
| Patient education | 0.769 | 0.714 | 0.644 | 0.473 | 0.084 |
| Promotion of self management | 0.653 | 0.530 | 0.299 | 0.051 | 0.004 |
| Patient reminders | 0.573 | 0.531 | 0.463 | 0.344 | 0.065 |
The results are presented as the probability that a QI component is cost-effective at a specific willingness-to-pay threshold. SeeAnalyses and interpretationfor an explanation of these results.
Table 15shows that there is considerable uncertainty in the results when considering which interventions are cost-effective compared with no intervention. In pairwise comparisons, a probability of > 0.95 could be considered highly likely to be cost-effective and a probability of > 0.7 could be considered to show some evidence of cost-effectiveness, but with significant uncertainty. There are areas of uncertainty that are not captured in these results but the results indicate which QI components are worth prioritising when developing interventions from a cost-effectiveness perspective. In the base case, with a baseline probability of DRS attendance of 0.7, there are no QI components with a probability of cost-effectiveness of > 0.9. Patient education has the highest probability of being cost-effective (0.64).
The greater scope for increasing DRS attendance with a lower baseline probability of screening attendance is reflected in the higher probabilities of cost-effectiveness for each QI component compared with no QI intervention at lower baseline probabilities of screening attendance. Even at a baseline probability of DRS attendance of 0.5, only ‘team changes’ and ‘patient education’ have a probability of being cost-effective of > 0.7. As the baseline probability of DRS attendance reduces from 0.7 to 0.5, the probability of being cost-effective increases more for ‘team changes’ than for ‘patient education’. This is because resource use is estimated to be much less for ‘patient education’ than for ‘team changes’ (see proportional RRs of resource use ranking inTable 13).
The probability of each QI intervention being cost-effective when all QI factors are compared simultaneously is reported inTable 16. The QI components with the highest probabilities of cost-effectiveness inTable 15also have the highest probabilities of cost-effectiveness inTable 16. WhatTable 16shows is that, among the QI components with the highest probabilities of being cost-effective, there is no single QI component that stands out as being far more likely to be cost-effective. Patient education has the highest probability of being cost-effective and this is only 0.31 in the base case.
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| No intervention | 0.034 | 0.063 | 0.121 | 0.270 | 0.783 |
| Audit and feedback | 0.015 | 0.009 | 0.008 | 0.005 | 0.000 |
| Case management | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Team changes | 0.133 | 0.092 | 0.042 | 0.011 | 0.001 |
| Electronic patient registry | 0.129 | 0.149 | 0.159 | 0.167 | 0.071 |
| Clinician education | 0.122 | 0.131 | 0.140 | 0.115 | 0.026 |
| Clinician reminders | 0.086 | 0.075 | 0.048 | 0.023 | 0.008 |
| Patient education | 0.280 | 0.293 | 0.314 | 0.261 | 0.062 |
| Promotion of self management | 0.119 | 0.097 | 0.048 | 0.018 | 0.003 |
| Patient reminders | 0.082 | 0.090 | 0.120 | 0.128 | 0.047 |
Sensitivity analyses for scenarios including different probabilities of monitoring following HES referral attendance, different DRS frequencies and different ages of the baseline cohort and including and excluding social care costs are presented inTable 17.
| Intervention | Screening every year, Pm = 0.78, cohort age 64 years, no care costs | Screening every year, Pm = 0.4, cohort age 64 years, no care costs | Screening every 2 years, Pm = 0.78, cohort age 64 years, no care costs | Screening every 3 years, Pm = 0.78, cohort age 64 years, no care costs | Screening every 3 years, Pm = 0.4, cohort age 64 years, no care costs | Screening every year, Pm = 0.78, cohort age 70 years, no care costs | Screening every year, Pm = 0.78, cohort age 56 years, no care costs | Screening every year, Pm = 0.78, cohort age 64 years, care costs |
|---|---|---|---|---|---|---|---|---|
| Audit and feedback | 0.109 | 0.126 | 0.374 | 0.453 | 0.422 | 0.079 | 0.066 | 0.181 |
| Case management | 0.000 | 0.001 | 0.027 | 0.075 | 0.048 | 0.000 | 0.000 | 0.001 |
| Team changes | 0.360 | 0.434 | 0.809 | 0.883 | 0.872 | 0.271 | 0.196 | 0.555 |
| Electronic patient registry | 0.418 | 0.439 | 0.549 | 0.570 | 0.555 | 0.415 | 0.393 | 0.468 |
| Clinician education | 0.491 | 0.517 | 0.693 | 0.729 | 0.705 | 0.442 | 0.415 | 0.562 |
| Clinician reminders | 0.246 | 0.294 | 0.590 | 0.664 | 0.660 | 0.194 | 0.163 | 0.374 |
| Patient education | 0.644 | 0.667 | 0.800 | 0.814 | 0.805 | 0.611 | 0.597 | 0.706 |
| Promotion of self management | 0.299 | 0.371 | 0.708 | 0.784 | 0.783 | 0.232 | 0.178 | 0.471 |
| Patient reminders | 0.463 | 0.488 | 0.596 | 0.616 | 0.618 | 0.434 | 0.405 | 0.505 |
Monitoring may occur if treatment is not provided after a positive diagnosis following HES referral attendance. In the base-case analysis it was assumed that 78% of people who are not treated following a positive diagnosis of preproliferative diabetic retinopathy, proliferative diabetic retinopathy or diabetic maculopathy are monitored. If this proportion is reduced to 40% then the probability of a given QI component being cost-effective compared with no QI intervention increases when DRS is scheduled every year.
The probability of an intervention being cost-effective is higher when DRS is carried out every 3 years than when it is carried out every 2 years, which in turn is higher than when DRS is carried out every year. This is consistent with the results of the Scanlonet al. 224cost–utility analysis, which identified screening every 3 years as being the most cost-effective frequency of screening.
In the base-case analysis it was assumed that the age of the cohort being screened was 64 years. If either a younger (age 56 years) or older (age 70 years) cohort is considered then the probability of an intervention being cost-effective compared with no intervention decreases. The cost-effectiveness of the intervention decreases at age 70 years because the potential cumulative benefit associated with the intervention decreases. Specifically, the likelihood of attending a referral to the HES decreases, the utility of an individual decreases with age and mortality increases. The quality of life benefits and potential cost savings increase with age. Starting DRS at an earlier age increases the duration that a benefit may be realised for an individual, but the greatest benefits occur a longer time after the start of DRS and these are discounted more heavily.
For other scenarios considered, the results described inTable 17indicate that there remains considerable uncertainty. However, for a cohort of people aged 64 years, increasing the screening interval to 2 or 3 years means that the probability that ‘team changes’ and ‘patient education’ would be considered cost-effective would be > 0.8 compared with no intervention. Similarly, increasing the screening interval to 3 years for the same cohort would mean that the probability that ‘promotion of self-management’ and ‘clinician education’ are cost-effective compared with no intervention would increase to > 0.7.
Behaviour change technique results
The base-case analysis includes a baseline probability of DRS attendance of 0.7. The probability that each BCT component would be more cost-effective than no BCT intervention in terms of improving attendance at DRS is presented inTable 18. The BCT components are listed as being either patient targeted or HCP targeted. All of these results are based on effectiveness and cost analyses that adjust for the other BCT components. They provide an indication of which BCT components are worth prioritising over which others when designing a DRS attendance intervention, which is likely to consist of a combination of BCTs. The results of the sensitivity analyses including baseline DRS attendance probabilities of 0.5, 0.6, 0.8 and 0.9 are also reported inTable 18. These results assume a societal willingness-to-pay threshold for a QALY of £20,000. The results assuming a threshold of £30,000 per QALY are reported inAppendix 10.
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| Patient-targeted BCTs | |||||
| Problem-solving | 0.677 | 0.556 | 0.264 | 0.023 | 0.001 |
| Goal-setting (outcome) | 0.999 | 0.992 | 0.964 | 0.558 | 0.151 |
| Feedback on outcomes of behaviour/biofeedback | 1.000 | 0.999 | 0.997 | 0.961 | 0.672 |
| Social support (unspecified) | 0.988 | 0.985 | 0.980 | 0.964 | 0.793 |
| Social support (practical) | 0.784 | 0.688 | 0.416 | 0.100 | 0.006 |
| Instruction on how to perform the behaviour | 0.514 | 0.335 | 0.117 | 0.012 | 0.000 |
| Information about health consequences | 1.000 | 1.000 | 0.999 | 0.994 | 0.936 |
| Prompts/cues | 0.877 | 0.873 | 0.841 | 0.748 | 0.400 |
| Credible source | 0.658 | 0.638 | 0.563 | 0.465 | 0.191 |
| Restructuring the social environment | 0.562 | 0.501 | 0.386 | 0.250 | 0.055 |
| HCP-targeted BCTs | |||||
| Feedback on outcomes of behaviour/biofeedback | 0.728 | 0.705 | 0.613 | 0.476 | 0.088 |
| Social support (practical) | 0.982 | 0.786 | 0.388 | 0.049 | 0.001 |
| Instruction on how to perform the behaviour | 0.534 | 0.477 | 0.350 | 0.191 | 0.014 |
| Prompts/cues | 0.676 | 0.599 | 0.362 | 0.089 | 0.009 |
| Credible source | 0.863 | 0.818 | 0.714 | 0.479 | 0.094 |
| Restructuring the social environment | 0.758 | 0.321 | 0.178 | 0.006 | 0.000 |
| Adding objects to the environment | 0.939 | 0.929 | 0.916 | 0.860 | 0.550 |
Similar interpretations of the probabilities presented for the cost-effectiveness of BCT components can be made as for the QI components. For the patient-targeted BCTs, at a probability of screening uptake of 0.7, ‘goal setting (outcome)’, ‘feedback on outcomes of behaviour’, ‘social support (unspecified)’ and ‘information about health consequences’ have extremely high probabilities of being cost-effective compared with no BCT intervention. To put this into context, this is crudely analogous to saying that a comparison is statistically significant in a one-sided test at conventional (5% levels) of statistical significance. The BCT ‘prompts/cues’ is also associated with a high probability of 0.84 of being cost-effective compared with no intervention.
For the HCP-targeted BCTs, only ‘adding objects to the environment’ has a probability of being cost-effective of > 0.9, with ‘credible source’ having a probability of being cost-effective of > 0.7.
When the baseline probability of DRS is decreased, then each BCT component has a higher probability of being considered cost-effective compared with no BCT intervention. At a baseline probability of DRS attendance of 0.5, ‘social support (practical)’ and ‘credible source’ are the only BCTs that have a probability of being cost-effective that rises above 0.8 compared with scenarios with a higher baseline probability of DRS attendance. When the baseline probability of DRS attendance is 0.9, however, all BCTs are less likely to be cost-effective and only the patient-targeted ‘information about health consequences’ has a probability of being cost-effective of > 0.9.
The probability of each BCT component being cost-effective when all BCT components are compared simultaneously is reported inTable 19. The BCT components with the highest probabilities of being cost-effective inTable 18also have the highest probabilities of being cost-effective inTable 19. WhatTable 19shows is that, among the BCT components with the highest probabilities of being cost-effective, there is no single BCT component that stands out as being far more likely to be cost-effective. Patient-targeted ‘information about health consequences’ has the highest probability of being cost-effective and this is only 0.47 in the base case. Patient-targeted ‘goal-setting (outcome)’, ‘feedback on outcomes of behaviour’, ‘social support (unspecified)’ and ‘information about health consequences’ all have a significant probability of being the most cost-effective isolated BCT component.
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| No intervention | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Patient-targeted BCTs | |||||
| Problem-solving | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Goal-setting (outcome) | 0.172 | 0.119 | 0.071 | 0.037 | 0.012 |
| Feedback on outcomes of behaviour/biofeedback | 0.311 | 0.305 | 0.278 | 0.197 | 0.092 |
| Social support (unspecified) | 0.105 | 0.127 | 0.151 | 0.199 | 0.256 |
| Social support (practical) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Instruction on how to perform the behaviour | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Information about health consequences | 0.371 | 0.419 | 0.465 | 0.520 | 0.521 |
| Prompts/cues | 0.001 | 0.002 | 0.004 | 0.007 | 0.021 |
| Credible source | 0.003 | 0.003 | 0.002 | 0.004 | 0.014 |
| Restructuring the social environment | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| HCP-targeted BCTs | |||||
| Feedback on outcomes of behaviour/biofeedback | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Social support (practical) | 0.021 | 0.004 | 0.001 | 0.000 | 0.000 |
| Instruction on how to perform the behaviour | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Prompts/cues | 0.001 | 0.002 | 0.001 | 0.001 | 0.000 |
| Credible source | 0.000 | 0.000 | 0.002 | 0.001 | 0.001 |
| Restructuring the social environment | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Adding objects to the environment | 0.013 | 0.017 | 0.026 | 0.034 | 0.067 |
Sensitivity analyses for scenarios including different probabilities of monitoring following HES referral attendance, different screening frequencies and different ages of the baseline cohort and including and excluding social care costs are presented inTable 20.
| Intervention | Screening every year, Pm = 0.78, cohort age 64 years, no care costs | Screening every year, Pm = 0.4, cohort age 64 years, no care costs | Screening every 2 years, Pm = 0.78, cohort age 64 years, no care costs | Screening every 3 years, Pm = 0.78, cohort age 64 years, no care costs | Screening every 3 years, Pm = 0.4, cohort age 64 years, no care costs | Screening every year, Pm = 0.78, cohort age 70 years, no care costs | Screening every year, Pm = 0.78, cohort age 56 years, no care costs | Screening every year, Pm = 0.78, cohort age 64 years, care costs |
|---|---|---|---|---|---|---|---|---|
| Patient-targeted BCTs | ||||||||
| Problem-solving | 0.264 | 0.346 | 0.791 | 0.849 | 0.830 | 0.186 | 0.130 | 0.478 |
| Goal-setting (outcome) | 0.964 | 0.976 | 1.000 | 1.000 | 1.000 | 0.955 | 0.918 | 0.984 |
| Feedback on outcomes of behaviour/biofeedback | 0.997 | 0.998 | 1.000 | 1.000 | 1.000 | 0.997 | 0.993 | 0.999 |
| Social support (unspecified) | 0.980 | 0.978 | 0.989 | 0.991 | 0.991 | 0.980 | 0.979 | 0.986 |
| Social support (practical) | 0.416 | 0.499 | 0.856 | 0.904 | 0.878 | 0.334 | 0.284 | 0.623 |
| Instruction on how to perform the behaviour | 0.117 | 0.174 | 0.631 | 0.740 | 0.708 | 0.071 | 0.044 | 0.275 |
| Information about health consequences | 0.999 | 0.999 | 0.999 | 1.000 | 1.000 | 0.999 | 0.998 | 1.000 |
| Prompts/cues | 0.841 | 0.846 | 0.891 | 0.896 | 0.902 | 0.819 | 0.810 | 0.867 |
| Credible source | 0.563 | 0.583 | 0.677 | 0.723 | 0.699 | 0.545 | 0.520 | 0.634 |
| Restructuring the social environment | 0.386 | 0.427 | 0.612 | 0.646 | 0.649 | 0.362 | 0.337 | 0.470 |
| HCP-targeted BCTs | ||||||||
| Feedback on outcomes of behaviour/biofeedback | 0.658 | 0.667 | 0.765 | 0.793 | 0.787 | 0.603 | 0.601 | 0.690 |
| Social support (practical) | 0.396 | 0.401 | 0.997 | 1.000 | 1.000 | 0.380 | 0.362 | 0.645 |
| Instruction on how to perform the behaviour | 0.377 | 0.401 | 0.573 | 0.617 | 0.625 | 0.325 | 0.316 | 0.455 |
| Prompts/cues | 0.398 | 0.469 | 0.766 | 0.829 | 0.824 | 0.320 | 0.286 | 0.545 |
| Credible source | 0.729 | 0.758 | 0.893 | 0.920 | 0.904 | 0.672 | 0.666 | 0.802 |
| Restructuring the social environment | 0.209 | 0.223 | 0.929 | 0.982 | 0.976 | 0.149 | 0.115 | 0.279 |
| Adding objects to the environment | 0.920 | 0.924 | 0.955 | 0.960 | 0.951 | 0.903 | 0.908 | 0.932 |
Monitoring may occur if treatment is not provided after a positive diagnosis following HES referral attendance. As in the QI analysis, in the base-case analysis it was assumed that 78% of people who are not treated following a positive diagnosis of preproliferative diabetic retinopathy, proliferative diabetic retinopathy or diabetic maculopathy are monitored. If this proportion is reduced to 40% then the probability of a given BCT component being cost-effective compared with no BCT intervention increases when DRS is scheduled every year.
As the frequency of DRS is reduced, the probability of a BCT component being considered cost-effective compared with no BCT intervention increases. When the screening interval is extended to 3 years, all BCTs have a probability of being cost-effective of > 0.8 except for ‘Instruction on how to perform the behaviour’ targeted at both the patient and the HCP, ‘credible source’ targeted at the patient, ‘restructuring the social environment’ targeted at the patient, ‘instruction on how to improve behaviour’ targeted at HCPs and ‘feedback on outcomes of behaviour’ targeted at HCPs. This is consistent with the results of the Scanlonet al. 224cost–utility analysis, which identified screening every 3 years as the most cost-effective screening frequency. However, when the age of the cohort is reduced from 64 years (the base case) to 56 years then the BCT components all become less cost-effective compared with no BCT intervention, although for some BCTs, such as ‘information about health consequences’ targeted at the patient, the change is very small (the probability that this BCT is cost-effective remains at 0.998, which is extremely high). When the age of the cohort is increased to 70 years then the probability that a BCT is cost-effective compared with no intervention also decreases. The quality-of-life benefits and potential cost savings increase with age. Starting DRS at an earlier age increases the duration that a benefit may be realised for an individual, but the greatest benefits occur a longer time after the start of DRS and these are discounted more heavily. The results do not change greatly in the other sensitivity analyses.
Threshold cost curve
The threshold intervention cost per patient was derived for a range of hypothetical RD effect estimates, using the base-case model and assuming a societal willingness-to-pay threshold for a QALY of £20,000 (Figure 21). The base-case analysis excludes the social care cost implications but the results of a model including the social care cost implications are also presented inFigure 21.
FIGURE 21.
The maximum cost per-patient-screened threshold below which a hypothetical DRS uptake intervention remains cost-effective compared with no intervention for a range of RD effect estimates for the intervention compared with no intervention.
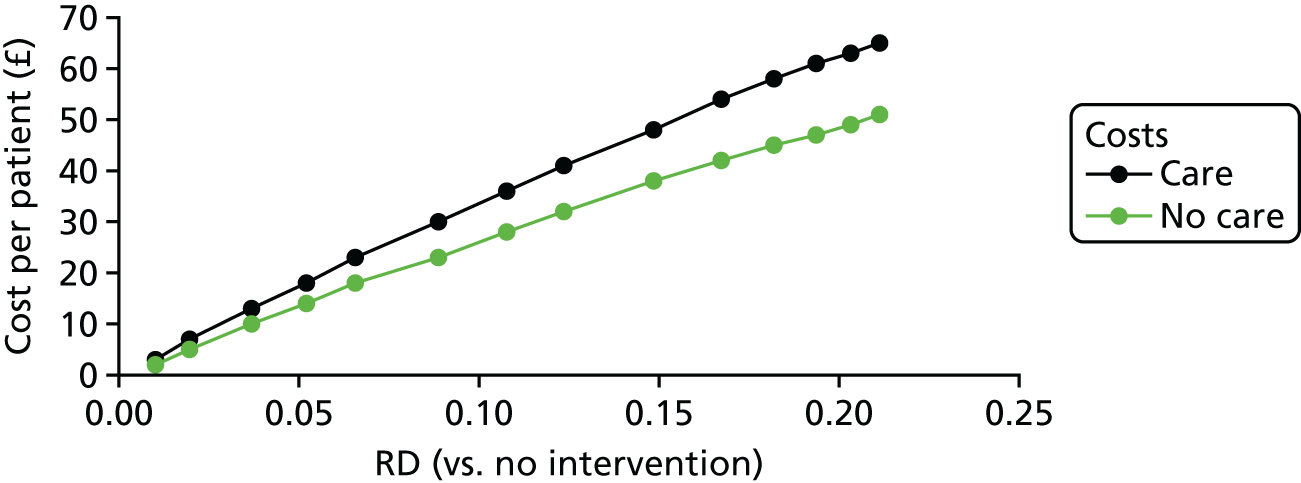
Similar results but with effectiveness reported as ORs are provided inFigure 22. As withFigure 21, the results for scenarios excluding and including social care cost implications are presented.
FIGURE 22.
The maximum cost per-patient-screened threshold below which a hypothetical DRS uptake intervention remains cost-effective compared with no intervention for a range of OR effect estimates for the intervention compared with no intervention.
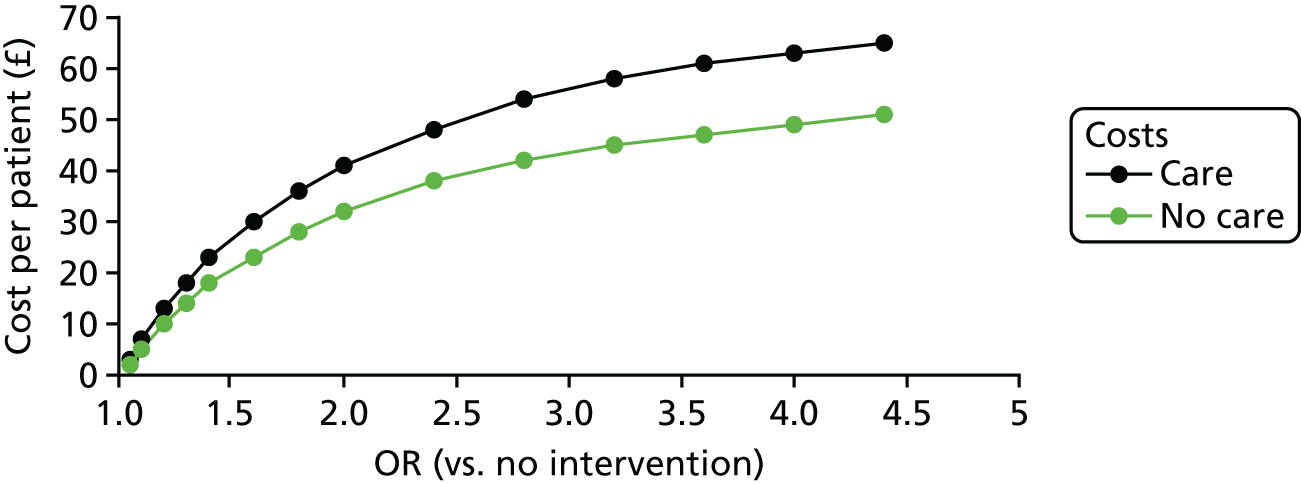
The cost thresholds for a range of RDs and RRs are presented inTable 21. The cost thresholds are presented using three different units: per patient, per general practice and for England as a whole. This table represents a lookup table indicating a plausible cost range over which an intervention with a specific level of effect may be cost-effective at a societal willingness-to-pay threshold for a QALY of £20,000.
| RD | RR | Cost threshold per patient (£) | |||||
|---|---|---|---|---|---|---|---|
| No care costs | Care costs | ||||||
| Per patient | Per general practice | England (000s) | Per patient | Per general practice | England (000s) | ||
| 0.3 | 1.43 | 73 | 5282 | 212,688 | 87 | 6295 | 253,478 |
| 0.28 | 1.40 | 68 | 4920 | 198,121 | 82 | 5933 | 238,910 |
| 0.26 | 1.37 | 63 | 4559 | 183,553 | 77 | 5572 | 224,342 |
| 0.24 | 1.34 | 58 | 4197 | 168,985 | 72 | 5210 | 209,775 |
| 0.22 | 1.31 | 53 | 3835 | 154,418 | 67 | 4848 | 195,207 |
| 0.2 | 1.29 | 48 | 3473 | 139,850 | 63 | 4559 | 183,553 |
| 0.18 | 1.26 | 45 | 3256 | 131,109 | 57 | 4124 | 166,072 |
| 0.16 | 1.23 | 40 | 2894 | 116,542 | 52 | 3763 | 151,504 |
| 0.14 | 1.20 | 36 | 2605 | 104,887 | 46 | 3329 | 134,023 |
| 0.12 | 1.17 | 31 | 2243 | 90,320 | 40 | 2894 | 116,542 |
| 0.1 | 1.14 | 26 | 1881 | 75,752 | 34 | 2460 | 99,060 |
| 0.08 | 1.11 | 21 | 1520 | 61,184 | 27 | 1954 | 78,666 |
| 0.06 | 1.09 | 16 | 1158 | 46,617 | 21 | 1520 | 61,184 |
| 0.04 | 1.06 | 11 | 796 | 32,049 | 14 | 1013 | 40,790 |
| 0.02 | 1.03 | 5 | 362 | 14,568 | 7 | 507 | 20,395 |
The maximum costs for the mean RD estimates for the QI and BCT components are presented inAppendix 11.
Discussion
The first objective of the economic analysis was to identify the BCT and QI components that are most likely to be cost-effective at established cost-effectiveness thresholds and are therefore worth prioritising in further research. A second objective was to estimate the maximum cost per person with diabetes eligible for screening that an intervention could have for a level of clinical effectiveness and still be considered cost-effective.
Summary of findings
In the base case there was considerable uncertainty in the cost-effectiveness of the QI components. The QI component most likely to be cost-effective at a societal willingness-to-pay threshold of £20,000 per QALY was patient education, which is perhaps the closest QI component to the most cost-effective BCT components. The probabilities of being cost-effective at a societal willingness-to-pay threshold of £20,000 per QALY were higher for BCTs than for QI components. Patient-targeted ‘goal-setting (outcome)’, ‘feedback on outcomes of behaviour’, ‘social support (unspecified)’ and ‘information about health consequences’ had extremely high probabilities of being cost-effective compared with interventions not containing this BCT. For the HCP-targeted BCTs, ‘adding objects to the environment’ had a probability of being cost-effective of > 0.9. The BCT components with the highest probabilities of being cost-effective were patient targeted. The sensitivity analyses that had a significant effect on the results were varying the baseline probability of screening uptake and reducing the frequency of screening. The change in the results was much more pronounced for the BCT components than for the QI components. A lower baseline probability of DRS attendance increased the probability of an intervention being cost-effective, whereas a higher baseline probability of DRS attendance reduced the probability of an intervention being cost-effective. Reducing the frequency of screening also increased the probability of an intervention being cost-effective.
The second objective was to estimate the cost threshold below which an intervention might be cost-effective over a range of effectiveness estimates. For a RD of 0.2, the cost threshold was £63 per patient or £4559 per general practice when including social care costs in the analysis.
Strengths, limitations and challenges
This economic analysis was concerned with estimating the cost-effectiveness of interventions designed to increase DRS attendance. Long-term cost and health outcomes (valued using QALYs) were incorporated using a published model to derive more useful estimates of the likelihood that QI and BCT components are cost-effective and of the cost threshold below which an intervention may be cost-effective. A number of assumptions were adopted within the Scanlonet al. 224model that we have not been able to review. In addition, the model used in our work was not a perfect reproduction of the Scanlonet al. 224model as simplifications were made. We relied on sensitivity analyses to test particularly significant changes to the model. It is possible that there are uncertainties that have not been evaluated, such as uncertainty associated with the outcomes of DRS attendance for different combinations of screening frequencies and interventions to encourage DRS attendance.
Although the objectives of the economic analysis were to evaluate the likelihood that individual QI components and BCTs are cost-effective, the interventions to increase DRS attendance that were studied are complex interventions consisting of multiple QI components and BCTs. The evidence on the effectiveness of the complex interventions was identified through a systematic review of the literature. The individual treatment effects and marginal costs of each QI component and BCT were estimated using multivariable meta-regressions. Although this is an appropriate method to estimate the individual effects, there is a possibility of confounding and spurious results when conducting meta-regression. Furthermore, there was heterogeneity in the study characteristics and most studies were not conducted in the UK, making firm conclusions around the cost-effectiveness of specific combinations of BCTs and QI components in specific UK populations difficult. Consequently, the economic analysis was limited to providing evidence on the likelihood that individual QI components and BCTs could be cost-effective. The focus of this was solely to help prioritise future research by focusing on QI components and BCTs that are most likely to be cost-effective when designing and studying interventions. It is possible that a complex intervention designed to incorporate a BCT or QI component with a high probability of being cost-effective may need to include a BCT or QI component that is less likely to be cost-effective.
Future research
This analysis suggests that future research should focus on designing and studying interventions consisting of one or more of the following patient-targeted BCTs: ‘goal-setting (outcome)’, ‘feedback on outcomes of behaviour’, ‘social support (unspecified)’ and ‘information about health consequences’. The HCP-targeted BCT, ‘adding objects to the environment’, should also be considered. The interventions studied may also include other BCTs.
Chapter 7 Discussion and conclusions
Discussion
The preceding chapters report a synthesis of the available evidence on the effectiveness and cost-effectiveness of interventions aiming to increase attendance for DRS. We have taken a global view of the evidence base and provide a summary of the intervention components that are most likely to be effective and cost-effective for improving DRS attendance. These interventions can be tailored and potentially applied to service models worldwide. We highlight uncertainties and provide evidence for the design of future studies in terms of the ‘best bet’ for behavioural components of interventions that could be tested in future research and in which patient groups.
To apply our findings to the UK NHS, we held a knowledge exchange event with key UK stakeholders (seeAppendix 2). The integrated findings are reported later in this chapter.
Based on evidence from randomised controlled trials, how effective are quality improvement interventions for increasing diabetic retinopathy screening attendance? (seeChapter 3)
We conducted a systematic review of the evidence from RCTs that investigated any QI intervention to improve attendance for DRS. Interventions could be directed at patients with diabetes, HCPs, the health-care system or any combination thereof. The primary outcome was attendance for one or more visits for DRS within a 2-year period following randomisation. We included data from 66 studies (analysing data for 352,879 participants). Fifty of the included studies (76%) were RCTs of general QI interventions that sought to improve a number of diabetes quality indicators and 16 studies (24%) tested interventions that specifically targeted DRS. The majority of studies were conducted in North America, with only three studies conducted in the UK. The interventions were usually multifaceted, consisting of multiple QI components (e.g. ‘patient education’, ‘team changes’ and ‘audit and feedback’). We used two validated taxonomies to describe the content of the interventions: a modified version of the taxonomy29originally developed by the Cochrane EPOC Group43to provide a general description of the intervention and the BCTTv136to describe the specific QI components present in each intervention. Interventions were further described in terms of their component BCTs, which provides a level of granularity that is better suited to describing the active ingredients of the interventions. BCTs were coded using the BCTTv136in terms of whether they targeted the patient, the HCP, or both.
Although our meta-analysis for the main comparison identified that the pooled effect estimate for studies that specifically targeted DRS was larger (17% absolute increase in DRS attendance) than that in studies of general QI interventions (12% increase), the difference between these subgroups was not statistically significant. This is an important finding given the previously demonstrated effectiveness of general QI interventions for the overall quality of diabetes care. For example, a systematic review of the effectiveness of such interventions by members of the current review team (NMI, JMG),29which incorporated 26 of the trials included in the current review, found improvements in glycated haemoglobin (HbA1c), diastolic and systolic blood pressure, low-density lipoprotein cholesterol and medication use and increased monitoring for other diabetes complications, for example foot screening.
The pooled effect estimates for studies comparing a more intensive (stepped) intervention with a less intensive intervention were smaller (5% increase in DRS attendance) than the pooled effect estimates for the comparisons with non-specified usual care. In terms of health economic outcomes, there was too much methodological variability in studies reporting comparative cost-effectiveness outcome measures to make a judgement on the relative cost-effectiveness of QI interventions compared with each other or with usual care. The effectiveness of interventions that included specific QI components or BCTs was also calculated for level of resource use, as measured on an ordered ranking scale, to explore whether or not more resource-intense interventions were more likely to be effective. The results indicate that there is no evidence of a clear relationship between effectiveness and intensity of resource use.
Sufficient studies were available to investigate the effectiveness of nine out of 12 QI components and 17 BCTs by subgroup analysis and meta-regression. All QI components analysed were associated with significantly higher DRS attendance. Larger pooled effect estimates were found for interventions directed at patients (promotion of self-management and patient education) or the organisation of the health system [team changes (e.g. use of multidisciplinary teams or expansion of professional roles) and introducing an electronic diabetes register]. All frequently used BCTs identified in at least 10 patient and/or HCP intervention arms were similarly associated with higher DRS attendance. Higher effect estimates were observed for the BCTs of ‘goal-setting (outcome)’ and ‘credible source’ (information on the importance of the behaviour from individuals/organisations with expertise in the field) in interventions targeting patients and for the BCTs of ‘restructuring the social environment’ (e.g. introducing diabetes link workers to facilitate the behaviour) and ‘credible source’ (e.g. persuasive argument from a respected colleague) in interventions targeting HCPs. Using univariate meta-regression we found evidence for larger effects in studies with poorer baseline DRS attendance.
The review highlighted a number of gaps within the evidence base. No data were available to assess the effectiveness of QI interventions to improve ongoing adherence to screening and limited data were available to assess the relative effectiveness of interventions in particular population subgroups, for example type 1 compared with type 2 diabetes and those of different ethnicities or socioeconomic status. Although we were able to show that interventions containing particular BCTs have a greater likelihood of success, given the multicomponent nature of interventions, it is likely that there is interaction with other BCTs or that other effect modifiers in the intervention arms may also be having an impact on effectiveness. The analysis conducted as part of this review did not attempt to evaluate the impact of individual BCTs in isolation; however, this was evaluated as part of the exploratory analysis (reported inChapter 6) to determine the likely cost-effectiveness of interventions in which multivariate meta-regression was used. This approach allows for more flexible modelling of arm-level covariates.
What are the theoretical determinants (e.g. barriers/enablers) of diabetic retinopathy screening attendance? (seeChapter 4)
We aimed to clarify how QI interventions might work and gain an understanding of factors influencing DRS attendance behaviour. This was achieved by identifying studies reporting primary data on barriers to and enablers of DRS attendance from the perspectives of people with diabetes and HCPs and framing these in terms of theoretical domains from the TDF (an integrated framework of behaviour change theories) and the CFIR (a comprehensive listing of constructs thought to influence implementation). We hypothesised that interventions that target these theoretical domains are more likely to be effective at increasing screening attendance.
From the analysis we identified the TDF domains ‘environmental context and resources’, ‘social influences’, ‘knowledge’, ‘memory, attention and decision processes’, ‘beliefs about consequences’ and ‘emotions’ as representing the most important factors potentially influencing screening attendance. We also identified that the other eight TDF domains – ‘social/professional role and identity’, ‘beliefs about capabilities’, ‘goals’, ‘intentions’, ‘behavioural regulation’, ‘optimism’, ‘reinforcement’ and ‘skills’ – may have less influence on screening behaviour.
These findings have two important implications for the design of effective interventions to promote DRS attendance: (1) they identify which theoretical domains to target in future QI interventions and which to avoid and (2) they can be used to assess the extent to which the active components (BCTs) identified in existing QI interventions are actually targeting the theoretical domains that are important in determining attendance.
The studies in this review predominantly identified barriers and enablers from the perspective of patients rather than the perspective of organisations or HCPs. Even the data from HCPs were mostly focused on patient barriers. Therefore, the CFIR did not offer additional insights over and above those gained from the TDF. Thematic synthesis within the TDF domains identified as being of high importance resulted in the identification of specific content themes that may help to identify potential targets for future QI interventions. There are four key recommendations based on these findings:
-
Reduce inconvenience to patients. Many of the barriers and enablers identified within the TDF domain ‘environmental context and resources’ relate to perceptions of convenience. Providing local screening facilities, ‘one-stop shops’, flexible appointment systems, childcare facilities and transportation might all be advantageous.
-
Increase awareness of the importance of DRS. HCP recommendations and information provision was reported to be an enabler within the TDF domain ‘knowledge’. Furthermore, within the TDF domain ‘social influences’, use of local media and local community networks was identified as another potential way to raise awareness.
-
Increase patients’ sense of comfort and support. A number of barriers were identified within the TDF domains ‘emotions’, ‘social influences’ and ‘beliefs about consequences’ that highlight the need to increase support for patients who struggle with issues such as communicating with HCPs, negative emotions (e.g. fear), a lack of social support and a lack of trust in the HCP or system. These barriers may be overcome by providing community-based clinics, ensuring that there is some social/cultural compatibility between the patient and the HCP and the HCP showing compassion to the patient.
-
Improve message content. Within the TDF domains ‘memory, attention and decision processes’, ‘beliefs about consequences’ and ‘knowledge’ we found a number of barriers that relate to health misconceptions. Many of the issues identified in this review, including those captured within recommendations 2 and 3, can perhaps be addressed through improved message content. Messages that highlight the asymptomatic nature of diabetic retinopathy, the safety of the screening procedure, the benefits of early detection (e.g. providing piece of mind) and the potential negative consequences if diabetic retinopathy is left unchecked are all potentially beneficial. Furthermore, messages that clarify the differences between routine eye tests and DRS, that offer reminders to attend for screening and which emphasise that DRS is a routine part of diabetes care could also be helpful.
The combination of deductive coding (informed by theoretical frameworks to guide barrier identification) and inductive analysis (to allow more granular content themes, unanticipated findings and patient insights to emerge) is a strength of this review. However, it is worth noting that the theoretical frameworks used in this review are limited in as much as they do not specify relationships between domains and hence the strength of the direct impact of barriers on behaviour is not known.
The majority of the studies included in this review were from North America. Additional UK studies are needed from the perspective of both patients and HCPs. Ideally, such additional research would focus on sampling the subgroups least likely to attend for screening.
Do the components of existing quality improvement interventions target the important theoretical determinants of diabetic retinopathy screening attendance? (seeChapter 5)
We hypothesised that QI interventions to increase DRS attendance would be more effective if they targeted the key barriers to/enablers of DRS attendance. To investigate this we integrated BCT components identified in existing QI interventions (seeChapter 3) with the identified important theoretical determinants of DRS attendance (seeChapter 4). Certain BCTs may be of greater or lesser relevance to addressing barriers/enablers within a given theoretical domain (i.e. may be of higher or lower theoretical coherence). We therefore examined the extent to which component BCTs in existing QI interventions are theoretically coherent with domains representing key barriers to/enablers of DRS attendance. This was achieved by applying published mapping tools that pair BCTs from taxonomies with domains from the TDF that experts agree represent theoretically coherent pairings.
We examined the extent of theoretical coherence for frequently used BCTs, identified in at least 10 intervention arms of existing QI interventions that target patients and/or HCPs (seeChapter 3). BCTs were classified as having high theoretical coherence if at least one domain with which they were paired in available mapping tools was classified as being important in the second review (seeChapter 4). Eight of the ten frequently used BCTs targeting patients had high theoretical coherence: (1) ‘feedback on outcomes (i.e. consequences) of behaviour (including biofeedback)’, (2) ‘credible source’ (i.e. persuasive argument presented by a respected person), (3) ‘prompts and cues’, (4) ‘social support (unspecified)’, (5) ‘restructuring the social environment’ (e.g. introducing a diabetes link worker), (6) ‘instruction on how to perform the behaviour’ (i.e. provide further details about how to make, accept or attend a DRS appointment), (7) ‘social support (practical)’ (e.g. assist with transport) and (8) ‘information about health consequences’ (e.g. provide an information leaflet on how DRS assists with the early detection and treatment of retinopathy). All seven frequently used BCTs targeting HCPs had high theoretical coherence: (1) ‘restructuring the social environment’, (2) ‘credible source’, (3) ‘adding objects to the environment’ (e.g. reminder systems), (4) ‘social support (practical)’, (5) ‘instruction on how to perform the behaviour’, (6) ‘prompts and cues’ and (7) ‘feedback on outcomes of behaviour’ (including biofeedback).
Our findings thus suggest that the majority of components that are frequently used in existing QI interventions do target the important determinants of DRS attendance. Although it was not possible to formally examine the association between theoretical coherence and effectiveness, the observation that nearly all frequently used BCTs associated with higher DRS attendance also had high theoretical coherence supports the notion that interventions are more likely to be effective if they target the key determinants of behaviour and behaviour change. Therefore, the design of future QI interventions, or the refinement of existing QI interventions, may benefit from the inclusion of the aforementioned BCTs with demonstrated effectiveness and high theoretical coherence.
However, existing QI interventions in this review were found to use a limited number of BCTs (median 4). Our mapping investigation revealed that there are numerous ‘missed opportunities’ for intervention design, in the form of BCTs that the mapping tools suggest are theoretically applicable to addressing the important domains representing identified barriers to/enablers of DRS attendance, but which are not frequently used in existing QI interventions. Missed opportunities for intervention design were identified for all six important TDF domains identified inChapter 4. These findings point to theoretically informed candidate BCTs that could be included in future QI interventions to increase the targeting of the important domains to DRS attendance. This could in turn increase intervention effectiveness and patient benefits. This is particularly true for the important theoretical domain ‘emotions’. None of the five BCTs that are agreed to be theoretically coherent with this domain was frequently identified in existing QI interventions (range 0–3 intervention arms). This suggests that there is a critical need for future QI interventions to include components that target the emotional influences on DRS attendance, such as the BCTs ‘reduce negative emotions’ (e.g. reassure the patient that any identified retinal pathology will be treated without delay), ‘information on emotional consequences’ (e.g. mention that a negative test will provide reassurance about eye health), ‘self-assessment of affective consequences’ (e.g. ask the person with diabetes to record their feelings after the DRS examination), ‘social support (emotional)’ (e.g. ask a friend to comfort the person with diabetes) and ‘conserving mental resources’ (e.g. arrange a DRS appointment at a time when the person with diabetes is less likely to be busy).
However, an important caveat to interpreting these findings concerns the applicability and validity of the methods and mapping tools used to pair BCTs with theoretically coherent TDF domains. Although this study is one of the first to provide an empirical test of the validity of available mapping tools, a number of limitations and discrepancies were identified in the tools, suggesting a need for further research to refine and expand these tools to enhance their validity and usability. The validity of any BCT and domain pairing recommended in each mapping tool depends on the nature of the behaviour being investigated and what intervention components are likely to be feasible, appropriate and acceptable to deliver in the context of DRS attendance. Lastly, our methods and available data did not enable investigation of which component BCTs may be more or less theoretically coherent for addressing barriers and enablers for different subgroups of patients attending DRS. As future evidence emerges concerning which barriers/enablers are of greater importance to different subgroups, there is the potential to apply the present mapping methods to develop more tailored interventions that include component BCTs that are theoretically coherent with the determinants of DRS attendance for specific patient subgroups.
Which quality improvement interventions designed to increase diabetic retinopathy screening attendance are most likely to be cost-effective and what are the potential cost consequences? (seeChapter 6)
At a baseline probability of DRS attendance of 0.7 and when the maximum that society is willing to pay for a QALY is £20,000, no QI components (coded using the modified EPOC Group taxonomy29) are likely to be cost-effective (judged on a probability of > 0.7) compared with no QI intervention. When the baseline probability of DRS attendance is decreased to 0.5, ‘team changes’ and ‘patient education’ have a probability of being cost-effective of > 0.7.
The probabilities of being cost-effective at a societal willingness-to-pay threshold of £20,000 per QALY were higher for BCTs (patient targeted or HCP targeted) than for QI components. Of the patient-targeted BCTs, ‘goal-setting (outcome)’, ‘feedback on outcomes of behaviour’, ‘social support’ and ‘information about health consequences’ had extremely high probabilities of being cost-effective compared with no BCT intervention. Of the HCP-targeted BCTs, ‘adding objects to the environment’ (e.g. decision support or monitoring tools such as diabetes passports) had a probability of being cost-effective of > 0.9. Nevertheless, the BCT components with the highest probabilities of being cost-effective were patient targeted. Likewise, the QI component with the highest probability of being cost-effective was ‘patient education’.
In sensitivity analyses, as the frequency of DRS was reduced, the cost-effectiveness of a BCT component compared with no BCT intervention increased. When the screening interval was increased to 3 years, all BCTs had a probability of cost-effectiveness of > 0.8 except for ‘instruction on how to perform the behaviour’ targeted at both patients and HCPs, ‘credible source’ and ‘restructuring the social environment’ targeted at patients, and ‘instruction on how to perform the behaviour’ and ‘feedback on outcomes of behaviour’ targeted at HCPs.
When the age of the cohort was reduced from 64 years (the base case) to 56 years then the BCT components all became less cost-effective compared with no BCT intervention. However, for some BCTs, such as ‘information about health consequences’ targeted at the patient, the change was very small (the probability that this BCT is cost-effective remained at 0.998, which is extremely high).
The economic analysis utilised the best available effectiveness and resource use data to evaluate the likelihood that individual QI and BCT components are cost-effective compared with no intervention and with each other at willingness-to-pay thresholds for a QALY normally considered to be acceptable. Resource use associated with the main intervention in each included study was recorded and valued so that both the effects of the QI and BCT components on DRS attendance and resource use could be estimated. Data from a recently published cost–utility model of screening frequency, conducted as part of a previous NIHR HTA study,224were used to model the long-term outcomes of screening attendance and non-attendance.
Interventions were assumed to target all people eligible for screening, that is, all people with diabetes. It may be possible to target a subset of the eligible population who would benefit from encouragement to attend screening. Included studies may have targeted different groups. This would require a baseline probability of screening uptake specific to that population, an intervention effect estimate specific to that population and cost estimates that account for a smaller targeted population. These data were not available from this study. 224
The economic analysis explores which individual BCTs and QI components are the most likely to be cost-effective given the available evidence; it does not consider the cost-effectiveness of multicomponent interventions. In reality, the interventions designed to encourage screening uptake are likely to be complex, incorporating several BCTs or QI components. The results of the economic analysis are therefore less useful in informing which interventions to adopt but provide an indication of which QI and BCT components are worth considering as a starting point in the design of a DRS attendance intervention.
The effectiveness evidence used in the economic model was derived from a multivariate meta-regression with all of the QI components and BCTs included as explanatory variables. These analyses are essentially based on observational data. The potential for the existence of uncontrolled confounding factors may affect the power of the statistical model to predict outcomes in other studies and settings. Furthermore, an additive model is assumed and the interaction effects between QI components and BCTs are unknown and are not captured in the model. This statistical approach is different from the analysis conducted as part of the phase 1 systematic review. The two approaches have different interpretations. The univariate meta-regression analysis conducted as part of the phase 1 intervention review compared studies that used multifaceted interventions that incorporate a particular QI/BCT component with studies using other multifaceted interventions that do not contain that component. This analysis estimates the average effect across a range of complex interventions. The additive (multivariate) model used in the economic analysis estimates the effects of individual QI components by adjusting for the other components in the intervention. There is far greater variation in the univariate model than in the multivariate model and some effects are significantly different. For example, using a RD outcome variable, the patient-targeted BCT ‘restructuring the social environment’ is ranked six out of 17 in the univariate analysis of BCTs with a coefficient of 0.051, whereas it is ranked 17 out of 17 in the multivariate model with a coefficient of –0.19.
There is considerable heterogeneity in the data and most of the studies were not conducted in the UK; therefore, it is difficult to make specific conclusions regarding people with diabetes in the UK. Consequently, the economic analysis was limited to providing evidence on the likelihood that individual QI components and BCTs are cost-effective to help prioritise future research by designing and studying interventions based around the QI components and BCTs most likely to be cost-effective. It is possible that a complex intervention designed to incorporate a BCT or QI component with a high probability of being cost-effective may need to include a BCT or QI component that is less likely to be cost-effective. It may be possible to develop an intervention based around a specific QI component or BCT that is more cost-effective than indicated here; the cost thresholds below which an intervention may be cost-effective may be a guide to the cost per patient of an intervention.
The evidence on the resources used to provide the QI/BCT components of interventions was based on an ordered logistic regression with all of the QI components and BCTs included as explanatory variables. A similar model was used to estimate the effectiveness of QI/BCT components and the same limitations apply. With respect to resource use, the weights used were developed in an explicit fashion and care was taken to check the scorings provided by the original raters. However, the resource use ordered rankings were derived using a small sample of cost estimates based on the intervention descriptions. Uncertainty associated with these cost estimates was not included in the analysis and further work could usefully be undertaken to provide a wider sample of cost estimates to place a value on the resource use rankings.
Overall, the economic evaluation does have a number of limitations (described in more detail inChapter 6), primarily linked to the limitations of the evidence base. However, the economic analysis identified the most promising BCT/QI components to take forward to enable further development of the evidence and theory-based interventions to increase attendance for DRS.
Conclusions
The results of this study suggest that a number of interventions are likely to improve attendance for DRS. We found that QI interventions targeted at those with diabetes, HCPs or the health-care system improve attendance by 12% on average compared with usual care. Compared with usual care, QI interventions aimed at improving the quality of diabetes care were as effective at improving attendance at DRS as those specifically targeting DRS attendance. There was some evidence to indicate that a larger effect size could be anticipated in poor attenders.
Recommendations for practice and applicability to the UK setting
The findings suggest that, among interventions that target the patient, promotion of self-management (provision of equipment or resources to promote self-management) and patient education (interventions designed to promote greater understanding of diabetic retinopathy) are more likely to be effective at increasing levels of DRS attendance. For interventions at a system or organisational level, team changes (changes to the structure or organisation of the primary health-care team, e.g. adding a team member or shared care, use of multidisciplinary teams or expansion or revision of professional roles) and the establishment of an electronic diabetes registry (e.g. general electronic medical record system or electronic tracking system for patients with diabetes) are associated with higher levels of DRS attendance.
Including behavioural interventions to support the uptake of DRS services could improve the uptake of DRS. Such interventions include providing feedback on the consequences of attendance or non-attendance; encouraging social (interpersonal) support to attend; providing more information on diabetic eye disease, including information about the health consequences, and on the process of screening; introducing reminder systems; and ensuring that patient information is provided by a credible source, such as respected national clinical guidelines. These interventions can be delivered at a patient level and at the level of the health system, including at the level of HCPs. We identified that these interventions are effective and are also likely to target the important factors associated with attendance at DRS.
Considering cost-effectiveness and theoretical coherence (seeChapter 5), we have identified BCTs that could be worthwhile for services with lower attendance levels. These are ‘feedback’ and ‘information about health consequences’, for example providing information on diabetic retinopathy and the consequences and benefits of DRS, explaining the difference between DRS and attendance for regular eye tests (e.g. enhanced patient information sheets), introducing processes to facilitate social support to attend and improving the screening environment [introducing processes to improve convenience for patients, e.g. online management/booking systems or monitoring tools (e.g. diabetes passports)].
Interventions should be judged by their cost-effectiveness, but it is also useful to know the relative resource implications that are likely to be associated with QI components and individual BCTs. The QI component with the highest probability of being cost-effective was ‘patient education’, but in general QI components were unlikely to be considered cost-effective. In terms of BCTs, among patient-targeted techniques, ‘problem-solving’, ‘goal-setting (outcome)’, ‘social support (practical)’ and ‘instruction on how to perform the behaviour’ were associated with relatively high resource use; ‘feedback on outcomes of behaviour/biofeedback’ and ‘restructuring the social environment’ were associated with moderately high resource use; and ‘social support (unspecified)’, ‘information about health consequences’, ‘prompts/cues’ and ‘credible source’ were associated with relatively low resource use. Among the HCP-targeted BCTs, ‘social support (practical)’, ‘prompts/cues’ and ‘restructuring the social environment’ were associated with relatively high resource use; ‘instruction on how to perform the behaviour’ and ‘credible source’ were associated with moderately high resource use; and ‘feedback on outcomes of behaviour/biofeedback’ and ‘adding objects to the environment’ were associated with relatively low resource use.
Implications for the UK NHS
In the UK, on average, 20% of those offered a DRS appointment fail to attend, with wide geographical variations in screening uptake. 20,23,24Living in areas of high social deprivation, a younger age and having a longer duration of diabetes are associated with lower attendance rates. Ethnicity is also an important determinant of DRS attendance, with black, Asian and minority ethnic groups less likely to attend for screening. How can attendance be improved for the missing 20%?
Integrating our findings to the specific context of the UK National Diabetic Eye Screening Programme, during a knowledge exchange event (seeAppendix 2), we agreed that interventions to increase the uptake of DRS should focus on low or non-attenders, especially those with multiple missed appointments, rather than attempting to achieve 100% for all. Special consideration is needed of working people, people with mental health issues, vulnerable groups including those on low incomes or those with special needs and people with multiple conditions and thus competing demands on their time. It was noted that there is great diversity across the UK and that initiatives should be tailored to local needs. The feasibility and ethical issues around focusing on low attenders and vulnerable groups need to be explored in future research.
Based on the study findings on key determinants of attendance, potentially effective and likely cost-effective interventions and the views of stakeholders, the following strategies could improve attendance at DRS.
-
Patient-focused interventions: improve patient information regarding DRS to improve patients’ knowledge about the condition and the screening process, in particular the differences between a regular eye test and a DRS appointment; work with other health professionals to use every potential health-care visit to provide information about eye health; work closely with diabetes teams and patient groups to review and update existing diabetes education programmes for both type 1 [Dose Adjustment for Normal Eating (DAFNE)235] and type 2 (X-PERT Health236and DESMOND237) diabetes patients so that they include relevant content on eye health and visual loss and its relation to general diabetes care; design and deliver media campaigns to ensure that the message is targeted and relevant to people with diabetes; strategies could include components that target the emotional influences on DRS attendance, which we identified as being under-represented in current interventions.
-
Team changes: expand the role of retinal screeners so that they provide patients with information about the findings at the time of screening and feed back to patients how the impact of these on vision can be lessened; involve pharmacists to provide information, including a point of contact, to patients when they attend a pharmacy for any diabetes-related medications or supplies; reassess and realise the importance of the GP in terms of information provision and encouraging patients to attend for screening [GPs (and general practice staff) are among the most trusted professions involved in patient care].
-
Environment: restructure the screening setting, for example the use of ‘one-stop shops’, so that all appointments related to diabetes occur in one place and during one patient visit; flexible timing of appointments, including appointments outside normal working hours; provide reminders; enable patients to self-book appointments, including the ability to cancel and reschedule; manage and update existing electronic registers in terms of completeness both by fostering better relationships with other health-care providers and through the use of health system intelligence such as electronic care records.
-
There were no data on the effectiveness of financial incentives to encourage DRS attendance. The UK stakeholder group proposed this as a possible intervention to improve uptake for those with a history of poor attendance, especially in areas where socioeconomic deprivation is high and for those for whom loss of income while attending appointments is a barrier. An abstract238for a UK-based RCT investigating the use of financial incentives to improve DRS attendance was identified, which did not meet our inclusion criteria. This study has now reported in full. 239
Recommendations for future research
The evidence synthesis identified interventions that could improve the uptake of DRS in different health systems including in the context of a national DRS programme as in the UK. We have identified a number of knowledge gaps that should be addressed by future research. We were unable to identify any RCTs that evaluated the effectiveness of QI interventions for ongoing attendance for DRS. There has been considerable debate on the optimal screening interval for diabetic retinopathy. A recent UK cost-effectiveness study concluded that annual screening was not cost-effective and recommended the introduction of personalised screening intervals. 224Nonetheless, periodic screening is essential to detect sight-threatening retinopathy and future trials should be of a sufficient length to capture multiple screening episodes (e.g. over 5 years). The longer timescale would also allow other important longer-term outcomes to be evaluated, for example the effect of an intervention on the progression of diabetic retinopathy and the impact on visual function.
Although the included studies provided data on a large number of participants, the characteristics of the study participants were often poorly described and data were not provided on the relative effectiveness of interventions in population subgroups. These data are important to illuminate health inequities. It was disappointing that < 5% of the included trials were conducted in the UK.
Through exploratory analyses of evidence from both randomised and non-randomised studies, we identified BCTs that we would regard as ‘best bets’ for evaluation in future RCTs, based on their theoretical coherence with identified barriers and enablers and their clinical effectiveness and cost-effectiveness. However, the complexity of the QI interventions made it difficult to completely disentangle the BCT components to identify the optimal combination of BCT components that would lead to a greater likelihood of DRS attendance. Future research could test particular BCTs and BCT combinations in adequately powered studies using head-to-head comparisons, including those theoretically coherent BCTs identified in our mapping exercise that have not been commonly used in previous RCTs. Particular gaps occur in relation to BCT components that target the emotional barriers to and enablers of screening attendance.
Research priorities
The evidence from this study can be used to inform the development of a future UK-based RCT evaluating QI interventions that use BCTs to improve DRS attendance. The design of the RCT can be framed using thepopulation,intervention,comparator,outcome(s) andtime frame (PICOT) format. Based on our findings we suggest the following design.
-
Population: the evidence on cost-effectiveness suggests that the focus should be on groups with a low baseline level of attendance.
-
Intervention: given that we identified that general (untargeted) QI interventions were statistically indistinguishable from targeted interventions in terms of improving DRS attendance, both types of intervention could be considered. Ideally, the intervention should be multifaceted and utilise BCTs that target the patient (e.g. ‘feedback on outcomes of behaviour’, ‘social support’ or ‘information about health consequences’) or the HCP (e.g. ‘adding objects to the environment’, such as decision support or monitoring tools such as diabetes passports). A number of additional BCTs that are not included in existing interventions may also be relevant to addressing important theoretical determinants of screening attendance. In particular, there are gaps around the inclusion of intervention components to address the emotional barriers to and enablers of screening attendance (such as anxiety about the experience of the screening test itself or fear of the screening result and sight loss).
-
Comparator: the findings of our evidence synthesis were based on comparisons with usual care. However, in included studies, usual care was either poorly described or not described at all. It is therefore important that usual care is specified in sufficient detail so that BCTs present in the control arm can be identified.
-
Outcome(s): the primary outcome should be attendance for DRS. Secondary outcomes could include other quality indicators of diabetes care, for example HbA1clevels, screening for other microvascular complications, ongoing attendance for DRS and the likely cost-effectiveness of the intervention.
-
Time frame: the study should be of a sufficient duration to capture ongoing attendance for DRS. The duration of the trial should be a minimum of 24 months (and ideally longer).
Before conducting the proposed RCT we recommend the following programme of research.
-
Identify the population with a low rate of DRS attendance: review electronic systems for recording DRS programme attendance, audit attendance records and analyse the characteristics of non-attenders, with special emphasis on those who have missed several appointments as these patients might be at the highest risk for visual loss.
-
Conduct a qualitative exploration of the key determinants of attendance in subgroups of low attendees to inform the design of meaningful interventions, with attention to ethical issues around identifying subgroups and targeting interventions to these subgroups.
-
Conduct empirical research to evaluate the validity of mapping tools to facilitate the selection of theoretically coherent BCTs (i.e. those that target important barriers and enablers) for future research and intervention design.
Acknowledgements
The project team acknowledges the help of:
Iris Gordon (Information Specialist for the Cochrane Eyes and Vision Group) for developing the electronic search strategies for the two systematic reviews.
Michael Bowen (Director of Research at the College of Optometrists) for facilitating a group discussion at the knowledge exchange event.
Katherine Cowan (Senior Adviser at the James Lind Alliance) for facilitating a group discussion at the knowledge exchange event.
Sally Crowe (Crowe Associates Ltd) for facilitating a group discussion at the knowledge exchange event and for writing the knowledge exchange report, which summarised the day’s event (seeAppendix 2).
Kristin Danko (Research Co-ordinator at the Ottawa Hospital Research Institute) for providing details of published studies on general QI strategies for diabetes care that included DRS outcomes.
Ana Quartilho (Senior Medical Statistician at Moorfields Eye Hospital NHS Foundation Trust) for contributing to the conceptualisation of the phase 1 review protocol.
Anupa Shah (Managing Editor at the Cochrane Eyes and Vision Group) for assisting with the publication of the Cochrane protocol for the phase 1 review.
All members of the project stakeholder advisory group for their input into the development of the project protocol.
All attendees at the knowledge exchange event for their contributions to the group discussions.
The following people from the Institute of Health and Society, Newcastle University, who contributed to the testing of the ordered ranking classification for resource requirements: Dimitrios Tzelis (Research Assistant), Kai Wai Lee (Research Assistant), Brenda Nyakang’o (Research Assistant), Kristoffer Halvorsrud (Research Associate) and Fiona Beyer (Information Specialist).
Contributions of authors
John G Lawrenson(Professor of Clinical Visual Science) was the principal investigator of the overall project and led the first systematic review. He screened, selected and quality appraised studies and coded the intervention content and resource use for the first systematic review and contributed to the quality appraisal for the second systematic review. He also led on the conceptualisation of the project protocol and wrote sections of and edited the final report.
Ella Graham-Rowe(Research Fellow) managed much of the day-to-day running of the overall project and led the second systematic review. She screened, selected and quality appraised studies and extracted and synthesised data for the second systematic review and contributed to extraction of data and coding of intervention content and resource use for the first systematic review. She also wrote sections of and edited the final report.
Fabiana Lorencatto(Research Fellow) led the mapping study in phase 3, contributed to coding of the intervention content in the first systematic review and contributed to the screening of studies and analysis of extracted data for the second systematic review. She also contributed to the conceptualisation of the project protocol and wrote sections of and edited the final report.
Stephen Rice(Senior Research Associate) conducted the economic modelling study in phase 3 and designed and developed an algorithm for resource requirements for the systematic review in phase 1. He also contributed to the conceptualisation of the project protocol and wrote sections of and edited the final report.
Catey Bunce(Reader in Medical Statistics) conducted the statistical analysis for the first systematic review. She also contributed to the conceptualisation of the project protocol and edited the final report.
Jill J Francis(Professor of Health Services Research) provided senior input into the second systematic review and the mapping study. She also contributed to the conceptualisation of the project protocol and edited the final report.
Jennifer M Burr(Reader) was the co-principal investigator for the overall project. She screened, selected and quality appraised studies for the first systematic review. She also contributed to the conceptualisation of the project protocol and wrote sections of and edited the final report.
Patricia Aluko(Research Assistant) conducted the economic evaluation review as part of the systematic review in phase 1. She also wrote sections of and edited the final report.
Luke Vale(Professor) provided senior input into the economic evaluation review in phase 1 and the economic modelling in phase 3. He also contributed to the conceptualisation of the project protocol and edited the final report.
Tunde Peto(Clinical Professor) provided clinical expertise, contributed to the conceptualisation of the project protocol and edited the final report.
Justin Presseau(Assistant Professor) provided behavioural science expertise to inform the BCT coding in the first review and the TDF coding in the second review and edited the final report.
Noah M Ivers(Family Physician and Clinical Scientist) provided methodological expertise and clinical expertise from the perspective of the management of diabetes in primary care. He also contributed to the conceptualisation of the project protocol and edited the final report.
Jeremy M Grimshaw(Senior Scientist) contributed to the conceptualisation of the project protocol, provided senior input into all aspects of the project and edited the final report.
Publications
Lawrenson JG, Graham-Rowe E, Lorencatto F, Burr J, Bunce C, Francis JJ,et al. Interventions to increase attendance for diabetic retinopathy screening. Cochrane Database Syst Rev2018;10:CD012054.
Graham-Rowe E, Lorencatto F, Lawrenson JG, Burr JM, Grimshaw JM, Ivers NM,et al. Barriers and enablers to diabetic retinopathy screening attendance: a systematic review of published and grey literature [published online ahead of print May 23 2018]. Diabet Med2018.
Data sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care..
References
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137-49. https://doi.org/10.1016/j.diabres.2013.11.002.
- Global Report on Diabetes. Geneva: WHO; 2016.
- Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol 2012;57:347-70. https://doi.org/10.1016/j.survophthal.2012.01.004.
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004015.
- Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD011234.pub2.
- Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 2014;6. https://doi.org/10.1002/14651858.CD007419.pub4.
- Wilson JMG, Jungner G. The Principles and Practice of Screening for Disease. Geneva: WHO; 1968.
- Scanlon PH. The English national screening programme for sight-threatening diabetic retinopathy. J Med Screen 2008;15:1-4. https://doi.org/10.1258/jms.2008.008015.
- Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med 2010;27:249-56. https://doi.org/10.1111/j.1464-5491.2009.02870.x.
- Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes 2017;9:320-4. https://doi.org/10.1111/1753-0407.12524.
- Boyd SR, Advani A, Altomare F, Stockl F. Canadian Diabetes Association Clinical Practice Guideline Expert Committee . Clinical Practice Guidelines: retinopathy. Can J Diabetes 2013;37:137-41. https://doi.org/10.1016/j.jcjd.2013.01.038.
- Quality Standards for Diabetes Care Toolkit. Wellington: Ministry of Health; 2014.
- Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Ophthalmology. Ophthalmology 2004;111:1055-62. https://doi.org/10.1016/j.ophtha.2004.02.004.
- Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology 2013;120:2604-10. https://doi.org/10.1016/j.ophtha.2013.06.029.
- Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al. The value of digital imaging in diabetic retinopathy. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7300.
- Silva PS, Cavallerano JD, Aiello LM. Ocular telehealth initiatives in diabetic retinopathy. Curr Diab Rep 2009;9:265-71. https://doi.org/10.1007/s11892-009-0041-6.
- Taylor CR, Merin LM, Salunga AM, Hepworth JT, Crutcher TD, O’Day DM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care 2007;30:574-8. https://doi.org/10.2337/dc06-1509.
- National Service Framework for Diabetes: Standards. London: Department of Health; 2001.
- Gulliford MC, Ashworth M, Robotham D, Mohiddin A. Achievement of metabolic targets for diabetes by English primary care practices under a new system of incentives. Diabet Med 2007;24:505-11. https://doi.org/10.1111/j.1464-5491.2007.02090.x.
- Millett C, Dodhia H. Diabetes retinopathy screening: audit of equity in participation and selected outcomes in South East London. J Med Screen 2006;13:152-5. https://doi.org/10.1258/096914106778440608.
- Paz SH, Varma R, Klein R, Wu J, Azen SP. Los Angeles Latino Eye Study Group . Noncompliance with vision care guidelines in Latinos with type 2 diabetes mellitus: the Los Angeles Latino Eye Study. Ophthalmology 2006;113:1372-7. https://doi.org/10.1016/j.ophtha.2006.04.018.
- Zhang X, Norris SL, Saadine J, Chowdhury FM, Horsley T, Kanjilal S, et al. Effectiveness of interventions to promote screening for diabetic retinopathy. Am J Prev Med 2007;33:318-35. https://doi.org/10.1016/j.amepre.2007.05.002.
- Leese GP, Boyle P, Feng Z, Emslie-Smith A, Ellis JD. Screening uptake in a well-established diabetic retinopathy screening program: the role of geographical access and deprivation. Diabetes Care 2008;31:2131-5. https://doi.org/10.2337/dc08-1098.
- Orton E, Forbes-Haley A, Tunbridge L, Cohen S. Equity of uptake of a diabetic retinopathy screening programme in a geographically and socio-economically diverse population. Public Health 2013;127:814-21. https://doi.org/10.1016/j.puhe.2013.04.015.
- Kliner M, Fell G, Gibbons C, Dhothar M, Mookhtiar M, Cassels-Brown A. Diabetic retinopathy equity profile in a multi-ethnic, deprived population in Northern England. Eye 2012;26:671-7. https://doi.org/10.1038/eye.2012.3.
- Gulliford MC, Dodhia H, Chamley M, McCormick K, Mohamed M, Naithani S, et al. Socio-economic and ethnic inequalities in diabetes retinal screening. Diabet Med 2010;27:282-8. https://doi.org/10.1111/j.1464-5491.2010.02946.x.
- Shojania KG, McDonald KM, Wachter RM, Owens DK. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 1: Series Overview and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2004.
- Worswick J, Wayne SC, Bennett R, Fiander M, Mayhew A, Weir MC, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews – what does the evidence tell us?. Syst Rev 2013;2. https://doi.org/10.1186/2046-4053-2-26.
- Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252-61. https://doi.org/10.1016/S0140-6736(12)60480-2.
- Behaviour Change: General Approaches. Manchester: NICE; 2007.
- Albarracin D, Gillette JC, Earl AN, Glasman LR, Durantini MR, Ho MH. A test of major assumptions about behavior change: a comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol Bull 2005;131:856-97. https://doi.org/10.1037/0033-2909.131.6.856.
- Noar SM, Zimmerman RS. Health behavior theory and cumulative knowledge regarding health behaviors: are we moving in the right direction?. Health Educ Res 2005;20:275-90. https://doi.org/10.1093/her/cyg113.
- Camilloni L, Ferroni E, Cendales BJ, Pezzarossi A, Furnari G, Borgia P, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-464.
- Brouwers MC, De Vito C, Bahirathan L, Carol A, Carroll JC, Cotterchio M, et al. What implementation interventions increase cancer screening rates? A systematic review. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-111.
- Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J. The determinants of screening uptake and interventions for increasing uptake: a systematic review. Health Technol Assess 2000;4.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. ‘Psychological Theory’ Group . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8060.
- Squires JE, Sullivan K, Eccles MP, Worswick J, Grimshaw JM. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals’ behaviours? An overview of systematic reviews. Implement Sci 2014;9. https://doi.org/10.1186/s13012-014-0152-6.
- Grimshaw JM, Presseau J, Tetroe J, Eccles MP, Francis JJ, Godin G, et al. Looking inside the black box: results of a theory-based process evaluation exploring the results of a randomized controlled trial of printed educational messages to increase primary care physicians’ diabetic retinopathy referrals [Trial registration number ISRCTN72772651]. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-86.
- Effective Practice and Organisation of Care (EPOC) Group . EPOC Taxonomy 2015. https://epoc.cochrane.org/epoc-taxonomy (accessed 18 July 2017).
- Presseau J, Ivers NM, Newham JJ, Knittle K, Danko KJ, Grimshaw JM. Using a behaviour change techniques taxonomy to identify active ingredients within trials of implementation interventions for diabetes care. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0248-7.
- Lawrenson JG, Graham-Rowe E, Lorencatto F, Presseau J, Burr J, Ivers N, et al. Interventions to increase attendance for diabetic retinopathy screening. Cochrane Database Syst Rev 2016;1. https://doi.org/10.1002/14651858.CD012054.
- Ivers N, Tricco AC, Trikalinos TA, Dahabreh IJ, Danko KJ, Moher D, et al. Seeing the forests and the trees – innovative approaches to exploring heterogeneity in systematic reviews of complex interventions to enhance health system decision-making: a protocol. Syst Rev 2014;3. https://doi.org/10.1186/2046-4053-3-88.
- Effective Practice and Organisation of Care (EPOC) Group . Data Collection Checklist 2017. https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC%20Data%20Collection%20Checklist.pdf (accessed 18 July 2017).
- O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol 2014;67:56-64. https://doi.org/10.1016/j.jclinepi.2013.08.005.
- World Bank . The World Bank Atlas Method – Detailed Methodology 2017. https://datahelpdesk.worldbank.org/knowledgebase/articles/378832-what-is-the-world-bank-atlas-method (accessed 18 July 2017).
- Centre for Reviews and Dissemination (CRD) . Improving Access to Cost-Effectiveness Information for Health Care Decision-Making: The NHS Economic Evaluation Database 2001. www.york.ac.uk/crd/publications/reports-books/ (accessed 24 October 2017).
- Cochrane Collaboration . Suggested Risk of Bias Criteria for Cochrane Effective Practice and Organisation of Care (EPOC) Group Reviews 2015. http://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 18 July 2017).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Higgins JPT, Deeks JJ, Altman DG, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). 2011.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) 2011. cochrane-handbook.org (accessed 18 July 2017).
- Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010;6:51-9. https://doi.org/10.1332/174426410X482999.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Peterson KA, Radosevich DM, O’Connor PJ, Nyman JA, Prineas RJ, Smith SA, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care 2008;31:2238-43. https://doi.org/10.2337/dc08-2034.
- Adair R, Wholey DR, Christianson J, White KM, Britt H, Lee S. Improving chronic disease care by adding laypersons to the primary care team: a parallel randomized trial. Ann Intern Med 2013;159:176-84. https://doi.org/10.7326/0003-4819-159-3-201308060-00007.
- Barceló A, Cafiero E, de Boer M, Mesa AE, Lopez MG, Jiménez RA, et al. Using collaborative learning to improve diabetes care and outcomes: the VIDA project. Prim Care Diabetes 2010;4:145-53. https://doi.org/10.1016/j.pcd.2010.04.005.
- Choe HM, Mitrovich S, Dubay D, Hayward RA, Krein SL, Vijan S. Proactive case management of high-risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. Am J Manag Care 2005;11:253-60.
- Clancy DE, Huang P, Okonofua E, Yeager D, Magruder KM. Group visits: promoting adherence to diabetes guidelines. J Gen Intern Med 2007;22:620-4. https://doi.org/10.1007/s11606-007-0150-3.
- Davis R, Hitch A, Salaam M, Herman W, Zimmer-Galler I, Mayer-Davis E. TeleHealth improves diabetes self-management in an underserved community diabetes telecare. Diabetes Care 2010;33:1712-17. https://doi.org/10.2337/dc09-1919.
- Dickinson WP, Dickinson LM, Nutting PA, Emsermann CB, Tutt B, Crabtree BF, et al. Practice facilitation to improve diabetes care in primary care: a report from the EPIC randomized clinical trial. Ann Fam Med 2014;12:8-16. https://doi.org/10.1370/afm.1591.
- Dijkstra RF, Braspenning JC, Huijsmans Z, Akkermans RP, van Ballegooie E, ten Have P, et al. Introduction of diabetes passports involving both patients and professionals to improve hospital outpatient diabetes care. Diabetes Res Clin Pract 2005;68:126-34. https://doi.org/10.1016/j.diabres.2004.09.020.
- Dijkstra R, Braspenning J, Grol R. Implementing diabetes passports to focus practice reorganization on improving diabetes care. Int J Qual Health Care 2008;20:72-7. https://doi.org/10.1093/intqhc/mzm051.
- Eccles MP, Whitty PM, Speed C, Steen IN, Vanoli A, Hawthorne GC, et al. A pragmatic cluster randomised controlled trial of a Diabetes REcall And Management system: the DREAM trial. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-6.
- Franco JM, de Chazournes P, Falcoff H. Impact of peer visits. Rev Prat 2007;57:1211-17.
- Frei A, Senn O, Chmiel C, Reissner J, Held U, Rosemann T. Implementation of the chronic care model in small medical practices improves cardiovascular risk but not glycemic control. Diabetes Care 2014;37:1039-47. https://doi.org/10.2337/dc13-1429.
- Frijling BD, Lobo CM, Hulscher ME, Akkermans RP, Braspenning JC, Prins A, et al. Multifaceted support to improve clinical decision making in diabetes care: a randomized controlled trial in general practice. Diabet Med 2002;19:836-42. https://doi.org/10.1046/j.1464-5491.2002.00810.x.
- Gabbay RA, Lendel I, Saleem TM, Shaeffer G, Adelman AM, Mauger DT, et al. Nurse case management improves blood pressure, emotional distress and diabetes complication screening. Diabetes Res Clin Pract 2006;71:28-35. https://doi.org/10.1016/j.diabres.2005.05.002.
- Gabbay RA, Anel-Tiangco RM, Dellasega C, Mauger DT, Adelman A, Van Horn DHA. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): results of a 2-year randomized controlled pragmatic trial. J Diabetes 2013;5:349-57. https://doi.org/10.1111/1753-0407.12030.
- Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, et al. Randomized effectiveness trial of a computer-assisted intervention to improve diabetes care. Diabetes Care 2005;28:33-9. https://doi.org/10.2337/diacare.28.1.33.
- Guldberg TL, Vedsted P, Kristensen JK, Lauritzen T. Improved quality of type 2 diabetes care following electronic feedback of treatment status to general practitioners: a cluster randomized controlled trial. Diabet Med 2011;28:325-32.
- Gutierrez N, Gimple NE, Dallo FJ, Foster BM, Ohagi EJ. Shared medical appointments in a residency clinic: an exploratory study among Hispanics with diabetes. Am J Manag Care 2011;17:e212-e4.
- Harris SB, Webster-Bogaert S, Van DM, O’Neill C, Leiter LA. Teleconferenced educational detailing: diabetes education for primary care physicians. J Contin Educ Health Prof 2005;25:87-9. https://doi.org/10.1002/chp.13.
- Hayashino Y, Suzuki H, Yamazaki K, Goto A, Izumi K, Noda M. A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: the Japan Diabetes Outcome Intervention Trial-2 (J-DOIT2). Diabet Med 2016;33:599-608. https://doi.org/10.1111/dme.12949.
- Hermans MP, Elisaf M, Michel G, Muls E, Nobels F, Vandenberghe H, et al. OPTIMISE International Steering Committee . Benchmarking is associated with improved quality of care in type 2 diabetes: the OPTIMISE randomized, controlled trial. Diabetes Care 2013;36:3388-95. https://doi.org/10.2337/dc12-1853.
- Herrin J, Nicewander DA, Hollander PA, Couch CE, Winter FD, Haydar ZR, et al. Effectiveness of diabetes resource nurse case management and physician profiling in a fee-for-service setting: a cluster randomized trial. Proc (Bayl Univ Med Cent) 2006;19:95-102.
- Hurwitz B, Goodman C, Yudkin J. Prompting the clinical care of non-insulin dependent (type II) diabetic patients in an inner city area: one model of community care. BMJ 1993;306:624-30. https://doi.org/10.1136/bmj.306.6878.624.
- Ilag LL, Martin CL, Tabaei BP, Isaman DJ, Burke R, Greene DA, et al. Improving diabetes processes of care in managed care. Diabetes Care 2003;26:2722-7. https://doi.org/10.2337/diacare.26.10.2722.
- Jacobs M, Sherry PS, Taylor LM, Amato M, Tataronis GR, Cushing G. Pharmacist Assisted Medication Program Enhancing the Regulation of Diabetes (PAMPERED) study. J Am Pharm Assoc (2003) 2012;52:613-21. https://doi.org/10.1331/JAPhA.2012.10183.
- Jansink R, Braspenning J, Keizer E, van der Weijden T, Elwyn G, Grol R. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial. Scand J Prim Health Care 2013;31:119-27. https://doi.org/10.3109/02813432.2013.797178.
- Kirwin JL, Cunningham RJ, Sequist TD. Pharmacist recommendations to improve the quality of diabetes care: a randomized controlled trial. J Manag Care Pharm 2010;16:104-13. https://doi.org/10.18553/jmcp.2010.16.2.104.
- Krein SL, Klamerus ML, Vijan S, Lee JL, Fitzgerald JT, Pawlow A, et al. Case management for patients with poorly controlled diabetes: a randomized trial. Am J Med 2004;116:732-9. https://doi.org/10.1016/j.amjmed.2003.11.028.
- Lafata JE, Baker AM, Divine GW, McCarthy BD, Xi H. The use of computerized birthday greeting reminders in the management of diabetes. J Gen Intern Med 2002;17:521-30. https://doi.org/10.1046/j.1525-1497.2002.10901.x.
- Litaker D, Mion L, Planavsky L, Kippes C, Mehta N, Frolkis J. Physician–nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients’ perception of care. J Interprof Care 2003;17:223-37. https://doi.org/10.1080/1356182031000122852.
- Maljanian R, Grey N, Staff I, Conroy L. Intensive telephone follow-up to a hospital-based disease management model for patients with diabetes mellitus. Dis Manag 2005;8:15-2. https://doi.org/10.1089/dis.2005.8.15.
- McCall N, Cromwell J. Results of the Medicare Health Support disease-management pilot program. N Engl J Med 2011;365:1704-12. https://doi.org/10.1056/NEJMsa1011785.
- McClellan WM, Millman L, Presley R, Couzins J, Flanders WD. Improved diabetes care by primary care physicians: results of a group-randomized evaluation of the Medicare Health Care Quality Improvement Program (HCQIP). J Clin Epidemiol 2003;56:1210-17. https://doi.org/10.1016/S0895-4356(03)00198-7.
- McDermott RA, Schmidt BA, Sinha A, Mills P. Improving diabetes care in the primary healthcare setting: a randomised cluster trial in remote indigenous communities. Med J Aust 2001;174:497-502.
- Meigs JB, Cagliero E, Dubey A, Murphy-Sheehy P, Gildesgame C, Chueh H, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care 2003;26:750-7. https://doi.org/10.2337/diacare.26.3.750.
- O’Connor PJ, Desai J, Solberg LI, Reger LA, Crain AL, Asche SE, et al. Randomized trial of quality improvement intervention to improve diabetes care in primary care settings. Diabetes Care 2005;28:1890-7. https://doi.org/10.2337/diacare.28.8.1890.
- Perria C, Mandolini D, Guerrera C, Jefferson T, Billi P, Calzini V, et al. Implementing a guideline for the treatment of type 2 diabetics: results of a cluster-randomized controlled trial (C-RCT). BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-79.
- Piette JD, Weinberger M, Kraemer FB, McPhee SJ. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial. Diabetes Care 2001;24:202-8. https://doi.org/10.2337/diacare.24.2.202.
- Prezio EA, Balasubramanian BA, Shuval K, Cheng D, Kendzor DE, Culica D. Evaluation of quality improvement performance in the Community Diabetes Education (CoDE) program for uninsured Mexican Americans: results of a randomized controlled trial. Am J Med Qual 2014;29:124-34. https://doi.org/10.1177/1062860613489165.
- Schnipper JL, Linder JA, Palchuk MB, Yu DT, McColgan KE, Volk LA, et al. Effects of documentation-based decision support on chronic disease management. Am J Manag Care 2010;16:P72-81.
- Simon SR, Trinacty CM, Soumerai SB, Piette JD, Meigs JB, Shi P, et al. Improving diabetes care among patients overdue for recommended testing: a randomized controlled trial of automated telephone outreach. Diabetes Care 2010;33:1452-3. https://doi.org/10.2337/dc09-2332.
- Simpson SH, Majumdar SR, Tsuyuki RT, Lewanczuk RZ, Spooner R, Johnson JA. Effect of adding pharmacists to primary care teams on blood pressure control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2011;34:20-6. https://doi.org/10.2337/dc10-1294.
- Sonnichsen AC, Winkler H, Flamm M, Panisch S, Kowatsch P, Klima G, et al. The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster-randomised controlled trial. BMC Fam Pract 2010;11. https://doi.org/10.1186/1471-2296-11-86.
- Steyn K, Lombard C, Gwebushe N, Fourie JM, Everett-Murphy K, Zwarenstein M, et al. Implementation of national guidelines, incorporated within structured diabetes and hypertension records at primary level care in Cape Town, South Africa: a randomised controlled trial. Global Health Action 2013;6. https://doi.org/10.3402/gha.v6i0.20796.
- Taylor CB, Miller NH, Reilly KR, Greenwald G, Cunning D, Deeter A, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care 2003;26:1058-63. https://doi.org/10.2337/diacare.26.4.1058.
- Varney JE, Weiland TJ, Inder WJ, Jelinek GA. Effect of hospital-based telephone coaching on glycaemic control and adherence to management guidelines in type 2 diabetes, a randomised controlled trial. Intern Med J 2014;44:890-7. https://doi.org/10.1111/imj.12515.
- Vidal-Pardo JI, Perez-Castro TR, Lopez-Alvarez XL, Santiago-Perez MI, Garcia-Soidan FJ, Muniz J. Effect of an educational intervention in primary care physicians on the compliance of indicators of good clinical practice in the treatment of type 2 diabetes mellitus (OBTEDIGA project). Int J Clin Pract 2013;67:750-8. https://doi.org/10.1111/ijcp.12145.
- Wagner EH, Grothaus LC, Sandhu N, Galvin MS, McGregor M, Artz K, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care 2001;24:695-700. https://doi.org/10.2337/diacare.24.4.695.
- Ward A, Kamien M, Mansfield F, Fatovich B. Educational feedback in the management of type 2 diabetes in general practice. Educ Gen Pract 1996;7:142-50.
- Welch G, Allen NA, Zagarins SE, Stamp KD, Bursell SE, Kedziora RJ. Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center. Diabetes Educ 2011;37:680-8. https://doi.org/10.1177/0145721711416257.
- Anderson RM, Musch DC, Nwankwo RB, Wolf FM, Gillard ML, Oh MS, et al. Personalized follow-up increases return rate at urban eye disease screening clinics for African Americans with diabetes: results of a randomized trial. Ethn Dis 2003;13:40-6.
- Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. Am J Public Health 1999;89:1878-82. https://doi.org/10.2105/AJPH.89.12.1878.
- Bush K, Thomas R, Raymond NT, Sankar S, Barker PJ, O’Hare JP. Cluster randomised controlled trial evaluation of a link worker-delivered intervention to improve uptake of diabetic retinopathy screening in a South Asian population. Diab Vasc Dis Res 2014;11:294-7. https://doi.org/10.1177/1479164114532964.
- Conlin PR, Fisch BM, Cavallerano AA, Cavallerano JD, Bursell SE, Aiello LM. Nonmydriatic teleretinal imaging improves adherence to annual eye examinations in patients with diabetes. J Rehabil Res Dev 2006;43:733-40. https://doi.org/10.1682/JRRD.2005.07.0117.
- Davis RM, Fowler S, Bellis K, Pockl J, Al Pakalnis V, Woldorf A. Telemedicine improves eye examination rates in individuals with diabetes: a model for eye-care delivery in underserved communities. Diabetes Care 2003;26. https://doi.org/10.2337/diacare.26.8.2476.
- Ellish NJ, Royak-Schaler R, Higginbotham EJ. Tailored and targeted interventions to encourage dilated fundus examinations in older African Americans. Arch Ophthalmol 2011;129:1592-8. https://doi.org/10.1001/archophthalmol.2011.190.
- Halbert RJ, Leung KM, Nichol JM, Legorreta AP. Effect of multiple patient reminders in improving diabetic retinopathy screening. A randomized trial. Diabetes Care 1999;22:752-5. https://doi.org/10.2337/diacare.22.5.752.
- Lian JX, McGhee SM, Gangwani RA, Hedley AJ, Lam CL, Yap MK, et al. Screening for diabetic retinopathy with or without a copayment in a randomized controlled trial: influence of the inverse care law. Ophthalmology 2013;120:1247-53. https://doi.org/10.1016/j.ophtha.2012.11.024.
- Mansberger SL, Sheppler C, Barker G, Gardiner SK, Demirel S, Wooten K, et al. Long-term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: a randomized clinical trial. JAMA Ophthalmol 2015;133:518-25. https://doi.org/10.1001/jamaophthalmol.2015.1.
- Pizzi LT, Zangalli CS, Murchison AP, Hale N, Hark L, Dai Y, et al. Prospective randomized controlled trial comparing the outcomes and costs of two eyecare adherence interventions in diabetes patients. Appl Health Econ Health Policy 2015;13:253-63. https://doi.org/10.1007/s40258-015-0159-4.
- Prela CM, Smilie JG, McInerney MJ, Harwell TS, Helgerson SD. Direct mail intervention to increase retinal examination rates in Medicare beneficiaries with diabetes. Am J Med Qual 2000;15:257-62.
- Rosenkranz S. Polaroid-fundus photography enhances patient compliance with screening for diabetic retinopathy. Diabetes Stoffwechsel 1996;5:69-75.
- Walker EA, Schechter CB, Caban A, Basch CE. Telephone intervention to promote diabetic retinopathy screening among the urban poor. Am J Prev Med 2008;34:185-91. https://doi.org/10.1016/j.amepre.2007.11.020.
- Weiss DM, Casten RJ, Leiby BE, Hark LA, Murchison AP, Johnson D, et al. Effect of behavioral intervention on dilated fundus examination rates in older African American individuals with diabetes mellitus: a randomized clinical trial. JAMA Ophthalmol 2015;133:1005-12. https://doi.org/10.1001/jamaophthalmol.2015.1760.
- Zangalli CS, Murchison AP, Hale N, Hark LA, Pizzi LT, Dai Y, et al. An education- and telephone-based intervention to improve follow-up to vision care in patients with diabetes: a prospective, single-blinded, randomized trial. Am J Med Qual 2016;31:156-61. https://doi.org/10.1177/1062860614552670.
- Zwarenstein M, Shiller SK, Croxford R, Grimshaw JM, Kelsall D, Paterson JM, et al. Printed educational messages aimed at family practitioners fail to increase retinal screening among their patients with diabetes: a pragmatic cluster randomized controlled trial (ISRCTN72772651). Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-87.
- Davis R, Mayer-Davis EJ. Cost effectiveness of a telehealth-based diabetes self-management (DSME) intervention in a rural community. Diabetes 2011;60:A325-6.
- Schechter CB, Basch CE, Caban A, Walker EA. Cost effectiveness of a telephone intervention to promote dilated fundus examination in adults with diabetes mellitus. Clin Ophthalmol 2008;2:763-8. https://doi.org/10.2147/OPTH.S3232.
- Prezio EA, Pagán JA, Shuval K, Culica D. The Community Diabetes Education (CoDE) program: cost-effectiveness and health outcomes. Am J Prev Med 2014;47:771-9. https://doi.org/10.1016/j.amepre.2014.08.016.
- Lawrenson JG, Graham-Rowe E, Lorencatto F, Burr J, Bunce C, Francis JJ, et al. Interventions to increase attendance for diabetic retinopathy screening. Cochrane Database Syst Rev 2018;10. https://doi.org/10.1002/14651858.CD012054.pub2.
- Jansink R, Braspenning J, van der Weijden T, Niessen L, Elwyn G, Grol R. Nurse-led motivational interviewing to change the lifestyle of patients with type 2 diabetes (MILD-project): protocol for a cluster, randomized, controlled trial on implementing lifestyle recommendations. BMC Health Serv Res 2009;9. https://doi.org/10.1186/1472-6963-9-19.
- Thorn JC, Noble SM, Hollingworth W. Timely and complete publication of economic evaluations alongside randomized controlled trials. PharmacoEconomics 2013;31:77-85. https://doi.org/10.1007/s40273-012-0004-7.
- Francis JJ, O’Connor D, Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: introducing a thematic series on the theoretical domains framework. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-35.
- Ferlie EB, Shortell SM. Improving the quality of health care in the United Kingdom and the United States: a framework for change. Milbank Q 2001;79:281-315. https://doi.org/10.1111/1468-0009.00206.
- Birken SA, Powell BJ, Presseau J, Kirk MA, Lorencatto F, Gould NJ, et al. Combined use of the Consolidated Framework for Implementation Research (CFIR) and the theoretical domains framework (TDF): a systematic review. Implement Sci 2017;12. https://doi.org/10.1186/s13012-016-0534-z.
- Wilkinson SA, McCray S, Beckmann M, Parry A, McIntyre HD. Barriers and enablers to translating gestational diabetes guidelines into practice. Pract Diabetes Int 2014;31:67-72a. https://doi.org/10.1002/pdi.1833.
- Heslehurst N, Newham J, Maniatopoulos G, Fleetwood C, Robalino S, Rankin J. Implementation of pregnancy weight management and obesity guidelines: a meta-synthesis of healthcare professionals’ barriers and facilitators using the theoretical domains framework. Obes Rev 2014;15:462-86. https://doi.org/10.1111/obr.12160.
- Lipworth W, Taylor N, Braithwaite J. Can the theoretical domains framework account for the implementation of clinical quality interventions?. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-530.
- Little EA, Presseau J, Eccles MP. Understanding effects in reviews of implementation interventions using the theoretical domains framework. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0280-7.
- Graham-Rowe E, Lorencatto F, Lawrenson JG, Burr J, Grimshaw JM, Ivers NM, et al. Barriers and enablers to diabetic retinopathy screening attendance: protocol for a systematic review. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0309-2.
- Diabetes care and research in Europe: the Saint Vincent declaration . Diabetes Med 1990;7. https://doi.org/10.1111/j.1464-5491.1990.tb01405.x.
- Anonymous . Critical Appraisal Skills Programme (CASP) n.d. http://media.wix.com/ugd/dded87_29c5b002d99342f788c6ac670e49f274.pdf (accessed 18 July 2017).
- Anonymous . Mixed Methods Appraisal Tool (MMAT) – Version 2011 n.d. http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/84371689/MMAT%202011%20criteria%20and%20tutorial%202011-06-29updated2014.08.21.pdf (accessed 18 July 2017).
- Helms C, Leask J, Robbins SC, Chow MY, McIntyre P. Implementation of mandatory immunisation of healthcare workers: observations from New South Wales, Australia. Vaccine 2011;29:2895-901. https://doi.org/10.1016/j.vaccine.2011.02.011.
- Hetrick S, Alvarez-Jimenez M, Parker A, Hughes F, Willet M, Morley K, et al. Promoting physical health in youth mental health services: ensuring routine monitoring of weight and metabolic indices in a first episode psychosis clinic. Australas Psychiatry 2010;18:451-5. https://doi.org/10.3109/10398561003731189.
- Nzinga J, Mbindyo P, Mbaabu L, Warira A, English M. Documenting the experiences of health workers expected to implement guidelines during an intervention study in Kenyan hospitals. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-44.
- Duncan EM, Francis JJ, Johnston M, Davey P, Maxwell S, McKay GA, et al. Learning curves, taking instructions, and patient safety: using a theoretical domains framework in an interview study to investigate prescribing errors among trainee doctors. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-86.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Patey AM, Islam R, Francis JJ, Bryson GL, Grimshaw JM, Canada PPT. Anesthesiologists’ and surgeons’ perceptions about routine pre-operative testing in low-risk patients: application of the theoretical domains framework (TDF) to identify factors that influence physicians’ decisions to order pre-operative tests. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-52.
- Lee DJ, Kumar N, Feuer WJ, Chou CF, Rosa PR, Schiffman JC, et al. Dilated eye examination screening guideline compliance among patients with diabetes without a diabetic retinopathy diagnosis: the role of geographic access. BMJ Open Diabetes Res Care 2014;2. https://doi.org/10.1136/bmjdrc-2014-000031.
- Al-Alawi A, Al-Hassan A, Chauhan D, Al-Futais M, Khandekar R. Knowledge, attitude, and perception of barriers for eye care among diabetic persons registered at employee health department of a tertiary eye hospital of central Saudi Arabia. Middle East Afr J Ophthalmol 2016;23:71-4. https://doi.org/10.4103/0974-9233.164629.
- Lindenmeyer A, Sturt JA, Hipwell A, Stratton IM, Al-Athamneh N, Gadsby R, et al. Influence of primary care practices on patients’ uptake of diabetic retinopathy screening: a qualitative case study. Br J Gen Pract 2014;64:e484-92. https://doi.org/10.3399/bjgp14X680965.
- Hwang J, Rudnisky C, Bowen S, Johnson JA. Socioeconomic factors associated with visual impairment and ophthalmic care utilization in patients with type II diabetes. Can J Ophthalmol 2015;50:119-26. https://doi.org/10.1016/j.jcjo.2014.11.014.
- Paksin-Hall A, Dent ML, Dong F, Ablah E. Factors contributing to diabetes patients not receiving annual dilated eye examinations. Ophthalmic Epidemiol 2013;20:281-7. https://doi.org/10.3109/09286586.2013.789531.
- Buonaccorso KM. Diabetic retinopathy screening: a clinical quality improvement project. J Healthc Qual 1999;21:35-8. https://doi.org/10.1111/j.1945-1474.1999.tb01002.x.
- Kiran T, Kopp A, Moineddin R, Victor JC, Campbell RJ, Shah BR, et al. Unintended consequences of delisting routine eye exams on retinopathy screening for people with diabetes in Ontario, Canada. CMAJ 2013;185:E167-73. https://doi.org/10.1503/cmaj.120862.
- Arora S, Kurji AK, Tennant MT. Dismantling sociocultural barriers to eye care with tele-ophthalmology: lessons from an Alberta Cree community. Clin Invest Med 2013;36:E57-63. https://doi.org/10.25011/cim.v36i2.19567.
- Hurrell DD, Donohoe S. The Barriers and Enablers that Affect Access to Primary and Secondary Eye Care Services – Glasgow Site Report. Glasgow: RNIB; 2012.
- Sachdeva A, Stratton IM, Unwin J, Moreton R, Scanlon PH. Diabetic retinopathy screening: study to determine risk factors for non-attendance. Diabetes Prim Care 2012;14:308-16.
- Strutton R, Du Chemin A, Stratton IM, Forster AS. System-level and patient-level explanations for non-attendance at diabetic retinopathy screening in Sutton and Merton (London, UK): a qualitative analysis of a service evaluation. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010952.
- Hipwell AE, Sturt J, Lindenmeyer A, Stratton I, Gadsby R, O’Hare P, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005498.
- Applebee E. The Barriers and Enablers that Affect Access to Primary and Secondary Eye Care Services – Bradford Site Report. London: RNIB; 2012.
- Hartnett ME, Key IJ, Loyacano NM, Horswell RL, Desalvo KB. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol 2005;123:387-91. https://doi.org/10.1001/archopht.123.3.387.
- Al-Malki . Barriers Prevent Diabetic Patients from Attending Diabetic Retinopathy Screening at Primary Eye Care Clinics at Primary Health Care in the State of Qatar 2009.
- Jones BJ, Mitra S, Price HC, Stratton IM. Factors affecting uptake of retinal screening in primary care. Diabet Med 2011;28.
- Tapp RJ, Zimmet PZ, Harper CA, de Courten MP, Balkau B, McCarty DJ, et al. Diabetes care in an Australian population: frequency of screening examinations for eye and foot complications of diabetes. Diabetes Care 2004;27:688-93. https://doi.org/10.2337/diacare.27.3.688.
- Hatef E, Vanderver BG, Fagan P, Albert M, Alexander M. Annual diabetic eye examinations in a managed care Medicaid population. Am J Manag Care 2015;21:e297-302.
- Dervan E, Lillis D, Flynn L, Staines A, O’Shea D. Factors that influence the patient uptake of diabetic retinopathy screening. Ir J Med Sci 2008;177:303-8. https://doi.org/10.1007/s11845-008-0192-5.
- Roy MS. Eye care in African Americans with type 1 diabetes: the New Jersey 725. Ophthalmology 2004;111:914-20. https://doi.org/10.1016/j.ophtha.2003.08.033.
- van Eijk KN, Blom JW, Gussekloo J, Polak BC, Groeneveld Y. Diabetic retinopathy screening in patients with diabetes mellitus in primary care: Incentives and barriers to screening attendance. Diabetes Res Clin Pract 2012;96:10-6. https://doi.org/10.1016/j.diabres.2011.11.003.
- Yuan Z. Risk Factors and Barriers to Eye Care Services Among Presenting Late Diabetic Retinopathy Patients in Shanxi Province in China 2007.
- John A. Barriers to diabetic retinopathy screening in South Asian groups. Prim Health Care 2014;24:25-30. https://doi.org/10.7748/phc.24.8.25.e897.
- Walker EA, Basch CE, Howard CJ, Zybert PA, Kromholz WN, Shamoon H. Incentives and barriers to retinopathy screening among African-Americans with diabetes. J Diabetes Complicat 1997;11:298-306. https://doi.org/10.1016/S1056-8727(96)00121-3.
- Peek M, Chin M, Tang H, Baker D, Wagner J. Perceived discrimination in healthcare and diabetes health outcomes. J Gen Intern Med 2010;25.
- Livingston PM, McCarty CA, Wood CA, Harper AC, Keeffe JE, Taylor HR. Use of focus groups to identify health promotion strategies for the early detection of diabetic retinopathy. Aust N Z J Public Health 1998;22:220-2. https://doi.org/10.1111/j.1467-842X.1998.tb01176.x.
- Silver K, Williams M, Macario E. The National Eye Health Education Program: increasing awareness of diabetic eye disease among American Indians and Alaska Natives. Ethn Dis 2006;16:920-5.
- Lewis K, Patel D, Yorston D, Charteris D. A qualitative study in the United Kingdom of factors influencing attendance by patients with diabetes at ophthalmic outpatient clinics. Ophthalmic Epidemiol 2007;14:375-80. https://doi.org/10.1080/09286580701375195.
- Peng P-H. Assessment the Factors Associated With the Acceptance of Retinal Screening Among Patients With Diabetes in Taiwan 2010.
- Gala SD, Wu WK. The impact of receiving diabetes self-management education (DSME) on preventive care practices among type-2 diabetes adults. Value Health 2013;16:A193-4. https://doi.org/10.1016/j.jval.2013.03.975.
- Will JC, German RR, Schuman E, Michael S, Kurth DM, Deeb L. Patient adherence to guidelines for diabetes eye care: results from the diabetic eye disease follow-up study. Am J Public Health 1994;84:1669-71. https://doi.org/10.2105/AJPH.84.10.1669.
- Byun SH, Ma SH, Jun JK, Jung KW, Park B. Screening for diabetic retinopathy and nephropathy in patients with diabetes: a nationwide survey in Korea. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0062991.
- Onakpoya OH, Adeoye AO, Kolawole BA. Determinants of previous dilated eye examination among type II diabetics in Southwestern Nigeria. Eur J Intern Med 2010;21:176-9. https://doi.org/10.1016/j.ejim.2010.01.009.
- Puent BD, Nichols KK. Patients’ perspectives on noncompliance with diabetic retinopathy standard of care guidelines. Optometry 2004;75:709-16. https://doi.org/10.1016/S1529-1839(04)70223-7.
- Rajput Y, Fisher M, Gu T, Singer J, Marshall A, Ryu S, et al. Patient and provider perspectives: why are patients with diabetes mellitus noncompliant with dilated eye exams?. Invest Ophthalmol Vis Sci 2015;56.
- Moss SE, Klein R, Klein BE. Factors associated with having eye examinations in persons with diabetes. Arch Fam Med 1995;4:529-34. https://doi.org/10.1001/archfami.4.6.529.
- Mackenzie J, Aldington SJ, Scanlon PH. Barriers and motivators for attendance at diabetic retinopathy screening. Eur J Ophthalmol 2015;25.
- Massaro L, Curry WJ, Quillen D. Screening for diabetic retinopathy: perceived barriers and patient acceptability of digital scans. J Clin Outcomes Manag 2010;17:17-22.
- Pasagian-Macaulay A, Basch CE, Zybert P, Wylie-Rosett J. Ophthalmic knowledge and beliefs among women with diabetes. Diabetes Educ 1997;23:433-7. https://doi.org/10.1177/014572179702300408.
- Hossen A, Zaman M, Chakrabarti R, Kawaski R, Critchley C, Shaw J, et al. Prevalence of Diabetic Retinopathy and the Barrier in Screening in a Rural District in Bangladesh n.d. https://researchbank.swinburne.edu.au/items/fee628f8–972f-499a-8c0a-843377e8fb96/1/ (accessed 18 July 2017).
- Chou CF, Sherrod CE, Zhang X, Barker LE, Bullard KM, Crews JE, et al. Barriers to eye care among people aged 40 years and older with diagnosed diabetes, 2006–2010. Diabetes Care 2014;37:180-8. https://doi.org/10.2337/dc13-1507.
- Lake AJ, Browne JL, Rees G, Speight J. Adults With Young-Onset Type 2 Diabetes: Exploring Factors Affecting Retinal Screening Uptake for Diabetic Retinopathy n.d. http://ads-adea-2015.m.asnevents.com.au/schedule/session/7242/abstract/26830 (accessed 17 October 2017).
- Laver FJ, Kennedy P, Scanlon PH. A grounded theory exploration of young adults’ non-attendance at diabetic retinopathy screening appointments. Diabet Med 2013;30.
- Njambi L. Prevalence of diabetic retinopathy and barriers to uptake of diabetic retinopathy screening at Embu Provincial General Hospital, Central Kenya. East Afr J Ophthalmol 2012;16:5-11.
- Khandekar R, Al Lawati J, Barakat N. A retrieval system for patients with avoidable blindness due to diabetic retinopathy who do not present for ophthalmic assessment in Oman. Middle East Afr J Ophthalmol 2011;18:93-7. https://doi.org/10.4103/0974–9233.80694.
- Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology 2001;108:563-71. https://doi.org/10.1016/S0161-6420(00)00600-X.
- Cano MR. Prevalence of diabetic retinopathy and barriers to uptake of eye care services by diabetic patients at the Social Security Institute Central Hospital in Asuncion, Paraguay. Community Eye Health 2007;20:10-1.
- Kovarik JJ, Eller AW, Willard LA, Ding J, Johnston JM, Waxman EL. Prevalence of undiagnosed diabetic retinopathy among inpatients with diabetes: the diabetic retinopathy inpatient study (DRIPS). BMJ Open Diabetes Res Care 2016;4. https://doi.org/10.1136/bmjdrc-2015-000164.
- Adriono G, Wang D, Octavianus C, Congdon N. Use of eye care services among diabetic patients in urban Indonesia. Arch Ophthalmol 2011;129:930-5. https://doi.org/10.1001/archophthalmol.2011.147.
- Jingi A, Ebana-Mvogo C, Ellong A. Primary care physicians and patients factors influencing eye care provision and utilisation in a group of diabetic patients. Diabetes Res Clin Pract 2014;103:S50-1. https://doi.org/10.1016/S0168-8227(14)70168-8.
- Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. How well do patients’ assessments of their diabetes self-management correlate with actual glycemic control and receipt of recommended diabetes services?. Diabetes Care 2003;26:738-43. https://doi.org/10.2337/diacare.26.3.738.
- Bell R, Arcury T, Grzywacz J, Edward IP, Kirk J, Saldana S, et al. Medical skepticism and diabetes self-management in rural older adults. Diabetes 2011;60.
- Centers of Disease Control . Eye-care utilization among women aged ≥ 40 years with eye diseases – 19 states, 2006–2008. MMWR 2010;59:588-91.
- Fisher M, Rajput Y, Gu T, Singer J, Marshall A, Ryu S, et al. Understanding barriers to dilated eye examinations in patients with diabetes. Diabetes 2015;64.
- Griffin-Shirley N, Trusty S, Kelley P, Siew-Jin LK, Macias EP. Barriers to eye care faced by adult Hispanics with diabetes. RE:View: Rehabilitation and Education for Blindness and Visual Impairment 2004;36:53-61. https://doi.org/10.3200/REVU.36.2.53-62.
- Karter AJ, Stevens MR, Herman WH, Ettner S, Marrero DG, Safford MM, et al. Out-of-pocket costs and diabetes preventive services: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 2003;26:2294-9. https://doi.org/10.2337/diacare.26.8.2294.
- Lee SJ, Sicari C, Harper CA, Livingston PM, McCarty CA, Taylor HR, et al. Examination compliance and screening for diabetic retinopathy: a 2-year follow-up study. Clin Exp Ophthalmol 2000;28:149-52. https://doi.org/10.1046/j.1442-9071.2000.00302.x.
- Lu Y, Serpas L, Genter P, Mehranbod C, Campa D, Ipp E. Disparities in diabetic retinopathy screening rates within minority populations: differences in reported screening rates among African American and Hispanic patients. Diabetes Care 2016;39:e31-2. https://doi.org/10.2337/dc15-2198.
- Mumba M, Hall A, Lewallen S. Compliance with eye screening examinations among diabetic patients at a Tanzanian referral hospital. Ophthalmic Epidemiol 2007;14:306-10. https://doi.org/10.1080/09286580701272079.
- Sheppler CR, Lambert WE, Gardiner SK, Becker TM, Mansberger SL. Predicting adherence to diabetic eye examinations: development of the compliance with Annual Diabetic Eye Exams Survey. Ophthalmology 2014;121:1212-19. https://doi.org/10.1016/j.ophtha.2013.12.016.
- Shukla R, Gudlavalleti MV, Bandyopadhyay S, Anchala R, Gudlavalleti AS, Jotheeswaran AT, et al. Perception of care and barriers to treatment in individuals with diabetic retinopathy in India: 11-city 9-state study. Indian J Endocrinol Metab 2016;20:S33-41. https://doi.org/10.4103/2230-8210.179772.
- Wang D, Ding X, He M, Yan L, Kuang J, Geng Q, et al. Use of eye care services among diabetic patients in urban and rural China. Ophthalmology 2010;117:1755-62. https://doi.org/10.1016/j.ophtha.2010.01.019.
- Yuen HK. Factors associated with preventive care practice among adults with diabetes. Prim Care Diabetes 2012;6:75-8. https://doi.org/10.1016/j.pcd.2011.12.002.
- Zhang X, Williams DE, Beckles GL, Gregg EW, Barker L, Luo H, et al. Diabetic retinopathy, dilated eye examination, and eye care education among African Americans, 1997 and 2004. J Natl Med Assoc 2009;101:1015-21. https://doi.org/10.1016/S0027-9684(15)31068-3.
- Consolidated Framework for Implementation Research . CFIR Constructs n.d. www.cfirguide.org/constructs.html (accessed 17 November 2017).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Michie S, Abraham C. Interventions to change health behaviours: evidence-based or evidence-inspired?. Psychol Health 2004;19:29-4. https://doi.org/10.1080/0887044031000141199.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles MP. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol Crim Justice 2008;57:660-80. https://doi.org/10.1111/j.1464-0597.2008.00341.x.
- Hardeman W, Sutton S, Griffin S, Johnston M, White A, Wareham NJ, et al. A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res 2005;20:676-87. https://doi.org/10.1093/her/cyh022.
- Cane J, Richardson M, Johnston M, Ladha R, Michie S. From lists of behaviour change techniques (BCTs) to structured hierarchies: comparison of two methods of developing a hierarchy of BCTs. Br J Health Psychol 2015;20:130-50. https://doi.org/10.1111/bjhp.12102.
- Cadogan CA, Ryan C, Francis JJ, Gormley GJ, Passmore P, Kerse N, et al. Improving appropriate polypharmacy for older people in primary care: selecting components of an evidence-based intervention to target prescribing and dispensing. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0349-3.
- Gardner B, Smith L, Lorencatto F, Hamer M, Biddle SJ. How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol Rev 2016;10:89-112. https://doi.org/10.1080/17437199.2015.1082146.
- Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-40.
- Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol 2010;29:1-8. https://doi.org/10.1037/a0016939.
- Steinmo SH, Michie S, Fuller C, Stanley S, Stapleton C, Stone SP. Bridging the gap between pragmatic intervention design and theory: using behavioural science tools to modify an existing quality improvement programme to implement ‘Sepsis Six’. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0376-8.
- Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull 1982;92:111-35. https://doi.org/10.1037/0033-2909.92.1.111.
- Gardner B, Whittington C, McAteer J, Eccles MP, Michie S. Using theory to synthesise evidence from behaviour change interventions: the example of audit and feedback. Soc Sci Med 2010;70:1618-25. https://doi.org/10.1016/j.socscimed.2010.01.039.
- Scanlon PH, Aldington SJ, Leal J, Luengo-Fernandez R, Oke J, Sivaprasad S, et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19740.
- Brailsford SC, Gutjahr WJ, Rauner MS, Zeppelzauer W. Combined discrete-event simulation and ant colony optimisation approach for selecting optimal screening policies for diabetic retinopathy. Comput Manag Sci 2007;4:59-83. https://doi.org/10.1007/s10287-006-0008-x.
- Davies R, Roderick P, Canning C, Brailsford S. The evaluation of screening policies for diabetic retinopathy using simulation. Diabet Med 2002;19:762-70. https://doi.org/10.1046/j.1464-5491.2002.00773.x.
- Vijan S, Hofer TP, Hayward RA. Cost–utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000;283:889-96. https://doi.org/10.1001/jama.283.7.889.
- UK National Screening Committee . The UK NSC Recommendation on Diabetic Retinopathy Screening in Adults n.d. https://legacyscreening.phe.org.uk/diabeticretinopathy (accessed 18 July 2017).
- UK National Screening Committee . UK NSC Diabetic Retinopathy Recommendation 2016. https://legacyscreening.phe.org.uk/policydb_download.php?doc=586 (accessed 17 October 2017).
- Diabetes UK . UK Diabetes Prevalence n.d. www.diabetes.co.uk/diabetes-prevalence.html (accessed 18 July 2017).
- NHS . General and Personal Medical Services 2013. http://content.digital.nhs.uk/catalogue/PUB09536/nhs-staf-2002–2012-gene-prac-rep.pdf (accessed 18 July 2017).
- National Institute for Health and Care Excellence . The Guidelines Manual 2012. www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness (accessed 18 July 2017).
- Public Health England . NHS Screening Programmes in England 2015. www.gov.uk/government/uploads/system/uploads/attachment_data/file/574716/Screening_in_England_2014_to_2015.pdf (accessed 18 July 2017).
- UK National Screening Committee . Interim Quality Standards and Performance Objectives for Diabetic Eye Screening Programmes Pending a Full Standards Review 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/600541/Interim_QA_standards.pdf (accessed 17 October 2017).
- DAFNE . Dose Adjustment for Normal Eating (DAFNE) n.d. www.dafne.uk.com/ (accessed 24 October 2017).
- X-PERT Health n.d. www.xperthealth.org.uk/ (accessed 24 October 2017).
- NHS . DESMOND n.d. www.desmond-project.org.uk/ (accessed 24 October 2017).
- Judah G, Vlaev I, Gunn L, King D, King D, Valabhji J, et al. Incentives in Diabetic Eye Assessment by Screening (IDEAS): study protocol of a three-arm randomized controlled trial using financial incentives to increase screening uptake in London. BMC Ophthalmol 2016;16. https://doi.org/10.1186/s12886-016-0206-4.
- Judah G, Darzi A, Vlaev I, Gunn L, King D, King D, et al. Incentives in Diabetic Eye Assessment by Screening (IDEAS) trial: a three-armed randomised controlled trial of financial incentives. Health Serv Deliv Res 2017;5.
- Davidson EM, Liu JJ, Bhopal R, White M, Johnson MR, Netto G, et al. Behavior change interventions to improve the health of racial and ethnic minority populations: a tool kit of adaptation approaches. Milbank Q 2013;91:811-51. https://doi.org/10.1111/1468-0009.12034.
Appendix 1 Stakeholder advisory group membership
We established a representative stakeholder reference group to capture the views of end-users of the outputs from this project. The group includes experts in diabetes care, representatives of the four nations screening programme, patients, practitioners, professional organisations and policy-makers. The following individuals were involved in the stakeholder reference group:
-
Professor Peter Scanlon (Clinical Director, Diabetic Retinopathy Screening Programme, England)
-
Dr Deborah Broadbent (Director of Diabetic Eye Screening, Liverpool)
-
Mr Andrew Crowder (Head of Diabetic Retinopathy Screening Wales)
-
Dr Caroline Styles (Lead Clinician for Diabetic Retinopathy Screening Scotland)
-
Mr Raymond Curran (Assistant Director, Directorate of Integrated Care, Health & Social Care Board, Northern Ireland)
-
Grant Duncan (British Association of Retinal Screening)
-
Simon O’Neil (Diabetes UK)
-
Helen Lee (RNIB)
-
Gozie Joe Adigwe (Eye Health Equalities Officer, RNIB Scotland).
Appendix 2 Report of the knowledge exchange event
Attendees
Facilitators
-
Katherine Cowan (James Lind Alliance).
-
Michael Bowen (College of Optometrists).
-
Sally Crowe (Crowe Associates Ltd).
Research team members
-
John G Lawrenson (City, University of London).
-
Jill J Francis (City, University of London City).
-
Fabiana Lorencatto (City, University of London).
-
Ella Graham-Rowe (City, University of London).
-
Tunde Peto (Queen’s University Belfast).
-
Stephen Rice (Newcastle University).
-
Luke Vale (Newcastle University).
-
Patricia Aluko (Newcastle University).
-
Jennifer M Burr (University of St Andrews).
Stakeholder members
-
Andrew Crowder (Diabetes UK, Wales).
-
Gozie Joe Adigwe (RNIB, Scotland).
-
Simon O’Neill (Diabetes UK).
-
Caroline Styles (NHS Fife, Scotland).
-
Helen Lee (RNIB, London).
-
David Owens (Diabetes Research Unit Cymru, Wales).
-
Phil Gardner (British Association of Retinal Screening).
-
Natalie Owen (Department of Health).
-
David Keene (NHS City & Hackney Clinical Commissioning Group).
-
David Parkins (College of Optometrists).
Patients
-
Vivienne Ruddock, Sheila Burston, Melissa Louis and David Beck.
Summary of stakeholder discussions
1. Introduction
This event on the 12 January 2017 brought together the research team, people with diabetes and experience of DRS, health professionals and service providers with an interest in DRS to hear about the early results of this project. It was also an opportunity for participants to discuss their experiences of barriers/enablers that affect regular DRS attendance and members of the research team were particularly interested in hearing ideas about how low rates of attendance could be improved.
The research team presented summaries of the methods and results in the morning, generating interesting questions and debate, and three smaller discussion groups were facilitated in the afternoon to reflect more deeply on the project results and capture ideas and feedback from participants. Discussion groups were facilitated by independent (from the research team) consultants, who took notes, and all discussions were recorded for accuracy.
This report describes these conversations and draws together the themes across the discussion groups, using the notes and recordings as primary material. In the final section the implications of the findings for UK screening programmes are summarised and suggestions are made for future research.
2. Discussion in groups
2.1 Group 1
The presentations were well received by this group who, although a little disappointed by the small percentage of UK studies represented in the review, thought that there was plenty of material to consider further. They suggested focusing on a UK breakdown of national/regional differences in barriers to and enablers of DRS attendance; learning from local initiatives; and subgroup analysis of young people, those from black and ethnic minority groups, those who travel extensively and stay for long periods in other countries (e.g. Pakistan) and those with mental health issues as well as diabetes, who may be less likely to attend screening. The overall message of the study that ‘doing something is better than doing nothing’ is an important finding and the group was encouraged by this.
Focusing more on the group experience, agreed effective initiatives to improve DRS screening included cleaning up patient databases, for example removing deceased people, ensuring that appointment slots are allocated to current patients and using text message reminders. Other ideas for which experience and/or the evidence base were less convincing included financial incentives to encourage attendance, sharing the accumulated cost of non-attendance and getting people to sign a promise/pledge to attend screening. It was noted that many of the interventions described in the study are behavioural or psychological interventions yet there is a dearth of psychologists in the diabetes field to help deliver change. It was also considered a challenge to comment on whether or not the initiatives in the study would work in the UK given the diversity of the populations and health-care settings. One of the challenges in this area is the diverse audiences to engage with in DRS, especially those at risk of low attendance. Another concern identified by this group was that successful initiatives piloted by the charity sector may not be taken up by mainstream screening services.
There was agreement in group 1 that there is varied understanding about DRS and confusion between DRS and routine eye tests and ophthalmology appointments. Added to this is the ‘duty of candour’, that is, telling people that screening is not foolproof may make them decide not to bother, communication problems such as actionable messages hidden in the text of patient information or being hard to find, overuse of medical language and the use of too-small print.
Group 1 identified those with potential low levels of attendance as working people, especially men, people from black and ethnic minority groups, people with type 1 diabetes, especially those struggling with the transition from paediatric to adult services, and people who do not have any symptoms and who may think that they do not have a problem. Vulnerable groups, people on low incomes and those with multiple conditions also need more attention with regard to screening. For example, people with many conditions may deprioritise DRS if the symptoms of other conditions are worse than the symptoms of diabetes.
Concerns were expressed that an increase in the prevalence of type 2 diabetes has led to more people with type 1 diabetes being missed for screening.
Experience in the group suggested that rural screening attendance rates can be higher than rates in urban areas because people appreciate the efforts made to reach out to them. However, mobile units are not always popular as they can be cramped and people often have to wait in their cars as the waiting area is so small. People also value the opportunity to use quality services that are more easily available in urban areas.
Group members thought that screening behaviour may be reinforced when people get into good habits within the first 3 months of a diabetes diagnosis. However, the evidence on this was questioned – is there is a correlation between attendance and behaviour within the first 3 months of diagnosis? The ways in which people might take responsibility for their health information were discussed, with an example given of Scotland’s ‘my diabetes my way’ portal where people can register and manage their diabetes appointments and processes. Other experiences of increasing screening rates included the choice of clinic day (Fridays are better than Mondays) and holding themed clinics (London).
This group had suggestions for further research, which are included in Section 3 of this report, but in summary their interests were in working more with groups with low attendance rates to understand their contexts and dynamics and whether or not existing BCTs could be adapted for these groups.
2.2 Group 2
This group noted and appreciated the scope and scale of the work, especially given the large numbers of participants from relatively few studies. Study team members clarified that the model used gave relatively lower weighting to the results from studies with smaller numbers of participants. Those in group 2 also wanted to know how the different UK health system might correlate with the findings, which were somewhat dominated by US study data. The differences between the US and UK systems in health care and screening were questioned and discussed, with the group concluding that the principal difference was in the importance given to the cost of attending screening, which was less of a barrier in the UK studies. Screening intervals and changes to these in the USA and UK were also discussed: although a 2-year interval is common in the USA, a 1-year interval has been standard in the UK (by consensus since inception), although the group noted that this was starting to vary in different areas. The other barriers/enablers were similarly ranked across UK and US studies in the review.
A discussion about the weighting of themes in the review analysis led to input from the research team that the frequency with which a theme occurred in the data may be a useful indicator of the importance/impact of a theme overall, but is not the only indicator. Clinicians in the group pointed out that the risk profile for someone who had attended two consecutive screening appointments and received a R0 result (no sign of retinopathy) were statistically at very low risk of sight-threatening complications. This was likely to drive the trend towards customised recall intervals in the UK and the extension of general intervals towards 2 years. Related to this are the risks associated with informing a patient who has attended screening that they are in the low-risk category; specifically, there was interest in whether this would make individuals more or less likely to attend future screening appointments.
There was interest in whether or not the review indicated that there would be potential cost savings to the system by moving from 1- to 2-year screening intervals. Clinicians and screening managers in the group noted that the growth in absolute numbers of people living with diabetes on the registers for screening meant that such an extension was more about delaying slightly the impact of these additional new costs than actually saving money overall (in their view). The collective view was that the interventions described in the review would probably work in the UK even if the majority of the data that supported them were US based.
Moving to interventions in the charity sector there is work under way to explore the benefits of central systems, in which a single appointment at a specialist centre could address all health screening needs for people with diabetes. Researchers in the group pointed out that one study had explored the impact of screening locations being geographically as close as possible to patients’ homes and the review shows that accessibility remains a key potential barrier to screening uptake.
The role of GPs in retinopathy screening might be less focused (not reminding patients to attend DRS or checking with them whether or not they have attended) as they consider the screening programme to be responsible for handling DRS. It was noted that information technology issues may prevent GPs from accessing DRS systems or that databases are not set up to notify GPs of patients’ attendance/non-attendance. In some areas it was possible for GPs to access screening system images for their patients using a code, but the group experience was that few used this facility. The exception seemed to be in practices in which there was at least one GP with a special interest in diabetes. A group member cited a review of general practices with low did-not-attend (DNA) rates for their patients within the screening system, which found that all of these practices had a diabetes special interest GP. 149It was noted that there was an undersupply of diabetes specialist nurses.
Patient members of this group felt that a GP (or other trusted professional) might carry more weight than a letter from the screening programme: ‘your GP is the one who knows you best of all’. Other barriers from the patient perspective were having multiple appointments at different locations and the times of available appointments (in this group the preference was for early morning, evening and weekend appointments), but these might differ by age and across other groups.
Those with direct experience of the DRS programme felt that older women were most likely to attend for screening. A low-attendance/at-risk group was teenagers as they approached adulthood, and then as young adults, in other words people in transition. Group members agreed that it was easy to fall through gaps in the system during this transition period. Although in theory it might seem easy for university students to attend for screening, the reality was that changing GP registration presented challenges.
In terms of looking to the future and the implications of the study, the group discussed that few of the studies had focused on interventions that sought to influence emotional and social factors that play a role (barriers or enablers) in DRS screening and that more understanding about these would be helpful. The role of large-scale national (television) advertising campaigns to increase public awareness of the importance of screening was discussed, in terms of both effectiveness and cost. It is vital that evidence from the project should inform practice and support the provision of better information for clinicians, commissioners and patients. Finally, there was consensus that this event had been very useful, with opportunities for patients, clinicians and representatives of stakeholder bodies to get together and talk. This should be encouraged on a regular basis!
2.3 Group 3
This group welcomed the research and enjoyed the presentations of the results so far. They welcomed a focus on the 20% or so who are low or non-attendees for DRS, rather than the ‘worried well’. People were surprised that some of the interventions had a limited impact and wanted to make the distinction between an outcome of attending screening and an outcome of improved diabetes eye health. The researchers added that some of the studies in the review were underpowered and were thus limited and unable to show significant outcomes. The setting of the studies (and differences in screening contexts and costs, for example) in the USA and UK was also noted and group members were interested in whether or not there was merit in isolating UK results. The researchers in this group suggested that it would be beneficial to extract the UK studies and explore any differences in results to try and determine what might be more likely to work in the UK.
People with diabetes in transition were identified as being potentially worth targeting for extra attention with regard to DRS, including young people and children with diabetes growing into adults; older people with diabetes (and multiple other conditions) becoming more in need of carers and who may not be able to self-manage; and people with diabetes with dependents. People with diabetes are not static and move through periods in their life in which there may be differing barriers and enablers affecting their awareness of screening and their motivation and ability to be screened.
Related to this theme was one of ‘getting lost in the DRS system’ and patients finding it hard to distinguish between eye health hospital appointments (diabetes related or other), DRS and more routine eye tests at high street opticians. Group members described how it can be easy to lose the thread, especially when in transition (child to adult services) or if your care is spread over different health systems and geographical areas. One person’s experience in the group showed how a gap of just 18 months in DRS communications resulted in significant grading changes to R3, that is, requiring closer surveillance or treatment. Other examples of change that might result in patients being ‘lost’ were changing GP or another significant health-care practitioner, moving appointments from fixed to variable appointments or significantly changing appointment times, losing the connection to a proactive system (automatic appointments and reminders) and reliance on patients making appointments.
Group members described (sometimes first hand) how people with diabetes find the most ‘suitable way’ to be screened that fits into their schedule and lifestyle (especially if they are economically active and in the 20–40 years age range). For example, they may experience a perfectly good interaction with DRS provided by an accredited high street optician at a convenient time on a Saturday morning, where they are ‘screened and get instant feedback and a picture to look at’, with this being a good user experience. What seems to be at the heart of this high street optician experience is convenience, a good feedback loop (compared with the DRS programme, in which patients receive a letter and are not allowed to see their images) and the opportunity to have a useful conversation with the operative. Group members reflected on what the DRS programme could learn from this, for example if there are ways that screeners in the DRS programme can indicate positive or negative changes (since the last visit) without disclosing the grading. Also, interestingly, there was a suggestion that silence on the part of the screener may be misconstrued by those with diabetes as ‘bad news’, especially if they are not aware that it is not the screener’s role to provide feedback. There was a sense that it is important to make every health contact count for people with diabetes and DRS was no exception to this and could be improved.
This group was concerned that people with diabetes may think that a regular ‘sight test’ provided by a high street optician is DRS when it is not and that they may not self-identify as not attending for the screening programme. What they are losing is a very specific and important screening and grading opportunity and the potential to be referred for further investigation if needed. The group felt that patient information should include simple information about how these services differ and why it is vital to undergo DRS in addition to other ophthalmic and eye health appointments.
The group discussed (at a researcher’s behest) the intervention and enabler of goal-setting – some group members had positive experiences of identifying screening as an attainable goal in self-care, but there was also caution about handling goal-setting holistically in a person-centred way, with an embedded feedback loop to assess progress.
Another potential intervention/enabler for DRS was the ‘credible source’ intervention. The group wondered if the DRS programme should make more of the enhanced training and skill of screeners and graders in the programme in the patient information (without being disrespectful to other providers). Pharmacists are also trusted professionals and potentially enablers for DRS. An example of placing business cards with information about DRS into bags of diabetic equipment was described and cited as a low-cost intervention (it was not clear if this was evaluated formally, but the group member was positive about the intervention).
The challenges of clinic times and locations were also discussed. One group member described the significant extra work carried out by a general practice, which went to great lengths to physically get patients to attend for DRS but which did not yield a higher turnout. This led the group to think about wider systems change – what can be changed and how? Some would value greater user design of screening services (e.g. for patients who cannot physically get to the screening equipment because of physical limitations) and exploring opportunities for more ‘one-stop shop’ or polyclinic approaches. Accessibility was identified as a major issue by the service providers in this group. Also, one group member had experience of a ‘learning health system’ approach to looking at data that are collected from both people with diabetes and diabetic services, so that more useful information can be obtained.
For the future this group felt that it had identified different ideas for further research (which are described in Section 3) but were concerned that outputs take the form of practical recommendations and interventions that are feasible to explore in the NHS and take account of where the DRS programme is and where it could be, that is, what can be changed and what cannot be changed.
3. Themes across the three discussion groups
3.1 Responses to the overall project results
-
Noted and appreciated the scope and scale of the work and appreciated that it is easier to identify barriers than solutions.
-
Welcomed the positive overall message that doing something is better than usual care or doing nothing; the differences between the USA and UK (as major sources of relevant data) merit further exploration, especially for the different contexts of screening and cost issues.
-
Interest from all groups in extracting the UK data from the overall data set to better understand what might work best in the UK.
-
The possible mismatch between the predominance of behavioural and psychological interventions and the prevalence of practitioners (specialist nurses and psychologists) able to provide support for these interventions.
-
Clarity about what really matters about outcomes of DRS – increase in overall uptake of DRS, increase in uptake of DRS in groups identified as being low or non-attendees (who may have more severe retinal disease), overall eye health outcomes and user experiences of DRS were suggestions.
-
Interest from all groups on focusing interventions and associated research in groups who are more likely to have low levels of attendance or to be non-attendees.
3.2 Views from diabetic retinopathy screening and other service providers
-
All groups identified with, and some had experienced, initiatives that were covered in the presentations.
-
These ranged from relatively simple approaches, for example cleaning up screening databases and the use of automatic appointment bookings, to more complex initiatives, such as GPs targeting those with low levels of attendance (driven by targets?).
-
It’s not just about interventions to and for patients, but also about systems rethink, for example customised recall intervals depending on the level of perceived risk to patients (those with two consecutive visits and no sign of retinopathy moving to 2-year recall) or more user input to the design of screening programmes.
-
Service providers felt (and in many cases patient representatives agreed) that there were misunderstandings about what the DRS programme is for and about. Examples were described in which people did not realise what sort of appointment they had had and in which there was confusion between DRS and other ophthalmology appointments and checks.
-
These issues may be a contributing factor as to why people seem to get lost in the system of DRS, with several examples shared in which a thread of continuity was lost or a system failed to identify people for screening.
-
The introduction of different screening intervals according to risk was discussed in two groups (evidence base for current consensus) and some participants wondered if this was within the scope of this project. These groups were interested in the effect of duty of candour, that is, informing a patient that he or she is in a low-risk category, and if this makes individuals more or less likely to attend future screening appointments.
3.3 Do the initiatives to improve attendance work in the UK?
-
Groups found this a little hard to answer because of the diversity of the populations and health-care settings in which DRS operates, for example the different cost issues and also the different recall times for screening. They thought that extracting the UK-specific data might add additional learning.
-
However, there was consensus that there are no reasons to think that any of the interventions described would not work in the UK.
-
Better evaluation of public campaigns to increase screening uptake was a theme in two groups who were aware of poorly evaluated projects, often funded as the result of spare financial reserves.
-
Two groups agreed that having access to screening close to home (or work) was important, but there were differing views about the value of mobile services in rural areas.
3.4 Perspectives of the charitable sector
-
There was some evidence across the groups of charity-funded initiatives to improve attendance, such as single appointments at specialist centres and appointments ‘close to home’ projects.
-
All groups cited accessibility of screening as a key barrier to screening uptake.
-
The groups seemed to think that there was a low prevalence of charity-led projects and patient events aimed at promoting attendance for DRS, and underfunding of such projects and events.
-
When there had been successful initiatives, these struggled to be implemented into mainstream services, which was discouraging.
3.5 Patient perspectives
-
There were consistent descriptions across groups of how people with diabetes mix up DRS with routine eye tests and maybe do not realise the important longer-term preventative nature of screening programmes.
-
The growth of the diabetic population means that there are more diverse populations with different levels of health literacy and additional vulnerabilities and needs, adding to the complexity involved in achieving high attendance rates for DRS.
-
The role of GPs was discussed at some length in all groups – for patients this can be a crucial relationship and when people change GP for whatever reason they can get lost in the system. This also applies to secondary services.
-
The role and effectiveness of diabetes special interest GPs and nurses were cited as important and as having an impact on screening uptake. However, examples were described of GPs who do not think that DRS is their responsibility, with the associated challenge of nudging them into changing their outlook.
-
Groups suggested that screeners and graders also belong to the group of people who have influence and credibility, but patients may not know this – is this something that could be communicated more clearly as a benefit of attending DRS?
-
The timing of DRS appointments was also a topic of conversation – all agreed that it was highly personal and contextual, but that there must be choice.
-
Two groups identified the concept of patients transitioning in life and that this can present an ideal opportunity to get ‘lost in the system’, for example moving from child to adolescent services or when patients have too much going on in life (children, care of elderly) to prioritise their retinopathy screening.
-
Reviewing existing diabetes education programmes (e.g. DAFNE235) for DRS information and prompts was suggested.
-
Conversations about risk–benefit with regard to DRS choices seem important to improve attendance for those with low rates of attendance or who are non-attendees.
-
Communication channels about DRS also seem important – exploring social media approaches, for example with younger people with diabetes, was suggested. It was acknowledged that the NHS is slow to change on these fronts.
3.6 Views on low or non-attendees
-
The following list draws from the discussions in all three groups about people with diabetes with low levels of attendance at screening or who do not attend for screening. The discussion groups felt that these groups merit special attention and interventions that may deliver rich returns in terms of preventing retinopathy disease (those mentioned most frequently are listed first):
-
teenagers and young people who find it hard to think in the long term, which is what a screening programme is all about.
-
vulnerable people and those living on the margins of society, for example those who are homeless, those in temporary accommodation, people in prison and offending units – all of these people move about a lot and may get lost in the DRS system
-
people with diabetes who are asymptomatic and who do not perceive themselves to be ‘at risk’ of retinopathy
-
‘highest-risk’ patients (people with type 1 diabetes)
-
linked to number 3, people with diabetes who are in denial about the long-term effects of the disease
-
young adults who are not really engaged with their condition, until they get pregnant and have dependents
-
people who use multiple health systems in different geographical areas
-
some black and ethnic minority groups
-
people who move for their work or who travel long distances for work and have very little time during the week
-
people who leave the country for long periods of time to see family (e.g. Pakistan)
-
people who change from 1- to 2-year checks – will they remember?
-
people in residential health and social care accommodation
-
elderly living on their own, especially without family members living close by
-
people on low incomes.
-
-
All groups strongly identified patients with multiple conditions requiring many appointments as being particularly at risk of low or no attendance, not least because of the practical challenges of attending multiple appointments at different locations, but also because of the difficulties of assimilating all of the important information and advice provided when attending DRS.
-
There was also a sense that emotional issues surrounding choices and decisions about DRS among people with diabetes, particularly vulnerable groups, is an area in which there could be more understanding – patients do not make information-led and rational choices, it is more complex than that.
-
There was a low awareness of particular initiatives to target vulnerable groups specifically for DRS attendance, but group 1 suggested that other successful programmes be explored and adapted for DRS target groups. A paper co-authored by Raj Bhopal was mentioned on adapting BCTs for ethnic minority groups. 240
3.7 Future directions
-
Focusing on the profiles of those with probable low levels of attendance or non-attendees was a clear message from this workshop – is it just DRS that they do not attend or do they show other similar behaviours and what does that tell us?
-
Can we rank the ‘best gain’ (in terms of reducing retinal disease) populations and put these central to the strategy, rather than achieving 100% screening attendance?
-
The focus on transitions in the discussions in all groups suggests that there may be a link between these transitional life events and attendance patterns – do they change over the life course and how can they be anticipated in reference to retinopathy screening?
-
All groups talked about the importance and role of behavioural and psychological (and knowledge underpinning this) interventions for screening; listening to the discussion recordings you can hear people with screening experience talking about the role of emotions and fears in choices.
-
There were compelling descriptions of systems failures and reflection on how much can be changed to target those who are less likely to attend rather than increasing the attendance of the ‘worried well’.
-
Groups were interested in the role of public awareness campaigns and patient information about screening (e.g. what it is and is not, role of specialist screeners and graders as distinct from that of opticians) and whether or not the results from the project could be used to inform the development of effective public (television, other media) awareness and information campaigns.
-
Groups agreed that vital evidence from the project should be used to inform practice and support the provision of better information for clinicians and commissioners, as well as patients.
-
Other sectors interested in diabetes and DRS should be targeted for project dissemination (e.g. optician services, industry, charities).
-
Outputs from the project needed to be disseminated beyond scientific journals and include less academic channels.
-
Outputs from the project should encompass practical recommendations and interventions that are feasible in the NHS.
-
Outputs and recommendations from the work also need to be realistic and take account of where the current DRS programme is, and where it could be, that is, what can be changed and what cannot be changed.
-
Final conversations highlighted how useful the event had been to participants in terms of providing an opportunity for patients, clinicians and representatives of stakeholder bodies to get together and talk. This should be encouraged on a regular basis.
4. Summary
4.1 What are the implications of the findings for UK screening programmes?
-
There is much to be learnt from the current project for the DRS programme. There is also a need for DRS to contribute to the further building of UK-based knowledge about screening uptake.
-
There is consensus from this event that a better understanding of, and focusing more on, groups of people with diabetes who have low levels of attendance at screening or who do not attend for screening might yield better outcomes for the programme than attempting to achieve 100% attendance.
-
Consider existing evaluated health promotion/screening programmes targeting people in the described groups with low levels of attendance or non-attendance and assess whether or not they can be adapted for DRS interventions.
-
Sector charities and campaigning organisations should consider targeting new campaigns at key groups at risk of low or no attendance.
-
Related to this is a review of DRS patient information, given the apparent misunderstandings and confusion about what DRS is and is not.
-
Communication channels for the provision of DRS information is also an important feature for the programme to consider.
-
The role of staff in primary care in DRS uptake seems important – understanding fully the potential of GPs, specialist nurses and pharmacists to achieve improved uptake of DRS seems a sensible output of this process.
-
The project findings should be translated into formats that make them accessible to patients/public and which renders them easily used by screening service commissioners/managers and clinicians seeking to optimise local services and implement interventions to improve attendance.
-
Given the usefulness of this event, screening programme leaders, clinicians involved in service delivery to those referred on from screening, patients and sector organisations with an interest in this area could meet annually to provide a forum for sharing good practice, discussing challenges and assessing progress.
4.2 What are the suggestions for future research?
UK perspectives
-
A UK breakdown of national/regional differences in barriers to and enablers of DRS attendance.
-
Gathering and learning from local initiatives (which may not be published and which may not have been studied in RCTs).
Focusing on low or non-attendees
-
Subgroup analysis of those with low levels of attendance and/or non-attendees: are they the same people as those not attending other appointments? Or is this just about their eyes?
Diabetes-related factors
-
Depression is common in diabetes and people often do not have a formal mental health diagnosis, but their behaviours are affected – it was felt that this did not come out of the review and therefore is an important research gap.
-
Do attendance patterns change over the life course and what life events and transitions affect the uptake of screening?
-
Do attendance data differ between people with type 1 diabetes and people with type 2 diabetes, that is, do those who have had diabetes since childhood or from a younger age develop better habits regarding managing and monitoring their diabetes and therefore are (a) at less risk generally and (b) more likely to be using screening services?
Behavioural interventions
-
Is there a correlation between attendance and behaviour within the first 3 months of diagnosis? Do people who do attend start attending early on?
-
Is there profit in reviewing BCTs for ethnic minority groups more generally and then applying this to DRS specifically?
-
More research to inform our understanding about social and emotional factors affecting screening uptake would be helpful.
The screening experience
-
How important and effective is the discussion at the time of screening? From patient discussions it seems that it is important and can affect subsequent screening attendance.
-
Related to this are the risks associated with informing a patient who has attended screening that he or she is in the low-risk category; specifically, there was interest in whether this would make individuals more or less likely to attend future screening appointments.
-
What can the DRS programme learn from the popular ‘high-street optician’ user experience, specifically in terms of accessibility (high street, choice of times and days), operator feedback and the provision of printed images that initiate conversation, feedback and advice.
Screening uptake campaigns
-
More robust evaluation of screening campaigns and investigating the reasons behind the common barrier of raising screening attendance above 80%.
Appendix 3 Example search strategy for the phase 1 systematic review: interventions to increase attendance for diabetic retinopathy screening
MEDLINE (via Ovid)
-
randomized controlled trial.pt.
-
random$.ab,ti.
-
placebo.ab,ti.
-
db.’s.
-
trial.ab,ti.
-
(group or groups).ab,ti.
-
or/1-6
-
exp animals/
-
exp humans/
-
8 not (8 and 9)
-
7 not 10
-
exp Randomized Controlled Trials as Topic/
-
11 or 12
-
exp Diabetes Mellitus/
-
exp Diabetes Complications/
-
exp Diabetic Retinopathy/
-
((diabet$ or proliferative or non-proliferative) adj4 retinopath$).tw.
-
diabetic retinopathy.kw.
-
(diabet$ adj3 (eye$ or vision or visual$ or sight$)).tw.
-
(retinopath$ adj3 (eye$ or vision or visual$ or sight$)).tw.
-
(DR adj3 (eye$ or vision or visual$ or sight$)).tw.
-
or/14-21
-
exp Mass Screening/
-
exp Vision Tests/
-
exp Telemedicine/
-
exp Photography/
-
exp Ophthalmoscopes/
-
exp Ophthalmoscopy/
-
(ophthalmoscop$ or fundoscop$ or funduscop$).ti.
-
((exam$ or photo$ or imag$) adj3 fundus).tw.
-
(photography or retinography).tw.
-
((mydriatic or digital or retina$ or fundus or steroscopic) adj3 camera).tw.
-
((mydriatic or digital or retina$ or fundus or steroscopic) adj3 imag$).tw.
-
screen$.tw.
-
((eye$ or retina$ or ophthalm$) adj4 exam$).tw.
-
((eye or vision or retinopathy or ophthalmic) adj4 test$).tw.
-
((eye$ or retina$ or ophthalm$) adj4 visit$).tw.
-
Office Visits/
-
(telemedicine$ or telemonitor$ or telescreen$ or telehealth or teleophthalmology).tw.
-
or/23-39
-
‘Quality of Health Care’/
-
Quality Improvement/
-
Delivery of Health Care/
-
Delivery of Health Care, Integrated/
-
service delivery.tw.
-
decision making.tw.
-
(consensus adj3 (process$ or discuss)).tw.
-
stakeholder$.tw.
-
Quality Control/
-
Total Quality Management/
-
Quality Indicators, Health Care/
-
Quality Assurance, Health Care/
-
quality assurance.tw.
-
(quality adj2 improv$).tw.
-
total quality.tw.
-
continuous quality.tw.
-
quality management.tw.
-
(organisation$ adj3 cultur$).tw.
-
Disease Management/
-
Program Evaluation/
-
((provider$ or program$) adj3 (monitor$ or evaluate$ or modif$ or practice)).tw.
-
(implement$ adj3 (improve$ or change$ or effort$ or issue$ or impede$ or glossary or tool$ or innovation$ or outcome$ or driv$ or examin$ or reexamin$ or scale$ or strateg$ or advis$ or expert$)).tw.
-
(need$ adj3 assess$).tw.
-
((education$ or learn$) adj5 (continu$ or material$ or meeting or collaborat$)).tw.
-
exp Medical audit/
-
(audit or feedback or compliance or adherence or training or innovation).ti.
-
(guideline$ adj3 (clinical or practice or implement$ or promot$)).tw.
-
exp Health Services Accessibility/
-
(outreach adj2 (service$ or visit$)).tw.
-
(intervention$ adj3 (no or usual or routine or target$ or tailor$ or mediat$)).tw.
-
usual care.tw.
-
exp Reminder Systems/
-
remind$.tw.
-
(improve$ adj3 (attend$ or visit$ or intervention$ or adhere$)).tw.
-
(increas$ adj3 (attend$ or visit$ or intervention$ or adhere$)).tw.
-
(appointment$ adj3 (miss$ or fail$ or remind$ or follow up)).tw.
-
Telephone/
-
telephone.tw.
-
Cell Phones/
-
Mobile Applications/
-
Remote Consultation/
-
(m-health or e-health or g-health or u-health).tw.
-
(phone$ adj1 (smart or cell)).tw.
-
(smartphone$ or cellphone$).tw.
-
(hand adj1 held device$).tw.
-
(mobile adj2 (health or healthcare or phone$ or device$ or monitor$ or comput$ or app or apps or application)).tw.
-
Internet/
-
Social Networking/
-
(email$ or text$ or message$).tw.
-
(letter or mail or mailed or print$ or brochure$ or newsletter$).tw.
-
Primary Health Care/
-
General Practitioners/ or Physicians, Family/ or Physicians, Primary Care/
-
Primary Prevention/
-
Preventive Health Services/
-
Community Health Services/
-
Community Health Nursing/
-
Health Services, Indigenous/
-
Rural Health Services/
-
Mobile Health Units/
-
(Ophthalmologist$ or Optometrist$ or Optician$ or Orthopist$ or Refractionists).tw.
-
((Ophthalmic or eye) adj3 (surgeon$ or nurse$ or technician$ or officer$ or assistant$ or staff$)).tw.
-
Physician’s Practice Patterns/
-
Professional Practice/
-
(professional adj3 (practice or develop$ or educat)).tw.
-
Education, Medical, Continuing/
-
exp nurses/
-
Specialties, Nursing/
-
Nurse’s Role/
-
Education, Nursing, Continuing/
-
(nurse or nurses).tw.
-
Pharmacists/
-
pharmacist$.tw.
-
((role or roles) adj3 expan$).tw.
-
(task$ adj3 shift$).tw.
-
exp Medical Records Systems, Computerized/
-
Management Information Systems/
-
Database Management Systems/
-
Computer Systems/
-
Point-of-Care Systems/
-
Hospital Information Systems/
-
((health or healthcare) adj4 (record or management system$)).tw.
-
(decision adj5 support).ti.
-
Economics/
-
‘costs and cost analysis’/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
Cost savings/
-
Cost of illness/
-
Cost sharing/
-
‘deductibles and coinsurance’/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp ‘fees and charges’/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
Uncompensated Care/
-
Reimbursement Mechanisms/
-
Reimbursement, Incentive/
-
(insurance adj3 (health$ or scheme$)).tw.
-
(financial or economic or pay or payment or copayment or paid or fee or fees or monetary or money or cash or incentiv$ or disincentiv$).tw.
-
or/41-159
-
exp Patient Acceptance of health Care/
-
exp Attitude to Health/
-
exp Health Behavior/
-
(barrier$ or obstacle$ or facilitat$ or enable$).tw.
-
(uptake or takeup or attend$ or accept$ or adhere$ or attitude$ or participat$ or facilitat$ or utilisat$ or utilizat$).tw.
-
(complie$ or comply or compliance$ or noncompliance$ or non compliance$).tw.
-
(encourag$ or discourage$ or reluctan$ or nonrespon$ or non respon$ or refuse$).tw.
-
(non-attend$ or non attend$ or dropout or drop out or apath$).tw.
-
Health Education/
-
exp Patient Education as Topic/
-
exp Health Promotion/
-
exp Counseling/
-
‘Attitude of Health Personnel’/
-
(health adj2 (promotion$ or knowledge or belief$)).tw.
-
(educat$ adj2 (intervention$ or information or material or leaflet)).tw.
-
Socioeconomic Factors/
-
exp Poverty/
-
Social Class/
-
Educational Status/
-
((school or education$) adj3 (status or level$ or attain$ or achieve$)).tw.
-
Employment/
-
Healthcare Disparities/
-
Health Status Disparities/
-
exp Medically Underserved Area/
-
Rural Population/
-
Urban Population/
-
exp Ethnic Groups/
-
Minority Groups/
-
Vulnerable Populations/
-
((health$ or social$ or racial$ or ethnic$) adj5 (inequalit$ or inequit$ or disparit$ or equit$ or disadvantage$ or depriv$)).tw.
-
(disadvant$ or marginali$ or underserved or under served or impoverish$ or minorit$ or racial$ or ethnic$).tw.
-
or/161-191
-
160 or 192
-
13 and 22 and 40 and 193
-
(ranibizumab or bevacizumab or avastin or aflibercept).ti.
-
(cataract$ or intraocular or glaucoma$ or phaco$ or photocoagulat$ or photodynamic or laser$ or vitrectom$).ti.
-
(macula$ adj2 (degener$ or oedema or edema)).ti.
-
nerve fiber layer.ti.
-
(coronary or cardiac or cardio$ or heart or myocardia$ or artery or aneurysm or atrial or echocardiography or hypertension or hypotension or stroke or pulmonary or COPD or lung$ or organ$ or smoking).ti.
-
(pregnan$ or gestational or neonat$ or perinatal or maternal or trimester or congenital or ovary or breast$).ti.
-
(kidney or liver or cirrhosis or renal or hepatitis or dialysis or pancrea$ or gastric or gastrectom$ or surg$ or duoden$).ti.
-
(blood glucose or blood pressure or ketoacidosis or hypoglycemi$ or rosiglitazone).ti.
-
(lipid$ or lipase$ or statin$ or hypercholesterolemia or microalbumin$ or albumin$ or platlet$ or plasma$ or hemoglobin$ or haemochromat$ or arterial).ti.
-
(cancer$ or carcinoma$ or neoplas$ or adenoma$ or metformin$).ti.
-
(urin$ or incontinence or bladder or constipat$ or bowel$ or faecal or colorectal or colon$).ti.
-
(gene$ or genotype$ or genome$ or genomic or phenotyp$ or biomarker$ or polymorphism$ or interleukin$).ti.
-
(cell$ or molecular or assay).ti.
-
(cystic or fibrosis or CF or tuberculosis or TB or lupus).ti.
-
(neuropath$ or nephropath$ or prematurity).ti.
-
($arthritis or steroid$ or osteoporosis or atherosclerosis or sclerosis).ti.
-
(apnea or sleep or limb or oral$ or celiac or coeliac or skin or MRSA or anesthesia or vitamin or HIV or testosterone or erectile or schizophren$ or bipolar or antipsychotic$ or psychotic$).ti.
-
prevalence.ti.
-
or/195-212
-
194 not 213
Appendix 4 Modified Effective Practice and Organisation of Care Group taxonomy of quality improvement intervention components
| QI component | Description |
|---|---|
| QI components targeting health-care systems | |
| Case management | Any system for co-ordinating diagnosis, treatment or routine management of patients (e.g. arrangement for referrals, follow-up of test results) by a person or multidisciplinary team in collaboration with, or supplementary to, the primary care clinician |
| Team changes | Changes to the structure or organisation of the primary health-care team (e.g. adding a team member or shared care, use of multidisciplinary teams or expansion or revision of professional roles) |
| Electronic patient registry | General electronic medical record system or electronic tracking system for patients with diabetes |
| Facilitated relay of information to clinicians | Clinical information collected from patients and transmitted to clinicians by means other than the existing medical record |
| Continuous QI | Interventions explicitly identified as involving the techniques of continuous QI, total quality management or any iterative process for assessing quality problems, developing solutions to those problems, testing their effects and then reassessing the need for further action |
| QI components targeting HCPs | |
| Audit and feedback | Summary of the clinical performance of health care delivered by an individual clinician or clinic over a specified period, which is then transmitted back to the clinician (e.g. the percentage of a clinician’s patients who underwent dilated eye examinations with a specified frequency) |
| Clinician education | Interventions designed to promote increased understanding of principles guiding clinical care or awareness of specific recommendations for a target disorder or population of patients |
| Clinician reminders | Paper-based or electronic systems intended to prompt a health professional to recall patient-specific information or carry out a specific task |
| Financial incentives | Interventions with positive or negative financial incentives directed at providers (e.g. linked to adherence to some process of care or achievement of some target outcome). This strategy also includes positive or negative financial incentives directed at patients |
| QI components targeting patients | |
| Education of patients | Interventions designed to promote greater understanding of a target disorder or to teach specific prevention or treatment strategies or provide specific in-person education |
| Promotion of self-management | Provision of equipment or access to resources to promote self-management |
| Reminder systems | Any effort (e.g. postcards or telephone calls) made to remind patients about upcoming appointments or important aspects of self-care |
Appendix 5 Behaviour change techniques used in included studies (Behaviour Change Technique Taxonomy version 1)
| Grouping and BCT | Description |
|---|---|
| Goals and planning | |
| Goal-setting (behaviour) | Set or agree on a goal defined in terms of the behaviour to be achieved |
| Problem-solving | Analyse, or prompt the person to analyse, factors influencing the behaviour and generate or select strategies that include overcoming barriers and/or increasing facilitators |
| Goal-setting (outcome) | Set or agree on a goal defined in terms of a positive outcome of wanted behaviour |
| Action-planning | Prompt detailed planning of performance of the behaviour. Context may be environmental (physical or social) or internal (physical, emotional or cognitive) |
| Review behaviour goals | Review behaviour goal(s) jointly with the person and consider modifying goal(s) or the behaviour change strategy in light of achievements |
| Discrepancy between current behaviour and goals | Draw attention to discrepancies between a person’s current behaviour (in terms of the form, frequency, duration or intensity of that behaviour) and the person’s previously set outcome goals, behavioural goals or action plans |
| Review outcome goals | Review outcome goal(s) jointly with the person and consider modifying goal(s) in light of achievements. This may lead to resetting the same goal, a small change in that goal or setting a new goal instead of, or in addition to, the first goal |
| Behavioural contract | Create a written specification of the behaviour to be performed, agreed on by the person and witnessed by another |
| Commitment | Ask the person to affirm or reaffirm statements indicating commitment to change the behaviour |
| Feedback and monitoring | |
| Monitoring of behaviour by others without feedback | Observe or record behaviour with the person’s knowledge as part of a behaviour change strategy |
| Feedback on behaviour | Monitor and provide informative or evaluative feedback on performance of the behaviour (e.g. form, frequency, duration, intensity) |
| Self-monitoring of behaviour | Establish a method for the person to monitor and record their behaviour(s) as part of a behaviour change strategy |
| Self-monitoring of outcomes of behaviour | Establish a method for the person to monitor and record the outcome(s) of their behaviour as part of a behaviour change strategy |
| Monitoring of outcomes of behaviour by others without feedback | Observe or record outcomes of behaviour with the person’s knowledge as part of a behaviour change strategy |
| Feedback on outcomes of behaviour/biofeedback | Monitor and provide feedback on the outcome of performance of the behaviour |
| Social support | |
| Social support (unspecified) | Advise on, arrange or provide social support (e.g. from friends, relatives, colleagues, ’buddies’ or staff) or non-contingent praise or reward for performance of the behaviour. Includes encouragement and counselling, but only when it is directed at the behaviour |
| Social support (practical) | Advise on, arrange or provide practical help (e.g. from friends, relatives, colleagues, ‘buddies’ or staff) for performance of the behaviour |
| Shaping knowledge | |
| Instruction on how to perform the behaviour | Advise or agree on how to perform the behaviour |
| Natural consequences | |
| Information about health consequences | Provide information (e.g. written, verbal, visual) about the health consequences of performing the behaviour |
| Salience of consequences | Use methods specifically designed to emphasise the consequences of performing the behaviour with the aim of making them more memorable |
| Information about social and environmental consequences | Provide information (e.g. written, verbal, visual) about the social and environmental consequences of performing the behaviour |
| Information about emotional consequences | Provide information (e.g. written, verbal, visual) about the emotional consequences of performing the behaviour |
| Comparison of behaviour | |
| Demonstration of the behaviour | Provide an observable sample of the performance of the behaviour, directly in person or indirectly (e.g. film, pictures), for the person to aspire to or imitate |
| Social comparison | Draw attention to others’ performance to allow comparison with the person’s own performance |
| Information about others’ approval | Provide information about what other people think about the behaviour. The information clarifies whether others will like, approve of or disapprove of what the person is doing or will do |
| Associations | |
| Prompts/cues | Introduce or define an environmental or a social stimulus with the purpose of prompting or cueing the behaviour. The prompt or cue would normally occur at the time or place of the performance |
| Reduce prompts/cues | Withdraw gradually prompts to perform the behaviour |
| Repetition and substitution | |
| Behavioural practice/rehearsal | Prompt practice or rehearsal of the performance of the behaviour one or more times in a context or at a time when the performance may not be necessary, to increase habit and skill |
| Graded tasks | Set easy-to-perform tasks, making them increasingly difficult but achievable, until the behaviour is performed |
| Comparison of outcomes | |
| Credible source | Present verbal or visual communication from a credible source in favour of or against the behaviour |
| Reward and threat | |
| Material incentive (behaviour) | Inform the person that money, vouchers or other valued objects will be delivered if and only if there has been effort and/or progress in performing the behaviour |
| Social reward | Arrange a verbal or non-verbal reward if and only if there has been effort and/or progress in performing the behaviour |
| Non-specific reward | Inform the person that a reward will be delivered if and only if there has been effort and/or progress in performing the behaviour |
| Antecedents | |
| Restructuring the physical environment | Change, or advise to change, the physical environment to facilitate performance of the wanted behaviour or create barriers to performance of the unwanted behaviour (other than prompts/cues, rewards and punishments) |
| Restructuring the social environment | Change, or advise to change, the social environment to facilitate performance of the wanted behaviour or create barriers to performance of the unwanted behaviour (other than prompts/cues, rewards and punishments) |
| Adding objects to the environment | Add objects to the environment to facilitate performance of the behaviour |
| Scheduled consequences | |
| Behaviour cost | Arrange for withdrawal of something valued if and only if an unwanted behaviour is performed |
| Self-belief | |
| Verbal persuasion about capability | Tell the person that they can successfully perform the wanted behaviour, arguing against self-doubts and asserting that they can and will succeed |
| Focus on past success | Advise the person to think about or list previous successes in performing the behaviour (or parts of it) |
Appendix 6 Summary of reported costs and resources needed to deliver interventions
| QI component | Study | DRS or GQI | Estimated costs of resources utilised | Resources utilised |
|---|---|---|---|---|
| Promotion of self-management | Davis 2011;124n = 85 patients | GQI | Staff cost per person = £625.25, costs of the other resources used = £476.35 over 12 months, direct cost per person = £1102 | 13 sessions (three individual and 10 group session) consisting of 15 minutes with nurses and 4 hours with a health educator per person |
| Wagner 2001;105n = 14 clinics,n = 278 patients | GQI | Not reported | 1-hour group session with relevant health professional every 3–6 months | |
| Team changes | Frei 2014;69n = 15 practices,n = 164 patients | GQI | Not reported | 6-day training for nurses, two 4-hour workshops for physicians and nurses |
| Wagner 2001;105n = 14 clinics,n = 278 patients | GQI | Not reported | 1-hour group session with relevant health professional every 3–6 months | |
| Litaker 2003;87n = 79 patients | GQI | Mean personnel costs for the intervention per month per patient = £130.15, total additional personnel costs = £10,281.97 | Average of 180 minutes with patients | |
| Case management | Krein 2004;85n = 123 | GQI | Not reported | 2 days of training for case managers, 20 hours per week spent with patients. Quarterly profiling for 24 months and subsequently every 6 months |
| Patient education | Prezio 2014;96n = 90 | GQI | Physician = £48.76 per hour, community health worker = £12.91 per hour, cost of intervention over 20 years = £3646.10 per patient | Seven sessions per patients, 1 hour of physician supervision for health workers |
| Pizzi 2015;117n = 117 for mailed intervention,n = 120 for telephone intervention | DRS | Staff time for 120 patients for telephone intervention over 1 month = £501.13, staff time for 117 patients for mailed intervention over 1 month = £173.17; £85.24 per hour for physician, £29.32 per hour for health services manager, £16.72 per hour for medical assistant; materials for telephone intervention = £30.25, materials for mailed intervention = £135.46; total cost of intervention = £577.64 for 120 patients in telephone intervention group over 1 month, £335.48 for 117 patients in mailed intervention group over 1 month; total cost per patient when appointment is made and kept: telephone intervention = £9.47, mailed intervention = £8.83 | 1 hour of supervision for every 20 hours of intervention delivered, two 1-hour meetings with medical assistants, health services manager and ophthalmologist | |
| Adair 2013;59n = 930 | GQI | Care guide cost for 120 patients = £375,917 at the rate of £11.77 per hour over 1 year, cost of two supervisory nurses = £85,847.24, training cost = £2228.99, cost of modular furniture and equipment for 12 stations = £79,422.81; total cost = £463,993; total cost of intervention per patient = £326 | 12 care guides, 2 weeks of training, two supervisory nurses, five visits on average to clinics, four contacts with patients, furniture and modular equipment | |
| McCall 2011;89n = approximately 20,000 | GQI | Not reported | Not reported | |
| Clancy 2007;62n = 96 | GQI | Deposit fee for group visit = £13.40 per visit, £160.60 for 12 visits | Monthly meeting for a year for 2 hours, including one primary care internal medicine physician and one registered nurse per visit, training for physicians and nurses, 3 hours of training for clinic staff | |
| Schechter 2008;125n = 305 for telephone intervention,n = 298 for print intervention | DRS | Health educator = £14,890.83, training and supervision = £2756.44, telephone charges = £679.67 for 305 patients, printing and mailing = £465.99 for 298 patients | Average of 3.2 calls for about 20 minutes + 5-minute call for preparation per patient over 6 months; 20 hours of training, 1 hour of supervision by a diabetes nurse educator, telephone calls | |
| Electronic patient register | Eccles 2007;67n = 30 practices,n = 1674 patients | GQI | Developing the guidelines = £10,208, software development = £12,519.36, educational activities = £2148.11, additional cost of running the system = £9964.46; annual cost per patient = £68.21 | Cost of guideline and software development, average of two follow-up contacts |
| Patient reminders | Schechter 2008;125n = 305 for telephone intervention,n = 298 for print intervention | DRS | Health educator = £14,890.83, training and supervision = £2756.44, telephone charges = £679.67 for 305 patients, printing and mailing = £465.99 for 298 patients | Average of 3.2 calls for about 20 minutes + 5-minute call for preparation per patient over 6 months; 20 hours of training, 1 hour of supervision by a diabetes nurse educator, telephone calls |
| Pizzi 2015;117n = 117 for mailed intervention,n = 120 for telephone intervention | DRS | Staff time for 120 patients for telephone intervention over 1 month = £501.13, staff time for 117 patients for mailed intervention over 1 month = £173.17; £85.24 per hour for physician, £29.32 per hour for health services manager, £16.72 per hour for medical assistant; materials for telephone intervention = £30.25, materials for mailed intervention = £135.46; total cost of intervention = £577.64 for 120 patients in telephone intervention group over 1 month, £335.48 for 117 patients in mailed intervention group over 1 month | 1 hour of supervision for every 20 hours of intervention delivered, two 1-hour meetings with medical assistants, health services manager and ophthalmologist | |
| Audit and feedback | Frijling 2002;70n = 62 clusters,n = 703 patients | GQI | Clinical decision making per practice = £341.51 | 80 hours of training for facilitator, 15 1-hour visits to the practice clinic, 3 hours of GP time for implementation of feedback |
| Clinician reminders | Litaker 2003;87n = 79 patients | GQI | Mean personnel costs for the intervention per month = £130.15, total additional personnel costs = £10,281.97 | Average of 180 minutes with patients over 12 months |
| Continuous QIs | Piette 2001;95n = 146 | GQI | Approximately £14–24 per year for automated calls | 13 nurses spending an average of 3.8 hours per patient, 15 automated calls |
Appendix 7 Theoretical Domains Framework: definitions and examples
| TDF domain and definition | Examples related to diabetic retinopathy |
|---|---|
| Knowledge: awareness of the existence of something |
|
| Skills: ability or proficiency acquired through practice |
|
| Social/professional role and identity: a coherent set of behaviours and displayed personal qualities of an individual in a social or work setting |
|
| Beliefs about capabilities: acceptance of the truth/reality about or validity of an ability, talent or facility that a person can put to constructive use |
|
| Optimism: confidence that things will happen for the best or that desired goals will be attained |
|
| Beliefs about consequences: acceptance of the truth/reality about or validity of outcomes of a behaviour in a given situation |
|
| Reinforcement: increasing the probability of a response by arranging a dependent relationship, or contingency, between the response and a given stimulus |
|
| Intentions: conscious decision to perform a behaviour or a resolve to act in a certain way |
|
| Goals: mental representation of outcomes or end states that an individual wants to achieve |
|
| Memory, attention and decision processes: ability to retain information, focus selectively on aspects of the environment and choose between two or more alternatives |
|
| Environmental context and resources: any circumstances of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence and adaptive behaviour |
|
| Social influences: interpersonal processes that can cause an individual to change their thoughts, feelings or behaviours |
|
| Emotion: a complex reaction pattern involving experiential, behavioural and physiological elements, by which the individual attempts to deal with a personally significant matter or event |
|
| Behavioural regulation: anything aimed at managing or changing objectively observed or measured actions |
|
Appendix 8 Consolidated Framework for Implementation Research: constructs and descriptions
| CFIR construct | Description |
|---|---|
| Intervention characteristics | |
| Intervention source | Perception of key stakeholders about whether the intervention is externally or internally developed |
| Evidence strength and quality | Stakeholders’ perceptions of the quality and validity of evidence supporting the belief that the intervention will have the desired outcomes |
| Relative advantage | Stakeholders’ perceptions of the advantages of implementing the intervention compared with an alternative solution |
| Adaptability | The degree to which an intervention can be adapted, tailored, refined or reinvented to meet local needs |
| Trialability | The ability to test the intervention on a small scale in the organisation and to be able to reverse the course (undo implementation) if warranted |
| Complexity | The perceived difficulty of implementation of an intervention, reflected by the duration, scope, radicalness, disruptiveness, centrality and intricacy and number of steps required to implement it |
| Design quality and packaging | Perceived excellence in how the intervention is bundled, presented and assembled |
| Cost | Cost of the intervention and costs associated with implementing the intervention, including investment, supply and opportunity costs |
| Outer setting | |
| Patient needs and resources | The extent to which patient needs, as well as barriers to and facilitators of meeting those needs, are accurately known and prioritised by the organisation |
| Cosmopolitanism | The degree to which an organisation is networked with other external organisations |
| Peer pressure | Mimetic or competitive pressure to implement an intervention, typically because most or other key peer or competing organisations have already implemented or are in a bid for a competitive edge |
| External policy and incentives | A broad construct that includes external strategies to spread interventions, including policy and regulations (governmental or other central entity), external mandates, recommendations and guidelines, pay for performance, collaborative and public or benchmark reporting |
| Inner setting | |
| Structural characteristics | The social architecture, age, maturity and size of an organisation |
| Networks and communications | The nature and quality of webs of social networks and the nature and quality of formal and informal communication within an organisation |
| Culture | Norms, values and basic assumptions of a given organisation |
| Implementation climate | The absorptive capacity for change, shared receptivity of involved individuals to an intervention and extent to which use of the intervention will be rewarded, supported and expected within the organisation |
| Tension for change | The degree to which stakeholders perceive the current situation to be intolerable or needing change |
| Compatibility | The degree of tangible fit between meaning and values attached to the intervention by involved individuals, how those align with individuals’ own norms, values and perceived risks and needs and how the intervention fits with existing workflows and systems |
| Relative priority | Individuals’ shared perceptions of the importance of implementation within the organisation |
| Organisation incentives and rewards | Extrinsic incentives such as goal-sharing awards, performance reviews, promotions and raises in salary and less tangible incentives such as increased stature or respect |
| Goals and feedback | The degree to which goals are clearly communicated, acted on and fed back to staff and alignment of that feedback with goals |
| Learning climate | A climate in which leaders express their own fallibility and need for team members’ assistance and input; team members feel that they are essential, valued and knowledgeable partners in the change process; individuals feel psychologically safe to try new methods; and there is sufficient time and space for reflective thinking and evaluation |
| Readiness for implementation | Tangible and immediate indicators of organisation commitment to its decision to implement an intervention |
| Leadership engagement | Commitment, involvement and accountability of leaders and managers with regard to implementation |
| Available resources | The level of resources dedicated for implementation and ongoing operations, including money, training, education, physical space and time |
| Access to knowledge and information | Ease of access to digestible information and knowledge about the intervention and how to incorporate it into work tasks |
| Characteristics of individuals | |
| Knowledge of beliefs about the intervention | Individuals’ attitudes towards and value placed on the intervention as well as familiarity with facts, truths and principles related to the intervention |
| Self-efficacy | Individuals’ belief in their own capabilities to execute the course of action to achieve implementation goals |
| Individual stage of change | Characterisation of the phase that an individual is in, as he or she progresses towards skilled, enthusiastic and sustained use of the intervention |
| Individual identification with the organisation | A broad construct related to how individuals perceive the organisation and their relationship with and degree of commitment to that organisation |
| Other personal attributes | A broad construct to include other personal traits such as tolerance of ambiguity, intellectual ability, motivation, values, competence, capacity and learning style |
| Process | |
| Planning | The degree to which a scheme or method of behaviour and tasks for implementing an intervention are developed in advance and the quality of those schemes or methods |
| Engaging | Attracting and involving appropriate individuals in the implementation and use of the intervention through a combined strategy of social marketing, education, role modelling, training and other similar activities |
| Opinion leaders | Individuals in an organisation who have formal or informal influence on the attitudes and beliefs of their colleagues with respect to implementing the intervention |
| Formally appointed internal implementation leaders | Individuals from within the organisation who have been formally appointed with responsibility for implementing an intervention as co-ordinator, project manager, team leader or other similar role |
| Champions | Individuals who dedicate themselves to supporting, marketing and ‘driving through’ an implementation, overcoming indifference or resistance that the intervention may provoke in an organisation |
| External change agents | Individuals who are affiliated with an outside entity that formally influences or facilitates intervention decisions in a desirable direction |
| Executing | Carrying out or accomplishing the implementation according to plan |
| Reflecting and evaluating | Quantitative and qualitative feedback about the progress and quality of implementation action accompanied with regular personal and team debriefings about progress and experience |
Appendix 9 Example search strategy for the phase 2 systematic review: barriers to and enablers of diabetic retinopathy screening attendance
MEDLINE (via Ovid)
-
exp Diabetic Retinopathy/
-
((diabet$ or proliferative or non-proliferative) adj4 retinopath$).tw.
-
diabetic retinopathy.kw.
-
(diabet$ adj3 (eye$ or vision or visual$ or sight$)).tw.
-
(retinopath$ adj3 (eye$ or vision or visual$ or sight$)).tw.
-
(DR adj3 (eye$ or vision or visual$ or sight$)).tw.
-
or/1-6)
-
exp Mass Screening/
-
exp Vision Tests/
-
exp Telemedicine/
-
exp Photography/
-
exp Ophthalmoscopes/
-
exp Ophthalmoscopy/
-
(ophthalmoscop$ or fundoscop$ or funduscop$).ti.
-
((exam$ or photo$ or imag$) adj3 fundus).tw.
-
(photography or retinography).tw.
-
((mydriatic or digital or retina$ or fundus or steroscopic) adj3 camera).tw.
-
((mydriatic or digital or retina$ or fundus or steroscopic) adj3 imag$).tw.
-
screen$.tw.
-
((eye$ or retina$ or ophthalm$) adj4 exam$).tw.
-
((eye or vision or retinopathy or ophthalmic) adj4 test$).tw.
-
((eye$ or retina$ or ophthalm$) adj4 visit$).tw.
-
Office Visits/
-
(telemedicine$ or telemonitor$ or telescreen$ or telehealth or teleophthalmology).tw.
-
or/8-24
-
exp Patient Acceptance of health Care/
-
exp Attitude to Health/
-
exp Health Behavior/
-
Motivation/
-
Fear/
-
exp Self Concept/
-
Personal Autonomy/
-
Self Care/
-
Behavior Therapy/
-
(barrier$ or obstacle$ or facilitat$ or enable$).tw.
-
(knowledge or skill$ or role$ or identity or capabilit$ or optimis$ or consequence$ or reinforcement or intention$ or goal$ or memory or attention or context$ or resources or emotion$).tw.
-
(decision adj2 process$).tw.
-
(social adj2 influence$).tw.
-
(behavioural adj2 regulation).tw.
-
(competence or self-efficac$ or self-confidence or incentiv$ or reward$ or anxiety fear$ or self-monitor$ or habits).tw.
-
(outcome adj2 expectanc$).tw.
-
(action adj2 plan$).tw.
-
(decision adj2 mak$).tw.
-
(social adj2 (support$ or norm)).tw.
-
((behaviour$ or behavior$) adj3 (change$ or modif$ or activat$ or control$ or amend$)).tw.
-
(uptake or takeup or attend$ or accept$ or adhere$ or attitude$ or participat$ or facilitat$ or utilisat$ or utilizat$).tw.
-
(motivat$ or satisf$ or promot$ or consent$ or self select$ or self referr$).tw.
-
(complie$ or comply or compliance$ or noncompliance$ or non compliance$).tw.
-
(encourag$ or discourage$ or reluctan$ or nonrespon$ or non respon$ or refuse$).tw.
-
(non-attend$ or non attend$ or dropout or drop out or apath$).tw.
-
Health Education/
-
exp Patient Education as Topic/
-
exp Health Promotion/
-
exp Counseling/
-
‘Attitude of Health Personnel’/
-
(health adj2 (promotion$ or knowledge or belief$)).tw.
-
(educat$ adj2 (intervention$ or information or material or leaflet)).tw.
-
Focus groups/
-
Interviews as Topic/
-
(focus adj3 group$).tw.
-
Socioeconomic Factors/
-
exp Poverty/
-
Social Class/
-
Educational Status/
-
((school or education$) adj3 (status or level$ or attain$ or achieve$)).tw.
-
Employment/
-
Uncompensated Care/
-
Reimbursement Mechanisms/
-
Reimbursement, Incentive/
-
(insurance adj3 (health$ or scheme$)).tw.
-
(financial or pay or payment or copayment or paid or fee or fees or monetary or incentiv$ or disincentiv$).tw.
-
Healthcare Disparities/
-
Health Status Disparities/
-
exp Medically Underserved Area/
-
Rural Population/
-
Urban Population/
-
exp Ethnic Groups/
-
Minority Groups/
-
Vulnerable Populations/
-
((health$ or social$ or racial$ or ethnic$) adj5 (inequalit$ or inequit$ or disparit$ or equit$ or disadvantage$ or depriv$)).tw.
-
(disadvant$ or marginali$ or underserved or under served or impoverish$ or minorit$ or racial$ or ethnic$).tw.
-
or/26-80
-
7 and 25 and 82)
-
limit 83 to yr = ‘1990 -Current’
-
(ranibizumab or bevacizumab or avastin or aflibercept).ti.
-
(cataract$ or intraocular or glaucoma$ or phaco$ or photocoagulat$ or photodynamic or laser$ or vitrectom$).ti.
-
(macula$ adj2 (degener$ or oedema or edema)).ti.
-
nerve fiber layer.ti.
-
(coronary or cardiac or cardio$ or heart or myocardia$ or artery or aneurysm or atrial or echocardiography or hypertension or hypotension or stroke or pulmonary or COPD or lung$ or organ$ or smoking).ti.
-
(pregnan$ or gestational or neonat$ or perinatal or maternal or trimester or congenital or ovary or breast$).ti.
-
(kidney or liver or cirrhosis or renal or hepatitis or dialysis or pancrea$ or gastric or gastrectom$ or surg$ or duoden$).ti.
-
(blood glucose or blood pressure or ketoacidosis or hypoglycemi$ or rosiglitazone).ti.
-
(lipid$ or lipase$ or statin$ or hypercholesterolemia or microalbumin$ or albumin$ or platlet$ or plasma$ or hemoglobin$ or haemochromat$ or arterial).ti.
-
(cancer$ or carcinoma$ or neoplas$ or adenoma$ or metformin$).ti.
-
(urin$ or incontinence or bladder or constipat$ or bowel$ or faecal or colorectal or colon$).ti.
-
(gene$ or genotype$ or genome$ or genomic or phenotyp$ or biomarker$ or polymorphism$ or interleukin$).ti.
-
(cell$ or molecular or assay).ti.
-
(cystic or fibrosis or CF or tuberculosis or TB or lupus).ti.
-
(neuropath$ or nephropath$ or prematurity).ti.
-
($arthritis or steroid$ or osteoporosis or atherosclerosis or sclerosis).ti.
-
(apnea or sleep or limb or oral$ or celiac or coeliac or skin or MRSA or anesthesia or vitamin or HIV or testosterone or erectile or schizophren$ or bipolar or antipsychotic$ or psychotic$).ti.
-
prevalence.ti.
-
or/85-102
-
84 not 103
Appendix 10 Results using a threshold of £30,000 per quality-adjusted life-year
Chapter 6reports the model results using a cost-effectiveness threshold of £20,000 per QALY. The model results using a threshold of £30,000 per QALY are reported in this appendix.
Probability that an intervention is cost-effective (compared with no intervention) for quality improvement interventions at a £30,000 per quality-adjusted life-year threshold
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| Audit and feedback | 0.415 | 0.351 | 0.259 | 0.102 | 0.013 |
| Case management | 0.035 | 0.022 | 0.006 | 0.000 | 0.000 |
| Team changes | 0.850 | 0.794 | 0.676 | 0.319 | 0.015 |
| Electronic patient registry | 0.577 | 0.527 | 0.500 | 0.417 | 0.202 |
| Clinician education | 0.712 | 0.670 | 0.619 | 0.470 | 0.186 |
| Clinician reminders | 0.640 | 0.554 | 0.452 | 0.232 | 0.038 |
| Patient education | 0.809 | 0.773 | 0.730 | 0.628 | 0.309 |
| Promotion of self management | 0.759 | 0.690 | 0.565 | 0.275 | 0.027 |
| Patient reminders | 0.619 | 0.584 | 0.546 | 0.456 | 0.210 |
Probability that an intervention is cost-effective (including all interventions) for quality improvement interventions at a £30,000 per quality-adjusted life-year threshold
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| No intervention | 0.015 | 0.022 | 0.053 | 0.146 | 0.411 |
| Audit and feedback | 0.018 | 0.014 | 0.011 | 0.012 | 0.003 |
| Case management | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Team changes | 0.201 | 0.157 | 0.097 | 0.038 | 0.006 |
| Electronic patient registry | 0.111 | 0.132 | 0.151 | 0.162 | 0.144 |
| Clinician education | 0.096 | 0.116 | 0.128 | 0.135 | 0.088 |
| Clinician reminders | 0.123 | 0.100 | 0.081 | 0.046 | 0.018 |
| Patient education | 0.213 | 0.257 | 0.285 | 0.294 | 0.204 |
| Promotion of self-management | 0.174 | 0.138 | 0.107 | 0.043 | 0.012 |
| Patient reminders | 0.049 | 0.064 | 0.085 | 0.125 | 0.113 |
Probability that an intervention is cost-effective (compared with no intervention) for behaviour change technique interventions at a £30,000 per quality-adjusted life-year threshold
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| Patient-targeted interventions | |||||
| Problem-solving | 0.822 | 0.748 | 0.597 | 0.267 | 0.010 |
| Goal-setting (outcome) | 1.000 | 0.999 | 0.997 | 0.961 | 0.191 |
| Feedback on outcomes of behaviour/biofeedback | 1.000 | 1.000 | 1.000 | 0.997 | 0.866 |
| Social support (unspecified) | 0.991 | 0.989 | 0.986 | 0.980 | 0.937 |
| Social support (practical) | 0.884 | 0.843 | 0.742 | 0.404 | 0.050 |
| Instruction on how to perform the behaviour | 0.694 | 0.590 | 0.436 | 0.112 | 0.005 |
| Information about health consequences | 1.000 | 1.000 | 0.999 | 0.999 | 0.986 |
| Prompts/cues | 0.896 | 0.881 | 0.880 | 0.846 | 0.666 |
| Credible source | 0.705 | 0.682 | 0.651 | 0.568 | 0.364 |
| Restructuring the social environment | 0.625 | 0.586 | 0.522 | 0.394 | 0.181 |
| HCP-targeted interventions | |||||
| Feedback on outcomes of behaviour/biofeedback | 0.776 | 0.742 | 0.730 | 0.634 | 0.325 |
| Social support (practical) | 0.998 | 0.994 | 0.901 | 0.396 | 0.003 |
| Instruction on how to perform the behaviour | 0.616 | 0.565 | 0.505 | 0.347 | 0.103 |
| Prompts/cues | 0.800 | 0.738 | 0.630 | 0.358 | 0.055 |
| Credible source | 0.917 | 0.884 | 0.852 | 0.715 | 0.339 |
| Restructuring the social environment | 0.964 | 0.879 | 0.459 | 0.168 | 0.002 |
| Adding objects to the environment | 0.946 | 0.947 | 0.937 | 0.914 | 0.798 |
Probability that an intervention is cost-effective (including all interventions) for behaviour change technique interventions at a £30,000 per quality-adjusted life-year threshold
| Intervention | Baseline probability of screening uptake | ||||
|---|---|---|---|---|---|
| 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | |
| No intervention | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Patient-targeted interventions | |||||
| Problem-solving | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Goal-setting (outcome) | 0.294 | 0.203 | 0.117 | 0.070 | 0.017 |
| Feedback on outcomes of behaviour/biofeedback | 0.282 | 0.292 | 0.313 | 0.253 | 0.158 |
| Social support (unspecified) | 0.081 | 0.103 | 0.130 | 0.160 | 0.231 |
| Social support (practical) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Instruction on how to perform the behaviour | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Information about health consequences | 0.268 | 0.353 | 0.411 | 0.486 | 0.528 |
| Prompts/cues | 0.000 | 0.001 | 0.002 | 0.002 | 0.011 |
| Credible source | 0.001 | 0.002 | 0.002 | 0.003 | 0.007 |
| Restructuring the social environment | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| HCP-targeted interventions | |||||
| Feedback on outcomes of behaviour/biofeedback | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Social support (practical) | 0.067 | 0.029 | 0.006 | 0.001 | 0.000 |
| Instruction on how to perform the behaviour | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Prompts/cues | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 |
| Credible source | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 |
| Restructuring the social environment | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Adding objects to the environment | 0.006 | 0.014 | 0.015 | 0.022 | 0.046 |
Appendix 11 The maximum cost per patient for the quality improvement and behaviour change technique components
This appendix presents the maximum costs per patient given the mean RD or RR estimated from the meta-regressions for the QI and BCT factors. These results were derived from the maximum cost curves presented inChapter 6.
Estimated maximum costs associated with the mean estimates of effectiveness and the lower and upper limits of the confidence intervals for different quality improvement components for the models assuming a 1-year screening interval, including and excluding care costs
| Intervention | RD (95% CI) | RR (95% CI) | Maximum cost per patient (95% CI) (£) | |||||
|---|---|---|---|---|---|---|---|---|
| No care costs | Care costs | |||||||
| Per patient | Per general practice (×1000) | England (×1,000,000) | Per patient | Per general practice (×1000) | England (×1,000,000) | |||
| Audit and feedback | 0.00 (–0.18 to 0.16) | 1.00 (0.75 to 1.23) | 0 (0 to 40) | 0 (0 to 2.89) | 0 (0 to 117) | 0 (0 to 52) | 0 (0 to 3.76) | 0 (0 to 152) |
| Case management | –0.07 (–0.26 to 0.10) | 0.91 (0.63 to 1.14) | 0 (0 to 26) | 0 (0 to 1.88) | 0 (0 to 76) | 0 (0 to 33) | 0 (0 to 2.39) | 0 (0 to 96) |
| Team changes | 0.11 (–0.03 to 0.21) | 1.16 (0.96 to 1.29) | 29 (0 to 50) | 2.10 (0 to 3.62) | 84 (0 to 146) | 37 (0 to 64) | 2.68 (0 to 4.63) | 108 (0 to 186) |
| Electronic patient registry | 0.04 (–0.20 to 0.18) | 1.05 (0.72 to 1.26) | 10 (0 to 45) | 0.72 (0 to 3.26) | 29 (0 to 131) | 13 (0 to 58) | 0.94 (0 to 4.20) | 38 (0 to 169) |
| Clinician education | 0.09 (–0.11 to 0.18) | 1.12 (0.85 to 1.25) | 22 (0 to 44) | 1.59 (0 to 3.18) | 64 (0 to 128) | 29 (0 to 57) | 2.10 (0 to 4.12) | 84 (0 to 166) |
| Clinician reminders | 0.06 (–0.13 to 0.20) | 1.09 (0.81 to 1.29) | 17 (0 to 49) | 1.23 (0 to 3.55) | 50 (0 to 143) | 22 (0 to 64) | 1.59 (0 to 4.63) | 64 (0 to 186) |
| Patient education | 0.10 (–0.08 to 0.19) | 1.14 (0.88 to 1.27) | 25 (0 to 47) | 1.81 (0 to 3.40) | 73 (0 to 137) | 32 (0 to 61) | 2.32 (0 to 4.41) | 93 (0 to 178) |
| Promotion of self-management | 0.09 (–0.07 to 0.21) | 1.13 (0.90 to 1.30) | 24 (0 to 51) | 1.74 (0 to 3.69) | 70 (0 to 149) | 31 (0 to 65) | 2.24 (0 to 4.70) | 90 (0 to 189) |
| Patient reminders | 0.05 (–0.14 to 0.16) | 1.07 (0.80 to 1.23) | 13 (0 to 40) | 0.94 (0 to 2.89) | 38 (0 to 117) | 17 (0 to 52) | 1.23 (0 to 3.76) | 50 (0 to 152) |
Estimated maximum costs associated with the mean estimates of effectiveness and the lower and upper limits of the confidence intervals for different behaviour change techniques for the models assuming a 1-year screening interval, including and excluding care costs
| Intervention | RD (95% CI) | RR (95% CI) | Maximum cost per patient (95% CI) (£) | |||||
|---|---|---|---|---|---|---|---|---|
| No care costs | Care costs | |||||||
| Per patient | Per GP practice (×1000) | England (×1,000,000) | Per patient | Per GP practice (×1000) | England (×1,000,000) | |||
| Patient-targeted interventions | ||||||||
| Problem-solving | 0.11 (–0.04 to 0.21) | 1.16 (0.95 to 1.30) | 30 (0 to 50) | 2.17 (0 to 3.62) | 87 (0 to 146) | 38 (0 to 64) | 2.75 (0 to 4.63) | 111 (0 to 186) |
| Goal-setting (outcome) | 0.23 (0.16 to 0.27) | 1.33 (1.23 to 1.38) | 58 (41 to 68) | 4.20 (2.97 to 4.92) | 169 (119 to 198) | 72 (52 to 83) | 5.21 (3.76 to 6.01) | 210 (152 to 242) |
| Feedback on outcomes of behaviour/biofeedback | 0.21 (0.12 to 0.25) | 1.30 (1.17 to 1.36) | 50 (31 to 64) | 3.62 (2.24 to 4.63) | 146 (90 to 186) | 64 (39 to 78) | 4.63 (2.82 to 5.64) | 186 (114 to 227) |
| Social support (unspecified) | 0.17 (0.04 to 0.24) | 1.24 (1.06 to 1.34) | 42 (11 to 59) | 3.04 (0.80 to 4.27) | 122 (32 to 172) | 54 (14 to 73) | 3.91 (1.01 to 5.28) | 157 (41 to 213) |
| Social support (practical) | 0.12 (–0.02 to 0.20) | 1.17 (0.97 to 1.29) | 30 (0 to 49) | 2.17 (0 to 3.55) | 87 (0 to 143) | 39 (0 to 63) | 2.82 (0 to 4.56) | 114 (0 to 184) |
| Instruction on how to perform the behaviour | 0.09 (–0.06 to 0.18) | 1.12 (0.91 to 1.26) | 23 (0 to 45) | 1.66 (0 to 3.26) | 67 (0 to 131) | 29 (0 to 58) | 2.10 (0 to 4.20) | 84 (0 to 169) |
| Information about health consequences | 0.20 (0.11 to 0.25) | 1.29 (1.15 to 1.35) | 48 (28 to 62) | 3.47 (2.03 to 4.49) | 140 (82 to 181) | 63 (36 to 77) | 4.56 (2.60 to 5.57) | 184 (105 to 224) |
| Prompts/cues | 0.10 (–0.05 to 0.19) | 1.14 (0.93 to 1.27) | 25 (0 to 46) | 1.81 (0 to 3.33) | 73 (0 to 134) | 33 (0 to 60) | 2.39 (0 to 4.34) | 96 (0 to 175) |
| Credible source | 0.06 (–0.14 to 0.19) | 1.09 (0.80 to 1.27) | 18 (0 to 47) | 1.30 (0 to 3.40) | 52 (0 to 137) | 23 (0 to 60) | 1.66 (0 to 4.34) | 67 (0 to 175) |
| Restructuring the social environment | 0.05 (–0.13 to 0.17) | 1.07 (0.81 to 1.25) | 13 (0 to 43) | 0.94 (0 to 3.11) | 38 (0 to 125) | 17 (0 to 55) | 1.23 (0 to 3.98) | 50 (0 to 160) |
| HCP-targeted interventions | ||||||||
| Feedback on outcomes of behaviour/biofeedback | 0.07 (–0.08 to 0.17) | 1.10 (0.89 to 1.24) | 19 (0 to 43) | 1.37 (0 to 3.11) | 55 (0 to 125) | 24 (0 to 55) | 1.74 (0 to 3.98) | 70 (0 to 160) |
| Social support (practical) | 0.25 (0.16 to 0.28) | 1.35 (1.23 to 1.40) | 61 (41 to 71) | 4.41 (2.97 to 5.14) | 178 (119 to 207) | 76 (53 to 86) | 5.50 (3.84 to 6.22) | 221 (154 to 251) |
| Instruction on how to perform the behaviour | 0.04 (–0.12 to 0.16) | 1.06 (0.83 to 1.23) | 12 (0 to 40) | 0.87 (0 to 2.89) | 35 (0 to 117) | 15 (0 to 51) | 1.09 (0 to 3.69) | 44 (0 to 149) |
| Prompts/cues | 0.12 (–0.07 to 0.22) | 1.17 (0.90 to 1.32) | 31 (0 to 54) | 2.24 (0 to 3.91) | 90 (0 to 157) | 40 (0 to 68) | 2.89 (0 to 4.92) | 117 (0 to 198) |
| Credible source | 0.11 (–0.03 to 0.20) | 1.16 (0.96 to 1.29) | 30 (0 to 49) | 2.17 (0 to 3.55) | 87 (0 to 143) | 38 (0 to 63) | 2.75 (0 to 4.56) | 111 (0 to 184) |
| Restructuring the social environment | 0.19 (0.08 to 0.25) | 1.27 (1.12 to 1.35) | 47 (22 to 62) | 3.40 (1.59 to 4.49) | 137 (64 to 181) | 61 (28 to 76) | 4.41 (2.03 to 5.50) | 178 (82 to 221) |
| Adding objects to the environment | 0.13 (–0.02 to 0.21) | 1.18 (0.98 to 1.31) | 33 (0 to 52) | 2.39 (0 to 3.76) | 96 (0 to 152) | 42 (0 to 66) | 3.04 (0 to 4.78) | 122 (0 to 192) |
Glossary
- Antiangiogenic therapy
- A procedure whereby drugs are injected into the eye to block the factors associated with the growth and leakage of abnormal blood vessels. Used in the treatment of proliferative diabetic retinopathy and diabetic maculopathy.
- Background diabetic retinopathy
- The earliest visible change to the retina in diabetes, in which small blood vessels show signs of damage. They may bulge slightly or leak blood (haemorrhage) or fluid.
- Behaviour change technique
- An observable and replicable component designed to change behaviour. It is the smallest component compatible with retaining the postulated active ingredients and can be used alone or in combination with other behaviour change techniques.
- Diabetes mellitus
- A lifelong condition that causes a person’s blood sugar level to become too high.
- Diabetic maculopathy
- A consequence of diabetic retinopathy. Maculopathy is damage to the macula, the part of the eye that provides us with our central vision.
- Diabetic retinopathy
- A complication of diabetes caused by high blood sugar levels damaging the back of the eye (retina). It can cause blindness if left undiagnosed and untreated.
- Dilated fundus examination
- A diagnostic procedure that uses eye drops to dilate or enlarge the pupil of the eye to obtain a better view of the retina.
- Laser photocoagulation
- A procedure used to treat proliferative diabetic retinopathy and maculopathy. Beams of bright laser light make tiny burns to stop the leaking and to stop the growth of new blood vessels.
- Non-mydriatic
- A term usually applied to cameras or imaging devices that take an image of the retina. Non-mydriatic means that eye drops that dilate the pupil of the eye are not needed during the procedure.
- Ophthalmologist
- A medical doctor who has specialised in the diagnosis and treatment of eye diseases.
- Optician
- A person qualified to prescribe and dispense glasses and contact lenses and to detect eye diseases (optometrist) or to make and supply glasses and contact lenses (dispensing optician).
- Optometrist
- Primary health-care professional trained to examine and detect diseases of the eye.
- Proliferative diabetic retinopathy
- The advanced stage of diabetic retinopathy in which new blood vessels grow along the inside surface of the retina. These blood vessels are very delicate and bleed easily. The bleeding (haemorrhage) causes scar tissue that starts to shrink and pull on the retina, leading to it becoming detached and possibly causing vision loss or blindness.
- Slit-lamp biomicroscopy
- A technique that uses an instrument (slit-lamp biomicroscope) to examine the front and back (retina) of the eye under magnification.
- Type 1 diabetes mellitus
- Occurs when the body cannot produce insulin, which is needed to control blood glucose levels.
- Type 2 diabetes mellitus
- Occurs when the body cannot produce enough insulin or the insulin is not working efficiently enough.
List of abbreviations
- BCT
- behaviour change technique
- BCTTv1
- Behaviour Change Technique Taxonomy version 1
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CFIR
- Consolidated Framework for Implementation Research
- CI
- confidence interval
- DFE
- dilated fundus examination
- DRS
- diabetic retinopathy screening
- EPOC
- Effective Practice and Organisation of Care
- GDP
- gross domestic product
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HbA1c
- glycated haemoglobin
- HCP
- health-care professional
- HES
- Hospital Eye Services
- HMIC
- Healthcare Management Information Consortium
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- NHS EED
- NHS Economic Evaluation Database
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROGRESS
- place, race, occupation, gender, religion, education, socioeconomic status, social status
- QALY
- quality-adjusted life-year
- QI
- quality improvement
- RCT
- randomised controlled trial
- RD
- risk difference
- RNIB
- Royal National Institute of Blind People
- RR
- relative risk
- TDF
- Theoretical Domains Framework
- WHO
- World Health Organization
