Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/116/13. The contractual start date was in October 2014. The draft report began editorial review in October 2016 and was accepted for publication in August 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Andrew Lewington has received honoraria from Alere, Inc. David Meads is a member of the Health Technology Assessment programme Emergency and Hospital Care panel. Patrick Hamilton has received funding from ChemoCentryx, Inc., outside this work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Hall et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background and introduction
Introduction
Acute kidney injury (AKI) has been identified as an area of unmet need affecting a sizeable population, with a health burden that has the potential for significant improvement. A large number of diagnostic tests and biomarkers feature in the published literature dating back many years but, as yet, they have failed to translate into clinical practice or meaningfully benefit patients. The AKI Diagnostics project was initiated as a direct response to this problem following initial attempts to quantify the evidence base for diagnostic tests in the specific setting of critical care. Its high-level objective was to map out a strategy for further research and development that will efficiently deliver patient benefits using this technological opportunity.
The clinical problem
The relevance of AKI (also referred to as acute kidney failure) as a major problem for public health has been recently emphasised in guidance issued by the National Institute for Health and Care Excellence (NICE). 1 NICE estimates that AKI costs the UK NHS £434–620M every year (which is more than the cost of breast, lung and skin cancer combined2). Moreover, according to NICE, adequate care of AKI could result in 42,000 deaths being avoided every year.
Acute kidney injury occurs in 30–70% of critically ill patients and is most commonly associated with multiorgan failure secondary to hypotension and sepsis. Patients who develop AKI have worse clinical outcomes, with a mortality rate of > 50%, which, despite advances in modern medicine, has remained unchanged for the last 30 years. Patients who develop severe AKI requiring renal replacement therapy (RRT) have a further increase in their risk of death. 3
The recognition of AKI currently relies on a rise in serum creatinine and/or a decrease in urine output, both of which are considered relatively poor biomarkers. Serum creatinine remains a non-specific marker of AKI, being a product of muscle metabolism, and does not indicate the site of the injury or distinguish between pre-renal (functional process) and intrinsic (damage process) AKI. The generation of creatinine is dependent on muscle mass and it is therefore a very poor marker of kidney function, particularly in malnourished patients or patients with liver disease. It is recognised that a person could lose 50% of their kidney function before the serum creatinine level rises above the normal range. The rise in serum creatinine is delayed in relation to the onset of the injury and the magnitude of rise does not correlate with the severity of injury. Likewise, serum creatinine levels do not correlate well with recovery of kidney function. More specific serum and urinary biomarkers of AKI are urgently needed. 4
The need for research
More recently it has been recognised that chronic kidney disease (CKD) occurs in 40% of survivors of AKI in critical illness and results in significant morbidity and expense. It is estimated that up to 10% of patients will not recover sufficient kidney function and will remain on RRT. 5 CKD in the UK costs £1.45B per year. 6 It is therefore important to identify patients at risk or who are developing AKI to reduce the impact, ameliorate the severity of the injury and thereby reduce its short- and long-term consequences. It was reported that, in 2009–10, patients who experienced an episode of AKI in the UK stayed in hospital an average of 4.7 days longer than patients without AKI. 2 AKI represents an important patient safety issue, as recognised by NHS England,7 and results in a significant financial burden on health-care services. There is a great potential to prevent AKI and reduce its severity and, therefore, the medical and financial burden to the NHS.
The development of better biomarkers to detect AKI would also benefit patients at risk outside of the intensive care unit (ICU) setting. Not all hospitals in the UK have renal units and the facility to deliver RRT; in these hospitals patients who develop severe AKI requiring RRT may have to be transferred to the ICU for RRT alone, which inappropriately utilises a precious NHS resource. Earlier detection of AKI with the potential to stratify prognosis would allow for prompt transfer of patients to the correct environment for their care.
Biomarker-based in vitro diagnostics (IVDs) offer an opportunity for early diagnosis, risk stratification and monitoring, enabling earlier specialist referral, targeted intervention or intensification of therapy when indicated by the test. There is some evidence that early RRT can improve outcomes from AKI, including reducing the duration of RRT, the length of the hospital stay and the rates of CKD and long-term RRT. 8 A number of pharmaceutical interventions are in late-phase development, which will increase the opportunity for targeted intervention in the coming years. Previous therapeutic interventions have been unsuccessful, in part because of the very crude approach to AKI and the failure to understand its complexity without the use of appropriate biomarkers.
The management of the majority of cases of AKI in the ICU remains supportive, with no proven pharmacological intervention for AKI secondary to hypoperfusion and sepsis. Currently, there is no robust evidence base to guide when to initiate RRT and this is determined empirically, dependent on the clinical context and utilising serum creatinine as a marker of severity of AKI. There is an ongoing research effort internationally to discover and develop biomarkers and diagnostics for AKI. The extent to which such tests can influence the clinical decisions and change the current management of patients admitted to critical care remains unknown. There is an urgent need to evaluate the extent to which AKI diagnostics have the potential to influence outcomes through a change in clinical practice. If a model-based analysis demonstrates that AKI diagnostics can potentially change practice in a way that results in more cost-effective care, there will be value in further investment in a development programme.
The sizeable waste within the historical research process has recently been highlighted. 4,9–14 Academic and commercial communities are at the start of a new era of diagnostics development for AKI. There is a time-limited opportunity to design this UK research programme efficiently and with appropriate upfront prioritisation.
Existing research
A number of different biomarkers have been investigated in small heterogeneous studies in critically ill patients with AKI, including neutrophil gelatinase-associated lipocalin (NGAL), interleukin (IL)-18, kidney injury molecule-1 (KIM-1), liver fatty acid-binding protein (L-FABP) and, more recently, the cell cycle arrest markers insulin-like growth factor-binding protein 7 (IGFBP-7) and tissue inhibitor of metalloproteinases 2 (TIMP-2). 4 More data are required from well-conducted trials to identify both improved patient outcomes and economic benefit to the NHS before their routine use can be recommended. It is unlikely that one biomarker will fit all because of the heterogeneity of causes of AKI and, therefore, a panel of biomarkers will be required. A number of biomarkers have already been commercialised as IVD test kits and are subject to active marketing campaigns, including IGFBP-7 and TIMP-2 (Nephrocheck®, Astute Medical, San Diego, CA, USA); the NGAL test (BioPorto, Hellerup, Denmark/Alpha Laboratories, Eastleigh, UK); urine NGAL (Abbott Laboratories, Chicago, IL, USA); and the Triage® NGAL Test (Alere, Waltham, MA, USA). There is therefore a real risk that these biomarkers could be adopted by NHS laboratories and clinicians prior to the development of robust supporting evidence for clinical effectiveness and cost-effectiveness.
In 2013 a diagnostic test based on the NGAL biomarker was considered by the NICE Diagnostics Advisory Committee (DAC). It concluded that more research was needed prior to adopting the test but that this was undoubtedly an area of considerable clinical need.
Adoption of diagnostic tests in the NHS
Diagnostic tests are the foundation of the new era of personalised medicine and, as such, are critical for future effective health care. The pipeline of research and development that brings new diagnostic tests to help patients within the NHS has been criticised for failing to deliver good-quality evidence-based technologies in a timely manner. It has been highlighted that progress in personalised medicine is slower than some had expected. 15 The reasons for this are likely multifactorial but may include inadequate science or insufficient economic incentives for investing in molecular diagnostics. What has recently become clear is that the methods employed in the development of evidence for new diagnostic technologies are inadequate for the task or are not used appropriately. Work is needed to develop new ways of rapidly bringing high-quality diagnostics to the front-line care of NHS patients.
The decision to reimburse a new technology on the NHS should require evidence for quality control, safety, effectiveness and cost-effectiveness; demonstration of these aspects represents the key hurdle in the process of health technology adoption. In the UK, decision-making for diagnostics may occur at a local or a national level. The gold standard process, however, is orchestrated by the NICE DAC and its methodological advisors have identified many problems and a lack of standardisation in the requirements for evidence on safety, efficacy, effectiveness and cost-effectiveness of diagnostic tests. This contrasts with the relatively well-defined evidence requirements for pharmaceuticals, which are driven by high financial stakes and a long history of safety scandals that have prompted a stepwise tightening of licensing and regulatory requirements.
The National Institute for Health Research Diagnostic Evidence Co-operatives
In response to these concerns, in 2013 the National Institute for Health Research (NIHR) in England commissioned a network of infrastructure support organisations called the Diagnostic Evidence Co-operatives (DECs). Their mission is to engage with commercial stakeholders, academic institutions and the NHS to bring high-value and high-impact diagnostic tests to patients in an efficient manner.
The two key objectives of the DECs that the AKI-Diagnostics project addressed were (1) to devise and refine methods in IVD study design, health economics and health informatics to improve and speed up the way that IVDs can be evaluated for NHS use and (2) to invite, select and prioritise specific IVD candidates across key clinical areas from partners and interested parties and help them develop and deliver appropriate evidence.
Technical background for the methodological approach
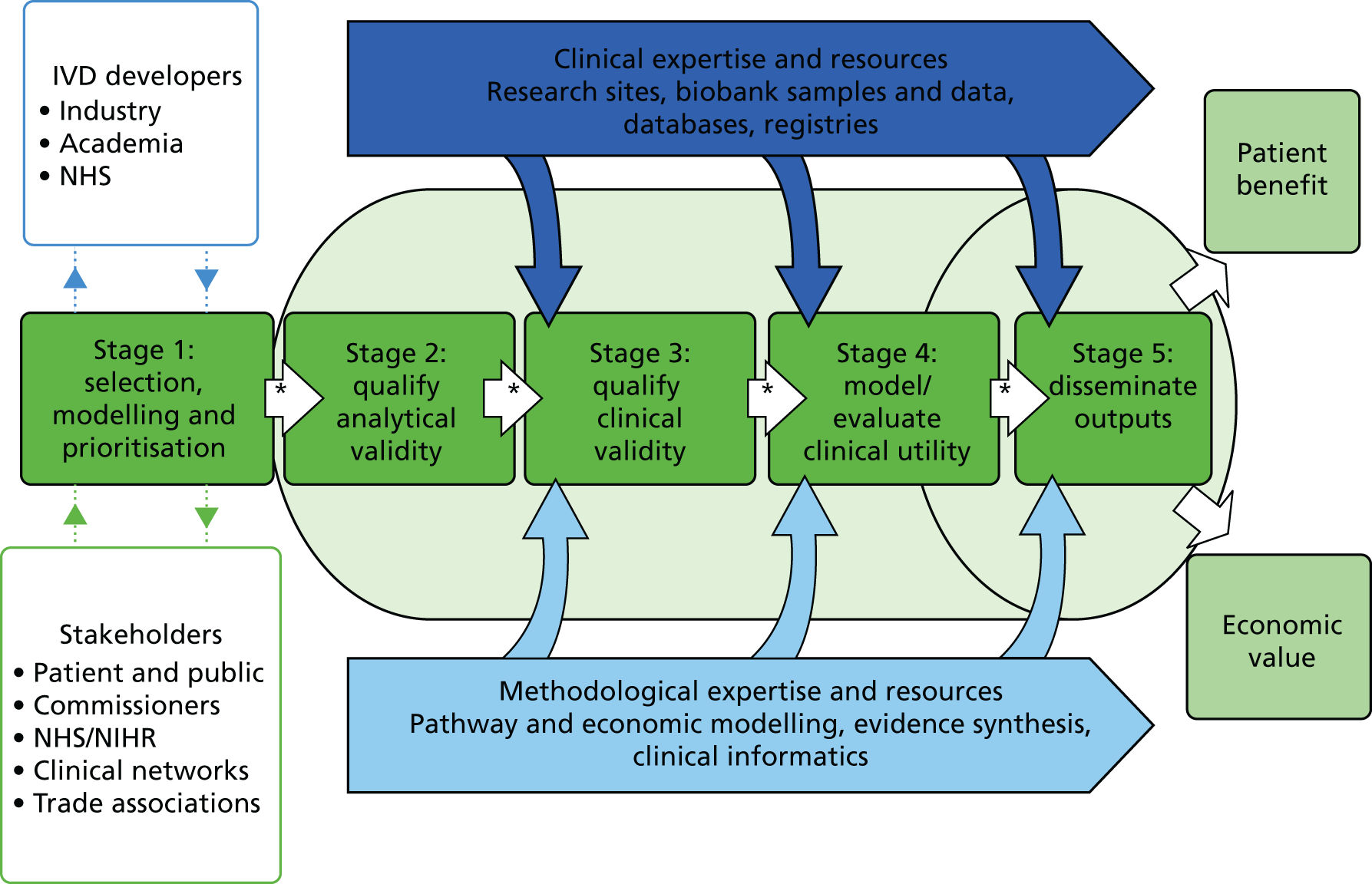
The DECs aim to promote efficient research design for new technologies within the NHS. They acknowledge that the gold standard for demonstrating clinical utility and cost-effectiveness (and accepted by NICE) is a model-based economic evaluation, informed when possible by randomised controlled trials (RCTs). For any given clinical context, modelling will therefore commence at the very start of technology evaluation, during the process that selects and prioritises technologies for inclusion within the DEC research pipeline (Figure 1). By introducing a model early, it is possible to characterise the potential impact of a diagnostic test on the clinical pathway, clinical decision points and expected clinical end economic outcomes. The optimal case definition threshold (cut-off point) for tests can be proposed for cost-effectiveness in addition to clinical validity alone. Models will be maintained and updated as IVD evaluation progresses, populated by meta-analysis of evidence generated both within and external to the DEC. Probabilistic modelling will be used to characterise areas of uncertainty in the evolving evidence between each phase of development, thus enabling iterative research design efficiency. Expected cost-effectiveness and value for the NHS can be established as well as commercial headroom for relevant manufacturers. It is possible to model the cost of a test under different commercialisation scenarios (e.g. large centralised laboratory vs. local hospital laboratory provision) and its impact on the value of alternative research activities can therefore be estimated. As an example, the trade-offs between investment in a large RCT and investment in alternative cheaper or quicker study designs can be described using the modelling process, including through the use of Bayesian decision modelling and value of information (VOI) analysis.
FIGURE 1.
The NIHR DEC research and development pipeline. *, strategic decision-making points.
Project objectives
Based on the DEC methodological approach, the objectives of the AKI-Diagnostics project, focusing on critical care, were to:
-
describe the care pathway followed by patients who are admitted to critical care and who are at risk of AKI, represented as a decision-analytic model
-
identify, through systematic review, candidate diagnostic tests for the early detection, risk stratification or therapy personalisation of AKI
-
systematically review and meta-analyse the evidence on the diagnostic properties, clinical validity and clinical utility of identified AKI diagnostic tests for AKI
-
identify decision points in the care pathway that might be influenced by the diagnostic tests
-
evaluate the potential clinical effectiveness and cost-effectiveness of the diagnostic tests, given their potential to change the care pathway
-
characterise uncertainties in the evidence, prioritise tests for development and identify efficient research designs.
Outline of the project components
To deliver these objectives the AKI-Diagnostics project was structured into five distinct phases:
-
phase 1: systematic review
-
phase 2: evidence synthesis and meta-analysis
-
phase 3: care pathway analysis
-
phase 4: decision-analytic model
-
phase 5: sensitivity analysis and VOI analysis.
Phase 1: systematic review
Phase 1 consisted of three distinct systematic literature searches and reviews. Review 1 was broad and inclusive, analogous to a horizon scan, and sought to identify candidate or in-development relevant diagnostic tests that could be used in critical care to identify AKI. Review 2 employed a focused search that identified current evidence on the analytical validity, clinical validity, clinical utility and cost-effectiveness of the prioritised tests identified in search 1. The purpose of review 3, which is reported in the health economics chapter (see Chapter 5), was primarily to inform the design and parameterisation of the economic model for assessing cost-effectiveness. Using a series of highly focused literature searches it aimed to identify information describing the clinical care pathway and standard care for AKI in critical care, including investigation, clinical management, interventions, health-care resource use, morbidity, mortality, quality of life and other relevant outcomes descriptors.
Phase 2: evidence synthesis and meta-analysis
Phase 2 aimed to combine the findings from phase 1 and enable summary estimation of the relevant metrics for the diagnostic properties of tests, predominantly sensitivity and specificity for relevant outcomes.
Phase 3: care pathway analysis
Phase 3 was conducted in parallel to literature search 3, using formal consultation with relevant experts and patients with the primary aim of defining standard care for patients at risk of and experiencing AKI in critical care. This was necessary as part of the economic model development process, including the identification of key decision points at which tests change the process of care, mechanisms for changing the process of care, relationships and downstream knock-on effects that may be influenced by tests and key surrogate end points. In addition to expert consultation and information obtained from literature search 3, recent UK clinical trial data sets and UK registration study data sets were identified and analysed to inform the model structure and parameters.
Phase 4: decision-analytic model
Phase 4 consisted of the construction, parameterisation and analysis of a health economic decision model based on the care pathway developed in phase 3. The model was initially designed to calculate expected costs and quality-adjusted life-years (QALYs) for the current AKI care pathway. AKI diagnostic tests were then incorporated into the model at key decision points, as indicated by the current evidence and recommendations from the specialist advisory group. Divergence in the clinical pathway consequent on test results was modelled based on the diagnostic properties and decision impact of the tests.
Phase 5: sensitivity analysis and value of information analysis
Phase 5 consisted of a series of sensitivity analyses undertaken to explore the impact of parameter and structural uncertainties on the expected cost-effectiveness of each test. In addition, VOI analysis was undertaken in an attempt to quantify the value of further publicly funded research into diagnostic tests. VOI analysis is a method based on Bayesian decision theory that can be used to characterise the burden of uncertainty on a NHS reimbursement decision-maker or commissioner. 16,17 The results were presented to guide a future research programme in this area, as needed prior to adoption of any new tests by the NHS.
Patient and public engagement in the study
Both the study research team and the specialist advisory group contained patient representation and through these two groups the patient perspective was taken into account in every aspect of the project.
As part of the development of the economic model structure, a focus group was held on 26 February 2015 with two clinicians (nephrologists) and two patient representatives with experience of chronic kidney failure. The primary aim of this session was to understand the care pathway for patients diagnosed with AKI in the ICU and the focus of the discussion was on developing a diagrammatic representation of the patient pathway for someone experiencing AKI in the ICU. The findings from this session were used to inform the development of the structure of the decision model. In addition to the focus group, the final structure and parameter estimates used for the decision model were determined via iterative feedback from the project advisory board, which included a patient representative.
Chapter 2 Systematic review
Aim
The overall aim of this systematic review was to provide evidence to evaluate the potential for AKI diagnostics to enhance the NHS care of patients admitted to critical care. The review involved three searches designed to meet specific project objectives:
-
search 1: to identify candidate or in-development relevant diagnostic tests that could be used in critical care to identify AKI (horizon scanning)
-
search 2: to identify current evidence on the clinical utility, analytical validity and cost-effectiveness of diagnostic tests identified in search 1
-
search 3: to gather information to describe the clinical care pathway and standard care for AKI in critical care, including investigation, clinical management, interventions, health-care resource use, morbidity, mortality, quality of life and other relevant outcome descriptors (methods and findings reported in Chapter 5).
Search 1: horizon scanning
Objective
The objective of this search was to identify candidate or in-development relevant diagnostic tests that could be used in critical care to identify AKI.
Identification of studies
A broad strategy was employed to support a horizon-scanning search for tests and biomarkers that are, or can be, used for AKI diagnosis but that are not currently used as standard practice in emergency and critical care. Our search included ongoing studies in trials registers, conference proceedings and recently published studies in PubMed to ensure that all emerging test and biomarker evidence was identified. Inclusion of the major databases such as MEDLINE, EMBASE and Science Citation Index (via Web of Science) enabled comprehensive identification of relevant literature and allowed assessment of the likely weight of evidence (volume of research) for different tests should they be included in the next stage (search 2, evidence on candidate tests). Searches were carried out in September 2014 in ClinicalTrials.gov (US National Institutes of Health) (accessed 29 September 2014), Cochrane Central Register of Controlled Trials (via Wiley Online Library) (Issue 9 of 12, September 2014), Cochrane Database of Systematic Reviews (via Wiley Online Library) (Issue 9 of 12, September 2014), Conference Proceedings Citation Index – Science (Thomson Reuters’ Web of Science) (1990 to September 2014), Database of Abstracts of Reviews of Effect (via Wiley Online Library) (Issue 3 of 4, July 2014), EMBASE Classic and EMBASE (via Ovid) (1947 to 25 September 2014), International Clinical Trials Registry Platform (World Health Organization) [www.who.int/ictrp/en/ (accessed 29 September 2014)], MEDLINE (via Ovid) (1946 to September Week 3 2014), MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (25 September 2014), metaRegister of Current Controlled Trials (mRCT) (accessed 29 September 2014), PubMed (US National Library of Medicine) (1946 to September 2014) and Science Citation Index (Thomson Reuters Web of Science) (1900 to September 2014).
The search terms included index terms, free-text words, abbreviations and synonyms. Terms for AKI and specific biomarkers were identified from known relevant papers, database thesauri and suggestions from clinical members of the team. Searches for diagnostic studies are known to often retrieve very large numbers of citations. Although the use of diagnostic search filters is cautioned against – because of poor reporting and indexing – for pragmatic reasons we included some diagnostic terms in these scoping searches. We combined our search concept for AKI with a ‘fluid’ search concept as only tests using fluid samples (rather than tissue samples) could be considered for use in emergency and critical care. A variety of terms, headings and subheadings were combined to identify ‘tests’. The concepts used were:
-
AKI or conditions that imply AKI (e.g. tubular necrosis)
-
diagnostic tests or biomarkers [generic ‘test’ terms; MeSH terms, e.g. Acute-Phase Proteins/du (Diagnostic use); specific test terms, e.g. NGAL]
-
plasma, blood, urine and serum samples or specimens
-
evaluation of a test to predict or diagnose (e.g. sensitivity, accuracy, monitor, etc.).
The research team agreed that biomarkers developed and evidence gathered during the previous 10 years would be most relevant for the scoping review. All sources were searched from 2004 onwards, except for Web of Science, which was searched from 2008 onwards. The complete search strategies are available in Appendix 1.
Selection of studies
Studies were included if the participants were adults or children with new or existing AKI and (1) they were based in critical care (or another clinical setting if using a candidate test for future use in clinical care), (2) they evaluated a fluid biomarker not currently used as part of routine care for AKI, (3) they used the biomarker for AKI diagnosis, risk stratification or prediction of treatment benefit and (4) they involved at least 52 subjects. The participant criterion was based on the hypothesis that useful biomarkers in the literature have been evaluated in underpowered studies. Thus, we made the following assumptions: in order to be published, markers have a sensitivity and specificity of 0.6; to be inclusive in terms of power (1 – β) and significance level (α), we assumed power to be 0.7 and the significance level to be 0.2; and ideally a marker would be most useful if it had a sensitivity and specificity of 0.7 or 0.8 (although 0.8 is unlikely). To identify the most relevant literature, we anticipated that the majority of early-stage clinical validation studies would have equal numbers of cases and controls. In addition, to include studies with some chance of identifying markers with a sensitivity/specificity of > 0.8, we selected papers that studied at least 52 patients (26 per arm in trials) (Table 1).
| Sensitivity and specificity | Number (non-AKI) | Number (AKI) | Number (total) |
|---|---|---|---|
| 0.65 | 467 | 467 | 934 |
| 0.70 | 113 | 113 | 226 |
| 0.75 | 48 | 48 | 96 |
| 0.80 | 26 | 26 | 52 |
| 0.85 | 15 | 15 | 30 |
| 0.90 | 10 | 10 | 20 |
Studies were excluded if the participants had CKD only, if they used a tissue biomarker or imaging technology for AKI diagnosis, or if the biomarker was used only for monitoring events subsequent to an AKI diagnosis (e.g. post discharge). Biomarker discovery and preclinical studies were also excluded, along with those evaluating the stability and/or storage of biomarker samples. No restrictions on language or study design were applied (Table 2).
| PICOS criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Interventions |
|
|
| Comparator |
|
|
| Outcomes |
|
|
| Study design |
|
|
Literature yield
Scoping searches conducted at the project proposal stage suggested a pool of around 2500 references; however, the final search identified 6329 articles, which was reduced to 4804 following exclusion of duplicates. Given this almost twofold increase in the volume of literature, and in a change to the protocol, the decision was taken to appraise articles in a single stage (title and abstract screen) rather than in two stages (title and abstract screen followed by full-text review). At the outset of the process, a training session was held to facilitate standardisation in screening and selection and ensure that each reviewer was aware of and understood the explicit inclusion and exclusion criteria. Abstracts were then screened for relevance by individual reviewers (with independent double assessment of 25% of articles), with 487 relevant articles identified.
Identification of candidate biomarkers
Data from each of the 487 studies were extracted by one reviewer (EDM) and used to produce a longlist of potential biomarkers. In total, 153 individual biomarkers (excluding serum creatinine) were identified. Non-novel biomarkers, that is, those that were already used as part of standard care in the diagnosis of kidney function, were excluded (n = 11), as were those for which the citation did not report complete details of the population studied (n = 19). The remainder (see Appendix 2) were then tabulated on four dimensions: volume of evidence, currency of evidence, total population included and biological or mechanistic plausibility (inflammatory marker, function marker, damage marker and cell cycle marker). Pragmatic limits were then set on each of the dimensions to enable the longlist to be reduced while trying to ensure that the focus of search 2 would be on those tests that would be likely to produce the most evidence. The limits and their rationales were as follows:
-
the biomarker must have been considered in six or more studies – to try to ensure that there were sufficient data to allow appropriate synthesis for each test
-
studies must have been published in the previous 5 years – to try to ensure that only the most promising biomarkers with recent evidence were included
-
the biomarker must have been used with ≥ 1500 subjects in total (i.e. across studies) – to try to ensure that the biomarker had wide clinical use and would provide sufficient data for synthesis
-
biomarkers from all four plausibility dimensions should be represented – to ensure that the focus did not exclude biomarkers with a specific biological or mechanistic function.
Application of these limits provided a shortlist of 10 candidate tests (Table 3), which, following review by the Project Delivery Board and ratification by the specialist advisory group, were used in search 2.
| Biomarker | Studies (n) | Subjects (n) | Biology/mechanism |
|---|---|---|---|
| BNP | 6 | 3402 | Functional marker |
| Cystatin C | 73 | 21,180 | Functional marker |
| IL-6 | 8 | 33,224 | Inflammatory marker |
| IL-18 | 40 | 15,965 | Inflammatory marker |
| KIM-1 | 40 | 12,959 | Damage marker |
| L-FABP | 28 | 7865 | Functional marker |
| NAG | 20 | 2982 | Cell cycle marker |
| Nephrocheck (TIMP-2 and IGFBP-7) | 6 | 1817 | Cell cycle marker |
| NGAL | 173 | 52,763 | Inflammatory/damage marker |
| TNF-α | 6 | 31,090 | Inflammatory marker |
Search 2: identification of evidence for candidate tests
Objective
The objective of this search was to identify current evidence on the analytical validity, clinical validity, clinical utility and cost-effectiveness of the diagnostic tests identified in search 1.
Identification of studies
The world literature from 2004 to November 2015 was reviewed to identify existing research describing the diagnostic accuracy, analytical validity or cost-effectiveness of the 10 candidate biomarkers. It became clear when developing our search strategy that we would identify considerably more literature than suggested by our prestudy scoping searches. We therefore took the decision to carry out a more sensitive search over a shorter time period rather than a precise search covering the full duration of the databases. As our aim was to identify novel tests, we believed that this approach would be more inclusive, identifying the diversity of newer biomarkers and reducing the identification of older, established biomarkers. We searched the following databases for published and unpublished literature: ClinicalTrials.gov (US National Institutes of Health) (accessed 30 November 2015), Cochrane Central Register of Controlled Trials (via Wiley Online Library) (Issue 10 of 12, October 2015), Cochrane Database of Systematic Reviews (via Wiley Online Library) (Issue 11 of 12, November 2015), Conference Proceedings Citation Index – Science (Thomson Reuters Web of Science) (1990 to November 2015), Database of Abstracts of Reviews of Effect (via Wiley Online Library) (Issue 2 of 4, April 2015), EMBASE Classic and EMBASE (via Ovid) (1947 to 24 November 2015), Health Technology Assessment database (via Wiley Online Library) (Issue 4 of 4, October 2015), Health Management Information Consortium database (1983 to November 2015), International Clinical Trials Registry Platform (World Health Organization) (accessed 30 November 2015), MEDLINE (via Ovid) (1946 to November Week 2 2015), MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (24 November 2015), NHS Economic Evaluation Database (via Wiley Online Library) (Issue 2 of 4, April 2015), PubMed (US National Library of Medicine) (1946 to November 2015) and Science Citation Index (Thomson Reuters Web of Science) (1900 to November 2015).
The search consisted of index terms and text words for AKI, AKI synonyms and name variants for the 10 biomarkers: brain natriuretic peptide (BNP), cystatin C, IL-18, IL-6, KIM-1, L-FABP, N-acetyl-beta-D-glucosaminidase (NAG), Nephrocheck, NGAL and tumour necrosis factor alpha (TNF-α). The searches were limited by date of publication (from 2004) but no restrictions on language were applied (non-English-language papers were included at this stage but would be included for full-text assessment only if translation of the study data would be possible within the project timescale). All study designs except for single case studies were included. A diagnostic search filter was tested but not used in the final searches as it excluded potentially relevant abstracts. Search strategies for each database are provided in Appendix 3.
Selection of studies
Studies were included if they evaluated at least one of the outcome areas of clinical validity (including utility), analytical validity or cost-effectiveness and:
-
the participants were adults or children with new or existing AKI
-
they were based in critical care or the emergency department or included patients undergoing cardiac surgery
-
they evaluated one or more of the 10 candidate biomarkers (fluid only)
-
they included serum creatinine or another candidate biomarker as a comparator
-
they assessed use of the biomarker for AKI-related decision-making
-
they involved at least 50 subjects (unless reporting an aspect of analytical validity or cost-effectiveness).
The emergency department and cardiac surgery (including presurgery) were included as the most important alternative settings to consider when trying to capture relevant tests used outside ICUs/high-dependency units that might be transferable to the critical care setting. A cut-off point of ≥ 50 participants was used to include studies with some chance of identifying markers with a sensitivity/specificity of > 0.75 (see Table 1). This threshold was lowered slightly from search 1 to be as inclusive as possible when obtaining evidence relevant to the candidate biomarkers.
Studies were excluded if they involved kidney transplant patients only, studied a tissue biomarker or imaging technology for AKI diagnosis or used the biomarker only for predicting transplant rejection. Studies considering risk factors for AKI itself or combining the results for individual tests (with the exception of algorithmic biomarkers) were also excluded, as were case studies and descriptive or commentary pieces (Table 4).
| PICOS criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Interventions |
|
|
| Comparator |
|
|
| Outcomes | Primary:
|
|
| Study design |
|
|
Identified citations were stratified according to the test under study and in relation to whether they considered single or multiple biomarkers and were reviewed on a group-by-group basis. Titles and abstracts were screened for eligibility by one reviewer, with a random sample (15%) of articles independently screened by a second reviewer. Full-text articles of potentially relevant studies were then obtained and independently assessed by two reviewers to determine their inclusion status. Differences of opinion were discussed until a consensus was reached. To facilitate consistency in appraisal, the three main reviewers (EDM, NC and NW) initially assessed a batch of 14 papers independently and then met to discuss outcomes and to ensure clarity around the inclusion and exclusion criteria.
Data extraction
Data extraction was carried out by a single reviewer using a bespoke proforma (see Appendix 4). Data extracted included study methodology, country of study, study duration, setting and patient population (including baseline characteristics), type of candidate test and parameters, details of the diagnostic ‘gold standard’ and AKI classification system used, outcome measure(s) studied and findings. Analytical and validation factors associated with the physical measurement of a biomarker [including sensitivity, specificity, precision, parallelism, recovery, selectivity, limit of quantitation (LOQ) and vulnerability to interferences] were also sought for review in line with current US Food and Drug Administration (FDA) best practice guidelines18 and Clinical and Laboratory Standards Institute (CLSI) principles (see Table 38). 29 Pre-analytical variables that might influence the quality, integrity or composition of samples, including biological factors (such as within-patient variability, sample timing, medical history and diet and lifestyle) and technical factors (such as sample collection, processing, shipping and storage conditions), were also obtained. Prior to use, the data extraction proforma was piloted by the reviewers and team statisticians on a small number of studies and refined where necessary. Once the process of data extraction began, the statisticians then reviewed a sample of completed proformas to ensure that the relevant data were being extracted.
Quality assessment
Quality assessment for the evidence synthesis was carried out by one main reviewer (EDM) using the QUADAS-2 (quality assessment of diagnostic accuracy studies) tool,30 a diagnostic test-specific approach to determine potential study bias. In this system, papers are evaluated on the basis of patient selection, interpretation of the index test, appropriateness and interpretation of the reference standard and flow of patients and timing of tests. The applicability of each study to the question under review is also assessed (see Appendix 5). In keeping with good practice, studies at risk of bias were not excluded from the meta-analysis. Instead, an appraisal of the strength of the existing evidence has been reported and the findings are interpreted in light of this.
Results of the systematic review
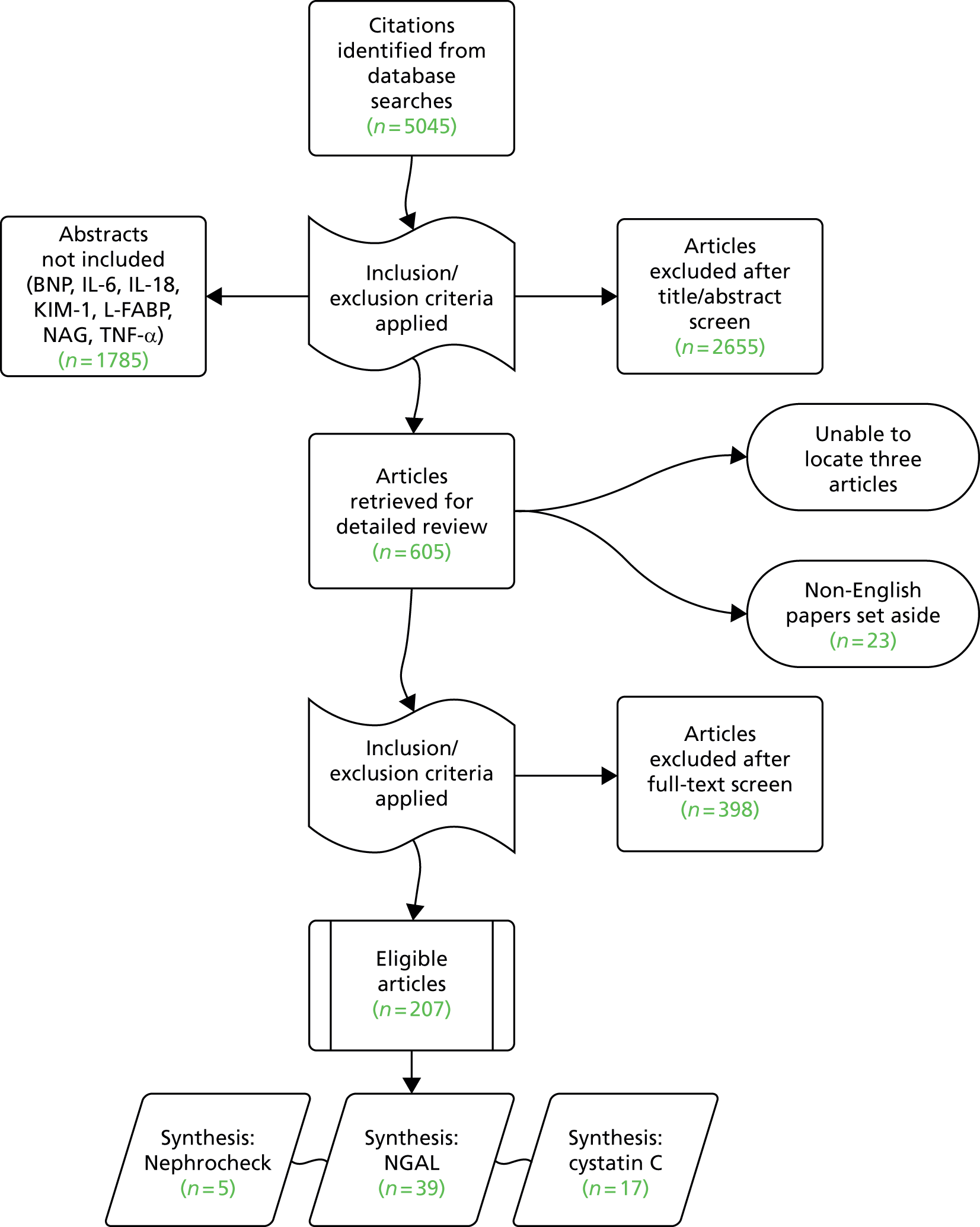
As was the case with search 1, broadening the search strategy to include tests used following cardiac surgery impacted significantly on the volume of literature identified compared with that anticipated by the original scoping strategy, with a total of 5045 articles identified. Given the time implications associated with assessment and data extraction in a complex review such as this, we took the decision to focus initially on three of the candidates: a recently developed test that is receiving considerable marketing and is the only FDA-licensed test for AKI (Nephrocheck, which detects two biomarkers, TIMP-2 and IGFBP-7) and the two biomarkers for which there was the greatest amount of evidence (cystatin C and NGAL). The choice of these three tests as the focus for study was ultimately a pragmatic one, determined with advice from the specialist advisory group. Although fewer studies have used Nephrocheck than some of the other shortlisted tests, it is one of the most recent biomarkers that has shown enough promise to be approved by the FDA and so there is much interest in its clinical utility. We were aware that there would be more publications on the older biomarkers; however, our approach seemed an intelligent approach that avoided ignoring a new and potentially novel biomarker. Between them, these markers were considered in 3260 of the identified citations (65%), with 605 articles meeting the inclusion criteria for detailed review (Figure 2). We were unable to locate three papers and 23 non-English-language papers were set aside. Most of the excluded studies were either conference abstracts with no subsequent publication or studies that did not focus on the population or setting under review (Table 5). In total, 207 eligible papers31–237 were included in the review (see Appendix 6).
FIGURE 2.
Flow of studies into the review.
| Reason for exclusion | Number of studies |
|---|---|
| Abstract only (no full text available) | 184 |
| Outside the review setting | 73 |
| Not used for AKI diagnosis or decision-making | 35 |
| Participant group < 50 | 27 |
| Non-English language | 23 |
| No discrete biomarker data | 12 |
| Duplicate paper or study | 10 |
| Review or meta-analysis (references checked) | 9 |
| No candidate biomarker studied | 4 |
| Editorial, commentary or letter | 3 |
| No comparator test included | 3 |
| Ongoing trial with full text identified | 3 |
| Study of transplant patients | 3 |
| Unable to obtain article | 3 |
| Animal study | 2 |
| Erratum only | 2 |
| Poster only (no full text available) | 1 |
| Study of patients with CKD | 1 |
Location and setting
The majority of the eligible studies were carried out in Europe (n = 114, 55%) and North America (n = 63, 30%), with the USA being the single most prolific country (Table 6). Fifteen studies involved centres in multiple countries. The most commonly reported clinical setting was cardiac care (n = 86, 42%) – including 11 studies on contrast-induced nephropathy – followed by critical care (n = 85; 41%), the emergency department (n = 20; 10%) and the laboratory (n = 11, 5%). The setting of five studies was unclear. Less than one-quarter of studies (n = 46, 22%) reported the involvement of multiple centres (median 3, range 2–35) (see Appendix 6). Study duration ranged from 1 month to 7 years and most papers were published between 2012 and 2015 (70%).
| Country | Number of papers (%) |
|---|---|
| Argentina | 1 (0.5) |
| Australia | 9 (4.3) |
| Austria | 3 (1.4) |
| Belgium | 6 (2.9) |
| Bosnia and Herzegovina | 1 (0.5) |
| Brazil | 3 (1.4) |
| Canada | 9 (4.3) |
| China | 14 (6.8) |
| Curaçao | 1 (0.5) |
| Denmark | 3 (1.4) |
| Egypt | 5 (2.4) |
| Finland | 5 (2.4) |
| France | 11 (5.3) |
| Germany | 17 (8.2) |
| Greece | 4 (1.9) |
| Hungary | 1 (0.5) |
| India | 2 (1.0) |
| Iran | 4 (1.9) |
| Ireland | 1 (0.5) |
| Italy | 12 (5.8) |
| Japan | 5 (2.4) |
| Republic of Korea | 10 (4.8) |
| Malaysia | 1 (0.5) |
| The Netherlands | 8 (3.9) |
| New Zealand | 5 (2.4) |
| Pakistan | 1 (0.5) |
| Poland | 3 (1.4) |
| Portugal | 3 (1.4) |
| Saudi Arabia | 1 (0.5) |
| Serbia | 3 (1.4) |
| Spain | 8 (3.9) |
| Sri Lanka | 1 (0.5) |
| Sweden | 10 (4.8) |
| Switzerland | 1 (0.5) |
| Taiwan | 2 (1.0) |
| Thailand | 1 (0.5) |
| Turkey | 7 (3.4) |
| UK | 7 (3.4) |
| USA | 54 (26.1) |
Population
Most studies were small in scale (mean 227, median 112 participants), with the smallest being an analytical validity study involving 17 patients undergoing surgery for congenital heart disease and the largest being a study of 1635 adults admitted to the emergency department (see Appendix 6). Two studies did not specify population size: one on the analytical validity of NGAL and the other using decision analysis to model the cost-effectiveness of NGAL following cardiac surgery. In more than three-quarters of studies the population was adult patients (n = 166, 80%), with three studies including both adults and children. The source of the samples was not specified in two studies on the analytical validity of NGAL.
Biomarkers and outcome areas
The most commonly studied biomarker was NGAL (n = 145, 70%) followed by cystatin C (n = 91, 44%) and Nephrocheck (n = 10, 5%). One study evaluated all three tests, 35 evaluated cystatin C and NGAL and three evaluated Nephrocheck and NGAL. The Nephrocheck test is carried out using urine only, but cystatin C and NGAL were evaluated on a range of sample matrices, most commonly serum for cystatin C and urine for NGAL (Table 7). Forty-one studies evaluated two different matrices for the same or multiple biomarkers (n = 14 for cystatin C, n = 35 for NGAL). Although there is a standard definition for the diagnosis of AKI, various classifications can be used in clinical practice to grade the level of injury (Table 8). In this review, the most commonly used criteria in studies determining clinical validity were the RIFLE (Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease) criteria,241 either alone or in conjunction with the Acute Kidney Injury Network (AKIN) criteria242 (n = 80, 42%). Almost one-fifth of studies did not report using one of the standard classifications.
| Sample matrix | Biomarker | ||
|---|---|---|---|
| Cystatin C | Nephrocheck | NGAL | |
| Plasma | 23 | – | 51 |
| Serum | 54 | – | 23 |
| Urine | 27 | 10 | 105 |
| Whole blood | – | – | 2 |
| Not specified | 1 | – | 1 |
| First author and year | Criteria | Studies,a n (%) |
|---|---|---|
| Definition of AKI | Absolute increase in SCr of ≥ 0.3 mg/dl (≥ 26.5 µmol/l) within 48 hours or a percentage increase of at least 50% (1.5 times the baseline value) or reduced urine output for > 6 hours (< 0.5 ml/kg/hour) | – |
| RIFLE241 | R: ≥ 1.5- and < 2-fold increase from baseline SCr or ≥ 25% fall in GFR from baseline or urine output < 0.5 ml/kg/hour for ≥ 6 and < 12 hours | 80 (41.5) |
| I: ≥ 2- and < 3-fold increase from baseline SCr or ≥ 50% fall in GFR from baseline or urine output < 0.5 ml/kg/hour for ≥ 12 hours and < 24 hours | ||
| F: ≥ 3-fold increase from baseline SCr or ≥ 75% fall in GFR from baseline or SCr ≥ 4 mg/dl with an acute rise of ≥ 0.5 mg/dl or urine output < 0.3 ml/kg/hour for ≥ 24 hours or anuria for ≥ 12 hours | ||
| L: complete loss of renal function for > 4 weeks | ||
| E: end-stage renal disease | ||
| AKIN242 | Stage 1: increase in SCr of ≥ 0.3 mg/dl (≥ 26.4 µmol/l) or increase in SCr to ≥ 150–200% (1.5- to 2-fold) of baseline value or urine output < 0.5 ml/kg/hour for ≥ 6 and < 12 hours | 62 (32.1) |
| Stage 2: increase in SCr to > 200–300% (> 2- to 3-fold) of baseline value or urine output < 0.5 ml/kg/hour for ≥ 12 hours and < 24 hours | ||
| Stage 3: increase in SCr to > 300% (3-fold) of baseline value or SCr ≥ 4.0 mg/dl (≥ 354 µmol/l) with an absolute increase of ≥ 0.5 mg/dl (≥ 44 µmol/l) or initiation of RRT or urine output < 0.3 ml/kg/hour for ≥ 24 hours or anuria for ≥ 12 hours | ||
| KDIGO (Kidney Disease: Improving Global Outcomes)238 | Stage 1: 1.5–1.9 × baseline SCr or ≥ 0.3 mg/dl (≥ 26.5 µmol/l) increase in SCr or urine output < 0.5 ml/kg/hour for ≥ 6 and < 12 hours | 26 (13.5) |
| Stage 2: 2.0–2.9 × baseline SCr or urine output < 0.5 ml/kg/hour for ≥ 12 hours and < 24 hours | ||
| Stage 3: 3.0 × baseline SCr or increase in SCr of ≥ 4.0 mg/dl (≥ 353.6 µmol/l) or initiation of RRT or, in patients aged < 18 years, decrease in eGFR to < 35 ml/minute/1.73 m2 or urine output < 0.3 ml/kg/hour for ≥ 24 hours or anuria for ≥ 12 hours | ||
| Otherb | – | 35 (18.1) |
| Not reported | – | 4 (2.1) |
Of the outcomes areas evaluated in this review, the most commonly considered was clinical validity (i.e. diagnostic accuracy; n = 193, 93%). A smaller number of studies focused on analytical validity (n = 12, 6%), with only two studies focusing on the cost-effectiveness of biomarker use (both in cardiac surgery, one looking at NGAL use in adults and the other looking at the use of cystatin C and NGAL in children). Almost one-quarter of studies (n = 46, 22%) reported on clinical validity alongside some aspect of analytical validity, usually related to brief details on limits of detection or inter-/intra-assay variation. In most of the studies dealing with clinical validity, the purpose of the use of the biomarker was AKI diagnosis, either solely (n = 126; 65%) or alongside risk prediction (n = 34, 18%) or prognosis (n = 21, 11%).
Quality assessment
With few exceptions, a cohort study design was the most frequently used study design in biomarker evaluation (n = 181, 87%), perhaps unsurprisingly given the topic under review. There were four RCTs, all of them relatively small scale (n = 71–204 adult patients), one in critical care and three involving cardiac surgery. One paper reported subgroup analysis from a larger trial but did not provide details of the parent study; the focus of two others was on the use of therapeutic drug treatment to prevent renal damage [Probucol (Sanofi Aventis, Paris, France) and erythropoietin].
Only 14% of studies (n = 29) reported a power calculation to justify the included sample size and less than half provided details of patient throughout [n = 88, 43%; Consolidated Standards of Reporting Trials (CONSORT) diagram, n = 38; written statement, n = 50]. For the most part, the reporting of methodology was poor and few studies stated that they had adhered to a quality standard when describing their study [Standards for Reporting Diagnostic Accuracy (STARD) guidance,243 n = 4; Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidance,244 n = 3; both STARD and STROBE, n = 1]. Fewer than two-thirds of clinical validity studies provided sensitivity and specificity data for a given biomarker cut-off threshold (n = 113, 59%). Only 42 eligible studies (20%) provided sufficient data to allow population of a confusion matrix and, therefore, their inclusion in meta-analysis: four studies evaluating Nephrocheck, 17 studies evaluating cystatin C and 35 studies evaluating NGAL (see Chapter 3).
Risk of bias among studies included in the evidence synthesis
When considered across the four domains – patient selection, index test, reference standard and flow and timing – only six45,48,50,54,58,66 of the studies included in the evidence synthesis had a low risk of bias for all; two studies49,72 did not report enough information to be able to allocate a level of bias for any of the domains (Table 9). Three studies had a high risk of bias for one domain: one36 that used a prespecified threshold for the biomarker cut-off point (potential index test bias), one in which the analysis did not include all patients39 and one that had a prolonged interval between the tests (both flow and timing bias). 57 In all of the remaining studies (n = 31, 74%), the level of bias was unclear in at least one of the domains. This was especially true for bias related to the reference standard, for which just over half of studies (n = 22, 52%) did not provide information on blinding, that is, whether or not the index test results were interpreted without knowledge of the results of the reference standard. There was little concern that the included studies were not applicable to this review.
| First author and year | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Ayodogdu 201332 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Bihorac 201433 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Chen 201234 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cho 201335 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Constantin 201036 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| de Geus 201137 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ghonemy 201438 | ? | ? | ✓ | ✓ | ✓ | ? | ✓ |
| Haase 200931 | ? | ? | ? | ✓ | ? | ? | ✓ |
| Haase-Fielitz 200939 | ? | ✓ | ? | ✗ | ? | ✓ | ? |
| Haase-Fielitz 200940 | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Han 200941 | ✓ | ? | ? | ✓ | ✓ | ? | ✓ |
| Herget-Rosenthal 200442 | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Hjortrup 201543 | ? | ✓ | ? | ? | ✓ | ✓ | ✓ |
| Hoste 201444 | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| Kashani 201345 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Kato 200846 | ✓ | ? | ? | ✓ | ✓ | ? | ✓ |
| Kidher 201447 | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Kokkoris 201248 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Legrand 201549 | ? | ? | ? | ? | ? | ? | ? |
| Liangos 200950 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Linko 201351 | ✓ | ? | ? | ? | ✓ | ✓ | ? |
| Liu 201352 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| McIlroy 201053 | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Meersch 201454 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Meersch 201455 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Munir 201356 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Nejat 201057 | ✓ | ? | ? | ✗ | ✓ | ✓ | ✓ |
| Oh 201258 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Palazzuoli 201559 | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Parikh 201160 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Park 201561 | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Perrotti 201562 | ✓ | ? | ? | ? | ✓ | ✓ | ✓ |
| Perry 201063 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Prowle 201564 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sargentini 201265 | ? | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Shum 201566 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tuladhar 200967 | ✓ | ? | ? | ? | ✓ | ✓ | ✓ |
| Tung 201568 | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Tziakas 201569 | ✓ | ? | ? | ? | ✓ | ✓ | ✓ |
| Varela 201570 | ? | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Villa 200571 | ? | ? | ? | ✓ | ✓ | ✓ | ✓ |
| Wagener 200872 | ? | ? | ? | ? | ✓ | ✓ | ✓ |
Discussion
This review was undertaken to provide a comprehensive picture of the current evidence around the clinical and analytical validity and cost-effectiveness of novel biomarkers for the diagnosis of AKI in critical care. There is undoubtedly a considerable amount of research in this area; however, issues related to the quality of reporting meant that less than one-quarter of the eligible studies identified were able to be included in meta-analysis. In addition, this made it difficult to determine the levels of potential bias across studies.
Several key issues were encountered when carrying out this piece of work. First, in the absence of published guidance, the test shortlisting criteria were developed by expert consultation and, as such, may not have captured all of the promising in-development tests because of the pragmatic focus on objective criteria (such as volume of evidence). Second, the literature yield was substantially greater than originally indicated by the prestudy scoping searches, largely because of the decision to broaden the final scope to include tests developed outside the critical care setting. This, combined with the number of candidate tests identified (including multiple tests evaluated in the same study) and the complexity of data extraction (which sought to determine both clinical and analytical validity), resulted in extended study timelines and an inability to complete the review for all 10 candidate biomarkers. Furthermore, differences in inclusion and exclusion criteria depending on whether the focus was on clinical or analytical validity made it more difficult to exclude potentially irrelevant studies at the abstract screening stage as this could not easily be achieved using sample size or the presence of a comparator (see Table 4). The decision to include an eligibility criterion based on sample size for studies with equal numbers of cases and controls could have been improved by stating that the group of interest (AKI in this case) had to be at least this size and the other group (no AKI) could have been larger.
As the number of biomarkers entering the health-care market continues to expand rapidly, the role of reviews to inform future research priorities is increasingly important. The two-stage search process outlined here represents a novel approach in this area; however, it is clear that further work is required to establish efficient and optimal search strategies and shortlisting criteria for such reviews.
Chapter 3 Meta-analysis of diagnostic tests for acute kidney injury
Introduction
In this chapter a meta-analysis of diagnostic accuracy studies is provided to evaluate the body of evidence available for three diagnostic tests of AKI and to provide input into the decision analysis in subsequent chapters. The primary health-care setting considered in the use of these tests was the critical care unit and the secondary health-care setting considered was cardiac surgery (pre and/or post intervention). The three diagnostic tests considered were the Nephrocheck test (which uses a combination of two proteins: TIMP-2 and IGFBP-7) and the biomarkers NGAL and cystatin C. The NGAL and cystatin C tests have been used in this setting for measurement of their concentration in samples of blood serum, blood plasma and urine and these media were considered separately. The pooled estimates of sensitivity and specificity and their variance from the meta-analyses directly informed the decision analysis in Chapter 5. In our searches we identified no previous reviews or meta-analyses considering Nephrocheck or cystatin C in this setting, but we did identify two relevant reviews of NGAL. 40,245
Methods
Primary objective
The primary objective was to estimate pooled means and variances of sensitivity and specificity for each of the diagnostic tests considered. When appropriate data were available, these estimates were obtained separately for each of the health-care settings considered and for each of the sample media considered. When only one study was available, no meta-analysis was undertaken.
Identification of studies
Details on the search strategies used to identify studies and the process for study screening and evaluation, data extraction and quality assessment are presented in Chapter 2.
Full papers were retrieved for studies of all patients (< 18 years, ≥ 18 years) in which AKI diagnosis had been evaluated using any one or multiples of the three diagnostic tests considered (Nephrocheck, NGAL, cystatin C), in any of the sample media considered (blood serum, blood plasma or urine), in either of the health-care settings considered (critical care unit or cardiac surgery). Studies were excluded if they were not primarily located in either of these health-care settings.
Study methods
The gold standard for determining AKI diagnosis was defined as diagnosis according to the RIFLE,241 AKIN242 or KDIGO (Kidney Disease: Improving Global Outcomes)238 diagnostic and classification system, based on an assessment of serum creatinine levels and urine output (see Chapter 2).
Outcome measurements
The primary outcomes were sensitivity (the probability of the test being positive given that the true diagnosis is positive) and specificity (the probability of the test being negative given that the true diagnosis is negative), which are determined by comparison of the results of the experimental diagnostic test with the results of the gold standard method used in the study. Studies were excluded if the gold standard method used to determine the outcome was not described in sufficient detail. Studies were not excluded if the cut-off point used to assess the positive and negative status of the outcome in the experimental diagnostic test was not reported.
Diagnostic and staging systems for acute kidney injury
A number of diagnostic and staging systems for AKI have been used in diagnostic accuracy studies. The most commonly used make use of repeated serum creatinine measurements and measurement of urine output to diagnose and stage AKI. Three commonly used systems are the RIFLE, AKIN and KDIGO systems.
RIFLE classification of acute kidney injury
The Acute Dialysis Outcome Initiative group proposed the RIFLE classification, which defines five categories of AKI,241 as shown in Table 8. AKI is staged for severity according to the criteria listed in Table 8, with any classification of risk or above being a diagnosis of AKI.
Acute Kidney Injury Network classification of acute kidney injury
The AKIN has defined diagnostic criteria for AKI and provided a staging system for the severity of AKI,242 as shown in Table 8. AKI is staged for severity according to the criteria listed in Table 8, with any classification of stage 1 or above being a diagnosis of AKI. In particular, in contrast to the RIFLE criteria, the absolute change in serum creatinine defining AKI is defined as an abrupt (within 48 hours) reduction in kidney function as defined by stage 1 or above.
KDIGO classification of acute kidney injury
The 2011 KDIGO Clinical Practice Guideline for AKI (Summary of Recommendation Statements, 2012)246 defined diagnostic criteria for AKI and provided a staging system for the severity of AKI, as shown in Table 8. AKI is staged for severity according to the criteria listed in Table 8, with any classification of stage 1 or above being a diagnosis of AKI. This classification system uses the same time frame for absolute changes as the AKIN criteria and clarifies that for the relative changes the baseline values should be known or presumed to have occurred within the previous 7 days.
Summary of staging methods
There is a similarity between the staging and diagnostic criteria proposed for AKI, which is demonstrated in Table 10. It has been shown that the AKIN criteria can diagnose more patients correctly with AKI than the RIFLE criteria (not unexpected given the additional criterion – the absolute change in serum creatinine level), but it has not been shown to have a better predictive ability for in-hospital mortality. 247 It has also been shown that the AKIN criteria do not improve the sensitivity of AKI diagnosis compared with the RIFLE criteria in the first 24 hours after admission to the critical care unit. 248 Similarly, it has been shown than a higher incidence of AKI can be diagnosed using the KDIGO criteria than using the RIFLE criteria and that the KDIGO criteria are more predictive for in-hospital mortality, but there was no significant difference between the AKIN criteria and the KDIGO criteria. 249 Other studies have suggested that the RIFLE, AKIN and KDIGO criteria are good tools for predicting mortality in critically ill patients and observe no evidence of a difference between them. 250
| Staging system | Stage or classification | ||||
|---|---|---|---|---|---|
| RIFLE | R | I | F | L | E |
| AKIN | 1 | 2 | 3 | ||
| KDIGO | 1 | 2 | 3 | ||
| RRT | |||||
| AKI ‘diagnosis’ | AKI ‘failure’ | ||||
Based on the definitions used in the different diagnostic and staging/classification systems and the evidence above we believe that there are broad similarities between the RIFLE, AKIN and KDIGO criteria and, for the purposes of this study, we defined a diagnosis of AKI, following the KDIGO criteria, as any of the following:
-
increase in serum creatinine of ≥ 0.3 mg/dl (≥ 26.5 µmol/l) within 48 hours
-
increase in serum creatinine to ≥ 1.5 × baseline, which is known or presumed to have occurred within the previous 7 days
-
urine volume < 0.5 ml/kg/hour for at least 6 hours.
Furthermore, in the studies identified for inclusion in the meta-analysis, studies that used either of the outcomes indicated by the shaded areas in Table 10 (RIFLE R, AKIN 1 or KDIGO 1 – a diagnostic-type outcome; RIFLE F, AKIN 3, KDIGO 3 or RRT – a failure-type outcome) were considered homogeneous for the purposes of the meta-analysis.
Key data extracted
The primary data extracted for inclusion in the meta-analysis are shown in Table 11. It is recommended in the STARD statement that a cross-tabulation of the index test results by the results of the reference standard is included in any study report,251,252 but it was anticipated that this information would not be present in all study reports. In this situation the elements of the confusion matrix were calculated using information describing the diagnostic outcomes and estimates of sensitivity and specificity. For example, if the sensitivity (s) and number of true diagnoses [given by the sum of the number of true positives (TPs) and the number of false negatives (FNs), i.e. (TP + FN)] were reported in the study then the number of TPs could be calculated as s.(TP + FN). A similar calculation for specificity (p) allowed the estimation of the number of true negatives (TNs): p.(FP + TN), where FP represents the number of false positives. Finally, given these estimates for TP and TN and the numbers of true outcomes [(TP + FN) and (FP + TN)], simple subtraction provided estimates for FN and FP.
| True outcome | Test outcome | Diagnostic property | True outcome | |
|---|---|---|---|---|
| Test+ | Test– | |||
| Disease+ (D+) | TP | FN | Sensitivity = TP/(TP + FN) | TP + FN |
| Disease– (D–) | FP | TN | Specificity = TN/(TN + FP) | FP + TN |
| TP + FP | FN + TN | |||
Study exclusion
Studies were excluded from the meta-analysis if it was not possible to estimate values for the elements of the confusion matrix or if other key data could not be extracted. Further reasons for the exclusion of studies were if diagnosis was carried out in the emergency department rather than in the critical care unit and if the biomarker was measured on a relative scale rather than an absolute scale, for example unit of biomarker per unit of serum creatinine.
Data analysis
Simple diagnostic accuracy summaries [sensitivity, specificity and the diagnostic odds ratio (DOR) and its components – positive likelihood ratio (LR+) and negative likelihood ratio (LR–)] were produced for each study included in the meta-analysis. The sensitivity of a diagnostic test (T) is defined formally as the probability that the test will give a positive result if the patient has the disease (D+), in this case AKI. This is often referred to as the TP rate for a diagnostic test and can be expressed as a conditional probability:
The specificity of a diagnostic test is the probability that the test will give a negative result if the patient does not have the disease (D–), which is equivalent to 1 minus the FP rate for the test and can be expressed as the conditional probability:
Confidence intervals (CIs) were estimated for sensitivity and specificity based on the Wilson score interval method. 253
The LR+ of a diagnostic test is the probability of a patient with disease (D+) having a positive test result divided by the probability of a patient without disease (D–) having a positive test result:
Similarly, the LR– of a diagnostic test is the probability of a patient with disease having a negative test result divided by the probability of a patient without disease having a negative test result:
Confidence intervals for the LR+ and LR– were estimated using the method of Koopman. 254
The DOR for a test is the ratio of the odds of a positive test result for a patient with disease relative to the odds of a positive test result for a patient without disease:
Confidence intervals for log(DOR) were estimated based on the assumption that, as an odds ratio, the DOR is normally distributed. Estimates for DOR were then obtained by back-transformation.
The method of meta-analysis for diagnostic accuracy studies used here was the bivariate meta-analysis proposed by Reitsma et al. ,255 based on the methodology of van Houwelingen et al. 256 Briefly, if logit sensitivity (µsi) and logit specificity (µpi) are
and
for each study i (with k studies included in the meta-analysis), the true logit sensitivity and logit specificity are then assumed to have a bivariate normal distribution across studies:
where σSPn is the covariance between logit sensitivity and logit specificity. This model is extended by incorporating the variability due to sampling through the variance of sensitivity (sS,i2) and specificity (sP,i2), as measured in each study:
and
assuming that 0 < p and s < 1 and that the number of subjects used to estimate sensitivity and specificity is large. 256 The final model is then a bivariate random-effects model of the form:
This model was estimated using likelihood-based methods using the mada package257 in the R Environment for Statistical Computing (The R Foundation for Statistical Computing, Vienna, Austria). It has been shown that this method is equivalent to the hierarchical regression meta-analysis proposed by and further developed by Rutter and Gatsonis when there are no study-level covariates. 258–260
Separate meta-analyses were conducted for each diagnostic test, sample media and health service setting. Pooled estimates of sensitivity, specificity, LR+, LR– and DOR can be estimated from back-transformed parameter estimates. Estimates from each study and pooled estimates from the meta-analysis are presented in forest plots. A summary receiver operating characteristic (SROC) curve was estimated, with estimates of the confidence and prediction region. 255,259 Approximate estimates of the variance of sensitivity and specificity for use in the economic model were determined using the delta method. 261
Tests of heterogeneity were not used, as such statistical methods (Cochran’s Q, I2) do not account for heterogeneity explained by phenomena such as positivity threshold effects and are not recommended by the Cochrane Diagnostic Test Accuracy Group. 262 Estimating the prediction region in the SROC curve is one way of examining the extent of heterogeneity by depicting a region within which, assuming that the model is correct, we have 95% confidence that the true sensitivity and specificity of a future study would lie. 260
Results
Papers selected for inclusion in the meta-analysis are described briefly in tabular summaries followed by a summary of diagnostic accuracy for each study. Pooled estimates of sensitivity and specificity and the SROC curve are also provided for each diagnostic test, health-care setting and sample type. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram that depicts the flow of information through the different phases of the systematic review to data extraction and final inclusion in the meta-analysis is shown in Chapter 2 (see Figure 2).
Nephrocheck
Critical care unit: plasma and serum
The searches identified no studies suitable for data extraction for this diagnostic test in either health-care setting for either plasma or serum.
Critical care unit: urine
Summaries of the baseline characteristics and test parameters for the included urinary Nephrocheck studies are shown in Table 12. Three studies were included,33,44,45 with a total of 1289 patients [199 patients (15.4%) with a diagnosis of AKI and 1090 patients (84.6%) without a diagnosis of AKI]. The sample for use in the test was taken on enrolment in all of the included studies. The outcome used to define the presence of AKI was consistent across each of the three studies (KDIGO stage 2 or 3). Similarly, the threshold used to define a positive test was consistent in all included studies [(TIMP-2) × (IGFBP-7) = 0.3]. Diagnostic accuracy summaries for the included studies are shown in Table 13.
| First author and year | Patient group | Age (years) | Timing of test sample | Threshold | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Kashani 201345 | Patients admitted to the critical care unit | Median (IQR): ± 64 (53, 73) | Within 18 hours of enrolment | 0.3 | KDIGO (2 or 3) within 12 hours | 101 | 627 |
| Bihorac 201433 | Patients admitted to the critical care unit | Mean (SD): ± 63 (17) | On enrolment (median 15 hours from admission) | 0.3 | KDIGO (2 or 3) within 12 hours | 71 | 337 |
| Hoste 201444 | Patients admitted to the critical care unit | Median (IQR): + 64 (54, 75); – 65 (54, 78) | On enrolment (within 24 hours of admission) | 0.3 | KDIGO (2 or 3) within 12 hours | 27 | 126 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Kashani 201345 | 90 | 313 | 11 | 314 | 0.891 (0.815 to 0.938) | 0.501 (0.462 to 0.540) | 1.785 (1.609 to 1.980) | 0.217 (0.124 to 0.382) | 8.21 (4.305 to 15.7) |
| Bihorac 201433 | 65 | 182 | 6 | 155 | 0.915 (0.828 to 0.961) | 0.460 (0.407 to 0.513) | 1.695 (1.502 to 1.914) | 0.184 (0.085 to 0.399) | 9.21 (3.891 to 21.9) |
| Hoste 201444 | 24 | 59 | 3 | 67 | 0.889 (0.719 to 0.961) | 0.532 (0.445 to 0.617) | 1.898 (1.510 to 2.387) | 0.209 (0.071 to 0.615) | 9.09 (2.602 to 31.7) |
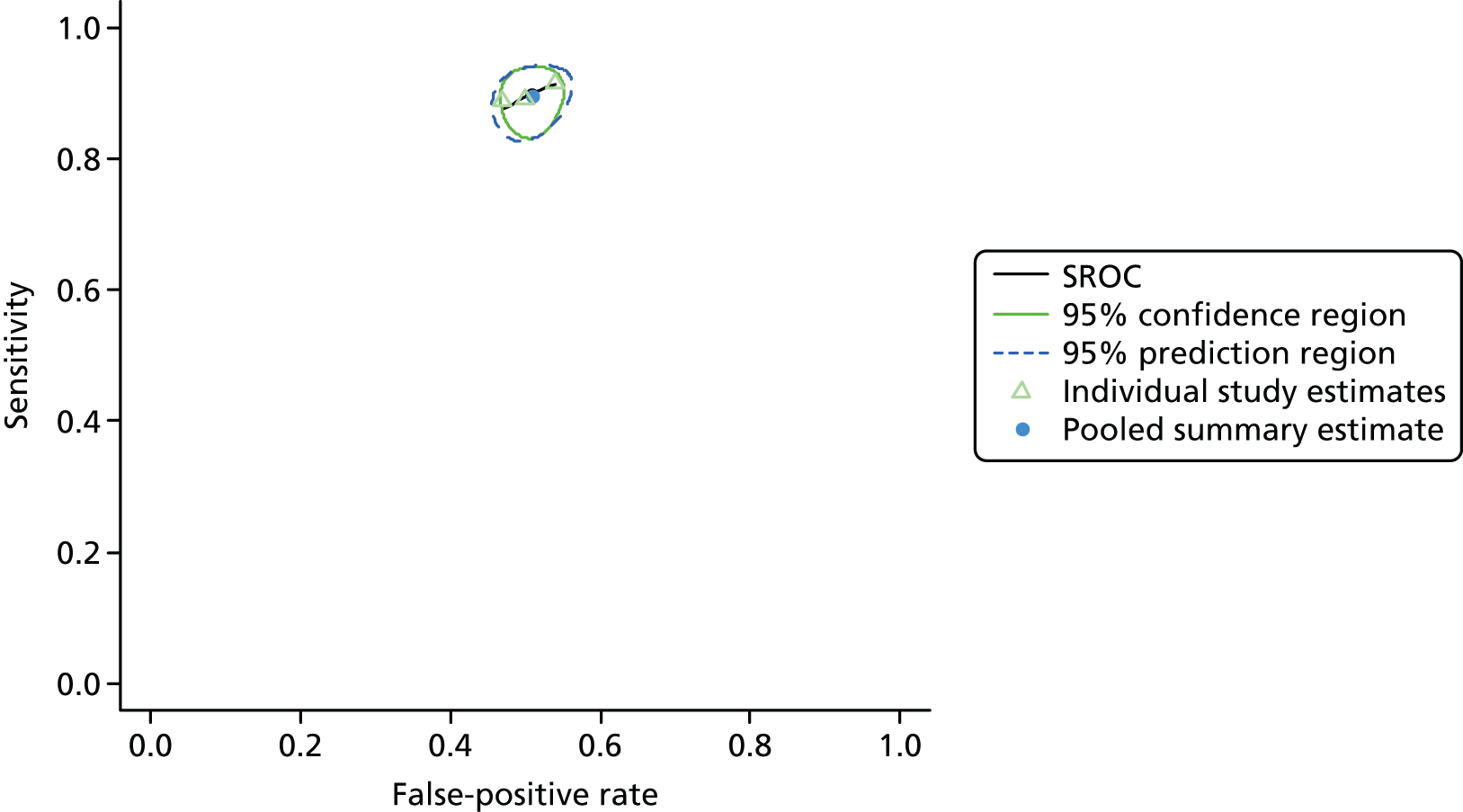
Figure 3 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for Nephrocheck in the critical care unit using patient urine samples. The pooled sensitivity estimate was 0.90 (95% CI 0.85 to 0.93) and the pooled specificity estimate was 0.49 (95% CI 0.46 to 0.53). Figure 4 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. The prediction and confidence regions are small, suggesting limited heterogeneity. This is to be expected given the highly controlled similarity in these studies.
FIGURE 3.
Forest plot for studies included in the meta-analysis and pooled estimates for sensitivity and specificity Nephrocheck in the critical care unit health-care setting using urine.
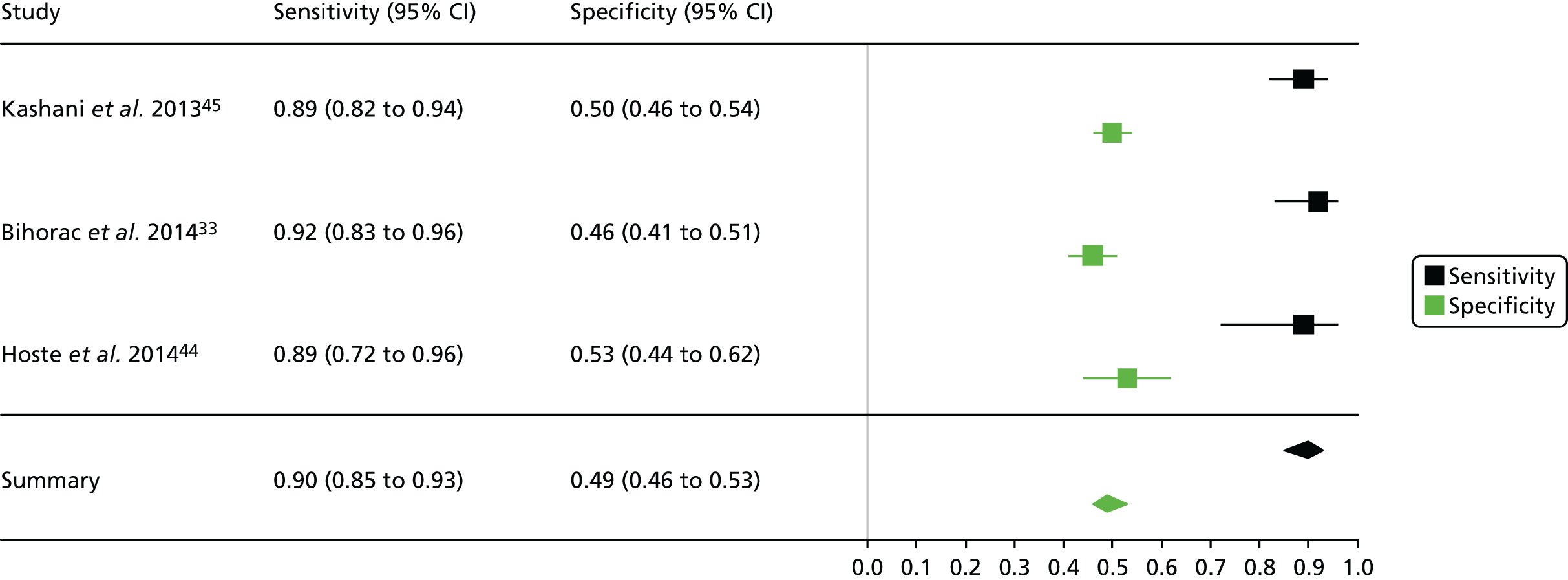
FIGURE 4.
Summary receiver operating characteristic curve for studies included in the meta-analysis for Nephrocheck in the critical care unit health-care setting using urine.
Cardiac surgery: urine
One study including 50 patients [26 patients (52.0%) with a diagnosis of AKI and 24 patients (48.0%) without a diagnosis AKI] was identified for the use of Nephrocheck in the cardiac surgery setting using urine samples. 54 A summary of the baseline characteristics and test parameters for the included study is shown in Table 14. The outcome used to define a diagnosis of AKI was a RIFLE classification of ≥ R within 72 hours of surgery. The threshold used to define a positive test was whether the maximum (TIMP-2) × (IGFBP-7) value in the 24 hours post cardiopulmonary bypass (CPB) was > 0.3. A diagnostic accuracy summary for the included study is shown in Table 15. No meta-analysis was performed for this single study.
| First author and year | Patient group | Age (years) | Timing of test sample | Threshold | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Meersch 201454 | Patients undergoing CPB | Mean (SD): ± 72 (11) | Maximum (TIMP-2) × (IGFBP7) in 24 hours post CPB | 0.3 | RIFLE (≥ R) within 72 hours of surgery | 26 | 24 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Meersch 201454 | 24 | 8 | 2 | 16 | 0.923 (0.759 to 0.979) | 0.667 (0.467 to 0.820) | 2.769 (1.556 to 4.929) | 0.115 (0.030 to 0.450) | 24.00 (4.502 to 128.0) |
Neutrophil gelatinase-associated lipocalin
Critical care unit: plasma
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 16. Eight studies were included,35,43,48,49,51,59,66,100 with a total of 1670 patients [381 patients (22.8%) with a diagnosis of AKI and 1289 patients (77.2%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was not consistent across the studies, with six studies35,43,49,59,66,100 using a diagnostic outcome and two studies48,51 using a failure-type outcome. There was also heterogeneity in the time at which the outcome assessment occurred (unclear, 48 hours and 7 days). Similarly, the threshold used to define a positive test was not consistent across the studies, ranging from 242 pg/ml49 to 558 ng/ml. 43 Diagnostic accuracy summaries for the included studies are provided in Table 17.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml)a | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Constantin 201036 | Patients admitted to the critical care unit | Mean (SD): ± 57 (16) | Within 2 hours of admission | 303 | RIFLE (≥ R) within 7 days of admission | 7 | 81 |
| de Geus 2011100 | Patients admitted to the critical care unit | Median (IQR): + 62 (50, 68); – 58 (43, 68) | On admission | 417 | RIFLE (≥ F) within 7 days of admission | 56 | 461 |
| Kokkoris 201248 | Patients admitted to the critical care unit | Median (IQR): + 63 (50.3, 80.8); – 49 (35.0, 66.3) | Within 13 hours of admission | 62 | RIFLE (≥ R) within 7 days of admission | 36 | 64 |
| Linko 201351 | Critically ill patients receiving ventilator support | Median (SD): ± 61 (51, 73) | Enrolment (at least 6 hours’ ventilator support) | 304 | RRT (AKIN 3, KDIGO 3) | 87 | 282 |
| Hjortrup 201543 | Patients admitted to the critical care unit with severe sepsis and in need of fluid resuscitation | Median (IQR): ± 66 (63, 85) | Enrolment | 558 | KDIGO (≥ 1) within 48 hours of enrolment | 31 | 100 |
| Legrand 201549 | Patients admitted to the critical care unit with oliguria (diuresis < 0.5 ml/hour/kg for > 6 consecutive hours) | Median (IQR): + 55 (41, 70); – 55 (41, 70) | At time of oliguria diagnosis | 242 pg/ml | KDIGO (≥ 1) within 7 days of admission | 41 | 70 |
| Palazzuoli 201559 | Acute heart failure (evidence of volume overload, pulmonary congestion or BNP greater than the ULN for age) | Mean (SD): + 78 (9); – 80 (8) | Within 24 hours of admission | 134 | AKIN (≥ 1) during the hospitalisation period | 78 | 125 |
| Shum 201566 | Patients admitted to the critical care unit and expected to stay for > 24 hours | Median (IQR): + 74 (60, 83); – 64 (54, 78) | 6 hours after admission | 230 | AKIN (≥ 1) within 48 hours of admission | 45 | 106 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Constantin 201036 | 43 | 1 | 9 | 35 | 0.827 (0.703 to 0.906) | 0.972 (0.858 to 0.995) | 29.7 (4.29 to 206.4) | 0.178 (0.098 to 0.323) | 167.2 (20.20 to 1384) |
| de Geus 2011100 | 39 | 46 | 17 | 415 | 0.696 (0.567 to 0.801) | 0.9 (0.869 to 0.924) | 6.98 (5.04 to 9.65) | 0.337 (0.227 to 0.502) | 20.70 (10.85 to 39.49) |
| Kokkoris 201248 | 26 | 14 | 10 | 50 | 0.722 (0.560 to 0.842) | 0.781 (0.666 to 0.865) | 3.302 (1.992 to 5.473) | 0.356 (0.207 to 0.612) | 9.286 (3.628 to 23.77) |
| Linko 201351 | 59 | 73 | 28 | 209 | 0.678 (0.574 to 0.767) | 0.741 (0.687 to 0.789) | 2.62 (2.05 to 1.60) | 0.434 (0.318 to 0.594) | 6.033 (3.577 to 10.18) |
| Hjortrup 201543 | 18 | 24 | 13 | 76 | 0.581 (0.408 to 0.736) | 0.76 (0.668 to 0.833) | 2.42 (1.53 to 3.83) | 0.552 (0.359 to 0.847) | 4.385 (1.877 to 10.24) |
| Legrand 201549 | 33 | 14 | 8 | 56 | 0.805 (0.660 to 0.898) | 0.8 (0.692 to 0.877) | 4.024 (2.460 to 6.583) | 0.244 (0.130 to 0.459) | 16.50 (6.259 to 43.50) |
| Palazzuoli 201559 | 66 | 25 | 12 | 100 | 0.846 (0.750 to 0.910) | 0.8 (0.721 to 0.861) | 4.231 (2.942 to 6.083) | 0.192 (0.113 to 0.326) | 22.00 (10.34 to 46.82) |
| Shum 201566 | 26 | 25 | 19 | 81 | 0.578 (0.433 to 0.710) | 0.764 (0.675 to 0.835) | 2.45 (1.60 to 3.74) | 0.553 (0.386 to 0.790) | 4.434 (2.111 to 9.134) |
Figure 5 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for NGAL in the critical care unit using patient plasma samples. The pooled sensitivity estimate was 0.72 (95% CI 0.65 to 0.79) and the pooled specificity estimate was 0.81 (95% CI 0.75 to 0.86). Figure 6 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. The prediction interval shows that there is a degree of heterogeneity in sensitivity and specificity, probably reflecting the variability in outcome measures and cut-off points, as well as other unidentified sources of heterogeneity.
FIGURE 5.
Forest plot for studies included in the meta-analysis and pooled estimates for NGAL in the critical care unit health-care setting using plasma.
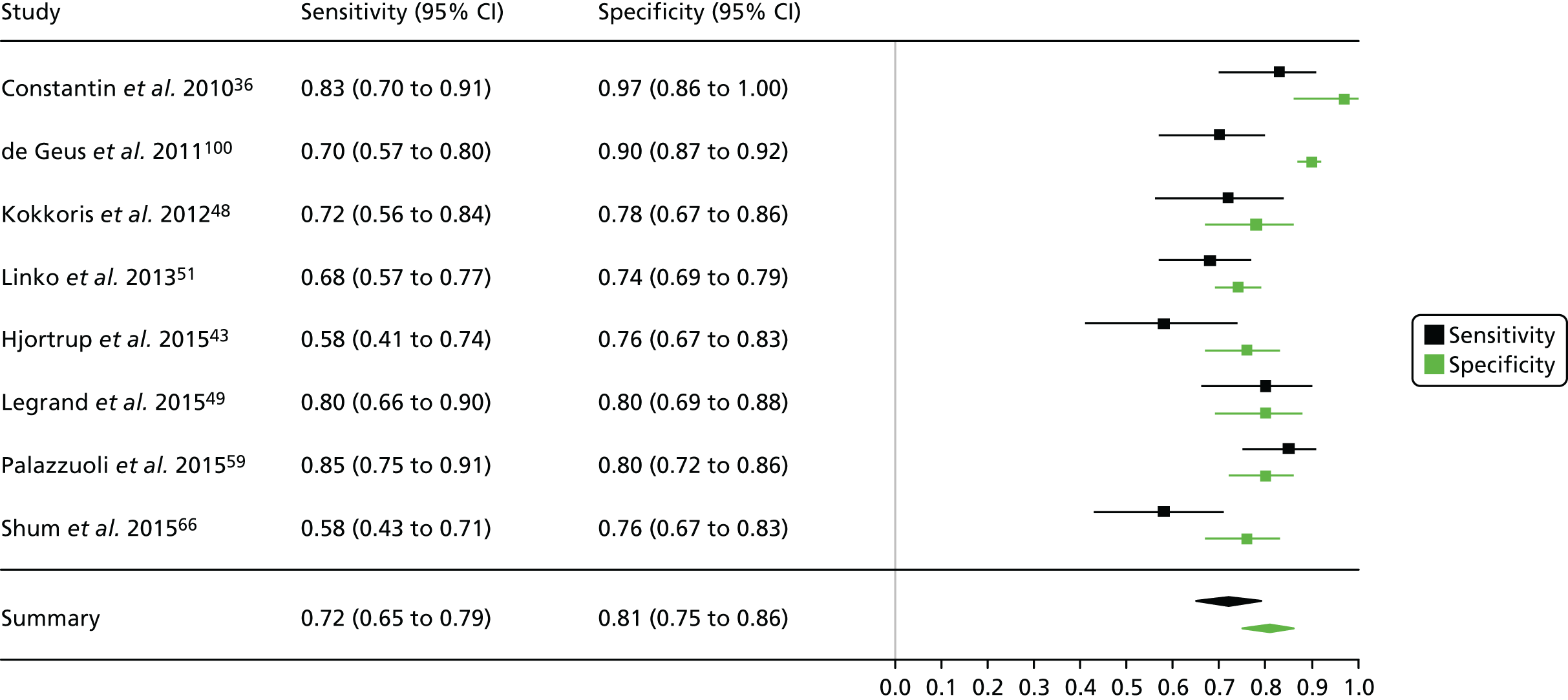
FIGURE 6.
Summary receiver operating characteristic curve for studies included in the meta-analysis for NGAL in the critical care unit health-care setting using plasma.
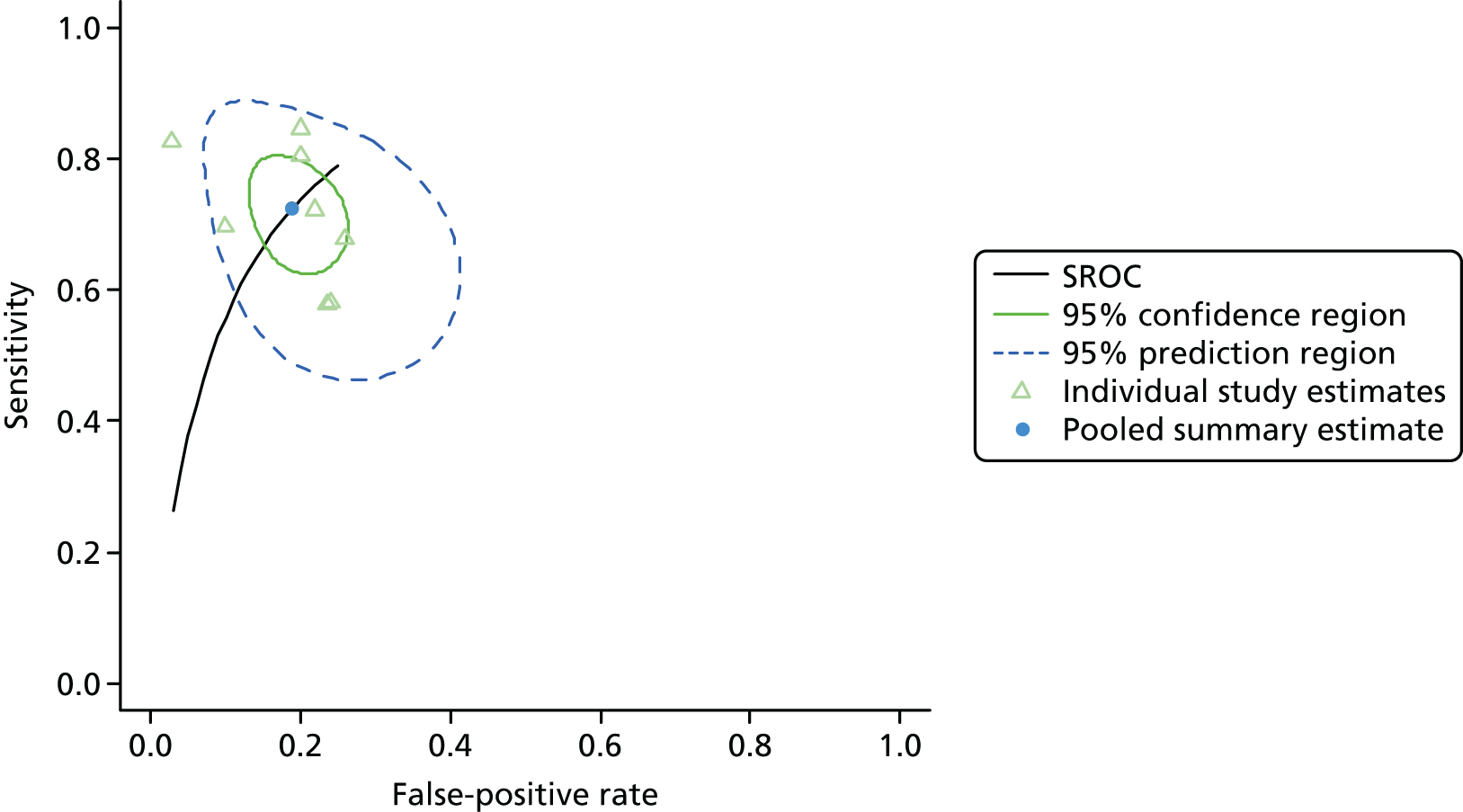
Critical care unit: serum
One study including 150 patients [43 patients (28.7%) with a diagnosis of AKI and 107 patients (71.3%) without a diagnosis of AKI] was identified for NGAL in the critical care unit setting using serum. 34 A summary of the baseline characteristics and test parameters for the included study is provided in Table 18. A diagnostic-type outcome was used to define the presence of AKI (AKIN stage 1 or above within 48 hours of admission). The threshold used to define a positive test was 110 ng/ml. A diagnostic accuracy summary for the included study is provided in Table 19. No meta-analysis was performed for this single study.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Chen 201234 | Patients admitted to the critical care unit | Mean (SE): 66 (1) | Admission | 110 | AKIN (≥ 1) within 48 hours of admission | 43 | 107 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Chen 201234 | 39 | 4 | 33 | 74 | 0.542 (0.427 to 0.652) | 0.949 (0.875 to 0.98) | 10.562 (3.973 to 28.084) | 0.483 (0.374 to 0.624) | 21.864 (7.221 to 66.195) |
Critical care unit: urine
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 20. Six studies were included,32,34,35,43,48,100 with a total of 1194 patients [283 patients (23.7%) with a diagnosis of AKI and 911 patients (76.3%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was not consistent across the studies. Of the studies included, five had a similar end point,32,34,35,43,48 being at least the least severe stage of the RIFLE, AKIN or KDIGO classification system. There was heterogeneity in the time up to which the outcome assessment occurred (from 28 hours up to the end of the hospital stay). Similarly, the threshold used to define a positive test was not consistent across the studies, ranging from 29.5 ng/ml32 to 1310 ng/ml. 100 Diagnostic accuracy summaries for the included studies are shown in Table 21.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| de Geus 2011100 | Patients admitted to the critical care unit | Median (IQR): + 62 (50, 68); – 58 (43, 68) | On admission | 1310 | RIFLE (≥ F) within 7 days of admission | 56 | 461 |
| Chen 201234 | Patients admitted to the critical care unit | Mean (SE): 66 (1) | On admission | 110 | AKIN (≥ 1) within 48 hours of admission | 43 | 107 |
| Kokkoris 201248 | Patients admitted to the ICU | Median (IQR): + 63 (50.3, 80.8); – 49 (35.0, 66.3) | Within 13 hours of admission | 58.5 | RIFLE (≥ R) within 7 days of admission | 36 | 64 |
| Aydogdu 201332 | Patients admitted to the ICU (without previous history of renal disease) | Mean (SD): + 70 (13) (sepsis); – 66 (10) (no sepsis); – 67 (15) (sepsis) | Daily throughout hospital stay | 29.5 | RIFLE (≥ R) during hospital stay | 63 | 88 |
| Cho 201335 | Patients admitted to the medical or surgical ICU | Mean (SD): + 65.4 (14.8); – 60.4 (17.4) | On admission to the ICU | 251 | AKIN (≥ 1) within 5 days of admission | 54 | 91 |
| Hjortrup 201543 | Patients admitted to the critical care unit with severe sepsis and in need of fluid resuscitation | Median (IQR): ± 66 (63, 85) | Enrolment | 558 | KDIGO (≥ 1) within 48 hours of enrolment | 31 | 100 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| de Geus 2011100 | 31 | 46 | 25 | 415 | 0.554 (0.424 to 0.676) | 0.900 (0.869 to 0.924) | 5.55 (3.87 to 7.96) | 0.496 (0.370 to 0.665) | 11.2 (6.09 to 20.6) |
| Chen 201234 | 28 | 17 | 15 | 90 | 0.651 (0.502 to 0.776) | 0.841 (0.760 to 0.898) | 4.098 (2.516 to 6.675) | 0.415 (0.273 to 0.629) | 9.882 (4.380 to 22.295) |
| Kokkoris 201234 | 28 | 18 | 8 | 46 | 0.778 (0.619 to 0.883) | 0.719 (0.599 to 0.814) | 2.765 (1.801 to 4.246) | 0.309 (0.165 to 0.581) | 8.944 (3.438 to 23.271) |
| Aydogdu 201332 | 55 | 24 | 8 | 64 | 0.873 (0.769 to 0.934) | 0.727 (0.626 to 0.809) | 3.201 (2.247 to 4.560) | 0.175 (0.090 to 0.338) | 18.333 (7.623 to 44.092) |
| Cho 201335 | 40 | 27 | 14 | 64 | 0.741 (0.611 to 0.839) | 0.703 (0.603 to 0.787) | 2.497 (1.753 to 3.555) | 0.369 (0.230 to 0.590) | 6.772 (3.177 to 14.435) |
| Hjortrup 201543 | 17 | 23 | 14 | 77 | 0.548 (0.378 to 0.708) | 0.770 (0.678 to 0.842) | 2.38 (1.48 to 3.85) | 0.587 (0.392 to 0.877) | 4.07 (1.74 to 9.48) |
Figure 7 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for NGAL in the critical care unit using patient urine samples. The pooled sensitivity estimate was 0.70 (95% CI 0.59 to 0.80) and the pooled specificity estimate was 0.79 (95% CI 0.71 to 0.86). Figure 8 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. The prediction region shows that there is a degree of heterogeneity in sensitivity and specificity, probably reflecting the variability in outcome measures and cut-off points, as well as other unidentified sources of heterogeneity.
FIGURE 7.
Forest plot for studies included in the meta-analysis and pooled estimates for NGAL in the critical care unit health-care setting using urine.
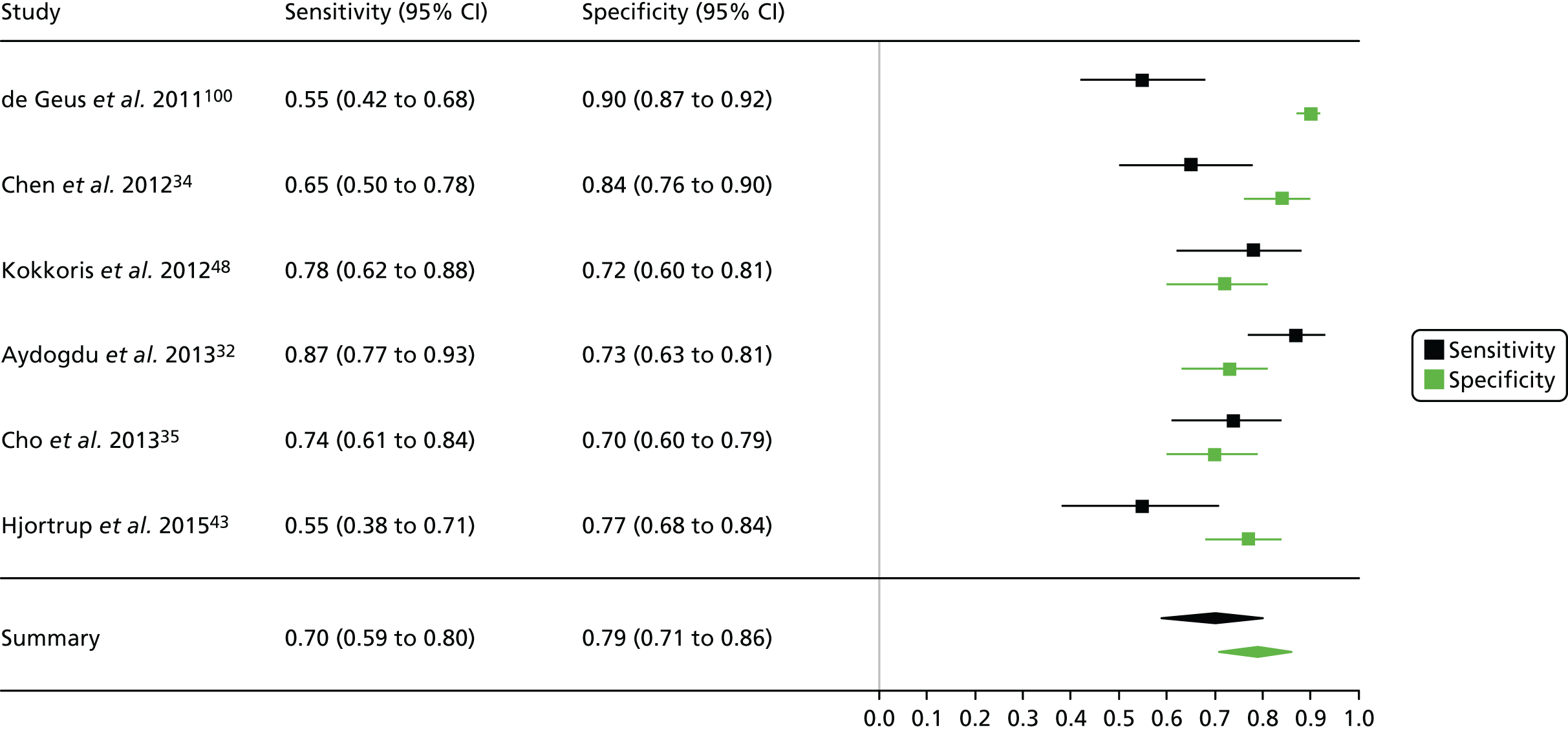
FIGURE 8.
Summary receiver operating characteristic curve for studies included in the meta-analysis for NGAL in the critical care unit health-care setting using urine.
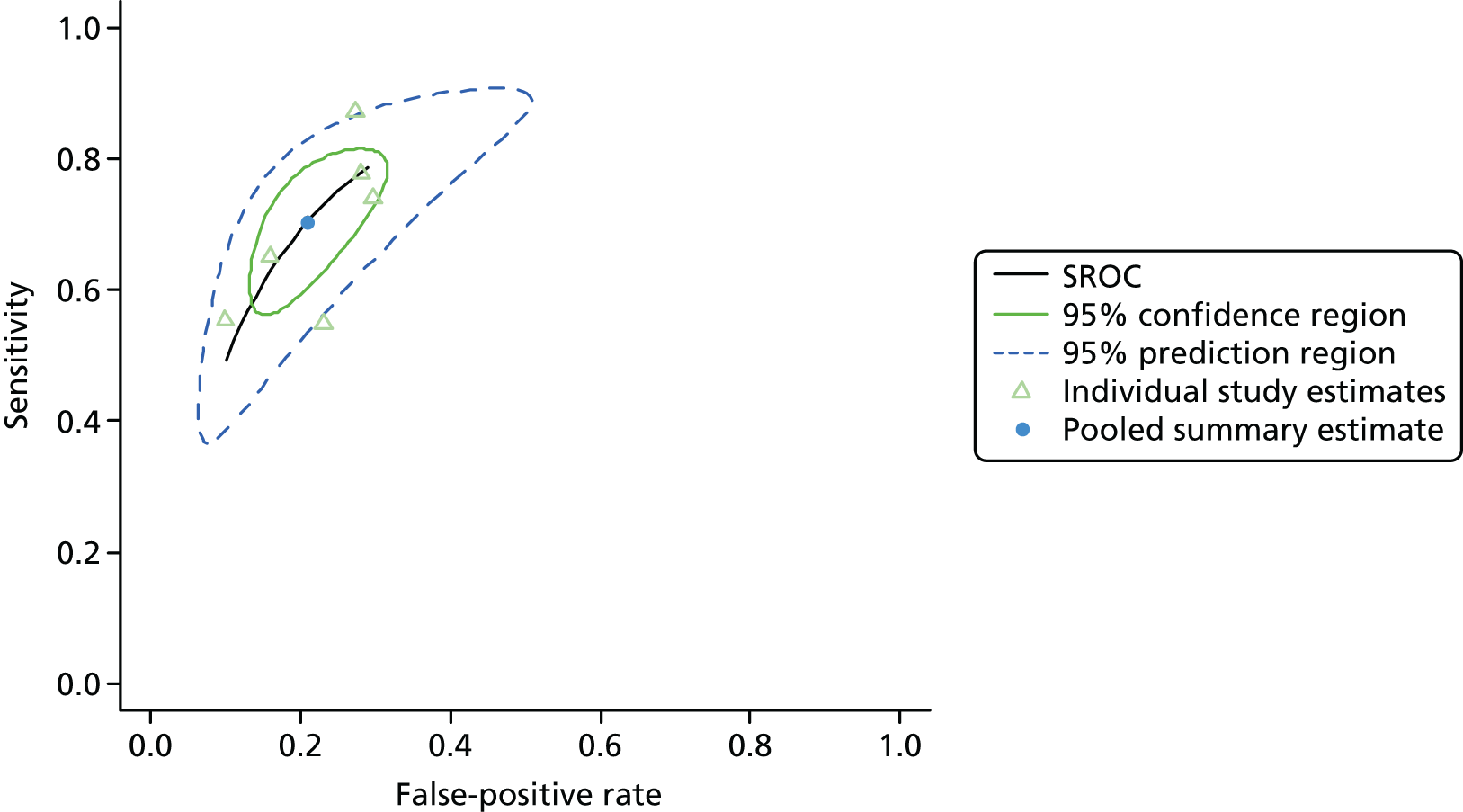
Cardiac surgery: plasma
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 22. Eight studies were included,31,40,47,60–63,67 with a total of 2644 patients [286 patients (10.8%) with a diagnosis of AKI and 2358 patients (89.2%) without a diagnosis of AKI]. The outcome used to define a diagnosis of AKI was largely consistent across the studies, although there was some heterogeneity in the time period in which the outcome was assessed. However, one study, used an end point that could not easily be mapped to the considered criteria. 67 Each of these could be considered to be somewhere between the least two severe categories of the RIFLE, AKN or KDIGO classification system. Similarly, the threshold used to define a positive test was not consistent across the studies, ranging from 150 ng/ml31 to 426 ng/ml. 67 Diagnostic accuracy summaries for the included studies are shown in Table 23.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Haase 200931 | Patients undergoing cardiac surgery | Mean (SD): + 74.2 (6.9); – 68.3 (10.3) | 6 hours post CPB surgery | 150 | AKIN (≥ 1) within 5 days of admission | 46 | 54 |
| Haase-Fielitz 200940 | Patients undergoing cardiac surgery with CPB | Mean (SD): + 75.9 (4.8); – 67.6 (9.9) | On arrival in the ICU (6 hours) | 150 | AKIN (≥ 1) within 5 days of admission | 23 | 77 |
| Tuladhar 200967 | Patients undergoing cardiac surgery | Median (IQR): + 70 (57–78); – 66 (41, 81) | 2 hours post surgery | 426 | An increase in SCr in the postoperative period by > 0.5 mg/dl from baseline | 9 | 41 |
| Perry 201063 | Patients undergoing CABG surgery | Mean (SD): + 65 (12); – 65 (10) | At CPB | 353.5 | AKIN (≥ 1) within 4 days postoperatively | 75 | 804 |
| Parikh 201160 | Patients undergoing cardiac surgery (CABG or valve surgery) | Mean (SD): ± 71 (10) | Soon after arrival in the ICU | 293 | AKIN (≥ 2) within 4 days postoperatively | 60 | 1159 |
| Kidher 201447 | Patients undergoing aortic valve replacement | Mean (SD): ± 71 (9) | 3 hours post surgery | 150 | RIFLE (≥ R) within 2 days postoperatively | 16 | 37 |
| Park 201561 | Patients undergoing cardiovascular surgery with CPB | Median (IQR): + 65 (50, 74); – 54 (40, 61) | On admission | 168.5 | RIFLE (≥ R) within 3 days postoperatively | 5 | 72 |
| Perrotti 201562 | Patients undergoing cardiac surgery | Mean (SD): ± 77 (6) | 15 minutes after interruption of extracorporeal circulation | 178 | RIFLE (≥ R) within 2 days postoperatively | 52 | 114 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Haase 200931 | 34 | 14 | 12 | 40 | 0.739 (0.597 to 0.844) | 0.741 (0.611 to 0.839) | 2.851 (1.760 to 4.618) | 0.352 (0.211 to 0.587) | 8.095 (3.303 to 19.84) |
| Haase-Fielitz 200940 | 18 | 17 | 5 | 60 | 0.783 (0.581 to 0.903) | 0.779 (0.675 to 0.857) | 3.545 (2.212 to 5.681) | 0.279 (0.127 to 0.611) | 12.706 (4.114 to 39.24) |
| Tuladhar 200967 | 7 | 14 | 2 | 27 | 0.778 (0.453 to 0.937) | 0.659 (0.505 to 0.784) | 2.278 (1.314 to 3.948) | 0.337 (0.097 to 1.168) | 6.750 (1.235 to 36.91) |
| Perry 201063 | 29 | 149 | 46 | 655 | 0.387 (0.285 to 0.500) | 0.815 (0.786 to 0.840) | 2.086 (1.515 to 2.873) | 0.753 (0.627 to 0.904) | 2.771 (1.685 to 4.558) |
| Parikh 201160 | 28 | 220 | 32 | 939 | 0.467 (0.346 to 0.591) | 0.810 (0.787 to 0.832) | 2.458 (1.830 to 3.304) | 0.658 (0.519 to 0.835) | 3.735 (2.203 to 6.332) |
| Kidher 201447 | 13 | 4 | 3 | 33 | 0.812 (0.570 to 0.934) | 0.892 (0.753 to 0.957) | 7.516 (2.892 to 19.53) | 0.210 (0.075 to 0.587) | 35.750 (7.013 to 182.23) |
| Park 201561 | 4 | 8 | 1 | 64 | 0.800 (0.376 to 0.964) | 0.889 (0.796 to 0.943) | 7.200 (3.278 to 15.81) | 0.225 (0.039 to 1.301) | 32.000 (3.172 to 322.8) |
| Perrotti 201562 | 28 | 33 | 24 | 81 | 0.538 (0.405 to 0.667) | 0.711 (0.621 to 0.786) | 1.860 (1.269 to 2.726) | 0.650 (0.474 to 0.891) | 2.864 (1.452 to 5.647) |
Figure 9 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for NGAL in the cardiac surgery setting using patient plasma samples. The pooled sensitivity estimate was 0.62 (95% CI 0.49 to 0.74) and the pooled specificity estimate was 0.78 (95% CI 0.75 to 0.81). Figure 10 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. The prediction region suggests limited heterogeneity when considering specificity, with far greater heterogeneity when considering sensitivity, confirming the observations that can be made from the forest plot (see Figure 9).
FIGURE 9.
Forest plot for studies included in the meta-analysis and pooled estimates for NGAL in the cardiac surgery health-care setting using plasma.
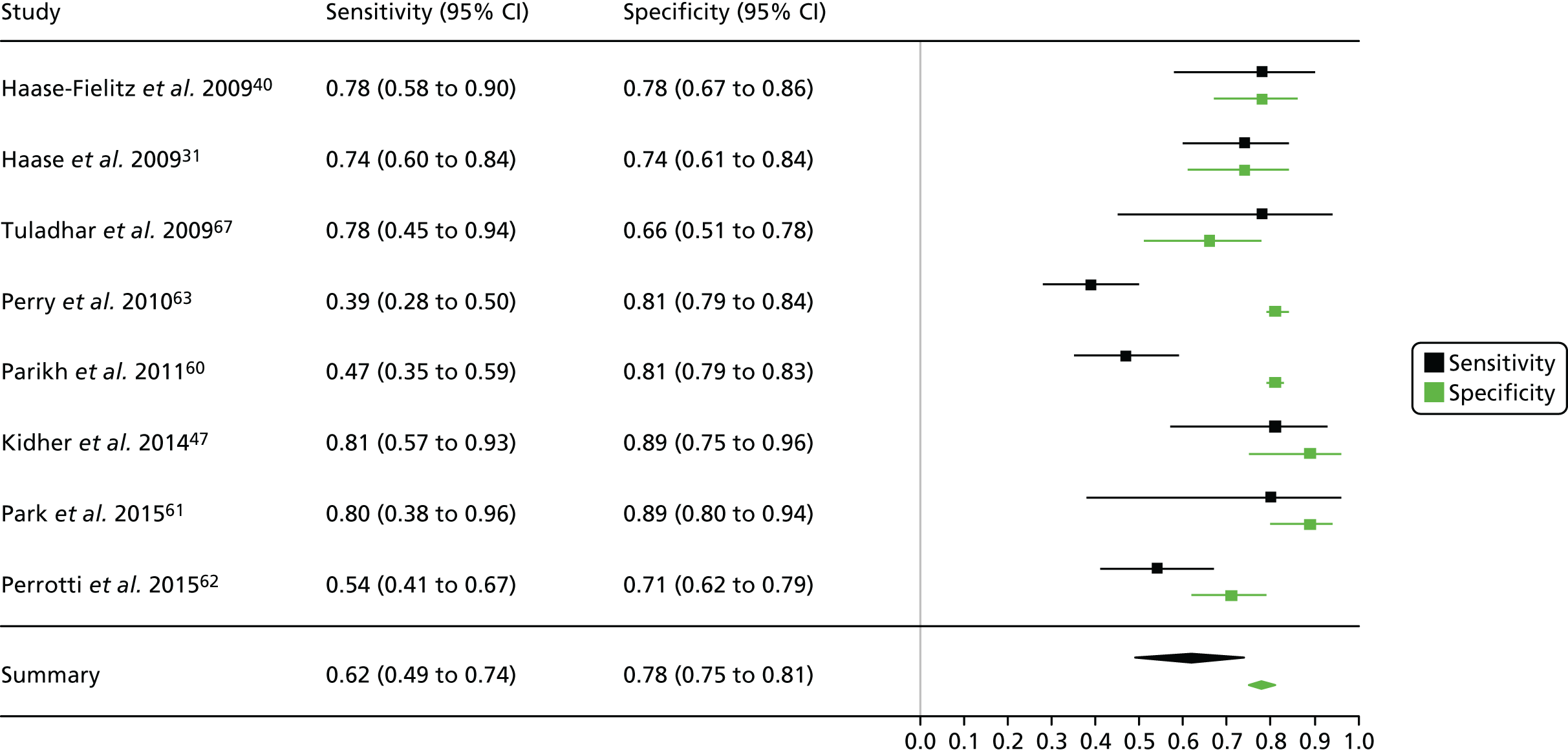
FIGURE 10.
Summary receiver operating characteristic curve for studies included in the meta-analysis for NGAL in the cardiac surgery health-care setting using plasma.
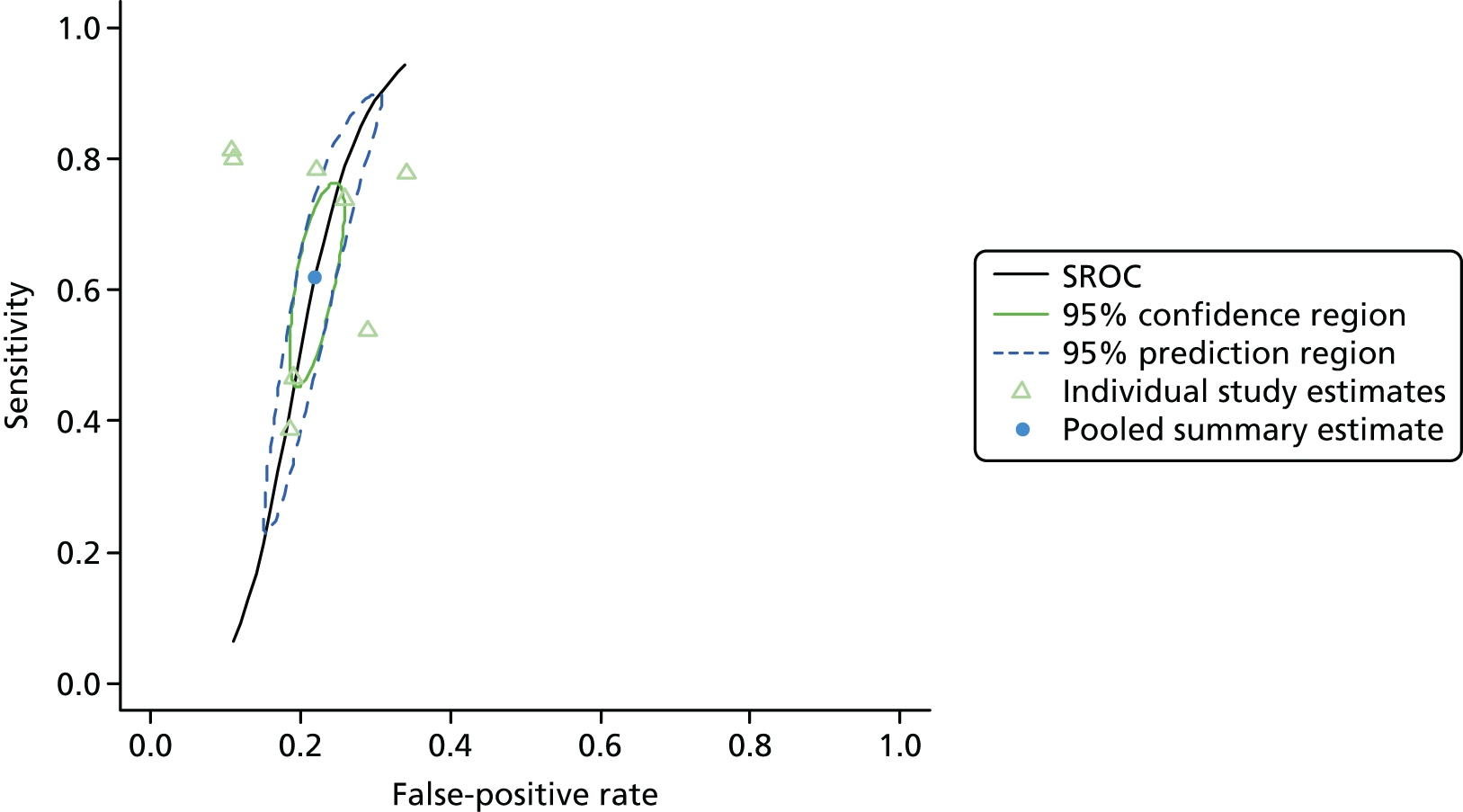
Cardiac surgery: serum
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 24. Two studies were included,38,68 with a total of 239 patients [53 patients (22.2%) with a diagnosis of AKI and 186 patients (77.8%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was not consistent across the studies, with one study38 using a less stringent version of the AKIN stage 1 classification without justification. Similarly, the threshold used to define a positive test was not consistent, being 0.62 ng/ml in one study38 and 133.7 ng/ml in the other study. 68 Diagnostic accuracy summaries for the included studies are shown in Table 25.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Ghonemy 201438 | Patients undergoing CABG surgery or valve replacement | Range: + 39–56; – 32–53 | 3 hours postoperatively | 0.62 | An increase in SCr either by 25% of the baseline or by 0.3 mg/dl above the baseline level within 24 hours postoperatively | 17 | 33 |
| Tung 201568 | Patients with STEMI receiving PCI | Mean (SD): + 68.14 (12.6); – 61.33 (13.9) | At presentation | 133.7 | AKIN (≥ 1) within 48 hours of admission | 36 | 153 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Ghonemy 201438 | 16 | 2 | 1 | 31 | 0.941 (0.730 to 0.990) | 0.939 (0.804 to 0.983) | 15.529 (4.032 to 59.81) | 0.063 (0.009 to 0.420) | 248.0 (20.87 to 2947) |
| Tung 201568 | 25 | 35 | 11 | 118 | 0.694 (0.531 to 0.820) | 0.771 (0.699 to 0.831) | 3.036 (2.112 to 4.363) | 0.396 (0.240 to 0.653) | 7.66 (3.432 to 17.12) |
Figure 11 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for NGAL in the cardiac surgery setting using patient serum samples. The pooled sensitivity estimate was 0.84 (95% CI 0.43 to 0.97) and the pooled specificity estimate was 0.87 (95% CI 0.59 to 0.97). Figure 12 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. There appears to be considerable heterogeneity in both sensitivity and specificity.
FIGURE 11.
Forest plot for studies included in the meta-analysis and pooled estimates for NGAL in the cardiac surgery health-care setting using serum.
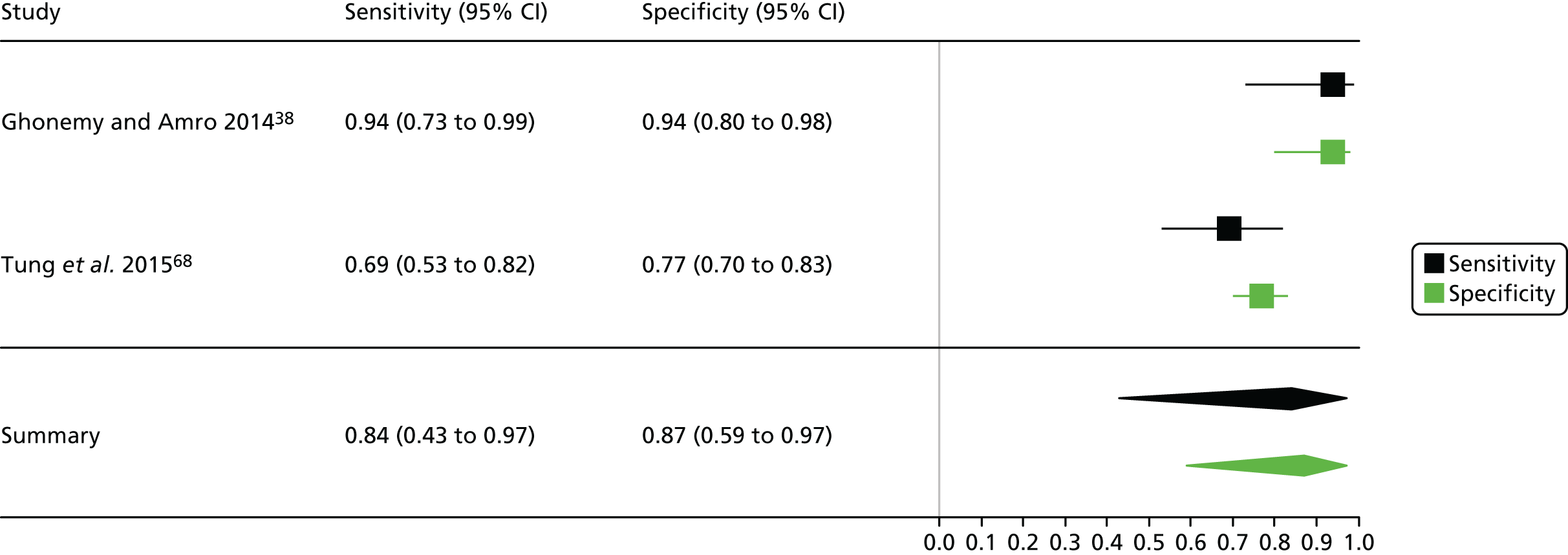
FIGURE 12.
Summary receiver operating characteristic curve for studies included in the meta-analysis for NGAL in the cardiac surgery health-care setting using serum.
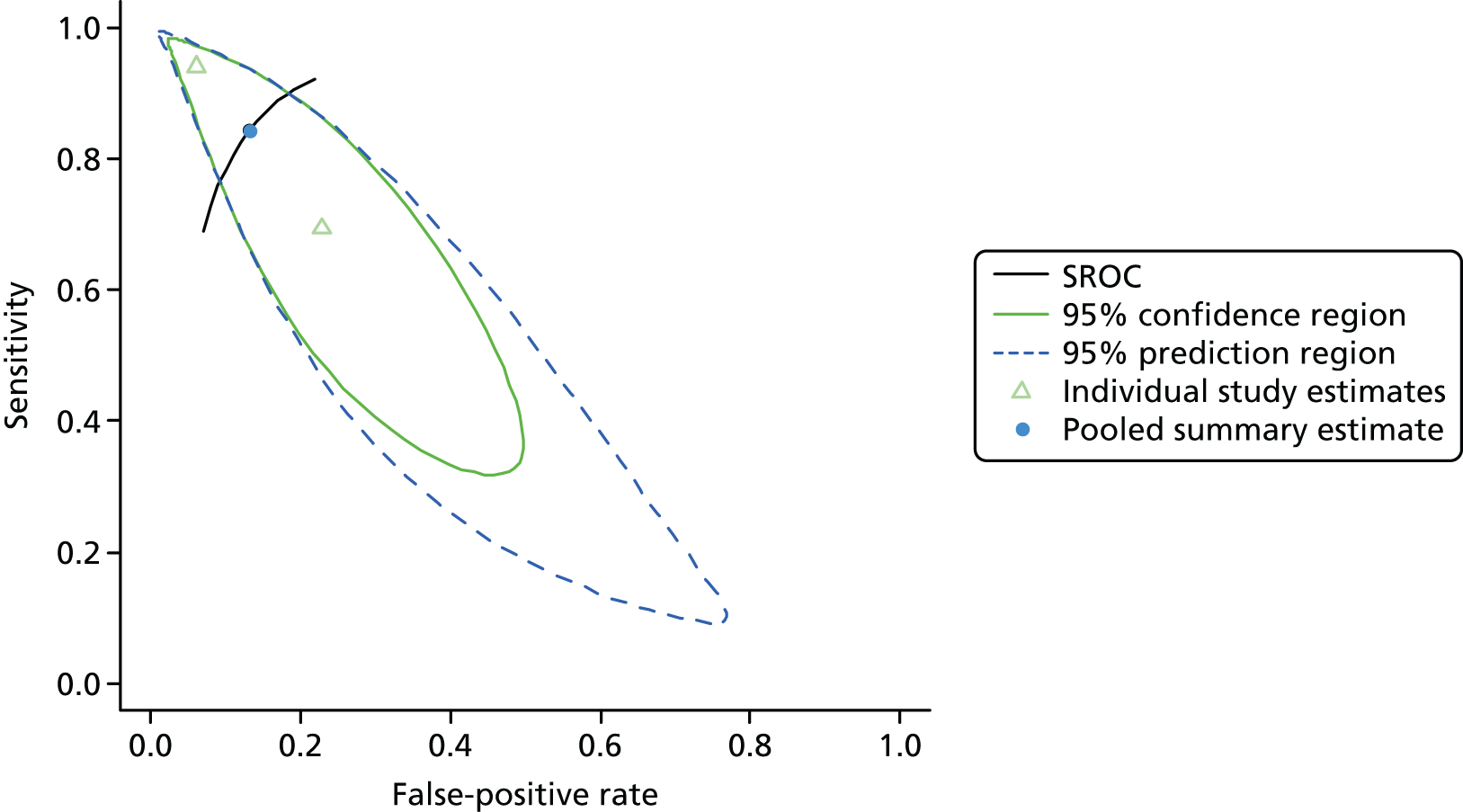
Cardiac surgery: urine
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 26. Thirteen studies were included,41,50,52,53,56,58,60,64,65,67,69,70,72 with a total of 3226 patients [444 patients (13.8%) with a diagnosis of AKI and 2782 patients (86.2%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was largely consistent across the studies, with one study67 using a non-standard definition and one study41 using only the serum creatinine assessment tool of the AKIN criteria. However, there was heterogeneity in the time to outcome assessment across the studies. The threshold used to define a positive test was not consistent across all of the studies, with some studies using raw concentration values and concentrations normalised by units of urine creatinine. Diagnostic accuracy summaries for the included studies are shown in Table 27.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml)a | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Wagener 200872 | Patients undergoing cardiovascular surgery | Mean (SD): + 69.5 (13.1); – 61.7 (14.9) | Immediately post surgery | 23.5 | AKIN (≥ 1) within 48 hours postoperatively | 85 | 341 |
| Han 200941 | Patients undergoing cardiovascular surgery | Mean (SD): + 68.3 (2.3); – 60.4 (1.98) | Immediately post surgery | 456.0 ng/mg of creatinine | Modified AKIN stages 1 and 2 within 72 hours postoperativelyb | 36 | 54 |
| Liangos 200950 | Patients undergoing on-pump cardiovascular surgery | Mean (SD): + 73 (9); – 67 (12) | 2 hours post surgery | 166 ng/mg of creatinine | RIFLE (≥ R) within 72 hours postoperatively | 13 | 90 |
| Tuladhar 200967 | Patients undergoing cardiac surgery | Median (IQR): + 70 (57, 78); – 66 (41, 81) | 2 hours post surgery | 393 ng/mmol of creatinine | An increase in SCr in the postoperative period by > 0.5 mg/dl from baseline | 9 | 41 |
| McIlroy 201053 | Hospitalised patients undergoing contrast-enhanced CT | – | Immediately post surgery | 8 | AKIN (≥ 1) within 48 hours postoperatively | 8 | 45 |
| Parikh 201160 | Patients undergoing cardiac surgery (CABG or valve surgery) | Mean (SD): ± 71 (10) | Soon after arrival in the ICU | 102 | AKIN (≥ 2) within 4 days postoperatively | 60 | 1159 |
| Oh 201258 | Patients undergoing CABG surgery | Median (IQR): placebo: + 73 (69, 77.5), – 68 (62, 72); EPO: + 70 (62, 75), – 62 (56.5, 72.5) | Immediately post surgery | 5 | AKIN (≥ 1) within 72 hours postoperatively | 21 | 50 |
| Sargentini 201265 | Patients undergoing cardiovascular surgery | Mean (SD): + 74 (6); – 67 (11) | 4 hours post ICU admission | 55.2 mg/g of creatinine | AKIN (≥ 1) within 48 hours postoperatively | 15 | 37 |
| Liu 201352 | Patients undergoing cardiovascular surgery | Mean (SD): ± 63.0 (11.3) | Immediately postoperatively | 131.1 | AKIN (≥ 1) within 48 hours postoperatively excluding urine output | 26 | 83 |
| Munir 201356 | Patients undergoing cardiovascular surgery | Median (IQR): + 56 (47–64); – 51 (45–61) | 4 hours post CPB | 109 | AKIN (≥ 1) within 48 hours postoperatively | 11 | 77 |
| Prowle 201564 | Patients undergoing cardiovascular surgery | Median (IQR): ± 70 (61–76) | Immediately postoperatively | 195 | RIFLE (≥ R) within 5 days postoperatively | 25 | 68 |
| Tziakas 201569 | Patients admitted with acute, spontaneous (type 1) AMI undergoing cardiovascular surgery | Mean (SD): ± 62 (13) | During admission | Unclear | AKIN (≥ 1) within 48 hours postoperatively | 118 | 687 |
| Varela 201570 | Patients undergoing cardiovascular surgery | Mean (SD): ± 68 (11) | 6 hours | Unclear | AKIN (≥ 1) within 48 hours postoperatively | 16 | 50 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Wagener 200872 | 26 | 65 | 59 | 276 | 0.306 (0.218 to 0.410) | 0.809 (0.764 to 0.848) | 1.605 (1.089 to 2.365) | 0.858 (0.738 to 0.997) | 1.871 (1.096 to 3.194) |
| Han 200941 | 26 | 33 | 10 | 21 | 0.722 (0.560 to 0.842) | 0.389 (0.270 to 0.522) | 1.182 (0.881 to 1.585) | 0.714 (0.383 to 1.333) | 1.655 (0.665 to 4.117) |
| Liangos 200950 | 9 | 80 | 4 | 10 | 0.692 (0.424 to 0.873) | 0.111 (0.061 to 0.193) | 0.779 (0.538 to 1.127) | 2.769 (1.016 to 7.551) | 0.281 (0.073 to 1.084) |
| Tuladhar 200967 | 8 | 9 | 1 | 32 | 0.889 (0.565 to 0.980) | 0.780 (0.633 to 0.880) | 4.049 (2.175 to 7.540) | 0.142 (0.022 to 0.910) | 28.444 (3.131 to 258.4) |
| McIlroy 201053 | 2 | 41 | 6 | 4 | 0.250 (0.071 to 0.591) | 0.089 (0.035 to 0.207) | 0.274 (0.082 to 0.914) | 8.437 (3.051 to 23.34) | 0.033 (0.005 to 0.218) |
| Parikh 201160 | 30 | 209 | 30 | 950 | 0.500 (0.377 to 0.623) | 0.820 (0.796 to 0.841) | 2.773 (2.093 to 3.673) | 0.610 (0.473 to 0.787) | 4.545 (2.682 to 7.705) |
| Oh 201258 | 19 | 26 | 2 | 24 | 0.905 (0.711 to 0.973) | 0.480 (0.348 to 0.615) | 1.740 (1.289 to 2.35) | 0.198 (0.051 to 0.765) | 8.769 (1.844 to 41.693) |
| Sargentini 201265 | 8 | 10 | 7 | 27 | 0.533 (0.301 to 0.752) | 0.730 (0.570 to 0.846) | 1.973 (0.970 to 4.020) | 0.640 (0.360 to 1.137) | 3.086 (0.887 to 10.74) |
| Liu 201352 | 20 | 15 | 6 | 68 | 0.769 (0.579 to 0.890) | 0.819 (0.723 to 0.887) | 4.256 (2.571 to 7.047) | 0.282 (0.139 to 0.572) | 15.111 (5.183 to 44.055) |
| Munir 201356 | 6 | 1 | 5 | 76 | 0.545 (0.280 to 0.787) | 0.987 (0.930 to 0.998) | 42.000 (5.57 to 316. 8) | 0.461 (0.241 to 0.880) | 91.200 (9.123 to 911.7) |
| Prowle 201564 | 19 | 27 | 6 | 41 | 0.760 (0.566 to 0.885) | 0.603 (0.484 to 0.711) | 1.914 (1.327 to 2.761) | 0.398 (0.193 to 0.821) | 4.809 (1.702 to 13.584) |
| Tziakas 201569 | 14 | 17 | 2 | 33 | 0.875 (0.640 to 0.965) | 0.660 (0.522 to 0.776) | 2.574 (1.677 to 3.949) | 0.189 (0.051 to 0.703) | 13.588 (2.763 to 66.830) |
| Varela 201570 | 89 | 378 | 30 | 309 | 0.748 (0.663 to 0.817) | 0.450 (0.413 to 0.487) | 1.359 (1.200 to 1.539) | 0.560 (0.407 to 0.772) | 2.425 (1.562 to 3.766) |
Figure 13 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for NGAL in the cardiac surgery setting using patient urine samples. The pooled sensitivity estimate was 0.66 (95% CI 0.54 to 0.76) and the pooled specificity estimate was 0.62 (95% CI 0.41 to 0.79). Figure 14 shows an estimate of the SROC with the 95% confidence region and 95% prediction region. The prediction interval covers almost all of the SROC space, suggesting that there is considerable heterogeneity between the studies included in this meta-analysis.
FIGURE 13.
Forest plot for studies included in the meta-analysis and pooled estimates for NGAL in the cardiac surgery health-care setting using urine.
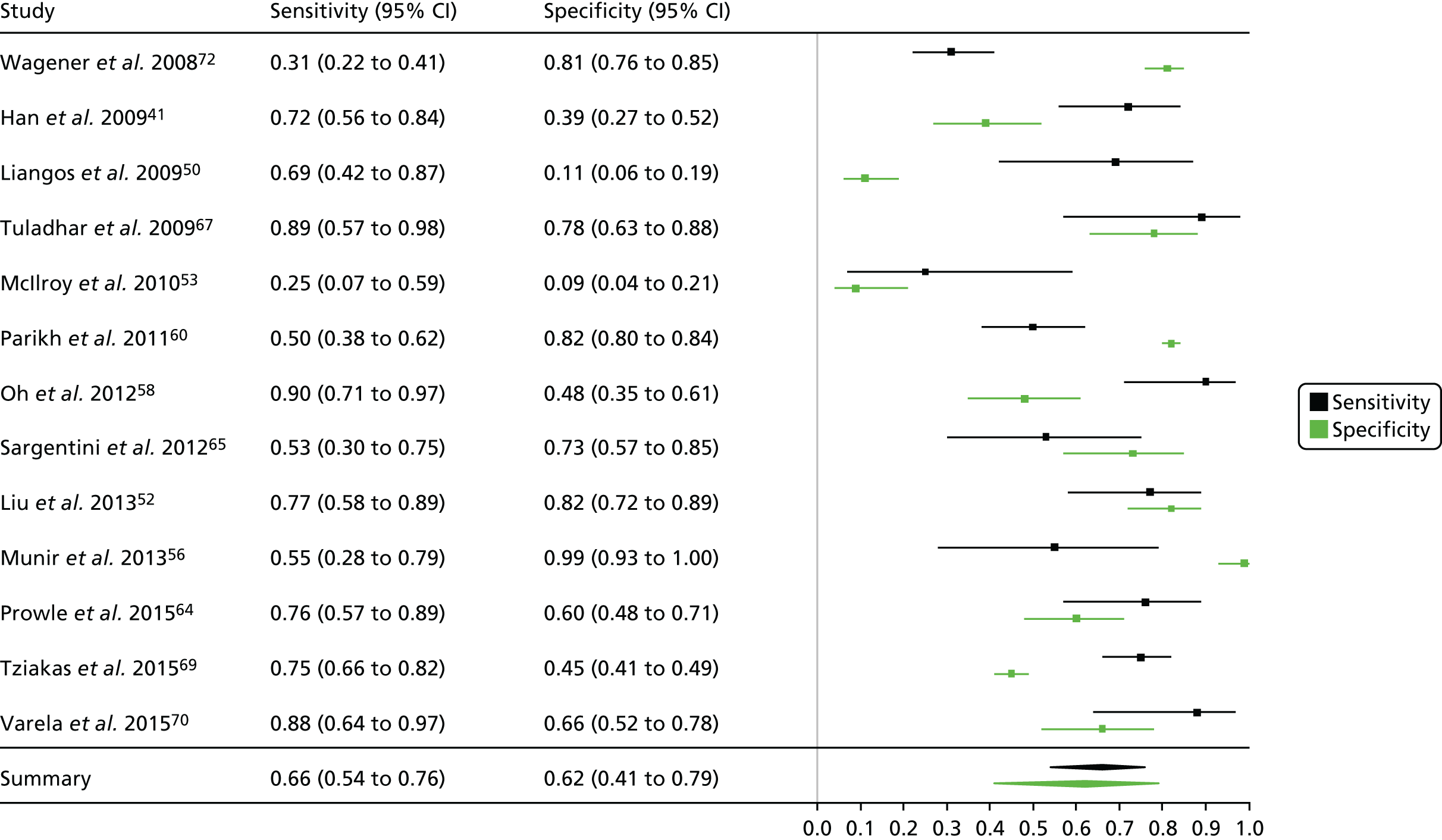
FIGURE 14.
Summary receiver operating characteristic curve for studies included in the meta-analysis for NGAL in the cardiac surgery health-care setting using urine.
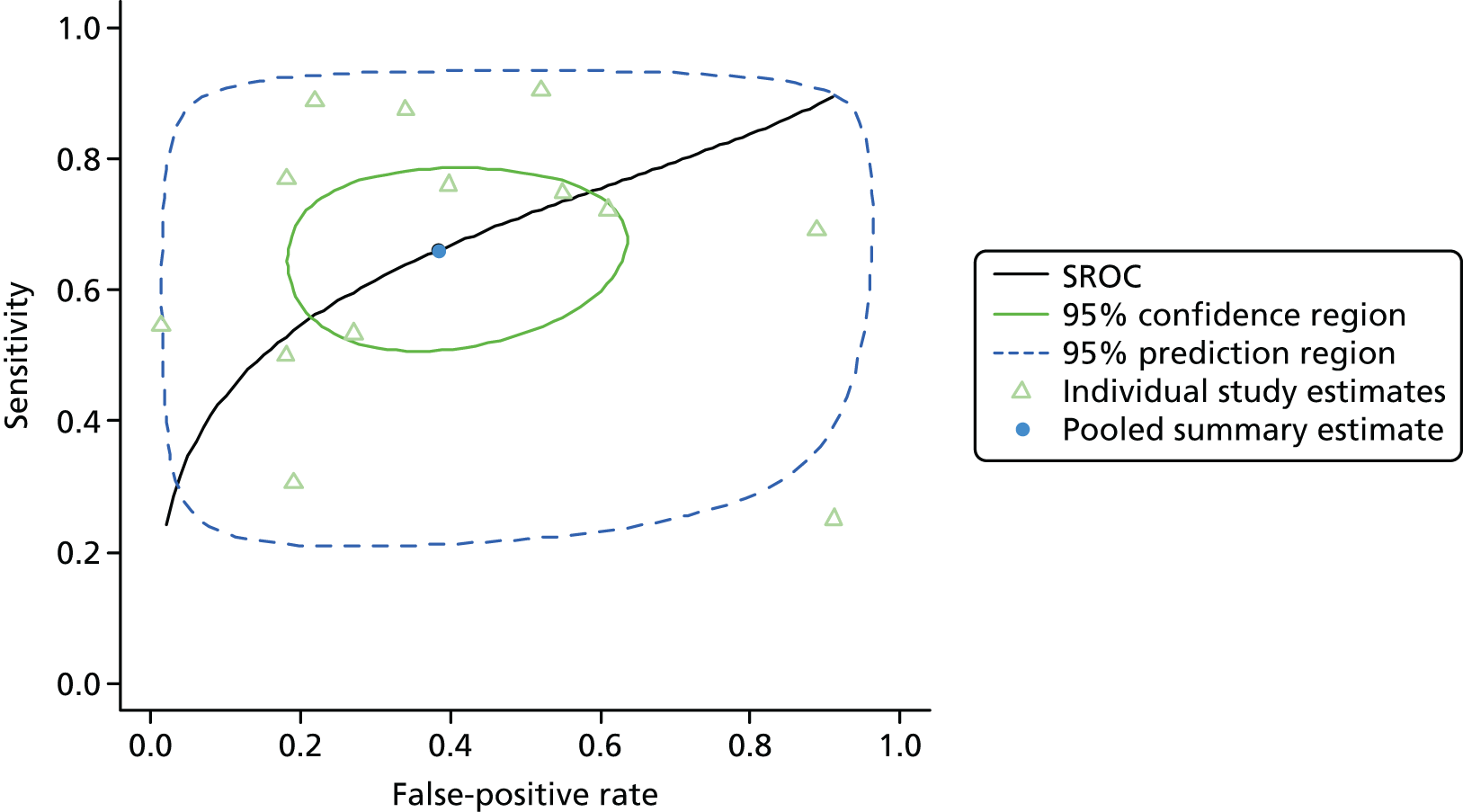
Cystatin C
Critical care unit: plasma
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 28. Three studies were included,32,48,49 with a total of 362 patients [140 patients (38.7%) with a diagnosis of AKI and 222 patients (61.3%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was not consistent across the studies. All of the studies can be considered to have used a similar end point, but there was heterogeneity in the time up to which the outcome assessment occurred (7 days post study entry up to during the hospital stay). Similarly, the threshold used to define a positive test was not consistent across the studies, ranging from 1040 ng/ml48 to 1500 ng/ml. 32 Diagnostic accuracy summaries for the included studies are shown in Table 29.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Kokkoris 201248 | Patients admitted to the critical care unit | Median (IQR): + 63 (50.3, 80.8); – 49 (35.0, 66.3) | Within 13 hours of admission | 1040 | RIFLE (≥ R) within 7 days of admission | 36 | 64 |
| Aydogdu 201332 | Patients admitted to the ICU (without previous history of renal disease) | Mean (SD): + 70 (13) (sepsis); – 66 (10) (no sepsis); – 67 (15) (sepsis) | Daily throughout hospital stay | 1500 | RIFLE (≥ R) during the hospital stay | 63 | 88 |
| Legrand 201549 | Patients admitted to the critical care unit with oliguria (diuresis < 0.5 ml/hour/kg for > 6 consecutive hours) | Median (IQR): + 55 (41, 70); – 55 (41, 70) | At the time of oliguria diagnosis | 1375 | KDIGO (≥ 1) within 7 days of admission | 41 | 70 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Kokkoris 201248 | 22 | 12 | 14 | 52 | 0.611 (0.449 to 0.752) | 0.812 (0.700 to 0.889) | 3.259 (1.838 to 5.779) | 0.479 (0.313 to 0.733) | 6.810 (2.719 to 17.055) |
| Aydogdu 201332 | 46 | 28 | 17 | 60 | 0.730 (0.610 to 0.824) | 0.682 (0.579 to 0.770) | 2.295 (1.632 to 3.226) | 0.396 (0.257 to 0.609) | 5.798 (2.838 to 11.848) |
| Legrand 201549 | 34 | 19 | 7 | 51 | 0.829 (0.687 to 0.915) | 0.729 (0.615 to 0.819) | 3.055 (2.031 to 4.595) | 0.234 (0.118 to 0.467) | 13.038 (4.946 to 34.364) |
Figure 15 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for cystatin C in the critical care unit using patient plasma samples. The pooled sensitivity estimate was 0.72 (95% CI 0.59 to 0.82) and the pooled specificity estimate was 0.74 (95% CI 0.65 to 0.81). Figure 16 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. Examining the forest plot and prediction region suggests that there is greater heterogeneity in sensitivity than in specificity.
FIGURE 15.
Forest plot for studies included in the meta-analysis and pooled estimates for cystatin C in the critical care unit health-care setting using plasma.
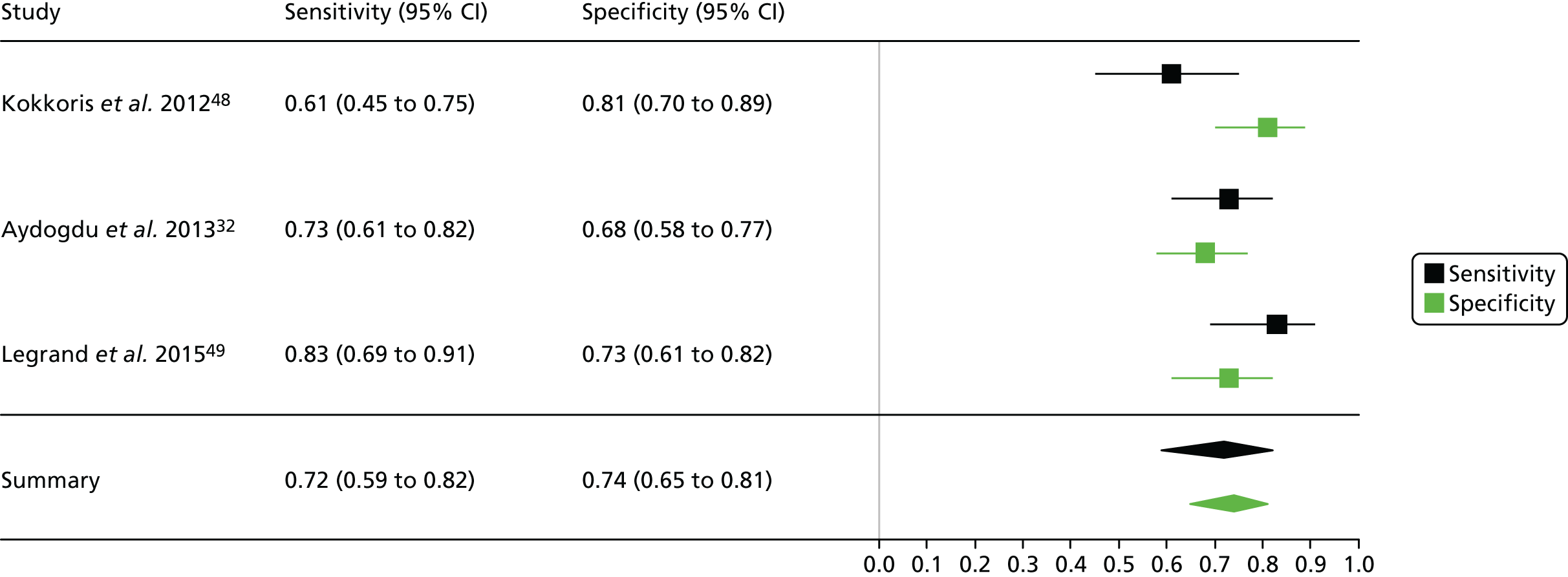
FIGURE 16.
Summary receiver operating characteristic curve for studies included in the meta-analysis for cystatin C in the critical care unit health-care setting using plasma.
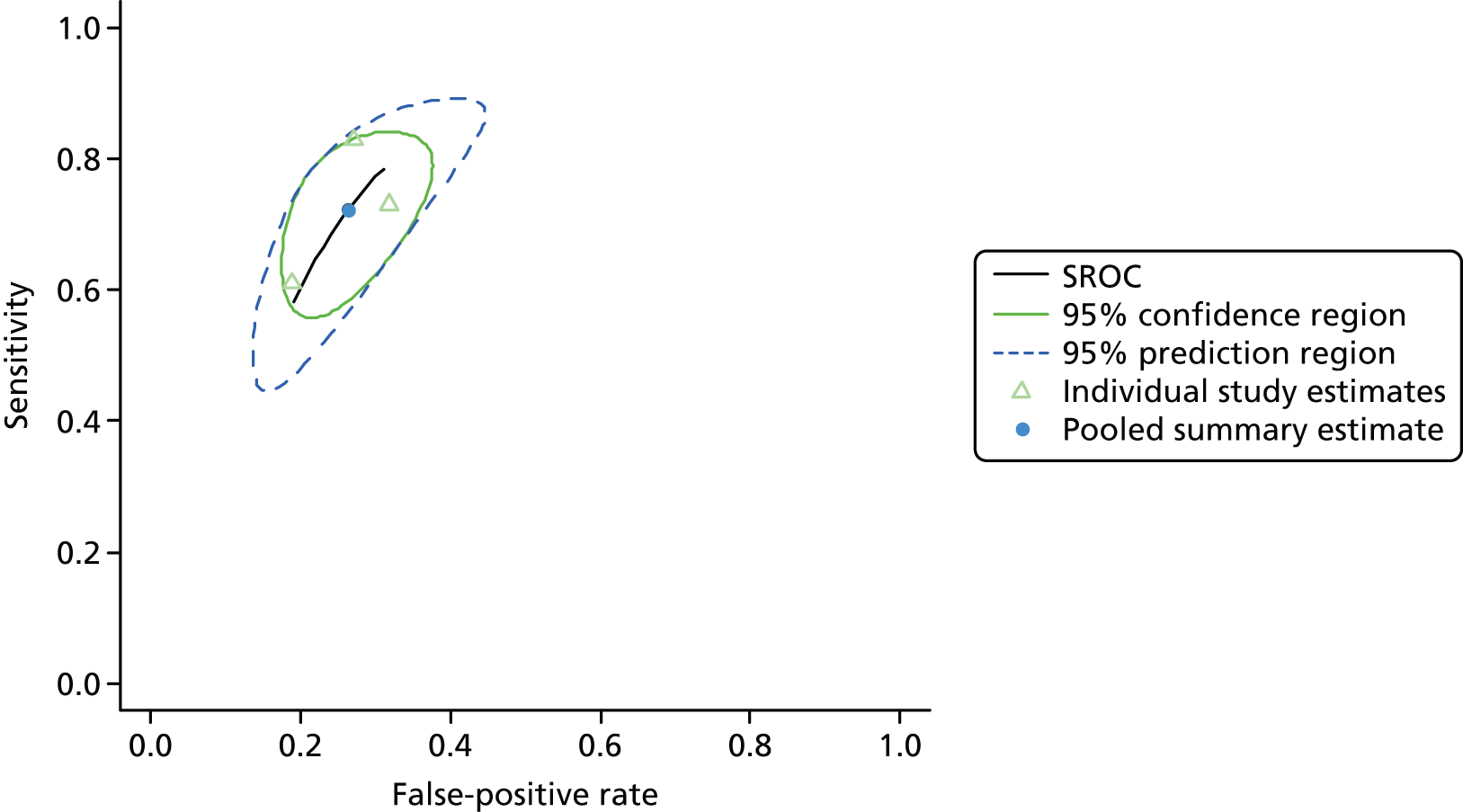
Critical care unit: serum
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 30. Four studies were included,34,42,46,71 with a total of 372 patients [110 patients (29.6%) with a diagnosis of AKI and 262 patients (70.4%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was not consistent across the studies, with one study using a definition that was less serious than the least serious stage of the RIFLE, AKIN or KDIGO classification system46 and one study basing the outcome on continuous urine collection. 71 Similarly, the threshold used to define a positive test was not consistent across all of the studies, ranging from absolute values of 1200 ng/ml46 to 1800 ng/ml34 and with other patient-specific relative thresholds used. Diagnostic accuracy summaries for the included studies are shown in Table 31.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Herget-Rosenthal 200442 | Patients predisposed to acute renal failure in the critical care unit | Mean (SD): + 70 (8); – 63 (11) | On admission | – | RIFLE (≥ R) within 48 hours | 24 | 61 |
| Villa 200571 | Risk of renal failure | Range 21–86 | In the morning | Elevated (no specific information) | Renal dysfunction (SCr < 80 ml/minute/1.73m2 – based on 24-hour urine sample) | 25 | 25 |
| Kato 200846 | All patients scheduled for elective CAG returning to the critical care unit | Range 43–86 | Prior to CAG | 1200 | An increase of > 25% from the baseline SCr value or an absolute increase of at least 0.5 mg/dl within 48 hours | 18 | 69 |
| Chen 201234 | Patients admitted to the critical care unit | Mean (SE): 66 (1) | On admission | 1800 | AKIN (≥ 1) within 48 hours of admission | 43 | 107 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Herget-Rosenthal 200442 | 13 | 3 | 11 | 58 | 0.542 (0.351 to 0.721) | 0.951 (0.865 to 0.983) | 11.014 (3.442 to 35.25) | 0.482 (0.311 to 0.747) | 22.848 (5.572 to 93.70) |
| Villa 200571 | 19 | 2 | 6 | 23 | 0.760 (0.566 to 0.885) | 0.920 (0.750 to 0.978) | 9.500 (2.469 to 36.55) | 0.261 (0.129 to 0.529) | 36.417 (6.575 to 201.7) |
| Kato 200846 | 17 | 10 | 1 | 59 | 0.944 (0.742 to 0.990) | 0.855 (0.753 to 0.919) | 6.517 (3.634 to 11.69) | 0.065 (0.010 to 0.438) | 100.300 (11.976 to 840.0) |
| Chen 201234 | 33 | 10 | 10 | 97 | 0.767 (0.623 to 0.868) | 0.907 (0.836 to 0.948) | 8.212 (4.450 to 15.15) | 0.257 (0.149 to 0.443) | 32.010 (12.239 to 83.72) |
Figure 17 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for cystatin C in the critical care unit using patient serum samples. The pooled sensitivity estimate was 0.76 (95% CI 0.57 to 0.88) and the pooled specificity estimate was 0.91 (95% CI 0.85 to 0.95). Figure 18 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region, which again shows greater heterogeneity in sensitivity than in specificity.
FIGURE 17.
Forest plot for studies included in the meta-analysis and pooled estimates for cystatin C in the critical care unit health-care setting using serum.
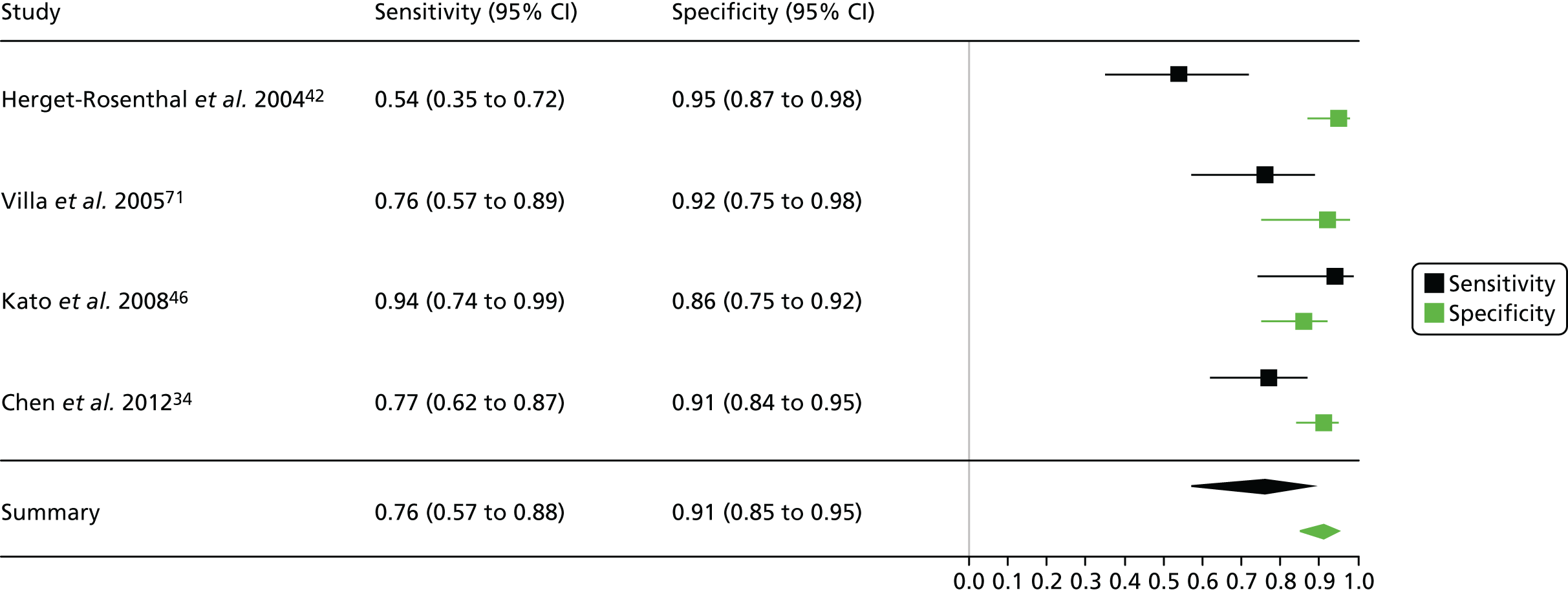
FIGURE 18.
Summary receiver operating characteristic curve for studies included in the meta-analysis for cystatin C in the critical care unit health-care setting using serum.
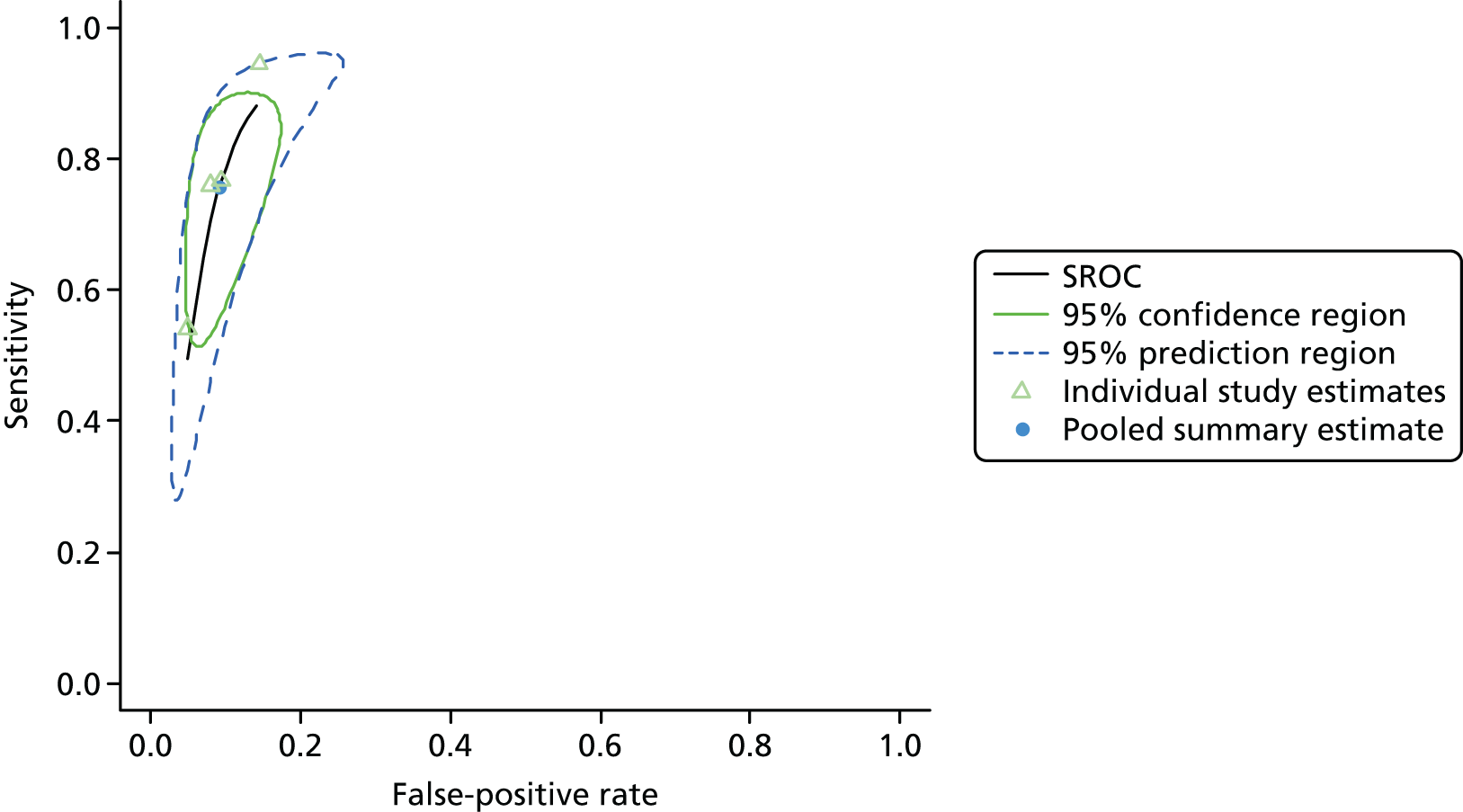
Critical care unit: urine
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 32. Three studies were included,32,34,175 with a total of 745 patients [231 patients (31.0%) with a diagnosis of AKI and 514 patients (69.0%) without a diagnosis of AKI]. The definition of AKI used was fairly consistent across the studies, but the time period within which the outcome was assessed varied from 48 hours to the entire length of the hospital stay. Similarly, the threshold used to define a positive test was not consistent across the studies, ranging from 106 ng/ml32 to 200 ng/ml. 34 Diagnostic accuracy summaries for the included studies are shown in Table 33.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Nejat 2010175 | Patients admitted to the critical care unit | Mean (SD): + 62 (15); – 58 (18) | On admission | 120 | AKIN (≥ 1) within 7 days of admission | 125 | 319 |
| Chen 201234 | Patients admitted to the critical care unit | Mean (SE): 66 (1) | On admission | 200 | AKIN (≥ 1) within 48 hours of admission | 43 | 107 |
| Aydogdu 201332 | Patients admitted to the ICU (without a previous history of renal disease) | Mean (SD): + 70 (13) (sepsis); – 66 (10) (no sepsis); – 67 (15) (sepsis) | Daily throughout the hospital stay | 106 | RIFLE (≥ R) during the hospital stay | 63 | 88 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Nejat 2010175 | 84 | 115 | 41 | 204 | 0.672 (0.586 to 0.748) | 0.639 (0.585 to 0.690) | 1.864 (1.540 to 2.256) | 0.513 (0.394 to 0.668) | 3.634 (2.346 to 5.631) |
| Chen 201234 | 20 | 17 | 23 | 90 | 0.465 (0.325 to 0.611) | 0.841 (0.760 to 0.898) | 2.927 (1.704 to 5.029) | 0.636 (0.476 to 0.850) | 4.604 (2.085 to 10.17) |
| Aydogdu 201332 | 54 | 18 | 9 | 70 | 0.857 (0.750 to 0.923) | 0.795 (0.700 to 0.867) | 4.190 (2.742 to 6.404) | 0.180 (0.097 to 0.332) | 23.333 (9.723 to 55.99) |
Figure 19 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for cystatin C in the critical care unit using patient urine samples. The pooled sensitivity estimate was 0.68 (95% CI 0.43 to 0.86) and the pooled specificity estimate was 0.76 (95% CI 0.62 to 0.86). Figure 20 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. Heterogeneity appears to be considerable and greater in terms of sensitivity than specificity.
FIGURE 19.
Forest plot for studies included in the meta-analysis and pooled estimates for cystatin C in the critical care unit health-care setting using urine.
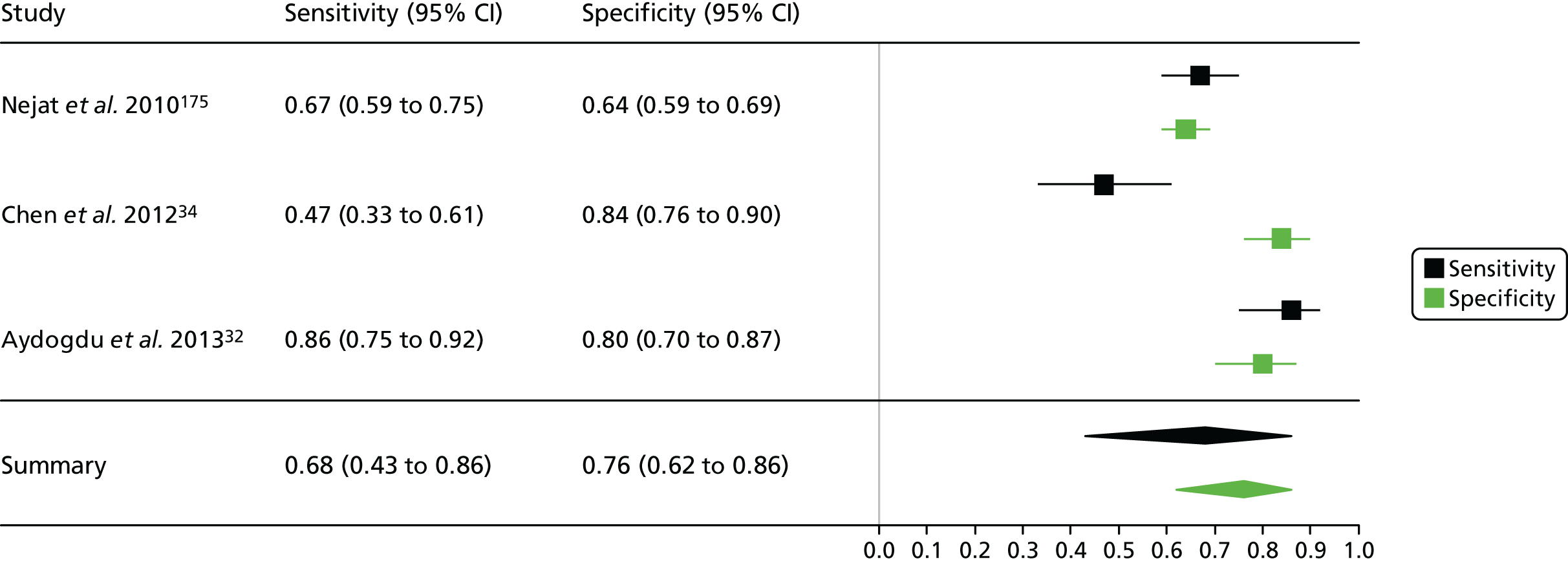
FIGURE 20.
Summary receiver operating characteristic curve for studies included in the meta-analysis for cystatin C in the critical care unit health-care setting using urine.
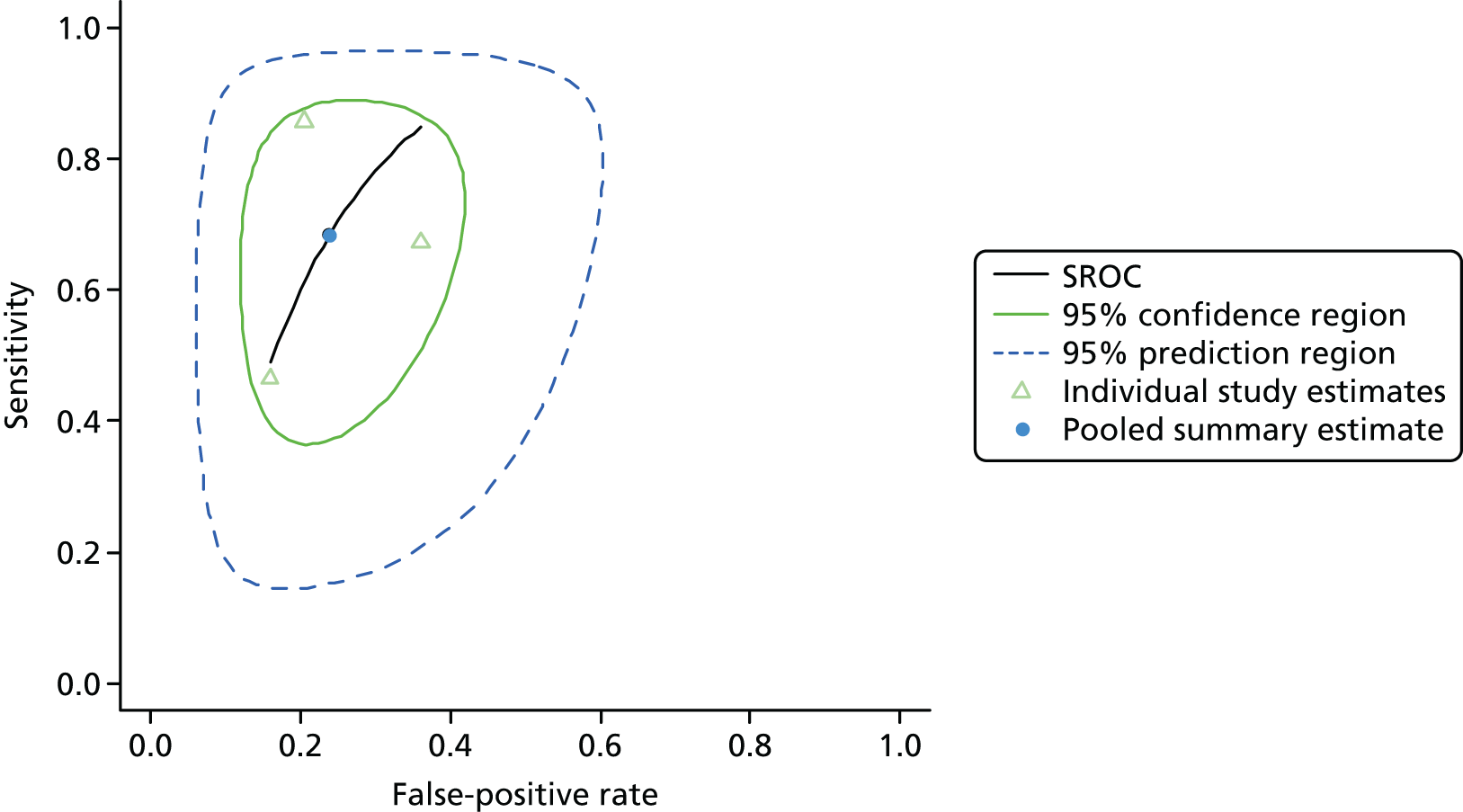
Cardiac surgery: plasma
The searches identified no studies suitable for data extraction for this diagnostic test, setting and sample type.
Cardiac surgery: serum
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 34. Five studies were included,31,38,39,64,68 with a total of 532 patients [147 patients (27.6%) with a diagnosis of AKI and 385 patients (72.4%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was not consistent across the studies, with one study38 using a definition that was less serious than the least serious stage of the RIFLE, AKIN or KDIGO classification system17 and one study basing the outcome on continuous urine collection (from 48 hours up to 4 days post study entry). Similarly, the threshold used to define a positive test was not consistent across the studies, ranging from 0.0265 ng/ml (26.5 pg/ml) to 1100 ng/ml. Diagnostic accuracy summaries for the included studies are shown in Table 35.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Haase 200931 | Patients undergoing cardiac surgery in a tertiary hospital | Mean (SD): + 74.2 (6.9); – 68.3 (10.3) | On arrival in the ICU (6 hours) | 1.1 | AKIN (≥ 1) within 5 days of admission | 46 | 54 |
| Haase-Fielitz 200939 | Patients undergoing cardiac surgery with CPB | Mean (SD): + 75.9 (4.8); – 67.6 (9.9) | On arrival in the ICU (6 hours) | 1100 | AKIN (≥ 1) within 5 days of admission | 23 | 77 |
| Ghonemy 201438 | Patients undergoing CABG surgery or valve replacement | Range: + 39–56; – 32–53 | 3 hours postoperatively | 0.0265 | An increase in SCr either by 25% of the baseline value or by 0.3 mg/dl above the baseline level within 24 hours postoperatively | 17 | 33 |
| Prowle 201564 | Patients undergoing cardiovascular surgery | Median (IQR): ± 70 (61–76) | Immediately postoperatively | 1.24 | RIFLE (≥ R) within 5 days postoperatively | 25 | 68 |
| Tung 201568 | Patients with STEMI receiving PCI | Mean (SD): + 68.14 (12.6); – 61.33 (13.9) | At presentation | 1.6 | AKIN (≥ 1) within 48 hours of admission | 36 | 153 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Haase 200931 | 34 | 18 | 12 | 36 | 0.739 (0.597 to 0.844) | 0.667 (0.534 to 0.778) | 2.217 (1.465 to 3.356) | 0.391 (0.232 to 0.659) | 5.667 (2.379 to 13.50) |
| Haase-Fielitz 200939 | 18 | 11 | 5 | 66 | 0.783 (0.581 to 0.903) | 0.857 (0.762 to 0.918) | 5.478 (3.043 to 9.863) | 0.254 (0.116 to 0.554) | 21.600 (6.646 to 70.20) |
| Ghonemy 201438 | 9 | 9 | 8 | 24 | 0.529 (0.310 to 0.738) | 0.727 (0.558 to 0.849) | 1.941 (0.950 to 3.968) | 0.647 (0.375 to 1.117) | 3.000 (0.884 to 10.18) |
| Prowle 201564 | 19 | 25 | 6 | 43 | 0.760 (0.566 to 0.885) | 0.632 (0.514 to 0.737) | 2.067 (1.411 to 3.028) | 0.380 (0.185 to 0.780) | 5.447 (1.922 to 15.44) |
| Tung 201568 | 28 | 47 | 8 | 106 | 0.778 (0.619 to 0.883) | 0.693 (0.616 to 0.760) | 2.532 (1.885 to 3.401) | 0.321 (0.173 to 0.596) | 7.894 (3.349 to 18.61) |
Figure 21 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for cystatin C in the cardiac surgery setting using patient serum samples. The pooled sensitivity estimate was 0.73 (95% CI 0.65 to 0.80) and the pooled specificity estimate was 0.72 (95% CI 0.63 to 0.79). Figure 22 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. The studies show limited evidence of heterogeneity, with a greater degree of heterogeneity with respect to specificity than sensitivity.
FIGURE 21.
Forest plot for studies included in the meta-analysis and pooled estimates for cystatin C in the cardiac surgery health-care setting using serum.
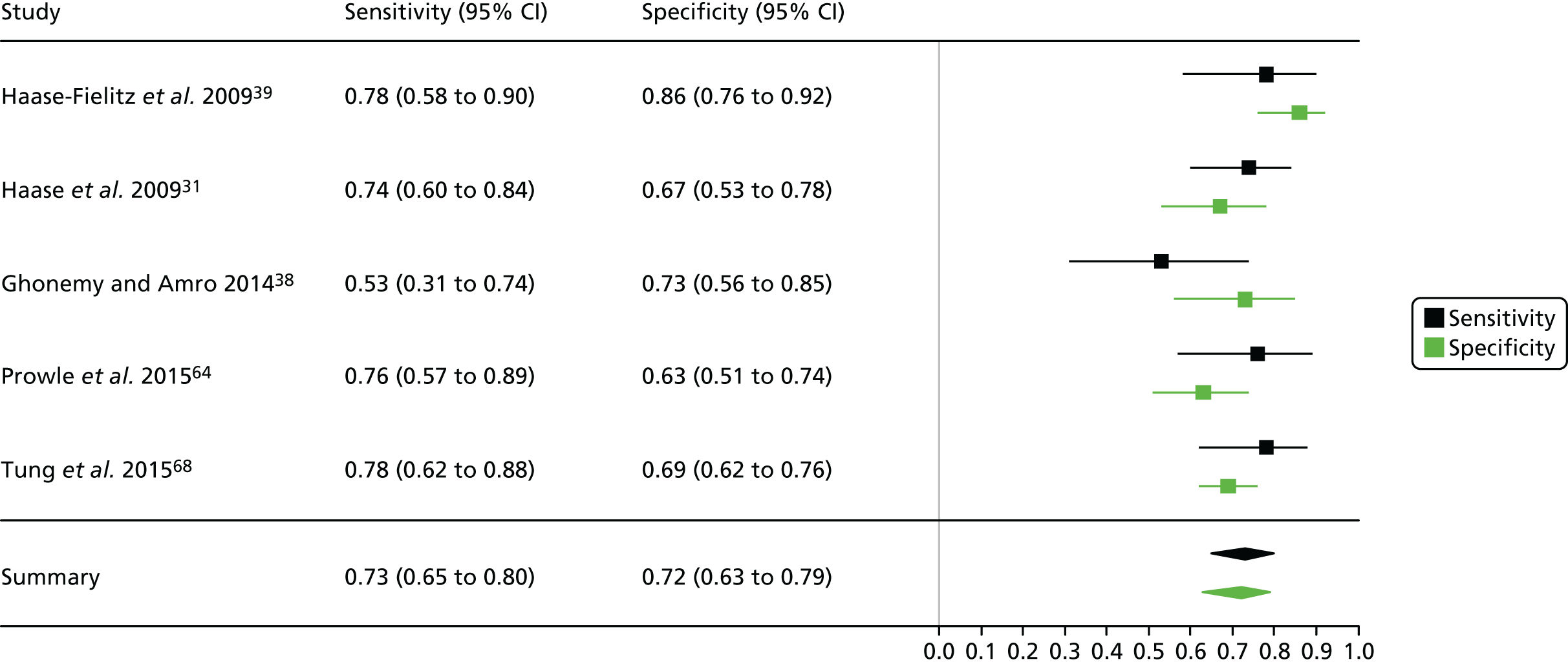
FIGURE 22.
Summary receiver operating characteristic curve for studies included in the meta-analysis for cystatin C in the cardiac surgery health-care setting using serum.
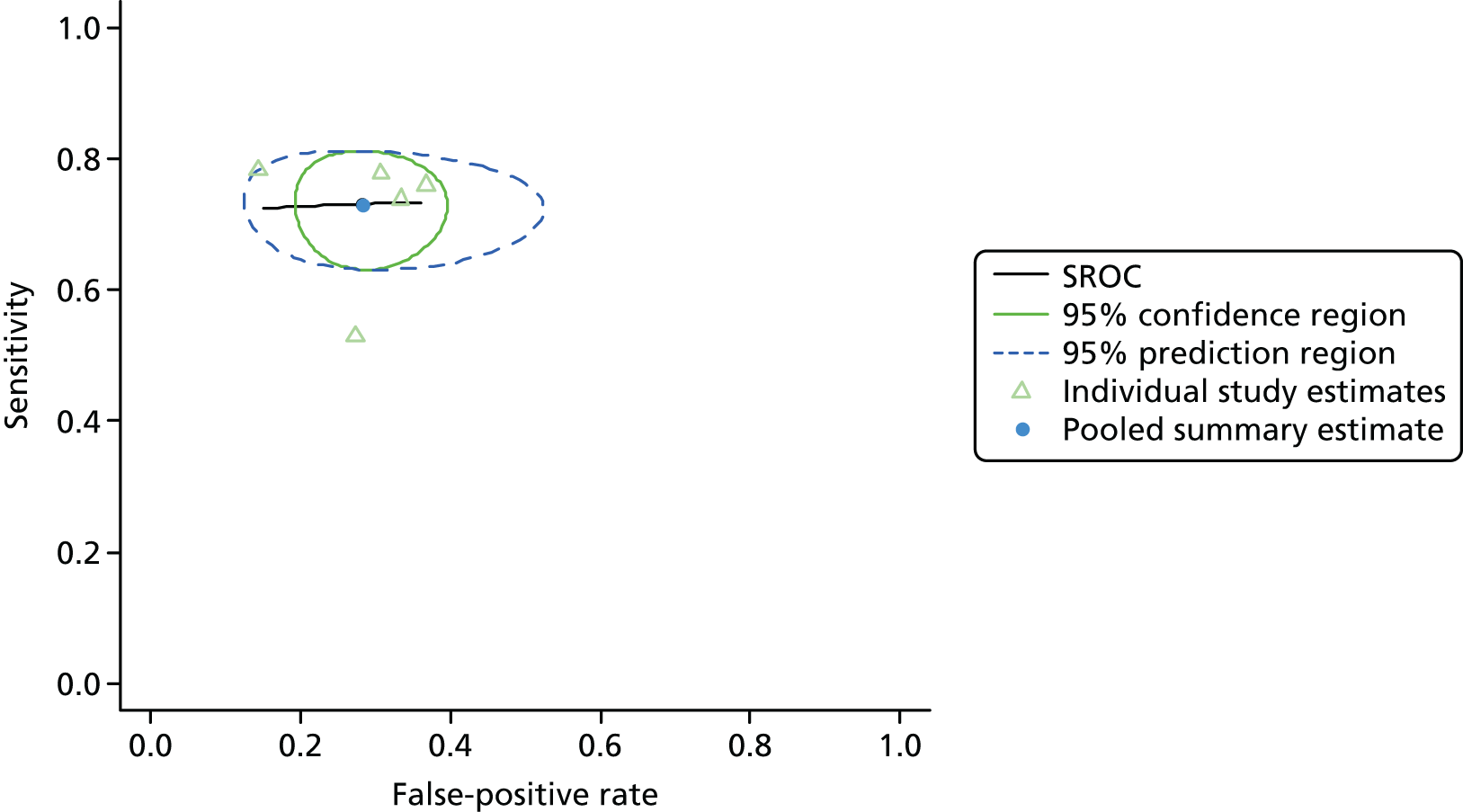
Cardiac surgery: urine
Summaries of the baseline characteristics and test parameters for the included studies are shown in Table 36. Two studies were included,50,69 with a total of 908 patients [131 patients (14.4%) with a diagnosis of AKI and 777 patients (85.6%) without a diagnosis of AKI]. The outcome used to define the presence of AKI was similar in both studies, although the time frame over which the outcome was assessed varied by 24 hours. The threshold used to define a positive test was not consistent across the studies, with one study69 not clearly reporting the threshold used. Diagnostic accuracy summaries for the included studies are shown in Table 37.
| First author and year | Patient group | Age (years) | Timing of sample | Threshold (ng/ml) | Outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| + | – | ||||||
| Liangos 200950 | Patients undergoing on-pump cardiovascular surgery | Mean (SD): + 73 (9); – 67 (12) | 2 hours post surgery | 192 ng/mg of creatinine | RIFLE (≥ R) within 72 hours postoperatively | 13 | 90 |
| Tziakas 201569 | Patients admitted with spontaneous (type 1) AMI undergoing cardiovascular surgery | Mean (SD): ± 62 (13) | During admission | Unclear | AKIN (≥ 1) within 48 hours postoperatively | 118 | 687 |
| First author and year | TP, n | FP, n | FN, n | TN, n | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Liangos 200950 | 5 | 13 | 8 | 77 | 0.385 (0.177 to 0.645) | 0.856 (0.768 to 0.914) | 2.663 (1.136 to 6.241) | 0.719 (0.464 to 1.115) | 3.702 (1.047 to 13.083) |
| Tziakas 201569 | 76 | 309 | 42 | 378 | 0.644 (0.554 to 0.725) | 0.550 (0.513 to 0.587) | 1.432 (1.223 to 1.676) | 0.647 (0.503 to 0.832) | 2.214 (1.475 to 3.321) |
Figure 23 shows point estimates of the sensitivity and specificity from individual studies and the pooled estimates for cystatin C in the cardiac surgery setting using patient serum samples. The pooled sensitivity estimate was 0.52 (95% CI 0.27 to 0.76) and the pooled specificity estimate was 0.72 (95% CI 0.36 to 0.92). Figure 24 shows an estimate of the SROC curve with the 95% confidence region and 95% prediction region. Examining the forest plot and prediction region in the SROC curve suggests that there is considerable heterogeneity between the studies in terms of sensitivity and specificity.
FIGURE 23.
Forest plot for studies included in the meta-analysis and pooled estimate for cystatin C in the cardiac surgery health-care setting using urine.
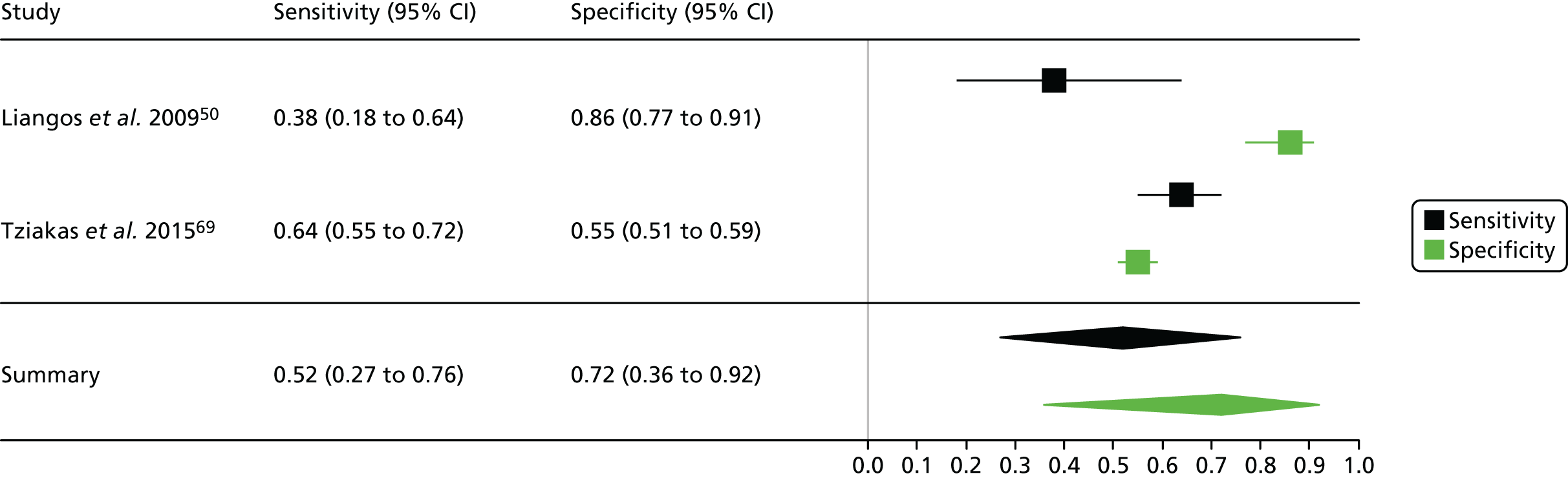
FIGURE 24.
Summary receiver operating characteristic curve for studies included in the meta-analysis for cystatin C in the cardiac surgery health-care setting using urine.
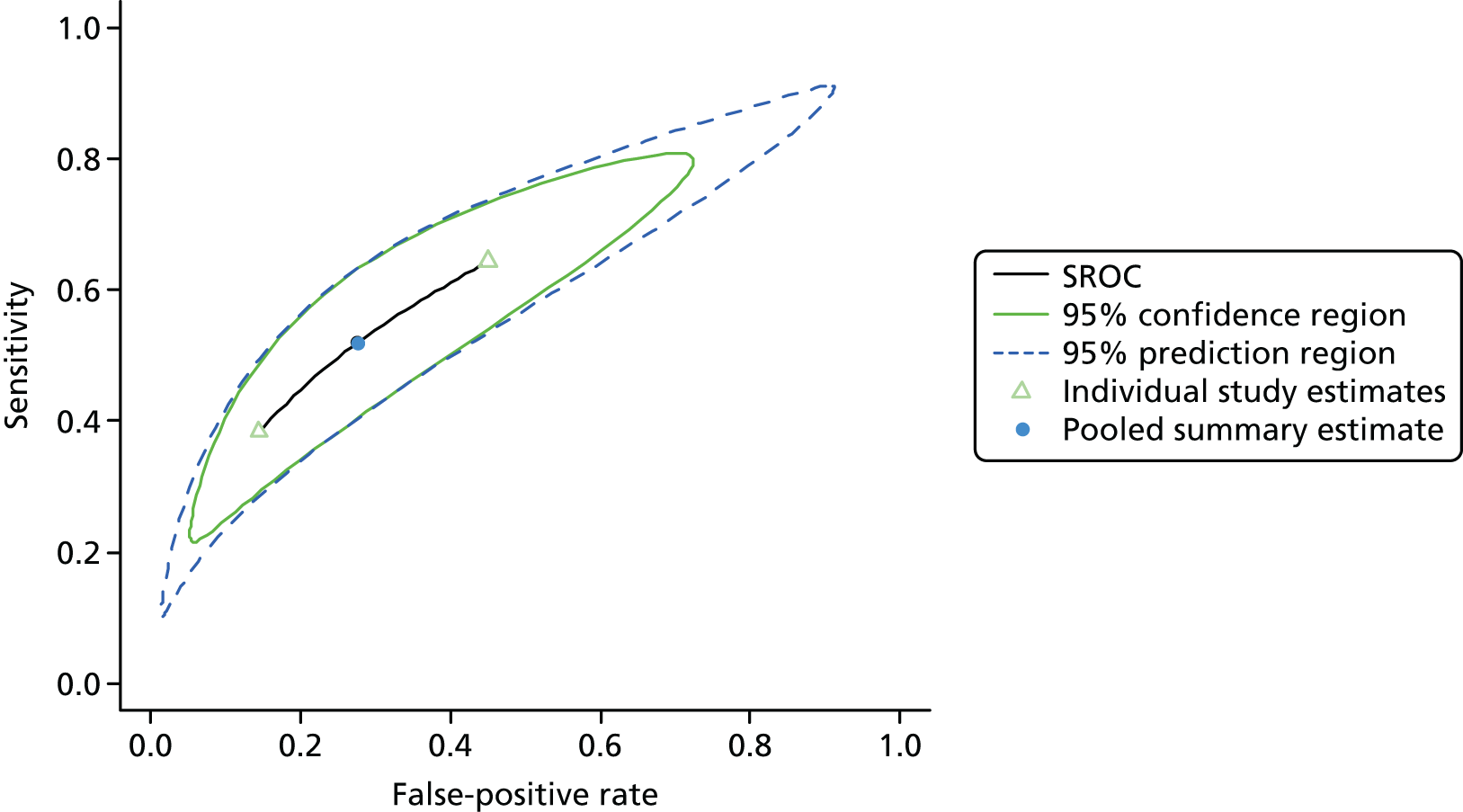
Limitations
A limitation of this work is the use of a number of similar criteria for diagnosing AKI based on the measurement of serum creatinine and urine output rather than a more direct and accurate determination of kidney function and injury. Criteria based on serum creatinine lack real-time sensitivity for kidney injury, as creatinine concentration has a slow rate of change and is affected by other factors such as sex and muscle mass. Current standard AKI criteria based on serum creatinine and urine output measures therefore represent an imperfect reference test for the early detection of AKI. Each of the studies considered in these meta-analyses used criteria based on changes in serum creatinine concentrations and is therefore affected equally by this limitation.
The method of meta-analysis used in this analysis is recommended by the Cochrane Screening and Diagnostic Tests Methods Group. 262 However, a similar method in the Bayesian paradigm, which has shown to be equivalent in simple cases, may have been a reasonable alternative. 259 Furthermore, this fully Bayesian approach has the advantage of potentially unifying the sensitivity analyses (assessment of uncertainty in the estimates in decision analysis) with the modelling step and of allowing predictions of test accuracies in future trials through a posterior predictive distribution. The hierarchical model approach may also have been flexible enough to include further modelling aspects related to analytical and biological variance of the diagnostic tests considered in this work, if enough studies were included to reasonably estimate models. This is not possible using the simple bivariate random-effects meta-analysis method and is a possible limitation of this work. Further work will consider in more detail the possibility of extending the hierarchical model to include these aspects and investigate if the hierarchical model can be estimated in meta-analyses of this size. Extension of this model that allows for imperfect gold reference standards may also be worthy of further investigation. 263
There is evidence of considerable heterogeneity in some of the included studies, which is clearly observed in the large prediction regions in the SROC space. Two of the sources of heterogeneity are the outcome measures used and the time within which the outcome is assessed. As mentioned earlier, a limitation of this work is the use of criteria based on measurement of serum creatinine and urine output rather than a more direct determination of kidney function. If more studies had provided data, meta-regression may have been useful for isolating and quantifying some of these sources of heterogeneity further. Further investigations may be conducted into modelling these sources of heterogeneity as part of future work described above related to investigating the possible extension of the hierarchical regression models.
Summary
A number of the diagnostic tests for AKI considered in these meta-analyses may have a role to play in certain health-care settings and using particular sample media. The Nephrocheck test using urine in the critical care unit setting appears overall to have the best sensitivity, albeit with low specificity. The estimates of sensitivity are high and there is low heterogeneity. The NGAL test using plasma shows moderate sensitivity and high specificity, but greater heterogeneity. Other health-care settings and sample types show evidence of considerable heterogeneity between studies. Two studies that were included in previously published NGAL meta-analyses were excluded here as they included patients who originated in the emergency room and who were subsequently released to other hospital departments. 245,264
Chapter 4 Measurement performance: a framework for the Quality Assessment of Measurement Procedures using in vitro diagnostic medical devices in clinical research
Introduction
It is generally accepted that between 60% and 80% of clinical decisions are influenced by the results of laboratory testing using IVDs. 265 A large proportion, although not all, of this testing is focused on the quantitative or semiquantitative measurement of biomarkers in patient samples. The accuracy and associated uncertainty of these measurements can have a major impact on the overall quality of clinical decisions and their subsequent clinical effectiveness and cost-effectiveness. 266,267
The main factors affecting measurement uncertainty are shown in Figure 25. Laboratories and regulators have historically focused on analytical factors associated with the measurement system, including analytical imprecision and trueness (accuracy). However, it is increasingly accepted that the major sources of error (uncertainty) associated with biomarker measurements are derived from either the patients themselves or the acquisition of their samples, referred to respectively as biological and pre-analytical factors. 268,269 The uncertainty introduced by these factors accumulates throughout the measurement system, eventually affecting test performance, such as diagnostic accuracy (Figure 26).
FIGURE 25.
Feather diagram depicting sources of uncertainty contributing towards the measurement uncertainty (UM).
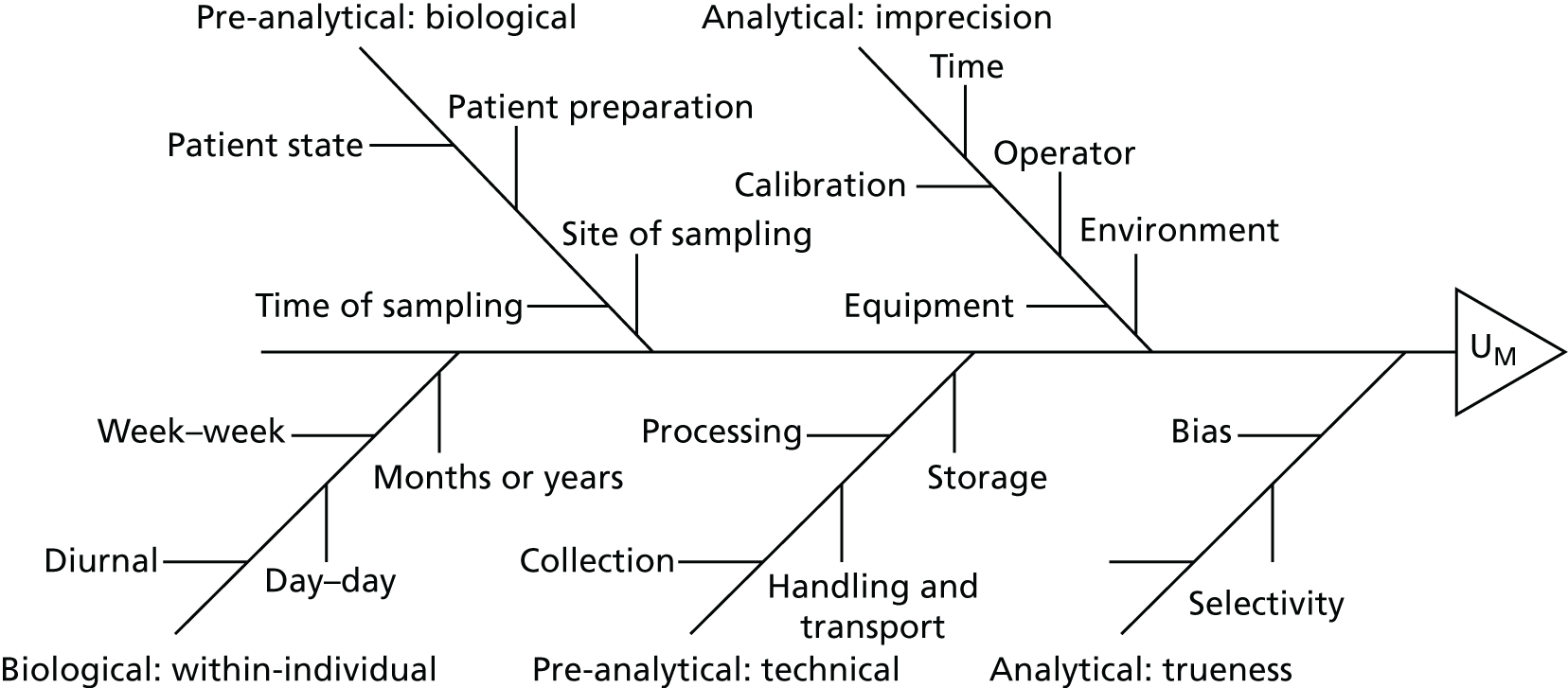
FIGURE 26.
Gaussian bell curves illustrating the interdependency of analytical uncertainty (UA), pre-analytical uncertainty (UPA), within-individual biological variation (CVwi) and within-group variation (CVG1 and CVG2). As previously illustrated by Rorass et al. 270
Although there has been significant progress in characterising and controlling for measurement factors in clinical care, they are almost always poorly accounted for in clinical research. 271 This has led to recent criticisms and calls for more rigorous methodology in the field. 272
Measurement factors can impact negatively on clinical studies and trials as follows:
-
Risk of bias. Subtle differences in measurement procedures between study arms can introduce systematic errors between groups of patients, which may be mistakenly interpreted as clinically relevant differences. 273 For example, a 2002 Lancet publication described a mass spectrometry-based proteomic signature for diagnosing ovarian cancer, with almost 100% sensitivity and specificity. 274 However, after the results proved difficult to reproduce, an independent analysis concluded that ‘procedural differences’ between the groups was the most likely explanation for the results. 275 Later studies have confirmed that mass spectrometry-based proteomic signatures are profoundly affected by pre-analytical sample-handling conditions, potentially leading to significant experimental bias. 276
-
Concerns regarding reproducibility. Differences in measurement procedures can introduce excessive variability between studies. This is a particular issue for systematic reviews and meta-analysis, in which reviewers do not generally take into account the robustness of measurement procedures or differences between studies. For example, evidence on the clinical validity and utility of the biomarker vascular endothelial growth factor (VEGF) has been complicated by differences in pre-analytical procedures between studies. 277,278
-
Concerns regarding the applicability of research findings to clinical practice. Often for reasons of pragmatism or cost, measurement procedures performed within a clinical study may differ substantially from those employed in clinical care. For example, it is not uncommon for trial samples to be frozen and analysed within a single batch. Although this reduces the study variance and makes a significant finding more likely, the results may not translate to clinical practice, where variability is usually higher as samples are measured over many days and using different batches of reagents. The effect of freeze–thawing may also introduce a systematic increase or decrease in biomarker concentration, invalidating clinical cut-off points and leading to a higher FP/FN rate in the clinic.
Although the updated STARD 2015251 and QUADAS-230 documents both address important methodological issues concerning studies of diagnostic accuracy, neither of them address the issues associated with measurement. Furthermore, although several reporting guidelines have been produced in specific areas of the field,279–281 we are not aware of any methods in use for evaluating the quality of measurement procedures within clinical studies. We suggest that this is limiting the ability of systematic reviewers and health technology assessors to fully evaluate risk and to model uncertainty within assessments. This has been highlighted in several recent NICE diagnostic assessment reports. 282–284
Development of a framework for the Quality Assessment of Measurement Procedures
A framework for the Quality Assessment of Measurement Procedures (QAMPs) was developed within this study. An initial framework was constructed through consultation with experts and this was subsequently tested and refined. The final framework was then validated and its utility explored by applying it to the literature included in the meta-analysis for Nephrocheck (see Chapter 3).
Three medical laboratory professionals (Rebecca Kift, Leeds Teaching Hospitals NHS Trust; Ashley Garner, Leeds Teaching Hospitals NHS Trust; Catherine Sturgeon, NHS Lothian) were consulted concerning the scope and parameters for inclusion. The group agreed that the scope should be limited to ‘in vitro diagnostic medical devices’ as the requirements for non-IVDs (e.g. imaging devices or devices for taking physical or clinical measurements) may differ. The defining features of ‘quality’ with respect to measurement procedures were agreed as ‘bias’, ‘reproducibility’ and ‘applicability’. It was agreed that parameters associated with biological within-individual variation, biological pre-analytical factors (also known as pre-pre-analytical factors), technical pre-analytical variation factors (also known simply as pre-analytical factors) and analytical factors would be included within the quality assessment framework. Existing standards and reviews in these areas were collated and reviewed to identify best practice in the field of IVD metrology. Once parameters had been identified, data extraction fields were created to capture the information required by a reviewer to quality assess each parameter within a research publication. The standards and guidelines identified and used are shown in Table 38.
| Organisation | Standard or guidance document | Type of uncertainty |
|---|---|---|
| ISO | BS EN ISO 15189:2012 Medical laboratories. Requirements for quality and competence285 | Pre-analytical and analytical |
| IFCC | Quality indicators in laboratory medicine: a fundamental tool for quality and patient safety286 | Pre-analytical and analytical |
| NIHR | RIPOSTE287 | Pre-analytical and analytical |
| EQUATOR | Biospecimen Reporting for Improved Study Quality (BRISQ)280 | Pre-analytical |
| EFLM | Standardization of collection requirements for fasting samples288 | Pre-analytical |
| EFLM | Preanalytical quality improvement. In pursuit of harmony289 | Pre-analytical |
| CLSI | H3-A6 Procedures for the Collection of diagnostic blood specimens by venepuncture290 | Pre-analytical |
| CLSI | H18-A4 Procedures for the handling and processing of blood samples291 | Pre-analytical |
| CLSI | GP16-A3 Urinalysis292 | Pre-analytical |
| ISBER | Pre-Analytical variables affecting the integrity of human biospecimens in biobanking293 | Pre-analytical |
| EQUATOR | STROBE-ME281 | Biological, pre-analytical and analytical |
| NACB | Tumor Marker Quality Requirements Guidelines294 | Biological, pre-analytical and analytical |
| EFLM | A checklist for critical appraisal of studies of biological variation279 | Biological |
| ISO | 17511:2003 In vitro diagnostic medical devices295 | Analytical |
| ISO | 13612:2002 Performance evaluation of in vitro diagnostic medical devices296 | Analytical |
| ISO | BS ISO 5725:1994 Accuracy (trueness and precision) of measurement methods and results297 | Analytical |
| ACB | Measurement verification in the clinical laboratory298 | Analytical |
| CLSI | EP32-R Metrological Traceability and Its Implementation19 | Analytical |
| CLSI | EP26-A User Evaluation of Between-Reagent Lot Variation20 | Analytical |
| CLSI | EP25-A Evaluation of Stability of In Vitro Diagnostic Reagents21 | Analytical |
| CLSI | EP21-A Estimation of Total Analytical Error for Clinical Laboratory Methods22 | Analytical |
| CLSI | EP15-A3 User Verification of Precision and Estimation of Bias23 | Analytical |
| CLSI | EP09-A3 Measurement Procedure Comparison and Bias Estimation Using Patient Samples24 | Analytical |
| CLSI | EP07-A2 Interference Testing in Clinical Chemistry25 | Analytical |
| CLSI | EP05-A3 Evaluation of Precision of Quantitative Measurement Procedures26 | Analytical |
| CLSI | I/LA30-A Immunoassay Interference by Endogenous Antibodies27 | Analytical |
| CLSI | EP14-A2 Evaluation of Matrix Effects28 | Analytical |
| FDA | Bioanalytical Method Validation18,299 | Analytical |
| LGC | Evaluating measurement uncertainty in clinical chemistry300 | Analytical |
| CLSI | Expression of Measurement Uncertainty in Laboratory Medicine29 | Analytical |
| AAPS | Fit-for-Purpose Method Development and Validation for Successful Biomarker Measurement301 | Analytical |
| AAPS | Recommendations for the Bioanalytical Method Validation of Ligand-binding Assays302 | Analytical |
The initial framework for assessing the quality of measurement procedures was developed and further refined through an iterative process of testing, consultation and updating. Additional fields were included by the group if they were thought to be beneficial to the process. The nomenclature ‘low risk’, ‘high risk’ and ‘unclear’ was adopted for rating quality criteria, as used by The Cochrane Collaboration303 and in the QUADAS-2 tool. 30 The QAMPs data extraction and quality assessment framework is shown in Table 39.
| Citation | ||||||
|---|---|---|---|---|---|---|
| Authors: | ||||||
| Title: | ||||||
| Journal: | Year: | Pages: | ||||
| Vol.: | ||||||
| Description of the measurement procedure | ||||||
| Features | Index | Reference | ||||
| Name of analyte | ||||||
| Test name | ||||||
| Test platform/method used | ||||||
| Manufacturer | ||||||
| Sample matrix used [e.g. urine, serum, plasma (including type)] | ||||||
| Pre-analytical biological | ||||||
| Patient state (e.g. fed/fasted, sitting/standing, rested) | ||||||
| Patient preparation | ||||||
| Anatomical site and/or mechanism | ||||||
| Time of sampling (e.g. before 0900, within 1 hour of reference test) | ||||||
| Pre-analytical technical | ||||||
| Sample collection (mechanism and use of stabilisation) | ||||||
| Preprocessing handling, temperature, transport and time | ||||||
| Sample processing (e.g. preservation, centrifugation conditions, timings and temperature) | ||||||
| Storage (e.g. volume, temperature, duration, freeze–thaw cycles) | ||||||
| Postprocessing handling and transport | ||||||
| Consideration of differences between groups | ||||||
| Standard operating procedures or quality assurance | ||||||
| Other | ||||||
| Analytical factors | ||||||
| Sample blinding procedure | ||||||
| Sample randomisation procedure | ||||||
| Batching procedure | ||||||
| Reference control materials | ||||||
| Quality assurance procedures | ||||||
| Patient inclusion/exclusion criteria | ||||||
| Test failure rate and reasons for test failure | ||||||
| Technical replication | ||||||
| Performance evaluation | ||||||
| Performance goals for: | ||||||
| Precision | ||||||
| Bias | ||||||
| (method of calculation) | ||||||
| Within-individual biological variation | ||||||
| Pre-analytical factors | ||||||
| Total measurement uncertainty | ||||||
| Analytical validation | ||||||
| Full validation or verification | ||||||
| Analytical sensitivity: | ||||||
| Method (brief description) | ||||||
| Limit of blank (LOB) | ||||||
| Limit of detection (LOD) | ||||||
| Limit of quantitation (LOQ) | ||||||
| Analytical selectivity: | ||||||
| Method (brief description) | ||||||
| Cross-reactivity | ||||||
| Interference | ||||||
| Carry-over | ||||||
| Trueness: | ||||||
| Method (brief description) | ||||||
| Bias | ||||||
| Precision: | ||||||
| Method (brief description including M-Factors: time, calibration, operator and equipment) | ||||||
| Repeatability (range), CV% | ||||||
| Intermediate Imprecision (range), CV% | ||||||
| Reproducibility (range), CV% | ||||||
| Linearity and working range: | ||||||
| Method (brief description) | ||||||
| Other (e.g. lot to lot, antibody validation profile) | ||||||
| Signalling questions | ||||||
| Were measurement procedures different between groups? | ||||||
| Were measurement procedures described in enough detail to be repeated? | ||||||
| Were measurement factors appropriately controlled for? | ||||||
| Were measurement procedures applicable to the final clinical setting? | ||||||
| Risk of bias (high, low, unclear) | ||||||
| Risk of irreproducibility (high, low, unclear) | ||||||
| Risk of inapplicability (high, low, unclear) | ||||||
To initially validate the QAMPs template and demonstrate its utility, two reviewers extracted and quality appraised the literature included in the meta-analysis for Nephrocheck from Chapter 2.
Testing the Quality Assessment of Measurement Procedures framework using Nephrocheck as a case study
In total, four studies were included in the quality assessment, as identified from the searches reported in Chapter 2. Studies were first data extracted and then supporting evidence was collated while answering the signalling questions. Finally, a quality judgement was reached using the supporting evidence as the basis for transparency and discussion. When evidence was identified, risk was judged as being either high or low; only when there was insufficient evidence was risk judged as being uncertain. The results of the signalling questions are presented in Box 1 and the quality assessment is summarised in Table 40. Full results from the data extraction are presented in Appendix 7.
Were measurement procedures different between groups?
-
Uncertain. Pre-analytical and analytical study procedures were not reported in enough detail to be confident that bias had been avoided, for example no details were reported concerning sample blinding, randomisation and batching.
-
In some patients, baseline creatinine level was used rather than creatinine level at time of enrolment; it is not clear if this was systematically different between patient groups.
Were measurement procedures described in enough detail to be repeated?
-
Limited details were provided concerning the Nephrocheck test; not enough details were provided to repeat the study.
-
Almost all parameters required to repeat the serum creatinine reference test were not described, even the name and manufacturer of the assay.
Were measurement factors appropriately controlled for?
-
No. Albumin, bilirubin and methylene blue are known Nephrocheck interferents, which were not controlled for in the urine samples.
-
Quality control procedures were not reported for the Nephrocheck test or for the creatinine reference test.
-
The method and traceability of the reference test were not described.
-
Performance characteristics of the reference test and index test were not described.
-
It is unclear whether the measurement systems were performing as specified by the manufacturer as no internal verification was reported.
-
No performance goals were reported.
Were measurement procedures applicable to the final clinical setting?
-
Unclear as the study procedures not described in enough detail.
-
Samples were frozen and thawed prior to measurement in the study, whereas samples are likely to be analysed immediately (fresh) in the acute clinical context. No data were available on freeze–thaw cycles, but the manufacturer suggests avoiding repeated freezing and thawing.
Were measurement procedures different between groups?
-
No, but it is not clear whether samples were measured randomly and/or in batches, which may have introduced a systematic bias.
Were measurement procedures described in enough detail to be repeated?
-
The urinary Nephrocheck test was generally reported in enough detail to be repeated.
-
Several parameters required to repeat the serum creatinine reference test were not described.
Were measurement factors appropriately controlled for?
-
No, the manufacturer’s kit insert identifies albumin and bilirubin as interferents and recommends ‘caution in interpreting Nephrocheck® results in patients with significant proteinuria or severe hyperbilirubinuria’. 304 Albumin (proteinuria and haematuria) and bilirubin (to a lesser extent) are associated with AKI. No attempt was made to identify and exclude these samples.
-
Quality control procedures were not reported for the creatinine reference test.
-
No validation or verification of the measurement systems was reported.
-
No performance goals were reported.
Were measurement procedures applicable to the final clinical setting?
-
No, measurements were conducted at three sites and the median of three measurements was used to determine diagnostic accuracy. Only a single measurement at a single site would be used in clinical practice, which may lead to less precise measurements.
-
Samples were freeze–thawed prior to measurement in the study, whereas samples are likely to be analysed immediately (fresh) in the acute clinical context. No data were available on freeze–thaw cycles, but the manufacturer suggests avoiding repeated freezing and thawing.
Were measurement procedures different between groups?
-
Uncertain. Pre-analytical and analytical study procedures were not reported in enough detail to be confident that systematic bias between groups had been avoided, for example no details were reported concerning sample randomisation and batching, although laboratory investigators were blinded to clinical outcomes.
-
Index and reference test samples were collected at different times; it was unclear whether this might introduce bias.
Were measurement procedures described in enough detail to be repeated?
-
No, very limited data were provided concerning Nephrocheck and only the analyte name, matrix and time points were provided for creatinine.
Were measurement factors appropriately controlled for?
-
No. Albumin, bilirubin and methylene blue are known Nephrocheck interferents, which were not controlled for in the urine samples.
-
Quality control procedures were not reported for either the index or the reference test.
-
The method and traceability of the reference test were not described.
-
Performance characteristics of the index test and reference test were not described.
-
It is unclear whether the measurement systems were performing as specified by the manufacturer as no internal verification was performed.
-
No performance goals were reported.
Were measurement procedures applicable to the final clinical setting?
-
No. Test was performed in patients aged < 18 years, which contradicts the instructions for use.
-
Samples were frozen and thawed prior to measurement in the study, whereas samples are likely to be analysed immediately (fresh) in the acute clinical context. No data were available on freeze–thaw cycles, but the manufacturer suggests avoiding repeated freezing and thawing.
Were measurement procedures different between groups?
-
Pre-analytical procedures were described in adequate detail and did not appear to differ between patient groups.
-
Although laboratory investigators were blinded to clinical outcomes, it is unclear whether samples were batched or randomised for analysis.
-
Index and reference test samples were collected at different times; it was unclear whether this might introduce bias.
Were measurement procedures described in enough detail to be repeated?
-
The urinary Nephrocheck test was reported in enough detail to be repeated, although it is not clear whether the measurements were performed in a single batch and randomised.
-
In addition, it is not clear how long samples were frozen for.
-
Several parameters required to repeat the serum creatinine reference test were not described.
Were measurement factors appropriately controlled for?
-
No; albumin, bilirubin and methylene blue are known Nephrocheck interferents, which were not controlled for in the urine samples. The manufacturer’s kit insert recommends ‘caution in interpreting Nephrocheck® results in patients with significant proteinuria or severe hyperbilirubinuria’. 304
-
Quality control procedures were not reported for the creatinine reference test. The method and traceability of the reference test were not described.
-
It is unclear whether the measurement systems were performing as specified by the manufacturer as no internal verification was performed.
-
It is also not clear whether the samples were processed within 1 hour of collection.
-
Performance characteristics and goals of the reference test and index test were not described.
Were measurement procedures applicable to the final clinical setting?
-
Unclear; samples were freeze–thawed prior to measurement in the study, whereas samples are likely to be analysed immediately (fresh) in the acute clinical context. No data were available on freeze–thaw cycles, but the manufacturer suggests avoiding repeated freezing and thawing.
Discussion
Quality assessment is now recognised as an essential component of systematic review and health technology assessment because of the marked heterogeneity between studies. Initiatives such as QUADAS305 and QUADAS-230 have been routinely adopted into these processes and have proven useful in identifying studies that are at high risk of bias or inapplicability. Similarly, the introduction of reporting guidelines such as STARD243 and STARD 2015251 have improved reporting in the areas covered, assisting reviewers to assess quality and differences between studies, while helping researchers to accurately report their findings. 306 However, factors associated with measurement have so far been excluded from such quality assessments, even though they are known to be a major source of bias, irreproducibility and inapplicability in clinical practice and increasingly in research studies. This could compromise patients’ health if inappropriate test adoption decisions are made based on biased or inaccurate results. As far as we are aware, this is the first such initiative to assess the quality of measurement procedures used in clinical studies or trials.
Application of this framework within the four Nephrocheck case studies identified several measurement parameters that present a high risk of irreproducibility, including a failure to exclude samples with known interferents, a lack of internal and external quality control and a complete lack of analytical measurement verification in all studies. It also highlighted several issues that might affect the clinical applicability of test results, including freeze–thawing of samples in the absence of validation data and against the recommendations of the manufacturer, potentially biasing clinical cut-off points and overestimating precision; use of a device in an unvalidated patient population (i.e. aged < 18 years); and reporting the median value of three measurements from different laboratories. Furthermore, it identified several issues that made assessment of the risk of bias uncertain.
A key finding of this assessment was that the reporting of critical measurement parameters was very poor in the majority of studies and this severely hindered the reviewers’ ability to assess their quality. Interestingly, all of the studies claimed to comply with the STARD reporting guidelines. In the absence of better reporting this framework will have limited utility, so this is an area that needs addressing. Unfortunately, the reporting guidelines already in existence [Biospecimen Reporting for Improved Study Quality (BRISQ)307 and (STPROBE-ME)308] have not been widely adopted or promoted by journals. It is important to note that the issues pertaining to poor measurement procedures and the application of the QAMPs framework apply not only to diagnostic, prognostic or predictive accuracy studies of IVDs but also to any clinical trial or study using IVDs as end points or inclusion criteria. This includes Clinical Trials of an Investigational Medicinal Product (CTIMPs), especially in the era of precision medicine, with an increasing number of CTIMPs basing their eligibility criteria and end points on molecular biomarkers measured using IVDs.
Because of the complexity and scale of the subject matter, it might be tempting to create a long and highly detailed data extraction template and include more signalling questions. However, in the interests of pragmatism and user friendliness we attempted to keep the number of fields and signalling questions to a minimum. We believe that this will probably change over time as experience of using the framework develops and our understanding of the requirements and knowledge of reviewers and the critical ‘at risk’ parameters develops. For example, following the review of the results we identified that it might prove useful in future to include a specific field for ‘sample exclusion’ procedures.
Although the work presented here demonstrates the value of such an approach to quality assessment, the authors accept that this is only the first step towards developing such a framework and that further work is required. Potential areas for future research include refining the parameters and signalling questions, validating the utility more widely and developing guidance for users. We have identified the following limitations that we hope to address in future work:
-
The framework has had limited input from the wider IVD community. To address this we plan to set up a workshop with key external stakeholders and apply a more systematic approach to development of the framework.
-
Interpretation is limited by prior knowledge of the measurement factors affecting an IVD. The instructions for use of IVDs must, by law, contain certain information on the analytical procedures and performance of a test. A formal data extraction process for instructions for use may provide a useful and pragmatic starting point against which to benchmark studies. However, additional parameters are often identifiable from the literature, perhaps requiring systematic review methods to be developed.
-
Limited evidence of utility. Further validation of the approach is required across diagnostic, prognostic, predictive and monitoring contexts in addition to investigating its application in RCTs of CTIMPs.
-
The framework is currently limited to quantitative measurements. There is a need to consider how to apply the framework to semiquantitative or qualitative measurements, for example next-generation sequencing.
-
Limited to IVDs. There are similar issues with all diagnostics including imaging, physical measurements and clinical support algorithms.
Chapter 5 Economic evaluation
Introduction
There is currently growing interest in the use of decision modelling earlier in the research and development process for new health-care technologies. 309–311 This is, in part, a response to the high costs and failure rates of pre-regulatory Phase III trials, which is a key issue for tests, in particular, because of the difficult and often financially prohibitive task of linking test outcomes with subsequent treatment pathways. A decision model can be used in the early phases of the research pathway to synthesise current evidence on the effectiveness and cost-effectiveness of emerging technologies. For tests, models are a key resource to enable linkage of diagnostic accuracy evidence with downstream health and cost impacts. Furthermore, models can be used to identify key uncertainties in the evidence base that pose the most risk for clinical, regulatory and reimbursement decision-makers; this information can then be used to tailor the ongoing trajectory and design of research to target and reduce critical uncertainties and to avoid unnecessary and expensive large-scale trials when possible. This approach thereby enables both optimisation of future research resources and maximisation of research outputs.
The objective of the economic evaluation in the AKI-Diagnostics study was to (1) assess the potential cost-effectiveness of AKI biomarkers in an acute care setting, based on an evaluation of the current evidence base, and (2) determine the value of conducting further research into such biomarkers. The focus of this chapter is on the development of a decision model to determine the expected cost-effectiveness of AKI biomarkers; the implications for future research are explored further in Chapter 6.
Overview of the economic evaluation
A de novo decision-analytic model was constructed to evaluate the potential impact of AKI biomarkers within a hospital critical care setting. The model structure was developed using the findings from a model literature review, a focus group and expert consultation; model parameters were subsequently derived using data from the literature, analysis of individual patient clinical trial data and expert opinion. The model adopts a UK NHS and Personal Social Services (PSS) perspective, with all costs reported in 2015 prices and patient health measured in terms of QALYs. Future cost and health outcomes were discounted at an annual rate of 3.5%, as per current NICE guidance. 312
Each of the tests included in the meta-analysis in Chapter 3 (Nephrocheck, NGAL and cystatin C) was compared both individually against standard care monitoring and in a multiway incremental analysis. Cost-effectiveness was assessed using the incremental cost-effectiveness ratio (ICER) and incremental net (health) benefit (INB) values and a range of sensitivity analyses was conducted to explore the impact of key model assumptions and parameter uncertainty on the results. The model was developed in line with current best practice standards312–314 and was built and analysed using R software version 3.0.3.
Methods
Population and perspective
The base-case primary evaluation focused on the assessment of AKI biomarkers for a population of adult all-comers (aged ≥ 18 years) admitted to the hospital critical care unit. A secondary analysis was also conducted to assess outcomes in a subgroup of patients admitted to critical care post cardiac surgery. These patients are known to be at particular risk of post-surgery renal failure and form a relatively homogeneous group who are managed in a controlled environment; they therefore represent a subgroup for whom there is a strong potential for management adjustments to lead to measurable changes in outcomes.
All analyses were conducted from a NHS and PSS perspective, as per the NICE reference case. 312 This aims to incorporate costs relating to NHS primary care, secondary care, community care and social care.
Testing strategies
Tests evaluated in the meta-analysis in Chapter 3 (Nephrocheck, cystatin C and NGAL) were included in the economic evaluation. Cystatin C and NGAL are currently available across three alternative media (plasma, urine and serum); each of these was considered separately in the analysis, which, together with the Nephrocheck test, resulted in a total of seven testing strategies.
All tests were assumed to be used once on entry to the critical care unit in addition to standard care, consisting of daily serum creatinine and urine output testing. Although these new tests could also be used sequentially or in a monitoring context, the majority of evidence on the diagnostic accuracy of these tests relates to single-time usage and this was the focus of the current systematic review. Assessment of sequential and monitoring testing strategies was therefore deemed beyond the scope of this analysis but remains a key area for future research.
Costs and health outcomes
All costs are reported in 2015 prices (Great British pounds) and were inflated when necessary using an online converter. 315
Health outcomes were measured in terms of QALYs. QALYs provide a generic measure of patient overall health and are a composite measure of patient survival weighted by quality of life (utility) over time, for example 1 year in full health is equivalent to 1 QALY, whereas 1 year at half-full health is equivalent to 0.5 QALYs. Expression of health benefit in terms of QALYs allows decision-makers to make a direct comparison between the cost-effectiveness of interventions across different disease areas and indications; NICE312 currently recommends the use of QALYs in cost-effectiveness analyses.
Literature review of acute kidney injury economic models
A literature search was conducted in February 2015 and updated in March 2016 to identify economic models of AKI to help inform the model structure and parameters.
The following databases were searched: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Cochrane Central register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and NHS EED (NHS Economic Evaluation Database). Search strategies included concepts for AKI and economic models (see Appendix 8 for the full database details and an example search strategy). All search results were screened by one health economist using a two-stage process: initial abstract screening to identify potentially relevant references followed by full-text screening to determine final inclusion.
The initial inclusion criteria aimed to identify any models including AKI health states, regardless of setting. For the updated search only economic models assessing AKI biomarker testing strategies were included, as these were deemed to be of most use to the project at that stage.
The original search identified 235 references (179 after removal of duplicates), which increased to 296 (48 after removal of duplicates) references in the update search. In total, 11 studies were included in the review,187,205,316–324 two of which were economic evaluations of AKI biomarkers187,205 (Figure 27).
FIGURE 27.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the AKI economic decision model search.
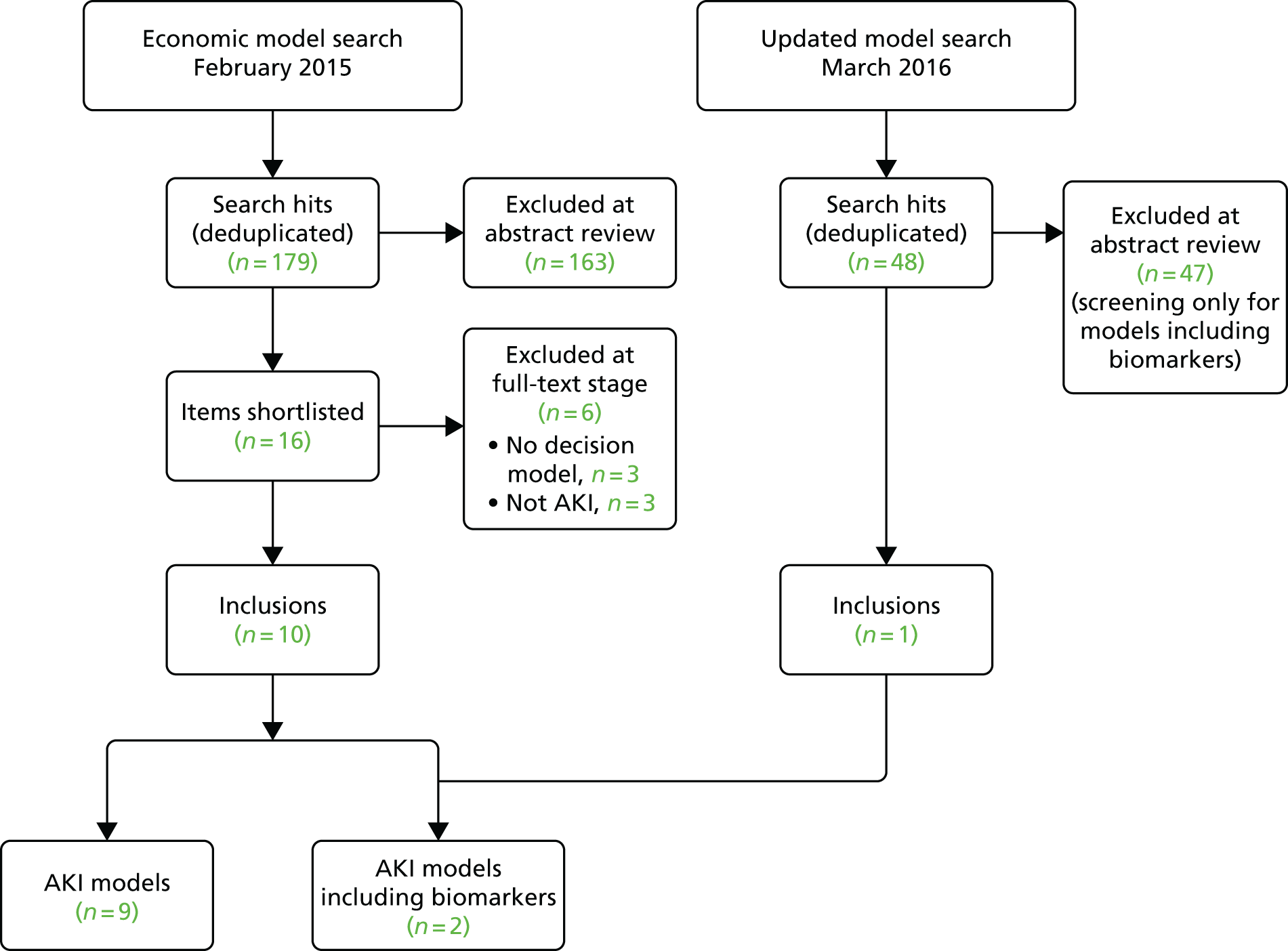
Details of the included models are presented in Table 41. The majority of studies not looking at biomarkers were concerned with assessing different dialysis modalities or contrast media to prevent or treat renal injury. These studies incorporated a range of health states, including within-hospital states such as dialysis/no dialysis, AKI/no AKI, AKI severity levels and hospital death, and post-discharge states, such as alive with/without dialysis, CKD, end-stage renal disease (ESRD), transplant and death.
| First author and year | Country | Intervention(s) | AKI status | Perspective | Time horizon | Model type | Health states included | Main result |
|---|---|---|---|---|---|---|---|---|
| Chicaiza-Becerra 2012316 | Colombia | Iso- and low-osmolality contrast media | Outpatients at high risk of renal injury | National Health System | Lifetime | Decision tree | Treatment + no AKI + death/no death; treatment + AKI + dialysis/no dialysis + death/no death | Lopamidol and lodixanol dominated all other alternatives. Lodixanol vs. lopamidol = US$14,660/life-year gained |
| De Smedt 2012317 | Belgium | CRRT, IRRT and conservative (CONS) treatment | AKI in the ICU | Payer perspective | 2 years | Area under the curve analysis | NA | CRRT was the most effective and costly strategy. CONS dominated IRRT. ICERs: CRRT vs. IRRT = €114,012 per QALY; CRRT vs. CONS = €590,410 per QALY |
| Desai 2008318 | USA | Daily vs. alternate-day haemodialysis | 60-year-old man in ICU with AKI | Societal | Lifetime | Markov model (annual cycle length) | Hospital death, post-discharge CKD ± haemodialysis, no CKD, transplant waiting list (± dialysis), transplant failure/success | ICER for daily vs alternate-day haemodialysis: US$5084 per QALY |
| Erstad 1999319 | USA | Albumin–furosemide complex vs. sequential therapy | Acute oliguric renal insufficiency | Teaching hospital | Short (approximately 6 months) | Decision tree | Treatment + successful (no dialysis); treatment + unsuccessful (dialysis) | Cost per averted dialysis: albumin–furosemide complex = US$28,807; sequential therapy = US$109,350 |
| Ethgen 2015320 | USA | CRRT vs. IRRT | AKI in the ICU | Third-party public payer | Lifetime | Markov model (daily cycle first 5 years; yearly thereafter) | CRRT in ICU, IRRT in ICU, post discharge ± dialysis, death | CRRT dominated IRRT (based on ICER) |
| He 2010321 | USA | Nesiritide Natrecor; Johnson & Johnson, New Brunswick, NJ, USA) vs. placebo for prevention of AKI | Post cardiac surgery | NA (no costs included) | NR | Decision tree | Hospital death ± dialysis, discharged alive ± dialysis | Absolute risk reductions for dialysis and hospital death for nesiritide vs. placebo = 1.3% and 3.3% respectively |
| Iannazzo 2014322 | Italy | Iodixanol vs. low-osmolar contrast media | Patients with intravenous contrast media CT | Health-care provider | Lifetime | Markov model (1-month cycle) | AKI free, AKI, myocardial infarction, death | Incremental cost per life-year gained: iodixanol dominated low-osmolar contrast media |
| Kerr 2014323 | UK | NA (assessment of costs and QALYs) | 72-year-old patients with AKI in hospital | English NHS | 2 years post discharge | Markov model (annual cycle) | Normal kidney function, AKI, CKD, ESRD ± RRT, transplant for ESRD, death | Lifetime cost for all AKI inpatients post-discharge care = £179M; lifetime QALY loss = 1.4 per person |
| Klarenbach 2009324 | Canada | Standard- or high-dose CRRT vs. IHD | ≈60-year-old requiring RRT in ICU | Health-care provider | Lifetime | Mixed decision tree and Markov model | Tree (hospital): dead, treatment ± recovery. Markov model (discharged): alive ± dialysis, dead | CRRT resulted in equivalent QALYs but was C$3679 more costly than IHD |
| Shaw 2011205 | UK | NGAL vs. standard care | 67-year-old man undergoing CABG surgery | Societal | Lifetime | Decision tree | AKI/no AKI, NGAL elevated/normal, AKI failure/injury, CKD/no CKD, treatment/no treatment, CKD/no CKD post discharge, death | NGAL dominated standard care |
| Petrovic 2015187 | USA | Cystatin C, urine NGAL and uL-FABP vs. standard care | Children with congenital heart disease post cardiac surgery | Health-care payer | Lifetime | Markov model | AKI/noAKI, AKI risk/injury/failure, discharged, death, long term no CKD, CKD, ESRD, transplantation, death | ICERs vs. standard care: US$5959 (uL-FABP), US$7077 (cystatin C), US$9315 (NGAL urine) |
Two studies assessed the cost-effectiveness of biomarkers for the early diagnosis of AKI in a critical care setting and are of greatest relevance to this study.
Shaw et al.205
This study assessed the lifetime cost-effectiveness of urinary NGAL for the early diagnosis of AKI after cardiac surgery compared with standard care testing of blood urea nitrogen, blood creatinine and urine output. The base case considered a 67-year-old man after coronary artery bypass graft surgery. The authors stated that they adopted a UK societal perspective; however, it was subsequently acknowledged that they could not include indirect costs because of difficulties in estimating them and the final analysis appears to have been restricted to a NHS perspective, with costs reported in 2008 Great British pounds.
The authors presented a simplified decision tree diagram of their model, in which patients in the testing arm were monitored using NGAL on four occasions (2 hours after surgery and then every 6 hours), with a single elevated result leading to early treatment for AKI. AKI severity was defined in terms of the RIFLE criteria, with each severity level being associated with a specific mortality risk, critical care length of stay and long-term CKD risk. The authors assumed a non-specific treatment modality for AKI including various dialysis modalities, avoidance of nephrotoxic agents, fluid management and an additional nephrologist visit. NGAL sensitivity (0.379) and specificity (0.812) were derived from two diagnostic studies of 72 and 426 patients (smaller studies were not considered) and, in the absence of data, the accuracy of sequential tests was assumed to be equivalent. Instigation of early AKI treatment in the risk stage was assumed to result in a 25% reduction in progression to the injury or failure state. 72,140 The cost of each NGAL test was £25. Discounting of future costs and outcomes was not reported.
The expected lifetime costs and QALYs were £4244 and 11.86 for urine NGAL and £4672 and 11.79 for usual diagnosis. NGAL dominated usual care, being more effective and less costly, and had a 100% probability of being cost-effective at a willingness-to-pay threshold of £30,000. NGAL remained the preferred strategy even in a ‘conservative scenario’ in which the treatment effect was halved from 25% to 12.5%. The most influential inputs identified from sensitivity analyses were the previous probability of AKI and the probability of developing CKD.
This study was funded by a grant from Abbott Diagnostics (Lake Forest, IL, USA), the manufacturer of an assay kit for the measurement of NGAL in urine.
Petrovic et al.187
This study assessed the lifetime cost-effectiveness of cystatin C (serum), NGAL (urine) and urine liver fatty acid-binding protein (uL-FABP) for the diagnosis of AKI in children (aged < 18 years) after cardiac surgery compared with standard care of serum creatinine monitoring. The analysis adopted a US third-party payer perspective (price year not reported).
The model consisted of an initial decision tree in which patients were divided into no AKI and AKI RIFLE severity groups. Patients in the testing arms were assumed to be tested 2 hours after surgery and were subsequently separated into TP/FP and TN/FN groups according to the test accuracies. Patients could then either die in hospital (with mortality dependent on AKI stage) or survive to discharge and then die or survive post discharge. A Markov model was used to capture the long-term risks of CKD (stages 1–4), ESRD, renal transplant and mortality for patients experiencing AKI. All cost and health outcomes were discounted by 3% per annum.
Sensitivity (cystatin C = 0.54; NGAL = 0.63; uL-FABP = 1) and specificity (cystatin C = 0.54; NGAL = 0.63; uL-FABP = 1) values were derived from a single study including 112 paediatric subjects. 184 Therapy as a result of early AKI diagnosis was assumed to result in a 25% improved outcome for AKI patients. Test costs were US$18.94 for cystatin C, US$17.81 for NGAL and US$24.38 for uL-FABP. Probabilistic sensitivity analysis was conducted; however, no variance data were presented for the parameter distributions.
The testing strategies were all more effective and more costly than standard care, with expected lifetime costs of US$18,463 for cystatin C, US$15,304 for NGAL and US$14,126 for uL-FABP compared with US$5608 for standard care and QALYs of 5.15, 5.16, 5.21 and 3.78 respectively. Cystatin C and NGAL were both dominated by uL-FABP, which produced an ICER of US$5959 per QALY compared with standard care and had a 100% probability of being cost-effective at a willingness-to-pay threshold of US$50,000 per QALY. The authors declared no conflicts of interest for this study.
Focus group
A focus group was held on 26 February 2015 with two clinicians (nephrologists) and two patient representatives with experience of chronic kidney failure. The primary aim was to understand the care pathway for patients diagnosed with AKI in a critical care setting, with secondary aims of understanding the impact of AKI and its treatments on patient outcomes and quality of life. The session was co-ordinated by two qualitative research officers (NW and KVC) and two health economists (DM and AS). The group discussion followed a topic guide, which was focused on developing a diagrammatic representation of the patient pathway for someone experiencing AKI in critical care. Interviews were audio recorded and transcribed verbatim by a third party and framework analysis was undertaken to systematically sift, chart and sort material by key themes.
The findings were outlined according to four subsections, briefly outlined below:
-
People at risk of developing AKI. There are many triggers for AKI and there are multiple groups at risk, including patients with long-term conditions (CKD/heart failure), patients with comorbidities and the elderly. In the hospital patients on any ward may develop AKI and may have previously had normal renal function.
-
Diagnosing AKI. Multiple tests and assessments can be used to diagnose AKI in hospital. Some hospitals now use electronic alert systems but there is currently a lack of standardised practice in this area.
-
Treating AKI. After diagnosis, treatment generally consists of a non-specific care bundle including fluid assessment, medication review and additional investigations and monitoring. Treatment procedures may vary depending on the cause of the AKI, individual patient characteristics and hospital procedures.
-
Long-term health consequences of AKI. Post treatment some patients completely recover whereas others may go on to develop CKD and require dialysis or renal transplantation.
The themes identified in the focus group generally support NICE-identified pathways for AKI. 1,325–327 Particular issues identified that were pertinent to the development of the decision model included (1) the multiple aetiologies of AKI and the associated heterogeneity of the AKI population, (2) the non-specific nature of AKI treatment and (3) the need to capture downstream risks of CKD.
Model structure
The model structure was developed using the findings from the literature review and focus group, NICE guidelines1,325–327 and expert feedback. A simplified representation of the model is presented in Figures 28 and 29.
FIGURE 28.
Model structure: initial decision tree. M, Markov model.
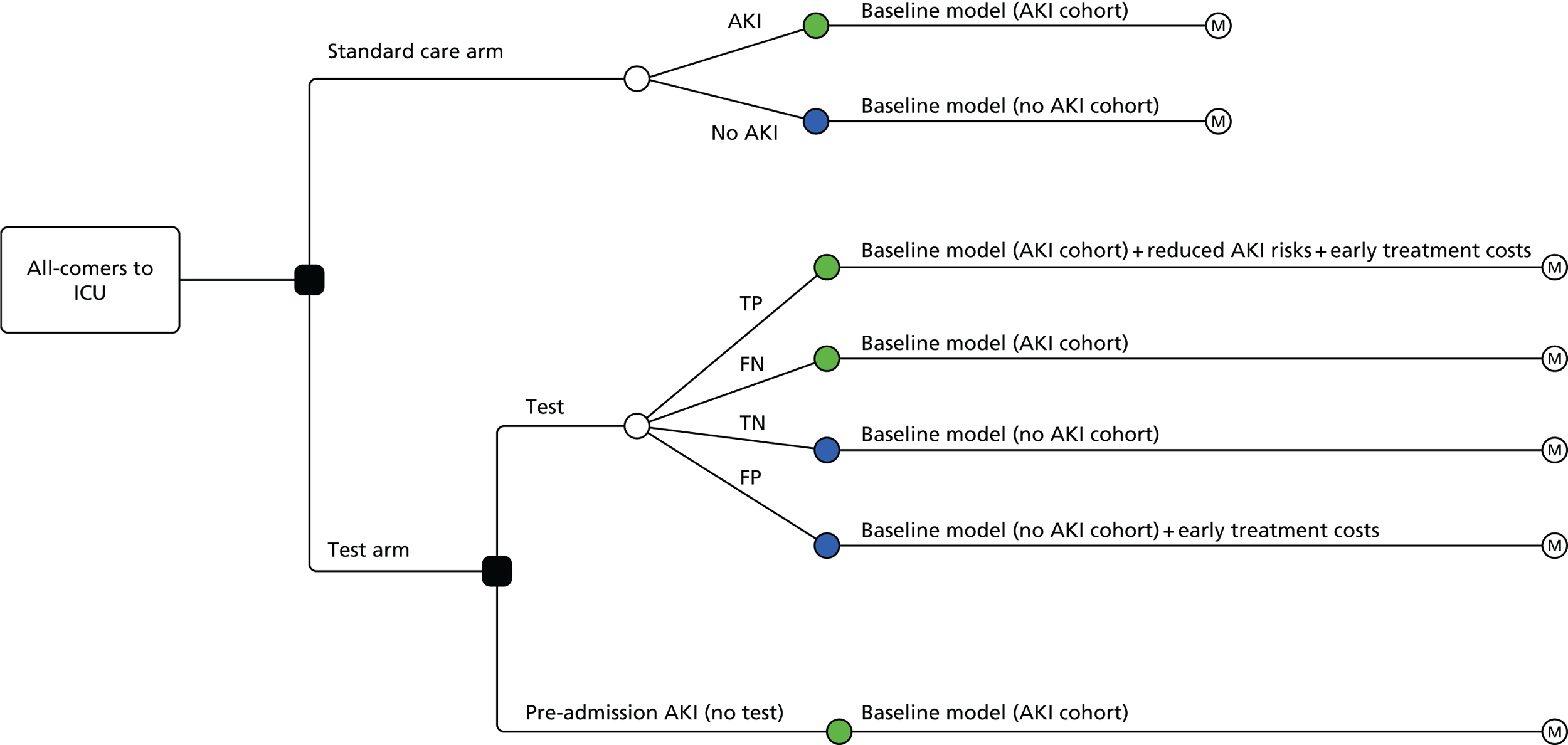
FIGURE 29.
Model structure: main Markov model.
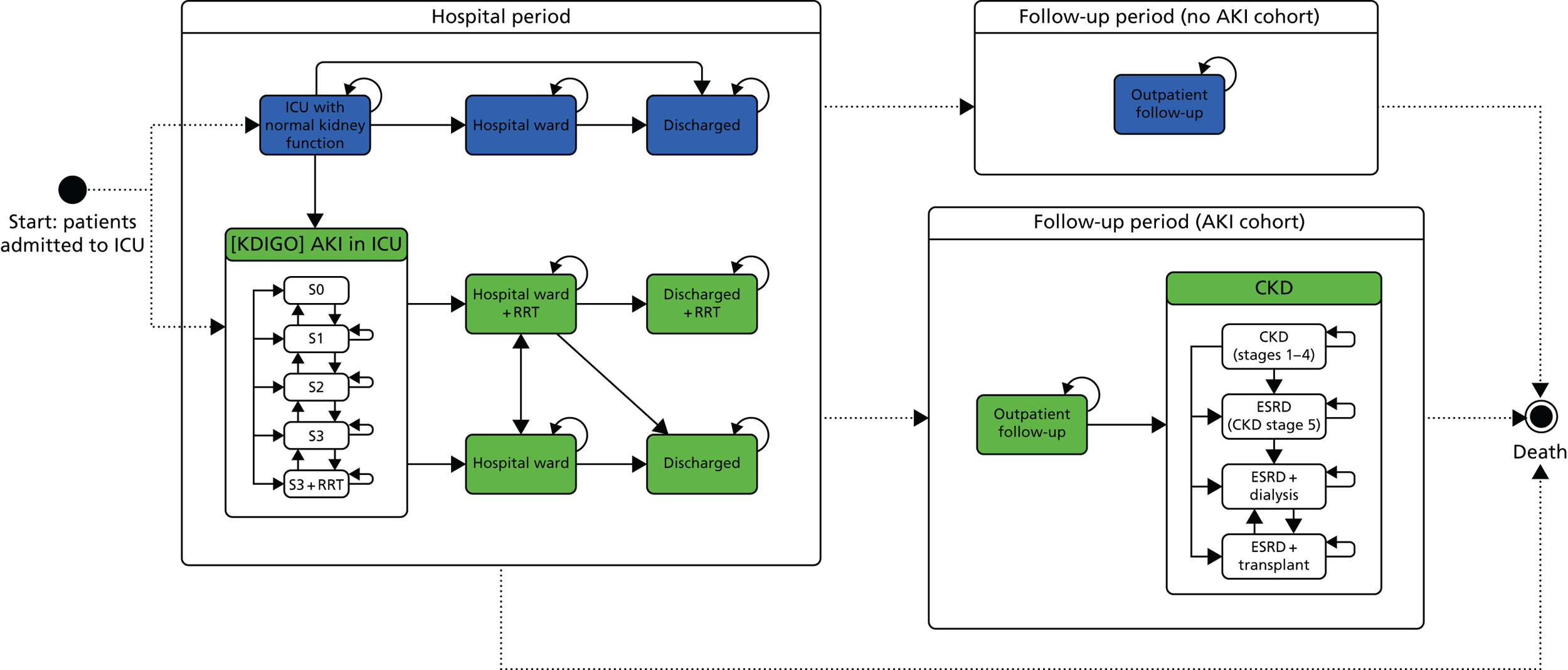
The model is split into an initial decision tree (see Figure 28) and a subsequent modified Markov model (see Figure 29). The decision tree separates patients into cohorts depending on their AKI status and test results; patients then enter the main Markov model, which describes patients’ health-care pathway over the duration of their hospital stay and post-discharge follow-up. In the Markov model patients occupy different health states and may move between those states over time, with movement triggered by events such as AKI onset, AKI progression, development of CKD and mortality. Each health state is associated with a specific cost and quality of life (utility) value such that over time patients accumulate costs and health benefits over model cycles of a defined length of time. The model is run separately for each of the specified cohorts within each of the arms (standard care and testing arms) to obtain an average cost and average QALYs (quality of life + survival), from which cost-effectiveness is calculated.
Initial decision tree model
The initial decision tree separates patients into cohorts depending on their AKI status and (for the intervention arms) their additional test results. In the standard care arm, patients are split into ‘AKI’ and ‘no AKI’ cohorts. Patients in the AKI cohort either arrive in the critical care unit (also hereafter referred to as the ICU) with pre-existing AKI or are destined to develop AKI at some point during their stay; patients in the no AKI cohort are those who maintain normal renal function throughout their critical care stay. Standard care testing is assumed to be perfect, such that all patients in the baseline model are correctly identified as either having or not having AKI.
Patients in the testing arms of the model are assumed to receive an additional test on admission to the critical care unit alongside standard care testing. It is expected that patients arriving in critical care with known moderate or severe AKI (KDIGO stages ≥ 2) would not receive additional testing; these patients follow the baseline AKI cohort pathway. All other patients are assumed to be tested and are separated into four cohorts according to the accuracy of the test results, that is, TPs, FNs, TNs and FPs.
Main Markov model
The Markov model consists of two periods: a hospital period, to assess patients’ short-term outcomes, and a follow-up period, to assess patients’ long-term outcomes post hospital discharge.
Hospital period (days 1–90)
The hospital period adopts daily cycles and runs from day 1 (entry to critical care unit) to day 90 to capture rapid changes in patients’ health during their critical care stay.
The model is separated into no AKI and AKI cohort states. Patients with no AKI start in the ‘ICU with normal kidney function’ state and over time may remain in critical care, be discharged directly into the community or be transferred to a general ward before being discharged. Patients in the AKI cohort are classified into one of five health states according to the severity of their AKI on entry to the critical care unit: current normal kidney function (but destined to get AKI) (stage 0; S0) and the four KDIGO AKI classifications – stage 1 (S1), stage 2 (S2), stage 3 (S3) and stage 3 plus RRT (S3 + RRT). Patients may deteriorate or improve over time (moving to higher or lower severity states). From the ICU health states, patients may be transferred to a hospital ward (with or without RRT) or be discharged home (with or without RRT). Post-discharge patients are assumed to remain in their discharged state for the remainder of the hospital period. By definition, the onset of CKD requires a minimum of 3 months of persistent renal failure;328 CKD is therefore not included within the hospital period, but is captured in the follow-up model.
Follow-up period
The follow-up period aimed to capture patients’ long-term outcomes post hospital discharge. It was run from 90 days post critical care admission for the patients’ lifetime (capped at 100 years) using annual cycles. A half-cycle correction was applied in the follow-up period of the Markov model to account for the continuous flow of patients between Markov states (i.e. in reality not all patients transition at the beginning/end of a year).
All patients in the no AKI cohort who are still alive at the end of the hospital period model are assumed to move to the ‘outpatient follow-up’ state in the follow-up model, where they remain, subject to an annual mortality risk.
Patients in the AKI cohort who are still alive and not on RRT at the end of the hospital period similarly move to a separate ‘outpatient follow-up’ state, where they experience elevated mortality and CKD risks compared with the no AKI cohort. Patients receiving RRT at the end of the hospital period (in the ICU, hospital ward or discharged plus RRT states) are assumed to have CKD and move to the ‘CKD (stages 1–4)’ state. From the CKD state, patients may go on to develop ESRD, with or without maintenance dialysis, or require a renal transplant. If the transplant is successful patients remain in the transplant state; if the transplant is unsuccessful patients are assumed to return to the ‘ESRD + dialysis’ state, but may go on to receive a subsequent transplant.
In all states for both cohorts, patients experience a mortality risk that is dependent on their current health state.
Impact of the tests
All patients in the testing arms without known pre-existing AKI (KDIGO stage ≥ 2) are assumed to be tested and, therefore, receive an additional cost of testing. Other baseline risks and costs in the model are adjusted according to the test result, as described in the following sections.
True positives
Patients with a TP test result are split into two subgroups. Patients with no or mild AKI (KDIGO stages ≤ 1) according to concurrent standard care test results are assumed to be able to benefit from early AKI intervention as a result of the positive test result. These patients follow the baseline AKI cohort but with an additional cost of early treatment and reduced risks of future AKI progressions [i.e. from (S0 or S1) to (S2, S3 or S3 +RRT)] and AKI-associated mortality (mortality in state S3 + RRT). Patients with moderate or severe AKI (KDIGO stages ≥ 2) according to concurrent standard care tests are assumed to not be able to benefit from early intervention. These patients follow the baseline AKI cohort model with no changes to risks or costs.
False positives
Patients with a FP result incur an unnecessary additional cost of early AKI intervention; however, in the base case this is assumed to have no detrimental impact on patients’ health. These patients follow the baseline no AKI cohort with no risk changes applied.
True negatives
Patients with a TN result follow the baseline no AKI cohort with no additional changes applied to the baseline risks or costs.
False negatives
Patients with a FN result receive a concurrent or eventual AKI diagnosis through standard care daily testing. In the base case these patients are assumed to incur no harm from the inaccurate test result and follow the baseline AKI cohort.
A range of alternative assumptions regarding the impact of testing are explored in the sensitivity analysis reported later in this chapter.
Model parameter literature review
Primary searches
To help inform model parameter estimation, a literature review was conducted in July and August 2015 across the following databases: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and The Cochrane Library. Six search strategies were devised to identify evidence on (1) AKI costs, (2) AKI utilities, (3) AKI risks, (4) CKD costs, (5) CKD utilities and (6) CKD risks. Date limits and UK filters were used in some searches to target studies with recent, UK-based data (i.e. of most relevance to the model). Full details are reported in Appendix 9.
For AKI searches, studies were included if they reported on relevant outcomes for adult patients with or recovering from AKI, or on dialysis, and had a hospital critical care or post-critical care setting. For CKD searches, studies were included if they reported on relevant outcomes for adult patients with a primary condition of CKD, who were on chronic dialysis or who had undergone a renal transplant, across any setting. Relevant outcomes were defined as follows:
-
cost searches: UK patient or health-care costs
-
utility searches: direct utilities reported on multiattribute utility indexes or through direct valuation exercises [i.e. EuroQol-5 Dimensions (EQ-5D),329 Short Form questionnaire-6 Dimensions (SF-6D)330 or time trade-off (TTO)/standard gamble (SG) utilities]
-
AKI risk searches: AKI incidence or progression, mortality, dialysis dependence, progression to CKD or ICU/hospital length of stay
-
CKD risk searches: incidence of CKD, ESRD or transplants post AKI, progression or recovery rates for these conditions, dialysis dependence, transplant success and mortality.
All screening and data extraction was conducted by one health economist (AS). Citations were initially screened by title and abstract, followed by full-text screening to determine inclusion. In cases in which the reviewer was unsure about study inclusion a second reviewer (DM or PH) was consulted. All papers screened at the full-text stage were hand-searched for additional relevant references. Search results were stored in six EndNote version X12 libraries [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and data extraction was conducted using a standard extraction form in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Across the six searches, 1153 potentially relevant citations were identified after removal of duplicates (Figure 30). In total, 129 references were included in the review: four for AKI costs,323,331–333 none for AKI utilities, 40 for AKI risks,323,331–369 33 for CKD costs,323,370–401 eight for CKD utilities402–409 and 44 for CKD risks. 370,373,410–451
FIGURE 30.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the model parameter searches. Reasons for exclusion: A = no relevant outcome reported; B = non-UK study (when this was applied as an additional exclusion criteria); C = inappropriate study type (e.g. editorial); D = date (when date restrictions were applied).
For the AKI utilities search, no UK studies or international evidence syntheses were identified according to the original scope of the search. Secondary screening and citation tracking of the search results was therefore conducted to identify any relevant non-UK studies, with no date restrictions, leading to four non-UK-relevant papers being included. 317,452–454
Tables 42–44 provide a summary of the included studies. The majority of evidence identified related to studies reporting outcomes for patients with CKD. Of these studies, the following were identified as being of particular use for model parameter estimation: (1) a comprehensive worldwide meta-analysis reporting CKD, dialysis and transplant health state utilities;402 (2) a large (n = 7246) UK randomised multicentre clinical trial reporting CKD, dialysis and transplant costs, as well as the probabilities of transition between CKD, dialysis and death;370 and (3) the UK Renal Registry annual report, which reports key data on annual transitions between ESRD, dialysis, transplant and mortality states410 (note that model parameters were derived from the subsequently published 2015 version of this report). 455
| First author and year | Country | n | Age (years) | Sex male (%) | AKI/CKD status | Utility measure | Reported utilities |
|---|---|---|---|---|---|---|---|
| AKI utilitiesa | |||||||
| Johansen 2010452 | USA | 377 | Mean 58 | 70.6 | AKI in ICU as a result of necrosis plus sepsis or organ failure, requiring RRT | HUI | Mean utility at 60 days post randomisation: 0.40 (SD 0.37) |
| De Smedt 2012317 | Belgium | 203 | Adults | NR | AKI in ICU requiring RRT with serum creatinine > 2 mg/dl | SF-36 | Mean utility at 20.1 months post hospital admission: 0.69 (SE 0.15, 95% CI 0.67 to 0.71) |
| Åhlström 2005453 | Finland | 153 | Mean 56 | 69 | AKI in ICU or ward requiring RRT | EQ-5D | Median utility at median follow-up of 2.4 years: 0.68 (IQR 0.53–0.85) for ICU patients vs. 0.86 (IQR 0.83–0.88) in matched general population |
| Hamel 1997454 | USA | 57 | Median 61 | 42 | AKI in ICU or ward requiring RRT with an average expected 6-month survival of 50% | TTO | Mean utility at 6 months follow-up: 0.84 (SD 0.25) |
| CKD utilities (all UK studies or reviews) | |||||||
| Neri 2012403 | UK and USA | 144 (UK patients) | Mean 52 | 61 | Kidney transplant patients. Mean time since transplant 5.3 years | EQ-5D | Median: 0.73 (IQR 0.23). Mean utility: transplant with CKD stages 1–2: 0.64; CKD stage 3: 0.58; CKD stage 4: 0.49; CKD stage 5: 0.28. Mean disutility of CKD stage 5 vs. CKD stages 1–2: –0.38 |
| Neri 2010404 | UK and USA | 209 (UK patients) | Mean 53 | NR | Kidney transplant patients. Mean time since transplant 5.6 years | EQ-5D | (Kidney transplant patients) Mean utility: transplant with CKD stages 1–2: 0.74; CKD stage 3: 0.69; CKD stage 4: 0.61; CKD stage 5: 0.39 |
| Wyld 2012402 | USA = 99, Europe = 151, other = 76 | Number of utility estimates: CKD pretreatment = 25, dialysis = 226, transplant = 66, conservative care = 3 | NR | NR | CKD, dialysis and transplant | Utilities on 0–1 scale (review) | Pretreatment CKD stages 3–5: 0.79 (95% CI 0.70 to 0.89); conservative care: 0.62 (95% CI 0.43 to 0.82); CKD stages 3–5 dialysis: 0.70 (95% CI 0.62 to 0.78); utility decrement vs. transplant: –0.02, –0.2 and –0.11 respectively; transplant: 0.85 |
| Wyld 2010405 | NR (170 studies included) | > 56,000 | NR | NR | CKD, dialysis and transplant | Utilities on 0–1 scale (review) | Pretreatment CKD: 0.56 (95% CI 0.52 to 0.60); conservative care: 0.66 (95% CI 0.30 to 1.00); CKD stages 3–5 dialysis: 0.52 (95% CI 0.50 to 0.53); home dialysis 0.57 (95% CI 0.53 to 0.61) vs. hospital 0.50 (95% CI 0.57–0.63); transplant: 0.60 (95% CI 0.57 to 0.63) |
| Blakeman 2014406 | UK | 436 | Mean 72.1 | 42 | CKD stage 3 | EQ-5D | Mean baseline utility CKD stage 3: 0.67 (SD 0.3) |
| Liem 2008407 | NR (27 studies included) | 27 studies included | Mean range 42.1–60 | Range 50–63 | ESRD requiring dialysis or transplant patients | TTO, SG or EQ-5D (review) | (ESRD dialysis) TTO values: HD: 0.61 (95% CI 0.54 to 0.68); PD: 0.73 (95% CI 0.61 to 0.85). EQ-5D values: HD: 0.56 (95% CI 0.49 to 0.62); PD: 0.58 (95% CI 0.50 to 0.67). SG values: HD: 0.75 (95% CI 0.57 to 0.92). TTO transplant: 0.78 (95% CI 0.63 to 0.93). EQ-5D transplant: 0.81 (95% CI 0.72 to 0.90) |
| Lung 2011408 | USA = 2, UK = 1, Japan = 1 | Mean of included studies = 184.6 | NR | NR | Diabetic patients with ESRD | Preference-based measures (review) | Diabetes + ESRD: 0.48 (95% CI 0.25 to 0.71) |
| Nafees 2014409 | UK | 100 general public | NR | NR | Patients with CHF and CKD (post hospital discharge) | TTO | CHF + CKD: 0.78 (SD 0.21) |
| First author and year | n | Age (years) | Sex male (%) | AKI/CKD status | Methods | Reported costs |
|---|---|---|---|---|---|---|
| AKI costs | ||||||
| Kerr 2014323 | 148,226 admissions | Adults | NR | AKI in critical care/general ward | Retrospective analysis of hospital records/database; economic Markov model. NHS perspective; 2010/11 prices | Annual inpatient cost (England) £380M (excluding critical care), £1.02B (including critical care). Cost of post-discharge care £179M |
| Kolhe 2014331 | 576 | Mean 76 | 57 | AKI in ICU/ward | Retrospective analysis of hospital records/database | Inpatient cost of AKI: overall £3748, AKIN 1 £3233, AKIN 2 £3206, AKIN 3 £4287, requiring RRT £8405, no RRT £3504 |
| Paterson 2013332 and 2014333 | 366 | Median 64 | 56 | AKI requiring dialysis in ICU | Retrospective single-centre before-and-after study | Low-volume RRT resulted in an annual 12% cost saving of £27,000 vs. high-volume RRT |
| CKD costs | ||||||
| Baboolal 2008371 | NA | NR | NR | Patients requiring dialysis | Multicentre retrospective cost analysis comparing dialysis modalities. NHS perspective; 2006 prices | Annual costs: automated PD £21,655, CAPD £15,570, hospital HD £35,023, satellite HD £32,669, home HD £20,746 |
| Black 2010372 | NA | Mean 72 | 44 | CKD (KDOQI) stages 1–4, excluding diabetes | Economic model comparing CKD referral strategies. NHS perspective; 35-year time horizon; 2006/7 prices | Annual CKD costs: CKD £344–492 (stage 3a–4), CKD + CVD £572–93. 35-year costs from £11,798 (current care) to £13,487 (referral at CKD stage 3a) |
| Chamberlain 2014373 | 370 | Mean 47–53 | 62–66 | Renal transplant recipients | Retrospective multicentre study of 3-year post-transplant costs. NHS perspective; 2010 euros (£1 = €1.16) | 3-year costs: GFR < 15: €19,230; 15 ≤ GFR ≥ 30: €16,215; 30 ≤ GFR ≥ 45: €13,943; 45 ≤ GFR ≥ 60: €9169; GFR ≥ 60: €8475 |
| Grün 2003374 | 171 | Mean 77 | 67 | Patients requiring dialysis | Prospective cohort study of post-transplant costs. Societal perspective; 1995 prices | Annual cost £22,740 (95% CI £21,467 to £24,015). Cost to social services £522 (2.3% of overall cost) |
| aIqbal 2014375 | NR | NR | NR | Patients requiring dialysis | Budget impact model to assess early cannulation arteriovenous grafts (ecAVGs) vs. tunnelled central venous catheters (TCVSs). Hospital perspective; price year NR | 6-month treatment costs: £5882 (TCVS) vs. £4954 (ecAVG) per patient |
| aJoseph 2010376 | NA | NR | NR | Patients requiring dialysis | Economic model to assess increasing the percentage of patients receiving home dialysis. NHS perspective; price year NR | Projected annual cost of current care in 2013 £35,048 vs. £31,584 for increased percentage of patients receiving home dialysis |
| Kadam 2013377 | 60,660 | All ≥ 40 | 36–39 | CKD + diabetes and CHF | Retrospective database analysis to assess the cost of CKD. NHS secondary care perspective; price year NR | 3-year A&E costs: CKD no diabetes £80, CKD + diabetes £105, CKD no CHF £75, CKD + CHF £164. Hospital costs: £2559, £3642, £2477 and £5344 respectively |
| Kent 2015370 | 7246 | Mean 63 | 64 | CKD stages 1–5 ± CVD and diabetes | Randomised multicentre clinical trial to assess the cost of CKD. Median follow-up 4.9 years; NHS perspective; 2011 prices | Annual hospital costs: CKD stages 1–3B £403, CKD stage 4 £393, CKD stage 5 £525, maintenance dialysis (year 1) £18,986, dialysis ongoing £23,326, kidney transplant (year 1) £24,602, transplant (ongoing) £1148 |
| Kerr 2014323 | NA (national databases) | NR | NR | CKD stages 3–5 | Retrospective database analysis and economic model to assess the cost of CKD. NHS perspective; 2009/10 prices | Annual cost £1.44–1.45B. Mean cost £27,000 on dialysis, £235 not on dialysis, £12,000 transplant recipients. Excess strokes and myocardial infarctions cost £174–8M per annum |
| Kirby 2001378 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Economic Markov model to assess the cost-effectiveness of HD vs. CAPD strategies. Lifetime horizon; hospital perspective; 1999 prices | Monthly costs: HD £827–923, CAPD £905, minor complications £2.50 (HD) and £8 (CAPD), major complications £1799 (HD) and £2111 (CAPD). Total cost: HD £63,370–79,478 (depending on scenario), CAPD £65,061–76,426 |
| Komenda 2012379 | NA | NR | NR | Patients requiring dialysis | Economic model to assess the home HD service. Payer perspective; 2010 US dollars | Home HD year 1: in-centre HD US$45,374, conventional home HD US$46,218, frequent home HD US$57,898. Subsequent years: US$45,034, US$37,762 and US$49,442 respectively |
| aLiu 2013380 | NA | NR | NR | Patients requiring dialysis | Budget impact model to assess dialysis modalities. NHS perspective; 5 year horizon; price year NR | Current 5-year cost: £4,380,678,000. Increasing prevalence of PD by 1.5% or 3% per year projected to save £18.5–63.6M |
| Liu 2014381 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Budget impact model to assess increasing the percentage of patients receiving home dialysis. NHS perspective; 5-year horizon; 2013/14 prices | Base-case 5-year per-patient cost: £23,187. In scenarios 5-year budget impact of increasing the percentage of patients receiving home dialysis ranged from a saving of £572 (£2.5%) to an increase in costs of £1043 (4.5%) |
| Liu 2015382 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Economic model to assess high-dose HD vs. conventional in-centre HD. Lifetime horizon; UK NHS perspective; 2013/14 prices | Lifetime discounted cost: base case (100% in-centre HD) £191,207; 100% high-dose in-centre HD £299,920 |
| McEwan 2006383 | NA | NR | NR | Renal transplant recipients | Economic model to assess sirolimus vs. tacrolimus for the prevention of graft failure. 20-year post-transplant horizon; NHS perspective; 2003 prices | Sirolimus £62,120, tacrolimus £75,265–81,972 (depending on data profiles used) |
| aMcEwan 2010384 | 879 | NR | NR | Renal transplant recipients | Retrospective single-centre study of post-transplant costs. NHS perspective; price year NR | 3-year costs: > 60 ml/minute/1.73 m2 group £497, 30–60 ml/minute/1.73 m2 group £1323, < 30 ml/minute/1.73 m2 group £1448 |
| aMcEwan 2012385 | NA | NR | NR | CKD requiring dialysis or transplantation | Economic model to assess the impact of graft survival time on transplantation cost-effectiveness. NHS perspective; 10-year time horizon | 10-year cost: remain on dialysis £394,379, functioning graft £118,049. 4-year graft survival was cost saving |
| Mowatt 2003386 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Economic model of home vs. in-centre HD. 5-year horizon; NHS perspective; 2001/2 prices | Annual costs: hospital HD £22,246, satellite HD 21,264, home HD 19,470. 5-year costs: £42,722, £46,001 and £41,250 respectively |
| Muduma 2014387 | Hypothetical n = 100 | NR | NR | Renal transplant recipients | Budget impact model of Prograf vs. Advagraf to prevent graft failure. 5-year horizon; NHS perspective; 2012/13 prices | 5-year cost: Advagraf £29,328, Prograf £33,061. Cost saving of £375,000 for 100 patients |
| Muduma 2014388 | NA | Model starting age = 45 | NR | Renal transplant recipients | Economic model of immunosuppressents to prevent graft failure. 25-year horizon; NHS perspective; 2012/13 prices | 25-year costs: Prograf £127,661, Advagraf £116,733, belatacept £116,733, ciclosporin £127,187, sirolimus I £103,896, sirolimus II £103,896 |
| Muduma 2014389 | NA | NR | NR | Renal transplant recipients | Budget impact model to assess Adgraf vs. Prograf for graft failure. NHS perspective; 5-year horizon; 2012/13 prices | 5-year costs: Advagraf £26,941, Prograf £30,356 |
| aMuduma 2015390 | Hypothetical n = 100 | NR | NR | Renal transplant recipients | Economic model of Prograf vs. Advagraf to prevent graft failure. NHS perspective; 2014 prices | Mean 5-year costs: Prograf £40,974, Advagraf £45,836 (cost saving of £4862) |
| Neil 2009391 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Budget impact model of dialysis costs. NHS perspective; 2007 prices | Projected 5-year cost of shift to HD : PD ratio of 70 : 30: £133M |
| NICE 2011392 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Economic model of dialysis settings and modalities. NHS perspective; 2008/9 prices | Monthly costs: hospital HD £2919, satellite HD £2722, home HD £1439, transplantation £10,250–13,627, transplant maintenance £583. 10-year costs: base £130,681, HD centred £136,146, PD £120,752 |
| NICE 2011393 | NA | NR | NR | Patients requiring dialysis (with ESRD) | Budget impact model of dialysis modalities. NHS perspective; 2011/12 prices | Unit costs: home HD £23,271, hospital HD £22,916, satellite HD £22,916, CAPD 17,411, APD 21,071. 5-year impact (1% PD increase per year): saving of £4,087,000 |
| Oates 2012394 | 78 | Mean 68 | 55 | Patients requiring dialysis | Prospective costing study to assess online haemo-diafiltration (OL-HDF) vs. high-flux HD. Perspective and price year NR | Dialysis session costs: high-flux HD £25.56, OL-HDF £24.78. Weekly drug costs 3 months prior to starting and 12 months post starting: high-flux HD £21 and £24 respectively, OL-HDF £16 and £21 respectively |
| aPollock 2013395 | NA | NR | NR | Renal transplant recipients | Budget impact model to assess Prograf vs. Advagraf to prevent graft failure. 5-year horizon; perspective NR; 2012/13 prices | 5-year cost per patient: Advagraf £29,290, Prograf £33,032. Total cost saving of £3742 |
| aPollock 2013396 | NA | NR | NR | Renal transplant recipients | Budget impact model of Prograf vs. Advagraf to prevent graft failure. Perspective NR; 5-year horizon; 2012/13 prices | 5-year cost per patient: Advagraf £26,958, Prograf £30,379. Total cost saving £3421 |
| Popat 2014397 | 45 | Mean 48–54 | 38–71 | Renal transplant recipients | Prospective single-centre study to assess the cost of IL2Mab vs. antithymocyte globulin (ATG) to prevent graft failure. Perspective and price year NR | Average cost in year after transplant: IL2Mab £18,929, ATG £14,904 (p = 0.002) |
| Roderick 2005398 | 736 | Mean 56–67 | 53–66 | Patients requiring dialysis | Cost study to assess renal satellite units (RSUs) vs. main renal units (MRUs). Health service and patient perspective; 2000/1 prices | Total annual (or since starting dialysis) cost of all hospitalisations: MRU mean range £31–138 (depending on assumptions), RSU £35–125 |
| aSun 2010399 | NA | NR | NR | Renal transplant recipients | Economic model to assess the cost of renal graft failure post transplant. Investment perspective | 1-year cost of renal graft failure approximately £58,847. Post graft failure cost £28,179 |
| Thompson 2013400 | Hypothetical n = 1000 | Mean 58 | 61 | CKD (stages 3–4) not on dialysis | Economic model to assess sevelamer vs. calcium carbonate. NHS perspective: lifetime horizon: 2011 prices | Model costs: HD session £161, PD session £53.15. Lifetime costs: calcium carbonate £46,117, sevelamer £83,399 |
| Treharne 2014401 | Hypothetical n = 100 | NR | NR | Patients requiring dialysis (with newly diagnosed ESRD) | Economic model to assess the cost-effectiveness of increasing the percentage of patients receiving PD. NHS perspective; 5- and 10-year horizon; 2013/14 prices | Current care (22% PD): 5-year horizon £96,307, 10-year horizon £133,339. Cost savings for increasing uptake to 39% PD: £3180 and £4102 respectively; cost savings for increasing uptake to 50% PD: £5238 and £6758 respectively |
| First author and year | Country | n | Age (years) | Sex male (%) | AKI/CKD status | Methods | Key risk results |
|---|---|---|---|---|---|---|---|
| AKI studies | |||||||
| Abosaif 2005343 | UK | 183 | Mean 65 | 68 | AKI in ICU on first day. Excluded patients with CKD or renal transplant | Retrospective review of medical notes. AKI patients compared with a randomly selected control group with decrease in eGFR of < 25% | AKI incidence: risk 60/183 (33%), injury 56/183 (31%), failure 43/183 (23%), control group 24 (13%). CVVH in ICU (percentage requiring): 39%, 28%, 50%, 58%, 4%. ICU mortality: 38%, 50%, 75% and 17% respectively. 6-month mortality: 43%, 54%, 86% and 25% respectively |
| Alassar 2012344,345 | UK | 79 | Mean 84 | 59 | AKI (Valve Academic Research Consortium criteria) after transcatheter aortic valve implantation. Excluded patients with CKD or on dialysis | Single-centre observational study | ICU AKI incidence: stage 1 9/79, stage 2 1/79. AKI resolved in 9/10 patients before hospital discharge. No patients required RRT in hospital and LOS was not affected by AKI; 3/10 patients died within 1 year vs. 13/79 for the total cohort |
| Ali 2007346 | UK | 5321 (37 received RRT) | NR | NR | Patients receiving RRT in hospital | Retrospective analysis of population hospital records | 37 (8%) AKI patients received RRT; 23 (62%) had first RRT in the ICU, 13 (35%) in the renal unit and one in a surgical high-dependency unit; 21 (57%) patients died within 6 months vs. 5 (45%) of patients with AKI on CKD who received RRT |
| aBarnes 2014347 | UK | 169 | NR | 83 | AKI (AKIN criteria) in ICU within 48 hours after off-pump CABG surgery | Retrospective analysis of hospital records | Incidence 46/169 (27.2%); 50% AKIN stage 1, 30% stage 2, 20% stage 3. Mean ICU LOS was 2.3 days for all patients and 4 days for patients requiring CRRT |
| Bastin 2013348 | UK | 1881 | > 16; mean 68 | 71 | AKI (AKIN, RIFLE, KDIGO) after cardiac surgery with CPB. Excluded patients on chronic dialysis or who died within 24 hours of surgery | Retrospective analysis of hospital records | (AKIN and KDIGO) no AKI 1394 (74.1%), stage 1 317 (16.9%), stage 2 34 (1.8%), stage 3 136 (7.2%); 122/1881 (6.5%) required RRT in hospital. ICU LOS (median): no AKI 1 day, stage 1 2 days, stage 2 4 days, stage 3 13 days; hospital LOS (median): 7, 9, 14 and 24 days respectively; hospital mortality: 4 (0.3%), 1 (0.3%), 0, 19 (14%) respectively |
| Baudouin 1993367 | UK | 35 | Mean 56 | 74 | Patients requiring RRT (continuous venovenous haemofiltration) after CPB surgery | Retrospective database/records review | 35/1300 (2.7%) patients required RRT in the ICU. Mean time from surgery to RRT was 8 days and mean time spent on RRT was 8 days; 3/9 ICU survivors died in hospital after ICU discharge |
| Bedford 2014349 | UK | 19,940 | Mean 62–76 | 45–52 | AKI (AKIN) in hospital. Excluded patients receiving chronic dialysis, maternity patients and day-case admissions | Retrospective analysis of hospital database | Mean ICU LOS: no AKI 3.0 days, AKIN 1 4.4 days, AKIN 2 4.5 days, AKIN 3 7.3 days; 77/588 (13.1%) patients with stage 3 AKI received RRT in hospital; 16/77 RRT patients remained on RRT 90 days post discharge |
| Bhandari 1996366 | UK | 1095 (cardiac 139) | NR | NR | AKI (acute uraemic emergency with serum creatinine ≥ 600 µmol/l and/or requiring dialysis) post cardiac surgery | Retrospective review of medical notes | Post-cardiac surgery severe AKI subgroup: 90-day survival 54 (38.8%), 90-day dialysis dependence 2 (1.4%) |
| aBrown 2014350 | UK | 2297 (306 with AKI) | NR | 58 | AKI requiring diffusive haemodialysis (CRRT) in ICU. Mixed non-surgical and surgical patients | Analysis of hospital records | 319/2297 (13.9%) patients admitted to the ICU required CRRT. Mean LOS (days): RRT patients 13, all patients 8.2. Mortality at hospital discharge was 56% for RRT patients and 20% for all patients |
| aChakkalakal 2013338 | UK | 71 | NR | 70–81 | Patients in ICU with creatine kinase > 5000 w/l | Retrospective analysis of hospital records | AKI incidence: 39/71 (55%); 19 (27%) patients required RRT. Hospital mortality: no AKI 9%, AKI stages 1–3 25%, RRT 79%. ICU mortality: RRT 79%, no RRT 15% |
| Challiner 2014351 | UK | 745 | NR | 55 | AKI (RIFLE, AKIN, AKIB) in ICU. Excluded maternity patients, admissions of < 24 hours, and patients on chronic dialysis | Retrospective audit | AKI incidence: RIFLE: risk 3 (2.9%), injury 5 (10.4%), fail 4 (20%); AKIN: stage 1 5 (4.3%), stage 2 4 (11.8%), stage 3 5 (23.8%). ICU AKI mortality 6/26 |
| de Mendonça 2000352 | 16 countries including the UK | 1411 | > 12; median 63–57 | AKI (serum creatinine ≥ 300 µmol/l and/or urine output < 500 ml/day) in ICU. Excluded patients with ICU stay of < 48 hours, a history of RRT or had had an elective operation | Prospective, multicentre, observational cohort analysis | AKI incidence: 348/1411 (24.7%). Median ICU LOS (days): AKI 7, no AKI 4. ICU LOS was the same for survivors and non-survivors. ICU mortality: AKI 149/348 (42.8%), no AKI 199/1068 (18.6%); hospital mortality: AKI 49.1%, no AKI 17.7% | |
| Grayson 2003353 | UK | 5132 | 50% < 65; 39% 65–74; 11% > 75 | 75 | AKI after cardiac operation involving CPB. Excluded patients with pre-existing significant renal impairment (serum creatinine > 200 mol/l) | Retrospective cohort analysis | AKI hospital incidence: 151 (2.9%); 105/151 patients with ARF did not require dialysis |
| aHurtado-Doce 2014354 | UK | 512 | Median 54 | 71 | AKI after cardiac surgery and requiring CRRT | Retrospective analysis of hospital records | 60/512 (12%) patients required CRRT for AKI. Mortality: all 23/512, no RRT 17/452, RRT 6/60 |
| aKarmali 2015355 | UK | 262 | NR | NR | AKI after CABG surgery using CPB (ONCAB, n = 131) or off-pump (OPCAB, n = 131). Excluded dialysed patients | Retrospective analysis of hospital records | AKI incidence: all 20/262 (7.6%), OPCAB 14/131 (10.7%), ONCAB 19/131 (14.5%). RRT in ICU: all 15/262 (5.7%), OPCAB 6/131 (4.6%), ONCAB 9/131 (6.9%). Mean ICU LOS (days): OPCAB 1.96, ONCAB 2.49 |
| Kerr 2014323 | UK | NR | Adults (age NR) | NR | AKI in hospital with ICU subgroup. Chronic RRT patients excluded from hospital data but not identifiable in HES | Retrospective database (HES) and records analysis and economic decision model | Mean LOS: no AKI 0.05 days, stage 1 0.17 days, stage 2 0.31 days, stage 3 1.57 days, all AKI 0.35 days. Ratio vs. no AKI: stage 1 2.60 days, stage 2 5.61 days, stage 3 18.2 days, all AKI 4.32 days |
| aKhawaja 2012337 | UK | 249 | Mean 83–82 | 62 | AKI (modified RIFLE score ≥ 2) after transcatheter aortic valve implantation. Excluded patients with history of RRT | Retrospective review of medical records | AKI in ICU: 89 (35.7%); 30-day mortality: AKI 13.5%, no AKI 3.8%. Mortality at mean follow-up (338 days): AKI 40.4%, no AKI 19.4% (p < 0.001) |
| Kirwan 2015356 | UK | 5544 | Median 64–66 | 78–82 | AKI in ICU requiring RRT. Excluded patients with known CKD, ESRD or transplant in the last 10 years | Retrospective review of hospital records | 781/5544 (14%) ICU admissions received RRT; 22/261 (8.4%) RRT survivors died within 3 months post hospital discharge; 7/261 commenced RRT within 3 months post discharge |
| Kolhe 2008341 | UK | 276,731 (17,326 with severe AKI) | Mean 63.2 | 66 | Severe AKI (serum creatinine ≥ 300 µmol/l and/or urea ≥ 40 mmol/l) in first 24 hours of ICU admission. Excluded patients with a history of RRT or an ICU stay of < 8 hours | Retrospective review of hospital records | AKI incidence: 6.3% (17,326/276,731). Median ICU LOS (days): AKI survivors 4.1, AKI non-survivors 2.0, whole cohort survivors 1.7, whole cohort non-survivors 2.0; median hospital LOS (days): 31, 8, 16 and 9 respectively |
| Kolhe 2014331 | UK | 576 | Mean 76 | 57 | Primary or secondary diagnosis of AKI in hospital according to International Classification of Diseases, 10th Edition (ICD-10) codes | Retrospective database and activity records analysis | 26 (4.5%) AKIN stage 3 patients required RRT in the ICU. AKI patients who needed RRT had a longer hospital LOS stay than those who did not need RRT: 16.7 vs. 10.3 days |
| aKolic 2013336 | UK | 282 | Median 50 | 68 | AKI and CKD in the ICU with an ICU stay of > 5 days | Retrospective review of hospital records | 180/282 (64%) patients had AKI in the ICU; 36/282 (12.8%) to 25/282 (8.9%) had CKD depending on the definition of CKD used |
| aKourliouros 2009342 | UK | 1072 | NR | NR | AKI after CABG surgery | Retrospective cohort study | Incidence of AKI in the ICU: 175/1072 (16%) |
| Metcalfe 2002357 | UK | 51 | Median 71.4 | 65 | Patients requiring RRT in hospital (either AKI or AKI on CKD) | Prospective observational study | 23/34 (67.6%) patients with AKI had RRT in the ICU. Hospital LOS: median 19 days. At 90 days: recovered: AKI cohort 8 (23.5%), AKI on CKD 3 (16.5%). Mortality: AKI 25 (73.5%), AKI on CKD 12 (67%), CKD 4 (11%), all 41 (46%) |
| Noble 2001363 | UK | 612 | Mean 57 | 61 | AKI requiring RRT and mechanical ventilation for respiratory failure in the ICU | Retrospective database review and telephone interviews | ICU median LOS: AKI 9.6 days, no AKI 2 days, AKI survivors 12 days, AKI non-survivors 8.3 days. Hospital mortality: 64.1% (392/612) |
| Ostermann 2000361 | UK | 2337 | Mean 65 | Female-to-male ratio from 4 : 14 to 5 : 16 | AKI (urine output ≤ 479 ml/24 hours or ≤ 159 ml/8 hours, serum urea ≥ 35 mmol/l, serum creatinine ≥ 300 mmol/l) after surgery with CPB | Prospective observational study compared with historical control subjects | 47/2337 (2.0%) patients needed CVVH vs. 2.7% from historical data; 21/39 (53.8%) patients vs. 74% in the historical data died in the ICU. Hospital mortality: current, 53.8% vs. historical, 83%. Hospital mean LOS: survivors 53 days vs. non-survivors 17.3 days. Duration of CVVH (days): survivors 11 vs. non-survivors 12.7 |
| Ostermann 2007362 | UK and Germany | 41,972 | Mean 61 | 64 | AKI (RIFLE) in the ICU. Excluded patients on dialysis at baseline | Retrospective database analysis | AKI incidence: risk 17.2%, injury 11%, failure 7.6%. ICU mortality: no AKI 5%, risk 14.7%, injury 36.5%, failure 47.6%. Hospital mortality: 8.4%, 20.9%, 45.6% and 56.8% respectively. Mortality odds ratio: risk 1.40, injury 1.96, failure 1.59 |
| Paterson 2013332 and 2014333 | UK | 366 | Mean 64–66 | 56–66 | Patients receiving RRT in the ICU. Excluded patients receiving dialysis for CKD at baseline and interhospital ICU transfers | Retrospective before-and-after study of high-volume vs. low volume RRT | Days on RRT for survivors: high-volume RRT 12, low-volume RRT 8. ICU RRT mortality: high-volume RRT 55 (29%), low-volume RRT 61 (34%). Hospital mortality: high-volume RRT 77 (41%), low-volume RRT 75 (42%). Death post-ICU discharge: high-volume RRT 22 (12%), low-volume RRT 14 (8%) |
| Prescott 2007358 | UK | 809 | > 15; median 65–72 | 61 | Patients requiring RRT (either AKI or AKI on CKD) in the ICU or renal ward. Excluded patients requiring RRT for ESRD or with renal transplants | Prospective observational study | 600 patients had AKI, 209 had AKI on CKD. Median days of RRT: AKI 5, AKI on CKD 8; 30% of AKI and 20% of AKI on CKD patients died within 10 days of starting RRT. Mortality at 90 days: AKI 50%, AKI on CKD 43% |
| Prowle 2014335 and 2014359 | UK | 700 | Median 46–51 | Range 62–70 | AKI (KDIGO) in the ICU with an ICU stay of > 5 days and patient surviving to hospital discharge. Excluded patients with new or pre-existing ESRD and transplant recipients | Retrospective analysis of hospital records | AKI incidence: 66% (459/700); stage 1 218 (31%), stage 2 75 (11%), stage 3 166 (24%). 121/700 (17.3%) patients received RRT. ICU median LOS (days): all 12, no AKI 8, stage 1 11.5, stage 2 11, stage 3 12. Hospital median LOS (days): no AKI 28, AKI 22 |
| Ricci 2008369 | Worldwide including the UK | 71,000 (8398 with relevant outcome) | NR | NR | AKI (RIFLE) in the ICU | Systematic review with meta-analysis | RR (vs. no AKI) for mortality in the ICU: risk 1.77, injury 2.35, failure 4.63. RR (vs. risk): injury 1.32, failure 2.33. RR (vs. injury): failure 1.74 |
| Saratzis 2015339 | UK | 149 | Mean 69 | 89 | AKI (AKIN and KDIGO) ≤ 48 hours post cardiac surgery (elective endovascular abdominal aneurysm repair). Excluded patients with ESRD or on dialysis at baseline | Prospective cohort study | AKI incidence: 28 (18.8%); stage 1 25, stage 2 3. Post-discharge mortality: AKI 32.1%, no AKI 1.7%. AKI hazard ratios: mortality 0.035, cardiovascular morbidity 0.021 |
| Susantitaphong 2013334 | Worldwide | 888,604 (ICU), 164,333 (cardiac) | Mean 23–80 | NR | AKI (KDIGO) in ICU or post cardiac surgery | Literature review of large cohort studies with meta-analysis | AKI incidence in the ICU: 31.7% (95% CI 28.6% to 35.0%), post cardiac surgery: 24.3% (95% CI 20.4% to 28.8%). AKI ICU mortality: 33.1% (95% CI 29.8% to 36.6%), post cardiac surgery: 8.3% (95% CI 6.6% to 10.4%) |
| aSyed 2011360 | UK | 373 | Adults (age NR) | NR | AKI stage 1 (AKIN) in ICU | Retrospective review of medical records to assess the impact of early oxygen delivery | ICU mortality for AKI stage 1 23.8%; hospital mortality for all 31.6%. No significant difference in hospital mortality between high and low oxygen monitoring groups. 48/249 (19.3%) patients with no monitoring progressed to AKI stage 3 |
| Thomson 2014340 | UK | 264 | Mean 70 | Female-to-male ratio 1 : 3 | AKI after CABG surgery and/or aortic valve surgery in cardiothoracic ICU | Prospective observational study to assess the impact of goal-directed therapy (GDT) | AKI incidence at day 3: GDT group 6.5%, control group 19.9% |
| Tsang 1996368 | UK | 48 | Mean 65 | 71 | AKI requiring continuous hemofiltration after cardiac surgery | Retrospective database/records review plus telephone interviews | 319/2297 (13.89%) patients admitted to the ICU required CRRT. Mean LOS: RRT patients 13, all patients 8.2. Mortality at hospital discharge: RRT patients 56%, all patients 20% |
| Uchino 2005364 | 23 countries including the UK | 29,269 (1738 with AKI; 52 UK patients) | Median 67 | 64 | Patients with AKI [urine output < 200 ml/12 hours and/or a marked blood urea nitrogen level > 84 mg/dl (30 mmol/l)] or treated with RRT in the ICU | Prospective observational study | AKI incidence: 1738/29,269 (5.8%), UK subgroup 20.6%; 1260 (4.2%) were treated with RRT in the ICU, 52% died in the ICU, 60.3% died in hospital (73.1% in the UK subgroup). Dialysis dependence at hospital discharge: 13.8% for AKI survivors |
| Uchino 2007365 | 23 countries including the UK | 1006 | Median 66 | 66 | Patients with AKI requiring CRRT in the ICU. Excluded patients on dialysis | Prospective observational study | ICU mortality: 555/1003 (55.3%). Hospital mortality: 641/999 (64.2%); 85.5% were dialysis independent at hospital discharge |
| CKD studies | |||||||
| aAnwar 2012411 | UK | 548 | NR | NR | Renal transplant recipients with at least 3 years of follow-up data | Single-centre observational study | 56/548 (10.2%) patients were on dialysis within 5 years post transplantation |
| aBabu 2014412 | UK | 155 (80 in CKD group) | Mean 61–69 | NR | Patients with CKD and primary prevention cardiac resynchronisation therapy devices | Single-centre observational study | Mean survival time: CKD 59.7 months, no CKD 81.2 months. CKD was an independent predictor of sustained ventricular arrhythmia |
| Balupuri 2000442 | UK | 47 | 16–60 | NR | Renal transplant recipients either from heart-beating donors (HBDs) or non-heart-beating donors (NHBDs) | Single-centre prospective study | In phase I, 19/21 (90.5%) HBD transplants were successful; in phase II (including non-HBDs), 5/11 (45.5%) transplants were successful; in phase III (non-HBDs including other departments), 12/13 (92.3%) transplants were successful |
| aBevins 2013413 | UK | 201 | NR | NR | CKD stage 4 | Retrospective analysis | Over a median follow-up of 1483 days, 60/201 patients progressed to RRT (i.e. ESRD) |
| Chamberlain 2014373 | Europe | 3181 (370 in the UK) | Mean 47–53 | 62–66 | Renal transplant recipients. Excluded multiorgan transplants | Retrospective multicentre study | Database results: over 3 years post-transplant outcomes for UK: delayed graft function 22.4%, acute rejection 37%, graft failure 10%, stroke 0%, death 1.9%. Questionnaire results: 29.9%, 20.6%, 11.5%, 2% and 2.7% respectively |
| aChan 2011414 | UK | 1288 | Mean 46 | 62 | Renal transplant recipients | Cohort analysis | 15-year patient and allograft survival was 84.6% and 66.8% respectively; 13/70 deaths were from malignancy |
| aCherukuri 2014415 | UK | 504 | NR | NR | Renal transplant recipients | Prospective cohort analysis | Transplant recipients who were vitamin D deficient had worse overall survival (77% vs. 92%) and death censored graft survival (89% vs. 96%) than those with normal vitamin D levels |
| Cherukuri 2010416 | UK | 94 | Mean 63 | 69 | Incident patients starting dialysis and on dialysis for at least 90 days | Prospective observational cohort study | 39/94 (41%) patients died during the study period, mostly from vascular disease (39%) or sepsis/infection (33%); 56% (n = 22) of deaths occurred in the first year |
| CKD Prognosis Consortium 2010444 | International including the UK | 105,872 | NR | NR | General population cohorts | Systematic review and meta-analysis | Compared with an eGFR of 95 ml/minute/1.73 m2, adjusted hazard ratios for all-cause mortality were 1.18 for 60 ml/minute/1.73 m2), 1.57 for 45 ml/minute/1.73 m2 and 3.14 for 15 ml/minute/1.73 m2). Similar findings were recorded for cardiovascular mortality |
| CKD Prognosis Consortium 2011445 | International including the UK | 21,688 | NR | NR | Kidney disease cohorts | Systematic review and meta-analysis | Below an eGFR of 45 ml/minute/1.73 m2, a 15-ml/minute/1.73 m2 drop in eGFR was significantly associated with mortality (hazard ratio 1.47) and ESRD (hazard ratio 6.24) |
| Coresh 2014417 | International | 1.7 million | Mean 51–74 | 49–80 | Mixed cohorts with and without CKD. Excluded patients with ESRD at baseline | Meta-analysis of studies from an international consortium consisting of 50 cohorts with > 1000 participants | A change of −57% in estimated GFR over 2 years was associated with adjusted hazard ratios for ESRD of 32.1 at lower eGFRs (< 60 ml/minute/1.73 m2) and 57.2 at higher eGFRs (≥ 60 ml/minute/1.73 m2). Mortality HRs were 3.7 and 3.8 respectively |
| Drey 2003451 | UK | 405,000 | NR | NR | Detected CKD in a general population cohort | Retrospective cohort study | Annual incidence of CKD: 1701 per million population with a median age of 77 years and male-to-female ratio of 1.6. CVD was the most common cause of death (46%); 4% were accepted for RRT |
| Ekberg 2009448 | International including the UK | 1645 | Median 47 | 65 | Renal transplant recipients with low-to-normal immunological risk | Randomised trial | Biopsy-proven acute rejection: 25% at 12 months, 26% at 24 months, 27% at 36 months; death censored graft survival: 94%, 92% and 91% respectively; uncensored graft survival: 92%, 89% and 88% respectively; patient survival: 97%, 96% and 95% respectively |
| aFarrugia 2013418 | UK | 19,103 | NR | NR | Renal transplant recipients | Retrospective database analysis | 2085/19,103 of patients died, 376 (18.0%) because of malignancy |
| aFavi 2015419 | UK | 639 | NR | NR | Renal transplant recipients | Single-centre observational study to assess polyomavirus-associated nephropathy (PVAN) | Death-censored graft loss rates: PVAN 42%, no viraemia 14% |
| Fellstrom 2005420 | International including the UK | 1052 | Mean 50 | 65.2 | Renal transplant recipients | RCT (but assessed placebo arm only in this analysis) | Over 5–6 years of follow-up: 54/1052 patients experienced cardiac death and 65/1052 experienced non-cardiac death and 66/1052 had definite myocardial infarction |
| Gansevoort 2011443 | International including the UK | 845,125 general population and 173,892 high risk | Mean 26.4–66.9 | 39.5–100 | General population and CKD high-risk cohorts | Systematic review and meta-analysis | Hazard ratios for ESRD at eGFRs of 60, 45 and 15 ml/minute/1.73 m2 (vs. 95 ml/minute/1.73 m2) in the general population cohort analysis: 3.69, 29.3 and 454.9 respectively |
| Ghazanfar 2010449 | UK | 201 | Mean 37.4 | Male-to-female ratio 6 : 1 | Renal transplant recipients (living donors) | Retrospective analysis | Graft survival at 1, 2 and 5 years post transplant: 93%, 89% and 87%, respectively, for multiple arteries; 94%, 91% and 89%, respectively, for single arteries. Patient survival rates at 1, 2 and 5 years post transplant: 97%, 93% and 92%, respectively, for multiple arteries; 95%, 94% and 91%, respectively, for single arteries. Mean graft and patient survival rates at 10 years were 71% and 79%, respectively, for multiple arteries and 77% and 83%, respectively, for single arteries |
| Hamed a2013421 and a2014422 | UK | 1090 | NR | NR | Renal transplant recipients | Single-centre observational study | 52/1090 (4.8%) patients experienced early graft loss and had an 8.5 times increased risk of death, with 1-year survival less than that for those on the waiting list (76.9% vs. 88.8%); 5-year survival in the early graft loss group was better than that for waiting list patients (69.3% vs. 51.4%). Retransplantation after early graft loss resulted in 1-year graft survival of 86.7% |
| Humar a2009423 and a2010424 | UK | 318 | NR | NR | High-risk renal transplant recipients | International RCT | 1-year rates of acute rejection (17.2% vs. 11%) and graft loss (1.8% vs. 1.9%) were comparable between the 100-day and the 200-day prophylaxis groups respectively. Patient survival was 100% and 97% respectively |
| Jardine 2005425 | UK | 1052 | Mean 50 | 65.2 | Low-risk renal transplant recipients | RCT (but assessed placebo arm only in this analysis) | Over a mean 65.3-month follow-up, 54/1052 patients experienced cardiac death and 65/1052 experienced non-cardiac death and 66/1052 had a definite myocardial infarction |
| Karim 2014426 | UK | 19,103 | Median 45 | 61 | Renal transplant recipients, excluding multiorgan transplants | Retrospective database linkage and review (population cohort analysis) | 2085/19,103 patients died over a median follow-up of 4.4 years. Repeat transplantations occurred in 635 recipients over the 11-year period |
| Kent 2015370 | International including the UK | 7246 | Mean 63 | 64 | CKD ± CVD; CKD stages 1–3: 1494; CKD stage 4: 2228; CKD stage 5 no dialysis: 1017; on dialysis: 2498 | Randomised prospective trial | Vascular deaths per patient-year: CKD stages 1–3b 36 (0.6%), CKD stage 4 92 (1%), CKD stage 5 86 (2.2%), CKD stage 5 + dialysis 235 (2.5%); non-vascular deaths per patient-year: 1.6%, 2.5%, 3.8% and 4.9% respectively; kidney transplant in current period: 0.2%, 1.6%, 5.4% and 6.7% respectively; kidney transplant in earlier period: 0.1%, 1.4%, 8.5% and 2.5% respectively; dialysis initiated in current period: 1.3%, 6.2%, 18.2% and 0.2% respectively; dialysis from earlier period: 0.7%, 6.9%, 33.8% and 80.6% respectively |
| aKrishnan 2013427 | UK | 13,167 | NR | 64 | Renal transplant recipients | Retrospective database analysis | 857 (18%) patients suffered rejection and 205 (3%) patients died |
| aMark 2010428 | UK | 199 | NR | NR | Patients with CKD stage 5, with contrast cardiovascular magnetic resonance imaging and assessed for renal transplant | Cohort analysis | Over a median follow-up of 61.6 months there were 61 (30.7%) deaths, of which 36 (59%) were cardiovascular deaths |
| Marks 2012447 | UK | 3414 | Median 78.6 | 44.2 | CKD (67% stage 3, 30% stage 4, 2% stage 5), excluded patients receiving RRT | Retrospective cohort study (case note review) | At 6 years’ follow-up, 170 (5%) patients had initiated RRT (with 77 subsequently dying), 59% died without initiating RRT and 36% were alive without RRT. Adjusted incident rate ratios for initiating RRT: CKD stage 4 vs. 3: 5.60, stage 5 vs. 3: 38.10; adjusted incident rate ratios for all-cause mortality: 1.41 and 1.55 respectively |
| Moore 2011429 | UK | 2763 | Mean 46 | 60 | Renal transplant recipients surviving at least 12 months post transplant and all treated with ciclosporin mircroemulsion | Retrospective database analysis | In a development data set, 196/2763 (7%) patients died and 225 (8%) experienced transplant failure. In a validation data set, 44/731 (6%) patients died and 101 (14%) experienced transplant failure |
| Nath 2015430 | UK | 1095 | Mean 27–48 | 40–66 | Obese patients receiving single organ renal transplantation with multiple renal arteries | Retrospective single-centre analysis | 1-year graft survival: all 996/1095 (91%), underweight 30/33 (91%), normal weight 378/403 (94%), overweight 358/394 (91%), obese 230/265 (87%). Patient 1-year survival: survival for all 99%, all 97%, underweight 99%, normal weight 99%, overweight 98% and obese 99% respectively |
| NHS Blood and Transplant 2015450 | UK | 8608 | NR | NR | Renal transplant recipients/patients on transplant waiting list | Kidney Activity Report (registry analysis) | Over 1 year (2014/15), of 8608 patients on the kidney transplant waiting list, 5713 (66%) remained on the list, 2183 (25%) were transplanted, 491 (6%) were removed from the list and 221 (3%) died. For patients transplanted in 2010–13, 1-year graft survival was 94% and 1-year patient survival was 96% |
| Raymond 2007431 | UK | 106,366 | Mean 58 | 44.5 | Community population with at least one serum creatinine measurement, retrospectively assigned to CKD stages | Retrospective database analysis | Annual mortality increased with CKD stage: stages 1 and 2 1.9%, stage 3A 6.4%, stage 3B 11.1%, stage 4 16.5%, stage 5 20.6%. RR for mortality: 4.0, 8.3, 16.2 and 43.5 for CKD stage 3A, 3B, 4 and 5 respectively. This impact reduced with age |
| Renal Association 2014410 | UK | 6680 | NR | NR | RRT patients | Analysis of registry data | After 5 years’ follow-up, of 5034 patients on HD, 31% remained on dialysis, 16% had a transplant and 51% died. Of 1297 patients on PD, 29% remained on dialysis, 37% had a transplant and 33% died. Of 349 patients on the transplant waiting list, 4% were on dialysis, 92% remained on the transplant waiting list and 5% died |
| Robinson 2012432 | International including the UK | 24,525 | Mean 61–63 | 54–58 | Patients with ESRD for > 180 days, receiving haemodialysis | Prospective cohort study | 5849/24,525 patients died over 42,174 patient-years of follow-up (rate 0.14 per year) |
| Roderick 2009433 | UK | 15,336 | Median 80.2 | 39 | Older patients (75+ years) with CKD in the community | Clinical trial | In the first 2 years of follow-up, adjusted hazard ratios for all-cause mortality for eGFR bands 45–49, 30–44 and < 30 ml/minute/1.73 m2 vs. > 60 ml/minute/1.73 m2 were 1.13, 1.69 and 3.87, respectively, for males and 1.14, 1.33 and 2.44, respectively, for females |
| aSeitz 2015434 | UK | 14,027 | NR | NR | First-time renal transplant recipients | Retrospective database analysis | Median time to rejection was 126 days in the alemtuzumab group and 35 days in the non-alemtuzumab group |
| Shabir 2014435 | UK | 651 | Mean 44.2–52.4 | 60–63 | Renal transplant recipients alive at 12 months post transplantation | Cohort analysis | Development cohort: for patients alive at 12 months post transplant, 31/651 died from 1–5 years post transplant and 91/651 had transplant failure. Validation cohort: for patients alive at 12 months post transplant, 58/787 (7.4%) died from 1–5 years post transplant and 62/787 (7.9%) had transplant failure |
| Taal 2007436 | UK | 35 | Median 63.8 | 60 | CKD stages 4 and 5 not yet on dialysis. Excluded patients with renal transplantation or on dialysis | Retrospective analysis of longitudinal study | After a median of 12.4 months, 22/35 (63%) patients with stage 4/5 CKD had commenced dialysis |
| Thomson 2007437 | UK | 263 | Median 66.7 | 51.3 | Patients receiving haemodialysis in a renal unit | Retrospective analysis | Over an 18-month follow-up period, 65/263 (24.7%) patients had died; 15 underwent renal transplantation and one recovered renal function |
| Udayaraj 2009438 | UK | 2770 | Median 58 | 58 | Patients with ESRD receiving PD therapy at 180 days from start of RRT | Retrospective analysis of UK Renal Registry | 1104/2770 patients died over a median follow-up of 3.7 years |
| van der Velde 2011446 | International including the UK | 266,975 | NR | NR | High-risk cohorts | Systematic review and meta-analysis | Risk for all-cause mortality was not associated with an eGFR between 60 and 105 ml/minute/1.73 m2 but increased at lower levels. Hazard ratios at eGFRs of 60, 45 and 15 ml/minute/1.73 m2 were 1.03, 1.38 and 3.11, respectively, compared with an eGFR of 95 ml/minute/1.73 m2. There were similar findings for cardiovascular mortality |
| Vivek 2010439 | UK | 126 | Mean 46–55 | Male-to-female ratio from 16 : 16 to 56 : 38 | Renal transplant recipients | Retrospective cross-sectional study | Over a mean of 91 months’ follow-up, 35 (27.8%) patients died. Cardiac deaths accounted for 12% of all deaths |
| Wen 2014440 | UK | 1,130,472 | NR | NR | General population cohort | Meta-analysis | In Asian, whites and black patients, compared with an eGFR of 90–104 ml/minute/1.73 m2, the hazard ratios for eGFR 45–59 ml/minute/1.73 m2 were 1.25, 1.09 and 1.33 for all-cause mortality, 1.59, 1.40 and 1.44 for cardiovascular mortality and 27.6, 11.2 and 4.05 for ESRD respectively |
| Woo 2002441 | UK | 434 | NR | 62.9 | Renal transplant recipients | Longitudinal cohort study | Age (hazard ratio 1.03), diabetes (hazard ratio 2.72), smoking (hazard ratio 1.81) and family history of premature CVD (hazard ratio 2.17) were independent risk factors for patient survival. Acute rejection (hazard ratio 2.38), smoking (hazard ratio 1.48) and age (hazard ratio 1.04) were independent predictors of graft failure |
Limited evidence on costs and utilities for AKI was identified: no UK utility studies were identified (compared with nine in the CKD search) and only three UK cost studies were identified (compared with 33 for CKD), none of which reported disaggregated or per-patient critical care costs that could be used to inform the model health state costs. For AKI risks, a worldwide systematic review334 was identified that reported an ICU KDIGO AKI incidence of 31.7% (95% CI 28.6 to 35.0) in an all-comer population and 24.3% (20.4 to 28.8) in a post-cardiac surgery population. 456 This was deemed to be the most reliable study to inform the estimates of AKI incidence. It was noted, however, that other clinical studies reported incidences both above and below the reported 95% CIs;334–342 this parameter was therefore included in the sensitivity analysis.
Additional searches
Two additional searches were conducted in an attempt to identify data on (1) cost and quality of life outcomes for patients treated in the ICU (AKI and no AKI cohorts) and (2) the impact of early AKI intervention.
Costs and utilities
Subsequent searching of BioMed Central Critical Care journal publications identified a high-quality recent publication reporting follow-up costs and mortality for a large cohort of all-comer patients (n = 5259) treated in ICUs across Scotland (published after the date of the review). 457 No further searches were therefore conducted for ICU/follow-up costs.
To help inform ICU utility estimates, an additional search was undertaken using the Cost-effectiveness Analysis (CEA) Registry458 and School of Health and Related Research Health Utilities Database (ScHARRHUD) databases,459 which provide specific health state utility search functions. Key terms relating to hospital wards and the ICU were searched to identify UK papers reporting direct utilities on multiattribute utility indexes or through direct valuation exercises (i.e. SF-6D or TTO/SG utilities). Three relevant studies were identified460–462 and are summarised in Table 45. The studies by Hernández et al. 460 and Cuthbertson et al. 461 measured EQ-5D outcomes for all-comer ICU populations and were deemed to be of most relevance for the model.
| First author and year | n | Age (years) | Sex male (%) | Methods | Utility |
|---|---|---|---|---|---|
| Latimer 2013462 | 123 | All > 80 | 0–32 | RCT of shock-absorbing floor for hospital falls. EQ-5D data collected 3 months post discharge for patients treated on a general ward | Mean utility for the ‘no fall’ group 0.38 (vs. 0.27–0.36 for fall categories) |
| Hernández 2014460 | 286 | Median 59–60 | 60 | Trial assessing ICU follow-up programmes. EQ-5D data collected at baseline (shortly after ICU discharge) and 6 and 12 months post discharge | Mean utility: baseline: 0.44 standard care vs. 0.49 for increased follow-up; 6 months: 0.62 vs. 0.63 respectively; 12 months: 0.60 vs. 0.58 respectively |
| Cuthbertson 2010461 | 300 | Median 61 | 59 | Prospective cohort study to assess long-term quality of life post ICU admission. EQ-5D data were collected at 1, 2.5 and 5 years after ICU admission. Scores were compared with those of hypothetical age- and sex-matched control subjects | Mean utility: 12 months post ICU admission: 0.666 vs. 0.82 in matched cohort; 2.5 years’ follow-up: 0.701 vs. 0.818 in matched cohort; 5 years’ follow-up: 0.677 vs. 0.817 in matched cohort |
Impact of early acute kidney injury intervention
A key parameter in the model concerns the impact of early AKI intervention on patient health outcomes, that is, the ‘treatment effect’ parameter linked to tests resulting from early identification of AKI. Two previous economic evaluations of AKI biomarkers187,205 identified in the model review assumed a uniform 25% reduction in subsequent AKI risks as a result of early diagnosis. However, in both studies no evidence base was cited to support this key parameter estimate.
A literature search was conducted in March 2015 to identify reviews of early treatment/preventative strategies for AKI in the ICU across the following databases: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and The Cochrane Library. The search aimed to identify UK reviews including early treatment/preventative strategies for adults with AKI in the ICU, published in the last 5 years. Search strategies included the search concepts acute kidney injury, critical care, early treatment and treatment effectiveness (see Appendix 10 for full details).
The database searches identified 689 records, with 496 remaining after removal of duplicates (Figure 31). In total, 37 relevant reviews and papers were initially identified. Within these 37 studies, four key recurring interventions were identified: early RRT, early nephrologist involvement, AKI e-alert systems and intravenous administration of alkaline phosphatase. Based on a review of the literature findings and expert consultation, evidence on the impact of early RRT was deemed to be currently contentious and this intervention was therefore not explored further. For the remaining interventions citation tracking was conducted to identify UK primary ICU studies. In total, eight primary studies463–470 were included for data extraction and are summarised in Table 46.
FIGURE 31.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the AKI early intervention search.
| First author and year | Country | n | AKI status | Methods | Key results |
|---|---|---|---|---|---|
| Early nephrologist involvement | |||||
| Flores-Gama 2013464 | Mexico | 1096 | AKI (RIFLE/AKIN) within 7 days of cardiac surgery in the cardiac ICU | Retrospective time series analysis of impact of nephrology on-demand vs. nephrology on-site (i.e. integrated into the daily ICU team) on renal recovery | Patients treated with nephrology on-site had a lower incidence of AKI (25.7% vs. 31.9%; adjusted OR 0.71) and in-hospital mortality for severe AKI (34.1% vs. 55.9%; adjusted OR 0.33) and higher renal recovery at hospital discharge (61.0% vs. 35.3%; adjusted OR 3.57) than patients treated with nephrology on-demand. No differences in ICU stay or mechanical ventilation |
| Costa e Silva 2013465 | Brazil | 366 | AKI (increase of ≥ 50% in baseline serum creatinine level according to the RIFLE risk stage) in the ICU and length of ICU stay > 48 hours | Prospective observational study to assess the impact of early (< 48 hours from AKI diagnosis day) vs. delayed (≥ 48 hours after AKI diagnosis) nephrology consultation | Of 53.6% who had a nephrologist consultation, those with a delayed consultation had a higher rate of hospital mortality (adjusted OR 3.39), increased dialysis dependence (adjusted OR 3.25), a longer ICU stay (19 vs. 13.5 days), a longer time on mechanical ventilation support (16.5 vs. 10 days) and a longer time from diagnosis to dialysis (7 vs. 2 days) |
| Ponce 2011466 | Brazil | 148 | AKI (AKIN) in the ICU | Prospective observational study of the impact of delayed nephrologist consultation (≥ 48 hours after AKI diagnosis) | Of patients who received a consultation, 29 received an early consultation and 48 received a late consultation. Delayed consultation was associated with increased ICU mortality (65.4% vs. 88.2%, adjusted OR 1.32) |
| Mehta 2002467 | USA | 215 | AKI in the ICU and hospital. AKI defined according to blood urea nitrogen and serum creatinine criteria | Prospective observational study to assess the impact of delayed (≥ 48 hours) nephrology consultation | Delayed consultation was associated with increased in-hospital mortality (adjusted OR 2.0), length of hospital stay (median 19 vs. 16 days) and length of ICU stay (17 vs. 6 days) |
| Electronic e-alerts | |||||
| Wilson 2015463 | USA | 1393 | Patients in hospital (including ICU) with stage 1 or above KDIGO AKI | Single-blind, parallel-group RCT of a text-based e-alert system. Patients were stratified by medical vs. surgical admission and ICU vs. non-ICU location | The e-alert system did not affect patient clinical outcomes (change in creatinine level, dialysis and death at 7 days post randomisation) |
| Colpaert 2012468 | Belgium | 951 | AKI (RIFLE) in the ICU | Prospective time series analysis of a telephone alert system. Three consecutive study phases: a 1.5-month pre-alert control phase, a 3-month intervention phase and a 1.5-month post-alert control phase | More patients in the alert group received intervention within 60 minutes of alert (28.7% vs. 7.9% and 10.4% in the pre- and post-alert control groups respectively) and received fluid therapy, diuretics and vasopressors (p < 0.001) and more patients in the alert group returned to a baseline kidney function within 8 hours of a ‘risk’ alert (p = 0.048). There was no impact on ICU length of stay, use of RRT or mortality |
| Alkaline phosphatase | |||||
| Pickkers 2012469 | The Netherlands | 36 | Patients with severe sepsis or septic shock and AKI (AKIN) in the ICU | Prospective RCT to assess the impact of intravenous infusion of alkaline phosphatase within 48 hours of AKI onset | Creatinine clearance (baseline to day 28) was significantly higher in the treated group (from a mean of 50 ± 27 to 108 ± 73 ml/minute vs. from a mean of 40 ± 37 to 65 ± 30 ml/minute for placebo). Reductions in RRT requirement and duration were not significant |
| Heemskerk 2009470 | The Netherlands | 36 | Patients with bacterial infection, more than two systemic inflammatory response syndrome criteria and < 12 hours end-organ dysfunction onset in the ICU | A multicentre RCT to assess treatment with an initial bolus intravenous injection of alkaline phosphatase followed by continuous infusion over the following 23 hours and 50 minutes | Over 28 days, mortality was 24% in the alkaline phosphatase group vs. 36% in the placebo group; RRT use was 24% in the alkaline phosphatase group vs. 36% in the placebo group. In patients with AKI, serum creatinine levels tended to decrease over the 2 days after the start of alkaline phosphatase treatment (p = 0.12), whereas there was a non-significant increase in the placebo group (p = 0.49) |
Evidence on the impact of e-alert systems was mixed, with the largest identified study finding no impact. 463 Alkaline phosphatase appears to be a potentially effective treatment for AKI but as of yet there are limited data to support use of this intervention in practice. The evidence on the impact of early nephrologist consultation was deemed to be of most relevance for this study as this can reasonably be assumed to represent a proxy for the non-specific bundle of early AKI treatments that patients would access as a result of biomarker-led early diagnosis. All of the four identified studies in this area reported some impact of early consultation on patient mortality and the largest study (n = 1096) reported a significant impact of early consultation on the incidence of AKI, with an adjusted odds ratio of 0.71 (95% CI 0.53 to 0.95; p = 0.02). 464
Acute kidney injury registry data
The Epidemiology of AKI in ICU study471 is an ongoing prospective observational international study with the primary aim of identifying the incidence and prevalence of AKI among critically ill patients. The study includes in the first instance an initial ‘screening period’ during which data on patient age, sex, weight, baseline creatinine level, daily creatinine level and urine output are collected over 7 days from patient admission to ICUs across participating sites. As a result this study provides a registry of daily individual patient data for patients experiencing AKI in the ICU. If a patient is found to meet AKI criteria within the screening period, informed consent is pursued to enable further research-specific activity. All patients admitted to the ICU are eligible for inclusion, with the exception of patients on chronic haemodialysis or peritoneal dialysis within the past 12 months, those with a functioning kidney transplant and prisoners. Currently, six hospitals in England have enrolled or begun enrolling patients, with the highest number of enrolled patients to date coming from the Leeds Teaching Hospitals NHS Trust (LTHT).
For the AKI-Diagnostics study, in July 2015 data were requested from the AKI registry on patients treated at LTHT. Data were provided on 60 patients who experienced AKI in the ICU, 13 of whom were in the ICU post cardiac surgery. An overview of the baseline characteristics of these patients is provided in Table 47. A summary of patients’ ICU and hospital length of stay and mortality is provided in Table 48.
| Characteristic | AKI cohort (n = 60) | AKI post cardiac surgery (n = 13) |
|---|---|---|
| Mean (SD) age (years) | 68 (13) | 71 (7) |
| Male (%) | 77 | 92 |
| Type of ICU (%) | ||
| General | 71.7 | 0.0 |
| Cardiac | 21.7 | 69.2 |
| Surgical | 6.7 | 30.7 |
| Admission diagnosis (%) | ||
| Trauma | 1.7 | – |
| Cardiac surgery | 21.7 | 100 |
| Vascular surgery | 3.3 | – |
| Other surgery | 10.0 | – |
| Sepsis/septic shock | 13.3 | – |
| Intoxication | 0.0 | – |
| Neurological diagnosis | 3.3 | – |
| Cardiac diagnosis | 18.3 | – |
| Respiratory diagnosis | 18.3 | – |
| Nephrological diagnosis | 0.0 | – |
| Gastrointestinal diagnosis | 10.0 | – |
| Haematological diagnosis | 0.0 | – |
| Oncological diagnosis | 0.0 | – |
| Endocrine diagnosis | 0.0 | – |
| Not reported | 0.0 | – |
| Surgery status (%) | ||
| Emergency | 18.3 | 7.7 |
| Elective | 35.0 | 92.3 |
| None | 46.7 | 0.0 |
| Not reported | 0.0 | 0.0 |
| Variable | ICU | Hospital (from ICU admission) |
|---|---|---|
| AKI cohort (n = 60) | ||
| LOS < 30 days, % (n) | 92 (55) | 80 (48) |
| LOS < 60 days, % (n) | 98 (59) | 92 (55) |
| LOS < 90 days, % (n) | 100 (60) | 95 (57) |
| LOS (days), minimum, maximum | 0, 65 | 0, 175 |
| Mean LOS (days) | 8 | 19 |
| Median LOS (days) | 4 | 10 |
| Mortality, % (n) | 53 (32) | 59 (35/59a) |
| AKI cohort post cardiac surgery (n = 13) | ||
| LOS < 30 days, n/N (%) | 11/13 (85) | 10/13 (77) |
| LOS < 60 days, n/N (%) | 13/13 (100) | 11/13 (85) |
| LOS < 90 days, n/N (%) | 13/13 (100) | 12/13 (92) |
| LOS (days), minimum, maximum | 2, 39 | 4, 123 |
| Mean LOS (days) | 10 | 25 |
| Median LOS (days) | 6 | 11 |
| Mortality, % (n) | 8 (n = 1) | 15 (2) |
Data on these patients were used to determine patient ICU daily status across the 10 possible AKI hospital period health states: AKI KDIGO S0, S1, S2, S3 and S3 + RRT, discharged to hospital ward, discharged to hospital ward + RRT, discharged home, discharged home + RRT and mortality. The daily statuses were used to derive daily Dirichlet transition probabilities between the health states for the model baseline AKI cohort. When AKI status could not be determined because of missing serum creatinine or urine output data, the closest observed AKI status value was carried forwards or backwards, as required. An adjustment of +0.01 was applied to all values to enable Dirichlet estimation in the presence of zero values.
A summary of the patients’ maximum ICU AKI status is provided in Table 49 and a summary of patients’ daily AKI status is provided in Figure 32 [note that, because of the size of the daily Dirichlet transition matrices (10 × 10 × 90), these are not reported].
| Maximum ICU AKI status | % (n) |
|---|---|
| AKI S1 | 50 (30) |
| AKI S2 | 20 (12) |
| AKI S3 | 7 (4) |
| AKI S3 + RRT | 23 (14) |
FIGURE 32.
Leeds Teaching Hospitals NHS Trust AKI registry: daily ICU AKI status over 7 days from admission.
Intensive care unit and hospital discharge and mortality data were also used to derive Kaplan–Meier survival curves for ICU length of stay (Figure 33) and survival (Figure 34), which were used to calculate daily discharge and mortality probabilities for the no AKI cohort by applying a relative risk (RR) (RR 0.30 for ICU and hospital mortality and RR 0.54 for ICU length of stay) to the AKI cohort survival curves (see Model parameters for more details).
FIGURE 33.
Leeds Teaching Hospitals NHS Trust AKI registry: Kaplan–Meier survival curve for AKI cohort ICU length of stay.
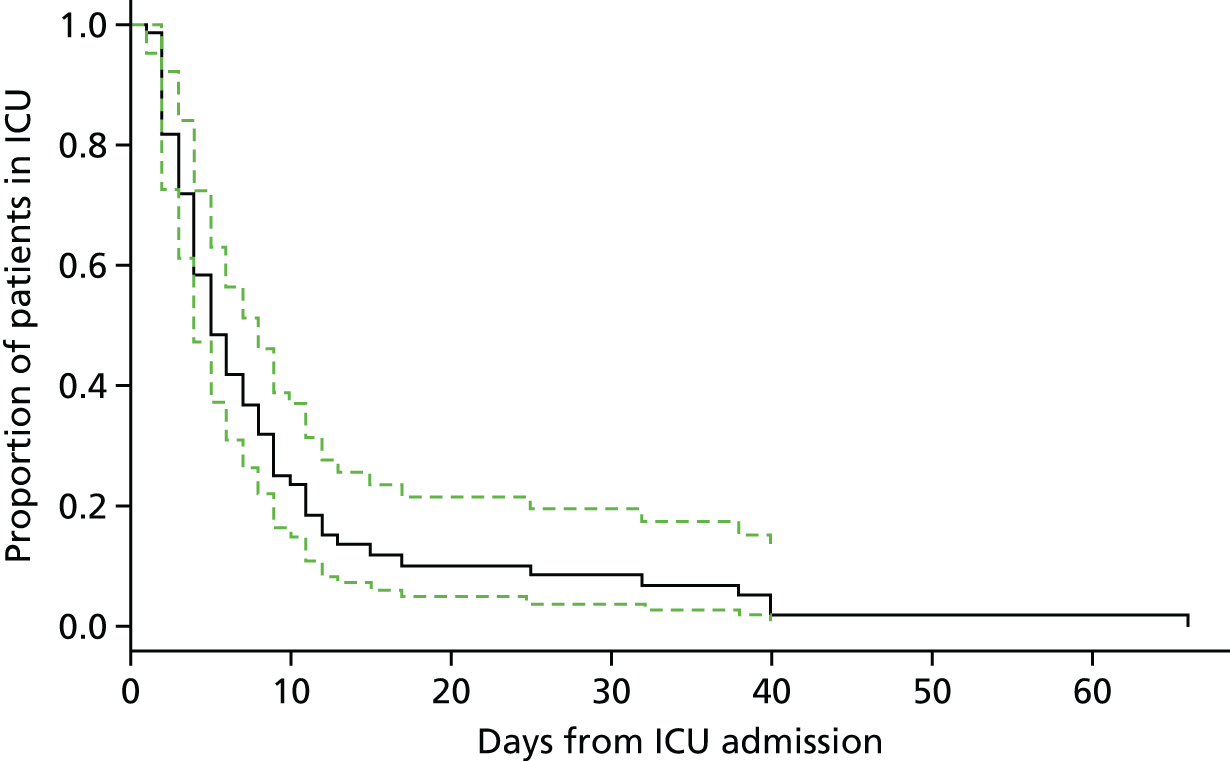
FIGURE 34.
Leeds Teaching Hospitals NHS Trust AKI registry: Kaplan–Meier survival curve for AKI cohort ICU survival.
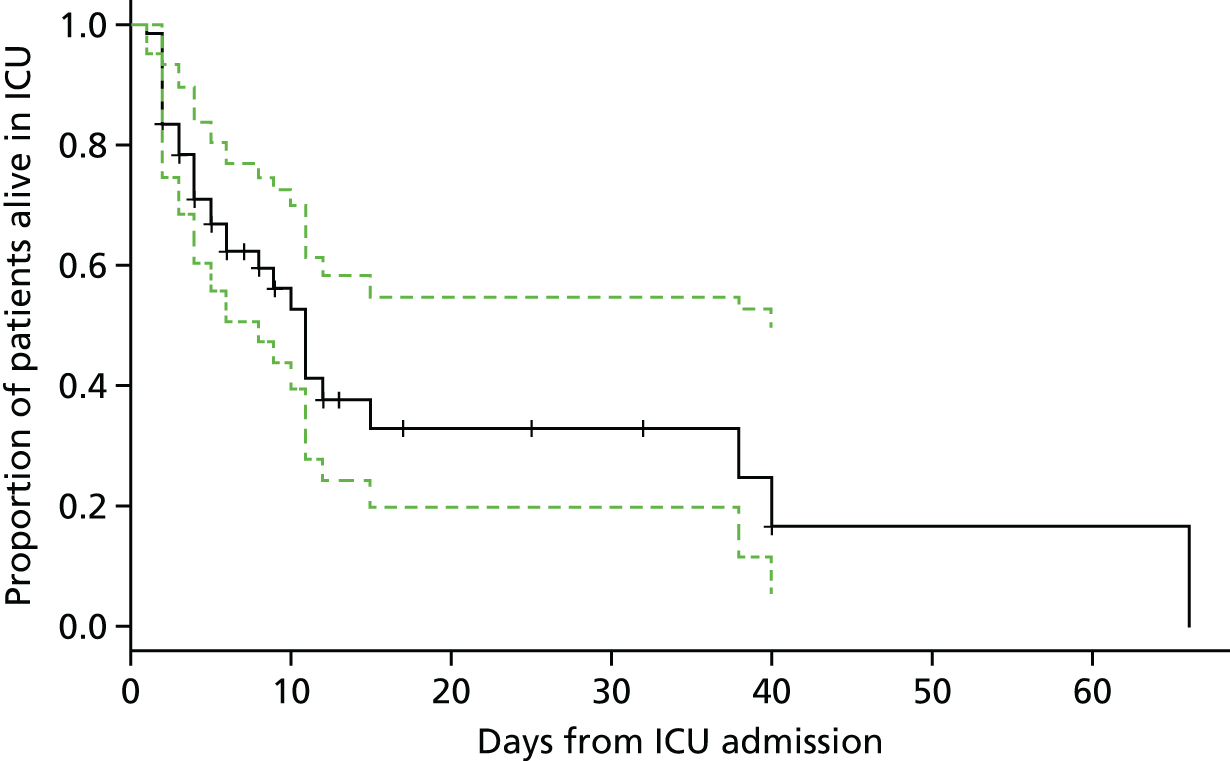
Model parameters
A summary of the model base-case parameters is provided in Table 50.
| Parameter | Base-case value | SD | Distribution | Source |
|---|---|---|---|---|
| Patient characteristics | ||||
| Starting age (years) of ICU cohort | 61 | – | Fixed | LTHT AKI registry data471 |
| Proportion male | 0.70 | – | Fixed | LTHT AKI registry data471 |
| Risks: hospital period | ||||
| Proportion who have or develop AKI in the ICU (AKI cohort) | 0.317 | 0.018 | Beta | Susantitaphong et al.334 |
| Proportion who have or develop AKI in the ICU post cardiac surgery (secondary analysis) | 0.243 | 0.021 | Beta | Susantitaphong et al.334 |
| AKI cohort: end day 1 with normal kidney function (as proportion of total ICU population) | 0.095 | 0.019 | Dirichlet | LTHT AKI registry data471 |
| AKI cohort: end day 1 with AKI S1 | 0.132 | 0.021 | Dirichlet | LTHT AKI registry data471 |
| AKI cohort: end day 1 with AKI S2 | 0.042 | 0.014 | Dirichlet | LTHT AKI registry data471 |
| AKI cohort: end day 1 with AKI S3 | 0.037 | 0.013 | Dirichlet | LTHT AKI registry data471 |
| AKI cohort: end day 1 on RRT | 0.011 | 0.007 | Dirichlet | LTHT AKI registry data471 |
| AKI cohort: daily transition probabilities for AKI cohort states (days 1–90) | – | – | Multiple Dirichlet | LTHT AKI registry data471 |
| AKI cohort: ICU mortality (used to inform no AKI cohort mortality only) | Survival curve | – | NA (10,000 curve simulations) | LTHT AKI registry data471 |
| AKI cohort: hospital ward (post ICU) daily mortality | 0.009 | 0.003 | Beta | LTHT AKI registry data471 |
| No AKI cohort: RR for ICU and hospital mortality in no AKI cohort vs. AKI cohort | 0.30 | 0.10 | Log-normal | Model calibration using LTHT AKI registry data,471 Susantiphong et al.,334 Chakkalakal et al.,338 de Mendonça et al.352 and Ostermann and Chang362 |
| AKI cohort: ICU length of stay (used to inform no AKI cohort discharge rates only) | Survival curve | – | NA (10,000 curve simulations) | LTHT AKI registry data471 |
| No AKI cohort: RR for ICU stay in no AKI cohort vs. AKI cohort | 0.542 | 0.15 | Log-normal | de Mendonça et al.,352 Prowle et al.,335 Ostermann and Chang362 and LTHT AKI registry data471 |
| No AKI cohort: probability discharged from ICU to hospital ward vs. home | 0.484 | 0.06 | Beta | LTHT AKI registry data471 |
| No AKI cohort: daily probability discharged from hospital | 0.089 | 0.03 | Beta | LTHT AKI registry data471 and Prowle et al.359 |
| Post-discharge mortality | 0.0003 | 1.33 × 10–5 | Beta | Lone et al.457 |
| On RRT post-discharge mortality | 0.0004 | 1.83 × 10–5 | Beta | Renal Association472 |
| Utilities: hospital period | ||||
| In ICU | –0.402 | 0.20 | Normal | Kind et al.473 (Appendix B) |
| In hospital ward (post ICU) | 0.44 | 0.31 | 1 – gamma | Hernández et al.460 |
| Discharged (post ICU) | 0.62 | 0.32 | 1 – gamma | Hernández et al.460 |
| Decrement for dialysis dependence (any time point post ICU discharge) | 0.11 | 0.02 | Normal | Wyld et al.402 |
| Daily costs: hospital period (£) | ||||
| Daily cost of ICU | 1306 | 290 | Log-normal | Department of Health474 |
| Daily cost of hospital ward | 304 | 111 | Log-normal | Curtis and Burns (p. 111)475 |
| Daily excess cost of AKI in hospital (any setting) | 265 | 77 | Log-normal | Department of Health474 |
| Daily excess cost of dialysis in hospital (any setting) | 691 | 1154 | Log-normal | Department of Health474 |
| Daily cost of discharged patient dialysis independent | 17 | 0.52 | Log-normal | Lone et al.457 |
| Daily cost of discharged patient dialysis dependent (excess cost) | 69 | 0.24 | Log-normal | Kent et al.370 |
| Utilities: follow-up period | ||||
| Post-discharge recovery (year 1) | 0.67 | 0.28 | 1 – gamma | Cuthbertson et al.461 |
| Post-discharge recovery (years 2–4) | 0.70 | 0.281 | 1 – gamma | Cuthbertson et al.461 |
| Post-discharge recovery (year 5 onwards, i.e. ‘recovered’) | 0.68 | 0.301 | 1 – gamma | Cuthbertson et al.461 |
| Successful kidney transplant | 0.68 | 0.301 | 1 – gamma | Assumed equivalent to ‘recovered’ |
| CKD stages 1–4 (decrement from ‘recovered’) | 0.02 | 0.03 | 1 – gamma | Wyld et al.402 |
| ESRD no dialysis (decrement from ‘recovered’) | 0.20 | 0.09 | 1 – gamma | Wyld et al.402 |
| ESRD maintenance dialysis (decrement from ‘recovered’) | 0.11 | 0.02 | Normal | Wyld et al.402 |
| Annual costs: follow-up period (£) | ||||
| Post-discharge follow-up (annual) year 1 | 6230 | 190 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 2 | 4010 | 156 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 3 | 3811 | 169 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 4 | 3618 | 182 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 5 | 3178 | 165 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 6 | 2739 | 165 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 7 | 2299 | 165 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 8 | 1860 | 165 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 9 | 1421 | 165 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 10 | 981 | 165 | Log-normal | Lone et al.457 |
| Post-discharge follow-up (annual) year 11+ | 542 | 165 | Log-normal | Lone et al.457 |
| Ratio for impact of AKI on follow-up costs | 1.15 | 0.074 | Log-normal | Lone et al.457 |
| CKD stages 1–4 (additional cost) | 579 | 56 | Log-normal | Kent et al.370 |
| ESRD no dialysis (additional cost) | 760 | 69 | Log-normal | Kent et al.370 |
| ESRD maintenance dialysis year 1 (additional cost) | 20,440 | 234 | Log-normal | Kent et al.370 |
| ESRD maintenance dialysis year 2+ (additional cost) | 25,035 | 87 | Log-normal | Kent et al.370 |
| Functioning transplant year 1 (additional cost) | 26,301 | 341 | Log-normal | Kent et al.370 |
| Functioning transplant follow-up years (additional cost) | 1467 | 122 | Log-normal | Kent et al.370 |
| Annual risks: follow-up period | ||||
| Starting distributions | From hospital model end states | – | – | From end state distributions from hospital period model (10,000 simulations) |
| Follow-up period mortality year 1 (AKI and no AKI) | 0.109 | 0.004 | Beta | Lone et al.457 |
| Follow-up period mortality years 2–5 (AKI and no AKI) | 0.066 | 0.002 | Beta | Lone et al.457 |
| Background age- and sex-standardised mortality (applied in year 6 onwards) | Mortality table | – | Fixed | Office for National Statistics476 |
| Baseline rate of CKD in post-ICU population | 0.0044 | – | – | Rimes-Stigare et al.477 |
| RR of CKD in AKI cohort vs. no AKI cohort | 7.6 | 1.25 | Log-normal | Rimes-Stigare et al.477 |
| CKD mortality (stages 1–4) | 0.03 | 0.002 | Beta | Kent et al.370 |
| CKD to ESRD + dialysis | 0.04 | 0.002 | Beta | Kent et al.370 |
| CKD to ESRD no dialysis | 0.01 | 0.001 | Beta | Kent et al.370 |
| ESRD (CKD stage 5) no dialysis mortality | 0.12 | 0.005 | Beta | Kent et al.370 |
| ESRD no dialysis to transplant | 0.09 | 0.004 | Beta | Kent et al.370 |
| ESRD no dialysis to ESRD + dialysis | 0.18 | 0.006 | Beta | Kent et al.370 |
| ESRD remain on dialysis | 0.784 | 0.006 | Dirichlet | Renal Association472 |
| ESRD + dialysis to transplant | 0.062 | 0.004 | Dirichlet | Renal Association472 |
| ESRD + dialysis to death | 0.154 | 0.006 | Dirichlet | Renal Association472 |
| Transplant success | 0.979 | 0.008 | Dirichlet | Renal Association472 |
| Transplant failure (move to dialysis) | 0.014 | 0.007 | Dirichlet | Renal Association472 |
| Transplant to death | 0.007 | 0.004 | Dirichlet | Renal Association472 |
| Test parameters | ||||
| Proportion who arrive in the ICU with pre-existing AKI (S2 and above) and who are therefore not tested | 0.066 | 0.017 | Beta | Communication with LTHT (January 2015) |
| Cost of the Nephrocheck test (£) | 71.27 | – | Fixed | Communication with manufacturer and LTHT (January 2015) |
| Cost of the NGAL test | 14.98 | – | Fixed | Communication with manufacturer and LTHT (January 2015) |
| Cost of the cystatin C test | 4.26 | – | Fixed | Communication with manufacturer and LTHT (January 2015) |
| Cost of early AKI intervention as a result of a positive test result (£) | 205 | 279 | Log-normal | Department of Health474 |
| RR for AKI risks as a result of early treatment | 0.782 | 0.255 | Log-normal | Flores-Gama et al.464 |
| Nephrocheck test: sensitivity | 0.90 | – | Multivariate normal | Meta-analysis |
| Nephrocheck test: specificity | 0.49 | – | Multivariate normal | Meta-analysis |
| NGAL test (plasma): sensitivity | 0.72 | – | Multivariate normal | Meta-analysis |
| NGAL test (plasma): specificity | 0.81 | – | Multivariate normal | Meta-analysis |
| NGAL test (urine): sensitivity | 0.70 | – | Multivariate normal | Meta-analysis |
| NGAL test (urine): specificity | 0.79 | – | Multivariate normal | Meta-analysis |
| NGAL test (serum): sensitivity | 0.92 | – | Multivariate normal | Chen et al.34 |
| NGAL test (serum): specificity | 0.69 | – | Multivariate normal | Chen et al.34 |
| Cystatin C test (plasma): sensitivity | 0.72 | – | Multivariate normal | Meta-analysis |
| Cystatin C test (plasma): specificity | 0.74 | – | Multivariate normal | Meta-analysis |
| Cystatin C test (urine): sensitivity | 0.68 | – | Multivariate normal | Meta-analysis |
| Cystatin C test (urine): specificity | 0.76 | – | Multivariate normal | Meta-analysis |
| Cystatin C test (serum): sensitivity | 0.76 | – | Multivariate normal | Meta-analysis |
| Cystatin C test (serum): specificity | 0.88 | – | Multivariate normal | Meta-analysis |
Hospital period parameters
For the hospital period of the model, the proportion of patients expected to experience AKI in the ICU (either on arrival or after) was derived from the worldwide systematic review (2004–12) of large cohort studies334 identified from the AKI risks literature search (see Model parameter literature review), which reported a pooled incidence rate across 41 studies (n = 888,604) for KDIGO AKI of 31.7% (95% CI 28.6% to 35.0%). For patients who experienced AKI in the ICU, the daily distribution and movement of patients between the AKI health states was derived using individual patient data obtained from the LTHT AKI registry (see Acute kidney injury registry data).
To determine the probability of ICU discharge and mortality in the no AKI cohort, RRs were applied to 10,000 simulations of the relevant survival curves from the AKI registry for the AKI cohort. For the probability of ICU discharge, the RR was determined by taking the mean ratio (0.542) of the median ICU length of stay for the no AKI group compared with the AKI group observed across three studies identified from the review, assuming a constant ratio over time. 349,352,359,362 The RR for ICU and hospital ward mortality was determined by model calibration, by setting the RR value such that the observed ratio of death for the AKI cohort compared with the no AKI cohort at day 10 in the model was equivalent to the mean ratio of ICU mortality (3.92) observed across three studies identified from the literature. 343,352,362
Daily hospital costs were derived from published NHS Reference Costs 2014 to 2015474 and Personal Social Services Research Unit (PSSRU) costs. 475 The daily cost of ICU (£1306) was determined by taking a weighted average of reported reference costs for critical care ‘Non-specific general adult critical care patients predominate’, across 0 to 6 organs supported (service code CCU01; Currency codes XC0[1–7]Z). The daily cost of the hospital ward was derived from the reported PSSRU cost for a non-elective inpatient short stay (£608), assuming that an average ‘short stay’ would be 2 days (i.e. daily cost £304). The daily excess cost of AKI in hospital (ICU or ward) was derived by taking the weighted average of reference costs for (long-stay) excess bed-days for non-elective inpatients with AKI either with or without interventions (service codes LA07[H/J/K/L/M/N/P]). This cost was assumed to be independent of the stage of AKI (for S1–S3 without RRT). A further excess cost of RRT was applied to all RRT states, which was taken as the weighted mean of reported reference costs for critical care ‘Renal Dialysis for Acute Kidney Injury’ using either haemodialysis or peritoneal dialysis for patients aged 19 years and over (service code RENALAKI; currency codes LE0[1/2]A).
In the absence of any identified data on patient utilities while in the ICU, patient utility was assumed to be equivalent to the utility of an unconscious patient reported in the EQ-5D scoring manual (–0.402)473 and to be independent of AKI status. As a result of the significant uncertainty around this parameter, it was included in the planned sensitivity analysis.
The utility of patients in the hospital ward and post-hospital discharge states was derived from a RCT460 identified from the review of utility databases (see Model parameter literature review), which evaluated a nurse-led ICU follow-up programme compared with standard care for 286 patients treated across three UK hospitals between 2006 and 2007. In the model, hospital ward utility is assumed to be equivalent to the reported baseline standard care arm utility (0.44) and post-discharge utility is assumed to be equivalent to the reported 6-month post-discharge utility (0.62). As for the ICU health states, because of a paucity of data, health state utilities for those on the general ward and those discharged were assumed to be independent of AKI status. However, a utility decrement was applied for anyone receiving RRT (–0.11), assuming a value equivalent to the reported disutility associated with chronic dialysis from a recent meta-analysis, which pooled data from 226 studies reporting dialysis utilities. 402
Follow-up period parameters
The starting distribution of patients across health states in the follow-up period of the model was taken from the end-state distribution of patients in the hospital period model. All patients in non-RRT health states were assumed to enter the relevant ‘follow-up’ state in the AKI or no AKI cohort follow-up model, whereas patients in the AKI cohort occupying a RRT health state at the end of the hospital period were assumed to transition to the CKD health state in the follow-up model. All patients who died in the hospital period transitioned to the corresponding ‘dead’ states in the follow-up period.
Post-discharge follow-up mortality and costs were derived from a recent large cohort study that provided 5-year follow-up mortality and hospital resource use data on 5259 patients surviving to hospital discharge after an ICU admission in Scotland. 457 After 5 years, mortality was assumed to return to population norm levels, which were sourced from UK Office for National Statistics data. 476 From year 6 to year 10, follow-up costs were assumed to continue to decrease at the same annual rate observed in the Lone et al. ,457 study with a constant value of standard deviation (SD); for year 11 onwards, costs were assumed to remain at the level calculated in year 11. For follow-up costs in the AKI cohort, a factor of 1.15 was applied to the baseline follow-up costs for the first 5 years. This value was derived from the Lone et al. 457 study, using the reported RR of 5-year follow-up hospital admission rates (used to derive costs in the study) for patients who required RRT during their ICU stay. This was assumed to be an appropriate proxy for the expected additional follow-up costs in the AKI cohort compared with the no AKI cohort as a result of renal injury in the first 5 years. Beyond 5 years, costs were assumed to be equal between the two cohorts.
The risk of progressing to the CKD state from the AKI cohort follow-up state was derived from a recent cohort study of 97,782 patients from the Swedish intensive care register (2005–11),370 which reported the incidence of CKD at 1 year post ICU discharge for AKI (6%) and no AKI (0.44%) survivors and the associated incident rate ratio (7.6). This additional risk was assumed to apply for the duration of the follow-up model (i.e. until death). Subsequent probabilities relating to progression from CKD to ESRD, renal transplantation and mortality were derived from the Renal Association472 and the study by Kent et al. ,370 which reported information on kidney disease progression and costs for 7246 patients included in the Study of Heart and Renal Protection (SHARP) international randomised trial. This study was also used to derive the annual costs of the CKD, ESRD and transplant health states.
Follow-up utility was derived from a prospective longitudinal cohort study that reported patient EQ-5D scores at 12 months (0.66), 2.5 years (0.701) and 5 years (0.677) post ICU admission for 300 patients treated within a UK university hospital ICU. Follow-up utility in year 1 of the follow-up model was assumed to be equivalent to the reported 12-month utility; utilities in years 2–4 were assumed to be equivalent to the reported 2.5-year utility; and utility in year 5 onwards was assumed to be equivalent to the reported 5-year utility. The utility for someone with a successful kidney transplant was assumed to be equivalent to the follow-up utility for year 5 onwards. Utility decrements for CKD, ESRD and chronic dialysis were applied to the successful transplant state value, using decrement values reported in a previous systematic review and meta-analysis of quality of life measures relating to CKD treatment modalities. 402
Test parameters
Test accuracy
Each test was assumed to be conducted on all patients arriving in the ICU without known AKI (KDIGO stages 2 and above). The proportion of patients arriving in the ICU with known AKI stage 2 or above (0.066) was estimated by communication with the LTHT (January 2015), who had previously requested these data from the Intensive Care National Audit & Research Centre (ICNARC) database.
Test accuracy (sensitivity and specificity) was based on the results of the AKI-Diagnostics systematic review and meta-analysis including papers on adult patients only (see Chapter 3). Using the pooled mean sensitivity and specificity values and variance–covariance matrices, test accuracies were determined by drawing from multivariate normal distributions to maintain parameter correlation, using the ‘mvrnorm’ function in the R ‘MASS’ package (version 3). 478 This effectively specifies that the logit sensitivity and specificity were generated from a bivariate normal distribution. When fewer than two papers were available to conduct a meta-analysis [i.e. for NGAL (serum) in the primary base-case analysis and Nephrocheck and cystatin C (plasma) in the post-cardiac surgery secondary analysis], sensitivity and specificity values were derived using the individual papers from the review (all tests had at least one paper for both the primary and the secondary analyses). For these three cases, because of a lack of reported data, variance–covariance values were assumed to be equivalent to the variance–covariance matrix from the meta-analysis of that specific test in the corresponding primary/secondary analysis. For example, for NGAL (serum) in the base case, the values for the test sensitivity and specificity variance–covariance matrix were assumed to be equivalent to those observed in the meta-analysis of NGAL (serum) in the post-cardiac surgery secondary analysis.
Using the multivariate normal distribution for test accuracy parameters can result in values > 1 (which are invalid) when the mean values are close to 1 and/or the variance around these parameters is large. In these cases, in the absence of any alternative methodology, affected distributions were truncated at 1. For most of the tests for which this occurred, the proportion of points on the distribution lying above 1 was minimal, with the exception of NGAL (serum) (Table 51). This is a limitation of the analysis and an area requiring future methodological research.
| Test | Population (primary/secondary analysis) | Proportion of points > 1 (%) | |
|---|---|---|---|
| Sensitivity distribution | Specificity distribution | ||
| Cystatin C (urine) | ICU all-comers | 00.42 | 00.01 |
| Post cardiac surgery | 00.07 | 03.55 | |
| Cystatin C (serum) | ICU all-comers | 00.15 | 00.00 |
| NGAL (serum) | ICU all-comers | 26.35 | 00.02 |
| Post cardiac surgery | 11.35 | 07.10 | |
Test costs
Direct test costs were derived by personal communication with each of the test manufacturers (January 2015). Laboratory staff and hospital overhead costs were derived by consultation with laboratory scientists and managers at LTHT (January 2015). The number of tests required to be conducted per year per hospital laboratory (n = 1253) was estimated based on the total number of ICU admission at St James’s University Hospital, Leeds, in 2015 (n = 1341) minus the proportion of patients expected to arrive with pre-existing moderate-to-severe AKI who would not be tested (6.5% of 1341 = 88). This was assumed to be broadly representative of the expected workload for a typical medium-sized NHS hospital laboratory.
A breakdown of each of the test costs is provided in the following sections.
Nephrocheck
Nephrocheck tests are currently provided by Astute Medical and Ortho Clinical Diagnostics (Raritan, NJ, USA; in partnership with Astute Medical). Nephrocheck can be run both on the Astute 140® Meter or using a high-throughput VITROS® platform. Currently, the evidence on the diagnostic accuracy of this test relates to its use on the Astute 140 Meter. In addition, there may be adoption barriers to using the VITROS platform because of the relative rarity of this platform in UK clinical laboratories; in particular, the recent trend in UK laboratories towards participation in managed service contracts is expected to result in limited opportunities for the utilisation of alternative platforms outside these contracts. It was therefore assumed in the model that the Astute 140 Meter would be used. Manufacturer-estimated costs, quoted in euros, were converted into pounds using an exchange rate of 0.84. 315
The cost of the Nephrocheck test consists of the:
-
kit cost:
-
kit cost = €1250 for 25 tests = €50 per test = £42 per test
-
liquid control kit = €120 (assumed one per kit) = €4.80 per test = £4.03 per test
-
kit paper roll = €2.90 (assumed one per kit) = €0.116 per test = £0.0974 per test
-
kit quality control and shipping costs included in the kit cost
-
total kit cost = £46.13
-
-
platform cost:
-
Astute 140 Meter platform acquisition cost (assumed one per laboratory) = €10,000 = £8400
-
platform maintenance for 5 years (assumed lifetime of platform) = €900 × 2 + €600 (cost of two 2-year warranties plus one 1-year warranty) = €2400 = £2016
-
platform external quality control device (assuming manufacturer-recommended four devices per year for 5 years) = €97 × 4 × 5 = €1940 = £1629.6
-
total platform cost (per test over 5-year lifetime) = £12,045.6/(5 × 1253) = £1.92 per test
-
-
indirect costs:
-
staff cost per test [assumed 30 minutes (top of the band) band 6 biomedical scientist time for non-automated test (£9.03) + 20% for headroom] = £10.85
-
trust overheads per test (assumed to be 21% of the total test cost) = (£46.13 + £1.92 + £10.85) × 0.21 = £12.37
-
-
total cost per Nephrocheck test = £46.13 + £1.92 + £10.85 + £12.37 = £71.26.
Neutrophil gelatinase-associated lipocalin
Neutrophil gelatinase-associated lipocalin tests are provided by two companies, BioPorto and Abbott Architect. Abbott Architect provides a specific platform for its test, whereas the BioPorto test can be run on several testing platforms. The BioPorto NGAL test kit is currently the cheaper option of the two and was therefore used in the cost calculation (assuming that the NHS would generally opt for the cheaper test). It was assumed that the test would be run using a Siemens ADVIA® 1800 platform (Siemens, Camberley, UK) as this is already used within several NHS laboratories (as are the Abbott Architect platforms). As these platforms are already in place and provide a significant turnover of tests under existing maintenance contracts, no additional platform maintenance or external quality control costs were included. In the absence of any data to indicate otherwise, costs for NGAL urine, plasma and serum tests were assumed to be equivalent. The cost of the NGAL tests consists of the:
-
kit cost:
-
kit cost = £3600 for 300 tests = £12.00 per test
-
kit quality control and calibration costs included in kit cost
-
shipping cost = £18.50 for 300 tests = £0.06 per test
-
-
platform cost:
-
not applicable
-
-
indirect costs:
-
staff cost per test [estimated cost of running automated assay using Genesys laboratory software (Daly City, CA, USA)] = £0.32
-
trust overheads per test (assumed to be 21% of the total cost) = (£12.06 + £0.32) × 0.21 = £2.60
-
-
total cost per NGAL test = £12.00 + £0.06 + £0.32 + £2.60 = £14.98.
Cystatin C
Several companies provide cystatin C test kits and the test may be run on several platforms. For this cost calculation, we assumed that the Siemens test and the ADVIA® Chemistry XPT Platform would be adopted, as this is currently used at the LTHT. As for the NGAL test, no platform maintenance or external quality control costs were applied and costs across the plasma, urine and serum tests were assumed to be equivalent. The cost of the cystatin C test consists of the:
-
kit cost:
-
kit cost = £250.63 for 400 tests = £0.63 per test
-
kit quality control costs per test (assuming 12 kits per year) = (£63.89 × 12)/1253 = £0.61
-
kit calibration costs per test (assuming six kits per year) = (£400 × 6)/1253 = £1.92
-
shipping cost assumed to be included in the kit cost
-
-
platform cost:
-
not applicable
-
-
indirect costs:
-
staff cost (estimated using Genesys laboratory software) = £0.32
-
trust overheads (assumed to be 21% of the total cost) = (£0.63 + £0.61 + £1.92 + £0.32) × 0.21 = £0.73
-
-
total cost per cystatin C test = £0.63 + £0.61 + £1.92 + £0.32 + £0.73 = £4.21.
Impact of early acute kidney injury Intervention
The impact of early AKI intervention [applied to patients with a TP test result and currently no AKI or early AKI (KDIGO stage ≤ 1) according to concurrent standard care tests] was derived from a Mexican study identified in the literature review of early AKI interventions. 464 This study assessed the impact of early nephrologist consultation in a group of 1096 patients within 7 days of cardiac surgery. The reported adjusted odds ratio (0.71) for AKI incidence was used to derive a RR, which was applied to all upwards AKI progressions from the S0 and S1 states and for mortality in the S3 + RRT state. This value was supported by independent consultation with the specialist advisory group.
Post-cardiac surgery subgroup population parameters
In the secondary analysis, alternative parameters specific to the post-cardiac surgery subgroup were adopted when possible (Table 52). When no post-cardiac surgery-specific evidence could be identified, parameter values were assumed to be equivalent to those used in the base-case analysis. It was assumed that the same patient pathway as adopted in the base case would apply for this subgroup.
| Parameter | Base-case value | SD | Distribution | Source |
|---|---|---|---|---|
| Risks: hospital period | ||||
| Proportion who have or develop AKI in the ICU | 0.243 | 0.021 | Beta | Susantitaphong et al.334 |
| No AKI cohort: RR for ICU and hospital mortality in the no AKI cohort vs. AKI cohort | 0.12 | 0.04 | Log-normal | Model calibration using LTHT AKI registry data471 and Bastin et al.348 |
| No AKI cohort: RR for ICU stay in the no AKI cohort vs. AKI cohort | 0.216 | 0.07 | Log-normal | Bastin et al.348 |
| No AKI cohort: daily probability discharged from hospital | 0.117 | 0.04 | Beta | LTHT AKI registry data471 and Bastin et al.348 |
| Daily costs: hospital period | ||||
| Daily cost of ICU (generic) | £1275 | get | Log-normal | Department of Health474 |
| Test parameters | ||||
| Nephrocheck: sensitivity | 0.80 | – | Multivariate normal | Meersch et al.54 |
| Nephrocheck: specificity | 0.83 | – | Multivariate normal | Meersch et al.55 |
| NGAL (plasma): sensitivity | 0.61 | – | Multivariate normal | Meta-analysis |
| NGAL (plasma): specificity | 0.77 | – | Multivariate normal | Meta-analysis |
| NGAL (urine): sensitivity | 0.66 | – | Multivariate normal | Meta-analysis |
| NGAL (urine): specificity | 0.62 | – | Multivariate normal | Meta-analysis |
| NGAL (serum): sensitivity | 0.84 | – | Multivariate normal | Meta-analysis |
| NGAL (serum): specificity | 0.87 | – | Multivariate normal | Meta-analysis |
| Cystatin C (plasma): sensitivity | 0.61 | – | Multivariate normal | Tziakas et al.69 |
| Cystatin C (plasma): specificity | 0.56 | – | Multivariate normal | Tziakas et al.69 |
| Cystatin C (urine): sensitivity | 0.52 | – | Multivariate normal | Meta-analysis |
| Cystatin C (urine): specificity | 0.72 | – | Multivariate normal | Meta-analysis |
| Cystatin C (serum): sensitivity | 0.73 | – | Multivariate normal | Meta-analysis |
| Cystatin C (serum): specificity | 0.72 | – | Multivariate normal | Meta-analysis |
For the estimation of AKI incidence, the same worldwide meta-analysis was used as in the base case,334 which reported a pooled KDIGO AKI incidence across 42 studies (n = 164,333 patients) in post-cardiac surgery populations of 24.3% (95% CI 20.4% to 28.8%). To inform the relative rate of ICU mortality and ICU and hospital discharge between the AKI cohort and the no AKI cohort, a single retrospective study was identified from the review that reported mortality and length of stay outcomes by AKI status for 1881 patients undergoing cardiac surgery necessitating CPB. 348 Daily ICU costs were derived as a weighted mean of critical care NHS Reference Costs 2014 to 2015 for ‘Cardiac surgical adult patients predominate’ services. 474
For test accuracies, sensitivity and specificity values for NGAL and cystatin C were derived from the post-cardiac surgery meta-analysis results, using the same methodology as outlined in the base case. For Nephrocheck, only one relevant paper was identified from the review, which did not report information on the parameter correlation. For this case we assumed the same variance–covariance matrix as observed in the meta-analysis of Nephrocheck studies conducted in all-comer ICU populations.
Summary of model assumptions
As with any economic evaluation, this study has limitations. Various simplifying assumptions were adopted in the analysis to produce an efficient and workable model. In particular:
-
The model is limited to an evaluation of the three tests judged currently to be of the highest priority. Many other tests are available and may represent cost-effective alternatives. The results of the multiway incremental analysis should therefore be interpreted with caution, as not all relevant comparators have been included.
-
The model considers only the use of one-off testing on entry to the ICU. Use of tests in a sequential or monitoring context have not been considered.
-
The model considers adult all-comers to the ICU only; paediatric populations were excluded from the analysis.
-
The model does not directly consider the case of AKI on top of CKD. The majority of evidence on the accuracy of the tests excludes patients with existing CKD and we have therefore not attempted to model these patients.
-
The model does not allow for re-entry to the ICU or hospital once a patient has been discharged. In reality, it is expected that in the short term a (potentially significant) proportion of patients would re-enter the ICU or hospital because of worsening health post discharge. This would be expected to have a noticeable impact on the results only if the biomarkers are expected to have an impact on the rate of re-admission.
-
The model does not allow for a hospital length of stay of > 90 days. Based on an analysis of the LTHT AKI registry data,471 it is expected that up to 5% of patients with AKI may stay in hospital for > 90 days (but 0% in the ICU).
-
Any difference between arms in the model in the downstream incidence of CKD is dictated by the proportion of patients ending the hospital period model in RRT states. As such, the model does not pick up the impact on CKD related to a reduced severity of AKI in terms of reduced rates of KDIGO S1–S3, or from a reduced duration of AKI. The impact of the tests on long-term CKD rates may be underestimated in this case.
-
The impact of early AKI intervention on patients’ risks of developing worse AKI in the model lasts for the whole hospital period (i.e. 90 days).
-
It was assumed that if patients are dialysis independent/dependent on hospital discharge, then they remain dialysis independent/dependent for the remainder of the hospital period of the model.
-
For the NGAL and cystatin C tests, diagnostic accuracy was determined by pooling data from studies using different cut-off point thresholds, because of the limited availability of data and reporting on alternative cut-off points. It is unclear, therefore, if these tests were to be adopted in practice, which cut-off point(s) should be used.
-
The analysis does not include societal costs because of the limited availability of data. Both the length of the ICU/hospital stay and the risk of CKD may have significant impacts on patient costs and productivity losses. This is therefore a key area for future research.
-
The secondary analysis of the subgroup of patients in the ICU post cardiac surgery is limited; only a few parameters were updated for this analysis based on data available from the literature reviews. In particular, the same ICU daily transition probabilities were applied for the AKI cohort, because of a limited number of data from the LTHT AKI registry on patients post cardiac surgery. Similarly, other key parameters in this analysis are not specific to this patient subgroup, for example early AKI treatment costs and impact, follow-up costs, mortality and utility and follow-up risk of CKD.
-
We have been unable to externally validate the model (i.e. compare the estimated model outputs to real-world data) because of a paucity of data with which to externally validate against. As a result, although we have conducted extensive internal validation and cross-validation (comparing the model outputs with those of other economic evaluations), the model results should be interpreted with caution.
Cost-effectiveness analysis
Cost-effectiveness is measured in terms of the ICER and the INB.
The ICER is calculated by dividing the difference in mean costs between two arms by the difference in mean health effects (QALYs) between the two arms:
where CT and ET are the expected cost and effectiveness of the intervention (i.e. test arm), CSC and ESC are the expected cost and effectiveness of the standard care arm and ΔC and ΔE are the incremental cost and effect, respectively, of the test arm compared with the standard care arm.
Assuming that the intervention is more costly and more effective than standard care, the ICER represents the additional cost required to be spent on the intervention to gain an additional unit of health. The cost-effectiveness of an intervention is determined by whether or not this ICER falls above or below the decision-maker’s willingness-to-pay per additional QALY (i.e. ‘threshold’). In the UK, NICE currently adopts a threshold value of £20,000 per QALY; if a new intervention has an ICER of < £20,000 per additional QALY then it is likely to be considered a cost-effective use of NHS resources, whereas an ICER of > £20,000 indicates that the intervention is not expected to be a cost-effective use of resources or is required to meet additional criteria. This decision rule is expressed using the following formula:
where λ is the adopted willingness-to-pay threshold.
When the threshold is known, we can divide the incremental cost by the threshold value to convert this value onto the QALY scale (or, conversely, multiply the QALYs by the threshold to express QALYs on the monetary scale). For example, if the threshold is £20,000 and the intervention has an additional cost of £10,000, we can calculate that this cost is equivalent to 0.5 QALYs. This allows us to rearrange the ICER formula to express the overall INB on the QALY scale or the incremental net monetary benefit (INMB) on the monetary scale:
Unlike the ICER, for which the exact interpretation of cost-effectiveness depends on whether or not the incremental cost and QALYs are positive or negative, the interpretation of the INB is straightforward: for any given set of strategies, the strategy with the greatest INB (or INMB) is the most cost-effective alternative. In addition, the favourable mathematical properties of the INB allow for more straightforward computation of the cost-effectiveness probabilities and, therefore, the INBs are presented alongside the ICERs for each of the analyses.
Cost-effectiveness was assessed by comparing each testing strategy with standard care in turn. In addition, a full incremental analysis, including all of the available tests within one evaluation, was conducted. In this approach testing strategies are ranked in order of increasing cost and any options that are either dominated (i.e. produce fewer QALYs for a greater cost than the next best alternative) or extendedly dominated (i.e. produce fewer QALYs for a greater cost than a linear combination of two alternatives) are removed. ICERs for the remaining strategies (now ordered in terms of increasing cost and QALYs) are then recalculated by comparing each strategy in turn to the next best alternative (i.e. the next most costly and effective strategy). 479
Sensitivity analysis
All model base-case analyses were run using probabilistic sensitivity analysis using 10,000 Monte Carlo simulations of the model. This accounts for joint parameter uncertainty in non-linear models by assigning probability distributions to each of the input parameters (using available variance data) and randomly drawing from these probabilities over the 10,000 simulations. The results are presented as a scatterplot on the cost-effectiveness plane (which presents incremental costs and QALYs for each intervention compared with standard care), with all base-case analyses assuming a willingness-to-pay threshold of £20,000. In addition, the results are presented using cost-effectiveness acceptability frontiers (CEAFs) to show (1) the probability that an intervention is cost-effective across different willingness-to-pay thresholds (range £0–150,000) and (2) which strategy is optimal based on having the highest mean net benefit (i.e. the ‘frontier’).
For each testing strategy a series of one-way sensitivity analyses was conducted, using 5000 simulations of the model. In the case of NGAL and cystatin C, for which multiple tests are available (i.e. plasma, urine and serum), the one-way sensitivity analyses were run only for the test found to have the highest probability of being cost-effective in the primary CEA.
The following one-way sensitivity analyses were conducted:
-
SA1: time horizon (base case: lifetime). A range of alternative time horizons were considered: 90 days (i.e. hospital period), 1 year (+90 days), 5 years (+90 days), 10 years (+90 days) and 20 years (+90 days).
-
SA2: test costs (base-case fixed costs: Nephrocheck £71.27, NGAL £14.99, cystatin C £4.62). A range of low and high test costs were explored, equal to 0.25, 0.5, 1.5, 2 and 5 times the base-case values.
-
SA3: AKI incidence (base case: mean 0.317, SD 0.018). A sensitivity analysis was conducted using a range of alternative values for the incidence of AKI in the ICU: 10%, 20%, 40% and 50%.
-
SA4: impact of the early AKI Intervention RR parameter (base case: mean RR 0.78, SD 0.25). Two sensitivity analyses were conducted around this key parameter:
-
Low-/high-impact scenarios – A sensitivity analysis was conducted assuming alternative values for the RR (0.2, 0.4, 0.6, 0.7, 0.9 and 0.95) to explore the possibility that early AKI intervention may be more or less effective than estimated in the base case.
-
Distribution adjustments – Ideally, a beta distribution would have been adopted for this parameter to ensure that the value was between 0 and 1 (i.e. that appropriate early intervention always resulted in improved outcomes). However, because of the high uncertainty around this parameter the beta distribution could not be used. In the base case this parameter was therefore drawn from a log-normal distribution, which maintains the expected ‘bell’ shape at the cost of allowing values > 1 (meaning that in some cases early AKI intervention leads to increased AKI risks). Two alternative parameterisations were explored: (1) truncating the distribution at 1 (i.e. recoding values > 1 to = 1) and (2) reducing the parameter uncertainty to allow beta estimation (SD 0.06).
-
-
SA5: cost of early AKI intervention (base case: mean £205, SD £279). A sensitivity analysis was conducted assuming alternative costs for early AKI intervention (£50, £100, £500, £800).
-
SA6: ICU utility (base case: mean –0.402, SD 0.201). A sensitivity analysis was conducted assuming alternative mean values of 0 and 0.2 for ICU utility, as there was a paucity of data for this value.
-
SA7: test accuracy adjustment (FP cases reset to TPs and overall incidence of AKI increased). The diagnostic accuracy studies informing the base-case sensitivity and specificity estimates for each of the tests were conducted using standard care testing as the reference standard, assuming that standard care perfectly identifies patients with and without AKI. However, standard care tests are known to be imperfect and, in particular, are expected to currently fail to identify many patients with early renal injury. An exploratory sensitivity analysis was conducted for each testing strategy assuming that 10%, 25%, 50% or 100% of test FP results are actually TP results (i.e. cases of early AKI that standard care testing failed to identify), with the associated reduced risks of AKI progression as a result of early AKI intervention. For simplicity, in the testing arms all of these extra cases of AKI were assumed to have a KDIGO AKI stage of S1. This analysis results in the overall incidence of AKI increasing by the proportion of FP results that have been recoded to TP results and the incidence of AKI is increased in the baseline arm to reflect this change.
-
SA8: negative impact of FP test results (increased mortality). The base-case analysis assumes no negative health consequences for patients falsely identified as having AKI. It is possible that these patients could suffer if, for example, access to required nephrotoxic agents, scans or other treatments is delayed. A sensitivity analysis was conducted assuming that, on average, patients with FP test results would experience a 5%, 10%, 30% or 50% increased risk of mortality while in the ICU.
-
SA9: impact of negative test results (reduction in monitoring leading to cost saving plus mortality risk for FNs). The focus of the base-case analysis was on the impact of testing on patients correctly diagnosed early. Testing may also enable reduced intensity of treatment or monitoring for patients with negative test results, at the expense of potentially worse outcomes for patients with FN results (as above). A sensitivity analysis was conducted, assuming that all patients with negative test results with early AKI (KDIGO ≤ S1) would have a reduced cost of ICU care (–£100) and that patients with FN results with early AKI would incur an increased mortality risk of 10%.
Cost-effectiveness results
Nephrocheck compared with standard care
Base case (adult intensive care unit all-comers)
The results for the base-case CEA of Nephrocheck testing compared with standard care are presented in Table 53. The addition of Nephrocheck testing on admission to the ICU for an all-comer adult population is expected to have a lifetime additional cost of £301 and an additional health benefit of 0.016 QALYs per patient. This results in an ICER of £19,324 per additional QALY, with a mean positive INB of 0.001 QALYs (equivalent to £20 in terms of INMB).
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Net benefit (QALYs) (95% CI) | INB (95% CI) | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | 32,596 (24,320 to 43,145) | 5.61 (0.46 to 9.09) | 3.979 (–1.14 to 7.34) | |||||||
| Nephrocheck | 32,897 (24,662 to 43,484) | 5.62 (0.46 to 9.12) | 301 (–1087 to 1713) | 0.016 (–0.16 to 0.20) | 19,324 | 3.985 (–1.15 to 7.33) | 0.001 (–0.12 to 0.13) | 0.57 | 0.32 | 0.48 |
The uncertainty around the model results can be seen in Figure 35. Although the mean ICER (£19,324) lies below the threshold line (indicating cost-effectiveness), the expected incremental cost and QALY results vary widely, with a significant proportion of points lying in non-cost-effective regions (i.e. above the threshold line).
FIGURE 35.
Scatterplot: incremental costs and QALYs for Nephrocheck vs. standard care (base case).
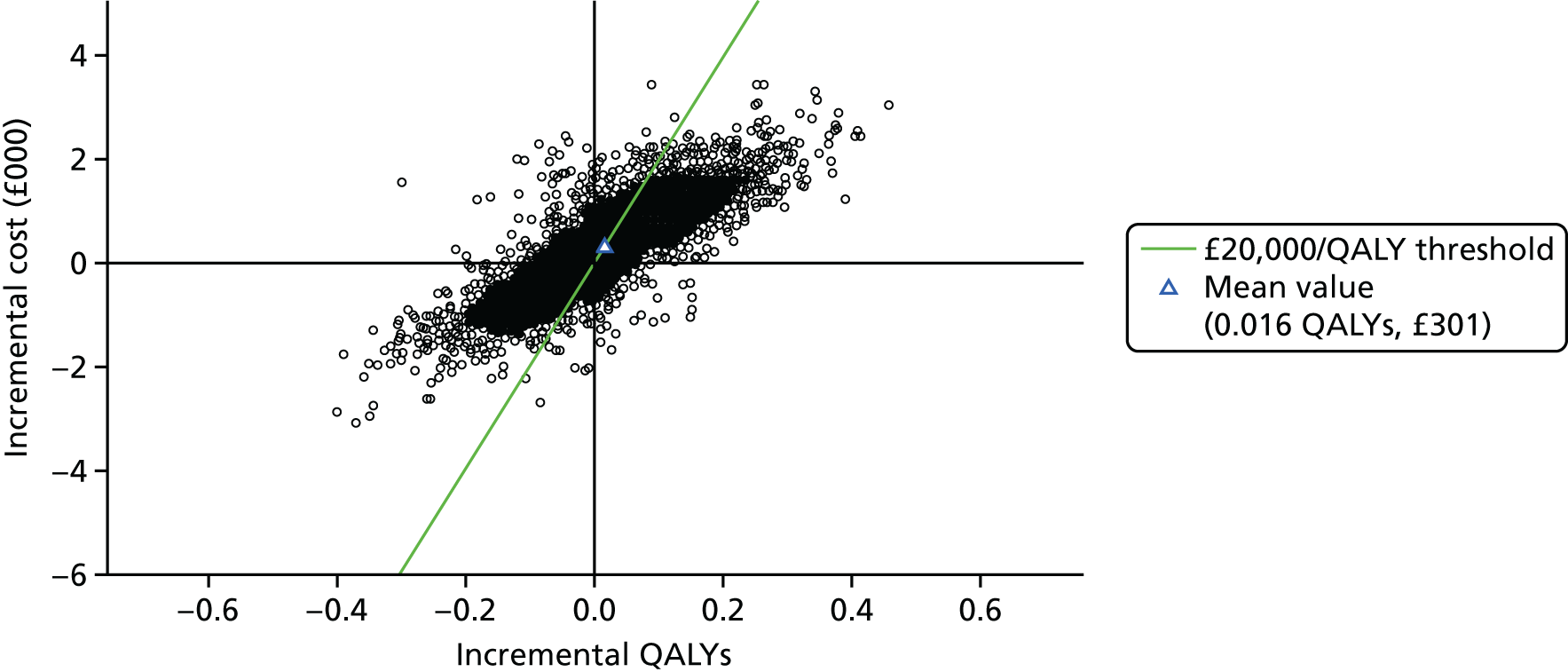
The uncertainty around the test cost-effectiveness over alternative thresholds is presented in the CEAF in Figure 36. Above a threshold of £19,400 per QALY, Nephrocheck testing is expected to be the most cost-effective strategy. The test has a 48% probability of being cost-effective at a £20,000 per QALY threshold, increasing to 54% at a £50,000 per QALY threshold.
FIGURE 36.
Cost-effectiveness acceptability frontier: Nephrocheck vs. standard care (base case).
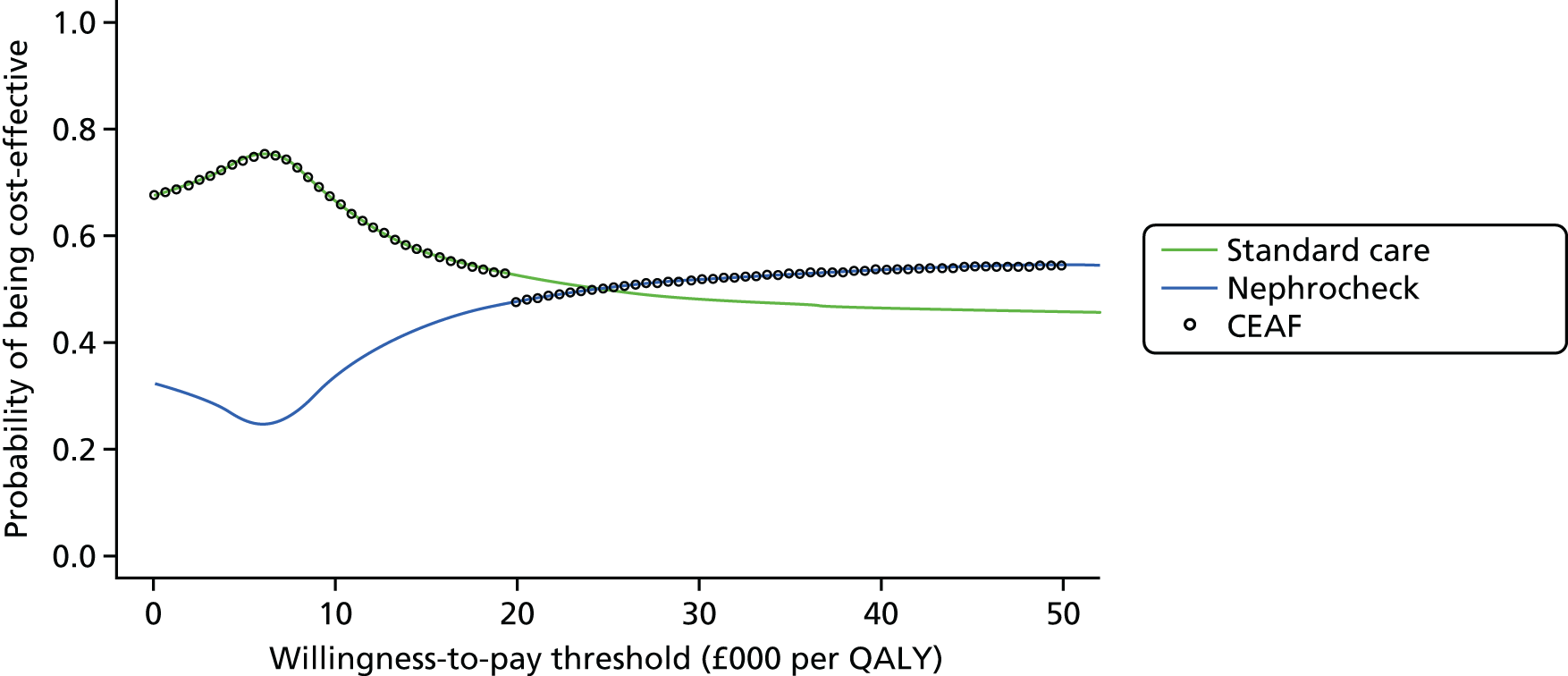
Secondary analysis (post-cardiac surgery subgroup)
The results of the secondary analysis are presented in Table 54. The addition of Nephrocheck testing in this setting is expected to have a lifetime additional cost of £205 and produce an additional 0.011 QALYs per patient. This results in an ICER of £18,617 per QALY, with a mean INB of 0.001 QALYs (equivalent to £20 in terms of INMB). At a £20,000 per QALY threshold there is a 50% probability that Nephrocheck testing is cost-effective in this subgroup, rising to 54% at a threshold of £50,000 (CEAF not shown).
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Net benefit (QALYs) (95% CI) | INB (95% CI) | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | 29,959 (22,738 to 37,726) | 6.50 (0.56 to 10.20) | 5.01 (–0.90 to 8.60) | |||||||
| Nephrocheck | 30,163 (22,893 to 37,942) | 6.52 (0.56 to 10.20) | 205 (–850 to 1249) | 0.011 (–0.13 to 0.15) | 18,617 | 5.01 (–0.91 to 8.60) | 0.001 (–0.09 to 0.10) | 0.57 | 0.34 | 0.50 |
Sensitivity analyses
The results of the one-way sensitivity analyses conducted for Nephrocheck testing compared with standard care are provided in Table 55. Nephrocheck testing is no longer cost-effective (i.e. has an ICER of > £20,000 per QALY) when (1) the time horizon is reduced to ≤ 20 years (SA1), (2) the test cost is increased by ≥ 50% (i.e. to ≥ £106.91) (SA2), (3) the incidence of AKI in the ICU is reduced to ≤ 20% (from 31.7% in the base case) (SA3), (4) the impact of early AKI intervention is limited to a ≤ 10% reduction in AKI risks (from 22% in the base case) (SA4), (5) the cost of early AKI intervention is increased to £500 or £800 (£205 in the base case) (SA5), (6) FP test results are assumed to lead to a ≥ 5% increased risk of ICU mortality (SA8) and (7) negative test results are assumed to lead to a £100 cost saving (because of diminished monitoring) and a simultaneous 10% increased mortality rate for FPs (SA9). In contrast, the only instances when the probability that Nephrocheck testing is cost-effective rises above 60% are when 25%, 50% or 100% of FP test results are assumed to have actually been TP results (with the incidence of AKI increasing to 40%, 49% and 66% respectively). In these cases the ICER falls below £10,000 per QALY (SA7).
| Sensitivity analysis | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) | Net benefit (QALYs) | INB | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Base-case analysis (for comparison) | ||||||||||
| Nephrocheck base case | 32,897 | 5.62 | 301 | 0.016 | 19,323 | 3.99 | 0.001 | 0.57 | 0.32 | 0.48 |
| SA1: time horizon (base case: lifetime, i.e. approximately 40 years) | ||||||||||
| 90 days | 13,928 | 0.05 | 210 | 0.000 | 1,679,292 | –0.64 | –0.010 | 0.54 | 0.26 | 0.25 |
| 1 year (+90 days) | 17,626 | 0.52 | 232 | 0.002 | 150,509 | –0.36 | –0.010 | 0.57 | 0.26 | 0.23 |
| 5 years (+90 days) | 25,732 | 2.02 | 264 | 0.006 | 43,783 | 0.73 | –0.007 | 0.57 | 0.29 | 0.30 |
| 10 years (+90 days) | 29,626 | 3.38 | 283 | 0.010 | 27,816 | 1.90 | –0.004 | 0.58 | 0.31 | 0.41 |
| 20 years (+90 days) | 31,859 | 5.04 | 302 | 0.015 | 20,163 | 3.45 | 0.000 | 0.57 | 0.32 | 0.47 |
| SA2: Nephrocheck test cost (base-case cost £71.27) | ||||||||||
| Test cost × 0.25 = £17.82 | 32,901 | 5.62 | 263 | 0.017 | 15,648 | 3.98 | 0.004 | 0.57 | 0.35 | 0.50 |
| Test cost × 0.50 = £35.64 | 32,918 | 5.62 | 279 | 0.017 | 16,640 | 3.98 | 0.003 | 0.57 | 0.34 | 0.49 |
| Test cost × 1.50 = £106.91 | 32,985 | 5.62 | 346 | 0.017 | 20,608 | 3.97 | –0.001 | 0.57 | 0.30 | 0.46 |
| Test cost × 2.0 = £142.54 | 33,018 | 5.62 | 379 | 0.017 | 22,591 | 3.97 | –0.002 | 0.57 | 0.29 | 0.45 |
| Test cost × 5.0 = £365.35 | 33,226 | 5.62 | 588 | 0.017 | 34,995 | 3.96 | –0.013 | 0.57 | 0.19 | 0.36 |
| SA3: ICU AKI incidence (base case: mean 0.317, SD 0.018) | ||||||||||
| AKI incidence = 0.10 | 32,558 | 6.26 | 237 | 0.005 | 45,241 | 4.63 | –0.007 | 0.59 | 0.16 | 0.33 |
| AKI incidence = 0.20 | 32,768 | 5.97 | 273 | 0.010 | 26,096 | 4.33 | –0.003 | 0.59 | 0.26 | 0.44 |
| AKI incidence = 0.40 | 33,187 | 5.39 | 346 | 0.021 | 16,523 | 3.73 | 0.004 | 0.59 | 0.33 | 0.50 |
| AKI incidence = 0.50 | 33,397 | 5.10 | 383 | 0.026 | 14,609 | 3.43 | 0.007 | 0.59 | 0.35 | 0.52 |
| SA4: impact of early AKI intervention on AKI risks (base case: RR 0.78, SD 0.25) | ||||||||||
| RR = 0.20 (i.e. 80% reduced risks) | 33,171 | 5.66 | 532 | 0.051 | 10,347 | 3.997 | 0.025 | 0.68 | 0.26 | 0.59 |
| RR = 0.40 (60% reduced risks) | 33,125 | 5.65 | 487 | 0.044 | 11,026 | 3.992 | 0.020 | 0.68 | 0.26 | 0.59 |
| RR = 0.60 (40% reduced risks) | 33,052 | 5.64 | 413 | 0.033 | 12,702 | 3.984 | 0.012 | 0.65 | 0.28 | 0.55 |
| RR = 0.70 (30% reduced risks) | 33,006 | 5.63 | 367 | 0.025 | 14,471 | 3.980 | 0.007 | 0.63 | 0.29 | 0.52 |
| RR = 0.90 (10% reduced risks) | 32,898 | 5.61 | 260 | 0.009 | 29,199 | 3.969 | –0.004 | 0.55 | 0.34 | 0.43 |
| RR = 0.95 (5% reduced risks) | 32,868 | 5.61 | 230 | 0.004 | 52,500 | 3.965 | –0.007 | 0.52 | 0.35 | 0.41 |
| RR values > 1 reset to 1 | 32,973 | 5.62 | 335 | 0.020 | 16,406 | 3.976 | 0.004 | 0.59 | 0.31 | 0.49 |
| RR SD reduced to 0.06 | 32,963 | 5.62 | 325 | 0.019 | 17,249 | 3.975 | 0.003 | 0.60 | 0.30 | 0.49 |
| SA5: cost of early AKI intervention (base case: mean cost £205, SD £279) | ||||||||||
| Cost of early AKI treatment = £50 | 32,843 | 5.62 | 205 | 0.017 | 12,180 | 3.979 | 0.007 | 0.57 | 0.38 | 0.53 |
| Cost of early AKI treatment = £100 | 32,871 | 5.62 | 232 | 0.017 | 13,824 | 3.978 | 0.005 | 0.57 | 0.37 | 0.51 |
| Cost of early AKI treatment = £500 | 33,092 | 5.62 | 453 | 0.017 | 26,975 | 3.967 | –0.006 | 0.57 | 0.24 | 0.41 |
| Cost of early AKI treatment = £800 | 33,257 | 5.62 | 619 | 0.017 | 36,839 | 3.958 | –0.014 | 0.57 | 0.17 | 0.34 |
| SA6: ICU utility (base case: mean –0.402, SD 0.201) | ||||||||||
| ICU utility = 0.00 | 32,947 | 5.63 | 309 | 0.017 | 18,704 | 3.982 | 0.001 | 0.57 | 0.33 | 0.47 |
| ICU utility = 0.20 | 32,947 | 5.63 | 309 | 0.017 | 18,690 | 3.987 | 0.001 | 0.57 | 0.33 | 0.47 |
| SA7: test accuracy adjustment (FPs reset to TPs and overall incidence of AKI increased) | ||||||||||
| 10% of FP results recoded as TPs (AKI S1) and overall incidence of AKI increased to 35.2% | 33,161 | 5.54 | 418 | 0.035 | 11,857 | 3.882 | 0.014 | 0.65 | 0.28 | 0.56 |
| 25% of FP results recoded as TPs (AKI S1) and overall incidence of AKI increased to 40.4% | 33,326 | 5.41 | 559 | 0.061 | 9228 | 3.740 | 0.033 | 0.72 | 0.24 | 0.66 |
| 50% of FP results recoded as TPs (AKI S1) and overall incidence of AKI increased to 49% | 33,751 | 5.20 | 774 | 0.102 | 7620 | 3.508 | 0.063 | 0.80 | 0.21 | 0.76 |
| 100% of FP results recoded as TPs (AKI S1) and overall incidence of AKI increased to 66.4% | 34,489 | 4.76 | 1217 | 0.184 | 6606 | 3.039 | 0.123 | 0.87 | 0.18 | 0.84 |
| SA8: increased mortality for FP results | ||||||||||
| 5% increased ICU mortality for FPs | 32,877 | 5.61 | £238 | 0.004 | 63,268 | 3.964 | –0.008 | 0.50 | 0.36 | 0.41 |
| 10% increased ICU mortality for FPs | 32,803 | 5.60 | £165 | –0.009 | Dominated | 3.955 | –0.017 | 0.44 | 0.41 | 0.34 |
| 30% increased ICU mortality for FPs | 32,517 | 5.54 | –121 | –0.060 | 2035a | 3.919 | –0.053 | 0.25 | 0.57 | 0.18 |
| 50% increased ICU mortality for FPs | 32,244 | 5.50 | –394 | –0.108 | 3646a | 3.884 | –0.088 | 0.15 | 0.69 | 0.11 |
| SA9: impact of negative test results (reduction in monitoring leading to cost saving + increased mortality risk for FNs) | ||||||||||
| One-off cost saving (–£100) + 10% increased ICU mortality for FNs | 32,558 | 5.58 | –80 | –0.020 | 4100a | 3.957 | –0.016 | 0.40 | 0.55 | 0.38 |
Neutrophil gelatinase-associated lipocalin compared with standard care
Base case (adult intensive care unit all-comers)
The results for the base-case CEA of NGAL testing compared with standard care are shown in Table 56. NGAL testing is associated with a lifetime additional cost of £164–215 (depending on the specific test) and an additional health benefit of 0.012–0.016 QALYs. This results in ICERs of £13,372–13,828 per additional QALY, with mean INBs of 0.004–0.005 (equivalent to £80–100 in terms of INMB). At a threshold value of £20,000 per additional QALY, NGAL (plasma) testing has the highest probability of being cost-effective (51.9%) and is therefore explored in the sensitivity analysis.
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Net benefit (QALYs) (95% CI) | INB (95% CI) | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | 32,596 (24,320 to 43,145) | 5.61 (0.46 to 9.09) | 3.979 (–1.14 to 7.34) | |||||||
| NGAL (urine) | 32,759 (24,521 to 43,351) | 5.62 (0.46 to 9.11) | 164 (–1179 to 1517) | 0.0119 (–0.16 to 0.19) | 13,742 | 3.985 (–1.15 to 7.33) | 0.004 (–0.11 to 0.13) | 0.56 | 0.40 | 0.514 |
| NGAL (plasma) | 32,759 (24,516 to 43,339) | 5.62 (0.46 to 9.11) | 164 (–1180 to 1510) | 0.0122 (–0.16 to 0.19) | 13,372 | 3.983 (–1.15 to 7.33) | 0.004 (–0.11 to 0.13) | 0.56 | 0.40 | 0.519 |
| NGAL (serum) | 32,811 (24,587 to 43,427) | 5.62 (0.46 to 9.11) | 215 (–1167 to 1619) | 0.0156 (–0.16 to 0.20) | 13,828 | 3.987 (–1.15 to 7.33) | 0.005 (–0.11 to 0.14) | 0.57 | 0.37 | 0.517 |
The uncertainty around the results for NGAL (plasma) testing is shown in Figures 37 and 38 [the results for NGAL (urine) and (serum) testing have a very similar distribution and are not presented]. As for Nephrocheck testing, although the mean ICER for NGAL (plasma) testing (£13,372) lies below the threshold line, the individual simulation points vary widely. Above a threshold of £13,400 per QALY, NGAL (plasma) testing is expected to be the most cost-effective strategy. The test has a 52% probability of being cost-effective at a £20,000 per QALY threshold, increasing to and levelling out at 56% at a £50,000 per QALY threshold.
FIGURE 37.
Scatterplot: incremental costs and QALYs for NGAL (plasma) vs. standard care (base case).
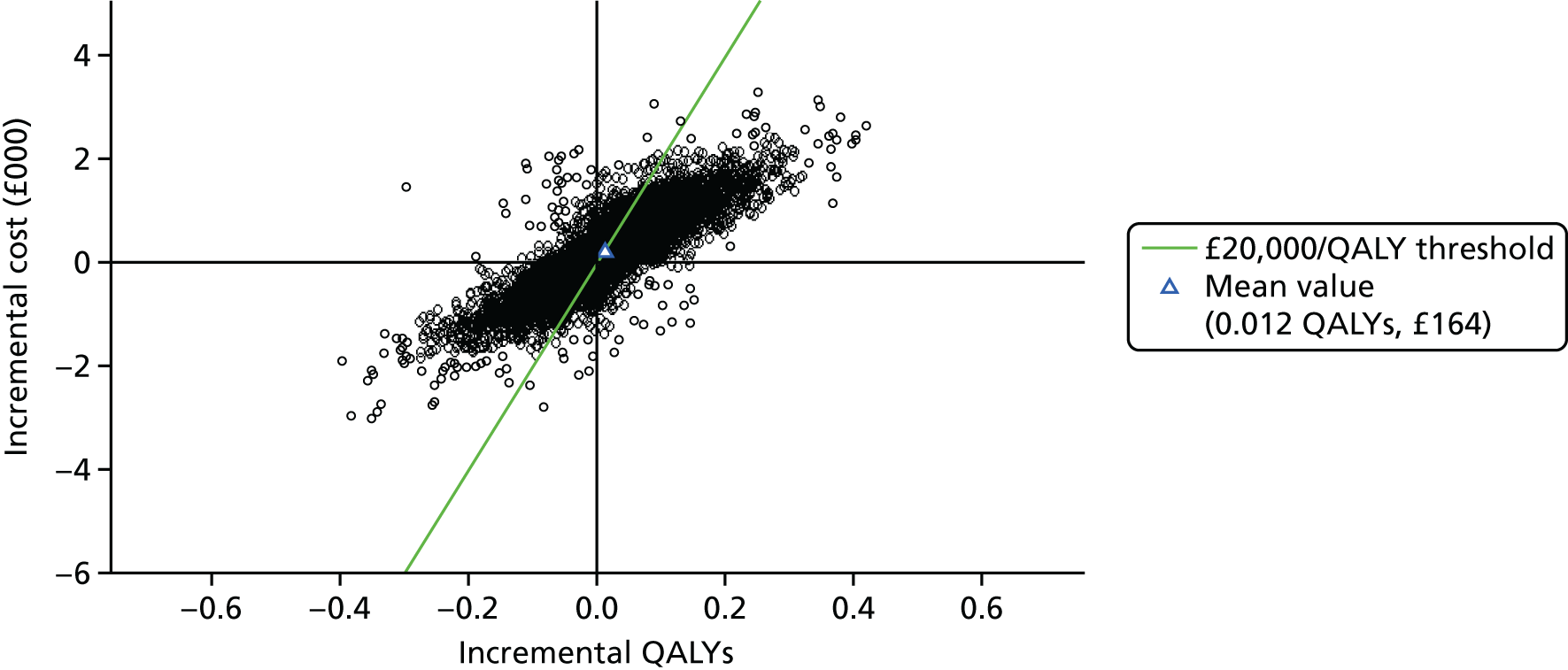
FIGURE 38.
Cost-effectiveness acceptability frontier: NGAL (plasma) vs. standard care (base case).
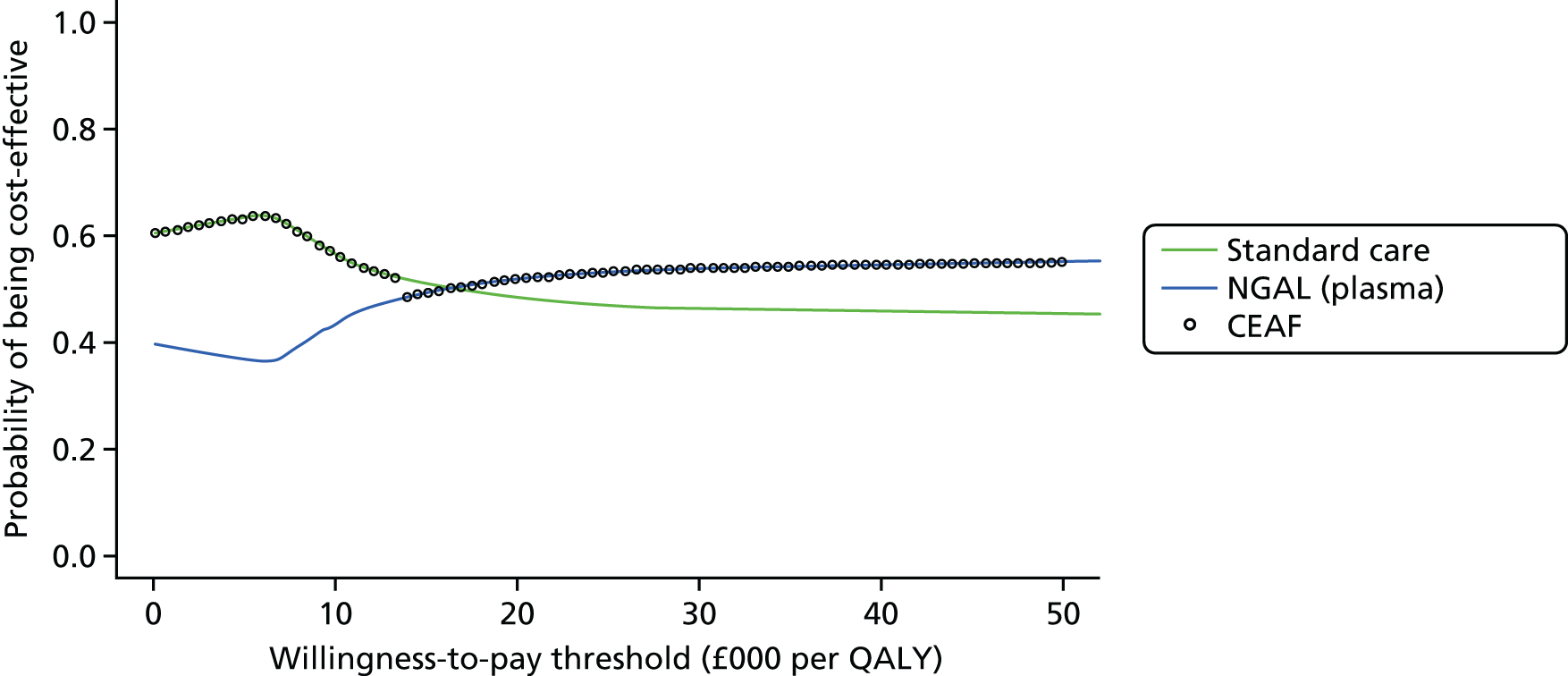
Secondary analysis (post-cardiac surgery subgroup)
The results of the secondary analysis conducted in a subgroup of patients after cardiac surgery are presented in Table 57. In this subgroup, NGAL testing is expected to have a lifetime additional cost of £137–172 and produce an additional health benefit of 0.008–0.012 QALYs per patient. This results in ICERs ranging from £13,051 to £19,287 per additional QALY, with mean INBs of 0.001–0.004 QALYs.
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Net benefit (QALYs) (95% CI) | INB (95% CI) | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | 29,959 (22,738 to 37,726) | 6.50 (0.56 to 10.20) | 5.007 (–0.90 to 8.60) | |||||||
| NGAL (plasma) | 30,096 (22,836 to 37,867) | 6.51 (0.56 to 10.21) | 137 (–895 to 1178) | 0.008 (–0.13 to 0.14) | 16,709 | 5.008 (–0.90 to 8.60) | 0.001 (–0.09 to 0.09) | 0.56 | 0.39 | 0.50 |
| NGAL (urine) | 30,131 (22,885 to 37,900) | 6.51 (0.56 to 10.21) | 172 (–875 to 1225) | 0.009 (–0.13 to 0.14) | 19,287 | 5.007 (–0.90 to 8.60) | 0.003 (–0.09 to 0.09) | 0.56 | 0.36 | 0.48 |
| NGAL (serum) | 30,108 (22,845 to 37,892) | 6.52 (0.56 to 10.22) | 149 (–905 to 1204) | 0.012 (–0.13 to 0.15) | 13,051 | 5.011 (–0.90 to 8.60) | 0.004 (–0.09 to 0.10) | 0.57 | 0.38 | 0.52 |
Sensitivity analyses
The results of the one-way sensitivity analyses conducted for NGAL (plasma) testing compared with standard care are provided in Table 58. NGAL testing is no longer cost-effective (i.e. has an ICER of > £20,000 per QALY) when (1) the time horizon is ≤ 5 years (SA1), (2) the incidence of AKI in the ICU is reduced to 10%, (from 31.7% in the base case) (SA3), (3) the impact of early AKI intervention is limited to a 5% reduction in AKI risks (from 22%) (SA4), (4) the cost of early AKI intervention is increased to £800 (from £205) (SA5), (5) FP test results result in a ≥ 10% increase in ICU mortality for those patients (SA8) and (6) negative test results are assumed to lead to a £100 cost saving (because of diminished monitoring) and a 10% increased mortality rate for FPs. In contrast, the only instances when the probability that NGAL testing is cost-effective rises above 60% are when the impact of early AKI intervention is increased to a ≥ 60% reduction in AKI risks (SA4) and when 25%, 50% or 100% of FP test results are assumed to have actually been TP test results (with corresponding increases in AKI incidence of 35%, 38% and 45%) (SA7).
| Sensitivity analysis | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) | Net benefit (QALYs) | INB | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Base-case analysis (for comparison) | ||||||||||
| NGAL (plasma) base case | 32,759 | 5.62 | 164 | 0.0122 | 13,372 | 3.983 | 0.004 | 0.56 | 0.40 | 0.52 |
| SA1: time horizon (base case: lifetime, i.e. approximately 40 years) | ||||||||||
| 90 days | 13,808 | 0.05 | 90 | 0.000 | 978,005 | –0.64 | –0.004 | 0.53 | 0.38 | 0.37 |
| 1 year (+90 days) | 17,504 | 0.52 | 110 | 0.001 | 89,683 | –0.35 | –0.004 | 0.56 | 0.37 | 0.35 |
| 5 years (+90 days) | 25,603 | 2.02 | 135 | 0.005 | 28,191 | 0.74 | –0.002 | 0.56 | 0.38 | 0.42 |
| 10 years (+90 days) | 29,493 | 3.38 | 150 | 0.008 | 18,517 | 1.90 | 0.001 | 0.56 | 0.39 | 0.49 |
| 20 years (+90 days) | 31,723 | 5.04 | 165 | 0.012 | 13,819 | 3.45 | 0.004 | 0.56 | 0.39 | 0.52 |
| SA2: NGAL test cost (base-case cost £14.98) | ||||||||||
| Test cost × 0.25 = £3.745 | 32,802 | 5.62 | 163 | 0.013 | 12,180 | 3.98 | 0.005 | 0.56 | 0.40 | 0.52 |
| Test cost × 0.50 = £7.49 | 32,805 | 5.62 | 167 | 0.013 | 12,442 | 3.98 | 0.005 | 0.56 | 0.40 | 0.52 |
| Test cost × 1.50 = £22.47 | 32,819 | 5.62 | 181 | 0.013 | 13,487 | 3.98 | 0.004 | 0.56 | 0.39 | 0.52 |
| Test cost × 2.0 = £29.96 | 32,826 | 5.62 | 188 | 0.013 | 14,010 | 3.98 | 0.004 | 0.56 | 0.38 | 0.51 |
| Test cost × 5.0 = £74.90 | 32,868 | 5.62 | 230 | 0.013 | 17,145 | 3.97 | 0.002 | 0.56 | 0.36 | 0.49 |
| SA3: ICU AKI incidence (base case: mean 0.317, SD 0.018) | ||||||||||
| AKI incidence = 0.10 | 32,420 | 6.26 | 98 | 0.004 | 23,909 | 4.63 | –0.001 | 0.58 | 0.31 | 0.46 |
| AKI incidence = 0.20 | 32,628 | 5.96 | 133 | 0.008 | 16,211 | 4.33 | 0.002 | 0.58 | 0.36 | 0.51 |
| AKI incidence = 0.40 | 33,045 | 5.38 | 204 | 0.016 | 12,363 | 3.73 | 0.006 | 0.58 | 0.39 | 0.53 |
| AKI incidence = 0.50 | 33,253 | 5.09 | 239 | 0.021 | 11,593 | 3.43 | 0.009 | 0.58 | 0.40 | 0.54 |
| SA4: impact of early AKI intervention on AKI risks (base case: RR 0.78, SD 0.25) | ||||||||||
| RR = 0.20 (i.e. 80% reduced risks) | 32,988 | 5.65 | 349 | 0.041 | 8497 | 3.996 | 0.024 | 0.66 | 0.33 | 0.61 |
| RR = 0.40 (60% reduced risks) | 32,951 | 5.64 | 313 | 0.035 | 8871 | 3.992 | 0.020 | 0.66 | 0.33 | 0.61 |
| RR = 0.60 (40% reduced risks) | 32,892 | 5.63 | 254 | 0.026 | 9780 | 3.986 | 0.013 | 0.62 | 0.35 | 0.58 |
| RR = 0.70 (30% reduced risks) | 32,856 | 5.62 | 217 | 0.020 | 10,729 | 3.982 | 0.009 | 0.60 | 0.36 | 0.56 |
| RR = 0.90 (10% reduced risks) | 32,770 | 5.61 | 131 | 0.007 | 18,587 | 3.973 | 0.000 | 0.54 | 0.41 | 0.49 |
| RR = 0.95 (5% reduced risks) | 32,746 | 5.61 | 107 | 0.003 | 31,140 | 3.971 | –0.002 | 0.52 | 0.43 | 0.46 |
| RR values > 1 reset to 1 | 32,830 | 5.62 | 191 | 0.016 | 11,762 | 3.979 | 0.007 | 0.58 | 0.38 | 0.53 |
| RR SD reduced to 0.06 | 32,822 | 5.62 | 183 | 0.015 | 12,208 | 3.978 | 0.006 | 0.58 | 0.38 | 0.53 |
| SA5: cost of early AKI intervention (base case: mean cost £205, SD £279) | ||||||||||
| Cost of early AKI treatment = £50 | 32,755 | 5.62 | 116 | 0.013 | 8669 | 3.980 | 0.008 | 0.56 | 0.43 | 0.55 |
| Cost of early AKI treatment = £100 | 32,769 | 5.62 | 131 | 0.013 | 9763 | 3.979 | 0.007 | 0.56 | 0.42 | 0.54 |
| Cost of early AKI treatment = £500 | 32,887 | 5.62 | 248 | 0.013 | 18,519 | 3.974 | 0.001 | 0.56 | 0.35 | 0.48 |
| Cost of early AKI treatment = £800 | 32,975 | 5.62 | 336 | 0.013 | 25,085 | 3.969 | –0.003 | 0.56 | 0.30 | 0.43 |
| SA6: ICU utility (base case: mean –0.402, SD 0.201) | ||||||||||
| ICU utility = 0.00 | 32,809 | 5.63 | 170 | 0.013 | 12,983 | 3.986 | 0.005 | 0.56 | 0.40 | 0.52 |
| ICU utility = 0.20 | 32,810 | 5.63 | 171 | 0.014 | 12,654 | 3.991 | 0.005 | 0.56 | 0.39 | 0.52 |
| SA7: test accuracy adjustment (FPs reset to TPs and overall incidence of AKI increased) | ||||||||||
| 10% of FP results recoded as TPs (AKI S1) and overall incidence of AKI increased to 33.0% | 32,926 | 5.59 | 221 | 0.021 | 10,335 | 3.945 | 0.010 | 0.61 | 0.37 | 0.56 |
| 25% of FP results recoded as TPs and overall incidence of AKI increased to 34.9% | 32,952 | 5.54 | 280 | 0.031 | 8932 | 3.890 | 0.017 | 0.64 | 0.34 | 0.60 |
| 50% of FP results recoded as TPs and overall incidence of AKI increased to 38.2% | 33,149 | 5.46 | 354 | 0.046 | 7678 | 3.804 | 0.028 | 0.69 | 0.32 | 0.66 |
| 100% of FP results recoded as TPs and overall incidence of AKI increased to 44.7% | 33,423 | 5.30 | 521 | 0.077 | 6752 | 3.629 | 0.051 | 0.77 | 0.27 | 0.75 |
| SA8: increased mortality for FP results | ||||||||||
| 5% increased ICU mortality for FPs | 32,784 | 5.61 | 146 | 0.009 | 17,108 | 3.974 | 0.001 | 0.53 | 0.41 | 0.49 |
| 10% increased ICU mortality for FPs | 32,757 | 5.61 | 118 | 0.004 | 32,218 | 3.970 | –0.002 | 0.51 | 0.43 | 0.46 |
| 30% increased ICU mortality for FPs | 32,647 | 5.59 | 8 | –0.016 | Dominated | 3.957 | –0.016 | 0.41 | 0.50 | 0.36 |
| 50% increased ICU mortality for FPs | 32,539 | 5.57 | –100 | –0.035 | 2884a | 3.943 | –0.030 | 0.32 | 0.56 | 0.27 |
| SA9: impact of negative test results (reduction in monitoring leading to cost saving + increased mortality risk for FNs) | ||||||||||
| One-off cost saving (–£100) + 10% increased ICU mortality for FNs | 31,753 | 5.52 | –885 | –0.088 | 10,064a | 3.929 | –0.044 | 0.15 | 0.91 | 0.25 |
Cystatin C compared with standard care
Base case (adult intensive care unit all-comers)
The results for the base-case CEA of cystatin C testing compared with standard care are shown in Table 59. Cystatin C testing is expected to have a lifetime additional cost of £149–166 (depending on the specific test) and produce an additional 0.012–0.013 QALYs. This results in ICERs ranging from £11,476 to £13,504 per additional QALY, with INBs of 0.004–0.006 QALYs (equivalent to £80–120 in terms of INMB). At a threshold of £20,000 per QALY, cystatin C (serum) testing has the highest probability of being cost-effective (54%) and was explored in the sensitivity analysis.
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Net benefit (QALYs) (95% CI) | INB (95% CI) | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | 32,596 (24,320 to 43,145) | 5.61 (0.46 to 9.09) | 3.979 (–1.14 to 7.34) | |||||||
| Cystatin C (urine) | 32,751 (24,524 to 43,347) | 5.62 (0.46 to 9.11) | 155 (–1187 to 1500) | 0.0115 (–0.16 to 0.19) | 13,449 | 3.985 (–1.15 to 7.33) | 0.004 (–0.11 to 0.13) | 0.56 | 0.40 | 0.52 |
| Cystatin C (plasma) | 32,761 (24,546 to 43,355) | 5.62 (0.46 to 9.11) | 166 (–1183 to 1524) | 0.0123 (–0.16 to 0.20) | 13,504 | 3.983 (–1.15 to 7.32) | 0.004 (–0.11 to 0.13) | 0.56 | 0.40 | 0.52 |
| Cystatin C (serum) | 32,744 (24,320 to 43,340) | 5.62 (0.46 to 9.11) | 149 (–1201 to 1496) | 0.0130 (–0.16 to 0.19) | 11,476 | 3.986 (–1.15 to 7.33) | 0.006 (–0.11 to 0.13) | 0.56 | 0.42 | 0.54 |
The uncertainty around the cost-effectiveness of cystatin C (serum) testing is illustrated in Figures 39 and 40 [the results for cystatin C (urine) and cystatin C (plasma) testing have a very similar distribution and are not presented]. Above a threshold of £11,500 per QALY, cystatin C (serum) testing is expected to be the most cost-effective strategy. The test has a 54% probability of being cost-effective at a £20,000 per QALY threshold, increasing to and levelling out at 56% at a £50,000 per QALY threshold.
FIGURE 39.
Scatterplot: incremental costs and QALYs for cystatin C (serum) vs. standard care (base case).
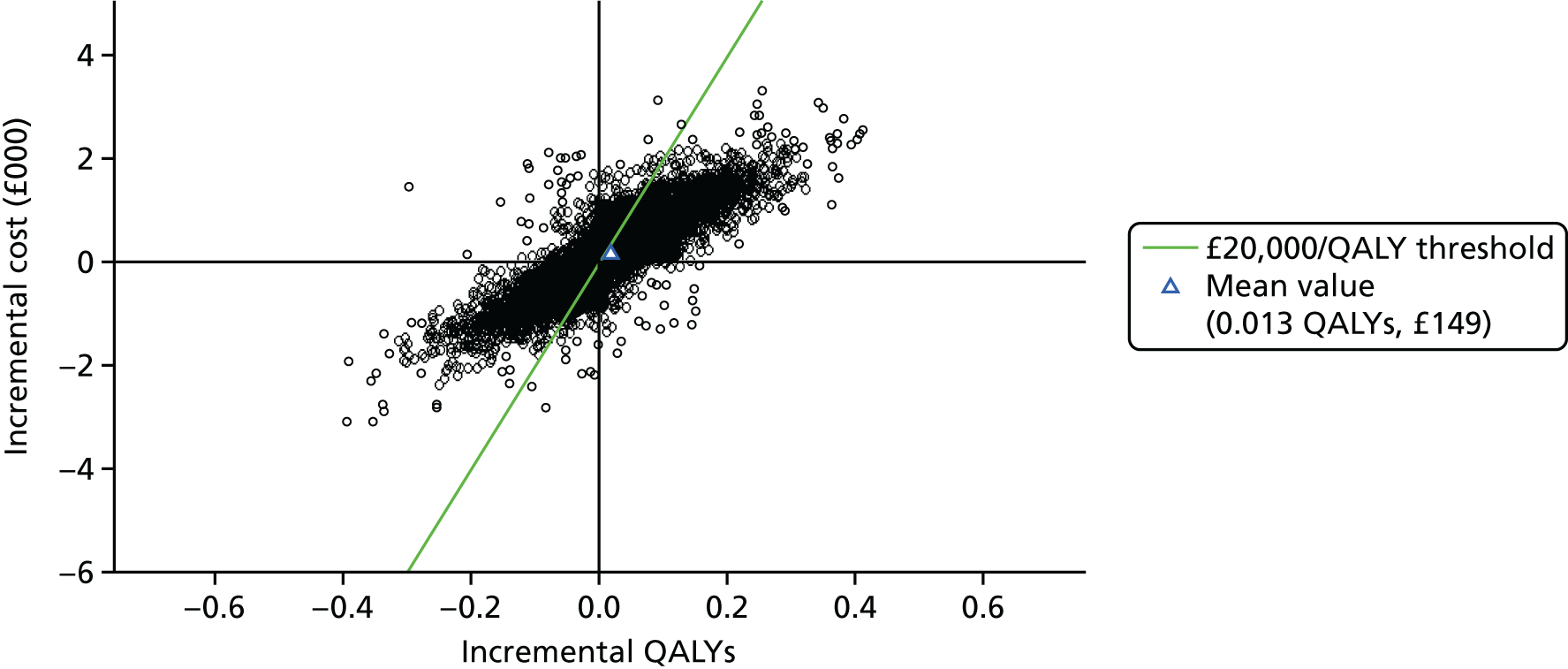
FIGURE 40.
Cost-effectiveness acceptability frontier: cystatin C (serum) vs. standard care (base case).
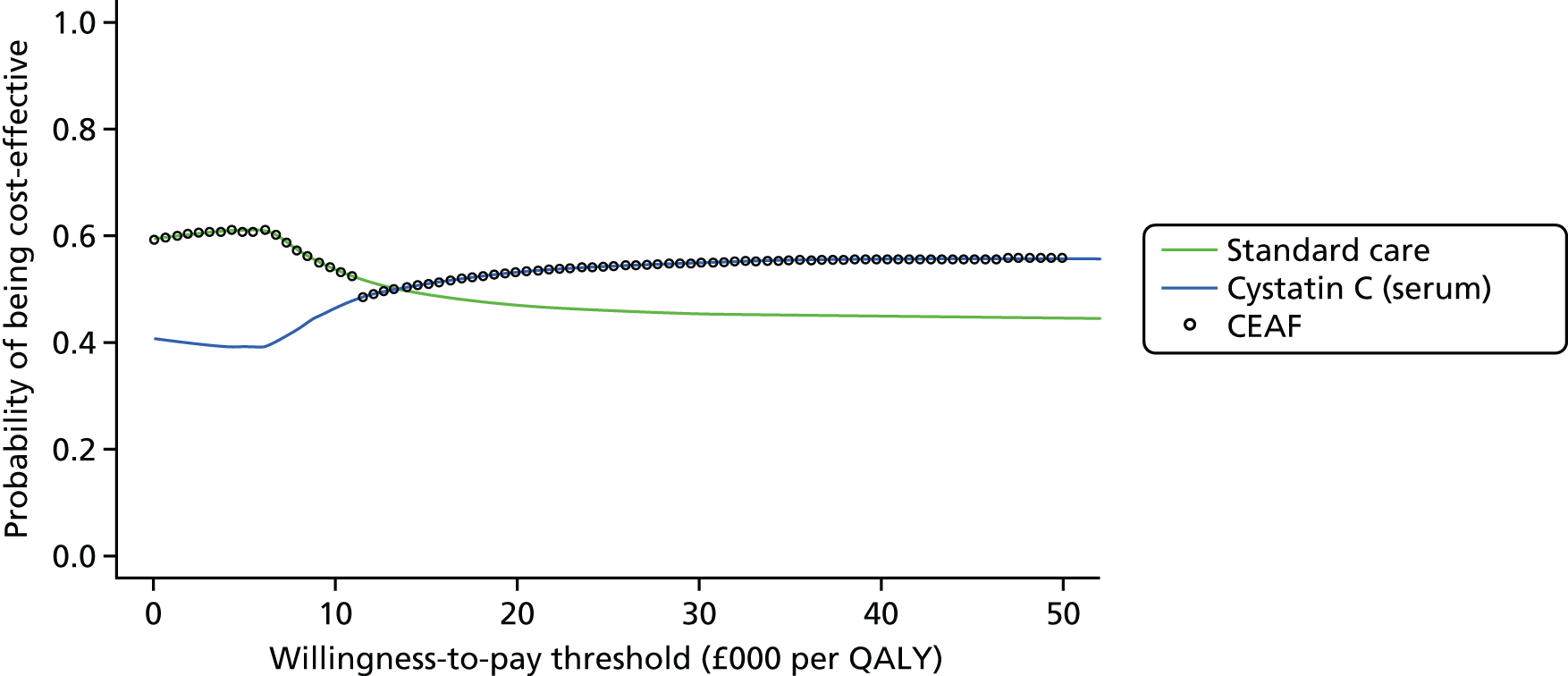
Secondary analysis (post-cardiac surgery subgroup)
The results of the secondary analysis conducted within a subgroup of patients after cardiac surgery in the ICU are presented in Table 60. In this subgroup, the addition of cystatin C testing is expected to have a lifetime additional cost of £124–166 and produce an additional health benefit of 0.007–0.01 QALYs per patient. This results in ICERs ranging from £15,337 to £20,435 per additional QALY, with mean INBs of –0.0002 to +0.002 QALYs.
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Net benefit (QALYs) (95% CI) | INB (95% CI) | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | 29,959 (22,738 to 37,726) | 6.50 (0.56 to 10.19) | 5.007 (–0.90 to 8.60) | |||||||
| Cystatin C (plasma) | 32,125 (22,879 to 37,889) | 6.51 (0.56 to 10.21) | 166 (–881 to 1229) | 0.008 (–0.13 to 0.14) | 20,435 | 5.007 (–0.90 to 8.60) | –0.0002 (–0.09 to 0.09) | 0.56 | 0.37 | 0.48 |
| Cystatin C (urine) | 30,082 (22,816 to 37,856) | 6.51 (0.56 to 10.21) | 124 (–908 to 1162) | 0.007 (–0.13 to 0.14) | 18,076 | 5.008 (–0.90 to 8.59) | 0.0007 (–0.09 to 0.09) | 0.55 | 0.40 | 0.49 |
| Cystatin C (serum) | 30,111 (22,847 to 37,869) | 6.51 (0.56 to 10.21) | 153 (–900 to 1204) | 0.010 (–0.13 to 0.15) | 15,337 | 5.009 (–0.90 to 8.60) | 0.0023 (–0.09 to 0.10) | 0.57 | 0.38 | 0.51 |
Sensitivity analyses
The results of the one-way sensitivity analyses conducted for cystatin C (serum) testing compared with standard care are provided in Table 61. Based on the mean expected cost and QALY results, cystatin C is no longer cost-effective (i.e. has an ICER of > £20,000 per QALY) when (1) the time horizon is ≤ 5 years (SA1), (2) the impact of early AKI intervention is limited to a 5% reduction in AKI risks (from 22% in the base case) (SA4), (3) the cost of early AKI intervention is increased to £800 (from £205) (SA5), (4) patients with a FP test result have a ≥ 30% increased ICU mortality rate (SA8) and (5) negative test results are assumed to lead to a £100 cost saving (because of diminished monitoring) and a 10% increased mortality rate for FPs (SA9). In contrast, the only instances in which the probability that cystatin C is cost-effective rises above 60% are when the impact of early AKI intervention is increased to a ≥ 40% reduction in AKI risks (SA4) and when ≥ 50% of FP test results are assumed to have actually been TP test results (SA7).
| Sensitivity analysis | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) | Net benefit (QALYs) | INB | P(more effective) | P(cost saving) | P(cost-effective) |
|---|---|---|---|---|---|---|---|---|---|---|
| Base-case analysis (for comparison) | ||||||||||
| Cystatin C (serum) base case | 32,744 | 5.62 | 149 | 0.013 | 11,476 | 3.986 | 0.006 | 0.56 | 0.42 | 0.54 |
| SA1: time horizon (base case: lifetime, i.e. approximately 40 years) | ||||||||||
| 90 days | 13,789 | 0.05 | 71 | 0.000 | 713,786 | –0.63 | –0.003 | 0.53 | 0.40 | 0.40 |
| 1 year (+90 days) | 17,486 | 0.52 | 92 | 0.001 | 71,008 | –0.35 | –0.003 | 0.57 | 0.39 | 0.38 |
| 5 years (+90 days) | 25,587 | 2.02 | 118 | 0.005 | 23,397 | 0.74 | –0.001 | 0.57 | 0.40 | 0.45 |
| 10 years (+90 days) | 29,477 | 3.38 | 134 | 0.009 | 15,710 | 1.90 | 0.002 | 0.57 | 0.40 | 0.51 |
| 20 years (+90 days) | 31,708 | 5.04 | 150 | 0.013 | 11,926 | 3.45 | 0.005 | 0.56 | 0.40 | 0.53 |
| SA2: cystatin C test cost (base case cost £4.26) | ||||||||||
| Test cost × 0.25 = £1.065 | 32,795 | 5.62 | 157 | 0.014 | 11,070 | 3.98 | 0.006 | 0.56 | 0.40 | 0.53 |
| Test cost × 0.50 = £2.13 | 32,796 | 5.62 | 158 | 0.014 | 11,140 | 3.98 | 0.006 | 0.56 | 0.40 | 0.53 |
| Test cost × 1.50 = £6.39 | 32,800 | 5.62 | 162 | 0.014 | 11,422 | 3.98 | 0.006 | 0.56 | 0.40 | 0.53 |
| Test cost × 2.0 = £8.52 | 32,802 | 5.62 | 163 | 0.014 | 11,563 | 3.98 | 0.006 | 0.56 | 0.40 | 0.53 |
| Test cost × 5.0 = £21.30 | 32,814 | 5.62 | 175 | 0.014 | 12,408 | 3.98 | 0.005 | 0.56 | 0.39 | 0.52 |
| SA3: ICU AKI incidence (base case: mean 0.317, SD 0.018) | ||||||||||
| AKI incidence = 0.10 | 32,395 | 6.26 | 74 | 0.00 | 17,035 | 4.64 | 0.001 | 0.58 | 0.35 | 0.50 |
| AKI incidence = 0.20 | 32,608 | 5.96 | 114 | 0.009 | 13,048 | 4.33 | 0.003 | 0.58 | 0.38 | 0.53 |
| AKI incidence = 0.40 | 33,033 | 5.38 | 192 | 0.017 | 11,055 | 3.73 | 0.008 | 0.58 | 0.40 | 0.54 |
| AKI incidence = 0.50 | 33,246 | 5.09 | 232 | 0.022 | 10,656 | 3.43 | 0.010 | 0.58 | 0.40 | 0.55 |
| SA4: impact of early AKI intervention on AKI risks (base case: RR 0.78, SD 0.25) | ||||||||||
| RR = 0.20 (i.e. 80% reduced risks) | 32,984 | 5.65 | 345 | 0.043 | 7958 | 3.999 | 0.026 | 0.67 | 0.33 | 0.62 |
| RR = 0.40 (60% reduced risks) | 32,945 | 5.64 | 307 | 0.037 | 8237 | 3.994 | 0.022 | 0.66 | 0.34 | 0.62 |
| RR = 0.60 (40% reduced risks) | 32,883 | 5.63 | 244 | 0.027 | 8911 | 3.988 | 0.015 | 0.63 | 0.35 | 0.60 |
| RR = 0.70 (30% reduced risks) | 32,844 | 5.63 | 205 | 0.021 | 9610 | 3.984 | 0.011 | 0.61 | 0.37 | 0.57 |
| RR = 0.90 (10% reduced risks) | 32,753 | 5.61 | 115 | 0.007 | 15,339 | 3.974 | 0.002 | 0.54 | 0.42 | 0.50 |
| RR = 0.95 (5% reduced risks) | 32,728 | 5.61 | 89 | 0.004 | 24,435 | 3.972 | –0.001 | 0.52 | 0.44 | 0.48 |
| RR values > 1 reset to 1 | 32,816 | 5.62 | 178 | 0.017 | 10,346 | 3.981 | 0.008 | 0.58 | 0.39 | 0.54 |
| RR SD reduced to 0.06 | 32,808 | 5.62 | 170 | 0.016 | 10,699 | 3.980 | 0.007 | 0.58 | 0.39 | 0.55 |
| SA5: cost of early AKI intervention (base case: mean cost £205, SD £279) | ||||||||||
| Cost of early AKI treatment = £50 | 32,748 | 5.62 | 109 | 0.014 | 7740 | 3.981 | 0.009 | 0.56 | 0.43 | 0.56 |
| Cost of early AKI treatment = £100 | 32,761 | 5.62 | 122 | 0.014 | 8643 | 3.981 | 0.008 | 0.56 | 0.42 | 0.55 |
| Cost of early AKI treatment = £500 | 32,863 | 5.62 | 224 | 0.014 | 15,860 | 3.976 | 0.003 | 0.56 | 0.36 | 0.50 |
| Cost of early AKI treatment = £800 | 32,939 | 5.62 | 301 | 0.014 | 21,273 | 3.972 | –0.001 | 0.56 | 0.32 | 0.46 |
| SA6: ICU utility (base case: mean –0.402, SD 0.201) | ||||||||||
| ICU utility = 0.00 | 32,795 | 5.63 | 157 | 0.014 | 11,202 | 3.987 | 0.006 | 0.56 | 0.40 | 0.53 |
| ICU utility = 0.20 | 32,795 | 5.63 | 157 | 0.014 | 11,194 | 3.992 | 0.006 | 0.56 | 0.40 | 0.53 |
| SA7: test accuracy adjustment (FPs reset to TPs and overall incidence of AKI increased) | ||||||||||
| 10% of FP results recoded as TPs and overall incidence of AKI increased to 32.5% | 32,893 | 5.60 | 194 | 0.020 | 9770 | 3.958 | 0.010 | 0.60 | 0.38 | 0.56 |
| 25% of FP results recoded as TPs and overall incidence of AKI increased to 33.8% | 32,887 | 5.57 | 236 | 0.026 | 8897 | 3.924 | 0.015 | 0.62 | 0.36 | 0.59 |
| 50% of FP results recoded as TPs and overall incidence of AKI increased to 35.8% | 33,029 | 5.52 | 278 | 0.035 | 7831 | 3.870 | 0.022 | 0.66 | 0.35 | 0.63 |
| 100% of FP results recoded as TPs and overall incidence of AKI increased to 39.9% | 33,206 | 5.42 | 382 | 0.055 | 6945 | 3.760 | 0.036 | 0.72 | 0.31 | 0.70 |
| SA8: increased mortality for FP results | ||||||||||
| 5% increased ICU mortality for FPs | 32,780 | 5.62 | 142 | 0.011 | 12,822 | 3.977 | 0.004 | 0.55 | 0.41 | 0.51 |
| 10% increased ICU mortality for FPs | 32,763 | 5.61 | 124 | 0.008 | 15,539 | 3.974 | 0.002 | 0.53 | 0.42 | 0.50 |
| 30% increased ICU mortality for FPs | 32,693 | 5.60 | 55 | –0.004 | Dominated | 3.966 | –0.007 | 0.46 | 0.47 | 0.43 |
| 50% increased ICU mortality for FPs | 32,624 | 5.59 | –14 | –0.016 | 886a | 3.957 | –0.016 | 0.41 | 0.52 | 0.37 |
| SA9: impact of negative test results (reduction in monitoring leading to cost saving + increased mortality risk for FNs) | ||||||||||
| One-off cost saving (–£100) + 10% increased ICU mortality for FNs | 31,881 | 5.53 | –758 | –0.072 | 10,471a | 3.938 | –0.034 | 0.21 | 0.85 | 0.31 |
Multiway incremental analysis
Base case (adult intensive care unit all-comers)
The results of the multiway incremental analysis for the base case are presented in Table 62 (the ICER for each strategy compared with standard care is shown in the last column for reference). After ranking all strategies in order of increasing costs, cystatin C (urine), cystatin C (plasma), NGAL (urine) and NGAL (plasma) were found to be dominated by cystatin C (serum), producing fewer QALYs for a greater cost. After removal of all dominated options, cystatin C (serum), NGAL (serum) and Nephrocheck remained, with ICERs of £11,476, £25,492 and £12,855,101 per additional QALY respectively. Based on this analysis, NGAL (serum) and Nephrocheck are no longer expected to be cost-effective, assuming a willingness-to-pay threshold of £20,000 per additional QALY.
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Comparatora | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Exclusion reason | ICER vs. standard care (£) |
|---|---|---|---|---|---|---|---|---|
| Standard care | 32,596 (24,320 to 43,145) | 5.6092 (0.46 to 9.09) | – | – | – | – | – | – |
| Cystatin C (serum) | 32,744 (24,320 to 43,340) | 5.6221 (0.46 to 9.11) | Standard care | 149 (–1201 to 1496) | 0.013 (–0.16 to 0.19) | 11,476 | – | 11,476 |
| Cystatin C (urine) | 32,751 (24,524 to 43,347) | 5.6207 (0.46 to 9.11) | Cystatin C (serum) | 6 (–111 to 114) | –0.0014 (–0.01 to 0.01) | –4405 | Dominated by cystatin C (serum) | 13,449 |
| NGAL (urine) | 32,759 (24,521 to 43,351) | 5.6211 (0.46 to 9.11) | Cystatin C (serum) | 15 (–65 to 84) | –0.0011 (–0.01 to 0.01) | –13,927 | 13,742 | |
| NGAL (plasma) | 32,759 (24,516 to 43,339) | 5.6214 (0.46 to 9.11) | Cystatin C (serum) | 15 (–55 to 80) | –0.0007 (–0.01 to 0.01) | –20,733 | 13,372 | |
| Cystatin C (plasma) | 32,761 (24,546 to 43,355) | 5.6214 (0.46 to 9.11) | Cystatin C (serum) | 17 (–59 to 106) | –0.0007 (–0.01 to 0.02) | –23,801 | 13,504 | |
| NGAL (serum) | 32,811 (24,587 to 43,427) | 5.6247 (0.46 to 9.11) | Cystatin C (serum) | 67 (–39 to 257) | 0.0026 (–0.01 to 0.02) | 25,492 | – | 13,828 |
| Nephrocheck | 32,897 (24,662 to 43,484) | 5.6248 (0.46 to 9.12) | NGAL (serum) | 86 (9 to 227) | 0.000007 (–0.01 to 0.01) | 12,855,101 | – | 19,324 |
The CEAF including all available strategies is shown in Figure 41a. Above a threshold value of £11,400, cystatin C (serum) testing is expected to be the most cost-effective strategy (based on the expected mean net benefit values, indicated by the cost-effectiveness frontier), with a probability of cost-effectiveness of 27%, falling to 23% at a £20,000 per QALY threshold. Above a threshold of £25,400, NGAL (serum) testing is expected to be the most cost-effective strategy, with a probability of cost-effectiveness of 20%, rising to 26% at a £50,000 per QALY threshold.
FIGURE 41.
Cost-effectiveness acceptability frontier: (a) multiway base-case analysis; (b) multiway secondary analysis (post cardiac surgery).
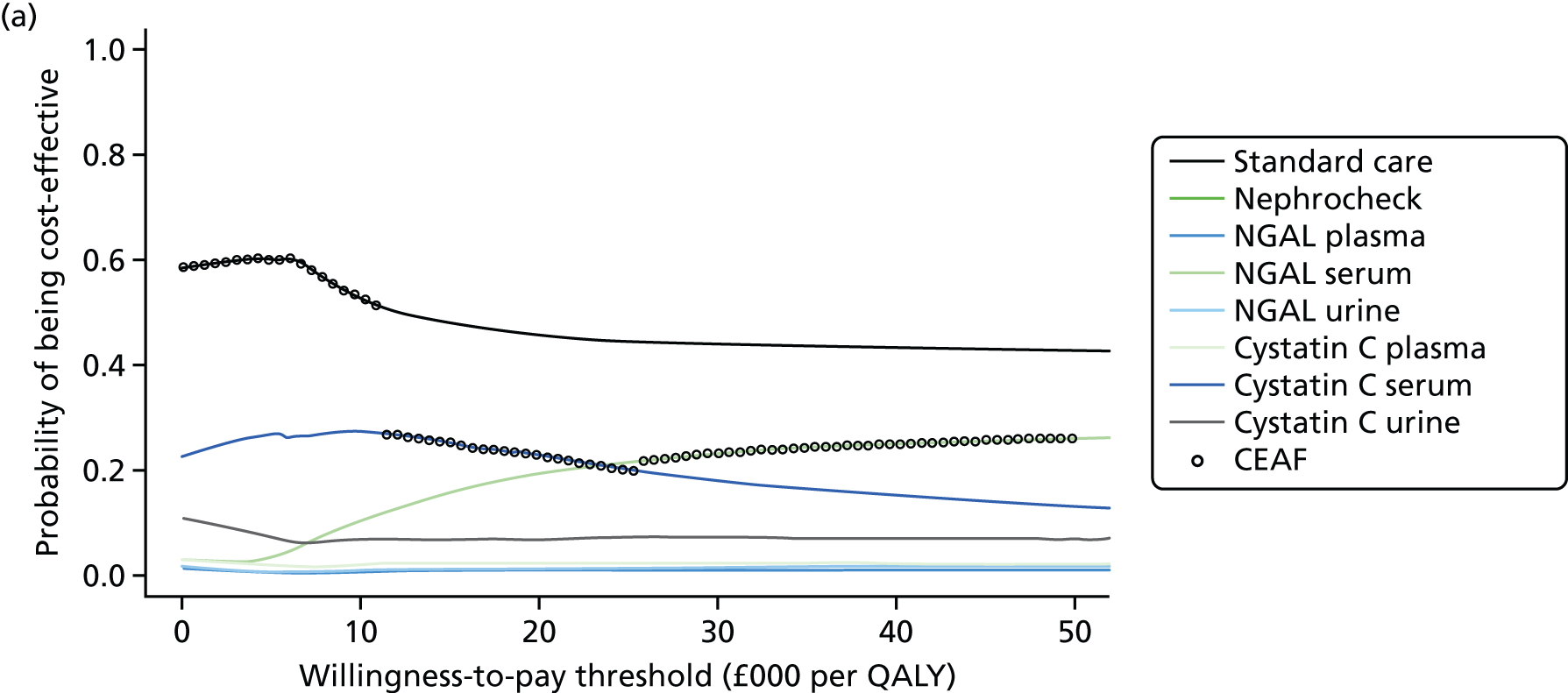
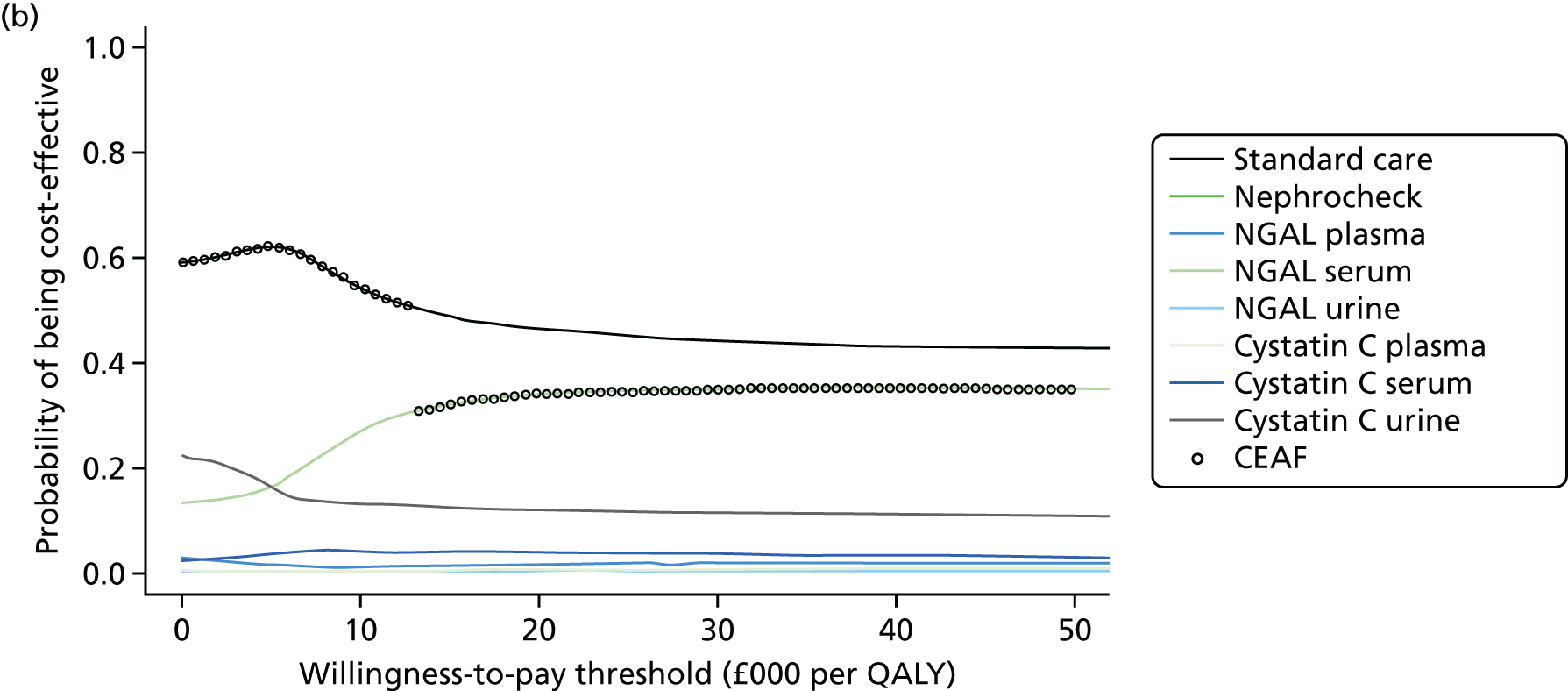
Secondary analysis (post-cardiac surgery subgroup)
The results of the multiway incremental analysis for the secondary analysis (post-cardiac surgery subgroup) are presented in Table 63 (the ICER for each strategy compared with standard care is shown in the last column for reference). After ranking all strategies in order of increasing costs, NGAL (serum) testing was found to dominate all other alternative strategies. Cystatin C (urine) and NGAL (plasma) testing are both extendedly dominated by NGAL (serum) testing, having higher ICERs, whereas the remaining tests are all strongly dominated, producing fewer QALYs at a greater cost than NGAL (serum) testing.
| Strategy | Total cost (95% CI) (£) | Total QALYs (95% CI) | Comparatora | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (£) | Exclusion reason | ICER vs. standard care (£) |
|---|---|---|---|---|---|---|---|---|
| Standard care | 29,959 (22,738 to 37,726) | 6.5048 (0.56 to 10.20) | – | – | – | – | – | – |
| Cystatin C (urine) | 30,082 (22,816 to 37,856) | 6.5116 (0.56 to 10.21) | Standard care | 124 (–908 to 1162) | 0.0068 (–0.13 to 0.14) | 18,076 | Extendedly dominated by NGAL (serum) | 18,076 |
| NGAL (plasma) | 30,096 (22,836 to 37,867) | 6.513 (0.56 to 10.21) | Standard care | 137 (–895 to 1178) | 0.0082 (–0.13 to 0.14) | 16,709 | 16,709 | |
| NGAL (serum) | 30,108 (22,845 to 37,892) | 6.5163 (0.56 to 10.22) | Standard care | 149 (–905 to 1204) | 0.0115 (–0.13 to 0.15) | 13,051 | – | 13,051 |
| Cystatin C (serum) | 30,111 (22,847 to 37,869) | 6.5148 (0.56 to 10.21) | NGAL (serum) | 3 (–110 to 129) | –0.0015 (–0.01 to 0.01) | –2145 | Dominated by NGAL (serum) | 15,337 |
| Cystatin C (plasma) | 30,125 (22,879 to 37,889) | 6.5129 (0.56 to 10.21) | NGAL (serum) | 17 (–138 to 205) | –0.0033 (–0.02 to 0.01) | –5120 | 20,435 | |
| NGAL (urine) | 30,131 (22,885 to 37,900) | 6.5137 (0.56 to 10.21) | NGAL (serum) | 23 (–116 to 194) | –0.0025 (–0.02 to 0.01) | –9050 | 19,287 | |
| Nephrocheck | 30,163 (22,893 to 37,942) | 6.5158 (0.56 to 10.20) | NGAL (serum) | 55 (–43 to 154) | –0.0004 (–0.01 to 0.01) | –123,202 | 18,617 |
The CEAF including all available strategies is shown in Figure 41b. Above a threshold value of £13,100, NGAL serum is expected to be the most cost-effective strategy (based on the expected mean net benefit values, indicated by the cost-effectiveness frontier), with a probability of cost-effectiveness of 31%, rising to 34% at a £20,000 per QALY threshold.
Summary
An economic evaluation was conducted to assess the cost-effectiveness of biomarkers compared with standard care for the early identification of AKI in the ICU. Three tests were assessed: Nephrocheck, cystatin C and NGAL. Cystatin C and NGAL tests are currently available across three alternative media (plasma, urine and serum); each of these was considered as a separate test in the analysis, resulting in a total of seven testing strategies together with Nephrocheck. The evaluation consisted of an economic decision model, in which cost-effectiveness was assessed over a lifetime horizon from a UK NHS and PSS perspective using individual patient trial data and information from the current literature. The primary analysis concerned the use of the tests in an all-comer ICU population; a secondary analysis was conducted to explore the impact of the tests on a subgroup of patients in the ICU post cardiac surgery.
In the primary analysis, based on the mean expected cost and QALY results only, each of the tests was cost-effective when compared in two-way analyses against standard care. Lifetime incremental QALYs ranged from 0.0115 [cystatin C (urine)] to 0.016 (Nephrocheck) and additional costs ranged from £149 [cystatin C (urine)] to £301 (Nephrocheck). The ICERs ranged from £11,476 to £13,504 per additional QALY for the cystatin C tests and from £13,372 to £13,828 for the NGAL tests; for Nephrocheck the ICER was £19,324. The corresponding INB values ranged from 0.001 QALYs for Nephrocheck to 0.006 QALYs for cystatin C (serum).
There is significant uncertainty around both the incremental cost results and the QALY results, leading to large uncertainty around the expected cost-effectiveness of the tests. For the incremental costs, all of the testing strategies had 95% CIs ranging from –£1000 to +£1400 and, for the incremental QALYs, all of the results ranged from –0.16 to +0.19 or +0.20. Compared with standard care alone, the probability that the tests are more effective than standard care was 56% for the cystatin C tests, 56–57% for the NGAL tests and 57% for Nephrocheck. The probability that the tests would be cost saving was 32% for Nephrocheck, 37–40% for the NGAL tests and 40–42% for the cystatin C tests. At a £20,000 per QALY threshold, the overall probability that the tests are cost-effective compared with standard care was 48% for Nephrocheck, 51–52% for the NGAL tests and 52–54% for the cystatin C tests. Raising the threshold value to £50,000 per QALY only slightly increased these probabilities.
The results of the multiway analysis indicate that, in the base case, between a threshold of £11,400 and a threshold of £25,400, cystatin C (serum) is the most cost-effective strategy, with a probability of cost-effectiveness of 23% at a £20,000 per QALY threshold. Above a £25,400 per QALY threshold, NGAL (serum) is expected to be the most cost-effective strategy, with a probability of cost-effectiveness of 20%. All other strategies either are found to be dominated by cystatin C (serum) or, in the case of Nephrocheck, have an ICER well above £20,000 per QALY [£12,855,101 compared with the next best alternative of NGAL (serum)]. This analysis suggests that, when taking into account the additional health impact that alternative tests could provide, several of the tests may no longer be cost-effective options.
Similar results were observed in the secondary analysis (i.e. in the post-cardiac surgery subgroup). All of the incremental costs and QALYs were slightly reduced compared with the base case, with incremental QALYs ranging from 0.007 [cystatin C (urine)] to 0.012 [NGAL (serum)] and additional costs ranging from £124 [cystatin C (urine)] to £205 (Nephrocheck). The ICERs were £13,051–19,287 per additional QALY for the cystatin C tests, £15,337–20,435 for the NGAL tests and £18,617 for Nephrocheck, with INB values ranging from –0.0002 to 0.004 QALYs. Again, there was substantial uncertainty around these results; at a £20,000 per QALY threshold there was a 48–52% probability that the tests would be cost-effective. In the multiway incremental analysis, only NGAL (serum) remained after removal of dominated or extendedly dominated alternatives (ICER £13,051 vs. standard care; probability of cost-effectiveness 35% at a £20,000 per QALY threshold).
The base-case results were highly sensitive to changes in key model parameters. Scenarios that lead to the tests becoming non-cost-effective (ICER > £20,000 per QALY) included shortening the time horizon of the analysis, reducing the incidence of AKI in the ICU, decreasing the impact or increasing the cost of early AKI intervention, applying a mortality risk for patients with FP test results, applying a cost saving for patients with negative test results and increased mortality for FN cases and increasing the cost of the Nephrocheck test. The only scenarios in which the probability that the tests were cost-effective increased to > 60% were increasing the impact of early AKI intervention to ≥ 40% and ≥ 60% risk reductions for cystatin C and NGAL respectively and assuming that at least 25%, 25% or 50% of FP test results were in fact TP test results for Nephrocheck, NGAL and cystatin C respectively.
Discussion
Interpretation of results
The results of the economic evaluation require careful interpretation. When analysed individually against standard care, all of the tests appear to be cost-effective in the base case and all except cystatin C (plasma) are cost-effective in the secondary analysis, based on an analysis of the overall expected costs and QALYs. However, the differences in each of the expected costs and QALYs are small and largely uncertain, resulting in probabilities of cost-effectiveness around 50%. Such uncertainty strongly indicates that further research is required before an informed adoption decision can be made. Furthermore, it necessitates a cautious interpretation of the subsequent multiway analysis, in which all tests are considered within the same evaluation; because the differences in the expected costs and QALYs across each of the tests are small and uncertain, marginal changes in any of these values could result in different test rankings, rendering these results potentially spurious. Perhaps the only exception to this is the Nephrocheck test, which is associated with extremely large ICERs compared with the alternative testing strategies because it has similar expected QALY outcomes but relatively higher costs. Assuming that the accuracy of the Nephrocheck test is not expected to improve significantly compared with the alternative tests, then the only way that this test is likely to represent a cost-effective strategy is if the cost of the test (currently estimated at > £70) is reduced to be in line with that of other competitor tests (i.e. £14.98 for NGAL and £4.26 for cystatin C).
Sensitivity analyses
The cost-effectiveness results are sensitive to changes in key parameters in the model. Most notably, all of the tests were sensitive to parameters related to the expected change in patient management and outcomes resulting from a positive or negative test result:
-
The cost and impact of early AKI intervention resulting from a TP test result. It is assumed that patients with a TP test result can benefit from some form of early AKI intervention; however, the exact nature of this intervention is unclear because of the fact that treatment for AKI currently relies on a heterogeneous and non-specific bundle of interventions. No diagnostic outcome studies that could inform an estimation of the impact of such early intervention on patient outcomes have yet been conducted. As such, data on the impact of early nephrologist consultation on AKI incidence in a population of Mexican patients treated post cardiac surgery were used as a proxy. 464 The appropriateness of this estimation is unclear and the model results were highly sensitive to both the expected impact of treatment on AKI risks and the cost of the intervention. Reducing the impact of early AKI intervention to a 10% AKI risk reduction or increasing the cost of the intervention to £500 (around the 90th percentile of the base-case cost distribution) results in the Nephrocheck test no longer being cost-effective, whereas reducing the risk reduction to 5% or increasing the cost to £800 (around the 95th percentile) results in all tests no longer being cost-effective. In contrast, increasing the risk reduction or reducing the intervention cost leads to significant increases in expected cost-effectiveness and the probability of cost-effectiveness of all of the tests.
-
The impact of a FP test result. In the base case it was assumed that no harm would result from a FP test result. Assuming instead that patients with a FP test result would incur a mortality impact (e.g. as a result of delayed access to necessary nephrotoxic agents) leads to reductions in the expected cost-effectiveness of the tests: a ≥ 5% mortality impact results in the Nephrocheck test no longer being cost-effective, a ≥ 10% mortality impact results in NGAL testing no longer being cost-effective and a ≥ 30% mortality impact results in cystatin C testing no longer being cost-effective.
-
The impact of a negative test result. In the base case it was assumed that a negative test result (either TN or FN) would have no impact on baseline treatment costs or QALYs. However, it is reasonable to assume that this may not be the case: a negative result may lead to reduced costs as a result of reduced patient monitoring and/or may result in harm for FN cases because of that same reduced monitoring. Sensitivity analysis indicates that if a one-off cost saving of £100 is applied to all negative results, and a 10% increased ICU mortality rate is applied to FN results, this would lead to all testing strategies becoming cost saving at the expense of producing fewer QALYs, resulting in all strategies no longer being cost-effective. Note that, in the absence of any data, these values were chosen purely arbitrarily.
Other parameters that were found to be influential in the sensitivity analysis include:
-
The time horizon of the analysis. Nephrocheck becomes cost-effective only if using a ≥ 20 year time horizon and cystatin C and NGAL become cost-effective only when using a ≥ 10-year time horizon. This indicates the importance of downstream health impacts (as a result of reduced CKD incidence and increased survival) relative to the short-term costs and health impacts in the model.
-
The incidence of AKI in the ICU. Reducing the incidence of AKI diminishes the opportunity for tests to improve patient outcomes (as the absolute proportion of TP cases is reduced) while at the same time increasing the relative cost of unnecessary testing. The impact of this appears to be greatest for the two most expensive tests: decreasing the AKI incidence to ≤ 20% results in Nephrocheck no longer being cost-effective, whereas decreasing the AKI incidence to 10% results in NGAL testing no longer being cost-effective (in contrast, cystatin C testing remains cost-effective throughout). Thus, the tests are less likely to be cost-effective when few cases of AKI are expected. This may not be a common occurrence within the UK ICU setting, where recorded rates of AKI are generally high. It may be more relevant in settings in which patients entering ICU may be less acutely ill because of higher critical care capacities, resulting in patients having an overall lower risk of AKI.
-
Adjusting the test accuracy to account for the imperfect reference test. Assuming that a proportion of FP test results are actually TP cases has a huge impact on the results. For example, recoding 10% of FP cases in NGAL and cystatin C testing, or 25% of FP cases in Nephrocheck testing, leads to ICERs of < £10,000; recoding 25% of FP cases results in all tests having a ≥ 60% probability of being cost-effective. The interpretation of this analysis depends on how much we are willing to believe that FP cases may actually be TP cases, as a result of the known deficiencies in standard care testing. We are not aware of any current evidence that could inform this expectation, thus this analysis should be considered purely exploratory. It does, however, highlight the importance of the methodological issue of how to account for bias in estimates of diagnostic accuracy resulting from the use of imperfect reference tests; although there is a growing body of methodological research in this area, the focus to date has been on methods that require access to individual-level data (which have limited application in this kind of study in which the main source of evidence is aggregate secondary data), and we are not aware of any work that has been conducted from a health economic perspective. This is, therefore, a key area for future research.
In general, of the three tests evaluated in the sensitivity analyses, Nephrocheck was the most sensitive to changes in key parameters, whereas cystatin C testing was the most resilient to parameter changes. Nevertheless, all of the tests were found to be sensitive to the key parameters listed above, indicating the need for further research across these areas. As the results of the VOI analysis provide further insight into the relative importance of the different areas of uncertainty, full research recommendations are discussed in Chapter 6.
Previous economic evaluations
Our review of economic decision models identified two previous economic evaluations of biomarkers for the early identification of AKI in the critical care setting. Both of these studies found that testing strategies were a cost-effective addition to standard care. However, there are notable differences in the overall and incremental cost and QALY outcomes between these studies and our analysis. In the study by Shaw et al. 205 (the most comparable study, which also considered a UK adult ICU population), the authors reported a mean lifetime cost of £4672 and 11.79 QALYs for standard care in a post-cardiac surgery population, whereas, in the current equivalent analysis, we expect a mean lifetime cost of £29,959 and 6.50 QALYs. A key reason for the lower cost in the study by Shaw et al. 205 is because the authors did not include any follow-up costs for patients post hospital discharge, which are expected to be significant (starting at > £6000 per year) based on a recent large cohort study. 457 Other key differences include the fact that the previous analysis assumed lower ICU length of stays and mortality, did not account for utility in the ICU (which was negative in the current model) or hospital, did not include a follow-up mortality risk post ICU discharge and did not include an elevated cost for ESRD (separate from that for CKD). We therefore believe that it is highly likely that previous assessments of the long-term costs and QALYs for patients treated in the ICU have been under- and overestimated respectively.
Shaw et al. 205 found that urinary NGAL testing dominated standard care, being more effective and less costly. This is in contrast to the current analysis in which the same test was associated with an additional cost of £172 and an increase in QALYs of 0.009, resulting in an ICER of £19,287 and a 48% probability of being cost-effective. Again, there are key differences between the studies that we believe are driving these alternative results. In particular, the analysis in the study by Shaw et al. 205 considered four sequential NGAL tests and assumed that test accuracy was independent of the sequence of the tests, which is likely to increase the overall accuracy of the tests. In addition, it appears that all patients with TP results were assumed to benefit from early AKI treatment, regardless of the concurrent standard care test results (in contrast we assumed that only patients without a concurrent diagnosis of moderate/severe AKI would be able to benefit from early AKI treatment in the testing strategies). Finally, Shaw et al. 205 reported limited details of the distributional assumptions applied in their probabilistic analysis and so it is unclear if uncertainty in the model parameters has been fully captured.
Overall, although all three economic evaluations of biomarkers for the early diagnosis of AKI in the ICU to date indicate that tests may be cost-effective, we believe that our analysis more accurately reflects the expected costs and QALYs associated with these strategies and, most importantly, more accurately depicts the level of uncertainty around these decisions.
Generalisability
This study has evaluated Nephrocheck, NGAL and cystatin C tests for the early identification of AKI within both a general all-comers ICU population and a post-cardiac surgery subgroup population. Based on the fact that this is an early analysis, with the results of the sensitivity analysis indicating that the results are sensitive to changes in key parameters including the incidence of AKI and the impact of early treatment, it is unlikely that these results can be extrapolated to the use of tests across other settings (e.g. the hospital ward or the community). In addition, as many of the costs are specific to the UK NHS, generalisability beyond the UK should also be considered with caution.
Although each of the three tests was shown to be cost-effective compared with standard care, there is significant uncertainty around the results, as highlighted by the wide distribution of the incremental costs and QALYs, the relatively low probabilities of cost-effectiveness and the sensitivity of the results to changes in key parameters. The potential value of future research as a means of reducing this uncertainty is explored further in Chapter 6.
It should be noted that combining CKD stages 1–4 was considered to be a necessary simplification within the model. The key objective of the model was to capture any impacts related to the intervention (i.e. in this case, the tests). Based on expert consultation, it was not apparent that the tests and resulting treatments would have any significant impact on patient outcomes once CKD has developed. The CKD portion of the model therefore aimed to capture the most significant cost and QALY impacts associated with CKD, which were assumed to be the use of dialysis, the risk of ESRD and transplantation. This simplification is in line with previous models outlined in the review of AKI models.
Chapter 6 Research prioritisation
Introduction
A key objective of the AKI-Diagnostics project was to inform a strategy towards the generation of evidence leading to the adoption of diagnostics tests in critical care in a manner that maximises patient benefit and value for the NHS. Subject to demonstration of clinical efficacy, the final step of any diagnostic test towards adoption is a reimbursement decision based on cost-effectiveness. As such, a specific metric – VOI – has been chosen as the main determinant of research recommendations arising from the AKI-Diagnostics project.
Value of information analysis provides a pertinent framework for setting priorities for further research. This approach relies on the fact that resources that are spent on a new intervention will not be available to spend on alternative interventions: if we invest in a new intervention that is not the most cost-effective option, we could lose health (or money) that could have been gained if we had invested in a more cost-effective intervention. Research (i.e. the gain of more information) therefore has value if it reduces the risk of adopting an intervention that is not cost-effective. This value can be quantified as either lost health (measured in QALYs) or lost health-care resources (measured in monetary units) within the VOI framework. This chapter outlines the methods and findings of the VOI analysis conducted as part of the AKI-Diagnostics economic evaluation.
It is important to note that the VOI analysis presented here relies on multiple two-way comparisons between standard care and specific tests. This is on the grounds that the decision problem, in reality, includes more than just the three tests subjected to detailed study within the project. As such, a four-way comparison such as that presented in Chapter 5 would be incomplete.
Methods
Value of information analysis
Value of information analysis was conducted for each of the shortlisted tests using the economic decision model outlined in Chapter 5. For the cystatin C and NGAL tests, which are available across multiple media (i.e. plasma, urine and serum), VOI analysis was conducted only for the individual test found to have the highest probability of being cost-effective based on the results of the CEA. This analysis therefore includes Nephrocheck, NGAL (plasma) and cystatin C (serum).
Within the VOI framework, several measures can be obtained to explore the impact of reducing uncertainty on the expected cost-effectiveness. The focus of this analysis was on the following measures:
-
Expected value of perfect information (EVPI). The EVPI represents the overall burden of uncertainty on the decision-maker for a defined decision. The economic model is used to determine the difference between the expected net benefit when a decision is made assuming perfect knowledge of all model parameters (estimated via model simulations assuming fixed input parameters sampling across the range of expected parameter values) and the net benefit under current uncertain information. The resulting EVPI provides an upper bound on the total amount that the decision-maker should be willing to invest in further research to eliminate all parameter uncertainty. A positive EVPI provides a necessary but not sufficient condition for the decision to invest in further research: a positive EVPI indicates that further research may be warranted; however, additional information including the expected cost of research, the likely reduction in uncertainty as a result of research and the opportunity cost of delayed adoption is required to make a definitive decision regarding future research requirements.
-
Expected value of perfect parameter information (EVPPI). The EVPPI is an extension of the EVPI in which the burden of uncertainty around particular individual parameters, or groups of parameters, is quantified. 480 The EVPPI indicates the maximum value that the decision-maker should be willing to invest to reduce all uncertainty around a specific parameter or group of parameters. As for the EVPI, the EVPPI presents a necessary but not sufficient condition for conducting further research. However, the EVPPI enables exploration of the key parameters driving the uncertainty, which can be used to inform the direction of further research.
Calculation of the VOI was conducted using non-parametric regression modelling approaches. 481,482 For single parameter EVPPI estimates and for groups of up to four parameters, regression was conducted using the generalised additive model. For parameter groups of five or above, the R ‘earth’ package was used, which utilises multivariate adaptive regression splines. 483
To estimate the VOI for the total population of patients expected to be affected by an adoption decision, a baseline population of 258,956 was assumed, based on the reported number of critical care records in the 2014–15 Hospital Episodes Statistics report (adult critical care). 484 An annual discount rate of 3.5% was applied to this number over a 10-year time period, assuming that this is the time over which the decision would remain relevant (e.g. before a comparator intervention would be adopted). This results in a total 10-year discounted population of 2,229,012. All VOI statistics are expressed in monetary terms using the net monetary benefit statistic.
Results
Expected value of perfect information
The distribution of the EVPI across different willingness-to-pay thresholds for the Nephrocheck, cystatin C (serum) and NGAL (plasma) tests is shown in Figures 42–44 respectively. At a willingness-to-pay threshold of £20,000 per QALY, the per-patient EVPI is £430 for Nephrocheck, £371 for NGAL (plasma) and £362 for cystatin C (serum). For the total 10-year discounted population of patients expected to be affected by this decision (n = 2,229,012), the corresponding 10-year population EVPI is £958M, £827M and £807M respectively. Population EVPPI for single model parameters, showing top ranked parameters only, is shown in Figure 45.
FIGURE 42.
Expected value of perfect information: Nephrocheck vs. standard care.
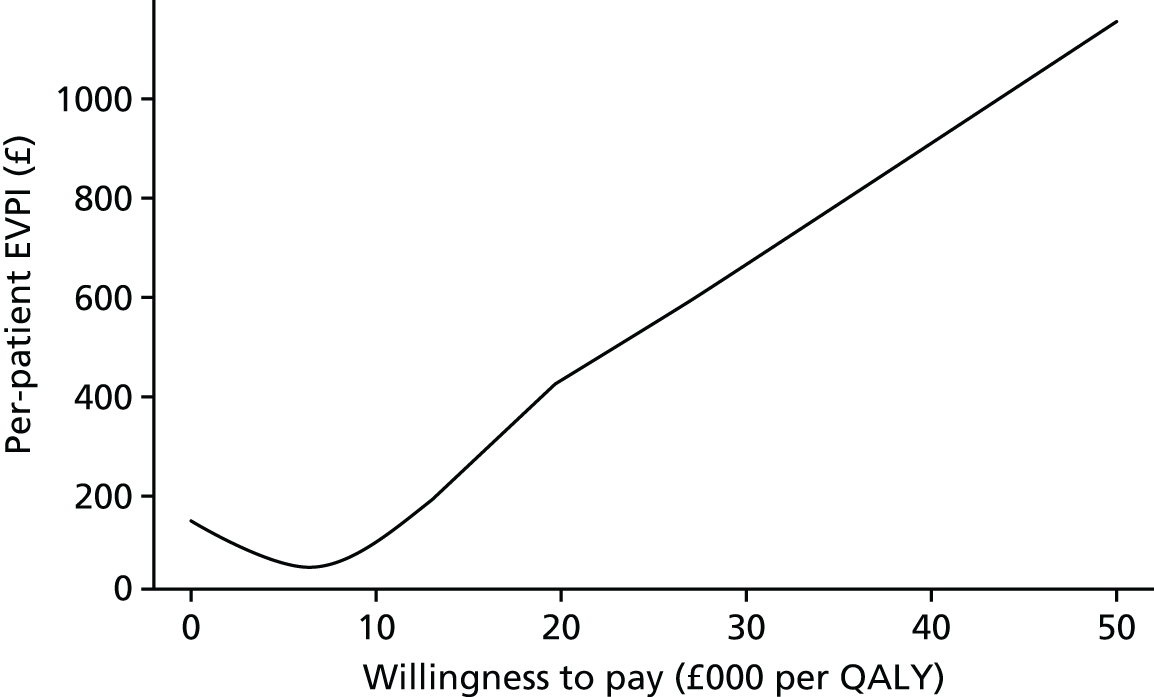
FIGURE 43.
Expected value of perfect information: cystatin C (serum) vs. standard care.
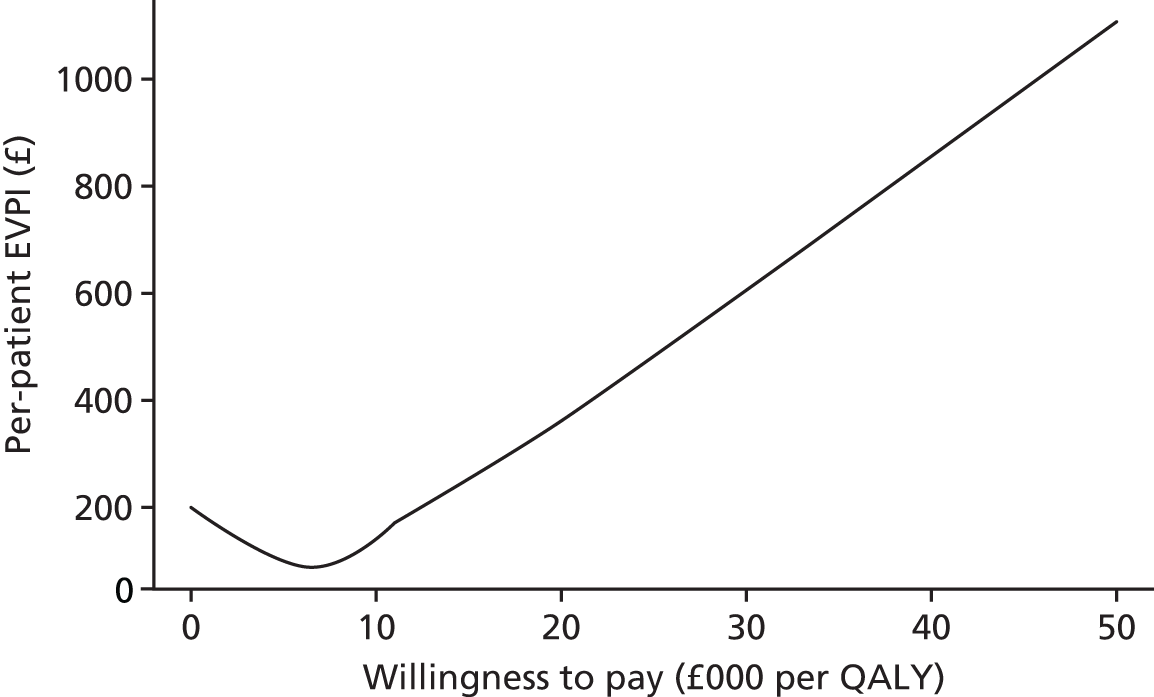
FIGURE 44.
Expected value of perfect information: NGAL (plasma) vs. standard care.
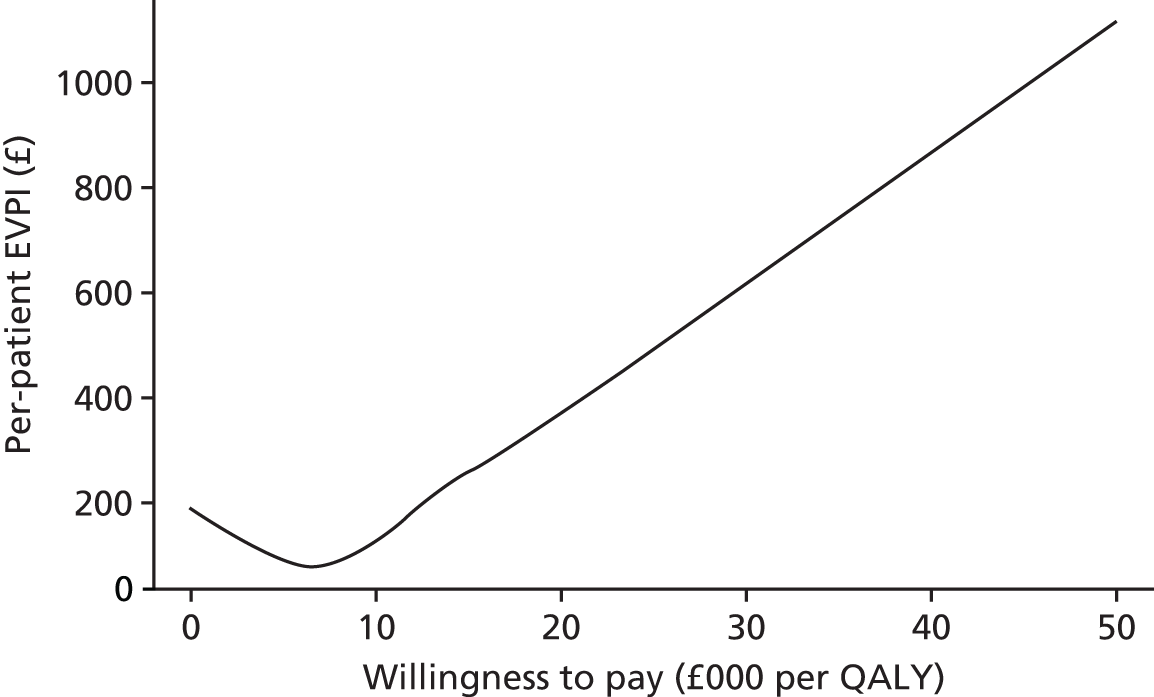
FIGURE 45.
Population EVPPI for single model parameters (showing top ranked parameters only).
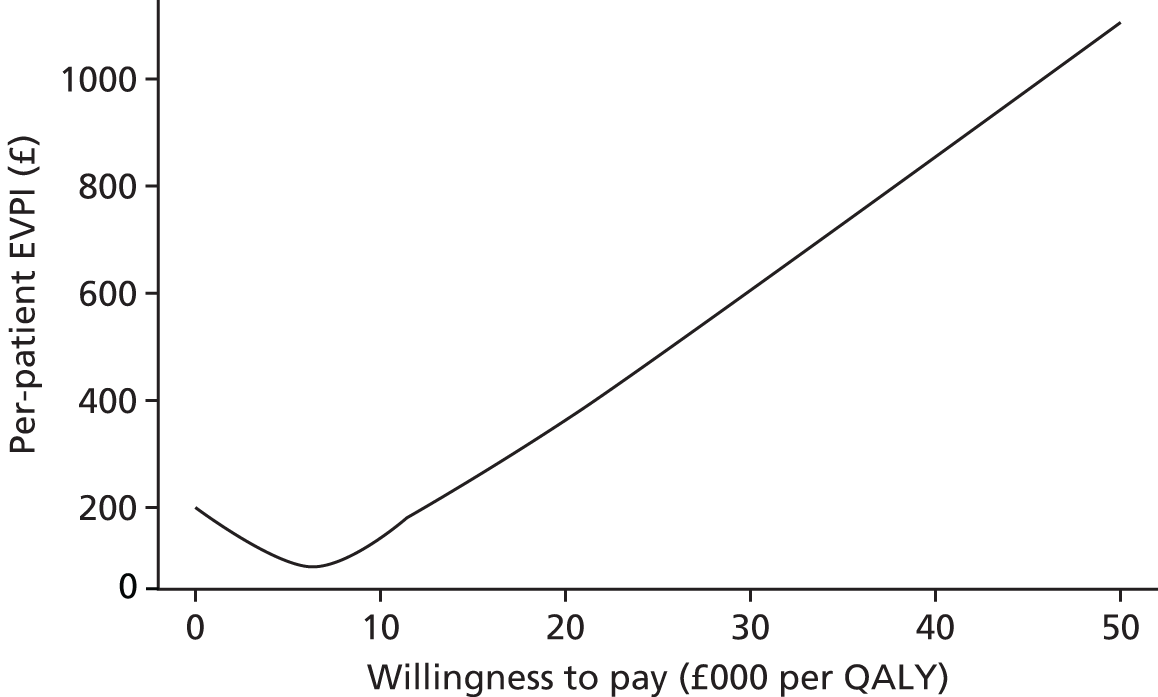
Expected value of perfect parameter information
The population EVPPIs for single model parameters are provided in Figure 46. For all tests the majority of top-ranked individual parameters relate to the proportion of patients arriving in the ICU with AKI, the proportion being diagnosed with AKI on the first day, the distribution of patients across health states at the end of the hospital period of the model and the impact of early AKI intervention on future AKI risks. Across all of the top ranked parameters shown, the Nephrocheck test is associated with the highest EVPPI, followed by the NGAL (plasma) test and the cystatin C (serum) test.
FIGURE 46.
Population EVPPIs for single model parameters (showing top-ranked parameters only). FUP, follow-up period; Tp, transition probability.
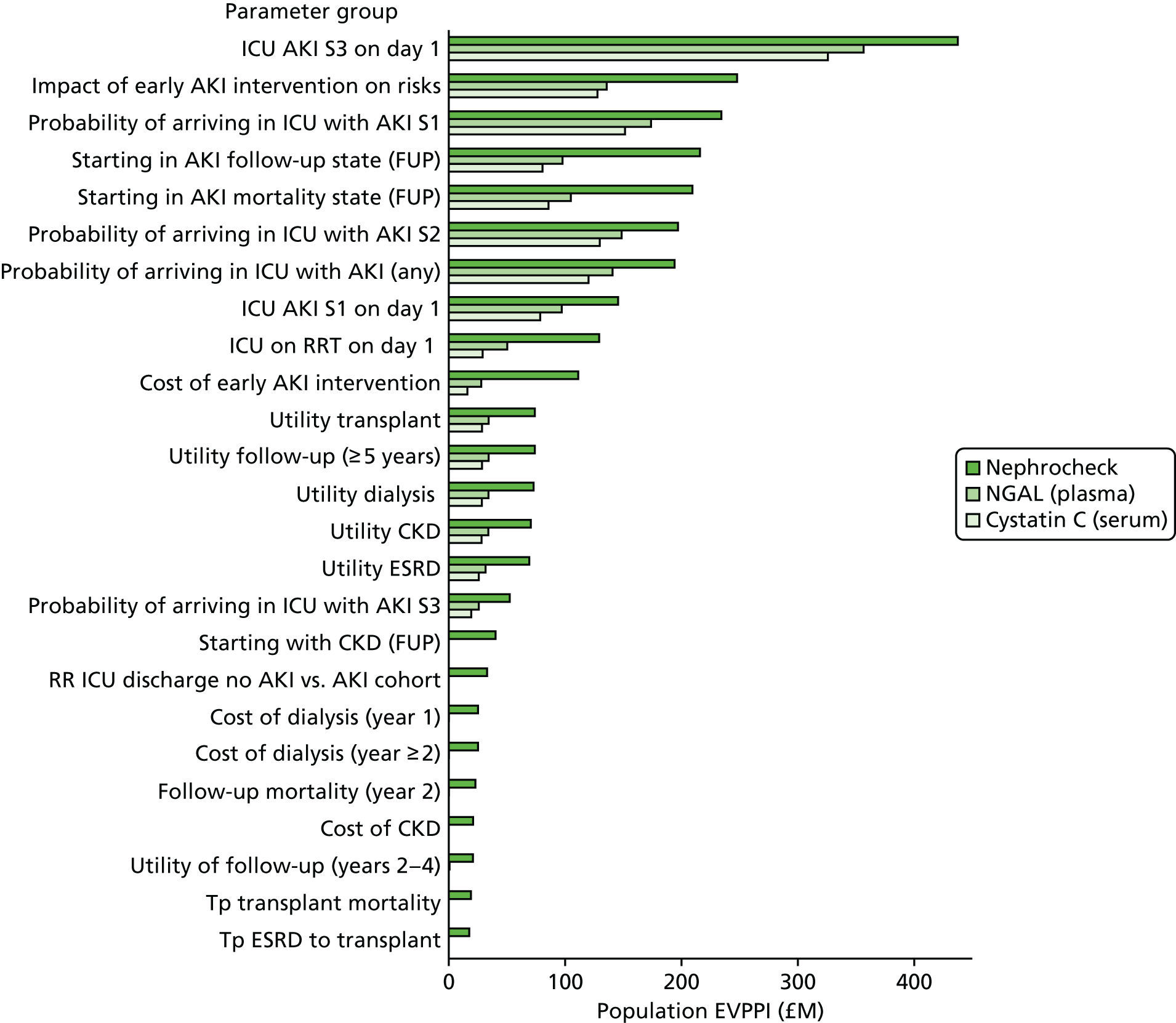
The population EVPPIs for groups of parameters are presented in Figure 47. The most influential groups of parameters are the incidence and starting stages of AKI, followed by the test parameters (test accuracy and impact and cost of early AKI intervention), and the starting distributions of patients entering the follow-up model (which are drawn from the end-state distribution of patients in the hospital period of the model). For all of the top-ranked parameter groups, the Nephrocheck test is associated with the highest VOI, followed by the NGAL (plasma) test and cystatin C (serum) test. The only parameter group for which this is not the case is ward mortality and discharge rates, for which NGAL (plasma) and cystatin C (serum) have positive EVPPIs (£1.53M and £0.48M respectively) and Nephrocheck has a zero value.
FIGURE 47.
Population EVPPIs for parameter groups.
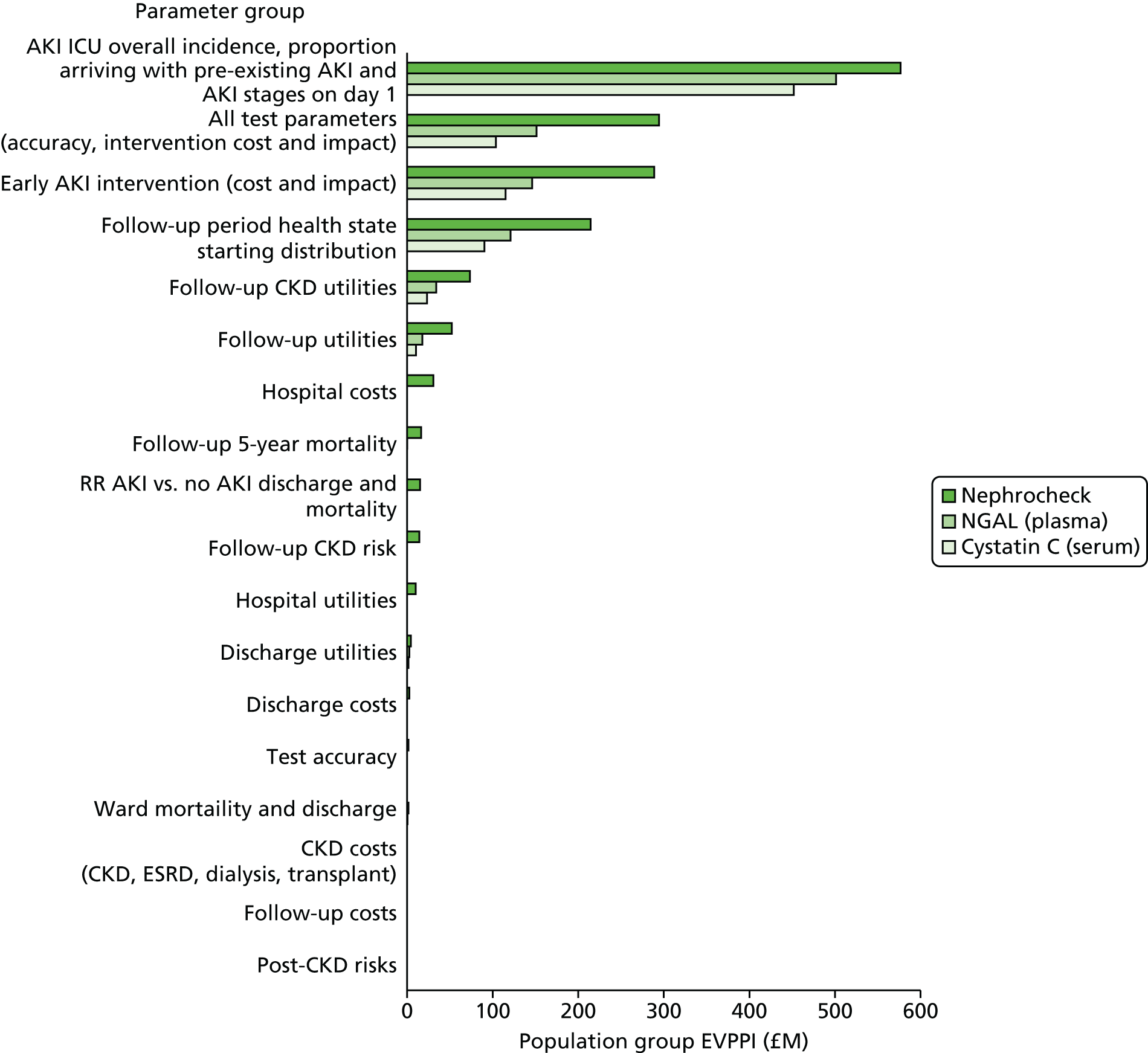
Summary
A VOI analysis was conducted to (1) inform a stop–go decision on further research into AKI diagnostic tests for the DEC programme and (2) guide the design of further research to ensure that it provides information that will reduce decision uncertainty in a targeted manner. Both the EVPI and the EVPPI metric were used to characterise the burden of decision uncertainty across and between the model parameters.
For the total 10-year discounted population of patients expected to be affected by this decision (n = 2,229,012), the 10-year population EVPIs are £958M, £827M and £807M for Nephrocheck, NGAL and cystatin C respectively. A positive EVPI is a necessary condition for further research being worthwhile, which has therefore been met for all three tests considered. Furthermore, the magnitude of these estimates suggests that the current burden of uncertainty puts us at significant risk of population health loss through an incorrect adoption decision.
When considering individual parameters, the top-ranked parameters in terms of population EVPPIs were the proportion of patients arriving in the ICU with pre-existing AKI, the proportion being diagnosed with AKI on the first day, the distribution of patients across health states at the end of the hospital period of the model and the cost and impact of early AKI intervention. Many of these values were > £100M.
When considering groups of parameters, the top-ranked EVPPIs (all also > £100M) were:
-
the incidence and starting stages of AKI
-
the impact and cost of early AKI intervention.
Discussion
Interpretation of the results and future research recommendations
The results of the VOI analysis indicate that there is a large population burden of decision uncertainty around the decision to reimburse the three tests that have been studied in detail. Conducting further research to reduce uncertainty around this decision problem is likely to be of high population value. In particular, two key areas for future research are highlighted as being particularly worthwhile: (1) the incidence and progression of AKI in the ICU and (2) the impact and cost of early AKI intervention. In the grouped EVPPI analysis, both of these parameter groups had values of > £100M across all three tests and ranked much higher than other parameters. This indicates that an investment of up to £100M would be worthwhile if it is able to eliminate uncertainty around these parameters. Although the test parameters grouping (composed of the test accuracy in addition to the impact and cost of early AKI intervention) also scored highly, it appears that this value is driven by the early AKI intervention parameters rather than the accuracy of the tests as these two group values are almost identical and the test accuracy parameters do not feature in the top-ranked single EVPPIs. It is also interesting to note that most of the top-ranked parameters relate to the short-term patient outcomes, suggesting that the downstream parameters are of less importance from the perspective of further research.
Although a formal RCT comparing a testing strategy with standard care would be necessary to provide definitive evidence for the clinical effectiveness and cost-effectiveness of the tests, these results suggest that expensive long-term follow-up may not be necessary. It may also be entirely possible to reduce our decision uncertainty substantially by undertaking some observational research to better understand the rates and trajectory of AKI in the ICU prior to undertaking a trial. It may even be possible to gain useful data from an observational study that looks at the use of the tests in real-world practice. This would enable valuable data to be gathered on how the tests may impact on clinical decision-making and change in the standard care pathway. It appears that, if knowledge is gained about these parameters, prominent sources of decision uncertainty could be addressed.
A single-centre study485 in patients after cardiac surgery using the Nephrocheck test has recently been reported, indicating that such studies are feasible. Further multicentre studies, ideally evaluating multiple tests, will be required to inform a robust assessment of the long-term health economic impact of such tests. These could be cluster or individually randomised interventions based on the complexities of the final study protocols and any concerns about contamination. Appropriate arguments would also need to be developed for the rationale of consenting patients (or not) in this setting.
It should be noted that the EVPPI results provide an upper bound on the amount that a decision-maker should be willing to spend on this research; additional factors need to be considered such as the cost of research; to what extent uncertainty would be diminished; the impact of treating patients in a research setting; and the impact of delaying access to treatment for NHS patients not taking part in a study. To make more precise statements about the value of research, a calculation of the expected value of sample information (EVSI) would be necessary, which has not been undertaken within the constraints of this project. Nevertheless, the magnitude of the VOI estimates observed strongly indicates that the required research would be achievable within this maximum budget (i.e. £100M). In addition to this form of study, better data on the incidence and progression of AKI in the ICU will continually become available from the AKI registry (see Chapter 5) and could be used to update the current evaluation, with a likely significant impact on uncertainty.
The results from the sensitivity analyses in Chapter 5 support these findings. In addition, the results of the sensitivity analyses also highlight the need for further evidence on the specific types of clinical management changes that would be implemented as a result of a positive or negative test result and the impact that they would have on patient short-term outcomes (i.e. not just limited to the impact of early AKI intervention for TPs). It appears that either a clinical consensus needs to be obtained here or the impact of alternative courses of action needs to be explored within a clinical study, which could be a fairly simple observational study. In addition, the meta-analysis of diagnostic studies could be extended to include a sensitivity analysis in which studies at high risk of bias are excluded. This would provide further information with respect to the robustness of estimates that are included in the economic evaluation.
Limitations
A key limitation of this analysis is the fact that the daily AKI transition probabilities could not be included as parameters of interest in the EVPPI analysis because of the size of these parameter matrices (which results in the VOI analysis taking an impractical amount of time to run, even using substantial parallel computing resources). However, the incidence of AKI, the proportion of patients with pre-existing AKI, the distribution of AKI on the first day and the end-state distribution of patients in the hospital period of the model all feature highly in the single- and grouped-parameter EVPPI analyses. It appears safe therefore to assume that the daily transitions between AKI states would similarly feature in the top-ranked parameters.
In addition to the EVPI and the EVPPI, the EVSI can be computed to determine the value of a defined research proposal, that is, a clinical trial of specified length and size that would provide information on specific parameters. EVSI calculation remains computationally expensive and was therefore not explored in this analysis but would be a useful addition if specific future study designs are proposed.
Chapter 7 Discussion
The AKI-Diagnostics project had the ambitious goal of establishing a recommended research and development strategy for diagnostic tests for AKI in critical care in the UK. This was undertaken within the NIHR DEC programme in acknowledgement that this is an area of significant health burden and is significantly underdeveloped as an area of technological application. As the first FDA-approved test, there was a risk that the Nephrocheck test would undergo widespread adoption without consideration of a robust evidence development strategy comparing it with other testing options and without it going through the necessary safeguard of a national health technology assessment.
The key clinical message from the project is that diagnostic tests for AKI in critical care patients who do not have AKI on admission have the potential to provide a meaningful albeit small benefit to patients. Although the number of studies supporting the Nephrocheck test is considerably smaller than those supporting the NGAL and cystatin C tests, the quality of these studies does appear to be high when faced with formal critical appraisal, particularly with regard to aspects of analytical validity.
All tests may represent strategies that may be cost-effective, but this is subject to uncertainty, which is particularly driven by assumptions regarding the impact of a diagnostic test on clinical care and the resulting change in clinical outcomes. Although these factors are both modelled in detail in the economic model, observational or experimental studies are required to ratify the link between possible care process changes and AKI rates and their longer-term implications.
The results of this project may be interpreted by some as providing sufficient evidence for adoption of the tests in the NHS; however, we would recommend that this is undertaken only within the framework of careful observational study, audit and an exit strategy at the point of evidence re-evaluation. Such an approach would allow many of the assumptions on which the economic model relies to be tested or better informed by data. There is interest by national reimbursement decision-makers such as NICE in new models of reimbursement that introduce conditionality on a positive reimbursement decision. We consider AKI diagnostic tests to be an ideal test case for such a model. There is interest by national reimbursement decision-makers such as NICE in new models of reimbursement that introduce conditionality on a positive reimbursement decision. We consider AKI diagnostic tests to be a suitable test case for such a model. This could be achieved by clearly defining the indication and putting in place a prospective audit framework that captures key data items that are currently uncertain or absent from the economic model.
The methods for quantitatively establishing an evidence-based and value-based research and development strategy for diagnostic testing are not established in a universally accepted or methodologically adequate manner. As such, the AKI-Diagnostics project represents a methods experiment that brings together many evaluation components. The structure of this report is intended to separate these components into their own sections for clarity, while acknowledging that they are interlinked and co-dependent. Most components ultimately feed into the economic model and this can be considered the summarising output from the project. The limitations of the individual components are discussed in detail in the individual chapters. It should be noted that, although our consideration of analytical validity goes further than what is normally required by health technology assessment authorities, the uncertainties around analytical validity parameters are not captured within the economic model and, therefore, do not feature in the VOI analysis. Development of improved methods for the incorporation of analytical validity in economic models should be considered an additional priority for further research.
Many learning points have emerged and will inform the design and conduct of similar attempts to map out a research strategy for the DECs and the wider NIHR in other diseases or scientific areas of study. The horizon scan identified a very large long list of candidate biomarkers and tests. We employed a ranking system based on numbers of publications, the combined sample size and mechanism of action, combined with expert opinion, using this as a surrogate for trajectory and novelty. Based on this ranking, we focused on three tests for detailed study, consuming the remaining resources available within the AKI-Diagnostics project. It is unknown to us whether these three tests represent the best options for further research investment compared with the many other putative tests that also require detailed study. Indeed, our relatively crude ranking strategy may specifically have missed novel or upcoming tests that have appeared only recently in the literature. We feel that further investment in methodology research to improve the methods for horizon scanning and the ranking of new technologies for public sector research investment would be very worthwhile.
There were two important limitations in the meta-analysis of sensitivity and specificity, the most important of which was the issue of an imperfect reference standard for an AKI definition based on urine output and serum creatinine; the other limitation was the issue of heterogeneity between studies, particularly with regard to the choice of case definition threshold. Our ability to explore both of these issues was severely hampered by a lack of data, particularly for NGAL and cystatin C, for which, despite a large volume of published studies, reporting standards were simply inadequate in the large majority for this purpose. We attempted to look at the potential impact of an imperfect reference standard in a fairly crude way using the economic model. In this analysis we showed that, if it is assumed that ≥ 25% of the FP results are actually TP results, tests have the potential to be notably more cost-effective (ICERs of <£10,000 per QALY and > 60% probability of being cost-effective). There should therefore be a strong incentive for researchers and manufacturers to study this further.
Several issues emerged during the construction and analysis of the economic model and the establishment of the clinical pathway on which it relies. A key challenge for the project was modelling the clinical impact and consequent outcomes from the results of the diagnostic tests. In the AKI-Diagnostics economic model these were derived from the confusion matrix, which allocates simulated patients as TPs, TNs, FPs and FNs. For the TP results we assumed that, for patients with early AKI, patients will benefit from some form of early AKI intervention, but the exact nature of this intervention is unclear because treatment for AKI currently relies on a non-specific bundle of ‘treatments’. No studies were available to inform the nature or impact of such early intervention on patient outcomes and as such we have had to use data on early nephrologist consultation as a proxy, with substantial uncertainty around the impact and cost of this intervention. All of the sensitivity and VOI analyses strongly indicate that the results are highly sensitive to changes in these parameters. Furthermore, we do not know what impact this early intervention would have on FP cases. It is also unclear if any management changes will result from a negative test result. In the base-case analysis we assumed no impact for FP or for all negative test results; however, it is completely reasonable to assume that this may not be the case. For example, FP results may result in harm because of delayed access to necessary treatments, whereas negative results may result in cost savings because of reduced monitoring costs and FN results may result in harm because of that same reduced monitoring. Again, the sensitivity analyses indicate that uncertainty in the impact of early intervention would have a significant impact. More information on all of these factors is needed if we want to make an informed adoption decision. In the face of emerging pharmaceutical treatments, further analysis could use the AKI-Diagnostics model to evaluate the potential cost-effectiveness of new drugs for AKI in light of hypothesised treatment effect sizes.
Uncertainty around the incidence and movement of patients between AKI stages in the model is another key driver of uncertainty. Our decision to model the transition of patients between AKI stages on a daily basis is data intensive, but we consider this level of detail to be necessary because of the importance of this time period in the natural history of AKI in a critical care setting. At the time of analysis, data from only 60 patients were available from the AKI registry. As time passes, a greater number of data will become available from the AKI registry and should help resolve much of this uncertainty.
A number of future research recommendations may build on or expand the work of the AKI-Diagnostics project. These include (1) a repeat meta-analysis excluding studies at high risk of bias and (2) testing the reliability and validity of the framework for assessing the quality of analytical validity studies.
We propose that biomarkers will help us stratify the many different types of AKI and better understand the underlying pathophysiology. A better understanding of the pathophysiology and the identification of the progression of functional AKI, in which there is no tubular damage, to tubular damage will further our ability to target therapies appropriately. We propose that we already have the capacity to introduce care pathways for patients with AKI and that some of the significant issues that we experience with this patient group relate to the delay in recognition of the condition. With regard to elevated creatinine values, some of this delay is associated with the transfer of data from the laboratory to the practitioner. However, even if the time delay associated with this pathway was reduced to a minimum the serum creatinine level would still represent a delayed marker that does not distinguish between functional AKI and damage AKI and does not indicate the severity of the damage. The early recognition by a biomarker of damage occurring in the kidney will trigger a clinical review for signs of sepsis, the avoidance of toxins, for example intravenous iodinated contrast and non-steroidal anti-inflammatory drugs, and a more robust response in terms of volume assessment, fluid replacement and consideration for the use of vasopressors. This would represent a personalised approach to a patient identified as developing AKI and it would be anticipated that this would reduce the injury and the chances of progression from early injury to more severe injury, with the associated increased risks of morbidity and mortality. Looking to the future it is hoped that, through the identification of new biomarkers that are able to distinguish tubular injury in the kidneys from functional AKI without kidney injury having yet occurred, therapies could be studied that target the underlying pathophysiological processes.
It may be considered that there will be no immediate added value to clinical practice from the AKI-Diagnostics project; however, this project has provided a comprehensive overview of work that has already been performed on biomarkers and, importantly, what work needs to be carried out going forward for them to be of clinical utility. There is a great deal of literature on these biomarkers and there is much confusion about their applicability in the NHS and outside in the global health-care community. This report has provided a summary of what is currently understood about the clinical utility of three high-profile biomarkers. We are regularly asked by clinical colleagues if and how we should utilise biomarkers in the critical care unit. We think that this report urges caution in investing in new biomarkers until the correct studies have been carried out that link the use of a particular biomarker with a clinical care pathway, so that its effect on patient outcomes through a plausible mechanism of clinical impact can be investigated.
Going forward, the development of a fit-for-purpose economic decision model in the AKI-Diagnostics project has provided a platform for the DECs and the NIHR to further develop a UK research strategy in this area. With relatively little funding, extra tests or biomarkers could be reviewed and evaluated in the economic model. It would also be possible to investigate tests used in settings beyond the immediate admission period to look at risk stratification or sequential monitoring. It is entirely possible that companies or academic institutions that wish to further evaluate in-development tests may take advantage of the opportunity presented by building on the work of the AKI-Diagnostics project or it may be that NICE or other national decision-makers wish to fund the evaluation of tests when they emerge from their respective pipelines.
Acknowledgements
We would like to thanks the contributions of the Specialist Advisory Committee for the AKI-Diagnostics project: Professor Linda Sharples, Professor Chris McCabe, Dr Chris Smith, Dr Cathie Sturgeon, Professor Douglas Thompson, Professor Walter Gregory, Dr Nick Selby, Dr Marlies Ostermann, Dr Lui G Forni, Professor Duncan Young, Dr Hayley Jones, Professor Mark Bellamy, Catherine Hall and the late Dr Rick Jones. We would also like to thank the contribution of the Project Delivery Board: Professor Peter Selby, Dr David Kluth, Professor Claire Hulme, Professor Doug Altman, Paul Weinburger and Barbara Hartley.
We would like to thank the members of the research team who did not qualify for authorship of this report but whose hard work, competence and dedication to the project made it possible: Nicola Calder (NC), Nyantara Wickramasekera (NW), Rebecca Kift (RK), Aleksandra Sobota (AS), Denise Womersley (DW) and Ashley Garner (AG).
Contributions of authors
Peter S Hall (Senior Clinical Lecturer) was the methods lead and provided expertise in health economics.
Elizabeth D Mitchell (Senior Research Fellow) was the lead for the systematic reviews and project management.
Alison F Smith (Research Fellow) was the project health economist.
David A Cairns (Principal Statistician) was the supervising project statistician.
Michael Messenger (Deputy Director of the NIHR DEC Leeds, Healthcare Scientist) was the lead for performance measurement.
Michelle Hutchinson (Biostatistician) was the project statistician.
Judy Wright (Senior Information Specialist) was the lead for the search strategies.
Karen Vinall-Collier (Research Fellow) was the lead for the qualitative methods and project co-ordination.
Claire Corps was the patient representative on the study.
Patrick Hamilton (Clinical Research Fellow) was the advising nephrologist.
David Meads (Senior Lecturer in Health Economics) was the supervising health economist.
Andrew Lewington (Consultant Nephrologist) was the clinical lead and provided expertise in nephrology.
Publications
Mitchell E, Smith A, Wright J, Calder N, Wickramasekera N, Messenger M, et al. Review Strategies to Inform Research Prioritization of Biomarkers: AKI-Diagnostics Case Study. Methods for Evaluating Medical Tests and Biomarkers Symposium, Birmingham, 2016 (abstract).
Smith A, Hall P, Meads D, Hutchinson M, Mitchell E, Wright J, et al. Cost-effectiveness of NephroCheck® for Acute Kidney Injury in Critical Care. American Society of Nephrology Annual Conference, Chicago, 2016 (abstract).
Mitchell E, Smith A, Wright J, Calder N, Wickramasekera N, Messenger M, et al. Diagnostic Accuracy of the NephroCheck Test for Acute Kidney Injury in Critical Care: Systematic Review and Meta-analysis. American Society of Nephrology Annual Conference, Chicago, 2016 (abstract).
Smith A, Hall P, Meads D, Hutchinson M, Mitchell E, Wright J, et al. Using Cost-effectiveness and Value of Information Analysis to Inform Research Prioritisation: Findings from the AKI-Diagnostics Study. iHEA, London, 2017 (abstract).
Data sharing statement
As a secondary research project, no data are available for sharing; however, the corresponding author will consider requests to share the model programming code.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Acute Kidney Injury: Prevention, Detection and Management. London: NICE; 2013.
- Unknown . Calculating the cost. Health Serv J 2011;121.
- Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med 2009;37:2552-8. https://doi.org/10.1097/CCM.0b013e3181a5906f.
- Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, et al. Current use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative Consensus Conference. Kidney Int 2014;85. https://doi.org/10.1038/ki.2013.374.
- Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 2011;79:1361-9. https://doi.org/10.1038/ki.2011.42.
- Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27:iii73-80. https://doi.org/10.1093/ndt/gfs269.
- NHS England . Acute Kidney Injury Programme – Think Kidneys n.d. www.england.nhs.uk/akiprogramme/ (accessed 25 May 2018).
- Kidney Disease: Improving Global Outcomes (KDIGO) Working Group . KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012;2.
- Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65. https://doi.org/10.1016/S0140-6736(13)62229-1.
- Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383:166-75. https://doi.org/10.1016/S0140-6736(13)62227-8.
- Al-Shahi Salman R, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85. https://doi.org/10.1016/S0140-6736(13)62297-7.
- Chan AW, Song F, Vickers A, Jefferson T, Dickersin K, Gøtzsche PC, et al. Increasing value and reducing waste: addressing inaccessible research. Lancet 2014;383:257-66. https://doi.org/10.1016/S0140-6736(13)62296-5.
- Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76. https://doi.org/10.1016/S0140-6736(13)62228-X.
- Moher D, Glasziou P, Chalmers I, Nasser M, Bossuyt PM, Korevaar DA, et al. Increasing value and reducing waste in biomedical research: who’s listening?. Lancet 2016;387:1573-86. https://doi.org/10.1016/S0140-6736(15)00307-4.
- Towse A, Ossa D, Veenstra D, Carlson J, Garrison L. Understanding the economic value of molecular diagnostic tests: case studies and lessons learned. J Pers Med 2013;3:288-305. https://doi.org/10.3390/jpm3040288.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. https://doi.org/10.1016/S0167-6296(98)00039-3.
- Hall P, McCabe C, Hulme C, Edlin R, Dunn J, Cameron D, et al. Efficient design of a Phase III trial of competing tests for personalised cancer treatment in the absence of gold standard outcome data: challenges and potential solutions. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-S1-O5.
- Guidance for Industry: Bioanalytical Method Validation. Silverspring, MD: US Food and Drug Administration; 2001.
- EP32-R Metrological Traceability and Its Implementation. Wayne, PA: Clinical and Laboratory Standards Institute; 2006.
- EP26-A User Evaluation of Between-Reagent Lot Variation. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
- EP25-A Evaluation of Stability of In Vitro Diagnostic Reagents. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
- Estimation of Total Analytical Error for Clinical Laboratory Methods; Approved Guideline. NCCLS document EP21-A. Wayne, PA: NCCLS; 2003.
- EP15-A3 User Verification of Precision and Estimation of Bias. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
- EP09-A3 Measurement Procedure Comparison and Bias Estimation Using Patient Samples. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
- EP07-A2 Interference Testing in Clinical Chemistry. Wayne, PA: Clinical and Laboratory Standards Institute; 2005.
- EP05-A3 Evaluation of Precision of Quantitative Measurement Procedures. Wayne, PA: Clinical and Laboratory Standards Institute; 2014.
- I/LA30-A Immunoassay Interference by Endogenous Antibodies. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
- EP14-A Evaluation of Matrix Effects; Approved Guideline – Second Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2005.
- EP29A Expression of Measurement Uncertainty in Laboratory Medicine. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Möckel M, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 2009;88:124-30. https://doi.org/10.1016/j.athoracsur.2009.04.023.
- Aydogdu M, Gursel G, Sancak B, Yeni S, Sari G, Tasyurek S, et al. The use of plasma and urine neutrophil gelatinase associated lipocalin (NGAL) and cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers 2013;34:237-46. https://doi.org/10.1155/2013/740351.
- Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014;189:932-9. https://doi.org/10.1164/rccm.201401-0077OC.
- Chen TH, Chang CH, Lin CY, Jenq CC, Chang MY, Tian YC, et al. Acute kidney injury biomarkers for patients in a coronary care unit: a prospective cohort study. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0032328.
- Cho E, Yang HN, Jo SK, Cho WY, Kim HK. The role of urinary liver-type fatty acid-binding protein in critically ill patients. J Korean Med Sci 2013;28:100-5. https://doi.org/10.3346/jkms.2013.28.1.100.
- Constantin JM, Futier E, Perbet S, Roszyk L, Lautrette A, Gillart T, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care 2010;25. https://doi.org/10.1016/j.jcrc.2009.05.010.
- de Geus HR, Woo JG, Wang Y, Devarajan P, Betjes MG, le Noble JL, et al. Urinary neutrophil gelatinase-associated lipocalin measured on admission to the intensive care unit accurately discriminates between sustained and transient acute kidney injury in adult critically ill patients. Nephron Extra 2011;1:9-23. https://doi.org/10.1159/000330428.
- Ghonemy TA, Amro GM. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl 2014;25:582-8. https://doi.org/10.4103/1319-2442.132194.
- Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery – a prospective cohort study. Crit Care Med 2009;37:553-60. https://doi.org/10.1097/CCM.0b013e318195846e.
- Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, et al. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant 2009;24:3349-54. https://doi.org/10.1093/ndt/gfp234.
- Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2009;4:873-82. https://doi.org/10.2215/CJN.04810908.
- Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int 2004;66:1115-22. https://doi.org/10.1111/j.1523-1755.2004.00861.x.
- Hjortrup PB, Haase N, Treschow F, Møller MH, Perner A. Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiol Scand 2015;59:25-34. https://doi.org/10.1111/aas.12427.
- Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014;29:2054-61. https://doi.org/10.1093/ndt/gfu292.
- Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17. https://doi.org/10.1186/cc12503.
- Kato K, Sato N, Yamamoto T, Iwasaki YK, Tanaka K, Mizuno K. Valuable markers for contrast-induced nephropathy in patients undergoing cardiac catheterization. Circ J 2008;72:1499-505. https://doi.org/10.1253/circj.CJ-07-1006.
- Kidher E, Harling L, Ashrafian H, Naase H, Chukwuemeka A, Anderson J, et al. Pulse wave velocity and neutrophil gelatinase-associated lipocalin as predictors of acute kidney injury following aortic valve replacement. J Cardiothorac Surg 2014;9. https://doi.org/10.1186/1749-8090-9-89.
- Kokkoris S, Parisi M, Ioannidou S, Douka E, Pipili C, Kyprianou T, et al. Combination of renal biomarkers predicts acute kidney injury in critically ill adults. Ren Fail 2012;34:1100-8. https://doi.org/10.3109/0886022X.2012.713279.
- Legrand M, Jacquemod A, Gayat E, Collet C, Giraudeaux V, Launay JM, et al. Failure of renal biomarkers to predict worsening renal function in high-risk patients presenting with oliguria. Intensive Care Med 2015;41:68-76. https://doi.org/10.1007/s00134-014-3566-3.
- Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers 2009;14:423-31. https://doi.org/10.1080/13547500903067744.
- Linko R, Pettila V, Kuitunen A, Korhonen AM, Nisula S, Alila S, et al. Plasma neutrophil gelatinase-associated lipocalin and adverse outcome in critically ill patients with ventilatory support. Acta Anaesthesiol Scand 2013;57:855-62. https://doi.org/10.1111/aas.12112.
- Liu S, Che M, Xue S, Xie B, Zhu M, Lu R, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers 2013;18:95-101. https://doi.org/10.3109/1354750X.2012.740687.
- McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol 2010;5:211-19. https://doi.org/10.2215/CJN.04240609.
- Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0093460.
- Meersch M, Schmidt C, Van Aken H, Rossaint J, Görlich D, Stege D, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0110865.
- Munir MU, Khan DA, Khan FA, Shahab Naqvi SM. Rapid detection of acute kidney injury by urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass surgery. J Coll Physicians Surg Pak 2013;23:103-6.
- Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant 2010;25:3283-9. https://doi.org/10.1093/ndt/gfq176.
- Oh SW, Chin HJ, Chae DW, Na KY. Erythropoietin improves long-term outcomes in patients with acute kidney injury after coronary artery bypass grafting. J Korean Med Sci 2012;27:506-11. https://doi.org/10.3346/jkms.2012.27.5.506.
- Palazzuoli A, Ruocco G, Pellegrini M, De Gori C, Del Castillo G, Franci B, et al. Comparison of neutrophil gelatinase-associated lipocalin versus B-type natriuretic peptide and cystatin C to predict early acute kidney injury and outcome in patients with acute heart failure. Am J Cardiol 2015;116:104-11. https://doi.org/10.1016/j.amjcard.2015.03.043.
- Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011;22:1748-57. https://doi.org/10.1681/ASN.2010121302.
- Park CM, Kim JS, Moon HW, Park S, Kim H, Ji M, et al. Usefulness of plasma neutrophil gelatinase-associated lipocalin as an early marker of acute kidney injury after cardiopulmonary bypass in Korean cardiac patients: a prospective observational study. Clin Biochem 2015;48:44-9. https://doi.org/10.1016/j.clinbiochem.2014.09.019.
- Perrotti A, Miltgen G, Chevet-Noel A, Durst C, Vernerey D, Bardonnet K, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015;99:864-9. https://doi.org/10.1016/j.athoracsur.2014.10.011.
- Perry TE, Muehlschlegel JD, Liu KY, Fox AA, Collard CD, Shernan SK, et al. Plasma neutrophil gelatinase-associated lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth Analg 2010;110:1541-7. https://doi.org/10.1213/ANE.0b013e3181da938e.
- Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Bagshaw SM, et al. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren Fail 2015;37:408-16. https://doi.org/10.3109/0886022X.2014.1001303.
- Sargentini V, Mariani P, D’Alessandro M, Pistolesi V, Lauretta MP, Pacini F, et al. Assessment of NGAL as an early biomarker of acute kidney injury in adult cardiac surgery patients. J Biol Regul Homeost Agents 2012;26:485-93.
- Shum HP, Leung NY, Chang LL, Tam OY, Kwan AM, Chan KC, et al. Predictive value of plasma neutrophil gelatinase-associated lipocalin for acute kidney injury in ICU patients after major non-cardiac surgery. Nephrology (Carlton) 2015;20:375-82. https://doi.org/10.1111/nep.12400.
- Tuladhar SM, Puntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol 2009;53:261-6. https://doi.org/10.1097/FJC.0b013e31819d6139.
- Tung YC, Chang CH, Chen YC, Chu PH. Combined biomarker analysis for risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0125282.
- Tziakas D, Chalikias G, Kareli D, Tsigalou C, Risgits A, Kikas P, et al. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol 2015;197:48-55. https://doi.org/10.1016/j.ijcard.2015.06.019.
- Varela CF, Greloni G, Schreck C, Bratti G, Medina A, Marenchino R, et al. Assessment of fractional excretion of urea for early diagnosis of cardiac surgery associated acute kidney injury. Ren Fail 2015;37:327-31. https://doi.org/10.3109/0886022X.2015.1087800.
- Villa P, Jiménez M, Soriano MC, Manzanares J, Casasnovas P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care 2005;9:R139-43. https://doi.org/10.1186/cc3044.
- Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis 2008;52:425-33. https://doi.org/10.1053/j.ajkd.2008.05.018.
- Adademir T, Ak K, Aljodi M, Elci ME, Arsan S, Isbir S. The effects of pulsatile cardiopulmonary bypass on acute kidney injury. Int J Artif Organs 2012;35:511-19. https://doi.org/10.5301/ijao.5000097.
- Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail 2010;16:49-54. https://doi.org/10.1016/j.cardfail.2009.07.003.
- Åhlström A, Tallgren M, Peltonen S, Pettila V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol 2004;62:344-50. https://doi.org/10.5414/CNP62344.
- Al-Beladi F, Al-Shabassy A. Cystatin C as a predictor of contrast-induced nephropathy in critically-ill patients. Bahrain Med Bull 2014;36:81-5. https://doi.org/10.12816/0004481.
- Alcaraz AJ, Gil-Ruiz MA, Castillo A, Lopez J, Romero C, Fernandez SN, et al. Postoperative neutrophil gelatinase-associated lipocalin predicts acute kidney injury after pediatric cardiac surgery. Pediatr Crit Care Med 2014;15:121-30. https://doi.org/10.1097/PCC.0000000000000034.
- Alharazy SM, Kong N, Saidin R, Gafor AH, Maskon O, Mohd M, et al. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after coronary angiography. Angiology 2014;65:216-23. https://doi.org/10.1177/0003319712474947.
- Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 2014;85:431-8. https://doi.org/10.1038/ki.2013.333.
- Ataei N, Bazargani B, Ameli S, Madani A, Javadilarijani F, Moghtaderi M, et al. Early detection of acute kidney injury by serum cystatin C in critically ill children. Pediatr Nephrol 2014;29:133-8. https://doi.org/10.1007/s00467-013-2586-5.
- Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med 2010;36:452-61. https://doi.org/10.1007/s00134-009-1724-9.
- Bargnoux AS, Pieroni L, Cristol JP. Analytical study of a new turbidimetric assay for urinary neutrophil gelatinase-associated lipocalin (NGAL) determination. Clin Chem Lab Med 2013;51:E293-E6. https://doi.org/10.1515/cclm-2013-0391.
- Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 2014;9:654-62. https://doi.org/10.2215/CJN.09720913.
- Bell M, Granath F, Martensson J, Lofberg E, Ekbom A, Martling CR, et al. Cystatin C is correlated with mortality in patients with and without acute kidney injury. Nephrol Dial Transplant 2009;24:3096-102. https://doi.org/10.1093/ndt/gfp196.
- Bell M, Larsson A, Venge P, Bellomo R, Martensson J. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers 2015;2015. https://doi.org/10.1155/2015/158658.
- Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008;3:665-73. https://doi.org/10.2215/CJN.04010907.
- Bojan M, Vicca S, Lopez-Lopez V, Mogenet A, Pouard P, Falissard B, et al. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol 2014;9:285-94. https://doi.org/10.2215/CJN.04730513.
- Bojic S, Kotur-Stevuljevic J, Kalezic N, Stevanovic P, Jelic-Ivanovic Z, Bilanovic D, et al. Diagnostic Value of Matrix Metalloproteinase-9 and Tissue Inhibitor of Matrix Metalloproteinase-1 in Sepsis-Associated Acute Kidney Injury. Tohoku J Exp Med 2015;237:103-9. https://doi.org/10.1620/tjem.237.103.
- Breidthardt T, Socrates T, Drexler B, Noveanu M, Heinisch C, Arenja N, et al. Plasma neutrophil gelatinase-associated lipocalin for the prediction of acute kidney injury in acute heart failure. Crit Care 2012;16. https://doi.org/10.1186/cc10600.
- Briguori C, Visconti G, Rivera NV, Focaccio A, Golia B, Giannone R, et al. Cystatin C and contrast-induced acute kidney injury. Circulation 2010;121:2117-22. https://doi.org/10.1161/CIRCULATIONAHA.109.919639.
- Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol 2010;5:2229-35. https://doi.org/10.2215/CJN.00980110.
- Cantinotti M, Storti S, Lorenzoni V, Arcieri L, Moschetti R, Murzi B, et al. The combined use of neutrophil gelatinase-associated lipocalin and brain natriuretic peptide improves risk stratification in pediatric cardiac surgery. Clin Chem Lab Med 2012;50:2009-17. https://doi.org/10.1515/cclm-2012-0125.
- Cemil K, Elif C, Serkan YM, Fevzi Y, Deniz AE, Tamer D, et al. The value of serum NGAL in determination of dialysis indication. J Pak Med Assoc 2014;64:739-42.
- Cho E, Lee JH, Lim HJ, Oh SW, Jo SK, Cho WY, et al. Soluble CD25 is increased in patients with sepsis-induced acute kidney injury. Nephrology 2014;19:318-24. https://doi.org/10.1111/nep.12230.
- Chung HJ, Pellegrini KL, Chung J, Wanigasuriya K, Jayawardene I, Lee K, et al. Nanoparticle detection of urinary markers for point-of-care diagnosis of kidney injury. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0133417.
- Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 2010;36:444-51. https://doi.org/10.1007/s00134-009-1711-1.
- Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem 2012;49:190-3. https://doi.org/10.1258/acb.2011.011105.
- Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care 2015. https://doi.org/10.1186/s13054-015-0941-6.
- De Berardinis B, Gaggin HK, Magrini L, Belcher A, Zancla B, Femia A, et al. Comparison between admission natriuretic peptides, NGAL and sST2 testing for the prediction of worsening renal function in patients with acutely decompensated heart failure. Clin Chem Lab Med 2015;53:613-21. https://doi.org/10.1515/cclm-2014-0191.
- de Geus HR, Bakker J, Lesaffre EM, le Noble JL. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 2011;183:907-14. https://doi.org/10.1164/rccm.200908-1214OC.
- de Geus HR, Betjes MG, Schaick R, Groeneveld JA. Plasma NGAL similarly predicts acute kidney injury in sepsis and nonsepsis. Biomark Med 2013;7:415-21. https://doi.org/10.2217/bmm.13.5.
- de Geus HR, Fortrie G, Betjes MG, van Schaik RH, Groeneveld AB. Time of injury affects urinary biomarker predictive values for acute kidney injury in critically ill, non-septic patients. BMC Nephrol 2013;14. https://doi.org/10.1186/1471-2369-14-273.
- Delanaye P, Cavalier E, Morel J, Mehdi M, Maillard N, Claisse G, et al. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol 2014;15. https://doi.org/10.1186/1471-2369-15-9.
- Delcroix G, Gillain N, Moonen M, Radermacher L, Damas F, Minon JM, et al. NGAL Usefulness in the Intensive Care Unit Three Hours after Cardiac Surgery. ISRN Nephrol 2013;2013. https://doi.org/10.5402/2013/865164.
- Demirtas S, Caliskan A, Karahan O, Yavuz C, Guclu O, Cayir MC, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for acute kidney injury in patients undergoing coronary artery bypass grafting. Exp Clin Cardiol 2013;18:107-9.
- Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care 2007;11. https://doi.org/10.1186/cc6192.
- Di Somma S, Magrini L, De Berardinis B, Marino R, Ferri E, Moscatelli P, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care 2013;17. https://doi.org/10.1186/cc12510.
- Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 2011;39:2464-9. https://doi.org/10.1097/CCM.0b013e318225761a.
- Doi K, Urata M, Katagiri D, Inamori M, Murata S, Hisagi M, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013;17. https://doi.org/10.1186/cc13104.
- Doi K, Noiri E, Nangaku M, Yahagi N, Jayakumar C, Ramesh G. Repulsive guidance cue semaphorin 3A in urine predicts the progression of acute kidney injury in adult patients from a mixed intensive care unit. Nephrol Dial Transplant 2014;29:73-80. https://doi.org/10.1093/ndt/gft414.
- Ejaz AA, Kambhampati G, Ejaz NI, Dass B, Lapsia V, Arif AA, et al. Post-operative serum uric acid and acute kidney injury. J Nephrol 2012;25:497-505. https://doi.org/10.5301/jn.5000173.
- El-Farghali OG, El-Raggal NM, Mahmoud NH, Zaina GA. Serum neutrophil gelatinase-associated lipocalin as a predictor of acute kidney injury in critically-ill neonates. Pak J Biol Sci 2012;15:231-7. https://doi.org/10.3923/pjbs.2012.231.237.
- Fan H, Zhao Y, Zhu JH, Song FC. Urine neutrophil gelatinase-associated lipocalin in septic patients with and without acute kidney injury. Ren Fail 2014;36:1399-403. https://doi.org/10.3109/0886022X.2014.945184.
- Fanning N, Galvin S, Parke R, Gilroy J, Bellomo R, McGuinness S. A Prospective Study of the Timing and Accuracy of Neutrophil Gelatinase-Associated Lipocalin Levels in Predicting Acute Kidney Injury in High-Risk Cardiac Surgery Patients. J Cardiothorac Vasc Anesth 2016;50:76-81. https://doi.org/10.1053/j.jvca.2015.07.034.
- Felicio ML, Andrade RR, Castiglia YM, Silva MA, Vianna PT, Martins AS. Cystatin C and glomerular filtration rate in the cardiac surgery with cardiopulmonary bypass. Rev Bras Cir Cardiovasc 2009;24:305-11. https://doi.org/10.1590/S0102-76382009000400008.
- Fouad M, Boraie M. Cystatin C as an early marker of acute kidney injury and predictor of mortality in the intensive care unit after acute myocardial infarction. Arab J Nephrol Transplant 2013;6:21-6.
- Gaipov A, Solak Y, Turkmen K, Toker A, Baysal AN, Cicekler H, et al. Serum uric acid may predict development of progressive acute kidney injury after open heart surgery. Ren Fail 2015;37:96-102. https://doi.org/10.3109/0886022X.2014.976130.
- Glassford NJ, Schneider AG, Xu S, Eastwood GM, Young H, Peck L, et al. The nature and discriminatory value of urinary neutrophil gelatinase-associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med 2013;39:1714-24. https://doi.org/10.1007/s00134-013-3040-7.
- Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0120863.
- Grenier FC, Ali S, Syed H, Workman R, Martens F, Liao M, et al. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem 2010;43:615-20. https://doi.org/10.1016/j.clinbiochem.2009.12.008.
- Guo Y, Yan KP. Prognostic significance of urine neutrophil gelatinase-associated lipocalin in patients with septic acute kidney injury. Exp Ther Med 2011;2:1133-9. https://doi.org/10.3892/etm.2011.339.
- Hamed HM, El-Sherbini SA, Barakat NA, Farid TM, Rasheed EA. Serum cystatin C is a poor biomarker for diagnosing acute kidney injury in critically-ill children. Indian J Crit Care Med 2013;17:92-8. https://doi.org/10.4103/0972-5229.114829.
- Hansen YB, Damgaard A, Poulsen JH. Evaluation of NGAL TestTM on Cobas 6000. Scand J Clin Lab Invest 2014;74:20-6. https://doi.org/10.3109/00365513.2013.855943.
- Hassinger AB, Backer CL, Lane JC, Haymond S, Wang D, Wald EL. Predictive power of serum cystatin C to detect acute kidney injury and pediatric-modified RIFLE class in children undergoing cardiac surgery. Pediatr Crit Care Med 2012;13:435-40. https://doi.org/10.1097/PCC.0b013e318238b43c.
- Heise D, Waeschle RM, Schlobohm J, Wessels J, Quintel M. Utility of cystatin C for assessment of renal function after cardiac surgery. Nephron 2009;112:c107-14. https://doi.org/10.1159/000213898.
- Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 2007;22:2089-95. https://doi.org/10.1007/s00467-007-0601-4.
- Hoffman SB, Massaro AN, Soler-Garcia AA, Perazzo S, Ray PE. A novel urinary biomarker profile to identify acute kidney injury (AKI) in critically ill neonates: a pilot study. Pediatr Nephrol 2013;28:2179-88. https://doi.org/10.1007/s00467-013-2524-6.
- Hong DY, Lee JH, Park SO, Baek KJ, Lee KR. Plasma neutrophil gelatinase-associated lipocalin as early biomarker for acute kidney injury in burn patients. J Burn Care Res 2013;34:e326-32. https://doi.org/10.1097/BCR.0b013e31827d1f36.
- In JW, Kim JE, Jeong JS, Song SH, Kim HK. Diagnostic and prognostic significance of neutrophil gelatinase-associated lipocalin in disseminated intravascular coagulation. Clinica Chimica Acta 2014;430:145-9. https://doi.org/10.1016/j.cca.2014.01.022.
- Jayaraman R, Sunder S, Sathi S, Gupta VK, Sharma N, Kanchi P, et al. Post cardiac surgery acute kidney injury: a woebegone status rejuvenated by the novel biomarkers. Nephrourol Mon 2014;6. https://doi.org/10.5812/numonthly.19598.
- Johansson M, Nozohoor S, Bjursten H, Kimblad PO, Sjogren J. Acute kidney injury assessed by cystatin C after transcatheter aortic valve implantation and late renal dysfunction. J Cardiothorac Vasc Anesthe 2014;28:972-7. https://doi.org/10.1053/j.jvca.2013.08.008.
- Kambhampati G, Ejaz NI, Asmar A, Aiyer RK, Arif AA, Pourafshar N, et al. Fluid balance and conventional and novel biomarkers of acute kidney injury in cardiovascular surgery. J Cardiovasc Surg 2013;54:639-46.
- Khosravi MB, Milani S, Kakaei F. Serum Neutrophil Gelatinase-Associated Lipocalin versus Serum Creatinine for the Prediction of Acute Kidney Injury after Liver Transplantation. Int J Organ Transplant Med 2013;4:102-9.
- Kiessling AH, Dietz J, Reyher C, Stock UA, Beiras-Fernandez A, Moritz A. Early postoperative serum cystatin C predicts severe acute kidney injury following cardiac surgery: a post-hoc analysis of a randomized controlled trial. J Cardiothorac Surg 2014;9. https://doi.org/10.1186/1749-8090-9-10.
- Kift RL, Messenger MP, Wind TC, Hepburn S, Wilson M, Thompson D, et al. A comparison of the analytical performance of five commercially available assays for neutrophil gelatinase-associated lipocalin using urine. Ann Clin Biochem 2013;50:236-44. https://doi.org/10.1258/acb.2012.012117.
- Kim S, Sung J, Kang WC, Ahn SY, Kim DK, Chin HJ, et al. Increased plasma osmolar gap is predictive of contrast-induced acute kidney injury. Tohoku J Exp Med 2012;228:109-17. https://doi.org/10.1620/tjem.228.109.
- Kim H, Hur M, Cruz DN, Moon HW, Yun YM. Plasma neutrophil gelatinase-associated lipocalin as a biomarker for acute kidney injury in critically ill patients with suspected sepsis. Clin Biochem 2013;46:1414-18. https://doi.org/10.1016/j.clinbiochem.2013.05.069.
- Koch AM, Dittrich S, Cesnjevar R, Ruffer A, Breuer C, Glockler M. Plasma neutrophil gelatinase-associated lipocalin measured in consecutive patients after congenital heart surgery using point-of-care technology. Interact Cardiovasc Thorac Surg 2011;13:133-6. https://doi.org/10.1510/icvts.2011.269647.
- Koeijers J, De Lima N, Kotzebue C, Ajubi N. NGAL as a marker of early diagnosis of acute kidney injury in ICU patients: The sehos-ICU study. Crit Care Med 2012;1.
- Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 2008;74:1059-69. https://doi.org/10.1038/ki.2008.341.
- Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 2010;5:2154-65. https://doi.org/10.2215/CJN.00740110.
- Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 2012;23:905-14. https://doi.org/10.1681/ASN.2011090907.
- Koyner JL, Garg AX, Shlipak MG, Patel UD, Sint K, Hong K, et al. Urinary cystatin C and acute kidney injury after cardiac surgery. Am J Kidney Dis 2013;61:730-8. https://doi.org/10.1053/j.ajkd.2012.12.006.
- Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, et al. Tissue Inhibitor Metalloproteinase-2 (TIMP-2) IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol 2015;26:1747-54. https://doi.org/10.1681/ASN.2014060556.
- Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J Am Soc Nephrol 2015;26:2023-31. https://doi.org/10.1681/ASN.2014060535.
- Krawczeski CD, Vandevoorde RG, Kathman T, Bennett MR, Woo JG, Wang Y, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol 2010;5:1552-7. https://doi.org/10.2215/CJN.02040310.
- Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 2011;58:2301-9. https://doi.org/10.1016/j.jacc.2011.08.017.
- Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr 2011;158:1009-15.e1. https://doi.org/10.1016/j.jpeds.2010.12.057.
- Lacquaniti A, Buemi F, Lupica R, Giardina C, Mure G, Arena A, et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy?. Radiology 2013;267:86-93. https://doi.org/10.1148/radiol.12120578.
- Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennett MR, Sabbisetti V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine?. Pediatr Nephrol 2015;30:665-76. https://doi.org/10.1007/s00467-014-2987-0.
- Lassus JP, Nieminen MS, Peuhkurinen K, Pulkki K, Siirila-Waris K, Sund R, et al. Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J 2010;31:2791-8. https://doi.org/10.1093/eurheartj/ehq293.
- Legrand M, De Berardinis B, Gaggin HK, Magrini L, Belcher A, Zancla B, et al. Evidence of Uncoupling between Renal Dysfunction and Injury in Cardiorenal Syndrome: Insights from the BIONICS Study. PLOS One 2014;9. https://doi.org/10.1371/journal.pone.0112313.
- Lewandowska L, Matuszkiewicz-Rowinska J, Jayakumar C, Oldakowska-Jedynak U, Looney S, Galas M, et al. Netrin-1 and semaphorin 3A predict the development of acute kidney injury in liver transplant patients. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0107898.
- Li Q, Fang JY, Wang WP, Liu JH, Wang KK. Cystatin C and serum creatinine in estimating acute kidney injury of shock patients. World J Emerg Med 2010;1:185-9.
- Li N, Zhao WG, Xu FL, Zhang WF, Gu WT. Neutrophil gelatinase-associated lipocalin as an early marker of acute kidney injury in patients with traumatic brain injury. J Nephrol 2013;26:1083-8. https://doi.org/10.5301/jn.5000282.
- Liebetrau C, Dorr O, Baumgarten H, Gaede L, Szardien S, Blumenstein J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of cardiac surgery associated acute kidney injury. Scand J Clin Lab Invest 2013;73:392-9. https://doi.org/10.3109/00365513.2013.787149.
- Liebetrau C, Gaede L, Doerr O, Blumenstein J, Rixe J, Teichert O, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of contrast-induced nephropathy after percutaneous coronary intervention. Scand J Clin Lab Invest 2014;74:81-8. https://doi.org/10.3109/00365513.2013.860615.
- Macdonald S, Arendts G, Nagree Y, Xu XF. Neutrophil Gelatinase-Associated Lipocalin (NGAL) predicts renal injury in acute decompensated cardiac failure: a prospective observational study. BMC Cardiovasc Disord 2012;12. https://doi.org/10.1186/1471-2261-12-8.
- Magro MC, Vattimo Mde F. Impact of cystatin C and RIFLE on renal function assessment after cardiac surgery. Biol Res Nurs 2013;15:451-8. https://doi.org/10.1177/1099800412446742.
- Makris K, Nikolaki E, Nanopoulos K, Pirgakis KM, Maltezos CK. Measurement of cystatin C in human urine by particle-enhanced turbidimetric immunoassay on an automated biochemistry analyzer. Clin Biochem 2013;46:1128-30. https://doi.org/10.1016/j.clinbiochem.2013.05.072.
- Malyszko J, Bachorzewska-Gajewska H, Koc-Zorawska E, Malyszko JS, Kobus G, Dobrzycki S. Midkine: a novel and early biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/879509.
- Manzano-Fernandez S, Januzzi JL, Boronat-Garcia M, Bonaque-Gonzalez JC, Truong QA, Pastor-Perez FJ, et al. Beta-trace protein and cystatin C as predictors of long-term outcomes in patients with acute heart failure. J Am Coll Cardiol 2011;57:849-58. https://doi.org/10.1016/j.jacc.2010.08.644.
- Marcelino P, Tavares I, Carvalho D, Marques C, Silvestre MJ, Perdigoto R, et al. Is urinary -glutamyl transpeptidase superior to urinary neutrophil gelatinase-associated lipocalin for early prediction of acute kidney injury after liver transplantation?. Transplant Proc 2014;46:1812-8. https://doi.org/10.1016/j.transproceed.2014.05.052.
- Martensson J, Martling CR, Oldner A, Bell M. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrol Dial Transplant 2012;27:576-81. https://doi.org/10.1093/ndt/gfr358.
- Martensson J, Glassford NJ, Jones S, Eastwood GM, Young H, Peck L, et al. Urinary neutrophil gelatinase-associated lipocalin to hepcidin ratio as a biomarker of acute kidney injury in intensive care unit patients. Minerva Anestesiol 2014.
- Matsa R, Ashley E, Sharma V, Walden AP, Keating L. Plasma and urine neutrophil gelatinase-associated lipocalin in the diagnosis of new onset acute kidney injury in critically ill patients. Crit Care 2014;18. https://doi.org/10.1186/cc13958.
- McCullough PA, Williams FJ, Stivers DN, Cannon L, Dixon S, Alexander P, et al. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol 2012;35:509-14. https://doi.org/10.1159/000339163.
- McIlroy DR, Farkas D, Matto M, Lee HT. Neutrophil gelatinase-associated lipocalin combined with delta serum creatinine provides early risk stratification for adverse outcomes after cardiac surgery: a prospective observational study. Crit Care Med 2015;43:1043-52. https://doi.org/10.1097/CCM.0000000000000927.
- Merrikhi A, Gheissari A, Mousazadeh H. Urine and serum neutrophil gelatinase-associated lipocalin cut-off point for the prediction of acute kidney injury. Adv Biomed Res 2014;3. https://doi.org/10.4103/2277-9175.125847.
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231-8. https://doi.org/10.1016/S0140-6736(05)74811-X.
- Mortara A, Bonadies M, Mazzetti S, Fracchioni I, Delfino P, Chioffi M, et al. Neutrophil gelatinase-associated lipocalin predicts worsening of renal function in acute heart failure: methodological and clinical issues. J Cardiovasc Med 2013;14:629-34. https://doi.org/10.2459/JCM.0b013e3283629ca6.
- Murty MS, Sharma UK, Pandey VB, Kankare SB. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol 2013;23:180-3. https://doi.org/10.4103/0971-4065.111840.
- Naruse H, Ishii J, Kawai T, Hattori K, Ishikawa M, Okumura M, et al. Cystatin C in acute heart failure without advanced renal impairment. Am J Med 2009;122:566-73. https://doi.org/10.1016/j.amjmed.2008.10.042.
- Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 2012;81:1254-62. https://doi.org/10.1038/ki.2012.23.
- Nejat M, Pickering JW, Walker RJ, Westhuyzen J, Shaw GM, Frampton CM, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care 2010;14. https://doi.org/10.1186/cc9014.
- Nemes B, Zadori G, Gelley F, Gaman G, Gorog D, Doros A, et al. Can a cutoff value for cystatin C in the operative setting be determined to predict kidney function after liver transplantation?. Transplant Proc 2010;42:2323-6. https://doi.org/10.1016/j.transproceed.2010.05.009.
- Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Int Med 2008;148:810-19. https://doi.org/10.7326/0003-4819-148-11-200806030-00003.
- Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 2012;59:246-55. https://doi.org/10.1016/j.jacc.2011.10.854.
- Nisula S, Yang R, Kaukonen KM, Vaara ST, Kuitunen A, Tenhunen J, et al. The urine protein NGAL predicts renal replacement therapy, but not acute kidney injury or 90-day mortality in critically ill adult patients. Anesth Analg 2014;119:95-102. https://doi.org/10.1213/ANE.0000000000000243.
- Omerika L, Rasic S, Serdarevic N. Importance of determination of urine neutrophile gelatinase associated lipocalin in early detection of acute kidney injury. Coll Antropol 2014;38:161-6.
- Ortuno-Anderiz F, Cabello-Clotet N, Vidart-Simon N, Postigo-Hernandez C, Domingo-Marin S, Sanchez-Garcia M. Cystatin C as an early marker of acute kidney injury in septic shock. Rev Clin Esp 2015;215:83-90. https://doi.org/10.1016/j.rceng.2014.09.001.
- Ozkan S, Durukan P, Kavalci C, Duman A, Sayhan MB, Salt O, et al. Importance of neutrophil gelatinase-associated lipocalin in differential diagnosis of acute and chronic renal failure. Iran Red Crescent Med J 2014;16. https://doi.org/10.5812/ircmj.14133.
- Park MY, Choi SJ, Kim JK, Hwang SD, Lee YW. Urinary cystatin C levels as a diagnostic and prognostic biomarker in patients with acute kidney injury. Nephrology 2013;18:256-62. https://doi.org/10.1111/nep.12037.
- Peco-Antic A, Ivanisevic I, Vulicevic I, Kotur-Stevuljevic J, Ilic S, Ivanisevic J, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem 2013;46:1244-51. https://doi.org/10.1016/j.clinbiochem.2013.07.008.
- Pedersen KR, Ravn HB, Hjortdal VE, Norregaard R, Povlsen JV. Neutrophil gelatinase-associated lipocalin (NGAL): validation of commercially available ELISA. Scand J Clin Lab Invest 2010;70:374-82. https://doi.org/10.3109/00365513.2010.486868.
- Perianayagam MC, Seabra VF, Tighiouart H, Liangos O, Jaber BL. Serum cystatin C for prediction of dialysis requirement or death in acute kidney injury: a comparative study. Am J Kidney Dis 2009;54:1025-33. https://doi.org/10.1053/j.ajkd.2009.05.022.
- Petrovic S, Bogavac-Stanojevic N, Lakic D, Peco-Antic A, Vulicevic I, Ivanisevic I, et al. Cost-effectiveness analysis of acute kidney injury biomarkers in pediatric cardiac surgery. Biochemia Medica 2015;25:262-71. https://doi.org/10.11613/BM.2015.027.
- Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury. Blood Purif 2013;35:295-302. https://doi.org/10.1159/000351542.
- Pipili C, Ioannidou S, Tripodaki ES, Parisi M, Douka E, Vasileiadis I, et al. Prediction of the renal replacement therapy requirement in mechanically ventilated critically ill patients by combining biomarkers for glomerular filtration and tubular damage. J Crit Care 2014;29:692.e7-13. https://doi.org/10.1016/j.jcrc.2014.02.011.
- Ralib AM, Pickering JW, Shaw GM, Than MP, George PM, Endre ZH. The clinical utility window for acute kidney injury biomarkers in the critically ill. Crit Care 2014;18. https://doi.org/10.1186/s13054-014-0601-2.
- Rewa O, Wald R, Adhikari NKJ, Hladunewich M, Lapinsky S, Muscedere J, et al. Whole-blood neutrophil gelatinase-associated lipocalin to predict adverse events in acute kidney injury: A prospective observational cohort study. J Crit Care 2015;30:1359-64. https://doi.org/10.1016/j.jcrc.2015.08.019.
- Ribichini F, Gambaro G, Graziani MS, Pighi M, Pesarini G, Pasoli P, et al. Comparison of serum creatinine and cystatin C for early diagnosis of contrast-induced nephropathy after coronary angiography and interventions. Clin Chem 2012;58:458-64. https://doi.org/10.1373/clinchem.2011.170464.
- Ricci Z, Netto R, Garisto C, Iacoella C, Favia I, Cogo P. Whole blood assessment of neutrophil gelatinase-associated lipocalin versus pediatric RIFLE for acute kidney injury diagnosis and prognosis after pediatric cardiac surgery: cross-sectional study. Pediatr Crit Care Med 2012;13:667-70. https://doi.org/10.1097/PCC.0b013e3182601167.
- Ristikankare A, Poyhia R, Kuitunen A, Skrifvars M, Hammainen P, Salmenpera M, et al. Serum cystatin C in elderly cardiac surgery patients. Ann Thorac Surg 2010;89:689-94. https://doi.org/10.1016/j.athoracsur.2009.11.018.
- Royakkers AA, Bouman CS, Stassen PM, Korevaar JC, Binnekade JM, van de Hoek W, et al. Systemic and urinary neutrophil gelatinase-associated lipocalins are poor predictors of acute kidney injury in unselected critically ill patients. Crit Care Res Pract 2012;2012. https://doi.org/10.1155/2012/712695.
- Royakkers AA, Korevaar JC, van Suijlen JD, Hofstra LS, Kuiper MA, Spronk PE, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med 2011;37:493-501. https://doi.org/10.1007/s00134-010-2087-y.
- Ruf B, Bonelli V, Balling G, Horer J, Nagdyman N, Braun SL, et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case-control study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0760-9.
- Rybi-Szuminska A, Wasilewska A, Litwin M, Kulaga Z, Szuminski M. Paediatric normative data for urine NGAL/creatinine ratio. Acta Paediatr 2013;102:e269-e72. https://doi.org/10.1111/apa.12200.
- Sagheb MM, Namazi S, Geramizadeh B, Karimzadeh A, Oghazian MB, Karimzadeh I. Serum cystatin C as a marker of renal function in critically Ill patients with normal serum creatinine. NephroUrol Mon 2014;6. https://doi.org/10.5812/numonthly.15224.
- Schaub JA, Garg AX, Coca SG, Testani JM, Shlipak MG, Eikelboom J, et al. Perioperative heart-type fatty acid binding protein is associated with acute kidney injury after cardiac surgery. Kidney Int 2015;88:576-83. https://doi.org/10.1038/ki.2015.104.
- Schinstock CA, Semret MH, Wagner SJ, Borland TM, Bryant SC, Kashani KB, et al. Urinalysis is more specific and urinary neutrophil gelatinase-associated lipocalin is more sensitive for early detection of acute kidney injury. Nephrol Dial Transplant 2013;28:1175-85. https://doi.org/10.1093/ndt/gfs127.
- Schnell D, Deruddre S, Harrois A, Pottecher J, Cosson C, Adoui N, et al. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock 2012;38:592-7. https://doi.org/10.1097/SHK.0b013e318271a39c.
- Seitz S, Rauh M, Gloeckler M, Cesnjevar R, Dittrich S, Koch AM. Cystatin C and neutrophil gelatinase-associated lipocalin: biomarkers for acute kidney injury after congenital heart surgery. Swiss Med Wkly 2013;143. https://doi.org/10.4414/smw.2013.13744.
- Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. The diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin in the prediction of acute kidney injury in emergency department patients with suspected sepsis. Ann Emerg Med 2010;56:52-9.e1. https://doi.org/10.1016/j.annemergmed.2010.02.010.
- Shaw AD, Chalfin DB, Kleintjens J. The economic impact and cost-effectiveness of urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Clin Ther 2011;33:1713-25. https://doi.org/10.1016/j.clinthera.2011.09.014.
- Shi HP, Xu DM, Wang GE. Prognostic indicators of patients with acute kidney injury in intensive care unit. World J Emerg Med 2010;1:209-11.
- Shlipak MG, Coca SG, Wang Z, Devarajan P, Koyner JL, Patel UD, et al. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis 2011;58:366-73. https://doi.org/10.1053/j.ajkd.2011.03.015.
- Shrestha K, Shao Z, Singh D, Dupont M, Tang WH. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol 2012;110:1329-35. https://doi.org/10.1016/j.amjcard.2012.06.035.
- Siew ED, Ware LB, Bian A, Shintani A, Eden SK, Wickersham N, et al. Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int 2013;84:786-94. https://doi.org/10.1038/ki.2013.174.
- Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 2009;20:1823-32. https://doi.org/10.1681/ASN.2008070673.
- Sohrabian A, Noraddin FH, Flodin M, Fredricsson A, Larsson A. Particle enhanced turbidimetric immunoassay for the determination of urine cystatin C on Cobas c501. Clin Biochem 2012;45:339-44. https://doi.org/10.1016/j.clinbiochem.2011.12.027.
- Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S, et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol 2010;5:1745-54. https://doi.org/10.2215/CJN.00690110.
- Soto K, Papoila AL, Coelho S, Bennett M, Ma Q, Rodrigues B, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol 2013;8:2053-63. https://doi.org/10.2215/CJN.12181212.
- Soyler C, Tanriover MD, Ascioglu S, Aksu NM, Arici M. Urine neutrophil gelatinase-associated lipocalin levels predict acute kidney injury in acute decompensated heart failure patients. Ren Fail 2015;37:772-6. https://doi.org/10.3109/0886022X.2015.1033324.
- Spahillari A, Parikh CR, Sint K, Koyner JL, Patel UD, Edelstein CL, et al. Serum cystatin C- versus creatinine-based definitions of acute kidney injury following cardiac surgery: a prospective cohort study. Am J Kidney Dis 2012;60:922-9. https://doi.org/10.1053/j.ajkd.2012.06.002.
- Svenmarker S, Haggmark S, Holmgren A, Naslund U. Serum markers are not reliable measures of renal function in conjunction with cardiopulmonary bypass. Interact Cardiovasc Thorac Surg 2011;12:713-17. https://doi.org/10.1510/icvts.2010.259432.
- Tasanarong A, Hutayanon P, Piyayotai D. Urinary Neutrophil Gelatinase-Associated Lipocalin predicts the severity of contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. BMC Nephrol 2013;14. https://doi.org/10.1186/1471-2369-14-270.
- Torregrosa I, Montoliu C, Urios A, Elmlili N, Puchades MJ, Solis MA, et al. Early biomarkers of acute kidney failure after heart angiography or heart surgery in patients with acute coronary syndrome or acute heart failure. Nefrologia 2012;32:44-52.
- Volpon LC, Sugo EK, Carlotti A. Diagnostic and Prognostic Value of Serum Cystatin C in Critically Ill Children With Acute Kidney Injury. Pediatr Crit Care Med 2015;16:E125-E31. https://doi.org/10.1097/PCC.0000000000000403.
- Wacker-Gusmann A, Buhren K, Schultheiss C, Braun SL, Page S, Saugel B, et al. Prediction of contrast-induced nephropathy in patients with serum creatinine levels in the upper normal range by cystatin C: a prospective study in 374 patients. AJR Am J Roentgenol 2014;202:452-8. https://doi.org/10.2214/AJR.13.10688.
- Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006;105:485-91. https://doi.org/10.1097/00000542-200609000-00011.
- Wai K, Soler-Garcia AA, Perazzo S, Mattison P, Ray PE. A pilot study of urinary fibroblast growth factor-2 and epithelial growth factor as potential biomarkers of acute kidney injury in critically ill children. Pediatr Nephrol 2013;28:2189-98. https://doi.org/10.1007/s00467-013-2543-3.
- Wald R, Liangos O, Perianayagam MC, Kolyada A, Herget-Rosenthal S, Mazer CD, et al. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol 2010;5:1373-9. https://doi.org/10.2215/CJN.06350909.
- Wang QP, Gu JW, Zhan XH, Li H, Luo XH. Assessment of glomerular filtration rate by serum cystatin C in patients undergoing coronary artery bypass grafting. Ann Clin Biochem 2009;46:495-500. https://doi.org/10.1258/acb.2009.009065.
- Wang F, Pan W, Wang H, Zhou Y, Wang S, Pan S. The impacts of thyroid function on the diagnostic accuracy of cystatin C to detect acute kidney injury in ICU patients: a prospective, observational study. Crit Care 2014;18. https://doi.org/10.1371/journal.pone.0143628.
- Wang X, Che M, Xie B, Xue S, Yan Y. Preoperative serum cystatin C combined with dipstick proteinuria predicts acute kidney injury after cardiac surgery. Ren Fail 2014;36:1497-503. https://doi.org/10.3109/0886022X.2014.949759.
- Westhoff JH, Tonshoff B, Waldherr S, Poschl J, Teufel U, Westhoff TH, et al. Urinary Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) * Insulin-Like Growth Factor-Binding Protein 7 (IGFBP7) Predicts Adverse Outcome in Pediatric Acute Kidney Injury. PLOS One 2015;10.
- Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 2008;36:1297-303. https://doi.org/10.1097/CCM.0b013e318169245a.
- Xiang D, Zhang H, Bai J, Ma J, Li M, Gao W, et al. Particle-enhanced turbidimetric immunoassay for determination of serum neutrophil gelatinase-associated lipocalin on the Roche Cobas c501 analyzer. Clin Biochem 2013;46:1756-60. https://doi.org/10.1016/j.clinbiochem.2013.09.007.
- Yin L, Li G, Liu T, Yuan R, Zheng X, Xu G, et al. Probucol for the prevention of cystatin C-based contrast-induced acute kidney injury following primary or urgent angioplasty: a randomized, controlled trial. Int J Cardiol 2013;167:426-9. https://doi.org/10.1016/j.ijcard.2012.01.017.
- Yoon HJ, Kim H, Lee JP, Choi SW, Cho HO, Shin HW, et al. The efficacy of the cystatin C based glomerular filtration rate in the estimation of safe contrast media volume. Sunhwangi 2013;43:622-7.
- Youssef DM, Esh AM, Helmy Hassan E, Ahmed TM. Serum NGAL in Critically Ill Children in ICU from a Single Center in Egypt. ISRN Nephrol 2013;2013. https://doi.org/10.5402/2013/140905.
- Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 2007;11. https://doi.org/10.1186/cc6089.
- Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int 2011;80:655-62. https://doi.org/10.1038/ki.2011.123.
- Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR, et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 2015;169:583-91. https://doi.org/10.1001/jamapediatrics.2015.54.
- Zheng JY, Xiao YY, Yao Y, Han L. Is serum cystatin C an early predictor for acute kidney injury following cardiopulmonary bypass surgery in infants and young children?. Kaohsiung J Med Sci 2013;29:494-9. https://doi.org/10.1016/j.kjms.2013.01.004.
- Zwiers AJM, de Wildt SN, van Rosmalen J, de Rijke YB, Buijs EAB, Tibboel D, et al. Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: a prospective cohort study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0910-0.
- Acute Kidney Injury Work Group . Kidney Disease: Improving Global Outcomes (KDIGO) – Clinical Practice Guideline for Acute Kidney Injury. Kidney Int 2012;2:1-138.
- Ronco C, Kellum JA, Mehta R. Acute dialysis quality initiative (ADQI). Nephrol Dial Transplant 2001;16:1555-8. https://doi.org/10.1093/ndt/16.8.1555.
- Kidney Disease: Improving Global Outcomes (KDIGO) . Acute Kidney Injury (AKI) n.d. http://kdigo.org/guidelines/acute-kidney-injury/ (accessed 19 March 2018).
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. workgroup. ADQI . Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. https://doi.org/10.1186/cc2872.
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11. https://doi.org/10.1186/cc5713.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem 2003;49:7-18. https://doi.org/10.1373/49.1.7.
- Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500-24. https://doi.org/10.1016/j.ijsu.2014.07.014.
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012-24. https://doi.org/10.1053/j.ajkd.2009.07.020.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. https://doi.org/10.1159/000339789.
- Xiong J, Tang X, Hu Z, Nie L, Wang Y, Zhao J. The RIFLE versus AKIN classification for incidence and mortality of acute kidney injury in critical ill patients: a meta-analysis. Sci Rep 2015;5. https://doi.org/10.1038/srep17917.
- Bagshaw SM, George C, Bellomo R. ANZICS Database Management Committe . A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008;23:1569-74. https://doi.org/10.1093/ndt/gfn009.
- Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X. Beijing Acute Kidney Injury Trial (BAKIT) workgroup . A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014;18. https://doi.org/10.1186/cc13977.
- Levi TM, de Souza SP, de Magalhães JG, de Carvalho MS, Cunha AL, Dantas JG, et al. Comparison of the RIFLE, AKIN and KDIGO criteria to predict mortality in critically ill patients. Rev Bras Ter Intensiva 2013;25:290-6. https://doi.org/10.5935/0103-507X.20130050.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351. https://doi.org/10.1136/bmj.h5527.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-4. https://doi.org/10.1136/bmj.326.7379.41.
- Wilson EB. Probable inference, the law of succession, and statistical inference. JASA 1927;22:209-12. https://doi.org/10.1080/01621459.1927.10502953.
- Koopman P. Confidence intervals for the ratio of two binomial proportions. Biometrics 1984;40:513-17. https://doi.org/10.2307/2531405.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Van Houwelingen HC, Zwinderman KH, Stijnen T. A bivariate approach to meta-analysis. Stat Med 1993;12:2273-84. https://doi.org/10.1002/sim.4780122405.
- Doebler P, Holling H. Meta-Analysis of Diagnostic Accuracy With Mada 2014. https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf (accessed 3 November 2017).
- Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293-316. https://doi.org/10.1002/sim.4780121403.
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865-84. https://doi.org/10.1002/sim.942.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. https://doi.org/10.1093/biostatistics/kxl004.
- Oehlert GW. A note on the delta method. Am Stat 1992;46:27-9.
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. London: Cochrane; 2010.
- Dendukuri N, Schiller I, Joseph L, Pai M. Bayesian meta-analysis of the accuracy of a test for tuberculous pleuritis in the absence of a gold standard reference. Biometrics 2012;68:1285-93. https://doi.org/10.1111/j.1541-0420.2012.01773.x.
- Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem 2014;51:335-51. https://doi.org/10.1177/0004563214521795.
- Hallworth MJ. The ‘70% claim’: what is the evidence base?. Ann Clin Biochem 2011;48:487-8. https://doi.org/10.1258/acb.2011.011177.
- Klee GG. Establishment of outcome-related analytic performance goals. Clin Chem 2010;56:714-22. https://doi.org/10.1373/clinchem.2009.133660.
- Hyltoft Petersen P, Klee GG. Influence of analytical bias and imprecision on the number of false positive results using Guideline-Driven Medical Decision Limits. Clin Chim Acta 2014;430:1-8. https://doi.org/10.1016/j.cca.2013.12.014.
- Fraser CG. Biological Variation: from Principles to Practice. Washington, DC: American Association for Clinical Chemistry; 2001.
- Lippi G, Becan-McBride K, Behúlová D, Bowen RA, Church S, Delanghe J, et al. Preanalytical quality improvement: in quality we trust. Clin Chem Lab Med 2013;51:229-41. https://doi.org/10.1515/cclm-2012-0597.
- Roraas T, Petersen PH, Sandberg S. Confidence intervals and power calculations for within-person biological variation: effect of analytical imprecision, number of replicates, number of samples, and number of individuals. Clin Chem 2012;58:1306-13. https://doi.org/10.1373/clinchem.2012.187781.
- Lippi G, Banfi G, Church S, Cornes M, De Carli G, Grankvist K, et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med 2015;53:357-70. https://doi.org/10.1515/cclm-2014-1051.
- Ioannidis JP. Biomarker failures. Clin Chem 2013;59:202-4. https://doi.org/10.1373/clinchem.2012.185801.
- Ransohoff DF. Bias as a threat to the validity of cancer molecular-marker research. Nat Rev Cancer 2005;5:142-9. https://doi.org/10.1038/nrc1550.
- Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet 2002;359:572-7. https://doi.org/10.1016/S0140-6736(02)07746-2.
- Baggerly KA, Morris JS, Edmonson SR, Coombes KR. Signal in noise: evaluating reported reproducibility of serum proteomic tests for ovarian cancer. J Natl Cancer Inst 2005;97:307-9. https://doi.org/10.1093/jnci/dji008.
- Banks RE, Stanley AJ, Cairns DA, Barrett JH, Clarke P, Thompson D, et al. Influences of blood sample processing on low-molecular-weight proteome identified by surface-enhanced laser desorption/ionization mass spectrometry. Clin Chem 2005;51:1637-49. https://doi.org/10.1373/clinchem.2005.051417.
- Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer 2007;97:978-85. https://doi.org/10.1038/sj.bjc.6603923.
- Hetland ML, Christensen IJ, Lottenburger T, Johansen JS, Svendsen MN, Horslev-Petersen K, et al. Circulating VEGF as a biological marker in patients with rheumatoid arthritis? Preanalytical and biological variability in healthy persons and in patients. Dis Markers 2008;24:1-10. https://doi.org/10.1155/2008/707864.
- Bartlett WA, Braga F, Carobene A, Coşkun A, Prusa R, Fernandez-Calle P, et al. A checklist for critical appraisal of studies of biological variation. Clin Chem Lab Med 2015;53:879-85. https://doi.org/10.1515/cclm-2014-1127.
- Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, et al. Biospecimen reporting for improved study quality (BRISQ). J Proteome Res 2011;10:3429-38. https://doi.org/10.1021/pr200021n.
- Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JP, Kirsch-Volders M, et al. STrengthening the Reporting of OBservational studies in Epidemiology – Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. PLOS Med 2011;8. https://doi.org/10.1371/journal.pmed.1001117.
- Freeman K, Connock M, Cummins E, Gurung T, Taylor-Phillips S, Saunders M, et al. Diagnostic Assessment Report Commissioned by the NIHR HTA Programme on Behalf of the National Institute for Health and Care Excellence – Final Report. Fluorouracil Plasma Monitoring: the My5-FU Assay for Guiding Dose Adjustment in Patients Receiving Fluorouracil Chemotherapy by Continuous Infusion. Southampton: National Institute for Health Research; 2014.
- Therapeutic Monitoring of TNF-alpha Inhibitors in Crohn’s Disease (LISA-TRACKER ELISA Kits, IDKmonitor ELISA Kits, and Promonitor ELISA Kits). London: NICE; 2016.
- Diagnosing Prostate Cancer: PROGENSA PCA3 Assay and Prostate Health Index. London: NICE; 2015.
- Medical Laboratories: Requirements for Quality and Competence (ISO 15189:2012). London: BSI Standards Limited; 2012.
- Plebani M, Sciacovelli L, Marinova M, Marcuccitti J, Chiozza MK. Quality indicators in laboratory medicine: a fundamental tool for quality and patient safety. Clin Biochem 2013;46:1170-4. https://doi.org/10.1016/j.clinbiochem.2012.11.028.
- Masca NGD, Hensor EMA, Cornelius VR, Buffa FM, Marriott HM, Eales JM, et al. Science Forum: RIPOSTE: a framework for improving the design and analysis of laboratory-based research. ELife 2015;4. https://doi.org/10.7554/eLife.05519.
- Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M. Standardization of collection requirements for fasting samples. Clin Chim Act 2014;432:33-7. https://doi.org/10.1016/j.cca.2013.11.008.
- Lippi G, Banfi G, Church S, Cornes M, De Carli G, Grankvist K, et al. Preanalytical quality improvement. In pursuit of harmony. Clin Chem Lab Med 2015;53:357-70.
- H3-A6 Procedures for collection of diagnostic blood specimens by venipuncture; approved guideline, 6th ed. Wayne, PA: Clinical Laboratory Standards Institute; 2007.
- H18-A4 Procedures for the Handling and Processing of Blood Specimens for Common Laboratory Tests; Approved Guideline – Fourth Edition. Wayne, PA: Clinical and Laboratory Standards Institute; 2010.
- GP16-A3 Urinalysis; Approved Guideline – Third edition. Wayne, PA: Clinical Laboratory Standards Institute; 2009.
- Ellervik C, Vaught J. Preanalytical variables affecting the integrity of human biospecimens in biobanking. Clin Chem 2015;61:914-34. https://doi.org/10.1373/clinchem.2014.228783.
- Sturgeon CM, Hoffman BR, Chan DW, Ch’ng S-L, Hammond E, Hayes DF, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in clinical practice: quality requirements. Clin Chem 2008;54:e1-e10. https://doi.org/10.1373/clinchem.2007.094144.
- In Vitro Diagnostic Medical Devices – Measurement of Quantities in Biological Samples – Metrological Traceability of Values Assigned to Calibrators and Control Materials. 17511:2003. Geneva: International Organization for Standardization; 2003.
- Performance Evaluation of In Vitro Diagnostic Medical Devices. London: BSI Standards Limited; 2002.
- Accuracy (Trueness and Precision) of Measurement Methods and Results. London: BSI Standards Limited; 1995.
- Khatami Z, Hill R, Sturgeon C, Kearney E, Breadon P, Kallner A. Measurement Verification in the Clinical Laboratory: A Guide to Assessing Analytical Performance during the Acceptance Testing of Methods (Quantitative Examination Procedures) and/or Analysers. London: The Association for Clinical Biochemistry & Laboratory Medicine; 2009.
- Guidance for Industry: Bioanalytical Method Validation. Silverspring, MD: US Food and Drug Administration; 2013.
- Barwick V. Evaluating Measurement Uncertainty in Clinical Chemistry: Case Studies. Teddington: LGC; 2012.
- Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharma Res 2006;23:312-28. https://doi.org/10.1007/s11095-005-9045-3.
- DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, Lee B, et al. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res 2003;20:1885-900. https://doi.org/10.1023/B:PHAM.0000003390.51761.3d.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Astute Medial . NEPHROCHECK® Test Kit Package Insert 2017. www.astutemedical.com/documents/US-English/NephroCheck-Test-Kit-Package-Insert-US-PN-300152.pdf.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. https://doi.org/10.1186/1471-2288-3-25.
- Maclean EN, Stone IS, Ceelen F, Garcia-Albeniz X, Sommer WH, Petersen SE. Reporting standards in cardiac MRI, CT, and SPECT diagnostic accuracy studies: analysis of the impact of STARD criteria. Eur Heart J Cardiovasc Imaging 2014;15:691-700. https://doi.org/10.1093/ehjci/jet277.
- Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, et al. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol 2011;119:92-101. https://doi.org/10.1002/cncy.20147.
- Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JP, Kirsch-Volders M, et al. STrengthening the Reporting of OBservational studies in Epidemiology – Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. Eur J Clin Invest 2012;42:1-16. https://doi.org/10.1111/j.1365-2362.2011.02561.x.
- Buisman L, Rutten-van Mölken M, Postmus D, Luime J, Uyl-de Groot C, Redekop W. The early bird catches the worm: early cost-effectiveness analysis of new medical tests. Int J Technol Assess Health Care 2016;32:46-53. https://doi.org/10.1017/S0266462316000064.
- Girling A, Lilford R, Cole A, Young T. Headroom approach to device development: current and future directions. Int J Technol Assess Health Care 2015;31:331-8. https://doi.org/10.1017/S0266462315000501.
- Brandes A, Sinner MF, Kääb S, Rogowski WH. Early decision-analytic modeling – a case study on vascular closure devices. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-1118-3.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices – overview: a report of the ISPOR-SMDM modeling good research practices task force – 1. Med Decis Making 2012;32:667-77. https://doi.org/10.1177/0272989X12454577.
- Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Health Technology Assessment. London: Springer; 2015.
- CCEMG – EPPI-Centre Cost Converter. London: Social Science Research Unit, UCL Institute of Education; 2016.
- Chicaiza-Becerra LA, Garcia-Molina M, Gamboa O. Cost-effectiveness of iso- versus low-osmolality contrast media in outpatients with high risk of contrast medium-induced nephropathy. Biomedica (Bogota) 2012;32:182-8.
- De Smedt DM, Elseviers MM, Lins RL, Annemans L. Economic evaluation of different treatment modalities in acute kidney injury. Nephrol Dial Transplant 2012;27:4095-101. https://doi.org/10.1093/ndt/gfs410.
- Desai AA, Baras J, Berk BB, Nakajima A, Garber AM, Owens D, et al. Management of acute kidney injury in the intensive care unit: a cost-effectiveness analysis of daily vs alternate-day hemodialysis. Arch Intern Med 2008;168:1761-7. https://doi.org/10.1001/archinte.168.16.1761.
- Erstad BL. Pharmacoeconomic comparison of an albumin-furosemide complex versus sequential therapy for renal insufficiency. Clin Ther 1999;21:1380-6. https://doi.org/10.1016/S0149-2918(99)80038-1.
- Ethgen O, Schneider AG, Bagshaw SM, Bellomo R, Kellum JA. Economics of dialysis dependence following renal replacement therapy for critically ill acute kidney injury patients. Nephrol Dial Transplant 2015;30:54-61. https://doi.org/10.1093/ndt/gfu314.
- He J, Winterstein AG, Beaver TM. Projecting the effect of nesiritide on dialysis and hospital mortality in cardiac surgery patients. Value Health 2010;13:643-8. https://doi.org/10.1111/j.1524-4733.2010.00710.x.
- Iannazzo S, Vandekerckhove S, De Francesco M, Nayak A, Ronco C, Morana G, et al. Economic evaluation of intravenous iodinated contrast media in Italy. Int J Technol Assess Health Care 2014;30:69-77. https://doi.org/10.1017/S0266462313000706.
- Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014;29:1362-8. https://doi.org/10.1093/ndt/gfu016.
- Klarenbach S, Manns B, Pannu N, Clement FM, Wiebe N, Tonelli M. Economic evaluation of continuous renal replacement therapy in acute renal failure. Int J Technol Assess Health Care 2009;25:331-8. https://doi.org/10.1017/S0266462309990134.
- Acutely Ill Adults in Hospital: Recognising and Responding to Deterioration. London: NICE; 2007.
- Acute Kidney Injury. London: NICE; 2014.
- Chronic Kidney Disease in Adults: Assessment and Management. London: NICE; 2015.
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089-100. https://doi.org/10.1111/j.1523-1755.2005.00365.x.
- van Reenen M, Janssen B. EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument n.d. https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf (accessed 19 March 2018).
- The University of Sheffield . Measuring and Valuing Health n.d. www.sheffield.ac.uk/scharr/sections/heds/mvh/sf-6d (accessed 19 March 2018).
- Kolhe NV, Eldehni MT, Selby NM, McIntyre CW. The reimbursement and cost of acute kidney injury: a UK hospital perspective. Nephron Clin Pract 2014;126:51-6. https://doi.org/10.1159/000358435.
- Paterson AL, Johnston AJ, Gokhale R, Kingston D, Mahroof R. Clinical and economic impacts of a switch from a high to low intensity continuous renal replacement therapy in patients with acute kidney injury. Intensive Care Med 2013;39.
- Paterson AL, Johnston AJ, Kingston D, Mahroof R. Clinical and economic impact of a switch from high- to low-volume renal replacement therapy in patients with acute kidney injury. Anaesthesia 2014;69:977-82. https://doi.org/10.1111/anae.12706.
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. Acute Kidney Injury Advisory Group of the American Society of Nephrology . World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482-93. https://doi.org/10.2215/CJN.00710113.
- Prowle JR, Kolic I, Purdell-Lewis J, Taylor R, Pearse RM, Kirwan CJ. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol 2014;9:1015-23. https://doi.org/10.2215/CJN.11141113.
- Kolic I, Purdell-Lewis J, Kirwan CJ, Prowle JR. Estimated glomerular filtration rate based on hospital discharge creatinine may significantly overestimate renal function and underestimate chronic kidney disease in survivors of critical illness. Crit Care 2013;17. https://doi.org/10.1186/cc12356.
- Khawaja M, Joshi A, Asrress KN, Wilson K, Macgillivray K, Young CP, et al. Risk factors associated with acute kidney injury after TAVI using bioprosthesis. EuroIntervention 2012;8.
- Chakkalakal N, Taylor R, Marshall G, Healy M, Kirwan CJ, Prowle JR. Rhabdomyolysis and acute kidney injury: Incidence, treatments and outcomes in the ICU. Intensive Care Med 2013;39.
- Saratzis A, Melas N, Mahmood A, Sarafidis P. Incidence of acute kidney injury (AKI) after endovascular abdominal aortic aneurysm repair (EVAR) and impact on outcome. Eur J Vasc Endovasc Surg 2015;49:534-40. https://doi.org/10.1016/j.ejvs.2015.01.002.
- Thomson R, Meeran H, Valencia O, Al-Subaie N. Goal-directed therapy after cardiac surgery and the incidence of acute kidney injury. J Crit Care 2014;29:997-1000. https://doi.org/10.1016/j.jcrc.2014.06.011.
- Kolhe NV, Stevens PE, Crowe AV, Lipkin GW, Harrison DA. Case mix, outcome and activity for patients with severe acute kidney injury during the first 24 hours after admission to an adult, general critical care unit: application of predictive models from a secondary analysis of the ICNARC Case Mix Programme database. Crit Care 2008;12. https://doi.org/10.1186/cc7003.
- Kourliouros A, Valencia O, Phillips SD, Collinson PO, Van Besouw JP, Jahangiri M. Low cardiopulmonary bypass perfusion temperature is an independent risk factor for acute kidney injury after coronary artery bypass surgery. Eur Heart J 2009;30.
- Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis 2005;46:1038-48. https://doi.org/10.1053/j.ajkd.2005.08.033.
- Alassar A, Abdulkareem N, Valencia O, Brecker S, Jahangiri M. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictive factors and prognostic effects. Innovations 2012;7.
- Alassar A, Roy D, Abdulkareem N, Valencia O, Brecker S, Jahangiri M. Acute kidney injury after transcatheter aortic valve implantation: incidence, risk factors, and prognostic effects. Innovations 2012;7:389-93.
- Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007;18:1292-8. https://doi.org/10.1681/ASN.2006070756.
- Barnes TC, Hernandez-Caballero C, Hurtado-Doce AI, Hall D. Risk factors for acute kidney injury post off pump coronary artery bypass graft (OP-CABG) surgery and its impact on shortterm mortality. Intensive Care Med 2014;40:S256-S7.
- Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 2013;28:389-96. https://doi.org/10.1016/j.jcrc.2012.12.008.
- Bedford M, Stevens PE, Wheeler TW, Farmer CK. What is the real impact of acute kidney injury?. BMC Nephrol 2014;15. https://doi.org/10.1186/1471-2369-15-95.
- Brown K, Challis M, Mikhail A. Continuous renal replacement therapy (CVVHD) for acute kidney injury in critical care: incidence and outcome across South West Wales. Crit Care 2014;18. https://doi.org/10.1186/cc13581.
- Challiner R, Ritchie JP, Fullwood C, Loughnan P, Hutchison AJ. Incidence and consequence of acute kidney injury in unselected emergency admissions to a large acute UK hospital trust. BMC Nephrol 2014;15. https://doi.org/10.1186/1471-2369-15-84.
- de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med 2000;26:915-21. https://doi.org/10.1007/s001340051281.
- Grayson AD, Khater M, Jackson M, Fox MA. Valvular heart operation is an independent risk factor for acute renal failure. Ann Thorac Surg 2003;75:1829-35. https://doi.org/10.1016/S0003-4975(03)00166-8.
- Hurtado-Doce AI, Barnes TC, Hernandez-Caballero C, Hall D. Incidence and outcomes of cardiac surgery-associated acute kidney injury (CSA-AKI) requiring continuous renal replacement therapy (CRRT). Intensive Care Med 2014;40.
- Karmali A, Walker C, Kuppurao L. Does cardiopulmonary bypass increase the risk of postoperative acute kidney injury after coronary artery bypass grafting?. Crit Care 2015;19. https://doi.org/10.1186/cc14373.
- Kirwan CJ, Blunden MJ, Dobbie H, James A, Nedungadi A, Prowle JR. Critically ill patients requiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but rarely receive specialist nephrology follow-up. Nephron 2015;129:164-70. https://doi.org/10.1159/000371448.
- Metcalfe W, Simpson M, Khan IH, Prescott GJ, Simpson K, Smith WC, et al. Scottish Renal Registry . Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM 2002;95:579-83. https://doi.org/10.1093/qjmed/95.9.579.
- Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, et al. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant 2007;22:2513-19. https://doi.org/10.1093/ndt/gfm264.
- Prowle JR, Kolic I, Purdell-Lewis J, Kirwan CJ. Acute kidney injury of all severity is associated with extended hospitalization after critical illness. Crit Care 2014;18. https://doi.org/10.1186/cc13558.
- Syed Y, Bennett D, Whiteley C, Ostermann M. High oxygen delivery in early AKI I is associated with a lower risk of progression to AKI III. Intensive Care Med 2011;37.
- Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med 2000;26:565-71. https://doi.org/10.1007/s001340051205.
- Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007;35:1837-43. https://doi.org/10.1097/01.CCM.0000277041.13090.0A.
- Noble JS, MacKirdy FN, Donaldson SI, Howie JC. Renal and respiratory failure in Scottish ICUs. Anaesthesia 2001;56:124-9. https://doi.org/10.1046/j.1365-2044.2001.01841.x.
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294:813-18. https://doi.org/10.1001/jama.294.7.813.
- Uchino S, Bellomo R, Kellum JA, Morimatsu H, Morgera S, Schetz MR, et al. Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs 2007;30:281-92.
- Bhandari S, Turney JH. Survivors of acute renal failure who do not recover renal function. QJM 1996;89:415-21. https://doi.org/10.1093/qjmed/89.6.415.
- Baudouin SV, Wiggins J, Keogh BF, Morgan CJ, Evans TW. Continuous veno-venous haemofiltration following cardio-pulmonary bypass. Indications and outcome in 35 patients. Intensive Care Med 1993;19:290-3. https://doi.org/10.1007/BF01690550.
- Tsang GM, Khan I, Dar M, Clayton D, Waller D, Patel RL. Hemofiltration in a cardiac intensive care unit: time for a rational approach. ASAIO J 1996;42:M710-13. https://doi.org/10.1097/00002480-199609000-00079.
- Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 2008;73:538-46. https://doi.org/10.1038/sj.ki.5002743.
- Kent S, Schlackow I, Lozano-Kühne J, Reith C, Emberson J, Haynes R, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease?. BMC Nephrol 2015;16. https://doi.org/10.1186/s12882-015-0054-0.
- Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting – a multicentre study. Nephrol Dial Transplant 2008;23:1982-9. https://doi.org/10.1093/ndt/gfm870.
- Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14210.
- Chamberlain G, Baboolal K, Bennett H, Pockett RD, McEwan P, Sabater J, et al. The economic burden of posttransplant events in renal transplant recipients in Europe. Transplantation 2014;97:854-61. https://doi.org/10.1097/01.TP.0000438205.04348.69.
- Grün RP, Constantinovici N, Normand C, Lamping DL. North Thames Dialysis Study Group . Costs of dialysis for elderly people in the UK. Nephrol Dial Transplant 2003;18:2122-7. https://doi.org/10.1093/ndt/gfg354.
- Iqbal K, Aitken E, Thompson P, Kingsmore D. Early cannulation arteriovenous vascular grafts for haemodialysis: a cost-saving alternative to tunneled central venous catheters? An estimated budget impact analysis in a singlecentre. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.03.1694.
- Joseph J, Laplante S. Impact of home dialysis on UK healthcare budget. NDT Plus 2010;3:iii91-2.
- Kadam UT, Uttley J, Jones PW, Iqbal Z. Chronic disease multimorbidity transitions across healthcare interfaces and associated costs: a clinical-linkage database study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003109.
- Kirby L, Vale L. Dialysis for end-stage renal disease. Determining a cost-effective approach. Int J Technol Assess Health Care 2001;17:181-9. https://doi.org/10.1017/S0266462300105045.
- Komenda P, Gavaghan MB, Garfield SS, Poret AW, Sood MM. An economic assessment model for in-center, conventional home, and more frequent home hemodialysis. Kidney Int 2012;81:307-13. https://doi.org/10.1038/ki.2011.338.
- Liu FX, Leipold R, Arici M, Farooqui U. Budget impact analysis of peritoneal dialysis vs. conventional in-center hemodialysis in the united kingdom. Nephrol Dial Transplant 2013;28.
- Liu FX, Treharne C, Culleton B, Crowe L, Arici M. The financial impact of increasing home-based high dose haemodialysis and peritoneal dialysis. BMC Nephrol 2014;15. https://doi.org/10.1186/1471-2369-15-161.
- Liu FX, Treharne C, Arici M, Crowe L, Culleton B. High-dose hemodialysis versus conventional in-center hemodialysis: a cost-utility analysis from a UK payer perspective. Value Health 2015;18:17-24. https://doi.org/10.1016/j.jval.2014.10.002.
- McEwan P, Dixon S, Baboolal K, Conway P, Currie CJ. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. PharmacoEconomics 2006;24:67-79. https://doi.org/10.2165/00019053-200624010-00006.
- McEwan P, Baboolal K, Cerri K. The economic burden of post-transplant events in renal transplant patients (portrait study) in a single UK centre. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(11)73044-3.
- McEwan P, Lebmeier M. Evaluating the minimum renal allograft survival time required for transplantation to remain cost saving in the UK. Value Health 2012;15. https://doi.org/10.1016/j.jval.2012.08.1460.
- Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7020.
- Muduma G, Odeyemi I, Smith-Palmer J, Pollock RF. Budget impact of switching from an immediate-release to a prolonged-release formulation of tacrolimus in renal transplant recipients in the UK based on differences in adherence. Patient Prefer Adherence 2014;8:391-9.
- Muduma G, Shaw J, Hart WM, Odeyemi A, Odeyemi I. Cost utility analysis of immunosuppressive regimens in adult renal transplant recipients in England and Wales. Patient Prefer Adherence 2014;8:1537-46.
- Muduma G, Odeyemi I, Pollock RF. A UK analysis of the cost of switching renal transplant patients from an immediate-release to a prolonged-release formulation of tacrolimus based on differences in trough concentration variability. J Med Econ 2014;17:520-6. https://doi.org/10.3111/13696998.2014.916713.
- Muduma G, Odeyemi IA, Pollock RF. Evaluating the economic implications of nonadherence and antibody-mediated rejection in renal transplant recipients: the role of once-daily, prolonged-release tacrolimus in the UK setting. Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.03.1081.
- Neil N, Walker DR, Sesso R, Blackburn JC, Tschosik EA, Sciaraffia V, et al. Gaining efficiencies: resources and demand for dialysis around the globe. Value Health 2009;12:73-9. https://doi.org/10.1111/j.1524-4733.2008.00414.x.
- Cost Effectiveness of Peritoneal Dialysis Provision. London: NICE; 2011.
- Kidney Disease: Peritoneal Dialysis. Costing Report. London: NICE; 2011.
- Oates T, Cross J, Davenport A. Cost comparison of online haemodiafiltration with high-flux haemodialysis. J Nephrol 2012;25:192-7. https://doi.org/10.5301/jn.5000046.
- Pollock RF, Smith-Palmer J, Odeyemi IA, Muduma G. Budget impact of switching from an immediate-release to a prolonged-release formulation of tacrolimus in renal transplant recipients in the UK based on differences in adherence. Value Health 2013;16. https://doi.org/10.1016/j.jval.2013.08.1871.
- Pollock RF, Smith-Palmer J, Odeyemi IA, Muduma G. An analysis of the cost of switching renal transplant patients from an immediate-release to a prolonged-release formulation of tacrolimus based on differences in trough concentration variability in the United Kingdom. Value Health 2013;16. https://doi.org/10.1016/j.jval.2013.08.1873.
- Popat R, Syed A, Puliatti C, Cacciola R. Outcome and cost analysis of induction immunosuppression with IL2Mab or ATG in DCD kidney transplants. Transplantation 2014;97:1161-5. https://doi.org/10.1097/01.tp.0000442505.10490.20.
- Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al. An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9240.
- Sun Y, Cerri K. The economic impact of renal graft failure: a cost analysis in a UK setting. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(11)73028-5.
- Thompson M, Bartko-Winters S, Bernard L, Fenton A, Hutchison C, Di Iorio B. Economic evaluation of sevelamer for the treatment of hyperphosphatemia in chronic kidney disease patients not on dialysis in the United Kingdom. J Med Econ 2013;16:744-55. https://doi.org/10.3111/13696998.2013.792267.
- Treharne C, Liu FX, Arici M, Crowe L, Farooqui U. Peritoneal dialysis and in-centre haemodialysis: a cost–utility analysis from a UK payer perspective. Appl Health Econ Health Policy 2014;12:409-20. https://doi.org/10.1007/s40258-014-0108-7.
- Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001307.
- Neri L, McEwan P, Sennfalt K, Baboolal K. Characterizing the relationship between health utility and renal function after kidney transplantation in UK and US: a cross-sectional study. Health Qual Life Outcomes 2012;10. https://doi.org/10.1186/1477-7525-10-139.
- Neri L, McEwan P, Cerri K, Baboolal K. Characterizing the relationship between health utility in kidney transplant recipients in UK and US with different levels of kidney function. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(11)73045-5.
- Wyld M, Morton R, Hayen A, Howard K, Webster A. A meta-analysis of quality of life estimates in chronic kidney disease. Nephrology 2010;15.
- Blakeman T, Blickem C, Kennedy A, Reeves D, Bower P, Gaffney H, et al. Effect of information and telephone-guided access to community support for people with chronic kidney disease: randomised controlled trial. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0109135.
- Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health 2008;11:733-41. https://doi.org/10.1111/j.1524-4733.2007.00308.x.
- Lung TW, Hayes AJ, Hayen A, Farmer A, Clarke PM. A meta-analysis of health state valuations for people with diabetes: explaining the variation across methods and implications for economic evaluation. Qual Life Res 2011;20:1669-78. https://doi.org/10.1007/s11136-011-9902-y.
- Nafees B, Cowie MR, Patel C, Deschaseaux C, Brazier J, Lloyd AJ. Health state utilities in chronic heart failure in the UK. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.1462.
- Renal Association . UK Renal Registry: 17th Annual Report 2014. www.renalreg.org/reports/2014-seventeenth-annual-report/ (accessed 3 November 2017).
- Anwar S, Pruthi R, Kenchayikoppad S. Hypercalcemia a risk factor for renal transplant dysfunction. Nephrol Dial Transplant 2012;27.
- Babu GG, Webber M, Kumar S, Gopalamurugan A, Ahsan S, Khan F, et al. Chronic kidney disease – an independent risk factor for sustained VT/VF in heart failure patients with primary prevention CRT-D devices. Europace 2014;16. https://doi.org/10.1093/europace/euu237.4.
- Bevins A, Assi L, Ritchie J, Jesky M, Stringer S, Kalra P, et al. A simple scoring algorithm using serum free light chains for the risk assessment of progression of chronic kidney disease. Nephrol Dial Transplant 2013;28.
- Chan K, Goodall D, Lawrence C, Galliford J, Charif R, Duncan N, et al. Risk factors for malignancy post renal transplantation in patients receiving tacrolimus. Am J Transplant 2011;11:511-12.
- Cherukuri A, Balasubramanian S, Abheygunaratne T, Baker R. Vitamin D deficiency is a risk factor for adverse graft outcomes in kidney transplant recipients (KTR). Transplantation 2014;98:126-7.
- Cherukuri A, Bhandari S. Analysis of risk factors for mortality of incident patients commencing dialysis in East Yorkshire, UK. QJM 2010;103:41-8. https://doi.org/10.1093/qjmed/hcp164.
- Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518-31. https://doi.org/10.1001/jama.2014.6634.
- Farrugia D, Mahboob S, Begaj I, Ray D, Sharif A. Risk factors for death from malignancy after kidney transplantation in England – an observational cohort study. Transpl Int 2013;26.
- Harris L, Worsfold J, Favi E, Cacciola R, Puliatti C, Sammartino C, et al. Incidence and outcomes of polyomavirus infection in 639 kidney transplant recipients: are high immunological risk characteristics more relevant than specific induction or maintenance immunosuppressive regimens?. Am J Transplant 2015;23.
- Fellstrom B, Jardine AG, Soveri I, Cole E, Neumayer HH, Maes B, et al. Renal dysfunction is a strong and independent risk factor for mortality and cardiovascular complications in renal transplantation. Am J Transplant 2005;5:1986-91. https://doi.org/10.1111/j.1600-6143.2005.00983.x.
- Hamed M, Pasea L, Bradley JA, Pettigrew GJ, Saeb-Parsy K. Early graft loss post kidney transplantation is an important risk factor for patient mortality. Transpl Int 2013;26.
- Hamed M, Pasea L, Bradley J, Pettigrew G, Saeb-Parsy K. Early graft loss after kidney transplantation: risk factors and consequences. Transplantation 2014;98:629-30.
- Humar A, Lebranchu Y, Vincenti F, Punch J, Abramowicz D, Blumberg E, et al. The IMPACT study: valganciclovir prophylaxis for until 200 days post-transplant in high risk kidney recipients substantially reduces the incidence of CMV disease. Am J Transplant 2009;9:248-9.
- Humar A, Lebranchu Y, Vincenti F, Blumberg E, Punch JD, Limaye AP, et al. The IMPACT study: extending valganciclovir prophylaxis in kidney transplant high risk (D+/R–) recipients is associated with long-term benefits. Transplantation 2010;90. https://doi.org/10.1097/00007890-201007272-00046.
- Jardine AG, Fellström B, Logan JO, Cole E, Nyberg G, Grönhagen-Riska C, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) study. Am J Kidney Dis 2005;46:529-36. https://doi.org/10.1053/j.ajkd.2005.05.014.
- Karim A, Farrugia D, Cheshire J, Mahboob S, Begaj I, Ray D, et al. Recipient age and risk for mortality after kidney transplantation in England. Transplantation 2014;97:832-8. https://doi.org/10.1097/01.TP.0000438026.03958.7b.
- Krishnan N, Higgins R, Short A, Zehnder D, Pitcher D, Hudson A, et al. High BMI is not a risk factor for mortality or rejection following renal transplantation. Am J Transplant 2013;13.
- Mark PB, Patel RK, Johnston N, Weir RAP, Steedman T, Foster JE, et al. Myocardial fibrosis detected with cardiovascular MRI and risk of cardiovascular mortality in end stage renal disease patients assessed for renal transplantation. Am J Transplant 2010;10.
- Moore J, He X, Shabir S, Hanvesakul R, Benavente D, Cockwell P, et al. Development and evaluation of a composite risk score to predict kidney transplant failure. Am J Kidney Dis 2011;57:744-51. https://doi.org/10.1053/j.ajkd.2010.12.017.
- Nath J, Mastoridis S, van Dellen D, Guy AJ, McGrogan DG, Krishnan H, et al. Complex kidneys for complex patients: the risk associated with transplantation of kidneys with multiple arteries into obese patients. Transplant Proc 2015;47:373-8. https://doi.org/10.1016/j.transproceed.2015.01.006.
- Raymond NT, Zehnder D, Smith SC, Stinson JA, Lehnert H, Higgins RM. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant 2007;22:3214-20. https://doi.org/10.1093/ndt/gfm396.
- Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2012;82:570-80. https://doi.org/10.1038/ki.2012.136.
- Roderick PJ, Atkins RJ, Smeeth L, Mylne A, Nitsch DD, Hubbard RB, et al. CKD and mortality risk in older people: a community-based population study in the United Kingdom. Am J Kidney Dis 2009;53:950-60. https://doi.org/10.1053/j.ajkd.2008.12.036.
- Seitz A, Robb M, Balasubramanian S, McLean A, Taube D, Johnson R, et al. Alemtuzumab induction has been safe in the United Kingdom and achieves long-term steroid avoidance in more than 80% of low risk renal transplant recipients. Am J Transplant 2015;15.
- Shabir S, Halimi JM, Cherukuri A, Ball S, Ferro C, Lipkin G, et al. Predicting 5-year risk of kidney transplant failure: a prediction instrument using data available at 1 year posttransplantation. Am J Kidney Dis 2014;63:643-51. https://doi.org/10.1053/j.ajkd.2013.10.059.
- Taal MW, Sigrist MK, Fakis A, Fluck RJ, McIntyre CW. Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract 2007;107:c177-81. https://doi.org/10.1159/000110678.
- Thomson PC, Stirling CM, Geddes CC, Morris ST, Mactier RA. Vascular access in haemodialysis patients: a modifiable risk factor for bacteraemia and death. QJM 2007;100:415-22. https://doi.org/10.1093/qjmed/hcm040.
- Udayaraj UP, Steenkamp R, Caskey FJ, Rogers C, Nitsch D, Ansell D, et al. Blood pressure and mortality risk on peritoneal dialysis. Am J Kidney Dis 2009;53:70-8. https://doi.org/10.1053/j.ajkd.2008.08.030.
- Vivek V, Bhandari S. Prevalence of modifiable cardiovascular risk factors in long-term renal transplant patients. Int J Nephrol Renovasc Dis 2010;3:175-82.
- Wen CP, Matsushita K, Coresh J, Iseki K, Islam M, Katz R, et al. Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney Int 2014;86:819-27. https://doi.org/10.1038/ki.2013.553.
- Woo YM, McLean D, Kavanagh D, Ward L, Aitken S, Miller GJ, et al. The influence of pre-operative electrocardiographic abnormalities and cardiovascular risk factors on patient and graft survival following renal transplantation. J Nephrol 2002;15:380-6.
- Balupuri S, Buckley P, Snowden C, Mustafa M, Sen B, Griffiths P, et al. The trouble with kidneys derived from the non heart-beating donor: a single center 10-year experience. Transplantation 2000;69:842-6. https://doi.org/10.1097/00007890-200003150-00029.
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339-52. https://doi.org/10.1016/S0140-6736(13)60595-4.
- CKD Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073-81. https://doi.org/10.1016/S0140-6736(10)60674-5.
- CKD Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with mortality and end-stage renal disease: a collaborative meta-analysis of kidney disease cohorts. Kidney Int 2011;79. https://doi.org/10.1038/ki.2010.550.
- van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341-52. https://doi.org/10.1038/ki.2010.536.
- Marks A, Black C, Fluck N, Smith WCS, Prescott GJ, Clark LE, et al. Translating chronic kidney disease epidemiology into patient care – the individual/public health risk paradox. Nephrol Dial Transplant 2012;27:iii65-72. https://doi.org/10.1093/ndt/gfr746.
- Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, Demirbas A, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant 2009;9:1876-85. https://doi.org/10.1111/j.1600-6143.2009.02726.x.
- Ghazanfar A, Tavakoli A, Zaki MR, Pararajasingam R, Campbell T, Parrott NR, et al. The outcomes of living donor renal transplants with multiple renal arteries: a large cohort study with a mean follow-up period of 10 years. Transplant Proc 2010;42:1654-8. https://doi.org/10.1016/j.transproceed.2009.12.067.
- Organ Donation and Transplant: Activity Report 2014/15. Bristol: NHS Blood and Transplant; 2015.
- Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003;42:677-84. https://doi.org/10.1016/S0272-6386(03)00916-8.
- Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM, et al. Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol 2010;5:1366-72. https://doi.org/10.2215/CJN.02570310.
- Åhlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 2005;31:1222-8. https://doi.org/10.1007/s00134-005-2681-6.
- Hamel MB, Phillips RS, Davis RB, Desbiens N, Connors AF, Teno JM, et al. Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med 1997;127:195-202. https://doi.org/10.7326/0003-4819-127-3-199708010-00003.
- Caskey F, Castledine C, Dawnay A, Farrington K, Fogarty D, Fraser S, et al. UK Renal Registry 18th Annual Report of the Renal Association. Nephron 2016;132.
- Worldwide Systematic Review n.d.
- Lone NI, Gillies MA, Haddow C, Dobbie R, Rowan KM, Wild SH, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med 2016;194:198-20. https://doi.org/10.1164/rccm.201511-2234OC.
- Center for the Evaluation of Value and Risk in Health . CEA Registry n.d. http://healtheconomics.tuftsmedicalcenter.org/cear4/ (accessed 28 May 2018).
- University of Sheffield . ScHARRHUD n.d. www.scharrhud.org/ (accessed 28 May 2018).
- Hernández RA, Jenkinson D, Vale L, Cuthbertson BH. Economic evaluation of nurse-led intensive care follow-up programmes compared with standard care: the PRaCTICaL trial. Eur J Health Econ 2014;15:243-52. https://doi.org/10.1007/s10198-013-0470-7.
- Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010;14. https://doi.org/10.1186/cc8848.
- Latimer N, Dixon S, Drahota AK, Severs M. Cost–utility analysis of a shock-absorbing floor intervention to prevent injuries from falls in hospital wards for older people. Age Ageing 2013;42:641-5. https://doi.org/10.1093/ageing/aft076.
- Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 2015;385:1966-74. https://doi.org/10.1016/S0140-6736(15)60266-5.
- Flores-Gama C, Merino M, Baranda F, Cruz DN, Ronco C, Vazquez-Rangel A. The impact of integrating nephrologists into the postoperative cardiac intensive care unit: a cohort study. Cardiorenal Med 2013;3:79-88. https://doi.org/10.1159/000350545.
- Costa e Silva VT, Liaño F, Muriel A, Díez R, de Castro I, Yu L. Nephrology referral and outcomes in critically ill acute kidney injury patients. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0070482.
- Ponce D, Zorzenon Cde P, dos Santos NY, Balbi AL. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dial Transplant 2011;26:3202-6. https://doi.org/10.1093/ndt/gfr359.
- Mehta RL, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual MT, et al. Nephrology consultation in acute renal failure: does timing matter?. Am J Med 2002;113:456-61. https://doi.org/10.1016/S0002-9343(02)01230-5.
- Colpaert K, Hoste EA, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 2012;40:1164-70. https://doi.org/10.1097/CCM.0b013e3182387a6b.
- Pickkers P, Heemskerk S, Schouten J, Laterre PF, Vincent JL, Beishuizen A, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care 2012;16. https://doi.org/10.1186/cc11159.
- Heemskerk S, Masereeuw R, Moesker O, Bouw MP, van der Hoeven JG, Peters WH, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med 2009;37. https://doi.org/10.1097/CCM.0b013e31819598af.
- University of Alabama . Epidemiology of AKI in ICU 2016. www.obriendata.org/EP1AKI/Welcome.aspx (accessed 2 May 2018).
- Renal Association . UK Renal Registry: 18th Annual Report 2015. www.renalreg.org/reports/2015-eighteenth-annual-report/ (accessed 3 November 2017).
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- NHS Reference Costs 2014 to 2015. London: Department of Health; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Office for National Statistics . How Have Mortality Rates by Age Changed over the Last 50 Years? 2013. www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2013/sty-mortality-rates-by-age.html (accessed 3 November 2017).
- Rimes-Stigare C, Frumento P, Bottai M, Mårtensson J, Martling C-R, Walther SM, et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 2015;19. https://doi.org/10.1186/s13054-015-0920-y.
- Venables WN, Ripley BD. Modern Applied Statistics with S. New York: Springer-Verlag; 2002.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Claxton KP, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. PharmacoEconomics 2006;24:1055-68. https://doi.org/10.2165/00019053-200624110-00003.
- Strong M, Oakley JE. An efficient method for computing single-parameter partial expected value of perfect information. Med Decis Making 2013;33:755-66. https://doi.org/10.1177/0272989X12465123.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- Milborrow S, Hastie T, Tibshirani R. Earth: Multivariate Adaptive Regression Spline Models 2014. https://rdrr.io/cran/earth (accessed 2 May 2018).
- NHS . Hospital Episode Statistics, Admitted Patient Care – England, 2014 –15 2015. http://digital.nhs.uk/catalogue/PUB19124 (accessed 3 November 2017).
- Oezkur M, Magyar A, Thomas P, Stork T, Schneider R, Bening C, et al. TIMP-2* IGFBP7 (Nephrocheck®) measurements at intensive care unit admission after cardiac surgery are predictive for acute kidney injury within 48 hours. Kidney Blood Press Res 2017;42:456-67. https://doi.org/10.1159/000479298.
Appendix 1 AKI-Diagnostics search strategies: search 1 – horizon scanning
ClinicalTrials.gov (via the US National Institutes of Health)
Searched 29 September 2014.
-
biomarker* or marker* | acute kidney injury (89)
-
identify | acute kidney injury (20)
-
diagnose | acute kidney injury (70)
-
prognosis | acute kidney injury (15)
-
detect | acute kidney injury OR acute renal injury (15)
-
predict | acute kidney injury OR acute renal injury (27)
-
monitor | acute kidney injury OR acute renal injury (2)
-
stratify | acute kidney injury OR acute renal injury (2)
-
accuracy | acute kidney injury OR acute renal injury (12)
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9
Cochrane Central Register of Controlled Trials (via Wiley Online Library) (Issue 9 of 12, September 2014)
Searched 29 September 2014.
Search strategy
#1 Biomarker* or ‘bio* marker*’ or test or tests or factor or diagnostic*:ti,ab,kw (Word variations have been searched)
#2 MeSH descriptor: [Biological Markers] explode all trees
#3 MeSH descriptor: [Acute Kidney Injury] explode all trees and with qualifier(s): [Diagnosis - DI]
#4 MeSH descriptor: [Acute-Phase Proteins] explode all trees and with qualifier(s): [Diagnostic use - DU]
#5 MeSH descriptor: [Insulin-Like Growth Factor Binding Proteins] explode all trees and with qualifier(s): [Diagnostic use - DU]
#6 (NGAL or ‘Neutrophil gelatinase-associated lipocalin’ or ‘lipocalin 2’ or lcn2 or ‘TIMP-2’ or ‘TIMP 2’ or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’ or KIM-1 or TIMD1 or ‘TIM-1’ or ‘Hepatitis A virus cellular receptor 1’ or ‘kidney injury molecule 1’ or Cystatin C or ‘Cystatin C’ or L-FABP or (Liver near/2 ‘Fatty acid-binding protein’) or ‘IL-18’ or ‘IL 18’ or Interleukin-18 or ‘Interleukin 18’ or MIOX or ‘myo-inositol oxygenase’ or NTN1 or ‘netrin-1’ or IGFBP7 or IBP-7 or ‘IGF-binding protein 7’ or ‘Insulin-like growth factor-binding protein 7’ or potassium or Creat or Crea or creatinine or ‘T Prot’ or ‘Total Protein’ or ‘Acute-Phase Protein*’ or Alb or Albumin or BUN or ‘Blood Urea Nitrogen’ or AAP or ‘AP-N’ or hAPN or AP-M or ‘EC=3.4.11.2’ or ‘microsomal aminopeptidase’ or ‘Alanine aminopeptidase’ or ‘ALP-1’ or GCAP or ‘PLAP-like’ or ‘Alkaline phosphatase’ or ‘Alkaline phosphatase’ or ‘placental-like’ or GST or ‘glutathione transferase’ or ‘glutathione-S-transferase’ or ‘Glutathione S-transferase A2’ or ‘EC = 2.5.1.18’ or ‘GGT 5’ or GGT5 or ‘gamma glutamyltransferase’ or ‘gamma glutamyl transferase’ or ‘glutamyl transpeptidase’ or (‘N-acetyl’ near/2 glucosaminidase) or ‘n acetylglucosaminyltransferase’ or ‘n acetylglucosamine’ or ‘histone acetyltransferase’ or ‘Bifunctional protein*’ or ncoat* or NAG or OGA or microglobulin or AMBP or ‘retinol binding protein 4’ or ‘plasma retinol binding protein’ or microalbumin or clusterin or ‘Apo-J’ or ‘TRPM-2’ or ‘Cysteine-rich protein’ or ‘Cysteine-rich motor neuron 1 protein’ or ‘CYR-61’ or ‘CRIM-1’ or ‘SPP-1’ or Osteopontin or NHE3 or NHE or ‘exchanger 9B2’ or ‘exchanger isoform’ or ‘proton sodium exchange’ or ‘sodium proton exchange protein 3’ or ‘Alpha-2-HS-glycoprotein’ or ‘fetuin A’ or Klotho or CALB1 or calbindin or ‘h-FABP’ or MDGI or ‘M-FABP’ or ‘Fatty acid-binding protein’ or renin or ‘ec=3.4.23.15’):ti,ab,kw
#7 {or #1-#6}
#8 MeSH descriptor: [Acute Kidney Injury] explode all trees
#9 MeSH descriptor: [Kidney Tubular Necrosis, Acute] explode all trees
#10 MeSH descriptor: [Acute Disease] explode all trees
#11 MeSH descriptor: [Kidney Diseases] explode all trees
#12 #10 and #11
#13 (Acute near/3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)):ti,ab,kw (Word variations have been searched)
#14 (Acute near/3 (renal disease* or renal injury or renal failure or renal dysfunction)):ti,ab,kw
#15 ((Acute near/3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’):ti,ab,kw
#16 (AKI):ti,ab,kw
#17 (‘contrast induced nephropathy’):ti,ab,kw
#18 (renal or kidney* or nephr*):ti,ab,kw or {or #8-#17}
#19 (reperfusion near/5 (injur* or isch?emi*)):ti,ab,kw
#20 MeSH descriptor: [Reperfusion Injury] explode all trees
#21 (‘delayed graft function*’):ti,ab,kw
#22 MeSH descriptor: [Delayed Graft Function] explode all trees
#23 {or #19-#22} and #18
#24 #23 or {or #8-#17} Publication Year from 2004 to 2014
#25 Sera or Serum or Serologic* or urine or urinary:ti (Word variations have been searched)
#26 sample* or specimen*:ti,ab,kw (Word variations have been searched)
#27 MeSH descriptor: [Analytic Sample Preparation Methods] explode all trees
#28 MeSH descriptor: [Blood Specimen Collection] explode all trees
#29 MeSH descriptor: [Urine Specimen Collection] explode all trees
#30 MeSH descriptor: [Specimen Handling] explode all trees
#31 plasma or blood or urine or urinary:ti,ab,kw (Word variations have been searched)
#32 MeSH descriptor: [Blood] explode all trees
#33 MeSH descriptor: [Blood] this term only
#34 MeSH descriptor: [Plasma] explode all trees
#35 MeSH descriptor: [Serum] explode all trees
#36 MeSH descriptor: [Urine] this term only
#37 ({or #31-#36} and {or #26-#30}) or #25
#38 MeSH descriptor: [Acute Kidney Injury] explode all trees and with qualifier(s): [Blood - BL, Urine - UR]
#39 MeSH descriptor: [Kidney Tubular Necrosis, Acute] explode all trees and with qualifier(s): [Blood - BL, Urine - UR]
#40 Any MeSH descriptor with qualifier(s): [Blood - BL, Urine - UR]
#41 #23 and #40
#42 {or #37-#39} or #41
#43 #42 and #24
#44 MeSH descriptor: [Biological Markers] explode all trees
#45 (discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif* or accura* or marker* or biomarker* or sensitivity):ti,ab,kw (Word variations have been searched)
#46 {or #44-#45} and #43
#47 MeSH descriptor: [Animals] explode all trees
#48 MeSH descriptor: [Veterinary Medicine] explode all trees
#49 MeSH descriptor: [Animal Experimentation] explode all trees
#50 MeSH descriptor: [Humans] explode all trees
#51 {or #47-#49} not #50
#52 #7 and #46
#53 #52 not #51
Cochrane Database of Systematic Reviews (via Wiley Online Library) (Issue 9 of 12, September 2014)
Searched 29 September 2014.
Same search as for Cochrane Central Register of Controlled Trials.
Conference Proceedings Citation Index – Science (via Thomson Reuters’ Web of Science) (2008–present)
Searched 29 September 2014.
Search strategy
-
TS=(‘acute kidney injury’ or AKI) OR TS=((Acute NEAR/3 (‘kidney disease*’ or ‘kidney injury’ or ‘kidney failure’ or ‘kidney dysfunction’))) OR TS=((Acute NEAR/3 (‘renal disease*’ or ‘renal injury’ or ‘renal failure’ or ‘renal dysfunction’))) OR TS=(((Acute NEAR/3 (‘Tubular Necrosis’ or nephrotoxic*)) or ‘nephrotoxic injur*’)) OR TS=(‘contrast induced nephropath*’) OR TS=((reperfusion NEAR/5 (injur* or isch?emi*)) AND (renal or kidney* or nephr*)) OR TS=((‘Delayed Graft Function’) AND (renal or kidney* or nephr*))
-
TI=((Sera or Serum or Serologic* or urine or urinary)) OR TS=((sample* or specimen*) AND (plasma or blood or urine or urinary or serum or sera or serologic)) OR TS=(Urinalysis)
-
#2 AND #1
-
TS=(discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif* or accura* or marker* or biomarker* or sensitivity).
-
TS=(NGAL or ‘Neutrophil gelatinase-associated lipocalin’ or ‘lipocalin 2’ or lcn2 or ‘TIMP-2’ or ‘TIMP 2’ or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’ or KIM-1 or TIMD1 or ‘TIM-1’ or ‘Hepatitis A virus cellular receptor 1’ or ‘kidney injury molecule 1’ or Cystatin C or ‘Cystatin C’ or L-FABP or (Liver NEAR/2 ‘Fatty acid-binding protein’) or ‘IL-18’ or ‘IL 18’ or Interleukin-18 or ‘Interleukin 18’ or MIOX or ‘myo-inositol oxygenase’ or NTN1 or ‘netrin-1’ or IGFBP7 or IBP-7 or ‘IGF-binding protein 7’ or ‘Insulin-like growth factor-binding protein 7’ or potassium or Creat or Crea or creatinine or ‘T Prot’ or ‘Total Protein’ or ‘Acute-Phase Protein*’ or Alb or Albumin or BUN or ‘Blood Urea Nitrogen’ or AAP or ‘AP-N’ or hAPN or AP-M or ‘EC=3.4.11.2’ or ‘microsomal aminopeptidase’ or ‘Alanine aminopeptidase’ or ‘ALP-1’ or GCAP or ‘PLAP-like’ or ‘Alkaline phosphatase’ or ‘Alkaline phosphatase’ or ‘placental-like’ or GST or ‘glutathione transferase’ or ‘glutathione-S-transferase’ or ‘Glutathione S-transferase A2’ or ‘EC=2.5.1.18’ or ‘GGT 5’ or GGT5 or ‘gamma glutamyltransferase’ or ‘gamma glutamyl transferase’ or ‘glutamyl transpeptidase’ or (‘N-acetyl’ NEAR/2 glucosaminidase) or ‘n acetylglucosaminyltransferase’ or ‘n acetylglucosamine’ or ‘histone acetyltransferase’ or ‘Bifunctional protein*’ or ncoat* or NAG or OGA or microglobulin or AMBP or ‘retinol binding protein 4’ or ‘plasma retinol binding protein’ or microalbumin or clusterin or ‘Apo-J’ or ‘TRPM-2’ or ‘Cysteine-rich protein’ or ‘Cysteine-rich motor neuron 1 protein’ or ‘CYR-61’ or ‘CRIM-1’ or ‘SPP-1’ or Osteopontin or NHE3 or NHE or ‘exchanger 9B2’ or ‘exchanger isoform’ or ‘proton sodium exchange’ or ‘sodium proton exchange protein 3’ or ‘Alpha-2-HS-glycoprotein’ or ‘fetuin A’ or Klotho or CALB1 or calbindin or ‘h-FABP’ or MDGI or ‘M-FABP’ or ‘Fatty acid-binding protein’ or renin or ‘ec=3.4.23.15’)
-
TS=(biomarker* or ‘bio* marker*’ or ‘marker*’) OR TS=(diagnostic or test or tests or factor)
-
#4 AND #3
-
#6 OR #5
-
#8 AND #7
-
TS= (rat or rats or swine or pigs or pig or mice or mouse) NOT TS=(human* or patient* or neonate* or child*or woman or women or men or man or adolescen*)
-
#9 NOT #10
-
TITLE: (((‘case stud*’ or ‘a case of’ or ‘case report*’ or ‘in a patient’ or girl or woman or man or boy or child or female or male or ‘a patient of’) not ‘case control’))
-
#11 NOT #12
Database of Abstracts of Reviews of Effect (via Wiley Online Library) (Issue 3 of 4, July 2014)
Searched 29 September 2014.
Same search as for Cochrane Central Register of Controlled Trials.
EMBASE Classic + EMBASE (via Ovid) (1947 to 25 September 2014)
Searched 26 September 2014.
Search strategy
-
exp *acute kidney failure/ (25,170)
-
exp *kidney tubule necrosis/ (1745)
-
exp acute disease/ and exp *kidney disease/ (2964)
-
exp *kidney injury/ (9663)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (11,879)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (30,030)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (4742)
-
aki.tw. (6336)
-
‘contrast induced nephropathy’.tw. (1830)
-
exp *contrast induced nephropathy/ (1310)
-
exp *reperfusion injury/ (20,312)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (53,348)
-
exp *delayed graft function/ or ‘delayed graft function*’.tw. (3768)
-
((renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. or (or/1-10)) and (11 or 12 or 13) (9795)
-
or/1-10,14 (70,674)
-
limit 15 to yr=‘2004 -Current’ (37,141)
-
(Sera or Serum or Serologic* or urine or urinary).ti. (492,367)
-
(sample* or specimen*).tw. (1,773,754)
-
(Sera or Serum or Serologic*).tw. (1,231,104)
-
plasma.tw. (883,935)
-
blood.tw. (1,964,937)
-
(Urine or Urinary).tw. (527,090)
-
exp blood/ (2,032,361)
-
exp plasma/ (150,511)
-
exp serum/ (238,704)
-
exp urine/ (175,881)
-
(18 and (or/19-26)) or 17 (971,361)
-
exp blood analysis/ (120,308)
-
exp urinalysis/ (73,802)
-
27 or 28 or 29 [ serum blood urine sample or analysis ] (1,096,197)
-
(NGAL or ‘Neutrophil gelatinase-associated lipocalin’ or ‘lipocalin 2’ or lcn2).tw. (3221)
-
exp neutrophil gelatinase associated lipocalin/ (3536)
-
(TIMP-2 or ‘TIMP 2’ or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’).tw. (4071)
-
exp ‘tissue inhibitor of metalloproteinase 2’/ (4696)
-
exp kidney injury molecule 1/ [ used for tim-1 / kim 1 ] (1011)
-
(KIM-1 or TIMD1 or TIM-1 or ‘Hepatitis A virus cellular receptor 1’).tw. (965)
-
(Cystatin C or ‘Cystatin C’).tw. (4931)
-
exp cystatin C/ (6100)
-
(L-FABP or (Liver adj2 ‘Fatty acid-binding protein’)).tw. (962)
-
exp fatty acid binding protein/ (5723)
-
(IL-18 or ‘IL 18’ or Interleukin-18 or ‘Interleukin 18’).tw. (7934)
-
exp interleukin 18/ (9525)
-
(MIOX or ‘myo-inositol oxygenase’).tw. (75)
-
exp inositol oxygenase/ (74)
-
(NTN1 or netrin-1).tw. (717)
-
exp netrin 1/ (743)
-
(IGFBP7 or IBP-7 or ‘IGF-binding protein 7’ or ‘Insulin-like growth factor-binding protein 7’).tw. (274)
-
exp somatomedin binding protein/ [ synonym for Insulin-Like Growth Factor Binding Proteins?] (5151)
-
exp potassium/ or exp potassium blood level/ (111,615)
-
potassium.tw. (144,039)
-
exp creatinine blood level/ or exp creatinine urine level/ or exp creatinine/ [11] (127,699)
-
(Creat or Crea or creatinine).tw. (116,882)
-
(‘T Prot’ or ‘Total Protein’).tw. (29,574)
-
exp protein/ [12] (408,671)
-
(Alb or Albumin).tw. (156,441)
-
exp serum albumin/ or exp albumin blood level/ or exp human albumin/ or exp albumin/ or exp human serum albumin/ (124,522)
-
exp acute phase protein/ [ broader than albumin and includes more such as retinol binding protein] (7283)
-
exp urea nitrogen blood level/ (21,193)
-
(BUN or Blood Urea Nitrogen).tw. (15,003)
-
(AAP or AP-N or hAPN orAP-M or ‘EC=3.4.11.2’ or ‘Alanine aminopeptidase’).tw. (3381)
-
exp microsomal aminopeptidase/ [Alanine aminopeptidase] (4941)
-
(ALP-1 or GCAP or PLAP-like or Alkaline phosphatase or Alkaline phosphatase or placental-like).tw. (72,967)
-
exp alkaline phosphatase/ [17 emtree only] (84,260)
-
(GST or glutathione-S-transferase or ‘Glutathione S-transferase A2’ or ‘EC=2.5.1.18’).tw. [18] (32,592)
-
exp glutathione transferase/ (30,078)
-
exp gamma glutamyltransferase/ or exp gamma glutamyl transferase blood level/ [20] (22,684)
-
(‘GGT 5’ or GGT5 or ‘gamma glutamyltransferase 5’ or ‘glutamyl transpeptidase’).tw. (5744)
-
((‘N-acetyl’ adj2 glucosaminidase) or ‘Bifunctional protein*’ or ncoat* or NAG or OGA).tw. (6637)
-
exp acetylglucosaminidase/ [21] (2190)
-
‘glucosaminidase’.tw. (5217)
-
exp n acetylglucosaminyltransferase/ or exp n acetylglucosamine/ or exp histone acetyltransferase/ (12,481)
-
exp beta 2 microglobulin/ [22] (13,632)
-
(microglobulin or AMBP).tw. (14,268)
-
exp alpha 1 microglobulin/ [23] (1314)
-
exp retinol binding protein/ [24] (3435)
-
‘retinol binding protein 4’.tw. (800)
-
microalbumin.tw. [25] (974)
-
exp microalbuminuria/ (12,250)
-
(clusterin or ‘Apo-J’ or ‘TRPM-2’).tw. or exp clusterin/ (3246)
-
(‘CYR-61’ or ‘CRIM-1’ or ‘Cysteine-rich protein’ or ‘Cysteine-rich motor neuron 1 protein’).tw. or exp cysteine rich protein 61/ [27] (1377)
-
(‘SPP-1’ or Osteopontin).tw. or exp osteopontin/ (10,784)
-
(NHE3 or NHE or ‘exchanger 9B2’ or ‘exchanger isoform’).tw. or exp proton sodium exchange/ or exp sodium proton exchange protein 3/ [29] (6082)
-
‘Alpha-2-HS-glycoprotein’.tw. or exp fetuin A/ (1182)
-
Klotho.tw. or exp Klotho protein/ (1512)
-
exp calbindin 1/ or exp calbindin/ or (CALB1 or calbindin).tw. [32] (5621)
-
exp fatty acid binding protein/ or (h-FABP or MDGI or M-FABP or ‘Fatty acid-binding protein’).tw. [33] (7116)
-
(renin or ‘ec=3.4.23.15’).tw. [34] (54,978)
-
exp kidney injury/di [Diagnosis] (1567)
-
exp acute kidney failure/di [Diagnosis] (3599)
-
(biomarker* or ‘bio* marker*’ or marker*).tw. (770,398)
-
diagnostic*.tw. (718,123)
-
factor.tw. (1,463,890)
-
(test or tests).tw. (1,841,173)
-
exp biological marker/ (131,802)
-
or/31-94 [ specific biomarkers SHs, txtwords, and biomarker general terms ] (5,223,158)
-
exp biological marker/ (131,802)
-
(discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif* or accura* or marker* or biomarker* or sensitivity).tw. (10,694,092)
-
96 or 97 [ detect filter ] (10,708,959)
-
(exp animals/ or exp nonhuman/ or animal experiment/ or exp veterinary medicine/ or exp experimental animal/) not exp human/ (5,908,462)
-
(16 and 30 and 95 and 98) not 99 (2664)
-
100 not ((‘case stud*’ or ‘a case of’ or ‘case report*’ or ‘in a patient’ or girl or woman or man or boy or child or female or male or ‘a patient of’) not ‘case control’).ti. (2502)
International Clinical Trials Registry Platform (via the World Health Organization)
Searched 29 September 2014.
Search strategy
-
(acute kidney injury OR AKI) AND biomarker* [standard search] (282 results)
-
(acute kidney injury OR AKI OR Acute renal failure) AND (identif* OR recogni* OR diagnos* OR indicat* OR correlat* OR prognosis OR predict* OR subclinical OR detect* OR monitor* OR stratif* OR accuracy OR sensitivity) [Advanced search] (49 results)
MEDLINE (via Ovid) (1946 to week 3 September 2014)
Searched 26 September 2014.
Search strategy
-
acute kidney injury/ or kidney tubular necrosis, acute/ (34,230)
-
Acute Disease/ and exp Kidney Diseases/ (7675)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (6635)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (21,345)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3418)
-
aki.tw. (3076)
-
‘contrast induced nephropathy’.tw. (944)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. or reperfusion injury/ or reperfusion/ (46,033)
-
exp Delayed Graft Function/ or ‘delayed graft function*’.tw. (2299)
-
((renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. or (or/1-7)) and (8 or 9) (7053)
-
or/1-7,10 [AKI] (56,812)
-
limit 11 to yr=‘2004 -Current’ (22,491)
-
(Sera or Serum or Serologic* or urine or urinary).ti. (366,534)
-
(sample* or specimen*).tw. (1,272,203)
-
exp Analytic Sample Preparation Methods/ or exp Blood Specimen Collection/ or exp Urine Specimen Collection/ or exp Specimen Handling/ or exp Specimen Handling/ (281,322)
-
(Sera or Serum or Serologic*).tw. (877,395)
-
plasma.tw. (675,552)
-
blood.tw. (1,343,744)
-
(Urine or Urinary).tw. (352,430)
-
blood/ (48,286)
-
exp plasma/ (16,374)
-
exp serum/ (61,384)
-
urine/ (33,925)
-
or/14-23 [ broadest search ] (3,896,894)
-
((or/16-23) and (or/14-15)) or 13 [refined search] (702,912)
-
exp Acute Kidney Injury/bl, ur or kidney tubular necrosis, acute/bl, ur (2902)
-
(bl or ur).fs. and (10 or 9) (1624)
-
exp Urinalysis/ (4768)
-
or/25-28 [refined search OR subheading bl ur] (708,078)
-
biomarker*.tw. (87,588)
-
‘bio* marker*’.tw. (18,195)
-
exp Biological Markers/ (646,975)
-
marker*.tw. (480,182)
-
diagnostic*.tw. (479,993)
-
factor.tw. (1,110,955)
-
(test or tests).tw. (1227167)
-
exp Acute Kidney Injury/di [Diagnosis] (3604)
-
Acute-Phase Proteins/du [Diagnostic Use] (19)
-
Insulin-Like Growth Factor Binding Proteins/du (3)
-
(NGAL or ‘Neutrophil gelatinase-associated lipocalin’ or ‘lipocalin 2’ or lcn2).tw. (1694)
-
(TIMP-2 or ‘TIMP 2’ or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’).tw. (3387)
-
(KIM-1 or TIMD1 or TIM-1 or ‘Hepatitis A virus cellular receptor 1’ or ‘kidney injury molecule 1’).tw. (670)
-
(Cystatin C or ‘Cystatin C’).tw. (3195)
-
(L-FABP or (Liver adj2 ‘Fatty acid-binding protein’)).tw. (769)
-
(IL-18 or ‘IL 18’ or Interleukin-18 or ‘Interleukin 18’).tw. (5940)
-
(MIOX or ‘myo-inositol oxygenase’).tw. (61)
-
(NTN1 or netrin-1).tw. (575)
-
(IGFBP7 or IBP-7 or ‘IGF-binding protein 7’ or ‘Insulin-like growth factor-binding protein 7’).tw. (170)
-
potassium.tw. or exp Potassium/ (160,692)
-
(Creat or Crea or creatinine).tw. (75,931)
-
(‘T Prot’ or ‘Total Protein’).tw. (20,553)
-
(Alb or Albumin).tw. (109,497)
-
(BUN or Blood Urea Nitrogen).tw. (9374)
-
(AAP or AP-N or hAPN orAP-M or ‘EC=3.4.11.2’ or ‘Alanine aminopeptidase’).tw. (2431)
-
(ALP-1 or GCAP or PLAP-like or Alkaline phosphatase or Alkaline phosphatase or placental-like).tw. (52,614)
-
(GST or glutathione-S-transferase or ‘Glutathione S-transferase A2’ or ‘EC=2.5.1.18’).tw. (27,618)
-
((‘N-acetyl’ adj2 glucosaminidase) or ‘Bifunctional protein*’ or ncoat* or NAG or OGA).tw. (5209)
-
((alpha* adj2 microglobulin) or AMBP).tw. (1206)
-
clusterin.tw. (1491)
-
(‘Apo-J’ or ‘TRPM-2’).tw. (185)
-
(‘Cysteine-rich protein’ or ‘Cysteine-rich motor neuron 1 protein’).tw. (640)
-
(‘CYR-61’ or ‘CRIM-1’).tw. (17)
-
(SPP-1 or Osteopontin).tw. (6369)
-
(NHE3 or NHE or ‘exchanger 9B2’ or ‘exchanger isoform’).tw. (2922)
-
‘Alpha-2-HS-glycoprotein’.tw. (231)
-
Klotho.tw. (832)
-
(CALB1 or calbindin).tw. (4203)
-
(h-FABP or MDGI or M-FABP or ‘Fatty acid-binding protein’).tw. (3785)
-
(renin or ‘ec=3.4.23.15’).tw. (42,939)
-
or/30-69 [additional biomarkers] (3,740,487)
-
(exp animals/ or exp Veterinary Medicine/ or exp Animal Experimentation/) not exp humans/ (4,020,113)
-
(discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif* or accura* or marker* or biomarker* or sensitivity).tw. (7,855,298)
-
exp Biological Markers/ (646,975)
-
72 or 73 [predict / diagnose filter] (8,047,219)
-
12 and 29 and 70 and 74 [ all concepts] (2309)
-
75 not 71 [ not animal studies ] (1830)
-
limit 76 to case reports (159)
-
76 not 77 [not case studies] (1671)
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (25 September 2014)
Searched 26 September 2014.
Search strategy
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (1403)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (1002)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (144)
-
aki.tw. (741)
-
‘contrast induced nephropathy’.tw. (144)
-
((reperfusion adj5 (injur* or isch?emi*)) or ‘delayed graft function*’).tw. (2530)
-
(renal or kidney* or nephr*).tw. or (or/1-5) (36,995)
-
6 and 7 (476)
-
or/1-5,8 [AKI] (2887)
-
limit 9 to yr=‘2004 -Current’ (2719)
-
(sample* or specimen*).tw. (139,336)
-
(Sera or Serum or Serologic*).tw. (45,535)
-
plasma.tw. (39,175)
-
blood.tw. (74,094)
-
(Urine or Urinary).tw. (18,581)
-
(or/11-15) or biomarker*.tw. [ broadest search, also biomarker*.tw added ] (272,537)
-
biomarker*.tw. (15,361)
-
‘bio* marker*’.tw. (1368)
-
marker*.tw. (38,812)
-
diagnostic*.tw. (39,369)
-
factor.tw. (85,140)
-
(test or tests).tw. (109,362)
-
(NGAL or ‘Neutrophil gelatinase-associated lipocalin’ or ‘lipocalin 2’ or lcn2).tw. (330)
-
(TIMP-2 or ‘TIMP 2’ or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’).tw. (172)
-
(KIM-1 or TIMD1 or TIM-1 or ‘Hepatitis A virus cellular receptor 1’ or ‘kidney injury molecule 1’).tw. (115)
-
(Cystatin C or ‘Cystatin C’).tw. (363)
-
(L-FABP or (Liver adj2 ‘Fatty acid-binding protein’)).tw. (53)
-
(IL-18 or ‘IL 18’ or Interleukin-18 or ‘Interleukin 18’).tw. (417)
-
(MIOX or ‘myo-inositol oxygenase’).tw. (4)
-
(NTN1 or netrin-1).tw. (48)
-
(IGFBP7 or IBP-7 or ‘IGF-binding protein 7’ or ‘Insulin-like growth factor-binding protein 7’).tw. (32)
-
potassium.tw. (8454)
-
(Creat or Crea or creatinine).tw. (5047)
-
(‘T Prot’ or ‘Total Protein’).tw. (1430)
-
(Alb or Albumin).tw. (6036)
-
(BUN or Blood Urea Nitrogen).tw. (818)
-
(AAP or AP-N or hAPN orAP-M or ‘EC=3.4.11.2’ or ‘Alanine aminopeptidase’).tw. (173)
-
(ALP-1 or GCAP or PLAP-like or Alkaline phosphatase or Alkaline phosphatase or placental-like).tw. (2787)
-
(GST or glutathione-S-transferase or ‘Glutathione S-transferase A2’ or ‘EC=2.5.1.18’).tw. (1498)
-
(‘GGT 5’ or GGT5 or (‘gamma glutamyltransferase 5’ or ‘glutamyl transpeptidase’)).tw. (205)
-
((‘N-acetyl’ adj2 glucosaminidase) or ‘Bifunctional protein*’ or ncoat* or NAG or OGA).tw. (229)
-
(microglobulin or AMBP).tw. (329)
-
(‘retinol binding protein 4’ or ‘plasma retinol binding protein’).tw. (93)
-
microalbumin.tw. (55)
-
clusterin.tw. (105)
-
(‘Apo-J’ or ‘TRPM-2’).tw. (5)
-
(‘Cysteine-rich protein’ or ‘Cysteine-rich motor neuron 1 protein’).tw. (34)
-
(‘CYR-61’ or ‘CRIM-1’).tw. (1)
-
(SPP-1 or Osteopontin).tw. (516)
-
(NHE3 or NHE or ‘exchanger 9B2’ or ‘exchanger isoform’).tw. (164)
-
‘Alpha-2-HS-glycoprotein’.tw. (10)
-
Klotho.tw. (117)
-
(CALB1 or calbindin).tw. (146)
-
(h-FABP or MDGI or M-FABP or ‘Fatty acid-binding protein’).tw. (301)
-
(renin or ‘ec=3.4.23.15’).tw. (1387)
-
or/17-55 [biomarkers] (273,001)
-
(discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif* or accura* or marker* or biomarker* or sensitivity).tw. (725,049)
-
10 and 16 and 56 and 57 (704)
-
((rat or rats or swine or pigs or pig or mice or mouse) not (human* or patient*)).tw. (63,154)
-
((‘case stud*’ or ‘a case of’ or ‘case report*’ or ‘in a patient’ or girl or woman or man or boy or child or female or male or ‘a patient of’) not ‘case control’).ti. (57,135)
-
58 not (59 or 60) (578)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction) adj3 (discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif*)).ti. (68)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction) adj3 (discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif*)).ti. (6)
-
(((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’) adj3 (discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif*)).ti. (0)
-
(aki adj3 (discriminat* or identif* or recogni* or diagnos* or indicat* or correlat* or prognos* or predict* or subclinical or detect* or monitor* or stratif*)).ti. (6)
-
(or/62-65) not (59 or 60) (77)
-
66 not 61 (25)
-
61 or 67 (603)
metaRegister of Controlled Trials (mRCT)
Searched 29 September 2014.
Search strategy
-
‘Acute kidney’ (161)
-
‘acute renal’ AND biomarker (6)
-
‘acute renal’ AND detect (10)
-
1 OR 2 OR 3
PubMed (via the US National Library of Medicine) (1946 to September 2014)
Searched 29 September 2014.
Search strategy
-
#1 (((‘acute kidney[Title] OR ‘acute renal’[Title] OR ‘acute tubular necrosis’[Title] OR ‘aki’[Title] OR ‘delayed graft function’[Title] OR ‘reperfusion injur*’[Title]))) (17,542)
-
#2 ((discriminat*[Title] OR identif*[Title] OR recogni*[Title] OR diagnos*[Title] OR indicat*[Title] OR correlate*[Title] OR prognot*[Title] OR predict*[Title] OR subclinical[Title] OR detect*[Title] OR monitor*[Title] OR stratif*[Title] OR accura*[Title] OR marker*[Title] OR biomarker*[Title] OR sensitivity[Title])) (1,769,745)
-
#3 ((pubstatusaheadofprint[sb] OR publisher[sb] OR pubmednotmedline[sb])) (1,826,775)
-
#4 #1 AND #2 AND #3 (111)
Science Citation Index (via Thomson Reuters’ Web of Science) (2008 to present)
Searched 29 September 2014.
Same search as for Conference Proceedings Citation Index – Science.
Appendix 2 AKI-Diagnostics horizon-scanning biomarker longlist
| Biomarker | Number of papers | Timescale |
|---|---|---|
| NGAL | 241 | 2005–14 |
| Cystatin C | 115 | 2004–14 |
| IL-18 | 73 | 2005–14 |
| KIM-1 | 72 | 2006–14 |
| L-FABP | 39 | 2006–14 |
| NAG | 37 | 2004–14 |
| B2M | 16 | 2006–14 |
| IL-6 | 11 | 2007–14 |
| α1-microglobulin | 10 | 2005–14 |
| GST | 8 | 2010–13 |
| IGFBP-7 (Nephrocheck) | 8 | 2013–14 |
| FENa | 7 | 2005–14 |
| BNP | 7 | 2012–14 |
| TIMP-2 (Nephrocheck) | 6 | 2013–14 |
| TNF-α | 6 | 2012–14 |
| CRP | 5 | 2013–14 |
| FEUrea | 5 | 2006–14 |
| GGT | 5 | 2006–13 |
| IL-10 | 4 | 2009–14 |
| Procalcitonin | 4 | 2009–14 |
| HGF | 4 | 2008–10 |
| MCP-1 | 4 | 2008–13 |
| IL-8 | 4 | 2009–12 |
| Lactate dehydrogenase | 4 | 2013–14 |
| AAP | 3 | 2012–14 |
| AP | 3 | 2006–14 |
| Hepcidin | 3 | 2011–13 |
| H-FABP | 3 | 2013–14 |
| hsCRP | 3 | 2011–14 |
| Renin | 3 | 2013–14 |
| SAA | 3 | 2011–13 |
| ADMA | 3 | 2012–13 |
| Angiotensinogen | 3 | 2013 |
| VEGF | 3 | 2008–14 |
| Adiponectin | 2 | 2013–14 |
| α-GST | 2 | 2013 |
| AOPP | 2 | 2010–12 |
| CXCR1 | 2 | 2012–14 |
| EPO | 2 | 2013–14 |
| F2-isoprostanes | 2 | 2011–12 |
| FB | 2 | 2012–13 |
| GT | 2 | 2011–14 |
| HDL | 2 | 2013 |
| LDH | 2 | 2013–14 |
| LVEF | 2 | 2013 |
| MMP-9 | 2 | 2009 |
| Osteopontin | 2 | 2009–10 |
| sRAGE | 2 | 2012–13 |
| SUA | 2 | 2012–13 |
| Vitamin D | 2 | 2012–13 |
| vWF | 2 | 2014 |
| Clusterin | 2 | 2013 |
| EGF | 2 | 2013 |
| FLC | 2 | 2013 |
| PAI-1 | 2 | 2012 |
| pi-GST | 2 | 2013 |
| Semaphorin 3A | 2 | 2014 |
| AGP | 1 | 2009 |
| ALP | 1 | 2013 |
| Angiopoietin-1 | 1 | 2014 |
| Angiopoietin-2 | 1 | 2014 |
| Apolipoprotein M | 1 | 2014 |
| Aprotinin | 1 | 2008 |
| AQP 1 | 1 | 2006 |
| AQP 2 | 1 | 2006 |
| ARC | 1 | 2012 |
| CD14 | 1 | 2009 |
| CXCL10 | 1 | 2008 |
| D-dimer | 1 | 2014 |
| Dihydroneopterin | 1 | 2010 |
| EA | 1 | 2013 |
| ELA-2 | 1 | 2014 |
| enRAGE | 1 | 2013 |
| EO | 1 | 2014 |
| EPC | 1 | 2014 |
| ESAM | 1 | 2014 |
| E-selectin | 1 | 2014 |
| esRAGE | 1 | 2012 |
| FEK | 1 | 2005 |
| FEMg | 1 | 2005 |
| FGF | 1 | 2009 |
| FGF-2 | 1 | 2013 |
| FGF-23 | 1 | 2013 |
| FST | 1 | 2014 |
| GA | 1 | 2012 |
| Hb | 1 | 2013 |
| HMGB-1 | 1 | 2013 |
| HO-1 | 1 | 2014 |
| Hyaluronic acid | 1 | 2014 |
| ICAM-1 | 1 | 2014 |
| IL-19 | 1 | 2011 |
| Kynurenine | 1 | 2014 |
| LDL | 1 | 2013 |
| MicroRNA-21 | 1 | 2013 |
| MIOX | 1 | 2014 |
| MMP-8 | 1 | 2014 |
| Neopterin | 1 | 2010 |
| Netrin-1 | 1 | 2014 |
| NT-ProBNP | 1 | 2014 |
| PAPP-A | 1 | 2013 |
| PDGF | 1 | 2009 |
| PGF2alpha | 1 | 2011 |
| PIGF | 1 | 2013 |
| Plasma homocysteine | 1 | 2005 |
| ProANP | 1 | 2014 |
| Pro-ENK | 1 | 2013 |
| RBP | 1 | 2013 |
| RCG-32 | 1 | 2014 |
| s2-microglobulin | 1 | 2013 |
| sCD25 | 1 | 2014 |
| SDMA | 1 | 2012 |
| Serum uric acid | 1 | 2012 |
| TGF-β1 | 1 | 2012 |
| Thrombomodulin | 1 | 2014 |
| TIE-2 | 1 | 2014 |
| TIMP-1 | 1 | 2013 |
| TLR4 | 1 | 2012 |
| Uric acid | 1 | 2011 |
| Urinary glutamyl | 1 | 2014 |
| Vancomycin trough | 1 | 2013 |
| VCAM-1 | 1 | 2014 |
| α1-antitrypsin | 1 | 2010 |
| α1-GST | 1 | 2010 |
Appendix 3 AKI-Diagnostics sample search strategy: search 2 – evidence for candidate tests
ClinicalTrials.gov (via the US National Institutes of Health; Advanced Search Interface)
Searched 30 November 2015.
Search strategy
-
Search Terms: NGAL OR Lipocalin OR LCN2 OR uNGAL OR sNGAL OR siderocalin OR ‘oncogene 24p3’ OR ‘cystatin c’ OR ‘gamma trace’ OR ‘Neuroendocrine basic polypeptide’ OR ‘Post-gamma-globulin’
-
Search Terms: ‘IL 18’ OR ‘Interleukin 18’ OR ‘Iboctadekin’ OR ‘Interferon gamma-inducing factor’ OR ‘Interleukin-1 gamma’ OR KIM-1 OR TIMD1 OR TIM-1 OR ‘Hepatitis A virus cellular receptor 1’ OR ‘kidney injury molecule 1’
-
Search Terms: L-FABP OR ‘Fatty acid-binding protein 1’ OR (Liver AND Fatty acid-binding protein) OR NAG OR ‘Bifunctional protein’ OR ncoat OR (‘N-acetyl’ AND glucosaminidase) OR OGA OR O-GlcNAcase OR ‘Meningioma-expressed antigen 5’ OR MGEA5 OR ‘Hexosaminidase C’
-
Search Terms: ‘Interleukin 6’ OR IL-6 OR BSF-2 OR IFN-beta-2 OR ‘B-cell stimulatory factor 2’ OR ‘CTL differentiation factor’ OR ‘Hybridoma growth factor’ OR ‘Interferon beta-2’ OR BNP OR ‘B-type natriuretic peptide’
-
Search Terms: nephrocheck OR ‘Metalloproteinase inhibitor 2’ OR ‘tissue inhibitor of metalloproteinase-2’ OR timp-2 OR IGFBP7 OR IBP-7 OR IGFBP-rP1 OR ‘IGF-binding protein 7’ OR ‘MAC25 protein’ OR ‘PGI2-stimulating factor’ OR ‘Prostacyclin-stimulating factor’
-
Search Terms: ‘Tumor-derived adhesion factor’ OR ‘Tumour-derived adhesion factor’ OR ‘Tissue Necrosis Factor’ OR ‘Tumor necrosis factor’ OR ‘Tumour necrosis factor’ OR ‘TNF-alpha’ OR ‘TNF-a’
-
1 or 2 or 3 or 4 or 5 or 6
-
Conditions: Acute AND (renal OR kidney OR ‘tubular necrosis’)
-
7 AND 8
Cochrane Central Register of Controlled Trials (via Wiley Online Library) (Issue 10 of 12, October 2015)
Searched 30 November 2015.
Search strategy
#1 MeSH descriptor: [Acute Kidney Injury] explode all trees
#2 MeSH descriptor: [Acute Disease] explode all trees
#3 MeSH descriptor: [Kidney Diseases] explode all trees
#4 #2 and #3
#5 (Acute near/3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)):ti,ab
#6 (Acute near/3 (renal disease* or renal injury or renal failure or renal dysfunction)):ti,ab
#7 ((Acute near/3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’):ti,ab
#8 (AKI):ti,ab
#9 (‘contrast induced nephropathy’):ti,ab
#10 {or #1, #4-#9}
#11 (renal or kidney* or nephr*):ti,ab
#12 #1 or #4
#13 #11 or #12
#14 (reperfusion near/5 (injur* or isch?emi*)):ti,ab
#15 MeSH descriptor: [Reperfusion Injury] explode all trees
#16 (‘delayed graft function*’):ti,ab
#17 MeSH descriptor: [Delayed Graft Function] explode all trees
#18 {or #14-#17} and #13
#19 #10 or #18 Publication Year from 2004 to 2014
#20 MeSH descriptor: [Lipocalins] this term only
#21 (NGAL or uNGAL or sNGAL or ‘Neutrophil gelatinase-associated lipocalin’ or ‘neutrophil gelatinase lipocalin’ or ‘lipocalin 2’ or lcn2 or ‘Oncogene 24p3’ or siderocalin):ti,ab
#22 MeSH descriptor: [Cystatin C] this term only
#23 (‘cystatin c’ or ‘Gamma trace’ or ‘Neuroendocrine basic polypeptide’ or ‘Post-gamma-globulin’):ti,ab
#24 MeSH descriptor: [Interleukin-18] this term only
#25 (‘IL 18’ or ‘Interleukin 18’ or ‘Iboctadekin’ or ‘Interferon gamma-inducing factor’ or ‘Interleukin-1 gamma’):ti,ab
#26 (KIM-1 or TIMD1 or TIM-1 or ‘Hepatitis A virus cellular receptor 1’ or ‘kidney injury molecule 1’):ti,ab
#27 MeSH descriptor: [Fatty Acid-Binding Proteins] explode all trees
#28 (L-FABP or ‘Fatty acid-binding protein 1’ or (Liver near/2 (‘Fatty acid-binding protein’))):ti,ab
#29 MeSH descriptor: [Acetylglucosaminidase] this term only
#30 (NAG or ‘Bifunctional protein*’ or ncoat* or ((‘N-acetyl’) near/3 (glucosaminidase)) or OGA or O-GlcNAcase or ‘Meningioma-expressed antigen 5’ or MGEA5 or ‘Hexosaminidase C’ or ‘Histone acetyltransferase’):ti,ab
#31 MeSH descriptor: [Interleukin-6] this term only
#32 (‘Interleukin 6’ or IL-6 or BSF-2 or IFN-beta-2 or ‘B-cell stimulatory factor 2’ or ‘CTL differentiation factor’ or ‘Hybridoma growth factor’ or ‘Interferon beta-2’):ti,ab
#33 MeSH descriptor: [Natriuretic Peptide, Brain] this term only
#34 (‘B-type natriuretic peptide*’ or BNP):ti,ab
#35 MeSH descriptor: [Tissue Inhibitor of Metalloproteinase-2] this term only
#36 (nephrocheck or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’ or timp-2 or IGFBP7 or IBP-7 or IGFBP-rP1 or ‘IGF-binding protein 7’ or ‘MAC25 protein’ or ‘PGI2-stimulating factor’ or ‘Prostacyclin-stimulating factor’ or ‘Tumo*r-derived adhesion factor’):ti,ab
#37 MeSH descriptor: [Tumor Necrosis Factor-alpha] explode all trees
#38 (‘Tissue Necrosis Factor’ or ‘Tumo*r necrosis factor’ or ‘TNF-alpha’ or ‘TNF-a’):ti,ab
#39 {or #20-#38}
#40 #19 and #39
#41 MeSH descriptor: [Animals] explode all trees
#42 MeSH descriptor: [Veterinary Medicine] explode all trees
#43 MeSH descriptor: [Animal Experimentation] explode all trees
#44 MeSH descriptor: [Humans] explode all trees
#45 {or #41-#43} not #44
#46 #40 not #45
Cochrane Database of Systematic Reviews (via Wiley Online Library) (Issue 11 of 12, November 2015)
Searched 30 November 2015.
Same search as for Cochrane Central Register of Controlled Trials.
Database of Abstracts of Reviews of Effects (via Wiley Online Library) (Issue 2 of 4, April 2015)
Searched 30 November 2015.
Same search as for Cochrane Database of Systematic Reviews.
EMBASE Classic + EMBASE (via Ovid) (1947 to 24 November 2015)
Searched 29 November 2015.
Search strategy
-
exp *acute kidney failure/ (25,748)
-
exp *kidney tubule necrosis/ (1746)
-
exp acute disease/ and exp *kidney disease/ (2978)
-
exp *kidney injury/ (9863)
-
exp *contrast induced nephropathy/ (1397)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (13,009)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (30,609)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (4801)
-
aki.tw. (7002)
-
‘contrast induced nephropathy’.tw. (1935)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (65,453)
-
exp *reperfusion injury/ (20910)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (55,051)
-
exp *delayed graft function/ (526)
-
‘delayed graft function*’.tw. (3794)
-
or/13-15 (58,543)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (1,017,273)
-
1 or 2 or 3 or 4 or 5 or 17 (1,020,779)
-
16 and 18 (9998)
-
11 or 19 (72,803)
-
11 or 19 [AKI] (72,803)
-
neutrophil gelatinase associated lipocalin/ (3844)
-
(NGAL or uNGAL or sNGAL).tw. (2362)
-
(‘Neutrophil gelatinase-associated lipocalin’ or ‘neutrophil gelatinase lipocalin’ or ‘lipocalin 2’ or lcn2).tw,tn. (2938)
-
Oncogene 24p3.tw,tn. (5)
-
siderocalin.tw,tn. (51)
-
or/22-26 [NGAL] (4430)
-
cystatin C/ (6428)
-
cystatin c.tw. (5201)
-
Gamma trace.tw. (20)
-
Neuroendocrine basic polypeptide.tw. (0)
-
Post-gamma-globulin.tw. (7)
-
or/30-34 [Cystatin C] (6843)
-
interleukin 18/ (9916)
-
IL 18.tw. (7741)
-
Interleukin 18.tw. (2711)
-
Iboctadekin.tw. (3)
-
Interferon gamma-inducing factor.tw. (28)
-
Interleukin-1 gamma.tw. (2)
-
or/38-43 [IL-18] (12,384)
-
kidney injury molecule 1/ (1117)
-
(KIM-1 or TIMD1 or TIM-1).tw. (1045)
-
Hepatitis A virus cellular receptor 1.tw. (20)
-
kidney injury molecule 1.tw. (653)
-
‘T-cell immunoglobulin and mucin domain-containing protein 1’.tw. (0)
-
‘T-cell immunoglobulin mucin receptor 1’.tw. (0)
-
‘T-cell membrane protein 1’.tw. (0)
-
or/47-53 [KIM-1] (1689)
-
fatty acid binding protein/ (5893)
-
L-FABP.tw. (662)
-
Fatty acid-binding protein 1.tw. (42)
-
(Liver adj2 ‘Fatty acid-binding protein’).tw. (782)
-
or/58-61 [L-FABP] (6065)
-
n acetyl beta glucosaminidase/ (4605)
-
Bifunctional protein*.tw. (836)
-
ncoat*.tw. (8)
-
NAG.tw. (4070)
-
(‘N-acetyl’ adj3 glucosaminidase).tw. (4830)
-
OGA.tw. (365)
-
O-GlcNAcase.tw. (408)
-
Meningioma-expressed antigen 5.tw. (2)
-
MGEA5.tw. (34)
-
Hexosaminidase C.tw. (24)
-
‘EC=3.2.1.169’.tw. (0)
-
‘EC=2.3.1.48’.tw. (0)
-
Histone acetyltransferase.tw. (2444)
-
or/65-77 [NAG] (12,925)
-
interleukin 6/ (136,429)
-
Interleukin 6.tw. (40,597)
-
IL-6.tw. (94,548)
-
BSF-2.tw. (102)
-
IFN-beta-2.tw. (21)
-
B-cell stimulatory factor 2.tw. (86)
-
CTL differentiation factor.tw. (4)
-
Hybridoma growth factor.tw. (55)
-
Interferon beta-2.tw. (30)
-
or/81-89 [IL-6] (149,048)
-
brain natriuretic peptide/ (17,988)
-
B-type natriuretic peptide*.tw. (6572)
-
BNP.tw. (13,985)
-
or/93-95 [BNP] (24,772)
-
nephrocheck.tw. (2)
-
‘tissue inhibitor of metalloproteinase 2’/ (4826)
-
Metalloproteinase inhibitor 2.tw. (15)
-
tissue inhibitor of metalloproteinase-2.tw. (609)
-
TIMP 2.tw. (3952)
-
somatomedin binding protein/ (5232)
-
(IGFBP7 or IBP-7 or IGFBP-rP1).tw. (330)
-
IGF-binding protein 7.tw. (12)
-
Insulin-like growth factor-binding protein 7.tw. (146)
-
MAC25 protein.tw. (5)
-
PGI2-stimulating factor.tw. (8)
-
Prostacyclin-stimulating factor.tw. (30)
-
or/99-110 [Nephrocheck TIMP-2 IGFBP7] (11,601)
-
tumor necrosis factor alpha/ (159,697)
-
Tissue Necrosis Factor.tw. (150)
-
Tumo?r necrosis factor.tw. (116,825)
-
TNF-alpha.tw. (103,358)
-
TNF-a.tw. (5983)
-
Tumour necrosis factor.tw. (19,564)
-
or/116-121 [TNF-a] (227,621)
-
27 or 35 or 44 or 54 or 62 or 78 or 90 or 96 or 111 or 122 (374,119)
-
21 and 125 (5514)
-
limit 126 to yr=‘2004 -Current’ (4843)
-
(exp animals/ or exp nonhuman/ or animal experiment/ or exp veterinary medicine/ or exp experimental animal/) not exp human/ (5,985,269)
-
127 not 131 (3236)
Health Technology Assessment database (via Wiley Online Library) (Issue 4 of 4, October 2015)
Searched 30 November 2015.
Same search as for Cochrane Database of Systematic Reviews.
Health Management Information Consortium (HMIC) (1983 to November 2015)
Searched 30 November 2015.
Same search as for MEDLINE In-Process & Other Non-Indexed Citations (via Ovid).
International Clinical Trials Registry Platform (via the World Health Organization, Advanced Search)
Searched 30 November 2015.
Search strategy
-
Title: NGAL OR Lipocalin OR LCN2 OR uNGAL OR sNGAL OR siderocalin OR ‘oncogene 24p3’ OR ‘cystatin c’ OR ‘gamma trace’ OR ‘Neuroendocrine basic polypeptide’ OR ‘Post-gamma-globulin’
-
Title: ‘IL 18’ OR ‘Interleukin 18’ OR ‘Iboctadekin’ OR ‘Interferon gamma-inducing factor’ OR ‘Interleukin-1 gamma’ OR KIM-1 OR TIMD1 OR TIM-1 OR ‘Hepatitis A virus cellular receptor 1’ OR ‘kidney injury molecule 1’
-
Title: L-FABP OR ‘Fatty acid-binding protein’ OR NAG OR ‘Bifunctional protein’ OR ncoat OR (‘N-acetyl’ AND glucosaminidase) OR O-GlcNAcase OR ‘Meningioma-expressed antigen 5’ OR MGEA5 OR ‘Hexosaminidase C’
-
Title: ‘Interleukin 6’ OR IL-6 OR BSF-2 OR IFN-beta-2 OR ‘B-cell stimulatory factor 2’ OR ‘CTL differentiation factor’ OR ‘Hybridoma growth factor’ OR ‘Interferon beta-2’ OR BNP OR ‘B-type natriuretic peptide’
-
Title: nephrocheck OR ‘Metalloproteinase inhibitor 2’ OR ‘tissue inhibitor of metalloproteinase-2’ OR timp-2 OR IGFBP7 OR IBP-7 OR IGFBP-rP1 OR ‘IGF-binding protein 7’ OR ‘MAC25 protein’ OR ‘PGI2-stimulating factor’ OR ‘Prostacyclin-stimulating factor’
-
Title: ‘Tumor-derived adhesion factor’ OR ‘Tumour-derived adhesion factor’ OR ‘Tissue Necrosis Factor’ OR ‘Tumor necrosis factor’ OR ‘Tumour necrosis factor’ OR ‘TNF-alpha’ OR ‘TNF-a’
-
1 or 2 or 3 or 4 or 5 or 6
-
Condition: acute kidney OR acute renal OR acute tubular necrosis OR AKI
-
7 and 8
MEDLINE (via Ovid) (1946 to November Week 2 2015)
Searched 29 November 2015.
Search strategy
-
Acute Disease/ and exp Kidney Diseases/ (7632)
-
acute kidney injury/ (32,719)
-
kidney tubular necrosis, acute/ (2173)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (6843)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (21,178)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3393)
-
aki.tw. (3147)
-
‘contrast induced nephropathy’.tw. (971)
-
or/1-8 (51,426)
-
reperfusion injury/ (20,176)
-
reperfusion/ (4154)
-
exp Delayed Graft Function/ (663)
-
‘delayed graft function*’.tw. (2118)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (39,781)
-
or/10-14 (48,027)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (689,988)
-
1 or 2 or 3 or 16 (697,761)
-
15 and 17 (7094)
-
9 or 18 [AKI] (56,709)
-
Lipocalins/ (2456)
-
(NGAL or uNGAL or sNGAL).tw. (1018)
-
(‘Neutrophil gelatinase-associated lipocalin’ or ‘neutrophil gelatinase lipocalin’ or ‘lipocalin 2’ or lcn2).tw,nm. (1885)
-
Oncogene 24p3.tw,nm. (4)
-
siderocalin.tw,nm. (42)
-
or/20-24 [NGAL] (2982)
-
Cystatin C/ (2620)
-
cystatin c.tw,nm. (3499)
-
Gamma trace.tw,nm. (56)
-
Neuroendocrine basic polypeptide.tw,nm. (0)
-
Post-gamma-globulin.tw,nm. (11)
-
or/26-30 [Cystatin C] (3531)
-
Interleukin-18/ (3995)
-
IL 18.tw,nm. (5449)
-
Interleukin 18.tw,nm. (4423)
-
Iboctadekin.tw,nm. (0)
-
Interferon gamma-inducing factor.tw,nm. (72)
-
Interleukin-1 gamma.tw,nm. (6)
-
or/32-37 [IL 18] (6372)
-
Hepatitis A virus cellular receptor 1.tw,nm. (19)
-
(KIM-1 or TIMD1 or TIM-1).tw,nm. (539)
-
kidney injury molecule 1.tw,nm. (381)
-
‘T-cell immunoglobulin and mucin domain-containing protein 1’.tw,nm. (0)
-
‘T-cell immunoglobulin mucin receptor 1’.tw,nm. (0)
-
‘T-cell membrane protein 1’.tw,nm. (0)
-
or/39-44 [KIM-1] (700)
-
fatty acid-binding proteins/ or myelin p2 protein/ (4046)
-
L-FABP.tw. (499)
-
Fatty acid-binding protein 1.tw,nm. (30)
-
(Liver adj2 ‘Fatty acid-binding protein’).tw,nm. (621)
-
or/46-49 [L-FABP] (4232)
-
Acetylglucosaminidase/ (4857)
-
Bifunctional protein*.tw,nm. (770)
-
ncoat*.tw,nm. (7)
-
NAG.tw. (2998)
-
(‘N-acetyl’ adj3 glucosaminidase).tw,nm. (4250)
-
OGA.tw. (194)
-
O-GlcNAcase.tw,nm. (242)
-
Meningioma-expressed antigen 5.tw,nm. (1)
-
MGEA5.tw. (21)
-
Hexosaminidase C.tw,nm. (163)
-
‘EC=3.2.1.169’.tw,nm. (0)
-
‘EC=2.3.1.48’.tw,nm. (0)
-
Histone acetyltransferase.tw,nm. (2068)
-
or/51-63 [NAG] (11,007)
-
Interleukin-6/ (46701)
-
Interleukin 6.tw,nm. (57,499)
-
IL-6.tw. (63,124)
-
BSF-2.tw. (101)
-
IFN-beta-2.tw,nm. (103)
-
B-cell stimulatory factor 2.tw,nm. (82)
-
CTL differentiation factor.tw,nm. (5)
-
Hybridoma growth factor.tw,nm. (55)
-
Interferon beta-2.tw,nm. (80)
-
or/65-73 [IL-6] (82,477)
-
Natriuretic Peptide, Brain/ (10,025)
-
B-type natriuretic peptide*.tw,nm. (4073)
-
BNP.tw. (6475)
-
or/75-77 [BNP] (12,102)
-
nephrocheck.tw,nm. (0)
-
Metalloproteinase inhibitor 2.tw,nm. (8)
-
‘Tissue Inhibitor of Metalloproteinase-2’/ (2890)
-
tissue inhibitor of metalloproteinase-2.tw,nm. (3043)
-
TIMP 2.tw. (3181)
-
(IGFBP7 or IBP-7 or IGFBP-rP1).tw. (208)
-
IGF-binding protein 7.tw,nm. (10)
-
Insulin-like growth factor-binding protein 7.tw,nm. (84)
-
MAC25 protein.tw,nm. (5)
-
PGI2-stimulating factor.tw,nm. (6)
-
Prostacyclin-stimulating factor.tw,nm. (28)
-
Tumor-derived adhesion factor.tw,nm. (14)
-
or/79-90 [Nephrocheck TIMP 2 IGFBP7] (4440)
-
Tumor Necrosis Factor-alpha/ (97,576)
-
Tissue Necrosis Factor.tw,nm. (115)
-
‘Tumor necrosis factor’.tw,nm. (136,471)
-
TNF-alpha.tw. (79,914)
-
TNF-a.tw. (1226)
-
or/92-96 [TNF-a] (156,262)
-
25 or 31 or 38 or 45 or 50 or 64 or 74 or 78 or 91 or 97 [10 Selected Biomarkers] (242,639)
-
19 and 98 (2776)
-
limit 99 to yr=‘2004 -Current’ (2219)
-
(exp animals/ or exp Veterinary Medicine/ or exp Animal Experimentation/) not exp humans/ (3,990,735)
-
100 not 101 [10 Biomarkers and AKI, 2004+, not animals studies] (1383)
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) (24 November 2015)
Searched 29 November 2015.
Search strategy
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (1612)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (1032)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (150)
-
aki.tw. (872)
-
‘contrast induced nephropathy’.tw. (147)
-
or/1-5 (2805)
-
‘delayed graft function*’.tw. (169)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (2578)
-
7 or 8 (2733)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (39,482)
-
9 and 10 (526)
-
6 or 11 [AKI] (3193)
-
(NGAL or uNGAL or sNGAL).tw. (235)
-
(‘Neutrophil gelatinase-associated lipocalin’ or ‘neutrophil gelatinase lipocalin’ or ‘lipocalin 2’ or lcn2).tw,nm. (312)
-
Oncogene 24p3.tw,nm. (0)
-
siderocalin.tw,nm. (2)
-
or/13-16 [NGAL] (351)
-
cystatin c.tw,nm. (397)
-
Gamma trace.tw,nm. (0)
-
Neuroendocrine basic polypeptide.tw,nm. (0)
-
Post-gamma-globulin.tw,nm. (0)
-
or/18-21 [Cystatin C] (397)
-
IL 18.tw,nm. (419)
-
Interleukin 18.tw,nm. (134)
-
Iboctadekin.tw,nm. (0)
-
Interferon gamma-inducing factor.tw,nm. (1)
-
Interleukin-1 gamma.tw,nm. (0)
-
or/23-27 [IL-18] (455)
-
Hepatitis A virus cellular receptor 1.tw,nm. (0)
-
(KIM-1 or TIMD1 or TIM-1).tw,nm. (95)
-
kidney injury molecule 1.tw,nm. (73)
-
‘T-cell immunoglobulin and mucin domain-containing protein 1’.tw,nm. (0)
-
‘T-cell immunoglobulin mucin receptor 1’.tw,nm. (0)
-
‘T-cell membrane protein 1’.tw,nm. (0)
-
or/29-34 [KIM-1] (122)
-
L-FABP.tw. (39)
-
Fatty acid-binding protein 1.tw,nm. (2)
-
(Liver adj2 ‘Fatty acid-binding protein’).tw,nm. (41)
-
or/36-38 [L-FABP] (59)
-
Bifunctional protein*.tw,nm. (19)
-
ncoat*.tw,nm. (0)
-
NAG.tw. (171)
-
(‘N-acetyl’ adj3 glucosaminidase).tw,nm. (133)
-
OGA.tw. (25)
-
O-GlcNAcase.tw,nm. (25)
-
Meningioma-expressed antigen 5.tw,nm. (0)
-
MGEA5.tw. (1)
-
Hexosaminidase C.tw,nm. (0)
-
‘EC=3.2.1.169’.tw,nm. (0)
-
‘EC=2.3.1.48’.tw,nm. (0)
-
Histone acetyltransferase.tw,nm. (132)
-
or/40-51 [NAG] (417)
-
Interleukin 6.tw,nm. (2351)
-
IL-6.tw. (5580)
-
BSF-2.tw. (0)
-
IFN-beta-2.tw,nm. (1)
-
B-cell stimulatory factor 2.tw,nm. (1)
-
CTL differentiation factor.tw,nm. (0)
-
Hybridoma growth factor.tw,nm. (1)
-
Interferon beta-2.tw,nm. (1)
-
or/53-60 [IL-6] (6473)
-
B-type natriuretic peptide*.tw,nm. (433)
-
BNP.tw. (572)
-
or/62-63 [BNP] (800)
-
nephrocheck.tw,nm. (0)
-
Metalloproteinase inhibitor 2.tw,nm. (2)
-
tissue inhibitor of metalloproteinase-2.tw,nm. (24)
-
TIMP 2.tw. (168)
-
(IGFBP7 or IBP-7 or IGFBP-rP1).tw. (29)
-
IGF-binding protein 7.tw,nm. (1)
-
Insulin-like growth factor-binding protein 7.tw,nm. (14)
-
MAC25 protein.tw,nm. (0)
-
PGI2-stimulating factor.tw,nm. (0)
-
Prostacyclin-stimulating factor.tw,nm. (0)
-
Tumo?r-derived adhesion factor.tw,nm. (1)
-
or/65-75 [Nephrocheck TIMP-2 IGFBP7] (206)
-
Tissue Necrosis Factor.tw,nm. (15)
-
‘Tumo?r necrosis factor’.tw,nm. (5964)
-
TNF-alpha.tw. (6211)
-
TNF-a.tw. (108)
-
or/77-80 [TNF-a] (9432)
-
17 or 22 or 28 or 35 or 39 or 52 or 61 or 64 or 76 or 81 [10 biomarkers] (15,155)
-
12 and 82 [AKI and Biomarkers] (319)
-
((rat or rats or swine or pigs or pig or mice or mouse) not (human* or patient*)).tw. (66,543)
-
83 not 84 (230)
-
limit 85 to yr=‘2004 -Current’ [10 biomarkes and AKI, 2004+, not animals] (227)
NHS Economic Evaluation Database (via Wiley Online Library) (Issue 2 of 4, April 2015)
Searched 30 November 2015.
Same search as for Cochrane Database of Systematic Reviews.
PubMed (via the US National Library of Medicine) (1946 to November 2015)
Searched 30 November 2015.
Search strategy
#1 Search (‘acute kidney’[Title] OR ‘acute renal’[Title] OR ‘acute tubular necrosis’[Title] OR ‘aki’[Title] OR ‘delayed graft function’[Title] OR ‘reperfusion injur*’[Title]) 18,005
#2 Search (((((NGAL[Title] OR Lipocalin[Title] OR LCN2[Title] OR uNGAL[Title] OR sNGAL[Title] OR siderocalin[Title] OR ‘oncogene 24p3’[Title] OR ‘cystatin c’[Title] OR ‘gamma trace’[Title] OR ‘Neuroendocrine basic polypeptide’[Title] OR ‘Post-gamma-globulin’[Title] OR ‘IL 18’[Title] OR ‘Interleukin 18’[Title] OR ‘Iboctadekin’[Title] OR ‘Interferon gamma-inducing factor’[Title] OR ‘Interleukin-1 gamma’[Title] OR KIM-1[Title] OR TIMD1[Title] OR TIM-1[Title] OR ‘Hepatitis A virus cellular receptor 1’[Title] OR ‘kidney injury molecule 1’[Title])) OR (L-FABP[Title] OR ‘Fatty acid-binding protein 1’[Title] OR (Liver[Title] AND Fatty acid-binding protein)[Title] OR NAG[Title] OR ‘Bifunctional protein’[Title] OR ncoat[Title] OR (‘N-acetyl’[Title] AND glucosaminidase)[Title] OR OGA[Title] OR O-GlcNAcase[Title] OR ‘Meningioma-expressed antigen 5’[Title] OR MGEA5[Title] OR ‘Hexosaminidase C’[Title])) OR (‘Interleukin 6’[Title] OR IL-6[Title] OR BSF-2[Title] OR IFN-beta-2[Title] OR ‘B-cell stimulatory factor 2’[Title] OR ‘CTL differentiation factor’[Title] OR ‘Hybridoma growth factor’[Title] OR ‘Interferon beta-2’[Title] OR BNP[Title] OR ‘B-type natriuretic peptide’[Title])) OR (nephrocheck[Title] OR ‘Metalloproteinase inhibitor 2’[Title] OR ‘tissue inhibitor of metalloproteinase-2’[Title] OR timp-2[Title] OR IGFBP7[Title] OR IBP-7[Title] OR IGFBP-rP1[Title] OR ‘IGF-binding protein 7’[Title] OR ‘MAC25 protein’[Title] OR ‘PGI2-stimulating factor’[Title] OR ‘Prostacyclin-stimulating factor’[Title])) OR (‘Tumor-derived adhesion factor’[Title] OR ‘Tumour-derived adhesion factor’[Title] OR ‘Tissue Necrosis Factor’[Title] OR ‘Tumor necrosis factor’[Title] OR ‘Tumour necrosis factor’[Title] OR ‘TNF-alpha’[Title] OR ‘TNF-a’[Title]) 59,070
#3 Search (pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]) 1,881,815
#4 Search (#1 and #2 and #4) 45
Science Citation Index (via Thomson Reuters’ Web of Science) (1900 to November 2015)
Searched 30 November 2015.
Search strategy
#1 TS=(‘acute kidney injury’ or AKI) OR TS=((Acute NEAR/3 (‘kidney disease*’ or ‘kidney injury’ or ‘kidney failure’ or ‘kidney dysfunction’))) OR TS=((Acute NEAR/3 (‘renal disease*’ or ‘renal injury’ or ‘renal failure’ or ‘renal dysfunction’))) OR TS=(((Acute NEAR/3 (‘Tubular Necrosis’ or nephrotoxic*)) or ‘nephrotoxic injur*’)) OR TS=(‘contrast induced nephropath*’) OR TS=((reperfusion NEAR/5 (injur* or isch?emi*)) AND (renal or kidney* or nephr*)) OR TS=((‘Delayed Graft Function’) AND (renal or kidney* or nephr*))
#2 TS=(NGAL or uNGAL or sNGAL or ‘Oncogene 24p3’ or siderocalin or ‘Neutrophil gelatinase-associated lipocalin’ or ‘neutrophil gelatinase lipocalin’ or ‘lipocalin 2’ or lcn2)
#3 TOPIC: (‘cystatin c’ or ‘Gamma trace’ or ‘Neuroendocrine basic polypeptide’ or ‘Post-gamma-globulin’)
#4 TOPIC: (‘IL 18’ or ‘Interleukin 18’ or ‘Iboctadekin’ or ‘Interferon gamma-inducing factor’ or ‘Interleukin-1 gamma’)
#5 TOPIC: (KIM-1 or TIMD1 or TIM-1 or ‘Hepatitis A virus cellular receptor 1’ or ‘kidney injury molecule 1’)
#6 TOPIC: (L-FABP or ‘Fatty acid-binding protein 1’ or ((Liver near/2 (‘Fatty acid-binding protein’))))
#7 TOPIC: (NAG or ‘Bifunctional protein*’ or ncoat* or ((‘N-acetyl’) near/3 (glucosaminidase)) or OGA or O-GlcNAcase or ‘Meningioma-expressed antigen 5’ or MGEA5 or ‘Hexosaminidase C’ or ‘Histone acetyltransferase’)
#8 TOPIC: (‘Interleukin 6’ or IL-6 or BSF-2 or IFN-beta-2 or ‘B-cell stimulatory factor 2’ or ‘CTL differentiation factor’ or ‘Hybridoma growth factor’ or ‘Interferon beta-2’)
#9 TOPIC: (‘B-type natriuretic peptide*’ or BNP)
#10 TS=(nephrocheck or ‘Metalloproteinase inhibitor 2’ or ‘tissue inhibitor of metalloproteinase-2’ or timp-2 or IGFBP7 or IBP-7 or IGFBP-rP1 or ‘IGF-binding protein 7’ or ‘MAC25 protein’ or ‘PGI2-stimulating factor’ or ‘Prostacyclin-stimulating factor’ or ‘Tumo#r-derived adhesion factor’)
#11 TOPIC: (‘Tissue Necrosis Factor’ or ‘Tumo#r necrosis factor’ or ‘TNF-alpha’ or ‘TNF-a’)
#12 #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
#13 #12 AND #1
#14 TS= (mice or mouse or rat or rats or hamster* or sheep or animal* or rabbit* or dog or dogs or cat or cats or chicken* or poultry or pig or pigs or swine or swines or horse or horses or cow or cows or bovine*) NOT TS=(human* or patient* or neonate* or child*or woman or women or men or man or adolescen*)
#15 TI= (mice or mouse or rat or rats or hamster* or sheep or animal* or rabbit* or dog or dogs or cat or cats or chicken* or poultry or pig or pigs or swine or swines or horse or horses or cow or cows or bovine*)
#16 #15 OR #14
#17 #13 not #16
Conference Proceedings Citation Index – Science (via Thomson Reuters’ Web of Science) (1990 to November 2015)
Searched 30 November 2015.
Same as search for Science Citation Index.
Appendix 4 AKI-Diagnostics systematic review data extraction proforma
| Ref ID: | Reviewer initials: | Data entered: | ||||
| Citation | ||||||
|---|---|---|---|---|---|---|
| Authors: | ||||||
| Title: | ||||||
| Journal: | Year: | Pages: | ||||
| Vol: | ||||||
| Study | ||||||
| Design:a | Aim: | |||||
| Location:b | ||||||
| Funding: | Industry | Charity | Conflict of interest declared: | Yes | ||
| Government | Other | No | ||||
| Research council | Not reported | |||||
| Reporting standard: | REMARK | STARD | Other (detail) | |||
| STROBE | TRIPOD | Not reported | ||||
| Population | ||||||
| Setting: | Critical care unit | Single site | Area evaluated: | Clinical validity/utility | ||
| Emergency dept. | Multisited | Analytical validity | ||||
| Cardiac surgery | n | Cost-effectiveness | ||||
| Laboratoryc | Other (specify) | |||||
| Other (specify) | ||||||
| Study duration (months): | Inclusion criteria: | |||||
| Recruitment period: | ||||||
| Recruitment method (consecutive, random, matched, retro or prospective, matched, etc.): | Exclusion criteria: | |||||
| Details of study throughput: | CONSORT diagram | Sample size/power calculation reported: | Yes | |||
| Written statement | No | |||||
| Other (detail) | ||||||
| Not reported | ||||||
| Characteristics | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| Patient group (detail) | ||||||
| Total number | ||||||
| Number (%) with previous AKI | ||||||
| Age range | ||||||
| Mean (SD) | ||||||
| Median (IQR) | ||||||
| Number male (%) | ||||||
| Number female (%) | ||||||
| Ethnicity (%) | ||||||
| White | ||||||
| Asian | ||||||
| Black | ||||||
| Other | ||||||
| Comorbidities | ||||||
| Relevant lifestyle factors | ||||||
| Between group difference at baseline: | Yes | Baseline difference adjusted: | Yes | Follow-up duration: | ||
| No | No | |||||
| Unclear | Unclear | Median follow-up: | ||||
| Biomarker details | ||||
|---|---|---|---|---|
| Features | Biomarker 1 | Biomarker 2 | Biomarker 3 | Reference method or comparator test |
| Name of analyte | ||||
| Test name | ||||
| Manufacturer | ||||
| Sample matrix used [e.g. urine, serum, plasma (incl. type)] | ||||
| Collection timing and frequency (e.g. on admission, every 2 hours) | ||||
| Test platform/method used | ||||
| Test purpose (e.g. diagnosis, prognosis, risk prediction) | ||||
| Cut-off threshold applied | ||||
| Prespecified threshold (e.g. reported in methods) | Y/N | Y/N | Y/N | Y/N |
| Method to determine cut-off (e.g. level recommended for assay) | ||||
| Time between index and reference tests | ||||
| Assessor blind to reference test results | Y/N | Y/N | Y/N | Y/N |
| Number of samples per patient | ||||
| Number of laboratoriess used | ||||
| Test timing to AKI diagnosis | ||||
| Main findings | ||||
|---|---|---|---|---|
| Study endpoints: | ||||
| Patient outcomesa | Biomarker 1b | Biomarker 2b | Biomarker 3b | Reference method/comparatorb |
| Patient group (detail) | ||||
| Baseline kidney function | ||||
| Classification system used: (RIFLE, AKIN, KDIGO or details if other method used) | ||||
| Number (%) diagnosed with AKI | ||||
| Cause(s) of AKI [e.g. cardiac surgery (specify if on- or off-pump), low blood pressure, etc.] | ||||
| Number (%) patients at (from classification above): | ||||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| Other (as above) | ||||
| Number (%) of patients with: | ||||
| Recovery of kidney function | ||||
| Chronic kidney disease | ||||
| Renal replacement therapy | ||||
| Re-admission | ||||
| Death | ||||
| Average length of stay | ||||
| Related costing data (incl. cost of biomarker, laboratory costs, staff time, QALY data, etc.). If unsure, add page reference | ||||
| Test validity | Biomarker 1a | Biomarker 2a | Biomarker 3a | Reference method/comparatora | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total number of tests | ||||||||||
| Test failure rate | ||||||||||
| Distribution of analyte (across the population): | ||||||||||
| Mean | ||||||||||
| SD | ||||||||||
| CV% | ||||||||||
| Range | ||||||||||
| Median | ||||||||||
| Interquartile range | ||||||||||
| True positive (TP), false positive, true negative (TN), false negative (FN) vs. gold standard (or comparator) | ||||||||||
| GS + | GS – | GS + | GS – | GS + | GS – | Include only if table presented | ||||
| Test + | TP | FP | TP | FP | TP | FP | ||||
| Test – | FN | TN | FN | TN | FN | TN | ||||
| Test validity | Biomarker 1a | Biomarker 2a | Biomarker 3a | Reference method/comparatora | ||||||
| Sensitivity, 95% CIb | ||||||||||
| Specificity, 95% CIb | ||||||||||
| Likelihood ratios, 95% CIb | ||||||||||
| ROC curve present | Y/N | Y/N | Y/N | Y/N | ||||||
| AUC and 95% CIb | ||||||||||
| Univariate analysis: | ||||||||||
| Odds/hazard ratios, 95% CIb | ||||||||||
| Dichotomised or continuous | D/C | D/C | D/C | D/C | ||||||
| Multivariate analysis: | ||||||||||
| Odds/hazard ratios, 95% CIb | ||||||||||
| Dichotomised or continuous | D/C | D/C | D/C | D/C | ||||||
| Variables adjusted for (detail) | ||||||||||
| Brief summary of key findings | Notes (incl. key limitation of study) |
|---|---|
Appendix 5 QUADAS-2 assessment
Phase 1: state the review question
| Patients (setting, intended use of index test, presentation, prior testing): | ICU/ED/cardiac surgery; adult and child populations; diagnosis or prognosis of acute kidney injury |
| Index test(s): | Nephrocheck; NGAL; cystatin C |
| Reference standard and target condition: | AKI diagnosed on the basis of creatinine/urine output criteria → classification systems: KDIGO, AKIN, RIFLE |
Phase 2: draw a flow diagram for the primary study
Phase 3: risk of bias and applicability judgments
QUADAS-2 is structured so that four key domains are each rated in terms of the risk of bias and the concern regarding applicability to the research question (as defined above). Each key domain has a set of signalling questions to help reach the judgments regarding bias and applicability.
Domain 1: patient selection
A. Risk of bias
Describe methods of patient selection:
-
Was a consecutive or random sample of patients enrolled? Yes/no/unclear
-
Was a case–control design avoided? Yes/no/unclear
-
Did the study avoid inappropriate exclusions? Yes/no/unclear
Could the selection of patients have introduced bias? Risk: low/high/unclear
B. Concerns regarding applicability
Describe included patients (prior testing, presentation, intended use of index test and setting):
Is there concern that the included patients do not match the review question? Concern: low/high/unclear
Domain 2: index test(s)
If more than one index test was used, please complete for each test.
A. Risk of bias
Describe the index test and how it was conducted and interpreted:
-
Were the index test results interpreted without knowledge of the results of the reference standard? Yes/no/unclear
-
If a threshold applicability was used, was it prespecified? Yes/no/unclear
Could the conduct or interpretation of the index test have introduced bias? Risk: low/high/unclear
B. Concerns regarding applicability
Is there concern that the index test, its conduct or interpretation differ from the review question? Concern: low/high/unclear
Domain 3: reference standard
A. Risk of bias
Describe the reference standard and how it was conducted and interpreted:
-
Is the reference standard likely to correctly classify the target condition? Yes/no/unclear
-
Were the reference standard results interpreted without knowledge of the results of the index test? Yes/no/unclear
Could the reference standard, its conduct, or its interpretation have introduced bias? Risk: low/high/unclear
B. Concerns regarding applicability
Is there concern that the target condition as defined by the reference standard does not match the review question? Concern: low/high/unclear
Domain 4: flow and timing
A. Risk of bias
Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram):
Describe the time interval and any interventions between the index test(s) and the reference standard:
-
Was there an appropriate interval between the index test(s) and the reference standard? Yes/no/unclear
-
Did all patients receive a reference standard? Yes/no/unclear
-
Did patients receive the same reference standard? Yes/no/unclear
-
Were all patients included in the analysis? Yes/no/unclear
Could the patient flow have introduced bias? Risk: low/high/unclear
Appendix 6 Characteristics of eligible studies
| Study | Population | Biomarker | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | First author | Location | Outcome area | Setting (sites) | Population | Classification | Analyte | Subjects (AKI) | Sensitivity/specificity |
| 524 | Adademir 201273 | Turkey | Clinical | Cardiac (single) | Adults | Other | NGAL (urine) | 55 | N/N |
| 983 | Aghel 201074 | USA, Belgium | Clinical | Unclear (single) | Adults | Other | NGAL (serum) | 91 | Y/Y |
| 1390 | Åhlström 200475 | Finland | Clinical | Critical care (single) | Adults | ADQI Group consensus | Cystatin C (serum) | 202 | N/N |
| 7818 | Al-Beladi 201476 | Saudi Arabia | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (serum) | 84 | N/N |
| 117 | Alcaraz 201477 | Spain | Clinical/analytical | Critical care (single) | Children | RIFLE | NGAL (urine) | 106 | Y/Y |
| 125 | Alharazy 201478 | Malaysia | Clinical/analytical | Cardiac: contrast (single) | Adults | Other | NGAL (serum) | 100 | Y/Y |
| 122 | Arthur 201479 | USA | Clinical | Cardiac (multisite, n = 4) | Adults | AKIN | Cystatin C (urine)/NGAL (urine) | 105 | N/N |
| 149 | Ataei 201480 | Iran | Clinical/analytical | Critical care (single) | Children | RIFLE | Cystatin C (serum) | 107 | Y/Y |
| 349 | Aydogdu 201332 | Turkey | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 151 | Y/Y |
| 982 | Bagshaw 201081 | Australia | Clinical | Critical care (multisite, n = 2) | Adults | RIFLE | NGAL (plasma, urine) | 83 | Y/Y |
| 5501 | Bargnoux 201382 | France | Analytical | Laboratory (multisite, n = 2) | Not specified | – | NGAL (urine) | 100 | N/N |
| 20 | Basu 201483 | USA | Clinical | Critical care (multisite, n = 17) | Children | KDIGO | NGAL (plasma) | 214 | Y/Y |
| 1030 | Bell 200984 | Sweden | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (serum) | 271 | N/N |
| 11252 | Bell 201585 | Sweden | Clinical | Critical care (single) | Adults | KDIGO | Nephrocheck (urine)/NGAL (urine)/cystatin C (urine) | 94 | N/N |
| 1170 | Bennett 200886 | USA | Clinical/analytical | Cardiac (single) | Children | Other | NGAL (urine) | 196 | Y/Y |
| 82 | Bihorac 201433 | USA | Clinical | Critical care (multisite, n = 23) | Adults | KDIGO | Nephrocheck (urine) | 408 | Y/Y |
| 112 | Bojan 201487 | France | Clinical | Cardiac (single) | Children | AKIN | NGAL (urine) | 200 | Y/Y |
| 11386 | Bojic 201588 | Serbia | Clinical | Critical care (single) | Adults | KDIGO | NGAL (serum, urine) | 103 | N/N |
| 532 | Breidthardt 201289 | Switzerland | Clinical | Emergency department (multisite, n = 3) | Adults | AKIN | NGAL (plasma) | 207 | Y/Y |
| 926 | Briguori 201090 | Italy | Clinical | Cardiac: contrast (single) | Adults | AKIN | Cystatin C (serum) | 410 | Y/Y |
| 826 | Cai 201091 | Sweden | Analytical | Laboratory (single) | Not specified | – | NGAL (urine) | 38 | N/N |
| 450 | Cantinotti 201292 | Italy | Clinical | Cardiac (single) | Children | RIFLE | NGAL (urine) | 135 | Y/Y |
| 52 | Cemil 201493 | Turkey | Clinical | Emergency department (single) | Adults | Not reported | NGAL (serum) | 60 | Y/Y |
| 597 | Chen 201234 | Taiwan | Clinical | Critical care (single) | Adults | AKIN | Cystatin C (serum, urine)/NGAL (serum, urine) | 150 | Y/Y |
| 390 | Cho 201335 | South Korea | Clinical | Critical care (single) | Adults | AKIN | NGAL (urine) | 145 | Y/Y |
| 23 | Cho 201494 | South Korea | Clinical | Critical care (single) | Adults | RIFLE | NGAL (serum, urine) | 82 | N/N |
| 11582 | Chung 201595 | Sri Lanka, USA | Analytical | Laboratory (multisite, n = 2) | Adults | – | Cystatin C (urine) | 42 | N/N |
| 967 | Constantin 201036 | France | Clinical | Critical care (single) | Adults | RIFLE | NGAL (plasma) | 88 | Y/Y |
| 981 | Cruz 201096 | Italy | Clinical | Critical care (single) | Adults | RIFLE | NGAL (plasma) | 301 | Y/Y |
| 599 | Cullen 201297 | Ireland | Analytical | Laboratory (single) | Adults | – | NGAL (urine) | 174 | N/N |
| 11388 | Dai 201598 | China | Clinical | Critical care (single) | Adults | KDIGO | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 112 | N/N |
| 11338 | De Berardinis 201599 | USA, Italy, France | Clinical/analytical | Emergency department (multisite, n = 3) | Adults | RIFLE | NGAL (plasma) | 530 | Y/Y |
| 1645 | de Geus 201137 | The Netherlands | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 510 | N/N |
| 768 | de Geus 2011100 | The Netherlands | Clinical | Critical care (single) | Adults | RIFLE | NGAL (plasma, urine) | 632 | Y/Y |
| 302 | de Geus 2013101 | The Netherlands | Clinical | Critical care (single) | Adults | AKIN | NGAL (plasma) | 663 | Y/Y |
| 179 | de Geus 2013102 | The Netherlands | Clinical/analytical | Critical care (single) | Adults | AKIN | NGAL (urine) | 481 | N/N |
| 139 | Delanaye 2014103 | France, Belgium | Clinical | Critical care (multisite, n = 3) | Adults | KDIGO | Cystatin C (serum) | 51 | N/N |
| 1611 | Delcroix 2013104 | Belgium | Clinical | Cardiac (single) | Adults | KDIGO | NGAL (plasma, urine) | 50 | Y/Y |
| 1624 | Demirtas 2013105 | Turkey | Clinical | Cardiac (single) | Adults | Not reported | NGAL (not specified) | 72 | N/N |
| 1219 | Dent 2007106 | USA | Clinical/analytical | Cardiac (single) | Children | RIFLE | NGAL (plasma) | 120 | Y/Y |
| 1600 | Di Somma 2013107 | Italy | Clinical | Emergency department (multisite, n = 3) | Adults | AKIN/RIFLE | NGAL (plasma) | 665 | Y/Y |
| 669 | Doi 2011108 | Japan | Clinical | Critical care (single) | Adults | RIFLE | NGAL (urine) | 339 | N/N |
| 1594 | Doi 2013109 | Japan | Clinical | Cardiac (multisite, n = 2) | Adults | AKIN | NGAL (plasma) | 146 | Y/Y |
| 141 | Doi 2014110 | Japan | Clinical | Critical care (single) | Adults | RIFLE | NGAL (urine) | 339 | N/N |
| 525 | Ejaz 2012111 | USA | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 100 | N/N |
| 408 | El-Farghali 2012112 | Egypt | Clinical | Critical care (single) | Children | AKIN | NGAL (serum) | 60 | Y/Y |
| 1511 | Fan 2014113 | China | Clinical | Critical care (single) | Adults | RIFLE | NGAL (urine) | 126 | Y/Y |
| 11667 | Fanning 2015114 | New Zealand | Clinical | Cardiac (single) | Adults | KDIGO | NGAL (serum, urine) | 50 | N/N |
| 997 | Felicio 2009115 | Brazil | Clinical | Cardiac (single) | Adults | Not reported | Cystatin C (serum) | 50 | N/N |
| 393 | Fouad 2013116 | Egypt | Clinical | Critical care (single) | Adults | AKIN/RIFLE | Cystatin C (serum) | 100 | N/N |
| 1432 | Gaipov 2015117 | Turkey | Clinical | Cardiac (single) | Adults | KDIGO | NGAL (serum, urine) | 60 | Y/Y |
| 75 | Ghonemy 201438 | Egypt | Clinical | Cardiac (single) | Adults | RIFLE | Cystatin C (serum)/NGAL (serum) | 50 | Y/Y |
| 242 | Glassford 2013118 | Australia | Clinical/analytical | Critical care (single) | Adults | RIFLE | NGAL (plasma, urine) | 102 | N/N |
| 11804 | Gocze 2015119 | Germany | Clinical | Critical care (single) | Adults | OTHER | Nephrocheck (urine) | 107 | N/N |
| 961 | Grenier 2010120 | USA, Canada, the Netherlands | Analytical | Laboratory (multisite, n = 3) | Adults | – | NGAL (urine) | Unclear | N/N |
| 4025 | Guo 2011121 | China | Clinical | Critical care (multisite, no numbers reported) | Adults | RIFLE | NGAL (urine) | 92 | N/N |
| 1049 | Haase 200931 | Australia | Clinical | Cardiac (single) | Adults | AKIN | Cystatin C (plasma)/NGAL (urine) | 100 | Y/Y |
| 1072 | Haase-Fielitz 200939 | Australia | Clinical/analytical | Cardiac (single) | Adults | RIFLE | Cystatin C (plasma)/NGAL (urine) | 100 | Y/Y |
| 1020 | Haase-Fielitz 200940 | Australia | Clinical/analytical | Cardiac (single) | Adults | AKIN/RIFLE | NGAL (plasma) | 100 | Y/Y |
| 1622 | Hamed 2013122 | Egypt | Clinical | Critical care (single) | Children | RIFLE | Cystatin C (serum) | 62 | Y/Y |
| 1062 | Han 200941 | USA | Clinical/analytical | Cardiac (single) | Adults | AKIN (modified) | NGAL (urine) | 90 | Y/Y |
| 142 | Hansen 2014123 | Denmark | Analytical | Laboratory (multisite, n = 2) | Adults | – | NGAL (plasma, urine) | 68 | N/N |
| 526 | Hassinger 2012124 | USA | Clinical | Cardiac (single) | Children | RIFLE | Cystatin C (serum) | 100 | N/N |
| 1059 | Heise 2009125 | Germany | Clinical | Cardiac (single) | Adults | National Kidney Foundation guideline | Cystatin C (serum) | 50 | N/N |
| 1403 | Herget-Rosenthal 200442 | Germany | Clinical/analytical | Critical care (single) | Adults | RIFLE | Cystatin C (serum) | 85 | Y/Y |
| 1236 | Hirsch 2007126 | USA | Clinical/analytical | Cardiac (single) | Children | Other | NGAL (plasma, urine) | 91 | Y/Y |
| 1440 | Hjortrup 201543 | Denmark | Clinical | Critical care (multisite, n = 3) | Adults | KDIGO | NGAL (plasma, urine) | 222 | Y/Y |
| 225 | Hoffman 2013127 | USA | Clinical/analytical | Critical care (single) | Children | Other | NGAL (urine) | 62 | Y/Y |
| 207 | Hong 2013128 | South Korea | Clinical/analytical | Emergency department/critical care (single) | Adults | RIFLE | NGAL (plasma) | 45 | Y/Y |
| 1488 | Hoste 201444 | USA, Canada, Europea | Clinical | Critical care (multisite, n = 35) | Adults | KDIGO | Nephrocheck (urine) | 153 | Y/Y |
| 1574 | In 2014129 | South Korea | Clinical | Critical care (single) | Adults | RIFLE | NGAL (plasma) | 126 | Y/Y |
| 1587 | Jayaraman 2014130 | India | Clinical | Cardiac (single) | Adults | RIFLE | NGAL (urine) | 100 | N/N |
| 1531 | Johansson 2014131 | Sweden | Clinical | Cardiac (single) | Adults | RIFLE | Cystatin C (serum) | 64 | N/N |
| 247 | Kambhampati 2013132 | USA | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 100 | N/N |
| 1601 | Kashani 201345 | USA, Europea | Clinical | Critical care (multisite, n = 35) | Adults | KDIGO | Nephrocheck (urine) | 728 | N/N |
| 1139 | Kato 200846 | Japan | Clinical | Critical care (single) | Adults | Other | Cystatin C (serum) | 87 | Y/Y |
| 1609 | Khosravi 2013133 | Iran | Clinical | Critical care (single) | Adults | RIFLE | NGAL (serum) | 90 | Y/Y |
| 67 | Kidher 201447 | UK | Clinical/analytical | Cardiac (single) | Adults | RIFLE | NGAL (plasma) | 53 | Y/Y |
| 137 | Kiessling 2014134 | Germany | Clinical/analytical | Cardiac (single) | Adults | AKIN | Cystatin C (serum) | 70 | N/N |
| 296 | Kift 2013135 | UK | Analytical | Laboratory (single) | Adults | AKIN | NGAL (urine) | 78 | N/N |
| 2189 | Kim 2012136 | South Korea | Clinical | Cardiac: contrast (single) | Adults | Other | Cystatin C (serum) | 89 | Y/Y |
| 232 | Kim 2013137 | South Korea | Clinical | Emergency department/critical care (single) | Adults/children | AKIN | NGAL (plasma) | 231 | N/N |
| 720 | Koch 2011138 | Germany | Clinical | Cardiac (single) | Children | RIFLE | NGAL (plasma) | 218 | N/N |
| 1889 | Koeijers 2012139 | Curaçao | Clinical | Critical care (single) | Adults | AKIN | NGAL (urine) | 88 | Y/Y |
| 473 | Kokkoris 201248 | Greece | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (plasma)/NGAL (plasma, urine) | 100 | Y/Y |
| 1127 | Koyner 2008140 | USA | Clinical | Cardiac (single) | Adults | Other | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 72 | Y/Y |
| 827 | Koyner 2010141 | USA | Clinical | Cardiac (single) | Adults | AKIN | Cystatin C (urine)/NGAL (urine) | 123 | N/N |
| 2483 | Koyner 2012142 | USA | Clinical | Cardiac (multisite, n = 6) | Adults | AKIN | NGAL (plasma, urine) | 380 | N/N |
| 341 | Koyner 2013143 | USA | Clinical | Cardiac (multisite, n = 8) | Adults/children | AKIN/RIFLE | Cystatin C (urine) | 1502 | N/N |
| 3999 | Koyner 2014144 | USA, Europea | Clinical | Critical care (multisite, n = 35) | Adults | KDIGO | Nephrocheck (urine) | 692 | N/N |
| 11469 | Koyner 2015145 | USA | Clinical | Critical care (unclear) | Adults | AKIN | Nephrocheck (urine)/NGAL (plasma, urine) | 77 | N/N |
| 871 | Krawczeski 2010146 | USA | Clinical | Cardiac (single) | Children | RIFLE | Cystatin C (serum) | 374 | Y/Y |
| 657 | Krawczeski 2011147 | USA | Clinical | Cardiac (single) | Children | AKIN/RIFLE | NGAL (urine) | 220 | N/N |
| 745 | Krawczeski 2011148 | USA | Clinical/analytical | Cardiac (single) | Children | AKIN/RIFLE | NGAL (plasma, urine) | 373 | Y/Y |
| 1961 | Lacquaniti 2013149 | Italy | Clinical | Cardiac: contrast (single) | Adults | Other | NGAL (serum, urine) | 120 | Y/Y |
| 4002 | Lagos-Arevalo 2014150 | Canada | Clinical/analytical | Critical care (single) | Children | KDIGO | Cystatin C (serum, urine)/NGAL (urine) | 160 | Y/Y |
| 840 | Lassus 2010151 | Finland | Clinical | Emergency department (multisite, n = 14) | Adults | AKIN/RIFLE | Cystatin C (serum) | 292 | Y/Y |
| 5108 | Legrand 2014152 | USA, Italy | Clinical | Emergency department (multisite, n = 2) | Adults | RIFLE | Cystatin C (urine)/NGAL (plasma) | 87 | N/N |
| 5087 | Legrand 201549 | France | Clinical | Critical care (single) | Adults | KDIGO | Cystatin C (plasma)/NGAL (plasma, urine) | 111 | Y/Y |
| 1500 | Lewandowska 2014153 | Poland | Clinical/analytical | Critical care (single) | Adults | RIFLE | NGAL (urine) | 63 | Y/Y |
| 1650 | Li 2010154 | China | Clinical | Emergency department (single) | Adults | RIFLE | Cystatin C (serum) | 71 | N/N |
| 1607 | Li 2013155 | China | Clinical | Critical care (single) | Adults | AKIN | NGAL (urine) | 55 | Y/Y |
| 1035 | Liangos 200950 | USA | Clinical/analytical | Cardiac (multisite, n = 2) | Adults | AKIN (modified) | Cystatin C (urine)/NGAL (urine) | 103 | Y/Y |
| 263 | Liebetrau 2013156 | Germany | Clinical/analytical | Cardiac (single) | Adults | KDIGO | Cystatin C (plasma)/NGAL (urine) | 141 | N/N |
| 97 | Liebetrau 2014157 | Germany | Clinical/analytical | Cardiac: contrast (single) | Adults | KDIGO | Cystatin C (plasma)/NGAL (urine) | 128 | N/N |
| 283 | Linko 201351 | Finland | Clinical | Critical care (multisite, n = 25) | Adults | RIFLE | NGAL (plasma) | 369 | Y/Y |
| 392 | Liu 201352 | China | Clinical/analytical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 109 | Y/Y |
| 591 | Macdonald 2012158 | Australia | Clinical/analytical | Emergency department (multisite, n = 2) | Adults | RIFLE | NGAL (plasma) | 102 | Y/Y |
| 237 | Magro 2013159 | Brazil | Clinical | Critical care (single) | Adults | AKIN/RIFLE | Cystatin C (serum) | 121 | N/N |
| 275 | Makris 2013160 | Greece | Analytical | Laboratory (single) | Adults | – | Cystatin C (urine) | 130 | N/N |
| 1434 | Malyszko 2015161 | Poland | Clinical | Cardiac: contrast (single) | Adults | Other | Cystatin C (serum)/NGAL (serum, urine) | 89 | N/N |
| 793 | Manzano-Fernandez 2011162 | Spain | Clinical/analytical | Unclear (single) | Adults | Other | Cystatin C (plasma) | 20 | Y/Y |
| 1528 | Marcelino 2014163 | Portugal | Clinical | Critical care (single) | Adults | AKIN | NGAL (urine) | 61 | Y/Y |
| 607 | Martensson 2012164 | Sweden | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (serum) | 327 | N/N |
| 4001 | Martensson 2014165 | Australia | Clinical | Critical care (single) | Adults | RIFLE | NGAL (urine) | 102 | N/N |
| 1519 | Matsa 2014166 | UK | Clinical | Critical care (single) | Adults | RIFLE | NGAL (plasma, urine) | 194 | Y/Y |
| 534 | McCullough 2012167 | USA | Clinical | Cardiac: contrast (multisite, n = 3) | Adults | Other | NGAL (plasma) | 63 | N/N |
| 979 | McIlroy 201053 | USA | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 426 | Y/Y |
| 11593 | McIlroy 2015168 | USA | Clinical | Cardiac (single) | Adults | KDIGO | NGAL (urine) | 603 | Y/Y |
| 1568 | Meersch 201454 | Germany | Clinical | Cardiac (single) | Adults | KDIGO | Nephrocheck (urine)/NGAL (urine) | 50 | Y/Y |
| 1485 | Meersch 201455 | Germany | Clinical | Cardiac (single) | Children | RIFLE | Nephrocheck (urine)/NGAL (urine) | 51 | Y/Y |
| 1592 | Merrikhi 2014169 | Iran | Clinical | Critical care (single) | Children | RIFLE | NGAL (plasma, urine) | 50 | Y/Y |
| 1369 | Mishra 2005170 | USA | Clinical | Cardiac (single) | Children | Other | NGAL (serum, urine) | 71 | Y/Y |
| 5578 | Mortara 2013171 | Italy | Clinical/analytical | Emergency department (single) | Adults | Other | NGAL (serum) | 30 | Y/Y |
| 381 | Munir 201356 | Pakistan | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 88 | Y/Y |
| 1628 | Murty 2013172 | India | Clinical | Unclear (single) | Adults | RIFLE | Cystatin C (serum) | 330 | N/N |
| 1058 | Naruse 2009173 | Japan | Clinical/analytical | Unclear (single) | Adults | K/DOQI | Cystatin C (serum) | 328 | N/N |
| 864 | Nejat 201057 | New Zealand | Clinical/analytical | Critical care (multisite, n = 2) | Adults | AKIN | Cystatin C (plasma) | 444 | N/N |
| 885 | Nejat 2010174 | New Zealand | Clinical/analytical | Critical care (multisite, n = 2) | Adults | AKIN | Cystatin C (plasma, urine) | 444 | Y/Y |
| 544 | Nejat 2012175 | Australia | Clinical | Critical care (multisite, n = 2) | Adults | AKIN | Cystatin C (urine)/NGAL (urine) | 489 | N/N |
| 6561 | Nemes 2010176 | Hungary | Clinical | Critical care (single) | Adults | Other | Cystatin C (serum) | 105 | Y/Y |
| 1166 | Nickolas 2008177 | USA | Clinical/analytical | Emergency department (single) | Adults | RIFLE | NGAL (urine) | 635 | Y/Y |
| 624 | Nickolas 2012178 | USA, Germany | Clinical/analytical | Emergency department (multisite, n = 3) | Adults | RIFLE | Cystatin C (urine)/NGAL (urine) | 1635 | Y/Y |
| 64 | Nisula 2014179 | Finland | Clinical | Critical care (multisite, no numbers reported) | Adults | KDIGO | NGAL (urine) | 1042 | Y/Y |
| 554 | Oh 201258 | South Korea | Clinical | Cardiac (single) | Adults | Other | NGAL (urine) | 71 | Y/Y |
| 72 | Omerika 2014180 | Bosnia Herzegovina | Clinical | Cardiac (single) | Adults | RIFLE | NGAL (urine) | 150 | N/N |
| 4004 | Ortuno-Anderiz 2015181 | Spain | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (plasma) | 50 | N/N |
| 1578 | Ozkan 2014182 | Turkey | Clinical | Emergency department (single) | Adults | Not reported | NGAL (serum) | 100 | N/N |
| 11344 | Palazzuoli 201559 | Italy | Clinical | Unclear (single) | Adults | AKIN | Cystatin C (plasma)/NGAL (plasma) | 203 | Y/Y |
| 695 | Parikh 201160 | USA, Canada | Clinical | Cardiac (multisite, n = 6) | Adults | RIFLE | NGAL (plasma, urine) | 1219 | Y/Y |
| 348 | Park 2013183 | South Korea | Clinical | Unclear (single) | Adults | AKIN | Cystatin C (plasma, urine) | 213 | Y/Y |
| 1453 | Park 201561 | South Korea | Clinical | Cardiac (single) | Adults | RIFLE | NGAL (plasma) | 189 | Y/Y |
| 252 | Peco-Antic 2013184 | Serbia | Clinical | Cardiac (single) | Children | RIFLE | Cystatin C (serum)/NGAL (serum, urine) | 112 | N/N |
| 875 | Pedersen 2010185 | Denmark | Analytical | Cardiac (single) | Adults/children | – | NGAL (plasma, urine) | 17 | N/N |
| 1004 | Perianayagam 2009186 | USA | Clinical/analytical | Critical care (multisite, n = 2) | Adults | Other | Cystatin C (serum) | 200 | N/N |
| 3997 | Perrotti 201562 | France | Clinical | Cardiac (single) | Adults | Other | NGAL (plasma) | 166 | Y/Y |
| 921 | Perry 201063 | USA | Clinical | Cardiac (multisite, n = 2) | Adults | ADQI Group consensus | NGAL (plasma) | 879 | Y/Y |
| 11359 | Petrovic 2015187 | Serbia | CEA | Cardiac (single) | Children | RIFLE | Cystatin C (serum)/NGAL (urine) | 112 | Y/Y |
| 260 | Pickering 2013188 | New Zealand | Clinical | Critical care (multisite, n = 2) | Adults | KDIGO | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 528 | N/N |
| 13 | Pipili 2014189 | Greece | Clinical | Critical care (single) | Adults | RIFLE | Cystatin C (serum)/NGAL (urine) | 106 | N/N |
| 11327 | Prowle 201564 | Australia | Clinical | Cardiac (single) | Adults | RIFLE | Cystatin C (serum)/NGAL (urine) | 93 | Y/Y |
| 11325 | Ralib 2014190 | New Zealand | Clinical | Emergency department (single) | Adults | KDIGO | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 77 | N/N |
| 11863 | Rewa 2015191 | Canada | Clinical | Critical care (multisite, n = 5) | Adults | KDIGO (modified) | NGAL (whole blood) | 227 | Y/Y |
| 615 | Ribichini 2012192 | Italy | Clinical/analytical | Cardiac: contrast (single) | Adults | Other | Cystatin C (serum) | 166 | Y/Y |
| 442 | Ricci 2012193 | Italy | Clinical | Cardiac (single) | Children | RIFLE | NGAL (whole blood) | 160 | Y/Y |
| 978 | Ristikankare 2010194 | Finland | Clinical | Cardiac (single) | Adults | RIFLE | Cystatin C (serum) | 110 | N/N |
| 790 | Royakkers 2011195 | The Netherlands | Clinical | Critical care (multisite, n = 5) | Adults | RIFLE | Cystatin C (serum, urine) | 151 | N/N |
| 1638 | Royakkers 2012196 | The Netherlands | Clinical | Critical care (multisite, n = 5) | Adults | RIFLE | NGAL (serum, urine) | 140 | N/N |
| 11514 | Ruf 2015197 | Germany | Clinical | Cardiac (single) | Children | RIFLE | Cystatin C (serum)/NGAL (plasma, urine) | 59 | N/N |
| 5666 | Rybi-Szuminska 2013198 | Poland | Analytical | Laboratory (multisite, no numbers reported) | Children | – | NGAL (urine) | 172 | N/N |
| 10309 | Sagheb 2014199 | Iran | Clinical | Critical care (single) | Adults | Other | Cystatin C (serum) | 80 | N/N |
| 465 | Sargentini 201265 | Italy | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 52 | Y/Y |
| 11626 | Schaub 2015200 | USA, Canada | Clinical | Cardiac (multisite, n = 6) | Adults | AKIN | NGAL (urine) | 959 | N/N |
| 316 | Schinstock 2013201 | USA | Clinical/analytical | Emergency department (single) | Adults | AKIN | NGAL (urine) | 363 | Y/Y |
| 432 | Schnell 2012202 | France | Clinical | Critical care (multisite, n = 2) | Adults | AKIN | Cystatin C (serum, urine) | 58 | N/N |
| 386 | Seitz 2013203 | Germany | Clinical | Cardiac (single) | Children | RIFLE | Cystatin C (serum)/NGAL (urine) | 139 | Y/Y |
| 899 | Shapiro 2010204 | USA | Clinical | Emergency department (multisite, n = 10) | Adults | RIFLE | NGAL (plasma) | 661 | Y/Y |
| 654 | Shaw 2011205 | USA | CEA | Cardiac (unclear) | Adults | RIFLE | NGAL (urine) | Unclear | Y/Y |
| 1649 | Shi 2010206 | China | Clinical | Critical care (multisite, n = 2) | Adults | AKIN | Cystatin C (serum) | 98 | N/N |
| 705 | Shlipak 2011207 | USA | Clinical | Cardiac (multisite, n = 6) | Adults | AKIN | Cystatin C (serum) | 1147 | N/N |
| 463 | Shrestha 2012207 | USA | Clinical | Critical care (single) | Adults | AKIN/RIFLE (simplified) | NGAL (serum, urine) | 93 | Y/Y |
| 3995 | Shum 201566 | China | Clinical | Critical care (single) | Adults | AKIN | NGAL (plasma) | 151 | Y/Y |
| 1040 | Siew 2009209 | USA | Clinical | Critical care (single) | Adults | AKIN | NGAL (urine) | 391 | N/N |
| 230 | Siew 2013210 | USA | Clinical/analytical | Critical care (single) | Adults | AKIN | Cystatin C (urine)/NGAL (urine) | 352 | N/N |
| 598 | Sohrabian 2012211 | Sweden | Analytical | Laboratory (single) | Adults | – | Cystatin C (urine) | 41 | N/N |
| 857 | Soto 2010212 | Portugal | Clinical | Emergency department (single) | Adults | AKIN/RIFLE | Cystatin C (plasma, urine) | 616 | Y/Y |
| 187 | Soto 2013213 | Portugal | Clinical | Emergency department (single) | Adults | AKIN/RIFLE | Cystatin C (serum)/NGAL (plasma) | 616 | Y/Y |
| 11842 | Soyler 2015214 | Turkey | Clinical | Emergency department (single) | Adults | AKIN | NGAL (urine) | 100 | Y/Y |
| 439 | Spahillari 2012215 | USA | Clinical | Cardiac (multisite, n = 6) | Adults | Other | Cystatin C (plasma) | 1150 | N/N |
| 750 | Svenmarker 2011216 | Sweden | Clinical | Cardiac (unclear) | Adults | Other | Cystatin C (serum) | 98 | N/N |
| 185 | Tasanarong 2013217 | Thailand | Clinical | Cardiac: contrast (single) | Adults | KDIGO | NGAL (urine) | 130 | Y/Y |
| 613 | Torregrosa 2012218 | Spain | Clinical/analytical | Critical care (single) | Adults | RIFLE | Cystatin C (urine)/NGAL (urine) | 135 | Y/Y |
| 1080 | Tuladhar 200967 | UK | Clinical | Cardiac (single) | Adults | ADQI Group consensus | NGAL (plasma, urine) | 50 | Y/Y |
| 11329 | Tung 201568 | Taiwan | Clinical | Cardiac (single) | Adults | AKIN | Cystatin C (serum)/NGAL (serum) | 189 | Y/Y |
| 11765 | Tziakas 201569 | Greece | Clinical/analytical | Cardiac (multisite, n = 3) | Adults | AKIN/RIFLE/KDIGO | Cystatin C (plasma, urine)/NGAL (plasma, urine) | 805 | Y/Y |
| 11253 | Varela 201570 | Argentina | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 66 | Y/Y |
| 1371 | Villa 200571 | Spain | Clinical | Critical care (single) | Adults | Other | Cystatin C (serum) | 50 | N/N |
| 11382 | Volpon 2015219 | Brazil | Clinical | Critical care (single) | Children | RIFLE | Cystatin C (serum) | 122 | Y/Y |
| 136 | Wacker-Gusmann 2014220 | Germany | Clinical | Cardiac: contrast (unclear) | Adults | AKIN/RIFLE | Cystatin C (serum) | 373 | N/N |
| 1307 | Wagener 2006221 | USA | Clinical | Cardiac (single) | Adults | ADQI Group consensus | NGAL (urine) | 81 | Y/Y |
| 1138 | Wagener 200872 | USA | Clinical | Cardiac (single) | Adults | AKIN | NGAL (urine) | 426 | Y/Y |
| 224 | Wai 2013222 | USA | Clinical | Critical care (single) | Children | RIFLE | NGAL (urine) | 60 | Y/Y |
| 882 | Wald 2010223 | USA, Canada | Clinical/analytical | Cardiac (multisite, n = 3) | Adults | AKIN (modified) | Cystatin C (plasma) | 150 | N/N |
| 6718 | Wang 2009224 | China | Clinical/analytical | Cardiac (single) | Adults | Other | Cystatin C (serum) | 61 | Y/Y |
| 1486 | Wang 2014225 | China | Clinical | Cardiac (single) | Adults | AKIN | Cystatin C (serum) | 616 | N/N |
| 1548 | Wang 2014226 | China | Clinical/analytical | Critical care (single) | Adults | AKIN | Cystatin C (serum) | 446 | Y/Y |
| 11832 | Westhoff 2015227 | Germany | Clinical | Critical care (single) | Children | RIFLE | Nephrocheck (urine) | 133 | Y/Y |
| 1183 | Wheeler 2008228 | USA | Clinical | Critical care (multisite, no numbers reported) | Children | Other | NGAL (serum) | 143 | Y/Y |
| 218 | Xiang 2013229 | China | Analytical | Laboratory (single) | Adults | – | NGAL (serum) | 454 | N/N |
| 291 | Yin 2013230 | China | Clinical | Cardiac (single) | Adults | Other | Cystatin C (serum) | 204 | Y/Y |
| 1619 | Yoon 2013231 | South Korea | Clinical | Cardiac: contrast (single) | Adults | Other | Cystatin C (not specified) | 723 | Y/Y |
| 1613 | Youssef 2013232 | Egypt | Clinical | Critical care (single) | Children | RIFLE | NGAL (serum) | 75 | Y/Y |
| 1222 | Zappitelli 2007233 | USA | Clinical | Critical care (single) | Children | RIFLE | NGAL (urine) | 140 | Y/Y |
| 700 | Zappitelli 2011234 | USA, Canada | Clinical/analytical | Cardiac (multisite, n = 3) | Children | AKIN | Cystatin C (plasma) | 288 | N/N |
| 11263 | Zappitelli 2015235 | USA, Canada | Clinical/analytical | Critical care (multisite, n = 3) | Children | KDIGO | Cystatin C (serum)/NGAL (urine) | 287 | N/N |
| 243 | Zheng 2013236 | China | Clinical/analytical | Cardiac (single) | Children | AKIN (modified) | Cystatin C (serum) | 43 | Y/Y |
| 11825 | Zwiers 2015237 | The Netherlands | Clinical/analytical | Critical care (single) | Children | RIFLE | NGAL (urine) | 100 | Y/Y |
Appendix 7 Full performance measures quality assessment forms for the Nephrocheck case studies
Bihorac et al.33
| Reference ID: | 82 | Reviewer initials: | MM/BK | Data entered: | 23 May 2016 | |||
| Citation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Authors: | Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, DeMuth GE, et al. | |||||||
| Title: | Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication | |||||||
| Journal: | American Journal of Respiratory and Critical Care Medicine | Year: | 2014 | Pages: | 932–9 | |||
| Vol.: | 189(8) | |||||||
| Description of the measurement procedure | ||||||||
| Features | Index | Reference | ||||||
| Name of analyte | Nephrocheck | Creatinine | ||||||
| Test name | Nephrocheck | Jaffe method | ||||||
| Test platform/method used | Astute 140 Meter | Roche COBAS Modular D | ||||||
| Manufacturer | Astute Medical | Roche | ||||||
| Sample matrix used [e.g. urine, serum, plasma (including type)] | Urine | Serum | ||||||
| Pre-analytical biological | ||||||||
| Patient state (e.g. fed/fasted, sitting/standing, rested) | Not described | Not described | ||||||
| Patient preparation | The presence of an indwelling urinary catheter was also a prerequisite | Not described | ||||||
| Anatomical site and/or mechanism | Catheter and urometer | Direct venepuncture via other available venous access or via an indwelling arterial line | ||||||
| Timing of sampling (e.g. before 0900) | Within 60 minutes of serum sample | Not described | ||||||
| Pre-analytical technical | ||||||||
| Sample collection (mechanism and use of stabilisation) | Urine samples were collected in standard (non-coated) specimen cups | Blood was collected in clot activator blood collection tubes (for serum) | ||||||
| Preprocessing handling, temperature, transport and time | For subjects with indwelling bladder catheters, the collection bag was first emptied and then a fresh sample of urine was collected; alternatively, the sample could be taken from a urometer, if present | Not described | ||||||
| Sample processing (e.g. preservation, centrifugation conditions, timings and temperature) | Urine was centrifuged (10 minutes at 1000g) to remove any cells or other debris and was then aliquoted and frozen | Serum was prepared by centrifugation for 10 minutes at a minimum of 1300g after clotting and was then aliquoted and frozen | ||||||
| Storage (e.g. volume, temperature, duration, freeze–thaw cycles) | Frozen (on dry ice or liquid nitrogen) ≤ 2 hours from collection and stored at ≤ –70 °C | Frozen (on dry ice or liquid nitrogen) ≤ 2 hours from collection and stored at ≤ –70 °C | ||||||
| Postprocessing handling and transport | Samples were shipped on dry ice. Samples were thawed immediately prior to analysis | Samples were shipped on dry ice. Samples were thawed immediately prior to analysis | ||||||
| Consideration of differences between groups | All samples were collected through the same prospective collection. No obvious difference between AKI and non-AKI | All samples were collected through the same prospective collection. No obvious difference between AKI and non-AKI | ||||||
| Standard operating procedures or quality assurance | Samples were excluded if they were not collected within 60 minutes of the blood sample, they were not processed and frozen within 2 hours, < 15 ml of urine was collected, they were not properly labelled or they were not properly frozen | Samples were excluded if they were of insufficient volume, the wrong collection tube was used, they were haemolysed, they were incorrectly labelled, they were not collected within 60 minutes of the urine samples, they were not processed within 2 hours or they were not properly frozen | ||||||
| Other | Sponsor banked samples; shipped to laboratories for analysis | Sponsor banked samples; shipped to laboratories for analysis | ||||||
| Analytical factors | ||||||||
| Sample blinding procedure | Technicians masked to clinical data | Not described | ||||||
| Sample randomisation procedure | Not described | Not described | ||||||
| Batching | Not described | Not described | ||||||
| Reference control materials | Traceable to reference standard solutions that contain defined mass (concentration) of TIMP-2 and IGFBP-7 in accordance with EN ISO 17511 | IDMS-traceable calibration | ||||||
| Quality assurance procedures | Two internal controls (one positive and one negative) were run automatically with every sample. If the automatic check of these internal controls showed that the control value results were not within predefined limits, the metre displayed an error message and the test result was not reported. These controls were in addition to external liquid controls (traceable to the same reference standard solutions as the test), which were run to verify test performance and operator proficiency | Not described | ||||||
| Patient inclusion/exclusion criteria | Critically ill adult patients within 24 hours of admission to an ICU. The presence of an indwelling urinary catheter was also a prerequisite for inclusion. Patients with documented AKI at the time of enrolment were excluded | Critically ill adult patients within 24 hours of admission to an ICU. The presence of an indwelling urinary catheter was also a prerequisite for inclusion. Patients with documented AKI at the time of enrolment were excluded | ||||||
| Test failure rate and reasons for test failure | 2.9% (12/420): eight invalid or missing test results, four sample processing deviations | Not described | ||||||
| Technical replication | Urine from each subject was analysed at each of the three testing sites, producing triplicate test values for each sample | Serum samples were analysed for creatinine at a central laboratory | ||||||
| Performance evaluation | ||||||||
| Performance goals (for precision and bias including method of calculation) | Not described | Not described | ||||||
| Within-individual biological variation | Not described | Not described | ||||||
| Pre-analytical factors | Not described | Not described | ||||||
| Total measurement uncertainty | Not described | Not described | ||||||
| Analytical verification or full validation | Not described – manufacturer’s data reported | Not described | ||||||
| Analytical sensitivity | ||||||||
| Method (brief description) | Manufacturer’s data reported | Not described | ||||||
| Limit of blank (LOB) | Manufacturer’s data reported | Not described | ||||||
| Limit of detection (LOD) | Manufacturer’s data reported | Not described | ||||||
| Limit of quantitation (LOQ) | Manufacturer’s data reported | Not described | ||||||
| Analytical selectivity | ||||||||
| Method (brief description) | Not described | Not described | ||||||
| Cross-reactivity | Not described | Not described | ||||||
| Interference | Not described | Not described | ||||||
| Carry-over | Not described | Not described | ||||||
| Trueness | ||||||||
| Method (brief description) | Not described | Not described | ||||||
| Bias | Not described | Not described | ||||||
| Precision | ||||||||
| Method (brief description including M-Factors: time, calibration, operator and equipment) | Manufacturer’s data reported | Not described | ||||||
| Repeatability (range), CV% | Not described | Not described | ||||||
| Intermediate Imprecision (range), CV% | Not described | Not described | ||||||
| Reproducibility (range), CV% | Manufacturer’s data reported | Not described | ||||||
| Linearity and working range | Manufacturer’s data reported | Not described | ||||||
| Method (brief description) | Manufacturer’s data reported | Not described | ||||||
| Other (e.g. lot to lot, antibody validation profile) | Not described | Not described | ||||||
| Signalling questions | ||||||||
Were measurement procedures different between groups?
|
||||||||
Were measurement procedures described in enough detail to be repeated?
|
||||||||
Were measurement factors appropriately controlled for?
|
||||||||
Were measurement procedures applicable to the final clinical setting?
|
||||||||
| Risk of bias (yes, no, uncertain) | ||||||||
| Unclear | ||||||||
| Risk of irreproducibility (yes, no, uncertain) | ||||||||
| High | ||||||||
| Risk of inapplicability (yes, no, uncertain) | ||||||||
| High | ||||||||
Meersch et al.
| Reference ID: | 1485 | Reviewer initials: | BK/MM | Data entered: | 13 May 2016 |
| Citation | |||||
|---|---|---|---|---|---|
| Authors: | Meersch M, Schmidt C, Van Aken H, Rossaint J, Görlich D, Stege D, et al. | ||||
| Title: | Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery | ||||
| Journal | PLOS ONE | Year: | 2014 | Pages: | e110865 |
| Vol: | 9 (10) | ||||
| Description of the measurement procedure | |||||
| Name of analyte | Nephrocheck | Creatinine | |||
| Test name | Nephrocheck | ||||
| Test platform/method used | Astute 140 Meter | ||||
| Manufacturer | Astute Medical | ||||
| Sample matrix used [e.g. urine, serum, plasma (including type)] | Urine | Serum | |||
| Pre-analytical biological | |||||
| Patient state (e.g. fed/fasted, sitting/standing, rested) | Not described | Not described | |||
| Patient preparation | Not described | Not described | |||
| Anatomical site and/or mechanism | Not described | Not described | |||
| Time of sampling (e.g. before 0900) | Immediately before and at 4 and 24 hours post CPB | Routinely measured before surgery, immediately after surgery and at least daily in the postoperative period | |||
| Pre-analytical technical | |||||
| Sample collection (mechanism and use of stabilisation) | Not described | Not described | |||
| Preprocessing handling, temperature, transport and time | Not described | Not described | |||
| Sample processing (e.g. preservation, centrifugation conditions, timings and temperature) | Not described | Not described | |||
| Storage (e.g. volume, temperature, duration, freeze–thaw cycles) | Stored in aliquots at –80 °C. Length of storage not described | Not described | |||
| Postprocessing handling and transport | Not described | Not described | |||
| Consideration of differences between groups | Nephrocheck and creatinine tests not performed at the same time | Nephrocheck and creatinine tests not performed at the same time | |||
| Standard operating procedures or quality assurance | Not described | Not described | |||
| Other | |||||
| Analytical factors | |||||
| Sample blinding | Laboratory investigators blinded to clinical outcomes | Laboratory investigators blinded to clinical outcomes | |||
| Sample randomisation | Not described | Not described | |||
| Batching | Not described | Not described | |||
| Reference control materials | Not described | Not described | |||
| Quality assurance procedures | Not described | Not described | |||
| Patient inclusion/exclusion criteria | All patients aged < 18 years undergoing cardiac surgery with CPB at the authors’ centre between July 2013 and December 2013 were approached for study inclusion. Patients with severe pre-existing renal insufficiency (serum creatinine two times the age-adjusted normal range) were excluded | All patients aged < 18 years undergoing cardiac surgery with CPB at the authors’ centre between July 2013 and December 2013 were approached for study inclusion. Patients with severe pre-existing renal insufficiency (serum creatinine two times the age-adjusted normal range) were excluded | |||
| Test failure rate and reasons for test failure | Not described | Not described | |||
| Performance evaluation | |||||
| Performance goals (for precision and bias including method of calculation) | Not described | Not described | |||
| Within-individual biological variation | Not described | Not described | |||
| Pre-analytical factors | Not described | Not described | |||
| Total measurement uncertainty | Not described | Not described | |||
| Analytical verification or full validation | Not described | Not described | |||
| Analytical sensitivity | |||||
| Method (brief description) | Not described | Not described | |||
| Limit of blank (LOB) | Not described | Not described | |||
| Limit of detection (LOD) | Not described | Not described | |||
| Limit of quantitation (LOQ) | Not described | Not described | |||
| Analytical specificity | |||||
| Method (brief description) | Not described | Not described | |||
| Cross-reactivity | Not described | Not described | |||
| Interference | Not described | Not described | |||
| Carry-over | Not described | Not described | |||
| Trueness | |||||
| Method (brief description) | Not described | Not described | |||
| Bias | Not described | Not described | |||
| Precision | |||||
| Method (brief description including M-factors: time, calibration, operator and equipment) | Not described | Not described | |||
| Repeatability (range), CV% | Not described | Not described | |||
| Intermediate imprecision (range), CV% | Not described | Not described | |||
| Reproducibility (range), CV% | Not described | Not described | |||
| Linearity and working range | Not described | Not described | |||
| Method (brief description) | Not described | Not described | |||
| Other (e.g. lot to lot, antibody validation profile) | Not described | Not described | |||
| Signalling questions | |||||
Were measurement procedures different between groups?
|
|||||
Were measurement procedures described in enough detail to be repeated?
|
|||||
Were measurement factors appropriately controlled for?
|
|||||
Were measurement procedures applicable to the final clinical setting?
|
|||||
| Risk of bias (high, low, unclear) | |||||
| Unclear | |||||
| Risk of irreproducibility (high, low, unclear) | |||||
| High | |||||
| Risk of inapplicability (high, low, unclear) | |||||
| High | |||||
Hoste et al.44
| Reference ID: | 1488 | Reviewer initials: | MM/BK | Data entered: | 18 May 2016 |
| Citation | |||||
|---|---|---|---|---|---|
| Authors: | Hoste EAJ, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. | ||||
| Title: | Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers | ||||
| Journal: | Nephrology Dialysis Transplantation | Year: | 2014 | Pages: | 2054–61 |
| Vol: | 29 | ||||
| Description of the measurement procedure | |||||
| Features | Index | Reference | |||
| Name of analyte | Nephrocheck | Creatinine | |||
| Test name | Nephrocheck | ||||
| Test platform/method used | Astute 140 Meter | Not described | |||
| Manufacturer | Astute Medical | Not described | |||
| Sample matrix used [e.g. urine, serum, plasma (including type)] | Urine | Serum | |||
| Pre-analytical biological | |||||
| Patient state (e.g. fed/fasted, sitting/standing, rested) | Not described | Not described | |||
| Patient preparation | Urinary catheter | Not described | |||
| Anatomical site and/or mechanism | Urinary catheter | Not described | |||
| Time of sampling (e.g. before 0900) | ‘At enrolment’ (unclear if within 1 hour) | Not described | |||
| Pre-analytical technical | |||||
| Sample collection (mechanism and use of stabilisation) | ‘Standard methods’ | Not described | |||
| Preprocessing handling, temperature, transport and time | Collected either directly from catheter or from collection bag (emptied first) | Not described | |||
| Sample processing (e.g. preservation, centrifugation conditions, timings and temperature) | Centrifugation (10 minutes at 1000g) | Not described | |||
| Storage (e.g. volume, temperature, duration, freeze–thaw cycles) | Frozen within 2 hours of collection, stored at ≤ 70 °C (length of storage not described) | Not described | |||
| Postprocessing handling and transport | Transport not described. Thawed immediately prior to analysis | Not described | |||
| Consideration of differences between groups | Prospective data used for Nephrocheck, sometimes retrospective data used for creatinine | In some patients the baseline creatinine value was used (two different methods for determining baseline) rather than the creatinine value at the time of enrolment | |||
| Standard operating procedures or quality assurance | Operators required to complete proficiency training using control samples. No other quality assurance described | Not described | |||
| Other | |||||
| Analytical factors | |||||
| Sample blinding | Technicians blinded to clinical data | Blinded to final determination of AKI status | |||
| Sample randomisation | Not described | Not described | |||
| Batching | Not described | Not described | |||
| Reference control materials | Traceable to reference standard solutions in accordance with ISO 17511 | Not described | |||
| Quality assurance procedures | Two detection zones used as internal controls (one positive and one negative control) and run automatically with every sample, in addition to external liquid controls (traceable to the same reference standard solutions as the test), which are run to verify test performance and operator proficiency | Not described | |||
| Patient inclusion/exclusion criteria | |||||
| Test failure rate and reasons for test failure | n = 7/744 Sapphire patients with invalid or missing test results | Not described | |||
| Performance evaluation | |||||
| Performance goals (for precision and bias including method of calculation) | Not described | Not described | |||
| Within-individual biological variation | Not described | Not described | |||
| Pre-analytical factors | Not described | Not described | |||
| Total measurement uncertainty | Not described | Not described | |||
| Analytical verification or full validation | Not described – manufacturer’s data reported | Not described | |||
| Analytical sensitivity | |||||
| Method (brief description) | Not described | Not described | |||
| Limit of blank (LOB) | Manufacturer’s data reported | Not described | |||
| Limit of detection (LOD) | Manufacturer’s data reported | Not described | |||
| Limit of quantitation (LOQ) | Manufacturer’s data reported | Not described | |||
| Analytical specificity | |||||
| Method (brief description) | Not described | Not described | |||
| Cross-reactivity | Not described | Not described | |||
| Interference | Not described | Not described | |||
| Carry-over | Not described | Not described | |||
| Trueness | |||||
| Method (brief description) | Not described | Not described | |||
| Bias | Not described | Not described | |||
| Precision | |||||
| Method (brief description including M-factors: time, calibration, operator and equipment) | Manufacturer’s data reported | Not described | |||
| Repeatability (range), CV% | Not described | Not described | |||
| Intermediate Imprecision (range), CV% | Not described | Not described | |||
| Reproducibility (range), CV% | Manufacturer’s data reported | Not described | |||
| Linearity and working range | Manufacturer’s data reported | Not described | |||
| Method (brief description) | Manufacturer’s data reported | Not described | |||
| Other (e.g. lot to lot, antibody validation profile) | Not described | Not described | |||
| Signalling questions | |||||
Were measurement procedures different between groups?
|
|||||
Were measurement procedures described in enough detail to be repeated?
|
|||||
Were measurement factors appropriately controlled for?
|
|||||
Were measurement procedures applicable to the final clinical setting?
|
|||||
| Risk of bias (high, low, unclear) | |||||
| Unclear | |||||
| Risk of irreproducibility (high, low, unclear) | |||||
| High | |||||
| Risk of inapplicability (high, low, unclear) | |||||
| Unclear | |||||
Meersch et al.54
| Reference ID: | 1568 | Reviewer initials: | MM/BK | Data entered: | 19 May 2016 |
| Citation | |||||
|---|---|---|---|---|---|
| Authors: | Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, et al. | ||||
| Title: | Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery | ||||
| Journal: | PLOS ONE | Year: | 2014 | Pages: | e93460 |
| Vol: | 9 | ||||
| Description of the measurement procedure | |||||
| Features | Index | Reference | |||
| Name of analyte | Nephrocheck | Creatinine | |||
| Test name | Nephrocheck | ||||
| Test platform/method used | Astute 140 Meter | ||||
| Manufacturer | Astute Medical | ||||
| Sample matrix used [e.g. urine, serum, plasma (including type)] | Urine | Serum | |||
| Pre-analytical biological | |||||
| Patient state (e.g. fed/fasted, sitting/standing, rested) | Not described | Not described | |||
| Patient preparation | Not described | Not described | |||
| Anatomical site and/or mechanism | Not described | Not described | |||
| Time of sampling (e.g. before 0900) | Pre CPB and 4, 12 and 24 hours after CPB | Pre CPB and 4, 12, 24, 48 and 72 hours post CPB and at time of discharge | |||
| Pre-analytical technical | |||||
| Sample collection (mechanism and use of stabilisation) | Not described | Not described | |||
| Preprocessing handling, temperature, transport and time | Not described | Not described | |||
| Sample processing (e.g. preservation, centrifugation conditions, timings and temperature) | Centrifuged immediately (further details not given) | Not described | |||
| Storage (e.g. volume, temperature, duration, freeze–thaw cycles) | Stored at ≤ –70 °C (duration not stated) | Not described | |||
| Postprocessing handling and transport | Thawed immediately prior to analysis | Not described | |||
| Consideration of differences between groups | Prospective data used for Nephrocheck, sometimes retrospective data used for creatinine | In some patients the baseline creatinine value was used rather than the creatinine value at the time of enrolment | |||
| Standard operating procedures or quality assurance | Not described | Not described | |||
| Other | |||||
| Analytical factors | |||||
| Sample blinding | Not described | Not described | |||
| Sample randomisation | Not described | Not described | |||
| Batching | Not described | Not described | |||
| Reference control materials | Not described | Not described | |||
| Quality assurance procedures | Not described | Not described | |||
| Patient inclusion/exclusion criteria | Those with a Cleveland Clinic Foundation Score of ≥ 6 were eligible for enrolment. Exclusion criteria included:
|
Those with a Cleveland Clinic Foundation Score of ≥ 6 were eligible for enrolment. Exclusion criteria included:
|
|||
| Test failure rate and reasons for test failure | Not described | Not described | |||
| Performance evaluation | |||||
| Performance goals (for precision and bias including method of calculation) | Not described | Not described | |||
| Within-individual biological variation | Not described | Not described | |||
| Pre-analytical factors | Not described | Not described | |||
| Total measurement uncertainty | Not described | Not described | |||
| Analytical verification or full validation | Not described | Not described | |||
| Analytical sensitivity | |||||
| Method (brief description) | Not described | Not described | |||
| Limit of blank (LOB) | Not described | Not described | |||
| Limit of detection (LOD) | Not described | Not described | |||
| Limit of quantitation (LOQ) | Not described | Not described | |||
| Analytical specificity | |||||
| Method (brief description) | Not described | Not described | |||
| Cross-reactivity | Not described | Not described | |||
| Interference | Not described | Not described | |||
| Carry-over | Not described | Not described | |||
| Trueness | |||||
| Method (brief description) | Not described | Not described | |||
| Bias | Not described | Not described | |||
| Precision | |||||
| Method (brief description including M-factors: time, calibration, operator and equipment) | Not described | Not described | |||
| Repeatability (range), CV% | Not described | Not described | |||
| Intermediate Imprecision (range), CV% | Not described | Not described | |||
| Reproducibility (range), CV% | Not described | Not described | |||
| Linearity and working range | Not described | Not described | |||
| Method (brief description) | Not described | Not described | |||
| Other (e.g. lot to lot, antibody validation profile) | Not described | Not described | |||
| Signalling questions | |||||
Were measurement procedures different between groups?
|
|||||
Were measurement procedures described in enough detail to be repeated?
|
|||||
Were measurement factors appropriately controlled for?
|
|||||
Were measurement procedures applicable to the final clinical setting?
|
|||||
| Risk of bias (high, low, unclear) | |||||
| Unclear | |||||
| Risk of irreproducibility (high, low, unclear) | |||||
| High | |||||
| Risk of inapplicability (high, low, unclear) | |||||
| High | |||||
Appendix 8 Search methods for the AKI-Diagnostics economic model literature review
Databases searched
-
Cochrane Central Register of Controlled Trials (via Wiley Online Library) (Issue 2 of 12, February 2016)
-
Database of Abstracts of Reviews of Effect (Wiley Online Library) (Issue 2 of 4, April 2015)
-
EMBASE Classic + EMBASE (via Ovid) (1947 to 26 February 2015)
-
Ovid MEDLINE (1946 to March Week 2 2016)
-
Ovid MEDLINE In-Process & Other Non-Indexed Citations (23 March 2016)
-
NHS Economic Evaluation Database (Wiley Online Library) (Issue 2 of 4, April 2015).
Example MEDLINE search
(Full search strategies used in all databases are available from the authors on request.)
Search strategy
-
Acute Disease/ and exp Kidney Diseases/ (8114)
-
acute kidney injury/ (35,209)
-
kidney tubular necrosis, acute/ (2292)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (8613)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (21,799)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3501)
-
aki.tw. (4196)
-
‘contrast induced nephropathy’.tw. (1099)
-
or/1-8 (55,186)
-
reperfusion injury/ (21,566)
-
reperfusion/ (4317)
-
exp Delayed Graft Function/ (832)
-
‘delayed graft function*’.tw. (2349)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (42,376)
-
or/10-14 (51,149)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (725,426)
-
1 or 2 or 3 or 16 (733,668)
-
15 and 17 (7807)
-
9 or 18 [AKI] (60,934)
-
models, economic/ or models, econometric/ (11,379)
-
markov chain/ (10,943)
-
Decision Trees/ (9378)
-
decision support techniques/ (14,411)
-
microsimulat*.tw. (484)
-
(patient level adj8 simulat*).tw. (44)
-
(simulat* adj3 model*).tw. and decision*.mp. (1428)
-
(discrete event* adj5 simulat*).tw. (429)
-
(discrete event* adj8 model*).tw. (346)
-
(decision* adj5 model*).tw. (9343)
-
(model* adj5 markov*).tw. (8445)
-
((econom* or cost or costs) adj6 model*).tw. (14,201)
-
‘state transition model*’.tw. (294)
-
(‘transition probabilit*’ and (state or states or model*)).tw. (1097)
-
or/20-33 [Econ models] (61,100)
-
19 and 34 (118)
Appendix 9 Search methods for the AKI-Diagnostics economic model parameters literature review
Databases searched
| Searches | Databases |
|---|---|
| AKI costs |
|
| AKI utilities |
|
| AKI risks |
|
| CKD costs |
|
| CKD utilities |
|
| CKD risks |
|
Example MEDLINE searches
(Full search strategies used in all databases are available from the authors on request.)
Acute kidney injury costs
Search strategy
-
Acute Disease/ and exp Kidney Diseases/ (7907)
-
acute kidney injury/ (34,725)
-
kidney tubular necrosis, acute/ (2253)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (7745)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (22,094)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3518)
-
aki.tw. (3679)
-
‘contrast induced nephropathy’.tw. (1025)
-
or/1-8 (54,260)
-
reperfusion injury/ (21,127)
-
reperfusion/ (4276)
-
exp Delayed Graft Function/ (713)
-
‘delayed graft function*’.tw. (2205)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (41,542)
-
or/10-14 (50,057)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (715,951)
-
1 or 2 or 3 or 16 (724,030)
-
15 and 17 (7499)
-
9 or 18 [AKI] (59,776)
-
exp Hospitalization/ (172,146)
-
Inpatients/ (14,321)
-
(ward or wards).tw. (39,848)
-
exp Hospitals/ (215,221)
-
hospital units/ or exp intensive care units/ (70,023)
-
hospital*.tw. (826,340)
-
‘high dependency’.tw. (785)
-
(critical adj2 care).tw. (17,692)
-
(intensive adj2 care).tw. (91,081)
-
(inpatient or inpatients).tw. (64,890)
-
or/20-29 [Hospital Wards] (1,093,337)
-
exp great britain/ (322,784)
-
(‘united king*’ or uk or ‘U.K.’ or ‘UK.’ or ‘U.K’ or britain).in,ti. (843,719)
-
(british or english or scottish or welsh or irish).in,ti. (81,221)
-
(scotland or ireland).in,ti. (85,992)
-
(england not ‘new england’).in,ti. (53,958)
-
(wales not ‘new south wales’).in,ti. (32,677)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).in,ti. (680,616)
-
((london adj2 ontario) or (london adj on) or new london).in,ti. (20,486)
-
(manchester adj3 (USA or massach*)).in,ti. (328)
-
(newcastle adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (6218)
-
(liverpool adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (1167)
-
or/38-41 (28,187)
-
37 not 42 (652,429)
-
(nhs or ‘national health service’).in,ti. (78,445)
-
or/31-36,43-44 (1,323,915)
-
19 and 30 and 45 (503)
-
exp ‘Costs and Cost Analysis’/ (191,630)
-
cost*.tw. (352,643)
-
budget*.tw. (18,103)
-
(price or prices or pricing).tw. (23,573)
-
(financial* adj4 burden*).tw. (2622)
-
(economic* adj4 burden*).tw. (5378)
-
expenditure*.tw. (36,926)
-
or/47-53 [Costs] (492,123)
-
19 and 30 and 45 and 54 [AKI Wards UK Costs] (49)
-
limit 55 to yr=‘2010 -Current’ (28)
-
19 and 30 and 54 (479)
-
limit 57 to ‘reviews (maximizes specificity)’ (20)
-
56 or 58 (48)
Acute kidney injury utilities
Search strategy
-
Acute Disease/ and exp Kidney Diseases/ (7907)
-
acute kidney injury/ (34,725)
-
kidney tubular necrosis, acute/ (2253)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (7745)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (22,094)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3518)
-
aki.tw. (3679)
-
‘contrast induced nephropathy’.tw. (1025)
-
or/1-8 (54,260)
-
reperfusion injury/ (21,127)
-
reperfusion/ (4276)
-
exp Delayed Graft Function/ (713)
-
‘delayed graft function*’.tw. (2205)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (41,542)
-
or/10-14 (50,057)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (715,951)
-
1 or 2 or 3 or 16 (724,030)
-
15 and 17 (7499)
-
9 or 18 [AKI] (59,776)
-
exp great britain/ (322,784)
-
(‘united king*’ or uk or ‘U.K.’ or ‘UK.’ or ‘U.K’ or britain).in,ti. (843,719)
-
(british or english or scottish or welsh or irish).in,ti. (81,221)
-
(scotland or ireland).in,ti. (85,992)
-
(england not ‘new england’).in,ti. (53,958)
-
(wales not ‘new south wales’).in,ti. (32,677)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).in,ti. (680,616)
-
((london adj2 ontario) or (london adj on) or new london).in,ti. (20,486)
-
(manchester adj3 (USA or massach*)).in,ti. (328)
-
(newcastle adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (6218)
-
(liverpool adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (1167)
-
or/27-30 (28,187)
-
26 not 31 (652,429)
-
(nhs or ‘national health service’).in,ti. (78,445)
-
or/20-25,32-33 [UK] (1,323,915)
-
exp Health Status/ (113,246)
-
‘health status’.tw. (37,086)
-
‘Quality of Life’/ (128,885)
-
(hql or hqol or h qol or hrqol or hr qol).tw. (8334)
-
‘quality of life’.tw. (149,495)
-
‘questionnaire*’.tw. (301,418)
-
or/35-40 (558,180)
-
(utility or utilities).ti. (18,669)
-
(qaly or ‘quality adjusted life year*’).tw. (7053)
-
42 or 43 (25,018)
-
41 and 44 (8176)
-
((utility or utilities) adj5 (hql or hqol or h qol or hrqol or hr qol or ‘quality of life’ or health* or score* or weight*)).ab. (4351)
-
(preference* adj5 (hql or hqol or h qol or hrqol or hr qol or ‘quality of life’ or health* or score* or weight*)).tw. (3981)
-
(sf6d or sf 6d or short form 6d or shortform 6d or sf sixd or sf six d).tw. (466)
-
(hui or hui1 or hui2 or hui3).tw. (904)
-
‘health utilities index*’.tw. (542)
-
*quality-adjusted life years/ (1625)
-
‘health related quality of life’.tw. (22,714)
-
quality-adjusted life years/ (7808)
-
QALY.tw. (4335)
-
(eq-5d* or eq5d* or euroqol*).tw. (4329)
-
or/45-55 [Utilities] (41,087)
-
19 and 34 and 56 [AKI and UK and Utilities] (9)
-
19 and 56 [AKI and Utilities] (54)
-
limit 58 to ‘reviews (best balance of sensitivity and specificity)’ (11)
-
limit 57 to yr=‘2010 -Current’ (3)
-
59 or 60 (14)
Acute kidney injury risks
Search strategy
-
Acute Disease/ and exp *Kidney Diseases/ (6067)
-
*acute kidney injury/ (26,477)
-
*kidney tubular necrosis, acute/ (1197)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (7787)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (22,104)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3519)
-
aki.tw. (3705)
-
‘contrast induced nephropathy’.tw. (1027)
-
or/1-8 (46,556)
-
*reperfusion injury/ (16,238)
-
*reperfusion/ (1132)
-
exp *Delayed Graft Function/ (373)
-
‘delayed graft function*’.tw. (2207)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (41,592)
-
or/10-14 (46,283)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (716,822)
-
1 or 2 or 3 or 16 (720,726)
-
15 and 17 (7033)
-
9 or 18 [AKI] (51,801)
-
‘high dependency’.tw. (785)
-
(critical adj2 care).tw. (17,716)
-
(intensive adj2 care).tw. (91,210)
-
(inpatient or inpatients).tw. (65,020)
-
critical care/ (25,394)
-
critical illness/ (18,693)
-
Intensive Care/ (16,540)
-
exp intensive care units/ (61,237)
-
critical illness*.tw. (4877)
-
ICU.tw. (29,797)
-
20 or 21 or 22 or 24 or 25 or 26 or 27 or 28 or 29 [ICU] (160,191)
-
likelihood functions/ (18,212)
-
markov chains/ (10,701)
-
odds ratio/ (66,036)
-
proportional hazards models/ (51,090)
-
risk/ (103,777)
-
logistic models/ (99,887)
-
risk assessment/ (189,789)
-
risk factors/ (612,509)
-
((risk or risks or odds or proportion*) adj5 (mortality or death or stage*)).ti. (9334)
-
((incidence or prevalen*) adj5 (mortality or death or stage*)).ti. (3039)
-
or/31-40 [Risks Odds Likelihood studies] (976,879)
-
meta-analysis/ (58,183)
-
sn.fs. (564,719)
-
42 or 43 [Data set cohort or meta analysis studies] (617,723)
-
*hospitalization/ or *’length of stay’/ or *patient admission/ or *patient discharge/ or *patient readmission/ or *patient transfer/ (58,042)
-
((risk or risks or odds or proportion* or incidence or prevalen*) adj8 (aki or ‘acute kidney*’)).ti. (394)
-
((risk or risks or odds or proportion* or incidence or prevalen*) adj8 (‘acute kidney*’ or ‘acute renal*’ or ‘acute nephr*’ or ‘acute tubular necrosis*’)).ti. (603)
-
46 or 47 [AKI Risks Title search] (634)
-
exp great britain/ (323,050)
-
(‘united king*’ or uk or ‘U.K.’ or ‘UK.’ or ‘U.K’ or britain).in,ti. (845,318)
-
(british or english or scottish or welsh or irish).in,ti. (81,404)
-
(scotland or ireland).in,ti. (86,152)
-
(england not ‘new england’).in,ti. (54,012)
-
(wales not ‘new south wales’).in,ti. (32,731)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).in,ti. (681,856)
-
((london adj2 ontario) or (london adj on) or new london).in,ti. (20,549)
-
(manchester adj3 (USA or massach*)).in,ti. (330)
-
(newcastle adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (6251)
-
(liverpool adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (1172)
-
or/56-59 (28,287)
-
55 not 60 (653,569)
-
(nhs or ‘national health service’).in,ti. (78,681)
-
or/49-54,61-62 [UK Filter] (1,326,014)
-
9 and 30 and 41 and 44 and 63 [AKI ICU Risks Data sets UK] (12)
-
19 and 30 and 45 and 63 [AKI ICU and Length of stay Discharged UK] (8)
-
48 and 63 [AKI Risks UK Titles] (34)
-
67 or/64–66 [Final AKI ICU Risks] (47)
Chronic kidney disease costs
Search strategy
-
Acute Disease/and exp Kidney Diseases/ (7918)
-
acute kidney injury/ (34,881)
-
kidney tubular necrosis, acute/ (2256)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (7865)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (22,132)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3525)
-
aki.tw. (3750)
-
‘contrast induced nephropathy’.tw. (1033)
-
or/1–8 (54,483)
-
reperfusion injury/ (21,228)
-
reperfusion/ (4287)
-
exp Delayed Graft Function/ (724)
-
‘delayed graft function*’.tw. (2217)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (41,727)
-
or/10–14 (50,280)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (718,566)
-
1 or 2 or 3 or 16 (726,662)
-
15 and 17 (7538)
-
9 or 18 [AKI] (60,029)
-
exp Hospitalisation/ (173,062)
-
Inpatients/ (14,410)
-
(ward or wards).tw. (39,983)
-
exp Hospitals/ (215,922)
-
hospital units/or exp intensive care units/ (70,293)
-
hospital*.tw. (830,289)
-
‘high dependency’.tw. (789)
-
(critical adj2 care).tw. (17,765)
-
(intensive adj2 care).tw. (91,464)
-
(inpatient or inpatients).tw. (65,272)
-
or/20–29 [Hospital Wards] (1,098,124)
-
exp great britain/ (323,690)
-
(‘united king*’ or uk or ‘U.K.’ or ‘UK.’ or ‘U.K’ or britain).in,ti. (850,035)
-
(british or english or scottish or welsh or irish).in,ti. (81,795)
-
(scotland or ireland).in,ti. (86,568)
-
(england not ‘new england’).in,ti. (54,183)
-
(wales not ‘new south wales’).in,ti. (32,822)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).in,ti. (685,504)
-
((london adj2 ontario) or (london adj on) or new london).in,ti. (20,635)
-
(manchester adj3 (USA or massach*)).in,ti. (339)
-
(newcastle adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (6295)
-
(liverpool adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (1182)
-
or/38–41 (28,436)
-
37 not 42 (657,068)
-
(nhs or ‘national health service’).in,ti. (79,247)
-
or/31–36,43–44 (1,331,854)
-
19 and 30 and 45 (513)
-
exp ‘Costs and Cost Analysis’/ (192,502)
-
cost*.tw. (354,814)
-
budget*.tw. (18,180)
-
(price or prices or pricing).tw. (23,711)
-
(financial* adj4 burden*).tw. (2651)
-
(economic* adj4 burden*).tw. (5444)
-
expenditure*.tw. (37,118)
-
or/47–53 [Costs] (494,870)
-
19 and 30 and 45 and 54 [AKI Wards UK Costs] (49)
-
limit 55 to yr = ’2010 -Current’ (28)
-
19 and 30 and 54 (479)
-
limit 57 to ‘reviews (maximises specificity)’ (20)
-
56 or 58 (48)
-
renal insufficiency, chronic/or exp kidney failure, chronic/ (90,923)
-
Chronic Disease/and exp Kidney Diseases/ (13,363)
-
(chronic kidney or chronic renal).tw. (50,765)
-
CKD.tw. (12,350)
-
(end stage renal or end stage kidney or endstage renal or endstage kidney).tw. (27,376)
-
(ESRF or ESKF or ESRD or ESKD).tw. (11,389)
-
exp Dialysis/ (22,640)
-
renal dialysis/or hemodiafiltration/or exp peritoneal dialysis/ (98,191)
-
renal replacement therapy/ (3833)
-
h?emodialysis.tw. (59,032)
-
peritonealdialysis.tw. (8)
-
dialysis.tw. (83,548)
-
(CAPD or CCPD).tw. (6461)
-
Kidney Transplantation/ (83,562)
-
((renal or kidney*) adj3 transplant*).tw. (65,170)
-
or/60–74 [CKD or End stage or dialysis or transplants] (299,294)
-
exp *’Costs and Cost Analysis’/ (50,115)
-
cost*.ti. (81,585)
-
budget*.ti. (4877)
-
(price or prices or pricing).ti. (6778)
-
financ*.ti. (12,421)
-
economic*.ti. (30,984)
-
expenditure*.ti. (7616)
-
or/76–82 [Cost Specific] (159,833)
-
45 and 83 and 75 (210)
-
limit 84 to yr = ’2010 -Current’ (69)
-
83 and 75 (2194)
-
limit 86 to ‘reviews (maximises specificity)’ (52)
-
85 or 87 [CKD Costs Reviews or Recent UK studies] (117)
-
88 not 59 [CKD not AKI costs] (112)
Chronic kidney disease utilities
Search strategy
-
exp great britain/ (323,690)
-
(‘united king*’ or uk or ‘U.K.’ or ‘UK.’ or ‘U.K’ or britain).in,ti. (850,035)
-
(british or english or scottish or welsh or irish).in,ti. (81,795)
-
(scotland or ireland).in,ti. (86,568)
-
(england not ‘new england’).in,ti. (54,183)
-
(wales not ‘new south wales’).in,ti. (32,822)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).in,ti. (685,504)
-
((london adj2 ontario) or (london adj on) or new london).in,ti. (20,635)
-
(manchester adj3 (USA or massach*)).in,ti. (339)
-
(newcastle adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (6295)
-
(liverpool adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (1182)
-
or/8–11 (28,436)
-
7 not 12 (657,068)
-
(nhs or ‘national health service’).in,ti. (79,247)
-
or/1–6,13–14 (1,331,854)
-
renal insufficiency, chronic/or exp kidney failure, chronic/ (90,923)
-
Chronic Disease/and exp Kidney Diseases/ (13,363)
-
(chronic kidney or chronic renal).tw. (50,765)
-
CKD.tw. (12,350)
-
(end stage renal or end stage kidney or endstage renal or endstage kidney).tw. (27,376)
-
(ESRF or ESKF or ESRD or ESKD).tw. (11,389)
-
exp Dialysis/ (22,640)
-
renal dialysis/or hemodiafiltration/or exp peritoneal dialysis/ (98,191)
-
renal replacement therapy/ (3833)
-
h?emodialysis.tw. (59,032)
-
peritonealdialysis.tw. (8)
-
dialysis.tw. (83,548)
-
(CAPD or CCPD).tw. (6461)
-
Kidney Transplantation/ (83,562)
-
((renal or kidney*) adj3 transplant*).tw. (65,170)
-
or/16–30 [CKD or End stage or dialysis or transplants] (299,294)
-
exp Health Status/ (113,928)
-
‘Quality of Life’/ (129,941)
-
‘health status’.tw. (37,306)
-
(hql or hqol or h qol or hrqol or hr qol).tw. (8435)
-
‘quality of life’.tw. (150,784)
-
‘questionnaire*’.tw. (303,559)
-
or/32–37 (562,053)
-
(utility or utilities).ti. (18,818)
-
38 and 39 (2240)
-
((utility or utilities) adj5 (hql or hqol or h qol or hrqol or hr qol or ‘quality of life’ or health* or score* or weight*)).ab. (4399)
-
(preference* adj5 (hql or hqol or h qol or hrqol or hr qol or ‘quality of life’ or health* or score* or weight*)).tw. (4018)
-
(sf6d or sf 6d or short form 6d or shortform 6d or sf sixd or sf six d).tw. (477)
-
(hui or hui1 or hui2 or hui3).tw. (913)
-
‘health utilities index*’.tw. (546)
-
quality-adjusted life-years/ (7915)
-
(qaly or ‘quality-adjusted life-year*’).tw. (7153)
-
‘health related quality of life’.tw. (22,942)
-
(eq-5d* or eq5d* or euroqol*).tw. (4398)
-
or/40–49 [Health Utilities] (41,544)
-
15 and 31 and 50 (98)
-
limit 51 to yr = ’2010 -Current’ (51)
-
31 and 50 (1140)
-
limit 53 to ‘reviews (maximises specificity)’ (52)
-
52 or 54 [CKD Health Utilities Search] (98)
-
Acute Disease/and exp Kidney Diseases/ (7918)
-
acute kidney injury/ (34,881)
-
kidney tubular necrosis, acute/ (2256)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (7865)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (22,132)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3525)
-
aki.tw. (3750)
-
‘contrast induced nephropathy’.tw. (1033)
-
or/56–63 (54,483)
-
reperfusion injury/ (21,228)
-
reperfusion/(4287)
-
exp Delayed Graft Function/ (724)
-
‘delayed graft function*’.tw. (2217)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (41,727)
-
or/65–69 (50,280)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (718,566)
-
56 or 57 or 58 or 71 (726,662)
-
70 and 72 (7538)
-
64 or 73 [AKI] (60,029)
-
15 and 50 and 74 (9)
-
50 and 74 (54)
-
limit 76 to ‘reviews (best balance of sensitivity and specificity)’ (11)
-
75 or 77 (19)
-
55 not 78 (96)
Chronic kidney disease risks
Search strategy
-
likelihood functions/ (18,331)
-
markov chains/ (10,769)
-
odds ratio/ (66,504)
-
proportional hazards models/ (51,509)
-
risk/ (104,119)
-
logistic models/ (100,507)
-
risk assessment/ (190,924)
-
risk factors/ (615,846)
-
((risk or risks or odds or proportion*) adj5 (mortality or death or stage*)).ti. (9407)
-
((incidence or prevalen*) adj5 (mortality or death or stage*)).ti. (3063)
-
or/1–10 [Risks Odds Likelihood studies] (982,161)
-
meta-analysis/ (58,864)
-
sn.fs. (568,088)
-
12 or 13 [Data set cohort or meta analysis studies] (621,726)
-
exp great britain/ (324,006)
-
(‘united king*’ or uk or ‘U.K.’ or ‘UK.’ or ‘U.K’ or britain).in,ti. (850,727)
-
(british or english or scottish or welsh or irish).in,ti. (81,862)
-
(scotland or ireland).in,ti. (86,638)
-
(england not ‘new england’).in,ti. (54,221)
-
(wales not ‘new south wales’).in,ti. (32,839)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).in,ti. (686,055)
-
((london adj2 ontario) or (london adj on) or new london).in,ti. (20,654)
-
(manchester adj3 (USA or massach*)).in,ti. (339)
-
(newcastle adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (6308)
-
(liverpool adj4 (australia* or ‘new south wales’ or nsw)).in,ti. (1187)
-
or/22–25 (28,473)
-
21 not 26 (657,582)
-
(nhs or ‘national health service’).in,ti. (79,391)
-
or/15–20,27–28 [UK Filter] (1,332,939)
-
renal insufficiency, chronic/or exp kidney failure, chronic/ (90,965)
-
Chronic Disease/and exp Kidney Diseases/ (13,364)
-
(chronic kidney or chronic renal).tw. (50,799)
-
CKD.tw. (12,359)
-
(end stage renal or end stage kidney or endstage renal or endstage kidney).tw. (27,394)
-
(ESRF or ESKF or ESRD or ESKD).tw. (11,393)
-
exp Dialysis/ (22,644)
-
renal dialysis/or hemodiafiltration/or exp peritoneal dialysis/ (98,226)
-
renal replacement therapy/ (3840)
-
h?emodialysis.tw. (59,057)
-
peritonealdialysis.tw. (8)
-
dialysis.tw. (83,581)
-
(CAPD or CCPD).tw. (6461)
-
Kidney Transplantation/ (83,595)
-
((renal or kidney*) adj3 transplant*).tw. (65,203)
-
or/30–44 [CKD] (299,421)
-
45 and 11 and 14 and 29 [CKD and risks and data set and UK] (259)
-
((risk or risks or odds or proportion* or incidence or prevalen*) adj8 (ckd or ‘chronic kidney’ or ‘chronic renal’)).ti. (1377)
-
((risk or risks or odds or proportion* or incidence or prevalen*) adj8 (end stage renal or end stage kidney or ESRF or ESKF or ESRD or ESKD)).ti. (599)
-
((risk or risks or odds or proportion* or incidence or prevalen*) adj8 (h?emodialysis or peritonealdialysis or dialysis or CAPD or CCPD)).ti. (1714)
-
((risk or risks or odds or proportion* or incidence or prevalen*) adj8 (renal or kidney*) adj3 transplant*).ti. (1572)
-
or/47–50 [CKD Risks Title search] (5157)
-
46 and 51 (29)
Appendix 10 Search methods for early treatment/preventative strategies for acute kidney injury in the intensive care unit
Databases searched
-
Cochrane Database of Systematic Reviews (via Wiley Online Library) (Issue 3 of 12, March 2016)
-
Cochrane Central Register of Controlled Trials (via Wiley Online Library) (Issue 2 of 12, February 2016)
-
EMBASE Classic+EMBASE (via Ovid) (1947 to 14 March 2016)
-
Ovid MEDLINE (1946 to March Week 1 2016)
-
Ovid MEDLINE In-Process & Other Non-Indexed Citations (14 March 2016).
Example MEDLINE search
(Full search strategies used in all databases are available from the authors on request.)
Search strategy
-
Acute Disease/and exp *Kidney Diseases/ (6286)
-
*acute kidney injury/ (26,908)
-
*kidney tubular necrosis, acute/ (1263)
-
(Acute adj3 (kidney disease* or kidney injury or kidney failure or kidney dysfunction)).tw. (8577)
-
(Acute adj3 (renal disease* or renal injury or renal failure or renal dysfunction)).tw. (21,792)
-
((Acute adj3 (Tubular Necrosis or nephrotoxic*)) or ‘nephrotoxic injur*’).tw. (3501)
-
aki.tw. (4174)
-
‘contrast induced nephropathy’.tw. (1095)
-
or/1–8 (47,456)
-
*reperfusion injury/ (16,539)
-
*reperfusion/(1162)
-
exp *Delayed Graft Function/ (429)
-
‘delayed graft function*’.tw. (2348)
-
(reperfusion adj5 (injur* or isch?emi*)).tw. (42,315)
-
or/10–14 (47,193)
-
(renal or kidney* or nephr* or ‘tubular necrosis’ or aki).tw. (724,886)
-
1 or 2 or 3 or 16 (729,011)
-
15 and 17 (7293)
-
9 or 18 [AKI] (52,903)
-
(HDU or ‘high dependency’).tw. (874)
-
(critical adj2 care).tw. (18,319)
-
(intensive adj2 care).tw. (93,030)
-
(inpatient or inpatients).tw. (66,467)
-
critical care/ (42,427)
-
critical illness/ (19,515)
-
Intensive Care/ (42,427)
-
exp intensive care units/ (62,277)
-
critical* ill*.tw. (31,178)
-
ICU.tw. (31,036)
-
or/20–29 [ICU] (235,133)
-
early diagnosis/ (18,720)
-
(early adj3 (treatment* or intervention* or involv*)).tw. (71,209)
-
(early adj3 onset).tw. (28,860)
-
(early adj3 (therapy or therapies)).tw. (12,709)
-
(early adj4 management).tw. (10,464)
-
early initiation.tw. (2380)
-
pre-emptive.tw. (1823)
-
or/31–37 [Early treatment] (138,435)
-
19 and 30 and 38 (232)
-
(prevent* adj3 (‘acute kidney injury’ or ‘acute kidney failure’ or ‘acute kidney disease’ or AKI)).tw. (406)
-
acute kidney injury/pc (2634)
-
40 or 41 [AKI prevention] (2824)
-
30 and 42 (299)
-
39 or 43 (505)
-
exp treatment outcome/ (738,812)
-
exp clinical trial/ (726,655)
-
effect*.ti. (1,483,694)
-
or/45–47 [Treatment effect] (2,629,847)
-
44 and 48 [AKI ICU Early intervention or Prevention Treatment Effect] (125)
-
limit 44 to ‘reviews (best balance of sensitivity and specificity)’ (213)
-
49 or 50 (310)
-
exp animals/not exp humans/ (4,201,019)
-
preconditioning.ti. (5874)
-
perioperative*.ti. (13,889)
-
or/52–54 [studies to remove] (4,216,305)
-
51 not 55 (296)
List of abbreviations
- AKI
- acute kidney injury
- AKIN
- Acute Kidney Injury Network
- BNP
- brain natriuretic peptide
- CEA
- cost-effectiveness analysis
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- CKD
- chronic kidney disease
- CLSI
- Clinical and Laboratory Standards Institute
- CONSORT
- Consolidated Standards of Reporting Trials
- CPB
- cardiopulmonary bypass
- CTIMP
- Clinical Trial of an Investigational Medicinal Product
- DAC
- Diagnostics Advisory Committee
- DEC
- Diagnostic Evidence Co-operative
- DOR
- diagnostic odds ratio
- EQ-5D
- EuroQol-5 Dimensions
- ESRD
- end-stage renal disease
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- FDA
- Food and Drug Administration
- FN
- false negative
- FP
- false positive
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IGFBP-7
- insulin-like growth factor-binding protein 7
- IL
- interleukin
- INB
- incremental net (health) benefit
- INMB
- incremental net monetary benefit
- IVD
- in vitro diagnostic
- KDIGO
- Kidney Disease: Improving Global Outcomes
- KIM-1
- kidney injury molecule-1
- L-FABP
- liver fatty acid-binding protein
- LR–
- negative likelihood ratio
- LR+
- positive likelihood ratio
- LTHT
- Leeds Teaching Hospitals NHS Trust
- NAG
- N-acetyl-beta-D-glucosaminidase
- NGAL
- neutrophil gelatinase-associated lipocalin
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QAMPs
- Quality Assessment of Measurement Procedures
- QUADAS-2
- quality assessment of diagnostic accuracy studies
- RCT
- randomised controlled trial
- RIFLE
- Risk, Injury, Failure, Loss of Kidney function and End-stage kidney disease
- RR
- relative risk
- RRT
- renal replacement therapy
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SG
- standard gamble
- SROC
- summary receiver operating characteristic
- STARD
- Standards for Reporting Diagnostic Accuracy
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- TIMP-2
- tissue inhibitor of metalloproteinases 2
- TN
- true negative
- TNF-α
- tumour necrosis factor alpha
- TP
- true positive
- TTO
- time trade-off
- uL-FABP
- urine liver fatty acid-binding protein
- VEGF
- vascular endothelial growth factor
- VOI
- value of information
