Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/145/04. The contractual start date was in June 2014. The draft report began editorial review in January 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Laura Jones reports personal fees from Quit51 Bionical outside the submitted work. Jonathan Mathers reports personal fees from Health Education England outside the submitted work. Melanie Calvert reports personal fees from Astellas Pharma Inc. and Ferring Pharmaceuticals outside the submitted work. Jonathan Deeks was a member of the National Institute for Health Research (NIHR) Health Technology Assessment Commissioning Board and the NIHR Efficient Study Designs Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Moiemen et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and background
Burn injury is the fourth most common type of trauma after road traffic accidents, falls and interpersonal intentional injury. 1 In 2004, it was estimated that 11 million people of all ages suffered a fire-related burn injury worldwide, that is, more than the number of people diagnosed with a human immunodeficiency virus (HIV) infection and tuberculosis combined. 2,3 Burns cause 265,000 deaths annually and are one of the leading causes of disability-adjusted life-years lost. 2 Seventy-one per cent of patients who survive suffer significant scarring. 4 Burn care during the last 30 years has seen a steep change in survival. 5 Fortunately, the increased survival has been paralleled by improved acute care and durable wound cover resulting in less deformity and scarring. However, there is still a great, and increasing, need for post-burn scar management. In a systematic review, Lawrence et al. 6 identified the need for further research to address the lack of a consistent definition of scarring, poor assessment methodology and the need for comprehensive scar outcome measures. In a comprehensive review of 703 burn survivors’ records, Gangemi et al. 4 identified the risk factors for post-burn hypertrophic scarring. These factors included being a young person, being female, having dark skin, sustaining a severe burn, the number of surgical procedures performed to achieve wound cover, the site on the body where the burn occurred (neck and upper limb in this study) and the time to the wound healing.
Post-burn hypertrophic scarring is typically treated non-invasively with the use of moisturiser, massage,7 pressure garments8,9 or silicone,10–13 or these modalities in various combinations. A survey of 19 paediatric NHS burn services in the UK showed that 18 services routinely use pressure garments for the prevention of hypertrophic scarring following burn injury. 14
History of hypertrophic scarring and pressure garment therapy
The first historical mention of keloid scarring dates back to the time of the ancient Egyptians, who recognised the condition and even realised at the time that treatment is difficult and that doing nothing is a wise strategy. The Edwin Smith Surgical Papyrus from 1700 BC, which predates Hippocrates by almost 1000 years, is thought to have been authored by an architect, a high priest and a physician of the Old Kingdom of ancient Egypt. The papyrus is a 4.88-metre scroll, with a detailed description of 84 case studies. It is considered to be a trauma manual of different parts of the body that is aimed at battle injuries. 15,16 In one case, there is a description of a growth on the chest of one of the patients and the author wisely recommended not to excise.
Although scarring was recognised in ancient times, the clear understanding and definition of the hypertrophic scar occurred much more recently. In 1962, Mancini and Quaife17 made the distinction between keloid and hypertrophic scarring in the way that we understand it today. In 1973, Madden and Peacock18 provided the first histological understanding of keloid and hypertrophic scarring.
In the sixteenth century, Ambroise Paré, a French barber surgeon who pioneered battlefield medicine, described pressure therapy for scarring. 19 In 1649, Thomas Johnson translated the original book The Work of that Famous Ambroise Paré20 that was first published by Paré in 1545. Paré recommended using pressure onto scars to improve their quality by applying a lead plate that is rubbed against mercury onto the scars. 21
It was not until the middle of the twentieth century that elasticated conforming garments were first used to improve surgical wound scars on the neck. In 1960, Fujumori et al. 22 published their experience of applying an adhesive foam mould to neck hypertrophic scars. At a similar time, Cronin23 and Gottlieb24 separately reported using cervical splints after the release of neck contractures and reconstruction with split-thickness skin grafts. Dr Duane Larson, the chief of staff of Shriners Hospitals for Children, and his team were the first to introduce the modern elasticated custom-made pressure garment, as we know it today, for burns. Several publications from their centre in the late 1960s and early 1970s publicised the technique in the USA and Canada and then worldwide. 20
Evidence of the effectiveness and efficacy of pressure garment therapy
Pressure garment therapy (PGT) for the prevention and treatment of hypertrophic scarring has become the standard of care in almost all burn centres globally. In spite of the widespread use, randomised controlled trials (RCTs) on PGT were not published until the 1990s, when Kealey et al. 25 reported a RCT of 110 patients comparing Tubigrip™ (Mölnlycke Health Care Ltd, Dunstable, UK; a cotton elastic garment) with elastic nylon pressure garments. The clinical outcome of the two groups was comparable, with significantly greater adherence and lower cost in the Tubigrip arm of the trial. In 1995, Chang et al. 26 randomised 122 patients, who required either skin grafting or their wounds to be healed over a period > 2 weeks, to either PGT or no PGT. The two groups were matched for age, percentage of total body surface area (TBSA) burned and length of hospital stay. Patients were followed up until their scars matured. The median time to scar maturation was 266 days in the PGT arm and 273 in the no-PGT arm, with no statistical difference between the two groups as assessed by the Vancouver Scar Scale (VSS).
In 2000, Groce et al. 27 and Moore et al. 28 independently presented two RCTs at the annual conference of the American Burn Association. Groce et al. 27 randomised 46 children from Galveston, TX, USA, who had a mean TBSA burn percentage of 11.2%, to receive PGT or no PGT. 27 The authors reported improvement of scar height at 6 months, but no significant difference in vascularity, pigmentation or pliability between the two arms of the trial. This work was presented as a preliminary study with only 6 months’ follow-up. In a separate publication, Groce et al. 29 presented the 18-month follow-up of the same cohort. They could not demonstrate any clinical improvement when they compared low- and high-pressure therapy after 18 months’ follow-up. Moore et al. 28 included 23 patients from Seattle, WA, USA, with forearm burns in a RCT. For each patient, half of their scar received pressure and the other half did not. The authors followed the patients every 3 months, until the scars matured, and objectively measured scar colour, softness and thickness. The authors reported improved scar softness, as measured by a durometer, at 6 months but the difference in improvement between PGT and no PGT disappeared at 12 months. Scar thickness, as measured by ultrasound, did not show any significant difference between the two groups at any time point. When three physicians assessed the scars, they incorrectly identified the part of the scar that had pressure therapy in 70%, 75% and 80% of the cases. The authors concluded that pressure garments did not alter the ultimate appearance of burn scars following skin grafting.
In 2005, Van den Kerckhove et al. 30 published a RCT of 60 patients with 3 months’ follow-up. They compared two types of pressure: 15 and 10 mmHg. Scarring was objectively assessed monthly for a total follow-up of 3 months, which is a very short follow-up period for the assessment of scar maturation. The authors found significant improvement of scar thickness and erythema when using a pressure of 15 mmHg compared with 10 mmHg.
In 2009, Anzarut et al. 31 conducted a systematic review of the available evidence for the use of PGT and assessed the quality of this available evidence. In the same article, the authors also conducted a meta-analysis to quantify the clinical effectiveness of PGT. The authors identified six studies in their review, one of which was unpublished [Tredget EE, Shankowsky HA, Mathey S and Anzarut A (editor), Edmonton, AB, Canada; 2004]26–30 which included a total of 316 patients. These six studies were considered to be of high methodological quality. The meta-analysis for global scar assessment did not show a difference between PGT and no PGT. However, scar height showed a small but statistically significant improvement when PGT was used. Other secondary outcome measures, including vascularity pliability and colour, failed to demonstrate a difference between the two therapies. The authors concluded that methodologically rigorous and adequately powered studies were required and called for a definitive trial of the clinical effectiveness of PGT for the prevention of abnormal scarring after burn injury.
Since the Anzarut et al. 31 systematic review and meta-analysis, there have been only a few RCTs of the use of PGT. 32–34 In 2010, Candy et al. 32 included a total of 53 patients in their trials, who had a mixture of post-burn, trauma and surgical wound scars that developed for 3–9 months before inclusion into the trial. 32 Participants were randomised into two trial arms of two pressures: 20–25 and 10–15 mmHg. The trial showed improvement in the redness and thickness, but no improvement in the pliability or colour of the scars of patients treated with the high-pressure garments. A four-arm RCT was published in same year from the same centre but had a different cohort of 104 Chinese patients, who had scars at variable stages of maturation that were caused by a mixture of burns, trauma or surgery. Patients were randomised to groups that used pressure garments, silicone gel sheets or a combination of pressure garments and silicone gel sheets, or a single-blinded control group. The treatment was given for 6 months. The results suggested that there was a benefit regarding scar thickness for both the pressure garment and the combined groups. 33
In 2010, Engrav et al. 34 published a follow-on trial from Seattle, WA, USA, from the Moore et al. 28 trial in 2000. As in the original study design, scars were divided into proximal or distal parts. A ‘treatment’ pressure of 17–24 mmHg was applied to one half of the scar and a ‘control’ pressure of < 5 mmHg was applied to the other half, enabling a ‘within-wound study’. The authors reported the results of 67 consecutive patients who were enrolled over the 12-year period with 12 months’ follow-up. They objectively measured scar hardness, colour and thickness, and the clinical appearance of the scars was also assessed by a panel of experts. Twenty-three patients from the Moore et al. trial from 2000 were included in this study. The authors stated that wounds treated with normal compression (> 15 mmHg) were significantly softer and thinner and had improved clinical appearance. They also concluded that these findings were evident only with moderate to severe scars and recommended the use of PGT for children. As the authors mentioned, there were changes in staffing, garment manufacturing and the hardware and software used to assess scars during the 12 years of the study. As an example, 11 of the 25 patients who received treatment between 2000 and 2007 received a pressure of ≥ 15 mmHg instead of < 5 mmHg as a control pressure. Regarding the recommendation for using PGT in children and for moderate and severe burns, there was no stratification of data by age or scar severity to justify this. The data that were presented came from only three children aged < 15 years. Eleven experts assessed the clinical appearance of the scars. The experts could identify the treatment zone in only 3 of the 41 patient photographs. Unfortunately, this trial was rated as having a high risk of bias.
Complications and adherence with pressure garment therapy
None of the clinical trials discussed in the previous section reported the adverse effects that may be associated with using PGT or the costs of the custom-made garments. Only one trial reported the problem of adherence in the use of the garment. 25 It is usually recommended that pressure garments are worn 23–24 hours a day from the time of the burn wound healing until scar maturation. Wearing the garment can be uncomfortable, especially in warm climates, and can cause overheating, wound breakdown or itching or appear unattractive. The garment can also cause skeletal and facial growth delay during the use of pressure garments for facial burns in children; however, this growth delay recovers after the therapy is discontinued. 35
In 2000, Hubbard et al. 36 reported two cases of children who received PGT for facial and upper trunk scars and developed obstructive sleep apnoea.
Pressure garment therapy is a high-demand treatment for burn survivors who may have very complex recovery and rehabilitation programmes. It is not surprising that adherence with PGT is a significant problem. 37,38 In 1994, Johnston et al. 39 conducted a structured adherence survey of 145 adult burn survivors. In total, 101 patients returned the questionnaire. The overall adherence rate was 41%. Adherence was better in men, in those with higher income (i.e. > US$30,000) and in those with burns of < 8% TBSA. The qualitative study conducted by Ripper et al. ,40 in 2009, gave an excellent insight into patients’ experience of PGT. Ninety-five per cent of respondents reported functional or physical problems in using the pressure garment, in the form of sweating, overheating, pain or itch, and 67% reported that additional effort is needed to manage the garment.
Evidence emerging during the time period of the PEGASUS study
During 2015–16, both a RCT protocol41 and a Cochrane review protocol42 were published. The RCT was designed to examine the effectiveness of low pressure (4–6 mmHg) on skin graft donor site scarring using Tubigrip, a low-cost elasticated cotton garment that is gentle to apply to fragile wounds. The Cochrane review protocol (which has recently been withdrawn) emphasised the need to clear the uncertainty that still exists regarding the clinical effectiveness of PGT, the use of which is advocated but has never been scientifically proven. Since the commissioning brief for this study was released, there has been little new evidence to support or discourage the use of PGT.
Rationale for the PEGASUS study
Although PGT is an established clinical treatment for post-burn hypertrophic scarring in almost all NHS burns service providers in the UK, there is limited evidence of PGT’s efficacy, clinical effectiveness and cost-effectiveness. There is also some evidence of poor adherence with the treatment, inconvenience and occasional complications. Although there appears to be adequate uncertainty of the value and effectiveness of PGT to consider the need to conduct a well-designed definitive trial,31 health-care professionals (HCPs) have been actively engaged or advocating this therapy for decades and a lack of clinical and personal equipoise may be a barrier to recruiting to an ethically acceptable RCT. In addition, patients, parents or their carers may have an expectation of receiving PGT for scar management, and these expectations may represent a barrier to their willingness to participate in a RCT. Identifying the barriers to, and the facilitators of, conducting a full-scale trial, or indeed if such a trial is feasible at all, is the main objective of the PEGASUS (A mixed-methods feasibility study including a randomised trial of PrEssure Garment therApy for the prevention of abnormal Scarring after bUrn injury in adultS and children) study.
The PEGASUS pilot feasibility trial will also inform the design of the full-scale trial. Burn survivors experience multifactorial problems related to scarring, function, psychological and social difficulties and reduced quality of life (QoL). Identifying core scar outcome domains is important to select patient-reported outcome measures (PROMs) that are specific, and unique, to both the patients’ and the HCPs’ experiences of post-burn scarring. A validated burn-specific measure for post-burn hypertrophic scarring should be considered as the primary outcome measure for a full-scale trial of PGT.
The PEGASUS study consists of a series of studies organised in two phases. In phase 1 (P1), national staff surveys and interviews with HCPs, patients and carers were conducted to assess the feasibility and acceptability of the trial in principle and to identify the suitability of potential outcome and economic assessments. A novel element of the study was the exploration of the feasibility of capturing costs and resource use from both NHS and societal perspectives and the feasibility of using contingent valuation (willingness to pay; WTP) to value outcomes and process utility from a patient perspective.
In phase 2 (P2), a pilot RCT tested the feasibility of running a RCT in practice and, particularly, whether or not it would be possible to recruit patients in sufficient numbers for a full-scale trial of PGT. Integrated qualitative research examines, in detail, the experiences of patients and clinicians of participating in, and delivering, the pilot trial. This research was designed to provide an opportunity to reflect upon trial participation and processes (e.g. recruitment and outcome assessment) and to understand how practice is influenced by staff and patients’ attitudes to PGT, as seen in P1.
Chapter 2 Aims and objectives of the PEGASUS study
Objectives of the different phases of the study
The aim of the PEGASUS study was to assess the feasibility of a full-scale RCT on the clinical effectiveness and the cost-effectiveness of PGT. If such a trial was feasible, the outcome of the PEGASUS study would inform the design of the full trial.
The PEGASUS study was organised in two phases: P1 and P2. In the following sections, we list the objectives addressed in each phase and briefly indicate the study method used. Full details of methods are included in each chapter.
Phase 1
Phase 1 consisted of a series of surveys and interviews with patients and health-care providers. The following objectives were assessed:
-
The current UK practice of PGT, including the number of patients receiving treatment, how garments are manufactured, costs of the garments to each NHS burns service and an estimate of the total costs to the NHS – data were collected by a web-based survey that was sent to all UK NHS burns units and data of the duration of PGT across the burn care providers were also collected. (Details and results are reported in Chapter 3.)
-
The number of children and adults eligible to be included in a PGT clinical trial in UK NHS burns units – data were collected by the same web-based survey used in objective 1. (Details and results are reported in Chapter 3.)
-
The views of HCPs [including consultants, nurses, physiotherapists and occupational therapists (OTs)] on conducting a RCT to compare PGT and no PGT, including attitudes to participation, perceived equipoise and barriers to, and facilitators of, undertaking and participating in a full-scale RCT of PGT – data were collected through a second web-based questionnaire, which was sent to individual HCPs, and in-depth interviews. (Results are reported in Chapter 4.)
-
The experience of patients and carers of PGT and their willingness to be randomised between PGT and no PGT – the impact of PGT on psychological and social functioning, body image and self-esteem and the psychological protection that some patients feel that PGT provides were explored. PGT treatment burden, perceived benefits and compliance (adherence to the treatment) were also assessed. Qualitative interviews were undertaken with patients who previously received PGT and/or their carers, either as face-to-face interviews or telephone interviews. (Details and results are reported in Chapter 5.)
-
Patient-centred outcomes that specifically measure post-burn scarring and appropriate methods of evaluating these outcomes – specific domains that are important to the patients, carers and HCPs were established in order to select the most suitable primary outcome for the full-scale trial. Data from the staff attitudes survey (objective 3) and from qualitative interviews with patients and carers (objective 4) and clinicians and HCPs were used. (Details and results are reported in Chapter 5.)
-
Appropriate methods for evaluating outcomes for the health economic analysis and resource use. (Details and results are reported in Chapter 6.)
Phase 2
Phase 2 was a multicentre pilot RCT that was conducted in four paediatric and six adult recruiting centres across England and Wales. This trial was an open two-arm trial with 12 months’ follow-up. Participants were randomised to PGT or no PGT, with both groups receiving other modalities of scar management, such as massage, moisturisation or silicone treatment.
The following objectives were assessed in the pilot RCT:
-
Recruitment rates and willingness to randomise between PGT and no PGT in multiple sites within the pilot RCT. (Details and results are reported in Chapter 7.)
-
Adherence with randomised allocation and PGT therapy. (Details and results are reported in Chapter 7.)
-
Possible processes for achieving blinded assessment of outcomes. (Details and results are reported in Chapter 7.)
-
Outcome distributions to inform a sample size calculation for a future trial. (Details and results are reported in Chapter 7.)
-
The perspectives, and experiences, of the patients, carers and HCPs who participated in the trial on trial participation and processes by qualitative process evaluation. (Details and results are reported in Chapter 8.)
-
The feasibility and success of the economic assessment to inform the assessment of cost-effectiveness in a future trial. (Details and results are reported in Chapter 9.)
The original study plan involved running P1 and P2 sequentially to allow findings from the qualitative work and surveys to influence the design of the pilot trial. However, the study duration was reduced on the request of the funding board and the two phases were run concurrently, which reduced the opportunity of findings from P1 to influence the design of the pilot trial in P2.
Chapter 3 UK survey of current practice of pressure garment therapy
Relevant objectives
The following objectives are assessed in this chapter:
-
to detail the manufacturers of pressure garments, the types of garments and the length of time that PGT is used, by patient group
-
to assess the number of children and adults eligible to be included in a trial of PGT.
Background
Pressure garments are designed to deliver a recommended pressure using an elastic fabric that contains Lycra® (INVISTA, Wichita, KS, USA). Scar management therapists are offered a choice of different fabrics and make their choice based on the functional requirements and comfort of the patient. Current recommendations, issued by suppliers of pressure garments, recommend regular wear time but do not stipulate a prescribed amount of time for the pressure garment to be effective. There is consensus, within the literature, for the length of time that patients should wear the pressure garment for, namely 23 hours a day. Following this guidance, scar management therapists recommend that their patients wear PGT for this length of time, removing garments to facilitate hygiene needs and for moisturisation and hydration of the burns scars.
Within the literature, the recommended number of months that patients should wear the pressure garment for, to achieve an optimally treated scar, ranges from 6 to 24 months. 31,43,44 The survey was designed to establish current practice concerning the length of time PGT is used and the rationale behind the recommendations.
Methodology
A web-based survey was sent to the lead OTs or physiotherapists at each of the UK NHS burns services across the UK.
The survey consisted of eight questions.
-
Who provides your burns pressure garments? (In-house/external.)
-
Approximately what is your monthly spend/cost of pressure garments for burns patients?
-
Do the general practitioners (GPs) fund the burns pressure garments?
-
How many new burns patients go into garments per month or year?
-
How many of these new patients are adults?
-
How many of these new patients are children?
-
Approximately how long do your burns patients wear garments for? (For example, 6–18 months.)
-
What guides you to select a particular garment?
The aim of the survey was to establish who provides the pressure garments for the burns patients throughout the UK, the current practice with regard to the length of time pressure garments are worn and the number of eligible patients that each NHS burns service could potentially recruit per month. In tallying the results, we have separated services for adults from services for children to enable separate enumeration of patient numbers and costs by age group. Across the existing 30 NHS burns services, 26 provide services for adults and 25 for children.
A total of 30 responses to the web-based survey were received from 15 services for adults and 15 services for children. Each of the remaining services was contacted again, via telephone and follow-up e-mails, but we were unable to obtain the required data. In calculating the total number of patients, we extrapolated the results obtained from the survey, stratifying by children and adults and by burns service type. Predicted annual NHS costs were computed assuming, on average, that children require 12 garments and adults require eight garments per year, with the average cost of an in-house garment being £50 and an externally sourced garment £81.
We were able to identify which of the NHS burns services manufacture in-house garments and which externally source them by reviewing the minutes from the Pressure Garment Meeting April 2016. We also contacted the external manufacturers by telephone to confirm which NHS burns services they currently supply and were able to identify from this the provider of pressure garments across the sites.
Results
Responses
A total of 26 NHS burns services were identified that provided care to adults and 25 that provided care to children. Responses were received from 15 of the adult services (58%) and 15 (60%) of the child services (Table 1).
| Burns unit’s tier level | Number of units in NHS | Results from survey of units | Extrapolation to NHS | |||||
|---|---|---|---|---|---|---|---|---|
| Number of units responding | Number of adults per year | Number of children per year | Garment spend per year (£) | Total number of adults per year | Total number of children per year | Total spend per year (£) | ||
| Children only | ||||||||
| Burns facility | 7 | 2 | 0 | 12 | 11,664 | 0 | 60 | 58,320 |
| Burns unit | 8 | 7 | 0 | 540 | 395,424 | 0 | 588 | 442,080 |
| Burns centre | 10 | 6 | 0 | 552 | 536,544 | 0 | 828 | 804,816 |
| Adults only | ||||||||
| Burns facility | 8 | 3 | 27 | 0 | 16,752 | 78 | 0 | 49,056 |
| Burns unit | 8 | 5 | 312 | 0 | 202,953 | 499 | 0 | 324,725 |
| Burns centre | 12 | 7 | 540 | 0 | 290,472 | 792 | 0 | 391,272 |
| Total | 53 | 30 | 879 | 1104 | 1,453,809 | 1369 | 1476 | 2,070,269 |
Numbers of patients receiving pressure garment therapy
Currently, within the UK, patients are treated for their burns injury within one of the three following NHS burns services:
-
burns facility – treatment of burns with a TBSA of < 10%
-
burns unit – treatment of burns with a TBSA of < 30% for adults and < 20% for children
-
burns centre – treatment of any size burn and all complex burns.
The number of eligible patients for PGT varied across each of the NHS burns services. From the 30 NHS burns services that responded to the survey, five (three for adults and two for children) are burn facilities (for a TBSA of < 10%), twelve (five for adults and seven for children) are burns units (for a TBSA of < 30% for adults and < 20% for children) and thirteen (seven for adults and six for children) are burns centres (for all percentages of TBSA).
Survey responses indicated that the number of eligible patients from the burn facilities ranged from 0 to 1 patients per month, burns units had 1–15 patients per month and burn centres had 2–18 patients per month. The services that responded to the survey estimated that, in total, 879 adults and 1104 children are treated each year, 55% in burns centres and 43% in burns units. Assuming similar patient numbers in facilities, units and centres that did not respond, we estimate that there are 1369 adults and 1476 children treated across the UK in burns services each year.
Source and cost of pressure garments
Information on garment source was available for all 26 services for adults and 25 of the services for children. Thirty-seven (72%) NHS burns services, the majority, choose to externally source pressure garments; of these services, 35 use the manufacturer Jobskin Ltd (Nottingham, UK), one DM Orthotics Ltd (Redruth, UK) and one Gottfried Medical, Inc. (Toledo, OH, USA). The remaining 14 (28%) NHS burns services provide an in-house service, which is mostly provided by the OT department, although one NHS burns service used their orthotics department.
Of the adult services, 31% produce pressure garments in-house; of the child services, 26% produce them in-house. The 30 services that completed the web-based survey consisted of eight with in-house pressure garment services and 22 with externally sourced pressure garment services, which were equally split between paediatric and adult services.
The approximate cost for the in-house garments per NHS burns service was £3237.50 per month, giving a yearly spend of £38,850 per service. The approximate cost for the external garments per NHS burns service was £4329.58 per month, giving a yearly spend of £51,995. We noted variation in the monthly spends per burns service, with a range of £300–5420 and a mean of £2986.
Extrapolating to all UK units based on the predicted patient numbers and standardised costs and garment numbers, and accounting for the split between internal and external provision, we estimate that the total NHS annual spend on pressure garments is £765,053 for adult services and £1,305,216 for paediatric services.
Funding for the provision of pressure garments varies depending on the burns unit and the hospital trust. The majority of the burns units obtain funding for pressure garments from the hospital budget, which is allocated to the following departments:
-
occupational therapy department
-
therapy department
-
burns and plastics
-
emergency budget.
Only three of the respondents received the pressure garment funding through their general practice.
Duration of pressure garment therapy
It is advised by the manufacturer and by the therapist that the patient wears the garment for 23 hours a day until the scars are fully mature. ‘Fully mature’ is a term that is used when the scars are no longer responding to pressure, appear pale in appearance when looking at vascularity, and do not alter in height when the garments are removed. When asking the question in regard to wear time, it was found that the time to scar maturity varied across the units and between patients. The length of time that patients are expected to wear pressure garments ranged across the burns centres from 3 months to 2 years, with the majority of burns centres recommending patients wear pressure garments for 12–18 months and with no difference noted between adult and paediatric recommendations.
Pressure garment selection
Pressure garment selection is routinely done by an experienced burns therapist who will consider many external factors (including physical and psychological factors) when selecting whether or not the patient is appropriate for PGT and the design of the garment. A wide range of designs are available both externally and internally, with bespoke garments often being designed and altered by the in-house technician or external manufacturer to ensure that the scar area is covered and the appropriate pressure is applied.
Within the survey we asked the NHS burns services ‘What guides you to select a particular garment?’. In 16 of the responses, the following criteria were listed as being important when selecting a particular garment:
-
burn location
-
burn size
-
patient’s age
-
functional ability
-
dexterity.
In addition to these factors, one site mentioned that all patients who have suffered a burn injury are prescribed PGT regardless of factors such as scar size and location. A blanket referral into pressure garments is unusual, as the therapist will usually complete a subjective assessment as part of their initial assessment that will determine the scar therapy requirements of the individual patient.
Factors for selecting a particular garment did vary between adults and paediatrics; one NHS burns service stated that it would consider the patient’s type of work and whether or not certain pressure garments would be suitable in some environments.
Two of the paediatric sites considered factors relating to children and their age, such as the need for current or future toilet training, when selecting particular pressure garments.
Local protocols for scar management using PGT vary across the service but are transferable within networks and the wider scar management service throughout the UK. The British Burn Association’s Burn Therapists’ Special Interest Group allows scar management therapists to meet annually to discuss current practice and benchmark their service to ensure that a gold standard of practice is available and delivered. Scar management guidelines are used by each of the networks for therapists to read and follow as their service allows; many different scar modalities are available but finance and staffing has an impact on whether or not these are available in each of the UK burns services. It is important to note that, as a result of staffing levels, three of the burns services that responded to the web-based survey only partially completed the survey.
Summary
-
Thirty out of 51 services in England, Scotland, Wales and Northern Ireland responded to the survey, representing 15 adult and 15 paediatric burns services. Missing data were obtained from conference reports and manufacturers.
-
We estimate that NHS burns services treat 2845 eligible patients over a 12-month period: 1476 who are aged < 16 years and 1369 aged ≥ 16 years.
-
Routinely, pressure garments are used for between 6 and 18 months and are either manufactured in-house or sourced externally (from three different companies). The majority of burns centres recommend that patients wear pressure garments for 12–18 months, with no difference noted between adult and paediatric recommendations.
-
Few UK trusts employ in-house technicians to manufacture pressure garments, with only between one-quarter and one-third choosing to provide an in-house service and the majority choosing to externally source. In-house garments are generally less expensive than externally sourced garments. Many of the individual services stated that they are aware of the difference in cost but are unable to apply for additional staff funding to employ pressure garment technicians, and so they continue to use external companies.
-
The funding streams of the pressure garment services varied across the UK, with many services not being able to provide their monthly garment costs initially because funding for the service was part of a much larger budget. Many services are still reliant on the ‘therapy budget’ or ‘plastics budget’ to fund the pressure garments, with others managing to get their general practice or health board to fund them. It is estimated that the cost of pressure garments to the NHS for burns patients is £2,171,184 per annum.
Chapter 4 National burns staff attitude survey and interviews
Relevant objective
The following objective is assessed in this chapter:
-
to understand the willingness of clinicians and therapists to randomise children and adults between PGT and no PGT, and to identify perceived barriers to participation in a RCT.
Methods
Online survey and analysis
A staff attitude survey was developed by the PEGASUS team in-house, given the lack of appropriate validated questionnaires in the literature (see Appendix 1). We sought input from our patient and public involvement (PPI) group during development, and pilot tested the survey with local burns staff, making revisions to language and ordering when necessary. The questionnaire, via fixed (e.g. yes/no, Likert scaling) and free-text responses, covered the following areas: (1) respondent demographics, (2) experience with PGT, (3) views on PGT for children and young people, and adults, (4) views on a full-scale RCT of PGT, (5) participation in a full-scale RCT of PGT, (6) patients’ (children and young people, parents, and adults) willingness to participate in a RCT and (7) clinical and non-clinical outcomes. Fixed-response data were analysed using simple descriptive statistics. Free-text responses were analysed using a simple content analysis (thematic) approach.
Survey sampling
Between October 2014 and March 2015, 30 burns services across England, Wales and Scotland were approached to participate in the survey (Figure 1). The survey link was sent out to all staff involved in PGT or scar management by the lead therapist in each service. Reminder e-mails were sent out up to three times, with all staff actively encouraged to participate within the data collection window.
FIGURE 1.
Map of PEGASUS P1 and P2 burns services in England, Scotland and Wales.
Qualitative telephone interviews and analysis
At the end of the online survey, participants were invited to provide their details to give permission for the PEGASUS research team to contact them to explore their survey responses in more detail. Respondents who left their details were then purposively sampled based on the following variables: (1) profession, (2) yes versus no response to a RCT for PGT and (3) agreement versus disagreement with attitudinal survey questions. A semistructured discussion guide was developed (see Appendix 2) based on the survey questions, the literature, discussions with our PPI group and the wider PEGASUS research team. Interviews were undertaken by telephone, audio-recorded, transcribed clean verbatim and analysed using a thematic approach. 45 Quotations in Results are identified using the participant’s unique identifier code and indicate whether the quotation was from an adult patient, a parent of a paediatric or adolescent patient or a member of NHS burns service staff. Staff quotations indicate profession and, when required, whether or not the quotation was from a survey free-text response.
Results
Sample characteristics and response rates of survey and interview participants
Responses were received from 245 burns service staff from 28 out of 30 NHS burns services in England, Scotland and Wales that were approached to participate. Following cleaning of the data, 223 surveys were usable for analysis. It was not possible to accurately calculate the number of staff who were approached to participate within each service to calculate the response rate for the survey, but the mean number of respondents from each service was eight (range 1–24). Fifteen survey respondents from 13 burns services also participated in a semistructured telephone interview (average interview length was 32 minutes, range 19–41 minutes). Table 2 provides a summary of respondent sample characteristics for the survey and interview participants. Participants were predominantly female, nurses or therapists and caring for both adults and children.
| Characteristics | Methodology, n (%) | |
|---|---|---|
| Survey (N = 223) | Interview (N = 15) | |
| Sex | ||
| Male | 29 (13.0) | 3 (20.0) |
| Female | 192 (86.1) | 12 (80.0) |
| Not stated | 2 (0.9) | 0 (0.0) |
| Profession | ||
| Doctors | 29 (13.0) | 3 (20.0) |
| Nurse/charge nurse | 89 (39.9) | 4 (26.7) |
| OT | 43 (19.3) | 5 (33.3) |
| Physiotherapist | 32 (14.3) | 2 (13.3) |
| Other | 30 (13.5) | 1 (6.7) |
| Patient group | ||
| Adults (aged ≥ 16 years) | 67 (30.0) | 4 (26.7) |
| Children (aged ≤ 15 years) | 56 (25.1) | 3 (20.0) |
| Adults and children | 100 (44.8) | 8 (53.3) |
| Pilot site | ||
| Yes | 88 (39.5) | 3 (20.0) |
| No | 127 (57.0) | 12 (80.0) |
| Missing | 8 (3.6) | 0 (0.0) |
Perspectives on pressure garments as part of scar management therapy
One hundred and sixty-seven of the survey respondents reported that they provided care either to adults only (n = 67) or to adults and children (n = 100). Table 3 provides a summary of staff perspectives on the use of PGT for scar management in adult patients. Staff reported that they agreed with the statements that PGT is one of the most important treatments (n = 123/167; 74%) and that it is beneficial to patients (n = 150/167; 90%), and just over half (n = 93/167; 56%) thought that it should remain the standard scar management treatment. However, 58% of staff (n = 97/167) were unsure about whether or not there was research evidence on the clinical effectiveness of PGT and some (n = 43/167; 26%) were uncertain of the benefits of PGT.
| Survey statements | Patients, n (%) | |
|---|---|---|
| Adults (N = 167) | Children (N = 156) | |
| PGT is one of the most important treatments | ||
| Strongly agree or agree | 123 (73.6) | 115 (73.7) |
| Neither agree nor disagree | 35 (20.9) | 34 (21.8) |
| Disagree or strongly disagree | 7 (4.2) | 6 (3.8) |
| Missing | 2 (1.2) | 1 (0.6) |
| PGT is a beneficial treatment | ||
| Strongly agree or agree | 150 (89.8) | 145 (92.9) |
| Neither agree nor disagree | 14 (8.4) | 7 (4.5) |
| Disagree or strongly disagree | 1 (0.6) | 1 (0.6) |
| Missing | 2 (1.2) | 3 (1.9) |
| There is research evidence that PGT is an effective treatment | ||
| Strongly agree or agree | 68 (40.7) | 62 (39.8) |
| Neither agree nor disagree | 67 (40.1) | 67 (42.9) |
| Disagree or strongly disagree | 30 (18.0) | 25 (17.0) |
| Missing | 2 (1.2) | 2 (1.3) |
| PGT should remain the standard treatment | ||
| Strongly agree or agree | 93 (55.7) | 77 (50.6) |
| Neither agree nor disagree | 65 (38.9) | 71 (45.5) |
| Disagree or strongly disagree | 7 (4.2) | 5 (3.2) |
| Missing | 2 (1.2) | 1 (0.6) |
| I am uncertain that PGT is a beneficial treatment | ||
| Strongly agree or agree | 43 (25.7) | 45 (28.8) |
| Neither agree nor disagree | 29 (17.4) | 38 (24.4) |
| Disagree or strongly disagree | 93 (55.7) | 57 (36.5) |
| Missing | 2 (1.2) | 16 (10.2) |
One hundred and fifty-six of the survey respondents reported that they provided care either to children only (n = 56) or to adults and children (n = 100). Table 3 provides a summary of staff perspectives of the use of PGT for scar management in paediatric patients. In a similar pattern to responses to the adult data, staff reported that they agreed with the statements that PGT is one of the most important treatments (n = 115/156; 74%) and that it is beneficial to patients (n = 145/156; 93%) and half (n = 77/156; 51%) thought that it should remain the standard scar management treatment. However, 60% of the staff (n = 92/156) were unsure about whether or not there was research evidence on the clinical effectiveness of PGT and some (n = 45/156; 29%) were uncertain of the benefits of PGT.
The majority of staff reported that they perceived pressure garments to be important and of benefit to patients, even though they were unsure whether or not there was research evidence of clinical effectiveness. Staff, often based on their own clinical experience, reported that pressure garments were important because of the benefits to scar management and the fact that PGT was better than other treatments, such as the use of silicone and massage. Reported benefits included (1) improving scar appearance, (2) helping to control symptoms such as itch, (3) helping functionality, (4) reducing the time it takes for the scar to mature and (5) providing physical (e.g. protection) and psychological (e.g. confidence and security) benefits:
It makes the scar paler, it makes it softer, it can help minimise itching, it can . . . where there are bands forming that make contractures it can hold those tightly against the body and minimise those, softens, flattens, pales, less lumpy, more pliable. So it’s about the colour, the texture and the height.
TLR05 OT
There were, however, some interviewees who articulated that they did not perceive any benefit of using pressure garments. Rather, they believed that the patients perceived a benefit as a result of the ‘scenario’ in clinical practice, in which patients are influenced by the treatment plan specified by the clinician and have an expectation of pressure garments:
Well, I don’t know whether they are benefits actually, I don’t think they are benefits. I think we have created a scenario where patients feel bad without them. So in other words if I gave them joss sticks and said if you use these three times a day and massage with some lavender and it’s really important you do that, and if you do that for 3 months non-stop they might get the same benefit, we don’t know. It’s that whole scenario about it. Even before the patient’s fully healed people start talking about we’ll measure you up for the pressure garments, and so it’s almost a done deal.
TEG15 consultant
A small number of interviewees expressed some uncertainty about the benefits of PGT. For example, benefits were reported as being dependent on the location of the scar, the type of scar and the characteristics of the patient. Some interviewees stated that the benefits were only apparent if the pressure garments were well fitted and the patients were compliant with treatment but for some patients, even if they were compliant, scar outcomes could be poor. There was discussion of the facts that scars ‘settle’ over time anyway, irrespective of the treatment, but that pressure garments, although perhaps not better than other treatments, were better than no treatment at all:
I think in some cases some patients do comply really well, and they still get some quite bad scarring and they are wearing a pressure garment. But you have no comparison to say well would that scarring have been a lot worse if they hadn’t worn the garment. There are some individual things I think does influence it, and I do think there are some quirks, so I think some children that absolutely comply to their pressure garment can still get a bad scar.
TSC06 OT
There was a split decision, to some extent, within the survey respondents about whether or not PGT should remain the standard treatment for scar management. Interviewees highlighted that they perceived there to be a role for pressure garments in scar management but that the decision to use pressure garments was dependent on clinical judgement and experience. This ‘judgement’ was also linked to the perceived benefits of PGT based on characteristics of the scar and the patient. The role of PGT in scar management and perceived benefits were particularly apparent for those working with children:
I think it’s a clinical judgement on what that scar is doing, how it’s behaving. But I definitely feel there’s a clear role for the use of pressure garments, and that for some, particularly I think the tricky more aggressive scars, I think pressure garments work better than some of the other options out there.
TSC06 OT, children only
Some interviewees also reflected that PGT should be part of a ‘toolkit’ of treatment options and that the pressure garments should only be used when required, based on clinical experience, rather than as standard care in which all patients are expected and expect to receive them:
I think it is [PGT] definitely a very important treatment modality to have in my toolkit, and I do use it.
TSA11 OT
When explored in further detail during the interviews, there was a range of knowledge about the research evidence on the clinical effectiveness of PGT. Some participants were fully aware that there is currently little evidence to show that PGT is clinically effective; however, others stated that the survey had prompted them to look at the evidence and led to the realisation that they had made an assumption about the existence of good evidence of clinical effectiveness. In addition, for some, they found in their own anecdotal experience that PGT was effective:
. . . this is proper research evidence as in . . . so this is the group I think in Canada that did a very good comprehensive review of pressure garment use a long time ago.
TEG15 consultant
This is very interesting, and I own this, this was a complete assumption that there was evidence, because everybody does it, and it was only after I had filled out your survey and then was having a conversation with one of my therapy colleagues who said actually that isn’t necessarily the case. So I was erroneously confident in my knowledge [laughs].
TCW12 clinical psychologist
Attitudes to a full-scale randomised controlled trial for pressure garment therapy
In response to the question ‘Do we need a full-scale RCT of PGT?’, 67% of staff participants (n = 149/223) answered yes, 8% (n = 17/223) answered no, and 26% (n = 57/223) were undecided. Table 4 shows the breakdown of responses to this question by professional group and pilot versus non-pilot site. Nearly all doctors (n = 26/29; 90%) and the majority of therapists (OT, n = 32/43, 75%; and physiotherapists, n = 24/32, 75%) identified a need for a trial; however, there was less certainty for nurses (n = 52/89; 58%) and other professional groups (n = 15/30; 50%). There was a similar proportion of pilot site (n = 61/88; 69%) and non-pilot site (n = 83/127; 65%) respondents reporting the need for a full-scale RCT of PGT. Although only 8% (n = 17/223) of survey respondents overall said that there was no need for a full-scale RCT, further exploration of these responses as part of the free-text survey and the interview data highlighted the complexity of the underpinning rationale. The rationale for responding yes to this question was centred, in the main, around providing evidence to support current PGT practice:
Yes, and what I really would like to see is a good trial that supports it, because my feeling is that if we run a good trial with enough numbers then we’ll give ourselves the evidence. So if we can get a NICE [National Institute for Health and Care Excellence] recommendation to use pressure garments on burns then we won’t face a situation in the future where the people holding the purse strings say well actually that’s not a proven technique to improve scar maturation so we’ll not pay for it. I am worried about the implications on our care in the future if we don’t get the evidence for doing it.
TCW01 consultant
| Survey question | Survey responses, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Professional group | Site (missing n = 8) | |||||||
| Total (n = 223) | Interview (n = 15) | Doctors (n = 29) | Nurse/charge nurse (n = 89) | OT (n = 43) | Physiotherapist (n = 32) | Other (n = 30) | Pilot (n = 88) | Non-pilot (n = 127) | |
| Is a full-scale RCT of PGT needed? | |||||||||
| Yes | 149 (66.8) | 8 (53.3) | 26 (89.7) | 52 (58.4) | 32 (74.4) | 24 (75.0) | 15 (50.0) | 61 (69.3) | 83 (65.4) |
| No | 17 (7.6) | 2 (13.3) | 1 (3.4) | 8 (9.0) | 3 (7.0) | 0 (0.0) | 5 (16.7) | 5 (5.7) | 11 (8.7) |
| Undecided | 57 (25.6) | 5 (33.3) | 2 (6.9) | 29 (32.6) | 8 (18.6) | 8 (25.0) | 10 (33.3) | 22 (25.0) | 33 (26.0) |
| Staff willingness to participate in a full-scale RCT of PGT | |||||||||
| Yes | 143 (64.1) | 9 (60.0) | 23 (79.3) | 60 (67.4) | 26 (60.5) | 18 (56.3) | 16(53.3) | 73 (83.0) | 68 (53.5) |
| No | 15 (6.7) | 0 (0.0) | 2 (6.9) | 4 (4.5) | 4 (9.3) | 2 (6.3) | 3 (10.0) | 3 (3.4) | 11 (8.7) |
| Do not know | 65 (29.1) | 6 (40.0) | 4 (13.8) | 25 (28.1) | 13 (30.2) | 12 (37.5) | 11 (36.7) | 12 (13.6) | 48 (37.8) |
| Service willingness to participate in a full-scale RCT of PGT | |||||||||
| Yes | 144 (64.6) | 10 (66.7) | 21 (72.4) | 61 (68.5) | 25 (58.1) | 21 (65.6) | 16 (53.3) | 73 (83.0) | 67 (52.8) |
| No | 4 (1.8) | 0 (0.0) | 1 (3.4) | 1 (1.1) | 1 (2.3) | 0 (0.0) | 1 (3.3) | 1 (1.1) | 3 (2.4) |
| Do not know | 75 (33.6) | 5 (33.3) | 7 (24.1) | 27 (30.3) | 17 (39.5) | 11 (34.4) | 13 (43.3) | 14 (15.9) | 57 (44.9) |
Staff did, however, acknowledge that there was a need to challenge embedded practice that was not evidence based, particularly given the cost and treatment burden of PGT:
I think that it would be very helpful for everyone if we had objective evidence that pressure garments worked, because as time goes on, funding, etc., is going to be more and more dependent on evidence-based treatment, and I think that having strong evidence to support the pressure garments and the use of pressure garments, and therapy time to make garments or to measure, etc., and carry out the treatments, it’s always easier to justify if we’ve got some hard evidence.
TSC07 consultant
One consultant reflected that, even though they did not see the benefits of PGT, they were engaged in the study because they were concerned about the potential impact of changes to burns scar management on staffing and morale within the team:
. . . we wouldn’t be doing this project if I wasn’t, but generally to some extent it’s a problem [lack of PGT evidence] because I could take a very authoritarian view in my department and just stop using pressure garments. That puts one person out of a job, possibly two, and it would in one single act turn all of the burns therapists against me, because they have been taught, fed, aligned that these are effective, and they have categorical anecdotal proof. So I tread a careful line, and I’ve taken a view that I will stop people from wearing them once I review them in my clinic. But it’s an awful expense as well, because if you think about the number of clinical attendances, and the number of times the patient is seen by highly specialised staff, the garments that are worn, then actually it’s a serious cost and we could use that on something else.
TEG15 consultant
It was perceived that having ‘hard’ evidence might help to placate burn service management, give a definitive answer whether or not PGT should be used and would also to help to boost staff confidence in talking to patients about treatment options:
I am confident in the way I explain the things [pressure garments] to patients, and to advise them as to how these things work, and what I’ve seen from my experience about the other members of the team. But to actually have that hard evidence would just I suppose give us another backup to say if that is the case that we can show that to patients.
TWY10 physiotherapist
There were two main reasons why participants said that there was not a need for a RCT of PGT: first, they had limited awareness of any lack of clinical effectiveness evidence; and, second, they were happy with PGT as a treatment and were concerned, from an ethical point of view, that there would be a no-pressure-garment arm in a trial:
I guess it was interesting because I hadn’t even thought about people not being offered pressure therapy, and perhaps that was my ignorance, is that I had assumed that it was something that was a treatment that had been researched and proved to be something that it was clinically effective and that we should be using. So I was very much, I’ll own it, I was coming from a position of ignorance that hadn’t actually realised that wasn’t necessarily the case . . . Yes, because I was ignorant before [when responded no to a RCT in survey], I thought we had one, why would you do it again?
TCW12 clinical psychologist
Those staff who were undecided about a RCT reflected that their reasoning centred around staffing and ethical concerns. The latter may highlight a lack of equipoise. Participants raised concerns about not having enough staff to participate in a RCT and around the specific design of the RCT (see Chapter 8 for further discussion). The ethical concerns were similar to those who said no to a RCT for PGT. Staff, in particular nurses, struggled to reconcile the fact that by undertaking a RCT there was a chance that patients, who they believed should be in pressure garments, might not receive them, that is, ‘withholding’ the best treatment. This was particularly true for patients with certain scar characteristics, such as very large or ‘severe’ scars. Furthermore, the staff were concerned that patients would want the ‘best treatment’, which in their view is PGT:
Yes, mine was mainly from an ethical background of if it is seen, and I don’t know if it is or not, as the gold standard treatment now, how the study would take place, some people not having pressure garments, some people having pressure garments, I don’t really . . . because obviously with a study you need to provide the treatment that’s at least as good as the treatment you . . . or think is as good as the treatment you’ve got now, so I couldn’t get my head around, I couldn’t understand what could be as good as having pressure garments, because otherwise you’re disadvantaging perhaps some patients who wouldn’t have pressure garments to measure against.
TSW14 nurse
Attitudes to undertaking and participating in a full-scale randomised controlled trial for pressure garment therapy
Table 4 shows the proportion of staff and perceived proportion of burns services who would or would not be willing to participate in a full-scale RCT of PGT, split by profession and pilot versus non-pilot site. Overall, two-thirds of respondents (n = 143/223, 64%) reported that they and their NHS burns service (n = 144/223, 65%) would be willing to participate in a full-scale of RCT for PGT; however, nearly one-third did not know (staff, n = 65/223, 29%; NHS burns services, n = 75/223, 33%). When split by professional group, doctors (n = 23/29, 79%) and nurses (n = 60/89, 67%) were more likely to say yes to participating than other professional groups, for example physiotherapists (n = 18/32, 56%). Respondents from pilot sites were also more likely to say that they would participate than those from non-pilot sites (pilot site, n = 73/88, 83%; non-pilot site, n = 68/127, 54%). When asked whether or not their NHS burns services would be willing to participate, doctors (n = 21/29, 72%), nurses (n = 61/89, 69%) and physiotherapists (n = 21/32, 66%) were more likely to say yes than other groups. Similar to staff participation, respondents from pilot sites were more likely to state that their NHS burns service would take part than those from non-pilot sites (pilot site, n = 73/88, 83%; non-pilot site, n = 68/127, 55%).
As part of the interviews, the barriers to, and facilitators of, staff and service participation in a full-scale RCT of PGT were explored (Figure 2). Many of the factors identified, for example appropriate resourcing for staff, worked in both directions; if they were present they acted as a facilitator of participation and if they were absent they acted as a barrier. Linking with the concerns given in Attitudes to a full-scale randomised controlled trial for pressure garment therapy concerning the need for a full-scale RCT, staff reported their general willingness to be part of a future RCT, although there were some reservations, particularly around what involvement would actually entail, as well as the feasibility and practicability of undertaking this type of clinical effectiveness study for an embedded clinical treatment:
FIGURE 2.
Barriers to, and facilitators of, NHS burns staff and service participation in a full-scale RCT of PGT.

I was a bit unsure in what my role would . . . say we were to participate exactly what my role would be. Would I be the therapist that then does the assessment and then sends that off somewhere for it all to be compiled together? Because there would need to be training to make sure that we’re all rating the scars in the same way to show that they are improving or not improving, and I think at the moment people use different scales, people do things slightly differently. So I think it would be training and then support throughout the process.
TSC06 OT, children only
Yes, I think that’s reasonable, because I think at the moment the staffing levels that we have are very . . . we are struggling to meet the need. Obviously by the time the trial comes round it would depend on staffing levels and the situation of the service at the time, the changes that we’re looking to making we’re looking to . . . there may be an investment needed there as well from what we’re just trying to do within the department let alone adding to that. But I know that there is a lot of support for research and development, and if that was the case perhaps that would be something that the trust would consider.
TSA11 OT
I think it’s a very tricky thing to do [run a RCT of PGT], I don’t know how. Again just with it being such an emotive thing with children, to see . . . every parent wants the best for their child. I would be . . . I think we need to do something like this.
TRH04 physiotherapist, children only
Key barriers included ethical concerns over the ‘usual-care’ treatment group, patient characteristics and burn type, whether or not patients would agree to participate and, for those who did consent, would they comply with treatment and trial processes:
I would have a problem with that, because if they are consented . . . if they know that if they do this they will get this benefit, and if they don’t do it they won’t get that benefit I don’t think people who are fully understanding are going to consent to be in it. I think you will probably only get people who don’t quite understand what they are signing up to . . . I would much rather the study design assessed patients who did not comply with the treatment in the first place and look retrospectively at their outcome, and the assessors not knowing whether they have pressure garments or not.
TLR05 OT
I think, I thought I can see where it’s coming from, my brain . . . I can’t see how you can get across all the practicalities of or the barriers that might come up to actually prove it, because I would have thought across different centres you’re going to have to make sure that how you’re going to rate the scar. There’s so many questions about how scarring is assessed anyway and what scales to use, I just don’t . . . Well we’d have to be very clear that didn’t, because otherwise it would bias the study. That would just be one of my questions, is whether it would be better just to do this study with adults rather than including children.
TSC06 OT, children only
Summary
-
The national survey and related interviews have allowed us to describe and explore the acceptability of a trial of PGT, in principle, across a broad sample of UK NHS burns services and staff.
-
The majority of staff reported that they perceived a need for a full-scale RCT of PGT; however, the rationale underpinning this was complex. The core issue was an apparent lack of clinical equipoise around the research question and PGT as a treatment.
-
A large proportion of staff stated that they want a full-scale RCT of PGT, not necessarily to provide evidence of the best treatment for patients, but rather to support current practice and the continued use of PGT for burns scar management.
-
In general, staff and services were receptive to the idea of a UK trial and stated that they would likely participate, demonstrating ‘buy-in’. However, it was clear that the strong views about the use of PGT have the potential to influence the conduct of a full-scale RCT, if those views are not recognised and taken into account as part of the trial design.
-
The exploration of the perceived barriers to, and facilitators of, a full-scale RCT of PGT highlighted that a future trial must take into account the following issues: the recruitment of parents and children, the definition of appropriate outcome measures and scar assessments and the scope and definition of eligibility criteria for severity of burns.
Chapter 5 Identification of patient-, parent- and clinician-reported outcomes
Relevant objectives
The following objectives are addressed in this chapter:
-
to assess the experience of patients and parents of PGT and assess willingness to be randomised between PGT and no PGT
-
to identify and select patient-centred outcomes.
Methods
Data sources
Data for this analysis of willingness to participate in a trial, patient-centred outcomes and subsequent outcome domains were drawn from three different sources: (1) the free-text responses in the staff attitude survey (n = 223; 28/30 NHS burns services), (2) semistructured telephone interviews with a sample of the staff survey respondents (n = 15; 13 NHS burns services) and (3) semistructured interviews with adult patients and parents (n = 40, 24 adults and 16 parents; four NHS burns services). Please see Chapter 3 for a detailed description of the methods used for data sources (1) and (2).
Sampling and recruitment for adult and parent interviews
To be eligible to participate in an interview, patients or parents of paediatric (aged 0–8 years) and adolescent (aged 9–15 years) patients had to have ≥ 6 months’ experience of PGT and have finished PGT no more than 2 years prior to the interview. We attempted to include a range of patients according to sex, age, ethnicity and type and severity of burn to facilitate a maximum variation sample. Participants were recruited by OTs and/or research nurses (RNs) working at four of the PEGASUS pilot trial sites in England: Queen Elizabeth Hospital, Birmingham (adults only); Birmingham Children’s Hospital (BCH) (parents only); St Andrews Centre for Plastic Surgery and Burns, Broomfield Hospital, Essex (adults only); and Queen Victoria Hospital, East Grinstead (adults and parents). Clinical staff provided information sheets to potential interviewees and took written consent to pass contact details on to the PEGASUS qualitative research team. A member of the qualitative research team then contacted potential interviewees, provided further information and answered questions as necessary, and then arranged a suitable time, date and venue for the interview. Interviews were mainly conducted in the patient’s home, which was the preferred venue; however, a small number took place on university and hospital premises or via telephone. Written informed consent was taken prior to the start of interview data collection.
Originally, we had planned to also conduct two discussion groups with older children aged 9–16 years. After initial delays with ethics and governance approvals and site initiation, and considering the logistics and time needed to arrange these discussion groups, it was decided to conduct a small number of further interviews with parents of older children instead. This decision was taken with the input of the Trial Management Group (TMG) and Trial Steering Committee.
Interview format and content
A semistructured discussion guide was developed (see Appendix 3) based on the literature, discussions with our PPI group and the wider PEGASUS research team. Interviews were conducted in a participant-focused manner, allowing issues and perspectives that were important to participants to emerge naturally. The topic guide and interview process was refined after reflection on a small sample of early interviews. Topics discussed included:
-
accounts of the accident and injury (when interviewees were happy to talk about these in order to provide context for the remainder of the data)
-
accounts of subsequent treatment
-
the experience of PGT and other scar management techniques
-
hopes and expectations for treatment, recovery and scar management
-
perspectives on a trial of PGT (e.g. would the interviewee have considered participating in a trial?)
-
patient-centred outcomes.
Analysis
Interviews were undertaken either face to face or via telephone, audio-recorded, transcribed clean verbatim and analysed using a thematic approach. 45 Early analytic findings were discussed among the members of the TMG and shared for discussion and feedback at PEGASUS investigator meetings, at which clinical staff delivering the pilot trial and at least one patient representative were present. Following agreement of the final themes, example quotations were identified from each data source. Quotations in Results are identified using the participant’s unique identifier code, and indicate whether the quotation was from an adult patient, a parent of a paediatric or adolescent patient or a member of NHS burns service staff. Staff quotations indicate profession and, when required, whether or not the quotation was from a survey free-text response.
Results
Sample characteristics
Table 5 describes and summarises respondent sample characteristics for the P1 adult patient and parents of paediatric or adolescent patient interviews. A total of 40 interviews with adults (n = 24) and parents (n = 16) were undertaken across four NHS burns services. Interviews lasted, on average, 51 minutes (range 19–108 minutes), with 33 conducted face to face and seven via telephone. Table 5 is supplemented with information about the burn injury, sex and age of the adult patients and the paediatric and adolescent patients whose parents were interviewed. The sample was approximately evenly split between males (52.5%) and females, across a range of ages. Most participants were white (77.5%), with the type of burn predominantly reported as flame (45.0% of total; 54.2% for adults and 31.3% for paediatric and adolescent patients) or scald (37.5% of total; 29.2% for adults and 50.0% for paediatric or adolescent patients).
| Adult and parent interviewee sample characteristicsa | Sample, n (%) | ||
|---|---|---|---|
| Total (n = 40)a | Adult patients (n = 24) | Parents of paediatric or adolescent patients (n = 16) | |
| Sex of adult patient or parent | |||
| Male | 21 (52.5) | 18 (75.0) | 3 (18.8) |
| Female | 19 (47.5) | 6 (25.0) | 13 (81.2) |
| Sex of paediatric or adolescent patient | |||
| Male | N/A | N/A | 11 (68.8) |
| Female | N/A | N/A | 5 (31.3) |
| Age of adult patient or parent (years) | |||
| < 21 | 1 (2.5) | 1 (4.2) | 0 (0.0) |
| 21–30 | 9 (22.5) | 4 (16.7) | 5 (31.3) |
| 31–40 | 9 (22.5) | 1 (4.2) | 8 (50.0) |
| 41–50 | 8 (20.0) | 6 (25.0) | 2 (12.5) |
| 51–60 | 7 (17.5) | 6 (25.0) | 1 (6.3) |
| > 60 | 6 (15.0) | 6 (25.0) | 0 (0.0) |
| Age of paediatric or adolescent patient (years) | |||
| < 1 | N/A | N/A | 0 (0.0) |
| 1–5 | N/A | N/A | 8 (50.0) |
| 6–9 | N/A | N/A | 5 (31.3) |
| 10–14 | N/A | N/A | 0 (0.0) |
| > 14 | N/A | N/A | 3 (18.8) |
| Site | |||
| Queen Elizabeth Hospital, Birmingham | 8 (20.0) | 8 (33.3) | 0 |
| BCH, Birmingham | 10 (25.0) | 0 | 10 (62.5) |
| Broomfield Hospital, Essex | 8 (20.0) | 8 (33.3) | 0 |
| Queen Victoria Hospital, East Grinstead | 14 (35.0) | 8 (33.3) | 6 (37.5) |
| Ethnicity | |||
| White | 31 (77.5) | 20 (83.3) | 11 (68.8) |
| Black African/Caribbean/black British | 3 (7.5) | 2 (8.3) | 1 (6.3) |
| Asian/Pakistani | 4 (10.0) | 2 (8.3) | 2 (12.5) |
| Unknown | 2 (5.0) | 0 (0.0) | 2 (12.5) |
| Type of burn | |||
| Flame | 18 (45.0) | 13 (54.2) | 5 (31.3) |
| Scald | 15 (37.5) | 7 (29.2) | 8 (50.0) |
| Contact | 4 (10.0) | 2 (8.3) | 2 (12.5) |
| Friction | 2 (5.0) | 1 (4.2) | 1 (6.3) |
| Electrical | 1 (2.5) | 1 (4.2) | 0 (0.0) |
| TBSA of burn (%) | |||
| < 10 | 11 (27.5) | 6 (25.0) | 5 (31.3) |
| 10–20 | 5 (12.5) | 0 (0.0) | 5 (31.3) |
| 21–30 | 8 (20.0) | 4 (16.7) | 4 (25.0) |
| 31–40 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 41–50 | 2 (5.0) | 2 (8.3) | 0 (0.0) |
| > 50 | 4 (10.0) | 4 (16.7) | 0 (0.0) |
| Do not know | 10 (25.0) | 8 (33.3) | 2 (12.5) |
Perspectives on a trial of pressure garment therapy
During interviews with adult patients and parents of paediatric or adolescent patients who had already experienced PGT, we discussed whether or not, in principle, they would have agreed to take part in a RCT had they been approached to do so following their initial acute treatment. Many of the participants stated that they believed that they would have agreed to take part, citing reasons that were similar to those later observed during the qualitative process evaluation of our pilot trial (see Chapter 8), for example altruism towards clinical staff and a desire to help generate knowledge that would benefit future patients. This was the case for most of the adult interviewees and approximately half of the parents that we spoke to:
I would have said yes, I would take part in it. I think because being in such a strange situation I think if it helps somebody else, and it’s going to do good whatever happens, so basically I see myself as lucky, because my scars are covered virtually. Seeing me without looking just about there you can’t really tell, so I’m a lucky person, that’s how I put myself down as. But if you have got on your face, or you have got somewhere that really stands out you need as much help as you can possibly get. If by doing a trial helps somebody with something facial or whatever well brilliant.
How would you have felt having this 50/50 chance?
Yes, fine, I would have taken that, I would yes, I would have said yes whatever.
CA04, adult patient
However, when considering this during interviews, several parents felt that they would have been reluctant to take part or would have definitely refused to do so. The reasons given reflect our observations later during the qualitative process evaluation of our pilot trial (see Chapter 8), and centre upon the need for parents to feel that their children were receiving the best treatment available. Interestingly, this parent reflected on the potential for differences in attitudes towards the trial concerning the participation of adults and children:
I would have said no.
That’s fine, I need to know.
I don’t like 50/50 chances [laughs].
So you’d have said no and you would have taken . . .
I think that idea is more better for adults, and then you would say well we’ve tried it on adults, we would like to try it on children, because for a child to do a 50/50 . . . I wouldn’t risk my child like that, I would be like you do what’s best for my child. I wouldn’t be thinking if that could have worked, and I didn’t use it I feel like I would regret it. So I wouldn’t want to make that decision for my child. I’d do it [take part in the trial] for probably me . . .
BC02, parent of paediatric patient
A small number of participants were unsure as to how exactly they would have reacted to a request to consider taking part in a trial.
General outcome measure and domain identification
A large number of burn scar-specific outcomes were reported by, and perceived to be important to, patients, parents and clinicians:
I feel all outcomes are important and valid and should include clinical observations and patient-reported outcomes. I think it is very important to look at scar appearance, pain and itch but also include function, body image and impact on life as using pressure garments affects all these aspects.
ID25, survey free-text response, physiotherapist
I think from the doctors themselves they measure the size of the scar, how deep it is, how firm it is, the colouring, and they go from that, and how he is, is he scratching it, is he pulling at the vest, is he doing . . . they ask all sorts of questions, so probably everything that they have asked is going to be asked in the trial. There’s nothing that I can think of on top of that.
EC03, parent of paediatric patient
Several of the NHS burns service staff were able to identify and articulate the importance of outcome measures that focus on how scar management affects the patient rather than ones that they perceived as being easy to measure objectively. One consultant, for example, highlighted the significance of identifying outcomes and using outcome measures that are important to the patient and their ability to return to ‘normal life’:
The main focus is really how does it affect the patient? So the things that I think I picked [in the survey: itch, function, ability to work, psychological state, social interaction with family and peers and cost] were related to the impact that it will have on the patient. It’s sometimes very easy to focus on . . . very easy to measure objective data, but if it actually makes no difference to the patient in terms of their ability to work, the symptoms that they have, the difficulties or the number of visits that they have to make, well then ‘so what?’ is what I would say. So if they score seven on a scar score but they’re working and they haven’t had to visit the hospital at all because they’re getting back on with their lives compared to scoring five, which is a better score, but they’ve had to make 17 visits and haven’t gone back to work, is that a success? I don’t think so. I think it’s about how we can pick outcomes that are related to returning to normal life, not getting good objective scores. So yes, I think what will actually . . . as with any study you need to balance what are things that are easy to measure with things that matter to the patient . . .
TEG15, consultant
Another member of staff, who responded to the free-text questions about outcomes in the survey, again highlighted the importance of focusing on patient-reported, rather than clinically reported, outcomes:
As with most outcomes, I believe patient-reported ones that focus more on well-being, function and psychosocial status, etc. should be more influential than pure[ly] ‘clinical’ ones.
ID28, survey free-text response, consultant
Identification of outcome domains
Five core outcome domains, covering the list of burn scar-specific outcomes, were identified within the data:
-
scar characteristics and appearance
-
movement and function
-
itch and pain
-
psychological and social functioning
-
treatment burden.
Scar characteristics and appearance
Patients, parents and staff identified a wide range of scar characteristics as being important outcomes, including height, texture, colour, size and shape and tightness/pliability. Table 6 provides a summary of the language used by participants to describe their own scars or the scars of their patients and the associated descriptive scar characteristic:
I think you need to focus on the physical parameters such as appearance, redness, scar height and function as these are easy to measure.
ID160, NHS burns service staff member, survey free-text response
Probably the colour, the texture of the scar, how raised it is and swollen, and that’s about it really, that’s all I can think of. I think the size of the scar, how big it is depends on how much has been damaged, and tends to stay that size, but the thickness I’ve noticed that has changed a lot from when I first got it, and the texture has changed, very firm and almost like a callus when I first had it, to now it’s soft and back to feeling like skin again.
CA06, adult patient
| Scar characteristic | Language used by interview participants |
|---|---|
| Height | Thinness, thin, thickness, thick, swelling, swollen, raised, flatness, flat, level, settle, height, bubbles up, skin held down/in, expanding, protuberance, solid, squashed down and bulky |
| Texture | Feels like normal skin, smoothness, dryness, firm, smooth, lumps, bumps, callus, soft, ridges, supple, scabby, shiny, softness, shine, sheen wrinkled and ridges |
| Colour | Colour, names of colours, pigment, blotchy, like steak, skin colour and flesh coloured |
The language used by patients and parents to describe scars, the impact of treatment (e.g. pressure garments) on scars and perceived outcomes relating to scars varied within the interviews depending on the context of the discussion at that point in time. For example, when describing the perceived impact of pressure garments on scarring and related outcomes, participants used terms such as ‘flatter’, ‘smoother’ and ‘paler’. As such, they identified changes to specific scar characteristics that they perceived as being easy to measure objectively. This may reflect the narratives and objective markers (outcomes) of scar maturation used by clinicians/therapists rather than the natural language of patients and parents:
It [the scar] is a lot flatter as they [clinician] said. I thought it wouldn’t really go down much but it actually has gone really flat, and it is a lot less noticeable.
ECO6, parent of paediatric patient
Even when reflecting on appearance, clinicians were typically focused on (perceived) objective measures of appearance:
It’s so difficult; it’s such a subjective thing appearance. But when you’re thinking about appearance we know that the thing behind it is vascularity, is height, is all of those things, and those we can measure objectively, and we can’t then measure how patients feel subjectively.
TSA11, OT
When talking in more general terms about scarring and treatment, patients and parents, as part of broader more discursive narratives, reflected on general appearance around the ‘look of the scar’, often without reference to specific scar characteristics. This may indicate that patients and parents may value a more subjective judgement of general scar appearance, over measures of singular specific scar characteristics, such as height. The measurement of scar characteristic outcomes may not resonate with patients and parents, who may subjectively assess the general overall appearance of the scar differently. Importantly, both patients and clinicians were able to identify and articulate the fact that outcomes around scar appearance need to reflect both the clinical and patient opinion, as they may differ:
How somebody feels their healing is coming, and how somebody in the scar clinic or burns unit feels it is, because obviously they could be two very different things couldn’t they? But it’s got to be down to the . . . it’s got to be both of those things, so it’s got to be a medical opinion, but the patient’s opinion as well, managing expectations.
EG08, adult patient
The visual appearance, what the patient feels their scars look like rather than us, because we tend to have different views than our patients on what their outcomes should be.
TQE09, nurse
There were also differences in the perceived importance of appearance based on factors such as the sex and age of the child, differences in the personality traits of adults and the location of the scarring:
Children are more acceptable with how they look until they get to a bit of an older age and then it’s about how other people see them, and it’s the same with adults as well, it’s how other people perceive what they look like and how they feel, how people are looking at them. So it’s about their self-image really. Teenagers again I think even more so, they actually want everything to stop this scarring happening, I think particularly.
TNC03, nurse
If it was my face or maybe my chest or neck or something like that, it might be more important to me, but I don’t think I’m a particularly narcissistic person, wrong word but you understand what I mean? I’m not particularly a vain person I don’t think I am, but it’s difficult, but no they don’t worry me. It’s something that’s happened, it’s a war wound, it’s a thing that everyone has a scar somewhere to a greater or lesser degree.
CA03, adult patient
Movement and function
Movement and function were perceived by clinicians, patients and parents as being important outcomes. The priorities of movement and function were also compared with appearance. For some, appearance was perceived as more important than movement and function, although for others it was the opposite:
Well I want to first 100% be assured in the future she will have no movement restriction, is the first thing. After that I will like to know as much as she can get help to make that look better, any help, and then after that any psychological way she can get help to be perfectly fine how she look . . . the look comes after, if she has perfectly fine then I will focus on her look, but if she is not or if she is restricted it’s more important.
BC04, parent of paediatric patient
However, those who were more focused on appearance did report that function was relatively good, and so it might be that their views on appearance could have been different if they had more limited function:
I’m going to say both really, yes, I’m pretty fortunate that I have got pretty much full mobility apart from like I said the tightness in my legs at times, and I can live with that, but for me it’s probably the appearance, if the appearance is a little bit better I would be a bit more confident and probably wear shorts and go swimming with the children.
CA09, adult patient
Appearance is very important but injury and treatment has never stopped [child] doing anything and didn’t affect function in any way.
EC04, parent of paediatric patient
One consultant argued that appearance and function were equally important from a clinical perspective, and that outcome preference will vary from patient to patient based on their individual circumstances:
The two outcomes from a clinical perspective with any burns patients are obviously the cosmetic appearance, but equally functional outcomes are just as important . . . I think that’s very difficult to say [which is more important] because the patients vary radically, some patients will accept quite significant cosmetic deficits, as long as their function is not impaired, and other patients find with what you looking at them might feel is not a particularly bad cosmetic outcome and they are devastated by it. So I think that’s a very individual thing for a patient, but the two issues that most patients are going to be concerned about are the cosmetic outcome, partly because of how they are perceived by the people, partly because any scar is going to remind them of what happened, and the less obvious that is the less likely they are to think about it and worry about it. And I think the functional outcome is very important to patients, particularly if they do have pain or discomfort, because of tightness or thick scars, that kind of thing.
TSC07, consultant
Although clinicians typically made a distinction between range of movement (ROM) and function, these terms were often used interchangeably by patients and parents. In the staff attitude free-text responses about outcomes, function was mentioned approximately 10 times more frequently than movement. Interpretation of the interview narratives indicated that function, in terms of being able to do things and including activities that the patient was doing prior to the burn injury, was perceived as more important than movement per se:
I think the most important was his arm, I think it was his arm, because my thing was even though you had these burns I want you to be able to do everything that a normal child could do that never had a burn, and without the range of his arm he couldn’t. He couldn’t reach for things, he couldn’t hold a lot of stuff, there’s a lot of stuff that he couldn’t do because of that arm, and he started to teach himself how to write with the left hand and use the left hand, but it was never the same. So my thing was I really wanted to get this arm back. Once he got that back I didn’t . . . that was my most important thing, seeing him do everything again, and I think for him it probably was the most important thing, because he seemed a lot more happier when he could play with other kids and use both arms than when he was just using the one.
BC02, parent of paediatric patient
The range of movement was the most important for me . . . because at the end of the day I am right handed, all of the jobs I’ve ever had have been manual work jobs which involve your hands, so to me being able to use it again was the most important thing.
CA06, adult patient
Many of the patients and parents reported that PGT had made a difference to their ability to move and, ultimately, function:
Well it [wearing pressure garments] gave me mobility back, because it was a killer for me personally to walk, because my legs just wouldn’t pass each other, didn’t matter whatever I was wearing my legs just stuck together. However weird that might sound just physically walking about and moving was just a no goer. I struggled even just to go outside to see the dogs, I couldn’t walk anywhere, and once we got them . . . once you put them on they actually stay where they are. So they feel like they’re holding you firm, but for me personally for my leg wounds they gave me mobility back.
CA05, adult patient
Itch and pain
Itch and pain were commonly highlighted as important sensory outcomes by patients, parents and clinicians:
Itchiness is actually probably a huge one which I didn’t mention for burns patients, is a major problem for them, so that’s probably another outcome that they may think of actually. Yes, scar itch is a real problem . . . yes, so that might be an outcome for a patient, but I think it would be something we would consider as well, because if they have got a lot of itching they tend to be on a lot of medication to try and control that.
TQE09, nurse
. . . so I think itching and pain control are critical [to measure], and obviously there’s a financial trade off because if we’re not prescribing them quite expensive things like gabapentin [Neurontin®, Pfizer] that that will help so these garments are controlling their pharmacology for itching and pain that’s good.
TCW01, consultant
Some parents reported that they found it difficult to estimate their child’s pain and so this may make it challenging to assess as an outcome; whereas other parents reported that they had a good understanding of their child’s discomfort and highlighted their perception that pressure garments had made a difference:
I think they do help with the itch because he can’t get to it, because he does cut himself occasionally doesn’t he when he scratches so hard. So I think they help him with protecting it so he can’t get to it. I don’t know so much about the pain or the itch, because it’s hard to say. I don’t think he’s had any pain with it as such. Since it’s healed he’s not actually had pain, it’s just itching.
BC03, parent of paediatric patient
Yes, measuring I suppose how it affected the child, or the patient, did it cause them distress? Is it worth putting them through all that distress for maybe only a little change?
Distress being?
Yes, but I think like you say to measure something, if it’s causing the patient discomfort and pain, like we say is it actually worth the pain and discomfort it’s going through then, but that varies from person to person as well. You know what my pain threshold is like.
EC05, parent of paediatric patient
Adults also reported that pressure garments helped with pain management, even for smaller burns:
I wore the pressure garment as much as I could tolerate it, and finding as I said the pain . . . that it helped with the pain. As I say I don’t . . . and even when I was just showing you that, it’s very tiny, but the pain it’s very difficult to describe the pain, even . . . and I can’t begin to imagine a whole body burn, how someone copes with the pain, and if these go . . . if the pressure garments go any way to helping with the pain for me that would . . . yes, I just can’t imagine what it would have been like without having one.
EG02, adult patient
Other sensory factors, such as scar sensitivity, were also identified as outcomes but less commonly than itch and pain:
It’s quite weird, the skin definitely does feel more tender on places on that arm, I say do feel tender, and I only have to touch my shin and I can soon break the skin on that shin.
CA05, adult patient
Psychological and social functioning
Three interlinking subdomains of the psychological and social functioning outcome domain were interpreted within the data: (1) body image and self-esteem, (2) engagement in activities and (3) return to routine.
Body image and self-esteem
As highlighted in Scar characteristics and appearance, the appearance of the scar following burn injury can affect body image and self-esteem. Patients, parents and clinicians highlighted that it was important to capture measures of confidence and self-esteem:
Does the garment cause any problems of self-esteem – appearing different from peers?
ID217, survey free-text response, nurse
Think the outcome should be patient focused – well-being, body image, etc.
ID112, survey free-text response, OT
One OT, who works with children, highlighted the need for a holistic outcome measure that captures both appearance and general QoL, as even if the scars look good (according to clinical judgement) they can still affect patients’ self-esteem and confidence every day:
Probably I was just thinking in general. I suppose it’s not all just about the physical how the scar affects movement or it’s how that affects that person, also doing things, so I’ve got a bunch of teenagers whose scars to me look fantastic and I tell them they look really good, but they still affect them every day, they still affect their esteem and confidence. So I think that also would need to be somehow measured in the study I guess. I don’t have a specific . . . we don’t use anything specific ourselves to look at that, it’s just one of the questions we talk about, but it’s not on an outcome measure as such.
TSC06, OT, children only
Patients and parents reported how other people, ‘react[ing]’, ‘staring’ or ‘making comments’ about the way they or their child looked could have a negative impact on self-esteem and that burns patients often try to keep their scarring and their pressure garment ‘hidden’:
For people who wear the pressure garments I think there’s some sort of psychological aspect in it as well, it’s not just a physical thing. I struggled immensely with keeping the thing hidden, and it’s not easy.
Keeping the pressure garments hidden from people?
Yes, they’re not the most attractive looking thing you’ve ever seen, so yes.
CA07, adult patient
[Wearing pressure garments] Just people don’t notice, people didn’t notice that my hands were all . . . not that I care now, but at the time they become a topic of conversation. As I say I’ve got a great family and great friends, and anybody looks at me in the wrong way they’re on them, but as I say it was the way I looked at it is I’ve got to live with this now, and I’ve never been what you call vain, but at the same time people’s perception of you and I think people’s lack of knowledge, it makes them act quite funny, makes them . . . I don’t think they mean to do it, but anything considered out of norm they will stare at.
EG06, adult patient
In contrast, for others, wearing pressure garments acted as a physical and emotional barrier, and offered perceived ‘protection’ and ‘security’:
It gave you a feeling of security . . . yes, a feeling of protection maybe, now whether that’s psychological or actual I don’t know . . .
CA03, adult patient
Engagement in activities
The desire to keep scarring and/or pressure garments hidden was linked with impact on engagement in activities, such as socialising with family and friends. One participant referred to this as ‘avoidance’ behaviour (TCW12 clinical psychologist). Having a burn injury may prevent some patients from engaging in social activities. However, this was more inhibiting for some patients than others, and it was perceived as an important outcome to capture:
So they are the physical aspects of things, and then obviously the psychological is what we said about body image. Social side of things, or wearing a garment, there is social side, you are wanting it to try and blend in as much as it can so it’s not obvious that people are wearing a garment, unless they’re not bothered about it being seen as a garment.
TEL13, OT
A combination of clinical outcomes and psychosocial outcomes. I think it is very important that psychosocial factors are included, as evidence shows no relationship between clinical features of burns and psychosocial well-being. Psychosocial factors could include: appearance-related distress, depression, anxiety, engagement in social situations.
ID18, survey free-text response, psychologist
Return to routine
Being able to measure when patients and/or their parents perceived that they have ‘returned to routine’, such as going back to employment/education following their burn injury, was identified by patients, parents and clinicians as important:
Return to normal level of function at work/education/leisure, etc.
ID201, survey free-text response, OT
Many of the participants, in particular adults, discussed various aspects of perceived normality and the desire to ‘return to normal’, including the impact that burn injuries can have on relationships with partners, family and friends:
Exactly, normality yes, you just want to do the things you done before . . . Just basically want to get back to normal, I only go twice a year now for my check ups and everything, so far it’s been OK, so yes just get back to normality and lead a normal life.
CA04, adult patient
I never thought I’d get back to normal ever. So I resigned myself to the fact that that’s it now, I’m just going to be like this, until the one they can do for me . . . all the future hospital operations which I know I’ll be having. So I just resign myself to it’s going to be a good few years of having treatment without thinking about . . . because they said, Don’t bother going back to work, you won’t be able to do much.
QA02, adult patient
Treatment burden
Much of the narrative within the interviews for patients and parents, and to some extent for clinicians, was constructed around the burden of care required for burn injuries and longer-term scar management, in particular, the use of PGT. Given the emphasis placed on this in the interviews, it has been interpreted as being a potentially important outcome domain and, therefore, an important outcome to measure in any future trial of scar management therapy. The treatment burden outcome domain was multifaceted and worked both positively and negatively, depending on the unique context and experience of each individual patient. For example, although nearly all patients and parents reflected on the importance of, and positive support provided by, NHS burns service staff, some patients reported positive experiences of wearing pressure garments but others articulated a much more negative perception of PGT:
No, to be honest just the parents and the staff at the hospital, they did enough for me, they reassured me that there’s nothing else I can do, and it does happen, you’re just in the wrong place at the wrong time. So yes they’re brilliant at the hospital, I couldn’t fault them at all.
BC03, parent of paediatric patient
It [PGT] was quite easy, I made it into a little game. She enjoyed . . . it sounds wrong but she enjoyed having the attention brought to her. Like if I forgot to do it she would come up to me and be like, Mummy you’ve got to do my hand, so I’d do her hand and everything. So it just became a day-to-day thing really, so it’s never affected her or anything, which I was quite happy for.
BC07, parent of paediatric patient
I vaguely felt relieved not to wear them because of the effort and the time spent in putting them on I think. So oh goodness I haven’t got to worry about that anymore sort of thing. I can remember vaguely feeling like that, relief really in not having to wear them. But it wasn’t anything to do with them being uncomfortable or anything like that, it’s more the difficulty in putting them on and the time and effort, and things like that, I think.
CA03, adult patient
The therapy burden was also reflected on by clinicians:
Along with pressure therapy is all the moisture and massaging, which clearly plays a massive role too. If you add it all up though for some patients, especially those with larger burn areas, and burns that are in areas they can’t physically get to, and need someone else to do those bits of treatment, you are potentially adding to what becomes a massive therapy burden, and I can also think of a couple of patients that I’ve worked with who have been close to giving up many times, because they just found it overwhelming, they are sick to death of the regime.
TWC12, clinical psychologist
One adult patient actually articulated that he himself felt like he was being a burden on the therapists and the wider NHS given that he had required 19 different garments as part of this treatment:
I felt like I said to [OT] I feel like a burden sometimes; I’ve had 19 of these [pressure garments]. So I didn’t judge whether it was good or not [scar], but I just feel like a burden sometimes, because it doesn’t hurt me at all or anything like that, it’s a little bit itchy every now and again, but I feel blessed that the NHS did what they could, and there’s no . . . I’m okay with having a scar there, I haven’t got a problem with it, I’m just glad to still be alive, because it could have been a lot worse. So I’m finding everything . . . I’m happy with everybody. I just feel like a burden sometimes, as if I’m draining the NHS on my own [laughs].
QA06, adult patient
Patients, parents and clinicians also reported the significant burden of care for scar management including, for example, the number of follow-up appointments required, the cost to the patient and the NHS and the impact on family, such as parents having to give up work to care for their children following a burn injury:
Yes, that’s it, you’re constantly hospital, in and out all the time, in and out. You was thinking I’ll get this, get back to work, and then something else you’ve got to have done, and just never bothered. I’m on incapacity benefit now, I’m not on disability, just incapacity, and income support, that’s what I’m on.
QA02, adult patient
The other I suppose outcome is cost, and when we analyse cost it’s not just a cost of a garment but it’s a cost in terms of numbers of visits and the travel times, and the travel costs associated with it as well, because if I saw a patient at 1 month and said, ‘You’re getting a bit of a thick scar if it dries out put a bit of cream on it, otherwise just keep on working and if you need some . . . if you’ve got an itch well massage it or take some [antihistamine] or something, and then come back in a month’s time’, that’s different from spending an enormous amount of time massaging and doing various things, and the repeat visits for pressure garments and so on, but that might actually cost not just the NHS, but the patient an enormous amount of money.
TEG15, consultant
Summary
-
We have explored the perspectives of NHS burns service staff, adult patients and parents of paediatric and adolescent patients who have experienced PGT of the outcome domains that should be captured in a future trial of PGT for scar management.
-
The results demonstrate that a patient-centred assessment of the outcomes of PGT should be holistic in nature. The impact of scar management is complex, involving a range of outcome domains, for example perceptions of appearance, specific scar characteristics, function, pain and itch, and broader psychosocial outcomes. Priorities for outcomes may vary from patient to patient and over time.
-
These outcome domains reflect a complex holistic patient experience of scar management and treatments, such as PGT.
-
When asked hypothetically about participation in a RCT in which they would have been randomised between PGT and no PGT, most P1 interviewees stated that they would have been willing to take part.
Chapter 6 Identifying methods for evaluating outcomes and resource use for health economic analysis
Relevant objective
The following objective is addressed in this chapter:
-
the appropriate methods for evaluating outcomes for the health economic analysis and resource use.
Method
Appropriate methods were identified from the literature, relevant policy guidelines and with the help of PPI and clinician feedback. Validated health-related quality-of-life (HRQoL) instruments were selected when possible. The resource use questionnaire was developed to facilitate detailed costing of the two treatment arms, from both NHS and societal perspectives.
Results
Health-related quality of life
The wide age range of patients recruited for the study was recognised as a complication from the outset, necessitating the use of multiple instruments and piloting of HRQoL instruments that are not yet widely used in health economics. Furthermore, it was acknowledged that the small sample of patients completing each instrument (i.e. falling into the age range for which each instrument had been validated) would limit full evaluation of these instruments.
In the National Institute for Health and Care Excellence (NICE) reference case, the EuroQol-5 Dimensions (EQ-5D) is specified as the preferred measure of HRQoL in adults. 46 The EuroQol-5 Dimensions, five-level version (EQ-5D-5L), was therefore chosen as the sole health economics instrument in patients aged ≥ 17 years. The timing of the data collection would coincide with the release of the value set for the new EQ-5D-5L instrument and it was therefore planned to use corresponding preference values from that publication. 47
In the case of assessing HRQoL in children, NICE acknowledges that ‘when necessary, consideration should be given to alternative standardised and validated preference-based measures of HRQoL that have been designed specifically for use in children’. 46
A 2014 systematic review48 of UK economic evaluations of paediatric interventions was critical of published economic evaluations for failing to make use of instruments specifically developed for use with children, and identified the Child Health Utility Index 9D (CHU-9D) as a potentially useful measure for future research. Although CHU-9D had not been used in the economic evaluation studies in this review, empirical work from the West Midlands (UK) demonstrated the superiority of CHU-9D over the EuroQol-5 Dimensions, Youth (EQ-5D-Y) for children aged 6–7 years, and CHU-9D has been validated for use with children aged 11–17 years. 49,50 We therefore selected the CHU-9D for use in this study as the sole health economics instrument for patients aged 5–6 years with proxy completion by the parent, for both child and parent/proxy completion for ages 7–10 years and for child completion only in patients aged 11–15 years. Young people aged 16 years also completed the CHU-9D alongside the EQ-5D-5L. Dual completion by parents and children in the 7–10 years age range presented us with the opportunity to assess whether or not child completion would be feasible as part of a full study.
Although there were initially no plans to include an instrument to assess HRQoL in patients aged < 5 years (the question on CHU-9D about ‘school work/homework’ suggests a natural cut-off point at 5 years as a minimum age in the UK), feedback from clinical colleagues stressed the significant prevalence of burn injuries within younger age groups (the original lower age range for the trial was removed as part of the first amendment, which was submitted to the Research Ethics Committee in September 2014). A decision was therefore taken to introduce the Pediatric Quality of Life Inventory (PedsQL), after the start date of the study, for proxy completion by parents on behalf of children aged 2–4 years. It should be noted that no HRQoL or health economics instrument was identified for ages 0–1 year.
Although there is currently no set of tariff or preference values for PedsQL, we were aware of plans to predict CHU-9D utility values from PedsQL scores using regression mapping algorithms, as part of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA)-funded PREDnisolone in NephrOtic Syndrome (PREDNOS) study (reference number 08/53/31). 51 It was therefore expected that a mapping algorithm would be in use in time for any full trial of PGT.
Costing/resource use
At the time of preparing the trial protocol, a search of the Database of Instruments for Resource Use Measurement (DIRUM) database (www.dirum.org; accessed 30 January 2014) revealed no previous costing/resource use questionnaires for burn treatments within the disease subcategories of ‘wounds’, ‘skin’ or ‘child health’. A new resource use questionnaire was therefore designed, but drew to some extent on previous work relating to leg ulcers and eczema (see Appendix 4). 52,53
A decision was taken to explore the feasibility of collecting cost data from a societal and a NHS perspective. It was expected that patients in both treatment arms may potentially need to travel long distances to receive care at central locations (particularly those travelling in from more rural areas) and that, outside of the trial environment, if PGT is not received, there may be fewer visits to the clinic. As well as direct travel costs, long journey times may necessitate time off work for both adult patients and parents. It was also expected that those patients not receiving PGT may purchase alternative over-the-counter dressings, bandages, tightly fitting clothing or ointments, hence shifting the cost, to some extent, from the NHS to patient or parent. Based upon feedback from our patient and public representatives, we considered the possibility that if patients deem a pressure garment to offer a degree of protection (versus no pressure garment), they may return to work or have increased productivity at work earlier, particularly in those in more manual professions. A decision was therefore taken to include questions relating to employment and presenteeism (reduced productivity in the workplace as a result of health problems).
A decision was taken not to ask patients or parents to self-report income, as there was concern that they may be reluctant to provide such personal information; we did, however, obtain data on occupation.
The wide range of ages necessitated the development of two versions of the resource use questionnaire, one for adults (defined in this context as aged ≥ 16 years to coincide with the adoption of the EQ-5D-5L), and one for parent completion in the case of children (aged ≤ 15 years).
The number of visits to the burns clinic was specified in the trial protocol, as was the management by the site of massage, moisturisation and the application of silicone gel. The location of the pressure garment and the date on which pressure garments were fitted was recorded by staff at the treatment centres via the case report form (CRF). It was planned that cost data relating to different types of pressure garment would be obtained from a commercial price list used by the majority of the sites.
Willingness to pay
The inclusion of the WTP questionnaire was intended both to (1) value the impact of the treatment using a single, monetary metric, across all age ranges, given that – ultimately – two different sets of utility values would be elicited (from EQ-5D-5L and from CHU-9D – directly and indirectly) and (2) allow patients/parents to value outcomes that they have reason to consider relevant and important. This was especially important as the HRQoL instruments could only crudely incorporate outcomes, such as the patient’s own perceptions of changes in scar appearance, or aspects of treatment such as itch; information and compassionate care provided by staff; and inconvenience. The value of such aspects of treatment (Mooney54 also suggests that these may include dignity and autonomy) can be thought of in terms of ‘process utility’. 54 This is not a limitation of the measures as such, but instead stems from a normative stance within cost–utility analysis that the sole objective is to maximise health outcomes.
Two versions of the WTP questionnaire were developed, for PGT and no-PGT patients (see Appendices 5 and 6), both to be administered at the 6-month follow-up. Both groups were asked to value the impact of the treatment that they had actually received (an ex post perspective), although the PGT patients were also provided with (or rather were reminded of, given that they had received an information leaflet at the time of recruitment) a short description of the difference between the two treatment arms, and asked the additional question: ‘If you could pay a smaller amount per month to receive the same treatment but without the tight fitting Lycra garment, would you choose to do so?’, with the follow-up question, if yes, of ‘I would pay £__ per month for this treatment’.
The 6-month follow-up was chosen to give sufficient time for patients or parents to experience treatment because not all patients were followed up at 12 months. Owing to resource constraints, it was planned that the questionnaire would be given out at the centre (by the usual centre staff) with the ‘standard’ questionnaires.
Summary
-
The wide age range included in the sample required that three different health economics instruments be chosen to capture health outcomes: EQ-5D-5L (for patients aged ≥ 16 years); CHU-9D (for ages 7–16 years, and with further differentiation within this age range relating to patient vs. proxy completion); and PedsQL (for ages 2–4 years, with solely proxy completion).
-
A WTP questionnaire was introduced to enable patients and parents to value outcomes broader than ‘just’ health. Two versions of the WTP questionnaire were developed (one for each treatment arm) so that patients could place a value on the care they had actually received.
-
A NHS and societal perspective was adopted to capture and record cost data. Information that was not routinely captured on the CRF would be elicited directly from the patient or parent. Costing for garments would be captured from a commercial price list.
Chapter 7 Pilot randomised controlled trial of pressure garment therapy
Relevant objectives
The following objectives are addressed in this chapter:
-
assess recruitment rates and willingness to randomise between PGT and no PGT in multiple sites
-
assess adherence with randomised allocation and PGT therapy
-
assess possible processes for achieving blinded assessment of outcomes.
A related objective, discussed in Chapter 10, was to consider outcome distributions to inform a sample size calculation for a future trial.
Methods
Trial design
The second phase of the PEGASUS study, P2, was an open, pilot, two-arm RCT with a maximum follow-up period of 12 months. Participants were randomised to PGT or no PGT and both groups were allowed standard massage, moisturisation and silicone treatments. The trial aimed to recruit in seven specialist burns units in the UK.
Participants
The study recruited participants of any age who were at risk of abnormal scarring following burn injury and had been referred to one of the specialist burns services. Following standard practice, consideration of scar management started before the wound had healed. When 90% wound healing had occurred (following visual assessment by a therapist), patients attended an initial appointment during which they were screened for eligibility by the local research team.
When the eligible patient’s burn was assessed as having healed, the clinical team introduced the concept of the PEGASUS study, explaining the treatment options available and the level of evidence behind each option. The clinical team provided the eligible patient or their parent or carer with the Research Ethics Committee-approved patient information leaflet (PIL) on NHS trust headed paper. Potential participants then had 24 hours to read the PIL, discuss it with their family, ask questions and decide whether or not they would like to consent to participate in the trial. Once consented, patients were randomised to either PGT or no PGT. All patients were allowed to receive other modalities of scar management such as massage, moisturisation or silicone treatment.
Inclusion criteria
Potential participants who were considered eligible for inclusion included:
-
adults and children with burn injuries covering > 1% of TBSA
-
those treated with split-thickness skin grafts or conservatively managed burn wounds or donor sites that had taken > 2 weeks to heal
-
those with potential for hypertrophic scarring
-
those considered suitable for scar management therapy.
Exclusion criteria
Potential participants were not considered eligible for the pilot trial if they:
-
could not read, write or understand English (as the outcomes identified from P1 may not have data collection tools in translatable versions for the future RCT, a patient’s understanding of English is essential)
-
had pre-existing skin conditions affecting wound healing
-
had a history of keloid scarring
-
had a known allergy to Lycra or any other component of pressure garments
-
were not, in the investigator’s opinion, suitable to participate in the trial because of a clinically relevant past medical history or other pertinent factors.
A screening log was kept to record numbers of patients meeting and not meeting the eligibility criteria and patients who were eligible to enter the study but declined to participate. Qualitative analysis of the reasons behind participants withdrawing and patients declining to participate in the trial is provided in Chapter 8.
Randomisation
After informed consent had been obtained, participants were randomised into the trial via a telephone randomisation service provided by the Birmingham Clinical Trials Unit (BCTU). The randomisation schedule was generated by computer using permuted random blocks of variable length to allocate eligible patients in a 1 : 1 ratio to either the intervention (PGT) or the control (no-PGT) arms. No stratification was employed in the allocation.
A trial number was allocated only after eligibility had been confirmed via the inclusion and exclusion criteria on the randomisation form and patient consent had been given. Once a trial number was allocated, a confirmatory e-mail was sent to the local principal investigator (PI) and the named RN or scar management therapist. With the participant’s permission, the GP was notified using the standard letter provided for this purpose.
Health technologies being assessed
Participants in both arms of the trial were allocated to receive standard education concerning scar management techniques from their clinical team. This typically involves demonstration of massage techniques, recommendation to undertake massage three or four times per day with moisturiser, stretching and other exercises, sun cream advice and a discussion concerning returning to normal functioning. To minimise treatment difference between the trial arms, participants were allowed to receive silicone products, including gels, sprays or sheets, from month 1 in the PGT arm and week 1 in the no-PGT arm, if deemed appropriate.
Participants in the PGT arm received pressure garments. Each centre in the trial used its standard supply route, either purchasing garments externally or manufacturing its own. Participants were measured for their garments after randomisation and were scheduled to return to the burns clinic for garment fitting 1 week later, at which point any alterations could be made. To avoid interruption of the application of the pressure garment for the recommended 23 hours a day, a second garment was supplied if needed. The fit and wear of the garments was checked throughout the trial at the routine clinic visits and garments were replaced as required.
All participants were required to attend routine follow-up scheduled appointments, with additional appointments allowed (for issues such as vulnerable adults and children), in line with standard management practice in each centre and not as part of the trial appointments.
Study setting
To evaluate the ability to recruit across multiple sites, seven specialist NHS burns centres were invited to participate: two centres that specialised in treating children, three sites that treated only adults and two that treated both. The five sites treating only adults or only children were:
-
BCH NHS Trust (children)
-
Central Manchester University Hospitals NHS Foundation Trust (Manchester) (children)
-
North Bristol NHS Trust (Bristol) (adults)
-
University Hospitals Birmingham (UHB) NHS Foundation Trust (adults)
-
The Welsh Centre for Burns and Plastic Surgery, Abertawe Bro Morgannwg University Health Board (Swansea) (adults).
The two sites treating both adults and children were:
-
Queen Victoria Hospital NHS Foundation Trust (East Grinstead)
-
Mid Essex Hospital Services NHS Trust (Essex).
Study procedures and schedule of assessments
The schedule of assessments for the trial is detailed below and summarised in Appendix 7, Table 27. In the original study protocol, it was intended that, as information on valid outcome measures emerged from P1 of this project, it would be desirable to refine the outcome measures collected in P2. However, with the delayed start to recruitment and the reduced follow-up time of the trial, there was insufficient separation between P1 and P2 to allow new outcome measures to be identified and included. The assessments made in the RCT were as follows.
Baseline burns assessments
The initial assessment of burn injury was captured during the baseline visit and consisted of the following.
-
Burn injury characteristics: the aetiology of the burn such as thermal (flash, contact, scald, radiation), electrical (low voltage or high voltage) or chemical (acid or alkali). The TBSA percentage was obtained from the patient’s clinical notes.
-
Fitzpatrick Scale score: a numerical classification of skin colour.
-
Medical history: a history of any significant comorbidities in the following categories – neurological, psychiatric/behavioural, musculoskeletal, dermatological and endocrinological.
Scar assessment
Scar assessment was completed throughout the trial on the same scars (up to a maximum of three) initially assessed as being suitable for scar management therapy. The assessment comprised three components:
-
Vancouver Scar Scale, a clinician-reported validated measure of global scar appearance. This consists of three domains – pigmentation, height and vascularity – that are scored from 0 to 3, and one domain, pliability, scored from 0 to 5. The total score, summed over the four domains, ranges from 0 to 14. Low scores are regarded as good. The burn scar index documented any change in scar appearance. Any non-trial scar requiring a VSS assessment followed local practice and was documented in the medical notes but was not recorded as trial data in the CRFs.
-
Patient and Observer Scar Assessment Scale (POSAS). The POSAS consists of two parts: a patient-reported scale and an observer-reported scale. Each part contains six items that are scored numerically from 1 to 10, with low scores being regarded as good. The relevant parts of POSAS were completed by the scar therapist and patient at each assessment.
-
The observer-reported section consists of six domains (vascularity, pigmentation, thickness, relief, pliability and surface area). There are also categorical responses for each domain, ranging from five possible levels for vascularity (pale, pink, red, purple and mixed) to two possible levels for thickness (thicker or thinner).
-
The patient-reported section consists of six questions covering pain, itch, colour, stiffness, thickness and irregular appearance and an overall opinion.
-
There is no appropriate POSAS patient-reported scale for paediatric patients. To record paediatric pain and itch, two scales were used, each with two age-specific versions: a Likert scale (younger patients) or a visual analogue scale (older patients). Low scores are regarded as good.
-
-
ROM. The ROM of joints affected by the burn injury was assessed by goniometry. The scar management therapist was asked to record the percentage of movement for each joint affected by the scars being followed in the trial. Higher percentages are regarded as good.
-
Cutometer® (Courage+Khazaka electronic GmbH, Cologne, Germany). For patients randomised at UHB and BCH, scar elasticity was assessed using a Cutometer. The percentage of elasticity was recorded on up to three scar sites and matching normal skin sites. Higher percentages are regarded as good.
Clinical photography
The scar being assessed at each trial visit might have been photographed (either with a digital camera provided by the PEGASUS trial or using the local NHS trust’s medical illustration service). Any photographs were stored securely in accordance with standard local practice.
Adherence
Adherence with allocated scar management therapy (including adherence with common components of massage, moisturisation and silicone gel) was assessed during each trial visit. Any required re-education on the importance of the scar management therapy was discussed with the participant and documented in the medical notes in accordance with local practice.
Adverse events
The review of adverse events (ulceration/wound breakdown, contractures/significant loss of joint ROM, interference with growth, intralesion steroid injection for aggressive hypertrophic scars and surgical intervention/scar revision) occurring since the previous visit was recorded in the medical notes as part of routine clinical care and was also entered onto the trial follow-up CRFs, as appropriate.
Medication review
Use of trial-related medications was recorded on the CRFs for the following categories:
-
antihistamines
-
analgesics
-
mild opioid analgesics
-
opioids
-
anticonvulsants (used for complex pain management).
Blinding
Owing to the nature of the intervention, it was not possible to blind either the participants or their parents/carers to the allocated treatment. The clinician/therapist scales (VSS and POSAS observer-reported scale) were completed by the therapist at the site, who is likely to have been aware of treatment allocation. The feasibility of potential methods for introducing blinding in a future trial was discussed at investigator meetings during the trial.
Sample size
As this is a feasibility study, it was not powered to detect any clinically meaningful difference between the PGT and no-PGT arms in any of the recorded outcomes. The principal objective of the feasibility component was to obtain reliable estimates of the rates of recruitment and retention ahead of a full-scale RCT. This study aimed to recruit at least 44 participants in each group, a total of 88 participants over an estimated 9-month recruitment period. This would allow the recruitment rate to be estimated with a 95% confidence interval (CI) and with a maximum width of 20%.
Statistical methods
Outcomes of interest are described using appropriate summary statistics. For continuous outcomes, means and standard deviations (SDs) are used if the distributions are approximately normal; medians and interquartile ranges (IQRs) are used if they are not. For categorical outcomes, summary statistics consist of numbers and percentages. The changes from baseline to the follow-up assessments at week 1 and at months 1, 3, 6 and 12 were calculated for each of the treatment arms, along with the IQRs. Estimates of the variability of any outcome measures identified as being suitable for a main trial are also calculated to inform sample size calculations for a future full-scale RCT. Recruitment rates are given as an average number of patients per centre per month. Dropout rates are expressed as a percentage separately for each arm, along with the reasons for dropout.
All analyses were performed on the intention-to-treat principle.
The data analyses were generated using SAS® software (version 9.4; SAS Institute Inc., Cary, NC, USA). SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates US registration.
Ethics approval and trial registration
The study was reviewed by the National Research Ethics Service Committee West Midlands – Coventry and Warwickshire on 28 May 2014. Following clarification of the content of the PIL, refinement of the adult consent form and further details of the composition of the monitoring committee, these amendments were submitted on 27 June 2014 and subsequently approved. The PEGASUS trial was given a favourable opinion by the committee on 8 July 2014.
Amendments to ethics approval
During the course of the PEGASUS study, three substantial amendments were submitted and approved by the West Midlands – Coventry and Warwickshire Research Ethics Committee. The first amendment was approved on 24 October 2014 and consisted of refinements to the inclusion and exclusion criteria and a minor rewording of the PIL for adults, young people, children and parents. New qualitative and pilot trial process evaluation documents were also submitted. The second amendment, to include an Independent Data Monitoring and Ethics Committee, as per instruction from the HTA programme, was approved on 1 May 2015. The third amendment updated the membership of the Trial Steering Committee and was approved on 6 November 2015.
Research and development approval
As a NIHR Portfolio study, the NIHR Coordinated System for gaining NHS permission was applied for via Northumberland Tyne and Wear Comprehensive Local Research Network. No difficulties were encountered with this process.
Trial registration
The trial was registered on the International Standard Randomised Controlled Trial Number (ISRCTN) registry on 3 October 2014 (registration number ISRCTN34483199), prior to any patients being recruited to the study.
Results
Participant screening and recruitment
The study was scheduled to open in September 2014 and to recruit for 9 months. Owing to delays in contract negotiations at sponsor level and gaining local approval at sites, the study opened to recruitment 4 months late, in December 2015. Support was obtained from the HTA programme manager in identifying strategies to resolve these protracted delays and engaging with the sponsor to help expedite the approval process. Recruitment opened on 1 December 2014 and ended, 9 months later, on 1 September 2015.
However, the original end date of the trial was not changed and follow-up ended for all participants by 31 May 2016. This meant that, although all participants were followed up for ≥ 6 months, only 56 out of the 88 participants (those recruited prior to 31 May 2015) were followed up as planned for 12 months.
The first participant was recruited to the trial on 6 January 2015, and six out of seven sites recruited their first participant within 3 weeks of opening. Recruitment of the 88 participants was completed on 19 August 2015, within the allotted 9 months, with 50% of the target attained in 4 months. During this 9-month period of recruitment, a total of 211 patients were screened, with 186 (88%) deemed eligible and 88 (47% of eligible patients) recruited into the trial (Figure 3). The eligibility rate of 88% (95% CI 83% to 92%) suggests that > 80% of screened patients would be eligible in a large-scale RCT. Of the 25 patients screened but deemed ineligible, nine (36%) were considered not suitable for PGT, seven (28%) were excluded because of their medical history (e.g. diabetes, memory loss, unstable personality), four (16%) were not suitable for screening, two (8%) required PGT, two (8%) were unlikely to comply with PGT and one (4%) was still undergoing treatment as the wound had not fully healed.
FIGURE 3.
Recruitment to the trial.
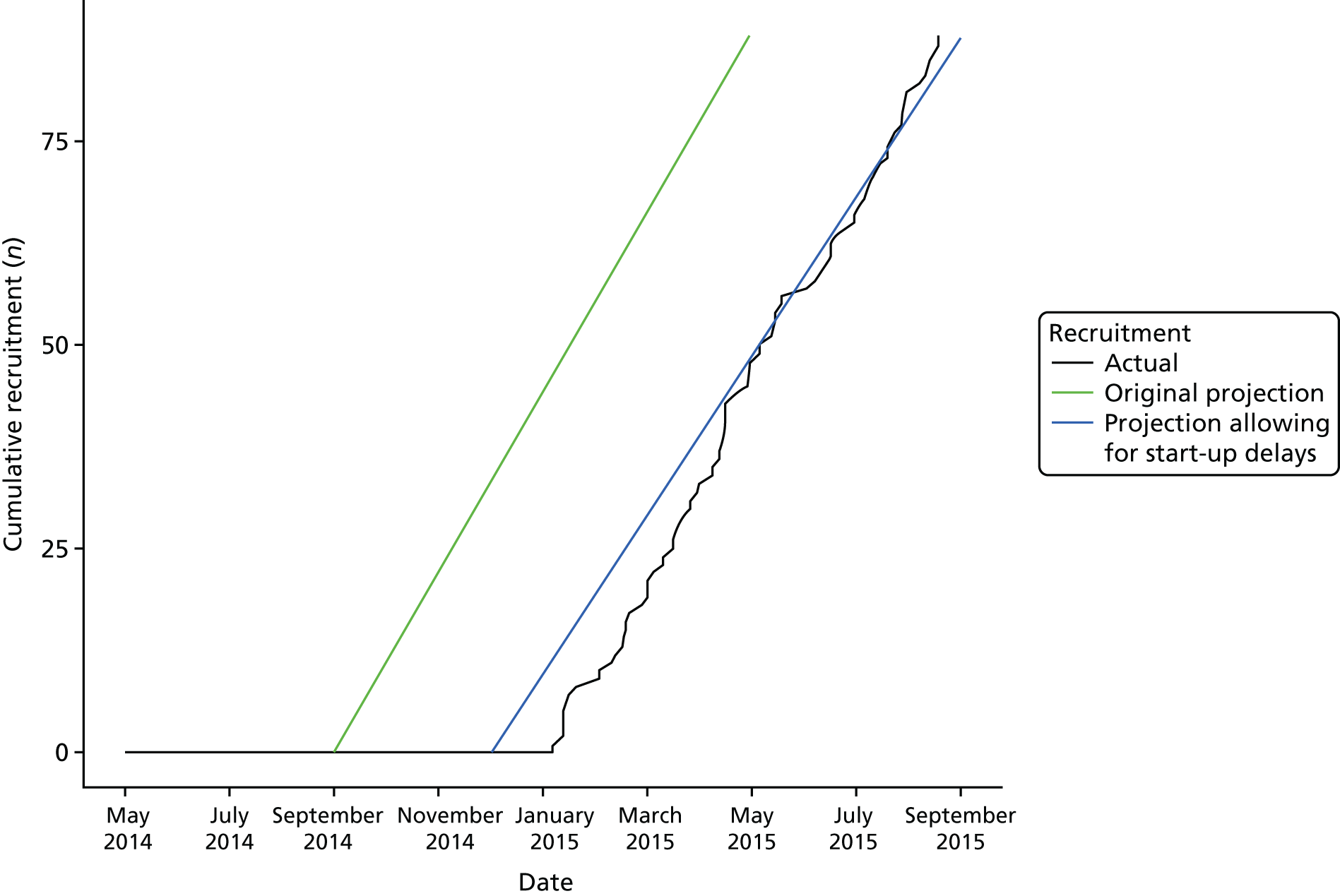
The recruitment of adult and paediatric patients is summarised in Table 7.
| Screening centre | Number screened (n) | Acceptance rate (%) | Number randomised (n) | Number LTFU, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | No PGT | PGT | Adult | Child | Total | No PGT | PGT | |||
| UHB NHS Foundation Trust | 51 | 43 | 22 | 9 | 13 | 22 | 0 | 4 (18) | 1 (11) | 3 (23) |
| BCH NHS Trust | 35 | 57 | 20 | 10 | 10 | 0 | 20 | 0 (0) | 0 (0) | 0 (0) |
| North Bristol NHS Trust | 38 | 37 | 14 | 10 | 4 | 14 | 0 | 5 (36) | 3 (30) | 2 (50) |
| Queen Victoria Hospital NHS Foundation Trust | 9 | 89 | 8 | 1 | 7 | 5 | 3 | 3 (38) | 1 (100) | 2 (29) |
| Mid Essex Hospital Services NHS Trust | 31 | 29 | 9 | 5 | 4 | 7 | 2 | 1 (11) | 1 (20) | 0 (0) |
| Central Manchester University Hospitals NHS Foundation Trust | 23 | 26 | 6 | 4 | 2 | 0 | 6 | 0 (0) | 0 (0) | 0 (0) |
| The Welsh Centre for Burns and Plastic Surgery, Abertawe Bro Morgannwg University Health Board | 24 | 38 | 9 | 5 | 4 | 9 | 0 | 1 (11) | 1 (20) | 0 (0) |
| Total | 211 | 42 | 88 | 44 | 44 | 57 | 31 | 14 (16) | 7 (16) | 7 (16) |
Consent to participation in the trial
The consent rate was 47% (95% CI 40% to 55%) among eligible patients and 42% (95% CI 35% to 49%) among all screened patients. Reasons for non-participation were offered by 19 out of the 98 eligible patients who did not give consent: six (6%) patients did not want PGT, six (6%) wanted PGT, one (1%) did not want to be randomised and six (6%) offered other individual reasons. The study team judged 10 (10%) further patients as unable to give informed consent, 12 (12%) were unable to speak/understand English and 10 (10%) were unlikely to complete follow-up. Thirty (31%) were recorded as simply declining participation and 12 (12%) as not stating any reason. A further five (5%) were not recruited because of staffing issues.
The main reason for failure at three of the sites recruiting paediatric patients (Central Manchester University Hospitals NHS Foundation Trust, Mid Essex Hospital Services NHS Trust and BCH NHS Trust) was because of the patient or parent declining to participate. This accounted for 41–59% of total screening failures at these centres compared with 13–24% at two of the sites recruiting adults only (North Bristol NHS Trust and UHB NHS Foundation Trust). The main reason for screening failure at these sites was lack of suitability for the trial. The other adult site, The Welsh Centre for Burns and Plastic Surgery, Abertawe Bro Morgannwg University Health Board, had 33% of screening failures because of staffing resources and 40% because patients declined to enter the trial. The Queen Victoria Hospital NHS Foundation Trust did not complete screening logs fully, so no information is available. Adequate staff provision and training is a key element to delivering any future full-scale trial of this type. Reasons for patients choosing to consent or decline their participation in the trial are analysed in detail through the qualitative work in Chapter 8.
Randomisation
Participants were equally allocated between study arms. Baseline demographic and clinical characteristics by allocated treatment are provided in Table 8. The majority of recorded burns were of either thermal flame or thermal scald aetiology (77% and 70% in the no-PGT and PGT arms, respectively). Few participants recorded any other injuries.
| Participant and burn injury characteristic | Treatment arm | |
|---|---|---|
| No PGT (n = 44) | PGT (n = 44) | |
| Age (years), median (IQR) | 31 (4.5–51.5) | 28 (6.5–52) |
| Patient, n (%) | ||
| Paediatric | 15 (34) | 16 (36) |
| Adult | 29 (66) | 28 (64) |
| Sex, n (%) | ||
| Male | 25 (57) | 24 (55) |
| Female | 19 (43) | 20 (45) |
| Height (cm) , median (IQR) | 169 (106–177) | 164 (111–176) |
| Weight (kg), median (IQR) | 61 (19–85) | 67 (18–84) |
| TBSA of burn (%), median (IQR) | 4 (1.8–7.5) | 4 (1.8–8) |
| Fitzpatrick Scale, n (%) | ||
| 1/a: white, always burns, never tans | 2 (5) | 5 (11) |
| 1/b: white, freckles/red hair/Celtic origin | 0 (0) | 2 (5) |
| 2: white, always burns, but tans with difficulty | 7 (16) | 6 (14) |
| 3: white, sometimes mildly burns, but tans | 21 (48) | 17 (39) |
| 4: moderate brown, very rarely burns, tans easily | 9 (20) | 10 (23) |
| 5: dark brown, very rarely burns, moderately pigmented, tans easily | 1 (2) | 1 (2) |
| 6: black, never burns, tans easily | 2 (5) | 3 (7) |
| Unknown | 2 (5) | 0 (0) |
| Centre, n (%) | ||
| UHB NHS Foundation Trust | 9 (20) | 13 (30) |
| BCH NHS Trust | 10 (23) | 10 (23) |
| North Bristol NHS Trust | 10 (23) | 4 (9) |
| Queen Victoria Hospital NHS Foundation Trust | 1 (2) | 7 (16) |
| Mid Essex Hospital Services NHS Trust | 5 (11) | 4 (9) |
| Central Manchester University Hospitals NHS Foundation Trust | 4 (9) | 2 (5) |
| The Welsh Centre for Burns and Plastic Surgery, Abertawe Bro Morgannwg University Health Board | 5 (11) | 4 (9) |
| Number of burns, n (%) | ||
| 1 | 20 (45) | 29 (66) |
| 2 | 15 (34) | 12 (27) |
| 3 | 7 (16) | 3 (7) |
| Unknown | 2 (5) | 0 (0) |
| Burn injury aetiology, n (%) | ||
| Thermal flame | 12 (27) | 15 (34) |
| Thermal flash | 4 (9) | 1 (3) |
| Thermal scald | 19 (43) | 14 (32) |
| Thermal flame and flash | 3 (7) | 1 (2) |
| Thermal flame and scald | 0 (0) | 1 (2) |
| Thermal contact | 4 (9) | 7 (16) |
| Chemical | 0 (0) | 4 (9) |
| Unknown | 2 (5) | 1 (2) |
| Other injuries, n (%) | ||
| Inhalation injury | 1 (2) | 1 (2) |
| Fractures/sprains | 0 (0) | 1 (2) |
| Other | 1 (2) | 1 (2) |
Withdrawals
Five out of 88 (6%) participants were withdrawn: four who were initially allocated to the no-PGT treatment arm and one to PGT treatment. The reasons for withdrawal for the four participants who were randomised to the no-PGT treatment arm were as follows:
-
The participant asked to be treated with pressure garments, as they felt that their scar was becoming lumpy and looking worse.
-
Study investigators at the site decided to withdraw the participant from the no-PGT treatment arm and started PGT for all three monitored scars.
-
The participant was elderly and relied on friends to travel to appointments. The participant did not wish to travel for any follow-up appointments and wished to choose the treatment that could be monitored at long intervals between follow-up appointments. The participant withdrew from the study before completion of baseline assessments.
-
The participant attended the initial baseline assessment but cancelled the first follow-up visit and then withdrew from the study and any further follow-up. The participant met the inclusion criteria but only had minimal evidence of scarring.
The one participant who withdrew from the PGT treatment arm withdrew from the study prior to the week 1 visit but gave no reason.
There was evidence of site confusion regarding the correct trial procedure for withdrawals in the event of withdrawal from treatment, as participants should have continued in the study (this applied to the first and second withdrawals listed above). Inadequate screening may also have led to the third participant inappropriately being recruited and randomised.
Lost to follow-up
Fourteen participants (16%) were lost to follow-up (LTFU): seven in each treatment arm. A further PGT participant did not attend follow-up at 6 months and was not seen at 12 months because the study had terminated before this follow-up point had been reached. Counting this participant as LTFU gives a LTFU rate of 17%. Reasons for being LTFU were known for only three of these participants: one was inappropriately included and two moved away from the area. Loss to follow-up occurred after month 1 in 11 out of the 15 participants (73%). Loss to follow-up showed variation between centres (see Table 7), although results are based on small numbers. The North Bristol NHS Trust and Queen Victoria Hospital NHS Foundation Trust had the highest losses to follow-up, of 36% and 38%, respectively, whereas both specialist children’s hospitals (BCH NHS Trust and Central Manchester University Hospitals NHS Foundation Trust) had no participants LTFU. The nature of the participant population at the centres (e.g. adult vs. paediatric participants) may relate to the levels of loss to follow-up, although at both the Mid Essex Hospital Services NHS Trust and The Welsh Centre for Burns and Plastic Surgery, Abertawe Bro Morgannwg University Health Board, only a single participant was LTFU. The possible impact of clinical equipoise on participant retention is discussed in detail in Chapter 8.
Owing to the reduced study timeline, all participants were able to be followed up for 6 months but not all could be followed up for the full 12-month period. Sixty-seven of the 88 participants (76%, 95% CI 66% to 85%) completed their 6-month visit: 32 (73%, 95% CI 57% to 85%) participants in the no-PGT arm and 35 (80%, 95% CI 65% to 90%) in the PGT arm. Of the 21 participants who were not assessed at 6 months, 14 were defined as LTFU, five were withdrawals, one had been discharged and one did not attend at 6 months and the study terminated before they reached 12 months. The estimated rate of loss to follow-up by 6 months is 16% (95% CI 9% to 25%). Aggregating losses to follow-up and withdrawals up to month 6, a total of 19 participants dropped out of the study; hence, the estimated drop-out rate is 22% (95% CI 14% to 32%).
The participant flow through the trial is shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 4).
FIGURE 4.
Consolidated Standards of Reporting Trials flow diagram.
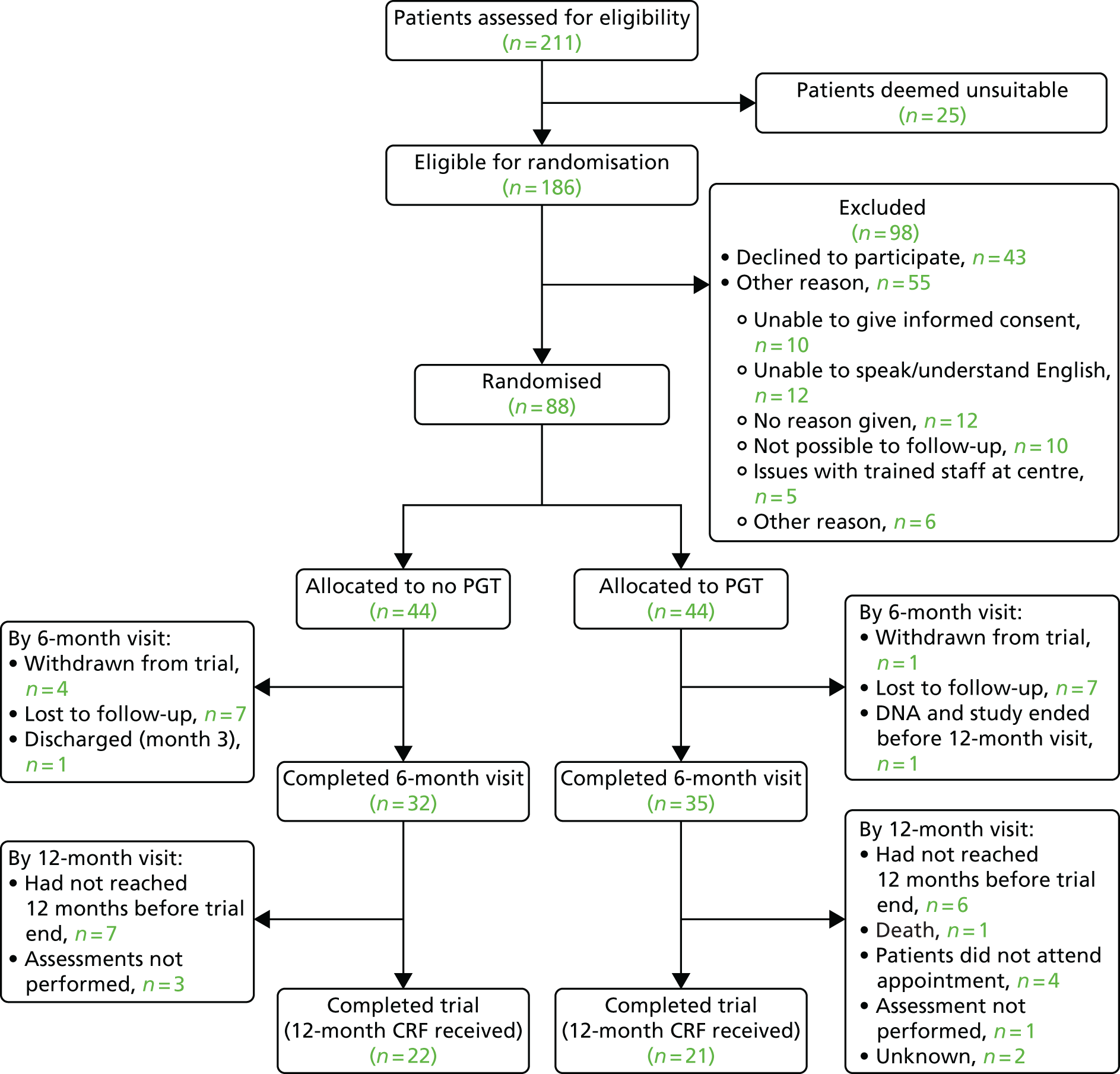
Adherence and crossover
Adherence
Table 9 summarises the participant-reported adherence with their allocated treatment (including PGT if in the PGT arm and other modes of therapy in both arms) at each follow-up appointment. Participants were asked if, since their last visit, they had complied with their scar management regime. Those participants reporting partial adherence were asked to estimate the percentage level of adherence they had achieved. Both treatment arms exhibit similar levels of full, partial and non-adherence across the follow-up visits.
| Time point | Treatment arm, adherence | |||||||
|---|---|---|---|---|---|---|---|---|
| No PGT (n = 44) | PGT (n = 44) | |||||||
| No | Partial | Yes, fully | Missing | No | Partial | Yes, fully | Missing | |
| Week 1 | ||||||||
| Number reporting adherence | 0 | 10 | 25 | 0 | 0 | 5 | 33 | 2 |
| % adherence (95% CI) | 100 (75 to 100) | 100 (100 to 100) | ||||||
| Month 1 | ||||||||
| Number reporting adherence | 0 | 13 | 23 | 1 | 0 | 16 | 22 | 0 |
| % adherence (95% CI) | 100 (75 to 100) | 100 (80 to 100) | ||||||
| Month 3 | ||||||||
| Number reporting adherence | 2 | 14 | 16 | 0 | 0 | 11 | 23 | 2 |
| % adherence (95% CI) | 97.5 (55 to 100) | 100 (90 to 100) | ||||||
| Month 6 | ||||||||
| Number reporting adherence | 0 | 14 | 18 | 0 | 0 | 12 | 21 | 1 |
| % adherence (95% CI) | 100 (72.5 to 100) | 100 (90 to 100) | ||||||
| Month 12 | ||||||||
| Number reporting adherence | 1 | 6 | 15 | 1 | 0 | 7 | 14 | 0 |
| % adherence (95% CI) | 100 (60 to 100) | 100 (80 to 100) | ||||||
Full adherence with their allocated treatment was reported by only 65 out of 75 (60%), 39 out of 68 (57%) and 39 out of 66 (59%) participants at months 1, 3 and 6, respectively. The estimated rate of full adherence by month 6 for a full trial is then 59% (95% CI 46% to 71%). Full adherence by month 6 was reported by 18 out of 32 (56%) and 21 out of 34 (62%) participants in the no-PGT and PGT arms, respectively. For participants reporting partial adherence, the levels are broadly similar across treatment arms, although those allocated to the PGT treatment arm tended to estimate a higher average level of adherence at months 1, 3 and 6 than those allocated to the no-PGT treatment arm. For the 26 participants supplying an estimate of partial adherence at 6 months, the estimated percentage and IQR was 75% (30–90%). Further discussion of issues with treatment adherence is provided in Chapter 8.
Participants who were allocated to the PGT arm were asked to estimate the number of hours per day they wore their pressure garments. For the visits at months 1, 3 and 6, participants reported wearing their garments for an average of 21 hours per day (Table 10). From notes made on the forms and discussion with the study investigators, there was some uncertainty about the phrasing of the questions; some therapists and participants felt that the definition of adherence was ambiguous and wanted to be able to define more precisely the extent to which components of the assigned treatment had been complied with (e.g. some may use the pressure garment but not the massage technique or silicone gel). At sites that used an external company to manufacture their pressure garment, many therapists found that the timing of the week 1 follow-up visit did not allow enough time for the garments to be made and fitted to measure the level of adherence.
| Time point (number who provided an estimate of the duration they wore the garment for) | Duration (number of hours), median (IQR) |
|---|---|
| Week 1 (n = 2) | Observed values of 6–23 |
| Month 1 (n = 12) | 20.5 (19–22.5) |
| Month 3 (n = 11) | 21.0 (15–23) |
| Month 6 (n = 13) | 20.0 (12–23) |
| Month 12 (n = 4) | 0.0 (0–7.5) |
Several reasons for the lack of adherence were collected at each follow-up visit. After week 1, the reasons given included having problems with the application of gel sheets, running out of silicone gel and not replacing it, use of Tubigrip instead of the pressure garment and removal in hot weather.
Crossover
There were eight participants who ‘crossed over’ to the other treatment arm and remained in the study. Three participants were in the no-PGT treatment arm and five in the PGT arm. Two participants in the no-PGT treatment arm were mistakenly withdrawn from the trial; including these participants in the analysis would mean that there were a total of 10 crossovers (11%), with five no-PGT and five PGT crossovers.
One participant crossed over from the no-PGT to the PGT treatment arm by month 1. Four crossed over by month 3: three from the no-PGT arm to the PGT arm and one from the PGT arm to the no-PGT arm. By month 6, there was one further crossover in each treatment arm. There are three participants without a clear date of crossover: one crossed over from the PGT arm to the no-PGT arm for two of their three scars, one stopped wearing pressure garments and the other requested a modified garment without a high neck.
The five participants who were assigned to the no-PGT arm but crossed over to the PGT arm were three paediatric participants (aged 2 years, 2 years and 3 years) and two adults (aged 20 years and 33 years). Their burns ranged from small to moderate in size (TBSA percentages of 2%, 3%, 4%, 9% and 13%, respectively) and were located on the hand, arm, leg and chest. The two adult participants who crossed over to the PGT treatment arm both did so at the recommendation of the consultant. One adult participant had a moderate-sized burn affecting their arm, wrist and hand and the other had a smaller burn that affected their chest and abdomen and was adjudged not to be healing sufficiently. One of the paediatric participants crossed over to the PGT treatment arm at the recommendation of the consultant. The other two paediatric participants did so following parental decisions.
There were five participants who were assigned to the PGT treatment arm but crossed over to the no-PGT arm. These participants included only one paediatric participant (aged 6 years) and four adults (aged 29 years, 46 years, 50 years and 55 years). Their burns were mostly small (TBSA percentages of 1%, 2%, 2%, 2% and 12%, respectively) and were on the foot, arm, leg and neck. The paediatric participant had a small scar (a TBSA of 1%) on their foot that was adjudged to have healed. Of the four adult participants, two had small scars (both with a TBSA of 2%) that were either adjudged to have healed by the clinician or by the participant themselves. The other two adult participants had difficulties wearing the pressure garments because of the position of their scars: one large scar (TBSA of 12%) on the neck, chest and back required a high-necked pressure garment that the participant did not think they could tolerate and one small scar (TBSA of 2%) on the leg and foot for which the participant struggled to wear the pressure garment, as it kept rolling down.
Pressure garment therapy assessments
For participants randomised to the PGT treatment arm of the trial, details regarding the manufacture and fitting/refitting of the pressure garments were collected at each visit. Table 11 summarises the pressure garment provision at baseline. The two participants who did not respond to this question were using version 1.0 of the baseline form, which did not include this particular question. Of the 42 respondents, all were measured for pressure garments, with most pressure garments being produced externally [24/42 (57%)] compared with being produced in-house [17/42 (40%)], with information not being recorded for one participant. Nearly half of the PGT treatment arm participants, 20 out of 42 (48%), had not been fitted with their pressure garments for their main scar (scar site 1) at baseline.
| Pressure garment fitting history | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 42) | Week 1 (n = 41) | Month 1 (n = 39) | Month 3 (n = 36) | Month 6 (n = 35) | Month 12 (n = 21) | |
| Were patients measured/fitted/refitted for their pressure garments?, n (%) | ||||||
| Yes | 42 (100) | 36 (88) | 17 (44) | 28 (78) | 23 (66) | 7 (33) |
| Where will pressure garments be made?, n (%) | ||||||
| In-house | 17 (40) | 16 (44) | 9 (53) | 10 (36) | 9 (39) | 2 (29) |
| Externally | 24 (57) | 19 (53) | 8 (47) | 18 (64) | 14 (61) | 5 (71) |
| Missing | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Were pressure garments (re)fitted?, n (%) | ||||||
| Scar site 1 (as per the VSS) | (n = 42) | (n = 36) | (n = 17) | (n = 28) | (n = 23) | (n = 7) |
| No, not required | 0 (0) | 0 (0) | 3 (18) | 6 (21) | 2 (9) | 1 (14) |
| No, other reason | 20 (48) | 2 (6) | 1 (6) | 0 (0) | 1 (4) | 6 (86) |
| Yes | 16 (38) | 29 (80) | 11 (65) | 21 (75) | 17 (74) | 0 (0) |
| Missing | 6 (14) | 5 (14) | 2 (12) | 1 (4) | 3 (13) | 0 (0) |
| Scar site 2 (as per the VSS) | (n = 15) | (n = 14) | (n = 9) | (n = 14) | (n = 11) | (n = 3) |
| No, not required | – | 2 (14) | 1 (11) | 2 (14) | 2 (18) | 0 (0) |
| No, other reason | 9 (60) | 1 (7) | 1 (11) | 0 (0) | 1 (9) | 0 (0) |
| Yes | 4 (27) | 10 (72) | 5 (56) | 10 (72) | 6 (55) | 3 (100) |
| Missing | 2 (13) | 1 (7) | 2 (22) | 2 (14) | 2 (18) | 0 (0) |
| Scar site 3 (as per the VSS) | (n = 3) | (n = 2) | (n = 2) | (n = 3) | (n = 2) | (n = 0) |
| No, not required | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No, other reason | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Yes | 1 (33) | 1 (50) | 2 (100) | 2 (67) | 1 (50) | 0 (0) |
| Missing | 0 (0) | 1 (50) | 0 (0) | 1 (33) | 1 (50) | 0 (0) |
Pressure garments were measured, fitted or refitted for 17 out of 39 (44%) participants at month 1, 28 out of 36 (78%) at month 3 and 23 out of 35 (66%) at month 6. The number of participants on the PGT treatment arm having a new pressure garment fitted to at least one of their scars was 17 out of 42 (40%) at baseline, 31 out of 41 (76%) at week 1, 15 out of 39 (38%) at month 1, 24 out of 36 (67%) at month 3, 20 out of 35 (57%) at month 6 and 7 out of 21 (33%) at month 12.
The question wording used in the pilot trial did not clearly distinguish between remeasurement, refitting and replacing garments, which caused some confusion in the responses. Assessment in a main trial requires separately recording these three different processes to fully assess the resource used. It is also important to distinguish between garments for the multiple scars that may be treated at the same time.
Blinding
Assessment of the feasibility of achieving blinded assessment of outcomes was one of the study objectives. The possibilities of undertaking each of the outcome assessments while being blinded to participant allocation was discussed with the therapists at the investigator meetings during the trial.
During the pilot trial, the physical presence of a pressure garment prohibited the blinding of either the participants or their parents/carers to the allocated treatment arm, and the study researcher who treated the participants at their follow-up assessments could not be blinded to treatment allocation because of the small number of adequately trained staff at each site. For most participants, a single researcher would measure and refit the pressure garment, if necessary, and conduct all assessments at follow-up appointments.
To perform a blinded assessment of scar appearance, a second study researcher, unaware of the treatment allocation, would need to be present at each site to complete the scar assessment forms. However, the therapists indicated that this would not guarantee blinded outcome assessment, as pressure garments are, by design, tight-fitting garments that leave indentations or markings on the skin. They would need to be removed for a considerable length of time before any blinded assessment could be made. This was viewed as being unnecessarily burdensome to the participants.
Although clinical photography was considered as a potential method of allowing blinded assessment of scar appearance, obtaining photographs was logistically difficult, as the variability in available facilities at different study sites (e.g. medical imaging vs. a handheld digital camera) meant that standardisation of the photographs was complex and lay outside the standard duties and experience of the study researcher performing the assessments. The requirement for use of medical imaging at some sites also proved prohibitively expensive for this small feasibility study. Moreover, clinical photography would still be subject to the problem of indentations or marks being left on the skin of participants who used pressure garments.
Outcomes
The PEGASUS study was not powered to provide definitive data to assess the clinical effectiveness of PGT compared with no PGT. However, we report the outcome data collected for incorporation in future meta-analyses, as well as to assess the feasibility of collecting outcome data on the chosen scales.
Global scar appearance
For all summaries of global scar appearance, the data are based on the scar that was identified as scar 1 on the baseline assessment. Results for other monitored scars were similar and are not presented here. Table 12 summarises global scar appearance at baseline by treatment arm, as measured using the clinician-reported VSS.
| VSS domain | Treatment arm | |
|---|---|---|
| No PGT (n = 42) | PGT (n = 43) | |
| VSS, median score (IQR) | ||
| Pigmentation (scored 0–3) | 2 (0–2) | 2 (0–3) |
| Pliability (scored 0–5) | 2 (1–2) | 2 (2–3) |
| Height (scored 0–3) | 1 (0–1) | 1 (1–1) |
| Vascularity (scored 0–3) | 2 (1–2) | 2 (1–2) |
| Total (scored 0–14) | 6 (4–7) | 7 (5–9) |
| VSS categories, n (%) | ||
| Pigmentation | ||
| Normal | 12 (28) | 11 (26) |
| Hypopigmented | 7 (17) | 6 (14) |
| Mixed | 13 (31) | 15 (35) |
| Hyperpigmented | 10 (24) | 11 (25) |
| Missing | 0 (0) | 0 (0) |
| Pliability | ||
| Normal | 4 (10) | 0 (0) |
| Supple | 8 (19) | 10 (23) |
| Yielding | 21 (50) | 18 (42) |
| Firm | 9 (21) | 15 (35) |
| Ropes | 0 (0) | 0 (0) |
| Contracture | 0 (0) | 0 (0) |
| Missing | 0 (0) | 0 (0) |
| Height | ||
| Flat | 13 (31) | 8 (19) |
| < 2 mm | 26 (62) | 29 (67) |
| 2–5 mm | 3 (7) | 5 (12) |
| > 5 mm | 0 (0) | 1 (2) |
| Missing | 0 (0) | 0 (0) |
| Vascularity | ||
| Normal | 1 (2) | 0 (0) |
| Pink | 17 (40) | 13 (30) |
| Red | 20 (48) | 22 (51) |
| Purple | 4 (10) | 19 (19) |
| Missing | 0 (0) | 0 (0) |
A summary of VSS domains on follow-up and for each domain individually is provided in Figure 5. Numerical values, as well as the proportion of participants for whom that domain improve from baseline, are presented in Appendix 7, Table 28. The VSS domains were similar at baseline. There were more participants with flat scars in the no-PGT treatment arm than in the PGT arm: 13 out of 42 (31%) versus 8 out of 43 (19%), but there was little difference in overall VSS score between the two treatment arms at any time point.
FIGURE 5.
Summary of baseline and follow-up scores on the VSS by visit and treatment arm for each VSS domain. Plots contain box plots (the central box depicting the IQR and median, whiskers depicting range with outliers indicated individually) and means (open circles) with their 95% CIs. (a) Pigmentation; (b) pliability; (c) height; (d) vascularity; and (e) total.
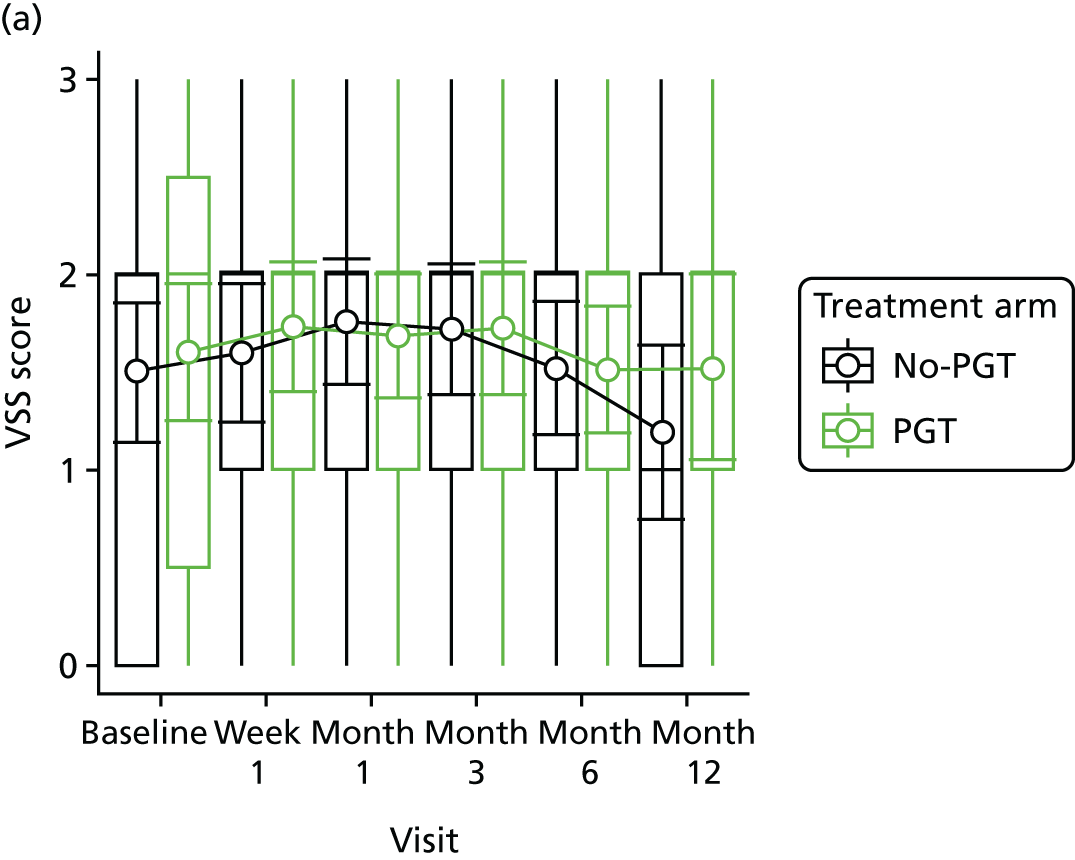
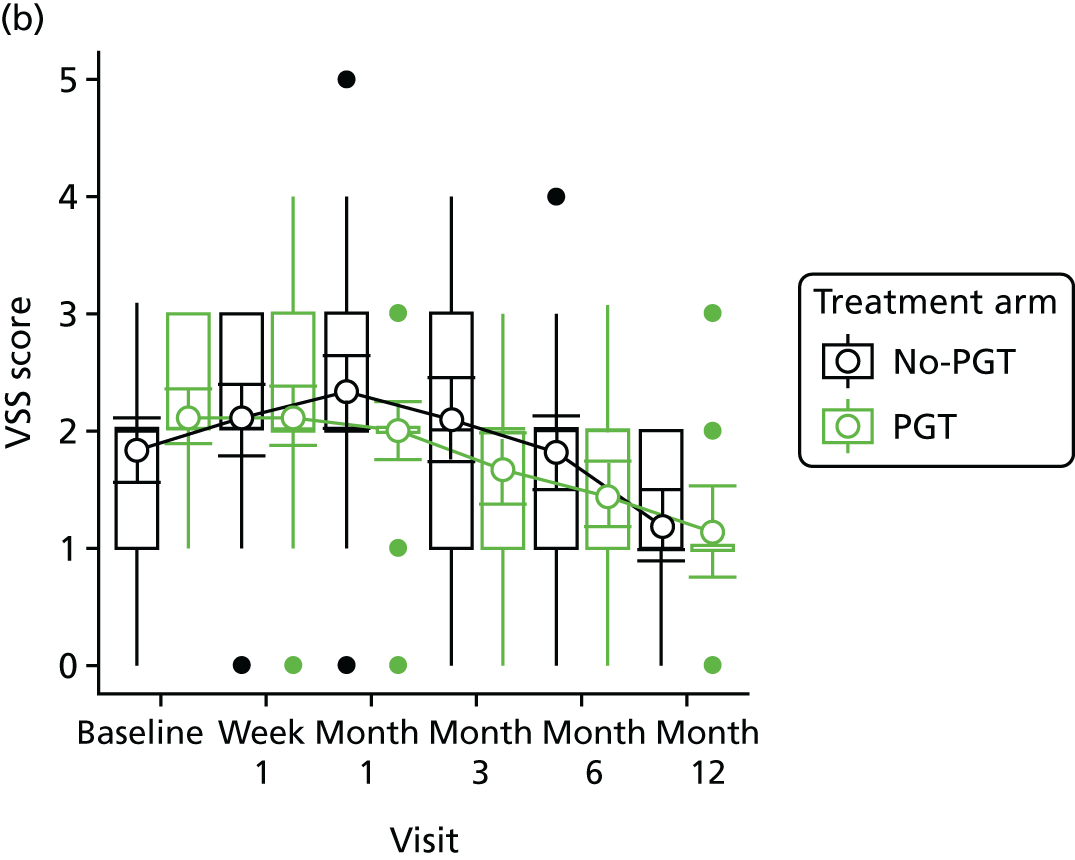
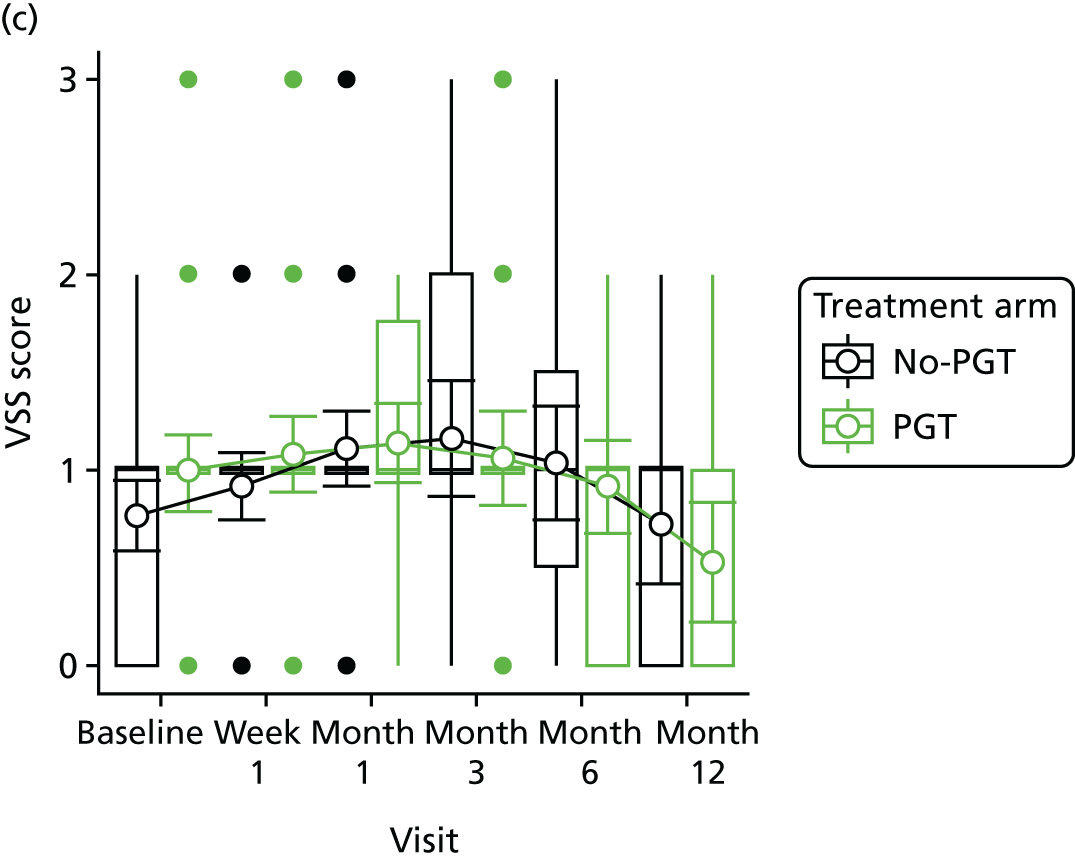
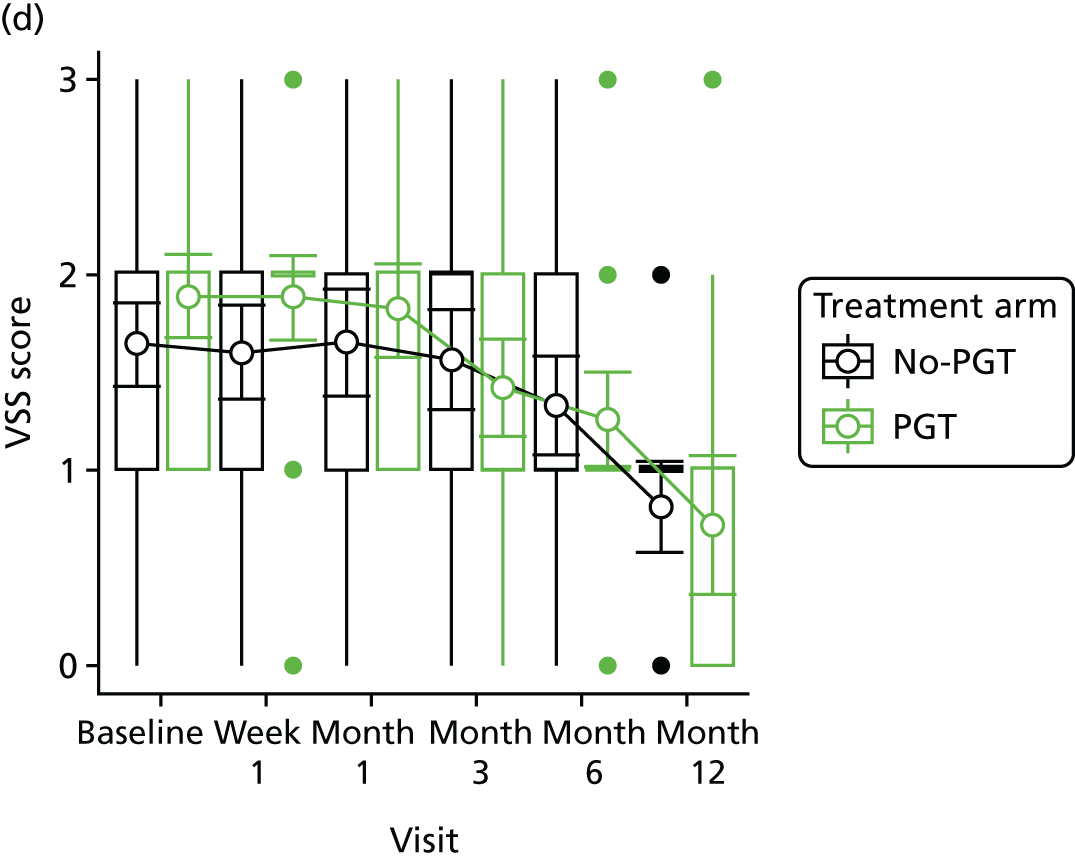
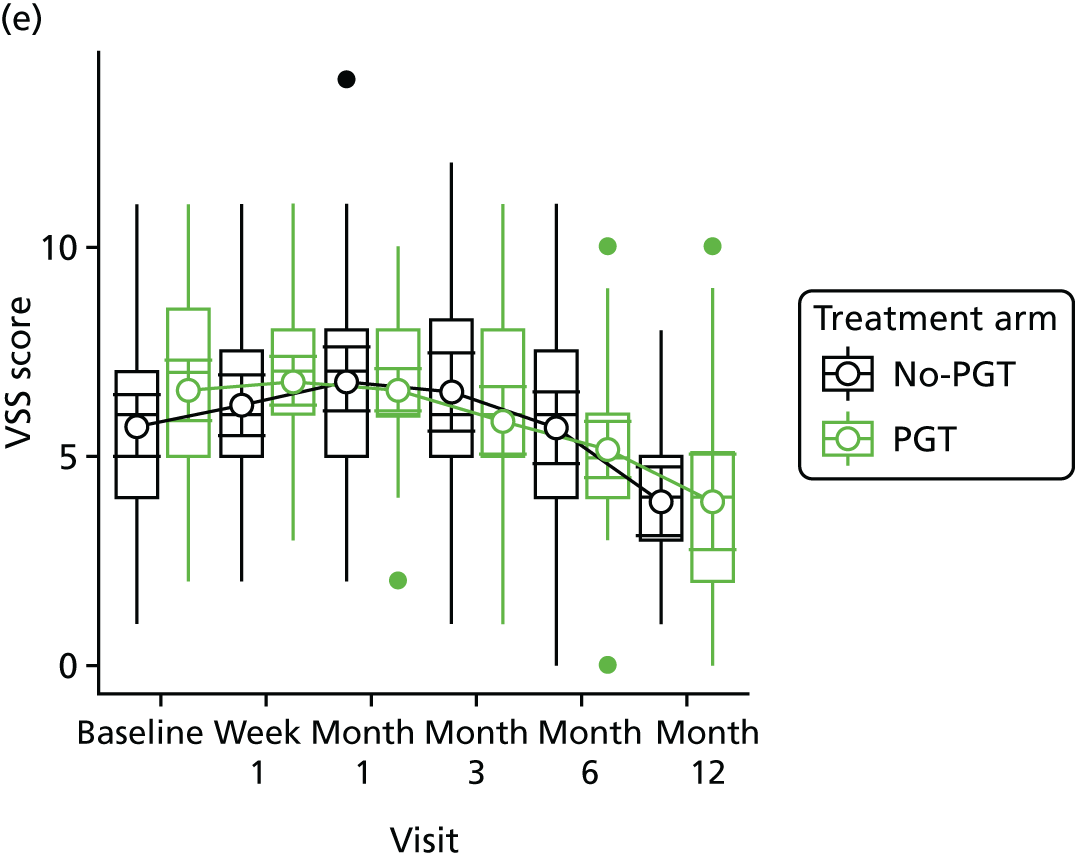
Tables 13–16 summarise the baseline POSAS domains on both the observer-reported and patient-reported scar scales. Results for surface area assessment demonstrated clumping as a result of the recording behaviour of a single therapist who argued that this domain was irrelevant as they would not expect most burns scars to contract or expand in surface area.
| POSAS observer scale (scored 1–10) | Treatment arm, median score (IQR) | |
|---|---|---|
| No PGT (n = 42) | PGT (n = 43) | |
| Vascularity | 6 (4–7) | 6 (4–7) |
| Pigmentation | 3 (2–6) | 4 (2–7) |
| Thickness | 3 (2–4) | 3 (2–5) |
| Relief | 3 (2–4) (n = 41) | 4 (2–5) |
| Pliability | 3 (3–4) | 4 (2–5) |
| Surface area | 1 (1–3) (n = 37) | 1 (1–2) (n = 39) |
| Overall opinion | 4 (3–5) | 5 (4–6) |
| POSAS observer scale domains | Treatment arm, n (%) | |
|---|---|---|
| No PGT (n = 42) | PGT (n = 43) | |
| Vascularity | ||
| Pale | 0 (0) | 0 (0) |
| Pink | 14 (33) | 7 (16) |
| Red | 19 (45) | 18 (42) |
| Purple | 2 (5) | 5 (12) |
| Mixed | 6 (14) | 13 (30) |
| Missing | 1 (2) | 0 (0) |
| Pigmentation | ||
| Hypopigmented | 6 (14) | 9 (21) |
| Hyperpigmented | 9 (22) | 9 (21) |
| Mixed | 26 (62) | 25 (58) |
| Missing | 1 (2) | 0 (0) |
| Thickness | ||
| Thicker | 36 (86) | 41 (95) |
| Thinner | 2 (5) | 0 (0) |
| Missing | 4 (9) | 2 (5) |
| Relief | ||
| More | 28 (67) | 25 (58) |
| Less | 1 (2) | 1 (2) |
| Mixed | 12 (29) | 16 (37) |
| Missing | 1 (2) | 1 (2) |
| Pliability | ||
| Supple | 10 (24) | 15 (35) |
| Stiff | 20 (48) | 21 (49) |
| Mixed | 11 (26) | 5 (11) |
| Missing | 1 (2) | 2 (5) |
| Surface area | ||
| Expansion | 5 (12) | 4 (9) |
| Contraction | 9 (21) | 4 (9) |
| Mixed | 19 (45) | 26 (61) |
| Missing | 9 (21) | 9 (21) |
| Adult patient scale (scored 1–10) | Treatment arm, median score (IQR) | |
|---|---|---|
| No PGT (N = 28) | PGT (N = 27) | |
| Has the scar been painful in the past few weeks? | 3 (1–6) (n = 27) | 3 (2–5) (n = 27) |
| Has the scar been itching in the past few weeks? | 4 (2–7) (n = 27) | 4 (2–7) (n = 27) |
| Is the scar colour different from the colour of your normal skin at present? | 8 (5–10) (n = 27) | 8 (6–10) (n = 27) |
| Is the stiffness of the scar different from your normal skin at present? | 8 (5–8) (n = 27) | 7 (5–8) (n = 27) |
| Is the thickness of the scar different from your normal skin at present? | 4 (3–6) (n = 26) | 5 (4–8) (n = 27) |
| Is the scar more irregular than your normal skin at present? | 5 (4–8) (n = 27) | 7 (4–9) (n = 27) |
| What is your overall opinion of the scar compared with normal skin? | 7 (5–8) (n = 27) | 9 (6–10) (n = 27) |
| Paediatric patient scale (scored 1–10) | Treatment arm, median score (IQR) | |
|---|---|---|
| No PGT (n = 14) | PGT (n = 16) | |
| Itch scale (younger patients) 1 (scored 0–4) | 1 (0–3) (n = 3) | 1.5 (1–2) (n = 8) |
| Itch scale (older patients) 2 (scored 0–10) | No data | 3 (n = 1) |
| Pain scale (younger patients) 3 (scored 0–10) | 0 (0–4) (n = 3) | 2 (0–2) (n = 9) |
| Pain scale (older patients) 2 (scored 0–10) | No data | No data |
The POSAS baseline scores are similar between treatment arms on both the patient-reported and observer-reported forms. The paediatric patient-reported scale pain and itch domains were poorly completed, with only 3 out of 14 (21%) and 9 out of 16 (56%) participants, in the no-PGT and PGT arms, respectively, giving any assessment of pain and itch.
A summary of POSAS observer-reported domain scores on follow-up and for each domain is provided in Figure 6. Numerical values, as well as the proportion of participants for whom that domain improved from baseline, are presented in Appendix 7, Table 29. The scores and trends are similar across most domains; scars are rated as getting slightly worse by month 1 before improving by months 3 and 6.
FIGURE 6.
Summary of baseline and follow-up scores on the POSAS observer-reported scale by visit and treatment arm for each POSAS domain. Plots contain box plots (the central box depicting the IQR and median, whiskers depicting range, with outliers indicated individually) and means (open circles) with 95% CIs. (a) Vascularity; (b) pigmentation; (c) thickness; (d) relief; (e) pliability; (f) surface area; and (g) overall opinion.
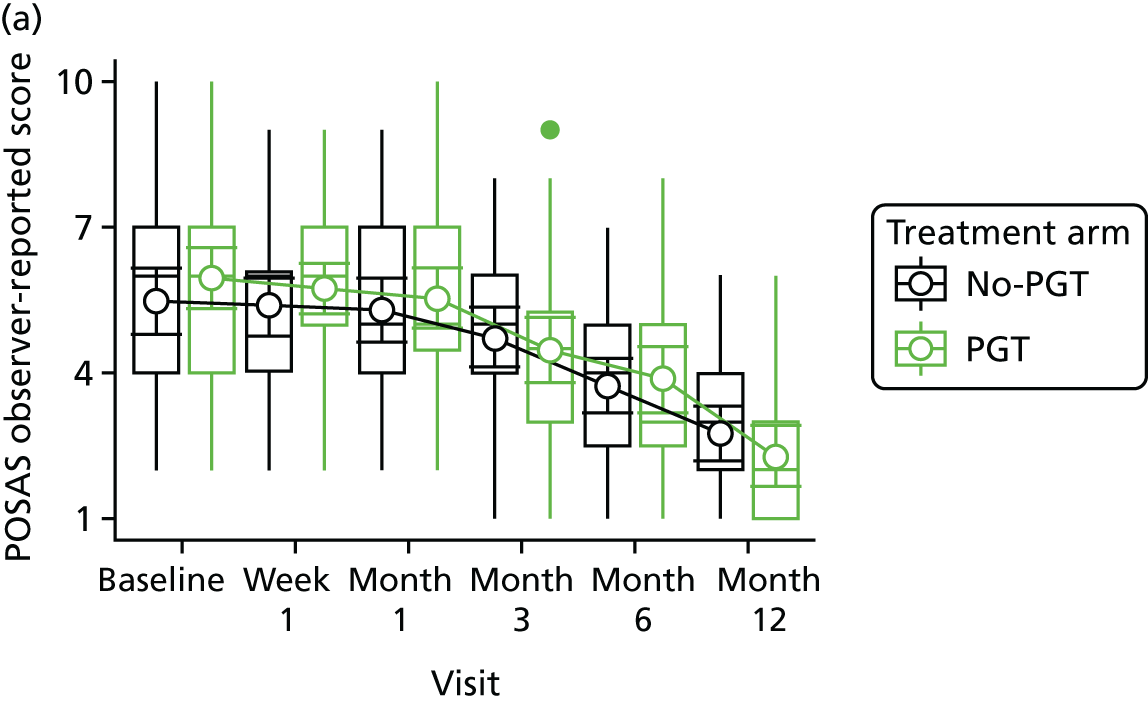
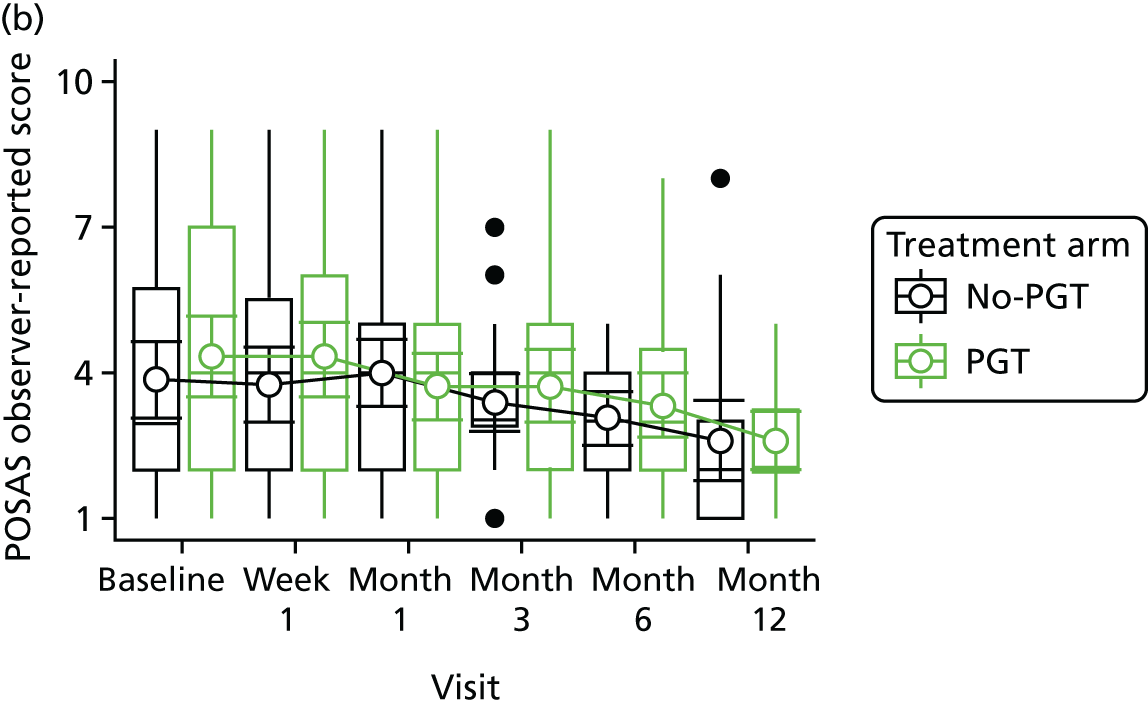
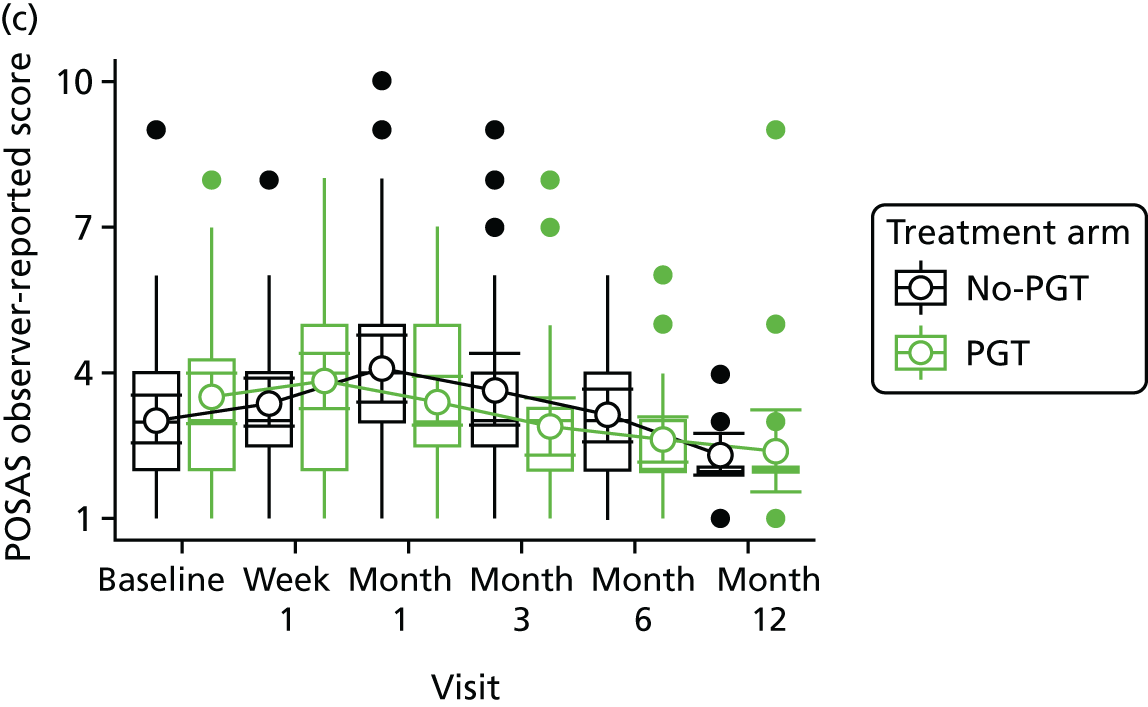
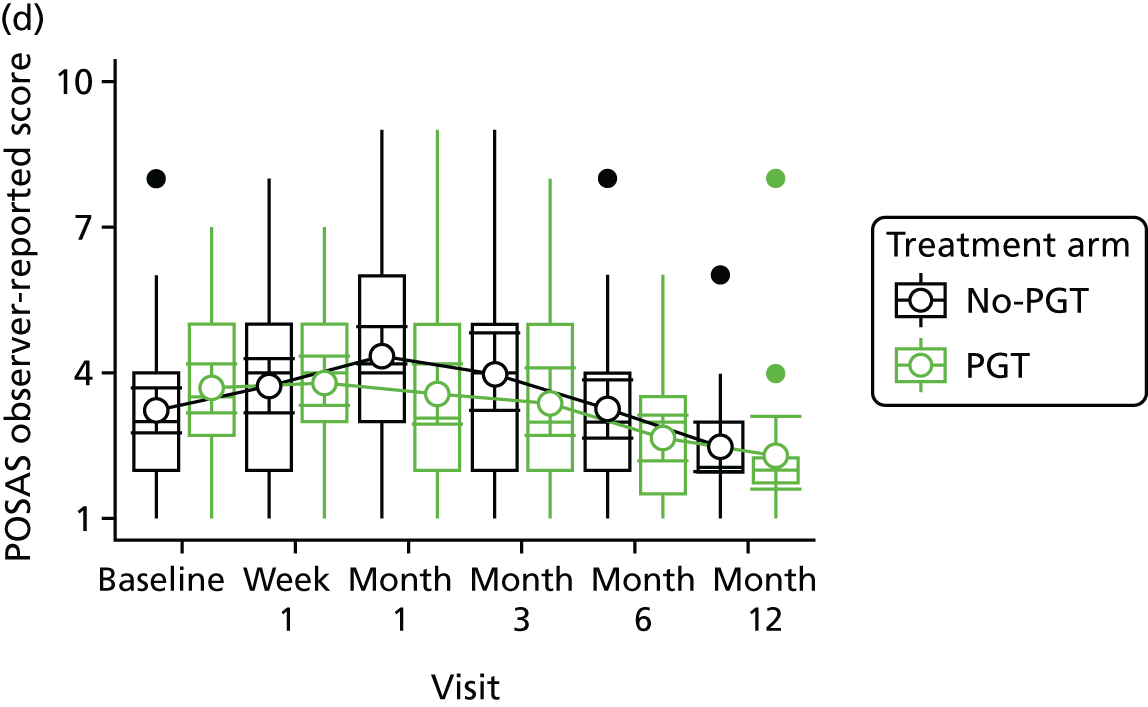
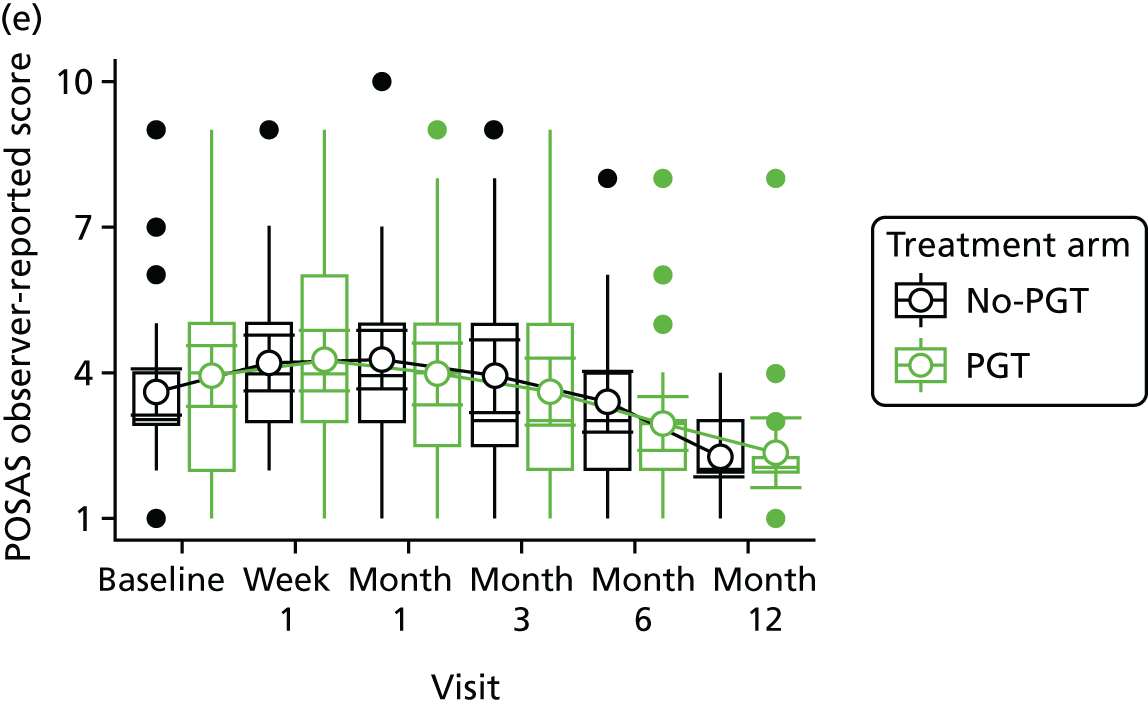
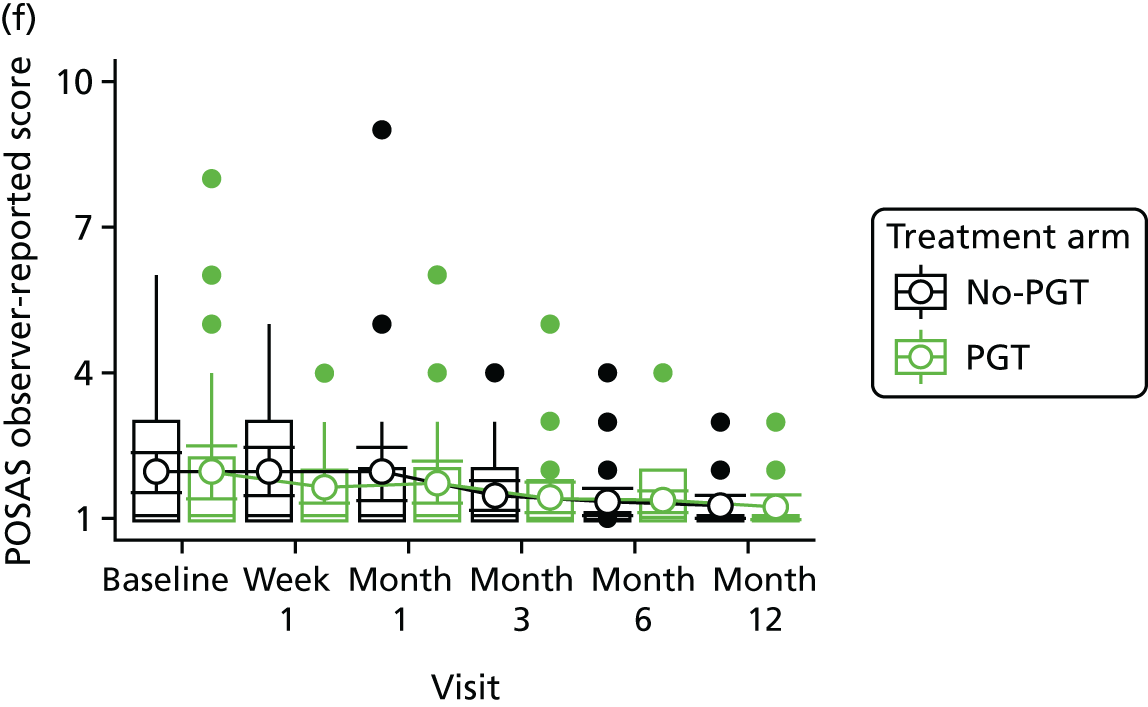
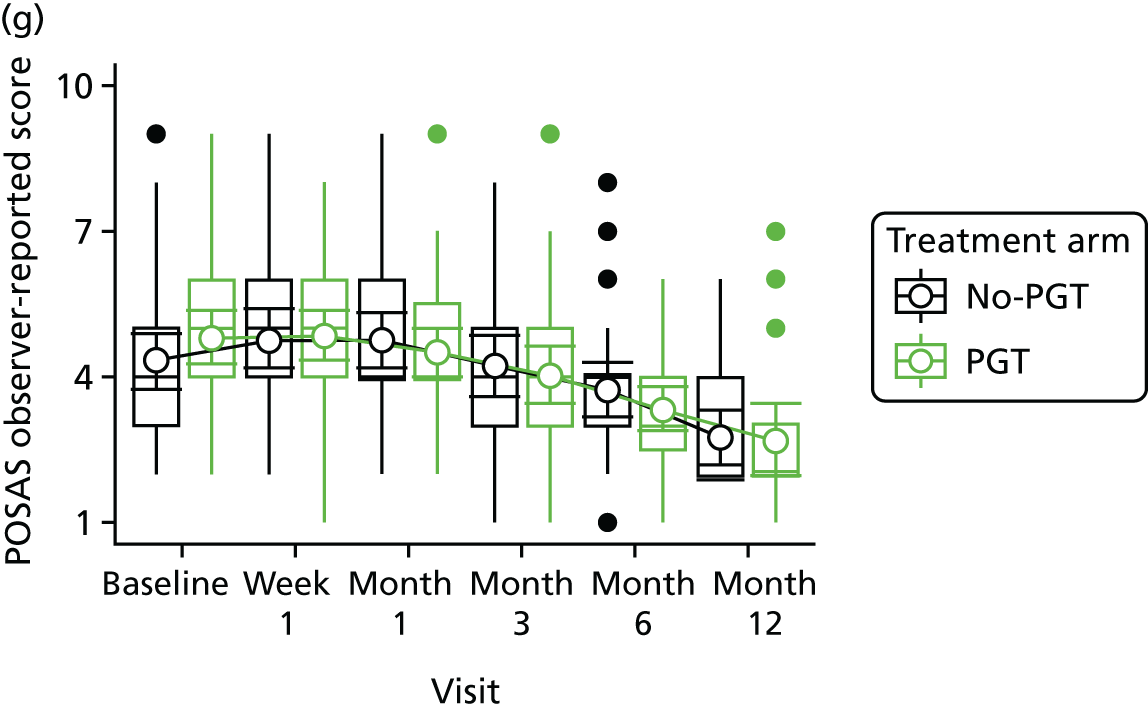
A summary of POSAS patient-reported domian scores on follow-up is provided in Figure 7. A summary of each domain, the numerical values and the proportion of participants for whom that domain improved is presented in Appendix 7, Table 30. Similar patterns are observed in the patient- and observer-reported scales. The response rate for the POSAS observer-reported form was higher than that for the patient-reported form, with 64 observer-reported forms returned at month 6, but only 36 patient-reported forms returned. The POSAS paediatric follow-up was completed very poorly, with very limited data supplied.
FIGURE 7.
Summary of baseline and follow-up scores on the POSAS patient-reported scale by visit and treatment arm for each POSAS domain. Plots contain box plots (the central box depicting the IQR and median, whiskers depicting range, with outliers indicated individually) and means (open circles) with 95% CIs. (a) Pain; (b) itch; (c) colour; (d) stiffness; (e) thickness; (f) irregular; and (g) overall.
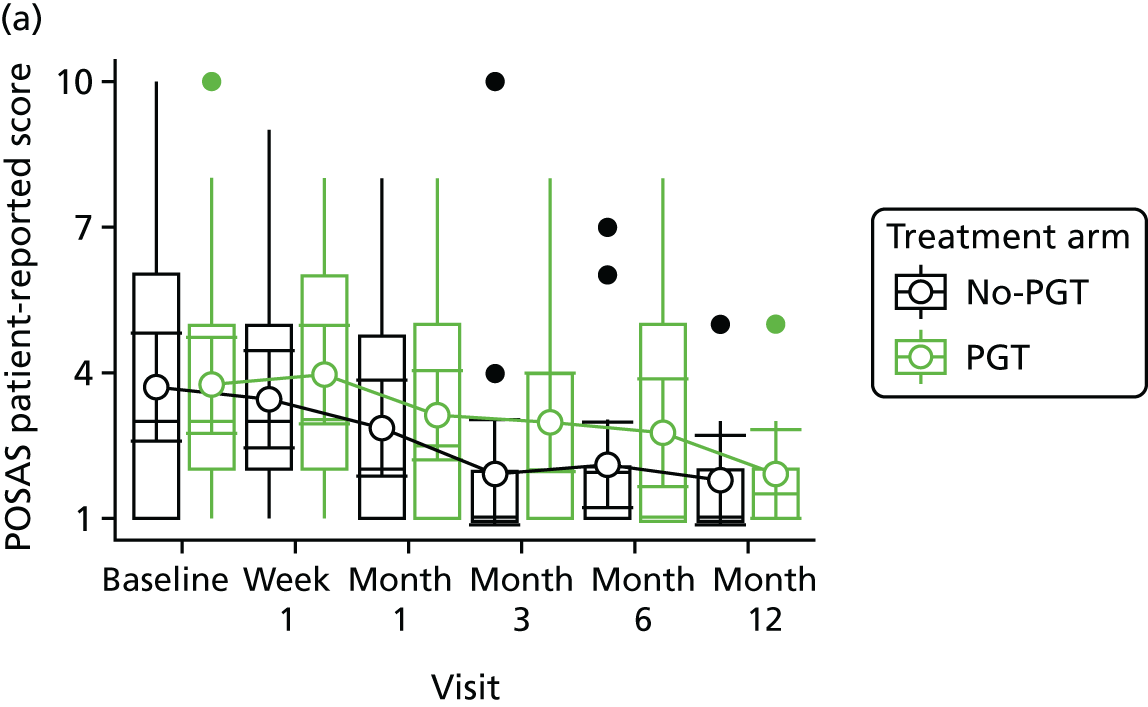
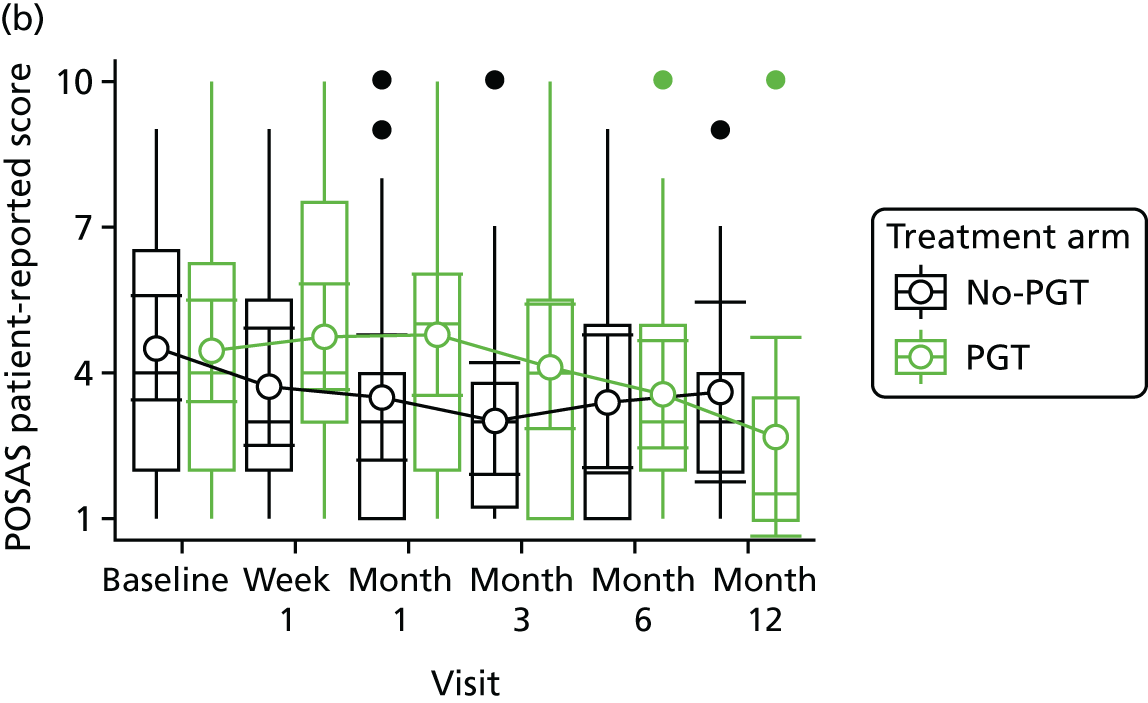
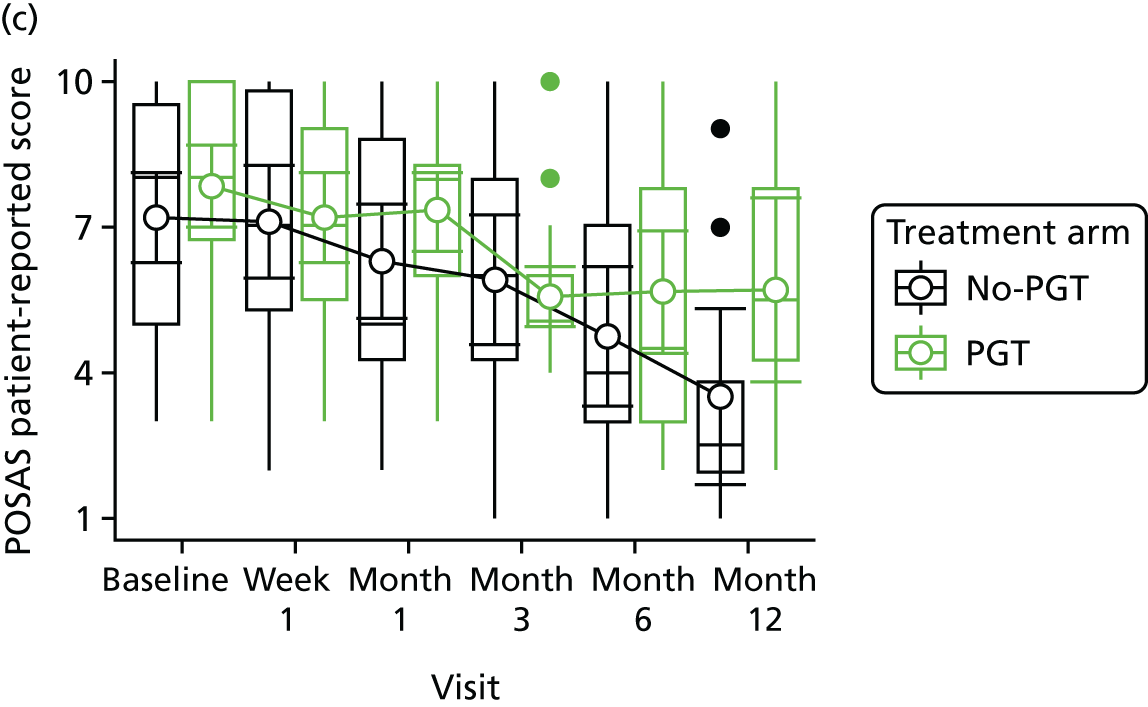
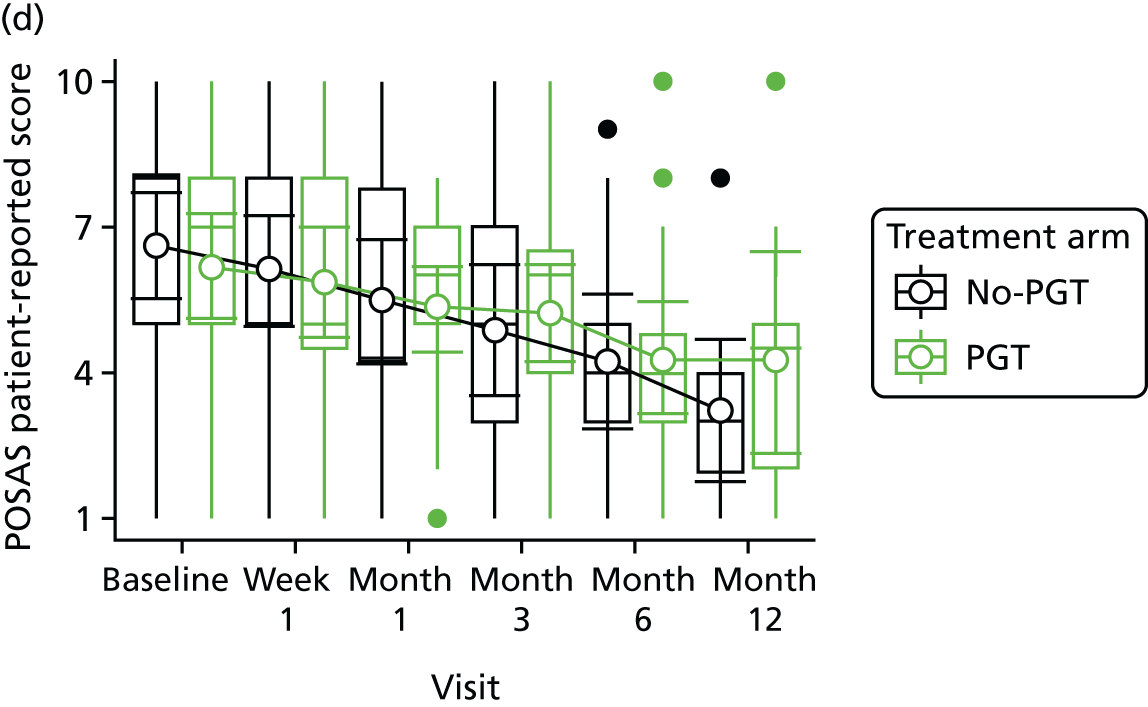
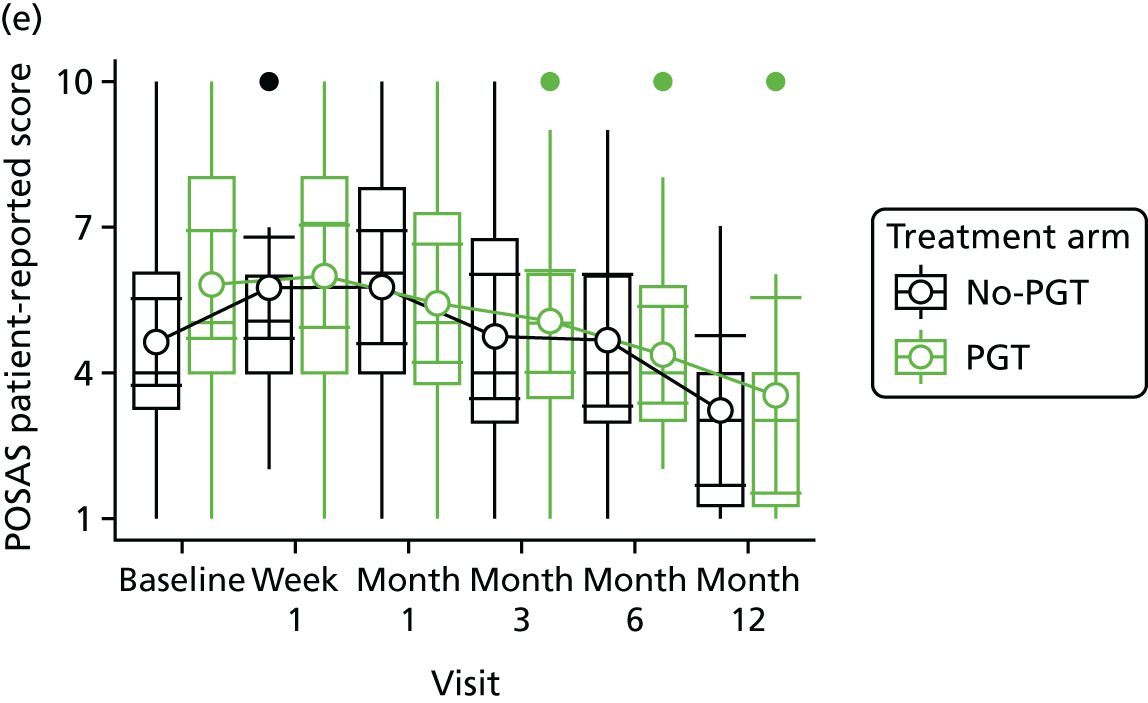
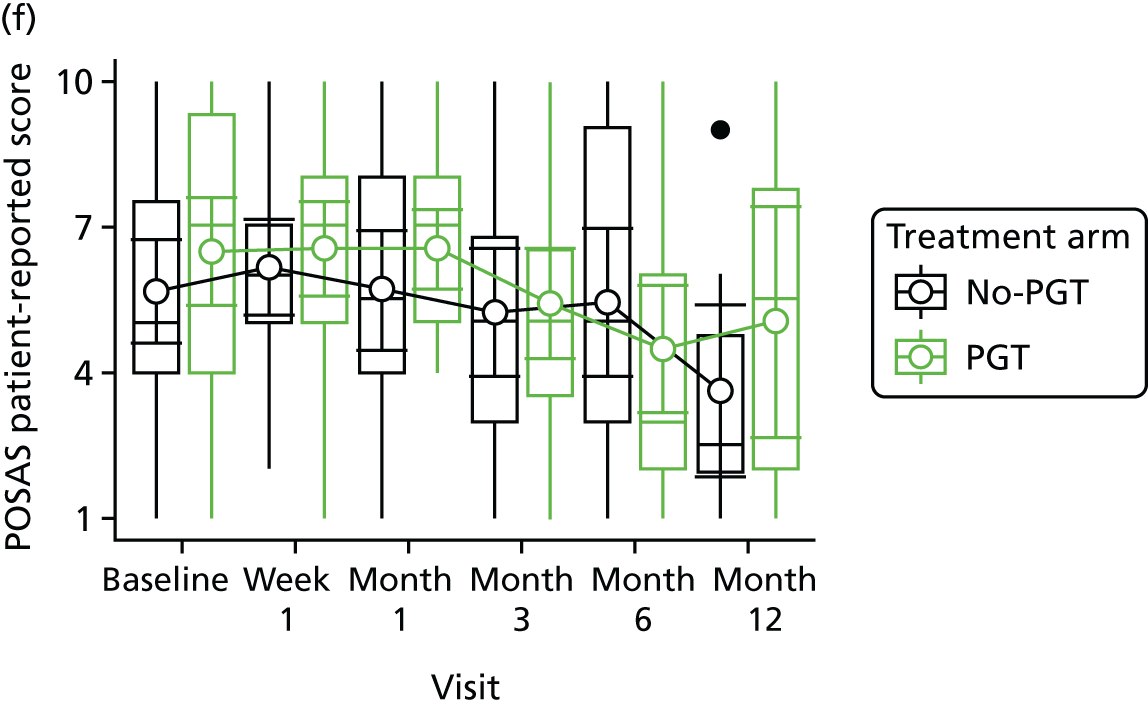
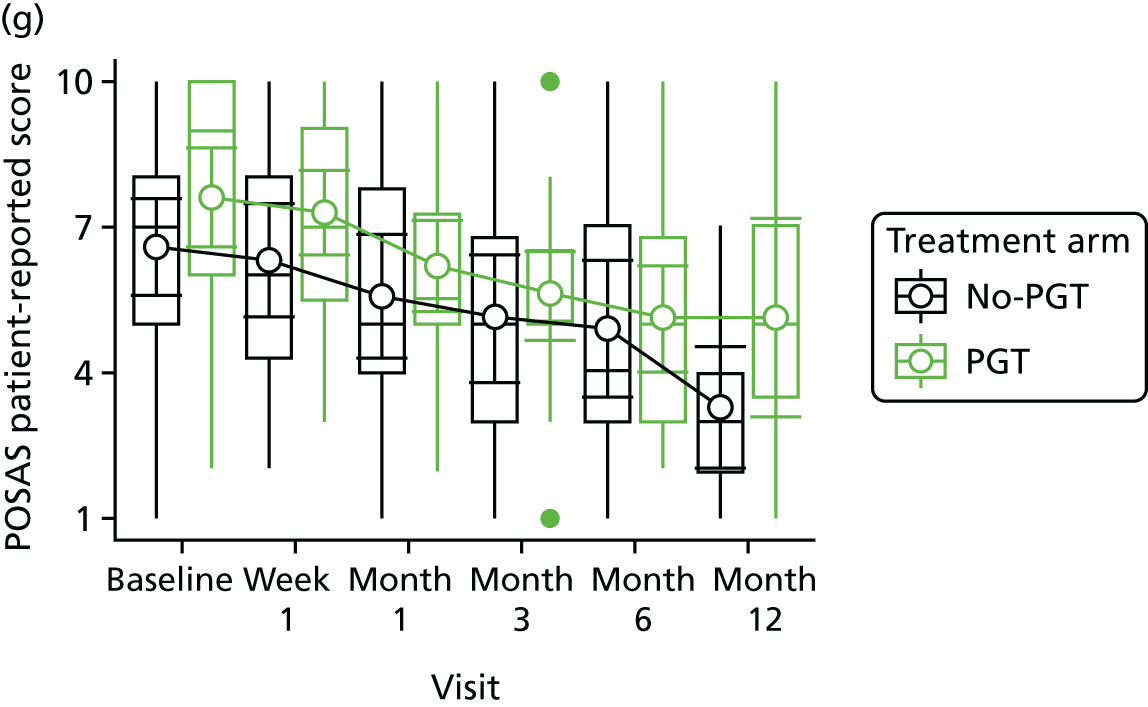
Overall scores for POSAS and VSS are presented in Appendix 7, Table 31.
Cutometer assessment
Assessments were provided by 18 participants from the UHB NHS Foundation Trust and eight participants from the BCH NHS Trust, with baseline elasticity recorded for 10 participants in the no-PGT arm and 16 in the PGT treatment arm (Table 17). In the follow-up assessments, scar elasticity was recorded for more participants than at baseline assessment. Scar elasticity appears to remain broadly similar across follow-up assessments and treatment arms, but this is based on very limited data and suggests that the use of the Cutometer requires appropriate training and support to produce useful data for any future trial.
| Time point | Treatment arm (%) | |||||
|---|---|---|---|---|---|---|
| No PGT (n = 10) | PGT (n = 16) | |||||
| Percentage elasticity, median (IQR) | Not applicable | Improved from baseline | Percentage elasticity, median (IQR) | Not applicable | Improved from baseline | |
| Baseline | 85 (82–90) (n = 10) | 0 (n = 0) | N/A | 86.5 (80–93.5) (n = 16) | 0 (n = 0) | N/A |
| Week 1 | 90 (–) (n = 1) | 0 (n = 0) | 10 (n = 1) | 86.5 (82.5–89.5) (n = 4) | 0 (n = 0) | 12.5 (n = 2) |
| Month 1 | 78 (–) (n = 1) | 0 (n = 0) | 0 (n = 0) | 70 (63–82) (n = 4) | 0 (n = 0) | 6.25 (n = 1) |
| Month 3 | 77.5 (69.5–86.5) (n = 4) | 0 (n = 0) | 0 (n = 0) | 78 (70–84) (n = 5) | 0 (n = 0) | 0 (n = 0) |
| Month 6 | 90 (85–95) (n = 5) | 0 (n = 0) | 30 (n = 3) | 81 (79–82) (n = 7) | 0 (n = 0) | 12.5 (n = 2) |
| Month 12 | 71 (70–91) (n = 3) | 0 (n = 0) | 10 (n = 1) | 61.5 (57.5–68.5) (n = 4) | 0 (n = 0) | 6.25 (n = 1) |
Range of movement
Response rates to the ROM assessments were very low for both treatment arms (Table 18), with only 16 out of 43 participants giving a response for upper limb movement (37%) and 10 out of 44 participants giving a response for hand and wrist movement (23%), which are the most commonly affected areas for no-PGT and PGT patients, respectively. With the limited response rates to this question, the numbers are too small to draw any meaningful conclusions regarding the number of participants with any ROM assessment that improved from baseline. Many of the therapists attempting to complete the ROM assessments commented on the restrictive complexity of the form. This outcome is unlikely to provide useful data in a main trial unless the data collection process can be considerably simplified.
| Time point | Treatment arm, % (n/N) | |||||
|---|---|---|---|---|---|---|
| No PGT | PGT | |||||
| Hand and wrist | Lower quadrant | Upper limb | Hand and wrist | Lower quadrant | Upper limb | |
| Week 1 | 20 (1/5) | 33 (2/6) | 25 (3/12) | 50 (3/6) | 11 (1/9) | 50 (2/4) |
| Month 1 | 25 (1/4) | 0 (0/3) | 55 (6/11) | 67 (4/7) | 0 (0/8) | 40 (2/5) |
| Month 3 | 0 (0/1) | 50 (1/2) | 56 (5/9) | 50 (3/6) | 0 (0/8) | 100 (3/3) |
| Month 6 | 50 (1/2) | 50 (1/2) | 29 (2/7) | 40 (2/5) | 14 (1/7) | 0 (0/3) |
| Month 12 | 0 (0/2) | 0 (0/1) | 0 (0/2) | 33 (1/3) | 0 (0/5) | 0 (0/1) |
Data completeness
Table 19 summarises the form-return rates and time to completion of the core CRFs by treatment arm at each study visit. Return rates exceeded 85% for the core CRFs up to month 6. The time to completion broadly stayed on target and was very similar across both treatment arms. Data collection for most aspects of the core CRFs seems timely, with a high rate of completion.
| Time point | Treatment arm | |||||
|---|---|---|---|---|---|---|
| No PGT | PGT | |||||
| Number of forms expected, n | Forms returned, n (%) | Median time to completion, number of days (IQR) | Number of forms expected, n | Forms returned, n (%) | Median time to completion, number of days (IQR) | |
| Baseline | 42 | 42 (100) | 0 (0–0) | 44 | 44 (100) | 0 (0–0) |
| Week 1 | 40 | 35 (88) | 8 (7–15) | 43 | 41 (95) | 7 (7–15) |
| Month 1 | 39 | 37 (90) | 36 (29–43) | 43 | 39 (91) | 37 (30–44) |
| Month 3 | 38 | 37 (97) | 98 (90–113) | 38 | 36 (95) | 100 (92–111) |
| Month 6 | 37 | 32 (86) | 199 (182–229) | 38 | 35 (92) | 195 (182–206) |
| Month 12 | 29a | 22 (76) | 365 (343–393) | 31b | 21 (68) | 372 (294–391) |
The core scar scale assessments of scar appearance and pain and itch were recorded with a high rate of completeness (approximately 85% up to month 6). Completion rates for paediatric pain and itch domains were poor, particularly for those aged < 4 years.
Outcome data collected on Cutometer assessments and ROM provided considerable evidence to suggest that they should be omitted from any future trial unless the data collection process can be greatly simplified. Both outcomes suffered from very poor completion rates and many study researchers found these assessments prohibitively onerous to complete.
Serious adverse events and other reported symptoms
Only two serious adverse events were reported in the study, one resulting in death and the other resulting in hospitalisation. Neither was deemed to be related to study treatment by the PI at the site. The chief investigator was also in agreement.
Adverse events
The adverse events by treatment arm and study visit are summarised in Table 20. At least 18% of participants in both treatment arms reported at least one adverse event at each study visit between baseline and month 12.
| Overall totals | Treatment arm | |||
|---|---|---|---|---|
| No PGT (n = 44) | PGT (n = 44) | |||
| Number of participants with at least one AE | 20 | 20 | ||
| Total number of AEs | 40 | 43 | ||
| Mean number of AEs per patient (95% CI) | 0.91 (0.38 to 1.43) | 0.98 (0.43 to 1.52) | ||
| Median number of AEs per patient (IQR) | 0 (0–1.5) | 0 (0–2) | ||
| Range of AEs per patient | 0–5 | 0–5 | ||
| By visit | Number of patients, n | Number of patients with at least one AE, n (%) | Number of patients, n | Number of patients with at least one AE, n (%) |
| Week 1 | 35 | 8 (23) | 41 | 11 (27) |
| Month 1 | 37 | 12 (32) | 39 | 9 (23) |
| Month 3 | 32 | 10 (31) | 36 | 9 (25) |
| Month 6 | 32 | 6 (19) | 35 | 10 (29) |
| Month 12 | 22 | 4 (18) | 21 | 4 (19) |
| By event type | Number of patients with at least one AE | Number of events, n | Number of patients with at least one AE | Number of events, n |
| Rash | 2 | 2 | 2 | 2 |
| Itch/pruritus | 12 | 22 | 12 | 29 |
| Infection | 2 | 2 | 2 | 2 |
| Wound breakdown | 5 | 6 | 3 | 3 |
| Steroid injection | 0 | 0 | 0 | 0 |
| Paraesthesia | 1 | 1 | 2 | 2 |
| Allergy to pressure garments | 0 | 0 | 0 | 0 |
| Contractures/joint deformity | 2 | 2 | 1 | 1 |
| Surgical scar revision | 1 | 1 | 1 | 1 |
| Blisters | 5 | 9 | 6 | 6 |
Table 20 also summarises the total adverse events by treatment arm over the entire study. Participants experienced similar levels of adverse events with means and 95% CIs of 0.91 (0.38 to 1.43) and 0.98 (0.43 to 1.52) in the no-PGT and PGT arms, respectively. This relatively high incidence of adverse events is mainly attributable to recording common side effects of scar management therapy. The incidence of itch/pruritus was very similar across both treatment arms, with 12 participants in each arm reporting at least one adverse event consisting of itch/pruritus and a total of 22 and 29 itch/pruritus events in the no-PGT and PGT treatment arms, respectively.
The large number of events collected in this study suggests that future data collection should perhaps focus on collecting the less typical or less expected adverse events.
Summary
-
The trial achieved the projected recruitment rate and randomised 88 participants over 9 months (with an average recruitment rate of just under 10 participants per month across all study sites).
-
To randomise 88 participants, a total of 211 patients were screened, of whom 186 were deemed eligible for the trial. The estimated eligibility rate is 88% (95% CI 83% to 92%) and the estimated recruitment rate of those eligible is 47% (95% CI 40% to 55%).
-
A total of 19 participants dropped out of the study by month 6, giving an estimated drop-out rate of 22% (95% CI 14% to 32%).
-
Adherence with allocated treatment was moderate – the estimated rate of full adherence by month 6 for a large RCT is 57% (95% CI 44% to 69%), with rates equal across the two treatment arms.
-
Rates of crossover were the same in each treatment arm with five participants crossing over from no PGT to PGT and five crossing over from PGT to no PGT. This gives an estimated total crossover rate for a future trial of 11% (95% CI 6% to 20%).
-
Blinded assessment of outcomes was not possible, even when an independent assessor was available or photography was used, because of the indentations left by pressure garments.
-
VSS forms were generally well completed. POSAS forms were better completed by observers than participants, and there were particular difficulties in obtaining complete data for children.
-
There were particular difficulties in completing ROM assessments and Cutometer measurements, suggesting that training and support would be required should they be considered for inclusion in a definitive trial.
-
Up to one-third of participants reported adverse events at each time point, which were, most frequently, itch/pruritus, wound breakdown or blistering.
Chapter 8 Integrated qualitative pilot trial process evaluation
Relevant objective
The following objective is addressed in this chapter:
-
to assess participant (patients and parents of patients) and clinician perspectives on trial participation and processes.
Methods
Sampling and recruitment: adult and parent interviews
We interviewed adult patients and parents of paediatric patients recruited to the pilot trial and allocated to the PGT and no-PGT treatment arms at two time points: time point 1 (T1) and time point 2 (T2). T1 interviews were undertaken soon after treatment allocation. T2 interviews were conducted approximately 12 months after recruitment to the pilot. We also attempted to recruit patients who had declined to participate in the pilot in order to explore that decision. The recruitment process was identical to that described in Chapter 5. T1 interviews were mainly conducted face to face in patient’s home, although a small number took place on hospital premises or via telephone. Owing to project staffing issues and timelines, the follow-up interviews for the non-Birmingham recruiting sites (two of the four sites) were conducted by telephone.
Interview format and content: adult and parent interviews
Interviews were semistructured according to a predevised topic guide and conducted in a participant-focused manner. The topic guide and interview process were refined after reflection on a small sample of early interviews. Topics included the following:
-
T1
-
accounts of the accident and injury
-
accounts of the initial approach regarding the pilot trial
-
reasons for agreeing to participate in the pilot trial (or declining participation for decliners)
-
understanding of the pilot trial
-
thoughts on treatment allocation received
-
hopes and expectations for treatment, recovery and scar management
-
early experience of scar management techniques
-
-
T2
-
update: progress with scar management and other treatments when applicable
-
experience of scar management techniques
-
adherence to scar management techniques
-
experience of participation in the pilot trial, including perspectives on the frequency and convenience of trial visits, the nature and content of scar assessment and other outcome measures
-
perceptions of scar outcomes achieved to date and broader impacts of burn injury and treatment.
-
Recruitment: staff interviews
We interviewed eight staff from six of the participating PEGASUS pilot sites. Of these, two were RNs and the remainder were front-line clinical staff who were delivering the pilot trial on a day-to-day basis (OTs/physiotherapists). Six interviews were conducted by telephone and two were carried out face to face in Birmingham. The quotations from staff interviews are identified using codes S1–S8.
Analysis
Interviews were audio-recorded, transcribed clean verbatim and analysed using a thematic approach. 45 Early analytic findings were discussed among the TMG and at PEGASUS investigator meetings, at which clinical staff delivering the pilot trial and at least one patient representative were present.
Findings
In total, we interviewed 34 patients at T1: 17 adult trial participants, eight parents, six adult decliners and three parent decliners. Of the 25 T1 pilot trial participants, 22 took part in a T2 interview: 15 adult patients and seven parents. In addition, we interviewed one parent of a paediatric patient being treated at the Central Manchester University Hospitals NHS Foundation Trust pilot site as a one-off interview at T2. We had hoped to interview further parents taking part in the pilot trial at sites other than the BCH NHS Trust, as we failed to recruit any parents to interviews at T1 from the Queen Victoria Hospital NHS Foundation Trust.
Sample characteristics
Sample characteristics for T1 interviews are given in Table 21.
| Phase 2 (T1) interview sample characteristics | Sample, n (%) | ||
|---|---|---|---|
| Total (N = 34)a | Adult patients (N = 23) | Parents of paediatric patients (N = 11) | |
| Sex of adult patient or parent | |||
| Male | 17 (50.0) | 14 (60.9) | 3 (27.3) |
| Female | 17 (50.0) | 9 (39.1) | 8 (72.7) |
| Sex of paediatric patient | |||
| Male | N/A | N/A | 9 (81.8) |
| Female | N/A | N/A | 2 (18.2) |
| Trial participant or decliner | |||
| PGT | 15 (44.1) | 11 (47.8) | 4 (36.4) |
| No PGT | 10 (29.4) | 6 (26.1) | 4 (36.4) |
| Decliner | 9 (26.5) | 6 (26.1) | 3 (27.3) |
| Age (years) | |||
| < 20 | 2 (5.9) | 2 (8.7) | 0 (0.0) |
| 21–30 | 8 (23.5) | 5 (21.7) | 3 (27.3) |
| 31–40 | 11 (32.4) | 3 (13.0) | 8 (72.7) |
| 41–50 | 3 (8.8) | 3 (13.0) | 0 (0.0) |
| 51–60 | 6 (17.6) | 6 (26.1) | 0 (0.0) |
| ≥ 61 | 4 (11.8) | 4 (17.4) | 0 (0.0) |
| Age of paediatric patient whose parent was interviewed (years) | |||
| < 1 | N/A | N/A | 0 (0.0) |
| 1–5 | N/A | N/A | 7 (63.6) |
| 6–9 | N/A | N/A | 3 (27.3) |
| 10–14 | N/A | N/A | 0 (0.0) |
| Not known | N/A | N/A | 1 (9.1) |
| Site | |||
| Queen Elizabeth Hospital Birmingham | 10 (29.4) | 10 (43.5) | N/A |
| BCH | 9 (26.5) | N/A | 9 (81.8) |
| Broomfield Hospital, Essex | 8 (23.5) | 7 (30.4) | 1 (9.1) |
| Queen Victoria Hospital, East Grinstead | 7 (20.6) | 6 (26.1) | 1 (9.1) |
| Ethnicity | |||
| White | 27 (79.4) | 20 (87.0) | 7 (63.6) |
| Black African/Caribbean/black British | 2 (5.9) | 1 (4.3) | 1 (9.1) |
| Asian/Pakistani | 1 (2.9) | 1 (4.3) | 0 (0.0) |
| Unknown | 4 (11.8) | 1 (4.3) | 3 (27.3) |
| Type of burn (of adult or paediatric patient whose parent was interviewed) | |||
| Flame | 14 (41.2) | 12 (52.2) | 2 (18.2) |
| Scald | 14 (41.2) | 6 (26.1) | 8 (72.7) |
| Contact | 2 (5.9) | 2 (8.7) | 0 (0.0) |
| Chemical | 2 (5.9) | 2 (8.7) | 0 (0.0) |
| Other | 1 (2.9) | 1 (4.3) | 0 (0.0) |
| Not known | 1 (2.9) | 0 (0.0) | 1 (9.1) |
| TBSA of burn (%) (of adult or paediatric patient whose parent was interviewed) | |||
| < 10 | 16 (47.1) | 10 (43.5) | 6 (54.5) |
| 10–20 | 5 (14.7) | 3 (13.0) | 2 (18.2) |
| 21–30 | 3 (8.8) | 3 (13.0) | 0 (0.0) |
| 31–40 | 1 (2.9) | 1 (4.3) | 0 (0.0) |
| 41–50 | 1 (2.9) | 1 (4.3) | 0 (0.0) |
| > 51 | 1 (2.9) | 1 (4.3) | 0 (0.0) |
| Do not know | 7 (20.6) | 4 (17.4) | 3 (27.3) |
Recruitment to pilot trial
Recruitment process and timing: staff perspectives
Although there was some variation in process across sites, RNs would predominantly identify and recruit potential participants in conjunction with other clinical staff. At one site, the RN worked closely with the OT until they were comfortable with the recruitment process and in discussions with patients about the pilot trial:
And I think from a support point of view research isn’t an OT’s main job normally, so I needed the support of having [RN] to come down to do the initial recruitment for the first few patients I would say, but then after when you get into the swing of things you find it a lot easier, and it’s almost like having someone there as your second person so that if you do forget something or if you are swaying towards too much information or too little information you’ve got someone else there that you can bounce off really.
Interviewee S6
For a number of front-line clinical staff, this was the first time that they had been involved in research and some lacked confidence when immediate support was absent.
The point of initial approach was also reported to vary, for instance according to whether patients were inpatients or being treated as an outpatient. However, many patients appear to have been approached relatively early during treatment, at early stages of wound healing, for example when they were inpatients following grafting:
If they were an inpatient and they met the criteria, so if they’d had a skin graft or they had taken longer than 2 weeks to heal then we could discuss it with them on the ward before their wounds had healed, so when they were about 90% healed we would be able to get involved with them at that point. But if they had been treated as an outpatient and they had not been an inpatient on the ward it would be at the point that their wounds had healed and they were referred to the outreach service.
Interviewee S5
Barriers to recruitment: staff perspectives
Strong views on pressure garment therapy among staff
A number of staff talked about the potential influence of their views regarding PGT on patients. Some reported feeling that PGT was the most effective therapy for certain patients. For example, one interviewee expressed concern that they may have subconsciously advocated the use of pressure garments, reflecting the influence of embedded clinical practice:
The recruitment actual process was quite straightforward, it was learning how to approach the patients in order not to sway them to the way that I would have probably subconsciously before, so trying not to sell pressure garments to my patients, which is quite difficult when I’m so used to probably doing that without even realising it.
Interviewee S8
Another interviewee acknowledged that their views regarding PGT had had an impact on their selection and screening of patients. Some patients, despite eligibility, were not approached regarding the pilot trial:
I was a bit naughty, there were a couple of patients that we didn’t recruit because the severity of their scars were that they . . . we’d already decided they needed to go into pressure before they even hit the point of going on the trial, like we had someone who was transferred over to us for rehab from one of the centre so she was healed and ready and scarring had already developed, so we had missed the healing point and clearly needed to go pressure therapy. So patients like that who in theory fit the criteria but we’d already knew we would have to go down a certain route. So if she had come into the non-pressure therapy we would have had to pull her off instantly, so there didn’t seem to be any point in enlisting her in the trial, and I don’t know if that’s my inexperience with research whether I did the right or the wrong thing there.
Interviewee S2
Another interviewee indicated that these views on PGT influenced interactions and conversations with patients; for example, patients were given reassurances that they would be withdrawn from the study if staff felt that a pressure garment was the best option:
We go through the consent forms with them, and they know they can withdraw at any time, and also we say to them that if the therapist thinks that even though we’re not 100% sure but we think that you may benefit from a pressure garment then the therapist would talk to you about coming out the study as well.
Interviewee S4
These accounts in the interviews echo the findings of similar research with staff recruiting to other clinical trials. 55 We have observed clinicians implicitly reporting (e.g. interviewee S2) emotional conflicts between their role as trial recruiter and their clinical role, resulting in discomfort when faced with the need to recruit certain patients who were deemed eligible for the pilot trial. Previous research examining the challenges of equipoise for clinicians recruiting to clinical trials has suggested that patients with certain clinical or other characteristics (‘edge patients’) may be perceived as being on the margins of a recruiter’s perceived core of eligibility. 56
Staff report strong views about pressure garment therapy among some patients
Although staff stated that they had expected patients to have strong views in favour of receiving PGT, several noted that some patients did not want to wear garments and, therefore, would avoid entry to the trial:
I think some of them just couldn’t be bothered. They just thought ‘no I can’t be bothered’ or ‘I don’t want a pressure garment, I will just do the massage, I don’t want to be involved’.
Interviewee S8
One interviewee noted that they had found the recruitment of patients with a lower TBSA burn percentage more difficult, as they did not want to receive garments:
The main hindrance I found was the fact that the inclusion criteria is anyone with a 1% burn or greater, just because prior to the study I think a lot of us were concerned that we would have difficulty recruiting patients because the patients would be worried about not receiving a pressure garment, I actually found the opposite problem, so the 1%/2%/3% burns were much higher in number than our big burns, which are a lot more of a rarity luckily, so I was trying to recruit a lot more patients with smaller burns who actually were reading the patient information leaflets, and not wanting to risk having a pressure garment. So I actually found that was a bigger barrier, which was the complete opposite to what we have imagined prior to the trial starting.
Interviewee S5
However, other patients were also reported to have strong views in favour of wearing garments and did not enter the trial for fear of not receiving them. One reason posited for this was the expectations set among patients by other clinical staff who were not involved in the pilot trial. For example, this interviewee describes how recruitment was sometimes difficult because of this:
I found it [recruitment] very difficult, I’m not sure if it’s because as a standard within our department people talk about pressure garments from quite early, so the whole team, and I don’t think we got the message across to the whole team early enough, so they were still giving the same messages that pressure garments are great and all this sort of thing. So the patients by the time we approached them already had that in their mind and so it was quite difficult with that.
Interviewee S3
Recruiting and consenting paediatric patients to the pilot trial
Interviewees reported that having time available to discuss the trial at length may be especially important with parents who were making decisions regarding treatment for their children. This was especially the case for patients who may have feelings of guilt associated with the accident and injury, particularly if they have young children, and who, therefore, may be less inclined to take part in a study in which treatment allocation is decided by chance:
But without a doubt the initial assessment and all that goes with the paperwork and the randomisation and all that takes an awful long time, a lot more than it would do for a normal therapy first assessment. So to expect a therapist just to be able to accommodate that in a normal clinic I don’t think it would work. You’ve got to have some sort of recognition of the time that is taken, the addition that parents want to know about. If they’re saying that they’re prepared to go in a trial they’re going to have an awful lot of questions in addition to their normal questions if you follow me? Because we’re not just dealing with a child, we’re dealing with a family . . . When you’re dealing with children you have the added thing that there’s quite often an awful lot of guilt tied up in the accident, because it’s usually that most of the children we see are very small children so therefore there is a responsibility to the adult isn’t there to care for them and to keep them safe?
Interviewee S1
Patient views on recruitment to the pilot trial
Interviews with patients and parents of paediatric patients illuminate their experience of recruitment to the pilot trial and the factors that have influenced their decision-making regarding participation.
The initial approach regarding the pilot trial: who and when
Trial participants reported that the initial approach about the pilot trial was often relatively early in treatment and made by RNs, research fellows or consultants when they, or their child, were still inpatients:
Can you remember when it was [name of staff member] or [name of staff member] spoke to you about PEGASUS?
Yes, when [name of child] was lying having his antibiotics and stuff in, because he was just lying on the bed, I think they almost came in to distract us, and sat and talked to us, so it was on that Monday morning, it was probably about 12 o’clock. He was laying on the bed having his plasma and what have you and they came in then, and said ‘it’s not a good time, but you know’ . . . And so we just sat and spoke to them and then [name of staff member] came in a bit later on and mentioned it again.
BCP06, parent of a paediatric patient, no PGT
Adult participants reported that the initial approach was often made at a time when they were in pain or receiving pain medication during acute inpatient stays. As a result, several interviewees questioned their ability to concentrate, understand and reflect on the study and what was being asked of them at this point:
I hadn’t realised that wearing a pressure garment was just a trial, so I was a bit confused. Sometimes, because of course they are talking to you during bandage change, or just after bandage change, and that’s a very painful experience to go through, when your bandages and your skin is being all moved around and you’re feeling distressed in yourself anyway because of the pain. So sometimes when somebody is talking to you, you can’t always actually absorb everything that they have said.
EAP01, adult, PGT
Some patients also found it difficult to recall the exact point at which the initial approach regarding the PEGASUS study had been made, and by whom. This may possibly reflect early approaches during inpatient stays when acute treatment is still ongoing.
However, parents of paediatric patients seem to display better recollection of this process:
Did they give you . . . did you have the patient information leaflet at that point [when child was an inpatient]?
Yes, they gave us a thick leaflet, and stood and explained about it was the case of they were trying to trial these pressure garments to see whether they actually do help in the way that they think that they do, and that they’re trying to get people to join the scheme so they can monitor it.
BCP06, parent of a paediatric patient, no PGT
As per the staff interviews, some patients described an initial approach about the pilot trial as being made by RNs and/or OTs when they were outpatients.
Although most patients said that they had received appropriate information and had time to make decisions regarding participation in the pilot trial, a small number did express some concern about feeling rushed into a decision:
I suppose I do have a slight feeling that it was a bit rushed, and it was like do you want to do it? Not in a nasty way.
CAP03, adult, PGT
Factors influencing decisions to participate in the pilot trial: patients’ perspectives
The most commonly cited influence on pilot trial participation was altruism, with patients expressing a desire to give something back to the staff and services who had helped them.
The notion of true or pure altruism is contested in the literature that has examined patients’ decision-making regarding trial participation, with recognition that altruism is more often conditional on judgements of benefit and lack of harm on the part of patients. 57,58 In this study, it was clear that several other factors influenced patients’ and parents’ attitudes towards the pilot trial. First, some interviewees talked about the role of previous experience or knowledge of PGT that influenced treatment preferences. One adult decliner had previous personal experience in PGT, as their partner had been treated using it. As a consequence, they had positive perceptions of the effect of PGT and did not want to participate in a trial in which they may not receive that treatment.
Other patients had researched options for scar management therapies, thereby influencing preferences for treatment:
[Name of staff member] said that we were the only people that declined to do it. I’m quite surprised that so many people have agreed to do it, because if they have done any research themselves they will see that pressure garments are really widely used. So I don’t know why people would not want to have them.
BCPD7, parent of a paediatric patient, decliner
However, not all patients had the inclination to do such research, preferring instead to rely on clinical expertise and recommendations:
Did you look up or find out about burns treatment at all after this accident?
No, the experts do that, not me. I suppose you get a lot of people that go on the internet and things, and look and think they’re not doing this right, they’re not . . . no, forget it. The doctors do their job, and I’ll leave them to it.
QAP17, adult, no PGT
Reflecting the same views that were apparent from the staff interviews, patients’ expectations relating to PGT and, therefore, attitudes towards the pilot trial may have been set by other health-care staff earlier in the care pathway. For example, one adult who had declined participation in the pilot described how they had already been measured for garments by an OT who was not involved in the trial before being approached to take part. Other interviewees also described conversations with staff earlier in the care pathway about the possibility of PGT.
Some interviewees who did not have set expectations to receive PGT prior to discussions about the pilot trial reported that they did not necessarily know or appreciate the distinction between the pilot trial and usual care:
OK, he’s had this injury, this is what’s going to happen now, and I think it was done backwards. It was like, OK, he’s had this injury, we’re doing this trial, and then you found out what was going to happen. I didn’t even know it was going to happen in terms of his care, I didn’t know [. . .] I think information, there should have been a hierarchy that information about your child’s care would have been more important than the information about the trial, and as I said it was only after I got . . . I think it was after I got the information about the trial that I knew more about the information in terms of his treatment if that makes sense.
BCP01, parent of a paediatric patient, no PGT
This can result in a misunderstanding of the relationship between the pilot trial and usual care, although, as demonstrated in the staff interviews, usual care is complex and dependent on a holistic assessment of patient circumstances and associated need:
What were your expectations once the graft had healed or started to heal for future treatment?
Because I’d gone on [search engine] I was expecting that she would have silicone sheets to try and flatten out the scars, and I was hoping that they would make her a pressure garment. But when I spoke to [names staff member] about it they said that they didn’t do that for everybody but there was a trial that might qualify and get her some garments or silicone and that’s why I signed up for it.
CPPD03, parent of a paediatric patient withdrawer
Patients, and especially the parents of paediatric patients, are making judgements about the best treatment options available. This judgement is most often made following interactions and discussions with HCPs, who are, therefore, key in determining perspectives. On occasion, strong views relating to the role and clinical effectiveness of PGT have influenced patients’ perceptions of best treatment and, therefore, perspectives on the pilot trial. This reflects some of the staff interviews, as described in Strong views on pressure garment therapy among staff. In the quotation below, one of the adult decliners explains how their decision not to participate was influenced by a clinician who was involved in the pilot trial:
Do you remember when someone spoke to you about this trial that we’re doing, this pressure garment trial?
Yes, it was the last time that I was at the hospital, I spoke to the lady when she was doing my dressings and she said that she was going to discharge me, and that the physio[therapist] was going to come round, and the physio mentioned the trials, and I read the piece of paper that she gave me, and I spoke to the nurse that was doing the dressings and I said to her that I didn’t mind doing it, and then I spoke to her and the physio together, and we agreed that it would be best that if I wore the pressure garments one for added [protection] and two because I have skin grafts on my toes, and right by my toes, and they were a bit worried about the scars spreading, so to make webbed feet.
EAD02, adult, decliner
A parent decliner gained a similar impression, which had influenced their decision-making:
Before you got the information about the trial had you been . . . had [physiotherapy] said that she would want to see [son] in pressure garments? Because you’d gone back in the February, they were concerned, and he’d got measured for pressure garments, so was she . . . she must have been wanting [son] to go into pressure garments?
Well no, she did explain from the offset that they’ve . . . that it’s not been proven how effective they are.
So it was left open?
Yes, so . . . but I did get the impression that if we’d said no to the trial that he would be in it [the pressure garment] anyway, but like I say we did feel that we really wanted to give it a try. But I think maybe she was championing them, well no like I say she was very honest and said it’s not been proven but this is the treatment we do at the hospital.
EPD01, parent of a paediatric patient, decliner
Some patients explicitly spoke about the perceived benefits of what they saw as increased surveillance by agreeing to take part in the pilot trial, again demonstrating conditional altruism. 58 This was more predominant for parents. We suspect that for parents this is linked to guilt and blame regarding the cause of their child’s accident or injury, which they have talked about extensively in these interviews. Naturally parents feel a strong need to ensure that their children receive the best possible treatment options:
I’d already decided with this particular trial I was going to take part. It’s a benefit that there’s always going to be a consultant, and a RN at the appointment.
BCP01, parent of a paediatric patient, no PGT
Judgements regarding participation were also made by patients based on reassurances that they would have the ability to withdraw at any point. Although this is a routine ethics principle and assurance for all patients entering any research study, here these assurances were linked to the concept of best treatment. Several interviewees reported that staff had assured them that if they judged that garments were required at a later stage, as the best treatment option, that they could be withdrawn from the study:
What did you feel about this idea of your child having a 50/50 chance of receiving pressure garments or not receiving pressure garments?
Rightly or wrongly, in relation to your study the staff in the hospital stated that regardless of the outcome of the study, whether he’d have pressure garments or not, they would treat him however they thought best, whether that was with pressure garments or not. When the response came through that yes he was to wear pressure garments’ the OT nodded and said, ‘Yes, that’s what we would have done anyway’. So they made that quite clear that whichever group he would have been put into in the trial would not in any way have prejudiced the treatment they were going to give him.
BCP07, parent of paediatric patient, PGT
Scar assessments and other outcome measures
Scar assessments: staff views
Some staff commented on the complexity of assessing multiple scar sites during the pilot trial, noting that this could potentially lead to an inconsistency in reporting of scar assessments between visits:
. . . just get myself a few times in a muddle with patients that had more than one scar site and just getting all the paperwork together and remembering to put them all in, and I had one who had three scar sites, so having to get them all in order, make sure I’m doing it the right one each time, and had to print off extra sheets and things and add them in. I found that in the rush of everything else we had to do clinically I found that quite at times a little bit taxing.
Interviewee S2
Interviewees also noted that some of the assessments required were complex and impractical, particularly multiple ROM assessments. Similarly, some doubts were voiced regarding the appropriateness and accuracy of the POSAS self-report measures for paediatric patients, particularly younger children.
Scar assessments and other outcome measures: patients’ views
Patient interviewees did not express any significant concerns regarding the nature of scar assessments undertaken as part of the pilot trial. Similarly, most patients were happy with the QoL questionnaires that they were asked to complete.
However, some interviewees did question aspects of the generic QoL measures and the validity of some of the self-report content of the paediatric scar assessments. One parent was keen to point out that some measures, including the Burn Man Itch Scale, were difficult for children to understand and complete accurately:
Yes, there were a couple of answer as well on that form which will have gone back into the data mix, which again that was [de-identified]’s answer and that was obviously what was recorded, but at no point to us apart from the first couple of days did he ever say that his feet were itchy, and yet one day we went again on the chart about the itchy man, I think he said it was 9 or 10, it was extraordinarily itchy, and I said, ‘Are you sure [de-identified]?’ He said yes, ‘Are you absolutely sure, because you never said your feet were so itchy before?’ ‘Yes, I think it’s that one,’ and he went for that, and it was in the car back where he said, ‘Daddy my feet don’t really itch,’ I thought, well why did you say that? ‘I like the look of the man’, or whatever it was, he just decided on a whim to go and pick that as his answer.
BCP07, parent of paediatric patient, PGT
One interviewee noted that the process of filling out the questionnaires could get repetitive, especially if they did not perceive any significant change between visits:
It would be all sorts of silly questions, and then it would be like on a scale of 1 to 10 how do you feel, happy or not? I’m like well 10 happy, and then [de-identified] would do the on a scale of 1 to 10 where would you compare your thickness between your scar and your normal skin, and you would be like well that’s soft, that’s fairly hard, so 5, stuff like that, all the time, every bloody time. I said to her, ‘Can’t you just photocopy it, photocopy my answers? Because it’s the same on a different day’.
Willingness-to-pay instrument
Staff concerns
All of the staff interviewed, almost universally, expressed significant concern over the WTP instrument that was administered at the 6-month follow-up. The staff thought that, conceptually, this was very difficult for patients to understand and also for them to try to explain to the patient or parent of a paediatric patient:
So I have got a comment here, the parents really struggled with the questionnaire at 6 months on willingness to pay, they really didn’t get it, and to be honest I’m not sure I thought it was that easily understood. Every single one without fail looked at me and said don’t really understand this, and obviously I had to try and explain it without affecting what they were going to write on there. So that one consistently was a problem.
Interviewee S1
Aside from the difficulty in understanding the concept underpinning the WTP instrument, staff felt that patients would be inhibited by the presence of their care provider, which would in turn influence the responses given:
And I think the nature of research patients is that they want to help so they don’t want to be saying things that we might think ‘What you think we’re only 50p?’ And I think that’s really hard, some of them were saying £500, and I was like would you . . . and it’s just a difficult concept isn’t it? And we really tried hard to explain it but it was hard.
Interviewee S7
Patients’ views
The interviews with patients demonstrated that, conceptually, the WTP exercise was difficult to grasp for the majority and, for many, it was not an acceptable exercise as administered in this context. For example, this adult patient stated that they simply did not understand the instrument and did not know how to rate their treatment, and so left the WTP questionnaire blank:
I think I left that blank, because I said I just don’t know, I just didn’t really know how to rate that. I do remember the question I think, yes, how much would I be prepared to pay for the same treatment, and I just said I don’t know, as I say my head had been pretty confused, and we left that one because I just really didn’t know how to rate that one at all sort of thing.
CAP03, adult, PGT
Another adult patient, similarly, did not know how best to complete the questionnaire and found the exercise difficult to understand:
The idea as a whole, it caught me by . . . yes, because I just didn’t know, I just couldn’t put a price to it really. I couldn’t put an amount there, and I’m not very good at judging how much things cost or how . . . so it took me quite a while to think of a total as such, and I’m just saying well look is it how much that I could afford on my wages? I’m very low-paid job, I could only afford a little amount. If I had to pay for these things, like a bigger amount, I don’t know how I would manage, so it really had to make me think about a set price. But that was the only question, yes exactly, that was the only question that really put me out a little bit.
EAP02, adult, PGT
Efforts to cost the treatment were implicit in the descriptions by interviewees of their attempts to fill out the questionnaire; for example, they tried to establish how much a pressure garment would be to manufacture. Some interviewees, parents in particular, expressly did not like the nature of the exercise:
I struggled with that one, I was like I don’t know, I struggled with having to pay. I was thinking I don’t really . . . I did struggle with that questionnaire. I didn’t like that questionnaire, no.
What was it about the . . .
Because you had to put in how much would you pay for your treatment, how much you pay for the garments and that, and you would pay but it was asking how much would you pay and all that, and didn’t like that. I don’t know if you could put a price on getting your child better. I didn’t like that questionnaire at all.
Did you fill it in?
I filled it in but I didn’t like it, and I said to [names staff member] I don’t know if I filled that in right but I didn’t like it.
What did [names staff member] say?
She didn’t really . . . she goes it’s a bit of a funny one, just one that we have to do . . . But I actually remember that and as soon as you said, I can’t remember everything but I can remember everything about how much would you pay. I didn’t like that because I didn’t think there was any . . . you can’t put a price . . . financially we would have struggled if we’d had to pay, but we would have found the money. But I didn’t really like that, didn’t like it.
I think it’s a questionnaire that’s been tried out to see what it’s like so that’s really useful feedback.
I can’t remember, I just remember I didn’t like that.
BCP08, parent of a paediatric patient, PGT
Furthermore, as per the staff interviews, some patients stated that they had found this difficult to complete for fear of harming relationships with clinical staff, for example if they felt that they had ‘undervalued’ the care received. None of the patients whom we interviewed commented positively on the exercise.
Volume of assessments and associated case report forms: staff perspectives
Although staff interviewees expressed some concerns about the content of some of the assessments, as described in Staff concerns, and that initially the volume of paperwork and time taken was considerable, most felt that over time, with familiarity, they became accustomed to it:
Actually it wasn’t too bad. To start with it seemed quite lengthy but once you had done it a couple of times it was quite easy.
Interviewee S3
Staff also reported that patients seemed to cope with the demands of completing the required paperwork and questionnaires:
. . . once they were on it [the trial] they were quite happy. They filled in their forms, went through the questionnaires, other than the ones who then just didn’t just come back. The ones who stayed on and come back have all been absolutely fine, no trouble at all.
Interviewee S2
PEGASUS assessment appointments: arrangements and impact on staff capacity
We discussed the frequency of PEGASUS study-related appointments with interviewees and how these were managed alongside routine care. Although there appear to be slight differences in routine scar management follow-up practice across the pilot sites, on the whole, interviewees did not report a need to arrange specific PEGASUS study appointments/clinics or that the number and frequency of follow-up assessments were difficult to manage. Longer appointment slots were allocated to the PEGASUS study patients in routine follow-up clinics:
Yes, they were fitted in, booked into our [mentions specific clinic] which is run once a week, and we just allotted an hour slot rather than the normal half-an-hour slot. So we didn’t see them outside of the clinic, we just put them into the clinic. I think initially we were going to be seeing them outside of the clinic up in the consultants’ clinic, but that didn’t work, because it was too disjointed, because sometimes we don’t attend that clinic, so it was a bit . . . it was easier just to slot them into the normal clinic and just make sure that you blocked out an hour for them, because then you knew you were going to see them and you were able to give the proper time to them.
Interviewee S8
We also discussed the impact of the PEGASUS study assessment appointments on staff time and clinical capacity. Views were mixed on this: some staff reported significant time requirements, whereas others reported minimal disruption and additional time requirements:
So it is time consuming. I don’t want to sound really negative about it because I think it’s been very interesting, and I’m all for studies that look at what we do and examine it. But I think that to get it to . . . in order to be able to do it properly and not just focus on the research and therefore not do what you would normally do in a treatment session, which certainly isn’t the objective is it? It’s supposed to be in addition, then you’ve got to appreciate that it takes time, and I would suggest it takes a lot more time when you’re dealing with children than it does when you’re dealing with adults.
Interviewee S1
One member of staff noted that, because of the relatively small number of patients who were recruited to the PEGASUS pilot at their site, the overall impact on time and capacity was minimal.
Patient perspectives on frequency of pilot trial appointments and assessments
On the whole, patients did not express any concern about the frequency of pilot trial follow-up appointments, and most understood this to be in line with routine follow-up practice. However, several patients discussed the practicalities of attending frequent follow-up appointments, for example the need for flexibility with working arrangements for those in employment or for parents of paediatric patients. Parents, in particular, noted how this could be an emotional burden, for example if only one parent was able to attend follow-up and trial appointments as a result of work commitments. Although the actual time taken at appointments was prolonged because of trial assessments, on the whole this was valued by patients because of the attention that was given to them and also as a consequence of very well-developed relationships with core clinical staff.
Loss to follow-up
The staff interviewed noted that they had been unable to follow up some patients throughout the whole PEGASUS pilot trial assessment period. Two main explanations for this were given. First, some patients with minor burns, who at lesser risk of poor scar outcomes, may not attend long-term follow-up. Second, interviewees noted that the social or socioeconomic circumstances of a proportion of their patient population could influence the likelihood of attendance for follow-up appointments:
Yes, so one of them was definitely . . . she was at so low risk for scarring issues, also didn’t want to come back to the hospital anyway whatever, because she got persuaded really, and probably wasn’t ever going to come back and follow through . . . Another one he probably would have benefited from scar management but equally because of his social setup, etc., you had an inkling that maybe he was not going to come back for follow-up appointments . . .
Interviewee S2
Withdrawals or crossover from the pressure garment therapy group
Some of the staff interviewees described situations in which participants who had originally been allocated to the no-PGT treatment arm were withdrawn from the trial. Predominantly, this was described as a result of clinical judgements that patients should be receiving PGT and may, therefore, be related to strong views in favour of PGT. Staff at both of the sites treating paediatric patients related instances in which this had occurred:
. . . one parent withdrew because she didn’t get pressure garments and she was really worried about her child’s scar and said that she didn’t want to be in the trial anymore, and there was another child who wasn’t in pressure garments whose scars got very hypertrophic and started restricting movement, so I got him to see the consultant in clinic and between the two of us we agreed that they needed to go into garments, and all the others stayed in the arms that they were assigned to.
Interviewee S1
Interestingly, in a further interview, one member of staff recalled that they had been encouraged to withdraw a patient from the PEGASUS pilot study because of concerns expressed by a more senior colleague. In this situation, the interviewee stressed that the patient did not report any functional issues or concerns with the appearance of his scars, or treatment received and, therefore, was continued in the trial:
. . . there was one patient who went into the non-pressure group who he clearly wouldn’t have used pressure garments anyway, so from that point it was good, and his scarring wasn’t great but he was doing alright and he was happy with it. But at one point I was being pushed by the [senior member of staff] to withdraw him, ‘Oh no he needs to come off, he needs to be put in pressure’, etc., but actually then he talked through with the patient and actually he wasn’t bothered, so again it helped perhaps challenge the [senior member of staff] back a little bit more when they were saying, ‘No we need this treatment’. ‘Well actually he’s on this trial, I’ve talked to him, it’s not causing him any functional problems, he’s not bothered by the look of his scars, the treatment he’s having he feels is working, leave it at that’.
Interviewee S2
Suggested improvements for a definitive trial
It is worth noting that none of the interviewees at any of the pilot sites stated that a further definitive trial of PGT is not feasible or that they or their patients would not participate in a further trial. Interviewees did, however, make some suggestions for improvements: first, in terms of capacity, on-site trial co-ordination and site initiation and, second, regarding some streamlining of the paperwork and assessments required.
Capacity, on-site trial co-ordination and site initiation
One interviewee suggested that, with the involvement of more than one therapist on-site, workload could be reduced:
. . . if the trial goes ahead, move on from this and goes forward, you need to make sure you have more than one therapist who can do the study, that was my learning point.
Interviewee S2
Other interviewees noted that the close working between therapists and RNs facilitated the smooth running of the pilot:
Yes, and if we were separate, if we were at different hospitals it would be difficult, and it probably would be more time consuming. But because we are in the same place after the appointments we can quite easily go off and do some of our other jobs and then come back and do it. Definitely need a RN and a therapist.
Interviewee S6
Similarly, co-ordination between therapy and burns services was also a key facilitator for the pilot trial:
. . . because our therapy department is separate from the burns unit so although they’re allocated staff to burns the therapist is run as a separate entity, so their manager [names staff member] perhaps should have been a little bit more involved.
Interviewee S4
A related issue was on-site awareness of the study and the role of site initiation in raising awareness of the study among all key staff, particularly to address potential barriers to recruitment and retention, as described in Patient views on recruitment to the pilot trial. One interviewee noted how site initiation had not been on-site and how a lack of broader involvement and awareness of staff locally of the pilot trial had been a barrier to conduct.
Streamlining of paperwork and assessments
As noted in Volume of assessments and associated case report forms – staff perspectives, staff interviewees had reflected on the range of assessments and associated CRFs. Some reiterated that streamlining of assessments and associated paperwork would help facilitate a definitive trial:
I think clarity of information, perhaps streamlining some paperwork, maybe taking out the range of movement or asking it in a different way, maybe just linking, maybe to have it, have they got a contracture or a range of movement problem[s], because actually knowing the range of what exactly the range of movement is, I’m not sure how helpful that is to the outcome. When they’ve got a problem it probably is, but what exactly the range is, is irrelevant.
Interviewee S2
Some interviewees also noted that there should be an opportunity to record other scar management treatments that patients are receiving. This was related to the range of scar management treatment options available to clinicians, as described in the following section.
‘Standard’ treatment options for scar management and associated clinical decision-making
Staff noted that there is a range of treatment options normally available for scar management, including moisturisers, silicone cream and sheets, massage, splints and PGT. On the whole, interviewees indicated that patients will be advised to use moisturisers and massage. When PGT is used in scar management, this will often be in combination with other treatments and often involves the addition of silicone:
You might get silicone stitched into the garment, you get pads stitched into the garments, you can get panels stitched into the garment, you can increase the pressure in the garment, so whole load of different things you can add in depending again on the problem that is presented in front of you, or something is not responding as well as you would hope then you might tackle it with extra things added in. I generally start simple and then add in if needed, unless again someone is perhaps presenting with a severe scar at that point.
Interviewee S2
Interviewees discussed factors that would influence clinical decision-making regarding treatment options for scar management and the use of PGT outside of the context of the pilot trial. Therapists described a complex holistic assessment of patients made on an individual basis. Components of this assessment included clinical and patient characteristics, as well as patient preferences. The main components that influenced decisions to use PGT were:
-
length of time to healing
-
location of scar
-
size of scar
-
skin type/tone
-
age of patient
-
social and family circumstances of patient
-
mobility.
Staff interviewees talked about time taken to heal and the location of the scar:
It’s experience, so signs of it raising at an early stage, if it’s had delayed healing, any history of scarring before or family history, sometimes the area where it is on the body as well, there might be some areas that are more likely to put a garment on than others. I would probably say the main thing would be how long it’s taken to heal.
Interviewee S3
Location of scarring could mean that function and movement were of particular concern and, therefore, patients were deemed suitable for PGT, for example, when a burn injury and scar involved joints and could affect mobility. Similarly, hand burns and potential complications, such as web creep, were mentioned specifically by interviewees as indications for PGT:
I think it is the length of time it’s taken to heal, and we’re talking well over 4 weeks or so, well over that, also whether it’s over a joint, the position of the . . . so hands I’m more prone to give pressure garments to, and really the depth the burn, the type of burn that it is. So that’s when I’ll lean towards pressure garments or think that might be a good idea.
Interviewee S8
But because we found that pressure garments really help to prevent web creep and maintain your webs . . .
Interviewee S6
As described in Strong views on pressure garment therapy among staff, when clinical staff may struggle emotionally to recruit patients whom they consider on the edge of eligibility, clinical characteristics, such as potential to restrict movement and function via involvement of joints, may influence recruitment behaviour.
Some interviewees indicated that the size of the scar would influence clinical decisions, with PGT deemed to be less suitable for smaller scars:
Smaller scars, which is why we had a bit of an issue with the 1% burns, they’re such tiny scars that patients don’t necessarily feel, and neither do I, that it warrants a pressure garment.
Interviewee S5
Associations between skin tone/type and scarring also influenced decisions, with non-white patients being seen as more prone to poor scar outcomes. Because of the potential difficulties of removing and applying a pressure garment, factors such as the patient’s age, the perceived importance of appearance and their mobility were also taken into account. Some staff suggested that garments were more likely to be used for children:
But even if they have taken 4 weeks to heal and it’s not a particularly deep burn, it’s not over a joint, I will . . . if it’s an adult . . . children it’s separate completely, I tend to be more . . . what’s the word? . . . giving pressure garments more than I would to an adult. With an adult I would be more watch and wait and let them have a look and take responsibility, and see. But with a child it’s slightly different. I’ll put more children into pressure garments maybe than I would an adult.
Interviewee S8
Finally, judgements may be made around the suitability of a treatment regime because of expected adherence based on family circumstances.
Patients’ understanding of the pilot trial
Hardly any of the patient interviewees were aware that the pilot trial was part of a feasibility study rather than a definitive trial to test the effectiveness of PGT. Perhaps this is unsurprising, as, despite the information materials being clear about the nature of the study, most knowledge and understanding will be consequent to discussions and interactions with HCPs, who were more likely to talk in terms of a trial of PGT, rather than a feasibility study or pilot trial. There were a very small number of exceptions to this. Generally, there was a good basic understanding of the treatment options in both treatment groups. For example, when asked about different scar management treatment options and how they relate to the trial, one parent gave the following response:
So my understanding is that there are a couple of different treatments available for the ongoing management of burn injuries, which is the silicone treatment and also pressure garments, or a combination of the two, or none of them.
BCP07, parent of paediatric patient, PGT
With regard to PGT specifically, again there was a good appreciation that garments were intended to be worn for up to 23 hours each day. However, there was some ambiguity about the length of time that patients would be in garments. This perhaps reflects variation in clinical practice and the variable length of time that patients may be asked to wear garments for.
Patients’ reaction to group allocation
We discussed patients’ and parents’ feelings about the group that they had been allocated to. Reactions were varied and there did not seem to be a clear pattern to reactions that could be associated with allocation to the PGT or no-PGT treatment arm. Many interviewees seemed happy with the treatment allocation they had received, although this may be related to any preconceived preferences for PGT or no PGT. For example, this parent reflected on the point at which they were told which treatment (no PGT) their child had been allocated to:
How did you find the randomisation process? . . .
Well we were busy talking to [name of staff member] and [name of staff member] came in and said he’s been randomised this way, and we said great, so that was fine. I’m sure there was someone with a random number generator at the end of a phone or a big red button that lights up a particular light somewhere and tells you which way it’s going to go, but that’s fine.
BCP05, parent of paediatric patient, no PGT
This interviewee went on to state that they (the mother and father) were pleased that their child had been allocated to the no-PGT treatment arm, as they felt it would allow the child to get back to normal as soon as possible:
I think we both liked the idea of a non-pressure garment treatment because he would feel more normal sooner. If he went with a pressure garment it would be extending that bandage feeling, and that there’s still it feels like there’s still something special going on here, there’s something out of the ordinary happening with the pressure garment. [. . .] So from that perspective we didn’t mind, but we liked the idea of a return to normality for him and his psychological adjustment to what he had been through. Kids are great, they bounce back really well anyway, and [name of child] is testament to that. He’s been completely unfazed by the whole thing, but we like the idea of him being able to get back to normal as quickly as possible. So the idea of a pressure garment was not ideal for us, we would have preferred an alternative, and we were lucky that we were randomised that way.
BCP05, parent of paediatric patient, no PGT
However, several interviewees expressed some disappointment with the treatment allocation, in both the PGT and no-PGT arms. For example, one adult patient with severe injuries said that they were disappointed when they had learned that they had been allocated to receive PGT and described those in the no-PGT treatment arm as ‘lucky’. This patient was concerned about the burden of wearing garments. Some parents expressed similar views with reasoning along the lines of that expressed by interviewee BCP05 in the previous quotation.
Some of the interviewees allocated to no PGT were disappointed by this. One adult interviewee stated that they had expected to receive the garments as part of the trial, even though they understood the nature of the treatment allocation. Allocation to no PGT therefore seemed to come as a surprise:
I was, I don’t know why, I suppose I think just in my head I’d thought well I’m in this trial, I reckon they’re going to use it on me to see whether it works. I hadn’t thought about the fact that I might not be wearing one. I hadn’t really give it any consideration, so that’s probably why it came as a surprise to me, that was not the fault of anybody, that was just the way my mind had gone, that I would be wearing one.
CAP04, adult, no PGT
One parent decided to withdraw her child from the trial when they were told that they had been allocated to no PGT:
How did you feel at that time of being allocated in the no pressure garment group?
Quite upset really, because I’d got my hopes up and I thought that I’d been led to believe that she would get them. I’d gone all the way there especially from here thinking that we were going to get them for her, and then to be told that we wouldn’t I thought well there’s no point doing the trial if I’ve got to go all that way, and all we’re going to do is just use cream. So at that point I started to look myself as to how much it would be to get the garments and pay for them myself.
CPPD03, parent of paediatric patient withdrawer
Patient reports of adherence to allocated treatment
Interviewees in both arms described variable adherence to their allocated treatment. Some interviewees who were allocated to receive PGT, described being able to maintain regimes that accommodated complete adherence to the PGT regime, for example wearing garments for 23 hours a day, and appropriate washing and care of the garments. Some interviewees allocated to the no-PGT treatment arm also seemed happy with the process and routine of massage and creaming, although this could involve some experimentation with the types of cream used. However, other patients, and parents of paediatric patients, described variable adherence and non-adherence to prescribed PGT regimes, but also to massage and creaming as part of the no-PGT treatment arm. Factors that appear to influence adherence include:
-
the need for, and availability of, social support (e.g. spouse, family) to enable scar management regimes
-
the fit and design of pressure garments
-
discomfort or pain associated with allocated treatment
-
emotional burden of allocated treatment
-
feelings of physical and emotional protection offered by pressure garments.
For some adult participants, especially those with more severe burn injuries, the physical and emotional support from the family was paramount in treatment regimes, influencing adherence:
All day, I wouldn’t take it off, it would just be on for a couple of days and then I would change it. I would have a shower and then my wife would cream on my back and my arm and up the side, and then it would all go back on again, and it would stay on for another 2 days and then I would have a shower again. The only thing is with the garments and the wounds, obviously showering is a nightmare, because I’ve got to wait for somebody to be here, my wife, to cream me afterwards. That was the major thing, and to help me put my garments back on.
QAP16, adult, PGT
This patient, among others, also described how the original fit and design of one of the pressure garments had influenced adherence, as it had not stayed in position and, therefore, it had had to be altered over time. Both adult patients and parents of paediatric patients frequently mentioned this issue, and some interviewees had been through several iterations of garment design and fit.
Other interviewees admitted to not having worn allocated garments or to having stopped wearing a garment after a short period of time. One adult patient, again with more severe injuries, stopped wearing one foot pressure garment soon after allocation to treatment as it was causing significant pain and discomfort and also discontinued wearing another arm garment after 2–3 months:
. . . I just started getting fed up of wearing, because like my feet hypersensitive, so of course you’re trying to get this bloody tight pressure garment over the top of it, and it was just a nightmare . . . My foot it’s like hypersensitive it is, it’s just you touch it and it buzzes like mad, it’s alright in the boot, and I’m alright standing up on my own, but if you touch the bottom of it or the side of it, the toes or whatever, it just buzzes like pins and needles, but it’s like a whole new level. So I give up with that. . . . So I just decided not to wear it on my leg. I wore my arm one, and then eventually I just thought well it’s looking quite tidy now, so I just didn’t bother wearing that one either.
QAP13, adult, PGT
This interviewee also described priorities, such as the need to regain movement and function, that meant that garments and appearance were less of an immediate priority. They said that this influenced their attitude towards adherence to PGT.
One parent, whose child had crossed over to wearing garments from the no-PGT arm, described how the perceived emotional burden of the PGT on their child influenced adherence to treatment, with a designated ‘break’ during 1 day each week:
I give him a day’s grace, so his day as I said is Saturdays.
So he gets Saturdays as a break?
He gets Saturdays and I just think he goes swimming, so wake up in the morning, he will put on his swimming things, he goes swimming, he comes out, has a shower at the swimming baths, by the time we come home I usually give him a bath when we get home, so I wash his hair properly and wash him properly, and then I just let him have a day’s grace without wearing it, and then on a Sunday he puts it on usually Sunday afternoon, and that’s because usually in the mornings we don’t really do anything, and then he has another bath in the afternoon, and then I put it back on. But I have never told anybody at the hospital that, but I feel that, and I am . . . like I say he’s not going to have it on today. It’s now got into a bit of a routine that Saturdays he doesn’t wear it.
So just a bit of a break?
Yes, just a bit of a break for him, because sometimes you take it off and you can see him doing that, and then you just think of my gosh the sweat [. . .] He just curls up, you know a release, you released me.
BCP01, parent of paediatric patient, no PGT
However, some interviewees, particularly adults, described how the garments could provide a feeling of both physical protection, as a barrier to knocks and injury, and also emotional protection in hiding some of the visible injuries and scarring. These were a positive influence on adherence to treatment.
As already mentioned, non-adherence was also evident in the no-PGT arm, with interviewees describing similar variability in adherence to advised treatment regimes and also similar influences on adherence. For some of the parents we have interviewed, the process of massage and creaming, particularly with young children, was described as an emotional burden:
. . . I found it difficult to do as well, because it meant that I had to have contact with quite a sensitive area emotionally and physically. So I found that daily routine of doing that time consuming, and hard emotionally and I felt physically on [de-identified]. So I found that difficult as well, and it meant that I had to look at the scar, and I had troubles at the beginning looking at the scar every day twice a day, really looking at it, because I had to then massage it, plus he was a 1 year old, trying to keep him still to do something on an area that’s [. . .] I was probably a bit more reluctant [to look at the scar] so I probably wasn’t massaging it as much as I should have done, just because I found it hard.
BCP01, parent of paediatric patient, no PGT
Subjective patient perceptions of effectiveness
When we discussed progress with scarring and impressions of the effect of treatments, most of the interviewees held positive views about their scar management treatments, regardless of whether it was PGT or no PGT (massage and creaming). As per the P1 interview work with patients concerning key outcome domains, interviewees would often discuss aspects of scar characteristics, including thickness, texture and colour, as illustrated in the following quotations:
The colour has gone down. All these parts here that have . . . veiny looking things, you can see it’s all red like this, all this was like that and really red, so it’s gone a lot paler now, more towards my skin colour.
QAP16, adult, PGT
So you’re happy with the massage and the creaming for your hands and how it’s gone?
Yes, couldn’t want for any better, but I think that’s just fantastic [. . .]
It sounds like you were really keen on the massage and . . .
Yes, I am really keen and it does work. I didn’t think it would ever get as good as this, what stage you’re at now, no way, not this time last year when I’m looking at my hands just thinking Jesus, they’re a right mess they are, they ain’t going to get much better than that, but they have, so surprised, so it’s well worth doing. Get stuck in and get the massaging done.
QAP12, adult, no PGT
However, some patients’ perspectives on progress and outcomes and the relationship with scar management treatment varied over time, and for those patients with extensive burns, comparisons would be made between different areas and scars:
Are you pleased yourself with how things are progressing?
I am now, but I wasn’t at the beginning. I just didn’t think the garments were doing anything for me. But I found at times when I have taken them off and I’ve really missed them, and I can feel my lumps and bumps, and then I put my garments back on for a while and only took them off when I should have done, and it felt smoother. So it’s mainly when late at night or when I get home from work and sit down I only have to have a little tingling sensation down on the scar and I start scratching, and I just scratch myself to pieces.
Is that all the scars or is there one in particular which is . . .
It’s the two big scars that I’ve got on my left leg that are playing me up. On the other leg which we’re monitoring as well, that’s doing really well, and that doesn’t scratch, itch, nothing, and it’s very smooth at the moment, and it’s fading in colour, so I’m really pleased with that side. But my left leg was very bad, and I’ve got one scar which is really bad, quite deep. The other one above it, which was bad as well, seems to be that is progressing nicely now, and it’s not so raised and hard and thick.
EAP02, adult, PGT
The views of clinicians and their feedback to patients are obviously key in the judgements of progression and outcome that patients are making:
The lady I saw last time I’d not seen for I think maybe I can’t remember if it was 3 months or 6 months, but a big chunk of time, and she was really impressed, because she had seen it right from the beginning when I first was in hospital with it, and she couldn’t quite believe how good it was looking. So definitely really pleased.
EAP05, adult, PGT
There are a small number of examples in which interviewees were wearing, or had worn, garments on one burn area and had not worn them on another. In the following example quotation, the patient compares outcomes between these, questioning the difference and, therefore, the effect of PGT on scarring:
It makes them softer, I can . . . but literally you have the pressure garment on, you take it off, and then it’s real smooth, and it’s real soft, give it about 4 hours and it will be back to being rough and bumpy again. So to me while you’re wearing it it’s doing it good, but the second you take it off it’s reverting back to its usual form if you like. So it would almost be like you’d have to wear them for a lifetime, and I ain’t doing that, no way mate. No literally you would wear it, but to be fair that’s no different. When you take it off that is smooth and soft, so it used to be just like that when I took off, and I ain’t wore this garment now for probably nearly 5/6 months, and it’s exactly like just the second I took it off, it’s no different, and these used to be . . . these here, this line used to be real thick, again I haven’t worn the pressure garment now for 5 or 6 months and it’s flat. It used to be real ridgy, like a real thick old ridge it would be, gone.
QAP13, adult, PGT
Summary
-
Staff report that strong views about PGT can influence perspectives on the trial and, subsequently, recruitment. Positive views may be held by staff and patients, although some patients expressed strong preferences against PGT. Some staff may face emotional challenges associated with the recruitment of patients who they consider on the edge of eligibility because of clinical and other characteristics. Staff may be most concerned about burns that involve joints and that could affect mobility.
-
Patients were often approached regarding the trial during the acute phase of treatment. Several adult patients reported that this was at a time of severe pain and/or when they were under the influence of painkillers.
-
Several factors influenced adult patients’ and parents’ decision-making regarding participation in the pilot trial. Most commonly, patients stated a sense of altruism and wanting to give back to staff, although this was often conditional. In addition, expectations were sometimes set around the role of PGT in scar management. The concept of best treatment is implicit in this decision-making process and influenced by interactions with clinical staff.
-
Staff had some concerns about the complexity of certain outcome assessments in the pilot trial, for example ROM, and the complexity of assessing multiple scar sites at repeat visits. However, the timing and number of assessments, on the whole, was not deemed prohibitive by staff or patients.
-
Both staff and patient interviewees expressed serious reservations about the application of the WTP instrument within this context.
-
Staff suggested that some loss to follow-up may be seen in patients with lower TBSA burns, who are less likely to attend all follow-up visits.
-
Strong views regarding therapy among staff have influenced some crossover and withdrawals of participants allocated to the no-PGT treatment arm.
-
Staff suggested that more concentrated site initiation and rationalisation of outcome assessment were key concerns for a future trial.
-
Clinical decision-making regarding the use, and utility, of PGT is complex and influenced by a number of factors that may influence staff views on the suitability of patients for PGT.
-
Participant adherence to allocated treatment was reported to be variable and influenced by available social support for treatment, fit and design of garments, pain, discomfort, treatment burden and positive feelings of physical and psychological protection afforded by garments.
Chapter 9 Economic measures that will inform the design of a cost–utility analysis and cost–benefit analysis in a definitive trial
Relevant objective
The following objective is addressed in this chapter:
-
to assess the feasibility and success of the economic assessment to inform the assessment of cost-effectiveness in a future trial.
Methods
The health economics element of the study explored the feasibility of conducting a cost–utility analysis in a future trial, following as closely as possible methods recommended by NICE. 46 A secondary aim was to explore the feasibility of using cost–benefit analysis as a means of valuing broader outcomes.
There were three components:
-
piloting the use of generic health economics instruments (selected in P1) in the pilot RCT to define and, when possible, ‘look up’ an associated population-based preference value for the health profile of patients (of all feasible ages) at the different follow-up points, allowing the calculation of quality-adjusted life-years
-
piloting a resource use questionnaire in the RCT to see which costs incurred as a result of the burn injury may be of most relevance in this patient group
-
exploring the feasibility of using WTP within the trial for cost–benefit analysis, hence allowing patients or parents to value non-health aspects associated with the two treatment arms.
The HRQoL instruments, selected in P1, were completed face to face in the clinic, at the follow-up points specified in the trial protocol. An overview of the content and motivation for using each of the HRQoL instruments was provided to centre staff at yearly investigator meetings, together with a reminder of the different age ranges covered by the different instruments. Table 22 shows the HRQoL questionnaires that were administered at each visit, depending on age at randomisation. It should be noted that, although recruitment occurred between December 2014 and September 2015, the PedsQL tool was not available for use at the centres until June 2015 as a result of licensing issues.
| Age (years) at randomisation | Questionnaire | |||
|---|---|---|---|---|
| PedsQL (parent reported) | CHU-9D (parent version) | CHU-9D (child version) | EQ-5D-5L | |
| < 2 | Not available at this age range | |||
| 2–4 | Yes | No | No | No |
| 5–6 | No | Yes | No | No |
| 7–10 | No | Yesa | Yesa | No |
| 11–15 | No | No | Yesa | No |
| 16 | No | No | Yesa | Yes |
| 17 | No | No | Noa | Yes |
| ≥ 18 | No | No | No | Yesa |
As detailed in Chapter 6, two versions of a WTP questionnaire were developed, one for use in each treatment arm. Patients (or parents) completed the WTP questionnaire (within the clinic) at the 6-month follow-up and valued the impact of the treatment that they had actually received.
Two versions of the resource use questionnaire were used, one for completion by adult patients (defined as being aged ≥ 16 years), and one for completion by parents in the case of young patients (aged ≤ 15 years).
Results
Pediatric Quality of Life Inventory
Parents of 7 out of the 10 patients aged between 2 and 4 years completed at least part of the PedsQL at one or more visits. Accounting for the unavailability of PedsQL at visits prior to June 2015, PedsQL completion rates were two out of three (67%) at baseline, two out of three (67%) at visit 1 (week 1), three out of three (100%) at visit 2 (month 1), six out of seven (86%) at visit 3 (month 3) and six out of eight (75%) at visit 4 (month 6). There was unnecessary proxy completion of the PedsQL in a further seven cases for patients aged < 2 years at the point of randomisation (i.e. completion was not in accordance with the study protocol).
Table 23 reports the completion rate, by item, of the PedsQL for all time points. Completion rates for items relating to the physical and emotional functioning were high (range 95–100%), but decreased for the social functioning domain (range 77–86%). Completion of the final item on PedsQL is optional, with the instruction, ‘Please complete this section if your child attends nursery or day care’, which is likely to explain the fact that the completion rate for this final item on the PedsQL was 77%.
| Item name | Responses (n) | Completion rate (%) | |
|---|---|---|---|
| Complete | Missing | ||
| PedsQL (aged 2–4 years, proxy) | |||
| Physical | |||
| Walking | 22 | 0 | 100 |
| Running | 22 | 0 | 100 |
| Active play | 21 | 1 | 95 |
| Lifting | 22 | 0 | 100 |
| Bathing | 22 | 0 | 100 |
| Pick up toys | 22 | 0 | 100 |
| Aches pains | 21 | 1 | 95 |
| Tired | 22 | 0 | 100 |
| Emotional | |||
| Afraid | 22 | 0 | 100 |
| Sad | 22 | 0 | 100 |
| Angry | 22 | 0 | 100 |
| Sleeping | 22 | 0 | 100 |
| Worrying | 22 | 0 | 100 |
| Social | |||
| Playing | 19 | 3 | 86 |
| Other children playing | 18 | 4 | 82 |
| Teased | 18 | 4 | 82 |
| Unable to do | 19 | 3 | 86 |
| Keeping up | 19 | 3 | 86 |
| Same as peers | 17 | 5 | 77 |
| Nursery | |||
| Missing nursery (unwell) | 17 | 5 | 77 |
| Missing nursery (doctor appointment) | 17 | 5 | 77 |
| CHU-9D (aged 5–10 years, proxy) | |||
| Worried | 24 | 0 | 100 |
| Sad | 24 | 0 | 100 |
| Pain | 24 | 0 | 100 |
| Tired | 24 | 0 | 100 |
| Annoyed | 24 | 0 | 100 |
| School work | 23 | 1 | 96 |
| Sleep | 24 | 0 | 100 |
| Daily routine | 24 | 0 | 100 |
| Able to join in activities | 24 | 0 | 100 |
| CHU-9D (aged 7–16 years, patient) | |||
| Worried | 25 | 0 | 100 |
| Sad | 25 | 0 | 100 |
| Pain | 25 | 0 | 100 |
| Tired | 25 | 0 | 100 |
| Annoyed | 25 | 0 | 100 |
| School work | 25 | 0 | 100 |
| Sleep | 25 | 0 | 100 |
| Daily routine | 25 | 0 | 100 |
| Able to join in activities | 25 | 0 | 100 |
| EQ-5D-5L (aged ≥ 16 years, patient) | |||
| Mobility | 236 | 2 | 99 |
| Self-care | 237 | 1 | 99 |
| Usual activities | 236 | 2 | 99 |
| Pain/discomfort | 234 | 4 | 98 |
| Anxiety/depression | 237 | 1 | 99 |
Child Health Utility Index 9D: parent version
The CHU-9D was at least partially completed at one or more visits on behalf of six out of a total of seven eligible patients who were aged between 5 and 10 years at randomisation [baseline, n = 6/7, 86%; visit 1 (week 1), n = 4/7, 57%; visit 2 (month 1), n = 5/7, 71%; visit 3 (month 3), n = 5/7, 71%; visit 4 (month 6), n = 4/7, 57%]. In the case of proxy completion of the CHU-9D, there was one single missing response (to one item) by one single parent (see Table 23) at one single time point. The CHU-9D was unnecessarily completed by two parents of patients aged < 5 years at the point of randomisation (i.e. completion was not in accordance with the study protocol).
Child Health Utility Index 9D: child version
The CHU-9D was fully completed (no item non-response) at one or more visits by six young patients out of a total of eight eligible patients aged between 7 and 16 years at randomisation [baseline, n = 5/8, 63%; visit 1 (week 1), n = 6/8, 75%; visit 2 (month 1), n = 5/8, 63%; visit 3 (month 3), n = 4/8, 50%; visit 4 (month 6), n = 5/8, 63%]. The CHU-9D was completed unnecessarily by two patients who were aged < 7 years at the time of randomisation (i.e. the child completed the instrument in addition to the parent).
In the case of two patients aged between 7 and 10 years, both patient and parent (proxy) completed the CHU-9D in accordance with the study protocol. The UK value set was used to convert the CHU-9D profiles into utility values. 59 Scores for the proxy and patient matched exactly in the case of one child across all five time points (0.963 at baseline, and at visits 1 and 2; and then 1.0 at visits 3 and 4). Scores differed for the second child across observations, with no consistency in terms of the magnitude or direction of the discrepancies. No values are available for this second patient at baseline; at visit 1 values were 0.699 (child) and 0.752 (parent); at visit 2 values were 0.915 (child) and 0.595 (parent); at visit 3 values were 0.882 (child) and 0.915 (parent); and at visit 4 values were 0.652 (child) and 0.925 (parent). Several of these differences were substantial – and raise some concerns about the validity of the responses.
EuroQol-5 Dimensions, five-level version
The EQ-5D-5L was completed by all 28 PGT-arm participants at one or more visits [baseline 25/28, 89%; visit 1 (week 1) 25/28, 89%; visit 2 (month 1) 23/28, 82%; visit 3 (month 3) 22/28, 79%; and visit 4 (month 6), 18/28, 64%]. Out of a total of 29 eligible no-PGT participants, the EQ-5D-5L was completed by 27 participants at one or more visits [baseline 23/27, 85%; visit 1, 22/27, 81%; visit 2, 17/27, 63%; visit 3, 16/27, 59%; and visits 4, 18/27, 67%]. There was an appropriate completion rate of at least 98% on every item of EQ-5D-5L (see Table 23). ‘Appropriate completion’ indicates that a tick was placed against a single level/box for a specific item. If more than one level was selected for a single item, this was classed as an error. One participant ticked all of the boxes on the form.
Mean utility scores by treatment arm and by visit are presented in Table 24. Aggregated (mean) utility in the PGT treatment arm was greater at baseline, but the utility values within both treatment arms remain fairly stable across all time points (albeit with slightly more fluctuation in the no-PGT arm). Looking at the different dimensions of the EQ-5D-5L at baseline for both treatment arms, participants rated their health as worse in relation to the pain/discomfort and usual activities items compared with other items.
| Trial visit: time point | Treatment arm, mean score (SD) [n] | |
|---|---|---|
| PGT | No PGT | |
| Baseline | 0.830 (0.149) [n = 25] | 0.794 (0.194) [n = 23] |
| Visit 1: week 1 | 0.835 (0.123) [n = 25] | 0.777 (0.161) [n = 22] |
| Visit 2: month 1 | 0.837 (0.136) [n = 23] | 0.845 (0.153) [n = 20] |
| Visit 3: month 3 | 0.835 (0.166) [n = 22] | 0.855 (0.137) [n = 16] |
| Visit 4: month 6 | 0.841 (0.152) [n = 22] | 0.803 (0.213) [n = 18] |
Resource use questionnaire
Forty-seven out of a total of 57 patients (82%) aged ≥ 16 years filled out the adult version of the resource use questionnaire. The breakdown by occupation at week 1 was as follows: 14 out of 47 (30%) adult patients reported being employed full-time, 5 out of 47 (11%) reported being employed part-time, 7 out of 47 (15%) were self-employed, 6 out of 47 (13%) were retired, 1 out of 47 (2%) patients reported being a full-time parent/guardian, 1 out of 47 (2%) reported being a full-time carer, 9 out of 47 (19%) were unemployed, 3 out of 47 (6%) were studying in full-time education and 1 out of 47 (2%) patients recorded their occupation type as ‘other’. In the case of this last patient, free-text comments revealed a mixture of self-employment and time as an employee.
Eleven of these 47 adult patients (23%) recorded a change in their employment status over the duration of the study. One (no-PGT arm) self-employed patient transitioned into full-time employment, and one (PGT arm) patient did the opposite. Two patients who were self-employed (one patient in the PGT arm and one in the no-PGT arm) and one employed full time (PGT arm) reported being unemployed at later time points. Two (no-PGT arm) patients transitioned from self-employment to ‘not actively working’, then back to self-employment; one (no-PGT arm) patient transitioned from part-time employment to full-time student. Two (no-PGT arm) patients who undertook a mixture of self-employment and part-time employment changed to full-time employment. One (PGT arm) patient temporarily switched from working full time to self-employment.
Qualitative work (see Chapter 5) confirmed that return to work was important to at least some trial participants, as evidenced by the following quotation:
The range of movement was the most important for me . . . because at the end of the day I am right-handed, all of the jobs I’ve ever had have been manual work jobs which involve your hands, so to me being able to use it again was the most important thing.
CA06, adult
Some patients responding to the question ‘Since your last appointment at this clinic, how many days of work have you missed because of your burn injury?’ appear to have recorded responses that are implausible, in the sense that days off work reported exceed the number of days between visits. This potentially indicates that patients misinterpreted the question as being the days off work since the start of the burn injury. This issue could be addressed in a full trial with careful amendment, and possible piloting, of the wording of this particular question.
Thirty-four patients recorded that they were prescribed medicines or creams by their GP for their burn injury at some point during the trial. The most prescribed product was moisturiser (29 reported prescriptions), followed by prescriptions relating to pain relief (12 reported prescriptions). Sun cream was only prescribed twice, and three prescriptions for antibiotics were reported. Thirty patients reported that they purchased over-the-counter medicines or creams at some time during the trial period; again, this was mostly moisturiser (22 purchases). Sun cream was purchased 17 times and painkillers nine times. Eleven patients (23%) purchased clothing specifically as a result of their burn injury (six in the no-PGT arm and five in the PGT arm). Evidence from the CRFs (in this specific case, notes relating to non-adherence), appear to confirm the fact that at least some patients randomised to the no-PGT arm personally purchased items intended to act in the same way as the tight-fitting Lycra garment:
Patient has started using Tubigrip on scar. He feels scar looks better and is protected by using Tubigrip.
Notes from a CRF on non-adherence
Most patients reported driving to the clinic. The mean distance travelled (as a return journey) varied by centre, with, on average, patients travelling the farthest (113.4 miles) distance to attend St Andrew’s Centre for Plastic Surgery and Burns, Broomfield Hospital, Essex, and patients attending Manchester’s Children’s Hospital travelling the shortest (25.1 miles) distance. Mean distances at other centres were: Bristol’s Southmead Hospital, 32.1 miles; Swansea’s Morriston Hospital, 50.7 miles; BCH, 55.6 miles; UHB, 60.3 miles; and East Grinstead’s Queen Victoria Hospital, 76.3 miles.
Pressure garment costs
The costs for the initial fitting of pressure garments ranged from £36 to £141 for outsourced garments (mean cost £63, median cost £45) and from £34 to £114 for garments manufactured in-house (mean cost £55, median cost £40). The cost of each specific garment type (e.g. glove, sock, etc.) is not reproduced here, as the costing information was taken from a commercial price list and hence is likely to be commercially sensitive. As it was unclear from some of the CRFs (as completed by staff at the treatment centres) whether or not a refitting had occurred at a specific visit, the average number of refittings for pressure garments (across the PGT sample) was not calculated. It was necessary to retrospectively obtain data from sites as to the type of garment fitted, as this was not recorded on the CRF and there was some ambiguity as to which type of garment would best fit certain burn sites on the body.
A minority of sites manufactured and supplied garments in-house, although cost differences were small. In a full trial, the impact of manufacturing garments in-house versus purchasing from a commercial supplier could be explored through sensitivity analysis.
Willingness to pay
A total of 47 patients (70% of patients still participating in the trial at the 6-month follow-up) completed the WTP questionnaire, 26 in the PGT arm (out of 35 PGT patients followed up at 6 months) and 21 in the no-PGT arm (out of 32 no-PGT patients followed up at 6 months). The amount patients were willing to pay ranged from £0 to £500 per month in the PGT arm (mean £110, median £100) and from £20 to £1000 per month in the no-PGT arm (mean £174, median £100). There was one zero amount and there was evidence that it was a true value and not a ‘protest vote’, as the participant recorded the following free-text comment:
Had I been a young man I may have been willing to pay, however, at my age and with the size of the scar I wouldn’t have bothered paying for any scar treatment.
The £1000 value reported by a single participant in the no-PGT arm increased the mean value (from £133 to £174) and may not be an affordable monthly amount.
Additional free-text comments indicated that one patient/parent was responding in terms of actual monetary cost, and not expressing a value:
I had to work out the prices of the amount of each box of [silicone adhesive tape] that I use on my daughter’s scars and the cream.
Parents in particular found the WTP question challenging:
Very difficult to answer – how do you place a monetary value on the care of your child?
These free-text comments support the qualitative findings, reported in Chapter 8.
As supported by the qualitative work, the free-text comments also gave some indication that the experience of receiving care from staff was of high value to patients/parents:
It’s not specific treatments so much as the care of the hospital staff that really helps.
Patients in the PGT arm were asked two additional, follow-up questions: ‘If you could pay a smaller amount per month to receive the same treatment but without the tight-fitting Lycra garment, would you choose to do so?’ and ‘What would be the lower amount that you would be willing to pay in this case?’. Five of the 26 PGT patients/parents indicated that they would be willing to pay less for treatment that did not include the pressure garment, although two of those five failed to record the amount that they would be willing to pay in that case. Three did report a lower amount: £50 rather than £100, £5 rather than £20 and £140 rather than £180.
Summary
-
There were high completion rates for the EQ-5D-5L with negligible levels of missing data, although little change in EQ-5D-5L values was noted across the follow-up period in either trial arm. Completion rates were also high for CHU-9D, with few missing data, although fewer patients fell into the appropriate age range. There were insufficient observations to draw meaningful conclusions in relation to the appropriateness of child versus proxy completion.
-
PedsQL was completed for only a small number of patients, reflecting the low numbers falling into that specific and narrow age range. Completion rates across the 21 items were frequently lower than those for EQ-5D-5L and CHU-9D. One section of the questionnaire was irrelevant for some patients, making the instrument non-comparable across patients.
-
Questions relating to resource use appear broadly relevant, but some proved difficult to analyse or interpret, and it is unclear whether missing responses are a true indication of no resource use or of respondents having simply avoided questions that were complex or time-consuming to answer. A future trial needs to ensure that participants provide a response (zero or a quantity) for each item of resource use.
-
Although the WTP questionnaire provided monetary estimates, it proved to be problematic and the values measured may not be trustworthy. Qualitative work with patients (see Chapter 8) clearly found that patients and parents struggled in terms of understanding the underlying concept and of considering an appropriate monetary response, and patients and parents reported guessing or estimating the actual cost of the care received (including staff time in some cases), with some being anxious not to undervalue the time and efforts of centre staff. The task (in the way it was administered here) added significantly to the burden at the treatment centre (for both staff and patients/parents).
-
On a number of occasions the wrong QoL questionnaire was filled out by the patient or parent, most likely caused by the inclusion of three separate and age-specific HRQoL questionnaires in the study CRF. The wide range of ages for the feasibility study undoubtedly increased the complexity and need for different validated QoL instruments. A further trial would benefit from using an enhanced electronic CRF system that ensured that only the single appropriate tool is completed for each patient at each visit.
-
Uncertainty about the type of pressure garment and the number of refittings (as reported by staff at the treatment centres using the CRF) meant that the total cost of pressure garments could not be reliably estimated. A future CRF should ensure that accurate data on the type of pressure garment and the number of refittings (at or outside the trial visit) for each scar site are recorded to help ascertain the true costs of pressure garments. Alternatively, in a full trial, these data could be collected from electronic medical records.
-
The similar costs for making the garments in-house and via outsourcing means that either could be used for costing in a future trial.
-
Measurement of resource use beyond the NHS perspective proved problematic, and it was unclear how the data obtained could be included in a meaningful analysis. Presenteeism (reduced productivity in the workplace as a result of health problems) was irrelevant to the 43% of patients/parents who were not employed. Fluctuations in terms of employment status do not indicate a clear trend and cannot directly be linked to burn injury or the nature of the treatment. Despite evidence from the qualitative work that return to normality (including usual tasks at work) is important to patients and clinicians, we would suggest that employment status, days off work and presenteeism are not assessed in a full trial with an analysis undertaken, instead, from a NHS perspective.
-
The most significant disadvantage of adopting only a NHS perspective would be the loss of questions relating to travel costs, medicines and clothing that have been bought privately by the patient or parent (i.e. the costs falling directly on the patient or parent, as opposed to broader, societal costs). It is, however, our belief that a shorter and simplified resource use questionnaire would provide more reliable data, place less burden on patients at a busy and emotionally difficult time and place less burden on staff in the busy clinical environment.
-
The adoption of a NHS perspective is currently the priority for organisations, such as NICE, and our recommendation (as given above) is that costing be limited to a NHS perspective in a full trial. The perspective should be consistent for both costs and benefits, meaning that the restriction of the costing to a NHS perspective should be matched by a restriction of the scope of the consequences to health gain, as per standard cost–utility analysis.
Chapter 10 Discussion and conclusions
The PEGASUS study was undertaken to determine whether or not it is feasible to undertake a RCT to assess the clinical effectiveness and cost-effectiveness of pressure garments for scar management and, if found to be feasible, to inform the design and execution of a future definitive trial. A feasibility study was considered necessary before embarking on a large-scale trial as pressure garments have become a routine component of scar management and may be perceived as effective, despite the absence of convincing evaluations, which raises concerns that efforts to recruit to a definitive RCT would be hindered by lack of willingness from both patients and clinicians to risk foregoing PGT.
In the PEGASUS study, we used web-based surveys of NHS burns services and health-care workers, in-depth interviews with health-care workers, adult patients and parents of paediatric and adolescent patients and the running of a pilot RCT with an embedded process evaluation and health economic analysis to assess whether or not we could recruit and retain adequate numbers of patients in a definitive trial and to inform thinking about the patients, interventions, outcomes, trial processes and sample size that a definitive trial would require. We approached the evaluation by devising a set of objectives to be addressed in the separate components of the study (Chapter 2), which we have reported on, sequentially, in Chapters 3–9.
Can a definitive trial be run?
The two primary tests of the ability to run a definitive trial are whether or not we could recruit and randomise a sufficient number of patients in a pilot trial and whether or not we could retain a sufficient proportion of them in their allocated intervention.
The pilot trial demonstrated that staff were willing to recruit to the RCT, and 88 patients were willing to be randomised to scar management strategies with and without PGT. Recruitment to the trial followed the planned trajectory, with 88 participants being recruited across the seven sites that were opened, giving a recruitment rate of 1.7 participants per centre per month. Despite running the trial with a pragmatic allowance for variations in scar management to occur, only 11% of participants were seen to switch to or from PGT. A further 22% of participants left the trial, some mistakenly because they were determined to no longer need therapy before the planned end of follow-up.
Successful completion of the pilot trial supports the conclusion that, in principle, it would be feasible to deliver a large definitive trial.
Is there equipoise?
Owing to the embedded nature of PGT within current clinical practice, doubts about the acceptability of this trial among burns staff and patients were a key focus of feasibility testing. We have explored this issue via the national staff attitudes survey and also in the qualitative research that has been integrated within the pilot trial. The national survey has allowed us to describe and explore the acceptability of this trial, in principle, across a broad sample of UK NHS burns services and staff members. Our integrated qualitative research with adult patients, parents of patients and staff in the pilot trial has explored how attitudes and acceptability play out in practice. Survey findings between pilot and non-pilot sites were similar, suggesting that observations gained from the pilot study are likely to be generalisable beyond the pilot sites.
The majority of staff reported that they perceived a need for a full-scale RCT of PGT; however, the rationale underpinning this response was complex. Despite the success of recruitment to the pilot trial, the web-based survey of staff attitudes and qualitative interviews with NHS burns service staff indicated an apparent lack of clinical equipoise around the research question and PGT as a treatment. Although a large proportion of staff stated that they want a full-scale RCT of PGT, their first response was that this was not necessarily to provide evidence of the best treatment for patients but rather to support current practice and the continued use of PGT for burns scar management. Many had been working under the presumption that the evidence base for PGT use already exists.
On the one hand, staff were receptive to the idea of a UK trial and stated that it was likely that they would participate and some non-pilot site services and individuals have expressed an interest in becoming a site for a future trial, based on their participation in the survey, demonstrating ‘buy-in’. On the other hand, strong views about the use of PGT have the potential to influence the conduct of a trial if those views are not recognised and taken into account at the design stage. In our trial process evaluation, interviews with staff and patients demonstrated that strong views about PGT among staff can influence their perspectives on the trial and, subsequently, recruitment and retention in allocated treatment.
Several factors influenced adult patients’ and parents’ decision-making regarding participation in the pilot trial. Most commonly, patients stated that they had a sense of altruism and wanted to give back to staff. However, often this altruism was conditional and expectations were sometimes set around receiving PGT in scar management. The concept of best treatment was implicit in the decision-making of patients and the perception of the best treatment was influenced by interactions with clinical staff.
Our interviews with patients suggest that although some may have preferences for scar management therapies that will mean that they would not agree to participate in a trial – for example, because of strong preferences for or against PGT – this is not an issue that is highly prevalent. Rather, and as observed in previous research examining trial recruitment, staff attitudes towards a future trial and associated behaviours are likely to be a stronger influence on acceptability to patients, who look to clinical staff for information and guidance regarding the best treatment options. We would suggest that a future trial should include a specific targeted recruitment investigation as part of a trial process evaluation, for example the QuinteT Recruitment Intervention devised by Professor Jenny Donovan at the University of Bristol. 60
What patients should be eligible?
There were three main questions that were raised during the study concerning patient eligibility: (1) the recruitment of infants and children, (2) the importance of burn severity (TBSA burn percentage) and (3) whether or not patients with particular burn injuries were unsuitable for recruitment to the trial because PGT was either infeasible for their injury or perceived to be necessary.
There was a strong rationale for including infants and children as well as adults in the trial. Our projected numbers of patients who are being treated annually in NHS burns services showed that more children (n = 1476) than adults (n = 1369) are treated each year, indicating the high burden of paediatric cases. Interviews with staff and parents indicated the higher perceived importance of successful scar maturation in children than adults and the impact of adverse effects of PGT on children. Thus, addressing the question of whether or not PGT is effective in children is of high priority. Although we successfully recruited 31 children to the RCT, logistical challenges in evaluating outcomes (both for clinical effectiveness and cost-effectiveness) in children were encountered, particularly in infants for whom suitable outcome tools did not exist.
The spectrum of disease severity judged to be eligible for the trial was broad, as all burn injuries of any size, with a TBSA of > 1%, judged at risk of hypertrophic scarring and that were considered suitable for scar management therapy were recruited. Of those recruited to the trial, the median TBSA of the burn injury was 4%, with over one-quarter of burn injuries having a TBSA of < 2%. We identified that, because of the low TBSA threshold, some of the patients who were recruited and randomised in the trial had scar injuries for which PGT may not have been considered in current care. Thus, the trial tested the extension of PGT to less severe burns than would be seen in routine practice, as well as testing the effectiveness question in patients who would currently receive PGT. Patients with smaller burns were also less likely to attend all follow-up visits and complete the study, as they often perceived that their scar had healed adequately before the planned end of follow-up.
An important consideration for a future trial is whether or not the study should be designed to investigate a differential benefit of PGT according to burn severity (as measured by percentage of TBSA). There was considerable support for the view that the expected benefits of PGT would be greater in larger, more severe burns than in smaller, less severe burns. Certainly, any future trial would need to stratify recruited patients by burn severity, but it may also usefully consider including a sufficiently powered preplanned analysis of effectiveness according to burn severity.
Burn location was also identified as a determinant of the perceived value of PGT. For some body locations, PGT was considered unlikely to be effective as a result of the difficulties of constructing PGT garments that would be able to deliver adequate pressure [e.g. on the central chest (pre-sternal)]. PGT for other body areas was considered indispensable by some therapists, particularly when scars have the potential to restrict function and ROM (e.g. burns on hands and feet, where scarring may cause contractures) and there is a risk that only a small number of patients with such injuries are included, as they may be considered on the edge of eligibility by staff.
It may be wise in a future trial to encourage and monitor the recruitment of patients with severe burns to ensure that the hypothesis concerning the benefit of PGT is properly tested. It appeared that therapists in the trial were making holistic judgements in determining which patients they would insist received PGT (and thus not enter the pilot trial) combining multiple aspects of the nature of the injury with each patient’s expectations and needs, including consideration of the patient’s personal circumstances (e.g. complex home environment), which might prevent them from engaging in the required follow-up care.
What interventions should be compared?
The study protocol specified that PGT in the intervention arm should be delivered as in current practice and it pragmatically allowed for variation in practice. This allowed units to continue to use their current PGT supplier and follow local treatment protocols, which eased the delivery of the trial and ensured that it evaluated the interventions as they are routinely used. Our survey and experience in the trial indicated that there was adequate similarity between units in PGT delivery for this approach to be valid. The majority of NHS burns centres recommend that patients wear pressure garments for 12–18 months, with no difference noted between adult and paediatric recommendations. Pressure garments were manufactured either in-house or externally (by three different companies, with the majority of centres using a single supplier).
Pressure garment therapy is one component of scar management and is used alongside moisturisation, silicone and massage, which, importantly, were available to patients in both arms of the trial. The acceptability of the trial to health-care workers and patients may rely on the trial being described as the comparison of two active scar management interventions (with and without PGT). We would suggest that a future trial may benefit from being described more neutrally as a ‘scar management trial’ rather than a ‘PGT trial’. Patients reported that they greatly valued the psychological support obtained from regular contact with health-care workers at routine visits (when PGT fit was checked in the intervention arm). Beyond the initial PGT fitting session, these were scheduled identically. This contact should be considered (and measured) as part of any scar management intervention.
Adherence with both PGT and other aspects of scar management was assessed by self-report. We estimate that full adherence with the intervention at month 6 for a large RCT would be around 60%, but we observed equal adherence across the two arms. We did not assess adherence with individual components of the intervention, but this may be considered in a future study. Patient adherence to allocated treatment was reported to be variable and influenced by available social support for treatment, fit and design of garments, pain, discomfort, treatment burden and positive feelings of physical and psychological protection afforded by garments.
Some of the apparent lack of adherence identified occurred because participants and health-care workers determined that no further treatment was necessary before the official end of trial, particularly for participants with smaller scars. A future trial would need to include a method to ensure that end-of-trial assessments are completed in these participants and that they are not classified as being non-adherents and LTFU. Collecting information about the reasons for termination of planned therapy would help to identify those participants stopping because of treatment success, treatment failure, intolerance of treatment or unwillingness to continue as a research study participant.
Our interviews with adult patients, parents and health-care workers identified that strong views regarding therapy among staff influenced some crossover of participants allocated to the no-PGT arm, with the likelihood of crossover being raised with participants before treatment allocations were known. Clearly, a lack of willingness to initially adhere to the assigned treatment contraindicates suitability for randomisation. This would need to be addressed in site setup training in a future trial and monitored closely via a recruitment investigation as part of a process evaluation. In the event, observed rates of crossover were the same in each treatment arm, with five participants crossing over from no PGT to PGT and five crossing over from PGT to no PGT. This gives an estimated total crossover rate for a future trial of 11% (range 6–20%).
What outcomes should be measured?
In P1 of the feasibility study, we undertook interviews with patients and parents of paediatric and adolescent patients who had experience of PGT for burns scar management in order to understand patient perspectives of the outcome domains that should be captured in a trial of PGT. Similarly, via the national staff survey and follow-up telephone interviews, we have gathered staff perspectives on this issue. This work demonstrates that a patient-centred assessment of the outcomes of PGT should be holistic in nature. The impact of scar management is complex, involving a range of outcome domains.
The following domains were identified as being core to outcome assessment: scar characteristics and appearance, movement and function, itch and pain, psychological and social functioning and the burden of any treatment. Priorities for outcomes may vary from patient to patient and over time. These outcome domains reflect a complex holistic patient experience of scar management and treatments such as PGT.
We also assessed the feasibility of undertaking blinded assessment of outcomes, and judged that this would not be possible, even when an independent assessor was available or photography used, as a result of the indentations left by pressure garments. Although blinding using a sham pressure garment (a garment indistinguishable from a pressure garment but that did not apply adequate pressure across the wound) may be successful, inclusion of such a sham garment would change the nature of the control intervention and likely affect important patient outcomes, such as treatment burden. We deemed this to be inappropriate and an invalidation of a pragmatic assessment of the holistic impact of PGT.
When the trial commenced, we were able to include two scar assessment scales (the VSS and the POSAS) and used three HRQoL assessment scales across the age groups (EQ-5D-5L, CHU-9D and PedsQL). An assessment of how the core outcome domains identified via our qualitative and survey work map against these measures is presented in Table 25.
| PEGASUS: core outcome domains (qualitative and survey work) | PEGASUS: pilot trial assessments | Burns HRQoL scales (not available to PEGASUS) | ||||
|---|---|---|---|---|---|---|
| Scar assessment scales (VSS and POSAS) | Other clinical assessments [ROM; Cutometer (elasticity)] | EQ-5D-5L | PedsQL | CHU-9D | BBSIP | |
| Scar characteristics | ✓ | ✓ (elasticity only) | ✗ | ✗ | ✗ | ✓ |
| Appearancea | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Movement | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ |
| Function | ✗ | ✗ | ✓ (walking and self-care) | ✓ (walking, running, sports/exercise, lifting, bath/shower, chores) | ✓ (bath/shower, getting dressed, sports) | ✓ |
| Itch and pain | ✓ (POSAS only) | ✗ | ✓ (pain/discomfort) | ✓ (hurts/aches) | ✓ (pain) | ✓ |
| Psychological and social functioning | ✗ | ✗ | ✓ [questions on usual activities (non-specific); anxiety/depression] | ✓ (emotional, social and school functioning) | ✓ (worried, sad, school work/homework, sleep, activities) | ✓ |
| Treatment burden | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ (impact of scar treatments) |
Taking each domain in turn, some key observations emerge:
-
Although the scar assessment scales used in PEGASUS (VSS and POSAS) assess various scar characteristics, none of the pilot trial measures assesses the overall judgements of patients of scar appearance. We suggest that this is a core outcome domain emerging from our qualitative and survey work that is particularly important to patients and their carers.
-
Range of movement has been assessed clinically but may be less important than the measurement of functional ability (changes in ROM may not correlate with clinically significant functional improvement). The QoL measures used (EQ-5D-5L, PedsQL and CHU-9D) all include items related to function and, therefore, cover some aspects of this domain.
-
Scar-specific itch and pain are covered by POSAS and each of the QoL tools used in the pilot measure non-specific pain/discomfort.
-
Psychological and social functioning is covered by the pilot QoL measures (EQ-5D-5L, PedsQL and CHU-9D) to a limited extent.
-
Treatment burden was not assessed directly in any of the pilot trial assessments.
In addition to failing to cover the core domains, the VSS and the observer-reported component of the POSAS were assessed by clinicians who were unblinded and whose responses were considered to have a risk of bias, particularly given the strong views about garments expressed by some health-care workers. Completion of POSAS was also problematic, with questions being irrelevant for some children. Cutometer and ROM assessments were often impractical to complete from a staff perspective; should they be included in a future study, training and support would be required.
In summary, when this feasibility study was commissioned and designed, there were no available scar management-specific HRQoL measures available for inclusion in the study and pilot trial and no core outcome set for burns or scar management in burns that might have been drawn upon for a trial. Although we included the two most commonly used assessment tools and commonly used physical measures, these do not provide the detailed holistic assessment required to assess the outcome domains identified by patients and clinicians.
However, we have identified two PROMs at advanced stages of development that would be suitable for inclusion in a future definitive trial, subject to successful completion of validation studies. The first of these, the Brisbane Burn Scar Impact Profile (BBSIP) tool, assesses burn scar-specific HRQoL, although it has not been validated in a UK population. 61 The BBSIP tool has been developed by the Centre for Children’s Burns and Trauma Research in Brisbane, Australia (www.coolburns.com.au/brisbane-burn-scar-impact-profile; accessed 1 October 2017). Four separate versions of the scale have been developed: for adults; for children aged 8–18 years; for caregivers of children aged 8–18 years; and for caregivers of children aged < 8 years. These are currently undergoing validation (Dr Catrin Griffiths, University of West England, 2017, personal communication) and manuscripts detailing the results of validation studies of the adult version have been submitted for publication. Testing of the other scales is ongoing. The BBSIP tool covers all of the outcome domains that have been identified by our qualitative and survey work (see Table 25), although treatment burden is assessed by only a single question pertaining to the perceived impact of scar treatments. The PEGASUS trial team is gaining experience in using pilot versions of the BBSIP tool in their current practice.
The second set of burns PROMs for adults and children is currently being developed and validated by a team based at the Centre for Appearance Research (CAR) at the University of the West of England, Bristol (Dr Catrin Griffiths, personal communication). A multicentre validation study is under way and the team expect that measures will be available for use in mid-2018. A version of this PROM was unavailable to the PEGASUS research team to map against the findings of our qualitative and survey research at this stage.
How many patients are needed?
We estimated that the NHS burns services treat 2845 patients with PGT over a 12-month period, with 1476 aged < 16 years and 1369 aged ≥ 16 years of age. The vast majority of these patients are treated in burns centres and burns units, with too few being treated in burns facilities to justify the setup of these as recruitment sites for a definitive trial. We do not have direct information on what proportion of the 2845 patients would be eligible for a trial but, given that the pilot trial achieved the projected recruitment rate, randomising 88 participants over 9 months (average recruitment rate of just under 10 patients per month across seven study sites), we can estimate potential recruitment through extrapolation. In total, there are 36 burns units and centres in the UK – if half of these were recruited to a definitive trial at the same rate as the pilot trial, 300 patients could be recruited in a year. Given our observed consent rate of 42% among those screened, we estimate 700 patients would need to be screened to recruit 300. Further allowance for an estimated 20–25% loss to follow-up would suggest that 225–240 of the 300 recruited patients would provide evaluable data.
Although we propose the use of an outcome such as the BBSIP tool in a definitive trial, data are not currently available on the minimal clinically important difference (MCID) or the SD of measurements for this tool required to compute a sample size for a definitive trial. We are currently using this tool in practice in order to obtain these data from a UK site, as well as from the ongoing international validation studies. To provide insight into the likely size of a definitive trial, we have computed sample sizes using the VSS score, the POSAS overall observer-reported score and the POSAS overall adult patient-reported score at two alternative primary end points: 6 and 12 months post randomisation (Table 26).
| Time point | Correlation | SD | Power (%) | Effect size (expressed as a z-score) | |||
|---|---|---|---|---|---|---|---|
| z = 0.5 | z = 0.4 | z = 0.3 | z = 0.2 | ||||
| VSS | |||||||
| 6 months | 0.326 | 1.912 | MCID 1.0 | MCID 0.8 | MCID 0.6 | MCID 0.4 | |
| 80 | 114 | 176 | 312 | 702 | |||
| 90 | 152 | 236 | 418 | 940 | |||
| 12 months | 0.494 | 2.548 | MCID 1.3 | MCID 1.0 | MCID 0.8 | MCID 0.5 | |
| 80 | 96 | 150 | 264 | 594 | |||
| 90 | 128 | 200 | 354 | 796 | |||
| POSAS observer reported | |||||||
| 6 months | 0.601 | 1.259 | MCID 0.6 | MCID 0.5 | MCID 0.4 | MCID 0.3 | |
| 80 | 82 | 126 | 224 | 502 | |||
| 90 | 108 | 168 | 300 | 672 | |||
| 12 months | 0.587 | 1.593 | MCID 0.8 | MCID 0.6 | MCID 0.5 | MCID 0.3 | |
| 80 | 84 | 130 | 230 | 516 | |||
| 90 | 112 | 174 | 308 | 690 | |||
| POSAS patient reported | |||||||
| 6 months | 0.545 | 2.409 | MCID 1.2 | MCID 1.0 | MCID 0.7 | MCID 0.5 | |
| 80 | 90 | 138 | 246 | 552 | |||
| 90 | 120 | 186 | 330 | 740 | |||
| 12 months | 0.670 | 2.846 | MCID 1.4 | MCID 1.1 | MCID 0.9 | MCID 0.6 | |
| 80 | 70 | 110 | 194 | 434 | |||
| 90 | 94 | 146 | 258 | 580 | |||
Sample sizes are computed for a statistical analysis that adjusts for baseline values using analysis of covariance, exploiting the efficiencies gained from the strong correlation between baseline and follow-up values. Correlations at 6 and 12 months post randomisation were 0.326 and 0.494, respectively, for VSS scores, 0.601 and 0.587, respectively, for observer-reported POSAS scores, and 0.545 and 0.670, respectively, for patient-reported POSAS scores.
Sample sizes are computed for effect sizes expressed as z-scores between 0.2 and 0.5 (also expressed as MCID in the units of measurement by multiplication by the observed SD). Sample sizes are computed for a two-sided test of superiority at the 5% level, and are equivalent for a 2.5% one-sided test of non-inferiority presuming no treatment difference between groups. We display samples sizes for both 80% and 90% power.
Values in the table indicate that a trial of around 400 participants is likely to have 90% power to detect a difference (or lower limit of equivalence) of z = 0.3, and a trial of 700–800 participants would be required to have 90% power to detect a difference (or with a lower limit of equivalence) of z = 0.2. Sample sizes would need to be rechecked when the distributional and psychometric properties of the BBSIP tool and/or CAR burns PROMs are known.
Further efficiencies may be gained by exploiting the repeated measurements structure of the data and including follow-up measurements from multiple time points. For example, for the VSS at the 6-month analysis, the n = 418 sample size for 90% power for detecting an effect size of z = 0.3 would decrease to n = 262 if a second outcome time point was included, and to n = 210 if a third time point was included, assuming the same correlation between outcome time points as with the baseline value.
What outcomes should be used for health economic evaluation?
The wide age range included in the sample required three different health economics instruments to capture quality-adjusted life-year-focused health outcomes: EQ-5D-5L (for patients aged ≥ 16 years), CHU-9D (for ages 7–16 years, and with further differentiation within this age range relating to patient vs. proxy completion) and PedsQL (for ages 2–4 years, with solely proxy completion). Completion rates were high for the EQ-5D-5L and CHU-9D; the completion rate for the 21 items of the PedsQL was lower. One section of the PedsQL was irrelevant for some patients, making the instrument non-comparable across patients. Inclusion of the EQ-5D-5L and CHU-9D to record the health profiles of patients aged ≥ 7 years are recommended for a future trial. It is difficult to justify the inclusion of PedsQL, as the optional response adds complexity and there are no utility values available for the tool (although future work may make it feasible).
Although the WTP questionnaire provided monetary estimates, it proved problematic, and responses may not have reflected preferences relating to treatment and observed/perceived outcomes. Our qualitative work demonstrated that many patients and parents attempted to estimate or guess the actual cost of the care received (including staff time in some cases), with some being anxious not to undervalue the time and efforts of centre staff. This qualitative finding was supported by the participant free-text comments on the questionnaire itself. Although it is entirely in keeping with the conceptual basis and motivation for using the WTP questionnaire that patients and parents had an opportunity to express a positive value for something they consider to be important (i.e. the compassion of staff), it appears that patients focused on cost rather than value and some may have exaggerated the value they reported as a way of expressing gratitude or showing support to staff. This problem was exacerbated by the fact that the WTP questionnaire was returned to the staff at the clinic. In some cases, those interviewed as part of the qualitative work reported that they were self-conscious about sharing their reported value with those same members of staff. The question (in the way it was administered here) added significantly to the burden at the treatment centre (for both staff and patients/parents).
The three problems – of burden at the treatment centre, of misunderstanding of the nature of the task and of the value to be reported – together with respondents feeling uncomfortable about reporting a monetary value for the care they receive in front of the staff providing that care could potentially be resolved through administering the WTP questionnaire as a telephone survey. Given the degree of struggle reported and observed here, trained interviewers would be needed in order to stand the best chance of obtaining valid results, which may not be feasible and acceptable in a definitive trial.
How should resource use be measured?
The funding streams of the pressure garment services varied across the UK, with many services not initially able to provide their monthly garment costs as a result of it being part of a much larger budget. Many services are still reliant on the ‘therapy budget’ or ‘plastics budget’ to fund the pressure garments, although others succeeded in funding them through their GP or health board. It is estimated that the cost of pressure garments to the NHS for burns patients is £2,171,184 per annum.
A NHS and societal perspective was adopted to capture and record cost data. Information not routinely captured on the CRF was elicited directly from the patient or parent. Costing for garments was captured from a commercial price list. Measurement of resource use beyond the NHS proved problematic, and it was unclear how the data obtained could be included in a meaningful analysis. Presenteeism was irrelevant to any patient or parent who was not employed. Fluctuations in terms of employment status and the need to account for external economic factors make information on employment status difficult to meaningfully interpret. In addition, employment presenteeism may be determined more by the nature and magnitude of the accident and injury itself than the impact of the scar. Despite evidence from the qualitative work that return to normality (including usual tasks at work) is important to patients and clinicians, we would suggest that employment status, days off work and presenteeism are not assessed in a full trial and that the analysis is undertaken from a NHS perspective rather than from a societal perspective.
The most significant disadvantage of adopting ‘just’ a NHS perspective would be losing the questions relating to medicines and clothing bought privately by the patient or parent. It is, however, our belief that a shorter and simplified resource use questionnaire would provide more reliable data, place less burden on patients at a busy and emotionally difficult time and place less burden on staff in the busy clinical environment.
Uncertainty about the type of pressure garment and the number of refittings meant that the total cost of pressure garments could not be reliably estimated. A future CRF should ensure that accurate data on the type of pressure garment and the number of refittings (at or outside the trial visit) for each scar site is recorded to help ascertain the true costs of pressure garments. The similar costs for making the garments in-house or via outsourcing means that either could be used for costing in a future trial.
Study logistics: lessons learned
Sites and recruitment
Interviews with staff, parents and patient participants in the pilot trial further demonstrate that established views regarding the role of PGT in burns scar management have the potential to influence trial conduct. Staff may influence patients’ and parents’ perspectives on the trial if expectations are set in favour of the need for PGT, either by the staff who are recruiting to the trial or by other burns service staff whom they interact with earlier in the care pathway. There is evidence from interviews with patients and parents that staff who were not involved in the pilot trial have discussed PGT with them, thereby influencing expectations and attitudes towards the trial. This is unsurprising, given the embedded nature of PGT in clinical practice. Some interviewees did also report that they felt that the recruiting clinician favoured the PGT option, and there is an isolated example of an interviewee reporting that they were told that their child would have been withdrawn from the pilot had they not been allocated to PGT. Although the latter was not a common feature in the patient and parent interviews, when discussing recruitment to the pilot trial some staff interviewees did also indicate that they had been selective on occasion about which patients to approach regarding the trial. We believe that these data reflect the findings from the staff attitudes survey and that, although recruitment to the pilot has been successful, these observations need to be taken into account during the design and delivery of a definitive trial.
With regard to recruitment to the pilot study, our other main finding from the qualitative research was that several of the adult patients reported approaches regarding entry into the trial relatively early in the patient care pathway, during acute treatment and at a time when many were in pain and/or under the influence of painkillers. Although some parents were similarly approached early on, their recollection of the process and knowledge and understanding of the trial generally seemed to be more complete. Although we cannot say definitively that this is related to the point at which recruitment takes place in the care pathway, some consideration of this is needed for a definitive trial. In terms of feasibility, this is not straightforward, as approaches later in the pathway, prior to scar management, may mean that patient expectations for treatment at that stage, and particularly regarding the role of PGT, could be different.
We would recommend that the whole burns service is involved in recruitment and delivery of a trial of PGT. Staff who are not investigators or recruiting to the trial should also be involved in site initiation activities to raise awareness of the trial and the rationale for it. Site initiation visits need to be face to face and multiple site initiation visits may be required, for example when the burns and therapy units are separate. This should be resourced appropriately. Any definitive study should be designed with an integrated mixed-methods process evaluation that has a targeted recruitment investigation. This should include clear reporting and feedback to staff recruiting to the trial, the study team, the clinical trials unit and study centres and investigators.
A component of site initiation should be clear training, and information given to recruiting staff and clinicians should include the evidence and rationale for the trial, relevant findings from the feasibility study, including observations regarding recruitment, and strategies for the discussion of the trial and its relationship with established routine scar management practice. Consideration should be given to calling this a ‘scar management trial’, which would enable more neutral conversations with patients about treatment options included in the trial.
Multiple burns
Patients with multiple burns raised challenging issues in the trial. We indicated that all burns suitable for PGT should be assessed in the trial and pressure garments used on these if the participant was allocated to the PGT arm, with a maximum of three scars being selected for inclusion in the outcome assessment. Difficulties were experienced in keeping track of the selected scars, which may have been a source of data errors.
Given the proposed emphasis in a future trial on holistic assessment, it would be preferable to consider treatment of a patient rather than of a single scar site, with outcome assessments based across all scars. Evaluation of the suitability of the chosen patient-reported outcome assessment tool to achieve this is required.
Crossovers, drop-out and adherence to therapy
We identified confusion at sites in the definitions of crossover and withdrawal from the trial. Patients who had a successful early response to therapy (with or without PGT) were misclassified as crossovers and/or study withdrawals. Clear instruction and training on how individuals should be classified and followed up once therapy is complete is required for a future trial to ensure that final outcome assessments are completed and data available for analysis.
Data collection
The patients and clinic staff experienced some confusion in completion of the outcome assessments. For example, there were a number of times when the wrong QoL questionnaire was filled out by the patient or parent, which was most likely caused by the inclusion of three separate and age-specific HRQoL questionnaires in the study CRF. Particular problems were experienced for children who crossed age boundaries during the duration of the trial. The wide range of ages for the feasibility study undoubtedly increased the complexity and need for different validated QoL instruments. A definitive trial would benefit from using an enhanced electronic CRF system that ensured that only the single appropriate tool is completed for each patient at each visit.
Questions relating to resource use appear broadly relevant but some proved difficult to analyse or interpret, and it is unclear if missing responses are a true indication of no resource use or if respondents simply avoided questions that were complex or time-consuming. A future trial needs to ensure that participants provide a response (zero or a quantity) for each item of resource use.
Strengths and limitations
The PEGASUS study constituted a mixed-methods research project, which provided quantitative evidence from one of the larger (if only a pilot) trials in scar management, with insights obtained from surveys and qualitative interviews with patients, health-care workers, trial participants and non-participants. This has greatly enriched our understanding of the context in which a future trial could be run and the barriers and facilitators that will ensure that it is a success and has provided clarity on what a future trial would need to be successful in determining the clinical effectiveness of scar management care for patients.
The trial was run across seven centres in the UK Burns network, providing evidence of an ability to engage with different sites and recruit both adults and children to a multicentre study. The experience gained provides confidence in the ability to recruit to a future definitive trial.
The study has limitations because of the constraints of the time frame within which it could be run. Originally, we planned to start the pilot trial (P2) after much of the work of the surveys and qualitative research (P1) was complete, which would have allowed the lessons learned in P1 to influence the design of the pilot trial. However, we were directed to complete the study in a shorter time period, which required the phases to run in parallel and reduced the opportunity for the qualitative research findings to influence the design and delivery of the pilot trial. Similarly, the unavoidable delays in site set-up led to early curtailment of follow-up; thus, we have fewer data on the follow-up to 12 months than we had originally planned. The time frame also led us to be unable to evaluate the BBSIP tool within the trial, which could have been useful to accelerate the tool’s validation.
Conclusions
The successful recruitment to the pilot study to time and target indicate that completion of a definitive trial of the use of pressure garments in scar maturation is feasible in the UK NHS context. However, we identified that current positive attitudes among staff towards the use of pressure garments may lead to biases in recruitment and retention in treatment. Provision of training and support to sites and ongoing assessment of trial processes as part of a recruitment investigation and process evaluation are required to ensure that a future trial is executed according to protocol and will deliver valid results.
Inclusion of children and infants in a future trial is desirable because of the high number and the impact of burns injuries in these age groups. Identification of valid outcome assessments, particularly for cost-effectiveness analysis, is challenging. Similarly, recruitment of patients with both severe and minor burns is essential to ensure that the hypothesis that the benefits of PGT depend on severity of injury is tested.
Although we faced a barrier of failing to include an outcome measure that covered the domains that were identified as important to patients and clinicians in the trial, the BBSIP tool or CAR burn PROMs that are currently in development and validation are likely to provide a suitable holistic outcome assessment tool for inclusion as a primary outcome in a future trial.
Implications for health care
It is not possible to propose any modifications to current health care based on the findings of this feasibility study.
Recommendations for research
The feasibility study has demonstrated that it is possible to complete a definitive randomised evaluation of the value of PGT in the management of burn scars within the UK NHS context. Key aspects that need to be defined in such a study include the recruitment of infants and children in addition to adults, specificity of the suitability for inclusion in a trial based on the degree of burn severity (i.e. TBSA burn percentage), whether or not the clinical effectiveness of PGT should be assessed according to burn severity, the selection of an appropriate outcome measure and the choice of tools for the assessment of cost-effectiveness in infants and children.
Although the BBSIP tool appears to be a suitable outcome measure, evaluations of the tool in a UK context are necessary to ensure that it has validity and to obtain estimates of measurement distributions and the clinically important differences required to compute the sample size for a trial.
Acknowledgements
The PEGASUS team
Trial development and management group
Naiem Moiemen (Chief Investigator/Applicant), University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Birmingham; e-mail: naiem.moiemen@uhb.nhs.uk.
Professor Jonathan Deeks (Co-investigator/Applicant, Biostatistics and Trial Design), BCTU, University of Birmingham, Birmingham.
Dr Emma Frew [Co-Investigator/Applicant, Health Economics (design phase)], Institute of Applied Health Research, University of Birmingham, Birmingham.
Melanie Calvert, Jonathan Mathers and Laura Jones (Co-investigators/Applicants, PROMs work), Institute of Applied Health Research, University of Birmingham, Birmingham.
Dr Philip Kinghorn (Co-Investigator/Applicant, Health Economics), School of Health and Population Sciences, University of Birmingham, Birmingham.
Amy Bamford (Current Practice Research, RN), University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Birmingham.
Fay Gardiner (Current Practice Research, OT), University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Birmingham.
Liz Coombes (Counselling Psychologist), University Hospitals Birmingham NHS Foundation Trust, Birmingham.
Karen Turner (née Doyle) (PPI involvement), Cancer Research UK Clinical Trials Unit, University of Birmingham, Birmingham.
Jonathan Bishop (Trial Statistician), BCTU, University of Birmingham, Birmingham.
Trial co-ordination and trial management (day to day)
Margaret Grant (Unit Co-ordination), Gemma Slinn (Senior Trial Co-ordinator), Dawn Brant (Trial Co-ordinator), Dana Bridges [Trial Co-ordinator (until May 2015)] and Hannah Watson (née Lack) (Data Manager), BCTU, University of Birmingham, Birmingham.
Patient-reported outcome measures research (not mentioned above)
Sue Wright, Ian Lichfield and Nicole Andrews (qualitative research), Institute of Applied Health Research, University of Birmingham, Birmingham.
Health economics research (not mentioned above)
Mark Monahan (health economic evaluation), School of Health and Population Sciences, University of Birmingham, Birmingham.
Participating centres
Central Manchester University Hospitals NHS Foundation Trust, Royal Manchester Children’s Hospital
Mamta Shah (PI), Katrina Keating (RN), Julie Walmsley (charge nurse), Lorna Stanyer (OT) and Anna Nixon (physiotherapist).
Abertawe Bro Morgannwg University Health Board, Morriston Hospital
William Dickson (previous PI), Tom Potokar (PI), Janine Evans (OT), Natalie Blytt Jordens (RN) and Jane Griffiths (RN).
University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital
Naiem Moiemen (chief investigator), Amy Bamford (senior RN), Fay Gardiner (OT), Elizabeth Chipp (consultant), Kwang Chear Lee (trial management) and Laura Mee (administration).
Mid Essex Hospital Services NHS Trust, St Andrews Centre for Plastic Surgery and Burns, Broomfield Hospital
Peter Dziewulski (PI), Helen Gerrish (RN), Natalie Whybro (RN), Victoria Dudman (OT) and Karen Cranmer (Research Co-ordinator).
Queen Victoria Hospital NHS Trust, Queen Victoria Hospital
Bajlit Dheansea (PI), Simon Booth (RN), Louise Rodgers (OT) and Poppy Cole (physiotherapist).
North Bristol NHS Trust, Southmead Hospital
Ian Mackie (PI), Anthony Sack (RN), Alison Guy (OT) and Sankhya Sen (Surgeon).
Birmingham Children’s Hospital NHS Trust, Birmingham Children’s Hospital
Yvonne Wilson (PI), Kate Whiting (OT), Sarah Payne (RN) and Federica D’Asta (Burns Fellow).
Others to be acknowledged
Trial Steering Committee
Stuart Watson (Independent Chairperson, Consultant Burns and Plastic Surgeon), Glasgow Royal Infirmary, North Glasgow University Hospitals NHS Trust, Glasgow.
Jamie Kirkham [Independent (Biostatistics), Lecturer in Medical Statistics], Institute of Translational Medicine, University of Liverpool, Liverpool.
Annemarie Nelson [Independent (PROMs), Department Director Marie Curie Palliative Care Research], Marie Curie Palliative Care Research Centre, Cardiff.
Sukhbir Rayatt [Independent (Consultant), Consultant Burns and Plastic Surgeon] University Hospitals of North Midlands NHS Trust, Stoke-on-Trent.
David Udale [Independent Member (PPI representative)].
Naiem Moiemen (on behalf of the TMG, Chief Investigator).
Jonathan Deeks (Professor of Biostatistics).
Data Monitoring and Ethics Committee
Alan Phipps (Chairperson, Consultant Plastic Surgeon), Department of Plastic Surgery, Mid Yorkshire Hospitals NHS Trust, Wakefield.
Vikram Sharma (Independent member, Consultant Plastic Surgeon), Department of Plastic and Reconstructive Surgery, Cambridge University Hospitals NHS Foundation Trust, Cambridge.
Sarah Brown (Independent member, Senior Medical Statistician), Clinical Trials Research Unit, University of Leeds, Leeds.
Contributions of authors
Naiem Moiemen was the Chief Investigator and senior clinical lead for the project, jointly designed the study plan and the grant application, provided leadership to all aspects of the study including the design, delivery, analysis and interpretation, wrote significant sections of the study report and commented on the final version of the full report.
Jonathan Mathers jointly designed the survey of staff attitudes, the qualitative research and process evaluation, undertook interviews for the qualitative research studies, jointly undertook the thematic analysis for the qualitative research and production of drafts of reports of the staff attitudes survey, qualitative research studies and the process evaluation. He also commented on the final version of the full report.
Laura Jones jointly designed the survey of staff attitudes, the qualitative research and process evaluation, undertook interviews for the qualitative research studies, jointly undertook the thematic analysis for the qualitative research and production of drafts of reports of the staff attitudes survey, qualitative research studies and the process evaluation. She also commented on the final version of the full report.
Jonathan Bishop contributed to the design of the pilot trial, conducted the pilot trial analysis, drafted the report of the pilot trial and commented on the full report.
Philip Kinghorn led the health economics elements of the study, including contributing to the design of the pilot trial; produced a drafted report of the health economic methods and results; and commented on the final version of the full report.
Mark Monahan contributed significantly to the data analysis relating to the health economics elements of the study and also contributed to the write up of the health economics results and discussion.
Melanie Calvert contributed to the evaluation of patient outcomes in the qualitative research studies and the trial, and commented on these sections of the report.
Gemma Slinn contributed to the design of the pilot trial and the study protocol, developed the CRFs, and managed delivery of the pilot trial.
Fay Gardiner undertook and analysed the web-based survey of current practice of PGT, drafted the report and contributed to the design, execution and interpretation of the qualitative research and pilot trial.
Amy Bamford undertook and analysed the web-based survey of current practice of PGT, drafted the report and contributed to the design, execution and interpretation of the qualitative research and pilot trial.
Susan Wright jointly designed and undertook data collection for the survey of staff attitudes and undertook interviews and initial analysis for the qualitative research study.
Ian Litchfield undertook interviews and analysis for the qualitative research study.
Nicole Andrews undertook analysis for the qualitative research study.
Karen Turner provided patient input into the design and execution of the studies.
Margaret Grant provided senior trials unit oversight of the design and execution of the trial.
Jonathan Deeks was the senior methodological lead for the project; jointly designed the study plan and the grant application; provided methodological oversight of the design, analysis and interpretation of the studies; wrote significant sections of the study report; and produced the final version of the trial report.
Publications
Jones LL, Calvert M, Moiemen N, Deeks JJ, Bishop J, Kinghorn P, et al. Outcomes important to burns patients during scar management and how they compare to the concepts captured in burn-specific patient reported outcome measures. Burns 2017;43:1682–92.
Andrews N, Jones LL, Moiemen N, Calvert M, Kinghorn P, Litchfield I, et al. Below the surface: parents’ views on the factors that influence treatment adherence in paediatric burn scar management — a qualitative study [published online ahead of print October 12, 2017]. Burns 2018.
Data sharing statement
For the pilot randomised controlled trial
Outcome data will be made available, on request to the BCTU, for incorporation in future meta-analyses.
For the qualitative research studies
This is a qualitative study and, therefore, the data generated are not suitable for sharing beyond that contained within the report to protect the identities of the participants.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Peck MD. Epidemiology of burns throughout the world. Part II: intentional burns in adults. Burns 2012;38:630-7. https://doi.org/10.1016/j.burns.2011.12.028.
- World Health Organization . Burns n.d. www.who.int/mediacentre/factsheets/fs365/en/ (accessed 12 January 2018).
- Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011;37:1087-100.
- Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo M, Bollero D, et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 2008;10:93-102. https://doi.org/10.1001/archfaci.10.2.93.
- Klein MB, Goverman J, Hayden DL, Fagan SP, McDonald-Smith GP, Alexander AK, et al. Benchmarking outcomes in the critically injured burn patient. Ann Surg 2014;259:833-41. https://doi.org/10.1097/SLA.0000000000000438.
- Lawrence JW, Mason ST, Schomer K, Klein MB. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res 2012;33:136-46. https://doi.org/10.1097/BCR.0b013e3182374452.
- Shin TM, Bordeaux JS. The role of massage in scar management: a literature review. Dermatol Surg 2012;38:414-23. https://doi.org/10.1111/j.1524-4725.2011.02201.x.
- Atiyeh BS, El Khatib AM, Dibo SA. Pressure garment therapy (PGT) of burn scars: evidence-based efficacy. Ann Burns Fire Disasters 2013;26:205-12.
- Sharp PA, Pan B, Yakuboff KP, Rothchild D. Development of a best evidence statement for the use of pressure therapy for management of hypertrophic scarring. J Burn Care Res 2016;37:255-64. https://doi.org/10.1097/BCR.0000000000000253.
- Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, et al. International clinical recommendations on scar management. Plast Reconstr Surg 2002;110:560-71. https://doi.org/10.1097/00006534-200208000-00031.
- Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel: a new treatment for hypertrophic scars. Surgery 1989;106:781-6.
- Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, et al. Updated international clinical recommendations on scar management: part 2-algorithms for scar prevention and treatment. Dermatol Surg 2014;40:825-31.
- O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.CD003826.pub3.
- Liuzzi F, Chadwick S, Shah M. Paediatric post-burn scar management in the UK: a national survey. Burns 2015;41:252-6. https://doi.org/10.1016/j.burns.2014.10.017.
- Breasted JH. The Edwin Smith Surgical Papyrus, Volume 1: Hieroglyphic Transliteration, Translation and Commentary. Chicago, IL: The University of Chicago Press; 1930.
- Breasted JH. The Edwin Smith Surgical Papyrus, Volume 2: Facsimile Plates and Line for Line Hieroglyphic Transliteration. Chicago, IL: The University of Chicago Press; 1930.
- Mancini RE, Quaife JV. Histogenesis of experimentally produced keloids. J Invest Dermatol 1962;38:143-81. https://doi.org/10.1038/jid.1962.29.
- Madden JW, Peacock EE. Studies on the biology of collagen during wound healing. 3. Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann Surg 1971;174:511-20. https://doi.org/10.1097/00000658-197109000-00017.
- Lee KC, Joory K, Moiemen NS. History of burns: the past, present and the future. Burns Trauma 2014;2:169-80. https://doi.org/10.4103/2321-3868.143620.
- Linares HA, Larson DL, Willis-Galstaun BA. Historical notes on the use of pressure in the treatment of hypertrophic scars or keloids. Burns 1993;19:17-21. https://doi.org/10.1016/0305-4179(93)90095-P.
- Paré A. The Workes of that Famous Chirurgion Ambrose Parey Translated out of the Latine and Compared with the French by Th. Johnson. Ann Arbor, MI: Text Creation Partnership; 1649.
- Fujimori R, Hiramoto M, Ofuji S. Sponge fixation method for treatment of early scars. Plast Reconstr Surg 1968;42:322-7. https://doi.org/10.1097/00006534-196810000-00006.
- Cronin TD. The use of a molded splint to prevent contracture after split skin grafting on the neck. Plast Reconstr Surg 1961;27:7-18. https://doi.org/10.1097/00006534-196101000-00002.
- Gottlieb E. Prolonged postoperative cervical pressure as an adjunct to plastic surgery on the neck. Plast Reconstr Surg 1963;32:600-6. https://doi.org/10.1097/00006534-196312000-00003.
- Kealey GP, Jensen KL, Laubenthal KN, Lewis RW. Prospective randomized comparison of two types of pressure therapy garments. J Burn Care Rehabil 1990;11:334-6. https://doi.org/10.1097/00004630-199007000-00012.
- Chang P, Laubenthal KN, Lewis RW, Rosenquist MD, Lindley-Smith P, Kealey GP. Prospective, randomized study of the efficacy of pressure garment therapy in patients with burns. J Burn Care Rehabil 1995;16:473-5. https://doi.org/10.1097/00004630-199509000-00002.
- Groce A, Herndon DN, McCauley RL, Chinkes D. The effects of pressure versus no pressure garments in the control hypertrophic scarring in children with small burns: a preliminary 6 month report: 88. J Burn Care Res 2000;21. https://doi.org/10.1097/00004630-200001001-00088.
- Moore ML, Engrav LH, Calderon J, Lezotte D, Ehde DM, Heimbach DM, et al. Effectiveness of custom pressure garments in wound management: a prospective trial within wounds and with verified pressure: 86. J Burn Care Res 2000;21. https://doi.org/10.1097/00004630-200001001-00086.
- Groce A, McCauley R, Serghiou M, Chinkes D, Herndon DN. The effects of high versus low pressure garments in the control of hypertrophic scar in the burned child: a final report: 196. J Burn Care Res 2001;22. https://doi.org/10.1097/00004630-200103002-00196.
- Van den Kerckhove E, Stappaerts K, Fieuws S, Laperre J, Massage P, Flour M, et al. The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns 2005;31:696-702. https://doi.org/10.1016/j.burns.2005.04.014.
- Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg 2009;62:77-84. https://doi.org/10.1016/j.bjps.2007.10.052.
- Candy LH, Cecilia LT, Ping ZY. Effect of different pressure magnitudes on hypertrophic scar in a Chinese population. Burns 2010;36:1234-41. https://doi.org/10.1016/j.burns.2010.05.008.
- Li-Tsang CWP, Zheng YP, Lau JCM. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res 2010;31:448-57. https://doi.org/10.1097/BCR.0b013e3181db52a7.
- Engrav LH, Heimbach DM, Rivara FP, Moore ML, Wang J, Carrougher GJ, et al. 12-Year within-wound study of the effectiveness of custom pressure garment therapy. Burns 2010;36:975-83. https://doi.org/10.1016/j.burns.2010.04.014.
- Fricke NB, Omnell ML, Dutcher KA, Hollender LG, Engrav LH. Skeletal and dental disturbances in children after facial burns and pressure garment use: a 4-year follow-up. J Burn Care Rehabil 1999;20:239-49. https://doi.org/10.1097/00004630-199905000-00016.
- Hubbard M, Masters IB, Williams GR, Chang AB. Severe obstructive sleep apnoea secondary to pressure garments used in the treatment of hypertrophic burn scars. Eur Respir J 2000;16:1205-7. https://doi.org/10.1034/j.1399-3003.2000.16f29.x.
- Brown CA. A comparison of the outcomes of two clinical audits of burn pressure garment satisfaction and compliance in Saudi Arabia. Burns 2001;27:342-8. https://doi.org/10.1016/S0305-4179(00)00139-X.
- Gallagher JM, Kaplan S, Maguire GH, Leman CJ, Johnson P, Elbaum L. Compliance and durability in pressure garments. J Burn Care Rehabil 1992;13:239-43. https://doi.org/10.1097/00004630-199203000-00012.
- Johnson J, Greenspan B, Gorga D, Nagler W, Goodwin C. Compliance with pressure garment use in burn rehabilitation. J Burn Care Rehabil 1994;15:180-8. https://doi.org/10.1097/00004630-199403000-00015.
- Ripper S, Renneberg B, Landmann C, Weigel G, Germann G. Adherence to pressure garment therapy in adult burn patients. Burns 2009;35:657-64. https://doi.org/10.1016/j.burns.2009.01.011.
- Donovan ML, Muller MJ, Simpson C, Rudd M, Paratz J. Interim pressure garment therapy (4-6 mmHg) and its effect on donor site healing in burn patients: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1329-x.
- Lu J, Xu T, Liu Y, Yang M, Jiang XH. Pressure garment therapy for preventing hypertrophic and keloid scarring after a major burn injury [Withdrawn]. Cochrane Database Syst Rev 2015;2. https://doi.org/10.1002/14651858.CD011543.
- Karimi H, Mobayen M, Alijanpour A. Management of hypertrophic burn scar: a comparison between the efficacy of exercise-physiotherapy and pressure garment-silicone on hypertrophic scar. Asian J Sports Med 2013;4:70-5.
- Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 2014;72:S198-201. https://doi.org/10.1097/SAP.0000000000000103.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Adlard N, Kinghorn P, Frew E. Is the UK NICE ‘Reference Case’ influencing the practice of pediatric quality-adjusted life-year measurement within economic evaluations?. Value Health 2014;17:454-61. https://doi.org/10.1016/j.jval.2014.02.007.
- Canaway AG, Frew EJ. Measuring preference-based quality of life in children aged 6-7 years: a comparison of the performance of the CHU-9D and EQ-5D-Y – the WAVES pilot study. Qual Life Res 2013;22:173-83. https://doi.org/10.1007/s11136-012-0119-5.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Webb NJA, Frew E, Brettell EA, Milford DV, Bockenhauer D, Saleem MA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid-sensitive nephroctic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-147.
- Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al. VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13550.
- Thomas KS, Koller K, Dean T, O’Leary CJ, Sach TH, Frost A, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: the Softened Water Eczema Trial (SWET). Health Technol Assess 2011;15. https://doi.org/10.3310/hta15080.
- Mooney G. Beyond health outcomes: the benefits of health care. Health Care Anal 1998;6:99-105. https://doi.org/10.1007/BF02678115.
- Donovan JL, Paramasivan S, Salis I, de Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15:5-17. https://doi.org/10.1186/1745-6215-15-5.
- Donovan JL, Sallis I, de Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. https://doi.org/10.1016/j.jclinepi.2014.03.010.
- Bidad N, MacDonald L, Winters ZE, Edwards SJL, Emson M, Griffin CL, et al. How informed is declared altruism in clinical trials? A qualitative interview study of patient decision-making about the QUEST trials (Quality of Life after Mastectomy and Breast Reconstruction). Trials 2016;17:431-41. https://doi.org/10.1186/s13063-016-1550-7.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-31.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Donovan J. QuinteT Recruitment Intervention. Bristol: University of Bristol; n.d.
- Tyack Z, Ziviani J, Kimble R, Plaza A, Jones A, Cuttle L, et al. Measuring the impact of burn scarring on health-related quality of life: development and preliminary content validation of the Brisbane Burn Scar Impact Profile (BBSIP) for children and adults. Burns 2015;41:1405-19. https://doi.org/10.1016/j.burns.2015.05.021.
Appendix 1 PEGASUS staff attitudes survey
Appendix 2 Staff interview discussion guide
Appendix 3 Phase 1 adult interview discussion guide
Appendix 4 Resource use tools
Appendix 5 Willingness-to-pay questionnaires (pressure garment therapy)
Appendix 6 Willingness-to-pay questionnaires (non-pressure garment therapy)
Appendix 7 Supplementary analyses
| Questionnaire/assessment | Screen | Time point | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 1 | Month 1 | Month 3 | Month 6 | Month 12g | ||
| Weeks 4–6 | Weeks 12–14 | Weeks 24–26 | Weeks 48–52 | ||||
| Issue PIL | ✗ | ||||||
| Informed consenta | ✗ | ||||||
| Patient QoL questionnaire(s)b | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Randomisation | ✗ | ||||||
| Baseline burns assessments | ✗ | ||||||
| Scar assessmentc | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Clinical photography (certain sites only) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Scar management techniquesd | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Cutometere | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Patient adherence | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Adverse events | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Medication review | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Health Resource Utilization Questionnairef | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Pressure garment measurement | ✗ | ||||||
| Fitting of the pressure garment | ✗ | ||||||
| WTP exercise | ✗ | ||||||
| Time point | Treatment arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No PGT (n = 42) | PGT (n = 43) | |||||||||
| Pigmentation | Pliability | Height | Vascularity | Total | Pigmentation | Pliability | Height | Vascularity | Total | |
| Baseline | ||||||||||
| n | 42 | 42 | 42 | 42 | 42 | 43 | 43 | 43 | 43 | 43 |
| Median score (IQR) | 2 (0–2) | 2 (1–2) | 1 (0–1) | 2 (1–2) | 6 (4–7) | 2 (0–3) | 2 (2–3) | 1 (1–1) | 2 (1–2) | 7 (5–9) |
| Week 1 | ||||||||||
| n | 35 | 35 | 35 | 35 | 35 | 41 | 41 | 41 | 41 | 41 |
| Median score (IQR) | 2 (1–2) | 2 (2–3) | 1 (1–1) | 2 (1–2) | 6 (5–8) | 2 (1–2) | 2 (2–3) | 1 (1–1) | 2 (2–2) | 7 (6–8) |
| Improvement (%) | 10 (n = 4) | 10 (n = 4) | 0 (n = 0) | 12 (n = 5) | 14 (n = 6) | 14 (n = 6) | 30 (n = 13) | 7 (n = 3) | 18 (n = 8) | 32 (n = 14) |
| Month 1 | ||||||||||
| n | 37 | 37 | 37 | 37 | 37 | 38 | 38 | 38 | 37 | 38 |
| Median score (IQR) | 2 (1–2) | 2 (2–3) | 1 (1–1) | 1 (1–2) | 7 (5–8) | 2 (1–2) | 2 (2–2) | 1 (1–2) | 2 (1–2) | 6 (6–8) |
| Improvement (%) | 14 (n = 6) | 5 (n = 2) | 2 (n = 1) | 21 (n = 9) | 12(n = 5) | 14 (n = 6) | 20 (n = 9) | 16 (n = 7) | 16 (n = 7) | 27 (n = 12) |
| Month 3 | ||||||||||
| n | 32 | 32 | 32 | 32 | 32 | 36 | 36 | 36 | 36 | 36 |
| Median score (IQR) | 2 (1–2) | 2 (1–3) | 1 (1–2) | 2 (1–2) | 6 (5–8.5) | 2 (1–2) | 2 (1–2) | 1 (1–1) | 1 (1–2) | 6 (5–8) |
| Improvement (%) | 12 (n = 5) | 17 (n = 7) | 5 (n = 2) | 14 (n = 6) | 21 (n = 9) | 20 (n = 9) | 41 (n = 18) | 18 (n = 8) | 39 (n = 17) | 50 (n = 22) |
| Month 6 | ||||||||||
| n | 31 | 31 | 31 | 31 | 31 | 35 | 35 | 35 | 35 | 35 |
| Median score (IQR) | 2 (1–2) | 2 (1–2) | 1 (0–2) | 1 (1–2) | 6 (4–8) | 2 (1–2) | 1 (1–2) | 1 (0–1) | 1 (1–1) | 5 (4–6) |
| Improvement (%) | 24 (n = 10) | 26 (n = 11) | 12 (n = 5) | 31 (n = 13) | 48 (n = 20) | 27 (n = 12) | 45 (n = 20) | 25 (n = 11) | 45 (n = 20) | 55 (n = 24) |
| Month 12 | ||||||||||
| n | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| Median score (IQR) | 1 (0–2) | 1 (1–2) | 1 (0–1) | 1 (1–1) | 4 (3–5) | 2 (1–2) | 1 (1–1) | 0 (0–1) | 1 (0–1) | 4 (2–5) |
| Improvement (%) | 21 (n = 9) | 29 (n = 12) | 12 (n = 5) | 33 (n = 14) | 40 (n = 17) | 20 (n = 9) | 34 (n = 15) | 25 (n = 11) | 32 (n = 14) | 39 (n = 17) |
| Time point | Treatment arm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No PGT (n = 42) | PGT (n = 44) | |||||||||||||
| Vascularity | Pigmentation | Thickness | Relief | Pliability | Surface Area | Overall Opinion | Vascularity | Pigmentation | Thickness | Relief | Pliability | Surface Area | Overall Opinion | |
| Baseline | ||||||||||||||
| n | 42 | 42 | 42 | 41 | 42 | 37 | 42 | 43 | 43 | 43 | 43 | 43 | 39 | 43 |
| Median score (IQR) | 6 (4–7) | 3 (2–6) | 3 (2–4) | 3 (2–4) | 3 (3–4) | 1 (1–3) | 4 (3–5) | 6 (4–7) | 4 (2–7) | 3 (2–5) | 4 (2–5) | 4 (2–5) | 1 (1–2) | 5 (4–6) |
| Week 1 | ||||||||||||||
| n | 35 | 35 | 35 | 35 | 35 | 31 | 34 | 41 | 41 | 41 | 41 | 41 | 39 | 41 |
| Median score (IQR) | 6 (4–6) | 4 (2–6) | 3 (2–4) | 4 (2–5) | 4 (3–5) | 1 (1–3) | 5 (4–6) | 6 (5–7) | 4 (2–6) | 4 (2–5) | 4 (3–5) | 4 (3–6) | 1 (1–2) | 5 (4–6) |
| Improvement (%) | 24 (n = 10) | 24 (n = 10) | 17 (n = 7) | 12 (n = 5) | 17 (n = 7) | 7 (n = 3) | 17 (n = 7) | 39 (n = 17) | 27 (n = 12) | 20 (n = 9) | 25 (n = 11) | 30 (n = 13) | 16 (n = 7) | 23 (n = 10) |
| Month 1 | ||||||||||||||
| n | 37 | 37 | 37 | 37 | 37 | 35 | 35 | 39 | 39 | 39 | 39 | 39 | 38 | 39 |
| Median score (IQR) | 5 (4–7) | 4 (2–5) | 4 (3–5) | 4 (3–6) | 4 (3– 5) | 1 (1–2) | 4 (4–6) | 5 (4–7) | 4 (2–5) | 3 (2–5) | 3 (2–5) | 4 (2–5) | 1 (1–2) | 4 (4–6) |
| Improvement (%) | 31 (n = 13) | 21 (n = 9) | 12 (n = 5) | 10 (n = 4) | 21 (n = 9) | 14 (n = 6) | 17 (n = 7) | 39 (n = 17) | 39 (n = 17) | 34 (n = 15) | 36 (n = 16) | 30 (n = 13) | 14 (n = 6) | 32 (n = 14) |
| Month 3 | ||||||||||||||
| n | 31 | 31 | 31 | 31 | 31 | 30 | 31 | 36 | 36 | 36 | 36 | 36 | 35 | 36 |
| Median score (IQR) | 5 (4–6) | 3 (3–4) | 3 (2–4) | 4 (2–5) | 3 (2–5) | 1 (1–2) | 4 (3–5) | 4.5 (3–5.5) | 4 (2–5) | 3 (2–3.5) | 3 (2–5) | 3 (2–5) | 1 (1–1) | 4 (3–5) |
| Improvement (%) | 45 (n = 19) | 29 (n = 12) | 7 (n = 3) | 19 (n = 8) | 24 (n = 10) | 21 (n = 9) | 36 (n = 15) | 64 (n = 28) | 43 (n = 19) | 45 (n = 20) | 43 (n = 19) | 41 (n = 18) | 18 (n = 8) | 45 (n = 20) |
| Month 6 | ||||||||||||||
| n | 31 | 31 | 31 | 31 | 31 | 29 | 31 | 35 | 35 | 35 | 35 | 35 | 34 | 35 |
| Median score (IQR) | 4 (2–5) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 1 (1–1) | 4 (3–4) | 3 (2–4) | 3 (2–5) | 2 (2–3) | 3 (1–4) | 3 (2–3) | 1 (1–2) | 3 (2–4) |
| Improvement (%) | 57 (n = 24) | 40 (n = 17) | 21 (n = 9) | 29 (n = 12) | 36 (n = 15) | 24 (n = 10) | 48 (n = 20) | 59 (n = 26) | 48 (n = 21) | 45 (n = 20) | 50 (n = 22) | 52 (n = 23) | 18 (n = 8) | 66 (n = 29) |
| Month 12 | ||||||||||||||
| n | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 20 | 20 | 20 | 20 | 20 | 20 |
| Median score (IQR) | 3 (2–4) | 2 (1–3) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 1 (1–1) | 2 (2–4) | 2 (1–3) | 2 (2–3.5) | 2 (2–2) | 2 (1.5–2.5) | 2 (2–2.5) | 1 (1–1) | 2 (2–3) |
| Improvement (%) | 45 (n = 19) | 29 (n = 12) | 21 (n = 9) | 26 (n = 11) | 31 (n = 13) | 14 (n = 6) | 36 (n = 15) | 45 (n = 20) | 32 (n = 14) | 34 (n = 15) | 39 (n = 17) | 36 (n = 16) | 9 (n = 4) | 41 (n = 18) |
| Time point | Treatment arm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No PGT (n = 42) | PGT (n = 44) | |||||||||||||
| Painful | Itching | Colour | Stiffness | Thickness | Irregular | Overall | Painful | Itching | Colour | Stiffness | Thickness | Irregular | Overall | |
| Baseline | ||||||||||||||
| n | 27 | 27 | 27 | 27 | 26 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| Median score (IQR) | 3 (1–6) | 4 (2–7) | 8 (5–10) | 8 (5–8) | 4 (3–6) | 5 (4–8) | 7 (5–8) | 3 (2–5) | 4 (2–7) | 8 (6–10) | 7 (5–8) | 5 (4–8) | 7 (4–9) | 9 (6–10) |
| Week 1 | ||||||||||||||
| n | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| Median score (IQR) | 3 (2–5) | 3 (2–6) | 7 (5–10) | 5 (5–8) | 5 (4–6) | 6 (5–7) | 6 (4–8) | 3 (2–6) | 4 (3–8) | 7 (5–9) | 5 (4–8) | 7 (4–8) | 7 (5–8) | 7 (5–9) |
| Improvement (%) | 32 (n = 9) | 39 (n = 11) | 29 (n = 8) | 39 (n = 11) | 25 (n = 7) | 32 (n = 9) | 39 (n = 11) | 32 (n = 9) | 21 (n = 6) | 46 (n = 13) | 39 (n = 11) | 32 (n = 9) | 32 (n = 9) | 32 (n = 9) |
| Month 1 | ||||||||||||||
| n | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Median score (IQR) | 2 (1–5) | 3 (1–4) | 5 (4–9) | 5.5 (4–8) | 6 (4–8) | 5.5 (4–8) | 5 (4–8) | 2.5 (1.5–5) | 5 (2–6) | 8 (6–8.5) | 6 (5–7) | 5 (3.5–7.5) | 7 (5–8) | 5.5 (5–7.5) |
| Improvement (%) | 36 (n = 10) | 43 (n = 12) | 32 (n = 9) | 43 (n = 12) | 18 (n = 5) | 36 (n = 10) | 43 (n = 12) | 43 (n = 12) | 32 (n = 9) | 39 (n = 11) | 43 (n = 12) | 39 (n = 11) | 32 (n = 9) | 54 (n = 15) |
| Month 3 | ||||||||||||||
| n | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 23 | 23 | 23 | 23 | 23 | 23 | 23 |
| Median score (IQR) | 1 (1–2) | 3 (1–4) | 6 (4–8) | 5 (3–7) | 4 (3–7) | 5 (3–7) | 5 (3–7) | 2 (1–4) | 4 (1–6) | 5 (5–6) | 6 (4–7) | 5 (3–6) | 5 (3–7) | 5 (5–7) |
| Improvement (%) | 39 (n = 11) | 46 (n = 13) | 36 (n = 10) | 50 (n = 14) | 29 (n = 8) | 29 (n = 8) | 43 (n = 12) | 39 (n = 11) | 32 (n = 9) | 61 (n = 17) | 46 (n = 13) | 50 (n = 14) | 50 (n = 14) | 54 (n = 15) |
| Month 6 | ||||||||||||||
| n | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 21 | 21 | 22 | 22 | 22 | 22 | 22 |
| Median score (IQR) | 2 (1–2) | 2 (1–5) | 4 (3–7) | 4 (3–5) | 4 (3–6) | 5 (3–9) | 4 (3–7) | 1 (1–5) | 3 (2–5) | 4.5 (3–8) | 4 (3–5) | 4 (3–6) | 3 (2–6) | 5 (3–7) |
| Improvement (%) | 39 (n = 11) | 39 (n = 11) | 50 (n = 14) | 46 (n = 13) | 39 (n = 11) | 36 (n = 10) | 46 (n = 13) | 43 (n = 12) | 36 (n = 10) | 54 (n = 15) | 57 (n = 16) | 57 (n = 16) | 57 (n = 16) | 57 (n = 16) |
| Month 12 | ||||||||||||||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Median score (IQR) | 1 (1–2) | 3 (2–4) | 2.5 (2–4) | 3 (2–4) | 3 (1–4) | 2.5 (2–5) | 3 (2–4) | 1.5 (1–2) | 1.5 (1–4) | 5.5 (4–8) | 4.5 (2–5) | 3 (1–4) | 5.5 (2–8) | 5 (3–7) |
| Improvement (%) | 25 (n = 7) | 21 (n = 6) | 36 (n = 10) | 25 (n = 7) | 21 (n = 6) | 25 (n = 7) | 36 (n = 10) | 25 (n = 7) | 25 (n = 7) | 21 (n = 6) | 21 (n = 6) | 21 (n = 6) | 25 (n = 7) | 29 (n = 8) |
| Time point | Scale | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VSS (0–14) | POSAS | ||||||||
| Observer (1–10) | Adult patient (1–10) | ||||||||
| No PGT | PGT | Difference in mean scores | No PGT | PGT | Difference in mean scores | No PGT | PGT | Difference in mean scores | |
| Baseline | |||||||||
| Mean score | 5.74 (n = 42) | 6.58 (n = 43) | –0.84 | 4.33 (n = 42) | 4.82 (n = 44) | –0.48 | 6.56 (n = 27) | 7.57 (n = 28) | –1.02 |
| 95% CI | 5.00 to 6.48 | 5.90 to 7.27 | –1.84 to 0.15 | 3.76 to 4.91 | 4.28 to 5.35 | –1.26 to 0.29 | 5.55 to 7.56 | 6.55 to 8.60 | –2.42 to 0.39 |
| Week 1 | |||||||||
| Mean score | 6.20 (n = 35) | 6.80 (n = 41) | –0.60 | 4.79 (n = 34) | 4.85 (n = 41) | –0.06 | 6.27 (n = 22) | 7.26 (n = 27) | –0.99 |
| 95% CI | 5.49 to 6.91 | 6.21 to 7.40 | –1.51 to 0.30 | 4.18 to 5.41 | 4.35 to 5.36 | –0.83 to 0.71 | 5.10 to 7.44 | 6.41 to 8.11 | –2.40 to 0.42 |
| Month 1 | |||||||||
| Mean score | 6.84 (n = 37) | 6.58 (n = 38) | 0.26 | 4.77 (n = 35) | 4.51 (n = 39) | 0.26 | 5.55 (n = 22) | 6.17 (n = 24) | –0.62 |
| 95% CI | 6.06 to 7.61 | 6.06 to 7.10 | –0.65 to 1.17 | 4.22 to 5.32 | 4.01 to 5.01 | –0.47 to 0.99 | 4.28 to 6.81 | 5.23 to 7.10 | –2.13 to 0.89 |
| Month 3 | |||||||||
| Mean score | 6.53 (n = 32) | 5.86 (n = 36) | 0.67 | 4.23 (n = 31) | 4.03 (n = 36) | 0.20 | 5.11 (n = 18) | 5.57 (n = 23) | –0.45 |
| 95% CI | 5.59 to 7.47 | 5.06 to 6.66 | –0.53 to 1.87 | 3.59 to 4.86 | 3.44 to 4.61 | –0.65 to 1.04 | 3.84 to 6.39 | 4.67 to 6.46 | –1.92 to 1.01 |
| Month 6 | |||||||||
| Mean score | 5.68 (n = 31) | 5.14 (n = 35) | 0.53 | 3.74 (n = 31) | 3.34 (n = 35) | 0.40 | 4.88 (n = 17) | 5.09 (n = 22) | –0.21 |
| 95% CI | 4.82 to 6.54 | 4.49 to 5.80 | –0.51 to 1.58 | 3.19 to 4.29 | 2.91 to 3.78 | –0.28 to 1.08 | 3.49 to 6.28 | 4.02 to 6.16 | –1.87 to 1.46 |
| Month 12 | |||||||||
| Mean score | 3.90 (n = 21) | 3.90 (n = 21) | 0.00 | 2.76 (n = 21) | 2.70 (n = 20) | 0.06 | 3.30 (n = 10) | 5.10 (n = 10) | –1.80 |
| 95% CI | 3.09 to 4.72 | 2.75 to 5.06 | –1.37 to 1.37 | 2.21 to 3.32 | 1.95 to 3.44 | –0.83 to 0.96 | 2.04 to 4.56 | 3.06 to 7.14 | –4.03 to 0.43 |
List of abbreviations
- BBSIP
- Brisbane Burn Scar Impact Profile
- BCH
- Birmingham Children’s Hospital
- BCTU
- Birmingham Clinical Trials Unit
- CAR
- Centre for Appearance Research
- CHU-9D
- Child Health Utility Index 9D
- CI
- confidence interval
- CRF
- case report form
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HCP
- health-care professional
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LTFU
- lost to follow-up
- MCID
- minimal clinically important difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OT
- occupational therapist
- P1
- phase 1
- P2
- phase 2
- PedsQL
- Pediatric Quality of Life Inventory
- PEGASUS
- A mixed-methods feasibility study including a randomised trial of PrEssure Garment therApy for the prevention of abnormal Scarring after bUrn injury in adultS and children
- PGT
- pressure garment therapy
- PI
- principal investigator
- PIL
- patient information leaflet
- POSAS
- Patient and Observer Scar Assessment Scale
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- QoL
- quality of life
- RCT
- randomised controlled trial
- RN
- research nurse
- ROM
- range of movement
- SD
- standard deviation
- T1
- time point 1
- T2
- time point 2
- TBSA
- total body surface area
- TMG
- Trial Management Group
- UHB
- University Hospitals Birmingham
- VSS
- Vancouver Scar Scale
- WTP
- willingness to pay
