Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/32/02. The contractual start date was in March 2012. The draft report began editorial review in June 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lee David is the director of 10 Minute CBT. She helped to develop Pedometer And Consultation Evaluation-UP (PACE-UP) patient resources and training for the PACE-UP nurses and received personal fees from 10 Minute CBT during the conduct of this study. Tess Harris is a member of the National Institute for Health Research (NIHR) Health Technology Assessment Primary Care and Community Preventative Interventions panel. Julia Fox-Rushby reports grants from the NIHR and membership of the Public Health Research Research Funding Board during the conduct of the study. Katy Morgan’s salary was funded through a NIHR research methods fellowship (reference number MET-12-16), during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Harris et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
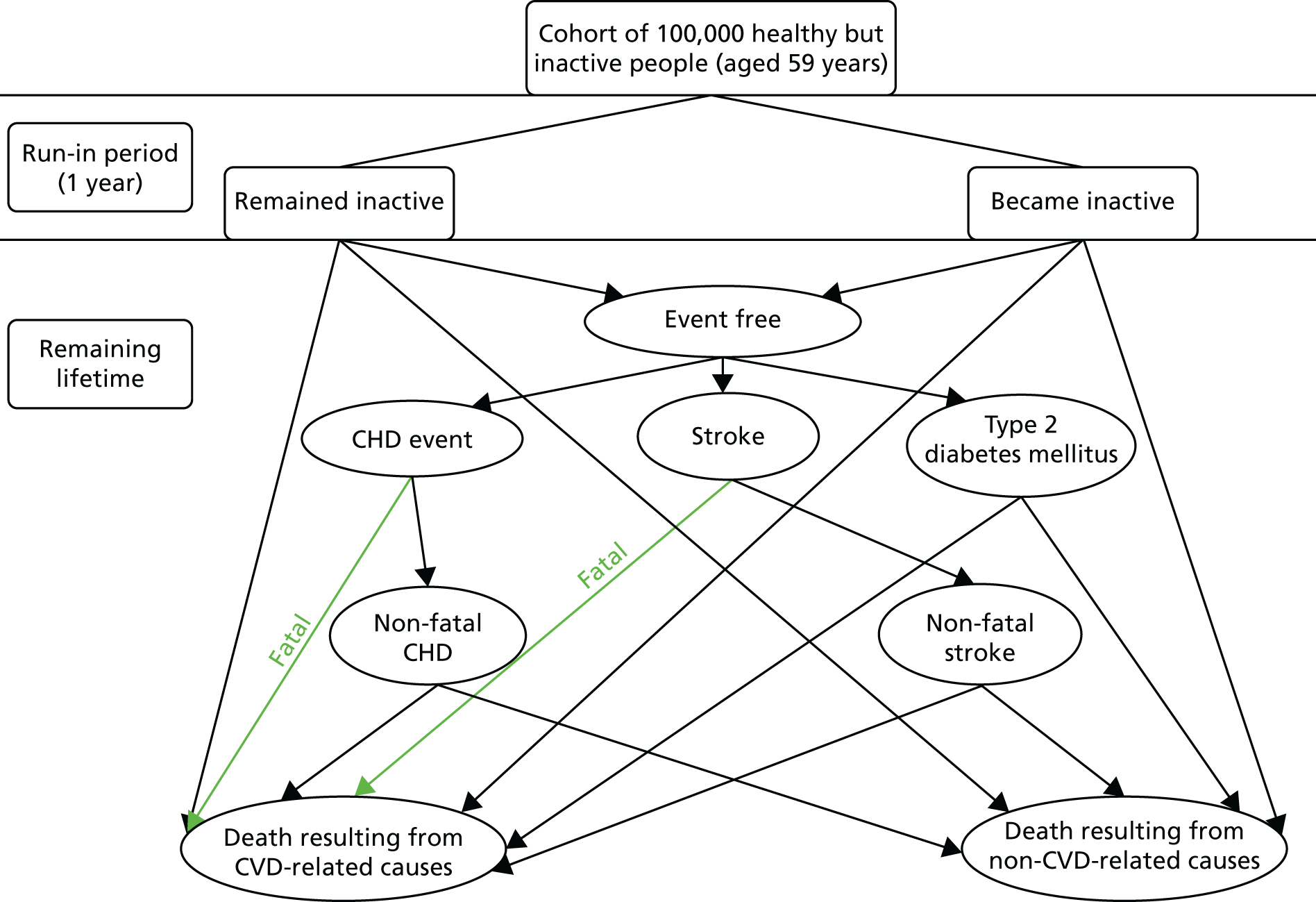
Chapter 1 Introduction: why this study was needed
Benefits and risks of physical activity and current physical activity guidelines
What are the benefits of physical activity for adults and older adults?
Physical activity (PA) leads to reduced mortality, a reduced risk of > 20 diseases and conditions, and improved function, quality of life and emotional well-being. 1 Physical inactivity is the fourth leading risk factor for global mortality;2 in 2008, it was estimated to have caused 9% of premature mortality and 5.3 million deaths worldwide. 3 Physical inactivity is also a major cost burden on health services. 1,4,5
What are the current physical activity guidelines and who is achieving them?
Adults and older adults are advised to be active daily and, for health benefits, should achieve at least 150 minutes (2.5 hours) weekly of at least moderate-intensity activity [moderate to vigorous PA (MVPA)] or 75 minutes of vigorous-intensity PA, or an equivalent combination, achieved in bouts of at least 10 minutes’ duration. 1,2 Muscle-strengthening activities are also recommended on at least 2 days weekly,1,2 but are not part of our intervention, which is focused on increasing walking. One effective way to achieve the aerobic PA recommendations is by undertaking 30 minutes of moderate-intensity PA on at least 5 days weekly. 1,6,7 Regular walking is the most common PA of adults and older adults, and walking at a moderate pace (3 miles or 5 km per hour) qualifies as moderate-intensity PA. 8 Time spent being sedentary for extended periods should also be minimised, as this is an independent disease risk factor,1 which increases steeply with age from 45 years. 9 There is an increasing awareness that as a dose–response relationship exists for PA and health benefits, getting inactive people to do a little more PA is also important, rather than just relying on trying to achieve PA recommendations. 10,11 Emphasising that the 30 minutes can be built up from 10-minute bouts is an important message for older adults and those with disabilities, enabling them to increase their MVPA gradually. Among adults in England aged ≥ 19 years, 66% of men and 56% of women self-reported meeting the recommended PA levels, whereas only 58% of men and 52% of women aged 60–74 years did so. 12 Lower socioeconomic groups9 and Indian, Pakistani, Bangladeshi and Chinese ethnic groups are significantly less likely to report meeting the recommended PA levels, whereas the activity levels of other ethnic groups (black Caribbean, black African and Irish) are similar to that of the general population. 13 Over one-third of adults worldwide are insufficiently active, but there is large geographical variation. 10,14 However, PA, including walking, is very unreliably recalled, so surveys overestimate PA levels. 15 Objective accelerometry found that only 5% of men and 4% of women aged 35–64 years and 5% of men and 0% of women aged ≥ 65 years achieved the recommended PA levels, which is a fraction of those who self-report achieving them. 9
What are the risks from increasing physical activity?
The risks from a sedentary lifestyle far exceed the risks from regular PA. 6,16,17 Moderate-intensity PA carries a low injury risk,18 mainly musculoskeletal injury or falls. 19 Walking is very low risk, ‘a near perfect exercise’. 8 Screening participants for contraindications before participating in light- to moderate-intensity PA programmes is no longer advocated. 6,20 An important safety feature of our study is that individualised goals can be set from the participant’s own baseline, in line with the advice that older adults in particular should start with low-intensity PA and increase the intensity gradually: the ‘start low and go slow’ approach. 16,17 This worked well with our previous PA trial in older adults, which employed a similar approach and showed no increase in adverse events (AEs). 21
Strategies for increasing physical activity
How can adults and older adults increase their physical activity levels?
A systematic review of PA interventions reported moderately positive short-term effects, but the findings were limited by mainly unreliable self-report measures in motivated volunteers. 22 This review has recently been updated by three complementary Cochrane reviews assessing (1) face-to-face PA interventions,23 (2) remote (including postal and telephone) and web 2.0 interventions24 and (3) a direct comparison of these two approaches. 23 Evidence supports the effectiveness of both face-to-face interventions and remote or web 2.0 interventions for promoting PA. However, the reviewers called for future studies to provide greater detail of the components of face-to-face interventions and to assess the impact on quality of life, AEs and economic data,23 and to include participants from varying socioeconomic and ethnic groups. 24 Only one study25 met the review criteria to compare face-to-face interventions with remote or web 2.0 interventions (many trials were excluded as a result of having less than 1 year’s follow-up data or an inadequate control group); this study showed no effect on cardiorespiratory fitness25 and there were no reported data for PA, quality of life or cost-effectiveness. 23 The review concluded that there was insufficient evidence to assess whether face-to-face interventions or remote and web 2.0 approaches are more effective at promoting PA, and called for more high-quality comparative studies. 23 None of the studies included in the reviews provided objective PA measurement. 23,24 Other studies have concluded that exercise programmes in diverse populations can promote short- to medium-term increases in PA when interventions are based on health behaviour theoretical constructs, individually tailored with personalised activity goals and using behavioural strategies. 26,27 A critical review and a best-practice statement on older people’s PA interventions advised home-based rather than gym-based programmes, and behavioural strategies (e.g. goal-setting, self-monitoring, self-efficacy, support, relapse prevention training), rather than health education alone. 17,27 National Institute for Health and Care Excellence (NICE)’s guidance concluded that no particular behaviour change model was superior and that training should focus on generic competencies and skills, rather than on specific models. 28 More recent complementary NICE guidance specifically recommended that goals, planning, feedback and monitoring techniques should be included in behaviour change interventions. 29 Starting low, but gradually increasing to moderate intensity is promoted as best practice, with advice to incorporate interventions into the daily routine (e.g. walking). 17 A recent systematic review of walking interventions concluded that interventions tailored to people’s needs, targeted at the most sedentary people and delivered at the level of the individual or household, can be effective, although evidence directly comparing interventions targeted at individuals, couples or households was lacking. 30
Are pedometers helpful?
Pedometers are small, cheap devices, worn at the hip, providing direct step count feedback. A systematic review (of 26 studies) found that pedometers increased steps per day by 2491 steps [95% confidence interval (CI) 1098 to 3885 steps] and PA levels by 27%, with significant reductions in body mass index (BMI) and blood pressure. 31 A second review (of 32 studies) found an average increase of 2000 steps per day. 32 Step count goals and diaries were key factors. 31,32 Several limitations were recognised: study sizes were small and long-term effects were undetermined; many studies included several components (e.g. pedometer and support), so independent effects were difficult to establish; and the inclusion of older people and men was limited. 31,32 Recent studies have addressed some of these limitations. A pedometer plus behaviour change intervention increased PA at 3 months, but not at 6 months, in 210 older women, with pedometers providing no additional benefit. 33 Two trials in high-risk groups showed sustained step count increases at 12 months. 34,35 A recent study of 298 older adults found a significant increase in both step counts and time in MVPA at 3 months and 12 months from a practice nurse-delivered pedometer-based walking intervention, but did not separate out pedometer- and nurse support-related effects. 21 NICE recently updated its advice from advising the use of pedometers only as part of research36 to advising their use as part of packages, including support to set realistic goals, monitoring and feedback. 37
How do step count goals relate to physical activity recommendations?
Step count goals lead to more effective interventions, but no specific approach to goal-setting is favoured. 28 Goals are based on a fixed target (e.g. 10,000 steps per day)38,39 or on advising incremental increases from the baseline as a percentage (5% per week,40 10% biweekly41 or 20% monthly33) or on a fixed number of extra steps. Those advocating a fixed number of extra daily steps have developed step-based guidelines to fit with existing evidence-based guidelines with an emphasis on 30 minutes of MVPA on ≥ 5 days weekly. 42 Despite individual variation, moderate-intensity walking appears to be approximately equal to at least 100 steps per minute. 42,43 Multiplied by 30 minutes, this produces a minimum of 3000 steps per day, to be done over and above habitual activity, which is the ‘3000 in 30’ message. 43 Several studies have advocated adding in 3000 steps per day on most days weekly, either from the beginning34 or by increasing incrementally (initially an extra 1500 steps per day and increasing),44,45 or by increasing by 500 steps per day biweekly. 35 Studies that advised adding 3000 steps per day from the baseline produced significant improvements in step counts at 3 months and two measured outcomes at 12 months, and showed sustained improvements in step counts,34,35 waist circumference34 and fasting glucose levels,35 but no sustained improvements to date in MVPA levels. Although there is no evidence at present to inform a moderate-intensity cadence (steps per minute) in older adults, Tudor-Locke et al. 46 advocate using the adult cadence of 100 steps per minute in older adults (while recognising that this may be unobtainable for some individuals) and advising that the 30 minutes can be broken down into bouts of at least 10 minutes. This model was used in a primary care walking intervention in 41 older people, which found significant step count increases from baseline to week 12, which were maintained at week 24. 47,48
Could accelerometers be useful in a pedometer-based walking intervention?
Accelerometers are small activity monitors worn like pedometers, but are more expensive; however, they are able to provide a time-stamped record of PA frequency (step counts) and intensity (activity counts). They require computer analysis, function as blinded pedometers in objectively measuring baseline and outcome data, and provide objective data on time spent in different PA intensities, including time spent in MVPA and time spent being sedentary, two important public health outcomes. Pedometer studies without accelerometers have relied on self-reported measures of these outcomes. Accelerometers are valid and acceptable to adults9,49 and older adults. 9,21,50–53 Although both instruments measure step count and are highly correlated,50 pedometers usually record lower step counts, and accelerometers cannot reliably be substituted for pedometers at an individual level. 54 Thus, although we used the accelerometer to measure outcomes, including step count, MVPA and sedentary time, we used the blinded pedometer, worn simultaneously at baseline, to set individual step count targets.
Are pedometers cost-effective?
There is limited knowledge on the cost-effectiveness of pedometer-based interventions in the UK. Recent systematic reviews that considered the economic outcomes of pedometer-based interventions found no evidence,55,56 partly because of an insufficient number of data. 57 However, a recent study assessed the cost-effectiveness of giving an individualised walking programme and pedometer with or without a PA consultation alongside a community-based trial of 79 people. 58 The incremental cost-effectiveness ratios (ICERs) per persons achieving an additional 15,000 steps per week were £591 and £92 with and without the consultation, respectively. However, even with this highly selected sample, no data on quality of life were collected, and the impacts on long-term outcomes were not estimated.
What is the role of primary care in promoting physical activity?
Primary care centres (general practices) in the UK provide health care and health promotion, free at the point of access, to a registered list of local patients (for many of whom PA will be of benefit), using disease registers to provide annual or more frequent chronic disease reviews via a multidisciplinary health-care team providing continuity of care. NICE guidance found that brief interventions in primary care are cost-effective, and it therefore recommends that all primary care practitioners should take the opportunity to identify inactive adults and provide advice on increasing PA levels. 36 New 5-yearly NHS health checks include adults aged 40–74 years and incorporate advice on increasing PA, often from primary care nurses. 59 Primary care nurses are effective at increasing PA, particularly walking, in this age group. 60 Not only can PA advice through consultation with health professionals be individually tailored61 and have more impact than other PA advice,62 but this is particularly the case for older adults,63 especially given the uncertainty about the effectiveness of exercise referral schemes from primary care. 64 Exercise-prescribing guidance in primary care reinforces the importance of follow-up to chart progress, set goals, solve problems, and identify and use social support;65 this will be an important feature of the nurse PA consultations in this trial. Evaluation of the UK Step-O-Metre programme, delivering pedometers through primary care, showed self-reported PA increases, but advised investigation with a randomised controlled trial (RCT) design. 44 Two trials, both in older primary care patients, have assessed the effectiveness of pedometers plus primary care PA consultations; one small trial (n = 41) showed a significant effect on step counts at 3 months, which was maintained at 6 months,47,48 and the other was our recent Pedometer Accelerometer Evaluation-LIFT (PACE-LIFT) trial21 (n = 298), which showed differences in both step counts and time in MVPA in bouts of at least 10 minutes, at 3 and 12 months for the nurse intervention compared with the control group. Neither trial separated out pedometer effects from the support provided. 21,47,48
Theoretical base, piloting and preparatory work to develop the intervention
The pedometer-based intervention is based on work cited above,31,32 showing that pedometers can increase step counts and PA intensity. 31,32 It extends current understanding by also including older adults and men, having a 12-month follow-up and ensuring that the pedometer and support components could be evaluated separately. The patient handbook, diary (both available on the journals library website: www.journalslibrary.nihr.ac.uk/programmes/hta/103202/#/) and practice nurse PA consultations use behaviour change techniques (BCTs; e.g. goal-setting, self-monitoring, feedback, boosting motivation, encouraging social support, addressing barriers or relapse anticipation). These techniques have been successfully used by non-specialists in primary care after brief training,66 and are emphasised in the Improving Health: Changing Behaviour: NHS Health Trainer Handbook,67 based on evidence from a range of psychological methods and intended for NHS behaviour change programmes, with local adaptation. 67 They also include techniques specifically recommended to be included in more recent NICE guidance (goals, planning, feedback and monitoring). 29 We adapted the Improving Health: Changing Behaviour: NHS Health Trainer Handbook67 for use in this trial into Pedometer And Consultation Evaluation-UP (PACE-UP) nurse and patient handbooks, to focus specifically on PA using pedometers. The BCTs were classified in accordance with the refined taxonomy of BCTs for PA interventions by Michie et al. 68 Diary recording of pedometer step counts provides clear material for PA goal-setting, self-monitoring and feedback, and should fit well with this approach. We have adopted the approach used by others44,45 of advocating adding in 3000 steps per day to an individual’s baseline on most days weekly in an incremental manner, and of advising on gradually increasing PA intensity to achieve more time in MVPA, with the message that 3000 steps in 30 minutes will help people to achieve PA guidelines. 43 Relevant pilot and preparatory work includes observational work using pedometers and accelerometers in primary care53 and a successful trial with older primary care patients developing the PA consultations and pedometer-based walking intervention (the PACE-LIFT trial; ISRCTN4212256121,69). The PACE-LIFT trial demonstrated that tailored support from practice nurse PA consultations combined with a pedometer-based walking programme (plus accelerometer feedback on PA intensity) led to an increase in both step counts and time in MVPA compared with the control group at both 3 and 12 months in 60- to 75-year-old primary care patients. The trial was limited in terms of both ethnic and socioeconomic diversity, has not yet published on sedentary time or cost-effectiveness and, as mentioned, was unable to separate out the effects of the pedometer (and accelerometer feedback) from the effects of nurse support. 21
Rationale for research
The PACE-UP trial aimed to fill the gaps in the current evidence base by evaluating the effect of a pedometer-based walking intervention, with and without additional nurse PA consultations in a population-based, primary care sample of inactive adults and older adults. The initial trial included follow-up to 1 year and aimed to ensure that adequate numbers of men, older adults and individuals from diverse socioeconomic and ethnic backgrounds were included. It also enabled the effectiveness of taking part as an individual or as a couple to be estimated. The intervention used step goals and diaries, and the PA consultations and patient handbook were based on BCTs, such as those used in the Improving Health: Changing Behaviour: NHS Health Trainer Handbook. 67 To objectively test the effectiveness of the intervention on important public health outcomes, such as time spent in MVPA and time spent being sedentary, PA outcomes were assessed by accelerometry. Anonymised practice demographic data were available for all those invited to participate, enabling the investigation of inequalities in trial participation. Qualitative evaluations were also needed to explore the reasons for trial non-participation, the acceptability of the intervention to both participants and practice nurses and the barriers to, and facilitators of, the intervention. An economic evaluation was performed alongside the trial and was also used to inform long-term cost-effectiveness.
Chapter 2 Methods
This chapter is a summary of the full study protocol for the trial as originally funded, except for the paragraph which describes changes to the published protocol. Some of the material, including the tables, has already appeared in publication,70 and is reproduced here under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article,70 unless otherwise stated. Further funding was later awarded by the National Institute for Health Research (NIHR) Health Technology Assessment programme for a 3-year follow-up of the trial cohort, the methods and results of which are described in Chapter 8.
Study design
The PACE-UP walking intervention trial was a pragmatic, three-arm parallel-cluster trial (randomised by household to allow individuals and couples to participate). It was based in primary care with 45- to 75-year-old inactive adults, with a 12-month follow-up period, and compared the following three groups: control (usual PA); pedometer and written instructions by post (pedometer by post); and pedometer, written instructions and practice nurse individually tailored PA consultations (pedometer plus nurse support).
Study aims and objectives
Study aims
The main hypotheses to be addressed were as follows:
-
Does a 3-month postal pedometer-based walking intervention increase PA in inactive 45- to 75-year-olds at the 12-month follow-up point?
-
Does providing practice nurse support through dedicated PA consultations provide additional benefit?
The study also aimed to assess the cost-effectiveness of both interventions, whether or not any factors modified the intervention effects and the effect of the interventions on patient-reported outcomes, anthropometric measures and primary care-recorded AEs.
Primary objectives (relating to the primary outcome of step counts)
In inactive adults aged 45–75 years, the primary objectives were to:
-
confirm that tailored support from practice nurse PA consultations combined with a pedometer-based walking programme can promote an increase in step counts compared with the control group at 12 months (pedometer plus nurse support vs. control)
-
determine whether or not the simple provision by post of pedometers plus written instructions for a pedometer-based walking programme can promote an increase in step counts compared with the control group at 12 months (pedometer by post vs. control)
-
estimate the effect of tailored support from practice nurse PA consultations combined with a pedometer-based walking programme compared with the postal pedometer-based walking programme, on step counts at 12 months (pedometer plus nurse support vs. pedometer by post).
Secondary objectives (relating to secondary outcomes of time in moderate to vigorous physical activity in bouts, sedentary time and cost-effectiveness)
In inactive adults aged 45–75 years, the secondary objectives were to:
-
confirm that tailored support from practice nurse PA consultations combined with a pedometer-based walking programme can promote an increase in steps counts at 3 months and time spent in MVPA in ≥ 10-minute bouts at 3 and 12 months, and a decrease in sedentary time at 3 and 12 months compared with control (pedometer plus nurse support vs. control)
-
determine whether or not the simple provision by post of pedometers plus written instructions for a pedometer-based walking programme can promote an increase in step counts at 3 months, an increase in time spent in MVPA in ≥ 10-minute bouts at 3 and 12 months and a decrease in sedentary time at 3 and 12 months compared with the control group (pedometer by post vs. control)
-
estimate the effect of tailored support from practice nurse PA consultations in addition to the pedometer-based walking programme alone on step counts at 3 months, and time spent in MVPA in ≥ 10-minute bouts and sedentary time at 3 and 12 months (pedometer plus nurse support vs. pedometer by post)
-
determine the cost-effectiveness of these alternative approaches to increasing PA levels at both 12 months and from a lifetime perspective from the viewpoint of the NHS and participants (see Chapter 4).
Other objectives
-
To determine the effect of the interventions on anthropometric measures (BMI, waist circumference and body fat) at 12 months.
-
To determine the effect of the interventions on patient-reported outcomes (self-reported PA levels, anxiety and depression score, exercise self-efficacy, quality of life, pain, AEs) and on primary care-recorded AEs at 3 months and 12 months.
-
To determine whether or not age groups (< 60 years vs. ≥ 60 years), sex, taking part as a couple, socioeconomic group, disability, pain, BMI and exercise self-efficacy modify the effect of the intervention on increasing step count at 3 months and 12 months (ethnic group was originally intended to be included as an effect modifier, but there was inadequate power for this analysis because of the low number of non-white participants; see Changes from the published protocol).
-
To compare the age, sex, socioeconomic group and ethnicity of those taking part in the trial with those invited but not participating, and to explore the reasons for not participating (see Chapter 5).
-
To assess the fidelity and quality of the intervention implementation over time, by the evaluation of patient diary step count goals and recorded step counts for both intervention groups at the 3-month assessment, and the number and timing of recorded practice nurse contacts for the nurse support group (see Chapter 6).
-
To explore the intervention’s acceptability to practice nurses and inactive adults, the reasons for dropout and the durability of effects, by qualitative interviews with participants after the 12-month follow-up, and a focus group with the nurses on study completion (see Chapter 7).
Practice and participant inclusion/exclusion criteria
Practice inclusion criteria
General practices were recruited through the Primary Care Research Network – Greater London. Practices were required to be in the south-west London cluster, have a practice list size of > 9000, give a commitment to participate over the duration of the study, have a practice nurse interested and with time to carry out the PA interventions and trial procedures, and have the availability of a room for the research assistant to recruit participants and carry out baseline and follow-up assessments.
Participant inclusion criteria
Participants were patients aged 45–75 years, who were registered with one of the recruited south-west London general practices, were able to walk outside the home and had no contraindications to increasing their MVPA levels.
Participant exclusion criteria
-
Physical activity based (by screening question on invitation letter). In order to maximise the benefits of the intervention to individuals and the NHS, the trial focused on less-active adults, using a single-item validated questionnaire measure of self-reported PA as a screening question to identify them. 60 Those who reported achieving a minimum of 150 minutes of MVPA weekly1 on their response letter were excluded (participants who, on subsequent baseline accelerometer assessment, were found to be above this PA level were not excluded, as they would be included if this intervention were to be rolled out in primary care).
-
Health based [either by the Read code from primary care records or by general practitioner (GP)/practice nurse opinion, or from the telephone or face-to-face baseline assessment with the research assistant] for the following reasons:
-
housebound or living in a residential or nursing home
-
three or more falls in the previous year, or one or more falls in the previous year requiring medical attention
-
terminal illness
-
dementia or significant cognitive impairment
-
registered blind
-
new-onset chest pain, myocardial infarction, a coronary artery bypass graft or an angioplasty within the last 3 months
-
a medical or psychiatric condition that the GP (or practice nurse) considered to exclude the patient (e.g. acute systemic illness such as pneumonia, acute rheumatoid arthritis, unstable/acute heart failure, significant neurological disease/impairment, unable to move about independently, psychotic illness)
-
pregnancy.
-
Recruitment of practices and participants: informed consent
Practice recruitment
The Primary Care Research Network – Greater London identified practices that fitted the above practice inclusion criteria. Practice recruitment was challenging for a number of reasons, including difficulties in finding practices with sufficient space to accommodate a research assistant on a regular basis, finding practices with nurses willing and with sufficient time to be engaged in delivering the intervention and finding practices that were prepared to provide administrative support. The Primary Care Research Network – Greater London provided us with strong support to recruit practices. Initially, six practices were recruited, with an additional practice added half-way through to boost recruitment. This was necessary, as recruitment at that point was running at just below 10% and we were concerned that we would not achieve the target recruitment from the original six practices within 12 months. The practices were selected to include a range of sociodemographic factors and geographical circumstances based on the practice postcode Index of Multiple Deprivation71 (IMD) scores (at least one practice from each quintile).
Participant recruitment
Practice staff identified patients aged 45–74 years on their primary care electronic patient record system, and, using Read codes and local care home knowledge, excluded ineligible patients (patients were aged 45–74 years when selected, but some were aged 75 years by the time of recruitment or randomisation). A list of potentially eligible patients was produced and ordered by household, with a unique household identifier number. An anonymised list was then used by the research team to create at least four random samples of 400 individuals at each practice. A maximum of two people per household were selected (we were aiming to select couples). If a household had two individuals, one was selected at random, and if the second individual had an age difference of ≤ 15 years, they were also selected; if they fell outside this age range, they were not included. If a household had more than two individuals, one was selected at random, and if there was a second individual aged within ≤ 15 years they were also selected; if there was not a second individual aged within ≤ 15 years, this became an individual household. Each sample list was examined by practice nurses or GPs to ensure trial suitability prior to invitation (see Participant exclusion criteria). Participants were recruited between September 2012 and October 2013, and follow-up was completed by October 2014.
Non-responders and non-participants
See Chapter 5 for more details.
Informed consent
Patients were sent an invitation letter from their own practice, along with a participant information sheet and a screening question on self-reported PA. A reminder invitation was sent if no reply was received after 6 weeks. A log was kept of the response rates from each practice. The decision regarding participation in the study was entirely voluntary. Those interested in participating returned the reply slip, including a response to a single screening question about their usual PA levels. If the participant self-reported as not achieving the PA guidelines,1 the research assistant arranged a baseline appointment for them and ran through the participant information sheet, and handled any questions or concerns that they had. If they were happy to proceed, they signed the study consent form; this form included consent to be contacted for qualitative interviews and consent for their general practice records for the year of the trial to be downloaded after trial follow-up was completed. Participants who had difficulty understanding, speaking or reading English were accompanied by a family member or friend during the research assistant appointment. Participants within a couple could attend together or separately.
Changes from the published protocol
We planned to recruit from six general practices, but to enable target recruitment, a seventh practice was added in December 2012. Changes from protocol-planned analyses70 were approved by the Trial Steering Committee (TSC), prior to analysis. We report MVPA in ≥ 10-minute bouts, as this relates more closely to PA guidelines. 1,2 Only 20% of participants were non-white; ethnic group was therefore excluded from the subgroup analyses, as a result of low power.
Interventions
Table 1 shows the components of the intervention provided to the postal and nurse groups. Table 2 shows the content of the patient handbook and the patient diary and the BCTs that were included in each of them, rated according to Michie et al. ’s CALO-RE taxonomy. 68 Table 3 shows the timing and session content for the three dedicated nurse PA consultations and the BCTs intended to be covered in each session. Figure 1 provides a summary of the 12-week walking programme in terms of steps per day or time spent walking, to be added to each individual’s baseline average daily steps. The training received by the practice nurses in order to deliver the interventions is described in Chapter 6. A figure summarising the trial procedures and complex intervention components is shown in Appendix 1, Figure 17.
| Component | What was provided | Trial arm receiving | Additional details on components |
|---|---|---|---|
| Pedometer | Yamax Digi-Walker (Yamasa Tokei Keiki Co., Ltd, Tokyo, Japan), SW-200 model |
|
Provided direct step count to participants. Required daily manual recording and resetting |
| PACE-UP handbook, 12-week walking plan and step count diarya | Handbook to support the 12-week walking programme. Individualised walking plan (see Figure 1). Diary to record weekly step count and walks for 12 weeks |
|
Baseline average daily step counts (from the blinded pedometer assessment) were used to create individual targets. The 12-week walking programme gradually increased targets to achieve an additional 3000 steps per day (approximately 30 minutes of brisk walking) on ≥ 5 days weekly. Daily step counts and target achievement were recorded in the diary. Table 2 lists BCTs in the PACE-UP handbook and diary |
| Practice nurse-dedicated PA consultations | Three individually tailored consultations. Participants could be seen individually or as a couple | Nurse-support group only | Session timings, content and planned BCTs are shown in Table 3. Sessions reinforced the intervention defined in the diary and the handbook. The nurse consultation allowed some additional BCTs to be used and provided an opportunity to individually tailor the intervention to participants’ needs |
| Component | Guide to content | aBCTs68 |
|---|---|---|
| Patient handbook |
|
1 and 2 4 7, 9 and 16 19 21 4 16, 1, 2, 26, 29 and 35 |
| Patient diary |
|
16 and 21 7, 9, 19 and 26 10, 12 and 13 20 and 29 20 2, 20 and 35 12, 13 and 29 12, 16 and 29 8 38, 17 and 11 9, 21 and 35 16, 29 and 36 8 and 35 1, 2, 7 and 23 16, 20 and 29 11, 16 and 17 1, 16 and 29 |
| Sessions | Guide to session content | aBCTs68 |
|---|---|---|
| Session1: week 1, first steps (30 minutes) |
|
1 and 2 2 4 and 21 19 21 and 26 20 7, 9 and 16 12 and 13 37 |
| Session 2: week 5, continuing the changes (20 minutes) |
|
10 and 19 12 and 13 8 7, 9 and 16 8 and 29 9 and 35 18, 29 and 36 37 |
| Session 3: week 9, building lasting habits (20 minutes) |
|
10 and 19 11 and 17 12 and 13 35 7, 23, 29 and 35 7, 9, 16 and 26 37 |
FIGURE 1.
A summary of the PACE-UP walking programme. Adapted from Harris et al. 70 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

Procedure for the postal intervention group
The participants received (by post) a pedometer (Yamax Digi-Walker, SW-200 model; Yamasa Tokei Keiki Co., Ltd, Tokyo, Japan), instructions and a 12-week step count diary for the 12-week walking intervention (see Figure 1). The research assistant contacted the participant to check that the pedometer had been received and to resolve any difficulties with the equipment. At the end of the 12-month follow-up period, the postal group were offered a single practice nurse PA appointment, if they wanted it.
Procedure for the nurse intervention group
Three dedicated PA consultations (week 1, week 5 and week 9) were arranged with the practice nurse, to individually tailor and support the 12-week pedometer-based walking programme (see Figure 1). At their first appointment, participants were given the same pedometer, diary and handbook that the postal group received. Participants were asked to wear a pedometer and keep a diary record of daily steps for 4 weeks between appointments, in order to review targets and goals at their next appointment. Participants were seen individually or as a couple.
Procedure for the control group
The participants were advised to continue their usual activity levels and were not offered the 12-week walking intervention, but were free to participate in any other PA, just as they would if they were not enrolled in the trial. At the end of the 12-month follow-up period, the control group was offered to receive a pedometer and the PACE-UP 12-week walking programme handbook and diary, either by post or as part of a single PA practice nurse appointment, as preferred.
Outcome measures
Primary and secondary outcome measures
These were selected to reflect the needs of the target population, helping adults and older adults to increase their PA, particularly through walking, and to inform UK public health policy. The primary outcome was the change in average daily step count, measured over 7 days, between baseline and 12 months, assessed objectively by accelerometry (GT3X+; ActiGraph LLC, Pensacola, FL, USA). Secondary outcomes were as follows: changes in step counts between baseline and 3 months; changes in time spent weekly in MVPA in ≥ 10-minute bouts between baseline and 3 months, and between baseline and 12 months; and time spent sedentary between baseline and 3 months, and between baseline and 12 months. All of these secondary outcomes were also assessed objectively by accelerometry. Cost-effectiveness was also a secondary outcome in our protocol [incremental cost per change in step count and per quality-adjusted life-year (QALY)]; this is presented in Chapter 4.
Ancillary outcomes
-
Change in self-reported PA, measured over the same 7 days as accelerometry using the short International Physical Activity Questionnaire (IPAQ)72 and the General Practice Physical Activity Questionnaire [(GPPAQ)73 as part of the 7-day PA questionnaire; see Appendix 1].
-
Change in other patient-reported outcomes (from the health and lifestyle questionnaires at baseline, 3 months and 12 months; see Appendix 1): confidence in ability to do PA, as measured by exercise self-efficacy;74 anxiety and depression, as measured by the Hospital Anxiety and Depression Scale (HADS);75 perceived health status (health-related quality of life), as measured by the EuroQol-5 Dimensions, five-level version (EQ-5D-5L);76 and self-reported pain, measured by two items from the Medical Outcomes Study 36-item short-form health survey. 77
-
Change in anthropometric measurements (weight, BMI, waist circumference, body fat; see Ascertainment of outcomes, Anthropometry, for measurement details).
-
Adverse outcomes [falls, fractures, injuries, exacerbation of pre-existing conditions, major cardiovascular events and deaths from serious AEs (SAEs)] were collected as part of safety monitoring for the trial, by questionnaire self-report items designed by us at 3 and 12 months, and from primary care records after the 12-month follow-up period, for those giving consent.
-
Health service use for those giving consent to primary care record access for the 12-month trial period, numbers of the following occurrences were collected for health economic evaluations (see Chapter 4): primary care consultations, accident and emergency (A&E) attendances, emergency and elective hospital admissions and outpatient referrals.
Ascertainment of outcomes
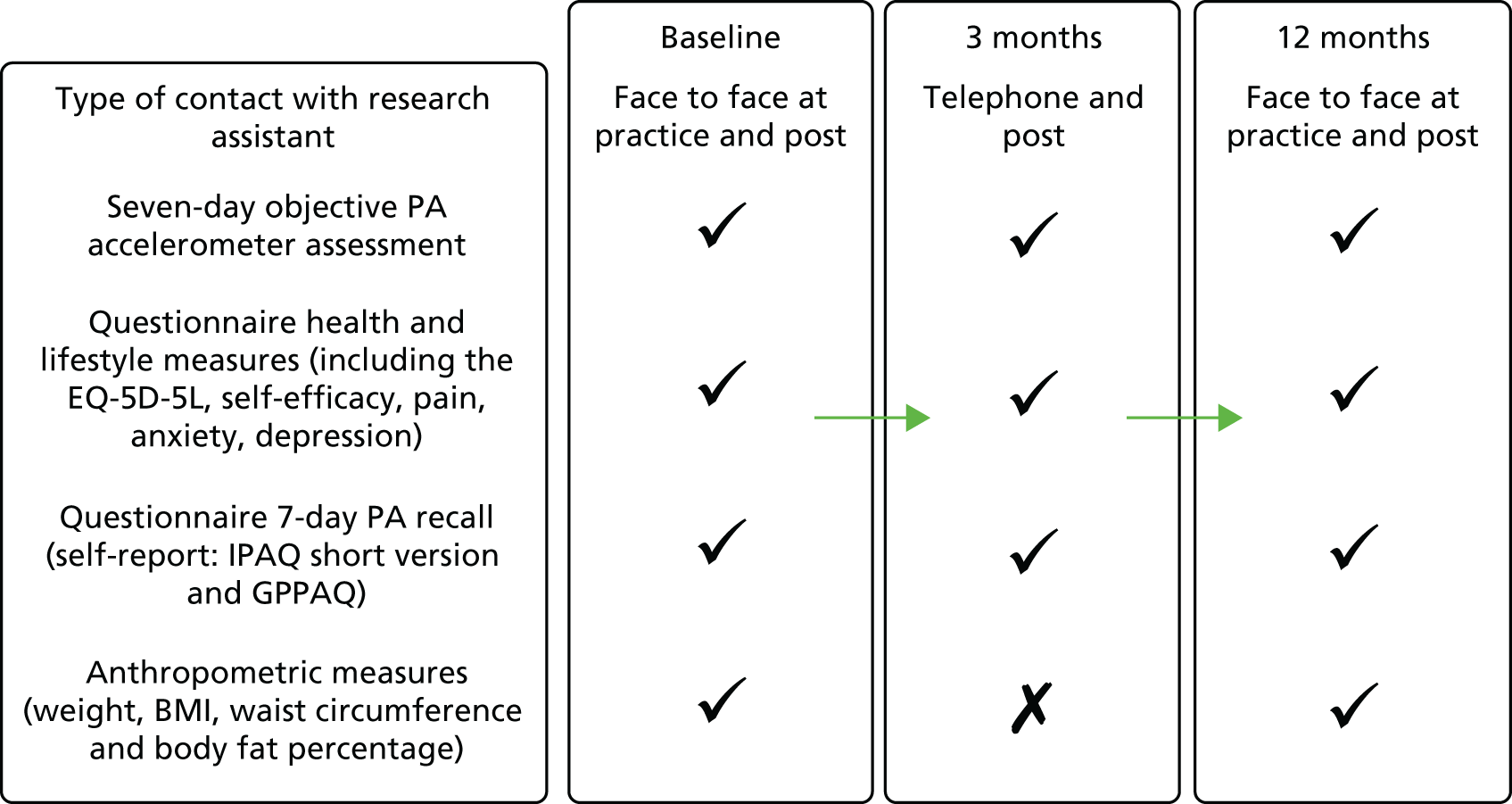
See Figure 2 for the schedule for outcome assessments and measures.
Accelerometry
Participants were asked to carry on with their usual PA levels and to wear an accelerometer (GT3X+, ActiGraph LLC) on a belt over one hip, during waking hours (from rising until going to bed) for 7 days, only removing it for bathing, at baseline, 3 months and 12 months. Participants were offered the option of text messaging to remind them to wear the accelerometer each day and to return it after the 7 days. A diary was provided to record what activities were done and for how long. The monitor, belt and diary were posted back on completion. Once returned, the participants received a £10 gift voucher.
Anthropometry
At the baseline and 12-month face-to-face assessments, the following measurements were taken: height (measured in bare feet to the nearest 0.5 cm using a stadiometer), weight (measured to the nearest 0.1 kg), body fat, bioimpedence [using the Tanita body composition analyser BC-418 MA (Tanita Corporation, Tokyo, Japan)] and waist and hip circumference (using a standard technique and tape measure with a clear plastic slider).
Questionnaire measures
Questionnaire measures were collected using validated tools (detailed under Outcome measures, Ancillary outcomes), as part of self-completed questionnaires at 3 months and 12 months.
In terms of the self-reported PA in the previous week, for the IPAQ72 we used the measure of MVPA (total minutes of vigorous and moderate PA weekly) and the measure of walking (total minutes of walking weekly). The GPPAQ73 provides a PA index (PAI), which is calculated from a combination of PA from both work and leisure activities. Active individuals are those who self-report ≥ 3 hours of MVPA per week on the PAI. However, walking is not included in the calculation of the PAI, although it is asked about in the questionnaire. Analysis of similar GPPAQ data compared with objective accelerometry in our earlier PACE-LIFT trial21 demonstrated that a modified PAI, which also included walking at a brisk pace for at least 3 hours per week, improved validity and repeatability compared with the standard GPPAQ. 78 A modified index, GPPAQ-Walk, was therefore generated.
In addition, the following were also recorded at baseline: demographic information, based on 2011 census questions79 (e.g. marital status, ethnic group, occupation, employment, household composition, home ownership); a list of common self-reported chronic conditions (e.g. heart disease, lung disease, arthritis, stroke, diabetes mellitus, depression); disability, as measured by the Townsend score;80 limiting long-standing illness;79 current medications; smoking; and alcohol consumption.
Several other questionnaire variables were collected at all three time periods, but were not considered to be ancillary trial outcomes in the trial protocol:70 loneliness, measured by a single item;81 risk of falls, measured using the Falls Risk Assessment Tool,82 was assessed using a combination of both self-reported items and direct observation of the ability to rise from a chair without using arms; and self-reported usual PA, as measured by the modified Zutphen Physical Activity Questionnaire. 83 Data from these variables are not presented in this report.
All study groups were asked about falls, injuries, fractures, exacerbation of any pre-existing conditions and the costs of any treatments in the 3- and 12-month questionnaires. Questions on the financial costs of participating in walking and other PAs were asked in the 3- and 12-month questionnaires.
Primary care computerised record measures
For participants who gave written consent, the following data were collected from their electronic primary care records, for the 12-month duration of the trial, after the 12-month follow-up:
-
adverse events potentially relating to trial participation [Read codes relating to falls, fractures, injuries, cardiovascular events (myocardial infarction, coronary artery bypass graft, coronary angioplasty, transient ischaemic attack, stroke) and death]
-
health service use – GP consultations, practice nurse consultations (excluding those for the trial), A&E attendances, emergency and elective hospital admissions and outpatient referrals.
These data were downloaded and pseudoanonymised before removal from the practice.
Baseline and follow-up data collection
Baseline data collection
At baseline, a face-to-face assessment with the research assistant occurred at the participant’s general practice, and questionnaire and anthropometric data were collected (see Ascertainment of outcomes). Participants were then given a belt with an accelerometer (GT3X+, ActiGraph LLC) and a blinded pedometer (Yamax Digiwalker CW200) on it, and asked to wear this for 7 days. The CW200 pedometer model was used to enable the baseline target-setting of the pedometer step count, because of its 7-day memory of consecutive daily steps. However, it is bulky to wear and complicated to use, so this model was not used for the intervention.
Follow-up data collection
Follow-up data collection was conducted in the same way for all trial groups (Figure 2): (1) 3 months (postal) after randomisation (questionnaires and accelerometry) and (2) 12 months (face to face) after the baseline assessment (questionnaires, accelerometry and anthropometry). Participants were also contacted by the research assistant at 6 and 9 months after randomisation by telephone or e-mail to check on falls for trial safety reporting and contact details. For those in the intervention groups, a replacement pedometer or batteries were offered at each contact point, if required. The intervention groups were asked to return their 12-week step count diary following the intervention at 3 months. This was then photocopied and sent back to participants.
FIGURE 2.
Schedule of outcome assessment measures used in the PACE-UP trial.
Accelerometer data reduction
The accelerometer measured vertical accelerations in magnitudes from 0.05 to 2.0 g sampled at 30 Hz, then summed over a 5-second epoch time period. ActiGraph data were reduced using Actilife software (v 6.6.0; ActiGraph LLC, Pensacola, FL, USA), ignoring runs of ≥ 60 minutes of zero counts. 70 Vertical counts were used, as these are the basis of the validated step count and MVPA algorithms. The analysis summary variables used were step counts, accelerometer wear time, time spent in MVPA (≥ 1952 counts per minute, equivalent to ≥ 3 metabolic equivalents),84 time spent in ≥ 10-minute MVPA bouts and time spent being sedentary (≤ 100 counts per minute, equivalent to ≤ 1.5 metabolic equivalents). 85
Adverse events and serious adverse events
An AE was defined as any unfavourable and unintended sign, symptom, syndrome or illness that developed or worsened during the observation period of the trial. This included:
-
exacerbation of a pre-existing illness
-
an increase in frequency or intensity of a pre-existing condition
-
a condition detected or diagnosed after the trial started (but which might have been present at baseline)
-
a persistent disease or symptoms present at baseline that worsened following the start of the trial.
A SAE was defined as any AE occurring during the trial for any of the three groups that resulted in any of the following outcomes:
-
death
-
a life-threatening AE
-
inpatient hospitalisation or prolongation of existing hospitalisation
-
a new disability/incapacity.
All AEs were assessed for seriousness, expectedness and causality. All AEs were recorded and closely monitored until resolution or stabilisation, or until it had been shown that the study intervention was not the cause. Participants were asked to contact the trial site immediately in the event of any SAE. The chief investigator was informed immediately and determined seriousness and causality in conjunction with two other medically trained trial investigators. A SAE that was determined to be directly or possibly trial related was reported, within agreed time frames, to the TSC and the ethics committee. All SAEs were reported annually to the TSC, ethics committee and the trial sponsor.
Although it was important to record AEs contemporaneously for trial safety monitoring during the trial, there was a risk of bias in their reporting, with those having nurse contact having more opportunities for reporting falls, injuries and illnesses. For analyses and reporting, we therefore concentrated on measures for which there were fewer risks of bias between groups: (1) spontaneously reported SAEs, (2) falls, fractures and injuries from questionnaire self-report at 3 and 12 months and (3) falls, fractures, injuries, cardiovascular events (new episode of any of the following: myocardial infarction, angioplasty, coronary artery bypass, stroke, transient ischaemic attack, new-onset angina, ischaemic heart disease) and deaths from primary care records after the 12-month follow-up point.
Sample size
A total of 217 patients in each of the three trial arms would allow a difference of 1000 steps per day to be detected between any two arms of the trial, with 90% power at the 1% significance level. However, we planned to randomise households. Assuming an intracluster correlation of 0.5 and an average household size of 1.6 eligible patients, we needed to analyse 282 patients per trial arm. Allowing for approximately 15% attrition, we needed to randomise a total of 993 patients (331 control participants, 331 participants receiving a pedometer by post and 331 participants receiving a pedometer plus nurse support). We initially planned on six practices to each recruit approximately 166 patients (approximately 55 participants to each of the three groups), but to enable target recruitment, a seventh practice was added.
We anticipated a 20% recruitment rate among eligible participants, based on other PA interventions (including with pedometers) among middle-aged and older adults in primary care, where the recruitment rate was between 17% and 35%. 21,33,86–89 We estimated that, even if our recruitment rate was as low as 10%, we would have enough eligible participants at practices. In fact, the recruitment rate dipped to below 10%, so a seventh practice was added.
Randomisation, concealment of allocation, contamination and treatment masking
Randomisation and concealment of allocation
Following completion of the baseline assessment (including providing accelerometry data on ≥ 5 complete days of ≥ 9 hours/540 minutes), each participant was allocated to a trial group using the King’s College London clinical trials unit internet randomisation service, to ensure independence of the allocation. If participants were unable to provide at least 5 days of ≥ 540 minutes wear time on accelerometry, they were asked to wear the accelerometer for a further 7 days, or they were excluded if this was not possible. Randomisation was at a household level. Randomisation of a group household took place only after both members of the household had completed the baseline assessment. Block randomisation was used within the practice with randomly sized blocks (2, 4 or 6) to ensure balance in the groups and an even nurse workload. Participants were informed by telephone which group they had been allocated to.
Contamination
Contamination could occur between partners in a household; we minimised this by ensuring that, if two household members were recruited, they were allocated to the same group (i.e. randomisation was at a household level). Contamination would have occurred if the control group used a pedometer to increase their walking during the 12-month trial follow-up. We tried to discourage participants in the control group from buying a pedometer, by ensuring that they knew that they would receive one at the end of follow-up. A question was included in the 12-month questionnaire to ask if they had used a pedometer during the course of the trial.
Treatment masking
Participants were randomised only after the successful return of accelerometers with 5 days’ recording. It was not possible to mask participants to their intervention group. The research assistants who carried out follow-up assessments were not masked to group allocation for pragmatic reasons alone: the study was funded to support only enough researchers to carry out recruitment and follow-up simultaneously. However, the main outcome was assessed objectively through accelerometry, and the assessment of the quality of the outcome data was done blind to intervention group: days with < 9 hours of data were excluded. Weight and body fat were also assessed objectively using the Tanita scales, which provided electronic printouts of results, and other outcomes were assessed using standardised measures (e.g. patient-reported outcomes from questionnaires). The statistician carrying out the primary analyses was masked to group allocation as far as possible.
Withdrawals, losses to follow-up and missing data
Withdrawals and losses to follow-up
Participants could withdraw from the trial at any point. Participants who withdrew following informed consent, and prior to randomisation, were replaced with another participant. Participants who withdrew after randomisation were not replaced and were asked if they were prepared to contribute to further data collection on outcomes at 3 and 12 months. Participants were made aware that withdrawal from the trial would not affect future care and that information on those who withdrew or were lost to follow-up that had already been collected would still be used, unless consent for this was withdrawn.
Procedure for accounting for missing data
Only days with at least 540 minutes of registered time on an accelerometer on a given day were used, which was consistent with previous work (the PACE-LIFT trial,21 Trost et al. 90 and Miller et al. 91). Participants were randomised only if they provided at least 5 such days of accelerometer data at baseline. A multilevel linear regression model was used, taking account of repeated days within individuals to estimate the baseline average daily step count for each subject, adjusted for the day of the week and the day order of wearing the accelerometer. The same approach was used to estimate the average daily step count at 3 months and 12 months. The main covariates – age, sex, practice, month of baseline accelerometry and whether or not participants were taking part as a couple – were known for all participants, and most patients had complete data for other measures. To lessen attrition bias, the primary analysis included all participants with at least 1 satisfactory day of accelerometry recording at 12 months (i.e. a wear time of ≥ 540 minutes). The main analysis assumed that, depending on the model covariates, outcome data were missing at random. This was likely to be true for missing data as a result of accelerometer failure, and was plausible for missing days and participants who did not return accelerometers. However, an alternative plausible assumption is that participants who failed to provide outcome data were less active. Multiple imputation was used to impute values for those with no accelerometer data at 12 months (see Statistical methods, Sensitivity analyses). Further sensitivity analyses examined the impact of assuming that missing step counts at 12 months in the control group were equal to their baseline values and, in the two intervention groups, varied between 1500 steps lower and 1500 steps higher than their baseline values.
Statistical methods
The analysis and reporting was in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines, with the primary analysis on an intention-to-treat basis. That is, all participants with outcome data were included, regardless of their adherence to the interventions. All participants were included in the primary analysis if they had at least 1 satisfactory day of accelerometer recording (≥ 540 minutes) of registered time during a day, out of 7 days, at 12 months. The adequacy of the randomisation process to achieve balanced groups was checked by comparing participant characteristics in the three arms (e.g. sex, age, socioeconomic group, baseline PA level or BMI). Stata®, version 12 (StataCorp LP, College Station, TX, USA) was used for all analyses.
Primary analysis
The primary outcome measure was change in average daily step count from baseline to the 12-month follow-up point measured over 7 days. However, to overcome Lord’s paradox,92 the analytic approach regressed 12-month outcomes on baseline measures, thus allowing for regression to the mean. Eligibility was defined on the basis of ≥ 5 days with ≥ 540 minutes’ activity at baseline. If the participant was asked to wear the accelerometer for a second time, the second 7 days was used in the analysis. If there were > 7 days’ wear on the accelerometer, then the first 7 days were used and later readings were discarded. The primary analysis used all participants providing at least 1 day of ≥ 540 minutes accelerometry wear time at 12 months (i.e. a complete-case analysis).
All analyses were carried out using Stata, version 12. The xtmixed procedure was used for regression models. A two-stage process was used for accelerometry data. Stage 1 estimated the average daily step count at both baseline and 12 months, using a multilevel model in which daily step counts were regressed on day of the week and day order of wearing the accelerometer (from day 1 to day 7) as fixed effects, and with day within individual as the random effect (i.e. level 1 was the day within individual and level 2 was individual). In stage 2, average daily step count at 12 months was regressed on baseline average daily step count, sex, age, general practice, month of baseline accelerometry and treatment group as fixed effects, and household as the random effect, to allow for clustering at a household level (i.e. level 1 was individual and level 2 was household). This method effectively measured the change in step count from baseline to 12 months, minimising bias and maintaining power. Adjusting for baseline steps controlled for many factors that predict the number of steps in cross-sectional analyses (e.g. BMI, socioeconomic group, health status). The reference group for the intervention group comparisons was the control group. The post-estimation command pwcompare was used to obtain the estimates of change with 95% CIs and p-values for the difference in change in steps for the postal group versus the control group, the nurse-support group versus the control group and the nurse-support group versus the postal group. This last comparison provided information on whether or not the nurse intervention promoted a worthwhile increase in activity compared with a pedometer alone. It should be noted that, although this estimate can be obtained from the difference of the first two estimates, pwcompare also provided 95% CIs for this comparison. Checks were carried out to confirm that the distribution of residuals from the regression model for change in steps was normally distributed.
Secondary and ancillary outcome analyses
Secondary PA outcome measures from accelerometry were total weekly minutes of MVPA in ≥ 10-minute bouts, average daily sedentary time at 12 months and steps, MVPA in bouts and sedentary time at 3 months. These data were processed and analysed in the same way as described for the step counts (see Primary analysis). MVPA was highly positively correlated with step counts and sedentary time was negatively correlated with step counts.
Other ancillary outcomes were changes in exercise self-efficacy, anxiety, depression, perceived health status (health-related quality of life, as measured using the EQ-5D-5L), self-reported pain, anthropometric measures (weight, BMI, waist circumference, body fat) and self-reported PA from the IPPAQ and GPAQ questionnaires. Changes in these outcomes from baseline to 3 and 12 months were analysed using identical models to stage 2, as described in Primary analysis (i.e. level 1 was individual and level 2 was household).
Adverse event analyses
The number of participants who suffered an AE between 0 and 3 months or between 0 and 12 months (a spontaneously reported SAE or a systematically reported SAE from the 3- or 12-month questionnaire, or a SAE collected from the primary care record data) was compared between groups using exact tests for categorical tables.
Subgroup analyses
Sex, age groups (< 60 years or ≥ 60 years), taking part as a couple, socioeconomic subgroups, BMI, disability, pain and exercise self-efficacy were examined as potential effect modifiers by adding interaction terms to the regression model for the primary outcome, which was changes in step counts at 12 months.
Sensitivity analyses
Sensitivity analyses were carried out for the primary outcome (change in step counts from baseline to 12 months). The effects of using different criteria for defining satisfactory wear at 12 months were examined as follows: (1) at least 5 days of ≥ 540 minutes’ wear time, (2) ≥ 1 day of ≥ 600 minutes’ wear time and (3) ≥ 5 days of ≥ 600 minutes’ wear time. The effect of adjusting for change in wear time between baseline and 12 months was also examined.
Additional sensitivity analyses assessed whether participants lost to follow-up or who failed to provide a single adequate day’s recording might have introduced bias. This was first done by assuming that outcome data were missing at random, depending on the model covariates, using the Stata procedure mi impute. The first model used the standard model covariates to impute missing step counts at 12 months (treatment group, baseline steps, sex, age, general practice, month of baseline accelerometry and household as a random effect) and the second model added in the National Statistics Socio-economic Classification (NS-SEC), self-reported pain and fat mass as additional covariates. Further analyses explored the possible impact of outcomes not being missing at random, using the following assumptions: among those with missing data in the control group, the change in mean steps from baseline to 12 months was 0, and among those with missing data in each of the intervention groups, the change in mean steps from baseline to 12 months was –1500, 0 or +1500.
Ethics approval and research governance
Ethics approval was granted for the trial from London, Hampstead Research Ethics Committee (reference number 12/LO/0219). The NHS Research and Development approval was granted by the Clinical Commissioning Groups in south-west London, through the Primary Care Research Network, to cover all the practice sites.
Management of the trial
The trial progress, including recruitment, safety, finance and data management, was reviewed regularly by the trial management group (TMG). This was made up of the chief investigator, two trial investigators, the trial statistician and the trial manager. The TMG met on a monthly basis. All of the trial investigators met as a group (the trial investigator group) on a biannual basis, and the TSC met prior to participant recruitment, and then annually or biannually as necessary. Minutes were kept of all TMG, trial investigator group and TSC meetings. The TSC included a patient advisor and more details of their role in terms of patient and public involvement are given in Appendix 1.
Further trial follow-up at 3 years
After the initial trial results were analysed, funding was obtained to follow up the trial cohort at 3 years. Details of the methods and results for this further follow-up are given in Chapter 8.
Chapter 3 Results
The main results from the PACE-UP trial are published,93 and are reproduced here under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided that the original author and source are credited.
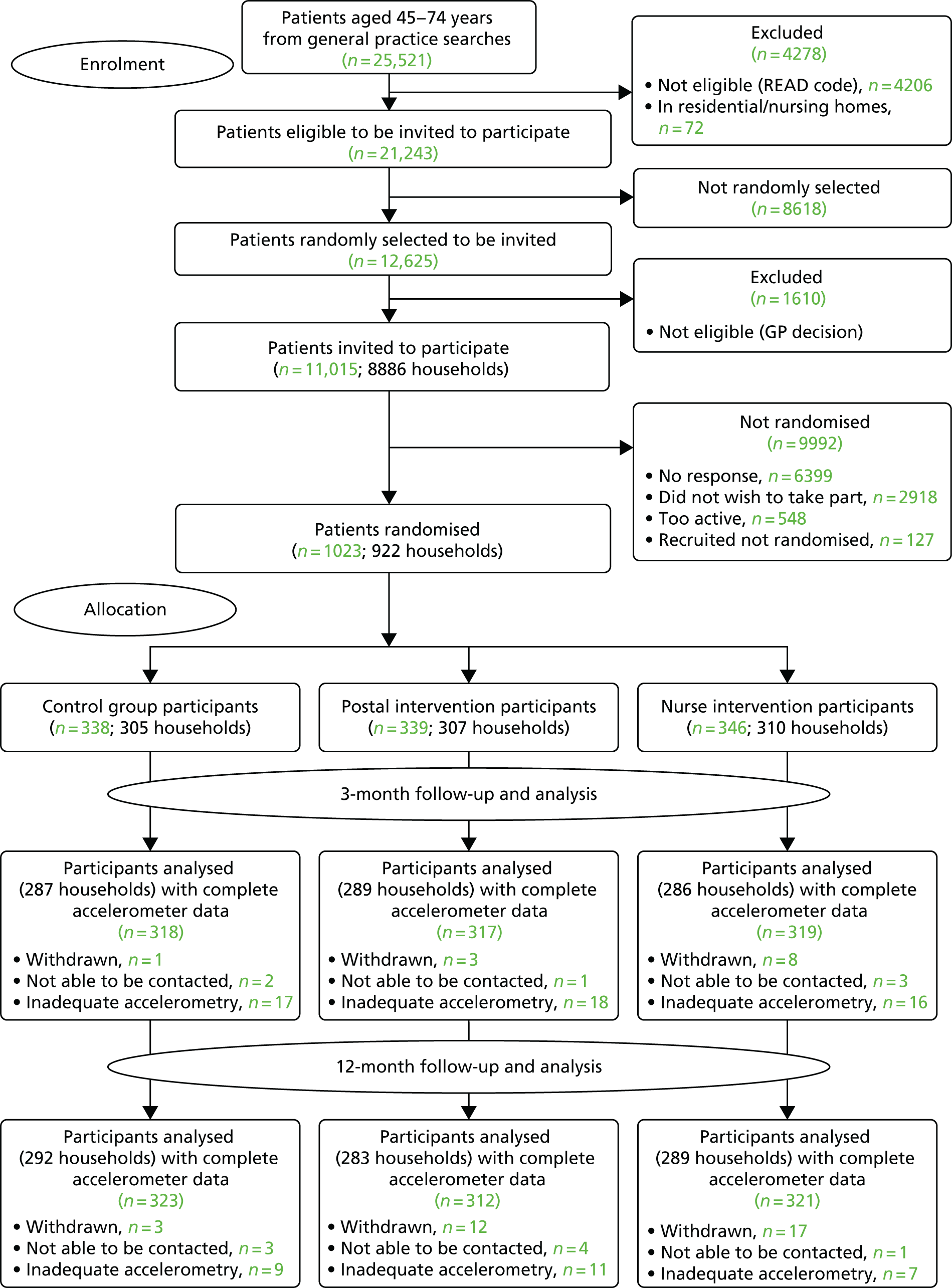
Recruitment of participants
The CONSORT diagram of participant flow (Figure 3) shows that of the 11,015 people invited to participate, 6399 did not respond and 548 were excluded as a result of self-reported PA guideline achievement; therefore, 1023 out of 10,467 (10%) were randomised.
FIGURE 3.
The PACE-UP trial CONSORT flow diagram. Adapted from Harris et al. 93 This is an open access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0.
Baseline characteristics of the study population (Table 4)
Participants recruited to the trial were evenly spread across the age groups. Just over one-third of those recruited were men, around two-thirds were married and around one-fifth took part in the trial as a couple. The majority were in full- or part-time employment, mostly in high-level manual, administrative or professional jobs, with a minority in intermediate or routine and manual occupations. About 80% of those recruited were of white ethnicity, around 10% were black/African/Caribbean or black British and approximately 7% were Asian or Asian British. In terms of health factors, just under 10% were current smokers, around 80% reported their health as being good or very good, the majority had one or more chronic disease and some self-reported pain, around 60% reported no current disability, around 10% had a high depression score, around 20% had a high anxiety score, and around two-thirds of participants were overweight or obese. Recruitment occurred throughout all four seasons, but was slightly higher in summer and slightly lower in winter. All of these factors were well balanced between the three randomised groups.
| Characteristic | Trial arm | ||
|---|---|---|---|
| Control (N = 338) | Postal (N = 339) | Nurse (N = 346) | |
| Age (years) at randomisation, n (%) | |||
| 45–54 | 101 (30) | 118 (35) | 121 (35) |
| 55–64 | 138 (41) | 125 (37) | 124 (36) |
| 65–75 | 99 (29) | 96 (28) | 101 (29) |
| Sex (male) , n (%) | 115 (34) | 124 (37) | 128 (37) |
| Marital status (married), n (%) | 213 (64) | 215 (65) | 230 (68) |
| Randomised as a couple,a n (%) | 66 (20) | 68 (20) | 73 (21) |
| Employment status,79 n (%) | |||
| In full- or part-time employment | 190 (57) | 193 (59) | 190 (56) |
| Retired | 102 (31) | 96 (29) | 101 (30) |
| Other | 39 (12) | 39 (12) | 50 (15) |
| NS-SEC (current or previous job),79 n (%) | |||
| High-level managerial, administrative, professional | 199 (62) | 191 (60) | 184 (56) |
| Intermediate occupations | 70 (22) | 85 (27) | 95 (29) |
| Routine and manual occupations | 51 (16) | 44 (14) | 52 (16) |
| Ethnicity,79 n (%) | |||
| White | 253 (78) | 270 (83) | 267 (80) |
| Black/African/Caribbean/black British | 30 (9) | 31 (10) | 40 (12) |
| Asian/Asian British | 26 (8) | 20 (6) | 22 (7) |
| Other | 15 (5) | 4 (1) | 6 (2) |
| Current smoker, n (%) | 27 (8) | 29 (9) | 26 (8) |
| General health:79 very good or good, n (%) | 265 (80) | 277 (84) | 277 (82) |
| Chronic diseases, n (%) | |||
| None | 129 (39) | 135 (41) | 117 (35) |
| One or two | 183 (55) | 171 (51) | 188 (55) |
| ≥ 3 | 21 (6) | 27 (8) | 34 (10) |
| Presence of self-reported pain,77 n (%) | 220 (66) | 236 (71) | 234 (70) |
| Limiting long-standing illness,79 n (%) | 76 (23) | 73 (22) | 74 (22) |
| Townsend disability score,80 n (%) | |||
| None (0) | 190 (57) | 196 (59) | 210 (62) |
| Slight or some disability (1–6) | 127 (38) | 130 (39) | 124 (36) |
| Appreciable or severe disability (7–18) | 15 (5) | 8 (2) | 7 (2) |
| HADS depression score:75 borderline or high, n (%) | 36 (11) | 33 (10) | 42 (12) |
| HADS anxiety score:75 borderline or high, n (%) | 65 (19) | 64 (19) | 71 (21) |
| Low self-efficacy score,74 n (%) | 102 (31) | 96 (29) | 117 (35) |
| Month of baseline measure, n (%) | |||
| March–May | 80 (24) | 75 (22) | 76 (22) |
| June–August | 105 (31) | 106 (31) | 110 (32) |
| September–November | 88 (26) | 82 (24) | 92 (27) |
| December–February | 65 (19) | 76 (22) | 68 (20) |
| Physical characteristics | |||
| Overweight/obese: BMI of ≥ 25 kg/m2, n (%) | 227 (67) | 221 (65) | 233 (67) |
| Fat mass (kg), mean (SD) | 26 (10) | 27 (11) | 26 (11) |
| Waist circumference (cm), mean (SD) | 93 (14) | 94 (14) | 93 (13) |
| PA data | |||
| Accelerometry | |||
| Adjusted baseline step count per day, mean (SD) | 7379 (2696) | 7402 (2476) | 7653 (2826) |
| Total weekly minutes of MVPA in ≥ 10-minute bouts, mean (SD) | 84 (97) | 92 (90) | 105 (116) |
| Average daily sedentary time (minutes), mean (SD) | 613 (68) | 614 (71) | 619 (78) |
| Average daily wear time (minutes), mean (SD) | 789 (73) | 787 (78) | 797 (84) |
| 150 minutes of MVPA in ≥ 10-minute bouts (yes), n % | 61 (18) | 68 (20) | 89 (26) |
| IPAQ score72 | |||
| IPAQ MVPA score: total weekly minutes of MVPA in ≥ 10-minute bouts (N = 909), mean (SD) | 197 (314) | 147 (256) | 172 (279) |
| IPAQ walking score: total weekly minutes of walking in ≥ 10-minute bouts (N = 888), mean (SD) | 333 (333) | 330 (338) | 312 (277) |
| 150 weekly minutes of IPAQ MVPA score (N = 909): yes, n (%) | 110 (37) | 91 (30) | 109 (35) |
| 150 weekly minutes of IPAQ walking score (N = 888): yes, n (%) | 193 (65) | 190 (66) | 208 (69) |
| GPPAQ score,73 n (%) | |||
| PAI score (N = 973) | |||
| Inactive | 159 (49) | 153 (48) | 156 (47) |
| Moderately inactive | 69 (21) | 66 (21) | 83 (25) |
| Moderately active | 50 (16) | 63 (20) | 60 (18) |
| Active | 44 (14) | 36 (11) | 34 (10) |
| PAI score, including walking (GPPAQ walking score; N = 973) | |||
| Inactive | 129 (40) | 134 (42) | 133 (40) |
| Moderately inactive | 57 (18) | 56 (18) | 63 (19) |
| Moderately active | 43 (13) | 49 (15) | 47 (14) |
| Active | 93 (29) | 79 (25) | 90 (27) |
In terms of objectively measured baseline PA levels, the nurse-support group had a slightly higher baseline-adjusted average daily step count [7653 steps, standard deviation (SD) 2826 steps] and minutes spent weekly in MVPA in bouts of ≥ 10 minutes (105 minutes, SD 116 minutes) than the postal group (7402 steps, SD 2476 steps; 92 minutes, SD 90 minutes) and the control group (7379 steps, SD 2696 steps; 84 minutes, SD 97 minutes). A higher proportion of the nurse-support group participants were achieving the guidelines of ≥ 150 minutes per week of MVPA in bouts of ≥ 10 minutes (26%, 89/346) than participants in the postal group (20%, 68/339) and those in the control group (18%, 61/338). The three groups were similar in terms of average daily sedentary time, at around 10 hours per day.
In terms of self-reported PA levels the patterns were different, with the control group reporting the highest number of weekly minutes of MVPA on the IPAQ, not including walking; however, the nurse-support group reported higher levels of MVPA if walking was included. A slightly higher proportion of participants in the control group than those in the intervention groups reported being active on the GPPAQ PAI, both excluding and including walking.
Losses to follow-up
Figure 3 shows the losses to follow-up. Of the 1023 people randomised, 32 (3%) withdrew and eight (1%) were unable to be contacted at 12 months. In total, 956 out of 1023 participants (93%) provided at least 1 day of 540 minutes’ wear time accelerometer data and were included in the 12-month primary analyses.
Data completeness for accelerometry
Accelerometer wear time was similar between the groups at baseline, the 3-month follow-up point and the 12-month follow-up point (see Tables 4 and 5). Over 90% of all groups provided ≥ 5 days of ≥ 540 minutes’ wear time at 12 months (see Appendix 2, Table 23).
Effect of the intervention on accelerometer-assessed physical activity outcomes (Table 5)
Three-month (interim) outcomes
There were significant differences for the change in average daily step counts from baseline to 3 months between intervention groups and the control group: additional step counts (steps per day) were 692 steps (95% CI 363 to 1020 steps; p < 0.001) for the postal group and 1173 steps (95% CI 844 to 1501 steps; p < 0.001) for the nurse-support group, and the difference between the intervention groups was statistically significant (481 steps, 95% CI 153 to 809 steps; p = 0.004). Findings for the change in time in MVPA levels showed a similar pattern: additional MVPA in bouts of ≥ 10 minutes (minutes per week) was 43 minutes (95% CI 26 to 60 minutes; p < 0.001) for the postal group and 61 minutes (95% CI 44 to 78 minutes; p < 0.001) for the nurse-support group, and the difference between intervention groups was 18 minutes (95% CI 1 to 35 minutes; p = 0.04). There was no difference between the groups for the change in sedentary time. Summary data for the 3-month PA outcomes are shown in Appendix 2, Table 24.
| Outcome | Comparison between trial arms | |||||
|---|---|---|---|---|---|---|
| Postal vs. control | Nurse support vs. control | Nurse support vs. postal | ||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | |
| Daily step count | ||||||
| 3 months | 692 (363 to 1020) | < 0.001 | 1173 (844 to 1501) | < 0.001 | 481 (153 to 809) | 0.004 |
| 12 months | 642 (329 to 955) | < 0.001 | 677 (365 to 989) | < 0.001 | 36 (–277 to 349) | 0.82 |
| Total weekly minutes of MVPA in ≥ 10-minute bouts | ||||||
| 3 months | 43 (26 to 60) | < 0.001 | 61 (44 to 78) | < 0.001 | 18 (1 to 35) | 0.04 |
| 12 months | 33 (17 to 49) | < 0.001 | 35 (19 to 51) | < 0.001 | 2 (–14 to 17) | 0.83 |
| Daily sedentary time (minutes) | ||||||
| 3 months | –2 (–12 to 7) | 0.59 | –7 (–16 to 3) | 0.16 | –4 (–13 to 5) | 0.38 |
| 12 months | 1 (–8 to 10) | 0.83 | –0.2 (–9 to 9) | 0.96 | –1 (–10 to 8) | 0.79 |
| Daily wear time (minutes) | ||||||
| 3 months | 2 (–8 to 12) | 0.69 | 4 (–6 to 14) | 0.40 | 2 (–8 to 12) | 0.65 |
| 12 months | 9 (–1 to 19) | 0.08 | 9 (–0.8 to 19) | 0.07 | 0.3 (–10 to 10) | 0.96 |
Twelve-month (main) outcomes
Both intervention groups increased their step counts between baseline and 12 months compared with the control group: additional step counts (steps per day) were 642 steps (95% CI 329 to 955 steps; p < 0.001) for the postal group and 677 steps (95% CI 365 to 989 steps; p < 0.001) for the nurse-support group, with no statistically significant difference between intervention groups (36 steps, 95% CI –277 to 349 steps; p = 0.82). Time spent in MVPA in bouts showed a similar pattern, that is, both intervention groups increased at 12 months compared with the control group: additional MVPA in bouts (minutes per week) was 33 minutes (95% CI 17 to 49 minutes; p < 0.001) for the postal group and 35 minutes (95% CI 19 to 51 minutes; p < 0.001) for the nurse-support group, with no statistically significant difference between the two intervention groups (2 minutes, 95% CI –14 to 17 minutes; p = 0.83). Again, there was no difference between the groups for the change in sedentary time. Summary data for the 12-month PA outcomes are shown in Appendix 2, Table 24.
Residuals from the 12-month models for steps and weekly MVPA in ≥ 10-minute bouts were plotted, and the distribution of residuals from both models was normally distributed (see Appendix 2, Figure 18).
Effect of the intervention on self-reported physical activity outcomes at 12 months (Table 6)
At 12 months, the IPAQ weekly minutes of MVPA (not including walking) did not show any effect of the intervention for either intervention group compared with the control group. However, weekly minutes of walking from the IPAQ at 12 months compared with baseline showed significant increases for both groups compared with controls: 69 minutes (95% CI 19 to 119 minutes) in the postal group and 55 minutes (95% CI 5 to 105 minutes) in the nurse-support group; there was no difference between the intervention groups (–14 minutes, 95% CI –64 to 37 minutes). This was also reflected in the odds ratio (OR) for achieving ≥ 150 minutes of activity in a week, conditional on the baseline state, which was not significant for IPAQ MVPA for either intervention group, but was for IPAQ walking (postal group vs. control group: OR 2.1 minutes, 95% CI 1.3 to 3.3 minutes; nurse-support group vs. control group: OR 1.7 minutes, 95% CI 1.1 to 2.6 minutes); there was no difference between the intervention groups (OR 0.8 minutes, 95% CI 0.5 to 1.3 minutes).
| Questionnaire outcome | Comparison between trial arms | |||||
|---|---|---|---|---|---|---|
| Postal vs. control | Nurse support vs. control | Nurse support vs. postal | ||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | |
| IPAQ72 | ||||||
| Change in weekly minutes of activity | ||||||
| Vigorous and moderate activity (n = 775) | –10 (–58 to 38) | 0.67 | –32 (–80 to 16) | 0.19 | –22 (–70 to 27) | 0.38 |
| Walking (n = 750) | 69 (19 to 119) | 0.01 | 55 (5 to 105) | 0.03 | –14 (–64 to 37) | 0.59 |
| OR for achieving ≥ 150 minutes of activity in a week at follow-up, conditional on baseline state | ||||||
| Vigorous and moderate activity (n = 775) | 1.0 (0.5 to 2.0) | 0.99 | 0.6 (0.3 to 1.3) | 0.18 | 0.6 (0.3 to 1.3) | 0.19 |
| Walking (n = 750) | 2.1 (1.3 to 3.3) | 0.001 | 1.7 (1.1 to 2.6) | 0.01 | 0.8 (0.5 to 1.3) | 0.41 |
| GPPAQ73 | ||||||
| OR for being active at follow-up, conditional on baseline state | ||||||
| PAI (n = 892) | 1.2 (0.7 to 2.1) | 0.46 | 0.9 (0.5 to 1.6) | 0.80 | 0.8 (0.4 to 1.3) | 0.32 |
| PAI, including walking (n = 892) | 1.1 (0.6 to 1.8) | 0.81 | 0.9 (0.5 to 1.5) | 0.64 | 0.8 (0.5 to 1.4) | 0.48 |
For the GPPAQ, the OR for being active at the 12-month follow-up, conditional on the baseline state, did not show a significant effect for either of the intervention groups compared with the control group, whether or not walking was included in the GPPAQ PAI.
Summary data for the effect of the intervention on the IPAQ and the GPPAQ are given in Appendix 2, Table 25.
Effect of the intervention on other health-related outcomes (Table 7)
Fat mass was slightly reduced at 12 months in both intervention groups, but this did not differ significantly from the control group. There was no change in BMI or waist circumference between baseline and 12 months. The interventions had no significant effects on anxiety, depression, quality of life (EQ-5D-5L) or pain scores at either 3 months or 12 months. The exercise self-efficacy score significantly increased in both intervention groups at 3 months compared with the control group, and there was a greater effect in the nurse-support group than in the postal group. By 12 months, the self-efficacy score was significantly higher in the nurse group than in the control group, and the postal group was intermediate between, but not significantly different from, either of the other groups.
| Health-related outcome | Comparison between trial arms | |||||
|---|---|---|---|---|---|---|
| Postal vs. control | Nurse support vs. control | Nurse support vs. postal | ||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | |
| BMI (kg/m2) | ||||||
| 12 months | –0.1 (–0.3 to 0.1) | 0.24 | –0.03 (–0.2 to 0.1) | 0.71 | 0.07 (–0.1 to 0.3) | 0.42 |
| Fat mass (kg) | ||||||
| 12 months | –0.4 (–0.8 to 0.07) | 0.10 | –0.2 (–0.7 to 0.2) | 0.30 | 0.1 (–0.3 to 0.6) | 0.54 |
| Waist circumference (cm) | ||||||
| 12 months | –0.04 (–0.8 to 0.7) | 0.92 | 0.08 (–0.6 to 0.8) | 0.23 | 0.1 (–0.6 to 0.8) | 0.74 |
| HADS anxiety score75 | ||||||
| 3 months | –0.3 (–0.7 to 0.1) | 0.13 | –0.3 (–0.7 to 0.1) | 0.16 | 0.01 (–0.4 to 0.4) | 0.94 |
| 12 months | –0.2 (–0.6 to 0.2) | 0.28 | –0.2 (–0.6 to 0.2) | 0.28 | 0.0006 (–0.4 to 0.4) | 1.00 |
| HADS depression score75 | ||||||
| 3 months | –0.2 (–0.6 to 0.1) | 0.12 | –0.2 (–0.5 to 0.1) | 0.19 | 0.04 (–0.3 to 0.3) | 0.82 |
| 12 months | –0.1 (–0.5 to 0.2) | 0.44 | –0.02 (–0.4 to 0.3) | 0.91 | 0.1 (–0.2 to 0.5) | 0.51 |
| EQ-5D-5L score76 | ||||||
| 3 months | –0.005 (–0.02 to 0.01) | 0.60 | –0.01 (–0.03 to 0.01) | 0.26 | –0.006 (–0.03 to 0.01) | 0.54 |
| 12 months | –0.01 (–0.03 to 0.01) | 0.30 | –0.01 (–0.03 to 0.01) | 0.23 | –0.002 (–0.02 to 0.02) | 0.87 |
| Exercise self-efficacy score74 | ||||||
| 3 months | 1.1 (0.2 to 2.0) | 0.01 | 2.3 (1.4 to 3.2) | < 0.001 | 1.2 (0.3 to 2.1) | 0.01 |
| 12 months | 0.6 (–0.3 to 1.6) | 0.20 | 1.2 (0.3 to 2.2) | 0.01 | 0.6 (–0.4 to 1.5) | 0.22 |
| Self-reported pain score | ||||||
| 3 months | 0.05 (–0.06 to 0.17) | 0.37 | 0.05 (–0.07 to 0.16) | 0.42 | –0.004 (–0.12 to 0.11) | 0.94 |
| 12 months | 0.05 (–0.06 to 0.17) | 0.35 | 0.02 (–0.10 to 0.13) | 0.76 | –0.04 (–0.15 to 0.08) | 0.53 |
Effect of the intervention on adverse events and serious adverse events (Table 8)
The number of total AEs did not differ between the groups at either 3 months or 12 months, whether using higher numbers of events self-reported from the patient questionnaire (falls, fractures, sprains or injuries) or lower numbers of events from primary care records (any AE – cardiovascular, fracture, sprains/injuries, falls or pain from back or lower limb). There was also no between-group difference in trial SAEs reported for safety monitoring. Self-reported falls were lower in the nurse-support group at 12 months (43/318, 14%) than in the postal group (57/310, 18%) or the control group (71/318, 22%; p = 0.02). Falls reported in primary care records over 12 months were fewer in number, but also in the same direction, although the differences were non-significant (p = 0.13). Primary care-recorded cardiovascular events over 0–12 months were lower in the nurse-support group (2/340, 0.6%) and the postal group (1/331, 0.3%) than in the control group (8/334, 2.4%; p = 0.04).
| AEs | Time frame in each trial arm | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–3 months | 0–12 months | |||||||
| Control, n (%) | Postal, n (%) | Nurse support, n (%) | p-valuea | Control, n (%) | Postal, n (%) | Nurse support, n (%) | p-valuea | |
| AEs reported on the questionnaire | (N = 931) | (N = 946) | ||||||
| Fall, fracture, sprain or injury | 59/313 (19) | 70/310 (23) | 65/308 (21) | 0.51 | 113/318 (36) | 99/310 (32) | 96/318 (30) | 0.34 |
| Fall | 25 (8) | 24 (8) | 24 (8) | 0.99 | 71 (22) | 57 (18) | 43 (14) | 0.02 |
| Fracture | 3 (1) | 3 (1) | 7 (2) | 0.28 | 15 (5) | 10 (3) | 11 (3) | 0.57 |
| Sprain or injury | 49 (16) | 54 (17) | 47 (15) | 0.74 | 66 (21) | 68 (22) | 63 (20) | 0.81 |
| (N = 911) | (N = 924) | |||||||
| Deterioration in health problems already present since the start of the study | 33/311 (11) | 30/303 (10) | 39/297 (13) | 0.42 | 68/313 (22) | 67/300 (22) | 65/311 (21) | 0.91 |
| AEs from primary care recordsb | (N = 1005) | (N = 1005) | ||||||
| Any AE | 29/334 (8.7) | 23/331 (7.0) | 20/340 (5.9) | 0.36 | 85/334 (25.5) | 75/331 (22.7) | 77/340 (22.7) | 0.62 |
| Cardiovascularc | 2 (0.6) | 0 | 1 (0.3) | 0.55 | 8 (2.4) | 1 (0.3) | 2 (0.6) | 0.04 |
| Fracture | 4 (1.2) | 2 (0.6) | 2 (0.6) | 0.68 | 11 (3.3) | 4 (1.2) | 4 (1.2) | 0.11 |
| Sprain/injury | 2 (0.6) | 1 (0.3) | 2 (0.6) | 1.00 | 8 (2.4) | 4 (1.2) | 5 (1.5) | 0.51 |
| Fall | 0 | 0 | 0 | 8 (2.4) | 4 (1.2) | 2 (0.6) | 0.13 | |
| Pain (back or lower limb) | 23 (6.9) | 20 (6.0) | 16 (4.7) | 0.48 | 65 (19.5) | 65 (19.6) | 70 (20.6) | 0.93 |
| SAE spontaneously reportedd | (N = 1023) | (N = 1023) | ||||||
| SAE | 3/338 (0.9) | 1/339 (0.3) | 3/346 (0.9) | 0.65 | 10/338 (3.0) | 5/339 (1.5) | 11/346 (3.2) | 0.30 |
Subgroup analyses (Figure 4)
There was no evidence of effect modification on the change in step count at 12 months for either of the intervention groups versus the control group for any of the following: age, sex, taking part as a couple, BMI, disability, pain, socioeconomic group and exercise self-efficacy.
FIGURE 4.
Subgroup analyses. (a) Postal group and control group; and (b) nurse-support group and control group.
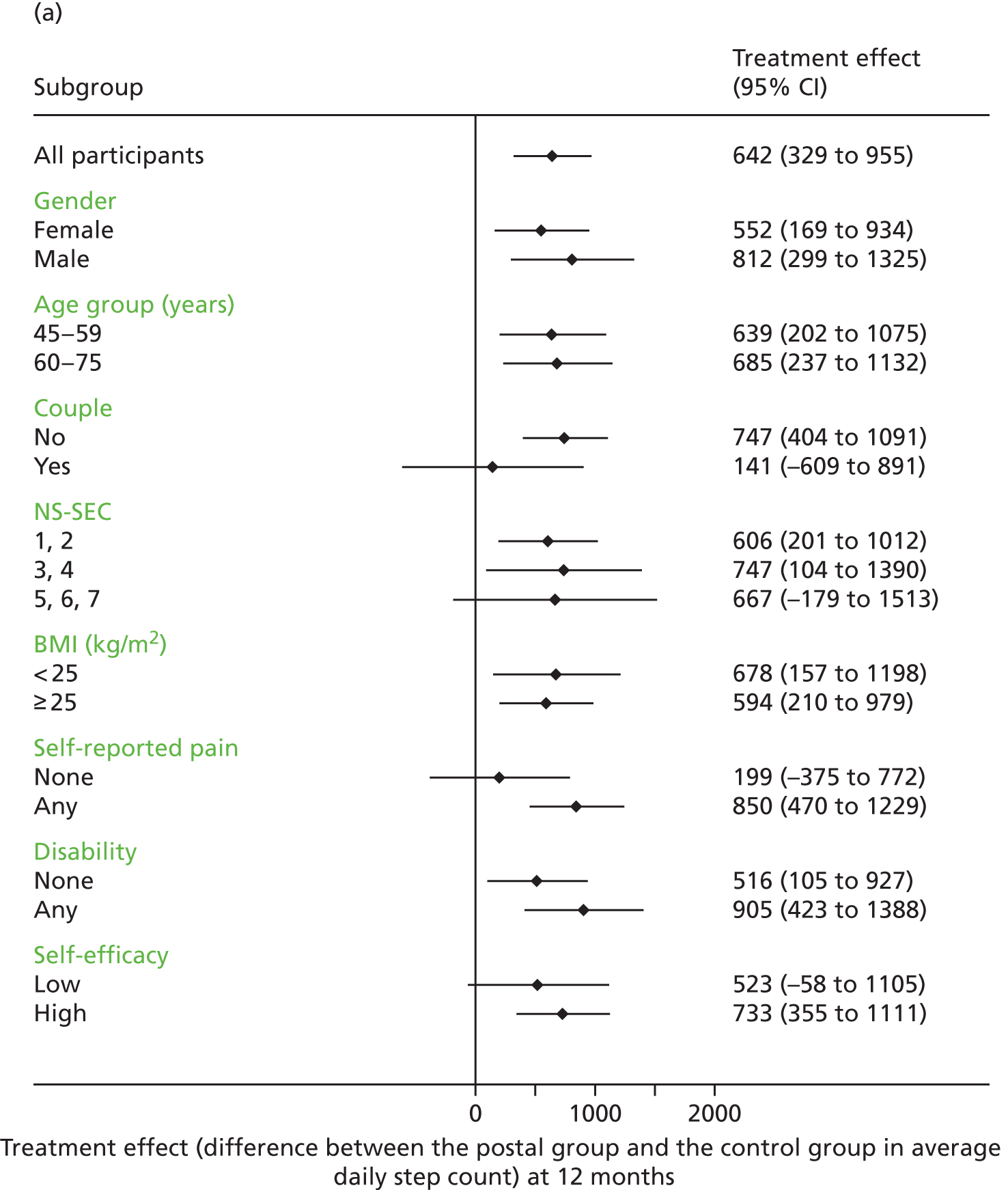
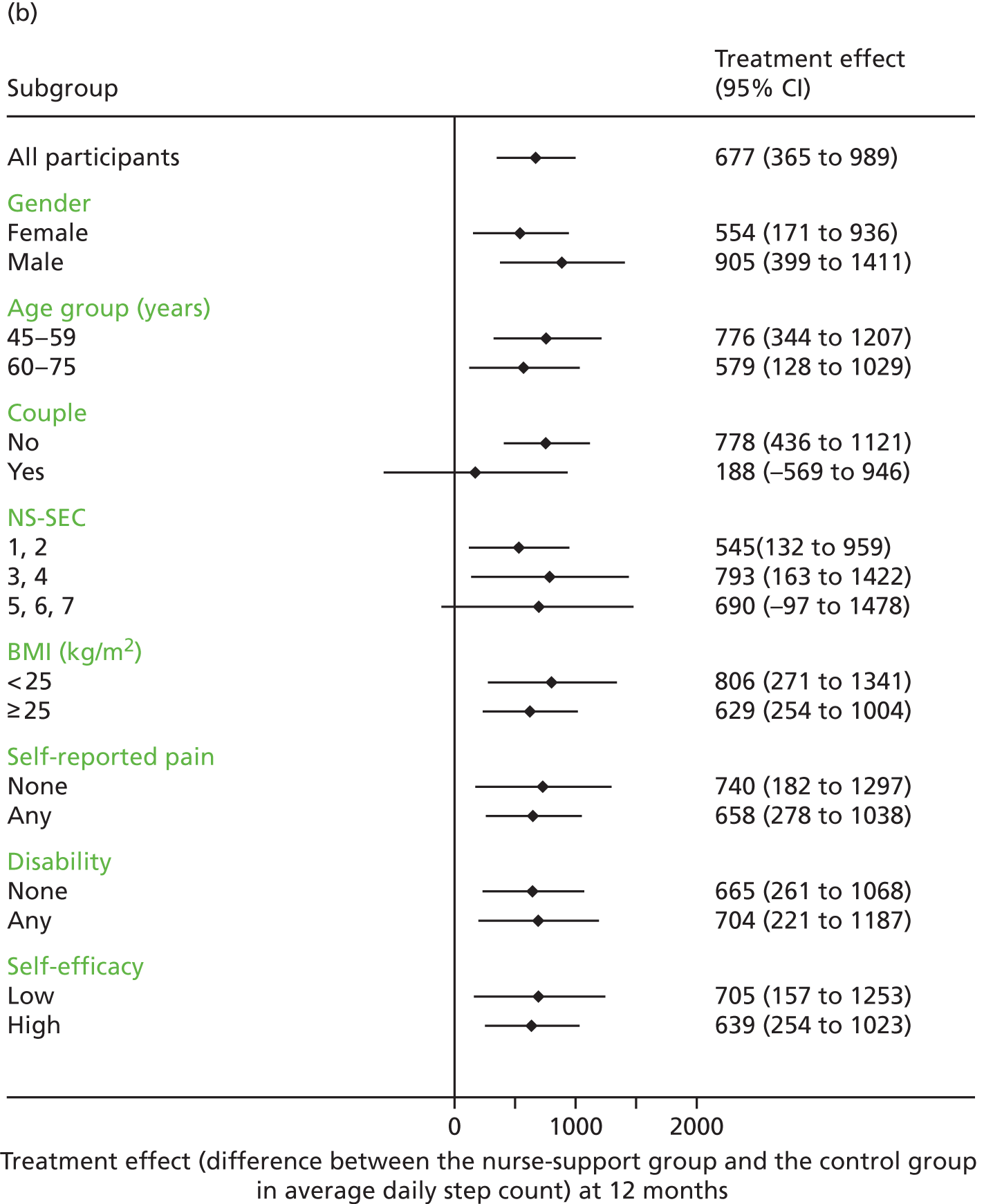
Sensitivity analyses and imputations (see Appendix 2, Table 26)
The sensitivity analyses on the primary outcome measure (change in average daily step count at 12 months), restricted to those with ≥ 600 minutes’ daily wear time, increased the effect size for both intervention groups versus the control group, but did not change the interpretation (both intervention groups had a significant effect compared with the control group, but there was no significant difference between the interventions). Similarly, imputations with both missing-at-random and missing-not-at-random assumptions made some difference to the effect sizes for both interventions compared with the control group and with each other, but, again, made no difference to the overall interpretation.
Summary of the main trial findings
Overall, 10% (1023/10,467) of those invited participated in the trial, and we had primary outcome data on 93% (956/1023) of participants at 12 months. Although the nurse-supported intervention had a greater effect on objective PA outcomes at 3 months, by the main 12-month outcome, both the postal and nurse-supported pedometer interventions significantly increased step counts by around 10%, and time in MVPA in bouts by around one-third compared with the control group, with no statistically significant difference between the interventions. There was no significant effect of the interventions on sedentary time or anthropometric measures. In terms of the effects on self-reported PA levels, the IPAQ MVPA questions did not show any intervention effect, but both the nurse-supported intervention and the postal intervention showed a significant effect on the IPAQ walking question. The GPPAQ score did not show an intervention effect, even when walking was included in the score. The interventions had no effect on most other patient-reported outcomes, except that exercise self-efficacy was increased in both intervention groups at 3 months, and in the nurse-support group at 12 months, compared with the control group. AEs were not increased by the interventions; some individual AEs were lower in the intervention groups, but this was based on small numbers of events. No important subgroup effects were demonstrated, and the sensitivity analyses and imputations did not change the interpretation of the trial results.
The following chapters present the results relating to other aspects of the trial: the economic evaluation (see Chapter 4), generalisability and representativeness (see Chapter 5), the process evaluation (see Chapter 6), the qualitative evaluation (see Chapter 7) and the 3-year trial follow-up (see Chapter 8). Chapter 9 discusses the trial findings in detail.
Chapter 4 Economic evaluation
Introduction
Evidence on the short- and long-term cost-effectiveness of pedometer-based interventions could support the development of policy and practice encouraging increased PA. Calls for evidence on ‘what works’ have been made, in both primary and secondary prevention policy documents, along with appeals to link interventions to clear health outcomes and ensure that resources are used most efficiently. 94,95
To date, only one published estimate of the cost-effectiveness of pedometer programmes for the UK was based on primary evidence. 58 A small (n = 79), highly selected (80% of women from one GP practice in Glasgow) sample was used. A ‘maximal’ pedometer-based walking programme, which included two 30-minute consultations (based on the transtheoretical model of behaviour change), was compared with a waiting list control group, which was asked to wait for 12 weeks, after which the group members received a ‘minimal’ walking programme, which included a pedometer and two 5-minute slots of brief advice. 45,96 Compared with the 12-week waiting list control group, it cost an additional £92 per person to achieve an additional eight people meeting the target of 15,000 steps per day over a 12-week period. Comparing the maximal walking programme with the minimal walking programme, it cost a further £591 for one additional person to achieve the same target. No data were collected on QALYs, and long-term cost-effectiveness was not modelled.
Elsewhere, in Australia, New Zealand and the Netherlands, evidence on the benefits of pedometer-based interventions for primary prevention has been assessed for community-based adults with low PA levels. 97–99 Interventions such as pedometer prescriptions and pedometer-based telephone coaching were compared with time-based activity prescriptions or usual practice. Evidence suggests that pedometers may be cost-effective in the long term, but estimates vary widely (from being cost-saving and having fewer disability-adjusted life-years in Australia in the long term to €11,110 per QALY gained). The generalisability of results to other contexts has also not been considered. 100
This chapter examines the short- and long-term cost-effectiveness, from the NHS perspective, of alternative pedometer-based walking programmes to increase PA levels using PA outcomes for comparison with other PA programmes and QALYs to aid decision-making beyond PA programmes. The interventions are compared against usual practice, in inactive adults aged 45–75 years from south London, and are as described in Chapter 2:
-
provision, by post, of pedometers with written instructions
-
pedometers provided with tailored support from a practice nurse.
This chapter is structured into two sections: (1) a within-trial analysis with a time horizon of 1 year, and (2) beyond-trial modelling that takes a lifetime perspective. In each section, the methods and results are presented. This is followed by a discussion of the findings in the context of the strengths and weaknesses of the study, as well as current literature.
Within-trial cost-effectiveness analysis
The population, interventions and comparator are identical to Chapter 2. Harris et al. 70 set out the protocol for methods, including for the economic evaluation. This section covers methods used to measure, value and aggregate costs and outcomes, the treatment of missing data and methods of assessing cost-effectiveness.
Methods
Identification, measurement, valuation and aggregation of cost
To identify NHS resource use, meetings with the trial team established ‘who did what, to whom and how often’,101 accounting for events that are likely to have high unit costs, be frequent or differ between trial arms, and excluding research-focused costs. NHS resources identified for collection included:
-
Set-up of service (e.g. design, setting up the intervention in GP practices and staff training, but excluding trial set-up; see Appendix 3, Table 27).
-
Delivery of service, including, for example, pedometers, post/telephone services, handbooks and staff time; all costs fell within months 0–3, except research assistant contacts with participants (4–12 months; see Appendix 3, Table 28).
-
Health service use in primary care (GP and nurse consultations, excluding nurse PA consultations undertaken as part of the trial) and secondary care (hospital admissions, A&E, outpatients), as changes could occur from treating AEs and changes in lifestyle (see Appendix 3, Table 29); the health service data were available on the 1005 out of 1023 randomised participants who gave written informed consent for their primary care data to be downloaded. Of these, 956 participants (323 in the control group, 312 in the postal group and 321 in the nurse-support group) also had 12-month outcome data and, therefore, were included in the health economic analyses relating to health service use.
Resource use was measured using administrative/trial management records, electronic diaries and interviews of the trial manager and the principal investigator. Participant-level health service use (e.g. GP visits, referrals) was collected through a one-time download of GP records, for those who gave explicit consent for this, at the end of the trial (see Appendix 3, Tables 27–29).
NHS resources were valued using national costs102,103 (see Appendix 3, Tables 27–33) to increase generalisability. Where national unit costs were not available, local unit costs from St George’s Hospital, London, were used. All costs were expressed in 2013–14 pounds sterling, inflated to the same base year when appropriate, using the Hospital and Community Health Service inflation index. 103 As the study covers 1 year, costs and outcomes were not discounted. All resources and costs were collected at, or apportioned to, the trial participant level. The total cost per participant was the sum of each resource use multiplied by the relevant unit cost over 0–3 and 4–12 months, with costs censored at 12 months.
To support a sensitivity analysis of an alternative wider viewpoint that included participants, as participation can be affected by economic barriers, three types of costs borne by participants were collected: participation in the intervention (e.g. time and money spent accessing the intervention for months 0–3; see Appendix 3, Table 30), money shown to contribute to the costs of ‘walking’ and other PA (e.g. membership/event fees, shoes/clothing, food/drink)104 and money spent as a result of falls/fractures/sprains/injuries. These data were collected for months 1–3 using participant-completed questionnaires at 3 months and for months 10–12 using the participant-completed questionnaires at 12 months (see Appendix 1). As the data for months 10–12 were follow-up data beyond the intervention, costs from months 10–12 were multiplied by three (to approximate annual costs when added to the cost from months 1–3) and added to the costs from months 1–3 for an annual participant cost.
Measurement, valuation and aggregation of outcomes
The economic analysis uses indicators of PA outcomes. The use of cost per additional step aids comparison of inputs with directly intended and objectively measured outputs, which the trial was specifically powered to detect, and which therefore relates the economics to the main trial outcome. As objectively measured weekly minutes of MVPA in bouts of ≥ 10 minutes were statistically significantly different, have important health impacts, provide a second point of comparison to other studies and link directly to the longer-term model, these were also included as an additional outcome measure for assessing cost-effectiveness.
To facilitate the comparison of the PACE-UP trial with other health interventions using the quality-of-life measure recommended for evaluating health interventions in England, participants also completed the EQ-5D-5L questionnaire at baseline, 3 and 12 months. The EQ-5D-5L, rather than the EQ-5D-3L (EuroQol-5 Dimensions, three-level version), was selected as the EQ-5D-3L is known to suffer from ‘ceiling effects’ and the five-level version was expected to be more sensitive to differences in health among healthier people and, therefore, less subject to suffer from ceiling effects. This has been subsequently shown to be the case in England, especially for older populations, and, therefore, the EQ-5D-5L has been recommended for use in general population surveys (despite the ceiling effects of dimensions ranging from 58–90%). 105 Utility weights were assigned at each time point, based on an interim scoring ‘cross-walk’ function106 linked to the standard UK-based weights. 107 EuroQol-5 Dimensions (EQ-5D) utilities were converted to QALYs over the trial period using the ‘area under the curve’ method. 108
Methods of analysis
Missing data
Data were investigated for patterns of missingness. 109 Mean imputation was used when the proportion of missing data was ≤ 5%. 110,111 Missing EQ-5D-5L data were replaced using an index, rather than domain imputation, as the sample size was > 500. 112 Multiple imputation by chained equations was fitted to replace item non-response. In line with Rubin’s rules113 and other recommendations,114,115 the point estimate for imputations was derived by averaging estimates of the imputed data based on results from five imputations. The point estimate for categorical data was rounded up to the nearest decimal point. The imputation model included variables used in the main model for the analysis, while also including the predictors of missingness. The dependent variable was included in the imputation model to ensure that the imputed values have the same relationship to the dependent variable as the observed values. 116
Incremental cost-effectiveness analyses
Incremental cost-effectiveness analyses were based on multiple regression models to adjust for variations not accounted for by randomisation, and to provide more robust estimates. Generalised linear models (GLMs) were fitted separately for costs and QALYs,117 accounting for the cluster effect (identified as household identifier) via clustered standard errors. 118 Models used for step count and MVPA have been described in Chapter 2. The cost models used the Poisson distribution and the QALY models used the binomial 1 family, equivalent to beta regression. 119 Although the generalised linear models do not account for the correlation between costs and QALYs, the efficiency loss (i.e. higher standard errors) will be minimal, as the inclusion of the cluster effect provides robust standard errors and mitigates the effects of potential inaccuracies in the family distribution used. The choice of distributional family rested on the modified Park test120 and the comparison of observed and predicted values. Covariates included the baseline level (for QALY-based models), as recommended,116 practice and variables found to be correlates of PA-related outcomes – that is, demography (age, sex, ethnicity, marital status, education, employment, socioeconomic status, cohabitation), health (number of disease conditions) and other lifestyle behaviours (smoking and alcohol intake). 121 Reduced models were generated using Wald tests to examine the joint significance of variables found to be insignificant in the base model. Significance levels were set at 5%.
To provide more precise estimates of uncertainty, the ‘margins method’ was used to generate sample means for trial arms and incremental point estimates for costs and QALYs. 116,122 A different standard error and calculated CIs123 accounted for the cluster design.
Sensitivity analyses
To reflect the stochastic uncertainty surrounding the mean incremental cost-effectiveness, cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs) were constructed using 2000 non-parametric samples from the base-case estimates. Bootstrapping used a new unique identifier for the clusters in addition to the original cluster identifier (household ID). 116
Deterministic sensitivity analyses performed included:
-
all randomised participants (not only those who provided accelerometry data)
-
varying exclusion of costs of health service use beyond the immediate intervention
-
a method of accounting for AEs
-
only spontaneously self-reported SAEs
-
only GP data relating to AEs; these were predefined by GP investigators and collaborators (TH, SI, SDW, JI) as possibly being related to increased walking, and included musculoskeletal events (falls requiring medical attention, fractures, sprains or injuries, pain in back or lower limb) and cardiovascular events [myocardial infarction, coronary artery bypass graft, angioplasty, stroke, transient ischaemic attack or new-onset ischaemic heart disease (angina)]
-
a perspective of analysis (i.e. NHS with or without users)
-
variation in the length of the life of a pedometer (between 1 and 4 years)
-
scenario combinations (excluding all health service use costs, and including participant costs related to participation in PA and interventions, except the health service use cost borne by participants), to ensure that the long-run model could use evidence of ‘worst-case’ findings.
Results
Table 9 summarises the data on costs, EQ-5D-5L utility scores and quality of life (see Appendix 3, Tables 31–35). At 3 months, the average cost per participant was highest in the nurse group (£249), followed by the postal group (£122) and the control group (£107). The mean cost and distribution is affected considerably by the inclusion of health service use, which resulted in the control group costing £36 more per participant than the postal group and £12 more than the nurse-support group. QALYs varied marginally, with the gap being the greatest between the control and postal groups at +0.002. At 12 months, the average cost per participant was highest in the nurse group (£603), followed by the control group (£461) and the postal group (£375). The inclusion of health service use resulted in the control group costing £86 more per participant than the postal group, but £142 less than the nurse-support group. QALYs were marginally higher in the postal group (0.843 QALYs) than in the control group (0.837 QALYs) and the nurse-support group (0.836 QALYs) group.
| Cost and quality of life (EQ-5D-5L) | Trial arm, mean (SD) | ||
|---|---|---|---|
| Control | Postal | Nurse support | |
| 0–3 months | (N = 318) | (N = 317) | (N = 319) |
| Costs (£) | |||
| Total cost | 107 (254) | 122 (107) | 249 (215) |
| Set-up cost | 0 (0) | 45 (0) | 105 (0) |
| Delivery of intervention | 0 (0) | 7 (0) | 50 (18) |
| Health service use | 107 (254) | 71 (107) | 95 (214) |
| Quality of life | |||
| EQ-5D-5L scores at baseline | 0.839 (0.14) | 0.853 (0.12) | 0.851 (0.12) |
| EQ-5D-5L scores at 3 months | 0.844 (0.14) | 0.848 (0.14) | 0.841 (0.14) |
| QALYs, 0–3 months | 0.194 (0.03) | 0.196 (0.03) | 0.195 (0.03) |
| 0–12 months | (N = 323) | (N = 312) | (N = 321) |
| Costs (£) | |||
| Total cost | 461 (916) | 375(611) | 603 (987) |
| Set-up cost | 0 (0) | 45 (0) | 105 (0) |
| Delivery of intervention | 0 (0) | 10 (0) | 52 (18) |
| Health service use | 461 (916) | 320 (611) | 447 (987) |
| Quality of life | |||
| EQ-5D-5L scores at baseline | 0.837 (0.14) | 0.850 (0.12) | 0.849 (0.13) |
| EQ-5D-5L scores at 3 months | 0.840 (0.14) | 0.847 (0.13) | 0.837 (0.14) |
| EQ-5D-5L scores at 12 months | 0.833 (0.15) | 0.836 (0.13) | 0.831 (0.14) |
| QALYs, 0–12 months | 0.837 (0.13) | 0.843 (0.11) | 0.836 (0.13) |
The main results (Table 10), which are adjusted for baseline differences, show that, at 3 months, the cost of the nurse-support group was statistically significantly higher than that of the control group (£135, 95% CI £99 to £171), whereas this was not the case for the postal group (£15, 95% CI –£15 to £45). Table 10 also shows that there was a statistically significant increase in daily steps and minutes of MVPA in ≥ 10-minute bouts for the intervention groups compared with the control group. The ICER per additional minute of MVPA in ≥ 10-minute bouts was £0.35 for the postal group and £2.21 for the nurse-support group, compared with the control group. However, both intervention groups accrued slightly fewer QALYs than the control group (postal group: –0.0005 QALYs; nurse-support group –0.0004 QALYs), although this difference was not statistically significant. Nevertheless, it contributed to the dominance (lower costs and more QALYs) of the control group compared with both intervention groups at 3 months.
| Cost, effects or cost-effectiveness | Trial arm, mean (95% CI) | |||
|---|---|---|---|---|
| Control | Postal deliverya | Nurse supporta | Nurse support vs. postal delivery, mean (95% CI) | |
| Costs and effects over 3 months | ||||
| Total cost per participant (£) | 108 (80 to 136) | 123 (111 to135) | 244 (221 to 266) | – |
| Incremental cost (£) | – | 15 (–15 to 45) | 135 (99 to 171) | 120 (95 to 146) |
| Total QALYs per participant | 0.1957 (0.1936 to 0.1978) | 0.1952 (0.1930 to 0.1974) | 0.1948 (0.1926 to 0.1970) | – |
| Incrementala QALYs | – | –0.0005 (–0.0027 to 0.0016) | –0.0009 (–0.0031 to 0.0012) | –0.0004 (–0.0026 to 0.0018) |
| Incremental daily steps | – | 692 (363 to 1020) | 1172 (844 to 1501) | 481 (153 to 809) |
| Incremental weekly minutes of MVPA in bouts of ≥ 10 minutes | – | 43 (26 to 60) | 61 (44 to 78) | 18 (1 to 35) |
| Costs and effects over 12 months | ||||
| Total cost per participant (£) | 467 (365 to 569) | 376 (307 to 445) | 593 (473 to 714) | – |
| Incremental cost (£) | – | –91 (–215 to 33) | 126 (–37 to 290) | 217 (81 to 354) |
| Total QALYs per participant | 0.842 (0.832 to 0.853) | 0.838 (0.827 to 0.849) | 0.836 (0.824 to 0.847) | – |
| Incremental QALYs | – | –0.004 (–0.017 to 0.009) | –0.007 (–0.020 to 0.007) | –0.002 (–0.016 to 0.011) |
| Incremental daily steps | – | 642 (329 to 955) | 677 (365 to 989) | 36 (–227 to 349) |
| Incremental weekly minutes of MVPA in bouts of ≥ 10 minutes | – | 33 (17 to 49) | 35 (19 to 51) | 2 (–14 to 17) |
| ICERa at 3 months | ||||
| Cost per additional QALY (£) | – | Postal delivery dominated by control | Nurse support dominated by control | Nurse support dominated by postal delivery |
| Cost per additional step count (£) | – | 0.02 | 0.12 | £0.25 |
| Cost per additional minute of MVPA in a bout of ≥ 10 minutes (£) | 0.35 | 0.35 | 2.21 | £6.67 |
| ICERa at 12 months | ||||
| Cost per additional QALY (£) | – | Postal delivery is less costly but has fewer QALYs. £21,162 saved per QALY lost | Nurse support dominated by control | Nurse support dominated by post |
| Cost per additional step count (£) | – | Postal delivery dominates control | 0.19 | 6.03 |
| Cost per additional minute of MVPA in a bout of ≥ 10 minutes (£) | – | Postal delivery dominates control | 3.61 | 109.00 |
Comparing the two interventions at 3 months (see Table 10) shows that the nurse group achieved 481 more steps (95% CI 153 to 809 steps) and 18 more minutes of MVPA (95% CI 1 to 35 minutes) per person than the postal group, at a statistically significant additional cost of £120 (95% CI £95 to £146). The estimated cost per additional step and additional MVPA minute (in bouts of ≥ 10 minutes) was £0.25 and £6.67, respectively. The nurse-support group had slightly fewer QALYs, although this was not significantly different (0.0004 QALYs, 95% CI –0.0026 to 0.0018 QALYs); however, it contributed to the nurse-support group being dominated (higher costs and fewer QALYs) by the postal group.
The main results at 12 months (see Table 10) were somewhat different from those at 3 months. Although the mean costs were lower for the postal group (–£91, 95% CI –£213 to £33) and higher for the nurse-support group (£126, 95% CI –£37 to £290) than the control group, neither was statistically significantly different. However, the increase in the cost of moving from postal delivery to nurse-support delivery was statistically significantly higher (£217, 95% CI £81 to £354). Although both interventions were associated with a statistically significant increase in both step count and weekly minutes of MVPA in ≥ 10-minute MVPA bouts compared with the control group, the difference between intervention groups was not statistically different at 12 months. The postal group took more steps on average (+ 642 steps) and cost less on average (–£91) than the control group, and dominated the control group in terms of the PA outcomes. Compared with the control group, the nurse-support group cost an additional £0.19 per step and £3.61 per additional minute of MVPA in bouts of ≥ 10 minutes. None of the small decrements in QALYs at each incremental comparison (–0.0009 QALYs for the postal group vs. the control group, –0.0007 QALYs for the nurse-support group vs. the control group and –0.0004 QALYs for the nurse-support group vs. the postal group) was statistically significantly different. Compared with the control group, the postal group had fewer QALYs (although this was not statistically significantly different) and lower costs (also not statistically significantly different). However, the magnitude of the cost-savings is such that they outweigh the forgone QALYs at a threshold of £20,000 per QALY, and would be considered cost-effective. Using QALYs, the nurse-support group is dominated by both the control group and the postal group.
Comparing the two interventions at 12 months (see Table 10) shows that the estimated cost per additional step and additional MVPA minute was £6 and £109, respectively, and that, in terms of QALYs, the nurse-support group was still dominated by the postal delivery group.
The cost-effectiveness planes (Figures 5 and 6; see also Appendix 3, Figure 19) broadly confirm the findings that the postal delivery group had a strong likelihood of lower costs, but also fewer QALYs, and that the nurse-support group tended to have fewer QALYs and higher costs than the control group. However, different levels of uncertainty surround these mean estimates, as reflected in the CEACs (Figure 7; see also Appendix 3, Figure 20). At £20,000 per QALY, the postal delivery group has a 50% chance of being cost-effective compared with the control group, which falls to 42% at £30,000 per QALY. This is because, as the willingness-to-pay threshold increases (and, therefore, the higher the value that is placed on forgone QALYs), the value of QALYs lost begins to outweigh the cost-savings (see Figure 9). This is reflected in the CEAC, on which the probability moves towards zero. The nurse-support group had only a 5.5% chance of being cost-effective compared with the control group at a willingness-to-pay threshold to gain, or give up, a QALY of £20,000, and this fell to 4.9% when compared with the postal delivery group.
FIGURE 5.
Cost-effectiveness plane for the postal delivery group vs. the control group at 12 months.
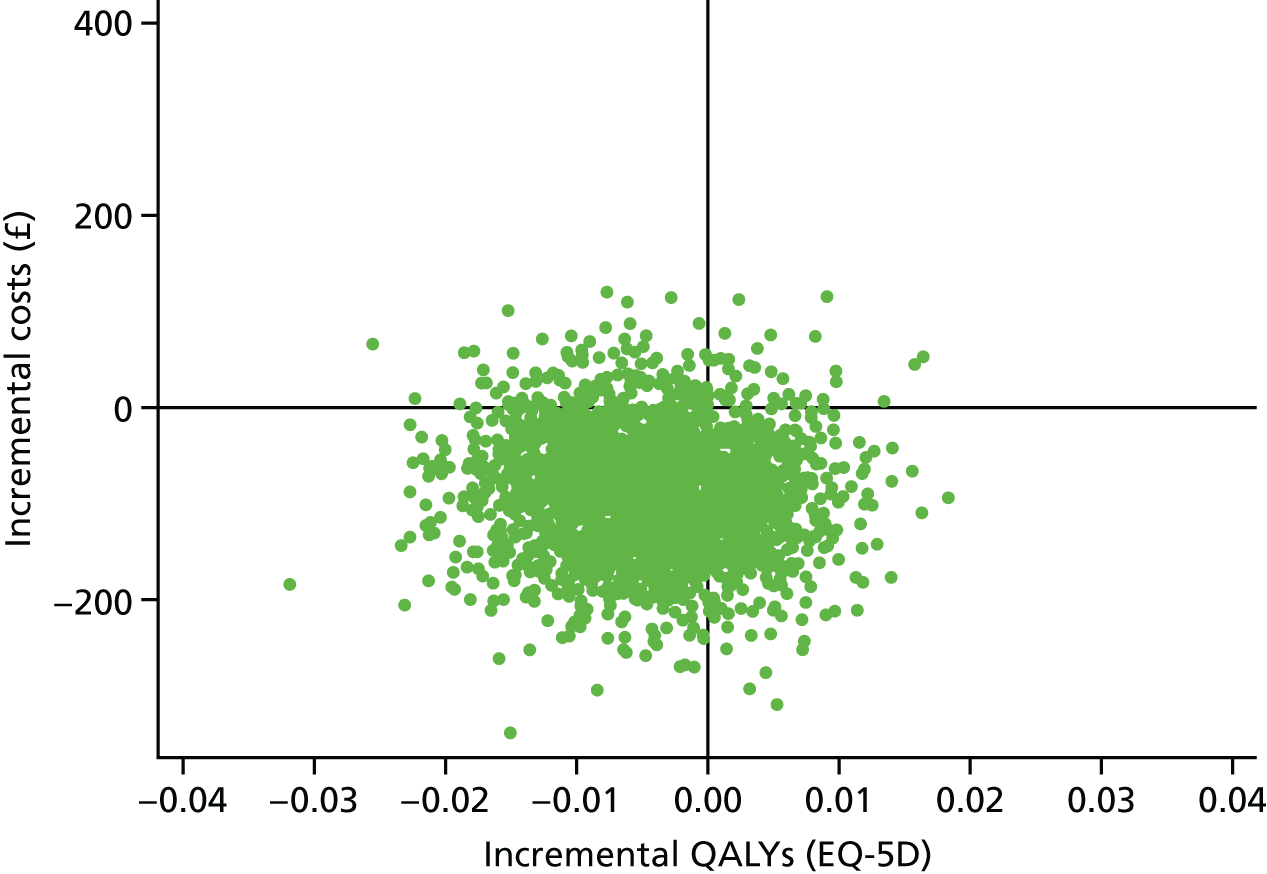
FIGURE 6.
Cost-effectiveness plane for the nurse-support group vs. the control group at 12 months.
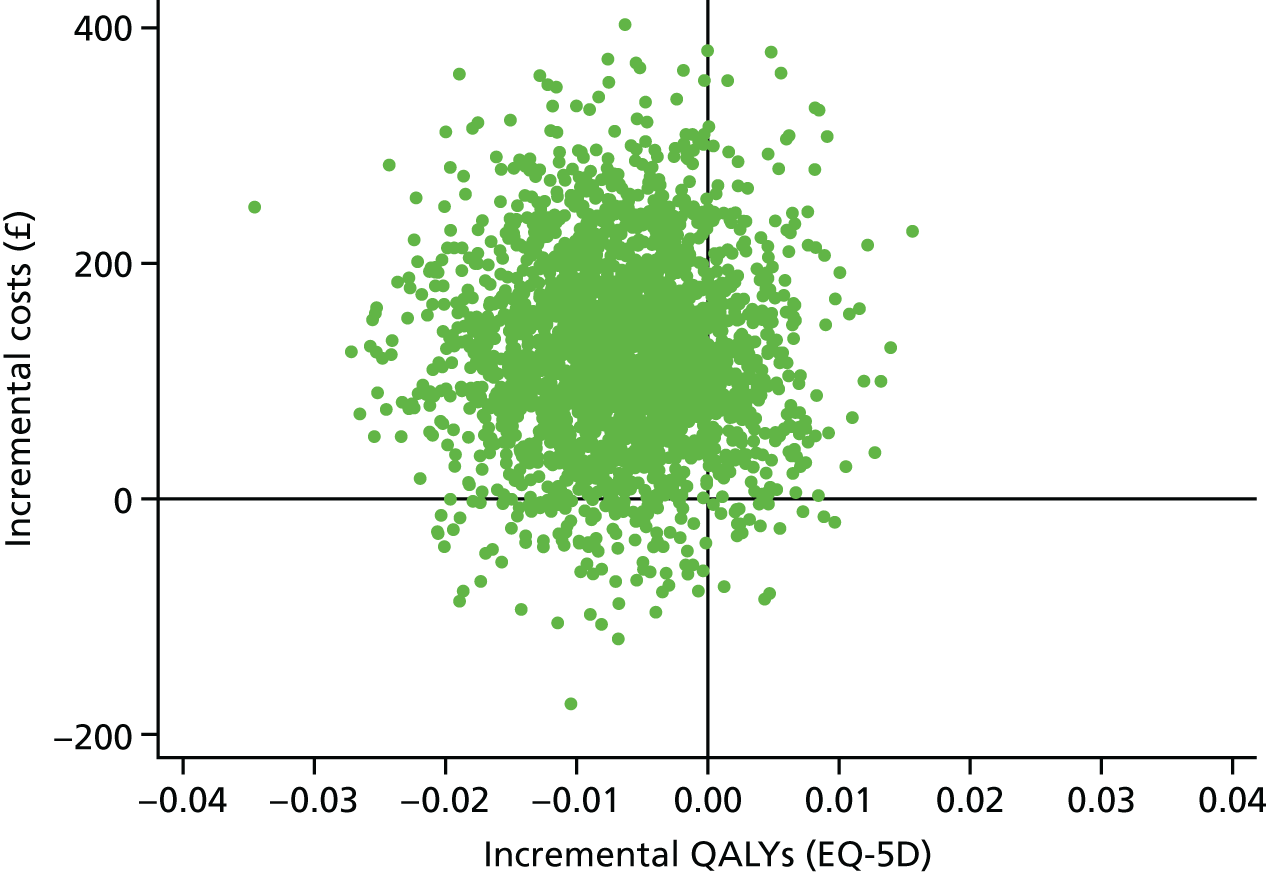
FIGURE 7.
Cost-effectiveness acceptability curve for the postal delivery and nurse-support groups (vs. the control group) at different willingness-to-pay-per-QALY thresholds.
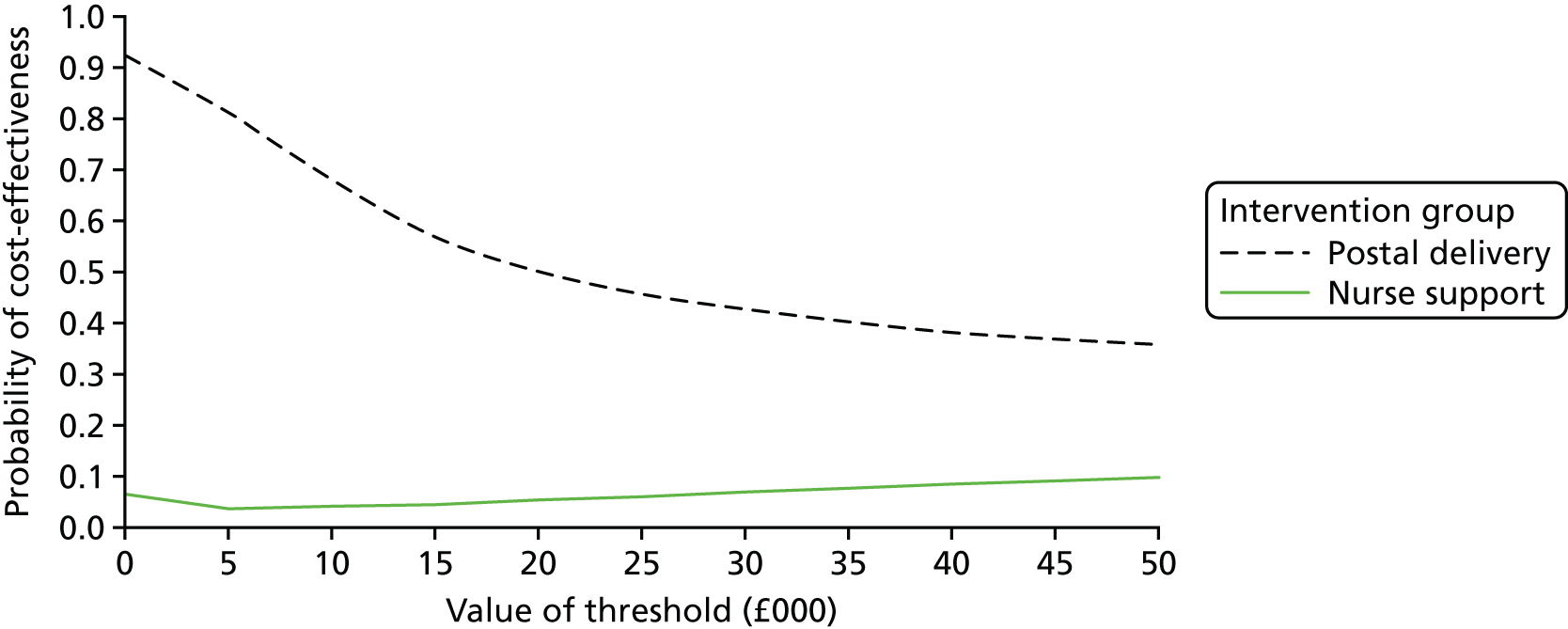
The deterministic sensitivity analyses (see Appendix 3, Table 36) show that, with the exceptions of (1) using health service use including only self-reported serious adverse effects, (2) excluding all health service costs, (3) changing perspective (including all participant costs) and (4) the worst-case ‘combined scenario’, the sensitivity analyses produced results that were consistent with the base-case findings. For the exceptions, the postal delivery group was dominated by the usual-care control group at 12 months.
Beyond-trial modelling
Systematic reviews have indicated the positive influence that PA has on primary prevention for a range of conditions, including coronary heart disease (CHD), stroke, type 2 diabetes mellitus and cancers, 124–128 and, more recently, for improving cognition in older adults. 129 Carlson et al. 5 have separately shown that increasing amounts of leisure time PA is associated with decreasing health expenditure. Therefore, by reducing the risk of disease, increased PA can increase future QALYs, as well as lower future costs. The next section provides the methods used to estimate the long-term cost-effectiveness of the PACE-UP trial.
Methods
An existing Markov model,130 designed to assess the cost-effectiveness of PA interventions, was used. This model has evolved from assessing the cost-effectiveness analysis of exercise referral schemes131–133 to brief interventions129 and beyond. 134 It has been used by NICE to update national guidance (PH44 on brief interventions, PH2 on exercise referral schemes guidance)36,135 and is the basis for the NICE Return on Investment Tool136 used by local authorities.
The model is driven by evidence of the impact that PA interventions have on the proportion of people meeting the recommended PA levels, short-term quality-of-life gains (associated with meeting the recommended PA levels) and the impact of PA on future rates of CHD, stroke and type 2 diabetes mellitus. Figure 8 shows the pathways within the model. In the original Markov model,129 a cohort of 100,000 33-year-old people were followed in annual cycles over their lifetime. At the end of the first year of the model, the cohort would be either ‘active’ (doing 150 minutes of MVPA per week) or ‘inactive’, and could have had one of three events (non-fatal CHD, non-fatal stroke, type 2 diabetes mellitus), remain event free (i.e. without CHD, stroke or diabetes mellitus) or die, either from cardiovascular disease (CVD) or from non-CVD-related causes. Active individuals have a better life expectancy and quality of life, attributable to lower risks of developing CHD, stroke and type 2 diabetes mellitus. People who become active in the first year (irrespective of the trial arm) accrue a one-off utility gain associated with achieving the recommended level of PA. QALYs reflect the health outcomes from a reduced risk of disease. A discount rate of 3.5% per annum is used for costs and QALYs, as recommended by NICE, and the analysis is conducted from a NHS perspective. Full details of the model are provided in Appendix 3 and elsewhere. 130
The model by Anokye et al. 130 was adapted for use in five ways:
-
The population begins with the mean age of the trial population (i.e. 59 years rather than 33 years) and was followed to 88 years (average life expectancy at 59 years in the UK). 137 This change was also reflected in the age-specific estimates used.
-
The intervention was either pedometer plus nurse support or a posted pedometer.
-
Within-trial costs were used, with a second year of annuitised values added for the intervention arms (£5.03 per person for the postal delivery group and £4.14 per person for the nurse-support group – the number of pedometers to the postal delivery group was relatively higher as a result of more replacements), as the trial analysis had assumed pedometers lasted 2 years.
-
Effectiveness estimates from the PACE-UP trial were used as follows: the probability of moving from an inactive to an active state was based on estimated relative risks (RRs) for achieving 150 minutes of MVPA in ≥ 10-minute bouts over 7 days at 12 months. RRs of achieving ≥ 150 minutes of MVPA in ≥ 10-minute bouts at 12 months were estimated from ORs using the formula OR/[(1 – Pref) + (Pref × OR)], in which Pref is the proportion of all subjects achieving 150 minutes of MVPA in ≥ 10-minute bouts at baseline (i.e. 218/1023 = 0.21). The OR was derived from a logistic regression model in which achieving 150 minutes of MVPA in bouts of ≥ 10 minutes at 12 months was regressed on baseline minutes of MVPA in bouts of ≥ 10 minutes, month of baseline accelerometry, age, sex, general practice and treatment group, with household as a cluster.
-
The short-term psychological benefits associated with achieving 150 minutes of MVPA per week used trial data; incremental EQ-5D-5L scores (at 12 months) for active people were regressed, via beta regression, on EQ-5D-5L scores at baseline, ethnicity, education, employment status, intervention group, practice and disability.
Other parameters were informed by estimates from the original model. Cost and utility estimates for disease conditions were originally sourced from literature reviews of economic evaluations conducted for NICE on CVD and diabetes mellitus. 5 Estimates for the health impacts of PA were taken from national/international evidence-based guidance on PA and health, such as the US Physical Activity Guidelines Advisory Committee Report, 2008. 138 Appendix 3, Table 37 details all parameter values.
The probabilistic sensitivity analysis (PSA) addressed the uncertainties around all parameters in the model (except for baseline mortality, as the mortality census data have little uncertainty). A total of 10,000 Monte Carlo simulations were used to generate stable estimates. Given that the within-trial sensitivity analysis showed the decisional influence of some assumptions, a deterministic sensitivity analysis using the lifetime model explored two alternative, conservative scenarios:
-
Scenario 1 – combined exclusion of all health service use costs during the trial period (year 1 of the model), with no short-term QALY gain associated with achieving the recommended level of PA. This was considered as a result of the uncertainty around short-term changes to health service use, and because previous studies found the exclusion of short-term QALY gain associated with being physically active to the recommended level to be decisional. 5,129
-
Scenario 2 – scenario 1 plus user costs related to participation in PA and the interventions. This combination represented a ‘worst-case’ scenario in the trial. Although this perspective is not one adopted by NICE, it represents the most conservative scenario based on this evidence.
Results
Table 11 shows that the postal delivery group dominated both usual practice and the nurse-support group, as the lifetime costs were lower and the number of QALYs was greater. The stochastic uncertainty associated with the mean ICER (Appendix 3, Figure 21) indicates that these findings are robust, as there is a 100% likelihood, at a willingness-to-pay threshold of £20,000 per QALY, that the postal delivery group is cost-effective compared with the control group and with the nurse-support group (Figure 9). This is consistent with the net monetary benefit estimates, which show that, although we can be 95% confident that the postal delivery group is better than the control group and the nurse-support group, we cannot be 95% confident that the nurse-support group is better than the control group.
| Costs, effects and cost-effectiveness | Trial arm, mean (95% CI) | |||
|---|---|---|---|---|
| Control | Postal deliverya | Nurse supporta | Nurse support vs. postal delivery | |
| Cost | ||||
| Lifetime total cost (£M)b | 340 (307 to 371) | 329 (296 to 361) | 351 (318 to 384) | – |
| Lifetime incremental cost (£M) | – | –11 (–12 to –10) | 11 (10 to 12) | 22 (21 to 23) |
| QALYs | ||||
| Lifetime total QALYs (million) | 1.07 (0.88 to 1.30) | 1.07 (0.88 to 1.30) | 1.07 (0.89 to 1.30) | – |
| Lifetime incremental QALYs | – | 759 (400 to 1247) | 671 (346 to 1071) | –108 (–223 to –10) |
| Lifetime ICER for QALYs (£) | – | Postal delivery dominates control | 16,368 | Postal delivery dominates nurse support |
| Lifetime incremental net monetary benefit (£M, @ £20,000 per QALY) | – | 26 (18 to 36) | 2 (–5 to 11) | –24 (–27 to –21) |
FIGURE 9.
Cost-effectiveness acceptability curve showing the probability of lifetime cost-effectiveness for the postal delivery group and the nurse-support group (vs. the control group) at different willingness-to-pay threshold levels.
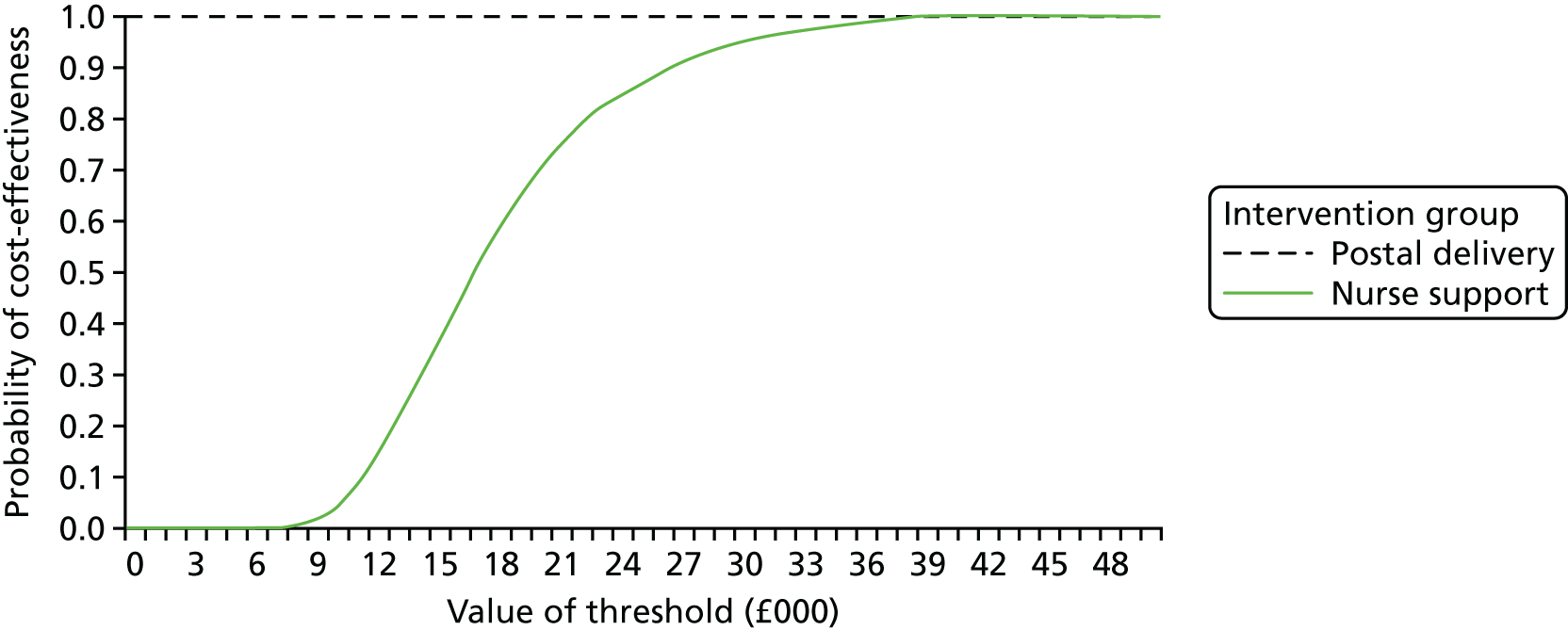
The results for scenario 1 of the sensitivity analyses for the 100,000 cohort were as follows:
-
postal delivery group versus control group – postal delivery moved from a dominant position to being a more expensive option (+£4M) with greater QALY gains (+609 QALYs) and an ICER of £6100
-
nurse-support group versus control group – the ICER increased from £16,000 to £26,000 (+£14M, +538 QALYs)
-
nurse-support versus postal delivery group – the nurse-support group remained dominated by the postal delivery group (+£10M, –87 QALYs).
For scenario 2, the sensitivity analyses for the 100,000 cohort showed the following:
-
postal delivery group versus control group – the postal delivery group moved from a dominant position to being more expensive (+£16M) with greater QALY gains (+609 QALYs) and an ICER of £26,600
-
nurse-support group versus control group – the ICER increased from £16,000 to £25,400 (+£13.7M; +538 QALYs)
-
nurse-support group versus postal delivery group – the nurse-support group moved from a dominated position to a cost-effective position (–£2M, –87 QALYs).
Discussion
The within-trial analysis shows that, at 3 months, compared with the control group, both interventions cost something to achieve increases in PA. Compared with the control group, the postal delivery group cost an additional £0.02 per additional step gained to an average of + 692 steps per day, which was a cheaper buy than the nurse-support group (at £0.12 per additional step). However, the nurse-support group achieved more steps on average, and the additional 448 steps were achieved at an incremental cost of £0.25 per step. Although this pattern of results was replicated for additional minutes of MVPA (in bouts of 10 minutes), the results for QALYs were very different as both intervention groups were dominated by the control group (i.e. the control group cost less and had more QALYs).
The main results at 12 months were different, leaning more favourably towards the postal intervention. Compared with the control group, the postal delivery group achieved statistically significantly better PA outcomes, and did so at a lower cost. This was much better than the results for the nurse-support group, and the insignificant difference in PA outcomes between the nurse-support and postal delivery groups at 12 months implied very high costs per additional step (£6.00). The analysis of the costs per QALY confirmed that the nurse-support group was not a cost-effective alternative. It also showed that, although the postal delivery group has fewer QALYs (although not statistically different) and lower costs (with costs saved higher than £20,000 per QALY), the postal delivery group could be considered to be cost-effective. Assuming a value of £20,000 per QALY, there was a 50% probability that the postal delivery group was cost-effective compared with the control group, and a 5% probability that the nurse-support group was cost-effective compared with the control group or the postal delivery group at 1 year. The sensitivity analyses did not change the conclusions, except in three cases (using self-reported SAEs, excluding health service use, but including all participant costs), when the postal delivery group was dominated by the usual-care control group.
The lack of evidence on effectiveness in terms of quality-of-life outcomes is not necessarily evidence of no effect, as the trial was not powered to detect a change in quality of life. The results indicate a lot of variation around the change in QALYs (95% CI –0.017 to 0.009 QALYs) and we are aware of some ceiling effects at baseline (98% self-care, 83% usual activities, 79% mobility, 73% anxiety, 43% pain). Although this might contribute to raising questions about the relevance of general quality-of-life measures for capturing the quality-of-life impacts of public health interventions within the first year, it also serves to highlight the importance of capturing the QALY impact of public health interventions on disease avoidance in longer-term economic models. Cost-per-QALY results from short-term public health trials have the potential to mislead decision-makers on the efficiency of investments in the context of changes that lead to longer-term reductions in the risk of disease.
A lifetime cost-effectiveness model characterised the long-term impact of PA interventions on CHD, stroke and type 2 diabetes mellitus. 129 This showed that the postal delivery group would dominate both the control group and the nurse-support groups, as quality-of-life gains (759 QALYs, 95%CI 400 to 1247 QALYs) add to increased cost-savings (–£11M, 95% CI –£12M to –£10M), resulting in an incremental net monetary benefit of £26M (95% CI £18M to £36M) for a 100,000 cohort. There was a 100% likelihood that the postal intervention was cost-effective compared with the control group and the nurse-support group. The conservatively framed deterministic scenario analyses showed that excluding both the short-term reduction in health service use and the utility gain seen in the trial would not alter the main conclusion that the postal delivery group would be an extremely cost-effective intervention (ICER of £6100 per QALY). Even taking the unusual step of including participant costs did not raise the ICER beyond a threshold value of £30,000 per QALY.
Strengths and weaknesses
To our knowledge, this is the first published study of short- and long-term costs and effects of a pedometer intervention. Its strengths are the use of detailed individual-level cost and effectiveness data from a well-designed population-based RCT, which had nearly complete (93.4%) follow-up data to 1 year, to estimate the cost per QALY at 1 year, and also to input into a lifetime model of cost-effectiveness. It also included both provider and user perspectives in costing service provision and participation, which allowed the impact of perspective to be investigated, and which provides a basis for further investigation of the role of cost in its association with participation in PA. The estimates of uncertainty extended commonly used techniques to account for increased precision in the context of clustered data. The sensitivity analysis pushed both the short- and long-term analyses to very conservative outcomes given the trial data and, therefore, provides a good indication of the robustness of findings based on the current evidence.
The weakness of the within-trial cost-effectiveness study connects to the cost of health service use over the period of the trial; no information was available on the severity or procedures used for hospital admissions, or cause of admission to the A&E department. We relied on the principal investigator’s ‘best guess’ or ‘nearest appropriate code’ (while blind to the treatment group) and on averaging across elective/non-elective admissions and we therefore explored alternative assumptions in our sensitivity analyses. There was considerable variation in costs in each trial arm, and the trial was not powered to detect a difference. Data were also not collected on costs to participants for months 4–9, and the last 3 months were multiplied to represent the missing data. These may have over- or underestimated participant costs and, if significantly underestimated, could be decisionally important. With respect to the long-term modelling, the model assumes that people would revert to PA patterns observed in long-term cohort studies. This estimate could be improved with longer-term trial data. All the challenges set out in previous work130 are also relevant here (e.g. some diseases, such as cancer and AEs, are not accounted for, which could lead to either over- or under-estimation of cost-effectiveness).
The study feeds into an area sparse of primary data139,140 populated only by small studies. 95,96 Leung et al. 97 showed a 95% likelihood that pedometers would be a cost-effective addition to green prescriptions (in New Zealand) at 12 months, which is much higher than the 50% likelihood that we found. Our study also provides long-term estimates based on the population-level primary data for comparison with the larger body of cost-effectiveness estimates134 from decision models. 97,98,141,142 Some have identified cost-savings and an improved quality of life at a population level from pedometers in the long term. 98,137 Others97,138 have indicated high probabilities that pedometers will be cost-effective in the long term, with Brennan et al. 142 indicating that, even with long-term support at £25 per year (for monitoring and support), ICERs fell well below £10,000 per QALY gained. Our study provides further support to indicate that pedometer-based programmes are a cost-effective method of improving health.
Conclusion
A range of sensitivity analyses of both short- and long-term cost-effectiveness confirmed the view that postal delivery of pedometer interventions to people aged 45–75 years through primary care has a high chance of being cost-effective in the long term and has a 50% likelihood of being cost-effective, through resource-savings from changes in health service use, within 1 year. Further research is needed to ascertain the level of maintenance of PA beyond 1 year and the impact of PA on quality of life and general health service use in both the short term and long term.
Chapter 5 Generalisability
Introduction
Although the numbers of adults achieving the recommended PA levels are generally low,9 there are marked differences between groups, with lower PA levels in women, older people and those from socioeconomically deprived areas and of Asian ethnicity. 9,143 Walking interventions aiming to increase PA levels ideally need to try to ensure that these groups are well represented. When participation rates are low,144–146 there may be systematic differences between those who participate and those who do not, but to whom the intervention could reasonably be applied. Failure to include certain groups of people for whom PA levels are lower may lead to the implementation of interventions that are likely to increase health inequalities. When there are differences between participants and non-participants, exploring the reasons for non-participation using a qualitative approach can be instructive.
The population-based sampling frame used in this study provided an opportunity to assess whether or not there were differences in terms of age, sex, ethnicity and area-level deprivation between general practice patients who replied to the invitation letter compared with those who did not. We also compared the health, lifestyle, education and social factors of those who agreed to participate in the trial with those who agreed to complete a questionnaire, but who did not wish to participate (see Appendix 4 for the non-participant questionnaire). These findings are now published. 147 Qualitative interviews with a sample of this latter group allowed us to investigate the reasons for non-participation in the trial, and these findings are also published. 148
Methods
Data collection for quantitative comparisons
The sex, age and IMD score of all those invited were collected from general practice records. The IMD score is an anonymised measure of deprivation based on postcode. 71 To avoid the possibility of individuals being identified, aggregated practice-recorded ethnicity was exported from the practice in 10-year age bands for all batches where everyone was mailed, less exclusions (n = 10,155). Those not wanting to participate in the trial were asked in their invitation letter if they were willing to complete a shortened version of the trial baseline questionnaire, including questions on demographics, health, PA levels and a question on reasons for not participating. The following categories were offered, based on previous research, with space to add other reasons for trial non-participation: (1) I do not have time, (2) I cannot increase my PA, (3) I am not interested in increasing my PA, (4) I am already very physically active, (5) I am not interested in research and (6) I do not want to be put in a group by chance.
Comparison groups
Individuals whose invitation letters were ‘returned to sender’ were excluded from the analyses before calculating response rates. ‘Responders’ are defined as those who replied to the invitation letter, regardless of whether or not they wanted to take part. Individuals could respond by post, e-mail or telephone. ‘Participants’ are those who completed the baseline assessment, although not all were randomised as some provided inadequate accelerometry data. ‘Non-participants’ are those who completed a questionnaire but did not wish to participate in the trial (Figure 10).
FIGURE 10.
Flow chart to show the recruitment process in the PACE-UP trial. All percentages are out of all of those whose age and sex were matched with GP records (n = 10,927).
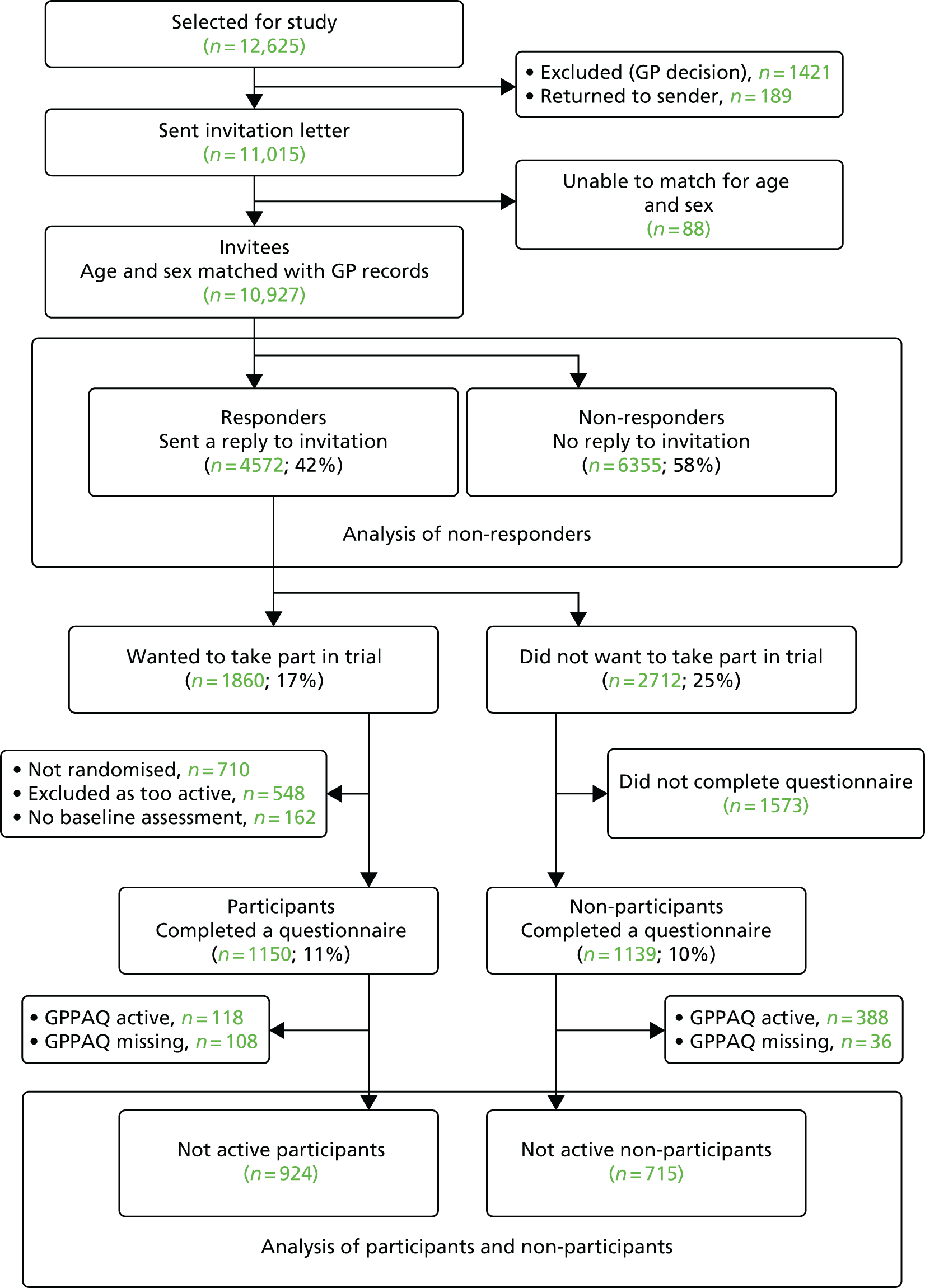
As the PACE-UP trial targeted inactive adults, participants who attended a baseline appointment were selected on the basis of their low PA levels. Non-participants were not selected in this way. In order to minimise selection bias, the quantitative analysis of participants and non-participants was therefore restricted to those categorised as ‘not active’ according to a self-reported primary care PA questionnaire, the GPPAQ,73 which was the only PA measure available for both groups.
Statistical analysis
Age- and sex-standardised rates were used to compare IMD quintiles for responders. Similarly, sex-standardised rates were used to compare age groups and age-standardised rates were used to compare sexes. The full population of invitees was used as a standard population throughout. No further analysis on non-responders was possible because they did not provide any questionnaire data on ethnicity or other factors.
Practice ethnicity data were available in 10-year age bands for 10,155 invitees, which was effectively a random sample of the 11,015 people invited to participate. The proportion of patients belonging to each ethnicity category within each age band and within each practice was calculated, and the number of invitees in each ethnicity in each practice and age band was estimated. Overall, 1903 invitees had ethnicity recorded as ‘unknown’. These are assumed to be missing at random in the main results, but sensitivity analyses were performed assuming that these were all white people or all non-white people. Age-standardised participation rates for not-active participants and non-participants completing questionnaires were calculated, assuming that invitees gave the same ethnicity on the questionnaire as was recorded in their practice records. Participation rates by age, sex and IMD score were calculated for not-active participants versus not-active non-participants completing questionnaires, as in the analysis of responders.
Not-active participants and non-participants completing the questionnaire were compared for additional demographic and social characteristics, and health and lifestyle factors, using logistic regression. All data came from the questionnaires. Models were adjusted for clustering by practice and household, by including fixed effects for practice and using robust standard errors for household.
Methods for the interview study of non-participants
This is fully described elsewhere. 148 Non-participants completing the questionnaire were asked if they could be contacted to discuss their reasons in more detail. A purposive sample of those willing to be contacted was selected to provide men and women of varying ages, ethnicities and employment status from the initial six participating practices. To maximise participation, we used focused telephone interviews; permission was gained for interviews to be audio-recorded. The topic guide was developed from the literature, from the previous PACE-LIFT trial qualitative evaluation21,149 and discussions between the authors, and is published148 and provided in Appendix 4. Approximately 30 interviews were planned, with recruitment continuing until no new themes were identified and a demographically balanced sample had been achieved. Open questions were asked about what influenced their decision not to participate and their opinions on the trial information received. Responses given on the completed questionnaires were used as a starting point to further explore their reasons. They were asked general questions about the perception of the trial design and were invited to make any final comments.
Methods for analysing interviews
Audio-recordings were transcribed verbatim and checked for accuracy. After 10 interviews, researchers (Rebecca Holmes, TH and CV) read the transcripts and discussed the interviews. The interview technique was then modified slightly to ensure that interviewees understood the trial randomisation process, as several participants had appeared not to understand the question about whether or not being put in a group by chance had influenced their decision not to participate. On completion of the interviewing, the transcripts were read and re-read for familiarisation by the researchers, who assigned codes (RN and TH), before a thematic framework was produced. 150 Coding discrepancies between researchers were resolved by discussion. The framework produced was informed both by a priori issues, mostly related to trial design, and by emerging themes. Themes were refined further by discussion between authors (Rebecca Holmes, TH, RN and CV), and broader categories, encompassing several subthemes, were generated. Reasons for declining given by all non-participants were also compared with those given at interview, to put our findings in a wider context and assess the generalisability to all of those actively declining.
Results
Results from quantitative comparisons
Of the 12,625 individuals selected for screening (see Figure 10), 1421 (11.3%) were excluded by practice staff and 189 (1.5%) had invitation letters that were returned, as they had moved away; both of these groups were classified as ‘not invited’. In the 44 households where one person refused the invitation and the other did not respond, it was impossible to match the response to individual invitees within the household, so age and sex are unknown. These 88 people have been excluded from further analyses. Of the remaining 10,927, 4572 (42%) responded to the invitation letter, mainly by post, and 1150 (11%) completed the baseline assessments.
Of all invitees, 5229 (48%) were aged 45–54 years. Although all quintiles of deprivation were represented, only 7% were in the most deprived quintile. Response rates were higher in older people, women and those living in less deprived areas (Table 12). As individual ethnicity was available only for the participants and non-participants who completed a questionnaire, it was not possible to estimate response rates by ethnicity for all responders.
| Characteristic | All invitees (N = 10,927), n (%) | Responders to the invitation (N = 4572) | ||
|---|---|---|---|---|
| n | Standardised percentage responsea (95% CI) | Ratio of response rates (95% CI) | ||
| Age (years) | ||||
| 45–54 | 5229 (47.8) | 1698 | 33.4 (32.1 to 34.7) | 0.57 (0.54 to 0.60) |
| 55–64 | 3367 (30.8) | 1535 | 46.2 (44.5 to 47.9) | 0.79 (0.76 to 0.84) |
| 65–75 | 2331 (21.3) | 1339 | 57.8 (55.8 to 59.8) | 1.00 |
| Sex | ||||
| Female | 5604 (51.3) | 2638 | 46.7 (45.4 to 48.0) | 1.00 |
| Male | 5323 (48.7) | 1934 | 36.8 (35.5 to 38.1) | 0.80 (0.76 to 0.84) |
| IMD national quintileb | ||||
| 1 (most deprived) | 712 (6.8) | 207 | 29.5 (26.2 to 32.8) | 0.55 (0.50 to 0.61) |
| 2 | 2768 (26.4) | 995 | 36.1 (34.4 to 37.9) | 0.67 (0.63 to 0.72) |
| 3 | 2960 (28.2) | 1242 | 41.2 (39.8 to 43.2) | 0.77 (0.73 to 0.82) |
| 4 | 2328 (22.2) | 1060 | 45.6 (43.6 to 47.5) | 0.85 (0.80 to 0.90) |
| 5 (least deprived) | 1711 (16.3) | 914 | 53.4 (51.4 to 56.0) | 1.00 |
Although the GPPAQ was not used to assess PA levels for trial inclusion, it was the only PA measure available for both participants and non-participants. A total of 118 participants and 388 non-participants were classified as active by the GPPAQ, and 134 people did not complete the GPPAQ. These people were excluded from further analysis, leaving 924 participants and 715 non-participants.
Similar to the response rates, participation rates were higher in older people, women and those living in less deprived areas. (Table 13). Ethnicity was extracted from the practice for 10,155 invitees. Of these, 5991 were recorded as white (59%), 893 (9%) were recorded as Asian or British Asian and 915 (9%) were recorded as black Caribbean, black British or black African. A total of 1903 (18.7%) were recorded as ‘unknown’. The percentage of people with ‘unknown’ ethnicity varied by practice from 3% to 48%. Table 13 shows the estimated number in each ethnicity category for all 10,927 invitees, assuming that those for whom ethnicity was recorded as ‘unknown’ and those for whom we were not able to collect ethnicity have the same ethnicity distribution as the group with known ethnicity.
| Characteristic | All invitees (N = 10,927), n | Participants (N = 9241)a | Non-participants (N = 7152)b | ||||
|---|---|---|---|---|---|---|---|
| n | Standardised completion ratea (95% CI) | Ratio of completion rates (95% CI) | n | Standardised completion ratec (95% CI) | Ratio of completion rates (95% CI) | ||
| Age (years) | |||||||
| 45–54 | 5229 | 331 | 6.4 (5.7 to 7.1) | 0.60 (0.51 to 0.71) | 231 | 4.6 (4.0 to 5.1) | 0.41 (0.34 to 0.49) |
| 55–64 | 3367 | 342 | 10.1 (9.1 to 11.1) | 0.94 (0.81 to 1.10) | 213 | 6.3 (5.5 to 7.1) | 0.56 (0.47 to 0.67) |
| 65–74 | 2331 | 251 | 10.8 (9.4 to 11.9) | 1.0 | 264 | 11.2 (10.0 to 12.6) | 1.00 |
| Sex | |||||||
| Female | 5604 | 597 | 10.6 (9.8 to 11.4) | 1.0 | 408 | 7.2 (6.5 to 7.9) | 1.00 |
| Male | 5323 | 327 | 6.2 (5.6 to 6.9) | 0.59 (0.52 to 0.67) | 308 | 5.9 (5.2 to 6.5) | 0.82 (0.71 to 0.94) |
| IMD national quintiled | |||||||
| 1 (most deprived) | 712 | 40 | 5.5 (3.8 to 7.2) | 0.52 (0.39 to 0.70) | 31 | 4.5 (3.0 to 6.0) | 0.51 (0.37 to 0.70) |
| 2 | 2768 | 183 | 6.7 (5.7 to 7.6) | 0.63 (0.52 to 0.78) | 128 | 4.6 (3.8 to 5.4) | 0.52 (0.41 to 0.66) |
| 3 | 2960 | 288 | 9.6 (8.6 to 10.7) | 0.92 (0.77 to 1.10) | 213 | 7.1 (6.2 to 8.0) | 0.80 (0.65 to 0.98) |
| 4 | 2328 | 206 | 8.8 (7.7 to 10.0) | 0.84 (0.69 to 1.02) | 172 | 7.4 (6.3 to 8.4) | 0.83 (0.67 to 1.03) |
| 5 (least deprived) | 1711 | 179 | 10.5 (9.1 to 11.9) | 1.0 | 150 | 8.9 (7.5 to 10.2) | 1.00 |
| Ethnicity | |||||||
| White | 81,295e | 709 | 8.7 (8.1 to 9.3) | 1.0 | 638 | 7.9 (7.3 to 8.4) | 1.00 |
| Asian | 11,315e | 61 | 5.4 (4.1 to 6.7) | 0.62 (0.50 to 0.76) | 27 | 2.4 (1.5 to 3.3) | 0.31 (0.24 to 0.38) |
| Black | 10,845e | 90 | 8.5 (6.7 to 10.2) | 0.97 (0.79 to 1.20) | 20 | 1.9 (1.1 to 2.8) | 0.24 (0.19 to 0.31) |
| Other | 5835e | 22 | 3.8 (2.2 to 5.4) | 0.44 (0.33 to 0.59) | 20 | 3.9 (2.2 to 5.6) | 0.59 (0.36 to 0.68) |
Of the white invitees, 709 (8.7%) agreed to participate in the trial and were not active, and a further 638 (7.9%) completed a non-participant questionnaire and were not active. Both Asian and black invitees had very low non-participant questionnaire completion (2.4% and 1.9%), but black invitees were as willing to participate as white invitees (8.5% vs. 8.7%), whereas only 5.4% of Asian invitees participated. The sensitivity analyses assuming that all ethnicities recorded as ‘unknown’ were white or non-white people showed similar results, and the same patterns were also seen in practices with nearly complete ethnicity coding.
Those providing questionnaire data were more likely to be working part-time, married or living with a partner, and less likely to have finished their education aged ≤ 16 years (Table 14). Participation was associated with recent primary care contact and with some degree of health problems (general health, long-standing illness and comorbidities), although those more severely affected were less likely to participate (see Table 14). This is consistent with the EQ-5D-5L (health-related quality-of-life) domains, whereby participants were more likely to have problems with pain and mobility, but less likely to have problems with self-care, which is likely to indicate greater disability. Forty-five per cent of the sample gave insufficient time (n = 327) or already being physically active (n = 325) as reasons for non-participation, even though those classified on the GPPAQ as active were excluded from this analysis. Less commonly, 152 (21%) people answered that they could not or were not interested in (n = 122, 17%) increasing their PA. Randomisation was cited as a reason for non-participation only by 88 respondents (12%).
| Variables | Participants with baseline information (N = 924),a n (%) | Non-participants who completed a questionnaire (N = 715),a n (%) | OR for participation adjusted for clustering (95% CI)b | OR for participation adjusted for clustering, age and sex (95% CI) |
|---|---|---|---|---|
| Demographic factors | ||||
| Invited as a couple | 393 (42.3) | 314 (43.9) | 0.98 (0.79 to 1.21) | 0.99 (0.79 to 1.23) |
| Married/living together as a couple | 595 (65.8) | 439 (62.5) | 1.20 (0.96 to 1.49) | 1.25 (1.01 to 1.56)* |
| Age (years) finished full-time education | ||||
| ≤ 16 | 238 (26.4) | 246 (35.6) | 0.64 (0.49 to 0.83) | 0.67 (0.51 to 0.87) |
| 17 or 18 | 204 (22.6) | 122 (16.2) | 1.39 (1.10 to 1.76) | 1.23 (0.93 to 1.64) |
| ≥ 19 | 334 (48.3) | 334 (48.3) | 1.0** | 1.0** |
| Employment status | ||||
| Full-time | 334 (37.1) | 248 (35.4) | 1.0*** | 1.0** |
| Part-time | 175 (19.4) | 83 (11.8) | 1.60 (1.17 to 2.19) | 1.57 (1.13 to 2.18) |
| Retired | 274 (30.4) | 269 (38.4) | 0.77 (0.60 to 0.99) | 0.87 (0.63 to 1.21) |
| Other | 118 (13.1) | 101 (14.4) | 0.87 (0.64 to 1.19) | 0.85 (0.62 to 1.17) |
| Residential status | ||||
| Home owner | 734 (82.7) | 587 (84.2) | 0.92 (0.69 to 1.23) | 0.91 (0.68 to 1.21) |
| Health and lifestyle factors | ||||
| Contact with GP or nurse in the last 3 months | 591 (65.4) | 409 (59.3) | 1.31 (1.61 to 1.06)* | 1.34 (1.09 to 1.65)** |
| Current smoker | 74 (8.4) | 62 (9.0) | 0.90 (0.63 to 1.29) | 0.87 (0.60 to 1.24) |
| General health level | ||||
| Very good/good | 727 (81.0) | 579 (84.0) | 1.0* | 1.0* |
| Fair | 154 (17.2) | 88 (12.8) | 1.34 (1.01 to 1.79) | 1.40 (1.05 to 1.86) |
| Poor/very poor | 16 (1.8) | 22 (3.1) | 0.54 (0.28 to 1.04) | 0.56 (0.29 to 1.09) |
| Limiting long-standing illness | ||||
| Yes, a lot | 24 (2.7) | 46 (6.7) | 0.40 (0.24 to 0.66) | 0.41 (0.24 to 0.70) |
| Yes, a little | 194 (21.7) | 113 (16.4) | 1.35 (1.04 to 1.77) | 1.40 (1.07 to 1.84) |
| No | 678 (75.7) | 528 (76.9) | 1.0*** | 1.0*** |
| One or more comorbidities | 568 (58.6) | 401 (41.4) | 1.23 (1.01 to 1.51)* | 1.29(1.05 to 1.59)* |
| One or more different medications taken per day | 517 (57.6) | 384 (55.5) | 1.07 (0.87 to 1.31) | 1.17 (0.95 to 1.46) |
| EQ-5D measurements | ||||
| Mobility – some problems | 202 (22.4) | 122 (17.4) | 1.36 (1.05 to 1.76)* | 1.44 (1.10 to 1.87)** |
| Self-care – some problems | 23 (2.6) | 31 (4.4) | 0.53 (0.30 to 0.93)* | 0.56 (0.32 to 0.99)* |
| Usual activities – some problems | 163 (18.3) | 121 (17.2) | 1.06 (0.81 to 1.38) | 1.09 (0.83 to 1.43) |
| Pain/discomfort – some problems | 522 (58.0) | 326 (46.4) | 1.61 (1.31 to 1.97)*** | 1.62 (1.32 to 2.00)*** |
| Anxiety/depression some problems | 247 (27.8) | 169 (24.0) | 1.20 (0.96 to 1.52) | 1.19 (0.94 to 1.50) |
| Health factors relating to exercise | ||||
| Balance problems | 106 (11.7) | 64 (9.3) | 1.26 (0.91 to 1.76) | 1.27 (0.90 to 1.78) |
| One or more falls in the past year | 157 (17.5) | 123 (18.0) | 0.97 (0.74 to 1.26) | 0.98 (0.75 to 1.27) |
| Brisk/fast walking pace | 256 (27.9) | 342 (48.2) | 0.42 (0.34 to 0.51)*** | 0.39 (0.32 to 0.49)*** |
| Someone to walk with | ||||
| Sometimes/often/always | 791 (87.2) | 600 (84.2) | 1.25 (0.93 to 1.69) | 1.20 (0.88 to 1.63) |
Results from qualitative comparisons
Fifty-five trial non-participants were telephoned from March to July 2013: 21 could not be contacted and four declined to be interviewed. Thirty trial non-participants representing the six initial participating practices were interviewed. Data saturation was achieved prior to completing 30 interviews, but we continued to 30 to ensure a more ethnically diverse sample. The demographic details of interviewees and their main reasons for non-participation are published. 141 Those interviewed were not selected on the basis of inactivity, and a slightly higher percentage (67%; 20/30) reported being too active as a reason for non-participation compared with those who were included in the main quantitative analysis.
Most interviewees gave one primary reason for declining participation, consistent across sex, ethnicity and age groups. The majority (n = 18) said that they were too active either because they felt that their activity exceeded the trial’s target levels or because others would benefit more. Less frequently cited main reasons included existing medical problems (n = 4), travel from home (n = 3), work/other commitments (n = 3), concerns about potential equipment problems (n = 1) and reluctance to be randomised (n = 1). To further understand the reasons for non-participation, we categorised the emerging themes into three domains: internal, external and trial related. Short quotations illustrating all of these reasons are shown in Table 15 and more detailed quotations are given in our published paper. 148
| Category | Subcategory | Theme | Quotations with non-participant (NP) number |
|---|---|---|---|
| Internal | Already active | Personal activity | I tend to walk quite a lot anyway, so I didn't think a pedometer would probably be likely to increase my walking at all really.NP12 |
| Work activity | I actually work as a postman, so I do a hell of a lot of walking . . . and that was basically the reason that I didn’t think I’d need to actually join the programme.NP06 | ||
| Medical problems | Stroke | I had the stroke in ‘94. So that limited my walking.NP07 | |
| Pain | If I walk for more than half an hour at a time, I get incredibly stiff and painful.NP16 | ||
| Heart condition | I’m always at the hospital seeing a cardiologist.NP18 | ||
| Multiple medical problems | I don’t need anything else going on to do with health . . . I certainly would have thought . . . that they would have thought, oh, she would not want to do this because she’s got lots of other problems.NP18 | ||
| No wish to increase activity | Not interested/does not like PA | And I do not really like running . . . and I certainly will not join a gym. I hate exercising.NP02 | |
| Already doing enough | No, I think I do enough. I’m fine with what I do.NP17 | ||
| Not interested in walking | ‘More interesting than walking’ | Cycling’s nice, swimming . . . any form of recreation thing, like ice skating or horse riding or bicycle riding, anything like that . . . Walking’s quite boring.NP02 | |
| Team sport | Well, it would be hard for you to organise team sports I should think wouldn’t it? I mean I used to play badminton quite a lot which I enjoyed.NP19 | ||
| Running | If anybody’s doing research into people who have had heart attacks and then trying to get back into running, that I’d be extremely interested in.NP24 | ||
| Not the right person | For younger people | You get to a stage in your life and you think, that’s it . . . I’m relaxing now. I exercise my mind instead.NP02 | |
| For lonely people | These sort of things people take them up if they are lonely and I’m not lonely.NP18 | ||
| For overweight people | Unless you were a really fat person, which I’m not.NP18 | ||
| Altruism | . . . an opportunity for someone else, you know, that it may be more useful to.NP13 | ||
| External | Work commitments | It’s bad enough trying to get . . . a day off for a normal appointment.NP02I just did not think I’d have time. . .because I know how important walking is, and I love walking, and if I have an hour or two free, I would prefer to walk than talk to the nurse.NP21 | |
| Travel difficulties | If I had time, I’d love to be part of your research and go to the surgery and all the rest of it, but I think, actually . . . the awkwardness of the journey . . .NP22 | ||
| Other commitments | Travel from home | I’m going away so much, I could not really tie myself down to anything like that.NP01 | |
| Caring for family member | I’m a carer for my father. I think most of it is just being there.NP04 | ||
| Chores/’life’ | I’ve got grandchildren. I’ve got a husband. I like to do my gardening. I’ve got a four bedroom house to keep clean. I feel my load is more than enough to keep me going.NP08 | ||
| Advice from others | I did mention it to my daughter actually and she said that sounds crazy! She said it’s not for me, so I did not go any further.NP07 | ||
| Trial related | Length of programme | It does sound a bit on the lengthy side does not it really . . . some people could be put off by that.NP10 | |
| Trial material | Too long | . . . there was a lot to read. Bullet points are good. Just make it simple.NP19 | |
| Aimed at older people | I just remember thinking, actually, I do not think I’m in that age group yet. It kind of seemed to be geared to people who really were in their 70s and over.NP09 | ||
| Equipment problems | Pedometer/accelerometer | Well I mean I have actually used a pedometer but I would not sort of particularly want to do it for a week.NP09 | |
| Randomisation | Did not like concept | I think if you’re doing research then you should be able to choose . . . within reason . . . what club you’re willing to join really.NP13 | |
| Did not want to be in nurse-support group | . . . I could probably commit to the other two groups, but possibly not to the nurse support.NP09 | ||
| Did not want to be in the control group | Well . . . I could not see the point of being in a group that did nothing.NP04 | ||
| Venue | Fitness-related venue better | If you’re going to do a fitness programme, you should do it in a fitness venue.NP04 | |
| Does not like the GP surgery | I have to go there when I’m not well. I certainly am not going to go to the surgery when I’m well.NP18 | ||
| Walking environment | Boring | Walking’s quite boring. Unless you’re walking somewhere on an outing somewhere, you know, in the country or something, seaside. You should have more trips.NP02 | |
| Wrong season | As the weather gets better, then I might go for a walk in the evening . . . it was really due to the seasons as well.NP28 | ||
| Preferred group | I think you get more encouragement if you are in a group.NP05 | ||
| Trial design | . . . that is not something I wanted to be part of I think I’d have found it incredibly boring.NP18 |
Internal reasons for non-participation included already being active, medical problems (pain, heart conditions, stroke and multimorbidity), no wish to increase activity, no interest in walking, feeling incorrectly ‘targeted’ and altruistic reasons. The dominant reason in this category was a belief in already being sufficiently active. When explored in more depth, it seemed that, on self-report, many were achieving, with some significantly exceeding, the recommendations. This is supported by the finding that non-participants were found to be more active on the GPPAQ. Of those citing medical reasons, it was less clear whether or not these problems constituted a definite contraindication, especially as those with predefined medical conditions contraindicating an increase in walking should have been excluded. A small number of people suggested that they did not enjoy PA, were not interested in walking or suggested a different activity or a team sport.
The external theme related to factors ‘external’ to the potential participant, including work and other commitments, travel problems, being a carer and advice from others. Work and work-related travel were frequently given as reasons for not participating and many feared that they could not commit to the trial. Family and home life commitments were also important barriers to increasing activity, including being a full-time carer for family members. We were interested in finding out whether or not advice from friends or family affected the decision not to participate. Very few interviewees discussed participation; for those who did, it did not influence their decision, except for one interviewee whose daughter strongly agreed that she should decline.
Reasons related to trial design included programme length, trial material, equipment problems, being randomised, the venue, the walking environment, the nurse interaction and the overall trial design. For some interviewees, the trial duration, at 3 months, was too long and it was difficult to commit for this period. One interviewee reported a previous negative experience with pedometers as her primary reason for declining. Several felt that not being able to choose their allocated group was a disadvantage. Two interviewees expressed concern about the trial promoting walking as an exercise, because the local walking environment was ‘boring’, and another stated that it was ‘the wrong season’ for walking outdoors; however, most interviewees thought that walking was an appropriate and inclusive activity. Some expressed interest in a group intervention rather than one to one with a nurse, feeling that this would improve motivation and sociability; however, others felt that interacting directly with a nurse was preferable to being in a group. Most interviewees approved of the choice of their GP surgery as the location for a PA intervention, describing their surgery as ‘lovely’, ‘pleasant’, ‘convenient’ and ‘appropriate’. Many interviewees expressed a positive attitude towards PA and research, and regretted not being able to participate.
Summary of the findings
The PACE-UP trial recruited 11% of patients aged 45–75 years who were invited by post from their registered general practice, although not all were randomised because of failure to provide adequate accelerometry data. Those not replying were younger and more likely to be male and from deprived postcode areas. Asian patients were less likely to participate. Participation was associated with mild or moderate health impairment, although those with more severe problems were less likely to participate. Not having enough time and being already physically active were the most common reasons for non-participation, even among patients who were classified as not active. Interview findings supported the questionnaire findings and gave more detail about the reasons behind the lack of time (work, travel, family, caring commitments, etc.) and the type of health impairments that stopped people from taking part. Despite not wanting to participate, almost all interviewees were positive about the trial, aware of the benefits of PA and the importance of research, and supported primary care as a venue for such programmes. The design of the trial and intervention was not stated as a key reason for declining to participate.
Strengths and limitations
The PACE-UP trial is a large trial recruiting from a clearly defined invited population, based on GP lists, enabling us to assess the potential reach of the intervention in terms of age, sex and deprivation. Our estimate of 11% participation may underestimate the true rate, particularly in areas of high mobility where patients may have moved away and not informed the practice, inflating the number of patients counted as invited. Although based on limited data, the PACE-UP trial offers a rare opportunity to examine demographic differences between participants and non-participants. We were able to estimate participation within different ethnicities using pooled data. However, we were not able to match at an individual level and some participants may have categorised themselves in a different ethnic group to that on the GP register. Ethnicity was also poorly recorded in some practices and we needed to make assumptions about whether or not those with ‘unknown’ ethnicity were similar to those with recorded ethnicity. In a sensitivity analysis, even under extreme assumptions, the difference between Asian and black ethnic groups persisted. The trial excluded individuals who self-reported being active, but the non-participants were not selected in this way. Our quantitative analysis attempted to mitigate this difference by restricting analysis to all those who self-reported as not being active. However, some residual bias may remain.
The interview study represents an innovative attempt to systemically explore the reasons for non-participation with a purposive sample of those who were invited but declined. Our aim was to further understand the perceived barriers to participation to enhance recruitment to future trials and exercise programmes. We were also able to explore non-participants’ perception of the trial design and research in general. This sample spanned six out of seven of the practices involved and included both sexes, a range of ages, ethnicities, employment and educational backgrounds. The telephone interviews allowed in-depth exploration of the barriers to participation not possible from a questionnaire alone and allowed us to compare the interview findings with the non-participant questionnaire responses. The main limitation of the interview study is that this was based on a self-selected group of people who both returned the non-participant questionnaire and agreed to be interviewed. It is possible that some of our sample would have been excluded in any case on the basis of their pre-existing activity levels and, therefore, their decision to decline may have been entirely appropriate. In addition, despite our attempt to sample interviewees from non-white British backgrounds, these groups were under-represented when compared with the ethnic diversity of the population invited.
Comparisons with previous work
A systematic review of 47 studies of walking interventions151 showed that recruitment methods and participation rates were poorly reported. Of 25 RCTs, participation rates could be calculated for only five. We recruited by post to reduce the burden on practice staff and to obtain response rate data. Postal invitations are used in primary care for other preventative activities, making this a pragmatic approach. 152 Other walking intervention trials using postal invitations in primary care33,48,144,153 had similar response rates of 10–20%; those with higher rates (37% and 39%)154,155 recruited individuals who were older and more frequent attenders in primary care, and invited individuals in the primary care consultation, as well as by post. Our previous trial21 used similar recruitment strategies to the PACE-UP trial, but, unlike other trials, included active people. This trial had a recruitment rate of 30%, but was conducted among older people in an affluent setting with few non-white residents.
Non-responders were followed up with one reminder letter, but because of data protection constraints we were unable to contact patients by telephone. Although only 1% of invitation letters were returned to sender, this may underestimate those who did not receive the letter as we did not use registered post. A previous London study using registered post found that 26% of letters were not delivered. 156 Our findings of greater participation in women,157,158 older people158 and those in affluent areas157 are supported by other studies. Attwood et al. 158 found no association with deprivation or ethnicity, but this trial was based in an area of high deprivation with few non-white patients. Among Asian patients, our response rate was similar to postal invitations in the PODOSA (Prevention of Diabetes and Obesity in South Asians) trial (5.2%), in which community-based approaches,159 through partnership with local South Asian groups, were found to be more effective. Wilbur et al. 160 found that social networking was the most effective method for recruiting African American women from low-income areas.
The finding that declining participation in this PA trial was attributable to interviewees considering themselves to be already sufficiently active is in line with other literature. 157,161–163 Importantly, objective measurement of PA reveals that most people overestimate their activity levels,9 and that their assessment of their personal activity levels is likely to be influenced by a social context. 161 However, this interview series allowed activity levels to be explored in more detail and revealed that, at least on self-report, this was a relatively active cohort for some of whom the trial may not have been appropriate.
Our finding that participation was associated with some degree of health problems, but that severe impairment reduced participation, is more nuanced than previous work, which has suggested that declining participation in PA programmes or trials is attributable to medical problems, including pain,157,161,162,164 particularly in studies with older participants. 165 Lack of time because of work and other commitments has also been identified as an important barrier,157,161–163,166,167 particularly in younger and middle-aged participants. 165 A lack of interest in PA has also been reported in the literature,157,161–163,166 but travel away from home has not been reported prominently. This may reflect the high proportion of our interviewees still in full- or part-time work and the seasonal migration of the diverse south-west London population.
Implications
Guidelines published by NICE36 concluded that more research was needed to determine which interventions are effective and cost-effective in increasing activity levels among lower socioeconomic and high-risk groups, and that there is little evidence on differential effects of interventions. In our trial, those groups for which more evidence is required tended to be those with the lowest recruitment rates, such as Asians and those in more deprived areas. 143 It has been suggested168 that specific cultural groups may respond better to interventions directly targeted at their needs, rather than to universal interventions. Reasons for non-participation were often related to individuals not wanting to increase activity or feeling that they were sufficiently active. It is likely that such resistance will similarly apply to any intervention roll-out and may apply more widely to other public health interventions. Low participation rates mean that policy-makers should be cautious about the intervention’s potential reach and the possibility that it could increase activity inequalities, but these are not a reason not to implement an intervention shown to be effective in 11%93 of the population. We were successful in recruiting older people, women and those with comorbidities or some degree of health limitation. These groups have lower PA levels and are likely to benefit more from increased PA. However, those with more severe disability, people who have had falls and those with a fear of falling were not over-represented, indicating a rational choice by individuals.
Only 12% of non-participants cited randomisation as a factor for not participating, whereas 45% cited time constraints. The nurse intervention required three additional visits to the practice on top of the three data collection visits, which may deter working people or those with child-care and other commitments. However, the PACE-UP trial showed that both the nurse-support and postal delivery groups performed similarly at the main 12-month outcome. 93 An intervention offering pedometers with brief advice, without the need to provide research data, may be more acceptable.
Both the PACE-LIFT and the PACE-UP trials recruited to target, achieved follow-up rates of over 90% and demonstrated that the interventions were effective in increasing PA levels. 21,93 However, considerably more research effort was required (e.g. more contacts from research assistants) per randomised participant in the PACE-UP trial than in the PACE-LIFT trial, resulting from a lower uptake rate. In spite of the effort, we still had limited power to investigate ethnic and socioeconomic subgroups. Trials with greater reach are likely to be more expensive in terms of recruitment, and gains in generalisability need to be balanced with greater costs. Our findings have important implications for those planning PA trials, as well as for those commissioning community PA programmes. As the cohort we interviewed appeared to be relatively physically active, it may be necessary to tailor some interventions to maintaining, rather than increasing, activity; this may also be important to mitigate the decrease in PA levels that often occurs with ageing. Equally, education about the levels of activity that optimise health gain may prevent potential participants from declining because of overestimation of their actual levels of activity. Measurements using pedometers or accelerometers provide an easy approach to validating PA levels.
Lack of time was an important barrier, so it may be helpful to reiterate that activity can be broken up into 10-minute bouts throughout the day (this can also help those individuals limited by pain or disability). Tailoring interventions to an individual’s travel and work commitments and for their specific health problems may also increase uptake. Promotional material should be inclusive and explicitly state that pre-existing medical conditions do not necessarily prevent participation and dispel myths about the risks of moderate-intensity exercise. Information about the value of PA, particularly walking, for many different health conditions1 should be emphasised in the invitation to participate. RCTs inevitably involve randomisation, but emphasising that, in some trials (including the PACE-UP trial), the control group can receive the intervention after the trial may help recruitment. Most interviewees felt that primary care was an appropriate, convenient location for delivering a walking-based PA intervention, indicating that further primary care-based trials and programmes are likely to be well received.
Conclusions
Participation in an effective PA trial among adults and older adults in a socially and ethnically diverse population was only 11%, with lower rates in more deprived and Asian subgroups, limiting the trial’s ability to investigate differential effects in these important subgroups. Trials with greater reach are likely to be more expensive in terms of recruitment, and gains in generalisability need to be balanced with greater costs. Differential uptake of interventions found to be successful in trials may increase inequalities in PA levels and should be monitored.
Chapter 6 Process evaluation
Introduction
Why is process evaluation necessary in the PACE-UP trial?
The PACE-UP RCT is a complex intervention comprising multiple interacting components (pedometer, handbook, diary, practice nurse PA consultations and BCTs – as part of both written materials and consultations). Although the RCT design is able to establish the effectiveness of the intervention (see Chapter 3), it does not provide information on how it works, or whether or not there are contextual factors that could be associated with variation in outcome in different settings. 169 Conducting a process evaluation of the PACE-UP trial enables a detailed examination of the mechanisms of change by gaining an understanding of how the intervention was delivered and received and how this may have affected the variation in outcomes. The process evaluation investigates the relationship between the fidelity and quality of implementation, the context of the intervention and the main trial outcomes. The evaluation also helps to draw conclusions on the replicability and generalisability of the intervention. The main findings from the process evaluation are published170 and are reproduced here under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0).
Medical Research Council’s guidance on process evaluations in complex interventions
In 2014, the Medical Research Council (MRC) published new guidance for process evaluations of complex interventions. 169 The guidance draws on the experiences of researchers and wider stakeholders who have conducted process evaluations within trials of complex public health interventions. We have used the guidance to provide the framework for the process evaluation of the PACE-UP trial. Process evaluation is accomplished through investigating aspects such as implementation, mechanisms of impact and context, and the relations between these, as described in Figure 11.
FIGURE 11.
The key functions of process evaluation and the relationships between these. Green boxes represent the components of process evaluation, which are informed by the causal assumptions of the intervention, and inform the interpretation of outcomes. Adapted from Moore et al. 169 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Implementation refers to the structures, resources and methods through which delivery is realised, and comprises the following factors: implementation process, reach, fidelity, dose and adaptations. The implementation process describes how the delivery is achieved, through training, support, resources, etc. Reach refers to coverage and the degree to which the intervention is delivered to those for whom it was intended, that is, who receives the intervention. The other aspects of implementation are related to what is delivered. Fidelity is the degree to which the intervention was delivered as intended (content) and includes assessment of the quality of the intervention. Dose denotes the quantity of the intervention implemented. Adaptations are participant and implementer adjustments, which may impede or strengthen the intervention and which arise in response to the intervention itself.
Mechanisms of impact refer to how the intervention activities and participants’ responses to them cause change and adaptations.
Context refers to external factors which may influence, and be influenced by, implementation mechanisms and outcomes.
The first stage of designing the process evaluation was to describe the intervention and to clarify casual assumptions, which was accomplished through the use of a logic model, shown in Figure 12.
FIGURE 12.
Logic model for the PACE-UP (Pedometers and Consultation Evaluation – UP) PA trial.
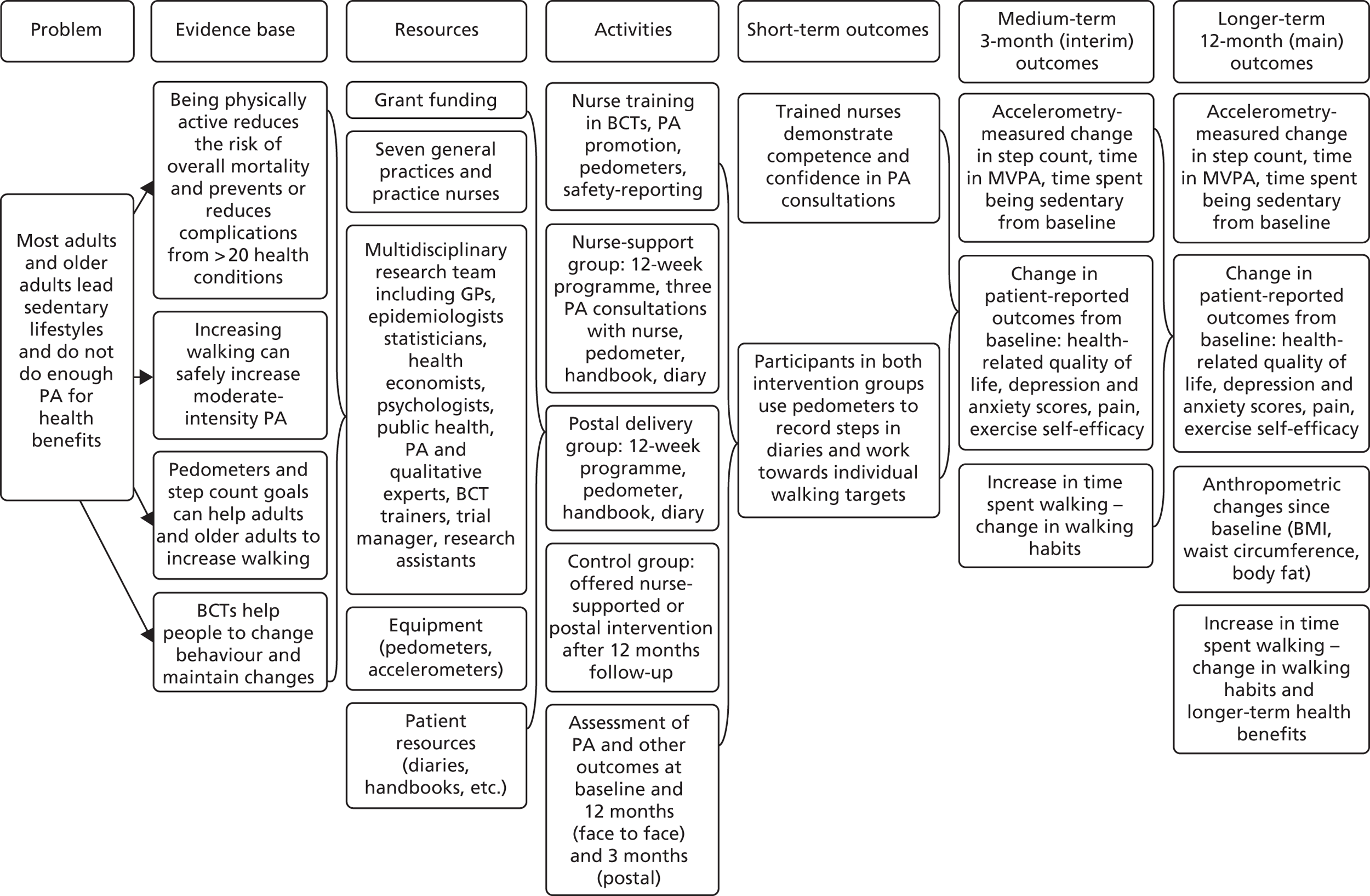
Methods and results
The PACE–UP trial process evaluation was conducted alongside the effectiveness evaluation, included both qualitative and quantitative components and was undertaken by the same team that carried out the effectiveness evaluation.
In accordance with the MRC guidance, the methods were selected through following the key functions model (see Figure 11) and are summarised in Table 16, which details the process evaluation components, the data sources, the trial group to which they refer and the measures used. The nurse-supported intervention was the most complex to deliver as it involved eight nurses from seven practices delivering three consultations over a 3-month period. Most of the process evaluation was therefore designed to evaluate the nurse-supported intervention group. When process evaluation occurred for other groups, this is clearly described.
| Process evaluation component | Data source | Trial groups evaluated | Measures |
|---|---|---|---|
| Implementation | |||
|
Implementation process How was it delivered? |
Training:
|
Nurse-support group | Time spent on training activities |
Resources:
|
Nurse-support and postal delivery groups | Cost of delivering intervention components | |
|
Reach Who was it delivered to? |
All groups and non-participants |
|
|
|
Fidelity (content and quality) What was delivered? |
|
Nurse-support group |
|
|
Nurse-support and postal delivery groups |
|
|
|
Dose What was delivered? |
|
Nurse-support group |
|
|
Adaptations What was delivered? |
|
Nurse-support group |
|
|
Nurse-support and postal delivery groups | ||
| Mechanisms of impact | |||
| Participant responsiveness |
|
Nurse-support group |
|
|
Nurse-support and postal delivery groups | Qualitative themes and subthemes | |
| Context | |||
| Contextual factors | Nurse training session records | Nurse-support group |
|
| Participant interviews and nurse focus groups and interviews (see Chapter 7) | Nurse-support and postal delivery groups | ||
To reduce duplication, and for ease of reading, the methods and results for each aspect of the process evaluation are presented together. The main results are summarised in a further key functions model after all of the results have been presented (Figure 13). Several aspects of the process evaluation are dealt with appropriately in other chapters of this report, such as reach in Chapter 5 and participant responsiveness in Chapter 7. They are referred to in Table 16 for completeness and the relevant chapter in which the methods and results for this aspect are presented are clearly shown.
FIGURE 13.
The key functions of process evaluation and the relationships between them for the PACE-UP trial. Green boxes represent components of process evaluation, which are informed by the causal assumptions of the intervention and inform the interpretation of outcomes. Adapted from Moore et al. 169 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
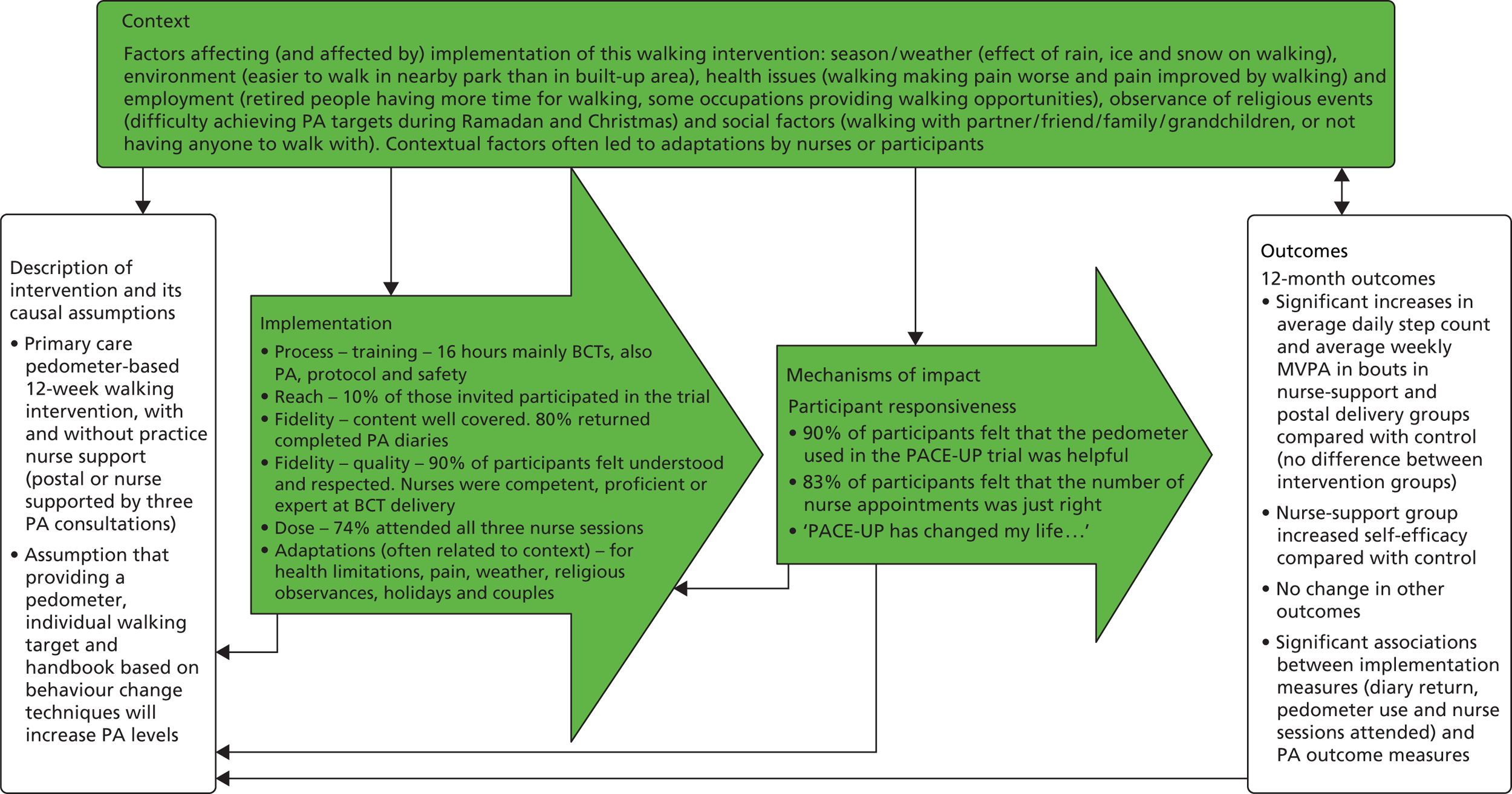
We have selected three quantitative aspects of the process evaluation to relate directly to PA outcome measurements at 12 months (change in step counts and change in time in MVPA in bouts): the number of nurse appointments attended whether or not completed step count diaries were returned by participants at 3 months in the nurse-support and postal delivery groups, and the use of pedometers in the nurse-support and postal delivery groups during the 12-week intervention. These analyses relating process to outcome measures are described at the end of Methods and results.
Implementation
Implementation process
Training
Nurses delivering the intervention were provided with training in PA guidance (1 hour and 25 minutes), trial protocols (4 hours), safety reporting (1 hour and 10 minutes) and BCTs (9 hours and 25 minutes) across the duration of the trial (see Appendix 5, Table 38). Data on nurse training were obtained from training-day agendas and BCT trainer telephone feedback records and trial administrative records. The total training time was approximately 16 hours; most of this was allocated to delivering BCTs as the active ingredient in the intervention.
Resources
Resources for the trial include the trial materials [patient handbooks and diaries, see the NIHR Journals Library website: www.journalslibrary.nihr.ac.uk/programmes/hta/103202/#/ (accessed 25 May 2018)], trial equipment (pedometers and accelerometers) and payment of nurse time and room hire. These are all fully costed in Chapter 4 and are not further commented on in this chapter.
Reach
The overall trial recruitment rate was 10% (1023/10,467). Details on how practices and participants were selected and recruited is described in Chapter 2. The methods for assessing trial representativeness and generalisability, by comparison of non-responders, non-participants and participants, and by interviews of non-participants, are described in Chapter 5.
Fidelity (content and quality)
Content
Nurse sessions
Nurse session attendance and session content delivered was recorded by the nurses after each session (see Appendix 5). There were 11 compulsory items to be covered in session 1 and six items to be covered in sessions 2 and 3. The level of nurse session attendance was high; approximately three-quarters of participants attended all three sessions (n = 255/346; 74%). Adherence to content delivered was high in all sessions; the mean number of items delivered in each session was 11 (range 10–11) and six (range 5–6) in sessions 2 and 3, respectively. Of those participants who attended session 3, most reported still using the pedometer and diary (n = 258/263; 98%) (Appendix 5, Table 39).
Physical activity diaries
Physical activity diaries (see Appendix 5, Table 40) returned by participants after the intervention provided data on the achievement of weekly walking targets for the intervention groups. Eighty per cent of participants (n = 549) returned completed diaries; there was similar return across both groups. One-third of participants in the nurse-support group altered their step count targets (89/346; 32%) and the majority were decreased (n = 80). In comparison, just four participants in the postal delivery group altered their step count targets and all were decreased. The relationship between diary return at 3 months and the trial outcome measures was explored (see the association between the process evaluation measures and the trial outcomes at the end of Methods and results).
Pedometer use
All participants were asked about their pedometer use during the 12-week intervention period (see Appendix 5, Table 41). During the 12-week intervention, a high proportion of both the postal delivery and nurse-support groups reported using their pedometer either every day or most days: 238 out of 294 (81%) in the postal delivery group and 269 out of 303 (89%) in the nurse-support group. The relationships between pedometer use during the intervention and the trial PA outcomes at 3 and 12 months were explored for both intervention groups (see the association between the process evaluation measures and the trial outcomes at the end of Methods and results).
Quality
Nurse and patient alliance questionnaire
Following the intervention, both the nurse and the participant independently completed a 12-item nurse and patient alliance questionnaire (see Appendix 5) covering different intervention aspects (e.g. working together and goal-setting, number of appointments). The questionnaires were developed by BCT trainers and trial investigators, and questions 1–11 were adapted from the Working Alliance Inventory,171,172 which is a validated alliance measure used in cognitive–behavioural therapy-based studies, and the Outcome Rating Scale. 173 Item 12 was added to specifically ask about the number of PACE-UP trial appointments. The questionnaire was posted to the participant and returned to the researcher, so that the nurse was blind to participant responses. Three directly comparable items (questions 1, 3 and 4) from both the patient questionnaire and the nurse questionnaire provided data on the quality of intervention delivery (Table 17). Seven directly comparable items directly relate to participant responsiveness (questions 5, 6, 8, 9, 10, 11 and 12). Two items (questions 2 and 7) were discounted as they did not relate to quality of participant responsiveness.
| Delivery and responsiveness | Questionnaire | |||||
|---|---|---|---|---|---|---|
| Patient alliance | Nurse alliance | |||||
| N | ‘Agree’ or ‘strongly agree’, n (%) | Missing items | N | ‘Agree’ or ‘strongly agree’, n (%) | Missing items | |
| Quality of delivery | ||||||
| Q1: the patient and I worked together on setting goals that were important to the patient | 287 | 231 (80) | 8 | 250 | 221 (88) | 1 |
| Q3: the patient felt heard, understood and respected | 287 | 259 (90) | 8 | 249 | 234 (94) | 2 |
| Q4: in our meetings together, the patient discussed everything they wanted to discuss | 285 | 267 (94) | 10 | 249 | 234 (94) | 2 |
| Participant responsiveness | ||||||
| Q5: the patient understands how to make lasting changes in activity levels | 289 | 259 (90) | 6 | 249 | 215 (86) | 2 |
| Q6: the approach to making change suited the patient | 287 | 247 (86) | 8 | 249 | 182 (73) | 2 |
| Q8: the patient feels confident to continue to make positive changes in PA on their own | 288 | 238 (83) | 7 | 246 | 191 (78) | 5 |
| Q9: the patient feels confident about overcoming obstacles to increasing activity levels in the future | 257 | 169 (66) | 38 | 190 | 124 (65) | 70 |
| Q10: the pedometer used in the PACE-UP study was helpful to the patient | 288 | 260 (90) | 7 | 246 | 209 (85) | 5 |
| Q11: the diary used in the PACE-UP study was helpful to the patient | 284 | 229 (81) | 11 | 247 | 203 (83) | 4 |
| Q12: the number of appointments with the PA nurse was just right . . . | 278 | 232 (83) | 17 | 241 | 178 (74) | 10 |
The questionnaires were completed by 295 out of 346 participants (85%) in the nurse-supported intervention group and by the nurses for 251 out of 346 nurse-support group participants (73%).
There was strong agreement between the participant and nurse results for all of the items relating to quality and 80% or more of both nurses and participants agreed or strongly agreed with all of these statements, suggesting high quality of delivery.
The following are some examples of participant and nurse comments relating to quality from the questionnaires:
Nurse was encouraging, supportive. Encouraged me to set goals that were achievable for me and not to put too much pressure on myself.
Female, aged 47 years, nurse-support group
My nurse was lovely and encouraged me all the way through, even when some days I couldn’t do what I needed, we discussed alternatives. My nurse was a diamond. Thanks to PACE-UP and the nurse my walking has really improved.
Female, aged 47 years, nurse-support group
Client pleased with programme. Learning curve. Would recommend to others.
Practice nurse
Enjoyed patient, good discussions and understanding around increasing exercise.
Practice nurse
Audio-recordings from nurse sessions
Nurses were asked to audio-record a sample of their sessions so that these could be listened to by the BCT trainer and rated according to their skill in six different communication skill competencies. Ratings were made by the BCT trainer against a primary care consultation rating scale (range 0–6) in six domains (Figure 14) and used for both fidelity (quality) evaluation and supervision purposes. The rating scale used was developed from the Cognitive Behavioural Therapy Techniques for Palliative Care Practitioners Rating Scale174 and the Department of Health and Social Care’s The Ten Essential Shared Capabilities – A Framework for the Whole of the Mental Health Workforce. 175 The nurses were each asked to provide three audio-recorded sessions, one each for sessions 1, 2 and 3. They were asked to try to ensure that one of the recorded sessions was from a session where a couple were seen together. The mean scores and ranges for all nurses are shown across all domains in Table 18.
FIGURE 14.
Behaviour change technique competency level.

| Scores | Communication skill competency | |||||
|---|---|---|---|---|---|---|
| 1. Framing, pacing, focus and use of time | 2. Empowering explanations | 3. Collaboration and active listening; interpersonal effectiveness | 4. Setting goals, agreeing actions and motivational techniques | 5. Feedback, reviewing and summarising | 6. Building self-efficacy | |
| Average (mean) score | 5.3 | 4.7 | 5.0 | 5.2 | 5.3 | 5.2 |
| Range of scores | 3–6 | 3–6 | 4–6 | 4–6 | 5–6 | 4–6 |
The range of scores illustrates that even the lowest ratings were competent, and the highest scores were expert, across all six competencies. The lowest scoring competency was given for empowering explanations (mean score 4.7), whereas all the other competencies had mean scores above 5, demonstrating proficiency, with very good features.
Qualitative perspective
Semistructured individual interviews with participants and focus groups with nurses provided a qualitative perspective of the intervention, including the quality of delivery; this is presented in detail in Chapter 7. Overall, the nurses and participants described the intervention in a positive manner, as highlighted by the following quotations:
. . . they kept saying how well I was doing, and all this sort of thing, so it made me want to continue. I think it was . . . a part motivation, yes, because I knew I had to face somebody and I didn’t want to fail.
Female, aged 63 years, nurse-support group
. . . if you had, in your drawer, you had like a set . . . a package, programme, you could do, and if through the NHS Health Check you identified someone who was suitable, you could then discuss it with them and say, ‘Would this be something you’d be wanting to look at? . . . and go from there.
Practice nurse
Dose
For the purpose of the PACE-UP trial, the dose delivered to the postal delivery group was fixed, as they all received the same handbook, diary and pedometer. The dose could vary for the nurse group according to the number of sessions attended and the length of each of the sessions. Nurses were required to complete checklists at the end of each session (see Appendix 5, Table 39), providing details on attendance and the duration of sessions. The duration of sessions was also captured from the audio-recorded sample of intervention sessions; this allowed a comparison with session durations calculated from nurse checklists. Overall, three-quarters of participants in the nurse-support group attended all three sessions. Ninety-five per cent of participants (330/346) attended session 1, 86% (296/346) attended session 2 and 76% (263/346) attended session 3. The relationship between the number of sessions attended and trial PA outcomes was also explored (see the association between process evaluation measures and trial outcomes at the end of Methods and results).
Trial protocols detailed the following approximate duration of each nurse intervention session: 30 minutes for session 1 and 20 minutes each for sessions 2 and 3. A summary of nurse intervention session durations from nurse self-report and audio-recordings is available in Appendix 5, Table 42 (19 recordings, relating to 22 participants, as some were couples). There was good agreement between the planned protocol session length and the nurse self-report durations: mean of 30 minutes (SD 4 minutes) for session 1, mean of 24 minutes (SD 3 minutes) for session 2 and mean of 22 minutes (SD 4 minutes) for session 3. The duration of consultations from audio-recordings was based on much smaller numbers (n = 22 participants) but had a shorter mean duration: mean of 21 minutes (SD 6 minutes) for session 1, mean of 21 minutes (SD 7 minutes) for session 2 and mean of 14 minutes (SD 5 minutes) for session 3.
Adaptations
Details of nurse and participant adaptations made to the intervention were provided from nurse training session records. There were many examples presented relating to step count target adaptation and tailoring the intervention to individual circumstances. Adjustments were made to the intervention to accommodate religious observances, such as Ramadan and Christmas. Step count targets were adapted to be more achievable to reflect participants’ reduced energy/activity levels in advance of holidays, when there were expected to be reductions or increases in PA, during periods of participant illness and pain and in response to changing weather conditions. Nurses also explained the need for flexibility with participants who experienced difficulties with equipment use; for example, a small number of participants who did not like using the pedometer were advised to use time to measure their walking, rather than measuring step count (e.g. extra walks of 10–15 minutes per day, rather than an extra 1000–1500 steps per day). At the second training session, it became clear that the nurses did not find the optional handouts provided for use in consultations to supplement the patient handbook helpful and, as a consequence, were not using them. From these discussions, it was decided that these materials would be discontinued. Another adaptation revealed by the nurses was adapting targets and advice for participants taking part as a couple, particularly if they had very different PA levels and targets; this sometimes related to one individual of the couple having health problems that had an impact on mobility. Nurses adapted the intervention to encourage both participants to meet their targets, often encouraging a mixture of walking together and walking apart to achieve this.
Physical activity diaries also provided data on adaptions to the prescribed intervention through alterations of participants’ walking targets (see Appendix 5, Table 40). Few participants in the postal intervention group altered their targets in the diary (1%; 4/339), whereas 32% (89/346) of the nurse-supported intervention group altered their walking targets, mainly by decreasing the target [29% (80/346)].
Additional details of nurse and participant adaptations to the intervention by both intervention groups, obtained as part of the qualitative evaluations of the trial, are presented in Chapter 7. Some examples are given here.
The nurse quotations below illustrate the flexibility in intervention delivery during Ramadan and also during bad weather:
. . . they couldn’t walk or increase on their walking at that time because they hadn’t eaten and then they weren’t feeling too good, and all that, so we did it a different way then, and what I did with them was we relaxed it and then I said to them, when Ramadan’s over, we made the appointment so that their actual trial went on a bit longer.
Practice nurse
. . . if the weather was bad, or it was cold, or there were obstacles that got in the way . . . they would do things like, you know . . . activities indoors where they could not always go outside.
Practice nurse
Mechanisms of impact
Participant responsiveness
Seven items reflecting participant responsiveness were identified in both the patient alliance questionnaire and the nurse alliance questionnaire (see Table 17). There was a good degree of consistency between participant and nurse responses for all of the items relating to participant responsiveness and high levels of agreement with all of the statements. For example, 90% of participants said that the pedometer was helpful and 83% said that the number of nurse appointments was just right, suggesting that there was a good level of participant responsiveness to the intervention.
Some examples of both participant and nurse comments relating to participant responsiveness from the alliance questionnaires are shown below:
PACE-UP has changed my life. I use the car less when I go about. Although I drive to work I park about 1 km away from work, then walk all the way to and from.
Female, aged 47 years, nurse-support group
Wearing the pedometer really raised my awareness of how far I walked each day. I will continue to use it.
Female, aged 68 years, nurse-support group
Positive, liked study book. Was a very reflective/honest person regarding his exercise. Positives/negatives easily identified by patient.
Practice nurse
Patient has own excel monitor of readings/pedometer count. Also converts to miles daily.
Practice nurse
Information about participant responsiveness also came from the qualitative evaluations of participants from both the postal delivery and the nurse groups (from individual interviews) and from practice nurses (from a focus group and individual interviews), and is presented in Chapter 7.
The following quotations from the qualitative evaluation illustrate participant responsiveness and engagement from a nurse and participant perspective:
. . . the only other thing I’d say about the diary is that the people that really liked filling it in found it a really good motivator. When they came to the last appointment, they wanted another one.
Practice nurse
There’s nothing like the fact that you know you’re going to meeting someone and talk about it to make you do it, you know, . . . It’s basically the routine of being checked up on by someone else . . .
Male, aged 61 years, nurse-support group
. . . well having something which counts the steps makes one conscious of it and filling out a little booklet every day, likewise, it just creates some personal pressure.
Male, aged 59 years, postal delivery group
Context
Comments made by nurses during training sessions relevant to contextual factors were noted down. There was overlap with the factors mentioned in the section on adaptation of the intervention, as contextual factors often required the nurses to consider adapting the intervention or targets after discussion with participants. Examples of contextual factors mentioned are as follows: the difficulty of walking in bad weather, the effect of taking part in the intervention as a couple, health issues that required a slower, more gradual approach and undertaking the intervention during Ramadan or Christmas.
How contextual factors may have affected (and been affected by) the implementation, such as season, environment, health status, employment and social and religious factors, were explored as part of the qualitative evaluation of participants (from individual interviews) and nurse perspectives (from focus group and individual interviews), and are described more fully in Chapter 7. The following factors were described: season/weather (problems with rain or snow and ice), environment (ease of walking in parks, more difficult in built-up areas), health issues (examples both of pain getting worse with walking and of walking improving pain), employment (retired people saying they have increased time for walking, some occupations providing the opportunity for walking or at least walking to work), religious factors (difficulty with walking during Ramadan when fasting and having low energy levels) and social factors (walking with family/grandchildren, etc. or not having anyone to walk with). This last factor is illustrated by the following quotation taken from the qualitative evaluation:
. . . it’s something I want to keep up, because I just felt that it was such a benefit, and even the kids would come out with me sometimes.
Nurse-support group participant
Association between process evaluation measures and trial outcome measures
Although the trial was powered only for analysis of the difference in outcome measures between the three groups, and not for exploration of the effect of process evaluation measures, we felt that it was interesting to explore if there was any relationship between adherence to the intervention and the change in outcomes. We have focused on three quantitative measures of process evaluation in relation to the PA outcome measures at 3 months and 12 months (changes in average daily step count and weekly time in MVPA in bouts). The three measures were all to do with the implementation of the intervention:
-
Dose – nurse session attendance (0, 1, 2 or 3 sessions attended; nurse-support group).
-
Fidelity – return of completed diaries after the 3-month intervention (yes/no; postal delivery and nurse-support groups).
-
Fidelity – pedometer use – how often did you wear the pedometer? Every day or most days (yes/no; postal delivery and nurse-support groups) during the 12-week intervention (0–3 months).
All measures described were considered as independent variables in the models, with (1) change in average daily step count and (2) change in total weekly MVPA in bouts as outcomes. All analyses were adjusted for age, sex, practice, month of baseline accelerometry, household identifier (to account for clustering by household) and trial group (as in the main trial analyses; see Chapter 2). Table 19 shows the results of the models.
| Intervention components | PA outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Daily step count | Total weekly minutes of MVPA in ≥ 10-minute bouts | |||||||
| 3 months | 12 months | 3 months | 12 months | |||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | |
| Nurse session attendance | ||||||||
| Attended all three nurse sessions: yes vs. no | 1197 (627 to 1766) | <0.001 | 605 (74 to 1137) | 0.03 | 74 (45 to 103) | <0.001 | 30 (3 to 57) | 0.03 |
| Diary returned | ||||||||
| Postal delivery group: yes vs. no | 1458 (854 to 2061) | <0.001 | 1114 (538 to 1689) | <0.001 | 64 (33 to 94) | <0.001 | 47 (17 to 75) | 0.002 |
| Nurse-support group: yes vs. no | 873 (190 to 1555) | 0.01 | 323 (–278 to 925) | 0.29 | 50 (15 to 85) | <0.001 | 3 (–27 to 33) | 0.89 |
| Pedometer use every day or most days during the 12-week intervention | ||||||||
| Postal delivery group: yes vs. no | 1029 (383 to 1675) | <0.001 | 606 (22 to 1190) | 0.04 | 40 (6 to 73) | 0.02 | 26 (–2 to 55) | 0.07 |
| Nurse-support group: yes vs. no | 337 (–525 to 1198) | 0.44 | 394 (–321 to 1109) | 0.28 | 24 (–20 to 68) | 0.28 | 10 (–25 to 45) | 0.58 |
Nurse session attendance and physical activity outcomes
In the nurse group at 3 months and 12 months there was a positive association between the number of sessions attended and the PA outcomes. Participants attending all three sessions increased their step count and their time in MVPA in bouts at 3 and 12 months by significantly more than those attending between 0 and 2 sessions.
Diary return and physical activity outcomes
In the postal delivery group, there was a strong positive association between returning a diary and on both change in steps and MVPA in bouts at both 3 and 12 months compared with those in the postal delivery group, who did not return a diary. In the nurse-support group, there was a positive association between returning the diary and on change in step count and MVPA at 3 months, compared with those in the nurse-support group who did not return a diary. However, by 12 months, there was no significant association for either PA outcome of returning a diary within the nurse-support group.
Pedometer use and physical activity outcomes
In the postal delivery group, reported use of a pedometer every day or most days during the 12-week intervention period was associated with a significant change in step count at 3 months and 12 months, and with change in MVPA at 3 months (borderline effect at 12 months).
Within the nurse-support group, there were no significant associations between regular pedometer use during the 12-week intervention and change in step count or MVPA at 3 months and 12 months. This lack of significant effect could be explained by the very small numbers of participants in the nurse-support group who reported not having used a pedometer regularly during the 12-week intervention (n = 34; 11%).
Overall, the analysis of the association between process measures and PA trial outcomes exhibits a clear pattern of positive associations (i.e. increased nurse appointments, diary return and pedometer use were associated with increased objective PA levels). This provides clear evidence of the engagement with the trial process and outcomes, but cannot be interpreted as causality.
Discussion
Main findings
Figure 13 summarises the key findings from the PACE-UP trial process evaluation, which followed the MRC guidance for process evaluation of complex interventions. 169 We gathered a number of positive data on implementation, suggesting good-quality intervention delivery and adherence to the protocol, despite the low reach of the trial. Nurse training was an important element of the trial, with nurses receiving approximately 16 hours of training, predominantly around delivery of BCTs. We demonstrated good coverage of the proposed session content by the nurses and also good-quality delivery, with the audio-recording of nurse sessions demonstrating high levels of competency in communication skills. High-quality delivery was also reflected in comments from participants who felt heard, understood and respected. Three-quarters of the nurse group attended all three PA consultations, and around 80% of both the nurse-support and the postal delivery groups engaged with the self-monitoring aspects of the trial and returned completed step count diaries at 3 months. In terms of the mechanisms of impact, we demonstrated high levels of participant responsiveness. Context was important and factors affecting the implementation of the walking intervention were suggested by nurses and participants from both intervention arms, and included the effects of weather, the environment, health issues, pain, employment, observance of religious events and social factors. The nurses worked with participants to help them to make adaptations, either to the intervention or their individual targets, or to encourage participants to come up with solutions when possible, when there were contextual challenges. Several process evaluation measures (number of nurse sessions attended, return of a completed diary and regular pedometer use) showed significant associations with PA outcomes at 3 and 12 months.
Strengths and limitations
Strengths
Despite the PACE-UP trial process evaluation having been designed before the publication of the MRC guidance,169 we were able to use the data that we collected and fit them into the framework, which has provided a useful structure for reporting process evaluation findings. We have a number of different data sources that reflect three different perspectives in the trial: participant, practitioner and observer. This provided a broader picture of the process than many studies have reported and allowed comparison of results from different methods (e.g. for consultation duration). We tried to reduce the burden of participant measurement, trial costs and duplication of effort, by collecting as many routine trial data as we could for the evaluation (from administrative records, nurse training records, nurse checklists, nurse audio-recordings from supervision sessions, etc.), but we supplemented this with data collected specifically for the evaluation (e.g. the nurse and patient alliance questionnaires or the 12-month pedometer use questionnaire). We have used a mixed-methods approach to the process evaluation, as recommended, combining quantitative data from key process variables from all participants with in-depth qualitative data from purposively selected samples. 169 The qualitative element is described in full in Chapter 7; however, we have used relevant quotations in this chapter to illustrate the quality of delivery, adaptations, participant responsiveness and context, and these have provided a voice to participants and nurses and added a richness and depth to the evaluation. The data were collected longitudinally and contemporaneously throughout the trial, which is seen to be the most complete and accurate method of data collection, and also allows any change in intervention delivery over the course of the trial to be detected. 169 The data are comprehensive, with a high response rate and completeness of data sources, strengthening the robustness of the findings. The process evaluation analysis was conducted before the outcome analysis to avoid a biased interpretation of the process data. Only the final analyses examining the effects of process evaluation variables on outcome data were carried out following the main outcome analyses.
Limitations
The process evaluation was conducted by the trial team while the trial was ongoing. This allowed efficient data collection in a contemporaneous manner, but could have led to bias in evaluation. We tried to minimise the bias by using objective instruments when possible (e.g. nurse and patient alliance questionnaires, 12-month pedometer use questionnaires, return of patient diaries). In addition, the qualitative evaluation was led by Christina Victor, who was not involved in the day-to-day trial conduct. Some process measures were not filled out by everyone (patient alliance questionnaire: 85% completion; nurse alliance questionnaire: 73% completion); this could have led to a more positive assessment of statements, if those who felt negatively about the programme did not reply. Nurses could have selected more positive consultations to audio-record, inflating the BCT competency levels, although they were encouraged to record cases as they occurred. Not all of the nurses submitted audio-recorded consultations with couples, meaning that we were unable to look separately at the quality of these consultations. The content delivery for the nurse consultations was evaluated from checklists filled out by the nurses because they may have overestimated what they had achieved. We tried to compensate for this by collecting additional data from both the participant and observer perspective to help to corroborate these data; both participant and observer data suggested a high degree of quality in the consultations. The consultation durations were shorter from observer data than from self-report, but data on observed consultations were based on a much smaller sample. The study was not powered to look at the effects of adherence to different aspects of the protocol on trial outcomes; we therefore have reduced power for these analyses, which limits the interpretation of the findings, which cannot be taken to be casual.
Comparison with other complex intervention process evaluations
A number of studies have examined intervention implementation fidelity, with a large variety of process structures and methods; therefore, it is difficult to draw direct comparisons. Process evaluations have become increasingly important, but the purposes and design of studies have been mixed. Many process evaluations are completed independently of trial data collection and are observations of a random subsample of participants or practitioners. 176,177 The process evaluation of the PACE-UP intervention provides both participant and nurse perspectives and identifies a link between contextual factors and adaptations in intervention delivery and acceptance. The study allowed us to look at both perceived and observed behaviour change in the nurse intervention delivery and participant responsiveness; this is unlike many other studies, which have tended to focus on only one perspective, which is most often the person delivering the intervention. 176,178–181 Nurse comments collected at training, individual session checklists and nurse and participant comments from the qualitative work illustrate the intervention delivery and adaptations in context. The intervention was designed to bring about change at an individual level when delivered in a primary care environment; we have observed that context influences the delivery and implementation of the intervention through adaptation. This is similar to findings from other PA and dietary complex intervention studies with process evaluation. 182,183 Specifically, Fitzgerald et al. 182 identified that negotiation and flexibility play an integral role in overcoming the barriers and resistance to change in a dietary intervention. Previous studies have collected data at an organisational or practice level;176,181 there are few studies that have captured the evaluation of behaviour change at an individual level and from two perspectives. A previous study that did look at both patient and practitioner perspectives, however, reported much greater variations in dose and adherence to protocols than seen in the PACE-UP trial, therefore making it difficult to establish which elements of the intervention were effective. 184 Berendsen et al. 184 reported that many health-care professionals deviated from the protocol of a lifestyle intervention to accommodate individuals and reduce fallout, which was associated with increased patient satisfaction for the intervention sessions. This perhaps suggests that adaptations and tailoring of an intervention have a strong influence on retention, adherence and, possibly, effectiveness in lifestyle and behaviour change interventions. The PACE-UP intervention allowed nurses to adapt the sessions as necessary to each individual, while maintaining the key deliverables in each nurse-led session; although we have not looked at the effect of adaptation on retention, we have seen that dose (nurse session attendance) was associated with effectiveness of the intervention. This, in turn, promotes the consideration of building adaptations and flexibility into intervention design. Our finding of an association between the return of a completed step count diary and a change in PA outcomes is consistent with the findings of a systematic review,31 which suggested that the use of a step count diary was common to many successful pedometer interventions.
Implications of the process evaluation for the interpretation of the PACE-UP trial
We have demonstrated that the PACE-UP trial had good adherence to the protocol, the intervention was acceptable and was rated positively by both the nurse-support group participants and the postal delivery group participants, and both groups engaged in self-monitoring using the pedometer and step count diary. It is not possible to infer causality directly from the process evaluation data, but the high level of engagement with pedometers and diaries by both intervention groups suggests that these were important factors in helping people to make the PA changes observed. This is supported by the associations demonstrated between increased PA levels and the following process measures: number of consultations attended, return of a completed step count diary and pedometer use. The careful description and documentation of the trial processes, the collection of additional data for the process evaluation and the publication of the resources used as appendices mean that our intervention and process evaluation would be easy for others to replicate, from training through delivery, to follow-up and evaluation. Use of the MRC framework gave a logical and coherent structure for reporting,169 which is also easy for others to follow. The PACE-UP trial had a positive and significant effect on PA outcomes, but had this not been the case, the positive process evaluation with high levels of fidelity would have enabled us to have confidence that any negative trial effect would not have been because of poor trial implementation. The trial demonstrated a stronger effect on the main PA outcomes and on exercise self-efficacy at 3 months in the nurse-support group than in the postal delivery group, although the effects on PA outcomes were similar between the groups at 12 months (self-efficacy remained higher at 12 months in the nurse-support group). The process evaluation demonstrated that the nurses were delivering BCTs in their PA consultations in accordance with the protocol and with high levels of competence (in addition to the BCTs provided in the handbook and diary for both groups). This suggests that the nurse-delivered BCT elements of the intervention have strong short-term effects on PA levels (and, possibly, longer-term effects on self-efficacy). The possible effects on longer-term maintenance are examined in Chapter 8. The implications of the low reach of the trial for generalisability and the public health impact have been discussed in Chapter 5 and will be considered further in the main discussion in Chapter 9. The process evaluation demonstrated important contextual factors that had an impact on participants’ ability to engage with a walking intervention; these should be considered in any future roll-out of the programme, particularly regarding how the programme may need adaptations to be made in these circumstances.
Conclusion
The PACE-UP trial process evaluation demonstrated that the trial was well delivered by the trial team and well received by participants. The MRC framework was a useful vehicle for reporting the evaluation. An association between adherence to the trial protocol and main trial PA outcomes has been demonstrated. Important contextual factors were shown that may need adaptations to be considered in any roll-out of the intervention.
Chapter 7 What did the nurses and participants think about the intervention?
Introduction
It is important, within the delivery of a behaviour change intervention trial, to understand the experiences of those involved in the delivery and receipt of the intervention. In order to address these important issues, two qualitative studies were embedded within the trial protocol. In these studies, we sought to gain insights into two important issues: (1) the views and experiences of the nurses delivering the intervention and (2) the experiences of trial participants. Summaries of the nurses’ perspectives on the trial185 and the participants’ evaluation of the trial in helping them to increase their levels of PA162 have been published and are reproduced here under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0). In this chapter, we focus upon the perceptions of the nurses and participants of the trial; some of the quotations have been published previously in the publications detailed above.
It is of fundamental importance to understand the perspectives of both those involved in the delivery of the intervention and those who received the intervention. In Chapter 6, we provided a comprehensive process evaluation of the trial which described, in detail, the training and support given to the trial nurses. In Chapter 6, we also addressed issues related to the fidelity of the trial delivery by the nurses. In this chapter, we focus on the nurses’ perspective on their experience of participating in the trial, with a view to understanding how we can better plan and deliver primary care-based trials and then implement them more widely. There is an extensive literature that examines adherence to behaviour change interventions in adults and which establishes the barriers to, and facilitators of, for example, increasing PA. For example, Picorelli et al. ,186 focusing on older adults, reported that adherence to exercise interventions was associated with key demographic factors (higher socioeconomic status and not living alone), health status (fewer health conditions, taking fewer medications and better self-rated health) and psychological factors (fewer depressive symptoms). 186 For trial participants, a novel part of our qualitative study addressed a related, but much less well researched, issue of trying to understand why the intervention did, or did not, work as a means of evaluating the individual elements of the intervention in facilitating behaviour change.
Recruiting trial participants for the qualitative study
At initial recruitment into the trial, participants were asked for consent to participate in follow-up telephone interviews, such as those included in this aspect of the study. The trial statistician prepared a spreadsheet of all participants who had completed the 12-month follow-up in January 2014 and who had given consent to be approached to participate in the telephone interviews (this list was updated in March 2014). We purposely recruited participants who had, and had not, increased their PA levels from both nurse-supported and pedometer-only intervention groups. We defined an increase as ≥ 200 steps per day; anyone who either did not achieve this or decreased their PA was defined as a non-improver. This gave us four interview groups: (1) nurse support/increase, (2) nurse support/no increase, (3) postal delivery/increase and (4) postal delivery/no increase. We also ensured that we sampled participants with a range of ages, from both sexes and from all six of the initial participating practices. As noted in Chapter 2, a novel feature of our trial was the option for participants to take part as a couple and we wanted to ensure their inclusion in our qualitative study. We purposely targeted potential participants from demographic groups under-represented in our main sample (e.g. ethnic minority community participants) to ensure that we explored the widest range of views possible.
Between February and April 2014, we identified 96 trial participants who had been selected on the basis of the criteria described above. We made contact with 44 of these participants, of whom only one declined to be interviewed. We were unable to make contact after three attempts with the remaining 52 participants, who had been initially identified as potential participants. The 43 participants we recruited broadly approximated to the demographic parameters specified, with 20 participants in the 45- to 59-year-old age group, 29 participants being female, 21 participants in the nurse-supported intervention group and 23 participants who did not increase their step counts. We interviewed seven participants who took part as a couple, but we did not interview both partners. In terms of ethnicity, 29 were white British and a further five were from other white ethnicity groups, with nine participants from black and Asian ethnic minority groups. Appendix 6, Table 43 shows the demographic and step count details of individual participants.
We used a semistructured interview guide tailored for each group to reflect the nature of the interventions and the classification of participants as improvers/non-improvers. 162 The 43 interviews completed ranged in duration from 9 to 44 minutes, with an average (mean) duration of 21 minutes. Interviews were recorded and transcribed verbatim. Full details of the analysis strategy for our qualitative interviews is provided elsewhere,162 but are briefly summarised here. All transcripts were read by four authors (RN, JS, CV and TH), codes were assigned independently and discrepancies were resolved by discussion. Codes were grouped into themes, which were further refined by discussion to produce broader themes, encompassing several subthemes. Theoretically informed BCTs were an important element of the trial and we were interested in understanding which of these had been of most use to participants. We performed an additional analysis of the data to specifically draw out themes relevant to these techniques. We have reported the reasons for trial non-participation and the barriers to, and facilitators of, increasing PA elsewhere. 148,162 We have noted elsewhere that, although we defined our groups by the quantitative increase in walking, responses from participants did not demonstrate this distinction. In this, and in our previous qualitative evaluation from the PACE-LIFT study,149 almost all participants interviewed felt that they had benefited from the trials, even if this had not been manifested by an increase in their step count. In this chapter, we focus on what participants told us about their motivation for participating in the trial, their experiences of the various components of the trial and the longer-term impact of trial participation.
The role of nurses in the PACE-UP intervention
As described in detail in Chapter 6, the trial intervention was delivered by eight nurses across seven practices. They delivered three PA consultations to participants in their arm of the trial. These took place in weeks 1, 5 and 9 of the 12-week pedometer-based walking programme. Participants developed an individualised PA plan with the practice nurses, based around their current level of activity, with the goal of increasing both step count and time spent in MVPA. The nurses provided each participant with an individual PA diary, including step count targets for the 12 weeks, based on their own baseline PA measures, but this could be tailored further in the nurse PA consultations through joint discussions between nurse and participant. Five PACE-UP trial nurses participated in a focus group that was led by two of the research team (CB and CV); additionally, two nurses were interviewed individually by Rebecca Normansell (who also attended the focus group) and one further nurse was not available to participate in this phase of the research. A further focus group was also carried out with nurses involved in the previous PACE-LIFT trial of a pedometer-based walking intervention with older people. 21 The focus groups lasted for, on average, 106 minutes and the individual interviews lasted for 50 minutes. The published evaluation of nurse experiences of the interventions includes data from both trials. 185 In this chapter, we have limited the results presented to those from the PACE-UP trial nurses. A semistructured interview guide was used to elicit the nurses’ views on their participation in the trial (see Appendix 6). The interviews/focus groups were audio-recorded and transcribed verbatim. Full details of the methods used are available elsewhere,185 but are briefly summarised here. Coding the transcript themes was guided by thematic analysis for both the group and individual interviews, and areas of disagreement were discussed to ensure a consensus. Researchers were mindful that group interviews reflect a generalised understanding, whereas individual interviews provide more personal views. However, similar interpretations and themes emerged from both types of interview, and referral to field notes throughout the process enhanced the trustworthiness of the findings through data triangulation. 185
In the rest of this chapter, we combine the perspectives of both the nurses and the trial participants to provide an overview of their experiences of being part of the PACE-UP trial. We present our results in terms of the three key phases of the intervention: preparing for the trial, delivering or receiving the intervention and after the trial/implementation.
Preparing for the trial
A key theme that emerged from our work with the nurses was the importance of the pre-trial training programme. As documented in Chapter 6, before the trial was implemented the nurses received training in the BCTs that underpinned the intervention. Support was then ongoing once the trial had started. Although these were experienced practice nurses, we could not presume detailed familiarity with all of the techniques that our study involved. An additional and important feature of the training was the importance of trial fidelity. One unique element of our trial was the delivery to couples. This is not a familiar service delivery model for our nurses and so it was one area where we provided specific support/training. As comprehensively demonstrated in Chapter 6, the consensus from our nurses was that they felt appropriately trained and prepared to deliver the intervention as per the protocol: ‘all the training was really excellent’ (PACE-UP nurse, focus group).
For participants, the key pre-trial activity focused upon the decision to participate. This is described in detail in Chapter 5, in which we discuss the recruitment and participation rates. In Chapter 5, and in Normansell et al. ,148 we present both the sociodemographic profile of participants and examine why individuals opted not to participate in the trial. Although we have details of the sociodemographic profiles of those who take part in PA trials, we have less information on what motivated participants to take part in the trial. This was not explicitly explored in our interview, as the focus was on the trial and its impact on levels of PA. However, some participants talked explicitly about their reasons for taking part in the trial. Key individually based motivations to participate were concerns about weight and helping to manage existing long-term conditions (especially diabetes mellitus). Others noted the commitment they were making in signing up for the intervention, even though they were not aware of which group they were in when they agreed to participate in the trial: ‘Well I must admit, when I first signed up for it, I was thinking what I have done to commit myself to this for quite a long time’ (ID30).
Delivering and participating in the trial
Our focus in this chapter is on the experiences of participating in the trial, rather than on the outcomes or the factors that facilitated changes in PA. Our trial had three arms: usual care (n = 338), postal pedometer intervention (n = 339) and nurse-supported pedometer intervention (n = 346). Of our 43 interviewees, 21 were in the nurse-supported intervention group and 22 were in the postal delivery group. We first consider the experiences of participants who were in the nurse-support group and compare these with the postal delivery group in terms of how they perceived their engagement with the trial.
Perceived value of nurse consultations
The nurse-led consultations were well accepted by the participants in that part of the trial, with 74% (255/346) attending all three sessions. Overall, those who were allocated to the nurse-supported intervention arm were very happy with their meetings with the nurse, as illustrated thus:
Yes, the nurse was very helpful . . . it was really good for me.
ID12
Conversely, those who had not been in the nurse-support group were, overall, confident that they did not need the support of the nurse:
I think I was happy doing it on my own. I don’t think . . . I mean it was very . . . it was very easy to follow your instructions and what you wanted us to do and so I don’t think meeting a nurse . . .
ID13
No, I don’t think it [visiting the nurse] would have been useful really.
ID17
Some were sceptical of what advice the nurse could give:
No, not really. What would she say, walk a bit quicker, eat a bit less? It’s common sense, I knew in the first place.
ID23
I know what I should be doing.
ID8
Others were confident in their own internal motivation:
I think if I’ve agreed to do something, then I will try and achieve that target, whether somebody tells me face to face or by post, so I think it’s dependent on the individuals maybe, individual choice.
ID38
There were two key caveats to the confidence of the postal delivery group in increasing their PA without the support of a nurse. These were existing health problems and overcoming barriers. Several participants in the postal delivery group observed that if they had had a long-standing health problem, they may have preferred the security and support of the nurse, as illustrated in this comment:
If I did have health problems, I may have wanted to see a nurse, and say, look, I’ve been doing this and I’ve had an ache and a pain here, shall I stop or . . . you know, if it was that situation, and I think yes, you might need to speak to someone you know who could advise you medically, but I didn’t need it.
ID13
In addition, some participants in the postal delivery group thought that being able to see the nurse might have helped them to overcome the difficulties and challenges they experienced when trying to increase their PA, for example:
I would have found that better [to see a nurse] because, if I’d have talked to her about the steps, she might have been able to umm introduce something else . . . [participant was concerned that the target step count was unachievable]. I think if I’d have been seeing the nurse regularly then, during that summer, umm, we would have found another way I feel, you know.
ID19
Behaviour change techniques
Our intervention included over 20 distinct behaviour change activities, as defined by Michie et al. ,68 embedded within the PACE-UP trial handbook and diary (received by both the postal delivery group and the nurse-support group) and, additionally, within the protocol for the nurse consultations. Reference to these specific BCTs were then extracted from the interview transcripts to determine what elements of behaviour change were viewed as being most helpful by the participants. 162 There were 152 examples of these factors in our interviews: 54 in the postal delivery group and 98 in the nurse-support group. With the exception of self-monitoring, the BCTs were more frequently noted in the nurse-support group compared with the postal delivery group. The elements of Michie et al. ’s68 typology that were especially evident in our participants’ comments were (1) the provision of information, (2) monitoring and feedback and (3) strategies for relapse prevention/overcoming challenges. Rewards, an important element of the typology, were not seen as being of great importance by our participants. Both of our intervention groups appreciated receiving information about the link between behaviour and health. However, a key and important type of information provided, especially in the nurse-support group, was specifically tailored and personalised information about how, where and when to increase walking, for example:
She gave me a printout of umm . . . some . . . walks that you could do, group walks and things like that.
ID18
In terms of monitoring and feedback, nurses played an important role for their group, as exemplified by comments such as the following:
She was very encouraging.
ID39
Oh I felt really happy [with the nurse] and she was very happy too with me and I did really like my nurses, yes. That was one of my reasons because, each week I go, I ask her if they think I am doing well, am doing well, yes [laughs].
ID12
Importantly, the nurses were seen to provide motivation and encouragement when the ‘novelty’ of the intervention was waning and participants were at risk of lapsing, for example:
I think there was a point where they sort of said, you know, don’t give up now, or something like that, you know, at the point where . . . the novelty might have worn off . . .
ID35
The postal delivery group largely felt that they could self-monitor their activity:
I felt like it was enough to know [the step count] . . . I think it was fine, just to sort of keep . . . I was very good about filling the diary in and it was sort of for me that was enough to keep me going really.
ID43
Adapting the trial protocol versus fidelity
Given that this was a trial, it was essential that the nurses delivered the intervention as per the protocol; therefore, before the trial started, we provided extensive training to the nurses and emphasised the importance of adherence to the prescribed protocol:
The equipment was excellent, the pedometers, the accelerometers, excellent, excellent, excellent.
However, given that the trial ran over 12 months, pragmatic adaptations were made by the nurses (in consultation with the team) in response to the specific circumstances of participants, for example, working around holiday periods (e.g. Christmas) or periods of religious observance, such as Ramadan:
. . . forget about the 2 months around Christmas . . . you can’t get appointments and they don’t want to wear it [pedometer].
This serves to remind us that delivering behaviour change interventions in real-world practice requires ‘fine tuning’ in the delivery to reflect the complexity of people’s daily lives. We assessed fidelity by audio-recording a minimum of three consultations for each nurse, as described in detail in Chapter 6. However, this also enabled us to provide specific feedback to the nurses on their use of the BCTs employed. This was universally welcomed and improved their practice during the trial, but also provided them with enhanced skills to take into practice beyond and after the trial:
I actually changed my practice from thereon, so yes, it was exceptionally helpful.
Trial materials and equipment
Both nurses and participants remarked on the materials that supported the trial, namely a pedometer, a PA diary and a series of optional handouts, as described in Chapter 2. From the participants’ perspective, each element of the trial had both advantages and disadvantages. However, the focus of the comments about equipment from the participants’ perspective was upon the accuracy (or otherwise) of the pedometer. Typical of the more critical comments of the pedometer was:
That day we went for a really long walk round the common, I was really disappointed when I come back, it didn’t seem to register very many steps. I’ve obviously done a lot more than . . . registered about ,000 or something, well walked around the whole of Tooting, is a bit more than that I think.
ID7
There were fewer comments about the diary, which was seen as being motivating:
I was very good about filling the diary in and it was sort of for me that was enough to keep me going really.
ID43
However, for others it was chore:
Like I say, it is an effort, it’s umm . . . you have to know what you are in for, and then really maintain it. I had to record it every day, yes, you are busy, sometimes you are out and you go for dinner and then you come home and then you had a few drinks and you can’t remember what day it is.
ID1
Actually writing down the activities and things, it . . . after about the first day, I got bored with that.
ID3
Nurse satisfaction
One feature of the trial noted by the nurses (but not the participants) was the time that they had to devote to the PA consultations compared with their normal activities, as this comment illustrates:
You know, we don’t have any protected time for health promotion . . . the health promotion is the add-on. It’s giving us the time, because we don’t have the time.
From the nurses’ perspective, this engagement provided considerable job satisfaction in seeing their participants embrace change and become more physically active. A feature not experienced prior to the trial by most of the nurses was delivering the intervention to couples rather than individuals. This presented a unique challenge for some of the nurses as, in normal practice, it is unusual to be working with two patients simultaneously. Sometimes the dynamic worked well:
. . . most couples, they enjoy doing it together because they’d go . . . they could go out walking together and, even if it was through the winter, at least if they were both going, they had each other . . . they use to encourage each other. So if one didn’t want to go the other one would encourage them and they’d make sure they went.
At other times, the problems encountered were a significant barrier:
I’m not actually overly sure how couples worked. I don’t know if I had, I don’t know if it caused more issues sometimes, in the fact with the pedometers, because they got so focused sometimes on the fact that their pedometers didn’t match up.
After the trial and implementation
All participants felt that they had benefited, regardless of changes in their objective PA levels, and had developed skills in terms of embedding PA in their daily lives and routines and by developing strategies to overcome challenges when they arose:
Yes, everyone in my house now, we don’t drive to the shops, we all walk to the shops . . . it was easier for me just to jump in to the car, now I have to think twice, do I really have to?
ID21
Yes, setting my own targets and now . . . well, it’s something that I’ve got used to now and I’m determined to keep it up.
ID11
All of our nurses were very positive about how participation in the trial had developed and enhanced their knowledge and skills, which they were applying across a wide range of routine lifestyle consultations, not just related to PA, but also for smoking cessation, weight loss management and the prevention of chronic diseases. From the perspective of primary care and the nurses specifically, participation in the trial generated a legacy and the project was, in a small way, able to support the development of expertise in primary care of the use of BCTs and in working with patients in different ways (e.g. couples).
Our intervention was an individual as opposed to a group-based intervention. Some participants firmly believed that this was most appropriate:
If it involved each person reporting back on their success or failure at meeting the previous targets, it might be a bit awkward in a group possibly.
ID2
However, others identified the potential benefits of a group intervention:
When you are with other people, and then you see the same problems they are facing, some of them might come up with other ideas . . . you can form a team, support network.
ID21
Ultimately, this suggests that we need a repertoire of interventions that mesh with the circumstances and preferences of the different populations.
There was virtually uniform support for the location of the trial within a primary care context, with many participants recognising the convenience of getting to their GP surgery:
. . . you wouldn’t want someone to have to travel and people know how to get to their doctors don’t they?
ID 29
Yes, that was good, because obviously it was very near home so it was ideal.
ID15
From the nurses’ perspective, although acknowledging that the intervention would be beneficial to their patients, they observed that within the time constraints of routine practice, they would not be able to replicate the full intervention as it stood within a routine nurse consultation. The nurses made suggestions for modifying the intervention to focus on the pedometer and printed materials for use opportunistically within ‘normal’ practice and, perhaps, to be available on prescription. The suggestion that health advisors, or a related role, could deliver the intervention that we evaluated was not supported by our nurses.
Discussion
From this phase of our study, several key points arise. Almost without exception, both the nurses and the participants enjoyed taking part in the study and felt that it had provided them with important and enduring benefits. For the nurses, these benefits were couched in terms of new skills that they could transfer into their routine practice. For participants, there was an increase in their awareness of the benefits of walking as a means of enhancing health by increasing PA. Among our intervention groups, this perceived benefit was articulated irrespective of the objective changes in PA.
For nurses and intervention group participants, a key feature of the trial was the preparatory work before the intervention started. This was especially important for the practices who participated and the nurses. We opted to deliver this trial in ‘ordinary’ general practice settings to test out the potential of the intervention to be implemented in routine primary care settings. Participants and nurses alike felt that primary care is the appropriate setting from which to run such interventions. However, the nurses provided a caveat to this with comments about the constraints of time within ‘real-world’ primary care. Prior to the implementation of the intervention, the research team and our behaviour change experts worked extensively with the practices and nurses and provided training in the intervention, feedback on the delivery of the intervention and support across the trial to the nurses delivering the intervention. One example of a challenge with which some nurses needed support was in working with couples. This is not something that usually occurs within their general working regime and support from the research team in dealing with these challenges was important.
The nurses were supported to adapt how materials were introduced and used within the consultations to make these materials relevant to the participant and thereby personalise the intervention more. This is a key challenge in effectively delivering standardised interventions, in both trial settings and every day primary care – how to ensure the consistency of information provision and support, while also making it relevant to individuals. Empowering nurses, and other primary care staff, to make ‘patient-centred’ adaptations to standard behaviour change programmes is likely to result in improved outcomes. This links to the important issue of tailoring support to make changes in health behaviours to match the circumstances and preferences of individuals. Thus, support needs to be appropriate to external factors, such as seasons or the time of year, or key events in peoples’ lives, such as retirement, but also reflect individual circumstances such as preferences for group or individual activities and the relevance of written or other types of digital materials and current health problems. Our study has included two types of intervention that offered varying levels of support, both of which generated increased levels of PA. A key challenge for future studies is to determine which groups would benefit from the ‘minimal support’ pedometer by post-type intervention, and those for whom the more intense nurse-led intervention is the most appropriate.
Chapter 8 Three-year follow-up to assess the maintenance of physical activity levels
Introduction
The PACE-UP analyses showed positive effects on 12-month PA levels (see Chapter 3). We wanted to see if this effect was maintained at 3 years, as this has important implications for the NHS; specifically, would any future pedometer programme require a ‘top-up’ after 12 months? Our interventions led to an extra 33–35 minutes weekly of MVPA in bouts (an increase of about one-third from baseline) in a predominantly inactive cohort. Delivery via three nurse PA consultations had the same effect on 12-month outcomes as the simpler, cheaper postal delivery. These are exciting findings, as they show that a low-cost postal pedometer intervention increases PA levels in sedentary adults and older adults. However, it is vital to know whether 12-month effects persist at 3 years or if a further intervention boost is needed. We therefore successfully applied for additional funding from NIHR’s Health Technology Assessment programme to follow-up the trial cohort at 3 years (2 years after the previous 12-month follow-up) with both quantitative and qualitative evaluations. Both the qualitative187 and the quantitative findings188 have been published and are reproduced here under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0).
To date, little is known about the long-term sustainability of PA interventions. A meta-analysis of interventions (including pedometers) to increase PA levels in 55- to 70-year-olds included only four trials with data beyond 12 months (all self-reported). They found a limited evidence base beyond 12 months, and called for more trials with a longer follow-up period and objective PA measures. 26 These findings were supported by a Cochrane systematic review23 and recent NICE guidance on PA interventions. 29 The ProAct 65 + trial189 of a PA intervention found that between-group differences persisted at 2 years post intervention, but only for self-reported PA.
As well as a lack of long-term objective PA data after interventions, there is also a lack of qualitative evidence on maintenance. A literature review suggested that PA disengagement usually occurs 6 months after an intervention has ended, but called for more research to distinguish the factors that lead to successful and unsuccessful PA maintenance. 190 A very small primary care study followed up participants 6 months after a pedometer-based intervention, and found some useful insights to explain how this pedometer intervention worked and how it may be developed,44 but further qualitative studies on longer-term effects are lacking.
The PACE-UP trial 3-year follow-up provides evidence on objective PA levels, 2 years after the 12-month follow-up. After the 12-month follow-up, 212 out of 322 controls (66%) received a pedometer, handbook and diary by post. They had no further input (unlike the original postal delivery group, who were telephoned by a research assistant 1 week after being sent the pedometer, to check that it had arrived, and who were asked to return their completed PA diaries for review at 3 months). The control participants being sent the pedometer by post mimics what would happen if this simple, pragmatic intervention were to be rolled out by post through routine primary care, without any further input. As described in our protocol,70 a further 64 out of 322 control participants (20%) attended a single nurse appointment after the main trial, in which they were given a pedometer, diary and handbook, but, again, received no further contact. The other intervention groups received no further intervention after the 12-month follow-up [apart from a small number of the postal delivery group, 50/312 (16%), who had a single nurse PA appointment].
Anthropometric measures did not show differences at 12 months; therefore, data on these were not collected at the 3-year follow-up. Therefore, our follow-up study focused on establishing evidence of effectiveness at 3 years in objective PA measures and had the following objectives:
-
to investigate if the original nurse-support and postal delivery groups showed any persistent intervention effect (change in step count and time in MVPA in bouts) at 3 years, compared with the baseline levels
-
to investigate if there were any differences between the original nurse-support and postal delivery groups in their change in objective PA levels (step count and time in MVPA in bouts) between baseline and 3 years
-
to investigate if the simple postal pedometer intervention at 12 months increased objective PA levels (step count and time in MVPA in bouts) in the control group compared with baseline.
We also felt that it was important to explore how participants in the nurse-support and postal delivery groups felt about the interventions in terms of maintenance of any increase in PA levels, and what factors might help to encourage this further or to overcome barriers they had to increasing or maintaining their PA levels. Furthermore, we were interested in how the initial control group felt about the minimalist intervention that they received. Our qualitative evaluation therefore had the following objectives:
-
qualitative evaluation of both the nurse-support and postal delivery intervention groups to look at factors affecting PA levels and maintenance of any increase in PA levels at 3 years
-
qualitative evaluation of the control group to see the effect of the minimal intervention on PA levels.
Methods for quantitative physical activity evaluation
The 3-year follow-up focused on collecting the objective PA accelerometry data and other questionnaire-based self-reported outcomes by post to minimise data collection costs. To allow for seasonal variation in PA levels, the baseline, 12-month and 3-year outcomes needed to be assessed in the same calendar month; therefore, follow-up ran from October 2015 to November 2016.
Ethics approval and research governance
Ethics approval for the 3-year follow-up was granted to the trial from London, Hampstead Research Ethics Committee (reference number 12/LO/0219). NHS local research and development approval was granted to cover all of the practice sites.
Participants eligible for the 3-year follow-up
All trial participants who had not withdrawn from the trial were eligible to be followed up, even if they had not provided 3- or 12-month follow-up data. Lists of eligible participants were organised by practice and practices were asked to check whether any participants had died, moved away or developed a terminal illness or dementia since trial participation. These patients were then excluded.
Contacting participants
Eligible participants were contacted with a letter explaining the trial 3-year follow-up. A participant information sheet, consent form and a Freepost return envelope were also included, as was information on the main 12-month trial results. The letter explained that a research assistant would contact the participants by telephone in around 1 week’s time to discuss the 3-year follow-up. If they were happy to take part without further discussion, they were invited to post back the signed consent form.
Informed consent
The research assistant contacted participants by telephone approximately 1 week after sending out the letter about the 3-year follow-up to discuss any questions they might have after reading the participant information sheet. Part of the consent form included consent to contact participants for an interview to discuss their current PA levels in more detail. If the participants were happy to proceed with the 3-year follow-up, they signed and dated the informed consent sheet and returned the top copy to us.
Data collection
Once the informed consent for follow-up was agreed, the research assistant arranged a time to post out the accelerometer (GT3X+) to participants to measure their current usual PA levels for 1 week. Instructions about how to wear the accelerometer were included (on a belt, over one hip) and participants were asked to wear it for 7 consecutive days, from getting up until going to bed, as they had done previously. A diary was also provided to record what activities were done and for how long. They were also sent a health and lifestyle questionnaire to complete (see Appendix 7; this was similar to that completed previously) and a short questionnaire about self-reported PA levels to complete (the 7-day PA questionnaire used previously) after they had finished wearing the accelerometer. They were provided with a Freepost return envelope to send the accelerometer and both questionnaires back. If the accelerometer did not record at least 5 days with at least 540 minutes per day, participants were asked to rewear it for a further week. The set of recordings with the greatest number of days with at least 540 minutes per day was included in the analysis. Once accelerometers with adequate data were received, participants were posted out a £10 gift voucher.
Outcome measures
The main outcome measures (all accelerometry) used to evaluate the 3-year follow-up were as follows:
-
change in average daily step count, measured over 7 days between baseline and 3 years
-
change in time spent weekly in MVPA in ≥ 10-minute bouts between baseline and 3 years
-
change in time spent sedentary weekly between baseline and 3 years.
Although patient-reported outcome measures were collected from the health and lifestyle questionnaire (e.g. depression,75 anxiety,75 quality of life,76 self-efficacy,74 pain,77 disability80) and from the 7-day PA questionnaire (IPAQ72 and GPPAQ73), these have not been assessed further as part of this report.
Accelerometer data reduction
ActiGraph data were reduced, as described previously in Chapter 2, for the main trial. Analysis summary variables were also identical to those used in the main trial, described fully in Chapter 2.
Procedure for accounting for missing data
Only days with at least 540 minutes of registered time on the accelerometer on a given day were used. The main analysis of effect included all subjects with at least 1 satisfactory day of recording at 3 years.
Statistical methods
Statistical methods for the analysis of the 3-year follow-up are largely as described in Chapter 2 for the primary analysis at 12 months. Average daily step count at 3 years was computed from a random-effects model, allowing for day of the week and day order of wearing the accelerometer as fixed effects and participant as a random effect. The average daily step count at 3 years was then regressed on average daily step count at baseline with treatment group, age, sex, practice and month of baseline accelerometry as fixed effects and household as a random effect, in a multilevel model. The same analyses were carried out for MVPA in ≥ 10-minute bouts and daily minutes of sedentary time.
The primary analyses used the 681 participants who provided accelerometry data at 3 years. Sensitivity analyses were carried out to assess the effect of missingness:
-
Multiple imputation methods were used to impute outcome data for those missing at 3 years, assuming that outcomes were missing at random, conditional on variables in the model. We used the Stata procedure mi impute.
-
Missing-not-at-random analyses were used when it was assumed that changes in the control group from baseline to 3 years were missing at random, but the change in each of the intervention groups was ± 500 and ± 1000 steps from their missing-at-random estimate.
For this analysis, we used a mean score method,191 which has been implemented in a Stata module, rctmiss, available from Statistical Software Components at https://ideas.repec.org/s/boc/bocode.html (accessed 25 May 2018).
Methods for qualitative evaluation
Sampling
Participants consented to be contacted for a telephone interview at the same time as they consented to take part in the 3-year follow-up. In February 2016, the research assistant produced three lists of participants who had already provided 3-year accelerometry data and who had given consent to be interviewed (one list for each arm of the trial – nurse support, postal delivery, control). The trial statistician randomly sorted these three lists ready for the qualitative researchers to start approaching participants for interviews. The interviewers were blinded to participants’ previous and current PA levels.
One of the aims of the qualitative evaluation was to explore the success, or otherwise, of the minimalist intervention provided to the control group after the main trial; therefore, the following participants were excluded from this qualitative evaluation:
-
control group participants who had attended a nurse consultation after the main trial
-
control group participants who opted not to receive the postal pedometer after the main trial.
At 12 months, participants in the postal delivery group were also offered a nurse consultation. Again, those who attended the nurse consultation were excluded from the sampling procedure, so that all those sampled from the postal delivery group had received just the postal intervention.
The aim was to interview approximately 15–20 people from each arm of the trial (45–60 people in total), but continuing further if required, until saturation was reached. As two researchers (CB and CW) were interviewing participants, they met regularly to discuss the sampling, interview schedule and any emerging themes. Interviews were conducted until saturation of new information was reached. By looking at the participants’ demographic information it was possible to ensure that the study group included both males and females and also represented a range of different ages and ethnicities, to ensure that a wide range of views were explored.
Recruitment and informed consent
Participants were initially contacted via e-mail to arrange an appropriate time to contact them for a telephone interview. Participants without an e-mail address were called and the interview was either conducted then or arranged for later. To assess response, a detailed record was kept of when each participant was contacted, including information on who agreed, who refused and who could not be contacted. Charlotte Wahlich and Carole Beighton conducted the guided interviews with participants using a topic guide (see Appendix 7). Before the interview commenced, the participants were reminded of their initial consent to be approached for an interview; if the participants were happy to go ahead, their consent was then sought for the interview to be audio-recorded. Once the recording had started, the qualitative researchers stated the participant’s ID number to ensure confidentiality and anonymity in the subsequent transcript. On interview completion, the participants were offered a £10 high-street gift voucher to thank them for their time.
Transcribing
Interviews were promptly transcribed verbatim by an external source. Once the transcripts were received back, they were double-checked against each audio-recording by the qualitative researchers. Transcripts were also circulated to the research team to ensure consistency between the interviewers and to help assess when theme saturation had been reached.
Interview schedule
The interview schedules were developed through discussions with Tess Harris, Charlotte Wahlich, Carole Beighton, Christina Victor and Rebecca Normansell. Slightly different questions were used for participants in the intervention groups (postal delivery and nurse support) and participants in the control group (see Appendix 7). For those in the intervention groups, the aim was to explore participants’ views about PA maintenance and whether or not a ‘top-up’ intervention was required, whereas for those in the control group, the aim was to explore their views about the minimalist intervention. The interview schedules were revised slightly during data collection to ensure that the questions were clear and to include additional questions to gain a better understanding of the participants’ experiences.
Analysis
All verbatim transcripts were read repeatedly by Charlotte Wahlich and Carole Beighton. Initial line-by-line coding was conducted independently to assign conceptual ideas to important episodes within the data. Through discussion with Rebecca Normansell, Tess Harris and Christina Victor, any discrepancies were resolved; this helped to ensure that the interpretation and categorisation of the data were valid. After further discussions between Charlotte Wahlich and Carole Beighton, these codes were then refined and grouped into emergent and anticipated themes.
Results for the quantitative physical activity evaluation
Follow-up rate
Of the 1023 original trial participants, 32 had withdrawn by the end of the 12-month follow-up, a further two had died between the 12-month and the 3-year follow-up and one was excluded by their practice for health reasons. Therefore, we approached 988 participants and 681 provided adequate accelerometry data (≥ 1 day with ≥ 540 minutes wear time) for analysis, giving a 3-year follow-up rate of 69% (681/988). However, in relation to the initial trial participants providing 3-year outcome data, the 3-year follow-up rate was 681 out of 1023 (67%). The CONSORT flow diagram with 3-year follow-up data is shown in Figure 15, by randomised group.
FIGURE 15.
The PACE-UP CONSORT flow diagram with 3-year follow-up data.
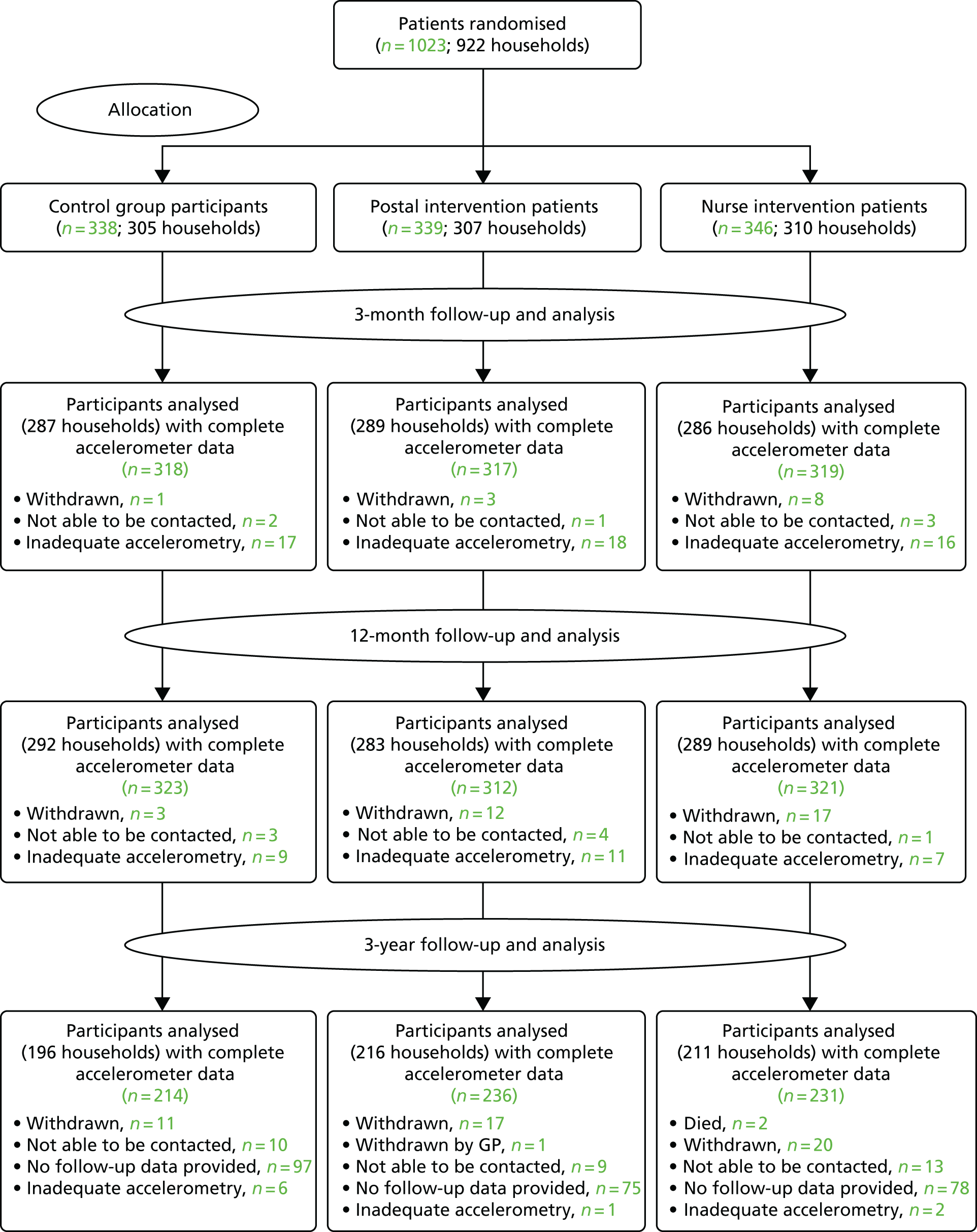
Data completeness
Table 20 shows that 92% of participants (625/681) overall provided ≥ 5 days of accelerometry data at 3 years (88% of control participants, 94% of postal delivery participants and 93% of nurse-support group participants).
| Accelerometry data | Randomised group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Postal delivery | Nurse-support | ||||||||||
| Baseline | 3 months | 12 months | 3 years | Baseline | 3 months | 12 months | 3 years | Baseline | 3 months | 12 months | 3 years | |
| Number of participants | 338 | 318 | 323 | 214 | 339 | 317 | 312 | 236 | 346 | 319 | 321 | 231 |
| Number of participants with ≥ 5 days wear (%) | 338 (100) | 286 (90) | 300 (93) | 188 (88) | 339 (100) | 282 (89) | 287 (92) | 222 (94) | 346 (100) | 296 (93) | 302 (94) | 215 (93) |
| Daily step count, mean (SD) | 7379 (2696) | 7327 (2688) | 7246 (2671) | 7281 (2721) | 7402 (2476) | 8086 (3014) | 8010 (2922) | 7896 (2853) | 7653 (2826) | 8707 (3206) | 8131 (3228) | 8131 (3410) |
| Total weekly minutes of MVPA in ≥ 10-minute bouts, mean (SD) | 84 (97) | 87 (101) | 89 (94) | 94 (102) | 92 (90) | 136 (125) | 129 (124) | 132 (124) | 105 (116) | 164 (154) | 138 (141) | 138 (161) |
| Daily sedentary time (minutes), mean (SD) | 613 (68) | 614 (70) | 616 (72) | 615(71) | 614 (71) | 614 (74) | 617 (71) | 617 (75) | 619 (78) | 613 (77) | 620 (79) | 620 (69) |
| Daily wear time (minutes), mean (SD) | 789 (73) | 795 (78) | 791 (76) | 789 (78) | 787 (78) | 798 (84) | 800 (80) | 798 (86) | 797 (84) | 805 (85) | 807 (89) | 805 (81) |
Objective physical activity findings
Table 20 shows the summary measures for all three groups at each time point and Table 21 shows the estimates of effect for the different groups. For the main trial outcome of steps per day, both intervention groups were still doing more than the original trial control group: 627 steps (95% CI 198 to 1056 steps) in the postal delivery group and 670 steps (95% CI 237 to 1102 steps) in the nurse-support group. The nurse-support and postal delivery groups combined did 648 steps per day (95% CI 272 to 1024 steps). The pattern was similar for the total weekly MVPA in bouts (minutes per week): 28 minutes (95% CI 7 to 49 minutes) in the postal delivery group and 24 minutes (95% CI 3 to 45 minutes) in the nurse-support group. The nurse-support and postal delivery groups combined did 26 minutes (95% CI 8 to 44 minutes). There was no difference between the groups at 3 years for sedentary time or daily wear time.
| Outcome | Comparison of change from baseline between randomised groups | |||||
|---|---|---|---|---|---|---|
| Postal delivery vs. control | Nurse support vs. control | Nurse support and postal delivery vs. control | ||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | |
| Daily step count | ||||||
| 3 months | 692 (363 to 1020) | < 0.001 | 1173 (844 to 1501) | < 0.001 | – | |
| 12 months | 642 (329 to 955) | < 0.001 | 677 (365 to 989) | < 0.001 | 660 (389 to 930) | < 0.001 |
| 3 years | 627 (198 to 1056) | 0.004 | 670 (237 to 1102) | 0.002 | 648 (272 to 1024) | < 0.001 |
| Total weekly minutes of MVPA in ≥ 10-minute bouts | ||||||
| 3 months | 43 (26 to 60) | < 0.001 | 61 (44 to 78) | < 0.001 | – | |
| 12 months | 33 (17 to 49) | < 0.001 | 35 (19 to 51) | < 0.001 | 34 (20 to 48) | < 0.001 |
| 3 years | 28 (7 to 49) | 0.009 | 24 (3 to 45) | 0.026 | 26 (8 to 44) | 0.006 |
| Daily sedentary time (minutes) | ||||||
| 3 months | –2 (–12 to 7) | 0.59 | –7 (–16 to 3) | 0.16 | – | |
| 12 months | 1 (–8 to 10) | 0.82 | 0 (–9 to 9) | 0.96 | 0 (–7 to 8) | 0.92 |
| 3 years | –1 (–12 to 11) | 0.90 | –2 (–14 to 9) | 0.69 | –1 (–11 to 8) | 0.77 |
| Daily wear time (minutes) | ||||||
| 3 months | 2 (–8 to 12) | 0.69 | 4 (–6 to 14) | 0.39 | – | |
| 12 months | 9 (–1 to 19) | 0.08 | 9 (–1 to 19) | 0.07 | 9 (0 to 18) | 0.04 |
| 3 years | 8 (–5 to 20) | 0.23 | 7 (–6 to 19) | 0.32 | 7 (–4 to 18) | 0.21 |
Missing data analyses
Imputation analyses (see Table 22) presents the results for missing at random, using imputations based on the different assumptions detailed in the methods section. The imputation analyses show that making adjustments for missing values has only a small effect on the primary outcome, that is, the step count estimate, and does not change the interpretation.
| Missing at random imputation analyses | N | Randomised group(s) vs. randomised group | |||||
|---|---|---|---|---|---|---|---|
| Postal delivery vs. control | Nurse support vs. control | Nurse support and postal delivery vs. control | |||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | ||
| All participants with follow-up data | 681 | 627 (198 to 1056) | 0.004 | 670 (237 to 1102) | 0.002 | 648 (272 to 1024) | < 0.001 |
| Imputed using treatment group, baseline steps, sex, age, practice, month of baseline accelerometry | 1023 | 597 (174 to 1020) | 0.006 | 679 (268 to 1089) | 0.001 | 649 (295 to 1003) | < 0.001 |
| Imputed using treatment group, baseline steps, sex, age, practice, month baseline accelerometry, NS-SEC, baseline self-reported pain and baseline body fat massa | 996 | 634 (211 to 1057) | 0.003 | 735 (293 to 1178) | 0.001 | 637 (239 to 1034) | 0.002 |
| Imputed using treatment group, baseline steps, sex, age, practice, month baseline accelerometry and 12-month stepsb | 965 | 625 (217 to 1033) | 0.003 | 683 (270 to 1095) | 0.001 | 655 (305 to 1005) | < 0.001 |
The missing-not-at-random analyses make a bigger impact, but only when we assume that there is a strong differential departure between the non-random effects in the control and treatment groups (solid lines in Figure 16). Even then, it is only when we assume that the missing data in the treatment groups are 1000 steps below their missing-not-at-random values, while the values in the control group are at their missing-not-at-random values, that the treatment effects become non-significant; even then, the CI is still largely positive.
FIGURE 16.
Sensitivity analyses for different values of missing step counts. (a) Postal delivery group vs. control group; (b) nurse-support group vs. control group; and (c) postal delivery and nurse-support groups combined vs. control group. The figures show how different values for the missing step counts changes the treatment effects. The starting point where all missing step counts are replaced by ‘missing-at-random’ estimates in each group is represented by the zero difference estimate. Estimates in the different groups are then altered differentially over a range of scenarios. Control group – missing step counts are the same as the 3-year missing-at-random estimates. Treatment groups – missing step counts are 500 or 1000 steps lower than the 3-year missing-at-random estimates. Control group – missing step counts are 500 or 1000 steps higher than the 3-year missing-at-random estimates. Treatment group – missing step counts are the same as the 3-year missing-at-random estimates. Missing step counts are 500 or 1000 steps lower or higher than the 3-year missing-at-random estimates in all control and treatment groups (see White191 for methods). The vertical lines are the 95% CIs for the estimated treatment effects. The treatment effect becomes statistically not significant when the CI crosses the horizontal line at zero.
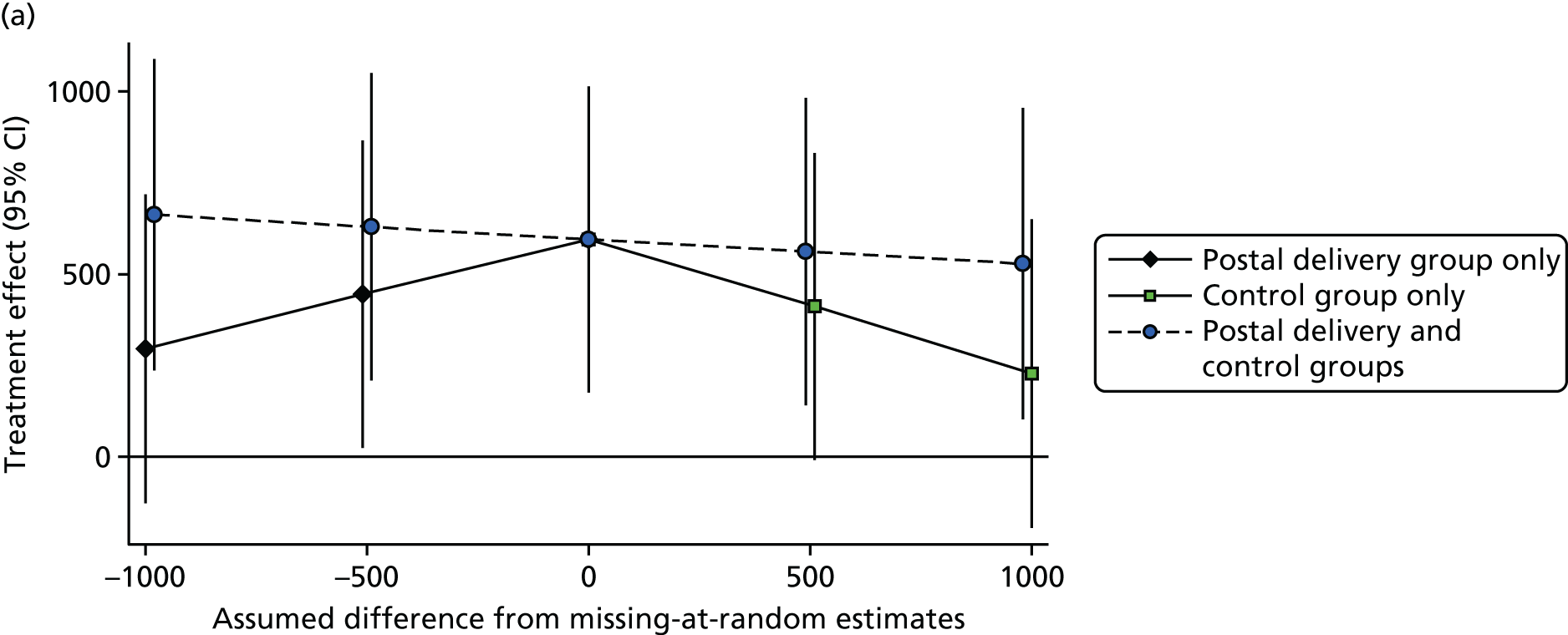
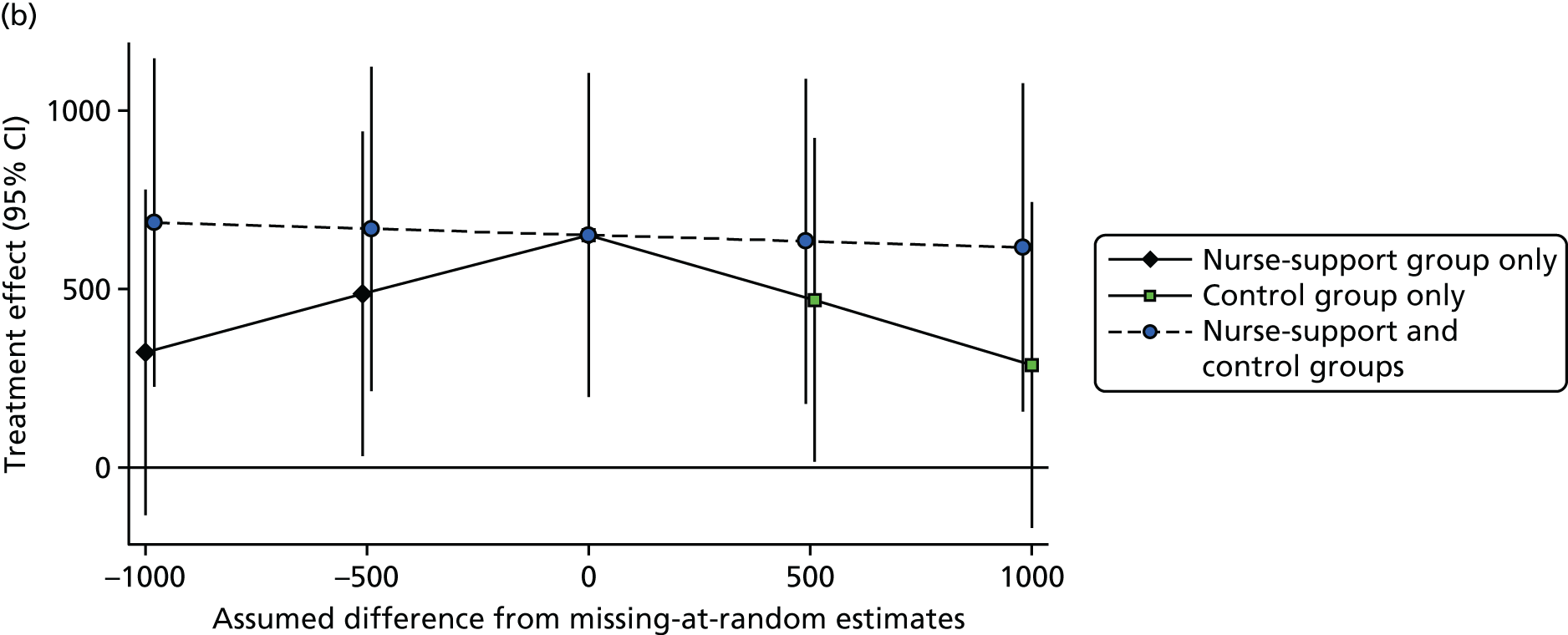
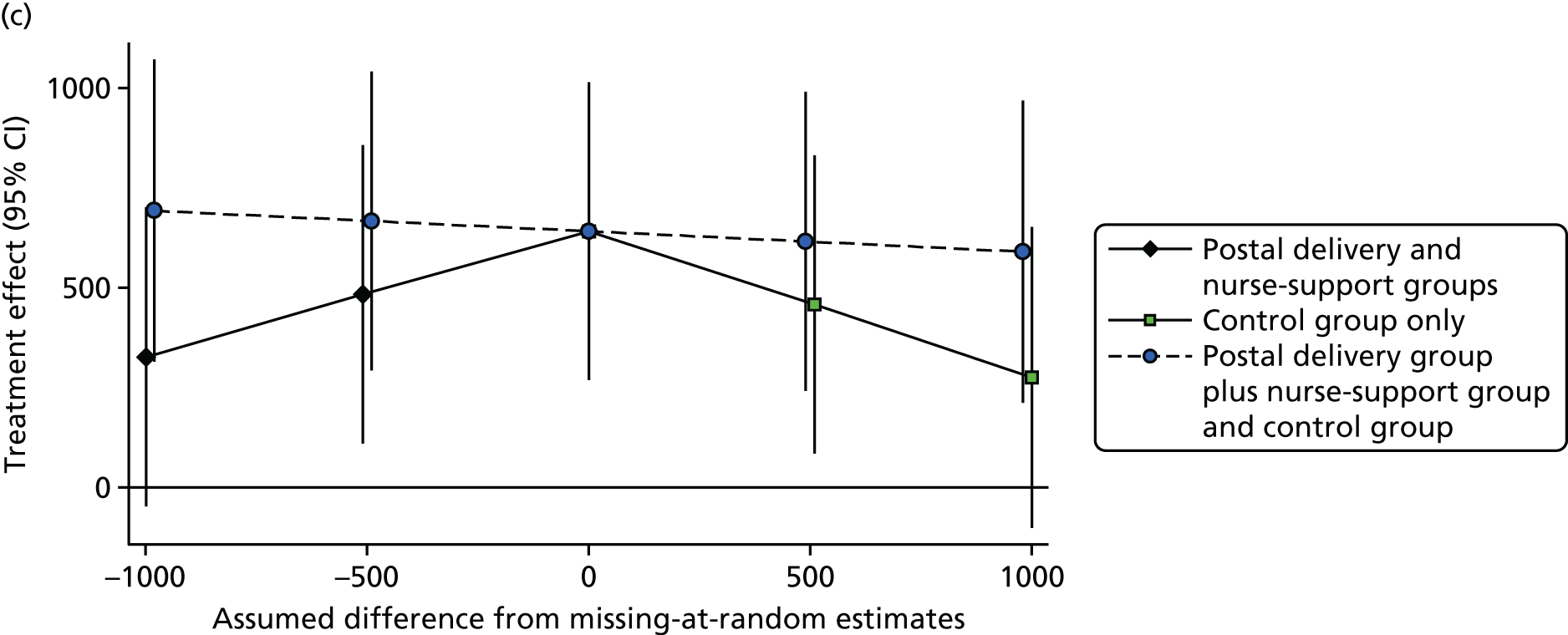
Results for the qualitative evaluation
Between March and April 2016, 105 participants were randomly selected, 96 were contacted and all agreed to participate. Telephone interviews were arranged and undertaken with 60 participants (20 from each trial arm). Fifty-two participants were white and eight were non-white. Interviews lasted between 4 and 22 minutes (median 10 minutes). One participant who had difficulty hearing was e-mailed the questions to complete. In the quotations that follow, ID3Y_ refers to the participant’s ID number, F/M refers to the participant’s sex, the number following this refers to their age and N, P or C refers to whether the participant is in the nurse-support group, the postal delivery group or the control group, respectively.
Factors affecting physical activity levels and maintenance at 3 years
A key theme that emerged from our interviews was the impact that the PACE-UP trial had on participants. Most participants, regardless of what group they were in, reported an increased awareness of PA. Participants described an increased understanding about the importance of PA for health, as well as an awareness about the amount of PA required to meet their daily step count target:
It’s made me more aware of the need to actually commit to doing some exercise a day, just strolling around the house, and going to the shops occasionally doesn’t really make much difference. It doesn’t meet the sort of threshold that you need to reach to ensure that you lead a healthy lifestyle.
ID3Y27M56P
Participants felt that taking part in the PACE-UP trial and using a pedometer had ‘kick-started’ regular activity:
It was the PACE-UP trial that helped get me started and I think that did make a huge difference to me.
ID3Y47F51N
Participants highlighted different barriers to, and facilitators of, being able to stay physically active in the longer term. These barriers and facilitators were often the opposite of each other; for example, some participants saw good weather as a motivator to engage in PA, whereas others saw bad weather as a barrier to being physically active. Other important facilitators and barriers included health, self-motivation, ageing and social support. These factors were considered to be important by participants, regardless of which group they were in. For some participants, they felt that engaging in regular PA helped them to manage their health condition:
The more active I am, the better the arthritis is.
ID3Y25F60P
Conversely, others felt that having a health condition was a contraindication to PA:
I’ve got an ongoing problem where I get pain, so there’s no way I’m going to be going out walking if I don’t have to.
ID3Y24F61P
Self-motivation was seen to be an important determinant to PA:
I think it’s got to come from inside.
ID3Y19F62C
Some participants attributed the PACE-UP trial to providing them with this motivation:
Before the PACE-UP trial, I had no incentive. And that really did help me. That put me/gave me the first steps as it were, got me on the right track.
ID3Y47F51N
On the other hand, a few participants felt that since the PACE-UP trial had ended, their PA levels had decreased as a result of a lack of self-motivation:
I’m not covering anything like what I was covering when I was on the programme, but I don’t know how . . . to put myself in that mind set . . .
ID3Y55M68N
Some participants chose to engage in PA as a way to stay young and slow down the ageing process:
I’ve got nieces and a nephew . . . I need to be active to keep up with them because they are young. I just need to keep up with everyone else really. I don’t want to slow down and become old. Unfortunately, I’m not really motivated by anything else.
605027F65N
Other participants felt that their age had become a barrier to PA and were less likely to do as much as they did when they were younger:
If anything I’m getting a bit older and I’m beginning to find it a little bit more of a strain.
ID3Y42F59N
Many participants spoke about the importance of having friends and family to motivate them to participate in PA. Support from, and accountability to, family and friends were therefore seen as common facilitators of PA:
Maybe my motivation is not only my health, but having somebody to do it with . . . to maybe be paired up with somebody who was like-minded . . . if I’ve promised/only promised myself, then I might find excuses not to do it.
ID3Y50F63N
Lack of social support meant that some participants did not engage in PA:
I haven’t got anyone to walk with.
ID3Y37F67P
Lack of time was the most frequently cited barrier to maintaining PA. Unlike the other factors previously mentioned, ‘having time’ to engage in PA was not mentioned as a facilitator. The reasons for a lack of time included having ‘family responsibilities’ (looking after children, caring for older relatives), ‘work commitments’ or simply being ‘too busy’:
I’ve got plenty of things that encourage me, it’s just the time I find because I work full time, I just find it difficult to come home, sort of prepare meals, go to the gym, go for a walk . . . I don’t think I need any more actual motivation, I just need a bit more time!
ID3Y43F54N
Some participants spoke about strategies they adopted to overcome the barrier of lack of time, either by incorporating PA into their daily routine or by building up their daily PA in short bouts of activity:
I walk up the escalators to get my little bit of exercise.
ID3Y15M62C
As opposed to the mind set of, oh, I’ve got to do an hour in the gym. Actually 10 minutes solid walking somewhere, several times a day, actually builds stuff up.
ID3Y56F68N
At the end of the interview, participants were asked for their views on what additional support could be provided to aid PA maintenance. Participants were offered examples to comment on, which included regular text messages, online resources, annual nurse appointments and walking groups. Participants had varying preferences over which additional resources they would find the most beneficial. Although some participants liked the idea of a regular text message or ‘jolly little reminders’ (ID3Y47F51N) encouraging PA, others felt that these would be ‘too intrusive’ (ID3Y27M56P). Similarly, some participants liked the idea of having PA resources online to ‘open at their own time’ (ID3Y9F62C), whereas others felt that obtaining this information online would require ‘a more proactive’ (ID3Y47F51N) approach. On the whole, walking groups and nurse appointments were considered to be favourable. Some participants felt that walking groups would provide ‘more motivation to go out’ (ID3Y17M68C) and a regular nurse or other appointment would provide ‘external accountability’ (ID3Y52M66N).
As well as providing feedback on suggested possible resources, we proposed that participants also come up with additional suggestions. These suggestions included holistic appointments with a nurse to discuss both diet and PA, and more opportunities for older people:
There is not much for people over 60, there’s no real places that are easy on the doorstep.
ID3Y2F64C
One participant spoke about being afraid to increase their PA levels, as they were unsure of whether or not it was safe. This participant sought more guidance around riskless ways to increase PA levels:
I’m doing quite well with what I’m doing, so what’s the point of sort of having a risk of a heart attack or something like that, suddenly break in to a stride and start running, so maybe a bit of guidance on what’s going to happen to you if you do step up your exercise plan.
ID3Y23M67P
The effect of the minimal intervention on physical activity levels of participants in the control group
Of the 20 control group participants interviewed, 17 received the pedometer, handbook and diary after 12 months. Thirteen of these participants reported using these resources when they first received them; however, at 3 years, only four participants were continuing to monitor their steps [two with a pedometer, one with a Fitbit (Fitbit, San Francisco, CA, USA) and one with a mobile phone].
Although most of the control group participants who were interviewed felt that the PACE-UP trial had not increased their PA levels, they still stated that the PACE-UP trial had increased their awareness:
It’s made me aware that I do not do as much as I should be doing.
ID3Y20F66C
Some, however, did talk about changes they had made to their daily lives as a result of their increased awareness:
I’m more likely to walk to work now, rather than going on the bus.
ID3Y11M54C
For those who did not utilise the pedometer, some reported difficulties in using it:
I could not work the pedometer . . . could not get it going.
ID3Y9F63C
A few others who used the pedometer stated that discontinued use was either because it had a negative impact on them psychologically or because they had fallen out of the habit of using it regularly:
It’s quite distressing to see how little I do.
ID3Y1M58C
. . . there are lots of other things [that] intrude and you tend to slip back to old patterns.
ID3Y5F68C
There was also a suggestion that, as well as the pedometer, you needed to have someone to report back to:
It always needs to have someone keeping you aware I think.
ID3Y5F68C
Discussion
Main quantitative findings from the 3-year follow-up
We followed up just over two-thirds of the original trial cohort with accelerometry outcome data at 3 years (and over 90% provided ≥ 5 days of data). Compared with baseline, those in the original nurse-support and postal delivery groups were still doing significantly more steps per day and weekly time in MVPA in bouts at 3 years than the control group. There were no significant differences in outcomes between the postal delivery and nurse-supported intervention groups (as was also the case at 12 months), and there were no significant differences between the three groups in terms of wear time or sedentary time. Our sensitivity analyses looking at the potential impact of missing outcome data at 3 years suggest that it is highly unlikely that missing data have substantially biased our results. Fairly extreme departures from the missing-at-random analyses were needed to result in non-significant effects and, even then, the 95% confidence limits were largely positive. This suggests that the trial interventions had a persistent effect on objectively measured PA levels at 3 years, with no difference between intervention groups. The fact that the minimal intervention given to the control group at 12 months was not effective at increasing the participants’ PA levels suggests that the additional support given to the original trial postal intervention group (with a follow-up telephone call after 1 week and encouragement to return the completed PA diary after 3 months) was an important component of this group’s success. Both the follow-up telephone call and the encouragement to return the completed PA diaries after the intervention were not part of the intended intervention package, but, rather, were research measures as part of the process evaluation to ensure fidelity of the intervention delivery. However, this minimal support, which was not provided face to face or by a health-care professional, seems to have been important to the success of the postal intervention. The original trial postal delivery group also received the postal pedometer intervention when they had just been recruited to the PA trial, when motivation may have been higher, and they had step count targets set for them based on their baseline blinded pedometer use, whereas those receiving the materials at 12 months needed to wear the pedometer again for 1 week to set their target step count. These factors may also have been important to the success of the trial postal intervention group.
Main qualitative findings from the 3-year follow-up interviews
A key finding was that most participants discussed their increased awareness of PA, irrespective of which group they were in and regardless of whether or not they thought that the PACE-UP trial had actually increased their PA levels. Key barriers to, and facilitators of, maintaining PA were reported that were often the inverse of one another and included health, weather, self-motivation, ageing and social support. Lack of time was the most frequently cited barrier. Some participants were able to overcome lack of time by incorporating PA into their daily routine or by breaking PA down into smaller, more manageable bouts throughout the day. Participants gave us mixed feedback on how useful they thought text messages and online resources would be to help to inform future interventions to increase and maintain PA, but walking groups and nurse or other appointments to provide external accountability were broadly welcomed. Additional suggestions provided included more holistic appointments with a nurse and more opportunities for PA for older people. Participants had differing opinions over the resources they would find most beneficial, emphasising the importance of individual tailoring of some aspects of PA interventions. Only a few of the control group participants interviewed were continuing to use the pedometer provided at 12 months. Some participants were not sure how it worked, and others felt that, as well as using the pedometer, it was important to have someone to report back to. This highlights the importance of the extra contact that participants in the postal delivery group received as part of the main trial: a follow-up telephone call to check that they knew how to work the device and encouragement to send back their completed PA diaries with step count recordings after the 12-week programme.
Further discussion of the strengths and weaknesses of both the quantitative and qualitative approaches, and the implications of the findings for health care and future research, are detailed in Chapter 9.
Chapter 9 Discussion
Summary of the findings
The PACE-UP trial demonstrated that both the postal delivery and the nurse-supported pedometer interventions, based on trying to gradually add in ‘3000 steps in 30 minutes’ on most days, increased objectively assessed PA (step counts by around one-tenth and MVPA in bouts by around one-third) among predominantly inactive 45- to 75-year-olds at 12 months. Although the nurse-led delivery had a greater effect than the postal delivery at 3 months, by 12 months this difference was not sustained. The interventions had no effect on sedentary time, anthropometry or other outcomes and did not increase AEs. No effect modification was demonstrated (by age, sex, taking part as a couple, self-efficacy, disability, socioeconomic status, pain or BMI). Questionnaire-based outcome measures tended to support the conclusions of accelerometer measures, but only if walking was an explicit part of the questionnaire. Thus, the IPAQ MVPA question did not show any intervention effect, but the IPAQ walking question showed a significant effect of both the nurse-supported and the postal delivery interventions with no difference between the interventions, although with less precision than the accelerometry data.
Both interventions were well accepted and the trial had high fidelity; three-quarters of the nurse group attended all three sessions and around 80% of both the postal delivery and the nurse-support groups returned completed step count diaries. Increase in step count was positively associated with both nurse session attendance and completed diary return.
Incremental cost per step was £0.19 and £3.61 per minute in a ≥ 10-minute MVPA bout for the nurse-support group, whereas the postal delivery group took more steps and cost less than the control group. The postal delivery group had a 50% chance of being cost-effective at a £20,000 per QALY threshold within 1 year. The QALY-based conclusion changed to the control group dominating the postal delivery group when four alternative assumptions were made (using the 3-month outcome data, extending the perspective to participants, excluding health service use and using self-reports of AEs), although this was not the case for cost-effectiveness ratios based on step count and MVPA. The postal delivery group was significantly more cost-effective than both the nurse-support group and the control group in the long term and this finding was robust to changes in assumptions.
Nurses and intervention group participants described the intervention in a positive way and confirmed that primary care was an appropriate setting. Nurses believed that participating in the trial, especially in the BCT training, enhanced the quality and delivery of the advice and support they provided within routine consultations. Participants described important facilitators of increasing PA, including the desire for a healthy lifestyle, improved physical health, enjoyment of walking in the local environment, having a flexible routine, appropriate self-monitoring and external monitoring and support from others. Important barriers included physical health problems, having an inflexible routine, work and other commitments and poor weather. Several BCTs were highlighted as having an important impact, including self-monitoring and review of goals and outcomes, planning social support/change and relapse prevention. Although most participants in the postal delivery group were confident in increasing their PA without nurse support, two key caveats were existing health problems and overcoming barriers.
The follow-up of over two-thirds of the trial cohort at 3 years demonstrated persistent increases in both step count and time in MVPA for the nurse-support and postal delivery groups compared with the control group, with no difference between intervention groups. The postal intervention given to the control group at 12 months, with no follow-up telephone call after 1 week and no requirement to post back the diary to be reviewed after 3 months, was not effective at increasing participants’ PA levels. This suggests that these ‘minimal support’ components of the postal delivery intervention, which were not face to face or not provided by a health-care professional, may have been important to its success. Qualitative evaluation found that most participants felt that the PACE-UP trial had increased their awareness of the importance of PA, irrespective of their intervention group and whether or not they felt that their PA had actually increased. Many of the barriers to, and facilitators of, PA maintenance were the inverse of each other and most were similar to those found to be important for increasing PA during the actual trial (health conditions, weather, ageing, social support, time). Participants varied in the resources that they would find most beneficial to help them maintain their PA levels, emphasising the importance of individual tailoring of some aspects of PA interventions.
The PACE-UP trial was novel in clearly separating out the effects of pedometer provision and nurse support in a general population sample of adults and older adults and in demonstrating the effects on both step counts and MVPA in bouts, thus making the outcome assessment relevant to current national and international PA guidelines.
Strengths and limitations
Study strengths
The PACE-UP study had many important strengths. It was large and population based with primary care sampling, allowing response and any bias in response to be assessed, rather than relying on recruiting volunteers. It was designed to have household randomisation, which allowed two members of a couple to take part together if they wished to, enabling a comparison of individual and couple effects. It had three trial arms, allowing the separation of nurse support and pedometer/handbook/diary effects. The intervention was pragmatic, using practice nurses who worked in the practices to deliver the nurse PA consultations, rather than external researchers or exercise specialists. There was a very good uptake rate of nurse appointments and return of completed step count diaries, showing participant engagement with the interventions. The main PA outcomes were objectively measured and were relevant to the PA guidelines. AEs were measured in a number of ways to minimise bias, both self-reported from questionnaires and objectively from primary care records. The trial achieved a follow-up rate of over 90% with complete primary outcome data. The trial also included embedded process, qualitative and economic evaluations, with the economic evaluations using trial results in a simulation of long-term cost-effectiveness. An extended 3-year follow-up allowed maintenance of any intervention effects beyond 12 months to be studied.
Study limitations
There were also some important study limitations. The 10% recruitment into the trial is considered in detail below, in Generalisability. At the baseline assessment, 218 out of 1023 participants (21%) achieved the PA guideline targets based on their accelerometry. These participants were not excluded from the trial because if the intervention were to be rolled out in primary care, self-reported PA levels would define participation. Our nurse-supported intervention group had slightly higher baseline PA levels; however, the trial results were not biased, as analyses were based on individual change, controlling for baseline PA level. It was impossible to mask participants and nurses to the intervention group and, pragmatically, research assistants recruited and followed up the same participants, so were unmasked to group at the outcome assessment. However, all the primary and secondary PA outcomes were assessed objectively by accelerometry. It is possible that participants might have tried harder with their PA when monitored, but this would also have affected control participants and would be reduced by using a 7-day protocol for data collection. 31 In addition, our intervention groups increased their MVPA in bouts of ≥ 10 minutes, implying that participants made changes suggested by the programme. Despite recruiting to target and having excellent follow-up, our CIs for the difference between intervention groups cannot rule out a small 12-month difference. Interpretation of our 3-year follow-up findings was potentially limited by the fact that two-thirds of the control group participants received a pedometer, handbook and diary, and 20% of them also received a single nurse appointment after their 12-month follow-up. However, any contamination appears to be minimal, as there was no evidence of a change in the control group and the intervention estimates at 3 years were very similar to those at 12 months. These findings are of potential importance as, in combination, they suggest that the minimal contact with the postal delivery group after participants were sent their pedometer packs (telephone contact after 1 week and encouragement to return their completed step count diaries at 3 months) was important in stimulating an effect. Timing may have been important too; as the control group was offered the intervention 12 months after the participants had initially expressed an interest in participating in the trial, they would have had to have worn the pedometer to measure their step count and set targets, whereas the trial postal delivery group had targets set based on wearing a blinded pedometer at baseline. These added factors may have also been important to the success of the trial postal delivery intervention group.
Generalisability
Overall, only 10% of people invited to participate in the trial ended up being recruited and randomised. This is similar to other primary care PA trials,33,192 but lower than the 30% that we achieved in our recent older adult PA trial. 21 However, 10% of a population sample is still a useful percentage to participate in a public health intervention, and this trial shows the potential of primary care to contribute to PA public health goals, particularly within an urban context. As well as monitoring overall recruitment, using primary care as a sampling framework allowed us to look for any selection bias in recruitment to the trial. Primary care record comparisons showed that participation rates were significantly lower in men, in those aged < 55 years, in those who were living in the most socioeconomically deprived quintile and among Asian rather than white or black ethnic groups. Despite selecting practices from deprived, ethnically diverse areas, few participants were from lower socioeconomic and ethnic minority groups, limiting both the subgroup analysis power and the generalisability to more diverse populations. Failure to include socioeconomically deprived or ethnic minority groups, in which PA levels were lower, could also increase health inequalities. In a RCT of an intervention, it is not possible to separate out reluctance to participate in the intervention from reluctance to participate in the trial itself, with requirements for informed consent, randomisation and rigorous follow-up and evaluation. If the intervention were to be rolled out in routine primary care, uptake could be higher and less prone to selection bias. Handing out the intervention materials (pedometer, handbook and diary) in primary care consultations in which advice to increase low PA levels is already being offered may also increase the intervention’s reach (e.g. in relevant chronic disease consultations, or as part of NHS Health Checks, which cover a similar age group and aim to reduce cardiovascular risk59). The intervention could also be a valuable addition to diabetes mellitus prevention strategies, such as the NHS Diabetes Prevention Programme, whereby primary care is being used to identify patients at a high risk of developing diabetes mellitus, many of whom are inactive193 and with higher proportions from ethnic minority groups. Using the PACE-UP trial intervention in these ways would need further evaluation and monitoring, but this may have the potential to improve generalisability and either decrease, or at least not increase, inequalities.
Comparison with other studies
We believe that this is the largest population-based trial of a pedometer-based walking intervention with 12-month follow-up findings and the only pedometer trial with objective PA data on time in MVPA, which is relevant to PA guidelines at 3 years. The results are consistent with, and extend, our findings in 60- to 75-year-olds that were achieved in the smaller PACE-LIFT trial21 and also support the recent change in NICE guidance to promoting pedometers as part of packages including support to set realistic goals, monitoring and feedback. 37 The intervention used in the PACE-LIFT trial also included pedometer feedback, use of a step count diary and practice nurse PA consultations based around BCTs. However, the PACE-LIFT trial intervention comprised four longer practice nurse consultations, which also included individual accelerometer feedback on PA intensity. The PACE-LIFT trial was a two-arm trial with only a single intervention arm, and was therefore unable to separate out PA monitor effects from those of the nurse-support group. Despite including a much less intense intervention, the PACE-UP trial has delivered similar levels of effect at both 3 and 12 months in PA outcomes and, furthermore, has shown what can be achieved via a postal route. It is also reassuring that our interventions did not increase sedentary time, given its potential harm,194,195 as compensation can sometimes occur. The absolute step count increase achieved in the PACE-UP trial was modest compared with that reported in systematic reviews. 26,31,32 However, most trials with 12-month data have been based on small numbers and recruited either volunteers196 or high-risk groups,35 or reported only self-reported PA data;97 all of these factors are likely to lead to larger effect sizes. Although PA guidelines focus on time in MVPA in bouts, not on step counts, the systematic reviews presented no data on this important outcome. 26,31,32 The PACE-UP trial results confirm the PACE-LIFT trial findings,21 with significant 12-month increases in MVPA in bouts. Based on the ‘3000 in 30’ formula, 33–35 extra minutes of MVPA per week in bouts corresponds to approximately 500 extra steps per day. Thus, approximately three-quarters of the extra steps achieved in the PACE-UP trial (650–700 per day) contributed to an increase in MVPA in bouts. We believe that our trial is the first to show that the ‘3000-in-30’ message43 can lead to an approximately one-third increase in weekly MVPA in bouts at 12 months, as was achieved across both intervention groups. The ‘3000 steps in 30 minutes’ formula neatly captures intensity43 and could become an important new public health goal, particularly as many people now have the ability to measure steps easily with their mobile phones. Based on a systematic review, which has quantified the strength of association between walking and the risk of developing CHD,197 the increase of 33 minutes per week in the postal delivery group in our study at 12 months, if sustained, would be expected to reduce the risk of CHD by approximately 4.5% (95% CI 3% to 6%; see Appendix 8 for details). A cohort study that has related pedometer-measured steps to mortality198 has similarly allowed us to estimate that a sustained increase of 642 steps per day would be expected to lead to a decrease in all-cause mortality of approximately 4% (95% CI 1% to 7%; see Appendix 8 for details). Recalculating these estimates for the effect estimates in the postal delivery group at 3 years makes little difference, with the resultant decreases being 4% (95% CI 3% to 5%) for CHD and 4% (95% CI 1% to 5%) for total mortality.
Most pedometer-based interventions have not separated out the effects of the pedometer itself from the effects of the additional support provided. 21,24,31 The Healthy Steps trial97 showed that pedometers achieved an additional effect compared with a primary care green prescription, but the PA outcomes presented were based on self-report. The PACE-UP trial demonstrates that although the nurse intervention group had a significantly greater effect on both step counts and time in MVPA at 3 months, by 12 months both the nurse-supported and postal delivery interventions still had a significant effect, but with no evidence of a difference between them. This stronger effect during the period of contact with the nurse, which was not sustainable in the longer term, has also been shown in other interventions with health professionals. 199 Both the nurse-support and postal delivery groups received a pedometer, diary and handbook as part of the PACE-UP trial package; therefore, it is not possible to know how much the individual components contributed. A systematic review suggested that step count diaries were common to successful pedometer interventions,31 and approximately 80% of both of our intervention groups returned completed step count diaries. In addition, our process evaluation showed that returning a completed diary was significantly associated with an increase in step counts for both of the intervention groups. Qualitative findings also confirmed that participants from both groups valued the handbook and diary, as well as the pedometer. 162 Control group participants provided with the pedometer, diary and handbook by post at 12 months did not significantly increase their PA levels; however, they were not asked to return completed step count diaries after 3 months, which may have contributed to the lack of effect of the materials in this group.
We found no effect of the interventions on anthropometric measures, such as BMI or fat mass; this is consistent with other similar studies. 21,196 Our interventions also did not affect anxiety or depression scores, which is consistent with other primary care pedometer-based interventions, suggesting either no effect or insensitivity of these measures to change, particularly when levels are in the normal range for most people. 21,33 Although a few participants mentioned that they had negative effects from overdoing walking, most intervention participants talked about feeling fitter, sleeping better, improved mood, having more energy, less pain and keeping more active into older age. 162 There is currently a lack of data comparing individual, couple or household participation in walking studies. 21,30 Household sampling allowed us to investigate this in the PACE-UP trial, but, unfortunately, only 20% of the participants took part in the study as a couple; therefore, we had reduced power for our subgroup analysis, which showed no effect of taking part as a couple, similar to the findings in our PACE-LIFT trial. 21
The self-efficacy differences that we demonstrated between both intervention groups and the control group at 3 months and between the nurse-support group and control group participants at 12 months are consistent with the positive relationship between changing self-efficacy and PA behaviour that others have reported. 200 The BCTs most associated with self-efficacy and successful PA outcomes are goal- and action-planning, prompting self-monitoring and feedback and planning of social support/change. 200 All of these BCTs were specifically recommended in recent guidance29 and were included in our study in written materials for both intervention groups and as a focus of nurse PA consultations. 70 Our qualitative interviews found that more BCT comments were made by the nurse support group than by the postal delivery group, apart from around self-monitoring. 162 Increased self-efficacy has also been shown to be important for long-term PA adherence;201 however, we found no difference in 3-year PA maintenance between intervention groups, despite the nurse-support group having a higher level of self-efficacy than the postal group at 12 months.
Walking is a safe intervention, which is indicated in many chronic diseases,1,8 although empirical data on the safety of walking interventions are limited. 24 A large trial based on 40- to 74-year-old women, which encouraged a single 30-minute brisk walk 5 days weekly, reported increased falls and injuries. 60 Our findings in the PACE-UP trial, showing no increase in AEs, builds on similar evidence from the PACE-LIFT trial,21 using both self-reported and objective primary care data, and highlights the potential importance of building up MVPA gradually, particularly in older adults, those who are inactive or those who have comorbidities. 1,11 The suggestion of a protective effect of the interventions in the PACE-UP trial on both falls and cardiovascular events at 12 months is plausible, but not definitive, as it is based on only a small number of events.
We demonstrated a persistent intervention effect at 3 years, in terms of both step counts and time in MVPA in bouts. This adds to the limited evidence from the systematic review by Hobbs et al. ,26 who found only two trials with objective PA measurement data beyond 12 months in this age group. One trial reported a significant intervention effect on step counts at 18 months, but suffered high attrition bias,202 and another trial found no effect at the 24-month follow-up on either step count or accelerometry-assessed vector magnitude. 203 Recent NICE guidance29 and a Cochrane systematic review23 also called for PA interventions with a longer follow-up period and objective PA measures. Our qualitative evaluations at 3 years also add to the limited evidence base on factors that lead to successful and unsuccessful PA maintenance190 and suggest that many of the factors that were important barriers to, or facilitators of, increasing PA levels in the original trial (e.g. health conditions, weather, ageing, social support, time) are still important when considering maintenance. This provides support for the credibility of our work and suggests that barriers and facilitators may be similar for both PA adoption and maintenance. 204 Our findings support others in suggesting that future interventions should focus on techniques to transform PA barriers into facilitators, for example by demonstrating the value of PA for many chronic health conditions, as well as safe ways in which individuals can increase their PA levels to change the presence of chronic health conditions from inhibiting to promoting PA as people age. 157,205,206
Our results on cost-effectiveness provide new evidence in a research area that reflects a dearth of primary evidence. 139,140 The evidence that the postal intervention has a 50% chance, and the nurse intervention a 5% chance, of being cost-effective within 12 months is new. Although lower than the 95% likelihood of green prescriptions being cost-effective in New Zealand at 12 months,97 it is still a reasonably high percentage for a behaviour change intervention to achieve at 12 months. The expectation that the postal intervention is most cost-effective over a lifetime is very strong and is comparable to other findings from models. 141,142
Interpretation of the results
Primary care patients aged 45–75 years can achieve important increases in their PA levels using a 12-week pedometer-based walking intervention, including handbooks and PA diaries (available at www.journalslibrary.nihr.ac.uk/programmes/hta/103202/#/), delivered either by post with minimal support or through practice nurse PA consultations, with both methods achieving similar 12-month effects. An important part of the intervention was to try and gradually add in 3000 steps in 30 minutes most days weekly. The persistent effect at 3 years suggests a long-term beneficial effect. This is a safe intervention that is acceptable to patients and nurses. The postal delivery group was significantly more cost-effective than the nurse-support and control groups in the long term, thus providing a cost-effective way of delivering long-term quality-of-life benefits. The lack of an increase in PA levels at 3 years in the control group, which received a simple postal intervention without further contact after the 12-month follow-up, suggests that contacting participants after posting the intervention components to them and encouraging the return of PA diaries may be important factors for success for the postal intervention route, but this minimal support does not need to be face to face or provided by a health-care professional.
Conclusions
Implications for health care
-
A primary care pedometer-based walking intervention, delivered by post with minimal support, could provide an effective and cost-effective approach to addressing the public health physical inactivity challenge.
-
The ‘3000 steps in 30 minutes’ formula neatly captures intensity and could become a useful new public health goal, particularly as many people can measure steps easily with their mobile phones.
-
The PACE-UP 12-week pedometer-based walking intervention could be considered for inclusion into the NHS Health Check programme, aimed at a similar age group (40- to 74-year-olds), and/or the NHS Diabetes Prevention Programme.
Recommendations for research
-
The PACE-UP trial generalisability is limited by the 10% overall recruitment rate and a lower recruitment rate in Asian and socioeconomically deprived patients. Further research into different recruitment methods is needed, as is research assessing the recruitment rate achievable if this programme were offered outside a trial setting over a more prolonged time period.
-
Although the overall postal intervention outcomes were as effective and more cost-effective than the nurse-supported intervention outcomes, further research is required to understand who would benefit most from the individual tailoring offered by a nurse-supported intervention.
-
There has been a recent dramatic increase in the use of wearables to monitor personal PA levels, including through smartphones, wrist-worn devices, online monitoring and mobile apps. Further research into how the PACE-UP 12-week PA programme could be integrated into the use of these devices (with/without a pedometer) is needed.
Acknowledgements
All the authors would like to pay tribute to Dr Sunil Shah, who was one of the trial investigators and helped to conceive the idea for the trial, and was involved in its planning, delivery and evaluation until his death in September 2015.
We are grateful to the south-west London general practices, their practice nurses who supported this study and all of the patients from these practices who participated: Upper Tooting Road Practice, Tooting; Chatfield Practice, Battersea; Wrythe Green Surgery, Carshalton; Francis Grove Surgery, Wimbledon; Putneymead Group Medical Practice, Putney; Heathfield Practice, Putney; and Cricket Green Medical Practice, Mitcham.
We would like to thank our supportive TSC: Professor Sarah Lewis (chairperson), Professor Paul Little (GP representative) and Mr Bob Laventure (patient and public involvement representative).
We would also like to provide our grateful thanks to the following people for their input in the study: Dr Iain Carey, who helped with processing the downloaded general practice data; Debbie Brewin, who helped with practice nurse training and handbook and diary content; and Becca Homes, who conducted the non-participant interviews.
Contributions of authors
Tess Harris (GP and Professor of Primary Care Research) conceived the idea for the study with the other study co-investigators; led the protocol development and the funding application; co-designed the behaviour change intervention, the patient handbook and the patient diary; supervised the running of the trial; contributed to the analysis and led the drafting of the report.
Sally Kerry (Reader in Medical Statistics) helped to conceive the idea for the study, participated in the study design and the development of the research protocol and the funding application, sat on the TMG, wrote the statistics analysis plan, performed the quantitative analyses and contributed to the drafting of the report.
Christina Victor (Professor of Gerontology and Health Services Research) helped to conceive the idea for the study, developed the research protocol and funding application, helped with the questionnaire development, led on the qualitative aspects of the study and contributed to the drafting of the report.
Steve Iliffe (GP and Professor of Primary Care for Older People) helped to conceive the idea for the study, developed the research protocol and funding application, advised on the trial safety aspects, helped with the questionnaire development and contributed to the drafting of the report.
Michael Ussher (Professor of Behavioural Medicine) helped to conceive the idea for the study, developed the research protocol and funding application, co-designed the behaviour change intervention, co-designed and helped to carry out the nurse training, advised on the questionnaire development and contributed to the drafting of the report.
Julia Fox-Rushby (Professor of Health Economics) developed the research protocol and funding application, designed the health economics procedures and data collection tools, supervised the health economics data collection, analysis and reporting and contributed to the drafting of the report.
Peter Whincup (Professor of Cardiovascular Epidemiology) helped to conceive the idea for the study, developed the research protocol and funding application, helped with the questionnaire development, advised on the anthropometric assessment and contributed to the drafting of the report.
Ulf Ekelund (Professor of Physical Activity Epidemiology) helped to conceive the idea for the study, developed the research protocol and funding application, advised on PA measurement, reporting and analysis and contributed to the drafting of the report.
Cheryl Furness (Trial Manager) provided the day-to-day overall management of the trial, sat on the TMG, co-designed and carried out the nurse training, co-ordinated the recruitment of practices and participants, took responsibility for data management, led on the process evaluation and contributed to the drafting of the report.
Elizabeth Limb (Study Statistician) sat on the TMG, helped to write the statistics analysis plan, arranged the random sampling of households, performed the quantitative analyses, prepared the tables and flow diagrams and contributed to the drafting of the report.
Nana Anokye (Health Economist) designed the health economics procedures and data collection tools, carried out the health economics analyses, prepared the tables and appendices relating to the health economics results and contributed to the drafting of the report.
Judith Ibison (GP and Senior Lecturer in Primary Care) helped with the recruitment of practices, recruitment of research staff, downloading of data in practices, questionnaire development and planning of the intervention delivery and contributed to the drafting of the report.
Stephen DeWilde (GP and Senior Lecturer in Primary Care) helped with the recruitment of practices, advising on GP searches, downloading of data in practices, interpretation of GP data and contributed to the drafting of the report.
Lee David (GP and Director of 10 Minute CBT) co-designed the behaviour change intervention, co-designed the patient handbook and diary, advised on the nurse and patient feedback forms, co-designed the nurse training, led on the behaviour change aspects of training, provided feedback to the nurses on BCT delivery and contributed to the drafting of the report.
Emma Howard (Research Assistant) was involved in compiling patient information and data collection packs, conducted recruitment and follow-up of participants (including informed consent, randomisation and data collection), collated and analysed data for the process evaluation and contributed to the drafting of the report.
Rebecca Dale (Research Assistant) was involved in compiling patient information and data collection packs, conducted recruitment and follow-up of participants (including informed consent, randomisation and data collection) and contributed to the drafting of the report.
Jaime Smith (Research Assistant) recruited and followed up participants (including informed consent, randomisation and data collection), carried out qualitative interviews with the intervention group participants, undertook the qualitative analyses and contributed to the drafting of the report.
Rebecca Normansell (GP and Deputy Coordinating Editor of the Cochrane Airways Group) carried out qualitative interviews with the intervention group participants and practice nurses, undertook the qualitative analyses and contributed to the drafting of the report.
Carole Beighton (Senior Lecturer in Nursing) conducted a focus group with the practice nurses, carried out qualitative interviews with participants as part of the 3-year follow-up, undertook the qualitative analyses and contributed to the drafting of the report.
Katy Morgan (Statistician) carried out analyses relating to the generalisability and representativeness of the sample and contributed to the drafting of the report.
Charlotte Wahlich (Research Assistant) contacted participants for the 3-year follow-up (including informed consent and data collection), carried out the qualitative interviews with participants as part of the 3-year follow-up, undertook the qualitative analyses and contributed to the drafting of the report.
Sabina Sanghera (Health Economist) carried out the economic analyses relating to the health services use data and contributed to the drafting of the report.
Derek Cook (Professor of Epidemiology) helped to conceive the idea for the study; participated in the study design, development of the research protocol and the funding application; sat on the TMG; wrote the statistical analysis plan; performed the quantitative analyses and contributed to the drafting of the report.
Publications
Harris T, Kerry SM, Victor CR, Shah SM, Iliffe S, Ussher M, et al. PACE-UP (Pedometer And Consultation Evaluation – UP) – a pedometer-based walking intervention with and without practice nurse support in primary care patients aged 45-75 years: study protocol for a randomised controlled trial. Trials 2013;14:418.
Normansell R, Smith J, Victor C, Cook DG, Kerry S, Iliffe S, et al. Numbers are not the whole story: a qualitative exploration of barriers and facilitators to increased physical activity in a primary care based walking intervention. BMC Public Health 2014;14:1272.
Beighton C, Victor C, Normansell R, Cook D, Kerry S, Iliffe S, et al. ‘It’s not just about walking . . . it’s the practice nurse that makes it work’: a qualitative exploration of the views of practice nurses delivering complex physical activity interventions in primary care. BMC Public Health 2015;15:1236.
Normansell R, Holmes R, Victor C, Cook DG, Kerry S, Iliffe S, et al. Exploring non-participation in primary care physical activity interventions: PACE-UP trial interview findings. BMC Trials 2016;17:178.
Harris T, Kerry SM, Limb ES, Victor CR, Iliffe S, Ussher M, et al. Effect of a primary care walking intervention with and without nurse support on physical activity levels in 45- to 75-year-olds: the Pedometer And Consultation Evaluation (PACE-UP) cluster randomised clinical trial. PLOS Med 2017;14:e1002210.
Wahlich C, Beighton C, Victor C, Normansell R, Cook D, Kerry S, et al. ‘You started something . . . then I continued by myself’: a qualitative study of physical activity maintenance. Prim Health Care Res Dev 2017;18:574–90.
Furness C, Howard E, Limb E, Cook DG, Kerry S, Wahlich C, et al. Relating process evaluation measures to complex intervention outcomes: findings from the PACE-UP primary care pedometer-based walking trial. Trials 2018;19:58.
Harris T, Kerry SM, Limb ES, Furness C, Wahlich C, Victor CR, et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care. PLOS Med 2018;15:e1002526.
Kerry SM, Morgan KE, Limb E, Cook DG, Furness C, Carey I, et al. Interpreting population reach of a large, successful physical activity trial delivered through primary care. BMC Public Health 2018;18:170.
Data sharing statement
Anonymised individual patient data may be available for the trial effectiveness and cost-effectiveness analyses. These will be stored in a secure data repository at St George’s, University of London. All queries and requests should be submitted to the corresponding author for initial consideration.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Start Active, Stay Active: A Report on Physical Activity for Health from the Four Home Countries’ Chief Medical Officers. London: Department of Health; 2011.
- Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010.
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Lancet Physical Activity Series Working Group . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219-29. https://doi.org/10.1016/S0140-6736(12)61031-9.
- Oldridge NB. Economic burden of physical inactivity: healthcare costs associated with cardiovascular disease. Eur J Cardiovasc Prev Rehabil 2008;15:130-9. https://doi.org/10.1097/HJR.0b013e3282f19d42.
- Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK. Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis 2015;57:315-23. https://doi.org/10.1016/j.pcad.2014.08.002.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. https://doi.org/10.1249/MSS.0b013e318213fefb.
- O’Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, et al. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci 2010;28:573-91. https://doi.org/10.1080/02640411003671212.
- Morris JN, Hardman AE. Walking to health. Sports Med 1997;23:306-32. https://doi.org/10.2165/00007256-199723050-00004.
- Health Survey for England – 2008: Physical Activity and Fitness. London: NHS Digital; 2009.
- de Souto Barreto P. Global health agenda on non-communicable diseases: has WHO set a smart goal for physical activity?. BMJ 2015;350. https://doi.org/10.1136/bmj.h23.
- Sparling PB, Howard BJ, Dunstan DW, Owen N. Recommendations for physical activity in older adults. BMJ 2015;350. https://doi.org/10.1136/bmj.h100.
- Health Survey for England – 2012. London: NHS Digital; 2013.
- Health Survey for England – 2004. London: Department of Health; 2005.
- World Health Organization . Global Health Observatory Data Repository n.d. www.who.int/gho/ncd/risk_factors/physical_activity_text/en/ (accessed 25 May 2018).
- Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med 2001;31:91-100. https://doi.org/10.2165/00007256-200131020-00002.
- Morey MC, Sullivan RJ. Medical assessment for health advocacy and practical strategies for exercise initiation. Am J Prev Med 2003;25:204-8. https://doi.org/10.1016/S0749-3797(03)00180-6.
- Cress ME, Buchner DM, Prohaska T, Rimmer J, Brown M, Macera C, et al. Best practices for physical activity programs and behavior counseling in older adult populations. J Aging Phys Act 2005;13:61-74. https://doi.org/10.1123/japa.13.1.61.
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003;107:3109-16. https://doi.org/10.1161/01.CIR.0000075572.40158.77.
- Hootman JM, Macera CA, Ainsworth BE, Martin M, Addy CL, Blair SN. Association among physical activity level, cardiorespiratory fitness, and risk of musculoskeletal injury. Am J Epidemiol 2001;154:251-8. https://doi.org/10.1093/aje/154.3.251.
- Ory M, Resnick B, Jordan PJ, Coday M, Riebe D, Ewing Garber C, et al. Screening, safety, and adverse events in physical activity interventions: collaborative experiences from the behavior change consortium. Ann Behav Med 2005;29:20-8. https://doi.org/10.1207/s15324796abm2902s_5.
- Harris T, Kerry SM, Victor CR, Ekelund U, Woodcock A, Iliffe S, et al. A primary care nurse-delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)-Lift cluster randomised controlled trial. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001783.
- Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev 2005;1. https://doi.org/10.1002/14651858.CD003180.pub2.
- Richards J, Thorogood M, Hillsdon M, Foster C. Face-to-face versus remote and web 2.0 interventions for promoting physical activity. Cochrane Database Syst Rev 2013;9. https://doi.org/10.1002/14651858.CD010393.pub2.
- Foster C, Richards J, Thorogood M, Hillsdon M. Remote and web 2.0 interventions for promoting physical activity [published online ahead of print September 30 2013]. Cochrane Database Syst Rev 2013. https://doi.org/10.1002/14651858.CD010395.pub2.
- King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group- vs home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA 1991;266:1535-42. https://doi.org/10.1001/jama.1991.03470110081037.
- Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-75.
- King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adults. A critical review and recommendations. Am J Prev Med 1998;15:316-33. https://doi.org/10.1016/S0749-3797(98)00085-3.
- Richards D. Behaviour change guidance. Evid Based Dent 2007;8:98-100. https://doi.org/10.1038/sj.ebd.6400520.
- Behaviour Change: Individual Approaches. London: NICE; 2014.
- Ogilvie D, Foster CE, Rothnie H, Cavill N, Hamilton V, Fitzsimons CF, et al. Interventions to promote walking: systematic review. BMJ 2007;334. https://doi.org/10.1136/bmj.39198.722720.BE.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. https://doi.org/10.1001/jama.298.19.2296.
- Kang M, Marshall SJ, Barreira TV, Lee JO. Effect of pedometer-based physical activity interventions: a meta-analysis. Res Q Exerc Sport 2009;80:648-55. https://doi.org/10.1080/02701367.2009.10599604.
- McMurdo ME, Sugden J, Argo I, Boyle P, Johnston DW, Sniehotta FF, et al. Do pedometers increase physical activity in sedentary older women? A randomized controlled trial. J Am Geriatr Soc 2010;58:2099-106. https://doi.org/10.1111/j.1532-5415.2010.03127.x.
- Houle J, Doyon O, Vadeboncoeur N, Turbide G, Diaz A, Poirier P. Innovative program to increase physical activity following an acute coronary syndrome: randomized controlled trial. Patient Educ Couns 2011;85:e237-44. https://doi.org/10.1016/j.pec.2011.03.018.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 2009;32:1404-10. https://doi.org/10.2337/dc09-0130.
- Four Commonly Used Methods to Increase Physical Activity: Brief Interventions in Primary Care, Exercise Referral Schemes, Pedometers and Community Based Exercise Programmes for Walking and Cycling. London: NICE; 2006.
- Walking and Cycling. Local Measures to Promote Walking and Cycling as Forms of Travel or Recreation. London: NICE; 2012.
- Schneider PL, Bassett DR, Thompson DL, Pronk NP, Bielak KM. Effects of a 10,000 steps per day goal in overweight adults. Am J Health Promot 2006;21:85-9. https://doi.org/10.4278/0890-1171-21.2.85.
- Lindberg R. Active living: on the road with the 10,000 Steps program. J Am Diet Assoc 2000;100:878-9. https://doi.org/10.1016/S0002-8223(00)00251-0.
- Croteau KA, Richeson NE, Farmer BC, Jones DB. Effect of a pedometer-based intervention on daily step counts of community-dwelling older adults. Res Q Exerc Sport 2007;78:401-6. https://doi.org/10.1080/02701367.2007.10599439.
- Prochaska JJ, Hall SM, Humfleet G, Munoz RF, Reus V, Gorecki J, et al. Physical activity as a strategy for maintaining tobacco abstinence: a randomized trial. Prev Med 2008;47:215-20. https://doi.org/10.1016/j.ypmed.2008.05.006.
- Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-79.
- Marshall SJ, Levy SS, Tudor-Locke CE, Kolkhorst FW, Wooten KM, Ji M, et al. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med 2009;36:410-15. https://doi.org/10.1016/j.amepre.2009.01.021.
- McKay J, Wright A, Lowry R, Steele K, Ryde G, Mutrie N. Walking on prescription: the utility of a pedometer pack for increasing physical activity in primary care. Patient Educ Couns 2009;76:71-6. https://doi.org/10.1016/j.pec.2008.11.004.
- Baker G, Gray SR, Wright A, Fitzsimons C, Nimmo M, Lowry R, et al. The effect of a pedometer-based community walking intervention ‘Walking for Wellbeing in the West’ on physical activity levels and health outcomes: a 12-week randomized controlled trial. Int J Behav Nutr Phys Act 2008;5. https://doi.org/10.1186/1479-5868-5-44.
- Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-80.
- Macmillan F, Fitzsimons C, Black K, Granat MH, Grant MP, Grealy M, et al. West End Walkers 65+: a randomised controlled trial of a primary care-based walking intervention for older adults: study rationale and design. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-120.
- Mutrie N, Doolin O, Fitzsimons CF, Grant PM, Granat M, Grealy M, et al. Increasing older adults’ walking through primary care: results of a pilot randomized controlled trial. Fam Pract 2012;29:633-42. https://doi.org/10.1093/fampra/cms038.
- Ekelund U, Tingström P, Kamwendo K, Krantz M, Nylander E, Sjöström M, et al. The validity of the Computer Science and Applications activity monitor for use in coronary artery disease patients during level walking. Clin Physiol Funct Imaging 2002;22:248-53. https://doi.org/10.1046/j.1475-097X.2002.00426.x.
- Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc 2009;41:1392-402. https://doi.org/10.1249/MSS.0b013e31819b3533.
- Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol 2007;100:581-9. https://doi.org/10.1007/s00421-006-0320-8.
- Jefferis BJ, Sartini C, Lee IM, Choi M, Amuzu A, Gutierrez C, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-382.
- Harris TJ, Owen CG, Victor CR, Adams R, Cook DG. What factors are associated with physical activity in older people, assessed objectively by accelerometry?. Br J Sports Med 2009;43:442-50. https://doi.org/10.1136/bjsm.2008.048033.
- Tudor-Locke C, Ainsworth BE, Thompson RW, Matthews CE. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc 2002;34:2045-51. https://doi.org/10.1097/00005768-200212000-00027.
- A Rapid Review of the Effectiveness of Pedometer Interventions to Promote Physical Activity in Adults. London: NICE; 2006.
- Talib H, Sabirin J. Pedometer and Accelerometer in Assessing Physical Activity Among Children and Adolescents. Kuala Lumpur, Malaysia: Health Technology Assessment Unit; 2007.
- Modelling the Cost-effectiveness of Physical Activity Interventions. London: NICE; 2006.
- Shaw R, Fenwick E, Baker G, McAdam C, Fitzsimons C, Mutrie N. ‘Pedometers cost buttons’: the feasibility of implementing a pedometer based walking programme within the community. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-200.
- Putting Prevention First: NHS Health Checks: Vascular Risk Assessment and Management Best Practice Guidelines. London: NHS; 2009.
- Lawton BA, Rose SB, Elley CR, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BMJ 2008;337. https://doi.org/10.1136/bmj.a2509.
- Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med 2003;25:172-83. https://doi.org/10.1016/S0749-3797(03)00182-X.
- Tai SS, Gould M, Iliffe S. Promoting healthy exercise among older people in general practice: issues in designing and evaluating therapeutic interventions. Br J Gen Pract 1997;47:119-22.
- Goodman C, Davies S, Tai SS, Dinan S, Iliffe S. Promoting older peoples’ participation in activity, whose responsibility? A case study of the response of health, local government and voluntary organizations. J Interprof Care 2007;21:515-28. https://doi.org/10.1080/13561820701637204.
- Pavey TG, Taylor AH, Fox KR, Hillsdon M, Anokye N, Campbell JL, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ 2011;343. https://doi.org/10.1136/bmj.d6462.
- Khan KM, Weiler R, Blair SN. Prescribing exercise in primary care. BMJ 2011;343. https://doi.org/10.1136/bmj.d4141.
- David L. Using CBT in General Practice. The 10 Minute Consultation. Bloxham, Oxfordshire: Scion; 2006.
- Improving Health: Changing Behaviour: NHS Health Trainer Handbook. London: Department of Health and Social Care; 2008.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. https://doi.org/10.1080/08870446.2010.540664.
- Harris T, Kerry S, Victor C, Ekelund U, Woodcock A, Iliffe S, et al. Randomised controlled trial of a complex intervention by primary care nurses to increase walking in patients aged 60–74 years: protocol of the PACE-Lift (Pedometer Accelerometer Consultation Evaluation - Lift) trial. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-5.
- Harris T, Kerry SM, Victor CR, Shah SM, Iliffe S, Ussher M, et al. PACE-UP (Pedometer and consultation evaluation – UP) – a pedometer-based walking intervention with and without practice nurse support in primary care patients aged 45-75 years: study protocol for a randomised controlled trial. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-418.
- English Indices of Deprivation 2010: Guidance Document. London: Department for Communities and Local Government; 2011.
- Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport 2000;71:114-20. https://doi.org/10.1080/02701367.2000.11082794.
- The General Practice Physical Activity Questionnaire (GPPAQ). London: Department of Health and Social Care; 2009.
- Jette AM, Rooks D, Lachman M, Lin TH, Levenson C, Heislein D, et al. Home-based resistance training: predictors of participation and adherence. Gerontologist 1998;38:412-21. https://doi.org/10.1093/geront/38.4.412.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- Ahmad S, Harris T, Limb E, Kerry S, Victor C, Ekelund U, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60-74 year old primary care patients. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0324-8.
- The English and Welsh 2011 Census. Newport: Office for National Statistics; 2012.
- McGee MA, Johnson AL, Kay DW. The description of activities of daily living in five centres in England and Wales. Medical Research Council Cognitive Function and Ageing Study. Age Ageing 1998;27:605-13. https://doi.org/10.1093/ageing/27.5.605.
- Tunstall J. Old and Alone. London: Routledge and Kegan; 1957.
- Nandy S, Parsons S, Cryer C, Underwood M, Rashbrook E, Carter Y, et al. Development and preliminary examination of the predictive validity of the Falls Risk Assessment Tool (FRAT) for use in primary care. J Public Health 2004;26:138-43. https://doi.org/10.1093/pubmed/fdh132.
- Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol 1991;133:1078-92. https://doi.org/10.1093/oxfordjournals.aje.a115821.
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777-81. https://doi.org/10.1097/00005768-199805000-00021.
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol 2008;167:875-81. https://doi.org/10.1093/aje/kwm390.
- Little P, Dorward M, Gralton S, Hammerton L, Pillinger J, White P, et al. A randomised controlled trial of three pragmatic approaches to initiate increased physical activity in sedentary patients with risk factors for cardiovascular disease. Br J Gen Pract 2004;54:189-95.
- Stevens W, Hillsdon M, Thorogood M, McArdle D. Cost-effectiveness of a primary care based physical activity intervention in 45-74 year old men and women: a randomised controlled trial. Br J Sports Med 1998;32:236-41. https://doi.org/10.1136/bjsm.32.3.236.
- Munro JF, Nicholl JP, Brazier JE, Davey R, Cochrane T. Cost effectiveness of a community based exercise programme in over 65 year olds: cluster randomised trial. J Epidemiol Community Health 2004;58:1004-10. https://doi.org/10.1136/jech.2003.014225.
- Sugden JA, Sniehotta FF, Donnan PT, Boyle P, Johnston DW, McMurdo ME. The feasibility of using pedometers and brief advice to increase activity in sedentary older women – a pilot study. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-169.
- Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:531-43. https://doi.org/10.1249/01.mss.0000185657.86065.98.
- Miller GD, Jakicic JM, Rejeski WJ, Whit-Glover MC, Lang W, Walkup MP, et al. Effect of varying accelerometry criteria on physical activity: the look ahead study. Obesity 2013;21:32-44. https://doi.org/10.1002/oby.20234.
- Senn S. Change from baseline and analysis of covariance revisited. Stat Med 2006;25:4334-44. https://doi.org/10.1002/sim.2682.
- Harris T, Kerry SM, Limb ES, Victor CR, Iliffe S, Ussher M, et al. Effect of a primary care walking intervention with and without nurse support on physical activity levels in 45- to 75-year-olds: the Pedometer And Consultation Evaluation (PACE-UP) cluster randomised clinical trial. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002210.
- Healthy Lives, Healthy People: Our Strategy for Public Health in England. London: Department of Health and Social Care; 2010.
- Improving Outcomes: A Strategy for Cancer. London: Department of Health and Social Care; 2011.
- Fitzsimons CF, Baker G, Wright A, Nimmo MA, Ward Thompson C, Lowry R, et al. The ‘Walking for Wellbeing in the West’ randomised controlled trial of a pedometer-based walking programme in combination with physical activity consultation with 12 month follow-up: rationale and study design. BMC Public Health 2008;8. https://doi.org/10.1186/1471-2458-8-259.
- Leung W, Ashton T, Kolt GS, Schofield GM, Garrett N, Kerse N, et al. Cost-effectiveness of pedometer-based versus time-based Green Prescriptions: the Healthy Steps Study. Aust J Prim Health 2012;18:204-11. https://doi.org/10.1071/PY11028.
- Over EA, Wendel-Vos GW, van den Berg M, Reenen HH, Tariq L, Hoogenveen RT, et al. Cost-effectiveness of counseling and pedometer use to increase physical activity in the Netherlands: a modeling study. Cost Eff Resour Alloc 2012;10. https://doi.org/10.1186/1478-7547-10-13.
- Cobiac LJ, Vos T, Barendregt JJ. Cost-effectiveness of interventions to promote physical activity: a modelling study. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000110.
- Vijay GC, Wilson ECF, Suhrcke M, Hardeman W, Sutton S. Are brief interventions to increase physical activity cost-effective?. Br J Sports Med 2016;50:408-17. https://doi.org/10.1136/bjsports-2015-094655.
- Drummond M SM, Klaxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- NHS Reference Costs 2014–15. London: Department of Health and Social Care; 2014.
- Curtis LA. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Anokye NK, Pokhrel S, Fox-Rushby J. Economic analysis of participation in physical activity in England: implications for health policy. Int J Behav Nutr Phys Act 2014;11. https://doi.org/10.1186/s12966-014-0117-9.
- Feng Y, Devlin N, Herdman M. Assessing the health of the general population in England: how do the three- and five-level versions of EQ-5D compare?. Health Qual Life Outcomes 2015;13. https://doi.org/10.1186/s12955-015-0356-8.
- Rabin R, Oemar M, Oppe M, Janssen B, Herdman M. EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument. Rotterdam: EuroQol Group; 2011.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Lydick E, Epstein RS, Himmelberger D, White CJ. Area under the curve: a metric for patient subjective responses in episodic diseases. Qual Life Res 1995;4:41-5. https://doi.org/10.1007/BF00434382.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing. presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. https://doi.org/10.1177/096228029900800102.
- Bennett DA. How can I deal with missing data in my study?. Aust N Z J Public Health 2001;25:464-9. https://doi.org/10.1111/j.1467-842X.2001.tb00294.x.
- Simons CL, Rivero-Arias O, Yu LM, Simon J. Multiple imputation to deal with missing EQ-5D-3L data: should we impute individual domains or the actual index?. Qual Life Res 2015;24:805-15. https://doi.org/10.1007/s11136-014-0837-y.
- Rubin D. Multiple Imputation for Nonresponse in Surveys United States of America. Hoboken, NJ: John Wiley & Sons; 1987.
- Allison PD. Multiple imputation for missing data: a cautionary tale. Sociol Method Res 2000;28:301-9. https://doi.org/10.1177/0049124100028003003.
- Carpenter JR, Kenward MG, Carpenter JR, Kenward MG. Missing Data In Randomised Controlled Trials: A Practical Guide. Birmingham: Health Technology Assessment Methodology Programme; 2007.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Vu T, Harris A, Duncan G, Sussman G. Cost-effectiveness of multidisciplinary wound care in nursing homes: a pseudo-randomized pragmatic cluster trial. Fam Pract 2007;24:372-9. https://doi.org/10.1093/fampra/cmm024.
- Basu A, Manca A. Regression estimators for generic health-related quality of life and quality-adjusted life years. Med Decis Making 2012;32:56-69. https://doi.org/10.1177/0272989X11416988.
- Manning WG, Mullahy J. Estimating log models: to transform or not to transform?. J Health Econ 2001;20:461-94. https://doi.org/10.1016/S0167-6296(01)00086-8.
- Smith L, Gardner B, Fisher A, Hamer M. Patterns and correlates of physical activity behaviour over 10 years in older adults: prospective analyses from the English Longitudinal Study of Ageing. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007423.
- Henderson C, Knapp M, Fernández JL, Beecham J, Hirani SP, Cartwright M, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f1035.
- Graubard BI, Korn EL. Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst 1999;91:1005-16. https://doi.org/10.1093/jnci/91.12.1005.
- Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity – a systematic review of longitudinal studies. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-813.
- Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789-95. https://doi.org/10.1161/CIRCULATIONAHA.110.010710.
- Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care 2007;30:744-52. https://doi.org/10.2337/dc06-1842.
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801-9. https://doi.org/10.1503/cmaj.051351.
- Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke 2003;34:2475-81. https://doi.org/10.1161/01.STR.0000091843.02517.9D.
- Olanrewaju O, Kelly S, Cowan A, Brayne C, Lafortune L. Physical activity in community dwelling older people: a systematic review of reviews of interventions and context. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0168614.
- Anokye NK, Lord J, Fox-Rushby J. Is brief advice in primary care a cost-effective way to promote physical activity?. Br J Sports Med 2014;48:202-6. https://doi.org/10.1136/bjsports-2013-092897.
- Campbell F, Holmes M, Everson-Hock E, Davis S, Buckley Woods H, Anokye N, et al. A systematic review and economic evaluation of exercise referral schemes in primary care: a short report. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19600.
- Pavey TG, Anokye N, Taylor AH, Trueman P, Moxham T, Fox KR, et al. The clinical effectiveness and cost-effectiveness of exercise referral schemes: a systematic review and economic evaluation. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15440.
- Anokye NK, Trueman P, Green C, Pavey TG, Hillsdon M, Taylor RS. The cost-effectiveness of exercise referral schemes. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-954.
- Anokye N, Anagnostou A, Lord J, Vali Y, Taylor S, Foster C, et al. An Individual-Level Simulation Model for Assessing Physical Activity Interventions n.d.
- Physical Activity: Brief Advice for Adults in Primary Care. London: NICE; 2013.
- Matrix . Estimating Return on Investment for Interventions and Strategies to Increase Physical Activity-Technical Report 2014.
- Office for National Statistics . Life Expectancy 2015. http://visual.ons.gov.uk/how-long-will-my-pension-need-to-last/ (accessed 27 November 2017).
- Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: Department of Health and Human Services; 2008.
- Laine J, Kuvaja-Köllner V, Pietilä E, Koivuneva M, Valtonen H, Kankaanpää E. Cost-effectiveness of population-level physical activity interventions: a systematic review. Am J Health Promot 2014;29:71-80. https://doi.org/10.4278/ajhp.131210-LIT-622.
- Garrett S, Elley CR, Rose SB, O’Dea D, Lawton BA, Dowell AC. Are physical activity interventions in primary care and the community cost-effective? A systematic review of the evidence. Br J Gen Pract 2011;61:e125-33. https://doi.org/10.3399/bjgp11X561249.
- De Smedt D, De Cocker K, Annemans L, De Bourdeaudhuij I, Cardon G. A cost-effectiveness study of the community-based intervention ‘10 000 Steps Ghent’. Public Health Nutr 2012;15:442-51. https://doi.org/10.1017/S1368980011001716.
- Brennan A, Blake L, Hill-McManus D, Payne N, Buckley Woods H, Blank L. Walking and Cycling: Local Measures to Promote Walking and Cycling As Forms of Travel or Recreation: Health Economic and Modelling Report. London: NICE; 2012.
- Health Survey for England – 2004: The Health of Ethnic Minorities, Headline Results. London: NHS Digital; 2006.
- Lamb SE, Bartlett HP, Ashley A, Bird W. Can lay-led walking programmes increase physical activity in middle aged adults? A randomised controlled trial. J Epidemiol Community Health 2002;56:246-52. https://doi.org/10.1136/jech.56.4.246.
- Copeland RJ, Horspool K, Humphreys L, Scott E. Booster trial team . Recruiting to a large-scale physical activity randomised controlled trial - experiences with the gift of hindsight. Trials 2016;17. https://doi.org/10.1186/s13063-016-1229-0.
- Caperchione CM, Duncan M, Kolt GS, Vandelanotte C, Rosenkranz RR, Maeder A, et al. Examining an Australian physical activity and nutrition intervention using RE-AIM. Health Promot Int 2016;31:450-8. https://doi.org/10.1093/heapro/dav005.
- Kerry SM, Morgan KE, Limb E, Cook DG, Furness C, Carey I, et al. Interpreting population reach of a large, successful physical activity trial delivered through primary care. BMC Public Health 2018;18. https://doi.org/10.1186/s12889-018-5034-4.
- Normansell R, Holmes R, Victor C, Cook DG, Kerry S, Iliffe S, et al. Exploring non-participation in primary care physical activity interventions: PACE-UP trial interview findings. Trials 2016;17. https://doi.org/10.1186/s13063-016-1299-z.
- Victor CR, Rogers A, Woodcock A, Beighton C, Cook DG, Kerry SM, et al. What factors support older people to increase their physical activity levels? An exploratory analysis of the experiences of PACE-Lift trial participants. Arch Gerontol Geriatr 2016;67:1-6. https://doi.org/10.1016/j.archger.2016.06.006.
- Ritchie J, Spencer L, Bryman AB, Burgess RG. Analysing Qualitative Data. London: Routledge; 1994.
- Foster CE, Brennan G, Matthews A, McAdam C, Fitzsimons C, Mutrie N. Recruiting participants to walking intervention studies: a systematic review. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-137.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Warner ET, Glasgow RE, Emmons KM, Bennett GG, Askew S, Rosner B, et al. Recruitment and retention of participants in a pragmatic randomized intervention trial at three community health clinics: results and lessons learnt. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-192.
- Dubbert PM, Cooper KM, Kirchner KA, Meydrech EF, Bilbrew D. Effects of nurse counseling on walking for exercise in elderly primary care patients. J Gerontol A Biol Sci Med Sci 2002;57:M733-40. https://doi.org/10.1093/gerona/57.11.M733.
- Dubbert PM, Morey MC, Kirchner KA, Meydrech EF, Grothe K. Counseling for home-based walking and strength exercise in older primary care patients. Arch Intern Med 2008;168:979-86. https://doi.org/10.1001/archinte.168.9.979.
- Cappuccio FP, Cook DG, Atkinson RW, Wicks PD. The Wandsworth Heart and Stroke Study. A population-based survey of cardiovascular risk factors in different ethnic groups. Methods and baseline findings. Nutr Metab Cardiovasc Dis 1998;8:371-85.
- Rogers A, Harris T, Victor C, Woodcock A, Limb E, Kerry S, et al. Which older people decline participation in a primary care trial of physical activity and why: insights from a mixed methods approach. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-46.
- Attwood S, Morton K, Sutton S. Exploring equity in uptake of the NHS Health Check and a nested physical activity intervention trial. J Public Health (Oxf) 2016;38:560-8. https://doi.org/10.1093/pubmed/fdv070.
- Douglas A, Bhopal RS, Bhopal R, Forbes JF, Gill JM, Lawton J, et al. Recruiting South Asians to a lifestyle intervention trial: experiences and lessons from PODOSA (Prevention of Diabetes & Obesity in South Asians). Trials 2011;12. https://doi.org/10.1186/1745-6215-12-220.
- Wilbur J, Buchholz SW, Ingram DM, Braun LT, Johnson TJ, Fogg L, et al. Effectiveness, efficiency, duration, and costs of recruiting for an African American women’s lifestyle physical activity program. Res Nurs Health 2013;36:487-99. https://doi.org/10.1002/nur.21550.
- Crombie IK, Irvine L, Williams B, McGinnis AR, Slane PW, Alder EM, et al. Why older people do not participate in leisure time physical activity: a survey of activity levels, beliefs and deterrents. Age Ageing 2004;33:287-92. https://doi.org/10.1093/ageing/afh089.
- Normansell R, Smith J, Victor C, Cook DG, Kerry S, Iliffe S, et al. Numbers are not the whole story: a qualitative exploration of barriers and facilitators to increased physical activity in a primary care based walking intervention. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-1272.
- Chinn DJ, White M, Howel D, Harland JO, Drinkwater CK. Factors associated with non-participation in a physical activity promotion trial. Public Health 2006;120:309-19. https://doi.org/10.1016/j.puhe.2005.11.003.
- Costello E, Kafchinski M, Vrazel J, Sullivan P. Motivators, barriers, and beliefs regarding physical activity in an older adult population. J Geriatr Phys Ther 2011;34:138-47. https://doi.org/10.1519/JPT.0b013e31820e0e71.
- Cohen-Mansfield J, Marx MS, Guralnik JM. Motivators and barriers to exercise in an older community dwelling population. J Ageing Phys Act 2003;11:242-53. https://doi.org/10.1123/japa.11.2.242.
- Justine M, Azizan A, Hassan V, Salleh Z, Manaf H. Barriers to participation in physical activity and exercise among middle-aged and elderly individuals. Singapore Med J 2013;54:581-6. https://doi.org/10.11622/smedj.2013203.
- Booth ML, Bauman A, Owen N, Gore CJ. Physical activity preferences, preferred sources of assistance, and perceived barriers to increased activity among physically inactive Australians. Prev Med 1997;26:131-7. https://doi.org/10.1006/pmed.1996.9982.
- Bock C, Jarczok MN, Litaker D. Community-based efforts to promote physical activity: a systematic review of interventions considering mode of delivery, study quality and population subgroups. J Sci Med Sport 2014;17:276-82. https://doi.org/10.1016/j.jsams.2013.04.009.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Furness C, Howard E, Limb E, Cook DG, Kerry S, Wahlich C, et al. Relating process evaluation measures to complex intervention outcomes: findings from the PACE-UP primary care pedometer-based walking trial. Trials 2018;19. https://doi.org/10.1186/s13063-017-2428-z.
- Horvath AO, Greenberg LS. Development and validation of the working alliance inventory. J Couns Psychol 1989;36:223-33. https://doi.org/10.1037/0022-0167.36.2.223.
- Andrusyna TP, Tang TZ, DeRubeis RJ, Luborsky L. The factor structure of the working alliance inventory in cognitive-behavioral therapy. J Psychother Pract Res 2001;10:173-8.
- Low DC, Miller SD, Squire B. The Outcome Rating Scale (ORS) and Session Rating Scales (SRS): Feedback Informed Treatment in Child and Adolescent Mental Health Services (CAMHS) 2012. www.aft.org.uk/SpringboardWebApp/userfiles/aft/file/Events/2012/David%20Low%20paper%20for%20CYP-IAPT.pdf (accessed 18 June 2018).
- Mannix KA, Blackburn IM, Garland A, Gracie J, Moorey S, Reid B, et al. Effectiveness of brief training in cognitive behaviour therapy techniques for palliative care practitioners. Palliat Med 2006;20:579-84. https://doi.org/10.1177/0269216306071058.
- Hope R. The Ten Essential Shared Capabilities – A Framework for the Whole of the Mental Health Workforce. London: Department of Health and Social Care; 2004.
- Ramsay CR, Thomas RE, Croal BL, Grimshaw JM, Eccles MP. Using the theory of planned behaviour as a process evaluation tool in randomised trials of knowledge translation strategies: a case study from UK primary care. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-71.
- Clarke DJ, Godfrey M, Hawkins R, Sadler E, Harding G, Forster A, et al. Implementing a training intervention to support caregivers after stroke: a process evaluation examining the initiation and embedding of programme change. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-96.
- Tomasone JR, Martin Ginis KA, Estabrooks PA, Domenicucci L. Changing minds, changing lives from the top down: an investigation of the dissemination and adoption of a Canada-wide educational intervention to enhance health care professionals’ intentions to prescribe physical activity. Int J Behav Med 2015;22:336-44. https://doi.org/10.1007/s12529-014-9414-6.
- Tomasone JR, Arbour-Nicitopoulos KP, Pila E, Lamontagne ME, Cummings I, Latimer-Cheung AE, et al. Exploring end user adoption and maintenance of a telephone-based physical activity counseling service for individuals with physical disabilities using the theoretical domains framework. Disabil Rehabil 2017;39:1332-40. https://doi.org/10.1080/09638288.2016.1193231.
- Hasson H, Blomberg S, Dunér A. Fidelity and moderating factors in complex interventions: a case study of a continuum of care program for frail elderly people in health and social care. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-23.
- Grimshaw JM, Zwarenstein M, Tetroe JM, Godin G, Graham ID, Lemyre L, et al. Looking inside the black box: a theory-based process evaluation alongside a randomised controlled trial of printed educational materials (the Ontario printed educational message, OPEM) to improve referral and prescribing practices in primary care in Ontario, Canada. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-38.
- Fitzgerald TM, Williams PA, Dodge JA, Quinn M, Heminger CL, Moultrie R, et al. Program implementation approaches to build and sustain health care coordination for type 2 diabetes. Health Promot Pract 2017;18:306-13. https://doi.org/10.1177/1524839916643705.
- Campbell-Scherer DL, Asselin J, Osunlana AM, Fielding S, Anderson R, Rueda-Clausen CF, et al. Implementation and evaluation of the 5As framework of obesity management in primary care: design of the 5As Team (5AsT) randomized control trial. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-78.
- Berendsen BA, Kremers SP, Savelberg HH, Schaper NC, Hendriks MR. The implementation and sustainability of a combined lifestyle intervention in primary care: mixed method process evaluation. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0254-5.
- Beighton C, Victor C, Normansell R, Cook D, Kerry S, Iliffe S, et al. ‘It’s not just about walking . . . it’s the practice nurse that makes it work’: a qualitative exploration of the views of practice nurses delivering complex physical activity interventions in primary care. BMC Public Health 2015;15. https://doi.org/10.1186/s12889-015-2568-6.
- Picorelli AM, Pereira LS, Pereira DS, Felício D, Sherrington C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother 2014;60:151-6. https://doi.org/10.1016/j.jphys.2014.06.012.
- Wahlich C, Beighton C, Victor C, Normansell R, Cook D, Kerry S, et al. ‘You started something . . . then I continued by myself’: a qualitative study of physical activity maintenance. Prim Health Care Res Dev 2017;18:574-90. https://doi.org/10.1017/S1463423617000433.
- Harris T, Kerry SM, Limb ES, Furness C, Wahlich C, Victor CR, et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: long-term follow-up of participants from two randomised controlled trials in UK primary care. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002526.
- Iliffe S, Kendrick D, Morris R, Masud T, Gage H, Skelton D, et al. Multicentre cluster randomised trial comparing a community group exercise programme and home-based exercise with usual care for people aged 65 years and over in primary care. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18490.
- Marcus BH, Dubbert PM, Forsyth LH, McKenzie TL, Stone EJ, Dunn AL, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychol 2000;19:32-41. https://doi.org/10.1037/0278-6133.19.Suppl1.32.
- White IR, Carpenter J, Horton N. A Mean Score Method for Sensitivity Analysis to Departures from the Missing at Random Assumption in Randomised Trials 2017. www.stat.sinica.edu.tw/statistica/ (accessed 25 May 2018).
- Tully MA, Cupples ME, Chan WS, McGlade K, Young IS. Brisk walking, fitness, and cardiovascular risk: a randomized controlled trial in primary care. Prev Med 2005;41:622-8. https://doi.org/10.1016/j.ypmed.2004.11.030.
- NHS England Public Health England Diabetes UK . Healthier You: NHS Diabetes Prevention Programme (NHS DPP) 2015. www.england.nhs.uk/ourwork/qual-clin-lead/action-for-diabetes/diabetes-prevention (accessed 27 November 2017).
- Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: a meta-analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0080000.
- Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123-32. https://doi.org/10.7326/M14-1651.
- Fitzsimons CF, Baker G, Gray SR, Nimmo MA, Mutrie N. Scottish Physical Activity Research Collaboration (SPARColl) . Does physical activity counselling enhance the effects of a pedometer-based intervention over the long-term: 12-month findings from the Walking for Wellbeing in the west study. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-206.
- Zheng H, Orsini N, Amin J, Wolk A, Nguyen VT, Ehrlich F. Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol 2009;24:181-92. https://doi.org/10.1007/s10654-009-9328-9.
- Dwyer T, Pezic A, Sun C, Cochrane J, Venn A, Srikanth V, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the tasped prospective cohort study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0141274.
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res 2005;7:871-80. https://doi.org/10.1080/14622200500266056.
- Ashford S, Edmunds J, French DP. What is the best way to change self-efficacy to promote lifestyle and recreational physical activity? A systematic review with meta-analysis. Br J Health Psychol 2010;15:265-88. https://doi.org/10.1348/135910709X461752.
- Cox KL, Flicker L, Almeida OP, Xiao J, Greenop KR, Hendriks J, et al. The FABS trial: a randomised control trial of the effects of a 6-month physical activity intervention on adherence and long-term physical activity and self-efficacy in older adults with memory complaints. Prev Med 2013;57:824-30. https://doi.org/10.1016/j.ypmed.2013.09.010.
- Kuller LH, Kinzel LS, Pettee KK, Kriska AM, Simkin-Silverman LR, Conroy MB, et al. Lifestyle intervention and coronary heart disease risk factor changes over 18 months in postmenopausal women: the Women On the Move through Activity and Nutrition (WOMAN study) clinical trial. J Womens Health 2006;15:962-74. https://doi.org/10.1089/jwh.2006.15.962.
- Opdenacker J, Boen F, Coorevits N, Delecluse C. Effectiveness of a lifestyle intervention and a structured exercise intervention in older adults. Prev Med 2008;46:518-24. https://doi.org/10.1016/j.ypmed.2008.02.017.
- Tulloch H, Sweet SN, Fortier M, Capstick G, Kenny GP, Sigal RJ. Exercise facilitators and barriers from adoption to maintenance in the diabetes aerobic and resistance exercise trial. Can J Diabetes 2013;37:367-74. https://doi.org/10.1016/j.jcjd.2013.09.002.
- Kaewthummanukul T, Brown KC. Determinants of employee participation in physical activity: critical review of the literature. AAOHN J 2006;54:249-61. https://doi.org/10.1177/216507990605400602.
- Sharma M, Sargent L, Stacy R. Predictors of leisure-time physical activity among African American women. Am J Health Behav 2005;29:352-9. https://doi.org/10.5993/AJHB.29.4.7.
- Annual survey of hours and earnings. ONS Digital; 2015.
- Sharples L, Glover M, Bennett M, Jordan J, Clutterbuck-James A, Davies M, et al. TOMADO: a crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18670.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self-management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4093.
- Richardson G, Kennedy A, Reeves D, Bower P, Lee V, Middleton E, et al. A Cost effectiveness of the Expert Participants Programme (EPP) for participants with chronic conditions. J Epidemiol and Community Health 2008;62:361-7. https://doi.org/10.1136/jech.2006.057430.
- Hu G, Qiao Q, Silventoinen K, Eriksson JG, Jousilahti P, Lindström J, et al. Occupational, commuting, and leisure-time physical activity in relation to risk for Type 2 diabetes in middle-aged Finnish men and women. Diabetologia 2003;46:322-9. https://doi.org/10.1007/s00125-003-1031-x.
- Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke 2005;36:1994-9. https://doi.org/10.1161/01.STR.0000177868.89946.0c.
- Hu G, Jousilahti P, Borodulin K, Barengo NC, Lakka TA, Nissinen A, et al. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis 2007;194:490-7. https://doi.org/10.1016/j.atherosclerosis.2006.08.051.
- Briggs AS, Sculpher MJ, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Hu G, Jousilahti P, Antikainen R, Tuomilehto J. Occupational, commuting, and leisure-time physical activity in relation to cardiovascular mortality among Finnish subjects with hypertension. Am J Hypertens 2007;20:1242-50. https://doi.org/10.1016/j.amjhyper.2007.07.015.
- Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Savage PJ, Levy D, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation 2009;120:212-20. https://doi.org/10.1161/CIRCULATIONAHA.108.846519.
- Ward SL, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary. Health Technol Assess 2007;11.
- Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. London: NICE; 2011.
- Jamrozik K. Estimate of deaths attributable to passive smoking among UK adults: database analysis. BMJ 2005;330. https://doi.org/10.1136/bmj.38370.496632.8F.
- Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Danish MONICA Study Group . Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6. https://doi.org/10.1161/hs0901.094253.
- Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728-35. https://doi.org/10.1161/CIRCULATIONAHA.108.829176.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. Statins for the prevention of coronary events. Technology assessment report commissioned by the HTA programme on behalf of The National Institute for Clinical Excellence. London: NICE; 2005.
- Hypertension: the clinical management of primary hypertension in adults. Clinical guideline: methods, evidence and recommendations. London: NICE; 2011.
- Gonzalez ELM, Johansson S, Wallander MA. Trends in the prevalence and incidence of diabetes in the UK, 1996–2005. J Epidemiol Community Health 2009;63:332-6. https://doi.org/10.1136/jech.2008.080382.
- Health Survey for England – 2011, Health, Social Care and Lifestyles. London: Department for Health and Social Care; 2012.
Appendix 1 Methods
Explanation of patient and public involvement across the study
Patient and public involvement across the study is described in our publication of the main results,93 and is reproduced here under the terms of the Creative Commons Attribution Licence (CC BY 4.0).
Pilot work with older primary care patients from three general practices was carried out ahead of seeking trial funding, with focus groups at each practice discussing ideas for a pedometer-based PA intervention. Patients were enthusiastic about the study and felt that the postal approach to recruitment and the interventions offered would be acceptable. They had input into aspects of the study design; for example, they encouraged us to offer participants in the usual-care arm a pedometer at the end of the follow-up period, and they encouraged us to recruit couples as well as individuals, and to allow couples to attend nurse appointments together.
One of the patient advisors was a TSC member, and was involved in discussions about recruitment and study conduct, as well as advising about patient materials, the dissemination of results to participants and safety reporting mechanisms.
The burden of the intervention was assessed by all participants in the nurse group with a questionnaire as part of the process evaluation, and by samples of both intervention groups as part of the qualitative evaluation of intervention participants. 162
All participants were provided with timely feedback of their individual trial results after completion of the 12-month follow-up, including their PA and body size measurements over the trial duration. Summaries of results for the whole trial were disseminated to all trial participants as A4 feedback sheets after completion of the baseline assessments and after analysis of the main results. A trial website (www.paceup.sgul.ac.uk/) has been created, and details have been circulated to participants. This also provides a summary of the trial results and details about further trial follow-up. All publications relating to the trial are provided on the website.
Appendix 2 Results
FIGURE 17.
Trial procedures and complex intervention components.
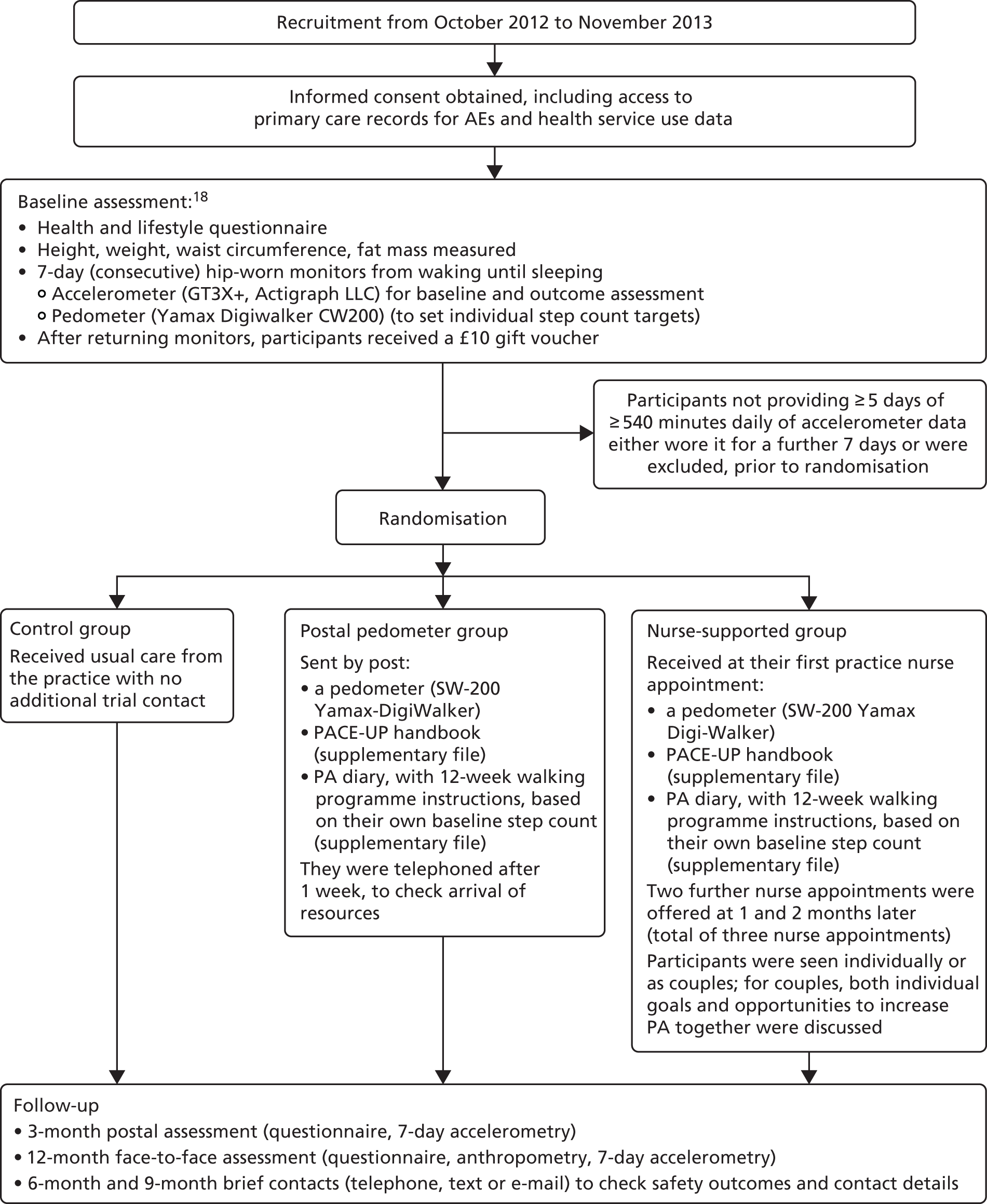
FIGURE 18.
Residuals from the 12-month models for steps and weekly MVPA in ≥ 10-minute bouts. (a) Steps model: distribution of residual; (b) steps model: standardised normal probability plot; (c) weekly MVPA in ≥ 10-minute bouts; and (d) weekly MVPA in ≥ 10-minute bouts.
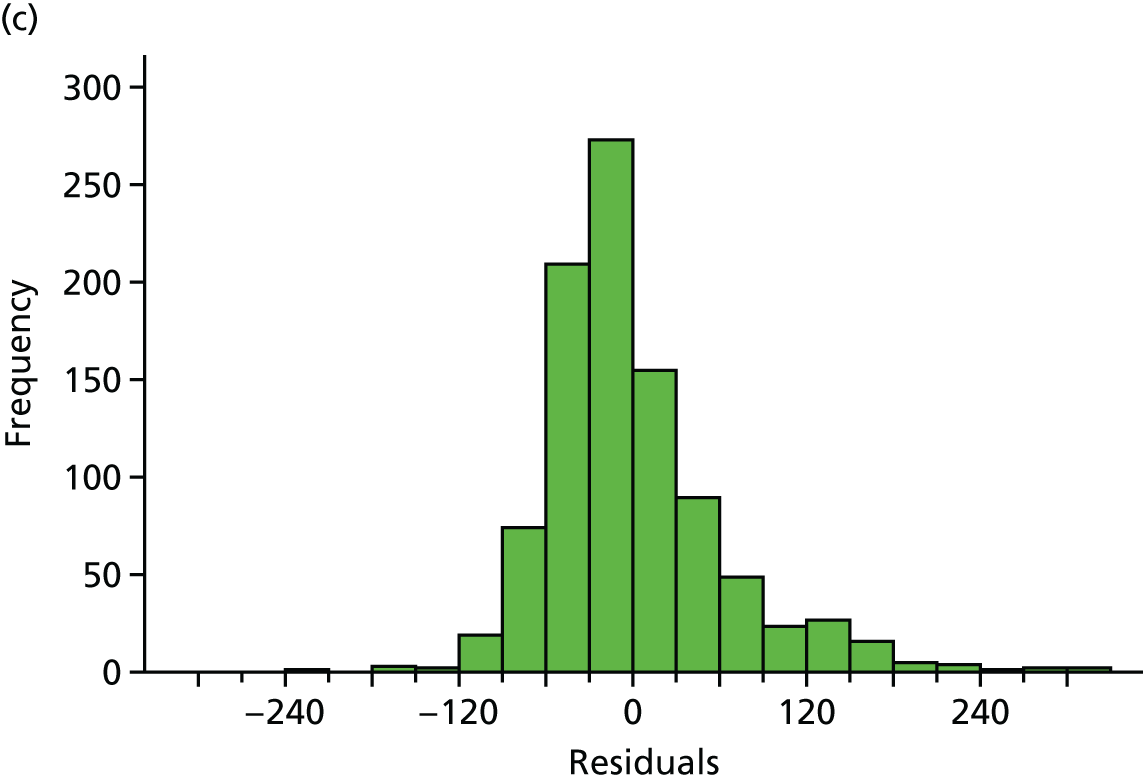
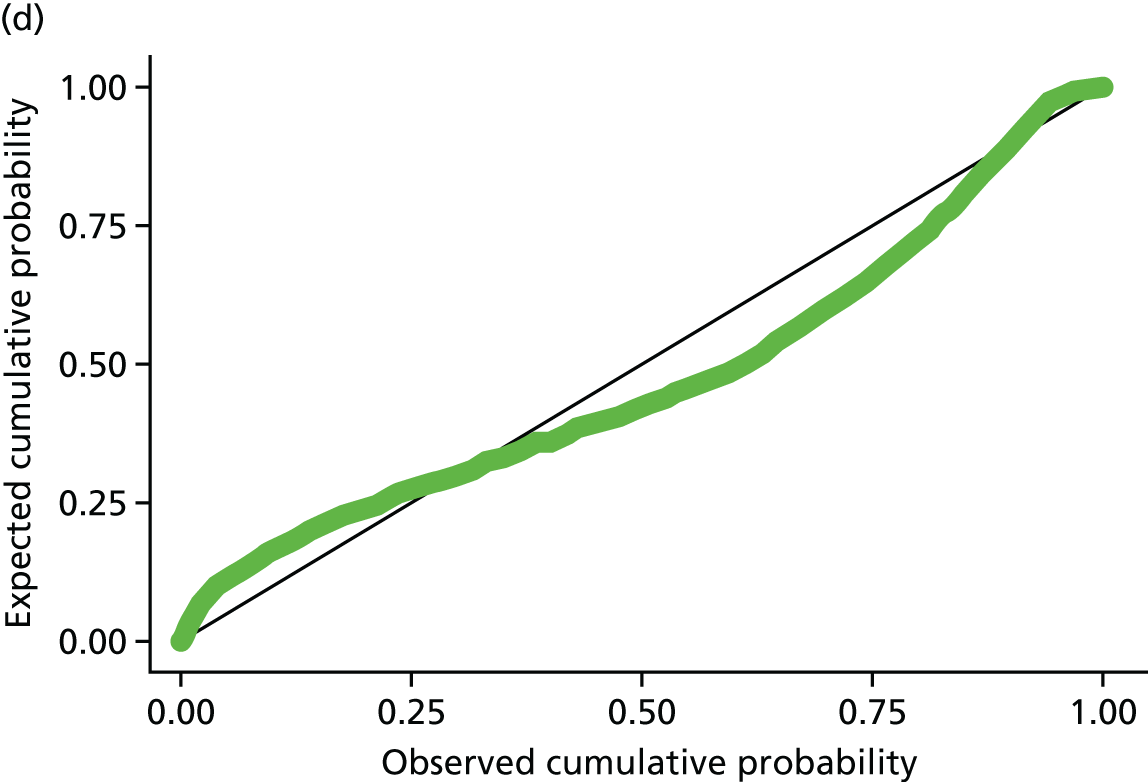
| Number of days with ≥ 540 minutes’ wear time | Time point, number of participants in each trial arm (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | |||||||
| Control (n = 338) | Postal delivery (n = 339) | Nurse support (n = 346) | Control (n = 318) | Postal delivery (n = 317) | Nurse support (n = 319) | Control (n = 323) | Postal delivery (n = 312) | Nurse support (n = 321) | |
| 1 | 0 | 2 (0.6) | 3 (1) | 1 (0.3) | 0 | 1 (0.3) | |||
| 2 | 2 (0.6) | 9 (3) | 6 (2) | 1 (0.3) | 1 (0.3) | 2 (0.6) | |||
| 3 | 9 (3) | 8 (3) | 3 (1) | 5 (2) | 4 (1) | 2 (0.6) | |||
| 4 | 21 (7) | 16 (5) | 11 (3) | 16 (5) | 20 (6) | 14 (4) | |||
| 5 | 29 (9) | 39 (12) | 40 (12) | 37 (12) | 25 (8) | 35 (11) | 42 (13) | 38 (12) | 35 (11) |
| 6 | 85 (25) | 83 (24) | 84 (24) | 64 (20) | 79 (25) | 67 (21) | 78 (24) | 57 (18) | 79 (25) |
| 7 | 224 (66) | 217 (64) | 222 (64) | 185 (58) | 178 (56) | 194 (61) | 180 (56) | 192 (62) | 188 (59) |
| ≥ 5 | 338 (100) | 339 (100) | 346 (100) | 286 (90) | 282 (89) | 296 (93) | 300 (93) | 287 (92) | 302 (94) |
| Outcome | Time point, mean number of participants in each trial arm (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control group | Postal delivery group | Nurse-support group | |||||||
| Baseline | 3 months | 12 months | Baseline | 3 months | 12 months | Baseline | 3 months | 12 months | |
| Accelerometry data (n) | 338 | 318 | 323 | 339 | 317 | 312 | 346 | 319 | 321 |
| Daily step count | 7379 (2696) | 7327 (2688) | 7246 (2671) | 7402 (2476) | 8086 (3014) | 8010 (2922) | 7653 (2826) | 8707 (3206) | 8131 (3228) |
| Total weekly minutes of MVPA in ≥ 10-minute bouts | 84 (97) | 87 (101) | 89 (94) | 92 (90) | 136 (125) | 129 (124) | 105 (116) | 164 (154) | 138 (141) |
| Daily sedentary time (minutes) | 613 (68) | 614 (70) | 616 (72) | 614 (71) | 614 (74) | 617 (71) | 619 (78) | 613 (77) | 620 (79) |
| Daily wear time (minutes) | 789 (73) | 795 (78) | 791 (76) | 787 (78) | 798 (84) | 800 (80) | 797 (84) | 805 (85) | 807 (89) |
| Clinical measurements (n) | 338 | 323 | 339 | 314 | 346 | 321 | |||
| BMI (kg/m2) | 27.7 (5.4) | 27.5 (5.2) | 28 (5.5) | 27.7 (5.6) | 27.6 (5.2) | 27.5 (5.2) | |||
| Fat mass (kg) | 26 (10.3) | 25.8 (9.8) | 26.8 (11.0) | 26.5 (11.2) | 26 (10.6) | 25.6 (11.1) | |||
| Waist circumference (cm) | 93.1 (14.3) | 93.4 (14.7) | 94.1 (13.9) | 94.3 (14.1) | 93.2 (13.2) | 93.7 (13.4) | |||
| Questionnaire data (n) | 335 | 316 | 318 | 335 | 312 | 311 | 342 | 310 | 319 |
| HADS anxiety score | 4.8 (3.3) | 4.7 (3.4) | 4.7 (3.4) | 4.6 (3.3) | 4.4 (3.3) | 4.4 (3.4) | 4.6 (3.6) | 4.4 (3.5) | 4.5 (3.9) |
| HADS depression score | 3.9 (2.8) | 2.7 (2.6) | 2.6 (2.9) | 3.8 (2.6) | 2.4 (2.7) | 2.4 (2.6) | 3.9 (2.9) | 2.4 (2.9) | 2.6 (3.0) |
| EQ-5D score | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.2) | 0.9 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.8 (0.1) |
| Exercise self-efficacy score | 22.3 (7.0) | 20.4 (6.7) | 21.0 (7.1) | 22.1 (7.2) | 21.4 (6.7) | 21.7 (7.0) | 22.0 (7.3) | 22.9 (6.7) | 22.4 (7.1) |
| Self-reported pain | 0.9 (0.8) | 0.9 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 0.9 (0.8) | 1.0 (0.8) | 1.0 (0.8) |
| Questionnaire | Trial arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control group | Postal delivery group | Nurse-support group | |||||||
| Baseline | 3 months | 12 months | Baseline | 3 months | 12 months | Baseline | 3 months | 12 months | |
| IPAQ | (N = 279) | (N = 234) | (N = 274) | (N = 270) | (N = 225) | (N = 251) | (N = 284) | (N = 241) | (N = 257) |
| Total weekly minutes of, mean (SD) | |||||||||
| Vigorous and moderate activity in ≥ 10-minute bouts | 194 (310) | 242 (387) | 237 (365) | 159 (266) | 191 (315) | 204 (294) | 167 (259) | 227 (340) | 214 (361) |
| Walking in ≥ 10-minute bouts | 323 (327) | 370 (336) | 365 (336) | 316 (326) | 357 (292) | 389 (320) | 307 (275) | 417 (308) | 371 (307) |
| Achieved at least 150 minutes of, n (%) | |||||||||
| Vigorous + moderate activity in ≥ 10-minute bouts | 102 (37) | 93 (40) | 112 (41) | 86 (32) | 85 (38) | 108 (43) | 101 (36) | 89 (37) | 99 (39) |
| Achieved at least 150 minutes of walking in ≥ 10-minute bouts | 178 (64) | 160 (68) | 192 (70) | 176 (65) | 168 (75) | 198 (79) | 193 (68) | 201 (83) | 197 (77) |
| GPPAQ | (N = 322) | (N = 308) | (N = 315) | (N = 318) | (N = 296) | (N = 303) | (N = 333) | (N = 305) | (N = 318) |
| Physical Activity Index, n (%) | |||||||||
| Active | 44 (14) | 37 (12) | 49 (16) | 36 (11) | 36 (12) | 51 (17) | 34 (10) | 39 (13) | 41 (13) |
| Including walking: active | 93 (29) | 94 (31) | 103 (33) | 79 (25) | 92 (31) | 101 (33) | 90 (27) | 102 (33) | 96 (30) |
| Analysis | Participants (N) | Comparison between trial arms | |||||
|---|---|---|---|---|---|---|---|
| Postal delivery vs. control | Nurse support vs. control | Nurse support vs. postal delivery | |||||
| Effect (95% CI) | p-value | Effect (95% CI) | p-value | Effect (95% CI) | p-value | ||
| Analysis based on all participants with follow-up data | |||||||
| Minimum daily wear time 540 minutes | |||||||
| At least 5 days at baseline and 1 day at 12 months | 956 | 642 (329 to 955) | < 0.001 | 677 (365 to 989) | < 0.001 | 36 (–277 to 349) | 0.82 |
| At least 5 days at baseline and 5 days at 12 months | 889 | 607 (285 to 930) | < 0.001 | 732 (412 to 1051) | < 0.001 | 124 (–198 to 446) | 0.45 |
| Minimum daily wear time of 600 minutes | |||||||
| At least 5 days at baseline and 1 day at 12 months | 877 | 675 (352 to 997) | < 0.001 | 714 (392 to 1036) | < 0.001 | 39 (–283 to 362) | 0.81 |
| At least 5 days at baseline and 5 days at 12 months | 760 | 752 (411 to 1093) | < 0.001 | 796 (456 to 1136) | < 0.001 | 44 (–295 to 384) | 0.80 |
| Model adjusting for change in wear time between baseline and 12 months | 956 | 579 (273 to 885) | < 0.001 | 637 (332 to 941) | < 0.001 | 58 (–248 to 363) | 0.71 |
| Analyses based on all randomised participants: missing step counts imputed for participants with no follow-up data at 12 months | |||||||
| Missing at random | |||||||
| Imputed using treatment group, baseline steps, sex, age, practice, month baseline accelerometry | 1023 | 638 (324 to 953) | < 0.001 | 679 (367 to 992) | < 0.001 | 41 (–270 to 352) | 0.80 |
| Imputed using treatment group, baseline steps, sex, age, practice, month baseline accelerometry, NS-SEC, self-reported pain and fat massa | 1013 | 673 (356 to 989) | < 0.001 | 686 (372 to 1000) | < 0.001 | 13 (–303 to 330) | 0.94 |
| Missing not at random, using extreme assumptions for missing data | |||||||
| Control group: 12-month step count equal to baseline step count | |||||||
| Both intervention groups: 12-month step count changes by | |||||||
| –1500 steps | 1023 | 651 (338 to 964) | < 0.001 | 783 (472 to 1095) | < 0.001 | 132 (–181 to 445) | 0.41 |
| Same as baseline step count | 1023 | 771 (458 to 1084) | < 0.001 | 892 (580 to 1204) | < 0.001 | 121 (–192 to 434) | 0.45 |
| +1500 steps | 1023 | 890 (577 to 1203) | < 0.001 | 1000 (688 to 1312) | < 0.001 | 110 (–203 to 423) | 0.49 |
Appendix 3 Economic evaluation
| Activity (trial arm applicable to) | Sources of data | Trial arm | |
|---|---|---|---|
| Resource use | Unit cost (£) | ||
| Design | |||
| Designing of intervention | Time spent by designers (diaries, administrative records) | Salary cost (PSSRU, administrative records) | Two intervention arms |
| Designing of participants’ handbooks and diaries | |||
| Designing of nurse trainers’ handbooks |
|
|
Pedometer plus nurse group |
| Setting up GP practices | |||
| Planning for recruitment of practices | Time spent by recruiters (diaries, administrative records) | Salary cost (PSSRU, administrative records) | All trial arms |
| Visits to recruit six practices |
|
|
|
| Searching practice computers to identify participants | Time spent by practice managers and trial staff (diaries, administrative records) | Salary cost (PSSRU, administrative records) | |
| Identify households from anonymised address list | Time spent by trial staff (diaries, administrative records) | Salary cost (administrative records) | |
| Practice staff members review lists for exclusion | Time spent by practice staff (administrative records) | Salary cost (PSSRU) | |
| Printing letters at practice |
|
|
|
| Packing envelopes with leaflets and letters |
|
|
All trial arms |
| Preparing rooms at practices for trial |
|
|
|
| Training | |||
| Training of trial manager |
|
|
All trial arms |
| Preparation of nurse training course | Time spent by course trainers (diaries, administrative records) | Salary cost (administrative records) | Pedometer plus nurse group |
| Mini training day of nurses |
|
|
|
| Full training day of nurses |
|
|
|
| Training for an absentee nurse |
|
|
Pedometer plus nurse group |
| Discussion of nurses’ recorded sessions (nurse group) |
|
|
|
| Follow-up half-day training (nurse group) |
|
|
|
| Training of research assistants (all trial arms) |
|
Salary cost (administrative records) | |
| Components | Sources of data | Trial arm | |
|---|---|---|---|
| Resource use | Unit cost (£) | ||
| Envelopes for posting pedometers | Number used (administrative records) | Price per envelope (invoice) | Two intervention arms |
| Stamps for posting pedometers | Number used (administrative records) | Price per stamp (invoice) | |
| Pedometers posted to participants (including replacements) | Number used (administrative records) | Price per pedometer (invoice) | |
| Replacement batteries for pedometer | Number used (administrative records) | Price per battery (invoice) | |
| Patient handbooks posted to participants | Number given out (administrative records) | Cost per handbook (administrative records) | |
| Walking plan/diary posted to participants | Number posted (administrative records) | Cost per walking plan (administrative records) | |
| Time of nurse consultation with participants | Duration of each (n = 3) consultation (nurse data base) | Salary cost for nurse (PSSRU 2014)103 | Pedometer plus nurse-support arm |
| Time of research assistants to arrange a consultation appointment for participants | Time spent by three research assistants (diary) | Salary cost (administrative records) | |
| Telephone calls by research assistants to arrange a consultation appointment for participants | Duration of telephone calls (administrative records) | Telephone charge per minute [BT tariff guide (average of landline/mobile phone)] | |
| Components | Source of data | Trial arm | |
|---|---|---|---|
| Resource use | Unit cost (£)a | ||
| GP consultations | Number of GP consultations (GP data) | Cost per GP consultation (PSSRU 2014)103 | All three trial arms |
| Nurse consultationsb | Number of nurse consultations (GP data) | Cost per nurse consultation (PSSRU 2014)103 | |
| Hospital admissions | Number of elective and emergency hospital admissions by diagnosis or procedure (GP data) | Cost of hospital admission [determined by type of diagnosis and/or related procedure (NHS Reference Costs 2014–15)]102 | |
| A&E visits | Number of A&E visits (GP data) | Cost per A&E admission (NHS Reference Costs 2014–15)102 | |
| Outpatient referrals | Number of outpatient visits by department (GP data)c | Cost per outpatient visit (NHS Reference Costs 2014–15)102 | |
| Components | Source of data | Trial arm | |
|---|---|---|---|
| Resource use | Unit cost | ||
| Time working out how to use pedometer | Duration (3-month questionnaire) | Wage rate of participants (ONS 2015207) | Both intervention arms |
| Time planning how to increase walking/step count | |||
| Time filling in PACE-UP diary | |||
| Parking fees to visit nurse | Number of nurse visits (nurse database) | Parking charge per last visit (3-month questionnaire) | Pedometer plus nurse support group |
| Time spent in consultation with nursea | Duration of the three consultations (nurse database) | Wage rate of participants (ONS 2015) | |
| Time travelling (irrespective of mode of transport) to visit nurseb | Duration travelling (3-month questionnaire); number of nurse visits (nurse database) | ||
| Transportation cost (for those who took public transport) of attending the nurse visit | Number of nurse visits (nurse database) | Fare for last nurse visit (3-month questionnaire) | |
| Time waiting time prior to consultation with nurse | Duration of waiting time at last visit to nurse (3-month questionnaire); number of nurse visits (nurse database) | Wage rate of participants (ONS 2015) | |
| Child care during nurse visits | Number of nurse visits (nurse database) | Child care charge for last nurse visit (3-month questionnaire) | |
| Activity (trial arm applicable to) | Resource | Total quantity | Group, cost (£) per participant | |
|---|---|---|---|---|
| Nurse support | Postal delivery | |||
| Designb | ||||
| Designing of intervention (both intervention arms) | Professor × 1 | 0.5 days | 4.43 | 4.43 |
| Readers × 3 | 1 day | |||
| Senior lecturers × 3 | 3.5 days | |||
| Consultants × 2 | 1 day | |||
| Designing of participants’ handbooks and diaries (both intervention groups) | Professor × 3 | 1.5 days | 3.56 | 3.56 |
| Readers × 2 | 1 day | |||
| Senior lecturers × 3 | 2 days | |||
| Consultants × 2 | 0.5 days | |||
| Designing of nurse trainers handbooks (nurse-support group) | Senior lecturers × 1 | 1 day | 2.74 | 0.00 |
| Consultants × 1 | 0.5 days | |||
| Handbooks | 9 handbooks | 0.19 | 0.00 | |
| Setting up GP practices | ||||
| Planning for recruitment of practices (all trial arms) | Professor × 1 | 1 hour | 0.99 | 0.99 |
| Senior lecturer × 1 | 5 hours | |||
| Consultants × 2 | 5 hours | |||
| Visits to recruit six practices (all trial arms) | Senior lecturers × 2 | 13 hours | 1.47 | 1.47 |
| Trial manager × 1 | 7 hours | |||
| Consultant × 1 | 5 hours | |||
| Round trips to practices (by all) | 25 hours | 0.10 | 0.10 | |
| Searching practice computers to identify participants (all trial arms) | Senior lecturer × 1 | 6 hours | 0.71 | 0.71 |
| Trial manager × 1 | 6 hours | |||
| Practice manager × 6 | 6 hours | |||
| Identify households from anonymised address list (all trial arms) | Senior lecturer × 1 | 32 hours | 2.28 | 2.28 |
| Trial manager × 1 | 32 hours | |||
| Practice staff reviews lists for exclusion (all trial arms) | GP × 5 (for sorting out two practices) | 20 hours | 4.50 | 4.00 |
| Nurse × 10 (for sorting out other five practices) | 50 hours | 1.96 | 1.96 | |
| Printing letters at practice (all trial arms) | Trial manager × 1 | 64 hours | 1.57 | 1.57 |
| Practice administrative staff × 2 | 4 hours | |||
| Number of printed letters | 24,000 | 0.94 | 0.94 | |
| Packing envelopes with leaflets and letters (all trial arms) | Trial manager × 1 | 240 hours | 7.04 | 7.04 |
| Research assistants × 2 | 56 hours | |||
| Practice administration staff × 11 | 27.5 hours | |||
| Cost of envelopes | £497.30 | 0.49 | 0.49 | |
| Cost of Postal stamps | £5530.50 | 5.41 | 5.41 | |
| Cost of Information leaflets | £5973.00 | 5.84 | 5.84 | |
| Preparing rooms at practices for trial (all trial arms) | Round trip to practices by research assistant | 14 trips | 0.04 | 0.04 |
| Research assistants × 2 | –c | 0.11 | 0.11 | |
| Training | ||||
| Training of trial manager (all trial arms) | Trial manager × 1 | 4 days | 1.51 | 1.51 |
| Senior lecturer × 1 | 2 days | |||
| Preparation of nurse training course (nurse-support group) | Trial manager × 1 | 1 day | 9.63 | 0.00 |
| Senior lecturer × 1 | 2 days | |||
| Reader × 1 | 0.5 days | |||
| Consultants × 2 | 2 days | |||
| Mini training day of nurses (nurse-support group) | Nurses × 11 | 33 hours | 7.46 | 0.00 |
| Trial manager × 1 | 17.33 hours | |||
| Senior lecturer × 1 | 17.33 hours | |||
| Round trips to the training centre (by tutors) | 16 hours | 0.19 | 0.00 | |
| Pedometers given to nurses | 12 hours | 0.04 | 0.00 | |
| Full training day of nurses (nurse-support group) | Nurses × 10 | 107.5 hours | 22.99 | 0.00 |
| Reader × 1 | 1 hour | |||
| Senior lecturer × 1 | 10 hours | |||
| Consultants × 2 | 22.5 hours | |||
| Round trips for training by nurses × 10 | 10 trips | 0.12 | 0.00 | |
| Round trips for training by consultants × 2 | 2 trips | 0.13 | 0.00 | |
| Refreshments | 1 set | 0.26 | 0.00 | |
| Training for an absentee nurse (nurse-support group) | Nurse × 1 | 10 hours | 2.47 | 0.00 |
| Trial manager × 1 | 11.33 hours | |||
| Research assistant × 1 | 11.33 hours | |||
| Round trips to training centre | 2 trips | 0.02 | 0.00 | |
| Discussion of nurses’ recorded sessions (nurse-support group) | Senior lecturer × 1 | 0.5 days | 3.78 | 0.00 |
| Consultants × 2 | 1 day | |||
| Nurses × 9 | 4.5 days | 0.99 | 0.00 | |
| Senior lecturer × 1 | 0.5 days | |||
| Consultants × 2 | 1 day | |||
| Duration of telephone calls | 270 minutes | 0.09 | 0.00 | |
| Follow-up half-day training (nurse-support group) | Nurses × 9 | 4.5 days | 7.70 | 0.00 |
| Trial manager × 1 | 0.5 days | |||
| Senior lecturer × 1 | 0.5 days | |||
| Consultants × 2 | 1 day | |||
| Nurse time travelling × 9 | 6.75 hours | 0.78 | 0.00 | |
| Round trips to training centre (nurses) | 9 trips | 0.10 | 0.00 | |
| Refreshment | 1 set | 0.15 | 0.00 | |
| Training of research assistants (all trial arms) | Research assistant × 3 | 6.6 days | 1.91 | 1.91 |
| Senior lecturer × 1 | 0.5 days | |||
| Reader × 1 | 0.5 days | |||
| Trial manager × 1 | 4 days | |||
| Total cost per participant | 104.64 | 44.83 | ||
| Components | Resource (from administrative records) | Quantity of resource | Unit cost (£) (data source) | Analysis | |||
|---|---|---|---|---|---|---|---|
| 3-month | 12-month | ||||||
| Total cost (£) | Cost (£) per participant | Total cost (£) | Cost (£) per participant | ||||
| Envelopes for posting pedometers (including replacements) | Number of envelopes | 426 | 0.03 (invoice) | 12.78 | 0.04 | 12.78 | 0.04 |
| Stamps for posting pedometer | Number of stamps | 426 | 2.50 (invoice) | 1065.00 | 3.14 | 1065 | 3.14 |
| Pedometers (including replacements) given to participants | Number of pedometers | 426 | 1/4a (invoice) | 426.00 | 1.26 | 1704 | 5.03 |
| Replacement batteries for pedometer | Number of replacement batteries | 11 | 0.67 (invoice) | 7.37 | 0.02 | 7.37 | 0.02 |
| Patient handbooks | Number of handbooks | 339 | 0.80 (administrative records) | 271 | 0.80 | 271 | 0.80 |
| Step count diary | Number of diaries | 339 | 1.30 (administrative records) | 440.70 | 1.30 | 440.70 | 1.30 |
| Total cost (£) per participant | 6.56 | 10.33 | |||||
| Components | Resource (data source) | Quantity of resource | Unit cost (£) (data source) | Analysis | |||
|---|---|---|---|---|---|---|---|
| 3-month | 12-month | ||||||
| Total cost (£) | Cost (£) per participant | Total cost (£) | Cost (£) per participant | ||||
| Pedometers given to participants | Number of pedometers (administrative records) | 346 | 1/4a (invoice) | 346.00 | 1.00 | 1384 | 4.00 |
| Patient handbooks | Number of handbooks (administrative records) | 346 | 0.80 (administrative records) | 277.00 | 1.00 | 277 | 1.00 |
| Step count diary | Number of diaries (administrative records) | 346 | 1.30 (administrative records) | 449.80 | 1.30 | 449.80 | 1.30 |
| Research assistants’ time to arrange consultation | Time spent by research assistants (diary) | 50.46 hours | 16.51 (administrative records) | 833.07 | 2.41 | 833.07 | 2.41 |
| Telephone calls by research assistants to arrange consultation | Duration of telephone calls (administrative records) | 3027.5 minutes | 0.11 (BT tariff)a | 333.03 | 0.96 | 333.03 | 0.96 |
| Cost of nurse visit per participant (project database for nurse group) | 43.00 | 42.00 | |||||
| Total cost per participant | 49.67 | 51.67 | |||||
| Intervention-related participant costs | Trial arm, mean cost (£) (SD) | ||
|---|---|---|---|
| Control (N = 323) | Postal delivery (N = 312) | Nurse support (N = 321) | |
| Time working out how to use pedometer | 0 (0) | 2 (6) | 1 (3) |
| Time planning how to increase walking/step count | 0 (0) | 5 (15) | 3 (4) |
| Time filling in the PACE-UP diary | 0 (0) | 51 (80) | 58 (122) |
| Parking fees to visit nurse | 0 (0) | 0 (0) | 0.11 (0.73) |
| Time spent in consultation with nurse | 0 (0) | 0 (0) | 10 (5) |
| Time travelling (irrespective of mode of transport) to visit nurse | 0 (0) | 0 (0) | 11 (10) |
| Transportation cost (for those who took public transport) of attending the nurse visit | 0 (0) | 0 (0) | 0.13 (1.33) |
| Time waiting time prior to consultation with nurse | 0 (0) | 0 (0) | 3 (4) |
| Child care during nurse visits | 0 (0) | 0 (0) | 0.3 (3.21) |
| Personal costs of participation in PA | 411 (817) | 492 (1293) | 333 (684) |
| Personal costs from falls/fractures/sprains/injuries | 17 (103) | 22 (184) | 6 (40) |
| Health service use | Trial arm (quantity) | Unit cost (£), weighted average (Q1–Q3) | Source for unit cost | ||
|---|---|---|---|---|---|
| Control (N = 323) | Postal delivery (N = 312) | Nurse support (N = 321) | |||
| Outpatient referrals (total)a | 164 | 158 | 186 | ||
| Ophthalmology | 10 | 18 | 15 | 86 (70–99) | NHS Reference Costs 2014–15 102 |
| Urology | 4 | 3 | 6 | 99 (76–116) | |
| General medicine | 4 | 0 | 2 | 157 (120–187) | |
| ENT | 9 | 6 | 12 | 92 (70–109) | |
| Podiatry | 9 | 7 | 7 | 44 (27–45) | |
| Trauma and orthopaedics | 14 | 13 | 10 | 113 (88–133) | |
| Physiotherapy | 26 | 33 | 37 | 46 (35–50) | |
| Nephrology | 0 | 1 | 0 | 145 (94–178) | |
| Oral surgery | 0 | 2 | 0 | 115 (85–142) | |
| Gynaecology | 6 | 7 | 14 | 134 (104–164) | |
| Audiology | 4 | 6 | 7 | 104 (55–174) | |
| Colorectal surgery | 1 | 5 | 1 | 117 (83–135) | |
| Neurology | 8 | 8 | 5 | 174 (136–204) | |
| Cardiology | 12 | 5 | 4 | 131 (92–154) | |
| Gastroenterology | 6 | 2 | 6 | 130 (99–153) | |
| Rheumatology | 4 | 6 | 7 | 135 (99–150) | |
| Dermatology | 1 | 8 | 7 | 98 (74–109) | |
| General surgery | 4 | 1 | 3 | 125 (98–165) | |
| Endocrinology | 2 | 1 | 2 | 144 (100–167) | |
| Neurosurgery | 2 | 0 | 0 | 181 (138–228) | |
| Oncology | 8 | 5 | 11 | 133 (97–165) | |
| Psychotherapy | 1 | 0 | 0 | 100 (47–217) | |
| Respiratory medicine | 4 | 6 | 3 | 150 (107–181) | |
| Clinical neurophysiology | 2 | 0 | 1 | 165 (107–197) | |
| Programmed pulmonary rehabilitation | 0 | 0 | 1 | 20 (12–31) | |
| Pain management | 2 | 0 | 4 | 135 (82–164) | |
| Allergy service | 0 | 1 | 0 | 149 (126–175) | |
| Dietetics | 2 | 2 | 3 | 62 (38–76) | |
| Vascular surgery | 2 | 1 | 4 | 149 (100–176) | |
| Mental illness | 1 | 1 | 1 | 234 (181–256) | |
| Clinical genetics | 1 | 0 | 1 | 429 (248–601) | |
| Clinical haematology | 2 | 1 | 0 | 160 (93–189) | |
| Spinal surgery services | 0 | 1 | 0 | 142 (112–164) | |
| Maxillofacial surgery | 0 | 0 | 1 | 111 (70–133) | |
| Plastic surgery | 1 | 1 | 1 | 93 (68–109) | |
| Clinical immunology | 0 | 1 | 0 | 215 (140–243) | |
| Interventional radiology | 1 | 0 | 0 | 192 (88–260) | |
| Breast surgery | 9 | 4 | 5 | 139 (103–166) | |
| Tropical medicine | 0 | 1 | 0 | 202 (203–203) | |
| Clinical psychology | 1 | 0 | 3 | 177 (116–245) | |
| Old age psychiatry | 0 | 1 | 2 | 108 (108–108) | |
| Referral to A&E | 1 | 0 | 0 | 135 (54–166) | |
| Community-based referrals (total)b | 27 | 19 | 21 | ||
| District nurse | 1 | 3 | 2 | 39 (31–43) | PSSRU’s Unit Costs of Health and Social Care 2014103 |
| Community podiatrist | 4 | 3 | 8 | 42 (35–58) | PSSRU’s Unit Costs of Health and Social Care 2014103 |
| Community dietitian | 0 | 2 | 0 | 80 (53–96) | NHS Reference Costs 2014–15 102 |
| Smoking cessation (nurse-support group) | 5 | 3 | 4 | 14 | 15.5 minutes’ nurse time; PSSRU’s Unit Costs of Health and Social Care 2014103 |
| Healthy lifestyle (nurse-support group) | 0 | 2 | 0 | 14 | 15.5 minutes’ nurse time; PSSRU’s Unit Costs of Health and Social Care 2014103 |
| Community gynaecologist | 5 | 1 | 0 | 134 (104–164) | NHS Reference Costs 2014–15 102 |
| Community physiotherapist | 7 | 4 | 1 | 52 (44–58) | PSSRU’s Unit Costs of Health and Social Care 2014103 |
| Community diabetic | 1 | 0 | 0 | 69 (38–93) | NHS Reference Costs 2014–15 102 |
| DESMOND diabetes programme | 4 | 0 | 6 | 230 | Gillett et al. (2010)209 (inflated to 2014) |
| Expert patient programme | 0 | 1 | 0 | 302 | Richardson et al. (2008)210 (inflated to 2014) |
| Primary care – excludes practice visits related to delivery and participation in the intervention (total)c | 2074 | 1748 | 2094 | ||
| GP (11.7 minutes) | 1743 | 1436 | 1729 | 42 | PSSRU’s Unit Costs of Health and Social Care 2014103 |
| GP nurse (15.5 minutes) | 331 | 312 | 365 | 14 | PSSRU’s Unit Costs of Health and Social Care 2014103 |
| A&E visitd | 49 | 36 | 46 | 124 | NHS Reference Costs 2014–15 102 |
| Non-elective hospital admissions (total)e,f | 12 | 4 | 20 | ||
| Biliary acute pancreatitis | 0 | 0 | 3 | 2037 (1247–2492) | NHS Reference Costs 2014–15 102 |
| Cardiac catheterisation for coronary artery disease | 1 | 0 | 1 | 2643 (1980–3028) | |
| Chest pain | 0 | 1 | 0 | 490 (370–563) | |
| Abdominal pain | 0 | 0 | 1 | 718 (922–1298) | |
| Acute ST segment elevation myocardial infarction | 2 | 0 | 0 | 1497 (1102–1740) | |
| Transient ischaemic attack | 0 | 0 | 1 | 878 (643–994) | |
| Guillain–Barré syndrome | 0 | 0 | 1 | 1571 (1069–1792) | |
| Pneumonia | 1 | 0 | 0 | 1894 (1406–2238) | |
| Epilepsy | 1 | 0 | 0 | 1125 (788–1266) | |
| Stroke and cerebrovascular accident | 1 | 0 | 0 | 2817 (2018–3396) | |
| UTI | 0 | 0 | 1 | 1530 (1187–1755) | |
| Detached retina | 0 | 0 | 1 | 908 (303–1935) | |
| Anxiety states | 0 | 0 | 1 | 1393 (984–1628) | |
| Infective endocarditis | 1 | 0 | 0 | 4480 (2351–5906) | |
| Acute appendicitis | 0 | 0 | 1 | 3017 (2459–3365) | |
| IUD removed | 0 | 0 | 1 | 1780 (1142–2135) | |
| Ankle fracture | 1 | 0 | 0 | 3762 (3109–4271) | |
| No procedure (NES) | 4 | 3 | 8 | 611 (408–726) | |
| Elective hospital admissions (total)e,g | 10 | 2 | 3 | ||
| Cardiac catheterisation | 2 | 0 | 0 | 2086 (1185–2709) | NHS Reference Costs 2014–15 102 |
| Percut translum balloon angioplasty multicoronary | 1 | 0 | 0 | 1813 (880–2233) | |
| Inguinal hernia | 0 | 1 | 0 | 2121 (1682–2392) | |
| Coronary artery bypass graft operations | 0 | 1 | 0 | 9310 (7369–9929) | |
| Laparoscopic cholecystectomy | 3 | 0 | 0 | 2567 (2082–2924) | |
| Endarterectomy of femoral artery (NEC) | 0 | 0 | 2 | 6028 (4593–7209) | |
| Malignant neoplasm of female breast for chemotherapy | 1 | 0 | 0 | 1780 (856–2139) | |
| Endarterectomy of carotid artery (NEC) | 1 | 0 | 0 | 3911 (2986–4497) | |
| Neurophysiological operation (NOS) | 2 | 0 | 0 | 1497 (1111–2118) | |
| Ovarian cancer | 0 | 0 | 1 | 1469 (741–1966) | |
| Total resource use (all health service use) | 2336 | 1967 | 2370 | ||
FIGURE 19.
Cost-effectiveness plane for nurse support vs. postal delivery at 12 months.
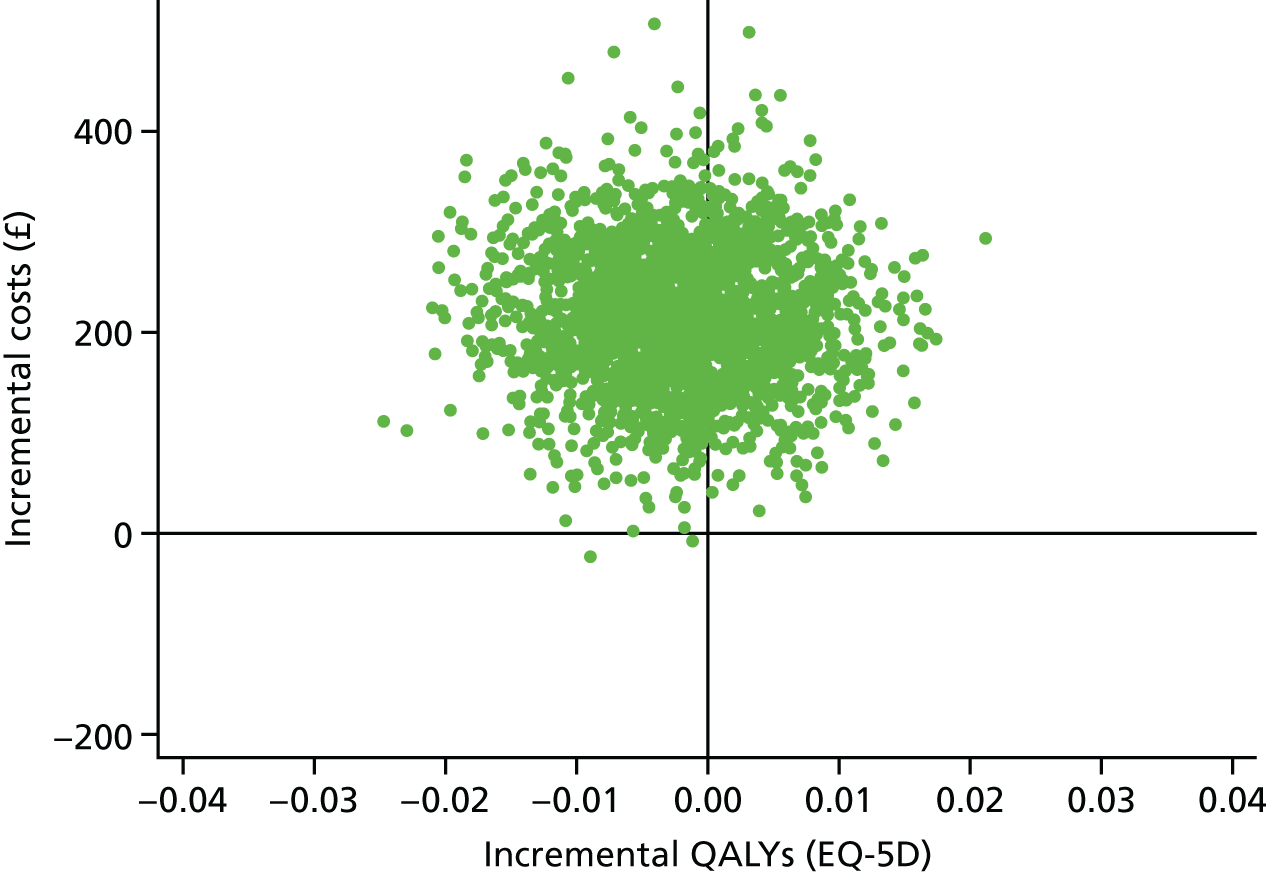
FIGURE 20.
Cost-effectiveness acceptability curve showing the probability of within-trial cost-effectiveness for nurse support vs. postal delivery at different willingness-to-pay threshold levels.
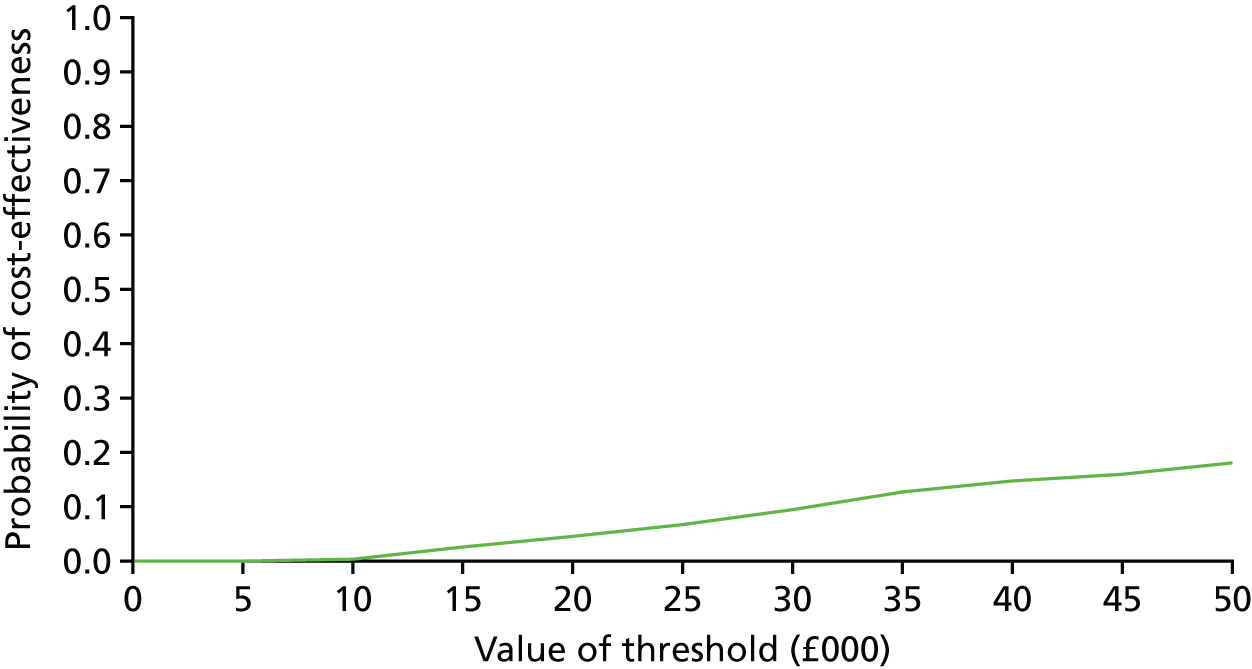
| Parameter | Comparison by trial arm, mean (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Postal delivery vs. control | Nurse support vs. control | Nurse support vs. postal delivery | |||||||
| Incremental cost (£) | Incremental QALY | ICER | Incremental cost (£) | Incremental QALY | ICER | Incremental cost (£) | Incremental QALY | ICER | |
| Base case | –91 (–215 to 33) | –0.0043 (–0.0172 to 0.0087) | Less costly, but less effective than control | 126 (–37 to 290) | –0.0066 (–0.0201 to 0.0068) | Intervention dominated by control | 217 (8 to 354) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Whole sample (all randomised) | –40 (–169 to –89) | –0.0070 (–0.0195 to 0.0054) | Less costly, but less effective than control | 150 (–6 to 306) | –0.0093 (–0.0222 to 0.0036) | Intervention dominated by control | 190 (48 to 332) | –0.0023 (–0.0148 to 0.0102) | Nurse support dominated by postal delivery |
| Health service use, including only GP data on referrals and admissions | –55 (–166 to –56) | –0.0043 (–0.0172 to 0.0087) | Less costly, but less effective than control | 129 (–17 to 275) | –0.0066 (–0.020 to 0.0068) | Intervention dominated by control | 184 (61 to 307) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Health service use, including only self-reported serious adverse effects | 21 (–65 to 107) | –0.0043 (–0.0172 to 0.0087) | Intervention dominated by control | 144 (65 to 224) | –0.0066 (–0.020 to 0.0068) | Intervention dominated by control | 123 (47 to 200) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Health service use, including only GP data on adverse effects | –11 (–107 to 85) | –0.0043 (–0.0172 to 0.0087) | Less costly, but less effective than control | 64 (–15 to 142) | –0.0066 (–0.020 to 0.0068) | Intervention dominated by control | 74 (13 to 135) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Excluding all health service use costs | 55.2 (55 to 55.4) | –0.0043 (–0.0172 to 0.0087) | Intervention dominated by control | 156.2 (–154 to 158) | –0.0066 (–0.0201 to 0.0068) | Intervention dominated by control | 101 (99 to 103) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Changing cost perspective (both participant – all participant costs – and NHS costs) | 36 (–177 to 250) | –0.0043 (–0.0172 to 0.0087) | Intervention dominated by control | 107 (–97 to 311) | –0.0066 (–0.020 to 0.0068) | Intervention dominated by control | 71 (–150 to 291) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Changing cost perspective [both participant (part; this excludes time costs of working out how to use pedometer, diary and planning to increase work) and NHS costs] | –22 (–235 to 191) | –0.0043 (–0.0172 to 0.0087) | Less costly, but less effective than control | 47 (–157 to 250) | –0.0066 (–0.020 to 0.0068) | Intervention dominated by control | 69 (–152 to 289) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Combination of excluding all health service use cost and including all participant costs (minus health service use cost borne by participants) | 179 (–1 to 361) | –0.0043 (–0.0172 to 0.0087) | Intervention dominated by control | 153 (24 to 281) | –0.0066 (–0.020 to 0.0068) | Intervention dominated by control | –27 (–203 to 149) | –0.0024 (–0.0156 to 0.0109) | Less costly but less effective than control |
| Pedometer lasts for 1 year (equivalent to pedometers not being reusable and the full cost of pedometer borne in year 1) | –86 (–210 to 38) | –0.0043 (–0.0172 to 0.0087) | Less costly, but less effective than control | 130 (–33 to 294) | –0.0066 (–0.0201 to 0.0068) | Intervention dominated by control | 216 (80 to 353) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
| Pedometer lasts for 4 years (double length of life considered in the base case) | –93 (–218 to 31) | –0.0043 (–0.0172 to 0.0087) | Less costly, but less effective than control | 124 (–39 to 287) | –0.0066 (–0.0201 to 0.0068) | Intervention dominated by control | 218 (81 to 354) | –0.0024 (–0.0156 to 0.0109) | Nurse support dominated by postal delivery |
Summary of methods of the economic model by Anokye, Lord and Fox-Rushby130
Anokye et al. 130 developed a Markov model to follow a cohort of physically inactive but healthy adults over their remaining lifetime. To see the impact of an intervention on costs and effects, the model is run twice – once with the intervention and once without the intervention, as a control. The model adopted a NHS perspective, based costs in 2010–11 prices (£) and used a 3.5% discount rate.
For the intervention, a cohort of 100,000 people aged 33 years (the average age of people in trials of brief interventions designed to increase PA) are ‘run in’ for 1 year, with the proportion of people becoming active in each arm reflecting evidence on effectiveness [i.e. achieving a minimum of 150 minutes of at least moderately intensive PA or at least 75 minutes of vigorously intensive PA per week (current guidance of sufficient PA)].
The model represented the most robustly evidenced risk reductions achievable from PA (i.e. in CHD, stroke and type 2 diabetes mellitus). Those people who are physically active at the end of the ‘run in’ period went on to have a longer life expectancy and better quality of life as a result of risk reductions. The model assumed that no one developed disease during the run-in period, although deaths from other causes could occur.
From the beginning of year 1 (cycle 2), each person in each cycle could be in one of six states: (1) event free (no CHD, stroke or type 2 diabetes mellitus), (2) non-fatal CHD, (3) non-fatal stroke, (4) type 2 diabetes mellitus, (5) death related to CVD and (6) death from non-CVD causes, each of which had defined annual risks of moving to another state. Data on the RRs (Relative Risks) of developing each disease condition were estimated from epidemiological studies measuring baseline PA (exposure) and related to subsequent onset of CHD, stroke or type 2 diabetes mellitus (outcomes) over a 10-year period.
Although PA can change over time, this was not explicitly modelled, as the impact of changing habits is captured in the cohort RR estimates. Three Finnish studies211–213 followed up people who were either inactive or active for a number of years, and found that the relationship between activity and outcomes somewhat diluted as people moved in and out of PA over time.
The model assumed that a proportion of CHD and stroke events were immediately fatal, whereas this was not the case for a diagnosis of type 2 diabetes mellitus, although all of those surviving either a CVD-related event or diagnosis with type 2 diabetes mellitus faced increased CVD-related and non-CVD-related mortality risks. For simplicity, the model assumed that individuals experienced only one type of disease (although they could face multiple events within this disease).
Estimates of lifetime costs and QALYs were derived from the model through weighting time spent in each health state for different parts of the cohort by annual costs and utility values associated with each state. To these, we added the costs of intervention delivery and a short-term (1-year) gain in QALYs, through mental health improvement, arising from participation in PA (see Pavey et al. 132 for estimation).
Data to populate the model were derived from a variety of sources:
-
effectiveness and cost of brief advice (to increase PA) delivered in primary care – from systematic literature review and meta-analysis
-
cost and utility estimates of disease conditions – economic evaluations conducted for existing guidelines for CVD and diabetes mellitus, and science-based guidelines on PA and health; rates of disease were converted to probabilities214
-
relative risks of each disease for physically active people and inactive people were based on cohorts that were followed up for 19 (CHD, stroke) and 12 (diabetes mellitus) years;211,212,215 RRs were assumed to hold for 10 years, in the base case, after which no benefit was derived
-
probabilities for developing disease conditions in inactive people were derived by adjusting the UK general population age-specific incidence rates216–218 using the attributable risk fraction;219 probabilities for active people used the RR data211,212,215
-
the probability that a primary stroke or CHD event was fatal216 was assumed to be independent of PA, as a result of a lack of data
-
probability of CVD and non-CVD mortality – RRs of stroke and CHD220 and type 2 diabetes mellitus216 mortality among people with stroke, CHD or type 2 diabetes mellitus were used to adjust age-specific probabilities for ‘healthy people’, as represented in UK interim life tables.
Deterministic sensitivity analyses explored the effectiveness of the intervention, the health impacts of PA, the starting age of the cohort, the discount rate and costs. Uncertainties around all parameters in the model (except for baseline mortality) were addressed simultaneously using PSA (with 10,000 Monte Carlo simulations).
The main results concluded that additional QALYs through brief advice could be bought at an average cost of £1730 for a cohort of 100,000 people from the age of 33 years over their remaining lifetime. Conclusions that this was a ‘good buy’ only changed when the assumption of short-term QALYs gained was dropped, when the cost-effectiveness ratio fell to £27,000 per QALY. Further details can be seen in Anokye et al. 130
| Parameter | Value (95% CI) | Source of data |
|---|---|---|
| RRs of | ||
| Becoming active (at year 1) | This study (PACE-UP) | |
| Postal delivery vs. control | 1.8 (1.4 to 2.3) | |
| Nurse support vs. control | 1.7 (1.3 to 2.2) | |
| Nurse support vs. postal delivery | 0.9 (0.7 to 1.3) | |
| Disease (active vs. inactive) | ||
| CHD | 0.90 | Hu et al. (2003)211 |
| Stroke | 0.86 | Hu et al. (2005)212 |
| Diabetes mellitus | 0.67 | Hu et al. (2007)213 |
| Non-CVD-related mortality after: | ||
| Non-fatal CHD | 1.71 | Brønnum-Hansen et al. (2001)220 |
| Non-fatal stroke | 1.71 | |
| Diabetes mellitus | 1.49 | Pries et al. (2009)221 |
| CVD-related mortality after: | ||
| Non-fatal CHD | 3.89 | Brønnum-Hansen et al. (2001) |
| Non-fatal stroke | 3.89 | |
| Diabetes mellitus | 2.61 | Pries et al. (2009)221 |
| Fatality cases (%) | ||
| CHD | ||
| 59–64 | 11.55 | Ward et al. (2005)222 |
| 65–74 | 21.07 | |
| ≥ 75 | 14.76 | |
| Stroke | ||
| 55–64 | 23.28 | Ward et al. (2005)222 |
| 65–74 | 23.47 | |
| ≥ 75 | 23.42 | |
| Incidence rates for (%) | ||
| CHD | ||
| 59–64 | 0.63 | Ward et al. (2005); National Clinical Guideline Centre (2011)223 |
| 65–74 | 0.97 | |
| ≥ 75 | 0.97 | |
| Stroke | ||
| 59–64 | 0.29 | |
| 65–74 | 0.69 | |
| ≥ 75 | 1.43 | |
| Diabetes mellitus | ||
| 59 | 0.06 | Gonzalez et al. (2009)224 |
| 60–69 | 0.10 | |
| 70–79 | 0.11 | |
| ≥ 80 | 0.11 | |
| Quality of life | ||
| Age-specific quality of life | ||
| 59–64 | 0.82 | Health Survey for England (2011)225 |
| 65–74 | 0.78 | |
| ≥ 75 | 0.72 | |
| Health state utility weight | ||
| Healthy | 1.00 | Ward et al. (2005); National Clinical Guideline Centre (2011)222,223 |
| CHD (first event) | 0.80 | |
| Post CHD (first event) | 0.92 | |
| Stroke (first event) | 0.63 | |
| Post stroke (first event) | 0.65 | |
| Diabetes mellitus | 0.90 | |
| Short-term psychological benefit of achieving 150 minutes of MVPA per week | 0.01 | This study (PACE-UP) |
| Annual cost (£) | ||
| Control | 467 (365 to 569) | This study (PACE-UP) |
| Post | 376 (307 to 445) | |
| Nurse | 593 (473 to 714) | |
| CHD (first event) | 4248 | National Clinical Guideline Centre (2011)223 |
| Post CHD (first event) | 485 | |
| Stroke (first event) | 10,968 | |
| Post stroke (first event) | 2409 | |
| Diabetes mellitus | 979 | |
FIGURE 21.
Cost-effectiveness acceptability curve showing the probability of lifetime cost-effectiveness for the nurse support group compared with the postal delivery group at different willingness-to-pay threshold levels.
Appendix 4 Generalisability
Adapted from Normansell et al. 148 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Telephone interview schedule for non-participants
Introduction:
Clarify purpose of interview, gain verbal consent and confirm anonymity and confidentiality.
Opening questions:
-
What did you think of the information that we originally sent you about the PACE-UP study?
-
Can you tell me a bit more about what influenced your decision not to take part in the study?
-
Did you discuss participating in research with anyone else?
Reasons for not participating:
Using the completed questionnaire, explore the reasons already given, including:
-
I do not have time
-
I cannot/am not interested in increasing my physical activity
-
I am already very physically active
-
I am not interested in research
-
I do not want to be put in a group by chance.
Additional possible reasons for not-participating:
Offer a number of other predefined reasons for non-participation and explore further any positive responses:
-
Lack of time.
-
Unable or nor interested in increasing PA.
-
Already active.
-
Not interested in research.
-
Do not want to be put in a group by chance.
-
Length of programme.
-
Travel difficulties.
-
Wearing a physical activity monitor.
-
Unpleasant/unsafe walking environment.
-
Programme is not relevant to you.
-
Programme is not for your age group.
-
Programme would clash with work/being away from home.
-
Medical problems prevent participation.
Trial design questions:
-
Venue.
-
Exercise type.
-
Group activity.
-
Anything else that would have facilitated participation?
End:
Summary and invite any final comments.
Non-participant health and physical activity survey
Appendix 5 Process evaluation
| Training event | Time spent in minutes | Total, time spent in minutes | |||
|---|---|---|---|---|---|
| PA guidance | Trial protocols | Safety reporting | BCT | ||
| Pre-trial visit | 20 | 30 | 0 | 0 | 50 |
| Pre-trial reading | 20 | 10 | 10 | 20 | 60 |
| Nurse reflection | 15 | 0 | 0 | 15 | 30 |
| Training day – 13 September 2012 | 10 | 40 | 30 | 250 | 330 |
| Training half-day – 18 January 2013 | 0 | 60 | 10 | 95 | 165 |
| Training half-day – 21 May 2013 | 10 | 40 | 10 | 60 | 120 |
| Training half-day – 24 September 2013 | 10 | 60 | 10 | 95 | 175 |
| BCT trainer individual feedback | 0 | 0 | 0 | 30 | 30 |
| Total time (minutes) | 85 | 240 | 70 | 565 | 960 |
| Total time (hours and minutes) | 1 hour 25 minutes | 4 hours 0 minutes | 1 hour 10 minutes | 9 hours 25 minutes | 16 hours |
| Fidelity item | Session | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Attended session, n/N (%) | 330/346 (95) | 296/346 (86) | 263/346 (76) |
| Mean number of items completed (range) | 11 (10–11) | 6 (5–6) | 6 (5–6) |
| Participant reported that they had used pedometer and diary ‘every day’ or ‘sometimes’, n/N (%) | 285/296 (96) | 258/263 (98) | |
| Diary return and use | Trial arm, n (%) | |
|---|---|---|
| Postal delivery group (N = 339) | Nurse-support group (N = 346) | |
| Number of diaries returned | 268 (79) | 281 (81) |
| Targets altered | 4 (1) | 89 (32) |
| Target increased | 0 (0) | 9 (3) |
| Target decreased | 4 (1) | 80 (29) |
| Used pedometer during the 12-week intervention | Trial arm, n (%) | |
|---|---|---|
| Postal delivery group (N = 294) | Nurse-support group (N = 303) | |
| Every day or most days | 238 (81) | 269 (89) |
| A few days or occasionally | 44 (15) | 30 (10) |
| Never | 12 (4) | 4 (1) |
| Session | Nurse self-report | Audio-recording | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Participants | Mean time in minutes (SD) | Range (minutes) | Median time in minutes (IQR) | Number of Participants | Mean time in minutes (SD) | Range (minutes) | Median time in minutes (IQR) | |
| 1 | 320 | 30 (4) | 10–55 | 25 (20–30) | 10 | 21 (6) | 12–29 | 22 (15–26) |
| 2 | 211 | 24 (3) | 7.5–45 | 20 (15–25) | 7 | 21 (7) | 10–29 | 23 (17–24) |
| 3 | 256 | 22 (4) | 5–60 | 20 (15–25) | 5 | 14 (5) | 9–21 | 12 (10–16) |
Nurse and patient alliance questionnaires
Nurse session checklists
Appendix 6 Qualitative evaluation
Initial thoughts for nurse focus group: interview schedule
Introductions
Introduce ourselves. Explain one will lead while the other takes notes, just in case the recording fails, etc.
The main point of this session is to find out what it was like to be involved in this study and to help patients to increase their physical activity using this particular method and this schedule of visits. It’s important that you tell us what it was like, warts and all – so that we can let the team know what went well and what could have been better both for you and for your study patients. It’s also important that we have all of your views – so if you disagree with what someone has said, then make sure we hear your perspective too. Hopefully, it will be a discussion between all of you, with us just throwing in a few questions to keep things going. OK?
Anonymity and confidentiality. Now say we won’t use their names if we extract something they said for a paper – also, nobody but us will know who said what, and it won’t be passed on to other members of the team.
Tell them we’re turning on the recorders.
Ask the nurses to introduce themselves for the recording.
PTO (please turn over) for the schedule itself.
The schedule
First of all, the training:
-
What stood out most for you from the actual training sessions?
-
What specific parts of the training do you recall as being particularly useful? (Challenge if they say ‘all of it!’ – must be precise).
-
Was anything less useful or could be improved?
-
Would you have liked anything more in the way of training or materials?
-
What do you feel about the number of training sessions? (too many/not enough/about right?)
-
What did you feel about the balance of the training sessions between communication/behaviour change techniques and practical trial aspects (physical activity guidelines/using pedometers/handbooks/reporting adverse events etc.).
-
-
Did your physical activity consulting change as a result of the training or from being involved in the trial?
-
If so, how? If not, why not?
-
Have any other aspects of your work changed?
-
Moving on to the nurse handbook and patient handbook and diary.
-
What was it like using the handbook/diary? (remind the nurses about the handbook/diary by showing it to them – have one copy to look at together, or else it will turn into individual silent reading sessions).
-
How did you find using it?
-
How did the patients find it? What were the best bits? Which bits caused most difficulty? How did you get round this?
-
How could the handbook be improved?
-
And now the pedometer and setting targets.
-
What about using the pedometer?
-
How easy was it to explain to people how to use it?
-
How common were difficulties with the pedometer? What kind of difficulties did people have?
-
Were most people happy to wear the pedometer while coming to see you & keep a step-count record?
-
Were targets that we had suggested realistic for most people? Did a lot of people change their targets? If people changed them did they tend to set higher or lower targets?
-
Patient engagement.
-
How acceptable did patients find the intervention?
-
Were any patients more responsive to the PA intervention than others?
-
– What were the characteristics of someone who really ‘went for it’?
-
– What about the characteristics of someone who really didn’t get on with it?
-
– Did anyone say anything to you that hinted at why they didn’t like it?
-
– Did you have experience of working with couples in the trial? How did you find this? What were the positive aspects? And the negative?
-
-
The trial protocol.
-
How about the trial protocol – the schedule for seeing patients (3 visits a month apart, first visit approximately 30 minutes, others approximately 20 minutes).
-
Was it possible to do what was required of you in the time prescribed?
-
Were there enough sessions/too many?
-
Did most patients actually get the 3 visits at the right time? How did it vary between patients?
-
Did you have problems with non-attenders? How did you manage this?
-
If things went wrong, how easy was it for you to get help/support from the study team?
-
How did you feel about having some of your sessions recorded? Was recording them or receiving feedback on the sessions helpful?
-
-
Some of you are also involved in NHS health checks at your practices, do you see this intervention as something that could be useful for those identified in health checks as needing to increase their physical activity levels?
-
If yes, how could this work? If no, why not?
-
-
From the nurse perspective, if we were to do the trial again with different practices, or try to put the intervention into your routine practice . . .
-
What would be the main things to keep?
-
The main things to change?
-
-
And from the patients’ perspective, as far as you can tell . . .
-
What would be the main things to keep?
-
The main things to change?
-
-
Anything else that you think we have missed / that you want to tell us?
| ID | Sex | Self-reported ethnicity | Group | Age (years) | Change in average steps per day from baseline to 12 months |
|---|---|---|---|---|---|
| 1 | Female | Any other white background | Nurse | 48 | +1697 |
| 2 | Male | White British | Nurse | 45 | +113 |
| 3 | Male | White British | Pedometer | 53 | +3708 |
| 4 | Male | Bangladeshi | Pedometer | 52 | –234 |
| 5 | Female | White British | Pedometer | 57 | +1718 |
| 6 | Female | White British | Pedometer | 51 | –2141 |
| 7 | Female | White British | Pedometer | 60 | –1808 |
| 8 | Female | White British | Pedometer | 65 | –1781 |
| 9 | Female | Black Caribbean | Pedometer | 69 | +243 |
| 10 | Male | Black African | Nurse | 64 | –1920 |
| 11 | Male | White British | Pedometer | 70 | +1543 |
| 12 | Female | White and black Caribbean | Nurse | 66 | +1211 |
| 13 | Female | White British | Pedometer | 66 | –446 |
| 14 | Female | Any other white background | Nurse | 49 | +4756 |
| 15 | Female | Any other white background | Nurse | 49 | –1097 |
| 16 | Female | White British | Nurse | 47 | +1573 |
| 17 | Female | Any other white background | Pedometer | 66 | –1027 |
| 18 | Female | White British | Nurse | 62 | –2836 |
| 19 | Female | White British | Pedometer | 66 | –1797 |
| 20 | Male | White British | Nurse | 52 | +3924 |
| 21 | Female | Black African | Nurse | 47 | +2962 |
| 22 | Male | White British | Nurse | 63 | –2652 |
| 23 | Female | White British | Pedometer | 64 | +226 |
| 24 | Female | Any other white background | Pedometer | 50 | +1031 |
| 25 | Male | White British | Pedometer | 67 | –955 |
| 26 | Female | White British | Nurse | 65 | –2013 |
| 27 | Male | White and Asian | Pedometer | 61 | –611 |
| 28 | Female | Chinese | Nurse | 72 | +4062 |
| 29 | Male | White British | Nurse | 59 | –493 |
| 30 | Female | White British | Nurse | 51 | +3269 |
| 31 | Male | White British | Pedometer | 59 | –756 |
| 32 | Female | White British | Nurse | 63 | +1966 |
| 33 | Female | White British | Nurse | 49 | –746 |
| 34 | Female | Black Caribbean | Pedometer | 73 | +403 |
| 35 | Female | White British | Nurse | 64 | +2100 |
| 36 | Female | White British | Pedometer | 64 | +1639 |
| 37 | Female | Indian | Pedometer | 51 | –1720 |
| 38 | Female | White British | Pedometer | 59 | +539 |
| 39 | Female | White British | Nurse | 61 | –1425 |
| 40 | Male | White British | Nurse | 48 | –3826 |
| 41 | Male | White British | Nurse | 65 | –43 |
| 42 | Male | White British | Pedometer | 72 | –2133 |
| 43 | Female | White British | Pedometer | 48 | +2253 |
Appendix 7 Three-year follow-up
Qualitative interview schedule: intervention group 3-year follow-up
Introduction:
-
Introduce self, explain you are calling from PACE-UP trial, confirm the name of the person.
-
Remind of initial consent to be approached for an interview in the 3-year follow-up consent letter.
-
Explain this is a telephone interview to discuss in a bit more detail their physical activity experiences since being involved in the trial. It should take no more than 20–25 minutes but explain that if they have lots of things they would like to feedback, then you have more time.
-
If participant happy to continue, thank them for their participation.
-
Explain that you would like to record the interview with their permission.
(Start recorder)
-
Explain you are now going to say their participant number for the benefit of the tape and ask them just to verbally confirm again that they happy to be interviewed and for a recording to be made.
-
Explain that everything they say will be kept confidential and any comments they make will be linked to an anonymous number rather than a name.
-
Explain that if the participant wants to stop the interview at any time it’s not a problem at all and to just let the interviewer know.
Main questions:
-
Can you tell me about what physical activity you did last week? Was that a typical week for you?
-
Do you think taking part in the PACE-UP trial has changed the physical activity you are doing now?
-
Is there anything about the PACE-UP trial that you particularly remember? i.e. take home message.
-
Do still you use the pedometer, diary or handbook given to you after the PACE-UP trial? If so, how often do you use them? If no, do you use anything else? i.e. phone, Fitbit.
-
What normally motivates you to be physically active? Is that different to how it was before you participated in PACE-UP?
-
Would you recommend the PACE-UP trial to family and friends?
-
Are there any additional resources or support that you could suggest that might help to keep you physically active? i.e. family, friends, text messages, online resources, annual visit to nurse, walking groups . . .
Closing:
That was all the questions I had for you, is there anything else you would like to add or think I’ve missed?
Thank you for taking the time out to answer our questions, as a token of our thanks we will be sending a £10 high-street voucher to you within the next week.
Prompts
Pedometer use
-
In what way do you think the pedometer influences your physical activity?
Barriers to being physically active
-
Are there any barriers or difficulties you’ve had to overcome when it comes to physical activity?
Motivation
-
Do you set yourself any physical activity targets/goals? If yes, prompt more.
-
Do you adopt any strategies to help you stay motivated to be physically active? If yes, prompt more.
Peer/social support and physical activity
-
Under question 5 if not discussed.
Qualitative interview schedule: control group 3-year follow-up
Introduction:
-
Introduce self, explain you are calling from PACE-UP trial, confirm the name of the person.
-
Remind of initial consent to be approached for an interview in the 3 year follow-up consent letter.
-
Explain this is a telephone interview to discuss in a bit more detail their physical activity experiences since being involved in the trial. It should take no more than 20–25 minutes but explain that if they have lots of things they would like to feedback, then you have more time.
-
If participant happy to continue, thank them for their participation.
-
Explain that you would like to record the interview with their permission.
(Start recorder)
-
Explain you are now going to say their participant number for the benefit of the tape and ask them just to verbally confirm again that they happy to be interviewed and for a recording to be made.
-
Explain that everything they say will be kept confidential and any comments they make will be linked to an anonymous number rather than a name.
-
Explain that if the participant wants to stop the interview at any time it’s not a problem at all and to just let the interviewer know.
Main questions:
-
Can you tell me about what physical activity you did last week? Was that a typical week for you?
-
Do you think taking part in the PACE-UP trial has changed the physical activity you are doing now?
-
Is there anything about the PACE-UP trial that you particularly remember? i.e. take home message.
-
What normally motivates you to be physically active? Is that different from how it was before you participated in PACE-UP?
-
As a participant in the study you will have received a pedometer, diary and handbook after the main trial was over. Did you find these resources helpful? Have you continued to use any of these resources? Do you use anything else? i.e. phone, Fitbit . . .
-
Would you recommend the PACE-UP trial to family and friends?
-
Are there any additional resources or support that you could suggest that might help to keep you physically active?’ i.e. family, friends, text messages, online resources, annual visit to nurse, walking groups . . .
Closing:
That was all the questions I had for you, is there anything else you would like to add or think I’ve missed?
Thank you for taking the time out to answer our questions, as a token of our thanks we will be sending a £10 high-street voucher to you within the next week.
Prompt sheet
Pedometer use
-
In what way do you think using a pedometer influences your physical activity?
Barriers to being physically active
-
Are there any barriers or difficulties you’ve had to overcome when it comes to physical activity?
Motivation
-
Do you set yourself any physical activity targets/goals? If yes, prompt more.
-
Do you adopt any strategies to help you stay motivated to be physically active? If yes, prompt more.
Peer/social support and physical activity
-
Under question 5 if not discussed.
Three-year health and lifestyle questionnaire
Appendix 8 Discussion
What is the potential benefit of our intervention on coronary heart disease and all-cause mortality?
Several systematic reviews have assessed the health benefits of walking based on pooling data from cohort studies. Typically, the RRs are 0.8 in people who are physically more active compared with those who are much less active. The difficulty of interpreting such analyses is their focus on comparing two extreme groups: the physically active versus those who are inactive. Zheng et al. 197 recognised the importance of studying the functional form of the dose–response effect of walking on the risk of developing CHD. They concluded that the risk of developing CHD decreases as the amount of brisk walking increases. 197 Specifically, Zheng et al. 197 concluded that 150 minutes of brisk walking per week reduces the incidence of CHD by 19% (a RR of 0.81, 95% CI 0.77 to 0.86); RR estimates were similar in both sexes and in older and younger subjects. From this, we can estimate (see below*) that the increase of 33 minutes per week in the postal delivery group in our study at 12 months would be expected to reduce the participants’ risk of developing CHD by 4.5% (95% CI 3.3% to 5.6%) if sustained. In a prospective study assessing the benefits of walking in a free-living population sample,198 it was found that a higher daily step count measured by pedometer was linearly associated with reductions in all-cause mortality. Using the same method (see below**) we estimate that the 643 step increase in our postal delivery group would result in a 4% (95% CI 1% to 7%) decrease in mortality.
*From the paper by Zheng et al. ,197 we can take the fact that log (risk) increases linearly with minutes of MVPA and that increasing MVPA per week by 150 minutes reduces the risk by 19% (a RR of 0.81), to estimate that increasing MVPA by 33 minutes per week would result in a RR of 0.81(33/150) = 0.810.22 (95% CI 0.770.22 to 0.860.22) = 0.955 (95% CI 0.944 to 0.967). That is a 4.5% (95% CI 3% to 6%) reduction in the risk of developing CHD.
**From the paper by Dwyer et al. ,198 the adjusted hazard ratio for all-cause mortality associated with an additional 1000 steps was 0.94 (95% CI 0.90 to 0.98). Using the same approach as above, an increase of 642 steps is estimated to reduce the risk by 0.94(642/1000) = 0.94(.642) (95% CI 0.90(.642) to 0.98(.642)) = 0.96 (95% CI 0.93 to 0.99). That is a 4% (95% CI 1% to 7%) reduction in all-cause mortality.
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- BCT
- behaviour change technique
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CVD
- cardiovascular disease
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- GPPAQ
- General Practice Physical Activity Questionnaire
- HADS
- Hospital Anxiety and Depression Scale
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IPAQ
- International Physical Activity Questionnaire
- MRC
- Medical Research Council
- MVPA
- moderate to vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NS-SEC
- National Statistics Socio-economic Classification
- OR
- odds ratio
- PA
- physical activity
- PACE-LIFT
- Pedometer Accelerometer Evaluation-LIFT
- PACE-UP
- Pedometer And Consultation Evaluation-UP
- PAI
- physical activity index
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- TMG
- trial management group
- TSC
- Trial Steering Committee