Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/22/50. The contractual start date was in October 2011. The draft report began editorial review in August 2016 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Elizabeth Ball declares UK travel reimbursement from Shire Medical (Lexington, MA, USA) outside the submitted work. Jonathan J Deeks was Deputy Chairperson of the National Institute for Health Research Health Technology Assessment (HTA) Commissioning Board (2011–16) and the HTA Efficient Study Designs Board (2016).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Khan et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Definition and prevalence of chronic pelvic pain
Chronic pelvic pain (CPP) may be defined as pain in the pelvic and lower abdominal region that lasts for 6 months or longer. 1 Idiopathic CPP is defined as CPP with an uncertain or unknown structural cause, that is, CPP that is unrelated to gynaecological organs, after the exclusion of other recognisable gynaecological pathology. 2 Symptoms include dysmenorrhoea (painful periods), dyspareunia (pain during sexual intercourse), dyschesia (painful bowel motions), dysuria (painful micturition) and constant or intermittent pelvic pain, which may or may not be related to the menstrual cycle. 3
In primary care, the annual prevalence of CPP is 38 out of 1000 in women aged 15–73 years,4 a rate comparable with that of asthma (37/1000) and chronic back pain (41/1000). 5,6 Unusually, no effective management policy exists for CPP. Only 20–25% of women respond to conservative treatment. 7 CPP remains the single most common indication for referral to a gynaecology clinic, accounting for 20% of all outpatient appointments. 8
Personal and societal costs of chronic pelvic pain
Chronic pelvic pain can have a substantial negative effect on a woman’s quality of life (QoL). Women with CPP tend to report reduced general physical health scores than those without pain. 9,10 Women with CPP describe loss, social isolation and effects on relationships. They have a high incidence of comorbidity, sleep disturbance and fatigue. 3 They tend to cope outside the health system and usually do not see a health-care provider. 8 Pain affects daily activities; around 18% of employed women in the UK take at least 1 day off work each year because of such pain. 9 The annual direct cost of health care for women with CPP was estimated to be £158M, with a further £24M in indirect costs, in 1992. 11 More recent data for endometriosis estimated that the total annual societal burden of endometriosis-related symptoms was approximately £8.2B, with 1.5 million women affected. 12
Pathogenesis of chronic pelvic pain
The pathogenesis of CPP is poorly understood. CPP may be a symptom of a single or multiple coexisting structural pathologies. The source of the pain can be either visceral (including the reproductive, urinary and gastrointestinal tracts) or somatic (including the pelvic bones, ligaments, muscles and fascia). The most recent taxonomy of pain from the International Society for the Study of Pain (ISSP) lists conditions such as endometriosis, fibroids, adenomyosis, cystic ovaries and pelvic inflammatory disease (PID) as pathological causes of CPP. 13 Adhesions, frequently associated with endometriosis and pelvic congestion syndrome, are not defined within the ISSP taxonomy and, indeed, the relationship between CPP and pelvic vein insufficiency is controversial. 14 The severity of pain may not be related to the severity of the underlying pathology, as illustrated by endometriosis, when the stage of disease is poorly correlated with reported pain. 15 Pelvic pain may not be gynaecological in origin, however. Musculoskeletal dysfunction can include muscle laxity, manifesting as pelvic organ prolapse, muscle spasms (which can potentially lead to nerve entrapment) and trigger points (hyperirritable regions within skeletal muscle). Finally, pelvic pain may have origins in the bowel or the bladder. Irrespective of the underlying cause, women with CPP are more likely to have structural and functional changes to the central nervous system, and an increased risk of psychological distress and dysfunctional stress responses, than pain-free control women. 16
A significant proportion of women with CPP appear to have no obvious underlying pathology identified during laparoscopy. 17 Differences in the clinical thresholds for performing laparoscopy may vary over time or location, but data from the UK-based LUNA (Laparoscopic Uterosacral Nerve Ablation) trial17 showed that clinicians did not identify any condition at laparoscopy in 54% of participants. In these cases, the CPP may have had an unknown cause that was not related to gynaecological organs and could be referred to as ‘idiopathic’. The ISSP and European Association of Urology (EAU) define a subgroup of CPP, in which no obvious disease is found, to be CPP syndrome. 13,18 Endometriosis-associated pain, vulval pain, bladder pain, primary dysmenorrhea and CPP with cyclical exacerbations are differentiated as subtypes of CPP syndromes by the ISSP. The EAU notes that, when pain is localised to a single organ, some specialists designate the pain accordingly, for example bladder pain syndrome, but when pain is localised to more than one site, the term ‘CPP syndrome’ should be used. Categorisation of the symptoms of idiopathic CPP syndrome into functional and psychological categories may then be appropriate. In a proportion of women, there may be a psychosomatic component, and psychological symptoms may be both causative and associative.
For this study, we used a working definition of idiopathic CPP with an uncertain or unknown structural cause after the exclusion of any other recognisable gynaecological pathology. This was derived by a nominal group method using an expert independent panel (EIP). A diagnosis of idiopathic CPP was arrived at once all other organic causes of pain were excluded by various diagnostic technologies or empirical treatment. 2
Variation in the presentation of chronic pelvic pain
Symptoms experienced in CPP are variable and non-specific, so establishing a differential diagnosis can be hard. With this chronic condition, women present repeatedly over several years. 19 It is possible in these cases that a previously diagnosed condition (e.g. endometriosis) has recurred or a new condition has developed (e.g. depression in a woman previously diagnosed with endometriosis). At present, there is wide variation in clinical practice concerning the diagnosis and management of CPP. 20 Women go from pillar to post, seeing several health professionals before eventually having their underlying condition identified. This wastes both the patient’s time and NHS resources. The diagnosis of endometriosis may be delayed by over 8 years after first presentation with CPP symptoms,19,21,22 potentially demoralising the patient and missing the opportunity to improve their QoL and fertility through early effective treatment.
Diagnosis of the cause of chronic pelvic pain
A troublesome clinical issue is the lack of accurate tools to efficiently diagnose and direct cases of women. 23 The Royal College of Obstetricians and Gynaecologists (RCOG) guidelines on the management of CPP provide a number of suggested initial investigations, including history, microbiological screening and vaginal examination, all with weak evidence (levels B or C) for recommendations. 20 If no cause of pain is found, the first port of call is often to perform a diagnostic laparoscopy.
Clinical assessment and ultrasound
The order of tests in current practice can vary between clinicians and according to presentation, but the RCOG’s clinical guidelines20 suggest that a clinical history is taken and a vaginal examination is performed. When there is suspicion of PID or a potential need, screening for chlamydia and gonorrhoea in women aged < 25 years may be performed. A transvaginal ultrasound may be performed,24 followed by a laparoscopy under general anaesthetic.
Laparoscopy
Diagnostic laparoscopy is a surgical procedure that allows a clinician to view the contents of the abdomen or pelvis to inform a diagnosis of CPP. A therapeutic laparoscopy is a surgical procedure used by a clinician to treat various conditions that may cause CPP. Endometriosis, pelvic adhesions, chronic PID and ovarian cysts are often observed via laparoscopy in women with CPP. 8 In a cohort of 487 women recruited into a trial of neuroablation for CPP, 54% of women had no identifiable pathology at laparoscopy, whereas 31% had endometriosis, 5% had PID and 17% had adhesions. 17 Approximately 11% had more than one finding. Those women with moderate to significant pathology were excluded from this trial. This is in broad agreement with other surveys. 8
The role of laparoscopy in the differential diagnosis of chronic pelvic pain
There is evidence to suggest that deep-infiltrating endometriosis, bladder pain syndrome/interstitial cystitis25 and irritable bowel syndrome26,27 are causally related to CPP, whereas, for adhesions, there is fair evidence. The associations between severe endometriosis and pelvic pain, and between endometriosis in general and infertility, are confirmed. However, there is little or no association between minimal endometriosis, pelvic adhesions or dilated pelvic veins and pain. 28 Debate remains over the relation between observed superficial peritoneal endometriosis and the extent of the laparoscopic finding,29 or with the degree of reported pain. 28
A combination of the non-invasive tests for the diagnosis of endometriosis can sometimes provide insufficient or poor-quality evidence. 30 However, of all conditions associated with CPP, many are not amenable to either laparoscopic diagnosis or treatment. Of those conditions for which laparoscopy has a role, most are managed by gynaecologists, and this probably explains the common use of laparoscopy for evaluating women with CPP. Over 40% of laparoscopies are performed solely as a diagnostic test to identify the causes of CPP. 31 The decision to perform a laparoscopy should be based on the history, physical examination and ultrasound scans suggesting a visceral origin.
The accuracy of laparoscopy in the diagnosis of chronic pelvic pain
The value of laparoscopy as a diagnostic tool for CPP has been considered in several papers,8,31,32 including a review of published reports of laparoscopically diagnosable conditions, in which an average of 61% of women undergoing laparoscopy for CPP had an identifiable pathology, compared with pathology in 28% of those without CPP. 8 However, invisible (occult) endometriosis can be present in a seemingly normal peritoneum. 33 A ‘negative’ laparoscopy for some women may lead to them feeling disappointed that no diagnosis has been made34 and disengage them from the care pathway. 35
However, a visual diagnosis of endometriosis during laparoscopy has been demonstrated to be unreliable. Only 54–67% of suspected endometriotic lesions are confirmed histologically, and 18% of women clinically suspected to have endometriosis have no evidence of endometriosis in biopsy samples. 36 A meta-analysis found that a positive finding through the use of laparoscopy will not be verified by histology in half of the cases (assuming a prevalence of 20%), although a negative laparoscopy is highly accurate for excluding endometriosis. 37 Other conditions identifiable through laparoscopy are listed in Table 1.
| Target conditions | Diagnostic criteria | Diagnosis by | |
|---|---|---|---|
| MRI | Laparoscopy | ||
| Endometriosis | Visualisation of endometriotic lesions with histological confirmation of biopsies from lesions37 |
Uncertain. MRI had a sensitivity of 85% and a specificity of 89% for detecting biopsy-proven endometriosis38 MRI had a sensitivity and a specificity for diagnosis of rectovaginal deep-infiltrating endometriosis of 55% and 99%, respectively. Sensitivity for other locations was considerably higher, for example, on uterosacral ligaments it was 85%39 |
Negative laparoscopy was accurate for excluding endometriosis (pooled LR –0.06, 95% CI 0.01 to 0.47) compared with biopsy, but positive findings were not as accurate (pooled LR 4.30, 95% CI 2.45 to 7.55)37 |
| Adhesions | Visualisation, directly or by absence of movement between adjacent organs | Evidence from a single trial: sensitivity of 88% and specificity of 93%40 | Gold standard |
| Chronic PID | Laparoscopic visualisation or histology from fimbrial mini biopsy | Ultrasonography represents preferable initial non-invasive diagnostic method. MRI sensitive for tubal, ovarian and pelvic abscesses41 | Can confirm tubo-ovarian abscesses and the presence of Fitz-Hugh–Curtis syndrome, but may fail to detect early disease or those with endosalpingitis only42,43 |
| Adenomyosis | Presence of diffuse endometrial tissue in myometrium at post-hysterectomy biopsy | MRI had a pooled sensitivity of 77% and a specificity of 89% against histological diagnosis44 | Uncertain. May observe bulky uterus |
Magnetic resonance imaging in the diagnosis of chronic pelvic pain
Advances in imaging techniques suggest that magnetic resonance imaging (MRI) may be a useful non-invasive tool for diagnosing some conditions that cause CPP, such as adenomyosis44 and deep-infiltrating endometriosis. 45 MRI has an established role in the diagnosis of adenomyosis and is currently the most accurate available non-invasive test in use. 46 The role of MRI in diagnosing small deposits of endometriosis20 is less established. A Cochrane review reported that no imaging techniques met the criteria for a replacement or triage test for detecting superficial pelvic endometriosis. 30 Several factors contribute to this poor accuracy of MRI: non-pigmented lesions will not be hyperintense on T1 scans, small focal lesions may have variable signal intensity, plaque-like lesions are hard to delineate and adhesions cannot be directly identified, but are implied when the normal anatomy is distorted. Other conditions, such as fibroids and congenital uterine anomalies, may co-exist and are accurately diagnosed with MRI. 47
Magnetic resonance imaging is currently not recommended in guidelines nor used in routine practice for the investigation of CPP; the important question is whether or not a normal MRI scan has a high enough negative predictive value to replace and avoid laparoscopy. In CPP women, MRI may potentially have an important role in eliminating the need for surgery.
There are currently no agreed standardised protocols for MRI of the pelvis in the evaluation of pelvic pain. Radiologists determine the protocol used based on the clinical information and the suspected pathology, as well as personal preference and experience. Various bodies, such as the European Society of Urogenital Radiology (ESUR), have recommended protocols for pelvic assessment for various conditions,48 but none specifically addresses CPP.
Treatment of chronic pelvic pain
As diagnoses emerge through careful history and examination and directed investigation, so may treatment strategies. These should be tailored to the needs of individual women, whatever the cause. A multidisciplinary approach is considered ideal for achieving this. Chronic symptoms need long-term management and a multimodal approach.
Some clinicians prefer that a diagnostic and therapeutic investigation is performed as part of a single procedure – sometimes referred to as ‘see-and-treat’ laparoscopy. 49,50 For example, adhesiolysis may be performed in what started as a diagnostic procedure. 51 Depending on the severity and corresponding patient consent, endometriosis may be treated at the time of diagnosis by either electrocoagulation, laser vaporisation or excision. 52 Endometrioma and benign ovarian cysts are usually excised. 53 The type of treatment offered may depend on the extent of the disease and surgical expertise. Surgical removal of deep-infiltrating endometriosis is highly specialised and, in the UK, undertaken in accredited specialist centres. Preoperative MRI scans can aid the planning of this complex surgery.
Other conditions observed at laparoscopy are not amenable to immediate laparoscopic treatment, for example myomectomy for subserosal fibroids. 54 Few surgeons attempt uterine-sparing surgery for adenomyosis, as a result of it frequently being diffused throughout the myometrium.
A diagnostic laparoscopy may also provide the opportunity to undertake other investigations, such as a cystoscopy, if symptoms are suggestive of bladder or intrauterine conditions. Some gynaecologists may also use the cover of a general anaesthetic to perform investigations capable of being performed as an outpatient procedure, such as hysteroscopy or insertion of a levonorgestrel-releasing intrauterine system.
Medical treatments are also options for CPP, irrespective of surgical intervention. The combined oral contraceptive pill is used post laparoscopy for prevention of the recurrence of endometriosis, as are long-acting reversible contraceptives. 55–57 Gabapentin is used for women with no identifiable cause of pain, although evidence for its efficacy is limited. 58
Overview of the Magnetic resonance imaging for Establishing Diagnosis Against Laparoscopy study
The MRI for Establishing Diagnosis Against Laparoscopy (MEDAL) study recruited women from 26 UK hospitals (as listed in Appendix 1, Table 29). An outline of the test-accuracy study design is shown in Figure 1. MRI was undertaken before laparoscopy, but the resulting report and images were not to be provided to the gynaecologist, unless there was a critical finding, such as suspected malignancy. This blinding was necessary in order not to distort clinical practice and avoid verification bias arising from knowledge of this index test. A diagnostic laparoscopy was performed and, together with information from the history, examination and ultrasound, produced a post-laparoscopy diagnosis (Figure 2). Information was collected from those who were not eligible for the test-accuracy study. Follow-up information was elicited directly from participants at 6 months.
FIGURE 1.
The MEDAL study flow chart. IRRC, Independent Radiology Review Committee.
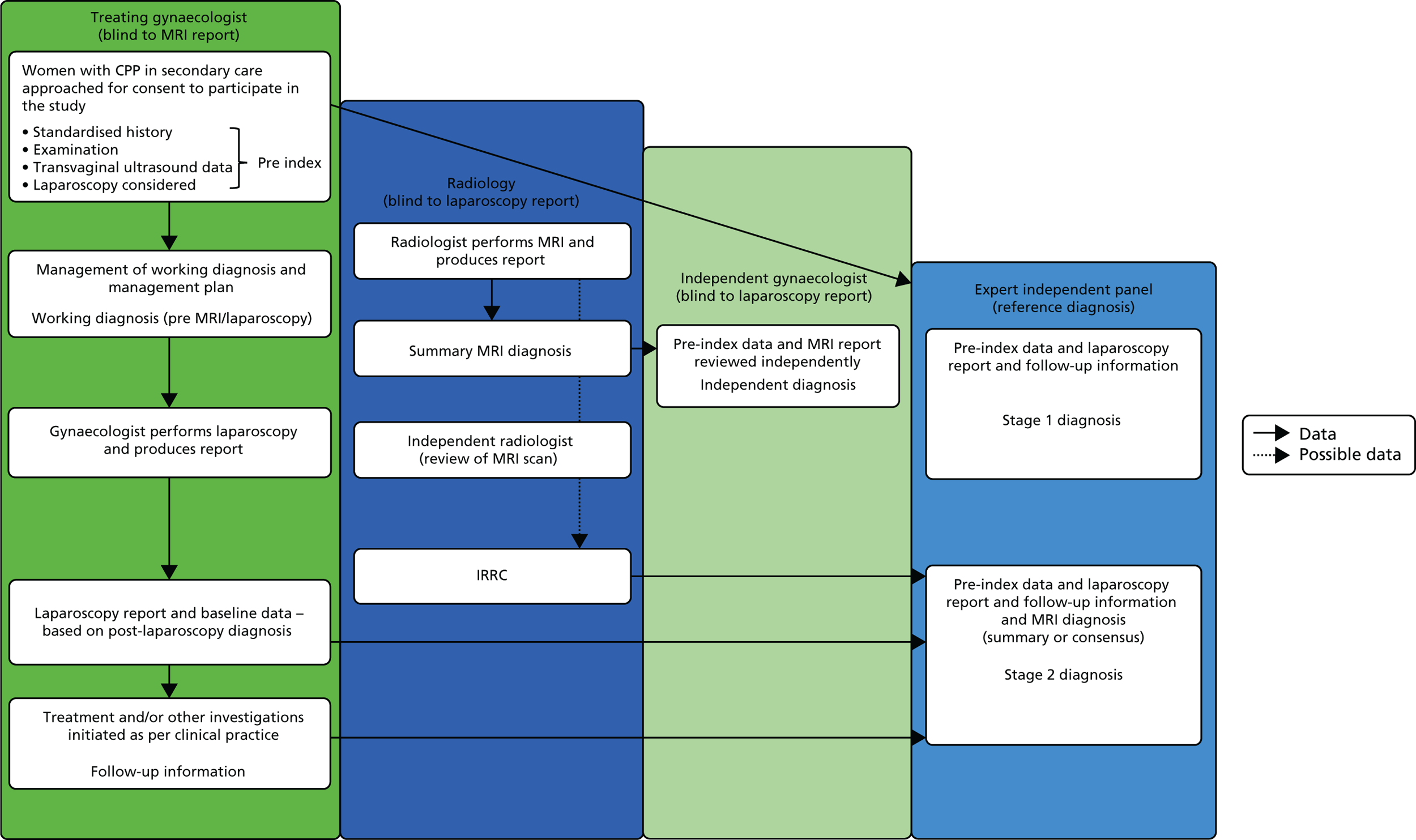
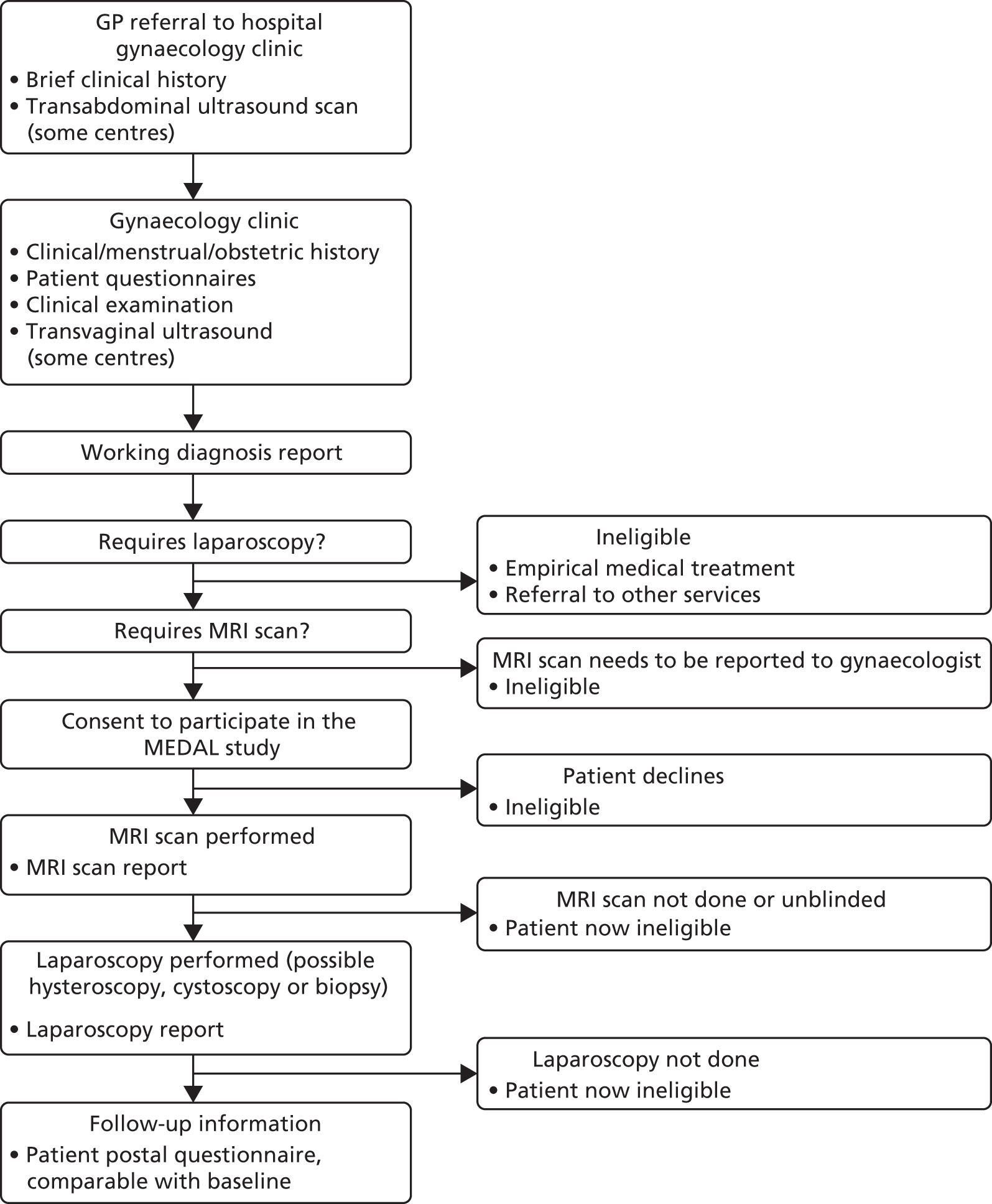
FIGURE 2.
Patient pathway and data points through the MEDAL study. GP, general practitioner.
An economic evaluation was performed to establish the cost-effectiveness of MRI as an alternative to laparoscopy for the sensitivity and specificity values of the various target conditions.
Rationale for the Magnetic resonance imaging for Establishing Diagnosis Against Laparoscopy study
There is a perception that diagnostic laparoscopy, an invasive, expensive and potentially risky procedure, is used far too frequently in the NHS, as a significant proportion of women have no pathology identified. 59 The procedure is associated with an approximate 3% risk of minor complications (e.g. nausea and vomiting, shoulder-tip pain) and a 0.24% risk of unanticipated injury causing major complications (e.g. bowel perforation), resulting in two-thirds of women requiring laparotomy. 59–61 There is an estimated risk of death of 3.3 to 8 per 100,000 women,59,61 and payments in medical negligence cases totalled £24.3M in one survey published in 2000. 62
Magnetic resonance imaging is easily accessible, less invasive and cheaper than surgery. In some circumstances, MRI may add a diagnostic benefit over laparoscopy, such as in the cases of severe deep-infiltrating endometriosis and adenomyosis. MRI may also be used to triage cases, so that therapeutic laparoscopy could be better planned. The aim of the MEDAL study was to determine the proportion of women for whom MRI could remove the need for a laparoscopy or, in other words, for whom MRI could be a replacement for laparoscopy.
For MRI to be used in routine clinical practice for the evaluation of CPP, imaging protocols and reporting need to be standardised to allow homogeneous MRI performance between centres and to limit acquisition and interobserver variability. Currently, there are no routinely used standardised reporting guidelines for CPP in the UK. 63 Guided by the MEDAL study, members of the study panel have proposed a template for the standardised reporting of CPP. 63
Following various treatment options, such as the oral contraceptive pill or hormone treatment, some women will undergo laparoscopy resulting in 50% of women having a negative laparoscopy with no pathological cause of pain identified. 8 Although a negative laparoscopy may fail to identify the pathology, it may have a reassuring effect on the patient. A laparoscopy may also have the potential to be a ‘see-and-treat’ laparoscopy if superficial peritoneal endometriosis is observed; MRI would add no benefit in that case.
The choice of study design
Test-accuracy studies are designed to generate measures of accuracy by comparison of the index test with a reference standard, a test that confirms or refutes the presence or absence of disease beyond reasonable doubt. Classical test-accuracy studies require that the target condition is independently verified by the reference test, which must provide a definitive diagnosis, be applicable in all cases and preferably be performed alongside the index test. Complete verification of the presence or absence of a target condition is essential to reduce bias and to maximise the statistical power of the study. By comparing the index test with the reference standard, the result of the index test can be categorised as a true positive, a false positive, a true negative or a false negative. Measures of index test accuracy, including sensitivity, specificity, positive and negative likelihood ratios, and diagnostic odds ratios, can be computed. 64
Verification of the reference standard is frequently dichotomised as disease present/disease absent. In the MEDAL study, we aimed to determine the accuracy of MRI in the differential diagnosis of the causes of CPP. There is a range of potential target conditions and, correspondingly, of reference diagnoses. MRI will visualise the various conditions with different degrees of accuracy (Table 2). To determine the sensitivity of MRI for each pathology would require a large number of participants, to accommodate the low prevalence of some conditions. Furthermore, some pathologies are not independent of each other and could frequently be concurrently observed; for example, endometriosis can give rise to adhesions from fibrotic tissue. Therefore, the principal research question is how frequently does a MRI scan correctly predict the reference diagnosis, thereby removing the need for a laparoscopy or, conversely, what diagnoses cannot be accurately ruled out by a MRI scan and require laparoscopic investigation?
| Radiological feature | Criteria used in analysis to indicate the presence of a target condition | Reference or justification |
|---|---|---|
| Anatomy of uterus, ovaries, adnexal structure, bladder and bowel | Not used for analysis | |
| Uterine size, appearance, JZ thickness, adjacent myometrial thickness, endometrial thickness | Adenomyosis is diagnosed in two ways:
|
|
| Presence of fibroids, location, number and maximum size | Presence of fibroids reported, irrespective of location, number or size | Fibroids are seen on the MRI scan |
| Ovarian size, ovarian cysts, and, if present, their size and signal intensity | Endometrioma of the ovary is diagnosed if the ovary had a cystic area or deposit with:
|
|
| Presence and location of other masses |
|
Bazot et al.,39 Bazot et al.65 and Chamié et al.66 |
| Presence of free or loculated fluid | Diagnosis of PID if either free fluid or tubal masses are seen, with low or intermediate signal intensity on T1, T2 and T1-FS images | |
| Adhesions | Diagnosis of adhesions if reported as:
|
|
| Bladder status, including wall thickness if abnormal | Not used for analysis | |
| Presence of small bowel in pelvis, location and description | Not used for analysis | |
| Other observations; for example, lumbar spine abnormalities | Not used for analysis |
In the context of the MEDAL study, there were a number of target conditions to be considered, not all of which had a perfect reference standard of identification against which to make a differential diagnosis. There was a risk of partial or differential verification of the underlying causes, with their inherent biases. There are several proposed study designs that overcome the problems of an imperfect reference diagnosis. 67 In the absence of a single ‘gold standard’ test, the results of several imperfect tests or observations can be combined to create a composite reference standard. These can be combined according to a predefined algorithm or a consensus diagnosis obtained from considering all the information.
We employed a panel (consensus) diagnosis in which a group of experts determined the presence or absence of the target condition based on several sources of information captured along the patient pathway. This was an acceptable way of addressing the problem of achieving a diagnosis from multiple sources of information and of subjective assessment of that information, as a diagnosis is achieved by consensus. 68 A final diagnosis can be obtained for all women and the panel method reflects the clinical reality, in which several items of information are synthesised by the clinician. The results of this methodology can be viewed as being generalisable to clinical practice.
To establish whether or not MRI can replace laparoscopy, a paired design was employed, in which both tests were compared with the reference standard. As sensitivity and specificity can vary across subgroups, the two tests and the reference standard are best performed in the same population. 68 It is feasible to perform MRI and laparoscopy in women with CPP and, although MRI does not interfere with laparoscopy, the paired design is preferable to a randomised trial. 69 As MRI would precede laparoscopy, it can also be viewed as a triage test, directing only women with a specific condition towards a laparoscopic confirmation or operative laparoscopic procedure. A fully paired study design is also appropriate for this type of diagnostic situation.
The certainty with which gynaecologists have made their diagnoses will be measured and compared as a secondary measure of diagnostic efficacy. In order to be clinically effective, tests should contribute to the diagnostician’s decision-making,70 for example, by changing a differential diagnosis, strengthening an existing hypothesis or simply reassuring the clinician. Although accuracy is the chief concern, the extent to which clinicians make use of test results also relies on their confidence that the test has contributed usefully to a diagnosis. The MEDAL study therefore evaluated the diagnostic impact of MRI and of laparoscopy by conducting a:
-
before-and-after comparison of diagnostic certainty for having diagnosed the cause of CPP
-
before-and-after comparison of diagnostic certainty for the leading diagnosis
-
before-and-after comparison of the number of differential diagnoses considered per patient
-
retrospective survey of the test’s perceived usefulness.
On the basis of clinical history, examination and ultrasound findings only, treating gynaecologists were asked to state whether or not a pathological cause for CPP was identified, and to express their certainty regarding this decision. They were also asked to list their differential diagnoses, to state whether each was thought to be a cause of CPP and to express their certainty regarding these opinions. After the laparoscopy was performed, clinicians were asked for a revised differential diagnosis and associated certainty using identical questions. A comparison between pre- and post-laparoscopy diagnostic certainty was made, primarily to determine the utility of laparoscopy for identifying a pathological cause of CPP, and secondarily to compare changes in the certainty surrounding the leading differential diagnosis. The number of differential diagnoses considered before laparoscopy was also compared directly with the number considered after the test results were known.
An identical process was followed with independent non-treating gynaecologists (who were blind to the laparoscopic findings) who used MRI to arrive at a diagnosis. Pre-MRI diagnoses and associated certainty based on the same clinical history, examination and ultrasound findings were compared with post-MRI revised diagnoses and certainties. Independent gynaecologists were also asked whether or not they believed that the patient required a laparoscopy.
The gynaecologist’s subjective assessment of the usefulness of MRI or laparoscopy was evaluated after disclosure of the relevant test results, by asking clinicians to select one of four statements that best reflected their perception of the contribution of the test to each case. These statements were initially drafted with reference to those used in published before-and-after diagnostic confidence studies,71–73 and modified for their relevance to a CPP diagnosis following consultation with eight practising gynaecologists.
Aim and objectives
Aim
The aim was to assess if MRI could add value to the decision to perform a laparoscopy in women presenting with CPP. In doing so, we wanted to determine the number of women for whom MRI is sufficiently accurate to avoid the need for a laparoscopy in the investigation of CPP, following evaluation of the presenting characteristics with a detailed history, clinical examination and ultrasound.
Objectives
-
To estimate the accuracy of post-MRI diagnoses with respect to:
-
the absence of any observed condition or cause, that is, idiopathic CPP
-
the main gynaecological conditions or causes of CPP as target conditions, using post-laparoscopy diagnoses and EIP consensus (with and without incorporating MRI findings) as reference standards.
-
-
To determine the added value of MRI in a care pathway involving baseline history, clinical examination and ultrasound before performing laparoscopy.
-
To quantify the impact that a pre-laparoscopy MRI scan can have on decision-making with respect to triaging for therapeutic laparoscopy.
-
To determine the cost-effectiveness of MRI compared with laparoscopy.
Chapter 2 Methods
Introduction
We conducted a test-accuracy study in a prospective cohort of women with CPP symptoms, subjected them to thorough investigations, including our index tests (MRI scan and laparoscopy), and sought expert panel consensus to establish a reference diagnosis. We performed a comparative test-accuracy study with panel consensus for determining the reference standard.
Oversight
The study was conducted in accordance with the protocol, which received a favourable ethics opinion from East Midlands (Nottingham 1) Research Ethics Committee (reference number 11/EM/0281). NHS trust research governance approval was obtained from 26 recruiting hospitals in the UK. The study sponsor was the Queen Mary University of London. Independent oversight for the MEDAL study was requested by the funding body; however, how this oversight should be arranged or what it should entail were unclear, as the study was not considered to be distinct from that of randomised controlled trials. For the MEDAL study, the Data Monitoring Committee was defined as a subgroup of the Study Steering Committee (SSC). All members were independent of the MEDAL study management group. The chairperson of the SSC (along with the lay member) was not included in the Data Monitoring Committee.
Study oversight was provided by an independent steering committee and an independent data monitoring committee (see Acknowledgements for membership).
Study participants and setting
Any woman aged ≥ 16 years, who had been referred to a gynaecologist with CPP of at least 6 months’ duration, was potentially eligible. Women were assessed by a gynaecologist, which involved a standardised assessment and a clinical examination. If clinically indicated, women were referred for an ultrasound scan. If a laparoscopy was indicated and the patient wished to proceed with the laparoscopy, and was prepared to have a MRI scan, eligibility to participate in the study was confirmed. Women were excluded if they had had a hysterectomy, were pregnant or were unable to give written informed consent, or if they definitely had a clinical indication for a MRI scan, based on examination, history or ultrasound. Women were also excluded if they had an identifiable cause of CPP for which treatment could be initiated without a laparoscopy. Eligible women who provided written informed consent were committed to the study by telephone registration service at Birmingham Clinical Trials Unit (University of Birmingham, Birmingham, UK).
Pre-index tests
History and patient-completed questionnaires
A structured assessment template for use by all gynaecologists was provided, which first collected data from the participant and then allowed the clinician to create a clinical record. The assessment incorporated (1) demographic details; (2) menstrual, contraceptive and obstetric history; (3) tobacco and alcohol use; and (4) previous investigations and treatments for pelvic pain. Visual analogue scales were used to elicit scores (from 0 to 10) for pain symptoms of (1) dysmenorrhea (including pain occurring pre menses, during menstruation, post menses and mid-cycle); (2) dyspareunia (at the point of penetration, deep pain, burning pain, post-coital pain); (3) dysuria (full bladder, micturition); (4) muscle/joint pain in the pelvis; and (5) backache and migraine. A body map to illustrate the location of pain was also provided. The assessment also included standardised patient-completed questionnaires, previously validated in either women with pelvic pain or other clinical symptom groups. The domains captured by the questionnaire components of the assessment were:
-
neuropathic pain symptoms, measured using the short-form McGill Pain Questionnaire74
-
bowel symptoms, measured using the Rome III diagnostic criteria75
-
urinary problems, measured using the Pelvic Pain and Urgency/Frequency (PUF) Patient Symptom Scale76
-
sexual activity, measured using the Sexual Activity Questionnaire77
-
personality characteristic, measured using the Big Five Inventory scale78
-
psychological coping strategies, measured using the Pain Catastrophizing Scale79
-
endometriosis-related pain, measured using the Short Form Endometriosis Health Profile Questionnaire (EHP-5)80
-
risk of depression, measured using the Patient Health Questionnaire depression module (PHQ-2)81
-
sexual and physical abuse history, measured using the Sexual and Physical Abuse History questionnaire82
-
QoL and well-being, measured using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) instrument and the Investigating Choice Experiments CAPability (ICECAP)-A measure. 83,84
Clinical examination and ultrasound
Each woman underwent a clinical examination by the recruiting gynaecologist. A data collection form helped to direct the examination, which aimed to describe the location of any abdominal tenderness, and any tenderness, erythema, discharge or lesions on the external genitalia. A bimanual examination aimed to identify vaginismus, bladder wall or uterosacral ligament tenderness, cervical excitation, uterine size, position, contour, consistency and mobility, ovarian tenderness, mobility and presence of masses. A speculum examination of the vagina was used to identify prolapse, vaginal atrophy, polyps and cervicitis, and also allowed swabs to be taken if indicated and if the woman consented. A urine sample was provided to perform a dipstick test to detect urinary tract infection.
The intention was for all women to have a detailed ultrasound scan, in accordance with the study protocol, scanning by both the transabdominal route on a full bladder and then the transvaginal approach on an empty bladder. However, in some centres, the ultrasound was scheduled with a sonographer prior to the first appointment with the gynaecologist. In these cases, it was not possible to repeat the ultrasound, and an incomplete data set for the study was extracted from the report. The intention was to describe the uterine size by both approaches, and also to examine the uterine position, myometrial appearance and endometrial width, as well as ovarian size, location and presence of ovarian cysts and, finally, bladder wall thickness, all by transvaginal ultrasound. Further information sought included the presence of free or loculated fluid and organ- or site-specific tenderness or restricted mobility.
Index tests
Magnetic resonance imaging scan
Women were asked to fast for 2–4 hours prior to the scan and not to empty their bladder immediately prior to the scan. Antiperistaltic agents were contraindicated, but antispasmodic drugs were acceptable.
The MRI standard protocol comprised T1 and T2 axial, T2 sagittal, T2 coronal and T1-fast-spin (FS) axial, sagittal and coronal sequences, using standardised anatomical landmarks, slice thicknesses and field of view. If unexpected abnormalities were detected, additional sequences, potentially using the contrast media gadolinium, could be added to the scanning protocol to enable further clarification and assessment.
To ensure blinding of assessments, a MRI scan was performed before diagnostic laparoscopy, and treating gynaecologists and investigators were kept blind to the MRI reports and images. To identify radiological parameters for inclusion in the MRI report, a two-generational Delphi survey with an expert panel of 28 radiologists specialising in gynaecological MRI from across the UK was undertaken. 63 The MRI report collected data described in Table 2.
The radiologists’ assessments of the quality of the scan were captured, and they were asked to summarise the observations and provide a radiological diagnosis in free-text form.
The MRI scan was also independently reported by a second radiologist who was blinded to the initial MRI report. This would be a radiologist involved in reviewing scans for the study, but based at a different site from the initial reporting radiologist. Where the local and independent radiologists agreed on the summary diagnosis, the findings were accepted as a consensus and provided the ‘post-MRI diagnosis’ data for analysis. Lack of agreement between local and independent radiologists prompted a review by the Independent Radiology Review Committee (IRRC), made up of three experienced radiologists who were not involved in imaging participants in the study. The IRRC agreed a consensus MRI report. The finalised report was provided to the independent gynaecologist for determining the post-MRI diagnosis, and to the EIP for the second stage in the reference diagnosis.
The diagnostic criteria used for the analysis of the accuracy of MRI are given in Table 2. These were defined by the lead radiological investigator and confirmed by a second radiologist otherwise not involved in the study. There were potential variations in diagnosis, for example in relation to whether an intermediate signal from a T1 and T1-FS image of a bowel mass was indicative of deep-infiltrating endometriosis; therefore, the different diagnostic criteria were considered in alternative or sensitivity analyses.
The MRI reports were not provided to the gynaecologist unless unexpected significant findings were identified by the local reporting radiologist. We prespecified the circumstances in which this may happen, such as identification of an unexpected cancer, abscess or non-gynaecological abnormality requiring immediate attention. In the case of unblinding, the participant was excluded from the diagnostic accuracy study and managed appropriately.
Laparoscopy
The diagnostic laparoscopy was performed under general anaesthetic in accordance with the gynaecologist’s standard practice. After a pneumoperitonium was established, a laparoscope was introduced to visualise the abdominal and pelvic structures, and any pathology. Laparoscopy was performed by an experienced gynaecologist who was capable of identifying all potential target conditions. Surgical findings were reported using a standardised proforma, which collected the information described in Table 3.
| Laparoscopic feature | Criteria used in analysis to define the observation of the target condition |
|---|---|
| Presence, location and severity of endometriosis | Presence of endometriosis in any location, categorised as either superficial or deep. Biopsies may have been taken for histological confirmation, but the results were not considered for the criteria. AFS grading was used85 |
| Presence, location and appearance (filmy, dense/vascular or an absent plane) of adhesions | Presence of adhesions in any location, irrespective of appearance |
| Presence of adenomyosis | Presence of adenomyosis observed. It is acknowledged that adenomyosis cannot be directly observed, but other features might be suggestive |
| Presence, location, type (simple, dermoid, cancerous, other) and size of ovarian cysts | Presence of any ovarian cysts, regardless of type |
| Presence, location and maximum size of fibroids | Presence of any fibroids, regardless of location. It is acknowledged that submucosal and small intramural fibroids cannot be directly observed, but other features may be suggestive or they may have been observed by hysteroscopy |
| Presence of PID | Presence of features indicative of PID |
| Pelvic congestion syndrome | Observation of dilated pelvic veins |
When clinically indicated, additional procedures were performed at the time of surgery. Information was collected on procedures, including ablation or excision of superficial endometriosis, ovarian cystectomy, adhesiolysis, hysteroscopy (noting the presence of fibroids or the insertion of the levonorgestrel-releasing intrauterine system), endometrial biopsies and histological confirmation of pathology and cystoscopy. If bladder biopsies were collected, the histological confirmation of pathology was recorded.
Follow-up
Some of the target conditions may respond well to treatment; therefore, information was collected at 6 months following laparoscopy, using a postal questionnaire. The questionnaire contained all of the elements of the initial patient questionnaire, including the visual analogue scales, validated questionnaires and questions regarding further tests and treatments, but omitted the demographic and history questions, as the answers to these questions would not have changed. Women were sent reminders if they failed to respond to the initial questionnaire. The information gained from follow-up was considered by the EIP when assigning the reference standard.
Assigning the cause of pain
At several stages in the study path for each patient, the treating gynaecologist and the independent gynaecologist reviewed the diagnostic evidence available. To establish a possible diagnosis for each target condition (including idiopathic CPP), they indicated their level of certainty that the condition was causing the pelvic pain on a numerical rating scale, ranking from 10 being certain (expressed as a 99 in 100 chance), through 5 being a fairly good possibility (a 5 in 10 chance) to 0, considered to be no chance or less than a 1 in 100 chance.
The first condition was the absence of structural cause, defined as idiopathic or unknown. There then followed seven structural gynaecological causes (superficial peritoneal endometriosis, deep-infiltrating endometriosis, endometrioma of the ovary, adhesions, ovarian cysts, adenomyosis, fibroids), together with the option to specify another gynaecological cause. The non-gynaecological causes offered were psychological or psychosexual, gastrointestinal, urinary, musculoskeletal, neurological or another pathological cause, with the requirement to specify the diagnosis further. Definitions for idiopathic CPP, superficial peritoneal and deep-infiltrating endometriosis, adhesions and pelvic congestion syndrome were provided.
Determining the role of magnetic resonance imaging by independent assessment
The treating gynaecologists were kept blind to the MRI scan report so that they could make an unbiased assessment of the laparoscopic findings. If MRI was found to be useful in the MEDAL study, an MRI scan would precede laparoscopy in the investigative pathway in the future. In order to model the impact of using the MRI scan report, an independent gynaecologist reviewed each case. This gynaecologist was randomly selected from another hospital recruiting to the MEDAL study, so was familiar with the study aims, processes and data collection. The independent gynaecologist was provided with all the data collection forms for the pre-index tests and clinical history, together with the laparoscopy report. The information was anonymised. The independent gynaecologists were asked to complete the certainty scales for each condition, which constituted the pre-MRI independent diagnosis and should be highly correlated with the recruiting gynaecologists’ diagnoses post laparoscopy, being derived from the same information. The independent gynaecologists were then asked to read the MRI scan report and repeat the process, completing the certainty scales. There were three extra hypothetical questions at this second post-MRI stage: if the gynaecologist was treating that particular patient, would they have scheduled a laparoscopy (having reviewed the MRI scan report), if they would have scheduled a therapeutic laparoscopy and, if so, would they anticipate that the diagnostic and therapeutic elements would be performed as part of a single procedure?
Reference standard
The analysis considers two sets of reference standard. The first identified was to assess the accuracy of the MRI scan for observing a condition, and the second was to assess the accuracy of the MRI scan for identifying the condition(s) causing pelvic pain. The first set used findings from laparoscopy for the reference standard, and the second used consensus from an EIP. It was expected that not all women who were observed to have each condition would have it categorised as being the source of pelvic pain.
Piloting the expert independent panel
There is little evidence on how panels should be convened or how information should be presented. Given the amount of information to be considered by the panel and the associated review time, the study management group, in conjunction with the EIP chairperson, considered the use of a summary report. To ensure that the diagnosis based on the summary report was the same as the diagnosis based on all of the data collection forms (initial patient assessment, including patient questionnaire, clinical examination, ultrasound, post-laparoscopy report and, finally, the MRI scan report), a pilot EIP panel was convened to pilot and review the process. The pilot EIP panel consisted of three clinicians not involved in the study recruitment.
Ten cases were presented to the three members. For the first five cases, two of the three members used the summary report and the third member used the data forms. For the last five cases, two members, who had previously used the summary report, used the data collection forms. For each case, the members defined a single or multiple diagnosis. Results from the meeting indicated that the summary report and data collection forms allowed the members to produce the same diagnosis. Data collection forms were preferred, or used in conjunction with the summary report, when cases were considered to be complex (e.g. when a patient had multiple conditions and various confounding factors).
To elicit a diagnosis, a form with a list of the target conditions was provided. This was comparable to the form used by the treating and independent gynaecologist, except that the certainty scale was not used and simple yes/no answers were allowed. During the pilot EIP meeting, members were allowed to record multiple causes. This was considered unsatisfactory as a reference standard, as they would complicate accuracy assessments, so the initial guidance regarding the first eight cases presented to the main EIP members was to diagnose what they believed to be the single main cause of pain. The process was performed in two stages: once with all of the data, except those from the MRI report, and then a second time, including the data from the MRI report. The first-stage diagnosis avoided incorporation bias from knowledge of the MRI scan results, whereas the second-stage diagnosis provided information to determine the added value of the MRI scan. Two further yes/no questions were posed to the panel: (1) does the laparoscopy add anything to your diagnosis that was not available from other investigations and (2) could this laparoscopy have a therapeutic purpose?
Feedback from these eight cases suggested that, in some instances, it was necessary to select more than one condition, given that two or more conditions could equally be the cause of pain. The criteria and guidance were changed so that EIP members could select any clinically appropriate conditions they believed to be the cause of pain, provided that they were at least 50% certain. The diagnosis form still allowed only yes/no answers, but multiple reasons could be given. The first eight cases were reviewed again at a later meeting using the revised criteria.
Expert panel membership and meeting format
The panel membership consisted of 15 consultant gynaecologists who were not involved in the study recruitment. Each face-to-face meeting was made up of three members who provided the reference diagnosis for each case presented based on the summary report of the data, with the individual data forms available if required.
The first-stage reference diagnosis was based on patient history and reported symptoms, clinical examination, ultrasound, laparoscopy and follow-up. For the second-stage reference diagnosis, the MRI scan report was provided. For both stages, each EIP member individually recorded the conditions that they were more than 50% certain were the cause of pain, and addressed the two questions regarding the potential gain from the laparoscopy, prior to a group discussion. The meeting chairperson documented the discussion and how agreement was reached, and the final consensus diagnosis constituted the reference standard.
Sample size
A sample size of 250 women was chosen a priori to address the primary research question of determining the proportion of women for whom MRI is sufficiently accurate to replace laparoscopy in the investigation of CPP, following evaluation of the presenting characteristics. With this sample size, the study was anticipated to have > 90% power (at p = 0.05) to detect a reduction of 10% in the number of laparoscopies needed (i.e. from 100% down to 90%). This difference would be cost-effective if laparoscopy was at least 10 times more expensive than MRI. Estimates used at the start of the study placed laparoscopy at being 7.4 times more expensive than MRI (£1274 vs. £173); however, these NHS tariffs may not necessarily reflect the true cost of the total investigative pathway, which will be estimated through primary data collection as part of this study.
Having 250 women as the sample size was also expected to provide a reasonable number of cases of each of the more common target conditions from which to estimate the sensitivity of MRI for diagnosis. We anticipated a high sensitivity of MRI for detecting common pathological causes of CPP; therefore, we based our calculations on an anticipated sensitivity of 80% for any particular condition (a sensitivity of 90% has also been provided for comparison; Table 4). We then computed the 95% confidence intervals (CIs) for these sensitivities for a range of prevalences of any particular condition (see Table 4). These figures could equally apply to specificity. For a target condition with a prevalence of 30% or more, we expected to be able to reliably rule out a sensitivity or specificity of < 70% if the ‘true’ sensitivity or specificity was > 80%.
| Assumed sample size of 250 women | Assumed sensitivity (%) | ||
|---|---|---|---|
| Prevalence (%) | Number of cases | 80 | 90 |
| 50 | 125 | 72–87 | 84–95 |
| 40 | 100 | 71–87 | 82–95 |
| 30 | 75 | 69–88 | 82–96 |
| 20 | 50 | 66–90 | 78–97 |
In May 2013, when the target sample size had already been slightly exceeded (269 women), the trial team, together with the input of the independent oversight committees, decided to extend recruitment until the end of September 2013, with a revised target of 340 women, and the protocol was amended accordingly. At this stage, the study was recruiting faster than was originally planned. The aim of extending recruitment was to improve the statistical precision of the accuracy estimates for the less prevalent gynaecological causes, and this revised plan was ratified by the external Trial Steering Committee. For example, a sensitivity of 90% would have a lower 95% CI boundary of 80% for conditions occurring in 30% of women in a sample size of 250, and in conditions occurring in 20% of women in a sample size of 340. However, between May 2013 and September 2013, recruitment to the study unexpectedly started to slow and this revised target was not met, with a final sample size of 291 participants at the start of the diagnostic study.
Data analysis
Reliability estimates
Agreement between the three individual members of the EIP in their diagnosis (for ‘any gynaecological cause’ and each gynaecological cause separately) was analysed using the methodology proposed by Fleiss,72 which is a generalisation of the Cohen’s kappa statistic to the measurement of agreement among multiple raters. In addition, the agreement between the consensus panel diagnosis, made with and without the MRI findings, was analysed using the Cohen’s kappa agreement statistic. In both cases, the kappa value was presented alongside its 95% CI. We also reported the number of cases in which disagreements occurred.
Accuracy estimates
By comparing the index test with the reference standard, the results of the index test were categorised as a true positive, a false positive, a true negative or a false negative. The sensitivity of each test was computed as the proportion of all positive results on the reference standard that were true positives, and the specificity was computed as the proportion of all negative results on the reference standard that were true negatives.
The prevalence of all gynaecological and non-gynaecological conditions in relation to whether or not they were believed to be causing pain (as determined by the reference standard established by the consensus of the EIP) was calculated, along with the 95% CIs, using binomial exact methods. 86 For gynaecological conditions, estimates of prevalence for whether or not the condition was also observed at laparoscopy were also produced.
The initial analysis looked at the diagnostic accuracy of MRI in being able to detect if a condition actually existed (regardless of whether or not it was judged to be the cause of pain), using the laparoscopy report as the reference standard; MRI data were taken directly from the MRI scan report using the set of rules described in Table 2. Accuracy estimates for this additional analysis (sensitivity and specificity, along with 95% CIs) were calculated, and Fisher’s exact test was used to explore if MRI and laparoscopy detected the same women with a presence of each target condition.
The primary analysis involved calculations of sensitivity and specificity (and the associated 95% CIs) for the presence or absence of each structural gynaecological cause of pain; data were taken from the binary yes/no responses of the reference standard before and after the MRI data were revealed. Receiver operating characteristic (ROC) curves were also constructed for each test for each condition, using the certainty estimates (0 = ‘no chance/almost no chance’ to 10 = ‘certain, practically certain’) provided by the clinician and the binary responses of the reference standard. The area under the ROC (AUROC) curve and the 95% CI were estimated for each test. Differences between the AUROC estimates of each of the four diagnoses were then calculated using a non-parametric approach for correlated data,71 and these differences are presented along with their 95% CIs. The same analysis was done to compare the responses after the MRI data were revealed (from the independent gynaecologist) with the responses after the laparoscopy had been undertaken (from the treating gynaecologist), to compare the accuracy of MRI-based diagnoses with laparoscopy-based diagnoses. Only participants with laparoscopic, MRI and EIP diagnoses were included in the analysis.
The proportion of women for whom a diagnostic and/or therapeutic laparoscopy could be avoided was calculated by adding up the number of women needing treatment other than laparoscopy (women with adenomyosis, fibroids or PID) and the number of women not needing any treatment (as a result of the absence of any gynaecological cause), which were correctly identified from the MRI scan.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Chapter 3 Diagnostic study results
Recruitment
The recruitment of participants started in December 2011 and closed in September 2013. A total of 1667 women were screened. A total of 435 women, who consented to participate, were recruited into the study from 26 centres (see Appendix 1, Table 29). Ultimately, 287 women contributed to the analysis (Figure 3).
FIGURE 3.
Flow chart of participants, showing the numbers who provided data at each stage.
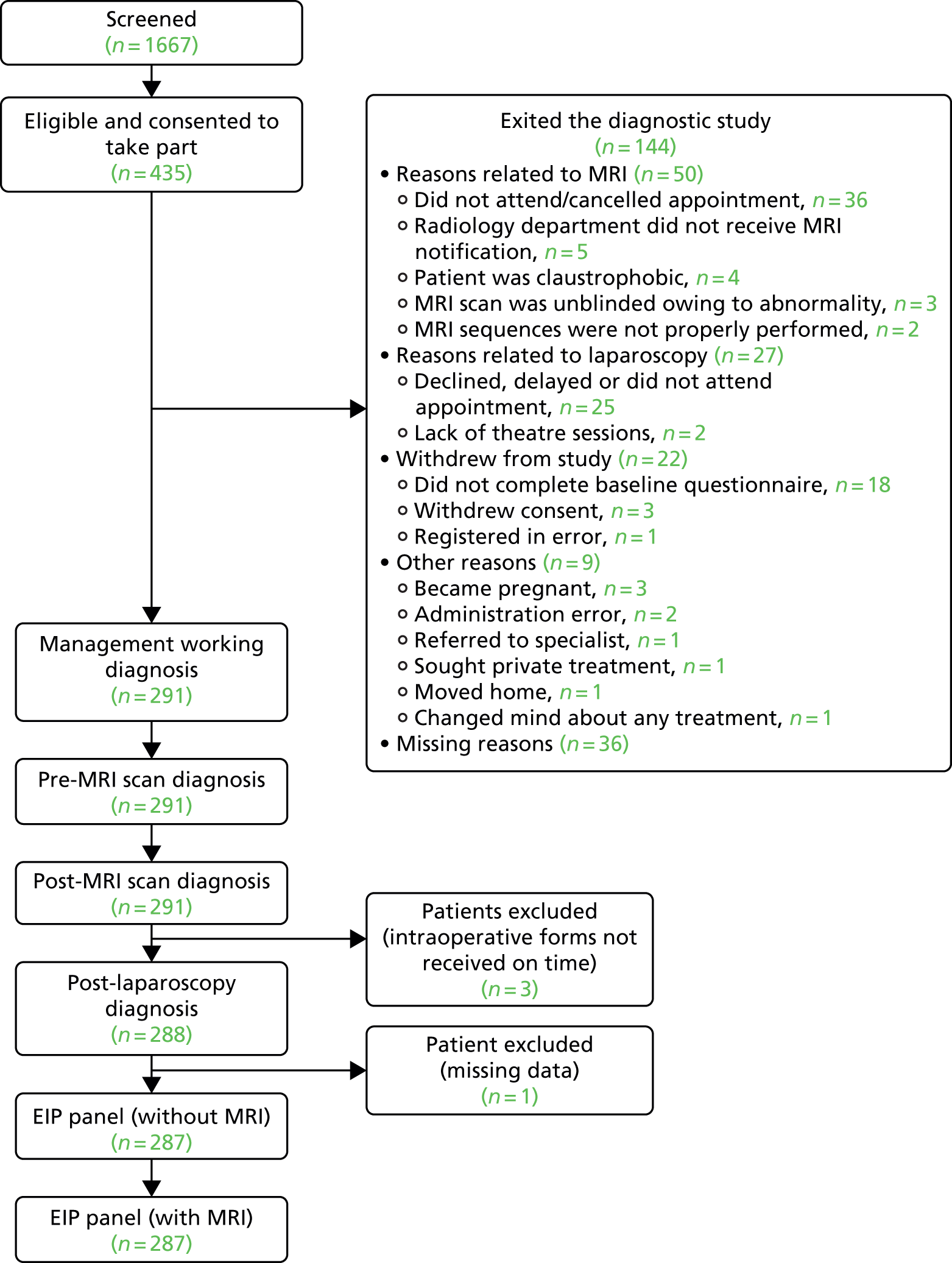
The original sample size of 250 participants was reached ahead of schedule, so the sample size was revised to 340 participants. The sample size was increased to improve the precision of estimates for all target conditions, but most notably for the less common target conditions.
Characteristics of participants
The mean age of the women in our sample was 31.6 years [standard deviation (SD) 8.3 years]. Of these, 197 women (68.5%) were in employment and 96 (33.5%) had received a university-level education. There were 216 white women (74.5%). The duration of pain was, on average, 4.2 years (SD 4.8 years); pain was present on at least 1 day every week in 226 women (79.5%), with a mean pain score of 7.1 points (SD 2.8 points) during menstrual periods and 6.4 points (SD 2.6 points) before menstrual periods. The average age at menarche was 12.8 years (SD 1.7 years). Heavy periods were a feature in 147 women (58.6%). Around three-quarters of women (n = 212; 73.4%) were sexually active, with pain during intercourse featuring over the last 20.3 months (SD 20.7 months). Contraceptive hormones had been prescribed for pain to 162 women (55.7%). Chlamydia testing was undertaken in 202 women (70.4%), of whom 176 (89.3%) had received negative results. Laparoscopy and MRI had been used for diagnosis in the past in 80 (27.5%) and 30 women (10.3%), respectively. Infertility was not a complaint in 206 women (85.1%). Further details of participant characteristics are shown in Table 5 and Appendix 1, Tables 30 and 31.
| Characteristic | Value |
|---|---|
| Age (years), mean (SD, n) | 31.6 (8.3, 291) |
| Marital status,a n (%) | |
| Single | 96 (33.2) |
| Living together | 66 (22.8) |
| Married | 110 (38.1) |
| Separated/divorced | 17 (5.9) |
| Employment,b n (%) | |
| Full-time employment | 128 (44.4) |
| Part-time employment | 50 (17.4) |
| Self-employed | 19 (6.6) |
| Caring for children | 35 (12.2) |
| Student | 22 (7.6) |
| Unemployed | 34 (11.8) |
| Highest level of education achieved,c n (%) | |
| No qualifications | 17 (5.9) |
| GCSE/O level/NVQ1–2 | 94 (32.9) |
| A level/BTEC qualification/NVQ3–4 | 79 (27.6) |
| University degree | 67 (23.4) |
| Postgraduate degree | 29 (10.1) |
| Ethnic group,d n (%) | |
| Asian/Asian British | 33 (11.3) |
| Black/black British | 18 (6.2) |
| White | 216 (74.5) |
| Mixed | 12 (4.1) |
| Any other ethnic group | 9 (3.1) |
| Do not wish to say | 2 (0.7) |
The median duration between the MRI scan and the diagnostic laparoscopy was 26 days (interquartile range of 9–54 days).
Reliability of the expert independent panel diagnoses
We investigated the reliability of the EIP reference diagnoses by looking first at the consensus ratings made with and without access to the MRI report and, second, at the agreement between individual raters in the independent expert group.
Table 6 reports the prevalence of each condition as defined by the EIP with and without access to the MRI report. We considered the primary reference standard to be the EIP consensus statement without the MRI report, as this would reduce the risk of incorporation bias in assessing the accuracy of the MRI report. However, we were interested in noting when the reference standard consensus diagnoses differed, as it was known that an MRI scan may be the only test to identify several of the conditions in some women.
| Cause of pelvic pain | Prevalence (N = 287), n (%) | Reliability | ||
|---|---|---|---|---|
| Reference diagnosis without MRI findings | Reference diagnosis with MRI findings | Assessors’ kappaa (95% CI) | Number of cases disputedb | |
| Idiopathic for gynaecological conditions | 156 (54.4) | 153 (53.3) | 0.75 (0.69 to 0.84) | 40 |
| Superficial peritoneal endometriosis | 71 (24.7) | 71 (24.7) | 0.74 (0.67 to 0.81) | 32 |
| Deep-infiltrating endometriosis | 40 (13.9) | 42 (14.6) | 0.96 (0.89 to 0.99) | 3 |
| Endometrioma of the ovary | 11 (3.8) | 13 (4.5) | 0.80 (0.73 to 0.87) | 6 |
| Adhesions | 24 (8.4) | 25 (8.7) | 0.54 (0.47 to 0.61) | 35 |
| Ovarian cysts | 1 (0.3) | 0 (0) | – | 0 |
| Adenomyosis | 11 (3.8) | 16 (5.6) |
0.32 (0.26 to 0.40) 0.60 (0.53 to 0.67)# |
24 22# |
| Fibroids | 4 (1.4) | 5 (1.7) | 0.53 (0.46 to 0.60) | 7 |
| PID | 10 (3.5) | 10 (3.5) | 0.52 (0.45 to 0.69) | 10 |
| Urinary causes | 18 (6.3) | 16 (5.6) | 0.35 (0.28 to 0.42) | 35 |
| Musculoskeletal causes | 48 (16.7) | 47 (16.4) | 0.51 (0.44 to 0.58) | 39 |
| Gastrointestinal causes | 24 (8.4) | 23 (8.0) | 0.51 (0.44 to 0.58) | 34 |
| Psychological/psychosexual causes | 78 (27.2) | 75 (26.1) | 0.53 (0.46 to 0.59) | 59 |
Table 6 shows that the difference in the number of cases detected was between zero and two for all conditions other than adenomyosis (difference of five cases detected), and cases with psychological and psychosexual causes (difference of three cases detected). Although there are clinical reasons why adenomyosis can only be detected by a MRI scan, which are likely to explain the difference, there are no reasons to explain the difference for cases with psychological and psychosexual causes. We therefore report comparisons with the second-stage MRI scan-informed EIP consensus reference diagnosis for analysis of adenomyosis, but not for any other conditions.
To assess the reliability of the EIP diagnoses, we considered the agreement between the three individual ratings made by the panel members prior to any group discussion. We computed the kappa statistic across the three raters to describe the agreement beyond that explained by chance. Kappa values above 0.80 were considered to indicate excellent agreement, whereas those between 0.60 and 0.79 indicate good agreement and those below 0.60 indicate poor or moderate agreement.
The panel were in excellent agreement in identifying deep-infiltrating endometriosis and endometrioma of the ovary as causes of pelvic pain, and in good agreement in identifying superficial peritoneal endometriosis and adenomyosis when the MRI reports were used in the reference standard. Lower levels of agreement were noted for deciding that adhesions, fibroids and PID were the cause of pelvic pain. The expert panel failed to demonstrate adequate reliability in determining whether any of the non-gynaecological causes were the cause of pelvic pain for further detailed examination of these causes.
Prevalence of conditions being the cause of pelvic pain or observed on magnetic resonance imaging scans or through laparoscopy
Table 7 reports the prevalence of the causes of pelvic pain as judged by the EIP and observed through laparoscopy and MRI scans. For many conditions (superficial peritoneal endometriosis, endometrioma of the ovary, adhesions, ovarian cysts, adenomyosis and fibroids), the prevalence of observing the condition through MRI scans and/or laparoscopy was higher than the prevalence according to the judgements of the EIP, reflecting that, in many women, the conditions may be observed but not judged to be the cause of pelvic pain.
| Cause of pelvic pain | Judged as being the cause of pelvic pain, n (%) | Observation by technique, n (%) | ||
|---|---|---|---|---|
| Reference diagnosis without MRI | Reference diagnosis with MRI | Laparoscopy (N = 287) | MRIa (N = 287) | |
| Idiopathic (no structural gynaecological cause) | 156 (54.4) | 153 (53.3) | 75 (26.1) |
49 (17.1)b 148 (51.6)c |
| Superficial peritoneal endometriosis | 71 (24.7) | 71 (24.7) | 120 (41.8) | No criteria |
| Deep-infiltrating endometriosis | 40 (13.9) | 42 (14.6) | 37 (12.9) | 3 (1.0) |
| Endometrioma of the ovary | 11 (3.8) | 13 (4.5) | 68 (23.7) | 31 (10.8) |
| Adhesions | 24 (8.4) | 25 (8.7) | 109 (39.6) | 37 (12.9) |
| Ovarian cysts | 1 (0.3) | 0 (0) | 56 (19.7) | 27 (9.4) |
| Adenomyosis | 11 (3.8) | 16 (5.6) | 21 (7.3) |
193 (67.2)b 34 (11.8)c |
| Fibroids | 4 (1.4) | 5 (1.7) | 24 (8.4) | 56 (19.5) |
| PID | 10 (3.5) | 10 (3.5) | 10 (3.5) | 15 (5.2) |
| Urinary causes | 18 (6.3) | 16 (5.6) | Not assessed | No criteria |
| Musculoskeletal causes | 48 (16.7) | 47 (16.4) | Not assessed | No criteria |
| Gastrointestinal causes | 24 (8.4) | 23 (8.0) | Not assessed | No criteria |
| Psychological/psychosexual causes | 78 (27.2) | 75 (26.1) | Not assessed | No criteria |
Superficial peritoneal endometriosis was identified as the most common gynaecological cause of pelvic pain in 25% of women, followed by deep-infiltrating endometriosis in 14%, adhesions in 8%, endometrioma of the ovary, adenomyosis and PID each in 4% and fibroids and ovarian cysts in ≤ 1%. Of the non-gynaecological causes, psychological/psychosexual causes were the most common in 27% of women, musculoskeletal causes in 17%, gastrointestinal causes in 8% and urinary causes in 6%. Fifty-four per cent of women were judged to have idiopathic pelvic pain with no structural gynaecological cause identified.
The three endometriosis conditions (superficial peritoneal, deep-infiltrating and endometrioma of the ovary) and ovarian cysts were observed twice as often with laparoscopy, and adhesions three times as often, as with MRI scans. Adenomyosis, fibroids and PID were noted more often through MRI than through laparoscopy. No MRI diagnostic criteria were available for the non-gynaecological conditions.
Figure 4 shows the relationships between the causes of pelvic pain based on the EIP diagnoses without the MRI report for structural gynaecological causes. Superficial peritoneal endometriosis, deep-infiltrating endometriosis and endometrioma of the ovary often occurred together: 17 out of 40 women with deep-infiltrating endometriosis also had superficial peritoneal endometriosis, 7 out of 11 women with endometrioma of the ovary also had deep-infiltrating endometriosis and 7 out of 11 women with endometrioma of the ovary also had superficial peritoneal endometriosis. Adhesions also appeared in combination with the three types of endometriosis.
FIGURE 4.
Relationships between the structural gynaecological causes.
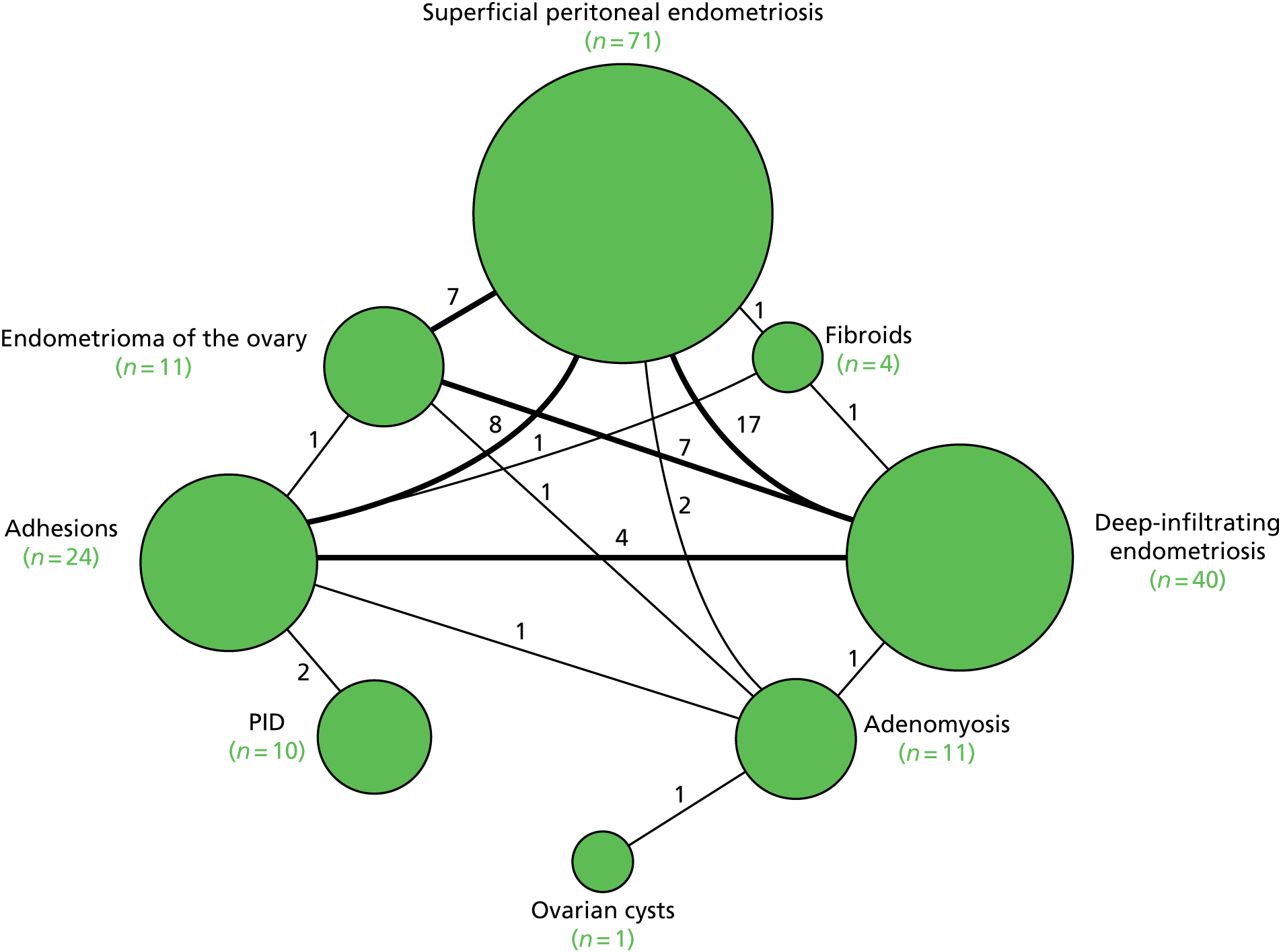
Connecting lines indicate the number of women with both diagnoses. A total of 131 women (46%) had structural gynaecological causes and are included in Figure 4.
Comparison of observations on magnetic resonance imaging scans against a reference standard of observations made through laparoscopy
If MRI were to be useful at identifying a condition as being the cause of pelvic pain, it is a prerequisite that the condition has to be observed in the MRI image. Thus, before investigating the accuracy of MRI for identification of each condition as a cause, we assessed the accuracy of MRI for observing each condition, using a reference standard of the condition being observed through laparoscopy. Although this reference standard was thought to be suitable for identifying the three endometriosis conditions, ovarian cysts and adhesions, it was unsuitable for use as a reference standard for adenomyosis and fibroids, as it was likely to miss many cases that could not be identified from inspection of the outside of the uterus. The estimates of test accuracy for adenomyosis and fibroids should therefore be considered alongside the likelihood of misclassification of cases on the reference standard, as apparent false positives and false negatives could be caused by misclassification errors in the reference standard, as well as misclassification errors in the index test. The same concern applied to interpretation of MRI scans judged to be idiopathic, as this was defined as being the absence of all conditions, including fibroids and adenomyosis. In addition, no criteria for identifying superficial peritoneal endometriosis or the non-gynaecological conditions on MRI reports were available, and PID was excluded because few cases were detected.
Table 8 shows that MRI had poor sensitivity for detecting many conditions. MRI detected only 1 of the 36 cases of deep-infiltrating endometriosis that had been observed through laparoscopy (with a sensitivity of 3%), and picked up 20 of the 107 cases of adhesions (with a sensitivity of 19%), 11 of the 56 cases of ovarian cysts (with a sensitivity of 20%) and 22 of the 66 cases of endometrioma of the ovary (with a sensitivity of 33%). The number of false positives (reported on the MRI scan but not seen on laparoscopy) for these conditions were low, with specificities between 93% and 99%.
| Cause of pelvic pain | MRI finding | Laparoscopy finding, n | Sensitivity (95% CI) | Specificity (95% CI) | Likelihood ratio (95% CI) | Diagnostic odds ratio (95% CI) | Test of association | ||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Positive | Negative | ||||||
| Idiopathica | Yes | 16 | 33 | 21.3% (12.7% to 32.3%) | 84.4% (78.8% to 89.0%) | 1.4 (0.8 to 2.3) | 0.9 (0.8 to 1.1) | 1.5 (0.8 to 2.8) | p = 0.3 |
| No | 59 | 179 | |||||||
| Idiopathicb | Yes | 55 | 93 | 73.3% (61.9% to 82.9%) | 56.1% (49.2% to 62.9%) | 1.7 (1.4 to 2.1) | 0.5 (0.3 to 0.7) | 3.5 (2.0 to 6.3) | p < 0.0001 |
| No | 20 | 119 | |||||||
| Superficial peritoneal endometriosis | Yes | 0 | 0 | 0% | 100% | – | – | – | – |
| No | 120 | 167 | |||||||
| Deep-infiltrating endometriosis | Yes | 1 | 2 | 2.8% (0.1% to 14.5%) | 99.2% (97.1% to 99.9%) | 3.5 (0.3 to 37.2) | 0.98 (0.93 to 1.04) | 3.5 (0.3 to 39.9) | p = 0.33 |
| No | 35 | 247 | |||||||
| Endometrioma of the ovary | Yes | 22 | 9 | 33.3% (22.2% to 46.0%) | 95.8% (92.2% to 98.1%) | 8.0 (3.9 to 16.5) | 0.7 (0.6 to 0.8) | 11.5 (5.0 to 26.7) | p < 0.0001 |
| No | 44 | 207 | |||||||
| Adhesions | Yes | 20 | 11 | 18.7% (11.8% to 27.4%) | 93.3% (88.4% to 96.6%) | 2.8 (1.4 to 5.6) | 0.9 (0.8 to 1.0) | 3.2 (1.5 to 6.9) | p = 0.003 |
| No | 87 | 154 | |||||||
| Ovarian cysts | Yes | 11 | 16 | 19.6% (10.2% to 32.4%) | 92.9% (88.7% to 95.9%) | 2.8 (1.4 to 5.6) | 0.9 (0.8 to 1.0) | 3.2 (2.2 to 4.5) | p = 0.009 |
| No | 45 | 208 | |||||||
| Adenomyosisa | Yes | 15 | 175 | 75.0% (51.0% to 91.0%) | 31.9% (26.0% to 38.0%) | 1.1 (0.8 to 1.4) | 0.8 (0.4 to 1.7) | 1.4 (0.5 to 4.0) | p = 0.6 |
| No | 5 | 82 | |||||||
| Adenomyosisb | Yes | 2 | 31 | 10.0% (1.2% to 31.7%) | 87.8% (83.1% to 91.5%) | 0.8 (0.2 to 3.2) | 1.0 (0.9 to 1.2) | 0.8 (0.2 to 3.6) | p = 0.9 |
| No | 18 | 222 | |||||||
| Fibroids | Yes | 21 | 35 | 87.5% (67.6% to 97.3%) | 86.4% (81.6% to 90.4%) | 6.5 (4.6 to 9.1) | 0.1 (0.05 to 0.4) | 44.6 (12.3 to 157.4) | p < 0.0001 |
| No | 3 | 223 | |||||||
We considered two different MRI definitions of adenomyosis (as defined in Table 2), the first of which was met by 193 women (69%) and the second by 34 women (12%). MRI did not show any relationship with the findings on laparoscopy for either definition of adenomyosis (p = 0.6 and p = 0.9, respectively). It was not possible to ascertain if this was attributable to the poor ability of laparoscopy or MRI to detect this condition. Observing fibroids on MRI scans was closely linked with observing fibroids on laparoscopy with a sensitivity of 88%, but 35 women had fibroids noted on MRI scans that were not observed on laparoscopy, which could be explained by the inability of laparoscopy to observe fibroids inside the uterus.
We defined an idiopathic MRI scan to be one in which no criteria for any of the above gynaecological conditions were met. As we considered two alternative MRI definitions of adenomyosis, there were two corresponding definitions of ‘idiopathic’. The first MRI definition categorised the majority of women as having adenomyosis, with only 49 women (18%) judged to have an idiopathic MRI report, and this finding showed no relationship with observing no structural gynaecological cause on laparoscopy (p = 0.3; see Appendix 2, Table 32).
Using the second definition, 148 women (52%) were categorised as having an idiopathic MRI report, of whom 93 had findings on laparoscopy. Twenty women, defined as idiopathic on laparoscopy, were noted to meet the criteria for a gynaecological condition using MRI. Overall, the idiopathic diagnoses made through laparoscopy and MRI were in agreement in only 61% of women (see Appendix 2, Table 33).
Comparison of observations made using magnetic resonance imaging against the expert panel reference standard for the cause of pelvic pain
In a further analysis, we considered whether or not MRI findings were diagnostic of the cause of pelvic pain as stated by the EIP. We compared with the EIP consensus diagnosis based on information excluding MRI for all conditions other than adenomyosis where consensus diagnoses including information from MRI was used (Table 9). Most conditions on MRI reports were less likely to be regarded as a cause of pelvic pain than conditions observed on laparoscopy; using MRI reports, superficial peritoneal endometriosis was rated as a cause of pain in 71 women, whereas it was observed in 120 women on laparoscopy; endometrioma of the ovary was noted as a cause in 11 women, but observed in 66 women; adhesions was a cause in 24 women, while noted in 107 women; ovarian cysts was a cause in only 1 woman, but noted in 56 women; adenomyosis was a cause in 16 women, but noted in 20 women; and fibroids was a cause in 4 women, while noted in 24 women. The only exceptions were deep-infiltrating endometriosis, which was regarded as a cause of pain in 39 women, but only noted in 36 women, and idiopathic (lack of a gynaecological structural cause), which was concluded in only 75 women on laparoscopy, but was the conclusion of the EIP in 156 women.
| Cause of pelvic pain | MRI finding | Expert panel consensus, n | Sensitivity (95% CI) | Specificity (95% CI) | Likelihood ratio (95% CI) | Diagnostic odds ratio (95% CI) | Test of association | ||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Positive | Negative | ||||||
| Idiopathica | Yes | 26 | 23 | 16.7% (11.2% to 23.5%) | 82.4% (74.8% to 88.5%) | 0.9 (0.6 to 1.6) | 1.0 (0.9 to 1.1) | 0.9 (0.5 to 1.7) | p = 0.9 |
| No | 130 | 108 | |||||||
| Idiopathicb | Yes | 88 | 60 | 56.4% (48.3% to 64.3%) | 54.2% (45.3% to 62.9%) | 1.2 (0.9 to 1.5) | 0.8 (0.6 to 1.0) | 1.5 (0.9 to 2.4) | p = 0.08 |
| No | 68 | 71 | |||||||
| Superficial peritoneal endometriosis | Yes | – | – | – | – | – | – | – | – |
| No | 71 | 216 | |||||||
| Deep-infiltrating endometriosis | Yes | 1 | 2 | 2.6% (0.1% to 13.5%) | 99.2% (97.1% to 99.9%) | 3.15 (0.29 to 34.0) | 0.98 (0.93 to 1.03) | 3.21 (0.28 to 36.30) | p = 0.36 |
| No | 38 | 244 | |||||||
| Endometrioma of ovary | Yes | 9 | 22 | 81.8% (48.2% to 97.7%) | 91.9% (88.0% to 94.8%) | 10.1 (6.2 to 16.4) | 0.2 (0.1 to 0.7) | 50.9 (10.4 to 250.5) | p < 0.0001 |
| No | 2 | 249 | |||||||
| Adhesions | Yes | 9 | 28 | 37.5% (18.8% to 59.4%) | 89.2% (84.8% to 92.7%) | 3.5 (1.9 to 6.5) | 0.7 (0.5 to 0.9) | 5.0 (2.0 to 12.4) | p = 0.001 |
| No | 15 | 232 | |||||||
| Ovarian cysts | Yes | 0 | 27 | 0% (0% to 97.5%) | 90.4% (86.3% to 93.6%) | – | – | – | p = 0.9 |
| No | 1 | 254 | |||||||
| Adenomyosisa [post MRI EIPc] | Yes | 9 (15) | 184 (178) | 81.8% (48.2% to 97.7%) | 31.6% (26.1% to 37.5%) | 1.2 (0.9 to 1.6) | 0.6 (0.2 to 2.0) | 2.0 (0.4 to 9.8) | p = 0.5 |
| No | 2 (1) | 85 (86) | [93.8% (70.0% to 99.8%)] | [32.6% (27.0% to 38.6%)] | [1.4 (1.2 to 1.6)] | [0.2 (0.03 to 1.3)] | [7.2 (0.9 to 55.8)] | [p = 0.03] | |
| Adenomyosisb [post MRI EIPc] | Yes | 0 (7) | 34 (27) | 0% (0% to 30.8) | 87.2% (82.6% to 91.0%) | 0 (–) | 1.15 (1.1 to 1.2) | 0 (–) | p = 0.6 |
| No | 10 (8) | 232 (234) | [46.7% (21.3% to 73.4%)] | [89.7% (85.3% to 93.1%)] | [4.5 (2.4 to 8.6)] | [0.6 (0.4 to 0.9)] | [7.6 (2.6 to 22.6)] | [p = 0.001] | |
| Fibroids | Yes | 4 | 52 | 100% (39.8 to 100) | 81.4% (76.4 to 85.8) | 5.4 (4.2 to 6.9) | 0 (–) | – (–) | p = 0.001 |
| No | 0 | 228 | |||||||
The accuracy of MRI to identify the cause of pelvic pain was inestimable for superficial peritoneal endometriosis and non-gynaecological causes, as no MRI definitions were available. Estimates of test sensitivity for ovarian cysts and fibroids were based on only one and four cases, respectively, and thus were very imprecise.
The sensitivity of MRI for detecting deep-infiltrating endometriosis as a cause of pelvic pain was very low (3%), but close to 80% for endometrioma of the ovary (albeit with wide CIs). The sensitivity for adhesions and adenomyosis using the second definition was between 40% and 50%. For all these conditions, specificity was around 90%.
There was no significant relationship between the MRI findings and the expert panel findings drawing an idiopathic conclusion for the cause of pelvic pain for either definition (p = 0.9 and p = 0.08, respectively). For the second definition (using the more stringent definition of adenomyosis), 159 out of 287 MRI findings and expert consensus classifications of whether the chronic pain was idiopathic or structural (55%) were in agreement.
The impact of magnetic resonance imaging on confidence and accuracy in making diagnoses
The independent gynaecologist rated the likelihood of each cause of CPP first when provided with all baseline data (including ultrasound reports) and, second, when additionally provided with the report from the MRI scan. This allows an assessment to be made of the degree to which the MRI scan provided additional diagnostic information over and above that available from the baseline assessments. The independent gynaecologist was not provided with the MRI criteria stated in Table 2, but was provided wih only the measurements made on MRI and the textual report. Each independent gynaecologist rated their certainty that each condition was causing pelvic pain on a scale of 0 (no chance) to 10 (certain). We deduced the certainty that the diagnosis was idiopathic by subtracting the highest score for the gynaecological structural causes from 10. The results for scores of 0–3, 4–6 and 7–10 are presented in Table 9, along with the likelihood ratios computed to indicate the degree to which high (or low) scores rule each condition in (or out). We measured the diagnostic accuracy across all scores by plotting ROC curves for each condition (Figure 5) and summarised the accuracy as the AUROC value. The likelihood ratio and AUROC values were computed for assessments made on baseline evidence alone, and then made with the additional provision of the MRI report; the incremental diagnostic value of the MRI scan is summarised using the change in the AUROC value and by comparing the likelihood ratio values.
FIGURE 5.
Accuracy of assessments of the cause of pelvic pain pre and post MRI and laparoscopy: ROC curves for comparison. (a) Idiopathic; (b) superficial peritoneal endometriosis; (c) deep-infiltrating endometriosis; (d) endometrioma of the ovary; (e) adhesions; and (f) adenomyosis (reference standard with MRI).
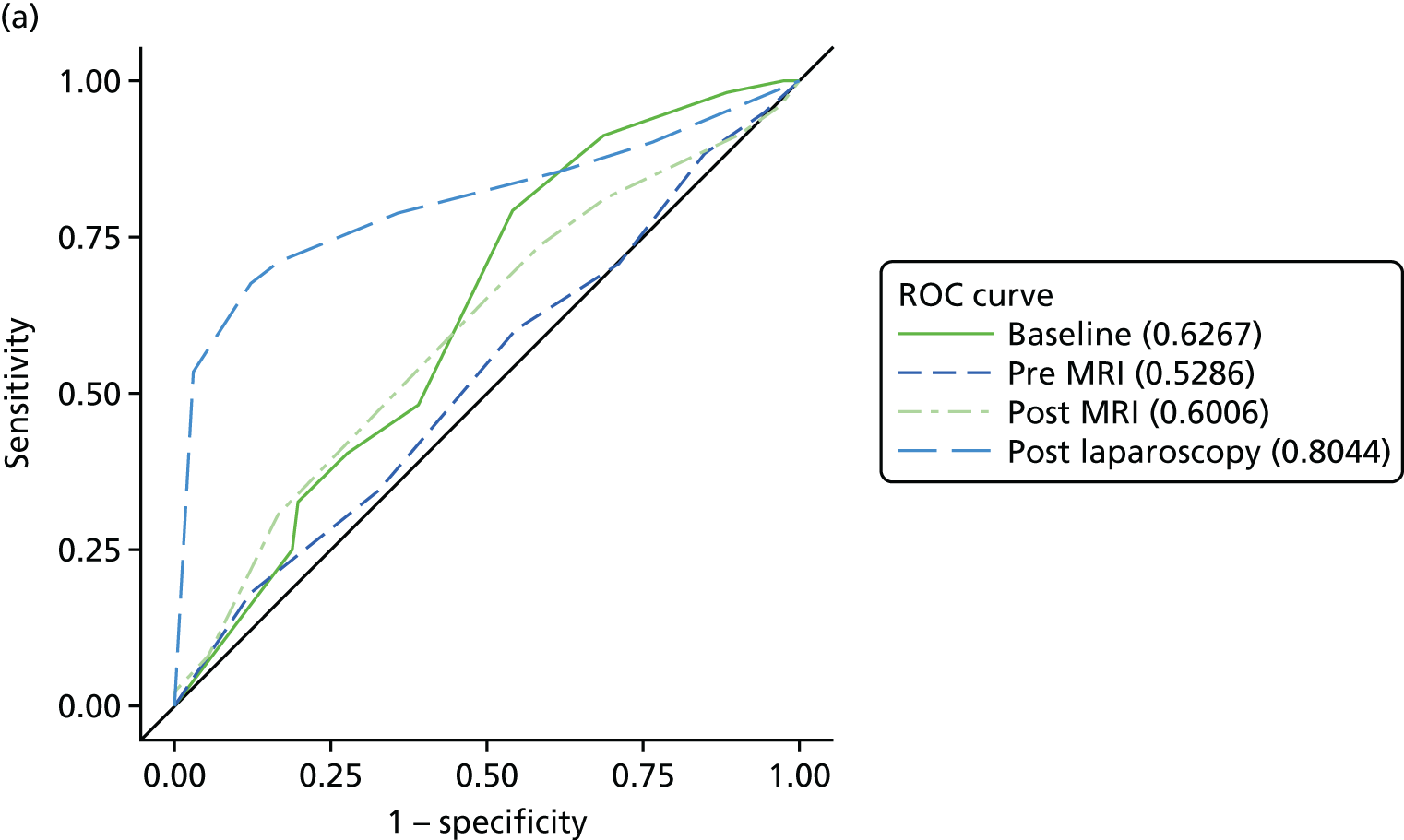
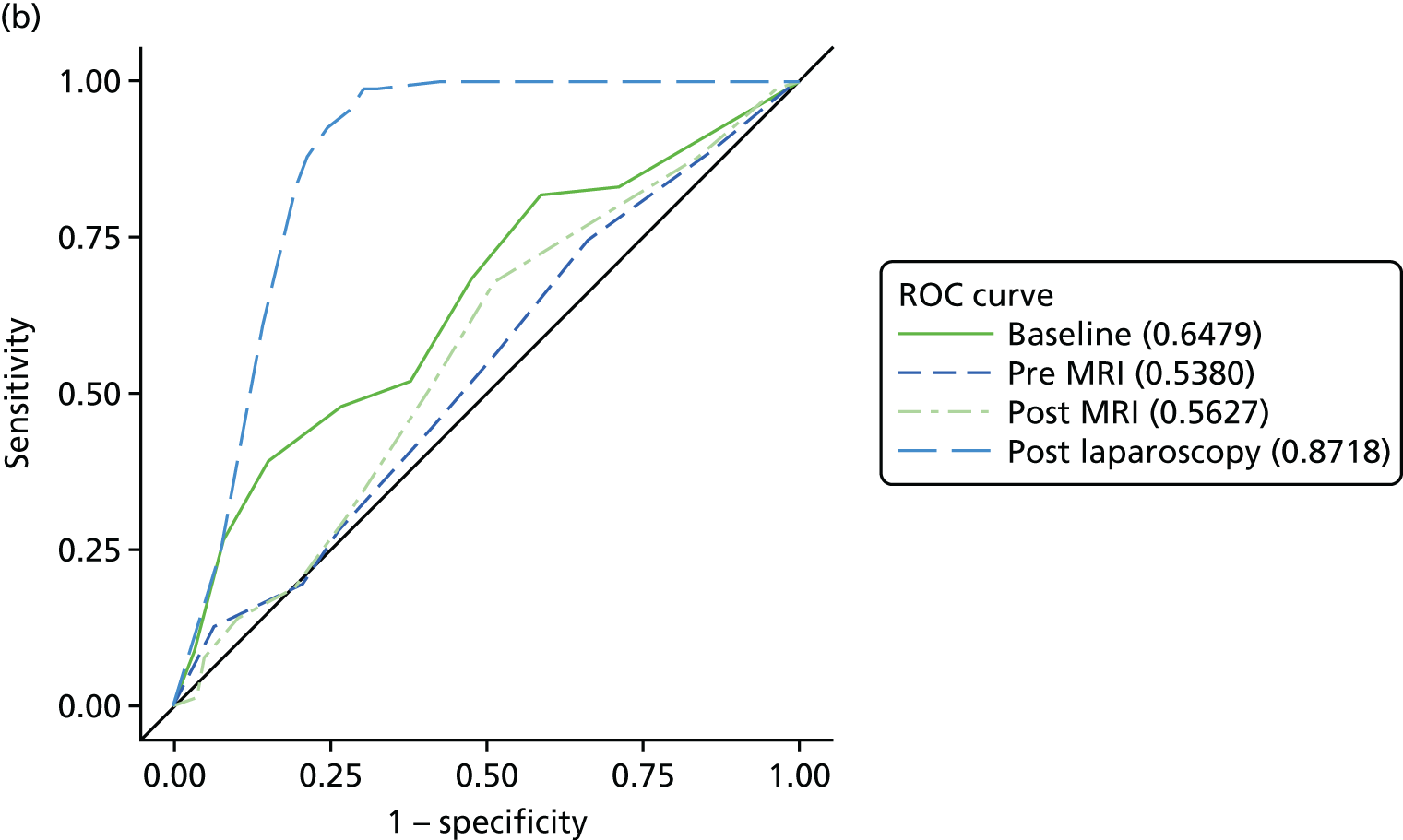
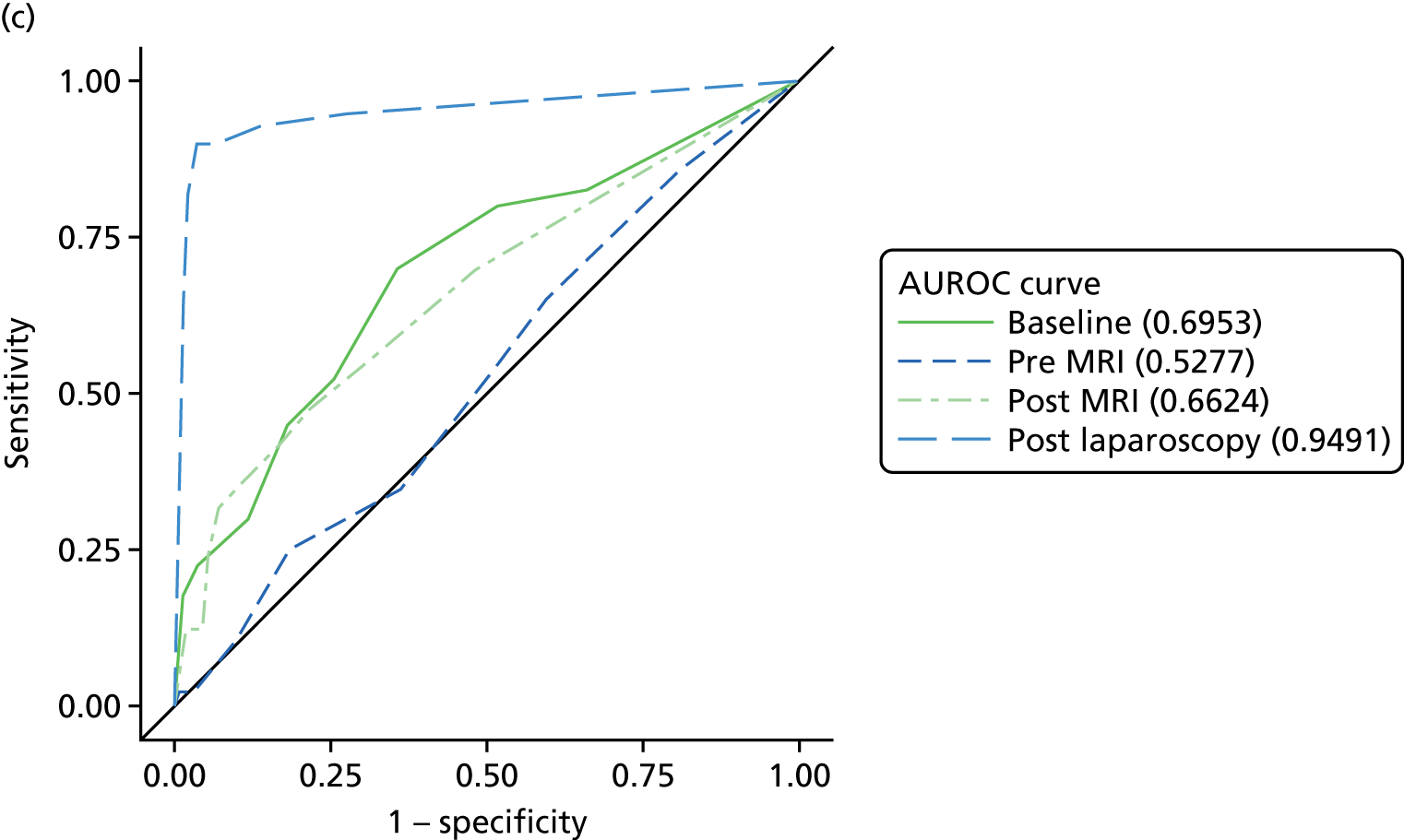
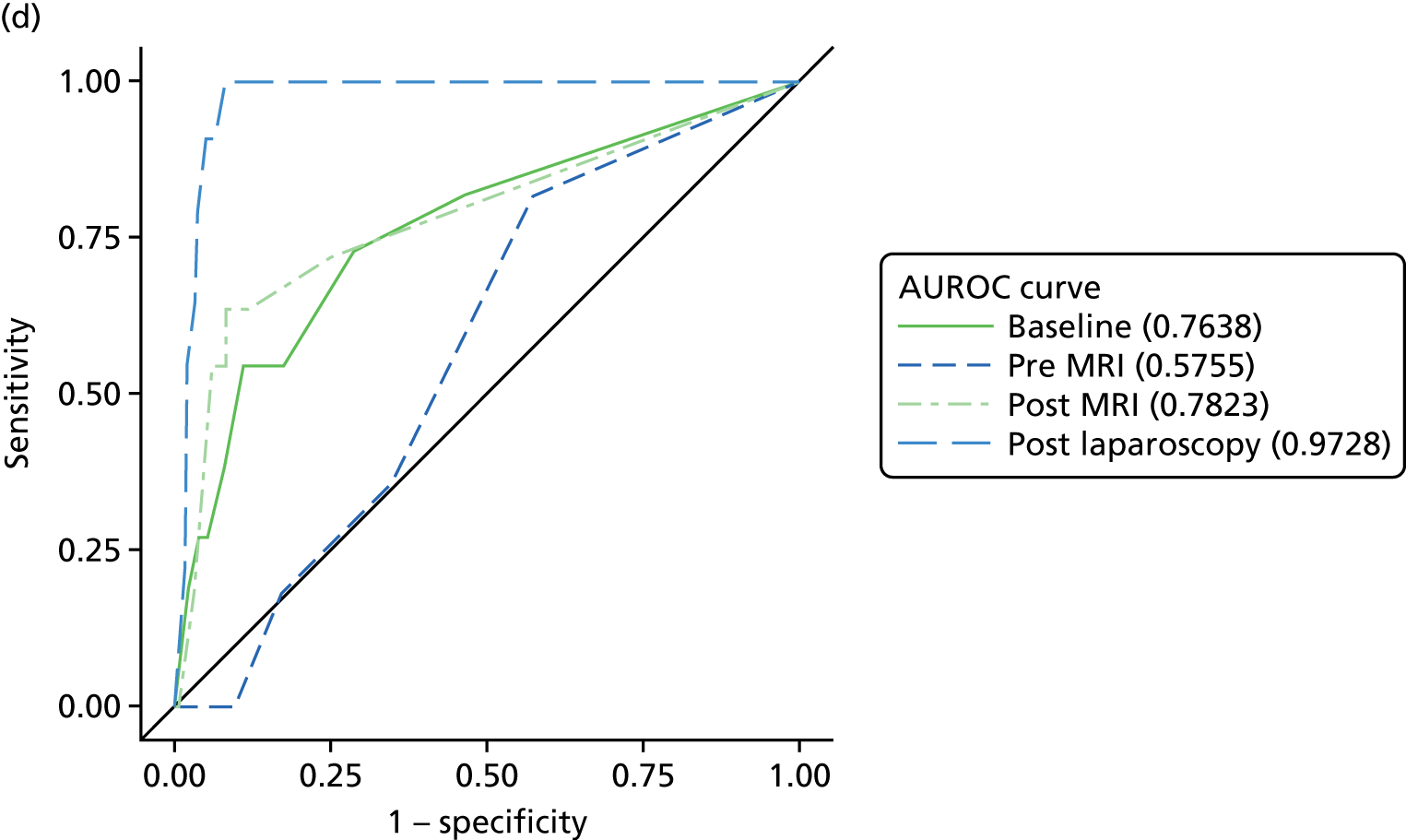
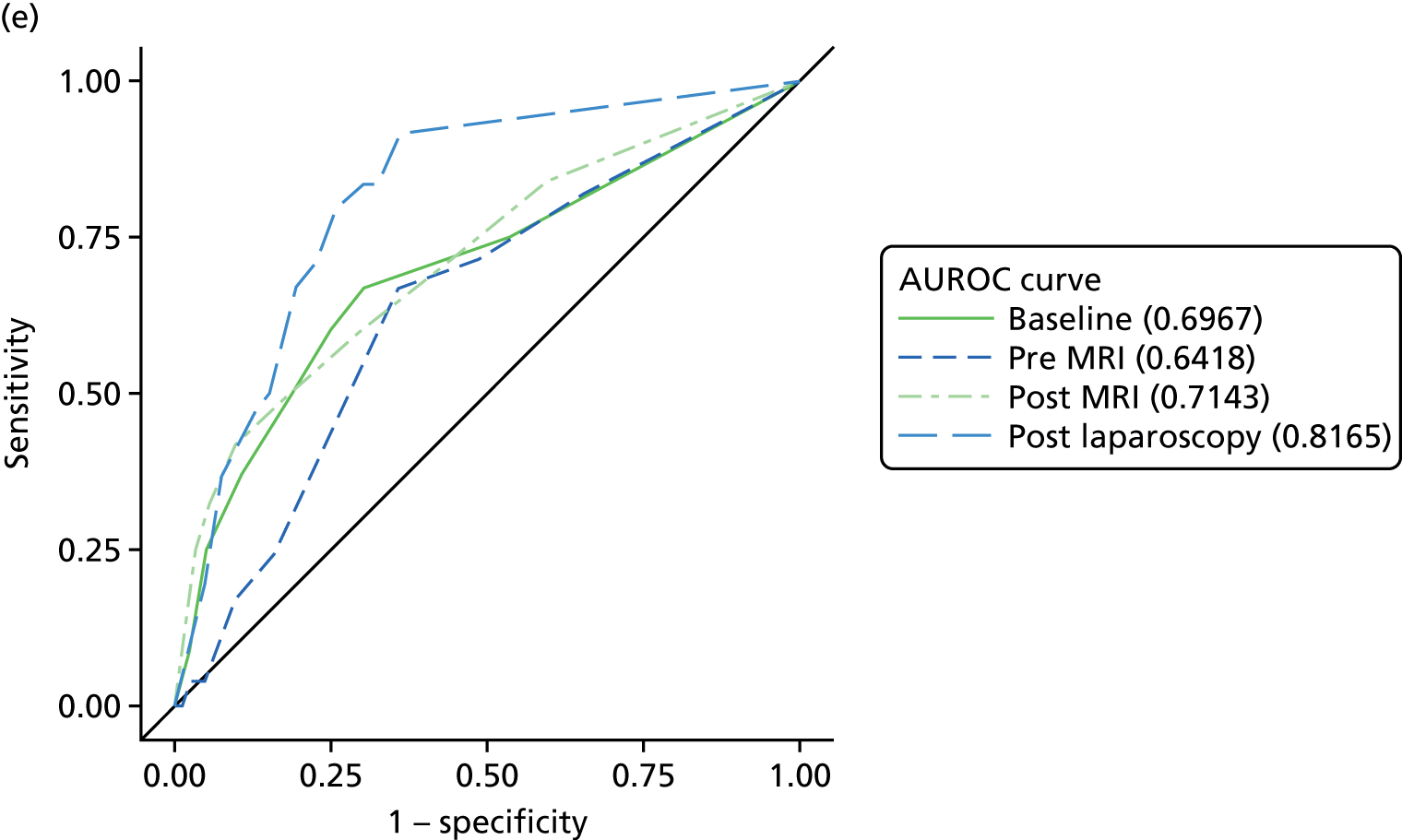
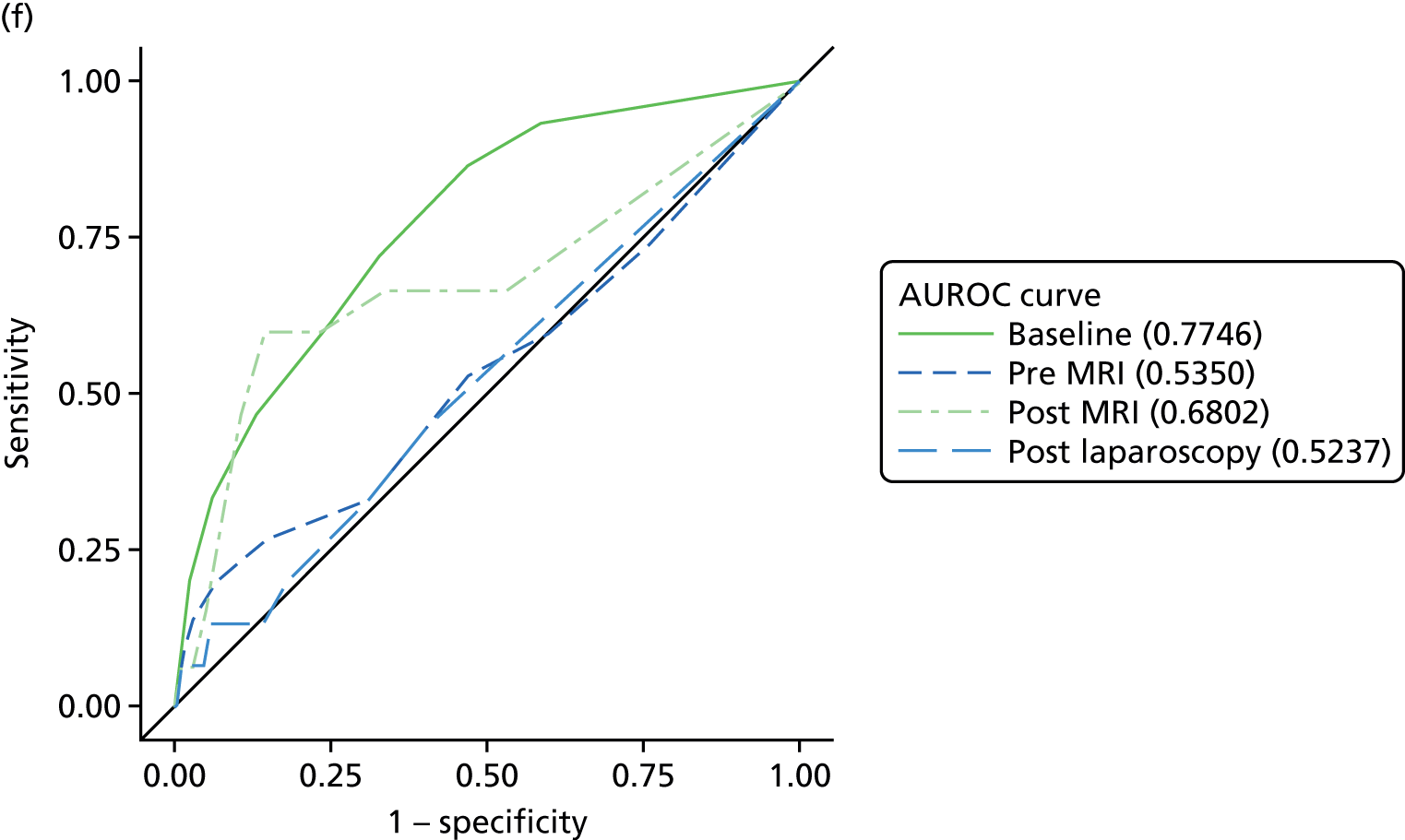
An AUROC value of 0.5 would be obtained if diagnoses were made by chance, whereas a test with perfect discrimination would have a value of 1.0. The baseline values of the AUROC were not significantly different from 0.5 for all conditions except adhesions (AUROC value of 0.64), indicating that the independent gynaecologist was not significantly better than chance in producing a diagnosis that agreed with that of the EIP, and only had a weak relationship for adhesions. Provision of the MRI report improved the accuracy of all diagnoses, but the increase in AUROC value was only statistically significant for deep-infiltrating endometriosis (p = 0.006) and endometrioma of the ovary (p = 0.02). Likelihood ratios for scores in the 4–6 and 7–10 categories deep-infiltrating endometriosis and endometrioma of the ovary were around 4–7, which are not high enough to convincingly rule in these diagnoses. Although scores of 7–10 for adhesions had the highest likelihood ratio, at 7.3, they were made in only 15 women, and detected in only 30% of those with adhesions as a cause of pelvic pain (Table 10).
| Cause of pelvic pain | Diagnostic confidence score | Rating | Change in AUROC (95% CI; p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before MRI results revealed | After MRI results revealed | |||||||||
| Expert panel consensus, n | Likelihood ratio (95% CI) | AUROC (95% CI) | Expert panel consensus, n | Likelihood ratio (95% CI) | AUROC (95% CI) | |||||
| Yes | No | Yes | No | |||||||
| Idiopathic | 7–10 | 53 | 43 | 1.0 (0.7 to 1.4) | 0.53 (0.46 to 0.60) | 77 | 44 | 1.5 (1.1 to 2.0) | 0.60 (0.53 to 0.67) | +0.07 (–0.01 to 0.15; p = 0.06) |
| 4–6 | 86 | 69 | 1.0 (0.8 to 1.3) | 49 | 47 | 0.9 (0.6 to 1.2) | ||||
| 0–3 | 17 | 19 | 0.8 (0.4 to 1.4) | 30 | 40 | 0.6 (0.4 to 1.0) | ||||
| Superficial peritoneal endometriosis | 7–10 | 9 | 13 | 2.1 (0.9 to 4.7) | 0.54 (0.46 to 0.61) | 5 | 10 | 1.5 (0.5 to 4.3) | 0.56 (0.49 to 0.64) | +0.02 (–0.05 to 0.10; p = 0.5) |
| 4–6 | 28 | 92 | 0.9 (0.7 to 1.3) | 19 | 54 | 1.1 (0.7 to 1.7) | ||||
| 0–3 | 34 | 111 | 0.9 (0.7 to 1.2) | 47 | 152 | 0.9 (0.8 to 1.1) | ||||
| Deep-infiltrating endometriosis | 7–10 | 1 | 2 | 3.1 (0.3 to 33.3) | 0.53 (0.43 to 0.62) | 5 | 6 | 5.1 (1.7 to 9.4) | 0.66 (0.57 to 0.76) | +0.13 (0.04 to 0.23; p = 0.006) |
| 4–6 | 9 | 42 | 1.3 (0.7 to 2.5) | 8 | 12 | 4.1 (1.8 to 9.4) | ||||
| 0–3 | 30 | 203 | 0.9 (0.8 to 1.1) | 27 | 229 | 0.7 (0.6 to 0.9) | ||||
| Endometrioma of the ovary | 7–10 | 0 | 2 | 0 | 0.58 (0.44 to 0.71) | 2 | 8 | 6.3 (1.5 to 26.1) | 0.78 (0.62 to 0.95) | +0.20 (0.02 to 0.39; p = 0.02) |
| 4–6 | 0 | 24 | 0 | 4 | 14 | 7.2 (2.8 to 18.2) | ||||
| 0–3 | 11 | 250 | 1.1 (1.0 to 1.2) | 5 | 254 | 0.5 (0.3 to 0.9) | ||||
| Adhesions | 7–10 | 1 | 5 | 2.2 (0.3 to 18.0) | 0.64 (0.53 to 0.75) | 6 | 9 | 7.3 (2.8 to 18.8) | 0.72 (0.60 to 0.83) | +0.08 (–0.03 to 0.18; p = 0.2) |
| 4–6 | 5 | 39 | 1.4 (0.6 to 3.2) | 6 | 37 | 1.8 (0.8 to 3.8) | ||||
| 0–3 | 18 | 219 | 0.9 (0.7 to 1.1) | 12 | 217 | 0.6 (0.4 to 0.9) | ||||
| Adenomyosis (post-MRI EIP consensus) | 7–10 | 2 | 7 | 4.8 (1.1 to 21.4) | 0.53 (0.36 to 0.71) | 6 | 22 | 4.6 (2.2 to 9.8) | 0.68 (0.50 to 0.85) | +0.15 (–0.03 to 0.32; p = 0.1) |
| 4–6 | 3 | 80 | 0.6 (0.2 to 1.8) | 4 | 41 | 1.7 (0.7 to 4.0) | ||||
| 0–3 | 11 | 184 | 1.0 (0.7 to 1.4) | 6 | 208 | 0.5 (0.3 to 0.9) | ||||
Accuracy of assessments of the cause of pelvic pain pre and post MRI and laparoscopy
Table 11 allows comparisons to be made between the findings from MRI and laparoscopy. As for assessment of the incremental diagnostic value of MRI, we report values for the AUROC, the change in AUROC and the likelihood ratios to describe diagnostic accuracy.
| Cause of pelvic pain | Diagnostic confidence score | Rating after | Change in AUROC (95% CI; p-value) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRI results are revealed | Laparoscopy | |||||||||
| Expert panel consensus, n | Likelihood ratio (95% CI) | AUROC (95% CI) | Expert panel consensus, n | Likelihood ratio (95% CI) | AUROC (95% CI) | |||||
| Yes | No | Yes | No | |||||||
| Idiopathic | 7–10 | 77 | 44 | 1.5 (1.1 to 2.0) | 0.60 (0.53 to 0.67) | 81 | 4 | 17.6 (6.6 to 46.6) | 0.80 (0.75 to 0.86) | +0.20 (0.12 to 0.28; p < 0.0001) |
| 4–6 | 49 | 47 | 0.9 (0.6 to 1.2) | 26 | 18 | 1.3 (0.7 to 2.2) | ||||
| 0–3 | 30 | 40 | 0.6 (0.4 to 1.0) | 44 | 109 | 0.4 (0.3 to 0.5) | ||||
| Superficial peritoneal endometriosis | 7–10 | 5 | 10 | 1.5 (0.5 to 4.3) | 0.56 (0.49 to 0.64) | 54 | 38 | 4.2 (3.1 to 5.7) | 0.87 (0.83 to 0.91) | +0.31 (0.22 to 0.39; p < 0.0001) |
| 4–6 | 19 | 54 | 1.1 (0.7 to 1.7) | 12 | 15 | 2.4 (1.2 to 4.8) | ||||
| 0–3 | 47 | 152 | 0.9 (0.8 to 1.1) | 5 | 156 | 0.1 (0.04 to 0.2) | ||||
| Deep-infiltrating endometriosis | 7–10 | 5 | 6 | 5.1 (1.7 to 9.4) | 0.66 (0.57 to 0.76) | 33 | 6 | 33.0 (14.7 to 73.3) | 0.95 (0.90 to 0.99) | +0.29 (0.16 to 0.35; p < 0.0001) |
| 4–6 | 8 | 12 | 4.1 (1.8 to 9.4) | 3 | 5 | 3.6 (0.9 to 14.4) | ||||
| 0–3 | 27 | 229 | 0.7 (0.6 to 0.9) | 4 | 228 | 0.1 (0.04 to 0.3) | ||||
| Endometrioma of ovary | 7–10 | 2 | 8 | 6.3 (1.5 to 26.1) | 0.78 (0.62 to 0.95) | 9 | 10 | 21.8 (11.2 to 42.6) | 0.97 (0.95 to 0.99) | +0.19 (0.03 to 0.36; p = 0.02) |
| 4–6 | 4 | 14 | 7.2 (2.8 to 18.2) | 1 | 7 | 3.5 (0.5 to 25.7) | ||||
| 0–3 | 5 | 254 | 0.5 (0.3 to 0.9) | 1 | 250 | 0.1 (0.01 to 0.6) | ||||
| Adhesions | 7–10 | 6 | 9 | 7.3 (2.8 to 18.8) | 0.72 (0.60 to 0.83) | 12 | 39 | 3.3 (2.0 to 5.4) | 0.82 (0.74 to 0.90) | +0.10 (–0.05 to 0.26; p = 0.2) |
| 4–6 | 6 | 37 | 1.8 (0.8 to 3.8) | 7 | 28 | 2.7 (1.3 to 5.4) | ||||
| 0–3 | 12 | 217 | 0.6 (0.4 to 0.9) | 5 | 189 | 0.3 (0.1 to 0.6) | ||||
| Adenomyosis (post-MRI EIP consensus) | 7–10 | 6 | 22 | 4.6 (2.2 to 9.8) | 0.68 (0.50 to 0.85) | 1 | 12 | 1.5 (0.2 to 10.5) | 0.52 (0.38 to 0.67) | –0.15 (–0.33 to 0.02; p = 0.09) |
| 4–6 | 4 | 41 | 1.7 (0.7 to 4.0) | 1 | 25 | 0.7 (0.1 to 4.8) | ||||
| 0–3 | 6 | 208 | 0.5 (0.3 to 0.9) | 13 | 226 | 1.0 (0.8 to 1.2) | ||||
The accuracy of diagnoses of the causes of pelvic pain made by laparoscopy was high for superficial peritoneal endometriosis (AUROC = 0.87), deep-infiltrating endometriosis (AUROC = 0.95) and endometrioma of the ovary (AUROC = 0.97). Certainty scores of 7 and above could accurately rule for deep-infiltrating endometriosis [LR (likelihood ratio) = 33] and endometrioma of the ovary (LR = 22), and low scores of 0–3 could rule out all three conditions (LR = 0.1).
Poorer performance was noted for laparoscopy diagnoses of idiopathic pelvic pain (AUROC = 0.80) and of adhesions being the cause of pelvic pain (AUROC = 0.82). However, a high score for idiopathic pelvic pain (computed as 10 minus the highest score for any other condition) provided convincing evidence (LR = 18) to rule in an idiopathic diagnosis. Laparoscopy was no better than chance in diagnosing adenomyosis as the cause of pelvic pain (AUROC = 0.52).
For four diagnoses (idiopathic pelvic pain, superficial peritoneal endometriosis, deep-infiltrating endometriosis and endometrioma of the ovary), laparoscopy was significantly more accurate than MRI-based diagnoses in identifying the cause of pelvic pain.
However, there are two reasons to consider these comparisons as being at risk of bias, and that they may potentially overestimate the magnitude of differences between the tests in favour of laparoscopy. First, Figure 5 shows that the ROC curves for baseline assessments made by the treating gynaecologist (labelled ‘baseline’) and the independent gynaecologist (labelled ‘pre MRI’), based on the same information obtained from history, examination and ultrasound, are not comparable. For all six conditions presented in Figure 5, the treating gynaecologist had superior diagnostic accuracy to the independent gynaecologist, which possibly reflects that the diagnoses of the treating clinician benefited from direct interaction with the patient, which the blinded independent gynaecologist lacked (more detail on idiopathic CPP diagnoses is given in Appendix 2, Tables 34 and 35). Given that the post-laparoscopy and post-MRI diagnoses were made by the treating gynaecologist and the independent gynaecologist, respectively, the bias introduced by this is likely to have led to overestimation of the difference.
Second, the EIP had access to the laparoscopy findings when making their reference diagnoses for all conditions, which is likely to have introduced a degree of incorporation bias, leading to overestimation of the accuracy of laparoscopy.
The diagnoses of adhesions showed no significant differences in accuracy between any of the four diagnoses for adhesions. This is likely, in part, to be explained by misclassification in the reference standard, which is suspected through the low agreement rates observed between the assessors (see Table 6).
Adenomyosis was the only diagnosis of CPP for which the post MRI test accuracy was superior to the post laparoscopy accuracy, despite the difference not being statistically significant. Intriguingly, the diagnosis of adenomyosis made by the recruiting clinician prior to either test was found to be the most accurate.
Use and recommendations for therapeutic laparoscopy
For the final analyses, women were categorised according to whether or not they would benefit from therapeutic laparoscopy. A test pathway incorporating MRI prior to laparoscopy would be of benefit to women should it correctly identify women who do not require therapeutic laparoscopy, as this is commonly undertaken during the diagnostic laparoscopic procedure.
Table 12 compares the accuracy of diagnoses made post MRI for the key conditions indicating the need for therapeutic laparoscopy, and the recommendations for therapeutic laparoscopy by the independent gynaecologist, following their assessment of the MRI report. Fifty-two out of the 112 women (46%) with the key conditions indicating the need for therapeutic laparoscopy were rated with diagnostic confidence scores of 5 or higher, although the independent gynaecologist rated more women (78/112; 70%) as potentially benefiting from a therapeutic laparoscopy, having seen the MRI report. Women with superficial peritoneal endometriosis were the most poorly identified.
| Cause of pelvic pain | Prevalence, % (reference standard) | Therapeutic laparoscopy, n (%) | Diagnostic confidence score post MRI test, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Recommended by the EIP | Performed (two missing) | ≥ 3 | ≥ 5 | ≥ 7 | Therapeutic laparoscopy recommended following MRI test | ||
| Conditions for which therapeutic laparoscopy is recommended | |||||||
| Superficial endometriosis | 71 (24.7) | 69 (97) | 58 (83) | 48 (68) | 13 (18) | 5 (7) | 49 (69) |
| Deep-infiltrating endometriosis | 40 (13.9) | 38 (95) | 31 (78) | 14 (35) | 9 (23) | 5 (13) | 30 (75) |
| Endometrioma of the ovary | 11 (3.8) | 11 (100) | 10 (91) | 7 (64) | 6 (55) | 2 (18) | 10 (91) |
| Any endometriosis | 96 (33.5) | 92 (96) | 78 (82) | 70 (73) | 35 (36) | 16 (17) | 67 (70) |
| Adhesions | 24 (8.4) | 23 (96) | 18 (75) | 14 (58) | 10 (42) | 6 (25) | 18 (75) |
| Any endometriosis, adhesions or ovarian cysts | 112 (39.0) | 106 (95) | 92 (83) | 91 (81) | 52 (46) | 31 (28) | 78 (70) |
| Conditions for which therapeutic laparoscopy is not recommended | |||||||
| Idiopathic | 72 (25.0) | 13 (18) | 34 (47) | 60 (83) | 36 (50) | 8 (11) | 49 (68) |
| Non-gynaecological cause only | 90 (31.0) | 7 (8) | 36 (40) | 81 (90) | 41 (46) | 13 (14) | 59 (66) |
| Anything other than endometriosis or adhesions or ovarian cysts | 175 (61.0) | 21 (12) | 75 (43) | 165 (94) | 104 (59) | 44 (25) | 116 (66) |
Of the 175 women with gynaecological conditions for which therapeutic laparoscopy would have no clear benefit, non-gynaecological conditions or no structural cause, the post-MRI diagnosis rated 104 (59%) with confidence scores of 5 or above for these conditions (with lower ratings for any endometriosis or adhesions). However, 116 out of the 175 women (66%) were rated as being likely to benefit from therapeutic laparoscopy, based on the interpretation of the MRI report by the independent gynaecologist.
Table 13 compares the ability of the independent gynaecologist to correctly identify women who would, and women who would not, benefit from therapeutic laparoscopy for three situations: (1) if a woman received an EIP diagnosis of superficial endometriosis, deep-infiltrating endometriosis, endometrioma of the ovary or adhesions; (2) if the EIP directly rated a woman as being likely to benefit from a therapeutic laparoscopy; and (3) if a woman was recorded on the operative sheet as having received a therapeutic component during the study laparoscopy.
| Therapeutic laparoscopy recommended following MRI test | EIP-identified condition amenable to therapeutic laparoscopy | Therapeutic laparoscopy recommended by the EIP | Therapeutic laparoscopy performed | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Yes, n | 78 | 116 | 88 | 106 | 117 | 76 |
| No, n | 34 | 59 | 39 | 54 | 50 | 42 |
| Sensitivity (95% CI) | 69.6% (60.2% to 78.0%) | 69.3% (60.5% to 77.2%) | 70.1% (62.5% to 76.9%) | |||
| Specificity (95% CI) | 33.7% (26.8% to 41.2%) | 33.8% (26.5% to 41.6%) | 35.6% (27.0% to 44.9%) | |||
| PPV (95% CI) | 40.2% (33.2% to 47.5%) | 45.4% (38.2% to 52.6%) | 60.6% (53.3% to 67.6%) | |||
| NPV (95% CI) | 63.4% (52.8% to 73.1%) | 58.1% (47.4% to 68.2%) | 45.7% (35.2% to 56.4%) | |||
For all three comparisons, the MRI assessment identified 70% of women categorised as requiring therapeutic laparoscopy (sensitivity). However, the MRI assessment wrongly suggested that two-thirds of women not meeting these criteria did require therapeutic laparoscopy (specificity).
Inspection of the predictive values provides information on the number of errors in decision-making that would be made should the MRI report be used to inform the decision to progress to laparoscopy. As the three criteria were met by increasing proportions of women [the first criterion was met by 112 women (39%), the second by 127 women (44%) and the third by 167 women (58%)], the predictive values of MRI recommendations changed according to the comparison that was made (see Table 13).
First, compared with an EIP recommendation that a woman would benefit from therapeutic laparoscopy, a recommendation based on the MRI findings to proceed to laparoscopy would be correct only for 45% of women: 55% would undergo the procedure unnecessarily. In comparison, the alternative pathway of all women receiving laparoscopy leads to 56% of women receiving the procedure unnecessarily. A recommendation to not proceed to laparoscopy would be correct in 58% of women, whereas 42% of women not receiving laparoscopy would have benefited from it.
Extrapolating from these figures, and assuming the same prevalence of conditions, in a cohort of 1000 women, deciding to proceed to laparoscopy based on MRI findings would lead to 369 women who did not require laparoscopy receiving it, and 136 women who did require laparoscopy not receiving it. In a ‘laparoscopy for all’ strategy, 557 women who did not require laparoscopy would receive it.
If we compare the recommendation for therapeutic laparoscopy from the MRI findings against the actual diagnoses made by the EIP, the number of women receiving laparoscopy unnecessarily would increase to 404 per 1000 women, and the number of women not receiving laparoscopy who did require it would decrease to 118. If the comparison was made against the actual use of therapeutic laparoscopy, the number of women receiving laparoscopy unnecessarily would decrease to 264 per 1000 women, and the number not receiving laparoscopy who did require it would increase to 174.
Chapter 4 Economic evaluation
Introduction
One of the factors that contributes to the lack of speedy diagnosis of CPP among women is that laparoscopy is perceived to be an invasive, expensive and potentially risky procedure, and that it is used far too frequently in the NHS, as a significant proportion of women have no pathology identified. 73 MRI represents a less invasive and, potentially, less expensive procedure than laparoscopy, and as such may be the preferred option for the woman or for the health-care provider. However, as shown in previous chapters of this report, the test accuracy of diagnostic laparoscopy in detecting the structural causes of CPP is greater than that for MRI, meaning that women are more likely to be properly diagnosed and then access the appropriate treatment following a diagnostic laparoscopy than with a MRI scan. In this chapter, all of these factors are considered as part of a wider cost-effectiveness analysis, the objective of which is to examine the cost-effectiveness of MRI compared with diagnostic laparoscopy in women with undiagnosed CPP. However, the study design used in the research meant that all women received all tests, and from the data collected, ROC curves were obtained for both MRI and laparoscopy which could then inform their sensitivity and specificity values based on alternative cut-off points. This meant that the actual sensitivity and specificity values for laparoscopy, as used in usual practice, could not be informed by this study, and so consequently it was not possible to parameterise a usual-practice scenario in this economic analysis. Therefore, we consider MRI and laparoscopy with alternative sensitivity and specificity pairs (as informed by the ROC curves) as separate strategies and examine which test and sensitivity and specificity values is the most cost-effective for women with CPP. For completeness, we also compare these strategies with a no-testing scenario, in which a patient receives neither MRI nor laparoscopy.
Methods
Developing the model structure
The comparison of the pathways undertaken by women in this study is best represented in a modelling framework, in which the alternative pathways that women can follow are compared in order to diagnose the cause of their CPP. As all women in the MEDAL study were tested with MRI scans and diagnostic laparoscopy, a model was required to simulate the outcomes of each of the patient pathways when only one test was administered. A model allows explicit representation of the impact of the accuracy of the tests, the costs to the health service and the impact on the health-related QoL experienced by the women undergoing a particular pathway.
A model was developed via consultation with the research team, drawing on key clinical and modelling expertise. A decision tree was applied using TreeAge Pro 2001 software (TreeAge Software, Inc., Williamstown, MA, USA). A 6-month time horizon was adopted, as this was the period of data collection, and, given that there was very little chance of events recurring on the patient pathways (such as a patient receiving multiple laparoscopies), it was felt that this approach was the most appropriate.
Women entered the model having been identified as having CPP and being eligible for diagnostic laparoscopy. Two different patient pathways were compared, which describe alternative approaches to the diagnosis and treatment of the causes of CPP, a summary of which can be found in Figure 6. Figures 7–10 show the patient pathways considered in this analysis, which together form the model structure for the decision tree used in the cost-effectiveness analysis. For both pathways, a baseline examination was conducted, which comprised a patient history followed by a physical examination, and an ultrasound scan when clinically indicated. The remainder of the pathway followed either the laparoscopy pathway or the MRI pathway.
FIGURE 6.
Women with CPP who were eligible for laparoscopy, who received either MRI or laparoscopy.
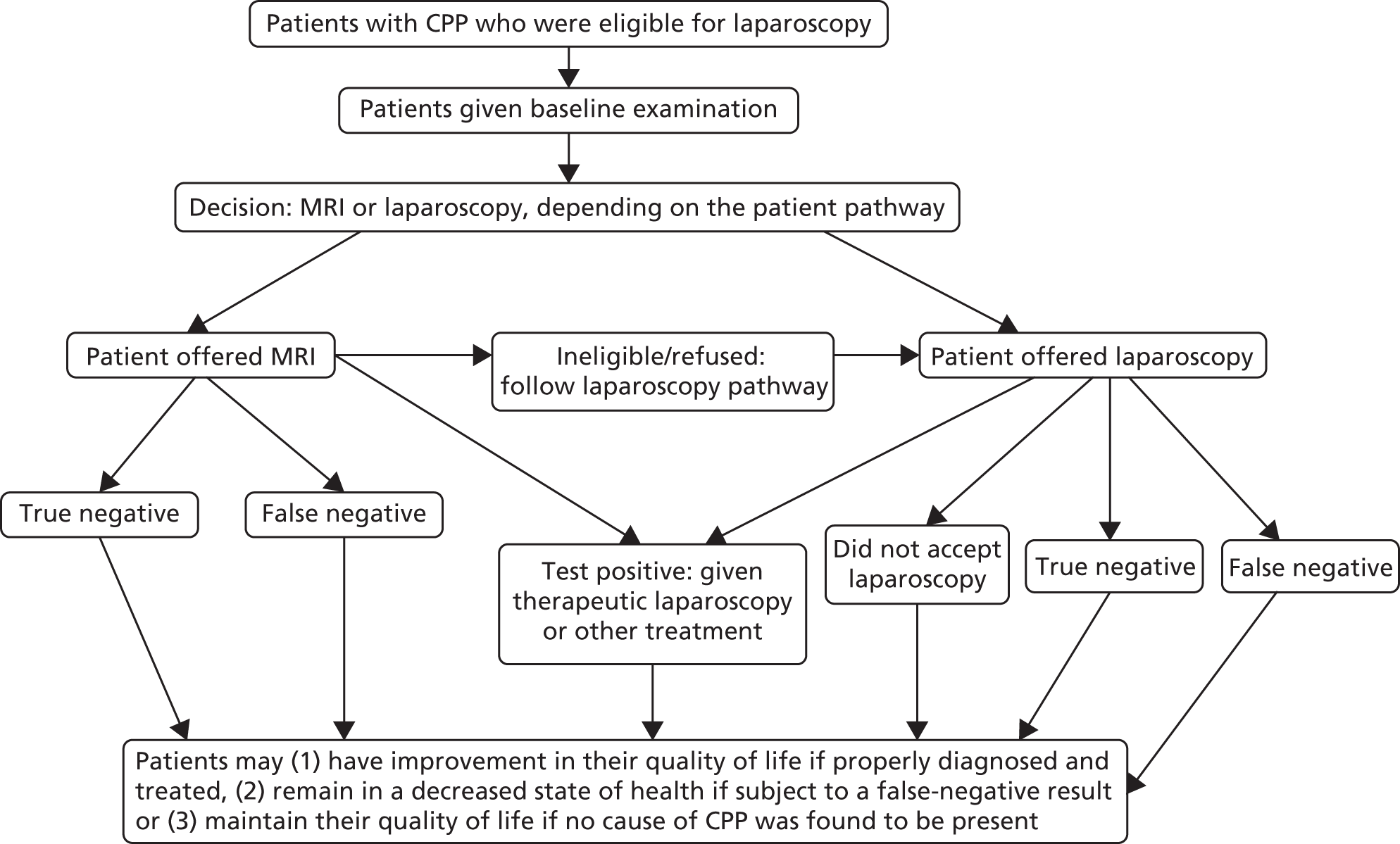
FIGURE 7.
Model structure for the laparoscopy treatment arm for women who tested positive for a structural cause of CPP. LNG-IUS, levonorgestrel-releasing intrauterine system.
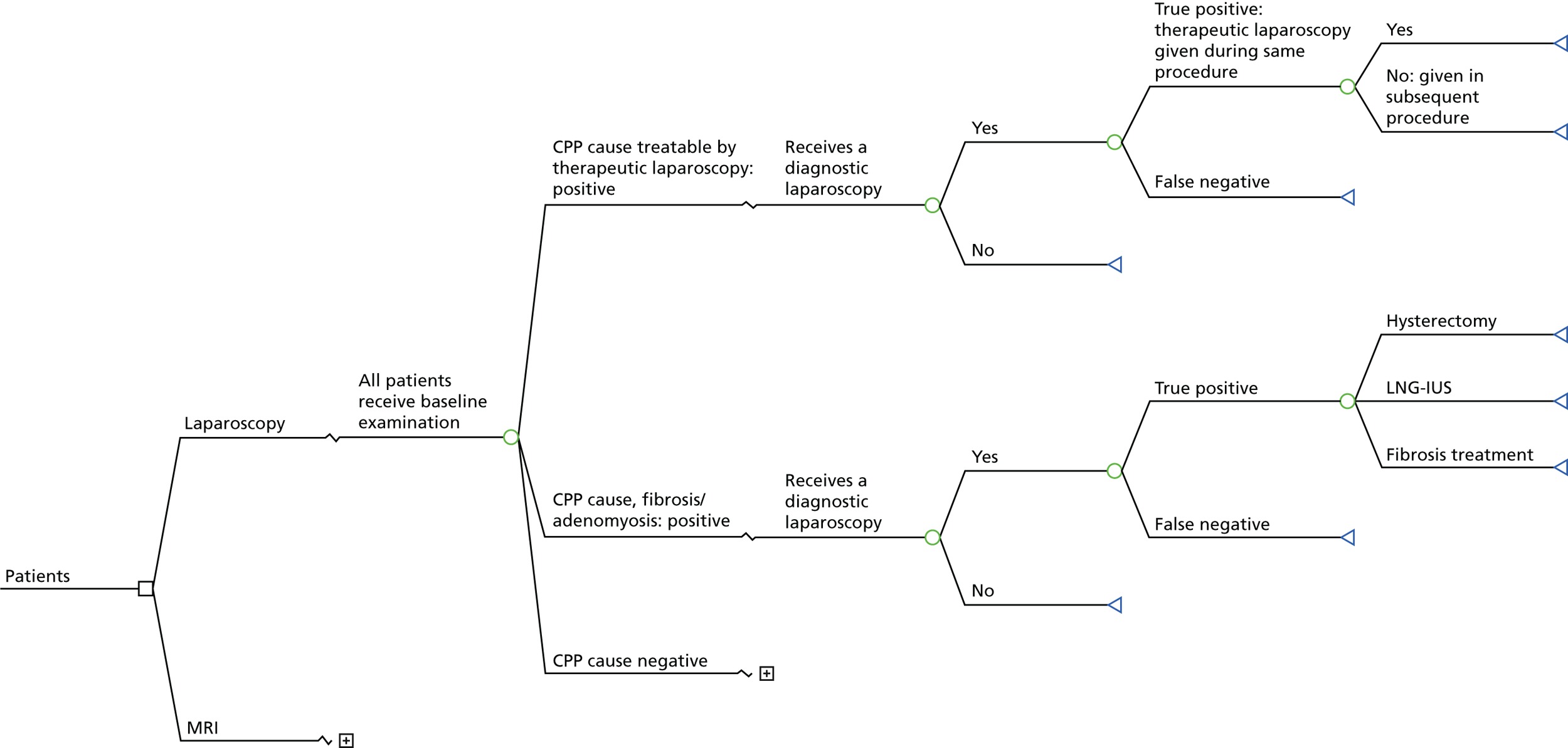
FIGURE 8.
Model structure for the MRI treatment arm for women who tested positive for a structural cause of CPP. LNG-IUS, levonorgestrel-releasing intrauterine system.
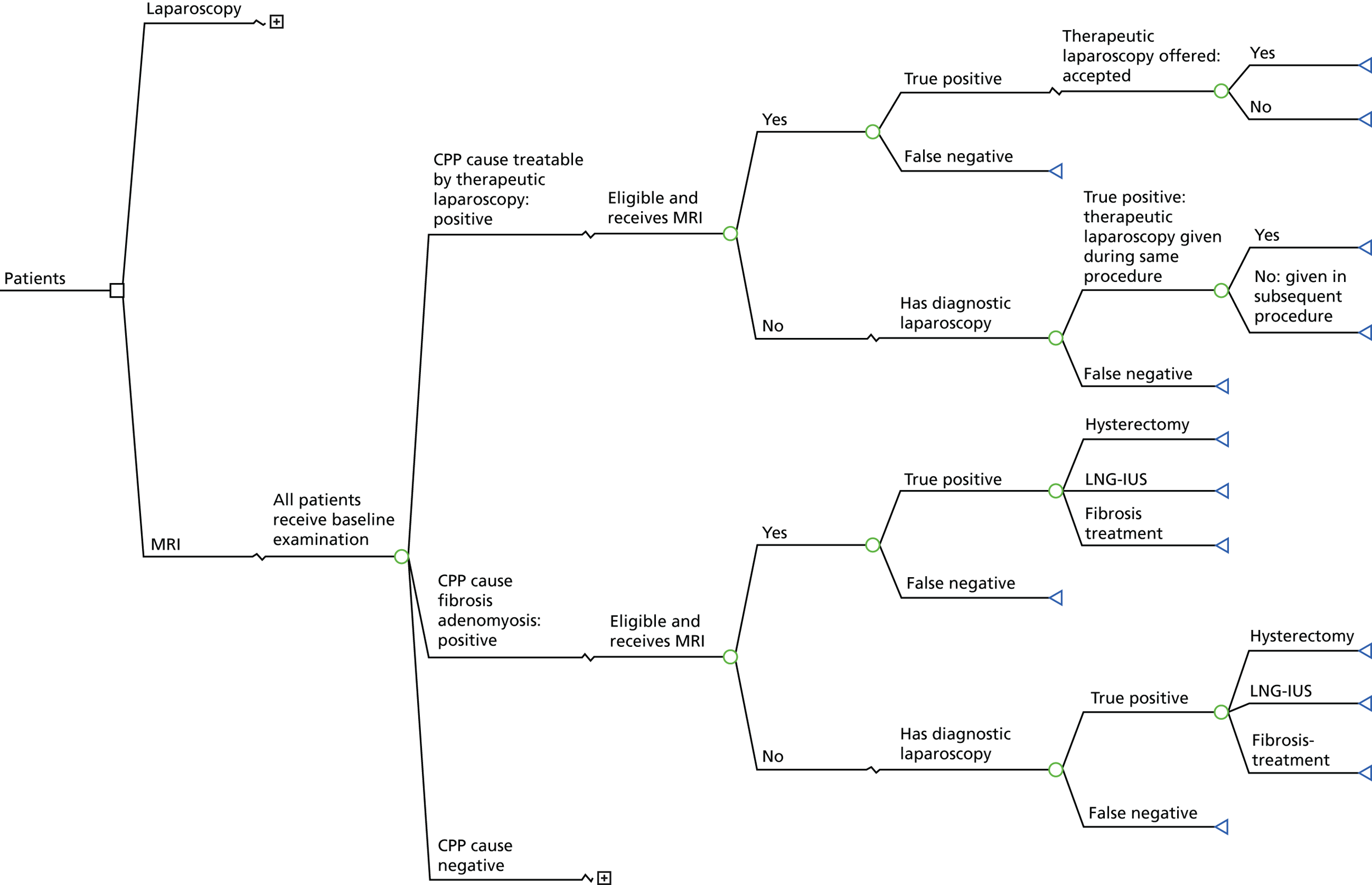
FIGURE 9.
Model structure for the laparoscopy treatment arm for women who tested negative for a structural cause of CPP. LNG-IUS, levonorgestrel-releasing intrauterine system.
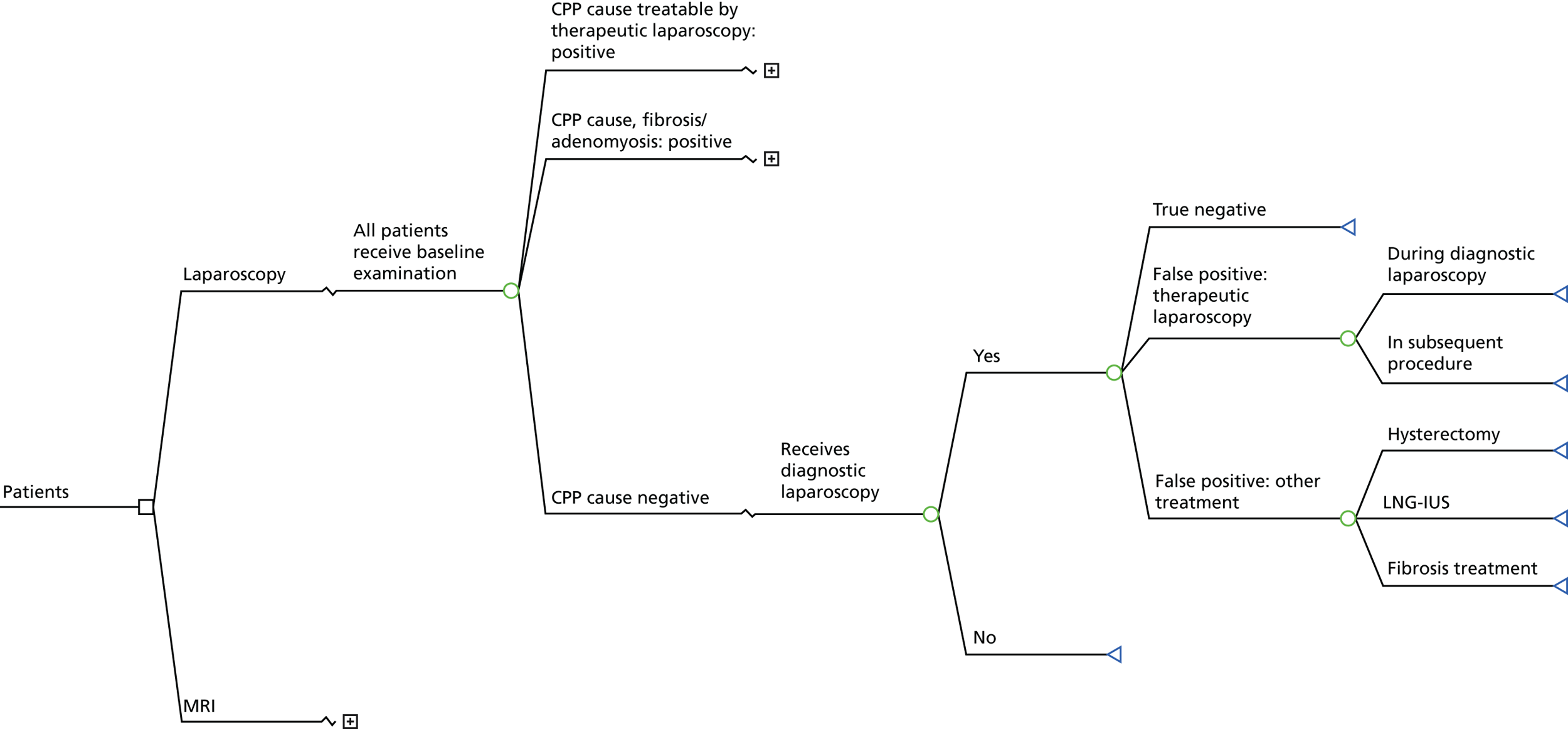
FIGURE 10.
Model structure for the MRI treatment arm for women who tested negative for a structural cause of CPP. LNG-IUS, levonorgestrel-releasing intrauterine system.
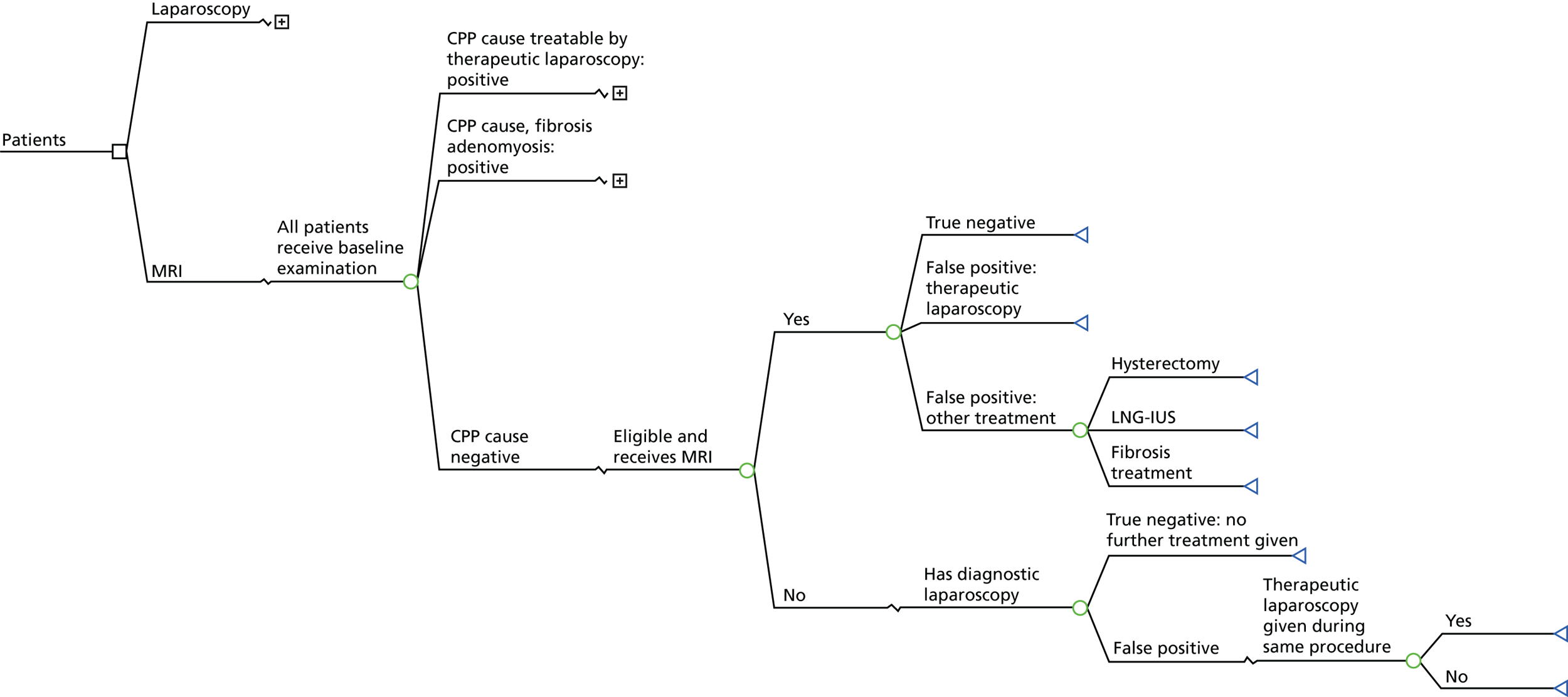
Laparoscopy pathway
Women were offered a diagnostic laparoscopy, which may or may not have been accepted. For those women who received the diagnostic laparoscopy, this would give either a positive or a negative result for the presence of a structural cause of CPP. For those who tested positive for a cause that required a therapeutic laparoscopy, this would be given during the same or a subsequent procedure. For women who tested positive for a structural cause that required other treatment, in this case fibroids or adenomyosis, this treatment would be subsequently administered. For those women who tested negative, no further tests or treatment would be administered, and if this was a false-negative test result, then the patient would remain in a decreased state of health (see Figures 7 and 9).
Magnetic resonance imaging pathway
Women were offered a MRI scan that may or may not have been accepted; women who rejected or were ineligible for a MRI scan would then follow the laparoscopy pathway (see Figures 8 and 9). Women who received a MRI scan and a positive test result were given a therapeutic laparoscopy or other treatment, as appropriate. For women who tested negative, no further tests or treatments were administered and, again, if the test result was false negative, these women would remain in a decreased state of health (see Figure 10).
No testing (neither magnetic resonance imaging nor laparoscopy)
A no-testing scenario was incorporated into this analysis, which was assumed to consist of a baseline examination (see Model assumptions). Although we acknowledge that this strategy would not be considered in practice for this patient group, the inclusion of no testing is important for comparative purposes, and can show whether or not a testing strategy is more harmful than no testing.
Model assumptions
In order to implement a working model structure and enable this analysis to be carried out, a number of model assumptions were necessary. These assumptions were broadly categorised as being related to resource use/costs, diagnostic pathway, course of treatment and QoL, and are described, as follows, in the next sections.
Resource use/costs
-
The baseline examination given to all women at the start of each strategy (including no testing) consisted of the following procedures:
-
patient history taken by a research nurse and consultant, which takes 20 minutes
-
physical examination undertaken by a consultant, which takes 20 minutes
-
an abdominal and vaginal ultrasound (for two-thirds of women).
-
-
A patient would receive one consultation prior to each laparoscopy, and then one consultation post laparoscopy, each taking 20 minutes with a consultant.
-
To allow for the increased likelihood of complications related to therapeutic laparoscopy, therapeutic laparoscopy was assumed to cost 10% more than diagnostic laparoscopy.
Diagnostic pathway
-
Only women with a structural cause of CPP were regarded as having a true-positive diagnosis and being in need of treatment.
-
A patient who received a false-positive result from a diagnostic laparoscopy would receive therapeutic laparoscopy during the same or a subsequent procedure.
-
A patient who received a false-negative test result would receive no further testing or treatment and would remain in a decreased state of health for the remainder of the time horizon of the analysis.
-
Women would always accept a laparoscopy following a positive MRI result.
-
A patient would always accept a diagnostic laparoscopy after having refused or having been ineligible for a MRI scan.
Course of treatment
-
For women with adenomyosis, 50% had a hysterectomy and 50% had a levonorgestrel-releasing intrauterine system.
-
Among those with deep-infiltrating endometriosis, 50% would have a second therapeutic laparoscopy.
-
It was assumed that all women only required one type of treatment, with women who had more than one condition being assumed to need a therapeutic laparoscopy if any one of their conditions required this treatment.
Quality of life
-
Women who dropped out after the baseline assessment, and did not go on to receive testing or treatment, had the same QoL estimates at 6 months as were measured at baseline.
-
For women who received treatment, their QoL changed in response to treatment 4 weeks after entering the model.
-
The impact on QoL of therapeutic laparoscopy was assumed to be the same as for all other treatments.
Data requirements
The data requirements for the economic evaluation were fulfilled through the use of the patient-level data collected as part of the MEDAL study.
Prevalence of the structural causes of chronic pelvic pain
Only women who were found to have a structural cause of CPP were considered to require treatment, with the remaining women assumed to be negative for structural causes. In this model, the treatments for structural causes of CPP were presented in two groups:
-
therapeutic laparoscopy – for women who required a therapeutic laparoscopy to resolve their structural cause of CPP
-
other treatment – for women who had a structural cause of CPP, but did not require a therapeutic laparoscopy, which, in this case, are women with fibroids and adenomyosis.
As shown in Table 14, data collected during the MEDAL study found that some women had more than one structural cause. In this case, if any of the women’s structural causes were considered to be an appropriate justification for a therapeutic laparoscopy, they would be incorporated into this group (see Model assumptions). The prevalence of structural causes by treatment group is shown in Table 15.
| Condition | Number of cases | Prevalence, % (95% CI) (N = 287) | Treatment group |
|---|---|---|---|
| None | 164 | 57.1 (51.2 to 62.9) | – |
| Superficial peritoneal endometriosis only | 46 | 16.0 (12.0 to 20.8) | TL |
| Deep-infiltrating endometriosis only | 20 | 7.0 (4.3 to 10.6) | TL |
| Adhesions only | 15 | 5.2 (3.0 to 8.5) | TL |
| Superficial peritoneal endometriosis and deep-infiltrating endometriosis | 10 | 3.5 (1.7 to 6.3) | TL |
| Adenomyosis only | 8 | 2.8 (1.2 to 5.4) | Other |
| Superficial peritoneal endometriosis and adhesions | 5 | 1.7 (0.6 to 4.0) | TL |
| Superficial peritoneal endometriosis, deep-infiltrating endometriosis and endometrioma of the ovary | 4 | 1.4 (0.4 to 3.5) | TL |
| Fibroids only | 3 | 1.1 (0.2 to 3.0) | Other |
| Deep-infiltrating endometriosis and endometrioma of the ovary | 2 | 0.7 (0.1 to 2.5) | TL |
| Endometrioma of the ovary only | 2 | 0.7 (0.1 to 2.5) | TL |
| Superficial peritoneal endometriosis and endometrioma of the ovary | 2 | 0.7 (0.1 to 2.5) | TL |
| Deep-infiltrating endometriosis and adhesions | 1 | 0.4 (0.01 to 1.9) | TL |
| Deep-infiltrating endometriosis, superficial peritoneal endometriosis and adhesions | 1 | 0.4 (0.01 to 1.9) | Tl |
| Deep-infiltrating endometriosis, superficial peritoneal endometriosis, adhesions and fibroids | 1 | 0.4 (0.01 to 1.9) | TL |
| Deep-infiltrating endometriosis, superficial peritoneal endometriosis, endometrioma of the ovary, adhesions and adenomyosis | 1 | 0.4 (0.01 to 1.9) | TL |
| Ovarian cysts and adenomyosis | 1 | 0.4 (0.01 to 1.9) | TL |
| Superficial peritoneal endometriosis and adenomyosis | 1 | 0.4 (0.01 to 1.9) | TL |
| Prevalence category | Number of cases (% of total) | Notes |
|---|---|---|
| No structural cause | 164 (57.14) | |
| Structural cause requires therapeutic laparoscopya | 112 (39.02) | |
| Structural cause requires other treatmentb | 11 (3.83) | |
| Total | 287 |
Test accuracy
The test accuracy of diagnostic laparoscopy and MRI was based on the sensitivity and specificity for the detection of structural causes that required either a therapeutic laparoscopy or other treatment, and were informed by data collected during the MEDAL study.
The test accuracy of MRI and diagnostic laparoscopy, respectively, was explored through the use of ROC curves for structural causes that required a therapeutic laparoscopy, and for those that required other treatment (Figure 11). The ROC curve was created by plotting the true-positive rate (true-positive rate or sensitivity) against the false-positive rate (false-positive rate or 1 – specificity) at various threshold settings.
FIGURE 11.
Receiver operating characteristic curves for laparoscopic and MRI diagnoses. (a) Diagnostic laparoscopy for structural causes of CPP that required treatment using a therapeutic laparoscopy; (b) diagnostic laparoscopy for structural causes of CPP that required other treatment (not therapeutic laparoscopy); (c) MRI scan for structural causes of CPP that required treatment using a therapeutic laparoscopy; and (d) MRI scan for structural causes of CPP that required other treatment (not therapeutic laparoscopy).
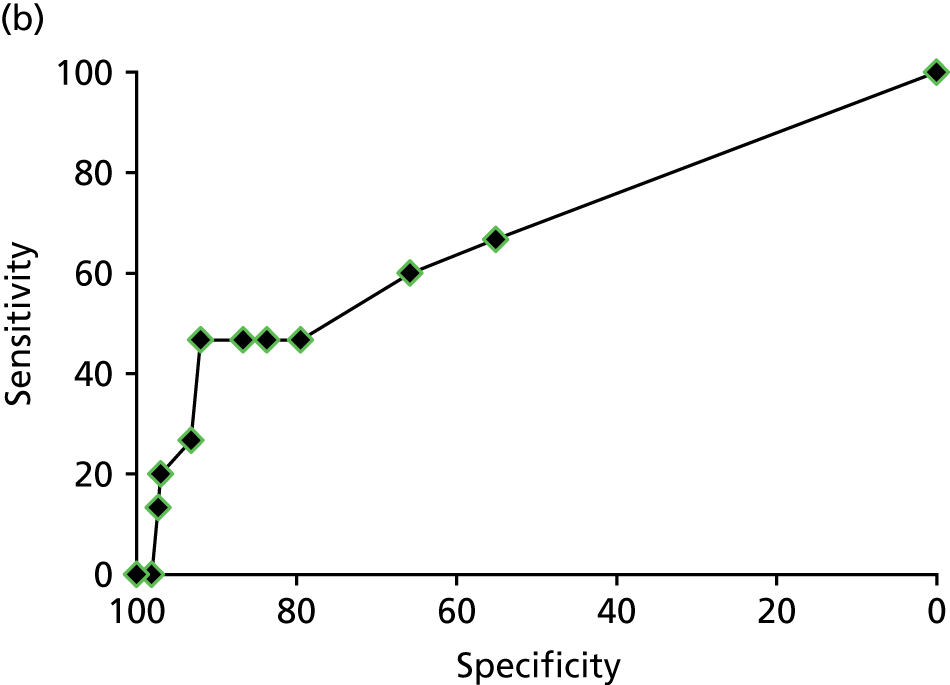
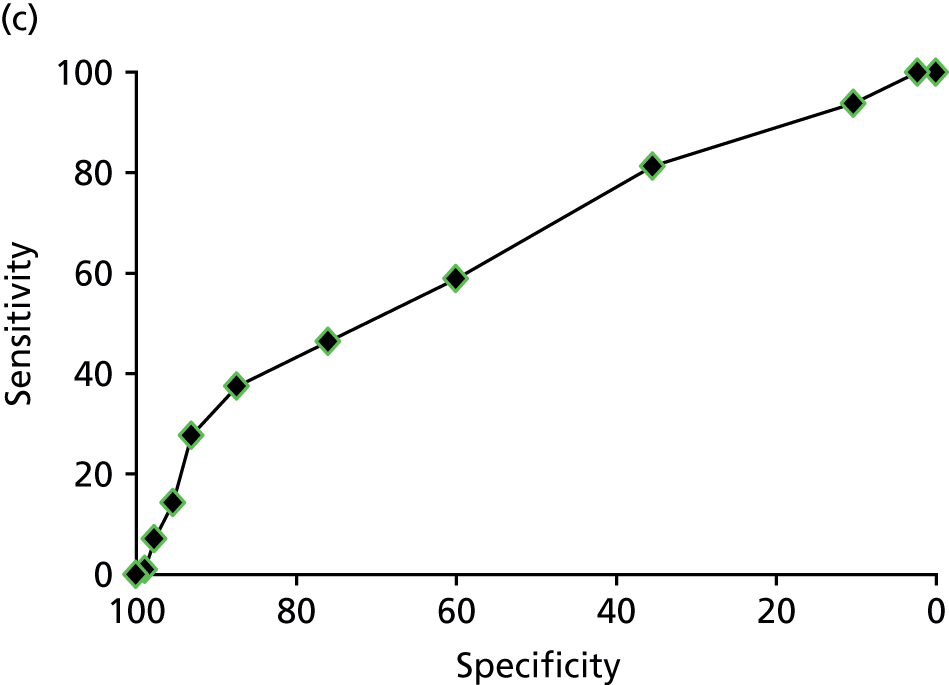
In our analysis, we refer to a low cut-off point as one that sets a high sensitivity and low specificity (more positive tests), and a high cut-off point as one that sets a low sensitivity and high specificity (more negative tests).
Calculation of test-accuracy parameters
In order to incorporate the impact of the test results on patient outcomes into the model, it was necessary to combine the prevalence of CPP with the sensitivity and specificity of the tests.
A patient in the model could be in one of three disease states:
-
patient has a structural cause that requires therapeutic laparoscopy
-
patient has a structural cause that requires other treatment
-
patient tests negative for any structural cause of CPP.
The prevalence of disease based on these groupings was combined with the test-accuracy characteristics of the tests through the following equations to give the proportion of women who test true or false positive or negative for a structural cause of CPP:
in which Sens is the sensitivity of the test, Spec is the specificity of the test, TP is a true-positive test result, FP is false positive, TN is true negative, FN is false negative and P is the prevalence of women with a structural cause subdivided into those women who require a therapeutic laparoscopy (TL) and those who require other treatment (OT).
Further model parameters
Parameters describing the uptake of MRI and laparoscopy are shown in Table 16 below. These were informed by the data collection conducted in the MEDAL study. As described in Model assumptions, 50% of women with deep-infiltrating endometriosis were assumed to receive two therapeutic laparoscopies. This was incorporated into the analysis for the purpose of calculating costs, but had no influence on patient outcomes, and the impact of this assumption was examined during the sensitivity analysis (see Sensitivity analyses).
| Parameters | Value | References | Notes |
|---|---|---|---|
| Proportion of eligible women who received a laparoscopy when offered | 288/309 | Data | Intraoperative form missing for one patient, and so was not included in the final analysis |
| Proportion of eligible women who received a MRI scan when offered | 291/329 | Data | Intraoperative form missing for four women, and so were not included in the final analysis |
| Proportion of women who had a therapeutic laparoscopy during the same procedure as a diagnostic laparoscopy | 92/112 | Data | Remainder of women had therapeutic laparoscopy during a subsequent procedure |
Cost and resource data
All costs used in this evaluation are in UK pounds sterling (at the 2013 value). NHS Reference Costs 2013–1487 were used to attribute costs to the resource use. The Unit Costs of Health and Social Care 201488 from the Personal Social Services Research Unit were used to obtain the costs for staff time. The resource costs are shown in Table 17.
| Item | Code | Cost (£) | Reference | Notes |
|---|---|---|---|---|
| Consultant/hour | Consultant medical (with qualification costs) | 140 | Unit Costs of Health and Social Care 2014 88 | |
| Nurse/hour | Nurse, day ward [includes staff nurse, registered nurse, registered practitioner)/hour of patient contact (with qualification costs)] | 100 | ||
| Ultrasound | RA24Z ultrasound scan, ≥ 20 minutes (diagnostic imaging) | 59 | NHS Reference Costs 2013–14 87 | |
| Baseline examination | Twenty minutes of consultation time with a consultant and a nurse, followed by 20 minutes of physical examination by a consultant, and then two-thirds of women received an ultrasound scan | 166 | Assume mean is equal to standard error for nurse and consultant time89 | |
| Diagnostic laparoscopy | MA10Z minor laparoscopic or endoscopic procedure, upper genital tract procedure (day case) | 1217 | NHS Reference Costs 2013–14 87 | Therapeutic laparoscopy assumed to be 10% more expensive than diagnostic laparoscopy (see assumptions) |
| MRI | RA03Z MRI scan, one area, pre and post contrast (diagnostic imaging) | 203 | ||
| Hysterectomy | MA07G major open upper genital tract procedures with a CC score of 0–2 | 3299 | Applied to 50% of those women with adenomyosis | |
| Implantation of an intrauterine device | MA35Z implementation of an intrauterine device | 311 | Applied to 50% of those women with adenomyosis | |
| Fibroids | MB09C non-malignant gynaecological disorders with interventions with a CC score of 0–2 | 2520 |
Outcomes
The primary outcome of this economic evaluation was the quality-adjusted life-year (QALY). In addition, the outcomes of cost per patient correctly diagnosed, and cost per patient correctly treated, were examined.
The QALY outcome measure was informed by QoL estimates obtained from the MEDAL study, through the EQ-5D-3L questionnaire, which was administered at baseline and 6 months post laparoscopy. This was not a randomised controlled trial in which women followed different patient pathways, and so it was not possible to obtain the QoL values for all the different patient pathways. Instead, the baseline values were used to inform the impact of a patient either having a structural cause of CPP or not having one, and then 6-month values were used to inform the impact on QoL of having a treatment with or without a cause of CPP. As no information was collected describing the impact of treatment on fibrosis or adenomyosis at 6 months, only the impact of therapeutic laparoscopy on QoL was considered. This QoL effect was assumed to be the same as the impact of treatment for fibrosis or adenomyosis (see Model assumptions). The QoL values used in this study are shown in Table 18.
| QoL estimate | Time point, EQ-5D-3L mean score (SD, n) | Application in the model | |
|---|---|---|---|
| Baseline | 6 months | ||
| Have a cause of CPP that requires therapeutic laparoscopya | 0.56 (0.32, 112) | QoL of women with a cause of CPP at baseline, and those who tested FN and, thus, were not treated | |
| Do not have a cause of CPPb | 0.53 (0.32, 151) | QoL of women who tested negative for a cause of CPP at baseline | |
| Do not have any cause of CPP and receive therapeutic laparoscopyc | 0.55 (0.35, 53) | QoL of negative women who tested FP and received unnecessary treatment | |
| Do not have any cause of CPP and do not receive therapeutic laparoscopyd | 0.69 (0.26, 71) | QoL of negative women at 6 months who tested TN and did not receive treatment | |
| Have cause of CPP that requires therapeutic laparoscopy and receives therapeutic laparoscopye | 0.74 (0.28, 68) | QoL of women who tested TP and received treatment | |
Analysis
This model-based economic evaluation takes the form of a cost–utility analysis based on the primary outcome of cost per QALY. The secondary outcomes of the cost per patient correctly diagnosed and cost per patient appropriately treated were also considered as part of the wider cost-effectiveness analyses. The perspective of the analysis is the health-care provider perspective (the UK NHS) in a secondary care (hospital) setting. Given the 6-month time horizon, no discounting was applied. The results in this study are described using the incremental cost-effectiveness ratio (ICER); this is defined as the difference between the costs of the two strategies divided by the difference in their outcomes.
In this analysis, for each of the strategies, MRI and laparoscopy, the cut-off value for sensitivity and specificity is treated as a decision variable, rather than an endogenous characteristic of each test. Given that there are 12 cut-off points for each ROC curve, this leads to 24 alternative strategies for MRI and laparoscopy, and, with the no-testing strategy, 25 alternative strategies were considered here.
Sensitivity analyses
A range of one-way sensitivity analyses were undertaken to gain further insights into the impact of reasonable changes to key parameters in the model, as follows:
-
To examine the impact of the 6-month time horizon on the model results, this was extended beyond the period of data collection up to 3 years. However, as a result of a lack of data being collected in this study beyond 6 months, this required two assumptions to be made. The first was that the QoL informed by data at 6 months was constant up to the time horizon under consideration, and the second was that no costs were incurred beyond the 6 months.
-
To examine the impact of changing the prevalence of structural causes among women with CPP in this model, this was varied between 0% and 100%. However, when applying the sensitivity analysis, it was assumed that the ratio of positive women in the therapeutic laparoscopy group to the other treatment group remained the same, as informed by the MEDAL study data.
-
To examine the impact of the assumption that 50% of women with deep-infiltrating endometriosis required two therapeutic laparoscopies, this value was varied between 0% and 100%.
-
To examine the impact of the assumption that 50% of women with adenomyosis had a hysterectomy and 50% had a levonorgestrel-releasing intrauterine system, these values were varied from 0% to 100% (always adding up to 100%).
In order to incorporate the uncertainty that is inherent in many of the parameters in this analysis, a probabilistic sensitivity analysis (PSA) was undertaken, with each parameter value being assigned a distribution and the model being run 10,000 times, sampling on each occasion from these distributions. The distributions applied to the parameters for the PSA are shown in Appendix 3.
Results
This section starts by examining the cost-effectiveness of MRI, laparoscopy, and no testing, whereby the different cut-off values to inform the sensitivity and specificity for each test was treated as a separate strategy. At baseline, a 6-month time horizon was utilised.
Quality-adjusted life-year outcome
Using the outcome of the QALYs, the cost-effectiveness of MRI and laparoscopy across varying cut-off points is shown in Table 19.
| Strategy and cut-off pointa | Cost (£) | QALY gain |
|---|---|---|
| MRI – 2 | 1856 | 0.3077 |
| MRI – 1 | 1908 | 0.3082 |
| MRI – 0 | 2097 | 0.3083 |
| Laparoscopy – 0 | 2231 | 0.3084 |
| MRI – 10 | 538 | 0.3094 |
| No testing | 166 | 0.3095 |
| MRI – 11 | 497 | 0.3095 |
| Laparoscopy – 11 | 1387 | 0.3095 |
| MRI – 3 | 1691 | 0.3097 |
| MRI – 4 | 1397 | 0.3100 |
| MRI – 9 | 623 | 0.3103 |
| MRI – 8 | 729 | 0.3109 |
| MRI – 5 | 1171 | 0.3121 |
| Laparoscopy – 1 | 2017 | 0.3134 |
| MRI – 7 | 862 | 0.3137 |
| MRI – 6 | 1002 | 0.3140 |
| Laparoscopy – 10 | 1481 | 0.3140 |
| Laparoscopy – 2 | 1973 | 0.3166 |
| Laparoscopy – 9 | 1593 | 0.3176 |
| Laparoscopy – 3 | 1904 | 0.3188 |
| Laparoscopy – 8 | 1664 | 0.3200 |
| Laparoscopy – 4 | 1873 | 0.3212 |
| Laparoscopy – 5 | 1839 | 0.3215 |
| Laparoscopy – 7 | 1747 | 0.3227 |
| Laparoscopy – 6 | 1793 | 0.3235 |
As shown in Table 20, the baseline results for strategies on the cost-effectiveness frontier, using the outcome of the QALY for most scenarios and excluding the scenarios for the more extreme cut-off points, shows that laparoscopy is more effective than MRI in terms of QALYs gained. For both MRI and laparoscopy, for some of the highest and lowest cut-off values that appear at the extreme ends on the ROC curve, in terms of QALYs gained, these are no better than no testing, and, in some cases, are even worse than no testing. This can be explained, as, for some of these strategies, women are given expensive unnecessary treatment as a result of false-negative test results. Finally, it is noted that for most scenarios, excluding the scenarios for the more extreme cut-off points, laparoscopy is more effective than MRI in terms of QALYs gained. The cost-effectiveness frontier for the results in Table 20 is shown in Figure 12.
| Strategy | Cost (£) | Incremental cost (£) | QALYs gained | Incremental effectiveness | ICER (cost/QALY), £ |
|---|---|---|---|---|---|
| No testing | 166 | 0.3095 | |||
| Laparoscopy (cut-off value of 6) | 1793 | 1627 | 0.3235 | 0.014 | 116,618 |
FIGURE 12.
Cost-effectiveness frontier for the baseline strategies, using the QALY outcome and the 6-month time horizon.
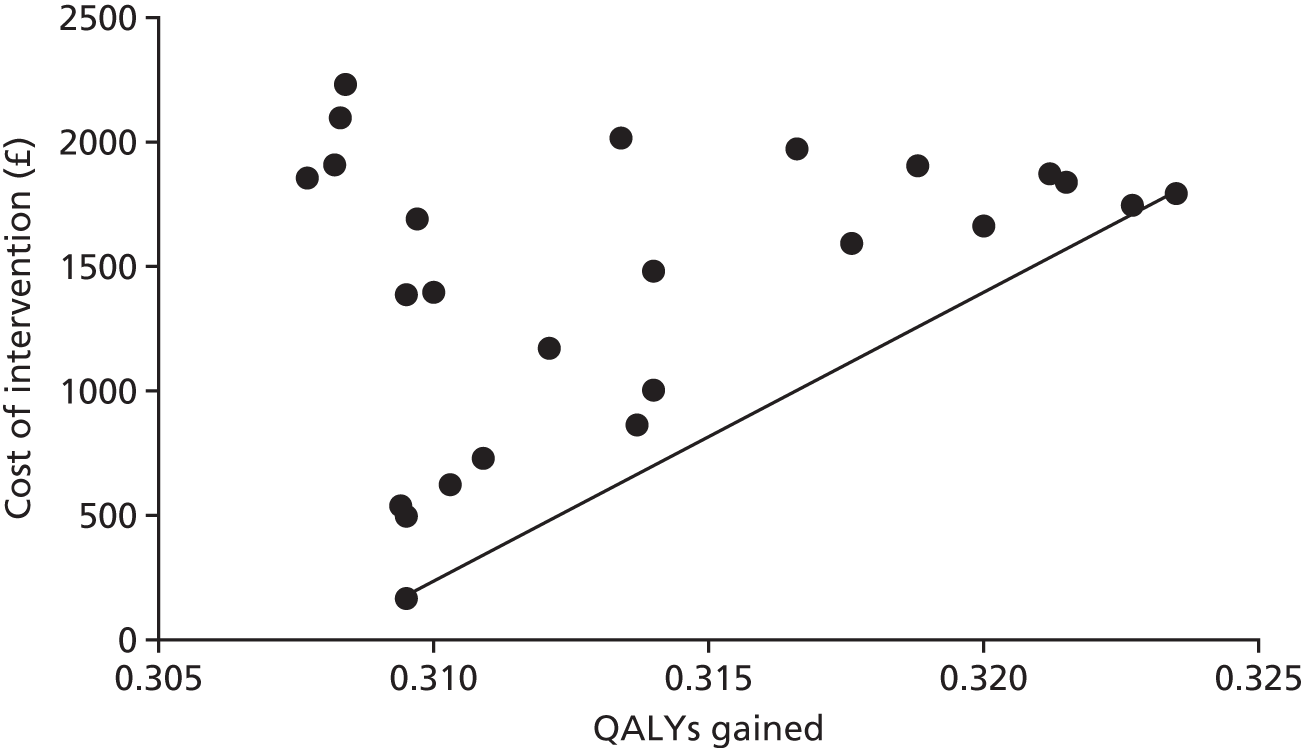
As can be seen in Figure 12, all the strategies were either dominated (less effective and more costly) or subject to extended dominance (another strategy provides more units of benefit at a lower cost per unit of benefit), except for no testing and laparoscopy, at a cut-off value of 6, which therefore lies on the cost-effectiveness frontier.
As shown in Table 20, for a 6-month time horizon, using the outcome of the QALY, laparoscopy (with a cut-off value of 6) is more expensive (£1793 vs. £166) and more effective (0.3235 vs. 0.3095) than no testing. In addition, the ICER for laparoscopy (with a cut-off value of 6) compared with no testing is £116,618, which means that one extra QALY gained from laparoscopy (with a cut-off value of 6) will cost £116,618 compared with no testing.
Correctly diagnosed outcome
Using the outcome measure of a correct diagnosis, the cost-effectiveness results for MRI and laparoscopy at various cut-off points, for a 6-month time horizon, are shown in Table 21.
| Strategy and cut-off pointa | Cost (£) | Correctly diagnosed |
|---|---|---|
| No testing | 166 | 0.0000 |
| Laparoscopy – 0 | 2231 | 0.3994 |
| MRI – 0 | 2097 | 0.4286 |
| MRI – 2 | 1856 | 0.4303 |
| MRI – 1 | 1908 | 0.4310 |
| MRI – 3 | 1691 | 0.4770 |
| Laparoscopy – 1 | 2017 | 0.4897 |
| MRI – 4 | 1397 | 0.5051 |
| Laparoscopy – 11 | 1387 | 0.5326 |
| Laparoscopy – 2 | 1973 | 0.5447 |
| MRI – 5 | 1171 | 0.5547 |
| MRI – 10 | 541 | 0.5654 |
| MRI – 8 | 729 | 0.5709 |
| MRI – 11 | 497 | 0.5714 |
| MRI – 9 | 623 | 0.5717 |
| Laparoscopy – 10 | 1498 | 0.5801 |
| Laparoscopy – 3 | 1904 | 0.5838 |
| MRI – 6 | 1002 | 0.5956 |
| MRI – 7 | 862 | 0.6022 |
| Laparoscopy – 9 | 1593 | 0.6195 |
| Laparoscopy – 4 | 1873 | 0.6255 |
| Laparoscopy – 5 | 1839 | 0.6369 |
| Laparoscopy – 8 | 1664 | 0.6421 |
| Laparoscopy – 7 | 1747 | 0.6679 |
| Laparoscopy – 6 | 1793 | 0.6733 |
As shown in Table 21, for the outcome of correctly diagnosed, the strategies at the extreme ends of the ROC curve were the least effective, with a few exceptions. The most effective strategies were the laparoscopy strategies around the mid-point of the ROC curve, that is, the cut-off values 4–9. The cost-effectiveness frontier for the results in Table 21 is shown in Figure 13.
FIGURE 13.
Cost-effectiveness frontier for baseline strategies, using the correctly diagnosed outcome and a 6-month time horizon.
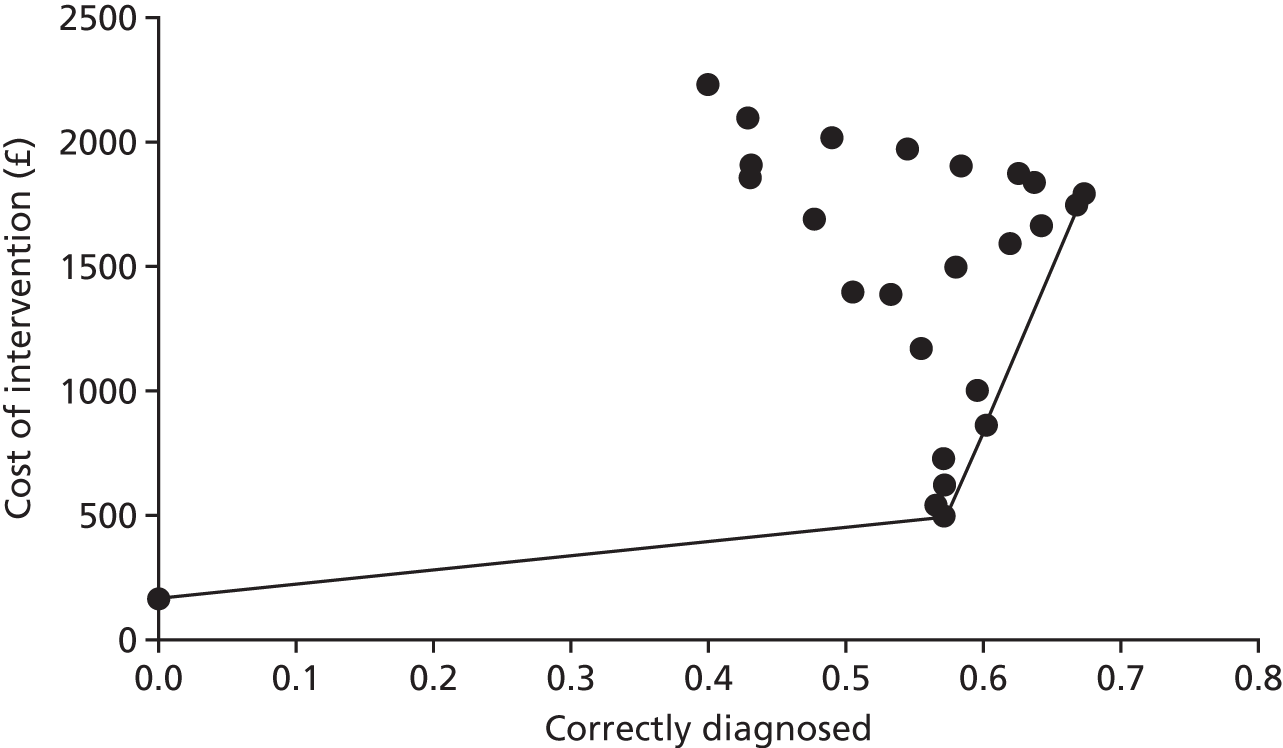
As can be seen in Figure 13, all the strategies were either dominated or subject to extended dominance, except for the four strategies on the cost-effectiveness frontier, these being no testing, MRI (cut-off value of 11), MRI (cut-off value of 6) and laparoscopy (cut-off value of 6).
As shown in Table 22, for a 6-month time horizon and using the outcome of correctly diagnosed, MRI (cut-off value of 11) was more expensive and more effective than no testing. MRI (cut-off value of 7) was more expensive and more effective than MRI (cut-off value of 11), and laparoscopy (cut-off value of 6) was more expensive and more effective than MRI (cut-off value of 7). In the case of the comparison between laparoscopy (cut-off value of 6) and MRI (cut-off value of 7), the ICER was £13,092, which means that it would cost £13,092 for one extra correct diagnosis for laparoscopy (cut-off value of 6) compared with MRI (cut-off value of 7).
| Strategy | Cost (£) | Incremental cost (£) | Correctly diagnosed | Incremental effectiveness | ICER (cost/correctly diagnosed), £ |
|---|---|---|---|---|---|
| No testing | 166 | 0 | |||
| MRI (cut-off value of 11) | 497 | 331 | 0.5714 | 0.5714 | 579 |
| MRI (cut-off value of 7) | 862 | 365 | 0.6022 | 0.0308 | 11,864 |
| Laparoscopy (cut-off value of 6) | 1793 | 1627 | 0.6733 | 0.0711 | 13,092 |
Correctly treated outcome
Using the outcome measure of a patient being positive for a structural cause of CPP and receiving the correct treatment, the model results for MRI and laparoscopy at various cut-off points, for a 6-month time horizon, are shown in Table 23.
| Strategy and cut-off valuea | Cost (£) | Correctly treated |
|---|---|---|
| No testing | 166 | 0.0000 |
| MRI – 0 | 2097 | 0.4286 |
| MRI – 2 | 1856 | 0.4303 |
| MRI – 1 | 1908 | 0.4310 |
| Laparoscopy – 0 | 2231 | 0.4383 |
| MRI – 3 | 1691 | 0.4770 |
| MRI – 4 | 1397 | 0.5051 |
| Laparoscopy – 1 | 2017 | 0.5285 |
| MRI – 5 | 1171 | 0.5547 |
| MRI – 10 | 541 | 0.5654 |
| MRI – 8 | 729 | 0.5709 |
| MRI – 11 | 497 | 0.5714 |
| Laparoscopy – 11 | 1387 | 0.5714 |
| MRI – 9 | 623 | 0.5717 |
| Laparoscopy – 2 | 1973 | 0.5835 |
| MRI – 6 | 1002 | 0.5956 |
| MRI – 7 | 862 | 0.6022 |
| Laparoscopy – 10 | 1498 | 0.6189 |
| Laparoscopy – 3 | 1904 | 0.6226 |
| Laparoscopy – 9 | 1593 | 0.6583 |
| Laparoscopy – 4 | 1873 | 0.6643 |
| Laparoscopy – 5 | 1839 | 0.6757 |
| Laparoscopy – 8 | 1664 | 0.6810 |
| Laparoscopy – 7 | 1747 | 0.7068 |
| Laparoscopy – 6 | 1793 | 0.7121 |
Table 23 shows that the least effective scenarios were the MRI strategies with low cut-off points, that is, those with high sensitivity and low specificity. The most effective scenarios were the laparoscopy scenarios for the cut-off values 3–10. The cost-effectiveness frontier for the results in Table 23 is shown in Figure 14.
FIGURE 14.
Cost-effectiveness frontier for baseline strategies, using the correctly treated outcome with a 6-month time horizon.
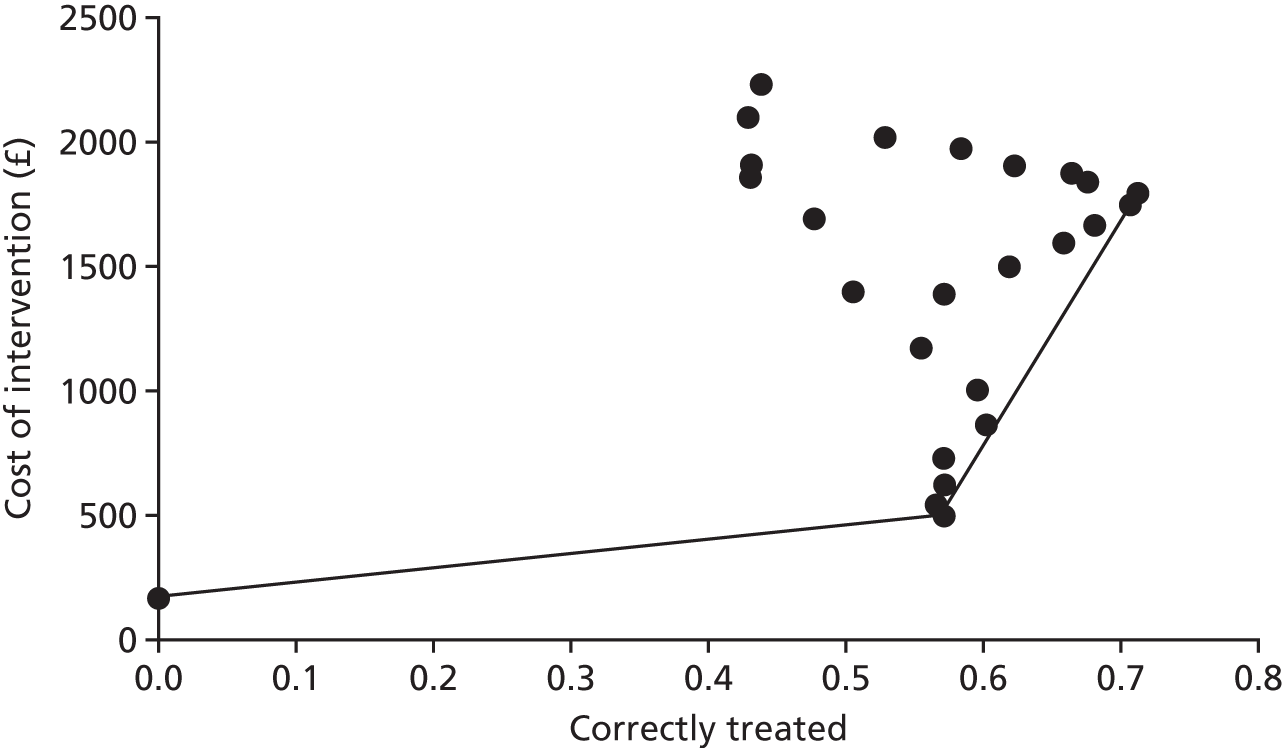
As can be seen in Figure 14, all the strategies were either dominated or subject to extended dominance, except for the three strategies on the cost-effectiveness frontier, which were no testing, MRI (cut-off value of 11), and laparoscopy (cut-off value of 6).
As shown in Table 24, for a 6-month time horizon, using the outcome of a women being correctly treated with MRI (cut-off value of 11) was more expensive (£497 vs. £166) and more effective (0.5714 vs. 0) than no testing, and the ICER for MRI (cut-off value of 11) compared with no testing was £579, which means that one extra patient correctly treated with MRI (cut-off value of 11) would cost £579 compared with no testing. Likewise, laparoscopy (cut-off value of 6) was more expensive (£1793 vs £497) and more effective (0.7121 vs. 0.5714) than MRI (cut-off value of 11) with an ICER of £9211, meaning that one extra woman correctly treated with laparoscopy (cut-off value of 6) will cost £9211 compared with MRI (cut-off value of 11).
| Strategy | Cost (£) | Incremental cost (£) | Correctly diagnosed | Incremental effectiveness | ICER (cost/correctly treated), £ |
|---|---|---|---|---|---|
| No testing | 166 | 0 | |||
| MRI (cut-off value of 11) | 497 | 331 | 0.5714 | 0.5714 | 579 |
| Laparoscopy (cut-off value of 6) | 1793 | 1296 | 0.7121 | 0.1407 | 9211 |
Sensitivity analysis
Time horizon
The baseline analysis has a time horizon of 6 months. The impact of extending the time horizon up to 3 years for the outcome of the QALY on model results is shown in Table 25.
| Time horizon and strategy | Cost (£) | Incremental cost (£) | QALYs gained | Incremental effectiveness | ICER (cost/QALY), £ |
|---|---|---|---|---|---|
| 6-month time horizon | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy (cut-off value of 6) | 1793 | 1627 | 0.3235 | 0.0140 | 116,618 |
| 1-year time horizon | |||||
| No testing | 166 | 0.6267 | |||
| Laparoscopy (cut-off value of 6) | 1793 | 1627 | 0.6574 | 0.0307 | 53,000 |
| 2-year time horizon | |||||
| No testing | 166 | 1.2610 | |||
| Laparoscopy (cut-off value of 6) | 1793 | 1627 | 1.3251 | 0.0641 | 25,384 |
| 3-year time horizon | |||||
| No testing | 166 | 1.8952 | |||
| Laparoscopy (cut-off value of 6) | 1793 | 1627 | 1.9929 | 0.0977 | 16,654 |
The results when varying the time horizon are shown in Table 25. In each case, only the strategies that lie on the cost-effectiveness plane are shown (dominated and subject to extended dominance scenarios not shown). It can be seen that, as the time horizon of the analysis is increased, laparoscopy (cut-off value of 6) becomes more cost-effective than no testing. However, it is noted that, although the effectiveness of the interventions increases over time, as a result of the model assumptions, the costs remain the same.
Prevalence
The impact of varying the overall prevalence of structural causes among women with CPP was examined, with the results shown in Table 26. Note that only the results for the scenarios that lie on the cost-effectiveness frontier are described.
| Prevalence | Strategy (with cut-off value) | Cost (£) | Incremental cost (£) | QALY gain | Incremental QALY | ICER (cost/QALY), £ |
|---|---|---|---|---|---|---|
| 0% | No testing | 166 | 0.3317 | Dominates | ||
| 10% | No testing | 166 | 0.3264 | Dominates | ||
| 20% | No testing | 166 | 0.3214 | Dominates | ||
| 30% | No testing | 166 | 0.3162 | |||
| 30% | Laparoscopy – 7 | 1730 | 1564 | 0.3198 | 0.0036 | 430,292 |
| 40% | No testing | 166 | 0.3110 | |||
| 40% | Laparoscopy – 6 | 1788 | 1622 | 0.3227 | 0.0117 | 138,407 |
| Baseline (42.86%) | No testing | 166 | 0.3095 | |||
| Baseline (42.86%) | Laparoscopy – 6 | 1793 | 1627 | 0.3235 | 0.0140 | 116,618 |
| 50% | No testing | 166 | 0.3058 | |||
| 50% | Laparoscopy – 6 | 1805 | 1639 | 0.3256 | 0.0197 | 83,032 |
| 60% | No testing | 166 | 0.3007 | |||
| 60% | Laparoscopy – 6 | 1820 | 1654 | 0.3285 | 0.0278 | 59,389 |
| 70% | No testing | 166 | 0.2955 | |||
| 70% | Laparoscopy – 6 | 1836 | 1670 | 0.3315 | 0.0360 | 46,398 |
| 70% | Laparoscopy – 4 | 1896 | 60 | 0.3327 | 0.0012 | 48,842 |
| 80% | No testing | 166 | 0.2903 | |||
| 80% | Laparoscopy – 4 | 1905 | 1739 | 0.3369 | 0.0467 | 37,255 |
| 80% | MRI – 0 | 2061 | 156 | 0.3388 | 0.0018 | 85,124 |
| 90% | No testing | 166 | 0.2852 | |||
| 90% | MRI – 1 | 1959 | 1793 | 0.3445 | 0.0593 | 30,248 |
| 90% | MRI – 0 | 2051 | 91 | 0.3467 | 0.0022 | 40,899 |
| 100% | No testing | 166 | 0.2800 | |||
| 100% | MRI – 1 | 1970 | 1804 | 0.3523 | 0.0723 | 24,966 |
| 100% | MRI – 0 | 2042 | 72 | 0.3550 | 0.0027 | 26,169 |
Table 26 shows the model results with variations in the prevalence of a structural cause of CPP. At low prevalence rates (0–20%), no testing is cheaper and more effective than all other strategies. As the prevalence increases, laparoscopy (cut-off value of 6) becomes increasingly cost-effective up to 60%, and then laparoscopy (cut-off value of 4) is preferred for prevalences of 70–80%. At the highest prevalence rates, MRI with a low cut-off value becomes the preferred option. This can be explained through the fact that, with a high prevalence rate, it is better to offer a cheap test that has a high sensitivity and low specificity, as there will be few false-positive test results.
Deep-infiltrating endometriosis
It was assumed that at baseline 50% of women with deep-infiltrating endometriosis required a second therapeutic laparoscopy. The results in Table 27 show the impact of varying this assumption from 0% to 100%.
| Proportion of women with deep-infiltrating endometriosis who required two therapeutic laparoscopies | Cost (£) | Incremental cost (£) | QALY gain | Incremental QALY | ICER (cost/QALY), £ |
|---|---|---|---|---|---|
| 0% | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy – 6 | 1730 | 1564 | 0.3235 | 0.014 | 112,109 |
| Baseline | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy – 6 | 1793 | 1627 | 0.3235 | 0.014 | 116,618 |
| 100% | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy – 6 | 1856 | 1690 | 0.3235 | 0.014 | 121,126 |
As shown in Table 26, varying the parameter describing the percentage of women with deep-infiltrating endometriosis who required two therapeutic laparoscopies had very little impact on the ICER values, and did not impact on the conclusions drawn from the model. All the ICER values lie close to the baseline ICER of £116,618 and remain above the £20,000–30,000 acceptance threshold for the cost/QALYs, as recommended by the National Institute for Health and Care Excellence (NICE). 90
Hysterectomy for adenomyosis
It was assumed at baseline that 50% of women with adenomyosis are treated with a hysterectomy. The results in Table 28 show the impact of varying this assumption from 0% to 100%.
| Proportion of women with ademomysis who were treated with a hysterectomy | Cost (£) | Incremental cost (£) | QALY gain | Incremental QALY | ICER (cost/QALY), £ |
|---|---|---|---|---|---|
| 0% | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy – 6 | 1682 | 1516 | 0.3235 | 0.014 | 108,624 |
| Baseline | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy – 6 | 1793 | 1627 | 0.3235 | 0.014 | 116,618 |
| 100% | |||||
| No testing | 166 | 0.3095 | |||
| Laparoscopy – 6 | 1905 | 1739 | 0.3235 | 0.014 | 124,612 |
As shown in Table 28, varying the parameter describing the percentage of women with adenomyosis who are treated with hysterectomy had very little impact on the ICER values, and did not impact on the conclusions drawn from the model. All the ICER values lie close to the baseline ICER of £116,618, and remain above the £20,000–30,000 acceptance threshold for the cost/QALYs, as recommended by NICE.
Probabilistic sensitivity analysis
The results for the PSA for 10,000 model runs are shown in the cost-effectiveness acceptability curves in Figure 15, and those results for no testing and laparoscopy (cut-off value of 6) are shown in Figure 16. These two strategies were chosen because they appear on the cost-effectiveness frontier at baseline. From Figure 15, no testing is always the most likely strategy to be cost-effective across willingness-to pay-values for the QALY from £0 to £200,000. In Figure 16, it can be seen that no testing is more likely to be cost-effective up to a willingness to pay for a QALY of approximately £150,000, at which point laparoscopy (cut-off value of 6) is more likely to be cost-effective.
FIGURE 15.
Cost-effectiveness acceptability curve showing the results of the PSA for a 6-month time horizon, for the strategies likely to be cost-effective across a range of willingness-to-pay thresholds for the QALY.
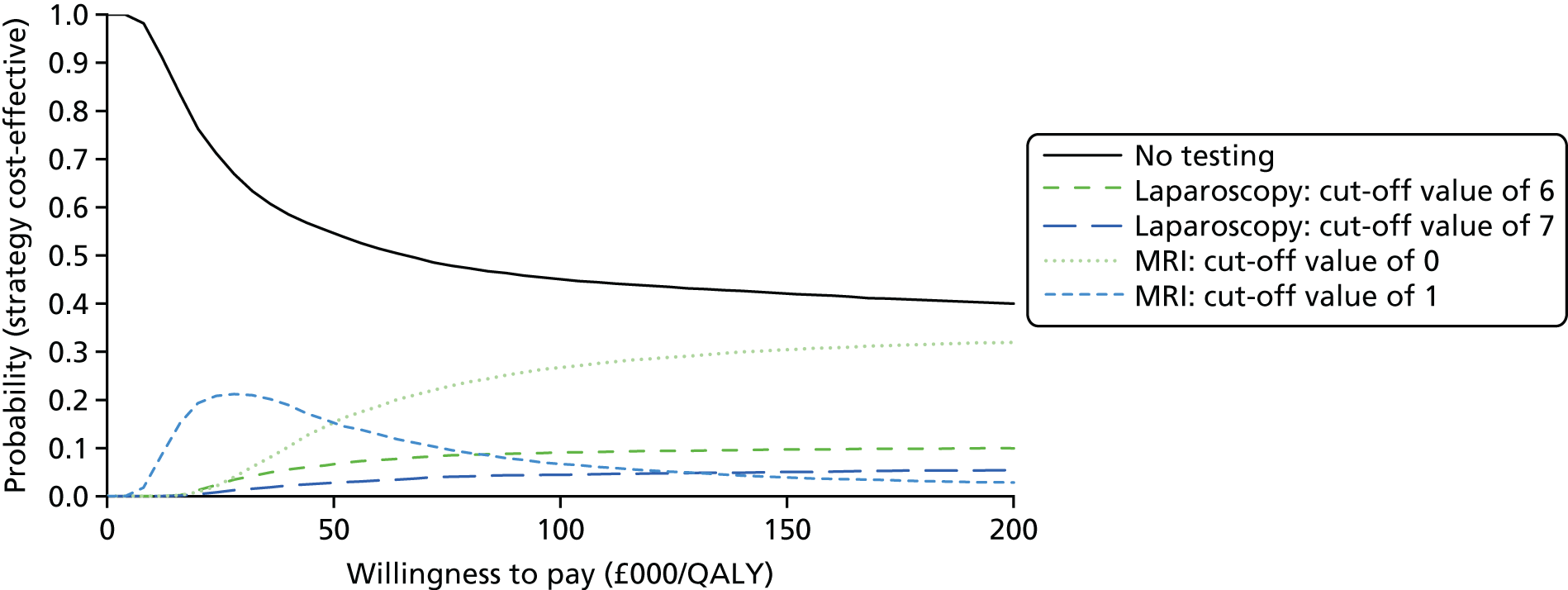
FIGURE 16.
Cost-effectiveness acceptability curve showing the uncertainty in the difference between costs and effectiveness (QALYs gained) for no testing and laparoscopy (cut-off 6) for 10,000 model runs using a 6-month time horizon.
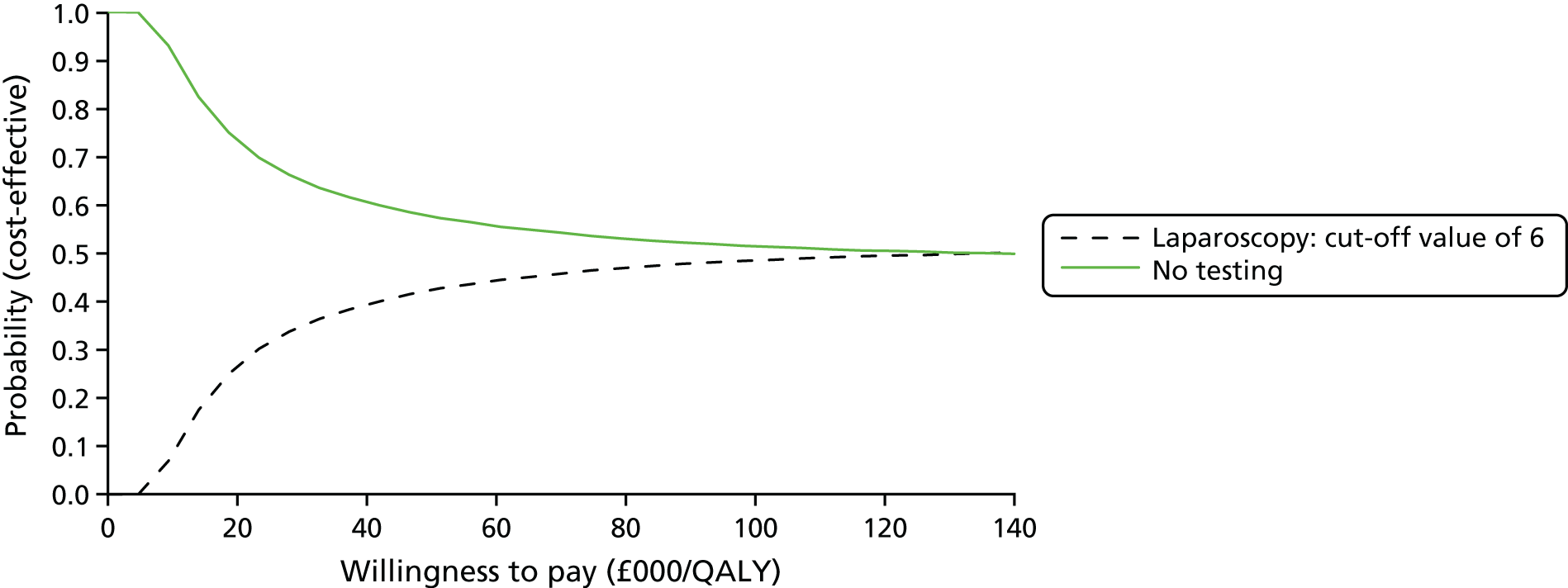
Chapter 5 Discussion
Principal findings
The diagnostic study demonstrated that, compared with the reference standard diagnosis verified at laparoscopy, MRI had high specificity but poor sensitivity for observing deep-infiltrating endometriosis (3%), endometrioma (33%), adhesions (19%) and ovarian cysts (20%). Sensitivity was higher for detecting these conditions as a cause of pelvic pain as categorised by the EIP, but not high enough to be useful. MRI correctly identified 56% of those women judged to have idiopathic CPP, but missed 46% of those women considered to have a gynaecological structural cause of CPP.
Laparoscopy was significantly more accurate than MRI in diagnosing idiopathic CPP (p < 0.0001), superficial peritoneal endometriosis (p < 0.0001), deep-infiltrating endometriosis (p < 0.0001) and endometrioma of the ovary (p = 0.02) as the cause of pelvic pain. The accuracy of laparoscopy appeared to be able to rule in these diagnoses. Laparoscopy was not able to make accurate diagnoses that adhesions were the cause of pelvic pain, nor was it able to diagnose adenomyosis.
Using MRI to identify those who require therapeutic laparoscopy would lead to 369 women in a cohort of 1000 receiving laparoscopy unnecessarily, and 136 women who required laparoscopy not receiving it. A strategy of all women receiving laparoscopy would lead to 557 women receiving unnecessary laparoscopic treatment.
Feeding these diagnostic performance data into a decision-analytic model, using a 6-month time horizon and taking the test cut-off value to inform the sensitivity and specificity values as a decision variable, it was found that diagnostic laparoscopy (with a cut-off value of 6) has an ICER value of £116,223 (cost/QALY) compared with a no-testing scenario, in which women only have a baseline examination and are then left untreated. This means that one extra QALY gained from the diagnostic laparoscopy will cost an average of £116,618 compared with no testing. This ICER is above the NICE acceptance threshold of £20,000–30,000 for the QALY. However, this value is based on a short time horizon (only 6 months) and strong assumptions, particularly regarding QALY gain in women treated with different strategies which were not actually observed in the study, which must be considered alongside these limitations, and does not provide strong enough evidence to inform clinical care.
Notably, all other scenarios, including all of the MRI scenarios across all cut-off values, were found to be either dominated (more costly and less effective) or subject to extended dominance (another strategy provides more units of benefit at a lower cost per unit of benefit). This means that MRI is not cost-effective in this setting.
As part of an extensive sensitivity analysis, the time horizon was extended to 3 years. Under these circumstances, the ICER for laparoscopy (with a cut-off value of 6) was £16,654 (cost/QALY) compared with the no-testing scenario, which would not be considered cost-effective. However, it is noted that extending the time horizon beyond the data collection period of 6 months does require further strong assumptions to be made. Nevertheless, this result does suggest that a time horizon of > 6 months should be adopted for data collection in this setting in future studies, in order for the impact of these interventions on patient QoL to be fully evaluated.
The ICER values for the outcome of cost per correct diagnosis were £579 for MRI (with a cut-off value of 11) versus no testing, £11,864 for MRI (with a cut-off value of 7) versus MRI (with a cut-off value of 11) and £13,092 for laparoscopy (with a cut-off value of 6) versus MRI (with a cut-off value of 7). In addition, the costs per patient correctly treated were £579 for MRI (with a cut-off value of 11) versus no testing, and £9211 for laparoscopy (with a cut-off value of 6) versus MRI (with a cut-off value of 11). Although it is difficult to draw conclusions as to which strategy should be preferred based on these outcomes, as there is no acceptable threshold for either outcome, it is notable that laparoscopy (with a cut-off value of 6) continues to be the most cost-effective strategy for each.
Strengths and weaknesses of the diagnostic study
The MEDAL study examined the diagnostic value and cost-effectiveness of MRI placed before laparoscopy in a care pathway for women presenting with CPP (incorporating several target conditions) in a gynaecology clinic. If found to be accurate and cost-effective, a strategy involving use of MRI triage to direct laparoscopy could lead to more selective use of the latter, an invasive, costly test requiring use of general anaesthesia in a day-case operating theatre. Construction of an appropriate study designed to produce valid, reliable and generalisable results to guide decision-making proved to be challenging from the outset. The challenges included, among others, variation in gynaecological practice; multiplicity of target conditions with respect to cause of CPP; incongruence between observed conditions and attribution of cause to CPP symptoms; a lack of consensus on radiology standards and reporting of pelvic MRI for benign gynaecological conditions; the need to blind MRI results to avoid bias in assessment of their value; and a lack of objective reference standards for the various target conditions.
The MEDAL study was a multicentre study with recruitment from 26 gynaecology outpatient clinics in the UK. The units represented the spectrum of settings, from busy district general hospitals to specialised tertiary centres, covering the whole range of existing women’s health referral networks, to hospitals with MRI facilities. These units served large, socioeconomically and ethnically diverse populations, which served to maximise the generalisability of the findings. The MEDAL study sought to approach all consecutive eligible women to obtain a suitably representative sample of women with CPP. The median interval between a MRI scan and a diagnostic laparoscopy was less than 1 month, making it unlikely that disease progression bias could influence the results, given the slow and chronic nature of the gynaecological conditions under consideration. The variation inherent in clinical and radiological practice in this mixture of settings also posed a challenge with respect to baseline data collection and MRI reporting. Establishing a standard operating procedure for MRI reporting was a key issue. We established a reporting template for CPP through a survey of an expert panel of radiologists specialising in gynaecological MRI from across the UK. 63 This format would facilitate the applicability of the findings of our study.
The use of laparoscopy for diagnostic evaluation in CPP was well established as the standard practice in these settings. Variations existed in the recording of history, gynaecological examination and pelvic ultrasound, before performing diagnostic laparoscopy. We standardised these during the course of the study using piloted data collections forms, which all had the input of consumers and experts to ensure sensitivity and relevance to women and their presenting complaint. These data were necessary for the care provided by the gynaecologist (who had to be kept blind to the MRI results), but their standardised collection was also necessary for replicable use by the independent gynaecologist assessing the information gained by MRI and the EIP, for establishing a reference diagnosis by consensus.
To determine the accuracy of MRI for each condition separately would require a large number of participants to accommodate the low prevalence of some conditions, such as ovarian cysts. Some pathologies are not independent of each other and could frequently be concurrently observed; for example, endometriosis can give rise to adhesions from fibrotic tissue. Furthermore, conditions observed are not always causes of the CPP symptoms. Therefore, our principal research question was developed to ascertain how MRI could be judiciously employed in a care pathway to minimise the use of laparoscopy, while maximising the correct identification of target conditions for treatments (diagnostic study) that improve QoL with the least investigative burden (decision-analytic cost-effectiveness analysis). Taking this approach, MRI test accuracy for each of the observed conditions served only as a secondary aim. Understanding the difference between diagnostic accuracy and diagnostic value in a care pathway is key to the interpretation of our findings.
To establish whether or not MRI can minimise the use of laparoscopy in a diagnostic pathway, a paired design was employed whereby both tests were performed in the study cohort. During the conduct of the study, it was necessary to blind the MRI report so that clinicians providing care remained uninfluenced by its results in their decision-making, otherwise there was a risk that the assessment of the MRI test performance in the study may be biased. The MRI test preceded laparoscopy in the study, so its findings can be interpreted for development of a triage test that can be used to direct only women with specific conditions towards laparoscopic confirmation or an operative laparoscopic procedure. By providing a MRI test prior to laparoscopy, any conditions that MRI can identify as a cause of CPP with sufficient accuracy can be treated medically without laparoscopy, or a therapeutic laparoscopy can be planned without the need for an initial diagnostic laparoscopy after the findings of the study are known. As it was feasible to perform MRI and laparoscopy in the same women with CPP in a blinded fashion, so that MRI did not interfere with laparoscopy, the paired design offered statistical efficiency over a randomised trial comparing two diagnostic strategies.
The most challenging issue was establishment of a reference diagnosis that attributed cause to the CPP symptoms. For this, we employed a panel consensus method. The panel reflected the clinical reality in which several items of information are synthesised to make a treatment decision. In this assessment, the reference standard in the MEDAL study was kept independent of the MRI test to confirm or refute the presence or absence of disease beyond reasonable doubt. A judgement was made by the expert panel about whether or not the conditions observed were a cause of the CPP symptoms without the use of MRI results, avoiding incorporation bias (except in the evaluation of adenomyosis as a target condition, as other means for this diagnosis are inadequate).
There is little evidence on how panels should be convened, how information should be presented or what the best methods for consensus are. We used a standardised approach to minimise variation in delineation of the reference standard. We established an expert panel of consultant gynaecologists who were not involved in the care of women recruited in the study. Although non-gynaecological conditions were considered as potential diagnoses, we did not include experts from other specialities, which may have contributed to the poor consensus regarding the attribution of pain to non-gynaecological causes. Using an EIP comprised solely of gynaecologists does, however, produce a diagnosis reflecting the clinical decision point, as the decision as to whether or not to perform a laparoscopy is made by gynaecologists.
Given the large amount of information to be considered per case, a piloted structured report was produced for the panel’s consideration. Each panel meeting involved three experts who provided the reference diagnosis for each case presented based on the structured summary of all the data collected, with the various case report forms available in their entirety, if required. Each member individually recorded their reference diagnosis. The meeting chairperson documented the discussion that followed and how agreement was reached through consensus. Understanding the difference between the presence of a condition and a target condition being the cause of pain proved to be the main challenge. After running pilot panels, the procedures for the panel meetings were reviewed and finalised. During the course of the panel assessments, we undertook formal repeatability testing for inter-rater agreement. We employed the agreement statistics in determining the reliability of the reference standard for the interpretation of our findings. We found that agreement among experts for the assignment of gynaecological conditions as causes of CPP was either good or very good, although it was weak for non-gynaecological conditions.
The MEDAL study has been reported in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) statement. Not all items apply to the MEDAL study, as it is not a typical test-accuracy evaluation, but this statement provides for a minimum requirement to ensure completeness and transparency of reporting.
Strengths and weaknesses of the economic evaluation
The strength of this model-based analysis is that it has utilised contemporary data informing the test accuracy of diagnostic laparoscopy and MRI in their application to women with CPP. In addition, these data have provided information for the model on the impact of successfully testing and treating women with structural causes of CPP on their QoL. Moreover, as part of this analysis, the cut-off value on the ROC curve to inform the sensitivity and specificity of each test was treated as a decision variable, meaning that the most cost-effective test at its optimum cut-off value could be identified.
This analysis has a number of weaknesses that need to be acknowledged. QoL data were collected from women over a 6-month period only, meaning that a time horizon of only 6 months could be adopted in the model, unless strong assumptions were made about the QoL and costs incurred beyond 6 months. During the sensitivity analysis, the time horizon was extended up to 3 years, but this relied on the assumptions that QoL did not change beyond 6 months and no costs were incurred by either strategy beyond that time. It is acknowledged that CPP among women is a problem that can often take many medical consultations to resolve, and so it is likely that the positive benefits of properly diagnosing and treating a structural cause of CPP will be felt long after the 6-month time horizon considered here.
Given that this model utilised data from a test-accuracy study in which all women received both tests, it was difficult to tease out the impact of the different test results and the resulting treatment on patient QoL. This meant that assumptions had to be made regarding how negative test results might affect women. This is really an unavoidable issue with model-based analyses that examine medical tests, but is acknowledged here nonetheless.
This economic analysis has considered only the diagnosis and treatment of structural causes of CPP, and has not included other possible causes of CPP, such as PID. Including more possible causes of CPP would make the model more cumbersome, and would also require the different treatment and test characteristics for the new causes to be incorporated into the model. This means that the full patient benefit of laparoscopy and MRI has not been considered here. Nevertheless, varying the prevalence of structural causes of CPP was undertaken in the sensitivity analysis presented here, and slightly increasing the prevalence could be regarded as a proxy for the inclusion of other causes of CPP, and thus this does help to show how their inclusion might impact on the conclusions drawn from the model.
In this model-based economic evaluation, a decision tree was utilised rather than a Markov model. This was attributable to the short time horizon and the fact that women acquiring new cases of CPP over time were not considered in the study. However, it is acknowledged that an individual sampling model could have been used, whereby the different structural causes of CPP would be described for each patient. However, given that the testing and treatment pathways for many of the causes of CPP considered in this study are the same, this approach was considered unnecessary as these pathways could easily be described using a decision tree.
Public and patient involvement
We have been supported throughout the project by the Pelvic Pain Support Network (PPSN) and, in particular, its chairperson. Public and patient involvement was crucial in improving the acceptability of the MEDAL study and promoting recruitment. We engaged with the PPSN chairperson throughout, developing an appreciation of the apprehension and uncertainty surrounding MRI and laparoscopy among women, as well as the opinions of clinicians and barriers to accessing MRI that the chairperson had encountered. This prompted us to provide a study-specific MRI information leaflet to potential participants. We also believed that it was important to establish a thorough understanding of all aspects of CPP and the potential underlying conditions, and so we designed the initial patient questionnaire with input from the PPSN.
We will engage with the PPSN regarding the dissemination of our findings, providing a plain English summary of the findings and the uncertainties around the evidence we have discussed here. This will be distributed via the PPSN website and e-newsletter. Any future research groups taking forward the research recommendations from this project would benefit from engaging with the PPSN.
Interpretation of the findings
To our knowledge, this is the first study that has examined the diagnostic value and cost-effectiveness of MRI compared with diagnostic laparoscopy in women with CPP.
The risks of MRI revolve around changing radiofrequencies and magnetic fields, which can theoretically produce heat that is absorbed by the body tissue; this is not known to produce any side effects. Conversely, like any surgery, a laparoscopy is not without its risks. It involves day care admission and general aesthesia. Laparoscopy involves minimal invasion of body tissues, but its complications can include damage to organs inside the abdomen and wound infections. Therefore, if MRI could minimise the use of laparoscopy, it could be beneficial to women and health services. Our diagnostic study found that MRI tests were not accurate for detecting structural gynaecological conditions. Furthermore, it did not add value over and above baseline history, examination and pelvic ultrasound in verifying the absence of a cause of CPP symptoms (before performing diagnostic laparoscopy).
With respect to specific gynaecological conditions, MRI was most accurate for endometrioma, adhesions and fibroids when compared with observations at laparoscopy but, even so, its performance was too inadequate to be useful. Evaluation of non-gynaecological conditions suffered from poverty of agreement over verification of the diagnosis by an expert panel, and this limits the useful interpretation of these data.
The health economic analysis found that MRI was never more cost-effective than laparoscopy. Given the poor accuracy of MRI, this conclusion was expected. Our extended analysis comparing laparoscopy and MRI with a theoretical no-testing scenario revealed the interesting observation that, at the 6-month follow-up, a strategy of no laparoscopy or MRI would be the most cost-effective – this finding is likely to be strongly affected by the short follow-up period (the benefits of successful treatment of CPP are likely to last many years), and the assumptions made about QoL without treatment (which could not be directly observed in the study).
Implications for practice
Magnetic resonance imaging was dominated by laparoscopy in the differential diagnosis of women presenting to gynaecology clinics with CPP. It did not add value to the information, already gained from history, examination and ultrasound, about idiopathic CPP and various gynaecological conditions before considering a laparoscopy. The little value it did show for a few conditions, such as endometrioma, could be obtained only at the cost of undertaking large numbers of scans, and was not cost-effective, as the small diagnostic gain came tagged with additional costs.
Unanswered questions and further research
The evaluation of subjective tests for differential diagnosis in care pathways targeting various conditions for treatment requires methodological development. Further data on the individual and joint distributions of the prevalence of causes of pain would be beneficial. In CPP, the development of diagnostic prediction models incorporating biomarkers into baseline data may improve the use of diagnostic laparoscopy; however, research will first be required to determine objective diagnostic criteria for the various target conditions and valid biomarkers for endometriosis.
Acknowledgements
The Magnetic resonance imaging for Establishing Diagnosis Against Laparoscopy study group
The MEDAL study collaborators (principal investigators/investigators/research nurses).
Basildon University Hospital
Moboladale Ojutiku, Andrew Hails, Sami Khan, Kerry Goodsell, Emily Redman, Stacey Pepper and Marco Bondoc.
Birmingham Women’s Hospital
Moji Balogun, Elizabeth Ewers, Aamir Khan, Tina Verghuse, Karen Davies, Amy Wilbraham, Chloe O’Hara, Natalie Cooka, Natalie Nunes, Yousri Afifi and Pallavi Latthe.
Chelsea and Westminster
Robert Richardson, Amer Raza, Priya Narayanan, Rhian Bull, Kylie Norrie, Sarah Ladd and Paul Cofie.
Cumberland Infirmary
Nalini Munjuluri, Toni Wilson, Job Berry, Rachel England, Linda Telford and Alexa Lilley.
Dorset County Hospital
Mamdouh Shoukrey, Karen Hogben, Andrew Gibbins, Katheryn Lawrence and Sue O’Kerwin.
Royal Infirmary of Edinburgh
Andrew Horne, Ann Doust, Scott Semple, Graham McKillop, Hayley Cuthbert, Pamela Haig, Lisa Derr, Fiona Steel and Helen Dewart.
Furness General Hospital
Vincent Bamigboye, Julia Alcide, Ali Ansarai, Amoah Christian, Burns Karen and Sanjay Sinha.
Homerton University Hospital
Sandra Watson, Akosua Aboagaye, Emma Goldstraw and Nicola Jenkerson.
Liverpool Women’s Hospital
Adel Soltan, Amy Smith, Gillian Smith, Kathie Cooke and Lesley Harris.
Medway Maritime Hospital
Sadoon Sadoon, Abu Ahmed, Dorothy Chakani, Emily Tam, Eunice Emeakaraoha, John Duckett, Jonathan Duckett, Ibrahim Ahmed, Pam Arkhurst and Sarah Burrows.
Musgrove Park Hospital
Guy Fender, Korinna Andrews, Paul Burn, Ann England and Michelle Farrar.
Newham Hospital
Antonios Antoniou, Joanne Hutchinson and Rashna Chenoy.
Ninewells Hospital
Refaat Youssef, Alison Boyle, Audrey Lyall, Deborah Forbes, Pamela Duthie, Audrey Lyall, Thiru Sudarshan, Jennifer Brady and John Brunton.
North Devon District Hospital
Seumas Eckford, Amanda Skinner, Colin Barratt, Emad Megaly, Osama Eskandar, Fiona Hammonds, Geraldine Belcher, Helen Shelton, Afaf Diyaf, Jennifer Macpherson and Natalie Taylor.
Nottingham University Hospital
Nick Raine-Fenning, Ann Selby, Anna Molnar, Corah Ohadike, Maruti Kumaran, Elizabeth Barnes, Gill Kirkwood, Kannamannadiar Jayaprakasan, Martin Powell and Maruti Kimaran.
Royal Hallamshire Hospital
Mary Connor, Andrew Baxter, Chris Wragg, Clare Pye, Sheila Duffy, Julie Metherall, Mary Connor, Shahram Abdi and Sheila Duffy.
The Royal London Hospital
Elizabeth Ball, Andrea Rockall, Seema Anoushka Tirlapur, Mathew Hogg, Mun Lim, Natasha Kaba, Shoreh Beksi and Teresita Beeston.
Royal Preston Hospital
Sanjeev Prashar, Sajuta Gupta, Patrick Keating, Sujata Gupta, Mike Dobson, Khalil Abdo, Himansuskhar Mallik, Sean Hughes, Pauline McDonald and Julie Butler.
South Tyneside District Hospital
Steve Orife, Oliver Schulte, Richard Cooper and Linda McNamee.
Southend University Hospital
Justin Winston, Venkat Raman, Cheng Lee, Gemma Ogden, Maryam Zare, Jane Hollywood, Onie Hove, Narayanaswamy Venkat Raman, Sid Liyanage and Neil Quinn.
Stafford Hospital
Kirk Chin, Elizabeth Gunning, Tracey Harrison, Susan Hendy, Lynne Ball, Dawn Sirdefield and Jill Stacey.
Sunderland Royal Hospital
Menem Yossry, Judith Ormonde, Karen Armstrong, Jemma Fenwick, Kim Hinshaw, Ahma Ahmed, Aarti Ulal, Gill Campbell, Denise Milford, Eileen Walton, Lesley Hewitt and Jemma Fenwick.
University Hospital Crosshouse
Inna Sokolova, Danielle Gilmour, Karen Gray and Margo Henry.
University Hospital of North Durham
Partha Sengupta, Chooi May Lee, Jean Dent and Partha Sengupta.
University Hospital of North Staffordshire
Jason Cooper, Anne Harrison, Bruce Jarvest, Chan, Junny, Cooper Jason, El-Gizawy Zeiad, Gourab Misra, Emma Hubball, Suzanne Jerreat, Junny Chan, Joanne Lakin, O’Brien Shaughn, Robha Ramakoti and Amanda Redford.
Whipps Cross University Hospital
Funlayo Odejinmi, Zandile Maseko and Oladimeji Olowu.
Expert independent panel (for establishing a consensus reference diagnosis)
Mr Christian Becker, John Radcliffe Hospital, Oxford; Mr Alfred Cutner, University College Hospital, London; Mr Patrick Chien (Expert Panel Chairperson), Ninewells Hospital, Dundee; Mr Justin Clark, Birmingham Women’s Hospital, Birmingham; Mr Yemi Coker, Queen’s Hospital, Romford; Mr Shane Duffy, Chelsea and Westminster Hospital, London; Mr Jed Hawe, Countess of Chester Hospital, Chester; Mr Kevin Phillips, Castle Hill Hospital, Cottingham; Dr Caroline Overton, University Hospitals Bristol NHS Foundation Trust, Bristol; Mr Adam Rosenthal, St Bartholomew’s Hospital, London; Mr Ertan Saridogan, University College Hospital, London; Mr Tom Smith Walker, Royal Cornwall Hospital, Truro; Mr Jim Thornton, Nottingham City Hospital, Nottingham; Mr Sanjay Vyas, Spire Bristol Hospital, Bristol; and Miss Ephia Yasmin, University College London Hospital, London.
Independent radiology review committee (for establishing a consensus diagnosis from the magnetic resonance imaging scan)
Dr Mark Blakeman, New Cross Hospital, Wolverhampton; Dr Claire Keaney, Sandwell General Hospital, West Bromwich/Birmingham City Hospital, Birmingham; and Dr Carolina Lopez, Bedford Hospital, Bedford.
The Study Steering and Data Monitoring Committees
The SSC provided independent supervision for the study, providing advice to the investigators and the sponsor on all aspects of the study and affording protection for women by ensuring that the study was conducted as applicable to the MRC guidelines for good clinical practice in clinical trials.
For the purposes of this study, the SSC nominated and convened a three-member independent Data Monitoring and Ethics Committee (DMEC) from within its membership, which did not include study researchers:
-
Professor Ovrang Djahanbakhch (SSC Chairperson and Head of the Unit for Women’s Health, The Royal London Hospital, London)
-
Professor Ben Willem Mol (DMEC Chairperson and Professor of Obstetrics and Gynaecology, University of Adelaide, Adelaide, SA, Australia)
-
Professor Javier Zamora (until 2013, Senior Lecturer, Barts and The London School of Medicine and Dentistry, London)
-
Dr Teresa Perez (replaced Dr Zamora in 2013; Complutense University of Madrid, Madrid, Spain)
-
Dr George Harrison (Consultant in Pain, Queen Elizabeth Hospital Birmingham, Birmingham, UK)
-
Ms Judy Birch (Chief Executive, Pelvic Pain Support Network, Poole, UK).
Study management group
The MEDAL study was co-ordinated by the Birmingham Clinical Trials Unit at the University of Birmingham and we acknowledge all of the hard work of all of the staff involved in the study, including Lee Priest (Trial Co-ordinator) and Julia Seeley (Data Manager). We thank Neil Winkles for designing and developing the study database. We thank Rita Champaneria and Bella Harris for the systematic review and reference support. We would also like to thank Christopher Webb for the organisation of the study meetings. The MEDAL study sponsor organisation was the Queen Mary University of London, and we acknowledge Kathline Mulligan (Senior Clinical Trials Manager until 2012), Julie Dodds (Senior Clinical Trials Manager from 2012), Tracy Holtham and Camille Paulsen for their support.
The study management group comprised Khalid Khan (Queen Mary University of London); Elizabeth Ball (The Royal London Hospital); Jon Deeks (University of Birmingham); Jane Daniels (University of Birmingham); Judy Birch (Pelvic Pain Support Network); Lee Middleton (University of Birmingham); Lee Priest (University of Birmingham); Julia Seeley (University of Birmingham); Seema Anushka Tirlapur (Queen Mary University of London); Julie Dodds (Queen Mary, University of London); and Teresita Beeston (Queen Mary University of London).
We are grateful to the British Society of Gynaecological Endoscopists.
Research governance
The study was conducted in accordance with the principles of MRC Guidelines for Good Clinical Practice in Clinical Trials (1998) and the appropriate NHS Research Governance Frameworks.
All centres will be required to sign an Investigator’s Agreement, detailing their commitment to accrual, compliance, good clinical practice, confidentiality and publication. Deviations from the agreement will be monitored and the SSC will decide if any action needs to be taken, such as withdrawal of funding or suspension of the centre. Proof of training in the principles of good clinical practice and informed consent may be required.
Study advisors
Dr Pallavi Latthe (Consultant Obstetrician and Gynaecologist, Birmingham Women’s Hospital) was a recruiting investigator and helped to develop the working diagnosis form.
Dr Susan Mallet (Senior Lecturer in Medical Statistics in the Test Evaluation Research Group, University of Birmingham) advised on the analysis of the study.
Dr Nigel Cowan (Consultant Clinical Radiologist, Nuffield Health Oxford Hospital) provided guidance on the criteria for the assessment of the target conditions investigated in the study.
Professor Charles Knowles (Clinical Professor of Surgical Research, Barts and the London School of Medicine and Dentistry Unit) provided guidance on bowel symptoms.
Professor Kasim Aziz (Professor of Neurogastroenterology, Queen Mary University of London) provided guidance on bowel symptoms.
Dr Jonathan Hill (Keele University) provided guidance on physiotherapy and musculoskeletal aspects.
Finally, we would like to thank all of the women who consented to participate in this study. The study would not have been possible without them.
Contributions of authors
Professor Khalid S Khan (Consultant Gynaecologist and lead applicant) conceived the idea for the study, contributed to the design, delivery, analysis and interpretation, recruited women, and, as the chief investigator, had overall responsibility for the MEDAL study.
Mr Konstantinos Tryposkiadis (Statistician) performed the analyses for the accuracy study and contributed to the interpretation and drafting of the report.
Dr Seema A Tirlapur (Clinical Research Fellow) recruited participants, developed the process for the EIP and contributed to the delivery of the accuracy study and the development of the economic model.
Mr Lee J Middleton (Senior Statistician) contributed to the design, analysis and interpretation of the accuracy study.
Dr Andrew J Sutton (Senior Economic Research Fellow) contributed to the design and interpretation of the economic evaluation, performed the analysis and drafted the economic study chapter (see Chapter 4).
Mr Lee Priest (Senior Trial Co-ordinator) was responsible for the day-to-day management and delivery of all study components, and developed the process for the EIP panel and edited the report.
Dr Elizabeth Ball (Consultant Gynaecologist and co-applicant) contributed to the design and delivery of the accuracy study and the development of the economic model, and recruited participants.
Dr Moji Balogun (Consultant Radiologist and co-applicant) contributed to the design of the accuracy study and the development of the MRI reporting standards.
Dr Anju Sahdev (Consultant Radiologist) contributed to the development of the MRI reporting standards.
Professor Tracy Roberts (Professor of Health Economics and co-applicant) contributed to the design, analysis and interpretation of the economic evaluation.
Mrs Judy Birch (Chairperson, Pelvic Pain Support Network and co-applicant) contributed to the design of the accuracy study and commented on the acceptability of participant-facing material.
Dr Jane P Daniels (Deputy Director of Birmingham Clinical Trials Unit and co-applicant) contributed to the design, delivery and interpretation of the MEDAL study, the first draft and overall editing of the final report.
Professor Jonathan J Deeks (Professor of Medical Statistics and co-applicant) defined the design and statistical analysis of the accuracy study and contributed to the interpretation of the accuracy study and economic model.
Publications
Tirlapur SA, Priest L, Daniels JP, Khan KS on behalf of the MEDAL study management group. How do we define the term idiopathic? Curr Opin Obstet Gynecol 2013;25:468–7.
Tirlapur SA, Priest L, Wojdyla D, Khan KS on behalf of the MEDAL Study. Bladder pain syndrome: validation of simple tests for diagnosis in women with chronic pelvic pain: BRaVADO study protocol. Reprod Health 2013;10:61.
Tirlapur SA, Daniels JP, Khan K on behalf of the MEDAL Study. Chronic pelvic pain: how does noninvasive imaging compare with diagnostic laparoscopy? Curr Opin Obstet Gynecol 2015;27:445–8.
Bharwani N, Tirlapur SA, Balogun M, Priest L, Khan KS, Zamora J, Sadhev A. MRI reporting standard for chronic pelvic pain: consensus development. Br J Radiol 2016;89:20140615.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by women and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Williams RE, Hartmann KE, Steege JF. Documenting the current definitions of chronic pelvic pain: implications for research. Obstet Gynecol 2004;103:686-91. https://doi.org/10.1097/01.AOG.0000115513.92318.b7.
- Tirlapur SA, Priest L, Daniels JP, Khan KS. MEDAL Study Management Group . How do we define the term idiopathic?. Curr Opin Obstet Gynecol 2013;25:468-73. https://doi.org/10.1097/GCO.0000000000000025.
- Daniels JP, Khan KS. Chronic pelvic pain in women. BMJ 2010;341. https://doi.org/10.1136/bmj.c4834.
- Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician 2014;17:E141-7.
- Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Prevalence and incidence of chronic pelvic pain in primary care: evidence from a national general practice database. Br J Obstet Gynaecol 1999;106:1149-55. https://doi.org/10.1111/j.1471-0528.1999.tb08140.x.
- Zondervan K, Barlow DH. Epidemiology of chronic pelvic pain. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:403-14. https://doi.org/10.1053/beog.1999.0083.
- Henzl MR. Dysmenorrhoea; achievements and challenge. Sex Med Today 1985;9:7-11.
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv 1993;48:357-87. https://doi.org/10.1097/00006254-199306000-00001.
- Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, et al. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract 2001;51:541-7.
- Grace V, Zondervan K. Chronic pelvic pain in women in New Zealand: comparative well-being, comorbidity, and impact on work and other activities. Health Care Women Int 2006;27:585-99. https://doi.org/10.1080/07399330600803725.
- Aslam N, Harrison G, Khan K, Patwardhan S. Visceral hyperalgesia in chronic pelvic pain. BJOG 2009;116:1551-5. https://doi.org/10.1111/j.1471-0528.2009.02305.x.
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292-9. https://doi.org/10.1093/humrep/des073.
- Baranowski A, Abrams P, Berger RE. Taxonomy of Pelvic Pain. Classification of Chronic Pain. Washington, DC: International Association for the Study of Pain; 2012.
- Champaneria R, Shah L, Moss J, Gupta JK, Birch J, Middleton LJ, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20050.
- Gruppo Italiano per lo Studio dell'Endometriosi . Relationship between stage, site and morphological characteristics of pelvic endometriosis and pain. Hum Reprod 2001;16:2668-71. https://doi.org/10.1093/humrep/16.12.2668.
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007;55:377-91. https://doi.org/10.1016/j.neuron.2007.07.012.
- Daniels J, Gray R, Hills RK, Latthe P, Buckley L, Gupta J, et al. Laparoscopic uterosacral nerve ablation for alleviating chronic pelvic pain: a randomized controlled trial. JAMA 2009;302:955-61. https://doi.org/10.1001/jama.2009.1268.
- Fall M, Baranowski AP, Elneil S, Engeler D, Hughes J, Messelink EJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol 2010;57:35-48. https://doi.org/10.1016/j.eururo.2009.08.020.
- Ballard K, Lowton K, Wright J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril 2006;86:1296-301. https://doi.org/10.1016/j.fertnstert.2006.04.054.
- The Initial Management of Chronic Pelvic Pain: Green-top Guideline No. 41. London: Royal College of Obstetricians and Gynaecologists; 2005.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod 1996;11:878-80. https://doi.org/10.1093/oxfordjournals.humrep.a019270.
- Matsuzaki S, Canis M, Pouly JL, Rabischong B, Botchorishvili R, Mage G. Relationship between delay of surgical diagnosis and severity of disease in patients with symptomatic deep infiltrating endometriosis. Fertil Steril 2006;86:1314-16. https://doi.org/10.1016/j.fertnstert.2006.03.048.
- Prentice A. Medical management of chronic pelvic pain. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:495-9. https://doi.org/10.1053/beog.1999.0087.
- Okaro E, Condous G, Khalid A, Timmerman D, Ameye L, Huffel SV, et al. The use of ultrasound-based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain – can we reduce the need for laparoscopy?. BJOG 2006;113:251-6. https://doi.org/10.1111/j.1471-0528.2006.00849.x.
- Cervigni M, Natale F. Gynecological disorders in bladder pain syndrome/interstitial cystitis patients. Int J Urol 2014;21:85-8. https://doi.org/10.1111/iju.12379.
- Walker EA, Gelfand AN, Gelfand MD, Green C, Katon WJ. Chronic pelvic pain and gynecological symptoms in women with irritable bowel syndrome. J Psychosom Obstet Gynaecol 1996;17:39-46. https://doi.org/10.3109/01674829609025662.
- Longstreth GF. Irritable bowel syndrome and chronic pelvic pain. Obstet Gynecol Surv 1994;49:505-7. https://doi.org/10.1097/00006254-199407000-00027.
- Thornton JG, Morley S, Lilleyman J, Onwude JL, Currie I, Crompton AC. The relationship between laparoscopic disease, pelvic pain and infertility; an unbiased assessment. Eur J Obstet Gynecol Reprod Biol 1997;74:57-62. https://doi.org/10.1016/S0301-2115(97)00082-1.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 2005;11:595-606. https://doi.org/10.1093/humupd/dmi029.
- Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 2016;7. https://doi.org/10.1002/14651858.CD012281.
- Howard FM. The role of laparoscopy as a diagnostic tool in chronic pelvic pain. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:467-94. https://doi.org/10.1053/beog.1999.0086.
- Howard FM. Laparoscopic evaluation and treatment of women with chronic pelvic pain. J Am Assoc Gynecol Laparosc 1994;1:325-31. https://doi.org/10.1016/S1074-3804(05)80797-2.
- Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Occult microscopic endometriosis: undetectable by laparoscopy in normal peritoneum. Hum Reprod 2014;29:462-72. https://doi.org/10.1093/humrep/det438.
- Moore J, Ziebland S, Kennedy S. ‘People sometimes react funny if they’re not told enough’: women’s views about the risks of diagnostic laparoscopy. Health Expect 2002;5:302-9. https://doi.org/10.1046/j.1369-6513.2002.00192.x.
- McGowan L, Luker K, Creed F, Chew-Graham CA. How do you explain a pain that can’t be seen? The narratives of women with chronic pelvic pain and their disengagement with the diagnostic cycle. Br J Health Psychol 2007;12:261-74. https://doi.org/10.1348/135910706X104076.
- Walter AJ, Hentz JG, Magtibay PM, Cornella JL, Magrina JF. Endometriosis: correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol 2001;184:1407-11. https://doi.org/10.1067/mob.2001.115747.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG 2004;111:1204-12. https://doi.org/10.1111/j.1471-0528.2004.00433.x.
- Stratton P, Winkel C, Premkumar A, Chow C, Wilson J, Hearns-Stokes R, et al. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil Steril 2003;79:1078-85. https://doi.org/10.1016/S0015-0282(03)00155-9.
- Bazot M, Bornier C, Dubernard G, Roseau G, Cortez A, Daraï E. Accuracy of magnetic resonance imaging and rectal endoscopic sonography for the prediction of location of deep pelvic endometriosis. Hum Reprod 2007;22:1457-63. https://doi.org/10.1093/humrep/dem008.
- Katayama M, Masui T, Kobayashi S, Ito T, Sakahara H, Nozaki A, et al. Evaluation of pelvic adhesions using multiphase and multislice MR imaging with kinematic display. AJR Am J Roentgenol 2001;177:107-10. https://doi.org/10.2214/ajr.177.1.1770107.
- Tukeva TA, Aronen HJ, Karjalainen PT, Molander P, Paavonen T, Paavonen J. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology 1999;210:209-16. https://doi.org/10.1148/radiology.210.1.r99ja04209.
- Munday PE. Pelvic inflammatory disease – an evidence-based approach to diagnosis. J Infect 2000;40:31-4. https://doi.org/10.1053/jinf.1999.0609.
- Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet 2014;289:1263-9. https://doi.org/10.1007/s00404-014-3150-7.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand 2010;89:1374-84. https://doi.org/10.3109/00016349.2010.512061.
- Hsu AL, Khachikyan I, Stratton P. Invasive and non-invasive methods for the diagnosis of endometriosis. Clin Obstet Gynecol 2010;53:413-19.
- Dueholm M, Lundorf E. Transvaginal ultrasound or MRI for diagnosis of adenomyosis. Curr Opin Obstet Gynecol 2007;19:505-12. https://doi.org/10.1097/GCO.0b013e3282f1bf00.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol 2002;186:409-15. https://doi.org/10.1067/mob.2002.121725.
- Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol 2017;27:2765-7. https://doi.org/10.1007/s00330-016-4673-z.
- Younas K, Majoko F, Sheard K, Edwards C, Bunkheila A. Select and treat at laparoscopy and dye test improves the spontaneous pregnancy. Hum Fertil 2014;17:56-9. https://doi.org/10.3109/14647273.2014.880522.
- Ball E, Koh C, Janik G, Davis C. Gynaecological laparoscopy: ‘see and treat’ should be the gold standard. Curr Opin Obstet Gynecol 2008;20:325-30. https://doi.org/10.1097/GCO.0b013e32830002bb.
- Cheong YC, Reading I, Bailey S, Sadek K, Ledger W, Li TC. Should women with chronic pelvic pain have adhesiolysis?. BMC Womens Health 2014;14. https://doi.org/10.1186/1472-6874-14-36.
- Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, Garry R, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev 2014;3. https://doi.org/10.1002/14651858.CD011031.
- Farquhar C, Sutton C. The evidence for the management of endometriosis. Curr Opin Obstet Gynecol 1998;10:321-32. https://doi.org/10.1097/00001703-199808000-00007.
- Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev 2014;10. https://doi.org/10.1002/14651858.CD004638.pub3.
- Wu L, Wu Q, Liu L. Oral contraceptive pills for endometriosis after conservative surgery: a systematic review and meta-analysis. Gynecol Endocrinol 2013;29:883-90. https://doi.org/10.3109/09513590.2013.819085.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD005072.pub3.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol 2010;50:273-9. https://doi.org/10.1111/j.1479-828X.2010.01152.x.
- Lewis SC, Bhattacharya S, Wu O, Vincent K, Jack SA, Critchley HO, et al. Gabapentin for the management of chronic pelvic pain in women (GaPP1): a pilot randomised controlled trial. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0153037.
- Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: a prospective multicentre observational study. Br J Obstet Gynaecol 1997;104:595-600. https://doi.org/10.1111/j.1471-0528.1997.tb11539.x.
- Kontoravdis A, Chryssikopoulos A, Hassiakos D, Liapis A, Zourlas PA. The diagnostic value of laparoscopy in 2365 patients with acute and chronic pelvic pain. Int J Gynaecol Obstet 1996;52:243-8. https://doi.org/10.1016/0020-7292(95)02611-8.
- Chapron C, Querleu D, Bruhat MA, Madelenat P, Fernandez H, Pierre F, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod 1998;13:867-72. https://doi.org/10.1093/humrep/13.4.867.
- Laparoscopic Injury Study. Rockville, MD: Physician Insurers Association of America; 2000.
- Bharwani N, Tirlapur SA, Balogun M, Priest L, Khan KS, Zamora J, et al. MRI reporting standard for chronic pelvic pain: consensus development. Br J Radiol 2016;89. https://doi.org/10.1259/bjr.20140615.
- Honest H, Khan KS. Reporting of measures of accuracy in systematic reviews of diagnostic literature. BMC Health Serv Res 2002;2. https://doi.org/10.1186/1472-6963-2-4.
- Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril 2009;92:1825-33. https://doi.org/10.1016/j.fertnstert.2008.09.005.
- Chamié LP, Blasbalg R, Gonçalves MO, Carvalho FM, Abrão MS, de Oliveira IS. Accuracy of magnetic resonance imaging for diagnosis and preoperative assessment of deeply infiltrating endometriosis. Int J Gynaecol Obstet 2009;106:198-201. https://doi.org/10.1016/j.ijgo.2009.04.013.
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11500.
- Irwig L, Bossuyt P, Glasziou P, Gatsonis C, Lijmer J. Designing studies to ensure that estimates of test accuracy are transferable. BMJ 2002;324:669-71. https://doi.org/10.1136/bmj.324.7338.669.
- Bossuyt PM, Irwig L, Craig J, Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ 2006;332:1089-92. https://doi.org/10.1136/bmj.332.7549.1089.
- Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making 1991;11:88-94. https://doi.org/10.1177/0272989X9101100203.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. https://doi.org/10.2307/2531595.
- Fleiss J. Statistical Methods for Rates and Proportions. New York, NY: John Wiley & Sons, Inc.; 1981.
- Walker JJ, Irvine G. How should we approach the management of pelvic pain?. Gynecol Obstet Invest 1998;45:6-10. https://doi.org/10.1159/000052846.
- Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191-7. https://doi.org/10.1016/0304-3959(87)91074-8.
- Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, et al. Rome. III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates, Inc.; 2006.
- Parsons CL. Diagnosing chronic pelvic pain of bladder origin. J Reprod Med 2004;49:235-42.
- Stead ML, Crocombe WD, Fallowfield LJ, Selby P, Perren TJ, Garry R, et al. Sexual activity questionnaires in clinical trials: acceptability to patients with gynaecological disorders. Br J Obstet Gynaecol 1999;106:50-4. https://doi.org/10.1111/j.1471-0528.1999.tb08084.x.
- Rammstedt B, John OP. Measuring personality in one minute or less: a 10-item short version of the Big Five Inventory in English and German. J Res Pers 2007;41:203-12. https://doi.org/10.1016/j.jrp.2006.02.001.
- Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524-32. https://doi.org/10.1037/1040-3590.7.4.524.
- Jones G, Jenkinson C, Kennedy S. Development of the Short Form Endometriosis Health Profile Questionnaire: the EHP-5. Qual Life Res 2004;13:695-704. https://doi.org/10.1023/B:QURE.0000021321.48041.0e.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. https://doi.org/10.1097/01.MLR.0000093487.78664.3C.
- Leserman J, Drossman DA, Li Z. The reliability and validity of a sexual and physical abuse history questionnaire in female patients with gastrointestinal disorders. Behav Med 1995;21:141-50. https://doi.org/10.1080/08964289.1995.9933752.
- EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Al-Janabi H, Flynn T, Coast J. Development of a self-reported measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2010;21:167-76.
- The American Fertility Society . Classification of endometriosis. Fertil Steril 1979;32:633-4. https://doi.org/10.1016/S0015-0282(16)44409-2.
- Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404-13. https://doi.org/10.1093/biomet/26.4.404.
- NHS Reference Costs 2013–14. London: DHSC; 2014.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2008.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9 (accessed November 2014).
Appendix 1 Additional baseline data from the diagnostic study
| Recruiting centre | n (%) |
|---|---|
| Basildon University Hospital | 3 (1) |
| Birmingham Women’s Hospital | 6 (2) |
| Chelsea and Westminster | 24 (8) |
| Cumberland Infirmary | 6 (2) |
| Dorset County Hospital | 3 (1) |
| Royal Infirmary of Edinburgh | 5 (2) |
| Furness General Hospital | 6 (2) |
| Homerton University Hospital | 19 (7) |
| Liverpool Women’s Hospital | 11 (4) |
| Medway Maritime Hospital | 2 (1) |
| Musgrove Park Hospital | 9 (3) |
| Newham University Hospital | 5 (2) |
| Ninewells Hospital | 11 (4) |
| North Devon District Hospital | 7 (2) |
| Nottingham University Hospital | 4 (1) |
| Royal Hallamshire Hospital | 14 (5) |
| The Royal London Hospital | 80 (27) |
| Royal Preston Hospital | 6 (2) |
| South Tyneside District Hospital | 2 (1) |
| Southend University Hospital | 7 (2) |
| Stafford Hospital | 8 (3) |
| Sunderland Royal Hospital | 6 (2) |
| University Hospital Crosshouse | 10 (3) |
| University Hospital of North Durham | 17 (6) |
| University Hospital of North Staffordshire | 16 (6) |
| Whipps Cross University Hospital | 4 (1) |
| Total | 291 (100) |
| Demographic variable | Value |
|---|---|
| Height (cm), mean (SD, n) | 1.64 (0.07, 283) |
| Weight (kg), mean (SD, n) | 71.1 (15.3, 274) |
| Smoking | |
| Smoked > 100 cigarettes during lifetime, n (%, N responses) | 117 (40.6, 288) |
| Current smoker, n (%, N responses) | 67 (57.3, 117) |
| Units of alcohol drunk per week, mean (SD, n) | 4.4 (6.5, 275) |
| Participant affected by special social circumstances, n (%, N responses) | 17 (6.0, 283) |
| Participant characteristics | Value |
|---|---|
| Pain problems | |
| Duration of pain | |
| Duration of pain (years), mean (SD, n) | 4.2 (4.8, 287) |
| Event associated with the onset of pain, n (%, N responses) | 136 (48.1, 283) |
| Change in pain since onset, n (%) | |
| Got a lot worse | 155 (53.8) |
| Got a little worse | 57 (19.8) |
| Not changed | 59 (20.5) |
| Got a little better | 12 (4.2) |
| Got a lot better | 2 (0.7) |
| Do not know | 3 (1.0) |
| Missing | 3 (1.0) |
| Number of days with pelvic pain in previous month | |
| < 1 day per month | 4 (1.4) |
| 1 day per month | 7 (2.5) |
| 2–3 days per month | 47 (16.6) |
| 1 day per week | 12 (4.2) |
| > 1 day per week | 100 (35.2) |
| Every day | 114 (40.1) |
| Missing | 7 (2.4) |
| Location of pain | |
| Severity of pain in each area (0 = no pain, 10 = most severe pain imaginable), mean (SD, n) | |
| Left upper back quadrant | 0.1 (0.9, 291) |
| Central upper back | 0.2 (1.0, 291) |
| Right upper back quadrant | 0.1 (0.7, 291) |
| Left lumbar back region | 1.2 (2.8, 291) |
| Central back | 1.5 (3.0, 291) |
| Right lumbar back region | 1.2 (2.7, 291) |
| Left lower back region | 1.0 (2.6, 291) |
| Central lower back | 1.3 (3.0, 291) |
| Right lower back | 1.1 (2.8, 291) |
| Left outer posterior thigh | 0.3 (1.5, 291) |
| Left inner posterior thigh | 0.5 (1.8, 291) |
| Right outer posterior thigh | 0.3 (1.5, 291) |
| Right inner posterior thigh | 0.4 (1.7, 291) |
| Right hypochondriac upper | 0.1 (0.9, 291) |
| Epigastric region | 0.1 (0.9, 291) |
| Left hypochondriac upper | 0.1 (0.9, 291) |
| Right lumbar | 1.0 (2.5, 291) |
| Umbilical region | 1.3 (2.9, 291) |
| Left lumbar | 1.0 (2.6, 291) |
| Right iliac | 3.8 (4.1, 291) |
| Hypogastric/suprapubic | 4.5 (4.1, 291) |
| Left iliac | 3.7 (4.1, 291) |
| Right outer anterior thigh | 0.5 (1.8, 291) |
| Right inner anterior thigh | 0.5 (1.9, 291) |
| Left inner anterior thigh | 0.5 (1.7, 291) |
| Left outer anterior thigh | 0.4 (1.7, 291) |
| Urethral region | 0.4 (1.7, 291) |
| Vulval | 0.7 (2.2, 291) |
| Perianal | 0.5 (1.9, 291) |
| Right inner thigh | 0.2 (1.3, 291) |
| Right buttock | 0.1 (0.7, 291) |
| Left inner thigh | 0.2 (1.3, 291) |
| Left buttock | 0.02 (0.4, 291) |
| Pain just before period (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 6.4 (2.6, 264) |
| Duration in months, mean (SD, n) | 24.1 (22.5, 201) |
| Pain during period (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 7.1 (2.8, 266) |
| Duration in months, mean (SD, n) | 25.3 (23.7, 202) |
| Pain when period is over (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 4.8 (2.8, 263) |
| Duration in months, mean (SD, n) | 22.8 (22.0, 184) |
| Pain mid-cycle (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 5.4 (2.9, 263) |
| Duration in months, mean (SD, n) | 23.7 (22.2, 193) |
| Sexual intercourse in the last month, n (%, N responses) | 212 (73.4, 289) |
| If yes, pain at the point of vaginal penetration (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 3.6 (3.3, 209) |
| Duration in months, mean (SD, n) | 20.3 (21.4, 114) |
| If yes, deep pain during intercourse (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 6.0 (3.3, 212) |
| Duration in months, mean (SD, n) | 20.3 (20.7, 146) |
| If yes, burning vaginal pain during intercourse (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 2.9 (3.4, 210) |
| Duration in months, mean (SD, n) | 19.8 (18.5, 77) |
| If yes, pelvic pain lasting hours or days after (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 5.1 (3.3, 211) |
| Duration in months, mean (SD, n) | 20.1 (21.1, 134) |
| Pain when bladder is full (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 4.1 (3.3, 286) |
| Duration in months, mean (SD, n) | 18.5 (21.1, 146) |
| Pain with urination (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 2.1 (2.8, 283) |
| Duration in months, mean (SD, n) | 17.1 (19.7, 95) |
| Muscle/joint pain in pelvic region (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 4.2 (3.6, 278) |
| Duration in months, mean (SD, n) | 22.5 (22.7, 137) |
| Pain in pelvis when lifting (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 3.5 (3.4, 282) |
| Duration in months, mean (SD, n) | 21.3 (22.7, 133) |
| Pain when sitting (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 4.0 (3.3, 282) |
| Duration in months, mean (SD, n) | 19.2 (18.1, 147) |
| Backache (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 5.1 (3.3, 286) |
| Duration in months, mean (SD, n) | 22.7 (24.5, 168) |
| Migraine headache (0 = no pain, 10 = worst pain imaginable), mean (SD, n) | 3.4 (3.7, 222) |
| Duration in months, mean (SD, n) | 23.8 (23.5, 93) |
| Treatments for pain, n (%) | |
| Acupuncture | 13 (4.5) |
| If used, was considered helpful, n (%, N responses) | 7 (53.8, 13) |
| Anti-seizure medications | 7 (2.4) |
| If used, was considered helpful, n (%, N responses) | 2 (28.6, 7) |
| Antidepressants | 39 (13.4) |
| If used, was considered helpful, n (%, N responses) | 17 (47.2, 36) |
| Biofeedback | 1 (0.3) |
| If used, was considered helpful, n (%, N responses) | 1 (100, 1) |
| Botox injection | 2 (0.7) |
| If used, was considered helpful, n (%, N responses) | 1 (50, 2) |
| Contraceptive pills/patch/ring | 162 (55.7) |
| If used, was considered helpful, n (%, N responses) | 51 (32.7, 156) |
| Exercise, yoga or pilates | 131 (45.0) |
| If used, was considered helpful, n (%, N responses) | 38 (30.4, 125) |
| Hormonal therapy for endometriosis | 27 (9.3) |
| If used, was considered helpful, n (%, N responses) | 10 (40, 25) |
| Herbal medicine | 27 (9.3) |
| If used, was considered helpful, n (%, N responses) | 8 (32, 25) |
| Homeopathic medicine | 14 (4.8) |
| If used, was considered helpful, n (%, N responses) | 3 (25, 12) |
| Massage | 80 (27.5) |
| If used, was considered helpful, n (%, N responses) | 32 (42.7, 75) |
| Meditation or relaxation exercises | 57 (19.6) |
| If used, was considered helpful, n (%, N responses) | 22 (40, 55) |
| Strong painkillers | 237 (81.4) |
| If used, was considered helpful, n (%, N responses) | 137 (60.9, 225) |
| Nerve blocks | 5 (1.7) |
| If used, was considered helpful, n (%, N responses) | 2 (40, 5) |
| Non-prescription medicine | 68 (23.4) |
| If used, was considered helpful, n (%, N responses) | 26 (41.3, 63) |
| Nutrition/diet | 90 (30.9) |
| If used, was considered helpful, n (%, N responses) | 28 (33.7, 83) |
| Physiotherapy | 27 (9.3) |
| If used, was considered helpful, n (%, N responses) | 5 (19.2, 26) |
| Psychological (talking) therapy | 20 (6.9) |
| If used, was considered helpful, n (%, N responses) | 6 (33.3, 18) |
| TENS | 12 (4.1) |
| If used, was considered helpful, n (%, N responses) | 6 (55.5, 11) |
| Other | 28 (9.6) |
| If used, was considered helpful, n (%, N responses) | 15 (57.7, 26) |
| Menstrual history | |
| Age at menarche, mean (SD, n) | 12.8 (1.7, 290) |
| Currently having menstrual periods, n (%, N responses) | 252 (86.9, 290) |
| Pelvic pain with periods in at 3 months, n (%, N responses) | |
| No | 13 (5.2) |
| Occasionally (with 1 in 3 of my periods) | 19 (7.6) |
| Often (with 2 in 3 of my periods) | 32 (12.9) |
| Always (every period) | 185 (74.3) |
| Missing | 42 |
| Pelvic pain at times other than with periods or sexual intercourse in the last 3 months, n (%, N responses) | 223 (90.6, 246) |
| If yes, pain before period, n (%, N responses) | 197 (88.3, 223) |
| If yes, pain after period, n (%, N responses) | 145 (66.1, 221) |
| Period regularity, n (%) | |
| Regular, I know when to expect my period | 89 (35.9) |
| Fairly regular, my period starts within a few days of when I expect | 75 (30.2) |
| Irregular, I cannot predict when my period will start | 53 (21.4) |
| I have bleeding on and off all the time | 31 (12.5) |
| Missing | 43 |
| Period heaviness, n (%) | |
| Light | 28 (11.2) |
| Moderate | 76 (30.3) |
| Heavy | 95 (37.9) |
| Bleed through protection | 52 (20.7) |
| Missing | 40 |
| Duration of period (days), mean (SD, n) | 5.3 (2.0, 244) |
| Average interval between periods (days), mean (SD, n) | 28.0 (12.3, 211) |
| Clots in menstrual flow, n (%, N responses) | 198 (80.5, 246) |
| Pain on day flow starts, n (%, N responses) | 53 (22.2, 239) |
| If no, how many days before flow, mean (SD, n) | 4.3 (3.3, 152) |
| Previous diagnoses, n (%) | |
| Endometriosis | 76 (26.7) |
| Adhesions | 20 (7.0) |
| Fibroids | 35 (12.3) |
| Adenomyosis | 10 (3.5) |
| Uterine polyps | 10 (3.5) |
| Ovarian cysts | 105 (36.8) |
| Appendicitis | 29 (10.2) |
| Hernia | 10 (3.5) |
| Infertility or low fertility | 25 (8.8) |
| Uterine or bladder prolapse | 2 (0.7) |
| Vulva pain/vulvodynia | 8 (2.8) |
| Irritable bowel syndrome | 49 (17.2) |
| Nerve entrapment in pelvis/pudendal neuropathy | 1 (0.4) |
| Fibromyalgia | 7 (2.5) |
| Painful bladder syndrome (interstitial cystitis) | 12 (4.2) |
| Sexually transmitted infection | 36 (12.7) |
| Female genital mutilation/cutting | 2 (0.7) |
| Previous tests | |
| Previous cervical screening test, n (%, N responses) | 229 (79, 290) |
| If test performed, outcome, n (%) | |
| Normal | 210 (92.5) |
| Abnormal | 17 (7.5) |
| Missing | 2 (0.9) |
| Previous chlamydia test, n (%, N responses) | 202 (70.4, 287) |
| If test performed outcome, n (%) | |
| Negative for chlamydia | 176 (87.1) |
| Positive for chlamydia and treated | 21 (10.4) |
| Positive for chlamydia, not treated | 0 (–) |
| Missing | 5 (2.4) |
| Previous investigations/operations, n (%) | |
| Previous investigations/operations | |
| Laparoscopy | 80 (27.5) |
| Cystoscopy | 16 (5.5) |
| Colonoscopy | 36 (12.4) |
| Hysteroscopy | 24 (8.3) |
| MRI | 30 (10.3) |
| Laparotomy | 9 (3.1) |
| Transvaginal ultrasound | 199 (68.4) |
| Transabdominal ultrasound | 220 (75.6) |
| Nerve transmission test | 1 (0.3) |
| Allergy tests | 20 (6.9) |
| Other | 7 (2.4) |
| Contraceptive user, n (%) | 172 (71.4) |
| Of those using contraception, method(s) used | |
| Female sterilisation (clips) | 9 (5.2) |
| Female sterilisation (implants) | 1 (0.6) |
| Male partner sterilisation | 11 (6.4) |
| Contraceptive pill | 71 (41.3) |
| Mini pill | 12 (7.0) |
| Injection | 3 (1.7) |
| Patch | 1 (0.6) |
| Implant | 10 (5.8) |
| Levonorgestrel-releasing intrauterine system | 19 (11.1) |
| Condom | 67 (39.0) |
| Diaphragm/cap | 1 (0.6) |
| Vaginal ring | – |
| Natural method | 7 (4.1) |
| Obstetric history | |
| Fertility, n (%) | |
| Currently trying to get pregnant, n (%, N responses) | 36 (14.8, 242) |
| Of those trying to get pregnant, n (%) | |
| Trying for < 1 year | 8 (22.2) |
| Trying for > 1 year | 28 (77.8) |
| Pregnancies, n (%) | |
| Number of times been pregnant previously | |
| 0 | 113 (40.0) |
| 1 | 46 (16.0) |
| 2 | 51 (18.0) |
| 3 | 33 (12.0) |
| 4 | 20 (7.0) |
| ≥ 5 | 19 (7.0) |
| Missing | 9 |
| If at least one previous pregnancy, outcome | |
| Live birth | |
| 0 | 38 (22.4) |
| 1 | 41 (24.2) |
| 2 | 58 (34.3) |
| 3 | 23 (13.6) |
| 4 | 5 (2.9) |
| ≥ 5 | 1 (0.6) |
| Missing | 3 (1.8) |
| Termination | |
| 0 | 110 (65.1) |
| 1 | 47 (27.8) |
| 2 | 8 (4.7) |
| 3 | 3 (1.8) |
| 4 | – |
| ≥ 5 | – |
| Missing | 1 (0.6) |
| Stillbirth | |
| 0 | 158 (93.5) |
| 1 | 7 (4.1) |
| 2 | 1 (0.6) |
| 3 | – |
| 4 | 1 (0.6) |
| ≥ 5 | – |
| Missing | 2 (1.2) |
| Miscarriage | |
| 0 | 112 (66.3) |
| 1 | 35 (20.7) |
| 2 | 10 (5.9) |
| 3 | 3 (1.8) |
| 4 | 4 (2.4) |
| ≥ 5 | 3 (1.7) |
| Missing | 2 (1.2) |
| If at least one live birth, route of delivery | |
| Vaginal | |
| 0 | 79 (47.6) |
| 1 | 27 (16.3) |
| 2 | 41 (24.7) |
| 3 | 14 (8.4) |
| 4 | 4 (2.4) |
| ≥ 5 | 1 (0.6) |
| Caesarean section (planned) | |
| 0 | 151 (91.0) |
| 1 | 10 (6.0) |
| 2 | 4 (2.4) |
| 3 | 1 (0.6) |
| 4 | – |
| ≥ 5 | – |
| Caesarean section (emergency) | |
| 0 | 135 (80.8) |
| 1 | 26 (15.6) |
| 2 | 5 (3.0) |
| 3 | – |
| 4 | 1 (0.6) |
| ≥ 5 | – |
| Forceps/ventouse | |
| 0 | 146 (88.0) |
| 1 | 18 (10.8) |
| 2 | 2 (1.2) |
| 3 | – |
| 4 | – |
| ≥ 5 | – |
| Did any of the following occur during pregnancy, labour or just after giving birth (yes) | |
| Abdominal pain (other than labour contractions) | 56 (37) |
| Perineal tear | 53 (35) |
| Episiotomy | 37 (25) |
| Pelvic girdle pain | 26 (17) |
| Interventions for peri-partum haemorrhage | 23 (15) |
| Participant-reported questionnaires | |
| Pelvic Pain and Urgency/Frequency Patient Symptom Scale | |
| Summary score (points) (SD, n) | 13.3 (5.5, 167) |
| Assessment of sexual activity | |
| Section I, n (%) | |
| Are you currently married or having an intimate relationship with someone? | 238 (83) |
| Have you changed your sexual partner in the last 6 months? | 24 (8) |
| Do you engage in sexual activity with anyone at the moment? | 222 (78) |
| Section II, n (%) | |
| I am not sexually active at the moment because | |
| I do not have a partner at the moment | 37 (60) |
| I am too tired | 6 (10) |
| My partner is too tired | 1 (2) |
| I am not interested in sex | 10 (16) |
| My partner is not interested in sex | 3 (5) |
| I have a physical problem that makes sexual relations difficult or uncomfortable | 13 (22) |
| My partner has a physical problem that makes sexual relations difficult or uncomfortable | 1 (2) |
| Other reason | 13 (22) |
| Section III | |
| Pleasure score (0 = worst outcome, 18 = best outcome) (SD, n) | 7.4 (4.5, 104) |
| Discomfort score (0 = worst outcome, 6 = best outcome) (SD, n) | 3.1 (1.7, 204) |
| Habit score (0 = less than usual, 1 = the same as usual, 2 = somewhat more than usual, 3 = much more than usual) (SD, n) | 2.2 (0.8, 213) |
| BFI score | |
| Extraversion (0 = worst outcome, 10 = best outcome), mean (SD, n) | 7.1 (2.0, 283) |
| Agreeableness (0 = worst outcome, 10 = best outcome), mean (SD, n) | 7.6 (1.8, 284) |
| Conscientiousness (0 = worst outcome, 10 = best outcome), mean (SD, n) | 8.4 (1.6, 279) |
| Neuroticism (0 = worst outcome, 10 = best outcome), mean (SD, n) | 6.2 (2.1, 286) |
| Openness (0 = worst outcome, 10 = best outcome), mean (SD, n) | 6.9 (1.6, 282) |
| Pain Catastrophizing Scale | |
| Over the last month, how much have you been affected by your pain (0 = least affected, 20 = worst affected), mean (SD, n) | – |
| Of all the problems and stresses in your life, how does your pain compare in importance? (0 = just one of many problems, 10 = the most important thing), mean (SD, n) | – |
| Depression (6 = worst outcome, 0 = best outcome), mean (SD, n) | 2.2 (1.8, 289) |
| Rumination (16 = worst outcome, 0 = best outcome), mean (SD, n) | 8.5 (4.8, 285) |
| Magnification (12 = worst outcome, 0 = best outcome), mean (SD, n) | 5.4 (3.4, 284) |
| Helplessness (24 = worst outcome, 0 = best outcome), mean (SD, n) | 11.6 (6.4, 284) |
| EQ-5D-3L | |
| Summary score (–0.59 = worst outcome, 1.0 = best outcome) (SD, n) | 0.6 (0.3, 286) |
| Health state (0 = worst outcome, 100 = best outcome) (SD, n) | 63.4 (19.4, 270) |
| ICECAP | |
| Capabilities, as a measure of well-being (0 = worst outcome, 1.0 = best outcome) (SD, n) | 0.3 (0.2, 198) |
Appendix 2 Additional results from the diagnostic study
| Reference standard | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Laparoscopy assessment of absence of any gynaecological cause | EIP A assessment of absence of any gynaecological cause | EIP B assessment of absence of any gynaecological cause | |||||||
| Features of absence of any gynaecological cause in the MRI report | Yes | No | Yes | No | Yes | No | |||
| Yes | 16 | 33 | Yes | 26 | 23 | Yes | 27 | 22 | |
| No | 59 | 179 | No | 130 | 108 | No | 126 | 112 | |
| p-value (Fisher’s exact test) | p = 0.3 | p = 0.9 | p = 0.9 | ||||||
| Accuracy estimates (95% CI) |
Sens = 21.3 (12.7 to 32.3) Spec = 84.4 (78.8 to 89.0) LR+ = 1.4 (0.8 to 2.3) LR– = 0.9 (0.8 to 1.1) DOR = 1.5 (0.8 to 2.9) |
Sens = 16.7 (11.2 to 23.5) Spec = 82.4 (74.8 to 88.5) LR+ = 0.9 (0.6 to 1.6) LR– = 1.0 (0.9 to 1.1) DOR = 0.9 (0.5 to 1.7) |
Sens = 17.7 (12.0 to 24.6) Spec = 83.6 (76.2 to 89.4) LR+ = 1.1 (0.6 to 1.8) LR– = 1.0 (0.9 to 1.1) DOR = 1.1 (0.6 to 2.0) |
||||||
| Reference standard | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Laparoscopy assessment of absence of any gynaecological cause | EIP A assessment of absence of any gynaecological cause | EIP B assessment of absence of any gynaecological cause | |||||||
| Features of absence of any gynaecological cause in the MRI report | Yes | No | Yes | No | Yes | No | |||
| Yes | 55 | 93 | Yes | 88 | 60 | Yes | 88 | 60 | |
| No | 20 | 119 | No | 68 | 71 | No | 65 | 74 | |
| p-value (Fisher’s exact test) | p < 0.0001 | p = 0.08 | p = 0.03 | ||||||
| Accuracy estimates (95% CI) |
Sens = 73.3 (61.9 to 82.9) Spec = 56.1 (49.2 to 62.9) LR+ = 4.7 (3.3 to 6.6) LR– = 0.3 (0.2 to 0.5) DOR = 14.9 (8.4 to 26.6) |
Sens = 56.4 (48.3 to 64.3) Spec = 54.2 (45.3 to 62.9) LR+ = 1.2 (0.9 to 1.5) LR– = 0.8 (0.6 to 1.0) DOR = 1.5 (0.9 to 2.4) |
Sens = 57.5 (49.3 to 65.5) Spec = 55.2 (46.4 to 63.8) LR+ = 0.4 (0.3 to 0.6) LR– = 1.5 (1.3 to 1.8) DOR = 0.3 (0.2 to 0.4) |
||||||
| Confidence score | Pre-index data diagnosis | Pre-index data and MRI report diagnosis | ||||
|---|---|---|---|---|---|---|
| EIP A judgement: absence of any gynaecological cause of pain, n (%) | Likelihood ratio (95% CI) | EIP A judgement: absence of any gynaecological cause of pain, n (%) | Likelihood ratio (95% CI) | |||
| Yes | No | Yes | No | |||
| 7–10 | 53 (34.0) | 43 (32.8) | 1.0 (0.7 to 1.4) | 77 (49.4) | 44 (33.6) | 1.5 (1.1 to 2.0) |
| 4–6 | 86 (55.1) | 69 (52.7) | 1.0 (0.8 to 1.3) | 49 (31.4) | 47 (35.9) | 0.9 (0.6 to 1.2) |
| 0–3 | 17 (10.9) | 19 (14.5) | 0.8 (0.4 to 1.4) | 30 (19.2) | 40 (30.5) | 0.6 (0.4 to 1.0) |
| Total | 156 | 131 | p* = 0.5 | 156 | 131 | p* = 0.004 |
| Confidence score | Pre-index data diagnosis | Pre-index data and laparoscopy report | ||||
|---|---|---|---|---|---|---|
| EIP A judgement: absence of any gynaecological cause of pain, n (%) | Likelihood ratio (95% CI) | EIP A judgement: absence of any gynaecological cause of pain, n (%) | Likelihood ratio (95% CI) | |||
| Yes | No | Yes | No | |||
| 7–10 | 62 (40.0) | 37 (28.2) | 1.5 (1.1 to 2.1) | 81 (53.6) | 4 (3.1) | 17.6 (6.6 to 46.6) |
| 4–6 | 60 (38.7) | 34 (26.0) | 1.5 (1.1 to 2.1) | 26 (17.2) | 18 (13.7) | 1.3 (0.7 to 2.2) |
| 0–3 | 33 (21.3) | 60 (45.8) | 0.5 (0.3 to 0.7) | 44 (29.1) | 109 (83.2) | 0.4 (0.3 to 0.5) |
| Total | 155 | 131 | p* = 0.0002 | 151 | 131 | p* < 0.0001 |
Appendix 3 Economic evaluation sensitivity analyses
The distributions used in the PSA for the economic model are shown in Table 36.
| Parameter | Distribution | Alpha | Beta |
|---|---|---|---|
| % of eligible women who receive a laparoscopy when offered | Beta | 287 | 20 |
| % of eligible women who receive a MRI when offered | Beta | 287 | 35 |
| % of women who have a therapeutic laparoscopy during the same procedure as a diagnostic laparoscopy | Beta | 92 | 20 |
| % of women who receive a second therapeutic laparoscopy during a separate procedure following their first therapeutic laparoscopy | Beta | 30 | 82 |
| Prevalence (negative, positive for therapeutic laparoscopy, other treatment) | Dirichlet (164,112,11) | ||
| Proportion of women who require other treatment who have fibroids (remainder of women have adenomyosis) | Beta | 3 | 8 |
| Time for consultant and nurse to conduct baseline examination | Gamma | 1 | 0.33 |
List of abbreviations
- AUROC
- area under the receiver operating characteristic
- CI
- confidence interval
- CPP
- chronic pelvic pain
- EAU
- European Association of Urology
- EIP
- expert independent panel
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FS
- fast spin
- GP
- general practitioner
- ICECAP
- Investigating Choice Experiments CAPability measure
- ICER
- incremental cost-effectiveness ratio
- IRRC
- Independent Radiology Review Committee
- ISSP
- International Society for the Study of Pain
- LR
- likelihood ratio
- MEDAL
- Magnetic resonance imaging for Establishing Diagnosis Against Laparoscopy
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- PID
- pelvic inflammatory disease
- PPSN
- Pelvic Pain Support Network
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCOG
- Royal College of Obstetricians and Gynaecologists
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SSC
- Study Steering Committee
