Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/110/01. The contractual start date was in March 2012. The draft report began editorial review in April 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All contributors participated in this trial, to which GlaxoSmithKline plc donated free patches. Paul Aveyard is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and CLAHRC. Peter Hajek and Hayden J McRobbie have provided research and consultancy to several manufacturers of smoking cessation treatments and have been paid for doing so. Tim Coleman is a member of the NIHR Health Technology Assessment Clinical Evaluation and Trials Board. Sarah Lewis is a member of the NIHR Health Services and Delivery Research funding board. Peter Hajek reports a research grant to Queen Mary University of London from Pfizer and personal consultancy fees from Pfizer. Andy McEwen reports grants from Pfizer and hospitality from North 51. Hayden J McRobbie reports a grant and honorarium from Pfizer for speaking at education meetings and an honorarium from Johnson & Johnson for speaking at education meetings and an advisory board meeting.
Disclaimer
Authors are listed in alphabetical order by centre. The list does not reflect relative contribution.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Aveyard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Smoking cessation and cessation medication
Most people who try to stop smoking fail within the first few weeks of the attempt. 1 This is because they experience urges to smoke that drive them to return to smoking. The aim of smoking cessation treatment, which typically starts just before or on the quit day, is to reduce the intensity of urges so that more people can overcome the difficult first few weeks. 2,3 Over time, the urges to smoke reduce in frequency and intensity and treatment can stop. 4
There are three medications for smoking cessation that are licensed widely around the world: (1) nicotine replacement therapy (NRT), (2) bupropion and (3) varenicline (Champix®; Pfizer Inc., New York, NY, USA). All three have strong evidence of effectiveness and are safe, although bupropion has rather more contraindications to its use and results in more severe adverse effects than the other two medications. 5 In the UK, bupropion fell out of favour because of unjustified concerns about its safety and is now rarely used. Thus, when people who smoke use medication to assist them, they typically use either varenicline or NRT.
Conventionally, people stopping smoking commence NRT on quit day. However, people stopping smoking with the aid of either bupropion or varenicline begin to take the medication about 1 week prior to quit day. This allows the dose to be escalated in order to reduce the number of adverse events (AEs) that can occur with rapid escalation of the dose of either medication. Thus, the original aim of this period serves simply to ensure that people are comfortable and are taking the full dose of the medication by the time of quit day.
The most effective medication for supporting smoking cessation is varenicline. 6 One trial7 examined the mechanisms of action of smoking cessation medications, comparing varenicline, bupropion and placebo. Varenicline reduced the intensity of urges and reduced the satisfaction obtained from a lapse (temporary smoking episode during a quit attempt) to a greater extent than bupropion. Most other mood symptoms occurred with a similar intensity. This suggests that reducing urge intensity and reducing satisfaction with smoking are key mechanisms of action. Varenicline is usually started 1 week before quit day and it could be that its use prior to quit day undermines the rewarding value of cigarettes and underlies its greater effectiveness. This raises the possibility that NRT, which is usually started on the quit day, may be effective if used in this way, as it too reduces satisfaction from smoking when used concurrently with cigarettes. 8 Using nicotine in this way is termed ‘nicotine preloading’. The reason that this may help achieve smoking cessation is because nicotine from NRT desensitises the nicotinic receptors and blocks the effects of further nicotine from cigarettes. This, in turn, undermines the learnt association between smoking and brain reward, making extinction of the reinforced behaviour more likely. 9
Nicotine preloading effectiveness
Two previous reviews10,11 have examined the effectiveness of nicotine preloading and provided evidence that it approximately doubles the chance of achieving abstinence. However, our own review12 showed no strong evidence that preloading was effective, principally because some larger trials showing no benefit had been published since the earlier reviews were conducted. The relative risk (RR) for short-term abstinence was 1.05 [95% confidence interval (CI) 0.92 to 1.19; p = 0.49], with a high level of heterogeneity (I2 = 69%; p = 0.002). The RR for long-term abstinence was 1.16 (95% CI 0.97 to 1.38), with a lower level of heterogeneity (I2 = 36%; p = 0.14). The principal aim of this review was to pool all forms of NRT, although we hypothesised that nicotine patches may be more effective in the context of preloading. There is a plausible reason why patches may be more effective for preloading than other forms of NRT. Smoking while using a short-acting NRT tends not to raise the concentration of nicotine in the blood, whereas smoking and using a patch does. 8 It may be this higher concentration of nicotine that undermines the reward from cigarettes. Indeed, there was some evidence of this, with patches having a RR of 1.26 (95% CI 1.03 to 1.55) for longer-term abstinence.
Mechanisms of action of preloading
We can understand the potential mechanism of preloading by understanding the development of tobacco addiction (Figure 1).
FIGURE 1.
Development of tobacco addiction.
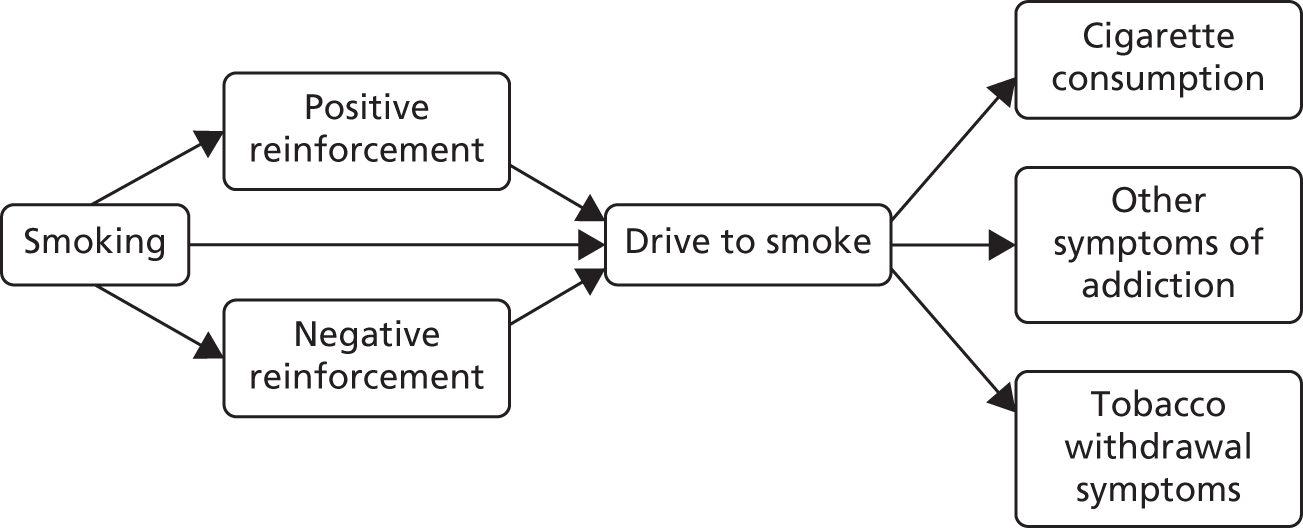
Tobacco smoking creates a positive reward, such as feelings of pleasure, which leads to the reinforcement of subsequent smoking. After a period of regular smoking, neuroadaptation to regular doses of nicotine means that people who smoke experience negative moods when nicotine concentrations in the brain drop; these feelings are relieved by smoking and, thus, smoking is negatively reinforced. Independently of any reward that people may experience, mechanisms in the nucleus accumbens set up associative learning that results in the drive to smoke. These mechanisms create an acquired drive to smoke and tobacco dependence, manifested by the regular consumption of cigarettes, the occurrence of tobacco withdrawal symptoms when unable to smoke and difficulty in stopping smoking if a person were to choose to do so.
Preloading may reduce the drive to smoke and both positive and negative reinforcement. By keeping the concentration of nicotine in the blood high, the nicotine patch can mitigate the need for nicotine and also reduce the impact of further exogenous nicotine from cigarettes, thus reducing the positive reinforcement of smoking. Likewise, if the need for nicotine is low, this may lower any impact of smoking on reducing withdrawal discomfort (negative reinforcement). All three actions, especially the reduced drive to smoke, would reduce smoke intake during the pre-quit period. The net effect of the changes in the drive to smoke mean that a person will not smoke when he or she normally would, usually when cued by the environment to do so. Not smoking when cued to smoke will begin to extinguish the learnt association between the action of smoking a cigarette and reinforcement in the brain. Alternatively, smoking when cued to smoke but receiving no reinforcement from the nicotine may also undermine the drive to smoke by weakening the association. Consequently, after cessation, urges to smoke should be lower and this makes cessation more likely to be successful. 13
Aside from this addiction-based mechanism, three other mediational hypotheses have been advanced for the mechanism of action of preloading. The first relates to medication adherence, which some evidence suggests is poor in smoking cessation. 14 The hypothesis here is that preloading enables the person to become used to using medication at a time when failure to use it does not undermine the success of the quit attempt. After quit day, when failure to use medication does undermine abstinence,15 adherence will be superior in people using preloading than in people who did not use preloading.
The second hypothesis is that the reduced level of smoking that occurs when people preload increases a person’s confidence that they can abstain from smoking after quit day; the confidence that one can achieve abstinence is associated with achieving abstinence. 16
A third hypothesis was developed from a recent trial17 in which participants were interviewed about their experiences using preloading. A large proportion reported feeling nauseous and having an aversion to cigarettes during preloading. Aversive smoking is a process in which people smoke excessively to the point of nausea and vomiting. Although it is rarely used as a treatment to enhance cessation, it is effective at increasing the rate of cessation. 18 It is, therefore, plausible that nausea and aversion to smoking mediate the effect of preloading, and we tested this in the Preloading trial.
In our review,12 we examined the putative mechanisms of action of preloading to find evidence to support or refute these possibilities. We examined the effects on both positive and negative reward, urge intensity and markers of consumption and addiction. There was scanty and mixed evidence on whether or not preloading reduced the positive reward from smoking. There was more evidence that preloading did not reduce the negative rewarding effects of smoking, that is, preloading did not appear to remove the ability of smoking to relieve tobacco withdrawal symptoms. Overall, four studies examined the effect on pre-quit urge intensity and two showed evidence of a modest reduction. There was clear evidence that preloading reduced cigarette consumption without instruction to do so. The mechanism of reduced intensity of urges suggests that we might expect reduced tobacco withdrawal symptoms after quit day, but there was reasonable evidence that this does not occur.
Cost-effectiveness
Interventions are approved by the National Institute for Health and Care Excellence (NICE) for use in the NHS only if they are cost-effective, defined as achieving a cost per quality-adjusted life-year (QALY) of < £20,000. Interventions for smoking cessation are typically highly cost-effective. This is because smoking is particularly harmful to health and cessation is remarkably effective at ameliorating the effects of many years of smoking. 19 In fact, West20 calculates that increments of as little as 1% in 6-month prolonged abstinence rates are highly likely to be cost-effective according to the NICE standard.
Arguably, the most reasonable way to plan a trial is to define a limit at which the intervention is cost-effective enough to change the decision on an intervention from non-use to use. In this case, the question would be: what increment in 6-month prolonged abstinence would produce a cost per QALY of < £20,000? The answer, argued by West,20 is around a 1% increment. The problem with using this to calculate a trial sample size is that it is impractically small. Any trial that would detect an effect of this size would be one or two orders of magnitude larger than most ‘large’ smoking cessation trials today. Such a trial would never be funded and would not be able to recruit to target. Therefore, cost-effectiveness, although providing a logical rationale for calculating a sample size, is not useful in this regard.
One compelling rationale for the cost-effectiveness analysis in this trial is the political realities of the health economy today. The NHS is under severe financial pressure. Preloading will inevitably cost more than the comparator, usual care, which, at this point in a quit attempt, provides no intervention. In the current NHS, additional investment in a treatment such as smoking cessation therapy is likely to be made only if the intervention is cost saving. As there are no data on the cost-effectiveness from previous trials of preloading, we set out to assess the cost-effectiveness of preloading in this trial.
Aims of the trial
It is clear, therefore, that an adequately powered trial of nicotine preloading is required to address the uncertainty about the effectiveness and cost-effectiveness of treatment and could usefully explore potential mechanisms of action, which could be practically as well as theoretically useful. A trial of varenicline preloading showed that people who spontaneously suppressed their smoking in response to varenicline were much more likely to achieve abstinence than those who did not. 21 Other trials have reported similar associations with NRT. 21–24 In clinical use, therefore, one could suggest stopping preloading in those who do not suppress their smoking and bringing forward the quit day. However, subsequent planned replication found evidence that the effect was much less strongly predictive of the ability to quit smoking during nicotine preloading. 25 We, therefore, planned to examine the effectiveness of preloading in a trial powered to detect modest effects on long-term abstinence and investigate potential mediators that could allow therapists to judge whether to continue or abandon preloading. The objectives were to:
-
examine the relative efficacy of a nicotine patch worn for 4 weeks prior to quitting plus standard NHS care post quit compared with standard NHS care only in smokers undergoing NHS treatment for tobacco dependence (addressed in Chapter 3)
-
examine the adverse effects and safety of nicotine preloading (addressed in Chapter 3)
-
examine the incremental cost-effectiveness of nicotine preloading (addressed in Chapters 6 and 7)
-
examine possible mediating pathways between nicotine preloading and outcomes (addressed in Chapter 4)
-
examine moderators of the effects of preloading, including the use of varenicline and baseline levels of dependence (addressed in this chapter and in Chapter 4)
-
investigate opinions of the preloading intervention (addressed in Chapter 5)
-
assess adherence to preloading treatment and subsequent standard smoking cessation pharmacotherapy (addressed in Chapter 3).
Chapter 2 Methods
Design
This was an open-label, multicentre, pragmatic superiority trial in which participants were randomised 1 : 1 to non-use or use of a nicotine patch for 4 weeks prior to quit day. Participants used standard pharmacotherapy and behavioural support for stopping smoking thereafter. The primary outcome was prolonged biochemically validated abstinence, measured 6 months after quitting. The protocol was published26 and implemented with one change, which related to participants who had moved from the area during follow-up and were not available to attend in person for carbon monoxide (CO) measurement to confirm abstinence. We, therefore, asked such participants to provide a saliva sample and measured cotinine or anabasine concentrations to confirm abstinence from smoking. Anabasine is a tobacco-specific alkaloid that will indicate smoking abstinence in a person who is using NRT or e-cigarettes. Cotinine is a metabolite of nicotine with a much longer half-life, making it a good indicator of nicotine consumption within the last week, and will be in low concentrations in people who do not smoke or use nicotine.
In the original proposal to the National Institute for Health Research (NIHR), we did not suggest collecting genetic samples or assessing the impact of quitting on weight gain. However, these additional non-trial-related hypotheses were added to the published protocol. 26 In the event, it turned out to be impractical to collect genetic samples and this ambition was abandoned. The observational analyses of the impact of quitting and failed quit attempts on weight gain will be reported separately.
Participants and settings
In three recruitment centres, Birmingham, Bristol and Nottingham, general practitioners (GPs) spoke to, wrote to, e-mailed or texted patients listed as smokers on the electronic health record. GPs encouraged their patients who wished to quit smoking with the help of pharmacotherapy and behavioural support to enrol in the trial. The final centre, London, was an existing NHS smoking cessation clinic, which asked participants seeking treatment if they would also like to take part in the trial. Thus, in all centres, patients were recruited from those who sought treatment at their own instigation, by referral from their GP or by advertising in various online and print media.
Potential participants telephoned the research team to learn more about the trial and were screened for eligibility and entered into our online trial database. If a potential participant appeared to be eligible and wanted to participate, we made an appointment and sent out the participant information sheet. At this initial appointment, we again described the trial and obtained witnessed consent with a signature and confirmed eligibility. The researcher, typically a research nurse, saw participants at their own general practice or at the London centre. We included people who were:
-
regular smokers of cigarettes, cigars and/or roll-up tobacco cigarettes, with or without marijuana, aged ≥ 18 years
-
suitable for preloading in the judgement of the researcher (see below)
-
seeking support to stop smoking from the NHS Stop Smoking Service
-
willing to set a quit day in 4 weeks
-
able to understand and willing to adhere to study procedures.
We excluded:
-
pregnant or breastfeeding women
-
people with extensive dermatitis or another skin disorder that precluded patch use
-
people who had acute coronary syndrome or who had had a stroke in the past 3 weeks
-
people with an active phaeochromocytoma or uncontrolled hyperthyroidism that would increase the risk of arrhythmias from the nicotine patch.
We judged if people would be suitable for preloading by assessing whether or not they were addicted to smoking. This was based on a clinical judgement of the following factors, with no specific cut-off points used:
-
time to first cigarette in the morning, with earlier use reflecting a higher level of addiction
-
number of cigarettes smoked per day, with a greater number reflecting a higher level of addiction
-
higher levels of exhaled CO, which reflect a higher level of addiction
-
failure of previous quit attempts despite use of appropriate pharmacotherapy.
Interventions
Intervention group
In the intervention group, participants wore a 21-mg nicotine patch daily for an intended 4 weeks prior to quit day. The intention was that this would be worn for 24 hours per day. However, any participants who had experienced previous AEs at night from 24-hour patch use were advised to wear the patch during waking hours only.
We asked participants to smoke as normal and not reduce nicotine consumption. Reducing consumption would probably lower the nicotine concentration in the blood, which could have resulted in cigarettes being more rewarding and, thus, have undermined the supposed benefits of preloading. 27
We referred patients to the NHS Stop Smoking Service to set a quit day, obtain behavioural support and receive a prescription of pharmacotherapy to support cessation. Although we aimed for this quit day to be 4 weeks from the start of preloading, in keeping with the pragmatic design, we asked participants and NHS clinics to set this date within a window from 3 to 5 weeks after starting preloading. Preloading was allowed to continue for up to 8 weeks in exceptional circumstances. Preloading was also allowed to restart once, if necessary, for example if this was interrupted because a participant was admitted to hospital. In such cases, participants aimed to complete 3–5 weeks of preloading from the date of recommencement. We dispensed NRT in two lots, one of 2 weeks and a second, 1 week later, of 3 weeks. We made arrangements to dispense additional lots of NRT if preloading was extended.
The researchers explained the rationale of preloading and prompted action planning to maximise adherence to the patches, that is, they talked through participants’ daily routine and assisted them to use it and environmental cues to minimise the chance of forgetting to use the patches. They provided reassurance about concerns that participants may have about side effects and how to manage them. A booklet containing this information was supplied to participants. We aimed to see participants 1 week after commencing preloading. At this visit, we asked participants about their experiences and their understanding of the necessity of preloading and again addressed these beliefs as well as concerns about preloading.
We offered participants lower-strength patches from the beginning if they reported experiencing adverse reactions to the 21-mg patch or if, during the treatment course, they experienced symptoms of nicotine overdose such as nausea, salivation and/or pounding heartbeat or other symptoms that they believed were due to the patch strength. We stopped preloading if a participant requested it, if it was not possible to alleviate AEs by reducing the dose or if an intervening health state or contraindication to preloading emerged.
Control group
The research team were keen to offer participants in the control group a placebo, but the NIHR Health Technology Assessment (HTA) Board would not allow this. We were concerned that offering no treatment would lead to disengagement and, to counteract this, we developed a behavioural intervention that could seem plausible to participants but had no evidence that it would increase abstinence. We asked participants to consider their smoking pattern and the triggers for particular cigarettes (e.g. the first cigarette of the day, the first after dinner) and to plan ways to reduce these cues. Such a process is standard in smoking cessation support, so participants in the intervention group were likely to engage in this after preloading and in preparation for quitting. The control group received a booklet outlining this process, which was designed to be comparable to the booklet supplied to the intervention group. As in the intervention group, participants in the control group were referred to the NHS Stop Smoking Service to commence a quit attempt between 3 and 5 weeks after enrolment.
Standard smoking cessation treatment common to both arms
In both arms, at the first and second contacts, we facilitated contact between participants and the local NHS Stop Smoking Service and wrote a referral letter to encourage the NHS Stop Smoking Service to work with us on encouraging participants to continue preloading. In particular, we asked the NHS Stop Smoking Service advisors to ignore the presence or absence of preloading when discussing and recommending the use of pharmacotherapy to support the quit attempt. We especially asked advisors to feel free to use varenicline, which starts prior to quit day and, hence, would be used concurrently with nicotine preloading. This is because we were keen that the treatment provided by the NHS Stop Smoking Services did not differ between trial arms. NICE guidance has specifically recommended against this combination of NRT and varenicline,28 which advisors wrongly assumed was because of safety concerns about concurrent use. We therefore addressed this in the referral letter, by telephone and in face-to-face discussions with the NHS Stop Smoking Service.
The NHS trains Stop Smoking Service advisors to give weekly behavioural support starting 1–2 weeks prior to quit day and continuing until at least 4 weeks after quit day. This support addresses issues such as planning for the quit day, the ‘not a puff’ rule and how to deal with difficult situations, such as others smoking around the person who has quit. It also provides monitoring of behaviour and validation of abstinence through CO testing. The support is largely withdrawal-orientated therapy. 29
Our protocol26 allowed all other medication to be used concurrently with preloading.
Outcomes
We followed up participants on five occasions for outcome assessment. These were named in relation to quit day and are shown in Table 1.
| Visit name | Timing in relation to baseline | Main purpose |
|---|---|---|
| –3 weeks | 1 week after baseline |
AE assessment Data on mediators Data on adherence |
| +1 week | ≈5 weeks after baseline |
AE assessment Data on mediators Data on adherence |
| +4 weeks | ≈9 weeks after baseline | Data from NHS Stop Smoking Service on abstinence |
| +6 months | ≈7 months after baseline | Abstinence |
| +12 months | ≈13 months after baseline | Abstinence |
The first follow-up appointment was a face-to-face appointment, when possible, and occurred 1 week after baseline and 3 weeks before quit day. One week after quit day we telephoned participants to collect data on AEs, adherence to preloading and use of, and adherence to, other smoking cessation pharmacotherapy. We obtained data on smoking cessation from the NHS Stop Smoking Service at 4 weeks or, failing this, from a telephone call to the participant. At 6 and 12 months, we telephoned participants to obtain data on smoking status and health service use. We invited participants who claimed to be abstinent for at least 1 week to a meeting where we asked them to provide an exhaled CO concentration reading. Participants were compensated £15 for their time for attending this meeting. Therefore, if they attended a meeting at both 6 and 12 months’ follow-up, participants were compensated a total of £30.
Primary outcome
The primary outcome was 6-month prolonged abstinence, defined according to the Russell Standard criteria. 30 This allows a grace period of 2 weeks following quit day when lapses do not count against abstinence. Thereafter, we counted a person as abstinent if they smoked fewer than five cigarettes or cigars up to the 6-month assessment and were biochemically confirmed abstinent by an exhaled CO concentration of < 10 parts per million (p.p.m.).
Secondary outcomes
The secondary outcomes were 12-month Russell Standard abstinence and biochemically confirmed 7-day point prevalence abstinence at 4 weeks, 6 months and 12 months.
Adverse events
We defined AEs as those that occurred after baseline and until 1 week after quit day, thereby covering the period of preloading and an additional week for AEs to emerge. This excluded planned events, such as scheduled surgery. In the protocol,26 we deemed that we would report only AEs of moderate severity or above, that is, those events that hindered or prevented the person going about their normal activities. This is because these events matter more to patients and because NRT has been used for many years and so many common minor AEs are known already.
We assessed the AEs arising from preloading in two ways. First, we elicited AEs from participants in both arms at the contacts at –3 weeks (1 week after baseline) and +1 week (5 weeks after baseline and 1 week after quit day). Spontaneously occurring AEs were coded at the level of preferred term and system organ class using the MedDRA (Medical Dictionary for Regulatory Activities) coding system. 31 However, spontaneously occurring reports may introduce bias. It seemed illogical to participants in the control group and to treating staff to discuss AEs when the participant was taking no medication at all. Therefore, second, at 1 week after baseline we also gave all participants a questionnaire concerning symptoms of nicotine overdose in the previous 24 hours (such as nausea and excessive salivation).
We defined serious adverse events (SAEs) as those leading to hospitalisation, death, permanent disability or congenital abnormality and reported these separately. We obtained the medical history of participants experiencing SAEs and these were classified by an independent assessment panel blinded to allocation as unrelated or unlikely to be, possibly, probably or definitely related to the use of nicotine patches.
Sample size
We determined the sample size from plausible estimates of 6-month abstinence. We estimated that 15% of participants in the control group would attain 6-month abstinence based on data from similar trials. 17,32,33 We felt that a RR of 1.4 was plausible based on our meta-analysis12 and would make preloading valuable to NHS Stop Smoking Service commissioners. 12 This gave us a sample size of 893 per group, or 1786 in total, to achieve 90% power (Table 2).
| Prolonged abstinence in control group, % | Prolonged abstinence in intervention group, % | Trial with 80% power, number per group | Trial with 90% power, number per group |
|---|---|---|---|
| RR = 1.3 | |||
| 14 | 18.2 | 1249 | 1655 |
| 15 | 19.5 | 1150 | 1524 |
| 16 | 20.8 | 1064 | 1409 |
| 20 | 26.0 | 805 | 1065 |
| RR = 1.4 | |||
| 14 | 19.6 | 734 | 970 |
| 15 | 21.0 | 676 | 893 |
| 16 | 22.4 | 625 | 825 |
| 20 | 28.0 | 471 | 622 |
| RR = 1.5 | |||
| 14 | 21.0 | 490 | 646 |
| 15 | 22.5 | 451 | 594 |
| 16 | 24.0 | 416 | 549 |
| 20 | 30.0 | 313 | 412 |
Randomisation
The research team randomised participants at the baseline visit following consent, assessment of eligibility and enrolment. An independent statistician used Stata® 14.2 (StataCorp LP, College Station, TX, USA) to generate a randomisation list, stratified by treatment centre and using randomly permuted blocks of varying sizes, using a 1 : 1 ratio. This sequence was incorporated into an online database and the sequence remained concealed from all research staff.
Blinding
This was an open-label trial so participants, research staff and NHS Stop Smoking Service personnel knew to which group participants were assigned. Blinded follow-up was impossible as all staff had been involved in recruitment.
Statistical analysis
The analysis of all cessation outcomes was performed using the intention-to-treat (ITT) principle as outlined in the Russell Standard. 30 Thus, everyone was included in the denominator and presumed to be smoking if this information remained unknown. This presumption was shown to be true in one trial that went to extraordinary lengths to establish the true status of those who did not respond to standard follow-up. 30 For the primary analysis, we calculated adjusted odds ratios (ORs) using multivariable logistic regression in Stata 14.2. We also calculated the percentage achieving abstinence, the risk difference (RD), RRs and 95% CIs using the post-estimation adjrr procedure in Stata 14.2. As the randomisation was stratified by centre, we adjusted for this covariate in the analysis and this was considered the primary analysis. In sensitivity analyses, we adjusted for two well-known predictors of abstinence to improve precision: the longest previous abstinence and the degree of addiction measured by the strength of urges to smoke at baseline. 34–36 Finally, because we envisaged that the use of NRT would deter NHS Stop Smoking advisors from prescribing varenicline, which is more effective than other pharmacotherapy,6 we also adjusted for varenicline use.
We had pre-planned subgroup analyses that were assessed by including multiplicative interaction terms in the calculations described in the previous paragraph. The presumed mechanism of action of preloading suggests that preloading could be more effective in people who are more addicted to nicotine. This was assessed by investigating whether or not outcomes varied with baseline Fagerström Test for Cigarette Dependence (FTCD) score or exhaled CO concentration readings and is reported in Chapter 3. In another analysis, we examined whether or not the effect of preloading was less pronounced in people who used varenicline in the pre-quit period. This is because varenicline includes a period of use of at least 1 week prior to quit day and this appears to have similar effects to preloading. 21
The population in whom we analysed the occurrence of AEs was all those who provided data on such events. The analysis of spontaneously elicited AEs was confined to events of moderate severity and above. We anticipated that most minor AEs would relate to well-established adverse reactions to nicotine patches; confining the analysis to AEs of moderate severity and above may identify novel adverse reactions to preloading specifically that were of more concern to patients. The analysis used analogous statistical models to those applied for the primary and secondary outcomes.
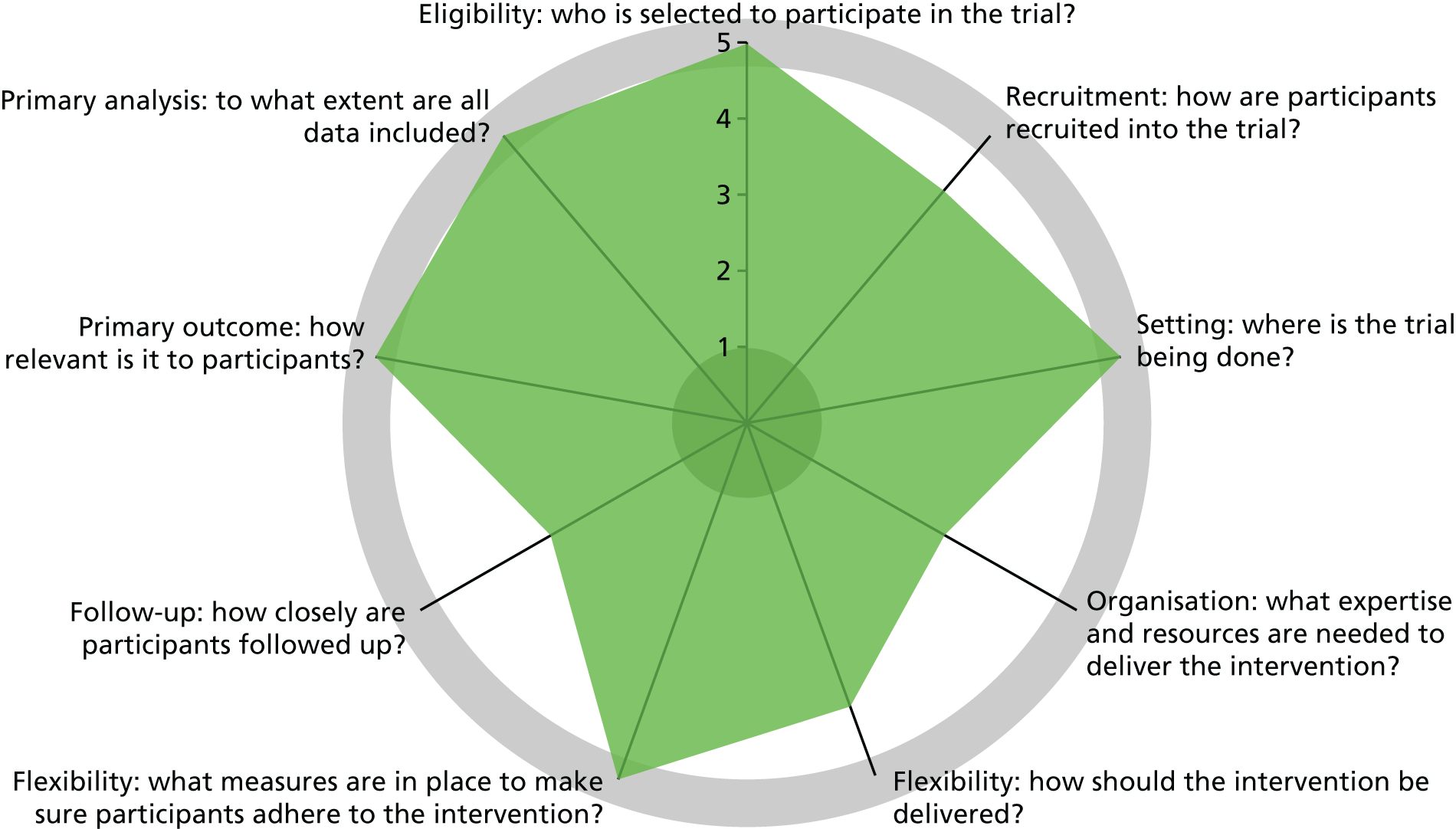
Design features classified by PRECIS-2
Trials vary on an explanatory to pragmatic continuum; this can be assessed and represented by a tool called PRECIS-2 (PRagmatic Explanatory Continuum Indicator Summary). 37 The aim of this trial was to examine the effectiveness of preloading in everyday clinical practice. In the UK, this was in the context of the NHS Stop Smoking Service. However, the aim was also to examine AEs and safety, which meant that these were assessed by researchers trained to assess these outcomes appropriately. Furthermore, NHS Stop Smoking Services do not routinely follow up people who try to stop smoking beyond 4 weeks, which meant that we instituted special follow-up measures to assess smoking abstinence. It was because of these aims that the trial design scored less than the maximum for pragmatic design features on the PRECIS-2 wheel (Figure 2).
Chapter 3 Results of the trial
Participant flow
Between 13 August 2012 and 10 March 2015, 3837 people telephoned about enrolment. Of these, 1805 (47.0%) attended the initial appointment and 1792 (99.3%) of those were eligible and were enrolled in the study. Overall, 490 (12.8%) people who had telephoned were ineligible; the most common reasons for ineligibility were skin problems and an objection to using preloading patches (Figure 3).
FIGURE 3.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. MI, myocardial infarction. Adapted from the Preloading Investigators. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See http://creativecommons.org/licenses/by/4.0/.
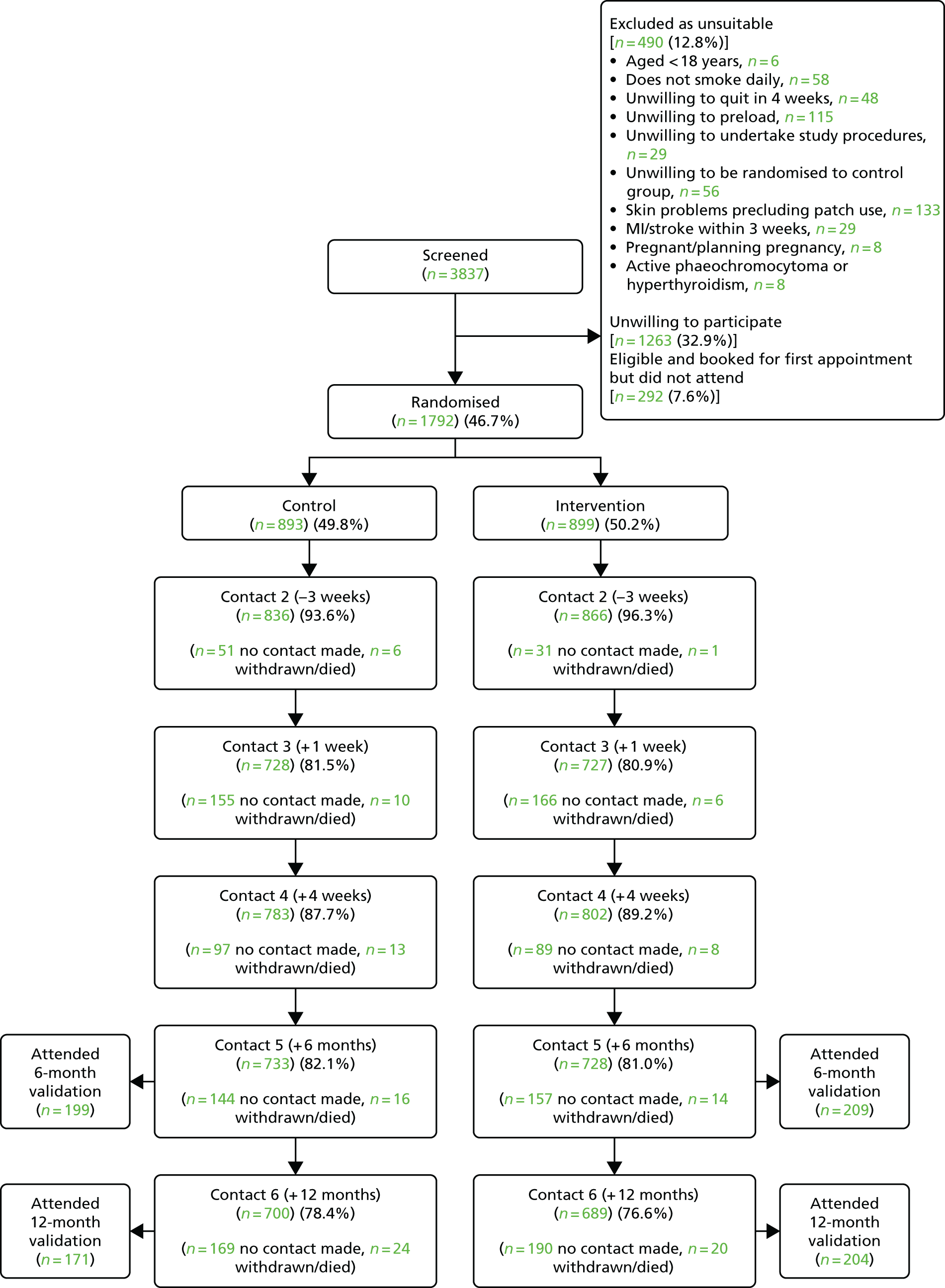
One week after baseline, we followed up 1702 (95.0%) participants; and 5 weeks after baseline, 1 week after quit day, we obtained data from 1456 (81.3%) participants. These assessments provided data on AEs.
In total, 312 (17.4%) participants never made a quit attempt, 157 (17.5%) in the intervention group and 155 (17.4%) in the control group. We obtained data on 1585 (88.5%) participants at 4 weeks, 1461 (81.5%) at 6 months and 1389 (77.5%) at 12 months after quit day. The proportion successfully followed was similar in each group. Although 331 (18.5%) participants did not provide data at 6 months, we knew that 97 of these participants were smoking at 4 weeks post quit day and could not, therefore, be classified as abstinent at 6 months and that 54 of these participants never made a quit attempt and likewise could not be classified as abstinent at 6 months. Thus, altogether, we were certain of the primary outcome in 1612 (90.0%) participants.
Baseline characteristics
The baseline characteristics were well balanced between the trial arms (Table 3). The mean age of participants was 48.9 [standard deviation (SD) 13.4] years. Men constituted 52.6% of the participants and 75.6% identified as white British. The proportion of participants with an advanced level of education was lower than the UK average. 40 Fifty-two per cent were in employment. Participants smoked a mean of 18.9 (SD 9.3) cigarettes per day at baseline, had a mean nicotine dependence score of 5.2 (SD 2.2), indicating moderate addiction, and a mean exhaled CO concentration of 23.7 (SD 12.5) p.p.m. One-third (32.5%) had used behavioural support or pharmacotherapy to try to quit in the previous 6 months.
| Characteristic | Trial group | ||
|---|---|---|---|
| Control (N = 893) | Intervention (N = 899) | Total (N = 1792) | |
| Age (years), mean (SD) | 48.8 (13.4) | 49.1 (13.3) | 48.9 (13.4) |
| Gender, n (%) | |||
| Male | 469 (52.6) | 473 (52.6) | 942 (52.6) |
| Female | 422 (47.3) | 426 (47.4) | 848 (47.4) |
| Ethnicity, n (%) | |||
| White: British | 675 (75.6) | 680 (75.6) | 1355 (75.6) |
| White: Irish | 36 (4.0) | 25 (2.8) | 61 (3.4) |
| White: other | 57 (6.4) | 55 (6.1) | 112 (6.3) |
| White and black Caribbean | 17(1.9) | 15 (1.7) | 32 (1.8) |
| White and black African | 3 (0.3) | 5 (0.6) | 8 (0.5) |
| White and Asian | 8 (0.9) | 6 (0.7) | 14 (0.8) |
| Mixed other | 7 (0.8) | 8 (0.9) | 15 (1.8) |
| Indian | 11 (1.2) | 10 (1.1) | 21 (1.2) |
| Pakistani | 9 (1.0) | 6 (0.7) | 15 (0.8) |
| Bangladeshi | 2 (0.2) | 13 (1.5) | 15 (0.8) |
| Asian: other | 3 (0.3) | 3 (0.3) | 6 (0.3) |
| Black: Caribbean | 29 (3.3) | 34 (3.8) | 63 (3.5) |
| Black: African | 8 (0.9) | 13 (1.5) | 21 (1.2) |
| Black: other | 4 (0.5) | 3 (0.3) | 7 (0.4) |
| Chinese | 3 (0.3) | 2 (0.2) | 5 (0.3) |
| Other | 12 (1.3) | 14 (1.6) | 26 (1.5) |
| More than one option | 0 | 4 (0.4) | 4 (0.2) |
| Missing | 9 (1.0) | 7 (0.8) | 16 (0.9) |
| Educational qualifications, n (%) | |||
| Degree or equivalent and above | 201 (22.5) | 218 (24.3) | 419 (23.4) |
| A levels or vocational level 3 and above | 198 (22.2) | 207 (23.0) | 405 (22.6) |
| Other qualifications below A level or vocational level 3 | 230 (25.8) | 212 (23.6) | 442 (24.7) |
| Other qualifications (e.g. foreign) | 52 (5.8) | 52 (5.8) | 104 (5.8) |
| No formal qualifications | 204 (22.8) | 199 (22.1) | 403 (22.5) |
| Missing | 8 (0.9) | 11 (1.2) | 19 (1.06) |
| Occupation, n (%) | |||
| Employed | 467 (52.3) | 468 (52.1) | 935 (52.3) |
| Unemployed | 126 (14.1) | 116 (12.9) | 242 (13.5) |
| Looking after home and family | 33 (3.7) | 44 (4.9) | 77 (4.3) |
| Student | 17 (1.9) | 22 (2.5) | 39 (2.2) |
| Retired | 153 (17.1) | 152 (16.9) | 305 (17.1) |
| Long-term sick or disabled | 26 (2.9) | 26 (2.9) | 52 (2.9) |
| Missing | 4 (0.5) | 8 (0.9) | 12 (0.7) |
| Smoking history | |||
| Type of cigarette smoked, n (%) | |||
| Manufactured cigarettes | 615 (68.9) | 607 (67.5) | 1222 (68.2) |
| Tobacco roll-ups | 272 (30.5) | 284 (31.6) | 556 (31.0) |
| Cigars | 6 (0.7) | 8 (0.9) | 14 (0.8) |
| Cigarettes per day (continuous) | |||
| Mean (SD) | 18.7 (9.0) | 19.1 (9.6) | 18.9 (9.3) |
| Median | 19 | 18 | 18 |
| Range | 0–60 | 0–80 | 0–80 |
| IQR | 12–30 | 14–20 | 13–20 |
| Dependence (FTCD), mean (SD) | 5.2 (2.2) | 5.2 (2.2) | 5.2 (2.2) |
| CO concentration reading (contact 1) | |||
| Mean (SD) | 23.8 (12.8) | 23.5 (12.3) | 23.7 (12.5) |
| Median | 21 | 21 | 21 |
| Range | 0–100 | 0–100 | 0–100 |
| IQR | 15–30 | 15–30 | 15–30 |
| Longest previous abstinence (continuous, days) | |||
| Mean | 358.4 | 442.3 | 400.3 |
| SD | 750.7 | 993.7 | 881.4 |
| Median | 90 | 90 | 90 |
| IQR | 21–330 | 14–365.3 | 21–330 |
| Smoking cessation support in last 6 months, n (%) | |||
| Yes | 304 (34.0) | 279 (31.0) | 583 (32.5) |
| No | 588 (65.9) | 619 (68.9) | 1207 (67.4) |
| Missing | 1 (0.1) | 1 (0.1) | 2 (0.1) |
Medication adherence
One week after baseline, nearly three-quarters of participants in the active group reported using the patch daily; in the subsequent 3 weeks of preloading, > 80% of participants in the active group reported using the patch daily (Table 4). In total, 49 (5.5%) participants discontinued preloading prematurely. The majority who ceased preloading did so during the first week of treatment.
| Variable, n | Number (%a) of participants |
|---|---|
| Week –3 (N = 866) | |
| How many days was the patch worn over the last week? | |
| 0 (0%) | 5 (0.6) |
| 1–3 | 34 (3.9) |
| 4–6 | 177 (20.4) |
| 7 (100%) | 645 (74.5) |
| Missing | 5 (0.6) |
| How many patches were used over the last week? (Median 7; range 0–14) | |
| 0 | 4 (0.5) |
| 1–6 | 179 (20.7) |
| 7 | 510 (58.9) |
| > 7 | 168 (19.4) |
| Missing | 5 (0.6) |
| Week +1 (N = 727) | |
| How many days was the patch worn over the last 3 weeks? | |
| 0–7 | 41 (5.6) |
| 8–14 | 47 (6.5) |
| 15–21 | 617 (84.9) |
| Missing | 22 (3.0) |
| How many patches were used over the last 3 weeks? (Median 21; range 0–42) | |
| 0–7 | 40 (5.5) |
| 8–14 | 46 (6.3) |
| 15–21 | 513 (70.6) |
| > 21 | 102 (14.0) |
| Missing | 26 (3.6) |
Post-quit day medication in those who made a quit attempt after preloading was also assessed. At 1 week after quit day, 11.8% of the control group and 8.2% of the intervention group were not using any medication. It is possible that these participants made a quit attempt without medication or that they made a quit attempt but had abandoned the attempt, and, hence, the medication to support the attempt, by the time of the assessment at 1 week after quit day (Table 5).
| Medication used | Trial group, n (%) | ||
|---|---|---|---|
| Control (N = 738)a | Intervention (N = 742)a | Total (N = 1480)a | |
| None | 87 (11.8) | 61 (8.2) | 148 (10.0) |
| Varenicline | 218 (29.5) | 164 (22.1) | 382 (25.8) |
| Bupropion | 6 (0.8) | 12 (1.6) | 18 (1.2) |
| Nicotine patches only | 99 (13.4) | 169 (22.8) | 268 (18.1) |
| Acute nicotine onlyb | 74 (10.0) | 44 (5.9) | 118 (8.0) |
| Combined nicotine | 156 (21.1) | 170 (22.9) | 326 (22.0) |
| Missing | 113 (15.3) | 135 (18.2) | 248 (16.8) |
Primary and secondary outcomes
The primary outcome, biochemically validated 6-month abstinence, was achieved by 157 (17.5%) participants in the intervention group and 129 (14.5%) participants in the control group, a difference of 3.02 (95% CI –0.37 to 6.41).
The secondary outcomes showed similar modest differences. At 4 weeks, 319 (35.5%) participants in the intervention group achieved 7-day point prevalence abstinence, whereas 288 (32.3%) participants in the control group did so. At 12 months, 126 (14.0%) participants in the intervention group achieved validated prolonged abstinence, whereas 101 (11.3%) participants in the control group achieved it (Tables 6 and 7).
| Outcome | Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Adjustedb | Adjustedc | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Primary outcome: 6-month Russell Standard abstinence | ||||||||
| Intervention | 1.25 (0.97 to 1.62) | 0.081 | 1.25 (0.97 to 1.62) | 0.081 | 1.26 (0.97 to 1.62) | 0.081 | 1.34 (1.03 to 1.73) | 0.028 |
| Secondary outcomes | ||||||||
| 4 weeks, Russell Standard abstinence | ||||||||
| Intervention | 1.21 (1.00 to 1.48) | 0.052 | 1.21 (1.00 to 1.48) | 0.053 | 1.22 (1.00 to 1.48) | 0.051 | 1.32 (1.08 to 1.62) | 0.007 |
| 4 weeks, 7-day point prevalence abstinence | ||||||||
| Intervention | 1.16 (0.95 to 1.41) | 0.148 | 1.16 (0.95 to 1.41) | 0.149 | 1.16 (0.95 to 1.41) | 0.150 | 1.26 (1.03 to 1.54) | 0.027 |
| 6 months, 7-day point prevalence abstinence | ||||||||
| Intervention | 1.13 (0.90 to 1.41) | 0.306 | 1.13 (0.90 to 1.41) | 0.306 | 1.14 (0.90 to 1.43) | 0.275 | 1.20 (0.95 to 1.51) | 0.129 |
| 12 months, Russell Standard abstinence | ||||||||
| Intervention | 1.28 (0.97 to 1.69) | 0.085 | 1.28 (0.97 to 1.69) | 0.085 | 1.27 (0.96 to 1.69) | 0.091 | 1.36 (1.02 to 1.80) | 0.036 |
| 12 months, 7-day point prevalence abstinence | ||||||||
| Intervention | 1.22 (0.97 to 1.54) | 0.083 | 1.23 (0.97 to 1.54) | 0.082 | 1.22 (0.97 to 1.54) | 0.087 | 1.28 (1.01 to 1.62) | 0.038 |
| Outcome | Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Adjustedb | Adjustedc | |||||
| RD/RR (95% CI) | p-value | RD/RR (95% CI) | p-value | RD/RR (95% CI) | p-value | RD/RR (95% CI) | p-value | |
| Primary outcome: 6-month Russell Standard abstinence | ||||||||
| Estimated risks | 17.5 and 14.5 | |||||||
| RR | 1.25 (0.97 to 1.62) | 0.081 | 1.21 (0.98 to 1.50) | 0.081 | 1.21 (0.98 to 1.50) | 0.082 | 1.27 (1.03 to 1.57) | 0.029 |
| RD | 3.02 (–0.37 to 6.41) | 0.081 | 3.02 (–0.37 to 6.41) | 0.081 | 3.03 (–0.37 to 6.43) | 0.080 | 3.80 (0.41 to 7.18) | 0.029 |
| Secondary outcomes | ||||||||
| 4 weeks, Russell Standard abstinence | ||||||||
| Estimated risks | 36.3 and 31.9 | |||||||
| RR | 1.14 (1.00 to 1.29) | 0.053 | 1.14 (1.00 to 1.29) | 0.053 | 1.14 (1.00 to 1.29) | 0.051 | 1.19 (1.05 to 1.35) | 0.007 |
| RD | 4.35 (–0.04 to 8.73) | 0.052 | 4.33 (–0.04 to 8.70) | 0.052 | 4.37 (–0.01 to 8.75) | 0.050 | 5.89 (1.60 to 10.19) | 0.007 |
| 4 weeks, 7-day point prevalence abstinence | ||||||||
| Estimated risks | 35.5 and 32.3 | |||||||
| RR | 1.10 (0.97 to 1.25) | 0.149 | 1.10 (0.97 to 1.25) | 0.149 | 1.10 (0.97 to 1.25) | 0.151 | 1.15 (1.02 to 1.31) | 0.027 |
| RD | 3.23 (–1.15 to 7.61) | 0.148 | 3.22 (–1.15 to 7.59) | 0.149 | 3.22 (–1.17 to 7.60) | 0.150 | 4.86 (0.58 to 9.14) | 0.026 |
| 6 months, 7-day point prevalence abstinence | ||||||||
| Estimated risks | 22.3 and 20.3 | |||||||
| RR | 1.10 (0.92 to 1.31) | 0.306 | 1.10 (0.92 to 1.31) | 0.306 | 1.10 (0.92 to 1.32) | 0.276 | 1.15 (0.96 to 1.37) | 0.129 |
| RD | 1.98 (–1.81 to 5.77) | 0.306 | 1.98 (–1.81 to 5.76) | 0.306 | 2.11 (–1.68 to 5.91) | 0.275 | 2.93 (–0.85 to 6.71) | 0.129 |
| 12 months, Russell Standard abstinence | ||||||||
| Estimated risks | 14.0 and 11.3 | |||||||
| RR | 1.24 (0.97 to 1.58) | 0.086 | 1.24 (0.97 to 1.58) | 0.086 | 1.24 (0.97 to 1.58) | 0.093 | 1.30 (1.02 to 1.66) | 0.036 |
| RD | 2.71 (–0.37 to 5.78) | 0.085 | 2.71 (–0.37 to 5.78) | 0.085 | 2.66 (–0.43 to 5.75) | 0.091 | 3.31 (0.22 to 6.39) | 0.036 |
| 12 months, 7-day point prevalence abstinence | ||||||||
| Estimated risks | 22.4 and 19.0 | |||||||
| RR | 1.17 (0.98 to 1.41) | 0.083 | 1.17 (0.98 to 1.41) | 0.083 | 1.17 (0.98 to 1.41) | 0.088 | 1.21 (1.01 to 1.45) | 0.038 |
| RD | 3.32 (–0.43 to 7.07) | 0.082 | 3.32 (–0.42 to 7.06) | 0.082 | 3.28 (–0.48 to 7.04) | 0.087 | 3.98 (0.23 to 7.73) | 0.037 |
Adjustment for predictors of abstinence left the results essentially unchanged, except for adjustment for the use of post-quit day varenicline use, which changed the results noticeably. The effect of preloading was larger than in the unadjusted results and was statistically significant.
Subgroup analysis
There was no evidence that people who used varenicline as their post-cessation medication received less benefit from nicotine preloading than people using other cessation medication. For the effect of preloading compared with control for participants using varenicline, the OR was 1.42 (95% CI 0.90 to 2.26). For participants not using varenicline, the OR was 1.30 (95% CI 0.95 to 1.77; p = 0.740) for the interaction.
Adverse events
Adverse events that were moderate or severe in intensity were uncommon in both arms. Therefore, the absolute difference between arms was small with an absolute difference in the percentage of participants suffering an AE in the intervention group compared with the control group of up to 4% (Table 8).
| Event | Trial group | Percentage point difference (95% CI) | |
|---|---|---|---|
| Control (N = 860) | Intervention (N = 880) | ||
| Gastrointestinal disorders | 19 (2.2) | 55 (6.2) | 4.0 (2.2 to 5.9) |
| Abdominal pain | 3 (0.3) | 6 (0.7) | 0.3 (–0.3 to 1.0) |
| Diarrhoea | 3 (0.3) | 8 (0.9) | 0.6 (–0.2 to 1.3) |
| Nausea | 8 (0.9) | 30 (3.4) | 2.5 (1.1 to 3.8) |
| Vomiting | 3 (0.3) | 14 (1.6) | 1.2 (0.3 to 2.2) |
| General disorders | 11 (1.2) | 30 (3.3) | 2.1 (0.7 to 3.5) |
| Asthenia | 5 (0.6) | 10 (1.1) | 0.6 (–0.3 to 1.4) |
| Fatigue | 0 (0.0) | 6 (0.7) | 0.7 (0.1 to 1.2) |
| Injuries, poisoning and procedural complications | 8 (0.9) | 4 (0.5) | –0.5 (–1.2 to 0.3) |
| Musculoskeletal and connective disorders | 7 (0.8) | 10 (1.1) | 0.3 (–0.6 to 1.2) |
| Nervous system | 16 (1.9) | 56 (6.4) | 4.5 (2.7 to 6.4) |
| Abnormal dreams | 1 (0.1) | 9 (1.0) | 0.9 (0.2 to 1.6) |
| Dizziness | 6 (0.7) | 15 (1.7) | 1.0 (0.0 to 2.0) |
| Headache | 3 (0.3) | 14 (1.6) | 1.2 (0.3 to 2.2) |
| Poor-quality sleep | 3 (0.3) | 20 (2.3) | 1.9 (0.9 to 3.0) |
| Psychiatric | 7 (0.8) | 17 (1.9) | 1.1 (0.0 to 2.2) |
| Depressed mood | 4 (0.5) | 5 (0.6) | 0.1 (–0.6 to 0.8) |
| Respiratory | 21 (2.4) | 15 (1.7) | –0.7 (–2.1 to 0.6) |
| Chest infection | 4 (0.5) | 1 (0.1) | –0.3 (–0.8 to 0.2) |
| Influenza-like illness | 3 (0.3) | 7 (0.8) | 0.4 (–0.3 to 1.2) |
| Nasopharyngitis | 7 (0.8) | 4 (0.5) | –0.4 (–1.1 to 0.4) |
| Skin and subcutaneous tissue disorders | 4 (0.5) | 7 (0.8) | 0.3 (–0.4 to 1.1) |
| Skin irritation | 2 (0.2) | 5 (0.6) | 0.3 (–0.3 to 0.9) |
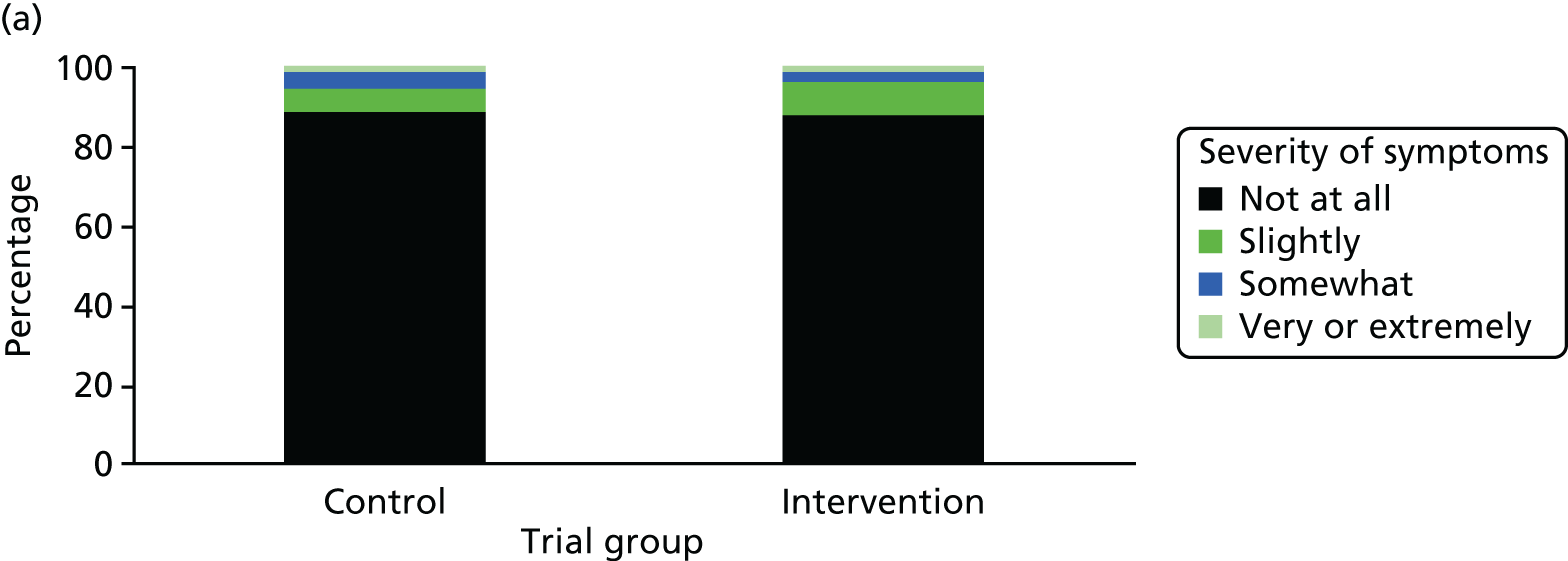
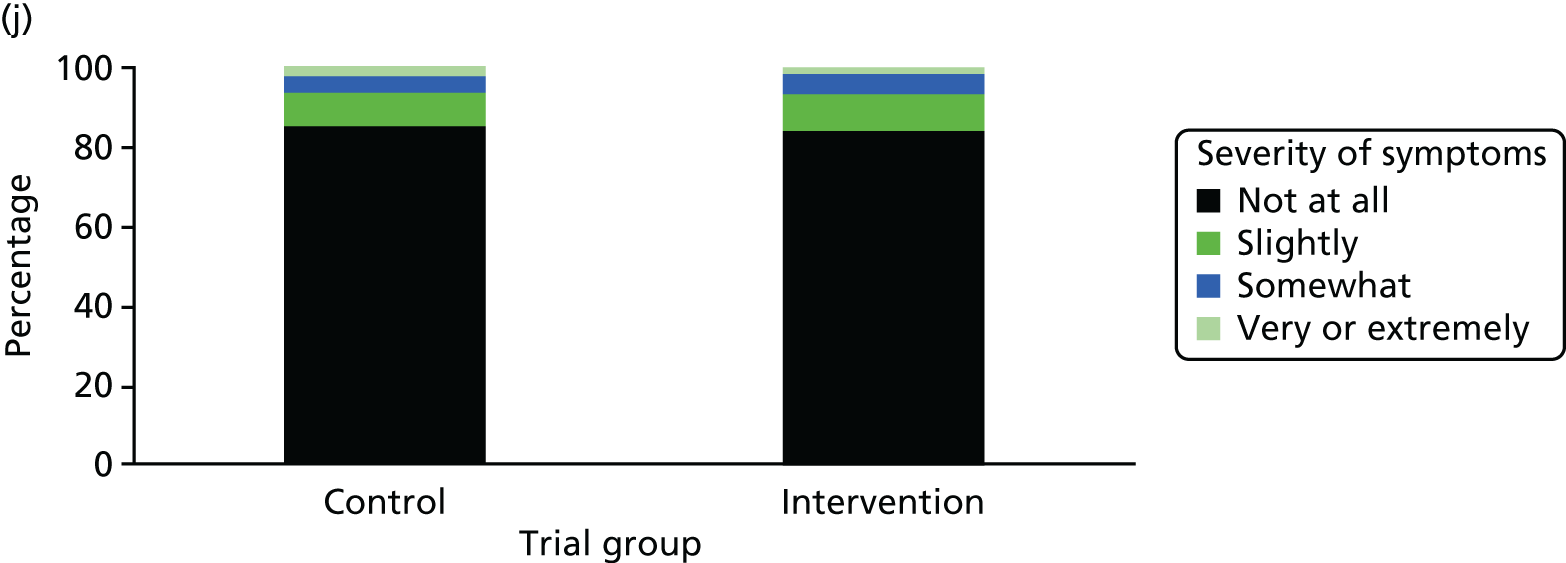
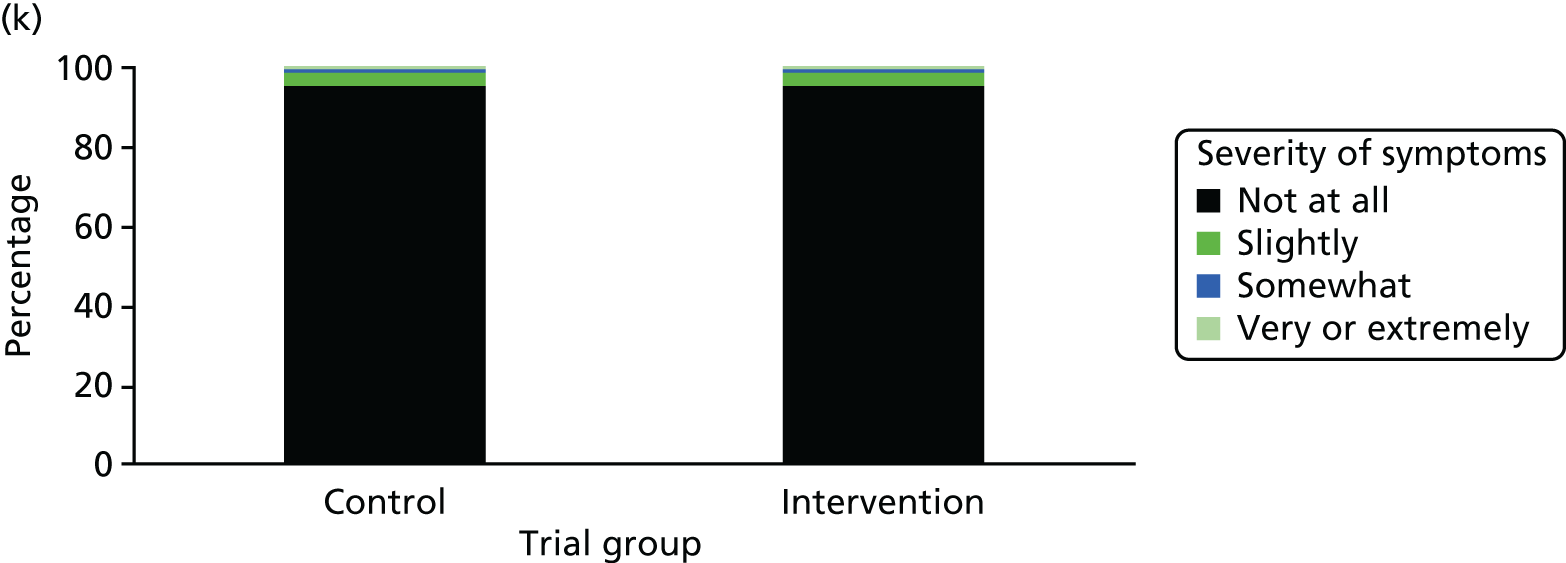
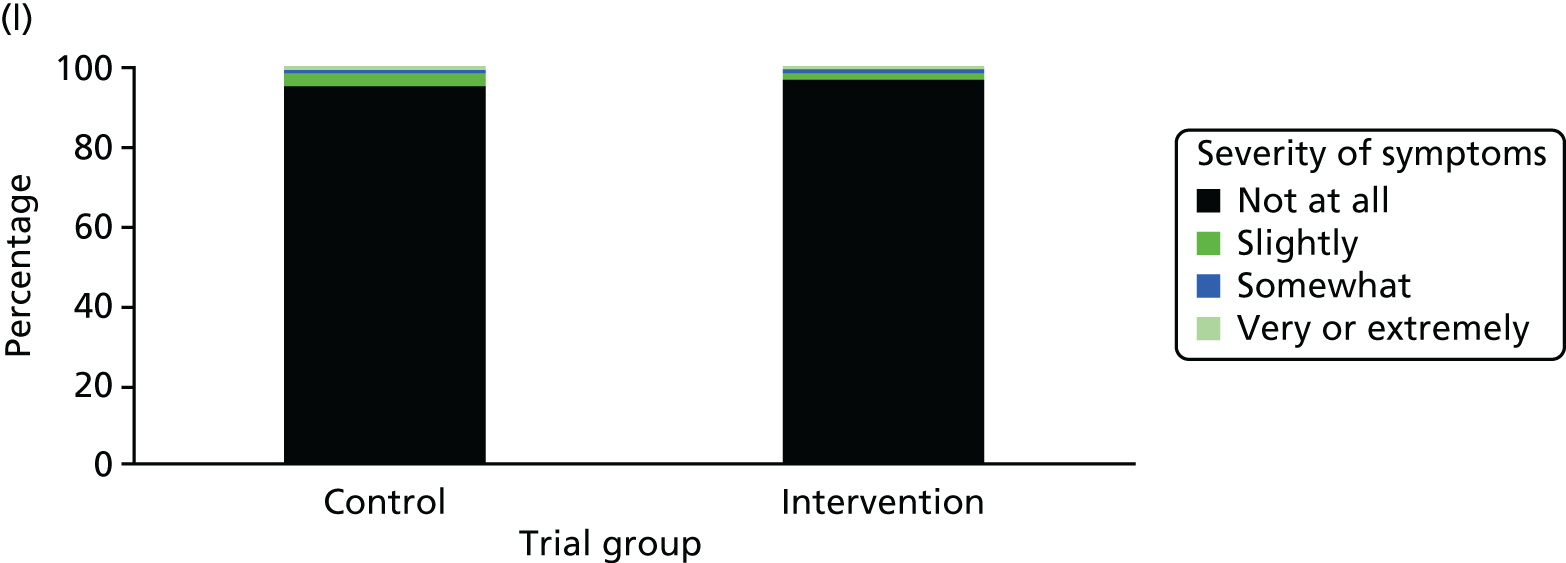
One week after baseline, 394 (45.5%) participants in the intervention group and 271 (32.4%) participants in the control group reported at least one symptom from the checklist of symptoms of nicotine excess (p < 0.001 for the difference). The only three symptoms for which there was statistically significant evidence that they were more common in the intervention group were dizziness, palpitations and nausea. Of these symptoms, 5.6% and 3.0% were somewhat or very dizzy in the intervention and control groups, 3.9% and 1.9% had palpitations and 8.1% and 3.1% experienced nausea, respectively (Figure 4).
FIGURE 4.
Prevalence and severity of symptoms experienced in the last 24 hours, reported after having been in the preloading group or control group for 1 week. (a) Stomach pain; (b) cold sweat; (c) diarrhoea; (d) palpitations; (e) dizziness; (f) nausea; (g) vomiting; (h) headaches; (i) watery mouth; (j) weakness; (k) tremor; and (l) pallor.
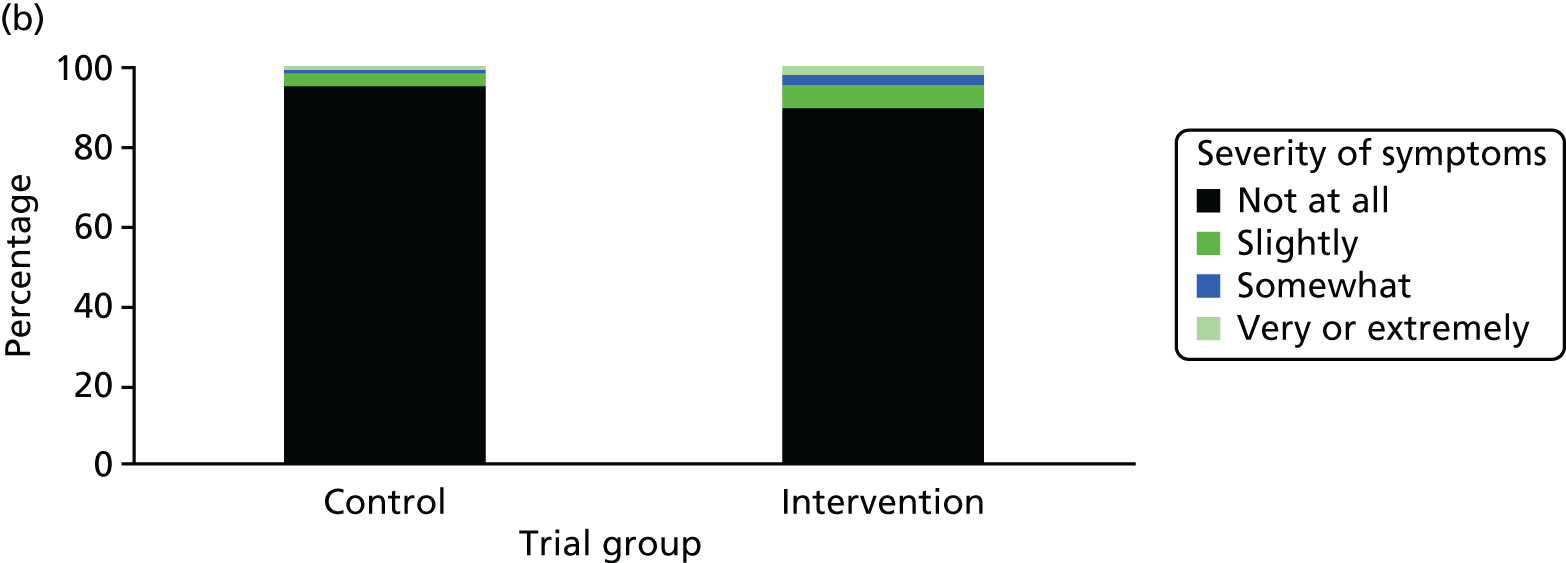
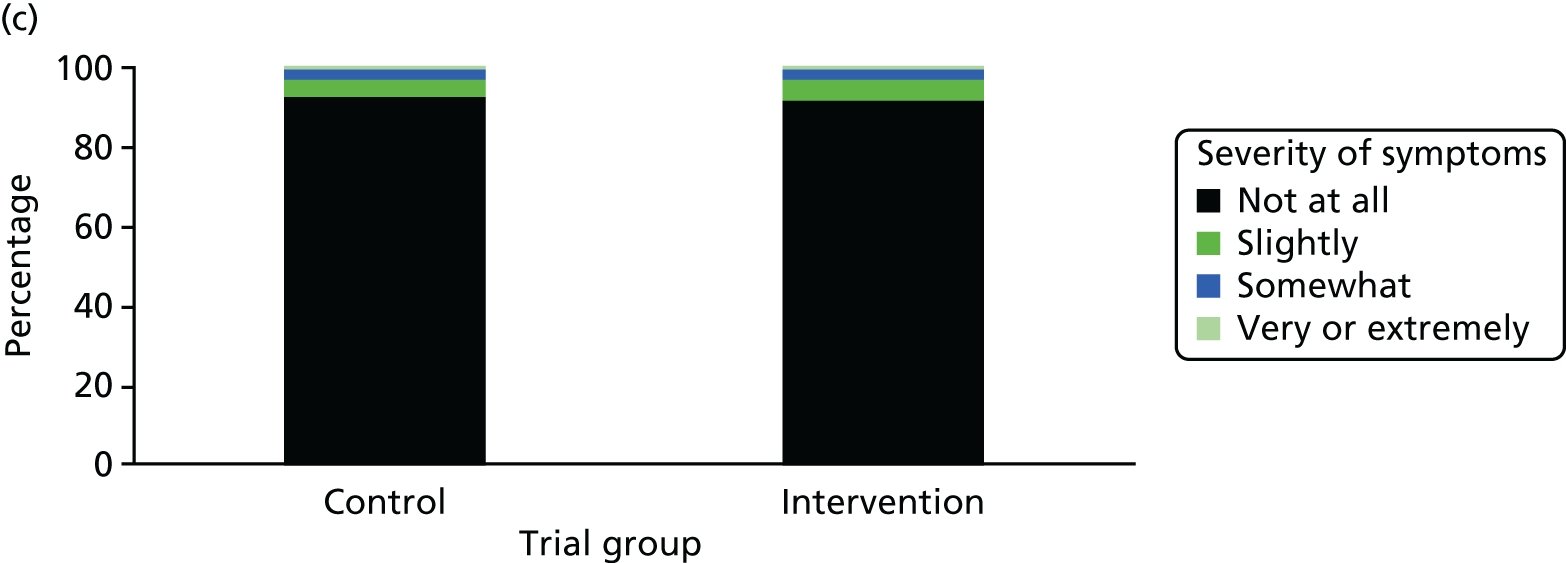
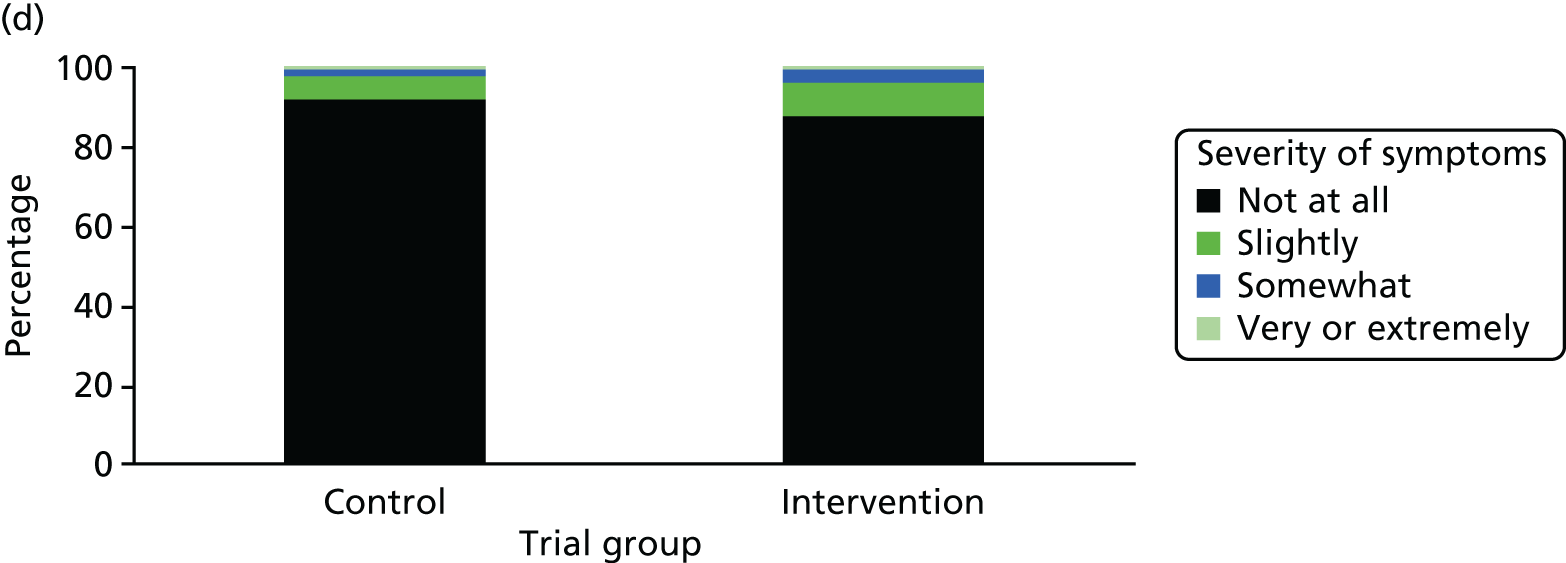
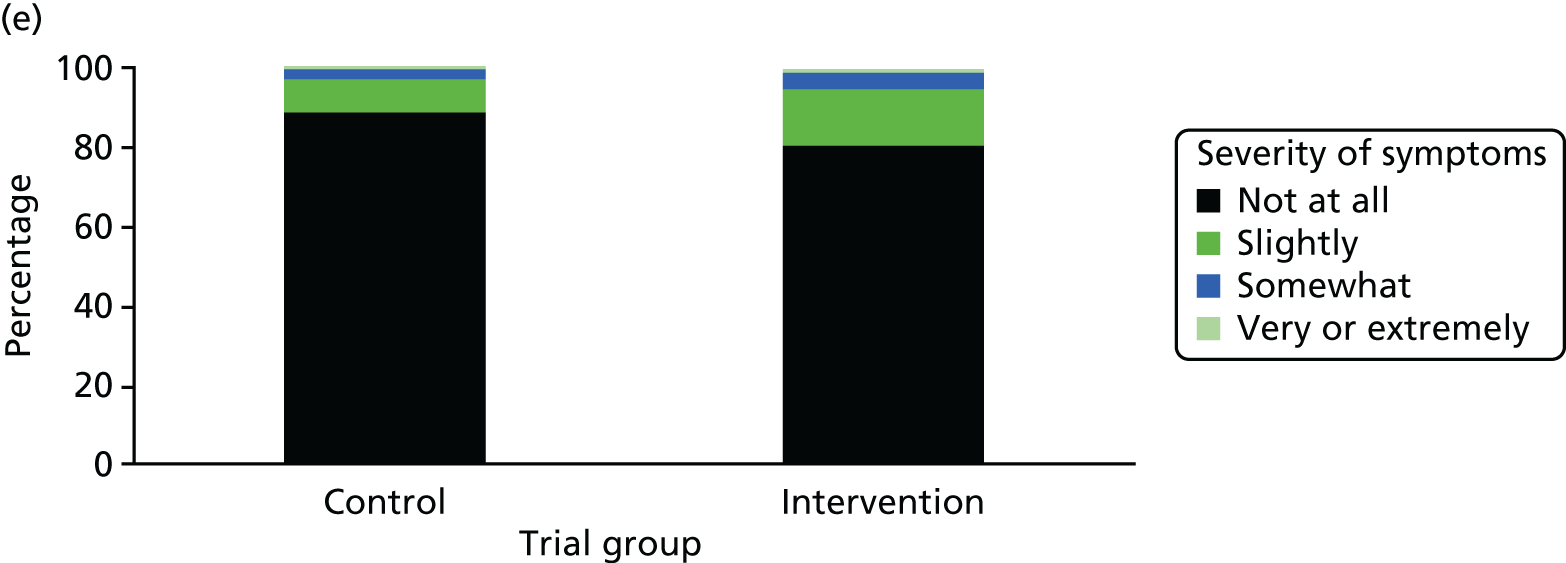
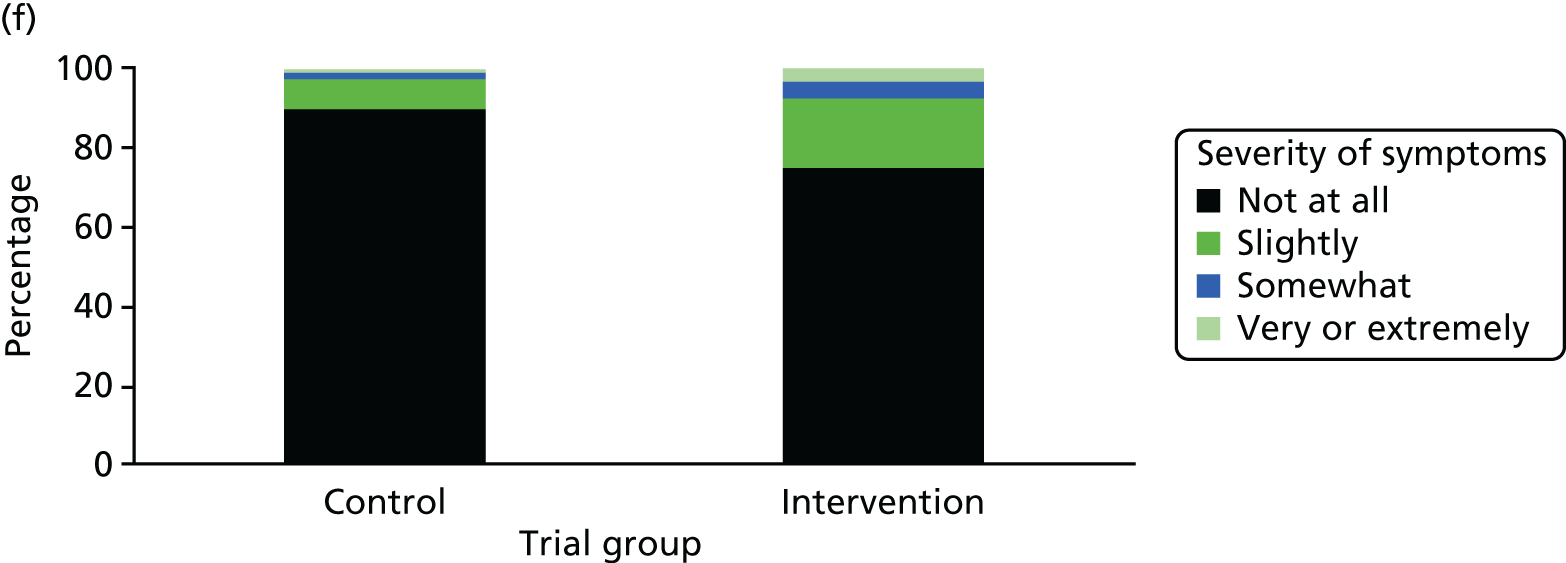
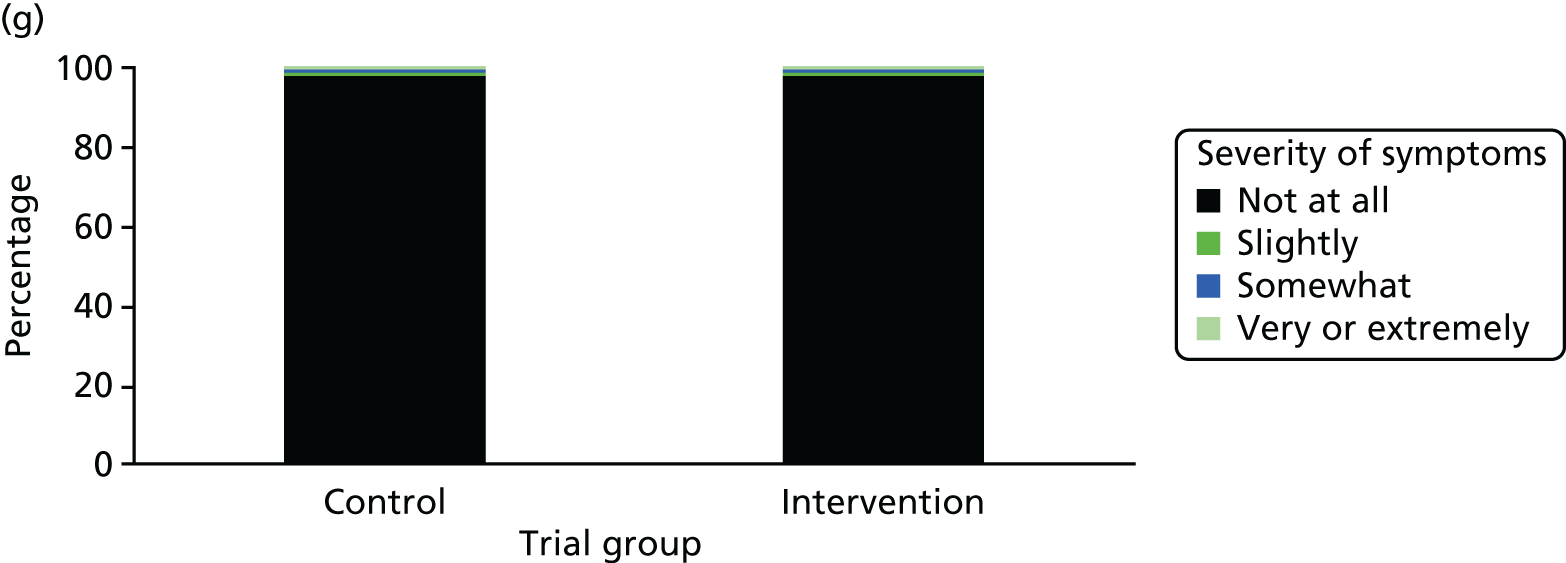
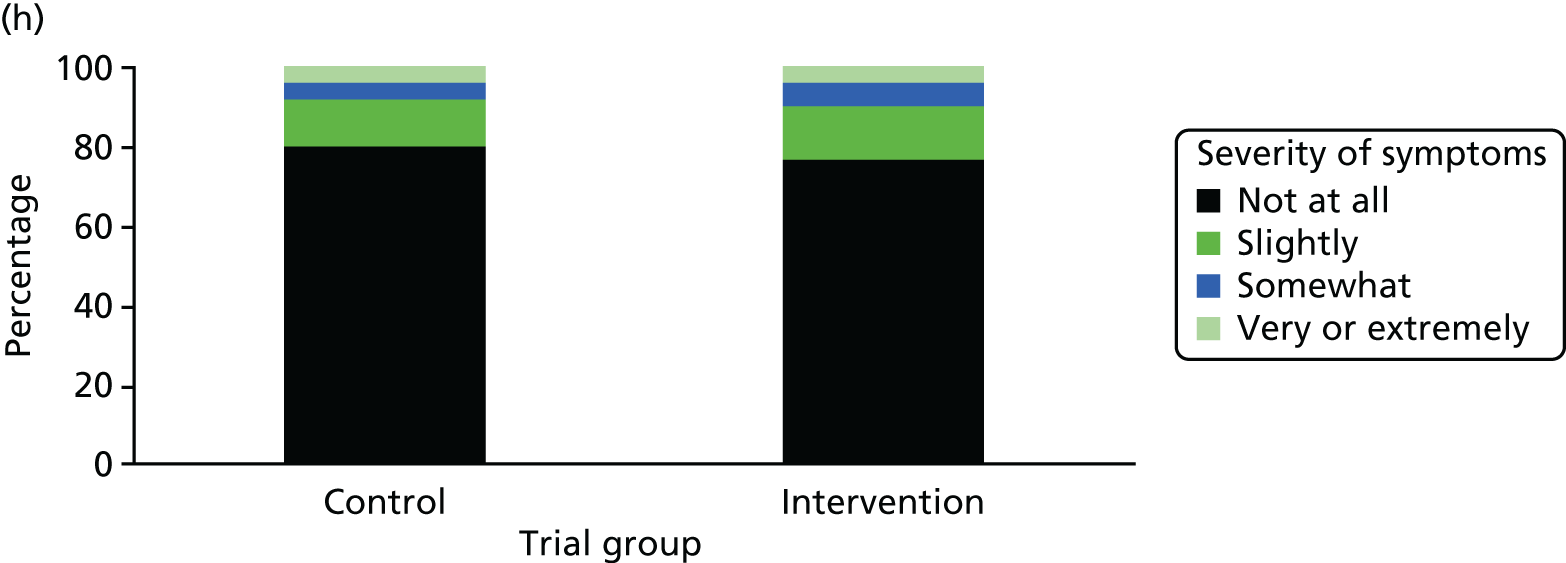
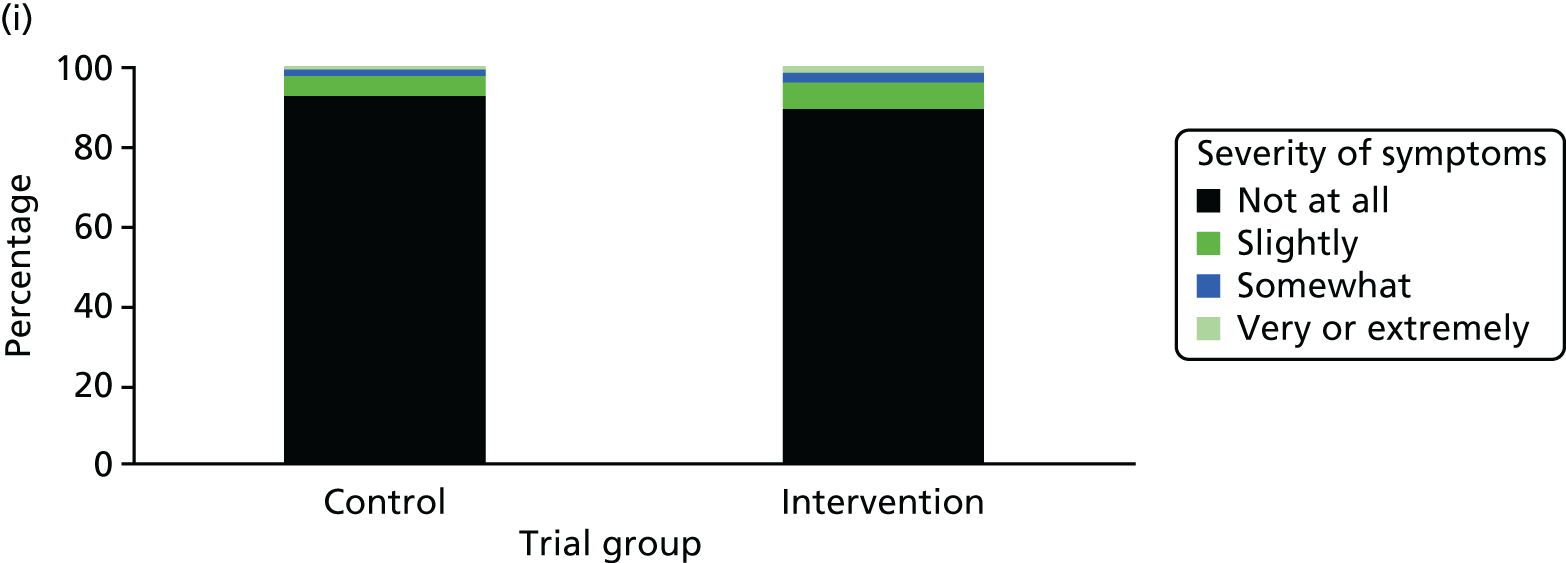
There were 17 SAEs during the 5-week period, nine in the intervention group and eight in the control group, giving an OR of 1.12 (95% CI 0.42 to 3.03). Of these, one was judged to be possibly caused by preloading: acute coronary syndrome in a 64-year-old woman. The SAEs reported are summarised in Table 9.
| Age and gender | Trial group | Relevant medical history | Event |
|---|---|---|---|
| 68-year-old man | Intervention | Chronic myeloid leukaemia | Hospitalised with chest infection |
| 85-year-old woman | Intervention | Osteoporosis | Hospitalised with pelvic fracture following accidental fall |
| 72-year-old woman | Intervention | None | Hospitalised for aspiration of malignant pleural effusion |
| 65-year old woman | Intervention | Cardiovascular disease, hypertension, pacemaker and history of blackouts | Hospitalised for a blackout |
| 27-year-old woman | Intervention | Psychotic illness, illicit drug use | Psychotic episode following period of illicit drug use leading to anxiety attacks |
| 64-year-old woman | Intervention | None | Acute coronary syndrome |
| 54-year-old woman | Intervention | Angina | Hospitalised with acute coronary syndrome or non-cardiac chest pain |
| 45-year-old woman | Intervention | Self-harming | Hospitalised for increased self-harming and suicidal ideation |
| 68-year-old man | Control | Reflux oesophagitis | Hospitalised with cancer of the oesophagus |
| 55-year-old woman | Control | COPD and type 2 diabetes mellitus | Death due to COPD |
| 59-year-old man | Control | Alcohol dependence | Death as a result of an accidental house fire |
| 52-year-old man | Control | Two previous hernia repairs | Hospitalised for hernia repair |
| 47-year-old woman | Control | None | Hospitalised for pyelonephritis |
| 38-year-old woman | Control | Asthma | Hospitalised with chest infection |
| 64-year-old woman | Control | COPD | Exacerbation of COPD |
| 25-year-old man | Control | None | Pneumonia |
Chapter 4 Moderators and mediators of the effect of nicotine preloading
Introduction
Chapter 3 showed no evidence that, in the primary analysis, preloading was an effective strategy for promoting smoking cessation in the current NHS setting. This is because preloading deterred the use of varenicline, the most effective smoking cessation medication, as a post-quit day medication. 6 However, when adjusted for the use of varenicline, there was evidence of an independent effect of preloading as an effective strategy for promoting smoking cessation. However, in the future, it is plausible that this deterrent effect could be overcome by changing the guidelines or the everyday customs and practices of smoking cessation therapists. Therefore, it is appropriate to understand better how preloading may work and the people for whom it may work best. This may allow us to maximise the benefit for patients and, in those who choose preloading, to monitor the effectiveness of the strategy and discontinue preloading if benefits are unlikely.
Effect modification
This chapter addresses two questions. The first examines effect modification or moderation of the possible treatment effect of preloading. It is possible that preloading was effective only for a subgroup of the population of smokers seeking help to quit. Given its proposed mechanism of action on undermining the reward from smoking and the drive to smoke, we hypothesised that preloading would be more effective for people who showed evidence of higher dependence on cigarettes. This might be evidenced by a higher cigarette consumption at baseline (shown by cigarettes per day or exhaled CO concentration at baseline) or a higher dependence score (FTCD). Understanding this may help us target preloading to the group who stand to gain a greater than average benefit from preloading, thereby maximising the cost-effectiveness of the treatment approach.
Mechanism of action of preloading
The second question addresses the question of how preloading may work.
As discussed in Chapter 1, Mechanisms of action of preloading, our review of the mechanisms of action of preloading revealed scant evidence for any of these mechanisms explaining the possible effectiveness of this strategy. This is important for clinical practice. Nicotine preloading may not help everyone who is trying to stop smoking. Therapists could monitor whether or not preloading is achieving its intermediate effects and, if not, abandon the strategy early, saving resources on behalf of the NHS. In this chapter, we examine the possible mediating pathways between nicotine preloading and outcomes.
Method
Design
The trial protocol and main outcomes are described in Chapters 2 and 3. For the moderation analysis, we followed the same analysis strategy as outlined in Chapter 2, Statistical analysis, but added multiplicative interaction terms to the equation between two markers of dependence assessed at baseline and trial group. As markers of dependence we used FTCD, the standard measure of baseline dependence, and exhaled CO concentration. The latter is a measure of smoke intake in the preceding hours; more dependent smokers draw more heavily on their cigarettes or smoke more cigarettes per day and, hence, tend to have higher CO measurements.
For the mediation analysis, we followed the regression framework described by Kenny. 41 The steps are as follows:
-
show that the preloading variable causes smoking cessation (Chapter 3)
-
show that preloading causes change in the mediator
-
show that change in the mediator is associated with smoking cessation
-
to establish complete mediation, show that controlling for change in the mediator abolishes the association between preloading and cessation.
Given that Chapter 3 showed that, on adjustment for the effect of post-cessation varenicline, there was evidence for the efficacy of preloading, this chapter deals with steps 2–4.
Outcomes
For the moderation analyses, the outcome was the primary outcome: Russell Standard 6-month abstinence.
We assessed the impact of preloading on potential mediators of effect outlined in Figure 5. These were measured 1 week after starting preloading or the control condition (3 weeks prior to quit day) and were:
-
positive reinforcement – modified Cigarette Evaluation Questionnaire (mCEQ) satisfaction subscale42 and noticing whether cigarettes were more or less enjoyable than previously
-
negative reinforcement – mCEQ reward subscale
-
drive to smoke – self-reported change in urge strength and change in frequency of the urge to smoke combined, assessed using the Mood and Physical Symptoms Scale – Craving (MPSS-C) subscale;43 smoking stereotypy taken from the Nicotine Dependence Syndrome Scale;44 the mCEQ craving question; and a question used by Hajek et al. 21 in a similar trial that asked participants to rate their urge to smoke compared with usual
-
cigarette consumption – cigarettes per day and exhaled CO concentration
-
symptoms of addiction – modified score on the FTCD,45 modified by removing cigarette consumption from the scale to ensure that symptoms of addiction were distinct from cigarette consumption.
FIGURE 5.
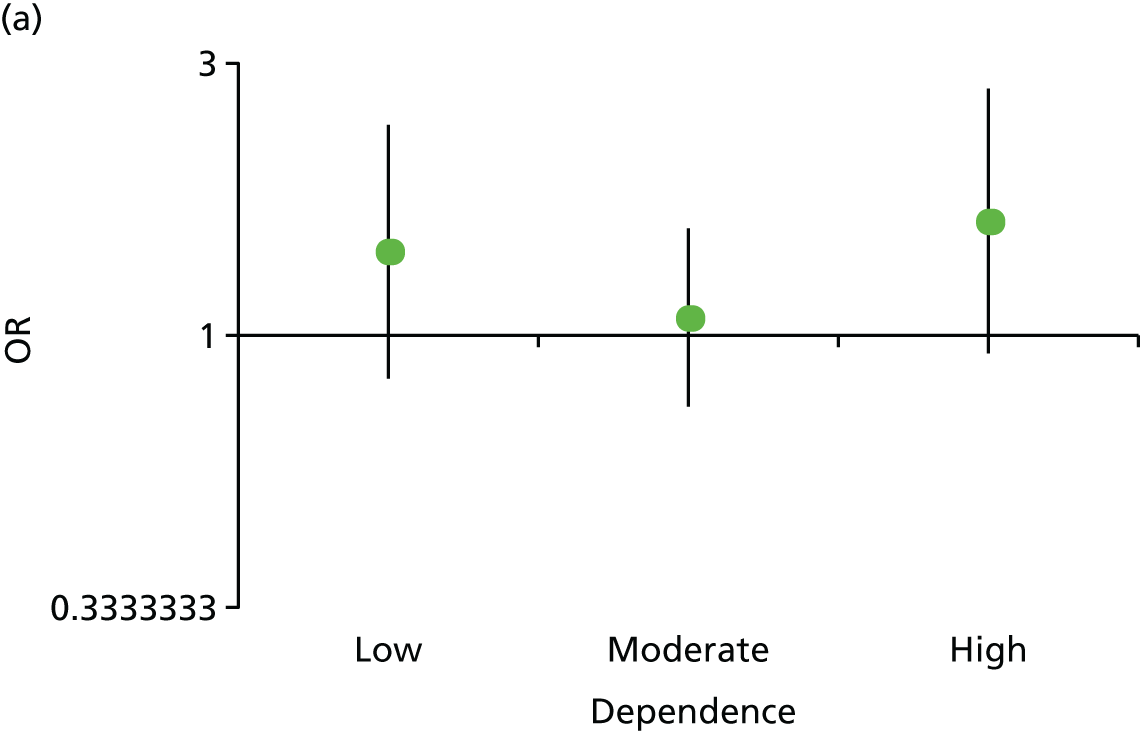
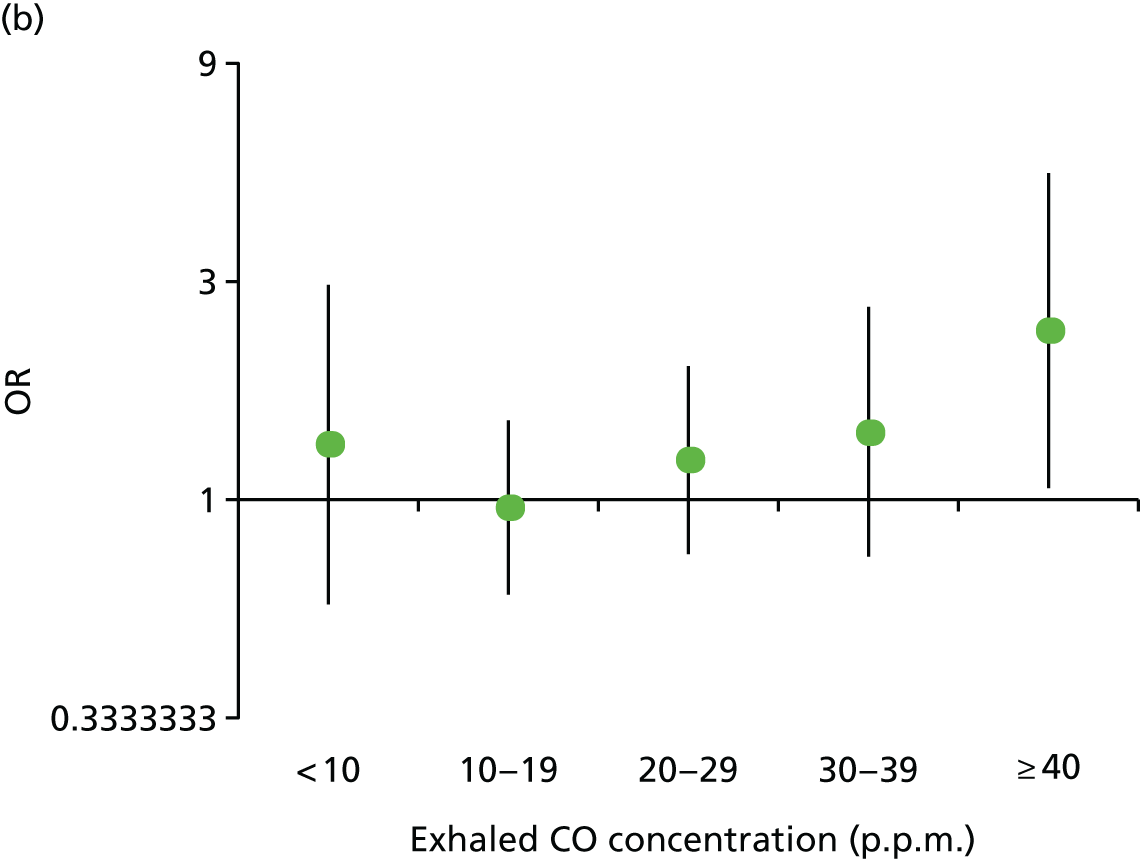
Effect of preloading compared with control on the primary outcome, depending on baseline tobacco dependence. (a) FTCD score (0–3, 4–6 and 7–10); and (b) baseline exhaled CO concentration in p.p.m.
The hypothesis is that, by undermining the learned addiction to cigarettes during the pre-quitting period, preloading reduces the intensity of craving and withdrawal during the post-quit period. For this, we examined the effect of preloading on the intensity of urges and mood symptoms at the end of the first week of abstinence. We used the MPSS-C and the Mood and Physical Symptoms Scale – Mood (MPSS-M) subscales to measure these. The results of assessments of craving and withdrawal symptoms in those who are smoking can be hard to interpret,46 so we analysed these once in those who had been perfectly abstinent and once in those who were continuing to try to be abstinent.
For our other three mediational hypotheses, we assessed the impact of the possible mediators measured at 1 week after commencement. These were as follows:
-
Medication adherence: days of use of post-quit day medication measured at the +1-week visit.
-
Confidence question: ‘How high would you rate your chances of giving up smoking for good at this attempt?’, measured on a five-point scale (with 1 being ‘not at all’ and 5 being ‘extremely good’).
-
Nausea and aversion: we assessed nausea using the mean scores for two questions derived from themes from participant interviews in a previous trial. 47 These were ‘Over the past week how nauseous have you felt when you have seen cigarettes or lighters?’ and ‘Over the past week how nauseous have you felt when you have smelt cigarette smoke?’ and were measured on five-point scales from not at all to extremely. Aversion was measured using the aversion subscale of the mCEQ.
To maximise the sensitivity to detect mediation in the mediator analyses, our outcomes were Russell Standard 4-week and 6-month abstinence. Given that 4-week abstinence is more proximate to the mediators, it should have a stronger association with the mediators than 6-month abstinence.
Statistical analysis
The moderator analysis used the same model as for the primary analysis outlined in Chapter 2, Statistical analysis, but added, in turn, our two markers of dependence (FTCD and exhaled CO concentration) and the multiplicative term representing the baseline marker of dependence multiplied by the trial group indicator variable.
In all of the mediator analyses, we excluded people who had missing data on the mediator. In the analyses, we examined for evidence of mediation on variables assessed at –3 weeks (1 week after baseline) and at +1 week (5 weeks after baseline, 1 week after quit day).
For the second step of the mediation analysis, we assessed the effect of treatment group on mediators descriptively by comparing means between treatment groups and then by analysis of covariance, with adjustment for centre and for the baseline value of the mediator when applicable; thus, effectively examining the effect of preloading on change in the mediator. We checked the residuals to ensure that there was no marked deviation from normality.
We proceeded to the third step of mediation analysis only for those variables for which there was reasonable statistical evidence that preloading influenced the change in the mediator. We assessed the effect of mediators on abstinence outcomes at 4 weeks and 6 months using logistic regression. We looked at this first in unadjusted analysis and then with adjustment for treatment group, as advocated for mediation analysis by Kenny. 41 As previously, these analyses were adjusted for stratification by centre.
In the fourth step of mediation, we examined whether or not potential mediators (i.e. those correlated with both the treatment group and the outcome) did indeed mediate the relationship between treatment group and each outcome; this was done by adding each to a logistic regression model including the mediator and the treatment group. Given that there was strong statistical evidence for the efficacy of preloading only when adjusted for use of varenicline, we used the model adjusted for varenicline as the primary model in this analysis. We added each potential mediator singly and then all of the mediators in the same model.
Results
Modification of the effect of preloading on abstinence by baseline tobacco dependence
There was no evidence that people who were more dependent on smoking received a greater benefit from preloading. The p-values for multiplicative interaction terms for the effect of preloading in those with higher dependence scores and a higher exhaled CO concentration were 0.831 and 0.171, respectively. Per unit increase in each variable, the ORs were 1.01 (95% CI 0.90 to 1.14) and 1.01 (95% CI 0.99 to 1.04), respectively (see Figure 5).
Effect of preloading on potential mediators
Mediators measured at –3 weeks
This assessment took place 1 week after baseline and 3 weeks before quit day.
There was evidence that preloading reduced both positive and negative reward (Table 10). Preloading should reduce the drive to smoke and the reduction of reward should also reduce the drive to smoke. There was evidence that three of the four measures of drive to smoke reduced because of preloading. The exception was withdrawal mood symptoms, but measures of drive to smoke specifically all reduced. If this model is correct, then consumption of cigarettes should decrease and this was indeed observed, with a mean modest reduction of 2.6 cigarettes per day (from a baseline mean of 18.9 cigarettes per day) and a reduction in exhaled CO concentration of 3.2 p.p.m. (from a baseline mean of 23.7 p.p.m.). Likewise, perhaps reflecting a decreased drive to smoke, symptoms of addiction (FTCD, excluding daily cigarette consumption) were also shown to have decreased.
| Potential mediator | Trial group, mean (SD) | Difference between intervention and controla (95% CI) | p-value | |
|---|---|---|---|---|
| Control | Intervention | |||
| At –3 weeks | ||||
| Positive reinforcement | ||||
| mCEQ satisfaction subscale | 4.132 (1.425) | 3.584 (1.351) | –0.547 (–0.651 to –0.443) | < 0.001 |
| Enjoyment more or less than usual | 2.673 (0.650) | 2.149 (0.725) | –0.524 (–0.590 to –0.459) | < 0.001 |
| Negative reinforcement | ||||
| mCEQ reward subscale | 2.945 (1.473) | 2.608 (1.338) | –0.354 (–0.459 to –0.249) | < 0.001 |
| Drive to smoke | ||||
| MPSS-C | 2.613 (0.941) | 2.141 (0.797) | –0.485 (–0.555 to –0.415) | < 0.001 |
| MPSS-M | 1.893 (0.726) | 1.904 (0.724) | 0.009 (–0.043 to 0.062) | 0.724 |
| Smoking stereotypy | 2.185 (0.761) | 2.285 (0.762) | 0.102 (0.036 to 0.168) | 0.003 |
| Urges stronger or weaker than usual | 2.911 (0.657) | 2.160 (0.722) | –0.752 (–0.818 to –0.686) | < 0.001 |
| Cigarette consumption | ||||
| Cigarettes per day | 15.7 (8.7) | 13.4 (8.3) | –2.6 (–3.2 to –2.1) | < 0.001 |
| Exhaled CO concentration | 23.58 (12.8) | 20.41 (11.7) | –3.17 (–4.0 to –2.3) | < 0.001 |
| Symptoms of addiction | ||||
| FTCD excluding cigarettes per day | 3.938 (1.789) | 3.636 (1.790) | –0.2997 (–0.403 to –0.196) | < 0.001 |
| Confidence in quitting | ||||
| How do you rate your chances? | 3.811 (0.788) | 3.853 (0.824) | 0.028 (–0.036 to 0.092) | 0.384 |
| Aversion | ||||
| Nausea | 1.334 (0.594) | 1.533 (0.736) | 0.186 (0.129 to 0.242) | < 0.001 |
| mCEQ aversion subscale | 1.339 (0.717) | 1.589 (0.954) | 0.241 (0.168 to 0.314) | < 0.001 |
| At +1 week | ||||
| Drive to smoke | ||||
| MPSS-C (in those abstinent) | 1.279 (1.046) | 1.0231 (0.958) | –0.285 (–0.438 to –0.132) | < 0.001 |
| MPSS-C (in those still trying to quit) | 1.504 (1.082) | 1.311 (1.053) | –0.214 (–0.335 to –0.093) | 0.001 |
| MPSS-M (in those abstinent) | 1.751 (0.634) | 1.719 (0.636) | –0.035 (–0.126 to 0.056) | 0.454 |
| MPSS-M (in those still trying to quit) | 1.789 (0.687) | 1.770 (0.682) | –0.007 (–0.079 to 0.065) | 0.848 |
| Confidence in quitting | ||||
| How do you rate your chances? (in those abstinent) | 4.371 (0.746) | 4.360 (0.658) | –0.010 (–0.117 to 0.097) | 0.858 |
| How do you rate your chances? (in those abstinent or still trying to quit) | 4.102 (0.916) | 4.190 (0.787) | 0.092 (–0.006 to 0.189) | 0.066 |
| Aversion (in those trying to quit) | 1.831 (1.199) | 1.976 (1.392) | 0.203 (–0.018 to 0.424) | 0.072 |
| Nausea (in those abstinent) | 0.683 (0.333) | 0.679 (0.319) | –0.011 (–0.060 to 0.038) | 0.649 |
| Nausea (in those abstinent or trying to quit) | 0.676 (0.317) | 0.695 (0.331) | 0.017 (–0.019 to 0.053) | 0.365 |
| Medication adherence | ||||
| Number of days of medication use in the last week (in those who are still trying to quit), n (%) | ||||
| 0 | 82 (14.2) | 68 (11.7) | 0.333 | |
| 1–6 | 78 (13.5) | 72 (12.3) | ||
| 7 | 419 (72.4) | 443 (76.0) | ||
Two alternative hypotheses were that preloading improves confidence in quitting and that preloading works through creating an aversion to smoking. There was no evidence that confidence in quitting improved because of preloading but there was strong evidence that both markers of aversion to smoking increased because of preloading (see Table 10). The medication adherence hypothesis could be tested only after quit day.
Mediators measured at +1 week
This assessment took place 1 week after quit day, and potentially after stopping preloading treatment, and 5 weeks after baseline.
As participants were trying to achieve abstinence and many were abstaining, we did not measure positive or negative reward from smoking cigarettes at this assessment. In the main hypothesis, reduced urges to smoke during preloading and a reduced level of smoking mean that people are experiencing cues to smoke that do not prompt smoking, thus beginning the process of extinguishing the learnt drive to smoke. This would manifest as reduced urges to smoke or craving experienced after quit day and there was evidence that this did occur, although there was no evidence that withdrawal mood symptoms were reduced. At this assessment, when participants were abstinent, it was not sensible to ask about any other potential mediators on this pathway.
As in the pre-quit period, there was no evidence that preloading increased participants’ confidence in their ability to quit smoking. Unlike in the pre-quit period, there was no evidence of a difference in aversion to cigarettes after the quit day (see Table 10).
The final mediating hypothesis is that preloading improves adherence to post-cessation medication but there was no evidence to support this (see Table 10).
Association between mediators and outcome (smoking abstinence)
We analysed the association between mediators and outcome for those variables for which there was evidence that preloading influenced the mediator (Table 11).
| Potential mediator | Abstinence | |||
|---|---|---|---|---|
| At 4 weeks, OR (95% CI); p-value | At 6 months, OR (95% CI); p-value | |||
| Unadjusted for treatment group | Adjusted for treatment group | Unadjusted for treatment group | Adjusted for treatment group | |
| Change from baseline at –3 weeks | ||||
| Positive reinforcement | ||||
| mCEQ satisfaction subscale | 0.974 (0.901 to 1.054); 0.521 | 0.987 (0.910 to 1.070); 0.746 | 0.915 (0.828 to 1.012); 0.083 | 0.928 (0.837 to 1.028); 0.153 |
| Enjoyment more or less than usual | 0.864 (0.761 to 0.981); 0.024 | 0.880 (0.761 to 1.017); 0.082 | 0.818 (0.695 to 0.963); 0.016 | 0.832 (0.691 to 1.003); 0.053 |
| Negative reinforcement | ||||
| mCEQ reward subscale | 0.996 (0.919 to 1.080); 0.932 | 1.006 (0.927 to 1.091); 0.885 | 1.073 (0.967 to 1.191); 0.182 | 1.089 (0.980 to 1.209); 0.112 |
| Drive to smoke | ||||
| MPSS-C | 0.959 (0.860 to 1.069); 0.445 | 0.980 (0.876 to 1.097); 0.727 | 1.060 (0.921 to 1.219); 0.416 | 1.099 (0.951 to 1.270); 0.202 |
| Smoking stereotypy | 1.021 (0.903 to 1.155); 0.741 | 1.015 (0.897 to 1.148); 0.819 | 1.080 (0.923 to 1.264); 0.338 | 1.072 (0.915 to 1.255); 0.390 |
| Urges stronger or weaker than usual | 0.864 (0.761 to 0.981); 0.024 | 0.880 (0.761 to 1.017); 0.082 | 0.818 (0.695 to 0.963); 0.016 | 0.832 (0.691 to 1.003); 0.053 |
| Cigarette consumption | ||||
| Cigarettes per day | 1.008 (0.993 to 1.023); 0.311 | 1.011 (0.995 to 1.027); 0.173 | 1.002 (0.983 to 1.022); 0.825 | 1.006 (0.986 to 1.026); 0.582 |
| CO concentration | 0.986 (0.976 to 0.996); 0.007 | 0.987 (0.977 to 0.997); 0.012 | 0.988 (0.975 to 1.001); 0.062 | 0.989 (0.976 to 1.002); 0.094 |
| Symptoms of addiction | ||||
| FTCD | 1.003 (0.918 to 1.095); 0.954 | 1.012 (0.925 to 1.106); 0.799 | 1.016 (0.907 to 1.138); 0.780 | 1.029 (0.918 to 1.153); 0.625 |
| Aversion | ||||
| Nausea | 0.888 (0.763 to 1.035); 0.128 | 0.872 (0.748 to 1.017); 0.082 | 1.072 (0.884 to 1.300); 0.481 | 1.052 (0.866 to 1.277); 0.611 |
| mCEQ aversion subscale | 1.001 (0.898 to 1.115); 0.989 | 0.990 (0.888 to 1.103); 0.856 | 1.012 (0.882 to 1.162); 0.863 | 1.000 (0.871 to 1.147); 0.992 |
| Change from baseline at +1 week | ||||
| MPSS-C | 0.750 (0.690 to 0.817); < 0.001 | 0.754 (0.692 to 0.820); < 0.001 | 0.774 (0.699 to 0.858); < 0.001 | 0.779 (0.703 to 0.863); < 0.001 |
In the analyses assessing the main hypothesis for the mechanism of action, at –3 weeks, there was no evidence of an association between change in either positive or negative reinforcement from smoking and subsequent abstinence from smoking at 4 weeks or 6 months. Although two of the three measures of drive to smoke were not associated with smoking abstinence, one of these, noticing a change in the strength of urges, was associated with abstinence. Objective reduction in consumption, measured by a reduced CO concentration over the week, was associated with later abstinence, but self-reported reduction in cigarettes smoked per day was not associated with later abstinence. There was no evidence that change in symptoms of addiction was associated with later abstinence (see Table 11). At +1 week, during the abstinence period, reduction in urge strength was associated with later abstinence.
After the second step in the mediation analysis, the only surviving competing hypothesis to our main hypothesis was that the effect of preloading was mediated by creating an aversion to smoking. However, in this third step, there was no evidence that a change in aversion (measured by nausea and the mCEQ aversion subscale at –3 weeks) predicted abstinence.
Effect of preloading on the outcome, controlled for mediators
In the final step of the mediation analysis, we considered whether or not the strength of association between use of preloading and abstinence was diminished after adding three potential mediators. These mediators, all of which showed evidence of potential mediation in the previous steps, were (1) noticing that urges were weaker than usual while still smoking, (2) reduced exhaled CO concentration while still smoking and (3) reduced urge strength after abstinence. As the clear evidence of efficacy of preloading emerged only in the analyses adjusting for post-cessation varenicline use, we used this as the primary comparison for this final stage of mediation analysis. In the data on abstinence, we included all participants in the analysis with the presumption that people who dropped out were smoking. However, where people were not followed up, the value of their mediator was not imputed and so these participants were dropped from the analysis. To ensure that the ORs were comparable across analyses, the number with valid data was used on the mediator in all analyses, including those analyses that did not include the mediator.
There was evidence that each of the mediators explained the effect of preloading to some extent (Table 12). Controlling for a reduction in urge strength reduced the effect of preloading on abstinence by 47% for 4-week abstinence and 59% for 6-month abstinence. Controlling for a reduction in exhaled CO concentration during the pre-quit period reduced the effect of preloading on abstinence by 23% for 4-week abstinence and 14% for 6-month abstinence. Controlling for a reduction in craving after quitting reduced the effect of preloading on abstinence by 28% for 4-week abstinence and 21% for 6-month abstinence. Controlling for all three potential mediators reduced the effect of preloading on abstinence by 71% for 4-week abstinence and 78% for 6-month abstinence.
| Potential mediator | OR (95% CI) for effect of treatment on smoking status at 4 weeks; p-value | OR (95% CI) for effect of treatment on smoking status at 6 months; p-value | ||||
|---|---|---|---|---|---|---|
| In model adjusted for centre and varenicline use onlya (n = 1792) | In model adjusted for centre and varenicline use only (n as available for the mediator) | In model adjusted for centre and varenicline use and mediator (n as available for the mediator) | In model adjusted for centre and varenicline use onlya (n = 1792) | In model adjusted for centre and varenicline use only (n as available for the mediator) | In model adjusted for centre and varenicline use and mediator (n as available for the mediator) | |
| Urges stronger or weaker than usual (n = 1696) | 1.32 (1.08 to 1.61); 0.007 | 1.26 (1.03 to 1.55); 0.026 | 1.13 (0.90 to 1.43); 0.303) | 1.33 (1.03 to 1.73); 0.028 | 1.29 (0.99 to 1.67) 0.056 | 1.11 (0.82 to 1.49); 0.501 |
| Change in exhaled CO concentration at –3 weeks (n = 1642) | 1.32 (1.08 to 1.61); 0.007 | 1.24 (1.01 to 1.53); 0.042 | 1.18 (0.96 to 1.46); 0.118 | 1.33 (1.03 to 1.73); 0.028 | 1.26 (0.97 to 1.64); 0.08 | 1.22 (0.93 to 1.59); 0.14 |
| Change in MPSS-C at +1 week (n = 1408) | 1.32 (1.08 to 1.61); 0.007 | 1.29, (1.03 to 1.60); 0.024 | 1.20 (0.96 to 1.50); 0.103 | 1.33 (1.03 to 1.73); 0.028 | 1.30 (0.99 to 1.70); 0.056 | 1.23 (0.94 to 1.61); 0.14 |
| All of the above (n = 1327) | 1.32 (1.08 to 1.61); 0.007 | 1.22 (0.98 to 1.53); 0.079 | 1.06 (0.82 to 1.38); 0.661 | 1.33 (1.03 to 1.73); 0.028 | 1.25 (0.95 to 1.64); 0.108 | 1.05 (0.77 to 1.44); 0.757 |
Chapter 5 People’s reactions to nicotine preloading
Introduction
It is important to understand how people feel about medical interventions, particularly in the case of behavioural issues, such as smoking cessation. People believe that they have direct insight into behavioural issues and behaviour change by virtue of their past and current experience and, therefore, need to feel that the treatment they are receiving is appropriate or they will not take it.
Methods
At week +1 (1 week after quit day) we asked participants to tell us their views on nicotine preloading. We did not ask similar questions to the control group. We used an open-ended question to ask participants for their views. However, to aid categorisation, the researcher collecting the data ticked one or more statements on a list of beliefs, attitudes and experiences that we had prepared and added to the case report form (CRF). This list was taken from qualitative interviews carried out in a previous study of preloading. 17 In addition, we asked participants to rate the helpfulness of preloading and whether or not they would recommend this strategy to another person trying to quit smoking.
Results
The results are shown in Table 13. Overall, 87.2% found preloading to be helpful and 90.5% would recommend preloading to others. A mixed set of beliefs and experiences was reported, most of which reflected a generally positive view of the intervention.
| Question | Number (%) of participants (N = 727) |
|---|---|
| How helpful was the preloading intervention? | |
| Very helpful | 488 (67.13) |
| Slightly helpful | 146 (20.08) |
| Not very helpful | 76 (10.45) |
| Missing | 17 (2.34) |
| Would you recommend the preloading intervention to someone else? | |
| Yes | 658 (92.8) |
| No | 51 (7.02) |
| Unprompted responses to thoughts on preloading | Number (%) of participants mentioning response |
| Experience of smoking | |
| Did not feel urge to smoke | 225 (30.95) |
| Reduced smoking to control nausea | 41 (5.64) |
| Experienced nausea | 87 (11.97) |
| Felt no effect of preloading | 66 (9.08) |
| Lack of enjoyment when smoking | 253 (34.80) |
| Smoking rate reduced | 473 (65.06) |
| Smoking rate stayed the same | 69 (9.49) |
| Smoking rate increased | 16 (2.20) |
| Relief to stop smoking | 107 (14.72) |
| Smoking intolerable | 31 (4.26) |
| Ready to quit on quit day | 254 (34.94) |
| Nausea aided quit attempt | 50 (6.88) |
| Overall evaluation | |
| Helpful | 495 (68.09) |
| Disappointed with preloading | 38 (5.23) |
| Surprised asked to preload | 93 (12.79) |
| Continued patch use until quit day | 314 (43.19) |
| Discontinued patch use | 64 (8.80) |
| Belief nicotine not harmful | 4 (0.55) |
| Preloading is aversion therapy | 31 (4.26) |
| Worried about preloading | 62 (8.53) |
| Trusted guidance of research team | 198 (27.24) |
| Preloading dangerous | 14 (1.93) |
| Did not accept reason for preloading | 19 (2.61) |
| Aware of previous warnings not to preload | 41 (5.64) |
| Patch AEs | |
| Skin irritation | 122 (16.78) |
| Sleep disturbance | 125 (17.19) |
| Trouble getting patches to stay on | 86 (11.83) |
Discussion
Participants in this trial were generally happy to preload, reported it as a positive experience and would recommend the strategy to others.
This evaluation was only ever meant to give some overall sense of participants’ reactions to preloading and clearly this lacks depth. In particular, we did not record or note the verbatim thoughts of participants and, for practical reasons, the research team interpreted what participants said and ticked one or more boxes that they felt captured their thoughts. This process is likely to be somewhat inaccurate, depending on the interpretation in the moment of the conversation, as well as not capturing the full extent of participants’ thoughts. Nevertheless, in the absence of a full qualitative analysis, which was not planned nor funded, we believe that it reinforces data presented in Chapters 1 and 2. In particular, it suggests that preloading was well tolerated and that most participants perceived changes in their smoking as a result of preloading. In the preloading trial group, 65% of participants perceived the reduction in consumption and 35% felt that smoking was less enjoyable. These spontaneously reported effects reinforce what we captured quantitatively in the mediation analysis.
Conclusion
These limited reflections on participants’ feelings about preloading suggest that most participants found it helpful and that the large majority would recommend preloading.
Chapter 6 A within-trial cost-effectiveness analysis of nicotine preloading
Introduction
The Preloading trial tested the effectiveness of a smoking cessation intervention. Such interventions typically have a very low cost per QALY and are among the most cost-effective in health care. 48,49 This is because continued smoking is dangerous but cessation is surprisingly effective at reducing the effects of many previous years of smoking. 19,50 As a consequence, one analysis suggests that, at any reasonable cost, improving the 6-month prolonged abstinence percentage by 1% should be cost-effective. 20 In this chapter, we examine the cost-effectiveness of preloading relative to the control condition.
Methods
Cost-effectiveness analysis was conducted alongside the Preloading trial. Following NICE guidance, the analysis was performed from the NHS and Personal Social Services perspective, to reflect the NHS England decision-making framework, and on a ITT basis. 51 All costs were presented in 2014/15 Great British pounds (£).
Costs
Total costs comprised the intervention costs, NHS Stop Smoking Services costs, AE costs, health resource use costs and medication costs.
Intervention costs
The intervention consisted of 4 weeks of NRT preloading (i.e. the use of NRT before quit day) and two sessions of behavioural support for the preloading group. For the control group, only two sessions of similar-intensity behavioural support were provided. For both arms, the participants were also given a four-page booklet containing information on smoking cessation pertinent to each group.
The NRT used in the intervention was a 21-mg patch every 24 hours provided by GlaxoSmithKline plc (GSK House, Middlesex, UK) at no cost, but, for the purposes of this analysis, we costed this as if it had been purchased by the NHS. The number of patches dispensed was extracted from the trial dispensing form. The cost of NRT for the intervention was calculated by multiplying the amount dispensed to the participants by the weighted average cost per patch. We assumed that the few participants with no record of dispensing had not been given any patches.
The behavioural support sessions were provided as part of the intervention at baseline and at –3 weeks. We included costs only for sessions that were attended so that not everyone incurred the cost of attending a second appointment. The behavioural support sessions were costed by adapting the existing cost template of NHS Stop Smoking Services according to the length of the sessions delivered in the trial. 52
NHS Stop Smoking Services costs
After the intervention period, the participants were referred to NHS Stop Smoking Services for standard behavioural support and pharmacotherapies. NHS Stop Smoking Services were unable to supply participants’ attendance data at the NHS Stop Smoking Services behavioural support sessions, so several assumptions were made to estimate the NHS Stop Smoking Services behavioural support cost. We assumed that people had received support if they had an appointment date and a service provider noted on the CRF. People who had no appointment date were assumed not to have attended. If provider data were missing or an appointment was missing, participants’ attendance at NHS Stop Smoking Services was considered missing. Based on the NHS Stop Smoking Services costing report,52 the average number of sessions was multiplied by the inflated cost per session (£16) to calculate the cost of NHS Stop Smoking Services behavioural support sessions for those who attended.
Data on the use of post-quit date pharmacotherapy were collected through self-report at +1 week (1 week after quit date), with respondents reporting the type of medication or could record a response of ‘none’. If no response was recorded, these data on pharmacotherapy were considered missing. The pharmacotherapies were costed using the weighted average cost per prescribed item for the form specified. 53 Table 14 lists the weighted average cost per prescription item for each type of pharmacotherapy used in the trial. Because this information was collected only at one time point and no other details were recorded, only one prescription item in the analysis was taken into account.
| Type of pharmacotherapy | Cost per prescription item (£) |
|---|---|
| Gum | 13 |
| Patch | 25 |
| Lozenge | 15 |
| Inhaler | 32 |
| Mist (mouth spray) | 25 |
| Nasal spray | 39 |
| Strips | 15 |
| Microtab | 17 |
| Unidentified spraya | 27 |
| Acute NRTb | 24 |
| Varenicline | 34 |
| Bupropion | 38 |
Adverse event costs
Adverse events resulting in hospitalisation were recorded according to the protocol. 26 For the purpose of costing, only the AEs that were determined to be at least possibly related to the intervention were included. The cost of an AE was estimated using the weighted average cost of all Healthcare Resource Groups (HRGs) related to the cause of the hospitalisation.
Health resource use costs
Information on health resource use was collected through a self-reported questionnaire at baseline and 6 and 12 months’ follow-up, covering the 6-month period prior to each time point. The health resources reported included help for smoking cessation outside of the study, hospital visits and use of primary and community services. If an entire section was left blank, the data were coded as missing. If some of the questions were answered and others were left blank, these blanks were assumed to represent nil use of those services. A set of national average costs were applied to the quantities reported by the participants (Table 15). For home visits from all professionals, travel time and expenses were not available. We assumed that the consultation time was the same as that in a general practice; 12 minutes of travel time was added in addition to account for the time spent by the professional travelling to the appointment. However, travel expenses or any other reimbursement were not included. Overheads were included when applicable.
| Cost item | Unit cost (£) | Source(s) |
|---|---|---|
| Help for smoking cessation | ||
| GP (per consultation) | 36 | Curtis and Burns54 |
| Practice nurse (per consultation) | 7 | Curtis and Burns54 |
| Pharmacist (per consultation) | 10 | Curtis and Burns54 |
| NHS Stop Smoking Services cessation class (per session) | 16 | NICE52 and Curtis and Burns54 |
| NHS smoking helpline (per call) | 6 | Curtis and Burns54 and Parrott et al.55 |
| Prescription for nicotine patches (per item) | 25 | Prescribing and Medicines Team53 |
| Prescription of acute NRT (per item) | 24 | Prescribing and Medicines Team53 |
| Prescription of bupropion (per item) | 38 | Prescribing and Medicines Team53 |
| Prescription of varenicline (per item) | 34 | Prescribing and Medicines Team53 |
| Hospital visits | ||
| Accident and emergency department (per visit) | 132 | DHSC56 |
| Emergency ambulance (per use) | 231 | Curtis and Burns54 |
| Outpatient (per attendance) | 113 | Curtis and Burns54 |
| Day case (per case) | 710 | Curtis and Burns54 |
| Non-emergency ambulance (per use) | 180 | DHSC56 |
| Patient transport services (per journey) | 35 | Curtis and Burns54 and DHSC56 |
| Hospital inpatient stay (per night) | 734 | DHSC56 |
| Primary and community services | ||
| GP, in practice (per consultation) | 37 | Curtis and Burns54 |
| Practice nurse, in practice (per consultation) | 12 | Curtis and Burns54 |
| GP, home visit (per visit) | 75 | Curtis and Burns54 |
| Practice/district nurse or health visitor, home visit (per visit) | 26 | Curtis and Burns54 |
| Community psychiatric nurse, home visit (per visit) | 27 | Curtis and Burns54 |
| Other health-care professionals, home visit (per visit) | 29 | DHSC56 |
| NHS walk-in centre (per visit) | 54 | Curtis and Burns54 |
Medication costs
Medication data, excluding smoking cessation medication, were collected alongside the trial by self-report, including medication name, start date and end date. If participants were still taking the medication at the 12-month follow-up, an option of ‘ongoing’ was provided. Owing to the extreme complexity of the records, several simplifying assumptions were made. All medication usage was assigned to the corresponding period based on the following assumptions.
-
All medicines recorded, unless they were specified as not prescribed, were assumed to be on prescription.
-
Because the number of prescriptions and the amount taken were not recorded, it was assumed that one prescription would last a maximum of 1 month.
-
If the start date was missing, there was no reasonable way to make assumptions about usage. The cost of the medicine was, therefore, considered missing.
-
If the start date of a medicine was earlier than the quit date, we assumed that the patient had had one prescription of the medicine during the intervention period.
-
If a person was recorded as using a medicine at the 12-month follow-up, the cost of this was calculated for the period starting from the recorded start date to the 12-month follow-up date. Depending on the start date, it was then allocated to the intervention period, 6 months post quit date and 12 months post quit date. Otherwise:
-
If the end date was missing, the cost of the medicine was assumed to be missing at 6 months post quit date and 12 months post quit date, because there was no reasonable way to make assumptions about usage.
-
If the end date was not missing, the cost was calculated according to the start and end dates and then allocated to the corresponding period.
-
As smoking cessation medications specifically were recorded, we excluded these from this medication list. It was also assumed that the participants with no medication recorded, but who had been followed up, had not taken any medication during the trial period.
The costs of medications were estimated by matching their generic name to that in the Prescription Cost Analysis, England 201553 and extracting a weighted average cost per item for that generic medicine. The form and dosage were used when available, but most records did not contain this information and so average costs were used.
Outcome
The outcome used in the cost-effectiveness analysis was 6-month prolonged Russell Standard abstinence, which was the primary outcome of the trial. The 4-week quit rate and 12-month prolonged abstinence, measured according to the Russell Standard, were also used for the secondary analysis to provide comparison with national statistics. The methods of calculation were the same as for the primary statistical analysis.
Analysis
Handling missing data
Following the Russell Standard, it was assumed that people who were missing from follow-up were smoking. Missing data for health resource use costs were handled by multiple imputation using chained equations as the primary method. A set of imputation models was specified, one for each variable with missing data. Each variable was then regressed on to all other variables, including completely recorded variables, and stratified by randomised group. Imputations were performed using predictive mean matching with 10 nearest neighbours for all cost variables on an aggregated level instead of individual usage. For continuous variables, imputations were performed using linear regression. Logistic regression was used for binary variables. Ordered logistic regression was used for categorical variables when the order was meaningful. The analysis models were fitted to each imputed data set separately and the results were estimated using Rubin’s rules. 57
Base-case analysis
Combining the costs and outcomes, a cost-effectiveness analysis was performed at 6 months post quit date, producing an incremental cost-effectiveness ratio (ICER) in the form of the incremental cost per additional 6-month abstinence, comparing the intervention group with the control group.
Similar analyses were performed at the 4-week and 12-month follow-ups. The ICER measuring cost per participant who quit for 4 weeks could be used to compare the results of the intervention with national statistics on NHS Stop Smoking Services.
Sensitivity analyses
A non-parametric bootstrap resampling technique was adopted to assess the uncertainty around the primary analysis at 6 months post quit date. Bootstrapping has been proposed as an efficient approach for calculating the confidence limits for the ICER as its validity does not depend on any specific form of underlying distribution. 58–61 A cost-effectiveness acceptability curve (CEAC) was plotted based on the outcomes of the 5000 bootstrap iterations. 62
A complete-case analysis was performed on the participants who had complete data on costs at both baseline and 6 months’ follow-up, as well as on other baseline characteristics (age, gender, research centre, medical history). This was to examine the impact of multiple imputation.
Results
Costs
Intervention costs
The weighted average cost per patch for all 21-mg nicotine patches was £1.40, extracted from Prescription Cost Analysis, England 2015. 53 During the trial, some participants received lower-dosage (14-mg) patches at a later stage of the intervention period. The extracted weighted average cost per unit for all 14-mg nicotine patches was the same as for 21-mg patches. Hence, all nicotine patches dispensed were costed at the same price (£1.40), regardless of the dosage.
Behavioural support sessions, provided by the trial team at baseline and at –3 weeks, were estimated to last 10 minutes per session for both arms. Based on the NHS Stop Smoking Services’ costing report,52 a 30-minute individual session, delivered by a band 5 or 6 smoking cessation adviser, was estimated to cost £14.82. By inflating this cost to 2014/15 prices54 and allocating the cost to a 10-minute session, we estimated the cost per session in the trial at £5.42.
Booklets used in the trial were printed in house and no precise costs were recorded. For the NHS, printing services are either included in overheads or outsourced to professional printing companies. The cost per booklet was estimated to be £0.15, based on an academic institution’s print services price list. 63 However, the marginal cost of printing is likely to reduce as the amount of printing increases, so this estimate is probably higher than the cost that would be incurred by the NHS.
In total, the intervention cost amounted to £59 per participant in the intervention group and £11 per participant in the control group (Table 16).
| Cost item | Trial group, cost (£) | |
|---|---|---|
| Intervention (n = 899) | Control (n = 893) | |
| Booklet | 0.15 | 0.15 |
| Behavioural support (SD) | 11 (1) | 11 (1) |
| Nicotine patches (SD) | 48 (14) | 0 (2) |
| Total (SD) | 59 (14) | 11 (2) |
NHS Stop Smoking Services costs
Table 17 shows the utilisation and mean cost of standard NHS Stop Smoking Services behavioural support and pharmacotherapies before the quit date. Of the 813 responding participants in the intervention group, 98.4% indicated that they had received standard NHS Stop Smoking Services behavioural support. In the control group, 97.6% of 798 responding participants indicated that they had received standard NHS Stop Smoking Services behavioural support. A slightly higher proportion of responding participants in the intervention group than in the control group had taken some form of pharmacotherapy as a smoking cessation aid (83.8% of 717 vs. 80.3% of 722). Costs were similar between arms.
| Standard services of NHS Stop Smoking Services | Trial group | |
|---|---|---|
| Intervention | Control | |
| Behavioural support, n | 813 | 798 |
| Participants receiving support, % (n) | 98.4 (800) | 97.6 (779) |
| Mean cost (£) (SD) | 94 (12) | 94 (15) |
| Pharmacotherapies, n | 717 | 722 |
| Participants using pharmacotherapy, % (n) | 83.8 (601) | 80.3 (580) |
| Mean cost (£) (SD) | 30 (18) | 30 (19) |
Adverse event costs
During the trial, there was one SAE that was identified as possibly being related to the intervention, which occurred in the intervention group. The SAE was a heart attack that required hospitalisation, with the number of bed-days not recorded. The weighted average cost of all HRGs related to myocardial infarction (all complication and comorbidity scores) was included in the analysis to account for the adverse effect of the intervention. This cost was estimated to be £1469 per episode. 56
Health resource use costs
Health resource use was recorded in three sections of the CRF: (1) help for smoking cessation, (2) hospital visits and (3) primary and community services. The completeness of these three sections did not vary much at the same time point within arms, indicating that the missing values were probably the result of loss to follow-up. Tables 18–20 summarise the usage of each service and the mean cost of each cost item. The low mean usage and large SDs suggested the uneven use of services among participants in both arms. Although a small group of participants used these services frequently, the majority used them only a little. For instance, at baseline, the maximum number of visits to a GP in the previous 6 months was 26 in both arms. However, over half of the participants visited a GP only once or not at all (54% in the intervention group and 51% in the control group).
| Help sources | Time point, mean number of uses or cost (SD) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||||
| Intervention (n = 897) | Control (n = 889) | Intervention (n = 692) | Control (n = 700) | Intervention (n = 644) | Control (n = 643) | |
| GP | 0.08 (0.43) | 0.10 (0.53) | 0.06 (0.39) | 0.09 (0.50) | 0.06 (0.35) | 0.10 (0.60) |
| Practice nurse | 0.11 (0.75) | 0.07 (0.42) | 0.45 (1.42) | 0.40 (1.31) | 0.09 (0.65) | 0.10 (0.70) |
| Pharmacist | 0.07 (0.59) | 0.09 (0.64) | 0.14 (0.90) | 0.20 (1.11) | 0.09 (0.83) | 0.06 (0.51) |
| NHS smoking cessation support | 0.07 (0.57) | 0.07 (0.49) | 1.75 (3.11) | 1.60 (2.78) | 0.24 (1.42) | 0.21 (1.24) |
| NHS smoking helpline | 0.01 (0.09) | 0.01 (0.09) | 0.04 (0.34) | 0.01 (0.09) | 0.01 (0.12) | 0.00 (0.04) |
| Prescription of nicotine patches | 0.07 (0.45) | 0.10 (0.54) | 0.66 (1.60) | 0.63 (1.68) | 0.18 (1.08) | 0.13 (0.80) |
| Prescription of acute forms of NRT | 0.14 (.072) | 0.13 (0.71) | 0.98 (1.98) | 0.76 (1.88) | 0.22 (1.17) | 0.13 (0.83) |
| Prescription of bupropion | 0.00 (0.03) | – | 0.01 (0.18) | 0.02 (0.23) | 0.00 (0.08) | – |
| Prescription of varenicline | 0.06 (0.42) | 0.06 (0.40) | 0.60 (1.48) | 0.62 (1.43) | 0.11 (0.66) | 0.09 (0.54) |
| Cost (£) of smoking cessation help | 13 (47) | 14 (45) | 96 (122) | 89 (122) | 21 (81) | 18 (70) |
| Hospital visits | Time point, mean number of use per cost (SD) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||||
| Intervention (n = 896) | Control (n = 890) | Intervention (n = 690) | Control (n = 703) | Intervention (n = 647) | Control (n = 649) | |
| Accident and emergency visit | 0.11 (0.39) | 0.15 (0.47) | 0.17 (0.54) | 0.13 (0.42) | 0.09 (0.34) | 0.14 (0.63) |
| Emergency ambulance | 0.04 (0.24) | 0.07 (0.34) | 0.06 (0.31) | 0.05 (0.28) | 0.03 (0.19) | 0.08 (0.54) |
| Outpatient | 0.77 (2.84) | 0.92 (3.91) | 0.75 (2.44) | 0.80 (4.46) | 0.67 (2.42) | 1.09 (4.69) |
| Day case | 0.11 (1.02) | 0.09 (0.40) | 0.08 (0.51) | 0.10 (0.43) | 0.10 (0.44) | 0.11 (0.61) |
| Non-emergency ambulance | 0.00 (0.05) | 0.01 (0.16) | 0.01 (0.15) | 0.00 (0.08) | 0.01 (0.08) | 0.02 (0.27) |
| Patient transport services | 0.00 (0.09) | 0.10 (2.62) | 0.01 (0.09) | 0.16 (3.63) | 0.01 (0.19) | 0.11 (2.59) |
| Hospital stay (nights) | 0.23 (1.74) | 0.38 (2.58) | 0.36 (3.64) | 0.47 (3.32) | 0.18 (1.37) | 0.65 (7.40) |
| Cost (£) of hospital-related services | 359 (1645) | 489 (2097) | 446 (2782) | 544 (2700) | 296 (1170) | 722 (5570) |
| Services | Trial group, mean number of use per cost (SD) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||||
| Intervention (n = 894) | Control (n = 891) | Intervention (n = 689) | Control (n = 701) | Intervention (n = 645) | Control (n = 647) | |
| GP (in practice) | 2.18 (2.95) | 2.37 (3.08) | 1.99 (2.77) | 2.06 (3.34) | 1.95 (3.86) | 2.34 (3.88) |
| Practice nurse (in practice) | 0.86 (2.45) | 0.84 (1.54) | 0.98 (3.29) | 0.93 (2.50) | 0.69 (1.95) | 0.86 (2.60) |
| GP (home visit) | 0.01 (0.13) | 0.03 (0.40) | 0.01 (0.16) | 0.02 (0.19) | 0.01 (0.08) | 0.02 (0.31) |
| Practice/district nurse or health visitor (home visit) | 0.02 (0.37) | 0.14 (2.03) | 0.02 (0.61) | 0.20 (1.52) | 0.11 (2.49) | 0.29 (5.23) |
| Community psychiatric nurse (home visit) | 0.02 (0.33) | 0.07 (1.15) | 0.02 (0.35) | 0.03 (0.58) | 0.08 (1.24) | 0.01 (0.17) |
| Other health-care professionals (home visit) | 0.09 (1.22) | 0.10 (1.26) | 0.05 (0.67) | 0.07 (1.18) | 0.02 (0.22) | 0.05 (0.52) |
| NHS walk-in centre | 0.08 (0.40) | 0.09 (0.45) | 0.09 (0.36) | 0.08 (0.33) | 0.08 (0.31) | 0.11 (0.72) |
| Cost (£) of primary and community services | 100 (134) | 113 (164) | 94 (130) | 101 (154) | 91 (204) | 114 (221) |
The unadjusted mean cost of services among the available cases did not appear to be different between arms at the same time point, except for hospital-related services. The consistently higher mean cost of hospital-related services in the control group was likely to be driven by the longer hospital stays.
Medication costs
Based on the assumptions described earlier in Methods, over half of the participants were prescribed medication during the intervention period. Among the participants providing data, the majority were on medication at each time point (Table 21). The mean cost of medication did not differ much between arms.
| Variable | Time period | |||||
|---|---|---|---|---|---|---|
| Within intervention period (4 weeks) | Months 1–6 | Months 7–12 | ||||
| Intervention (N = 855) | Control (N = 835) | Intervention (N = 611) | Control (N = 570) | Intervention (N = 594) | Control (N = 565) | |
| Participants taking medication, % (n) | 62.3 (533) | 66.6 (556) | 56.5 (345) | 55.3 (315) | 54.2 (322) | 52.4 (296) |
| Mean cost (£) of medication (SD) | 30 (142) | 35 (111) | 140 (404) | |||
Analysis
Multiple imputation
Table 22 shows the missing data percentages for the variables included in the imputation model. Although the primary outcome time point was 6 months, in order to provide as much information as possible some baseline covariates and the 12-month variables are also included. As the highest percentage of missing data was 35.32%, the number of imputations was set to be 35. 64
| Variable | Number of missing values, n | Proportion of missing values, % |
|---|---|---|
| Gender | 2 | 0.11 |
| Age | 12 | 0.67 |
| Research centre | 0 | 0 |
| Medical condition at baseline | 4 | 0.22 |
| Longest abstinence in days | 0 | 0 |
| Strength of urge to smoke at baseline | 8 | 0.45 |
| Cigarettes smoked per day at baseline | 0 | 0 |
| Cigarettes smoked per day at 6 months | 460 | 25.67 |
| Cigarettes smoked per day at 12 months | 583 | 32.53 |
| Cost of standard NHS Stop Smoking Services behavioural support | 181 | 10.10 |
| Cost of standard NHS Stop Smoking Services pharmacotherapy | 353 | 19.70 |
| Cost of help for smoking cessation at baseline | 6 | 0.33 |
| Cost of help for smoking cessation at 6 months | 400 | 22.32 |
| Cost of help for smoking cessation at 12 months | 505 | 28.18 |
| Cost of hospital-related services at baseline | 6 | 0.33 |
| Cost of hospital-related services at 6 months | 399 | 22.27 |
| Cost of hospital-related services at 12 months | 496 | 27.68 |
| Cost of primary and community services at baseline | 7 | 0.39 |
| Cost of primary and community services at 6 months | 402 | 22.43 |
| Cost of primary and community services at 12 months | 500 | 27.90 |
| Cost of intervention | 0 | 0 |
| Cost of medication within intervention period | 102 | 5.69 |
| Cost of medication in months 1–6 post quit date | 611 | 34.10 |
| Cost of medication in months 7–12 post quit date | 633 | 35.32 |
| Smoking status according to Russell Standard at 4 weeks post quit date | 0 | 0 |
| Smoking status according to Russell Standard at 6 months post quit date | 0 | 0 |
| Smoking status according to Russell Standard at 12 months post quit date | 0 | 0 |
| Experience of SAEs resulting in hospitalisation | 0 | 0 |
| Cost of possibly related SAEs | 0 | 0 |
Base-case analysis
At 6 months post quit date, the intervention group had a higher proportion of participants remaining abstinent than the control group (17.5% vs. 14.4%). The difference between arms was 3.1 percentage points (95% CI –0.3 to 6.4 percentage points). The total costs at 6 months post quit date included the intervention costs, NHS smoking cessation support during the intervention period, health resource use costs and medication costs in the 6 months after the quit date. The breakdown of the results is shown in Table 23. Although the unadjusted mean total costs in the intervention group were lower than in the control group, after adjusting for health resource use costs at baseline, age, gender, research centre and presence of medical conditions at baseline, the mean total costs were £4 higher in the intervention group than in the control group (95% CI –£188 to £222). Combining the adjusted difference in mean total costs and difference in abstinence rate at 6 months post quit date, the ICER was calculated as £129 per additional 6-month abstinence. The bootstrapped CI for both the adjusted difference in mean total costs and the ICER showed a wide uncertainty.
| Variable | Trial group | |
|---|---|---|
| Intervention (n = 899) | Control (n = 893) | |
| Quit rate at 4 weeks post quit date according to Russell Standard, mean (95% CI), % | 36.3 (33.1 to 39.4) | 31.9 (28.9 to 35.0) |
| Difference in quit rate at 4 weeks post quit date according to Russell Standard, mean (95% CI), percentage points | 4.4 (–0.1 to 8.6) | |
| Abstinence rate at 6 months post quit date according to Russell Standard, mean (95% CI), % | 17.5 (15.0 to 20.0) | 14.4 (12.1 to 16.8) |
| Difference in abstinence rate at 6 months post quit date, mean (95% CI), percentage points | 3.1 (–0.3 to 6.4) | |
| Abstinence rate at 12 months post quit date, mean (95% CI), % | 14.0 (11.7 to 16.3) | 11.3 (9.2 to 13.4) |
| Difference in abstinence rate at 12 months post quit date, mean (95% CI), percentage points | 2.7 (–0.3 to 5.8) | |
| Health resource use cost in the 6 months prior to baseline, mean (SE), £ | 471 (56) | 615 (72) |
| Intervention cost (£), mean (SE) | 58 (0) | 11 (0) |
| NHS Stop Smoking Services cost (£) during the intervention period, mean (SE) | 124 (1) | 122 (1) |
| Smoking cessation cost (£) before quit date, mean (SE) | 182 (1) | 133 (1) |
| Difference in smoking cessation cost (£) before quit date, adjusted for age, gender, research centre and urge to smoke at baseline, mean (95% CI) | 49 (47 to 51) | |
| Health resource use cost (£) in the 6 months post quit date, mean (SE) | 640 (104) | 770 (106) |
| Medication cost (£) in the 6 months post quit date, mean (SE) | 154 (14) | 186 (19) |
| Cost (£) of possibly related SAEs, mean (SE) | 2 (2) | 0 (0) |
| Total costs (£) at 6 months’ follow-up, mean (SE) | 978 (105) | 1090 (110) |
| Adjusted difference in total costs (£) at 6 months,a mean (95% CI) | 4 (–188 to 222) | |
| Health resource use cost (£) from 6 months to 12 months post quit date, mean (SE) | 414 (46) | 804 (186) |
| Medication cost (£) from 6 months to 12 months post quit date, mean (SE) | 165 (22) | 188 (22) |
| Total costs (£) at 12 months’ follow-up, mean (SE) | 1557 (125) | 2082 (231) |
| Adjusted difference in total costs (£) at 12 months,a mean (95% CI) | –233 (–747 to 42) | |
| ICER (£) at 4 weeks post quit date, mean (95% CI) | 1114 (–2259 to 7439) per additional person who quits | |
| ICER (£) at 6 months post quit date, mean (95% CI) | 129 (–16,527 to 17,782) per additional abstinence | |
| ICER (£) at 12 months post quit date | Intervention dominates control (preloading being more effective and less costly) (–95,487 to 37,133) | |
In the national statistics for the NHS Stop Smoking Services,65 only the smoking cessation costs per person who quit were reported, including for behavioural support and pharmacotherapy if applicable. For the purpose of comparison, a smoking cessation cost was also calculated by adding the cost of the nicotine patches and/or the behavioural support cost to the NHS Stop Smoking Services cost incurred before the quit date. As expected, owing to the additional cost of the intervention, the intervention group had a higher mean cost than the control group (£182 vs. £133). The cost per participant who was proven to have quit at 4 weeks was £501 in the intervention group and £417 in the control group. The adjusted difference was almost the same as the unadjusted difference, with the intervention group costing £49 more than the control group. The incremental cost per additional participant who was proven to have quit at 4 weeks was, therefore, £1114.
At the 12-month follow-up, the difference between total costs was greater than at the 6-month follow-up, with the costs in the intervention group being much lower than those in the control group (£1557 vs. £2082). The intervention group had lower total costs, by £233, even after adjusting for baseline health resource use costs and other covariates. As abstinence was higher in the intervention group than in the control group (14.0% vs. 11.3%), the results demonstrated a dominance condition, in which the intervention group was less costly and more effective than the control group. Similar to the results at the 6-month follow-up, the bootstrapped CIs showed a wide range of uncertainty.
Sensitivity analyses
To explore the uncertainty surrounding the results, 5000 bootstrap iterations were used to plot the cost-effectiveness plane and CEAC. Figure 6 presents the cost-effectiveness plane, which shows a cluster of ICERs scattering across all four quadrants. Although the overall majority fell to the right side of the zero incremental abstinence rate line, indicating a more effective intervention, over half of those concentrated in the north-east quadrant, indicating a more expensive intervention.
FIGURE 6.
Cost-effectiveness plane of incremental costs and incremental abstinence rate at 6 months post quit date, comparing the preloading group with the control group.
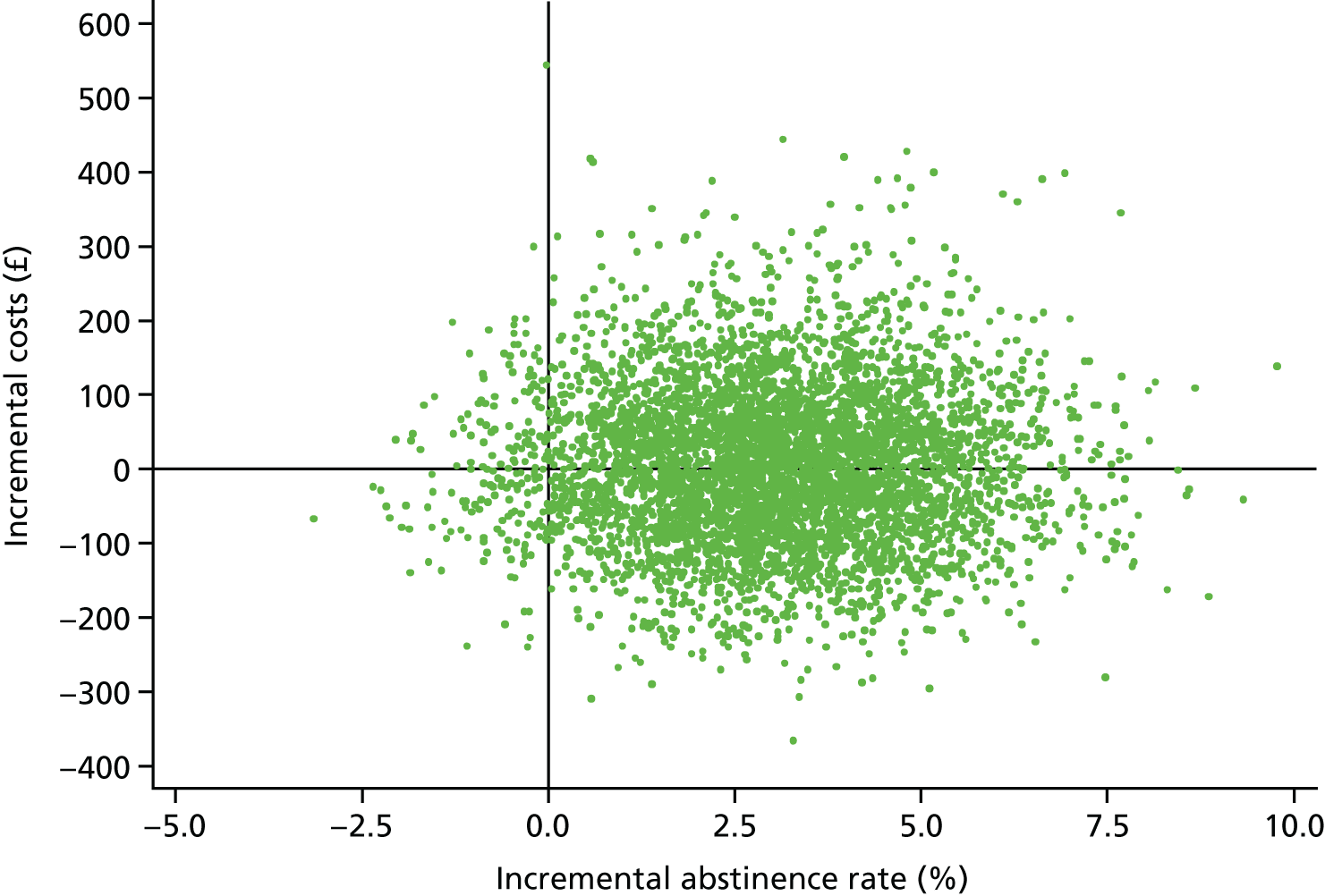
Figure 7 presents the CEAC to illustrate the bootstrapping iterations. The CEAC shows that the probability of preloading being cost-effective, compared with the control group, increased with the increase in costs (i.e. uncertainty of the results reduced). When the costs reached £3600, the probability of preloading being cost-effective was approximately 80%. For the probability to reach 90%, another £3300 would be required. Soon after this point, the curve became flat, stabilising at about 96%.
FIGURE 7.
Cost-effectiveness acceptability curve of the probability of preloading being cost-effective compared with the control group.
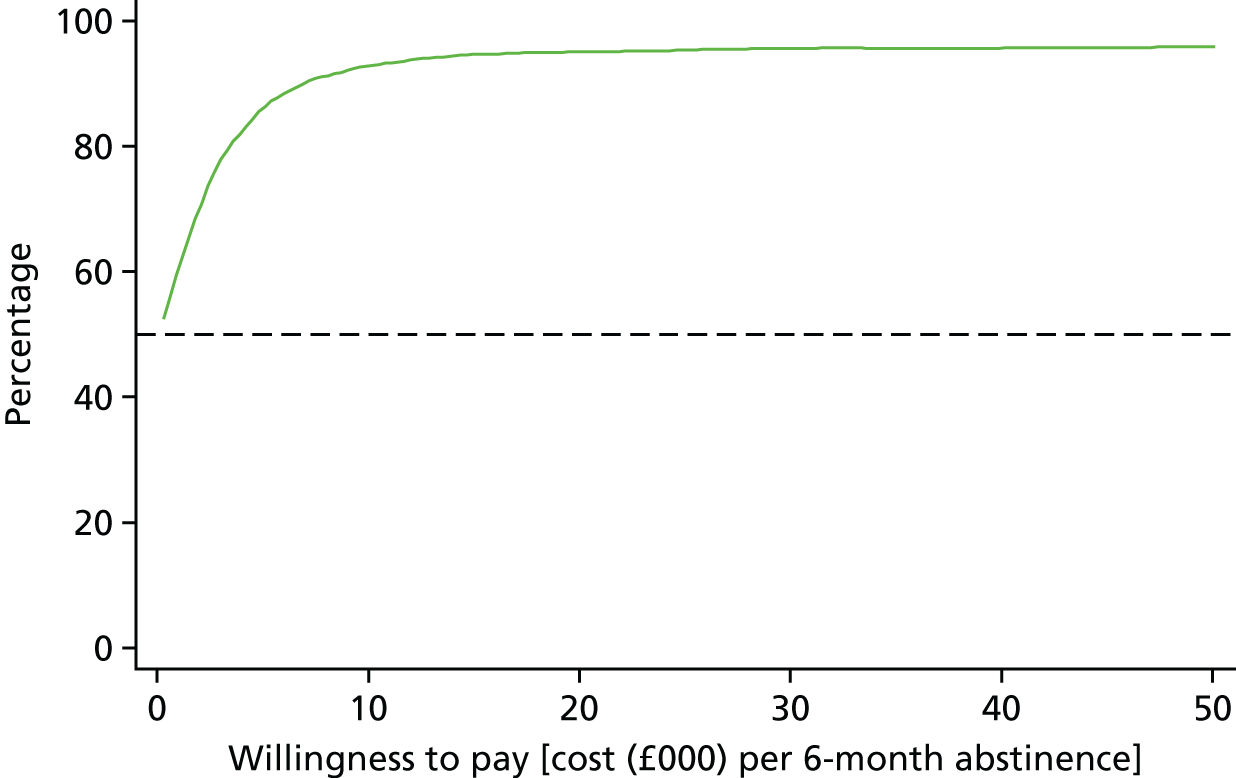
Complete-case analysis
To assess the impact of multiple imputation on the results, an analysis of the outcomes at the primary outcome time point (6 months post quit date) was conducted on complete cases only. In this analysis, only the participants who had complete data on costs both at baseline and at 6 months’ follow-up, as well as other baseline covariates, were included. Therefore, 462 participants in the intervention group and 426 participants in the control group were not included in the complete-case analysis.
Among the complete cases, the 6-month prolonged abstinence rate was 25.3% in the intervention group and 23.0% in the control group. The difference between arms was slightly smaller than in the imputed data set (2.3 vs. 3.1 percentage points). Although the health resource use cost in the 6 months prior to baseline was similar to that in the imputed data set, the health resource use cost in the 6 months post quit date was much lower than in the imputed data set. In the intervention group, it was £507 among the complete cases, compared with £640 in the imputed data set. The difference was more prominent in the control group, with a cost of £458 among the complete cases and £770 in the imputed data set. This led to a reverse of the relative position. In the imputed data set, the health resource use cost in the 6 months post quit date was higher in the control group, whereas among the complete cases it was higher in the intervention group. The only SAE was excluded in the complete case analysis because of missing data following that event. There was, therefore, no cost for the SAE incurred among complete cases. As a result, the adjusted difference in total costs at 6 months’ follow-up was estimated as £138, which is larger than in the imputed data set. The ICER was calculated as £6000 per additional 6-month prolonged abstinence, with a much wider CI (Table 24).
| Variable | Trial group | |
|---|---|---|
| Intervention (N = 462) | Control (N = 426) | |
| Number of 6-month prolonged abstinence cases | 117 | 98 |
| Abstinence rate (%) at 6 months post quit date, mean (95% CI) | 25.3 (21.4 to 29.5) | 23.0 (19.1 to 27.3) |
| Difference in abstinence rate (percentage points) at 6 months post quit date, mean (95% CI) | 2.3 (–3.3 to 8.0) | |
| Health resource use cost (£) in the 6 months prior to baseline, mean (SD) | 454 (1486) | 627 (2551) |
| Intervention cost (£), mean (SD) | 59 (12) | 11 (1) |
| NHS Stop Smoking Services cost (£) during the intervention period, mean (SD) | 126 (21) | 124 (23) |
| Health resource use cost (£) in the 6 months post quit date, mean (SD) | 507 (1432) | 458 (1430) |
| Medication cost (£) in the 6 months post quit date, mean (SD) | 153 (445) | 165 (608) |
| Cost (£) of possibly related SAEs | – | – |
| Total costs (£) at 6 months’ follow-up, mean (SD) | 844 (1548) | 758 (1641) |
| Difference in total costs (£), adjusted for baseline health-care services cost, age, gender, research centre and presence of medical conditions at baseline, mean (95% CI) | 138 (–13 to 323) | |
| ICER (£) at 6 months follow-up, mean (95% CI) | 6000 (–2440 to 1,276,267) per additional 6-month prolonged abstinence | |
As the smoking cessation costs before quit date did not differ much between the base case and complete cases, a comparison was made of health resource use costs. In addition, the health resource use cost among the available cases (i.e. the cost variable at the single time point is not missing) was also provided for reference (Table 25). It is apparent that the mean cost drawn from imputed data is closer to that of the available cases. This suggests that the complete-case analysis was likely to have underestimated the mean health resource use cost because of the requirement of completeness on multiple variables, especially for the control group at 6 months’ follow-up.
| Analysis | n | Intervention (£) | n | Control (£) |
|---|---|---|---|---|
| 6 months prior to baseline | ||||
| Complete | 462 | 454 | 426 | 627 |
| Available | 892 | 471 | 886 | 617 |
| Imputed | 899 | 471 | 893 | 615 |
| 6 months post quit date | ||||
| Complete | 462 | 507 | 426 | 458 |
| Available | 688 | 637 | 696 | 738 |
| Imputed | 899 | 640 | 893 | 770 |
Chapter 7 The modelled cost-effectiveness of nicotine preloading
Introduction
The objective of the current analysis was to compare the long-term cost-effectiveness of pharmacotherapy for 4 weeks prior to setting a smoking quit date with no pharmacotherapy before the quit date through extrapolation of the results of the clinical trial. The analysis was conducted using a modified version of the European-study on Quantifying Utility of Investment in Protection from Tobacco (EQUIPT) return on investment (ROI) tool. 66 At the core of the EQUIPT ROI tool is a validated economic model, which forecasts the short- and long-term outcomes for smokers who cease smoking within the next calendar year or who continue smoking. 67 The model allows the estimation of the impact of smoking cessation interventions based on these outcomes. As detailed below, the economic model has been modified slightly to allow the estimation of the comparative costs and effectiveness of the interventions within the current clinical trial, which compared the use of nicotine patches for 4 weeks prior to an attempt to quit smoking with no use of pharmacotherapy prior to the quit attempt.
Methods
Clinical trial
The clinical trial compared treatment with nicotine patches for 4 weeks prior to a planned quit date (intervention) with no pre-treatment prior to a planned quit date (control). Both the intervention and control groups also received standard behavioural counselling prior to the quit date. Biochemically validated 12-month abstinence was achieved by 14.0% of the intervention group and 11.3% of the control group. In the primary analysis, this gave an unadjusted OR of 1.24 (95% CI 0.97 to 1.58; p = 0.086) and a RD of 2.71 (95% CI –0.37 to 5.78; p = 0.085). As expected, there was an imbalance between arms in the frequency of use of varenicline for post-cessation medication; adjustment for this gave an OR of 1.30 (95% CI 1.02 to 1.66; p = 0.036) and a RD of 3.31 (95% CI 0.22 to 6.39; p = 0.036) between the intervention and control groups.
Economic model
A Markov model (EQUIPTMOD), developed as part of EQUIPT, was used to estimate the long-term costs and effects of using NRT pre-treatment compared with no pre-treatment in a hypothetical cohort of smokers with the same age and sex distribution as in the trial, who make a one-time attempt at quitting smoking. EQUIPTMOD allows the determination of the impact of either continuing or stopping smoking on both morbidity and mortality outcomes and costs. In this chapter, we estimate the lifetime costs, QALYs and life-years associated with NRT pre-treatment compared with no pre-treatment.
Methods
Design of the model
There are three primary states within the model: (1) current smoker, (2) former smoker and (3) dead (Figure 8).
FIGURE 8.
Model schematic.
With each 1-year cycle, the cohort is subjected to a set of transition probabilities that allow them to either stay within their current state or move to one of the other two states. Death is an absorbing state, meaning that those who enter this state remain within the state. We ran the model until participants reached the age of 100 years or died.
The model is replicated for different population cohorts based on the age and sex distribution of patients within the clinical trial. To obtain trial population-level estimates, these cohort-level estimates are weighted by the percentage of the trial population falling into each age and sex cohort.
To estimate the impact of the intervention on costs and smoking-related outcomes, two separate models were created: one that simulates a cohort of smokers who quit in the first year and one that simulates a cohort of smokers who do not quit in the first year. The results of the two models are combined by weighting the outputs by the trial population and the effectiveness of the intervention and the control.
Transition probabilities
Transition probabilities incorporated within the model include:
-
the probability of quitting smoking in the first cycle (year) based on the effectiveness of the intervention and the control within the trial
-
the age- and sex-specific annual probability of mortality associated with current smoking and being a former smoker
-
the probability of smoking cessation and relapse over the long term in current and former smokers.
The probabilities of smokers successfully quitting in the first year after the intervention were based on the biochemically validated 12-month abstinence rates from the clinical trial. Based on this measure, 14.0% of those in the intervention group and 11.3% of those in the control group were abstinent at 12 months. In the base-case analysis, the 12-month abstinence rate was assumed to be predictive of long-term cessation. This was tested within sensitivity analyses through applying a rate of relapse of 30% in the second year after the intervention. 68 Smokers who did not quit smoking, or who relapsed, were subjected to the same background quit rate in all subsequent years, which is reflective of the balance of quitting and relapsing over a lifetime. 69
The probability of death by age, sex and smoking status was calculated using the attributable risk of death based on smoking status. The following was required for this calculation: age- and sex-specific probabilities of death in the general population; the age- and sex-specific prevalences of smoking status (current, former or never smokers); and the RR of death by smoking status. Mortality rates were based on actuarial life tables and the age- and sex-specific prevalences of smoking were derived from the survey data. 70,71 The RRs of death for former and current smokers compared with never smokers were derived from published data19 (see Appendix 1, Table 29).
Long-term cessation and relapse were modelled through the application of an underlying quit rate, which applies to all age- and sex-specific cohorts after the first year. This was estimated at a value of 2% per annum and represents the balance between those quitting smoking each year and those who start smoking or relapse. 69
Prevalence of smoking-related diseases
The smoking-related population attributable fraction relating to current and former smokers for smoking-related diseases was estimated based on epidemiological data. The model estimates, for each cycle, the prevalence of smoking-related diseases based on the proportion of the cohort who are either current smokers or former smokers.
Four diseases, lung cancer, coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD) and stroke, were found to be responsible for the majority of the smoking-related disease burden and were, therefore, incorporated within the model. 72,73 The number of cases each year of these diseases was derived through calculation of the attributable fraction based on the prevalence of smoking-related diseases in England, the prevalence of smoking status in England (current, former or never smokers) and the relative effect of smoking on the prevalence of each disease49,74–78 (see Appendix 1, Tables 30–36).
Costs
Within the model, costs comprised both the smoking cessation costs of the intervention and control arms of the clinical trial and the health-care costs associated with smoking-related diseases.
Prior to the planned quit date, both the control group and the intervention group received standard behavioural counselling. In addition, those in the intervention group also received 4 weeks of NRT. Based on the within-trial analysis, this resulted in a mean cost per person of £58 [standard error (SE) £0] in the intervention group and £11 (SE £0) in the control group. Furthermore, prior to the quit date, both treatment arms received NHS Stop Smoking Services, with a mean cost per person of £124 (SE £1) in the intervention group and £122 (SE £1) in the control group. The cost of the NHS Stop Smoking Services accessed over the 12 months after the quit date was also included in the analysis: £133 (SE £6) for the intervention group and £103 (SE £5) for the control group, based on the within-trial analysis. This resulted in total costs overall for smoking cessation of £295 (SE £6) for the intervention group and £236 (SE £6) for the control group.
The annual costs for an individual with each of the diseases were estimated based on a systematic literature review. Costs relate to prevalent cases of disease. The annual cost for COPD is £1042.57, for lung cancer is £7002.65, for CHD is £1247.79 and for stroke is £5886.6679–82 (see Appendix 1, Table 36).
All costs were inflated to the year 2016 using the Hospital and Community Health Services Index83 (see Appendix 1, Table 37).
Utility values
Utility decrements associated with smoking-related diseases were derived from the US Medical Expenditure Panel Survey. 84 Utility values based on current smoking status (current or former smoker) were derived from the Health Survey for England adjusted for relevant covariates including disease prevalence. 85 The EuroQol-5 Dimensions, a validated instrument for measuring health-related quality of life, was used for both sets of utility values and UK tariffs were used for calculating utility scores86 (see Appendix 1, Table 38).
Outcomes
The costs, life-years and QALYs associated with both the intervention and the control groups were estimated over the lifetime of the cohort. In the base case, both costs and effects were discounted at a rate of 3.5% per annum as per NICE guidance. 87 A 1.5% per annum discount rate for costs and outcomes was tested in the sensitivity analyses. The analysis was conducted from the perspective of the NHS.
The incremental cost–utility ratio, expressed as the incremental cost per QALY gained, was reported to assess the cost-effectiveness of the intervention group compared with the control group.
Key assumptions
As is the case with all models, a number of assumptions were required to estimate the economic impact of tobacco-control interventions; these have been detailed in a previous publication67 and are summarised in Box 1.
The population-based mortality rates are adjusted using the RRs of death in smokers and former smokers, which are derived from the literature. 19 Although the reference is dated and absolute mortality may have changed, the assumption that the relative effect was likely to be maintained, and the choice of study, was justified based on the prospective nature of the study, the sample size (n = 34,439) and the years of follow-up (40 years).
The current model does not explicitly adjust for the time since quitting because of the absence of distributional data regarding time since quitting; duration of smoking; and the risk of smoking-related disease and mortality. Rather, an average risk of smoking-related disease and mortality is applied to former smokers. As this is a cohort rather than an individual patient simulation model, the impact of this assumption may be limited.
Given the lack of data to support an alternative assumption, the prevalence of each disease is assumed to be independent of the prevalence of other diseases. Also, the model assumes that, in the case of multiple diseases, the disutility associated with the disease with the greatest disutility is applied. This is a conservative approach in that it provides a lesser estimate of the QALY gains from smoking cessation than either a multiplicative or an additive approach.
For all diseases, in people aged < 35 years, the risk of smoking-related disease was assumed to be equal across smoking groups. This was deemed to be the most appropriate assumption given that data regarding the differential rate of all diseases by smoking status were not available for this age group. This assumption is both conservative and, given the very low prevalence of disease in this group, unlikely to have a significant impact on the results.
The underlying quit rate, which applies to all cohorts after the first year, represents the balance between those who quit smoking each year and those who start smoking or relapse. This assumption is supported by a meta-analysis, which showed that there was no difference in relapse rates after 12 months, regardless of whether the patients used or did not use an intervention to quit smoking. 68
Handling uncertainty
The effect of parameter uncertainty on the calculated outcomes was assessed through probabilistic sensitivity analyses involving Monte Carlo simulation. 88 Standard probability distributions were included for the natural history parameters, RRs and ORs, costs and utilities. Beta distributions were used for transition probabilities and utility values by smoking status, normal distributions were used for utility decrements due to disease, log-normal distributions were used for RRs and ORs and gamma distributions were used for costs. Population-level data were assumed to be fixed. Distribution parameters can be found in Appendix 1, Tables 35–38. The background quit rate was parameterised using a beta distribution. As distributional data were not available we assumed a conservative sample size of 50 for the parameterisation.
A set of 5000 outcome estimates were obtained, with the results displayed using a scatterplot of incremental costs versus QALYs and a CEAC that graphically presents the probability that the intervention is cost-effective as a function of the willingness of decision-makers to pay for an outcome (QALY). 88
Results
Base-case analysis
The total lifetime costs within the intervention group were £13,111 and within the control group were £13,177 (a difference of £66). The costs associated with smoking cessation, which included both behavioural and pharmacological interventions, were higher in the intervention group (£295) than in the control group (£236; a difference of £59). Health-care costs associated with smoking-related diseases over a lifetime, however, were lower in the intervention group (£12,816) than in the control group (£12,941; a difference of £125) (Table 26).
| Variable | Trial group | Difference (intervention – control) | |
|---|---|---|---|
| Intervention | Control | ||
| Costs (£) per smoker | |||
| Smoking cessation costs | 295 | 236 | 59 |
| Health-care costs | 12,816 | 12,941 | –125 |
| Total costs | 13,111 | 13,177 | –66 |
| Effectiveness | |||
| Quitters per 1000 smokers | 139 | 112 | 27 |
| Life-years per smoker | 17.977 | 17.957 | 0.020 |
| QALYs per smoker | 14.300 | 14.267 | 0.033 |
| Cost-effectiveness | |||
| ICER | Intervention is dominant | ||
A greater number of individuals quit smoking in the intervention group than in the control group (139 per 1000 smokers vs. 112 per 1000 smokers, a difference of 27 individuals). The greater number of individuals quitting smoking in the intervention group resulted in more discounted life-years lived in the intervention group than in the control group (17.977 life-years vs. 17.957 life-years) and more QALYs (14.300 vs. 14.267). The intervention is dominant over the control as the intervention was both more effective, producing both more QALYs and more life-years, and less costly.
Additional analysis
An analysis was conducted adjusting for an imbalance in the use of varenicline post quit date within the two groups (Table 27). The adjusted RD for cessation at 12 months was 3.31 (95% CI 0.22 to 6.39), compared with a RD of 2.71 (95% CI –0.37 to 5.78) for the base case. The adjusted difference in smoking cessation costs was £63 (95% CI £52 to £78), compared with £59 (95% CI £44 to £74) in the base case.
| Variable | Trial group | Difference (intervention – control) | |
|---|---|---|---|
| Intervention | Control | ||
| Costs (£) per smoker | |||
| Smoking cessation costs | 299 | 236 | 63 |
| Health-care costs | 12,787 | 12,941 | –153 |
| Total costs | 13,086 | 13,177 | –90 |
| Effectiveness | |||
| Quitters per 1000 smokers | 145 | 112 | 33 |
| Life-years per smoker | 17.982 | 17.957 | 0.025 |
| QALYs per smoker | 14.307 | 14.267 | 0.040 |
| Cost-effectiveness | |||
| ICER | Intervention is dominant | ||
After adjustment for varenicline imbalance, there were 33 more people who quit in the intervention group than in the control group; this is six more people who quit than in the unadjusted analysis. The difference in total costs was also greater, with the intervention costs being £90 lower than the control costs. The intervention also resulted in more QALYs (a difference of 0.040) and life-years (a difference of 0.025) than the control. As in the base-case analysis, the intervention is dominant over the control as it is both less costly and more effective.
Deterministic sensitivity analysis
The cost-effectiveness results were robust to an alternative discount rate of 1.5% per annum for costs and outcomes, with the intervention remaining cost saving (£19,273 for the intervention group vs. £19,384 for the control group) and resulting in more QALYs (19.091 for the intervention group vs. 19.043 for the control group) (Table 28).
| Scenario | Trial group | Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|---|
| 1.5% discount rate | Control | 19,385 | 19.043 | Reference | Reference | |
| Intervention | 19,273 | 19.091 | –111.72 | 0.049 | Intervention is dominant | |
| 30% relapse | Control | 13,333 | 14.226 | Reference | Reference | |
| Intervention | 13,303 | 14.250 | –29.02 | 0.023 | Intervention is dominant |
With a relapse rate of 30% after the first year, the cost difference between the intervention group and the control group was reduced, as were the QALY and life-year differences. However, the intervention remained less costly and more effective than the control and is, therefore, still dominant over the control (Table 28).
Probabilistic sensitivity analysis
As can be seen from the scatterplot of incremental costs compared with incremental QALYs (Figure 9), the majority of the estimates (80%) are within the lower right quadrant, indicating that the intervention is dominant over the control. As is evident from the CEAC (Figure 10), at a willingness-to-pay (WTP) threshold of £20,000 per QALY there is a 94% probability that the intervention is cost-effective. There is a 95% probability that the intervention is cost-effective at a WTP threshold of £30,000 per QALY.
FIGURE 9.
Scatterplot of incremental costs vs. incremental QALYs for the intervention group vs. the control group.
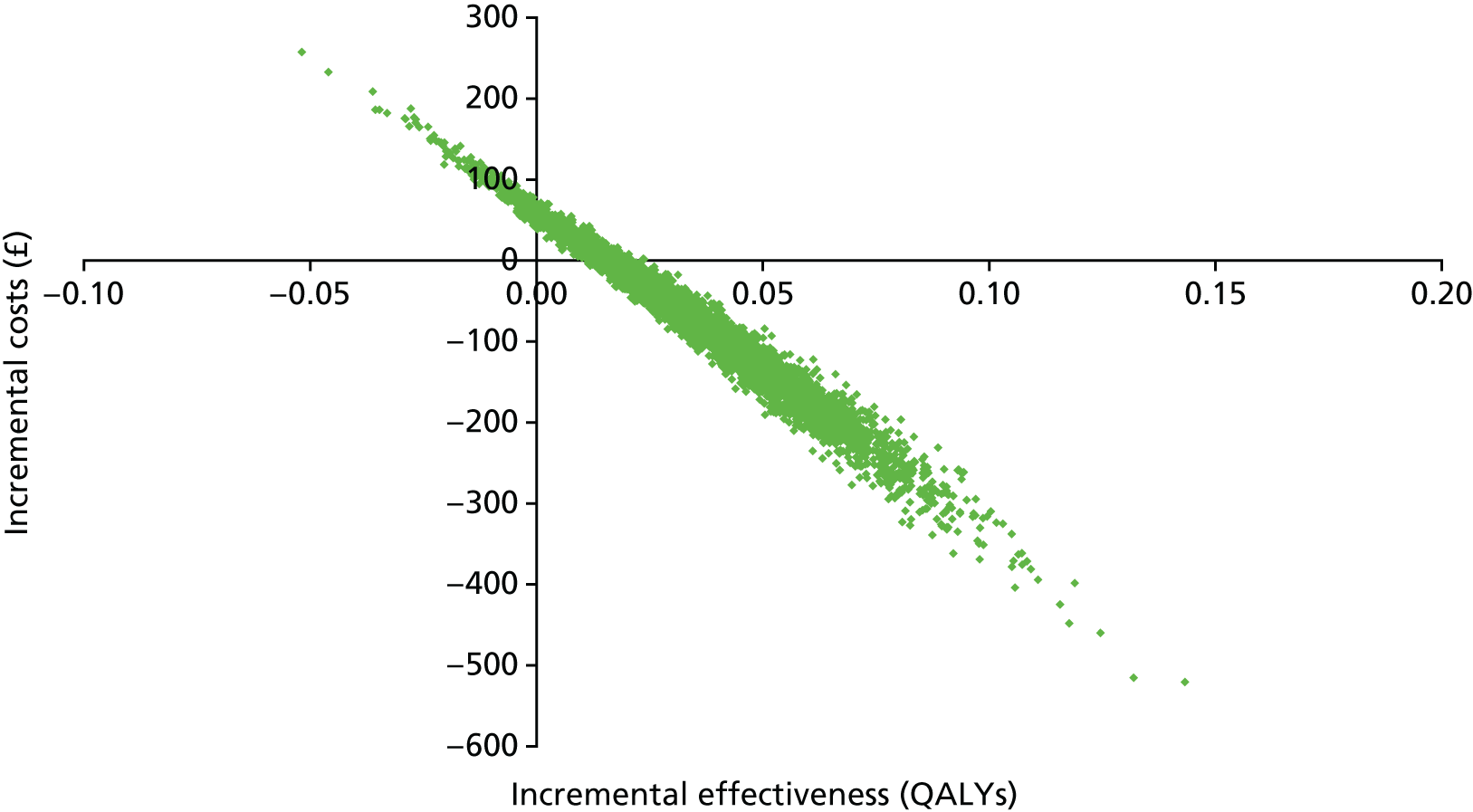
FIGURE 10.
Cost-effectiveness acceptability curve for the probability that the intervention is cost-effective with a relapse rate of 0% after year 1.
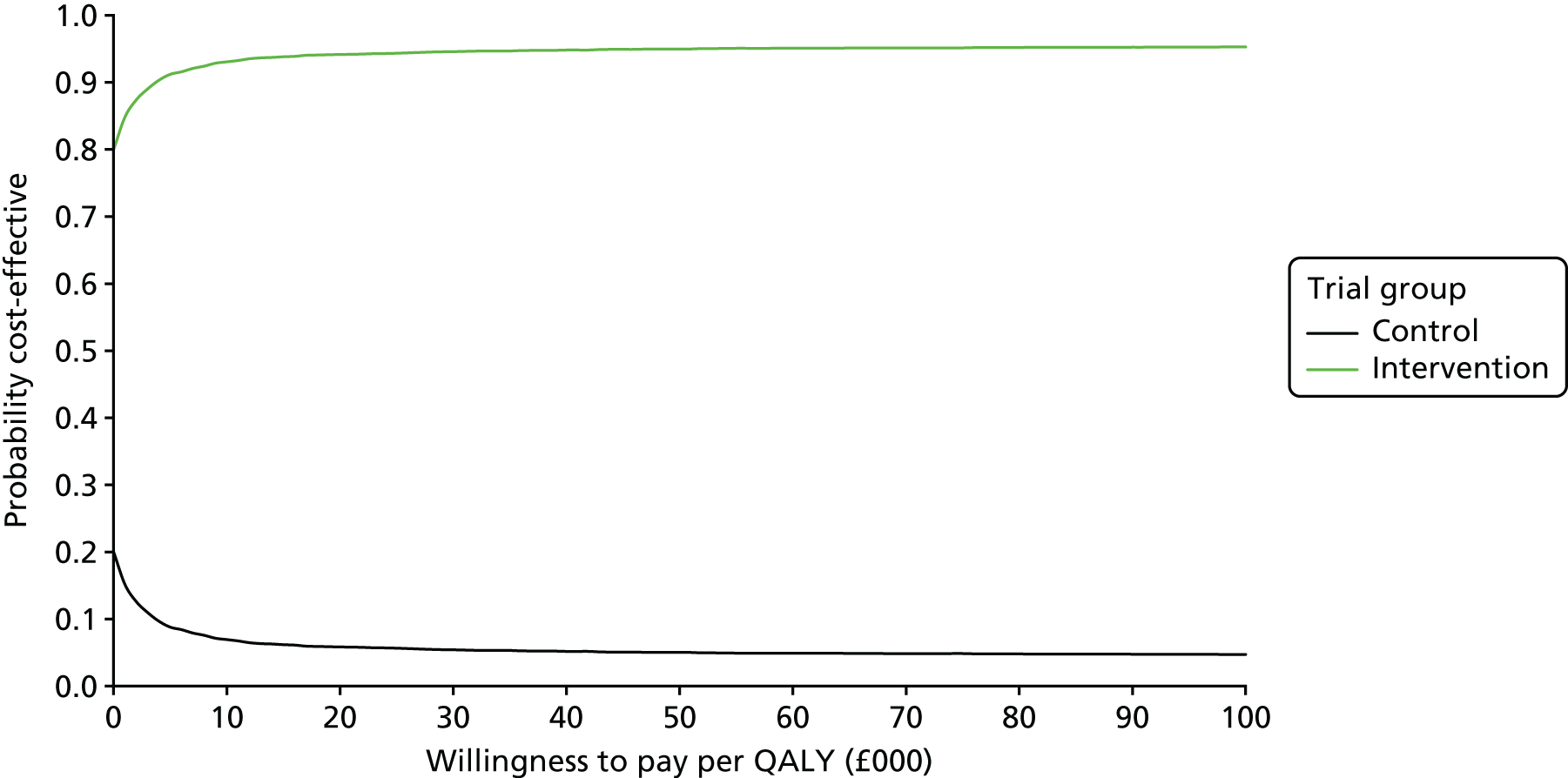
If 30% of smokers who quit in the first year are assumed to relapse, there is a 93% probability that the intervention is cost-effective at a WTP threshold of £20,000 per QALY and a 94% probability that the intervention is cost-effective at a WTP threshold of £30,000 per QALY (Figure 11).
FIGURE 11.
Cost-effectiveness acceptability curve for the probability that the intervention is cost-effective with a relapse rate of 30% after year 1.
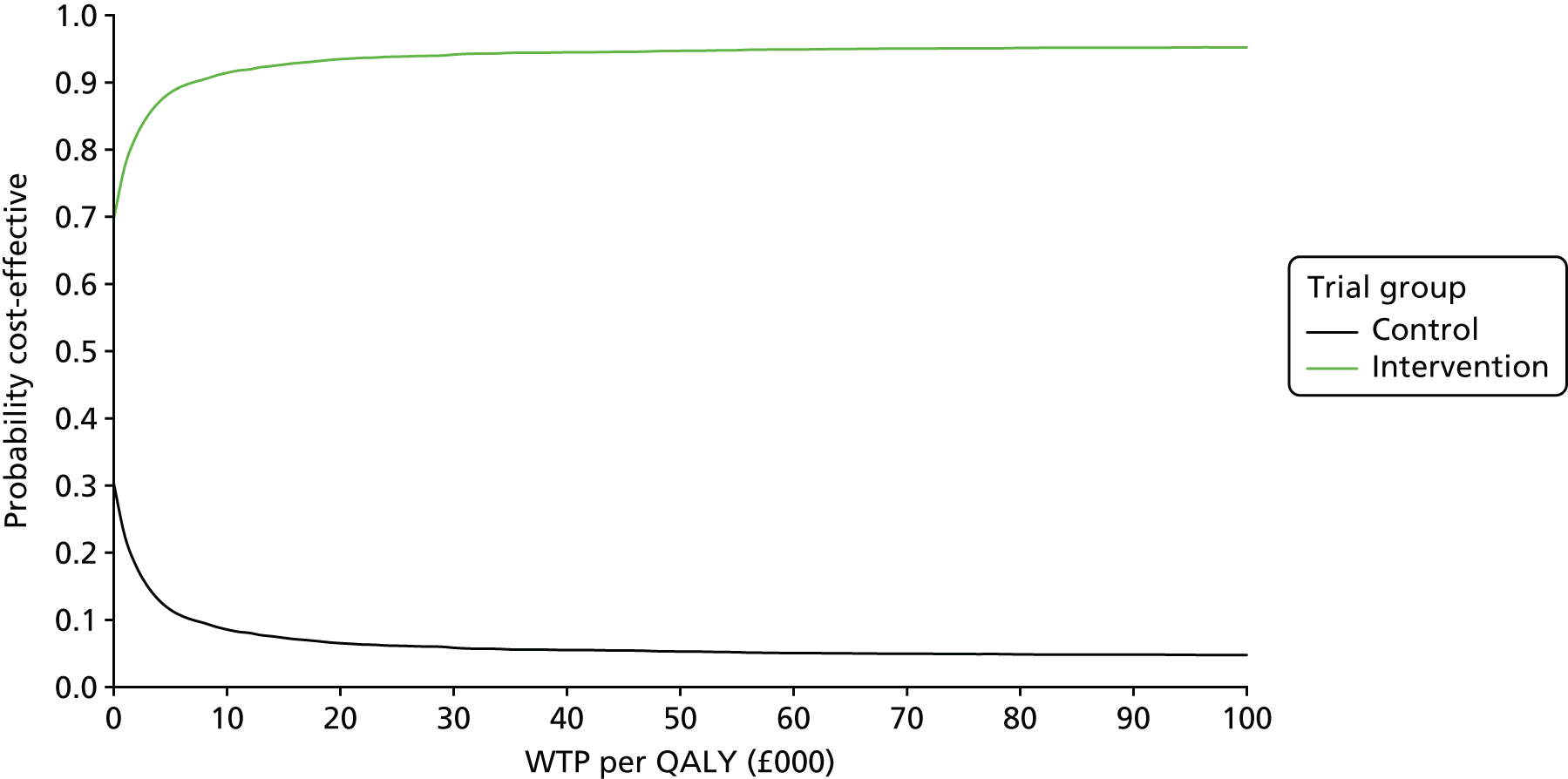
Chapter 8 Discussion and conclusions
Effectiveness of preloading
Summary of main findings
In this pragmatic open-label trial testing the effect of nicotine preloading for 4 weeks prior to a quit day, there was no evidence that preloading increased the proportion of people achieving smoking abstinence. Adherence to the nicotine patch used for preloading was good, with the large majority using treatment as prescribed. Preloading was tested in a clinical setting where smokers could opt to use either NRT or non-nicotine pharmacotherapies for continued cessation treatment after preloading had ended. Preloading encouraged the use of NRT for cessation treatment or deterred the use of varenicline as post-quit day medication. Varenicline is more effective than other medications, so in a planned analysis we adjusted for its use after quit day. This led to clear evidence that preloading itself did increase the likelihood of achieving abstinence. There was no evidence that use of varenicline undermined the effect of preloading. Most people continued with the preloading treatment, implying that it is well tolerated. Around 1 in 20 people suffered AEs of moderate severity and above as a consequence of the treatment, principally nausea. There was no evidence of an excess of SAEs.
Public involvement
The study was run under the auspices of the UK Centre for Tobacco and Alcohol Studies (UKCTAS). 89 UKCTAS has a standing panel of public members who guide the research programme, helping select topics that matter to people who smoke and shaping the way that research is carried out. Before the study started, we discussed the study and the methods with the panel. One member of the panel joined the trial steering committee that provided oversight to the trial.
In the early days of the study, we encountered a much slower rate of recruitment than we had anticipated. We used the method of recruitment that we had employed in three of the four centres in previous trials. 17,32,36 In the past, around 5–10% of people who received an invitation letter from their GP had enrolled in our studies. As in this trial, in the previous trials the invitation letter invited people wanting to stop smoking to seek support, rather than emphasising the novel treatment. We know from discussions with previous participants that it is the opportunity to receive support to quit that is the main draw for potential participants. In the Preloading trial, only around 1% of people who were invited enrolled in the trial. We asked GPs to write to batches of participants, 100 at a time, to ensure that we had a reasonable number of initial appointments to fill the clinic but did not generate a waiting list. However, initial recruitment was much slower than anticipated and, therefore, accrual into the study was much slower than planned.
As a consequence, we consulted with eight members of the UKCTAS panel to review our recruitment material and two other members of the public from the Clinical Research Network. They commented on the documents and answered questions about our material. Our recruitment material in the Preloading trial, as in previous trials, was very brief, which is probably important in attracting people who smoke. Consequently, only a few minor amendments were suggested, which we implemented after approval from the ethics committee. However, the response to the recruitment letter remained as poor as it had been. In fact, the Preloading trial coincided with a downturn in interest in NHS Stop Smoking Services and a rise in the use of e-cigarettes; it is thought that these two factors may be linked. We enhanced our recruitment rate by sending more letters per practice in larger batches and using Docmail® (CFH Docmail Ltd, Radstock, UK) to send the invitations, easing the burden on general practices. We also added other means of recruitment including text messages from practices and advertising.
At the time of writing, the results of this trial have only just become available. We plan to return to the UKCTAS panel with the results and discuss them with the members. To help guide implementation into policy and practice, it will be important to obtain the public’s views on what the results mean and what would make a person who wants to stop smoking take up preloading.
Strengths and limitations
This trial has several strengths. The trial had power to detect the modest benefits that may have been expected, given the previous meta-analyses. 12 It was conducted with rigour, with a prespecified protocol and analysis plan; it followed standards set out in CONSORT (Consolidated Standards of Reporting Trials) guidelines90 and achieved high follow-up rates compared with many trials of smoking cessation interventions, increasing confidence in the findings. It is the first trial of preloading to assess AEs to standards set out in Good Clinical Practice. In an open-label trial such as this, it is non-intuitive, both to participants and to trial staff, to report and enquire about AEs occurring in people on no medication. We trained our staff to enquire in the same way regardless of trial group, but it remains possible that the open-label design may have led to over-reporting of AEs in the preloading group. By also actively soliciting reports of likely AEs by questionnaire in all participants, we may have removed some of this bias. However, both methods revealed a somewhat similar profile, with nausea being the most common adverse consequence of preloading specifically. Patients also reported other well-established AEs of using a nicotine patch relating to its stimulant (poor sleep and dreaming) and adhesive (skin irritation) properties, but these were uncommon. However, taken together, both methods showed that severe AEs are rare and that the medication is well tolerated. A further strength of the study is that we collected data on potential mechanisms of action of preloading; these are addressed in Chapter 4.
The open-label design could be viewed as a limitation. Most notably, the open-label design led to 29.5% of participants in the control group choosing to use varenicline for post-quit day medication compared with 22.1% in the preloading group. This is a modest absolute difference, but, in this study, use of varenicline more than doubled the odds of achieving the primary outcome compared with the use of an alternative pharmacotherapy (chiefly NRT) and, in our pre-stated analysis, adjustment for this difference led to a statistically significant effect of preloading on the primary and secondary outcomes. There were also more people in the preloading group who used nicotine patches only, possibly because they received 5 weeks’ supply of the medication. The two study arms also had different expectations, which may have affected their smoking behaviour and their responses to various subjective ratings meant to assess the effects of preloading on smoking reward, enjoyment and urges to smoke. A blinded design would probably have shown more clearly an effect of preloading on smoking abstinence and would allow an unbiased analysis of mediators.
The open-label design could be seen as a strength because it produced an outcome that would not have been detected in a blinded design. If patch preloading reduces the use of varenicline, this would suggest that, within the NHS Stop Smoking Services, preloading is an efficacious strategy, but not an effective one, because preloading would be used in an open-label manner in that context. It is unclear, however, whether the difference in varenicline use arose because patients who were happy on NRT opted to continue it or because smoking cessation therapists or prescribers (mainly GPs) were unhappy to prescribe varenicline to someone already using NRT. We sought to counter the latter effect in discussions and with a letter of referral from the research team to the NHS Stop Smoking Services, which took on the pre- and post-quit support and prescribing. However, we know of several instances where prescribers could not be persuaded that co-prescribing the medications was safe, as NICE guidance was viewed as ‘trumping’ this. Revised NICE guidance could easily remove the apparent prohibition on concurrent use. If the difference was due to patients feeling content with patches and disinclined to add another medication, informing patients that adding varenicline improves outcomes would be another potential remedy.
Thus, the open-label design may be viewed as a weakness because it clouded the answer to the scientific question of efficacy and mechanisms of action of preloading, and as a strength because it revealed the effect of preloading as it would play out in the current UK NHS or similar health systems. That said, it is likely that we would be able to overcome the effect on use of varenicline subsequently through changes to guidelines and discussion with patients.
Comparison with other literature
Our previous meta-analysis yielded a RR of 1.16 (95% CI 0.97 to 1.38) for prolonged abstinence, but a RR of 1.26 (95% CI 1.03 to 1.55) for nicotine patches. 12 In this trial, the RR for the primary outcome (also prolonged abstinence) was very similar, at 1.27 (95% CI 1.03 to 1.57), in the analysis adjusted for the use of varenicline. The previous meta-analysis of nicotine patches alone was from an exploratory subgroup analysis and the main analysis showed no evidence of effectiveness, with a RR of 1.16 (95% CI 0.97 to 1.38). Thus, compared with the current literature, the trial has strengthened the evidence for the efficacy of preloading. However, our trial is the only trial to be carried out in a context in which the therapists who prescribed the preloading (our trial team) were different from those who prescribed the post-cessation support (the NHS Stop Smoking Service). Arguably, the context of this trial is more likely to reflect that of most health systems, where there is less behavioural support and dedicated smoking cessation expertise available to people who want to stop smoking than has been the case in the previous efficacy trials, thus revealing an effect that could negatively affect the impact of preloading.
One other factor also deserves attention. In this trial, we asked centres to record the proportion of people who did not make a quit attempt. We were concerned that deferring a quit attempt may mean that a person loses motivation to make one, thereby undermining the effect of preloading. This variable was incompletely recorded by centres, and it was clear that practice with respect to recording also differed in a way that makes relying on this variable unwise. From cross-tabulating other relevant data recorded, it appears that 112 (6.3%) people did not make a quit attempt: 60 (6.7%) of these were in the preloading group and 52 (5.8%) were in the control group. As the proportion was similar in both arms, this is not an effect of preloading per se, but it could reflect the fact that participants waited 4 weeks to quit smoking and we know that plans to quit smoking change quickly. 91 Not many smoking-related trials record the proportion of participants who do not make attempts to quit smoking. However, some of our previous trials have recruited in largely similar ways to this trial and, in these trials, 3% and 12% made no attempt to quit smoking, with a much shorter delay (1–2 weeks and 1 day) between trial enrolment and the target quit day. 33,36 A more recent trial reported that 17% of people who had undertaken 2 weeks of preloading failed to make a quit attempt, but this was defined as not achieving 24 hours of abstinence after quit day. 17 Evidence suggests that around half of all quit attempts do not achieve this milestone, meaning that perhaps only 8–9% failed to make a quit attempt. 92 Taken with these other trials, it appears that preloading and the necessary delay in making a quit attempt is not deterring people from following through with that attempt.
Interpretation
This trial has clarified the role of preloading in the treatment of tobacco dependence. Preloading is an efficacious strategy that could lead to around 3% of people who are seeking help to quit smoking achieving 12-month prolonged abstinence, who might not otherwise have done so. This effect may seem small, but the current 12-month abstinence rate in the UK specialist Stop Smoking Services is 8%93 and so an additional 3% would represent a worthwhile improvement. A failed quit attempt is likely to cost a person about 3–5 years of life expectancy. 34 Thus, around 12 people need to use preloading to gain around 1 year of life. However, within the specific setting of the NHS Stop Smoking Services, without changing NICE guidelines and informing advisors, the efficacy of preloading could be undermined by the effect it has on deterring use of the most effective cessation medication (varenicline) and, possibly, by delaying quit attempts in those motivated to quit immediately. Thus, it seems sensible to confine it as a strategy for use in highly controlled circumstances. If used in NHS Stop Smoking Services, we would recommend that NHS advisors discuss the choice of post-cessation treatment at the start of any period of preloading so that experience of preloading does not deter the use of varenicline.
Our study is not the only study that has produced observational evidence of the superiority of varenicline in routine NHS practice. The recent HTA-funded ELONS (Evaluating Long-term Outcomes of NHS Stop Smoking Services) study showed that the OR for use of varenicline compared with non-use of varenicline was 1.7 (95% CI 1.3 to 2.3),93 very similar to the OR of 2.2 in this study. A Cochrane network meta-analysis of trials gave an OR of 1.57 (95% credible interval 1.29 to 1.91) for varenicline compared with single-form NRT and an OR of 1.59 (95% credible interval 1.29 to 1.96) for varenicline compared with bupropion. 6 A subsequent very large trial produced ORs of 1.68 (95% CI 1.46 to 1.93) for varenicline compared with the NRT patch and 1.75 (95% CI 1.52 to 2.01) for varenicline compared with bupropion. 94 The randomised trials suggest that the superiority of varenicline in these observational data is probably because varenicline is more effective both within and outside the context of a clinical trial. This suggests that a way to improve the outcome of the NHS Stop Smoking Services is to increase the proportion of patients who use varenicline. The ORs of 1.7 and 2.2 for use of varenicline compared with non-use of varenicline compare favourably with those for preloading (1.25 for the primary analysis and 1.34 for the varenicline-adjusted analysis); the use of varenicline seems simpler, and requires less service change, than implementing a new strategy of preloading.
It is important to note that these conclusions relate to preloading as we implemented it in this trial. It remains possible that preloading performed in other ways or in contexts other than the UK NHS Stop Smoking Services, which provide a range of medications and behaviour support free of charge, may improve outcomes to a greater extent. We used a 4-week period of preloading, which, theoretically, ought to be more effective than the 2 weeks used in several previous studies. However, there is no clear evidence that this is so; therefore, limiting preloading to a shorter period could overcome the possible disadvantages that significantly delaying a quit attempt may entail. Theoretically, it is also possible that higher doses of nicotine could lead to greater effectiveness. 95 The additional 21 mg of nicotine per 24 hours preceding quit day was generally well tolerated and other research shows that 63 mg of nicotine per day appears to be well tolerated too. 91 In addition, the higher-dose NRT reduced consumption to a greater extent than the 21-mg patch that was used, suggesting that higher doses may be effective.
It is also possible that varenicline preloading may be effective. One small trial showed that varenicline preloading more than doubled the number achieving 12-week abstinence. 21 A further trial produced somewhat less positive results, but the trial was too small to offer a precise estimate of effect. 96 Consequently, there is no evidence that varenicline preloading improves long-term abstinence, but, given that the RR of achieving abstinence through use of a smoking cessation medication appears to be constant, whether measured early or late in a quit attempt,97 we might expect that it does. Thus, future trials of preloading might want to examine whether or not this promising effect of varenicline holds in a larger trial and manifests in an increased proportion of participants achieving long-term abstinence.
Mediation
Summary of findings
There was no evidence that preloading was more effective for those showing a greater dependence on smoking. Participants allocated to preloading compared with the control condition showed a reduced reward from smoking, a reduced urge to smoke and reduced smoke inhalation prior to quitting and lowered their cigarette consumption. After attempting abstinence, participants using preloading experienced a reduced craving for cigarettes. Of these possible mediators, only a reduced urge to smoke before and after quit day and reduced consumption while using preloading were associated with abstinence. Together, these changes appeared to mediate most of the effect of preloading on abstinence. Although preloading increased feelings of aversion to smoking, these feelings were not associated with an increased likelihood of achieving abstinence and, thus, there is no evidence that this was an important mediating mechanism. There is also no evidence that the effect of preloading on abstinence was explained by increased confidence in quitting or increased adherence to post-cessation medication.
Strengths and limitations
This study has several strengths. Chief among these are that we planned a comprehensive evaluation of the mechanism of action, assessing all steps in the pathway, and we included analyses of competing hypotheses that were advanced to explain the preloading effect. Being the largest trial to date and the trial with the most comprehensive data on mediators means that the pathway of effect has been clarified. The trial also has some limitations. Our follow-up of participants to assess the effect of preloading on mediators while smoking was carried out only 1 week after commencing treatment. This follow-up was timed to maximise adherence to preloading and to allow the dose to be adjusted if needed. However, previous trials have suggested that the effect of preloading on smoking increases with duration;21,96 therefore, we may have seen larger associations between preloading and change in mediators and between change in mediators and abstinence if this assessment had taken place later. Another issue is that our analysis does not account for the multistep nature of mediation. Our hypothesis was that preloading reduces the strength of urges to smoke while in the pre-quit phase and these reduced urges reduce cigarette consumption. However, we modelled these mediators as separate ‘parallel’ mediators rather than in a structural equation model. Finally, the open-label trial leaves open the possibility that some of these effects are subject to bias, such as participant expectations. Participants in the intervention group were told about the proposed mechanism of preloading to motivate them to adhere to the medication. Thus, the effect of preloading on the proposed mediators could at least partly be caused by this effect rather than by the pharmacological action of the additional nicotine. However, we might have seen an increase in confidence in the preloading group if this was driven by expectations of a positive outcome and this was not apparent. It, therefore, seems less likely that participants’ expectations could explain the increase in abstinence and, therefore, this may be an unlikely explanation of the apparent mediation.
Comparison with other studies
Our analysis of whether or not the effect of preloading depends on baseline tobacco dependence is unique. We used two markers of dependence, FTCD and exhaled CO concentration. In our study, as in previous studies, for example Aveyard et al. ,36 FTCD predicted the likelihood of achieving abstinence. The ORs were 0.85 (95% CI 0.62 to 1.17) for moderate dependence compared with low dependence and 0.61.(95% CI 0.42 to 0.90) for moderate dependence compared with high dependence. The p-value for trend through those categories is 0.013. Thus, in this population of people who seek help to stop smoking and who are more dependent than the average person who smokes, this marker functions as a predictor of abstinence and might, therefore, be expected to moderate the effect of preloading. However, exhaled CO concentration did not function as a useful predictor of the likelihood of abstinence; compared with a CO concentration of < 10 p.p.m. at baseline, for the categories in Figure 5b, the ORs were 0.67, 0.63, 0.79 and 0.74, with a p-value for trend of 0.84. This may, therefore, explain why exhaled CO concentration did not moderate the association.
Our mediation analysis is complemented by previous analyses in other trials of preloading that examined the effect of preloading on several potential mediators. Unlike previous analyses,12 we found that preloading did reduce both positive and negative reward from smoking, but our study is much larger than the previous studies that have examined these mediators, so these trials may have missed this association. As two previous studies have also discovered,12 we found a small reduction in pre-quit urges to smoke. Most previous trials examined changes in cigarette consumption caused by preloading and, as we found, noted reductions in the pre-quit period. 12 Those trials that examined reduction in dependence reported the same kind of reductions that we observed in this trial. Previously, we concluded that preloading did not appear to reduce craving after quit day,12 but there was clear evidence in this trial that preloading did reduce craving. Although previous studies examined the effect of preloading on potential mediators, none undertook a full mediation analysis, as we have done here. Doing so revealed new insights. Although people’s perception of reward may have changed, the main apparent mechanism seems to be through reduction in the urge to smoke. It is possible that this effect is indeed mediated by reward, but that people’s perception of it may not be very sensitive. Alternatively, the drive to smoke may weaken in smokers saturated with nicotine from an alternative source without any effect on their subjective reactions when they do smoke. The main drive to smoke does not require conscious appreciation for it to be experienced. 98 No previous studies have examined whether or not there is effect modification by strength of dependence on tobacco.
Interpretation
This study and previous literature together have produced clear evidence that preloading reduces the intensity of urges to smoke and that it reduces the intensity of smoking during the pre-quit period. The reduced drive to smoke appears to be the best candidate mechanism for the effect of preloading, with reduced smoke intake being one of its consequences.
This has important implications for clinical practice. If preloading were to be used routinely in NHS Stop Smoking Services, then advisors could monitor its effect using markers of urge strength, asking users whether or not they have experienced reduced urge intensity, and also by measuring exhaled CO concentration, which is routine in NHS clinics. This could allow clinicians to advise their patients to abandon preloading if it appears to be having little effect, confident that the evidence now supports this.
Many trials of treatment for tobacco dependence enrol people who smoke a minimum of 10 cigarettes per day, but around one-third of the population who smoke consume fewer cigarettes than this per day. We enrolled such participants if they were seeking help to quit and smoked at least one cigarette per day, providing they manifested other features of dependence, including difficulty attaining abstinence. This may have contributed to including a greater range of participants than in some trials of pharmacotherapy. Including a wide range of participants should make it easier to detect effect modification between markers of dependence. Although there was no clear evidence of effect modification, both point estimates suggested a greater relative benefit in people with a higher level of dependence. As we did not plan for the study to have power to detect this interaction, it may be that we simply failed to find evidence for an effect, rather than this representing clear evidence against an effect. Given the mechanisms of action of preloading and this evidence together, it would seem appropriate for smoking cessation advisors to consider this strategy in anyone seeking treatment, but perhaps particularly for people with a higher level of dependence.
Within-trial cost-effectiveness
Summary of main findings
The primary outcome of the cost-effectiveness analysis was the incremental cost per additional case of 6-month prolonged abstinence. The prolonged abstinence rate at 6 months post quit date according to the Russell Standard was 17.5% in the intervention group and 14.4% in the control group, a difference of 3.1% (95% CI –0.3% to 6.4%). Mean total costs, from randomisation to 6 months post quit date, were £978 (SE £105) in the intervention group and £1090 (SE £110) in the control group. The adjusted incremental costs were £4 (95% CI –£188 to £222). The incremental costs per additional 6-month prolonged abstinence were, therefore, £129 (95% CI –£16,527 to £17,782). The wide CI crossed the zero point, which amounts to no clear evidence that the intervention was more cost-effective than the control condition. The analysis of data at 12 months’ follow-up suggested a possible reduction in health service costs in the intervention group (–£233, 95% CI –£747 to £42). This pointed to a potential cost-saving scenario in the long run, given that the abstinence rate at 12 months was not significantly different between arms. Sensitivity analysis did not change the pattern of these results. There were indications that the costs might have been underestimated in the complete-case analysis.
Strengths and limitations
The strength of this analysis lies in the comprehensive analysis of all health and social care costs, thus providing a clear picture for the NHS when deciding on treatment. As in all such studies, however, we suffered from missing data. In particular, people who try to quit smoking with support and fail to do so are always somewhat reluctant to return for follow-up. For the sake of the primary outcome, we maximised the return of data on smoking status among participants who were reluctant to be followed up. This sometimes meant that we excused people from answering the health resource questions and, as a result, these data were more often missing than data on smoking status. As the data showed, people with complete data were more likely to be abstinent, but there is no reason to imagine that health service use differed between arms among people who were not abstinent and this is unlikely to bias the results.
With the complicated nature of health resource use data, it was inevitable that some assumptions had to be made before any analysis could be undertaken. The less than reliable approach of self-reporting only added to the uncertainties. However, short of accessing participants’ usage directly from the providers, it was the best way to collect this information. The assumption that no response equates to no use is generally seen to be compatible with the way that people complete health resource use questionnaires. The assumptions made about monthly prescriptions are less certain. The prescribing patterns varied between GPs and, without more detailed information, it was not possible to estimate the cost of medication more accurately. Collecting these data from participants directly in a trial of this kind would add a considerable burden and probably increase withdrawal rates. Even if this burden could be disregarded, participants’ reports of medication use are often less than satisfactory for the purpose of costing. In spite of these uncertainties, the assumptions were applied to both arms and, therefore, should not bias the results towards either group.
Participants with complete information on health resource use had much lower use than that suggested from the imputed data. This is not surprising: participants with greater health needs may find it more burdensome to complete questionnaires, be less likely to achieve abstinence and be more likely to drop out of follow-up. However, this effect is unlikely to differ between arms.
The data presented are a within-trial analysis of cost-effectiveness. The reason that people try to stop smoking, and the reason the NHS invests in smoking cessation services, is to support long-term abstinence and to avoid the costs of smoking-related illnesses in the future. It is not possible to collect such data in trials, but it is possible to model them and we present these data in Chapter 7. A second issue relates to the design of the trial itself and the constraints that this imposed on the cost-effectiveness analysis. To be able to assess AEs and the mechanism of action of preloading in an unbiased way, those in the control group delayed their quit attempt to the same extent as those in the preloading group. To ensure that people returned to give us these data, we gave behavioural support to those in the control group and set this group up to believe that this would be useful to them. There is no clear evidence that this is the case and, in any case, the support that we gave is commonly incorporated into standard NHS smoking cessation support, which both the intervention group and the control group received later. In this within-trial analysis, we included the cost of the behavioural support in the control group. The NHS decision problem, however, was to assess the difference between preloading and standard NHS smoking cessation support, with only a minimal period of preparation before quitting. Thus, the control group behavioural support sessions would not happen in any future NHS scenario. Assuming that the outcomes remain the same, the simple exclusion of two behavioural support sessions from the control group would result in an increase in the adjusted difference in costs between arms and an increase in the ICER in turn. For the complete-case analysis, the difference in costs between trial arms would increase from £138 to £155, resulting in a modest change in the ICER of around £700, whereas the change in the adjusted difference in costs between arms in the imputed data (from £4 to £17) would lead to an increase in the ICER of £550. However, the assumption that extra behavioural support in preparation to quit has no impact on outcomes cannot be easily made. These numbers are only estimates and further assessment is required to provide supporting or opposing evidence. In addition, the cost-effectiveness analysis appropriately focuses on the primary analysis of the primary outcome. However, this is a biased estimate of the efficacy of preloading because it is confounded by differences in the use of varenicline. Had we used the estimate for the difference in the effect of preloading adjusted for varenicline, then the cost-effectiveness might have more strongly favoured preloading over the control.
Interpretation
This is the first assessment of the cost-effectiveness of nicotine preloading. Indeed, relatively few trials have conducted within-trial analyses of the cost-effectiveness of smoking cessation medication. One comparator is the NHS Stop Smoking Service, which collects data on the cost per person who has quit smoking and remained abstinent for 4 weeks. Statistics on NHS Stop Smoking Services in England April 2014 to March 201565 showed, among local authorities, a cost per person who quit smoking ranging from £98 to £1185, with a mean of £456. The cost per person who quit smoking at 4 weeks in this trial was £501 in the preloading group and £417 in the control group, somewhat in the middle of the distribution. That said, the NHS counts self-reported abstinence as abstinence, which we excluded; if we had included self-reported abstinence, it would reduce the cost per quitter by nearly one-third.
Interpreting the data here is harder because there is no available WTP threshold for 6-month prolonged abstinence; therefore, it is not possible to draw clear conclusions with regard to whether or not the intervention can be seen to be cost-effective. An incremental cost of £129 per additional 6-month abstinence might be considered cost-effective if the policy-makers judge the abstinence to be beneficial, given the impact of smoking cessation on long-term quality of life. What may encourage policy-makers to consider preloading are the data showing that, at 12 months, preloading reduced overall health service costs. In the short term at least, smoking cessation should reduce the demand for health services by reducing the incidence of both minor and major ill-health consequences of smoking. Future modelling may provide further data to support this.
Modelling the long-term cost-effectiveness of preloading
Summary of findings
The smoking cessation costs within the intervention group over the course of the first year were higher than those in the control group, with intervention group costs of £295, compared with control group costs of £265. However, more smokers quit smoking in the intervention group (n = 139) than in the control group (n = 112) and consequently health-care costs associated with smoking were lower in the intervention group over the course of the lifetime. Overall, the total costs were £29 lower in the intervention group. The intervention was also more effective than the control condition, resulting in 14.0% of smokers in the intervention group quitting at 1 year compared with 11.3% of smokers in the control group, and consequently produced more QALYs and life-years. The intervention would be considered cost-effective as it resulted in greater efficacy at a lower cost than the control condition. The results were robust to changes in assumptions regarding discount rates and relapse after the first year of follow-up.
Strengths and limitations
The major strength of this approach is that it builds on the comprehensive within-trial cost-effectiveness assessment presented in Chapter 6. However, it takes the same perspective as people who are stopping smoking: that the main reason to stop smoking is to avoid future smoking-related disease. 99 As such, it better represents the views of possible service users of NHS Stop Smoking Services and of the community at large. The major weakness is that, as with practically all long-term cost-effectiveness studies, the results are modelled on the future likelihood of disease. Confidence in this modelling is supported by the robust epidemiological evidence available on the association between smoking and incidence of and mortality from disease and the difference in risk arising as a result of stopping smoking at different ages. The model has been extensively discussed and described elsewhere (forthcoming supplement in the journal Addiction) and is used by NICE to examine the cost-effectiveness of smoking cessation medications. 67 However, nobody has prospectively validated models of incidence, mortality and health-care expenditure of cohorts of people who continue or stop smoking, so ultimately the data should be regarded as somewhat conjectural. This emphasises the importance of sensitivity analyses and a strength of the findings here is that they appear largely unaltered by both deterministic and probabilistic sensitivity analyses; all answers converged to suggest that preloading is dominant over the comparator in the large majority of model runs.
Research recommendations
-
Will changes to NICE guidance and interventions with clinicians and/or patients overcome the effect of preloading on the subsequent use of varenicline?
-
Are higher doses of nicotine preloading more effective, especially in those who do not seem to suppress the urge to smoke in response to the standard dose?
-
Are people who reduce their urge to smoke in response to preloading more likely to stop smoking than those who experience no reduction in the urge to smoke?
-
Is reduced urge to smoke translated into reduced consumption of cigarettes? Would this work as a marker of the effectiveness of nicotine preloading?
-
Is response to use of cessation pharmacotherapy while in the period prior to quit day an effective way of personalising treatment for people seeking help to stop smoking?
-
Does varenicline preloading increase the proportion of people achieving long-term abstinence? Is varenicline preloading cost-effective in the long term?
Recommendations for practice
Using nicotine preloading requires a person to make a planned quit attempt. Our trial shows that, when there is dislocation between the person prescribing the NRT preloading and the subsequent smoking cessation service, the choice of subsequent pharmacotherapy undermines the benefit of preloading. The most likely scenario for implementation in the NHS is that a GP would refer a patient to NHS Stop Smoking Services, which replicates the scenario in our trial. In such a circumstance, preloading is unlikely to add greatly to the effectiveness of standard care and we would not recommend its use.
In other circumstances, it is possible that preloading would prove to be effective. A clinician looking to maximise the chances of successful cessation in a patient would prescribe varenicline as post-cessation medication. Both observational data from our trial, other observational data and trial data (discussed in Modelling the long-term cost-effectiveness of preloading) testify to the superiority of this medication. Suggesting this to a patient first and gaining commitment to its use before discussing the use of preloading may overcome the deterrent effect of preloading on the subsequent use of varenicline.
Conclusion
Use of nicotine patch preloading for 4 weeks prior to attempting to stop smoking can increase the proportion of people who stop successfully, but its benefit is undermined because it reduces the use of varenicline after preloading. If this latter effect could be overcome, then nicotine preloading appears to reduce health service costs in the long term. Using the primary outcome of 6-month prolonged abstinence, there is no clear evidence that preloading is more cost-effective than the control condition, but there is a suggestion that over a 12-month period it may be more effective and it is very likely to be cost-effective over a lifetime perspective.
Nicotine preloading appears to work because it reduces the strength of urges to smoke during preloading and after preloading has finished. This seems to be the central mechanism underlying its effect. None of the other theories proposed for its mechanism of action have any support and are likely to be part of the mechanism of action.
Acknowledgements
This trial was funded by the NIHR HTA programme (reference number 09/110/01). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
The trial protocol is published in the journal Trials. 26
Trial Steering Committee
We are very grateful to the following members of the Trial Steering Committee:
-
Professor Michael Ussher PhD (chairperson)
-
Dr Lion Shahab MA MSc PhD CPsychol (expert in tobacco dependence)
-
Dan Griffin (smokers panel representative/lay member)
-
Jane Wright (NHS Stop Smoking Service manager)
-
Dr Helen J Stokes-Lampard FRCGP PhD (academic GP)
-
Dr Tess Harris (academic GP)
-
Dr Rumana Omar PhD (statistician)
-
Professor Paul Aveyard (chief investigator, non-voting member)
-
Dr Nicola Lindson (trial manager, non-voting member).
Contributions of authors
We agreed as a group that authorship would be corporate – the Preloading Investigators – and would prefer this to be how the report was described when published. The investigators were as follows:
Paul Aveyard (Professor of Behavioural Medicine) was the chief investigator and principal investigator of both the Bristol and the Birmingham centres.
Nicola Lindson (Cochrane Managing Editor) was a trial manager and co-applicant.
Sarah Tearne (Clinical Trial Manager) was a clinical trial manager.
Rachel Adams (Research Nurse) was responsible for recruitment and follow-up.
Khaled Ahmed (Data Entry Clerk) was responsible for data entry.
Rhona Alekna (Research Nurse) was responsible for recruitment and follow-up.
Miriam Banting (Research Nurse) was responsible for recruitment and follow-up.
Mike Healy (Administrator) was responsible for study administration.
Shahnaz Khan (Administrator) was responsible for study administration.
Gurmail Rai (Senior Analyst Programmer) was responsible for database design and maintenance.
Carmen Wood (Administrator) was responsible for study administration.
Emma C Anderson (Research Assistant) was responsible for recruitment and follow-up.
Alia Ataya-Williams (Administrator) was responsible for study management and interpretation of the data.
Angela Attwood (Research Fellow) was responsible for recruitment and follow-up.
Kayleigh Easey (Research Assistant) was responsible for recruitment and follow-up.
Megan Fluharty (Research Assistant) was responsible for recruitment and follow-up.
Therese Freuler (Research Assistant) was responsible for recruitment and follow-up.
Megan Hurse (Apprentice) was responsible for study administration, recruitment and follow-up.
Jasmine Khouja (Research Assistant) was responsible for recruitment and follow-up.
Lindsey Lacey (Data Entry Clerk), was responsible for data entry and also acted as a research assistant.
Marcus Munafò (Professor of Biological Psychology) was the lead researcher at the Bristol centre and a co-applicant.
Deborah Lycett (Reader in Nutrition, Dietetics and Spiritual Health) was a co-investigator and was responsible for study management and the interpretation of data.
Andy McEwen [National Centre for Smoking Cessation and Training (NCSCT) Executive Director] was responsible for study management and interpretation of the data.
Tim Coleman (Professor of Primary Care) was the principal investigator at the Nottingham centre.
Anne Dickinson (Research Assistant) was responsible for recruitment and follow-up.
Sarah Lewis (Professor of Medical Statistics) was the trial statistician with responsibility for the statistical analysis.
Sophie Orton (Research Fellow) was responsible for the statistical analysis.
Johanna Perdue (Administrator) was a trial administrator, with responsibility for study administration.
Clare Randall (Administrator) was a trial administrator, with responsibility for study administration.
Rebecca Anderson (Data Entry Clerk) was responsible for site set-up, recruitment, intervention delivery, data collection and data entry.
Natalie Bisal (Data Entry Clerk) was responsible for data collection, data cleaning and study closure.
Peter Hajek (Professor of Clinical Psychology and Director of the Wolfson Institute of Preventive Medicine’s Tobacco Dependence Research Unit) was a co-applicant and a site principal investigator, responsible for study design, data analysis and write-up and supervision of site activities.
Celine Homsey (Data Entry Clerk) was responsible for site set up, recruitment, intervention delivery, data collection and data entry.
Hayden J McRobbie (Senior Clinical Researcher) was a co-applicant (study design) and was responsible for study management, design and interpretation of data.
Katherine Myers-Smith (Research Fellow) was responsible for site set up, recruitment, intervention delivery, data collection and data entry.
Anna Phillips (Data Entry Clerk) was responsible for recruitment, data collection and study closure.
Dunja Przulj (Data Entry Clerk) was responsible for recruitment, data collection and study closure.
Jinshuo Li (Health Economist) was responsible for the health economic analysis.
Doug Coyle (Health Economic Analyst) was responsible for the health economic analysis.
Katherine Coyle (Health Economic Analyst) was responsible for the health economic modelling.
Subhash Pokhrel (Health Economic Analyst) was responsible for the health economic analysis.
Publications
The Preloading Investigators. Effects on abstinence of nicotine patch treatment before quitting smoking: parallel, two arm, pragmatic randomised trial. BMJ 2018;361:k2164. https://doi.org/10.1136/bmj.k2164
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and if appropriate agreements are in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29-38. https://doi.org/10.1111/j.1360-0443.2004.00540.x.
- West R, Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology 2001;155:115-22. https://doi.org/10.1007/s002130100712.
- Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology 2003;166:343-50. https://doi.org/10.1007/s00213-002-1338-1.
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007;9:315-27. https://doi.org/10.1080/14622200701188919.
- Hartmann-Boyce J, Aveyard P. Drugs for smoking cessation. BMJ 2016;352. https://doi.org/10.1136/bmj.i571.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD009329.pub2.
- West R, Baker C, Cappelleri J, Bushmakin A. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2008;197:371-7. https://doi.org/10.1007/s00213-007-1041-3.
- Fagerström KO, Hughes JR. Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: a review. Nicotine Tob Res 2002;4:73-9. https://doi.org/10.1080/1462220021000032753.
- West R, Corrigall WA. Understanding Nicotine and Tobacco Addiction. Chichester: John Wiley & Sons, Ltd; 2006.
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD000146.pub3.
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther 2008;30:1852-8. https://doi.org/10.1016/j.clinthera.2008.09.016.
- Lindson N, Aveyard P. An updated meta-analysis of nicotine preloading for smoking cessation: investigating mediators of the effect. Psychopharmacology (Berl) 2011;214:579-92. https://doi.org/10.1007/s00213-010-2069-3.
- West R, Brown J. Theory of Addiction. Chichester: John Wiley & Sons, Ltd; 2013.
- Foulds J, Hughes J, Hyland A, Le Houezec J, McNeill A, Melvin C, et al. Barriers to Use of FDA-approved Smoking Cessation Medications: Implications for Policy Action. Madison, WI: Society for Research on Nicotine and Tobacco; 2009.
- Shiffman S. Use of more nicotine lozenges leads to better success in quitting smoking. Addiction 2007;102:809-14. https://doi.org/10.1111/j.1360-0443.2007.01791.x.
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: a meta-analysis. Psychol Addict Behav 2009;23:56-6. https://doi.org/10.1037/a0013529.
- Lindson-Hawley N, Banting M, West R, Michie S, Shinkins B, Aveyard P. Gradual versus abrupt smoking cessation: a randomized, controlled noninferiority trial. Ann Intern Med 2016;164:585-92. https://doi.org/10.7326/M14-2805.
- Hajek P, Stead LF. Aversive smoking for smoking cessation. Cochrane Database Syst Rev 2001;3. https://doi.org/10.1002/14651858.CD000546.pub2.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328. https://doi.org/10.1136/bmj.38142.554479.AE.
- West R. The clinical significance of ‘small’ effects of smoking cessation treatments. Addiction 2007;102:506-9. https://doi.org/10.1111/j.1360-0443.2007.01750.x.
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med 2011;171:770-7. https://doi.org/10.1001/archinternmed.2011.138.
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res 2006;8:89-101. https://doi.org/10.1080/14622200500431866.
- Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res 2009;11:1067-75. https://doi.org/10.1093/ntr/ntp103.
- Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score. Mol Med 2010;16. https://doi.org/10.2119/molmed.2009.00159.
- Lindson-Hawley N, Shinkins B, West R, Michie S, Aveyard P. Does cigarette reduction while using nicotine replacement therapy prior to a quit attempt predict abstinence following quit date?. Addiction 2016;111:1275-82. https://doi.org/10.1111/add.13330.
- Lindson-Hawley N, Coleman T, Docherty G, Hajek P, Lewis S, Lycett D, et al. Nicotine patch preloading for smoking cessation (the preloading trial): study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-296.
- Flaxman J. Quitting smoking now or later: gradual, abrupt, immediate, and delayed quitting. Behav Ther 1978;9:260-70. https://doi.org/10.1016/S0005-7894(78)80111-7.
- Guidance on Smoking Cessation. London: NICE; 2008.
- Hajek P. Withdrawal-oriented therapy for smokers. Br J Addict 1989;84:591-8. https://doi.org/10.1111/j.1360-0443.1989.tb03474.x.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. https://doi.org/10.1111/j.1360-0443.2004.00995.x.
- Medical Dictionary for Regulatory Activities . Welcome to MedDRA n.d. www.meddra.org (accessed 23 January 2018).
- Marteau TM, Aveyard P, Munafò MR, Prevost AT, Hollands GJ, Armstrong D, et al. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0035249.
- Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ 2008;336:1223-7. https://doi.org/10.1136/bmj.39545.852616.BE.
- Fidler J, Ferguson SG, Brown J, Stapleton J, West R. How does rate of smoking cessation vary by age, gender and social grade? Findings from a population survey in England. Addiction 2013;108:1680-5. https://doi.org/10.1111/add.12241.
- West R, Ussher M. Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures?. Psychopharmacology 2010;208:427-32. https://doi.org/10.1007/s00213-009-1742-x.
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafò MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62:898-903. https://doi.org/10.1136/thx.2006.071837.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- PRagmatic Explanatory Continuum Indicator Summary (PRECIS)-2 . PRECIS-2 n.d. http://precis-2.org (accessed 23 January 2018).
- The Preloading Investigators . Effects on abstinence of nicotine patch treatment before quitting smoking: parallel, two arm, pragmatic randomised trial. BMJ 2018;361. https://doi.org/10.1136/bmj.k2164.
- Education at a Glance 2014. France: OECD; 2014.
- Kenny DA. Mediation 2016. http://davidakenny.net/cm/mediate.htm (accessed 3 January 2017).
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav 2007;32:912-23. https://doi.org/10.1016/j.addbeh.2006.06.028.
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl) 2004;177:195-9. https://doi.org/10.1007/s00213-004-1923-6.
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine Tob Res 2004;6:327-48. https://doi.org/10.1080/1462220042000202481.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-27. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- Shiffman S, West R, Gilbert D. SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials . Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res 2004;6:599-614. https://doi.org/10.1080/14622200410001734067.
- Lindson N, Aveyard P, Ingram JT, Inglis J, Beach J, West R, et al. Rapid reduction versus abrupt quitting for smokers who want to stop soon: a randomised controlled non-inferiority trial. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-69.
- Aveyard P. The place of varenicline in smoking cessation treatment. Thorax 2008;63:666-8. https://doi.org/10.1136/thx.2008.096081.
- Parrott S, Godfrey C, Raw M, West R, McNeill A. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Health Educational Authority. Thorax 1998;53:S1-38.
- The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Smoking Cessation Services Costing Report – Implementing NICE Guidance. London: NICE; 2008.
- Prescription Cost Analysis, England 2015. Leeds: NHS Digital (formerly the Health and Social Care Information Centre); 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Parrott S, Godfrey C, Kind P. Cost-effectiveness of Brief Intervention and Referral for Smoking Cessation. York: Centre for Health Economics, University of York; 2006.
- Reference Costs 2014–15. London: DHSC; 2015.
- Rubin DB. Statistical matching using file concatenation with adjusted weights and multiple imputations. J Bus Econ Stat 1986;4:87-94. https://doi.org/10.2307/1391390.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Stat Med 1996;15:1447-58. https://doi.org/10.1002/(SICI)1097-0258(19960715)15:13<1447::AID-SIM267>3.0.CO;2-V.
- Willan AR, O’Brien BJ. Confidence intervals for cost-effectiveness ratios: an application of Fieller’s theorem. Health Econ 1996;5:297-305. https://doi.org/10.1002/(SICI)1099-1050(199607)5:4<297::AID-HEC216>3.0.CO;2-T.
- Severens JL, De Boo TM, Konst EM. Uncertainty of incremental cost-effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 1999;15:608-14.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Design & Print Solutions . Business Stationery 2017. www.york.ac.uk/media/design-and-print/print-solutions/pricing/price_list.pdf (accessed 14 February 2017).
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Statistics on NHS Stop Smoking Services in England April 2014 to March 2015. Leeds: NHS Digital; 2015.
- Pokhrel S, Evers S, Leidl R, Trapero-Bertran M, Kalo Z, Vries H, et al. EQUIPT: protocol of a comparative effectiveness research study evaluating cross-context transferability of economic evidence on tobacco control. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006945.
- Coyle K, Coyle D, Lester-George A, West R, Nemeth B, Hiligsmann M, et al. Development and application of an economic model (EQUIPTMOD) to assess the impact of smoking cessation [published online ahead of print August 18 2017]. Addiction 2017. https://doi.org/10.1111/add.14001.
- Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 2006;15:280-5. https://doi.org/10.1136/tc.2005.015487.
- West R. Background Smoking Cessation Rates in England. London: Smoking in England; 2006.
- National Life Tables, United Kingdom: 2012–2014. Newport: ONS; 2014.
- Public Health England . Local Tobacco Control Profiles for England 2014.
- Thun MJ, Apicella LF, Henley SJ. Smoking vs. other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA 2000;284:706-12. https://doi.org/10.1001/jama.284.6.706.
- The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Centre for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics: A Compendium of Health Statistics. 2012 Edition. London: British Heart Foundation; 2012.
- Association of Public Health Observatories (APHO) . Modelled Estimate of Prevalence of COPD in England 2011.
- Health Survey for England – 2013. Leeds: NHS Digital; 2013.
- Maddams J, Brewster D, Gavin A, Steward J, Elliott J, Utley M, et al. Cancer prevalence in the United Kingdom: estimates for 2008. Br J Cancer 2009;101:541-7. https://doi.org/10.1038/sj.bjc.6605148.
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351-64. https://doi.org/10.1056/NEJMsa1211127.
- Britton M. The burden of COPD in the UK: results from the Confronting COPD survey. Respir Med 2003;97:71-9. https://doi.org/10.1016/S0954-6111(03)80027-6.
- McMurray J, Hart W, Rhodes G. An evaluation of the cost of health failure to the National Health Service in the UK. J Med Econ 1993;6:99-110.
- Sanderson H, Spiro S, Stevens A, Raftery J, Mant J, Simpson S. Health Care Needs Assessment: The Epidemiologically Based Needs Assessment Reviews. Abingdon: Radcliffe Publishing; 2004.
- Simpson EL, Stevenson MD, Scope A, Poku A, Minton J, Evans P. Echocardiography in newly diagnosed atrial fibrillation patients: a systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17360.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-203.
- Brazier J, Czoski-Murray C, Roberts J, Brown M, Symonds T, Kelleher C. Estimation of a preference-based index from a condition specific measure: the King’s Health Questionnaire. Med Decis Making 2008;28:113-26. https://doi.org/10.1177/0272989X07301820.
- National Institute for Health and Care Excellence . Discounting of Health Benefits in Special Circumstances n.d. www.nice.org.uk/guidance/ta235/resources/osteosarcoma-mifamurtide-discounting-of-health-benefits-in-special-circumstances2 (accessed 23 January 2018).
- Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics 2000;17:479-500. https://doi.org/10.2165/00019053-200017050-00006.
- UK Centre for Tobacco and Alcohol Studies . UKCTAS n.d. www.ukctas.ac.uk (accessed 23 January 2018).
- Consolidated Standards of Reporting Trials . Welcome to the CONSORT Website n.d. www.consort-statement.org (accessed 23 January 2018).
- Hughes JR, Keely JP, Fagerstrom KO, Callas PW. Intentions to quit smoking change over short periods of time. Addict Behav 2005;30:653-62. https://doi.org/10.1016/j.addbeh.2004.08.011.
- Borland R, Partos TR, Yong H-H, Cummings KM, Hyland A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control 4-Country cohort survey. Addiction 2012;107:673-82. https://doi.org/10.1111/j.1360-0443.2011.03685.x.
- Bauld L, Hiscock R, Dobbie F, Aveyard P, Coleman T, Leonardi-Bee J, et al. English Stop-Smoking Services: one-year outcomes. Int J Environ Res Public Health 2016;13. https://doi.org/10.3390/ijerph13121175.
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507-20. https://doi.org/10.1016/S0140-6736(16)30272-0.
- Benowitz NL, Zevin S, Jacob P. Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther 1998;287:958-62.
- Hawk LW, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther 2012;91:172-80. https://doi.org/10.1038/clpt.2011.317.
- Stapleton J. Cigarette smoking prevalence, cessation and relapse. Stat Methods Med Res 1998;7:187-203. https://doi.org/10.1177/096228029800700206.
- West R, Shiffman S. Fast Facts: Smoking Cessation. Abingdon: Health Press Ltd; 2016.
- McEwen A, West R, McRobbie H. Motives for smoking and their correlates in clients attending Stop Smoking treatment services. Nicotine Tob Res 2008;10:843-50. https://doi.org/10.1080/14622200802027248.
- Leaviss J, Sullivan W, Ren S, Everson-Hock E, Stevenson M, Stevens JW, et al. What is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? A systematic review and economic evaluation. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18330.
Appendix 1 Supplementary tables for the long-term cost-effectiveness modelling
The material presented here is adapted from the adapted from the Preloading Investigators. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See http://creativecommons.org/licenses/by/4.0/.
| Age range (years) | Smoking status | ||
|---|---|---|---|
| Current smoker | Former smoker | Non-smoker | |
| 0–34 | 0.11 | 0.11 | 0.11 |
| 35–44 | 2.80 | 2.00 | 1.60 |
| 45–54 | 8.10 | 4.90 | 4.00 |
| 55–64 | 20.30 | 13.40 | 9.50 |
| 65–74 | 47.00 | 31.60 | 23.70 |
| 75–84 | 106.00 | 77.30 | 67.40 |
| ≥ 85 | 218.70 | 179.70 | 168.60 |
| Age range (years) | Sex (%) | |
|---|---|---|
| Male | Female | |
| 0–44 | 0.002 | 0.003 |
| 45–54 | 0.089 | 0.076 |
| 55–64 | 0.089 | 0.076 |
| 65–74 | 0.748 | 0.331 |
| ≥ 75 | 0.748 | 0.331 |
| Age range (years) | Sex (%) | |
|---|---|---|
| Male | Female | |
| 16–44 | 1.28 | 1.28 |
| 45–64 | 4.15 | 4.15 |
| 65–74 | 8.13 | 8.13 |
| ≥ 75 | 8.94 | 8.94 |
| Age range (years) | Sex (%) | |
|---|---|---|
| Male | Female | |
| 16–24 | 0.1 | 0.1 |
| 25–34 | 0.2 | 0.1 |
| 35–44 | 0.6 | 0.3 |
| 45–54 | 3.6 | 1.3 |
| 55–64 | 10.6 | 3.5 |
| 65–74 | 20.8 | 10.0 |
| ≥ 75 | 28.6 | 19.3 |
| Age range (years) | Sex (%) | |
|---|---|---|
| Male | Female | |
| 0–44 | 0.10 | 0.11 |
| 45–54 | 0.85 | 0.75 |
| 55–64 | 2.60 | 1.80 |
| 65–74 | 6.08 | 4.16 |
| ≥ 75 | 14.55 | 12.17 |
| Age range (years) | Smoking status, prevalence (%) | |||||
|---|---|---|---|---|---|---|
| Current smokers | Former smokers | Never smokers | ||||
| Male | Female | Male | Female | Male | Female | |
| 16–24 | 27.5 | 18.9 | 4.3 | 7.0 | 68.2 | 74.1 |
| 25–34 | 37.4 | 24.5 | 16.2 | 18.0 | 46.3 | 57.5 |
| 35–44 | 25.1 | 18.6 | 27.2 | 22.3 | 47.7 | 59.1 |
| 45–54 | 24.6 | 19.2 | 26.9 | 23.2 | 48.5 | 57.5 |
| 55–64 | 21.4 | 17.2 | 35.2 | 28.6 | 43.3 | 54.2 |
| 65–74 | 11.7 | 11.6 | 47.2 | 34.3 | 41.1 | 54.1 |
| ≥ 75 | 7.0 | 4.9 | 56.3 | 32.5 | 36.7 | 62.6 |
| Disease | Age range, years | Sex | Smoking status | RR | Distribution (alpha, beta) | Reference |
|---|---|---|---|---|---|---|
| Lung cancer | 35–54 | Male | Current | 14.33 | Log-normal (13.00, 15.80) | Parrott et al.49 |
| Female | Current | 13.3 | Log-normal (12.26, 14.42) | Parrott et al.49 | ||
| Male | Former | 4.4 | Log-normal (4.05, 4.75) | Parrott et al.49 | ||
| Female | Former | 2.64 | Log-normal (2.52, 2.77) | Parrott et al.49 | ||
| ≥ 55 | Male | Current | 24.97 | Log-normal (22.2, 28.09) | Thun et al.78 | |
| Female | Current | 25.66 | Log-normal (23.17, 28.40) | Thun et al.78 | ||
| Male | Former | 6.75 | Log-normal (6.06, 7.52) | Thun et al.78 | ||
| Female | Former | 6.7 | Log-normal (6.09, 7.36) | Thun et al.78 | ||
| COPD | 35–54 | Male | Current | 1.0 | Parrott et al.49 | |
| Female | Current | 1.0 | Parrott et al.49 | |||
| Male | Former | 1.0 | Parrott et al.49 | |||
| Female | Former | 1.0 | Parrott et al.49 | |||
| ≥ 55 | Male | Current | 25.61 | Log-normal (21.68, 30.25) | Thun et al.78 | |
| Female | Current | 22.35 | Log-normal (19.55, 25.55) | Thun et al.78 | ||
| Male | Former | 7.05 | Log-normal (6.07, 8.19) | Thun et al.78 | ||
| Female | Former | 8.09 | Log-normal (7.19, 9.1) | Thun et al.78 | ||
| CHD | 35–54 | Male | Current | 3.88 | Log-normal (3.53, 4.27) | Parrott et al.49 |
| Female | Current | 4.98 | Log-normal (4.44, 5.59) | Parrott et al.49 | ||
| Male | Former | 1.83 | Log-normal (1.71, 1.95) | Parrott et al.49 | ||
| Female | Former | 2.23 | Log-normal (2.02, 2.46) | Parrott et al.49 | ||
| 55–64 | Male | Current | 2.5 | Log-normal (2.34, 2.66) | Thun et al.78 | |
| Female | Current | 2.86 | Log-normal (2.65, 3.08) | Thun et al.78 | ||
| Male | Former | 1.43 | Log-normal (1.37, 1.48) | Thun et al.78 | ||
| Female | Former | 1.44 | Log-normal (1.38, 1.51) | Thun et al.78 | ||
| ≥ 65 | Male | Current | 2.5 | Log-normal (2.34, 2.66) | Thun et al.78 | |
| Female | Current | 2.86 | Log-normal (2.65, 3.08) | Thun et al.78 | ||
| Male | Former | 1.43 | Log-normal (1.37, 1.48) | Thun et al.78 | ||
| Female | Former | 1.44 | Log-normal (1.38, 1.51) | Thun et al.78 | ||
| Stroke | 35–54 | Male | Current | 1.00 | Parrott et al.49 | |
| Female | Current | 1.00 | Parrott et al.49 | |||
| Male | Former | 1.00 | Parrott et al.49 | |||
| Female | Former | 1.00 | Parrott et al.49 | |||
| 55–64 | Male | Current | 1.92 | Log-normal (1.66, 2.21) | Thun et al.78 | |
| Female | Current | 2.1 | Log-normal (1.87, 2.36) | Thun et al.78 | ||
| Male | Former | 1.16 | Log-normal (1.07, 1.25) | Thun et al.78 | ||
| Female | Former | 1.15 | Log-normal (1.07, 1.22) | Thun et al.78 | ||
| ≥ 65 | Male | Current | 1.92 | Log-normal (1.66, 2.21) | Thun et al.78 | |
| Female | Current | 2.1 | Log-normal (1.87, 2.36) | Thun et al.78 | ||
| Male | Former | 1.16 | Log-normal (1.07, 1.25) | Thun et al.78 | ||
| Female | Former | 1.15 | Log-normal (1.07, 1.22) | Thun et al.78 |
| Disease | Costs (£) | Distributiona |
|---|---|---|
| CHD | 1247.79 | Gamma |
| COPD | 1642.57 | Gamma |
| Lung cancer | 7002.65 | Gamma |
| Stroke | 5886.66 | Gamma |
| Year | Inflation multiplier | Pay and Price Index |
|---|---|---|
| 2001 | 1.5115 | 195.5 |
| 2002 | 1.4383 | 206.5 |
| 2003 | 1.3898 | 213.7 |
| 2004 | 1.3212 | 224.8 |
| 2005 | 1.2785 | 232.3 |
| 2006 | 1.2329 | 240.9 |
| 2007 | 1.1890 | 249.8 |
| 2008 | 1.1556 | 257.0 |
| 2009 | 1.1124 | 267.0 |
| 2010 | 1.1057 | 268.6 |
| 2011 | 1.0734 | 276.7 |
| 2012 | 1.0513 | 282.5 |
| 2013 | 1.0338 | 287.3 |
| 2014 | 1.0224 | 290.5 |
| 2015 | 1.0133 | 293.1 |
| 2016 | 1.0000 | 297.0 |
| Model state/disease | Utility value, mean (SE) | Distribution (alpha, beta) | Reference |
|---|---|---|---|
| Current smokers | 0.8497 (0.0005) | Beta(477,912, 84,525) | Vogl et al.85 |
| Former smokers | 0.8695 (0.0014) | Beta(47,833, 7180) | Vogl et al.85 |
| Lung cancer | 0.5600 (0.0430) | Beta(74, 58) | Sullivan et al.84 |
| CHD | 0.6210 (0.0110) | Beta(1207, 737) | Sullivan et al.84 |
| COPD | 0.7320 (0.0070) | Beta(2930, 1073) | Sullivan et al.84 |
| Stroke | 0.5500 (0.0160) | Beta(531, 435) | Sullivan et al.84 |
List of abbreviations
- AE
- adverse event
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CRF
- case report form
- EQUIPT
- European-study on Quantifying Utility of Investment in Protection from Tobacco
- FTCD
- Fagerström Test for Cigarette Dependence
- GP
- general practitioner
- HRG
- Healthcare Resource Groups
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- mCEQ
- modified Cigarette Evaluation Questionnaire
- MPSS-C
- Mood and Physical Symptoms Scale – Craving
- MPSS-M
- Mood and Physical Symptoms Scale – Mood
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- p.p.m.
- parts per million
- PRECIS-2
- PRagmatic Explanatory Continuum Indicator Summary
- QALY
- quality-adjusted life-year
- RD
- risk difference
- ROI
- return on investment
- RR
- relative risk
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- UKCTAS
- UK Centre for Tobacco and Alcohol Studies
- WTP
- willingness to pay