Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/104/24. The contractual start date was in October 2012. The draft report began editorial review in November 2017 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Philip M Bath reports grants from the British Heart Foundation and National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study, and he is a shareholder in Platelet Solutions Ltd (Nottingham, UK). He also notes personal fees from Diamedica (Minneapolis, MN, USA), Nestlé (Vevey, Switzerland), Phagenesis Ltd (Manchester, UK), ReNeuron (Bridgend, UK), Athersys (Cleveland, OH, USA), and Covidien (Zurich, Switzerland) outside the submitted work. Robert A Dineen reports a grant from the British Heart Foundation during the conduct of the study. Hugh S Markus reports grants from the NIHR HTA programme during the conduct of the study. Alan A Montgomery is a member of the NIHR HTA Clinical Evaluation and Trials Funding Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Bath et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Stroke is devastating to patients, carers and society through high mortality (1 in 4 patients by the first year), morbidity (dependency in 1 in 3 patients by the first year, many of whom need long-term care) and cost (NHS and social care costs of £1.7B a year in England). 1 Both stroke incidence and prevalence will increase as the UK population ages. Following stroke or transient ischaemic attack (TIA), the risk of recurrence is high, especially immediately after the event (approximately 10% over the first few weeks), after which it falls to a total of about 40% by 5 years. Typically, recurrent strokes are more severe than earlier events. 2,3
A TIA (‘mini stroke’) is defined as ‘an acute loss of focal cerebral or ocular function with symptoms lasting less than 24 hours and which is thought to be due to inadequate cerebral or ocular blood supply as a result of low blood flow, thrombosis or embolism associated with diseases of the blood vessels, heart, or blood’. 4 TIAs are important because they are a key risk factor for subsequent stroke. Patients presenting with specific TIA features are at a particularly high risk of a subsequent stroke, as assessed by the ABCD2 score5 (as derived from the ABCD score6), which takes account of age (A), blood pressure (B), clinical symptomology (C), duration of symptoms (D), and presence of diabetes (D). An important caveat is that data for the training databases used to derive and validate the ABCD2 score were collected up to 1998 and 2005, respectively, and so the absolute risk rates of stroke are now lower as enhanced secondary prophylaxis has become standard practice. Although some research groups have validated the ABCD2 scoring system,7,8 its value in diagnosis and prognosis after TIA has more recently been questioned. 9
The risk of recurrence can be reduced, but not abolished, with lifestyle changes; drug interventions comprising antithrombotics, antihypertensives and statins; and carotid endarterectomy (after large artery stroke/TIA). 10–12 Although oral anticoagulants are established for cardioembolic stroke,13,14 other patients with non-cardioembolic ischaemia (the majority) need antiplatelet therapy. 15,16 These interventions are all cost-effective.
The archetypal antiplatelet, aspirin (which is an inhibitor of cyclo-oxygenase), reduces recurrence [i.e. relative risk reduction (RRR)] by 17% in patients with prior stroke or TIA. 17 Clopidogrel (which is an adenosine diphosphate receptor antagonist) was slightly more efficacious than aspirin in the Clopidogrel vs. Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial. 18 Importantly, the relative difference in efficacy between aspirin and clopidogrel was highest in patients with prior stroke or myocardial infarction (MI). 19 No trials comparing clopidogrel with control or aspirin have been reported in patients with TIA. Dipyridamole (which inhibits the phosphodiesterase inhibitor-5 and red blood cell uptake of adenosine) reduced recurrence by 16% in comparison with placebo, and was comparable to aspirin, in the European Stroke Prevention Study-2 (ESPS-2) trial. 20 Evidence now suggests that stroke prevention is dependent on the number of antiplatelets [e.g. combined aspirin and dipyridamole reduce events by 23% in comparison to aspirin (or dipyridamole) alone without increasing the risk of bleeding, as seen in the ESPS-2 and European/Australasian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) trials]. 20,21 As with clopidogrel alone, the difference in efficacy between aspirin and dipyridamole versus aspirin alone was greatest in patients with the highest baseline risk. 22 Similarly, aspirin and clopidogrel was superior to aspirin in cardiac patients. 23,24 However, the superiority of clopidogrel-based dual therapy was not seen in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial,25 probably because the apparent benefit in those with prior stroke or MI (who had the highest risk of recurrence) was diluted by lack of efficacy in those with no previous vascular events (who were at lower risk). The risk of bleeding with aspirin and clopidogrel versus aspirin was 30–40% higher in these three trials. The Management of ATherothrombosis with Clopidogrel in High-risk patients (MATCH) trial (aspirin and clopidogrel vs. clopidogrel) found that dual aspirin and clopidogrel also increased bleeding. 26,27
On the basis of these trials and taking account of the prices of branded clopidogrel and dipyridamole-ER [£37 and £10 per month, respectively, British National Formulary (BNF)],28 the National Institute for Health and Care Excellence (NICE) recommended in 2005 that patients should take combined aspirin and dipyridamole after ischaemic stroke or TIA [Technology Appraisal (TA) number 90]. 29 In late 2010, NICE updated its recommendation to aspirin and dipyridamole for TIA, and clopidogrel for ischaemic stroke (TA210);30 these decisions take account of the large drop in the price of clopidogrel, reflecting its generic status (£3.40, BNF 6128) but the lack of significant randomised data and a licence for clopidogrel in patients with TIA. Former and current guidelines have not recommended dual aspirin and clopidogrel because of increased bleeding. 31,32 The preference for combined aspirin and dipyridamole, or clopidogrel alone, over aspirin alone was also recommended by the European Stroke Organisation in its 2008 guidelines. 33 In contrast, the 2011 American Stroke Association secondary prevention guidelines still gave equal recommendations for aspirin (50–325 mg daily) alone, dual aspirin and dipyridamole, and clopidogrel (75 mg daily) alone,34 thereby ignoring the results of recent trials. 18,20,21,35
The above data for stroke reflect long-term prophylaxis, a very different situation from the situation immediately after an event when the risk of recurrence is much higher. Conventional acute antiplatelet therapy is based on aspirin alone for ischaemic stroke reflecting the results of the International Stroke Trial and Chinese Aspirin Stroke Trial (CAST) megatrials. 36,37 However, the effect size is small (absolute risk reduction ≈1.1%) and, until recently, the acute treatment of TIA had not been investigated. As the risk of recurrence falls quickly after stroke or TIA, intensive antiplatelet specific treatment is likely to be needed only for a short period so that the exposure time to hazard (mainly bleeding) is limited. Although clopidogrel-based dual therapy has not proved effective/safe in long-term stroke prophylaxis, early and short-term dual antiplatelet therapy based on clopidogrel or dipyridamole may be useful, at least after TIA/minor stroke, as suggested by several trials [Fast Assessment of Stroke and TIA to prevent Early Recurrence (FASTER),38 EARLY,39 Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) early]40 and observational studies [Early use of EXisting Preventive Strategies for Stroke (EXPRESS),41 SOS-TIA42]. In the FASTER trial (n = 392), 90 days of aspirin and clopidogrel (vs. aspirin) showed a trend to reduce stroke by absolute 3.7% (not significant), and increased symptomatic intracranial haemorrhage (sICH) by absolute 1% (not significant) leading to a net absolute benefit of 2.7%. 38 Similarly, the EARLY trial (n = 543, acute ischaemic stroke/TIA) found a tendency to reduced vascular events at day 90 with aspirin and dipyridamole (vs. aspirin, not significant) but no effect on functional outcome. 39 A pattern of observations also seen with aspirin and dipyridamole (vs. clopidogrel) in the PRoFESS early subgroup (n = 1360, mild acute ischaemic stroke). 40
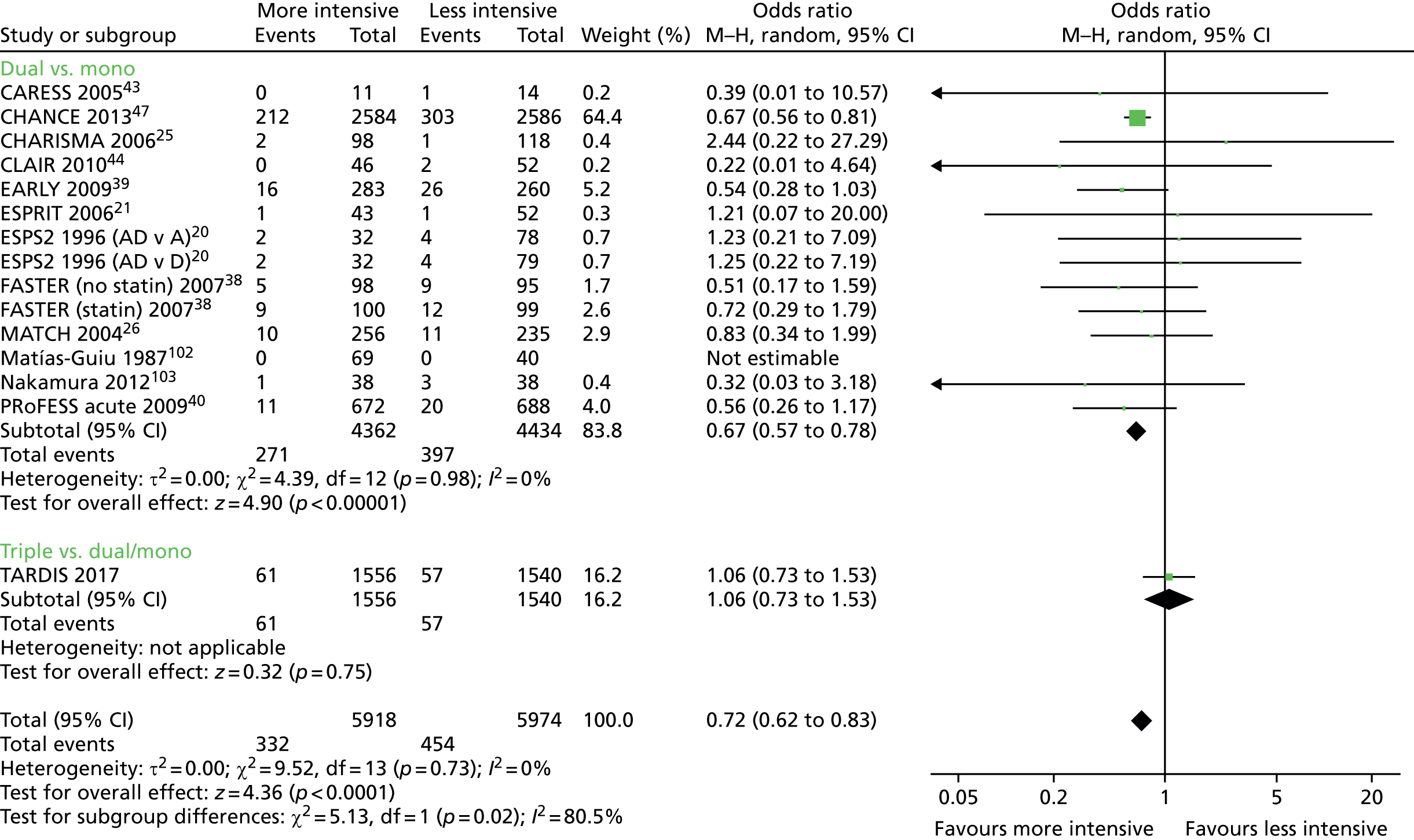
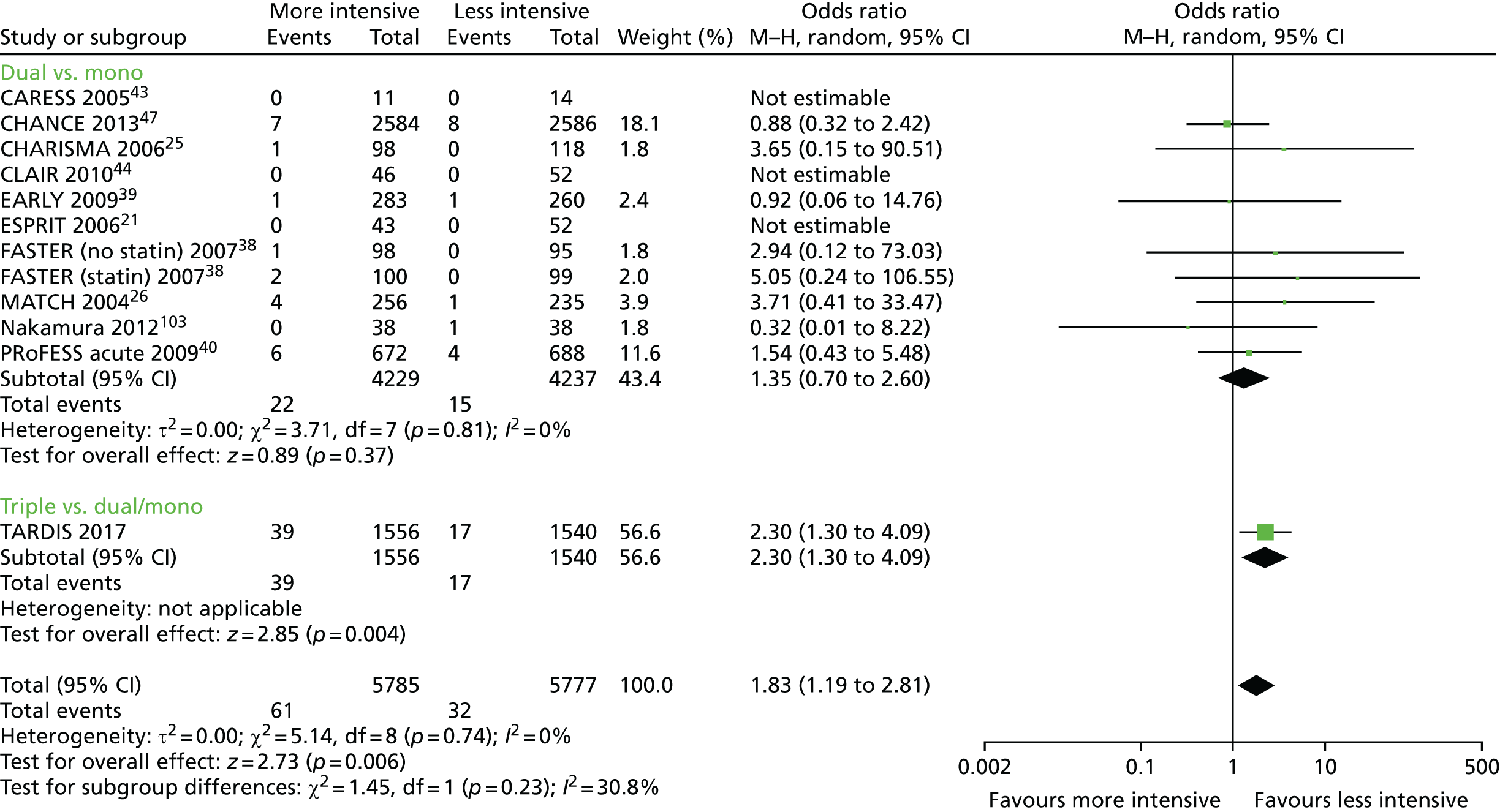
In a meta-analysis of all trials comparing dual with mono antiplatelet therapy in patients with acute stroke or TIA [including ESPS-2, ESPRIT, CHARISMA, MATCH, PRoFESS early, EARLY, FASTER, Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) and Clopidogrel plus Aspirin for Infarct Reduction in acute stroke/TIA patients with large artery stenosis and microembolic signal (CLAIR)20,21,25,26,38–40,43,44], acute dual therapy versus monotherapy within 3 days of ictus significantly reduced stroke recurrence and a composite of vascular events. 45 No significant differences were seen for MI, sICH, major bleeding or death (but there were few events, Table 146). No heterogeneity existed in any analysis, which suggested that the composition of dual and monotherapy was not of primary importance. None of the trials was large enough (each < 1400 patients) to show individual significant differences in stroke or vascular events. Importantly, the magnitude of effect appeared to decline with time from ictus so trials recruiting earlier had greater reductions in their point estimates (albeit non-significant because of small sample size) than those recruiting later: range of odds ratios (ORs) for stroke – early, OR 0.51 to 0.71 (EARLY,39 FASTER,38 PRoFESS early40); later, OR 0.83 to 2.44 (CHARISMA,25 MATCH26). The large Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial47 compared combined aspirin and clopidogrel with aspirin alone in 5170 Chinese patients with acute minor stroke or TIA recruited within 24 hours of the event. The results are similar to the meta-analysis and showed that dual therapy was superior with reduced stroke recurrence [hazard ratio (HR) 0.68, 95% confidence interval (CI) 0.57 to 0.81] and no difference in moderate or severe haemorrhage. 47 The above meta-analysis45 was updated with CHANCE47 and summarises all available data for acute stroke and TIA involving trials comparing dual versus mono antiplatelet therapy;46 a summary of the main findings is given in Table 1. The large POINT trial,48 which also compared combined aspirin and clopidogrel for acute minor stroke and TIA (within 12 hours) in a Western population, reported similar findings to CHANCE.
| Outcome | Number of data sets | Number of events | Number of patients | Risk ratio (95% CI) | p-value | Heterogeneity I2 (%) |
|---|---|---|---|---|---|---|
| Stroke recurrence | 14 | 668 | 8796 | 0.69 (0.60 to 0.80) | < 0.001 | 0 |
| Stroke, TIA, ACS, all death | 9 | 826 | 8174 | 0.71 (0.63 to 0.81) | < 0.001 | 0 |
| Major bleeding | 11 | 37 | 8466 | 1.35 (0.70 to 2.59) | 0.37 | 0 |
Current stroke prevention is far from perfect. Stroke is heterogeneous in type (ischaemic vs. haemorrhage; lacunar vs. cardioembolic vs. large artery), severity and outcome, and treatments reduce, but do not abolish, events (‘treatment failure’). In addition, patients may be (relatively) insensitive to treatment (‘treatment resistance’, as identified for aspirin and clopidogrel49); hence, improvements in secondary prevention are still needed.
If combined aspirin and dipyridamole is superior to aspirin for long-term secondary prevention,20,21,50 and aspirin and clopidogrel are probably superior to aspirin in acute minor stroke/TIA,38,41,46,47 then triple antiplatelet therapy (combined aspirin, clopidogrel and dipyridamole) might be better still, providing that the risk of recurrence is high and bleeding does not become excessive. In this respect, the risk of bleeding when adding clopidogrel to aspirin and dipyridamole is likely to be similar to that of when adding clopidogrel to aspirin alone, as dual aspirin and dipyridamole does not increase bleeding over aspirin. 20,21 We have performed a series of ‘proof-of-concept’ laboratory and clinical studies investigating this approach. 51–55 Studies in vitro found that triple therapy was most effective in inhibiting aggregation, platelet–leucocyte conjugation and leucocyte activation. 51,54,55 In multiway crossover Phase I and II trials comparing short-term administration of mono, dual and triple antiplatelet platelet therapies, the combination of aspirin and clopidogrel, with or without dipyridamole, was most potent in inhibiting platelet function ex vivo in both normal volunteers (n = 11) and patients with previous stroke/TIA (n = 11). 52,53
In the only parallel group trial of triple therapy in patients with stroke (Phase II trial, n = 17), was triple therapy feasible to administer for up to 24 months. 56 The comparator was aspirin, chosen as this was the UK standard of care at trial commencement. The trial was stopped early on publication of the ESPRIT trial,21 confirming the superiority of dual aspirin and dipyridamole over aspirin (i.e. it was unethical to continue patients on aspirin alone). Predictably, there was increased bleeding with long-term triple therapy versus aspirin. Although unintended, the participants were at a low risk of recurrence (young age/recruited months after the event/many lacunar strokes), a problem that was also seen in the MATCH and CHARISMA trials. 26,57 A conclusion was that future trials of triple antiplatelet therapy would need to target patients at a high risk of recurrence so that benefit was likely to outweigh hazard. We have also used chronic triple antiplatelet therapy in clinical practice in patients at a very high risk of recurrence, defined as recurrence on dual antiplatelet therapy, and this appeared to be safe. 58
Short-term randomised controlled trials of triple antiplatelet therapy have been reported in patients with acute coronary syndromes (ACSs) or to cover stent insertion (25 studies,59 17,383 patients). In our published meta-analysis, and in comparison with dual antiplatelet therapy, glycoprotein IIb/IIIa-based triple therapy reduced MI in patients with non-ST elevation myocardial infarction (NSTEMI) (OR 0.70, 95% CI 0.56 to 0.88) and ST-elevation myocardial infarction (STEMI) (OR 0.26, 95% CI 0.17 to 0.38) patients, and vascular events in NSTEMI (OR 0.69, 95% CI 0.55 to 0.86) and STEMI (OR 0.39, 95% CI 0.30 to 0.51) patients. 59 Death was also reduced after STEMI; major bleeding and transfusions were non-significantly increased and were few in number such that benefit outweighed hazard in absolute numbers of patients. The number of stroke events were too few to assess any statistical trends, and minimal or no data were available for other antiplatelets (cilostazol, clopidogrel, dipyridamole). 59
Rationale for trial
The Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke (TARDIS) trial was predicated on the following.
-
There is a high early risk of recurrent cerebral ischaemic events after stroke and TIA.
-
Some patients ‘fail’ on monotherapy with aspirin, clopidogrel or dipyridamole.
-
Dual therapy with aspirin and dipyridamole is superior to aspirin alone after stroke.
-
Clopidogrel, or dual aspirin and dipyridamole, is the standard of care in the UK (NICE).
-
If dual therapy is superior, then triple therapy may be better still.
-
Laboratory studies, Phase I/II trials, and routine clinical use support the use of intensive antiplatelet therapy with three agents. 51–55
Hence, triple therapy may be better still in high-risk patients providing the benefits exceed the risk of bleeding.
Chapter 2 Methods
Objectives
The primary objective was to assess ordinal stroke severity at 90 days after short-term administration (1 month) of triple antiplatelet therapy (aspirin/clopidogrel/dipyridamole) versus standard dual therapy (aspirin/dipyridamole) in patients with very recent ischaemic stroke or TIA.
Secondary objectives
The secondary objectives were to:
-
assess the safety of short-term administration (1 month) of triple antiplatelet therapy (aspirin/clopidogrel/dipyridamole) versus standard dual therapy (aspirin/dipyridamole) in patients with very recent ischaemic stroke or TIA
-
further assess, in high-risk patients with stroke/TIA, whether or not the addition of clopidogrel to aspirin/dipyridamole (1) is feasible to administer acutely and tolerable to take for 1 month, (2) is superior in respect of surrogate markers such as emboli [with transcranial Doppler (TCD)] and platelet function and (3) improves functional outcome
-
assess whether or not ordinal outcomes are superior to binary events.
Design
The TARDIS trial was an international Prospective Randomised Open-label Blinded End point (PROBE) study that recruited from 106 sites in four countries (Denmark, Georgia, New Zealand and the UK). The protocol can be found online. 60
Study settings
The trial was carried out in the stroke services of 106 hospitals in four countries: the UK, Denmark, Georgia and New Zealand. Stroke services were housed in a range of settings including teaching/tertiary and district/secondary hospitals (see list in Appendix 1).
Participants
Adult patients aged ≥ 50 years were eligible for inclusion if they were at risk of a recurrent ischaemic stroke, and had either a non-cardioembolic ischaemic stroke or a non-cardioembolic TIA. Randomisation had to be performed within 48 hours of symptom onset unless the participants had received intravenous thrombolysis, in which case randomisation had to be performed once 24 hours had elapsed after the end of this treatment and post-treatment neuroimaging excluded secondary cerebral bleeding. Patients gave written consent, or written proxy consent was obtained from a relative or carer if the patient lacked capacity. The consent form and patient information sheet are available at www.journalslibrary.nihr.ac.uk/programmes/hta/1010424/#/ (accessed July 2018). The full criteria follow here.
Inclusion criteria
Adults at a high risk of recurrent ischaemic stroke who:
-
were aged ≥ 50 years
-
were within 48 hours of ictus (24–48 hours if thrombolysed)
-
had TIA with limb weakness and/or dysphasia lasting between 10 minutes and < 24 hours with no residual symptoms and presenting with any of the following:
-
an ABCD2 score of ≥ 4
-
crescendo TIA
-
already be on dual antiplatelet therapy with aspirin and dipyridamole
-
positive neuroimaging evidence to support the new event, ischaemic stroke on magnetic resonance diffusion imaging.
-
– Patients who were on monotherapy (e.g. aspirin alone, clopidogrel alone or dipyridamole alone) were eligible for recruitment. Similarly, patients who were on combined therapy aspirin and dipyridamole were eligible for recruitment if they fulfilled the above criteria.
-
– Patients with posterior fossa events were eligible if they fulfilled the above criteria.
-
– Neuroimaging was not necessary for TIA. Crescendo TIA was defined as more than one TIA in the immediate previous week, and the time of onset of the last TIA was taken as the time of ictus.
-
-
-
Ischaemic non cardioembolic stroke presenting with any of the following:
-
ongoing limb weakness of more than one hour duration and/or
-
ongoing dysphasia of > 1 hour duration and/or
-
resolved limb weakness of > 1 hour duration with ongoing facial weakness and/or
-
ongoing isolated hemianopia of > 1 hour duration with positive neuroimaging evidence to support the new event (e.g. ischaemic stroke in the occipital lobe) and/or
-
limb weakness that resolves between 24–48 hours after onset and/or
-
dysphasia that resolves between 24–48 hours after onset and/or
-
positive neuroimaging to support the new ischaemic event with magnetic resonance diffusion and/or
-
already on dual antiplatelet therapy with aspirin and dipyridamole:
-
– Neuroimaging was essential for ischaemic stroke to exclude intracranial haemorrhage and non-stroke diagnoses. If the patient received thrombolysis, a post-thrombolysis/pre-TARDIS brain CT scan had to be done to exclude new thrombolysis associated bleeding prior to enrolment. Typically, this was done as ‘standard of care’, but if it was not done routinely then it had to be done prior to enrolment.
-
-
-
Patients thrombolysed for stroke with full recovery in < 24 hours from the onset of symptoms were eligible for inclusion providing neuroimaging post-thrombolysis excluded intracranial haemorrhage.
-
Informed consent from participant. If the participant was unable to give meaningful consent (e.g. owing to dysphasia, confusion or reduced conscious level, proxy consent could be obtained from a relative, carer, friend or legal representative).
Exclusion criteria
-
Aged < 50 years.
-
Isolated sensory symptoms or vertigo/dizziness or facial weakness.
-
Isolated hemianopia without positive neuroimaging evidence.
-
Intracranial haemorrhage.
-
Baseline neuroimaging showing a parenchymal haemorrhage (PH) transformation (I/II) of infarct, subarachnoid haemorrhage or other non-ischaemic cause for symptoms.
-
Presumed cardioembolic stroke [e.g. a history of or current atrial fibrillation (AF), MI within 3 months].
-
Participants with contraindications to, or intolerance of, aspirin, clopidogrel or dipyridamole.
-
Participants with definite need for treatment with aspirin, clopidogrel or dipyridamole individually or in combination (e.g. aspirin and clopidogrel for recent MI/ACS).
-
Definite need for full dose orally [e.g. warfarin, dabigatran (Pradaxa®, Boehringer Ingelheim, Bracknell, UK)] or medium- to high-dose parenteral (e.g. heparin) anticoagulation. Note that low-dose heparin for deep-vein thrombosis (DVT) prophylaxis was allowed.
-
Definite need for glycoprotein IIb–IIIa inhibitors.
-
Patients who had received thrombolysis within 24 hours.
-
No enteral access.
-
Pre-morbid dependency [modified Rankin Scale (mRS) score of > 2].
-
Severe high blood pressure (> 185/110 mmHg).
-
Haemoglobin levels of < 10 g/dl.
-
Platelet count > 600 × 109/l or < 100 × 109/l.
-
White cell count of > 30 × 109/l or < 3.5 × 109/l.
-
Major bleeding within 1 year (e.g. peptic ulcer, intracerebral haemorrhage).
-
Planned surgery during 3-month follow-up (e.g. carotid endarterectomy).
-
Concomitant STEMI or NSTEMI.
-
Stroke secondary to a procedure (e.g. carotid or coronary intervention).
-
Coma [Glasgow Coma Scale (GCS) score of < 8).
-
Non-stroke life expectancy < 6 months.
-
Dementia.
-
Participation in another drug or devices trial concurrently or within 30 days (participants may take part in observational studies or non-drug or devices trials).
-
Geographical or other factors that may interfere with follow-up (e.g. no fixed address or telephone contact number, not registered with a general practitioner, or overseas visitor).
-
Females of childbearing potential, pregnancy or breastfeeding.
-
Patients who have not had post-thrombolysis neuroimaging.
-
Patients on aspirin and clopidogrel prior to the underlying event.
Data collected at baseline
Baseline data collected immediately prior to randomisation included demographic information (age, sex and ethnicity), medical history including vascular risk factors, current antiplatelet therapy (none, aspirin, clopidogrel, dipyridamole, other), and pre-morbid dependency (mRS, scores range from 0 to 6, with a score of 0 indicating no symptoms, 5 indicating severe dependency and 6 indicating death61,62). Clinical information included blood pressure and stroke syndrome [Oxfordshire Community Stroke Project (OCSP)63]. Neurological impairment was recorded in stroke patients using the National Institutes of Health Stroke Scale (NIHSS), for which scores range from 0 to 42 with higher scores indicating a more severe neurological deficit. 64,65 The risk of recurrence after index TIA was assessed using the ABCD2 scale (scores range from 0 to 7 with higher scores indicating a higher risk of recurrence5).
Interventions
Participants randomised to the intervention (intensive, triple antiplatelets) group received combined aspirin (load 300 mg, maintenance 50–150 mg daily, typically 75 mg, given orally, nasogastrically or rectally), clopidogrel (load 300 mg, maintenance 75 mg daily, given orally or nasogastrically) and dipyridamole (200 mg twice daily modified release, given orally; or 100 mg three or four times daily, given orally or nasogastrically). Those randomised to guideline antiplatelet therapy received either combined aspirin and dipyridamole or clopidogrel alone, using the loading and maintenance doses given above.
The two approaches for guideline therapy arose because of a change in clinical guidelines. At the start of the trial, NICE recommended the use of aspirin and dipyridamole for secondary prevention29 and the initial trial protocol defined this as the guideline comparator. Once clopidogrel became generic (and its price fell), NICE updated their guidance in 2010 with a recommendation that clopidogrel should be used first line for secondary prophylaxis after ischaemic stroke (but not TIA because clopidogrel was not licensed for TIA and there was an absence of randomised trial data for this indication)30 and the protocol was updated accordingly.
Randomised antiplatelet drugs were given for 30 days so as to influence the time period of high risk of recurrence without intending to accrue significant bleed. After 30 days, participants were treated in accordance with local/national guidelines, typically with combined aspirin and dipyridamole or clopidogrel alone. Drugs were sourced by each participating hospital with supply from any licensed manufacturer (including generic sources).
Detailed information on compliance with randomised treatment was collected up to 7 days, but not during the subsequent 21 days to end of treatment.
At the start of the trial, clopidogrel was available as a branded drug, Plavix® (Sanofi Guildford, UK). Subsequently, multiple generic versions became available with the advantage of a much lower cost; as a result, recruiting sites increasingly used generic clopidogrel. A number of studies and a meta-analysis have compared Plavix with generic clopidogrel and these do not report any significant difference in antiplatelet responses or effects on vascular outcomes. 66–69
Randomisation
Demographic and baseline clinical characteristics were entered online into a secure web-based database system,70 which was a bespoke system that also provided randomisation and was based on that used in the Efficacy of Nitric Oxide in Stroke (ENOS) trial and the Prevention of Decline in Cognition After Stroke Trial (PODCAST). 71,72 Baseline data were checked to confirm the patient’s eligibility and the system then assigned the participant to intensive or guideline antiplatelet therapy, with a 1 : 1 allocation.
Patients randomised to the guideline group received either combined aspirin and dipyridamole, or clopidogrel alone, in accordance with local policy and guidelines, and antiplatelets taken prior to randomisation.
-
Each site chose what comparator(s) they wished to use for ischaemic stroke and TIA separately. They could elect to use one comparator only, or randomise between the comparators. The principal investigator could change the choice of comparison group(s) via the database at any stage during the trial, but changes took 48 hours to take effect to avoid them being made for individual participants. The elected comparator option of each site (irrespective of whether a participant was eventually randomised to intensive or guideline treatment) has been included as a covariate in all adjusted analyses and investigated as part of the prespecified subgroup analysis.
-
As aspirin and clopidogrel have irreversible effects on platelets while platelets circulate for 7–10 days, randomisation also took account of which antiplatelet(s) had been taken shortly before stroke onset or in hospital prior to randomisation. The concern was that the comparator group might amount to triple antiplatelet therapy once pre-randomisation treatment was taken into account. However, because aspirin is widely taken both before stroke and immediately following scanning in hospital, it was allowed in all choices prior to randomisation. For example, a patient on clopidogrel prior to stroke and given aspirin in hospital could not be randomised to aspirin and dipyridamole as this would functionally result in them being exposed to all three drugs for the first week after randomisation. As a result, the following rules were followed at the time of randomisation when determining an appropriate comparator (Table 2).
| Pre stroke | Post stroke | Allowed comparator(s) |
|---|---|---|
| Nil | Nil | Clopidogrel vs. aspirin and dipyridamole |
| Nil | Aspirin | Clopidogrel vs. aspirin and dipyridamole |
| Aspirin | Aspirin | Clopidogrel vs. aspirin and dipyridamole |
| Clopidogrel | Nil | Clopidogrel alone |
| Clopidogrel | Aspirin | Clopidogrel alone |
| Dipyridamole | Nil | Aspirin and dipyridamole |
| Dipyridamole | Aspirin | Aspirin and dipyridamole |
Randomisation comprised stratification on country and index event (stroke vs. TIA), and minimisation on key prognostic baseline factors [age, sex, pre-morbid function (using the mRS), systolic blood pressure, syndrome (cortical vs. lacunar63), previous antiplatelet therapy (none/mono vs. dual), use of gastroprotection, use of low dose heparin and time to randomisation]. For TIA participants, minimisation also included presence of crescendo TIAs (more than one TIA in the previous week) and ABCD2 score;5 for those with stroke, minimisation also included NIHSS and treatment with alteplase.
Definition of events (updated from the statistical analysis plan)73
Stroke, MI and bleeding (major, moderate) are adjudicated independently by two adjudicators; if they differ in type of vascular event or severity of bleeding, a third adjudicator also assesses the event and a majority view is recorded for analysis. Serious adverse events (SAEs) are assessed by a single adjudicator. All adjudicators are blinded to treatment assignment.
Adherence
Details on tablets taken were only recorded over the first 7 days after randomisation. Hence, adherence was judged as whether or not the first treatment was received, and whether or not all of the first week’s treatment was taken. Reasons for non-adherence during the first 7 days were recorded.
Asymptomatic intracerebral haemorrhage
Any haemorrhage seen on computed tomography (CT)/magnetic resonance imaging (MRI) scanning, if done after randomisation, with no neurological deterioration (as defined here).
Bleeding
Major bleed74
-
Fatal bleeding and/or
-
symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or intramuscular with compartment syndrome and/or
-
bleeding causing fall in haemoglobin of ≥ 20 g/l (≥ 1.24 mmol/l), or leading to transfusion of ≥ 2 units of whole blood or red blood cells.
Moderate bleed
-
Not major bleed and
-
bleeding causing fall in haemoglobin of ≥ 10 g/l (≥ 0.62 mmol/l) but < 20 g/l (< 1.24 mmol/l) and leading to no transfusion, or transfusion of only 1 unit of whole blood or red blood cells.
Minor bleed
-
Not major or moderate bleed and
-
comprising bruising, ecchymoses, gingival bleed or similar other type of bleeding. Note: this excludes asymptomatic intracranial haemorrhage.
Deep-vein thrombosis, symptomatic
Clinical suspicion of DVT will need confirmation by either venography or ultrasound examination.
Disposition
Disposition is categorised as death, institution or home:
-
Institution refers to warden controlled, residential home, care home, nursing home, still an inpatient, or readmitted to hospital.
-
Home refers to home alone, home with spouse/carer or at carer’s home.
Extracranial haemorrhage, major
An extracranial bleed that is major in severity (see Major bleed).
Feeding status
Feeding status is defined as:
-
oral – normal diet, soft diet
-
non-oral – nasogastric tube fed, percutaneous endoscopic gastrostomy (PEG)-tube fed, intravenous/subcutaneous fluids, no feeding/fluids.
Headache, requiring treatment or cessation of treatment
A headache occurring during treatment that necessitates intervention, including withdrawing antiplatelet treatment.
Intracerebral haemorrhage, secondary on computed tomography/magnetic resonance imaging scanning
Haemorrhagic infarct (HI): petechial infarction without space occupying effect –
-
HI1: small petechiae
-
HI2: more confluent petechiae.
Parenchymal haemorrhage: haemorrhage with mass effect –
-
PH1: < 30% of the infarcted area with mild space occupying effect
-
PH2: > 30% of the infarcted area with significant space occupying effect.
Note: patients with PH should not be enrolled into the trial.
Intracerebral haemorrhage, symptomatic
Neurological deterioration or death, associated with intracerebral haemorrhage found on CT/MRI scan or autopsy. The haemorrhage must be the predominant cause of the neurological deterioration.
Note: this excludes other forms of intracranial haemorrhage, including extradural, subdural and subarachnoid haemorrhage, which will be reported separately.
Intracranial haemorrhage
Symptomatic intracranial haemorrhage, extradural haemorrhage, subdural haemorrhage and/or subarachnoid haemorrhage.
Myocardial infarction
Either one of the following criteria satisfies the diagnosis for an acute, evolving or recent MI:75
-
Typical rise and fall of biochemical markers of myocardial necrosis with at least two of the following criteria –
-
ischaemic symptoms
-
development of pathologic Q waves on the electrocardiography (ECG)
-
ECG changes indicative of ischaemia (ST segment elevation or depression) or
-
coronary artery intervention (e.g. coronary angioplasty).
-
-
Pathological findings of an acute MI –
-
STEMI: MI with ST elevation on ECG
-
NSTEMI: MI with no ST elevation on ECG.
-
Neurological deterioration
An increase in NIHSS by ≥ 4 points over the baseline value.
Pulmonary embolism, symptomatic
The clinical suspicion of pulmonary embolism will need confirmation by either high-probability ventilation-perfusion lung scintigraphy, pulmonary angiography, inconclusive V/Q scan and DVT, or lead to death.
Recurrent stroke, symptomatic
A stroke, defined as below, occurring after the qualifying stroke, or a progression of neurological symptoms or signs (increase in NIHSS score of ≥ 4) in the same vascular territory as the index event. Classified as haemorrhagic or ischaemic (if documented by CT/MRI scan or autopsy), or of unknown type. The time from stroke onset and lesion side will be noted.
Note: this definition deliberately does not attempt to differentiate true recurrence from extension of the presenting lesion as this is clinically and radiologically difficult unless recurrence occurs in a new arterial territory.
Stroke
A clinical syndrome characterised by rapidly developing clinical symptoms and/or signs of focal (and at times global) loss of cerebral function with symptoms lasting ≥ 24 hours or leading to death, with no apparent cause other than that of vascular origin.
Transient ischaemic attack
A sudden focal neurological deficit of the brain or eye, presumed to be of vascular origin and lasting < 24 hours.
Note: the tissue diagnosis of TIA based on the results of MRI scanning will not be used as MRI scanning is not routinely available out of hours at many participating hospitals.
Time at home
Calculated as time from date of discharge to day 90 or death if earlier; those who die in hospital or are discharged to a non-home setting are given a score of zero. Readmission to hospital is not counted in this time.
Unstable angina
Presence of acute cardiac chest pain at rest without ST elevation on ECG and without elevation in cardiac enzymes.
Venous thromboembolism, symptomatic
Symptomatic DVT and/or symptomatic pulmonary embolism.
Assessments after randomisation
Participants were seen in clinic at days 7 (on treatment) and 35 (end of treatment plus 5–7 days to allow for wash-out) to ascertain whether any outcome or bleeding events had taken place (this included performing a full blood count) and to determine compliance with treatment. Final follow-up was performed centrally at 90 days by telephone from the co-ordinating centre in each country, with the assessor blinded to treatment allocation. If the participant could not be contacted (following multiple attempts), a questionnaire covering the same outcome measures was sent by post. Identification of recurrent cerebrovascular events used multiple sources of information: assessment by the investigator at days 7 and 35, or via serious adverse event (SAE) reporting, patient reporting at day 90 telephone follow-up, and by the general practitioner following a questionnaire posted to them shortly after day 90. The primary outcome, bleeding and investigator-reported SAEs (including cause-specific case fatality) were validated and categorised by expert adjudicators who were blinded to treatment assignment. Participants who did not receive their assigned treatment or who did not adhere to the protocol were still followed up in full at day 90.
Primary efficacy outcome
The primary efficacy outcome was the incidence and severity of recurrent stroke and TIA occurring by 90 days. The timing of 90 days was chosen to emulate the usual follow-up period in acute stroke trials. Severity of recurrent stroke was assessment of the mRS, a six-level ordered categorical scale:76,77 fatal stroke, non-fatal severe stroke (mRS score of 4–5), moderate stroke (mRS score of 2–3), mild stroke (mRS score of 0–1), TIA and no stroke or TIA. 77 The primary outcome was a balance of ischaemic stroke (potential benefit if reduced) and intracerebral haemorrhage (potential harm if increased).
Originally, the intention was to analyse across nine levels of severity comprising the individual seven levels of mRS, TIA and no event (protocol version 1.1, dated 17 October 2008; protocol version 1.2, dated 20 May 2009). Subsequently, the scale was reduced to five levels: fatal stroke, moderate to severe stroke (mRS score of 2–5), mild stroke (mRS score of 0–1), TIA and no event (protocol version 1.3, dated 20 December 2011; protocol version 1.4, dated 26 February 2013; protocol version 1.5, dated 28 February 2014). Finally, the scale was increased to six levels. 73 The changes resulted from multiple discussions among the statistical and clinical teams on simplifying the scale (from nine levels) but not overmerging mRS levels, in particular, keeping mild, moderate and severe stroke separate. These changes occurred during enrolment when the database was locked. A post hoc analysis comparing these three approaches (5, 6, 9 levels) was performed.
Choice of the primary efficacy outcome
Clinical trials are increasingly having to increase their sample size, largely because major advances have been made in preventing cardiovascular events (including stroke and TIA), which has meant that the risk of recurrent events has fallen. Furthermore, the number of randomised trials has also increased. As a result, it is increasingly difficult to find sufficient participants to enter trials. Vascular prevention studies typically count outcomes as dichotomous events (e.g. event vs. no event) although this is inefficient statistically and gives no indication regarding the severity of the recurrent event. Recurrent vascular events, such as stroke, could therefore be polychotomised with ordering of outcome events determined by severity.
The purpose of using an ordinal outcome was twofold. First, it allowed the effect of treatment to be assessed on the severity of recurrent events as well as their rate. In general, interventions that reduce the risk of recurrence (such as antithrombotics, blood pressure- and lipid-lowering therapies, and carotid surgery) also reduce the severity of those events that do occur;76,78 similarly, interventions that increase recurrence (e.g. hormone replacement therapy) also increase the severity of events. 79 Second, ordinalising dichotomous events improves statistical power so that sample size can be reduced for a given power, or power increased for a given sample size. Such an approach could reduce trial costs while improving statistical efficiency and amplifying the potential to demonstrate a treatment effect.
The approach can be used with any dichotomous outcome that can encompass a measure of severity, such as recurrence of stroke/TIA, bleeding and SAEs (as done here). 76 Other outcomes, such as MI, heart failure and venous thromboembolism, can be analysed in the same way. Although this approach has been tested empirically using published data,76,78,79 the TARDIS trial is the first trial to use the method prospectively.
Secondary efficacy outcomes
Prespecified secondary outcomes at day 9080 included activities of daily living [Barthel Index (BI)81], cognition [modified telephone Mini-Mental State Examination (t-MMSE),82 Telephone Interview for Cognition Scale – modified (TICS-M),83 categorical verbal fluency using animal naming84], health-related quality of life [EuroQol-5 Dimensions, three-level version (EQ-5D-3L),85 from which health status utility values (HSUVs) were calculated; EuroQoL Visual Analogue Scale (EQ-VAS)], and mood [short Zung Depression Scale (ZDS)86]. At discharge from initial hospitalisation, duration of hospital stay and discharge disposition (institution or home) were recorded.
Safety outcomes
The main safety outcome was bleeding comprising a five-level ordered categorical scale: fatal, major, moderate, minor and none. 76 The definitions of fatal, major and moderate bleeding were those of the International Society on Thrombosis and Haemostasis and are based on severity, site, fall in haemoglobin and need for transfusion. 74 Additional safety outcomes included all-cause and cause-specific case fatality, early neurological deterioration (defined as an increase from baseline to day 7 of at least 4 points on the NIHSS and/or decrease in the consciousness component of the NIHSS), and SAEs.
Information on recurrent stroke and TIA, MI and bleeding was collected using the SAE reporting forms, with additional information specific to the outcome.
Net balance in efficacy and safety
To assess the net balance between efficacy and hazard, composite end points of any stroke or major (including fatal) bleeding, and death, stroke, MI or major bleeding, were analysed.
Study oversight
The trial was conceived and designed by the grant applicants who wrote the protocol (available at http://tardistrial.org/jevpybki.htm). The study was approved by national and/or local ethics committees in each participating country and site, was registered (ISRCTN47823388) and adopted in the UK by the National Institute for Health Research (NIHR) Stroke Research Network. The trial was overseen by a Trial Steering Committee (TSC) (which included five independent members and a patient–public representative) and an International Advisory Committee (comprising each national co-ordinator). The day-to-day conduct of the trial was run by a Trial Management Committee based at the TARDIS co-ordinating centre in the Stroke Trials Unit in Nottingham. Study data were collected, monitored and analysed in Nottingham. Analysis, interpretation and report writing were performed independent of the funders and sponsor, and no pharmaceutical companies were involved in any part of the trial. The corresponding author wrote the first draft of this report; this and subsequent drafts were edited by the grant applicants, who all approved the decision to submit the manuscript for publication. The corresponding author and two statisticians (LJW, KF) had full access to all the data in the study. In addition, the corresponding author had final responsibility for the decision to submit for publication and is the guarantor for the study.
Data Monitoring Committee
An independent Data Monitoring Committee (DMC) reviewed unblinded data in confidence every 6 months; altogether they met on 13 occasions and recommended trial continuation for all but the last data review. The DMC was responsible for safeguarding the interests of trial participants, assessing the safety and efficacy of the intervention during the trial, assessing data integrity and monitoring the overall conduct of the trial. The DMC reviewed the recruitment of participants and assessed safety and efficacy measures by treatment group.
The DMC followed a pre-defined charter and were charged with informing the TSC if, at any time, the data showed evidence beyond reasonable doubt of a difference between the randomised groups in the primary outcome. They also considered data in the light of external information such as results from completed trials. One interim analysis was performed. In addition, the DMC could perform statistical assessments whenever they deemed it necessary.
The DMC were given specific stopping rules for efficacy and hazard but not futility; stopping criteria were based on the Haybittle–Peto rule (i.e. a difference of three standard errors was to be considered as clear evidence of a treatment effect):
The balance between safety and efficacy should be considered.
With respect to safety, the following outcomes in particular will initiate discussion and minuting of detailed reasons for recommending early stopping or continuation of the study:
-
The primary outcome (‘shift’ in mRS in participants having a recurrent stroke event or TIA) favours the control group (who receive standard antiplatelet therapy but not clopidogrel); p < 0.01 (nominal, two-sided).
-
Combined outcome of fatal or non-fatal stroke or major bleeding favours the control group, p < 0.01 (nominal, two-sided).
-
The overall rate of sICH exceeds 2%.
-
During the vanguard phase, major bleeding favours the control group; p < 0.01 (nominal, two-sided).
In making any decision, the committee will consider the overall internal and external evidence, the multiplicity of testing and the possibility that the trends in the data might be reversed with longer follow-up or increased recruitment.
With respect to efficacy, the committee will conduct formal interim analyses, after 40% and 70% of the target number of participants have been enrolled and had their 90-day outcome assessed, based on the following outcome.
-
Combined outcome of fatal or non-fatal stroke or major bleeding event favours the clopidogrel group; p < 0.001 (two-sided).
In making any decision, the committee will consider the overall internal and external evidence.
Missing data
As outcomes such as mRS, EQ-5D-3L/HSUV and BI include scores for death (6, 0 and –5, respectively) and in case treatment was associated with asymmetric effects on death and other outcome measures (e.g. more death and less impairment), an extreme value for death was added to the other outcome scales: EQ-VAS = –1, t-MMSE = –1, SSS = –1, TICS-M = –1, verbal fluency = –1, and ZDS = 102.5. 77,87 Including death in these secondary outcome scores has the additional advantage of increasing statistical power and of anchoring the scales to one another.
Statistical analyses
Statistical analyses were performed according to the published statistical analysis plan73 by Lisa J Woodhouse and Katie Flaherty (with oversight by SP) using SAS® software (version 9.3) (SAS Institute Inc., Cary, NC, USA; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration). Analyses were by intention to treat for all comparisons (i.e. according to the treatment group participants were allocated to and irrespective of the treatment they actually received). Data are shown as number (%), median [interquartile range (IQR)], mean [standard deviation (SD)] and OR (95% CIs).
Analysis of the effect of treatment on the primary efficacy outcome was analysed as a shift in stroke and its severity [fatal stroke, non-fatal severe stroke (mRS 4–5), moderate stroke (mRS 2–3), mild stroke (mRS 0–1), TIA and no stroke or TIA] with adjustment for the factors used in stratification and minimisation at the time of randomisation (index event – ischaemic stroke, TIA; country; guideline randomisation choice – aspirin/dipyridamole, clopidogrel, either; age; sex – female, male; pre-morbid mRS; time from onset to randomisation; number of antiplatelets before index event; stroke syndrome – lacunar syndrome (LACS), posterior circulation syndrome (POCS), partial anterior circulation syndrome (PACS), total anterior circulation syndrome (TACS) systolic blood pressure; gastroprotection – yes, no; use of heparin – yes, no; stroke severity – NIHSS; treated with recombinant tissue plasminogen activator – yes, no; ABCD2 score; number of TIA in last week88) and is reported as an adjusted common odds ratio (acOR) with 95% CIs. The common odds ratio (cOR) represents the odds of a patient on treatment moving categories of outcome as compared with a patient on control; cOR < 1 suggests benefit and cOR > 1 hazard. The OR and significance were calculated using ordinal logistic regression following a check (using the likelihood ratio test) that the assumption of common proportional odds was not violated. For sensitivity purposes, the primary outcome was also analysed without adjustment and as a binary outcome of fatal or major stroke. The heterogeneity of the treatment effect on the primary outcome was assessed in prespecified subgroups by adding an interaction term in an unadjusted ordinal logistic regression model. Similarly, the effect of treatment on the main safety outcome was analysed as a shift in bleeding and its severity (fatal, major, moderate, minor and none) with adjustment for the stratification and minimisation factors. For sensitivity purposes, bleeding was also analysed unadjusted, as a binary outcome of fatal and major bleeding, heterogeneity within subgroups was also assessed. The composite outcomes of stroke or major bleeding, and death, stroke, MI or major bleeding were compared between treatment groups using adjusted Cox regression.
Death was analysed using Kaplan–Meier and Cox regression models. Other outcomes were analysed using adjusted multiple linear regression (BI, ZDS, t-MMSE, TICS-M, verbal fluency, EQ-5D/HSUV and EQ-VAS). All analyses were also performed unadjusted for completeness. The nominal level of significance for all analyses, including interaction testing, was p < 0.05. No adjustment was made for multiplicity of testing for secondary analyses.
Sample size
The TARDIS trial was designed with a vanguard phase to assess safety, feasibility and tolerability [funded by the British Heart Foundation (BHF)] and a main phase to assess safety and efficacy (funded by NIHR Health Technology Assessment).
The null hypothesis (H0) was that intensive antiplatelets would not alter the frequency and severity of stroke/TIA in participants with previous ischaemic stroke or TIA. The alternative hypothesis was that the frequency and severity of stroke/TIA would differ between those participants randomised to intensive versus guideline antiplatelets. A total sample size89,90 of 4100 (2050 per group) participants with ischaemic stroke or TIA was required, assuming overall type I error rate (significance, alpha) = 0.05 with two-sided significance test. Power (1-beta) = 0.90, OR = 0.68 (equivalent to an OR = 0.57 and RRR = 0.31 for binary stroke), distribution in outcome as in Table 3, treatment crossovers 5% and losses to follow-up 2%, and a reduction of 20% for baseline covariate adjustment. 90,91 No adjustment was made for two interim analyses in view of the stringent stopping rules given to the DMC (see above).
| mRS (%) | No event | TIA | 0–1 | 2–3 | 2–5 | 4–5 | 6 (death) |
|---|---|---|---|---|---|---|---|
| Initial assumption | 93.82 | 3.57 | 1.53 | 0.77 | 0.51 | ||
| Final assumption | 93.15 | 3.22 | 1.23 | 1.30 | 0.55 | 0.55 |
Originally, a five-level stroke/TIA ordered outcome was planned with distribution based on data from the TARDIS trial during the vanguard phase (n = 392) (see Table 3). However, this was changed to a six-level scale to allow discrimination of moderate and severe non-fatal stroke outcomes, and to keep together mRS scores of 2 and 3 (which can be challenging to separate clinically). Distribution of mRS came from blinded data from a TSC report (based on n = 1460 participants).
Protecting against bias, including blinding
Multiple measures were taken to minimise bias: recruitment on the basis of predefined inclusion/exclusion criteria, with exclusion of patients enrolled in other trials; central data registration with real-time data validation and concealment of allocation; blinded central telephone assessment of day 90 outcomes by the National Coordinating Centre (NCC) staff; assessment of patient recall of treatment;92 blinded adjudication of outcomes (stroke/TIA, MI, bleeding), SAEs and CT/MRI scans; research staff trained in trial protocol and processes; analysis by intention to treat; analyses adjusted for baseline prognostic variables, including minimisation factors; adjustment for non-randomised acute treatment (thrombolysis with alteplase).
Neuroimaging scan adjudication
The CT or MRI brain scans were performed in accordance with local site practice at baseline in all patients with ischaemic stroke to confirm the diagnosis. Investigators decided whether or not to perform neuroimaging in patients with TIA depending on clinical need. Sites could also perform follow-up scans at any time point after enrolment according to clinical need (e.g. if a neurovascular outcome or bleeding was suspected). For this publication, scan-derived information is based on radiological reporting from local sites.
Future reports will utilise information based on central adjudication of neuroimages. Neuroimages were submitted to the International Coordinating Centre in Nottingham using one of two methods:
-
Uploaded onto the trial website as uncompressed encrypted non-anonymised digital imaging and communications in medicine (DICOM) files. Once the trial system had validated the files against the expected patient details, the files were then anonymised.
-
Sent by courier on a compact disc read-only memory (CD-ROM) or digital versatile disc (DVD), with files in DICOM format with pseudo-anonymisation of patient details; the patients were identified with their unique study number and initials.
When reviewed, some images were in non-DICOM format [e.g. portable network graphic (PNG), Joint Photographic Experts Group (JPG)] and these were converted to DICOM. The anonymised image files were then presented to a panel of expert adjudicators using a browser-based system driven from the trial database. Adjudicators were trained and assessed using the ACCESS system [https://sirs2.ccbs.ed.ac.uk/sirs2 (accessed August 2018)]93,94 and reviewed scans blinded to treatment assignment. Adjudication parameters were derived from the International Stroke Trial-3 and ENOS trial image adjudication systems71,95 and included information on:
-
the presence of an acute stroke lesion – location, mass effect and presence of secondary ischaemia or haemorrhage
-
the presence of pre-stroke changes – atrophy, white matter hyperintensities, old stroke.
Information from adjudication was used to inform the final diagnosis for all participants with a received scan. When clinical and radiological information were incongruent, Robert A Direen performed a second adjudication to confirm imaging findings.
Sites, investigators and monitoring
Training of investigators
All TARDIS investigators were trained in good clinical practice (through their host institution), the protocol and use of three assessment scales: NIHSS, mRS and BI. In addition, national telephone outcome assessors were trained and then tested with case scenarios in the mRS.
Schedule for monitoring of sites and data integrity
Site monitoring was performed by each NCC with the aim of ensuring quality control of the delivery of the protocol, collection of data and adherence with national regulations and ethics. Each recruiting site had a start-up visit for training and at least one monitoring visit; further visits were performed as deemed necessary by the NCC. Monitoring visits confirmed the presence of the participant and their consent, eligibility criteria, selected data critical to the trial (demographics, prescription of interventions and blood pressure) and reported SAEs.
Central statistical monitoring of the data was performed according to Buyse et al. 96 during the trial and prior to the locking of the data. Checks included logic and range checks, digit preference, comparison of univariate data between sites and comparison of multiple variable models between countries. The monitoring procedures were compliant with the requirements of the sponsor, the national ethics committees and regulatory authorities in the participating countries, and fulfilled good clinical practice requirements.
Protocol amendments
Five protocol amendments were made during the trial, these started from version 1.1.
Protocol version 1.1 to 1.2 (20 May 2009)
-
Investigational medicinal product (IMP): only clopidogrel-considered IMP, not aspirin and dipyridamole which are considered standard/routine treatment.
-
Secondary end points added: incidence and type of infection.
-
Inclusion criteria added:
-
All strokes must have motor weakness or dysphasia at time of randomisation.
-
All TIAs must have motor weakness or dysphasia lasting at least 10 minutes.
-
The ABCD score of > 4 changed from a score of > 5.
-
-
Exclusion criteria changed:
-
Aged < 50 years changed from < 40 years.
-
Motor weakness or dysphasia lasting < 10 minutes changed from < 30 minutes.
-
Patients with definite need for treatment with clopidogrel (e.g. recent MI).
-
Definite need for full-dose oral (e.g. warfarin) or parenteral (e.g. heparin or glycoprotein IIb IIIa inhibitors) anti-coagulation. (Low-dose heparin for DVT prophylaxis is allowed.)
-
Definite need for, or currently on, triple antiplatelet therapy or anticoagulation.
-
Received thrombolysis within the last 30 hours changed from indication for, or received (in last week), thrombolysis.
-
Pre-morbid dependency mRS score of > 2 changed from a mRS score of > 3.
-
Known haemoglobin < 10 g/dl.
-
Known platelet count < 100 × 109/l.
-
Known white cell count < 3.5 × 109/l.
-
Planned surgery during the first month post stroke (e.g. carotid endarterectomy) deleted.
-
-
Statistics: added – the effect of the intervention on the primary outcome will be performed within the following subgroups of subjects by:
-
Age < 75 years, > 75 years.
-
Sex – male or female.
-
Stroke/TIA.
-
Stroke subtype – lacunar, posterior fossa or cortical.
-
Stroke severity – severe, moderate/mild; NIHSS score of < 10, > 10.
-
Baseline systolic blood pressure of > 160 mmHg, 140–160 mmHg or < 140 mmHg.
-
Treatment delay of > 24 hours, < 24 hours.
-
Patients enrolled into TCD substudy.
-
Patients enrolled into P-selectin substudy.
-
Patients on antiplatelet therapy at randomisation – mono, dual.
-
Aspirin naive versus aspirin.
-
Heparin – none, unfractionated, low-molecular-weight heparin.
-
Number of TIAs in the last week.
-
Thrombolysis – yes, no.
-
An ABCD2 score of 4, > 4.
-
Protocol version 1.2 to 1.3 (20 December 2011)
-
Investigational medicinal product: aspirin and dipyridamole considered as IMP in addition to clopidogrel.
-
Known side effects: adverse reactions and drug interactions added for aspirin and dipyridamole.
-
Purpose and primary objective: updated to reflect use of either clopidogrel or aspirin/dipyridamole as guideline therapy owing to updated NICE guidance. 30
-
Trial duration changed: start-up phase 4 years changed from 3 years.
-
The main phase will recruit in the order of ≈ 3100 patients (depending on the rate and distribution of ordinal events) and will last an additional 5 years.
Protocol version 1.3 to 1.4 (26 February 2013)
-
Funding source: BHF (start-up phase), NIHR Health Technology Assessment (HTA) programme (main phase).
-
Investigational medicinal product changed:
-
Aspirin loading dose to 300 mg, then 50–150 mg per day. Aspirin may be given in combination with dipyridamole as Asasantin® (Boehringer Ingelheim) or equivalent changed from loading dose 300 mg, then 75 mg once a day.
-
Dipyridamole 225 mg to 450 mg daily, including 200 mg modified-release twice daily changed from 200 mg modified-release twice a day.
-
-
Inclusion criteria:
-
Patients who are on combined therapy of aspirin plus dipyridamole or on monotherapy (e.g. aspirin alone, clopidogrel alone or dipyridamole alone) are eligible for recruitment changed from Already on dual anti platelet therapy.
-
Neuroimaging is essential for ischaemic stroke to exclude intracranial haemorrhage and a non-stroke diagnosis. If the patient received thrombolysis, a post-thrombolysis/pre-TARDIS scan needs to be carried out to exclude new thrombolysis-associated bleeding prior to enrolment. Typically, this is done routinely as ‘standard of care’ but, if it is not done, then it must be done prior to enrolment.
-
Patients thrombolysed for stroke with full recovery in < 24 hours from the onset of symptoms are eligible for inclusion as a TIA providing neuroimaging post thrombolysis excludes intracranial haemorrhage. Changed from Neuroimaging is essential for ischaemic stroke to exclude intracranial haemorrhage and/or non-stroke diagnosis.
-
-
Exclusion criteria:
-
Deleted participant has taken clopidogrel or dipyridamole after the index event but prior to randomisation (aspirin is allowed between ictus onset and randomisation).
-
Added patients who have not had post thrombolysis neuroimaging.
-
-
Comparators: the remaining decision is dependent on what the patient could be randomised to, and the general rule is they cannot have something that may confound the guideline group:
-
aspirin, clopidogrel and dipyridamole versus clopidogrel versus aspirin and dipyridamole – aspirin only before randomisation (i.e. no clopidogrel or dipyridamole)
-
aspirin, clopidogrel and dipyridamole versus clopidogrel – aspirin or clopidogrel only before randomisation (i.e. no dipyridamole)
-
aspirin, clopidogrel and dipyridamole versus aspirin and dipyridamole – aspirin or dipyridamole only before randomisation (i.e. no clopidogrel added).
-
-
Protocol violations:
-
Added patients who do not have a post thrombolysis scan.
-
An ABCD2 score of < 4, not a crescendo TIA and not on dual antiplatelet therapy or monotherapy antiplatelets changed from an ABCD2 score of < 4, not a crescendo TIA and not on dual antiplatelet therapy.
-
Patient receives > 450 mg of dipyridamole daily changed from patient receives > 400 mg daily of dipyridamole.
-
-
Sponsor: sponsorship of the trial is undertaken in each participating country. The University of Nottingham will hold a contract with each sponsor. The University of Nottingham is the trial sponsor in the UK but not in other countries and will delegate responsibility for the design and conduct of the trial to the chief investigator via our sponsor/chief investigator agreement. Changed from the University of Nottingham is the trial sponsor in the UK and will delegate responsibility for design and conduct of the trial to the chief investigator via our sponsor/chief investigator agreement.
-
Appendix J: trial inclusion flow chart – already on mono/dual antiplatelet therapy changed from already on dual antiplatelet therapy.
Protocol version 1.4 to 1.5 (28 February 2014)
-
Investigational medicinal product: aspirin (loading dose of 300 mg, then 50–100 mg daily) and dipyridamole (between 225 mg and 450 mg daily), or guideline antiplatelet therapy (aspirin and dipyridamole or clopidogrel, doses as above) changed from aspirin (loading dose of 300 mg, then 75 mg daily), and dipyridamole (modified-release 200 mg twice daily).
-
Study duration: 8 years changed from 5 years.
-
Inclusion criteria added:
-
Positive neuroimaging to support the new ischaemic event with resonance imaging diffusion.
-
Already on combined dual antiplatelet therapy (aspirin and dipyridamole).
-
-
Exclusion criteria:
-
Added patients on aspirin and clopidogrel prior to the underlying event.
-
Removed TCD (as not in main study).
-
-
Protocol violations:
-
Participant has taken dipyridamole between index event and prior to stroke randomisation, when clopidogrel is the control treatment.
-
Participant has taken clopidogrel between index event and prior to stroke randomisation, when aspirin and dipyridamole is the control treatment changed from participant has taken dipyridamole or clopidogrel following the index event and prior to stroke randomisation.
-
Added patient does not receive the correct loading dose.
-
Substudies
Three substudies were funded by the BHF in the TARDIS start-up phase.
Platelet function
Platelet expression of P-selectin was used to monitor antiplatelet effects of the interventions. Blood was taken from all patients at baseline and day 7, fixed, posted by Royal Mail (Royal Mail Plc, London, UK) to Nottingham, and the surface platelet expression of P-selectin measured using flow cytometry, with blinding to patient and treatment identity. The effect of pre-randomisation antiplatelet agents on P-selectin has been published. 97 The effect of randomised antiplatelet agents on P-selectin, and the relationship between P-selectin and outcomes (recurrence, bleeding) is being analysed. Continuation of this substudy in the main phase of the TARDIS trial was not funded by the HTA programme but was continued, ad hoc, with local funding. As a result, platelet function was only assessed in a minority of patients.
Cerebral emboli
The TCD recordings were performed from the middle cerebral artery at baseline and day 2 using Nicolet/EME TCD systems (Pioneer Medical Devices AG, Berlin, Germany) with a 2 MHz transducer, as we have previously described. 98 One-hour recordings were stored digitally and transferred to London for analysis by Hugh S Markus, as before,43 with blinding to patient and treatment identity. Recording protocols were similar to those successfully used in the CARESS study. 43 Unfortunately, the lack of TCD machines across UK sites, and lack of incentives for NIHR research staff to perform substudies, meant that few participants had TCD performed (baseline – 39, day 3–21, with 19 participants at both time points), which was deemed insufficient to analyse. Continuation of this substudy in the main phase of the TARDIS trial was not funded by the HTA programme and the substudy was closed for the main phase.
Health economic analyses
These analyses, and the necessary data collection, were not funded by the HTA programme and were not performed.
Chapter 3 Results
Recruitment
The trial was designed in 2007 and initial funding was confirmed in 2008. The trial commenced recruitment on 7 April 2009 and enrolment was halted on the advice of the independent DMC on 18 March 2016, after a total of 3096 participants (out of a planned target of 4100; 75.5%) had been enrolled. Prior to this, the DMC had met on 12 occasions and recommended trial continuation. The advice to stop was based on a combination of three observations:
-
the presence of a significant increase in major (including fatal) bleeding in participants randomised to intensive antiplatelet therapy
-
intensive antiplatelet therapy was associated with a non-significant reduction in the primary outcome, but not enough to numerically outweigh the increase in bleeding
-
a conditional power analysis suggested that the trial was highly unlikely to demonstrate a significant difference in the primary outcome.
Once the decision to halt further recruitment had been made, patients randomised to intensive antiplatelet therapy were telephoned and asked to switch treatment to guideline therapy. The TSC reviewed the same data as well as additional analyses on 12 April 2016 and additionally noted that there was no difference in the net balance between benefit and hazard based on the composite of death, stroke, MI and major bleeding. The TSC stopped recruitment on the basis of futility.
Participant flow
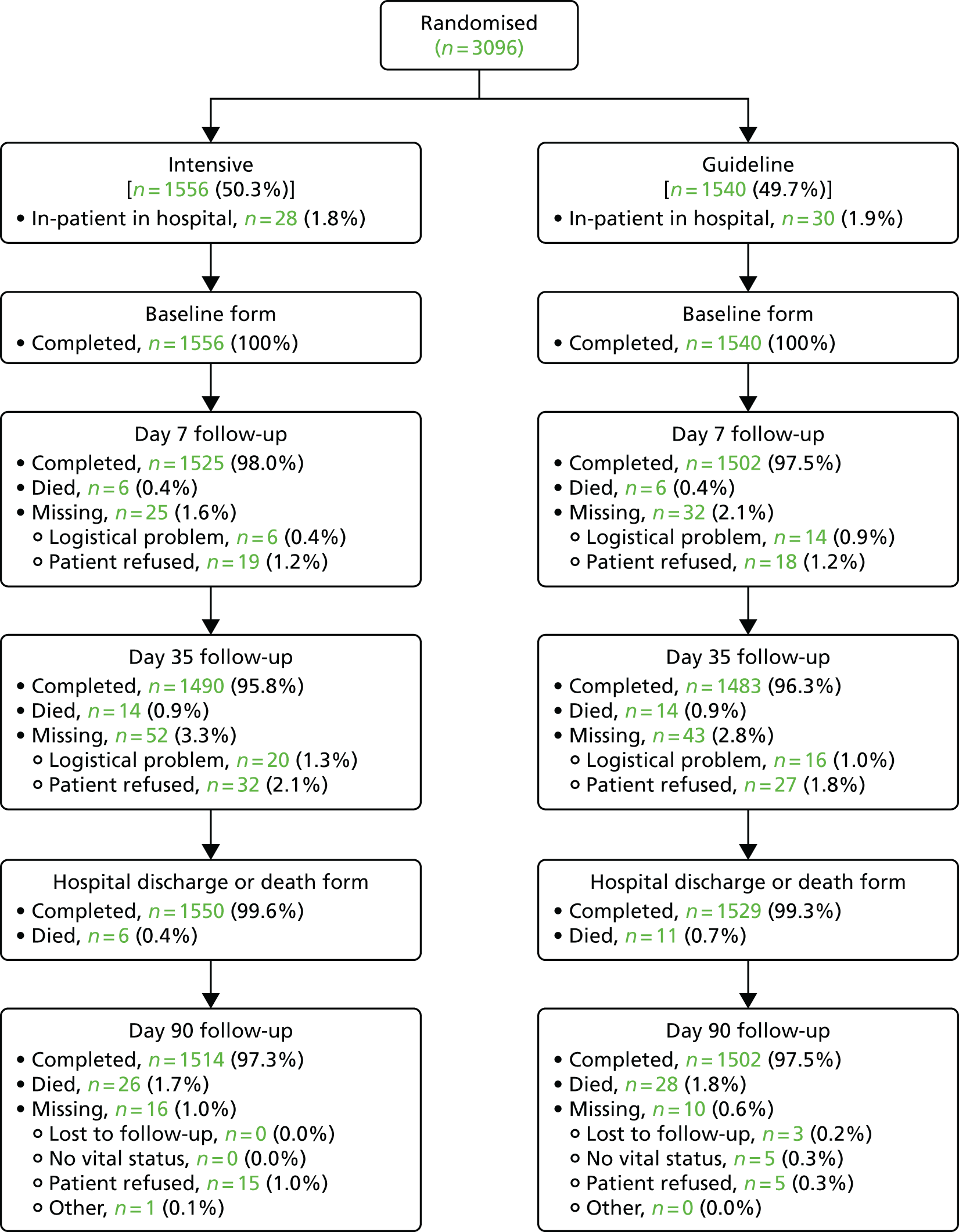
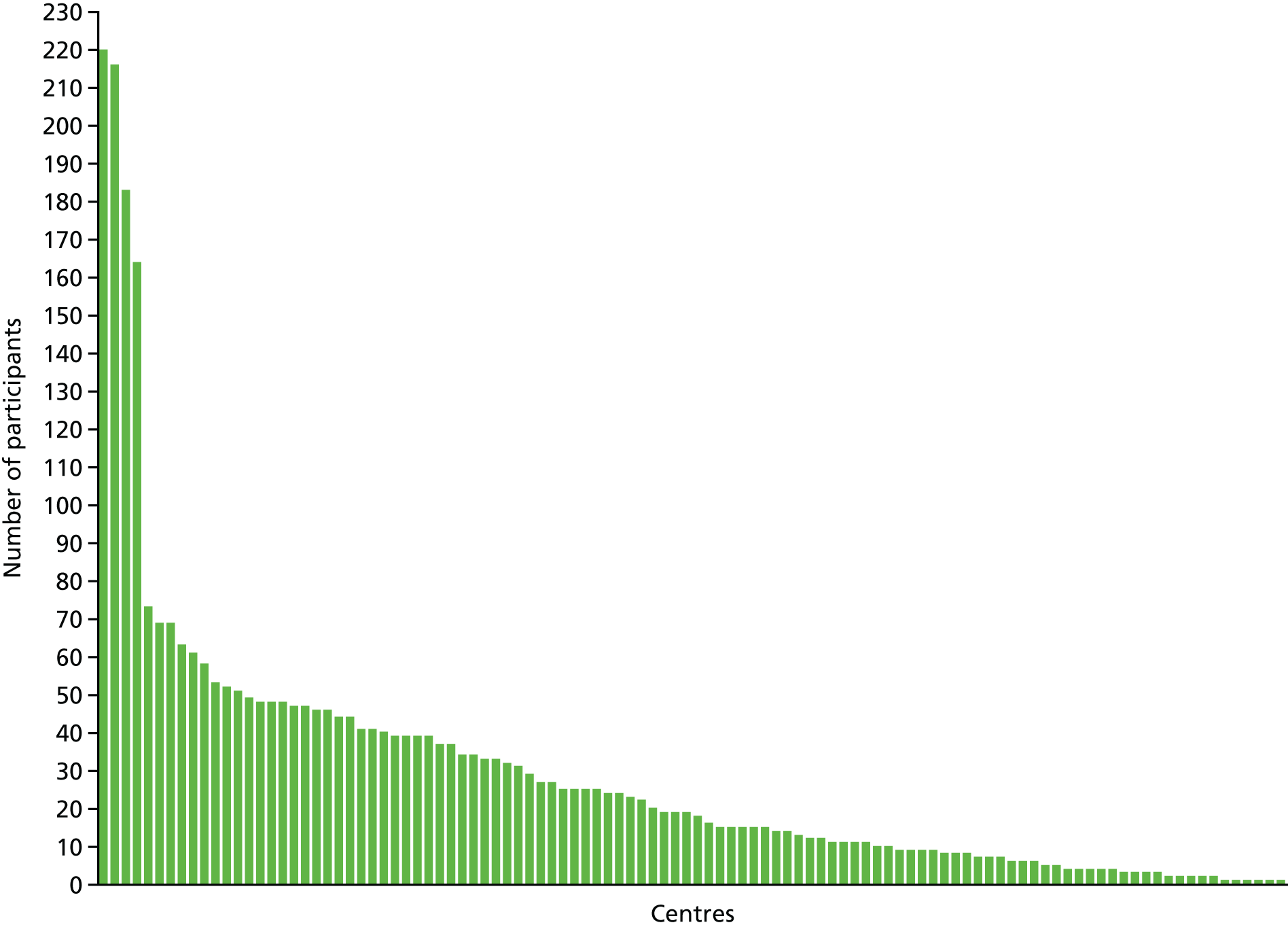
The recruitment curve is shown in Figure 1 and shows three phases of recruitment: an initial start-up phase with very low rate, a vanguard phase funded by British Heart Foundation with an intermediate rate and a main phase with high rate and funded by the NIHR’s HTA programme. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 2. By 90 days, 54 (1.7%) patients had died and data were missing from 26 (0.8%) patients. A majority of participants (2955, 95.4%) were recruited in the UK, with others from Denmark and Georgia (134, 4.3%) and New Zealand (7, 0.2%) (Table 4). Four sites recruited a far higher proportion of patients than other sites (‘waterfall plot’; Figure 3).
FIGURE 1.
Recruitment graph by time. Updated from Bath et al. 99 © 2016 World Stroke Organization. This article is distributed under the terms of the Creative Commons Attribution 3.0 License (http://www.creativecommons.org/licenses/by/3.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
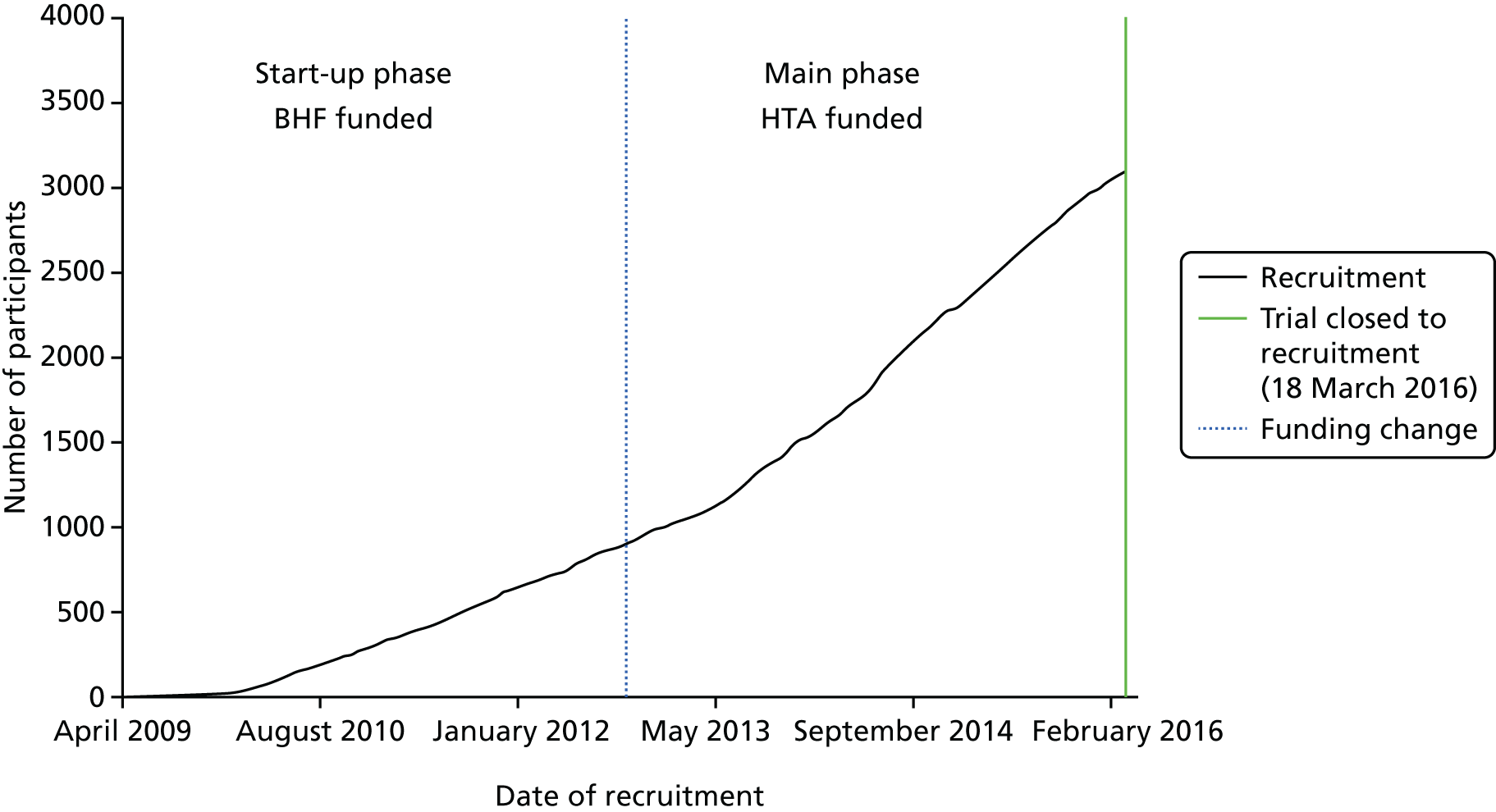
FIGURE 2.
The CONSORT flow diagram showing the flow of participants in the trial.
| Countries | Number of sites (%) | Number of patients (%) |
|---|---|---|
| UK | 100 (94.3) | 2955 (95.4) |
| Georgia | 3 (2.8) | 83 (2.7) |
| Denmark | 2 (1.9) | 51 (1.6) |
| New Zealand | 1 (0.9) | 7 (0.2) |
| Total | 106 (100) | 3096 (100) |
FIGURE 3.
Recruitment by hospital sites (‘waterfall plot’). Updated from Bath et al. 99 © 2016 World Stroke Organization. This article is distributed under the terms of the Creative Commons Attribution 3.0 License (http://www.creativecommons.org/licenses/by/3.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Baseline data
Table 5 shows the baseline characteristics of study participants, which were well matched except for a difference in ‘other antiplatelets’ (absolute difference of 0.5%). The mean age was 69.0 (SD 10.1) years and 1945 (62.8%) participants were male. Clinical information at the time of randomisation, and subsequent investigations, judged the qualifying event to be ischaemic stroke in 2220 participants (71.7%), TIA in 838 participants (27.1%) and non-ischaemic stroke/TIA (mimic) in 38 participants (1.2%). The median time from ictus to randomisation was 29.3 (IQR 21.8–39.6) hours; 314 (10.1%) and 651 (21.0%) participants were recruited within 12 and 13–24 hours of onset, respectively. In participants recruited with an ischaemic stroke, the mean NIHSS score was 4.0 (SD 3.8) and 336 participants (15.7%) received thrombolysis. In participants who were thrombolysed, the median times to this commencing from ictus and randomisation were 2.3 (IQR 1.8–3.0) hours and 36.5 (IQR 28.4–42.6) hours, respectively. In TIA participants, the median ABCD2 score was 5.0 (IQR 5.0–6.0), 155 participants (20.1%) presented with a crescendo TIA (defined as more than one TIA over the previous week), and 36 participants (4.3%) were already taking two antiplatelet agents. Among all participants, the mean blood pressure was 143.5 (SD 18.2)/79.5 (SD 11.4) mmHg and the clinical syndrome was cortical (combined total and partial anterior syndromes) in 1593 participants (51.5%) and lacunar in 1288 participants (41.6%). 63
| Characteristic | Total | Intensive | Guideline |
|---|---|---|---|
| Number of participants | 3096 | 1556 | 1540 |
| Mean age (years) (mean SD)a | 69.0 (10.1) | 69.1 (9.9) | 68.9 (10.3) |
| Male sex, n (%)a | 1945 (62.8) | 982 (63.1) | 963 (62.5) |
| Geographical region, n (%)b | |||
| UK | 2955 (95.4) | 1482 (95.2) | 1473 (95.6) |
| Denmark | 51 (1.6) | 26 (1.7) | 25 (1.6) |
| Georgia | 83 (2.7) | 45 (2.9) | 38 (2.5) |
| New Zealand | 7 (0.2) | 3 (0.2) | 4 (0.3) |
| Ethnicity, n (%) | |||
| White | 2939 (94.9) | 1489 (95.7) | 1450 (94.2) |
| Black | 63 (2.0) | 27 (1.7) | 36 (2.3) |
| East Asian | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| South Asian | 46 (1.5) | 18 (1.2) | 28 (1.8) |
| South East Asian | 1 (0.0) | 1 (0.1) | 0 (0.0) |
| Other Asian | 11 (0.4) | 7 (0.4) | 4 (0.3) |
| Mixed: white and black | 9 (0.3) | 3 (0.2) | 6 (0.4) |
| Mixed: other | 2 (0.1) | 2 (0.1) | 0 (0.0) |
| Other | 12 (0.4) | 4 (0.3) | 8 (0.5) |
| Not stated | 11 (0.4) | 4 (0.3) | 7 (0.5) |
| Dominant hand, right, n (%) | 2798 (91.5) | 1396 (90.8) | 1402 (92.2) |
| Source of referral, n (%) | |||
| Emergency department | 2057 (66.4) | 1037 (66.6) | 1020 (66.2) |
| Outpatient clinic | 130 (4.2) | 66 (4.2) | 64 (4.2) |
| Ambulance | 600 (19.4) | 302 (19.4) | 298 (19.4) |
| General practitioner | 157 (5.1) | 72 (4.6) | 85 (5.5) |
| Inpatient ward | 58 (1.9) | 28 (1.8) | 30 (1.9) |
| Other | 94 (3.0) | 51 (3.3) | 43 (2.8) |
| mRS score, pre-stroke [/6], median (IQR)a | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| 0, n (%) | 2604 (84.1) | 1308 (84.1) | 1296 (84.2) |
| 1–2, n (%) | 490 (15.8) | 247 (15.9) | 243 (15.8) |
| 3, n (%) | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| Medical history, n (%) | |||
| Prior antiplatelet agents | |||
| Aspirin | 816 (26.4) | 412 (26.5) | 404 (26.2) |
| Aspirin and dipyridamole | 85 (2.7) | 43 (2.8) | 42 (2.7) |
| Clopidogrel | 162 (5.2) | 89 (5.7) | 73 (4.7) |
| Other | 17 (0.5) | 13 (0.8) | 4 (0.3) |
| Prior heparin | 7 (0.2) | 2 (0.1) | 5 (0.3) |
| Glycoprotein inhibitor | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastroprotection (PrPI/H2RA)a | 803 (25.9) | 405 (26.0) | 398 (25.8) |
| Hypertension | 1824 (58.9) | 930 (59.8) | 894 (58.1) |
| Antihypertensives | 1739 (56.2) | 880 (56.6) | 859 (55.8) |
| Hyperlipidaemia | 1317 (44.3) | 655 (43.8) | 662 (44.8) |
| Lipid lowering | 1381 (44.6) | 679 (43.6) | 702 (45.6) |
| Diabetes mellitus | 590 (19.1) | 280 (18.0) | 310 (20.1) |
| Insulin | 59 (1.9) | 32 (2.1) | 27 (1.8) |
| Oral agents | 347 (11.2) | 155 (10.0) | 192 (12.5) |
| Both (insulin and oral agents) | 79 (2.6) | 40 (2.6) | 39 (2.5) |
| Neither (insulin or oral agents) | 105 (3.4) | 53 (3.4) | 52 (3.4) |
| AFc | 1 (0.0) | 0 (0.0) | 1 (0.1) |
| Stroke | 348 (11.2) | 189 (12.1) | 159 (10.3) |
| TIA | 337 (10.9) | 174 (11.2) | 163 (10.6) |
| Ischaemic heart disease | 403 (13.0) | 196 (12.6) | 207 (13.4) |
| PAD | 70 (2.3) | 40 (2.6) | 30 (2.0) |
| Family history of young stroke | 170 (5.9) | 89 (6.2) | 81 (5.6) |
| Smoking, current, n (%) | 784 (25.7) | 404 (26.4) | 380 (24.9) |
| Alcohol intake, high (> 21 units per week), n (%) | 291 (9.7) | 150 (10.0) | 141 (9.4) |
| Feeding status, n (%) | |||
| Normal diet | 2874 (92.8) | 1444 (92.8) | 1430 (92.9) |
| Soft diet | 168 (5.4) | 84 (5.4) | 84 (5.5) |
| Nasogastric tube fed | 51 (1.6) | 26 (1.7) | 25 (1.6) |
| PEG/RIG fed | 3 (0.1) | 2 (0.1) | 1 (0.1) |
| IV/SC fluids | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nothing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Qualifying event, n (%)b | |||
| Ischaemic stroke | 2220 (71.7) | 1121 (72.0) | 1099 (71.4) |
| TIA | 838 (27.1) | 413 (26.6) | 425 (27.6) |
| Crescendoa,d | 155 (20.1) | 72 (18.6) | 83 (21.6) |
| Dual antiplatelets | 36 (4.3) | 23 (5.6) | 13 (3.1) |
| Non-ischaemic stroke/TIAe | 38 (1.2) | 22 (1.4) | 16 (1.0) |
| Side of lesion, right, n (%) | 1428 (51.2) | 698 (50.1) | 730 (52.3) |
| Weakness, n (%) | 2789 (90.1) | 1392 (89.5) | 1397 (90.8) |
| Sensory loss, n (%) | 1066 (34.4) | 511 (32.8) | 555 (36.0) |
| Dysphasia, n (%) | 1007 (32.5) | 522 (33.5) | 485 (31.5) |
| Isolated, n (%) | 160 (5.2) | 88 (5.7) | 72 (4.7) |
| Neglect, n (%) | 331 (10.7) | 154 (9.9) | 177 (11.5) |
| Hemianopia, n (%) | 304 (9.8) | 146 (9.4) | 158 (10.3) |
| Isolated, n (%) | 16 (0.5) | 6 (0.4) | 10 (0.6) |
| Dysarthria, n (%) | 1279 (41.3) | 650 (41.8) | 629 (40.8) |
| Posterior circulation, n (%) | 287 (9.3) | 147 (9.4) | 140 (9.1) |
| NIHSS score (/42), mean (SD) | 2.8 (3.6) | 2.9 (3.7) | 2.7 (3.5) |
| ABCD2 score [/7], median (IQR)a | 5.0 (5.0–6.0) | 5.0 (5.0–6.0) | 5.0 (5.0–6.0) |
| GCS score [/15], median (IQR) | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) |
| Classification, n (%)a | |||
| Total anterior | 181 (5.9) | 86 (5.5) | 95 (6.2) |
| Partial anterior | 1412 (45.6) | 714 (45.9) | 698 (45.4) |
| Lacunar | 1288 (41.6) | 646 (41.5) | 642 (41.7) |
| Posterior | 213 (6.9) | 110 (7.1) | 103 (6.7) |
| TOAST, n (%)f | |||
| Cardioembolicc | 134 (4.4) | 65 (4.2) | 69 (4.5) |
| Large vessel | 490 (16.0) | 268 (17.5) | 222 (14.6) |
| Small vessel | 1224 (40.0) | 621 (40.5) | 603 (39.6) |
| Mixed | 22 (0.7) | 8 (0.5) | 14 (0.9) |
| Other | 1182 (38.7) | 569 (37.1) | 613 (40.2) |
| SBP (mmHg),a mean (SD) | 143.5 (18.2) | 143.4 (17.8) | 143.6 (18.5) |
| DBP (mmHg), mean (SD) | 79.5 (11.4) | 79.4 (11.3) | 79.6 (11.5) |
| Heart rate, mean (b.p.m.), mean (SD) | 73.1 (12.6) | 72.9 (12.5) | 73.2 (12.7) |
| Haemoglobin (g/l), mean (SD) | 141.8 (14.7) | 141.9 (15.1) | 141.6 (14.3) |
| Blood sugar (mmol/l), mean (SD) | 6.8 (2.5) | 6.8 (2.6) | 6.8 (2.5) |
| Temperature (Celsius), mean (SD) | 36.5 (0.5) | 36.5 (0.5) | 36.5 (0.5) |
| Weight (approximate, in kg), mean (SD) | 75.3 (16.6) | 75.5 (16.7) | 75.1 (16.5) |
| Brain imaging | |||
| Normal/no lesion, n (%) | 1550 (50.1) | 770 (49.5) | 780 (50.7) |
| Ischaemic stroke, n (%) | 1390 (45.0) | 702 (45.1) | 688 (44.8) |
| Non-stroke, n (%) | 6 (0.2) | 4 (0.3) | 2 (0.1) |
| No brain scan, n (%) | 146 (4.7) | 79 (5.1) | 67 (4.4) |
| Time OTR (hours),a median (IQR) | 29.3 (21.8–39.6) | 29.3 (21.7–39.7) | 29.3 (21.9–39.5) |
| Index event | |||
| Ischaemic stroke, median (IQR) | 32.1 (24.7–41.2) | 32.2 (24.6–41.7) | 32.0 (24.8–41.0) |
| TIA, median (IQR) | 24.2 (17.5–29.7) | 24.3 (17.5–29.5) | 24.2 (17.5–30.0) |
| Time (hours), n (%) | |||
| ≤ 12 | 314 (10.1) | 147 (9.4) | 167 (10.8) |
| 13–24 | 651 (21.0) | 342 (22.0) | 309 (20.1) |
| 25–48 | 2123 (68.6) | 1063 (68.3) | 1060 (68.8) |
| > 48c | 8 (0.3) | 4 (0.3) | 4 (0.3) |
| Thrombolysisa, n (%) | 341 (11.0) | 169 (10.9) | 172 (11.2) |
| Time to thrombolysis (hours), median (IQR) | 2.3 (1.8–3.0) | 2.3 (1.7–3.1) | 2.4 (1.9–3.0) |
Antiplatelet treatment
Out of the 3096 recruited participants, 1556 were randomised to intensive antiplatelet therapy and 1540 to the guideline group (see Figure 2). More participants were randomised to clopidogrel alone (n = 849, 55.1%) than combined aspirin and dipyridamole (n = 691, 44.9%) in the control group. Adherence to randomised treatment over the first 7 days was fair in both treatment groups: initial treatment was received in 83.5% of patients and 98.4% received at least some randomised treatment during the first week (Table 6). Participants randomised to intensive antiplatelets were more likely to receive all treatment in the first week than those randomised to guideline treatment.
| Adherence, n (%) | All | Intensive | Guideline | p-value |
|---|---|---|---|---|
| Participants randomised | 3096 | 1556 | 1540 | |
| Adherence during first 7 days | ||||
| First treatment | 2584 (83.5) | 1391 (89.4) | 1193 (77.5) | < 0.001 |
| Any treatment | 3045 (98.4) | 1525 (98.0) | 1520 (98.7) | 0.14 |
| Incomplete treatment by day 7 | 974 (31.5) | 459 (29.5) | 515 (33.4) | 0.030 |
| Discharged | 652 (21.1) | 299 (19.2) | 353 (22.9) | 0.026 |
| Lost to follow-up | 51 (1.6) | 23 (1.5) | 28 (1.8) | 0.45 |
| Recurrent stroke/TIA | 52 (1.7) | 22 (1.4) | 30 (1.9) | 0.33 |
| Bleeding event | 93 (3.0) | 57 (3.7) | 36 (2.3) | 0.032 |
| Adverse event, unacceptable | ||||
| Headache | 27 (0.9) | 21 (1.3) | 6 (0.4) | 0.002 |
| Rash | 5 (0.2) | 3 (0.2) | 2 (0.1) | 0.65 |
| SAE | 95 (3.1) | 50 (3.2) | 45 (2.9) | 0.63 |
| Death | 11 (0.4) | 5 (0.3) | 6 (0.4) | 0.72 |
| Anticoagulation for AF | 31 (1.0) | 18 (1.2) | 13 (0.8) | 0.51 |
| Carotid endarterectomy | 37 (1.2) | 19 (1.2) | 18 (1.2) | 0.94 |
| Other | 185 (6.0) | 84 (5.4) | 101 (6.6) | 0.13 |
The rate of treatment crossover was low: 6 (0.4%) participants randomised to the intensive group did not receive triple antiplatelets and 30 (1.9%) participants randomised to the guideline group received intensive antiplatelets at some point in the treatment phase. During treatment, 37 (1.2%) participants had carotid endarterectomy leading to temporary cessation of one or more antiplatelets (Table 6). A further 31 (1.0%) participants had antiplatelet therapy replaced with oral anticoagulation following identification of AF. Gastroprotection was recommended in the protocol and was provided in 803 (25.9%) patients: intensive for 405 patients (26.0%) and guideline for 398 patients (25.8%). Following the final DMC review and early closure to recruitment, participants randomised to intensive antiplatelet therapy were immediately asked to switch treatment to guideline therapys.
Outcome assessment
Final follow-up (at day 90) was completed for 3016 (97.4%) participants and vital status was available for all but five (0.1%) (see Figure 2). Outcomes were determined by telephone in 2889 (93.3%) participants and by post in 67 (2.2%) participants.
Primary efficacy outcome
The primary outcome (recurrent stroke or TIA and their severity by day 90) was determined in 3070 (99.2%) participants. Overall, a total of 198 (6.4%) participants had a recurrent stroke or TIA (intensive, n = 93, vs. guideline, n = 105; Table 7), which comprised 118 strokes (ischaemic, n = 96; haemorrhagic, n = 19; unknown type due to absence of neuroimaging, n = 5; both an ischaemic and haemorrhagic stroke, n = 1; and both an ischaemic and unknown type of stroke, n = 1) and 80 TIAs. There was no difference in the incidence and severity of stroke or TIA (acOR 0.90, 95% CI 0.67 to 1.20; p = 0.47) or fatal stroke (aOR 1.95, 95% CI 0.76 to 4.99; p = 0.16) between intensive and guideline antiplatelet therapies (Figure 4 and see Table 7). The likelihood ratio test for the proportional odds assumption was not significant (χ2 = 0.178, degrees of freedom = 8; p = 1.00). The primary efficacy outcome by time to recruitment is shown in Table 8.
| Outcome | All, n (%) | Intensive, n (%) | Guideline, n (%) | Adjusted cOR/OR (95% CI) | p-value | Unadjusted cOR/OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Number | 3096 | 1556 | 1540 | ||||
| Primary outcome | 3070 | 1540 | 1530 | ||||
| Ordinal stroke/TIA | 198 (6.4) | 93 (6.0) | 105 (6.9) | 0.90 (0.67 to 1.20) | 0.47 | 0.88 (0.66 to 1.18) | 0.39 |
| Death (mRS score of 6) | 20 (0.7) | 13 (0.8) | 7 (0.5) | 1.95 (0.76 to 4.99) | 0.16 | 1.85 (0.74 to 4.66) | 0.19 |
| mRS score of 4–5 | 20 (0.7) | 11 (0.7) | 9 (0.6) | ||||
| mRS score of 2–3 | 45 (1.5) | 22 (1.4) | 23 (1.5) | ||||
| mRS score of 0–1 | 33 (1.1) | 15 (1.0) | 18 (1.2) | ||||
| TIAa | 80 (2.6) | 32 (2.1) | 48 (3.1) | ||||
| No stroke/TIA | 2872 (93.6) | 1447 (94.0) | 1425 (93.1) | ||||
| Sensitivity analyses | |||||||
| Ordinal, per protocol | 124 (5.9) | 65 (6.0) | 59 (5.9) | 1.07 (0.74 to 1.55) | 0.72 | 1.03 (0.71 to 1.48) | 0.89 |
| Recurrent stroke or TIA | 198 (6.4) | 93 (6.0) | 105 (6.9) | 0.88 (0.65 to 1.18) | 0.38 | 0.87 (0.65 to 1.16) | 0.35 |
| Recurrent stroke | 118 (3.8) | 61 (4.0) | 57 (3.7) | 1.07 (0.74 to 1.56) | 0.71 | 1.07 (0.74 to 1.54) | 0.73 |
| Ischaemic | 96 (3.1) | 46 (3.0) | 50 (3.3) | 0.91 (0.61 to 1.38) | 0.66 | 0.91 (0.61 to 1.37) | 0.65 |
| Haemorrhagic | 19 (0.6) | 14 (0.9) | 5 (0.3) | 2.89 (1.02 to 8.19) | 0.046 | 2.80 (1.01 to 7.79) | 0.049 |
| Unknown | 5 (0.2) | 2 (0.1) | 3 (0.2) | 0.42 (0.04 to 4.61) | 0.48 | 0.66 (0.11 to 3.97) | 0.65 |
| mRS score of > 2 | 62 (2.0) | 34 (2.2) | 28 (1.8) | 1.23 (0.74 to 2.05) | 0.42 | 1.21 (0.73 to 2.01) | 0.46 |
| TIA, alla | 88 (2.9) | 34 (2.2) | 54 (3.5) | 0.62 (0.39 to 0.96) | 0.033 | 0.62 (0.40 to 0.95) | 0.030 |
| Death | 54 (1.7) | 26 (1.7) | 28 (1.8) | 0.88 (0.50 to 1.54) | 0.65 | 0.91 (0.53 to 1.57) | 0.75 |
FIGURE 4.
Primary outcome as distribution of recurrent stroke and TIA, and their severity, at day 90. Comparison by ordinal logistic regression adjusted for baseline factors.
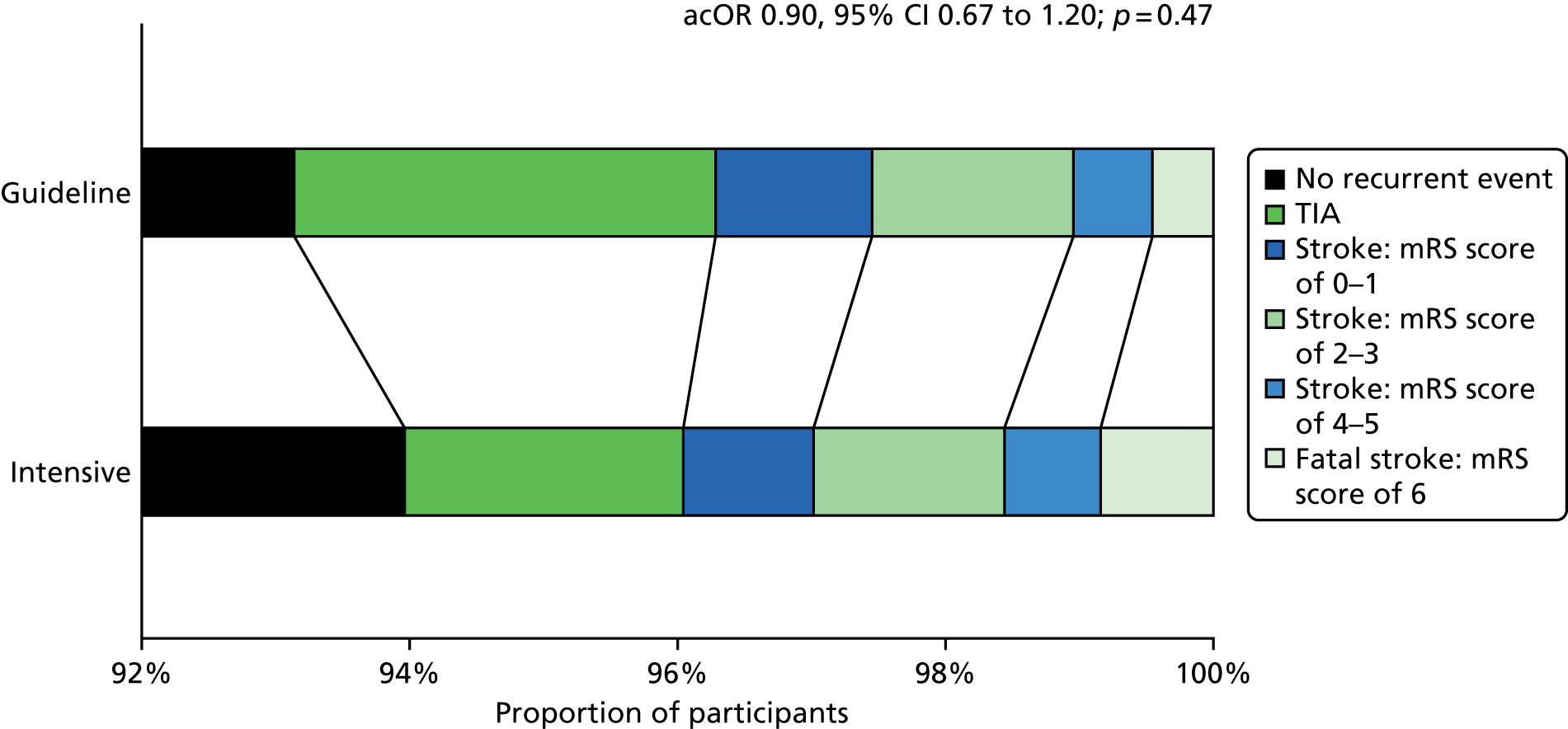
| Outcome | Hours, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensive | Guideline | |||||||||
| ≤ 12 | 12.1–24 | 24.1–36 | 36.1–48 | > 48a | ≤ 12 | 12.1–24 | 24.1–36 | 36.1–48 | > 48a | |
| Number | 144 | 340 | 537 | 516 | 3 | 166 | 305 | 561 | 494 | 4 |
| No event, n (%) | 131 (91.0) | 322 (94.7) | 504 (93.9) | 488 (94.6) | 2 (66.7) | 147 (88.6) | 277 (90.8) | 531 (94.7) | 467 (94.5) | 3 (75.0) |
| TIA, n (%) | 3 (2.1) | 7 (2.1) | 12 (2.2) | 9 (1.7) | 1 (33.3) | 9 (5.4) | 13 (4.3) | 14 (2.5) | 11 (2.2) | 1 (25.0) |
| mRS score of 0, n (%) | 3 (2.1) | 1 (0.3) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.7) | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| mRS score of 1, n (%) | 1 (0.7) | 2 (0.6) | 4 (0.7) | 3 (0.6) | 0 (0.0) | 2 (1.2) | 3 (1.0) | 4 (0.7) | 5 (1.0) | 0 (0.0) |
| mRS score of 2, n (%) | 3 (2.1) | 2 (0.6) | 4 (0.7) | 3 (0.6) | 0 (0.0) | 2 (1.2) | 3 (1.0) | 3 (0.5) | 3 (0.6) | 0 (0.0) |
| mRS score of 3, n (%) | 1 (0.7) | 1 (0.3) | 4 (0.7) | 4 (0.8) | 0 (0.0) | 3 (1.8) | 2 (0.7) | 3 (0.5) | 4 (0.8) | 0 (0.0) |
| mRS score of 4, n (%) | 1 (0.7) | 0 (0.0) | 4 (0.7) | 1 (0.2) | 0 (0.0) | 1 (0.6) | 2 (0.7) | 3 (0.5) | 2 (0.4) | 0 (0.0) |
| mRS score of 5, n (%) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 3 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| mRS score of 6, n (%) | 1 (0.7) | 5 (1.5) | 2 (0.4) | 5 (1.0) | 0 (0.0) | 2 (1.2) | 2 (0.7) | 1 (0.2) | 2 (0.4) | 0 (0.0) |
| Total, n (%) | 13 (9.0) | 18 (5.3) | 33 (6.1) | 28 (5.4) | 1 (33.3) | 19 (11.4) | 28 (9.2) | 30 (5.3) | 27 (5.5) | 1 (25.0) |
Originally, the trial intended to assess the effect of intervention on a nine-level ordinal outcome for stroke and TIA, and subsequently a five-level outcome. However, these were felt to be over- or under-granular, respectively, and the six-level outcome was chosen for the final analysis. Decisions on these changes were made blinded to treatment assignment. In post hoc analyses, the original outcomes were analysed and gave comparable results (Table 9).
| Levels | Ordinal or binary stroke/TIA | Adjusted cOR/HR (95% CI) | p-value | Analysis |
|---|---|---|---|---|
| 2 | mRS score of 0–6 and TIA/no event | 0.87 (0.66 to 1.16) | 0.34 | Sensitivity |
| 5 | mRS score of 6/2–5/0–1/TIA/no event | 0.92 (0.67 to 1.27) | 0.61 | Post hoc |
| 6 | mRS score of 6/4–5/2–3/0–1/TIA/no event | 0.90 (0.67 to 1.20) | 0.47 | Primary |
| 9 | mRS score of 6/5/4/3/2/1/0/TIA/no event | 0.90 (0.67 to 1.20) | 0.47 | Post hoc |
When assessed in prespecified subgroups (Figure 5), there were no significant interactions between the primary outcome and treatment. In the intensive arm, there was a tendency for fewer and less severe strokes/TIAs in participants presenting with a mild stroke, and worse outcomes in participants with a more severe stroke, but this interaction was not significant (p = 0.070). In a sensitivity analysis, the rate of stroke or TIA by day 90 did not differ between participants receiving intensive versus guideline antiplatelet therapy (aOR 0.88, 95% CI 0.65 to 1.18; p = 0.38) (Figure 6 and see Table 7). Similarly, alternative cut-off points were tested for the measures of severity, onset to randomisation and degree of carotid stenosis. These post hoc analyses, and the original results, are shown in Table 10; all the interaction tests remain non-significant.
FIGURE 5.
Primary outcome at day 90 in prespecified subgroups. Unadjusted common odds ratio. a, Depending on local policy and guidelines, sites were given the option to choose which treatment they would give as the comparator, that is, aspirin/dipyridamole, clopidogrel alone or either treatment. NC, not calculable.
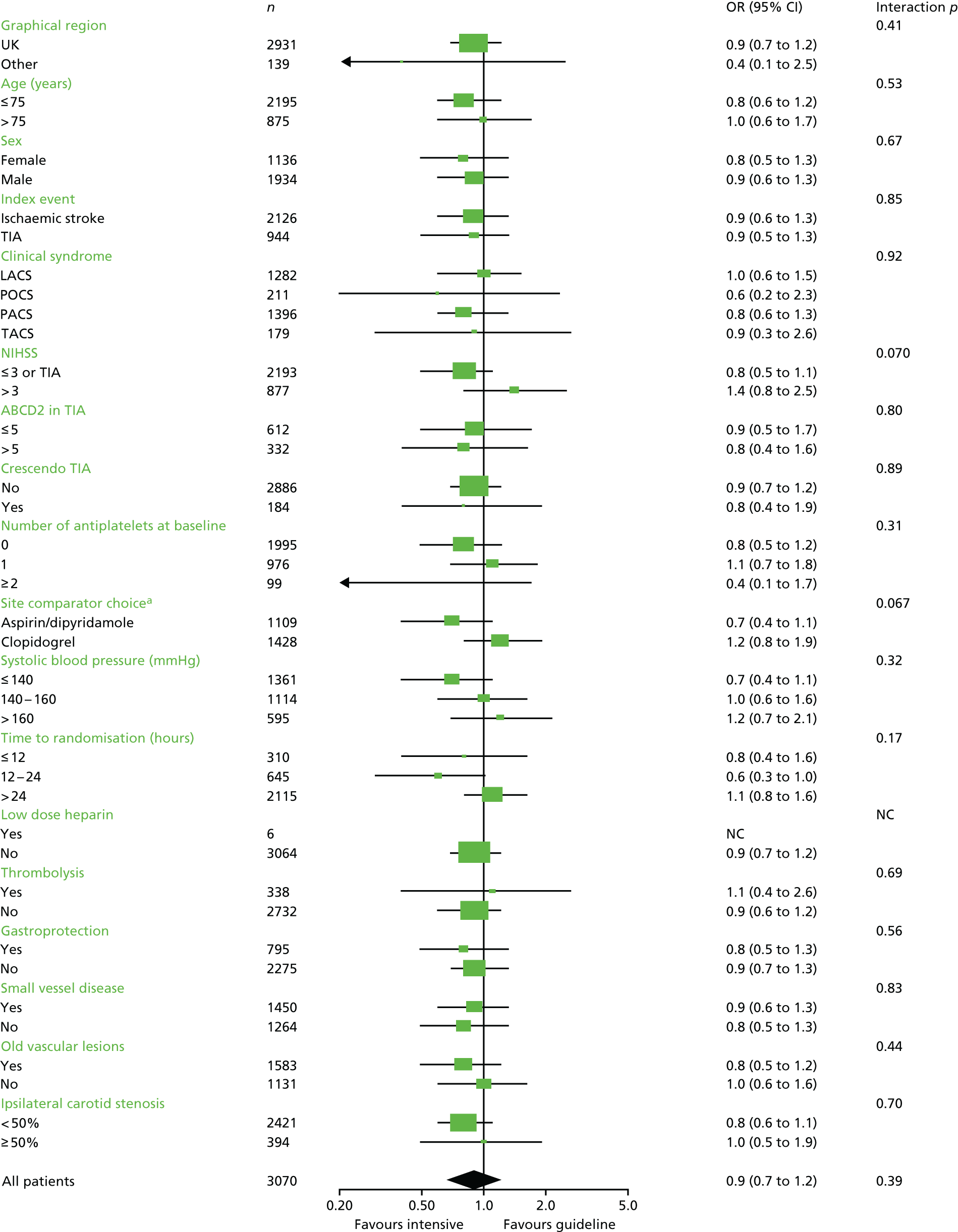
FIGURE 6.
Kaplan–Meier curve for recurrent stroke or TIA over 90 days. Comparison by Cox proportional regression adjusted for baseline factors.
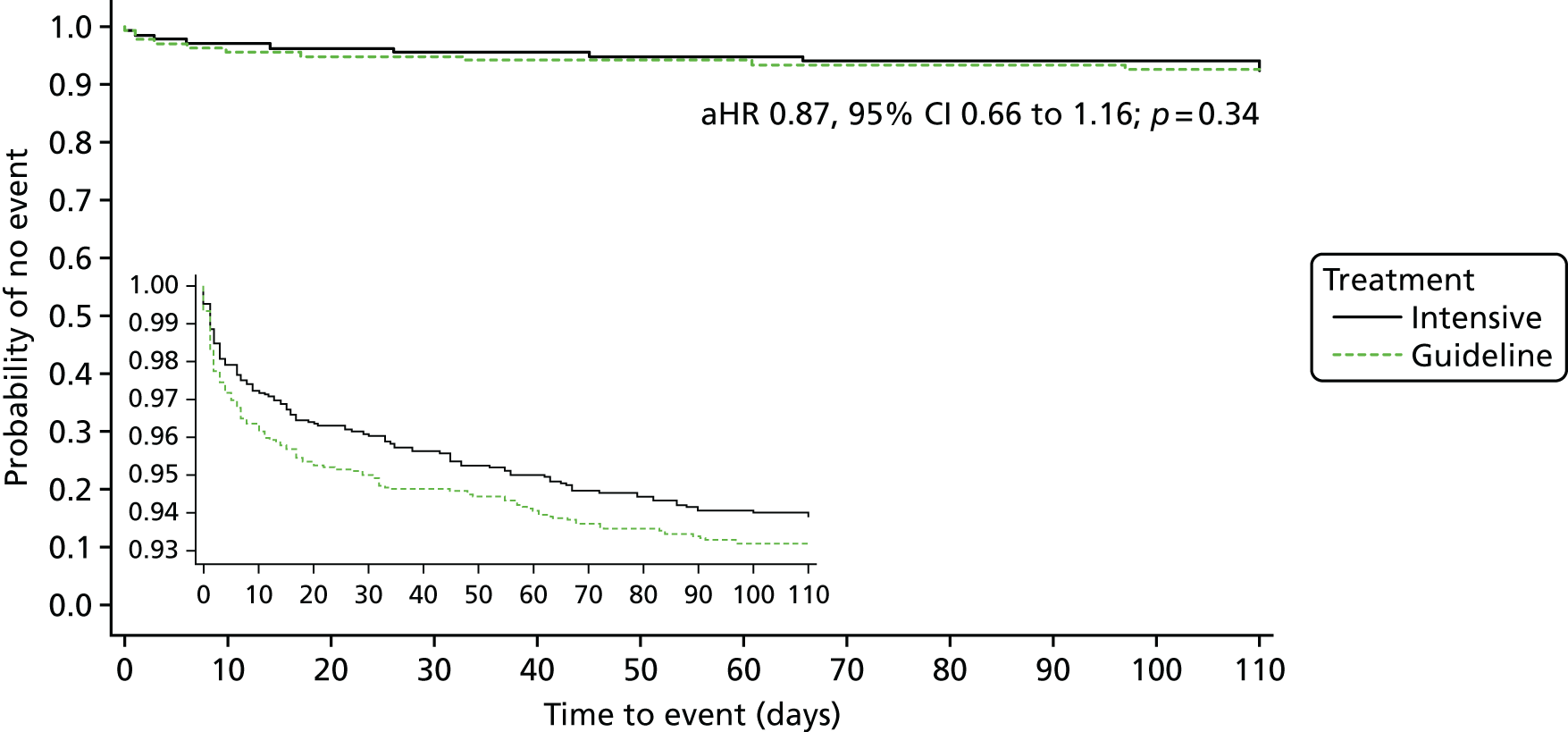
| Analysis | Total number | Intensive, n (%) | Guideline, n (%) | OR (95% CI) | p-value | Interaction p |
|---|---|---|---|---|---|---|
| Stroke severity | ||||||
| Stroke/TIA (NIHSS score) | ||||||
| Original | 0.070 | |||||
| > 3 | 877 | 442 (28.7) | 435 (28.4) | 1.41 (0.78 to 2.54) | 0.26 | |
| ≤ 3 or TIA | 2193 | 1098 (71.3) | 1095 (71.6) | 0.76 (0.54 to 1.06) | 0.10 | |
| Post hoc | 0.18 | |||||
| > 6 | 326 | 179 (11.6) | 147 (9.6) | 1.38 (0.49 to 3.88) | 0.55 | |
| 4–6 | 551 | 263 (17.1) | 288 (18.8) | 1.45 (0.71 to 2.98) | 0.31 | |
| ≤ 3 or TIA | 2193 | 1098 (71.3) | 1095 (71.6) | 0.76 (0.54 to 1.06) | 0.10 | |
| Post hoc | 0.53 | |||||
| > 5 | 435 | 234 (15.2) | 201 (13.1) | 1.16 (0.48 to 2.80) | 0.75 | |
| ≤ 5 or TIA | 2635 | 1306 (84.8) | 1329 (86.9) | 0.86 (0.63 to 1.17) | 0.33 | |
| Major bleeding (NIHSS score) | ||||||
| Original | 0.64 | |||||
| > 3 | 877 | 442 (28.7) | 435 (28.4) | 2.66 (1.77 to 4.01) | < 0.0001 | |
| ≤ 3 or TIA | 2195 | 1099 (71.3) | 1096 (71.6) | 2.39 (1.86 to 3.07) | < 0.0001 | |
| Post hoc | 0.43 | |||||
| > 6 | 326 | 179 (11.6) | 147 (9.6) | 3.92 (1.75 to 8.77) | 0.00089 | |
| 4–6 | 551 | 263 (17.1) | 288 (18.8) | 2.34 (1.44 to 3.79) | 0.00060 | |
| ≤ 3 or TIA | 2195 | 1099 (71.3) | 1096 (71.6) | 2.39 (1.86 to 3.07) | < 0.0001 | |
| Post hoc | 0.44 | |||||
| > 5 | 435 | 234 (15.2) | 201 (13.1) | 3.10 (1.61 to 5.96) | 0.00071 | |
| ≤ 5 or TIA | 2637 | 1307 (84.8) | 1330 (86.9) | 2.40 (1.91 to 3.02) | < 0.0001 | |
| Time | ||||||
| Stroke/TIA (OTR) | ||||||
| Original (hours) | 0.17 | |||||
| > 24 | 2115 | 1056 (68.6) | 1059 (69.2) | 1.09 (0.75 to 1.57) | 0.65 | |
| 12.1–24 | 645 | 340 (22.1) | 305 (19.9) | 0.56 (0.30 to 1.03) | 0.063 | |
| ≤ 12 | 310 | 144 (9.4) | 166 (10.8) | 0.78 (0.37 to 1.64) | 0.51 | |
| Post hoc (hours) | 0.069 | |||||
| > 24 | 2115 | 1056 (68.6) | 1059 (69.2) | 1.09 (0.75 to 1.57) | 0.65 | |
| ≤ 24 | 955 | 484 (31.4) | 471 (30.8) | 0.63 (0.39 to 1.00) | 0.052 | |
| Major bleeding (OTR) | ||||||
| Original (hours) | 0.098 | |||||
| > 24 | 2116 | 1057 (68.6) | 1059 (69.2) | 2.77 (2.14 to 3.58) | < 0.0001 | |
| 12.1–24 | 646 | 340 (22.1) | 306 (20.0) | 2.26 (1.38 to 3.68) | 0.0011 | |
| ≤ 12 | 310 | 144 (9.3) | 166 (10.8) | 1.24 (0.62 to 2.46) | 0.54 | |
| Post hoc (hours) | 0.10 | |||||
| > 24 | 2116 | 1057 (68.6) | 1059 (69.2) | 2.77 (2.14 to 3.58) | < 0.0001 | |
| ≤ 24 | 956 | 484 (31.4) | 472 (30.8) | 1.86 (1.25 to 2.75) | 0.0020 | |
| Carotid stenosis | ||||||
| Stroke/TIA | ||||||
| Original | 0.70 | |||||
| ≥ 50% | 394 | 196 (13.9) | 198 (14.1) | 0.96 (0.48 to 1.91) | 0.90 | |
| < 50% | 2421 | 1211 (86.1) | 1210 (85.9) | 0.82 (0.59 to 1.15) | 0.25 | |
| Post hoc | 0.43 | |||||
| > 0% | 1440 | 735 (52.2) | 705 (50.1) | 0.76 (0.51 to 1.13) | 0.17 | |
| 0% | 1375 | 672 (47.8) | 703 (49.9) | 0.97 (0.61 to 1.53) | 0.88 | |
| Major bleeding | ||||||
| Original | 0.25 | |||||
| ≥ 50% | 395 | 197 (14.0) | 198 (14.1) | 1.69 (0.98 to 2.91) | 0.059 | |
| < 50% | 2422 | 1211 (86.0) | 1211 (85.9) | 2.50 (1.95 to 3.20) | < 0.0001 | |
| Post hoc | 0.42 | |||||
| > 0% | 1441 | 736 (52.3) | 705 (50.0) | 2.13 (1.58 to 2.87) | < 0.0001 | |
| 0% | 1376 | 672 (47.7) | 704 (50.0) | 2.61 (1.86 to 3.67) | < 0.0001 | |
Although there was no difference between the treatment groups for stroke alone as an outcome, there was a tendency for more recurrent strokes of haemorrhagic type in the group randomised to intensive antiplatelet therapy (aOR 2.89, 95% CI 1.02 to 8.19; p = 0.046) (see Table 7). The rates of TIA were lower in the intensive treatment group (aOR 0.62, 95% CI 0.39 to 0.96; p = 0.033) (Figure 7 and see Table 7). Analyses based on unadjusted comparisons did not differ qualitatively for the primary outcome.
FIGURE 7.
Kaplan–Meier curve for recurrent TIA over 90 days. Comparison by Cox proportional regression adjusted for baseline factors.
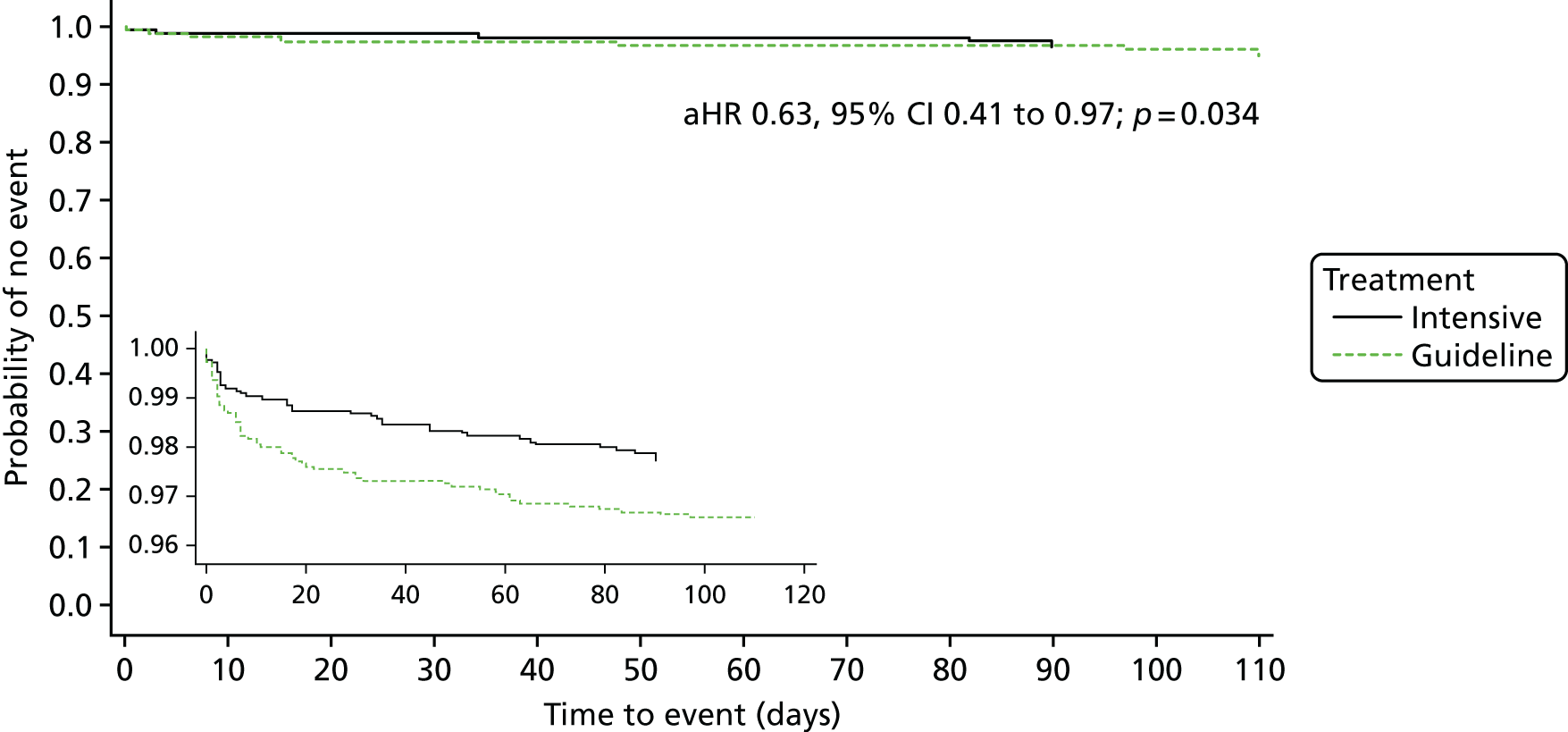
Secondary efficacy outcomes
Hospital-based assessments
Across all patients, there were no differences in death, recurrence, impairment (assessed using the NIHSS) or neurological deterioration by day 7 (Table 11). However, headache was more common in participants assigned to the intensive group (all of whom had been randomised to take dipyridamole, a known cause of headache101) than the guideline group (of whom 44.9% had been randomised to dipyridamole); in a post hoc analysis, headache only differed when the intensive group was compared with clopidogrel (32, 2.1% vs. 1, 0.1%; p < 0.001) but not when compared with combined aspirin and dipyridamole (32, 2.1% vs. 8, 1.2%; p = 0.63). Qualitatively, the above observations were similar for events by day 35 (see Table 10). There was no difference in venous thromboembolism at day 35. Similarly, length of stay in hospital and discharge disposition (comprising return home, discharge to an institution, or death in hospital), did not differ between the groups.
| Outcome | Number | Intensive | Guideline | Adjusted acOR/aMD/aHR (95% CI) | p-value | Unadjusted cOR/MD/HR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Patient | 3096 | 1556 | 1540 | ||||
| Day 7 | 3096 | 1556 | 1540 | ||||
| Death, n (%) | 3096 | 6 (0.4) | 6 (0.4) | 0.99 (0.28 to 3.54) | 0.99 | 0.99 (0.32 to 3.08) | 0.99 |
| Recurrent stroke or TIA, n (%) | 2753 | 41 (3.0) | 57 (4.2) | 0.72 (0.47 to 1.10) | 0.12 | 0.71 (0.47 to 1.06) | 0.096 |
| NIHSS score (/42), mean (SD) | 2992 | 1.9 (4.1) | 1.9 (4.1) | 0.0 (–0.2 to 0.2) | 0.94 | 0.1 (–0.2 to 0.4) | 0.67 |
| ND, n (%)a | 3056 | 26 (1.7) | 24 (1.6) | 1.08 (0.61 to 1.92) | 0.79 | 1.07 (0.61 to 1.87) | 0.82 |
| Headache, n (%) | 3072 | 32 (2.1) | 9 (0.6) | 4.47 (2.03 to 9.83) | < 0.001 | 3.59 (1.71 to 7.54) | < 0.001 |
| Day 35 | 3084 | 1550 | 1534 | ||||
| Death, n (%) | 3096 | 14 (0.9) | 14 (0.9) | 0.93 (0.41 to 2.08) | 0.86 | 0.99 (0.47 to 2.08) | 0.98 |
| Recurrent stroke or TIA, n (%) | 3013 | 70 (4.6) | 83 (5.5) | 0.83 (0.60 to 1.16) | 0.28 | 0.83 (0.60 to 1.15) | 0.26 |
| NIHSS score (/42), mean (SD) | 2918 | 1.5 (4.7) | 1.5 (4.6) | 0.0 (–0.3 to 0.3) | 0.83 | 0.1 (–0.3 to 0.4) | 0.65 |
| NDa, n (%) | 3016 | 32 (2.1) | 33 (2.2) | 0.96 (0.58 to 1.58) | 0.87 | 0.96 (0.59 to 1.58) | 0.88 |
| Headache, n (%) | 3072 | 40 (2.6) | 12 (0.8) | 4.13 (2.09 to 8.15) | < 0.001 | 3.37 (1.76 to 6.46) | < 0.001 |
| VTE, n (%) | 3070 | 7 (0.5) | 8 (0.5) | 0.91 (0.31 to 2.66) | 0.87 | 0.87 (0.31 to 2.40) | 0.79 |
| Fatal PE | 0 (0.0) | 0 (0.0) | |||||
| PE | 4 (0.3) | 4 (0.3) | |||||
| DVT | 3 (0.2) | 4 (0.3) | |||||
| None | 1533 (99.5) | 1522 (99.5) | |||||
| Hospital discharge | 3096 | 1556 | 1540 | ||||
| Length of stay (days), mean (SD) | 2767 | 5.4 (11.6) | 5.2 (11.1) | 0.2 (–0.5 to 0.9) | 0.62 | 0.2 (–0.7 to 1.0) | 0.67 |
| Discharge disposition, n (%) | |||||||
| Home, alone | 3061 | 334 (21.7) | 342 (22.5) | 1.05 (0.90 to 1.22) | 0.56 | 1.07 (0.92 to 1.25) | 0.38 |
| Home, spouse/carer | 1079 (70.1) | 1068 (70.2) | |||||
| Carer’s home | 8 (0.5) | 9 (0.6) | |||||
| Warden-aided flat | 1 (0.1) | 2 (0.1) | |||||
| Residential home | 8 (0.5) | 6 (0.4) | |||||
| Nursing home | 10 (0.6) | 8 (0.5) | |||||
| Rehabilitation hospital | 82 (5.3) | 72 (4.7) | |||||
| Still an inpatient | 12 (0.8) | 3 (0.2) | |||||
| Died in hospital, n (%) | 3061 | 6 (0.4) | 11 (0.7) | 0.41 (0.14 to 1.21) | 0.11 | 0.54 (0.20 to 1.45) | 0.22 |
| Day 90 | 3096 | 1556 | 1540 | ||||
| Death, n (%) | 3091 | 26 (1.7) | 28 (1.8) | 0.89 (0.51 to 1.55) | 0.69 | 0.99 (0.57 to 1.70) | 0.96 |
| Vascular death, n (%) | 3091 | 14 (0.9) | 10 (0.7) | 1.42 (0.62 to 3.24) | 0.41 | 1.38 (0.61 to 3.11) | 0.43 |
| MI, n (%) | 3070 | 11 (0.7) | 12 (0.8) | 0.87 (0.38 to 2.01) | 0.74 | 0.91 (0.40 to 2.06) | 0.82 |
| Fatal MI | 0 (0.0) | 1 (0.1) | |||||
| Non-fatal MI | 4 (0.3) | 9 (0.6) | |||||
| Unstable angina | 1 (0.1) | 0 (0.0) | |||||
| Stable angina | 6 (0.4) | 2 (0.1) | |||||
| No MI/angina | 1529 (99.3) | 1518 (99.2) | |||||
| mRS score, median (IQR) | 3070 | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.96 (0.85 to 1.10) | 0.58 | 0.98 (0.87 to 1.12) | 0.79 |
| BI,b mean (SD) | 2980 | 92.4 (19.8) | 93.2 (18.7) | –0.6 (–1.9 to 0.6) | 0.30 | –0.8 (–2.2 to 0.6) | 0.26 |
| BI < 60, n (%) | 2980 | 83 (5.6) | 68 (4.6) | 1.29 (0.89 to 1.87) | 0.18 | 1.23 (0.89 to 1.71) | 0.21 |
| ZDS,b mean (SD) | 2506 | 46.1 (17.0) | 46.6 (17.5) | –0.4 (–1.6 to 0.9) | 0.59 | –0.5 (–1.9 to 0.8) | 0.46 |
| t-MMSE,b mean (SD) | 2360 | 18.3 (4.2) | 18.3 (4.4) | 0.0 (–0.3 to 0.3) | 0.94 | 0.0 (–0.3 to 0.4) | 0.98 |
| TICS-M,b mean (SD) | 2389 | 21.1 (6.2) | 21.0 (6.5) | 0.0 (–0.5 to 0.5) | 0.93 | 0.0 (–0.5 to 0.5) | 0.93 |
| EQ-VAS,b mean (SD) | 2859 | 72.8 (21.5) | 72.0 (22.4) | 0.9 (–0.6 to 2.5) | 0.23 | 0.8 (–0.8 to 2.4) | 0.34 |
| EQ-5D-3L/HSUV,b mean (SD) | 2991 | 0.7 (0.3) | 0.7 (0.3) | 0.0 (0.0 to 0.0) | 0.43 | 0.0 (0.0 to 0.0) | 0.63 |
Central follow-up at day 90
Across all patients, there was no difference between the treatment groups in outcome at day 90 assessed as dependency (mRS, Figure 8), disability (BI), mood (Zung Depression Scale), cognition (t-MMSE, telephone interview cognition status – modified, verbal fluency) or quality of life (EQ-VAS) (see Table 11). Similarly, the rate of MI did not differ between the treatment groups. Analyses based on unadjusted comparisons did not differ qualitatively for any of the secondary outcomes (see Table 11).
FIGURE 8.
Distribution of mRS in all patients at day 90 comparison, by ordinal logistic regression adjusted for baseline factors.
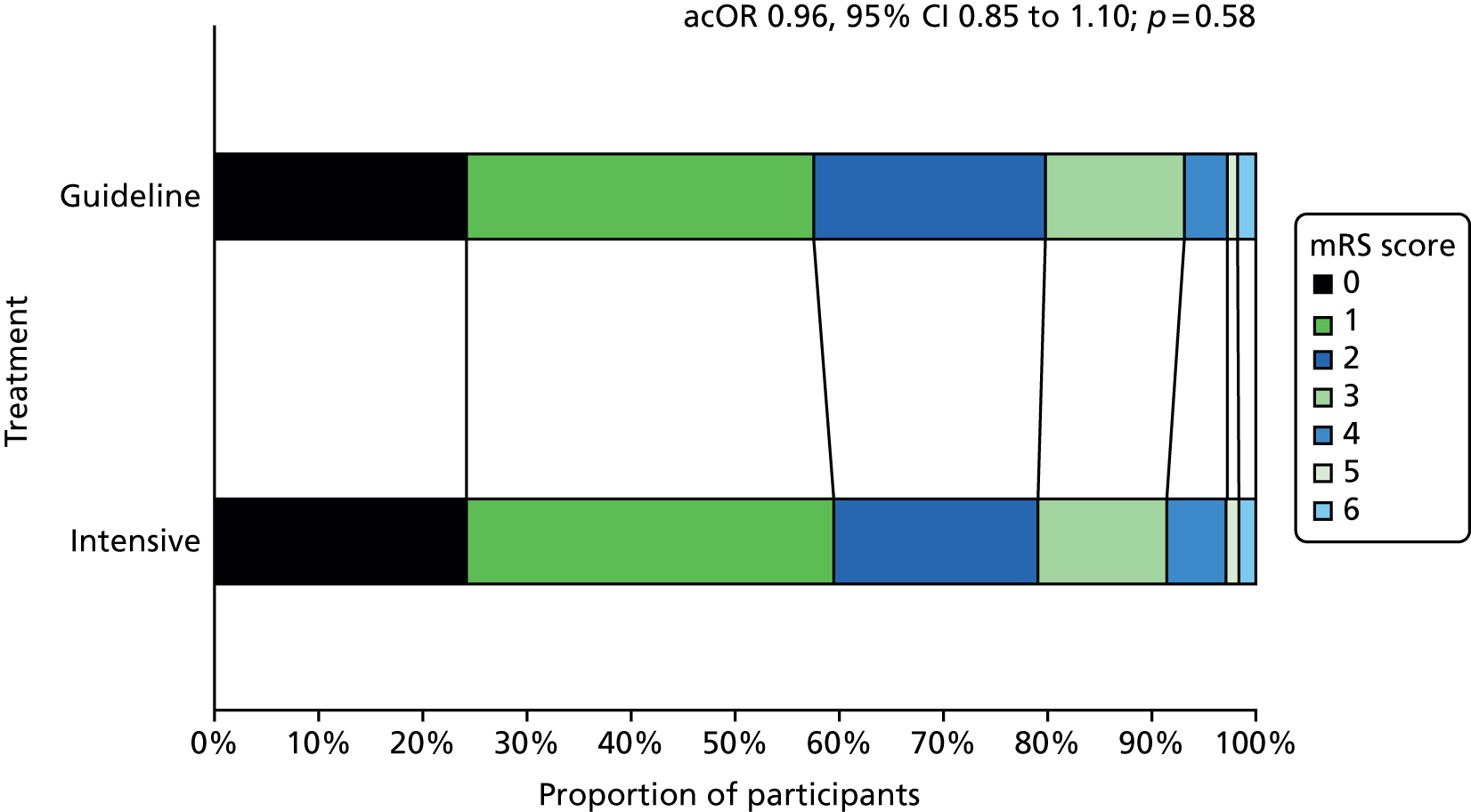
Safety: bleeding
The main safety outcome was the distribution of risk and severity of bleeding (using the ordinal scale of fatal, major, moderate, mild or no bleed) at day 90; this was shifted to more bleeding and bleeding of greater severity in participants randomised to intensive antiplatelet therapy (acOR 2.54, 95% CI 2.05 to 3.16; p < 0.001) (Table 12 and Figure 9). When the distribution of bleeding and its severity was assessed in prespecified subgroups, a statistically significant interaction between bleeding and the site comparator choice was present (Figure 10). Intensive treatment was associated with more bleeding than guideline treatment when compared within sites that chose aspirin and dipyridamole as the comparator arm than when compared within sites that chose clopidogrel alone as the comparator. An interaction was also seen for patients who received thrombolysis; intensive antiplatelets were associated with higher bleeding rates in those who received thrombolysis than those who did not. The effect of treatment on ordinal bleeding by time to recruitment is shown in Table 13.
| Outcome | Intensive, n (%) | Guideline, n (%) | Adjusted acOR/aHR (95% CI) | p-value |
|---|---|---|---|---|
| Participants with data | 1541 | 1531 | ||
| Safety, bleeding | ||||
| Ordinal bleeding | 305 (19.8) | 139 (9.1) | acOR 2.54 (2.05 to 3.16) | < 0.001 |
| Fatal74 | 8 (0.5) | 3 (0.2) | 3.48 (0.89 to 13.63) | 0.074 |
| Major | 31 (2.0) | 14 (0.9) | ||
| Moderate | 25 (1.6) | 13 (0.8) | ||
| Mild | 241 (15.6) | 109 (7.1) | ||
| None | 1236 (80.2) | 1392 (90.9) | ||
| Sensitivity analyses | ||||
| Fatal or major74 | 39 (2.5) | 17 (1.1) | 2.23 (1.25 to 3.96) | 0.006 |
| Bleeding | ||||
| Intracranial bleeding | 16 (1.0) | 5 (0.3) | 3.14 (1.14 to 8.61) | 0.026 |
| Intracerebral | 13 (0.8) | 4 (0.3) | 3.26 (1.05 to 10.06) | 0.040 |
| Subdural or extradural | 2 (0.1) | 0 (0.0) | NC | |
| Fatal | 6 (0.4) | 3 (0.2) | 2.43 (0.59 to 10.01) | 0.22 |
| Major | 9 (0.6) | 1 (0.1) | 8.79 (1.10 to 69.95) | 0.040 |
| Fatal or major | 15 (1.0) | 4 (0.3) | 3.84 (1.26 to 11.63) | 0.018 |
| Extracranial | 293 (19.0) | 135 (8.8) | 2.37 (1.93 to 2.91) | < 0.001 |
| Gastrointestinal | 48 (3.1) | 34 (2.2) | 1.39 (0.89 to 2.16) | 0.15 |
| Other | 255 (16.5) | 104 (6.8) | 2.70 (2.14 to 3.39) | < 0.001 |
| Fatal | 2 (0.1) | 0 (0.0) | NC | |
| Major | 24 (1.6) | 13 (0.8) | 1.71 (0.86 to 3.38) | 0.13 |
| Fatal or major | 26 (1.7) | 13 (0.8) | 1.89 (0.96 to 3.71) | 0.064 |
FIGURE 9.
Distribution of ordinal bleeding at day 90. Comparison by ordinal logistic regression adjusted for baseline factors.
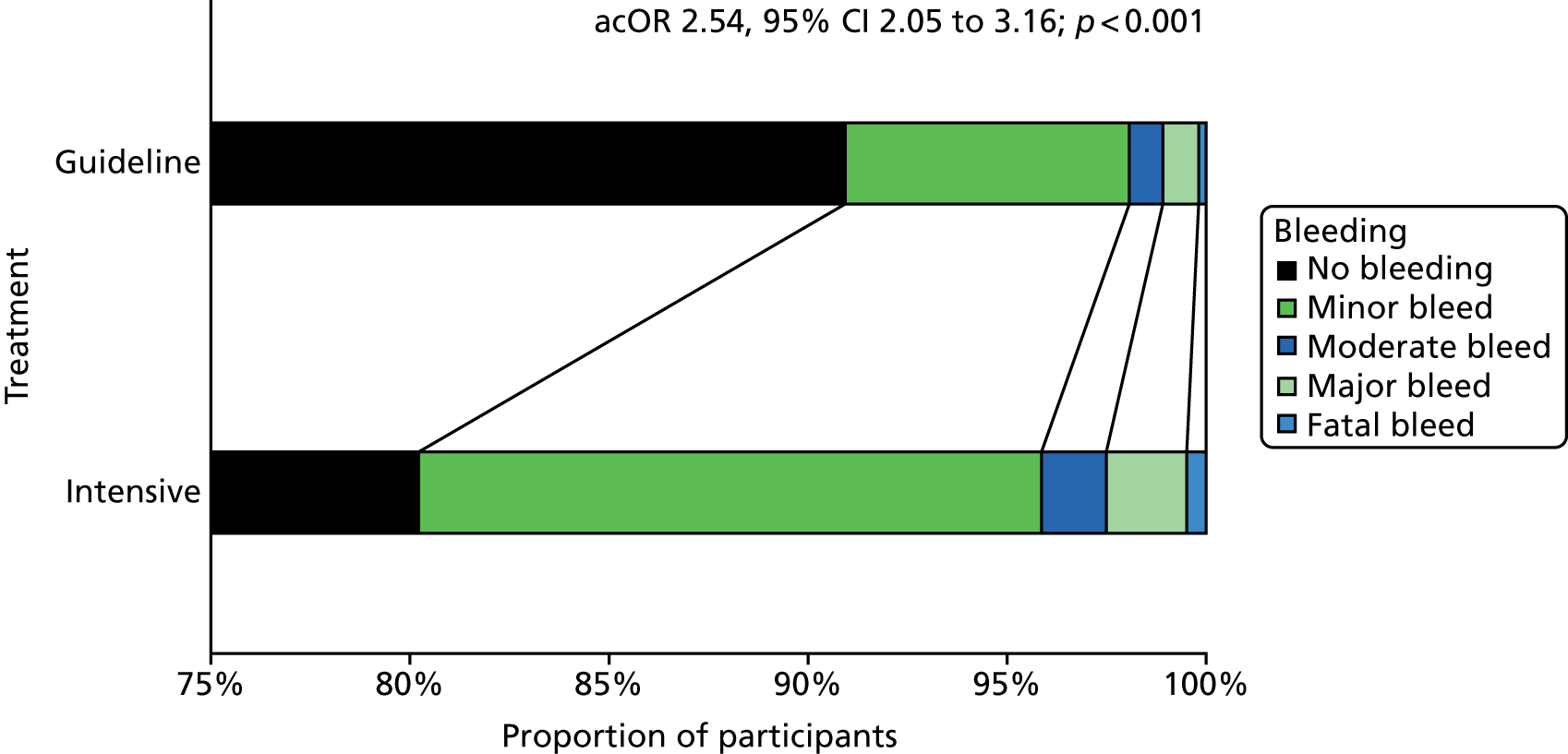
FIGURE 10.
Ordinal bleeding at day 90 in prespecified subgroups. Unadjusted common odds ratio. a, Depending on local policy and guidelines, sites were given the option to choose which treatment they would give as the comparator, that is, aspirin/dipyridamole, clopidogrel alone or either treatment. NC, not calculable.
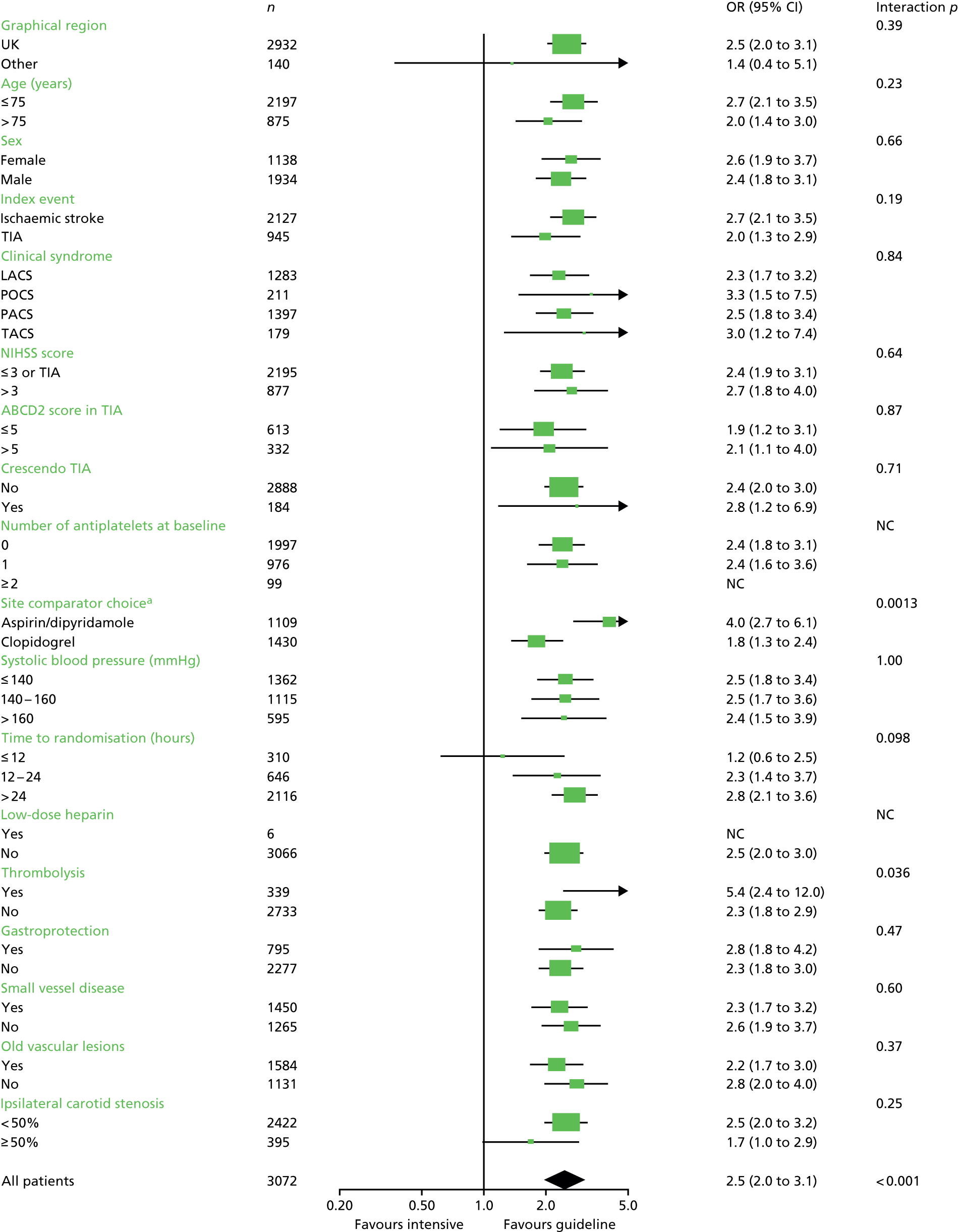
| Bleeding | Hours, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensive | Guideline | |||||||||
| ≤ 12 | 12.1–24 | 24.1–36 | 36.1–48 | > 48a | ≤ 12 | 12.1–24 | 24.1–36 | 36.1–48 | > 48a | |
| Number | 144 | 340 | 537 | 516 | 4 | 166 | 306 | 561 | 494 | 4 |
| None | 125 (86.8) | 282 (82.9) | 429 (79.9) | 403 (78.1) | 1 (25.0) | 148 (89.2) | 280 (91.5) | 522 (93.0) | 440 (89.1) | 4 (100.0) |
| Minor | 14 (9.7) | 44 (12.9) | 86 (16.0) | 92 (17.8) | 1 (25.0) | 12 (7.2) | 20 (6.5) | 34 (6.1) | 41 (8.3) | 0 (0.0) |
| Moderate | 2 (1.4) | 6 (1.8) | 8 (1.5) | 7 (1.4) | 2 (50.0) | 2 (1.2) | 2 (0.7) | 3 (0.5) | 6 (1.2) | 0 (0.0) |
| Major | 2 (1.4) | 5 (1.5) | 13 (2.4) | 11 (2.1) | 0 (0.0) | 3 (1.8) | 3 (1.0) | 2 (0.4) | 6 (1.2) | 0 (0.0) |
| Fatal | 1 (0.7) | 3 (0.9) | 1 (0.2) | 3 (0.6) | 0 (0.0) | 1 (0.6) | 1 (0.3) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| Total | 19 (13.2) | 58 (17.1) | 108 (20.1) | 113 (21.9) | 3 (75.0) | 18 (10.8) | 26 (8.5) | 39 (7.0) | 54 (10.9) | 0 (0.0) |
The rate of severe (fatal or major) bleeding by day 90 was 2.5% in those assigned to intensive antiplatelet therapy and 1.1% among participants receiving guideline antiplatelets [adjusted hazard ratio (aHR) 2.23, 95% CI 1.26 to 3.96; p = 0.006] (Figure 11 and see Table 13). The rates of bleeding increasingly diverged between the treatment groups up to the end of treatment at day 30 but not thereafter. Combined fatal and major intracranial bleeding was increased with intensive antiplatelet therapy (aHR 3.84, 95% CI 1.26 to 11.63; p = 0.018). A non-significant tendency to more fatal or major extracranial bleeding was also seen with intensive antiplatelets (aHR 1.89, 95% CI 0.96 to 3.71; p = 0.064) (see Table 13).
FIGURE 11.
Kaplan–Meier curve for major bleeding over 90 days. Comparison by Cox proportional regression adjusted for baseline factors.
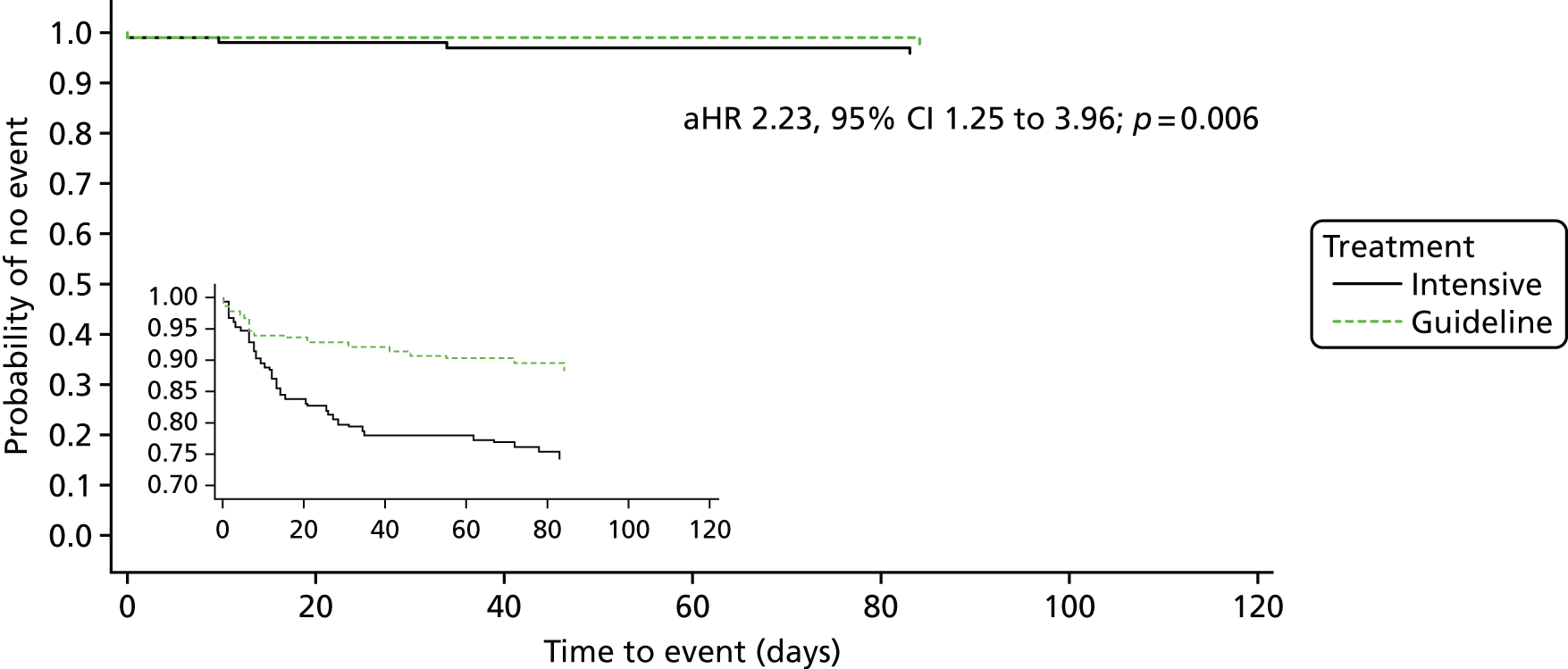
The above differences in ordinal bleeding between intensive and guideline antiplatelets at day 90 were apparent by day 7, and ordinal bleeding, major extracranial bleeding and fatal/major bleeding were different at day 35 (see Table 13). At day 90, intensive antiplatelet was associated with more, and more severe, bleeding when assessed as the worst (see Table 13) or first (see Table 12 and Table 14) bleed. Similarly, intensive antiplatelet therapy was associated, in post hoc analyses, with a higher rate of major bleeding by day 14 of treatment (Table 15).
| Outcome | Number | Intensive, n (%) | Guideline, n (%) | OR/MD (95% CI), adjusted | p-value |
|---|---|---|---|---|---|
| Patients | 3072 | 1541 | 1531 | ||
| Day 7 (or discharge) | |||||
| Bleeding, most severe | 3072 | 149 (9.7) | 53 (3.5) | 2.91 (2.13 to 3.99) | < 0.001 |
| Ordinal | 3.05 (2.20 to 4.23) | < 0.001 | |||
| Fatal74 | 3072 | 5 (0.3) | 2 (0.1) | 6.14 (0.79 to 47.67) | 0.083 |
| Major | 8 (0.5) | 7 (0.5) | |||
| Moderate | 14 (0.9) | 2 (0.1) | |||
| Mild | 122 (7.9) | 42 (2.7) | |||
| None | 1392 (90.3) | 1478 (96.5) | |||
| sICH | 3070 | 6 (0.4) | 4 (0.3) | 1.48 (0.39 to 5.58) | 0.56 |
| mECB | 3070 | 8 (0.5) | 6 (0.4) | 1.30 (0.44 to 3.80) | 0.64 |
| Haemoglobin (g/dl), mean (SD), [range] | 2912 | 14.0 (1.5), [8.7–20.0] | 14.0 (1.4), [9.4–20.9] | MD 0.0 (–0.1 to 0.1) | 0.71 |
| Fatal/major bleeding | 3072 | 13 (0.8) | 9 (0.6) | 1.49 (0.63 to 3.53) | 0.37 |
| Day 35 | |||||
| Bleeding, most severe | 3072 | 277 (18.0) | 103 (6.7) | 2.88 (2.30 to 3.62) | < 0.001 |
| Ordinal | 3.11 (2.44 to 3.95) | < 0.001 | |||
| Fatal74 | 3072 | 6 (0.4) | 2 (0.1) | 5.78 (0.92 to 36.17) | 0.061 |
| Major | 28 (1.8) | 10 (0.7) | |||
| Moderate | 22 (1.4) | 10 (0.7) | |||
| Mild | 221 (14.3) | 81 (5.3) | |||
| None | 1264 (82.0) | 1428 (93.3) | |||
| sICH | 3070 | 10 (0.6) | 4 (0.3) | 2.36 (0.72 to 7.69) | 0.16 |
| mECB | 3070 | 24 (1.6) | 9 (0.6) | 2.55 (1.18 to 5.53) | 0.018 |
| Haemoglobin (g/dl), mean (SD), [range] | 2806 | 13.6 (1.6), [6.8–18.5] | 13.7 (1.4), [7.8–19.5] | MD –0.1 (–0.2 to 0.0) | 0.031 |
| Fatal/major bleeding | 3072 | 34 (2.2) | 12 (0.8) | 2.79 (1.43 to 5.41) | 0.003 |
| Day 90 | |||||
| Bleeding, first | 3072 | 305 (19.8) | 139 (9.1) | 2.40 (1.96 to 2.94) | < 0.001 |
| Ordinal | 2.54 (2.04 to 3.15) | < 0.001 | |||
| Fatal74 | 3072 | 8 (0.5) | 3 (0.2) | 3.47 (0.89 to 13.62) | 0.074 |
| Major | 25 (1.6) | 14 (0.9) | |||
| Moderate | 23 (1.5) | 13 (0.8) | |||
| Mild | 249 (16.2) | 109 (7.1) | |||
| None | 1236 (80.2) | 1392 (90.9) | |||
| Fatal/major bleeding | 3072 | 33 (2.1) | 17 (1.1) | 1.93 (1.07 to 3.47) | 0.029 |
| Analysis | Day | Adjusted HR (95% CI) | p-value |
|---|---|---|---|
| Post hoc | 07 | 1.49 (0.63 to 3.53) | 0.37 |
| Post hoc | 14 | 2.48 (1.14 to 5.41) | 0.023 |
| Post hoc | 21 | 2.30 (1.13 to 4.69) | 0.021 |
| Post hoc | 30 | 2.71 (1.35 to 5.43) | 0.005 |
| Main | 90 | 2.23 (1.25 to 3.96) | 0.0063 |
Serious adverse events
There was no evidence of a mortality difference between the treatment groups (Figure 12 and see Table 7). Excluding primary outcome and bleeding events, the overall incidence of SAEs was similar in the two treatment groups: intensive 21.7% versus guideline 21.4% (acOR 1.02, 95% CI 0.86 to 1.22; p = 0.80) (Table 16 and Figure 13); similarly, the rate of fatal SAEs did not differ between the treatment groups (intensive 0.8%, guideline 1.4%, aHR 0.52, 95% CI 0.25 to 1.05; p = 0.070).
FIGURE 12.
Kaplan–Meier curve for death over 90 days. Comparison by Cox proportional regression adjusted for baseline factors.
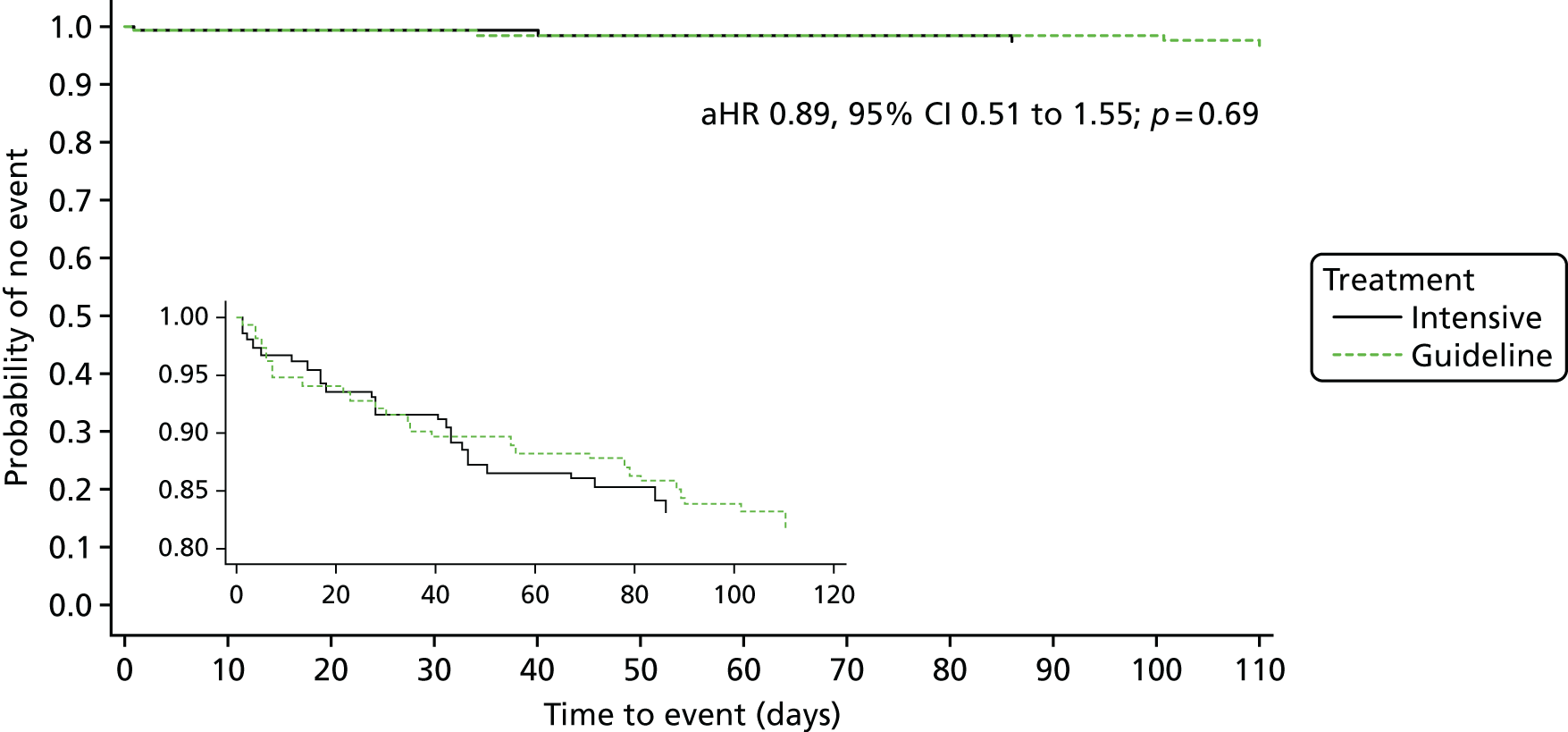
| Cause | All, n (%) | Fatal, n (%) | ||||
|---|---|---|---|---|---|---|
| Intensive | Guideline | p-value | Intensive | Guideline | p-value | |
| Severity | ||||||
| SAE (CPHR) | 335 (21.7) | 327 (21.4) | 0.60 | |||
| Ordinal | 0.69 | |||||
| Fatal | 13 (0.8) | 22 (1.4) | 0.13 | 13 (0.8) | 22 (1.4) | 0.13 |
| Severe | 54 (3.5) | 39 (2.5) | ||||
| Moderate | 167 (10.8) | 148 (9.7) | ||||
| Mild | 101 (6.5) | 118 (7.7) | ||||
| None | 1208 (78.4) | 1204 (78.7) | ||||
| Site | ||||||
| Neurological | 106 (6.9) | 84 (5.5) | 0.11 | 3 (0.2) | 7 (0.5) | 0.22 |
| Initial stroke | ||||||
| Complication | 3 (0.2) | 5 (0.3) | 0.48 | 0 (0.0) | 3 (0.2) | NC |
| Extension | 26 (1.7) | 20 (1.3) | 0.39 | 1 (0.1) | 1 (0.1) | 1.00 |
| Cardiac | 82 (5.3) | 84 (5.5) | 0.84 | 2 (0.1) | 2 (0.1) | 0.99 |
| AF | 52 (3.4) | 64 (4.2) | 0.24 | 0 (0.0) | 1 (0.1) | NC |
| Failure | 5 (0.3) | 2 (0.1) | 0.28 | |||
| Hypertension | 3 (0.2) | 3 (0.2) | 0.99 | |||
| Hypotension | 4 (0.3) | 7 (0.5) | 0.37 | |||
| Sudden death | 2 (0.1) | 0 (0.0) | NC | 2 (0.1) | 0 (0.0) | NC |
| Gastrointestinal | 43 (2.8) | 15 (1.0) | 0.00042 | 2 (0.1) | 1 (0.1) | 0.57 |
| Respiratory | 32 (2.1) | 38 (2.5) | 0.45 | 2 (0.1) | 3 (0.2) | 0.65 |
| Pneumonia | 16 (1.0) | 8 (0.5) | 0.11 | 1 (0.1) | 2 (0.1) | 0.57 |
| PE | 5 (0.3) | 7 (0.5) | 0.56 | 1 (0.1) | 0 (0.0) | NC |
| Other | 131 (8.5) | 158 (10.3) | 0.083 | 4 (0.3) | 9 (0.6) | 0.17 |
| Unattended death | 1 (0.1) | 0 (0.0) | NC | 1 (0.1) | 0 (0.0) | NC |
| DVT | 7 (0.5) | 4 (0.3) | 0.38 | |||
| Malignancy | 8 (0.5) | 13 (0.8) | 0.27 | 2 (0.1) | 7 (0.5) | 0.12 |
| Septicaemia | 0 (0.0) | 1 (0.1) | NC | 0 (0.0) | 1 (0.1) | NC |
| UTI | 12 (0.8) | 13 (0.8) | 0.83 | |||
| Renal failure | 2 (0.1) | 2 (0.1) | 0.99 | |||
| Other | 11 (0.7) | 15 (1.0) | 0.42 | |||
| Timing | ||||||
| By day 7 | 191 (12.4) | 150 (9.8) | 0.022 | 2 (0.1) | 4 (0.3) | 0.42 |
| By day 35 | 278 (18.0) | 255 (16.7) | 0.32 | 7 (0.5) | 10 (0.7) | 0.46 |
| By day 90 | 335 (21.7) | 327 (21.4) | 0.81 | 13 (0.8) | 22 (1.4) | 0.13 |
FIGURE 13.
Distribution of SAEs at day 90. Comparison by ordinal logistic regression adjusted for baseline factors.
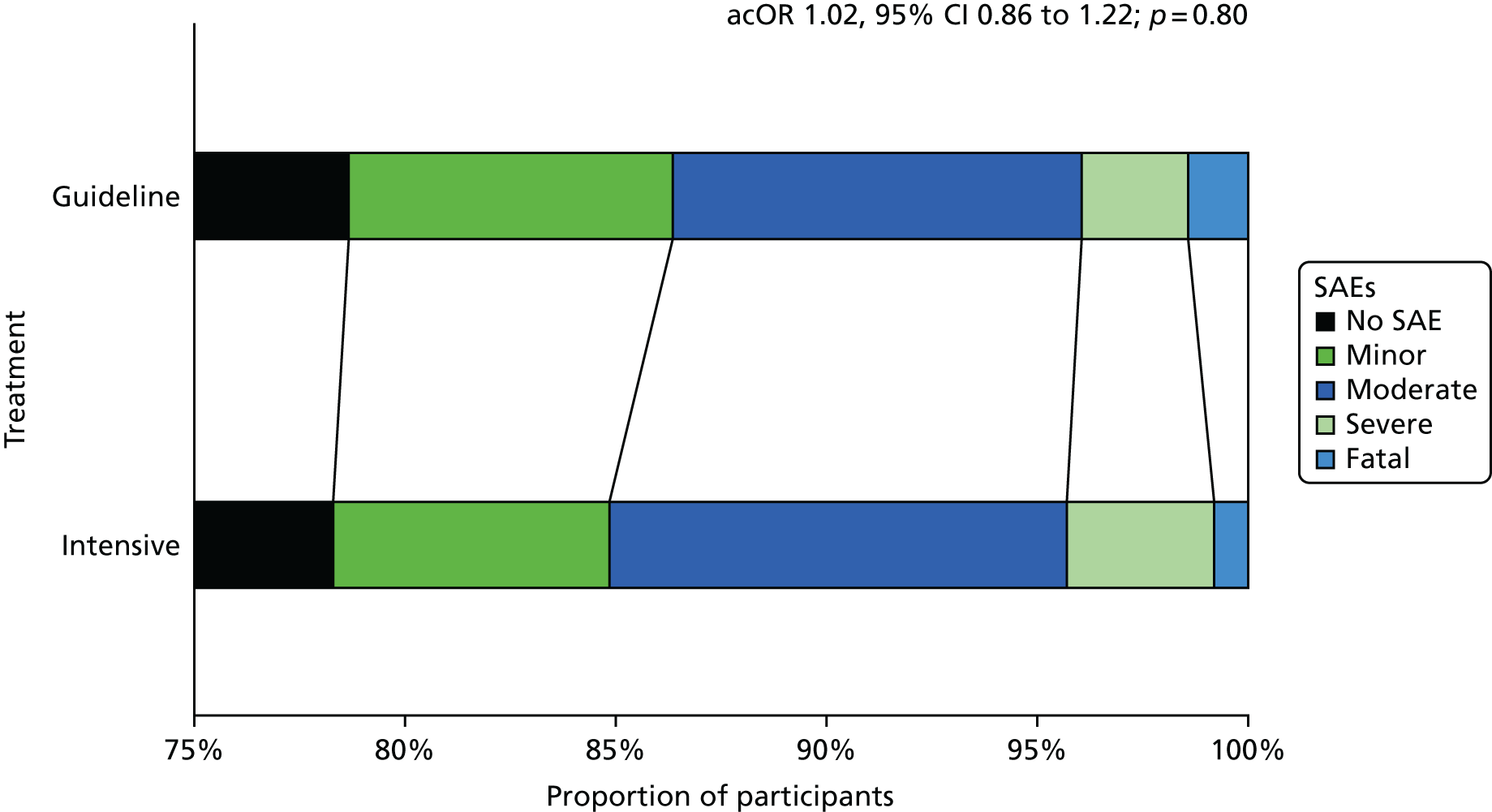
The severity of SAEs, as judged by the reporting investigator, did not differ in their frequency or distribution (see Table 16). When assessed by time in trial, SAEs were more common by day 7 in the intensive group than in the guideline group, although this difference had disappeared by day 35 (Figure 14). When considered by organ, no differences were apparent except that gastrointestinal-based SAEs (excluding reported bleeding events) were more frequent in the intensive than guideline group despite gastroprotection being recommended (2.8% vs. 1.0%; p < 0.001; see Table 16). In a post hoc analysis, gastrointestinal-based SAEs (excluding reported bleeding events) differed for both comparisons of the intensive group with clopidogrel [43 (2.8%) vs. 8 (0.9%); p = 0.005] and with combined aspirin and dipyridamole [43 (2.8%) vs. 7 (1.0%); p = 0.012], suggesting that the presence of dipyridamole was not the only explanation for the increase in SAEs.
FIGURE 14.
Kaplan–Meier curve for SAEs over 90 days. Comparison by Cox proportional regression adjusted for baseline factors.
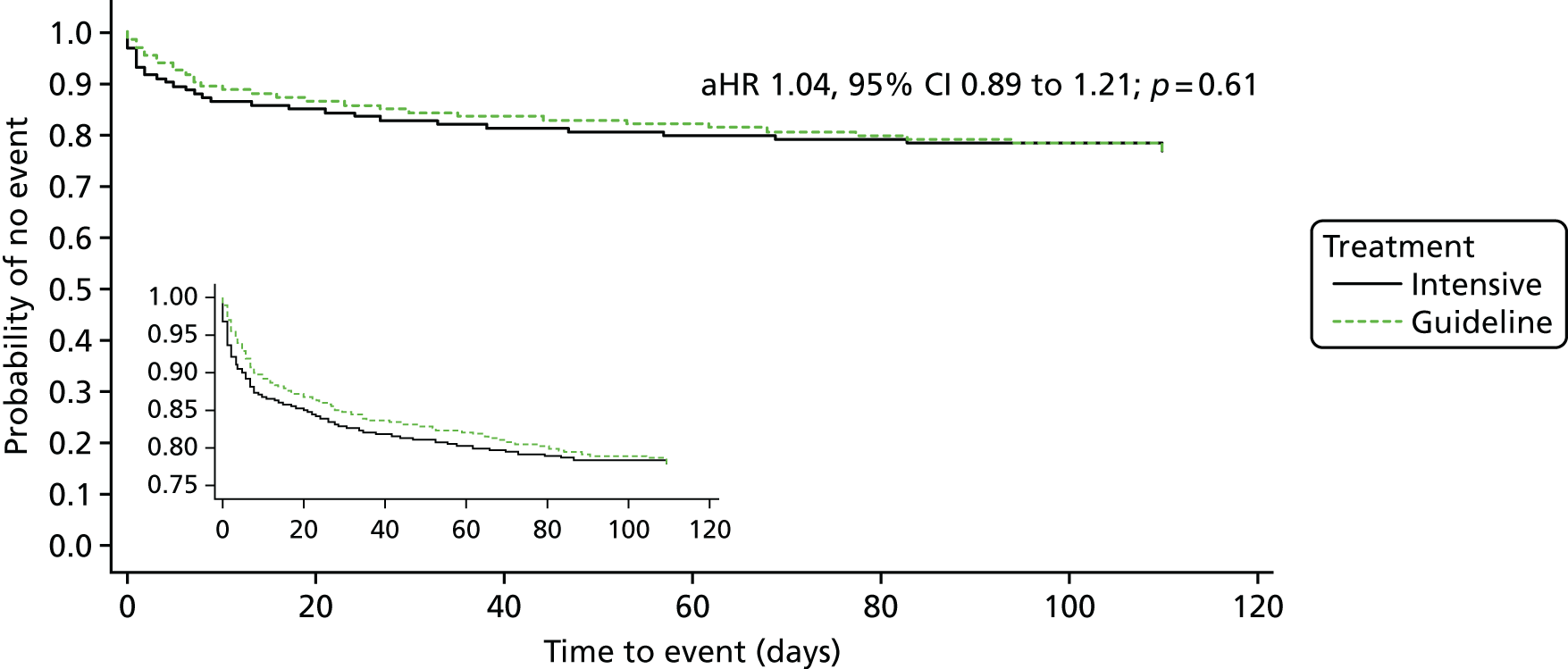
Composite outcomes
The composite end point of any stroke or fatal and major bleeding occurred in 87 (5.6%) participants in the intensive group and 69 (4.5%) participants in the guideline group (aHR, 1.24, 95% CI 0.90 to 1.70; p = 0.19) (Table 17). Similarly, the composite end point of death, stroke, MI or fatal and major bleeding did not differ between the treatment groups: intensive group 102 (6.6%) vs. guideline group 98 (6.4%) (aHR, 1.02, 95% CI 0.77 to 1.35; p = 0.88). Figure 15 shows a bar chart of the relative frequencies of different measures of net benefit and risk; no differences are apparent.
| Outcome | Intensive, n (%) | Guideline, n (%) | Adjusted aHR (95% CI) | p-value |
|---|---|---|---|---|
| Participants | 1556 | 1540 | ||
| Death | 26 (1.7) | 28 (1.8) | 0.89 (0.51 to 1.55) | 0.69 |
| Stroke or major bleed | 87 (5.6) | 69 (4.5) | 1.24 (0.90 to 1.70) | 0.19 |
| Stroke, TIA, ACS or death | 111 (7.2) | 134 (8.8) | 0.82 (0.64 to 1.06) | 0.14 |
| Vascular death, stroke, MI or major bleed | 94 (6.1) | 81 (5.3) | 1.14 (0.84 to 1.53) | 0.40 |
| Death, stroke or MI | 79 (5.1) | 86 (5.6) | 0.91 (0.67 to 1.24) | 0.55 |
| Death, stroke, MI or major bleed | 102 (6.6) | 98 (6.4) | 1.02 (0.77 to 1.35) | 0.88 |
FIGURE 15.
Bar chart for net benefit: risk at day 90. All comparisons non-significant.
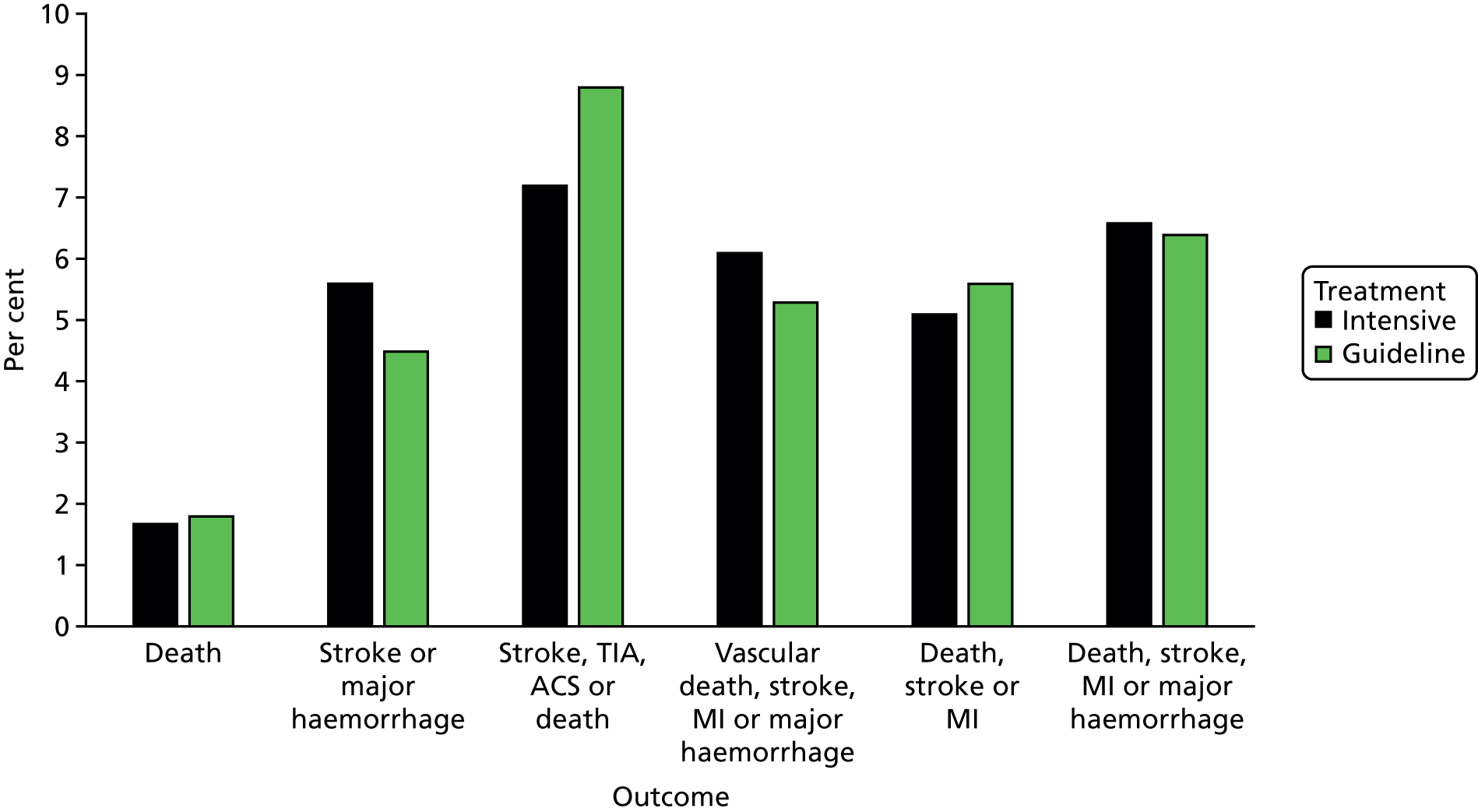
Protocol violations
One or more protocol violations were present in 414 (13.4%) participants, with more present in the intensive (n = 246, 15.8%) than guideline (n = 154, 10.0%) group (p < 0.001) (Table 18). When considered as subtypes of violations, the difference between the treatment groups was maintained for matters of trial practice such as not administering the intervention loading dose or not giving the interventions for at least 16 days.
| Protocol violation | All, n (%) | Intensive, n (%) | Guideline, n (%) | p-value |
|---|---|---|---|---|
| Patients | 3096 | 1556 | 1540 | |
| Total number of patients with violations | 411 (13.3) | 244 (15.7) | 167 (10.8) | < 0.001 |
| Total number of violations | 476 | 290 | 186 | |
| Baseline characteristics – patients | 52 (10.9) | 28 (9.7) | 24 (12.9) | 0.27 |
| Randomisation > 48 hours from onset of symptoms | 7 (1.5) | 4 (1.4) | 3 (1.6) | |
| Failure to obtain appropriate consent prior to randomisation | 6 (1.3) | 3 (1.0) | 3 (1.6) | |
| Pre-morbid dependency (mRS) score of > 2 | 2 (0.4) | 1 (0.3) | 1 (0.5) | |
| On anticoagulation therapy except low dose low-molecular-weight heparin | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Participant received inappropriate antiplatelet treatment prior to randomisation | 23 (4.8) | 12 (4.1) | 11 (5.9) | |
| Thrombolysis < 24 hours prior to randomisation | 1 (0.2) | 0 (0.0) | 1 (0.5) | |
| Presumed cardioembolic stroke or history of AF | 6 (1.3) | 4 (1.4) | 2 (1.1) | |
| Concomitant STEMI or NSTEMI | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Baseline SBP reading of > 185 mmHg or DBP of > 110 mmHg | 2 (0.4) | 1 (0.3) | 1 (0.5) | |
| Planned surgery within the 90-day follow-up period | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Known history of dementia | 1 (0.2) | 0 (0.0) | 1 (0.5) | |
| Unavailable for follow-ups | 1 (0.2) | 0 (0.0) | 1 (0.5) | |
| Baseline characteristics (stroke) – patients | 4 (0.8) | 2 (0.7) | 2 (1.1) | 0.65 |
| No cranial imaging results available prior to randomisation | 3 (0.6) | 2 (0.7) | 1 (0.5) | |
| Isolated sensory symptoms, vertigo or dizziness or facial weakness as presenting symptoms of the index event | 1 (0.2) | 0 (0.0) | 1 (0.5) | |
| Baseline characteristics (TIA) – patients | 10 (2.1) | 3 (1.0) | 7 (3.8) | 0.043 |
| Limb weakness and/or dysphasia lasting < 10 minutes | 3 (0.6) | 1 (0.3) | 2 (1.1) | |
| ABCD2 score of < 4, not a crescendo TIA and not on dual antiplatelet therapy | 7 (1.5) | 2 (0.7) | 5 (2.7) | |
| Practice during the trial – patients | 343 (72.1) | 221 (76.2) | 122 (65.6) | 0.012 |
| Subsequent randomisation into another drug or devices trial | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Patient has received treatment that they are not randomised to | 40 (8.4) | 8 (2.8) | 32 (17.2) | |
| Failure to complete SAEs when appropriate | 1 (0.2) | 0 (0.0) | 1 (0.5) | |
| Failure to complete outcomes when appropriate | 2 (0.4) | 0 (0.0) | 2 (1.1) | |
| Patient does not receive correct loading dose | 116 (24.4) | 76 (26.2) | 40 (21.5) | |
| Patient receives high dose of treatment post loading | 6 (1.3) | 3 (1.0) | 3 (1.6) | |
| Patient does not receive 5 days of randomised treatment in the first 7 days | 138 (29.0) | 101 (34.8) | 37 (19.9) | |
| Patient does not receive 16 days of randomised treatment in the first 21 days | 37 (7.8) | 28 (9.7) | 9 (4.8) | |
| Patient received more than 28 days of randomised treatment | 5 (1.1) | 4 (1.4) | 1 (0.5) | |
| Full consent was not obtained – proxy consent was given prior to randomisation | 3 (0.6) | 3 (1.0) | 0 (0.0) | |
| Follow-up assessments performed | 55 (11.6) | 31 (10.7) | 24 (12.9) | 0.46 |
| Day 7 follow-up or FBC not done on correct date | 24 (5.0) | 14 (4.8) | 10 (5.4) | |
| Day 35 follow-up or FBC not done on correct date | 31 (6.5) | 17 (5.9) | 14 (7.5) | |
| Miscellaneous | 6 (1.3) | 2 (0.7) | 4 (2.2) | 0.16 |
| Other protocol violations | 6 (1.3) | 2 (0.7) | 4 (2.2) |
The TARDIS trial in context of earlier antiplatelet intensity trials
Findings from earlier large trials and meta-analyses suggested that acute dual antiplatelet therapy (either aspirin and dipyridamole, or aspirin and clopidogrel) was superior to one agent in preventing early recurrent cerebral ischaemic events. 45,46 The results of the TARDIS trial, which involved combined aspirin, clopidogrel and dipyridamole, are statistically different (p = 0.02; I2 80.5%) from those earlier trials that involved two agents when considering the outcome of recurrent stroke (Figure 16). As a result, the summary statistic at the bottom of the figure is irrelevant. In contrast, there was no difference between the TARDIS trial and earlier trials for major/fatal bleeding (p = 0.23; I2 30.8%) (Figure 17).
Chapter 4 Discussion
Interpretation
The TARDIS trial met its main objective of comparing the safety and efficacy of intensive triple antiplatelet therapy with guideline-based treatment. In this group of patients with acute non-cardioembolic ischaemic stroke or TIA, intensive antiplatelet therapy did not reduce stroke recurrence or its severity when compared with guideline antiplatelet therapy. Guideline antiplatelet therapy comprised either clopidogrel alone or combined aspirin and dipyridamole. Furthermore, intensive antiplatelet therapy did not improve measures of disability, cognition, quality of life or mood. However, intensive antiplatelet therapy was associated with both an increase in, and more severe, bleeding. There was no difference in mortality or the composite end point of stroke or major bleeding.
Previous meta-analyses of trials of antiplatelets in acute stroke/TIA have suggested that it is the number of drugs (i.e. two vs. one), rather than which drugs are used, that is important when determining efficacy, at least when considering aspirin, clopidogrel and dipyridamole. 45,46 If two agents are better than one, then three might be better still, providing that bleeding is not overly increased. However, the TARDIS trial demonstrated that the addition of a third agent does not reduce recurrent stroke but does increase bleeding. Because the primary outcome included haemorrhagic stroke, the failure to reduce stroke recurrence and its severity overall appears to reflect the combination of increased secondary intracranial haemorrhage and a tendency to reduced cerebral ischaemic stroke. Several factors appear to explain the results. First, participants with a severe stroke (presenting with cortical strokes) tended to do better on guideline therapy whereas intensive antiplatelets favoured those with mild stroke. The explanation for this observation is not obvious, as stroke severity did not appear to influence the effect of treatment on bleeding. Second, the type of guideline comparator appeared to be important, as there was a tendency, albeit just non-significant, for intensive therapy to have beneficial effects on the primary outcome in comparison with combined aspirin and dipyridamole, but not when compared with clopidogrel alone. In parallel, intensive antiplatelet therapy was more likely to cause bleeding when compared with combined aspirin and dipyridamole than when compared with clopidogrel. Nevertheless, these comparisons are indirect as most sites did not elect to randomise participants between the guideline groups.
A confounding factor was the use of thrombolysis that may have increased the difference in bleeding between intensive versus guideline antiplatelet therapy. Large trials such as CHANCE and Acute Stroke or Transient Ischaemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes (SOCRATES) excluded patients who received thrombolysis because of shorter recruitment time windows and inclusion of patients with only mild stroke. 47,104 In the TARDIS trial, randomised antiplatelet therapy commenced 24 hours after the completion of any alteplase maintenance dose, which might have promoted bleeding. A treatment–thrombolysis interaction was present for bleeding, which is surprising as the circulating half-life of alteplase is a few minutes (although the tissue and biological half-lives may be longer). Furthermore, antiplatelet agents were given with a loading dose following thrombolysis, as recommended in guidelines, and it is possible that this acceleration of antiplatelet activity contributed to the risk of bleeding in the presence of recent thrombolysis. Whatever the reason, this finding suggests that bleeding risk is higher following intravenous thrombolysis in spite of an interval of ≥ 24 hours between completing alteplase and randomising into the TARDIS trial.
The TARDIS trial was the first trial designed to use ordered categorical primary and safety outcomes of the type fatal event/severe non-fatal event/mild non-fatal event/no event. Empirical analyses of this approach using published data from existing trials of antiplatelets and other prophylactic interventions suggested that studies would have more statistical power, or the same power for a smaller sample size. 76 A key secondary aim of the TARDIS trial was to test this methodological approach and to compare it with analyses based on binary outcomes. As the primary efficacy analysis was non-significant, the relative merits of ordinal versus binary analysis could not be adequately assessed for stroke. However, ordinal bleeding gave comparable, if not more pronounced, results in comparison with the outcome of ‘fatal or major bleeding’.
Strengths
The present trial has several strengths, especially generalisability owing to wide inclusion criteria. In addition to motor presentations, patients with acute ischaemic stroke included those with severe stroke, dysphasia or neuroimaging-positive hemianopia. Similarly, patients with TIA included those with crescendo TIA or who were already on dual antiplatelet agents. Hence, groups of patients who are typically excluded in stroke prevention trials could be enrolled. Inclusion of patients with severe stroke meant that those with cortical syndromes, often a minority in such trials, could participate. The wide time window of 48 hours meant that patients could be enrolled after intravenous thrombolysis. Furthermore, the trial had a large sample size of > 3000 patients, concealment of treatment assignment, prospective assessment of multiple outcomes including safety measures such as haemorrhage, very high rates of follow-up (99.2% of participants had their primary outcome determined), care in specialist stroke services, and use of locally sourced aspirin, clopidogrel and dipyridamole from a variety of manufacturers (thus increasing the external validity of the trial). An additional strength is that the results define clearly that three agents do not add further efficacy over and above standard care based on one or two agents.
Limitations
Nevertheless, several limitations apply. First, the broad population might have included groups that were more likely to either respond (e.g. those with minor stroke or TIA, or atherosclerotic disease105) or have a major bleed (e.g. those receiving thrombolysis or having small vessel disease or microbleeds106), and this might explain the neutral results. Future trials of antiplatelets may need to be more specific and focus on individuals with atherosclerotic disease, who do not have significant numbers of microbleeds on MRI scanning, and who are known to respond to the antiplatelet agent(s) under test. Second, the antiplatelet agents were administered in an open-label design and participants knew which drug(s) they were on. This may have driven the reporting of known adverse events such as headache with dipyridamole and bleeding with intensive antiplatelet therapy. In mitigation, outcomes at day 90 were assessed centrally and blinded to treatment assignment to reduce the potential for bias. Third, the comparator group involved different antiplatelet agents, a situation reflecting changes in national and international guidelines that added monotherapy with clopidogrel to the existing recommendation of combined aspirin and dipyridamole. The PRoFESS mega trial compared these two strategies in 20,332 patients with chronic (not acute) ischaemic stroke or TIA and found no differential effect on stroke recurrence, although major haemorrhage occurred more frequently with combined aspirin and dipyridamole. 107 It should be noted that the TARDIS trial did not allow aspirin monotherapy (as shown to be effective for ischaemic stroke in two mega trials36,37) as a comparator because this was not recommended in UK guidelines for secondary prevention in either 2005 or 2010, largely because both aspirin and dipyridamole, and clopidogrel alone, had previously been shown to be superior to aspirin alone in three large trials. 18,20,21 Fourth, the time window for recruitment was longer than in other trials such as POINT,48 CHANCE47 and SOCRATES,104 and this led to a lower overall event rate because the risk of recurrence falls with time, even over the first 48 hours. Fifth, randomised treatments were given for 30 days and this might have been too long in view of the identified haemorrhage risk. Importantly, the risk of haemorrhage for intensive and guideline antiplatelets diverged from the start of randomised treatment and was significantly different by 14 days; hence, it is not apparent that a shorter period of intensive antiplatelets would have avoided the risk of bleeding. Last, the trial was stopped early following recommendation by the independent DMC and the results may represent a false-neutral finding related to the lower than planned statistical power. The trial recruited > 70% of its planned target of 4100 participants and the post hoc statistical power remained high at 85%. Furthermore, the prespecified effect size of 0.68 was almost ruled out (with 95% confidence). As such, it is likely that the trial’s main findings are correct (i.e. that intensive antiplatelet therapy does not appear to reduce recurrent cerebral ischaemic events but does increase the risk of haemorrhage).
Patient and public involvement
The trial was designed and delivered with active patient and public involvement (PPI) involvement by three patients, including in design, helping writing patient information sheets, membership of the TSC and interpretation of the results.
Attendance at TSCs was challenging for PPI members both in attending physically (in the presence of continuing physical impairments) and in discussion (in the presence of continuing dysphasia). Although joining by telephone resolved physical issues it was complicated by partial hearing loss. Nevertheless, these concerns were outweighed by the active involvement of PPI members, and they enjoyed the experience.
Conclusions
Implications for health care
The TARDIS trial showed that among patients with acute ischaemic stroke or TIA who were recruited within 48 hours after symptom onset, treatment with intensive antiplatelet therapy as compared with guideline antiplatelet therapy did not reduce stroke recurrence and its severity but did increase bleeding and its severity. Hence, there is no evidence to support the use of intensive treatment based on three standard antiplatelets (aspirin, clopidogrel, dipyridamole).
Recommendations for research
Trials are needed to confirm whether or not dual antiplatelet therapy with aspirin and clopidogrel is superior to aspirin alone in patients with acute stroke or TIA; the POINT trial48 addresses this question. Intensive treatment with three antiplatelet agents should only be studied in the context of randomised controlled trials, although there is no obvious reason for further testing of this approach. Future trials examining potent antiplatelets should consider whether or not it would be safe to use them with existing antiplatelets in patients with acute cerebral ischaemia. Future trials need to assess the value of establishing individual patient responses to different antiplatelet regimes, and better ways of identifying patients at risk of bleeding complications.
Acknowledgements
We thank the participants, investigators and research staff at the participating sites and members of the TSC and independent DMC for their involvement in, and support for, the study. We also thank the NIHR Stroke Research Network/Clinical Research Network (Stroke) for its support for recruitment in the UK, which would not have been possible otherwise.
Contributions of authors
Philip M Bath (Stroke Association Professor of Stroke Medicine, Stroke) was chief investigator, is guarantor for the study and wrote the first draft of the manuscript. He is also a NIHR senior investigator.
Lisa J Woodhouse (Statistical Research Fellow, Stroke) ran data checks during the trial and prepared the results for publication. She revised the manuscript.
Jason P Appleton (Clinical Research Fellow, Stroke) supported medical questions and problems during the trial. He revised the manuscript.
Maia Beridze (Professor of Neurology, Neurology) was national co-ordinator for Georgia. She revised the manuscript.
Hanne Christensen (Professor of Neurology, Neurology) was national co-ordinator for Denmark. She revised the manuscript.
Robert A Dineen (Associate Professor of Neuroradiology, Neuroradiology) led neuroimaging. He revised the manuscript.
Katie Flaherty (Medical Statistician, Stroke) supported preparation of the results for publication. She revised the manuscript.
Lelia Duley (Professor of Clinical Trials Research, Clinical Trials Unit) advised on trial design and delivery. She revised the manuscript.
Timothy J England (Associate Professor of Stroke, Vascular Medicine) cowrote the protocol and supported medical questions and problems during the trial. He revised the manuscript.
Diane Havard (Senior Trials Manager, Stroke) managed the International Coordinating Centre. She revised the manuscript.
Stan Heptinstall (Professor of Haemostasis and Thrombosis, Stroke) led the P-selectin substudy. He revised the manuscript.
Marilyn James (Professor of Health Economics, Rehabilitation and Ageing) led the proto health economics substudy. She revised the manuscript.
Chibeka Kasonde (Patient Representative, Nottingham) advised on patient–carer issues, interpretation of the results and dissemination. She revised the manuscript.
Kailash Krishnan (Consultant Stroke Physician, Stroke) supported medical questions and problems during the trial, and adjudicated outcomes and SAEs. He revised the manuscript.
Hugh S Markus (Professor of Neurology, Neurology) led the TCD substudy. He revised the manuscript. He is also a NIHR senior investigator.
Alan A Montgomery (Professor of Medical Statistics and Clinical Trials, Clinical Trials Unit) supported the analyses. He revised the manuscript.
Stuart Pocock (Professor of Medical Statistics, Medical Statistics) led on statistical design and supported the analyses. He revised the manuscript.
Marc Randall (Consultant Neurologist, Neurology) adjudicated outcomes and SAEs. He revised the manuscript.
Annamarei Ranta (Associate Professor of Neurology, Neurology) was national co-ordinator for New Zealand. She revised the manuscript.
Thompson G Robinson (Professor of Stroke Medicine, Stroke) advised on trial design and delivery. He revised the manuscript. He is also a NIHR senior investigator.
Polly Scutt (Statistical Research Fellow, Stroke) supported preparation of the results for publication. She revised the manuscript.
Graham S Venables (Consultant Neurologist, Neurology) advised on trial design and delivery. He revised the manuscript.
Nikola Sprigg (Professor of Stroke Medicine, Stroke) was deputy chief investigator, and led adjudication of outcomes and SAEs. She revised the manuscript.
Publications
Bath PM, Robson K, Woodhouse LJ, Sprigg N, Dineen R, Pocock S, et al. Statistical analysis plan for the ‘triple antiplatelets for reducing dependency after ischaemic stroke’ (TARDIS) trial. Int J Stroke 2015;10:449–51.
TARDIS Trial Investigators. Safety and efficacy of intensive vs. guideline antiplatelet therapy in high-risk patients with recent ischemic stroke or transient ischemic attack: rationale and design of the Triple Antiplatelets for reducing Dependency after Ischaemic Stroke (TARDIS) trial (ISRCTN47823388). Int J Stroke 2015;10:1159–65.
Bath PM, Appleton JP, Beridze M, Christensen H, Dineen RA, Duley L, et al. Baseline characteristics of the 3096 patients recruited into the ‘Triple Antiplatelets for Reducing Dependency after Ischemic Stroke’ trial. Int J Stroke 2017;12:524–38.
Bath PM, May J, Flaherty K, Woodhouse LJ, Dovlatova N, Fox SC, et al. Remote assessment of platelet function in patients with acute stroke or transient ischaemic attack. Stroke Res Treat 2017;7365684.
TARDIS. Wiki Journal Club 2017 November. URL: www.wikijournalclub.org/wiki/TARDIS (accessed 8 August 2018).
Bath PM, Woodhouse LJ, Appleton JP, Beridze M, Christensen H, Dineen RA, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet 2018;391:850–9.
Data-sharing statement
Anonymised data will be deposited with the Virtual International Stroke Trials Archive. 108 All queries and data requests should be submitted to the corresponding author for consideration in the first instance.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Stroke Health Economics: Cost and Cost-effectiveness Analysis 2016. London: Royal College of Physicians; 2016.
- PROGRESS Collaborative Group . Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41. https://doi.org/10.1016/s0140-6736(01)06178-5.
- PATS Collaborating Group . Post-stroke antihypertensive treatment study. A preliminary result. Chin Med J 1995;108:710-7.
- Hankey GJ, Warlow CP. Transient Ischaemic Attacks of the Brain and Eye. London: WB Saunders; 1994.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-92. https://doi.org/10.1016/S0140-6736(07)60150-0.
- Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005;366:29-36. https://doi.org/10.1016/S0140-6736(05)66702-5.
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack – a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease: the American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-93. https://doi.org/10.1161/STROKEAHA.108.192218.
- Perry JJ, Sharma M, Sivilotti ML, Sutherland J, Symington C, Worster A, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. CMAJ 2011;183:1137-45. https://doi.org/10.1503/cmaj.101668.
- Wardlaw J, Brazzelli M, Chappell F, Miranda H, Shuler K, Sandercock P, et al. ABCD2 score and secondary stroke prevention. Neurology 2015;85:373-80. https://doi.org/10.1212/WNL.0000000000001780.
- Lees KR, Bath PM, Naylor AR. ABC of arterial and venous disease. Secondary prevention of transient ischaemic attack and stroke. BMJ 2000;320:991-4. https://doi.org/10.1136/bmj.320.7240.991.
- Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741-8. https://doi.org/10.1161/01.STR.0000092488.40085.15.
- Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549-59. https://doi.org/10.1056/NEJMoa061894.
- EAFT (European Atrial Fibrillation Trial) Study Group . Secondary prevention in non-rheumatic atrial fibrillation after TIA or minor stroke. Lancet 1993;342:1255-62.
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62. https://doi.org/10.1016/S0140-6736(13)62343-0.
- Bath PM, Lees KR. ABC of arterial and venous disease. Acute stroke. BMJ 2000;320:920-3. https://doi.org/10.1136/bmj.320.7239.920.
- Zhao L, Heptinstall S, Bath P. Antiplatelet therapy for stroke prevention. BJC 2005;12:57-60.
- Antithrombotic Trialists Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. https://doi.org/10.1136/bmj.324.7329.71.
- CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329-39. https://doi.org/10.1016/S0140-6736(96)09457-3.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W. Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators . Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004;35:528-32. https://doi.org/10.1161/01.STR.0000110221.54366.49.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1-13. https://doi.org/10.1016/S0022-510X(96)00308-5.
- Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. ESPRIT Study Group . Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006;367:1665-73. https://doi.org/10.1016/S0140-6736(06)68734-5.
- Halkes PH, Gray LJ, Bath PM, Diener HC, Guiraud-Chaumeil B, Yatsu FM, et al. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry 2008;79:1218-23. https://doi.org/10.1136/jnnp.2008.143875.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. https://doi.org/10.1056/NEJMoa010746.
- Steinhubl SR, Berger PB, Mann JT, Fry ETA, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary invention: a randomized controlled trial. JAMA 2002;288:2411-20. https://doi.org/10.1001/jama.288.19.2411.
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-17. https://doi.org/10.1056/NEJMoa060989.
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331-7. https://doi.org/10.1016/S0140-6736(04)16721-4.
- Bath P. Role of aspirin in MATCH. Lancet 2004;364. https://doi.org/10.1016/S0140-6736(04)17342-X.
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press; n.d.
- Clopidogrel and Modified Release Dipyridamole in the Prevention of Occlusive Vascular Events. Technology Appraisal Guidance (TA90). London: NICE; 2005.
- Clopidogrel and Modified Release Dipyridamole in the Prevention of Occlusive Vascular Events. Technology Appraisal Guidance (TA210). London: NICE; 2010.
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2004.
- Leys D, Kwiecinski H, Bogousslavsky J, Bath P, Brainin M, Diener HC, et al. Prevention. European Stroke Initiative. Cerebrovasc Dis 2004;17:15-29. https://doi.org/10.1159/000074817.
- Hacke W, Ringleb PA, Bousser MG, Ford G, Bath P, Brainin M, et al. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008 – the European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee. Cerebrovasc Dis 2008;25:457-50. https://doi.org/10.1159/000131083.
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227-76. https://doi.org/10.1161/STR.0b013e3181f7d043.
- Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 2008;39:1647-52. https://doi.org/10.1161/STROKEAHA.107.189063.
- International Stroke Trial Collaborative Group . The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997;349:1569-81. https://doi.org/10.1016/S0140-6736(97)04011-7.
- CAST (Chinese Acute Stroke Trial) Collaborative Group . CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997;349:1641-9. https://doi.org/10.1016/S0140-6736(97)04010-5.
- Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. FASTER Investigators . Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961-9. https://doi.org/10.1016/S1474-4422(07)70250-8.
- Dengler R, Diener HC, Schwartz A, Grond M, Schumacher H, Machnig T, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:159-66. https://doi.org/10.1016/S1474-4422(09)70361-8.
- Bath PM, Cotton D, Martin RH, Palesch Y, Yusuf S, Sacco R, et al. Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke 2010;41:732-8. https://doi.org/10.1161/STROKEAHA.109.564906.
- Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007;370:1432-42. https://doi.org/10.1016/S0140-6736(07)61448-2.
- Lavallée PC, Meseguer E, Abboud H, Cabrejo L, Olivot JM, Simon O, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007;6:953-60. https://doi.org/10.1016/S1474-4422(07)70248-X.
- Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005;111:2233-40. https://doi.org/10.1161/01.CIR.0000163561.90680.1C.
- Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489-97. https://doi.org/10.1016/S1474-4422(10)70060-0.
- Geeganage CM, Diener HC, Algra A, Chen C, Topol EJ, Dengler R, et al. Dual or mono antiplatelet therapy for patients with acute ischemic stroke or transient ischemic attack: systematic review and meta-analysis of randomized controlled trials. Stroke 2012;43:1058-66. https://doi.org/10.1161/STROKEAHA.111.637686.
- Wong KS, Wang Y, Leng X, Mao C, Tang J, Bath PM, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis. Circulation 2013;128:1656-66. https://doi.org/10.1161/CIRCULATIONAHA.113.003187.
- Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11-9. https://doi.org/10.1056/NEJMoa1215340.
- Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215-2. https://doi.org/10.1056/NEJMoa1800410.
- Michelson AD, Cattaneo M, Eikelboom JW, Gurbel P, Kottke-Marchant K, Kunicki TJ, et al. Aspirin resistance: position paper of the Working Group on Aspirin Resistance. J Thromb Haemost 2005;3:1309-11. https://doi.org/10.1111/j.1538-7836.2005.01351.x.
- Leonardi-Bee J, Bath PM, Bousser MG, Davalos A, Diener HC, Guiraud-Chaumeil B, et al. Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke 2005;36:162-8. https://doi.org/10.1161/01.STR.0000149621.95215.ea.
- Scholz T, Zhao L, Temmler U, Bath P, Heptinstall S, Lösche W. The GPIIb/IIIa antagonist eptifibatide markedly potentiates platelet-leukocyte interaction and tissue factor expression following platelet activation in whole blood in vitro. Platelets 2002;13:401-6. https://doi.org/10.1080/0953710021000024367.
- Bath PMW, Zhao L, Heptinstall S. Current status of stroke prevention in patients with atrial fibrillation. Eur Heart J 2005;7:C12-18. https://doi.org/10.1093/eurheartj/sui015.
- Zhao L, Gray L, Leonardi-Bee J, Weaver CS, Heptinstall S, Bath PM. Effect of aspirin, clopidogrel and dipyridamole on soluble markers of vascular function in normal volunteers and patients with prior ischaemic stroke. Platelets 2006;17:100-4. https://doi.org/10.1080/09537100500235966.
- Zhao L, Bath P, Heptinstall S. Effects of combining three different antiplatelet agents on platelets and leukocytes in whole blood in vitro. Br J Pharmacol 2001;134:353-8. https://doi.org/10.1038/sj.bjp.0704248.
- Zhao L, Bath PM, Fox S, May J, Judge H, Lösche W, et al. The effects of GPIIb-IIIa antagonists and a combination of three other antiplatelet agents on platelet-leukocyte interactions. Curr Med Res Opin 2003;19:178-86. https://doi.org/10.1185/030079903125001721.
- Sprigg N, Gray LJ, England T, Willmot MR, Zhao L, Sare GM, et al. A randomised controlled trial of triple antiplatelet therapy (aspirin, clopidogrel and dipyridamole) in the secondary prevention of stroke: safety, tolerability and feasibility. PLOS ONE 2008;3. https://doi.org/10.1371/journal.pone.0002852.
- Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke – a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists (Reprinted from Stroke 2007;38:1655–711). Circulation 2007;115:E478-534. https://doi.org/10.1161/circulationaha.107.181486.
- Willmot M, Zhao L, Heptinstall S, Bath P. Triple antiplatelet therapy for secondary prevention of recurrent ischemic stroke. J Stroke Cerebrovasc Dis 2004;13:138-40. https://doi.org/10.1016/j.jstrokecerebrovasdis.2004.03.001.
- Geeganage C, Wilcox R, Bath PM. Triple antiplatelet therapy for preventing vascular events: a systematic review and meta-analysis. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-36.
- University of Nottingham . TARDIS Protocol Version 1.5 n.d. http://tardistrial.org/SA0314%20TARDIS%20Protocol%20v15.pdf (accessed August 2018).
- Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scott Med J 1957;2:200-15. https://doi.org/10.1177/003693305700200504.
- Lees KR, Bath PM, Schellinger PD, Kerr DM, Fulton R, Hacke W, et al. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke 2012;43:1163-70. https://doi.org/10.1161/STROKEAHA.111.641423.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. https://doi.org/10.1016/0140-6736(91)93206-O.
- Adams RJ, Meador KJ, Sethi KD, Grotta JC, Thomson DS. Graded neurologic scale for use in acute hemispheric stroke treatment protocols. Stroke 1987;18:665-9. https://doi.org/10.1161/01.STR.18.3.665.
- Adams HP, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126-31. https://doi.org/10.1212/WNL.53.1.126.
- Khosravi AR, Pourmoghadas M, Ostovan M, Mehr GK, Gharipour M, Zakeri H, et al. The impact of generic form of clopidogrel on cardiovascular events in patients with coronary artery stent: results of the OPCES study. J Res Med Sci 2011;16:640-50.
- Sambu N, Radhakrishnan A, Curzen N. A randomized crossover study comparing the antiplatelet effect of plavix versus generic clopidogrel. J Cardiovasc Pharmacol 2012;60:495-501. https://doi.org/10.1097/FJC.0b013e31826f36bc.
- Caldeira D, Fernandes RM, Costa J, David C, Sampaio C, Ferreira JJ. Branded versus generic clopidogrel in cardiovascular diseases: a systematic review. J Cardiovasc Pharmacol 2013;61:277-82. https://doi.org/10.1097/FJC.0b013e31827e5c60.
- McGregor GP. Pivotal bioequivalence study of Clopacin®, a generic formulation of clopidogrel 75 mg film-coated tablets. Adv Ther 2016;33:186-98. https://doi.org/10.1007/s12325-016-0290-0.
- University of Nottingham . TARDIS Trial n.d. https://nottingham.ac.uk/∼nszwww/tardis/tardistrialdb/tardis_login.php (accessed August 2018).
- Bath P, Woodhouse L, Scutt P, Krishnan K, Wardlaw J, Bereczki D, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015;385:617-28. https://doi.org/10.1016/S0140-6736(14)61121-1.
- Blackburn DJ, Krishnan K, Fox L, Ballard C, Burns A, Ford GA, et al. Prevention of Decline in Cognition after Stroke Trial (PODCAST): a study protocol for a factorial randomised controlled trial of intensive versus guideline lowering of blood pressure and lipids. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-401.
- Bath PM, Robson K, Woodhouse LJ, Sprigg N, Dineen R, Pocock S. TARDIS Trialists . Statistical analysis plan for the ‘Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke’ (TARDIS) trial. Int J Stroke 2015;10:449-51. https://doi.org/10.1111/ijs.12445.
- Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4. https://doi.org/10.1111/j.1538-7836.2005.01204.x.
- Anonymous . Myocardial infarction redefined – a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502-13. https://doi.org/10.1053/euhj.2000.2305.
- Bath PMW, Geeganage CM, Gray LJ, Collier T, Pocock S. Use of ordinal outcomes in vascular prevention trials: comparison with binary outcomes in published stroke trials. Stroke 2008;39:2817-23. https://doi.org/10.1161/STROKEAHA.107.509893.
- Krishnan K, Beridze M, Christensen H, Dineen R, Duley L, Heptinstall S, et al. Safety and efficacy of intensive vs. guideline antiplatelet therapy in high-risk patients with recent ischemic stroke or transient ischemic attack: rationale and design of the Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke (TARDIS) trial (ISRCTN47823388). Int J Stroke 2015;10:1159-65. https://doi.org/10.1111/ijs.12538.
- Bath PM, Geeganage CM, Gray LJ. Ordinal reanalysis of the SHEP trial. Stroke 2008;39. https://doi.org/10.1161/STROKEAHA.108.527044.
- Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J 2008;29:2031-41. https://doi.org/10.1093/eurheartj/ehn299.
- Schellinger PD, Bath PM, Lees KR, Bornstein NM, Uriel E, Eisert W, et al. European Stroke Organisation Outcomes Working Group . Assessment of additional endpoints for trials in acute stroke – what, when, where, in who?. Int J Stroke 2012;7:227-30. https://doi.org/10.1111/j.1747-4949.2012.00773.x.
- Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538-41.
- Metitieri T, Geroldi C, Pezzini A, Frisoni GB, Bianchetti A, Trabucchi M. The Itel-MMSE: an Italian telephone version of the Mini-Mental State Examination. Int J Geriatr Psychiatry 2001;16:166-7. https://doi.org/10.1002/1099-1166(200102)16:2<166::AID-GPS290>3.0.CO;2-M.
- Desmond DW, Tatemichi TK, Hanzawa L. The telephone interview for cognitive status (TICS): reliability and validity in a stroke sample. Int J Geriatr Psychiatry 1994;9:803-7. https://doi.org/10.1002/gps.930091006.
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167-77.
- Brooks R. EuroQoL: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry 1965;13:508-15. https://doi.org/10.1001/archpsyc.1965.01730060026004.
- Ankolekar S, Renton C, Sare G, Ellender S, Sprigg N, Wardlaw JM, et al. ENOS Trial Investigators . Relationship between poststroke cognition, baseline factors, and functional outcome: data from ‘efficacy of nitric oxide in stroke’ trial. J Stroke Cerebrovasc Dis 2014;23:1821-9. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.04.022.
- Weir CJ, Lees KR. Comparison of stratification and adaptive methods for treatment allocation in an acute stroke clinical trial. Stat Med 2003;22:705-26. https://doi.org/10.1002/sim.1366.
- Weaver CS, Leonardi-Bee J, Bath-Hextall FJ, Bath PM. Sample size calculations in acute stroke trials: a systematic review of their reporting, characteristics, and relationship with outcome. Stroke 2004;35:1216-24. https://doi.org/10.1161/01.STR.0000125010.70652.93.
- Optimising Analysis of Stroke Trials (OAST) Collaboration . Calculation of sample size for stroke trials assessing functional outcome: comparison of binary and ordinal approaches. Int J Stroke 2008;3:78-84. https://doi.org/10.1111/j.1747-4949.2008.00184.x.
- Gray LJ, Bath PM, Collier T. Optimising the Analysis of Stroke Trials (OAST) Collaboration . Should stroke trials adjust functional outcome for baseline prognostic factors?. Stroke 2009;40:888-94. https://doi.org/10.1161/STROKEAHA.108.519207.
- Rashid P, Weaver C, Leonardi-Bee JA, Fletcher S, Bath FJ, Bath PMW. The effects of transdermal glyceryl trinitrate, a nitric oxide donor on blood pressure, cerebral and cardiac haemodynamics and plasma nitric oxide levels in acute stroke. J Stroke Cerebrovasc Dis 2003;13:143-51. https://doi.org/10.1016/S1052-3057(03)00037-5.
- Wardlaw JM, Farrall AJ, Perry D, von Kummer R, Mielke O, Moulin T, et al. Factors influencing the detection of early CT signs of cerebral ischemia: an internet-based, international multiobserver study. Stroke 2007;38:1250-6. https://doi.org/10.1161/01.STR.0000259715.53166.25.
- Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0015757.
- Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352-63. https://doi.org/10.1016/S0140-6736(12)60768-5.
- Buyse M, George SL, Evans S, Geller NL, Ranstam J, Scherrer B, et al. The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials. Stat Med 1999;18:3435-51. https://doi.org/10.1002/(SICI)1097-0258(19991230)18:24%3C3435::AID-SIM365%3E3.0.CO;2-O.
- Bath PM, May J, Flaherty K, Woodhouse LJ, Dovlatova N, Fox SC, et al. Remote assessment of platelet function in patients with acute stroke or transient ischaemic attack. Stroke Res Treat 2017;2017. https://doi.org/10.1155/2017/7365684.
- King A, Bath PM, Markus HS. Clopidogrel versus dipyridamole in addition to aspirin in reducing embolization detected with ambulatory transcranial Doppler: a randomized trial. Stroke 2011;42:650-5. https://doi.org/10.1161/STROKEAHA.110.601807.
- Bath PM, Appleton JP, Beridze M, Christensen H, Dineen RA, Duley L, et al. Baseline characteristics of the 3096 patients recruited into the ‘Triple Antiplatelets for Reducing Dependency after Ischemic Stroke’ trial. Int J Stroke 2017;12:524-38. https://doi.org/10.1177/1747493016677988.
- Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke 1993;24:35-41.
- Davidai G, Cotton D, Gorelick P, Bath PMW, Lipton RB, Sacco R, et al. Dipyridamole-induced headache and lower recurrence risk in secondary prevention of ischaemic stroke: a post hoc analysis. Eur J Neurol 2014;21:1311-17. https://doi.org/10.1111/ene.12484.
- Matías-Guiu J, Dávalos A, Picó M, Monasterio J, Vilaseca J, Codina A. Low-dose acetylsalicylic acid (ASA) plus dipyridamole versus dipyridamole alone in the prevention of stroke in patients with reversible ischemic attacks. Acta Neurol Scand 1987;76:413-21. https://doi.org/10.1111/j.1600-0404.1987.tb03596.x.
- Nakamura T, Tsuruta S, Uchiyama S. Cilostazol combined with aspirin prevents early neurological deterioration in patients with acute ischemic stroke: a pilot study. J Neurol Sci 2012;313:22-6. https://doi.org/10.1016/j.jns.2011.09.038.
- Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016;375:35-43. https://doi.org/10.1056/NEJMoa1603060.
- Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017;16:301-10. https://doi.org/10.1016/S1474-4422(17)30038-8.
- Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817-25. https://doi.org/10.1056/NEJMoa1204133.
- Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008;7:875-84. https://doi.org/10.1016/S1474-4422(08)70198-4.
- Ali M, Bath PM, Curram J, Davis SM, Diener HC, Donnan GA, et al. The Virtual International Stroke Trials Archive. Stroke 2007;38:1905-10. https://doi.org/10.1161/STROKEAHA.106.473579.
Appendix 1 – Study investigators
Study investigators
Writing committee
Philip M Bath, Lisa J Woodhouse, Jason P Appleton, Maia Beridze, Hanne Christensen, Robert A Dineen, Lelia Duley, Timothy J England, Katie Flaherty, Diane Havard, Stan Heptinstall, Marilyn James, Kailash Krishnan, Hugh S Markus, Alan A Montgomery, Stuart Pocock, Marc Randall, Annamarei Ranta, Thompson Robinson, Polly Scutt, Graham S Venables and Nikola Sprigg, on behalf of the TARDIS triallists.
Trials committee
Independent members: Helen Rodgers (Newcastle, TSC chairperson), Ahamad Hassan (Leeds), Christine Roffe (Stoke-on-Trent), Craig Smith (Salford) and William Toff (Leicester).
Grant holders: Philip Bath (Nottingham, chief investigator), Rob Dineen (Nottingham, neuroradiology lead), Lelia Duley (Nottingham), Stan Heptinstall (Nottingham, platelet expert), Marilyn James (Nottingham, health economic lead), Hugh Markus (Cambridge), Stuart Pocock (London, statistical lead), Thompson Robinson (Leicester), Nikola Sprigg (Nottingham, deputy chief investigator) and Graham Venables (Sheffield).
Patient–public representative (PPI, Nottingham): Oswald Newell (2008–14) and Chibeka Kasonde (2014–16).
Sponsor’s representative: Angela Shone (University of Nottingham).
International advisory committee
Denmark: Hanne Christensen (Copenhagen); Georgia: Maia Beridze (Tblisi); New Zealand: Annamarei Ranta (Wellington); and UK: Philip Bath (chairperson, Nottingham).
Data monitoring committee
Ian Ford (Glasgow, UK; chairperson), Didier Leys (Lille, France), Cathie Sudlow (Edinburgh, UK) and Matthew Walters (Glasgow, UK).
Independent events (outcome, serious adverse event) adjudicator
Nikola Sprigg (Nottingham, UK), Marc Randall (Leeds, UK), Wayne Sunman (Nottingham, UK) and Kailash Krishnan (Nottingham, UK).
Neuroimaging adjudicators
Rob Dineen (Nottingham, UK), Alessandro Adami (Verona, Italy), Lesley Cala (Perth, WA, Australia), Ana Casado (Edinburgh, UK), Rebecca Gallagher (Derby, UK), David Swienton (Leicester, UK) and Satheesh Ramalingam (Birmingham, UK).
Platelet substudy
Stan Heptinstall, Sue Fox and Jane May (Nottingham, UK).
Trial management committee
Senior trial managers: Sally Utton (2012–14) and Diane Havard (2015–17).
Trial manager: Hayley Foster (2013–16).
UK co-ordinators: Margaret Adrian (2009–17), Tanya Payne (2009–15), Alice Durham (2013–14), Harriet Howard (2014–15), Michael Stringer (2014–17) and Jamie Longmate (2015–17).
International co-ordinators: Sharon Ellender (2009–13), Alice Durham (2013–14), Sarah Grant (2013–14) and Joanne Keeling (2014–16).
Outcome co-ordinators: Sharon Ellender (2009–13), Lynn Stokes (2010–12), Patrick Cox (2011–13), Judith Clarke (2012), Lyndsey Cobane (2012–14), Kathy Whittamore (2013–14), Joanne Keeling (2013–16), Jennifer Smithson (2014–16), James Kirby (2015–16) and Gemma Walker (2015–16).
Statisticians: Michael Tracy (2009–10), Cheryl Renton (2009–12), Cyrille Correia (2011), Lydia Fox (2012–13), Aimee Houlton (2012–13), Katie Flaherty (2013–17), Polly Scutt (2013–17) and Lisa J Woodhouse (2013–17).
Programming/database management: Graham Watson (2009–11), Liz Walker (2009–17) and Richard Dooley (2013–17).
Physicians: Tim England (2009), Chamilla Geeganage (2009–12), Sandeep Ankolekar (2009–11), Kailash Krishnan (2012–15) and Jason Appleton (2015–17).
Data managers: Lida Kaur (2009–10), Tanya Jones (2010–13), Clare Randall (2011–12), Dawn Hazle (2012–16) and Mark Sampson (2013–17).
Finance: Wim Clarke (2009–17).
Secretaries: Marilyn Stonham (2011), Susan Blencowe (2012–13), Yvonne Smallwood (2012–17), Lauren Dunn (2013–14), Monika Kowalczyk (2014–16) and Patricia Robinson (2015–16).
Administrators (temporary): Sarah Bull (2013), Georgina Phillips (2013), Harry Banks (2013–14), Esther Akanya (2014), Sean McLoughlin (2015) and Richard Barks (2015–17).
Participating countries, sites and investigators
The numbers in brackets refer to number of participants recruited.
Denmark
Co-ordinating centre: H Christensen (national co-ordinator) and L M Christensen (follow-up investigator).
Copenhagen University Hospital, Bispebjerg (33): H Christensen, L Bentsen, L M Christensen, C Krarup Hansen and T T Thomsen.
Herlev Hospital (91): C Kruuse, H H Jensen, S S Hansen and V Petrovic.
Georgia
Co-ordinating Centre: M Beridze (national co-ordinator) and N Beridze (follow-up investigator).
Tblisi State Medical Institute (15): N Kakabadze, M Beridze, N Beridze, T Kherkheulidze and D Kakabadze.
Khechinashvili Medical University Hospital (46): I Toidze, N Beridze, N Lobjanidze and N Akiashvili.
Tblisi Central Hospital (22): A Tevdoradze, N Beridze, T Kherkheulidze, N Khizanishvili and T Tsanava.
New Zealand
Co-ordinating Centre: A Ranta (national co-ordinator) and R Taylor (follow-up investigator).
Palmerston North Hospital (7): I Iniesta, J Kok, J Duignan, M Funnell, A Ranta, P Cariga, M Rodriguez, R Taylor, I J Watson and S Tennant.
United Kingdom
Aberdeen Royal Infirmary (58): M Macleod, J Furnace, H Gow, J Irvine, A Joyson, S Nelson and V Taylor.
Airedale NHS Foundation Trust (1): M Smith, R Bellfield and B Hairsine.
Arrowe Park Hospital (4): R Davies and A Dodd.
Atnagelvin Hospital, Londonderry (1): J Corrigan and M Doherty.
Barnsley District General Hospital (39): A Ahmed, C Denniss, S Johnson-Holland and K A Kay.
Barts Health NHS Trust – Royal London Hospital (39): R Icart Palau, G Auld, P Daboo, R Erande, G Grimwood, D Hove, L Howaniec and O Redjep.
Basildon University Hospital (2): R Rangasamay and G Butt.
Birmingham Heartlands Hospital (3): D Sandler, J Reddan and S Stafford.
Blackpool Victoria Hospital (1): J McIlmoyle.
Bradford Royal Infirmary (4): S Maguire and R Bellfield.
Bristol Royal Infirmary (5): P Murphy, J Chambers, L Guthrie, M Osborn and A Steele.
Buckinghamshire Healthcare NHS Trust, High Wycombe (31): M Burn, A Benford, A Misra and D Hilton.
Cambridge University Hospitals NHS Foundation Trust (8): E O’Brien, E Amis, S Finlay and J Mitchell.
Charing Cross Hospital, Imperial (32): O Geraghty, K Harvey, B Hazel, S Mashate and P Wilding.
Chesterfield Royal Hospital NHS Foundation Trust (25): M Sajid, M Ball and R Gascoyne.
Colchester General Hospital (44): R Sivakumar and A Wright.
Countess of Chester NHS Foundation Trust (73): K Chatterjee, S Booth, H Eccleson, C Kelly, S Leason and C Perkins.
County Durham and Darlington NHS Foundation Trust (34): D Bruce, E Brown, S Clayton, M Garside and G Rogers.
Croydon University Hospital (10): E Lawrence, S Mahmood and C Watchurst.
Doncaster and Bassetlaw Teaching Hospitals. NHS Foundation Trust (29): D Chadha, L Glover, L Holford, K Smith and D Walstow.
Dorset County Hospital NHS Foundation Trust, Dorchester (1): R Williams, L O’Shea and J Goodsell.
Eastbourne General Hospital (40): C Athulathmudali and E Barbon.
Fairfield General Hospital (7): R Namushi, P Jacob, L Johnson and D Morse.
Forth Valley Royal Hospital (11): M Macleod and C McGhee.
Frimley Park Hospital NHS Foundation Trust (5): O Speirs, S Atkinson and A Peacocke.
Glasgow Royal Infirmary (19): P Langhorne, R Graham, F Wright and C McAlpine.
Good Hope Hospital (3): A Ravindrane, J Reddan and S Stafford.
Great Western Hospitals NHS Trust (4): M Bajoriene, L Matter and S Windebank.
Hampshire Hospitals NHS Foundation Trust (24): E Giallombardo, D Dellafera, C Eglinton and J Wilson.
James Cooke University Hospital (46): D Broughton, K Chapman and L Dixon.
James Paget University Hospital NHS Foundation Trust (1): M Zaidi.
Kettering General Hospital (1): K Ayes and J Kessell.
King’s College Hospital (164): D Manawadu, O Adegbaju, J Aeron-Thomas, K Anderson, A Brigden, E Cattermole, J Good, S Hassan, E Khoromana, L Lee-Carbon, K Marks, E Mckenzie and N Sikondari.
Kings Mill Hospital (47): M Cooper, K Whysall and I Wynter.
Leeds General Infirmary (48): J Bamford, A Hassan, P Wanklyn, M Kambafwile, L Makawa, M Randall, D Waugh and E Veraque.
Lincoln County Hospital (14): MGG Soliman, S Arif, R Brown, S Butler, C Hewitt and J Hindle.
Lister Hospital (20): A Pusalkar, H Beadle, K Chan, M Siddiqui, P Dangri, S Buddha and A Asokanathan.
Leicester United Hospitals NHS Trust (Leicester Royal Infirmary) (33): A Mistri, D Eveson, K Musarrat, L Manning, S Anand, P Christian, S Khan and C Patel.
Macclesfield District General Hospital (16): M Sein, J Banns, E Gibson, T Gordon, Y Gruenbeck and S Wong.
Mid Yorkshire Hospitals NHS Trust (Dewsbury and Pinderfields) (61): P Datta, G Bateman, L Jackson and A Needle.
Milton Keynes Hospital NHS Foundation Trust (4): Y Duodu, R Oliver and C Padilla-Harris.
Monklands Hospital (48): M Barber, D Esson, F Brodie and C McInnes.
New Cross Hospital, Wolverhampton (25): K Fotherby, D Butler, D Morgan, K Preece and A Willberry.
North Devon District Hospital (2): M Dent, F Hammonds, J Hunt and C Vernon.
Northampton General Hospital NHS Trust (8): D O’Kane, F Faola, P Lai, J O’Callaghan and C Smith.
Northumbria Specialist Emergency Care Hospital (23): C Price, R Lakey, V Riddell, A Smith and G Storey.
Nottingham University Hospitals (220): S Munshi, P Bath, N Sprigg, A Buck, J Clarke, N Gilzeane, M Godfrey, F Hammonds, R Keshvara, C Richardson, J Roffe, L Ryan, F Shelton, W Sunman, A Tittle, J Tomlinson, K Whittamore and G Wilkes.
Peterborough and Stamford Hospitals NHS Foundation Trust (7): P Owusu-Agyei and N Temple.
Pilgrim Hospital (34): D Mangion, A Hardwick and K Netherton.
Plymouth Hospitals NHS Trust (15): A Mohd Nor, C Eglinton, B Hyams, S Norman and N Persad.
Poole Hospital NHS Foundation Trust (15): S Ragab, C Dickson, J Dube, E Jinks, K Knops and B Wadams.
Princess Royal Hospital, Haywards Heath (4): K Ali, J Gaylard and G Spurling.
Princess Royal University Hospital (39): L Sztriha, T Ajao, M Alao, F K Chan and P Webster.
Queen Alexandra Hospital, Portsmouth (2): P Howard, T J Dobson and L Hyatt.
Queen Elizabeth Hospital Birmingham (12): D Sims and J Cunningham.
Queen Elizabeth Hospital (Gateshead) (37): B Esisi, T Cassidy, M Bokhari, B McClelland and B Mokoena.
Queen Elizabeth, the Queen Mother Hospital (51): G Gunathilagan, S Jones, M Reader, G Thomas and S Tilby.
Raigmore Hospital (37): P Findlay, F Barrett, F Leslie, S Ross and I Shread.
Rotherham District General Hospital (14): J Okwera and J Howe.
Royal Cornwall Hospitals NHS Trust (19): F Harrington, G Courtauld and C Schofield.
Royal Derby Hospital (69): T England, R Donnelly, M Maddula, J Scott, J Beavan, K Muhidden, I Memon, J Clarke, M Clarke, A Hedstrom and L Mills.
Royal Devon and Exeter NHS Foundation Trust (63): A Hemsley, A Bowring, L Boxall, H Kingwell, S Keenan and C Roughan.
Royal Liverpool University NHS Trust (69): A Manoj, P Cox, G Fletcher and P Lopez.
Royal Preston Hospital (39): H Emsley, B Gregary, A McLoughlin and S Raj.
Royal Stoke University Hospital (216): C Roffe, N Abano, A Barry, A Butler, R Carpio, K Castro, K Finney, S Gomm, J Hiden, J Grocott, S Lyjko, H Maguire, A Remegoso, R Sanyal, S Stevens, I Natarajan, J Chembala, G Muddegowda and A Warusevitane.
Royal Surrey County Hospital NHS Trust (13): A Blight, O Balazikova and C Lawlor.
Royal United Hospital Bath NHS Trust (25): L Shaw, D Button, D Howcroft, S Lucas, B Madigan and S McCann.
Royal Victoria Infirmary, Newcastle-upon-Tyne (53): A Dixit, A Barkat, J Davis, M Fawcett, L Finlay, H Guy, C Hays, V Hogg, E Horsley, C Hubbuck, C Pringle, C Stevenson, K Storey, T Thompson and S Woodward.
Russells Hall Hospital (12): A Banerjee, C Allcock and S Merotra.
Salford Royal NHS Foundation Trust, Salford Royal (10): C Douglass, E Campbell, R Jarapa, M Johnes, C Keaveney, T Marsden, Z Naing, J Perez and K Shaw.
Salisbury District Hospital (15): T Black, A Anthony and C Clarke.
Scarborough General Hospital (9): J Paterson, K Deighton and E Temlett.
Sheffield Teaching Hospitals NHS Foundation Trust (41): C Blank, C Doyle, S Duty, K Gill, K Harkness, J Howe, C Kamara and E Richards.
Solihull Hospital (2): K Elfandi and S Stafford.
Southend University Hospital NHS Foundation Trust (9): P Guyler, P Harman, C Khuoge, S Kunhunny and S Tysoe.
St Georges Healthcare NHS Trust (183): B Moynihan, T Adedoyin, N Chopra, N Dayal, R Ghatala, N Jeyaraj, I Jones, F Kennedy, L Kerin, N Khanom, S Lewis, S Maheswaran, L Montague, M Niemierko, J O’Reilly, S Trippier, C Watchurst and F Watson.
St Peters Hospital, Chertsey (9): P Wilkinson and E Young.
Stepping Hill Hospital (11): K Dizayee, H Cochrane and D Morse.
Sunderland Royal Hospital (9): J O’Connell, L Mokoena, E Osborne and A Smith.
The Calderdale Royal Hospital (41): A Nair and J Greig.
The County Hospital, Hereford (8): C Jenkins, J Powell and F Price.
The Ipswich Hospital NHS Trust (48): M Chowdhury, S Brixey, L Hunt, N Rands, G Rose and S Stoddart.
The Princess Royal Hospital, Telford (2): M Srinivasan and N Motherwell.
The Queen Elizabeth Hospital, King’s Lynn (27): R Shekhar, T Fuller, A Lankester, P Lingwood, C Rankin and H Webb.
The Royal Bournemouth Hospital (19): B Jupp, J Bell, G Hann, B Longland and C Ovington.
Torbay District Hospital (49): B Bhaskaran, G Ayres, C Bailey, H Bearne, J Buxton, P Fitzell, C Hilaire, D Kelly, S Szabo and D Tomlin.
Univ Hosp of South Manchester (11): E Gamble and B Charles.
University Hospital Aintree (52): R Kumar, T Fluskey, Z Mellor, J Peters and V Sutton.
University Hospital Coventry (11): A Kenton, I Martin and S Nyabadza.
University Hospital of Ayr (3): S Ghosh and M Henry.
University Hospital of North Tees (47): B Kumar, D Bruce, C Ambulo, S Crawford, T Nozedar and M Platton.
Victoria Hospital/Queen Margaret Hospital (27): V Cvoro, M Couser, K McCormick and D Wilkinson.
Walsall Manor Hospital (6): K Javaid and S Hurdowar.
Watford General Hospital (6): T Attygalle and S Sundayi.
West Cumberland Hospital (15): O Orugun, H Crowther, R Jolly and U Poultney.
West Suffolk NHS Hospital Trust (3): A Azim, M Krasinska-Chavez and J White.
Western Sussex Hospitals (6): N Sengupta, J Margalef and M G Metiu.
Whiston Hospital (25): S Meenakshisundaram and S Dealing.
William Harvey Hospital (44): D Hargroves, E Beranova, L Cowie, H Rudenko, A Thomson and A Verrion.
Yeovil District Hospital NHS Foundation Trust (24): K Rashed, S Board, C Buckley, D Hayward, K Jenkins, E Keeling, R Rowland-Axe, C Vickers and D Wood.
Repatriation sites
Homerton University Hospital (11 repatriated patients): A Lehman, O Redjep, R Erande, G Grimwood and D Hove.
Lewisham Healthcare NHS Trust (four repatriated patients): M Patel and H Russell.
Royal Albert Edwards Infirmary, Wigan (three repatriated patients): H Rehman, D Forrest and P Farren.
List of abbreviations
- acOR
- adjusted common odds ratio
- ACS
- acute coronary syndrome
- AD
- aspirin and dipyridamole
- AF
- atrial fibrillation
- aHR
- adjusted hazard ratio
- BHF
- British Heart Foundation
- BI
- Barthel Index
- BNF
- British National Formulary
- CARESS
- Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis
- CHANCE
- Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events
- CHARISMA
- The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- cOR
- common odds ratio
- CT
- computed tomography
- DICOM
- digital imaging and communications in medicine
- DMC
- Data Monitoring Committee
- DVT
- deep-vein thrombosis
- ECG
- electrocardiography
- ENOS
- Efficacy of Nitric Oxide in Stroke
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-VAS
- EuroQoL Visual Analogue Scale
- ESPRIT
- European/Australasian Stroke Prevention in Reversible Ischaemia Trial
- ESPS-2
- European Stroke Prevention Study-2
- FASTER
- Fast Assessment of Stroke and TIA to prevent Early Recurrence
- GCS
- Glasgow Coma Scale
- HI
- haemorrhagic infarct
- HR
- hazard ratio
- HSUV
- health status utility value
- HTA
- Health Technology Assessment
- IMP
- investigational medicinal product
- IQR
- interquartile range
- LACS
- lacunar syndrome
- MATCH
- Management of ATherothrombosis with Clopidogrel in High-risk patients
- MI
- myocardial infarction
- MRI
- magnetic resonance imaging
- mRS
- modified Rankin Scale
- NCC
- National Coordinating Centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- NSTEMI
- non-ST elevation myocardial infarction
- OCSP
- Oxfordshire Community Stroke Project
- OR
- odds ratio
- PACS
- partial arterior circulation syndrome
- POCS
- posterior circulation syndrome
- PEG
- percutaneous endoscopic gastrostomy
- PH
- parenchymal haemorrhage
- PPI
- patient and public involvement
- PRoFESS
- Prevention Regimen for Effectively Avoiding Second Strokes
- RRR
- relative risk reduction
- SAE
- serious adverse event
- SD
- standard deviation
- sICH
- symptomatic intracranial haemorrhage
- SOCRATES
- Acute Stroke or Transient Ischaemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes
- STEMI
- ST-elevation myocardial infarction
- TACS
- total anterior circulation syndrome
- TARDIS
- Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke
- TCD
- transcranial Doppler
- TIA
- transient ischaemic attack
- TICS-M
- Telephone Interview for Cognition Scale – modified
- t-MMSE
- telephone Mini-Mental State Examination
- TA
- Technology Appraisal
- TSC
- Trial Steering Committee
- ZDS
- Zung Depression Scale