Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/127/12. The contractual start date was in July 2014. The draft report began editorial review in July 2017 and was accepted for publication in March 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Maureen Coggrave reports personal fees from Hollister Incorporated (Libertyville, IL, USA) and Wellspect HealthCare (Weybridge, UK), both outside this study. John Norrie was a member of the National Institute for Health Research (NIHR) Commissioning Board from 2010 to 2016, is currently an editor on the NIHR Journals Editorial Board and is Deputy Chairperson of the NIHR Health Technology Assessment General Board. Peter Donnan reports grants from Shire plc (Dublin, Ireland), Novo Nordisk A/S (Bagsværd, Denmark), GlaxoSmithKline plc (London, UK), AstraZeneca plc (Cambridge, UK) and Gilead Sciences Inc. (Foster City, CA, USA), outside this study. He is also a member of the New Drugs Committee of the Scottish Medicines Consortium.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by McClurg et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
The prevalence of multiple sclerosis (MS) is increasing in the UK, and MS is the most common neurological condition in young adults (the average age at onset is 34 years), affecting > 100,000 people at present. 1 It is estimated that 60% of people with multiple sclerosis (PwMS) have problematic neurogenic bowel dysfunction (NBD);2 increased life-expectancy rates, as a result of advances in health care, present additional challenges of the ageing bowel. 3 NBD is rated as one of the most distressing scenarios affecting these patients and includes the symptoms of constipation and faecal incontinence (FI). 4 Constipation can lead to the individual becoming housebound, spending hours trying to empty their bowels and limiting their ability to work; whereas FI is often described as the most devastating event imaginable, leading to social and emotional issues. 5 A MS Trust report6 published in April 2017 revealed that emergency admissions (many of which are thought to be preventable) to hospital for PwMS have increased by 12.7% over the 2 years 2015/16, with overall admissions for bladder- and bowel-related issues, for example impaction, costing £10.4M in 2015/16.
Aetiology of neurogenic bowel dysfunction
Aetiology of NBD in PwMS is multifactorial; reduced mobility and polypharmacy may have a contributory causative role. Coincidental pelvic nerve lesion, occurring during childbirth, could also contribute to FI in women with MS. 7 Spinal cord lesions appear to be most important in the pathogenesis of NBD symptoms in MS. 8 The pathways of neural control of defecation are not fully defined; however, cortical and pontine centres may play a pivotal role in the regulation of sacral segments. 8,9
Conduction times of central motor pathways to sphincteric sacral neurons and pelvic floor striated muscle have been shown to be prolonged in MS. 10 Impaired anorectal sensation may also contribute to the symptoms; somatosensory-evoked potentials were delayed in PwMS compared with controls in one study. 11 Loss of central modulation on spinal cord segments may lead to sympathovagal imbalance, which, in turn, can lead to constipation, characterised by lengthened colon transit time. 12 Constipation has thus been attributed to rectal outlet obstruction, absent or incomplete puborectalis, anal canal and sphincter musculature relaxation, and prolonged colonic transit time. 10,13 Regarding FI, studies have described reduced sensation of rectal filling, reduced rectal compliance, low anal sphincter pressures and hyper-reactivity of the rectal wall. The coexistence of FI and constipation can be explained by inco-ordinated action of the external/internal anal sphincter during expulsion; poor pelvic musculature relaxation may cause incomplete emptying of the rectum, which precipitates FI when anal sphincter weakness and anorectal hyposensitivity are present. 14,15
Current treatment/management options
Management of NBD in PwMS has been little explored and lacks supporting evidence. 16 It is costly both in terms of patient time and to the NHS (e.g. PwMS have two to three times more admissions to hospital for bowel complications than non-MS patients). 17 It also has an impact on the families and carers of PwMS. 18 PwMS use laxatives, suppositories, prolonged digital rectal stimulation and/or rectal irrigation, but often these interventions have inconsistent results. For example, one patient in our previous study would take laxatives two evenings per week, but then could not leave the house the next day as he had no control over when he would pass stool. 5
Evidence for the clinical effectiveness of abdominal massage for constipation
A Cochrane systematic review19 has been undertaken to determine the effects of abdominal massage for the relief of symptoms of chronic constipation in comparison with no treatment or other treatment options. Nine randomised controlled trials (RCTs) (12 randomised comparisons) involving 427 participants were included in the review. 19 The study populations were small (with a maximum of 32 participants per group), heterogeneous and all were rated as having a moderate or high risk of bias. Findings from two trials suggested that abdominal massage compared with advice from a physician provided significant additional relief of symptoms from constipation in the short term. Two trials found no significant differences between groups. One trial, which had the three groups (aroma massage, plain massage and control), reported that both the aroma massage group and the plain massage group had an improved quality of life. The review concluded that there were insufficient data to allow reliable conclusions to be drawn on the effects of abdominal massage in the management of constipation. There was some evidence to suggest that there might be a therapeutic effect; however, larger, more rigorous trials are required to provide evidence of both the clinical effectiveness and the cost-effectiveness of abdominal massage.
How the intervention might work
There is some evidence to suggest that abdominal massage will reduce colonic transit time and enable predictable complete evacuation; however, the possible mechanism of action is not yet fully understood. 20,21 The function of the gastrointestinal tract is influenced by, among other things, activity in the parasympathetic division in the autonomic nervous system. Stimulation of the parasympathetic division increases the motility of the muscles, increases the digestive secretions and relaxes sphincters in the gastrointestinal canal. 22–24 Massage is thought to stimulate peristalsis in the gut by producing rectal muscular waves that stimulate the somatoautomatic reflex and initiate bowel sensation, thereby reducing colonic transit time. 25 Furthermore, the active massaging action may result in a softening of stool consistency, allowing the stool to be passed more easily. 26
Development of the intervention
The abdominal massage intervention used within this trial was based on massage as formally taught in physiotherapy training within the UK and used by an expert in the area in earlier studies. 27–29 This expert was involved in the development of the training materials and in the training of the clinicians undertaking the massage. All clinicians involved with teaching the massage to participants underwent 1 half day of training in the massage technique, as well as presentations on NBD and good bowel care. Information provided to participants on bowel care was based on the MS Society’s handbook on bowel management. A description of the intervention has previously been published. 30 A copy of the training materials is available in Appendix 1.
Who delivers the intervention
In previous studies, the massage was delivered by a health-care professional (HCP) with experience in massage, a family carer or by the patient themselves. It was discovered, however, that the amount of training and support received by participants is poorly described. In this trial, the massage was designed and taught to be either self-massage or undertaken by a ‘carer’. Likewise, the training of the ‘trainers’ (HCPs involved in seeing the patient and teaching the massage) was such that it was delivered in one half-day session, but also required individuals to undertake further practice, to consolidate their training. If this limited training plus support materials for HCPs and patients were to prove effective, then the abdominal massage could potentially be implemented in many settings to patient populations who experience constipation.
Hypothesis
A 6-week intervention of abdominal massage and bowel management advice (intervention group) will improve symptoms and quality of life in PwMS who have NBD compared with advice alone (control group).
Chapter 2 Trial design and methods design
The Abdominal Massage for Bowel Dysfunction Effectiveness Research (AMBER) trial was designed to evaluate whether or not abdominal massage is an effective treatment in reducing the symptoms of NBD, particularly constipation, in PwMS. This trial was a multicentre, patient-randomised, superiority trial comparing the following in PwMS who have stated that their constipation is ‘bothersome’: an intervention of optimised bowel care with once-daily abdominal massage for 6 weeks with the control of optimised bowel care without massage. A description of the trial protocol has already been published. 30
The main trial was supported by a process evaluation to explore the possible mediating factors that may affect the clinical effectiveness of the intervention, how these mediating factors influence clinical effectiveness, and whether or not the factors differ between the randomised groups. Trial processes were evaluated to provide evidence of potential importance in the future implementation of the intervention (see Chapter 5).
The main study objectives were to:
-
establish if an optimised bowel care programme (i.e. provision of advice/information on bowel management) with abdominal massage, compared with an optimised bowel care programme alone, is more clinically effective and cost-effective in reducing the symptoms of NBD at week 24 in PwMS
-
identify and investigate, via a process evaluation, the possible mediating factors that affect the clinical effectiveness of the intervention (including intervention fidelity), how these mediating factors influence clinical effectiveness and whether or not the factors differ between the randomised groups (see Chapter 5)
-
undertake a formal economic evaluation of the interventions from a NHS and a patient perspective (see Chapter 4)
-
undertake a feasibility study relating to the mechanisms of action using anal manometry and colonic transit tests at one tertiary bowel centre where these tests are routinely undertaken
-
record data to validate the responsiveness of a questionnaire to change in quality of life following an intervention.
Ethics approval and research governance
Ethics approval for the trial was granted by the West of Scotland Research Ethics Committee (REC) 4 on 11 June 2014 (reference number 14/WS/0111). NHS approval was granted for 10 different trusts/foundation trusts in England, and two local health boards granted approval for the two sites in Scotland. The trial sponsor was Glasgow Caledonian University (GCU) and the AMBER trial office was based in the Nursing, Midwifery and Allied Health Professions Research Unit (NMAHP RU) at GCU. The AMBER trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) registry (ISRCTN85007023) and on ClinicalTrials.gov (NCT03166007).
Participants
The trial recruited PwMS who reported that they were ‘bothered’ (in their own judgement) by their NBD symptoms at 12 sites across UK (n = 2 in Scotland and n = 10 in England).
Inclusion criteria
People were eligible for the trial if they met the following inclusion criteria:
-
bothered by their NBD
-
aged ≥ 18 years
-
diagnosis of MS (in a stable phase, i.e. no MS relapse in the previous 3 months)
-
no major change of medication in the previous 1 month [e.g. introduction of disease-modifying treatments (DMTs)]
-
not used abdominal massage in the previous 2 months.
Exclusion criteria
-
Being unable to undertake the massage themselves and did not have a carer willing to do it.
-
Being unable to understand the trial processes in order to give informed consent.
-
Contraindications to abdominal massage, which included the following: history of abdominal/pelvic cancer, hiatus, inguinal or umbilical hernia, rectal prolapse, inflammatory bowel disease, volvulus and pregnancy.
-
Abdominal scars, abdominal wounds or skin disorders that may make abdominal massage uncomfortable.
If a potential participant reported recent sudden and severe changes in bowel habits or rectal bleeding, these symptoms were first discussed with the consultant at the relevant site to determine suitability.
Recruitment procedure
The research team at each trial site were responsible for identifying potential participants. Following identification of potentially eligible individuals, a letter of introduction and an ‘expression of interest’ form was either posted or given to patients at their routine clinic appointment. Each patient approached about the trial was allocated a unique participant identifier number. This consisted of six characters: three letters that were an abbreviation of the site name followed by three numbers that were allocated on a consecutive basis (e.g. 001 for first participant). This unique identifier was used throughout the trial and was added to all participant paperwork. Once a completed ‘expression of interest’ form was returned, a member of the research team telephoned the individual to provide further information and assess eligibility. If eligible and willing to take part, the individual was sent a baseline appointment letter along with a 7-day bowel diary for completion. The participant completed the bowel diary the week before the baseline appointment. Participants were also asked to bring someone who was willing to do the massage to this appointment, if required.
Informed consent
Informed, written consent was obtained for all participants at the baseline appointment and included consent to any site-specific tests. The REC agreed to the completion of bowel diaries before the baseline appointment, as the participants’ consent was implied by them willingly completing the diary. There was no use of these data until participants had provided written informed consent; this method aided recruitment to the trial because it meant that there was only one clinic visit required. Participants were made aware that the treatment was allocated at random, regardless of any personal preference they had. They had the right to withdraw from the trial at any time and for any reason; all participants were also made aware that withdrawal would not affect their routine care.
The consent form also had the option for the participant to be contacted if they were interested in taking part in the process evaluation interviews. Chapter 5 explains the consent method followed for this part of the study. The general practitioners (GPs) of all those who took part in the trial were informed of their involvement.
Randomisation, concealment and blinding
Participants who provided written informed consent were randomly allocated to one of two treatment groups during their baseline appointment: (1) advice to optimise bowel care (control group) or (2) advice to optimise bowel care and abdominal massage (intervention group). The web-based randomisation service was provided by the Tayside Clinical Trials Unit (CTU), a UK Clinical Research Collaboration (UKCRC)-registered trials unit, and research staff at sites carried out the randomisation. In a few instances, the AMBER trial central office would assist with the randomisation remotely. This took place when there may have been issues for the staff when connecting to the web-based randomisation system (room allocation with no computer or web connectivity issues). Group allocation was relayed by telephone to the site and copies of the relevant randomisation paperwork were sent to all of those involved. Owing to the nature of the intervention, it was not possible to blind the participants or site staff to the allocation. Participant group allocation was unknown to the data analysis team. Randomisation was stratified by site and minimised on level of disability (walking unaided, aided or wheelchair bound).
Treatment group allocation
Both trial groups
Participants in both the intervention and the control group received a 6-week intervention consisting of one face-to-face consultation (baseline appointment) followed by weekly telephone calls to review adherence and any changes/difficulties with their bowel management. This meant that both groups had the same number of contacts with a HCP. Both groups received advice to optimise bowel care, as described in the following section.
Control group (advice to optimise bowel care)
During the baseline appointment, the participants’ existing routine bowel care was reviewed and discussed with them by a member of the site research team. Dietary and fluid advice was provided, and participants were encouraged to be more active and to use a correct defaecation position, which was described to them. Participants were given a copy of the bowel care advice leaflet of the MS Society that reinforced this advice [see project web page URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1212712/#/ (accessed 30 October 2017)].
Intervention group (abdominal massage and advice to optimise bowel care)
In addition to optimised bowel care as described for the control group, staff delivering the intervention (local HCPs all fully trained in the massage technique) taught the participant and/or his or her carer how to deliver the abdominal massage. This teaching included the following:
-
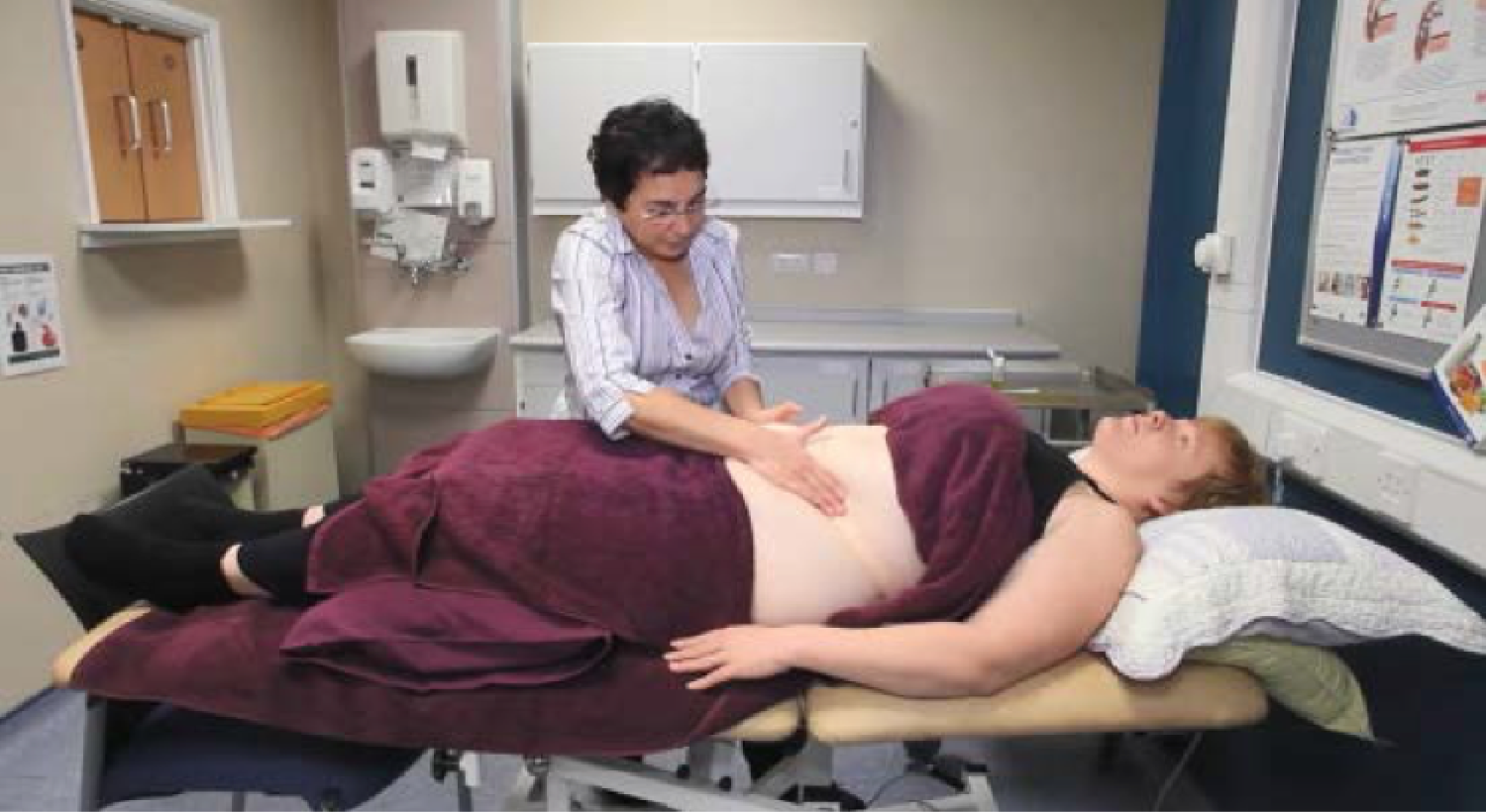
Viewing a short trial-specific digital versatile disc (DVD) that demonstrated the massage techniques for carer and self-massage (Figure 1 shows a picture captured from the training DVD).
-
Provision of a study-specific abdominal massage training booklet.
-
A demonstration of the massage technique on the participant.
-
Practice of the various strokes by the carer or participant.
-
An opportunity for the participant and carer to ask questions. Possible adaptations to accommodate a participant’s disability were also discussed. A daily massage of 10 minutes duration was recommended.
FIGURE 1.
A picture from the AMBER trial training DVD, which demonstrates the massage strokes to be used.
Participants in this group were given an information pack that consisted of the following:
-
the MS Society’s bowel care advice booklet
-
the massage DVD
-
patient abdominal massage training information leaflets.
In order to standardise the intervention delivery across all sites, training for all site staff delivering the intervention was provided by one individual with clinical expertise in the area. Staff attended a trial training day and/or they were trained during the site initiation visits. Each staff member had to perform practical demonstrations and be deemed proficient in the technique before being signed off as fully competent. Sites were contacted after the baseline appointment of their first participant from the intervention group to discuss how staff found delivering the massage and to answer any questions. Further training was available at this point, but all sites felt confident in the delivery of the intervention. Any questions/feedback from the individual sites were shared with all research site staff via monthly update teleconferences. The weekly telephone calls to participants were done either by the staff member who delivered the massage training, or by another member of staff on the delegation log.
Participants randomised to the control group were informed that they would be given access to the massage training materials at the end of their follow-up (week 24). In addition, some of the sites offered to hold training sessions with the control group participants after they had completed the study.
A description of how to perform the massage technique can be found in Appendix 1, along with the training material given to participants as an aide memoire.
Mechanistic evaluation
One of the sites in the AMBER trial [University College Hospital (UCH), London] is a regional NBD centre where standard anorectal physiology and colonic transit tests are routinely undertaken. AMBER trial participants recruited at this site underwent the following tests before the intervention, and then again at 24 weeks:
-
Anorectal pressure test – this test involves the insertion of a small probe into the rectum to measure the strength of the anal muscles.
-
Anal and rectal sensation and capacity was measured by using a tiny amount of current and inflating a small balloon within the rectum (a balloon was inserted via a tube, and sensory thresholds to progressive distension were established. The initial tube was then removed and a different catheter with a bipolar electrode inserted; the sensory threshold to 1 mA of current was then determined).
-
Transit tests – three sets of radiopaque capsules were posted to the participant, who ingested them in the order specified in the instructions on 3 consecutive days. Participants then attended for an abdominal radiography 2 days after the last capsule to determine total colonic transit time (not segmental transit).
This was a small substudy in the AMBER trial to look at possible mechanisms involved in NBD in PwMS and to look at the feasibility of undertaking such tests within this population and their compliance with attending the repeat tests.
Data collection and management
Data were collected and recorded on study-specific paper-based case report forms (CRFs) by either site staff or the participants (bowel diaries and patient-reported outcomes during weeks 1–6 and week 24). Sites were trained on completion of all the paperwork before recruitment commenced and a monthly teleconference, with all sites jointly, allowed any data issues/inconsistencies to be discussed and resolved. The AMBER trial central office entered all data into the OpenClinica database (OpenClinica, LLC, Waltham, MA, USA). This was set up and managed by Tayside CTU. A range of data validation checks was used to minimise erroneous and missing data.
Baseline assessment
Demographic data and information on participants’ MS, medical history and bowel symptoms were collected at the baseline assessment. Participants also completed a questionnaire booklet which contained five different questionnaires (including the primary and secondary outcome measures – see Outcome measures and Appendices 2 and 3). Information on current medication was recorded, including any laxative use. Participants were given the bowel diaries and questionnaires, which were to be completed during the 6-week intervention phase, at the baseline assessment, with an instruction sheet detailing how they should be completed. Baseline anorectal physiology and colonic transit time data were collected from the London participants using an anorectal physiology CRF.
Baseline assessments were conducted between 22 January 2015 and 19 July 2016.
Participant follow-up
The duration of follow-up was 24 weeks from the date of randomisation.
Outcomes were collected through the following documents, which were completed by participants:
-
a questionnaire booklet at weeks 6 and 24 (the same as the baseline questionnaire)
-
a 7-day bowel diary (control group) or bowel and massage diary (intervention group) at weeks 1 to 6 and during week 23
-
patient resource-use questionnaires at weeks 1–6, and weeks 12, 18 and 24.
All information completed by the participants was returned to the AMBER trial central office by reply-paid envelopes that were provided.
Anorectal physiology and colonic transit time data were collected at week 24 in UCH participants only.
Site research staff telephoned all participants weekly during weeks 1 to 6 and again at week 24 to collect additional information on any potential issues, changes in diet/exercise/fluid, adverse events (AEs) and any changes in medication. Any potential issues with bowel management or the massage were discussed and fed back to the AMBER trial central office if deemed necessary.
All AMBER trial follow-ups were completed by 19 January 2017.
Table 1 shows the AMBER trial matrix and data collected at each time point.
| Item | Time point | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screen | Week –1 | Baseline appointment | Telephone call | Post | Telephone call and post | Withdrawal of data collection | |||||||
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 12 | Week 18 | Week 24 | |||||
| Informed consent | 7 | ||||||||||||
| Inclusion/exclusion | 7 | ||||||||||||
| Medical history | 7 | ||||||||||||
| Current medications | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||||
| Randomisation | 7 | ||||||||||||
| 7-day bowel diary | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | |||
| Process evaluation/interviewsa | 7 | 7 | |||||||||||
| 7-day bowel and massage diarya | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||||
| Trial questionnairesb | 7 | 7 | 7 | 7 | |||||||||
| Physiology formsc | 7 | 7 | Visit | 7 | |||||||||
| Patient resource questionnaire | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||||
| AEs | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | |||||
Outcome measures
Primary outcome: Neurogenic Bowel Dysfunction Score
The Neurogenic Bowel Dysfunction Score (NBDS)31 is a 10-item questionnaire covering frequency of bowel movements (0–6 points); headache, perspiration or discomfort during defaecation (0–2 points); medication for constipation or faecal incontinence (0–4 points each); time spent on defaecation (0–7 points); frequency of digital stimulation or evacuation (0–6 points); frequency of faecal incontinence (0–13 points); flatus (0–2 points); and perianal skin problems (0–3 points). The maximum score is 47 points; the higher the score, the more severe the symptoms, with a score of ≥ 14 points rated as severe. In the AMBER trial, the primary outcome measure was the change in the NBDS from baseline to week 24.
Secondary outcomes
Bowel symptoms
The Constipation Scoring System (CSS)32 was completed at baseline and at weeks 6 and 24 to assess constipation symptoms. The CSS is an eight-item questionnaire with items on frequency of bowel movement, difficulty with evacuation, feeling of incomplete evacuation, pain, length of time for evacuation, assistance with evacuation, number of failed attempts and the duration of constipation. The maximum score is 30 points, with higher scores indicating greater severity. A 7-day bowel diary (designed for use in the AMBER trial) was used to record information on bowel symptoms, such as frequency of bowel movement, time spent defaecating, stool type (Bristol stool chart33), laxative use, additional interventions (such as digital stimulation) and if there were any episodes of bowel incontinence. The diary was completed prior to baseline, during weeks 1–6 and at week 23. In the intervention group, a 7-day massage diary was used to record daily information on massage compliance and duration and was completed prior to baseline, during weeks 1–6 and at week 23.
Bladder dysfunction
Bladder function was measured using the SF-Qualiveen, consisting of an eight-item questionnaire assessing bladder dysfunction, such as leakage and signs of incomplete voiding. 34 Often, if patients with MS are suffering from constipation, they report that their bladder symptoms are worse, especially urgency and frequency, which can lead to an increase in urinary incontinence. This outcome measure allowed the effect of the change in bowel function on the bladder to be assessed at baseline and at weeks 6 and 24. A higher score indicates a poorer quality of life.
Quality-of-life outcomes
To determine health status, the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), generic questionnaire was used. 35 Participants completed the EQ-5D 5L at baseline and at weeks 6 and 24.
A neurogenic bowel impact score (NBIS) questionnaire was completed at baseline and at weeks 6 and 24. This score was developed by one of the collaborators on the AMBER trial as part of a National Institute for Health Research (NIHR)-funded postdoctoral fellowship. The questionnaire has three subscores [(1) quality of life, (2) faecal incontinence and (3) symptoms] and includes four stand-alone items. It is intended for use with individuals with a range of conditions that result in NBD. The measure’s reliability and criterion validity were to be evaluated.
Economic outcomes
The cost and use of NHS services were collected via a patient resource questionnaire [see project web page URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1212712/#/ (accessed 30 October 2017)] during weeks 1–6 and at weeks 12, 18 and 24. From this information (along with the EQ-5D-5L data) the costs and quality-adjusted life-years (QALYs) were calculated for each group. A cost–utility analysis was conducted to calculate the incremental cost per QALY of abdominal massage compared with optimised bowel care; this is described in detail in Chapter 4.
Change of medication
Changes of medication were recorded using a current medication form. Any changes to a participant’s medications during their involvement in the trial were recorded. Also recorded were any reductions or stoppage of laxatives between baseline and week 24.
Radiopaque marker transit tests
Different parameters were collected on an anorectal CRF (see Report Supplementary Material 1) for the physiology and transit tests. The total number of markers remaining in the gut at abdominal radiography was analysed to determine any differences between baseline and week 24, and all other data were summarised.
Adverse events
Expected AEs arising from the treatments in the AMBER trial are noted below. These are common in individuals with constipation and thus were not collected as AEs but noted in the weekly follow-up data collection:
-
increased flatulence
-
abdominal cramps
-
stomach rumblings/noises
-
loose stool, which in some instances may lead to faecal incontinence.
All AEs (for which a participant sought intervention from a HCP) and SAEs (including death, life-threatening conditions, inpatient hospitalisation or prolongation of existing hospitalisation and persistent or significant disability or incapacity) were assessed for causality, severity and expectedness, and were reported to the relevant regulatory bodies. If a site was in doubt about whether or not an event was an AE, this was reported and discussed before data lock. Any AE that was deemed as ongoing at the end of the trial was reviewed and further clarification from the site was sought. If the AE was still ongoing after this (owing to the nature of the event, but not related to the intervention), this was when the follow-up ended.
Adverse events (including SAEs) were coded with the Medical Dictionary for Regulatory Activities (MedDRA)36 16.1 and reported by primary System Organ Class (SOC) and preferred term (PT). Participants were counted only once when calculating the incidence of AEs. An overview table was created counting the number of AEs by SOC and PT.
Sample size
The sample size for the RCT was based on the NBDS, using data from a pilot study37 that provided the only published data available on abdominal massage in this group of patients. That study37 found a difference of 4.21 points in the NBDS between those receiving the intervention {mean score of 6.86 points [standard deviation (SD) 3.8 points] at 8 weeks} and the comparison group [mean score of 11.07 points (SD 7.02 points) at 8 weeks]. Other outcomes in that study changed in favour of the intervention and participants anecdotally reported that the massage was relaxing and that they were keen to do it themselves. Using these data, we selected a minimum clinically important difference (MCID) of 4.21 points and selected the higher SD of 7.02 points, found in the comparison group, as the basis for our sample size calculation.
Using these data, 60 participants per group was calculated as the necessary number of participants to detect a difference between groups of 4.21 points (SD 7.02 points) at a 5% level of significance with 90% power. Thus, for a fully powered study, the total sample size, allowing for a 20% dropout rate, was 150. However, in response to suggestions from the funding body, the sample size was increased to 200 participants (100 per group), which allowed for greater attrition.
Statistical analyses
Statistical methods for analysis of the main primary and secondary outcomes are detailed in the following sections. This document was drawn up by the trial statisticians, reviewed by the Project Management Group and formally signed off by the chief investigator and trial statistician before analysis commenced.
Analysis populations
Analysis was performed for the intention-to-treat population and is reported in accordance with Consolidated Standards of Reporting Trials (CONSORT). 38
Subgroups
Subgroup analyses were carried out by first testing for a subgroup factor by intervention interaction. If this was significant at the 5% level, results were estimated separately by the different subgroups. This included a secondary analysis comparing those who undertook the massage themselves with those who had a carer massage them.
Missing data
The extent of missing data was explored in the outcomes, especially the primary outcome. Patterns of missing data were explored and predictors of missingness examined, especially if these varied by intervention. A table was constructed to assess differences in characteristics of those with complete data and those with missing data for the primary analysis. Multiple imputation (MI) was implemented for the primary outcome, assuming data were missing at random.
Summary of trial data
All continuous variables were summarised using the following descriptive statistics: non-missing sample size, number of missing records, mean, SD, median, maximum and minimum. The frequency and percentages (based on the non-missing sample size) of observed levels were reported for all categorical measures. In general, all data were listed, sorted by subject and treatment and, when appropriate, by visit number within subject.
All summary tables were structured with a column for each treatment group and an additional column for the total population relevant to that table/treatment, including any missing observations.
Demographic and baseline variables
The baseline characteristics of participants that were recorded comprised age, sex, body mass index (BMI), type of MS, site, number of years since diagnosis, cognitive symptoms of MS and level of mobility (walking unaided, aided or wheelchair bound).
Prior and current medications
Prior medications were all medications that were being taken by a participant before the trial started. Concomitant medications were all medications commenced during the trial and all changes to the dosing of prior medications. Prior medications were listed but not analysed. Concomitant medications were analysed by number of medications taken.
Treatment adherence
Treatment adherence was calculated from the weekly bowel diaries. The number of times the bowel massage was done per week was used in the main analysis. For the control group, this number was set to 0.
Efficacy analyses
Data for continuous outcome measures (both primary and secondary) were assessed for normality before analysis. Transformations of the outcome variables were used when necessary, if these were not normally distributed.
If data were normally distributed, outcome measures were assessed by multiple linear mixed-model regression. The primary analysis consisted of comparisons between treatment groups (bowel massage vs. no massage) at the final visit (week 24), adjusted for site, minimisation variable level of mobility (walking unaided, aided or wheelchair bound), as well as baseline measure of the outcome and sex.
In a secondary analysis of the primary outcome, additional baseline variables (age, sex, BMI, type of MS, number of years since diagnosis and cognitive symptoms of MS) were included in the model.
When data were not normally distributed and could not be transformed into a normal distribution, they were analysed using non-parametric methods in addition to multiple linear regression.
In addition to the comparison of baseline with week 24, a repeated measures mixed-model analysis was performed on the outcomes using all available visits.
Data for categorical outcome measures were assessed by logistic regression in the same way as described for continuous outcome measures.
Primary efficacy analysis
The primary outcome measure was the between-group difference in the change of NBDS at week 24 with the analysis adjusted, as described above.
Secondary efficacy analyses
Bowel outcomes
-
Between-group difference in change in constipation symptoms. Analysis variable is the total constipation score.
-
Bowel symptoms (7-day bowel diary). The percentage of normal stools per week was calculated and used for analysis. In addition, the number of days that a stool was passed and time spent passing stools was analysed.
-
Radiopaque marker transit tests. The number of total markers was used for the analysis.
-
Adherence to massage schedule (massage diary). As this is only available for the intervention treatment group, data are summarised in the descriptive statistics. No formal testing was done.
Urinary outcomes
-
Between-group difference in change in total score of bladder function (SF-Qualiveen).
Quality-of-life outcomes
-
Between-group difference in change in health-related quality of life, measured by the EQ- 5D-5L using both the EuroQol Visual Analogue Scale (EQ-VAS) and the index score.
-
Between-group difference in change of patient-reported quality of life. This consists of four scores derived from the NBD patient-reported outcomes tool.
-
Between-group difference in change in medication, analysed as all patients who stopped using laxatives at week 24. This was determined from the concomitant medication page.
-
Between-group difference in change in medication, analysed as the number of changes in usual laxative use at week 24. This was taken from the bowel diary as the number of changes from usual laxative use to use of fewer laxatives at week 24.
-
Between-group changes in the regular use of medications to counter constipation were assessed at weeks 6 and 24.
Reporting conventions
Values of ≥ 0.001 are reported to three decimal places; p-values of < 0.001 are reported as < 0.001. The mean, SD and any other statistics, other than quantiles, are reported to one decimal place greater than the original data. Quantiles, such as median or minimum and maximum, use the same number of decimal places as the original data. Estimated parameters not on the same scale as raw observations (e.g. regression coefficients) are reported to three significant figures.
All analyses were performed using SAS® 9.3 (SAS Institute Inc., Cary, NC, USA. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.) All data, analysis programs and output were kept on the Mackenzie Server and backed up according to the internal Tayside Clinical Trials Unit (TCTU) information technology standard operating procedures.
Analysis programs were required to run without errors or warnings. The analysis programs for outcomes were reviewed by a second statistician and any irregularities in the programs were investigated and fixed, and the date of finalised analysis programs was signed and recorded.
Economic analysis
The cost of abdominal massage and optimised bowel care relative to optimised bowel care alone in PwMS who have NBD was considered from NHS and patient perspectives. Health-care resource use by patients in both trial groups was collected at each of the follow-up time periods (weeks 1–6, 12, 18 and 24). This included contact with health professionals and medications prescribed. These were costed using NHS pay and prices or, when appropriate, other (e.g. market-based) sources. The economic analysis (including the methods used) is detailed in Chapter 4.
Important changes to protocol after trial commencement
All sites used protocol version 2, dated 18 November 2014, throughout the trial duration. The London site used an additional patient information leaflet (PIL) to describe the additional tests carried out at this site. After original approval of the PIL, one of the tests was no longer completed routinely at the London site, so this information was removed and the amended PIL was approved by all relevant regulatory bodies. This change was carried out before any patients were recruited at the site.
Another substantial amendment was to incorporate a substudy, entitled SWAT (study within a trial) 24, in the AMBER trial. Participants were randomised to receive either the original cover letter or an enhanced cover letter (sent with the questionnaire at week 24) to evaluate whether or not the wording used would increase return rates of the questionnaires.
Data from the substudy will contribute to the Trial Forge39 initiative to improve trial efficiency and to the Cochrane review of strategies to improve trial retention. 40
The results of the Trial Forge39 project will help to increase the evidence base on the retention of participants to trials. The only change to the AMBER trial was the way that the cover letter sent with questionnaires was written (no protocol change).
Other non-substantial changes included the:
-
addition of new sites as the trial progressed (original target was 10 sites but final total was 12 sites)
-
set-up of two patient-identifying centres to assist two sites with recruitment
-
sites collecting the NBDS during the telephone call with participants at week 24 to maximise the primary outcome data in the study.
Trial oversight
The trial was led by the chief investigator who, along with the trial management team members (consisting of a trial manager, a data co-ordinator and a process evaluation researcher), were employed by NMAHP RU.
The trial was overseen by a Project Management Group (PMG), a Trial Steering Committee (TSC) and a Data Monitoring and Ethics Committee (DMEC).
The PMG had a teleconference approximately every 4–6 weeks during the recruitment period and then bimonthly after this. The group’s role was to support any decision-making that the trial management team needed further advice on.
The TSC had both an independent chairperson and members but also consisted of the trial collaborators. The TSC had four meetings over the course of the trial, with additional updates on recruitment when requested. The TSC commended the team on recruiting to target and on time.
An independent DMEC, chaired by a statistician, had three meetings over the course of the study, and additional updates were provided when requested. All statistical reports to the DMEC were prepared by a statistician from the TCTU. The DMEC had no issues with the trial continuing at any time point and commended the team on the recruitment and management of the study. The DMEC charter can be reviewed in Appendix 2.
Patient and public involvement
The AMBER trial has had active participation with a group of PwMS (hereafter referred to as the MS focus group). Some of the MS focus group were involved with the development of the grant application, providing feedback on the lay summary, trial design and appropriate outcome measures and questionnaires. Several additional PwMS became involved during the very early stages of the trial and throughout implementation and dissemination of results. The MS focus group included males and females, with various levels of disability and of various ages, some with and some without NBD. Approximately 10 members of the MS focus group attended each of the meetings. Material to review was sent electronically before the meetings and was available in hard copy at the meetings; very helpful feedback and discussions took place. One of the members of the MS focus group has also attended each TSC meeting as a lay representative and has actively engaged in the conversations and discussions at each meeting. Before recruiting any participants to the trial, the MS focus group reviewed the massage training DVD and the massage training material that would be given to the participant to take home with them, and their input to this was extremely influential. The group’s opinion of the initial version of the training DVD was that the visual was excellent but the language used was ‘too clinical’. This was overcome by the chief investigator of the trial doing a voice-over on the DVD; the group reviewed this again and it was deemed much more acceptable and user friendly. Many of the trial participants subsequently commented that they thought the video was extremely useful and easy to follow.
We initially had two different training documents and we asked the group which would be better used as an aide memoire. The MS focus group had very mixed opinions on their preferences, and discussed the style, language and diagrams used. It was therefore concluded that both of these additional training materials would be provided to all participants in the intervention arm and they could decide which material they felt was better for them. However, there is the possibility of combining the information into one single training document if the intervention is rolled out to clinical care.
Participants in the AMBER trial were given quite a lot of information to take away with them at the baseline appointment, in the form of a ‘follow-up pack’. This pack included all the questionnaires and bowel diaries and study instructions on what they had to complete over the 6-week intervention period, and also the massage training DVD and reading materials (only if the participant was in the intervention group). The MS focus group reviewed this pack and thought that it was logical and clear and some further feedback from site staff implied that the participants ‘liked’ having this pack to take away with them.
We kept in touch with the MS focus group throughout the study, giving updates on recruitment, and there were discussions on dissemination plans. A dissemination day was held on 23 January 2018 in Glasgow Caledonian University, to which all the research staff involved in the study, and local participants, were invited. Representatives of the MS focus group also attended.
Throughout the active recruitment of the study, local and national MS charities were aware of our research and promoted the study where regulations allowed.
Chapter 3 Results
Trial recruitment
Recruitment overall was considered very successful; Figure 2 shows how well the actual recruitment met the expected monthly targets over the 18 months of active recruitment. The trial oversight committees agreed to stop recruitment on time at 191 participants after reviewing attrition information. Twelve sites recruited participants from 22 January 2015 to 19 July 2016 and each site recruited between 9 and 26 participants (see Appendix 3).
FIGURE 2.
Predicted recruitment vs. actual recruitment.
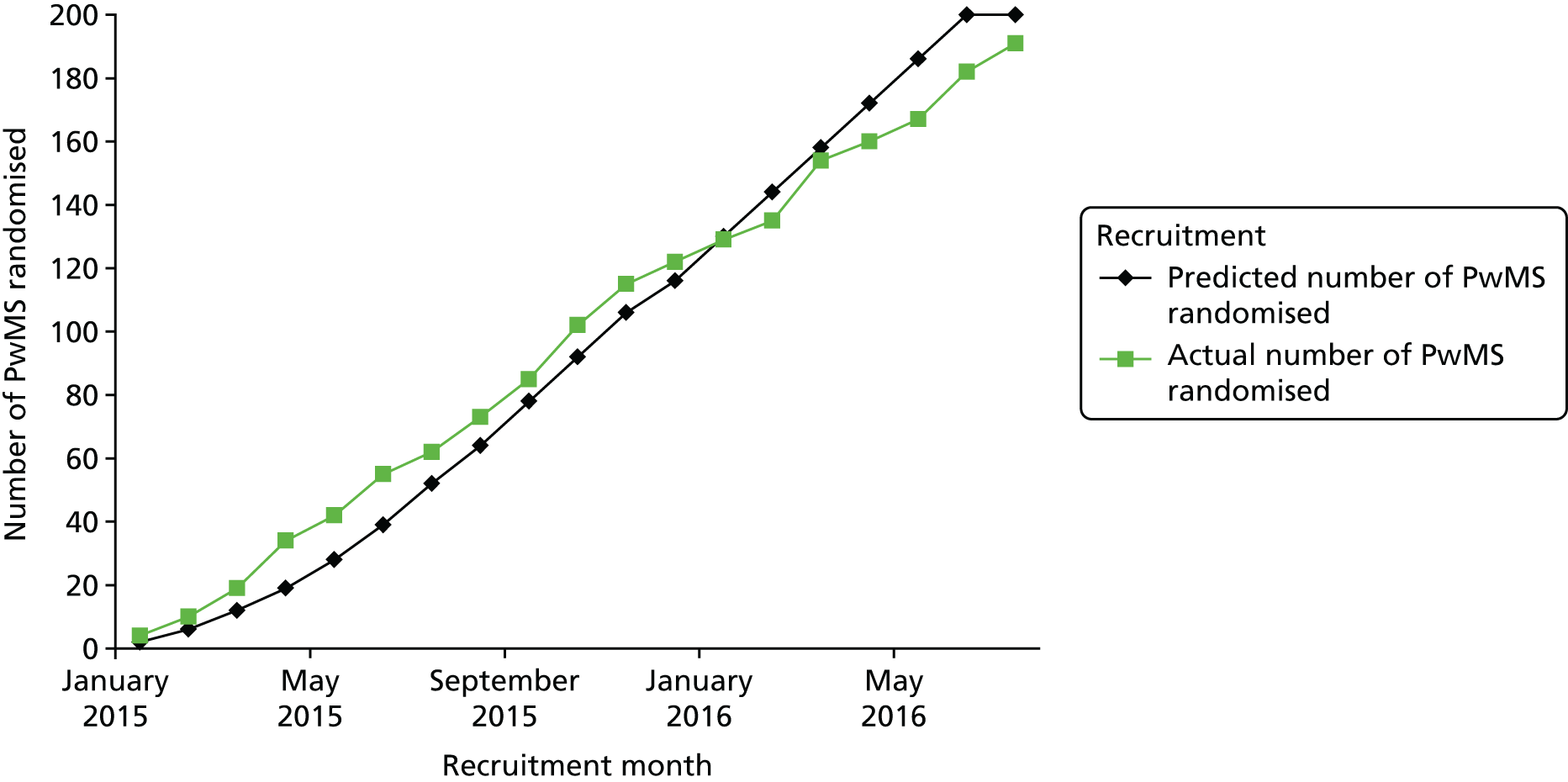
The CONSORT flow diagram (Figure 3) shows the movement of participants through the AMBER trial. There were 237 PwMS and possible bowel problems screened (≈61% of the 389 PwMS approached by the research staff) and 191 PwMS (81% of those screened; 49% of those approached) were randomised. The near 50% uptake on those approached versus those randomised was in accordance with the estimate of uptake stated in the protocol.
FIGURE 3.
The CONSORT flow diagram. Qu, questionnaire. a, In the enrolment and screening information, all percentages are calculated from the number of people approached about the study (n = 389); b, reasons for those who were eligible but did not take part vary from a patient’s personal circumstances changing to baseline appointments being made for a patient but he/she not attending; c, in each group, one participant was randomised and subsequently deemed ineligible for the trial when eligibility was reassessed. No data were collected for these two participants; and d, these sections show the breakdown for the primary outcome measure (NBDS) at each stage of data collection: baseline, week 6 and week 24. All percentages calculated are from n = 90 (intervention group) and n = 99 (control group).
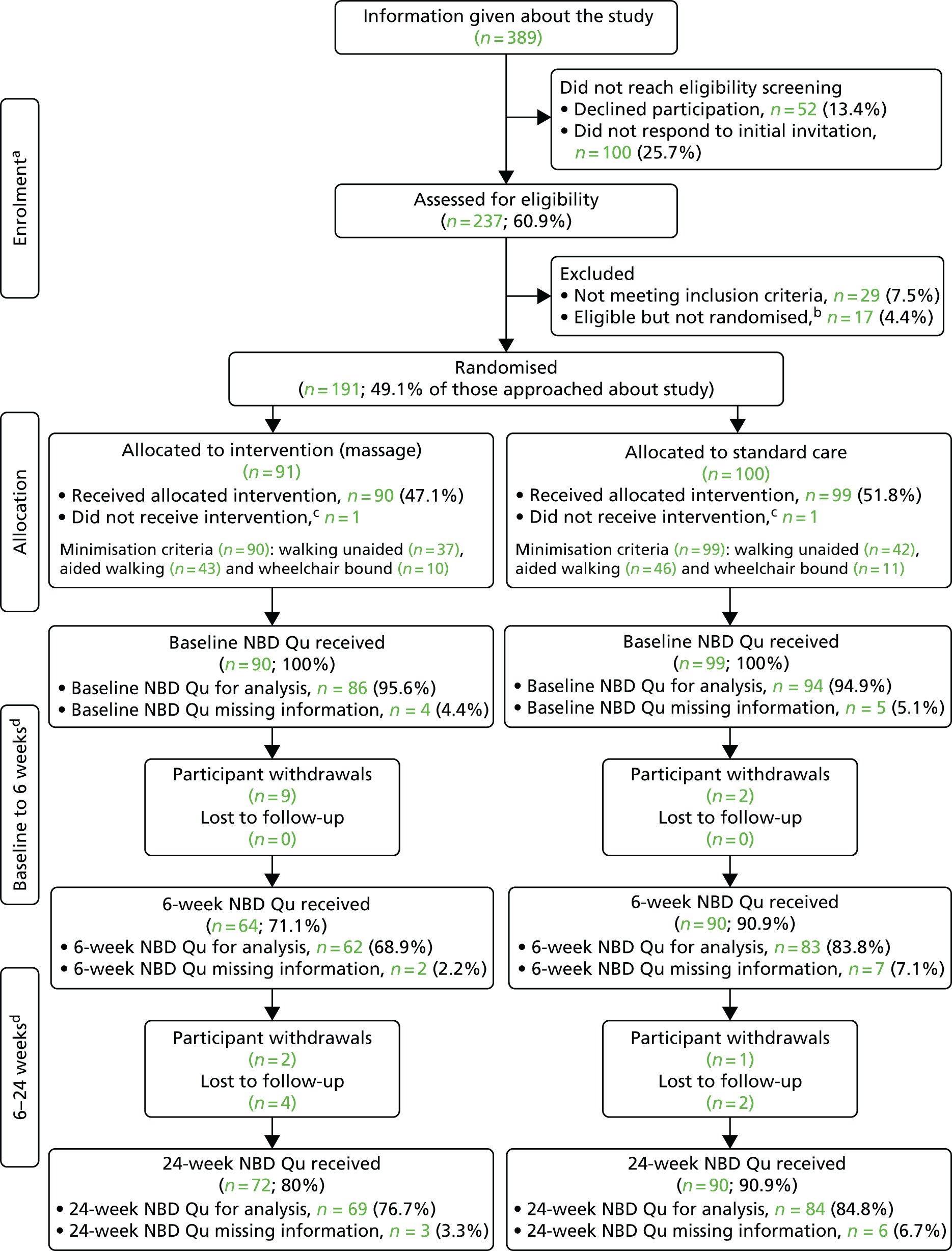
Of the randomised participants, 22 did not complete the study. Two of these were post-randomisation exclusions (essentially randomised in error) and data were not collected from these two participants. Thus, the analysis was based on 189 participants: 90 in the intervention group (abdominal massage plus advice on optimised bowel care) and 99 in the control group (only advice on optimised bowel care). The inequality in the number of participants per group was attributable to minimisation at site level.
For the 20 correctly randomised participants (intervention group, n = 15; control group, n = 5) who did not complete the study, baseline data were successfully collected and all participants agreed that their existing data could be used. Participants either withdrew (intervention group, n = 11; control group, n = 3) or were lost to follow-up (intervention group, n = 4; control group, n = 2). In some instances, if the data were not returned, then the week-6 and week-24 follow-up data collection was completed by the researcher during a telephone call. This explains the differing numbers for the NBDS in the CONSORT flow diagram at weeks 6 and 24 in relation to reported withdrawals or loss to follow-up.
Quality of participant-completed outcome data
Participant-completed outcomes were returned by post at weeks 6 and 24. Questionnaire and bowel diary data were checked for completeness and every effort was made to collect any missing information when acceptable to do so.
The CONSORT flow diagram (see Figure 3) shows the numbers available for the primary outcome analysis at baseline and weeks 6 and 24 (NBDS). During monitoring of the study attrition rates and primary and secondary outcome data received, it was evident that some participants were stating that they had returned their outcome measures by post but these were not received in the AMBER trial office. Thus, in order to maximise our available primary outcome data for analysis, from 22 March 2016 onwards, the research staff at each site completed the NBDS (10 questions) during the telephone call at week 24. This increased our available data for primary outcome analyses at 24 weeks, compared with 6 weeks, in both study groups (intervention group, 76.7%; control group, 84.8%).
There was a greater number of withdrawals/losses to follow-up in the intervention group (15/90) than in the control group (5/99).
For all outcome data, the numbers available for analysis and any reasons for missing data will be reported when discussing each outcome below.
Reasons for withdrawal/losses to follow-up
There were two randomisation failures and 20 participants who withdrew and were lost to follow-up (n = 14 intervention group, n = 16 control group; none withdrew consent for use of existing data). We have undertaken an analysis of the missing data (see Appendix 4) and it would seem that they do not suggest any major biases in the primary analysis. The reasons for withdrawal in the intervention group were varied and included change in diagnosis of MS, family circumstances, worsening of condition and too much paperwork. There is also the possibility that those in the control group remained in the study so that they would receive the training in the abdominal massage and had not yet experienced the potential disappointment of the intervention not working for them, which might increase the likelihood of withdrawing from the study. Interestingly, of those who took part in the interview study, none withdrew or were lost to follow-up, which may indicate that this was a more motivated group or that taking part in the interviews facilitated retention.
Missing primary outcome data
The missingness of the primary outcome data appeared to be relatively unrelated to baseline characteristics, apart from the following: trial group (more missing data in the intervention group), more missing data in those not wheelchair bound, slightly more missing data for women and for younger participants, and more missing data in some centres (see Appendix 4 and Chapter 6 for possible reasons for all of the above). However, using the characteristics at baseline to impute missing data, MI was carried out for the primary outcome and the primary analysis was repeated as a sensitivity analysis. This approach assumes that data are missing at random; this is discussed further in Chapter 6.
Baseline data
The mean age of participants was 53 years (SD 10.4 years) and 81% (154/189) were female. Mean time since diagnosis of MS was 14.3 years (SD 9.1 years). Baseline demographics and clinical data are summarised in Table 2. Demographics and symptom characteristics of the two groups were comparable at baseline.
| Characteristic | Trial group | |
|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |
| Clinical characteristics | ||
| Minimisation variable: walking aids, n (%) | ||
| Walking unaided | 37 (41.1) | 42 (42.4) |
| Aided walking | 43 (47.8) | 46 (46.5) |
| Wheelchair bound | 10 (11.1) | 11 (11.1) |
| Age (years), mean (SD) | 53.5 (11.32) | 51.3 (10.32) |
| Sex, n (%) | ||
| Male | 14 (15.6) | 21 (21.2) |
| Female | 76 (84.4) | 78 (78.8) |
| BMI (kg/m2), mean (SD) | 27.4 (6.207) | 26.22 (5.525) |
| Time since diagnosis of MS (years), mean (SD) | 14.8 (9.76) | 13.9 (8.64) |
| Type of MS, n (%) | ||
| Benign | 0 (0.0) | 2 (2.0) |
| Relapsing–remitting | 45 (50.0) | 61 (61.6) |
| Secondary progressive | 36 (40.0) | 23 (23.2) |
| Primary progressive | 9 (10.0) | 13 (13.1) |
| Severity of symptoms, n (%) | ||
| Cognitive | ||
| None | 39 (43.3) | 35 (35.4) |
| Moderate | 50 (55.5) | 61 (62.6) |
| Severe | 1 (1.1) | 3 (3.0) |
| Pain | ||
| None | 41 (46.6) | 46 (46.5) |
| Moderate | 43 (47.8) | 52 (52.5) |
| Severe | 6 (7.6) | 1 (1.0) |
| Spasm | ||
| None | 33 (36.7) | 31 (31.3) |
| Moderate | 58 (64.4) | 63 (64.7) |
| Severe | 17 (12.2) | 11 (11.1) |
| Depression | ||
| None | 41 (45.6) | 52 (52.5) |
| Moderate | 45 (50.0) | 42 (43.5) |
| Severe | 4 (4.4) | 4 (4.0) |
| Fatigue | ||
| None | 8 (8.9) | 5 (5.1) |
| Moderate | 58 (64.5) | 68 (68.7) |
| Severe | 24 (26.7) | 26 (26.3) |
| Bladder | ||
| None | 12 (13.3) | 15 (15.2) |
| Moderate | 57 (63.4) | 59 (59.6) |
| Severe | 29 (32.2) | 29 (29.3) |
Bowel symptoms
To be eligible to participate in the trial, participants had to be ‘bothered’ by their constipation. Bowel symptoms had commenced > 10 years ago in 37% of participants and < 1 year ago in 4% of participants. The main bowel symptoms reported by participants at baseline were a feeling of incomplete emptying, straining to pass stool and bloating (Table 3).
| Bowel symptoms | Trial group | |
|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |
| Pain: yes, n (%) | 59 (65.6) | 60 (60.6) |
| Bloating: yes, n (%) | 76 (84.4) | 86 (86.9) |
| Faecal incontinence: yes, n (%) | 39 (43.0) | 60 (60.6) |
| Successful opening of bowels 2–4 times a week, n (%) | 59 (65.6) | 53 (53.5) |
| Type of stool (Bristol stool chart) over the last week (%) | ||
| Types 1 and 2 | 21.8 | 19.4 |
| Types 3 and 4 | 20.2 | 23.6 |
| Types 5, 6 and 7 | 16.1 | 14.6 |
| No stool | 39.8 | 38.8 |
| Missing | 2.3 | 2.4 |
| Constipated (no stool + types 1 and 2) | 61.6 | 58.2 |
| Straining to pass stool: yes, ≥ 25% of the time, n (%) | 80 (88.9) | 74 (74.8) |
| Digital stimulation: yes, ≥ 25% of the time, n (%) | 28 (31.1) | 29 (29.3) |
| Feeling of incomplete emptying: yes, ≥ 25% of the time, n (%) | 83 (92.8) | 94 (94.9) |
Primary analyses
The primary analysis is the comparison between treatment groups (bowel massage vs. no massage) at the final visit (24 weeks), adjusted for site and minimisation variable level of mobility (walking unaided, walking aided, or wheelchair bound), as well as baseline measure of the outcome and sex.
Primary outcome measure
Neurogenic Bowel Dysfunction Score
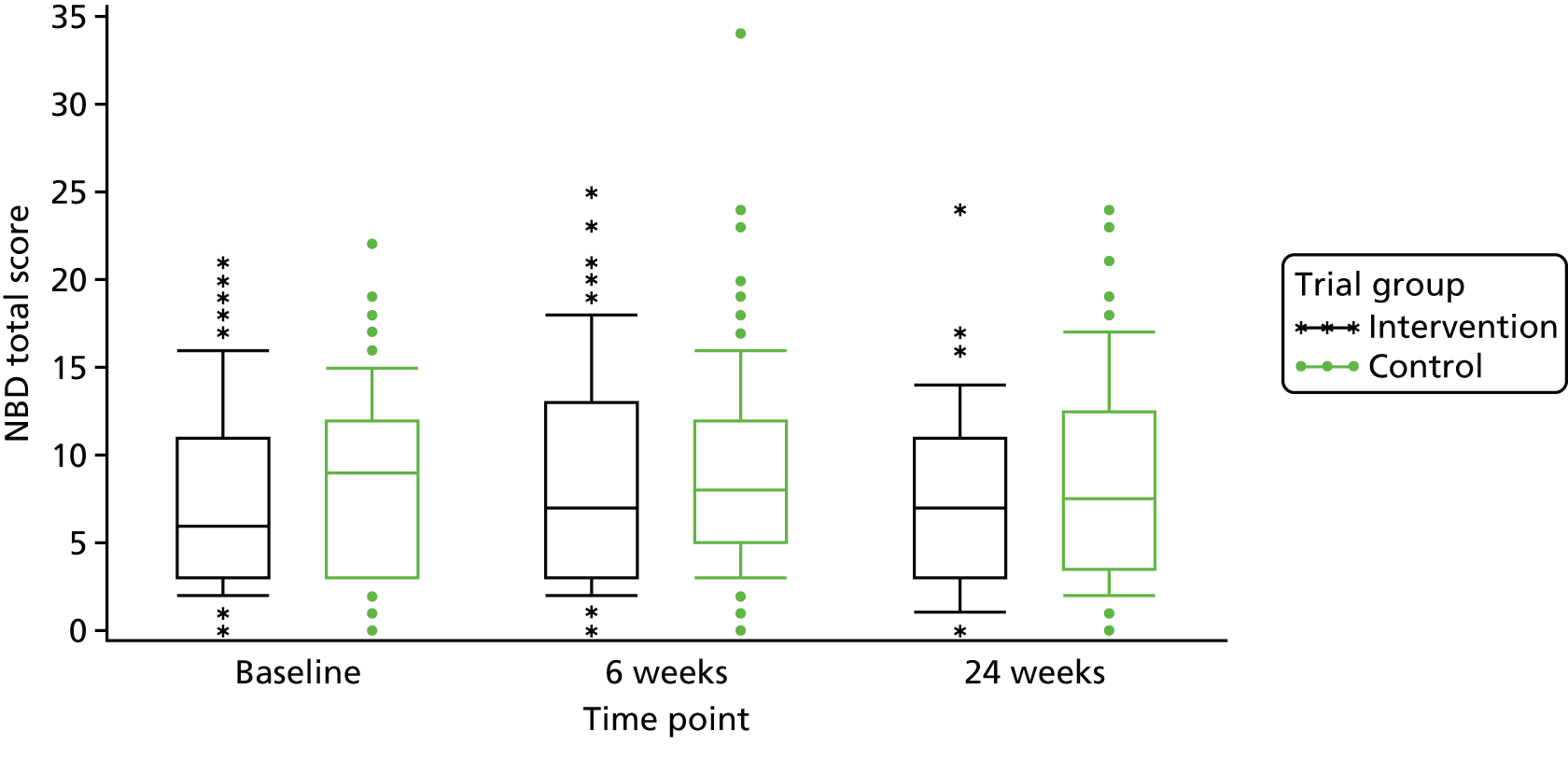
At baseline, the mean score for the intervention group was 7.6 points (SD 5.31 points) and the median was 6.0 points (range 0–21 points). For the control group at baseline, the mean NBDS was 8.6 points (SD 5.08 points) and the median was 9.0 points (range 0–22 points) (Table 4). These scores indicate that the NBD symptoms were having a minor impact on most participants. Scores of 7–11 points indicate minor impact and scores of ≥ 14 points indicate severe impact. The mean NBDS at week 24 was 7.4 points (SD 5.23 points) and the median was 7.0 points (range 0–24 points) for the intervention group; the mean NBDS was 8.7 points (SD 5.70 points) and the median was 7.5 points (range 0–24 points) for the control group at week 24. The mean adjusted difference in change between randomised groups from baseline to week 24 was not statistically significantly different [mean difference between groups (intervention – control): –1.6, 95% confidence interval (CI) –3.32 to 0.04; p = 0.0558; Table 5]. Figures 4 and 5 visually show the change over time within the two groups.
| Score | Trial group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | ||||||||
| Mean (SD) | n | Median (range) | Mean (SD) | n | Median (range) | ||||
| Primary outcome measure – symptom severity | |||||||||
| NBD score (points)a | |||||||||
| Baseline | 7.6 (5.3) | 86 | 6 (0–21) | 8.6 (5.1) | 94 | 9 (0–22) | |||
| Week 6 | 8.4 (6.2) | 62 | 7 (0–25) | 9.1 (5.7) | 83 | 8 (0–34) | |||
| Week 24 | 7.4 (5.2) | 69 | 7 (0–24) | 8.7 (5.7) | 84 | 7.5 (0–24) | |||
| Secondary outcome measure – symptom severity | |||||||||
| Constipation score (points)b | |||||||||
| Baseline | 11.7 (4.1) | 88 | 12 (1–25) | 11.5 (3.8) | 97 | 11 (3–21) | |||
| Week 6 | 10.6 (4.3) | 58 | 11 (1–22) | 10.8 (4.0) | 83 | 11 (1–22) | |||
| Week 24 | 10.1 (4.1) | 57 | 10 (2–22) | 11.1 (3.9) | 81 | 11 (3–27) | |||
| Bowel diary data | |||||||||
| Time spent on toilet (minutes per week) | |||||||||
| Baseline | 75.6 (69.6) | 80 | 57.5 (3–330) | 75.8 (74.4) | 87 | 55.0 (3–370) | |||
| Week 6 | 77.9 (73.3) | 66 | 55.0 (5–315) | 85.0 (88.5) | 85 | 50.0 (2–400) | |||
| Week 24 | 78.2 (92.4) | 53 | 45.0 (3–550) | 77.0 (68.5) | 78 | 56.5 (1–295) | |||
| Number of attempts per week to empty the bowels | |||||||||
| Baseline | 10.4 (6.7) | 86 | 9 (0–35) | 8.6 (5.2) | 91 | 8 (0–32) | |||
| Week 6 | 11.3 (7.1) | 65 | 9 (2–32) | 8.9 (6.0) | 88 | 8 (1–32) | |||
| Week 24 | 10.7 (7.2) | 53 | 10 (1–43) | 8.3 (5.1) | 77 | 7 (1–23) | |||
| Number of stools passed per week | |||||||||
| Baseline | 3.9 (1.7) | 88 | 4.0 (0–7) | 4.0 (1.7) | 98 | 4 (0–7) | |||
| Week 6 | 4.3 (1.9) | 68 | 4.5 (1–7) | 3.9 (1.8) | 89 | 3 (0–7) | |||
| Week 24 | 4.3 (1.9) | 57 | 4.0 (0–7) | 3.9 (1.9) | 81 | 4 (0–7) | |||
| Bladder symptom severity | |||||||||
| SF-Qualiveen total bladder scorec | |||||||||
| Baseline | 1.8 (1.10) | 90 | 1.8 (0–4) | 2.0 (1.20) | 99 | 1.8 (0–4) | |||
| Week 6 | 1.7 (1.13) | 61 | 1.6 (0–4) | 2.1 (1.15) | 85 | 2.1 (0–4) | |||
| Week 24 | 1.7 (1.10) | 57 | 1.8 (0–4) | 2.1 (1.12) | 81 | 2.0 (0–4) | |||
| QoL | |||||||||
| EQ-5D-5L VAS scored (maximum score of 100) | |||||||||
| Baseline | 60.6 (21.1) | 89 | 60 (3–100) | 55.7 (20.6) | 98 | 60 (3–100) | |||
| Week 6 | 59.4 (24.0) | 59 | 65 (5–97) | 55.4 (20.8) | 86 | 60 (5–100) | |||
| Week 24 | 59.8 (22.6) | 58 | 62.5 (10–95) | 51.3 (20.3) | 83 | 50 (10–90) | |||
| EQ-5D-5L health index scoree (maximum score of 1) | |||||||||
| Baseline | 0.50 (0.25) | 95 | 0.6 (–0–1) | 0.50 (0.28) | 99 | 0.6 (–0–1) | |||
| Week 6 | 0.50 (0.29) | 60 | 0.6 (–0–1) | 0.50 (0.27) | 84 | 0.5 (–0–1) | |||
| Week 24 | 0.50 (0.28) | 58 | 0.6 (–0–1) | 0.50 (0.28) | 83 | 0.5 (–0–1) | |||
| New QoL measure for validation (NBIS) | |||||||||
| NBISf (total score maximum of 52) | |||||||||
| Baseline | 20.2 (8.5) | 86 | 20 (6–41) | 20.8 (7.4) | 98 | 21 (5–38) | |||
| Week 6 | 19.9 (8.2) | 56 | 19 (5–42) | 21.4 (7.0) | 82 | 21 (3–42) | |||
| Week 24 | 19.0 (8.4) | 56 | 18 (5–50) | 20.9 (7.4) | 77 | 21 (1–40) | |||
| NBIS QoL (maximum score of 24) | |||||||||
| Baseline | 9.6 (5.1) | 88 | 9 (0–22) | 10.7 (4.7) | 99 | 11 (1–22) | |||
| Week 6 | 9.9 (4.9) | 56 | 10 (0–21) | 10.7 (4.3) | 83 | 11 (1–23) | |||
| Week 24 | 9.2 (4.8) | 58 | 8.5 (2–23) | 10.7 (4.7) | 80 | 11 (0–24) | |||
| NBIS faecal incontinence score (maximum score of 12) | |||||||||
| Baseline | 3.7 (2.4) | 90 | 3 (0–10) | 3.7 (2.0) | 99 | 3 (0–9) | |||
| Week 6 | 3.6 (2.3) | 61 | 3 (0–9) | 4.1 (2.1) | 87 | 4 (0–11) | |||
| Week 24 | 3.8 (2.5) | 57 | 4 (0–12) | 4.1 (1.8) | 82 | 4 (0–9) | |||
| NBIS symptom score (maximum score of 16) | |||||||||
| Baseline | 6.9 (2.9) | 88 | 7 (0–16) | 6.4 (3.1) | 98 | 6 (1–13) | |||
| Week 6 | 6.4 (3.2) | 60 | 6 (1–14) | 6.6 (2.5) | 84 | 6 (1–14) | |||
| Week 24 | 6.0 (2.8) | 57 | 6 (1–15) | 6.2 (2.8) | 80 | 6 (0–14) | |||
| Time point | Trial group | Mean difference in change between groups (intervention–control), mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change (95% CI) | n | Mean change (95% CI) | Adjusteda (95% CI) | p-value | |
| Baseline to week 6 | 61 | 0.6 (–0.73 to 1.98) | 80 | 0.9 (–0.5 to 2.22) | –0.58 (–2.38 to 1.22) | 0.5236 |
| Baseline to week 24 | 66 | –0.6 (–2.11 to 0.93) | 80 | 0.5 (–0.78 to 1.83) | –1.64 (–3.32 to 0.04) | 0.0558 |
FIGURE 4.
Change in NBDS over time.
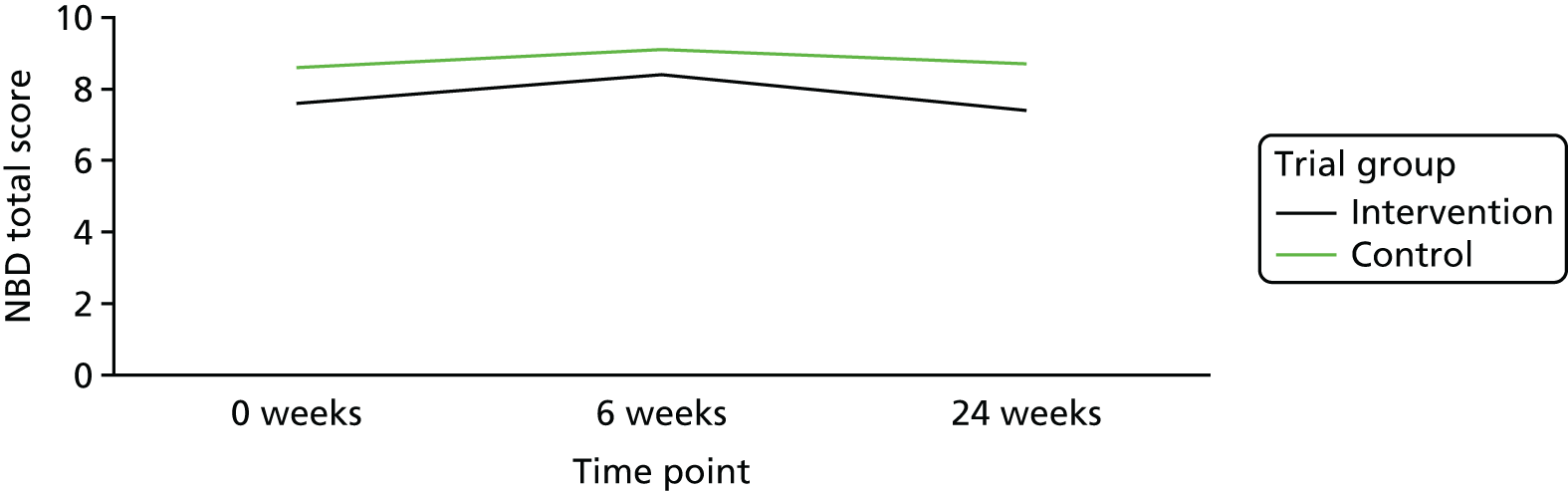
FIGURE 5.
Box-and-whisker plot for NBDS.
Secondary outcomes
Constipation Scoring System
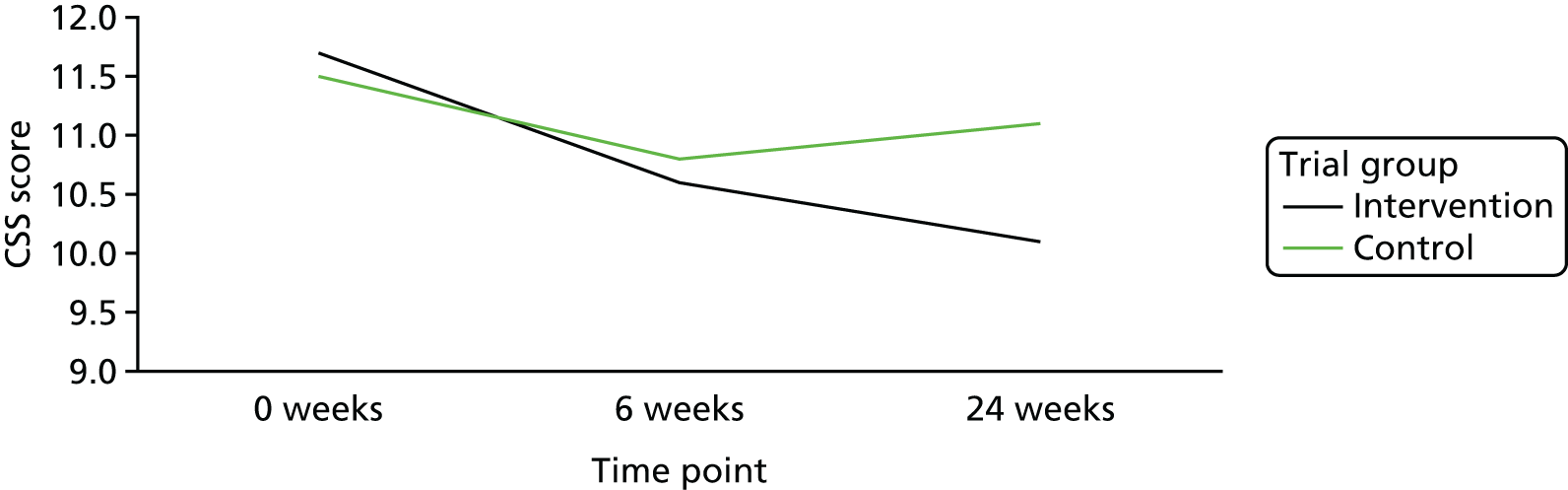
At baseline, the intervention group had a mean CSS score of 11.7 points (SD 4.05 points), and the control group had a mean CSS score of 11.5 points (SD 3.77 points). At week 24, the intervention group had a mean CSS score of 10.1 points (SD 4.10 points), and the control group had a mean CSS score of 11.1 points (SD 3.91 points). Figure 6 is a line graph showing the change over time in the two groups. There was no significant mean difference between the groups in change in the total CSS score between baseline and either time point (Table 6).
FIGURE 6.
Change in total CSS score over time.
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change (95% CI) | n | Mean change (95% CI) | Adjusteda (95% CI) | p-value | |
| Baseline to week 6 | 57 | –1.2 (–2.11 to –0.33) | 82 | –0.3 (–1.05 to 0.48) | –0.89 (–2.03 to 0.26) | 0.1273 |
| Baseline to week 24 | 56 | –1.1 (–2.15 to –0.1) | 81 | –0.3 (–1.08 to 0.45) | –0.88 (–2.03 to 0.27) | 0.1308 |
Short Form Qualiveen Bladder Questionnaire
At baseline, both groups demonstrated moderate effects of bladder dysfunction on their overall quality of life with a total SF-Qualiveen score in the intervention group of 1.8 points (SD 1.10 points) and the control group of 2.0 points (SD 1.20 points). The results in all four domains of the SF-Qualiveen, that is (1) bother with limitations, (2) frequency of limitations, (3) fears and (4) feelings related to urinary problems, were also similar in both groups There were no significant differences between groups in the change from baseline to weeks 6 or 24 in the SF-Qualiveen score (Table 7).
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change (95% CI) | n | Mean change (95% CI) | Adjusteda (95% CI) | p-value | |
| Baseline to week 6 | 61 | 0.8 (–0.74 to 2.38) | 85 | 1.4 (0.26 to 2.48) | –1.09 (–2.89 to 0.70) | 0.2306 |
| Baseline to week 24 | 57 | 0.6 (–1.2 to 2.43) | 81 | 0.5 (–0.89 to 1.96) | –0.58 (–2.74 to 1.58) | 0.5968 |
EuroQol-5 Dimensions, five-level version: quality-of-life outcomes
All the results of the EQ-5D-5L are summarised in Chapter 4.
Neurogenic bowel dysfunction patient-reported outcome measure
There were no significant changes in any of the outcomes from the NBIS. All results are summarised in Table 8.
| NBIS | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change (95% CI) | n | Mean change (95% CI) | Adjusteda (95% CI) | p-value | |
| Total score | ||||||
| Week 6 | 53 | 0.3 (–1.34 to 1.9) | 82 | 1.0 (–0.8 to 2.01) | –1.04 (–2.72 to 0.64) | 0.2216 |
| Week 24 | 53 | –0.8 (–2.62 to 1.04) | 77 | 0.3 (–0.85 to 1.47) | –1.46 (–3.43 to 0.52) | 0.1468 |
| Quality-of-life score | ||||||
| Week 6 | 54 | 0.6 (–0.21 to 1.47) | 83 | 0.4 (–0.22 to 1.01) | 0.04 (–0.93 to 1.01) | 0.9387 |
| Week 24 | 56 | –0.2 (–1.17 to 0.81) | 80 | 0.3 (–0.45 to 0.97) | –0.70 (–1.82 to 0.43) | 0.2239 |
| Faecal incontinence score | ||||||
| Week 6 | 61 | 0.0 (–0.51 to 0.48) | 87 | 0.3 (–0.03 to 0.68) | –0.49 (–1.03 to 0.05) | 0.0768 |
| Week 24 | 57 | –0.1 (–0.69 to 0.52) | 82 | 0.4 (–0.05 to 0.78) | –0.39 (–1.01 to 0.23) | 0.2117 |
| Symptom score | ||||||
| Week 6 | 59 | –0.2 (–0.89 to 0.45) | 84 | 0.2 (–0.2 to 0.68) | –0.42 (–1.12 to 0.29) | 0.2469 |
| Week 24 | 56 | –0.4 (–1.04 to 0.22) | 80 | –0.2 (–0.66 to 0.31) | –0.26 (–0.97 to 0.46) | 0.4779 |
Bowel diary data
Within the statistical analysis plan (SAP), we identified the most important data to be analysed in the bowel diary. These were the number of days passing stools, time spent on the toilet and percentage of normal stools.
Each participant was required to complete 8 weeks of bowel diaries (at baseline, weeks 1–6 and week 24). Overall, these were well completed and compliance was high. For example, frequency of passing of stool was completed by 88 out of 90 (97.7%) participants and 98 out of 99 (98.9%) participants at baseline; by 68 out of 90 (75.5%) and 89 out of 99 (89.8%) participants at 6 weeks; and by 57 out of 90 (63%) and 81 out of 99 (81%) participants at week 24 for the intervention and control groups, respectively.
Stools passed per week
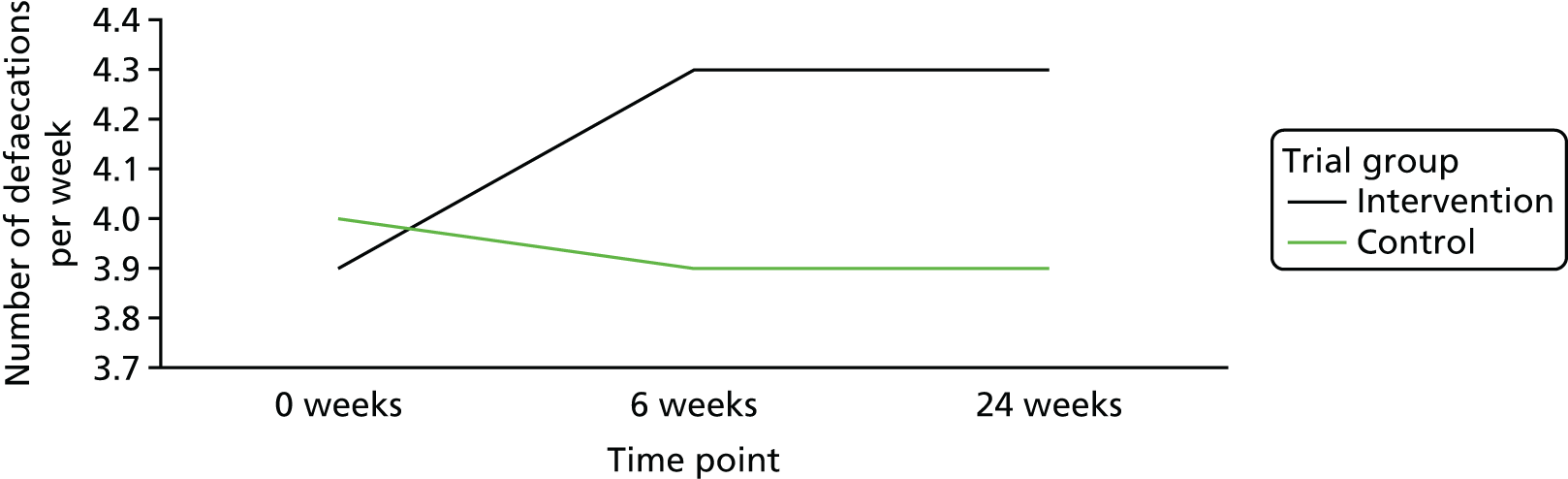
The mean frequency of stools passed per week at baseline was 3.9 (SD 1.68 stools passed per week) in the intervention group and 4.0 (SD 1.74 stools passed per week) in the control group. At week 6, this increased to 4.3 stools passed per week (SD 1.87) in the intervention group and decreased to 3.9 stools passed per week (SD 1.81) in the control group; at week 24, there was no change from week 6 in either group [i.e. mean frequency of stools passed per week was 4.3 (SD 1.88) in the intervention group and 3.9 (SD 1.89) in the control group]. Figure 7 is a line graph visually showing the change over time within the two groups.
FIGURE 7.
Change in the mean number of stools passed per week.
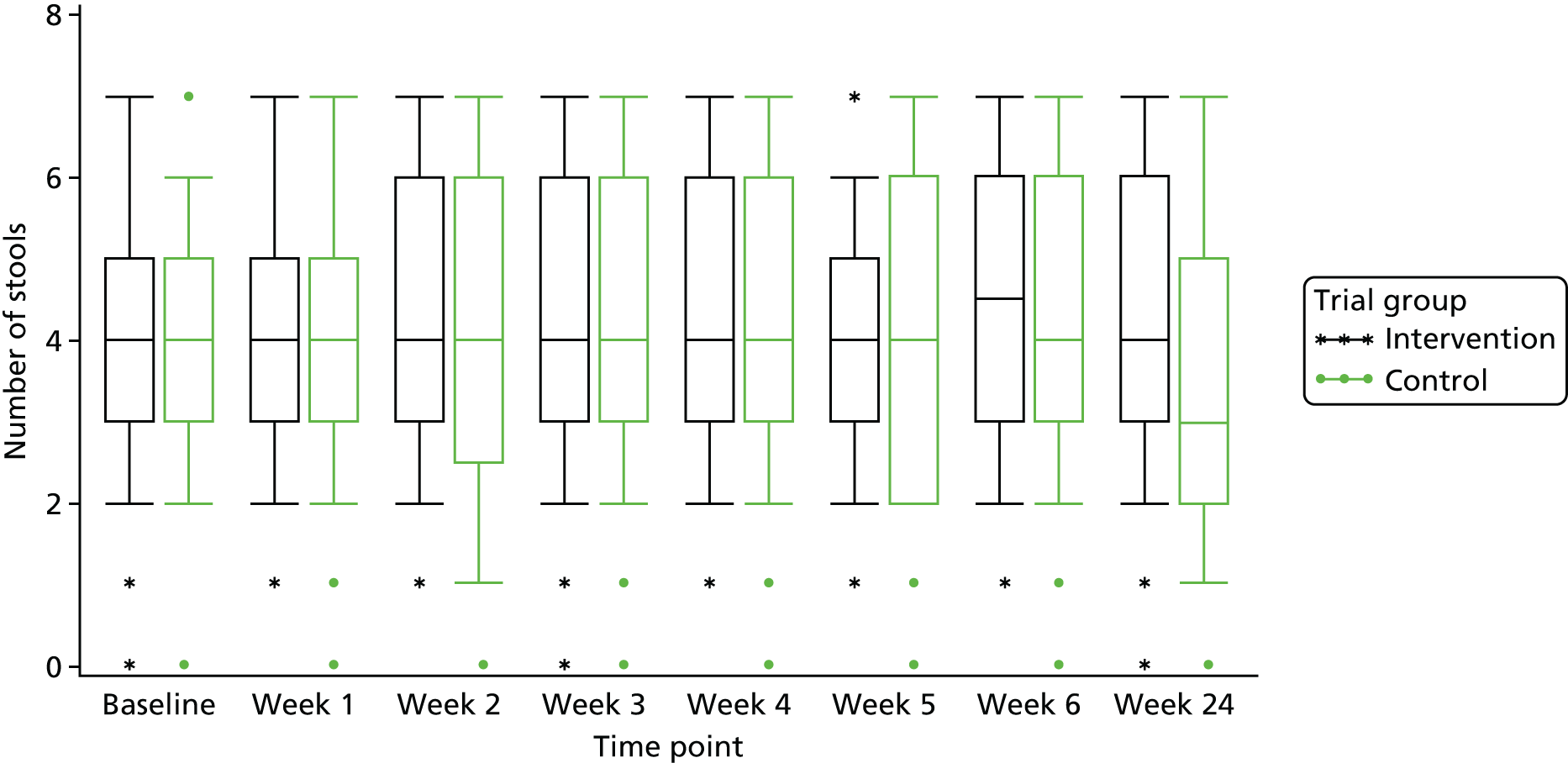
There was a statistically significant difference between trial groups in the change in number of stools passed from baseline to week 24 (Table 9). The difference between the trial groups was 0.62 (95% CI 0.03 to 1.21; p = 0.039). As there was some inconsistency in completion of the diaries, the analyses of change values were derived from a combination of two questions [(1) how often did you pass a stool? and (2) type of stool] to give one answer on frequency. If one or the other question was answered, this was taken as having passed a stool; if neither question was answered, this was taken as no stool passed. Figure 8 highlights very little change in the first 5 weeks.
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change (95% CI) | n | Mean change (95% CI) | Adjusteda (95% CI) | p-value | |
| Stools passed per week (adjusted to combine number and type of stool if data inconsistent) | ||||||
| Baseline to week 6 | 67 | 0.4 (0.07 to 0.68) | 88 | 0.0 (–0.34 to 0.39) | 0.38 (–0.08 to 0.85) | 0.1036 |
| Baseline to week 24 | 56 | 0.1 (–0.34 to 0.51) | 80 | –0.5 (–0.88 to 0.02) | 0.62 (0.03 to 1.21) | 0.039 |
FIGURE 8.
Box-and-whisker plot of number of stools passed per week.
Time spent on the toilet
At baseline, those in the intervention group spent a mean of 75.6 minutes (SD 69.60 minutes) per week on the toilet and those in the control group spent a mean of 75.8 minutes (SD 74.36 minutes) per week on the toilet. At week 6, this time was 77.9 minutes (SD 73.26 minutes) per week for the intervention group and 85.0 minutes (SD 88.52 minutes) per week for the control group. At week 24, these times were 78.2 minutes (SD 92.43 minutes) and 77.0 minutes (SD 68.51 minutes) per week for the intervention and control groups, respectively. There was no statistically significant difference between groups in the mean change in time spent going to the toilet between baseline and either time point (Table 10).
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change from baseline (95% CI) | n | Mean change from baseline (95% CI) | Adjusteda (95% CI) | p-value | |
| Time spent on the toilet (minutes per week) | ||||||
| Baseline to week 6 | 60 | –0.7 (–15.7 to 14.39) | 80 | 9.8 (–5.28 to 24.95) | –7.92 (–29.0 to 13.17) | 0.4588 |
| Baseline to week 24 | 50 | –6.5 (–21.86 to 8.78) | 71 | –3.8 (–19.71 to 12.17) | –3.35 (–23.1 to 16.4) | 0.7377 |
Type of stool
For each stool passed, participants were asked to indicate the type of the stool, as per the Bristol stool chart. 33 Using types 3 and 4 as normal, and types 1 and 2 and no stool per week as constipated, there was a reduction in the percentage of participants who were constipated in the intervention group (from 61.6% at baseline to 55.4% at week 24) and in the control group (from 59.0% to 58.9% at week 24). The percentage passing normal stools (types 3 and 4) was 20.2% in the intervention group and 17.5% in the control group at baseline; at week 24 this percentage was 23.9% in the intervention group and 22.8% in the control group (see Appendix 5).
Medications used in the AMBER trial for bowel management (information from medication form)
At baseline, 67 (74%) participants in the intervention group and 80 (80%) participants in the control group were on at least one medication, with numbers of medications ranging from 1 to 35. The number of participants on medication for management of their bowel symptoms was 44 (i.e. 44/67; 66%) in the intervention group and 54 (i.e. 54/80; 68%) in the control group. Laxido Orange (Almac Pharma Services Ltd, Craigavon, UK), Movicol (Norgrine Ltd, Hengoed, UK) and docusate sodium appeared to be the most popular medications used in both groups, with suppositories being used by approximately only 9% of participants in each group.
At the start of the trial, 44 participants were on zero medications (24 participants in the intervention group and 20 in the control group).
During the course of the study, 32 participants in the intervention group and 44 participants in the control group started new medications, with nearly twice as many new medication entries recorded in the control group compared with the intervention group (69 vs. 135). Thirteen participants in the intervention group had taken at least one additional medication for bowel management, with 19 different entries recorded in total (e.g. one participant had five entries for four different laxatives). In the control group, 12 participants started new medications for their bowels, with 33 different entries (several participants reported three or four additional bowel medications; one had 12 entries, eight for different bowel medications and four for glycerine suppositories).
At the end of the study, 15 participants in the intervention group (18 entries in total) and 13 participants in the control group (35 entries) had stopped taking some of their bowel management medications. There were still 38 participants who were not on any form of medication (intervention group, n = 20; control group, n = 18) at the end of the study.
Anorectal physiology and transit test results
University College Hospital, London, was the only site in the AMBER trial to recruit participants to a pilot substudy, to determine if any information about the mechanism of action of abdominal massage could be gleaned through anorectal physiology and colonic transit tests, which are routinely undertaken at this site. Participants had a test at baseline and a repeat test at week 24. All participants from UCH took part in the substudy.
A total of 26 participants were randomised at UCH; however, two post-randomisation exclusions occurred. All baseline outcomes for the main study were completed by 24 participants. Of these, 23 participants underwent baseline transit tests; the 23 participants comprised two male (8.7%) and 21 female (91.3%) participants, 11 from the intervention group and 12 from the control group, with a mean age of 53.5 years (SD 12.59 years). There were no baseline transit test data for one of the 24 participants. Three participants withdrew from the study during the 6 weeks of intervention and a further participant was lost to follow-up (could not be contacted for the week-24 follow-up and did not return any patient-reported outcomes). A further eight participants withdrew from having the repeat tests at week 24, leaving 12 participants with week-24 transit and physiology results. One of these participants had no baseline transit data; thus, 11 sets of baseline and week 24 transit test data were available for analysis.
There was no difference between the groups with respect to changes in the duration of bowel symptoms, faecal incontinence, infrequent emptying, pain or bloating (see Report Supplementary Material 1). The baseline data indicated that 65.2% (15/23) of the participants who underwent the transit test had slow transit, six (54.5%) in the intervention group and nine (75%) in the control group.
Table 11 shows that there was no significant difference in the change in the number of total markers between the groups; although there was a possibility that the groups were not well matched at baseline with a median number of markers of 17 (range 0–54) in the intervention group, while in the control group the median number of markers was 47 (range 0–60). The CI is wide because of the small number of participants. The markers in the rectosigmoid were relatively well matched at baseline, median 10 (range 0–46) in the intervention group and a median 12.5 (range 0–35) in the control group.
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| n | Mean change from baseline in the number of markers (95% CI) | n | Mean change from baseline in the number of markers (95% CI) | Adjusteda (95% CI) | p-value | |
| 24 weeks | 5 | 13.2 (–20.04 to 46.44) | 6 | –7.8 (–20.29 to 4.63) | 15.7 (–37.69 to 69.01) | 0.4846 |
Notwithstanding the substantial number of participants who failed to complete the test at week 24 (five in the intervention group and six in the control group), the change between pre treatment and week 24 was 16 (range –6 to 32) in the intervention group and –5.0 (range –13 to 17) in the control group. This may be explained by the fact that, in the intervention group, the massage was moving content to the distal colon, if not necessarily triggering evacuation.
The difference at baseline for the total markers and markers in the left and right colon, that is, groups not matched at baseline for these outcomes, as well as the numbers not completing the test at week 24, make it impossible to differentiate between changes as a result of regression to the mean and actual changes.
Anorectal pressure tests and anal and rectal sensation and capacity
The small number of participants who completed the tests at week 24 mean that the sample size is too small to draw conclusions.
Adverse events
A total of 84 AEs were noted in the trial: 28 in the intervention group and 56 in the control group. Table 12 summarises this information, along with the numbers of participants affected in each group.
| Category | Trial group (n) | Total (n) | |
|---|---|---|---|
| Intervention | Control group | ||
| All participants | 91 | 100 | 191 |
| Participants with AEs | 19 | 30 | 49 |
| AEs | 28 | 56 | 84 |
| Of which, SAEs | 3 | 6 | 9 |
Appendix 6 is a summary of all the AEs. Five AEs were reported as being possibly related to the intervention; however, two of these were reported in the control group [urinary tract infection (UTI) and diarrhoea]; as they had no intervention, the fact that there were two AEs in this group that were classed as possibly related to the intervention is deemed as an error on the part of the site reporting the AE. For the three possibly related AEs reported in the intervention group, two were for one participant who had two UTIs, both of which resolved within 1 week, and one participant had reflux and went to accident and emergency (A&E) but was not admitted. Both of these participants were still continuing with the massage at 24 weeks despite these reported AEs.
Additional information on all AEs (reported by primary SOC and PT) can be found in Report Supplementary Material 1.
Summary of serious adverse events
From the 84 reported AEs, nine were classified as a SAE. A summary of the SAEs reported is in Appendix 6. Two of these SAEs were a hospitalisation as a result of a MS relapse (one in each study group) and one was a fall, but, considering the study cohort, these are not surprising events. None was related to the trial and all were resolved.
Secondary analysis
In a secondary analysis of the primary outcome, using MI for the missing data, the following baseline variables were included in the model: age, sex, BMI, type of MS, number of years since diagnosis, cognitive symptoms of MS and minimisation variable level of mobility (walking unaided, walking aided or wheelchair bound). Table 13 shows the results adjusted for all variables; this indicated a similar result as found in the primary analysis, with no significant difference between the intervention and control groups. For assessing if changes in symptoms were dependent on site, with Edinburgh taken as the reference site, there was no statistically significant difference between the sites (p = 0.8326), although slightly better results were reported at sites in Sheffield, Salford, Lincoln, Leeds and the John Radcliffe Hospital than in Edinburgh. Regression analysis also indicated that there is more likely to be a benefit in the intervention group for those walking unaided or aided, as they responded better than wheelchair-bound participants, while those of greater age and higher BMI also did slightly better. The time since diagnosis of MS did not seem to be important, but those with relapsing–remitting MS responded more positively than those with primary progressive MS. Cognition severity indicated that those with mild or no impairment did better than those with severe impairment. Consistent with other findings, males responded significantly better (mean difference –2.789, 95% CI –5.179 to –0.399; p = 0.0226).
| Variable | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intervention vs. control | –1.060 | –2.976 to 0.855 | 0.2751 |
| Centre vs. Edinburgh | 0.8326 | ||
| Southern General Hospital, Glasgow | 0.135 | –4.334 to 4.604 | |
| Royal Victoria Hospital, Newcastle upon Tyne | 2.411 | –2.397 to 7.218 | |
| UCH, London | 0.997 | –3.614 to 5.608 | |
| Royal Preston Hospital, Preston | 1.868 | –2.996 to 6.732 | |
| The Walton Centre, Liverpool | 1.411 | –3.704 to 6.526 | |
| John Radcliffe Hospital, Oxford | –0.257 | –4.901 to 4.387 | |
| Leeds Community Healthcare NHS Trust | –0.326 | –5.321 to 4.668 | |
| United Lincolnshire Hospitals NHS Trust | –0.034 | –4.845 to 4.778 | |
| Salford Royal Hospital NHS Trust | –0.687 | –5.481 to 4.107 | |
| Sheffield Teaching Hospitals NHS Trust | –1.594 | –6.223 to 3.034 | |
| Northampton General Hospital, Northampton | 1.893 | –3.560 to 7.347 | |
| Baseline NBDS (+ 1 unit) | 0.327 | 0.137 to 0.516 | 0.0009 |
| Minimisation variable: mobility vs. wheelchair bound | 0.9560 | ||
| Walking unaided | –0.218 | –3.476 to 3.040 | |
| Walking aided | –0.425 | –3.525 to 2.674 | |
| Age (+ 1 year) | –0.097 | –0.211 to 0.018 | 0.0972 |
| BMI (+ 1 kg/m2) | –0.042 | –0.207 to 0.124 | 0.6171 |
| Male vs. female | –2.789 | –5.179 to –0.399 | 0.0226 |
| Time since diagnosis of MS (+ 1 years since diagnosis) | 0.046 | –0.069 to 0.160 | 0.4309 |
| Cognitive status vs. severe | 0.7719 | ||
| None | –0.754 | –6.832 to 5.323 | |
| Mild | –1.720 | –7.722 to 4.282 | |
| Moderate | –0.709 | –6.833 to 5.415 | |
| Type of MS vs. primary progressive | 0.5537 | ||
| Type of MS: benign | –1.515 | –10.071 to 7.041 | |
| Type of MS: relapsing–remitting | –2.525 | –6.031 to 0.980 | |
| Type of MS: secondary progressive | –1.749 | –5.325 to 1.826 | |
Sensitivity analysis
A sensitivity analysis was carried out using MI of the primary outcome NBDS, and a similar result to the primary analysis was found (difference in change between the intervention and control group: –1.266, 95% CI –2.936 to 0.403; p = 0.1371) (Table 14). Males, however, again demonstrated a greater beneficial effect (males vs. females: –2.280, 95% CI –4.478 to –0.083; p = 0.0419).
| Parameter | Estimate | SE | 95% CI | p-value |
|---|---|---|---|---|
| Intervention group vs. control group | –1.266 | 0.852 | –2.936 to 0.403 | 0.1371 |
| Centre | ||||
| John Radcliffe Hospital, Oxford | –0.084 | 2.289 | –4.570 to 4.402 | 0.9708 |
| Leeds Community Healthcare NHS Trust | –0.012 | 2.434 | –4.784 to 4.759 | 0.9959 |
| Northampton General Hospital, Northampton | 0.847 | 2.368 | –3.794 to 5.488 | 0.7206 |
| Royal Preston Hospital, Preston | 1.062 | 2.233 | –3.315 to 5.438 | 0.6345 |
| Royal Victoria Hospital, Newcastle upon Tyne | 1.225 | 2.290 | –3.263 to 5.712 | 0.5927 |
| Salford Royal Hospital NHS Trust | 0.090 | 2.254 | –4.328 to 4.508 | 0.9682 |
| Sheffield Teaching Hospitals NHS Trust | –1.622 | 2.247 | –6.026 to 2.783 | 0.4706 |
| Southern General Hospital, Glasgow | –0.083 | 2.159 | –4.313 to 4.148 | 0.9694 |
| The Walton Centre, Liverpool | 1.039 | 2.487 | –3.835 to 5.914 | 0.6760 |
| United Lincolnshire Hospitals NHS Trust | –0.468 | 2.304 | –4.984 to 4.048 | 0.8391 |
| UCH, London | 0.168 | 2.148 | –4.042 to 4.378 | 0.9377 |
| NBD total score at baseline | 0.356 | 0.090 | 0.181 to 0.532 | < 0.0001 |
| Minimisation variable: mobility vs. wheelchair bound | ||||
| Walking unaided | 0.320 | 1.449 | –2.521 to 3.161 | 0.8251 |
| Walking aided | –0.192 | 1.420 | –2.975 to 2.591 | 0.8924 |
| Male vs. female | –2.280 | 1.121 | –4.478 to –0.083 | 0.0419 |
The repeated-measures analysis (Table 15) was not significant for the primary outcome or all other outcomes, except for the increased number of times that participants felt that their bowel was emptied and the number of stools passed per week, which were in favour of the intervention. Between baseline and week 6, the mean difference in the number of stools for the intervention group was 0.98 (95% CI 0.36 to 1.61; p = 0.03902) and for the control group it was 0.56 (95% CI 0.03 to 1.10; p = 0.039). This effect was decreased for both outcomes at week 24, but was still statistically significant over all time periods.
| Outcome | Intervention – control | Overall p-valuea | |||
|---|---|---|---|---|---|
| Week 6 | Week 24 | ||||
| LS mean (95% CI)a | p-valuea | LS mean (95% CI)a | p-valuea | ||
| NBDS | –1.16 (–2.84 to 0.53) | 0.179 | –0.54 (–2.26 to 1.17) | 0.535 | 0.254 |
| Constipation score | –0.88 (–1.99 to 0.23) | 0.121 | –0.53 (–1.64 to 0.58) | 0.349 | 0.157 |
| Time spent on toilet | –0.42 (–21.5 to 20.6) | 0.969 | –9.70 (–29.3 to 9.9) | 0.331 | 0.545 |
| No attempts per week | 1.31 (–0.66 to 3.29) | 0.192 | 1.01 (–0.83 to 2.86) | 0.281 | 0.138 |
| Did not empty bowels | 0.98 (0.36 to 1.61) | 0.002 | 0.48 (–0.11 to 1.07) | 0.108 | 0.007 |
| No stools per week | 0.56 (0.03 to 1.10) | 0.039 | 0.32 (–0.18 to 0.82) | 0.215 | 0.026 |
| SF-Qualiveen score | –0.24 (–2.17 to 1.69) | 0.809 | –0.87 (–2.76 to 1.01) | 0.363 | 0.498 |
| EQ-5D-5L VAS score | 4.30 (–2.05 to 10.65) | 0.184 | 0.67 (–5.59 to 6.92) | 0.834 | 0.374 |
| EQ-5D-5L UK Health Index score | 0.003 (–0.05 to 0.065) | 0.916 | 0.004 (–0.056 to 0.065) | 0.885 | 0.888 |
| NBIS | |||||
| Faecal score | –0.37 (–0.95 to 0.20) | 0.203 | –0.43 (–0.99 to 0.13) | 0.136 | 0.107 |
| QoL | –0.67 (–1.68 to 0.34) | 0.192 | –0.06 (–1.07 to 0.95) | 0.907 | 0.403 |
| Symptom score | –0.29 (–0.99 to 0.41) | 0.415 | –0.43 (–1.12 to 0.25) | 0.214 | 0.226 |
| Total score | –1.40 (–3.15 to 0.35) | 0.117 | –1.05 (–2.79 to 0.68) | 0.234 | 0.106 |
Change in laxative use
At week 6, the outcome for the regular use of laxatives or drops at week 6 compared with no use of laxatives or drops had an odds ratio (OR) of 2.37 (95% CI 0.87 to 6.46; p = 0.092), using ordinal regression adjusted for all baseline variables (baseline use, centre, age, sex, mobility, BMI, time since diagnosis of MS, type of MS and cognitive status). At week 24, the OR for the same outcome was 1.62 (95% CI 0.74 to 3.55; p = 0.229) using ordinal regression adjusted for all the same baseline variables. Although the ORs were not significant, there is some evidence that the intervention group were twice as likely to achieve a lower level of laxative use than the control group (Table 16).
| Variable | Trial group, n (%) | Total, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| Pre intervention | |||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No regular use | 41 (45.6) | 48 (48.5) | 89 (47.1) |
| Regular use of laxative drops or tablets | 43 (47.8) | 47 (47.5) | 90 (47.6) |
| Regular use of both laxative drops and tablets | 6 (6.7) | 4 (4.0) | 10 (5.3) |
| Total | 90 (100.0) | 99 (100.0) | 189 (100.0) |
| Week 6 | |||
| Missing | 0 (0.0) | 1 (1.1) | 1 (0.6) |
| No regular use | 34 (53.1) | 48 (53.3) | 82 (53.2) |
| Regular use of laxative drops or tablets | 27 (42.2) | 37 (41.1) | 64 (41.6) |
| Regular use of both laxative drops and tablets | 3 (4.7) | 4 (4.4) | 7 (4.5) |
| Total | 64 (100.0) | 90 (100.0) | 154 (100.0) |
| Week 24 | |||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No regular use | 43 (59.7) | 51 (56.7) | 94 (58.0) |
| Regular use of laxative drops or tablets | 26 (36.1) | 35 (38.9) | 61 (37.7) |
| Regular use of both laxative drops and tablets | 3 (4.2) | 4 (4.4) | 7 (4.3) |
| Total | 72 (100.0) | 90 (100.0) | 162 (100.0) |
Post hoc analysis
The following descriptive analysis (and, when indicated, further statistical analysis) focuses on questions within questionnaires and on further bowel diary data on symptoms that were identified as being important to participants and/or were identified in other analyses as significantly changed between the two groups.
Neurogenic Bowel Dysfunction Score: those with constipation at baseline (i.e. with a score of ≥ 11 points)
As the overall level of constipation at baseline was rated as mild in both groups according to the NBDS [at baseline the total mean score for the intervention group was 7.6 points (SD 5.31 points) and the median was 6.0 points (range 0–21 points), whereas in the control group, the mean was 8.6 points (SD 5.08 points) and the median was 9.0 points (range 0–22 points)], the analysis was repeated for only those participants with a NBDS at baseline of ≥ 11 points. The results are presented in Table 17.
| Time point | Trial group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||||
| n | Mean SD | First quartile | Median | Third quartile | 95% CI | n | Mean SD | First quartile | Median | Third quartile | 95% CI | |
| Intervention | 20 | 12.3 | 7.0 | 13.0 | 18.5 | 9.3 to 15.2 | 28 | 10.7 | 7.5 | 10.5 | 13.5 | 8.9 to 12.5 |
| 6 weeks | 6.4 | 4.7 | ||||||||||
| Intervention | 22 | 9.0 | 4.0 | 9.5 | 12.0 | 6.2 to 11.8 | 28 | 9.9 | 6.0 | 9.5 | 13.0 | 7.8 to 12.2 |
| 24 weeks | 6.3 | 5.4 | ||||||||||
The univariate analysis and linear regression analysis can be reviewed in Report Supplementary Material 1. The numbers in both groups were reduced and the only evidence of any effect was at week 6 for those with a longer time since diagnosis of MS (F4,30, p = 0.043, estimate 0.153, t-test value 2.07, p = 0.0437, 95% CI 0.004 to 0.302).
Frequency of defaecation as per question 1 in the Neurogenic Bowel Dysfunction Score
There was an increase in the number of participants passing stools daily from baseline to week 24 in the intervention group (12.2% to 23.6%), with a smaller increase in the control group (16.2% to 20.0%). The intervention group had a small decrease in the percentage passing stools fewer times than once per week (6.7% to 5.6%), compared with an increase in the control group (7.1% to 12.2%).
Frequency of defaecation as per the question in the Constipation Scoring System
The percentage of participants in the intervention group who were passing stool two or more times per week by week 6 was 92.1% and in the control group it was 88.6%; at week 6, 6.6% in the intervention group and 10.1% in the control group were passing stool fewer times than once a week. At week 24, the percentage of participants passing stools more than two times per week had increased to 94.9% in the intervention group and had decreased in the control group to 80.7%; 3.4% of the intervention group were now passing stools fewer times than once per week, whereas in the control group this percentage had increased to 16.8%.
Feeling of incomplete evacuation as per the question in the Constipation Scoring System
At baseline, the percentage of participants who ‘never felt incomplete evacuation’ was 6.7% in the intervention group and 9.1% in the control group; at week 24, this increased to 15.5% in the intervention group and decreased to 8.4% in the control group. At baseline, 21.3% of participants in the intervention group and 20.3% in the control group ‘always felt incomplete evacuation’; at week 24 this decreased to 3.4% in the intervention group and 10.8% in the control group.
Bowel diary data
Attempts to pass stool
At baseline, the mean number of attempts to pass stool in the intervention group was 10.4 (SD 6.73 attempts), whereas for the control group this mean was 8.6 (SD 5.22 attempts). At week 6, the mean number of attempts was 11.3 (SD 7.31 attempts) for the intervention group and 8.9 (SD 6.00 attempts) for the control group; and at week 24 the mean number of attempts to pass stool was 10.7 (SD 7.16 attempts) and 8.3 (SD 5.09 attempts) for the intervention and control groups, respectively.
As can be seen in Table 18 and Figure 9, the mean changes between baseline and weeks 6 and 24 for attempts to empty the bowel were not statistically significant between groups.
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change from baseline (95% CI) | n | Mean change from baseline (95% CI) | Adjusteda (95% CI) | p-value | |
| 6 weeks | 62 | 0.2 (–1.49 to 1.84) | 84 | 0.4 (–0.83 to 1.66) | 1.08 (–0.81 to 2.96) | 0.2608 |
| 24 weeks | 52 | –0.9 (–2.82 to 1.02) | 75 | –0.5 (–1.96 to 1.0) | 1.14 (–0.92 to 3.19) | 0.2770 |
FIGURE 9.
Frequency of feeling of successful evacuation.
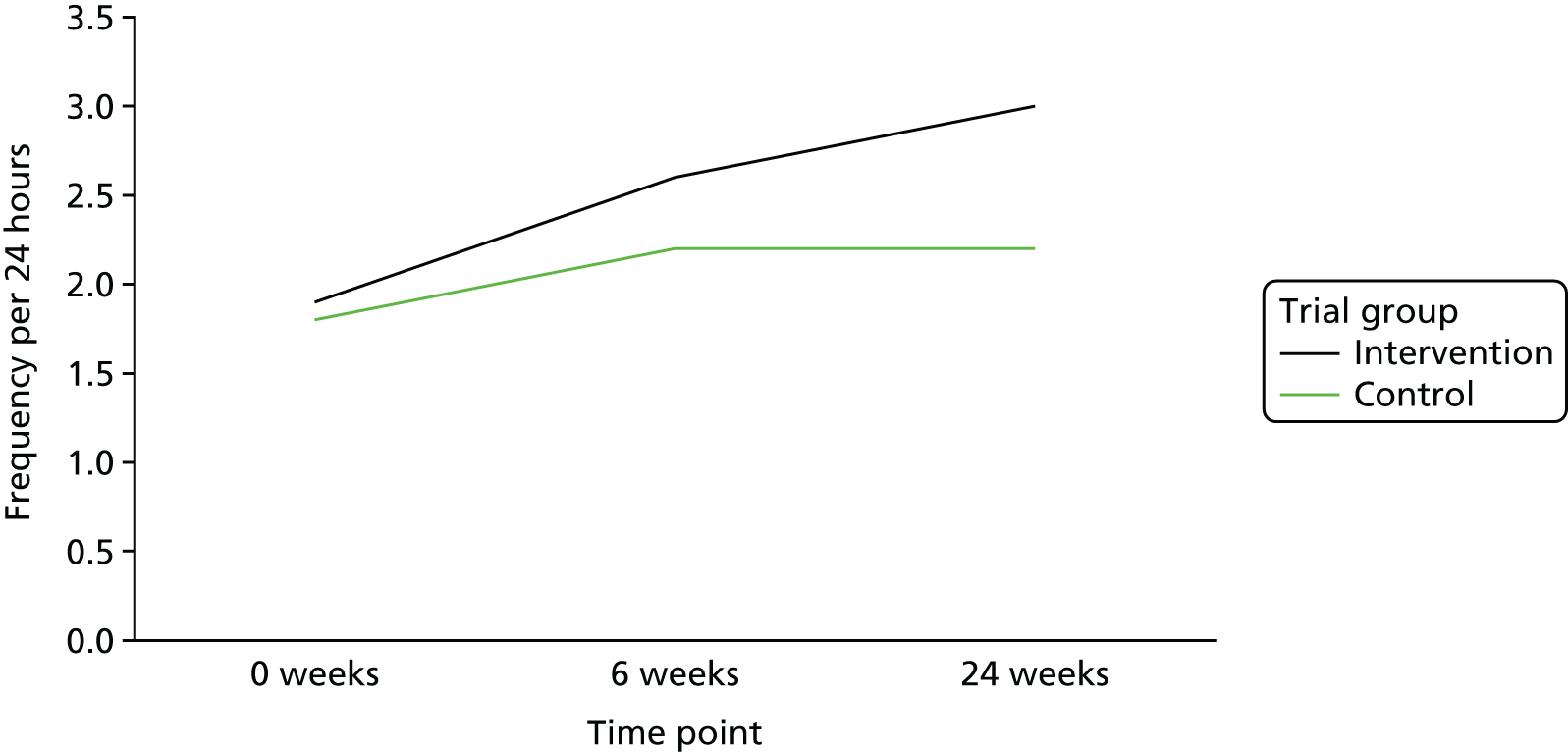
At baseline, the number of times per week that the participants felt that they had a complete evacuation was 1.9 (SD 2.2 times) and 1.8 (SD 1.73 times) for the intervention and control groups, respectively. At week 6, this was 2.6 times (SD 2.2 times) and 2.2 times (SD 2.0 times) for the intervention and control groups, respectively, and at week 24 this number was 3.0 times (SD 2.16) and 2.2 times (SD 2.14 times) for the intervention and control groups, respectively.
There was a statistically significant difference between groups in the change in number of complete evacuations per week from baseline to week 24 (p = 0.002) (Table 19). The intervention group had, on average, increased the number of complete evacuations per week by 1.08 more than the control group, although this was a post hoc analysis.
| Time point | Trial group | Mean difference in change between groups, mixed models | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 90) | Control (N = 99) | |||||
| n | Mean change from baseline (95% CI) | n | Mean change from baseline (95% CI) | Adjusteda (95% CI) | p-value | |
| 6 weeks | 62 | 0.8 (0.4 to 1.2) | 84 | 0.2 (–0.2 to 0.6) | 0.48 (–0.10 to 1.06) | 0.104 |
| 24 weeks | 52 | 1.2 (0.6 to 1.8) | 75 | 0.3 (–0.1 to 0.7) | 1.08 (0.41 to 1.76) | 0.002 |
Other diary data
Analysis of other data provided by the self-completed bowel diary was not statistically tested as per the SAP (e.g. frequency of faecal incontinence, use of digital stimulation, type of stool) and no change was identified between groups to warrant post hoc analysis. The bowel diary question on laxative use asked if laxative use was the same, less or more. This was difficult to analyse as it was not being compared with baseline but with the previous week.
Adherence to the intervention (massage diary and nurse weekly telephone calls)
All participants received weekly telephone calls from the research nurse during the 6 weeks of intervention and again at week 24. The aim of the calls was to support fidelity to the trial protocol. There was good response to the follow-up telephone calls made by research staff at sites; 81% of participants in the intervention group and 86% in the control group were reached at week 24 and data were collected during the telephone calls.
According to information collected during the follow-up calls, 72.6% to 83.3% of participants in the intervention group administered the massage themselves throughout the 6 weeks of intervention; for 10.3% to 14.1% of participants, a carer undertook the massage. Some data were missing or massage was not undertaken in 2.6% of cases.
According to the bowel diary records, which also recorded when the massage was undertaken in the intervention group, the mean number of days on which massage was performed per week was 5.2 (SD 1.88). This varied little over the 6 weeks of intervention [at week 6, the mean was 5.4 days (SD 1.75)]. At week 24 those still doing the massage (n = 57, 82%) were undertaking it on average 3.2 times per week (SD 2.83 times per week). Mean time spent on the massage during weeks 1–6 was 72.5 minutes (SD 4.0 minutes) and at week 24 it was 55.8 minutes (SD 40.0 minutes).
Participants were shown how to perform the massage lying down, semi-lying down or sitting up. Information on the choice of position utilised, in addition to the time of day the massage was performed, was collected during the 6 weeks of the intervention. During weeks 1–6, 58–64% of participants performed the massage lying down, 16–24% semi-lying down and 1–4% sitting up.
Morning massage administration seemed to be the preferred time (for 46–56% of all participants), then evening (26–31%), with afternoon administration being least common (4–6%).
Information from nurse weekly telephone calls
Reasons for discontinuing the intervention, as reported in the final week 24 telephone call with the research nurse (n = 77 interviews; 20 discontinued), included no benefit (n = 8; 10%), burden on carer (n = 1; 1.3%) and too difficult (n = 5; 6.5%); the rest gave no reason or data were missing.
Adherence to lifestyle
In the intervention and control groups, 20% and 30% of participants, respectively, stated that they made at least one change to their lifestyle as part of the optimised bowel care information. More participants in the control group than the intervention group changed their diet during weeks 1–4 but did not seem to continue with this; however, they did continue to alter their fluid intake and do more exercise. The number who changed their position for defaecation was similar in both groups.
Approximately 50% of participants in the intervention group reported a change in their bowel habits compared with 38% in the control group at week 1, and this difference was mirrored at week 24 with approximately 43% in the intervention group versus 31% in the control group reporting a change in bowel habits. Changes reported more frequently in the intervention group were more frequent bowel movements, less time spent on the toilet and softer stools than the control group at weeks 1 and 24.
For the AMBER trial full set of descriptive statistics, see Report Supplementary Material 1.
Neurogenic bowel impact score
Analysis of the new NBD symptom score was undertaken for further validation; the analysis carried out is in Report Supplementary Material 1.
The new neurogenic bowel patient-reported outcome measure (NBIS) showed only moderate repeatability in the control group for the AMBER trial. However, this was over a longer time period than is usually used for test–retest stability, and it is possible that the MS bowel symptoms had genuinely changed in the control group. It is known that MS bowel symptoms are variable over time. A repeat of this test–retest with a shorter time between the completions, and possibly asking participants if they perceive that their symptoms changed or not, is recommended.
When compared against other bowel symptom scores, such as the NBDS and the Wexner score, a high correlation was found for most items, suggesting good criterion validity for the new questionnaire. It was also highly correlated with the EuroQol-5 Dimensions (EQ-5D) quality-of-life score, but not the EuroQol Visual Analogue Scale (EQ-VAS). NBIS was strongly correlated with the primary outcome measure NBDS. As the new questionnaire was developed after extensive qualitative work with people with NBD, including those with MS, this lends credibility to both scores. However, neither score showed a significant difference between our intervention and control groups, so there is no evidence that one is more responsive to change that the other. Further work is needed to determine which score patients find better reflects what is important to them. As our trial found no significant difference between groups, we are not able to recommend either score as being more or less sensitive to change. We did not find anything to suggest an advantage to either questionnaire and both had similar completion rates.
However, some questions within the NBDS (primary outcome) were difficult for some patients to fully understand (e.g. digital stimulation, use of drops), whereas in the new questionnaire, the language was better but the whole questionnaire was felt to be too long.
Chapter 4 Health economic evaluation
Economic evaluation
The aim of the AMBER trial was to determine the clinical effectiveness and cost-effectiveness of abdominal massage (intervention) as part of an adjunct to the control of optimised bowel care in PwMS who have NBD. This chapter gives the results of a formal economic evaluation of the AMBER trial intervention compared with control from a NHS and patient cost perspective. Health-related quality-of-life (HRQoL) data and health-care resource-use data were used to examine the following:
-
the cost of delivery for the patient groups
-
the health-care costs for participants in both trial groups
-
HRQoL through calculation of utility values using the EQ-5D-5L.
Using the above information, we calculated an incremental cost-effectiveness ratio (ICER) and the probability of the intervention being cost-effective at different thresholds of willingness to pay (WTP) per QALY gained.
Data related to the economic analysis
Abdominal massage costs
The intervention is described in Chapter 2. In this chapter, only costs that would be observed if the intervention was delivered in practice will be considered; trial-related costs are not part of the analysis. In terms of the materials used for the training of the patients, it was estimated that the DVD production and the training materials provided would cost approximately £1 per patient. Research staff involved in the AMBER trial confirmed that the training of patients would take approximately 30–45 minutes. If abdominal massage becomes an established intervention in the NHS, it is expected that NHS staff involved in training patients would usually be at a NHS pay grade of 5, 6 or 7 (and could also be non-nursing staff).
For the purpose of this economic evaluation, and based on the above information, an ongoing cost of £90 per patient was calculated for the intervention and £0 costs were assumed for the controls. For the intervention, this is calculated assuming a hospital-based grade-6 nurse would provide this service for 50 minutes, which comes at £89 cost per patient contact, since contact will typically take < 50 minutes and this service can be provided by lower pay-grade staff and non-nursing staff. Duration of contact was not recorded in the trial. Therefore, the exact duration of these appointments could not be determined with certainty, and so the longest possible duration (50 minutes) was chosen for our baseline analysis. It was assumed that standard care involved no additional costs other than resource use; therefore, zero was taken as the baseline cost for the control group, even though, in practice, NBD patients would have access to services, which would involve some costs for the NHS. Moreover, in this trial, the control group did get follow-up calls and they were aware of the potential to be offered abdominal massage in the future. These calls were considered to be trial-only costs and were not included in the cost-effectiveness analysis.
Health-care resource-use data
Resources used by the participants were recorded in the outcome questionnaires at baseline and weeks 6 and 24. Participants were asked to record use of NHS services and all contacts (not just those they directly associated with bowel problems) with HCPs throughout the trial period. These are presented in Appendix 7.
Health-care costs
The information on resource use was combined with the unit cost of each resource to estimate the total cost of NHS resources used. Health service unit costs were valued using the most recent Department of Health and Social Care resource cost data, at 2015–16 UK prices. 41 The NHS resources that were included, and their unit costs, are shown in Appendix 8, along with the source of cost information. The cost of drugs consumed by participants only includes drugs prescribed by the participant’s GP (this is not shown in the Appendix 8). The British National Formulary42 was consulted for the unit cost of individual drugs prescribed to participants.
The NHS resource-use costs were calculated using unit costs, as shown in Appendix 8. Hospitalisation refers to the cost of an average inpatient stay in a hospital in England and Wales, as estimated in the Personal Social Services Research Unit (PSSRU). Patients did not report length of stay; they only reported if they had been admitted to hospital. Therefore, we assumed that, on average, patients stayed in hospital for the average duration of stay in England and Wales and we applied the cost of £1609 to each reported case of hospitalisation. The total resource-use-related costs for the NHS in each trial group at each time point are also shown in Appendix 8.
Between the two groups, NHS resource use and associated costs were not statistically different. Reported resource use suggests that there was little difference between the groups in terms of NHS costs related to resource use. The impact of the costs at weeks 6 and 24 is investigated further in the cost-effectiveness analysis. Costs at week 6 include all resource use from baseline up to the week-6 follow-up point. Costs at week 24 include all resource use from the week-6 follow-up point to the end of the trial at week 24. Baseline costs are not included in the cost-effectiveness analysis because they take place before the trial started, but they are used to show that there is no statistical difference between the two groups at baseline. As standard practice, and as part of the economic evaluation for this trial, we collected information on NHS resource use, prescribed medication costs and out-of-pocket costs. For reasons unknown, completion of prescribed medications and out-of-pocket costs was poor, resulting in many missing observations. Participants did have the option of reporting zero costs in these questions, but most did not respond. Given that the reported medication and out-of-pocket costs were very low, and similar between the two groups, we decided to use only intervention costs and NHS resource-use costs in the economic evaluation that follows, and excluded other costs. In general, few participants reported drug-related costs, resulting in many missing observations. These costs are not taken into account in the cost-effectiveness analysis that follows, because of the low numbers of participants who responded to these questions. Drug prescription costs in the intervention group were driven by one participant who was prescribed very expensive medicinal cannabis-based drugs. For example, if we remove this participant from the calculations below, the mean £187.25 cost per participant decreases to £18 cost per participant. This participant reported a cost of £1372 in the period between 6 and 24 weeks follow-up, vastly inflating the reported mean cost of £187.25 at week 24 in Table 20. Testing the equality of means at weeks 6 and 24 using t-tests (t = 1.16 and t = 0.79, respectively) suggests that there is no significant difference between the patients who responded to these questions on drug-related costs.
| Time point | Trial group, per-participant cost (£) | |
|---|---|---|
| Intervention | Control | |
| At baseline | 3.20 (0.00) | 5.08 (0.19) |
| At week 6 | 11.17 (6.00) | 22.83 (21.59) |
| Up to week 24, excluding baseline | 187.25 (169.33) | 52.48 (52.48) |
Patient costs
Information on out-of-pocket expenses was collected in the questionnaires. At each follow-up, participants were asked if they had bought medicines or other equipment related to their condition. They reported out-of-pocket expenses for medicines and the majority of participants who responded reported costs related to their NBD needs. The most common items reported were laxatives, suppositories and incontinence pads. The out-of-pocket expenses for incontinence pads and other items are summarised in Table 21. In terms of average out-of-pocket cost per participant, there is a very small difference between the two groups; the higher spending costs seen in the control group were driven by one individual only. We report only total costs for the two groups and a mean cost calculated by dividing by the number of participants who reported costs in each group. Overall, out-of-pocket spending reported per participant was very small in both groups and, again, because of the low numbers that responded to these questions, these costs were not taken into account in the cost-effectiveness analysis that follows.
| Time point | Trial group, out-of-pocket costs (£) | |
|---|---|---|
| Intervention | Control | |
| Baseline to week 6 | 244.96 | 409.98 |
| Week 6 to week 24 | 148.93 | 541.10 |
| Total | 393.89 | 951.08 |
| Mean, per patient through to week 24 | 6.57 | 11.46 |
EuroQol-5 Dimensions, five-level version, data
The EQ-5D-5L data were collected via participant-completed questionnaires at baseline, week 6 and week 24. The EQ-5D-5L responses were given in the two sections of the EQ-5D-5L questionnaire: the EQ-VAS and the EQ-5D Descriptive System. 43 The EQ-5D-5L Descriptive System was scored using the UK tariffs. 44 Table 4 provides a summary of the EQ-VAS and EQ-5D Descriptive System index score. Higher scores represent better quality of life. In the cost-effectiveness analysis that follows, HRQoL, as measured by the EQ-5D-5L index scores in Table 4, is combined with resource-use costs as shown in Appendix 8 and intervention costs (which were described in Abdominal massage costs and calculated as £90), and assigned to each participant in the intervention group.
Economic evaluation of abdominal massage (intervention) versus standard care (control)
The raw data indicate that there is little difference between the two groups in this trial both in HRQoL outcomes and costs. However, there were fewer quality-of-life data to analyse than the number of recorded participant withdrawals or losses to follow-up. Missing data occur frequently in RCTs as participants may withdraw, questionnaires may be unreturned and responses to individual questionnaire items may be impossible to use. In the AMBER trial, one reason for the differing numbers was the fact that, in some instances when missing outcome data were chased, participants said that they had already completed the outcomes and returned them by post, but these were never received (previously discussed in Chapter 3).
At the end of the trial, 58 participants in the intervention group and 83 participants in the control group had EQ-5D-5L data to analyse. To account for the missing data, and the highly imbalanced nature of the two groups that resulted in more missing values in the intervention group, the following statistical approaches45 were used: EQ-5D-5L data were analysed (Table 22) and then MI was performed. The imputed data sets were then bootstrapped to perform a cost-effectiveness analysis including NHS resource-use costs and interventions costs. In the economic evaluation that follows, NHS resource use included all costs related to both bowel issues and other health issues as reported in Appendix 9.
| EQ-5D-5L index scores | Trial group | Effect size (95% CI)a | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Baseline (n = 90) | Week 6 (n = 60) | Week 24 (n = 58) | Baseline (n = 99) | Week 6 (n = 84) | Week 24 (n = 83) | Week 6 | Week 24 | |
| Mean (SD) | 0.545 (0.246) | 0.520 (0.290) | 0.536 (0.276) | 0.498 (0.281) | 0.481 (0.271) | 0.458 (0.277) | 0.004 (–0.061 to 0.068) | 0.017 (–0.047 to 0.081) |
| p-value | ||||||||
| Median (range) | 0.615 (–0.183 to 1.000) | 0.570 (–0.245 to 1.000) | 0.592 (–0.245 to 1.000) | 0.555 (–0.467 to 1.000) | 0.527 (–0.213 to 1.000) | 0.548 (–0.130 to 1.000) | 0.916 | 0.605 |
The results in Table 22 are not adjusted for missing values. The economic analysis of the AMBER trial data employed methods described in another study to handle missing data by MI (to reduce bias and ensure that missing data are handled appropriately). 46 The economic evaluation, therefore, also included participants with only partial data. For the cost-effectiveness analysis, QALYs and total patient costs were calculated for the 6 months that the trial lasted. The imputation was run 60 times, resulting in 60 different data sets to be used in the cost-effectiveness analysis. The imputation was implemented separately for the intervention and control groups to account for differences in the missing values between the two groups.
Multiple imputation was performed using predictive mean matching. 47 The MI model uses baseline covariates (EQ-5D index scores, age, sex, time since diagnosis of MS and severity of MS symptoms), costs and QALYs at each follow-up to impute unobserved costs and QALYs, so that, for example, missing costs at week 24 are imputed using data on baseline covariates, costs at baseline and week 6 (if available) and QALYs between baseline and week 6 (if available). QALYs were imputed using EQ-5D index scores. One thousand bootstrap samples were drawn from each of the 60 multiply imputed data sets, analysed and the difference in net benefit between the treatment groups in each bootstrap sample was estimated (at a given threshold for cost per QALY). The proportion of bootstrap samples in which the net benefit is positive represents the probability that the treatment is cost-effective for each multiply imputed data set. This probability is then averaged across all multiply imputed data sets.
Tables 23 and 24 show resource-use spending at 24 weeks before and after imputation. As expected, resource use remains not statistically significantly different between the two groups after imputation. Table 25 shows the estimates of costs and QALYs per patient in the MI models. These costs include intervention costs and resource-use costs as shown in Table 25. QALYs were calculated from EQ-5D index score, taking into account the fact that the trial lasted only 6 months.
| Summary statistics | Trial group (£) | |
|---|---|---|
| Intervention | Control | |
| Mean cost per participant | 416.40 | 487.01 |
| Standard error | 130.70 | 83.84 |
| SD | 986.79 | 768.39 |
| 95% CI | 154.57 to 678.23 | 320.26 to 653.76 |
| t-test on the equality of means | 0.47 | |
| Summary statistics | Trial group (£) | |
|---|---|---|
| Intervention | Control | |
| Mean cost per participant | 427.49 | 500.46 |
| Standard error | 83.79 | 71.42 |
| SD | 794.87 | 710.64 |
| 95% CI | 261.01 to 593.98 | 358.72 to 642.19 |
| t-test on the equality of means | 0.67 | |
| Summary statistics | Trial group (£) | |
|---|---|---|
| Intervention | Control | |
| Mean costs per participant | 590.44 | 540.65 |
| Standard error | 127.44 | 96.28 |
| Mean QALYs per patient | 0.230 | 0.216 |
| Standard error | 0.015 | 0.012 |
Table 26 shows the average incremental costs and QALYs for every participant obtained from the multiply imputed sample and the bootstrapping. The ICER based on these data is negative at –£24,149 because the model estimates a negative incremental QALY.
| Summary statistics | Estimate | Standard error | 95% CI |
|---|---|---|---|
| QALYs | –0.002 | 0.009 | –0.029 to 0.027 |
| Costs | 56.50 | 116.99 | –372.62 to 415.68 |
This method accounts for the uncertainty around the mean estimates of both costs and QALYs; to make conclusions about the cost-effectiveness of abdominal massage compared with control, the probability of cost-effectiveness at the WTP threshold of £20,000 per QALY is calculated at 31.3%. That probability increases to 34.2% for a WTP threshold of £30,000 per QALY.
To test if the results were affected by the imputation or the bootstrapping processes, two extra models were estimated. First, it was estimated that a seemingly unrelated regression model was applied on the imputed data sets. 48 Second, a mixed model was employed using maximum likelihood estimation. 44,49 The mixed model did not require an imputation step; this approach is a good check to see if imputation affected the results. The results of the seemingly unrelated regression model and the mixed model are shown in Appendix 7.
These results are similar to the bootstrapping model, and all three methods predict a probability of cost-effectiveness that is < 50% at the WTP threshold of £20,000 per QALY gained. Both imputation models predict a negative QALY gain after controlling for baseline HRQoL in these models. For this reason, the ICER of these models is negative, making straightforward comparisons rather difficult. The mixed model also controlled for baseline HRQoL but the estimate on incremental QALYs and the ICER of this model was positive. In all three estimated models, the impact on quality of life is close to zero, with relatively large standard errors reflecting the uncertainty around patient utility scores. The mixed-model point estimate of the ICER is at £28,722, which is over the £20,000 WTP threshold.
In sensitivity testing, patient characteristics (age, sex, time since diagnosis of MS and severity of MS symptoms) were further controlled for, along with baseline HRQoL, in the three models, but this did not significantly change the results. In another sensitivity test, unit costs were varied by 20%, but this did not have a significant impact on the probability of cost-effectiveness, possibly because of similar resource use between the two groups. In a final sensitivity test, the impact of changing the intervention cost was explored. All three models were ran with the intervention halved and doubled. The results do appear sensitive to the intervention cost, as the probability of cost-effectiveness at the WTP threshold of £20,000 per QALY gained ranges from 21% for high intervention costs of £180 per patient to 55% probability of cost-effectiveness for low costs of £45 per patient. Notably, the point estimate of the ICER drops to between £6000 and £7000, which is in the cost-effective range given a WTP threshold of £20,000 per QALY gained. As described earlier, it is more likely that the intervention costs will be < £90 per patient than > £90, and the results in Appendix 7 should be considered as conservative. However, it should be noted that given the negative QALY increment, the control was bound to dominate as long as the mean cost was higher in the intervention group.
The economic evaluation results show that abdominal massage is less likely to be a cost-effective alternative to standard care than the other way around. However, all models predicted a probability of cost-effectiveness of > 30% and, in the mixed model, close to 47%. In the sensitivity analysis, this probability was > 55% assuming a low intervention cost per participant. The probability of cost-effectiveness can be seen as the probability that an individual (random) patient will have a positive individual incremental net benefit. It can also be seen as the proportion of all patients in the population who have positive individual incremental net benefits; therefore, it is reasonable to assume that our results suggest that there is a subset of patients who had a positive incremental net benefit from abdominal massage. 50 If there were no patients with a positive net incremental benefit, then the likelihood of cost-effectiveness would have been close to zero. Further research is needed to establish the type of patient that may have benefited from abdominal massage (discussed further in Chapter 6).
This economic evaluation has certain limitations. First, excluded drug costs and out-of-pocket costs were excluded because very few participants responded to these questions. The participants who responded reported very low costs per patient and it is not expected that inclusion of these would have a large impact on our results. Second, subgroup analysis, by estimating these models on a subset of participants, was not performed. This was decided against because regression results (available on request) did not show a statistically significant impact of participant characteristics, such as age and sex, on costs and QALYs gained. Finally, the results were not extrapolated over a horizon longer than 6 months. The results suggest that the impact of the intervention on quality of life as measured by the EQ-5D-5L is small and significant differences in the probability of cost-effectiveness are not expected if the trial lasted for a longer period.
Chapter 5 Process evaluation
Introduction
The AMBER trial included a process evaluation, in line with advice from the Medical Research Council guidance for evaluating complex interventions. 51,52 This was informed by realist evaluation methodology, which goes beyond the evaluation question ‘What works?’ to ‘What works, for whom and in what context?’53 The aim of this approach is to situate and explain outcomes within the contexts in which they are achieved in order to explain potential discrepancies between expected and observed outcomes, and to assess fidelity to implementation processes. Furthermore, results of the process evaluation also provide data to inform the optimisation of the intervention and seek to explore potential routes to sustainable implementation. The process evaluation follows a longitudinal case study design. 54,55
The objectives of the process evaluation are to explore:
-
fidelity to processes of implementation of the trial intervention
-
implementation contexts (including settings, demographics and implementation processes, delivery and take up of the intervention, and adherence and non-completion)
-
intervention optimisation and sustainability beyond the life of the funded project.
This chapter presents the research methods for the process evaluation, the results of the analysis of data from interviews, bowel diaries and telephone support recordings. The chapter ends with practice recommendations.
Methods
Recruitment and sampling
People with multiple sclerosis
A total of 20 PwMS taking part in the trial, and randomised to the intervention group, were selected via convenience sampling. 56 The small numbers recruited into the trial from each site meant that our intended purposive sampling strategy was not feasible. However, as Figure 1 illustrates, the sample achieved a variation in terms of geographical location, age, MS type and different stages of the disease progression. This sample variation facilitated the exploration of hypothesised context–mechanism–outcome (CMO) configurations. We did not adhere rigidly to definitions of ‘contexts’ or ‘mechanisms,’ since previous experience of using this approach informed us that contexts can become active mechanisms that promote change in some instances and, at other times, mechanisms of action can be better conceptualised as contexts. 57,58 For instance, sampling reflected the following hypotheses: that there could be sex differences in the acceptability of massage, severity of MS might have an impact on ability to do the massage and length of time living with MS may also contribute to attitudes towards self-care. This sample is reflective of the characteristics of trial participants as a whole, with those aged > 50 years and women being the most prominent. The mean age for the intervention group participants was 52.2 years (51.3 years for those in the control group); 84.4% of intervention participants were female (78.8% for the control group). Of the interview participants, 16 lived in the north of England, reflecting the fact that half of the 12 sites involved in the study were located in that part of the UK. Table 27 provides further details on the characteristics of those interviewed. Figure 10 shows the flow chart for the process evaluation.
| Characteristic | Number of participants |
|---|---|
| Age range (years) | |
| < 21 | 0 |
| 21–30 | 0 |
| 31–40 | 1 |
| 41–50 | 4 |
| 51–60 | 7 |
| > 60 | 8 |
| Sex | |
| Male | 1 |
| Female | 19 |
| Employment status | |
| Unemployed | 2 |
| Employed | 3 |
| Business owner | 1 |
| Retired (on ill-health basis) | 12 |
| Retired (reached retirement age) | 2 |
| Geographical location | |
| West Scotland | 3 |
| North-west England | 10 |
| North-east England | 6 |
| South-east England | 1 |
| Type of MS | |
| Benign | 0 |
| Relapsing–remitting | 11 |
| Secondary progressive | 8 |
| Primary progressive | 1 |
| Years with MSa | |
| < 5 | 3 |
| 5–10 | 3 |
| 11–20 | 6 |
| 21–30 | 3 |
| > 30 | 6 |
FIGURE 10.
Flow chart for the process evaluation.
By the time of the first interview, participants had been enrolled in the trial for ≈4 weeks. All those who took part in a first interview agreed to participate in a second interview. A total of 20 PwMS were interviewed twice, giving a total of 40 interviews.
Health-care professionals
Forty-two interviews were conducted with 25 different HCPs. One or two people involved in delivering the AMBER trial from each of the 12 sites were recruited to interviews. The range of involvement in the trial varied from those who delivered the massage training and/or the participant follow-up to the local principal investigators, whose role centred on identifying suitable patients for the trial. Appendix 10 documents the roles of the HCPs interviewed and the number of interviews. This sample was also designed to reflect the realist evaluation approach.
Initial interviews with HCPs were usually conducted within a few weeks of recruiting their first intervention participant. Owing to variation in site initiation and trial recruitment, initial interviews took place between January 2015 and March 2016. During this first stage, 23 staff members were interviewed.
Second interviews were sought with staff members who were actively involved in patient training and follow-up, in order to explore any problems that may have arisen since the first stage of interviewing. In a small number of cases, a different staff member who had since become more involved in the trial or delivery of the treatment was selected for interview at the second stage. Nineteen staff members were interviewed in the second stage of interviews.
Stakeholders
Six interviews were carried out with stakeholders purposefully selected for their expertise in neurological and incontinence treatment provision, policy-making and service development. The process of identifying suitable interviewees began with research into neurological and incontinence services throughout the UK. Snowball sampling was also used, meaning those interviewed recommended other potential interviewees. Appendix 10 illustrates the types of organisations that stakeholders came from and the number of interviews.
Fifteen potential interviewees were contacted and six people were interviewed after giving informed consent. The interviews took place from early to mid-2016, in order to capture their knowledge and insights into recent policy and clinical developments related to the treatment of MS.
Data collection
Interviews
Data collection consisted of qualitative semistructured interviews, allowing the views and ‘lived experiences’ of participants to be explored. 59 Interviews drew on topic guides (see Appendix 11), taking an iterative approach to allow unanticipated themes to be explored in subsequent interviews. 59 All interviews were conducted by telephone to save on travel costs, as interviewees lived in locations throughout the UK.
The purpose of the interviews with trial participants was to explore experiences of living with MS and bowel problems, as well as experiences of taking part in the trial. The second stage of interviews focused on exploring any change in symptoms, any adaptations that they had made to the massage, further thoughts on taking part in the trial and whether or not they intended to continue with abdominal massage at the end of the trial. Appendix 11 details the main topics covered in each stage of interviews and Appendix 12 details the interview schedules and site questionnaires.
The aim of the interviews with HCPs was to explore any issues with the training processes (both their own training in massage as well as delivering massage training to trial participants), trial delivery and implementation. The first stage of interviews aimed to detect any problems faced in delivering the trial and anything that worked well. The original interview transcripts were used to write the topic guide for the second stage of HCP interviewing. Second stage interviews gathered an overview of staff members’ experiences in participant recruitment, the delivery of massage training and advice and participant follow-up. Appendix 11 gives an indication of the topics explored during both stages of interviews with HCPs.
Stakeholder interviews explored ways to implement the abdominal massage intervention on a larger scale and any potential challenges to doing so. Background research was conducted into the organisations for which the interviewees worked. Current neurological and incontinence policy developments at local, regional and national levels were also extensively researched to ensure that appropriate, targeted questions were asked during the interviews. Although wider policy developments and trends were acknowledged during interviews, the focus remained on those that may facilitate or hinder the possible implementation of abdominal massage treatment. Appendix 11 gives an indication of the range of topics covered in stakeholder interviews; although this varied in accordance with the expertise of each organisation and interviewee.
Data analysis
Interview recordings were transcribed verbatim. Data were analysed by drawing on an adapted framework approach. 60 This followed an initial categorising and coding process similar to thematic analysis, using the qualitative data analysis software NVivo version 10 (QSR International, Warrington, UK). Coding and all further stages of analysis were conducted by Selina Doran (SD) and checked for consistency by Fiona Harris (FH). Analysis was conducted continuously with interviewing, contributing to the iterative process, allowing emerging themes of interest to be explored further in future interviews. The initial coding was then transferred into framework matrices for the three categories of interviewees. These summary tables explored within-case issues, in which each trial site was considered as a case, that is, the unit for analysis. During the analysis, attention was paid to any contextual differences that could have an impact on outcomes. Cross-case comparison then identified higher-level themes that explored facilitating contexts and mechanisms, and any barriers to successful implementation. The matrices also tracked the process of change over time, taking account of the longitudinal aspect of interviews. Interviews with PwMS also retained attention to case study sites to ensure that analyses were sensitive to any contextual variations. However, given the small numbers recruited from each site, participants have not been identified by recruitment location. Identifiers in quotes simply read as, for example, PwMS1 or HCP1. When second interviews were conducted with the same person, the identifier illustrates this by the additional number so that, for instance, the second interview with PwMS 1 would be identified as PwMS1_2. Bowel diaries were also analysed for those participants who reported either no improvement or only a temporary improvement in bowel symptoms. To ensure validity and reliability of the analyses, these tables were deliberated within the process evaluation team (SD and FH), and received further clinical input from the wider study team.
Results
This section presents the analysis of interviews with trial participants alongside bowel diary analysis, and analysis of interviews with HCPs and higher-level stakeholders. The within-case descriptive data informed the beginning of Chapter 5; here the focus is on presenting experiences of trial participation and a cross-case comparison of implementation issues drawn from interviews with HCPs.
Bowel dysfunction and treatment
We begin this section by exploring the experiences of interviewees, who provided some powerful narratives of how living with NBD affects their everyday lives. This is followed by views from HCPs and stakeholders on current treatments and the delivery of care for constipation.
Living with bowel dysfunction
Trial participants revealed the extent to which quality of life is impeded by severe constipation, which might range from passing a stool four times per week to passing a stool once a fortnight. Bowel problems, coupled with their impact on other symptoms, had a negative impact on the quality of their lives:
My whole life is ruled by my bowels – that’s all I think about every day, 24/7.
PwMS20_1
One person explained that they felt thus:
I can go for days without having to go to the toilet, it can be, like, a week, and of course my stomach ends up bloated away out to here and then you get worried that if you go out somewhere, that you’re going to have to make a quick dash to the toilet, and then when you’re there you can be there for ages. So if you’re out with friends and you disappear to the toilet, you’re stressed ‘cause you think, ‘God, they’re going to wonder where I am, what’s happened?’ and it becomes embarrassing then, and I have, on maybe two occasions, actually had an accident when I’ve been out and it’s just been an absolute nightmare, so you’ve got to try and plan ahead, you know, to work round it.
PwMS11_1
This sometimes resulted in taking extreme measures to cope with symptoms. For instance, some participants avoided eating at certain times because of the uncertainty of when they would next pass a stool:
I can’t eat because I’m scared, I can’t eat because I don’t know when I . . . if we’re going on holiday I can’t eat because I don’t know when I can go to the toilet again or what the toilet facilities are when you go away and things like that. And when I went [on holiday] . . . I only ate [for] 2 of those 6 days and I managed, even though I was really, really hungry.
PwMS20_1
Constipation was linked to laxative usage, which could also result in negative side effects. Interviewees reported that taking laxatives caused pain, sleeplessness, cramps and diarrhoea. The unpredictability of passing a stool when taking laxatives meant that a number of patients became very concerned about their potential for bowel accidents, sometimes not leaving home for days because of the lack of control. For some people, this can lead to social isolation:
At the moment it’s absolutely disastrous, like today I won’t even answer the doorbell if the doorbell rings. . . . That’s what happens when I have a day that I know I’m going to spend it hanging around, hovering, trying to go to the toilet, I can’t even answer the door, I can’t leave the toilet ‘cause I’m scared I’ll have an accident, . . . I have no control whatsoever.
PwMS20_1
Current delivery of care for constipation
Health-care professional and stakeholder interviews revealed consistent views that abdominal massage could provide a useful addition to current treatments that commonly involve oral therapies. Abdominal massage has a number of positive attributes as a form of treatment: it is cheap, low technology, simple, non-invasive and can be used anywhere.
However, stakeholder interviews revealed that most patients are underinformed about bowel matters. They stated that people do not fully understand constipation; thus, in some cases, they do not report it as an issue until it is quite advanced. Furthermore, HCPs reported that constipation is not discussed enough, and patients may not connect this to their MS. There is also a lack of evidence and education around treatment for bowel problems. In addition, the huge workloads of MS nurses have led to increasing numbers of MS patients being managed by general nurses (Stakeholder 1 and Stakeholder 4). As one stakeholder put it:
Excellence in continence care comes from a degree of specialists, who would be able to make better decisions and better treatment plans at a local level.
Stakeholder 6
Ten out of the 12 sites did not currently use abdominal massage as a form of treatment for bowel problems. Although one site had a bowel specialist who offered some form of abdominal massage, MS patients were only referred to her when their bowel problem was at a critical stage. The other site currently using abdominal massage offered specialist bowel clinics as part of a community service. This meant that this site struggled to find PwMS who had not used abdominal massage before to recruit into the trial. One further site had previously used abdominal massage as part of the continence service; however, only one member of staff had been trained in it and had since left, so massage treatment was no longer offered.
Experiencing and delivering the intervention
In this section, we explore how the intervention was experienced by participants, and issues around implementation. Interviews gathered data on various aspects of trial experience and delivery that may inform any future implementation of abdominal massage as a treatment for PwMS. This included experiences of massage training, supporting materials and telephone support. This is briefly reported in the following sections.
Recruitment and retention
By the end of recruitment, four sites had recruited to or exceeded their targets. The main reasons for failing to meet recruitment targets centred on understaffing and time constraints. At one site, recruitment was entirely reliant on one research nurse, who was also working on 15 other trials. This meant that she did not have the time to repeatedly chase patients and was sometimes not available to see them in clinic. The incorporation of the AMBER trial into the existing workloads of staff members also proved troublesome in some cases, with an already limited staff capacity further stretched unexpectedly by sickness absence or resignations.
Sites that did not meet their recruitment targets were, nevertheless, usually successful in retaining those that were enrolled in the trial. Retention was affected by being patient-centred in approach during baseline appointments. In one site, both staff members involved attended every baseline appointment: one demonstrated the massage technique while the other checked the paperwork. These were also all home visits, because that was more convenient for the participants. Another site that conducted home visits reported the unexpected benefit of establishing a better understanding of patients’ home contexts:
It helped to see them at home because you had an idea about their home set-up – how chaotic their lives were.
HCP19_2
Probably the most important contributor to retention was the relationships established between those delivering the trial and the participants. For some sites, this was facilitated by the fact that participants already knew the staff members involved. One way of enhancing the rapport with participants was to have the same staff member carrying out the weekly calls:
I think it’s just getting to know me and feeling comfortable with giving me information.
HCP21_2
Being supportive and offering advice during the first appointment and follow-up also appeared to enhance retention.
Massage training
Delivery of massage training by health-care professionals
The massage training delivered to participants during the baseline appointment was a critical component of the trial. Staff members had a variety of techniques: showing participants how to position their hands during the massage, administering the technique on them to give them an idea about pressure, watching participants do the massage themselves and commenting on it. A problem that arose during this initial training was that some people preferred the massage to be administered over their clothes or on body parts other than their stomach. This may have affected how the staff member was able to deliver the training, since the massage should be administered on the abdomen in order to allow them to gauge pressure. As per the AMBER trial protocol, the staff member advised that a carer or spouse could administer or assist with the massage, or that they could use their fist as opposed to a flat hand in order to provide more pressure.
Trial participants
All 20 trial participants agreed that the massage training and follow-up materials were very useful. For most participants the training comprised being given the AMBER trial DVD, having the massage demonstrated on them and then engaging in supervised practice. One-quarter of interviewees had their partner present at the baseline appointment to receive the training:
If there’s a day where I’m not capable of doing it or can’t remember a bit, then I’m covered.
PwMS3_1
Most interviewees were very positive about the massage video:
The DVD is brilliant. It is so simple and easy to understand.
PwMS12_1
Engagement with the DVD was enhanced by the woman demonstrating the massage in the video, who appeared to normalise the technique:
She’s a real person . . . when I saw her I immediately relaxed and felt I could do it.
PwMS3_1
Feedback on the video was positive and, after initial viewings, most people used the quick reference guides as aids during the administration of the massage. The quick reference guides were found to be helpful, clear, portable and ideal to use while administering the massage. The few negative comments about the DVD were related to the format, as some participants found that their copy of the DVD would not work on their DVD players and had to seek alternative copies. Others preferred to use the information leaflet as it was easier to carry around and refer to.
Experiences of doing the massage
The frequency of administering the massage varied, with participants fitting the massage around their daily routines. A number stated a preference for administering the massage last thing at night, as part of their bedtime routine:
It’s part of my ritual now, before I go to bed.
PwMS15_2
However, all participants adjusted the frequency and timing of their massage routine based on their stamina levels and personal circumstances. Circumstances that interfered with the massage routine included grandchildren visiting, a house refurbishment, family bereavement and festive periods, such as Christmas. Health problems that affected adherence included diarrhoea, vomiting and bladder infections.
Physical weakness and numbness in fingers, hands and arms caused by the MS posed another challenge, which led to several participants adapting the massage technique to suit their abilities or enlisting the help of a partner. Adaptations included administering the massage first thing in the morning (when feeling stronger), using one hand to guide the other and asking a partner to assist with parts or the whole of the massage routine.
As Table 28 illustrates, these results were drawn on to establish some key CMO configurations that might impede or facilitate positive impacts. Those contextual factors that might facilitate positive outcomes are linked to the adaptability of participants, with those who demonstrate an ability to adapt either the massage techniques or the massage oil to suit their own capabilities and preferences.
| Context | Mechanism/action | Outcome |
|---|---|---|
| Severity of MS | Ability to do massage | Impedes adherence or effective massage technique, unless administered by a carer |
| Physical weakness/mobility issues/fatigue | Adaptability and commitment to continue | Achieves adherence via adaptations to massage technique or enlisting help |
| Greasy massage oil leads to increased time involved (e.g. showering after massage); may reduce adherence | Adaptability and commitment to continue: use alternative massage products | Continued adherence; no lubricant being used may lead to poor massage technique and negative outcomes |
Supporting and experiencing the trial
Site staff made weekly calls to participants, in both the intervention and the control groups, for a period of 6 weeks and then again at the 24-week stage. Fourteen of these calls were audio-recorded from 8 out of the 12 sites in order to check fidelity to the AMBER trial support call protocol. Calls were not recorded at the remaining sites because of the timing of patient calls, the site not completing the calls (the AMBER trial office took over this task for one site) or the availability of the recording equipment. In the sample of recorded calls, one was in week 24 and the remainder took place in weeks 1–6. The support calls were structured by a CRF guide of questions and, at the 24-week stage, questions were asked about issues relating to the primary outcome measure. Questions covered the following three areas:
-
participant experience – changes to bowel habits, health condition and personal circumstances; self-administration of the massage (if applicable)
-
managing symptoms – usage of medication and laxatives; any changes to diet, fluid, exercise and positioning on the toilet seat
-
delivery of the trial – more practical aspects, such as time and staffing arrangements for weekly calls and completing and returning paperwork.
However, from the 18 recordings it was identified that, in some instances, these calls were not as supportive in discussing the lifestyle changes and/or the massage as had been anticipated and were more of a tick-box exercise. In an effort to improve the quality of support, the control and intervention participant calls completed by one site, which were deemed by the AMBER trial office to be exemplary, were transcribed, anonymised and circulated to all sites as a good practice guide.
Further review of these transcripts highlighted a number of strategies that may have enhanced participant engagement in the trial. Some HCPs referred to the results from the previous call or information in participants’ forms, highlighting the value of providing information about their condition. Callers gave positive reinforcement to encourage positive lifestyle change, explored diet and fluid intake and advised on massage technique. Some of the callers reminded participants to complete and return paperwork and advised on how to complete the questionnaire.
The weekly telephone calls from sites were positively received by participants. The additional support available from staff members seemed to be the main attraction, as one person stated:
It’s nice to think there’s somebody out there that’s listening and helping.
PwMS2_1
Participants found the advice useful and supportive; this may have been one of the reasons that trial retention was high within the control group. The only minor points of criticism came from a few trial participants who became anxious over missed calls and one person was initially given the wrong dates, which led to waiting in for calls that never came:
We had a wee bit of a hiccup with the first one, I was sitting waiting because it was written down as the 19th and it was written down as a Monday and I remember her saying about a Monday and I sat for about an hour and nobody phoned.
PwMS13_1
However, this initial mix up with dates was sorted out and subsequent calls were received when expected.
Participants’ reactions to the trial paperwork were mixed. Although some participants found the paperwork tiring and burdensome, a number of people felt that completing the bowel diary was invaluable for keeping track of progress and may have encouraged adherence to the massage:
You forget and think ‘did I go or did I not go?’ and then you have a look back and you’re like ‘oh, yeah, I did’.
PwMS9_1
During the second stage of interviews, some people said that they would have liked to have continued completing the bowel diaries, as this acted as a record.
The consensus from HCPs was that the paperwork was fairly straightforward for participants to complete, with the exception of one person who had severe visual impairment. In this case, a staff member completed the paperwork during the baseline appointment and the participant developed their own bowel diary. This issue may have been mitigated by large print materials for visually impaired participants.
A number of suggestions were made by HCPs to improve the bowel diaries. These included being more explicit about when they should be started and picking up an error in the reporting of stool frequency, which added up to > 100%. It was also reported that bowel diaries did not fully capture bowel habits; for instance, although it recorded the number of attempts to pass a stool, it did not record how many times this led to a bowel movement. Another area of confusion was the options for laxative usage of ‘usual,’ ‘less’ and ‘more’, and the lack of a category for no laxatives.
For whom does abdominal massage work?
Positive improvements related to massage
Three-quarters of those interviewed (n = 15) reported improvements in their bowels as a result of the massage. The main benefit seemed to be a feeling of empowerment and control over their bowel habits:
I know when I get to the toilet I’m going to have a bowel movement.
PwMS17_1
By the time of the second-stage interviews, one participant said:
I have to make myself look back to see how bad things were because there’s a terrific improvement.
PwMS1_2
This was the case for many people in the second stage of interviewing, with them passing the ‘ideal types’ of stools (type 3 or 4) more frequently, and with less pain. For example, one participant who mainly passed stool types 1 or 2 every 3 to 4 days or longer before the trial stated:
It was just awful. I’d sit on the toilet for ages and ages and ages just knowing that I had to do something and it was painful.
PwMS17_2
However, by the second interview, she had a bowel movement of stool type 3 or 4 every day. Others reported being able to stop taking laxatives and an increased frequency of bowel movements or, as one person reported, the time spent trying to pass a stool changed from 3 hours to only 5 minutes.
Although improvements were not so dramatic for others, one of the consequences of even small improvements was, for instance, a reduction in anxiety about potential impaction and hospitalisation. Notably, when participants stopped doing the massage because of personal circumstances, any improvements in symptoms were lost:
In some ways that was a positive thing because it proves that while I wasn’t doing it [the massage] things deteriorated.
PwMS3_2
Other reported benefits from doing the massage included feeling less bloated, clothing becoming looser and a decrease in sluggishness, which reduced fatigue levels. As one person reported:
I don‘t think I’ve been tired since I’ve been on this [trial].
PwMS14_1
Some participants were also able to stop or reduce laxative usage, which was reported to disrupt sleep patterns. Improved diet was also noted by some participants, which was particularly important for those who ate very little because of their bowel problems. One person, who had a diminished appetite at the beginning of the trial, stated that by the end:
It’s weird to say I feel hungry, even saying the word starving.
PwMS12_1
At the second stage of interviews, all 15 people who had reported some improvements agreed that participating in the trial had been worthwhile.
Exploring reasons for no improvement
Five interviewees reported no improvement arising from the massage. A complete set of bowel diaries for these participants was analysed alongside their interview data in order to determine whether or not this captured any changes in bowel habits, and to explore potential explanations for the lack of improvement.
A higher severity of MS and body numbness may well have interfered with the ability to administer the massage effectively. Indeed, one stakeholder interviewee reported that effectiveness of abdominal massage is likely to vary:
Some people find it beneficial and some people don’t find it that effective. I think it depends on how advanced the MS is.
Stakeholder 3
Furthermore, when delivering the massage training, one HCP expressed doubts as to the likely effectiveness of the massage, attributable to the reduced physical dexterity of some participants:
Some of them, when you’re watching them doing it and you’re thinking ‘how effective is that going to be?’
HCP19_2
Another person who found no improvement experienced a worsening of their MS during the course of the trial, perhaps impeding the potential to show improvement. Although bowel diary data support the reported lack of improvement, the interview data reveals that in fact this person, unlike before the massage, now had sensation and was able to feel the urge to pass a stool. This was perceived as a positive change related to the massage that, nevertheless, would not have been captured by trial outcome measures. Unfortunately, this person’s condition worsened during the course of the trial and they reported the impact of worsening symptoms thus:
I find now I’ve sort of got quite a bit worse and I find if I get down and do these massages I can’t get up again, I have real trouble getting up. So I’ve had to stop doing them which was a shame really.
PwMS5_2
Two further participants showed no improvement on the main indicators for constipation and consistently responded ‘no’ to the question ‘Do you feel you have emptied your bowel?’ However, both of these participants reported ‘ideal stool types’ at baseline, indicating that the severity of constipation was not significant enough to demonstrate any improvement.
Another participant felt that, although the treatment worked initially, this effect was not sustained:
It started to work a little bit, that was really good; unfortunately, it didn’t last.
PwMS16_2
The initial benefit was improved control over when bowel movements happened, which in her case meant that she no longer remained at home for 3 days at a time in fear of bowel accidents. Her bowel diaries revealed that she only ever passed type 1 stools and that there was no noticeable improvement in frequency, stool type or amount of time spent passing stool throughout the course of the trial. As this participant was wheelchair bound, mobility issues may well have played a part in her ability to effectively administer the massage.
Despite a perceived lack of impact, these five participants remained in the trial. This may be attributable to their overall trial experience being positive. Indeed, perhaps a ‘positive outlook’ could explain why three of these participants expressed the intention to continue with the massage after the trial, in case it did eventually work.
A coding matrix was applied to all interview transcripts from trial participants to explore attributes, such as severity of MS, severity of constipation and massage adherence. This revealed contextual factors that affected outcomes, as illustrated in Table 29.
| Context | Mechanism/impact | Outcome |
|---|---|---|
| High severity of MS: reduced mobility, fatigue, severe constipation, numbness and lack of sensation | Reduced ability to massage effectively or apply correct pressure unless carer administers the massage; high severity of bowel problem means it is difficult to show an improvement | No improvement (n = 5) |
| Bowel diary reports show ideal stool type, reasonable frequency and duration on toilet | Bowel diary cannot demonstrate improvement as baseline recorded as ‘ideal’ with no capacity to demonstrate benefit |
Further analysis was undertaken to explore whether or not expectations of the trial might have been linked to perceived impact, as a means of exploring whether or not ‘hopefulness’ might have an association with subsequent outcomes. The majority of those interviewed had no previous knowledge about abdominal massage and were unsure of what to expect. As a result of this, around two-thirds of participants initially felt doubt about whether or not the massage would help them. This was especially true for those for whom other forms of treatment had failed:
Because nothing else had worked like the Senna tablets and stuff, I was a bit like ‘well, I wonder if it is going to work?’
PwMS15_2
Although frequency data must be approached with extreme caution when reporting on such a small sample, Table 30 serves to illustrate the likelihood that there was no association between expectations and trial outcomes.
| Expectation (n) | |||
|---|---|---|---|
| Hopeful of improvement | Sceptical/no expectation of improvement | ||
| Positive impact | No impact | Positive impact | No impact |
| 6 | 2 | 9 | 3 |
Contextualising the trial primary outcome
One further piece of analysis was conducted in order to explore the discrepancy between the statistical analysis of the NBD total score and self-reported outcomes of the trial. The NBD total scores for all interviewees were matched up with data from interviews. Table 31 illustrates the discrepancies found by comparing these data. Although 15 interviewees reported some improvements related to the massage, only seven participants recorded an improved NBD score between baseline and week 24, six participants showed no change and seven worsened over the course of the trial. However, of those seven participants who showed improved NBDSs, two of these interviewees self-reported as not having experienced any improvement in their symptoms. There are several potential explanations for these discrepancies. First, both the interview and the NBDS questionnaire captured snapshots of participant experience tied into particular weeks. Although interviews roughly followed the same time point as the trial measures, they were within the same month rather than completed at the same time as the trial measures. Second, and perhaps more importantly, the NBDS questionnaire did not necessarily explore those aspects of participant experience that affected quality of life more generally, hence the discrepancy between self-reported outcomes and those measured by the NBD questionnaire.
| Identification | Type of MS | NBDS total score | Adherence to massage reported in interviews | Perceived impact |
|---|---|---|---|---|
| PwMS6 | Relapsing–remitting | Baseline, n = 13 |
|
No perceived impact. Continuing to take laxatives. Bowel diary shows ideal stool type and frequency of three or four stools per week |
| Week 6, n = 7 | ||||
| Week 24, n = 8 | ||||
| PwMS16 | Relapsing–remitting | Baseline, n = 6 | Daily for 18 weeks then stopped |
|
| Week 6, n = 13 | ||||
| Week 24, n = 9 | ||||
| PwMS18 | Secondary progressive | Baseline, n = 5 | Sporadic. A few times a week up to week 6 | No perceived improvement. On medication for bladder problems; bowel diary shows ideal stool, regular frequency but not feeling full evacuation |
| Week 6, n = 5 | ||||
| Week 24, n = 5 | ||||
| PwMS20 | Secondary progressive | Baseline, n = 12 | Daily | No perceived improvement. High severity of MS and body numbness means massage likely not to be effective; does not seek help with massage |
| Week 6, n = 21 | ||||
| Week 24, n = 24 | ||||
| PwMS5 | Secondary progressive | Baseline, n = 3 | Sporadic. Had problems with the massage oil and administering the massage |
|
| Week 6, n = NR | ||||
| Week 24, n = 3 | ||||
| PwMS8 | Secondary progressive | Baseline, n = 14 | Two or three times a week | Slight improvement. It still takes a long time to pass a stool but massage seems to help and she has reduced stomach pain |
| Week 6, n = 19 | ||||
| Week 24, n = 13 | ||||
| PwMS1 | Primary progressive | Baseline, n = 16 | Daily (carer assistance) | Improved: passing a stool daily. Decreased laxative usage. Better sleep |
| Week 6, n = 8 | ||||
| Week 24, n = 2 | ||||
| PwMS2 | Secondary progressive | Baseline, n = 1 | Two or three times a day |
|
| Week 6, n = NR | ||||
| Week 24, n = 8 | ||||
| PwMS3 | Relapsing–remitting | Baseline, n = 7 | Five times a week | Improved. Passes a stool every 2 or 3 days with ‘less bother.’ When stopped massage, improvements disappeared |
| Week 6, n = 7 | ||||
| Week 24, n = 7 | ||||
| PwMS4 | Relapsing–remitting | Baseline, n = 1 | Daily | Improved. Passes a stool every 2 or 3 days and she has managed to stop taking laxatives. Improved appetite |
| Week 6, n = 6 | ||||
| Week 24, n = NR | ||||
| PwMS7 | Relapsing–remitting | Baseline, n = 5 | Daily | Improved. Feels that if she starts to feel constipation, the massage clears it. Managed to stop taking oral constipation solution |
| Week 6, n = 6 | ||||
| Week 24, n = 5 | ||||
| PwMS9 | Relapsing–remitting | Baseline, n = 5 | Every second day | Improved. Passes stool three times a week and has stopped taking laxatives. Without massage, there is no movement at all |
| Week 6, n = NR | ||||
| Week 24, n = 3 | ||||
| PwMS10 | Secondary progressive | Baseline, n = 5 | Daily | Improved. Passes stool every second day. Greater sensation of emptying, less bloating, improved appetite and energy levels |
| Week 6, n = 11 | ||||
| Week 24, n = 3 | ||||
| PwMS11 | Relapsing–remitting | Baseline, n = 3 | Daily | Improved. Feels it is easier to pass a stool |
| Week 6, n = 11 | ||||
| Week 24, n = 8 | ||||
| PwMS12 | Relapsing–remitting | Baseline, n = 11 | Daily |
|
| Week 6, n = 13 | ||||
| Week 24, n = 11 | ||||
| PwMS13 | Secondary progressive | Baseline, n = 21 | Five times a week | Improved. Passes a stool once a week with more ease and it takes less time (reduced from 3 hours to 5 minutes). Has lost weight since being on the trial (regarded as positive) |
| Week 6, n = 13 | ||||
| Week 24, n = 8 | ||||
| PwMS14 | Relapsing–remitting | Baseline, n = 11 | Every second day | Improved. Passes a stool three or four times a week. Stopped massage during trial because of bladder infections but plans to resume as felt that it worked |
| Week 6, n = 7 | ||||
| Week 24, n = 14 | ||||
| PwMS15 | Secondary progressive | Baseline, n = 3 | Daily |
|
| Week 6, n = 11 | ||||
| Week 24, n = 7 | ||||
| PwMS17 | Relapsing–remitting | Baseline, n = 7 | Three times a week | Improved. Passes a stool every day with more ease and stopped laxatives. Feels lighter |
| Week 6, n = 0 | ||||
| Week 24, n = 4 | ||||
| PwMS19 | Relapsing–remitting | Baseline, n = 7 | Daily (carer-led massage) | Improved. Passes a stool every 6 days and has reduced abdominal pain, less bloating |
| Week 6, n = NR | ||||
| Week 24, n = 14 |
Post-trial intentions
From discussions with participants during second-stage interviews, it appeared that all but two of them intended to continue with the massage. This is either because it worked for them or they hoped that it would work in the future. The massage seemed to have been incorporated seamlessly into their routines.
Views on potential future implementation
This section explores the views of both HCPs and stakeholders regarding the potential to implement the intervention within the NHS. These data are situated within the larger body of work conducted by the process evaluation team, and interviews were done on the premise that the intervention may demonstrate effectiveness and were carried out before the results of the trial were known.
Health-care professionals in the majority of sites reported issues related to staff capacity, which represents a potential barrier to future implementation of abdominal self-massage within the NHS.
Across all services in NHS England and Scotland, there are threats to the specialist nurse role and other positions through despecialisation, redundancy and not replacing staff members when they leave. At one site, there was a problem with staff recruitment and retention, which resulted in a reliance on agency staff to deliver the service.
To successfully implement abdominal massage as a form of treatment, staff members would require reassurance regarding workload and capacity. There was a perception that this would require additional resources, although in many cases this might replace more invasive, time-consuming treatments and could reduce the ‘revolving door’ of hospital admission or attendance for severe constipation. There were also concerns that if massage training was extended to other patient groups, there could be a resulting marked increase in referrals:
We could potentially be inundated with patients.
HCP16_2
There was also some debate about which staff members would be best placed to deliver the massage training. It was suggested that massage training could be offered by the colorectal and continence team in hospitals, or built into existing MS or continence clinics. This could be coupled with the DVD and written training materials to assist people in learning the technique. Furthermore, it was suggested that the massage could possibly benefit patients on bed rest or in nursing homes:
That could be something we teach them, to look after more progressive patients.
HCP18_2
It was suggested by some sites that community and district nurses could become involved in training people in massage in their homes. Indeed, there were indications that abdominal massage could be introduced to a wider population in a range of health-care and community settings.
Enthusiasm for abdominal massage was such that a number of sites indicated a willingness to incorporate it into current services. For instance, a HCP from one site who had set up a bowel clinic during the trial said that, since the staff members who delivered the AMBER trial were already trained in the massage, this could be rolled out more widely. Interest was also expressed in distributing training materials and engaging in additional training.
Stakeholders indicated that there are a number of policy and capacity issues affecting the potential for abdominal massage to be successfully implemented within the NHS. Evidence of clinical effectiveness is required in order to convince commissioners and other services to support this new treatment option. Moreover, in the current NHS environment, health boards and trusts are ‘just looking for a short-term gain from cost savings’ (Stakeholder 6), thus evidence of cost-effectiveness is also important.
The case for abdominal massage needs to be persuasive to convince Clinical Commissioning Groups, HCPs and other services to adopt this intervention if it was found to be clinically effective. This might highlight, for instance, the potential for abdominal massage to free up capacity within GP, continence and MS services by dealing with bowel problems before they deteriorate (Stakeholder 4 and Stakeholder 5). Training in the massage could be framed as upskilling staff members:
It should be career-enhancing rather than career-threatening for those involved.
Stakeholder 6
It could also reduce the costs and capacity issues associated with long-term bowel damage: unplanned hospital admissions, bed occupancy and the prescribing of medications/other forms of treatment (Stakeholder 1, Stakeholder 2 and Stakeholder 6). Secondly, there would be the potential to improve quality of life for patients:
It’s the cost to the patient of the indignity of having bowel management such as a suppository.
Stakeholder 2
A potential barrier to implementation is the challenge of ‘getting something from best practice to common practice’ (Stakeholder 4). First, massage treatment would probably be most useful if administered at an early stage in the development of constipation problems, rather than when patients are at crisis point (Stakeholder 3). GPs are integral to this process:
They see the whole person and spot potential issues around bowel dysfunction at a much earlier stage.
Stakeholder 6
A health-care assessment for each individual needs to be carried out to determine what is causing the constipation and to ensure that abdominal massage is the right treatment for them (Stakeholder 2). Furthermore, one stakeholder suggested that abdominal massage should be part of an individual’s self-management programme, supplemented by advice around fluid intake, changes to diet and exercise suggestions (Stakeholder 5). The use of tracking tools like bowel diaries can help demonstrate how things have progressed (Stakeholder 2 and Stakeholder 3).
Stakeholders felt that abdominal massage could be taught by community nurses or other HCPs, such as general nurses, physiotherapists and continence advisors (Stakeholder 3). They also suggested that consideration should be given as to how this treatment could be taught to those who are severely disabled and unlikely to be able to self-massage (e.g. those in care homes). As reported by HCPs above, this suggests the need for staff members at care homes to be trained in the carer-led massage.
Stakeholder interviewees suggested ways of disseminating the massage training materials to reach the people who matter most: those with NBD and HCPs dealing with these issues. To that end, PwMS would need to be given access to videos and other training resources, and HCPs would need to be trained in the intervention and how to teach it. Stakeholders suggested that a number of key organisations should be included in approaches to dissemination of training and, when appropriate, training materials could be hosted on their websites:
-
National Institute for Health and Care Excellence (NICE)
-
NHS England
-
Royal College of Nursing
-
Association for Continence Advice
-
MS Society
-
MS Trust
-
Association of British Neurologists
-
UK MS Nurse Association.
Process evaluation discussion
The experiences of PwMS provide powerful illustrations of how constipation can have a wide-ranging, negative effect on their everyday lives. Quality of life is severely impaired, to the extent that some people are afraid to leave their homes and may end up socially isolated, anxious and in pain. Interviews revealed the vicious cycle of symptoms, such as reducing food intake and disturbed sleep as a result of laxative use, and the fatigue commonly associated with MS17 was exacerbated by constipation. Despite the symptom burden, there are few evidence-based treatments available and the cost to the NHS of dealing with constipation is substantial. 61
The findings from the process evaluation demonstrate the potential for abdominal massage to be adopted as a bowel management technique more widely within the NHS. Seventy-five per cent of interviewees (n = 15) experienced improvements in their symptoms, and we were able to offer possible explanations for the lack of improvement in others. In some instances, it would be more appropriate to report that there were only minor improvements rather than none, but these minor improvements were not detectable via primary and secondary outcome measures in the main trial. It seemed that only the more dramatic improvements were able to be captured. The training focused on supporting patients to administer the massage themselves and, although the AMBER trial protocol did include the option for carer-/partner-administered massage, this option may have been missed by those unaware at the outset that they did not have the physical dexterity to perform the massage effectively.
Most of the sites involved in the trial were not currently using abdominal massage as a form of treatment and the consensus, from both HCPs and stakeholders, was that bowel problems in PwMS had to be better managed. The interviews revealed that most participants intended to continue with the massage, demonstrating the acceptability of this noninvasive treatment.
The process evaluation presents results of an approach that draws on longitudinal interviews. Following both trial participants and trial delivery over time allowed us to track processes of change and offer explanations for trial outcomes. The finer nuance achieved by bringing together interviewee experiences with bowel diary data offered insights that suggest that the outcomes may actually be more positive than the statistical analysis suggests. Furthermore, arguably, the AMBER trial achieved greater value by incorporating insights on trial processes. While being mindful of the need to protect trial equipoise, the process evaluation team worked closely with the trial team to establish a feedback loop to report anything that could be easily addressed without affecting the intervention itself. For instance, the time burden associated with compiling information packs was reported back to the trial team and they subsequently changed the format of recruitment packs to address this. This ensured that HCPs within the sites felt listened to and it encouraged them to reflect on the trial and share their feedback with us.
Capacity issues at sites mainly affected recruitment levels in the trial. Understaffing, part-time contracts and hectic workloads meant that staff members were limited in the amount of time that they could spend on the trial. As some sites were already offering massage, this also meant that there were few eligible participants to recruit. Interestingly, it was reported across all sites that participants randomised to the control group of the trial were disappointed, but the retention rate was higher in this group. This may be because the control group had a greater incentive to remain in the trial until the end, when they would be offered the opportunity to learn the massage technique. Control group retention may also have been positively affected by the telephone support offered by site staff. This may also have contributed to fewer differences between intervention and control group outcomes, because site staff gave advice on medications as well as lifestyle.
What may be extrapolated from our process evaluation is that there is the potential for massage to improve the symptoms of NBD; this in turn may then lead to reduced hospitalisations, reduced prescriptions, amount of time HCP spend with patients and improved quality of life for PwMS. The main macrolevel changes from the massage are said, by interviewees, to be freeing up staff capacity in the long term and greater quality of life for PwMS. The argument would need to be made that the short-term work and costs associated with implementing massage training would save money in the long term.
Health-care professional and stakeholder interviewees expressed interest in implementing abdominal massage in the longer term. A number of sites were willing to adopt abdominal massage as a form of treatment and many of these interviewees felt that there was potential to roll this out to wider population groups, in a range of health and community settings. Finally, the words of our trial participants are worth repeating:
It made a terrific difference, in fact I have to make myself look back to see how bad things were because there’s a terrific improvement, yes. It’s made an awful lot of difference to me ‘cause things were really bad to start with, but they’re a lot better now.
PwMS1_2
There are some strengths and limitations to the process evaluation. Strengths include the fact that although we were unable to purposively sample, nevertheless the convenience sample yielded a participant mix that reflected our initial hypotheses regarding time since diagnosis, severity of symptoms and sex. This enabled us to capture robust data from which to explore potential variations in the experiences of trial participation (PwMS), trial delivery (HCPs) and to reflect on issues of potential future implementation and sustainability. The HCP interviews all included at least one staff member involved in delivering the trial at each site. This allowed the researcher to identify any challenges around recruitment or other trial processes that could be fed back to the trial management team. Another strength was the longitudinal nature of the research, thus facilitating scope for follow-up on any problems raised during the original interviews and exploration of the longer-term impact of abdominal massage. This is a methodological innovation rarely accomplished within process evaluations and is a major strength of the AMBER trial. Similarly, interviewing HCPs in two stages provided an opportunity to talk to additional staff members who had become involved in the trial later on, allowing for the exploration of contextual factors, such as change in capacity over time, that might have negatively affected trial delivery and implementation.
There are, however, some limitations to the process evaluation study. Owing to limited resources, only those in the intervention group were recruited for interview; therefore, we could not explore any contexts experienced by control group participants that might also have had an effect on outcomes. Another potential limitation was that those who agreed to be interviewed were likely to have had a higher level of engagement with the trial; thus, they may have been both more likely to fully participate in the intervention and to remain in the trial until its completion. However, our analysis of bowel diaries linked to interview participants revealed that they had variable levels of adherence to the massage. Although all interviewees remained in the trial, interviews with those that did withdraw would have been informative. Finally, participants’ expectations of the trial were not associated with positive or negative outcomes, suggesting that our conclusions regarding their trial experiences and outcomes can be used to interpret wider trial outcomes. As only 12 sites (hospitals in Scotland and England) were involved in the trial, and our stakeholder interview numbers were small, the results cannot be seen to be representative of health-care services across the whole of the UK.
Chapter 6 Discussion
Neurogenic bowel dysfunction in PwMS has a significant impact on quality of life as demonstrated by this quotation:
My whole life is ruled by my bowels – that’s all I think about every day, 24/7.
PwMS20
Despite this, it remains a topic that is often not discussed by patient or clinician and is perceived as an area with few evidence-based treatment options. 16
This is the first large RCT looking at the short- and long-term effects of a supported programme of advice on lifestyle and training in abdominal massage compared with lifestyle advice only. Moreover, this research adds to the evidence on the impact of NBD on quality of life, and identifies the most frequent symptoms and possible mechanisms; it also facilitates the triangulation of information from the quantitative and process evaluation substudies.
Overall, the study recruited to statistical target sample size; the 20% dropout rate was in alignment with that expected in our sample size calculation. The increment in the primary outcome measure, the NBDS,31 favoured the intervention group but was small and did not show a statistically significantly different change between groups at any time point, but we believe that findings of trials should not be described as ‘negative’ on the simplistic metric of whether the p-value is strictly < 0.05. There is a small effect (–1.64 units) that is around two-fifths of the specified MCID (4.21, as specified in the power calculation). We estimated with good precision (i.e. we have narrow 95% CIs) a treatment effect on the primary outcome that was around half the magnitude that we declared as being the estimated MCID, and we found a p-value of 0.0558 for this difference. The bulk of the 95% CI (–3.32 to 0.04) is in favour of the intervention, making benefit a more likely outcome than harm. Our lower 95% CI, at 3.32, rules out our original suggested MCID of 4.21. It would, therefore, seem appropriate to say that we have weak evidence of a small effect (< 2 units on the NBD measure) and our study has been able to rule out the larger MCID of 4.21. The conclusion could then boil down to a CI that is mostly in favour of benefit, but an effect that is smaller than the one the trial was designed to detect. Frequency of defaecation and feeling of more complete emptying are two secondary outcomes specifically mentioned as being of great importance in our interviews, and in both we did demonstrate statistically significant improvement in those that undertook the massage, with similar benefits being reported by the bowel diary and specific questions within the questionnaires.
As identified in Chapter 5, there does, however, appear to be some disparity between the quantitative and qualitative findings. This may be attributable to the questionnaires being insensitive in this population or could perhaps be a result of interviewees wishing to please the interviewer, or a mixture of the two. The NBDS31 is only validated for NBD in spinal cord-injured patients. We used this as our primary outcome measure because spinal cord-injury and MS patients have relatively similar NBD symptoms, the differences being that only some symptoms in PwMS correspond to the level of the spinal cord lesions, can fluctuate between constipation and faecal incontinence and may involve slow transit and/or pelvic floor dyssynergia. However, there are also some differences in the pathophysiology of the diseases and approaches to treatment.
Once stabilised and rehabilitated, spinal cord-injury patients tend to establish a bowel care routine often established while in hospital. Their ‘disease’ does not progress and the bowel symptoms relate to the level of injury. PwMS, on the other hand, have an ultimately progressive disease with many symptoms that can fluctuate in nature and severity. In addition, we do not know all the physiological implications of the disease on the bowel itself or on the neural control of the bowel. For this reason, the NBDS may not have been sensitive enough to detect changes in the symptoms that we have identified as mattering most to the patient, that is, empowerment and control:
I know when I get to the toilet I’m going to have a bowel movement.
PwMS17
Our other symptom questionnaire, the CSS,32 is validated in those with chronic but non-neurological constipation and, like the NBDS, uses terms that have been shown to be unfamiliar to the general public, such as defaecation, use of drops for evacuation, evacuation, digital stimulation, evacuation of the rectum, constipation, FI, flatus incontinence and perianal skin problems. A recent study has suggested that many of these terms are unrecognised by the public62 and this aligns with some of our incomplete questionnaires in which questions, for example, about digital stimulation were left incomplete, either because the patient did not know what this was or were too embarrassed to answer (information gleaned from nurses’ calls). There were also some participants who stated that completing the questionnaires was very tiring, especially the length of the NBIS questionnaire, which we were hoping to validate; this again may have attributed to poor or indeed inaccurate completion. Moreover, the EQ-5D-5L35 results indicated that, in both groups, MS symptoms worsened over the study period and this may have made it difficult to improve one symptom significantly. Following on from our analyses of the NBIS questionnaires’ repeatability and criterion reliability, further work is needed to determine which questions truly reflect what is important to PwMS who have NBD.
Throughout the quantitative results, there is evidence of greater improvement in the intervention group when compared with the control group in the symptoms of incomplete evacuation, infrequency and reduced laxative use (and, therefore, side effects), which were also identified in the interviews. The sensation of incomplete evacuation is a symptom that is rated as very common and causes a lot of distress with > 90% of participants indicating that they were distressed:
Sometimes it would get so bad that I could hardly sit down because I was so bagged up and just couldn’t go.
PwMS13
Data from the bowel diary indicated a significant benefit in this outcome [incomplete evacuation (mean change 1.08, 95% CI 0.41 to 1.76; p = 0.002, see Table 19)]. The response to the individual question in the CSS also indicated a benefit in this symptom (the percentage of participants in the intervention group who never felt incomplete evacuation increased from 6.7% at baseline to 15.5% at week 24, and in the control group this percentage decreased from 9.1% at baseline to 8.4% at week 24 (i.e. more participants in the intervention group improved). Those in the intervention group who always felt that there was incomplete evacuation decreased from 21.3% at baseline to 3.4% at week 24; in the control group, this percentage decreased from 20.2% at baseline to 10.8% at week 24. Moreover, the results of the transit study test identified that the markers in the intervention group moved more quickly to the distal colon, and so, if not triggering voiding, could potentially aid evacuation once defaecation was started. Change in stool type could also indicate a possible decrease in transit time with a move towards normal stool types 3 and 4. For example, one participant who mainly passed stool types 1 or 2 before the trial every 3 to 4 days or more stated:
It was just awful. I’d sit on the toilet for ages and ages and ages just knowing that I had to do something and it was painful.
PwMS17
By the second stage of interviews, however, she had a bowel movement of stool type 3 or 4 every day:
It’s back to what it used to be years ago before the MS . . . really, really quite consistent.
PwMS17
The results of stool consistency in the main study as recorded in the bowel diary also indicated a greater percentage in the intervention group passing stool type 3 or 4 at week 6, as well as reporting less total constipation at both weeks 6 and 24 (see Table 13).
An increase in stool frequency was evident from the responses in the NBDS, the CSS score and from the bowel diary. This bowel diary outcome was significant (p = 0.039) and indicated an increase of 0.7 stools per week [adjusted change from pre treatment versus week 6 was 0.5 stools per week (SD 1.55 stools per week) in the intervention group and –0.2 stools per week (SD 1.64 stools per week) in the control group; pre treatment versus week 24 in the intervention group was 0.4 stools per week (SD 1.79 stools per week) and in the control group it was 0.2 stools per week (SD 1.62 stools per week)]. In addition, within the repeated-measures analysis, the change in frequency of defaecation was significant at week 6 (OR 0.56, 95% CI 0.03 to 1.10; p = 0.039); although this effect was decreased at week 24. It is, however, worth noting that at baseline the frequency of defaecation in the bowel diary was not that severe, with a mean of 3.9 times a week (SD 1.71) and the NBD mean score of 8.2 (SD 5.2), indicating low to medium levels of constipation; this was also supported in the CSS.
An episode of FI can have a devastating impact on a person’s self-esteem and confidence, creating a reluctance to leave the house, and was described by one participant as an ‘absolute nightmare’ when it happens, especially if out of the house. FI may be frank and unexpected or may be attributable to the urgency created by the use of laxatives. There was no change in the frequency of FI episodes in the quantitative data, but participants in our qualitative study reported decreased episodes of FI and more confidence in leaving the house. Other reported benefits from doing the massage included feeling less bloated, clothing becoming looser and a decrease in sluggishness with reduced fatigue levels.
Adherence
According to the self-completed diary, which recorded the frequency of undertaking the massage, adherence to the massage intervention was good; 75% of participants administered the massage five times per week, although, interestingly, the vast majority were undertaking self-massage. At week 24, 66% of participants were continuing with the massage at least three times per week. In the qualitative interviews, participants reported that they practised the massage when it fitted into their routine and altered it according to effect, that is they did not necessarily do it every day. In fact, one person felt it to be too effective if undertaken every day.
The adherence to changing lifestyle, such as diet and exercise, was slightly greater in the control group, with 30% (20% in the intervention group) saying that they had made at least one lifestyle change. Such changes may have facilitated the changes reported within the control group. The overall uptake of the suggested modifications, supported and discussed at the weekly telephone calls, was perhaps lower than expected and is in contrast to what was reported in the qualitative interviews in which the participants said that they found the information to be helpful. To that end, discussing it with the nurse should have aided implementation in both groups. However, those in the control group were not interviewed (a limitation of the study), so there is no feedback as to how they felt about the delivery of the advice. It is a recognised feature that for some PwMS, the processing of such information is difficult and requires frequent, spaced reinforcement.
Who benefits and why?
Abdominal massage is thought to work by stimulating the bowel and decreasing the overall transit time; thus facilitating defaecation by the stronger propulsion of stools that are less hard and difficult to evacuate. Within our anorectal substudy, it was identified that 60% of this subgroup demonstrated slow transit; if we could extend this frequency of slow transit to the whole study group then it may, to some extent, explain why the response was varied and less than expected. Patients may also have anorectal dyssynergia, which can make it difficult to pass stool; if this was the primary reason for their constipation, then abdominal massage would have little effect. However, slow transit and dyssynergia often coexist and, unfortunately, owing to poor uptake of the follow-up anorectal outcomes, we can only speculate and identify this as an area requiring further investigation. Given the small improvement in the primary outcome but not in terms of QALYs, a low-cost version of the intervention might be considered worthwhile by some patients. For example, it could be a part of a supported self-management pathway for NBD in PwMS.
Our data indicate consistently that the male participants reported better outcomes from the intervention. Although only a small group (n = 14 in the intervention group and n = 21 in the control group), the reasons behind these findings are unclear (see Tables 18 and 19). It may be that the male participants were stronger and less fatigued and undertook the massage more effectively. To date, we do not know the amount of force that is needed to be effective in stimulating the bowel. Within our qualitative study, a theme emerged around the adaptations made as a result of fatigue and difficulties actually doing the massage; yet there was also a theme that this was something PwMS wanted to do themselves, so as not to increase care burden, and a way of improving symptoms without taking medications. The amount of pressure and an assistive device to help with self-massage are potentially other avenues that should be explored. There was also some evidence that those with a slightly higher BMI, those who were slightly older, those who were less cognitively impaired and those with relapsing–remitting MS improved with the abdominal massage more than those with a lower BMI, who were younger, more cognitively impaired and did not have relapsing–remitting MS. Potentially, the older participants may be retired and have more time; however, why the slightly heavier people should improve more is difficult to explain.
Medication
Constipation was linked to laxative usage, which could also result in negative side effects. Interviewees reported that taking laxatives caused pain, sleeplessness, cramps and diarrhoea. The unpredictability of passing a stool when taking laxatives meant that a number of patients were afraid to leave the house once they had taken laxatives. From the analysis of laxative use, it would seem that those in the intervention group were more likely to reduce their laxative use. Participants’ overall medication change was monitored and the number of medications seemed to change more in the control group; however, this was difficult to analyse as several participants had multiple changes and, in one instance, suppositories were individually mentioned. It was, however, interesting to see the history of the prescribing of laxatives, where it seemed to be the norm to increase the dose as well as prescribing additional laxatives within a short space of time.
This is an intervention for which we found no evidence of harm and which does not require extensive resources or training. There were a small number of AEs, none of which could be directly attributable to the massage. Moreover, this is the first time that the reduced training, to both clinicians and participants, was rolled out. In our earlier studies, those teaching the massage to the participants were experienced continence clinicians and the participants received weekly follow-up visits for support. In this trial, some of the clinicians had no experience of treating either bladder or NBD and only one had pre-trial experience with the massage. Feedback from clinicians was very supportive of the intervention and our recruitment is testimony to this. Some reported that further training may have been helpful and this is understandable if a ‘hands-on’ concept was new and also the lifestyle information was not within their usual scope (clinicians comprised MS nurses, continence nurses, a research nurse and one research assistant). However, as shown in our secondary and sensitivity analyses (see Tables 18 and 19), results were not significantly dependent on sites and the experience of the researchers.
If this intervention was to roll out within the NHS, then we suspect that it would depend on local services to facilitate staff training; yet, where these staff sit (e.g. the continence service or the MS nurse service) is undecided at the moment. Remarkably, there was no feedback from participants that they would have preferred more training or support with the massage. The interviews did highlight that, during the initial training, some people preferred the massage to be administered over their clothes or on body parts other than their stomach. This may have affected how the staff member was able to deliver training, because the massage should be administered on the abdomen in order to allow the patient to gauge pressure. The clinicians interviewed all remained enthusiastic about the intervention, which influenced the mainly positive feedback participants were giving to them. Those used to treating PwMS recognised that interventions to treat the symptoms of NBD are scarce and that this could be a part of their ‘toolkit’, as an adjunct or perhaps instead of, for instance, laxatives, rectal irrigation, biofeedback, none of which is evidence based. Stakeholders also recognised that there was a definite need for additional treatment modalities and that this is a relatively cheap intervention if, after appropriate assessment and training, it is undertaken by the patient themselves or a carer. Additional resources may, however, be required should NHS employees be required to administer the massage routinely.
Limitations
There are several limitations in this study. The differential numbers recruited to our two groups (attributable to minimisation by centre) coupled with the additional numbers in the intervention group who failed to complete the study meant that we ended up with a smaller number in the intervention group than we had hoped. The extent of missing data and the reasons behind it have been explored but do not seem to be related to any predictable factors. One site, UCH, London, which undertook the anorectal physiology tests, had a slightly lower retention rate (76.9%); this may have been because of the invasive nature of these tests and the unwillingness of participants to return for follow-up. Royal Preston and the Walton Centre both had a retention rate of 81%, but no specific pattern for loss to follow-up could be identified as reasons were varied and appeared unconnected to the intervention (e.g. family illness, moving location).
It may also have been better to use more stringent inclusion/exclusion criteria, such as Rome III,63 as some participants were not severely affected (according to the primary outcome measure) by their constipation and, therefore, had limited capacity to improve. These criteria had been used in a previous study by the authors, but were felt to be too stringent, focusing, as they do, on a clinical expert definition rather than a patient-based or a combined clinician–patient definition of constipation. Indeed, the suitability of the Rome III criteria for assessing symptoms of constipation has been challenged, as studies show that many patients who report constipation symptoms do not fulfil the Rome III criteria. 64–66 Patients’ perception of ‘bothersomeness’ seemed to fit better. A large number of participants undertook the massage themselves, but because of their weakness and fatigue the effectiveness may have been reduced. Several participants in our qualitative study stated some difficulty self-massaging because of fatigue, but tried to work out a way around it by, for example, taking rests. However, for some participants, the massage was probably undertaken at a suboptimal level. Within our process evaluation study, owing to financial constraints, the interviews were only conducted with those who were undertaking the abdominal massage, which poses a limitation on our knowledge of the uptake of the advice and perceptions of taking part in the trial as part of the control group. The limitations in the economic evaluation have already been discussed in Chapter 4.
Future research
It would seem from the results of the RCT, the qualitative study and the health economic component that, similar to other studies, abdominal massage is effective on some patients. Most likely, these would be patients who have primarily slow transit (potentially 60% in this study). Therefore, future research should focus on identifying those with slow transit, which, as our anorectal substudy showed, is not easy to do using invasive tests. There have been attempts to validate questionnaires (e.g. Kess Questionnaire66) that may indicate which type of constipation a person has, that is slow transit and/or dyssynergia, and these should first be validated in a MS population and then used for possible screening of those with predominant slow transit constipation. In addition, the optimum frequency and, importantly, intensity of massage should be explored with use of pressure-sensitive devices and massage devices.
The following is a list of recommendations for future research:
-
further mechanistic evaluation of NBD and ways of identifying those with predominantly slow transit
-
further validation of the NBIS questionnaire
-
development of a treatment algorithm that would aid clinicians in the treatment of NBD
-
development and evaluation of a device to aid abdominal self-massage
-
development and clinical trials of other interventions that may help with the symptoms of constipation/FI, for example the superfruit baobab, which has recently been developed into a drink, Baotic (Hippo & Hedgehog Ltd, Glasgow, UK).
Chapter 7 Conclusion
The benefits of using abdominal massage for the relief of symptoms in PwMS and NBD are not clearly demonstrated by the results of this methodologically high-quality trial. Moreover, there is uncertainty around cost estimates with domination by the control group. The research identified that, although the increment in the primary outcome favoured the intervention, it was not statistically significant. The analysis of the process evaluation component identified that most (15/20) participants reported benefit and felt it an attractive option as it was non-pharmacological and non-invasive. Feedback from clinicians teaching the massage was also positive, although in a few cases they expressed concern around the capability of the participant to do the massage effectively. Budget holders, albeit before the results of the trial were known, thought that the infrastructure and training required to introduce abdominal massage as an additional treatment option in this group of patients was implementable.
Based on the results of this programme of work, we believe that there are some PwMS and NBD who may benefit from undertaking abdominal massage as a low-cost, self-management intervention; the challenge is to identify these individuals and introduce it as part of a self-management bowel care pathway. Bowel dysfunction is not widely researched; there are fewer than 30 RCTs (n = 1300 participants)16 published relating to all neurogenic bowel conditions.
Work such as this should be used to develop a treatment algorithm to facilitate better management of FI and/or constipation, thus improving the lives of patients and their carers.
Acknowledgements
We would like to acknowledge the following in relation to the AMBER study.
The AMBER trial group
Principal Investigators: Dr Stewart Webb, Dr Martin Duddy, Dr Anton Emmanuel, Dr Roger Mills, Dr Carolyn Young, Mr Matt Craner, Mrs Nicki Stubbs, Professor Basil Sharrack, Dr David Rog, Mrs Emma Matthews and Dr Belinda Weller.
Tayside CTU for their assistance with randomisation, data management and statistical support.
All members of the DMEC and TSC for their advice and support throughout the trial.
Staff at all 12 UK sites for their hard work throughout the trial: Queen Elizabeth University teaching Hospital, Glasgow; Royal Victoria Hospital, Newcastle upon Tyne; UCH, London; Royal Preston Hospital, Preston; The Walton Centre, Liverpool; John Radcliffe Hospital, Oxford; St Mary’s Hospital, Leeds; Lincoln County Hospital and Pilgrim Hospital, Lincoln; Salford Royal Hospital, Salford; Royal Hallamshire Hospital, Sheffield; Northampton General Hospital, Northampton; and Anne Rowling Generative Neurology Clinic, Edinburgh.
Our PPI group who gave their time, views and opinions, and who remained enthused about the study throughout the 3 years of their involvement.
All the AMBER trial participants for the time and commitment they gave to the study.
Contributions of authors
Professor Doreen McClurg (Chief Investigator and lead grant holder and member of the PMG/TSC) conceived the study and led on the protocol development, analysis and interpretation of the data, and drafting and submitting the final report.
Dr Fiona Harris (Associate Professor, grant holder and member of the PMG/TSC) led and supervised the process evaluation component of the trial and contributed to the analysis and drafting of the final report.
Dr Kirsteen Goodman (Trial Manager and member of the PMG/TSC) contributed to the running of the trial, data management, interpretation of results and drafting the final report.
Dr Selina Doran (Qualitative Researcher and member of the PMG/TSC) conducted the process evaluation data collection and analysis and contributed to drafting the final report.
Professor Suzanne Hagen [Interventions Programme Director (NMAHP RU), grant holder and member of the PMG/TSC] contributed to the study design, provided clinical expertise throughout the study and reviewed the final report.
Professor Shaun Treweek (Professor of Health Services Research, grant holder and member of the PMG/TSC) contributed to the study design, provided clinical trial expertise throughout the study and reviewed the final report.
Professor Christine Norton (Professor of Nursing, grant holder and member of the PMG/TSC) contributed to the study design, provided clinical expertise throughout the study and reviewed the final report.
Dr Maureen Coggrave (Research Fellow, grant holder and member of the PMG/TSC) contributed to the study design, provided clinical expertise throughout the study and reviewed the final report.
Professor John Norrie (Chair of Medical Statistics and Trial Methodology, grant holder and member of the PMG/TSC) contributed to the study design, provided clinical trial expertise throughout the study and reviewed the final report.
Miss Petra Rauchhaus (Trial Statistician at TCTU and member of the PMG and TSC) contributed to the trial analysis and reporting of results.
Professor Peter Donnan (Co-Director of TCTU, Senior Trial Statistician, grant holder and member of the PMG/TSC) led on the statistical aspect of the trial design and analysis of data, contributed to the drafting and review of the statistical sections of the final report.
Dr Anton Emmanuel (Senior Lecturer and Consultant Gastroenterologist, grant holder, member of the PMG/TSC and a PI) contributed to the study design and provided clinical expertise throughout the study and reviewed the final report.
Dr Sarkis Manoukian (Health Economist and member of the PMG/TSC) led on the health economic aspects of the trial design, interpretation of the data and drafting the health economic section of the final report.
Dr Helen Mason (Reader in Health Economics, grant holder and member of the PMG/TSC) led on the health economic aspects of the trial design, interpretation of the data and drafting of the health economic section of the final report.
Publication
McClurg D, Goodman K, Hagen S, Harris F, Treweek S, Emmanuel A, et al. Abdominal massage for neurogenic bowel dysfunction in people with multiple sclerosis (AMBER – Abdominal Massage for Bowel Dysfunction Effectiveness Research): study protocol for a randomised controlled trial. Trials 2017;18:150.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry 2014;85:76-84. https://doi.org/10.1136/jnnp-2013-305450.
- Nortvedt MW, Riise T, Frugård J, Mohn J, Bakke A, Skår AB, et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler 2007;13:106-12. https://doi.org/10.1177/1352458506071210.
- Awad RA. Neurogenic bowel dysfunction in patients with spinal cord injury, myelomeningocele, multiple sclerosis and Parkinson’s disease. World J Gastroenterol 2011;17:5035-48. https://doi.org/10.3748/wjg.v17.i46.5035.
- Sze EH, Barker CD, Hobbs G. A cross-sectional survey of the relationship between fecal incontinence and constipation. Int Urogynecol J 2013;24:61-5. https://doi.org/10.1007/s00192-012-1851-7.
- McClurg D, Beattie K, Lowe-Strong A, Hagen S. The elephant in the room: the impact of bowel dysfunction on people with multiple sclerosis. J Assoc Chartered Physiotherapists in Women’s Health 2012;111:13-21.
- Multiple Sclerosis Trust . MS Trust Report Shows Emergency Admissions for People With MS Continue to Rise n.d. www.mstrust.org.uk/news/news-about-ms/ms-trust-report-shows-emergency-admissions-people-ms-continue-rise (accessed 13 June 2017).
- Swash M, Snooks SJ, Chalmers DH. Parity as a factor in incontinence in multiple sclerosis. Arch Neurol 1987;44:504-8. https://doi.org/10.1001/archneur.1987.00520170034018.
- Weber J, Grise P, Roquebert M, Hellot MF, Mihout B, Samson M, et al. Radiopaque markers transit and anorectal manometry in 16 patients with multiple sclerosis and urinary bladder dysfunction. Dis Colon Rectum 1987;30:95-100. https://doi.org/10.1007/BF02554940.
- Nathan PW, Smith MC. Spinal pathways subserving defaecation and sensation from the lower bowel. J Neurol Neurosurg Psychiatry 1953;16:245-56. https://doi.org/10.1136/jnnp.16.4.245.
- Mathers SE, Ingram DA, Swash M. Electrophysiology of motor pathways for sphincter control in multiple sclerosis. J Neurol Neurosurg Psychiatry 1990;53:955-60. https://doi.org/10.1136/jnnp.53.11.955.
- Snooks SJ, Swash M. Motor conduction velocity in the human spinal cord: slowed conduction in multiple sclerosis and radiation myelopathy. J Neurol Neurosurg Psychiatry 1985;48:1135-9. https://doi.org/10.1136/jnnp.48.11.1135.
- Haldeman S, Glick M, Bhatia NN, Bradley WE, Johnson B. Colonometry, cystometry, and evoked potentials in multiple sclerosis. Arch Neurol 1982;39:698-701. https://doi.org/10.1001/archneur.1982.00510230024008.
- Chia Y, Gill K, Jameson JS, Forti AD, Henry M, Swash M, et al. Paradoxical puborectalis contraction is a feature of constipation in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 1996;60:31-5. https://doi.org/10.1136/jnnp.60.1.31.
- Waldron DJ, Horgan PG, Patel FR, Maguire R, Given HF. Multiple sclerosis: assessment of colonic and anorectal function in the presence of faecal incontinence. Int J Colorectal Dis 1993;8:220-4. https://doi.org/10.1007/BF00290311.
- Pintér A, Cseh D, Sárközi A, Illigens BM, Siepmann T. Autonomic dysregulation in multiple sclerosis. Int J Mol Sci 2015;16:16920-52. https://doi.org/10.3390/ijms160816920.
- McClurg D, Norton C. What is the best way to manage neurogenic bowel dysfunction?. BMJ 2016;354. https://doi.org/10.1136/bmj.i3931.
- Thomas S, Mynors G, Simpson S, Meade N, Bowen A. Measuring the burden of hospitalisation in multiple sclerosis: a cross-sectional analyses of the English hospital episode statistics database 2009–2014. NHiS Commissioning Excellence and MS Trust 2015. www.nhis.com/ms-report (accessed 13 June 2017).
- Dibley L, Coggrave M, McClurg D, Woodward S, Norton C. ‘It’s just horrible’: a qualitative study of patients’ and carers’ experiences of bowel dysfunction in multiple sclerosis. J Neurol 2017;264:1354-61. https://doi.org/10.1007/s00415-017-8527-7.
- McClurg D, Hagen S, Dickinson L. Abdominal massage for the treatment of constipation (protocol). Cochrane Database Syst Rev 2011;4. https://doi.org/10.1002/14651858.CD009089.
- Sinclair M. The use of abdominal massage to treat chronic constipation. J Bodyw Mov Ther 2011;15:436-45. https://doi.org/10.1016/j.jbmt.2010.07.007.
- Sinclair M. The forgotten core – revisiting abdominal massage. Massage and Bodywork Magazine 2009:72-9.
- Smith DG. Abdominal massage for constipation: techniques, evidence base and safety. Gastrointest Nurs 2013;11:13-4. https://doi.org/10.12968/gasn.2013.11.1.13.
- Allan D, Montague S, Watson R, Herbert RA. Physiology for Nursing Practice. Edinburgh: Elsevier; 2005.
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psych Bull 2004;130:3-18. https://doi.org/10.1037/0033-2909.130.1.3.
- Purves D, Augustine GJ, Fitzpatrick D, Hall C, LaMantia AS, McNamara JO, et al. Neuroscience. Sunderland, MA: Sinauer Associates Inc; 2007.
- Liu Z, Sakakibara R, Odaka T, Uchiyama T, Yamamoto T, Ito T, et al. Mechanisms of abdominal massage for difficult defecation in a patient with myelopathy. J Neurol 2005;252:1280-2. https://doi.org/10.1007/s00415-005-0825-9.
- Harrington K, Haskvitz EM. Managing a patient’s constipation with physical therapy. Phys Ther 2006;86:1511-9. https://doi.org/10.2522/ptj.20050347.
- Emly M. Abdominal massage. Nurs Times 1993;89:34-6.
- Emly M, Wilson L, Darby J. Abdominal massage for adults with learning disabilities. Nurs Times 2001;97:61-2.
- McClurg D, Goodman K, Hagen S, Harris F, Treweek S, Emmanuel A, et al. Abdominal massage for neurogenic bowel dysfunction in people with multiple sclerosis (AMBER – Abdominal Massage for Bowel Dysfunction Effectiveness Research): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1890-y.
- Krogh K, Christensen P, Sabore S, Laurberg A. Neurogenic bowel dysfunction score. Spinal Cord 2006;44:625-31. https://doi.org/10.1038/sj.sc.3101887.
- Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 1996;39:681-5. https://doi.org/10.1007/BF02056950.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920-4. https://doi.org/10.3109/00365529709011203.
- Bonniaud V, Bryant D, Parratte B, Guyatt G. Development and validation of the short form of a urinary quality of life questionnaire: SF-Qualiveen. J Urol 2008;180:2592-8. https://doi.org/10.1016/j.juro.2008.08.016.
- Nowels D, McGloin J, Westfall JM, Holcomb S. Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res 2005;14:95-105. https://doi.org/10.1007/s11136-004-0614-4.
- Medical Dictionary for Regulatory Activities . Welcome to MedDRA n.d. www.meddra.org (accessed 30 October 2017).
- McClurg D, Hagen S, Hawkins S, Lowe-Strong A. Abdominal massage for the alleviation of constipation symptoms in people with multiple sclerosis: a randomized controlled feasibility study. Mult Scler 2011;17:223-33. https://doi.org/10.1177/1352458510384899.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-32.
- Treweek S, Altman DG, Bower P, Campbell M, Chalmers I, Cotton S, et al. Making randomised trials more efficient: report of the first meeting to discuss the Trial Forge platform. Trials 2015;16. https://doi.org/10.1186/s13063-015-0776-0.
- Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2013;12.
- NHS Reference Costs 2015 to 2016. London: Department of Health and Social Care; 2016.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. OHE Research Paper 16/01. London: Office of Health Economics; 2016.
- The Prevention and Treatment of Missing Data in Clinical Trials. Washington, DC: The National Academies Press; 2010.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45. https://doi.org/10.1002/sim.1794.
- Verbeke G, Fieuws S, Molenberghs G, Davidian M. The analysis of multivariate longitudinal data: a review. Stat Methods Med Res 2014;23:42-59. https://doi.org/10.1177/0962280212445834.
- Anderson R. New MRC guidance on evaluating complex interventions: clarifying what interventions work by researching how and why they are effective. BMJ 2008;337.
- O’Hagan A, Stevens JW. The probability of cost-effectiveness. BMC Med Res Methodol 2002;2. https://doi.org/10.1186/1471-2288-2-5.
- Craig N, Dieppe P, MacIntyre, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage; 1997.
- Yin R. Case Study Research: Design and Method. London: Sage; 2009.
- Kœnig G. Realistic evaluation and case studies: stretching the potential. Evaluation. Sage Journals 2009;15:9-30.
- Basset C, Basset C. Qualitative Research in Health Care. London: Whurr Publishers Ltd; 2004.
- Harris FM, Maxwell M, O’Connor R, Coyne JC, Arensman E, Coffey C, et al. Exploring synergistic interactions and catalysts in complex interventions: longitudinal, mixed methods case studies of an optimised multi-level suicide prevention intervention in four European countries (Ospi-Europe). BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-2942-z.
- Harris FM, Maxwell M, O’Connor RC, Coyne J, Arensman E, Székely A, et al. Developing social capital in implementing a complex intervention: a process evaluation of the early implementation of a suicide prevention intervention in four European countries. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-158.
- Arksey H, Knight PT. Interviewing for Social Scientists: An Introductory Resource. London: Sage; 1998.
- Ritchie J, Spencer L, Ritchie J, Spencer L. Analyzing Qualitative Data. London: Sage; 1994.
- Bakshi R. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Mult Scler 2003;9:219-27. https://doi.org/10.1191/1352458503ms904oa.
- Dimidi E, Dibley L, Cotterill N, Scott, M, Whelan K, Knowles CH, et al. Validated symptom and quality of life measures neither reflect patient or clinicians concerns nor use words familiar to patients. Gastrointest Nurs 2016;14:29-38. https://doi.org/10.12968/gasn.2016.14.8.29.
- Lämås K, Graneheim UH, Jacobsson C. Experiences of abdominal massage for constipation. J Clin Nurs 2012;21:757-65. https://doi.org/10.1111/j.1365-2702.2011.03946.x.
- Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol 2001;96:3130-7. https://doi.org/10.1111/j.1572-0241.2001.05259.x.
- Rey E, Balboa A, Mearin F. Chronic constipation, irritable bowel syndrome with constipation and constipation with pain/discomfort: similarities and differences. Am J Gastroenterol 2014;109:876-84. https://doi.org/10.1038/ajg.2014.18.
- Garrigues V, Gálvez C, Ortiz V, Ponce M, Nos P, Ponce J. Prevalence of constipation: agreement among several criteria and evaluation of the diagnostic accuracy of qualifying symptoms and self-reported definition in a population-based survey in Spain. Am J Epidemiol 2004;159:520-6. https://doi.org/10.1093/aje/kwh072.
- Knowles CH, Eccersley AJ, Scott SM, Walker SM, Reeves B, Lunniss PJ. Linear discriminant analysis of symptoms in patients with chronic constipation: validation of a new scoring system (KESS). Dis Colon Rectum 2000;43:1419-26. https://doi.org/10.1007/BF02236639.
Appendix 1 Massage training materials
Appendix 2 Data Monitoring and Ethics Committee charter
Appendix 3 Site and recruitment information
| Site | Type of facility | Governing body | Target, n | Participants recruited, n (% met) | Retention, n (%) | Trial group, n (%) | HCPs involved | Current bowel treatments offered | |
|---|---|---|---|---|---|---|---|---|---|
| Abdominal massage | Standard care | ||||||||
| Anne Rowling Clinic, Edinburgh | Neurological research facility | NHS Lothian Health Board | 10 | 9 (90) | 9 (100.0) | 4 (4.4) | 5 (5.1) | Consultant neurologist | None. This site is focused more on advancing MS research |
| John Radcliffe Hospital, Oxford | Tertiary teaching and research hospital | Oxford University Hospitals NHS Foundation Trust | 20 | 17 (85) | 15 (88.2) | 9 (10.0) | 8 (8.1) | MS specialist nurse and research nurse | Bowel problems are underrecognised and only referred to the bowel specialist nurse when explicitly raised |
| St Mary’s Hospital, Leeds | Community health-care service | Leeds Community Healthcare NHS Trust | 10 | 10 (100) | 10 (100.0) | 4 (4.4) | 6 (6.1) | Bowel specialist nurse and research nurse | Specialised bowel clinics and home visits for those with constipation, FI and irritable bowel dysfunction |
| Lincoln County Hospital, Lincoln | District general hospital | United Lincolnshire Hospitals NHS Trust | 10 | 13 (exceeded by n = 3) | 13 (100.0) | 7 (7.8) | 6 (6.1) | Consultant neurologist and research nurses | Advice from a MS specialist nurse |
| Northampton General Hospital, Northampton | General, specialist and teaching hospital | Northampton General Hospital NHS Trust | 20 | 14 (70) | 12 (85.7) | 7 (7.8) | 7 (7.1) | Research and Development team, research nurse and MS nurse specialist | Home visits and a bladder and bowel clinic, with bladder specialist nurse and bowel specialist nurse |
| Royal Hallamshire Hospital, Sheffield | Clinical research facility | Sheffield Teaching Hospitals NHS Trust | 10 | 17 (exceeded by n = 7) | 16 (94.1) | 8 (8.9) | 9 (9.1) | Research sister, clinical research nurse and consultant neurologist | Laxatives and diet advice in neurology department |
| Royal Preston Hospital, Preston | District general hospital | Lancashire Teaching Hospitals NHS Foundation Trust | 20 | 22 (exceeded by n = 2) | 18 (81.0) | 10 (11.1) | 12 (12.1) | Senior research nurse and neurologist | Laxatives, digital stimulation, medication and self-administration of enemas |
| Royal Victoria Infirmary, Newcastle upon Tyne | Tertiary teaching hospital | Newcastle upon Tyne NHS Foundation Trust | 20 | 17 (85) | 16 (94.1) | 8 (8.9) | 9 (9.1) | Consultant (50% neurology, 50% MS) and research nurse | MS patients only get to see the bowel specialist in Newcastle upon Tyne if there is a significant problem |
| Salford Royal Hospital, Salford | General, specialist and teaching hospital | Salford Royal Hospital NHS Foundation Trust | 20 | 15 (75) | 14 (93.3) | 7 (7.8) | 8 (8.1) | Research officer and senior research nurse | Dietary advice from MS specialist nurses. If bowel problems continue, they refer PwMS to colorectal specialists |
| Southern General Hospital, Glasgow | Tertiary referral centre | NHS Greater Glasgow and Clyde Health Board | 20 | 20 (100) | 18 (90.0) | 10 (11.1) | 10 (10.1) | Health-care support worker, MS nurse and consultant neurologist with specialist interest in MS | MS team can refer to gastroenterology department for them to investigate the problem and offer medication |
| The Walton Centre, Liverpool | Neuroscience centre | The Walton Centre NHS Foundation Trust | 20 | 11 (55) | 9 (81.8) | 5 (5.6) | 6 (6.1) | Consultant neurologist and research nurse | Bowel issues are discussed in multidisciplinary clinic and bowel management advice is given |
| UCH, London | National referral and teaching hospital | University College London Hospitals NHS Foundation Trust | 30 | 26 (87) | 20 (76.9) | 11 (12.2) | 13 (13.1) | Bowel management specialist nurse and gastroenterologist consultant | This is a specialist bowel centre. Sustainable provision for those with lower bowel conditions: irrigation feedback, pelvic floor physiotherapy, tailored drug regimes, irrigation therapy, surgery and diet advice |
Appendix 4 Differences in characteristics of missing Neurogenic Bowel Dysfunction Score at 24 weeks compared with complete data
| Variable | NBDS at week 24, n (%) | Total, n (%) | |
|---|---|---|---|
| Present | Not present | ||
| Intervention | |||
| Abdominal massage | 72 (44.4) | 18 (66.7) | 90 (47.6) |
| Standard care | 90 (55.6) | 9 (33.3) | 99 (52.4) |
| Total | 162 (100.0) | 27 (100.0) | 189 (100.0) |
| Centre | |||
| Southern General Hospital, Glasgow | 18 (11.1) | 2 (7.4) | 20 (10.6) |
| Royal Victoria Hospital, Newcastle upon Tyne | 15 (9.3) | 2 (7.4) | 17 (9.0) |
| UCH, London | 18 (11.1) | 6 (22.2) | 24 (12.7) |
| Royal Preston Hospital, Preston | 14 (8.6) | 8 (29.6) | 22 (11.6) |
| The Walton Centre, Liverpool | 9 (5.6) | 2 (7.4) | 11 (5.8) |
| John Radcliffe Hospital, Oxford | 15 (9.3) | 2 (7.4) | 17 (9.0) |
| St Mary’s Hospital, Leeds | 10 (6.2) | 0 (0.0) | 10 (5.3) |
| Lincoln Community Hospital, Lincoln | 13 (8.0) | 0 (0.0) | 13 (6.9) |
| Salford Royal Hospital, Salford | 14 (8.6) | 1 (3.7) | 15 (7.9) |
| Royal Hallamshire Hospital, Sheffield | 16 (9.9) | 1 (3.7) | 17 (9.0) |
| Northampton General Hospital | 11 (6.8) | 3 (11.1) | 14 (7.4) |
| Anne Rowling Centre, Edinburgh | 9 (5.6) | 0 (0.0) | 9 (4.8) |
| Total | 162 (100.0) | 27 (100.0) | 189 (100.0) |
| Minimisation criterion | |||
| Walking unaided | 66 (40.7) | 13 (48.1) | 79 (41.8) |
| Aided walking | 76 (46.9) | 13 (48.1) | 89 (47.1) |
| Wheelchair bound | 20 (12.3) | 1 (3.7) | 21 (11.1) |
| Total | 162 (100.0) | 27 (100.0) | 189 (100.0) |
| Sex | |||
| Male | 31 (19.1) | 4 (14.8) | 35 (18.5) |
| Female | 131 (80.9) | 23 (85.2) | 154 (81.5) |
| Total | 162 (100.0) | 27 (100.0) | 189 (100.0) |
Differences in continuous characteristics of missing Neurogenic Bowel Dysfunction Score at 24 weeks compared with complete data
| Variable | NBDS at week 24 | Total | |
|---|---|---|---|
| Present | Not present | ||
| Total NBDS at baseline | |||
| Complete, n | 155 | 25 | 180 |
| Missing, n | 7 | 2 | 9 |
| Mean (SD) | 8.1 (5.1) | 8.3 (6.0) | 8.2 (5.2) |
| Median (minimum, maximum) | 8.0 (0, 22) | 6.0 (1, 22) | 7.5 (0, 22) |
| Age (years) | |||
| n | 162 | 27 | 189 |
| Missing, n | 0 | 0 | 0 |
| Mean (SD) | 52.8 (10.7) | 49.4 (11.3) | 52.3 (10.8) |
| Median (minimum, maximum) | 52.0 (26, 79) | 48.0 (32, 70) | 51.0 (26, 79) |
| Time since diagnosis of MS (years) | |||
| n | 162 | 27 | 189 |
| Missing, n | 0 | 0 | 0 |
| Mean (SD) | 14.4 (9.4) | 14.1 (8.1) | 14.3 (9.2) |
| Median (minimum, maximum) | 13.0 (0, 51) | 14.0 (1, 32) | 13.0 (0, 51) |
| BMI | |||
| n | 157 | 25 | 182 |
| Missing, n | 5 | 2 | 7 |
| Mean (SD) | 26.7 (5.9) | 27.3 (5.8) | 26.8 (5.9) |
| Median (minimum, maximum) | 25.8 (14.7, 48.8) | 26.5 (19.8, 43.9) | 25.9 (14.7, 48.8) |
Appendix 5 Summary of stool type (Bristol stool chart information)
| Time point | Trial group (%) | |
|---|---|---|
| Intervention | Control | |
| Baseline | ||
| No stool | 39.8 | 40.1 |
| Types 1 and 2 | 21.8 | 18.9 |
| % constipated | 61.6 | 59.0 |
| Types 3 and 4 | 20.2 | 17.5 |
| Types 5, 6 and 7 | 16.0 | 19.6 |
| Missing | 2.3 | 3.8 |
| Week 6 | ||
| No stool | 35.0 | 38.8 |
| Types 1 and 2 | 22.4 | 19.0 |
| % constipated | 57.4 | 58.2 |
| Types 3 and 4 | 29.9 | 23.6 |
| Types 5, 6 and 7 | 11.4 | 14.6 |
| Missing | 0.9 | 2.4 |
| Week 24 | ||
| No stool | 32.0 | 41.4 |
| Types 1 and 2 | 23.4 | 17.5 |
| % constipated | 55.4 | 58.9 |
| Types 3 and 4 | 23.9 | 22.8 |
| Types 5, 6 and 7 | 15.5 | 12.0 |
| Missing | 5.0 | 5.7 |
Appendix 6 Summary of all adverse events
| Variable | Trial group, n (%) | Total, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| Severity | |||
| Mild | 10 (35.7) | 26 (46.4) | 36 (42.9) |
| Moderate | 14 (50.0) | 23 (41.1) | 37 (44.0) |
| Severe (not serious) | 4 (14.3) | 7 (12.5) | 11 (13.1) |
| Total | 28 (100.0) | 56 (100.0) | 84 (100.0) |
| Causality | |||
| Unrelated | 25 (89.3) | 54 (96.4) | 79 (94.0) |
| Possibly related | 3 (10.7) | 2 (3.6) | 5 (6.0) |
| Total | 28 (100.0) | 56 (100.0) | 84 (100.0) |
| Action taken | |||
| None | 14 (50.0) | 22 (39.3) | 36 (42.9) |
| Hospitalisation | 2 (7.1) | 4 (7.1) | 6 (7.1) |
| Medication(s) commenced | 6 (21.4) | 23 (41.1) | 29 (34.5) |
| Other | 2 (7.1) | 2 (3.6) | 4 (4.8) |
| Hospitalisation/medication(s) commenced | 1 (3.6) | 3 (5.4) | 4 (4.8) |
| Intervention reduced/other | 1 (3.6) | 0 (0.0) | 1 (1.2) |
| Medication(s) commenced/other | 2 (7.1) | 0 (0.0) | 2 (2.4) |
| None/other | 0 (0.0) | 2 (3.6) | 2 (2.4) |
| Total | 28 (100.0) | 56 (100.0) | 84 (100.0) |
| Outcome | |||
| Recovered | 17 (60.7) | 37 (66.1) | 54 (64.3) |
| Ongoing | 11 (39.3) | 19 (33.9) | 30 (35.7) |
| Total | 28 (100) | 56 (100) | 84 (100.0) |
Summary of serious adverse events
| SAE | Trial group (n) | Total (n) | |
|---|---|---|---|
| Intervention | Control | ||
| Breast cancer | 1 | 0 | 1 |
| Cholecystitis | 0 | 1 | 1 |
| Fall | 1 | 0 | 1 |
| Femoral neck fracture | 0 | 1 | 1 |
| Localised infection | 0 | 1 | 1 |
| MS relapse | 1 | 1 | 2 |
| Myocardial infarction | 0 | 1 | 1 |
| UTI | 0 | 1 | 1 |
Appendix 7 Cost-effectiveness analysis of abdominal massage versus standard care
| Measurement | MI with a seemingly unrelated regression model | Mixed model |
|---|---|---|
| Difference in costs (£) | ||
| Mean | 50.02 | 77.54 |
| SE | 156.84 | 143.8 |
| Difference in QALYs | ||
| Mean | –0.002 | 0.0026 |
| SE | 0.012 | 0.011 |
| ICER (£ per QALY) | –19,392.89 | 28,722.05 |
| Probability of cost-effectiveness at £20,000 per QALY gained (%) | 37 | 46.6 |
Appendix 8 Frequency of health-care contacts, by trial group, at baseline
| Service used | Trial group (n) | |||
|---|---|---|---|---|
| Intervention group | Control group | |||
| BR | HR | BR | HR | |
| GP at surgery | 0 | 5 | 0 | 6 |
| Nurse at surgery | 0 | 2 | 0 | 0 |
| GP on the telephone | 0 | 1 | 1 | 4 |
| Nurse on the telephone | 0 | 0 | 1 | 2 |
| GP at home | 0 | 0 | 0 | 0 |
| Nurse at home | 3 | 0 | 1 | 0 |
| Out-of-hours clinic | 0 | 0 | 0 | 0 |
| Outpatient department | 1 | 3 | 2 | 6 |
| Admitted to hospital | 0 | 0 | 0 | 0 |
| A&E visit | 0 | 0 | 0 | 0 |
| Allied Health Professionals | 0 | 8 | 0 | 9 |
| Continence Service | 0 | 0 | 1 | 1 |
Frequency of health-care contacts, by trial group, recorded at week 6
| Service used | Trial group (n) | |||
|---|---|---|---|---|
| Intervention group | Control group | |||
| BR | HR | BR | HR | |
| GP at surgery | 1 | 15 | 1 | |
| Nurse at surgery | 0 | 7 | 0 | |
| GP on the telephone | 1 | 7 | 1 | |
| Nurse on the telephone | 6 | 7 | 11 | 4 |
| GP at home | 0 | 0 | 0 | 1 |
| Nurse at home | 10 | 2 | 5 | 0 |
| Out-of-hours clinic | 0 | 0 | 0 | 2 |
| Outpatient department | 4 | 13 | 1 | |
| Admitted to hospital | 0 | 2 | 1 | 5 |
| A&E visit | 0 | 0 | 0 | 1 |
| Allied Health Professionals | 2 | 41 | 0 | |
| Continence Service | 3 | 2 | 0 | 2 |
Frequency of health-care contacts, by trial group, recorded at week 24
| Service used | Trial group (n) | |||
|---|---|---|---|---|
| Intervention group | Control group | |||
| BR | HR | BR | HR | |
| GP at surgery | 7 | 29 | 7 | 42 |
| Nurse at surgery | 4 | 15 | 1 | 27 |
| GP on the telephone | 7 | 18 | 2 | 20 |
| Nurse on the telephone | 12 | 0 | 3 | 9 |
| GP at home | 1 | 2 | 1 | 9 |
| Nurse at home | 6 | 7 | 14 | 10 |
| Out-of-hours clinic | 0 | 0 | 0 | 3 |
| Outpatient department | 3 | 20 | 4 | 40 |
| Admitted to hospital | 1 | 6 | 1 | 8 |
| A&E visit | 1 | 1 | 0 | 4 |
| Allied Health Professionals | 8 | 27 | 14 | 73 |
| Continence Service | 3 | 3 | 5 | 7 |
Total frequency of health-care contacts, by trial group, excluding baseline
| Service used | Trial group (n) | |||
|---|---|---|---|---|
| Intervention group | Control group | |||
| BR | HR | BR | HR | |
| GP at surgery | 8 | 44 | 8 | 60 |
| Nurse at surgery | 4 | 22 | 1 | 42 |
| GP on the telephone | 8 | 25 | 3 | 32 |
| Nurse on the telephone | 18 | 7 | 14 | 13 |
| GP at home | 1 | 2 | 1 | 10 |
| Nurse at home | 16 | 9 | 19 | 10 |
| Out-of-hours clinic | 0 | 0 | 0 | 5 |
| Outpatient department | 7 | 33 | 5 | 67 |
| Admitted to hospital | 1 | 8 | 2 | 13 |
| A&E visit | 1 | 1 | 0 | 5 |
| Allied Health Professionals | 10 | 68 | 14 | 117 |
| Continence Service | 6 | 5 | 5 | 9 |
Appendix 9 NHS unit costs
| Service | Unit cost (£) | Reference |
|---|---|---|
| GP at surgery | 44 | PSSRU |
| Nurse at surgery | 16 | PSSRU |
| GP on the telephone | 27 | PSSRU |
| Nurse on the telephone | 5 | PSSRU |
| GP at home | 114 | PSSRU |
| Nurse at home | 47 | PSSRU |
| Out-of-hours clinic | 66 | PSSRU |
| Outpatient department | 117 | DHSC reference costs41 |
| Admitted to hospital | 1609 | DHSC reference costs41 |
| A&E visit | 138 | DHSC reference costs41 |
| Allied Health Professionals | 24 | PSSRU |
| Continence (Hospital) Service | 108 | PSSRU |
Resource-use NHS costs per participant, by trial group, at baseline
| Summary statistics | Trial group (£) | |
|---|---|---|
| Intervention | Control | |
| Mean cost per participant | 14.59 | 17.73 |
| Standard error | 4.12 | 5.12 |
| SD | 35.41 | 48.82 |
| 95% CI | 6.39 to 22.80 | 7.56 to 27.89 |
| t-test on the equality of means | 0.46 | |
Resource-use NHS costs per participant, by trial group, at 6 weeks
| Summary statistics | Trial group (£) | |
|---|---|---|
| Intervention | Control | |
| Mean cost per participant | 120.65 | 186.78 |
| Standard error | 38.01 | 52.36 |
| SD | 299.29 | 485.60 |
| 95% CI | 82.67 to 290.89 | 82.67 to 290.89 |
| t-test on the equality of means | 0.95 | |
Resource-use NHS costs per participant, by trial group, at 24 weeks
| Summary statistics | Trial group (£) | |
|---|---|---|
| Intervention | Control | |
| Mean cost per participant | 312.14 | 313.18 |
| Standard error | 104.45 | 65.90 |
| SD | 795.49 | 600.41 |
| 95% CI | 102.98 to 521.30 | 182.08 to 444.28 |
| t-test on the equality of means | 0.01 | |
Appendix 10 Roles of health-care professionals interviewed
| Type of interviewee | Interviewees in total (n) | Interviewed in first stage (n) | Interviewed in second stage (n) |
|---|---|---|---|
| Principal investigator | 6 | 6 | 2 |
| Research nurse (including those in senior positions) | 7 | 6 | 6 |
| General nurse | 1 | 1 | 1 |
| MS specialist nurse | 3 | 3 | 3 |
| Health-care support worker | 1 | 0 | 1 |
| Bowel management specialist nurse | 2 | 2 | 2 |
| Research co-ordinator/officer/assistant | 5 | 5 | 4 |
Stakeholders selected for interview
| Type of organisation | Aim of organisation | Interviewees (n) | Expertise of interviewees |
|---|---|---|---|
| MS charities | Support and resources for PwMS | 2 | Policy and research |
| Incontinence foundations | Supporting people with continence problems; developing educational and commissioning guidelines | 3 | Continence service provision; commissioning |
| NHS Specialist Commissioner | Commissioning support for neurology services in the NHS | 1 | Neurology expertise; policy and research |
Appendix 11 Topics discussed during interviews
Intervention group
| First stage at week 4 | Second stage at end of study |
|---|---|
| Personal experiences with MS and bowel problems | Issues faced during first stage |
| Recruitment into trial | Trial paperwork |
| Massage training | Weekly nurse calls |
| Weekly nurse calls | Any other challenges to lifestyle |
| Trial paperwork | Impact of massage on bowel problems |
| Administering massage | Unexpected health benefits of massage |
| Initial impact of massage on bowel problems | Post-trial intentions with the massage |
| Any problems | Any problems |
| Advice for other participants/staff members | Advice for other participants/staff members |
Health-care professionals
| First stage | Second stage |
|---|---|
| Training delivered by the AMBER trial team | Issues faced in first stage |
| Recruiting participants | Participant recruitment (target met or not) |
| Training participants in massage | Training participants in massage |
| Dealing with control group participants | Dealing with control group participants |
| Participant follow-up (weekly calls) | Participant follow-up (weekly calls) |
| Current treatment options at the site | Any problems faced/advice for other sites |
| Other policy and clinical developments at the site | Implementing the treatment long term |
| Any problems faced/advice for other sites | Any problems faced/advice for other sites |
Stakeholders
-
The organisation’s role in neurological and/or incontinence services.
-
Current policy developments in this area (local, regional and national).
-
Current treatment options for bowel problems, particularly neurogenic ones.
-
Whether or not there is a need for an additional treatment option.
-
The long-term implementation of abdominal massage and any challenges involved.
Appendix 12 Process evaluation interview schedules and site questionnaire
Includes:
-
draft interview schedule – participant interviews (first stage)
-
interview schedule – participant interviews (second stage)
-
interview schedule – stakeholder interviews
-
draft interview schedule – HCP site interviews (first stage)
-
draft interview schedule – HCP site interviews (second stage)
-
six-month site-tracking questionnaire.
Appendix 13 Summary of ‘yes’ responses to questions asked during the weekly telephone call (weeks 1 and 24)
| Outcome | Time point, trial group (% of yes responses) | |||
|---|---|---|---|---|
| Week 1 | Week 24 | |||
| Intervention | Control | Intervention | Control | |
| Diet changed | 13.4 | 19.1 | 13.1 | 18.1 |
| Fluid intake changed | 20.4 | 27.7 | 15.5 | 25.1 |
| Defaecation position changed | 25.3 | 27.7 | 9.1 | 8.5 |
| More exercise | 15.1 | 18.1 | 12.9 | 18.8 |
| Concomitant medication(s) changed | 5.7 | 9.6 | 15.6 | 14.9 |
| Use of laxatives changed | 13.8 | 16.0 | 9.1 | 15.4 |
| Bowel habits changed | 49.4 | 38.3 | 42.9 | 30.9 |
| Bowel habits (more often) | 35.6 | 24.5 | 29.9 | 19.1 |
| Bowel habits (less time) | 6.9 | 4.3 | 18.2 | 6.4 |
| Bowel habits (less hard stool) | 20.7 | 5.3 | 20.8 | 14.9 |
List of abbreviations
- AE
- adverse event
- AMBER
- Abdominal Massage for Bowel Dysfunction Effectiveness Research
- BMI
- body mass index
- CI
- confidence interval
- CMO
- context–mechanism–outcome
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CSS
- Constipation Scoring System
- CTU
- Clinical Trials Unit
- DMEC
- Data Monitoring and Ethics Committee
- DMT
- disease-modifying treatment
- DVD
- digital versatile disc
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-VAS
- EuroQol-5 Dimensions Visual Analogue Scale
- FI
- faecal incontinence
- GCU
- Glasgow Caledonian University
- HCP
- health-care professional
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- MCID
- minimum clinically important difference
- MI
- multiple imputation
- MS
- multiple sclerosis
- NBD
- neurogenic bowel dysfunction
- NBDS
- Neurogenic Bowel Dysfunction Score
- NBIS
- neurogenic bowel impact score
- NHIS
- National Health Interview Survey
- NMAHP RU
- Nursing, Midwifery and Allied Health Professions Research Unit
- OR
- odds ratio
- PIL
- patient information leaflet
- PMG
- Project Management Group
- PT
- preferred term
- PwMS
- people with multiple sclerosis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SOC
- System Organ Class
- TCTU
- Tayside Clinical Trials Unit
- TSC
- Trial Steering Committee
- UCH
- University College Hospital
- UKCRC
- UK Clinical Research Collaboration
- UTI
- urinary tract infection
- WTP
- willingness to pay
