Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/212/02. The contractual start date was in February 2016. The draft report began editorial review in July 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bryony Beresford and Megan Thomas were authors of primary studies that are included in this review. Catriona McDaid is a member of the National Institute for Health Research Health Technology Assessment (HTA) and Efficacy and Mechanism Evaluation Editorial Board. Catherine Hewitt is a member of the HTA Commissioning Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Beresford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Introduction
This project was undertaken in response to a commissioning brief from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme. The call was for a systematic review to address the question of which interventions – pharmacological and non-pharmacological – are clinically effective for non-respiratory sleep disturbances in children with neurodisabilities (NDs) and which have generalisable, as opposed to disorder-specific, effects. The brief requested a broad systematic review to ‘take stock’ of the current evidence that is available on what works and for whom and to identify promising interventions for future primary research. The following two sections describe the definitions used to identify the scope of the review; we then move on to discuss, specifically, the issue of sleep disturbance in children with NDs.
Sleep disturbance
Sleep has been described as an active ‘restorative process’1 and is essential for optimal physical and mental functioning and well-being. It is a complex process: the timing, duration and quality of sleep is the outcome of the interplay of biological processes, socioenvironmental influences and behaviours. As a result, there is a wide range of reasons why an individual’s sleep may be affected in some way and, for children with NDs, the cause may be multifactorial. 2–9
The International Classification of Sleep Disorders – Third Edition (ICSD-3)10 lists the current diagnostic categorisation of sleep disorders as follows: insomnia, sleep-related breathing disorders, central disorders of hypersomnolence (e.g. narcolepsy), circadian rhythm sleep–wake disorders, parasomnias, sleep-related movement disorders and ‘other sleep disorders’.
The extent to which sleep is disturbed, or interrupted, is a diagnostic criterion for insomnias and circadian rhythm sleep–wake disorders. That said, it is accepted that there needs to be room for clinical judgement regarding the clinical significance of the extent of sleep disturbance. 11
However, although the ICSD-3 provides a classification and diagnostic framework, it is important to note that, in paediatric research at least, very few studies actually use the ICSD-3 criteria to define or screen research participants. 12,13 Instead, in both the clinical and research literature, a number of different phrases are used to describe the manifestations of a sleep disorder in terms of the impact that it has on an individual’s sleep: sleep disturbance, sleep problems and sleep difficulties. 2 Such terms have all been used for issues related to falling asleep (i.e. sleep initiation) and staying asleep, as opposed to night wakings or very early waking (i.e. sleep maintenance). The scope of this review was guided by the commissioning brief, which made two clear specifications: it should be concerned with ‘non-respiratory sleep disturbance’ and sleep disturbance (experienced by the child and/or parent) should be a feature of the presenting problem. The ICSD-3 classification was use to specify sleep disorders that were relevant, or not, to the review. Three types of sleep disorder were not relevant because disturbed sleep is not a diagnostic feature, namely sleep-related breathing disorders, central disorders of hypersomnolence (e.g. narcolepsy) and sleep-related movement disorders. Interventions that addressed sleep disturbances that aligned with the diagnostic features of insomnias or circadian sleep–wake cycle disorders were included. In addition, we included parasomnias because of the potential impact that they could have on parental sleep. However, for reasons noted above, we did not require studies to use the ICSD-3 to define or screen participants. We briefly set out the ICSD-3 definitions of these disorders in the following sections.
Insomnia disorders
The ICSD-3 definition of insomnia is as follows: a persistent difficulty with sleep initiation, duration, consolidation or quality, which occurs despite adequate opportunity and circumstances for sleep and results in some form of daytime impairment. It comprises three subcategories: (1) chronic insomnia disorder, (2) short-term insomnia disorder and (3) other insomnia disorders. In children, chronic insomnia also includes behavioural insomnia disorders, the aetiology of which is located in the practices of parents around bedtime/settling and responding to night/early-morning wakings. 12 Behavioural insomnias are common in childhood, with an even higher prevalence among children with NDs. 14
Circadian rhythm sleep–wake disorders
Circadian rhythm sleep–wake disorders are characterised by abnormalities in the length, timing and/or regularity of the sleep–wake cycle relative to the day–night cycle. It is caused by genetic, neurological or visual pathway damage/disorders affecting circadian rhythms, including melatonin release. 14,15
Parasomnias
Parasomnias were included in the review because of the impact that they have on parents’ sleep. The ICSD-3 defines parasomnias as sleep-related occurrences that represent undesirable physical or cognitive experiences (e.g. sleep terrors, sleep walking) occurring out of sleep, during the transition from sleep to the awake state or from the awake state to sleep. They are more common in children than in adults. 16
Childhood neurodisability
A consensus definition offered by Morris et al. 17 defines NDs as:
. . . congenital or acquired long-term conditions that are attributed to impairment of the brain and/or neuromuscular system and create functional limitations.
A wide range of conditions fall under this definition, including cerebral palsy, autism spectrum disorder (ASD), Down syndrome and other chromosome disorders, epilepsy, attention deficit hyperactivity disorder (ADHD), neurometabolic degenerative conditions (e.g. Batten disease), genetic disorders (e.g. Rett syndrome), as well as non-specific diagnoses such as ‘learning/intellectual disability’ and ‘developmental delay’. The involvement of the brain and/or neuromuscular system means that children can experience a range of impairments (e.g. sensory, learning, physical/motor function and speech and language) and health complications or clinical needs (e.g. respiratory, orthopaedic, gastroenterological and pain management). The severity of impairment can range from mild to profound. The fact that it is not uncommon for some conditions to co-occur adds to the complexity and severity of impairment. It is not surprising, therefore, that many children with NDs are frequent users of the health service at all levels: community, primary care inpatient and outpatient settings. 18
In terms of epidemiology, some NDs are quite common (e.g. autism affects ≈1 in 100 children, cerebral palsies affect ≈1 in 400 children and severe intellectual disabilities affect ≈3 in 1000 children). However, also within this ‘cluster’ of conditions are very rare syndromes [e.g. tuberous sclerosis (incidence of < 1 in 100,000) and ataxia telangiectasia (incidence of < 1 in 40,000)]. 19 Estimates of the overall prevalence of ND among the child population in England vary depending on the measure/indicator used and all are flawed in some way. However, it is generally accepted that ≈4 in 100 children have a ND and that children with NDs constitute the largest group of disabled children. 19
Sleep disturbances in children with neurodisabilities
Sleep disturbances in children with NDs are more common and more severe than in children with typical development. 14,15 However, non-respiratory sleep disturbance is very rarely a diagnostic criterion. Indeed, this is the case only with respect to four conditions: Rett syndrome, Angelman syndrome, Smith–Magenis syndrome and Williams syndrome. 20
The impacts of sleep disturbance
Sleep disturbance can have an impact on all members of the family: the child and their parents and siblings. Indeed, often it is the parents’ own sleep deprivation or poor sleep quality that precipitates them to seek help with their child’s sleep. 9,21 Child sleep problems are associated with poor outcomes for parents, such as heightened levels of parental stress and irritability,22,23 and for children, including poorer educational progress and daytime behaviour problems. 24 These outcomes in themselves increase demands on statutory services, as well as creating further additional support needs, such as respite care. 25,26 The wider association between sleep quality and economic consequences has also been described. 27,28 Parents consistently highlight the need for support with their child’s sleep problems,29,30 although, historically, little time has been allocated to training the relevant professionals to provide this kind of support. 31 Parents, practitioners and other stakeholders agree that research on sleep management interventions is a priority with respect to children with NDs. 32
Interventions for non-respiratory sleep disturbance
Interventions to address sleep disturbance among children with NDs examine both pharmacological and non-pharmacological approaches.
Pharmacological interventions act on an aspect of the physiological processes of sleep and/or the timing of the sleep–wake cycle; the most frequently used interventions are melatonin (a hormone playing a key role in the timing of the sleep–wake cycle), clonidine (which inhibits noradrenaline activity and hence has a soporific effect) and antihistamines [which inhibit neurotransmitters (histamines) that are involved in wakefulness/alertness]. 33–35 All are prescribed ‘off-label’. Other pharmacological interventions are used in relation to children’s sleep, such as medications to manage seizures and pain; however, these are outside the scope of this review because the primary purpose of the intervention is not sleep disturbance.
Non-pharmacological interventions address other causes of disturbances in sleep initiation, maintenance and/or scheduling and are wide-ranging in approach. Interventions that are available within and/or outside the NHS include:
-
behavioural and cognitive behavioural interventions – addressing behavioural aspects of sleep including parents’ management of sleep behaviours and routines
-
chronotherapy – intervening in the timing of sleep within the 24-hour cycle
-
phototherapy (or ‘bright light therapy’) – using light exposure to effect changes in the circadian rhythm
-
dietary interventions – removing stimulants and restricting to hypoallergenic food
-
sensory interventions, including weighted blankets36 and ‘safe space’ bed tents37
-
cranial osteopathy38
-
changing the bedroom environment, for example by removing any televisions or other stimulatory materials and adjusting heating and/or lighting.
Current guidance on the management of sleep disturbance in children advocates that once clinical (e.g. pain or seizures) or respiratory reasons for sleep disturbance are excluded, behavioural approaches that seek to change parents’ responses to sleep-related problems should be the ‘first port of call’ for any child,33,39–41 with pharmacological intervention (and to date, this is typically melatonin) suggested in cases in which such approaches prove ineffective, which should be used alongside behavioural parent-directed approaches. 2,42
The justification for this is that, in common with children with typical development, the origins of sleep difficulties for many disabled children are behavioural, located in the way in which parents address and manage their child’s sleep. 4,43,44 The intensity of behavioural intervention depends on the complexity of the sleep problem and/or child/family-centred factors. Sleep problems that cannot be resolved through low-intensity approaches (e.g. simple information leaflets, verbal information/guidance during a routine appointment, one-off ‘sleep management workshops’) may require a more tailored and sustained approach involving a detailed assessment of the sleep problem, the creation of a bespoke sleep management strategy (based on behaviour modification principles) and time-limited (typically face-to-face) support as parents implement the strategy. In recent years, there has also been an increasing interest in using groups, as opposed to a series of one-to-one sessions, to deliver behavioural sleep support to parents. 45 Furthermore, online ‘self-directed’ sleep management interventions for adults are now available (e.g. Sleepio.com), and the management of sleep disturbance also appears within the curricula of newly available online parenting interventions for parents of children with typical development and disabilities (e.g. Stepping Stones Triple P). Constrained resources and wider changes in the way in which health care is delivered mean that it is likely that these newer modes of delivering behavioural sleep management intervention, such as groups and self-management, will increasingly be considered and used by clinicians and parents. 46 It was important, therefore, that this review examined evidence of the impact that the mode of delivery has on outcomes.
The availability of sleep interventions and the organisation of sleep disturbance services
A number of different services deliver a sleep management intervention to children with NDs. They include community paediatric teams, general practitioners, health visitors, specialist paediatric neurology/autism/ADHD services, child and adolescent mental health services and tertiary sleep services. Within the UK, third-sector organisations (e.g. Sleep Scotland, Scope, the Children’s Sleep Charity) are also highly active in this area. Such organisations offer education/training to parents and professionals on sleep and behavioural approaches to managing sleep difficulties, as well as sleep intervention services. Some NHS trusts are commissioning services of this kind from third-sector organisations such as these.
Although there has been no systematic analysis of the way that sleep disturbance in children with NDs is managed by the NHS, a recent survey of paediatricians by the British Paediatric Respiratory Society led to the conclusion that services for sleep disorders in children were ‘chaotic and unplanned . . . often unfunded and frequently perceived as inadequate for local needs’. 47 Current practice in prescribing medicines such as melatonin has been described as haphazard48 and access to behavioural interventions as patchy. 49
There are a number of reasons for this current state of affairs, including the apparent absence of education on sleep disorders and sleep management in medical school education,50 a lack of recognition of the importance of assessing/checking for sleep disturbance, lack of knowledge/skills or resources to deliver non-pharmacological interventions, parental expectations regarding their child’s sleep, the complexity and range of conditions falling under the umbrella of NDs and the complexity of sleep disturbance and its potential causes. In addition, although there have been some attempts to develop sleep management pathways within paediatrics, these have been restricted to particular types of sleep disturbance and/or sleep intervention and/or diagnostic groups, for which the evidence is more plentiful and/or of higher quality. 47,51,52
Informing the development of a robust evidence base
A robust evidence base is clearly required to inform the development of a paediatric ND sleep management pathway for non-respiratory disturbance that integrates pharmacological and non-pharmacological interventions. A first step towards this, as noted in the commissioning brief, is to ‘take stock’ of the existing evidence base. Our preliminary scoping found that previous systematic reviews have mainly focused on either individual NDs51,53–57 and/or single interventions or pharmacological interventions only. 7,53,56,57 Three reviews that included pharmacological and non-pharmacological interventions were restricted by type of population: children with ASDs,51 children aged 0–12 years with cerebral palsy or traumatic brain injury58 and children with ADHD. 54 One review with a wide population of chronic health conditions in patients aged up to 19 years (including NDs) investigated non-pharmacological interventions (behavioural and non-behavioural);1 another review of behavioural interventions focused on disabled children aged up to 8 years. 59 The search end dates for the previous reviews we identified ranged from 2004 to 2013. None of the previous reviews addressed the research question in the commissioning brief and a new review was considered appropriate.
Given the complexity of sleep disturbance in children with ND, and in contrast to previous reviews, it was essential that our review evaluated pharmacological and non-pharmacological interventions, as much as existing evidence allows, across the range of NDs and age groups. This allows identification of evidence gaps, as well as the accumulation of knowledge on which sleep disturbance interventions work (solely or in combination with other interventions) and for whom they work within the diverse population of children with NDs.
Aims and objectives
There were two overarching aims of this review: (1) to identify the implications for practice for non-respiratory sleep disturbance in children with NDs, evidence permitting, and (2) to inform the focus and priorities of a future call by NIHR for primary research in this area. Unlike previous systematic reviews, we have sought to be holistic in terms of both the population (all children with a ND) and the types of intervention (i.e. pharmacological and non-pharmacological). The objectives were to:
-
evaluate and compare the clinical effectiveness of different intervention approaches to sleep disturbances for children with NDs and, when possible, to:
-
examine whether or not intervention clinical effectiveness differs for different types of ND, different causes of sleep disturbance and different types of sleep disturbance (i.e. sleep initiation, sleep maintenance and sleep scheduling)
-
review and evaluate evidence regarding the use of more than one intervention approach, sequentially or in combination, to manage a specific cause of sleep disturbance
-
review and evaluate evidence regarding the impact that the setting and/or skills/qualifications of practitioners have on intervention clinical effectiveness
-
-
describe and compare evidence regarding the acceptability and feasibility of sleep disturbance interventions
-
describe the settings in which sleep disturbance interventions are being delivered, and by whom
-
make recommendations, when appropriate, with respect to the management of sleep disturbance among children with ND generally and/or with respect to particular NDs
-
identify and describe interventions that look promising and are of relevance to and/or feasible for the NHS but that have not been robustly evaluated
-
make recommendations regarding priorities for future primary research on this topic
-
disseminate the findings in a timely and effective way.
Chapter 2 Methods
This systematic review was undertaken in accordance with Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care60 and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. 61
The review protocol was published prospectively and was registered with PROSPERO as CRD42016034067 (see www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=34067??).
Inclusion and exclusion criteria
Studies were assessed for eligibility based on the criteria detailed in the following sections.
Population
Studies of children and young people with NDs who were experiencing non-respiratory sleep disturbances were eligible for inclusion in the review.
-
Children and young people aged from 0 to 18 years were eligible. We did not expect to find many studies targeted at very young infants. Some previous reviews have used a lower age cut-off point of 3 months and others have not. Given the comprehensive nature of the review, we did not use a lower age cut-off point. ND was defined in accordance with the consensus definition developed by Morris et al. :17
. . . congenital or acquired long-term conditions that are attributed to impairment of the brain and/or neuromuscular system and create functional limitations.
-
Non-respiratory sleep disturbances, of any duration, related to the initiation, maintenance or scheduling of sleep, diagnosed by a health-care professional based on parental/carer or child report or sleep observation were eligible.
-
Excluded non-respiratory sleep disorders were central disorders of hypersomnolence (in which daytime sleepiness is not caused by nocturnal sleep disturbance or misaligned circadian rhythms) and sleep-related movement disorders.
We excluded studies of respiratory-related sleep disturbances. However, NDs are complex conditions and sleep disturbances may have multifactorial causes. Therefore, we included studies in which the respiratory-related component was being controlled and the focus of the intervention was another cause of sleep disturbance. We also excluded studies in which the main focus of the intervention was not treatment of the sleep disturbance (e.g. interventions to control seizures when sleep outcomes were also reported) and studies of mixed populations of children with and without a ND, unless the results were reported separately for the two groups or the sample was predominantly (> 90%) children with a ND.
Intervention
NHS-relevant pharmacological and non-pharmacological interventions targeted at improving sleep initiation, maintenance, scheduling or sleep quality in any setting were eligible for inclusion. For pharmacological studies, ‘NHS relevant’ was defined as relating to drugs that are licensed for use for this indication in children or that are currently used for this purpose in the NHS. For non-pharmacological studies, ‘NHS relevant’ was defined as those interventions meeting current practice standards; for example, behavioural interventions that used punishment were excluded. Multicomponent interventions were eligible.
The NHS-relevant pharmacological interventions were melatonin, clonidine and antihistamines.
The NHS-relevant non-pharmacological interventions included (but were not restricted to):
-
behavioural interventions delivered in a range of settings such as primary, secondary and tertiary or community, outpatient or inpatient that were delivered in groups or to individual children/families by health-care professionals
-
self-help booklets, web-based packages and other online support
-
behavioural/cognitive–behavioural interventions addressing behavioural aspects of sleep, including parents’ management of sleep behaviours and routines
-
chronotherapy – intervening in the timing of sleep within the 24-hour cycle
-
phototherapy (or ‘bright light therapy’) – using light exposure to effect changes in the circadian rhythm
-
dietary interventions – removing stimulants, restricting to hypoallergenic food
-
sensory interventions, including weighted blankets and ‘safe space’ bed tents
-
cranial osteopathy
-
changing the bedroom environment, for example by removing any televisions or other stimulatory materials and adjusting heating and/or lighting.
Comparator
Studies using no intervention, waiting list control, placebo or another NHS-relevant intervention were eligible for inclusion.
Outcomes
The following outcomes were assessed.
Primary outcomes:
-
child’s sleep-related outcomes – parent-/carer- and child-reported outcomes relating to the initiation, maintenance, scheduling or quality of sleep (using measures such as sleep diaries, standardised scales, e.g. the Composite Sleep Disturbance Index or Epworth Sleepiness Scale) and objective measures such as actigraphy (used to calculate outcomes such as total sleep duration, time taken to fall asleep or sleep efficiency)
-
parent sleep-related outcomes – quality of sleep
-
measures of perceived parenting confidence and/or efficacy and/or understanding of sleep/sleep management (which are particularly relevant for parent training/behavioural interventions that seek to change the way that parents manage sleep disturbance).
Secondary outcomes:
-
child-related quality of life, daytime behaviour and cognition
-
parent/carer quality of life and well-being, including global quality of life (e.g. Short Form questionnaire – 36 items) and more specific outcomes such as physical well-being, mental well-being, and mental health (e.g. stress, depression)
-
family functioning
-
adverse events, including side effects from medication.
Data on uptake of the intervention, retention and intervention adherence were used as indicators of the acceptability and feasibility of the intervention. Quantitative or qualitative data on parents’/children’s experiences of receiving a sleep disturbance intervention included:
-
the acceptability and feasibility of the intervention
-
other experiences of receiving the intervention
-
satisfaction with intervention outcomes and ‘fit’ with their priorities with regard to their child’s sleep disturbances; views/perspectives on the mechanisms by which outcomes were achieved.
Study design
Randomised controlled trials (RCTs) and non-randomised controlled studies, such as controlled before-and-after studies and cohort studies with a control group, were included. Both parallel and crossover RCTs were eligible for inclusion. Concerns have been expressed by others that a crossover design may be inappropriate owing to uncertainty about the duration of the effect of interventions on sleep patterns and circadian rhythm and, therefore, on the most appropriate duration for the washout period. 48 We agree with these concerns. However, given that the aim was to undertake a broad review and, as there were few RCTs likely to be available, we included crossover studies.
In order to achieve the second objective of the review, studies without a control group were included in the absence of controlled studies, that is, cohort studies and before-and-after studies. This was because they could include potentially promising interventions that were at an early stage of evaluation. Case studies were not eligible for inclusion.
Qualitative and quantitative studies were included if they reported data on parents’ or children’s experiences of receiving a sleep disturbance intervention (including intervention acceptability), such as the process of receiving the sleep intervention, satisfaction with intervention outcomes and ‘fit’ with their priorities with regard to their child’s sleep disturbances, and views/perspectives on the mechanisms by which outcomes were achieved. Data could be collected as part of studies of clinical effectiveness or studies that only sought to examine research questions on experiences and satisfaction.
Literature searches
The available evidence was identified by carrying out systematic searches of electronic databases, and reference checking of relevant reviews and included studies. The list of included studies identified from the electronic searches was shared with clinicians in the team to establish if there were any relevant studies that were missing. The searches were undertaken by an experienced information specialist and the search strategy was peer reviewed by a second information specialist.
Information sources
A range of databases were searched in February and March 2016 and updated in February 2017 to ensure coverage from the fields of health, nursing and allied health, and social care. We searched Applied Social Sciences Index and Abstracts (ASSIA), The Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews, Conference Proceedings Citation Index, Cumulative Index to Nursing & Allied Health, Database of Abstracts of Reviews of Effects, EMBASE, Health Management Information Consortium, MEDLINE, MEDLINE In-Process & Other Non-indexed Citations, PsycINFO, Science Citation Index, Social Care Online and Social Policy & Practice.
The Social Care Online, Social Policy & Practice, Health Management Information Consortium, Conference Proceedings Citation Index and PsycINFO all provide some coverage of reports and other unpublished documents; therefore, the available grey literature are represented in the search results.
In addition, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform and the UK Clinical Trials Gateway were searched for trials, both ongoing and completed. No limits on date, language or study design were applied in the searches. Full details of search strategies used and numbers of records retrieved are given for each database in Appendix 1.
While the search results were being scanned, some additional search terms that had not been included in the original search strategies were identified. Consequently, we carried out some further searches of each of the databases that incorporated these new terms alongside the original search strategy. Details of these additional search strategies can be found in Appendix 2.
The reference lists of relevant systematic reviews and included studies were also scanned.
Screening and study selection
The database search results were all loaded into EndNote bibliographic software [version 17.0.2.7390, Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and deduplicated.
There was a three-stage screening process to manage the large number of records. First, the titles of the records were screened for relevance. Two researchers did this jointly for 10% of the titles, and the remainder were screened independently by a single researcher. Records that were identified as potentially relevant based on their title were screened independently by two researchers. When there was no consensus, a third member of the team was consulted. The full texts of potentially relevant papers were ordered. Finally, full papers were independently screened against the eligibility criteria by two researchers. Disagreements were resolved by consensus or by consultation with a third team member if necessary.
Data extraction
A data extraction form for study details was developed and piloted. A Microsoft Excel 2010® spreadsheet (Microsoft Corporation, Redmond, WA, USA) was used to extract the outcome data. Owing to the various ways in which adverse events were described in papers, data for this outcome were extracted separately into a table using Microsoft Word 2010® (Microsoft Corporation, Redmond, WA, USA). All data were extracted by one researcher and checked by a second. Details of the data items extracted are available in Appendix 3. For the purposes of this review, we extracted follow-up data relating to the assessment time point closest to the end of the intervention.
Assessment of risk of bias
The Cochrane risk of bias tool62 was used to assess the quality of RCTs and the newly developed tool, A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI), was used to assess the non-randomised controlled before-and-after studies. 63 Uncontrolled before-and-after studies were assessed using questions adapted from ACROBAT-NRSI, as used in another HTA review. 64 Risk of bias was independently assessed by two researchers. Disagreements were resolved through consensus and through discussion with a third researcher if necessary. In addition, for crossover trials, we assessed whether or not an appropriate analysis using paired data was conducted and whether or not there was a treatment by period interaction, as undertaken in a previous systematic review including crossover studies. 65
A summary risk-of-bias score was calculated following guidance. 62 This score was calculated as follows: any study that had one or more of the domains on the risk-of-bias tool classified as ‘no’ was considered to be rated as having a high risk of bias. Any study that had one or more domains classified as ‘unclear’ on the risk-of-bias tool was considered to be rated as having an unclear risk of bias. To be considered to have a rating of a low risk of bias, a study needed to meet the criteria on all domains on the risk-of-bias tool and classify them as ‘yes’.
For studies containing qualitative and quantitative data on parents’ and/or children’s satisfaction with the intervention, take-up, retention and adherence to the intervention and experiences of the intervention, the quality of the study was assessed and reported using the quality appraisal checklist of Hawker et al. 66
It was not possible to blind the types of non-pharmacological interventions and comparators used in the studies under consideration. In addition, owing to the nature of the outcomes measured, robust, blinded outcome assessment was difficult. Although actigraphy-based child sleep outcomes are more objective than parent-reported measures, we did not consider these to be true objective outcomes, with non-blinding unlikely to introduce bias. Therefore, all of the measures were regarded as having the capacity to be influenced by lack of blinding.
Analysis
The synthesis aimed to:
-
Assess the clinical effectiveness of the interventions for sleep disturbance, in particular interventions that may work across conditions.
-
Inform future research by identifying gaps in the evidence and identifying interventions that are the most promising front runners to be considered for future primary research.
First, narrative and tabular summaries of key study characteristics were undertaken. This allowed a mapping of which interventions have been investigated for which ND and for which type of sleep disturbance (e.g. sleep initiation) in order to identify interventions that have been investigated across conditions. We also mapped information on the feasibility and acceptability of each of the interventions.
Synthesis involved paired meta-analyses and narrative synthesis.
Meta-analyses
Pharmacological intervention studies
When sufficient data for our primary and secondary outcomes were available, they were pooled in quantitative synthesis using a random-effects model (for continuous outcomes). As data sets often included both parallel and crossover trials, or just crossover trials, data were pooled using the generic inverse variance method in RevMan version 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). 67 Only crossover trials with a washout period were included in meta-analyses, for which data from both treatment periods were used. For trials without a washout period, we considered using data from the first period only; however, in the event, this was not possible, as data summaries were not provided by treatment period for these trials.
The recommendations provided in the Cochrane handbook were followed as closely as possible. 68 The mean difference (MD) between melatonin (M) and placebo (P) at the end point was either taken as reported in the article or calculated as the difference in means for each group/period:
When a 95% confidence interval (CI) for the group means was presented instead of a standard deviation (SD) (e.g. Garstang and Wallis69), the SD was calculated using the formula:
where the t-value was obtained by entering = tinv(1–0.95, N-1) in a cell in a Microsoft Excel spreadsheet.
For the crossover trials, when only means and SDs for the measurements of the intervention (melatonin) and the control (placebo) were available, the SD of within-participant differences between M and P measurements was estimated using the formula:
The correlation coefficient ρ was estimated from other studies reporting all three SDs for the same outcome or by using 0.5 when no other studies were available to use.
The standard error (SE) of the MD was calculated using:
where N is the sample size, or by dividing the MD by the t-statistic when this was presented for a two-sample t-test of the period differences (e.g. Weiss et al. 70).
To transform the parallel-group trial data for entry into the generic inverse variance facility, a two-sample t-test was conducted to calculate the unadjusted difference (and SE of the MD) between the groups at follow-up (post intervention) using raw group summary data (N, mean and SD).
Statistical heterogeneity between trials was assessed using the I2 statistic. 71 Two sources of potential clinical and methodological heterogeneity were identified for the pharmacological intervention trials, and subgroup analyses were conducted based on these, when appropriate:
-
type of neurological disorder – population primarily with ASD
-
receipt of prior intervention – whether or not participants were offered an additional intervention prior to the start of the study.
The risk of publication bias was not formally assessed.
When data could not be pooled, summaries of the findings for each trial and outcome are presented with a (estimated unadjusted) MD and 95% CI between melatonin and control at follow-up.
Adverse event data are summarised narratively.
Non-pharmacological intervention studies
Non-pharmacological intervention studies included parallel-group RCTs and before-and-after studies. Owing to insufficient data for each outcome and/or significant heterogeneity in study design and intervention, data were not pooled in meta-analyses. Narrative and quantitative summaries of the findings for each trial and outcome are presented. For continuous data in the RCTs, the preferred choice was difference in end-point data; however, when a (estimated unadjusted) MD between intervention and control at follow-up could not be calculated, the difference in change scores from baseline to follow-up was presented instead.
We had planned to undertake a mixed-treatment comparison of the multiple treatment options;72 however, this was not possible owing to the paucity of RCTs for interventions other than melatonin. Adverse event data are summarised narratively.
Narrative synthesis
Although we planned to undertake separate quantitative and qualitative syntheses, this was to a large extent not possible, as few studies could be pooled. With the exception of the melatonin trials, there was a large degree of variability between studies evaluating different classes of interventions. For example, for behavioural interventions, there was wide variability in aspects of the interventions such as mode of delivery, duration and intensity of interventions, as well as in the comparators used. There was also variability in the conditions being studied, outcomes reported and the measures used to assess individual outcomes, follow-up times and types of data reported. Consequently, there were few instances in which it became appropriate to pool the data. Thus, it became necessary to adopt a principally narrative synthesis to report the findings. We present a narrative synthesis as our main analysis, with comparisons made for melatonin versus placebo, which were the only interventions that we judged could be appropriately compared.
Narrative synthesis was undertaken when quantitative synthesis was not appropriate or there were insufficient data, and applied mainly to the non-pharmacological interventions. When possible, we display outcomes in a forest plot, even when studies are not statistically pooled, to aid exploration of study results. When feasible, we investigated the subgroup characteristics outlined previously. Non-pharmacological studies were grouped by type of intervention (i.e. comprehensive parent-directed tailored, comprehensive parent-directed non-tailored or non-comprehensive) and comparator if heterogeneous. We explored outcomes by type of sleep disturbance with the aim of identifying effects that may be transferable to other NDs. Results are discussed in the context of ratings of risk of bias in the individual studies.
In terms of the qualitative data analysis, the topic areas that were subject to review were well defined; we therefore adopted a thematic approach to data extraction, analysis and synthesis. 60,73 To start, studies were grouped into pharmacological, behavioural and other non-pharmacological studies. For each, a descriptive report of relevant studies, and topic areas covered, was produced. The tabulated data were then scrutinised and analytical notes were made that summarised findings across studies with respect to the topic areas. Part of this process involved testing for contradictions in the evidence. 74
The synthesis interrogated such data, when available, to assist in identifying interventions that could be generalisable across conditions and those that are condition specific. 75,76 Factors taken into consideration in identifying promising interventions included feasibility of delivery of the intervention in a NHS setting, acceptability to children and families, evidence of clinical effectiveness or in the direction of clinical effectiveness based on CIs (taking into consideration the clinical significance of the estimates).
Protocol changes
During the course of the review, we identified numerous RCTs for the melatonin studies. We therefore decided to include only RCTs for this intervention. For all other interventions, we have included any design except case studies, as per the original protocol. Some studies reported multiple case studies that were not eligible for inclusion in the review. We made the decision to exclude uncontrolled studies with fewer than 10 participants.
Patient and public involvement
Three parents of children with NDs (two mothers, one father) acted as project advisors. They were recruited from a permanent parent consultation group of the chief investigator’s research unit. These parents were invited to the project team meetings, which were held three times over the course of study and were attended by the research team and all co-applicants. Each parent attended at least one meeting. They were also consulted, via e-mail, regarding the implications of the findings of the review. The children’s diagnoses included autism and rare, genetic conditions. At the first meeting, an early item on the agenda was a presentation of an overview of systematic reviews as a research method. Throughout the meetings, the parents were encouraged to share their experiences and opinions, and their contributions provided useful contextual information.
Chapter 3 Results
Study selection
The searches identified 23,292 records: 21,529 records were identified from the original searches undertaken in February and March 2016, 1563 were identified from the updated searches undertaken in February 2017, 194 were identified from trial registries and 6 were identified from subsequent reference checking (Figure 1). After removing duplicate references, 15,745 titles were screened. On the basis of titles, 14,420 titles were excluded; a further 937 were excluded on reading the abstract.
FIGURE 1.
Flow chart of the study selection process.
Full-text articles were sought for the remaining 388 records. We could not obtain full-text articles for 30 of the records. Of these, 11 were conference abstracts that were not available in full text22,77–86 and five were trial registry entries that were recorded as ‘complete’ but had no study results available. 87–91 We contacted the authors to check whether or not there were any publications from the trials and received one response that the authors did not intend to publish the results as fewer than 10 participants had been recruited into the study. 91 Two trials were registered as ‘terminated’ with no published results (one owing to poor recruitment,92 and the other for unknown reasons 93 and our attempt to contact authors did not elicit a response). One trial was registered as ‘study status unknown’94 with no published results; we contacted the authors but received no response. Five trials were eligible but ongoing so were excluded from the review as results were not yet available. 95–99 A further six articles could not be obtained from the British Library. 100–105
A total of 358 full-text articles were, therefore, assessed, including two non-English language papers that required translation. Of these, 117 articles (33%) were excluded because the intervention was out of scope or sleep disturbance was not the target of the intervention, 110 (31%) were excluded based on study design, 55 (15%) were excluded owing to the population, 6 (2%) were excluded based on the outcomes evaluated and 5 trials (1%) identified from trial registries were related to studies that had been completed and had already been identified through the searches and included in the review. 36,48,49,106,107 Appendix 4 lists all studies excluded at full-text screening and reasons for exclusions.
Overview of included studies
There were 39 included studies reported in 64 articles. Although we sought to include studies published in any language, all the studies meeting the eligibility criteria were published in English. The included studies were from the UK (n = 13, 33%), the USA (n = 10, 26%), Australia (n = 6, 15%), Canada (n = 5, 13%), the People’s Republic of China (n = 1, 2.6%), the Netherlands (n = 1, 2.6%), Hong Kong (n = 1, 2.6%), Italy (n = 1, 2.6%) and Israel (n = 1, 2.6%).
Thirteen RCTs investigated a pharmacological intervention, which in all cases was oral melatonin (Table 1). We did not identify any eligible pharmacological studies investigating clonidine or antihistamines. Twenty-six studies investigated non-pharmacological interventions, of which 12 were RCTs. Of these, nine (35%) evaluated parent-directed tailored interventions; eight (31%) evaluated parent-directed non-tailored interventions; two (8%) evaluated non-comprehensive parent-directed interventions; and seven (27%) evaluated other non-pharmacological interventions – dietary interventions (n = 2, 5%), alternative medicine (n = 1, 3%), exercise-based intervention (n = 1, 3%), faded bedtime with response costs (n = 1, 3%), weighted blankets (n = 1, 3%) and a light therapy plus a behavioural programme (n = 1, 3%). Thirteen studies explored feasibility and/or acceptability and/or parent/clinician views of sleep disturbance interventions. 36,49,106,107,122–130
| Study details and design | Participants randomised (total N and by group) | Trial treatments | Sleep disturbance | Mean age (SD) | ND disorder | Previous sleep hygiene/behavioural interventions | Risk of bias (low, unclear, high) |
|---|---|---|---|---|---|---|---|
| Melatonin vs. placebo: parallel-group RCTs | |||||||
| Appleton et al. (2012)48 | |||||||
|
Associated publications: Gringras et al. (2012),108 Appleton et al. (2011),109 Appleton et al. (2012)48 UK |
N = 146 (melatonin, n = 70; placebo, n = 76) |
Melatonin: 0.5 mg, 45 minutes before bedtime for 12 weeks Placebo: matching capsule, 0.5 mg for 12 weeks Melatonin and placebo dose could be raised to 2 mg, 6 mg to 12 mg in first 4 weeks then maintained |
Problems with sleep initiation and sleep maintenance |
Melatonin: 106.0 months (34.8 months) Placebo: 100.7 months (37.4 months) |
DD alone, and DD + other | Yes | Low |
| Cortesi et al. (2012)110 | |||||||
| Italy | N = 160 (melatonin, n = 40; melatonin and CBT, n = 40; CBT, n = 40; placebo, n = 40) |
Melatonin: controlled release, 3 mg at 21.00 hours for 12 weeks CBT: four, weekly individual sessions Melatonin and CBT: as above Placebo: identical tablet, 3 mg at 21.00 hours for 12 weeks |
Problems with sleep initiation and sleep maintenance |
Melatonin: 6.8 years (0.9 years) Melatonin and CBT: 6.4 years (1.1 years) CBT: 7.1 years (0.7 years) Placebo: 6.3 years (1.2 years) |
ASD | None stated | High |
| Van der Heijden et al. (2007)111 | |||||||
|
Associated publications: Hoebert et al. (2009)112 The Netherlands |
N = 107 (melatonin, n = 54; placebo, n = 53) |
Melatonin: fast release, 3 mg (if < 40 kg), 6 mg (if > 40 kg) at 19.00 hours for 4 weeks Placebo: identical appearing placebo at 19.00 hours for 4 weeks |
Problems with sleep initiation |
Melatonin: 9.1 years (2.3 years) Placebo: 9.3 years (1.8 years) |
ADHD | None stated | Unclear |
| Crossover trials | |||||||
| Camfield et al. (1996)113 | |||||||
|
Individualised ‘N of 1 crossover trials’ Canada |
N = 6 |
Melatonin: 0.5 mg for three cases, 1.0 mg for three cases, taken at 18.00 hours Placebo: identical capsule Ten-week trial. For each of the five 2-week intervals, participants were randomised to receive placebo or melatonin for 1 week with the alternate agent given in the second week |
Problems with sleep initiation and sleep maintenance | 7.3 years (4.6 years) | Mixed | Yes | High |
| Dodge and Wilson (2001)114 | |||||||
|
Associated publications: Hoebert et al. (2009)112 USA |
N = 36 |
Melatonin: 5 mg at 20.00 hours for 2 weeks Placebo: capsule and filler packaged to be identical to the melatonin, 5 mg at 20.00 hours for 2 weeks Washout period: 1 week |
‘Chronic sleep problems’ | 89 months (NR) | Mixed (mainly cerebral palsy) | Yes | Unclear |
| Garstang and Wallis (2006)69 | |||||||
| UK | N = 11 |
Melatonin: 5 mg for 4 weeks Placebo: dose NR, for 4 weeks Washout period: 1 week |
Problems with sleep initiation and sleep maintenance | 8.6 years (3.1 years) | ASD only, and ASD + learning disability | Yes | High |
| Jain et al.115 (2015) | |||||||
|
Associated Publications: Jain et al. (2014)116 USA |
N = 11 |
Melatonin: sustained release, 9 mg 30 minutes before bedtime for 4 weeks Placebo: identical appearance to melatonin tablets, dose NR Washout period: 1 week |
A score of > 30 on the Sleep Behaviour Questionnaire | 8.4 years (1.3 years) | Epilepsy | None stated | High |
| Wasdell et al. (2008)117 | |||||||
|
Associated publications: Carr et al. (2007)118 Canada |
N = 51 |
Melatonin: controlled release, 5 mg, 20–30 minutes before bedtime for 10 days Placebo: identical to melatonin, 5 mg, 20–30 minutes before bedtime for 10 days ‘Placebo washout’: 3–5 days |
Problems with sleep initiation and sleep maintenance | 7.4 years (NR) | Mixed | Yes | Unclear |
| Weiss et al. (2006)70 | |||||||
| Canada | N = 23 |
Melatonin: short-acting, 5 mg, 20 minutes before bedtime for 10 days Placebo: for 10 days ‘Placebo washout’: 5 days |
Problems with sleep initiation | 10.3 years (NR) | ADHD | Yes | Unclear |
| Wirojanan et al. (2009)119 | |||||||
| USA | N = 18 |
Melatonin: 3 mg, 30 minutes before bedtime for 2 weeks Placebo: 3 mg, 30 minutes before bedtime for 2 weeks No washout period |
‘Sleep problem’ | 5.5 years (3.6 years) | Mixed (fragile X Syndrome/ASD) | None stated | Unclear |
| Wright et al. (2011)106 | |||||||
| UK | N = 20 |
Melatonin: standard release, 2 mg, 30–40 minutes before bedtime for 3 months Placebo: identical to melatonin, 2 mg, 30–40 minutes before bedtime for 3 months Melatonin and placebo dose increased by 2 mg every 3 nights to a maximum of 10 mg. Taken for 3 months Washout period: 1 month |
Problems with sleep initiation and sleep maintenance | 9.0 years (2.9 years) | Mixed (autism and Asperger syndrome) | Yes | Unclear |
| Melatonin vs. melatonin-crossover trials | |||||||
| Hancock et al. (2005)120 | |||||||
| UK | N = 8 (≤ 18 years, n = 5) |
Melatonin: 1 × 5 mg plus 1 × 5-mg placebo, 30 minutes before bedtime for 2 weeks Melatonin: 2 × 5 mg of melatonin (10 mg in total), 30 minutes before bedtime for 2 weeks Washout period: 2 weeks |
Quine sleep index score of at least 6 |
All: 12.1 years (10.0 years) ≤ 18 years: 6.9 years (4.0 years) |
Tuberous sclerosis | None stated | High |
| Jan et al. (2000)121 | |||||||
| Canada | N = 16 (≤ 18 years, n = 15) |
Melatonin: sustained release, variable doses from 2 mg to 10 mg, 30 minutes before bedtime for 11 days Control: fast-release melatonin, variable doses from 2 mg to 10 mg for 11 days No washout period |
‘Chronic sleep wake disorders’ |
All: 10.1 years (4.9 years) ≤ 18 years: 9.3 years (4.1 years) |
Mixed | None stated | High |
The mean age of the children included in the studies ranged from 2 to 12 years. In 16 studies (41%), participants were described in terms of a single ND diagnosis as follows: ADHD (n = 5), autism spectrum condition (ASC) (n = 3), ASD (n = 3), epilepsy (n = 1), tuberous sclerosis (n = 1), ‘mental retardations’ (we have replaced this term with ‘learning disability’; this is interchangeable with the term ‘intellectual disability’) (n = 2) and severe ND (n = 1). In 22 studies (56%), participants were reported to have two or more NDs. One study did not report participants’ NDs. There was also a range of sleep disturbance represented in the eligible studies. Owing to the different terminology used to describe sleep disturbances in the eligible studies, we have classified sleep disturbance under the following headings: sleep initiation (n = 30, 77%) (e.g. sleep latency, sleep association, settling, bedtime resistance and insomnia); sleep maintenance (n = 26, 67%) (e.g. night waking, waking time, parasomnia, co-sleeping and sleep fragmentations) and sleep scheduling (n = 1, 2.5%) (e.g. daytime sleepiness). Three studies (7%) reported that parents completed sleep questionnaires or assessment tools to determine their child’s eligibility, including the Sleep Behaviour Questionnaire,131 Quine sleep index132 and the Children’s Sleep Habits Questionnaire (CSHQ). 133 Five studies (13%) did not specify the types of sleep disturbance eligible for inclusion. Children often had multiple sleep disturbances.
Melatonin
Study characteristics
Table 1 is a summary of the characteristics of the melatonin studies, and Appendix 5 provides further details. Of the 13 RCTs evaluating oral melatonin, which were undertaken in Canada, Italy, the Netherlands, the UK and the USA, 10 compared melatonin with placebo only; one compared melatonin, cognitive–behavioural therapy and a combination of the two with placebo; and two compared two regimens of melatonin (5 mg vs. 10 mg, and fast release vs. sustained release). Ten were crossover trials and three were parallel-group RCTs. Observed sample sizes ranged from 6 to 160 participants.
Assessment of risk of bias
A summary assessment of risk of bias for each RCT is provided in Table 1 and the full risk-of-bias assessment involving each bias domain is provided in Appendix 6 and Report Supplementary Material 1. One trial was rated as having a low risk of bias. 48 The ratings of risk of bias in the remaining RCTs were high or unclear; therefore, the findings from these studies may not be robust.
We could not locate a registered protocol for nine trials69,70,106,110,113–115,117,119 and one trial protocol was registered retrospectively,111 making it unclear whether or not the studies were free of selective reporting. Seven studies provided no, or little, detail regarding sequence generation;70,106,111,113,114,117,119 and four studies provided little or no detail regarding allocation concealment. 110,113,114,119 For three studies, how blinding was undertaken was unclear. 69,70,113 In three studies, the analysis of incomplete outcome data was not considered69,110 or was unclear. 114
Melatonin versus placebo
Eleven trials (n = 589 randomised participants) compared melatonin with placebo: eight crossover trials,69,70,106,113–115,117,119 two two-armed parallel-group trials,48,111 and one four-armed trial of oral melatonin, cognitive–behavioural therapy, oral melatonin plus cognitive–behavioural therapy and placebo. 110
The washout period used by the crossover trials varied. One had no washout period,119 one had a 1-month washout period106 and the remaining five trials had a washout period of between 3 and 7 days. 69,70,114,115,117 Camfield et al. 113 reported six ‘N of 1’ crossover trials with no washout period.
Six of the trials varied drug dosages depending on the child’s age and/or weight and tolerance of dosage (see Table 1). The dose ranges were 0.5–1 mg,113 0.5–12 mg,48 3–6 mg111 and 2–10 mg. 106 Fixed dosages were 3 mg (n = 2),110,119 5 mg (n = 4)69,70,114,117 and 9 mg. 115 Matched placebos were used except for in one trial,70 in which the placebo was not explicitly described, although in this trial the authors reported that each patient received a blister card of 30 days’ supply of medication.
The length of time that melatonin was prescribed for varied between studies: 10 days,70,117 2 weeks,114,119 4 weeks,69,111,115 5 weeks113 and 12 weeks. 48,106,110
The age of participants included in these trials ranged from 1 to 18 years, although the mean age across studies was broadly similar, ranging from 5.5 to 10.3 years (see Table 1). The ND that was represented varied. Four trials included children with a mixed range of neurodevelopmental disabilities,48,113,114,117 three trials included only children with ASC,69,106,110 two trials included children with ADHD,70,111 one trial included children with ASD and/or fragile X syndrome119 and one trial included children with epilepsy. 115
The type of sleep disturbances reported in the participants also varied. Most trials had more than one criterion for inclusion in the trial and included children with a mix of sleep disturbances (see Table 1) relating to sleep initiation48,69,70,106,110,111,113,117 and sleep maintenance. 48,69,106,110,113,117 However, two trials focused on a single sleep problem (‘sleep onset insomnia’111 and ‘initial insomnia’70) and one trial required a score of > 30 in the Sleep Behaviour Questionnaire. 115 Two studies did not specify the type of sleep problems eligible for inclusion. 114,119
Seven trials specified in their inclusion criteria that a preceding psychoeducational or behavioural sleep management intervention had to have been ineffective. 48,69,70,106,113,114,117 In five of these trials, guidance on sleep was provided as part of the trial in the form of a behaviour therapy advice booklet,48 tailored sleep hygiene advice,117 a sleep hygiene intervention,70 a sleep hygiene advice leaflet69 and behaviour management and parenting support. 106 In four of these trials, only children who continued to experience sleep problems were randomised. 48,70,106,117
Across these 11 trials, 19 sleep-related outcomes were measured (see Appendix 7). The most commonly measured outcomes were total sleep time (TST) (n = 11), sleep onset latency (SOL) (n = 10), number of night wakings (n = 6) and sleep efficiency (n = 5). Additional outcomes included arousals, bedtime, CSHQ, difficulty falling asleep, sleep onset, time from drug to sleep, longest sleep episode, the Sleep Behaviour Questionnaire, percentage of sleep stages, wake after sleep onset (WASO), nights without awakening, wake-up time, naptime, moving time, ‘interdaily stability’, ‘interdaily variability’ and ‘L5’ (average activity during the least active 5 hours). Definitions of outcome measures can be found in Appendix 7.
All trials had follow-up periods that commenced immediately following the completion of the intervention: 10 days,70,117 2 weeks,114,119 4 weeks,69,111,115 10 weeks113 or 12 weeks. 48,106,110
Global measures and composite scores
Total sleep time
All 11 trials48,69,70,106,110,111,113–115,117,119 (n = 589 randomised participants) assessed TST (see Appendix 7); four measured this using parent-reported sleep diaries only,69,106,113,114 whereas the remaining seven48,70,110,111,115,117,119 used both actigraphy and sleep diaries. However, of these, three report only actigraphy-measured TST data,110,111,119 as the sleep diaries were used purely to inform, or verify, actigraphy data. This follows existing guidance on the interpretation of actigraphy data. 134 The remaining four trials48,70,115,117 reported TST derived from both parent-completed sleep diaries and actigraphy data.
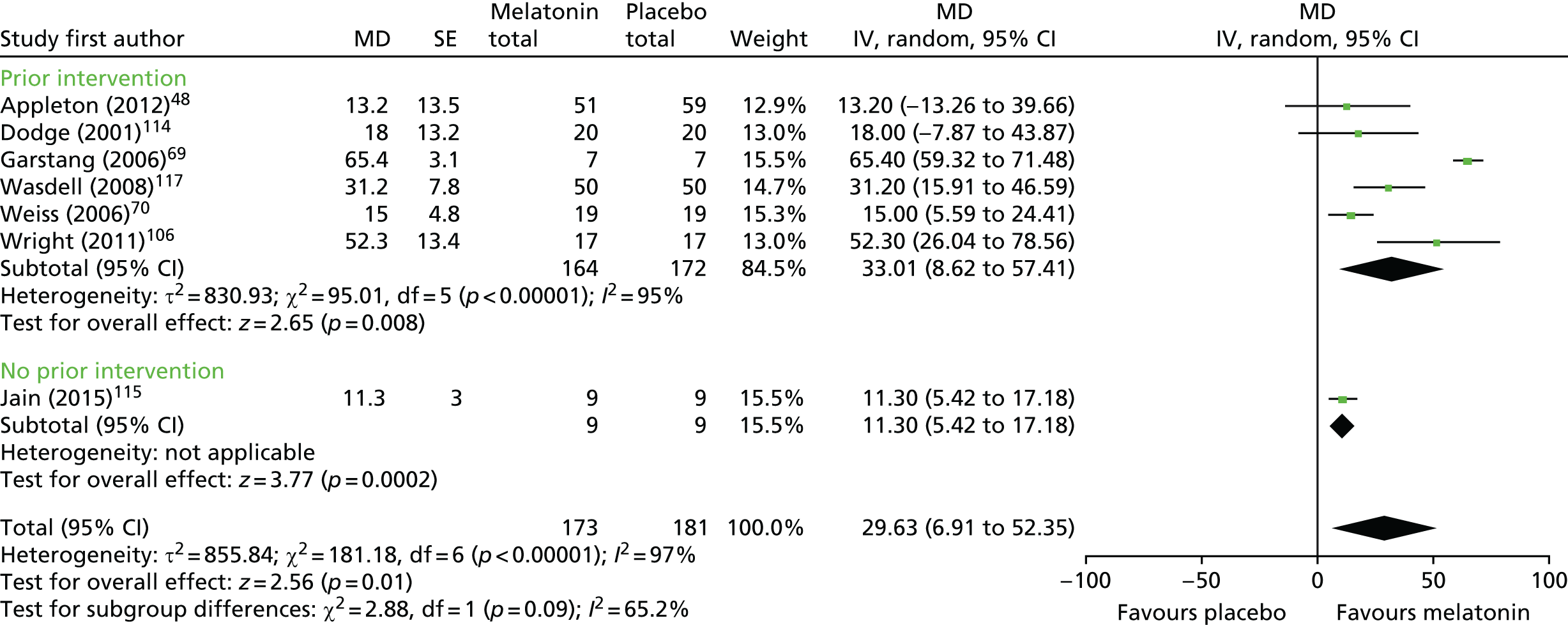
Data from seven trials using sleep diary-reported TST were pooled: six crossover trials with a washout period (n = 122 analysed participants),69,70,106,114,115,117 and one parallel-group trial (n = 110). 48 Note that in the forest plot (Figure 2), the sample sizes presented count participants in crossover trials twice (as being in the melatonin and placebo groups), so the figures reported in the text and shown in the figures may not match for this reason. There was a statistically significant increase in sleep diary-reported TST with melatonin compared with placebo (pooled MD 29.6 minutes, 95% CI 6.9 to 52.4 minutes; p = 0.01; see Figures 2 and 3). Statistical heterogeneity was high (I2 = 97%) and this treatment effect is unlikely to be generalisable, although the effect estimates were all in the direction of benefit with melatonin. Heterogeneity was reduced when studies were stratified based on whether or not the study population was exclusively ASD or not (test for subgroup differences: p < 0.001; I2 = 99%); there was a pooled MD of 64.7 minutes (95% CI 58.8 to 70.7 minutes, I2 = 0%) for the studies of ASD (n = 24), and a smaller pooled MD of 15.9 minutes (95% CI 9.2 to 22.6 minutes, I2 = 31%) for the studies of mixed or other populations (n = 208). There was only a single study (n = 9) in which participants had no prior sleep hygiene or behavioural intervention limiting the usefulness of this subgroup analysis; the overall results did not substantially change with removal of this study (pooled MD 33.0 minutes, 95% CI 8.6 to 57.4 minutes, n = 223; Figure 3). When the single trial rated as having a low risk of bias is considered alone, the increase in sleep time with melatonin was 13.2 minutes (95% CI –13.3 to 39.7 minutes). 48
FIGURE 2.
Sleep diary-reported TST: melatonin vs. placebo and ASD subgroup analysis. df, degrees of freedom; IV, instrumental variable.
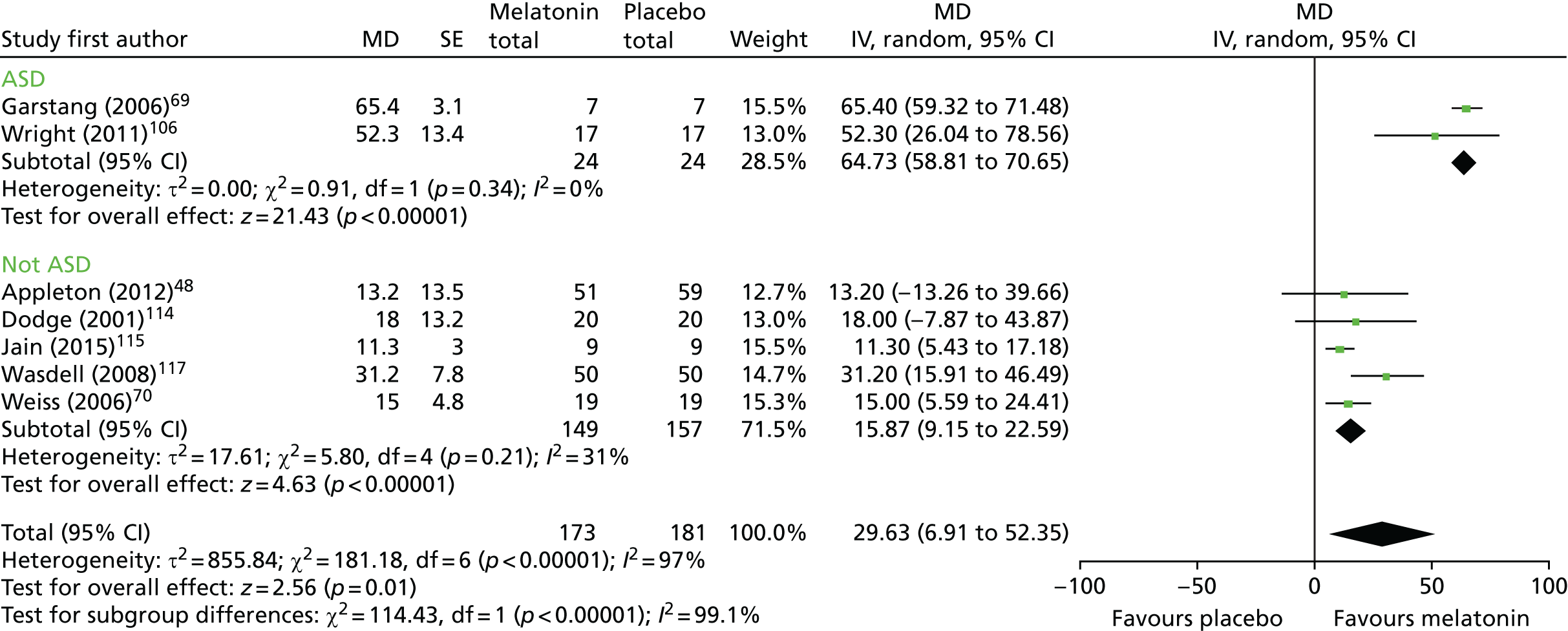
FIGURE 3.
Sleep diary-reported TST: melatonin vs. placebo and prior intervention subgroup analysis. df, degrees of freedom; IV, instrumental variable.
The study of six ‘N of 1’ trials in six participants also reported sleep diary TST but, owing to the unusual trial design, could not be included in the meta-analysis. 113 The authors reported no ‘notable difference in sleep pattern or daytime behaviour between melatonin and placebo weeks’113 and provided raw data for each participant. From this, we calculated the MD between melatonin and placebo for parent-reported TST to be 13.9 minutes in favour of the melatonin group (95% CI –6.8 to 34.6 minutes; p = 0.14).
Five trials48,110,111,115,117 (n = 266 analysed participants) were pooled for actigraphy-measured TST, comprising two crossover trials with a washout period (n = 60)115,117 and three parallel-group trials (n = 206). 48,110,111 Weiss et al. 70 used actigraphy to measure TST post intervention and reported that there was no significant difference between melatonin and placebo, but the study did not provide data on this outcome to allow it to be included in the meta-analysis.
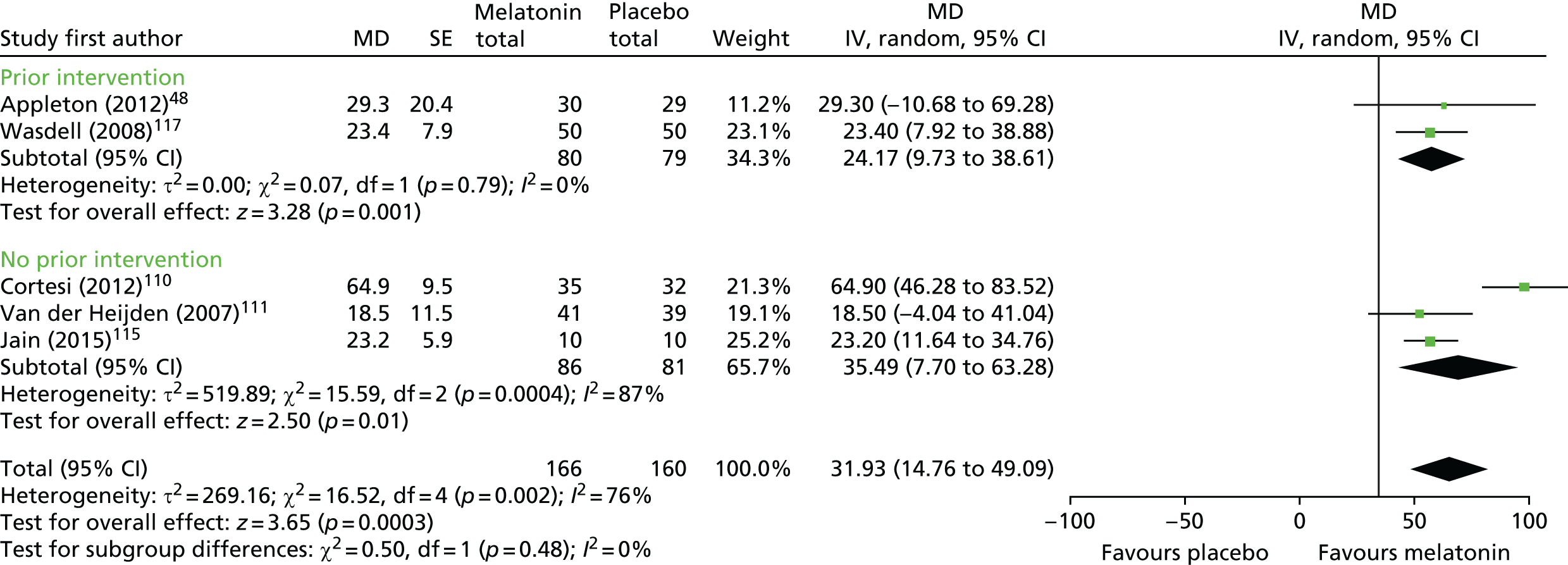
There was a statistically significant increase in actigraphy-measured TST with melatonin compared with placebo (pooled MD 31.9 minutes, 95% CI 14.8 to 49.1 minutes; p < 0.001) (Figure 4). Heterogeneity was high (I2 = 76%) and this treatment effect is unlikely to be generalisable, although the effect estimates were all in the direction of benefit with melatonin. There was no statistically significant difference in effect between the studies in which participants received or did not receive a prior intervention (test for subgroup differences, p = 0.48; I2 = 0%). Subgroup analysis by type of ND was not possible.
FIGURE 4.
Actigraphy-measured TST: melatonin vs. placebo. df, degrees of freedom; IV, instrumental variable.
One study without a washout period119 (n = 12 analysed participants) reported a MD in actigraphy-measured TST post intervention of 21 minutes between melatonin and placebo, favouring melatonin. Using non-parametric analysis methods, the authors report p-values of 0.0019 and 0.02 for this outcome, based on data sets produced using two different approaches for dealing with missing data (complete case and last observation carried forward). This outcome was also analysed using a paired t-test, giving a p-value of 0.057. We have estimated the 95% CI for the MD of 21 minutes as –0.7 to 42.7 minutes.
For TST based on polysomnography at 4 weeks immediately post intervention,115 there was no significant difference between melatonin and placebo, with a reported MD of 39.3 minutes (favouring placebo, 95% CI –34.7 to 113.3 minutes, n = 10).
Sleep efficiency
Five trials48,110,111,115,117 (n = 475 randomised participants) reported sleep efficiency (i.e. the ratio of TST to total time in bed): one trial used both actigraphy and parent report,117 three used actigraphy data only48,110,111 and one trial used polysomnography. 115
Cortesi et al. 110 also measured the percentage of children who achieved a sleep efficiency in the normative level of > 85% at the 12-week assessment.
Four trials48,110,117,135 (n = 255 analysed participants) for actigraphy-measured sleep efficiency were pooled, comprising three parallel trials (n = 205) and one crossover trial with a washout period (n = 50). There was no statistically significant difference in sleep efficiency with melatonin compared with placebo (pooled MD 4.76% favouring melatonin, 95% CI –0.95% to 10.47%; p = 0.10; Figure 5). Heterogeneity was high (I2 = 94%) and this treatment effect is unlikely to be generalisable; although the trials did consistently report very small differences between groups in the proportion of time spent in bed asleep, these are unlikely to be clinically meaningful.
FIGURE 5.
Actigraphy-measured sleep efficiency: melatonin vs. placebo. df, degrees of freedom; IV, instrumental variable.
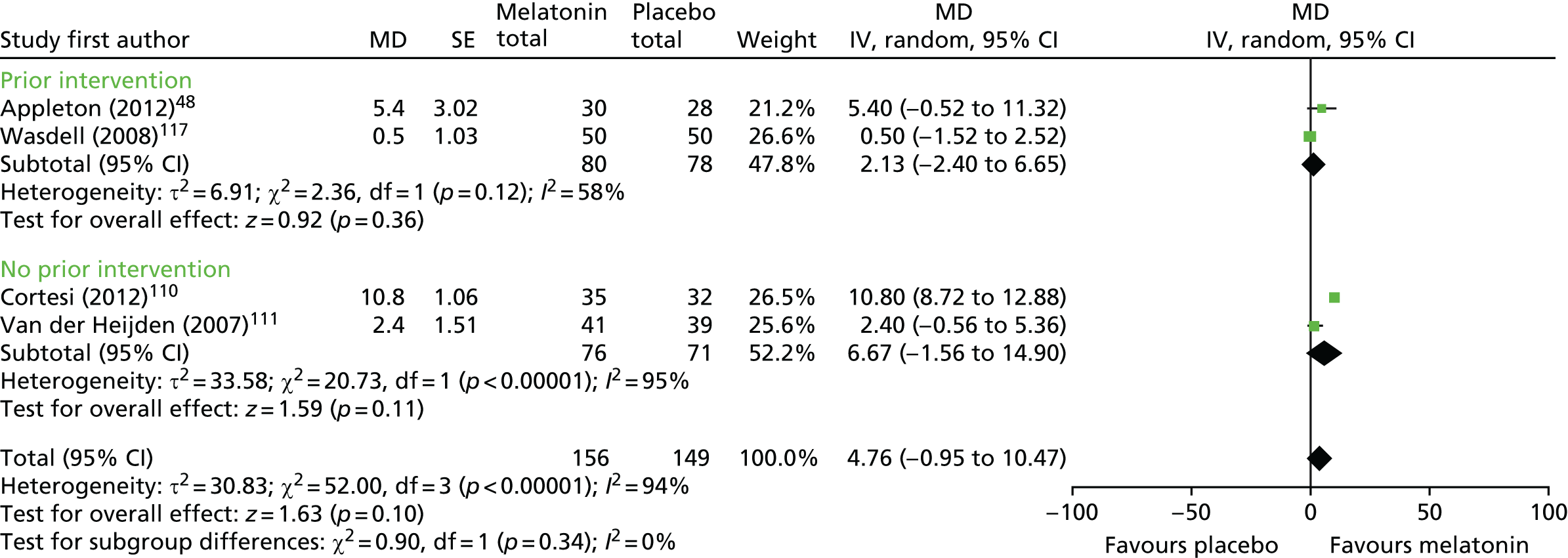
For the single trial117 reporting parent-reported sleep efficiency (n = 50 analysed participants), there was no statistically significant difference between groups (MD 0.30% favouring melatonin, 95% CI –0.90% to 1.49%; p = 0.62).
Cortesi et al. 110 reported the percentage of children who achieved a sleep efficiency in the normative range (> 85%) as 46.43% of the melatonin group compared with none of the placebo group.
Jain et al. 115 reported no difference in polysomnography-measured sleep efficiency (MD 3.8% favouring melatonin, 95% CI –2.5% to 10.1%, n = 10).
Sleep initiation
Sleep onset latency
Ten trials48,69,70,106,110,111,114,117,119 (n = 583 randomised participants) measured SOL, the time from bedtime to sleep onset time (see Appendix 7): three trials reported parent-reported SOL data only;69,106,114 two reported actigraphy-measured SOL data only;110,119 four reported both actigraphy-measured and parent-reported SOL data;48,70,111,117 and one used polysomnography- and actigraphy-measured data, but reported only the results of the polysomnography. 115 As with TST, trials reporting actigraphy data stated that it was informed, or verified, by parent-reported sleep diaries.
Cortesi et al. 110 also calculated the percentage of children who met either a standard sleep criterion for SOL of ≤ 30 minutes or a reduction of SOL by 50%. Wright et al. 106 used an additional measure of SOL defined as the duration of time between taking the medication and falling asleep.
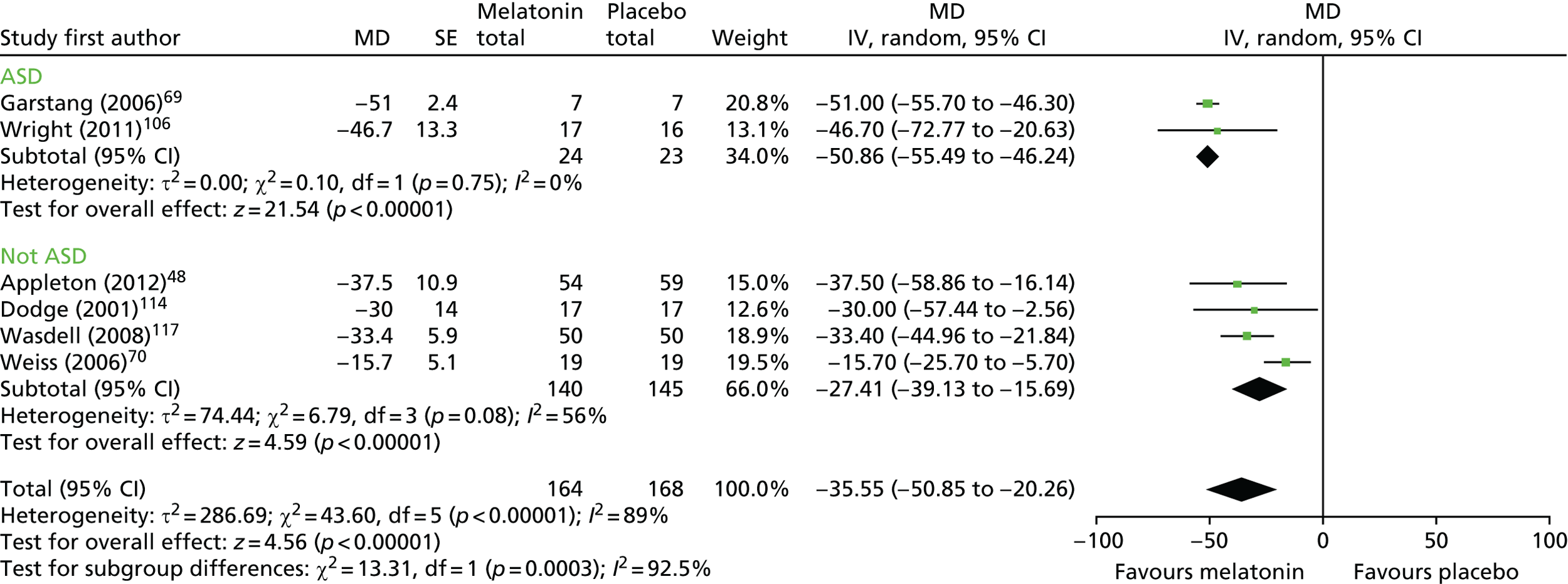
For parent-reported/sleep diary SOL, six trials48,69,70,106,114,117 (n = 223 analysed participants) were pooled, comprising five crossover trials with a washout period (n = 110)69,70,106,114,117 and one parallel-group trial (n = 113). 48 There was a statistically significant decrease (favouring melatonin) in SOL for melatonin compared with placebo (pooled MD –35.6 minutes, 95% CI –50.9 to –20.3 minutes; p < 0.001; Figure 6). Heterogeneity was high (I2 = 89%) and the treatment effect is unlikely to be generalisable, although the effect estimates were all in the direction of benefit with melatonin. There was a statistically significant difference in effect between the studies of children with ASD and those with mixed and other populations (test for subgroup differences, p < 0.001; I2 = 93%). There was a larger difference in the ASD group between melatonin and placebo, with a mean reduction in favour of melatonin of 50.9 minutes (95% CI –55.5 to –46.2 minutes) compared with 27.41 minutes (95% CI –39.1 to –15.7 minutes) in the other group. Subgroup analysis by whether or not participants had received a prior intervention was not possible.
FIGURE 6.
Sleep diary-measured SOL: melatonin vs. placebo. df, degrees of freedom; IV, instrumental variable.
For actigraphy-measured SOL, five trials48,70,110,111,117 (n = 265 analysed participants) were pooled, comprising three parallel trials (n = 196)48,110,111 and two crossover trials with a washout period (n = 69). 70,117 There was a statistically significant decrease (favouring melatonin) in actigraphy-reported SOL (pooled MD –23.4 points, 95% CI –30.9 to –15.8 points; p < 0.001; Figure 7). There was moderate heterogeneity (I2 = 48%). Based on the subgroup analysis, there was no statistically significant difference in effect between studies in which participants had or did not have a prior intervention (test for subgroup differences, p = 0.55; I2 = 0%). A subgroup analysis based on neurodevelopmental condition was not possible.
FIGURE 7.
Actigraphy-measured SOL: melatonin vs. placebo. df, degrees of freedom; IV, instrumental variable.
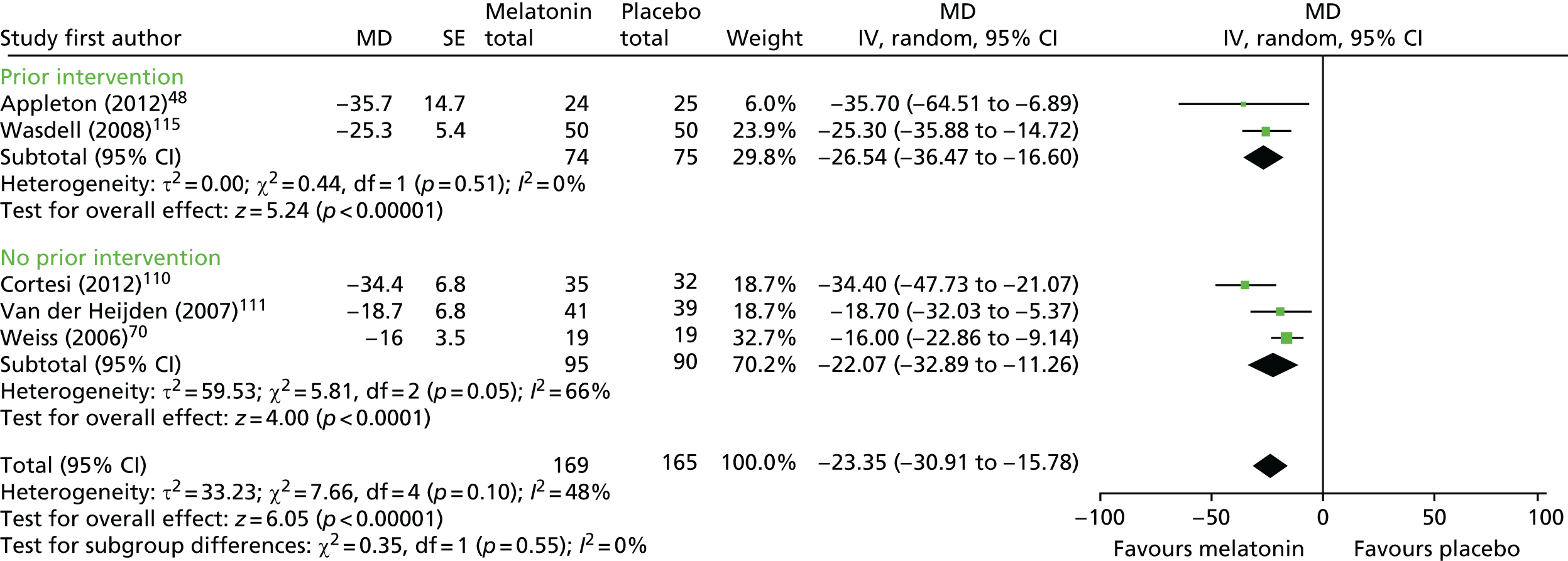
For both sleep diary-measured and actigraphy-measured SOL, the single study rated as having a low risk of bias48 reported a statistically significant improvement with melatonin compared with placebo (see Figures 6 and 7).
The crossover study without a washout period119 (n = 12 analysed participants) reported a statistically significant decrease in mean SOL for the melatonin period, compared with the placebo period, using a non-parametric analysis (complete-case analysis, p = 0.02; last observation carried forward, p = 0.0001), but this difference is not significant when tested using a paired t-test (MD –28.08 minutes; p = 0.10; estimated 95% CI –2.5 to 58.7 minutes).
Cortesi et al. 110 used an additional indicator of SOL (the percentage of children who met a criterion of SOL of ≤ 30 minutes or a reduction of SOL by 50% post intervention) and reported that 39% of the melatonin group versus 0% of the placebo group achieved these changes (n = 66 analysed participants). Jain et al. 115 (n = 10), using polysomnography, reported that melatonin significantly reduced the mean SOL compared with placebo, with a reported MD of –11.4 minutes (95% CI –17.2 to –5.6 minutes).
Wright et al. 106 (n = 17) reported a significant decrease in SOL with melatonin compared with placebo [MD 51.7 minutes (SD 71.9 minutes; p = 0.012; estimated 95% CI 16.5 to 86.9 minutes)].
Sleep maintenance
Number of night wakings
Six trials69,106,113,114,117,119 (n = 142 randomised participants) reported the number of night wakings.
Four crossover trials with a washout period were pooled (n = 94 analysed participants). 69,106,114,117 There was no difference in the mean number of night wakings with melatonin compared with placebo (pooled MD –0.04 points, 95% CI –0.22 to 0.13 points; p = 0.61; Figure 8). Heterogeneity was high (I2 = 84%), although the results were fairly consistent, with the exception of those of Dodge and Wilson. 114 Based on the subgroup analysis, there was no statistically significant difference in effect between studies based on ND (test for subgroup differences, p = 0.06; I2 = 71%).
FIGURE 8.
Parent-reported number of night wakings: melatonin vs. placebo. df, degrees of freedom; IV, instrumental variable.
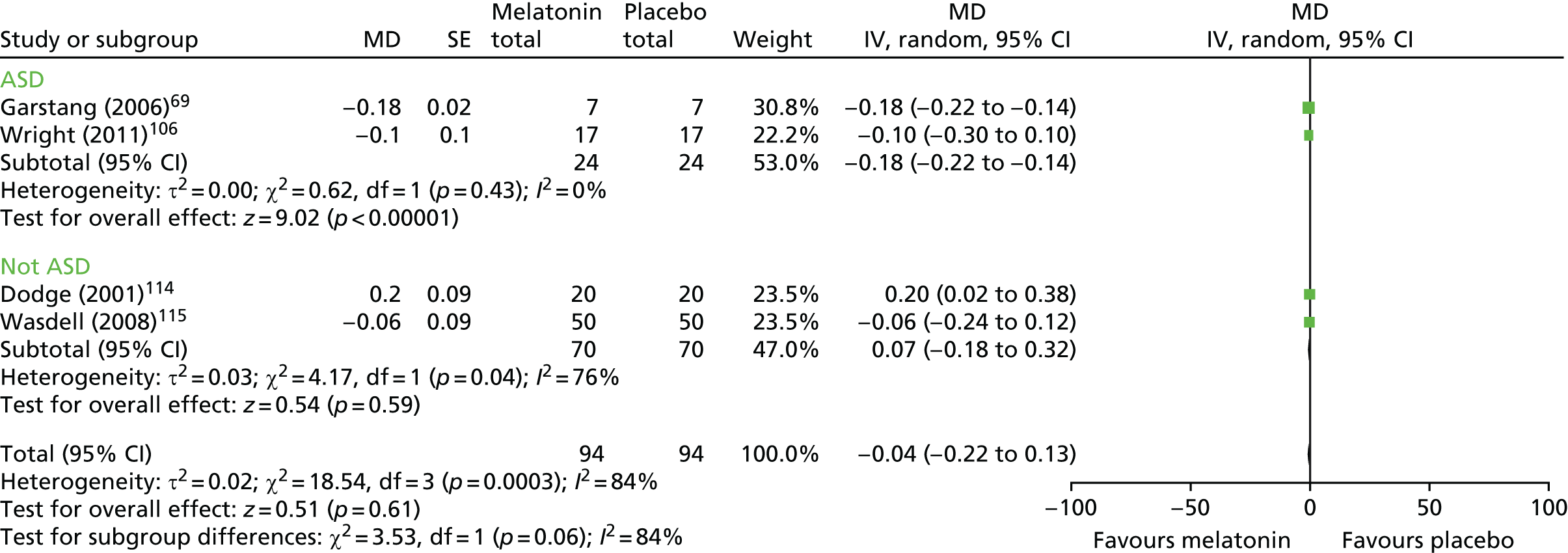
The crossover study without a washout period119 reported no significant difference for the melatonin period compared with the placebo period by either the non-parametric analyses or the paired t-test [MD by paired t-test –0.07 points (favours melatonin, p = 0.73; estimated 95% CI –0.44 to 0.30 points)]. Wasdell et al. 117 reported no statistically significant treatment difference (p = 0.48) for melatonin compared with placebo (estimated MD –0.41 points favouring melatonin, 95% CI –1.47 to 0.66 points).
Other outcomes
Six out of the 11 trials reported other outcomes of interest,110,111,113,115,117,119 although, with the exception of WASO (night waking duration and/or frequency after the child falls asleep), which was reported by two studies,110,115 each outcome measure was reported by only a single study. These data are summarised in Table 2.
| Study | Outcome | Time point, mean (SD) | MD between melatonin and placebo (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Global measures and composite scores | ||||
| Cortesi et al. (2012)110 | CSHQ (total points) |
Melatonin: 66.67 (8.55) Placebo: 64.20 (4.85) |
Melatonin only: 54.78 (6.22) Placebo: 64.80 (4.52) |
–10.02 (–12.71 to –7.33) |
| WASO (minutes) |
Melatonin: 73.71 (45.00) Placebo: 69.75 (45.21) |
Melatonin: 42.21 (22.35) Placebo: 70.15 (42.76) |
–27.94 (–44.58 to –11.30) | |
| Jain et al. (2015)115 | Sleep Behaviour Questionnaire (total points) | Overall: 59.2 (10.5 ) |
Melatonin: 52.4 (5.4) Placebo: 48.3 (7.4) |
3.4 (–2.2 to 9.0) |
| WASO (minutes) | Overall: 60.6 (24.0) |
Melatonin: 42.3 (30.3) Placebo: 57.3 (31.6) |
–22.2 (–34.1 to –10.3) | |
| Wasdell et al. (2008)117 | Somnolog longest sleep episode (minutes) | Overall: 415.41 (106.23) |
Melatonin: 453.30 (118.41) Placebo: 434.26 (109.09) |
18.3 (–5.0 to 41.6) |
| Actigraph longest sleep episode (minutes) | Overall: 185.17 (102.63) |
Melatonin: 199.37 (100.46) Placebo: 189.25 (99.98) |
7.9 (–16.6 to 32.4) | |
| Sleep initiation | ||||
| Cortesi et al. (2011)7 | Bedtime (units unclear) |
Melatonin: 23.45 (1.15) Placebo: 23.41 (1.19) |
Melatonin: 22.30 (1.10) Placebo: 23.51 (1.12) |
–1.21 (–1.76 to –0.66) |
| Van der Heijden et al. (2007)111 | Sleep onset (hour : minutes) |
Melatonin: 21:40 (0.59 minutes) Placebo: 21:38 (0.47 minutes) |
Melatonin: 21:13 (0:58) Placebo: 21:48 (0:48) |
–35 minutes (–59 to –11 minutes) |
| Difficulty falling asleepa (points) |
Melatonin: 3.4 (0.9) Placebo: 3.2 points (0.7 points) |
Melatonin: 2.2 (0.9) Placebo: 3.1 points (1.0 points) |
–0.9 (–1.3 to –0.5) | |
| Jain et al. (2015)115 | Bedtime (hour : minutes) | Overall: 21:44 (0.75 minutes) |
Melatonin: 21:57 (0.91 minutes) Placebo: 21:49 (0.72 minutes) |
6.8 minutes (–23.3 to 9.7 minutes) |
| Wirojanan et al. (2009)119 | Sleep onset time (hour : minutes) | NR |
Melatonin: 20:43 (1.39 minutes) Placebo: 21:25 (2.00 minutes) |
–42 minutes (–74.8 to –9.2 minutes) |
| Sleep maintenance | ||||
| Camfield et al. (1996)113 | Nights without awakening | NR |
Raw data for nights without awakening between 22:00 and 07:00 hours per day/the number of days of complete data: Melatonin: 2/25 (8%); 0/21 (0%); 7/35 (20%); 10/29 (34%); 4/33 (12%); 26/35 (74%) Placebo: 2/28 (7%); 0/25 (0%); 1/31 (3%); 4/30 (13%); 1/31 (3%); 24/35 (69%) |
8.9 (–0.1 to 17.9) |
| Van der Heijden et al. (2007)111 | Wake up timeb (hour : minutes) |
Melatonin: 07:25 (0.39) Placebo: 07:25 (0.34) |
Melatonin: 07:21 (0.40 minutes) Placebo: 07:33 (0.26 minutes) |
–12.0 minutes (–27.1 to 3.1 minutes) |
| Moving timeb (%) |
Melatonin: 11.95 (4.38) Placebo: 10.43 (3.69) |
Melatonin: 12.79 (8.20) Placebo: 12.30 (3.88) |
0.5 (2.4 to 3.4) | |
| Jain et al. (2015)115 | Wake time (hour : minutes) | Overall: 7:09 (1:04) |
Melatonin: 7:31 (1.09 minutes) Placebo: 7:11 (1.09 minutes) |
–18.1 minutes (–0.2 to –36.0 minutes) |
| Sleep scheduling | ||||
| Cortesi et al. (2012)110 | Naptime (units unclear) |
Melatonin: 33.57 (56.63) Placebo: 37.33 (56.19) |
Melatonin: 17.00 (33.11) Placebo: 36.10 (33.28) |
–19.10 (–35.43 to –2.77) |
| Other outcomes | ||||
| Van der Heijden et al. (2007)111 | Interdaily stabilityb,c (points) |
Melatonin: 0.65 (0.13) Placebo: 0.64 (0.15) |
Melatonin: 0.66 (0.16) Placebo: 0.68 (0.11) |
–0.02 (–0.08 to 0.04) |
| Intradaily variabilityb,d (points) |
Melatonin: 0.65 (0.18) Placebo: 0.67 (0.15) |
Melatonin: 0.69 (0.23) Placebo: 0.63 (0.14) |
0.06 (–0.03 to 0.15) | |
| L5 (average activity during the least active 5 hours) (points) |
Melatonin: 44.89 (26.72) Placebo: 36.01 (27.09) |
Melatonin: 39.57 (28.86) Placebo: 50.56 (28.67) |
–11.0 (–24.0 to 2.0) | |
| Jain et al. (2015)115 | Arousal index per hour of sleep | Overall: 12.9 (7.4) |
Melatonin: 10.3 (4.1) Placebo: 12.3 (6.8) |
2.5 (–2.5 to 7.5) |
| Percentage of sleep stages (%) | ||||
| N1 | Overall: 6.3 (2.9) |
Melatonin: 4.0 (2.0) Placebo: 4.8 (2.8) |
–0.1 (–0.5 to 0.3) | |
| N2 | Overall: 42.6 (5.2) |
Melatonin: 44.0 (9.2) Placebo: 42.6 (8.1) |
–2.9 (–10.5 to 4.7) | |
| N3 | Overall: 28.9 (3.4) |
Melatonin: 32.6 (8.3) Placebo: 27.4 (4.8) |
–5.5 (–7.6 to –3.4) | |
| REM stage (%) | Overall: 22.2 (3.7) |
Melatonin: 19.4 (4.2) Placebo: 25.2 (5.1) |
5.8 (4.2 to 7.4) | |
| REM latency (minutes) | Overall: 136.2 (43.6) |
Melatonin: 135.2 (53.1) Placebo: 98.1 (52.8) |
–58.3 (–36.2 to –80.4) | |
Melatonin versus melatonin
Two crossover trials120,121 (n = 24 randomised participants) compared melatonin with melatonin. Jan et al. 121 compared controlled-release with fast-release melatonin, and Hancock et al. 120 compared a dose regimen of 5 mg with a dose regimen 10 mg of melatonin.
Jan et al. 121 did not have a washout period between the two intervention phases, whereas Hancock et al. 120 had a 2-week washout period. Hancock et al. 120 compared a fixed-dose regimen of 5 mg with 10 mg of melatonin regardless of the child’s age. The authors did not report whether or not the melatonin was controlled or fast release. The final average dose for controlled-release melatonin for Jan et al. 121 was 5.7 mg (range 2–12 mg), and the final average dose for fast-release melatonin was 7 mg (range 2–15 mg). Jan et al. 121 reported that the dosage for fast-release melatonin varied for each child depending on the dosage deemed by the trial team to be most effective for that child. However, the controlled-release melatonin was approximately 50% of the fast-release dose, which the authors stated was to avoid possible adverse side effects, as they had no previous information on the use of controlled-release melatonin in children. The duration of the intervention was 11 days for Jan et al. 121 and 2 weeks for Hancock et al. 120
The age of participants included in the trials ranged from 18 months to 31 years for Hancock et al. 120 (eligible participants < 18 years, n = 5, mean age 6.9 years); and from 4 to 21 years for Jan et al. 121 (eligible participants < 18 years, n = 15, mean age 9.3 years). Children in Hancock et al. 120 had severe neurodevelopmental difficulties, whereas the ND in the Jan et al. 121 study was tuberous sclerosis. The sleep disturbance in both studies focused on both sleep initiation and maintenance: in Hancock et al. 120 ‘severe sleep problems’, confirmed by a Quine sleep index score of at least 6 out of a possible 8, and for Jan et al. 121 ‘chronic wake–sleep disorders’.
In Jan et al.,121 the inclusion criterion was that children had already been treated with fast-release melatonin for > 3 months but slept for < 5–6 hours. No additional guidance or leaflets on sleep management were provided to parents in either of the trials.
Across both trials, four sleep-related outcomes were measured (see Appendix 8). Hancock et al. 120 measured TST, SOL and number of night wakings, whereas Jan et al. 121 measured ‘changes in sleep pattern’ as their only outcome. The results of these outcomes are summarised in Table 3 for participants aged < 18 years. Each outcome measure was reported by a single study. There was no evidence of benefit on any of the sleep-related outcomes for the RCT that compared controlled-release melatonin with fast-release melatonin121 or the RCT that compared a dose of 5 mg of melatonin with 10 mg of melatonin. 120
| Study | Outcome | Time point, mean (SD) | MD between interventions (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Global measures and composite scores | ||||
| Hancock et al. (2005)120 | TST (minutes) | NR |
Melatonin, 5 mg: 548.6 (18.9) Melatonin, 10 mg: 548.0 (34.6) |
–0.6 (–35.8 to 34.6) |
| Jan et al. (2000)121 | Changes in sleep pattern | N/A | Improvements in the sleep patterns, to the satisfaction of the caregivers, were observed in 10 participants during the controlled-release period relative to the fast-release period | N/A |
| Sleep initiation | ||||
| Hancock et al. (2005)120 | SOL (minutes) | NR |
Melatonin, 5 mg: 70.0 (58.0) Melatonin, 10 mg: 68.4 (46.9) |
–1.6 (–37.4 to 34.2) |
| Sleep maintenance | ||||
| Hancock et al. (2005)120 | Number of night wakings | NR |
Melatonin, 5 mg: 0.8 (0.4) Melatonin, 10 mg: 1.0 (0.9) |
0.3 (–0.7 to 1.2) |
Adverse events
Eleven out of the 13 trials measured adverse events,48,70,106,110,111,114,115,117,119–121 which were collected and reported in different ways across the studies (Table 4).
| Study | AEs | Method of collection |
|---|---|---|
| Appleton et al. (2012)48 |
Melatonin: mild AEs, n = 151; moderate AEs, n = 35; severe AEs, n = 3 (waking up in the night because of nightmares, severe irritation to skin, seizure) Other non-treatment-emergent signs and symptoms: fatigue, n = 8; headache, n = 10; other, n = 31. Seizures: pre randomisation, n = 49; post randomisation, n = 211 Placebo: mild AEs, n = 195; moderate AEs, n = 28; severe AEs, n = 4 (dislocated elbow in accident at school, petechiae covering the dorsum of the right hand, choking on dinner, vomiting caused by viral illness, which caused dehydration) Other non-treatment-emergent signs and symptoms: fatigue, n = 8; headache, n = 7; other, n = 40. Seizures: pre randomisation, n = 61; post randomisation, n = 192 |
Treatment-emergent signs and symptoms tool |
| Camfield et al. (1996)113 | Not reported | Not reported |
| Cortesi et al. (2012)110 | No AEs were reported or observed | Recorded during face-to-face visits/telephone calls by study team |
|
Van der Heijden et al. (2007)111 Associated paper: Hoebert et al. (2009)112 |
Melatonin: one AE, n = 5, two AEs, n = 4, three AEs, n = 1. There were no discontinuations or withdrawals as a result of AEs. None of the AEs required treatment. At 3 weeks post intervention: headache, n = 3; hyperactivity, n = 3; dizziness, n = 2; abdominal pain, n = 2; nose bleeding, n = 1; itching lumps on the skin, n = 1; painful lumps on the skin, n = 1; diarrhoea, n = 1; decrease of mood, n = 1; maintenance of insomnia, n = 1. At 2 years after participation: 7 out of 24 parents reported one or more AE: bedwetting, n = 2, abnormal faeces, n = 2; drowsiness, n = 2; dizziness, n = 1; sleep maintenance problems, n = 1; skin pigment changes, n = 1; decreased mood, n = 1 Placebo: no AEs reported Long-term follow-up (mean follow-up time 3.66 years) with 94 parents of children who participated in the Van der Heijen et al. 111 trial: 19 children experienced AEs that they or their parents attributed to melatonin treatment. The majority of parents (63.2%) reported multiple AEs; seven reported one AE; four reported two AEs; four reported three AEs and four parents reported four AEs. Ten children had AEs that were self-limiting. In six children the AEs persisted and were a reason to discontinue treatment in three out of six children. In three children it was not mentioned in the questionnaire if the AEs were self-limiting or not. Dizziness, n = 4; visual disturbances, n = 2; melatonin treatment bedwetting, n = 3; excessive morning sedation, n = 2; sleep maintenance insomnia, n = 3; constipation, n = 1; headache, n = 2; profuse perspiration, n = 1; nausea, n = 2; decreased mood, n = 1; skin pigment changes, n = 2; daytime laziness, n = 1; nightmares, n = 2; and change in behaviour, n = 1 |
Open-ended interviews Parent-completed questionnaire |
| Dodge and Wilson (2001)114 |
Melatonin: more moody, n = 1; more ‘hyper’, n = 1. No reported changes in seizure frequency Placebo: more moody, n = 1; more ‘hyper’, n = 1 (different child). No reported changes in seizure frequency |
Parent-completed questionnaire |
| Garstang and Wallis (2006)69 | Not reported | Not reported |
| Hancock et al. (2005)120 |
No adverse effects were reported during the trial Seizures (n = 5). There was no change in the frequency (or type) of seizures seen compared with the baseline period before melatonin treatment at either dose |
‘Reported by parents’ |
| Jain et al. (2015)115 |
Melatonin: AEs (n = 4); increased severity of headache (n = 1). ‘Unrelated adverse events’: bronchitis and ear infection (n = 1), agitation (n = 1) and increased urinary frequency (n = 1 continued from placebo phase) Placebo: AEs n = 2. ‘Unrelated adverse events’: agitation (n = 1 continued from melatonin phase) and increased urinary frequency (n = 1) |
Recorded during face-to-face visits/telephone calls by study team |
| Jan et al. (2000)121 | No adverse effects were experienced during the trial | Not explicitly stated – response to melatonin was measured through sleep charts and parental history |
|
Wasdell et al. (2008)117 Associated paper: Carr et al. (2007)118 |
98 AEs reported across arms Melatonin: 36%. Most common AEs: seizures, n = 11; cold/flu/infection, n = 8; gastrointestinal illness, n = 5; agitation, n = 4; anxiety, n = 2 – considered consistent with patient’s medical history and not related to melatonin; headache, n = 2 – considered consistent with patient’s medical history and not related to melatonin. 40% of AEs in treatment group were from one patient: seizures, agitation, gagging and headaches, which were considered consistent with patient’s medical history ‘Treatment with controlled release melatonin was well tolerated and no treatment differences were evident on vital signs or physical examinations’117 Placebo: 40% (and 24% in placebo washout phases) Most common AEs: cold/flu/infection, n = 10; seizures, n = 8; gastrointestinal illness, n = 5; and behavioural problems (agitation, anxiety, irritability, emotional lability), n = 7. One serious AE consistent with participant’s medical history (aspiration pneumonia requiring hospitalisation) occurred during placebo treatment in the first period of the crossover trial After 3-month open-label phase: 16 AEs reported, consistent with pre-existing medical conditions. One serious AE: patient admitted to hospital for 3 days owing to an upper respiratory infection Prospective long-term, open-label follow-up of melatonin: mean (SD), frequency of AEs: nausea, 0.15 (0.65), 0–4; vomiting, 0.05 (0.22), 0–1; diarrhoea, 0.10 (0.37), 0–1; impaired appetite, 0.05 (0.31), 0; weight loss, 0.05 (0.31), 0–2; confusion, 0 (0), 0; excessive morning sedation, 0.12 (0.51), 0–3; depression, 0.10 (0.63), 0–4; irritability, 0.27 (0.71), 0–3; hyperactive behaviour, 0.10 (0.37), 0–2; deterioration of behaviour, 0.10 (0.49), 0–3; regression of development, 0 (0), 0; precocious puberty, 0 (0), 0; increase in seizures, 0.07 (0.26), 0–1; nasal allergy, 0.12 (0.40), 0–2; rash, 0.12 (0.46), 0–2; deterioration in asthma, 0.07 (0.35), 0–2; worsening of balance, 0.02 (0.16), 0–1; new tremor, 0.05 (0.22), 0–1; headache, 0.07 (0.35), 0–2; visual disturbance, 0.02 (0.16), 0–1; interference with other medications, 0 (0), 0; interference with other therapies, 0 (0), 0 |
Open-ended interviews Telephone call with caregivers |
| Weiss et al. (2006)70 |
Melatonin: 20% of all AEs reported during the melatonin phase Placebo: 23% of all AEs reported during the placebo phase All mild/moderate adverse effects with exception of one severe AE (migraine). Rash from actigraph (n = 2) No serious AEs and no clinically significant changes in vital signs or abnormalities on physical examination |
Recorded on a standardised form that included severity, timing and relationship to the study drug |
| Wirojanan et al. (2009)119 | No side effects were reported by parents | Unclear |
| Wright et al. (2011)106 |
Authors report that there were no statistically significant differences between treatment and placebo in the frequencies of reported side effects (but do not give actual numbers), including the following: daytime drowsiness, dizziness, headaches, vomiting, tummy aches, reduced appetite, low mood, anxiety, irritability, reduced alertness, confusion, tearfulness, diarrhoea, constipation, rashes, sort throat, ear aches, asthma, fit/seizure, mild tremor and ‘other’. Daytime drowsiness, reduced appetite, reduced alertness and diarrhoea were reported as ‘never present’ more often in the placebo than treatment group, but the difference was not statistically significant. There were no serious AEs One child stopped medication during an influenza episode and did not continue as sleep ‘continued to be good’. There were no known reports of seizures or asthma during the trial. One child displayed increased moodiness and self-injurious behaviour but clinicians reported that these were long-standing problems for the child |
Study-specific questionnaire |
One study reported adverse event data using the standardised assessment tool ‘treatment-emergent signs and symptoms’, which classified events into seven domains: somnolence, increased excitability, mood swings, seizures, rash, hypothermia and cough. 48 Signs and symptoms were graded as ‘no symptoms’, ‘mild symptoms’, ‘moderate symptoms’ and ‘severe symptoms’. Seriousness and causality were also assessed. In two studies,110,115 adverse events were determined at each in-person/telephone call visit by the study team and recorded in the participant’s chart; one study measured adverse events using study-specific questionnaires completed by parents;106 one reported using a standardised form;70 one reported using parent-completed questionnaires, in which the origin of the questionnaire was not reported;114 two reported measuring adverse events using open-ended interviews;111,117 one study reported only that adverse effects were reported by parents;120 and in one study it was unclear how the data were collected. 119
Three trials reported that no adverse events were observed or reported during the trial. 110,119,121 Further details of the adverse events reported can be found in Table 4. Overall, melatonin was tolerated and the adverse event profile appeared to be similar between the melatonin and placebo groups.
Summary
Eleven RCTs (eight crossover and three parallel trials) compared melatonin, of varying doses and duration, with placebo. The mean age of participants ranged from 5.5 to 10.3 years and some studies included children with a single ND, whereas others included mixed populations. Children had mostly sleep initiation and/or sleep maintenance problems and in the majority of studies had been offered a prior intervention, ranging from a sleep hygiene advice leaflet to behaviour management and parenting support. Only three child-related sleep outcomes were reported by more than half of the studies, with many of the additional outcomes assessed by a single study. None of the studies assessed parent-related outcomes.
One study was assessed as having a low risk of bias, with the remaining studies having an unclear or high risk of bias. When possible, studies were grouped in the synthesis by ND (ASD vs. mixed or other groups) and by whether or not they had received a prior intervention. However, the results of the subgroup analyses should be interpreted with caution, rather than be considered definitive, as they are based on summary data and are, therefore, observational rather than randomised comparisons as in a trial. In addition, some of the subgroups contained a particularly small number of studies.
There was evidence of benefit with melatonin compared with placebo, although the precise extent of the benefit, which children might benefit the most and the clinical importance of the benefit remain uncertain. The overall benefit was similar for diary-reported and actigraphy-recorded TST. Based on two small studies of children with ASD, there was a mean increase in diary-recorded TST of 64.78 minutes with melatonin compared with placebo (95% CI 58.8 to 70.7 minutes); for the five mixed or other ND studies, the mean increase was 15.9 minutes (95% CI 9.2 to 22.6 minutes). For the single study that was rated as having a low risk of bias (mixed population), the mean increase in TST was 13.2 minutes and the lower CI included the possibility of reduced sleep time (95% CI –13.3 to 39.7 minutes). The findings were similar for SOL. The benefit was greatest for the ASD studies in which there was a mean reduction of 50.9 minutes (95% CI –55.5 to –46.2 minutes) in diary-measured SOL, based on two small studies, whereas there was a smaller mean reduction of 27.4 minutes (95% CI –39.1 to –15.7 minutes) for the mixed and other population subgroup. For the single study with a low risk of bias (mixed population), the mean reduction in SOL was 37.5 minutes (95% CI –58.9 to –16.1 minutes). For other outcomes, there was some evidence of statistically significant benefit with melatonin for sleep initiation outcomes but not sleep maintenance outcomes, such as number of night wakings, although some of the single studies may not have been sufficiently powered to detect an effect.
A single RCT121 compared controlled-release and fast-release melatonin and one RCT120 compared a 5 mg and a 10 mg dose of melatonin, both with a high risk of bias. There was no evidence of benefit with either strategy.
Non-pharmacological studies
Study characteristics
Twenty-six studies evaluated non-pharmacological interventions: 12 RCTs, one controlled before-and-after study and 13 uncontrolled before-and-after studies. Sample sizes ranged from 5 to 244 participants. Studies were conducted in Australia, Canada, China, Hong Kong, Israel, the UK and the USA. A summary of key characteristics is provided in Table 5 and further details are provided in Appendix 9.
| Study details and design | Participants randomised (total N and by group) | Intervention details | Sleep disturbance | Mean age (SD) | ND disorder | Previous sleep hygiene/behavioural interventions | Risk of bias (low, unclear, high) |
|---|---|---|---|---|---|---|---|
| Parent-directed tailored interventions: RCTs | |||||||
| Beresford et al. (2012)21 | |||||||
|
UK Associated publications: Beresford et al. , (2013)136 Stuttard et al. (2015)45,137 Parallel RCT |
N = 13 Intervention group: n = 7 Comparison group: n = 6 |
Intervention group: two face-to-face sessions for assessment, development of sleep management strategy and training parent in strategy. Implementation support delivered via telephone calls Comparison group: usual approach to providing sleep management intervention – as above but implementation support delivered via home visits |
Problems with sleep initiation and maintenance |
Intervention group: 2.86 years (0.82 years) Comparison group: 2.67 years (1.07 years) |
Mixed | None stated | High |
| Hiscock et al. (2015)138 | |||||||
|
Australia Associated publications: Papadopoulos et al. (2015)139 Parallel RCT |
N = 244 Intervention group: n = 122 Comparison group: n = 122 |
Intervention group: one session for assessment, development of sleep management strategy and training parent in strategy. Implementation support delivered via one face-to-face session and one telephone call Comparison group: usual care |
Problems with sleep initiation |
Intervention group: 10.3 years (1.8 years) Comparison group: 9.9 years (2.1 years) |
ADHD + LD or ASD/Asperger syndrome | None stated | Higha |
| Johnson et al. (2013)107 | |||||||
|
USA Associated publications: Turner (2013)140 Parallel RCT |
N = 40 Intervention group: n = 20 Comparison group: n = 20 |
Intervention group: one session for assessment, development of sleep management strategy; five sessions training parent in strategy. Implementation support delivered via one face-to-face session Comparison group: non-sleep-related parent education delivered in identical manner to intervention group |
Problems with sleep initiation and maintenance |
Intervention group: 3.5 years (0.98 years) Comparison group: 3.6 years (1.12 years) |
Autism and ASD | None stated | High |
| Moss et al. (2014)124 | |||||||
|
Australia Associated publications: O’Connell et al. (2012 and 2010)141,142 Parallel RCT |
N = 26 Intervention group: n = 13 Comparison group: n = 13 |
Intervention group: two training workshops followed by a home visit for assessment and development of sleep-managed strategy. Implementation support delivered via one home visit followed by telephone calls as needed Comparison group: waiting list control |
Problems with sleep initiation, maintenance and scheduling and snoring | 11.74 years (2.53 years) (NR separately) | Mixed | One child taking melatonin | High |
| Sciberras et al. (2011)125 | |||||||
|
Australia Associated publications: Sciberras et al., (2010)143 Sciberras and Rinehart, (2015)144 Fulton et al. (2010)145 Parallel RCT |
N = 27 Intervention group: n = 14 Comparison group: n = 13 |
Intervention group: two sessions for assessment, development of sleep management strategy and training parent in the strategy. Implementation support delivered via a single telephone call followed by a further face-to-face session if needed Comparison group: single session for assessment, development of sleep management strategy and training parent in the strategy. No implementation support |
Problems with sleep initiation |
Intervention group: 12.1 years (2.2 years) Comparison group: 10.9 years (2.5 years) |
ADHD | None stated | High |
| Parent-directed tailored interventions: before-and-after studies | |||||||
| Austin et al. (2013)123 | |||||||
| Australia | N = 8 | Two training workshops followed by a home visit for assessment and development of sleep management strategy, followed by a third workshop. Implementation support delivered via weekly telephone calls | Problems with sleep initiation and maintenance | 4.0 years (1.9 years) | Mixed | None stated | High |
| Beresford et al. (2013)21 | |||||||
|
UK Associated publications: Beresford et al. , (2013)136 Stuttard et al. (2015)45,137 Intervention 2 |
N = 12 | Two sessions for assessment, development of sleep management strategy and training parent in strategy. Implementation support delivered via fortnightly face-to-face sessions | Problems with sleep initiation and maintenance | 2.88 years (1.25 years) | Mixed | None stated | High |
| Quine and Wade (1991)146 | |||||||
|
UK Associated publications: Wade and Wade (1991)147 |
N = 25 | Two sessions for assessment, development of sleep management strategy and training parent in strategy. Implementation support delivered via face-to-face sessions | Problems with sleep initiation and maintenance | Mean (SD) NR (range 3–21 years) | LD | None stated | High |
| Weiskop et al. (2005)126 | |||||||
|
Australia Before and after with multiple baseline |
N = 13 | Four sessions for assessment, development of sleep management strategy and training parent in strategy. Implementation support (via telephone calls) delivered from outset of intervention, continuing after training sessions completed with a face-to-face session and further telephone calls | Problems with sleep initiation and maintenance | 5.1 years (2.0 years) | Mixed | One child was taking medication for behaviour and sleep problems | High |
| Parent-directed non-tailored interventions: RCTs | |||||||
| Adkins et al. (2012)127 | |||||||
|
USA Associated publications: Malow et al. (2011)148 Parallel-group RCT |
N = 36 Intervention group: n = 18 Comparison group: n = 18 |
Intervention group: training curriculum contained in a booklet provided to parent Comparison group: no booklet provided |
Problems with sleep initiation | 6.4 years (2.6 years) (NR separately) | Mixed | None stated | High |
| Montgomery et al. (2004)49 | |||||||
|
UK Associated publications: Montgomery et al. (2004)49 Parallel-group RCT |
N = 66 Intervention group a: n = 22 Intervention group b: n = 34 Comparison group: n = 26 |
Intervention group a: training curriculum contained in a booklet provided to parent Intervention group b: training curriculum (identical to that contained in booklet) delivered via face-to-face session Comparison group: no intervention (waiting list) |
Problems with sleep initiation and maintenance | Mean (SD) NR (range 27–101 months, NR separately) | Mixed | None stated | High |
| Malow et al. (2014)128 | |||||||
|
USA Parallel-group RCT |
N = 80 Intervention group: n = 39 Comparison group: n = 41 |
Intervention group: training curriculum delivered via two group-delivered sessions. Implementation support delivered via telephone calls Comparison group: training curriculum delivered via single face-to-face session. Implementation support delivered via telephone calls |
Problems with sleep initiation |
Intervention group: 5.9 years (2.8 years) Comparison group: 5.6 years (2.6 years) |
Mixed | None stated | High |
| Parent-directed non-tailored interventions: before-and-after studies | |||||||
| Beresford et al. (2012)21 | |||||||
|
UK Associated publications: Beresford et al. (2013)136 Stuttard et al. (2015)45,137 Intervention 3 |
N = 22 | Group delivery of training curriculum over four sessions | Problems with sleep initiation | 8.91 years (3.25 years) | Mixed | None stated | High |
| Beresford et al. (2012)21 | |||||||
|
UK Associated publications: Beresford et al. (2013)136 Stuttard et al. (2015)45,137 Intervention 4 |
N = 25 | Training curriculum delivered via a single half-day workshop | Problems with sleep initiation and maintenance | 7.0 years (3.3 years) | Mixed | None stated | High |
| Bramble (1997)149 | |||||||
|
UK Associated publications: Bramble (1996)122 |
N = 15 | Training curriculum delivered via single session. Implementation support delivered via telephone calls | Problems with sleep initiation and maintenance | 7.2 years (2.6 years) | Mixed | Previous interventions indicated but not described, other than as sedatives | High |
| Reed et al. (2009)129 | |||||||
|
Canada Associated publications: Reed et al. (2008)150 |
N = 22 | Group delivery of training curriculum over three sessions | Problems with sleep initiation and maintenance | 5.8 years (2.7 years) | ASD | None stated | Unclear |
| Yu et al. (2015)151 | |||||||
| Hong Kong | N = 54 | Group delivery of training curriculum over three sessions, supported by weekly telephone calls. Implementation support delivered via telephone calls | Problems with sleep initiation and maintenance | 4.78 years (0.85 years) | ASD and Asperger syndrome | None stated | High |
| Non-comprehensive parent-directed interventions: RCTs | |||||||
| Wiggs and Stores (1998)152 | |||||||
|
UK Associated publications: Wiggs and Stores (1999 and 2001)153,154 Cluster RCT |
N = 30 Intervention group: n = 15 Comparison group: n = 15 |
Intervention group: tailored intervention. Single session for assessment, development of sleep management strategy and training parent in strategy. Implementation support delivered via telephone calls Comparison group: no intervention |
Problems with sleep initiation and maintenance |
Intervention group: 8.21 years (2.7 years) Comparison group: 10.77 years (3.8 years) |
Mixed | None stated | High |
| Non-comprehensive parent-directed interventions: before-and-after study | |||||||
| Peppers et al. (2016)155 | |||||||
| USA | N = 23 | Intervention group: prescriptive sleep hygiene intervention. One session delivered by a practitioner | Global measure of sleep disturbance (CSHQ) used to define eligibility to receive intervention | Mean (SD) NR (range 5–11 years) | NR | None stated | High |
| Other interventions – RCTs | |||||||
| Francis and Dempster (2002)156 | |||||||
|
Australia Crossover RCT |
N = 5 |
Intervention group: the intervention contained valerian of 500 mg per tablet, 30 mg per kg of body weight, single nightly dose ≥ 1 hour before bedtime, 2 weeks Comparison group: to give the same appearance and odour, placebo contained 25 mg of whole-root Valeriana edulis extract, 2 weeks |
Problems with sleep initiation and maintenance | 6.6 years (3.4 years) | Mixed | None stated | High |
| Gringras et al. (2014)36 | |||||||
|
UK Crossover RCT |
N = 73 |
Intervention group: weighted blanket 2.25 kg (small) or 4.5 kg (large). 12–16 days and were given by researchers at home/clinic visits Comparison group: placebo blanket |
Problems with sleep initiation and maintenance |
Weighted blanket first: 8.7 years (3.3 years) Control blanket first: 9.9 years (2.8 years) |
Mixed | None stated | High |
| Piazza et al. (1997)157 | |||||||
|
USA Parallel-group RCT |
N = 14 Intervention group: n = 7 Comparison group: n = 7 |
Intervention group: faded bedtime with response cost, 10 days. The study author delivered the face-to-face (home) visits and booklet intervention Comparison group: bedtime scheduling |
Problems with sleep maintenance and initiation |
Intervention group: 6.7 years (2.6 years) Comparison group: 8.3 years (3.0 years) |
Mixed | Children were excluded if receiving pharmacological interventions for sleep. No other previous interventions reported | High |
| Other non-pharmacological interventions: non-randomised study designs | |||||||
| Guilleminault et al. (1993)158 | |||||||
| USA | N = 14 | Light therapy and behavioural programme. Daily light exposure at 07.00 and 12.00 hours | Problems with sleep maintenance and ‘lack of sleep consolidation’ | 2.9 years (range 9 months to 4 years) | Moderate to severe learning disability | Eight children had attended sleep clinics and centres for treatment. Some children had had behavioural treatments | High |
| Oriel et al. (2016)159 | |||||||
|
USA A–B–A withdrawal design |
N = 8 | Aquatic exercise programme. 60 minutes of aquatic exercise two times per week | Parent/guardian report of sleep dysfunction | 8.9 years (SD NR, range 6–11 years) | ASD | None stated | High |
| Yehuda et al. (2011)160 | |||||||
|
Israel Controlled before and after |
N = 78 Intervention group: n = 40 Comparison group: n = 38 (Healthy control n = 22 not included) |
Intervention group: essential fatty acids supplement, 90 g of α-linolenic and 360 g of linoleic acid in mineral oil. Two capsules per day for 10 weeks Comparison group: placebo |
Sleep deprived | (All) mean (SD) NR (range 9–12 years) | ADHD | None stated | Unclear |
| Yu and Hong (2012)161 | |||||||
|
China Before and after |
N = 30 | Acupuncture and ear-point taping. Two courses of acupuncture treatment, once every other day, three times a week, with 36 sessions constituting one course. Ear-point taping was given three times a week, with 36 sessions constituting one course. Two courses were required | Problems with sleep initiation, maintenance and abnormal sleep state (including apnoea) | 6.9 years (3.1 years) | Learning disability | None stated | High |
We grouped studies, based on the intervention, into the following: parent-directed tailored interventions (n = 9), parent-directed non-tailored interventions (n = 8), non-comprehensive parent-directed interventions (n = 2) and ‘other’ non-pharmacological interventions (n = 7). Intervention and control details for non-pharmacological studies are outlined in Appendix 10.
Parent-directed interventions can be conceived as psychoeducational interventions that have the objective of ‘training’ parents to manage their child’s sleep disturbance by equipping them with the relevant knowledge and skills. These interventions vary in their intensity and different modes are used to deliver the training, including individual work, group work, teaching workshops and written material. Nineteen studies21,49,107,123–129,138,146,149,151,152,155,162,163 of parent-directed interventions were included in the review. The interventions varied considerably in terms of a number of characteristics. There was no consistency in the terminology used by study authors to describe the interventions, and the terms that were used to describe an intervention were not routinely defined. This was not unexpected. Generally, reporting of non-pharmacological interventions is acknowledged to be much poorer than for pharmacological studies. 164 However, it carries the risk of erroneous comparison of interventions or pooling of studies.
Therefore, a framework to describe the parent-directed interventions represented in this study was developed by the research team. It included intervention characteristics posited as being an ‘active ingredient’ of an intervention and/or having an impact on intervention effectiveness, for example, intensity, duration, mode of delivery and whether the intervention was condition specific or ND generic. There was incomplete reporting on these intervention characteristics across the studies. We defined tailored and non-tailored interventions as follows:
-
Tailored – a face-to-face clinical assessment by a trained practitioner guides clinical decision-making regarding the management of a specific child’s sleep disturbance. A sleep management plan specific to the child/family is developed, and training in implementing that plan is delivered. There is extended ‘implementation support’, that is, ongoing support and advice as the parents implement changes to sleep management strategies and practices. The intervention is typically delivered on a one-to-one basis. However, an intervention that used teaching workshops and one-to-one work was also categorised as tailored123,124 because it fulfilled the criteria defined above.
-
Non-tailored – the delivery of a standard ‘training curriculum’. The curriculum may include opportunities for a parent to be supported to operationalise the material learnt for their child’s sleep disturbance. Some implementation support may also be included. Among the studies included in this review, such interventions were delivered in a number of ways: written material, single-session workshops, group delivery and one-to-one work.
All but two of the parent-directed interventions were comprehensive in their content, that is, they included training across three topic areas: sleep and sleep processes, sleep hygiene and the management of specific problem behaviours (e.g. night wakings). The remaining two interventions, which were classified as non-comprehensive parent-directed interventions, focused on a single topic area related to managing sleep disturbance. One was concerned only with sleep hygiene training155 and the other focused on particular behavioural strategies to manage specific problem behaviours. 152
Assessment of risk of bias
A summary assessment of risk of bias for each RCT is provided in Table 5 and the full risk-of-bias assessment involving each bias domain is provided in Appendix 21. Overall, the studies were rated as having a risk of bias that was high (n = 24) or unclear (n = 2); therefore, the findings from these studies may not be robust. Following the guidance outlined in Chapter 2, Assessment of risk bias, one study was rated as having a high risk of bias, as blinded outcome assessment was not possible. 138 However, this study had no other important limitations and all other domains were rated as having a low risk of bias.
We were unable to find a registered protocol for 10 RCTs,21,36,49,107,124,125,127,128,152,156 making it unclear whether or not the studies were free of selective reporting. Eight trials provided little or no detail regarding sequence generation. 21,36,49,107,124,127,152,157 Ten RCTs provided little or no detail regarding allocation concealment. 21,36,49,107,124,125,127,128,152,156 In all RCTs, blinded outcome assessment was not undertaken21,36,49,107,125,127,128,138,152 or it was unclear whether or not blinding occurred. 124,156 In eight RCTs, the analysis of incomplete outcome data was not considered21,36,125,128,152 or it was unclear whether or not the authors had considered this. 107,124,127
Eleven non-randomised designs21,123,126,146,149,155,159–163 were reported as having a risk of bias owing to a probable or unclear risk of confounding of the effect of the intervention. Fourteen studies were reported as having a risk of bias because of how they selected participants, for example by not reporting inclusion and/or exclusion criteria, selecting participants from one setting only or including more males than females. 21,123,126,129,146,149,151,155,158–163 There was likely or unclear bias reported in the measurement of intervention outcomes in seven studies, for example few or no details provided about how outcomes were measured. 21,126,146,159–161 One further study160 was reported as having unclear bias because of departures from intended interventions, owing to missing data and to the selection of the reported results.
Parent-directed tailored interventions
Nine studies evaluated parent-directed, comprehensive tailored interventions (Table 6). Seven studies evaluated the clinical effectiveness of these interventions: three two-armed parallel-group RCTs107,124,138 with a range of no intervention comparators (i.e. usual care, waiting list control or non-sleep related parent education); and four before-and-after studies. 21,123,126,146 Two further RCTs21,125 evaluated alternative ways of delivering an intervention. One compared the mode by which implementation support was provided: home visit versus telephone call. 21 The other compared the intensity of practitioner involvement when delivering the intervention: brief versus extended. 125
| Study | Total duration of intervention (including period of implementation support) | Mode of delivery | Number of sessions and location | Mode of delivering implementation support, and intensity, once regular sessions with practitioner were completed | Intervention described as developed for specific ND? | Manual? | Follow-up, from baseline |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| Hiscock et al. (2015)138 | 4 weeks | Face to face | One (home or clinic) | Face to face (n = 1), later followed by a telephone call (n = 1) | Yes, ADHD | No | 3 and 6 months |
| Johnson et al. (2013)107 | NR | Face to face | Five (home and clinic) | Face to face (n = 1) | Yes, ASD | Yes | 1 and 2 months |
| Moss et al. (2014)124 | 15 weeks | Teaching workshops and face to face | Two workshops and one face-to-face session (home) | Home visit (n = 1), followed by telephone calls, ‘on a needs basis for approximately 2 months’ | No | Yes | 15 and 23 weeks |
| Before-and-after studies | |||||||
| Austin et al. (2013)123 | 15 weeks | Teaching workshops and face to face | Two workshops, one home visit and one workshop | Approximately weekly telephone call for 6-week period | No | Yes | 19 weeks |
| Beresford et al. (2012)21 | 12–16 weeks | Face to face | Two (clinic, home) | Fortnightly sessions at clinic | No | No | 12 and 24 weeks |
| Quine and Wade (1991)146 | 6–28 weeks | Face to face | Two (home) | Described as ‘weekly’ home visits, although study authors also report frequency decided between practitioner and parent and diminishing in intensity | No | Yes | 3 months |
| Weiskop et al. (2005)126 | Minimum 7 weeks | Face to face | Four (a mixture of home and clinic), plus at least weekly telephone contact between sessions | ‘Review session’ 5 weeks after session 4; telephone calls ‘gradually reduced’ after session 5 | No | Yes | 3 and 12 months |
| RCT comparisons of intervention delivery | |||||||
| Beresford et al. (2012)21 | 10 weeks | Face to face | One (home) | Home visit approximately weekly for 6–8 weeks vs. telephone call approximately weekly for 6–8 weeks | No | 10 and 22 weeks | |
| Sciberras et al. (2011)125 | 1 session vs. 4 weeks | Face to face | One (clinic) vs. two (clinic) | None vs. telephone call (n = 1) followed by a face-to-face session (clinic) if needed | Yes, ADHD | No | 2 months |
Six studies included children with a range of neurodevelopmental conditions,21,123,124,126,146 two included children with a diagnosis of ADHD125,138 and one included children with a diagnosis of ASD. 107
Interventions varied in terms of the total number of sessions delivered; the number of sessions spent in assessment; the development of, and training in, a sleep management plan; the duration of the intervention; and the mode of delivery (see Table 6). The number of assessment/training sessions ranged from one21,138 to five107 sessions in the RCTs; and from two21,146 to four sessions123,126 in the other study designs. In the RCTs, the intervention duration was one session,125 4 weeks138 and 10 weeks,21 and was not reported in two studies. 107,124 In the other studies,21,123,126,146 the duration of the intervention ranged from 6 to 28 weeks.
The intervention was delivered in the home and/or clinic for four RCTs107,124,125,138 and solely in the home for one. 21 All before-and-after study interventions were delivered in both homes and in clinics, apart from one study, in which the intervention was delivered solely in the home. 146 Implementation support in the RCTs, once regular sessions were completed, ranged from none,125 to one or two further contacts107,138 and to regular contacts over a period of several weeks. 21,124 All before-and-after studies had implementation support involving regular contact of between 6 and 28 weeks. 21,123,126,146
The age of participants varied, with a mean age ranging from 2.67 to 12.1 years for the RCTs21,107,124,125,138 and from 2.88 to 5.1 years for the before-and-after studies. 21,123,126,146 Most RCTs had more than one criterion for inclusion in the trial and included children with a mix of sleep disturbances (see Table 6) relating to sleep initiation only,125,138 sleep initiation and maintenance21,107 or sleep initiation, maintenance and scheduling. 124 All before-and-after studies included children with sleep initiation and maintenance disturbances. 21,123,126,146
There was limited reporting of prior interventions. Two RCTs excluded children who were already receiving specialist sleep input138 or who were using sleep supplements. 107 One RCT124 reported that children were taking medication prescribed for behaviour and sleep problems; however, it was not clear if melatonin was being taken to aid sleep. One before-and-after study126 reported that one child was taking medication for behaviour and sleep problems.
Twenty-seven different sleep-related outcomes were measured (see Appendices 11–13). First outcome measurement time points ranged from immediately post intervention to 2 months post intervention (see Table 6). Five trials measured additional follow-up time points; however, in this review we focus only on the outcomes closest to the end of the intervention.
Given the variation in interventions in terms of, for example, number of sessions, implementation support and mode of delivery, as well as differences in comparator and length of follow-up, a narrative synthesis was undertaken of the parent-directed tailored interventions.
Disorder global measures and composite scores
Total sleep time
Two RCTs (n = 284)107,138 reported actigraphy-measured TST and a before-and-after study126 presented parent-reported TST. However, because of the way in which data were reported, we are unable to calculate the MD from pre to post intervention or a CI. 126 Sleep management interventions seek to increase duration of TST.
There was no evidence of a statistically significant difference in change in actigraphy-measured TST from baseline to post intervention for a tailored parent-directed intervention compared with usual care (MD 10.9 minutes, 95% CI –19.0 to 40.8 minutes). 138 There was also no difference between the comparator and intervention groups post intervention for a group receiving a tailored parent-directed intervention compared with non-sleep-related parent education (MD 26.0 minutes, 95% CI –35.1 to 87.1 minutes). 107
Sleep efficiency
Two RCTs (n = 284)107,138 reported actigraphy-measured sleep efficiency, that is, the ratio of TST to total time in bed, informed by or verified using sleep diaries. Greater sleep efficiency is preferable. There was no evidence of a statistically significant difference in change in actigraphy-measured sleep efficiency from baseline to post intervention for a tailored parent-directed intervention compared with usual care (MD –1.6%, 95% CI –5.2% to 1.9%),138 or in the difference between groups post intervention for a tailored parent-directed intervention compared with non-sleep-related parent education (MD –1.0%, 95% CI –7.6% to 5.6%). 107
The Children’s Sleep Habits Questionnaire
Four RCTs (n = 310)21,124,125,138 and two before-and-after studies21,123 reported the CSHQ total score. The CSHQ is a validated parent-reported assessment of child sleep. 133 Higher scores on the CSHQ indicate a greater severity of the sleeping disturbance, either because of the frequency or number of different behaviours presenting.
There was a statistically significant reduction (i.e. improvement) in total CSHQ score in one RCT of a parent-directed tailored intervention compared with a usual care control. However, Hiscock et al. ,138 in another RCT, found no statistically significant improvement using a parent-directed tailored intervention compared with waiting list control,124 although the direction of effect was in a positive direction. No evidence of a difference was observed in the RCTs of extended versus brief intervention125 and face-to-face implementation support compared with telephone-delivered implementation support,21 although the direction of effect was the same (Table 7).
| Study | Time point, mean CSHQ score (SD) | MD (95% CI) | |
|---|---|---|---|
| Baseline | Follow-up | ||
| RCTs | |||
| Beresford et al. (2012)21 |
Intervention group: 59.50 points (11.82 points) Control group: 53.33 points (4.27 points) |
Intervention group: 52.17 points (11.44 points) Control group: 53.33 points (8.76 points) |
–1.16 points (–14.27 to 1.95 points)a |
| Hiscock et al. (2015)138 |
Intervention group: 57.8 points (8.8 points) Control group: 59.0 points (7.8 points) |
Intervention group: 50.1 points (8.3 points) Control group: 55.1 points (8.6 points) |
Adjusted: –6.6 points (–8.5 to –4.6 points)b –5.0 points (–7.6 to –2.4 points)a |
| Moss et al. (2014)124 |
Intervention group: 56.20 points (9.38 points) Control group: 51.38 points (7.54 points) |
Intervention group: 46.50 points (7.29 points) Control group: 51.12 (6.51) |
–4.62 points (–10.83 to 1.59 points)a |
| Sciberras et al. (2011)125 | NR |
Intervention group: (change score) 5.09 points (5.12 points) Control group: (change score) 6.82 points (8.02 points) |
–1.73 points (–7.11 to 3.65 points)c |
| Before-and-after studies | |||
| Austin et al. (2013)123 | 55.43 points (7.68 points) | 47.57 points (9.14 points) | –7.86 points (–14.39 to –1.33 points)a |
| Beresford et al. (2012)21 | 59.55 points (7.59 points) | 56.57 points (10.77 points) | Cannot be estimated.d Effect size given as 0.42 |
There was a decrease (i.e. an improvement) in total CSHQ score post intervention compared with pre intervention in one before-and-after study;123 however, it was not possible to estimate the MD between pre and post intervention in a second study as it was not a matched sample. 21
Other global measures and composite scores
Several further composite scores and global outcome measures were reported by single studies. One RCT138 (n = 244) of a tailored parent-directed intervention compared with usual care reported a statistically significant reduction in parent-reported moderate or severe sleep problems (Table 8). There appeared to be improvement at follow-up in three further outcomes measured in two before-and-after studies (see Table 8). 21,123
| Study | Outcome | Time period, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Hiscock et al. (2015)138 | Moderate or severe sleep problems – parent/caregiver reported (none/mild and moderate/severe) | NR but eligibility criteria of ‘parent reported moderate to severe sleep problems’ |
Intervention group: 30% Comparison group: 56% |
Adjusted odds ratio 0.30 (0.16 to 0.59)a Difference in absolute risk 25.7% (14.1% to 37.3%)a |
| Before-and-after studies | ||||
| Austin et al. (2013)123 | Sleep Disturbance Index | NR | NR | No data reported other than that pre- and post-treatment scores differed significantly (z = −2.37; p < 0.05) |
| Beresford et al. (2012)21 | Parent-set child sleep goal(s) | 2.64 (2.11) | 6.50 (2.14) | Cannot be estimatedb |
| Beresford et al. (2012)21 | Change in goal attainment rating | N/A |
Improved: 75.0% No change: 12.5% Deteriorated: 12.5% |
N/A |
Sleep initiation
Sleep onset latency
One RCT (n = 40)107 reported actigraphy-measured SOL, the time from bedtime to sleep onset time, which was verified using sleep diaries. One before-and-after study reported on SOL using sleep diaries123 and another reported SOL using parent-completed visual graphs. 126
There was no evidence of a statistically significant difference in SOL in the RCT107 of a tailored parent-directed intervention compared with non-sleep-related parent education (MD 4 minutes, 95% CI –15.0 to 23.0 minutes). One before-and-after study showed no statistically significant difference pre and post intervention (MD 43 minutes, 95% CI –30 to 116 minutes). 123 The results reported in the other study126 are based on visual graphs without any associated numerical data; therefore, we are unable to present a MD and CI.
Other sleep initiation outcomes
Two further before-and-after studies measured time to settle at night. One reported a statistically significant improvement in time to settle at night (MD –91.3 minutes, 95% CI –112.6 to –69.9 minutes)146 and the other did not report data for any sleep initiation outcomes. 126
Sleep maintenance
Night wakings
Three before-and-after studies123,126,146 reported night wakings using parent-reported diaries (n = 46), although each study defined the outcome differently.
One study reported a statistically significant reduction in the number of night wakings post intervention (MD –2.7, 95% CI –3.0 to –2.4). 146 Another showed a statistically significant reduction in the time in minutes it took to settle the child after night wakings (MD –24.0 minutes, 95% CI –44.7 to –3.3 minutes). 123 For the third study,126 which measured number of night wakings per week, the results reported by the authors are based on visual graphs without associated numerical data; therefore, we are unable to present the MD and CI.
Other sleep maintenance outcomes
A variety of other outcomes related to sleep maintenance were reported by single studies. One RCT138 (n = 244) reported WASO (night waking duration and/or frequency after the child falls asleep) and found no evidence of a statistically significant difference between a tailored parent-directed intervention and usual care (Table 9). Another RCT125 (n = 27) comparing an extended versus brief intervention found that there was a greater reduction (i.e. now ‘no’ or only a ‘mild’ sleep problem) in sleep problems at 2 months for the extended intervention group compared with the brief intervention group. One before-and-after study146 reported statistically significant improvements in mean minutes per night the child was awake, and number of nights the child does not sleep in their own bed. Another before-and-after study126 reported measuring the number of nights per week that the child fell asleep in their own bed and the number of nights per week that the child co-slept; however, the authors reported insufficient data to determine the effects of the intervention on these outcomes (see Table 9).
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs: sleep maintenance | ||||
| Hiscock et al. (2015)138 | WASO (minutes) | NR | NR | 1.5 (–16.9 to 19.9)a |
| Sciberras et al. (2011)125 | Parent/caregiver reported ‘no’ or ‘mild’ sleep problems | NR |
Intervention group: 25% Control group: 64% |
–39% (–74% to –4%)b |
| Before-and-after studies: sleep maintenance | ||||
| Quine and Wade (1991)146 | Mean minutes per night the child was awake | 70.2 (32.1) | 3.2 (4.7) | –67.0 (–90.4 to –43.6)c |
| Quine and Wade (1991)146 | Number of nights child does not sleep in own bed per week | 5.6 (0.9) | 0.1 (0.4) | –5.5 (–6.3 to –4.7)c |
| Weiskop et al. (2005)126 | Number of nights per week that child fell asleep in own bed | NR | NR | NR |
| Weiskop et al. (2005)126 | Number of nights per week that child co-slept | NR | NR | NR |
Child-related quality of life, daytime behaviour and cognition
Cognition outcomes
No studies reported child cognition outcomes.
Attention deficit hyperactivity disorder symptoms
Two RCTs (n = 271)125,138 reported on the severity of ADHD symptoms using the total score of the ADHD Rating Scale IV,165 which is a validated parent- or teacher-completed questionnaire that has been used to assess the presence and severity of symptoms by parents/carers of children with ADHD. A higher score indicates more severe problems.
The authors of a RCT125 comparing an extended versus brief parent-directed tailored intervention reported only that there was ‘minimal change in ADHD symptoms . . . across both groups at 2 and 5 months’. 125 There was a statistically significant reduction in symptom severity as measured by the parent-reported ADHD Rating Scale IV in another RCT138 comparing a tailored parent-directed intervention with usual care (adjusted MD –3.7 points, 95% CI –6.1 to –1.2 points; unadjusted MD –3.5 points, 95% CI –6.5 to –0.5 points). However, the difference in the teacher-reported ADHD Rating Scale IV score was not statistically significant at 3 months’ follow-up (adjusted MD –2.4 points, 95% CI –5.3 to 0.4 points; unadjusted MD –5.3 points, 95% CI –9.2 to –1.4 points).
Pediatric Quality of Life Inventory
Two RCTs125,138 (n = 271) reported on the child’s quality of life using the Pediatric Quality of Life Inventory (PedsQL) version 4.0. The PedsQL is a validated parent-completed questionnaire that assesses parents’ perception of quality of life in paediatric patients with chronic health conditions. Higher scores indicate better health-related quality of life.
There was a statistically significant improvement in the PedsQL in one RCT138 comparing a tailored parent-directed intervention with usual care (adjusted MD 9.4 points, 95% CI 5.6 to 13.2 points; unadjusted MD 10.6 points, 95% CI 6.0 to 15.2 points). In the other RCT, there was no statistically significant difference in change scores from baseline to 2 months between groups receiving the extended versus brief formats of a parent-directed tailored intervention on the PedsQL (MD 4.27 points, 95% CI –2.48 to 11.02 points). 125
Daytime behaviour
Three RCTs (n = 297) of parent-directed tailored interventions reported other child-related quality of life, daytime behaviour and cognition outcomes. 124,125,138 These data are summarised in Table 10. Hiscock et al. 138 reported statistically significant improvements in the Daily Parent Rating on the Evening and Morning Behaviour Scale score, in parent- and teacher-reported Strengths and Difficulties Questionnaire scores and in backwards digit recall, a parent-directed tailored intervention, compared with the usual care control group. 138 Another RCT124 reported that there was no statistically significant difference in the Developmental Behaviour Checklist when comparing a parent-directed tailored intervention with waiting list control. A third RCT125 found that there was no statistically significant difference between extended and brief format sessions in the Daily Parent Rating on the Evening and Morning Behaviour Scale score.
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Child-related quality of life, daytime behaviour and cognition | ||||
| RCTs | ||||
| Hiscock et al. (2015)138 | Daily parent rating of evening and morning behaviour (total) |
Intervention group: 22.6 points (5.0 points) Control group: 22.7 points (5.8 points) |
Intervention group: 16.6 (5.8 points) Control group: 21.0 (5.8 points) |
Adjusted: –4.7 points(–6.5 to –2.8 points)a –4.4 points(–6.2 to –2.6 points)b |
| Hiscock et al. (2015)138 | SDQ – parent report (total) |
Intervention group: 22.6 points (5.7 points) Control group: 21.9 points (5.4 points) |
Intervention group: 18.6 points (5.0 points) Control group: 21.4 points (5.4 points) |
Adjusted: –3.0 points (–4.3 to –1.7 points)a –2.8 points (–4.4 to –1.2 points)b |
| Hiscock et al. (2015)138 | SDQ – teacher report (total) |
Intervention group: 15.0 points (7.3 points) Control group: 17.2 points (6.8 points) |
Intervention group: 13.5 points (6.6 points) Control group: 17.2 points (6.8 points) |
Adjusted: –1.7 points (–3.4 to –0.1 points)a –3.7 points (–5.7 to –1.7 points)b |
| Hiscock et al. (2015)138 | Backwards digit recall | NR | NR | Adjusted: 5.2 points (0.03 to 10.4 points)a |
| Moss et al. (2014)124 | Developmental Behaviour Checklist (Parent Version) |
Intervention group: 66.20 points (25.66 points) Control group: 72.29 points (16.50 points) |
Intervention group: 57.70 points (27.10 points) Control group: 69.25 points (14.95 points) |
11.55 points (–31.61 to 8.51 points)b |
| Sciberras et al. (2011)125 | Daily Parent Rating on the Evening and Morning Behaviour scale | NR |
Intervention group: (change score) 4.13 points (9.63 points) Control group: (change score) 0.67 points (3.77 points) |
3.46 points (–2.43 to 9.35 points)c |
| Before-and-after studies | ||||
| Austin et al. (2013)123 | Developmental Behaviour Checklist (Parent Version) | 87.00 points (25.81 points) | 74.57 points (27.96 points) | 12.43 points (2.25 to 22.61 points)d |
| Quine and Wade (1991)146 | BPI | 13.0 points (4.6 points) | 9.7 points (4.3 points) | –3.3 points (–5.1 to –1.5 points)d |
| Weiskop et al. (2005)126 | Child’s sleep behaviour goals | NR | 12/25 goals (48%) were achieved with 100% success, and the mean achievement scale was 76.3% | N/A |
For the before-and-after studies, one123 reported a statistically significant improvement in the Developmental Behaviour Checklist166 post intervention. A second before-and-after study146 evaluated child daytime behaviour and cognition using the Behavior Problem Index (BPI)167 and reported a statistically significant reduction in total BPI score. A third before-and-after study126 evaluated children’s sleep behaviour goals and reported improvements post intervention, although we were unable to report the MD and CI, as the authors provided insufficient data to enable this.
Other child-related sleep outcomes
Parent help-seeking for sleep problem
One RCT138 (n = 244) collected parent reports of other professional help sought for their child’s sleep. Parents of children in the intervention group were less likely than parents of children in the control group to seek help for their child’s sleep from a health professional (e.g. general practitioner, paediatrician or psychologist) (14% vs. 20% at 3 months), but this difference was not statistically significant.
School attendance
Two RCTs (n = 271) reported on school attendance, defined as the number of days missed from school. 125,138 Both reported only that there was no difference in this outcome between the intervention groups. 125,138
Parent-related outcomes
Three RCTs124,125,138 and one before-and-after study146 reported parent-carer-related outcomes. The outcomes measured and methods of assessment are summarised in Appendix 12.
Depression Anxiety Stress Scales
Two RCTs (n = 271) reported on parental stress using the Depression Anxiety Stress Scales (DASS). 125,138 The DASS are a validated self-report instrument designed to measure the three related negative emotional states of depression, anxiety and tension/stress. Higher scores indicate worse depression, anxiety and stress.
There was no evidence of a statistically significant difference in the DASS for a parent-directed tailored intervention compared with usual care (adjusted MD –5.3 points, 95% CI –13.0 to 2.4 points; unadjusted MD –5.3 points, 95% CI –13.1 to 2.5 points). 138 Sciberras et al. 125 found no evidence of a statistically significant difference in change scores from baseline to month 5 (data not available for 2-month follow-up) between the extended and brief intervention groups (MD 1.82 points, 95% CI –1.03 to 4.67 points).
Other parent-/carer-related outcomes of parent-directed tailored interventions
Two RCTs (n = 53)124,125 and one before-and-after study146 reported other parent-/carer-related outcomes of parent-directed tailored interventions. These data are summarised in Table 11. These outcomes focused on parental stress and morale124,146 and parent work attendance. 125
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Moss et al. (2014)124 | Parenting Stress Index – Short Form |
Intervention group: 89.30 points (18.70 points) Control group: 103.63 points (20.04 points) |
Intervention group: 80.50 points (15.23 points) Control group: 103.00 points (21.84 points) |
–22.50 points (–39.02 to –5.98 points)a |
| Sciberras et al. (2011)125 | Parental work attendance | NR | NR | ‘At three months, intervention parents reported fewer days late for work as a result of their child’s behaviour than control parents (p = 0.02, non-parametric test for trend) and fewer missed days at work (p = 0.03)’ |
| Before-and-after studies | ||||
| Quine and Wade (1991)146 | Maternal stress (Malaise Inventory) | 6.4 points (4.1 points) | 3.8 points (2.8 points) | –2.6 points (–1.5 to –3.7 points)a |
| Quine and Wade (1991)146 | Maternal morale (Cantrill ladder) | 6.7 points (2.2 points) | 7.6 points (1.3 points) | 0.9 points (0.2 to 1.6 points)a |
There was a statistically significant reduction (i.e. improvement) in the Parenting Stress Index – Short Form when comparing a parent-directed tailored intervention with a waiting list control. 124 Another RCT125 found a statistically significant difference in parent work attendance for an extended versus a brief version of a parent-directed tailored intervention.
The before-and-after study found both a statistically significant reduction in maternal stress, as measured by the Malaise Inventory, and an improvement in maternal morale, as measured by the Cantrill ladder, from pre to post intervention. 146
Measures of perceived parenting confidence and/or efficacy and/or understanding of sleep/sleep management
One RCT21 and two before-and-after studies21,146 reported on measures of perceived parenting confidence and/or efficacy and/or understanding of sleep/sleep management. Details of how these were measured are summarised in Appendix 13.
Parenting Sense of Competence scale and satisfaction and efficacy subscales
One RCT (n = 13)21 and one before-and-after study (n = 12)21 used the Parenting Sense of Competence (PSOC) scale. 168 The PSOC scale is a validated self-completed questionnaire. A higher score indicates a higher sense of parenting competence.
No evidence of a statistically significant difference was found in the RCT comparing modes of delivering implementation support (face to face vs. telephone) for either the satisfaction (MD 6.17 points, 95% CI –5.75 to 18.09 points) or the efficacy (MD –0.50 points, 95% CI –9.66 to 8.66 points) subscales of the PSOC scale. 21 Both satisfaction and efficacy subscale scores increased post intervention in the before-and-after study; however, a MD and CI was not calculated, as the summary data presented were not reported for a matched sample pre and post intervention. 21
Other measures of perceived confidence and/or efficacy and/or understanding of sleep/sleep management
One before-and-after study146 measured improvement in parental knowledge of behavioural principles using the Knowledge of Behavioural Principles as Applied to Children test. 169 The Knowledge of Behavioural Principles as Applied to Children test is a validated multiple choice instrument that assesses verbal understanding of basic behavioural principles. Higher scores indicate better knowledge scores. There was a significant improvement between pre and post intervention in the parents’ knowledge scores (MD 2.2 points, 95% CI 1.7 to 2.7 points).
Summary
Nine studies evaluated parent-directed, comprehensive tailored interventions. 21,107,123–126,138,146 There were five RCTs,21,107,124,125,138 three of which107,124,138 were rated as having a high risk of bias and compared the intervention with usual care or other control, one125 of which was rated as having a high risk of bias and compared brief with extended versions of an intervention and another of which21 was rated as having a high risk of bias and compared different modes of delivering implementation support. There were also four before-and-after studies21,123,126,146 that were rated as having a high or an unclear risk of bias. The mean age of participants ranged from 2.7 to 12.1 years. The majority of studies21,123–126,138,146 included children with a mixed range of NDs, with three studies107,125,146 including children with a single condition. The majority of studies21,107,123,126,146 also included children with a mix of sleep disturbances, mainly sleep initiation and/or sleep maintenance problems.
Although all of the studies used face-to-face sessions with parents (at home or in a clinic) as the primary mode of delivery and were similar insofar as the content was classified as comprehensive, there was considerable variation in the duration of the intervention, the number of sessions delivered and the extent of implementation support when the sessions had finished, for example through follow-up telephone contact.
Overall, there was mixed evidence about the effects of parent-directed tailored interventions compared with usual care or other control, with limited evidence of benefit on more objective actigraphy child-related sleep outcomes and more evidence of benefit on parent-reported child outcomes and a few parent outcomes. Conclusions are hampered by the limited number of RCTs, the multitude of outcome measures and the risk of studies being underpowered to detect an effect. A single study138 was rated as having a low risk of bias for all domains except for blinding, as the outcomes may have been influenced by the lack of blinded outcome assessment. This RCT138 of a parent-directed comprehensive tailored intervention (with implementation support) compared with usual care, which provided the best available evidence identified, reported a small increase in TST in favour of the intervention, but the lower CI covered the possibility of worsening of TST (MD 10.9 minutes, 95% CI –19.0 to 40.8 minutes). A second RCT of a parent-directed comprehensive tailored intervention (with implementation support) compared with usual care had a similar finding (MD 26.0 minutes, 95% CI –35.1 to 87.1 minutes). 107 There was also no statistically significant improvement in other actigraphy-measured sleep outcomes with the intervention compared with control (sleep efficiency, WASO, SOL), measured by either or both studies. There was a statistically significant improvement in child sleep habits (CSHQ) and in parent-reported child sleep problems in one of these studies,138 as well as child behaviour and quality of life (parent-reported ADHD Rating Scale IV, PedsQL, Parent Daily Reporting of Morning and Evening Behaviour). The third RCT124 reported no statistically significant difference in child behaviour (Developmental Behaviour checklist). One RCT138 reported no statistically significant improvement in parental stress (DASS) with the intervention compared with control, whereas another RCT124 reported a statistically significant improvement (Parenting Stress Index – Short Form). The four before-and-after studies21,123,126,146 showed improvement across several child-related and parent-related outcomes.
Based on a single RCT,21 there was no statistically significant difference in the clinical effectiveness of home-delivered compared with telephone-based implementation support on child sleep outcomes (CSHQ) or parental outcomes (PSOC scale). Based on a single RCT,125 there was a statistically significant improvement in parental work attendance and parent-reported child sleep problems with an extended versus brief intervention but no difference for multiple other child-related and parent-related outcomes.
Parent-directed non-tailored interventions
Eight studies evaluated parent-directed non-tailored interventions (Table 12): two two-armed parallel-group RCTs,49,127 one three-armed parallel-group RCT49 and five before-and-after studies. 129,149,151,162,163 Non-tailored parent-directed intervention involves the delivery of a standard ‘training curriculum’. The curriculum may include opportunities for a parent to be supported to operationalise the material learnt for their child’s sleep disturbance. Some implementation support may also be included.
| Study | Total duration of intervention | Intervention details (active arms only) | Was the intervention described as having been developed for a specific ND? | Manual? | Length of follow-up, from baseline | |||
|---|---|---|---|---|---|---|---|---|
| Mode of delivery | Number of sessions over which the curriculum was delivered | Was there an opportunity to operationalise curriculum content for the child’s sleep problem? | Mode of delivering implementation support, and intensity, once curriculum was delivered: mode and intensity | |||||
| RCTs | ||||||||
| Adkins et al. (2012)127 | N/A | Booklet | N/A | No | None | Yes, ASD | N/A | 2 weeks |
| Before-and-after studies | ||||||||
| Beresford et al. (2012)162 | 5 weeks | Group | 4 | Yes | None (but included within curriculum for group session) | No | Yes | 5, 17 and 29 weeks |
| Beresford et al. (2012)163 | 1 session | Teaching workshop | 1 | No | None | No | Yes | 12 and 24 weeks |
| Bramble (1997)149 | 1 session | Face to face (clinic) | 1 | Minimal (‘only minor individual tailoring’) | Telephone calls on 3 consecutive days after session. Additional calls arranged if necessary | No | Yes | 2 weeks, 4 months and 18 months |
| Reed et al. (2009)129 | 3 weeks | Group | 3 | Yes | None (but included within curriculum for group sessions) | Yes, ASD | Yes | 7 weeks |
| Yu et al. (2015)151 | 7 weeks | Group, plus weekly telephone calls | 3 | Yes | Weekly for 4 weeks | Yes, ASD | Yes | 3, 7 and 11 weeks |
| RCT comparisons of intervention delivery | ||||||||
| Montgomery et al. (2004)49 | N/A vs. 1 session | Booklet vs. face to face | N/A vs. 1 | No vs. no | None vs. none | No | Yes | 6 weeks |
| Malow et al. (2014)128 | 2 weeks | Face to face vs. group | 1 vs. 2 | Yes | Weekly telephone call (n = 2) after sessions completed | Yes, ASD | Yes | 1 month |
The interventions varied in terms of the number of training sessions, the mode of delivery and the provision of implementation support. The number of sessions ranged from zero (leaflet provision only)127 to two for the RCTs49 and from one149,163 to three and four sessions for the before-and-after studies. 129,151,162 The duration of the intervention was relatively short for RCTs, up to 2 weeks,128 but was longer for the before-and-after studies, ranging from a single session149,163 to 7 weeks. 151 Interventions also varied in terms of whether or not ‘implementation support’ was provided to parents. Some did not offer any implementation support (n = 3), some offered it during training sessions only (n = 2) and others also offered it for a time-limited period after all the sessions had been completed (n = 3). The curricula of four of the interventions included the opportunity within sessions for parents to operationalise learning for their child’s specific sleep problem.
The RCTs investigated interventions delivered both via written materials and through individual work (face to face and telephone calls). The mode of delivery was in booklet format for two RCTs49,127 and was face to face for the third RCT, which was supplemented with two weekly telephone calls. 128 The mode of intervention delivery for the before-and-after studies was predominantly in group format, apart from in one study. 149 Implementation support was included in two before-and-after studies, which took the form of telephone calls for 3 days following the initial session for one study149 and weekly for 4 weeks for the other. 151
The comparators in the three trials were no intervention,127 individual versus group delivery of an intervention128 and, in a three-arm trial, written material versus face-to-face delivery of material versus no intervention control. 49
For four interventions, study authors reported that the content of the intervention was informed by the nature of the target population, namely children with ASDs.
One RCT and three before-and-after studies included children with a range of NDs. 49,149,162,163 Two RCTs and two before-and-after studies included children with ASD only. 127–129,151
The age of participants ranged from 2 to 5.9 years in the RCTs,49,127,128 and from 4.8 to 8.9 years for the before-and-after studies. 129,149,151,162,163 Children in the RCTs had problems with sleep initiation127,128 and problems with both sleep initiation and maintenance. 49 Children in the before-and-after studies had problems with both sleep initiation and maintenance, with the exception of one study. 162 Only one study149 reported on whether or not the child had received any prior interventions.
Table 12 provides an overview of the studies evaluating the non-tailored parent training interventions.
The RCTs49,127,128 reported on five outcomes and the before-and-after studies129,149,151,162,163 reported on 10 outcomes. The most commonly reported outcome measures used were the CSHQ,127–129,151,162,163 TST,127,128,151 SOL,127,128,149,151 WASO127,128,151 and child daytime behaviour. 128,129,149,151 No studies measured child cognition outcomes.
Sixteen different sleep-related outcomes were measured (see Appendices 14–16). The first outcome measurement time points ranged from 2 weeks to 7 weeks from baseline (see Table 12). Four trials149,151,162,163 measured additional follow-up time points; however, in this review, we focus only on the outcomes closest to the end of the intervention.
Given the variations in intervention characteristics (e.g. mode of delivery, number of sessions, availability of implementation support), study design and study objectives, it was not considered appropriate to pool the RCT data. Therefore, the studies are described in a narrative synthesis.
Child-related outcomes
The outcomes measured and methods of assessment are summarised in Appendix 14.
Global measures and composite scores
Total sleep time
Two RCTs (n = 116)127,128 and one before-and-after study (n = 25)129 evaluated actigraphy-measured TST informed by the use of sleep diaries following existing guidance on the interpretation of actigraphy data. 134
In one of the RCTs,127 no evidence of a statistically significant difference in actigraphy-measured TST was found for an intervention comprising the provision of written information compared with no intervention (MD 12.2 minutes, 95% CI –25.1 to 49.5 minutes). A similar finding was reported by the RCT that compared the modes of delivering a sleep management training curriculum to parents (i.e. face to face vs. group) [MD –7.2 minutes (favours group intervention), 95% CI –29.7 to 15.3 minutes]. 128 The authors also report the findings of a post hoc analysis, pre and post intervention, for the two groups combined, which are not described in this report.
The before-and-after study129 narratively reports that there were no ‘significant changes’ in TST following a group intervention.
Sleep efficiency
Two RCTs (n = 116)127,128 reported actigraphy-measured sleep efficiency (the percentage of time spent in bed asleep) informed or verified by sleep diaries. The authors of the RCT127 that evaluated the provision of sleep management training via written material, compared with no intervention, report a statistically significant difference in the mean change (baseline to treatment) for the two groups (p = 0.04); however, we were able to calculate the unadjusted difference between the two groups only post intervention and this difference was not statistically significant (MD 2.7%, 95% CI 2.0% to 7.4%). Similarly, no significant difference in this outcome was reported by the RCT128 that compared face-to-face delivery with group delivery of an intervention (MD –1.1%, 95% CI –3.6% to 1.4%). The authors also report the findings of a post hoc analysis for sleep efficiency, pre and post intervention, for the two groups combined, which are not described in this report.
The Children’s Sleep Habits Questionnaire
One RCT (n = 80)128 and four before-and-after studies (n = 126)129,151,162,163 reported the CSHQ total score. The CSHQ is a validated parent-reported assessment of child sleep. 133 Higher scores on the CSHQ indicate a greater severity of the sleeping disturbance resulting from either the frequency or number of different behaviours presenting. The RCT reported the CSHQ results only for all participants in the two treatment groups (combining participants receiving group and individual education) and not for each group separately.
For the RCT,128 there was no statistically significant difference in total CSHQ score post intervention between the group receiving face-to-face delivery and the group delivery arm (Table 13). In a post hoc analysis, the authors report merging the two arms and report combined before-and-after outcomes, which are not described here. Two before-and-after studies, one evaluating a three-session group-delivered intervention129 and the other evaluating a four-session group-delivered intervention with implementation support,151 report a statistically significant improvement (i.e. a decrease) in CSHQ total score post intervention compared with pre intervention. For two additional before-and-after studies,162,163 it was not possible to estimate the MD in total CSHQ score between pre and post intervention, as they were not matched samples; however, the studies report small or very small effect sizes.
| Study | Time point, mean CSHQ score (SD) | MD (95% CI) | |
|---|---|---|---|
| Baseline | Follow-up | ||
| RCTs | |||
| Malow et al. (2014)128 | NR | NR | NR |
| Before-and-after studies | |||
| Beresford et al. (2012)162 | 57.86 (9.76) | 51.79 (8.91) | Cannot be estimated.a Effect size given as 0.20 |
| Beresford et al. (2012)163 | 56.58 (9.50) | 55.56 (10.76) | Cannot be estimated.b Effect size given as 0.02 |
| Reed et al. (2009)129 | 56.63 (9.21) | 49.74 (9.24) | –6.89 points (–2.58 to –11.2 points)c |
| Yu and Hong (2015)151 | 55.11 (8.38) | 51.76 (7.53) | –3.34 points (–1.4 to –5.3 points)d |
Parent-set child sleep goals
Two before-and-after studies (n = 47) – one evaluating a single-session group-delivered intervention163 and the other evaluating a group-delivered intervention over four sessions162 – reported parent-set child sleep goals using a goal attainment rating scale. This was a 10-point rating scale used to indicate the extent to which a goal has been achieved, from 1 = very far from this goal to 10 = I have achieved my goal.
Both studies reported that, post intervention, the mean goal attainment rating had ‘significantly improved’ compared with the pre-intervention rating. It was not possible to estimate the MD between the scores at pre and post intervention, as neither study reported data for a matched sample.
These same before-and-after studies162,163 also reported the proportion of children that had improved, deteriorated or not changed in the goal attainment rating (improvement/deterioration based on whether or not the goal scores have moved 1 point in a positive or negative direction). Both studies reported that, post intervention, the majority of parents reported an improvement in goal attainment rating compared with pre intervention. In one study,162 93% of participants had improved and there was no change in the other 7% and, in the other study,163 65% improved, 19% had no change and 16% had deteriorated. 163
Family Inventory of Sleep Habits
One RCT (n = 80)128 and two before-and-after studies (n = 79)129,151 evaluated child sleep outcomes using the Family Inventory of Sleep Habits (FISH). FISH is a validated parent-completed scale to assess sleep hygiene in children with ASD170 and a higher score indicates better sleep hygiene.
The RCT128 reported the results of a post hoc analysis for all participants in the two treatment groups (group and individual education) combined and not for each group separately; therefore, these data are not reported here. One of the before-and-after studies129 also did not report data for the total score (only for each item separately) and, therefore, they are not reported here. The other before-and-after study151 of a group-delivered sleep management intervention reported statistically significant improvements (data reported only at week 11) in total FISH score post intervention compared with pre intervention (MD 1.38, 95% CI 0.05 to 2.71).
Composite sleep disturbance score
One RCT (n = 66)49 reported a composite sleep disturbance score. The composite sleep disturbance score was calculated from parent-completed diaries to identify sleep problems, covering settling frequency, settling duration, night waking frequency and night waking duration. A higher score on the composite sleep disturbance score indicates more severe sleep problems.
This study compared no intervention with either the intervention delivered in written format or via a face-to-face session and it reported that there was no statistically significant difference between these delivery methods post intervention (MD 0.15, 95% CI –1.38 to 1.68). The trial also included a third, ‘control’, arm that received no treatment for the first 6 weeks after randomisation, following which participants were re-randomised to receive either the conventional treatment or the booklet treatment. The authors compared the two active arms (written format and face to face) with the control and found statistically significant differences between the written format group and the control group (MD 3.20, 95% CI 1.89 to 4.51), and between the face-to-face format and the control group (MD 3.35, 95% CI 2.32 to 4.38). 49
Severity of sleep problems
One before-and-after study, evaluating an individually delivered parent-directed tailored intervention, used a visual analogue scale to capture parent-reported ‘severity of sleep problems’, ranging in score from 0 = no problem to 10 = very severe problem. 149 The author reported a statistically significant improvement for the group post intervention compared with pre intervention but does not present sufficient data to allow for a MD and 95% CI to be calculated.
Sleep initiation
Sleep onset latency
Two RCTs (n = 116)127,128 and two before-and-after studies (n = 40)129,149 listed SOL (the time from bedtime to sleep onset time) as an outcome. Bramble149 described SOL as ‘mean time to settle’, whereas the other studies described SOL as the time from bedtime to sleep onset time.
In the RCT evaluating the provision of written information, no evidence of a statistically significant difference in SOL was found between this intervention and no intervention (Table 14). 127 Similarly, the authors of the second RCT128 comparing modes of delivery (individual vs. group) reported that they found no evidence of a statistically significant difference between the two groups in terms of this outcome. The authors also report the findings of a post hoc analysis for SOL, pre and post intervention, for the two groups combined, which are not described in this report.
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Adkins et al. (2012)127 | SOL (minutes) |
Intervention group: 56.7 (27.1) Control group: 52.1 (25.1) |
Intervention group: 49.5 (26.7) Control group: 61.3 (47.0) |
–11.8 (–37.3 to 13.7) |
| Malow et al. (2014)128 | SOL (minutes) |
Individual: 59.8 (31.6) Group: 56.0 (25.2) |
Individual: 39.5 (21.6) Group: 39.7 (21.5) |
–0.2 (–9.9 to 9.5) |
| Before-and-after studies | ||||
| Reed et al. (2009)129 | SOL (minutes) | NR | NR | NR |
| Bramble (1997)149 | Mean time to settle (minutes) | 58.6 (24.6) | 15.8 (7.8) | –42.8 (–61.0 to –24.6) |
One before-and-after study129 only narratively reports that there were no ‘significant changes’ in SOL following a group intervention. The other before-and-after study149 that evaluated ‘mean time to settle’ reported a statistically significant reduction in mean time to settle following a face-to-face session.
Sleep maintenance
Wake after sleep onset
The outcome measure was WASO (night waking duration and/or frequency after the child falls asleep) in two RCTs (n = 116)127,128 and one before-and-after study. 129
The RCT127 comparing an intervention (provision of written material) with no intervention found that there was no evidence of a statistically significant difference at follow-up between the two groups (MD 0.5, 95% CI –18.9 to 19.9). The RCT128 comparing individual and group delivery of an intervention also reported that there was no statistically significant difference between the groups on this outcome measure (MD –0.2, 95% CI –9.9 to 9.5). When the two treatment groups were combined, the mean change from pre to post intervention was –3.5 minutes (95% CI –7.3 to 0.3 minutes). The before-and-after study only narratively reports that there were no ‘significant changes’ in WASO following a group intervention. 129
Child behaviour
Child behaviour was evaluated using four different outcome measures: (1) the Child Behavior Checklist (CBCL), (2) the Repetitive Behaviour Scale – Revised (RBS-R), (3) the Parental Concerns Questionnaire (PCQ) and (4) the BPI.
The Child Behavior Checklist
One RCT (n = 80)128 and one before-and-after study (n = 54)151 used the CBCL to investigate changes in daytime behaviour problems. 171 The CBCL is a validated parent-completed outcome measure for identifying problem behaviour in children, which is comprised of eight subscales. A higher score indicates more severe problems. Although the before-and-after study reported a total CBCL score, the RCT reported the following subscales of the CBCL only: anxious/depressed, attention, ADHD and withdrawn.
The RCT comparing individual with group delivery of an intervention reported that there was no difference between the two arms at follow-up, although data were not presented separately for the two groups. 128 With the two groups combined, the mean change from baseline to post intervention was as follows: anxious/depressed –2.2 (95% CI –3.7 to –0.7), attention –3.3 (95% CI –5.4 to –1.2), ADHD 0.54 (95% CI –1.3 to 2.4) and withdrawn –2.8 (95% CI –4.8 to –0.8).
The before-and-after study evaluated a group-delivered intervention (with implementation support offered via telephone) and did not find evidence of a statistically significant improvement in total CBCL scores post intervention (week 7) (MD –0.91, 95% CI –2.48 to 0.66). 151
The Repetitive Behaviour Scale – Revised
One RCT (n = 80)128 and one before-and-after study (n = 25)129 evaluated child daytime behaviour using the RBS-R (total). 172 A lower RBS-R score indicates fewer problems.
The authors of the RCT, comparing group and individual delivery, reported that they found no evidence of a statistically significant difference in the change from baseline in daytime behaviours between the two groups post intervention (data not provided). The authors also report the findings of a post hoc analysis for RBS-R, pre and post intervention, for the two groups combined, which are not described in this report. 128 In the before-and-after study129 that evaluated a group-delivered intervention, a statistically significant improvement (i.e. decrease) was observed post intervention compared with pre intervention (MD –1.22, 95% CI –2.06 to –0.38).
The Parental Concerns Questionnaire
Two before-and-after studies (n = 79)129,151 evaluated child daytime behaviour using the PCQ. 173 The PCQ is designed as a parent interview screening instrument assessing the severity of developmental and associated psychiatric symptomatology using a four-point scale. A higher PCQ score indicates more severe problems.
Both of the before-and-after studies129,151 evaluated group-delivered interventions, one of which included implementation support. 151 A total score could not be derived for one study,129 as the authors did not report this (data reported for each subscale of the PCQ separately). The other study151 found no evidence of a statistically significant change in the PCQ scores post intervention (data reported for week 11 only) compared with pre intervention (MD –1.38, 95% CI –2.82 to 0.06).
The Behavior Problem Index
One before-and-after study (n = 15)149 evaluated child daytime behaviour using the BPI. 167 The BPI is a validated parent-reported outcome measure of child behaviour. Higher scores indicate a greater level of behaviour problems.
The authors reported the BPI at 4 months’ follow-up only and reported a statistically significant improvement for the group post intervention, with a score of 22.1 (SD 12.4), compared with a pre-intervention score of 32.6 (SD 13.6) (MD –10.5, 95% CI 3.3 to 17.7). 149
Child’s health-related quality of life
One RCT (n = 80)128 measured health-related quality of life using the PedsQL. 174 The PedsQL is a validated parent-completed questionnaire that assesses parents’ perception of quality of life in paediatric patients with chronic health conditions. Higher scores indicate better health-related quality of life.
The authors of the RCT,128 comparing group delivery with individual delivery of a sleep management intervention, reported that they found no evidence of a statistically significant difference in the change from baseline in PedsQL score between the two groups post intervention (data not provided). The authors also report the findings of a post hoc analysis for PedsQL, both pre and post intervention, for the two groups combined, which are not described in this report.
Parent outcomes
Several studies assessed parent outcomes. The outcome domains assessed were mental well-being,129,149,151 quality of sleep149,151 and parents’ sense of competence. 128,162,163
Quality of sleep
A before-and-after study (n = 15)149 of an individually delivered intervention with implementation support measured self-reported quality of sleep using the Maternal Sleep Scale. 175 The Maternal Sleep Scale is a validated self-completed questionnaire that measures mothers’ sleep quality, with higher scores indicating better sleep quality.
The authors reported the Maternal Sleep Scale scores at the end of the treatment phase and reported a statistically significant improvement for the group post intervention, with a mean score of 7.1 (SD 2.3), compared with a pre-intervention mean score of 4.1 (SD 2.3) (MD 3, 95% CI 1.7 to 4.3). 149
A second before-and-after study (n = 54)151 of a group-delivered intervention with implementation support used the Pittsburgh Sleep Quality Index to measure parents’ self-reported quality of sleep. The Pittsburgh Sleep Quality Index is a validated self-report questionnaire that assesses sleep quality. 176 Lower scores denote a healthier sleep quality. No statistically significant change on this measure was reported following the intervention (week 7) (MD –0.51, 95% CI –1.37 to 0.35). 151
Parent/carer mental well-being
Two before-and-after studies (n = 79)129,151 assessed parental stress using the Parenting Stress Index – Short Form. 177 The Parenting Stress Index – Short Form is a validated self-completed outcome measure used for measuring parenting stress. A higher score indicates higher levels of parental stress.
Both studies evaluated group-delivered interventions, one with implementation support151 and one without. 129 Neither reported a statistically significant change on the Parenting Stress Index – Short Form in post-intervention compared with pre-intervention scores (Table 15).
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Reed et al. (2009)129 | Parental Stress Index – Short Form | 96.10 (23.40) | 94.00 (23.00) | –2.1 (–12.9 to 8.7) |
| Yu and Hong (2012)161 | Parental Stress Index – Short Form | 82.55 (8.15) | 81.73 (8.32) | 0.82 (–1.63 to 3.27) |
| Bramble (1997)149 | Malaise Inventory | 8.7 (4.3) | 4.7 (3.9) | –4.0 (–1.7 to –6.3) |
A further before-and-after study149 assessed psychological distress using the Malaise Inventory. The Malaise Inventory is a validated self-completed questionnaire that measures psychological distress. 178 A higher score on the Malaise Inventory indicates higher psychological distress. This study evaluating an individually delivered sleep intervention with implementation support reported a statistically significant improvement in scores on the Malaise Inventory post intervention compared with pre intervention (see Table 15). 149
Parenting sense of competence
One RCT (n = 80)128 and two before-and-after studies (n = 47)162,163 assessed parents’ sense of competence using the PSOC scale. 168 The PSOC scale is a validated self-completed questionnaire. A higher score indicates a higher parenting sense of competence. This scale comprises two subscales: sense of efficacy and parenting satisfaction.
The authors of the RCT,128 comparing group and individual delivery, reported that they found no evidence of a statistically significant difference in the change from baseline in PSOC between the two groups post intervention (data not presented). The authors also reported the findings of a post hoc analysis for parenting sense of competence, both pre and post intervention, for the two groups combined, which are not described in this report.
One before-and-after study162 reported a statistically significant improvement in the PSOC-efficacy (effect size 0.82), but not PSOC-satisfaction (effect size 0.38), from pre to post intervention, following a four-session group intervention; however, a MD and 95% CI could not be calculated, as data were not reported for a matched sample. The second before-and-after study163 reported very little change in scores on PSOC-efficacy and PSOC-satisfaction 12 weeks after attending a single-session workshop (effect sizes –0.15 and 0.11, respectively); however, a MD and 95% CI could not be calculated as data were not reported for a matched sample.
Summary
Eight studies49,127–129,149,151,162,163 evaluated parent-directed non-tailored interventions to manage sleep disturbance in children with NDs. We defined non-tailored interventions as those comprising the delivery of a standard ‘training curriculum’. All the non-tailored interventions were comprehensive in their content, covering material on sleep and sleep processes, sleep hygiene and the management of specific problem behaviours (e.g. night wakings). Within this set of interventions, the design of the curriculum may include opportunities for parents to be supported to operationalise the material learnt for their child’s sleep disturbance. Some implementation support (i.e. support to parents as they implement new ways of managing their child’s sleep disturbance) may also be included.
Compared with the tailored interventions reported in the previous section, the non-tailored interventions evaluated by studies included in this review were much more diverse in terms of mode of delivery [i.e. written material, group delivery (single vs. multiple sessions) and one-to-one work]. In addition, they differed in terms of the extent to which they could accommodate the specific information and training needs parents may have in terms of their own child’s condition and/or sleep problem. Furthermore, some included implementation support, but others did not. When implementation support was provided, it was always via telephone calls.
The eight studies included one RCT127 that compared intervention (written material) and no intervention and five before-and-after studies. 129,149,151,162,163 Three129,151,162 evaluated a group-delivered intervention comprising three or four sessions, one163 evaluated a single-session workshop and one evaluated an individually delivered intervention. 149 One RCT49 compared two modes of delivering the same training curriculum (written material vs. face-to-face session) with no intervention. The final RCT128 compared delivery of the same training curriculum via group and individual delivery. All the studies were rated as having a high risk of bias.
Four interventions127–129,151 were developed specifically for children with ASDs, and only children with these conditions were recruited to the studies. In the remaining four studies,49,149,162,163 the interventions were evaluated using samples of children with a range of NDs. The mean age of children recruited to the studies ranged from 2.0 to 8.9 years. The majority of studies reported that the children had problems with both sleep initiation and sleep maintenance.
The conclusions that can be drawn are limited, owing to the rating of the high risk of bias, the use of post-intervention follow-up time points and the risk of studies being underpowered to detect an effect. In addition, a large number of different outcome measures were used and these evaluated a range of outcome domains. We organise the findings in terms of mode of delivery and, when relevant, availability of implementation support.
A single trial evaluated the impact of an intervention comprising the provision of written information (and no implementation support) with no intervention. 127 There was no evidence of benefit at 2 weeks post intervention, as measured using actigraphy-collected sleep outcomes data (TST, Sleep Efficiency, SOL and WASO). Another three-armed trial49 compared the provision of written information or the delivery of the same material via a single face-to-face session with a waiting list control group. Neither active arm included the provision of implementation support. Evidence of benefit at 6 weeks post intervention, based on a parent-report measure (composite sleep disturbance score), was reported for both modes of intervention delivery compared with the control group (written information vs. control, MD 3.20, 95% CI 1.89 to 4.51; face-to-face delivery vs. control, MD 3.35, 95% CI 2.32 to 4.38). The study was not powered to detect a difference between the two active arms. No other child outcomes, or parent outcomes, were assessed by these studies. There is, therefore, mixed and very limited evidence regarding the impact that the provision of written information with no implementation support has on child sleep outcomes only. Given the differences in outcome measures, differences in post-intervention follow-up time points and the high risk of bias for both studies, it is not possible to draw further conclusions regarding this sort of intervention. Furthermore, no conclusions can be drawn regarding the relative benefits of written versus face-to-face delivery of training on sleep management.
One before-and-after study149 evaluated an intervention comprising a single face-to-face training session and implementation support available for up to 2 weeks after the session. The authors report significant improvements post intervention on non-standardised sleep outcome measures and a standardised measure of child behaviour (BPI) but data were not fully presented. Given the study limitations, no conclusions can be drawn from this evidence.
Two before-and-after studies evaluated multisession (n = 3 or 4) group-delivered interventions for which no implementation support was available after the group sessions were completed. 129,162 In one study, outcomes were reassessed immediately post intervention;129 in the other study, this took place 1 month post intervention. 162 Both studies report improvements in child sleep outcomes measured using the same standardised parent-report measure (CSHQ) (for Beresford et al. ,162 the pre- and post-intervention samples were not matched and the reported effect size was 0.2; for Reed et al. ,129 the MD was –6.89, with a 95% CI of –2.58 to –11.2) and, for one study (Beresford et al. 162), using achievement of parent-set sleep goals (progress towards goals, 93% of participants; no progress, 7% of participants). However, one of the studies129 also used actigraphy to collect sleep outcomes data (TST, SOL, WASO). No benefits were reported on these outcomes. Reed et al. 129 also reported evidence of benefit in terms of parent-reported child behaviour (RBS-R). A further before-and-after study151 also evaluated a multisession (n = 3) group-delivered intervention but also offered implementation support after the group sessions had finished via weekly telephone calls (duration unclear). This study also used the CSHQ to measure child sleep outcomes and reported significant improvements immediately post intervention (MD –3.34, 95% CI –1.4 to –5.3). Child behaviour outcomes were also assessed (CBCL and PCQ) and no benefits were reported. Beresford et al. 162 also report significant improvements in self-reported parental efficacy but not parenting satisfaction (PSOC scale); however, pre- and post-intervention samples were not matched so a MD and CI could not be calculated. Reed et al. 129 and Yu and Hong161 investigated parenting stress using the Parenting Stressing Index; neither study reported a change in this outcome between pre- and post-intervention time points. Yu and Hong161 also report no effect on parent-reported sleep quality (Pittsburgh Sleep Quality Index). Taken together, there is some limited evidence of benefit for group-delivered interventions in terms of parent-reported child sleep outcomes. We would again, however, note that these studies were rated as having a high risk of bias. It is not possible to draw further conclusions with respect to other child, or parent, outcomes.
A RCT128 compared delivery of the same training curriculum via a multisession (n = 2) group-delivered intervention and a single intervention; both arms included the same level of implementation support. No differences in outcomes (1 month post intervention) between the two modes of delivery were reported on actigraphy-derived sleep outcomes (TST, Sleep Efficiency, SOL and WASO) or parent-reported measures of child sleep (CSHQ and FISH), behaviour (CBCL and RBS-R) or quality of life (PedsQL). As this is a single study that was rated as having a high risk of bias, and it is not clear if it was adequately powered, no conclusions can be drawn.
The final mode of intervention delivery evaluated was a single workshop (5 hours) delivered to groups of up to 20 parents. 163 No implementation support was available. Parent-reported sleep outcome measures (CSHQ and attainment of parent-identified sleep goals) were readministered 3 months after the workshop. Benefits on these indicators were reported by the authors (PSOC-efficacy, effect size 0.82; PSOC-satisfaction, effect size 0.38). However, MD and CIs could not be calculated, as the data were not reported for a matched sample. Given that this mode of intervention delivery was evaluated by only a single study that was rated as having a high risk of bias, no further conclusions can be drawn.
Non-comprehensive parent-directed interventions
Two studies evaluated parent-directed sleep management interventions, which we classified as being non-comprehensive in their content, that is, the content of the training was focused on just one topic area related to sleep disturbance (whereas interventions that were classified as comprehensive addressed sleep and sleep processes, sleep hygiene and the management of specific problem behaviours, such as night waking).
One intervention, evaluated using a cluster RCT152 with an attention control arm, comprised a tailored intervention that was restricted to training parents on behavioural principles of managing problem sleep behaviours, including functional analysis and behaviour management strategies. A single session with a practitioner, delivered in families’ homes, was followed by telephone calls on at least a weekly basis to support the implementation of sleep management strategies. The total duration of implementation support was variable, continuing up to 3 months after the session with the practitioner. A description of the ‘attention control’ intervention was not provided. Although this appears to be a cluster trial, the description of the recruitment and randomisation processes is unclear and, so, for the purposes of this analysis, we have treated it as if it were an individually randomised trial. There were insufficient data to allow for any potential clustering and, in any case, the nature of the clustering was unclear.
A before-and-after study155 evaluated the second non-comprehensive intervention. This intervention was restricted to training parents on principles of sleep hygiene only and was a single session delivered in a clinic. No implementation support was provided.
The mean age of participants in the RCT152 was 9.49 years and the children had a mixed range of NDs. Neither the children’s ages nor their NDs were reported by the before-and-after study. 155 Neither study reported whether or not the child/parent had received any prior interventions.
A total of 37 sleep-related outcomes were measured in the RCT152 (see Appendices 15–17). Follow-ups were conducted post intervention at ‘visit 4’ (approximately 1 month post randomisation) and ‘visit 6’ (approximately 3 months post randomisation). Here, when data are available for both time points, we report data from visit 4 as, for the majority of the sample, contact with a sleep practitioner had ceased by this point. 152 A total of four sleep-related outcomes were measured in the before-and-after study155 (see Appendices 15 and 17). Follow-ups were conducted after 6 weeks, at the end of the intervention.
Table 16 provides an overview of the studies evaluating the non-comprehensive parent-directed interventions.
| Study | Total duration of intervention | Mode of delivery | Number of sessions and location | Opportunity to operationalise curriculum content for child’s sleep problem? | Mode of delivering implementation support, and intensity, once regular sessions with practitioner were completed | Intervention described as developed for specific ND? | Manual? |
|---|---|---|---|---|---|---|---|
| RCTs | |||||||
| Wiggs and Stores (1998)152 | Unclear | Face to face (home) | 1 (home) | Yes | Weekly telephone calls. Continued for at least 1 month, total duration unclear | No | Yes |
| Before-and-after studies | |||||||
| Peppers, et al. (2016)155 | 1 session | Face to face (clinic) | 1 (clinic) | Yes | None | Yes, ADHD | Unclear |
Child sleep-related outcomes
Global measures and composite scores
Both the RCT153 (n = 30) and the before-and-after study155 (n = 23) reported global sleep outcomes and composite scores, although they used different measures (Table 17).
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Wiggs and Stores (1998)152 | Composite Sleep Index |
Intervention group: 6.73 (2.31) Control group: 7.23 (2.26) |
Intervention group: 3.79 (1.89) Control group: 6.62 (1.89) |
–2.83 (–4.24 to –1.42)a |
| TST (hours) |
Intervention group: 9.2 (0.3) Control group: 9.1 (0.9) |
Intervention group: 9.6 (0.6) Control group: 9.4 (0.9) |
0.2 (–0.4 to 0.8)a | |
| Before-and-after studies | ||||
| Peppers et al. (2016)155 | CSHQ | 50.13 (7.16) | 43.74 (6.49) | 6.4 (4.3 to 8.5)b |
The RCT152 of the intervention restricted to training on behaviour modification principles found a statistically significant difference in Composite Sleep Index-measured child sleep compared with no intervention at ‘visit 4’. There was no statistically significant difference in actigraphy-measured TST for children in the intervention and control groups at visit 4. The before-and-after study,155 evaluating the intervention providing training in sleep hygiene, reported a statistically significant improvement in CSHQ total score at 6 weeks post intervention.
Sleep maintenance
The RCT152 (n = 30) of the intervention restricted to training on principles of behaviour modification reported a variety of actigraphy-measured sleep maintenance outcomes.
There were statistically significant reductions in fragmentation index (percentage of immobile phases during sleep period that were ≤ 30 seconds of duration), movement during sleep and movement index (percentage of sleep period spent moving) for both groups from pre intervention to visit 4; however, the differences between the groups at follow-up were not statistically significant (Table 18). 152
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Wiggs and Stores (1998)152 | Fragmentation index (%) |
Intervention group: 16.5 (3.7) Control group: 17.8 (5) |
Intervention group: 11.9 (4.1) Control group: 11.9 (5.7) |
0.0 (–3.7 to 3.7)a |
| Movement during sleep |
Intervention group: 1.9 (1.6) Control group: 2.7 (2.9) |
Intervention group: 1.4 (0.5) Control group: 1.3 (0.8) |
0.1 (–0.4 to 0.6)a | |
| Movement index (%) |
Intervention group: 11.3 (5.1) Control group: 13.1 (6.4) |
Intervention group: 9.0 (2.7) Control group: 8.8 (3.0) |
0.2 (–1.9 to 2.3)a | |
Other child outcomes
The RCT152 (n = 30) comparing the intervention restricted to training on behaviour modification principles with an attentional-control-measured child daytime behaviour using the parent-completed, and teacher-completed, Aberrant Behavior Checklist (ABC) (Table 19). 179 No statistically significant differences on ABC subscales (parent, or teacher, report) between the intervention and control arms were found post intervention, except for the parent-reported stereotypies subscale.
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Wiggs and Stores (1998)152 | ABC (parent report)a | |||
| Inappropriate speech |
Intervention group: 2.47 (2.88) Control group: 4.53 (5.13) |
Intervention group: 2.36 (1.95) Control group: 4.38 (3.64) |
–2.02 (–4.20 to 0.16)c | |
| Hyperactivity |
Intervention group: 21.13 (9.69) Control group: 24.20 (14.18) |
Intervention group: 18.07 (11.12) Control group: 22.31 (13.16) |
–4.24 (–13.35 to 4.87)c | |
| Stereotypies |
Intervention group: 3.93 (2.74) Control group: 6.07 (4.59) |
Intervention group: 2.86 (2.44) Control group: 6.46 (3.71) |
–3.60 (–5.95 to –1.25)c | |
| Lethargy |
Intervention group: 7.20 (6.03) Control group: 8.13 (6.63) |
Intervention group: 5.14 (7.10) Control group: 7.69 (8.78) |
–2.55 (–8.52 to 3.42)c | |
| Irritability |
Intervention group: 15.07 (7.40) Control group: 15.07 (7.79) |
Intervention group: 12.07 (8.46) Control group: 14.54 (8.35) |
–2.47 (–8.76 to 3.82)c | |
| ABC (teacher report)b | ||||
| Inappropriate speech |
Intervention group: 2.14 (3.51) Control group: 1.50 (1.95) |
Intervention group: 1.08 (1.93) Control group: 1.42 (2.02) |
–0.34 (–1.82 to 1.14)c | |
| Hyperactivity |
Intervention group: 13.36 (8.53) Control group: 14.43 (11.76) |
Intervention group: 10.75 (8.00) Control group: 8.33 (10.43) |
2.42 (–4.53 to 9.37)c | |
| Stereotypies |
Intervention group: 2.00 (2.39) Control group: 3.93 (5.88) |
Intervention group: 1.50 (2.35) Control group: 3.00 (4.57) |
–1.50 (–4.22 to 1.22)c | |
| Lethargy |
Intervention group: 5.29 (4.12) Control group: 10.36 (10.34) |
Intervention group: 4.00 (4.71) Control group: 4.42 (4.01) |
–0.42 (–3.69 to 2.85)c | |
| Irritability |
Intervention group: 7.93 (5.57) Control group: 11.29 (11.19) |
Intervention group: 6.83 (6.83) Control group: 5.42 (7.05) |
1.41 (–3.78 to 6.60)c | |
| Before-and-after studies | ||||
| Peppers et al. (2016)155 | Vanderbilt Assessment Scale – Parent Form (questions 1–9) | 11.39 (7.75) | 7.52 (8.41) | –3.87 (–7.37 to –0.37)c |
| Vanderbilt Assessment Scale – Parent Form (questions 10–18) | 9.30 (9.08) | 6.39 (8.51) | –2.91 (–4.81 to –1.01)c | |
The before-and-after study155 (n = 23) of an intervention training in sleep hygiene principles reported a statistically significant improvement in scores on an assessment tool capturing severity of ADHD symptoms.
Parent outcomes
The RCT152 (n = 30) comparing the intervention restricted to training on behaviour modification principles with an attentional control reported on a range of parent outcomes, including:
-
maternal TST (actigraphy)
-
maternal fragmentation index (actigraphy)
-
maternal movement during sleep (actigraphy)
-
maternal movement index (actigraphy)
-
maternal daytime sleepiness (Epworth Sleepiness Scale)
-
paternal daytime sleepiness (Epworth Sleepiness Scale)
-
maternal satisfaction with their own sleep (six-point Likert scale)
-
paternal satisfaction with their own sleep (six-point Likert scale)
-
maternal stress (Malaise Inventory)
-
paternal stress (Malaise Inventory)
-
maternal perceived ability to control difficult sleep-related behaviour of their child (visual analogue scale)
-
paternal perceived ability to control difficult sleep-related behaviour of their child (visual analogue scale)
-
maternal perceptions of their partner’s ability to control difficult sleep-related behaviour of their child (visual analogue scale)
-
paternal perceptions of their partner’s ability to control difficulty sleep-related behaviour of their child (visual analogue scale).
Further details of how these outcomes were measured are provided in Appendix 22. The data are summarised in Table 20.
| Outcome | Time period, mean (SD) | MD (95% CI) | |
|---|---|---|---|
| Baseline | Follow-up | ||
| Global measures | |||
| TST (maternal) |
Intervention group: 6.9 (0.9) Control group: 7.5 (0.8) |
Intervention group: 7.6 (0.7) Control group: 7.2 (1.4) |
0.4 (–0.4 to 1.2)a |
| Sleep maintenance | |||
| Fragmentation index (%) |
Intervention group: 10.3 (4.5) Control group: 10.4 (3.9) |
Intervention group: 9.7 (3.9) Control group: 9.8 (3.2) |
–0.1 (–2.8 to 2.6)a |
| Movement during sleep |
Intervention group: 1.6 (1.2) Control group: 1.5 (0.5) |
Intervention group: 1.2 (0.5) Control group: 1.6 (1.7) |
–0.4 (–1.3 to 1.9)a |
| Movement index (%) |
Intervention group: 9.6 (3.7) Control group: 10.6 (6.0) |
Intervention group: 7.6 (4.2) Control group: 7.2 (2.4) |
0.4 (–2.2 to 3.0)a |
| Sleep scheduling (Epworth Sleepiness Scale) | |||
| Mothers |
Intervention group: 5.67 (4.32) Control group: 7.47 (4.94) |
Intervention group: 4.07 (2.67) Control group: 6.31 (4.01) |
–2.24 (–4.79 to 0.31)a |
| Fathers |
Intervention group: 10.38 (5.03) Control group: 8.00 (4.71) |
Intervention group: 9.83 (5.06) Control group: 9.75 (3.77) |
0.08 (–3.26 to 3.42)a |
| Quality of sleep (satisfaction with own sleep) | |||
| Mothers |
Intervention group: 4.27 (1.16) Control group: 4.07 (1.44) |
Intervention group: 2.93 (1.27) Control group: 3.85 (1.57) |
–0.92 (–1.99 to 0.15)a |
| Fathers |
Intervention group: 3.08 (1.26) Control group: 2.67 (1.30) |
Intervention group: 2.17 (1.03) Control group: 2.50 (1.31) |
–0.33 (–1.21 to 0.55)a |
| Other outcomes | |||
| Maternal stress |
Intervention group: 8.36 (4.27) Control group: 8.73 (3.51) |
Intervention group: 6.14 (4.96) Control group: 8.69 (4.66) |
–2.55 (–6.15 to 1.05)a |
| Paternal stress |
Intervention group: 5.27 (2.61) Control group: 5.92 (3.85) |
Intervention group: 4.25 (2.38) Control group: 5.13 (4.16) |
–0.88 (–3.41 to 1.65)a |
| Satisfaction with child’s sleep (mother) |
Intervention group: 3.67 (1.53) Control group: 3.53 (1.36) |
Intervention group: 3.29 (1.44) Control group: 3.27 (1.27) |
0.02 (–1.00 to 1.04)a |
| Satisfaction with child’s sleep (father) |
Intervention group: 4.62 (1.19) Control group: 3.92 (0.90) |
Intervention group: 2.50 (1.31) Control group: 3.87 (1.36) |
–1.37 (–2.37 to -0.37)a |
| Satisfaction with ability to cope with child’s sleep (mother) |
Intervention group: 3.79 (1.53) Control group: 3.27 (1.10) |
Intervention group: 2.43 (0.94) Control group: 2.77 (1.30) |
–0.34 (–1.19 to 0.51)a |
| Satisfaction with ability to cope with child’s sleep (father) |
Intervention group: 3.83 (2.67) Control group: 2.67 (1.44) |
Intervention group: 3.58 (1.19) Control group: 3.38 (1.19) |
0.20 (–0.69 to 1.09)a |
| Perceived ability to control difficult sleep-related behaviour of own child (mother) |
Intervention group: 5.51 (2.52) Control group: 4.87 (2.96) |
Intervention group: 7.21 (2.15) Control group: 6.52 (2.00) |
0.69 (–0.86 to 2.24)a |
| Perception of partner’s ability to control difficult sleep-related behaviour of their child (mother) |
Intervention group: 5.61 (3.00) Control group: 4.38 (2.42) |
Intervention group: 5.05 (2.26) Control group: 6.19 (1.83) |
–1.14 (–2.68 to 0.40)a |
| Perceived ability to control difficult sleep-related behaviour of own child (father) |
Intervention group: 5.42 (3.26) Control group: 4.93 (2.96) |
Intervention group: 5.35 (2.65) Control group: 5.34 (2.50) |
0.01 (–1.92 to 1.94)a |
| Perception of partner’s ability to control difficult sleep-related behaviour of their child (father) |
Intervention group: 6.46 (2.53) Control group: 7.36 (2.26) |
Intervention group: 5.60 (2.98) Control group: 6.35 (2.51) |
–0.75 (–2.81 to 1.31)a |
We found no statistically significant differences between the groups at follow-up for any of these outcomes except father’s satisfaction with child’s sleep.
Measures of perceived confidence and/or efficacy and/or understanding of sleep/sleep management
Both the RCT152 and the before-and-after study155 reported on measures of perceived confidence and/or efficacy and/or understanding of sleep/sleep management.
The RCT152 reported on parents’ orientation to internal or external control beliefs as measured using the internality/externality control scale. These data are summarised in Table 21 and details of how these outcomes were measured are provided in Appendix 23. There were significant between-group differences for the father measures. The before-and-after study155 measured perceived confidence and efficacy using a parent satisfaction Likert-type scale. The authors narratively report that 87% of the responses were positive; however, it is unclear whether or not these positive responses related to child’s sleep and/or the intervention.
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Wiggs and Stores (1998)152 | Internality (mother) |
Intervention group: 10.71 (3.29) Control group: 9.60 (4.85) |
Intervention group: 11.71 (4.12) Control group: 9.85 (3.63) |
1.86 (–1.04 to 4.76)a |
| Externality (mother) |
Intervention group: 12.29 (3.29) Control group: 12.93 (4.71) |
Intervention group: 11.50 (4.05) Control group: 13.08 (3.64) |
–1.58 (–4.46 to 1.30)a | |
| Internality (father) |
Intervention group: 12.73 (5.14) Control group: 12.00 (4.77) |
Intervention group: 13.64 (4.59) Control group: 10.38 (2.97) |
3.26 (0.37 to 6.15)a | |
| Externality (father) |
Intervention group: 10.27 (5.14) Control group: 11.00 (4.77) |
Intervention group: 9.36 (4.59) Control group: 12.63 (2.97) |
–3.27 (–6.16 to –0.38)a | |
| Before-and-after studies | ||||
| Peppers et al. (2016)155 | Parent satisfaction | NR | NR | 87% of the responses were positive |
Summary
Two studies evaluated tailored interventions that were non-comprehensive in terms of their content. Thus, although comprehensive parent-directed interventions (tailored and non-tailored) covered material on sleep and sleep processes, sleep hygiene and the management of specific problem behaviours (e.g. night wakings), non-comprehensive interventions focused on just one of these aspects. Both non-comprehensive interventions were, however, tailored interventions, that is, the intervention was personalised to the specific child and family context.
A RCT152 evaluated a parent-directed intervention in which the content was restricted to behavioural principles of managing problem sleep behaviour. This intervention was delivered via a single face-to-face session followed by implementation support at least weekly for up to 3 months (duration as required). The comparator group was an attention control in which parents attended a single session with a practitioner (same duration as active arm, session content not described). We treated follow-up data collected 1 month after the face-to-face session as post-intervention outcomes. The sample included a range of NDs and the mean age of the children was 8.21 years. The rating of risk of bias was high.
Post intervention, an actigraphy-derived measure of sleep outcome (TST) was no different between the intervention and control arms, with similar findings for other actigraphy-derived measures (fragmentation index, movement during sleep and movement index). However, significant differences in scores post intervention on a parent-reported child sleep measure (Composite Sleep Index), favouring the intervention group, were found (MD –2.83, 95% CI –4.24 to –1.42). In addition, post intervention, fathers’ (but not mothers’) ratings of satisfaction with the child’s sleep (non-standardised measure) were higher for the intervention group than for the control group (MD –1.37, 95% CI –2.37 to 0.37).
The study also assessed child behaviour (parent and teacher report) using five subscales (inappropriate speech, hyperactivity, stereotypies, lethargy and irritability) of the ABC. Post intervention, no significant differences between the intervention and control arms were found on any subscale scores except parent-reported stereotypies. Here, a benefit to the intervention group was reported (MD –3.6, 95% CI –5.95 to 1.25).
In terms of mothers’ and fathers’ outcomes, sleep outcomes [actigraphy-derived (mothers only): TST, fragmentation index, movement during sleep; self-report: Epworth Sleepiness Scale, non-standardised measure of satisfaction with sleep] and other outcomes (psychological distress (Malaise Inventory), non-standardised measures of perceptions of own and partner’s ability to control child’s difficult sleep-related behaviour) were monitored. No differences between intervention and control arms were found post intervention for any of these outcomes.
Overall, given that there is just one trial – and it has a high risk of bias – no conclusions can be drawn regarding the effectiveness of parent-directed sleep management interventions in which the content was restricted to behavioural principles of managing problem sleep behaviour.
In the second non-comprehensive parent-directed intervention, the content was restricted to sleep hygiene. 155 This was evaluated using a before-and-after study design. The intervention, specific to children with ADHD, was delivered via a single session between parent and practitioner. No implementation support was offered. The ages of children recruited to the study were not reported. Risk of bias was high. Follow-up was 6 weeks post intervention.
Post intervention, scores on the parent-reported CSHQ had improved (MD 6.4, 95% CI 4.3 to 8.5). A measure of ADHD symptom severity was also used (Vanderbilt Assessment Scale), and improvements were also reported on this measure post intervention (Vanderbilt Assessment Scale questions 1–9, MD –3.87, 95% CI –7.37 to 0.37; Vanderbilt Assessment Scale questions 10–18, MD –2.91, 95% CI –4.81 to 1.01). (Changes in scores on a non-standardised measure of parent satisfaction were also reported, although it is unclear if this is capturing satisfaction with the child’s sleep and/or the intervention per se).
In terms of this intervention, given that the evidence is limited to a before-and-after study with a high risk of bias, no conclusions regarding its impact on children’s sleep or ADHD symptoms can be drawn.
Other non-pharmacological interventions
Seven studies36,156–161 evaluated other types of non-pharmacological interventions (see Table 9 and Appendix 18).
One crossover RCT compared valerian (dried and crushed whole root from the Valeriana edulis plant) with placebo;156 one crossover RCT compared weighted blankets with placebo blankets;36 one parallel RCT compared faded bedtime with response costs and bedtime scheduling;157 three uncontrolled before-and-after studies evaluated light therapy and a behavioural programme,158 an aquatic exercise programme159 and acupuncture and ear-point taping;161 and one controlled before-and-after study compared essential fatty acid supplements with placebo. 160
The total intervention duration ranged from 12 days to 36 weeks36,156,160,161 and was not specified in two studies. 158,159
Three studies included children with a mixed range of NDs. 36,156,157 Two included children with learning disabilities,158,161 one included children with a diagnosis of ASD159 and one included children with a diagnosis of ADHD. 160
The age of participants varied, with a mean age range of 6.7–10.8 years for the RCTs36,156,157 and a mean age range of 2.9–8.8 years for the uncontrolled before-and-after studies. 158,159,161 The controlled before-and-after study reported the participants’ ages as between 9 and 12 years. 160 All RCTs included children with sleep initiation and sleep maintenance disturbances. 36,156,157 The before-and-after studies included children with a mix of sleep disturbances (see Table 9) relating to sleep maintenance and ‘lack of sleep consolidation’,158 sleep dysfunction,159 ‘sleep deprived’160 and sleep initiation, maintenance and abnormal sleep state. 161
There was limited reporting of prior interventions. One parallel RCT reported that children were excluded if they were receiving pharmacological interventions for sleep. 157 One before-and-after study reported that over half of the study sample had attended sleep clinics and centres for treatment, with some children receiving behavioural treatments. 158
A total of 17 child sleep-related outcomes were measured by the RCTs (see Appendix 18). Commonly measured child sleep-related outcomes included SOL,36,156,157 night waking,36,156,157 TST,36,156 sleep quality36,156 and child daytime behaviour and cognition. 36,156 A total of 10 child sleep-related outcomes were measured by the before-and-after studies (see Appendix 18) and these were mainly measured by single studies. The majority of outcomes reported by before-and-after studies were measured by single studies. However, TST was measured by two studies. 158,159
In all studies, follow-up was conducted immediately following the completion of the intervention, and this varied between studies. For the RCTs, the follow-up times were at 2 weeks,156 at 4 weeks36 and after the last 10 days of an ‘on average 8-week’ treatment. 157 For the before-and-after studies, the follow-up times were at 10 weeks160 and at 6 months. 158 For one before-and-after study, the length of the follow-up was unclear and it was reported as ‘after treatment’. 161 The final study, Oriel et al. ,159 adopted an A–B–A withdrawal design, in which A was a control phase and B was a treatment phase. Outcomes were reported at 4 (A1), 8 (B) and 12 (A2) weeks from the start of the intervention. 159 We report all outcomes for Oriel et al. 159 at 8 weeks, which was when the intervention ceased.
Global measures and composite scores
Total sleep time
Two RCTs (n = 78)36,156 and two before-and-after studies158,159 (n = 22) reported TST (Table 22).
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Francis and Dempster (2002)156 | TST (hours) | Combined: 9.93 (0.56) |
Intervention group: 10.28 (0.43) Control group: 9.94 (0.7) |
0.34 (–0.42 to 1.10)a |
| Gringras et al. (2014)36 | TST (minutes) (actigraphy) | Combined: 452.8 (59.7) |
Intervention group: 452.8 (65.0) Control group: 455.4 (65.8) |
–4.2 (–13.6 to 5.2)a |
| TST (minutes) (sleep diary) | Combined: 531.8 (109.6) |
Intervention group: 528.9 (127.1) Control group: 513.0 (154.1) |
15.9 (–6.8 to 38.6)a | |
| Before-and-after studies | ||||
| Guilleminault et al. (1993)158 | TST (minutes) |
Responders: 204 (24) Non-responders: 192 (51) Overall: 196 (40) |
Responders: 425 (55) Non-responders: 202 (40) Overall: 281 (48) |
Responders: 221 (162 to 280)b Non-responders: 10 (–26 to 46)b Overall: 85 (59 to 111)b |
| Oriel et al. (2016)159 | TST (minutes) | 493.59 (60.51) | 576.91 (37.48) | 83.32 (17.55 to 149.09)c |
One study reported TST using actigraphy-verified sleep diary data,156 one study measured TST using sleep diary and actigraph data;36 and one measured TST via ‘semiweekly’ telephone calls from researchers to parents/guardians. Results show that there was no statistically significant difference in TST between valerian and placebo groups. 156 There was no statistically significant difference in actigraphy- or sleep diary-reported TST for weighted blankets compared with control blankets. 36 There was a statistically significant increase in TST between periods A1 and B following an aquatic exercise intervention159 and from pre to post intervention following light therapy and a behavioural programme. 158
Other global measures and composite scores
One RCT36 (n = 73) and two before-and-after studies161 (n = 38) reported further global measures and composite scores (Table 23). There was a statistically significant difference in Composite Sleep Disturbance Index score (but not sleep efficiency) with weighted blankets compared with placebo blanket. 36
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Gringras et al. (2014)36 | Sleep efficiency (%) | Combined: 72.7 (8.8) |
Intervention group: 73.6 (9.5) Control group: 74.2 (8.0) |
–0.3 (–1.7 to 1.1)a |
| CSDI | Combined: 12.2 (2.1) |
Intervention group: 10.8 (2.3) Control group: 11.4 (2.0) |
–0.7 (–1.3 to –0.1)a | |
| Before-and-after studies | ||||
| Yu and Hong (2012)161 | CSHQ (total) | 57.97 (4.58) | 46.47 (5.13) | –11.50 (–13.33 to –9.67)a |
Two before-and-after studies159,161 measured CSHQ total score (validated parent-reported assessment of child sleep, with higher scores indicating a greater severity of the sleeping disturbance),159,161 although one reported this only for baseline. 159 A statistically significant reduction in total CSHQ score was reported following acupuncture and ear-point taping post intervention. 161
Sleep initiation
Bedtime settling
Two RCTs36,156 (n = 78) and one before-and-after study159 (n = 8) reported SOL (Table 24). One RCT reported SOL using a sleep diary only156 and one used a parental diary and actigraphy. 36 The before-and-after study measured SOL via telephone calls from parents to researchers. 159
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Francis and Dempster (2002)156 | SOL (minutes) | Combined: 41.14 (20.99) |
Intervention group: 23.49 (13.42) Control group: 39.14 (34.68) |
–15.65 (–53.31 to 22.01)a |
| Gringras et al. (2014)36 | SOL (minutes) (actigraphy) | Combined: 76.5 (46.1) |
Intervention group: 71.4 (48.2) Control group: 70.6 (44.3) |
2.1 (–5.5 to 9.7)a |
| SOL (minutes) (sleep diary) | Combined: 69.9 (47.6) |
Intervention group: 55.6 (37.8) Control group: 57.2 (42.8) |
–1.6 (–6.7 to 3.5)a | |
| Before-and-after studies | ||||
| Oriel et al. (2016)159 | SOL (minutes) | 38.95 (21.19) | 21.76 (15.94) | 19.11 (–40.95 to 6.57)a |
There was no statistically significant difference in SOL with valerian treatment compared with placebo156 or following an aquatic exercise intervention. 159 There was no statistically significant difference in actigraphy- or parent-reported SOL for the RCT of weighted blankets. 36
One RCT narratively reported exactly what time the child fell asleep at night. 157 Among the eight participants who had issues with sleep initiation at baseline, trained observers reported that three out of the four participants who received the faded bedtime and response costs intervention experienced improved sleep initiation during the last 10 days of treatment, with one participant continuing to have issues falling asleep. Of the three participants who reported sleep initiation issues at baseline and received ‘bedtime scheduling’ (the control), two participants improved, one worsened and one saw little difference to their sleep initiation after 10 days of treatment. 157
Sleep maintenance
Night waking
Three RCTs36,156,157 (n = 92 participants randomised) and one before-and-after study159 (n = 8) reported night waking using actigraphy,156 sleep diary,36 observations157 and telephone calls from parents to researchers. 159
Results are summarised in Table 25. These show that there was no statistically significant reduction in time awake during the night (night waking) following valerian treatment. 156 There was no statistically significant difference in number of night wakings following an aquatic exercise programme,159 although the baseline number of wakings was very low, or for weighted blankets compared with placebo blankets. 36
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Francis and Dempster (2002)156 | Nocturnal time awake (minutes) | Combined: 17.92 (8.21) |
Intervention group: 6.85 (7.16) Control group: 8.38 (9.07) |
–1.53 (–11.83 to 8.77)a |
| Gringras et al. (2014)36 | Number of night wakings | Combined: 20.9 (8.0) |
Intervention group: 19.5 (7.0) Control group: 19.5 (6.8) |
–0.2 (–1.1 to 0.7)a |
| Before-and-after studies | ||||
| Oriel et al. (2016)159 | Number of night wakings | 0.99 (0.77) | 0.37 (0.41) | –0.62 (–1.45 to 0.21)a |
Piazza et al. 157 narratively reported that all five participants who received the faded bedtime and response costs intervention saw improvements to night wakings. Following bedtime scheduling of the six control participants who had issues with night wakings at baseline, two participants improved, two saw little difference and one had worsened night wakings. 157
Other sleep maintenance outcomes
Two RCTs36,157 (n = 87) and one before-and-after study158 (n = 14) reported other outcomes related to sleep maintenance (Table 26). The before-and-after study evaluating light therapy and a behavioural programme reported sleep diary-measured ‘longest wake and sleep periods during a 24-hour cycle’. Data for this outcome were reported only graphically and it was not possible to estimate results from the graph. 158
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Gringras et al. (2014)36 | Time awake after sleep onset (minutes) | Combined: 85.4 (45.1) |
Intervention group: 84.6 (42.6) Control group: 84.5 (41.5) |
–2.5 (–9.5 to 4.5)a |
| Proportion of nights with ≥ 1 waking | Combined: 0.3 (0.3) |
Intervention group: 0.2 (0.3) Control group: 0.2 (0.3) |
–0.01 (–0.06 to 0.04)a | |
| Piazza et al. (1997)157 | Hours of disturbed sleep |
Intervention group: 1.44 (SD NR) Control group: 1.37 (SD NR) |
Intervention group: 0.53 (SD NR) Control group: 1.10 (SD NR) |
0.57b |
One RCT narratively reported that all six of the participants who received the faded bedtime and response costs intervention and had problems with early waking at baseline had improved on this outcome during the last 10 days of treatment. In the control group, two out of the six participants reported improvements in early wakings, one worsened and three saw little or no change following bedtime scheduling. 157
There was no difference between the treatment groups at follow-up in Gringas et al. 36 in terms of time awake after sleep onset and the proportion of nights with one or more awakening.
Sleep quality
Two RCTs36,156 (n = 78) and one before-and-after study160 (n = 78) reported quality of sleep (Table 27).
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| RCTs | ||||
| Francis and Dempster (2002)156 | Sleep quality | Combined: 5.34 (1.49) |
Intervention group: 7.54 (1.47) Control group: 6.71 (1.29) |
0.83 (–0.90 to 2.56)a |
| Gringras et al. (2014)36 | Children’s perceptions of sleep qualityb | NR |
Intervention group: smiley face 56%, neutral face 30% and unhappy face 14% Control group: smiley face 35%, neutral face 47% and unhappy face 18% |
– |
| Parent’s perceptions of child’s sleep qualityc | – |
Intervention group: more agitated, 0%; no different from usual, 44%; calmer, 35%; and N/A as few awakenings, 21% Control group: more agitated, 5%; no different from usual, 62%; calmer, 14%; N/A as few awakenings, 20% |
– | |
| Sleep improvement (parent perceptions)d | NR |
Intervention group: very much improved, 15%; much improved, 36%; minimally improved, 31%; no change, 13%; minimally worse, 3%; much worse, 1%; very much worse, 0% Control group: very much improved, 1%; much improved, 15%; minimally improved, 24%; no change, 49%; minimally worse, 6%; much worse, 4%; very much worse, 0% |
– | |
| Before-and-after studies | ||||
| Yehuda et al. (2011)160 | Quality of sleep |
ADHD-FA: 1.0 (0.8) ADHD-vehicle: 1.2 (0.7) |
ADHD-FA: 3.8 (0.7) ADHD-vehicle: 1.4 (0.8) |
ADHD FA: 2.8 (2.6 to 3.0) ADHD vehicle: 0.2 (–0.05 to 0.4)e |
There was a statistically significant improvement in sleep diary-measured sleep quality from baseline for children who received both valerian treatment and placebo; however, the difference in this outcome at follow-up between the groups was not statistically significant. 156 There was also improvement in sleep quality for children who received fatty acids and placebo, as measured on a ‘short questionnaire’. 160
Gringras et al. 36 measured children’s perceptions of sleep quality using a smiley face rating scale. Parents’ perceptions of their child’s sleep quality were also measured. Parents were asked ‘Compared with before the trial, when my child was not using any special sensory blanket, if my child woke at night, he or she seemed: more agitated; no different from usual; calmer; and not applicable as so few awakenings’. Gringras et al. 36 also reported parent’s perceptions of their child’s sleep improvement. These data are summarised in Table 27.
One before-and-after study158 evaluating light therapy plus a behavioural programme reported sleep diary-measured distribution of sleep bouts during a 24-hour cycle. Data for this outcome were reported graphically, with no narrative discussion from the authors, and could not be extracted.
Child-related quality of life, daytime behaviour and cognition
Two RCTs36,156 (n = 78) reported on child daytime behaviour using a sleep diary156 and the ABC. 36
There was no statistically significant difference in daytime behaviour and cognition for total ABC score or subscale scores between the weighted blanket group and the control blanket group at 4 weeks from baseline (MD –2.3, 95% CI –5.4 to 0.8). 36 Francis and Dempster156 used the sleep diary data to narratively describe anecdotal changes in child behaviour, but quantitative data were not reported.
Other outcomes
One RCT (n = 73) and one controlled before-and-after study (n = 78) reported other outcomes of interest. 36,160 The RCT reported that there were no significant differences for the total Sensory Behaviour Questionnaire or its subscales between the weighted blanket group and the control blanket group. 36 The controlled before-and-after study that compared essential fatty acid supplements with placebo reported a variety of other child-related outcomes at the end of the 10-week intervention period. 160 These data are summarised in Table 28.
| Study | Outcome | Time point, mean (SD) | MD (95% CI) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Child-related quality of life | ||||
| Gringras et al. (2014)36 | Sensory Behaviour Questionnaire | Combined: 148.8 (42.4) |
Intervention group: 138.6 (41.3) Control group: 142.4 (46.2) |
–4.9 (–10.2 to 0.4)a |
| Other outcomes | ||||
| Yehuda et al. (2011)160 | Degree of fatigue in general during the day |
ADHD-FA: 2.3 (1.0) ADHD-vehicle 2.6 (0.8) |
ADHD-FA: 4.0 (0.3) ADHD-vehicle 3.0 (0.9) |
ADHD-FA: 1.7 (1.4 to 2.0)b ADHD-vehicle 0.4 (0.1 to 0.7)b |
| Level of good mood in general |
ADHD-FA: 2.2 (1.1) ADHD-vehicle 2.0 (0.9) |
ADHD-FA: 3.7 (0.9) ADHD-vehicle 2.4 (1.0) |
ADHD-FA: 1.5 (1.2 to 1.8)b ADHD-vehicle 0.4 (0.1 to 0.7)b |
|
| Level of ability to concentrate during the day |
ADHD-FA: 2.0 (1.3) ADHD-vehicle 1.9 (1.1) |
ADHD-FA: 3.8 (0.8) ADHD-vehicle 2.4 (1.2) |
ADHD-FA: 1.8 (1.4 to 2.2)b ADHD-vehicle 0.5 (0.1 to 0.9)b |
|
| Percentage of homework completed in general |
ADHD-FA: 2.0 (1.3) ADHD-vehicle 2.3 (1.5) |
ADHD-FA: 3.3 (1.2) ADHD-vehicle 2.9 (1) |
ADHD-FA: 1.3 (0.9 to 1.7)b ADHD-vehicle 0.6 (0.2 to 1.0)b |
|
Adverse events
Two out of the seven studies reported adverse events (Table 29). 36,160 One study36 reported how adverse events were measured, namely through parents reporting to a 24-hour telephone number and weekly face-to-face or telephone-based reviews with parents. Five studies did not report adverse events. 156–159,161
| Study | Adverse events reported | Measures used |
|---|---|---|
| Francis and Demspter (2002)156 | NR | |
| Gringras et al. (2014)36 |
No serious adverse events were reported Two-day skin rash, n = 1 (may have been related to the weighted blanket). The authors report that all other adverse events were unrelated illnesses such as colds, fever, chickenpox, broken bone in hand (number of children with other unrelated adverse events was not reported) |
A 24-hour telephone number was available to parents for reporting adverse events Weekly parent reviews (face to face or telephone) |
| Guilleminault et al. (1993)158 | NR. Heat caused by the artificial light was reported as the only complication to light therapy treatment | NR |
| Oriel et al. (2016)159 | NR | |
| Piazza et al. (1997)157 | NR | NR |
| Yehuda et al. (2011)160 | Fatty acid supplement: transient stomach upset and diarrhoea, n = 3; dizziness, n = 2 | NR |
| Yu and Hong (2012)161 | NR |
Summary
Seven studies evaluated seven types of non-pharmacological interventions. These interventions were all different from each other. Therefore, we summarise the findings from each study in turn.
Weighted blankets were evaluated using a crossover RCT, with a non-weighted blanket as the control intervention. 36 The intervention was developed for children with ASD, and all children recruited to the study had this diagnosis. They were aged between 5 and 16 years. The trial was rated as having a high risk of bias. Outcomes were measured over each 2-week treatment period. Data on 27 children in each arm were analysed.
No benefits in terms of TST and SOL (derived from actigraphy) or a number of other sleep outcomes derived from parent-completed sleep diaries (number of wakings/night, proportion of nights with more than one waking and total time awake after sleep onset) were reported for children in the weighted blanket group compared with the non-weighted blanket group. Similarly, no benefits from a weighted blanket were reported in terms of child behaviour (ABC) or increased sensitivities to sensory stimulation (Sensory Behaviour Questionnaire). There were no serious adverse events.
Valerian, a herb prepared in tablet form, was evaluated with a placebo using a RCT156 in a sample of five children with a range of NDs aged 7–14 years who had difficulties with sleep initiation and/or sleep maintenance. The rating of risk of bias was high.
At 2 weeks post intervention, there was no difference between arms in terms of TST, SOL and duration of time awake during the night. Parents’ perceptions of sleep quality (measured using a non-standardised visual analogue scale) did not differ significantly between trial arms post intervention. No other quantitative data on child outcomes were reported. Adverse events were not reported.
Essential fatty acid supplement preparation in tablet form was evaluated using a controlled before-and-after study. 160 All children (active arm, n = 40; placebo, n = 38), aged 9–12 years, recruited to the study were diagnosed with ADHD and were described as ‘sleep deprived’. The study was rated as having a high risk of bias.
At 10 weeks post intervention, scores on a non-standardised measure of sleep quality did not differ between active and placebo groups. A number of other parent-reported child outcomes were evaluated using non-standardised, parent-reported indicators (degree of fatigue in general during the day, level of good mood in general, level of ability to concentrate during the day and percentage of homework completed in general). No significant difference between active and placebo arms was found for these outcomes. Transient gastric problems were reported for some children in the active group.
An aquatic exercise programme for children with ASD (mean age 8.8 years) and ‘sleep dysfunction’ was evaluated using a before-and-after study design. 159 Eight children were recruited to the study. The study was rated as having a high risk of bias. The outcomes at 8 weeks post intervention were reviewed. Biweekly telephone interviews with parents were used to collect data, from which TST and SOL were calculated. Increased TST post intervention was reported (MD 83.3 minutes, 95% CI 17.55 to 149.09 minutes). No other changes to child sleep outcomes were reported. Adverse events were not reported.
Acupuncture and ear-point taping were also evaluated using a controlled before-and-after study design. 161 Thirty children (mean age 6.9 years) with sleep initiation or maintenance difficulties and/or an ‘abnormal sleep state’ were recruited to the study. All were described as having learning disabilities. It was not clear when post-intervention outcomes data were collected. The study was rated as having a high risk of bias. At post intervention, a significant improvement in scores on a parent-reported sleep outcome measure (CSHQ) was reported (MD –11.50, 95% CI –13.33 to –9.67).
A light therapy intervention combined with a programme of daytime schedules and activities was evaluated using a before-and-after study. 158 Fourteen children, aged 9 months to 4 years, were recruited to the study. They were described as having learning disabilities. The study was rated as having a high risk of bias. Outcomes were measured at 6 months post intervention. Five out of 14 children were reported to have ‘responded to treatment’ as measured by increases in TST (calculated from information collected by the study team during regular interviews with parents), which were reported to have improved post intervention. There were no serious adverse events.
Finally, the relative effectiveness of faded bedtime and response costs with bedtime scheduling was investigated using a parallel RCT. 157 The trial took place in an inpatient unit for children with very severe behaviour problems. The children (n = 14, 7 per arm) recruited to the trial had a range of NDs and sleep disturbances relating to sleep maintenance and ‘sleep consolidation’. They were aged 4–14 years. The trial was rated as having a high risk of bias. Outcomes were measured pre intervention and 10 days post intervention. Only narrative reports of sleep outcomes (based on observational data) are provided.
Overall, there is no evidence of benefit for weighted blankets, although we note that this was a single study that was rated as having a high risk of bias. Issues regarding study design and study bias mean that no conclusions can be drawn regarding the impact of the following interventions: valerian, fatty acid supplements, light therapy with daytime activities programme, acupuncture and ear-point taping, and an aquatic exercise programme. Finally, we note that the last two studies described157,158 were both reported almost 25 years ago, and no further studies replicating these interventions are reported. It is questionable whether or not they remain relevant or accepted approaches to managing sleep disturbance in children with NDs.
Issues of feasibility, acceptability and experiences of receiving and implementing a sleep management intervention
Sixteen of the interventions included in the clinical effectiveness review also investigated the feasibility, acceptability and/or parent/clinician views of sleep disturbance interventions. 36,49,106,107,122–130 Going forward, we use the term ‘family experience’ to refer to this topic area.
Fourteen of these interventions were parent-directed interventions,21,49,107,123–129,149 the remaining two were evaluations of melatonin106 and weighed blankets,36 respectively. For 11 out of the 16 interventions, data on family experience are reported in papers alongside the presentation of data on outcomes. For one intervention,149 these data are reported in a separate paper. 122 A further four interventions were collectively investigated with respect to the above issues and findings were reported separately to papers reporting intervention outcomes. 130
No additional studies relevant to this topic were identified through searches. We would note that separate but relevant literature on parents’ views and attitudes towards pharmacological and non-pharmacological interventions for sleep disturbance in children with NDs was identified (e.g. Goodday et al.,180 Keenan et al. 181). However, this body of work did not fall within the scope of this review, as these studies explored parental views of multiple sleep interventions.
A number of data collection methods were used, including questionnaires, structured interview and semistructured interviews (individual and group).
Ten studies36,49,107,122–125,127–129 devised an intervention evaluation questionnaire to explore family experience. One of these studies also included semistructured interviews with a subsample. 124 A further study used a modified version of a previously published intervention evaluation questionnaire. 126 All collected data from parents, apart from one study that sought children’s views about the weighted blanket they had been using. 36
A further study used semistructured interviews to collect data from parents via individual interviews or groups. 130 Finally, one study reported data on reasons for study dropout. Appendix 19 reports the methods used. 106
Quality appraisal
The quality appraisal of ‘family experience’ studies used the tool created by Hawker et al.,66 chosen because it was developed for use in reviews in which both qualitative and quantitative data are included and data in this review have been generated by studies using different paradigms. The tool was used in two ways. First, for studies (n = 2) in which a primary objective was to investigate family experience, and this is reported in a separate paper, a full quality appraisal was conducted (see Appendix 20). Second, for studies (n = 10) in which ‘family experience’ was a secondary study objective and was a minor element of evidence presented in any papers, the tool was used by the team to appraise, but not individually rate, the quality of the studies and evidence presented. One study106 reported data that were a part of routine data collection within a trial (i.e. the reason for study dropout). We did not subject this specific element of the study to a separate quality appraisal.
For the 10 studies36,49,107,123–129 for which ‘family experience’ was a secondary objective, the quality of studies and study reporting was very mixed. A number of issues were frequently noted that affected the quality of the ‘family experience’ objectives of these studies. First, family experience was sometimes not explicitly identified as a research objective. In addition, parent characteristics (e.g. gender, educational achievement, ethnicity and first language) were rarely reported. This is a significant omission, given that the majority of interventions represented in the ‘family experience’ data set were parent-directed interventions. Data collection tools were inadequately reported and/or study-specific tools were developed that, it would appear, had not undergone any piloting or been developed in consultation with parents. Descriptions of the analysis of family experience data were typically missing or very limited. Analyses were descriptive and opportunities to explore factors affecting experiences were not exploited. Based on the description of the data collection instruments, it appeared that, in some studies, not all findings were reported.
Findings
Findings on family experience can be found in Appendix 19.
Melatonin
One of the evaluations of melatonin reported that a child was withdrawn from the study owing to difficulties administering the medication to the child. No further data on family experience were identified with respect to the pharmacological interventions. 106
Weighted blanket
Children taking part in the RCT evaluating weighted blankets were asked to describe their feelings about using the study blanket using a three-point scale (‘really liked’, ‘just OK’ or ‘really disliked’). 36 A greater proportion of children in the intervention group reported liking their blanket, but the differences in responses between the intervention and control groups were not significant. 36
Parent-directed interventions
A number of themes, or topic areas, can be identified in the data reported:
-
parents’ experiences of accessing the intervention (e.g. time of day or competing demands on time)
-
parents’ experiences of implementing a sleep management strategy
-
parents’ views about the elements of the intervention that had an impact on the outcomes experienced
-
parents’ experiences as service users or of the process of receiving the intervention
-
recommending the intervention to other parents.
It should be noted that some data that were identified as potentially relevant to these topic areas were ambiguous and open to different interpretations; for example, ‘satisfaction’ may refer to satisfaction with outcomes achieved or satisfaction with the way that an intervention is delivered. Data of this nature, although extracted and presented in Appendix 19, are not reported in the following narrative.
Parents’ experiences of accessing the intervention
This topic was not widely or consistently explored. One study124 reported that the majority of participants (five out of six) in their interview subsample believed that time pressures on parents and the time needed to make the initial commitment to embark on the intervention were barriers to parents accessing and completing such an intervention. In addition, one interviewee spontaneously mentioned that the time of day of the intervention was a ‘least-liked’ element of the intervention. A further study126 reported that the time-consuming nature of the intervention was identified as something that was ‘least liked’ about the intervention they received. However, the authors do not clarify whether this refers to time attending sessions or the time required to implement a sleep management strategy. Johnson et al. 107 report data on very high levels of attendance at intervention sessions, which could be regarded as proxy evidence regarding the accessibility of the intervention.
Parents’ experiences of implementing the sleep management strategy
Five studies report parents’ views on the acceptability of the intervention, specifically the acceptability of the sleep management strategy that they were instructed to implement. Implementing new ways of managing sleep disturbance (e.g. not settling to sleep, night-time wakings) are likely to generate resistance and negative responses (e.g. crying) on the part of the child.
Bramble122 reported that a minority (3/15) of their sample found this process ‘rather tough’ but were willing to continue. Two out of the twelve participants in Weiskop et al. 126 identified ‘sticking to bedtime routine’ as the least-liked element of the intervention. Austin et al. 123 reports that three out of five parents in their study found that implementing sleep management strategies was stressful. However, Moss et al. 124 reported that 21 out of 26 study participants described the implementation of the treatment plans as acceptable or very acceptable, and no improvements to the intervention were suggested. Both Austin et al. 123 and Weiskop et al. 126 also asked study participants if they would recommend the intervention to other parents and all said that they would.
Beresford et al. 130 describes parents’ accounts of the demands that implementing, and sustaining, a sleep management strategy can place on them. A number of barriers to implementation were also identified. These included lack of consistency across caregivers, changes and disruptions in usual routines (e.g. owing to illness or holidays) and difficulties with the home environment, particularly if the child had to share with siblings. 130
Adherence to implementing a sleep management strategy is an additional facet to this topic. Two studies provided relevant data. 107,125 First, Scibberas et al. 125 reported that ‘Most caregivers reported that they could implement sleep management strategies “at least half of the time” ’. No further details are provided. Second, Johnson et al. 107 collected clinicians’ ratings of study participants’ adherence to the intervention (including evidence of implementation of sleep management strategies) based on their observations during intervention sessions. In this study, an attention control arm was used; the intervention delivered to this arm was purely educational and did not require parents to implement behaviour management strategies. Over 90% adherence was reported for both study arms (active arm, 93%, 75–100%; control arm, 98%, 75–100%).
Parents’ views about the elements of the intervention that had an impact on the outcomes experienced
Two approaches to exploring this topic were used. Some studies asked participants to rate the helpfulness, utility or relevance of a pre-set list of the different elements of the intervention they had received,107,122,123,125,128 although this varied in terms of how fine-grained the elements were. Overall, there is consistent evidence that parents report the training delivered via the interventions as relevant and useful. It is not clear from the way that the data are reported whether or not parents vary in which elements they find particularly helpful. Differences in data collection instruments and ambiguities of language make further synthesis of the data impossible.
Other studies elicited parents’ (spontaneous) views via interviews124,127,130 or free-text responses in questionnaires. 126 Across these studies, the following were identified by parents as being important to supporting the achievement of positive sleep outcomes: receiving education on various aspects of sleep and sleep management,124,130 training in specific strategies to manage problem behaviours (communication, behavioural or sensory),124,127,130 that the intervention is tailored to the child,124,130 provision of one-to-one instruction,126 peer support via group delivery,130 that attention is paid to developing parents’ confidence regarding their parenting,130 use of mechanisms for keeping track of progress (e.g. sleep diary)130 and giving implementation support. 124,126,130
Parents’ experiences as service users or of the process of receiving the intervention
The mode of delivery is a core element of the service user experience. This was evaluated in one of the trials included in the review. 128 This study also captured data on family experience, including preference regarding mode of delivery. A smaller proportion of study participants in the individual delivery arm reported that they would have preferred the alternative mode of delivery than those in the group delivery arm (3/41 vs. 8/39). 128
There were limited data on this topic area. A single participant (1/12) in the study by Weiskop et al. 126 found that the training sessions were too long. Reed et al. 129 reports that 10 out of 18 study participants believed that the duration of the intervention was sufficient.
Recommending the intervention to other parents
Five studies123,126,128,129,144 specifically collected data on whether or not study participants would recommend the intervention to other parents. Three studies123,126,129 reported that all parents receiving the intervention would recommend it to other parents. The other studies128,144 reported that the great majority of study participants said that they would recommend the intervention. Malow et al. 128 compared the responses of parents receiving the intervention via individual face-to-face sessions with those receiving it via group delivery. A greater proportion of study participants receiving the intervention face to face reported that they would recommend the intervention than those in the group delivery arm (38/41 vs. 32/39). 128
Summary
Thirteen studies concerning family experience of sleep management interventions were identified, reporting 16 sleep management interventions in total. 36,49,106,107,122–130 All but one106 of the studies concerned non-pharmacological interventions: 14 were parent-directed interventions49,107,122–130 and one was a study of weighted blankets. 36 For these studies, data were predominantly quantitative (total n = 225) and typically collected using a questionnaire specifically designed for the study. One study124 supplemented questionnaire data through semistructured interviews with a subsample (n = 6) of study participants. A further study130 (n = 35), which investigated the family experience of four parent-directed sleep management interventions, used entirely qualitative methods. Study quality varied.
The pharmacological study106 reported data relevant to family experience106 concerning difficulties administering the medication. This was reported within the context of reporting on reasons for study withdrawal, rather than the study explicitly seeking to include an exploration of the family experience along with the evaluation of the medicine. 106
Therefore, the most extensive data set concerns parents’ experiences of receiving and implementing a sleep management intervention (11 studies21,49,107,123–129,149). The data were organised around five themes: parents’ experiences of accessing the intervention, parents’ experiences of implementing a sleep management strategy, parents’ views about the elements of the intervention that had an impact on the outcomes experienced, parents’ experiences as service users and recommending the intervention to others. Across all these themes, the data are very limited. There are a number of reasons for this. First, the pool of studies is very small. Second, many studies collected very few data. Third, studies typically used their own data collection instrument, thus hindering the pooling of data.
With these caveats, the following comments are made. There is very little evidence on parents’ experiences of accessing sleep management interventions. One study reported that parents believed that the time requirements associated with receiving a sleep management intervention could be a barrier to parents accessing an intervention. 124 Another found that the time-consuming nature of the intervention was a feature of the intervention that was ‘least liked’ by some study participants. 126 However, across all studies, only one participant is reported as finding the duration of the intervention too long. 126
Parents’ experiences of implementing a sleep management strategy were explored by five studies. 107,122,123,126,130 A consistent theme across all – although it was not universally reported by study participants – is the challenges and demands placed on parents as they implement the strategy. Despite this, all of the studies that asked parents if they would recommend the intervention to others reported unanimous or high levels of recommendation. On a related point, a further study144 reported exploring parents’ judgements as to their adherence to a sleep management strategy and it suggests that consistent adherence to sleep management interventions should not be assumed. Another study identified a range of factors that may further interfere with implementing a sleep management strategy. 130
Support with implementing a sleep management intervention was consistently identified by studies that offer data on elements of interventions that parents believe support positive outcomes. In terms of mode of delivery, both individual work and group delivery are identified as supporting positive outcomes: one offering the opportunity for a highly tailored intervention and the other offering peer support.
Chapter 4 Discussion
Introduction
Overall, the evidence on the management of sleep disturbance in children with NDs is very thin – in volume, scope and quality – particularly given that this group of conditions or diagnoses represents the majority of disabled children, and when the incidence and severity of sleep disturbance is greater than that for children with typical development. The conclusions that we have been able to draw regarding intervention effectiveness are limited to melatonin. Here, the conclusion is drawn that there is evidence of benefit; however, the clinical importance of the benefit is not certain. Therefore, there are no implications for health-care practice.
This lack of evidence is more compelling – as is the argument for strategic investment in this topic – given our understanding of the health, social and economic impacts of sleep deprivation. It is not surprising that in a national research prioritisation exercise for children with NDs, the management of sleep disturbance was ranked in the top 10 research priorities,32 with both pharmacological and parent-directed interventions specified.
In this chapter, first, we present the strengths and limitations of the study and then public and patient involvement. Second, we discuss pharmacological interventions and principal findings with respect to this intervention approach. Third, we consider parent-directed interventions, providing an overview of these interventions before reporting principal findings and discussing the implications of these findings. Fourth, we move on to discuss other non-pharmacological interventions, presenting the principal findings with respect to this range of interventions. A final section discusses the issues and challenges for future research in this area.
Strengths and limitations of the study
We undertook thorough searches for eligible studies that included systematic searches of 16 databases, without language restrictions, and included sources for unpublished studies. We used standard methods, for example having two researchers undertake key study processes such as study selection, to reduce error and bias. The risk of bias in the included studies was assessed, although this assessment was often limited by poor reporting. We included small before-and-after studies that cannot provide a reliable estimate of the clinical effectiveness of an intervention because of the lack of a control group. These were included in order to help identify interventions that may be worth considering for evaluation in future RCTs. To militate against the limitations of some of the study designs included, we have clearly distinguished between randomised and non-randomised designs in the synthesis. We have also taken account of their limitations when drawing conclusions.
Given the number of interventions that were under consideration, a mixed-treatment comparison would have been the ideal statistical approach, permitting ranking of the benefits and harms of the different treatment options. 72 However, owing to the heterogeneity of the non-pharmacological studies, statistical pooling was not considered appropriate. As a result of the sparsity of combined and sequential interventions and poor reporting of any prior interventions received by participants in studies, a robust analysis of the impact that single, combined and sequential interventions had on clinical effectiveness was not possible. Similarly, any other planned subgroup analysis was possible only for the melatonin trials and this was limited by the small number of studies and the potential for confounding with other study characteristics.
It is not possible to blind the types of interventions and comparators used in the studies under consideration. In addition, owing to the nature of the outcomes measured, robust blinded outcome assessment is likely to be difficult. Although actigraphy-based child sleep outcomes are more objective than parent-reported measures, we did not consider these to be true objective outcomes, with non-blinding being unlikely to have introduced bias. Therefore, all of the included non-pharmacological RCTs were rated as having a high risk of bias, even though blinding of treatment and comparator is not possible. For one study that compared a parent-directed tailored intervention with control,138 this led to an overall rating of high risk of bias, despite all the other bias domains for that study being rating as having a low risk of bias. We acknowledge that applying the Cochrane criteria ‘less strictly’ would have led to this study having an overall rating of low risk of bias. This is an issue for all non-pharmacological studies in this area and we believe that it is unhelpful to have these studies rated as having a high risk of bias, as evidence from non-pharmacological interventions will always look weaker than that from pharmacological studies. Equally, we acknowledge that there is a risk of overestimating the clinical effectiveness of an intervention in which allocation is unblinded and outcomes have an element of subjectivity and may be influenced by lack of blinding. There is currently no established method of blinded outcome assessment in this field that we are aware of and further work in this area may be beneficial. There may also be value in further consideration by methodologists about how lack of blinding is graded in studies when blinding is not possible and the outcomes are subjective. Adoption of statistical approaches used in surgical studies, such as secondary statistical analyses taking into account participants’ treatment allocation, may also have some value. 182
Patient and public involvement
Three parents of children with ND (two mothers and one father) acted as project advisors. They were recruited from a permanent parent consultation group of the chief investigator’s research unit. The children’s diagnoses were autism and a rare, genetic condition.
The parents were invited to the project team meetings, which were held three times over the course of study and attended by the research team and all co-applicants. They were also occasionally consulted between meetings via e-mail.
Each parent attended at least one meeting. At the first meeting, an early item on the agenda was a presentation of an overview of systematic reviews as a research method. At the meetings, parents actively engaged in discussions. Their experiences also provided useful contextual information for the research team, some of whom had no prior experience of working in this topic area or with this particular group of children.
Pharmacological interventions
Research on the management of sleep disturbance affecting children with NDs is dominated by two intervention approaches: melatonin and parent-directed interventions. In terms of other pharmacological interventions, a small number of trials of other medicines were identified [acebutolol (Sectral®, Promius Pharma LLC, Princeton, NJ, USA ), eszopiclone (Lunesta®, Sunovion Pharmaceuticals, Inc., Marlborough, MA, USA), gabapentin (Neurontin®, Pfizer Inc., New York City, NY, USA), ramelteon (Rozerem®, Takeda Pharmaceutical Company Ltd, Osaka, Japan) and zolpidem (Ambien®, Sanofi S.A., Paris, France)] but fell outside the eligibility criteria set for this review (these criteria were decided in consultation with clinicians). The review was, therefore, restricted to melatonin, clonidine and antihistamines. No studies of clonidine or antihistamines that fulfilled our study inclusion criteria were identified.
Principal findings: pharmacological interventions
There was evidence of benefit of melatonin compared with placebo, although the precise extent of the benefit, which children may benefit the most and the clinical importance of the benefit remain uncertain.
Discussion of principal findings: pharmacological interventions
This conclusion concurs with the issues raised by the team that conducted the only trial of (fast-release) melatonin rated as having a low risk of bias included in the review. 48 In it, the study sample comprised children with developmental delay and some with additional diagnoses, including epilepsy, autism or a specific genetic or chromosomal disorder. The authors note that ‘the sheer heterogeneity of the population studied has inevitably limited our ability to accurately estimate the impact of melatonin treatment for individual groups of patients with specific clinical (genetic), behavioural or developmental presentations’. 48
A core inclusion criterion for the Appleton et al. 48 trial was that a parent-directed intervention – in the form of an advice booklet – had not successfully addressed the presenting sleep disturbance. For over half of the children recruited to the trial (56%), this intervention did not sufficiently address the sleep disturbance and they were randomised to the melatonin or placebo arms. This finding does lend support to the argument, and based on understandings of types and aetiologies of sleep disturbance in children with NDs, that pharmacological and non-pharmacological approaches may both be required. It can also be taken to suggest that for some types of sleep disturbance, recourse to melatonin should occur only once attempts to change the way that a family may be managing the sleep disturbance have been tried.
Parent-directed interventions
Behavioural insomnias (difficulties with bedtime settling and self-settling after night wakings) are defined as sleep patterns and behaviours developed through unhelpful sleep behaviours and sleep management practices. 183 Parent-directed interventions seek to provide parents with the knowledge and skills to change these patterns and behaviours.
This review adopted the term ‘parent-directed interventions’ to refer to sleep management interventions that involve training parents to respond to their child’s sleep problems in different ways. The use of the term ‘parent-directed’ was deliberate. It emphasises that the direct recipient of the intervention is the parent, not the child. The child is merely the recipient of the outcomes the intervention achieves with respect to the parent. This has implications for the way in which such interventions should be understood and evaluated.
We categorised parent-directed interventions as comprehensive and non-comprehensive. Comprehensive interventions included training parents in evaluating the bedroom environment, paying attention to daytime and bedtime activities and routines (or sleep hygiene practices) and providing training in the use of particular behavioural strategies to manage specific problems related to settling and night waking. The majority of interventions were comprehensive, with two ‘non-comprehensive’ interventions also being included: one evaluating training on particular behaviour management strategies only152 and the other providing sleep hygiene training for parents of children with ADHD. 155
We made a further distinction with respect to these interventions: tailored versus non-tailored. Tailored interventions consisted of an assessment of the child’s sleep and wider family context, which was then used to developed a personalised sleep management strategy. This approach necessarily meant that these interventions were delivered individually. They also all included implementation support, that is, contact between the practitioner and parent as the sleep management strategy in order to provide support or advice and/or suggest and supervise changes to the strategy.
Non-tailored interventions, in contrast, comprised the delivery of a standard ‘training curriculum’ in managing sleep disturbance in children with NDs. These were delivered via written material, in groups and/or individually. Some of these interventions had opportunities for parents to apply learning to their own child and/or implementation support.
This review identified some core intervention characteristics by which these interventions could be classified: comprehensive versus non-comprehensive; tailored versus non-tailored; mode(s) of delivery; number of practitioner–parent contacts; availability of implementation support; and condition-specific or generic ND.
Principal findings: parent-directed interventions
Studies of parent-directed interventions took two broad forms: (1) they were concerned with the clinical effectiveness of the intervention compared with no intervention or (2) they were testing different approaches to delivering the same intervention (e.g. varying mode of delivery or intensity of contact between the practitioner and the parent). There was variability between interventions in terms of intervention characteristics, such as mode of delivery, intensity, duration and availability of implementation support. Overall, the quality of the evidence was rated as having a high or unclear risk of bias in reporting and, therefore, the findings of these studies cannot be considered robust.
In terms of the tailored interventions, there was mixed evidence about the effects of these interventions on child (and parent) outcomes. There was limited evidence of benefit on more objective measures of sleep outcomes (actigraphy, verified or not with parent-completed sleep diaries). More evidence of benefit was found on parent-reported outcomes and a few parent outcomes. Conclusions are hampered by the limited number of RCTs, the multitude of outcome measures and the risk of studies being underpowered to detect an effect. Two small RCTs investigated specific intervention characteristics: mode of delivering implementation support21 and a brief versus extended (with implementation support) version of an intervention. 125 The rating of a high risk of study bias and the trials being underpowered mean that no conclusions can be drawn.
Compared with the tailored interventions, the non-tailored interventions evaluated were even more diverse in terms of a number of intervention characteristics, including mode of delivery [i.e. written material, group delivery (single vs. multiple sessions) and one-to-one work], the extent to which they accommodated parents’ individual learning and training needs and whether or not implementation support was available. Overall, the conclusions that can be drawn from this evidence are limited owing to the high risk of bias, the wide range in post-intervention follow-up time points and the risk of studies being underpowered to detect an effect. In addition, a large number of outcome measures were used, capturing a wide range of outcome domains.
There was mixed and very limited evidence regarding the impact that the provision of written information to parents had on managing their child’s sleep disturbance. No conclusions can be drawn regarding the relative benefits of written versus face-to-face delivery of sleep management training to parents. There is some limited evidence of benefit for group-delivered interventions in terms of parent-reported sleep outcomes (but not reported for more objective measures of sleep). A single RCT128 compared the individual delivery of an intervention via a single session with delivery of the same training via two group-delivered sessions. The rating of having a high risk of study bias and the trial being underpowered means that no conclusions can be drawn from the study findings. No RCTs were identified that evaluated a single-session workshop approach to delivering sleep management training to parents. It is useful to note that this mode of delivery is widely accessible to parents in the UK via statutory and non-statutory providers.
Two non-comprehensive parent-directed interventions are also reported by this study. The first is a trial (rated as having a high risk of bias) that evaluated an intervention in which the content was restricted to training on behavioural principles of managing problem behaviour compared with an attention control intervention. 152 The content of the second intervention was restricted to sleep hygiene principles and practices,155 which was evaluated using a before-and-after study design.
Discussion of principal findings: parent-directed interventions
It is relevant to briefly contextualise these findings on parent-directed interventions within the wider evidence base on the clinical effectiveness of parent-directed interventions for managing sleep in children with typical development. Here, a recent review, which identified 15 trials110,127,184–197 and 11 before-and-after studies,149,198–208 concluded that there was ‘moderate support’ for such interventions for young children (≤ 5 years). A paucity of trials that included older children and adolescents limited any conclusions being drawn with respect to these older age groups. 184 The authors recommend that, for young children who have typical development and are healthy, parent-directed interventions should be implemented with ‘no hesitation’. Furthermore, although noting the lack of evidence, the authors also recommend that they are used for older children and adolescents.
For children with NDs, however, additional factors may be at play in the development of behavioural insomnias, which may have implications for the content and scope of the training and advice given to parents, the duration of support parents may require to implement new sleep management approaches and strategies and the clinical effectiveness of a parent-directed intervention. 209 These include communication difficulties (both parental communication about bedtime and sleep and the child’s ability to understand sleep cues or communicate their needs),210,211 sensory sensitivities,209 cognitive delay and arousal associated with daytime behaviour problems. 212 This has implications for the design of future evaluations.
Parent-directed interventions as complex interventions: implications
Parent-directed interventions to manage sleep disturbance in children with NDs can be regarded as ‘complex intervention’, that is, they comprise a number of different interconnected elements. Early guidance, issued by the Medical Research Council on developing and evaluating complex interventions,213,214 has subsequently been developed and refined, including the need for intervention development and evaluation to be theory driven. 215 The theory underlying or informing an intervention will identify its ‘active ingredients’ and the factors that may moderate or mediate their therapeutic action. Although accepted as a fundamental feature of pharmacological studies, the notion of ‘active ingredients’ is as relevant and important to our understanding and evaluation of non-pharmacological interventions. 216,217 However, adoption of robust approaches to the evaluation of complex interventions, in which a theory of change has informed the study design and outcomes measured and the active ingredients are clearly defined and specified, remains patchy. 218
Specifying a theory of change, and the active ingredients, to complex interventions such as parent-delivered sleep management interventions requires looking not only at content of the ‘training’ delivered to parents, per se, but also at the features of the individuals involved in delivery, at the context, or mode, in which the training is delivered and in the implementation. 216,219 It would be fair to say that the influence of this thinking and methodological debate has, to date, had relatively little impact on evaluations of parent-directed, sleep management interventions for children with NDs. Behavioural theories (e.g. extinction, positive reinforcement) are explicitly identified as underpinning all the sleep management strategies on which parents were trained in the comprehensive parent-directed interventions and one of the non-comprehensive interventions. 152 In addition, the influence of social learning theory220 on choices about the techniques used to teach and train parents is apparent (e.g. the use of role-play, modelling, observation, group problem-solving). However, a theory of the process by which upskilling a parent in sleep management results in changes in a child’s sleep, and the factors that have an impact on that process, is left (relatively) undiscussed and unexplored. This is evidenced by the fact that only a small minority of the studies measured changes in parents’ knowledge of sleep management, perceived parenting confidence, collected data on parents’ characteristics and/or sought to assess the extent to which parents accurately or consistently applied their training.
The evidence reviewed regarding the ‘family experience’ of parent-directed sleep management interventions offers some, limited, insights into the process by which parent-directed interventions affect changes in the duration or quality of a child’s sleep and the factors that parents believe facilitate or hinder this process. However, the story appears complex. Thus, for example, although support with implementing new approaches to managing their child’s sleep disturbance was consistently identified as a valued or helpful aspect of the intervention received by parents, views on mode of delivery revealed that there were different benefits in group versus individual delivery. Exposure to other parents’ experiences and access to peer support were identified as supporting positive outcomes. In terms of individually delivered interventions, a key benefit reported by parents was the scope to personalise an intervention to the child and the family’s specific needs and context. It is also important to note that parents are likely to vary in their abilities, capacities and/or willingness to access the different modes of delivery. 221
Other non-pharmacological interventions
Seven other types of non-pharmacological intervention were included: valerian,156 weighted blankets,36 a sleep management intervention delivered in a specialist inpatient setting,157 light therapy alongside a daytime activities programme,158 an aquatic exercise programme,159 acupuncture and ear-point taping 161 and fatty acid supplements. 160 The evaluations of two of these interventions – the sleep management intervention delivered in a specialist inpatient setting157 and light therapy alongside a daytime activities programme158 – were both carried out ≥ 20 years ago. We have included them in this review but their current relevance may be open to question.
Principal findings
There was no evidence that weighted blankets – an intervention that parents can purchase and use without professional supervision or receive via a prescription from statutory health services – were clinically effective. Issues of study design and ratings of high risk of study bias mean that no conclusions can be drawn regarding the impact of the following interventions: valerian, fatty acid supplements, light therapy with daytime activities programme, acupuncture and ear-point taping and the aquatic exercise programme.
Future research: overarching issues and challenges
A key aim of this review was to make recommendations regarding priorities for future research on this topic. In this section, we consider some overarching issues and challenges.
Outcomes and outcome measurement
A number of issues need to be addressed in future research to generate robust and meaningful evidence that, in future reviews, can be subject to meta-analysis.
First, a large number of different child sleep outcomes and measurement tools were used by the studies included in this review. Our synthesis of the studies was partly hampered by this diversity of outcome measures in relation to both child sleep outcomes and other outcomes for children and their parents. Only TST was measured in the majority of studies and assessed in a similar way across studies. We would note that when evidence of benefit of a sleep management intervention was reported – and actigraphy and parent-report measures were used – benefit was more frequently observed with respect to parent-reported measures. It is not clear whether this is because of issues of objectivity versus subjectivity in outcome measurement or that these different measurement approaches are capturing qualitatively different outcomes.
Adverse events were reported in the majority of melatonin trials. However, the type of adverse event reported, data collection methods used and reporting of adverse events varied. Standardisation of the reporting and data collection methods for adverse events in future trials is important to understand the safety of pharmacological interventions. This also has relevance for non-pharmacological studies, in which interventions may have unintended consequences.
There were two additional issues: (1) variability in the outcome domains of interest, for example carer outcomes were assessed in some studies and not others and (2) variability in the measure used to assess specific outcome domains. Previous research has identified sleep as a key health outcome for children and young people with ND, their parents30 and health-care professionals. 222 However, based on searches of the Core Outcome Measures In Effectiveness database (www.comet-initiative.org; accessed 21 June 2017), no work has been undertaken to prioritise outcomes for children with NDs who experience sleep disturbances and their parents. A core outcome set would greatly assist the usefulness of research in this field, in which there are so many management options, allowing comparison between studies and also ensuring that the outcomes that are assessed are of relevance to children, their parents and also the health-care professionals involved in their care.
Second, further work with families to establish what constitutes a meaningful and worthwhile change in these outcome measures is important. Just one study49 adopted this approach, with a 50% reduction in the composite sleep disturbance score identified by parents participating in the study as the minimum change for the intervention to be considered worthwhile.
Meaningful and sufficient change may also have guided the duration of implementation support in interventions in which the duration of this was reported as variable, or needs-led,21,124,146 but the heuristic for ceasing implementation support was not explicitly reported. Furthermore, other studies reported an extended period of implementation support but it was not clear whether this duration was predetermined or simply what actually occurred when the intervention was delivered. An alternative, or additional, approach used by a small number of studies was the setting of parent-identified goals for their child’s sleep, with progress towards these goals tracked over follow-up time points. However, none of these studies used Goal Attainment Scaling methodology,223,224 which allows identification of, and tracking of progress towards, personalised intervention goals, with the creation of an aggregated score for each study participant, and allows robust comparisons between participants. This would appear to be a measurement approach worth considering in future research, alongside objective and other parent-reported outcome measures, as is the case in evaluations of complex interventions in other fields. 225
Third, consistency in follow-up time points based on an evidence-informed theory of change is required. This is particularly an issue for non-pharmacological interventions in which the implementation of newly acquired knowledge and skills in managing a child’s sleep may take time to have an effect. Thus, within the different types of parent-directed interventions, consensus is required as to the most clinically meaningful follow-up time points and whether or not evaluations should seek to also investigate maintenance of outcomes. This would also assist in comparisons between studies in future systematic reviews and meta-analyses.
Fourth, further work is required to identify other relevant child outcome measures and the time points at which such outcomes should be measured. Cognitive ability is an important example here. Little is understood about the benefits of (even small) cumulative gains in sleep in terms of arresting neuronal and cognitive loss. 226 Thus, the identification of appropriate follow-up time points and meaningful measures of cognition, an outcome that is challenging to accurately capture in children with severe learning disabilities, are essential. The selection of other child outcome measures also needs to be evidence based and informed by the intervention’s theory of change for that outcome.
Fifth, for parent-directed interventions, the development and application of a theory of change is required in the selection of appropriate outcomes. This is because children’s sleep outcomes will be mediated by the outcomes for parents achieved by the intervention, that is, the acquisition of new knowledge and understanding of sleep, and training in managing sleep disturbance. Furthermore, evidence from the wider literature on parenting interventions indicates that the extent to which these parent-centred outcomes are achieved are mediated/moderated by a number of factors, located in parent, practitioner and intervention characteristics. Only a minority of parent-directed interventions captured outcomes in this domain. Similarly, few studies report parent characteristics.
The design of evaluations
As we have noted earlier, evaluations need to be designed in such a way that the evidence generated addresses the key questions of what works, for whom and in what circumstances? Interestingly, a similar call for evidence has been made with respect to evaluation of similar interventions for children with typical development. 184 It would be informative to explore subgroup analyses within any future trials. In order to make any analyses more credible, it will be important to define these subgroups in advance, and the direction of effect, based on existing evidence. 227,228 Depending on the evaluation, subgroups may be in terms of sleep problem, child, parent or intervention characteristics.
In addition, for parent-directed interventions, RCTs need to incorporate measures of adherence and/or fidelity. These are loosely defined concepts. An evaluation of parent-directed interventions to manage autism symptoms usefully defined it as both the accuracy and comprehensiveness of the intervention delivered to the parents by the practitioner and the consistency and competency of the parent as they implement new approaches to managing their child’s sleep disturbance. 229 Our review of ‘family experience’ data identified that parents can find implementing a new sleep management strategy stressful and demanding. Only one study125 attempted to assess this in any way, relying on parents’ reports of the proportion of times that they implemented the new sleep management strategy. There are clearly significant challenges related to the feasibility and costs associated with measuring parent’s adherence and fidelity to a sleep management strategy given the time of day when parents will be implementing a sleep management strategy and the home setting. Methodological research will be required to inform incorporating this into study designs.
Finally, the final follow-up time point for most studies allowed consideration of short-term outcomes only. Future studies need to consider what longer-term follow-ups should be incorporated into study designs. Evidence on longer-term outcomes is particularly pertinent for parent-directed interventions that require significant investment in parents’, as well as practitioners’, time, physical and emotional resources (and potentially entail significant, if short-term, disruption to the family). If available, such evidence would support shared decision-making regarding the management of a child’s sleep disturbance.
Moving towards replication
At the moment, the evidence base predominantly comprises single evaluations of interventions. These are principally either investigations into the management of sleep disturbance by a particular clinic or are (typically) early evaluations of a newly developed intervention, prior to its implementation into routine practice. There are just two instances of some sort of replication study; however, in both,123,124,128,129 there are differences between the studies in the way the intervention was delivered. A taxonomy by which interventions should be defined and adherence to Template for Intervention Description and Replication (TIDieR) guidance164 in study reporting would support both replication and future evidence syntheses.
Chapter 5 Conclusions
Implications for health care
The poor quality of evidence and/or uncertainty regarding the clinical significance of findings mean that there are no implications for health-care practice.
Recommendations for research
Randomised controlled trials are required to assess a range of possible treatment options for sleep disturbance in children with ND. Future trials need to be informed by a greater understanding of the mechanisms by which non-pharmacological interventions may have an impact on a child’s sleep and on outcomes and outcome measurement. Developing an understanding of these mechanisms will require mixed-methods research.
Future research needs to take account of the range of types, or aetiologies, of sleep disturbance, including behavioural, physiological (e.g. heightened arousal, anxiety or atypical melatonin profiles) and disorders of the circadian rhythm,230 which will vary between NDs.
We would also highlight that reporting was poor across RCTs, before-and-after studies and in studies reporting parents’ views and experiences of sleep management interventions. This was particularly the case for the non-pharmacological interventions. Future research should follow the appropriate reporting standards for the specific study design (www.equator-network.org; accessed 21 June 2017), including giving detailed description of the interventions using the TIDieR checklist. 164 Within the TIDieR checklist, it is important that sufficient detail is given on the specific curricula/training content of each intervention and the techniques used to teach and train parents.
Bearing all of these issues in mind, we recommend that the topic areas listed below are priorities for future research. Generating robust evidence in these topic areas will support clinicians (and families) to make evidence-informed decisions about the identification, prevention and management of sleep disturbance in children with NDs. It will also support evidence-informed decision-making with respect to commissioning of services. The suggested areas for future research are quite diverse. Although necessarily presented in an order, it would seem important that all key stakeholders contribute to any prioritisation processes.
-
The development of a core outcome set would be beneficial. This should be developed in consultation with parents and carers, the children themselves, when possible, and health-care professionals and others involved in supporting parents and children, using a structured process such as that developed by the Core Outcome Measures In Effectiveness group. 231 Similarly, standardising the adverse events recorded, data collection methods used and level of detail reported is important for future evaluations of pharmacological and non-pharmacological interventions.
-
If not already recently conducted, a review of existing tools, practices and strategies to identify sleep disturbance in children with NDs that are appropriate and feasible for use in routine practice is recommended.
-
If available in a suitable preparation for the population under consideration, a trial comparing a slow-release formulation of melatonin with a fast-release formulation of melatonin is suggested.
-
There are a range of possible other pharmacological interventions for this population, such as clonidine, but eligible studies were not identified. Prioritisation of evaluations of alternative pharmacological options to melatonin (e.g. clonidine) is recommended.
-
No studies were identified that evaluated a combined or sequential use of melatonin (or other medicines) and parent-directed interventions. Studies of this nature are required because children may present with different types of sleep disturbance and/or sleep problems. Specifically, an apparent subclinical benefit of melatonin in managing sleep initiation difficulties may be beneficial in supporting the impact that a parent-directed intervention has. We recommend trials to investigate this.
-
Parent-directed interventions range considerably in the intensity of practitioner input, from the simple provision of written material through to extended one-to-one contact and incorporation of implementation support. Studies are required that will support clinician and parent decision-making regarding the appropriate intervention. Evaluations of parent-directed interventions that allow for comparison of the relative impacts/clinical effectiveness of different features of the interventions (e.g. mode of delivery, group vs. individual and the nature of implementation support) are recommended, which should incorporate an investigation into issues of feasibility and acceptability. Prioritisation of interventions to address sleep initiation is suggested.
-
None of the studies included in this review was presented as a preventative intervention. Rather, clinical cut-off points of sleep disturbance severity and/or parent reports of a sleep disturbance that had extended over some period of time were among study eligibility criteria. However, some types of sleep disturbance are preventable and/or amenable to early intervention. The brief, less-intense, parent-directed interventions included in this review (e.g. provision of information, single-session group intervention or face-to-face intervention) would, however, appear to align with a preventative or early intervention approach. Evaluating these types of intervention approaches in terms of the impact that they have on preventing the development of sleep disturbance, per se, or in preventing a newly emerging sleep disturbance increasing in severity is recommended.
-
Studies that map current practices and provision and research into families’ understanding of sleep disturbance and their experiences of seeking help are recommended. These would provide useful evidence to support the development of provision.
Finally, and across all topic areas, we would recommend that evaluations include a comprehensive and holistic economic evaluation, including costs to families.
Acknowledgements
We would like to thank our parent advisors for their interest in and enthusiasm for the project and their contributions at project meetings. We would also like to thank Kate Baxter for her contribution to the quality appraisal work. We would like to thank Katherine Chatterton for her assistance with sourcing full-text articles and Emma Turner for proofreading the report.
Contributions of authors
Bryony Beresford (Professor, Health and Care Services Research) was a joint co-applicant, contributed to all elements and, with Catriona McDaid, oversaw the delivery of the review and supervised junior members of the review team. She is joint lead author of this report.
Catriona McDaid (Senior Research Fellow, Systematic Reviews, Clinical Trials) was jointly responsible for writing the protocol and had shared responsibility for co-ordinating and leading the project, provided advice and input to all elements of the project and commented on drafts of the report.
Adwoa Parker (Research Fellow) contributed to study selection, data extraction, quality assessment and report writing.
Arabella Scantlebury (Research Fellow) contributed to study selection, data extraction, quality assessment and report writing.
Gemma Spiers (Research Fellow) worked on the project from February to September 2016. During that time, she was responsible for the day-to-day running of the project and led on screening, retrieval, data extraction and quality appraisal.
Caroline Fairhurst (Statistician) conducted the data analysis and contributed to the report writing.
Catherine Hewitt (Professor, Statistics) contributed to the protocol, provided methodological advice throughout the project and commented on drafts of the report.
Kath Wright (Research Fellow, Information Specialist) designed and undertook the literature searches and wrote related sections of the report.
Vicki Dawson (Founder and Chief Executive Officer, The Children’s Sleep Charity) provided expert clinical advice throughout the project, contributed to screening and data extraction processes and to drafts of the report.
Heather Elphick (Consultant in Paediatric Respiratory and Sleep Medicine; Visiting Professor, Sheffield Hallam University) provided expert clinical advice throughout the project, contributed to screening and data extraction processes and to drafts of the report.
Megan Thomas (Consultant Community Paediatrician) provided expert clinical advice throughout the project, contributed to screening and data extraction processes and to drafts of the report.
Publication
Scantlebury A, McDaid C, Dawson V, Elphick H, Fairhurst C, Hewitt C, et al. Non-pharmacological interventions for non-respiratory sleep disturbance in children with neurodisabilities: a systematic review [published online ahead of print 29 July 2018]. Dev Med Child Neurol 2018. https://doi.org/10.1111/dmcn.13972
Data-sharing statement
No primary data were produced. Most of the data extracted from the primary studies are available in the main body of the report and the appendices. Any further data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Brown CA, Kuo M, Phillips L, Berry R, Tan M. Non-pharmacological sleep interventions for youth with chronic health conditions: a critical review of the methodological quality of the evidence. Disabil Rehabil 2013;35:1221-55. https://doi.org/10.3109/09638288.2012.723788.
- Stores G. Multifactorial influences, including comorbidities, contributing to sleep disturbance in children with a neurodevelopmental disorder. CNS Neurosci Ther 2016;22:875-9. https://doi.org/10.1111/cns.12574.
- Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev 2012;16:129-36. https://doi.org/10.1016/j.smrv.2011.03.007.
- Wiggs L. Behavioural aspects of children’s sleep. Arch Dis Child 2009;94:59-62. https://doi.org/10.1136/adc.2007.125278.
- Montgomery P, Dunne D. Treatment of Sleep Problems in Children, Clinical Evidence. London: BMJ Books; 2006.
- Mazurek MO, Petroski GF. Sleep problems in children with autism spectrum disorder: examining the contributions of sensory over-responsivity and anxiety. Sleep Med 2015;16:270-9. https://doi.org/10.1016/j.sleep.2014.11.006.
- Hollway JA, Aman MG. Pharmacological treatment of sleep disturbance in developmental disabilities: a review of the literature. Res Dev Disabil 2011;32:939-62. https://doi.org/10.1016/j.ridd.2010.12.035.
- Breau LM, Camfield CS. Pain disrupts sleep in children and youth with intellectual and developmental disabilities. Res Dev Disabil 2011;32:2829-40. https://doi.org/10.1016/j.ridd.2011.05.023.
- Heaton J, Noyes J, Sloper P, Shah R. Families’ experiences of caring for technology-dependent children: a temporal perspective. Health Soc Care Community 2005;13:441-50. https://doi.org/10.1111/j.1365-2524.2005.00571.x.
- International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine; 2014.
- Sateia MJ. International classification of sleep disorders – third edition: highlights and modifications. Chest 2014;146:1387-94. https://doi.org/10.1378/chest.14-0970.
- Vriend J, Corkum P. Clinical management of behavioral insomnia of childhood. Psychol Res Behav Manag 2011;4:69-7. https://doi.org/10.2147/PRBM.S14057.
- Mindell JA, Emslie G, Blumer J, Genel M, Glaze D, Ivanenko A, et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics 2006;117:e1223-32. https://doi.org/10.1542/peds.2005-1693.
- Tietze AL, Blankenburg M, Hechler T, Michel E, Koh M, Schlüter B, et al. Sleep disturbances in children with multiple disabilities. Sleep Med Rev 2012;16:117-27. https://doi.org/10.1016/j.smrv.2011.03.006.
- Dorris L, Scott N, Zuberi S, Gibson N, Espie C. Sleep problems in children with neurological disorders. Dev Neurorehabil 2008;11:95-114. https://doi.org/10.1080/17518420701860149.
- Kotagal S. Parasomnias in childhood. Sleep Med Rev 2009;13:157-68. https://doi.org/10.1016/j.smrv.2008.09.005.
- Morris C, Janssens A, Tomlinson R, Williams J, Logan S. Towards a definition of neurodisability: a Delphi survey. Dev Med Child Neurol 2013;55:1103-8. https://doi.org/10.1111/dmcn.12218.
- NHS Standard Contract for Paediatric Neurosciences – Neurodisability. Schedule 2 – The Services. A Service Specification. Leeds: NHS England; 2013.
- Blackburn C, Read J, Spencer N. Children with Neurodevelopmental Disabilities. London: Department of Health and Social Care; 2012.
- Grigg-Damberger M, Ralls F. Treatment strategies for complex behavioral insomnia in children with neurodevelopmental disorders. Curr Opin Pulm Med 2013;19:616-25. https://doi.org/10.1097/MCP.0b013e328365ab89.
- Beresford B, Stuttard L, Clarke S, Maddison J, Beecham J. Managing Behaviour and Sleep Problems in Disabled Children: an Investigation into the Effectiveness and Costs of Parent-Training Interventions. London: Department for Education; 2012.
- Wiggs L, Stores G. A randomised controlled trial of behavioural intervention for sleeplessness in children with autism spectrum disorders. J Sleep Res 2006;15.
- Doo S, Wing YK. Sleep problems of children with pervasive developmental disorders: correlation with parental stress. Dev Med Child Neurol 2006;48:650-5. https://doi.org/10.1017/S001216220600137X.
- Simola P. Sleep Problems and Their Implications from Preschool to School Age 2014.
- McConkey R, Kelly F, Craig S. Access to respite breaks for families who have a relative with intellectual disabilities: a national survey. J Adv Nurs 2011;67:1349-57. https://doi.org/10.1111/j.1365–2648.2010.05586.x.
- Quach J, Gold L, Hiscock H, Mensah FK, Lucas N, Nicholson JM, et al. Primary healthcare costs associated with sleep problems up to age 7 years: Australian population-based study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002419.
- Colten H. Sleep Disorders and Sleep Deprivation: an Unmet Public Health Problem. Washington, DC: National Academies Press; 2006.
- Hillman DR, Murphy AS, Pezzullo L. The economic cost of sleep disorders. Sleep 2006;29:299-305. https://doi.org/10.1093/sleep/29.3.299.
- Beresford B. Expert Opinions: a National Survey of Parents Caring for a Severely Disabled Child. Bristol: Policy Press; 1995.
- Allard A, Fellowes A, Shilling V, Janssens A, Beresford B, Morris C. Key health outcomes for children and young people with neurodisability: qualitative research with young people and parents. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004611.
- Stores G. Children’s sleep disorders: modern approaches, developmental effects, and children at special risk. Dev Med Child Neurol 1999;41:568-73. https://doi.org/10.1017/S001216229900119X.
- Morris C, Simkiss D, Busk M, Morris M, Allard A, Denness J, et al. Setting research priorities to improve the health of children and young people with neurodisability: a British Academy of Childhood Disability–James Lind Alliance Research Priority Setting Partnership. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014–006233.
- Appleton RE, Gringras P. Melatonin: helping to MEND impaired sleep. Arch Dis Child 2013;98:216-17. https://doi.org/10.1136/archdischild-2012-303606.
- MacLeod R, Keen D. Innovations in practice: ‘off-label’ clonidine: UK paediatric and child and adolescent psychiatry prescribing practice for sleep problems. Child Adolesc Psychiatry Ment Health 2014;19:147-50. https://doi.org/10.1111/camh.12032.
- Heussler H, Chan P, Price AM, Waters K, Davey MJ, Hiscock H. Pharmacological and non-pharmacological management of sleep disturbance in children: an Australian Paediatric Research Network survey. Sleep Med 2013;14:189-94. https://doi.org/10.1016/j.sleep.2012.09.023.
- Gringras P, Green D, Wright B, Rush C, Sparrowhawk M, Pratt K, et al. Weighted blankets and sleep in autistic children – a randomized controlled trial. Pediatrics 2014;134:298-306. https://doi.org/10.1542/peds.2013-4285.
- Fjeldsted B, Hanlon-Dearman A. Sensory processing and sleep challenges in children with fetal alcohol spectrum disorder. Occupational Therapy Now 2009;11:26-8.
- Wyatt K, Edwards V, Franck L, Britten N, Creanor S, Maddick A, et al. Cranial osteopathy for children with cerebral palsy: a randomised controlled trial. Arch Dis Child 2011;96:505-12. https://doi.org/10.1136/adc.2010.199877.
- Galland BC, Mitchell EA. Helping children sleep. Arch Dis Child 2010;95:850-3. https://doi.org/10.1136/adc.2009.162974.
- Bruni O, Novelli L. Sleep disorders in children. BMJ Clin Evid 2010;2010.
- Autism: The Management and Support of Children and Young People on the Autism Spectrum. London: NICE; 2013.
- Owens JA, Moturi S. Pharmacologic treatment of pediatric insomnia. Child Adolesc Psychiatr Clin N Am 2009;18:1001-16. https://doi.org/10.1016/j.chc.2009.04.009.
- Newman CJ, O’Regan M, Hensey O. Sleep disorders in children with cerebral palsy. Dev Med Child Neurol 2006;48:564-8. https://doi.org/10.1017/S0012162206001198.
- Stores G, Stores R. Sleep disorders and their clinical significance in children with Down syndrome. Dev Med Child Neurol 2013;55:126-30. https://doi.org/10.1111/j.1469-8749.2012.04422.x.
- Stuttard L, Beresford B, Clarke S, Beecham J, Curtis J. A preliminary investigation into the effectiveness of a group-delivered sleep management intervention for parents of children with intellectual disabilities. J Intellect Disabil 2015;19:342-55. https://doi.org/10.1177/1744629515576610.
- Montgomery P, Bjornstad G, Dennis J. Media-based behavioural treatments for behavioural problems in children. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD002206.pub3.
- Working Party on Sleep Physiology and Respiratory Control Disorders in Childhood. Standards for Services for Children with Disorders of Sleep Physiology. London: Royal College of Paediatrics and Child Health; 2009.
- Appleton RE, Jones AP, Gamble C, Williamson PR, Wiggs L, Montgomery P, et al. The use of MElatonin in children with Neurodevelopmental Disorders and impaired Sleep: a randomised, double-blind, placebo-controlled, parallel study (MENDS). Health Technol Assess 2012;16. https://doi.org/10.3310/hta16400.
- Montgomery P, Stores G, Wiggs L. The relative efficacy of two brief treatments for sleep problems in young learning disabled (mentally retarded) children: a randomised controlled trial. Arch Dis Child 2004;89:125-30. https://doi.org/10.1136/adc.2002.017202.
- Mindell JA, Bartle A, Wahab NA, Ahn Y, Ramamurthy MB, Huong HT, et al. Sleep education in medical school curriculum: a glimpse across countries. Sleep Med 2011;12:928-31. https://doi.org/10.1016/j.sleep.2011.07.001.
- Malow BA, Byars K, Johnson K, Weiss S, Bernal P, Goldman SE, et al. A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics 2012;130:106-24. https://doi.org/10.1542/peds.2012-0900I.
- Autism: The Management and Support of Children and Young People on the Autism Spectrum. London: Royal College of Psychiatrists; 2013.
- Barrett JR, Tracy DK, Giaroli G. To sleep or not to sleep: a systematic review of the literature of pharmacological treatments of insomnia in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2013;23:640-7. https://doi.org/10.1089/cap.2013.0059.
- Cortese S, Brown TE, Corkum P, Gruber R, O’Brien LM, Stein M, et al. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2013;52:784-96. https://doi.org/10.1016/j.jaac.2013.06.001.
- Guénolé F, Godbout R, Nicolas A, Franco P, Claustrat B, Baleyte JM. Melatonin for disordered sleep in individuals with autism spectrum disorders: systematic review and discussion. Sleep Med Rev 2011;15:379-87. https://doi.org/10.1016/j.smrv.2011.02.001.
- Bendz LM, Scates AC. Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder. Ann Pharmacother 2010;44:185-91. https://doi.org/10.1345/aph.1M365.
- Cohen-Zion M, Ancoli-Israel S. Sleep in children with attention-deficit hyperactivity disorder (ADHD): a review of naturalistic and stimulant intervention studies. Sleep Med Rev 2004;8:379-402. https://doi.org/10.1016/j.smrv.2004.06.002.
- Galland BC, Elder DE, Taylor BJ. Interventions with a sleep outcome for children with cerebral palsy or a post-traumatic brain injury: a systematic review. Sleep Med Rev 2012;16:561-73. https://doi.org/10.1016/j.smrv.2012.01.007.
- McDaid C, Sloper P. Evidence on Effectiveness of Behavioural Interventions to Help Parents Manage Sleep Problems in Young Disabled Children: a Rapid Review. Working Paper no. C4EO 2296. York: Social Policy Research Unit: University of York; 2008.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination; 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Llewellyn A, Norman G, Harden M, Coatesworth A, Kimberling D, Schilder A, et al. Interventions for adult Eustachian tube dysfunction: a systematic review. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18460.
- McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13040.
- Hawker S, Payne S, Kerr C, Hardey M, Powell J. Appraising the evidence: reviewing disparate data systematically. Qual Health Res 2002;12:1284-99. https://doi.org/10.1177/1049732302238251.
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III: the issue of carry-over. Stat Med 2002;21:2161-73. https://doi.org/10.1002/sim.1207.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane; 2011.
- Garstang J, Wallis M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev 2006;32:585-9. https://doi.org/10.1111/j.1365-2214.2006.00616.x.
- Weiss MD, Wasdell MB, Bomben MM, Rea KJ, Freeman RD. Sleep hygiene and melatonin treatment for children and adolescents with ADHD and initial insomnia. J Am Acad Child Adolesc Psychiatry 2006;45:512-19. https://doi.org/10.1097/01 chi.0000205706.78818.ef.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. https://doi.org/10.1136/bmj.331.7521.897.
- Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8. https://doi.org/10.1186/1471–2288–8-45.
- Booth A, Carroll C, Ilott I, Low LL, Cooper K. Desperately seeking dissonance: identifying the disconfirming case in qualitative evidence synthesis. Qual Health Res 2013;23:126-41. https://doi.org/10.1177/1049732312466295.
- Anderson LM, Oliver SR, Michie S, Rehfuess E, Noyes J, Shemilt I. Investigating complexity in systematic reviews of interventions by using a spectrum of methods. J Clin Epidemiol 2013;66:1223-9. https://doi.org/10.1016/j.jclinepi.2013.06.014.
- Burford B, Lewin S, Welch V, Rehfuess E, Waters E. Assessing the applicability of findings in systematic reviews of complex interventions can enhance the utility of reviews for decision making. J Clin Epidemiol 2013;66:1251-61. https://doi.org/10.1016/j.jclinepi.2013.06.017.
- de Leersnyder H, Fabiano A, de Bodinat C. Agomelatine efficacy on major sleep disturbances in Smith–Magenis syndrome: an exploratory, open study in children. Fundamental Clin Pharmacol 2007;21.
- Fabiano A, de Leersnyder H. P.7.a.001 Agomelatine efficacy on major sleep disturbances in Smith–Magenis syndrome: an exploratory, open study in children. Eur Neuropsychopharmacol 2007;17. https://doi.org/10.1016/S0924-977X(07)70882-9.
- Gupta A, Varthamanan C. 1537 effectiveness of melatonin in treating sleep problems in children – parent satisfaction survey. Arch Dis Child 2012;97.
- Hall L, Shapiro C, Berall G, Hwang P. 5. Sleep disturbances in Prader–Willi syndrome and the effects of topiramate and modafinil. Clin Neurophysiol 2013;124:e2-e3. https://doi.org/10.1016/j.clinph.2012.08.043.
- Huerta R, Labra A, Sanchez-Narvaez F, Jimenez-Correa U, Medina-Chavez JH, Portillo E, et al. Improvement of sleep architecture in down syndrome children after a nutritional complement. J Sleep Res 2014;23:323-4.
- Haro R, Huerta R, Labra A, Sanchez-Narvaez F, IbarraCoronado F, Portillo E, et al. Effects of a dietary intervention on the sleep patterns in children with autistic spectrum disorders. J Sleep Res 2014;23.
- Haro RH. Effects of a dietary intervention on the sleep patterns in children with autistic spectrum disorders. Sleep 2014;37.
- Rodolico L. T-O-133 Sleep intervention therapy in children with neurodisabilities affected by sleep disturbances: a service evaluation. Sleep Med 2011;12. https://doi.org/10.1016/S1389-9457(11)70346-X.
- Rodolico L, Bem A. Sleep intervention therapy in children with neurodisabilities affected by sleep disturbances: a service evaluation. Arch Dis Child 2012;97.
- Tan X. Effectiveness of omega-3 fatty acids supplementation on sleep in children and adolescents with attention deficit hyperactivity disorder. Ann Acad Med Singapore 2014;1.
- ClinicalTrials.gov . Improving Sleep and Daytime Functioning Among Children Diagnosed With Attention Deficit Hyperactivity Disorder (ADHD) 2009. https://clinicaltrials.gov/ct2/show/NCT00867451 (accessed 9 February 2016).
- ISRCTN Registry . A Randomised Controlled Trial of Sleep Problems for Children With Autism 2004. https://doi.org/10.1186/ISRCTN74087708.
- ClinicalTrials.gov . Melatonin for Sleep in Children With Autism 2009. https://clinicaltrials.gov/ct2/show/NCT00927030.
- ClinicalTrials.gov . Trial of Melatonin to Improve Sleep in Children With Epilepsy and Neurodevelopmental Disabilities 2010. https://clinicaltrials.gov/ct2/show/NCT01161108 (accessed 9 February 2016).
- ClinicalTrials.gov . Telephone Care Management to Address Sleep Problems in Young Children With Autism 2012. https://clinicaltrials.gov/ct2/show/NCT01558180 (accessed 9 February 2016).
- ClinicalTrials.gov . Melatonin CR for the Treatment of Impaired Sleep Maintenance in 4–8 Year Old Children With Autism Spectrum Disorders 2009. https://clinicaltrials.gov/ct2/show/NCT01033565 (accessed 9 February 2016).
- ClinicalTrials.gov . Iron Treatment of Sleep Disorders in Children With Autism Spectrum Disorder 2012. https://clinicaltrials.gov/ct2/show/NCT01745497 (accessed 9 February 2016).
- ClinicalTrials.gov . Comparing Treatment With Melatonin to Treatment With Stimulants (Methylphenidate) in Children With Attention Deficit Hyperactivity Disorder and Sleep Difficulties 2011. https://clinicaltrials.gov/ct2/show/NCT01393574 (accessed 9 February 2016).
- ISRCTN Registry . Sleeping Sound With Attention Deficit Hyperactivity Disorder (ADHD) 2014. https://doi.org/10.1186/ISRCTN50834814.
- ClinicalTrials.gov . Treatment Strategies for Children With Smith–Magenis Syndrome 2007. https://clinicaltrials.gov/ct2/show/NCT01393574 (accessed 9 February 2016).
- ClinicalTrials.gov . Efficacy and Safety of Circadin® in the Treatment of Sleep Disturbances in Children With Neurodevelopment Disabilities n.d. https://clinicaltrials.gov/ct2/show/NCT01906866 (accessed 9 February 2016).
- ClinicalTrials.gov . Does Melatonin Restore Sleep Architecture in Autistic Children 2013. https://clinicaltrials.gov/ct2/show/NCT01993251 (accessed 9 February 2016).
- ClinicalTrials.gov . Sleep Intervention for Pediatric Epilepsy 2015. https://clinicaltrials.gov/ct2/show/ NCT02514291 (accessed 9 February 2016).
- De Graaf L. Sleep onset insomnia in children with ADHD due to disturbed wake–sleep rhythm: Melatonin puts the clock back. Pharm Weekbl 2007;142:46-9.
- Valdizan J. Nocturnal prolactin in complex partoal or generalized epilepsy of childhood. Revista Espanola De Neurologia 1990;5:239-42.
- Weiss MD, Wasdell MB, Bomben MM, Rea KJ, Freeman RD. Sleep Hygiene and Melatonin Treatment for ADHD Sleep-Onset Delay n.d.
- Yaghoobnezhad S. Effectiveness of psychological interventions on improving sleep quality of adolescents with Down syndrome. Iran J Psychiatry 2012;1.
- Frazier TW, Krishna J, Klingemier E, Beukemann M, Nawabit R, Ibrahim S. A randomized, crossover trial of a novel sound-to-sleep mattress technology in children with autism and sleep difficulties. J Clin Sleep Med 2016;20.
- Waldron AY, Spark MJ, Dennis CM. The use of melatonin by children: parents’ perspectives. J Clin Sleep Med 2016;12:1395-401. https://doi.org/10.5664/jcsm.6198.
- Wright B, Sims D, Smart S, Alwazeer A, Alderson-Day B, Allgar V, et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J Autism Dev Disord 2011;41:175-84. https://doi.org/10.1007/s10803-010-1036-5.
- Johnson CR, Turner KS, Foldes E, Brooks MM, Kronk R, Wiggs L. Behavioral parent training to address sleep disturbances in young children with autism spectrum disorder: a pilot trial. Sleep Med 2013;14:995-1004. https://doi.org/10.1016/j.sleep.2013.05.013.
- Gringras P, Gamble C, Jones AP, Wiggs L, Williamson PR, Sutcliffe A, et al. Melatonin for sleep problems in children with neurodevelopmental disorders: randomised double masked placebo controlled trial. BMJ 2012;345. https://doi.org/10.1136/bmj.e6664.
- Appleton RE, Gringras P. MENDS Study Group . MENDS: the use of melatonin in children with neuro-developmental disorders and impaired sleep – a randomised, double-blind, placebo-controlled, parallel trial. Arch Dis Child 2011;96. https://doi.org/10.1136/adc.2011.212563.1.
- Cortesi F, Giannotti F, Sebastiani T, Panunzi S, Valente D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J Sleep Res 2012;21:700-9. https://doi.org/10.1111/j.1365-2869.2012.01021.x.
- Van der Heijden KB, Smits MG, Van Someren EJ, Ridderinkhof KR, Gunning WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry 2007;46:233-41. https://doi.org/10.1097/01.chi.0000246055.76167.0d.
- Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res 2009;47:1-7. https://doi.org/10.1111/j.1600-079X.2009.00681.x.
- Camfield P, Gordon K, Dooley J, Camfield C. Melatonin appears ineffective in children with intellectual deficits and fragmented sleep: six ‘N of 1’ trials. J Child Neurol 1996;11:341-3. https://doi.org/10.1177/088307389601100414.
- Dodge NN, Wilson GA. Melatonin for treatment of sleep disorders in children with developmental disabilities. J Child Neurol 2001;16:581-4. https://doi.org/10.1177/088307380101600808.
- Jain SV, Horn PS, Simakajornboon N, Beebe DW, Holland K, Byars AW, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med 2015;16:637-44. https://doi.org/10.1016/j.sleep.2015.01.005.
- Jain SV, Horn PS, Simakajornboon N, Beebe DW, Holland K, Byars AW, et al. Melatonin improves sleep in children with epilepsy: results from a randomized, doubleblind, placebo-controlled, cross-over study. Epilepsy Currents 2014;14.
- Wasdell MB, Jan JE, Bomben MM, Freeman RD, Rietveld WJ, Tai J, et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res 2008;44:57-64.
- Carr R, Wasdell MB, Hamilton D, Weiss MD, Freeman RD, Tai J, et al. Long-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disorders. J Pineal Res 2007;43:351-9. https://doi.org/10.1111/j.1600-079X.2007.00485.x.
- Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome [Erratum appears in J Clin Sleep Med 2010;6(Suppl. 4):preceding 311]. J Clin Sleep Med 2009;5:145-50.
- Hancock E, O’Callaghan F, Osborne JP. Effect of melatonin dosage on sleep disorder in tuberous sclerosis complex. J Child Neurol 2005;20:78-80. https://doi.org/10.1177/08830738050200011302.
- Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M. Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders. J Pineal Res 2000;29:34-9. https://doi.org/10.1034/j.1600-079X.2000.290105.x.
- Bramble D. Consumer opinion concerning the treatment of a common sleep problem. Child Care Health Dev 1996;22:355-66. https://doi.org/10.1111/j.1365-2214.1996.tb00438.x.
- Austin K, Gordon JE, O’Connell A. Preliminary evaluation of sleepwise program for children with sleep disturbance and developmental delay. Child Fam Behav Ther 2013;35:195-211. https://doi.org/10.1080/07317107.2013.818886.
- Moss AH, Gordon JE, O’Connell A. Impact of sleepwise: an intervention for youth with developmental disabilities and sleep disturbance. J Autism Dev Disord 2014;44:1695-707. https://doi.org/10.1007/s10803-014-2040-y.
- Sciberras E, Fulton M, Efron D, Oberklaid F, Hiscock H. Managing sleep problems in school aged children with ADHD: a pilot randomised controlled trial. Sleep Med 2011;12:932-5. https://doi.org/10.1016/j.sleep.2011.02.006.
- Weiskop S, Richdale A, Matthews J. Behavioural treatment to reduce sleep problems in children with autism or fragile X syndrome. Dev Med Child Neurol 2005;47:94-104. https://doi.org/10.1017/S0012162205000186.
- Adkins KW, Molloy C, Weiss SK, Reynolds A, Goldman SE, Burnette C, et al. Effects of a standardized pamphlet on insomnia in children with autism spectrum disorders. Pediatrics 2012;130:139-44. https://doi.org/10.1542/peds.2012-0900K.
- Malow BA, Adkins KW, Reynolds A, Weiss SK, Loh A, Fawkes D, et al. Parent-based sleep education for children with autism spectrum disorders. J Autism Dev Disord 2014;44:216-28. https://doi.org/10.1007/s10803-013-1866-z.
- Reed HE, McGrew SG, Artibee K, Surdkya K, Goldman SE, Frank K, et al. Parent-based sleep education workshops in autism. J Child Neurol 2009;24:936-45. https://doi.org/10.1177/0883073808331348.
- Beresford B, Stuttard L, Clarke S, Maddison J. Parents’ experiences of psychoeducational sleep management interventions: a qualitative study of parents of children with neurodevelopmental disabilities. Clin Pract Pediat Psychol 2016;4:164-75. https://doi.org/10.1037/cpp0000144.
- Cortesi F, Giannotti F, Ottaviano S. Sleep Problems and daytime behavior in childhood idiopathic epilepsy. Epilepsia 1999;40:1557-65. https://doi.org/10.1111/j.1528-1157.1999.tb02040.x.
- Quine L. Sleep problems in children with mental handicap. J Ment Defic Res 1991;35:269-90.
- Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 2000;23:1043-51. https://doi.org/10.1093/sleep/23.8.1d.
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 2003;26:337-41. https://doi.org/10.1093/sleep/26.3.337.
- van der Heijden KB, Smits MG, Gunning WB. Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J Sleep Res 2006;15:55-62. https://doi.org/10.1111/j.1365-2869.2006.00491.x.
- Beresford B, Stuttard L, Beecham J. A group delivered sleep support programme for parents of children with learning disabilities: a preliminary investigation into effectiveness and costs. Dev Med Child Neurol 2013;55:11-25.
- Stuttard L, Clarke S, Thomas M, Beresford B. Replacing home visits with telephone calls to support parents implementing a sleep management intervention: findings from a pilot study and implications for future research. Child Care Health Dev 2015;41:1074-81. https://doi.org/10.1111/cch.12250.
- Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S, et al. Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ 2015;350. https://doi.org/10.1136/bmj.h68.
- Papadopoulos N, Sciberras E, Hiscock H, Mulraney M, McGillivray J, Rinehart N. The efficacy of a brief behavioral sleep intervention in school-aged children with ADHD and comorbid autism spectrum disorder [published online ahead of print 2 February 2015]. J Atten Disord 2015. https://doi.org/10.1177/1087054714568565.
- Turner K. Parental Knowledge of Behavioral Principles following Training to Address Sleep Problems in Children with Autism Spectrum Disorders: a Follow-up Study. Pittsburgh, PA: University of Pittsburgh; 2013.
- O’Connell A, Gordon J, Koppelman-Guthrie J, Moss A. Sleepwise – an evaluation of a multicomponent sleep education and home based intervention for older children and adolescents with developmental disabilities and sleep disturbance. Sleep Biol Rhythms 2012;10:1-74.
- O’Connell A. Addressing sleep disturbance in young children with developmental delay. Interconnections Quarterly Journal 2 2010;2:41-5.
- Sciberras E, Efron D, Gerner B, Davey M, Mensah F, Oberklaid F, et al. Study protocol: the sleeping sound with attention-deficit/hyperactivity disorder project. BMC Pediatr 2010;10. https://doi.org/10.1186/1471-2431-10-101.
- Sciberras E, Rinehart N. Treating behavioural sleep problems in children with ADHD and ASD: the sleeping sound program. Eur Child Adolesc Psychiatry 2015;24:S14-S5.
- Fulton M, Sciberras E, Oberklaid F, Efron D, Hiscock H. Treating sleep problems in school aged children with ADHD: evaluation of a behavioural sleep intervention. J Paediatr Child Health 2010;46.
- Quine L, Wade K. Sleep Disturbance in Children with Severe Learning Difficulties: an Examination and an Intervention Trial. Canterbury: University of Kent, Centre for Research in Health Behaviour; 1991.
- Wade K, Wade L. Solving sleep problems in children with mental and physical handicaps: report of an intervention trial. Behavioural Social Work Review 1991;13:25-34.
- Malow BA, Adkins K, Clemons T, Goldman SE, Mollow D, Wofford D, et al. Effects of a standardized pamphlet on sleep latency in children with autism. Sleep 2011;34.
- Bramble D. Rapid-acting treatment for a common sleep problem. Dev Med Child Neurol 1997;39:543-7.
- Reed HE, Artibee K, McGrew SG, Goldman SE, Frank K, Malow BA. Sleep education classes for parents of children with autism spectrum disorders. Sleep 2008;31.
- Yu XT, Lam HS, Au CT, Chan S, Chan D, Li AM. Extended parent-based behavioural education improves sleep in children with autism spectrum disorder. HK J Paediatr (New Series) 2015;20:219-25.
- Wiggs L, Stores G. Behavioural treatment for sleep problems in children with severe learning disabilities and challenging daytime behaviour: effect on sleep patterns of mother and child. J Sleep Res 1998;7:119-26. https://doi.org/10.1046/j.1365-2869.1998.00107.x.
- Wiggs L, Stores G. Behavioural treatment for sleep problems in children with severe learning disabilities and challenging daytime behaviour: effect on daytime behaviour. J Child Psychol Psychiatry 1999;40:627-35. https://doi.org/10.1111/1469-7610.00479.
- Wiggs L, Stores G. Behavioural treatment for sleep problems in children with severe intellectual disabilities and daytime challenging behaviour: effect on mothers and fathers. Br J Health Psychol 2001;6:257-69. https://doi.org/10.1348/135910701169197.
- Peppers KH, Eisbach S, Atkins S, Poole JM, Derouin A. An intervention to promote sleep and reduce ADHD symptoms. J Pediatr Health Care 2016;30:e43-e48. https://doi.org/10.1016/j.pedhc.2016.07.008.
- Francis AJ, Dempster RJ. Effect of valerian, Valeriana edulis, on sleep difficulties in children with intellectual deficits: randomised trial. Phytomedicine 2002;9:273-9. https://doi.org/10.1078/0944-7113-00110.
- Piazza CC, Fisher WW, Sherer M. Treatment of multiple sleep problems in children with developmental disabilities: faded bedtime with response cost versus bedtime scheduling. Dev Med Child Neurol 1997;39:414-18.
- Guilleminault C, McCann CC, Quera-Salva M, Cetel M. Light therapy as treatment of dyschronosis in brain impaired children. Eur J Pediatr 1993;152:754-9. https://doi.org/10.1007/BF01953995.
- Oriel KN, Kanupka JW, DeLong KS, Noel K. The impact of aquatic exercise on sleep behaviors in children with autism spectrum disorder. Focus Autism Other Dev Disabil 2016;31:254-61. https://doi.org/10.1177/1088357614559212.
- Yehuda S, Rabinovitz-Shenkar S, Carasso RL. Effects of essential fatty acids in iron deficient and sleep-disturbed attention deficit hyperactivity disorder (ADHD) children. Eur J Clin Nutr 2011;65:1167-9. https://doi.org/10.1038/ejcn.2011.80.
- Yu H-B, Hong Y-Y. Acupuncture combined with ear point taping and pressing for sleep disturbance in 30 children with mental retardation. World J Acupunct Moxibustion 2012;22:9-12. https://doi.org/10.1016/S1003-5257(13)60020-3.
- Beresford B, Stuttard L, Clarke S, Maddison J, Beecham J. Managing Behaviour and Sleep Problems in Disabled Children: an Investigation into the Effectiveness and Costs of Parent-Training Interventions. London: Department of Health; 2012.
- Beresford B, Stuttard L, Clarke S, Maddison J, Beecham J. Managing Behaviour and Sleep Problems in Disabled Children: an Investigation into the Effectiveness and Costs of Parent-Training Interventions. London: Department of Health; 2012.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Tomás Vila M, Aleu Pérez-Gramunt M, Beseler Soto B, Benac Prefasi M, Pantoja Martínez J, Pitarch Castellano I. Methylphenidate and sleep: results of a multicentre study on a population of children with attention deficit hyperactivity disorder. An Pediatr 2010;73:78-83. https://doi.org/10.1016/j.anpedi.2010.05.013.
- Dekker M, Nunn R, Koot H. Psychometric properties of the revised Developmental Behaviour Checklist scales in Dutch children with intellectual disability. J Intellect Disabil Res 2002;46:61-75. https://doi.org/10.1046/j.1365-2788.2002.00353.x.
- Zill N, Peterson J. Behaviour Problems Index. Washington, DC: Child Trends Inc.; 1990.
- Johnston C, Marsh EJ. A measure of parenting satisfaction and efficacy. J Clinical Child Psychol 1989;18:167-75. https://doi.org/10.1207/s15374424jccp1802_8.
- O’Dell SL, Tarler-Benlolo L, Flynn JM. An instrument to measure knowledge of behavioural principles as applied to children. J Behaviour Ther Exp Psychol 1979;10:29-34. https://doi.org/10.1016/0005-7916(79)90033-8.
- Malow BA, Crowe C, Henderson L, McGrew SG, Wang L, Song Y, et al. A sleep habits questionnaire for children with autism spectrum disorders. J Child Neurol 2009;24:19-24. https://doi.org/10.1177/0883073808321044.
- Manual for the Child Behaviour Checklist/ 4-18 and 1991 Profile. Burlington, VT: Department of Psychiatry, University of Vermont; 1991.
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord 2007;37:855-66. https://doi.org/10.1007/s10803-006-0213-z.
- Schroeder SR, Rojahn J, An X, Mayo-Ortega L, Oyama-Ganiko R, Leblanc J. The Parental Concerns Questionnaire: a brief screening instrument for potentially severe behavior problems in infants and toddlers at-risk for developmental delays. J Dev Phys Disabil 2014;26:237-47. https://doi.org/10.1007/s10882-013-9359-8.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. https://doi.org/10.1097/00005650-200108000-00006.
- De Diana I. Two stochastic sleep quality scales for self-rating of subject’s sleep. Sleep Res 1976;5.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-21. https://doi.org/10.1016/0165-1781(89)90047-4.
- Abidin RR. Parenting Stress Index – Short Form. Charlottesville, VA: Pediatric Psychology Press; 1990.
- Rutter M, Tizard J, Whitmore K. Education, Health and Behaviour. London: Longman Publishing Group; 1970.
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the aberrant behavior checklist. Am J Ment Defic 1985;89:492-50.
- Goodday A, Corkum P, Smith IM. Parental acceptance of treatments for insomnia in children with attention-deficit/hyperactivity disorder, autistic spectrum disorder, and their typically developing peers. Child Health Care 2014;43:54-71. https://doi.org/10.1080/02739615.2014.850879.
- Keenan RA, Wild MR, McArthur I, Espie CA. Children with developmental disabilities and sleep problems: parental beliefs and treatment acceptability. J Appl Res Intellect Disabil 2007;20:455-65. https://doi.org/10.1111/j.1468-3148.2007.00382.x.
- Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Martin BC, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. JAMA 2015;313:1037-47. https://doi.org/10.1001/jama.2015.1629.
- Thorpy MJ. Classification of sleep disorders. Neurotherapeutics 2012;9:687-701. https://doi.org/10.1007/s13311-012-0145-6.
- Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol 2014;39:932-48. https://doi.org/10.1093/jpepsy/jsu041.
- Adachi Y, Sato C, Nishino N, Ohryoji F, Hayama J, Yamagami T. A brief parental education for shaping sleep habits in 4-month-old infants. Clin Med Res 2009;7:85-92. https://doi.org/10.3121/cmr.2009.814.
- Adair R, Zuckerman B, Bauchner H, Philipp B, Levenson S. Reducing night waking in infancy: a primary care intervention. Pediatrics 1992;89:585-8.
- Eckerberg B. Treatment of sleep problems in families with small children: is written information enough?. Acta Paediatr 2002;91:952-9. https://doi.org/10.1111/j.1651-2227.2002.tb02861.x.
- Mindell JA, Du Mond C, Sadeh A, Telofski LS, Kulkarni N, Gunn E. Efficacy of an internet-based intervention for infant and toddler sleep disturbances. Sleep 2011;34:451-8. https://doi.org/10.1093/sleep/34.4.451.
- Mindell JA, Telofski LS, Wiegand B, Kurtz E. A nightly bedtime routine: impact on sleep problems in young children and maternal mood. Sleep 2009;32:599-606. https://doi.org/10.1093/sleep/32.5.599.
- Moore BA, Friman PC, Fruzetti AE, MacAleese K. Brief report: evaluating the bedtime pass program for child resistance to bedtime – a randomized controlled trial. J Pediatr Psychol 2007;32:283-7. https://doi.org/10.1093/jpepsy/jsl025.
- Scott G, Richards MP. Night waking in infants: effects of providing advice and support for parents. J Child Psychol Psychiatr 1990;31:551-67. https://doi.org/10.1111/j.1469-7610.1990.tb00797.x.
- Seymour FW, Brock P, During M, Poole G. Reducing sleep disruptions in young children: evaluation of therapist-guided and written information approaches: a brief report. J Child Psychol Psychiatr 1989;30:913-18. https://doi.org/10.1111/j.1469-7610.1989.tb00293.x.
- Stremler R, Hodnett E, Kenton L, Lee K, Weiss ST, Weston J, et al. Effect of behavioural-educational intervention on sleep for primiparous women and their infants in early postpartum: multisite randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f1164.
- Wolfson A, Lacks P, Futterman A. Effects of parent training on infant sleeping patterns, parents’ stress, and perceived parental competence. J Consult Clin Psychol 1992;60:41-8. https://doi.org/10.1037/0022-006X.60.1.41.
- Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther 2011;49:379-88. https://doi.org/10.1016/j.brat.2011.03.008.
- Quach J, Hiscock H, Ukoumunne OC, Wake M. A brief sleep intervention improves outcomes in the school entry year: a randomized controlled trial. Pediatrics 2011;128:692-701. https://doi.org/10.1542/peds.2011-0409.
- Stores R, Stores G. Evaluation of brief group-administered instruction for parents to prevent or minimize sleep problems in young children with Down syndrome. J Appl Res Intellect Disabil 2004;17:61-70. https://doi.org/10.1111/j.1360-2322.2004.00174.x.
- Blunden S. Behavioural treatments to encourage solo sleeping in pre-school children: an alternative to controlled crying. J Child Health Care 2011;15:107-17. https://doi.org/10.1177/1367493510397623.
- Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev 2005;25:629-44. https://doi.org/10.1016/j.cpr.2005.04.007.
- Eckerberg B. Treatment of sleep problems in families with young children: effects of treatment on family well-being. Acta Paediatr 2004;93:126-34. https://doi.org/10.1111/j.1651-2227.2004.tb00686.x.
- Johnson CM, Lerner M. Amelioration of infant sleep disturbances: effects of scheduled awakenings by compliant parents. Infant Mental Health J 1985;6:21-30. https://doi.org/10.1002/1097-0355(198521)6:1<21::AID-IMHJ2280060105>3.0.CO;2-Q.
- Leeson R, Barbour J, Romaniuk D, Warr R. Management of infant sleep problems in a residential unit. Child Care Health Dev 1994;20:89-100. https://doi.org/10.1111/j.1365-2214.1994.tb00856.x.
- Pritchard AA, Appleton P. Management of sleep problems in preschool children: effects of a behavioural programme on sleep routines, maternal depression and perceived control. Early Child Dev Care 1988;34:227-40. https://doi.org/10.1080/0300443880340117.
- Sadeh A. Assessment of intervention for infant night waking: parental reports and activity-based home monitoring. J Consult Clin Psychol 1994;62:63-8. https://doi.org/10.1037/0022-006X.62.1.63.
- Schlarb AA, Brandhorst I, Hautzinger M. Mini-KiSS – A multimodal group therapy intervention for parents of young children with sleep disorders: a pilot study. Z Kinder Jugendpsychiatr Psychother 2011;39:197-206. https://doi.org/10.1024/1422-4917/a000106.
- Schlarb AA, Brandhorst I. Mini-KiSS Online: an internet-based intervention program for parents of young children with sleep problems – influence on parental behavior and children’s sleep. Nat Sci Sleep 2012;4:41-52. https://doi.org/10.2147/NSS.S28337.
- Skuladottir A, Thome M. Changes in infant sleep problems after a family-centered intervention. Pediatric Nursing 2003;29:375-8.
- Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Studies 2005;42:843-50. https://doi.org/10.1016/j.ijnurstu.2004.12.004.
- Beebe DW. A brief primer on sleep for pediatric and child clinical neuropsychologists. Child Neuropsychol 2012;18:313-38. https://doi.org/10.1080/09297049.2011.602014.
- Goldman S, Malow B, Kothare S, Kotagal S. Sleep in Childhood Neurological disorders. New York, NY: Demos Medical Publishing; 2011.
- Jan JE, Owens JA, Weiss MD, Johnson KP, Wasdell MB, Freeman RD, et al. Sleep hygiene for children with neurodevelopmental disabilities. Pediatrics 2008;122:1343-50. https://doi.org/10.1542/peds.2007-3308.
- Owens J. The ADHD and sleep conundrum: a review. J Dev Behav Pediatr 2005;26:312-22. https://doi.org/10.1097/00004703-200508000-00011.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- De Silva MJ, Breuer E, Lee L, Asher L, Chowdhary N, Lund C, et al. Theory of Change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-267.
- Boutron I, Moher D, Altman D, Schulz K, Ravaud P. Extending the CONSORT statement to randomised trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-310. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Hoffmann TC, Erueti C, Glasziou PP. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ 2013;347. https://doi.org/10.1136/bmj.f3755.
- Craig P, Rahm-Hallberg I, Britten N, Borglin G, Meyer G, Köpke S, et al. Erratum to: Researching complex interventions in health: the state of the art. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1416-4.
- McCleary N, Duncan EM, Stewart F, Francis JJ. Active ingredients are reported more often for pharmacologic than non-pharmacologic interventions: an illustrative review of reporting practices in titles and abstracts. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-146.
- Bandura A. Social Learning Theory. New York, NY: General Learning Press; 1971.
- Beresford B, Stuttard L, Clarke S, Maddison J, Beecham J. Managing Behaviour and Sleep Problems in Disabled Children: an Investigation into the Effectiveness and Costs of Parent-Training Interventions. London: Department of Health; 2012.
- Janssens A, Williams J, Tomlinson R, Logan S, Morris C. Health outcomes for children with neurodisability: what do professionals regard as primary targets?. Arch Dis Child 2014;99:927-32. https://doi.org/10.1136/archdischild-2013-305803.
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method for evaluating comprehensive community mental health programs. Community Ment Health J 1968;4:443-53. https://doi.org/10.1007/BF01530764.
- Turner-Stokes L, Williams H, Johnson J. Goal attainment scaling: does it provide added value as a person-centred measure for evaluation of outcome in neurorehabilitation following acquired brain injury?. J Rehabil Med 2009;41:528-35. https://doi.org/10.2340/16501977-0383.
- Hurn J, Kneebone I, Cropley M. Goal setting as an outcome measure: a systematic review. Clin Rehabil 2006;20:756-72. https://doi.org/10.1177/0269215506070793.
- Jan JE, Reiter RJ, Bax MC, Ribary U, Freeman RD, Wasdell MB. Long-term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatr Neurol 2010;14:380-90. https://doi.org/10.1016/j.ejpn.2010.05.001.
- Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340. https://doi.org/10.1136/bmj.c117.
- Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405-11. https://doi.org/10.1001/jama.2013.285063.
- Pellecchia M, Connell JE, Beidas RS, Xie M, Marcus SC, Mandell DS. Dismantling the active ingredients of an intervention for children with autism. J Autism Dev Disord 2015;45:2917-27. https://doi.org/10.1007/s10803-015-2455-0.
- Esbensen AJ, Beebe DW, Byars KC, Hoffman EK. Use of sleep evaluations and treatments in children with Down syndrome. J Dev Behav Pediatr 2016;37:629-36. https://doi.org/10.1097/DBP.0000000000000333.
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18. https://doi.org/10.1186/s13063-017-1978-4.
- O’Connell A. Sleepwise: A Resource Manual: Positive Sleep Practices for Young Children with Developmental Delay. South Australia: Intellectual Disability Services Council (South Australia), Early Childhood Service; 2005.
- Sleep Solutions n.d. www.scope.org.uk/support/services-directory/sleep-solutions-training-for-families (accessed 26 July 2018).
- Piazza CC, Fisher W. A faded bedtime with response cost protocol for treatment of multiple sleep problems in children. J Appl Behav Anal 1991;24:129-40. https://doi.org/10.1901/jaba.1991.24-129.
- Griffin M, Hudson A. Parents as Therapists: the Behavioural Approach. Melbourne: PIT Press; 1978.
Appendix 1 Search strategies
Interface: Applied Social Sciences Index and Abstracts via ProQuest
Date range searched: no restriction.
Search date: 7 February 2016.
Records identified: 153.
Search strategy
((((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initial* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initial* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initial* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleepwalk*” OR “nighthawk*” .) OR (sleepwalk* OR sleepwalk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) AND ((SU.EXACT(“Children”) OR SU.EXACT(“adolescentce”) OR SU.EXACT(“Infants”)) OR (adolescent* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infancy* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”))) AND (((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperges* OR “cerebral palsy”) OR (“conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disability* OR delay*)) OR neurodisability* OR (neurodevelopment* NEAR/3 (delay* OR disability* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disability* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disability* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disability* OR disease* OR disorder* OR dysfunction)))
Interface: Cumulative Index to Nursing & Allied Health via EBSCOhost
Date range searched: no restriction.
Search date: 8 February 2016.
Records identified: 1157.
Search strategy
| Search terms | Search options | Results |
|---|---|---|
| S37 | S20 AND S36 | 1157 |
| S36 | S34 OR S35 | 66,730 |
| S35 | ( developmental N2 (disabilit* or delay*) ) OR neurodisabilit* OR ( neurodevelopment* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) OR ( neuromotor* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) OR ( neuropsychiatric* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) OR ( neuropsychol* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) | 10,885 |
| S34 | ( ADHD or (attention deficit) ) OR (angelman syndrome) OR ( autism or autistic or asperger* ) OR (cerebral palsy) OR (conduct disorder*) OR ( epilepsy or epileptic ) OR (Down* syndrome) OR (Fragile x syndrome) OR (Prader Willi Syndrome) OR (Rett syndrome) OR ( Smith-Magenis syndrome) OR (Williams syndrome) | 58,518 |
| S33 | (MH “Williams Syndrome”) | 374 |
| S32 | (MH “Smith-Magenis Syndrome”) | 41 |
| S31 | (MH “Rett Syndrome”) | 374 |
| S30 | (MH “Prader-Willi Syndrome”) | 493 |
| S29 | (MH “Fragile X Syndrome”) | 673 |
| S28 | (MH “Down Syndrome”) | 5123 |
| S27 | (MH “Cerebral Palsy”) | 8165 |
| S26 | (MH “Epilepsy+”) | 11,511 |
| S25 | (MH “Child Behavior Disorders”) | 6418 |
| S24 | (MH “Attention Deficit Hyperactivity Disorder”) | 10,817 |
| S23 | (MH “Angelman Syndrome”) | 136 |
| S22 | (MH “Developmental Disabilities”) | 6552 |
| S21 | (MH “Child Development Disorders”) OR (MH “Child Development Disorders, Pervasive”) | 3027 |
| S20 | S14 AND S19 | 9983 |
| S19 | S15 OR S16 OR S17 OR S18 | 793,040 |
| S18 | (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or young people or young person*) | 793,040 |
| S17 | (MH “Adolescence”) | 350,551 |
| S16 | (MH “Infant”) | 118,046 |
| S15 | (MH “Child”) | 313,998 |
| S14 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 | 41,776 |
| S13 | narcolepsy or nocturnal hyperkinesia | 992 |
| S12 | ( (sleepless* or insomnia* or parasomnia* or night terror* or nightterror* or night mare* or nightmare*) ) OR ( sleep-wak* or night-wak* ) OR ( sleepwalk* or sleep-walk* or sleep walk* or somnambulism ) | 9309 |
| S11 | ( (sleep* N3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*) ) OR ( (sleep* N3 (dysfunction* or disorder or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( sleep* N3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*) ) | 17,544 |
| S10 | ( (nocturnal* N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( (nocturnal* N3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*) ) | 366 |
| S9 | ( (night* N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( (night* N3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*) ) | 996 |
| S8 | ( (bed time*) N3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*) ) OR ( (bedtime*) N3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*) ) | 94 |
| S7 | ( (bed time*) N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( (bedtime*) N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) | 66 |
| S6 | (MH “Dreams”) | 1111 |
| S5 | (MH “Parasomnias”) | 662 |
| S4 | (MH “Narcolepsy”) | 826 |
| S3 | (MH “Somnambulism”) | 151 |
| S2 | (MH “Sleep+”) | 16,169 |
| S1 | (MH “Sleep Disorders+”) | 26,313 |
Interface: The Cochrane Central Register of Controlled Trials via Wiley Online Library
Date range searched: no restriction.
Search date: 8 February 2016.
Records identified: 458.
Search strategy
#1 MeSH descriptor: [Sleep Wake Disorders] explode all trees
#2 MeSH descriptor: [Sleep] explode all trees
#3 MeSH descriptor: [Somnambulism] explode all trees
#4 MeSH descriptor: [Narcolepsy] explode all trees
#5 sleep* near/3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*):ti,ab,kw or sleepless* or insomnia* or parasomnia* or “night terror*” or nightterror* or “night mare*” or nightmare*:ti,ab,kw or “sleep-wak*” or “night-wak*”:ti,ab,kw or “sleep-wak*” or “night-wak*” or sleepwalk* or “sleep-walk*” or “sleep walk*” or somnambulism:ti,ab,kw or narcolepsy or “nocturnal hyperkinesia”:ti,ab,kw (Word variations have been searched)
#6 nocturnal near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw or nocturnal near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw or sleep* near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw or sleep* near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw (Word variations have been searched)
#7 night* near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw or night* near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw (Word variations have been searched)
#8 bed* near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw and bed* near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw (Word variations have been searched)
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 MeSH descriptor: [Child] explode all trees
#11 MeSH descriptor: [Adolescent] explode all trees
#12 MeSH descriptor: [Infant] explode all trees
#13 adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or “young people” or “young person*”:ti,ab,kw (Word variations have been searched)
#14 #10 or #11 or #12 or #13
#15 #9 and #14
#16 MeSH descriptor: [Developmental Disabilities] explode all trees
#17 MeSH descriptor: [Child Development Disorders, Pervasive] explode all trees
#18 MeSH descriptor: [Angelman Syndrome] explode all trees
#19 MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] explode all trees
#20 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] explode all trees
#21 MeSH descriptor: [Conduct Disorder] explode all trees
#22 MeSH descriptor: [Epilepsy] explode all trees
#23 MeSH descriptor: [Cerebral Palsy] explode all trees
#24 MeSH descriptor: [Down Syndrome] explode all trees
#25 MeSH descriptor: [Fragile X Syndrome] explode all trees
#26 MeSH descriptor: [Prader-Willi Syndrome] explode all trees
#27 MeSH descriptor: [Rett Syndrome] explode all trees
#28 MeSH descriptor: [Smith-Magenis Syndrome] explode all trees
#29 MeSH descriptor: [Williams Syndrome] explode all trees
#30 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#31 ADHD or “attention deficit”:ti,ab,kw or “angelman syndrome”:ti,ab,kw or autism or autistic or asperger*:ti,ab,kw or “cerebral palsy”:ti,ab,kw or “conduct disorder*”:ti,ab,kw (Word variations have been searched)
#32 epilepsy or epileptic:ti,ab,kw or “Down* syndrome”:ti,ab,kw or “Fragile x syndrome”:ti,ab,kw or “Prader Willi Syndrome”:ti,ab,kw or “Rett syndrome”:ti,ab,kw (Word variations have been searched)
#33 “Smith-Magenis syndrome”:ti,ab,kw or “Williams syndrome”:ti,ab,kw (Word variations have been searched)
#34 developmental near/2 (disabilit* or delay*):ti,ab,kw or neurodisabilit*:ti,ab,kw or neurodevelopment* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw or neuromotor* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw (Word variations have been searched)
#35 neuropsychiatric* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw or neuropsychol* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw (Word variations have been searched)
#36 #31 or #32 or #33 or #34 or #35
#37 #30 or #36
#38 #15 and #37
Interface: Cochrane Database of Systematic Reviews via Wiley Online Library
Date range searched: no restriction.
Search date: 8 February 2016.
Records identified: 8.
Search strategy: the Cochrane Database of Systematic Reviews strategy was the same as the CENTRAL strategy above.
Interface: Conference Proceedings Citation Index via Web of Science
Date range searched: no restriction.
Search date: 9 February 2016.
Records identified: 262.
Search strategy
| # | Results | Search options |
|---|---|---|
| #13 | 262 |
#12 AND #9 Indexes=CPCI-S Timespan=All years |
| #12 | 28,755 |
#11 OR #10 Indexes=CPCI-S Timespan=All years |
| #11 | 2014 |
TOPIC: (developmental NEAR/2 (disabilit* or delay*)) OR TOPIC: (neurodisabilit*) OR TOPIC: (neurodevelopment* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuromotor* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychiatric* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychol* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) Indexes=CPCI-S Timespan=All years |
| #10 | 27,174 |
TOPIC: (ADHD or “attention deficit”) OR TOPIC: (“angelman syndrome”) OR TOPIC: (autism or autistic or asperger*) OR TOPIC: (“cerebral palsy”) OR TOPIC: (“conduct disorder*”) OR TOPIC: (epilepsy or epilepti) OR TOPIC: (“Down* syndrome”) OR TOPIC: (“Fragile x syndrome”) OR TOPIC: (“Prader Willi Syndrome”) OR TOPIC: (“Rett syndrome”) OR TOPIC: (“Smith-Magenis syndrome”) OR TOPIC: (“Williams syndrome”) Indexes=CPCI-S Timespan=All years |
| #9 | 1224 |
#8 AND #7 Indexes=CPCI-S Timespan=All years |
| #8 | 146,852 |
TOPIC: (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or “young people” or “young person*”) Indexes=CPCI-S Timespan=All years |
| #7 | 10,583 |
#6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=CPCI-S Timespan=All years |
| #6 | 4957 |
TS=(sleep* NEAR/3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*)) OR TS=(sleepless* or insomnia* or parasomnia* or “night terror*” or nightterror* or “night mare*” or nightmare*) OR TS=(sleep-wak* or night-wak*) OR TS=(sleepwalk* or sleep-walk* or “sleep walk*” or somnambulism) OR TS=(narcolepsy or “nocturnal hyperkinesia”) Indexes=CPCI-S Timespan=All years |
| #5 | 6374 |
TS=(nocturnal* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(nocturnal* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (dysfunction* or disorder or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) Indexes=CPCI-S Timespan=All years |
| #4 | 393 |
TS=(“bed time*” NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(bedtime* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) Indexes=CPCI-S Timespan=All years |
| #3 | 641 |
TS=(narcolepsy) Indexes=CPCI-S Timespan=All years |
| #2 | 20 |
TS=(somnambulism) Indexes=CPCI-S Timespan=All years |
| #1 | 3818 |
TS=(sleep disorder*) Indexes=CPCI-S Timespan=All years |
Interface: Database of Abstracts of Reviews of Effects via Wiley Online Library
Date range searched: no restriction.
Search date: 8 February 2016.
Records identified: 5.
Search strategy: the Database of Abstracts of Reviews of Effects strategy was the same as the CENTRAL strategy above.
Interface: EMBASE via Ovid
Date range searched: 1974 to 3 February 2016.
Search date: 4 February 2016.
Records identified: 10,288.
Search strategy
-
exp Sleep Disorder/ (162,095)
-
Sleep/ (81,239)
-
Sleep Walking/ (1490)
-
Narcolepsy/ (6862)
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (318)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (282)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (2451)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (3053)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1558)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1279)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (22,635)
-
(sleep$ adj3 (dysfunction$ or disorder or disorders or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (50,363)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (13,975)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (29,033)
-
(sleep-wak$ or night-wak$).ti,ab. (11 to 232)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (981)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (5221)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (242,035)
-
exp Infant/ or exp Child/ or exp Adolescent/ (2,934,245)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (2,021,633)
-
19 or 20 (3,492,441)
-
Childhood Disintegrative Disorder/ (128)
-
Developmental Disorder/ (28,670)
-
Asperger Syndrome/ (3660)
-
Attention Deficit Disorder/ or Attention Deficit Disorder with Hyperactivity/ or Attention Deficit Hyperactivity Disorder/ (43,642)
-
exp Autism/ (44,025)
-
Conduct Disorder/ (5234)
-
exp Epilepsy/ (194,464)
-
Cerebral Palsy/ (28,687)
-
Down Syndrome/ (29,198)
-
Fragile X Syndrome/ (6859)
-
Happy Puppet Syndrome/ (2139)
-
“Pervasive Developmental Disorder not otherwise specified”/ (790)
-
Prader-Willi Syndrome/ (4427)
-
Rett Syndrome/ (3903)
-
Smith Magenis Syndrome/ (480)
-
Williams Beuren Syndrome/ (2655)
-
(ADHD or attention deficit).ti,ab. (32,156)
-
angelman syndrome.ti,ab. (1370)
-
(autism or autistic or asperger$).ti,ab. (38,978)
-
cerebral palsy.ti,ab. (22,489)
-
conduct disorder$.ti,ab. (4762)
-
(epilepsy or epileptic).ti,ab. (137,265)
-
Down$ syndrome.ti,ab. (23,072)
-
Fragile x syndrome.ti,ab. (4278)
-
Prader Willi Syndrome.ti,ab. (3070)
-
Prader-Willi Syndrome.ti,ab. (3070)
-
Rett syndrome.ti,ab. (3072)
-
Smith-Magenis syndrome.ti,ab. (334)
-
Williams syndrome.ti,ab. (1688)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (18,243)
-
neurodisabilit$.ti,ab. (271)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (8726)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (20)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (428)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (11,567)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (2857)
-
23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 (415,764)
-
18 and 21 and 58 (10,326)
-
(animal/ or nonhuman/) not exp human/ (4,938,572)
-
59 not 60 (10,288)
Interface: Health Management Information Consortium via Ovid
Date range searched: 1979 to November 2015.
Search date: 8 February 2016.
Records identified: 10.
Search strategy
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (4)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (43)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (29)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (2)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (26)
-
(sleep$ adj3 (dysfunction$ or disorder or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (262)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (77)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (202)
-
(sleep-wak$ or night-wak$).ti,ab. (14)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (1)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (4)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (36 to 213)
-
(ADHD or attention deficit).ti,ab. (200)
-
angelman syndrome.ti,ab. (0)
-
(autism or autistic or asperger$).ti,ab. (546)
-
cerebral palsy.ti,ab. (144)
-
conduct disorder$.ti,ab. (85)
-
(epilepsy or epileptic).ti,ab. (372)
-
Down$ syndrome.ti,ab. (255)
-
Fragile x syndrome.ti,ab. (12)
-
Prader Willi Syndrome.ti,ab. (3)
-
Prader-Willi Syndrome.ti,ab. (3)
-
Rett syndrome.ti,ab. (5)
-
Smith-Magenis syndrome.ti,ab. (0)
-
Williams syndrome.ti,ab. (0)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (100)
-
neurodisabilit$.ti,ab. (13)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (31)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (24)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (7)
-
or/1-13 (559)
-
or/15-34 (1671)
-
14 and 35 and 36 (10)
Interface: MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) via Ovid
Date range searched: 1946 to present.
Search date: 4 February 2016.
Records identified: 4314.
Search strategy
-
exp Sleep Wake Disorders/ (67,333)
-
Sleep/ (41,262)
-
Somnambulism/ (561)
-
Narcolepsy/ (3034)
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (175)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (146)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1573)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1850)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1041)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (811)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (15,245)
-
(sleep$ adj3 (dysfunction$ or disorder or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (34,423)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (8810)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (17,408)
-
(sleep-wak$ or night-wak$).ti,ab. (7703)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (665)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (3478)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (125,134)
-
exp Infant/ or exp Child/ or Adolescent/ (3,027,976)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (1,603,721)
-
19 or 20 (3,390,703)
-
exp Child Development Disorders, Pervasive/ (23,521)
-
Developmental Disabilities/ (16,203)
-
Angelman Syndrome/ (996)
-
exp “attention deficit and disruptive behavior disorders”/ or attention deficit disorder with hyperactivity/ or asperger syndrome/ or autistic disorder/ (42,213)
-
Conduct Disorder/ (2623)
-
exp Epilepsy/ (138,055)
-
Cerebral Palsy/ (16,902)
-
Down Syndrome/ (21,645)
-
Fragile X Syndrome/ (4267)
-
Prader-Willi Syndrome/ (2411)
-
Rett Syndrome/ (1997)
-
Smith-Magenis Syndrome/ (107)
-
Williams Syndrome/ (1371)
-
(ADHD or attention deficit).ti,ab. (22,948)
-
angelman syndrome.ti,ab. (1106)
-
(autism or autistic or asperger$).ti,ab. (29,066)
-
cerebral palsy.ti,ab. (16,339)
-
conduct disorder$.ti,ab. (3664)
-
(epilepsy or epileptic).ti,ab. (92,832)
-
Down$ syndrome.ti,ab. (18,536)
-
Fragile x syndrome.ti,ab. (3622)
-
Prader Willi Syndrome.ti,ab. (2414)
-
Prader-Willi Syndrome.ti,ab. (2414)
-
Rett syndrome.ti,ab. (2494)
-
Smith-Magenis syndrome.ti,ab. (285)
-
Williams syndrome.ti,ab. (1375)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (13,041)
-
neurodisabilit$.ti,ab. (135)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (6427)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (11)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (304)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (8341)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (2022)
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 (303,873)
-
18 and 21 and 55 (4314)
Interface: PsycINFO via Ovid
Date range searched: 1806 to week 1 February 2016.
Search date: 4 February 2016.
Records identified: 1727.
Search strategy
-
exp Sleep Disorders/ (12,542)
-
Sleep/ (17,334)
-
Sleepwalking/ (384)
-
Narcolepsy/ (1251)
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (184)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (154)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (972)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1170)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (402)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (359)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (8609)
-
(sleep$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (17,897)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (5748)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (12,114)
-
(sleep-wak$ or night-wak$).ti,ab. (4320)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (749)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (1836)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (44,643)
-
(childhood birth 12 yrs or adolescence 13 17 yrs).ag. (657,693)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (760,813)
-
19 or 20 (950,005)
-
“3250”.cc. (35,406)
-
exp Pervasive Developmental Disorders/ (31,057)
-
exp Developmental Disabilities/ (12,448)
-
Neurodevelopmental Disorders/ (1374)
-
Attention Deficit Disorder/ (5012)
-
“Attention Deficit Disorder with Hyperactivity”/ (16,339)
-
exp Autism/ (23,157)
-
Aspergers Syndrome/ (2444)
-
Conduct Disorder/ (3750)
-
exp Epilepsy/ (22,293)
-
Cerebral Palsy/ (4098)
-
Down’s Syndrome/ (5404)
-
Fragile X Syndrome/ (1368)
-
Prader Willi Syndrome/ (454)
-
Rett Syndrome/ (694)
-
Williams Syndrome/ (848)
-
(ADHD or attention deficit).ti,ab. (26,445)
-
angelman syndrome.ti,ab. (269)
-
(autism or autistic or asperger$).ti,ab. (37,207)
-
cerebral palsy.ti,ab. (5354)
-
conduct disorder$.ti,ab. (6181)
-
(epilepsy or epileptic).ti,ab. (29,858)
-
Down$ syndrome.ti,ab. (6294)
-
Fragile x syndrome.ti,ab. (1495)
-
Prader Willi Syndrome.ti,ab. (568)
-
Prader-Willi Syndrome.ti,ab. (568)
-
Rett syndrome.ti,ab. (887)
-
Smith-Magenis syndrome.ti,ab. (75)
-
Williams syndrome.ti,ab. (990)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (10,810)
-
neurodisabilit$.ti,ab. (64)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (3603)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (5)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (115)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (5090)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (2035)
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 (135,560)
-
18 and 21 and 58 (1727)
Interface: Science Citation Index via Web of Science
Date range searched: no restriction.
Search date: 8 February 2016.
Records identified: 2831.
Search strategy
| # | Results | Search options |
|---|---|---|
| #13 | 2831 |
#12 AND #9 Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #12 | 225,596 |
#11 OR #10 Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #11 | 31,246 |
TOPIC: (developmental NEAR/2 (disabilit* or delay*)) OR TOPIC: (neurodisabilit*) OR TOPIC: (neurodevelopment* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuromotor* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC:(neuropsychiatric* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychol* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #10 | 203,758 |
TOPIC: (ADHD or “attention deficit”) OR TOPIC: (“angelman syndrome”) OR TOPIC: (autism or autistic or asperger*) OR TOPIC: (“cerebral palsy”)OR TOPIC: (“conduct disorder*”) OR TOPIC: (epilepsy or epilepti) OR TOPIC: (“Down* syndrome”) OR TOPIC: (“Fragile x syndrome”) OR TOPIC:(“Prader Willi Syndrome”) OR TOPIC: (“Rett syndrome”) OR TOPIC: (“Smith-Magenis syndrome”) OR TOPIC: (“Williams syndrome”) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #9 | 12,415 |
#8 AND #7 Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #8 | 1,497,016 |
TOPIC: (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or “young people” or “young person*”) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #7 | 76,127 |
#6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #6 | 36,633 |
TS=(sleep* NEAR/3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*)) OR TS=(sleepless* or insomnia* or parasomnia* or “night terror*” or nightterror* or “night mare*” or nightmare*) OR TS=(sleep-wak* or night-wak*) OR TS=(sleepwalk* or sleep-walk* or “sleep walk*” or somnambulism) OR TS=(narcolepsy or “nocturnal hyperkinesia”) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #5 | 47,890 |
TS=(nocturnal* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(nocturnal* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (dysfunction* or disorder or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #4 | 3852 |
TS=(“bed time*” NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(bedtime* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #3 | 4917 |
TS=(narcolepsy) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #2 | 297 |
TS=(somnambulism) Indexes=SCI-EXPANDED Timespan=1900-2016 |
| #1 | 33,513 |
TS=(sleep disorder*) Indexes=SCI-EXPANDED Timespan=All years |
Interface: Social Care Online via www.scie-socialcareonline.org.uk
Date range searched: no restriction.
Search date: 4 February 2016.
Records identified: 35.
Search strategy
Interface: Social Policy & Practice via Ovid
Date range searched: no restriction.
Search date: 8 February 2016.
Records identified: 48.
Search strategy
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (2)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (12)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (33)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (44)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (4)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (7)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (67)
-
(sleep$ adj3 (dysfunction$ or disorder or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (527)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (260)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (258)
-
(sleep-wak$ or night-wak$).ti,ab. (41)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (13)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (5)
-
(ADHD or attention deficit).ti,ab. (941)
-
angelman syndrome.ti,ab. (8)
-
(autism or autistic or asperger$).ti,ab. (2638)
-
cerebral palsy.ti,ab. (344)
-
conduct disorder$.ti,ab. (431)
-
(epilepsy or epileptic).ti,ab. (292)
-
Down$ syndrome.ti,ab. (509)
-
Fragile x syndrome.ti,ab. (49)
-
Prader Willi Syndrome.ti,ab. (32)
-
Prader-Willi Syndrome.ti,ab. (32)
-
Rett syndrome.ti,ab. (20)
-
Smith-Magenis syndrome.ti,ab. (3)
-
Williams syndrome.ti,ab. (12)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (590)
-
neurodisabilit$.ti,ab. (19)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (64)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (32)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (12)
-
or/1-13 (993)
-
or/14-33 (5507)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (139,979)
-
34 and 35 and 36 (48)
Trial registers
In addition to the searches of the bibliographic databases, searches of the following trials registers were carried out: ClinicalTrials.gov, WHO International Clinical Trials Registry Platform and the UK Clinical Trials Gateway.
ClinicalTrials.gov via https://clinicaltrials.gov
This resource was searched on 9 February 2016 using a number of small focused search strategies. The results (103 records) were loaded into bibliographic software and, after deduplication, there were a total of 70 records. The strategies and numbers identified are given below:
-
Sleep disorders & children & angelman (3)
-
Sleep disorders & children & attention deficit/ADHD (13)
-
Sleep disorders & children & autism (16)
-
Sleep disorders & children & conduct disorder (46)
-
Sleep disorders & children & epilepsy (10)
-
Sleep disorders & children & down syndrome (5)
-
Sleep disorders & children & cerebral palsy (1)
-
Sleep disorders & children & fragile x syndrome (0)
-
Sleep disorders & children & prader willi syndrome (2)
-
Sleep disorders & children & Rett syndrome (1)
-
Sleep disorders & children & Smith-Magenis syndrome (6)
-
Sleep disorders & children & Williams syndrome (0)
The World Health Organization International Clinical Trials Registry Platform via http://apps.who.int/trialsearch/
This resource was searched on 9 February 2016 using the search terms ‘sleep AND children’ and 114 records for 108 trials were identified.
UK Clinical Trials Gateway via www.ukctg.nihr.ac.uk
This resource was searched on 9 February 2016 using the search phrase ‘sleep disorders and children’ and 16 trials were identified.
Appendix 2 Additional searches using terms not included in original search strategies
Interface: Applied Social Sciences Index and Abstracts via Proquest
Date range searched: no restriction.
Search date: 17 March 2016.
Records identified: 154.
Search strategy
| Set number | Searched for | Databases | Results |
|---|---|---|---|
| S1 | ((((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleep-wak*” OR “night-wak*” .) OR (sleepwalk* OR sleep-walk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) AND ((SU.EXACT(“Children”) OR SU.EXACT(“Adolescence”) OR SU.EXACT(“Infants”)) OR (adolescen* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infanc* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”))) AND (((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperger* OR “cerebral palsy”) OR ( “conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disabilit* OR delay*)) OR neurodisabilit* OR (neurodevelopment* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction))) | ASSIA | 154 |
| S2 | (((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleep-wak*” OR “night-wak*” .) OR (sleepwalk* OR sleep-walk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) | ASSIA | 2877 |
| S3 | ((SU.EXACT(“Children”) OR SU.EXACT(“Adolescence”) OR SU.EXACT(“Infants”)) OR (adolescen* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infanc* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”)) | ASSIA | 151,572 |
| S4 | (((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperger* OR “cerebral palsy”) OR ( “conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disabilit* OR delay*)) OR neurodisabilit* OR (neurodevelopment* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction))) | ASSIA | 17,586 |
| S6 | (intellectual* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR (mental* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR (learning NEAR/2 (disability or disabled or difficult*)) | ASSIA | 11,198 |
| S7 | (((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleep-wak*” OR “night-wak*” .) OR (sleepwalk* OR sleep-walk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) AND ((SU.EXACT(“Children”) OR SU.EXACT(“Adolescence”) OR SU.EXACT(“Infants”)) OR (adolescen* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infanc* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”)) AND (((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperger* OR “cerebral palsy”) OR ( “conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disabilit* OR delay*)) OR neurodisabilit* OR (neurodevelopment* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction))) |
ASSIA These databases are searched for part of your query |
154 |
| S8 | (((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleep-wak*” OR “night-wak*” .) OR (sleepwalk* OR sleep-walk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) AND ((SU.EXACT(“Children”) OR SU.EXACT(“Adolescence”) OR SU.EXACT(“Infants”)) OR (adolescen* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infanc* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”)) AND ((((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperger* OR “cerebral palsy”) OR ( “conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disabilit* OR delay*)) OR neurodisabilit* OR (neurodevelopment* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction))) OR ((intellectual* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR (mental* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR (learning NEAR/2 (disability or disabled or difficult*)))) |
ASSIA These databases are searched for part of your query |
174 |
| S9 | ((((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleep-wak*” OR “night-wak*” .) OR (sleepwalk* OR sleep-walk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) AND ((SU.EXACT(“Children”) OR SU.EXACT(“Adolescence”) OR SU.EXACT(“Infants”)) OR (adolescen* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infanc* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”)) AND ((((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperger* OR “cerebral palsy”) OR (“conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disabilit* OR delay*)) OR neurodisabilit* OR (neurodevelopment* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction))) OR ((intellectual* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR (mental* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR (learning NEAR/2 (disability or disabled or difficult*))))) NOT ((((SU.EXACT(“Sleep disorders”) OR SU.EXACT(“Sleep problems”)) OR SU.EXACT(“Narcolepsy”)) OR ((“bed time*” NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (bedtime* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (“bed time*” NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*))) OR ((night* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (night* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (nocturnal NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (nocturnal NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*))) OR ((sleep* NEAR/3 (dysfunction* OR disorder* OR difficult* OR disrupt* OR disturb* OR delay* OR problem*)) OR (sleep* NEAR/3 (settle* OR settling OR wake* OR awake OR wakeful* OR waking* OR awaking* OR awakening* OR wakening*)) OR (sleep* NEAR/3 (initiat* OR pattern* OR routine* OR practice* OR maintain* OR intervention* OR schedule*)) OR (sleepless* OR insomnia* OR parasomnia* OR “night terror*” OR nightterror* OR “night mare*” OR nightmare*) OR (“sleep-wak*” OR “night-wak*” .) OR (sleepwalk* OR sleep-walk* OR “sleep walk*” OR somnambulism) OR (narcolepsy OR “nocturnal hyperkinesia”))) AND ((SU.EXACT(“Children”) OR SU.EXACT(“Adolescence”) OR SU.EXACT(“Infants”)) OR (adolescen* OR baby OR babies OR child OR children OR boy OR boys OR girl OR girls OR infant* OR infanc* OR juvenile* OR paediatric OR pediatric OR preschooler* OR schoolboy* OR schoolgirl* OR schoolchild* OR teens OR teenager* OR toddler* OR youth OR youths OR “young people” OR “young person*”)) AND (((SU.EXACT(“Developmentally disabled children”) OR SU.EXACT(“Developmentally delayed children”)) OR (SU.EXACT(“Developmental delays”) OR SU.EXACT(“Developmental disorders”)) OR SU.EXACT(“Angelman syndrome”) OR (SU.EXACT(“Attention deficit disorder”) OR SU.EXACT(“Attention deficit hyperactivity disorder”)) OR SU.EXACT(“Conduct disorders”) OR SU.EXACT(“Complex partial seizure disorder” OR “Epilepsy” OR “Idiopathic childhood epilepsy” OR “Landau-Kleffner syndrome” OR “Panayiotopoulos syndrome” OR “Temporal lobe epilepsy”) OR SU.EXACT(“Cerebral palsy”) OR SU.EXACT(“Down’s syndrome”) OR SU.EXACT(“Fragile X syndrome”) OR (SU.EXACT(“Prader - Willi syndrome”) OR SU.EXACT(“Prader-Willi syndrome”))) OR (SU.EXACT(“Rett syndrome”) OR SU.EXACT(“Smith-Magenis syndrome”) OR (SU.EXACT(“Williams-Beuren syndrome”) OR SU.EXACT(“Williams’ syndrome”)) OR (ADHD or “attention deficit” OR “angelman syndrome”) OR (autism or autistic or asperger* OR “cerebral palsy”) OR ( “conduct disorder*” OR epilepsy or epileptic) OR (“Down* syndrome” OR “Fragile x syndrome”) OR (“Prader Willi Syndrome” OR “Prader-Willi Syndrome”) OR (“Rett syndrome” OR “Williams syndrome”)) OR (developmental NEAR/2 (disabilit* OR delay*)) OR neurodisabilit* OR (neurodevelopment* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuromotor* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychiatr* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)) OR (neuropsychol* NEAR/3 (delay* OR disabilit* OR disease* OR disorder* OR dysfunction)))) | ASSIA | 20 |
Interface: The Cochrane Central Register of Controlled Trials via The Cochrane Library
Date range searched: no restriction.
Search date: 17 March 2016.
Records identified: 15.
Search strategy
#1 MeSH descriptor: [Sleep Wake Disorders] explode all trees
#2 MeSH descriptor: [Sleep] explode all trees
#3 MeSH descriptor: [Somnambulism] explode all trees
#4 MeSH descriptor: [Narcolepsy] explode all trees
#5 sleep* near/3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*):ti,ab,kw or sleepless* or insomnia* or parasomnia* or “night terror*” or nightterror* or “night mare*” or nightmare*:ti,ab,kw or “sleep-wak*” or “night-wak*”:ti,ab,kw or “sleep-wak*” or “night-wak*” or sleepwalk* or “sleep-walk*” or “sleep walk*” or somnambulism:ti,ab,kw or narcolepsy or “nocturnal hyperkinesia”:ti,ab,kw (Word variations have been searched)
#6 nocturnal near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw or nocturnal near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw or sleep* near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw or sleep* near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw (Word variations have been searched)
#7 night* near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw or night* near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw (Word variations have been searched)
#8 bed* near/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*):ti,ab,kw and bed* near/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*):ti,ab,kw (Word variations have been searched)
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 MeSH descriptor: [Child] explode all trees
#11 MeSH descriptor: [Adolescent] explode all trees
#12 MeSH descriptor: [Infant] explode all trees
#13 adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or “young people” or “young person*”:ti,ab,kw (Word variations have been searched)
#14 #10 or #11 or #12 or #13
#15 #9 and #14
#16 MeSH descriptor: [Developmental Disabilities] explode all trees
#17 MeSH descriptor: [Child Development Disorders, Pervasive] explode all trees
#18 MeSH descriptor: [Angelman Syndrome] explode all trees
#19 MeSH descriptor: [Attention Deficit Disorder with Hyperactivity] explode all trees
#20 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] explode all trees
#21 MeSH descriptor: [Conduct Disorder] explode all trees
#22 MeSH descriptor: [Epilepsy] explode all trees
#23 MeSH descriptor: [Cerebral Palsy] explode all trees
#24 MeSH descriptor: [Down Syndrome] explode all trees
#25 MeSH descriptor: [Fragile X Syndrome] explode all trees
#26 MeSH descriptor: [Prader-Willi Syndrome] explode all trees
#27 MeSH descriptor: [Rett Syndrome] explode all trees
#28 MeSH descriptor: [Smith-Magenis Syndrome] explode all trees
#29 MeSH descriptor: [Williams Syndrome] explode all trees
#30 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#31 ADHD or “attention deficit”:ti,ab,kw or “angelman syndrome”:ti,ab,kw or autism or autistic or asperger*:ti,ab,kw or “cerebral palsy”:ti,ab,kw or “conduct disorder*”:ti,ab,kw (Word variations have been searched)
#32 epilepsy or epileptic:ti,ab,kw or “Down* syndrome”:ti,ab,kw or “Fragile x syndrome”:ti,ab,kw or “Prader Willi Syndrome”:ti,ab,kw or “Rett syndrome”:ti,ab,kw (Word variations have been searched)
#33 “Smith-Magenis syndrome”:ti,ab,kw or “Williams syndrome”:ti,ab,kw (Word variations have been searched)
#34 developmental near/2 (disabilit* or delay*):ti,ab,kw or neurodisabilit*:ti,ab,kw or neurodevelopment* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw or neuromotor* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw (Word variations have been searched)
#35 neuropsychiatric* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw or neuropsychol* near/3 (delay* or disabilit* or disease* or disorder* or dysfunction*):ti,ab,kw (Word variations have been searched)
#36 #31 or #32 or #33 or #34 or #35
#37 #30 or #36
#38 #15 and #37
#39 MeSH descriptor: [Intellectual Disability] explode all trees
#40 intellectual* near/2 (disabilit* or disabled or deficit* or handicap* or retard*):ti,ab,kw (Word variations have been searched)
#41 (mental$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$))
#42 (learning adj (disability or disabled or difficult$))
#43 #39 or #40 or #41 or #42
#44 #37 or #43
#45 #9 and #14 and #44
#46 #45 not #38
Interface: Cumulative Index to Nursing & Allied Health via EBSCOhost
Date range searched: no restriction.
Search date: 17 March 2016.
Records identified: 53.
Search strategy
| Search identification number | Search terms | Results |
|---|---|---|
| S45 | s44 NOT s42 | 53 |
| S44 | S14 AND S19 AND S43 | 1375 |
| S43 | S38 OR S39 OR S41 | 96,086 |
| S42 | S14 AND S19 AND S41 | 1322 |
| S41 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 | 74,324 |
| S40 | S38 OR S39 | 33,365 |
| S39 | (MH “Intellectual Disability+”) | 22,711 |
| S38 | ( intellectual* N2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR ( mental* N2 (disabilit* or disabled or deficit* or handicap* or retard*) ) OR ( learning N2 (disabilit* or disabled or difficult*) ) | 26,110 |
| S37 | S20 AND S36 | Rerun |
| S36 | S34 OR S35 | Rerun |
| S35 | ( developmental N2 (disabilit* or delay*) ) OR neurodisabilit* OR ( neurodevelopment* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) OR ( neuromotor* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) OR ( neuropsychiatric* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) OR ( neuropsychol* N3 (delay* or disabilit* or disease* or disorder* or dysfunction*) ) | Rerun |
| S34 | ( ADHD or (attention deficit) ) OR (angelman syndrome) OR ( autism or autistic or asperger* ) OR (cerebral palsy) OR (conduct disorder*) OR ( epilepsy or epileptic ) OR (Down* syndrome) OR (Fragile x syndrome) OR (Prader Willi Syndrome) OR (Rett syndrome) OR ( Smith-Magenis syndrome) OR (Williams syndrome) | Rerun |
| S33 | (MH “Williams Syndrome”) | Rerun |
| S32 | (MH “Smith-Magenis Syndrome”) | Rerun |
| S31 | (MH “Rett Syndrome”) | Rerun |
| S30 | (MH “Prader-Willi Syndrome”) | Rerun |
| S29 | (MH “Fragile X Syndrome”) | Rerun |
| S28 | (MH “Down Syndrome”) | Rerun |
| S27 | (MH “Cerebral Palsy”) | Rerun |
| S26 | (MH “Epilepsy+”) | Rerun |
| S25 | (MH “Child Behavior Disorders”) | Rerun |
| S24 | (MH “Attention Deficit Hyperactivity Disorder”) | Rerun |
| S23 | (MH “Angelman Syndrome”) | Rerun |
| S22 | (MH “Developmental Disabilities”) | Rerun |
| S21 | (MH “Child Development Disorders”) OR (MH “Child Development Disorders, Pervasive”) | Rerun |
| S20 | S14 AND S19 | Rerun |
| S19 | S15 OR S16 OR S17 OR S18 | Rerun |
| S18 | (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or young people or young person*) | Rerun |
| S17 | (MH “Adolescence”) | Rerun |
| S16 | (MH “Infant”) | Rerun |
| S15 | (MH “Child”) | Rerun |
| S14 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 | Rerun |
| S13 | narcolepsy or nocturnal hyperkinesia | Rerun |
| S12 | ( (sleepless* or insomnia* or parasomnia* or night terror* or nightterror* or night mare* or nightmare*) ) OR ( sleep-wak* or night-wak* ) OR ( sleepwalk* or sleep-walk* or sleep walk* or somnambulism ) | Rerun |
| S11 | ( (sleep* N3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*) ) OR ( (sleep* N3 (dysfunction* or disorder or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( sleep* N3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*) ) | Rerun |
| S10 | ( (nocturnal* N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( (nocturnal* N3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*) ) | Rerun |
| S9 | ( (night* N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( (night* N3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*) ) | Rerun |
| S8 | ( (bed time*) N3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*) ) OR ( (bedtime*) N3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*) ) | Rerun |
| S7 | ( (bed time*) N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) OR ( (bedtime*) N3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*) ) | Rerun |
| S6 | (MH “Dreams”) | Rerun |
| S5 | (MH “Parasomnias”) | Rerun |
| S4 | (MH “Narcolepsy”) | Rerun |
| S3 | (MH “Somnambulism”) | Rerun |
| S2 | (MH “Sleep+”) | Rerun |
| S1 | (MH “Sleep Disorders+”) | Rerun |
Interface: Conference Proceedings Citation Index via Web of Science
Date range searched: no restriction.
Search date: 17 March 2016.
Records identified: 12.
Search strategy
| # | Search options | Results |
|---|---|---|
| #17 |
#16 not #13 Indexes=CPCI-S Timespan=All years |
12 |
| #16 |
#15 AND #9 Indexes=CPCI-S Timespan=All years |
274 |
| #15 |
#14 OR #11 OR #10 Indexes=CPCI-S Timespan=All years |
31,261 |
| #14 |
TOPIC: (intellectual* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*)) OR TOPIC: (mental* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*)) OR TOPIC: (learning NEAR/2 (disability or disabled or difficult*)) Indexes=CPCI-S Timespan=All years |
3386 |
| #13 |
#12 AND #9 Indexes=CPCI-S Timespan=All years |
262 |
| #12 |
#11 OR #10 Indexes=CPCI-S Timespan=All years |
28,814 |
| #11 |
TOPIC: (developmental NEAR/2 (disabilit* or delay*)) OR TOPIC: (neurodisabilit*) OR TOPIC: (neurodevelopment* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuromotor* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychiatric* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychol* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) Indexes=CPCI-S Timespan=All years |
2019 |
| #10 |
TOPIC: (ADHD or “attention deficit”) OR TOPIC: (“angelman syndrome”) OR TOPIC: (autism or autistic or asperger*) OR TOPIC: (“cerebral palsy”) OR TOPIC: (“conduct disorder*”) OR TOPIC: (epilepsy or epilepti) OR TOPIC: (“Down* syndrome”) OR TOPIC: (“Fragile x syndrome”) OR TOPIC: (“Prader Willi Syndrome”) OR TOPIC: (“Rett syndrome”) OR TOPIC: (“Smith-Magenis syndrome”) OR TOPIC: (“Williams syndrome”) Indexes=CPCI-S Timespan=All years |
27,231 |
| #9 |
#8 AND #7 Indexes=CPCI-S Timespan=All years |
1227 |
| #8 |
TOPIC: (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or “young people” or “young person*”) Indexes=CPCI-S Timespan=All years |
148,172 |
| #7 |
#6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=CPCI-S Timespan=All years |
10,610 |
| #6 |
TS=(sleep* NEAR/3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*)) OR TS=(sleepless* or insomnia* or parasomnia* or “night terror*” or nightterror* or “night mare*” or nightmare*) OR TS=(sleep-wak* or night-wak*) OR TS=(sleepwalk* or sleep-walk* or “sleep walk*” or somnambulism) OR TS=(narcolepsy or “nocturnal hyperkinesia”) Indexes=CPCI-S Timespan=All years |
4969 |
| #5 |
TS=(nocturnal* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(nocturnal* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (dysfunction* or disorder or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) Indexes=CPCI-S Timespan=All years |
6394 |
| #4 |
TS=(“bed time*” NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(bedtime* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) Indexes=CPCI-S Timespan=All years |
395 |
| #3 |
TS=(narcolepsy) Indexes=CPCI-S Timespan=All years |
641 |
| #2 |
TS=(somnambulism) Indexes=CPCI-S Timespan=All years |
20 |
| #1 |
TS=(sleep disorder*) Indexes=CPCI-S Timespan=All years |
3826 |
EMBASE via Ovid
Date range searched: 1974 to 16 March 2016.
Search date: 17 March 2016.
Records identified: 281.
Search strategy
-
exp Sleep Disorder/ (163,578)
-
Sleep/ (81,807)
-
Sleep Walking/ (1497)
-
Narcolepsy/ (6905)
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (321)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (284)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (2464)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (3072)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1566)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1285)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (22,772)
-
(sleep$ adj3 (dysfunction$ or disorder or disorders or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (50,790)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (14,079)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (29,260)
-
(sleep-wak$ or night-wak$).ti,ab. (11,301)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (987)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (5254)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (244,169)
-
exp Infant/ or exp Child/ or exp Adolescent/ (2,954,891)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (2,038,598)
-
19 or 20 (3,517,560)
-
Childhood Disintegrative Disorder/ (128)
-
Developmental Disorder/ (28,919)
-
Asperger Syndrome/ (3698)
-
Attention Deficit Disorder/ or Attention Deficit Disorder with Hyperactivity/ or Attention Deficit Hyperactivity Disorder/ (44,022)
-
exp Autism/ (44,576)
-
Conduct Disorder/ (5292)
-
exp Epilepsy/ (196,376)
-
Cerebral Palsy/ (29,042)
-
Down Syndrome/ (29,384)
-
Fragile X Syndrome/ (6913)
-
Happy Puppet Syndrome/ (2147)
-
“Pervasive Developmental Disorder not otherwise specified”/ (796)
-
Prader-Willi Syndrome/ (4459)
-
Rett Syndrome/ (3939)
-
Smith Magenis Syndrome/ (483)
-
Williams Beuren Syndrome/ (2672)
-
(ADHD or attention deficit).ti,ab. (32,439)
-
angelman syndrome.ti,ab. (1374)
-
(autism or autistic or asperger$).ti,ab. (39,494)
-
cerebral palsy.ti,ab. (22,786)
-
conduct disorder$.ti,ab. (4786)
-
(epilepsy or epileptic).ti,ab. (138,577)
-
Down$ syndrome.ti,ab. (23,196)
-
Fragile x syndrome.ti,ab. (4311)
-
Prader Willi Syndrome.ti,ab. (3090)
-
Prader-Willi Syndrome.ti,ab. (3090)
-
Rett syndrome.ti,ab. (3099)
-
Smith-Magenis syndrome.ti,ab. (335)
-
Williams syndrome.ti,ab. (1696)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (18,431)
-
neurodisabilit$.ti,ab. (275)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (8887)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (21)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (431)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (11,690)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (2874)
-
23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 (419,846)
-
18 and 21 and 58 (10,411)
-
(animal/ or nonhuman/) not exp human/ (4,980,395)
-
59 not 60 (10,373)
-
intellectual impairment/ (14,389)
-
(intellectual$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (13,554)
-
(mental$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (44,139)
-
(learning adj (disability or disabled or difficult$)).ti,ab. (7378)
-
62 or 63 or 64 or 65 (68,203)
-
58 or 66 (462,906)
-
18 and 21 and 67 (10,694)
-
(animal/ or nonhuman/) not exp human/ (4,980,395)
-
68 not 69 (10,654)
-
70 not 61 (281)
Interface: Health Management Information Centre via Ovid
Date range searched: 1979 to January 2016.
Search date: 17 March 2016.
Records identified: 4.
Search strategy
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (4)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (43)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (29)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (2)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (26)
-
(sleep$ adj3 (dysfunction$ or disorder or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (262)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (77)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (202)
-
(sleep-wak$ or night-wak$).ti,ab. (14)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (1)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (4)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (36,281)
-
(ADHD or attention deficit).ti,ab. (200)
-
angelman syndrome.ti,ab. (0)
-
(autism or autistic or asperger$).ti,ab. (554)
-
cerebral palsy.ti,ab. (145)
-
conduct disorder$.ti,ab. (85)
-
(epilepsy or epileptic).ti,ab. (373)
-
Down$ syndrome.ti,ab. (255)
-
Fragile x syndrome.ti,ab. (12)
-
Prader Willi Syndrome.ti,ab. (3)
-
Prader-Willi Syndrome.ti,ab. (3)
-
Rett syndrome.ti,ab. (5)
-
Smith-Magenis syndrome.ti,ab. (0)
-
Williams syndrome.ti,ab. (0)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (101)
-
neurodisabilit$.ti,ab. (13)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (31)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (24)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (7)
-
or/1-13 (559)
-
or/15-34 (1680)
-
14 and 35 and 36 (10)
-
(intellectual$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (318)
-
(mental$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (2397)
-
(learning adj (disability or disabled or difficult$)).ti,ab. (2394)
-
38 or 39 or 40 (4883)
-
36 or 41 (6387)
-
14 and 35 and 42 (14)
-
43 not 37 (4)
Interface: MEDLINE via Ovid Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R)
Date range searched: 1946 to present.
Search date: 17 March 2016.
Records identified: 220.
Search strategy
-
exp Sleep Wake Disorders/ (68,198)
-
Sleep/ (41,721)
-
Somnambulism/ (563)
-
Narcolepsy/ (3053)
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (182)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (149)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1593)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1872)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (1051)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (819)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (15,445)
-
(sleep$ adj3 (dysfunction$ or disorder or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (35,005)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (8934)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (17,638)
-
(sleep-wak$ or night-wak$).ti,ab. (7799)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (668)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (3509)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (126,691)
-
exp Infant/ or exp Child/ or Adolescent/ (3,049,474)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (1,619,190)
-
19 or 20 (3,417,108)
-
exp Child Development Disorders, Pervasive/ (23,981)
-
Developmental Disabilities/ (16,408)
-
Angelman Syndrome/ (1005)
-
exp “attention deficit and disruptive behavior disorders”/ or attention deficit disorder with hyperactivity/ or asperger syndrome/ or autistic disorder/ (42,816)
-
Conduct Disorder/ (2671)
-
exp Epilepsy/ (138,933)
-
Cerebral Palsy/ (16,993)
-
Down Syndrome/ (21,762)
-
Fragile X Syndrome/ (4312)
-
Prader-Willi Syndrome/ (2429)
-
Rett Syndrome/ (2027)
-
Smith-Magenis Syndrome/ (115)
-
Williams Syndrome/ (1394)
-
(ADHD or attention deficit).ti,ab. (23,416)
-
angelman syndrome.ti,ab. (1121)
-
(autism or autistic or asperger$).ti,ab. (29,748)
-
cerebral palsy.ti,ab. (16,486)
-
conduct disorder$.ti,ab. (3715)
-
(epilepsy or epileptic).ti,ab. (93,799)
-
Down$ syndrome.ti,ab. (18,660)
-
Fragile x syndrome.ti,ab. (3664)
-
Prader Willi Syndrome.ti,ab. (2435)
-
Prader-Willi Syndrome.ti,ab. (2435)
-
Rett syndrome.ti,ab. (2537)
-
Smith-Magenis syndrome.ti,ab. (286)
-
Williams syndrome.ti,ab. (1389)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (13,253)
-
neurodisabilit$.ti,ab. (143)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (6630)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (11)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (304)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (8556)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (2050)
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 (307,168)
-
18 and 21 and 55 (4385)
-
Intellectual Disability/ (49,399)
-
(intellectual$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (9558)
-
(mental$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (34,226)
-
(learning adj (disability or disabled or difficult$)).ti,ab. (5309)
-
or/57-60 (74,629)
-
55 or 61 (359,076)
-
18 and 21 and 62 (4605)
-
63 not 56 (220)
Interface: PsycINFO via Ovid
Date range searched: 1806 to week 2 March 2016.
Search date: 17 March 2016.
Records identified: 135.
Search strategy
-
exp Sleep Disorders/ (12,601)
-
Sleep/ (17,435)
-
Sleepwalking/ (384)
-
Narcolepsy/ (1260)
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (186)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (155)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (978)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (1173)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (402)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (359)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (8660)
-
(sleep$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (18,021)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (5781)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (12,168)
-
(sleep-wak$ or night-wak$).ti,ab. (4347)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (751)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (1850)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (44,891)
-
(childhood birth 12 yrs or adolescence 13 17 yrs).ag. (660,440)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (764,560)
-
19 or 20 (954,409)
-
“3250”.cc. (35,699)
-
exp Pervasive Developmental Disorders/ (701)
-
exp Developmental Disabilities/ (12,490)
-
Neurodevelopmental Disorders/ (1401)
-
Attention Deficit Disorder/ (5017)
-
“Attention Deficit Disorder with Hyperactivity”/ (16,484)
-
exp Autism/ (0)
-
Aspergers Syndrome/ (0)
-
Conduct Disorder/ (3766)
-
exp Epilepsy/ (22,533)
-
Cerebral Palsy/ (4159)
-
Down’s Syndrome/ (5416)
-
Fragile X Syndrome/ (1379)
-
Prader Willi Syndrome/ (454)
-
Rett Syndrome/ (701)
-
Williams Syndrome/ (853)
-
(ADHD or attention deficit).ti,ab. (26,638)
-
angelman syndrome.ti,ab. (269)
-
(autism or autistic or asperger$).ti,ab. (37,492)
-
cerebral palsy.ti,ab. (5404)
-
conduct disorder$.ti,ab. (6208)
-
(epilepsy or epileptic).ti,ab. (30,119)
-
Down$ syndrome.ti,ab. (6310)
-
Fragile x syndrome.ti,ab. (1506)
-
Prader Willi Syndrome.ti,ab. (572)
-
Prader-Willi Syndrome.ti,ab. (572)
-
Rett syndrome.ti,ab. (891)
-
Smith-Magenis syndrome.ti,ab. (75)
-
Williams syndrome.ti,ab. (998)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (10,871)
-
neurodisabilit$.ti,ab. (66)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (3656)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (5)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (115)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (5141)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (2044)
-
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 (136,113)
-
18 and 21 and 58 (1739)
-
exp intellectual development disorder/ (40,609)
-
(intellectual$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (10,964)
-
(mental$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (28,202)
-
(learning adj (disability or disabled or difficult$)).ti,ab. (14,016)
-
60 or 61 or 62 or 63 (65,071)
-
58 or 64 (183,477)
-
18 and 21 and 65 (1874)
-
66 not 59 (135)
Interface: Science Citation Index via Web of Science
Date range searched: no restriction.
Search date: 17 March 2016.
Records identified: 113.
Search strategy
| Set | Results | |
|---|---|---|
| #17 | 113 |
#16 not #13 Indexes=SCI-EXPANDED Timespan=All years |
| #16 | 2987 |
#15 AND #9 Indexes=SCI-EXPANDED Timespan=All years |
| #15 | 257,021 |
#14 OR #11 OR #10 Indexes=SCI-EXPANDED Timespan=All years |
| #14 | 45,206 |
TOPIC: (intellectual* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*)) OR TOPIC: (mental* NEAR/2 (disabilit* or disabled or deficit* or handicap* or retard*)) OR TOPIC: (learning NEAR/2 (disability or disabled or difficult*)) Indexes=SCI-EXPANDED Timespan=All years |
| #13 | 2874 |
#12 AND #9 Indexes=SCI-EXPANDED Timespan=All years |
| #12 | 227,721 |
#11 OR #10 Indexes=SCI-EXPANDED Timespan=All years |
| #11 | 31,704 |
TOPIC: (developmental NEAR/2 (disabilit* or delay*)) OR TOPIC: (neurodisabilit*) OR TOPIC: (neurodevelopment* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuromotor* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychiatric* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) OR TOPIC: (neuropsychol* NEAR/3 (delay* or disabilit* or disease* or disorder* or dysfunction*)) Indexes=SCI-EXPANDED Timespan=All years |
| #10 | 205,587 |
TOPIC: (ADHD or “attention deficit”) OR TOPIC: (“angelman syndrome”) OR TOPIC: (autism or autistic or asperger*) OR TOPIC: (“cerebral palsy”)OR TOPIC: (“conduct disorder*”) OR TOPIC: (epilepsy or epilepti) OR TOPIC: (“Down* syndrome”) OR TOPIC: (“Fragile x syndrome”) OR TOPIC:(“Prader Willi Syndrome”) OR TOPIC: (“Rett syndrome”) OR TOPIC: (“Smith-Magenis syndrome”) OR TOPIC: (“Williams syndrome”) Indexes=SCI-EXPANDED Timespan=All years |
| #9 | 12,558 |
#8 AND #7 Indexes=SCI-EXPANDED Timespan=All years |
| #8 | 1,509,601 |
TOPIC: (adolescen* or baby or babies or child or children or boy or boys or girl or girls or infant* or infanc* or juvenile* or paediatric or pediatric or preschooler* or schoolboy* or schoolgirl* or schoolchild* or teens or teenager* or toddler* or youth or youths or “young people” or “young person*”) Indexes=SCI-EXPANDED Timespan=All years |
| #7 | 76,873 |
#6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=SCI-EXPANDED Timespan=All years |
| #6 | 36,970 |
TS=(sleep* NEAR/3 (initiat* or pattern* or routine* or practice* or maintain* or intervention* or schedule*)) OR TS=(sleepless* or insomnia* or parasomnia* or “night terror*” or nightterror* or “night mare*” or nightmare*) OR TS=(sleep-wak* or night-wak*) OR TS=(sleepwalk* or sleep-walk* or “sleep walk*” or somnambulism) OR TS=(narcolepsy or “nocturnal hyperkinesia”) Indexes=SCI-EXPANDED Timespan=All years |
| #5 | 48,375 |
TS=(nocturnal* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(nocturnal* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) OR TS=(sleep* NEAR/3 (dysfunction* or disorder or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) Indexes=SCI-EXPANDED Timespan=All years |
| #4 | 3897 |
TS=(“bed time*” NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(bedtime* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (dysfunction* or disorder* or difficult* or disrupt* or disturb* or delay* or problem*)) OR TS=(night* NEAR/3 (settle* or settling or wake* or awake or wakeful* or waking* or awaking* or awakening* or wakening*)) Indexes=SCI-EXPANDED Timespan=All years |
| #3 | 4952 |
TS=(narcolepsy) Indexes=SCI-EXPANDED Timespan=All years |
| #2 | 299 |
TS=(somnambulism) Indexes=SCI-EXPANDED Timespan=All years |
| #1 | 33,931 |
TS=(sleep disorder*) Indexes=SCI-EXPANDED Timespan=All years |
Applied Social Sciences Index and Abstract via Proquest
Date range searched: no restriction.
Search date: 17 March 2016.
Records identified: 12.
Search strategy
-
((bed time or bedtime$) adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (2)
-
((bed time$ or bedtime$) adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (12)
-
(night$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (33)
-
(night$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (44)
-
(nocturnal$ adj3 (dysfunction$ or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (4)
-
(nocturnal$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (7)
-
(sleep$ adj3 (settle$1 or settling or wake$1 or awake or wakeful$ or waking$ or awaking$ or awakening$ or wakening$)).ti,ab. (67)
-
(sleep$ adj3 (dysfunction$ or disorder or disorder$ or difficult$ or disrupt$ or disturb$ or delay$ or problem$)).ti,ab. (527)
-
(sleep$ adj3 (initiat$ or pattern$ or routine$ or practice$ or maintain$ or intervention$ or schedule$)).ti,ab. (260)
-
(sleepless$ or insomnia$ or parasomnia$ or night terror$ or nightterror$ or night mare$ or nightmare$).ti,ab. (258)
-
(sleep-wak$ or night-wak$).ti,ab. (41)
-
(sleepwalk$ or sleep-walk$ or sleep walk$ or somnambulism).ti,ab. (13)
-
(narcolepsy or nocturnal hyperkinesia).ti,ab. (5)
-
(ADHD or attention deficit).ti,ab. (941)
-
angelman syndrome.ti,ab. (8)
-
(autism or autistic or asperger$).ti,ab. (2638)
-
cerebral palsy.ti,ab. (344)
-
conduct disorder$.ti,ab. (431)
-
(epilepsy or epileptic).ti,ab. (292)
-
Down$ syndrome.ti,ab. (509)
-
Fragile x syndrome.ti,ab. (49)
-
Prader Willi Syndrome.ti,ab. (32)
-
Prader-Willi Syndrome.ti,ab. (32)
-
Rett syndrome.ti,ab. (20)
-
Smith-Magenis syndrome.ti,ab. (3)
-
Williams syndrome.ti,ab. (12)
-
(developmental adj2 (disabilit$ or delay$)).ti,ab. (590)
-
neurodisabilit$.ti,ab. (19)
-
(neurodevelopment$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (64)
-
(neuro-motor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuromotor$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (0)
-
(neuropsychiatric$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (32)
-
(neuropsychol$ adj3 (delay$ or disabilit$ or disease$ or disorder$ or dysfunction$)).ti,ab. (12)
-
or/1-13 (993)
-
or/14-33 (5507)
-
(adolescen$ or baby or babies or child or children or boy or boys or girl or girls or infant$ or infanc$ or juvenile$ or paediatric or pediatric or preschooler$ or schoolboy$ or schoolgirl$ or schoolchild$ or teens or teenager$ or toddler$ or youth or youths or young people or young person$).ti,ab. (139,979)
-
34 and 35 and 36 (48)
-
(intellectual$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (2135)
-
(mental$ adj (disabilit$ or disabled or deficit$ or handicap$ or retard$)).ti,ab. (1252)
-
(learning adj (disability or disabled or difficult$)).ti,ab. (5838)
-
35 or 38 or 39 or 40 (13,791)
-
34 and 36 and 41 (60)
-
42 not 37 (12)
Appendix 3 The data extraction variables used for the study results
| Variable | Description |
|---|---|
| Study | Author, date |
| N_Treat | Number of participants in treatment arm |
| N_Treat2 | Number of participants in second treatment arm (if applicable) |
| N_Treat3 | Number of participants in third treatment arm (if applicable) |
| N_Control | Number of participants in control arm |
| Followup | Baseline ‘0’ |
| Followup1 | Final follow-up point for which subsequent data are being extracted (e.g. 12 weeks, 10 days) |
| Mean0_combined | Baseline mean score for crossover trials |
| Mean0_treat | Baseline mean score for treatment arm |
| LowerCI0_treat | Lower CI for baseline mean score for treatment arm |
| UpperCI0_treat | Upper CI for baseline mean score for treatment arm |
| SD0_combined | SD for baseline mean score for crossover trials |
| SD0_treat | SD for baseline mean score for treatment arm |
| SE0_treat | SE for baseline mean score for treatment arm |
| Median0_treat | Baseline median score for treatment arm |
| Min0_treat | Baseline minimum score for treatment arm |
| Max0_treat | Baseline maximum score for treatment arm |
| Range0_treat | Baseline range score for treatment arm |
| Q250_treat | Baseline lower quartile for treatment arm |
| Q750_treat | Baseline upper quartile for treatment arm |
| IQR0_treat | Baseline interquartile range for treatment arm |
| Mean0_plac | Baseline mean score for control/placebo arm |
| LowerCI0_ plac | Lower CI for baseline mean score for control/placebo arm |
| UpperCI0_ plac | Upper CI for baseline mean score for control/placebo arm |
| SD0_ plac | SD for baseline mean score for control/placebo arm |
| SE0_ plac | SE for baseline mean score for control/placebo arm |
| Median0_ plac | Baseline median score for control/placebo arm |
| Min0_ plac | Baseline minimum score for control/placebo arm |
| Max0_ plac | Baseline maximum score for control/placebo arm |
| Range0_ plac | Baseline range score for control/placebo arm |
| Q250_ plac | Baseline lower quartile for control/placebo arm |
| Q750_ plac | Baseline upper quartile for control/placebo arm |
| IQR0_ plac | Baseline interquartile range for control/placebo arm |
| Mean1_treat | Follow-up mean score for treatment arm |
| LowerCI1_treat | Lower CI for follow-up mean score for treatment arm |
| UpperCI1_treat | Upper CI for follow-up mean score for treatment arm |
| SD1_treat | SD for follow-up mean score for treatment arm |
| SE1_treat | SE for follow-up mean score for treatment arm |
| Median1_treat | Follow-up mean median score for treatment arm |
| Min1_treat | Follow-up mean minimum score for treatment arm |
| Max1_treat | Follow-up mean maximum score for treatment arm |
| Range1_treat | Follow-up mean range score for treatment arm |
| Q251_treat | Follow-up mean lower quartile for treatment arm |
| Q751_treat | Follow-up mean upper quartile for treatment arm |
| IQR1_treat | Follow-up mean interquartile range for treatment arm |
| Mean1_ plac | Follow-up mean score for control/placebo arm |
| LowerCI1_ plac | Lower CI for follow-up mean score for control/placebo arm |
| UpperCI1_ plac | Upper CI for follow-up mean score for control/placebo arm |
| SD1_ plac | SD for follow-up mean score for control/placebo arm |
| SE1_ plac | SE for follow-up mean score for control/placebo arm |
| Median1_ plac | Follow-up mean median score for control/placebo arm |
| Min1_ plac | Follow-up mean minimum score for control/placebo arm |
| Max1_ plac | Follow-up mean maximum score for control/placebo arm |
| Range1_ plac | Follow-up mean range score for control/placebo arm |
| Q251_ plac | Follow-up mean lower quartile for control/placebo arm |
| Q751_ plac | Follow-up mean upper quartile for control/placebo arm |
| IQR1_ plac | Follow-up mean interquartile range for control/placebo arm |
| TimeEffect_F | ANOVA f-value for time effect |
| TimeEffect_P | ANOVA p-value for time effect |
| GroupEffect_F | ANOVA f-value for group effect |
| GroupEffect_P | ANOVA p-value for group effect |
| GroupTimeEffect_F | ANOVA f-value for interaction effect |
| GroupTimeEffect_P | ANOVA p-value for interaction effect |
| ChangeBL1_Mean_Treat | Mean change from baseline to follow-up for treatment arm |
| ChangeBL1_SD_Treat | SD of mean change from baseline to follow-up for treatment arm |
| ChangeBL1_SE_Treat | SE of mean change from baseline to follow-up for treatment arm |
| ChangeBL1_range_Treat | Range score in change from baseline to follow-up for treatment arm |
| ChangeBL1_LowerCI_Treat | Lower CI in change value from baseline to follow-up for treatment arm |
| ChangeBL1_UpperCI_Treat | Upper CI in change value from baseline to follow-up for treatment arm |
| ChangeBL1_P_Treat | p-value for change from baseline to follow-up for treatment arm |
| ChangeBL1_%_Treat | Percentage change from baseline to follow-up for treatment arm |
| ChangeBL1_Mean_plac | Mean change from baseline to follow-up for control/placebo arm |
| ChangeBL1_SD_ plac | SD of mean change from baseline to follow-up for control/placebo arm |
| ChangeBL1_SE_ plac | SE of mean change from baseline to follow-up for control/placebo arm |
| ChangeBL1_range_ plac | Range score in change from baseline to follow-up for control/placebo arm |
| ChangeBL1_LowerCI_ plac | Lower CI in change value from baseline to follow-up for control/placebo arm |
| ChangeBL1_UpperCI_ plac | Upper CI in change value from baseline to follow-up for control/placebo arm |
| ChangeBL1_P_ plac | p-value for change from baseline to follow-up for control/placebo arm |
| ChangeBL1_%_ plac | Percentage change from baseline to follow-up for control/placebo arm |
| Diff_Treat_Plac | Raw value/score of the difference between the treatment and control/placebo arm |
| Diff_Treat_Plac_mean | MD between the treatment and control/placebo arm |
| Diff_Treat_Plac_SD | SD of the MD between the treatment and control/placebo arm |
| Diff_Treat_Plac_test_statistic(DF) | Test statistic of the difference between the treatment and control/placebo arm |
| Diff_Treat_Plac_ES | Effect size of the difference between the treatment and control/placebo arm |
| Diff_Treat_Plac_P | p-value of the difference between the treatment and control/placebo arm |
| CarryOverEffect_P | Carry-over effect for crossover trials |
| Period_effect_P | Period effect for crossover trials |
| Period_T-P_Mean | Mean period effect in order of treatment to control/placebo for crossover trials |
| Period_T-P_SD | SD of the mean period effect in order of treatment to control/placebo for crossover trials |
| Period_P-T_Mean | Mean period effect in order of control/placebo to treatment for crossover trials |
| Period_P-T_SD | SD of the mean period effect in order of control/placebo to treatment for crossover trials |
| Period_diff_P | p-value for period effect |
| DiffChange_Mean | Difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| DiffChange_SD | SD of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| DiffChange_SE | SE of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| DiffChange_LowerCI | Lower CI of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| DiffChange_UpperCI | Upper CI of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| DiffChange_P | p-value of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| DiffChange_PES | Effect size of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| AdjustedDiff_Mean | Adjusted difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| AdjustedDiff_SD | Adjusted SD of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| AdjustedDiff_SE | Adjusted SE of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| AdjustedDiff_LowerCI | Adjusted lower CI of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| AdjustedDiff_UpperCI | Adjusted upper CI of the difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| AdjustedDiff_P | p-value of the adjusted difference in mean change from baseline to follow-up between the treatment and control/placebo arm |
| Notes | Any additional notes of relevance |
Appendix 4 List of papers excluded after full-text review and reasons for exclusions
| Reference | Reason for exclusion |
|---|---|
| Records identified from trial registeries | |
| ISRCTN05534585, The use of MElatonin in children with Neuro-developmental Disorders and impaired Sleep; a randomised, double-blind, placebo-controlled, parallel study | Already included as Appleton et al.48 |
| ISRCTN92655217, Snuggledown – Use of sensory blankets for children with autistic spectrum disorder | Already included as Gringras et al.36 |
| NCT01322022, Treatment of sleep disturbances in young children with autism. 2009 | Already included as Johnson et al.107 |
| ISRCTN84194243, Development of effective primary care treatment of severe sleep disorders in children with a learning disability; a randomised controlled trial | Already included as Montgomery et al.49 |
| ISRCTN77884120, Melatonin treatment for sleep problems in children with autism: a randomised controlled crossover trial | Already included as Wright et al.106 |
| 2007-004664-46, Influence of methylphenidate on sleep and circadian rhythm in children with Attention-Deficit/Hyperactivity Disorder (ADHD) – MELMET | Intervention |
| 2011-003313-42, Agomelatine efficacy of the drug to improve sleep problems in autistic people | Intervention |
| ISRCTN31542578, A blinded randomised cross-over study of the effect of melatonin treatment of sleep disturbances on hypothalamic–pituitary-gonadal axis and leptin in pubertal children | Intervention |
| NCT00393042, Sleep and tolerability study: comparing the effects of Adderall XR and Focalin XR | Intervention |
| NCT00695136, The effect of Donepezil [Aricept (Registered Trademark)] on REM sleep in children with autism | Intervention |
| NCT00745030, Efficacy and tolerability of Ramelteon in patients with rapid eye movement (REM) behavior disorder and Parkinsonism | Intervention |
| NCT00807222, Effect of Vyvanse on sleep in children aged 6–12 years with attention deficit hyperactivity disorder (ADHD) | Intervention |
| NCT00989950, Study of the effect of individualizing Daytrana wear-times on sleep in children with ADHD | Intervention |
| NCT01156051, Effect of Guanfacine extended-release on attention deficit hyperactivity disorder (ADHD)-associated insomnia | Intervention |
| NCT01887132, A trial of the drug Donepezil for sleep enhancement and behavioral change in children with autism | Intervention |
| NCT02231008, Evaluating the effects of Tasimelteon vs Placebo on sleep disturbances in SMS | Intervention |
| NCT02487082, Pilot study of sleep therapy and biomarkers in children with autism spectrum disorders | Intervention |
| NCT02638168, Effects of evening dose of immediate release methylphenidate on sleep in children with ADHD | Intervention |
| NCT00152750, Study of Clonidine on sleep architecture in children with Tourette’s Syndrome (TS) and comorbid ADHD | Outcome |
| NCT01508793, Enhancing sleep duration: effects on children’s eating and activity behaviors | Outcome |
| NCT01903681, Assessment of the pharmacokinetics of Circadin® in children with neurodevelopmental disorders and sleep disturbances | Outcome |
| NCT02132273, Use of an educational story to prepare children with developmental disabilities for sleep study | Outcome |
| IRCT2015062222865N1, The effect of aromatherapy with Rosa Damascena on sleep quality of children | Population |
| NCT00005753, Pharmacological and behavioral treatment of insomnia | Population |
| NCT00133055, Parenting matters: helping parents with young children | Population |
| NCT00877162, The rocky sleep study | Population |
| NCT02195401, The effects of a clean room sleeping environment on elemental and chemical concentrations in children with autism | Population |
| NCT02398214, A sleep hygiene-based intervention program for infants and toddlers | Population |
| NCT02648568, Does hypnosis improve severe sleepwalking? | Population |
| NTR4045, Effects of melatonin treatment, light therapy, and sleep improvement in children with sleep onset problems | Population |
| NCT00691080, Understanding sleep problems in children with autism spectrum disorder | Study design |
| Records identified from databases | |
| Adams JB, Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complement Med 2004;10:1033–9 | Intervention |
| Ahmann PA, Theye FW, Berg R, Linquist AJ, Van Erem AJ, Campbell LR. Placebo-controlled evaluation of amphetamine mixture-dextroamphetamine salts and amphetamine salts (Adderall): efficacy rate and side effects. Pediatrics 2001;107:E10 | Intervention |
| Allen KD, Kuhn BR, DeHaai KA, Wallace DP. Evaluation of a behavioral treatment package to reduce sleep problems in children with Angelman Syndrome. Res Dev Disabil 2013;34:676–86 | Study design (before-and-after study with ≤ 10 participants) |
| Ashkenasi A. Effect of transdermal methylphenidate wear times on sleep in children with attention deficit hyperactivity disorder. Pediatr Neurol 2011;45:381–6 | Intervention |
| Askenasi A. O251. Effect of transdermal methylphenidate wear times on sleep in children with ADHD. 22nd meeting of the European Neurological Society, 9–12 June 2012, Prague, Czech Republic. J Neurol 2012;259:S33–34 | Intervention |
| Barlow J, Cullen L. Coming together through touch: the experiences of parents of children with disabilities learning the principles of massage. Early Child Dev Care 2000;161:93–106 | Intervention |
| Barlow J, Powell L, Cheshire A. The Training and Support Programme (involving basic massage) for parents of children with cerebral palsy: an implementation study. J Bodyw Mov Ther 2007;11:44–53 | Intervention |
| Becke SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 2016;37:395–404 | Intervention |
| Blumer JL, Findling RL, Shih WJ, Soubrane C, Reed MD. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/hyperactivity disorder in children 6 to 17 years of age. Pediatrics 2009;123:e770–6 | Intervention |
| Bovet-du Bois N. [Neuroleptic treatment in child psychiatry: analysis of a series of 100 cases treated with thioridazine.] Schweiz Rundsch Med Prax 1973;62:1556–64 | Intervention |
| Braam W, van Geijlswijk I, Keijzer H, Smits MG, Didden R, Curfs LM. Loss of response to melatonin treatment is associated with slow melatonin metabolism. J Intellect Disabil Res 2010;54:547–55 | Intervention |
| Brady A, Mpetha K, Humphreys S, Carney AM. Developing a sleep service for children with learning disabilities or autistic spectrum disorders aged 0–5: setting up the service and lessons from practice. Clinical Psychology Forum 2011;222:31–5 | Intervention |
| Briault M, Labrosse M, Verreault M, Berthiaume C, Lageix P, Turgeon L, Godbout R. Sleep in children with attention deficit disorders: effects of comorbid anxiety and response to treatment. 23rd Annual Meeting of the Associated-Professional-Sleep-Societies, 6–11 June 2009, Seattle, WA, USA. Sleep 2009;32:A65 | Intervention |
| Buckley AW, Sassower K, Rodriguez AJ, Jennison K, Wingert K, Buckley J, et al. An open label trial of donepezil for enhancement of rapid eye movement sleep in young children with autism spectrum disorders. J Child Adolesc Psychopharmacol 2011;21:353–7 | Intervention |
| Bussing R, Mason D, Garvan CW, Gurnani T, Koro-Ljungberg M, Noguchi K, Albarracin D. Willingness to use ADHD self-management: mixed methods study of perceptions by adolescents and parents. J Child Fam Stud 2016;25:562–73 | Intervention |
| Campbell M, Perry R, Polonsky BB, Deutsch SI, Palij M, Lukashok D. An open study of fenfluramine in hospitalized young autistic children. J Autism Dev Disord 1986;16:495–506 | Intervention |
| Campbell M, Deutsch SI, Perry R, Wolsky BB, Palij M. Short-term efficacy and safety of fenfluramine in hospitalized preschool-age autistic children: an open study. Psychopharmacol Bull 1986;22:141–7 | Intervention |
| Campbell M, Small AM, Palij M, Perry R, Polonsky BB, Lukashok D, Anderson LT. The efficacy and safety of fenfluramine in autistic children: preliminary analysis of a double-blind study. Psychopharmacol Bull 1987;23:123–7 | Intervention |
| Chen XQ, Zhang WN, Yang ZX, Zhao M, Cai FC, Huang SP, et al. Efficacy of levetiracetam in electrical status epilepticus during sleep of children: a multicenter experience. Pediatr Neurol 2014;50:243–9 | Intervention |
| Cheng Song J, Hiscock H, Scibberras E, Schuster T. Behavioural sleep problems in children with ADHD: cross–sectional associations with parenting and sleep hygiene. Sleep Biologic Rhythms 2015;13(Suppl. 1):1–98 | Intervention |
| Chung I, Han CH, Kim HW, Cho SC. P.7.c.008 The effect of prolonged-release methylphenidate on the sleep of children with attention-deficit/hyperactivity disorders. Eur Neuropsychopharmacol 2010;20(Suppl. 3):624–5 | Intervention |
| Kim HW, Yoon IY, Cho SC, Kim BN, Chung S, Lee H, et al. The effect of OROS methylphenidate on the sleep of children with attention-deficit/hyperactivity disorder. Int J Neuropsychopharmacol 2010;13:184 | Intervention |
| Clonidine for treatment of attention-deficit/hyperactivity disorder. Med Lett Drugs Ther 1996;38:109–10 | Intervention |
| Cocchi R. Drug therapies for sleep troubles, hyperactivity and aggression in young adult autistics. Ital J Intellective Impairment 1995;8:169–74 | Intervention |
| Cohen-Zion M. Sleep and circadian rhythms in children with attention deficit-hyperactivity disorder: before and after stimulant treatment. San Diego, CA: University of California, San Diego and San Diego State University; 2005 | Intervention |
| De Leersnyder H, de Blois M-C, Vekemans M, Sidi D, Villain E, Kindermans C, Munnich A. β1-adrenergic antagonists improve sleep and behavioural disturbances in a circadian disorder, Smith–Magenis syndrome. J Med Genet 2001;38:586–90 | Intervention |
| DeLeon IG, Fisher WW, Marhefka JM. Decreasing self-injurious behaviour associated with awakening in a child with autism and developmental delays. Behav Interv 2004;19:111–19 | Intervention |
| Duncan B, Barton L, Edmonds D, Blashill BM. Parental perceptions of the therapeutic effect from osteopathic manipulation or acupuncture in children with spastic cerebral palsy. Clin Pediatr 2004;43:349–53 | Intervention |
| Ediberidze T, Maisuradze L, Kasradze S. Influence of anticonvulsants on sleep difficulties in children with epileptic seizures of genetic aetiology (ESGE)-a questionnaire based study. J Sleep Res 2016;25:229 | Intervention |
| Rugino T. Effect of Guanfacine Extended-Release on Attention Deficit Hyperactivity Disorder (ADHD)-Associated Insomnia. Children’s Specialised Hospital; 2010. URL: https://clinicaltrials.gov/ct2/show/NCT01156051 (accessed 26 July 2018) | Intervention |
| Efron D, Lycett K, Sciberras E. Use of sleep medication in children with ADHD. Sleep Med 2014;15:472–5 | Intervention |
| Elbhrawy S, et al. Polysomnographic based study of sleep in juvenile myoclonic epilepsy patients. Epilepsia 2016;57(Suppl. 2):43 | Intervention |
| Esmaeli L, Abedi MR, Najafi MR, Aminjafari A, Afsar F, Moghtadaei M. Effectiveness of emotion regulation on anxiety, insomnia and social dysfunction of epileptic adolescent girls. Abstracts of 5th International Congress Of Child and Adolescent Psychiatry, 8–11 October 2012, Tehran, Iran. Iran J Psychiatry 2012;1:3 | Intervention |
| Faber S, Zinn GM, Boggess A, Fahrenholz T, Kern JC, Kingston HM. A cleanroom sleeping environment’s impact on markers of oxidative stress, immune dysregulation, and behavior in children with autism spectrum disorders. BMC Complement Altern Med 2015;15:71 | Intervention |
| Feinberg I, Hibi S, Braun M, Cavness C, Westerman G, Small A. Sleep amphetamine effects in MBDS and normal subjects. Arch Gen Psychiatry 1974;31:723–31 | Intervention |
| Fletcher F, Foster-Owens M, Conduit R, Cornish K. The impact of sleep hygiene on sleep onset issues in children with high functioning autism and Asperger syndrome. Sleep Biol Rhythms 11:12 | Intervention |
| Fletcher, Rinehart N, Conduit R, Rajaratnam S, Thomas D, Cornish K. Targeting sleep problems and anxiety in children with high functioning autism. Sleep Biol Rhythms 2012;10:63 | Intervention |
| Gastfriend DR, Biederman J, Jellinek MS. Desipramine in the treatment of adolescents with attention deficit disorder. Am J Psychiatry 1984;141:906–8 | Intervention |
| Gastfriend DR, Biederman J, Jellinek MS. Desipramine in the treatment of attention deficit disorder in adolescents. Psychopharmacol Bull 1985;21:144–5 | Intervention |
| Giblin JM, Strobel AL. Effect of lisdexamfetamine dimesylate on sleep in children with ADHD. J Atten Disord 2011;15:491–8 | Intervention |
| Goldman SE, Adkins KW, Calcutt MW, Carter MD, Goodpaster RL, Wang L, et al. Melatonin in children with autism spectrum disorders: endogenous and pharmacokinetic profiles in relation to sleep. J Autism Dev Disord 2014;44:2525–35 | Intervention |
| Grubar JC, Gigli GL, Colognola RM, Ferri R, Musumeci SA, Bergonzi P. Sleep patterns of Down’s syndrome children: effects of butoctamide hydrogen succinate (BAHS) administration. Psychopharmacology 1986;90:119–22 | Intervention |
| Gruber R, Grizenko N, Schwartz G, Ben Amor L, Gauthier J, de Guzman R, Joober R. Sleep and COMT polymorphism in ADHD children: preliminary actigraphic data. J Am Acad Child Adolesc Psychiatry 2006;45:982–9 | Intervention |
| Gupta M, Aneja S, Kohli K. Add-on melatonin improves quality of life in epileptic children on valproate monotherapy: a randomised, double-blind, placebo-controlled trial. Epilepsy Behav 2004;5:316–21 | Intervention |
| Hagerman RJ, Riddle JE, Roberts LS, Breese K, Fulton M. Survey of the efficacy of clonidine in fragile X syndrome. Dev Brain Dysfunc 1995;8:336–44 | Intervention |
| Hallböök T, Lundgren J, Köhler S, Blennow G, Strömblad LG, Rosén I. Beneficial effects on sleep of vagus nerve stimulation in children with therapy resistant epilepsy. Eur J Paediatr Neurol 2005;9:399–407 | Intervention |
| Hallböök T, Lundgren J, Rosén I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia 2007;48:59–65 | Intervention |
| Head TK. Evaluation of medication effects on academic performance, sleep, and core ADHD symptoms in children. Kalamazoo, MI: Western Michigan University; 2015 | Intervention |
| Henderson JA, Barry TD, Bader SH, Jordan SS. The relation among sleep, routines, and externalising behaviour in children with an autism spectrum disorder. Res Autism Spectr Disord 2011;5:758–67 | Intervention |
| Hewitt K. Behavioural approaches to sleeplessness. Br J Learning Disabil 1985;13:112–14 | Study design |
| Honomichl RD, Goodlin-Jones BL, Burnham MM, Hansen RL, Anders TF. Secretin and sleep in children with autism. Child Psychiatry Hum Dev 2002;33:107–23 | Intervention |
| Hoshino K. Effectiveness of low dose of L-dopamine in children with autism and attention-deficithyperactivity disorder. Dev Med Child Neurol 2012;54:5–212 | Intervention |
| Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Psychiatry 1985;24:617–29 | Intervention |
| Hunt RD, Minderaa RB, Cohen DJ. The therapeutic effect of clonidine in attention deficit disorder with hyperactivity: a comparison with placebo and methylphenidate. Psychopharmacol Bull 1986;22:229–36 | Intervention |
| Hvolby A. Does treatment of ADHD sleeping problems improve attention, hyperactivity and impulsiveness in children with attention deficit hyperactivity disorder? Eur Child Adolesc Psychiatry 2013;22:S87–323 | Intervention |
| Laboni A, Gibbons J, Hamilton J, Narang I. Sleep characteristics of prepubescent children with Prader–Willi syndrome before and after growth hormone treatment. Sleep 2009;32:A113 | Intervention |
| Jan JE, Connolly MB, Hamilton D, Freeman RD, Laudon M. Melatonin treatment of non-epileptic myoclonus in children. Dev Med Child Neurol 1999;41:255–9 | Intervention |
| Kent JD, Joseph C, Blader HS, Koplewicz HA, Foley CA. Effects of late-afternoon methylphenidate administration on behaviour and sleep in attention-deficit hyperactivity disorder. Pediatrics 1995;96:320–5 | Intervention |
| Kim HW, Yoon IY, Cho SC, Kim BN, Chung S, Lee H, et al. The effect of OROS methylphenidate on the sleep of children with attention-deficit/hyperactivity disorder. Int Clin Psychopharmacol 2010;25:107–15 | Intervention |
| Konafel E, Lecendreuz M, Bouvard MP, Mouren-Simeoni MC. Effects of vesperal methylphenidate (MPH) administration on diurnal and nocturnal activity in ADHD children: an actigraphic study. Amer Acad Sleep Med 2001:A215 | Intervention |
| Liboni F, Palagini L, Mandredi A, Tacchi A, Ricci F, Mauri M, et al. Effects of six-months methylphenidate treatment on sleep disturbances in children with attention-deficit/hyperactivity disorder. A pilot study. JOURNAL OF, 2014;23:322–2 | Intervention |
| Lyon MR, Kapoor MP, Juneja LR. The effects of L-theanine (Suntheanine) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): a randomised, double-blind, placebo-controlled clinical trial. Altern Med Rev 2011;16:348–54 | Intervention |
| Malow BA, MacDonald LL, Fawkes DB, Alder ML, Katz T. Teaching children with autism spectrum disorder how to sleep better: a pilot educational program for parents. Clin Pract Paediat Psychol 2016;4:125 | Study design (before-and-after study with ≤ 10 participants) |
| Malow BA, Katz T, Reynolds AM, Shui A, Carno M, Connolly HV, et al. Sleep difficulties and medications in children with autism spectrum disorders: a registry study. Pediatrics 2016;137(Suppl. 2):98–104 | Intervention |
| Miyamoto A, Fukuda I, Tanaka H, Oka R, Araki A, Cho K. [Treatment with ramelteon for sleep disturbance in severely disabled children and young adults.] No To Hattatsu 2013;45:440–4 | Intervention |
| Mostafavi S-A, Mohammadi MR, Hosseinzadeh P, Eshraghian MR, Akhondzadeh R, Hosseinzadeh-Attar MJ, et al. Dietary intake, growth and development of children with ADHD in a randomised clinical trial of Ritalin and Melatonin co-administration: Through circadian cycle modification or appetite enhancement? Iran J Psychiatry 2012;7:114–19 | Intervention |
| Nayak C, Sinha S, Ramachandraiah CT, Nagappa M, Thennarasu K, Taly AB, Satishchandra P. Differential improvement of the sleep quality among patients with juvenile myoclonic epilepsy with valproic acid: A longitudinal sleep questionnaire-based study. Ann Indian Acad Neurol 2015;18:403–7 | Intervention |
| Long Y, Tan J, Nie Y, Lu Y, Mei X, Tu C. Hyperbaric oxygen therapy is safe and effective for the treatment of sleep disorders in children with cerebral palsy. Neurol Res 2017;39:239–47 | Intervention |
| Ornitz EM, Forsythe AB, de la Peña A. Effect of vestibular and auditory stimulation on the REMs of REM sleep in autistic children. Arch Gen Psychiatry 1973;29:786–91 | Intervention |
| Owens J, Weiss M, Nordbrock E, Mattingly G, Wigal S, Greenhill LL, et al. Effect of aptensio XR (methylphenidate HCl extended-release) capsules on sleep in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2016;26:873–81 | Intervention |
| Petre-Quadens O, De Greef A. Effects of 5-HTP on sleep in Mongol children. Preliminary results. J Neurol Sci 1971;13:115–19 | Intervention |
| Larsen S, Harrington K, Hicks S. The LENS (Low Energy Neurofeedback System): a clinical outcomes study on one hundred patients at Stone Mountain Centre, New York. J Neurother 2006;10:69–78 | Intervention |
| Ramstad K, Jahnsen R, Lofterod B, Skjeldal OH. Continuous intrathecal baclofen therapy in children with cerebral palsy – when does improvement emerge? Acta Paediatr 2010;99:1661–5 | Intervention |
| Robinson AA, Malow BA. Gabapentin shows promise in treating refractory insomnia in children. J Child Neurol 2013;28:1618–21 | Intervention |
| Robinson AM, Richdale AL. Sleep problems in children with an intellectual disability: parental perceptions of sleep problems, and views of treatment effectiveness. Child Care Health Dev 2004;30:139–50 | Intervention |
| Rogozea R, Florea-Ciocoiu V. Orienting reaction in patients with night terrors. Biol Psychiatry 1985;20:894–905 | Intervention |
| Rugino TA. Effect on primary sleep disorders when children with ADHD are administered Guanfacine extended release. J Atten Disord 2014;22:14–24 | Intervention |
| Binay Safer V, Ozbudak Demir S, Ozkan E, Demircioglu Guneri F. Effects of botulinum toxin serotype A on sleep problems in children with cerebral palsy and on mothers sleep quality and depression. Neurosciences 2016;21:331–7 | Intervention |
| Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep 2006;29:1573–85 | Intervention |
| Sangal RB, Blumer JL, Lankford DA, Grinnell TA, Huang H. Eszopiclone for insomnia associated with attention-deficit/hyperactivity disorder. Pediatrics 2014;134:e1095–103 | Intervention |
| Sangal RB, Owens J, Allen A, Kelsey D, Sutton V, Schuh K. Effects of atomoxetine and methylphenidate on sleep in children with attention-deficit/hyperactivity disorder. Int J Neuropsychopharmacol 2004;7 | Intervention |
| Schelleman M, Richdale A. The relative and combined effects of a diet and a behavioural intervention for behaviour and sleep problems in children with significant challenging behaviours. Sleep Biol Rhythms 2010;8:A7 | Intervention |
| Shah T, Tse A, Gill H, Wong I, Sutcliffe A, Gringras P, et al. Administration of melatonin mixed with soft food and liquids for children with neurodevelopmental difficulties. Dev Med Child Neurol 2008;50:845–9 | Intervention |
| Sherwin I, Hooge JP. Comparative effectiveness of natural sleep and methohexital. Provocative tests in electroencephalography. Neurology 1973;23:973–6 | Intervention |
| Smits MG. Clinical experiences of DLMO measurements in insomnia patients at the Gelderse Vallei Hospital Sleep Centre. J Sleep Res 2010;19:97 | Intervention |
| Strawn JR, McReynolds D. An evidence-based approach to treating paediatric anxiety disorders. Current Psychiatry 2012;11:16–21 | Intervention |
| Tai A, Horne R, Davey M, Nixon G. The effects of growth hormone on sleep parameters in children with Prader–Willi syndrome. Sleep Biol Rhythms 2009;7:A56 | Intervention |
| Tajima S, Matsuzawa S, Takai K, Kawakami M, Nakashima M, Miike T. Poster presentations 1. Sleep Biol Rhythms 2011;9:254–342 | Intervention |
| Tatsumi Y, Mohri I, Shimizu S, Tachibana M, Ohno Y, Taniike M. Daytime physical activity and sleep in pre-schoolers with developmental disorders. J Paediatr Child Health 2015;51:396–402 | Intervention |
| Tilford JM, Payakachat N, Kuhlthau KA, Pyne JM, Kovacs E, Bellando J, et al. Treatment for sleep problems in children with autism and caregiver spillover effects. J Autism Dev Disord 2015;45:3613–23 | Intervention |
| Tirosh E, Sadeh A, Munvez R, Lavie P. Effects of methylphenidate on sleep in children with attention-deficit hyperactivity disorder: an activity monitor study. Am J Dis Child 1993;147:1313–15 | Intervention |
| Tizard B. A controlled study of all-night sleep in overactive imbecile children. Am J Ment Defic 1968;73:209–13 | Intervention |
| Valdizan JR, Almarcegui C, Brualla J, Alejos MV, Chulilla JL, Dolz I. The influence of gabapentin on sleep in children with secondarily generalised partial epilepsy. Rev Neurol 1999;29:718–21 | Intervention |
| De Weerd A, Van Den Bosschhe R. Sleep in very young children with Prader Willi syndrome before and during growth hormone substitution. J Sleep Res 2010;19:29 | Intervention |
| Van der Heijden KB, Smits MG, Gunning WB. Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J Sleep Res 2006;15:55–62 | Intervention |
| Verrillo E, Bruni O, Franco P, Ferri R, Thiriez G, Pavone M, et al. Analysis of NREM sleep in children with Prader–Willi syndrome and the effect of growth hormone treatment. Sleep Med 2009;10:646–50 | Intervention |
| Verrillo E, Bizzarri C, Bruni O, Ferri R, Pavone M, Cappa M, Cutrera R. Effects of replacement therapy on sleep architecture in children with growth hormone deficiency. Sleep Med 2012;13:496–502 | Intervention |
| Wachob D, Lorenzi DG. Brief report: influence of physical activity on sleep quality in children with autism. J Autism Dev Disord 2015;45:2641–6 | Intervention |
| Wang L, Liu Y. Behaviour improvement by diet and environmental interventions in brain injured children. CRTER 2007;11:5983–5 | Intervention |
| Wehmeier PM, Dittman RW, Schacht A, Helsbery K, Lehmkuhl G. Morning and evening behaviour in children and adolescents treated with atomoxetine once daily for attention-deficit/hyperactivity disorder (ADHD): Findings from two 24-week, open-label studies. Child Adolesc Psychiatry Ment Health 2009;3:5 | Intervention |
| Williams G, Sears L, Allard A. Parent perceptions of efficacy for strategies used to facilitate sleep in children with autism. J Dev Phys Disabil 2006;18:25–33 | Intervention |
| Vol’f MSh, Prokudin VN, Iurkova IA. [The place of nitrazepam in the complex treatment of epilepsy.] Zh Nevropatol Psikhiatr Im S S Korsakova 1973;73:1714–18 | Intervention |
| Wyatt K, Edwards V, Franck L, Britten N, Creanor S, Maddick A, Logan S. Cranial osteopathy for children with cerebral palsy: a randomised controlled trial. Arch Dis Child 2011;96:505–12 | Intervention |
| Zametkin A, Rapoport JL, Murphy DL, Linnoila M, Ismond D. Treatment of hyperactive children with monoamine oxidase inhibitors. I. Clinical efficacy. Arch Gen Psychiatry 1985;42:962–6 | Intervention |
| Zametkin AJ, Reeves JC, Webster L, Werry JS. Promethazine treatment of children with Attention Deficit Disorder with Hyperactivity – ineffective and unpleasant. J Am Acad Child Psychiatry 1986;25:854–6 | Intervention |
| Zandieh S, Khatwa U, Zarowski M, Kothare S. Utility of the maintenance of wakefulness test (MWT) in assessing treatment efficacy & optimising management in children with narcolepsy. 2012;35:A391 | Intervention |
| Zhou JY, Tang XD, Huang LL, Zhong ZQ, Lei F, Zhou D. The acute effects of levetiracetam on nocturnal sleep and daytime sleepiness in patients with partial epilepsy. J Clin Neurosci 2012;19:956–60 | Intervention |
| Beriault M, Turgeon L, Labrosse M, Berthiaume C, Verreault M, Berthiaume C, Godbout R. Comorbidity of ADHD and anxiety disorders in school-age children: impact on sleep and response to a cognitive-behavioural treatment. J Atten Disord 2015;22:22 | Outcome |
| Borusiak P, Bast T, Kluger G, Weidenfeld A, Langer T, Jenke ACW, Wiegand G. A longitudinal, randomized, and prospective study of nocturnal monitoring in children and adolescents with epilepsy: Effects on quality of life and sleep. Epilepsy Behav 2016;61:192–8 | Outcome |
| Bartlet L, Beaumont J. Treating the sleep disorders of children with disabilities and illness: a one-year project. Clin Child Psychol Psychiatry 1998;3:591–612 | Population |
| Bazil CW, Dave J, Cole J, Stalvey J, Drake E. Pregabalin increases slow-wave sleep and may improve attention in patients with partial epilepsy and insomnia. Epilepsy Behav 2012;23:422–5 | Population |
| Braam W, Didden R, Maas AP, Korzilius H, Smits MG, Curfs LM. Melatonin decreases daytime challenging behaviour in persons with intellectual disability and chronic insomnia. J Intellect Disabil Res 2010;54:52–9 | Population |
| Braam W, Didden R, Smits M, Curfs L. Melatonin treatment in individuals with intellectual disability and chronic insomnia: a randomised placebo-controlled study. J Intellect Disabil Res 2008;52:256–64 | Population |
| Brand S, Jossen S, Holsboer-Trachsler E, Pühse U, Gerber M. Impact of aerobic exercise on sleep and motor skills in children with autism spectrum disorders – a pilot study. Neuropsychiatr Dis Treat 2015;11:1911–20 | Population |
| Burke RV, Kuhn BR, Peterson JL. Brief report: a ‘storybook’ ending to children’s bedtime problems – the use of a rewarding social story to reduce bedtime resistance and frequent night waking. J Pediatr Psychol 2004;29:389–96 | Population |
| Chang YS, Lin MH, Lee JH, Lee PL, Dai YS, Chu KH, et al., Melatonin supplementation for children with atopic dermatitis and sleep disturbance a randomised clinical trial. JAMA Pediatrics 2016;170:35–42 | Population |
| Coppola G, Iervolino G, Mastrosimone M, La Torre G, Ruiu F, Pascotto A. Melatonin in wake-sleep disorders in children, adolescents and young adults with mental retardation with or without epilepsy: a double-blind, cross-over, placebo-controlled trial. Brain Dev 2004;26:373–6 | Population |
| Corkum P, Lingley-Pottie P, Davidson F, McGrath P, Chambers CT, Mullane J, et al. Better nights/better days – distance intervention for insomnia in school-aged children with/without ADHD: a randomised controlled trial. J Paediatr Psychology 2016;41:701–13 | Population |
| Cronin S, Gose L, Gottschlich MM, Kagan RJ. Retrospective examination of the effectiveness of zolpidem for sleep in paediatric burn patients with a known history of attention deficit/hyperactivity disorder. J Burn Care Res 2012;1:S117 | Population |
| Cronin SD, Gottschlich MM, Gose LM, Kagan RJ. Zolpidem and sleep in pediatric burn patients with attention deficit/hyperactivity disorder. Pediatr Nurs 2015;41:132–4, 140 | Population |
| Bruin EJ, Bogels SM, Oort FJ, Meijer A. Improvements of adolescent psychopathology after insomnia treatment: results from a randomised controlled trial over 1 year. J Sleep Res 2016;25:91 | Population |
| De Cock VC, Diene G, Molinas C, Masson VD, Kieffer I, Mimoun E, et al. Efficacy of modafinil on excessive daytime sleepiness in Prader–Willi syndrome. Am J Med Genet A 2011;155A:1552–7 | Population |
| Einfeld SL, Smith E, McGregor IS, Steinbeck K, Taffe J, Rice LJ, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A 2014;164A:2232–9 | Population |
| Freeman KA. Treating bedtime resistance with the bedtime pass: a systematic replication and component analysis with 3-year-olds. J Appl Behav Anal 2006;39:423–8 | Population |
| Gigli GL, Grubar JC, Colognola RM, Amata MT, Pollicina C, Ferri R, et al. Butoctamide hydrogen succinate and intensive learning sessions: effects on night sleep of Down’s syndrome patients. Sleep 1987;10:563–9 | Population |
| Gupta M, Gupta YK, Aneja S, Kohli K. Effects of add-on melatonin on sleep in epileptic children on carbamazepine monotherapy: a randomised placebo controlled trial. Sleep Biol Rhythms 2004;2:215–19 | Population |
| Gupta M, Aneja S, Kohli K. Add-on melatonin improves sleep behaviour in children with epilepsy: randomised, double-blind, placebo-controlled trial. J Child Neurol 2005;20:112–15 | Population |
| Holsboer-Trachsler E. Aerobic exercise and skill training improved objective sleep and motor skills in children suffering from autism spectrum disorder (ASD) – a pilot study. Biol Psychiatry 2015;77:10010–1710 | Population |
| Hvolby A, Bilenberg N. Use of Ball Blanket in attention-deficit/hyperactivity disorder sleeping problems. Nord J Psychiatry 2011;65:89–94 | Population |
| Ishizaki A, Sugama M, Takeuchi N. [Usefulness of melatonin for developmental sleep and emotional/behaviour disorders--studies of melatonin trial on 50 patients with developmental disorders.] No To Hattatsu 1999;31:428–37 | Population |
| Ivanenko A, Crabtree VM, Tauman R, Gozal D. Melatonin in children and adolescents with insomnia: a retrospective study. Clin Pediatr 2003;42:51–8 | Population |
| Keshavarzi Z, Bajoghli H, Mohamadi MR, Salmanian M, Kirov R, Gerber M, et al. In a randomised case – control trial with 10-years olds suffering from attention deficit/hyperactivity disorder (ADHD) sleep and psychological functioning improved during a 12-week sleep-training program. World J Biol Psychiatry 2014;15:609–19 | Population |
| Ledet D, Aplin-Kalisz C, Filter M, Dycus P. A pilot study to assess a teaching intervention to improve sleep-wake disturbances in parents of children diagnosed with epilepsy. J Neurosci Nurs 2016;48:2–14 | Population |
| McArthur AJ, Budden SS. Sleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatment. Dev Med Child Neurol 1998;40:186–92 | Population |
| Merrifield R. Evaluation of a health visitor-led sleep and behaviour clinic. Community Pract 2005;78:283–8 | Population |
| Mila M, Cecilia A, Gracia A, Antonio M, Oscar T, Patricio P. Evaluation of oral iron supplementation in paediatric maintenance insomnia. Sleep Med 2013;14:e207–8 | Population |
| Ming X, Gordon E, Kang N, Wagner GC. Use of clonidine in children with autism spectrum disorders. Brain Dev 2008;30:454–60 | Population |
| Mohammadi MR, Mostafavi SA, Keshavarz SA, Eshraghian MR, Hosseinzadeh P, Hosseinzadeh-Attar MJ, et al. Melatonin effects in methylphenidate treated children with attention deficit hyperactivity disorder: a randomised double blind clinical trial. Iran J Psychiatry 2012;7:87–92 | Population |
| Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Fatty acids and sleep in UK children: subjective and pilot objective sleep results from the DOLAB study – a randomised controlled trial. J Sleep Res 2014;23:364–88 | Population |
| O’Callaghan FJ, Clarke AA, Hancock E, Hunt A, Osborne JP. Use of melatonin to treat sleep disorders in tuberous sclerosis. Dev Med Child Neurol 1999;41:123–6 | Population |
| O’Connell A, Vannan K. Sleepwise: addressing sleep disturbance in young children with developmental delay. Aust Occup Ther J 2008;55:212–14 | Population |
| Paasch V, et al. Preparing children with autism spectrum disorders for overnight sleep studies: a case series. Clin Pract Pediatr Psychol 2016;4:153–63 | Population |
| Pelsser LM, Frankena K, Buitelaar JK, Rommelse NN. Effects of food on physical and sleep complaints in children with ADHD: a randomised controlled pilot study. Eur J Pediatr 2010;169:1129–38 | Population |
| Prince JB, Wilens TE, Biederman J, Spencer TJ, Wozniak JR. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psychiatry 1996;35:599–605 | Population |
| Ross C, Davies P, Whitehouse W. Melatonin treatment for sleep disorders in children with neurodevelopmental disorders: an observational study. Dev Med Child Neurol 2002;44:339–44 | Population |
| Schlarb AA, Brandhorst I, Hautzinger M. [Mini-KiSS – a multimodal group therapy intervention for parents of young children with sleep disorders: a pilot study.] Z Kinder Jugendpsychiatr Psychother 2011;39:197–206 | Population |
| Schroder C, Schmidt C, Kilic-huck U, Danion-Grilliat A, Bourgin P. Impact of long-term melatonin treatment for sleep disturbances in a child psychiatric population. Eur Child Adolesc Psychiatry 2015;24:1–303 | Population |
| Silva LM, Scalock M, Ayres R, Bunse C, Budden S. Qigong massage treatment for sensory and self-regulation problems in young children with autism: a randomised controlled trial. Am J Occupation Ther 2009;63:423–32 | Population |
| Simcock G. Sleep problems: assessing behavioural approaches. Community Pract 1999;72:128–30 | Population |
| Smits MG, Nagtegaal EE, van der Heijden J, Coenen AM, Kerkhof GA. Melatonin for chronic sleep onset insomnia in children: a randomized placebo-controlled trial. J Child Neurol 2001;16:86–92 | Population |
| Smits MG, van der Heijden KR, Meijer A, Coenen AM, Kerkhof GA. Melatonin improves health status and sleep in children with idiopathic chronic sleep-onset insomnia: a randomised placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 2003;42:1286–93 | Population |
| Stores R, Stores G. Evaluation of brief group-administered instruction for parents to prevent or minimise sleep problems in young children with Down syndrome. J Appl Res Intellect Disabil 2004;17:61–70 | Population |
| Szeinberg A, Borodkin K, Dagan Y. Melatonin treatment in adolescents with delayed sleep phase syndrome. Clin Pediatr 2006;45:809–18 | Population |
| Van Maanen A, Meijer AM, Smits MG, Oort FJ. Termination of short term melatonin treatment in children with delayed Dim Light Melatonin Onset: effects on sleep, health, behaviour problems, and parenting stress. Sleep Med 2011;12:875–9 | Population |
| Ward F, Nanjappa M, Hinder SA, Roy M. Use of melatonin for sleep disturbance in a large intellectual disability psychiatry service. Int J Developmental Disabil 2015;61:182–7 | Population |
| Williams TI. Evaluating effects of aromatherapy massage on sleep in children with autism: a pilot study. Evid Based Complement Alternat Med 2006;3:373–7 | Population |
| Abdollahian E, Mohareri F. Evaluation of the effect of melatonin on improvement of sleep quality in children with attention deficit/hyperactivity disorder whom received ritalin. Eur Psychiatry 2015;30(Suppl. 1):584 | Study design |
| Catherall C, Williams-Jones A. Managing sleep problems in children. Learning Disability Practice 2011;14:14–19 | Study design |
| Andersen IM, Kaczmarska J, McGrew SG, Malow BA. Melatonin for insomnia in children with autism spectrum disorders. J Child Neurol 2008;23:482–5 | Study design |
| Anonymous. Does melatonin improve sleep in children with neurodevelopmental disorders? DTB 2013;51:4 | Study design |
| Anonymous. Melatonin for primary insomnia? DTB 2009;47:74–7 | Study design |
| Anonymous. Melatonin improves sleep in children with ADHD and chronic insomnia: no effect found on behaviour, cognition or quality of life. CABL 2007;23:1–7 | Study design |
| Anonymous. Neurodevelopmental disorders: No evidence for efficiency of weighted blankets in improving sleep in children with autism spectrum disorder. Nat Rev Neurol 2014;10:428 | Study design |
| Arns M, Kenemans JL. Neurofeedback in ADHD and insomnia: vigilance stabilisation through sleep spindles and circadian networks. Neurosci Biobehav Rev 2014;44:183–94 | Study design |
| Ayyash HF, Preece P, Morton R, Cortese S. Melatonin for sleep disturbance in children with neurodevelopmental disorders: prospective observational naturalistic study. Expert Rev Neurother 2015;15:711–17 | Study design |
| Behavioural Interventions To Improve Sleep Disturbance in Typically Developing Children May be Effective for Children with Autism Spectrum Disorder. Clinician’s Research Digest: Adult Populations. 2012;30:6 | Study design |
| Bourdon GG. Treating insomnia in children with ADHD . . . ‘towards a psychology of understanding: attention-deficit/hyperactivity disorder’. JAAPA 1998;11:76 | Study design |
| Bouvier M, Claustrat B, Franco P. Melatonin treatment in autism spectrum disorders (ASD): preliminary results. J Sleep Res 2012;236 | Study design |
| Brown LW. Looking beyond the polysomnograph in ADHD. Sleep 2006;29:745–6 | Study design |
| Christodulu KV, Durand VM. Reducing bedtime disturbance and night waking using positive bedtime routines and sleep restriction. Focus Autism Other Dev Disabli 2004;19:130–9 | Study design |
| Christodulu KV. Reducing bedtime disturbances and night waking using positive bedtime routines and sleep restriction. Diss Abstr Int 2000;3:130–9 | Study design |
| Colville GA, Watters JP, Yule W, Bax M. Sleep problems in children with Sanfilippo syndrome. Dev Med Child Neurol 1996;38:538–44 | Study design |
| Cortese S, Brown TE, Corkum P, Gruber R, O’Brien LM. First step in sleep problems in ADHD is promoting healthy sleep habits. CABL Update 2013;15:8 | Study design |
| Damiani JM, Sweet BV, Sohoni P. Melatonin: an option for managing sleep disorders in children with autism spectrum disorder. Am J Health Syst Pharm 2014;71:95–101 | Study design |
| De Leersnyder H, Bresson JL, de Blois MC, Souberbielle JC, Mogenet A, Delhotal-Landes B, et al. Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith–Magenis syndrome. J Med Genet 2003;40:74–8 | Study design |
| De Leersnyder H, Zisapel N, Laudon M. Prolonged-release melatonin for children with neurodevelopmental disorders. Pediatr Neurol 2011;45:23–6 | Study design |
| Didden R, Curfs LM, van Driel S, de Moor JM. Sleep problems in children and young adults with developmental disabilities: home-based functional assessment and treatment. J Behav Ther Exp Psychiatry 2002;33:49–58 | Study design |
| Didden R, de Moor JMH, Curfs LMG, Behavioural treatment of sleep problems in three children with developmental disabilities. BJDD 2004;50:13–19 | Study design |
| Doan RJ. Risperidone for insomnia in PDDs. Can J Psychiatry 1998;43:1050–1 | Study design |
| Does melatonin improve sleep in children with neurodevelopmental disorders? Drug Ther Bull 2013;51:4 | Study design |
| Durand VM, Christodulu KV. Description of a sleep-restriction program to reduce bedtime disturbances and night waking. J Posit Behav Interv 2004;6:83–91 | Study design |
| Durand VM, Gernert-Dott P, Mapstone E. Treatment of sleep disorders in children with developmental disabilities. Res Pract Persons Severe Disabl 1996;21:114–22 | Study design |
| Durand VM. Treating sleep terrors in children with autism. J Posit Behav Interv 2002;4:66–72 | Study design |
| Duwez M, Rouby A, Renou M, Chanoine S, Sabourdy C, Vercueil L, et al. Effectiveness and safety of oral melatonin premedication in sleep EEG in paediatrics. Int J Clin Pharm 2016;38:580–1 | Study design |
| Efron D, Lycett K, Sciberras E. Response to: sleep medication in patients with attention-deficit/hyperactivity disorder. Sleep Med 2015;16:208 | Study design |
| Elkhayat HA, Hassanein SM, Tomoum HY, Abd-Elhamid IA, Asaad T, Elwakkad AS. Melatonin and sleep-related problems in children with intractable epilepsy. Pediatr Neurol 2010;42:249–54 | Study design |
| Esbensen AJ, Beebe DW, Byars KC, Hoffman EK. Use of sleep evaluations and treatments in children with Down syndrome. J Dev Behav Pediatr 2016;37:629–36 | Study design |
| Espie CA, Wilson A. Improving sleep-wake schedules among people with mental handicaps: some preliminary case material. Behav Cogn Psychother 1993;21:51–5 | Study design |
| Fauteck J, Schmidt H, Lerchl A, Kurlemann G, Wittkowski W. Melatonin in epilepsy: first results of replacement therapy and first clinical results. Biol Signals Recept 1999;8:105–10 | Study design |
| Gee BM, Peterson T, Buck A, Lloyd K. Improving sleep quality using weighted blankets among young children with an autism spectrum disorder. Int J Ther Rehabil 2016;23:173–81 | Study design |
| Giannotti F, Cortesi F, Cerquiglini A, Bernabei P. An open-label study of controlled-release melatonin in treatment of sleep disorders in children with autism. J Autism Dev Disord 2006;36:741–52 | Study design |
| Giannotti F, Cortesi F, Antonella C, Bernabei P. Long-term melatonin treatment for sleep disorders in autistic children. A two-year follow-up study. Amer Acad Sleep Med 2004:94–4 | Study design |
| Greydanus DE. Causes and management of sleep problems in children with developmental disabilities. Child Care Health Dev 2010;36:29 | Study design |
| Gupta R, Hutchins J. Melatonin: a panacea for desperate parents? (Hype or truth). Arch Dis Child 2005;90:986–7 | Study design |
| Hart-Santora D, Hart LL. Clonidine in attention deficit hyperactivity disorder. Ann Pharmacother 1992;26:37–9 | Study design |
| Hätönen T, Kirveskari E, Heiskala H, Sainio K, Laakso ML, Santavuori P. Melatonin ineffective in neuronal ceroid lipofuscinosis patients with fragmented or normal motor activity rhythms recorded by wrist actigraphy. Mol Genet Metab 1999;66:401–6 | Study design |
| Herrmann S. Counting sheep: sleep disorders in children with autism spectrum disorders. J Pediatr Health Care 2016;30:143–54 | Study design |
| Hodoba D, Schmidt D. Biperiden for treatment of somnambulism in adolescents and adults with or without epilepsy: clinical observations. Epilepsy Behav 2012;25:517–28 | Study design |
| Howlin P. A brief report on the elimination of long term sleeping problems in a 6-year-old autistic boy. Behav Cogn Psychother 1984;12:257–60 | Study design |
| Hvolby A, Bilenberg N. Use of ball blanket in attention deficit hyperactivity disorder sleeping problems. Eur Child Adolesc Psychiatry 2011;20:7–223 | Study design |
| Hylkema T, Vlaskamp C. Significant improvement in sleep in people with intellectual disabilities living in residential settings by non-pharmaceutical interventions. J Intellect Disabil Res 2009;53:695–703 | Study design |
| Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders – a case series. Eur Child Adolesc Psychiatry 2005;14:34–40 | Study design |
| Itoh M, Hayashi M, Hasegawa T, Shimohira M, Kohyama J. Systemic growth hormone corrects sleep disturbance in Smith–Magenis syndrome. Brain Dev 2004;26:484–6 | Study design |
| Jan JE, Espezel H. Melatonin treatment of chronic sleep disorders. Dev Med Child Neurol 1995;37:279–80 | Study design |
| Jan JE, O’Donnell ME. Use of melatonin in the treatment of paediatric sleep disorders. J Pineal Res 1996;21:193–9 | Study design |
| Jan JE, Espezel H, Goulden KJ. Melatonin in sleep disorders of children with neurodevelopmental disabilities. Melatonin in psychiatric and neoplastic disorders. Progress Psychiatry 1998;55:169–88 | Study design |
| Jan JE, Freeman RD, Fast DK. Melatonin treatment of sleep–wake cycle disorders in children and adolescents. Dev Med Child Neurol 1999;41:491–500 | Study design |
| Jan JE, Espezel H, Appleton RE. The treatment of sleep disorders with melatonin. Dev Med Child Neurol 1994;36:97–107 | Study design |
| Jan MM. Melatonin for the treatment of handicapped children with severe sleep disorders. Pediatr Neurol 2000;23:229–32 | Study design |
| Jin CS, Hanley GP, Beaulieu L. An individualized and comprehensive approach to treating sleep problems in young children. J Appl Behav Anal 2013;46:161–80 | Study design |
| Kawabe K, Horiuchi F, Oka Y, Ueno S. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioural symptoms in autistic disorder. Case Rep Psychiatry 2014;2014:561071 | Study design |
| Knight R, Johnson M. Using a behavioural treatment package for sleep problems in children with autism spectrum disorders. Child Fam Behav Ther 2014;36:204–21 | Study design |
| Konishi T, Masuko K, Naganuma Y, Hongou K, Yagi S. Flunitrazepam for sleep disturbance in children with intractable epilepsy. Brain Dev 1995;17:69–72 | Study design |
| Lerchl A, Reiter RJ. Treatment of sleep disorders with melatonin. BMJ 2012;345:e6968 | Study design |
| Malow B, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, Burnette C. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J Autism Dev Disord 2012;42:1729–37 | Study design |
| Malow BA, Adkins KW, McGrew SG, Wang L, Goldman SE, Fawkes D, Burnette C. Erratum to: Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J Autism Dev Disord 2012;42:1738 | Study design |
| Malow BA, Adkins KW, McGrew SG, Surdyka K, Goldman SE, Wofford D. Impact of supplemental melatonin on sleep and behaviour in children with autism spectrum disorders. 2009;32:A64 | Study design |
| Malow BA, Adkins K, Goldman SE, McGrew SG, Burnette C, Wofford D, et al. Supplemental melatonin decreases sleep latency in children with autism. Amer Acad Sleep Med 2011:A267 | Study design |
| Malow BA, Adkins KW, McGrew SG, Surdyka K, Wofford D. Supplemental melatonin improves sleep in children with autism spectrum disorders. Ann Neurol 2009;66:S31 | Study design |
| McArthur I, Maisey J, Zuberi SM. Does slow release melatonin (SR) prevent recurrent night wakenings in children with neurodisability? Amer Academy Sleep 2003;26:A122–3 | Study design |
| McArthur I, Maisey J, Zuberi SM. Impact of a nurse led sleep clinic on the use and evaluation of melatonin FR (Fast release) for sleep problems in children with neurodisability. Amer Academy Sleep 2003;126:A123 | Study design |
| McLay LLK, France K. Empirical research evaluating non-traditional approaches to managing sleep problems in children with autism. Dev Neurorehabil 2014;19:123–34 | Study design |
| Melatonin for insomnia in ADHD. Nurses’ Drug Alert. AJN 2010;34:3–4 | Study design |
| The Brown University Child and Adolescent Behaviour Letter (CABL) 2007;23:1–7. URL: https://onlinelibrary.wiley.com/doi/epdf/10.1002/cbl.20043 (accessed 26 July 2018) | Study design |
| Miyamoto A, Oki J, Takahashi S, Okuno A. Serum melatonin kinetics and long-term melatonin treatment for sleep disorders in Rett syndrome. Brain Dev 1999;21:59–62 | Study design |
| Moon EC, Corkum P, Smith IM. Case study: a case-series evaluation of a behavioural sleep intervention for three children with autism and primary insomnia. J Pediatr Psychol 2011;36:47–54 | Study design |
| Moore P. The use of social stories in a psychology service for children with learning disabilities: a case study of a sleep problem. Br J Learn Disabil 2004;32:133–8 | Study design |
| Mullane J, Corkum P. Case series: evaluation of a behavioral sleep intervention for three children with attention-deficit/hyperactivity disorder and dyssomnia. J Atten Disord 2006;10:217–27 | Study design |
| Nakano T, Koyama E, Taniike M, Unase K. Application of an infrared sensor to home-monitoring of rest-activity patterns in a child with sleep disturbance. Sleep Biol Rhythms 2003;1:173–4 | Study design |
| Narasingharao K, Pradhan B, Navaneetham J. Sleep Disorder, Gastrointestinal Problems and Behaviour Problems Seen in Autism Spectrum Disorder Children and Yoga as Therapy: A Descriptive Review. J Clin Diagn Res 2016;10:VE01–3 | Study design |
| Niederhofer H, Staffen W, Mair A, Pittschieler K. Brief report: melatonin facilitates sleep in individuals with mental retardation and insomnia. J Autism Dev Disord 2003;33:469–72 | Study design |
| Paavonen EJ, Nieminen-von Wendt T, Vanhala R, Aronen ET, von Wendt L. Effectiveness of melatonin in the treatment of sleep disturbances in children with Asperger disorder. J Child Adolesc Psychopharmacol 2003;13:83–95 | Study design |
| Palm L, Blennow G, Wetterberg L. Long-term melatonin treatment in blind children and young adults with circadian sleep-wake disturbances. Dev Med Child Neurol 1997;39:319–25 | Study design |
| Piazza CC, Fisher W. A faded bedtime with response cost protocol for treatment of multiple sleep problems in children. J Appl Behav Anal 1991;24:129–40 | Study design |
| Piazza CC, Fisher WW. Bedtime fading in the treatment of pediatric insomnia. J Behav Ther Exp Psychiatry 1991;22:53–6 | Study design |
| Piazza CC, Hagopian LP, Hughes CR, Fisher WW. Using chronotherapy to treat severe sleep problems: a case study. Am J Ment Retard 1998;102:358–66 | Study design |
| Piazza CC, Fisher W, Moser H. Behavioral treatment of sleep dysfunction in patients with the Rett syndrome. Brain Dev 1991;13:232–7 | Study design |
| Pillar G, Shahar E, Peled N, Ravid S, Lavie P, Etzioni A. Melatonin improves sleep-wake patterns in psychomotor retarded children. Pediatr Neurol 2000;23:225–8 | Study design |
| Pillar G, Etzioni A, Shahar E, Lavie P. Melatonin treatment in an institutionalised child with psychomotor retardation and an irregular sleep-wake pattern. Arch Dis Child 1998;79:63–4 | Study design |
| Reeve A, Miers S. Managing sleep problems in children with special needs. Health Visit 1994;67:230–1 | Study design |
| Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Dev Med Child Neurol 1999;41:60–6 | Study design |
| Richdale AL. Treatment of sleep problems in children with autism. J Intellect Disabil Res 2000;44:441–1 | Study design |
| Ross C, Morris B, Whitehouse W. Melatonin treatment of sleep-wake cycle disorders in children and adolescents. Dev Med Child Neurol 1999;41:850 | Study design |
| Rukovets O. Mending Children’s sleep habits, improving family relations. Neurol Today 2012;12:37 | Study design |
| Scott J. Melatonin an effective treatment for sleep problems in children with autism. Foods Matter (USA) 2009:15 | Study design |
| Snowden S. Question 1. Does melatonin improve sleep pattern in children with attention deficit hyperactivity disorder? Arch Dis Child 2009;94:321–2 | Study design |
| Stevinson C. Valerian may improve sleep of children with intellectual deficits. Focus Altern Complement Ther 2002;7:347–8 | Study design |
| Summers JA, Lynch PS, Harris JC, Burke JC, Allison DB, Sandler L. A combined behavioural/pharmacological treatment of sleep–wake schedule disorder in Angelman syndrome. J Dev Behav Pediatr 1992;13:284–7 | Study design |
| Thackeray EJ, Richdale AL. The behavioural treatment of sleep difficulties in children with an intellectual disability. Behav Interv 2002;17:211–31 | Study design |
| Tjon Pian Gi CV, Broeren JP, Starreveld JS, Versteegh FG. Melatonin for treatment of sleeping disorders in children with attention deficit/hyperactivity disorder: a preliminary open label study. Eur J Pediatr 2003;162:554–5 | Study design |
| Top tips. Learning Disability Today. 2013;13:20 | Study design |
| Toussaint FS, Baverstock AC. An audit of melatonin use and parental satisfaction in a district general hospital. Arch Dis Child 2012;97(Suppl. 1):A128 | Study design |
| Uberos J, Augustin-Morales MC, Molina Carballo A, Florido J, Narbona E, Munoz-Hoyos A. Normalisation of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin levels after a therapeutic trial with melatonin in children with severe epilepsy. J Pineal Res 2011;50:192–6 | Study design |
| Wassmer E, Carter PF, Quinn E, McLean N, Welsh G, Seri S, Whitehouse WP. Melatonin is useful for recording sleep EEGs: a prospective audit of outcome. Dev Med Child Neurol 2001;43:735–8 | Study design |
| Waters F. Natural alternative offers hope on sleep disorders: the National Network for Learning Disability Nurses held its annual conference in Pontypridd last week. Nurs Stand 2004;18:9–10 | Study design |
| Weiskop S, Matthews J, Richdale A. Treatment of sleep problems in a 5-year-old boy with autism using behavioural principles. Autism 2001;5:209–21 | Study design |
| Wilens TE, Spencer TJ, Swanson JM, Connor DF, Cantwell D. Combining methylphenidate and clonidine: a clinically sound medication option. J Am Acad Child Adolesc Psychiatry 1999;38:614–19 | Study design |
| Yamashita Y, Matsuishi T, Murakami Y, Kato H. Sleep disorder in Rett syndrome and melatonin treatment. Brain Dev 1999;21:570 | Study design |
| Zhdanova IV, Wurtman RJ, Wagstaff J. Effects of a low dose of melatonin on sleep in children with Angelman syndrome. J Pediatr Endocrinol Metab 1999;12:57–67 | Study design |
| Zolpidem not effective for ADHD-associated insomnia. CABL Update 2009;11:7 | Study design |
| Zotter H, Kerbl R, Millner M, Kurz R. Methylphenidate and melatonin for sleep disorder with optic glioma. J Am Acad Child Adolesc Psychiatry 2001;40:992–3 | Study design |
Appendix 5 Study details for pharmacological interventions
| Study details and study design | Trial treatments | Participant characteristics: number randomised, mean age (SD); % male, % diagnosis | Type of sleep disturbance/prior interventions | Risk of bias (low, unclear or high) |
|---|---|---|---|---|
| Melatonin vs. placebo | ||||
| Parallel trials | ||||
|
Appleton et al. 48 Associated publications: Gringras et al. 108 and Appleton et al. 48,109 UK |
Melatonin: capsules, starting dose of 0.5 mg, taken 45 minutes before bedtime for 12 weeks. Dose could be raised to 2, 6 and 12 mg in the first 4 weeks, then maintained Placebo: capsule-matching placebo, starting dose of 0.5 mg. Dose could be escalated through 2 mg and 6 mg to 12 mg in the first 4 weeks, then maintained |
N = 146 Melatonin (n = 70): 106.0 months (34.8 months), range 44–181 months 70% male DD, 19%; DD and epilepsy, 11%; DD and ASD, 43%; DD, ASD and epilepsy, 0; DD and ‘other’, 27% Placebo (n = 76): 100.7 months (37.4 months), range 37–186 months 63% male DD, 9%; DD and epilepsy, 7%; DD and ASD, 39%; DD, ASD and epilepsy, 3%; DD and ‘other’, 38% |
Sleep disturbance
|
Low |
|
Cortesi et al. 110 Italy |
Melatonin: controlled release, dose 3 mg, taken at 9 p.m. for 12 weeks CBT: 4 sessions, weekly individual sessions Melatonin and CBT: as above Placebo: identical placebo tablet (same appearance smell and flavour to active treatment), dose 3 mg, taken at 9 p.m. for 12 weeks |
N = 160 Melatonin (n = 40): 6.8 years (0.9 years) 82% male ASD, 100% Melatonin and CBT (n = 40): 6.4 years (1.1 years) 80% male ASD, 100% CBT (n = 40): 7.1 years (0.7 years) 83% male ASD, 100% Placebo (n = 40): 6.3 years (1.2 years) 84% male ASD, 100% |
Sleep disturbance
|
High |
|
Van der Heijden et al. 111 Associated publications: Hoebert et al. 112 The Netherlands |
Melatonin: fast release, 3 mg (if < 40 kg), 6 mg (if > 40 kg) at 19.00, 4 weeks Control: identical appearing placebo at 19.00, 4 weeks |
N = 107 Melatonin (n = 54): 9.1 years (2.3 years) 66% male ADHD subtype: ADHD-C, 77%; ADHD-I, 17%; ADHD-HI, 4% Placebo (n = 53): 9.3 years (1.8 years) 83% male ADHD subtype: ADHD-C, 69%; ADHD-I, 25%; ADHD-HI, 4% |
Sleep disturbance
|
Unclear |
| Crossover trials | ||||
|
Camfield et al. 113 Individualised ‘N of 1 crossover trial’ Canada |
Melatonin: capsules 0.5 mg for three cases and 1.0 mg for three cases, taken at 18.00, given for 1 week Placebo: capsules, identical-looking to intervention, given for 1 week Ten-week trial in which, for each of the five 2-week intervals, the child was randomised to receive daily placebo or melatonin for the first week with the alternate agent given in the second week |
N = 6 7.3 years, range 3–13 years 67% male Congenital optic nerve hypoplasia: 17%; moderate spastic quadriplegia 17%; schizencephaly, spastic diplegia and severe learning disabilities, 17%; moderate learning disabilities and severe athetoid cerebral palsy, 17%; developmental disorder with extreme hyperactivity and moderate to severe learning disabilities, 17%; autosomal recessive disorder with cerebellar hypoplasia and tapetoretinal degeneration but with stable moderate to severe learning disabilities, 17% |
Sleep disturbance
|
High |
|
Dodge and Wilson114 Associated publications: Hoebert et al. 112 USA |
Melatonin: capsules, dose 5 mg, taken at 20.00 for 2 weeks Placebo: capsule and filler packaged to be identical to the melatonin capsules, dose 5 mg, taken at 20.00 for 2 weeks Washout period of 1 week |
N = 36 89 months, range 13 months to 15 years Gender: not reported Cerebral palsy, 75%; autism, 10%; genetic syndrome, 10%; learning disabilities, 5% |
Sleep disturbance
|
Unclear |
|
Garstang and Wallis69 UK |
Melatonin: dose 5 mg, for 4 weeks (type of dose not reported) Placebo: capsule, dose not reported, for 4 weeks Washout period of 1 week |
N = 11 8.6 years (3.1 years), range 5–15 years 64% male All ASD plus: mild LD, 27%; moderate LD, 18%; severe LD, 9%; dyspraxia, 18%; no other diagnoses, 27% |
Sleep disturbance
|
High |
|
Jain et al. 115 Associated publications: Jain et al. 116 USA |
Melatonin: sustained release, tablet form, dose 9 mg, taken 30 minutes before bedtime for 4 weeks Placebo: tablet form, the same appearance as melatonin tablets, dose not reported Washout period of 1 week |
N = 11 8.4 years (1.3 years) 70% male Epilepsy type: focal, 70%; generalised, 20%; undetermined, 10% |
Sleep disturbance
|
High |
|
Wasdell et al. 117 Associated publications: Carr et al. 118 Canada |
Melatonin: controlled release, capsules, dose 5 mg, taken 20–30 minutes before bedtime for 10 days Placebo: identical capsules to melatonin, dose 5 mg, 20–30 minutes before bedtime for 10 days ‘Placebo washout’ period of 3–5 days |
N = 51 7.4 years, range 2.1–17.8 years; 62% Severe intellectual loss, 64%; cerebral palsy, 52%; epilepsy, 46%; visual impairment, 40%; lack of mobility, 36%; ASD, 32% |
Sleep disturbance
|
Unclear |
|
Weiss et al. 70 Canada |
Melatonin: short acting, dose 5 mg, taken 20 minutes before bedtime for 10 days Placebo: dose X mg for 10 days The 10-day treatment phases were separated by a ‘placebo washout’ period of 5 days |
N = 23 10.29 years, range 6.5–14.7 years 91% male All ADHD. Subtype: inattentive, 4%; ADHD-C, 96% |
Sleep disturbance
|
Unclear |
|
Wirojanan et al. 119 USA |
Melatonin: dose 3 mg, taken 30 minutes prior to bedtime for 2 weeks Placebo: dose 3 mg, taken 30 minutes before bedtime for 2 weeks No washout period |
N = 18 5.5 years (3.6 years), range 2–15.3 years 92% male Fragile X syndrome + ASD, 25%; fragile X syndrome, 25%; ASD, 42%; fragile X syndrome permutation, 8% |
Sleep disturbance
|
Unclear |
|
Wright et al. 106 UK |
Melatonin: standard release melatonin, dose 2 mg, taken 30–40 minutes before bedtime. Dose increased by 2 mg every 3 nights to a maximum of 10 mg. Taken for 3 months Placebo: capsules identical to melatonin, dose 2 mg, taken 30–40 minutes before bedtime. Dose increased by 2 mg every three nights to a maximum of 10 mg. Taken for 3 months Washout period of 1 month |
N = 20 9.0 years (2.9 years), range 4–16 years 80% male Autism, 70%; atypical autism, 20%; Asperger syndrome, 10% |
Sleep disturbance
|
Unclear |
| Melatonin vs. melatonin | ||||
| Crossover trials | ||||
|
Hancock et al. 120 UK |
Melatonin: 1 × 5 mg plus 1 × 5 mg placebo, 30 minutes before bedtime for 2 weeks Melatonin: 2 × 5 mg of melatonin (10 mg in total), taken 30 minutes before bedtime for 2 weeks Washout period of 2 weeks |
N = 8 12.1 years (10.0 years), range 1.5–31 years 57% male All tuberous sclerosis Of participants aged < 19 years 6.9 years (4 years), range 1.5–11 years; 40% All tuberous sclerosis |
Sleep disturbance
|
High |
|
Jan et al. 121 Canada |
Melatonin: sustained-release melatonin, variable doses from 2–10 mg, taken 30 minutes before bedtime, 11 days Control: fast-release melatonin, variable does from 2–10 mg, taken for 11 days No washout period |
N = 16 Age range: 4–21 years N = 15 eligible participants (under 18 years) 9.3 years, range 4–16 years Gender: not reported Multidisabled but not reported separately for RCT |
Sleep disturbance
|
High |
Appendix 6 Study quality: studies evaluating melatonin
| Study | Domain | ||||||
|---|---|---|---|---|---|---|---|
| 1: adequate sequence generation? | 2: allocation concealment? | 3: blinding? | 4: incomplete outcome data addressed? | 5: free of selective reporting? | 6: free of other bias? | Additional questions for crossover trials | |
| Melatonin vs. placebo | |||||||
| Parallel trials | |||||||
| Appleton et al.48 |
Yes Computer-generated sequence |
Yes Pharmacy led |
Yes Double blinding, using identical capsules and central allocation. In two cases participants were unblinded in order to treat serious adverse events |
Yes Similar numbers lost in each arm for similar reasons. Data analysed on intention-to-treat basis |
Yes Protocol available, amendments detailed, all outcomes reported |
Yes No other bias obvious |
N/A |
| Cortesi et al.110 |
Yes Computer-generated sequence |
Unclear Allocation was done by someone independent of treatment personnel and via computer, but it is not clear if the allocation was concealed to the independent person (e.g. via opaque envelopes) |
Unclear Authors argue that researchers and participants did not know which arm they were in (there were four arms in total). Although this is plausible for the melatonin only and placebo-only arms, in which the capsules were identical, it is less plausible for the melatonin combined with CBT and the CBT only, as they would obviously know if they were receiving CBT. Similarly, the principal investigators (PIs) observed tapes of the CBT for adherence to protocol, so they would know at least who was receiving CBT (even if they could not know if they were receiving this alone or with melatonin), so this is in agreement with the above judgement |
No None were lost to follow-up but 16 dropped out owing to difficulties in administering medication, non-compliance and lack of improvement. An additional 10 were excluded from analysis owing to missing actigraphy data. The final analysis excluded all above. There were no details of the potential implications of missing data/dropouts. All six dropouts in placebo group were because of lack of improvement; none of the dropouts in the other groups were for this reason |
Unclear No protocol is referenced in the paper and one was not found on searching. The study reports outcomes that one would expect. However, table 3 reports two outcomes that are not referred to anywhere else in the paper (Naptime and Bedtime). It is not clear what they are In the methods section, the paper only states what the primary outcomes were and so it is presumed that Naptime and Bedtime are secondary, but this is not stated so there is a low degree of selective reporting, as the authors state that groups and clinicians were unaware of what they were receiving and both groups were treated as per an identical protocol |
Yes | N/A |
| Van der Heijden et al.111 |
Unclear Randomisation in blocks of four to keep number in each treatment group closely balanced. No details of how sequence generated |
Yes Central allocation. Performed by a hospital pharmacist who was not connected to study |
Yes Reported as double-blind and the placebo tablets looked identical to intervention tablets. Investigators and participants unware of allocation. Code broken after all treatment completed and data recorded |
Yes No loss to follow-up. One child in placebo group and one in the melatonin group were withdrawn prior to treatment because they started other treatment without permission |
Unclear Protocol available on the International Standard Randomised Controlled Trial Number website (although it says it was retrospectively registered). Outcomes listed on trial record are: (primary) sleep onset, latency, total sleep duration (acting and sleep log) and Dim Light Melatonin Onset and (secondary) sustained attention and response inhibition, severity of ADHD symptoms, quality of life and side effects. The study paper reports these outcomes but also reports that difficulty falling asleep (rated in severity) was a primary outcome (p. 235). CBCL (behavioural problems) was also reported (which may be the measure of ADHD severity listed in protocol but this is not clear) An additional file that includes all raw data for study outcomes is available |
Yes The number of missing data on behaviour and quality-of-life measures was large but authors suggest that the risk of bias in the analysis is small because the missing data are equal in both groups |
N/A |
| Crossover trials | |||||||
| Camfield et al.113 |
Unclear No details are provided in the paper |
Unclear No details are provided in the paper |
Unclear Participants were blinded, although not clear how. Not clear if researchers blinded at analysis |
Yes No loss to follow-up and data presented for all six participants |
Unclear No protocol is referenced in the paper and one was not found on searching. Three outcome measures are used: average hours of sleep per 24 hours, number of awakenings between 9 p.m. and 7 p.m. per day and number of nights with no arousals from 8 p.m. to 7 a.m. The reported ‘nights without awakening’ was 10 p.m. to 7 a.m. rather than 9 p.m. to 7a.m. Not clear whether this was a typo or the outcome measure changed |
No Small sample and only descriptive data are reported |
|
| Dodge and Wilson114 |
Unclear Randomisation performed by pharmacy personal but sequence generation not described |
Unclear Randomisation and packaging of capsules done by research pharmacy personal but it is not clear if these research pharmacy personal were part of research team, and no detail is provided about whether or not allocation was concealed |
Yes Identical packaging. Parents asked to guess which treatment their children were on prior to unblinding. Randomisation by research pharmacists, not study team |
Unclear No flow chart. In total, 20 out of 36 participants who enrolled completed the study but it was not clear how missing data were addressed. Losses to follow-up were reported as having no significant difference on age, primary diagnosis, epilepsy or vision impairment compared with those completing study. Non-completion was because of lost sleep logs (n = 1), lost medication (n = 2), changed mind about participating (n = 4), intercurrent illness (n = 2), family emergency (n = 1), family lost to follow-up (n = 3) and medication not working (n = 2) |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without protocol, it is difficult to be certain |
Unclear Details of sample characteristics between treatment and placebo at baseline are not reported, making the comparability unclear |
N/A |
| Garstang and Wallis69 |
Yes Random numbers table |
Yes Pharmacy led |
Unclear Reported as double blind but there were few details as to how this was achieved. There was no reference to treatment and placebo capsules being identical |
No Dropouts: after drugs recall following empty placebo capsules, n = 3; owing to a house move, n = 1; and on the start of a child protection enquiry, n = 1. No detail on whether last two were receiving placebo or melatonin. Data were analysed for trial completers only |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
No There was an assumption that the child was asleep (and logged as asleep) if parents were not disturbed. Child could have been awake but did not disturb the parent. There was also a drugs recall and the trial was stopped early, so it is not clear if and how this affected subsequent procedure and blinding of treatment |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? Washout period used, but no analysis of carry-over effect Are unbiased data available? There does not appear to be any analysis of difference between the treatment and control groups |
| Jain et al.115 |
Yes Computer-randomised number generator |
Yes Pharmacy led |
No The appearance of capsules was identical but the statistician and pharmacist were unblinded |
Unclear In total, 1 out of 11 did not complete the study owing to being unable to swallow the capsules The limitations section states that there were some secondary outcome data lost for two participants and that this may have had an impact on the results. It is not clear what the missing data or impacts were |
No Protocol is available on clinical trials register. Outcomes listed on register include: (primary) sleep efficiency and improved lapse time on psychomotor vigilance task and (secondary) improvement in eleptiform discharges on electroencephalography and seizure frequency. However, in the paper, more outcomes are reported. The primary outcomes in the paper are SOL and wakefulness. Twenty secondary outcomes are listed, including the one listed as primary (sleep efficiency) on the trials register |
Yes |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? Carry-over effects observed for two REM variables and so adjusted analysis Are unbiased data available? When carry-over effects are not observed, authors report using Wilcoxon rank-sum test and two sample tests |
| Wasdell et al.117 |
Unclear Blocked randomisation method led by pharmacy, ‘in which every four patients had equal probability of receiving either of the two treatment sequences’ (p. 58). However, no detail given on how the randomisation sequence was generated |
Yes Pharmacy led |
Yes Patients, caregivers, the study investigator and clinical staff were blinded to the medication randomisation. Unblinding occurred at the end of the study |
Yes Overall, 50 out of 51 randomised participants completed trial. One withdrew owing to illness. Only the 50 who completed the trial were included in the analysis |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Yes |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? Washout period used and authors undertook analysis to test for carry-over effect and report that there were none Are unbiased data available? Yes. Paired t-test used |
| Weiss et al.70 |
Unclear No details are provided |
Yes Pharmacy led |
Unclear Authors state that all participants and study personal blinded to the order of treatment during randomisation phase. This implies that blinding may not have extended after randomisation (e.g. at data collection and analysis). However, no further detail is given so it is difficult to judge this |
Yes 3/22 discontinued because of protocol violations but outcomes unchanged whether included or not (were excluded in results presented in paper) |
Unclear No protocol referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without protocol, it is difficult to be certain. Furthermore, CADS-P was not reported, other than that no significant change was observed; therefore, the data cannot be included in a meta-analysis |
Yes |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Not clear Can it be assumed that the trial was not biased from carry-over effects? Washout period used and authors undertook analysis to test for carry-over effect and report there were none Are unbiased data available? Yes. Paired analysis used |
| Wirojanan et al.119 |
Unclear No details are provided |
Unclear Authors say that the allocation was concealed but do not say how |
Yes Authors say that assignment to the treatment condition was concealed until the end of the study. Allocation key with investigator (locked in file) until the end of the study |
Yes In total, 12 out of 18 participants completed trial. No details of characteristics of the six who did not complete. Reasons were sleep diaries not completed/actigraphy data not readable (n = 2 placebo, n = 1 melatonin); actigraphy watch not worn during first treatment phase (melatonin) (n = 1); actigraphy watch taken off in first phase (placebo) (n = 1); study protocol not followed (n = 1). Data only from the 12 were analysed Missing output data dealt with by complete-case analysis and last observation carried forward |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Unclear Authors report that normality assumption for parametric paired test was violated owing to highly skewed data and presence of outliers. The authors present both parametric and non-parametric analyses. The authors imply in the discussion that some families may have been practising sleep hygiene, with variability in this. There is no further information about this but the implication is that this was possible and not minimised or controlled. Without any further information (e.g. how many families practised sleep hygiene), it is difficult to judge whether or not this has introduced bias |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? No Can it be assumed that the trial was not biased from carry-over effects? No. No washout period was used between crossover. The authors say that this is because of the half-life of melatonin but is this conclusive? Different studies have used different approaches – some having a washout period and others not. It is not clear or certain if this could present a bias Are unbiased data available? Paired t-tests used, both parametric and non-parametric |
| Wright et al.106 |
Unclear No details are provided |
Yes Remote pharmacy led |
Yes Double-blind randomisation was undertaken by someone with no contact with the research team and the capsules were identical |
Yes There were four withdrawals (three in melatonin first, one in placebo first, but two while taking melatonin and two while taking placebo). Reasons: too difficult for parents to administer medication, n = 1; parents unable to complete sleep diary questionnaire measures, n = 1; very significant sleep benefits early in first arm, n = 1; and apparent ineffectiveness of medication, n = 1. The last completed one arm of trial and so data were included in some of analyses. This participant was in melatonin arm first and another clinician prescribed another drug Only one withdrawal (early very significant effects) was likely to be related to the true outcome |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Yes |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? No Can it be assumed that the trial was not biased from carry-over effects? A washout period of 1 month used Are unbiased data available? Paired t-tests were used |
| Melatonin vs. melatonin | |||||||
| Crossover trials | |||||||
| Hancock et al.120 |
Unclear The hospital pharmacy led randomisation but it is not clear how the sequence was generated The pharmacy department generated random numbers that determined whether individuals started with 5 mg or 10 mg of melatonin. This is assessed as LOW risk/Yes |
Yes Pharmacy led |
Yes The study was double blind, identical capsules were used and unblinding occurred after completion of trial |
Unclear In total, one out of eight sets of data was lost in the mail. There are no details of lost patient characteristics and the implications of this There is no detail of how other missing data are dealt with |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
No It had a small sample, but it is a pilot study |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? A washout period used but no analysis of carry-over effect Are unbiased data available? Paired t-tests used |
| Jan et al.121 |
Unclear No details are provided in the paper |
Unclear No details are provided in the paper |
Unclear Paper reports that both investigators and caregivers were blinded but no detail is given as to how (e.g. identical packs) |
Yes No loss to follow-up |
No No protocol was referenced and one was not found after searching. However, the only outcome reported is changes in sleep pattern, which is reported as whether or not there was a response to treatment (with little detail on what exactly was measured). There are no data presented regarding what this response was and it is, thus, rather vague. Perhaps this is because it was a dose-finding RCT, but even so, the lack of detail on the outcome makes the reporting appear selective and lacks transparency |
No Participants had already been treated with another form of melatonin. It is not clear how the 16 participants were selected; for example, whether or not they fit eligibility criteria |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? No, as there was no washout period between crossover. There was no analysis of carry-over effect Are unbiased data available? No. The type of analysis used was not clear |
Appendix 7 Child sleep-related outcomes in trials comparing melatonin with placebo
| Study | Child sleep-related outcome assessed | Method of assessmenta | Definitionsb |
|---|---|---|---|
| Global measures and composite scores | |||
| Appleton et al.48 | Sleep efficiency | Actigraphy | Number of minutes spent sleeping in bed/total number of minutes spent in bed × 100 |
| TST | Actigraphy, sleep diary | The amount of time between the time that the child went to sleep and the time that the child woke up the following morning minus any night-time awakenings | |
| Camfield et al.113 | TST | Sleep diary | Average sleep per day |
| Cortesi et al.110 | CSHQ | CSHQ (total score and subscales) | A 33-item parent questionnaire that includes items relating to a number of key sleep domains, which are grouped into the following subscales: bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness. The authors provide a reference |
| Sleep efficiency | Actigraphy | No definition provided, other than that ‘these variables were averaged over 7 nights for each assessment phase’110 | |
| TST | Actigraphyc | No definition provided, other than that ‘these variables were averaged over 7 nights for each assessment phase’110 | |
| WASO | Actigraphyc | Inclusion criteria defined WASO as wake after sleep onset > 30 minutes that occurred on ≥ 3 nights a week | |
| Van der Heijden et al.111 | Sleep efficiency | Actigraphyc | Percentage of time spent asleep in the period from lights out until the time of leaving bed |
| TST | Actigraphyc | Actual amount of sleep, calculated as the period from sleep onset to wake up time minus estimated time awake in the period from sleep onset until awake time | |
| Dodge and Wilson114 | TST | Sleep log | Duration of sleep |
| Garstang and Wallis69 | TST | Sleep chart | No definition provided |
| Jain et al.115 | Sleep efficiency | Polysomnography, actigraphyd | ‘The American Academy of Sleep Medicine standard definition was used’115 |
| TST | Polysomnography, actigraphy, sleep diary | ‘The American Academy of Sleep Medicine standard definition was used’115 | |
| WASO | Polysomnography, actigraphyd | The sum of wake time in minutes from sleep onset to the final awakening | |
| Sleep Behaviour Questionnaire | Sleep Behaviour Questionnaire | Sensory Behaviour Questionnaire includes a set of six questions related to sleep–wake habits and a 29-item Likert-type rating scale. The subscales include parasomnias, parent/child interaction, sleep fragmentation, daytime drowsiness and bedtime difficulties. The Sensory Behaviour Questionnaire total score is considered a global index of sleep problems, with higher scores representing more sleep problems | |
| Wasdell et al.117 | Sleep efficiency | Actigraphy, somnolog | No definition provided |
| TST | Actigraphy, somnolog | For somnolog-measured TST: ‘Total night-time sleep as recorded on care-giver completed somnologs which provided a running record of the time when the child was asleep or awake during the relevant measurement period’117 | |
| Longest sleep episode | Somnolog, actigraphy | No definition provided | |
| Weiss et al.70 | TST | Actigraphy, somnolog | Total sleep duration |
| Wirojanan et al.119 | TST | Actigraphyc | The time from sleep onset to wake-up time minus the time awake during the night |
| Wright et al.106 | TST | Sleep diary | No definition provided |
| Sleep initiation | |||
| Appleton et al.48 | SOL | Actigraphy, sleep diary | The time taken to fall asleep; the number of minutes between lights out/‘snuggle down’ time and sleep start time |
| Cortesi et al.110 | SOL | Actigraphyc | Inclusion criteria defined SOL as mixed sleep onset and maintenance insomnia |
| Bedtime | Actigraphyc | No definition provided | |
| Van der Heijden et al.111 | Difficulty falling asleep | Sleep log | Averaged score (over seven days) on an item asking parents how difficult it was for the child to fall asleep in the evening (1 = not difficult; 5 = very difficult) |
| Sleep onset | Actigraphyc | Start of a period of at least 10 minutes of consecutively recorded immobile actigraphy data following lights out | |
| SOL | Actigraphyc | Time from lights out until sleep onset | |
| Dodge and Wilson114 | SOL | Sleep log | Time taken to fall asleep |
| Garstang and Wallis69 | SOL | Sleep chart | No definition provided |
| Jain et al.115 | SOL | Polysomnography, actigraphy (data not reported) | The time in minutes from lights out to sleep onset |
| Bedtime | Sleep diary | No definition provided | |
| Wasdell et al.117 | SOL | Actigraphy, somnolog | No definition provided |
| Weiss et al.70 | SOL | Actigraphy, somnolog | The amount of time between when the child was put to bed and when he or she fell asleep |
| Wirojanan et al.119 | SOL | Actigraphyc | The time from bedtime to sleep onset time |
| Sleep onset | Actigraphyc | The clock time that the child fell asleep | |
| Wright et al.106 | SOL | Sleep diary | Time from start of bedtime routine to sleep |
| Sleep latency | Sleep diary | Time from drug to sleep | |
| Sleep maintenance | |||
| Camfield et al.113 | Number of night wakings | Sleep diary | Total number of awakenings between 9.00 p.m. and 7.00 a.m. per day/the number of days of complete data |
| Nights without awakening | Sleep diary | Nights without awakening between 10.00 p.m. and 7.00 a.m. per day/the number of days of complete data | |
| Van der Heijden et al.111 | Wake time | Actigraphy | Last epoch of actigraphically assessed immobility before the start of a 10-minute consecutive period of activity around the time of leaving bed |
| Non-specified night-time sleep disturbance: moving time | Actigraphy | Percentage of time spent moving during the assumed sleep period | |
| Dodge and Wilson114 | Number of night wakings | Sleep log | No definition provided |
| Garstang and Wallis69 | Number of night wakings | Sleep chart | No definition provided |
| Jain et al.115 | Wake time | Sleep diary | No definition provided |
| Wasdell et al.117 | Number of night wakings | Actigraphy, somnolog | No definition provided |
| Wirojanan et al.119 | Number of night wakings | Actigraphyc | No definition provided |
| Wright et al.106 | Number of night wakings | Sleep diary | No definition provided |
| Sleep scheduling | |||
| Cortesi et al.110 | Naptime | Actigraphyc | No definition provided |
| Other outcomes | |||
| Van der Heijden et al.111 | Interdaily stability | Actigraphy | Degree of resemblance between the activity patterns on individual days (range from 0 to 1, higher values indicating more stable rhythms) |
| Interdaily variability | Actigraphy | Fragmentation of periods of rest (or sleep) and activity (or wakefulness) (range from 0 to 2, with higher values indicating more fragmented rhythms) | |
| L5 (average activity during the least active 5 hours) | Actigraphy | Average activity during the least active 5-hour period in the average 24-hour activity rhythm | |
| Jain et al.115 | Arousal | Arousal index | American Academy of Sleep Medicine standard definitions were used for arousal index. The authors provide a reference |
| Percentage of sleep stages | Sleep diary | American Academy of Sleep Medicine standard definitions were used for percentage of sleep stages (N1, N2, N3 or REM %). The authors provide a reference | |
Appendix 8 Child sleep-related outcomes in trials comparing melatonin with melatonin
| Study | Child sleep-related outcome assessed | Method of assessmenta | Definitionsb |
|---|---|---|---|
| Global measures and composite scores | |||
| Hancock et al.120 | TST | Sleep diary | No definition provided |
| Jan et al.121 | Changes in sleep pattern | Sleep charts and parental history | Based on sleep onset, the number of awakenings, duration of sleep and how the children behaved the following day |
| Sleep initiation | |||
| Hancock et al.120 | Bedtime settling: SOL | Sleep diary | Time taken to fall asleep |
| Sleep maintenance | |||
| Hancock et al.120 | Night waking: number of night wakings | Sleep diary | The mean number of awakenings each night |
Appendix 9 Study details for non-pharmacological interventions
| Study details and study design | Intervention details | Participant characteristics: number randomised (N), mean age (SD); % male; % diagnosis | Type of sleep disturbance/prior sleep interventions | Risk of bias (low, unclear or high) |
|---|---|---|---|---|
| Parent-directed comprehensive tailored interventions | ||||
| Parallel trials | ||||
|
Beresford et al. 21 Associated publications: Beresford et al. 136 UK |
Intervention: two face-to-face sessions for the assessment and development of a sleep management strategy and parent training were given. Parents were supported to implement the strategy via telephone calls Control: the usual approach. As for the intervention but implementation support was delivered via home visits |
N = 13 Home visits (n = 6):Telephone support (n = 7): |
Sleep disturbance
|
High |
|
Hiscock et al. 138 Associated publications: Papadopoulos et al. 139 Australia |
Intervention: one session was given for the assessment and development of a sleep management strategy and parent training. Parents were supported to implement the strategy via one face-to-face session and one telephone call Control: usual care |
N = 244 Behavioural programme (n = 122):Usual care (n = 122): |
Sleep disturbance
|
High |
|
Johnson et al. 107 Associated publications: Turner140 USA |
Intervention: one session for assessment and the development of sleep management strategy was given; there were five sessions for training the parent in the strategy. Implementation support was delivered via one face-to-face session Control: non-sleep-related parent education delivered in an identical manner to the intervention group |
N = 40 Behavioural parent training programme (n = 20, data on n = 15):Non-sleep-related parent education (n = 20, data on n = 18): |
Sleep disturbance
|
High |
|
Moss et al. 124 Associated publications: O’Connell et al. 141,142 Australia |
Intervention: two parent training workshops, followed by a home visit for the assessment and development of a sleep management strategy were given. Implementation support was delivered via one home visit followed by telephone calls as needed Control: waiting list control |
N = 26 Intervention and control not reported separately |
Sleep disturbance
|
High |
|
Sciberras et al. 125 Associated publications: Sciberras et al. ,143 Fulton et al. 144 and Sciberras and Rinehart145 Australia |
Intervention: two sessions for the assessment and development of a sleep management strategy and training parents in the strategy were given. Implementation support was delivered via a single telephone call followed by further face-to-face session if needed Control: a single session for the assessment and development of a sleep management strategy and training parents in the strategy was given. No implementation support was given |
N = 27 Extended behavioural programme (n = 14):Brief behavioural programme (n = 13): |
Sleep disturbance
|
High |
| Before-and-after studies | ||||
|
Austin et al. 123 Australia |
Two parent training workshops were given, followed by a home visit for the assessment and development of sleep management strategy, followed by a third workshop. Implementation support was delivered via one face-to-face session and a telephone call |
N = 8 3.9 years 100% male Pervasive developmental disorder: |
Sleep disturbance
|
High |
|
Beresford et al. 21 Associated publications: Beresford et al. 136 and Stuttard et al. 45,137 UK |
Two sessions were given for the assessment and development of sleep management strategy and training the parents in the strategy. Implementation support was delivered via fortnightly face-to-face sessions |
N = 12 2.88 years (1.25 years) 50% male ASC, 25%; LD, 25%; and unknown/awaiting diagnosis, 50% |
Sleep disturbance
|
High |
|
Quine and Wade146 Associated publications: Wade and Wade147 UK |
Two sessions were given for the assessment and development of a sleep management strategy and training parents in the strategy. Implementation support delivered via face-to-face sessions |
N = 25 Range 3–21 years 69% male LD, 100% |
Sleep disturbance
|
High |
|
Weiskop et al. 126 Before-and-after study with multiple baseline Australia |
Four sessions were given for the assessment and development of a sleep management strategy and training parents in the strategy. Implementation support (via telephone calls) was delivered from outset of intervention. It continued after training sessions completed with face-to-face session and further telephone calls | N = 13
|
Sleep disturbance
|
High |
| Parent-directed comprehensive non-tailored interventions | ||||
| Parallel trials | ||||
|
Adkins et al. 127 Associated publications: Malow et al. 148 USA |
Intervention: training curriculum contained in a booklet provided to parents Control: no booklet provided |
N = 36 Overall age 6.4 years (2.6 years) Sleep education pamphlet (n = 18):Control (no pamphlet) (n = 18): |
Sleep disturbance
|
High |
|
Montgomery et al. 49 Associated publications: Montgomery et al. 49 UK |
Intervention a: a training curriculum was contained in a booklet provided to parent Intervention b: a training curriculum (content identical to intervention a) was delivered via a face-to-face session Control: there was no intervention for 6 weeks, at which point they were re-randomised into an active treatment group |
N = 66 Overall age range: 27–101 months 64% male Brief treatment (n = 22):Conventional treatment (n = 34):Control (n = 26): |
Sleep disturbance Present for at least 3 months and not because of physical problem: Prior interventions |
High |
|
Malow et al. 128 USA |
Intervention: the training curriculum was delivered via two group-delivered sessions. Implementation support was delivered via telephone calls Control: training curriculum delivered via single, face-to-face session. Implementation support was delivered via telephone calls |
N = 80 Group-delivered parent education programme (n = 39):Individual-delivered parent education programme (n = 41): |
Sleep disturbance
|
High |
| Before-and-after studies | ||||
|
Beresford et al. 21 Associated publications: Beresford et al. 136 and Stuttard et al. 45,137 UK |
Group delivery of a training curriculum over four sessions |
N = 22 8.91 years (3.25 years) 50% male ASC, 64%; LD, 27%; physical or sensory disability, 4%; and no diagnosis, 4% |
Sleep disturbance
|
High |
|
Beresford et al. 21 Associated publications: Beresford et al. 136 and Stuttard et al. 45,137 UK |
A training curriculum delivered via a single half-day workshop |
N = 25 7 years (3.30 years) 64% male ASC, 36%; LD, 16%; physical or sensory disability, 16%; and ASC other, 20% |
Sleep disturbance
|
High |
|
Bramble149 Associated publications: Bramble122 UK |
A training curriculum delivered via a single session. Implementation support was delivered via telephone calls |
N = 15 7.2 years (2.6 years) 67% male All severe learning disability, aetiological factor known for 60%: Down syndrome, 20%; macrocephaly, 7%; Angelman syndrome, 7%; Smith–Magenis syndrome, 7%; carcinuria:, 7%; perinatal cerebral anoxia, 7%; and cerebral leucodystrophy, 7% |
Sleep disturbance
|
High |
|
Reed et al. 129 Associated publications: Reed et al. 150 Canada |
Group delivery of a training curriculum over three sessions |
N = 22 5.8 years (2.7 years) 82% male ASD, 100% |
Sleep disturbance
|
Unclear |
|
Yu et al. 151 Hong Kong |
Group delivery of a training curriculum over three sessions, supported by weekly telephone calls. Implementation support was delivered by telephone calls |
N = 54 4.78 years (0.85 years) 79.6% male ASD, 96%; and Asperger syndrome, 2% |
Sleep disturbance
|
High |
| Non-comprehensive parent-directed interventions | ||||
| Cluster trials | ||||
|
Wiggs and Stores152 Associated publications: Wiggs and Stores153,154 UK |
Intervention: a tailored intervention. There was a single session for the assessment and development of a sleep management strategy and training the parent in the strategy. Implementation support was delivered via telephone calls Control: no intervention |
N = 30 Individually tailored behavioural programme (n = 15):Control (n = 15): |
Sleep disturbance ‘Severe problems’ defined as:Prior interventions |
High |
| Before-and-after study | ||||
|
Peppers et al. 155 USA |
Prescriptive sleep hygiene advice delivered in a single session |
N = 23 Prescriptive sleep hygiene intervention (n = 23):Control (n = 30): |
Sleep disturbance
|
High |
| Other non-pharmacological interventions | ||||
| Parallel trials | ||||
|
Piazza et al. 157 USA |
Intervention: FBRC, for 10 days. The study author delivered the face-to-face (home visits) and booklet intervention Control: bedtime scheduling |
N = 14 FBRC (n = 7):Bedtime scheduling (n = 7): |
Sleep disturbance Prior interventions |
High |
| Crossover trials | ||||
|
Francis and Dempster156 Australia |
Intervention: valerian: 500 mg per tablet, 30 mg per kilogram of body weight as a single nightly dose at least 1 hour before bedtime, taken for 2 weeks Control: to give the same appearance and odour, the placebo contained 25 mg of whole room V. edulis extract, taken for 2 weeks |
N = 5 Age range: 7–14 years 100% male Moderate intellectual disability, 40%; mild to moderate intellectual disability, 20%; genetic disorder, 20%; episodic fibril convulsions, 20%; hyperactivity and behaviour problems, 20%; ADD, 20%; and ADHD, 20% |
Sleep disturbance ‘Significant sleep problems’ including:Prior interventions |
High |
|
Gringras et al. 36 Associated publications: Gringras et al. 36 UK |
Intervention: a weighted blanket. The blanket weight was 2.25 kg (small) or 4.5 kg (large). Blankets used for 12–16 days and were given by researchers at home/clinic visits Control: a placebo blanket |
N = 73 Intervention + control (n = 36):Control and intervention (n = 37): |
Sleep disturbance
|
High |
| Before-and-after studies | ||||
|
Guilleminault et al. 158 USA |
Light therapy + behavioural programme. Light exposure was given daily at 07.00 and 12.00 for 45 minutes (the overall treatment duration, setting and practitioner unclear) |
N = 142.9 years, range 9 months to 4 years 57% male Moderate to severe learning disabilities, 100% |
Sleep disturbance
|
High |
|
Oriel et al. 159 A–B–A withdrawal design USA |
An aquatic exercise programme. 60 minutes of aquatic exercise was undertaken two times per week |
N = 8 8.88 years, range 6–11 years 63% male ASD, 100% |
Sleep disturbance
|
High |
|
Yehuda et al. 160 Controlled before-and-after study Israel |
Intervention: essential fatty acids supplement that comprised 90 g of α-linolenic and 360 g of linoleic acid in mineral oil. Two capsules per day for 10 weeks Control: placebo (and healthy control group) |
N = 78 Essential fatty acid supplement (n = 40):ADHD control (n = 38):Healthy control (n = 22): |
Sleep disturbance ‘Sleep deprived’ Prior interventions Not reported |
Unclear |
|
Yu and Hong161 China |
Acupuncture and ear-point taping. Two courses of acupuncture treatment were given once every other day, three times a week, with 36 sessions constituting one course. Ear-point taping was given three times a week, with 36 sessions constituting one course. Two courses were required |
N = 30 6.9 years (3.1 years) 77% male Learning disabilities, 100% |
Sleep disturbance Sleep restlessness including:Prior interventions |
High |
Appendix 10 Intervention and control details table: non-pharmacological studies
| Study | Mode of delivery: active arm(s) | Intervention overview: the chronological order of delivery | The intervention period; the duration of each parent–practitioner contact; intensity | Content of the intervention | Group delivery only: were parents supported to apply learning to their own child? | For a specific ND? | Location | Who delivered it? |
|---|---|---|---|---|---|---|---|---|
| Tailored interventions | ||||||||
| Study objective: evaluation of intervention clinical effectiveness | ||||||||
| Parallel trials | ||||||||
| Hiscock et al.138 | Individual (face to face and telephone) | Intervention:
|
Intervention:
|
Intervention:
|
N/A | ADHD | Paediatrican’s office, hospital clinic or home | Psychologist or consultant paediatrician |
| Johnson et al.107 | Individual (face to face) | Intervention:
|
Intervention:
|
Intervention:Session content tailored to the family’s specific needs and ‘a cumulative bedtime and sleep intervention plan developed’, modified as needed over the intervention period [A detailed overview of the sessions and content of the control were provided in the paper] |
N/A | ADHD | Clinic | Masters-level doctoral students or senior behavioural analyst |
| Moss et al.124 | Group and individual (face to face and telephone) | Intervention:232
|
Intervention:
|
Intervention:
|
N/A | No |
Workshop: not stated Face-to-face sessions: home |
Allied health workers |
| Before-and-after studies | ||||||||
| Austin et al.123 | Group and individual (face to face and telephone) | Intervention:232
|
Intervention period: 15 weeks Workshops (n = 3) at 3 hours each Face-to-face sessions (n = 1): at ≈2 hours (Workshops 1 and 2 were 2 weeks apart; the face-to-face session was a week later; and workshop 3 was a week after that) Telephone calls: duration of calls not reported; delivered weekly for 6 weeks |
|
N/A | No |
Workshops: not stated Face-to-face session: home |
Psychologist or manager of early intervention centre |
| Beresford et al.21 | Individual (face to face) |
2 × face-to-face sessions Implementation support: face-to-face sessions – number determined by progress |
Intervention period: variable, usually 12–16 weeks Face-to-face sessions (n = variable): duration not reported |
|
N/A | No | Community centre and home | Community-based disability link workers |
| Quine and Wade146 | Individual (face to face) |
2 × face-to-face sessions Implementation support: face-to-face sessions – number determined by progress |
Intervention period: variable, 6–28 weeks Face-to-face sessions (n = variable): duration not reported. They took place weekly, but after session 3, the authors sought to increase the spacing of the sessions |
|
N/A | No | Home | Health professional: health visitor, community nurse or district nurse, school nurse |
| Weiksop et al.126 | Individual (face to face and telephone) |
4 × face-to-face sessions Implementation support: 1 × face-to-face session Telephone calls weekly after session 4, diminishing in intensity after session 5 |
Intervention period: minimum 7 weeks Face-to-face session 1: duration not reported Face-to-face sessions 2–4: duration not reported; consecutive weeks Face-to-face session 5: duration not reported; delivered 5 weeks after session 4 |
|
N/A | No | Home and clinic | ‘Therapist’ |
| Study objective: comparative evaluation of modes of delivering implementation support [home visit (arm a) vs. telephone call (arm b)] | ||||||||
| Parallel trial | ||||||||
| Beresford et al.21 |
Arm A: individual (face to face) Arm B: individual (face to face and telephone) |
Arm A:
|
Arm A:
|
|
N/A | No | Home | Specialist health visitors |
| Study objective: comparative evaluation of intervention intensity (brief vs. extended) | ||||||||
| Parallel trial | ||||||||
| Sciberras et al.143 |
Brief intervention: individual (face to face) Extended intervention: individual (face to face and telephone) |
Brief intervention:
|
Brief intervention:
|
Brief and extended intervention:
|
N/A | ADHD | Hospital | Paediatric trainee or child psychologist |
| Non-tailored interventions | ||||||||
| Study objective: evaluation of intervention effectiveness | ||||||||
| Parallel trial | ||||||||
| Adkins et al.127 | Written material | Intervention:
|
N/A | Intervention:
|
N/A | ADHD | N/A | N/A |
| Before-and-after studies | ||||||||
| Beresford et al.21 | Group |
4 × workshops (maximum group size 8) Implementation support: none except that included in workshop curriculum |
Intervention period: 5 weeks Workshops (n = 4) at 3 hours; delivered over a 5-week period |
Workshop 1: ‘children’s sleep; behavioural approaches to behaviour management; positive and negative reinforcers; communication’21 Workshop 2: ‘sleep routines; structuring bedtime; using reinforcers to manage behaviour; planning bedtime routines: bedroom environment’21 Workshop 3: ‘principles of behavioural analysis then applied to children’s sleep problems’21 Workshop 4: ‘specific strategies to manage sleep problem behaviours; the use of medication’21 |
Yes | No | Community setting | Staff based in Learning Disability Child Mental Health Service |
| Beresford et al.21 | Group |
1 × workshop (maximum group size 20). 233 (www.scope.org.uk/support/services-directory/sleep-solutions-training-for-families; accessed 20 April 2016) Implementation support: none |
Intervention period: a single face-to-face session Face-to-face session (n = 1) at 5 hours |
Workshop topics: ‘the impact of sleep disorders; your existing routine; the bedroom environment; does your child’s impairment matter?; techniques, tips and resources; making a change, when the time is right’21 | No | No | Community setting | Staff trained in managing sleep disturbance and working for Scope UK (a UK charity for children with cerebral palsy and their families) |
| Bramble149 | Individual (face to face and telephone) |
1 × face-to-face session Implementation support: telephone calls delivered on 3 consecutive days after the face-to-face session, with additional calls arranged, if necessary, for up to 2 weeks |
Intervention period: up to 2 weeks Face-to-face session (n = 1): duration not reported Telephone calls (n = variable): duration not reported. Delivered 3 consecutive days after face-to-face session, with additional calls arranged if necessary for up to 2 weeks |
Session 1: education in children’s sleep and behaviour modification based on the following principles: ‘setting regular bed and wake times; establishing routines; setting appropriate mood for sleep; rapid settling of child at bedtime; parental withdrawal after settling; ignoring protestations; returning child to room with minimal contact; rewarding and praising improved morning and night-time behaviour’149 (p. 544) The content of the session was subject to minimal adaptation only in specific cases Telephone calls: provided support to parents as they changed the ways that they managed sleep disturbance |
N/A | No | Clinic or home | Consultant psychiatrist |
| Reed et al.129 | Group |
3 × workshops (group size 3–5) Implementation support: none except that included in workshop curriculum |
Intervention period: 3 weeks Workshops (n = 3): at 2 hours; delivered over consecutive weeks |
Workshop 1: establishing daytime and night-time habits and the child’s bedtime routine Workshop 2: minimising night wakings and early morning awakenings Workshop 3: addressing individualized sleep concerns129 (pp. 939–40) [A detailed overview of the workshop content is provided in the paper] |
Yes | ASD | Not reported | Neurology sleep specialist, with assistance from a nurse educator and educational consultant |
| Yu et al.151 | Group and individual (telephone) |
3 × workshops (maximum group size 8) Implementation support: telephone calls weekly for the entire intervention period |
Intervention period: 7 weeks Workshops (n = 3): duration not reported; delivered over consecutive weeks Telephone calls (n = 7): weekly for entire intervention period; duration of calls not reported |
Workshop 1: ASD and sleep; impacts; sleep hygiene Workshop 2: promoting sleep – behavioural approaches Workshop 3: other concerns; sleep medication for sleep problems Telephone calls: offer parents immediate support post workshop, answer enquiries and gain feedback |
Yes | ASD | Not reported | Trained research nurse |
| Study objective: comparative evaluation of mode of intervention delivery vs. no intervention | ||||||||
| Parallel trial | ||||||||
| Montgomery et al.49 |
Arm A: written material Arm B: individual (face to face) |
Arm A:
|
Arm A:
|
Arms A and B:
|
N/A | No | Arm A:
|
Arms A and B:
|
| Study objective: comparative evaluation of alternative modes of intervention delivery | ||||||||
| Parallel trial | ||||||||
| Malow et al.128 |
Arm A: group and individual (telephone) Arm B: individual (face to face and telephone) |
Arm A:
|
Arm A:
|
Arms A and B:
|
Group delivery only: were parents supported to apply learning to their own child?’: Yes | ADHD | Arm A:
|
Arms A and B:
|
| Non-comprehensive parent-directed interventions | ||||||||
| Tailored intervention: content restricted to behavioural principles of managing problem behaviour | ||||||||
| Study objective: evaluation of intervention clinical effectiveness (intervention vs. attention control) | ||||||||
| Parallel trial | ||||||||
| Wiggs and Stores152 |
Intervention: individual (face to face and telephone) Attention control: individual (face to face) |
Intervention:
|
Intervention:
|
Intervention:
|
N/A | No | Both arms:
|
‘The researcher’ |
| Tailored intervention: content restricted to sleep hygiene | ||||||||
| Before-and-after study | ||||||||
| Peppers155 | Individual (face to face) | Intervention:
|
Intervention period: a single face-to-face session Face-to-face session (n = 1): duration not reported |
Session content: 1. The parent/caregiver and child view 6-minute standardised sleep hygiene video (see p. e44 for content) 2. Opportunity to discuss the video and key aspects of the sleep hygiene routine with practitioner 3. Development of a patient specific sleep hygiene routine, then embedded in the electronic health record for documentation 4. Provision of a written copy of the patient specific sleep hygiene routine’155 (p. e46) |
N/A | ADHD | Clinic | Physician or nurse practitioner |
| Other non-pharmacological interventions | ||||||||
| Parallel trials | ||||||||
| Piazza et al.157 | Individual (face to face) | Intervention:
|
‘Average treatment length 8 weeks’ |
Faded bedtime with response cost involved establishing a bedtime in which it was likely that the child would fall asleep within 15 minutes. Response cost involved keeping the child awake for 1 hour if they did not fall asleep within 15 minutes of bedtime Bedtime reduced by half an hour each night, if the child fell asleep within 15 minutes of bedtime the previous night. Bedtime increased by half an hour if the child did not fall asleep within 15 minutes the previous night |
N/A | No | Hospital | Not specified |
| Crossover trials | ||||||||
| Francis and Dempster156 | Individual (face to face) | Intervention:
|
Treatment A: 2 weeks Washout period 1: 1 week Treatment B: 2 weeks Washout period 2: 1 week |
Intervention:
|
N/A | No | Tablets administered at home | Parent administered tablets to child |
| Gringas et al.36 | Not explicitly stated, reasons for using weighted blankets ‘the deep pressure and more consistent sensory input provided by weighted items reduces the body’s physiological level of arousal and stress, which might improve sleep’ | Intervention:
|
At baseline, participants received a weighted blanket, which was used for 12–16 days At 2-week follow-up, the initial blanket was removed and the alternative blanket was provided and used for 12–16 days |
Intervention:
|
N/A | No |
Blankets received at home or clinic Visits were at home or in a clinic. Blankets used at child’s home |
Researchers |
| Before-and-after studies | ||||||||
| Guilleminault et al.158 | Individual | Intervention:
|
Overall treatment duration: unclear Light exposure duration: daily at 7 a.m. and 12 a.m. for 45 minutes |
Light therapy and behavioural programme Children were exposed to bright light (sunlight or artificial) A behavioural programme was also implemented and involved scheduled parent child interaction, scheduled naps for younger children, avoidance of naps for older children, scheduled lunch and scheduled sleep time |
N/A | No | Unclear | Unclear |
| Oriel et al.159 | Group | Intervention:
|
Intervention: 60 minutes of aquatic exercise two times per week | Aquatic exercise programme:
|
No | No | Not specified | It is not clear who delivered exercise programme – each participant was paired with a researcher or volunteer |
| Yehuda et al.160 | Individual | Intervention:
|
Two capsules per day for 10 weeks | Intervention:
|
No | No | Not specified | Not specified |
| Yu and Hong161 | Individual | Intervention:
|
Two courses of acupuncture treatment were given once every other day, three times a week, with 36 sessions’ constituting one course Ear-point taping was given three times a week with 36 sessions constituting one course. Two courses were required |
See full paper for technical details of acupuncture and ear-point taping | No | No | Not specified | Not specified |
Appendix 11 Child-related outcomes for studies evaluating parent-directed tailored interventions
| Study | Child sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Global measures and composite scores | |||
| Austin et al.123 | CSHQ | CSHQ (total score) | The CSHQ was used to measure overall total sleep disturbance in children aged 4–12 years. Subscales include bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness. There were 33 items rated on a three-point Likert scale. The authors provide a reference |
| Beresford et al.21 | CSHQ | CSHQ (total score and subscales) | The CSHQ was used to assess the severity of sleep problems in children aged 4–10 years. Parent reported. Subscales include bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness. The authors provide a reference |
| Parent-set child sleep goal(s) | Parent-set child sleep goal(s) | A 10-point scale captured progress towards goals. Parents identified up to three goals during session 1. The authors provide a reference | |
| Beresford et al.21 | CSHQ | CSHQ (total score and subscales) | The CSHQ was used to assess the severity of sleep problems in children aged 4–10 years. Parent reported. Subscales include bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness. The authors provide a reference |
| Parent-set child sleep goal(s) | Parent-set child sleep goals | A 10-point scale captured progress towards goals. Parents identified up to three goals during session 1. The authors provide a reference | |
| Hiscock et al.138 | CSHQ | CSHQ (total score) | The CSHQ was used, a validated 33-item parent-reported measure of difficulties initiating and maintaining sleep over the past week. The maximum total score was 99. The authors provide a reference |
| Sleep efficiency | Actigraphy | The ratio of time asleep to time spent in bed | |
| TST | Actigraphy | The duration of night-time sleep | |
| Johnson, et al.107 | Composite sleep index (bedtime resistance, night waking, early waking, sleeping in places other than bed) | Composite sleep index of the modified version of the Simonds and Parraga Sleep Questionnaire | A modified version of the Simon and Parraga Sleep Questionnaire completed by child’s primary caregiver. The Total Composite Sleep Index score ranged from 0 to 12. It was calculated by assigning a score to the frequency of targeted sleep problems: bedtime resistance, night awakening, early awakening and sleeping in places other than bed. Additional scores were assigned to the duration of sleep latency and night awakenings. To calculate frequency, scores were as follows: 1 = problems occurring once or twice a week and 2 = problems occurring more than several times a week. To calculate duration, scores were as follows: 1 = sleep latency lasting up to 1 hour and 2 = over 1 hour. For night awakenings, scores were as follows: 1 = awakenings lasting a few minutes and 2 = lasted longer than a few minutes. The authors provide a reference for the Simond and Parraga Sleep Questionnaire |
| Sleep efficiency | Actigraphy and sleep diary (to verify actigraphy) | Percentage of time sleeping while in bed and lights off | |
| TST | Actigraphy and sleep diary (to verify actigraphy) | No definition provided | |
| Moss et al.124 | CSHQ | CSHQ total score and subscales | ‘A comprehensive parent-report sleep screening instrument designed for school-aged children’ was used to identify behaviourally and medically based sleep problems. It consists of a total score and eight subscale scores. The CSHQ includes additional questions relating to hours of daily sleep and length of night waking. Participants only completed the questions that were used to calculate total and subscale scores. The authors provide a reference |
| Sleep goals | Goal Attainment Scale | Families defined individual child sleep goals in conjunction with the facilitator to obtain an objective measure of sleep disturbance change. There were five levels of possible outcome levels: 0%, 25%, 50%, 75% and 100%. The child’s current sleep pattern was outlined as 0%. ‘For example: If the child did not settle to sleep in less than 30 minutes on any night in the week, a parent report of 100% level of success would have been achieved when the child settled to sleep in less than 30 minutes, 6 nights a week.’ No reference was provided | |
| Sciberras et al.125 | CSHQ | CSHQ | The CSHQ was used to measure caregiver report of sleep problems and three items from the CSHQ were used to screen children for sleep apnoea. The authors provide a reference but no further details are provided |
| Weiskop et al.126 | TST | Sleep diary | The average duration of night-time sleep per week. The length of night wakings was subtracted if these data were available |
| Child’s sleep behaviour goals | GAS | The GAS was used to assess the clinical significance of any changes in child’s sleep behaviour and to provide a quantifiable measure of intervention success. The GAS was based on parent-stated goals: a separate GAS was developed for each sleep behaviour identified by parents as a goal for behaviour change. For each behaviour, 0% success was set as the baseline rate of that behaviour. The parents and therapists decided what total success (100%) meant before the intervention. This did not have to mean elimination of the sleep problem but related to the level of improvement that the parents thought would make a difference to their lives and was developmentally appropriate. Post intervention, the change in each behaviour was expressed as a percentage of success over baseline. The authors provide a reference | |
| Sleep initiation | |||
| Austin et al.123 | Bedtime settling: bedtime resistance, bedtime routine and SOL | Sleep diary | No definition provided |
| Johnson et al.107 | Bedtime settling: SOL | Actigraphy, sleep diary (to verify actigraphy) | Time from lights off to sleep onset |
| Quine and Wade146 | Bedtime settling: child settling | Parent report – Behaviour Screening Questionnaire | Settling was assessed using the behaviour screening questionnaire. Severe settling problem = ≥ 3 times per week. Mild settling problem = once or twice a week. The authors provide a reference for the Behaviour Screening Questionnaire and a detailed description of the questionnaire is provided in an appendix to the Quine and Wade report |
| Weiskop et al.126 | Bedtime settling: | ||
| Number of pre-sleep disturbances/week | Sleep diary | Pre-sleep disturbance was defined as any disruption occurring between the time that the child was put to bed and the time of sleep onset, for example crying, leaving the room or calling out | |
| Number of nights that child fell asleep in own bed | Sleep diary | No further definition provided | |
| SOL | Sleep diary | Average sleep latency per week. Sleep latency was considered as the number of minutes between first being settled to bed and sleep onset | |
| Sleep maintenance | |||
| Austin et al.123 | Night waking | Sleep diary | No definition provided |
| Waking time | Sleep diary | No definition provided | |
| Non-specified sleep disturbance: co-sleeping and severity of child’s sleep disturbance | Sleep disturbance index | A parent-reported scale of 0–2 with a total maximum score of 8. Parents reported on the severity of their child’s sleep. The index focusses on difficulties settling the child to sleep, night-time wakening, parent’s attendance to the child during the night and parental sleep loss through co-sleeping | |
| Hiscock et al.138 | Night waking: WASO | ||
| Actigraphy | No definition was provided | ||
| Non-specified night-time sleep disturbance: sleep problems | Primary caregiver report of child sleep problems over past 4 weeks | Sleep problems were rated as none, mild, moderate or severe | |
| Quine and Wade146 | Waking time: number of night wakings and sleeping in parent’s bed | Parent reported-behaviour screening questionnaire | It was classified as a severe waking problem if it occurs > 3 times per week and the child wakes for more than a few minutes, disturbs parents or goes into the parents’ room or bed. The authors provide a reference for the behaviour screening questionnaire and a detailed description of the questionnaire is provided in an appendix to the Quine and Wade report |
| Sciberras et al.125 | Non-specified night-time sleep disturbance: sleep problems | CSHQ and caregiver report | The CSHQ was used to measure care-giver report of sleep problems and three items from the CSHQ were used to screen children for sleep apnoea. The authors provide a reference but no further details are provided |
| Weiskop et al.126 | Night waking: Number of night wakings | Sleep diary | Number of night wakings per week that parents were aware of |
| Non-specified night-time sleep disturbance: number of nights/week that child co-slept | Sleep diary | Co-sleeping was not coded when the parents only lay with the child until they fell asleep at bedtime | |
| Sleep scheduling | |||
| Austin et al.123 | Napping | Sleep diary | No definition provided |
| Child-related quality of life, daytime behaviour and cognition | |||
| Austin et al.123 | Behavioural and emotional disturbance | Developmental Behaviour Checklist – parent version | Used to assess behavioural and emotional disturbance in children with developmental and intellectual disabilities. 96 items are grouped into the following subscales: disruptive/antisocial, self-absorbed, communication disturbance, anxiety and social relating. Subscales are combined to determine a total behaviour problem score. The authors provide a reference |
| Hiscock et al.138 | Child-related quality of life | PedsQL | The PedsQL version 4.0 was used for parent proxy report. It is a validated 23-item measure of quality of life for children aged 2–18 years. Items were rated on a 5-point scale. 15 items contributed to a psychosocial health summary score. Scores ranged from 0 to 100. The authors provide a reference |
| Child daytime behaviour and cognition | |||
| ADHD symptoms | ADHD Rating Scale IV (parent and teacher reported) | The ADHD Rating Scale IV parent- and teacher-reported versions. It is a validated 18-item measure of ADHD symptoms rated on 4-point scale. Nine items assess inattentive symptoms and nine assess hyperactive symptoms. The authors provide a reference | |
| Daily functioning | Daily parent rating of evening and morning behaviour | A 11-item parent-reported measure of core ADHD symptoms and behavioural problems on a 4-point scale. Scores ranged from 0 to 33. The authors provide a reference | |
| Behaviour | Strengths and difficulties questionnaire | Parent and teacher versions. It is a validated 25-item measure of behavioural and emotional problems for children aged 4–16 years. Items rated on 3-point scale, with 20-item total problem score from 0 to 40. The authors provide a reference | |
| Working memory | Working memory test battery for children | Three subtests from the working memory test battery for children assessing the central executive working memory domain: backwards digital recall, counting recall and listening recall. These subsets provide a central executive composite. The authors provide a reference | |
| Moss et al.124 | Child daytime behaviour and cognition | Developmental Behaviour Checklist – Parent Version | Developmental Behaviour Checklist is used to measure the behavioural and emotional problems of children with developmental and intellectual disabilities aged 4–18 years. The checklist includes five subscales: disruptive/antisocial, self-absorbed, communication disturbance, anxiety and societal relating and a total score. The authors provide a reference |
| Quine and Wade146 | Child daytime behaviour and cognition | Daytime behaviour (BPI) | The BPI is an adaptation of the Behaviour Screening Questionnaire that includes a wider range of sleep problems that are deemed more appropriate for children with severe learning difficulties. A reference is provided as is a more detailed description of the Index and the Behaviour Screening Questionnaire in an appendix to the report |
| Sciberras et al.125 | Child related quality of life | PedsQL | Health-related quality of life was measured using PedsQL (version 4.0), psychosocial health summary score. The authors provide a reference |
| Child daytime behaviour and cognition | ADHD Rating Scale IV | ADHD symptom severity was measured using the ADHD Rating Scale IV. The authors provide a reference | |
| Daily parent rating on the Evening and Morning Behaviour Scale | The Evening and Morning Behaviour Scale | Daily functioning was measured via daily parent rating on the Evening and Morning Behaviour Scale. The authors provide a reference | |
| School attendance | Number of days missed or late for school over previous 6 months | No further definition provided. The authors provide a reference | |
| Other child-related sleep outcomes | |||
| Austin et al.123 | Meal times | Sleep diary | No definition provided |
| Hiscock et al.138 | School attendance | Parent report of child’s school attendance | Parent report of whether or not their child had missed or been late for school over the preceding 3 months |
| Sleep help | Parent report of other professional help sought for their child’s sleep – for example, general practitioner or psychologist | Parent report of other professional help sought for their child’s sleep | |
Appendix 12 Parent sleep-related outcomes for studies evaluating parent-directed tailored interventions
| Study | Parent sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Parent-carer-related quality of life | |||
| Hiscock et al.138 | Parental stress | DASS | Validated 21-item measure of adult mental health including scales assessing depression, anxiety and stress. Items rated on a 4-point scale. The authors provide a reference |
| Moss et al.124 | Parent stress | Parenting Stress Index – Short Form | The Parenting Stress Index enables a clinician or researcher to examine the relationship of parenting stress with child characteristics, parent characteristics and situations that are directly related to the role of being a parent. The index includes three subscales: parental distress, parent–child dysfunctional interaction and difficult child. A total stress score is also provided. The authors provide a reference |
| Quine and Wade146 | Parent stress | Maternal stress and morale – the Malaise Inventory | A 24-item binary choice questionnaire adapted from the Cornell Medical Index. Scores of 5 or 6 were outside the normal range and indicate stress. Scores of ≥ 7 were considered more critical. The authors provide a reference |
| Sciberras et al.125 | Mental health | DASS | Mental health was measured via the DASS. The authors provide a reference |
| Other outcomes | |||
| Hiscock et al.138 | Family functioning | Parent-reported missed work attendance | Parent report of whether or not they had missed or been late for work over the preceding 3 months and the number of days that they missed or were late for work during that period |
| Sciberras et al.125 | Parent work attendance | Parent/caregiver report | Number of days missed or late to work over the previous 6 months. The authors provide a reference |
Appendix 13 Measures of perceived confidence and/or efficacy and/or understanding of sleep/sleep management for studies evaluating parent-directed tailored interventions
| Study | Method of assessment | Definitiona |
|---|---|---|
| Behavioural | ||
| Beresford et al.21 | PSOC scale and satisfaction and efficacy subscales | A 16-item scale with two subscales. Parents respond to a series of questions about parenting, indicating their level of agreement or disagreement on a six-point Likert scale. The satisfaction subscale measures the extent that parents are satisfied with their role as a parent and so captures the affective dimension of parenting competence, including the extent of parental frustration, anxiety and motivation. The efficacy subscale measures the extent that parents feel that they are managing the role of being a parent and captures competence, problem-solving ability and capability in the parenting role. The authors provide a reference |
| Beresford et al.21 | PSOC scale and satisfaction and efficacy subscales | A 16-item scale with two subscales. Parents respond to a series of questions about parenting, indicating their level of agreement or disagreement on a six-point Likert scale. The satisfaction subscale measures the extent that parents are satisfied with their role as a parent and so captures the affective dimension of parenting competence, including the extent of parental frustration, anxiety and motivation. The efficacy subscale measures the extent that parents feel that they are managing the role of being a parent and captures competence, problem-solving ability and capability in the parenting role. The authors provide a reference |
| Quine and Wade146 | Improvement in knowledge of behavioural principles using Knowledge of Behavioural Principles as Applied to Children test | A 50-item multiple forced-choice test designed to assess understanding of the application of basic behavioural principles as they are applied to children, which takes about 30 minutes. Each item presents a problem situation to which the respondent is required to select the correct behavioural response. Criterion response for each question was selected on the basis of learning principles. The authors provide a reference |
Appendix 14 Child-related outcomes for studies evaluating parent-directed non-tailored interventions
| Study | Child sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Global measures and composite scores | |||
| Adkins et al.127 | Sleep efficiency | Actigraphy | Percentage of TST/time in bed |
| TST | Actigraphy | Actual time slept – the sum of all ‘sleep epochs’ measured in minutes within the interval between the time set on the actogram for night-time sleep and morning wake time | |
| Beresford et al.21 | CSHQ and subscales | CSHQ | The CSHQ was used to assess the severity of sleep problems in children aged 4–10 years. Parent reported. Subscales include bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness. The authors provide a reference |
| Parent-set child sleep goals | Parent-set child sleep goals | A 10-point scale captured progress towards goals. Parents identified up to three goals during session 1 (GAS). The authors provide a reference | |
| Beresford et al.21 | CSHQ | CSHQ | The CSHQ was used to assess the severity of sleep problems in children aged 4–10 years. Parent reported. Subscales include bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness. The authors provide a reference |
| Parent-set child sleep goals | Parent-set child sleep goals | A 10-point scale captured progress towards goals. Parents identified up to three goals during session 1 (GAS). The authors provide a reference | |
| Malow et al.128 | CSHQ | CSHQ | The CSHQ is a parent-completed questionnaire consisting of 33 questions on a 3-point scale. The CSHQ Is used to examine sleep behaviour in toddlers, preschool and school-aged children with a variety of conditions. Subscales of the CSHQ measure insomnia-related dimensions, such as bedtime resistance, sleep anxiety, sleep onset delay, sleep duration and night wakings. Other dimensions include daytime sleepiness, sleep disordered breathing and parasomnias. The authors provide a reference |
| FISH | FISH | FISH is a quantitative scale of sleep habits, including bedtime routine, sleep environment and parental interactions. FISH as used was a 12-item scale – the full version includes 22 items. The authors provide a reference | |
| Sleep efficiency | Actigraphy and sleep diary | Per cent of TST out of the total time in bed | |
| TST | Actigraphy | Actual time slept, the sum of all sleep epochs, measured in minutes with the interval between the time set on the actogram for night-time sleep and morning wake time | |
| Montgomery et al.49 | Composite Sleep Disturbance Score | Sleep diary | A Composite Sleep Disturbance Score was calculated by summing the score on each problem. Calculated from the sleep diary as follows: 4 = minimum entry score, representing, for example, a child with settling problems lasting > 30 minutes at least five times weekly and 8 = maximum score, representing a child who also wakes in the night for at least 30 minutes > 3 nights each week. No reference provided |
| Reed et al.129 | CSHQ | CSHQ and subscales | The CSHQ is a parent-completed questionnaire used to examine sleep behaviour in toddlers, preschool and school-aged children with a variety of conditions. Subscales measured insomnia-related dimensions including bedtime resistance, sleep anxiety, sleep onset delay, sleep duration and night wakings. Additional dimensions include daytime sleepiness, sleep disordered breathing and parasomnias. A total score is also calculated and the authors reference as previously reported the use of a modified totals core incorporating the insomnia domains – total of items compromising the bedtime resistance, sleep anxiety, sleep onset delay, sleep duration and night wakings scales. The CSHQ was used to measure changes in subscales and total scores after the behavioural intervention. The authors provide a reference |
| FISH | FISH | FISH is a parent-reported questionnaire that assesses sleep hygiene for their children. It includes giving attention to developing a structured consistent bedtime routine, sleep environment, daytime habits and parental interactions at bedtime and on night wakings. Parents rate the frequency of sleep habits over the last month on a 5-point scale. The authors previously validated a 12-item research version of the scale. In parent education classes, the authors used the 22-item scale that provides a comprehensive overview of sleep habits. The full version of the scale is provided in a table | |
| TST (time in bed) | Actigraphy | No definition provided | |
| Sleep efficiency | Actigraphy | No definition provided | |
| Yu et al.151 | CSHQ | CSHQ | The CHSQ is a sleep-screening instrument that is widely used to identify sleep problems in children with a range of problems, including ASD. It includes 50 questions, and each item is scored as 3 = usually (5–7 times/week), 2 = sometimes (2–4 times/week) or 1 = rarely (0–1 times/week). It produces a total score and eight subscale scores for sleep onset delay, night awakening, sleep duration, sleep resistance, sleep anxiety, parasomnia, daytime sleepiness and sleep disordered breathing, which reflect key sleep domains that encompass the major medical and behavioural sleep disorders. The authors provide a reference |
| FISH | FISH | FISH was developed to measure sleep hygiene and behaviours in children with ASD. There are 22 questions and each question is scored from 1 to 5 depending on the frequency of occurrence, namely never, rarely, sometimes, usually and always. The authors provide a reference | |
| Sleep initiation | |||
| Adkins et al.127 | Bedtime settling: SOL | Actigraphy | The number of minutes that it took for the child to fall asleep when the parent turned the lights out and expected them to fall asleep |
| Bramble149 | Bedtime settling: time to settle (SOL) | Sleep diary | Mean time to settle once the child was in bed |
| Malow et al.128 | Bedtime settling: SOL | Actigraphy and sleep diary | The number of minutes taken for the child to fall asleep when the parent turned the lights out and expected the child to fall asleep |
| Reed et al.129 | Bedtime settling: | ||
| SL | Actigraphy | No definition provided | |
| Bedtime | Sleep diary and event markers | No definition provided | |
| Sleep maintenance | |||
| Adkins et al.127 | Night waking: | ||
| WASO | Actigraphy | The total time that the child was awake during the night, after the SOL was excluded. WASO was measured as the sum of all wake epochs during the sleep period. WASO did not include wake time in bed before the final arising and terminal wakefulness was not encountered | |
| Fragmentation index | Actigraphy | The Fragmentation Index captures all movement regardless of the intensity of the movement. The Fragmentation Index is a measure of nocturnal movement that is calculated using: (number of movile epochs lasting four epochs + number of immobile epochs < 1-minute duration/number of immobile epochs > 1-minute duration) × 100 | |
| Bramble149 | Non-specified night-time sleep disturbance: severity of sleep problems | Parent/carer report on VAS | Efficacy of treatment assessed by parental reports of the severity of sleep problems using a VAS (0 = no problems to 10 = very severe problems, converted to %). The severity of sleep problems was also rated on a categorical scale (0 = usually sleeps and settles well to 9 = sleep is disturbed, settles late and wakes early most nights). These scales were derived from the two sleep-problem items of the Wing and Goulds Handicaps, Behaviours and Skills scale. The authors provide a reference |
| Malow et al.128 | WASO | Actigraphy and sleep diary (to confirm accuracy) | The total time that the child was awake during the night after the SOL was excluded. WASO was measured as the total of all wake epochs during the sleep period |
| Reed et al.129 | Night waking: WASO | Actigraphy, sleep diary and event markers | No definition provided |
| Child-related quality of life, daytime behaviour and cognition | |||
| Bramble149 | Child daytime behaviour and cognition | BPI | The BPI was derived for specific use with children with severe learning disabilities and was scored from 0 to 64. The authors provide a reference |
| Malow et al.128 | Child-related quality of life | PedsQL (total score) | The PedsQL is a 23-item instrument designed for children aged 2–18 years. PedsQL includes four domains of functioning: physical, emotional, social and school. Each domain has a subscore and a total score and a psychosocial health summary score. The authors provide a reference |
| Child daytime behaviour and cognition | CBCL | The parent-completed CBCL consists of two modules, one for ages 1.5–5 years and one for ages 6–18 years. For analysis, the authors selected muscles that were common to both modules and showed improvements in previous interventional studies (e.g. scales including anxious/depressed, withdrawn and withdrawn/depressed, attention and DSM-oriented scales attention deficit hyperactivity). The authors provide a reference | |
| RBS-R | RBS-R | The parent-completed RBS-R consists of six subscales: stereotyped, self-injurious, compulsive, ritualistic, sameness and restricted behaviours and a total scale. The scale is validated in children. Subscales were selected for analysis that had shown improvements in prior interventional studies (e.g. stereotyped, compulsive and restricted behaviours). The authors provide a reference | |
| Reed et al.129 | Child daytime behaviour and cognition | PCQ | The PCQ is a validated parent-completed questionnaire used to assess the presence and severity of 13 developmental and behavioural concerns expressed by parents of children with ASD. Domains include those related to core symptoms of ASD (e.g. language delay and social interaction) and related symptoms (e.g. hyperactivity and compulsive behaviours). The authors provide a reference |
| RBS-R | RBS-R | The RBS-R is an observer-completed questionnaire validated in adults with ASD. A total score and scores for the subscales of stereotyped, self-injurious, compulsive, ritualistic, sameness and restricted behaviour are calculated. The authors provide a reference | |
| Yu et al.151 | Child daytime behaviour and cognition | CBCL | The CBCL measured the daytime behaviour of each child and is a commonly used questionnaire to identify social/emotional and/or behavioural problems in children. It is also used to aid diagnosis and evaluate emotional and behavioural problems in children with ASD. The form includes 100 problem items – 99 closed-ended items and one open-ended item that requests respondents add any additional problems not previously listed. Parents rate each item as 0 not true, 1 for somewhat true and 2 for very true or often true. A total problem score is calculated by totalling the scores on all of the items, including emotional reaction, anxious/depressed syndrome, somatic complaints, withdrawal, sleep problems, attention problems and aggressive behaviour. The internalising score is the sum of the scores on items in the withdrawal, somatic complaints and anxious/depressed syndrome profiles. The externalising score is the sum of the scores on the items on attention problems and aggressive symptoms. The authors provide a reference |
| Daytime behaviour | PCQ | The PCQ is used to assess daytime behaviour. It is a 13-item parent-interview screening instrument assessing the severity of core developmental and associated psychiatric symptomology using a 4- point scale. Parents are asked to describe the extent that each symptom has been a problem with 1, 2, 3 and 4 representing no, mild, moderate and severe problems, respectively. The authors provide a reference | |
Appendix 15 Parent sleep-related outcomes for studies evaluating parent-directed non-tailored interventions
| Study | Parent sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Parent-carer-related quality of life | |||
| Bramble149 | Maternal stress | Rutters’ Malaise Inventory | Rutters’ Malaise Inventory measured changes in maternal stress and is scored from 0–11. The authors provide a reference |
| Reed et al.129 | Parental stress | Parenting Stress Index – Short Form | A 36-item abbreviated version of the Parenting Stress Index, which provides a Total Stress Score and subdomain scores of parental distress, difficult child and parent–child interactions. The measure has been used to measure parental stress in autism disorder. The authors provide a reference |
| Yu et al.151 | Parental stress | Parental Stress Index – Short Form | Used to assess parental stress. A validated reliable and widely used instrument for measuring parenting stress. Parents rate each of the 36 items on a 5-point scale ranging from strongly disagree to strongly agree. A total score is calculated. The authors provide a reference |
| Quality of sleep | |||
| Bramble149 | Maternal sleep quality | Maternal Sleep Scale | Mothers appraised their own sleep quality using an adapted version of De Diana’s175 sleep rating scale – the maternal sleep scale, which requires yes/no responses to 11 statements (e.g. ‘I usually sleep well during the night’) and is scored from 0 to 11. The authors provide a reference |
| Yu et al.151 | Sleep quality | Pittsburgh Sleep Quality Index | The Pittsburgh Sleep Quality Index is used to assess parental sleep habit, quality and quantity in a range of populations. It is a self-rated questionnaire consisting of 19 questions that generate a total score and seven subscores, including sleep quality, SOL, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction. The authors provide a reference |
Appendix 16 Measures of perceived confidence and/or efficacy and/or understanding of sleep/sleep management for studies evaluating parent-directed non-tailored interventions
| Study | Method of assessment | Definitionsa |
|---|---|---|
| Beresford et al.162 | PSOC (satisfaction and efficacy subscales) | The PSOC is a validated self-reported 17-item scale developed to assess parents’ self-esteem. Two subscales provide a measure of self-efficacy, indicative of the parent’s sense of his/her own problem-solving ability and capability as a parent, and a measure of satisfaction with parenting that reflects frustration, anxiety and motivation with the parenting role. A higher score indicates a higher parenting sense of competency |
| Beresford et al.163 | PSOC (satisfaction and efficacy subscales) | The PSOC is a validated self-reported 17-item scale developed to assess parents’ self-esteem. Two subscales provide a measure of self-efficacy, indicative of the parent’s sense of his/her own problem-solving ability and capability as a parent, and a measure of satisfaction with parenting that reflects frustration, anxiety and motivation with the parenting role. A higher score indicates a higher parenting sense of competency |
| Malow et al.128 | PSOC (satisfaction and efficacy subscales) | The PSOC is a validated self-reported 17-item scale developed to assess parents’ self-esteem. Two subscales provide a measure of self-efficacy, indicative of the parents’ sense of his/her own problem-solving ability and capability as a parent, and a measure of satisfaction with parenting that reflects frustration, anxiety and motivation with the parenting role. The authors provide a reference |
Appendix 17 Child-related outcomes for studies evaluating non-comprehensive parent-directed interventions
| Study | Child sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Global measures and composite scores | |||
| Peppers et al.155 | CSHQ | CSHQ total score | Parent completed measure used to assess sleep in children who met the inclusion criteria and agreed to participate. A sleep disturbance score of ≥ 42 indicated a paediatric sleep disorder. The authors provide a reference |
| Wiggs and Stores152 | Composite sleep index (bedtime resistance, night waking, early waking and sleeping in places other than bed) | Composite sleep index of the modified version of the Simonds and Parraga Sleep Questionnaire | Calculated from parental questionnaire information obtained through a modified version of the Simonds and Parraga Sleep Questionnaire. Scores ranged from 0 to 12. Settling and night waking were scored in terms of frequency and duration, and early waking and sleeping in the parents’ bed for frequency only. Frequency = problems occurring more than several times a week = 2. Duration was scored as following: settling problems lasting up to 1 hour = 1; > 1 hour = 2. Night wakings were scored as if lasting a few minutes = 1 and if they lasted longer = 2. The authors provide a reference |
| TST | Actigraphy | Time from sleep onset to the time the child woke up. Referred to as sleep period | |
| Sleep maintenance | |||
| Wiggs and Stores152 | Non-specified night-time sleep disturbance: | ||
|
Actigraphy | Defined as the sum of the epoch scores divided by total number of epochs in the sleep period | |
| Actigraphy | Defined as the number of thirties epochs with a value > 0 (i.e. with movement), divided by the total number of thirties epochs in the sleep period × 100 | ||
| Night waking: | |||
|
Actigraphy | Defined as the number of discrete thirties epochs with a value of 0 (i.e. with no movement) divided by the total number of immobile phases of any duration × 100 | |
| Child-related quality of life, daytime behaviour and cognition | |||
| Peppers et al.155 | ADHD symptoms | Parent Vanderblit ADHD symptom checklist | A parent-/caregiver-completed screening tool to assess ADHD behaviour. No further definition or reference provided |
| Wiggs and Stores152 | Child daytime behaviour and cognition | ABC parent and teacher versions reported | The ABC was used to assess the following types of challenging behaviour: self injury, aggression, screaming, temper tantrums, non-compliance and impulsivity. The authors provide a reference |
Appendix 18 Child sleep-related outcomes for studies evaluating other non-pharmacological interventions
| Study | Child sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Global measures and composite scores | |||
| Francis and Dempster156 | TST | Sleep diary | No definition provided |
| Gringras et al.36 | CSHQ | CSHQ (no data reported) | – |
| Composite sleep disturbance index; frequency and duration of sleep problems | Composite sleep disturbance index; frequency and duration of sleep problems | No definition provided, reference to key papers provided | |
| Sleep efficiency | Parental diary | The proportion of time spent in bed asleep | |
| TST | Parental diary, actigraphy | No definition provided | |
| Guilleminault et al.158 | TST | Parent report (actigraphy verified) | Nocturnal TST |
| Oriel et al.159 | CSHQ | CSHQ | No reference provided. The authors state that the CSHQ developed by researchers at Brown University was used to quantify sleep problems and consisted of eight subscales (bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing and daytime sleepiness) and 33 items for the total sleep disturbance score. Maximum score of 99 |
| TST | Telephone call from researcher | The average number of hours of sleep for each participant during each study phase | |
| Yu and Hong161 | CSHQ | CSHQ | No reference provided, the authors state the scale was used to assess the sleep state of patients before and after treatment. Scale included: the total time of sleep’ the time of getting into bed for sleep; sleep habit; sleep behaviour; wake state at night; state at getting up in the morning; sleep state at daytime and total score |
| Sleep initiation | |||
| Francis and Dempster156 | Bedtime settling: SL | Sleep diary | Time taken to fall asleep |
| Gringas et al.36 | Bedtime settling: SOL | Parental diary, actigraphy | No definition provided |
| Oriel et al.159 | Bedtime settling: SOL | Telephone call from researcher | The average number of minutes to fall asleep for each participant during each study phase |
| Piazza et al.157 | Bedtime settling: exactly what time the child fell asleep at night | Observations | No definition provided |
| Sleep maintenance | |||
| Francis and Dempster156 | Night waking: time spent awake during the night | Sleep diary | ‘Where nightime awakenings for the child fell in the parent’s sleep period, only those which disturbed the parent to awakening were recorded’156 |
| Gringas et al.36 | Night waking: number of night wakings | ||
| Time awake after sleep onset | Actigraphy and parental diary | No definition provided | |
| Proportion of nights with > 1 wakes | Actigraphy and parental diary | No definition provided | |
| Actigraphy and parental diary | No definition provided | ||
| Guilleminault et al.158 | Non-specified night-time sleep disturbance: longest wake and sleep periods during 24-hour cycle | Sleep diary (actigraphy-validated sleep diary) | No definition provided |
| Sleep diary (actigraphy-validated sleep diary) | No definition provided | ||
| Oriel et al.159 | Night waking: number of night wakenings | Telephone call from researcher | No definition provided |
| Piazza et al.234 | Night waking: time of night wakes and return to sleep | Observations | No definition provided |
| Non-specified night-time sleep disturbance: hours of disturbed sleep | Observations | No definition provided | |
| Waking time: exactly what time the child awakened in the morning | Observations | No definition provided | |
| Sleep scheduling | |||
| Francis and Dempster156 | Sleep quality | Sleep diary | No definition provided |
| Gringas et al.36 | Sleep improvement | Children and parent blanket scale | No definition provided |
| Quality of sleep | Child smiley-face rating and parent blanket scale | No definition provided | |
| Guilleminault et al.158 | Distribution of sleep bouts during 24-hour cycle | Parent report, actigraphy (validated sleep diaries) | No definition provided |
| Yehuda et al.160 | Degree of fatigue in general during the day | Parent blanket scale | No definition provided |
| Quality of sleep | Short questionnaire | No definition provided | |
| Child-related quality of life, daytime behaviour and cognition | |||
| Francis and Dempster156 | Child daytime behaviour and cognition | Sleep diary | No definition provided |
| Gringas et al.36 | Daytime behaviour and cognition | ABC (total score and subscales) | No definitions provided, reference to checklist manual provided |
| Sensory Behaviour Questionnaire | Sensory Behaviour Questionnaire; sensory stimuli response profile | Reference to unpublished original paper provided | |
| Yehuda et al.160 |
Level of good mood in general Level of ability to concentrate during the day, mainly at school Percentage of homework completed in general |
Short questionnaire | ‘5 point Likert scale’ |
Appendix 19 Studies evaluating family experience
| Study | Sample | Experiences of receiving or implementing the intervention (parent report unless specified) | Parents’ views on the value/relevance and/or usefulness of different elements of the intervention | Recommend the intervention? |
|---|---|---|---|---|
| Pharmacological interventions | ||||
| Wright et al.106 |
Sample: 20 study participants Data were recorded within usual trial data collection processes |
One parent (1/20) withdrew their child from trial because they found that it was too difficult to administer the medication (oral melatonin) | Not specifically asked | |
| Non-pharmacological: parent-directed tailored interventions | ||||
| Bramble149 |
Sample: 15/15 study participants Data collected: 4-month follow-up Fixed response question regarding style of intervention approach. Response options: too tough; rather tough; just right; rather soft; too soft Overall rating of helpfulness: visual analogue scale [0 (no help) to 10 (extremely helpful)] Checklist of 10 key components of intervention (referred to as ‘advice items’): respondents ticked those that they felt had changed the child’s sleep problems and rated helpfulness [+ 2 (very helpful) to –2 (very unhelpful)] |
12/15 reported the treatment was ‘just right’; 3/15 rated it ‘rather tough’ but were willing to continue |
Overall rating of helpfulness: mean score 8.9 (SD 1.9) Mean ratings of helpfulness of ‘advice items’: |
Not specifically asked |
| Austin et al.123 |
Sample: 5/6 study participantsa completed 23-item CATS designed for study Response format: five-point Likert Scale (maximum score 35) Domains: treatment goals, training and resources, appropriateness and acceptability of intervention plan, treatment compliance, outcomes and implications, most valued aspects of programme, programme difficulties and suggestions for future sleep interventions |
Domains of CATS:Mean score 27.6 (SD 4.73) of a maximum score of 35 Three out of five parents reported that using bedtime restriction and/or bedtime fading with response cost was stressful to implement |
Domains of CATS:
|
5/5 would recommend |
| Johnson et al.107 |
Sample: 13/15 (active arm) and 17/18 (attention control) Parent Satisfaction Questionnaire developed for study. Parents rated the quality of various elements of the intervention:Response format: three- or four-point Likert scales (higher scores reflecting greater levels of satisfaction) Adherence to intervention: |
Parent Satisfaction Questionnaire
|
Parents in the active arm only Helpfulness of specific elements of the intervention:All other responses were ‘okay’; there were no ratings of ‘not helpful’ |
Not specifically asked |
| Moss et al.124 |
Sample: 26/26 study participants Completed ‘an informal survey designed by study authors assessing acceptability of the programme’s goals, workshops and resources, treatment plans, and outcomes’. 124 Referred to as CATS. Response format: five-point Likert scale. Total number of items not reported. Not clear if same as used by Austin et al. 123 Subsample (6/26) participated in a semistructured interview. (20/26 not available for interview.) Method of data analysis not reported |
Parent report
|
Semistructured interviews
|
Not specifically asked |
| Scibberas et al.125 |
Sample: 27/27 study participants asked to complete a study-designed scale. The number completing the scale not reported The ‘study-designed scale’ captured parents’ reports of the helpfulness of the intervention and use sleep management strategies learnt during the intervention. No further information provided |
‘Most caregivers reported that they could implement sleep management strategies “at least half of the time” ’125 (p. 934). Note: ‘most’ not further specified | ‘Most . . . reported sleep strategies as helpful, including:
|
‘All but one’125 (p. 934) would recommend the intervention |
| Weiskop et al.126 |
Sample: 12/12 study participants Parents completed a modified version of the PEQ (Griffin and Hudson, 1978235), which comprised: |
Approval of techniques: 7/12 gave the maximum positive rating Overall satisfaction: mean score 13.8 (range 11–15) Least-liked elements were: |
‘Responses to the PEQ indicated that the best aspects of the programme were . . . the support provided, the telephone calls, and the method of instruction’126 (p. 102) | 12/12 would recommend |
| Non-pharmacological: parent-directed non-tailored interventions | ||||
| Adkins et al.127 |
Sample: 6/9 study participants in active arm (n = 9) Parents in the active arm (who received a sleep education pamphlet) were ‘asked a series of questions to collect parent feedback on pamphlet use’127 (p. S141) and what was ‘most useful about the pamphlet and what might have been more useful’127 (p. S140) |
Parents commented that the pamphlet contained good information, but that it would have been more useful to be given specific examples of how to take the information and put it into practice | Feedback from parents suggested that the pamphlet was useful as it contained good information, for example they liked that it included ‘basic rules for sleep’ and ‘important of consistent bedtime’. They commented that they would have found it more useful if it had included specific suggestions of how to take the information and put it into practice | Not specifically asked |
| Malow et al.128 |
Sample: 80 study participants. (individual-mode arm, n = 41; group-mode arm, n = 39) Parents completed an ‘anonymous survey’ at end of education element of intervention. A 4-point Likert scale was used to capture views on general satisfaction with content of intervention and educator, whether or not they would recommend it to others, whether or not the intervention had improved the child’s sleep habits and whether or not they would have preferred alternative mode of intervention delivery |
Preferences for alternative mode of delivery:
|
Response to statement: ‘The information covered was relevant and useful’
|
‘Strongly agreed’ that they would recommend:
|
| Reed et al.129 |
Sample: 18/20 study participants Parents completed an ‘anonymous evaluation’. No further details were given |
Duration of the workshop was sufficient: 10/18 | The information conveyed was relevant and useful: 17/18 | 18/18 would recommend |
| Montgomery et al.49 |
Sample: 23/33 study participants who received copy of booklet regarding managing sleep Brief questionnaire was given to evaluate the booklet in terms of relevance, ease with which it can be understood and usefulness on a four-point Likert scale (low = poor/negative experience; high = good/positive experience). The maximum score was 12 |
Mean total score on the questionnaire evaluating the booklet: 10.17 (SD 1.87) | Not specifically asked | |
| Non-pharmacological: parent-directed: two tailored and two non-tailored | ||||
| Beresford et al.130 | Sample: 35 parents purposively sampled (in terms of intervention outcome, child’s diagnosis, parents’ education and partner involvement in the intervention) from a total sample of parents (n = 74) who had received one of four parent-directed interventions included in this review:
|
Parents referred to the demands that the intervention places on parent/caregivers, including the challenge of changing and sustaining new sleep management strategies Implementing new approaches to managing their child’s sleep could be hampered by a number of factors: (1) a lack of consistency across caregivers, (2) changes and disruptions in usual routines (e.g. illness, holidays) and (3) issues with the home environment, particularly when the child shared a bedroom siblings) |
Features of the intervention identified by parents as supporting positive outcomes:
|
Not specifically asked |
| Other non-pharmacological interventions | ||||
| Gringras et al.36 | Sample: 73 study participants. Data recorded within usual trial data collection processes | Child report
|
Parents’ views on the value/relevance and/or usefulness of different elements of the intervention: parents favoured the weighted blanket | Not specifically asked |
Appendix 20 Study quality: studies of acceptability/feasibility and experiences of implementing sleep interventions
| Study | Abstract and title | Introduction and aims | Method and data | Sampling | Data analysis | Ethics and bias | Results | Transferability/generalisability | Implications and usefulness | Score (maximum 36) |
|---|---|---|---|---|---|---|---|---|---|---|
| Beresford et al. (2016)130 | Good | Good | Good | Fair | Good | Fair | Good | Good | Good | 34 |
| Comments: sample drawn from sample recruited to outcomes evaluations. The recruitment to the outcomes evaluations had limitations | ||||||||||
| Bramble (1996)122 | Poor | Fair | Fair | Poor | Poor | Poor | Fair | Fair | Fair | 23 |
| Comments: lacks clear description of presentation of questions and method of recording. No report of parent characteristics. No account given of data analysis. Ethics approval not reported. Some further analyses needed/reported, for example table 3. Evaluation of a specific intervention. Findings not (necessarily) transferable to other parent-directed interventions. No mention of authors’ biases – study conducted by one author with clinical experience | ||||||||||
Appendix 21 Study quality: quality assessment of non-pharmacological interventions
Summary of quality assessment using the Cochrane risk of bias for randomised controlled trials tool62
| Study | Domain | Additional questions for crossover trials | |||||
|---|---|---|---|---|---|---|---|
| 1. adequate sequence generation? | 2. allocation concealment? | 3. blinding? | 4. incomplete outcome data addressed? | 5. free of selective reporting? | 6. free of other bias? | ||
| Parent-directed tailored interventions | |||||||
| Beresford et al.21 |
Unclear No details are provided |
Unclear No details are provided |
No No details are provided, but it would have been difficult to achieve blinding given the nature of the intervention |
No Response rates were reported as 92% (post intervention) and 62% (12-week follow-up) (i.e. 5/12 lost to follow-up). No reasons for loss to follow-up were given or if/how this was dealt with in the analysis. No indication was given of which arm of the trial the participants were lost from |
Unclear No protocol but data analysis detailed in appendix E. It seems to be an explanation of what analyses were carried out rather than an a priori plan/protocol. This intervention is part of larger study of multiple interventions – the same analyses will be carried out on all interventions, as applicable. All measures appear to have been reported |
No Small study sample (n = 13). The intervention was suspended on two occasions, which affected the small sample size |
N/A |
| Hiscock et al.138 |
Yes Computer-generated random number sequence |
Yes Sealed opaque envelopes |
No Parents were aware of the intervention (see discussion in the paper). It would be difficult or impossible to blind given nature of intervention |
Yes Missing data were imputed, and both imputed and non-imputed reported Intervention and control group at baseline: n = 122 in each group Number lost to follow-up at the 3-month follow-up: intervention, n = 36; control, n = 33 Number lost to follow-up at 6-month follow-up: intervention, n = 16; control, n = 33 Numbers included in the primary analyses varied for parent-and teacher-reported ADHD symptoms (n = 99 and n = 83 in the intervention group; n = 85 and n = 77 in the control group, respectively) Multiple imputation used in intention-to-treat analysis. There were n = 122 in each group |
Yes Protocol available. All specified outcomes reported |
Yes | N/A |
| Johnson et al.107 |
Unclear The authors state that participants were equally randomised using block randomisation with a block size of 10, but no information is provided about how they generated a sequence |
Unclear No details of this are provided |
No Parents and therapists were not blinded |
No Only participants for whom baseline and 4-week follow-up data were available were included in the analysis |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Yes | N/A |
| Moss et al.124 |
Unclear No details are provided |
Unclear No details are provided. Allocation was by research team but it is not clear if any attempts were made to conceal this (e.g. opaque envelopes) |
No Owing to the nature of the intervention, participants and staff would know which group they were in |
Unclear A total of 26 children were recruited: post treatment, n = 22 (treatment group, n = 12; control group, n = 10); follow-up, n = 18 (treatment group, n = 10, control, n = 8). Reasons for dropout (from the control and intervention groups after treatment) were family work/carer commitment, child health problems or family tragedy. There was no mention of pre-intervention dropouts |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Yes One child in sample was taking melatonin, but we do not think this would be enough to introduce bias |
N/A |
| Sciberras et al.125 |
Yes Computer-generated random number sequence |
Unclear Allocation was done by independent statistician, but there is no detail on the process of this and if/how it was concealed |
No Owing to the nature of the intervention, the researchers and participants would know which group they were in |
Unclear The authors do not state how loss to follow-up was addressed (n = 8 at 2 months and n = 4 at 5 months) |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Yes | N/A |
| Parent-directed non-tailored interventions | |||||||
| Adkins et al.127 |
Unclear No details are provided |
Unclear No details are provided about allocation concealment |
No Blinding would have been difficult to achieve – both to parents and those taking the assessments/collecting data. First, parents receiving the leaflet intervention would know that they were in that intervention, and those not receiving a leaflet would know they were in the control group. In the discussion, the authors state they could have used a generic leaflet for the control group to promote better blinding. In addition, the clinicians/researchers knew who received the leaflet because they instructed parents to read it |
Unclear A total of 18 were randomised into each group (n = 36), but in table 2, it says there are n = 19 in the pamphlet condition and n = 17 in the no pamphlet condition. There is no explanation in the text about this discrepancy and, therefore, it is not clear if the loss of one participant in one group means that there were missing data, and if and how these were dealt with |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Unclear The authors say that the leaflet used for the intervention is available from a website. Is it possible that the leaflet was available on this website pre intervention and, therefore, parents could have accessed it before the trial, which would introduce bias? It is impossible to clarify this, as it is not clear if the leaflet was already in use and available publicly |
N/A |
| Malow et al.128 |
Yes Database software randomly assigned participants in a 1 : 1 block in each site |
Unclear Allocation was carried out using software but still not clear in the paper if this was concealed from the researchers |
No Participants were not blinded. It would have been difficult to do given nature of the intervention |
No A total of 114 participants were enrolled into study. Of these, 80 completed all study procedures. Only the 80 with complete data were included in analysis. Reasons for non-completion were that it was too time-consuming, SOL not confirmed by actigraphy, children could not tolerate the actigraphy device, the child started new medications or parents opted for other ways of addressing sleep problems In total, 41 were randomised to the group arm and 39 to the individual arm. Six families switched from the group arm to the individual arm because of logistical reasons. Analysis was made with and without switchover |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
Yes Six participants randomised to the individual arm were then moved to the group arm. This could introduce bias, but the authors analysed and present results both with and without these six participants in the individual arm. After reporting results from the two arms (both intervention groups, no control group), they combined data from the two and report together as before and after. However, this is not a bias in the design, but a possible bias in the presentation of the results |
N/A |
| Montgomery et al.49 |
Unclear No details are provided |
Unclear Opaque envelopes were used but there was no evidence that envelopes were sequentially numbered. Envelopes were ‘selected’ by an independent researcher |
No There was no blinding of participants, which would have been difficult given nature of the intervention |
Yes A total of 2/66 were missing at follow-up owing to families moving. Families moving is not related to the study treatment and so this is unlikely to affect fidelity of outcomes |
No No protocol was referenced in the paper and one was not found after searching. The paper reports Composite Sleep Disturbance Score as the main outcome and evaluation of the programme, but we would expect to see objective measures of sleep |
No No detail on age or gender of the two groups and, thus, comparability of groups. Not clear what type of statistical analysis was used in some places |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? There would be no possibility of carry-over effect as the only crossover is from the control group to the treatment group Are unbiased data available? A Kruskal–Wallis test is used |
| Other parent-directed interventions | |||||||
| Wiggs and Stores152 |
Unclear No details are provided, other than that the schools were randomised. The number of ‘clusters unclear’ |
Unclear No details are provided |
No Owing to the nature of the intervention, the researchers and participants would know which group they were in. The paper does not describe the study as blinded |
Unclear No details are provided as to whether or not there was loss to follow-up |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without a protocol, it is difficult to be certain |
No Reporting of the trial design was unclear. It was also unclear how the control group was ‘matched’, as the trial randomised schools (clusters) but matched intervention and control children (individuals) It is not clear if the analysis accounted for the study being a cluster trial. The authors state that there was no explicit hypotheses but data were analysed in an ‘exploratory manner’ |
N/A |
| Other non-pharmacological interventions | |||||||
| Piazza et al.157 |
Unclear No details are provided |
Unclear No details are provided |
No Owing to nature of the intervention, assessors would know which group (of the two treatment groups) participants were in. Data were collected in a sleep laboratory by trained observers and the potential for bias in recording was mitigated by having two observers on 86% of days across all clients |
Unclear Data were presented for 14 participants. There were no details about the number of participants who were initially approached/recruited or if it was ≥ 14 |
Unclear No protocol was referenced in the paper and one was not found after searching. The outcomes reported are those that are expected. However, without protocol, it is difficult to be certain |
Yes | N/A |
| Francis and Dempster156 |
Yes Random numbers table |
Unclear Allocation was done by a senior investigator. The paper reports that this senior investigator had no contact with the participating children and parents (who self-completed the data collection). Codes were known only to the senior investigator. But, it is not clear if this process was concealed to the investigator |
Unclear Investigator who held the codes had no contact with participants and the placebo and treatment capsules were similar in appearance; therefore, blinding to participants is plausible. It is possible that research staff (except the senior investigator) were blinded, as only the senior investigator had the key to the codes, but it is also not clear what involvement this investigator had in the subsequent research process and whether or not this would introduce bias The authors state that there was blinding by the senior investigator who had no contact with patients or parents. Outcomes were self-reported so the outcome is unlikely to be influenced |
Yes There was no loss to follow-up and a total of five participants |
Unclear No protocol was referenced and one was not found after searching. The study is listed on European Union Clinical Trials Register, but little information is provided on the study records and there is not enough to make a judgement about whether or not all outcomes are reported. However, relevant sleep outcomes are reported The description of measures other than primary outcomes is poor. There is no detail of what was included in end-of-study interviews; therefore, it is difficult to determine whether or not there was selective reporting |
No Only night wakings that occurred during parents sleep period were recorded; therefore, other night wakings outside the parent sleep period would not be recorded. Authors say these data must be regarded as conservative Some of the children were taking other medications for sleep The study had very small numbers (n = 5). There are no details of the recruitment process other than that it was through schools/organisations for children with LD. There is no indication of the total numbers approached or if these five patients self-selected |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? Yes, washout period used, but no analysis of carry-over effect Are unbiased data available? Yes. A repeated measures ANOVA was used |
| Gringras et al.36 |
Unclear Block randomisation with random variable block lengths of 2 and 4, stratified by centre, but no detail of sequence generation was given |
No Authors report that the trial investigators and the statistician were blind to treatment allocation, but researchers were not. Although randomisation was done remotely, the researchers dispensed the allocated treatments |
No Researchers were not blinded to initial allocation but trial investigators and the statistician were all blinded throughout the trial and analysis It is impossible to blind parents/children to the weight of the blankets but all other aspects of the blankets were the same |
No A total of N = 73 were randomised. The loss to follow-up n = 0. Discontinued intervention and not included in the analysis n = 6 The reasons were that the child could not tolerate the blanket (n = 4, not clear which arm of trial these participants were from), the child was ill (n = 1) or parent withdrew child (n = 1). There was no discussion of the effect on analyses of loss resulting from not being able to tolerate blanket but this is a key finding and so likely to have an impact on results A total of n = 13 were excluded from analysis owing to insufficient or missing data. Missing data for questionnaire were prorated if < 10% were missing, or excluded otherwise. There was no detail on the reasons for missing data |
Unclear Tried to access the protocol (the URL to this is listed in the paper), but when clicking on the link it takes you to a web page about ongoing projects. From this unable to find the protocol. The study reports all the outcomes expected, but it is difficult to be certain without protocol |
Yes |
Was use of a crossover design appropriate? Yes Is it clear that the order of receiving treatments was randomised? Yes Can it be assumed that the trial was not biased from carry-over effects? The authors report (p. 299) that there were no breaks between interventions, and so no ‘washout’ period. Although this is not a study of a drug, there might have been a carryover of the effects of the weighted blanket. For example, if the child had grown accustomed to better sleep? Are unbiased data available? Data were presented as baseline, weighted and control, regardless of order. Authors use an independent t-test to rule out a period effect and then undertook a paired t-test |
Summary of quality assessment for controlled before-and-after studies using ACROBAT-NRSI63
| Other non-pharmacological intervention (Yehuda et al.160) | |||
|---|---|---|---|
| Bias because of confounding | |||
| Domain | Reviewer 1 quality appraisal | Reviewer 2 quality appraisal | Reviewers 3 and 4 quality appraisals |
| 1.1 Is confounding of the effect of the intervention unlikely in this study? |
PN A total of 40 children received treatment and 38 received placebo. There are no details on why participants were allocated (if they were allocated) to each group. The characteristics of participants is not provided in detail (only overall age range, diagnosis of ADHD and reported sleep deprivation). A non-ADHD group served as a control group. The group was reported to have corresponding ages and socioeconomic statuses but details were not provided |
NI It is hard to judge given the little information about the sample. The sample were selected based on whether or not they had ADHD, were male and were sleep deprived. I cannot think of a prognostic factor (other than the above, which were the reasons for receiving the intervention) that would predict the participants receiving the sleep intervention (fatty acids). One possibility might be whether or not there was a nutritional intolerance to fatty acids (which might predict if someone would not get the intervention) but this is not reported |
Guidance for ACROBAT-NRSI, states that NI is not an option for this question. I would agree with KB that PN is appropriate as there is some information, albeit a limited amount of it is provided. Agree with KB and AS that PN is appropriate given that very limited information is provided on allocation and participants |
|
1.2. If N or PN to 1.1: Were participants analysed in accordance with their initial intervention group throughout follow-up? |
Y |
NI It appears so but there is no information about dropout or whether or not participants switched groups |
NI – insufficient information provided to enable judgement |
|
1.3. If N or PN to 1.2: Were intervention discontinuations or switches unlikely to be related to factors that are prognostic for the outcome? |
NI – insufficient information provided to enable judgement | N/A | NI – insufficient information provided to enable judgement |
| If Y or PY to 1.3, answer questions 1.4 to 1.6, which relate to baseline confounding. If N or PN to 1.1 and 1.2 and 1.3, answer questions 1.7 and 1.8, which relate to time-varying confounding | |||
| 1.4. Did the authors use an appropriate analysis method that adjusted for all the critically important confounding domains? | NI | N/A | N/A – in line with guidance |
|
1.5. If Y or PY to 1.4: Were confounding domains that were adjusted for measured validly and reliably by the variables available in this study? |
N/A | N/A |
N/A – as per above guidance N/A – in line with guidance |
| 1.6. Did the authors avoid adjusting for post-intervention variables? |
Y Authors presented before and after scores for each group with no adjustments |
N/A |
N/A – as per above guidance N/A – in line with guidance |
| 1.7. Did the authors use an appropriate analysis method that adjusted for all the critically important confounding domains and for time-varying confounding? | N/A | N/A |
N/A N/A – in line with guidance |
|
1.8. If Y or PY to 1.7: Were confounding domains that were adjusted for measured validly and reliably by the variables available in this study? |
N/A | N/A |
N/A N/A – in line with guidance |
| Bias in selection of participants into the study | |||
| 2.1. Was selection into the study unrelated to intervention or unrelated to outcome? |
N Children in the intervention and placebo groups were selected into study because of ADHD and sleep deprivation. In total, 6/7 outcomes related to ADHD and/or sleep deprivation The comparison group was unrelated to the intervention or the outcomes |
NI There is not enough information in the paper about recruitment to make a judgement on this. This applies to all the outcomes |
N – some but very limited information is provided explaining that children were recruited on the basis of ADHD and sleep deprivation |
| 2.2. Do start of follow-up and start of intervention coincide for most subjects? |
Y The intervention lasted 10 weeks. Questionnaires were completed on day 1 and at end of 10 weeks |
Y Participants answered a questionnaire/data collected on day 1 of study. This was applicable for all outcomes, which were assessed by the same questionnaire |
Y – participants completed a questionnaire on the first day of the study and at the end of the 10-week intervention period |
|
2.3. If N or PN to 2.1 or 2.2: Were adjustment techniques used that are likely to correct for the presence of selection biases? |
N/A Overall judgement = ?. Selection into the study was not related to intervention/essential fatty acids but was related to outcome, but when testing the impact it has on sleep, you have to select those having trouble with sleep |
N/A |
PN? N/A |
| Bias in measurement of interventions | |||
| 3.1 Is the intervention status well defined? |
Y Two capsules/day of an essential fatty acids mixture composed of alpha-linolenic 0.95 g/ml and linolenic 0.90 g/ml free fatty acids, both 99% pure. Each capsule contained 360 g of linolenic acid and 90 g of alpha-linolenic acid in mineral oil. The placebo was composed of mineral oil in an identical capsule |
Y Detail of the treatment is given on p. 1167. It applies to all outcomes |
Y – A specific mixture of essential fatty acids was used that were created in the researchers’ laboratory. Highly purified alpha-linolenic and linoleic acids were used to avoid the variations that occur in commercially prepared fatty acid oils, which may introduce possible confounding effects of other fatty acids or lipid mixtures. The essential fatty acids mixture was composed of alpha-linolenic (0.95 g/ml) and linoleic (0.90 g/ml) free fatty acids, both 99% pure. Each capsule contained 360 g of linoleic acid and 90 g of alpha-linoleic acid in mineral oil. The placebo was mineral oil in an identical capsule. The treatment lasted 10 weeks and each participant took two capsules per day Y |
| 3.2 Was information on intervention status recorded at the time of intervention? |
Y Given that capsules were created to be identical in the intervention and placebo, participants must have been allocated to intervention or placebo group; therefore, the group was known at start of intervention |
NI There is not enough detail in the paper to make a judgement about this |
PY – Given that capsules were created to be identical in control and placebo, participants must have been allocated to the intervention or placebo group; therefore, group was known at start of intervention PY |
| 3.3 Was information on intervention status unaffected by knowledge of the outcome or risk of the outcome? |
Y This was an experimental study with groups assigned |
NI There is not enough detail in the paper to make a judgement about this |
NI – there is not enough detail in the paper to make a judgement about this NI – insufficient information |
| Bias because of departures from intended interventions | |||
| 4.1. Were the critical cointerventions balanced across intervention groups? |
NI No data were available on possible cointerventions, that is, nothing on what other drugs/behavioural methods were being used at the same time/started during the 10-week study period |
NI Possible cointerventions include melatonin, sleep hygiene and/or behavioural techniques. However, there is no information in the paper about whether or not participants used such cointerventions |
NI – no data on possible co-interventions were provided NI |
| 4.2. Were numbers of switches to other interventions low? | NI | See above |
NI – as above NI |
| 4.3. Was implementation failure minor? | NI |
NI There is not enough information about implementation fidelity in the paper (e.g. no detail on compliance in taking the capsules) |
NI – there is not enough information about implementation fidelity in the paper (e.g. there is no detail on compliance in taking the capsules) NI |
|
4.4. If N or PN to 4,1, 4.2 or 4.3: Were adjustment techniques used that are likely to correct for these issues? |
NI | See above |
NI – as above NI |
| Bias because of missing data | |||
| 5.1 Are outcome data reasonably complete? |
NI There were no data about missing data |
NI There is little detail in the paper, other than that 40 participants received the intervention. It is not clear if the 40 participants were the original 40 recruited or if there were more recruited but only 40 proceeded to take the intervention. There is no detail on if there were incomplete outcome data |
NI – no information regarding missing data is provided NI |
| 5.2 Was intervention status reasonably complete for those in whom it was sought? |
Y It is clear who was in which group |
See above |
NI – as above NI – insufficient information |
| 5.3 Are data reasonably complete for other variables in the analysis? |
NI There were no data about missing data or other variables |
NI There is no detail about whether or not any participants were excluded from the analysis |
NI – there were no data about missing data or other variables |
|
5.4 If N or PN to 5.1, 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? |
See above | See above | |
|
5.5 If N or PN to 5.1, 5.2 or 5.3: Were appropriate statistical methods used to account for missing data? |
See above | See above | |
| Bias in measurement of outcomes | |||
| The outcomes for this study are as follows: co-operation, good mood, ability to concentrate, fatigue during the day, preparing homework, quality of sleep and haemoglobin | |||
| 6.1 Was the outcome measure objective? |
N All measures except the haemoglobin level were based on self-completed questionnaire |
N For all measures except the haemoglobin test |
N All outcomes were based on self-completed questionnaire data, with the exception of the haemoglobin test, which was measured by a blood assay on the first day of the study and at the end of the 10-week study period |
| 6.2 Were outcome assessors unaware of the intervention received by study participants? |
N Assume that the participants were blind to the treatment group (owing to identical capsules). If so, then as the participant assessed their own outcomes, then they were unaware of intervention received. It is not clear if the person who took and measured the haemoglobin level was blinded |
N Data were collected by self-report of participants and analysed/assessed by researchers. Neither group were blinded/unaware of the intervention received |
N No information regarding blinding was provided. The questionnaire was self-reported data for five or six outcomes. It was unclear if the haemoglobin measurement was undertaken by a blinded individual N – for all measures except haemoglobin level, as these were based on a self-completed questionnaire |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? |
Y All outcomes were measured through self-completed questionnaires at day 1 and 10 weeks plus a haemoglobin test |
Y |
Y – all outcomes were measured through self-complete questionnaires at day 1 and 10 weeks plus a haemoglobin test Y |
| 6.4 Were any systematic errors in measurement of the outcome unrelated to intervention received? | NI | Not sure how to judge this |
NI – no information regarding errors were provided. For instance, reliability of haemoglobin measurement? NI |
| Bias in selection of the reported result | |||
| Is the reported effect estimate unlikely to be selected, on the basis of the results, from . . . multiple outcome measurements within the outcome domain? |
?NI All domains are reported but there is no protocol |
NI The paper does not refer to a published protocol, and with little detail provided on the analysis (i.e. intended analysis and actual analysis), it is difficult to make a judgement. It is impossible to know whether or not there were multiple outcome measurements and whether or not those reported were in fact a subset (or not) of these |
NI – agree with reviewer 2 NI |
| Is the reported effect estimate unlikely to be selected, on the basis of the results, from . . . multiple analyses of the intervention–outcome relationship? |
?NI Two-way ANOVA reported |
NI As above. With little information and no protocol, it is impossible to judge whether or not the analysis reported are a selective subset of a wider analysis or not |
NI – agree with reviewer 2 NI |
| Is the reported effect estimate unlikely to be selected, on the basis of the results, from . . . different subgroups? |
?NI No subgroup analysis |
NI As above. With little information and no protocol, it is impossible to judge whether or not there were any subgroups analysed. No subgroup analysis is reported |
NI – agree with reviewer 2 NI |
Summary of quality assessment for before-and-after studies64
| Study | Were the selection/eligibility criteria adequately reported? | ls the sample likely to be representative? | If yes, was it a random sample? | Were patients recruited prospectively? | Were patients recruited consecutively? | Was the participation rate adequate (> 80% of those eligible)? | Was there ≥ 80% follow-up from baseline? |
|---|---|---|---|---|---|---|---|
| Questions 1–7 | |||||||
| Parent-directed tailored interventions | |||||||
| Austin et al.123 |
No Details of the sample were given, but they were not clearly stated in terms of whether or not these details were inclusion or exclusion criteria |
No The sample size was very small – six parents of seven children (but it was described as a preliminary evaluation) |
N/A |
Unclear No detail is given to be able to assess this |
Unclear No detail is given to assess this |
Unclear There is no detail given in the paper about the numbers of eligible children it cannot be assessed whether or not there was > 80% participation rate |
Yes 7/8 were followed up |
| Beresford et al.21 |
No The inclusion/exclusion criteria are not described |
No Small sample (n =12) |
N/A | Yes |
Unclear No detail is given to assess this |
Unclear There is no detail given in the paper about the numbers of eligible children so cannot assess whether or not there was a participation rate of > 80% |
Unclear Follow-up loss is not reported so unable to assess this |
| Quine and Wade146 |
Unclear Inclusion criteria are reported but no exclusion criteria were reported |
No Authors report that the sample was a ‘highly selective group’ owing to the bias towards males. They also compared the group to a previous sleep problem prevalence study that they undertook. From this, they noted that the sample had more sleep management problems and a longer duration of sleep problems than the prevalence study group, and that there was more marital unhappiness and maternal irritability than the prevalence study. In addition, the sample was selected from playgroups for preschool children, so although the ages of the sample are not reported they will be biased towards the younger ages |
N/A | Yes |
Unclear Participants were volunteers not referrals and so there was not really an ‘order’ to be consecutive from |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was > 80% participation rate |
Yes |
| Weiskop et al.126 | Yes |
No The age range of the sample is biased towards younger children. This was intentional as the authors felt that the intervention would be more suitable to younger ages. All but one were in specialist education services. This may make the sample quite specific in terms of representativeness. Furthermore, the sample was partly recruited through a disability newsletter, but there is no information given on who receives this newsletter and if it represents a specific subset of families |
N/A | Yes |
Unclear There is insufficient detail to make a judgement on this |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
No 77% were followed up |
| Parent-directed non-tailored interventions | |||||||
| Beresford et al.21 |
No The inclusion/exclusion criteria were not described |
No Small sample (n = 22) |
N/A | Yes |
Unclear No detail is given to assess this |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
No 70% (post intervention), 65% (12-week follow-up) and 78% (24-week follow-up) were followed up |
| Beresford et al.21 |
No The inclusion/exclusion criteria are not described |
No Small sample |
N/A | Yes |
Unclear No detail is given to assess this |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
No 69% were followed up at 12 weeks and 62% at 24 weeks |
| Bramble149 |
Unclear Inclusion criteria reported but exclusion criteria not reported |
No Small sample of 15 participants |
N/A | Yes | Yes |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
Yes Reported that there were no dropouts but there were missing data for at least five children on one score and seven on another |
| Reed et al.129 | Yes |
Unclear There was some variability in some demographics (e.g. ethnicity), but some were very homogenous. The authors report that child care was offered to minimise selection bias |
N/A |
Unclear Some participants were recruited via a medical centre with a record review used but it is unclear in the paper whether or not this record review was used after prospective recruitment or to select and recruit participants |
Unclear There is insufficient detail to make a judgement on this |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
Yes 80% follow-up |
| Yu et al.151 | Yes |
No The sample was biased towards younger children and, other than autism, it excluded those with neurological conditions that could have affected sleep (e.g. epilepsy) |
N/A | Yes |
Unclear There is insufficient detail to make a judgement on this |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
Yes 85% follow-up |
| Other parent-directed interventions | |||||||
| Peppers et al.155 | Yes |
No Small sample (n = 23), demographics of only intervention group reported |
N/A | Yes | Unclear | No | Yes |
| Other non-pharmacological interventions | |||||||
| Guilleminault et al.158 | No |
Unclear Little detail on the sample is given |
N/A |
Unclear Participants were those referred to a clinic but it is not clear if they were recruited at the time of referral or retrospectively |
Unclear No detail is given on this |
Unclear There is no detail given in the paper about the numbers of eligible children so it cannot be assessed whether or not there was a > 80% participation rate |
Yes N = 14. It is reported that 5/14 children responded to treatment. Non-responders to treatment described as five boys and four girls. The implication is that none was lost to follow-up |
| Oriel et al.159 | Yes |
Unclear Participants were recruited through letters sent home through local ASD support classrooms |
N/A | Yes | Unclear | Unclear | Yes |
| Yu and Hong161 | Yes | Unclear | N/A | Unclear | Unclear | Unclear | Yes |
| Study | Was loss to follow-up reported? | Were relevant prognostic factors reported? | Were other relevant confounding factors reported? (e.g. use of co-interventions) | Was an appropriate measure of variability reported? | Was there an appropriate statistical analysis? | Were there any other important limitations? |
|---|---|---|---|---|---|---|
| Questions 8–13 | ||||||
| Parent-directed tailored interventions | ||||||
| Austin et al.123 | Yes | No | No |
Yes It reports SDs |
Yes Repeated-measures t-tests |
Yes Small sample. Note: described as preliminary evaluation |
| Beresford et al.21 |
Unclear There is no detail in the report to assess this |
Unclear There is little detail about the sample to assess this |
Unclear There is little detail about the sample to assess this |
Yes It reports SDs |
Yes Appendix E notes that, owing to a very small sample size, tests of statistical significance were not applied |
No |
| Quine and Wade146 | Yes |
No Very little detail about the sample is reported, including possible prognostic factors |
No Very little detail is available on the sample |
Yes It reports SDs |
No Descriptive before-and-after data are reported, but a test of difference is reported for some but not for all variables. It is not always clear what type of test was used |
Yes The multiple baseline design data collection was stopped [i.e. the plan was to collect diary data for all from time of entering the study (all at same time point)] but as the parents were tired and because some had to wait a long time to start the intervention, for most families, data were only collected for the first and second weeks at baseline. Therefore, it was not possible to compare baselines and interventions in ‘real’ time. The follow-up period immediately after the intervention is not reported, so it is not clear what the ‘after’ measurement relates to. A further 3-month follow-up was used but most of the data reported pertain to the first (unknown) follow-up Note: the authors say they used an age-matched control group for comparison but this is not reported in the results and only before-and-after results are reported |
| Weiskop et al.126 | Yes |
No It is not clearly reported whether or not there were any comorbidities that could have influenced the outcome (e.g. if any participants experienced seizures or duration of sleep disturbance) |
Yes The paper reports that some children were taking medication for behaviour and/or sleep problems |
No |
No The authors report that emphasis is placed on clinical significance and use of goal attainment scaling and visual analysis of graphs. There is no analysis of difference using a particular test, but perhaps this is correct given the small sample size (n = 13) |
Yes Small sample (n = 13). Two studies were conducted (one with a sample of children with ASD and one with a sample of children with fragile X syndrome) and then combined ‘in the interest of brevity of results’126. It is not clear why two separate studies were conducted and so it is difficult to establish the validity of combining the two. One study had a 12-month follow-up, whereas the other did not. In addition, the treatment duration varied for participants, with a minimum of 7 weeks, but it was longer if treatment was interrupted (e.g. because of illness) |
| Parent-directed non-tailored interventions | ||||||
| Beresford et al.21 |
No The paper deals with missing data reported but not the characteristics of those lost to follow-up |
No | No |
Yes It reports SDs; CIs were plotted but not reported in text |
Yes Repeated measures ANOVA, paired t-tests if appropriate, effect sizes reported |
No |
| Beresford et al.21 |
No Procedures for dealing with missing data discussed (appendix E) but no details on characteristics of those lost to follow-up |
No | No |
Yes It reports SD; CIs were plotted but not reported in text |
Yes Repeated measures ANOVA, paired t-tests |
No |
| Bramble149 | Yes |
Yes Various descriptions of the sample, which may have influenced outcomes, are reported |
No It is not reported whether or not children were using other sleep aids/medications |
Yes It reports SD or SE of the mean |
Yes Comparisons of pre and post treatment, using Friedman and Wilcoxon signed-rank tests |
Yes Some of results were not presented very clearly (e.g. there were graphs but no tables) There were some incomplete data and exclusions from analysis |
| Reed et al.129 | Yes |
Yes Children were excluded if they had factors likely to contribute to disordered sleep |
Yes All medications taken by participants were reported |
Yes It reports SDs |
Yes Paired data, Wilcoxon signed-rank test |
Yes Small sample size (20–25). Some actigraphy data were lost and so data from only 12 participants were used for this outcome measure |
| Yu et al.151 | Yes |
Yes Children with medical conditions that could have affected sleep were excluded. For the sample it is not clear what the duration of the sleep disturbance was prior to the study and thus whether or not this would have affected outcomes |
Yes Medications use reported |
Yes It reports SDs |
Yes Repeated measures ANOVA |
No |
| Other parent-directed interventions | ||||||
| Peppers et al.155 | Yes |
Unclear Medication and comorbid diagnosis for the intervention group were reported |
Unclear Medications used by intervention group were reported |
Yes It reports SDs, SE and CIs |
Yes t-tests and descriptive statistics |
Yes |
| Other non-pharmacological interventions | ||||||
| Guilleminault et al.158 |
No It was not reported explicitly but can be deduced from the text that the study had data on response to treatment for all 14 participants |
Yes Various sample descriptions were reported |
Yes The use of other drugs was reported and standardised |
Yes It reports SDs |
Yes Repeated measures ANOVA |
Yes Chloral hydrate was being used concurrently but withdrawn over several weeks as a sleep pattern was established. There was no discussion of whether or not this would introduce bias Small sample of 14 children Not much detail was given on the analysis and measures: sleep logs were used (TST, distribution of sleep bouts, longest wake and sleep periods), and actigraphy, but only for some participants, although it is not clear how many. Later, the authors report that sleep logs were not being regularly kept in two cases, indicating that there were unreliable data |
| Oriel et al.159 |
Yes No loss to follow-up |
Unclear | Unclear | No | Yes |
Unclear Researchers were unable to stop children from engaging in aquatic exercise in the control phases. Researchers could encourage parents to avoid these activities but had no control over what they actually did |
| Yu and Hong161 |
No 100% |
N/A But there was none |
Unclear Patients who used other drugs for expectant treatments were not eligible. Interventions involved two processes – it is not clear on the relationship/interactions between the two |
Unclear It reports mean and SDs |
Unclear Not clear what statistical tests were used |
No |
Appendix 22 Parent sleep-related outcomes for studies evaluating non-comprehensive parent-directed interventions
| Study | Parent sleep-related outcome assessed | Method of assessment | Definitionsa |
|---|---|---|---|
| Global measures and composite scores | |||
| Wiggs and Stores152 | TST | Actigraphy | Time from sleep onset to the time the child woke up |
| Parent-carer-related quality of life | |||
| Wiggs and Stores152 | Parental stress | Malaise Inventory, reported separately for mothers and fathers | The Malaise Inventory is a 24-item binary choice questionnaire that has been used in a number of studies of disabled children to measure carers’ stress. Scores of 5 or 6 are considered to indicate stress outside the normal range. Scores of ≥ 7 are said to have critical implications for physical and mental health |
| Sleep maintenance | |||
| Wiggs and Stores152 | Parent sleep (sleep period, activity score, movement index and fragmentation index) | Activity monitors |
Sleep period: time from sleep onset to the time the child woke up. Referred to as sleep period Activity score: the sum of the epoch scores divided by total number of epochs in the sleep period Movement index: the number of thirties epochs with a value > 0 (i.e. with movement), divided by the total number of thirties epochs in the sleep period × 100 Fragmentation index: the number of discrete thirties epochs with a value of 0 (i.e. with no movement) divided by the total number of immobile phases of any duration × 100 |
| Sleep scheduling | |||
| Wiggs and Stores152 | Daytime sleepiness (mothers and fathers) | Epworth sleepiness scale | An 8-item self-report scale concerning the likelihood of falling asleep in everyday situations. Sleeping propensity was measured on a 4-point scale and scored as follows: 0 = would never sleep, 1 = slight chance of sleeping, 2 = quite likely that they would sleep, 3 = very likely that they would sleep. The possible score range was from 0 to 24. Scores of ≥ 11 may indicate hypersomnolescence. The authors provide a reference |
| Quality of sleep | |||
| Wiggs and Stores152 | Parental satisfaction with own sleep | A six-point Likert scale, reported separately for mothers and fathers | A six-point Likert scale; the responses were totally satisfied, satisfied but could be better, more often satisfied than not satisfied, more often unsatisfied than satisfied, unsatisfied but could be worse and totally unsatisfied |
| Other outcomes | |||
| Wiggs and Stores152 | Parental satisfaction with child’s sleep and parental satisfaction with their ability to cope with their child’s sleep pattern and daytime behaviours | A six-point Likert scale, reported separately for mothers and fathers | A six-point Likert scale; the responses were totally satisfied, satisfied but could be better, more often satisfied than not satisfied, more often unsatisfied than satisfied, unsatisfied but could be worse and totally unsatisfied |
Appendix 23 Measures of perceived confidence and/or efficacy and/or understanding of sleep/sleep management for studies evaluating non-comprehensive parent-directed interventions
| Study | Method of assessment | Definitionsa |
|---|---|---|
| Behavioural | ||
| Peppers et al.155 | Parent Satisfaction Likert Survey | No further definition or reference provided |
| Wiggs and Stores152 | The parents’ perceived control to manage own and partners’ sleep difficulties (VAS), reported separately for mother and father | VAS (100 mm) scored from 0 to 100. Parents rated their ability on a scale of not at all able to control it to totally able to control it. Parents also rated their partners |
| The parents’ locus of control: internally/externally control scale reported separately for mother and father | A 29-item forced choice questionnaire that includes six filler items to make the test purpose ambiguous. It was used to measure parents’ orientation to internal or external beliefs. External control beliefs: events are caused by some attribute of the environment including powerful others, fate or luck. Internal control beliefs: events are contingent on own actions or own relatively permanent characteristics. Individuals scoring highly on an internal belief scale tend to be more resilient to negative events and are less likely to develop subsequent psychological or physical characteristics. The authors provide a reference | |
List of abbreviations
- ABC
- Aberrant Behavior Checklist
- ACROBAT-NRSI
- A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions
- ADHD
- attention deficit hyperactivity disorder
- ASC
- autism spectrum condition
- ASD
- autism spectrum disorder
- ASSIA
- Applied Social Sciences Index and Abstracts
- BPI
- Behavior Problem Index
- CBCL
- Child Behavior Checklist
- CENTRAL
- The Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CSHQ
- Children’s Sleep Habits Questionnaire
- DASS
- Depression Anxiety Stress Scales
- FISH
- Family Inventory of Sleep Habits
- HTA
- Health Technology Assessment
- ICSD-3
- International Classification of Sleep Disorders – Third Edition
- MD
- mean difference
- ND
- neurodisability
- PCQ
- Parental Concerns Questionnaire
- PedsQL
- Pediatric Quality of Life Inventory
- PSOC
- Parenting Sense of Competence
- RBS-R
- Repetitive Behaviour Scale–Revised
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SOL
- sleep onset latency
- TIDieR
- Template for Intervention Description and Replication
- TST
- total sleep time
- WASO
- wake after sleep onset
- WHO
- World Health Organization
