Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/28/05. The contractual start date was in October 2013. The draft report began editorial review in October 2017 and was accepted for publication in February 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Richard IG Holt received fees for lecturing, consultancy work and attendance at conferences from the following companies: Boehringer Ingelheim (Ingelheim am Rhein, Germany), Eli Lilly and Company (Indianapolis, IN, USA), Janssen Pharmaceutica (Beerse, Belgium), Lundbeck (Copenhagen, Denmark), Novo Nordisk (Bagsværd, Denmark), Novartis (Basel, Switzerland), Otsuka Pharmaceutical Co., Ltd (Tokyo, Japan), Sanofi-Aventis (Paris, France) and Sunovion Pharmaceuticals, Inc. (Marlborough, MA, USA). Paul French is a member of the National Institute for Health Research (NIHR) Health Technology Assessment Mental, Psychological and Occupational Health funding panel. Melanie J Davies and Kamlesh Khunti are members of a NIHR Clinical Trials Unit. Melanie J Davies reports personal fees from Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Merck Sharp & Dohme (Kenilworth, NJ, USA), Boehringer Ingelheim, AstraZeneca (Cambridge, UK), Janssen Pharmaceutica, Servier Laboratories (Neuilly-sur-Seine, France), Mitsubishi Tanabe Pharma Corporation (Tokyo, Japan) and Takeda Pharmaceuticals International Inc. (Canton of Zürich, Switzerland) and grants from Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Boehringer Ingelheim and Janssen Pharmaceutica. Kamlesh Khunti has received fees for consultancy and being a speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Servier Laboratories and Merck Sharp & Dohme. He has received grants in support of investigator and investigator-initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Pfizer (New York, NY, USA), Boehringer Ingelheim and Merck Sharp & Dohme. Kamlesh Khunti has also received funds for research and honoraria for speaking at meetings, and has served on advisory boards for Eli Lilly and Company, Sanofi-Aventis, Merck Sharp & Dohme and Novo Nordisk. David Shiers is an expert advisor to the National Institute for Health and Care Excellence (NICE) centre for guidelines, a board member of the National Collaborating Centre for Mental Health (NCCMH) and a clinical advisor (on a paid consultancy basis) to the National Clinical Audit of Psychosis (NCAP); any views given in this report are personal and not those of NICE, NCCMH or NCAP. David Shiers reports personal fees from the Wiley-Blackwell publication Promoting Recovery in Early Psychosis, 2010 (ISBN 978-1-4051-4894-8), as he is a joint editor in receipt of royalties. John Pendelbury received personal fees for involvement in the study from a NIHR grant; he reports personal fees from the Greater Manchester Mental Health NHS foundation trust outside the submitted work and has published papers in the area of weight management and physical health related to mental health. Marian E Carey and Yvonne Doherty report being employed by the Leicester Diabetes Centre, an organisation (employer) jointly hosted by a NHS hospital trust and the University of Leicester and which is the holder (through the University of Leicester) of the copyright of the STEPWISE programme and of the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) suite of programmes, training and intervention fidelity framework that were used in this study. Fiona Gaughran reports personal fees from Otsuka and Lundbeck and personal fees and non-financial support from Sunovion, outside the submitted work, and has a family member with professional links to Eli Lilly and Company and GlaxoSmithKline (Brentford, UK), including shares.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Holt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Obesity and weight gain in people with schizophrenia
Schizophrenia, a psychotic illness, affects ≈1% of the population. Mortality rates in people with schizophrenia are increased twofold to fourfold, with life expectancy reduced by 10–20 years. 1–3 Around 75% of people with schizophrenia die from physical illness, most commonly cardiovascular disease. Weight gain is a key contributor to excess morbidity and mortality, with obesity being two or three times more prevalent in people with schizophrenia than in the general population. 4 The rates of obesity have increased substantially and have risen faster than in the general population over the past three decades. 5
The cause of the increase in obesity is multifactorial and includes environmental factors (such as poverty, urbanisation, poor diet6,7 and physical inactivity), disease effects (such as altered neuroendocrine functioning, altered reward perception) and treatment effects; antipsychotics cause weight gain in 15–72% of patients. 8 Weight gain is observed early in treatment; between 37% and 86% of people with a first episode of psychosis gain > 7% of their body weight over 12 months. 9 Much of this weight gain occurs within 12 weeks of treatment initiation10 but continues in the longer term, albeit at a slower rate. 11
Interventions to control or reduce antipsychotic-related weight gain
The British Association of Psychopharmacology has reviewed interventions designed to promote weight loss or attenuate weight gain in people taking antipsychotics. Given the underlying mechanisms associated with weight gain, these have taken three, usually exclusive, approaches: (1) lifestyle interventions to improve diet and physical activity, (2) adjustment of antipsychotic medication to minimise the use of drugs associated with the greatest weight change and (3) the use of adjunctive medications that may attenuate the antipsychotic effect. The STructured lifestyle Education for People WIth SchizophrEnia, schizoaffective disorder and first episode psychosis programme (STEPWISE) intervention was intended to promote weight loss through lifestyle modification. However, when the intervention was designed, the developers also considered the effect of the illness and treatment and how these could interact with lifestyle modification.
Prior to the STEPWISE project, a systematic review of randomised controlled trials (RCTs) reported that non-pharmacological interventions lead to a mean 3.12-kg weight reduction over a period of 8–24 weeks in people with schizophrenia, with commensurate change in other cardiovascular risk factors. 12 Programmes offered benefits regardless of treatment duration, modality (individual vs. group setting), behaviour change versus educational content or whether they aimed to prevent weight gain or promote weight loss. Most interventions contained cognitive behavioural elements as well as diet or exercise-based content. The sample size of RCTs was generally small (median 53, range 15–110) and most participants were not followed up beyond 12 weeks. Of the few studies with long-term follow-up, not all sustained long-term weight control. 13 The systematic review team called for larger trials with long-term follow-up and a focus on weight maintenance following initial intervention.
The 2014 National Institute for Health and Care Excellence (NICE) guidance on psychosis and schizophrenia in adults14 expanded this review to 24 studies with similar conclusions. Several of these studies included significant numbers of individuals with other types of mental illness, for whom the strategies needed to promote weight loss may be different. Consistent with the earlier systematic review, few studies examined weight change beyond 6 months, and only six studies included > 100 participants. No study was undertaken in the UK, most were rated as being at moderate risk of bias and there was substantial heterogeneity of effect size. NICE concluded that lifestyle interventions were effective in reducing and maintaining body weight in the short term, but, without longer-term data, the effects beyond 6 months were unknown.
The most recent systematic review published in August 2017 included 17 studies and 1968 participants. 15 Sixty-six per cent of participants had schizophrenia spectrum disorders. Consistent with previous publications, the 10 studies reporting short-term interventions of < 6 months’ duration showed greater weight loss than usual care [standardised mean difference (SMD) –0.20, 95% confidence interval (CI) –0.34 to –0.05], but with significant heterogeneity in response. The six studies that reported interventions lasting longer than 1 year showed a more consistent weight reduction (SMD −0.24, 95% CI −0.36 to −0.12). However, within this group, only two of the six studies achieved a statistically significant weight loss, both of which included people with other severe mental illnesses.
Although some weight loss programmes have promoted behaviour change through intensive one-to-one counselling strategies, resource scarcity in many health services makes the sustainability of such programmes challenging. 16 An alternative is structured education, which encompasses patient-centred, group educational programmes with a clear philosophy, a written curriculum and a basis in behaviour change theory and empirical data and is delivered by trained, quality-assessed educators. 17 The NICE diabetes mellitus prevention guidance advocates structured education as a potentially cost-effective method for the promotion of self-management and behaviour change in people with chronic disease. 18
The Leicester Diabetes Centre structured education approach
The STEPWISE intervention was developed by the Leicester Diabetes Centre (LDC), using the same process used in developing the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) programme. 19 The DESMOND programme and other adaptations have effectively promoted lifestyle change and induced weight loss in multicentre RCTs. The curricula promote physical activity, a healthy diet and weight loss by encouraging self-regulation through self-monitoring (feedback), relapse prevention (identifying and addressing barriers to change) and goal-setting strategies. It incorporates standardised educator training and a quality assurance programme.
Rationale and objectives
Rationale
Pragmatic interventions that offer long-term weight control or reduction in people with schizophrenia or first episode psychosis are needed. The STEPWISE trial aimed to evaluate if a structured lifestyle education programme, delivered in a community mental health setting, supported weight loss at 1 year in adults with schizophrenia, schizoaffective disorder or first episode psychosis.
Objectives
-
To develop a group-based, structured self-management lifestyle education programme for people with schizophrenia, schizoaffective disorder and first episode psychosis.
-
To conduct a multicentre RCT to investigate if the intervention leads to a clinically important difference in weight change, as well as physical activity and diet, compared with usual care.
-
To conduct a mixed-methods process evaluation to explore intervention delivery, participant and facilitator experiences and explain discrepancies between expected and observed outcomes.
-
To conduct an economic evaluation of the intervention.
-
To assess the fidelity of intervention delivery when undertaken at 10 different sites.
Chapter 2 Intervention development
The aim of the intervention development phase was to develop a sustainable evidence-based programme to support people with schizophrenia, schizoaffective disorder or first episode psychosis with their weight management in a way that is acceptable and feasible to deliver within NHS settings and available resources. The intervention was developed by a team from the LDC, together with expert colleagues, patients and public involvement.
Literature review
The meta-analysis of non-pharmacological interventions designed to address antipsychotic-associated weight gain by Caemmerer and colleagues12 was updated by re-running the search strategy across the PsycINFO, MEDLINE, PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Cochrane Library databases using the original search terms, and only one further paper was identified. 20
The literature review reported the benefits of non-pharmacological interventions, but the effectiveness did not differ between modalities or intervention duration, or between interventions that employed group-based versus individual approaches. The only difference was the benefit of outpatient over inpatient interventions. Some studies suggested that nutritional interventions were more effective than cognitive–behavioural therapy, but there was considerable overlap between interventions, making these distinctions difficult to determine. Most interventions ran as 12–24 weekly sessions, delivered either in groups or individually. Some interventions provided specific cardiovascular exercise training. Common dietary themes included:
-
reading food labels
-
switching drinks from full sugar to low calorie
-
eating healthy snacks
-
eating more slowly and deliberately
-
recognising satiety.
Several used ‘psychoeducation’, but further details were not specified, and a theoretical basis was reported in only one study that employed social cognition theory. 21
Development of a theoretical framework
As the theoretical basis of the interventions was specified in only one paper, despite this being widely considered to be good practice,22 we used the wider behaviour change and weight management literature to inform our approach. We considered three key areas that are core to weight management interventions in people with schizophrenia:
-
behaviour change theory, specifically with a focus on food and physical activity
-
the psychological processes underlying weight management
-
the challenges of living with psychosis and the impact on eating and weight.
These core factors determined the draft theoretical framework that guided the overall intervention development (Figure 1). We thus ensured a focus on key hypothesised problem behaviours, were clear about the receipt of the intervention by participants24 and thus applied appropriate behaviour change techniques. 25,26 The intervention was inspired by a number of theories, but systematically employed three (Table 1), and intervention components were coded using the behaviour change taxonomy. 25
FIGURE 1.
Theoretical framework of the STEPWISE intervention. Reproduced from Holt et al. 23 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
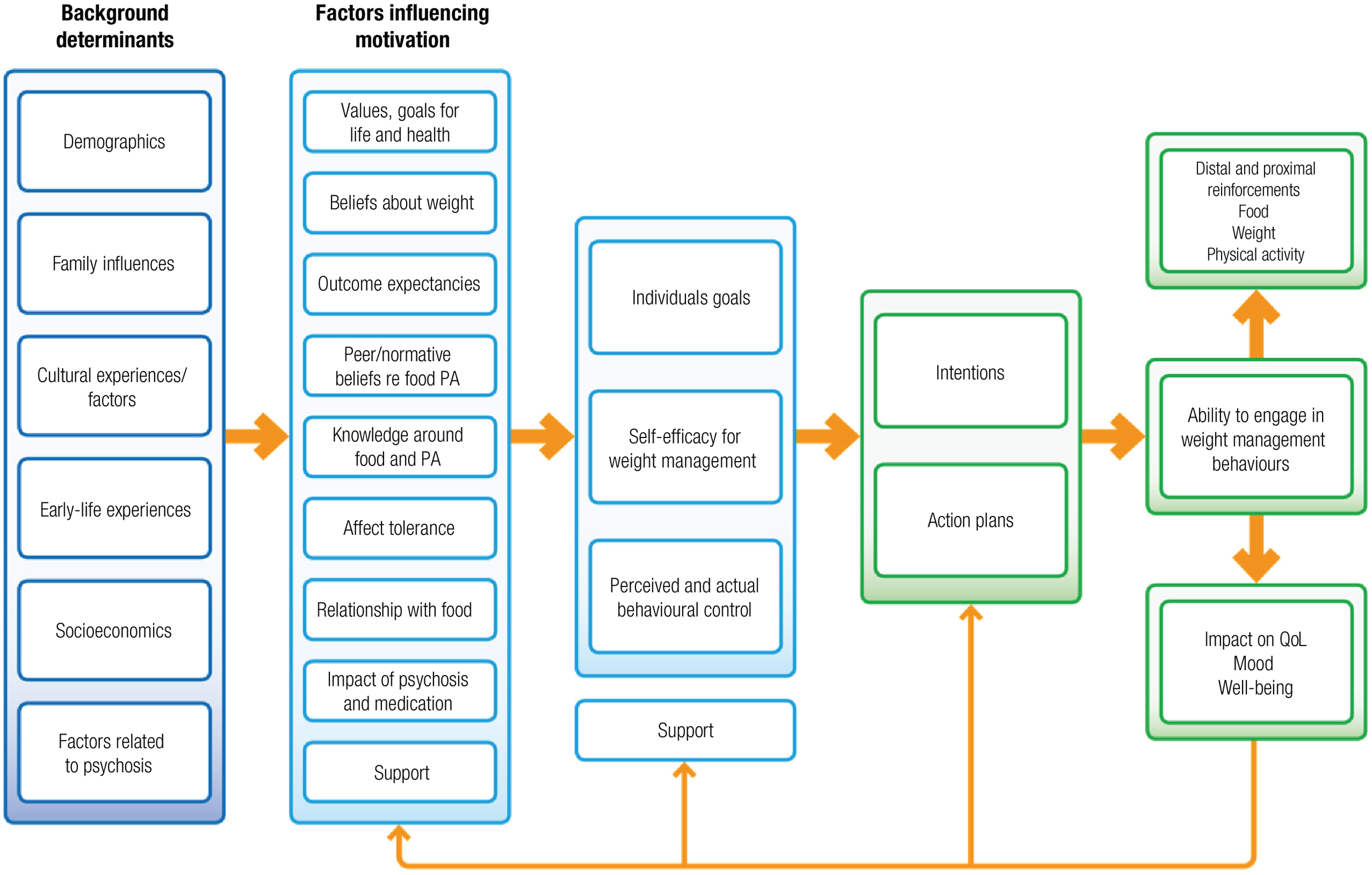
| Identified target behaviour/problem | Theory | Participant receipt and potential behavioural outcome | Intervention on the STEPWISE course | Mapping to behavioural taxonomy |
|---|---|---|---|---|
| Belief about weight problems (e.g. because of their medication, they can have no impact on their weight) | Self-regulation theory – specifically, illness representations around weight management
|
To have identified their own potential erroneous beliefs and questioned these in order to directly influence their decisions around weight management |
‘Your story’ session: elicit participants’ beliefs about what caused their weight problem, what ‘treatment’ would help to manage it, the consequences for them and their health Topic sessions: information sessions throughout the course, specifically the impact of medication on their weight and the strategies that they can employ to manage their weight |
Not completely specified, but included in:
|
| Low levels of confidence around being able to engage in successful weight management, possibly related to multiple unsuccessful attempts at sustained weight loss | Self-efficacy
|
Increased belief in their ability to engage successfully in weight management. Identified strategies to increase their self-efficacy and engage in behaviour change |
‘Sharing stories’ session: eliciting what has gone well in terms of behaviour change, problem-solving around challenges and observing and learning from others’ successes and problem-solving. Discussing feelings as activators of, and barriers to, change Next steps: action-planning, identifying barriers, problem-solving and setting small graded tasks |
|
| Maintenance of behaviour change, particularly as there are strong cues to previous behaviours and thus high likelihood of relapse | Relapse prevention model:
|
Reviewed the situations that would most likely result in relapse. Developed plans of how to manage these when they occur View relapse as a natural part of the change process and as an opportunity to learn rather than berate themselves and reinforce a potential negative self-perception |
Keeping it going: visual tools and interactive exercises to explore potential sources of relapse and develop plans to overcome this when it occurs |
|
Intervention development
Initially, we planned to adapt the STEPWISE programme from the DESMOND ‘Let’s Prevent Type 2 Diabetes programme’ intervention, which seeks to promote lifestyle changes in people who are at an increased risk of developing type 2 diabetes mellitus, and results in modest benefits in biomedical and lifestyle outcomes. Although significant weight change was not achieved, progression to diabetes mellitus in those who were at risk of developing it decreased. 27 However, it rapidly became apparent from the literature review, early meetings with stakeholders and the expert opinions of health-care professionals actively providing local weight management interventions in mental health settings that, although the underlying principles of the Let’s Prevent Type 2 Diabetes programme were relevant, the programme itself was not suitable for people with schizophrenia.
The STEPWISE intervention was therefore developed from first principles, using an established pathway and robust framework28 (Figure 2). The prototype STEPWISE intervention aimed to promote autonomous problem-solving around dietary and physical activity choices, with a specific focus on relapse prevention and weight improvement.
FIGURE 2.
The Leicester development pathway for self-management interventions. Reproduced with permission from Dr Sharon Spencer (Dr Sharon Spencer, University of Leicester, November 2017, personal communication).
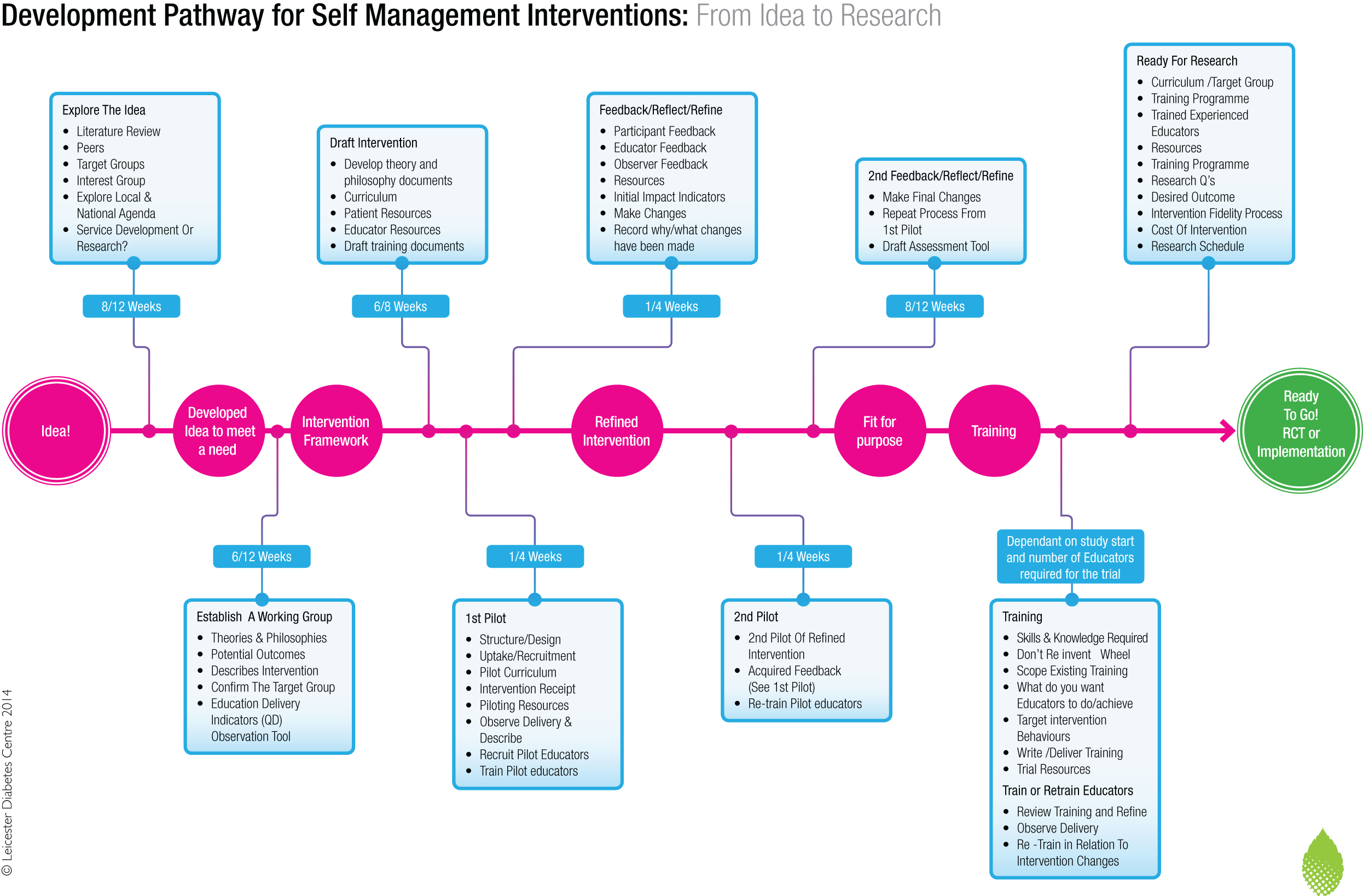
The intervention development incorporated four stages, the first three of which are described in this section:
-
foundation of the programme
-
prototype
-
pilot of prototype and incorporation of amendments and adaptation
-
facilitator training.
The programme was developed collaboratively by a team with expertise in the development of obesity and lifestyle programmes, and mental health-care professionals and researchers with specialist knowledge of schizophrenia and psychosis. Following the literature review, we sought the opinion of service users in Sheffield and Leicester between January and September 2014. Three meetings were held in Leicester at a user-led mental health support group; these were attended by 11 people with schizophrenia or other mental health conditions, who provided input to discussions of the suggested prototype curriculum, resources and subsequent iterations. Topics and suggestions were drawn directly from the group, but the researchers also raised issues that they wished to explore with service users. In addition, other service users, carers and investigators provided input at monthly meetings and in one-to-one interviews. We sought advice from expert practitioners with experience of delivering lifestyle management groups for people with psychosis in Salford, UK, and Sydney, Australia. The principles of the constant comparative approach based on grounded theory were used to analyse these meetings. 29
Six themes emerged from the service users:
-
transportation to and from the venue
-
venue and time
-
level of concentration
-
sessions
-
incentives and motivation
-
accompanying persons.
These are described in Testing the pilot intervention and informed the design of the prototype intervention, its content, delivery and logistics.
Prototype
The user group specifically contributed to the intervention format and played an important role in refining the logistics, content and delivery. The Sheffield mental health team and research team also suggested further changes to the prototype curriculum and resources; for example, appropriate supporting tools (incentives) for participants were introduced and the curriculum terminology was adjusted to improve its acceptability.
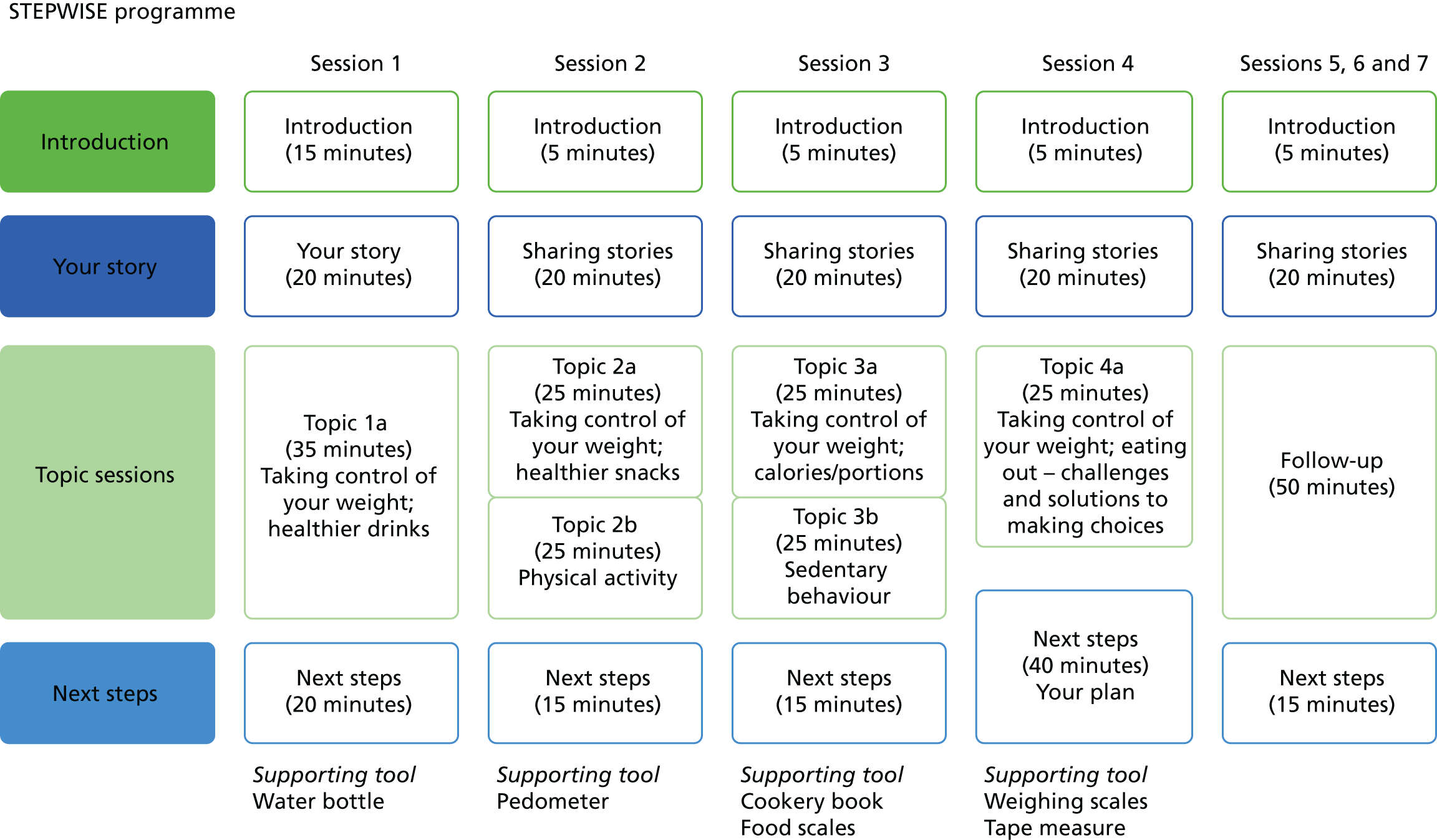
The prototype intervention comprised four 90-minute core group education sessions, delivered to small groups of six to eight participants over 4 consecutive weeks. A breakdown of each session can be found in Figure 3. The curriculum included individual personal stories, taking control of weight, healthier food and drink choices, the relationship between weight and medication, and the relationship between calories and portions and physical activity. The intervention had a written curriculum to ensure consistency and resources in line with its person-centred philosophy. Participants were not ‘taught’ in a formal way, but instead were supported to discover and work out knowledge for themselves to inform their goals and plans.
FIGURE 3.
Outline of the STEPWISE group sessions. Reproduced from Holt et al. 23 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
Although the intervention was designed to be delivered by two local trained facilitators, the prototype sessions were delivered in Sheffield by the development team and were watched by health-care professionals who had been identified as potential facilitators. Feedback from the developers and potential facilitators confirmed the appropriateness of content and resources, while challenging any assumptions made about style and content. We envisaged that at least one facilitator would be a registered mental health professional, whereas the other would have a professional background as a registered mental health professional, mental health support worker, health-care assistant or similar. Current experience of working with people with mental health issues and knowledge of antipsychotics were key facilitator attributes.
To provide ongoing support, a series of 110-minute follow-up ‘booster’ sessions were designed to occur every 3 months after the end of the core sessions. Fortnightly support telephone calls from the facilitator team were arranged and appropriate training was given to the facilitators.
Testing the pilot intervention
The pilot testing of the intervention was undertaken in a community setting and comprised a cycle of four cohorts between May and December 2014 (Figure 4). Biomedical and lifestyle outcomes were not collected during the pilot. The aims were to:
-
test the components of the intervention
-
assess the skills and knowledge required from facilitators and so inform the training programme content
-
understand the obstacles to, and enablers of, delivering the intervention in a real-world situation in accordance with intervention mapping, as described by Bartholomew et al. ,22 and following the Medical Research Council framework for evaluating complex interventions. 30
FIGURE 4.
Consolidated Standards of Reporting Trials flow diagram for the STEPWISE Intervention Development Study. a, Sheffield Health and Social Care NHS Foundation Trust advised that approximately 100 patients (60 patients at one Community Mental Health Team, 20 at another and 20 at a third Community Mental Health Team) from care co-ordinator lists were prescreened and deemed not to meet the basic inclusion criteria. A further cohort of patients was then prescreened for cycle 4, with a further 64 identified as potentially eligible; b, two participants recruited prior to the first Intervention Development Study cycle were unable to attend but willing to take part in the second cycle; however, they did not take part in the second cycle as a result of returning to work (n = 1) and not being contactable (n = 1). DNA, did not attend.
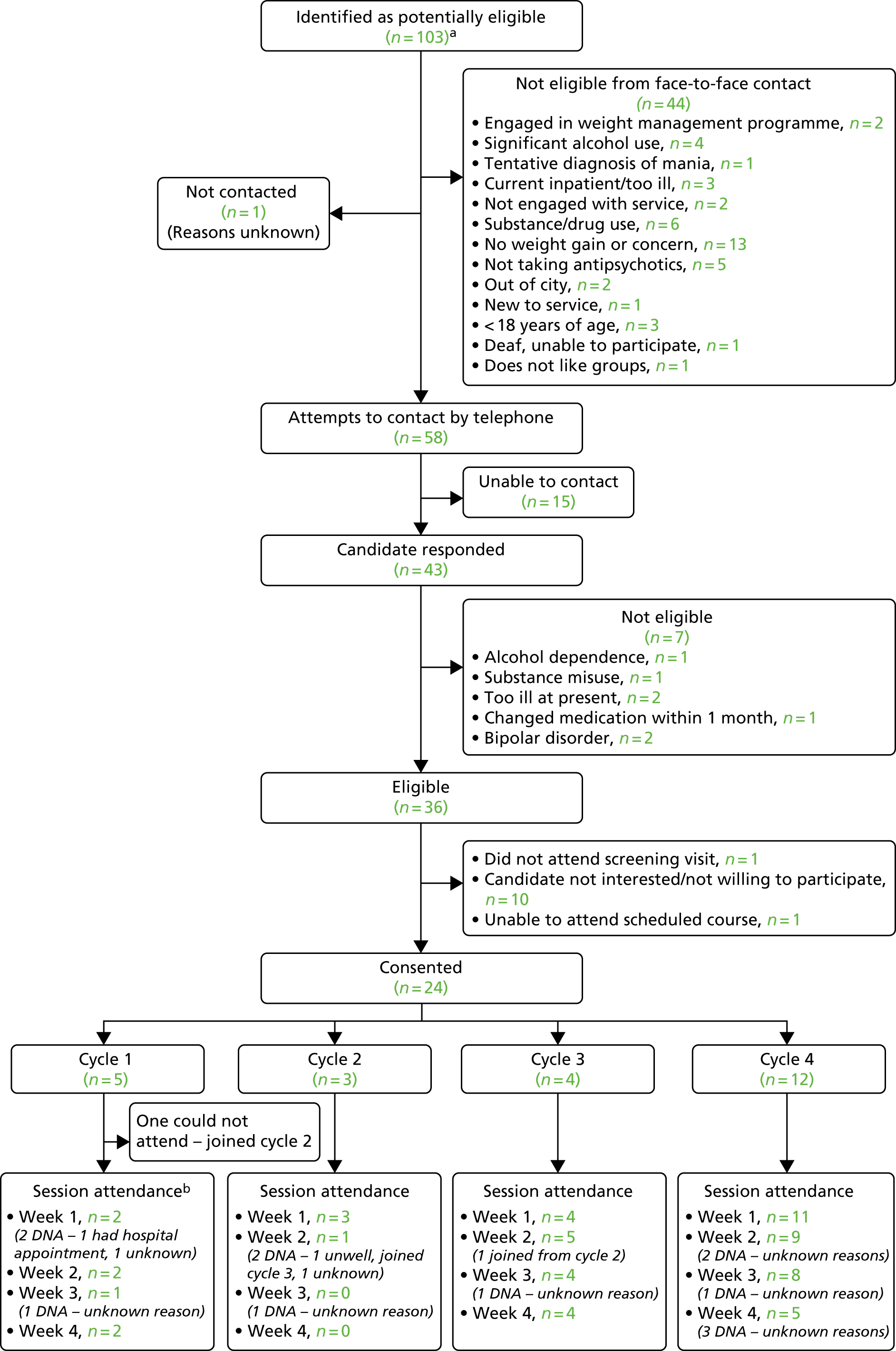
The participants were recruited through Sheffield Health and Social Care NHS Foundation Trust Community Mental Health Teams (CMHTs). The eligibility criteria were identical to those proposed for the future RCT (see Chapter 3, Participant selection and eligibility) to ensure that the intervention would meet the needs of potential trial participants.
Participants were identified during routine clinic appointments and case note review and given brief information about the study. A total of 103 service users with schizophrenia or first episode psychosis were approached by the mental health team, of whom 44 did not meet the eligibility criteria, 16 were non-contactable and 19 declined to participate. Twenty-four people consented to participate, and 20 attended at least one session.
The participants attended one of four pilot interventions at a local venue; these comprised the four weekly core sessions, each lasting ≈2 hours, including a refreshment break. Observation notes were taken at each session to supplement feedback from participants and facilitators in order to refine the programme content, resources and delivery as necessary in an iterative process.
Recruitment and reminders for attendance
Recruitment and retention to the first two cohorts were difficult, and novel recruitment strategies, such as the offer of transport, and text and telephone-call reminders, were needed to increase attendance. This led to improved participation in the third and fourth iterations.
Logistical considerations
Participant feedback showed that they valued the intervention and discussion mostly focused on practical ways of facilitating attendance and active engagement. Organisational issues were seen as key factors (e.g. arranging taxis helped participants to arrive in a timely manner, while reducing the anxiety of using public transport). The local mental health venue was described as a convenient and familiar place to reach. The participants reported that the number of sessions was reasonable.
Many people with schizophrenia have altered sleep patterns, which make early-morning appointments difficult, and so the time of the sessions (12.30 for a 13.00 start) was considered to be ideal. During the first cohort, participants struggled to attend on time; this was partly solved by providing taxis, but we also introduced a pre-session healthy lunch. This meant that the participants did not disrupt sessions if they arrived late, and also provided a practical demonstration of how to eat healthily on a limited budget.
Many people with schizophrenia may experience cognitive deficits and limited ability to concentrate over time. To overcome this, we incorporated sufficient and flexible breaks into the pilot intervention.
Sessions
Participants enjoyed the small-group setting, which enabled them to assimilate information more efficiently, share experiences with fellow participants and reinforce existing messages. Participants and facilitators found the resources, such as flipcharts, laminates and booklets, valuable and engaging. Participants commented on the benefit of using the same facilitators throughout the intervention. Contrary to our expectations, participants wished to attend alone and thought that they would not benefit from bringing an accompanying person to the sessions. Participants felt that undertaking the intervention was something they could accomplish on their own and that it would be easier to share information with fellow participants without the presence of people who did not have mental illness.
The use of supporting tools (e.g. samples of low-calorie drinks and snacks, kitchen scales, cookery books and pedometers) reinforced the messages provided to participants about the benefits of participation, improved internal motivation and supported engagement and attendance.
Chapter 3 Evaluation methods
Trial methods
Trial design
We undertook a multicentre, two-arm, parallel-group RCT of the STEPWISE structured lifestyle education programme compared with usual care plus written lifestyle advice in 10 NHS Mental Health Trusts in England. Participants were individually randomised to the STEPWISE programme or usual care. This report is concordant with the Consolidated Standards of Reporting Trials (CONSORT) statement (2010). 31
Important changes to methods after trial commencement
Trial recruitment commenced on 12 March 2015 (first patient, first visit) and, following this, in response to early observations and feedback from the intervention development study, a number of changes were made to the protocol. The protocol was published32 and the current version is available via the National Institute for Health Research (NIHR) Journals Library website. 33
Between the initial Research Ethics Committee approval and study commencement, there were two substantial amendments. These revised the sample size, clarified the eligibility criteria, described the control arm and updated the screening and consent process to ensure that recruitment could take place closer to scheduled intervention sessions.
In March 2015 (substantial amendment 3, protocol version 4.0), following feedback from the intervention development study, the option for participants in the intervention arm to bring along a friend, relative or carer to the intervention sessions was removed. This amendment also added details about referring any concerns or participant risk to the clinical care team. Amendments were made before delivery of the first foundation session (23 April 2015).
In June 2015 (substantial amendment 4, protocol version 5.0), the data collection windows for the Operational Criteria Checklist for Psychotic Illness and Affective Illness (OPCRIT+) and the outcome assessments were increased, to provide more opportunity for sites to follow up participants, allowing for missed appointments.
A further amendment in November 2015 (substantial amendment 5, protocol version 6.0) clarified the use of ‘Community Mental Health Teams’ in the eligibility criteria. This allowed potential participants within a variety of services, including those stepping down from inpatient to community services, to participate, provided that they could fully implement the learning from the intervention. This amendment also allowed 1 additional week to obtain the fasting blood sample at the 12-month follow-up.
In February 2016 (substantial amendment 6, protocol version 8.0), changes were made to the protocol to allow over-recruitment beyond the initial recruitment target, in order to allow centres to recruit in waves and run intervention groups with sufficient participants.
Participant selection and eligibility
The trial was co-ordinated from the Clinical Trials Research Unit (CTRU) in the Sheffield School of Health and Related Research (ScHARR). Delegated study staff located at participating centres identified and gained consent from potential participants. Eight of the 10 centres [and their principal investigators (PIs)] were involved at the proposal development stage, with two further centres identified through expressions of interest via the NIHR Clinical Research Network.
The trial was promoted within clinical teams and in community areas in which mental health services are delivered. Clinicians used a standardised script to ensure that potential participants received consistent information about the trial. The research team at participating sites worked with clinical teams to identify potentially eligible patients from clinic lists and other caseloads. Self-referrals were also accepted, following the use of information displayed on posters and leaflets.
The study took place within CMHTs, including early intervention services, in 10 mental health NHS trusts in a range of locations, urban and rural, in Sheffield, Leeds, York, Bradford, Greater Manchester, South London, Sussex, Hampshire, Devon, Somerset and Cornwall.
Adults were eligible for inclusion in the study if they:
-
were aged ≥ 18 years
-
had a diagnosis of schizophrenia or schizoaffective disorder [defined by the International Classification of Diseases, Tenth Revision (ICD-10) codes F20 and F25] or first episode psychosis (defined as < 3 years since presentation to the mental health team) using case note review
-
were being treated with an antipsychotic
-
were able to give written informed consent
-
were able and willing to attend and participate in a group education programme
-
were able to speak and read English
-
had a body mass index (BMI) of ≥ 25 kg/m2 or were concerned about their weight (in the case of participants from South Asian and Chinese backgrounds, the BMI threshold was reduced to ≥ 23 kg/m2).
People were excluded from the study if they:
-
had a physical illness that could seriously reduce their life expectancy or ability to participate in the trial
-
had a coexisting physical health problem that would, in the opinion of the PI, independently affect metabolic measures
-
had a mental illness that could seriously reduce their ability to participate in the trial
-
had a current pregnancy or were < 6 months post partum
-
had a condition associated with significant weight gain (e.g. Cushing syndrome)
-
engaged in significant alcohol or substance misuse which, in the opinion of the PI, would limit the patient’s ability to participate in the trial
-
had a diagnosis or a tentative diagnosis of psychotic depression or mania
-
had a primary diagnosis of a learning disability
-
were currently (or within the past 3 months) engaged in a systematic weight management programme, to ensure that other programmes did not have an impact on baseline measures.
During the trial, participants were not prevented from joining weight loss or physical activity programmes (intervention arm participants were encouraged to make positive behaviour change); however, any uptake of systematic programmes (other than STEPWISE) was captured at the 3- and 12-month follow-up by asking all participants if they had taken up any other weight management or physical activity programme outside the trial.
All potential participants had a minimum of 24 hours in which to decide whether or not they wished to participate, before attending a consent visit.
The CTRU co-ordinated the follow-up and data collection in collaboration with centres. Participant study data were collected and recorded on study-specific case report forms (CRFs) by site research staff and were entered onto a remote web-based data capture system at each site.
Interventions
All participants received written lifestyle advice on diet, physical activity and alcohol and smoking use before randomisation. Participants were randomised to receive either the STEPWISE education programme or usual care.
Research intervention (STEPWISE education programme)
Participants allocated to the research intervention were contacted by the session co-ordinator and provided with pre-course information, which included an introductory letter and leaflet confirming when and where the sessions would take place and what to expect.
The intervention took place over approximately a 12-month period post randomisation. Participants allocated to STEPWISE received a foundation course of four weekly 2.5-hour (including breaks) group sessions delivered by two trained facilitators. The protocol specified approximately 6–10 participants for each course, to allow for a good group size and to account for likely attrition.
The foundation course was followed by one-to-one support contact lasting for around 10 minutes, approximately every 2 weeks for the remainder of the intervention period. This contact was personalised and was mostly conducted by telephone, although it also took place in person on some occasions. The purpose of the support contact was to discuss participants’ progress towards their goals, highlight any issues and try and motivate participants to change their behaviours in line with the group-based session. When participants could not be contacted, a motivational postcard was sent to them to maintain this contact. Support contact was undertaken by a trained facilitator to support behaviour change and obtain feedback from the participant.
Participants were invited to attend 2.5-hour group-based booster education sessions at 4, 7 and 10 months post randomisation. Attendance at all intervention sessions and receipt of support contact were recorded.
Participants who attended education sessions were invited by facilitators to complete a ‘session feedback’ form at the end of each session. This was completed independently and sealed in an envelope to maintain anonymity. The envelopes were posted directly to the CTRU for data entry. Participants could choose whether or not to include their name on the feedback form, and the aim was to capture a self-report of empowerment and health belief during the intervention.
Between four and six health-care professionals or associated staff at each participating centre received facilitator training to deliver the STEPWISE intervention. This training covered the core DESMOND philosophy (3 days), specific content and delivery of the STEPWISE programme (1 day) and the content and delivery of booster sessions (1 day).
Control arm
Participants allocated to the control arm received treatment as usual (TAU), enhanced by the provision of written lifestyle advice. There is known variability in the provision of physical health care, despite NICE guidelines on the treatment and management of schizophrenia regarding healthy eating and physical health. 14 To standardise usual care in both groups as far as possible, participating centres provided printed guidance to all participants (regardless of allocation) before randomisation on the risk of weight gain and lifestyle advice, regarding diet, physical activity, smoking and alcohol use, when appropriate.
Usual care
It is appropriate in pragmatic trials for the comparison intervention to involve ‘usual practice’, offering study sites leeway in deciding what that should involve. 34 As the content of usual care can influence effect size,35 we undertook a survey of usual care at the participating centres at two time points. The methods for this exercise have been reported previously,36 but, in brief, sites were surveyed on their current practices regarding healthy eating and physical activity programmes offered to people with schizophrenia. The survey was based on current NICE guidance14 and included questions about any trust-offered healthy eating and physical activity programmes, what discussions take place with patients before antipsychotic initiation, the availability of smoking cessation support and how physical health reviews are completed, including what measures are reviewed and how often.
Measurement of outcomes
Following consent but before randomisation, research staff completed CRFs for each participant, which covered medical and psychiatric history, demographics and current medication information (Table 2). All assessments and questionnaires were undertaken either at the participant’s home or in the NHS trust. A fasting blood sample was taken, as well as measurements of vital signs and anthropometry. Baseline self-report instruments were completed, with research staff encouraged to read questions aloud to participants, with all available answer options, to ensure understanding. Participants were provided with a wrist-worn accelerometer to wear 24 hours per day for 7 days following the visit.
| Outcome measure | Time point | |||
|---|---|---|---|---|
| Pre-baseline | Baseline | 3 months | 12 months | |
| Eligibility criteria assessed by the clinical care team | ✗ | |||
| Medical history | ✗ | |||
| Psychiatric history | ✗ | |||
| OPCRIT+37 | ✗ | |||
| Renal function | ✗ | ✗ | ||
| Hepatic function | ✗ | ✗ | ||
| Height (to calculate BMI) | ✗ | |||
| Weight | ✗ | ✗ | ✗ | |
| Waist circumference | ✗ | ✗ | ✗ | |
| Physical activity [7-day wrist-worn GENEActiv (Activinsights, Kimbolton, UK) accelerometer] | ✗ | ✗ | ✗ | |
| Adapted DINE questionnaire38 | ✗ | ✗ | ✗ | |
| Blood pressure | ✗ | ✗ | ✗ | |
| Fasting glucose | ✗ | ✗ | ||
| Lipid profile | ✗ | ✗ | ||
| Glycated haemoglobin | ✗ | ✗ | ||
| EQ-5D-5L39 | ✗ | ✗ | ✗ | |
| SF-3640 | ✗ | ✗ | ✗ | |
| B-IPQ41 | ✗ | ✗ | ✗ | |
| BPRS42 | ✗ | ✗ | ✗ | |
| Smoking status | ✗ | ✗ | ✗ | |
| CSRI43 | ✗ | ✗ | ✗ | |
| Changes in medication (dose and side effects) | ✗ | ✗ | ✗ | |
| PHQ-944 | ✗ | ✗ | ✗ | |
| Use of weight loss programmes | ✗ | ✗ | ||
| Adverse events | ✗ | ✗ | ||
| Session feedback (intervention only; each group session) | ✗ | |||
Additional information, such as medication details and details of hospital admissions, was obtained and/or verified using the patient’s medical notes. At both the 3- and the 12-month follow-up visits, research staff who were masked to treatment allocation confirmed medication information with participants, particularly doses and side effects of antipsychotic medication. They took measurements of blood pressure, weight and waist circumference (and a fasting blood sample at 12 months only) and provided the participant with a wrist accelerometer to wear for the next 7 days. Participants were asked to complete the same questionnaires as at baseline, with the addition of a form to capture the uptake of any weight loss programmes outside the STEPWISE trial. The follow-up windows at 3 and 12 months were defined as minus 2 weeks and plus 4 weeks to allow time for missed and rearranged appointments.
Biomedical measures
The biomedical outcomes were:
-
Weight outcomes – change in weight, the proportion of participants who maintained or reduced weight, percentage change in weight, waist circumference and BMI. Standard operating procedures specified how to measure body weight (e.g. light clothing, shoes off) using the Class III approved Marsden 430-C portable scales (Marsden Weighing Machine Group Limited, Rotherham, UK) to the nearest 0.1 kg; height (e.g. standing tall, feet hip-width apart and head level) using the Class I approved Marsden HM-250P stadiometer (Marsden Weighing Machine Group Limited, Rotherham, UK) to the nearest 1 cm; and waist circumference (e.g. tape lying flat and level, taut but not tight) using the Class I approved Seca 201 tape measure (Seca, Birmingham, UK) to the nearest 0.1 cm. Participants who provided 12-month weight data in accordance with the protocol received a £20 shopping voucher.
-
Vital signs and laboratory measurements:
-
blood pressure (average of three measurements) and pulse, measured by electronic sphygmomanometer in the non-dominant arm after 5 minutes’ rest
-
fasting glucose, lipid profile and glycated haemoglobin (HbA1c) at baseline and 12 months only (all blood samples were analysed by local laboratories).
-
Physical activity
Physical activity was assessed by wrist-worn accelerometer (GENEActiv, Activinsights Ltd, Kimbolton, UK). Participants were asked to wear the accelerometer on their non-dominant wrist continuously (i.e. 24 hours per day) for 7 days and data were derived using the approach reported in da Silva et al. 45 The GENEActiv.bin files were analysed with R-package GGIR, version 1.5 (Cran-R Project, the Netherlands). 28,29 Signal processing in GGIR includes the following steps:
-
autocalibration, using local gravity as a reference28
-
detection of sustained abnormally high values
-
detection of non-wear
-
calculation of the average magnitude of dynamic acceleration (i.e. the vector magnitude of acceleration corrected for gravity [Euclidean norm minus one g (ENMO)] as:
over 5-second epochs, with negative values rounded up to zero.
Files were excluded from all analyses if the post-calibration error was > 0.02 g30 or < 16 hours of wear time as recorded by either monitor during the 24-hour day of interest. Detection of non-wear has been described in detail previously. 29 In brief, non-wear is estimated based on the standard deviation (SD) and value range of each axis, calculated for 60-minute windows with 15-minute moving increments. If for at least two out of the three axes the SD is < 13 mg (milligravity) or the value range is < 50 mg, the time window is classified as non-wear.
The average magnitude of dynamic wrist acceleration (ENMO) and time accumulated in moderate or vigorous physical activity (MVPA) were calculated. The threshold for determining MVPA was ≥ 100 mg.
Four measures were taken:
-
mean acceleration calculated per day
-
MVPA based on a 5-second epoch setting (i.e. total unbouted MVPA)
-
MVPA based on a 5-second epoch setting and a bout duration of 5 minutes, inclusion criteria based on > 80%
-
MVPA based on a 5-second epoch setting and a bout duration of 10 minutes, inclusion criteria based on > 80%.
Each of the above was calculated for weekdays (provided data were available for at least 3 of the 5 days), weekends (provided data were available for at least 1 day) and all days (when data were available for at least 4 of the 7 days).
Lifestyle factors
Three lifestyle measures were collected. These were the adapted Dietary Instrument for Nutrition Education (DINE) questionnaire,38 smoking status and use of weight loss programmes:
-
The adapted DINE questionnaire measures diet on six items – fibre intake, fat intake, unsaturated fat intake, sugar intake, alcohol intake and meal type. Scores and ratings were calculated only when all constituent items had been reported for the particular item.
-
Smoking status was measured by the number and percentage of participants who smoke, smoking category [light (< 10 cigarettes per day), moderate (10–19 cigarettes per day) or heavy (≥ 20 cigarettes per day)], interventions offered to aid smoking cessation and interventions taken to aid smoking cessation.
-
Use of weight loss programmes (follow-up only); the number and percentage of participants who reported enrolling in any weight loss programme.
Patient-reported outcome measures
Four patient-reported outcome measures were collected:
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L) health utility,46 comprising a health state and thermometer scale. Higher scores indicate a better health state.
-
The Short Form questionnaire-36 items (SF-36),47 from which eight domains of quality of life (QoL) were derived. Higher scores indicate a higher QoL.
-
The Patient Health Questionnaire-9 (PHQ-9),44 a measure of depressive symptoms. Higher scores indicate more severe depressive symptoms.
-
The adapted Brief Illness Perception Questionnaire (B-IPQ),41 a measure of illness perception. Higher scores reflect a more threatening view of the obesity.
Clinician-assessed outcome measure
Participants were assessed using the Brief Psychiatric Rating Scale (BPRS), a clinician-rated measure that evaluates the psychopathology of patients with schizophrenia. 42 Higher scores indicate greater psychiatric concern.
The OPCRIT+ was completed by the research team, using case note review, within 10 weeks of the baseline visit. This was to provide comparable baseline characteristics for all participants across the study. The protocol allowed baseline fasting blood samples and accelerometry data to be collected after randomisation when recruitment occurred close to a scheduled intervention course.
Cardiovascular and diabetes mellitus risk
The 10-year cardiovascular risk was calculated using the Framingham Cardiovascular Risk Score. 48 An analysis using a second cardiovascular risk score for people with severe mental illness (PRIMROSE)49 was planned but not used, as a problem emerged with the algorithm during the analysis. The 10-year type 2 diabetes mellitus risk was calculated by the Leicester score. 50
Derivation of outcome measures
The EuroQol-5 Dimensions (EQ-5D) tariff was scored using the EQ-5D-5L for UK population norms;51 no score was calculated if any of the five items were missing. The eight subscales of the SF-36 were calculated as per McHorney et al. ;40 subscores were calculated when at least half of the questions within the domain had been answered. When all eight domains were completed, the aggregate physical and mental component scores were calculated. The adapted B-IPQ was scored by summing the responses to items into a single score41 if at least six of the eight questions were answered. The BPRS was scored by summing up the responses into a single score42 and was calculated if at least 14 of the 18 items had been answered.
Sample size
The sample size calculation was based on data from two sources, both of which assessed behavioural interventions for weight loss in people prescribed antipsychotics for schizophrenia. First, a systematic review undertaken by Das et al. 52 examined both randomised and non-randomised controlled trials, and reported between-group differences of 1.5–6 kg (SD ≈5 kg). Data on overweight and obese UK patients with severe mental illness from a second study also contributed to the sample size calculation. A total of 51 people with schizophrenia were followed up for at least 1 year, and the weight change reported was 7.7 kg (SD 6.5 kg). 53
The sample size used in STEPWISE aimed to detect a difference of 4.5 kg, which is clinically meaningful (average ≈5% reduction in body weight)54 and also appears to be compatible based on previous work. A conservative estimate of a SD of 10 kg, 95% study power and a two-sided significance level of 5% was assumed, meaning that 130 participants per intervention arm (260 participants in total) were required to detect a minimum clinically important difference of 4.5 kg.
Owing to the group nature of the intervention, it is possible that the outcomes of participants within the same group may be correlated. Therefore, an average group size of seven participants was assumed, with an intraclass correlation of 5% in the intervention arm. For this reason, the sample size was inflated by a design effect of 1.3 in the intervention arm, which gives revised sample sizes of 169 participants in the intervention arm and 130 participants in the control arm (299 in total).
To ensure a 1 : 1 allocation, 158 participants were required per arm to reproduce this power. Assumptions were made for a conservative dropout rate of 20%, which is higher than that observed in similar studies,55 giving a final sample size of 198 participants per trial arm. This equates to 40–50 participants at each centre, with 20–25 of these receiving the intervention in up to four groups per site.
Randomisation
Sequence generation
The randomisation list was generated using the CTRU’s web-based system, which provided central randomisation and ensured that the study team was blinded to the allocation. The list was generated using permuted blocks of random sizes to allocate participants to either TAU plus the STEPWISE lifestyle education programme or TAU alone in a 1 : 1 ratio, stratified by site and time since the start of antipsychotic medication (< 3 months or ≥ 3 months). When the exact duration was unknown, an approximate duration was considered to be acceptable for the purposes of randomisation.
Implementation
After the baseline assessments were completed and consent was provided, participants were randomised using the STEPWISE randomisation system. Once randomised, an unblinded member of the site research team informed the participant and their general practitioner (GP) of the treatment allocation.
Blinding
All research team members performing outcome assessments were blind to treatment allocation. Blind (or suspected) breaks were recorded. Owing to the nature of the intervention, participants were not blinded.
Ethics aspects
The study received a favourable opinion from the National Research Ethics Committee, Yorkshire & the Humber – South Yorkshire on 4 February 2014 (reference 14/YH/0019).
Patient and public involvement
Angela Etherington (a person with severe mental illness and experience of taking antipsychotic medication) and David Shiers (the carer of a family member with schizophrenia) were involved in the design of the study and the intervention, management meetings, the qualitative research analysis and the drafting of the report. They reviewed, made changes to and approved the final lay summary.
Statistical methods
Outcome measures
Primary outcome measure
The primary end point of the STEPWISE trial was the change in weight (kg) at 12 months after randomisation.
Secondary outcome measures
Secondary outcomes included biomedical measurements, physical activity, dietary components and psychosocial factors, including QoL, health beliefs and cost-effectiveness. All secondary outcome measures were assessed at baseline and after 3 and 12 months (except when stated), to measure if there was an effect at the end of the intervention and, if so, whether or not this was sustained over the longer term.
Analysis of weight change
The primary objective (weight change at 1 year post randomisation) was assessed by fitting a marginal generalised estimating equation (GEE) model using robust standard errors and an exchangeable correlation structure. The difference between intervention and control arms was adjusted for baseline weight, site and years since antipsychotic medication initiation. The intraclass correlation coefficient (i.e. the ‘cluster’ term) was derived from the correlation matrix of the GEE model. The following preplanned sensitivity analyses were undertaken:
-
alternative covariates (in which any imbalanced baseline characteristics were added into the GEE model)
-
alternative model structure (multilevel model in place of the GEE to estimate the cluster effect)
-
alternative assumptions for missing data.
The last of these analyses used approaches proposed by von Hippel,56 Carpenter et al. 57 and White et al. 58 The complete-case analyses were augmented with a reanalysis assuming a missing-at-random (MAR) mechanism, achieved by incorporating all baseline covariates that were associated with the probability of missing weight at 12 months and/or with weight change among those who were followed up; these included baseline demographics, disease characteristics, recruiting site, treatment group, weight, BPRS score, B-IPQ score and physical domains of the SF-36 at baseline and 3 months.
The first model incorporated baseline measures as covariates, following which the treatment effect was re-estimated (model MAR 1). Following this, predictive mean matching multiple imputation via chained estimation incorporated all available baseline and 3-month values to impute missing 12-month weight using the original model covariates (model MAR 2). Thereafter, the sensitivity to missing not at random (MNAR) was assessed by adding a range of fixed quantities (delta) to the MAR prediction; for example, delta = 1 corresponds to the assumption that an individual with no 12-month data has a weight change of 1 kg more than predicted. The values of delta used ranged from –5 to + 5 kg and included different values of delta for the two treatment groups.
The treatment comparison was calculated in the following preplanned subgroups:
-
uptake of therapy (non-attender; attended one or two foundation sessions; attended three or four foundation sessions but no booster sessions; attended at least three foundation sessions and one booster session)
-
recruiting centre
-
clinical diagnosis (first episode vs. other diagnoses)
-
time since starting antipsychotic medication (≤ 12 months, > 12 to ≤ 24 months and > 24 months)
-
BMI (≤ 25 kg/m2, > 25 to ≤ 30 kg/m2, > 30 to ≤ 35 kg/m2, > 35 to ≤ 40 kg/m2 and > 40 kg/m2)
-
principal reason for dietary concern, as assessed by the B-IPQ.
Analysis of other outcomes
Other outcomes were analysed using a GEE model, with the covariates being treatment group, site, years since antipsychotic medication initiation and the baseline measurement of the respective outcome.
General considerations
A comprehensive statistical analysis plan was developed before the database freeze and while the statistician was blinded to treatment allocation. Data were reported and presented in accordance with the revised CONSORT statement. 31,59 Analyses were performed on an intention-to-treat basis, unless otherwise stated. Analyses of outcome in relation to protocol compliance were undertaken by looking at the level of course attendance (a subgroup analysis in Analysis of other outcomes) and by complier-average causal effect (CACE), using two-stage least squares regression; the latter defined compliance as attendance of at least one foundation course.
All statistical tests were two-tailed at a 5% significance level and CIs were two-sided, with 95% intervals. No adjustment was made for multiplicity, but the number of outcome measures used necessitates cautious interpretation of statistical significance. All analyses were performed in the Stata® version 14.2 (StataCorp LP, College Station, TX, USA) statistical software, using the user-written Stata package rctmiss for the first MNAR model. 60
Process evaluation
Overview
The process evaluation was undertaken ‘to explain discrepancies between expected and observed outcomes, to understand how context influences outcomes, and to provide insights to aid implementation’. 30 Specifically, we investigated whether or not (1) treatment is consistent with the underpinning behaviour change theories (treatment theory or theory of change) and (2) contextual factors affected implementation. The process evaluation used a pipeline logic model, showing causal links between resources, activities and outcomes, integrating the National Institutes for Health Behaviour Change Consortium (NIHBCC)’s approach to treatment fidelity24 and a modified version of Linnan and Steckler’s framework for process evaluation. 61 We described context qualitatively and took a mixed-methods approach to characterising recruitment, reach, dose delivered/received and fidelity both qualitatively and quantitatively, with triangulation between data sources. 62 Interviews were held with intervention designers, health professionals and RCT participants, and the analyses were combined with RCT data and quantitative fidelity data.
Researchers
The qualitative researchers, Rebecca Gossage-Worrall, a female graduate sociologist [Master of Arts (MA); Research Associate], and Daniel Hind, a male graduate anthropologist [Doctor of Philosophy (PhD), Reader], had 8 and 10 years’ experience of interviewing, respectively. No relationship was established with any participant outside or before the interview. The interview purpose was explained to participants twice, at consent to trial and at interview. Two facilitators had a prior relationship with Rebecca Gossage-Worrall (through their study research role) before participating in the interviews. Daniel Hind knew two intervention developers prior to interview via project meetings.
Theoretical and thematic framework
Rationale and worldview63/epistemology64
We incorporated qualitative research to understand the implementation of, and response to, the intervention,65–67 to propose causal pathways to success or failure. 65–68 However, our rationale was primarily pragmatic rather than explanatory;69 we were pursuing a basis for ‘organising future observations and experiences’70 by ‘investigating conceivable practical consequences’71 of future decisions, rather than advancing, building or testing social science theory. 68,72
Research design63/methodology64/approach73
We used a single-case design,74 with the unit of analysis variably at the participant level (n = 24 participants) and at the level of the experimental intervention programme (n = 20 facilitator interviews). In participant case studies, the embedded units of analysis were (1) post-course interview and (2) quantitative CRFs, especially weight at 0, 3 and 12 months.
Theory
We used the International Classification of Functioning (ICF) as a conceptual framework for describing the schizophrenia-specific context for implementation. 75,76 We used an a priori framework, based on similar studies,77,78 to inform the topic guide for interviews with participants (Table 3). Topic guides for interviews with health professionals were designed around the normalisation process theory (NPT). 80–83 We used the theoretical domains framework (TDF)84 to characterise stakeholder understandings of the intervention. Codes from the TDF were later mapped to constructs from the principal theories underpinning the STEPWISE intervention: Bandura’s self-efficacy theory,85 the self-regulation theory of Leventhal et al. 86 and Marlatt and George’s relapse prevention model. 87 We used the NIHBCC’s framework for understanding intervention fidelity79 and the framework of Sekhon et al. for understanding the acceptability of health-care interventions. 88
| A priori theme | Example interview question |
|---|---|
| Acceptability77,78 |
|
| Being in a group77,78 |
|
| Presentation of content78 |
|
| Changes in behaviour77,78 |
|
| Processes of change78 |
|
| Types of interventions78 |
|
| Fidelity79 |
|
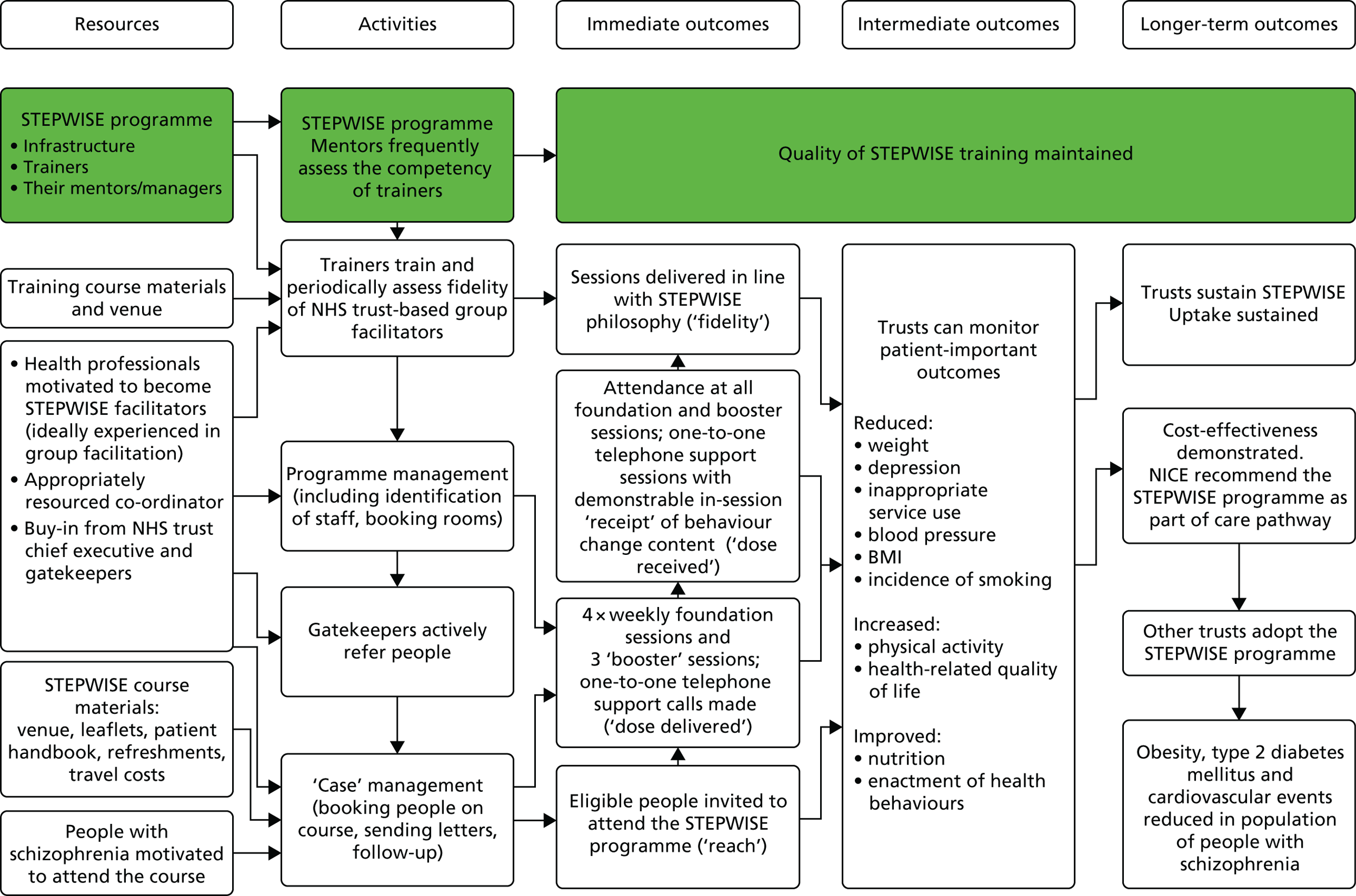
We developed a programme theory to identify essential elements for the successful replication and causes of failure in the contracts, actions, interactions and emergent relationships between people and organisations that surround the STEPWISE intervention. 89–91 The programme theory development was deductive, through literature review and by articulating mental models in discussion with the LDC team, and inductive, through interviews with participants and professionals. 92 To illustrate how sequences of events were to bring about desirable outcomes, as per the programme theory, we developed a logic model (Figure 5). 93,94
FIGURE 5.
Logic model for the implementation of the STEPWISE intervention.
Participant selection and setting
Consent for participant interviews was requested, face to face, at the time of consent to the RCT (when participants specified a preferred method of approach) and reconsented immediately before interview. The majority of participants were approached by telephone. Professionals were approached directly by telephone, e-mailed the information sheet and consented by telephone; intervention designers were approached during project meetings and by e-mail. The RCT intervention arm participants were purposively sampled (n = 24) from those consenting and allocated to the intervention (n = 188) to reflect different study centre, gender and age (Table 4). A total of 63 participants (34%) were sampled, of whom 22 (35%) were non-responsive, despite a minimum of three attempts to contact. Ten participants declined when invited to participate, one declined at (re-)consent and six consented but did not attend the appointment. Forty professionals were purposively sampled to reflect differences in site, occupation, sex and prior group facilitation characteristics (derived from a short survey after completing facilitator training) for invitation to study, of whom 20 were interviewed (Table 5); none formally declined, but many left their employment or were non-responsive, or it became unnecessary to interview them because of accrual of the target sample. All those involved with the intervention design were interviewed. All interviews were conducted by telephone.
| ID | Sex | Age (years) | Diagnosisa | Ethnicity | BPRS score | Sessions | Weight in kg | Weight change in kg | Interview took place after (session) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foundation | Booster | 0 months | 3 months | 12 months | 0–3 months | 3–12 months | 0–12 months | |||||||
| Clinically important weight loss over 12 months | ||||||||||||||
| S03/Q06 | M | 35 | F20 | White British | 34 | 4 | 3 | 115.5 | 111.7 | 98.3 | –3.8 | –13.4 | –17.2 | Booster 2 |
| S01/Q01 | F | 40 | F20 | African | 27 | 3 | 2 | 106.7 | 101.0 | 94.0 | –5.7 | –7.0 | –12.7 | Foundation |
| S08/Q05 | F | 28 | F25 | White British | 41 | 4 | 3 | 103.4 | 104.9 | 94.1 | 1.5 | –10.8 | –9.3 | Booster 1 |
| S04/Q02 | F | 33 | FEP | African | 34 | 4 | 0 | 92.2 | 93.4 | 86.1 | 1.2 | –7.3 | –6.1 | Foundation |
| S06/Q01 | M | 23 | F20 | White British | 27 | 3 | 2 | 92.7 | 92.6 | 87.5 | –0.1 | –5.1 | –5.2 | Foundation |
| S01/Q05 | M | 40 | F20 | White British | 24 | 4 | 2 | 110.4 | 105.4 | 105.2 | –5.0 | –0.2 | –5.2 | Foundation |
| S02/Q04 | F | 40 | F25 | White British | 31 | 4 | 3 | 74.0 | 71.0 | 69.3 | –3.0 | –1.7 | –4.7 | Booster 2 |
| Weight loss that is not clinically important | ||||||||||||||
| S10/Q03 | F | 39 | F20 | White British | 23 | 3 | 3 | 110.8 | 108.2 | 107.4 | –2.6 | –0.8 | –3.4 | Booster 1 |
| S04/Q09 | M | 48 | F20 | White British | 47 | 3 | 1 | 70.1 | 71.9 | 68.7 | 1.8 | –3.2 | –1.4 | Foundation |
| S09/Q04 | M | 43 | F20 | White British | 25 | 4 | 2 | 125.1 | 110.0 | 123.7 | –15.1 | 13.7 | –1.4 | Foundation |
| S09/Q02 | F | 49 | F25 | White British | 21 | 4 | 3 | 105.5 | 104.4 | 104.2 | –1.1 | –0.2 | –1.3 | Foundation |
| S08/Q06 | F | 44 | F20 | White British | 28 | 2 | 2 | 92.0 | 93.6 | 90.8 | 1.6 | –2.8 | –1.2 | Foundation |
| S01/Q04 | M | 43 | F20 | White British | 33 | 3 | 3 | 96.7 | 97.0 | 96.6 | 0.3 | –0.4 | –0.1 | Booster 1 |
| Weight gain that is not clinically important | ||||||||||||||
| S05/Q03 | F | 54 | F25 | White British | 53 | 4 | 3 | 92.4 | 90.0 | 92.9 | –2.4 | 2.9 | 0.5 | Foundation |
| S06/Q02 | M | 34 | FEP | White British | 32 | 3 | 2 | 121.8 | 126.0 | 123.9 | 4.2 | –2.1 | 2.1 | Booster 1 |
| S03/Q01 | F | 36 | F20 | Bangladeshi | 24 | 4 | 2 | 101.5 | 103.3 | 103.7 | 1.8 | 0.4 | 2.2 | Foundation |
| S04/Q10 | M | 19 | FEP | White British | 36 | 2 | 0 | 84.6 | 83.9 | 87.7 | –0.7 | 3.8 | 3.1 | Foundation |
| S08/Q09 | F | 31 | F25 | White British | 23 | 4 | 1 | 92.4 | 96.4 | 95.5 | 4.0 | –0.9 | 3.1 | Foundation |
| S09/Q01 | M | 54 | F20 | White British | 29 | 3 | 3 | 86.3 | 87.3 | 90.4 | 1.0 | 3.1 | 4.1 | Foundation |
| Clinically important weight gain | ||||||||||||||
| S04/Q12 | M | 30 | FEP | Indian | 26 | 4 | 3 | 120.0 | 124.3 | 125.5 | 4.3 | 1.2 | 5.5 | Foundation |
| S07/Q06 | M | 25 | FEP | White British | 34 | 3 | 2 | 131.6 | 140.0 | 142.9 | 8.4 | 2.9 | 11.3 | Foundation |
| S04/Q08 | F | 32 | FEP | White British | 44 | 4 | 3 | 126.2 | 139.8 | 155.9 | 13.6 | 16.1 | 29.7 | Booster 1 |
| Qualitative participants without weight data | ||||||||||||||
| S04/Q06 | F | 25 | FEP | White British | 34 | 4 | 0 | 86.0 | 94.0 | ND | 8.0 | ND | ND | Booster 1 |
| S06/Q06 | M | 21 | FEP | White other | 39 | 3 | 2 | 91.3 | 79.0 | ND | –12.3 | ND | ND | Booster 1 |
| ID | Professional category | Education | Worked in | Groups facilitated | Confidence in skills (0 = low; 5 = high) | |||
|---|---|---|---|---|---|---|---|---|
| Mental health | Physical health | |||||||
| Years | Months | Years | Months | |||||
| S01/F02 | Support worker | City and Guilds of London Institute | 29 | 0 | ND | ND | 1 | 4 |
| S01/F04 | Mental health nurse | PG degree | 8 | 0 | 0 | 0 | 0 | 3 |
| S02/F02 | Healthy living advisor | UG degree | ND | ND | ND | ND | 10 | 4 |
| S02/F03 | Physiotherapist | UG degree | 2 | 4 | 4 | 3 | 10 | 4 |
| S02/F06 | Dietitian | UG degree | 3 | 4 | 3 | 4 | 99 | 4 |
| S03/F02 | Occupational therapist | UG degree | 5 | 0 | 0 | 0 | 24 | 4 |
| S03/F04 | Mental health nurse | UG degree | 11 | 4 | ND | ND | ND | 5 |
| S03/F05 | Community development | UG degree | 8 | 10 | 3 | 11 | 15 | 4 |
| S04/F02 | Support worker | No data | 38 | 4 | 14 | ND | ND | 4 |
| S04/F03 | Clinical studies officer | PG diploma | 6 | 2 | 0 | 0 | 3 | 4 |
| S05/F02 | Mental health nurse | PG degree | 17 | 0 | 17 | 0 | 2 | 5 |
| S06/F01 | Research assistant | PG degree | 2 | 0 | ND | ND | 0 | 3 |
| S06/F04 | Mental health nurse | Diploma (college) | 24 | 11 | 0 | 0 | 1 | 4 |
| S06/F05 | Mental health nurse | PG diploma | 7 | 8 | ND | ND | 0 | 3 |
| S07/F05 | Mental health nurse | UG degree | 7 | 3 | 0 | 0 | 0 | 4 |
| S08/F03 | Mental health nurse | UG degree | 26 | 9 | ND | ND | 0 | 4 |
| S08/F05 | Occupational therapist | UG degree | 20 | 0 | ND | ND | ND | 4 |
| S09/F01 | Mental health nurse | Diploma (college) | 25 | 4 | ND | ND | 0 | 3 |
| S09/F03 | Occupational therapist | UG degree | 6 | 7 | ND | ND | 5 | 4 |
| S09/F04 | Pharmacy technician | BTEC degree in Pharmaceutical Science | 8 | 0 | ND | ND | 1 | 3 |
Data collection
Semistructured interview guides for participants (see Appendix 1) and professionals (see Appendix 2) were pilot-tested with the relevant project team members. No topic guide was used for the unstructured interviews with intervention designers, although sections from the behaviour change wheel,95 the logic model and the NIHBCC framework24 were used as prompts. Interviews were recorded on an encrypted digital recorder and field notes were made during and after the interview. The median (range) length of the interviews was 18:57 minutes (13:06–30:33 minutes) for participant interviews, 46:13 minutes (29:29–76:32 minutes) for facilitator interviews and 39:20 minutes (43:39–64:00 minutes) for intervention designer interviews. Data saturation was achieved in the participant, professional and intervention designer data sets. No repeat interviews were carried out and transcripts were not returned to participants for comment/correction.
Data analysis
Two coders (RG-W and DH) coded participant interviews systematically in NVivo version 11 (QSR International, Warrington, UK) to behaviour change theory, intervention functions, theoretical domains95 and dimensions of acceptability,88 as well as opportunistically to the NIHBCC framework79 (see Table 6) and logic model constructs (see Figure 5). Staff interviews were coded systematically to NPT constructs and opportunistically to the NIHBCC framework and logic model constructs. Developer interviews were coded systematically to intervention functions, logic model and NIHBCC framework constructs (see Table 6).
Fidelity assessment
Leicester Diabetes Centre staff monitored the fidelity of intervention delivery through direct observation of sessions, using two instruments. First, the STEPWISE Core Facilitator Behavioural Observation Sheet assesses the relative presence or absence of 35 behaviours in six domains: non-judgemental engagement of participants (five items); eliciting and responding to emotions/feelings (two items); facilitating reflective learning (eight items); behavioural change, planning and goal-setting (nine items); overall group management (nine items); and other behaviours (two items). Second, LDC staff objectively assessed participant–educator interaction during observation visits by means of the DOT (DESMOND Observation Tool). The coder sat at the back of the room, with a compact disc playing in a headphone, from which a beep sounded every 10 seconds, whereupon the coder recorded whether an educator or a participant was currently talking at that point. Silence, laughter or multiple conversations were classed as ‘miscellaneous’. In self-management programme research, a link has been proposed between less facilitator talk and a more effective participant receipt of the education process, defined as a less didactic/more facilitative approach. 96 The national and local infrastructure available for quality control and mentoring in education programmes such as DESMOND was not available for STEPWISE, so feedback of observations in pursuit of accreditation was conducted. Other steps to ensure intervention fidelity are described in Table 6.
| Goal | STEPWISE fidelity strategies |
|---|---|
| Design | |
| Ensure the same treatment dose within conditions |
|
| Ensure an equivalent dose across conditions | Not applicable (no active control) |
| Plan for implementation setbacks |
|
| Training | |
| Standardise training |
|
| Ensure provider skill acquisition |
|
| Minimise ‘drift’ in provider skills | Not applicable (no benchmark established) |
| Accommodate provider differences |
|
| Delivery | |
| Control for provider differences |
|
| Reduce differences within treatment |
|
| Ensure adherence to treatment protocol |
|
| Minimise contamination between conditions |
|
| Receipt | |
| Ensure participant comprehension |
|
| Ensure participant ability to use cognitive skills |
|
| Ensure participant ability to perform behavioural skills |
|
| Enactment | |
| Ensure participant use of cognitive skills |
|
| Ensure participant use of behavioural skills |
|
Triangulation protocol
Different methods and informants were used and a formal framework was employed for comparison of the findings to comprehensively address different questions, increase confidence in findings and provide a basis for feedback from participants and professionals on the project team. 97 No priority was granted to either quantitative or qualitative methods, which were used concurrently to assess the intervention. A modified triangulation protocol62 was employed for the methodological triangulation of data sets, in five stages:
-
Data sets were reviewed to compare for presence and examples (‘sorting’) of logic model constructs.
-
The level of convergence between data types was coded for each of the 22 logic model components as ‘agreement’ (full interpretive agreement between data sets); partial agreement (some disagreement within/between data sets); silence (a logic mode component covered by only one data set); and dissonance [disagreement between data sets (‘convergence coding’)].
-
The global level of convergence was characterised (‘convergence assessment’).
-
Differences in the data set contribution to the case study were summarised (‘completeness comparison’).
-
The triangulated results were shared and points of disagreement were discussed with stakeholders at a face-to-face meeting on 14 July 2017 (‘feedback’), with changes in interpretation being incorporated when supported by the data. A formal comparison of researcher coding was not conducted, owing to time constraints and a rapidly evolving analysis strategy.
Health economic methods
Introduction
The economic evaluation of the STEPWISE trial was conducted using both health and social care and societal perspectives. Individual participant data were used in the analysis to generate results for the cost-effectiveness of the intervention regarding its impact on cost-relative quality-adjusted life-years (QALYs), derived from the EQ-5D-5L and the SF-36 questionnaires, and weight change. The time horizon of the trial (3- and 12-month follow-up) was the same in the economic evaluation. The economic analyses included all trial participants. The comparison was between the group receiving the STEPWISE intervention and the group receiving a leaflet with advice. This analysis determined cost-effectiveness using the £20,000-per-QALY-gained threshold set by NICE. A sensitivity analysis was used to establish the effect of changes in costs and QoL measurement (comparing results using utility values from the EQ-5D and the SF-36)51,98 on the results. Value sets for health-related QoL measures related to value sets containing preferences from a population in England for the EQ-5D-5L51 and in the UK for the SF-36. 98
Health and social care costs
Health and social care resources were calculated using the unit costs from the Personal Social Services Research Unit (PSSRU)99 and NHS reference costs,100 with service use data. Responses to the Client Service Receipt Inventory (CSRI)101 questionnaire, which captured health and social care as well as other resource use, were collected for the 3 months before the baseline assessment and the 3-month follow-up and the 9 months before the 12-month follow-up. The CSRI includes information on hospital care (inpatient and outpatient), social care, primary care and criminal justice service contacts. Less frequently used health-care professional contacts were reported in the ‘other health-care professional’ category, including drug advisors, outreach workers, early intervention services, occupational therapists, drug service facilities and communities, using the CSRI. Medication prices from the British National Formulary102 in March 2017 were applied to the data on medication use by the participants in the study. All drugs for physical and mental health conditions, including those that could be bought over the counter, were included. Ongoing medications recorded on the CRF were used as a source of medication data.
Intervention costs
The intervention comprised four foundation sessions (2.5 hours each), three booster sessions (at 4, 7 and 10 months, of 2.5 hours each) and support contacts (telephone calls, texts and postcards) every 2 weeks. The base case was conducted to represent the scenario in which only four patients attended each session. This was to reflect lower attendance than the seven full sessions for the intervention. The cost of the accelerometer was omitted.
The foundation and booster sessions of the intervention were costed as group therapy sessions, using the mental health nurse wage level and related oncosts, combined with capital costs and other costs of the service at 2015–16 prices. The intervention unit costs, using the wages of a mental health nurse and a dietitian, were costed for a 2.5-hour session of the intervention. For most analyses, the results are reported for two mental health nurses facilitating a group session with six participants. The unit costs per person, calculated using the costs of a mental health nurse, were £88 for a group of four, £59 for a group of six and £44 for a group of eight (the costs for groups of six and eight were used in the sensitivity analyses). Support contacts by telephone were costed at the price of one nurse or dietitian per minute, multiplied by the length of the call. Text messages and postcards were costed at a nominal value of £5. The costs of the intervention were split between time periods, as the intervention booster sessions and support contacts continued after the 3-month period.
Societal costs
Average wage rates were applied to productivity losses and informal care time, which were obtained using data from the Office for National Statistics. 103 In the sensitivity analyses, we used minimum wage rates,104 which were also applied to productivity losses and informal care, and the unit costs for a home-care worker for informal care using the figure from the PSSRU. 99
Police officer contact time was calculated from police salary scales105 and workforce statistics. 106 A cost of police contacts was included, but no other criminal justice system costs were included. The cost of a lost education day was calculated as the cost of a working day; as it was assumed that as the participants were all adults, this was more appropriate. The CSRI also recorded time lost from work, education and informal care from friends and family.
No discount rate was applied to the costs and QoL measures, as the follow-up period for participants was 1 year.
Health-related quality of life
Health-related QoL was measured using the EQ-5D-5L107 and the SF-36/Short Form questionnaire-6 Dimensions (SF-6D). 98 The EQ-5D is the measure more commonly used in health technology assessments in England and is recommended by NICE. However, the EQ-5D is known to have a ceiling effect in schizophrenia, which is why the SF-6D was also used, as this measure is more likely to be normally distributed. The SF-36 questionnaire responses were converted to the SF-6D QoL measure for the analysis, using domain weightings from Brazier and Roberts. 108 QALYs were calculated from the tariff scores using the area under the curve method for both the EQ-5D-5L and the SF-6D QoL measures. It was assumed that there was a linear change between any two time points.
Statistical analyses
The net benefit approach was used in a cost–utility analysis of costs and both types of QALY data, and in a cost-effectiveness analysis with health/social care and societal costs. The net benefit is derived from the multiplication of a threshold value for a unit of outcome minus the cost.
Service use and cost differences between the two groups were described. The analysis of costs and outcomes was carried out in four combinations: (1) EQ-5D-5L QALY and health and social care costs; (2) EQ-5D-5L QALY and societal costs; (3) SF-6D QALY and health and social care costs; and (4) SF-6D QALY and societal costs.
Incremental cost-effectiveness ratios (ICERs) were computed by dividing incremental costs by incremental outcomes. Cost-effectiveness planes were constructed by obtaining, through bootstrapped regression models, 1000 pairs of cost and outcome differences and showing these on scatterplots. The proportion of replications in each quadrant could then be obtained.
Cost-effectiveness acceptability curves
Cost-effectiveness acceptability curves (CEACs) show the probability that one group is more cost-effective than the other for a chosen value of willingness to pay per unit of outcome. The STEPWISE economic evaluation uses the net benefit approach109 with the 12-month follow-up results for these CEACs. The willingness-to-pay value that is used is the £20,000 NICE threshold for total health and social care costs. CEACs are also produced for societal costs at the 12-month follow-up; however, the relevant analysis for NICE methodology is from the health and social care perspective. The value of the willingness-to-pay threshold was also varied in values of £10,000 between £0 and £200,000, to show how the probability of cost-effectiveness changes as the threshold rises.
Missing data
The multiple imputation (chained equations) method110 was applied to impute missing values for cost and QoL data, assuming that data were MAR before bootstrapping to carry out the cost-effectiveness analysis.
Sensitivity analysis
Three types of sensitivity analysis will be reported in the final analysis, with varying costs. First, costs of the intervention were used [mental health nurse (£1.18 per minute, £59 per session – two facilitators with a group of four patients)]. Second, informal care costs were calculated using two alternative unit costs: home care worker (£20 per weekday hour of a home care worker based on independent and social services home care) and minimum wage rate (£7.20 per hour in 2016). Third, productivity losses (lost employment time), valued using the average wage rate in the base case, are also calculated using the minimum wage rate104 (£7.20 per hour in 2016). Each of these sensitivity analyses were carried out using values from both the EQ-5D and the SF-6D. No adjustments were made to take into account deaths during the study, as the numbers were so low.
Chapter 4 Results of the trial
Recruitment and participant flow
The first participant consented to the trial on 10 March 2015 and was randomised on 14 March 2015. The trial closed as scheduled on 31 March 2017, when the last 12-month follow-up was completed (Figure 6).
FIGURE 6.
Randomisation by month. IDS, Intervention Development Study.
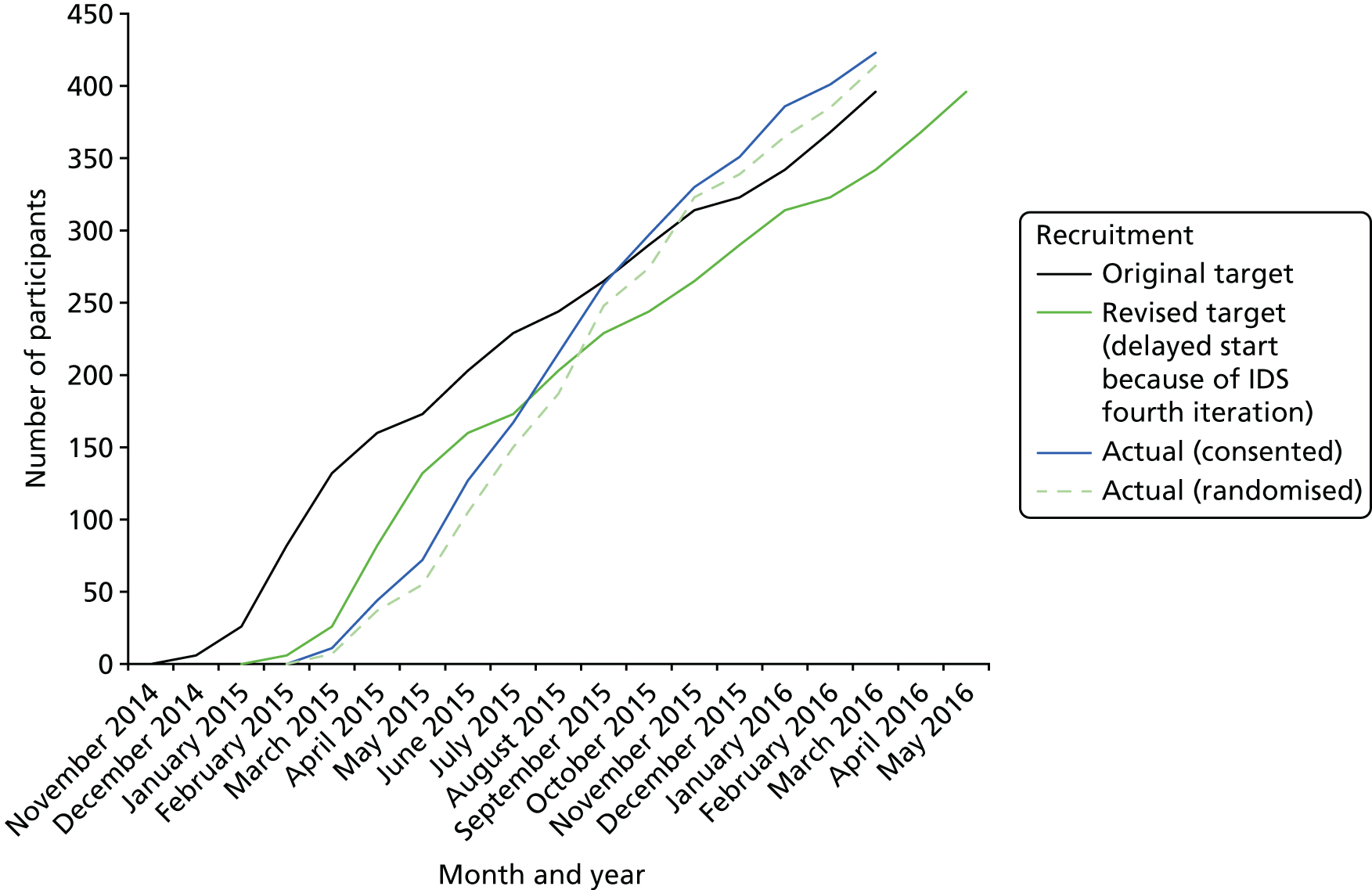
A total of 414 participants were randomised, of whom two were not included in any analyses: one was erroneously randomised, having previously withdrawn consent to randomisation, and a second withdrew all consent to use their data. Therefore, 412 participants (207 intervention, 205 control) were included in the following summaries.
Figure 7 shows that completion was slightly higher in the control group. There were three deaths, all in the intervention arm, owing to pulmonary embolism (n = 1), myocardial infarction (n = 1) and diabetic ketoacidosis in a person with type 2 diabetes mellitus previously treated with oral antidiabetes agents, leading to cardiac arrest (n = 1).
FIGURE 7.
Consolidated Standards of Reporting Trials flow diagram. a, Referred but not contacted before end of recruitment (n = 27), current inpatient (n = 18), work (n = 9), Intervention Development Study participant (n = 9), discharged from CMHT (n = 8), too busy/away a lot (n = 7), not able to travel/out of area (n = 5), did not attend consent/baseline visit (n = 5), mental health problem relating to weight (n = 5), unknown (n = 4), other (specified reasons) (n = 31). b, Receipt of intervention defined as attending at least one foundation. Reproduced from Holt et al. 23 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
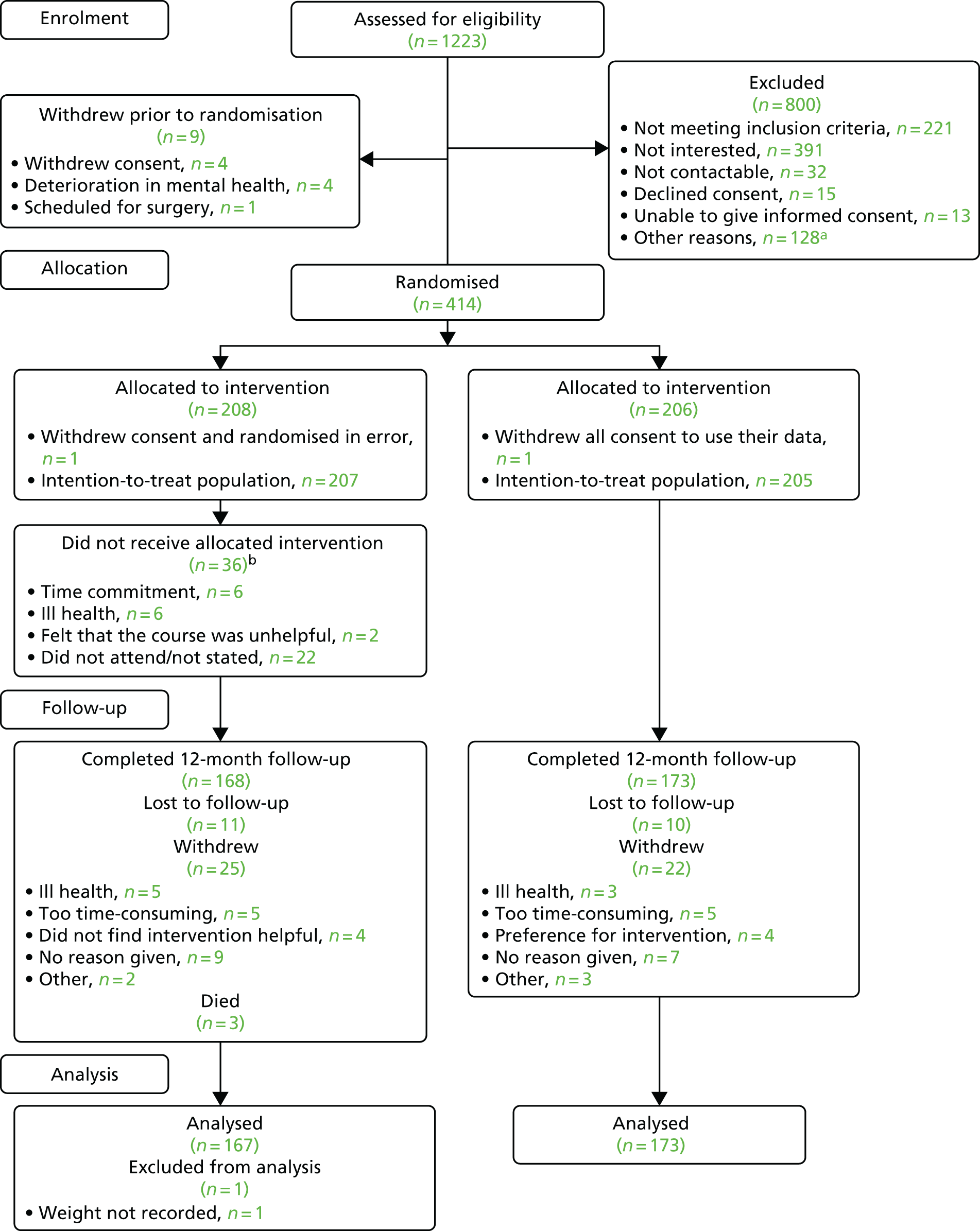
Intervention arm participants were categorised as non-attenders, attending one or two foundation (‘core’) sessions, attending three or four core sessions without any boosters or attending three or four core sessions with at least one booster. A total of 47 participants (22.7%) attended all core and booster sessions (Table 7). The mean number of sessions attended was 2.7 foundation sessions and 1.4 booster sessions (Table 8). The mean intervention group size as randomised was 6.3, but the mean number of participants who actually attended ranged from 4.0 to 4.4 during the foundation course and dropped to 2.7 to 3.0 during the booster sessions (see Table 9).
| Attendance | n (%) |
|---|---|
| Non-attender | 36 (17.4) |
| One or two core sessions | 34 (16.4) |
| ≥ 3 core and 0 booster sessions | 26 (12.6) |
| ≥ 3 core and ≥ 1 booster sessions | 111 (53.6) |
| Attended all courses | 47 (22.7) |
| Attendance and group size | Group size | |||
|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | |
| Group size at randomisation | 6.3 | 6 | 3 | 11 |
| Foundation coursesa | ||||
| Week 1 | 4.4 | 4 | 2 | 9 |
| Week 2 | 4.2 | 4 | 1 | 8 |
| Week 3 | 4.2 | 4 | 2 | 9 |
| Week 4 | 4.0 | 4 | 1 | 9 |
| Booster sessionsa | ||||
| Month 4 | 3.0 | 3 | 0 | 8 |
| Month 7 | 2.8 | 3 | 0 | 7 |
| Month 10 | 2.7 | 3 | 0 | 6 |
Overall, there were 3218 support contacts made with participants outside the group sessions, of which three-quarters were made by telephone. A total of 169 participants (81.6%) had one or more support contact; 167 participants (81%) had at least one telephone contact, among whom the average number of received telephone calls was 15, and 105 (50.7%) had at least one mail contact, with the average number being five. Face-to-face contact was less common, but 68 participants (32.9%) attended at least one session, with the average number of contacts being three and the average duration of these being just under 1 hour.
Table 9 compares support contact (i.e. contact other than group sessions) at the recruiting sites. Manchester made the most contacts overall, with the vast majority being by telephone (93.8%), with only one contact being face to face. Cornwall made the smallest percentage of contacts by telephone (41.1%), but had the largest percentage of contacts by mail and electronically. Leeds and York and Somerset had the largest percentages of face-to-face contacts – 11.5% and 12.6%, respectively, almost three times the sites’ average of 4.4%.
| Site | Contact type, n (%) | All contacts | |||
|---|---|---|---|---|---|
| Electronic | Face to face | Telephone | |||
| Overall | 88 (2.7) | 141 (4.4) | 555 (17.3) | 2434 (75.6) | 3218 |
| Bradford | 5 (2.5) | 7 (3.5) | 2 (1.0) | 189 (93.1) | 203 |
| Cornwall | 63 (15.2) | 22 (5.3) | 159 (38.4) | 170 (41.1) | 414 |
| Devon | 11 (4.2) | 10 (3.8) | 49 (18.6) | 193 (73.4) | 263 |
| Leeds and York | 0 | 30 (11.5) | 61 (23.3) | 171 (65.3) | 262 |
| Manchester | 0 | 1 (0.2) | 38 (6.1) | 588 (93.8) | 627 |
| Sheffield | 0 | 1 (0.4) | 43 (18.5) | 189 (81.1) | 233 |
| Somerset | 0 | 17 (12.6) | 34 (25.2) | 84 (62.2) | 135 |
| South London | 3 (1.4) | 20 (9.1) | 0 | 196 (89.5) | 219 |
| Southern Health | 5 (1.1) | 14 (3.0) | 126 (26.9) | 324 (69.1) | 469 |
| Sussex | 1 (0.3) | 19 (4.8) | 43 (10.9) | 330 (84.0) | 393 |
| Mean contact time (minutes) | 1.8 | 59.4 | 0.4 | 5.2 | 6.3 |
Baseline data
The characteristics of the randomised participants are given in Tables 10 and 11. The groups were largely well balanced, but the intervention arm participants were, on average, 3 kg heavier at baseline, which is partially explained by the higher proportion of men in the intervention arm (55.6% vs. 46.3%; the average weight among men was 109 kg vs. 98 kg among women). The prevalence of type 2 diabetes mellitus was higher in the intervention arm (16.4% vs. 11.7%), whereas the control group included a larger proportion of participants with current depression (30.2% vs. 26.1%) and previous depression (28.3% vs. 24.2%).
| Characteristic | Trial arm | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| Site, n (%) | ||
| Bradford | 17 (8.2) | 19 (9.3) |
| Cornwall | 21 (10.1) | 21 (10.2) |
| Devon | 21 (10.1) | 21 (10.2) |
| Leeds and York | 22 (10.6) | 20 (9.8) |
| Manchester | 31 (15.0) | 29 (14.1) |
| Sheffield | 18 (8.7) | 17 (8.3) |
| Somerset | 12 (5.8) | 14 (6.8) |
| South London | 16 (7.7) | 14 (6.8) |
| Southern Health | 27 (13.0) | 27 (13.2) |
| Sussex | 22 (10.6) | 23 (11.2) |
| Age (years) | ||
| Number of participants | 207 | 205 |
| Mean (SD) | 40.0 (11.3) | 40.1 (11.5) |
| Sex, n (%) | ||
| Male | 115 (55.6) | 95 (46.3) |
| Female | 92 (44.4) | 110 (53.7) |
| Ethnicity, n (%) | ||
| White | 179 (86.5) | 170 (82.9) |
| Asian | 9 (4.3) | 7 (3.4) |
| Black | 12 (5.8) | 19 (9.3) |
| Mixed | 4 (1.9) | 7 (3.4) |
| Other | 3 (1.4) | 2 (1.0) |
| Smoking status, n (%) | ||
| Ex-smoker | 55 (26.6) | 52 (25.4) |
| Never smoked | 54 (26.1) | 45 (22.0) |
| Current smoker | 98 (47.3) | 108 (52.7) |
| Light smoker | 18 (8.7) | 20 (9.8) |
| Moderate smoker | 37 (17.9) | 32 (15.6) |
| Heavy smoker | 43 (20.8) | 55 (26.8) |
| Missing | 0 | 1 (0.5) |
| Weight (kg) | ||
| n | 206 | 204 |
| Mean (SD) | 105.2 (22.2) | 102.1 (22.1) |
| Median (IQR) | 104.2 (89.0–116.2) | 99.3 (88.0–113.0) |
| BMI (kg/m2) | ||
| n | 206 | 204 |
| Mean (SD) | 36.1 (7.2) | 35.3 (7.2) |
| Median (IQR) | 35.1 (31.0–39.5) | 34.2 (29.9–39.1) |
| Waist circumference (cm) | ||
| n | 205 | 204 |
| Mean (SD) | 117.8 (15.6) | 116.1 (17.4) |
| Median (IQR) | 118.0 (106.0–128.2) | 116.8 (104.5–125.5) |
| Systolic blood pressure (mmHg) | ||
| n | 205 | 203 |
| Mean (SD) | 126.4 (15.9) | 124.0 (16.8) |
| Median (IQR) | 126.0 (116.0–135.0) | 123.0 (112.0–134.0) |
| Diastolic blood pressure (mmHg) | ||
| n | 205 | 203 |
| Mean (SD) | 82.5 (11.0) | 81.9 (12.5) |
| Median (IQR) | 82.0 (74.0–89.0) | 81.0 (74.0–90.0) |
| Pulse (b.p.m.) | ||
| n | 204 | 203 |
| Mean (SD) | 84.5 (13.9) | 83.8 (13.6) |
| Median (IQR) | 84.5 (75.0–95.0) | 84.0 (75.0–93.0) |
| HbA1c (mmol/mol) | ||
| n | 171 | 170 |
| Mean (SD) | 41.5 (13.3) | 39.9 (10.9) |
| Median (IQR) | 37.0 (35.0–42.0) | 38.0 (35.0–41.0) |
| HbA1c (%) | ||
| Mean (SD) | 5.9 (1.2) | 5.8 (1.0) |
| Median (IQR) | 5.5 (5.4–6.0) | 5.6 (5.4–5.9) |
| HbA1c category | ||
| < 6% (42 mmol/mol) | 133 (64.3%) | 139 (67.8%) |
| 6–6.5% (42–47 mmol/mol) | 10 (4.8%) | 17 (8.3%) |
| 6.5–7.5% (48–57 mmol/mol) | 9 (4.3%) | 7 (3.4%) |
| 7.5–8.5% (58–68 mmol/mol) | 9 (4.3%) | 2 (1.0%) |
| ≥ 8.5% (69 mmol/mol) | 10 (4.8%) | 6 (2.9%) |
| Missing | 36 (17.4%) | 34 (16.6%) |
| Total cholesterol (mmol/l) | ||
| n | 175 | 179 |
| Mean (SD) | 5.0 (1.2) | 5.1 (1.2) |
| Median (IQR) | 4.9 (4.2–5.8) | 4.9 (4.2–5.7) |
| HDL cholesterol (mmol/l) | ||
| n | 170 | 173 |
| Mean (SD) | 1.2 (0.5) | 1.2 (0.4) |
| Median (IQR) | 1.2 (1.0–1.4) | 1.2 (1.0–1.3) |
| Non-HDL cholesterol (mmol/l) | ||
| n | 169 | 174 |
| Mean (SD) | 3.8 (1.3) | 3.9 (1.4) |
| Median (IQR) | 3.8 (2.9–4.6) | 3.8 (2.9–4.6) |
| Triglycerides (mmol/l) | ||
| n | 172 | 172 |
| Mean (SD) | 2.5 (2.0) | 2.2 (1.7) |
| Median (IQR) | 1.9 (1.4–2.8) | 1.7 (1.2–2.8) |
| Fasting glucose (mmol/l) | ||
| n | 170 | 172 |
| Mean (SD) | 5.9 (2.2) | 5.8 (2.3) |
| Median (IQR) | 5.2 (4.8–6.1) | 5.3 (4.7–6.0) |
| eGFR (ml/minute/1.73 m2), n (%) | ||
| ≤ 60 | 2 (1.0) | 3 (1.5) |
| 60–90 | 58 (28.0) | 55 (26.8) |
| > 90 | 15 (7.2) | 9 (4.4) |
| Missing | 132 (63.8) | 138 (67.3) |
| Evidence of hepatic disease?, n (%) | ||
| No | 167 (80.7) | 167 (81.5) |
| Yes | 5 (2.4) | 7 (3.4) |
| Missing | 35 (16.9) | 31 (15.1) |
| Diagnosis | Trial arm | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| Schizophrenia diagnosis type, n (%) | ||
| ICD-10: F20 | 145 (70.0) | 138 (67.3) |
| ICD-10: F25 | 30 (14.5) | 36 (17.6) |
| First episode psychosis | 32 (15.5) | 31 (15.1) |
| OPCRIT+ diagnosis, n (%) | ||
| Schizophrenia [F20] | 37 (17.9) | 37 (18.0) |
| Schizoaffective disorder [F25.X] | 8 (3.9) | 7 (3.4) |
| Other non-organic psychosis [F28.X] | 72 (34.8) | 69 (33.7) |
| Affective disorder [F3X.X, F30X, F31, F32] | 11 (5.3) | 14 (6.8) |
| Other | 60 (29.0) | 62 (30.2) |
| Not met | 12 (5.8) | 12 (5.9) |
| Missing | 7 (3.4) | 4 (2.0) |
| Diabetes mellitus status, n (%) | ||
| Type 1 | 1 (0.5) | 1 (0.5) |
| Type 2 | 34 (16.4) | 24 (11.7) |
| Diagnosed chronic kidney disease, n (%) | ||
| Current | 0 (0.0) | 1 (0.5) |
| Atrial fibrillation, n (%) | ||
| Current | 0 (0.0) | 0 (0.0) |
| Past | 2 (1.0) | 1 (0.5) |
| Hypertension, n (%) | ||
| Current | 21 (10.1) | 17 (8.3) |
| Rheumatoid arthritis, n (%) | ||
| Current | 9 (4.3) | 5 (2.4) |
| Heart attack, n (%) | ||
| Past | 0 (0.0) | 3 (1.5) |
| Angina, n (%) | ||
| Ongoing | 1 (0.5) | 5 (2.4) |
| Past | 2 (1.0) | 1 (0.5) |
| Stroke or TIA, n (%) | ||
| Past | 4 (1.9) | 3 (1.5) |
| Peripheral vascular disease, n (%) | ||
| Ongoing | 0 (0.0) | 1 (0.5) |
| Past | 0 (0.0) | 0 (0.0) |
| Depression, n (%) | ||
| Current | 54 (26.1) | 62 (30.2) |
| Past | 50 (24.2) | 58 (28.3) |
| Any other relevant medical conditions, n (%) | ||
| Yes | 72 (34.8) | 70 (34.1) |
| Has a first-degree relative who has had angina or a heart attack before the age of 60 years, n (%) | ||
| No | 170 (82.1) | 158 (77.1) |
| Yes | 36 (17.4) | 44 (21.5) |
| Unknown | 1 (0.5) | 2 (1.0) |
| Has a first-degree relative with diabetes mellitus, n (%) | ||
| No | 158 (76.3) | 146 (71.2) |
| Yes | 47 (22.7) | 56 (27.3) |
| Unknown | 2 (1.0) | 2 (1.0) |
| Has a history of heavy drinking or an alcohol problem,a n (%) | ||
| No | 157 (75.8) | 153 (74.6) |
| Yes | 50 (24.2) | 52 (25.4) |
| Time since first contact with psychiatric services (years) | ||
| Median (IQR) | 14 (6–22) | 15 (6–22) |
| Time since first contact with psychiatric services (years), n (%) | ||
| < 1 | 7 (3.4) | 9 (4.4) |
| 1–2 | 8 (3.9) | 16 (7.8) |
| 2–5 | 29 (14.0) | 20 (9.8) |
| 5–10 | 27 (13.0) | 27 (13.2) |
| 10–20 | 66 (31.9) | 65 (31.7) |
| ≥ 20 | 70 (33.8) | 68 (33.2) |
| Years since started antipsychotics | ||
| Median (IQR) | 13 (5–20) | 12 (5–20) |
| Time since started antipsychotic medication (years), n (%) | ||
| < 1 | 12 (5.8) | 12 (5.9) |
| 1–2 | 11 (5.3) | 20 (9.8) |
| 2–5 | 28 (13.5) | 19 (9.3) |
| 5–10 | 28 (13.5) | 33 (16.1) |
| 10–20 | 71 (34.3) | 69 (33.7) |
| ≥ 20 | 57 (27.5) | 52 (25.4) |
| Time since first symptoms of psychosis (years) | ||
| Median (IQR) | 15 (7–22) | 15 (6–23) |
| Time since first symptoms of psychosis (years), n (%) | ||
| < 1 | 8 (3.9) | 9 (4.4) |
| 1–2 | 5 (2.4) | 11 (5.4) |
| 2–5 | 26 (12.6) | 20 (9.8) |
| 5–10 | 27 (13.0) | 26 (12.7) |
| 10–20 | 70 (33.8) | 70 (34.1) |
| ≥ 20 | 66 (31.9) | 69 (33.7) |
| Not recorded | 5 (2.4) | 0 (0.0) |
Medication use on entry is given in Table 12. All bar one participant were recorded as being currently prescribed antipsychotic medication and over half reported use of antidepressant medication, all of which factors were reasonably well balanced between the groups.
| Psychiatric medication | Participants in each trial arm, n (%) | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| Antipsychotic medication at baseline | ||
| Any antipsychotic | 207 (100.0) | 204 (99.5)a |
| Haloperidol | 7 (3.4) | 3 (1.5) |
| Amisulpride | 21 (10.1) | 16 (7.8) |
| Aripiprazole | 40 (19.3) | 34 (16.6) |
| Clozapine | 89 (43.0) | 81 (39.5) |
| Olanzapine | 31 (15.0) | 31 (15.1) |
| Quetiapine | 28 (13.5) | 24 (11.7) |
| Risperidone | 10 (4.8) | 21 (10.2) |
| Flupentixol | 8 (3.9) | 11 (5.4) |
| Zuclopenthixol | 10 (4.8) | 21 (10.2) |
| Paliperidone | 7 (3.4) | 8 (3.9) |
| Other antipsychotic | 19 (9.2) | 9 (4.4) |
| Antidepressant medication at baseline | ||
| Any antidepressant | 131 (63.3) | 129 (62.9) |
| Citalopram | 19 (9.2) | 13 (6.3) |
| Lithium | 8 (3.9) | 9 (4.4) |
| Mirtazepine | 9 (4.3) | 10 (4.9) |
| Other antidepressant | 107 (51.7) | 115 (56.1) |
Course delivery and protocol non-compliance
Although the courses were designed to be delivered by two trained facilitators, 16 of the 231 courses (eight foundation and eight booster) were led by a single facilitator owing to staff unavailability. At one centre, the wave 2 7-month booster session (with five participants allocated to the group) did not take place owing to lack of facilitator availability. At another centre, two booster sessions were delivered by the external (fully trained NHS employee) fidelity assessor who delivered the session on the day because neither facilitator was available owing to sickness. In addition, the support contacts were not always at the intended intervals of approximately every 2 weeks; 8% of contacts occurred within 1 week of the previous contact and 10% occurred more than 3 weeks after the previous contact.
Of the 54 individuals who facilitated at least one course, 47 completed a short survey describing their qualifications and experience before delivering the intervention. Twenty-two were mental health nurses (47%), six were support workers (13%), five were occupational therapists (11%) and the remainder were from other disciplines. Thirty-three (70%) were trained to graduate or postgraduate level; with the remainder mostly having vocational qualifications (n = 11, 23%). Facilitators had spent, on average, 13 years working in mental health (range 2–40 years); 13 facilitators reported a career of between 1 and 17 years (mean 6 years) working in physical health. Facilitators were asked to rate their confidence in group facilitation skills on a 5-point scale; the majority rated their abilities as ‘3’ (n = 16, 34%) or ‘4’ (n = 24, 51%), with six rating their confidence as ‘5’ (very confident) and one scoring themselves as ‘2’.
Of the 230 foundation and booster courses delivered, 162 (71%) included a mental health nurse as a facilitator, 80 (35%) involved a support worker and 31 (13%) involved an occupational therapist. Eight of the 10 sites utilised facilitators with substantial experience of mental health (> 10 years’ experience). By contrast, only two sites utilised facilitators with experience of physical health; the average experience (summed across all courses) was 10 years in Manchester, 7 years in London and < 2 years elsewhere.
Outcomes and estimation
Weight change (primary end point)
Among participants completing follow-up, the weight change was almost identical for the intervention and control arms. At 3 months, there was a reduction of 0.2 kg in the intervention arm and an increase of 0.4 kg in the control arm (difference = –0.55 kg, 95% CI –1.44 to 0.35 kg; p = 0.230). At 12 months (the timing of the primary comparison), the mean reduction in weight was 0.47 kg in the intervention group and 0.51 kg in the control group (difference = 0.04 kg, 95% CI –1.59 to 1.67 kg; p = 0.964). The distributions are shown in Figure 8 and further summaries are presented in Table 13. There was considerable variation in the weight change in both groups, ranging from –24 kg to +30 kg in the intervention arm and from –25 kg to +30 kg in the control arm.
FIGURE 8.
Weight change. (a) 3 months and (b) 12 months. Reproduced from Holt et al. 23 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
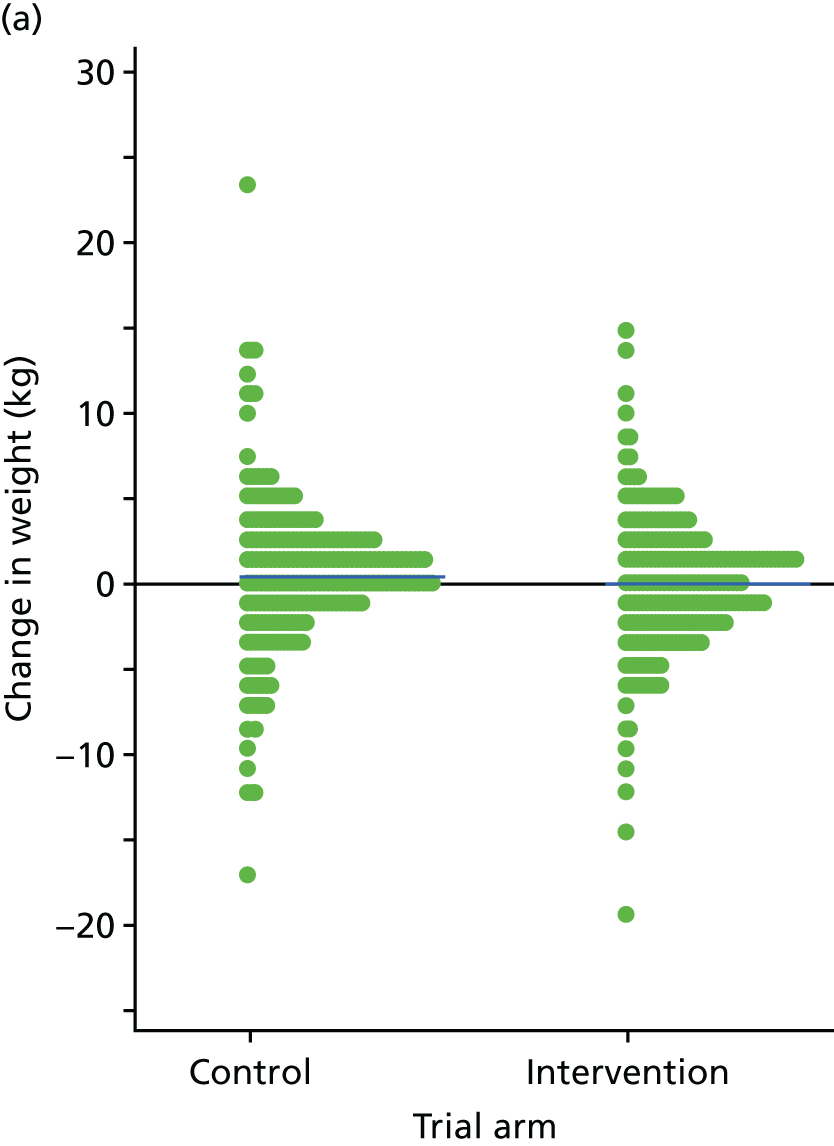
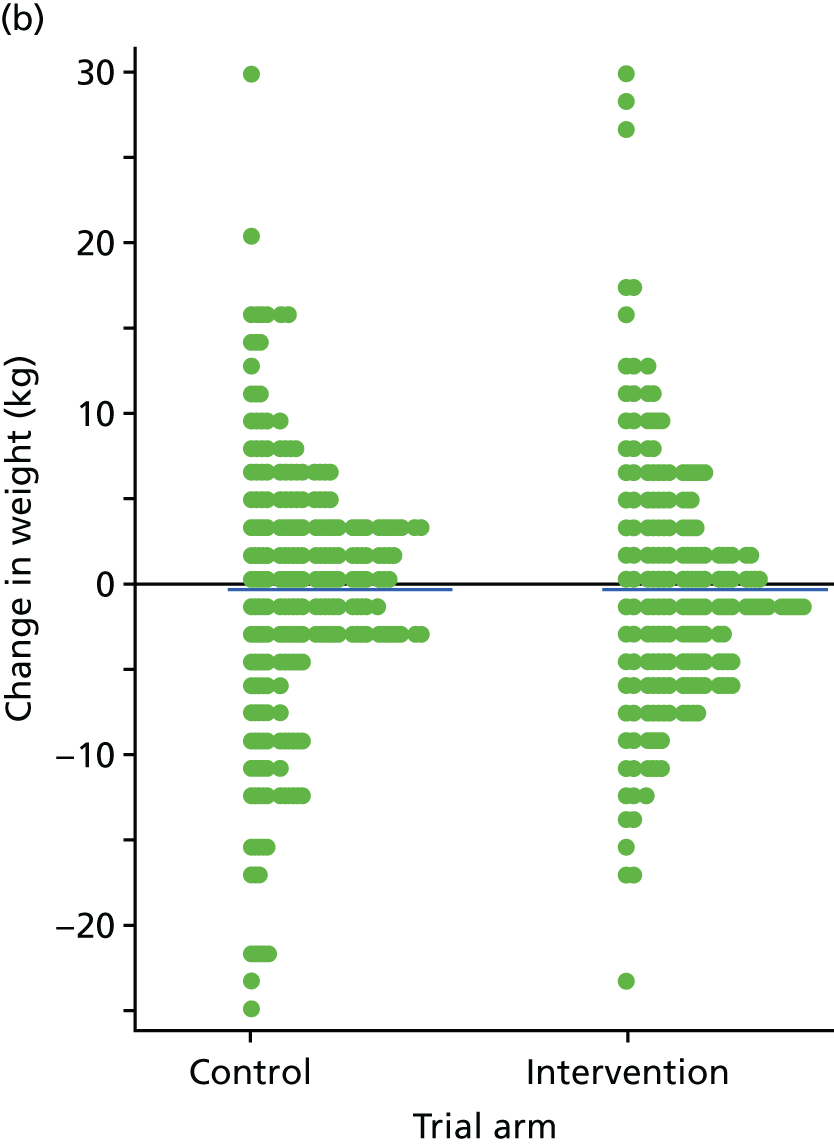
Figure 9 shows the mean and 95% CI for the adjusted mean weight change by time point and group. The means are adjusted for the primary covariates (recruiting site, years since starting antipsychotics, baseline weight and course identifier). The mean weight loss was similar between arms and was some distance from the 4.5 kg defined as the minimally clinically important difference in the sample size calculation.
FIGURE 9.
Mean and 95% CI for weight change.
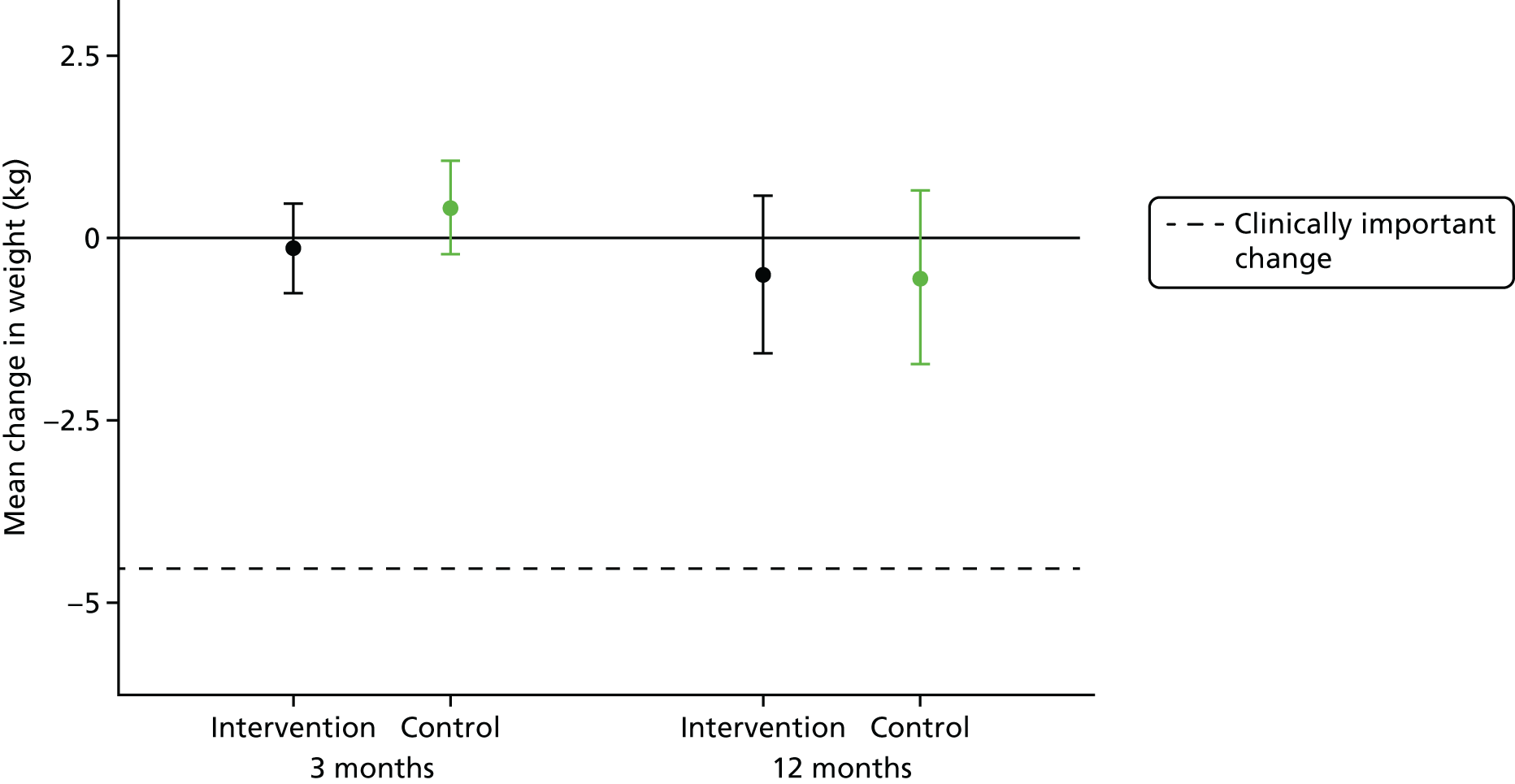
The weight change by recruiting centre is displayed in Figure 10. The difference between the intervention and control arms ranged from –2.6 kg to +7.0 kg. Adding an interaction term between site and treatment to the primary model gave a test statistic of χ2(9) = 14.9 (p = 0.094), indicating some evidence of a variation in the size of intervention effect between sites.
FIGURE 10.
Mean weight change by recruiting site.
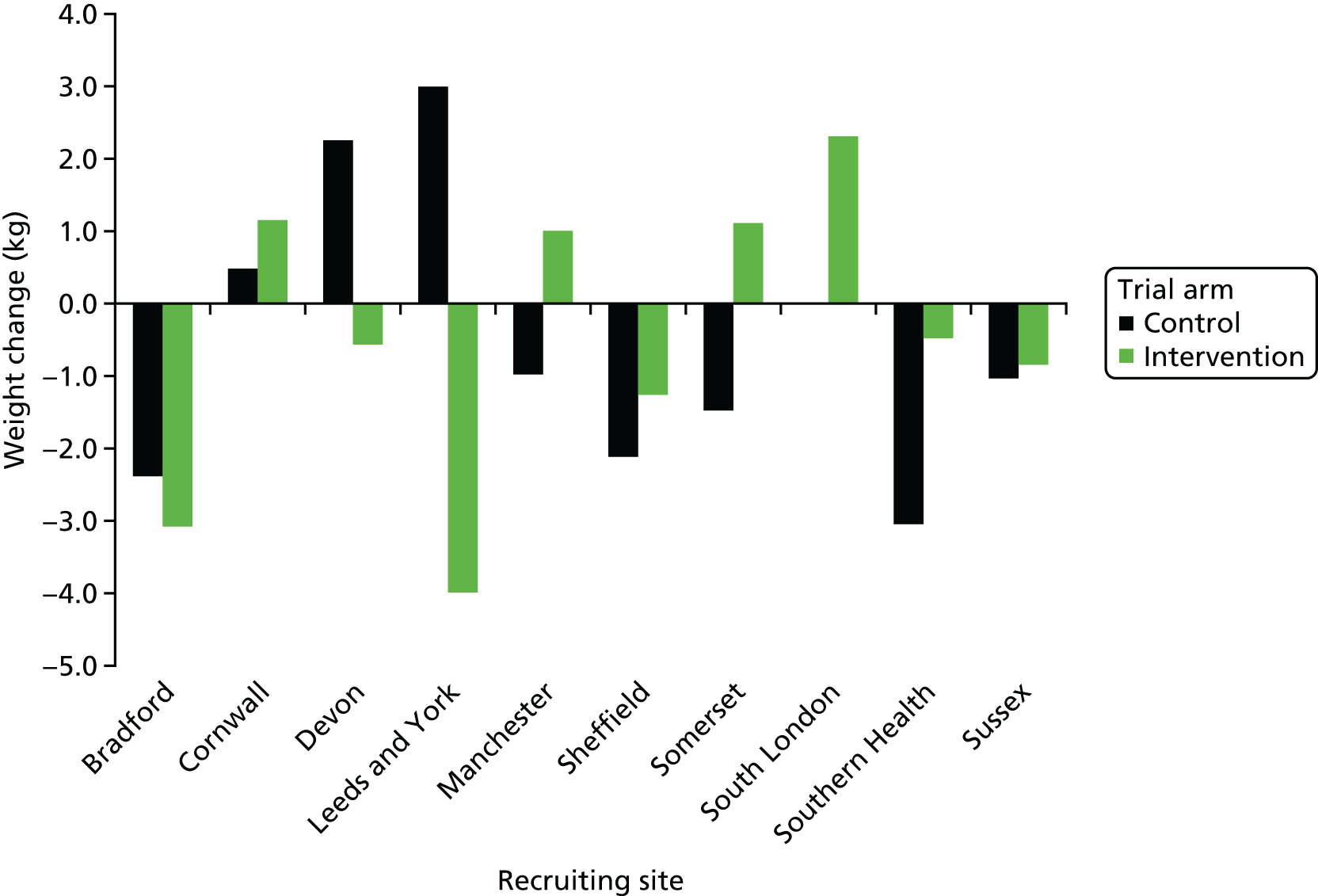
Other weight-related end points
The weight, BMI and waist circumference outcomes are also presented in Table 13. Although the proportion of participants reporting any weight reduction was slightly higher in the intervention arm, the magnitude of the difference was almost identical between the two trial arms. None of the comparisons reached statistical or clinical significance.
| Characteristic | Trial arm | Mean differencea (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Weight (kg) | ||||
| Baseline | ||||
| n | 206 | 204 | ||
| Mean (SD) | 105.2 (22.2) | 102.1 (22.1) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 104.7 (21.5) | 103.1 (23.5) | ||
| Change mean (SD) | –0.2 (4.4) | 0.4 (4.7) | –0.55 (–1.44 to 0.35) | 0.230 |
| 12 months | ||||
| n | 167 | 173 | ||
| Mean (SD) | 104.1 (21.1) | 101.3 (23.7) | ||
| Change mean (SD) | –0.5 (7.9) | –0.5 (8.3) | 0.04 (–1.58 to 1.66) | 0.963 |
| Change in weight (%) | ||||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | –0.0 (4.3) | 0.4 (4.4) | –0.4 (–1.3 to 0.5) | 0.350 |
| 12 months | ||||
| n | 167 | 173 | ||
| Mean (SD) | –0.4 (7.9) | –0.5 (8.2) | 0.0 (–1.6 to 1.7) | 0.964 |
| Maintained or lost weight | ||||
| 3 months | 93 (52.2%) | 80 (44.2%) | 1.35 (0.88 to 2.05)b | 0.169 |
| 12 months | 98 (58.3%) | 88 (50.9%) | 1.35 (0.85 to 2.14)b | 0.206 |
| BMI (kg/m2) | ||||
| Baseline | ||||
| n | 206 | 204 | ||
| Mean (SD) | 36.1 (7.2) | 35.3 (7.2) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 35.8 (7.1) | 35.5 (7.4) | –0.15 (–0.47 to 0.16) | 0.337 |
| 12 months | ||||
| n | 167 | 173 | ||
| Mean (SD) | 35.6 (7.2) | 34.8 (7.3) | 0.05 (–0.51 to 0.60) | 0.869 |
| Waist circumference (cm) | ||||
| Baseline | ||||
| n | 205 | 204 | ||
| Mean (SD) | 117.8 (15.6) | 116.1 (17.4) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 116.8 (15.2) | 115.4 (17.0) | 0.79 (–0.65 to 2.22) | 0.284 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 116.4 (16.1) | 114.0 (17.7) | 1.23 (–0.74 to 3.20) | 0.222 |
Sensitivity analyses of weight change
A number of sensitivity analyses were undertaken for weight change with regard to alternative model types, covariates and missing data assumptions (Figure 11). The weight change at 12 months was refitted with sex included as a covariate, as the groups were imbalanced in this; doing so made little difference, as sex was not associated with weight loss. The model assumptions were also reassessed using a mixed-effects model, by CACE (using the same covariates) to assess the impact of uptake, and with missing data assumed to be MAR using predictive mean matching imputation. In all cases, the difference between groups remained small.
FIGURE 11.
Sensitivity analyses for weight change. PMM, predictive mean matching.
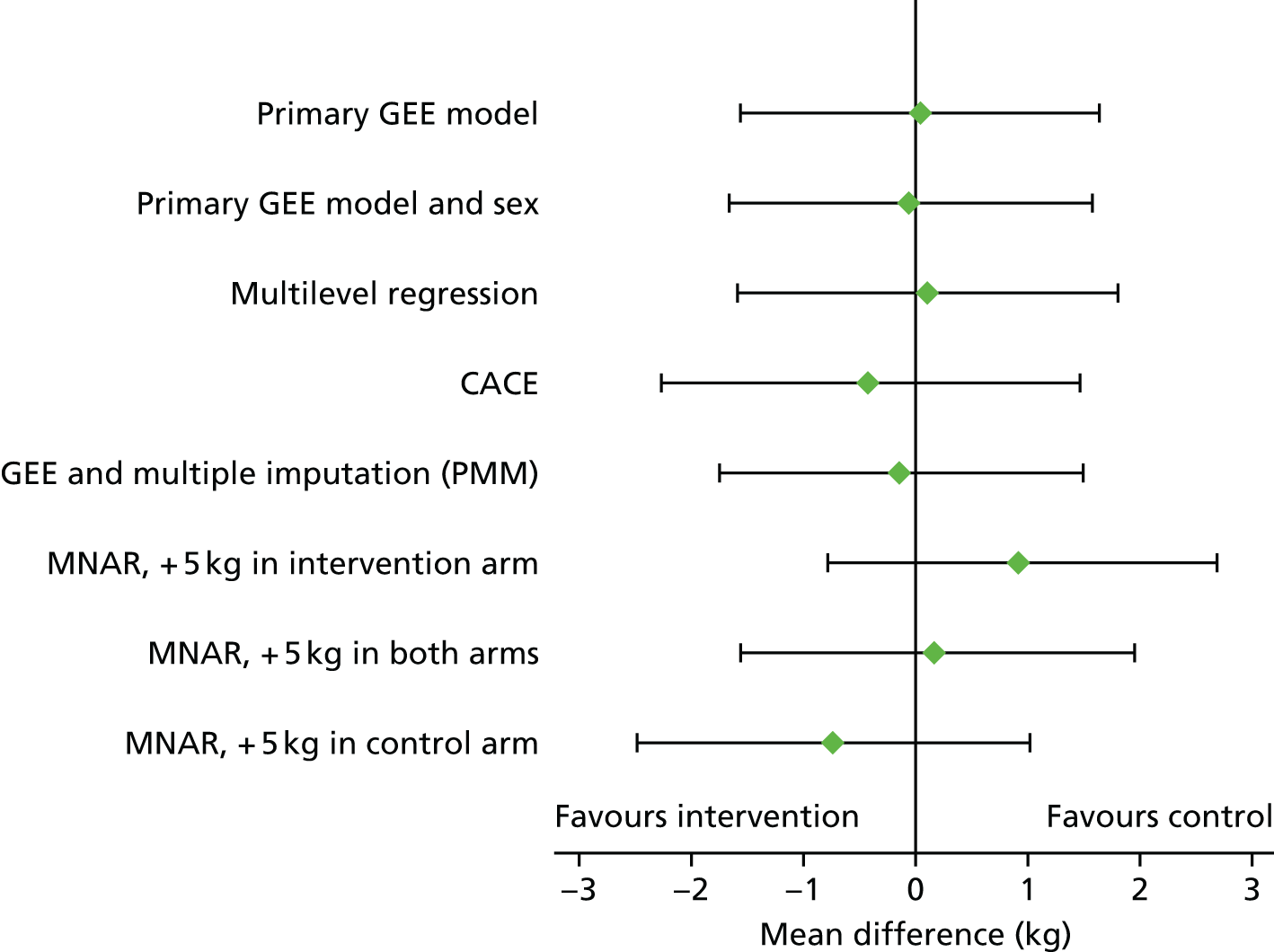
It is possible that some participants did not remain in the study because of their weight (i.e. informative dropout or MNAR), and the above models do not account for this. As a final sensitivity analysis, missing weight was imputed in which those who withdrew were assigned weights up to 5 kg more than their predicted value; this was done in the intervention arm only, the control arm only and both. Although this affected the estimated treatment differences, the differences remained small and were not statistically significant.
The impact of the intervention by subgroup was estimated in four subgroups, as described in Chapter 3, Analysis of other outcomes. In all cases, the difference between groups was small and not of clinical or statistical significance (Figure 12).
FIGURE 12.
Weight change by subgroup.
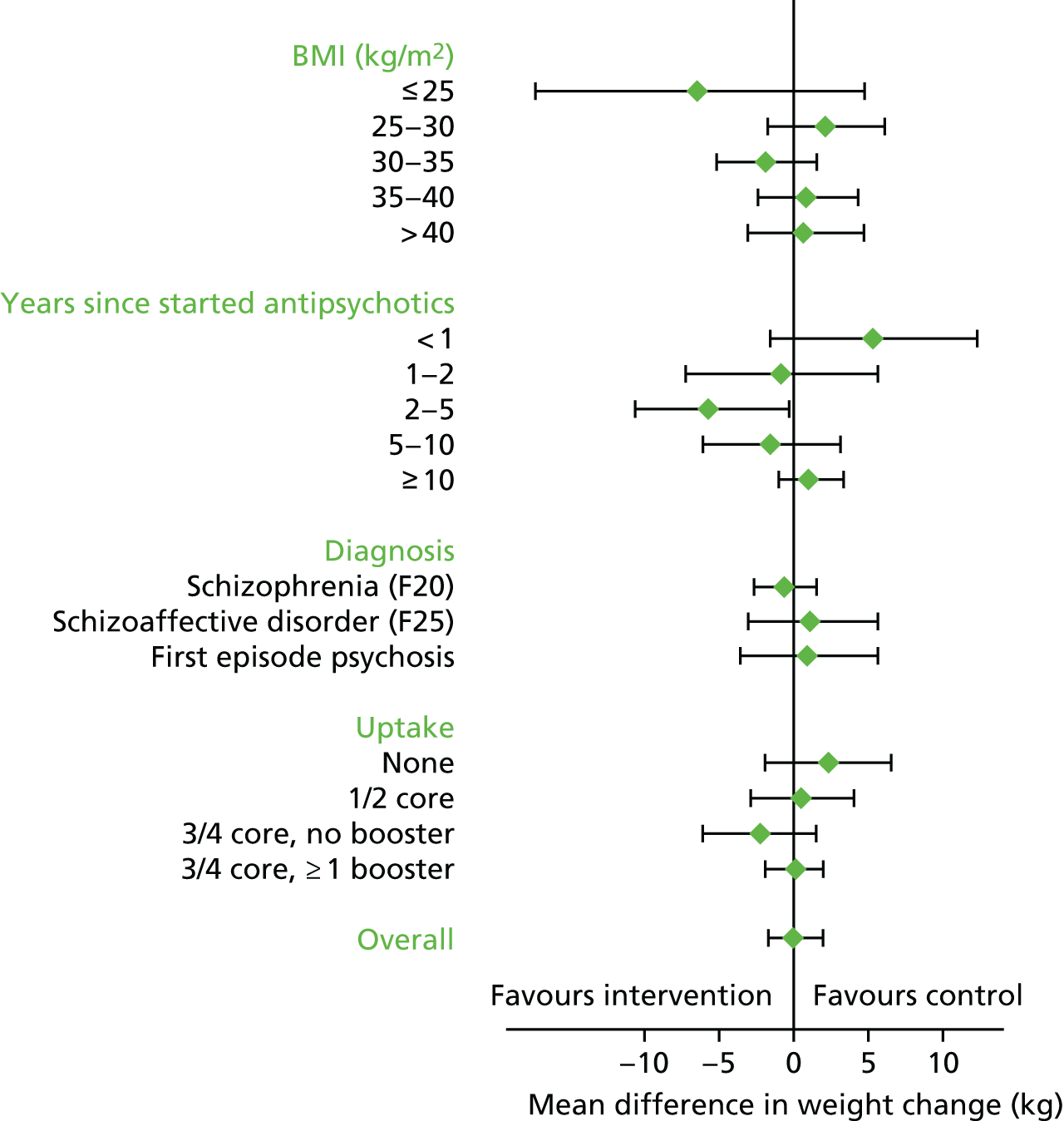
Predictors of weight change
A series of linear regressions were undertaken to assess whether or not any participant characteristics were associated with weight change. The univariate analyses are presented in Table 14. Few of the characteristics appeared to systematically explain weight gain or loss. Weight loss was modestly associated with age, with weight reduction increasing by 0.8 kg per 10 additional years (95% CI 0.0 to 1.5 kg; p = 0.042), participants with a diagnosis of schizoaffective disorder had a greater mean weight loss (2.7 kg) than those with first episode psychosis (0.3 kg) or schizophrenia (0.01 kg gain; global test, p = 0.075). Weight loss was greater among female participants and was, on average, greater among those with higher baseline weight and longer duration on antipsychotics, but none of these findings was statistically significant. Additional analyses based on non-linear associations, using fractional polynomial regression and multivariable regression models, did not reveal any important predictors of weight change. There was no association between total (group, telephone and face-to-face) contact time and weight change (see Figure 13).
| Predictor | Mean difference (95% CI) | p-value |
|---|---|---|
| Baseline demographics | ||
| Age (per 10-year increase) | –0.8 (–1.5 to –0.0) | 0.042 |
| Sex (female vs. male) | –1.2 (–2.9 to 0.6) | 0.182 |
| Weight at baseline (per 10 kg) | –0.1 (–0.5 to 0.3) | 0.587 |
| Disease characteristics and comorbidities | ||
| Diagnosis | ||
| Schizoaffective disorder vs. schizophrenia | –2.7 (–5.1 to –0.4) | 0.023 |
| First episode psychosis vs. schizophrenia | –0.4 (–2.8 to 2.1) | 0.774 |
| Time since started antipsychotics (per 10 years) | –0.5 (–1.3 to 0.4) | 0.253 |
| Presence of diabetes mellitus | –2.1 (–4.6 to 0.5) | 0.109 |
| Evidence of hepatic disease | –1.5 (–6.2 to 3.2) | 0.532 |
| Mental health at baseline | ||
| Diagnosis of clinical depression | 0.7 (–0.3 to 1.7) | 0.194 |
| BPRS | 0.0 (–0.1 to 0.1) | 0.845 |
| B-IPQ overall total score | –0.4 (–0.9 to 0.1) | 0.134 |
| PHQ-9 score | –0.0 (–0.2 to 0.1) | 0.525 |
| SF-36 QoL at baseline | ||
| Physical functioning | 0.0 (–0.0 to 0.1) | 0.106 |
| Role limitations owing to physical health | 0.0 (–0.0 to 0.0) | 0.746 |
| Role limitations owing to emotional problems | –0.0 (–0.0 to 0.0) | 0.529 |
| Energy/fatigue score | –0.0 (–0.1 to 0.0) | 0.469 |
| Emotional well-being | –0.0 (–0.1 to 0.0) | 0.380 |
| Social functioning | 0.0 (–0.0 to 0.0) | 0.763 |
| Bodily pain | 0.0 (–0.0 to 0.0) | 0.462 |
| General health | –0.0 (–0.0 to 0.0) | 0.876 |
| Antipsychotic medication at baseline | ||
| Amisulpride | –1.8 (–5.1 to 1.5) | 0.274 |
| Aripiprazole | 2.1 (–0.1 to 4.3) | 0.061 |
| Clozapine | 0.3 (–1.5 to 2.0) | 0.757 |
| Flupentixol | –2.1 (–6.0 to 1.7) | 0.276 |
| Haloperidol | –0.9 (–5.8 to 4.0) | 0.725 |
| Paliperidone | –3.1 (–7.7 to 1.6) | 0.199 |
| Olanzapine | –0.6 (–2.9 to 1.8) | 0.642 |
| Quetiapine | 0.1 (–2.4 to 2.6) | 0.941 |
| Risperidone | –0.2 (–3.6 to 3.1) | 0.893 |
| Zuclopenthixol | –1.2 (–4.5 to 2.1) | 0.471 |
| Other antipsychotic | 2.2 (–1.5 to 5.9) | 0.237 |
| Antidepressant medication at baseline | ||
| Any antidepressant | –0.2 (–2.0 to 1.6) | 0.811 |
| Citalopram | 1.3 (–1.9 to 4.5) | 0.415 |
| Lithium | –4.4 (–8.5 to –0.4) | 0.033 |
| Mirtazepine | 0.4 (–3.7 to 4.5) | 0.851 |
| Other antidepressant | –0.4 (–2.1 to 1.4) | 0.666 |
| Post-baseline medication and change in medication | ||
| Changed antipsychotics by 3 months | 3.4 (0.3 to 6.5) | 0.031 |
| Changed antipsychotics by 12 months | –0.2 (–2.6 to 2.2) | 0.856 |
| On antidepressants at 3 months | 0.4 (–1.4 to 2.2) | 0.660 |
| Changed antidepressants by 3 months | 6.1 (2.3 to 9.9) | 0.002 |
| On antidepressants at 12 months | 0.0 (–1.8 to 1.8) | 0.963 |
| Changed antidepressants by 12 months | 1.5 (–0.9 to 3.8) | 0.220 |
| Weight loss programme | ||
| Attended any weight loss programme over 12 months | –0.1 (–2.8 to 2.7) | 0.965 |
| Intervention uptake | ||
| Total contact time (hours) | –0.1 (–0.4 to 0.2) | 0.607 |
Participants who were prescribed lithium had a statistically significantly greater weight reduction than those who were not (mean difference = 4.4 kg, 95% CI 0.4 to 8.5 kg; p = 0.033), although this accounted for only 16 participants completing the trial. Among participants taking antipsychotics, weight loss was greatest among participants on paliperidone (n = 12, average weight loss 3.4 kg) and lowest among those on aripiprazole (n = 63, 1.2-kg gain) and other antipsychotics (n = 20, 1.6 kg gain). Participants who changed medication within 3 months of randomisation had a higher average weight gain than those who did not (antipsychotic change: n = 29, mean difference = 3.4 kg, 95% CI 0.3 to 6.5 kg; p = 0.031; antidepressant change: n = 18, mean difference = 6.1 kg, 95% CI 2.3 to 9.9 kg; p = 0.002), but changes over the full 12 months were not associated with weight change.
Finally, there was no association between total contact time (summed across foundation sessions, booster sessions and support contact) and weight loss (Figure 13).
FIGURE 13.
Weight loss at 12 months vs. total contact time (group session and support contact). The line is a locally weighted scatterplot smoother with bandwidth 0.4.
Other vital signs
The vital signs (blood pressure and pulse) at baseline, 3 months and 12 months are presented in Table 15. Systolic (but not diastolic) blood pressure was significantly higher in the intervention group at 3 months (difference = 2.4 mmHg, 95% CI 0.1 to 4.6 mmHg), but otherwise no change was apparent either between groups or across time points.
| Vital sign | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Systolic blood pressure (mmHg) | ||||
| Baseline | ||||
| n | 205 | 203 | ||
| Mean (SD) | 126.4 (15.9) | 124.0 (16.8) | ||
| 3 months | ||||
| n | 178 | 179 | ||
| Mean (SD) | 126.9 (16.2) | 122.8 (15.7) | 2.4 (0.1 to 4.6) | 0.040 |
| 12 months | ||||
| n | 164 | 168 | ||
| Mean (SD) | 124.7 (15.2) | 122.2 (16.4) | 1.7 (–1.1 to 4.6) | 0.237 |
| Diastolic blood pressure (mmHg) | ||||
| Baseline | ||||
| n | 205 | 203 | ||
| Mean (SD) | 82.5 (11.0) | 81.9 (12.5) | ||
| 3 months | ||||
| n | 178 | 179 | ||
| Mean (SD) | 82.2 (11.0) | 81.4 (12.2) | 0.4 (–1.5 to 2.3) | 0.696 |
| 12 months | ||||
| n | 164 | 168 | ||
| Mean (SD) | 82.2 (10.3) | 81.1 (10.5) | 1.1 (–0.7 to 3.0) | 0.231 |
| Pulse (b.p.m.) | ||||
| Baseline | ||||
| n | 204 | 203 | ||
| Mean (SD) | 84.5 (13.9) | 83.8 (13.6) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 86.1 (13.7) | 83.6 (16.0) | 2.1 (–0.5 to 4.6) | 0.108 |
| 12 months | ||||
| n | 164 | 168 | ||
| Mean (SD) | 84.4 (13.9) | 82.2 (14.0) | 1.2 (–0.8 to 3.3) | 0.245 |
Laboratory measurements
The laboratory measurements at baseline and 12 months are presented in Table 16. No change was apparent either between groups or across time points.
| Laboratory measurement | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| HbA1c (mmol/mol) | ||||
| Baseline | ||||
| n | 171 | 171 | ||
| Mean (SD) | 41.5 (13.3) | 39.9 (10.9) | ||
| 12 months | ||||
| n | 134 | 132 | ||
| Mean (SD) | 42.8 (15.4) | 41.1 (14.1) | ||
| Change from baseline | ||||
| n | 119 | 118 | ||
| Mean (SD) | 0.7 (7.9) | 0.6 (5.6) | 0.2 (–1.5 to 1.9) | 0.807 |
| HbA1c (%) | ||||
| Baseline | ||||
| n | 171 | 171 | ||
| Mean (SD) | 5.9 (1.2) | 5.8 (1.0) | ||
| 12 months | ||||
| n | 134 | 132 | ||
| Mean (SD) | 6.1 (1.4) | 5.9 (1.3) | ||
| Change from baseline | ||||
| n | 119 | 118 | ||
| Mean (SD) | 0.1 (0.7) | 0.1 (0.5) | ||
| Fasting glucose (mmol/l) | ||||
| Baseline | ||||
| n | 170 | 172 | ||
| Mean (SD) | 5.9 (2.2) | 5.8 (2.3) | ||
| 12 months | ||||
| n | 137 | 132 | ||
| Mean (SD) | 6.4 (3.0) | 6.0 (2.8) | ||
| Change from baseline | ||||
| n | 123 | 122 | ||
| Mean (SD) | 0.3 (2.1) | 0.1 (1.5) | 0.3 (–0.2 to 0.8) | 0.242 |
| Total cholesterol (mmol/l) | ||||
| Baseline | ||||
| n | 175 | 179 | ||
| Mean (SD) | 5.0 (1.2) | 5.1 (1.2) | ||
| 12 months | ||||
| n | 143 | 138 | ||
| Mean (SD) | 4.9 (1.2) | 5.1 (1.1) | ||
| Change from baseline | ||||
| n | 129 | 127 | ||
| Mean (SD) | –0.2 (1.2) | –0.1 (0.9) | –0.2 (–0.4 to 0.1) | 0.176 |
| HDL cholesterol (mmol/l) | ||||
| Baseline | ||||
| n | 170 | 173 | ||
| Mean (SD) | 1.2 (0.5) | 1.2 (0.4) | ||
| 12 months | ||||
| n | 143 | 137 | ||
| Mean (SD) | 1.2 (0.6) | 1.2 (0.3) | ||
| Change from baseline | ||||
| n | 125 | 122 | ||
| Mean (SD) | 0.0 (0.7) | –0.0 (0.3) | 0.0 (–0.1 to 0.1) | 0.824 |
| Triglycerides (mmol/l) | ||||
| Baseline | ||||
| n | 172 | 172 | ||
| Mean (SD) | 2.5 (2.0) | 2.2 (1.7) | ||
| 12 months | ||||
| n | 139 | 135 | ||
| Mean (SD) | 2.4 (1.4) | 2.4 (2.2) | ||
| Change from baseline | ||||
| n | 124 | 120 | ||
| Mean (SD) | –0.2 (1.8) | 0.2 (1.7) | –0.2 (–0.6 to 0.1) | 0.203 |
| Non-HDL cholesterol (mmol/l) | ||||
| Baseline | ||||
| n | 169 | 174 | ||
| Mean (SD) | 3.8 (1.3) | 3.9 (1.4) | ||
| 12 months | ||||
| n | 143 | 136 | ||
| Mean (SD) | 3.7 (1.2) | 3.8 (1.2) | ||
| Change from baseline | ||||
| n | 124 | 121 | ||
| Mean (SD) | –0.2 (1.4) | –0.2 (0.9) | –0.1 (–0.4 to 0.2) | 0.428 |
Accelerometry
Table 17 shows the results of the accelerometry. A total of 352 participants had baseline recordings for the primary accelerometry end point [(ENMO) i.e. average acceleration], of whom 302 had worn the GENEActiv for at least 4 days and therefore had a valid baseline measure. Of these, valid ENMO data were available in 222 participants at 3 months and in 209 participants at 12 months, meaning that the change in accelerometry could be estimated in 54% and 51% of the trial population at 3 and 12 months, respectively.
| Measure of physical activity | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Mean acceleration magnitude, mg (ENMO) | ||||
| Baseline | ||||
| n | 160 | 152 | ||
| Mean (SD) | 21.3 (7.9) | 20.8 (7.3) | ||
| 3 months | ||||
| n | 123 | 130 | ||
| Mean (SD) | 21.7 (9.0) | 19.8 (7.1) | –0.3 (–1.4 to 0.9) | 0.654 |
| 12 months | ||||
| n | 120 | 134 | ||
| Mean (SD) | 22.4 (8.1) | 20.5 (8.5) | 0.1 (–1.5 to 1.6) | 0.947 |
| Mean acceleration magnitude (all days measured) | ||||
| Baseline | ||||
| n | 160 | 152 | ||
| Mean (SD) | 21.3 (7.9) | 20.8 (7.4) | ||
| 3 months | ||||
| n | 123 | 130 | ||
| Mean (SD) | 21.7 (9.0) | 19.8 (7.1) | –0.4 (–1.5 to 0.8) | 0.555 |
| 12 months | ||||
| n | 120 | 134 | ||
| Mean (SD) | 22.4 (8.2) | 20.5 (8.5) | 0.2 (–1.4 to 1.7) | 0.844 |
| Mean acceleration magnitude (weekends) | ||||
| Baseline | ||||
| n | 167 | 154 | ||
| Mean (SD) | 19.6 (8.0) | 19.8 (8.3) | ||
| 3 months | ||||
| n | 128 | 139 | ||
| Mean (SD) | 20.4 (9.6) | 18.7 (6.9) | 1.0 (–0.3 to 2.4) | 0.140 |
| 12 months | ||||
| n | 126 | 137 | ||
| Mean (SD) | 20.9 (8.6) | 19.4 (8.8) | 0.3 (–1.5 to 2.1) | 0.708 |
| Mean acceleration magnitude (weekdays) | ||||
| Baseline | ||||
| n | 159 | 153 | ||
| Mean (SD) | 22.1 (8.3) | 21.1 (7.1) | ||
| 3 months | ||||
| n | 125 | 129 | ||
| Mean (SD) | 22.1 (9.2) | 20.2 (7.5) | –0.7 (–2.0 to 0.6) | 0.278 |
| 12 months | ||||
| n | 124 | 136 | ||
| Mean (SD) | 23.0 (8.5) | 20.9 (8.6) | 0.0 (–1.6 to 1.6) | 0.994 |
| MVPA, based on 5-second epochs (all days) | ||||
| Baseline | ||||
| n | 160 | 152 | ||
| Mean (SD) | 75.6 (45.9) | 72.4 (42.2) | ||
| 3 months | ||||
| n | 123 | 130 | ||
| Mean (SD) | 77.5 (53.3) | 64.1 (39.4) | –0.5 (–7.7 to 6.8) | 0.902 |
| 12 months | ||||
| n | 120 | 134 | ||
| Mean (SD) | 79.8 (50.3) | 68.9 (47.2) | 0.4 (–8.6 to 9.3) | 0.939 |
| MVPA, 5-second bout duration (weekends) | ||||
| Baseline | ||||
| n | 167 | 154 | ||
| Mean (SD) | 65.7 (44.7) | 67.5 (47.0) | ||
| 3 months | ||||
| n | 128 | 139 | ||
| Mean (SD) | 70.3 (55.1) | 57.9 (37.5) | 9.2 (1.7 to 16.7) | 0.016 |
| 12 months | ||||
| n | 126 | 137 | ||
| Mean (SD) | 71.9 (50.0) | 63.0 (48.3) | 2.1 (–7.9 to 12.1) | 0.677 |
| MVPA, based on 5-second epochs (weekdays) | ||||
| Baseline | ||||
| n | 159 | 153 | ||
| Mean (SD) | 21.6 (22.8) | 17.6 (17.8) | ||
| 3 months | ||||
| n | 125 | 129 | ||
| Mean (SD) | 20.6 (25.8) | 14.3 (17.7) | –2.3 (–10.0 to 5.5) | 0.563 |
| 12 months | ||||
| n | 124 | 136 | ||
| Mean (SD) | 23.6 (28.7) | 18.0 (23.3) | –0.4 (–9.7 to 8.8) | 0.926 |
| MVPA, > 5-minute bout duration, 80% inclusion (all days) | ||||
| Baseline | ||||
| n | 160 | 152 | ||
| Mean (SD) | 19.8 (20.7) | 16.8 (16.5) | ||
| 3 months | ||||
| n | 123 | 130 | ||
| Mean (SD) | 19.9 (25.5) | 13.5 (15.8) | 1.7 (–1.8 to 5.2) | 0.350 |
| 12 months | ||||
| n | 120 | 134 | ||
| Mean (SD) | 22.0 (25.5) | 17.0 (22.4) | 1.0 (–3.6 to 5.6) | 0.675 |
| MVPA, ≥ 5-minute bout duration, 80% inclusion (weekends) | ||||
| Baseline | ||||
| n | 167 | 154 | ||
| Mean (SD) | 14.7 (19.6) | 14.9 (18.7) | ||
| 3 months | ||||
| n | 128 | 139 | ||
| Mean (SD) | 17.2 (28.9) | 11.5 (15.5) | 7.0 (2.7 to 11.4) | 0.002 |
| 12 months | ||||
| n | 126 | 137 | ||
| Mean (SD) | 17.3 (25.2) | 14.2 (22.2) | 2.3 (–2.3 to 6.9) | 0.333 |
| MVPA, > 5-minute bout duration, 80% inclusion (weekdays) | ||||
| Baseline | ||||
| n | 159 | 153 | ||
| Mean (SD) | 21.6 (22.8) | 17.6 (17.8) | ||
| 3 months | ||||
| n | 125 | 129 | ||
| Mean (SD) | 20.6 (25.8) | 14.3 (17.7) | 0.2 (–3.4 to 3.7) | 0.924 |
| 12 months | ||||
| n | 124 | 136 | ||
| Mean (SD) | 23.6 (28.7) | 18.0 (23.3) | 0.4 (–5.0 to 5.9) | 0.871 |
| MVPA, ≥ 10-minute bouts, 80% inclusion (all days) | ||||
| Baseline | ||||
| n | 160 | 152 | ||
| Mean (SD) | 13.3 (16.8) | 11.0 (13.1) | ||
| 3 months | ||||
| n | 123 | 130 | ||
| Mean (SD) | 13.3 (20.4) | 8.8 (12.6) | 2.0 (–0.9 to 4.9) | 0.183 |
| 12 months | ||||
| n | 120 | 134 | ||
| Mean (SD) | 15.4 (21.7) | 11.8 (19.3) | 1.5 (–2.5 to 5.5) | 0.473 |
| MVPA, ≥ 10-minute bouts, 80% inclusion (weekends) | ||||
| Baseline | ||||
| n | 167 | 154 | ||
| Mean (SD) | 9.6 (16.6) | 9.6 (14.8) | ||
| 3 months | ||||
| n | 128 | 139 | ||
| Mean (SD) | 11.3 (24.9) | 7.4 (12.4) | 5.6 (2.0 to 9.3) | 0.003 |
| 12 months | ||||
| n | 126 | 137 | ||
| Mean (SD) | 11.9 (22.1) | 9.5 (19.2) | 2.2 (–1.8 to 6.2) | 0.274 |
| MVPA, ≥ 10-minute bouts, 80% inclusion (weekdays) | ||||
| Baseline | ||||
| n | 159 | 153 | ||
| Mean (SD) | 14.4 (18.5) | 11.6 (14.8) | ||
| 3 months | ||||
| n | 125 | 129 | ||
| Mean (SD) | 13.8 (20.3) | 9.5 (14.3) | 0.9 (–2.0 to 3.8) | 0.552 |
| 12 months | ||||
| n | 124 | 136 | ||
| Mean (SD) | 16.6 (24.5) | 12.6 (20.1) | 1.0 (–3.9 to 6.0) | 0.680 |
Overall baseline physical activity was low in both groups. Intervention arm participants were, on average, more active than control arm participants both before randomisation and after randomisation, but, after adjusting for baseline, few of the differences were statistically significant. The exception was MVPA, measured at weekends at the 3-month follow-up, which was modestly greater in the intervention group. The mean length of MVPA in the intervention and control groups was 70 versus 58 minutes per day (difference of 9.2 minutes per day, 95% CI 1.7 to 16.7 minutes per day; p = 0.016) for 5-minute bouts and 11 versus 7 minutes per day for 10-minute bouts (difference of 5.6 minutes per day, 95% CI 2.0 to 9.3 minutes per day; p = 0.003). The corresponding outcome for acceleration was not statistically significant (20 vs. 19 mg, difference = 1.0 mg, 95% CI –0.3 to 2.4 mg; p = 0.140). None of the remaining outcomes was different between the trial arms.
Dietary intake as assessed by the Dietary Instrument for Nutrition Education questionnaire
Figures 14–18 depict food intake as assessed by the DINE questionnaire (blue lines denote mean values). The measures (including the categorisations as low, medium and high) are reported in Table 18. The baseline self-reported diet of the participants in both groups indicated a high consumption of refined sugar from sugary drinks and low fibre. There was some evidence that alcohol intake had reduced in the intervention arm, although the intake was highly skewed and not robust to alternative models, including non-parametric analyses. No other dietary changes were observed as a result of the intervention or trial participation.
FIGURE 14.
Dietary Instrument for Nutrition Education fibre intake. (a) Baseline; (b) 3 months; and (c) 12 months.
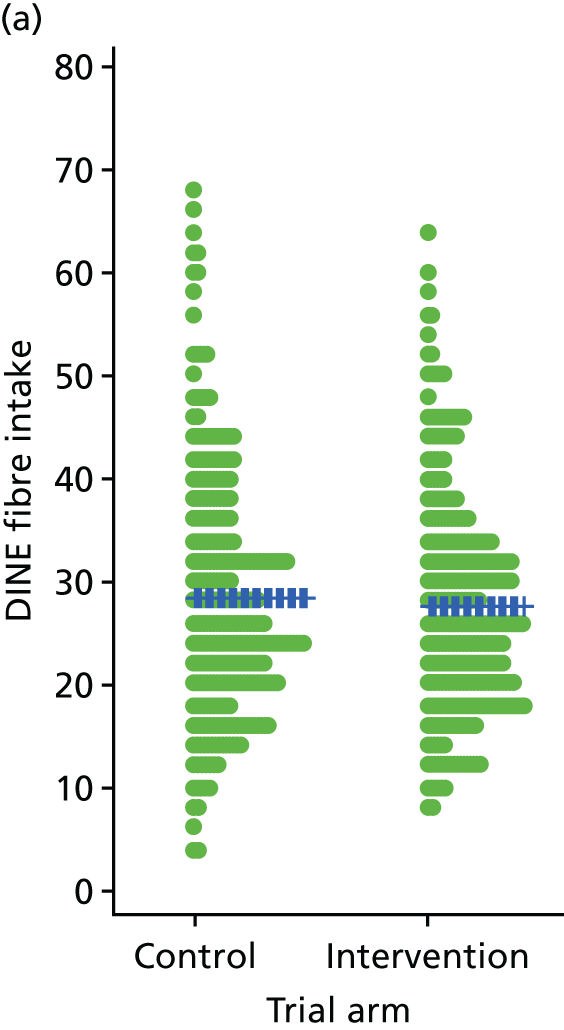
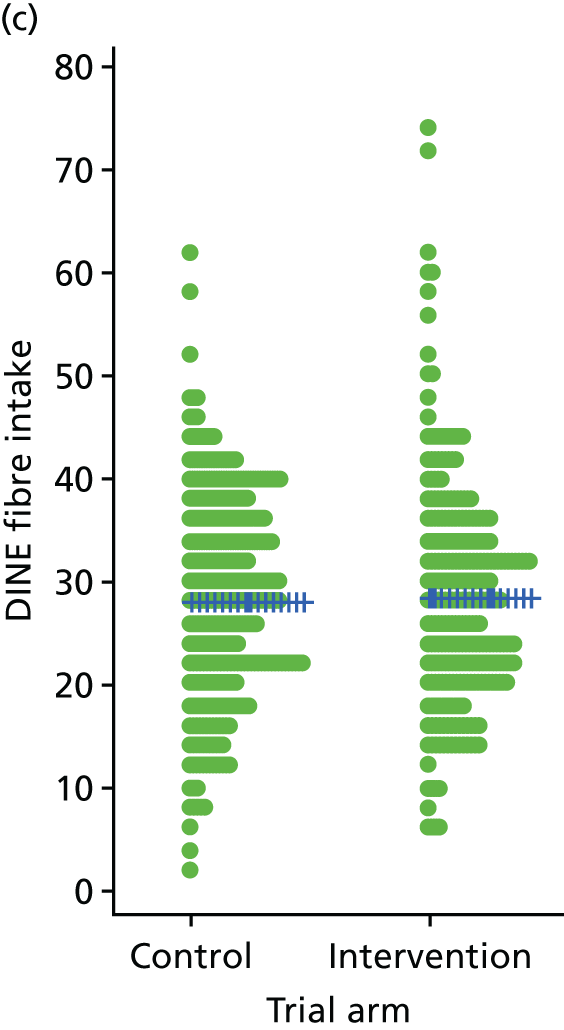
FIGURE 15.
Dietary Instrument for Nutrition Education fat intake. (a) Baseline; (b) 3 months; and (c) 12 months.
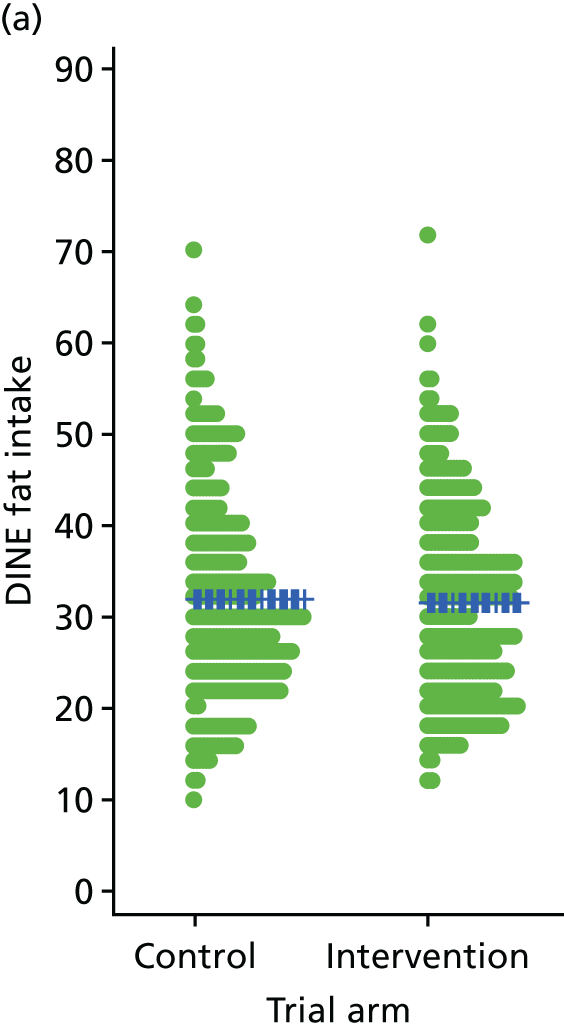
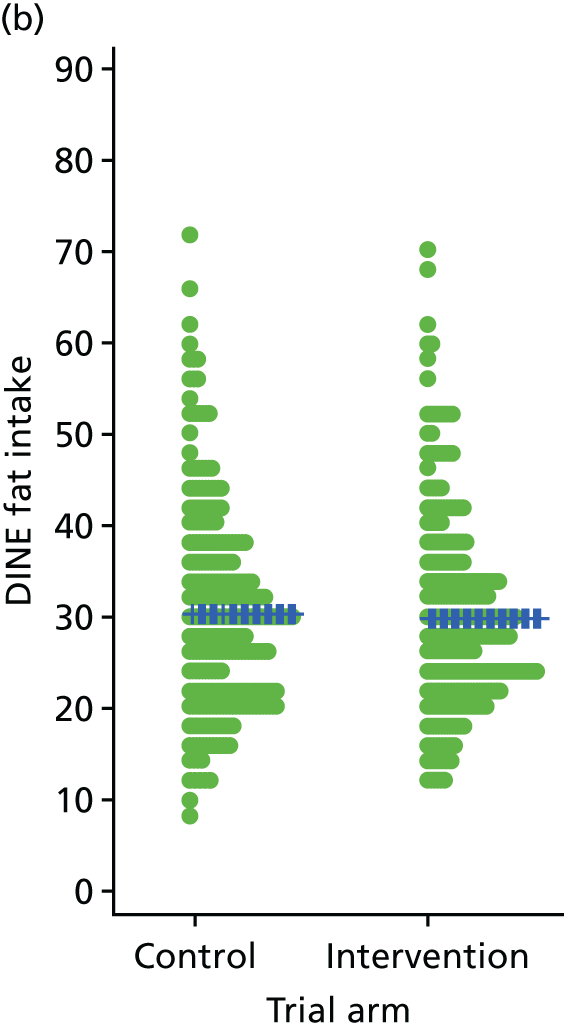
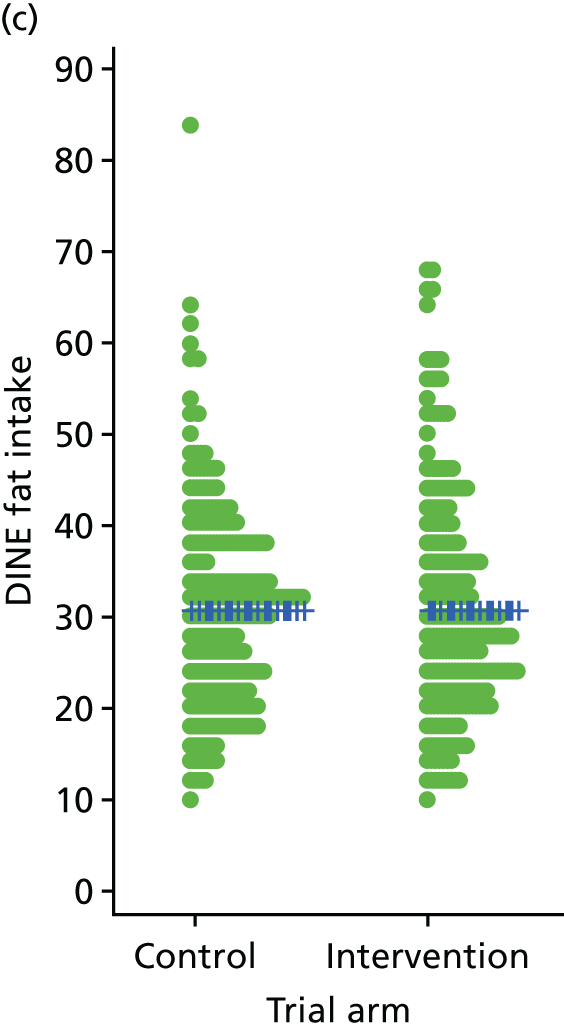
FIGURE 16.
Dietary Instrument for Nutrition Education unsaturated fat intake. (a) Baseline; (b) 3 months; and (c) 12 months.
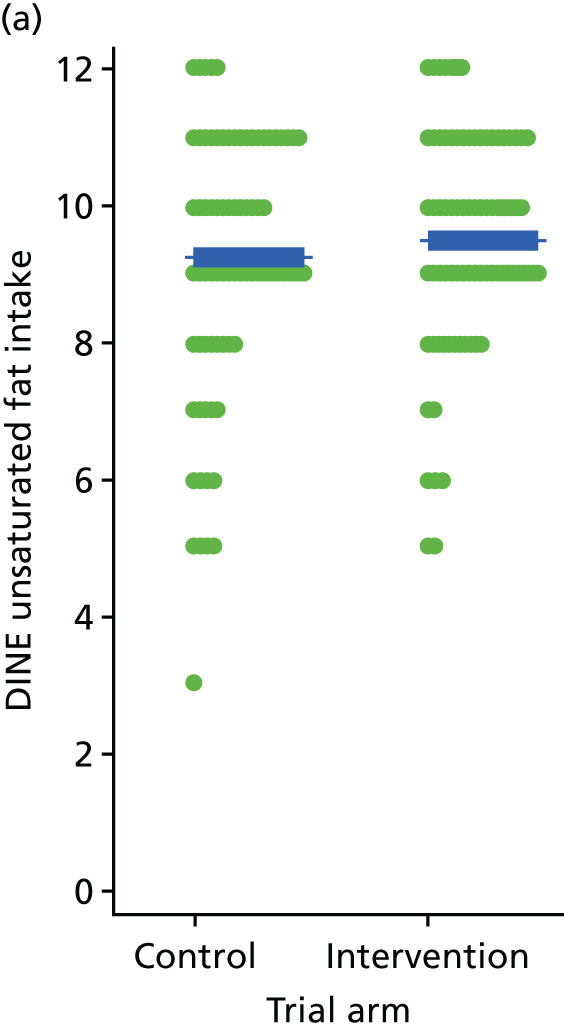
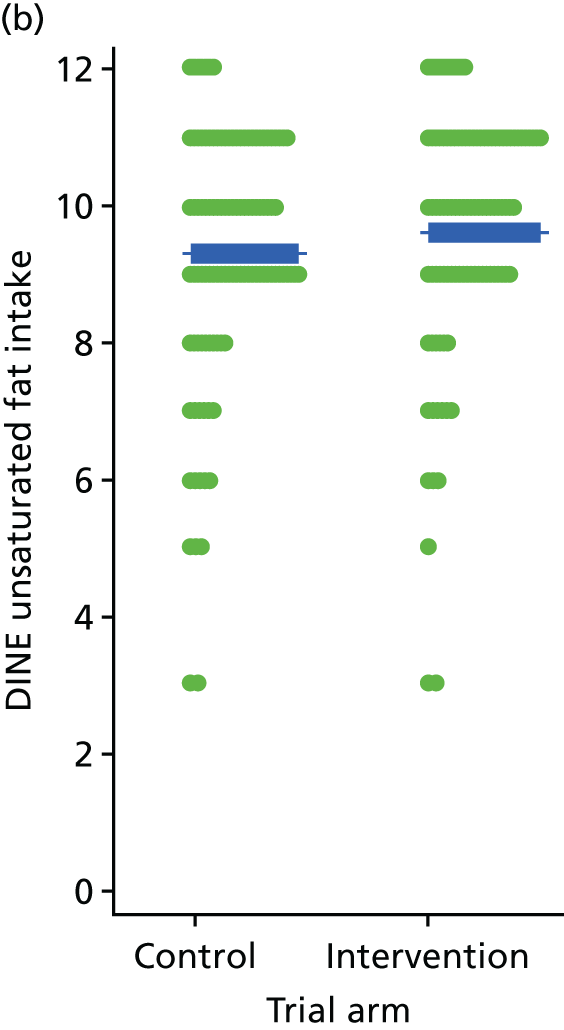
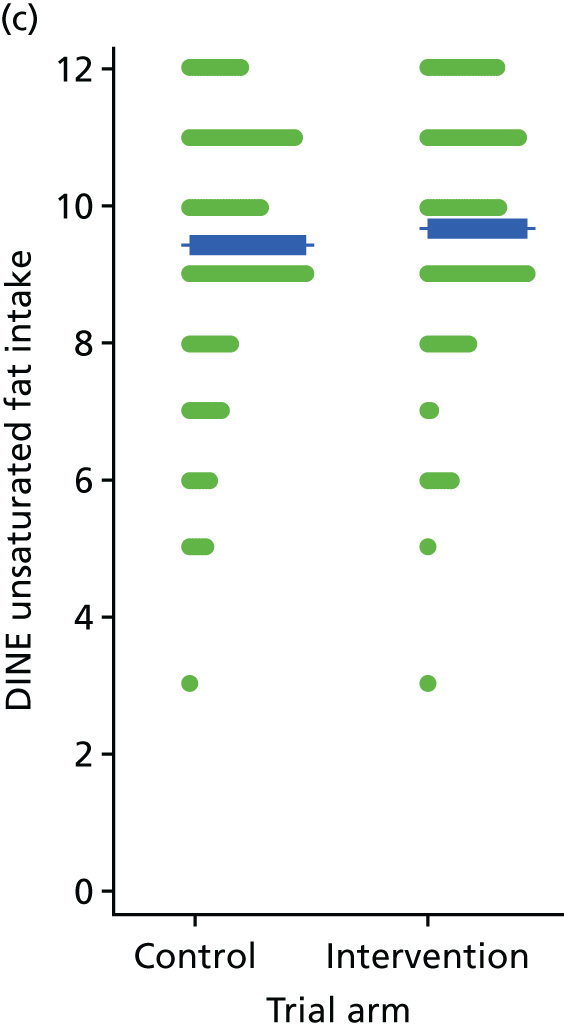
FIGURE 17.
Dietary Instrument for Nutrition Education daily sugar intake from drinks (g). (a) Baseline; (b) 3 months; and (c) 12 months.
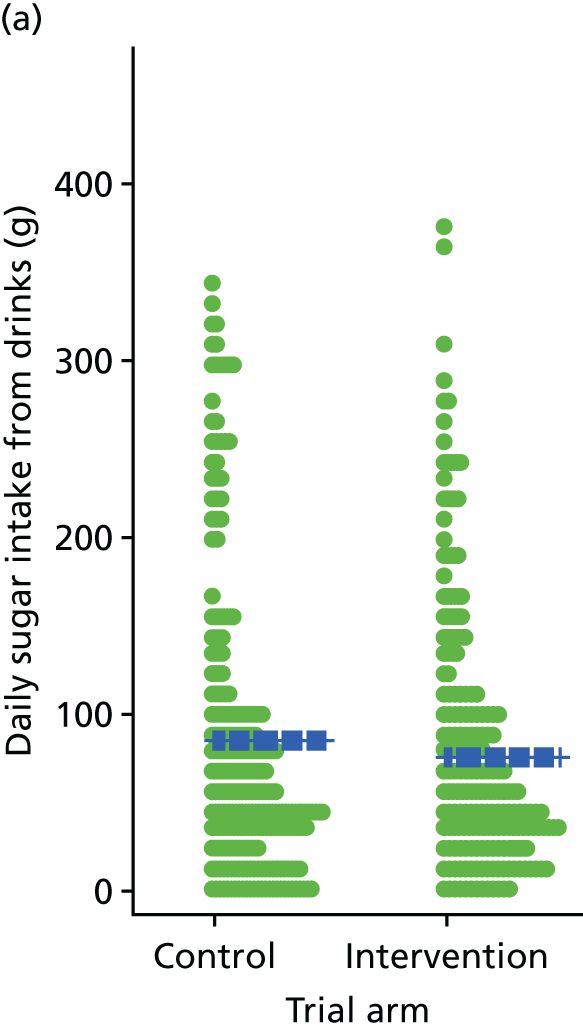
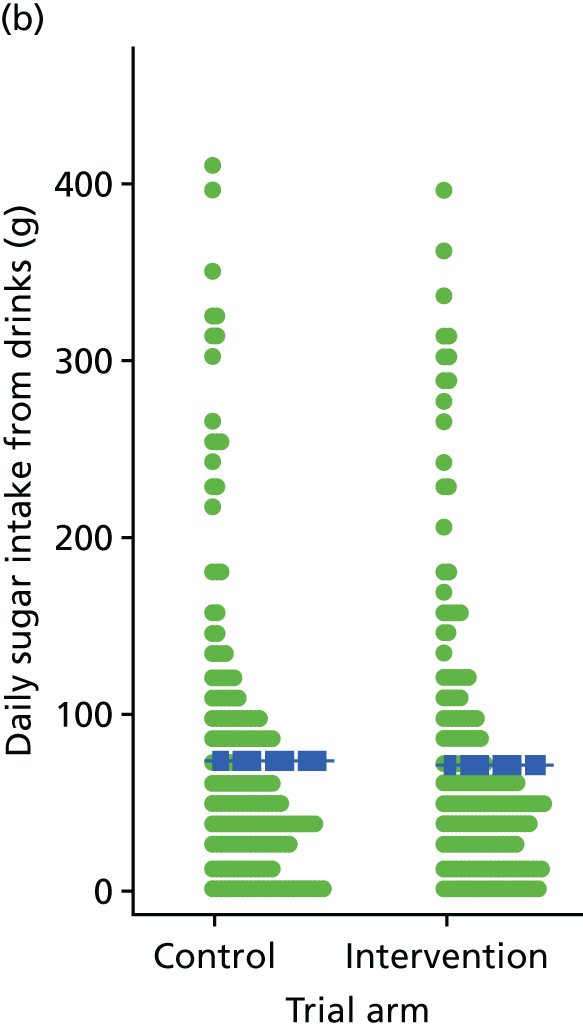
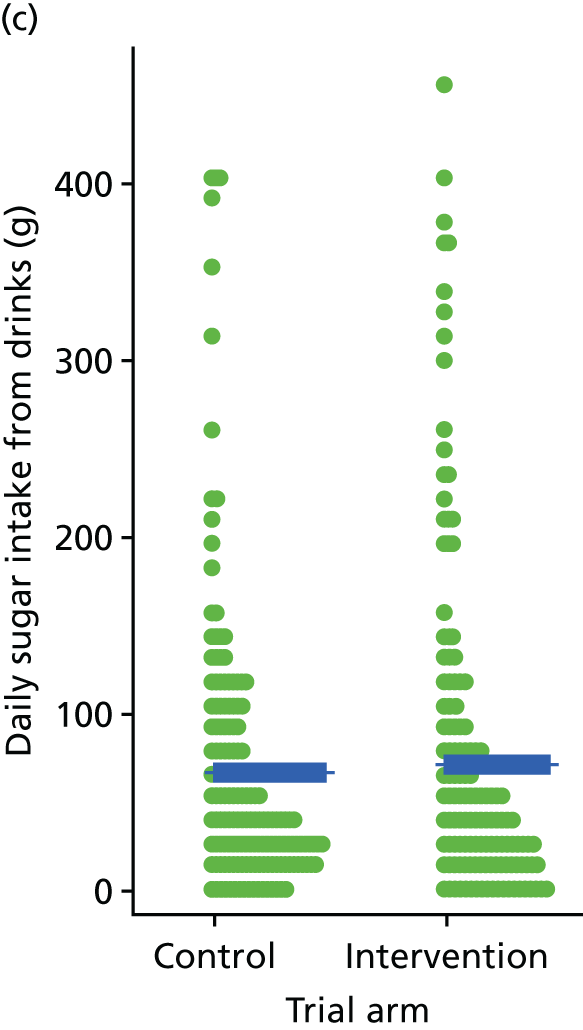
FIGURE 18.
Dietary Instrument for Nutrition Education weekly alcohol intake (units). (a) Baseline; (b) 3 months; and (c) 12 months.
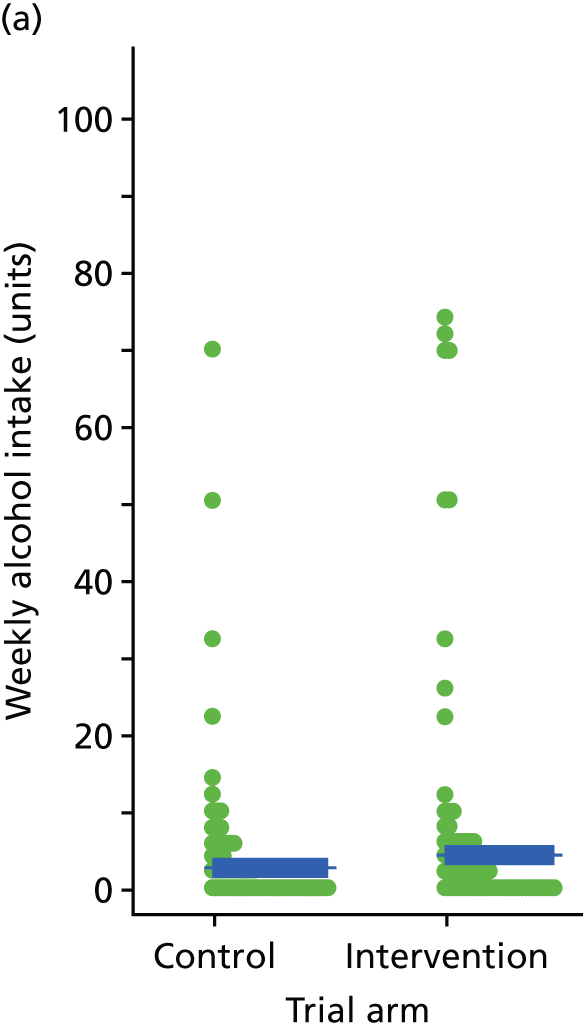
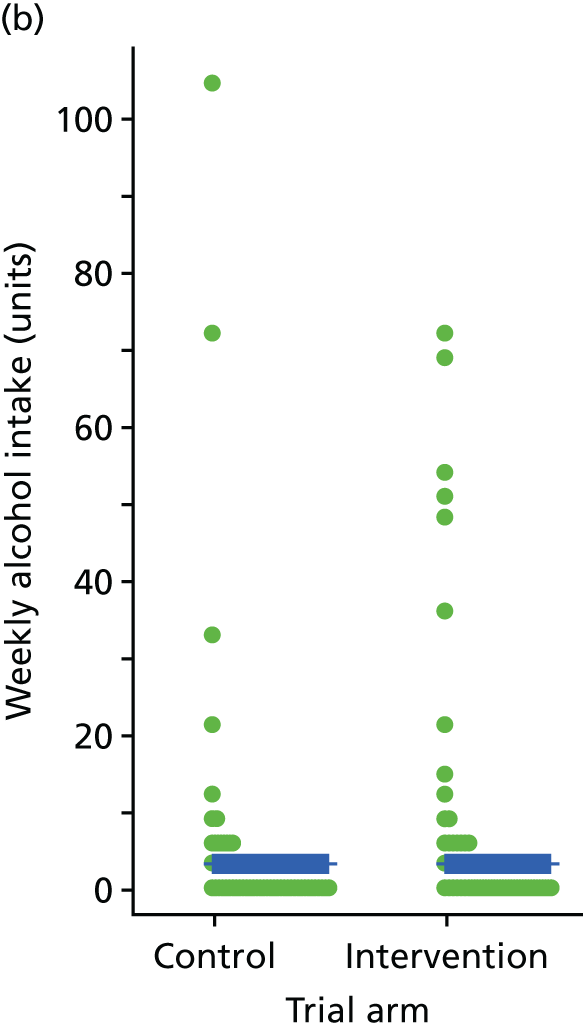
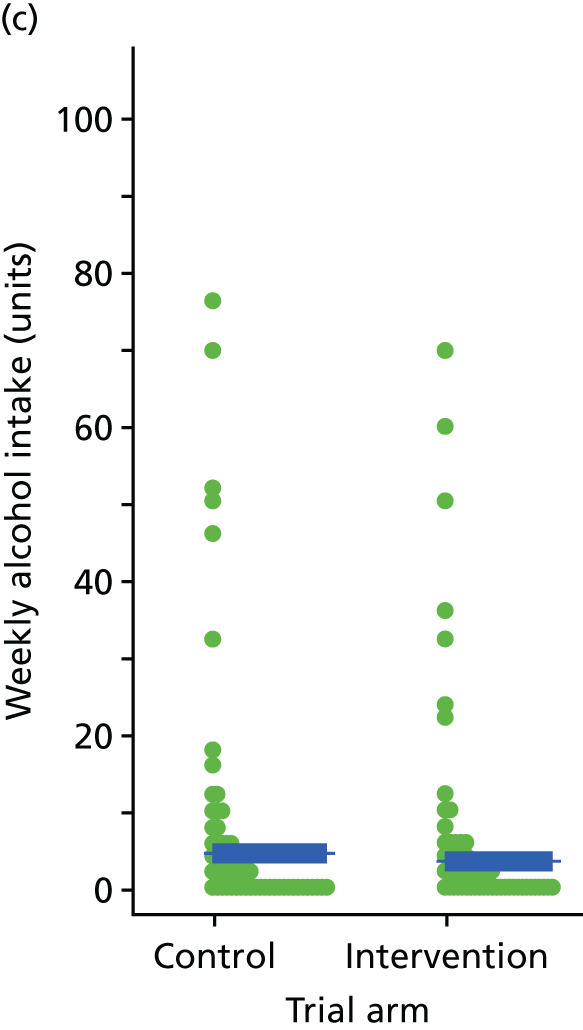
| Dietary intake | Trial arm | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| DINE fibre intake | ||
| Baseline | ||
| n | 207 | 205 |
| Mean (SD) | 27.6 (11.3) | 28.3 (12.6) |
| Median (IQR) | 26.0 (19.0 to 34.0) | 26.0 (20.0 to 36.0) |
| 3 months | ||
| n | 178 | 180 |
| Mean (SD) | 28.2 (12.0) | 28.0 (12.4) |
| Median (IQR) | 26.0 (19.0 to 36.0) | 26.5 (19.5 to 36.0) |
| 12 months | ||
| n | 165 | 172 |
| Mean (SD) | 28.6 (12.1) | 28.1 (10.6) |
| Median (IQR) | 28.0 (20.0 to 35.0) | 28.0 (21.0 to 36.0) |
| Baseline, n (%) | ||
| Low | 122 (58.9) | 120 (58.5) |
| Medium | 55 (26.6) | 50 (24.4) |
| High | 30 (14.5) | 35 (17.1) |
| 3 months, n (%) | ||
| Low | 101 (56.7) | 103 (57.2) |
| Medium | 41 (23.0) | 53 (29.4) |
| High | 36 (20.2) | 24 (13.3) |
| 12 months, n (%) | ||
| Low | 90 (54.5) | 93 (54.1) |
| Medium | 52 (31.5) | 61 (35.5) |
| High | 23 (13.9) | 18 (10.5) |
| DINE fat intake | ||
| Baseline | ||
| n | 207 | 205 |
| Mean (SD) | 31.5 (11.0) | 32.2 (11.9) |
| Median (IQR) | 31.0 (23.0 to 39.0) | 30.0 (23.0 to 39.0) |
| 3 months | ||
| n | 178 | 180 |
| Mean (SD) | 30.1 (11.4) | 30.2 (11.6) |
| Median (IQR) | 28.0 (22.0 to 36.0) | 29.0 (21.0 to 37.0) |
| 12 months | ||
| n | 165 | 172 |
| Mean (SD) | 30.6 (12.7) | 30.6 (11.6) |
| Median (IQR) | 28.0 (22.0 to 37.0) | 30.0 (22.0 to 37.5) |
| Baseline, n (%) | ||
| Low | 96 (46.4) | 96 (46.8) |
| Medium | 65 (31.4) | 63 (30.7) |
| High | 46 (22.2) | 46 (22.4) |
| 3 months, n (%) | ||
| Low | 99 (55.6) | 97 (53.9) |
| Medium | 49 (27.5) | 52 (28.9) |
| High | 30 (16.9) | 31 (17.2) |
| 12 months, n (%) | ||
| Low | 90 (54.5) | 80 (46.5) |
| Medium | 41 (24.8) | 62 (36.0) |
| High | 34 (20.6) | 30 (17.4) |
| DINE unsaturated fat intake | ||
| Baseline | ||
| n | 207 | 204 |
| Mean (SD) | 9.5 (1.6) | 9.2 (1.9) |
| Median (IQR) | 10.0 (9.0 to 11.0) | 9.0 (8.0 to 11.0) |
| 3 months | ||
| n | 178 | 179 |
| Mean (SD) | 9.6 (1.9) | 9.3 (1.9) |
| Median (IQR) | 10.0 (9.0 to 11.0) | 9.0 (9.0 to 11.0) |
| 12 months | ||
| n | 164 | 171 |
| Mean (SD) | 9.7 (1.8) | 9.4 (1.9) |
| Median (IQR) | 10.0 (9.0 to 11.0) | 10.0 (9.0 to 11.0) |
| Baseline, n (%) | ||
| Low | 6 (2.9) | 12 (5.9) |
| Medium | 93 (44.9) | 94 (45.9) |
| High | 108 (52.2) | 98 (47.8) |
| 3 months, n (%) | ||
| Low | 6 (3.4) | 9 (5.0) |
| Medium | 65 (36.5) | 81 (45.0) |
| High | 107 (60.1) | 89 (49.4) |
| 12 months, n (%) | ||
| Low | 3 (1.8) | 7 (4.1) |
| Medium | 68 (41.2) | 78 (45.3) |
| High | 93 (56.4) | 86 (50.0) |
| Daily sugar intake from drinks (g) | ||
| Baseline | ||
| n | 207 | 205 |
| Mean (SD) | 76.2 (73.1) | 85.4 (85.1) |
| Median (IQR) | 53.0 (25.0 to 102.0) | 56.0 (29.0 to 104.0) |
| 3 months | ||
| n | 178 | 180 |
| Mean (SD) | 70.9 (78.9) | 74.2 (81.0) |
| Median (IQR) | 49.0 (19.0 to 86.0) | 50.0 (22.0 to 93.0) |
| 12 months | ||
| n | 165 | 172 |
| Mean (SD) | 71.8 (90.6) | 66.3 (77.9) |
| Median (IQR) | 37.0 (12.0 to 90.0) | 41.0 (19.0 to 91.0) |
| Weekly alcohol intake (units) | ||
| Baseline | ||
| n | 207 | 205 |
| Mean (SD) | 4.6 (12.6) | 3.0 (7.4) |
| Median (IQR) | 0.5 (0.0 to 3.5) | 0.5 (0.0 to 3.0) |
| 3 months | ||
| n | 178 | 180 |
| Mean (SD) | 3.5 (10.3) | 3.5 (12.8) |
| Median (IQR) | 0.0 (0.0 to 2.5) | 0.0 (0.0 to 2.5) |
| 12 months | ||
| n | 165 | 172 |
| Mean (SD) | 3.7 (9.9) | 4.5 (12.2) |
| Median (IQR) | 0.5 (0.0 to 2.5) | 0.0 (0.0 to 3.5) |
Patient-reported outcome measures and disease severity
Health utility (EQ-5D) and QoL (SF-36) are reported in Tables 19 and 20. Self-reported QoL post randomisation was generally slightly higher in intervention participants (physical functioning, role limitations), but emotional well-being was slightly higher in the control group participants.
Health utility (EuroQol-5 Dimensions)
The main measure of the EQ-5D-5L showed little difference between groups at either time point, although the ‘thermometer’ health scale showed a greater improvement among control participants at 12 months, with a difference of 4.4 points (p = 0.028).
| Dimension | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| EQ-5D-5L health utility | ||||
| Baseline | ||||
| n | 206 | 203 | ||
| Mean (SD) | 0.793 (0.201) | 0.783 (0.187) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 0.815 (0.165) | 0.785 (0.214) | 0.02 (–0.02 to 0.05) | 0.259 |
| 12 months | ||||
| n | 165 | 170 | ||
| Mean (SD) | 0.793 (0.237) | 0.793 (0.239) | –0.02 (–0.06 to 0.03) | 0.495 |
| EQ-5D-5L ‘thermometer’ health state | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 57.9 (20.3) | 54.4 (20.4) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 60.3 (19.8) | 57.7 (19.8) | 0.1 (–3.1 to 3.3) | 0.956 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 58.5 (21.3) | 61.6 (21.1) | –4.4 (–8.4 to –0.5) | 0.028 |
Health utility (Short Form questionnaire-6 Dimensions)
Self-reported QoL using the SF-36 suggested higher QoL post randomisation in intervention participants for physical functioning and bodily pain, but emotional well-being was higher in the control group (Table 20).
| Dimension | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Physical functioning | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 72.8 (25.6) | 71.9 (24.6) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 76.9 (23.1) | 70.9 (26.2) | 4.4 (1.0 to 7.9) | 0.012 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 76.5 (25.6) | 70.9 (26.8) | 4.1 (0.2 to 8.0) | 0.038 |
| Role limitations owing to physical health | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 54.6 (42.0) | 54.2 (41.0) | ||
| 3 months | ||||
| n | 178 | 179 | ||
| Mean (SD) | 63.8 (39.7) | 60.2 (41.3) | 3.3 (–4.8 to 11.4) | 0.425 |
| 12 months | ||||
| n | 164 | 169 | ||
| Mean (SD) | 65.9 (41.8) | 60.8 (40.7) | 4.8 (–3.0, 12.7) | 0.226 |
| Role limitations owing to emotional problems | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 46.2 (42.6) | 44.0 (42.3) | ||
| 3 months | ||||
| n | 178 | 179 | ||
| Mean (SD) | 52.2 (43.8) | 50.1 (44.0) | 1.3 (–7.3 to 9.8) | 0.770 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 58.5 (43.9) | 52.5 (45.4) | 5.1 (–3.6 to 13.8) | 0.251 |
| Energy/fatigue score | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 43.2 (21.8) | 40.5 (23.1) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 44.9 (21.6) | 42.8 (23.6) | –0.6 (–3.6 to 2.5) | 0.720 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 44.9 (22.2) | 45.0 (24.6) | –2.7 (–6.3 to 0.9) | 0.140 |
| Emotional well-being | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 58.3 (21.4) | 56.4 (23.9) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 58.8 (22.3) | 57.0 (25.3) | –0.1 (–3.5 to 3.3) | 0.957 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 58.8 (23.4) | 62.2 (23.4) | –5.3 (–9.0 to –1.7) | 0.004 |
| Social functioning | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 60.5 (29.1) | 61.2 (28.7) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 64.5 (29.6) | 62.7 (31.5) | 2.8 (–2.7 to 8.4) | 0.311 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 66.1 (30.0) | 65.7 (29.5) | 1.5 (–4.2 to 7.1) | 0.615 |
| Bodily pain | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 71.6 (28.8) | 74.8 (27.4) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 75.2 (25.5) | 71.3 (28.4) | 5.5 (0.9 to 10.2) | 0.020 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 71.6 (30.7) | 70.6 (29.1) | 1.4 (–2.9 to 5.7) | 0.534 |
| General health | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 45.0 (20.3) | 44.8 (20.7) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 48.0 (21.8) | 46.8 (20.3) | –0.3 (–3.4 to 2.8) | 0.846 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 49.8 (23.1) | 46.8 (21.4) | 2.1 (–1.5 to 5.6) | 0.249 |
| Health change | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 57.4 (30.2) | 57.4 (29.0) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 62.1 (26.7) | 58.6 (28.9) | ||
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 62.5 (25.3) | 63.4 (26.7) | ||
Condition perception (Brief Illness Perception Questionnaire)
The B-IPQ measured participants’ perceptions of their ‘weight’ condition. The total score showed a small improvement in both groups over time, although the changes on the eight dimensions of the B-IPQ were mixed. There were no significant differences between groups on the total score or for each of the eight dimensions (Table 21).
| Perception | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| B-IPQ overall total | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 5.5 (1.5) | 5.5 (1.7) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 5.0 (1.7) | 5.3 (1.7) | –0.2 (–0.4 to 0.0) | 0.110 |
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 5.0 (1.9) | 5.0 (1.7) | –0.0 (–0.3 to 0.3) | 0.973 |
| How much does your weight problem affect your life? | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 6.1 (2.7) | 6.2 (2.8) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 5.9 (2.8) | 6.1 (2.9) | ||
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 5.9 (3.0) | 5.7 (2.9) | ||
| How long do you think your weight problem will continue? | ||||
| Baseline | ||||
| n | 206 | 202 | ||
| Mean (SD) | 6.1 (2.5) | 6.0 (2.7) | ||
| 3 months | ||||
| n | 176 | 180 | ||
| Mean (SD) | 5.9 (2.4) | 5.9 (2.6) | ||
| 12 months | ||||
| n | 163 | 169 | ||
| Mean (SD) | 5.7 (2.7) | 5.6 (2.9) | ||
| How much control do you feel you have over your weight problem? | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 4.4 (2.6) | 4.1 (2.9) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 5.4 (2.5) | 4.7 (2.8) | ||
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 5.4 (2.6) | 4.9 (3.0) | ||
| How much do you think lifestyle programmes can help your weight problem? | ||||
| Baseline | ||||
| n | 206 | 203 | ||
| Mean (SD) | 7.2 (2.1) | 6.7 (2.6) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 6.8 (2.7) | 5.9 (2.7) | ||
| 12 months | ||||
| n | 163 | 170 | ||
| Mean (SD) | 6.5 (2.7) | 6.0 (3.0) | ||
| How much do you experience symptoms from your weight problem? | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 5.4 (3.0) | 5.2 (3.1) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 4.6 (3.0) | 4.7 (3.2) | ||
| 12 months | ||||
| n | 162 | 170 | ||
| Mean (SD) | 4.9 (3.2) | 4.5 (3.2) | ||
| How concerned are you about your weight problem? | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 7.6 (2.6) | 7.4 (2.7) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 7.0 (2.9) | 7.0 (2.8) | ||
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 6.8 (2.8) | 6.4 (3.2) | ||
| How well do you feel you understand your weight problem? | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 6.4 (2.8) | 6.7 (2.8) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 7.2 (2.6) | 6.8 (2.8) | ||
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 7.1 (2.7) | 6.8 (2.9) | ||
| How much does your weight problem affect you emotionally? | ||||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 6.1 (2.9) | 6.1 (3.4) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 5.5 (3.3) | 6.0 (3.4) | ||
| 12 months | ||||
| n | 164 | 170 | ||
| Mean (SD) | 5.3 (3.2) | 5.2 (3.2) | ||
Psychiatric well-being (Brief Psychiatric Rating Scale and Patient Health Questionnaire-9)
Tables 22 and 23 show scores on the observer-rated BPRS and the patient-completed PHQ-9. The baseline scores of the BPRS of around 30 indicate ‘mild illness’, and the differences between the groups and over time were not clinically significant (see Table 22). Table 23 shows the percentage of participants who scored above the clinical cut-off point (i.e. ≥ 10) for depression on the PHQ-9 at each time point. Although the percentage who scored above the cut-off point reduced in both groups between baseline and 12 months, there were no significant differences between the groups. The mean baseline scores on the PHQ-9 were in the moderate depressive symptom range, and again the differences between the groups and over time were not clinically significant (see Table 23).
| BPRS | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 30.9 (8.8) | 31.5 (9.4) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 30.3 (9.0) | 30.4 (9.4) | 0.2 (–1.3 to 1.7) | 0.827 |
| 12 months | ||||
| n | 165 | 170 | ||
| Mean (SD) | 29.1 (9.7) | 28.3 (9.5) | 1.0 (–0.9 to 2.9) | 0.303 |
| PHQ-9 scores | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Baseline | ||||
| n | 207 | 204 | ||
| Mean (SD) | 10.6 (6.3) | 11.0 (6.8) | ||
| PHQ-9 score of ≥ 10, n (%) | 106 (51.2) | 116 (56.9) | ||
| 3 months | ||||
| n | 178 | 180 | ||
| Mean (SD) | 10.3 (6.3) | 10.1 (7.1) | 0.5 (–0.4 to 1.3) | 0.314 |
| PHQ-9 score of ≥ 10, n (%) | 94 (52.8) | 94 (52.2) | ||
| 12 months | ||||
| n | 165 | 170 | ||
| Mean (SD) | 9.9 (7.0) | 9.6 (6.6) | 0.5 (–0.4 to 1.5) | 0.299 |
| PHQ-9 score of ≥ 10, n (%) | 74 (44.8) | 79 (46.5) | ||
Smoking status
At baseline, approximately half of the participants were current smokers (Table 24). Smoking rates did not change during the trial in either group.
| Smoking status | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| Baseline | ||
| Smoking status | N = 207 | N = 205 |
| Current smoker | 98 (47.3) | 108 (52.7) |
| Light smoker | 18 (8.7) | 20 (9.8) |
| Moderate smoker | 37 (17.9) | 32 (15.6) |
| Heavy smoker | 43 (20.8) | 55 (26.8) |
| Amount not reported | 0 (0.0) | 1 (0.5) |
| Offered help to stop smoking | 89 (47.3) | 90 (47.1) |
| Brief intervention | 38 (20.2) | 53 (27.7) |
| Nicotine replacement | 58 (30.9) | 61 (31.9) |
| Drug treatment | 3 (1.6) | 4 (2.1) |
| Electronic cigarettes/vape | 5 (2.7) | 3 (1.6) |
| Other types | 8 (4.3) | 6 (3.1) |
| Currently using therapy to stop smoking | 24 (12.8) | 25 (13.1) |
| Nicotine replacement | 15 (8.0) | 013 (6.8) |
| Drug treatment | 0 | 0 |
| Electronic cigarettes/vape | 10 (5.3) | 12 (6.3) |
| Other types | 9 (4.8) | 12 (6.3) |
| 3 months | ||
| Smoking status | N = 178 | N = 180 |
| Current smoker | 85 (47.8) | 96 (53.3) |
| Light smoker | 13 (7.3) | 19 (10.6) |
| Moderate smoker | 28 (15.7) | 31 (17.2) |
| Heavy smoker | 44 (24.7) | 46 (25.6) |
| Offered help to stop smoking | 78 (47.0) | 71 (42.5) |
| Brief intervention | 39 (23.5) | 39 (23.4) |
| Nicotine replacement | 55 (33.1) | 54 (32.3) |
| Drug treatment | 4 (2.4) | 2 (1.2) |
| Electronic cigarettes/vape | 5 (3.0) | 1 (0.6) |
| Other types | 2 (1.2) | 2 (1.2) |
| Currently using therapy to stop smoking | 21 (12.7) | 19 (11.4) |
| Nicotine replacement | 11 (6.6) | 13 (7.8) |
| Drug treatment | 0 | 0 |
| Electronic cigarettes/vape | 10 (6.0) | 6 (3.6) |
| Other types | 9 (5.4) | 6 (3.6) |
| 12 months | ||
| Smoking status | N = 166 | N = 172 |
| Current smoker | 75 (45.2) | 88 (51.2) |
| Light smoker | 17 (10.2) | 13 (7.6) |
| Moderate smoker | 29 (17.5) | 27 (15.7) |
| Heavy smoker | 29 (17.5) | 48 (27.9) |
| Offered help to stop smoking | 64 (45.1) | 63 (42.9) |
| Brief intervention | 27 (19.0) | 27 (18.4) |
| Nicotine replacement | 47 (33.1) | 42 (28.6) |
| Drug treatment | 1 (0.7) | 3 (2.0) |
| Electronic cigarettes/vape | 3 (2.1) | 3 (2.0) |
| Other types | 6 (4.2) | 2 (1.4) |
| Currently using therapy to stop smoking | 22 (15.4) | 11 (7.5) |
| Nicotine replacement | 12 (8.4) | 6 (4.1) |
| Drug treatment | 0 | 0 |
| Electronic cigarettes/vape | 10 (7.0) | 4 (2.7) |
| Other types | 9 (6.3) | 5 (3.4) |
Weight loss programmes
During the trial, 25 participants [(7.4%) eight control, 17 intervention] reported attending one or more weight loss programmes outside the trial (Table 25).
| Use of weight loss programme | Trial arm | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| 3 months | ||
| Followed up | N = 178 | N = 180 |
| Attended any weight loss programme | 4 (2.5%) | 8 (4.4%) |
| Slimming World® (Alfreton, UK) | 2 | 1 |
| Weight Watchers® (New York, NY, USA) | 0 | 3 |
| Structured programme organised by GP/care team | 1 | 3 |
| Other | 1 | 1 |
| 12 months | ||
| Followed up | N = 165 | N = 170 |
| Attended any weight loss programme | 17 (10.3%) | 8 (4.7%) |
| Slimming World | 8 | 3 |
| Weight Watchers | 4 | 1 |
| Structured programme organised by GP/care team | 3 | 4 |
| Other | 4 | 0 |
Ten-year cardiovascular and diabetes risk
The Framingham cardiovascular risk scores are presented in Table 26. Twenty-six participants in each trial arm had a pre-existing cardiovascular diagnosis and 84 (40 intervention, 44 control) were aged < 30 years; the Framingham risk score is not defined for these subgroups. The mean ‘desk’ Framingham 10-year risk (which does not incorporate laboratory measures) was 10.9% for the intervention group and 10.6% for the control group at baseline, and remained similar at both 3 months and 12 months. The mean laboratory-based Framingham scores were substantially lower in the control group at baseline (7.7% vs. 8.6%), and both demonstrated minor change (< 1%) at 12 months. Only one participant in the intervention arm developed a cardiac event during the trial; this individual died.
| Risk measure | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Aged 30–74 years and no cardiovascular condition, n (%) | 142 (68.6) | 136 (66.3) | ||
| Framingham % cardiovascular risk score (excluding laboratory measures) | ||||
| Baseline | ||||
| n | 142 | 135 | ||
| Mean (SD) | 10.9 (9.5) | 10.6 (10.9) | ||
| 3 months | ||||
| n | 123 | 120 | ||
| Mean (SD) | 10.8 (8.6) | 10.7 (10.5) | ||
| Change at 3 months | ||||
| n | 123 | 120 | ||
| Mean (SD) | –0.5 (3.8) | –0.5 (4.4) | 0.0 (–0.8 to 0.9) | 0.986 |
| 12 months | ||||
| n | 114 | 123 | ||
| Mean (SD) | 10.9 (9.6) | 10.5 (10.5) | ||
| Change at 12 months | ||||
| n | 112 | 118 | ||
| Mean (SD) | –0.3 (3.9) | –0.3 (4.4) | –0.1 (–1.1 to 1.0) | 0.905 |
| Framingham % cardiovascular risk score: including laboratory measures | ||||
| Baseline | ||||
| n | 120 | 117 | ||
| Mean (SD) | 8.7 (8.2) | 7.6 (6.3) | ||
| 12 months | ||||
| n | 100 | 105 | ||
| Mean (SD) | 8.6 (7.7) | 7.6 (6.3) | ||
| Change at 12 months | ||||
| n | 87 | 90 | ||
| Mean (SD) | –0.7 (4.0) | –0.4 (4.1) | –0.2 (–1.2 to 0.8) | 0.690 |
Table 27 shows that 60 participants (35 in the intervention group and 25 in the control) group had diabetes on entry to the trial. Among the remainder, the 10-year risk of developing diabetes was 17.2% in both trial arms at baseline, with over half being in the ‘moderate’ risk category. By 12 months, the risk had decreased by 0.5% in the intervention arm and by 0.4% in the control arm. No participant was recorded as having developed diabetes during the trial, although one participant (intervention arm) was hospitalised twice because of diabetes-related complications.
| Risk measure | Trial arm | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Has diabetes mellitus, n (%) | 35 (16.9) | 25 (12.2) | ||
| Diabetes UK 10-year diabetes mellitus risk (%) | ||||
| Baseline | ||||
| n | 171 | 180 | ||
| Mean (SD) | 17.2 (6.2) | 17.2 (6.2) | ||
| Diabetes UK 10-year diabetes mellitus risk group, n (%) | ||||
| Low | 3 (1.5) | 6 (2.9) | ||
| Increased | 65 (31.6) | 68 (33.3) | ||
| Moderate | 111 (53.9) | 109 (53.4) | ||
| High | 27 (13.1) | 21 (10.3) | ||
| Diabetes UK 10-year diabetes mellitus risk (%): 3 months | ||||
| n | 150 | 159 | ||
| Mean (SD) | 16.8 (6.1) | 17.3 (6.2) | ||
| Change at 3 months | ||||
| n | 150 | 159 | ||
| Mean (SD) | –0.1 (1.8) | –0.1 (1.8) | –0.0 (–0.4 to 0.3) | 0.940 |
| Diabetes UK 10-year diabetes mellitus risk group: 3 months, n (%) | ||||
| Low | 5 (2.8) | 5 (2.8) | ||
| Increased | 57 (32.0) | 58 (32.0) | ||
| Moderate | 97 (54.5) | 100 (55.2) | ||
| High | 19 (10.7) | 18 (9.9) | ||
| Diabetes UK 10-year diabetes mellitus risk (%): 12 months | ||||
| n | 141 | 154 | ||
| Mean (SD) | 16.6 (6.6) | 16.9 (6.6) | ||
| Change at 12 months | ||||
| n | 141 | 154 | ||
| Mean (SD) | –0.5 (2.8) | –0.4 (2.9) | 0.0 (–0.7 to 0.8) | 0.896 |
| Diabetes UK 10-year diabetes mellitus risk group: 12 months, n (%) | ||||
| Low | 9 (5.4) | 10 (5.8) | ||
| Increased | 54 (32.1) | 54 (31.2) | ||
| Moderate | 86 (51.2) | 89 (51.4) | ||
| High | 19 (11.3) | 20 (11.6) | ||
Change in medications
During the trial, the vast majority of participants remained on antipsychotics (Table 28), although around one-fifth of participants in both groups experienced a change of antipsychotic treatment. Approximately half of participants in both groups were treated with antidepressants. By the end of the trial, 17% of intervention arm participants and 15% of control arm participants had experienced a change of antidepressant medication.
| Psychiatric medication | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |
| Antipsychotics | ||
| Antipsychotics changed by 3 months | 9 (5.0) | 21 (11.6) |
| Antipsychotics changed by 12 months | 24 (14.3) | 29 (16.7) |
| Antidepressants | ||
| On antidepressants at 3 months | 112 (62.9) | 111 (61.3) |
| Antidepressants changed by 3 months | 11 (6.1) | 10 (5.5) |
| On antidepressants at 12 months | 111 (66.1) | 105 (60.7) |
| Antidepressants changed by 12 months | 29 (17.3) | 27 (15.4) |
Session feedback forms and centre outcomes
Session feedback
Intervention group participants could provide anonymous feedback following each group session. It was not possible to link feedback to their clinical outcomes, but the feedback could be linked to the study centre results. Seven hundred and eight session feedback forms were returned after the seven group sessions across the 10 centres, although we did not know who responded or on how many occasions. Therefore, the numbers and percentages in the tables below are based on ‘forms returned’ rather than on ‘participants’. Five of the returned forms had no study centre identifiers and were excluded. When two responses were indicated (n = 3), the higher score was taken, and missing responses (n = 10) were replaced with the mean of the participants’ other responses.
Table 29 shows that the vast majority of responses were positive for all six statements, with almost 90% ‘agreeing’ with each statement either ‘strongly’ or scoring ‘2’ on a Likert scale of 5. A total of 87.2% of participants strongly agreed or agreed that the sessions had met their needs.
Participants had the opportunity to add comments to their forms, although only four did so. Three of these comments were positive:
I felt this has been a fantastic group and really looking forward to meeting up in 3 months’ time.
One thing that went well? Talking together about different weight control.
What went well. Everything except the cold.
The only negative comment made was:
Mental health as bad as ever which affects my answer.
Session feedback by study centre
Session feedback scores could range from 6 to 30, with 6 indicating the most positive response on all five items and 30 indicating the most negative response on all items. Table 29 shows the average feedback scores across the 10 centres and Table 30 shows the average scores by centre. Feedback was similar across the centres: in most centres > 50% of forms recorded the most positive score of 6 and in all centres at least 75% of forms recorded scores below 12 (equivalent to scoring 2 for each statement). There were no significant correlations between mean weight change and mean feedback scores for centres, at 3 months or 12 months (Spearman’s rank-order correlation = –0.20; p = 0.476, and Spearman’s rank-order correlation = 0.042; p = 0.454, respectively).
| Question | Responses (%) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| (strongly agree) | (neither agree nor disagree) | (strongly disagree) | |||
| The facilitator listened to me | 77.1 | 12.5 | 2.0 | 2.0 | 6.4 |
| I understood what we talked about | 74.4 | 13.9 | 3.0 | 2.3 | 6.4 |
| I found what we talked about useful | 71.8 | 17.1 | 2.7 | 2.6 | 5.8 |
| I felt the facilitator understood the challenges I face | 66.1 | 20.3 | 5.1 | 3.0 | 5.4 |
| What we talked about made sense to me | 76.7 | 12.4 | 2.4 | 3.3 | 5.3 |
| Overall the session met my needs | 67.3 | 19.9 | 4.7 | 3.1 | 5.0 |
| Site | Number of forms returned | Total score | IQR | Score of, % | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Median | 6 (most positive) | > 24 (most negative) | |||
| Sheffield | 51 | 7.9 (4.3) | 7 | 6–8 | 47.1 | 3.9 |
| Leeds and York | 39 | 8.1 (4.2) | 6 | 6–10 | 64.1 | 2.6 |
| Bradford | 70 | 10.6 (8.5) | 6 | 6–11 | 58.6 | 15.7 |
| Manchester | 84 | 7.7 (3.6) | 6 | 6–9 | 61.9 | 1.2 |
| South London | 61 | 10.1 (7.1) | 6 | 6–11 | 54.1 | 8.2 |
| Sussex | 105 | 9.6 (5.8) | 7 | 6–11 | 45.7 | 5.7 |
| Southern Health | 116 | 9.2 (6.3) | 6 | 6–9 | 57.8 | 7.8 |
| Devon | 69 | 9.7 (7.0) | 6 | 6–10 | 56.5 | 10.1 |
| Somerset | 26 | 7.6 (3.0) | 6 | 6–8 | 65.4 | 0 |
| Cornwall | 82 | 9.9 (6.1) | 8 | 6–11 | 41.5 | 7.3 |
| Overalla | 703 | 9.2 (6.1) | 6 | 6–10 | 54.1 | 6.8 |
Adverse events
The adverse events are summarised in Table 31. Most were expected and related to the psychiatric illness. The majority were hospitalisations for psychiatric reasons. Serious adverse events were assessed for relatedness and two were considered to be possibly related to the intervention, as described below.
| Adverse event | Trial arm | |||
|---|---|---|---|---|
| Intervention (N = 207) | Control (N = 205) | |||
| Number of events | Number (%) of participants | Number of events | Number (%) of participants | |
| Any adverse event | 46 | 37 (17.9) | 34 | 26 (12.7) |
| Psychiatric hospitalisation | 23 | 20 (9.7) | 17 | 16 (7.8) |
| Self-harm | 0 | 1 | 1 (0.55) | |
| Suicide attempt | 2 | 2 (1.0) | 2 | 2 (1.0) |
| Hospitalisation (not mental health related) | 13 | 11 (5.3) | 11 | 10 (4.9) |
| Death | 3 | 3 (1.4) | 0 | |
| Skin reaction to accelerometer | 4 | 4 (1.9) | 0 | |
| Other | 1 | 1 (0.5) | 3 | 3 (1.5) |
Thirty-six participants (20 in the intervention group and 16 in the control group) were admitted to hospital for mental health reasons (specified as ‘expected’ in the protocol) during the trial. One admission was deemed to be possibly trial related; the patient was admitted following a relapse after stopping their medication in an attempt to control their weight. The participant was randomised to the intervention arm, but did not attend any of the sessions.
Four participants (two in each trial arm) were reported to have attempted suicide (specified as ‘expected’ in the protocol) and 21 participants were hospitalised (11 in the intervention group and 10 in the control group) for non-mental health-related reasons. None of these events was considered to be related to the study procedures.
Participant deaths
Four deaths were reported, all in the intervention group. Three deaths occurred during the trial and one occurred several months after the trial ended. Detailed information is given below.
Patient 1 was a 53-year-old white man with a BMI of 34 kg/m2 and concurrent diagnoses of asthma and depression, who had been on antipsychotic medication for 32 years. His death occurred 264 days after randomisation, after he had attended four foundation sessions and two booster sessions. The cause of death was a pulmonary embolism, which is likely to have developed secondarily to a ruptured Achilles tendon.
Prior to the study, the participant reported walking a lot, achieving more than 10,000 steps per day, as assessed by pedometer. Objective accelerometry data indicated that he had not changed his physical activity after the intervention. Nevertheless, as a ruptured Achilles tendon could have occurred as a result of following advice from the intervention, the relationship was reported to the ethics committee as possibly intervention related. This death was therefore reported within 15 days to the research ethics committee, which determined that no further action was required.
Patient 2 was a 48-year-old white woman with a BMI of 40 kg/m2 and concurrent diagnoses of hypertension and dyslipidaemia. She had been taking antipsychotic medication for 22 years. Her death occurred 260 days after randomisation, after she had attended three foundation sessions. The cause of death was determined by the coroner as 1a) left ventricular hypertrophy, 1b) hypertension and obesity. The death was unrelated to the intervention.
Patient 3 was a 35-year-old black woman with a BMI of 38 kg/m2 and a concurrent diagnosis of type 2 diabetes mellitus who had been on antipsychotic medication for 7 years. Her death occurred 57 days after randomisation after four foundation sessions. Her diabetes mellitus had been treated with oral antidiabetes agents, but not sodium–glucose co-transporter-2 inhibitors. She was found unresponsive at her home and the death was determined as resulting from diabetic ketoacidosis leading to cardiac arrest. The death was unrelated to the intervention.
Patient 4 was a 36-year-old Asian woman with a BMI of 36 kg/m2 and a concurrent diagnosis of type 2 diabetes mellitus who had been taking antipsychotic medication for 10 years. She died from a myocardial infarction 1 month after she completed the study, while she was detained under section 3 of the Mental Health Act 1983. 111 She had been under the care of the local cardiology team, which advised that she stop taking clozapine, which precipitated the deterioration in her mental health. The death was unrelated to the intervention.
Chapter 5 Results of the process evaluation
Context
Context, sometimes defined as ‘settings, roles, interactions and relationships’,112 affects the implementation of complex interventions. Figure 19 shows contextual factors that affect the implementation of, and engagement with, the STEPWISE intervention, coded in accordance with the ICF conceptual framework. 76
FIGURE 19.
Context understood through the ICF conceptual framework. Reprinted from the WHO’s Towards a Common Language for Functioning, Disability and Health ICF;76 diagram entitled ‘model of disability’ on page 9. WHO reference number: WHO/EIP/GPE/CAS/01.3. Copyright 2002.
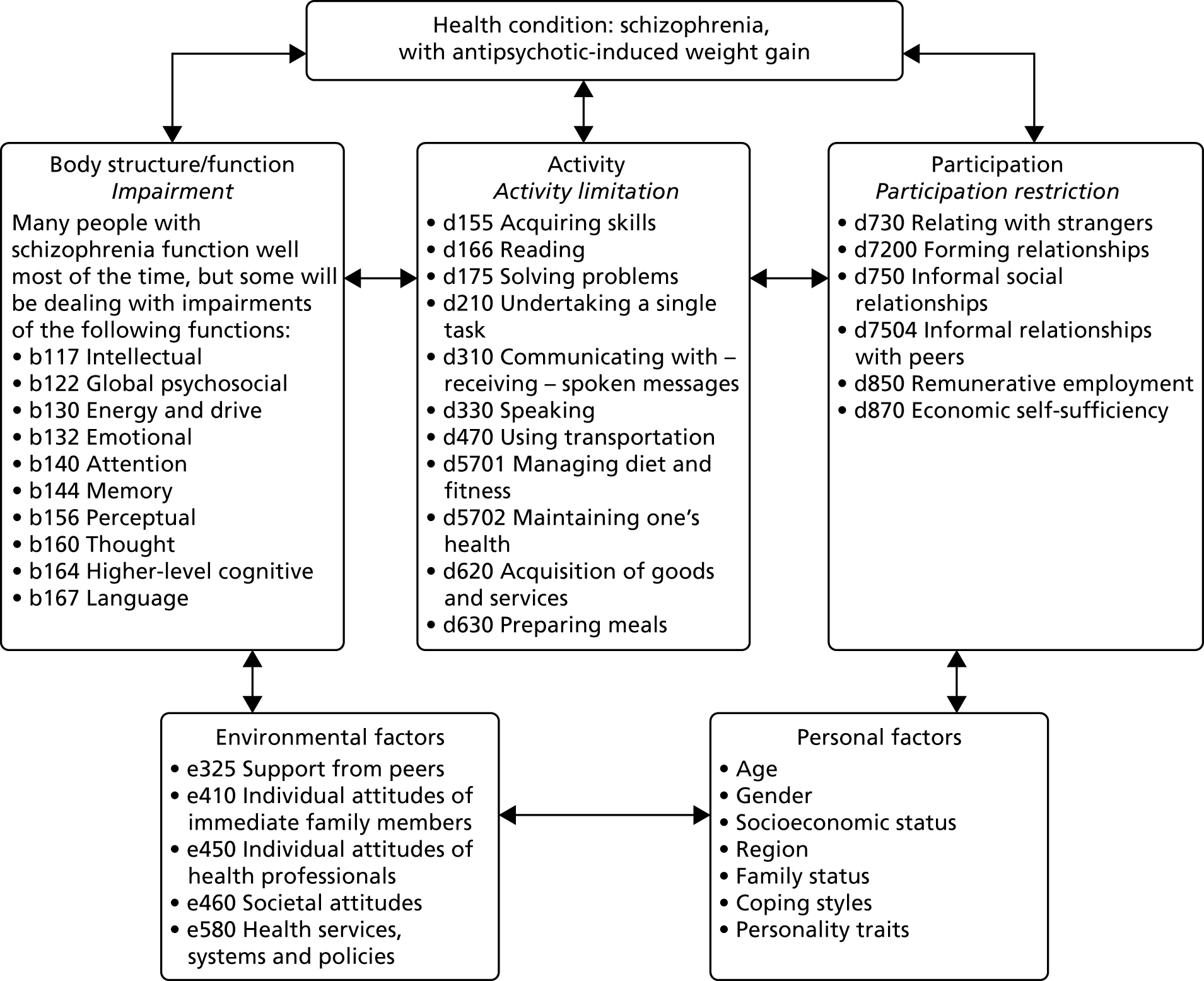
Body functions
Half of the facilitators interviewed commented that, although most participants were receptive to the intervention, an individual’s psychosocial functioning could affect how well they could engage with STEPWISE. Facilitators discussed this most frequently in terms of the transient severity of hallucinatory or delusional symptoms associated with paranoid schizophrenia; some also spoke of comorbid learning disabilities, implying problems with attention, memory, goal-directed/logical thought, insight, executive function or language, which are commonly associated with disorganised-type schizophrenia:
. . . when we’ve gone out together we’ve all been discussing . . . university degrees they’ve got . . . they are really quite intelligent people but they still do have a psychotic illness . . . they could be distracted if there’s something on their mind, or if they’re paranoid at the time . . . that has to be taken into consideration a little bit more when you’re planning.
Facilitator S03/F02
We’ve got quite a small group and one of the persons in the group . . . he has a mild learning difficulty and I’m not sure that he’s quite sort of clear about what, why he’s there particularly sometimes, which is difficult.
Facilitator S06/F05
I think some of them have mild learning disabilities and umm I’m not sure that you know they take in everything we tell them in the session.
Facilitator S09/F01
There could be like mild learning disabilities running alongside their mental illness as well and that, I have definitely noticed that that’s hindered . . . someone’s ability to engage.
Facilitator S09/F03
In addition, developers characterised this group as sometimes less interactive than other populations and occasionally somnolent or impulsive:
. . . there’s been a couple of occasions where people do seem a bit overmedicated . . . Asleep . . . or saying very random things that really have no you know no connection to what is being talked about.
Developer D05
We all said that the medication that we’re on, different people different reactions, but quite a few of them said you know, I’m sluggish from the sedation. And it’s hard to wake you up in the morning.
Participant S08/Q06
. . . so when the sandwiches came out there was definitely some people [sic.] it was literally . . . automatic that reaching for sandwiches and eating and reaching for another sandwich . . . and thinking do you know, ‘have you noticed how many of those sandwiches that you’ve eaten?’.
Developer D03
Activities and participation
Cognitive problems meant that some participants had problems learning and applying knowledge, specifically, acquiring skills, reading and problem-solving. Undertaking tasks could take longer than expected, and that could be difficult:
We did have to sort of adjust to the people that were in the group . . . the ones that were struggling with the reading . . . we would just sort of put more time in with them.
Facilitator S09/F01
Costs notwithstanding, facilitators felt that the use of taxis was essential for attendance; the barriers to using transportation were great for those with severe symptoms or poor cognition, and many lived in areas that were poorly served by public transport. Aside from managing diet and fitness, the principal objective of STEPWISE, a self-care theme that often arose was the importance of adherence to antipsychotic medication in the face of weight gain:
Quite a few service users get put off continuing to take their medication because of the side effects and this gives them a way to control it so it isn’t only kind of benefiting their physical health, but it will benefit their mental health as well if it’s helping them to stay on the medication.
Facilitator S06/F01
In some cases, family or residential care staff were responsible for shopping and food preparation. Meeting strangers and forming and conducting informal social relationships could cause anxiety. Some had never met others with their condition; others talked about the difficulty of discussing antipsychotic-induced weight gain at Weight Watchers owing to the stigma of mental illness:
. . . they are finally coming in to a group of people they have never met. It’s quite anxiety provoking for them, isn’t it?
Facilitator S06/F04
So, the STEPWISE programme was really the first time I had had exposure to other service users.
Participant S09/Q01
I would never have said anything [at Weight Watchers] about my medication sort of being a contributor to me putting on weight because they’d say ‘What is it?’ and . . . I would’ve felt pressure to tell them. And then it was like a ‘oh no I’ve told them I’m mentally ill now’ sort of thing.
Participant S10/Q03
Many STEPWISE attendees were not in remunerative employment and some were living in economically difficult circumstances:
You end up having more of a sedentary lifestyle because you’re told you’re not fit to work.
Participant S08/Q09
I was struck by some of the levels of deprivation . . . there was somebody who was living in a caravan. Their caravan had been washed away in the floods so they actually didn’t have anywhere to live . . . they were there at the group, which was pretty amazing, but if you think about . . . where this fits on their . . . continuum of needs . . . some people are just getting by.
Developer D03
Environmental factors
The attitudes of family members were not always supportive vis-à-vis lifestyle changes, and stigmatising treatment by other members of the public was reported:
They found these sessions were the opportunity for them do it for themselves instead of having someone else there . . . [one of] the participants brought someone with them . . . it was a relative and you could see, there was a lot of . . . judgemental attitude, like, ‘oh, you should be doing this,’ or, ‘you shouldn’t be doing that,’ and . . . ‘why are you doing this?’ and it was a lot of pressure on that participant.
Developer 01
The individual attitudes of facilitators were characterised by developers as being generally sensitive to the needs of an autonomy-enabled approach to behaviour change, with only a small minority being overly didactic in delivery. The following quotation from a facilitator is illustrative of how many facilitators characterised their roles, interactions and relationships with clients:
Going to an acute [psychiatric] ward and you’re saying to the person take these tablets, they’ll turn around and say to you, ‘why should I?’ Whereas, if you’ve got somebody who’s got a general illness they just sit there and put them in their mouth [laughs] . . . you get used to having to discuss things with people and in that respect I suppose it was natural for me to do things that way [i.e. in accordance with the STEPWISE philosophy] . . . you’ve got to be more open-minded, I think, with people with mental health . . . a lot of the people you’re talking to are extremely intelligent people and so therefore you do respect them . . . they’re not an illness, they’re a person [with] need, wants feelings, ways of doing things . . .
Facilitator S03/F02
However, some facilitators thought that health professionals who were meant to be referring patients to STEPWISE (‘gatekeepers’) sometimes erected barriers to patient engagement:
A lot of clinicians have said their clients don’t like group work or they don’t like to leave their home and they’ve put a lot of barriers in the way of people engaging.
Facilitator S06/F01
Interviewees described the availability of, and referral patterns associated with, existing lifestyle interventions as ad hoc, fragmented and locally idiosyncratic, often involving referral to other organisations. This was confirmed by our survey of usual care. 36 Monitoring of weight was not systematic for this population in all trusts, and, although all trusts talked about physical health as a priority, scarcity of resources was a problem, although quality-improvement initiatives were changing this in some cases (see also Sense-making, Operational work and Appraisal work).
How context might affect the success of the intervention
Facilitator views of STEPWISE and Implementation process concentrate on how organisational context affects outcome. To more vividly illustrate and explore potential context effects (see also Table 4), we present six vignettes of interviewees from the intervention arm who experienced clinically important weight gain (Box 1) and weight loss (Box 2). These vignettes suggest that, although weight control is possible in people with first episode psychosis and in people living in the most deprived neighbourhoods (S04/Q02), it is particularly difficult for those with first episode psychosis (S04/Q08, S04/Q12, S07/Q06). Those with clinically important weight loss may have less severe psychiatric symptoms and comorbidities (S04/Q02; BPRS score of 34) than those with clinically important weight gain (S04/Q08; BPRS score of 44). Those who experienced clinically important weight loss generally talk about needing more ongoing support or, especially, closer monitoring of behaviour change enactment and outcomes, as if they are more alert to the risk of relapse.
Woman, 32 years old, ethnic white British, unemployed, neighbourhood in the 20% most deprived. Gained 13.6 kg in the first 3 months and a further 16.1 kg thereafter (total weight gain of 29.7 kg). Diagnosed comorbidities: depression, asthma and an alcohol problem; first-degree relatives with diabetes mellitus or a heart condition. First episode psychosis (2 years since diagnosis). BPRS score of 44 (moderately ill). Moderately severe somatic concern, anxiety and hallucinatory behaviour. Moderate depressive mood. Mild suspiciousness. Very mild emotional withdrawal, conceptual disorganisation, tension, mannerisms, hostility, motor retardation, unco-operativeness, blunted affect and excitement. Reported a high level of acceptability of the intervention, focusing on self-monitoring and social support elements, but wanted more sessions. Enjoyed small group size owing to self-reported anxiety. Reported losing weight during the foundation course but putting on weight thereafter. Reported using the taxis provided, despite living 5 minutes’ drive from the venue.
S04/Q12Man, 30 years old, ethnic Indian, unemployed, neighbourhood in the 10% most deprived. Gained 4.3 kg in the first 3 months and a further 1.2 kg thereafter (total weight gain of 5.5 kg). No comorbidities. First episode psychosis (2 years since diagnosis). BPRS score of 26 (mildly ill). Moderate motor retardation. Mild emotional withdrawal and blunted affect. Very mild unco-operativeness. Reported finding the intervention acceptable, but remarked ‘I think it needed more talking about . . . all you can do in the gym, like, to lose weight faster’. Preferred smaller groups.
S07/Q06Man, 25 years old, ethnic white British, unemployed, neighbourhood in the 50% most deprived. Gained 8.4 kg in the first 3 months and a further 2.9 kg thereafter (total weight gain of 11.3 kg). First episode psychosis (9 months since diagnosis). Diagnosed with depression and irritable bowel syndrome. BPRS score of 34 (mildly ill). Severe hallucinatory behaviour. Moderate anxiety, guilty feelings, depressive mood. Mild somatic concern. Focused on dietary aspects of the intervention only.
Woman, 33 years old, ethnic African, unemployed, neighbourhood in the 10% most deprived. Gained 1.2 kg in the first 3 months and lost 7.3 kg thereafter (total weight loss of 6.1 kg). Diagnosed with hypertension and depression. First episode psychosis (7 months since diagnosis). BPRS score of 34 (mildly ill). Moderate somatic concern and blunted affect. Mild anxiety, emotional withdrawal, unco-operativeness. Very mild guilty feelings, mannerisms, depressive mood, suspiciousness. ‘It needs to be longer . . . maybe like 10 weeks . . . give us updates on how we are coping, on what we are doing . . . to monitor us more closely . . . it was becoming a routine and then it just stopped . . . the questions were important because they, we needed to know if there’s any downfalls . . . challenges . . .’.
S03/Q06Man, 35 years old, ethnic white British, unemployed, neighbourhood in the 10% most deprived. Lost 3.8 kg in the first 3 months and lost a further 13.4 kg thereafter (total weight loss of 17.2 kg). Diagnosed with asthma. ICD-10 F20. BPRS score of 34 (mildly ill). Moderate emotional withdrawal, blunted affect. Mild somatic concern, anxiety, conceptual disorganisation, depressive mood. Very mild guilty feelings, tension. ‘It helped . . . [to] be aware of my eating habits . . . it’s important to do exercise in order to lose the weight . . . it’s been quite a lot of time in between doing the course and the refresher course . . . so I’m feeling a bit less motivation with the dieting’.
S08/Q05Woman, 28 years old, ethnic white British, unemployed, neighbourhood in the 50% most deprived. Gained 1.5 kg in the first 3 months and lost a further 10.8 kg thereafter (total weight loss of 9.3 kg). Diagnosed with having an alcohol problem. ICD-10 F25. BPRS score of 41 (moderately ill). Moderately severe anxiety, hallucinatory behaviour, unusual thought content. Mild somatic concern, hostility. Very mild emotional withdrawal, guilt feelings, tension, grandiosity, depressive mood, suspiciousness, blunted affect. Talked in compelling detail about enactment of exercise strategies, demonstrating understanding and application of STEPWISE content; also, talked about the need for, ‘more regular support . . . see how you’re doing, because . . . the next session’s quite far away’.
The intervention’s theory of change
As part of the process evaluation, the intervention development team at LDC was asked to use a behaviour change techniques taxonomy25,95 to identify active ingredients within the STEPWISE intervention (Table 32). 95,113 Although the STEPWISE intervention is inspired by a range of behaviour change theories, in conversation with LDC staff, we established that it has a systematic basis in three in particular. 114 The first is self-efficacy theory (Figure 20), which posits that the central mechanism behind behaviour change is a person’s belief that they can change. 85 Interventions can boost those beliefs by confronting someone with personal experiences of their success, other people’s experiences of success, verbal persuasion about their capability and arousing their emotions. The second theory, the self-regulatory model of illness behaviour (Figure 21), proposes that the interpretation of symptoms and social messages, coping strategies and the appraisal of their effectiveness all interact, and are all targets for intervention. 115 The third and final theory, the relapse prevention model (Figure 22), aims to maintain abstinence from problem behaviours by helping the client to develop coping responses and perceived self-efficacy. 116 On a day-to-day basis, the LDC team talked rather more about empowerment philosophy than the three behaviour change theories. Empowerment philosophy is ‘based on the premise that human beings have the capacity to make choices and are responsible for the consequences of their choices’. 117 It is also the difference between what drives the style of delivery; empowerment philosophy maintains that people are autonomous decision-makers and not purely driven by information provision. Self-reflection supports change, whereas behaviour change theory supports the content of the intervention. It is better understood as a conceptual model rather than a middle-range theory118 of the kind associated with health psychology and behaviour change,114,119 being comparatively vague in terms of how change is to be effected. The section above, How context might affect the success of the intervention, and the vignettes in Box 2 suggest that some participants might welcome more sustained support, so care must be taken to ensure that empowerment does not translate to a demand for patients to self-manage in isolation. 120,121
| Behaviour change techniques | Behaviour change theory: construct | Demonstrated in the programme |
|---|---|---|
| 1. Goals and planning | ||
| 1.1 Goal-setting behaviour |
SET: VE (modelling) SET: VP (suggestion; self-instruction) SET: OE CSM: coping procedures (approach) RP: coping |
|
| 1.2 Problem-solving |
SET: VE (modelling) SET: VP (suggestion; self-instruction) CSM: coping procedures (approach) RP: coping |
|
| 1.4 Action-planning |
SET: VE (modelling) SET: VP (suggestion; self-instruction) CSM: coping procedures (approach) RP: coping |
|
| 1.5 Review behaviour goals |
SET: VE (modelling) SET: VP (suggestion; self-instruction) SET: EA (symbolic exposure/desensitisation) CSM: appraisal RP: review behaviour goals |
Sharing stories asks to review goals but not specifically reset unless it is revisited in the ‘next steps’ (sessions 2–4, booster sessions 1–3) |
| 2. Feedback and monitoring | ||
| 2.3 Self-monitoring of behaviour |
SET: EME (performance exposure; self-instructed performance) SET: VP (self-instruction) SET: EA (symbolic exposure) CSM: appraisal CSM: coping procedures (approach) RP: self-monitoring RP: behaviour assessment |
Encourage use of food diaries/pedometers and physical activity diaries
|
| 2.4 Self-monitoring of outcomes of behaviour |
SET: EME (performance exposure; self-instructed performance); VP (self-instruction) CSM: appraisal CSM: coping procedures (approach) |
|
| 3. Social support | ||
| 3.1 Social support (unspecified) | CSM: coping procedures (approach) |
|
| 4. Shaping knowledge | ||
| 4.1 Instruction on how to perform the behaviour |
SET: VE (symbolic modelling) SET: VP (suggestion) |
|
| 4.2 Information about antecedents |
SET: EME (performance exposure) SET: VE (live and symbolic modelling) CSM: emotion (food diary) CSM: emotion (high risk) RP: self-monitoring |
|
| 4.4 Behavioural experiments |
SET: EME (self-instructed performance) SET: OE SET: VE (live and symbolic modelling) CSM: appraisal |
|
| 5. Natural consequences | ||
| 5.1 Information about health consequences |
SET: VE (symbolic modelling) SET: PAS (symbolic exposure) SET: OE CSM: illness representations RP: education about effects of substance |
|
| 5.6 Information about emotional consequences |
SET: VE (symbolic modelling) SET: VP (suggestion) SET: PAS (attribution, symbolic exposure) SET: OE CSM: ER |
|
| 6. Comparison of behaviour | ||
| 6.2 Social comparison |
SET: VE (live and symbolic modelling) SET: OE |
|
| 7. Associations | ||
| 7.1 Prompts |
CSM: coping procedure (approach) RP: coping |
|
| 7.4 Remove access to the reward |
SET: VE (live and symbolic modelling) VP (self-instruction) RP: reminder – what to do if you slip |
|
| 8. Repetition and substitution | ||
| 8.2 Behavioural substitution |
SET: VP (suggestion; self-instruction) CSM: coping procedure (approach) |
Prompt group to come up with these throughout programme:
|
| 8.4 Habit reversal |
SET: VP (suggestion; self-instruction) CSM: coping procedure (approach) |
Elicit from group/person alternatives to behaviours: snack, drinks, physical activity, sedentary behaviour (sessions 1, 2 and 3) |
| 8.6 Generalisation of target behaviour |
SET: EME CSM: coping procedure (approach) |
Mindfulness – advised to put the magnet on the fridge Looking at menus (session 3 or 4) |
| 8.7 Graded tasks |
SET: EME (performance exposure) SET: VP (suggestion; self-instruction) |
|
| 9. Comparison of outcomes | ||
| 9.1 Credible source | SET: VP (suggestion) |
|
| 10. Reward and threat | ||
| 10.7 Self-incentive |
SET: INCENTIVE CSM: coping procedure (approach coping) |
|
| 10.9 Self-reward |
SET: VP (suggestion; self-instruction) CSM: coping procedure (approach coping) |
Encourage self-praise in the ‘sharing stories’ section |
| 11. Regulation | ||
| 11.2 Reduce negative emotions |
SET: VE (live and symbolling modelling) EA: (attribution; symbolic exposure; symbolic desensitisation) CSM: ER CSM: coping procedure (approach coping) RP: stress management |
|
| 12. Antecedents | ||
| 12.1 Restructuring physical environment | RP: skills training |
Keeping it going (booster sessions 1–3)/sharing stories – high-risk situations – strategies to manage (not having a big bag of crisps – not buying things in the first place) Magnet on the fridge |
| 12.2 Restructuring social environment | RP: skills training | Keeping it going (booster sessions 1–3) |
| 12.3 Avoidance/reducing exposure to cues for behaviour |
SET: VP (self-instruction) Relapse prevention model RP: skills training |
Keeping it going (booster sessions 1–3) |
| 12.4 Distraction |
SET: VP (self-instruction; suggestion) RP |
Keeping it going (booster sessions 1–3); sharing stories (sessions 1–4; all booster sessions) |
| 13. Identity | ||
| 13.2 Framing/reframing |
CSM: IR RP: cognitive restructuring |
Keeping it going (booster sessions 1–3) |
| 14. Self-belief | ||
| 14.1 Verbal persuasion about capacity |
SET: VP (suggestion; self-instruction) RP: cognitive restructuring |
Elicit (not tell) how they might challenge self-doubts
|
| 14.3 Focus on past success |
SET: EME CSM: appraisal RP: behaviour assessment |
|
FIGURE 20.
Use of self-efficacy theory in the STEPWISE intervention. BCT, behaviour-change theories.
FIGURE 21.
Use of the common-sense model in the STEPWISE intervention.
FIGURE 22.
Use of the relapse prevention model in the STEPWISE intervention.
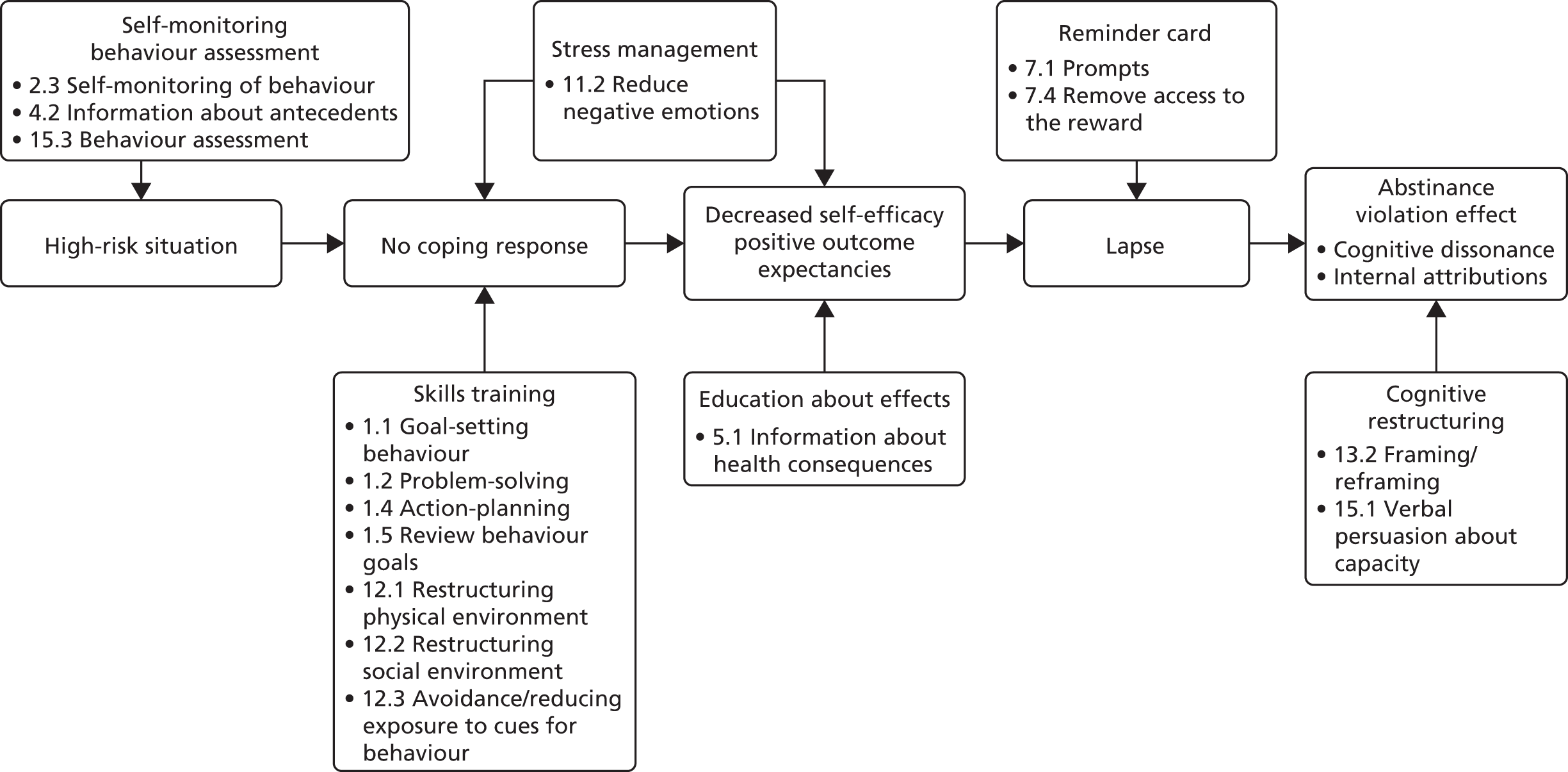
When we mapped participant views on the intervention to behaviour change wheel intervention functions (Table 33), we found that the majority focused on education, enablement (concordant with the developers’ views) and environmental restructuring (reflecting the fact that the topic guide asked about the support tools). A minority of participants (n = 8) talked about STEPWISE in terms of persuasion, but four of these were from the subset of people who experienced clinically important weight loss (n = 7).
| Study identifier | Function | ||||||
|---|---|---|---|---|---|---|---|
| Education | Enablement | Environmental restructuring | Incentivisation | Modelling | Persuasion | Training | |
| Intervention developers | |||||||
| D01 | • | • | ? | ? | • | ? | • |
| D02 | • | • | – | – | • | ? | • |
| D03 | • | • | • | ? | • | ? | • |
| D04 | • | • | ? | • | • | • | • |
| D05 | • | • | • | • | • | • | • |
| D06 | • | • | – | • | • | • | • |
| D07 | • | • | ? | ? | • | ? | • |
| Participants | |||||||
| Clinically important weight loss over 12 months | |||||||
| S03/Q06 | • | • | – | – | – | • | • |
| S01/Q01 | • | • | • | – | • | • | • |
| S08/Q05 | • | • | • | – | • | • | – |
| S04/Q02 | • | • | – | – | – | – | – |
| S06/Q01 | – | • | • | – | – | – | • |
| S01/Q05 | • | • | • | – | • | – | • |
| S02/Q04 | • | • | • | – | • | • | • |
| Weight loss that is not clinically important | |||||||
| S10/Q03 | • | • | • | – | • | – | • |
| S04/Q09 | • | • | – | – | – | – | – |
| S09/Q04 | • | • | • | – | – | – | – |
| S09/Q02 | • | • | • | – | • | – | – |
| S08/Q06 | • | • | • | – | – | • | • |
| S01/Q04 | • | • | • | – | • | – | • |
| Weight gain that is not clinically important | |||||||
| S05/Q03 | • | • | • | – | • | • | – |
| S06/Q02 | • | • | – | – | • | – | – |
| S03/Q01 | • | • | • | – | – | – | • |
| S04/Q10 | • | – | – | – | – | – | • |
| S08/Q09 | • | • | • | • | – | – | • |
| S09/Q01 | – | • | • | – | • | – | – |
| Clinically important weight gain | |||||||
| S04/Q12 | • | • | – | – | • | • | – |
| S07/Q06 | • | • | • | – | – | – | – |
| S04/Q08 | • | • | – | – | – | – | – |
| Qualitative participants without weight data | |||||||
| S04/Q06 | • | • | – | – | • | • | – |
| S06/Q06 | • | • | – | – | • | – | • |
When we mapped participant views on the intervention to behaviour change technique categories (Table 34), we found that the majority of interviews could be coded to feedback and monitoring, social support, shaping knowledge and antecedents (again, reflecting the salience of the support tools). The only participants for whom behaviour change techniques in the ‘identity’ category were salient were again from the subset of people who experienced clinically important weight loss (n = 3/7).
| Study identifier | Category | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01. Goals and planning | 02. Feedback and monitoring | 03. Social support | 04. Shaping knowledge | 05. Natural consequences | 06. Comparison of behaviour | 07. Associations | 08. Repetition and substitution | 09. Comparison of outcomes | 10. Reward and threat | 11. Regulation | 12. Antecedents | 13. Identity | 14.0 Scheduled consequences | 15.0 Self-belief | 16. Covert learning | |
| Clinically important weight loss over 12 months | ||||||||||||||||
| S03/Q06 | • | • | • | • | – | – | – | – | – | – | – | • | • | – | – | – |
| S01/Q01 | • | – | • | • | – | • | • | • | – | – | – | • | • | – | – | – |
| S08/Q05 | • | • | • | • | • | • | • | – | – | – | – | • | • | – | – | – |
| S04/Q02 | – | – | • | • | – | – | – | – | – | – | – | – | – | – | – | – |
| S06/Q01 | – | • | • | – | – | – | – | • | – | – | – | • | – | – | – | – |
| S01/Q05 | • | • | • | • | • | • | – | • | – | – | – | • | – | – | – | – |
| S02/Q04 | • | • | • | • | – | • | – | – | – | – | – | • | – | – | – | – |
| Weight loss that is not clinically important | ||||||||||||||||
| S10/Q03 | – | • | • | – | • | • | – | • | – | – | – | • | – | – | – | – |
| S04/Q09 | – | • | • | – | – | – | – | – | – | – | – | – | – | – | – | – |
| S09/Q04 | – | • | – | • | • | – | • | • | – | – | – | • | – | – | – | – |
| S09/Q02 | – | • | • | • | • | • | – | • | – | – | – | • | – | – | – | – |
| S08/Q06 | – | • | – | • | • | – | – | • | – | – | – | • | – | – | – | – |
| S01/Q04 | • | • | – | • | • | – | – | • | – | – | – | • | – | – | – | – |
| Weight gain that is not clinically important | ||||||||||||||||
| S05/Q03 | • | – | • | – | • | • | – | – | – | – | – | • | – | – | – | – |
| S06/Q02 | • | • | • | • | • | • | – | – | – | – | – | • | – | – | – | – |
| S03/Q01 | – | • | – | • | • | – | – | • | – | – | – | • | – | – | – | – |
| S04/Q10 | – | – | – | • | • | – | – | – | – | – | – | – | – | – | – | – |
| S08/Q09 | – | • | • | • | • | – | – | • | – | – | – | • | – | – | – | – |
| S09/Q01 | – | • | – | – | – | • | – | – | – | – | – | • | – | – | – | – |
| Clinically-important weight gain | ||||||||||||||||
| S04/Q12 | • | • | • | – | • | – | • | – | – | – | – | • | – | – | – | – |
| S07/Q06 | – | • | – | • | • | – | – | • | – | – | – | • | – | – | – | – |
| S04/Q08 | – | • | • | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Qualitative participants without weight data | ||||||||||||||||
| S04/Q06 | • | • | • | – | • | • | – | – | – | – | – | • | – | – | – | – |
| S06/Q06 | • | • | • | • | • | – | • | • | – | – | – | • | – | – | – | – |
Participant acceptability
Affective attitude
The majority of participants felt that the intervention was acceptable, mentioning that lunch, support tools (e.g. weighing scales) and being in a group were helpful.
Intervention coherence
Participants described the facilitators as welcoming, realistic and flexible and that they appeared to be confident. Some participants said that the facilitators helped their understanding and made the intervention more accessible:
Well it’s very friendly and informal, um and you could ask questions at any time. Um, I’ve tried to go to Weight Watchers . . . in the past, and it is quite awkward to sort of stop and say hang on a minute, what, ‘what did you mean about that’ . . . STEPWISE was really easy to understand and was put in a way that you could sort of take away with you sort of thing.
Participant S10/Q03
Burden
Participants generally felt that the length and frequency of sessions was acceptable and the intervention did not require significant effort to take part; however, several participants wanted more sessions (8–12) or more time within a session (≈3 hours):
Yeah. And what might that have erm, obviously if you’d have had the sessions for a couple of months, what would that have . . . given you?
Erm a bit more of a boost, erm like because at the minute I’ve been like, when I was going there, I was losing weight and since I’ve like not been going I’ve been like putting weight on. And like, not been like sticking to my routine and me diet and stuff like that, so it was giving me more confidence and more help and getting me out of me house and me flat and stuff like that so. Erm this month’s not been going, I’ve been like comfort eating, so, it got me out more.
Yeah it’s a bit tedious . . . too much time teaching people how to cook eggs to be fair . . . Not knocking the facilitators, they delivered it quite well but the material was patronising . . .
Participant S04/Q09
. . . the way that it was set out and done was good, you just weren’t given enough time on certain subjects.
Participant S02/Q04
Those who travelled by taxi to and from the venue reported how this made it easy to attend sessions, especially for those participants who travelled a long way.
Coherence
Generally, participants said that they understood the intervention and how it works:
Well I think it was useful, as I say, to reinforce what you’re doing . . . and also it was useful to have lunch as well because . . . and it also broke the silence . . .
Participant S01/Q04
Perceived effectiveness
Most participants felt that they benefited from participating in the programme, reporting (varying degrees of) weight loss, improved nutrition and increased exercise. Participants felt that being in a group with those in a similar situation was particularly useful. Several participants would have liked more content about physical activity (S04/Q12; S05/Q03):
Oh, yes, definitely, I’ve lost quite a bit of weight and I’m in much better shape. Like, I’m healthier because it’s taught me do a bit of exercise as well, yeah. Quite helpful. I’m in much better shape as well.
Participant S06/Q06
I found it quite useful, there’s been some interesting thoughts as well seeing what other people thought of the . . . what we was trying to aim for . . . like-minded and had similar views on medication and stuff as well [as] on gaining weight.
Participant S06/Q02
Yeah . . . I think it needed more talking about the gym like. You know. Like . . . you know to lose weight . . . and, evidence for like towards how you should go to the gym a lot more often.
Participant S04/Q12
Facilitator views of STEPWISE
Sense-making
Staff distinguished STEPWISE from other interventions (‘differentiation’) in terms of its use of the biopsychosocial (rather than the medical) model, with an autonomy-enabling, rather than a paternalistic, didactic, approach;122–124 its comparatively structured character (the commonest response); its content, including attention to antipsychotic weight gain; the group and peer-support elements; a greater time commitment; a comparatively short programme duration; its use of boosters and telephone calls; its external management; and its internal delivery – as lifestyle interventions often involve an external referral:
They’re encouraged to do our physical checks and look after people’s physical health including their weight but . . . it’s done more in a medicalised sort of way rather than the way the STEPWISE project offered which is better . . . it’s looking at a much more individualised person-centred approach.
Facilitator S06/F05
We don’t sort of tell them you can’t eat this . . . It’s more . . . giving them the opportunity to do what works for them. We are giving them the information for them to work it out for themselves.
Facilitator S04/F02
Only one facilitator, a community development worker, compared STEPWISE unfavourably with another manualised intervention:
[The other intervention] was more thorough in that it went right to the basics starting with food groups, etc. and then moving up, you know, into like portion sizes, about sugars . . . because there’s more time you’ve got more to explain . . . [whereas, with STEPWISE] the background information wasn’t there.
Facilitator S03/F05
Facilitators reported understanding what the intervention required of them (‘individual specification’) and sharing a sense of the aims of STEPWISE with other health professionals (‘communal specification’). This understanding was not immediately or always grasped by participants, either because of learning difficulties, cognitive deficit or an expectation of something more didactic involving closer monitoring:
And their functioning is quite different, you know, there’s really quite a high-level functioning lady there who sort of understood the thing straight away, and another guy . . . it’s very difficult to find out what his understanding actually is. Because he tends to talk in slightly psychotic terms.
Facilitator S05/F02
We do repeat, you know . . . it’s a lifestyle choice . . . we’re not saying that you’ve got to ban all bad food . . . they think they’re going to go there, get weighed, and they’ll go on a diet, we’re going to give them a diet sheet. And for some people that would work, and for other people empowering them works . . . sometimes the like peer pressure and going to Weight Watchers and knowing that you know there’s some expectation you’ve lost some weight, because it’s been marked down, that works very well for some people but not others.
Facilitator S01/F02
Although most facilitators constructed potential value for STEPWISE (‘internalisation’), this was conditional on the trial demonstrating an important difference in participant weight compared with usual care. Anticipated benefits, apart from lifestyle changes and broadly defined well-being, included:
-
the opportunity for often isolated individuals to interact, receive peer support, improve their social skills and grow new interests
-
an improved understanding of the participant’s own situation through modelling (intervention function) and self-esteem
-
better adherence to antipsychotics in the face of weight gain
-
mental health improvements brought on by improvements in physical health
-
improving the skillset, confidence and attitude toward physical health of health professionals
-
meeting local or national quality improvement targets and
-
downstream participant and health-system gains in terms of diabetes mellitus prevention.
Most facilitators agreed that STEPWISE fitted well with the goals of their organisation, although in some trusts they had faced apathy toward referral and scepticism that the intervention would be sustained beyond the end of the study:
I think it’s for the whole NHS really, because we are spending a lot of money on things like diabetes . . . it’s a good opportunity for service users to come together . . . they talk to each other and they share their own views, how they lost the weight, and again like engagement for them to get the social skills . . . when people are quite lonely they can’t talk to anybody . . . you can go there you can sit down, you can do a little bit of parkrun [parkrun Ltd, Twickenham UK] in the local park and you can talk to each other. So, it does benefit, it does benefit to the physically, mentally and socially.
Facilitator S03/F04
Quite a few service users get put off continuing to take their medication because of the side effects and this gives them a way to control it so it is not only kind of benefiting their physical health but it will benefit their mental health as well if it’s helping them to stay on the medication.
Facilitator S06/F01
Relational work
The key individuals who drove the intervention forward (‘initiation’) were different between organisations. Although many facilitators discussed having supportive clinical management, they frequently commented that the trust research departments, rather than clinical managers, were the drivers of implementation. In most trusts, the facilitators presented themselves as ultimately responsible for making STEPWISE happen and one developer flagged the general absence of effective change agents – individuals who influence innovation decisions:125
I know that people who are key in those sites are people who take part in [research project] meetings . . . they probably do not really see it as their job . . . to be that link with people on the ground . . . you do need senior people, very senior people in the trust to be supportive and to want something to happen . . . at [trust name] . . . the people who sat round that table were on board but practically speaking they were all a bit you know disconnected . . . you have got to have buy-in but you have got to have communication between all levels.
Developer 03
The facilitators all believed that it was right to deliver a structured lifestyle programme (‘legitimation’), reporting that they and most of their colleagues considered that it should be part of their work. One facilitator commented that this was now externally reinforced by the Commissioning for Quality and Innovation (CQUIN) payments framework, which was incentivising the promotion of physical health in mental health trusts during the study:
There is nobody who doesn’t perceive it as actually a very useful intervention and a useful way of working.
Facilitator S05/F02
Completely compatible really . . . it’s very valid . . . It’s not reinventing the wheel it’s just putting things in place that you need to know to make changes.
Facilitator S02/F03
I think that’s [STEPWISE is] definitely something that we haven’t been doing. And I think it’s something that we should be doing.
Facilitator S02/F06
By and large, motivated trust employees put themselves forward for intervention training and delivery (‘enrolment’), seeing it as a development opportunity as well as being good for patients; there were some instances in which this was not the case, although the first quotation here is comparably extreme:
I felt that a colleague was being slightly, mildly bullied into it . . . I had quite a threatening e-mail [after resigning] saying how . . . I was letting down the trust.
Facilitator S05/F02
There were some people who were ‘volunteered’ and . . . who were less positive but my general feeling when we did the training was that most people had seen STEPWISE and thought ‘oh, this looks really interesting, this would be nice thing to do, it fits’, you know, ‘I’m interested in health promotion’.
Developer 03
At some trusts, participants were easily engaged; at others, clinical ‘gatekeepers’ and patients presented barriers to filling STEPWISE course places. Although the current research context may have aggravated gatekeeping, health professionals in other settings employ tacit and ‘informal criteria when selecting individuals’ for routine clinical services. 126 Such gatekeeping was also reported to service user co-authors David Shiers and Angela Etherington, in their previous roles as project team members on the National Audit of Schizophrenia:127
We’ve got more people wanting to do it than we are able to put into our group.
Facilitator S08/F03
A lot of clinicians have said their clients don’t like group work or they don’t like to leave their home and they’ve put a lot of barriers in the way of people engaging . . . service users . . . the people who do want to take part are really positive about it . . . it’s something that they’ve been looking for . . . though you do also get some people who say . . . they wouldn’t want to talk about it in front of a group of other people, or . . . they don’t have the time to kind of stick to it . . . they’ve already engaged in other activities [gym or nutritionist] . . . the clinician has referred them because they know they’re overweight, but actually they don’t have concerns themselves . . . a lot of people live quite far away and that’s what puts them off.
Facilitator S06/F01
Service users are slightly put off by the thought of engaging in a research project, but I think that there would be a lot more individuals who’d be interested in engaging in STEPWISE if it wasn’t part of research, if it was just something that we’d rolled out.
Facilitator S09/F03
Although many facilitators continued to support STEPWISE (‘activation’), developers noted that there were high levels of facilitator attrition (Figure 23) compared with other interventions on the LDC portfolio. Although most of the facilitators had group facilitation experience and relevant experience of the clinical population, there were still concerns among the developers that staff selection was not always well considered:
There’s no point in training 10 facilitators if they only get to deliver one session . . . obviously at any one time you’ve only got a certain number of people with capacity and they may not be the right people and, obviously, there were some people who trained and then they went on and got other jobs . . . there was an awful lot of attrition in facilitators. I would have expected quite a bit anyway perhaps not quite as much as we had.
Developer 02
FIGURE 23.
Time to facilitator attrition from foundation training (weeks).
Facilitators saw the availability of transport costs as critical to maintaining participant engagement during the initial four sessions (see Table 7). Thereafter, there were problems with keeping participants involved during the follow-up telephone calls (see Table 9), because of participants’ social commitments and staff annual or sick leave:
A lot of times you speak to people about attending the sessions, they say ‘well, where’s it going to be? And, how am I gonna get there?’. So I think that’s been a really good way of keeping people attending the groups, knowing that they can get a taxi and not have to worry about going and getting a bus . . .
Facilitator S06/01
It tends to seem to have worked better . . . where they had one person that kind of took ownership of doing all of the calls . . . in most areas it tends to be either one of the facilitators . . . then takes them through their calls and things.
Developer D05
No one picks up, you know. And it’s how many times really do you try and get through to someone. Erm, but I’ve always left messages, erm. But I’ve tried to contact them if I haven’t got through. But it’s usually about half, we’ve managed to actually contact.
Facilitator S02/F06
It’s been quite mixed . . . some of the . . . service users that you’d speak to I think find it really useful . . . to actually reflect back on how the last few weeks have been for them . . . Whereas a couple of people it almost feels like you’re pestering them a little bit and they do not have so much of an interest in talking about their diet and exercise levels and it’s quite a short conversation that they just say yes everything’s fine, no I do not need support with anything else. So it is quite mixed in the response that you get from people.
Facilitator S06/F01
Operational work
Facilitators could perform the tasks required by the intervention (‘interactional workability’). They were universally complimentary about the training and printed materials they received, although:
It was a lot to take in just those 3 days initially . . . quite intense.
Facilitator S08/03
Facilitators described how they translated or adapted instructions from the manual to support participants to perform particular tasks. Although most found that delivering their first sessions was tricky, they reported becoming happier with group facilitation over time. Some reflected on the challenges of an autonomy-enabled, rather than a paternalistic, communication style, all believing that they were adapting well to this requirement. However, many facilitators complained about the logistical burden of programme management, in particular the purchase, transport, setting up, taking down and storage of materials, including sandwiches, other foodstuffs and flip charts. Intervention delivery and broader programme/case management could be made more difficult by sick leave or staff attrition. The workability of materials was problematic. For instance, one exercise involved showing pictures of foodstuffs to participants and asking them to discuss how many calories they contained; however, it did not say in the materials and facilitators who were not themselves dietitians did not know the right answer:
Had it not been for her [the administrator] . . . I don’t think half of the time I would have turned up with the right stuff. And it would have took me a full day. It’s only because she had everything organised . . . on a Wednesday morning she was getting me the stuff together. Erm, now some of the stuff was out of date. That meant that we then had to go to the shops and go and buy replacements, which we’re still waiting for the money, we paid for it out of our own pockets. And er I know £10, £13, doesn’t seem like a lot but erm when you’re waiting 4 months for it . . .
Facilitator S01/F02
Facilitators sometimes alluded to the repetitive nature of aspects of the intervention causing problems with some participants. They mentioned the problems of dealing with a client group in whom concentration could be poor. Sometimes, one facilitator had to break off discreetly to support participants who were struggling because of learning difficulties or symptoms. Small groups (≈4 participants) seemed to allow enough time for the delivery of course content and for participants to come up with their own solutions, whereas larger groups often felt rushed. One facilitator found that supporting participants to complete the action-planning task was the most difficult part, but that retaining flip chart notes from across the sessions helped to prompt and persuade participants to engage with the task. The timing of content delivery during the sessions, and completing within the allotted time, was a source of anxiety for facilitators – given the need for a smooth conclusion by the time taxis arrived:
It was a booster session I did recently, and erm this client said, halfway through she like put her hands, er her head on table, and she said ‘I’m going to have to go . . . I’ve lost the will, you know. Because everything’s been said over and over’ she said, ‘and I’ve just had enough.’ And I thought ‘well that’s how I were feeling actually’.
Facilitator S01/F02
In general, facilitators maintained trust in each other’s work and expertise during the delivery of STEPWISE (‘relational integration’). In particular, they described being reliant on each other to share the logistical burden and provide cover when things went wrong in session delivery. They felt that a wide range of other people would need to be involved in one way or another, aside from the facilitators and the senior management, to ensure the smooth running of STEPWISE:
GPs . . . community teams . . . nurses, social workers . . . care co-ordinators, medics involved in the community mental health teams, so they can, you know, have an overview of you know the blood results . . . and signpost people that might be having any issues with physical health . . . someone to arrange taxis . . . an admin person . . . people’s carers or support network . . .
Facilitator S01/F04
Two types of comments indicated that some facilitators felt that others might not be qualified to deliver the intervention; some felt that more specialist nutritional training should be required and one felt that overweight facilitators should not be delivering the course, although other facilitators had talked about being overweight themselves:
I’d say it’s the people who haven’t got the nutrition background that I worry about, not necessarily the . . . or not feeling competent, or not knowing that they’re not competent.
Facilitator S02/F06
[Facilitators] are like a role model, you know what I mean? . . . if I am overweight . . . they will think, oh my goodness, he or she is overweight and they are talking to me about this programme like to lose the weight, and he hasn’t or she has not lost any weight at all.
Facilitator S03/F04
Otherwise, facilitators agreed that the work of delivering STEPWISE was appropriately allocated (‘skill set compatibility’).
Every facilitator mentioned resource scarcity, with even paper and whiteboards at a premium. The venues provided for sessions were generally adequate, but the storage of materials was often a problem. Programme or case management and tasks, including preparation/documentation of sessions and writing up telephone support logs, were very time-consuming and added pressure to the facilitator’s job: facilitators at almost every trust said that the time required had been radically underestimated in what was allocated to them. More than one facilitator had to stop delivering STEPWISE because its delivery was affecting their clinical work and some commented that the intervention was fragile in the face of clinical emergencies and staff sickness, maternity leave and turnover.
Some facilitators implied that the intervention was not adequately supported by the host NHS trusts (‘contextual integration’); although the senior management supported the principle of addressing physical health, the resources were not forthcoming. Backfilling clinical time involved was sometimes a matter of immediate staff availability rather than financial constraints or management motivation. Facilitators at several trusts, said that, with inadequate backfill, clinical colleagues had missed their presence. Things were reported to be working better in trusts, which had clear and meaningful buy-in from the senior management and when CQUIN incentivised the delivery of something like STEPWISE (see Relational work):
[My time] didn’t get backfilled . . . I did actually get some . . . negativity . . . from some of my colleagues. Because, well, to quote one person, I was taking the piss because . . . I’d put a lot of this time, blanked it out in my diary . . . [STEPWISE has been] ignored since we’ve done the training . . . I think the system has . . . failed the programme a little bit . . . if it’s going to be successful then we’ve got to have resources for it.
Facilitator S01/F02
It’s just a case of senior management being completely blind . . . anything above team leader level . . . They all go, ‘yes it’s lovely’, but then . . . do not then provide us with the resources to be able to do it . . . there isn’t a system to allow members of staff to do something like this . . . They say there is, but there isn’t . . . if there is a system it is flawed and broken.
Facilitator S05/F02
It’s a big piece of work when you’re full-time somewhere else, and they would say well we’ve got money to backfill but there’s no staff to backfill.
S09/F03
Within this particular trust, one of the deputy directors had been involved right from the start and he was very keen for it to happen so it’s been sort of disseminated downwards from there . . .
Facilitator S03/F05
Appraisal work
The extent to which facilitators accessed information about the effects of the intervention (‘systematisation’) was limited. None was routinely accessing information on participant weight and this would have been possible only at a minority of trusts where weighing was routine and performed at regular intervals. Instead, most facilitators reported participant anecdotes involving the adoption of desirable behaviours by participants and (in terms of cooking) their carers:
No one seems to be taking weight! [laughs] We’re just getting, I’m not gathering data on individual clients . . . we do not even have an ongoing way of monitoring weight now. I think the trust would like it, but we keep telling them there is no way of actually monitoring people’s physical health on the system that we have got . . . you have to go through a hundred different things and write them all down separately, and then you can draw a graph . . . I think the trust is willing, and would like it, but they just do not know, they are just not willing to put the money and the investment into the IT [information technology] things.
Facilitator S05/F02
I am not sure whether when they leave our session whether they follow the advice . . . [one] service user has been telling to her mother not to add too much oil to curry and things like that . . . so there has been some insight there.
Facilitator S03/F04
I had one person that said ‘I was eating takeaways nearly every single night and I’ve stopped doing that now’.
Facilitator 03/F05
Few facilitators reported having the opportunity to assess the worth of the intervention together (‘communal appraisal’); however, when this did happen, the conclusion was usually positive:
The facilitators we’d spoke between ourselves, but there wasn’t anywhere where you could go really and feed back to someone.
Facilitator S01/F02
I did a talk at a . . . bite-sized physical health training game . . . about STEPWISE and it was a very positive response, and generated a lot of referrals for the group.
Facilitator S06/F04
Well I’ve actually had people come up who’ve heard about it. Who were talking to people that go to the clubs or clinics. And they’ve spoken to some people who have attended it and they are keen to know whether there are going to be other groups and things.
Facilitator S08/F03
Although they stressed that their final appraisal was contingent on the outcome of the RCT, facilitators individually assessed the intervention as worthwhile (‘individual appraisal’), with only two exceptions. The benefits for participants were expressed as:
-
having the opportunity to think through their lifestyle
-
having the opportunity to meet other people and openly discuss antipsychotic-related weight gain
-
maintaining self-esteem in the face of weight gain.
Some facilitators thought that these benefits would have positive effects on mental health. The benefits for professionals included:
-
raising the profile of physical health and the poverty of current services
-
new communication skills or knowledge they had learned that had applicability beyond STEPWISE, which, in turn would make them more confident in giving lifestyle advice.
Criticisms of STEPWISE included that elements were repetitive or patronising, that it lacked incentives for behaviour change, that it was not underpinned by adequate dietary or nutritional knowledge and that behaviour change momentum would not continue without group support:
One of the patients was saying, it’s all good when we’re doing it together, but I live alone and I find it difficult to cook just for myself. It would be good if we could you know once in a while meet again and you know, make a meal for, for each other, altogether sort of thing. So yeah, er, the philosophy is good, it’s whether people will be consistent and continue it afterwards . . .
Facilitator S07/F05
Many participants said that they would be modifying their work in response to their appraisal of STEPWISE. In particular, they valued the client-centred, autonomy-enabled communication style, which they had learned in training, and some planned to use that style and to insert more structure into other clinical encounters as a result of their experience. Some identified other populations, not targeted by the RCT – such as people with borderline personality disorder – who could benefit from a STEPWISE programme. On the other hand, some facilitators thought that, if STEPWISE was sustained, it would have to be modified, in particular to take account of its current resource intensity, with materials and lunches in particular jeopardy:
It’s actually made me think a little bit more about you know the style that I deliver my groups in for other service users.
Facilitator S02/F03
I know a number of people that would really benefit from STEPWISE but they can’t take part because . . . they don’t meet the criteria.
Facilitator S09/F03
If it works, I wouldn’t imagine that we’re going to be running these sessions, it’s going to become part of like the working practice for everybody. We won’t be giving out free gifts and you know weight scales and books and everything else. It will just be the programme as it is, as an ongoing thing. You know part of people’s care plan.
Facilitator S01/F02
Obviously it’s potentially quite expensive to run, that’s the only thing, because the lunches and support tools, so all those things would need to be considered, uhm . . . whether there were potential long-term benefits because cost is a huge implication for the trust.
Facilitator S08/F05
Summary
The findings are summarised in Figure 24. STEPWISE made sense to those who delivered it. They understood its objectives and believed that it could be worthwhile. At most trusts, change agents in senior management did not drive the intervention forward and, although facilitators felt that the intervention was the right thing to do and they became involved, competing clinical caseload demands and other factors resulted in high staff attrition. Facilitators understood how to deliver the intervention, gaining confidence with practice, maintaining trust in each other and drawing on their expertise. However, programme management was under-resourced and facilitators doubted the long-term commitment of senior management to what they perceived as a resource-intensive intervention. In no trust were facilitators accessing data on weight to understand the effects of STEPWISE; instead, they relied on ad hoc participant self-reported accounts of the enactment of cognitive and behavioural skills. Facilitators rarely collectively appraised STEPWISE; individual appraisals were generally positive. Many reported modifying their own practice based on STEPWISE training and thought that STEPWISE was applicable to other mental health populations, with some anticipating modifying STEPWISE, because it would have to be less resource intensive to be sustainable.
FIGURE 24.
Summary of the qualitative findings understood through the normalisation process theory.
Implementation process
Programme theory and logic model
The programme theory, elaborated in the logic model (see Figure 5), is described as follows. For the STEPWISE programme to work, certain resources need to be in place, including the facilitator training and quality assurance infrastructure and resource allocated by CMHT management for programme delivery. This being the case, certain activities can be delivered; LDC would train and quality assure trainers, who would, in turn, train and quality assure STEPWISE facilitators. Clinical gatekeepers would refer participants. Facilitators and local co-ordinators would perform case management and wider programme management functions. The immediate outcomes would be that the quality of every level of training is maintained, STEPWISE courses are delivered and eligible patients are referred to and attend the group. Through the participants’ receipt of the course content, the intermediate outcomes measured by the trial were brought about.
Resources
Programme infrastructure/trainers/mentors/managers
During the trial, LDC staff fulfilled these roles (excepting management), as they currently do for active services such as DESMOND and the Let’s Prevent Type 2 Diabetes programme (see Chapter 1, The Leicester Diabetes Centre structured education approach). Interviewee D02 highlighted that, should the STEPWISE intervention be sustained and commissioned more widely, a permanent infrastructure would be required. At the top end would be a steering group with a national director, educationalists, consultant clinical psychologists and quality assurance experts (n ≈ 6 in other programmes). Below the steering group would be a training and assessing team (n ≈ 6 in other programmes). At each site, there would be a STEPWISE lead linking with the steering group; if the lead does not have easy access to senior management, a separate champion might be required to ensure adequate resourcing (see Facilitator views, Operational work).
Facilitator training course and venue
During interviews with LDC staff and facilitators, no systemic problems were identified with resources for training, details of which are in Table 35. Course materials were well regarded by facilitators (see Facilitator views, Operational work).
| Facilitator training/resources | n | Cost (£) | Details |
|---|---|---|---|
| Original facilitator training | 48a | 4413.70 | 3–5 March and 10 June 2015 (Sheffield); 21–23 April and 24 June 2015 (Southampton); 28–30 April and 7 July 2015 (Exeter). Three sites trained six (rather than four) staff |
| Booster training day (original) | 35 | 3151.51 | Not all facilitators in the original training completed the booster training day (dates above) |
| Additional (full) training | 7 | 1932.23 | Four sites identified seven staff for training: 3–5 November 2015 (Leicester) |
| Additional booster training | 5 | 554.45 | Cost of staff attending alternative training dates (booster session content only). One out of five did not attend |
| Additional booster and support contact | 5 | 1250.00 | Five facilitators from three sites were trained: 6 and 7 June 2016 (Sheffield) |
| Core teaching kit and supporting kit | – | 17,395.34 | |
| Training subtotal | 28,697.23 | ||
| Delivery resources | n | Cost (£) | |
| Patient handbooks | – | 3908.32 | |
| Lunch | 4410 | Costs based on a price per head of £3, assuming that seven participants attended all seven group sessions × 3 waves (n = 21 intervention participants) at each site (n = 10). Paid directly to the University of Leicester by NHS trusts | |
| Resources subtotal | 8318.32 | ||
| NHS staff costs | Cost (£) | Assumptions/notes | |
| Facilitator/s time to deliver courses | 40 | 46,980.00 | Based on 4 × 0.05 WTE Agenda for Change band 7 (point 30) for 24 months |
| Administrator time to support delivery | 10 | 173,412.00 | Based on 0.1 WTE Agenda for Change band 3 (point 9) for 24 months. Activity: room bookings, pre-course material, booking taxis, processing expense claims and collating attendance logs |
| Staff subtotal | 220,392.00 | ||
| Total | 257,407.55 |
Health professionals motivated to become facilitators
Motivation was generally high in interviewees (see Facilitator views, Relational work) and, although facilitators had a wide range of exposure to group facilitation (see Table 5), the LDC team felt that appropriate individuals came forward. However, quantitative data highlighted problems with staff attrition from facilitation (see Figure 23).
Appropriately resourced co-ordinator
Interviews with facilitators and the LDC team indicated a lack of clarity about the roles of the co-ordinator and/or under-resourcing at some trusts:
There were sites where you had a very proactive co-ordinator . . . helping the facilitator to make sure that everything was there and they had everything they needed on the day and the taxis were booked and you know the logistics of running it went well . . . I got the impression in some other places that actually that wasn’t quite the case . . . it was very much left to them [facilitators].
Developer D03
Bought-in trust chief executives and gatekeepers
Senior management buy-in was often good, but sometimes did not translate into appropriate resourcing (see Facilitator views, Operational work). Similarly, gatekeepers referred patients well at some trusts and less well at others (see Facilitator views, Relational work). The budget for taxis was always available and deemed to be very necessary (Context, Activities and participation).
STEPWISE course materials
There were difficulties in arranging the storage of course materials (S01/F04). The necessary budget for consumables [paper, Blu Tack (Bostik, Paris, France), whiteboard] required for the course was sometimes not forthcoming (S01/F04). The necessity for facilitators to buy materials, in the absence of a properly funded co-ordinator, was problematic (see Facilitator views, Operational work).
People with schizophrenia motivated to attend
The study screened 1223 people for eligibility, of whom 800 did not participate. A total of 406 out of the 800 people excluded actively expressed a lack of interest or declined consent.
Activities
Mentors assessing the competency of trainers
As the training in the trial was conducted by DESMOND trainers, who are themselves mentors, this activity was not conducted.
Training of facilitators
Forty-nine facilitators were fully trained in 8 months by seven trainers, with additional booster/support contact training provided to a further five staff (see Table 35). Training was well regarded by facilitators (see Facilitator views, Operational work). The STEPWISE programme employed generic strategies24 to ensure intervention fidelity through training; these included standardised training and accommodating provider differences (see Table 6). LDC does not assess provider skill acquisition at baseline or attempt to minimise provider skills ‘drift’;24 rather, the DESMOND programme expect, gradual skill acquisition through ongoing observation, feedback and self-reflection – a process not simulated in the STEPWISE trial, because of the short-term study-specific commitment to the intervention, and the burden of additional procedures:
We would never, ever say because we train people for 2 days or 3 days that that means they are absolutely fantastic . . . it’s only the beginning of the journey . . . the process by which people get accredited is that, within 6 months after training, people get a mentorship visit from an experienced trainer/assessor . . . and they’ll sit and they’ll observe and they’ll use tools that are available to the educators themselves . . . and they will give some mentorship . . . the person does an action plan . . . and probably within the next 6 months they’ll have an accreditation visit where they will be observed delivering 50% of the programme . . . and, as a result of that, they’ll either be accredited or they’ll have to do a little bit more work. Most people get accredited within 18 months if they follow that process.
Developer D02
The LDC’s approach to minimising skills drift is to encourage facilitators:
To form a community of interest in their organisation, support each other, make links with neighbouring organisations . . . the reason that we don’t do any more competency [assessment] is because organisations won’t pay for it and . . . they’d never get the time released.
Developer D02
Programme management
The work taken to realise the client interactions was described universally as greater than anticipated. Although facilitators at many trusts rose to the challenge, the experience of programme management had distressed or overfaced some facilitators. Scheduling of courses was reported to be chaotic in some trusts, with delays and cancellations observed and facilitators being obliged to travel long distances to deliver courses in multisite trusts. Facilitators sometimes found it difficult to protect time to plan, to deliver the intervention uninterrupted by client emergencies associated with their usual clinical role or to debrief (see Facilitator views, Operational work). Under-resourcing of local co-ordinators or a lack of clarity about their role meant that facilitators spent undue time preparing refreshments, booking taxis, transporting or storing materials, and so on (D01):
I think they underestimated the amount of time doing these programmes . . . you have got to spend a lot of time reading through the material . . . And because the, the groups are run off site from where I work, I’ve got to get there, set the groups up . . . it is a big investment in time. And it does sort of create strains a lot of days . . .
Facilitator S02/F02
But for me there was a lot of work behind the scenes . . . obviously my caseload is my paramount priority so if there’s any kind of downtime, STEPWISE would slip into that . . . I’ve had to do some at home . . .
Facilitator S02/F03
It’s the calls that . . . take quite a lot of time. Because not everybody picks up the phone the first time, you need to call back . . . the groups are quite easy to, to manage, with workload, but I think the calls are definitely more difficult to fit in.
Facilitator S02/F06
It takes planning . . . the 4 weeks [foundation course] . . . it was almost impossible. Because I was suddenly having to put 5 days of work into 4 days of work. You know, but our caseload did not go down, I still had the same number of ward rounds to attend, same number of clients to see each week . . . I was told there would be backfill, I was then . . . told that the backfill would not actually cover the hours that I was actually working it . . . there was no acknowledgement of the fact that every e-mail takes 5, 10, 15 minutes to reply to, that there were extra meetings . . . they wanted us to buy our own tickets for . . . 3 days travel down [to training] . . . We absolutely refused, it was a nightmare getting those tickets . . . I cannot remember how many hours . . . I’d put into it. But it’s a lot more than other people thought . . . I think they thought . . . that delivering the session would be 3 hours of our worktime. No! It’s a day of our work time . . . far more than just 4 hours a week. Because . . . it was 2–3 hours of preparing each session at home.
Facilitator S05/F02
Gatekeepers actively refer people
Gatekeepers were rarely reported to be a barrier to participant recruitment (Facilitator views, Relational work). Health professionals encouraged involvement with STEPWISE, as they recognised that weight management was a huge problem for the client group.
Case management
Many facilitators described having an existing psychiatric caseload ‘over multiple wards in multiple locations’ (facilitator S02/F03). Inadequate resourcing and lack of protected time (see Facilitator views, Operational work and Implementation process, Activities) made individual-level, STEPWISE-specific participant interaction difficult to schedule and complete. Scheduling telephone calls could be difficult because of staff availability and participant commitment, as could arranging cover for staff leave to avoid missing calls that were due. Telephone calls were often described as resource intensive, owing to the number of calls that had to be made before contact was established and, for this reason, the workload of case management was easier in small groups. Facilitators recalled providing additional support to cognitively challenged participants who were struggling with content in the sessions. Many also felt that they needed to be responsive to non-weight management concerns brought up during booster sessions and one-to-one calls, which made calls longer:
I felt it would have been better to have people . . . specifically doing that role . . . being taken away for half a day . . . for the period for when you’re doing telephone calls . . . having a block of time not at your own desk, at another desk ‘cause . . . if I’m on this call . . . for STEPWISE I could easily get interrupted because . . . we respond quickly to our patients and somebody might come in and say, ‘OK I need you now!’ You know and then you’re having to stop.
Facilitator S01/F04
Patients also can be funny with a strange voice or someone they don’t know. I’ve had that problem with follow ups. The patients wouldn’t answer, or when they heard somebody’s voice sometimes they’d just put the phone down. And then I would then phone them . . . and then explain that my colleague would be doing the follow up, and he is trying to get in touch. There was one participant . . . she said yeah I haven’t been feeling well so I haven’t been answering my phone.
Facilitator S07/F05
Immediate outcomes
Eligible people invited to attend STEPWISE ‘recruitment’ and ‘reach’
Recruitment to STEPWISE was somewhat artificial, owing to the trial-specific consent and randomisation procedures. Recruitment to the STEPWISE trial and programme was reliant, primarily, on referral from direct care teams. A script was circulated for use by clinical team members in conversations with patients about recruitment, in order to provide a consistent message. Site PIs talked to clinical teams about the study and intervention around 1 month before recruitment commenced. Posters, including eligibility criteria, along with short information leaflets co-produced with patients, were displayed by clinical teams in reception areas. Given the engagement of gatekeepers and adequate co-ordination, it seems unlikely that recruitment in real-world circumstances would be problematic.
Estimates on the number of potential participants varied significantly between NHS trusts, partly because of different methods and definitions of ‘first episode psychosis’; for example, these ranged from 1233 (Sheffield; 2 June 2017), 2420 (Bradford; 28 March 2014) to 12,968 (Sussex; 11 April 2014). Target versus actual recruitment at these sites was 40 out of 36, 50 out of 45 and 42 out of 35, respectively. Work and family commitments affected the attendance at (or reach61 of) STEPWISE. Qualitative work identified two participants who were unable to make certain sessions because of child care responsibilities. Care providers rescheduled the start time of foundation sessions to accommodate participant child care responsibilities (S04/Q06) and for when booster sessions fell on a school holiday (S04/Q02). Information from screening logs showed that 11 people gave employment commitments as the reason for them being unable to participate. Many participants said that they were unable to attend, but did not provide specific reasons; however, one interviewed participant (S06/Q01) was able to attend STEPWISE because it fell on a non-work day.
Foundation, booster and telephone support calls: ‘dose delivered’
Foundation sessions and most booster sessions were delivered as planned. Not all sessions were delivered by two trained facilitators, and, on one occasion, a (7-month) booster session was not delivered. Further non-compliance was recorded as a result of only one facilitator delivering the sessions (see Chapter 4, Course delivery and protocol non-compliance). When this occurred, most centres provided the facilitator with an (untrained) member of staff to provide support in the sessions.
Sessions delivered in line with the STEPWISE philosophy: ‘fidelity’
When we interviewed LDC staff about their observations and read their notes, they generally indicated that facilitators had achieved a reasonable initial level of fidelity with the STEPWISE philosophy, although there was often room for improvement. For instance, most LDC interviewees identified that facilitators would give their own examples of a phenomenon, rather than allowing participants to remember and voice their own experiences. Equally, LDC staff felt that participants were sometimes not given sufficient thinking time to answer a question, which was particularly important given the cognitive challenges some participants face (see Body functions) and that aspects of the programme, such as action-planning, were not given adequate time:
I think overall with the ones I observed, they did overall well with the talking time, erm but I did observe a couple of times in terms of asking questions to the group they weren’t really giving them time to answer back, in terms of kinda letting them think about the answers.
Developer D01
It was done in a more didactic telling way than what we’d hoped . . . there were people who went off-piste . . . and added bits . . . there was one place where somebody . . . started doing CBT . . . because they’d been on the [CBT] course recently . . . it took it huge amount of time and there wasn’t enough time left for the, for the other elements.
Developer D03
The facilitators were very conscious of – and expressed mixed views on – how structured the intervention was, with some acknowledging departures from the course:
I think the level of structure has probably been the most structured that I’ve come across [in a manualised course].
Facilitator S08/F05
I’ll be quite honest and say we haven’t been quite so structured as the book will give it. And we sort of twisted things slightly in the way we might use some of the supporting tools.
Facilitator S08/F03
CPNs [community psychiatric nurses] tend to work very individually . . . tend to be very autonomous workers, and it was challenging . . . to follow specific guides and you know . . . leave gaps and things.
Facilitator S05/F02
Table 36 and Figure 25 show that the overall mean (SD) for the percentage of facilitator talk time (DOT scores) across four sections of sessions (‘Sharing stories’, ‘Topic a’, ‘Topic b’ and ‘Next steps’) was 47.6% (12.3%). Mean (SD) scores ranged from 36.8% (6.4%) in Devon to 63.2% (9.4%) in Cornwall. Although the DOT scores provide indicators of the level of competence of the facilitators, precise scores that would be considered acceptable cannot be determined by the trial, as a large sample would be needed and linked to outcomes.
| Centre | Group sessions observed (n) | ‘DOT’ scored session sections (n) | % of facilitator talk time | Facilitator behaviour scores, session sections (n) | % positive (‘left’) | % negative (‘right’) | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (range) | Mean (SD) | Median (range) | Mean (SD) | Median (range) | ||||
| Bradford | 2 | 8 | 46.6 (6.8) | 45.5 (39.0–57.0) | 8 | 46.4 (12.2) | 47.1 (25.7–62.9) | 26.8 (8.2) | 28.6 (14.3–37.1) |
| Cornwall | 2 | 8 | 63.2 (9.4) | 63.8 (50.0–78.0) | 8 | 31.8 (13.2) | 34.3 (14.3–54.3) | 38.9 (13.9) | 35.7 (20.0–62.9) |
| Devon | 2 | 7 | 36.8 (6.4) | 37.0 (28.0–44.0) | 7 | 59.6 (6.7) | 57.1 (54.3–71.4) | 3.7 (2.2) | 2.9 (0–5.7) |
| Leeds and York | 6 | 22 | 50.0 (13.1) | 52.5 (27.0–78.4) | 24 | 57.5 (10.2) | 57.1 (37.1–77.1) | 21.0 (11.5) | 20.0 (2.9–40.0) |
| Manchester | 2 | 8 | 41.6 (8.3) | 45.5 (29.0–50.0) | 8 | 64.6 (17.7) | 65.7 (42.9–85.7) | 7.1 (11.7) | 0.0 (0–28.6) |
| Sheffield | 1 | 4 | 42.5 (5.2) | 42.3 (36.4–48.8) | 4 | 63.6 (16.9) | 65.7 (42.9–80.0) | 20.7 (15.5) | 15.7 (8.6–42.9) |
| Somerset | 1 | 4 | 44.0 (8.8) | 45.5 (32.0–53.0) | 4 | 64.3 (6.8) | 61.4 (60.0–74.3) | 15.0 (4.9) | 15.7 (8.6–20.0) |
| Southern Health | 2 | 8 | 50.8 (8.2) | 49.0 (41.0–66.0) | 8 | 45.4 (11.1) | 45.7 (28.6–62.9) | 41.4 (14.6) | 40.0 (14.3–60.0) |
| South London | 6 | 23 | 49.3 (13.0) | 48.1 (25.0–75.0) | 23 | 48.1 (14.2) | 45.7 (20.0–71.4) | 31.4 (14.5) | 28.6 (11.4–65.7) |
| Sussex | 6 | 24 | 44.5 (12.9) | 44.5 (22.0–65.0) | 23 | 61.5 (12.2) | 57.1 (34.3–85.7) | 20.7 (12.4) | 20.0 (0–40.0) |
| Overall | 30 | 116 | 47.6 (12.3) | 47.4 (22.0–78.4) | 117 | 54.1 (15.0) | 54.3 (14.3–85.7) | 23.8 (15.4) | 22.9 (0–65.7) |
FIGURE 25.
Box plot for percentage of facilitator talk time (DOT scores) across sites.
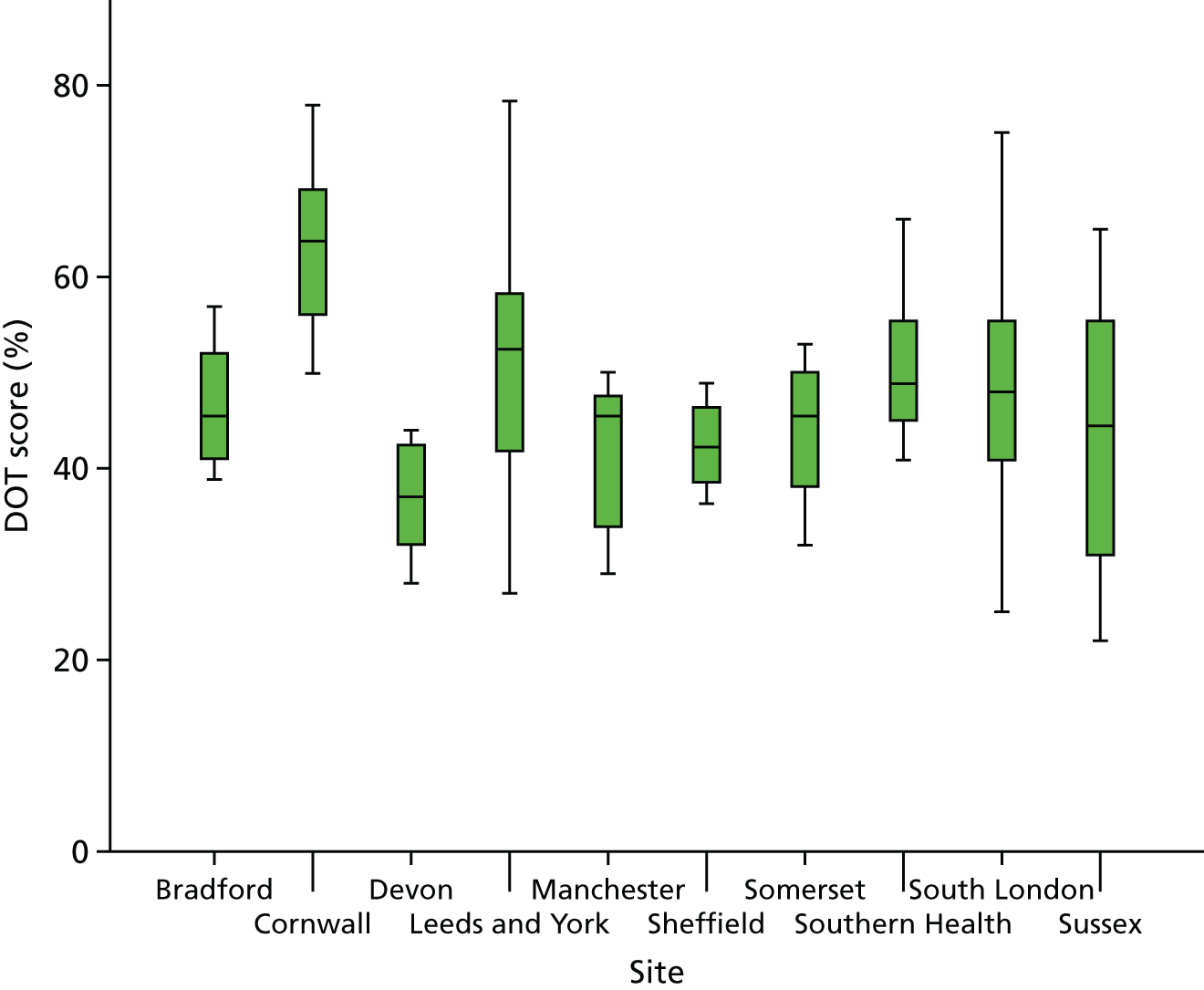
Facilitator behaviour was also assessed by direct observation and categorised as ‘positive’, reflecting a facilitative approach, and ‘negative’, reflecting more didactic teaching. Positive behaviour was observed for a mean (SD) of 54.1% (14.97%) of the time, with a range across centres of between 31.8% (13.18%) in Cornwall and 64.6% (17.67%) in Manchester (see Table 36 and Figure 25). Conversely, the mean (SD) time of negative behaviours observed was 23.8% (15.45), with a range between centres of 3.7% (2.16) in Devon and 41.4% (14.57) in Southern Health.
The STEPWISE intervention contained explicit strategies for assuring the fidelity of delivery in terms of reducing differences within the treatment and ensuring adherence to the protocol (see Table 6). It did not contain any explicit strategy for controlling provider differences; findings from the session feedback, interviews and observations were not fed back to facilitators during the study. Serious contamination seems unlikely, although facilitators from a number of trusts admitted lifting content or using the communication style that they had learned in STEPWISE training in interactions with other patients. When asked, they did not believe that this might have involved control arm participants, with one exception:
I care co-ordinate one of them . . . we’ve always discussed actually longer term what she needs to be doing to lose weight. To get motivation and things like that. So it just, that’s in use. I tried very hard not to bring STEPWISE stuff into it because honestly I don’t want to impact on her control group but in conversations that I’ve with her for a number of years.
Facilitator S08/F03
Attendance and ‘receipt’ of behaviour change content (‘dose received’)
Participants attended a median of four (0–4) group foundation sessions and two (0–3) booster sessions. Forty-seven out of 172 participants randomised to STEPWISE attended all seven group sessions, 96 attended all foundation sessions and 61 attended all three booster sessions. One hundred and sixty-nine participants (81.6%) had at least one support contact. Most (75.6%) were by telephone and 167 (81.0%) had at least one telephone contact. Participants received, on average, 15 contacts and the average time per contact was 5.2 minutes. Fewer participants, 68 (32.9%), had at least one face-to-face contact with an average of three contacts and an average time of 59.4 minutes per contact. Furthermore, 105 participants (50.7%) had at least one mail contact, with the average number being five, whereas 24 (11.6%) had at least one contact by e-mail.
Strategies to ensure ‘receipt’ of the intervention, in terms of ensuring participant comprehension or using cognitive and behavioural skills, were less robust than those to ensure delivery (see Table 6), with the intervention prioritising client autonomy. Some of the LDC team and a minority of facilitators acknowledged this as a potential problem, owing to the cognitive function of some of the participants (see Context, Body functions):
Sometimes it wasn’t explored to check understanding . . . for example when people were asked to do their plans . . . I would want to say with somebody, ‘summarise what key message you’ve taken away from that session’, and I didn’t see a lot of that . . . I don’t think we cover it in training so again, it’s something that could be emphasised more.
Developer D07
Their functioning is quite different, you know, there’s . . . [a] lady there who understood the thing straight away, and another guy who . . . I think he does have an understanding but it’s very difficult to find out what his understanding actually is. Because he tends to talk in slightly psychotic terms and . . . very concrete thinking.
Facilitator S05/F02
Triangulation
Convergence assessment
Table 37 shows how the quantitative and qualitative findings converge. There was agreement on three components, partial agreement on two, silence on six (all expected areas were amenable to only one form of assessment), and dissonance on seven. Some areas of dissonance indicated that data were being collected on quite different aspects of the construct, for instance the amount of resource committed versus how the result was valued. In other areas, qualitative research revealed nuances, for instance, although resource for co-ordination was allocated, it was insufficient.
| Logic model construct | Data | Convergence code | ||
|---|---|---|---|---|
| Quantitative | Qualitative | |||
| Resources (see the Resources section, except where stated otherwise) | ||||
| 1 | STEPWISE programme
|
Costs of infrastructure and mentors/managers not included in this experiment | Seven LDC mentors/managers played the role of trainers for this experiment. Infrastructure to be established if the trial is successful and the market allows | Agreement. This research does not provide evidence of effective infrastructure to sustain the STEPWISE programme |
| 2 | Training course materials and venue | Training costs: £2868 for 10 centres | Course materials are universally popular resources (see Relational work). Limited availability but good acceptability of venues | Dissonance. The meaning of items coded to this construct are different. Costs were committed; they were adequate and deployed successfully |
| 3 | Health professionals motivated to become STEPWISE facilitators (ideally experienced in group facilitation) and an appropriately resourced co-ordinator and a bought-in NHS trust chief executive and gatekeepers | Group facilitation experience was variable; co-ordinator excess treatment costs were £1734 per centre; facilitator excess treatment costs were £468 per centre (10 centres) for three courses of six to eight participants | Motivation generally good, with some exceptions (see Relational work). Co-ordinators assumed to be appropriately resourced – although administration load on facilitators was a common complaint (see Operational work). A minority of those interviewees did not believe that people of sufficient seniority drove the intervention forward (see Relational work) | Dissonance. The meaning of items coded to this construct are different. Costs were committed. In some cases, it was claimed that both monetary and wider organisational commitment was inadequate |
| 4 | STEPWISE course materials: venue, leaflets, patient handbook, refreshment, travel costs | Excess treatment costs for course materials: £832 per centre (10 centres); venue costs hidden in crude costs | Subsidised travel, course materials and refreshments were popular with participants (see Course delivery and protocol non-compliance), but facilitators sometimes doubted their sustainability (see Predictors of weight change) | Dissonance. The meaning of items coded to this construct are different. Resource was committed, popular with patients, but sometimes seen as unsustainable by professionals |
| 5 | People with schizophrenia were motivated to attend the course | The study screened 1223 people, of whom 800 did not participate. A total of 406 out of 800 exclusions expressed a lack of interest or declined consent | – | Silence. No qualitative data about the motivation of the wider population |
| Activities (see the Activities section, except where stated otherwise) | ||||
| 6 | STEPWISE programme mentors frequently assess the competency of trainers | – | The research project did not incorporate a system of mentorship located within trusts | Silence. Quantitative data did not speak to mentorship arrangements |
| 7 | Trainers train and periodically assess the fidelity of NHS trust-based group facilitators | Three facilitator training courses ran from March to July 2015; 48 facilitators attended core training, of whom 42 attended booster/support training. November 2015 to June 2016, seven staff attended full training; five were trained in booster/support contact only. Ten per cent of group sessions were observed using the DOT and facilitator behaviours checklist | LDC interviews confirmed training and fidelity assessment observations | Agreement. Both data types confirmed that action was completed |
| 8 | Programme management (including the identification of staff, booking rooms) | – | Programme management felt under-resourced and stressful for many facilitators (see Operational work) | Silence. Quantitative data did not speak to programme management |
| 9 | Gatekeepers actively refer people | Screening CRFs captured three incidents in which the care co-ordinator did not give permission to contact potentially eligible candidates, and two in which an assistant practitioner referred a candidate and the care co-ordinator blocked contact | Facilitators confirmed that gatekeepers sometimes presented barriers to participation (see Relational work) | Agreement. Quantitative and qualitative data confirmed the existence of clinical ‘gatekeeping’ |
| 10 | ‘Case’ management (booking people on the course, sending letters, follow-up) | – | Facilitators had trouble protecting time for individual-level case management, which was labour intensive | Silence. Quantitative data did not speak to case management |
| Immediate outcomes (see the section Immediate outcomes, except where stated otherwise) | ||||
| 11 | Quality of STEPWISE training maintained | – | The research project did not incorporate a system of continuous quality assurance | Silence. Quantitative data did not speak to quality assurance arrangements |
| 12 | Sessions delivered in line with the STEPWISE philosophy (‘fidelity’) | Mean (SD) facilitator talk time (DOT score) was 47.6% (12.26%). Mean percentage (SD) of facilitator behaviours that were deemed positive was 54.1% (17.67/35 items) | LDC staff indicated that facilitators sometimes gave insufficient time to action-planning or for the participant to answer questions – often offering, rather than eliciting, solutions. Facilitators reported that the highly-structured character of the intervention was challenging and acknowledged deviation | Partial agreement. DOT scores were difficult to interpret, but data of both types indicate that fidelity was not complete, with a highly structured intervention proving challenging to some staff, who were used to more professional autonomy |
| 13 | Attendance at all foundation and booster sessions; one-to-one telephone support sessions with demonstrable in-session ‘receipt’ of behaviour change content (‘dose received’) | 82.6% of participants (n = 171) attended at least two sessions. 36 (17.4%) allocated to the intervention were non-attenders, whereas 111 (53.6%) attended ≥ 3 core sessions and ≥ 1 booster session. 3218 support contacts were made, with 169 participants (81%) receiving one or more contact. 167 (81%) had at least one telephone contact (average of 15). 105 participants (50.7%) received at least one mail contact (average of five) and 68 (32.9%) received a minimum of one face-to-face support contact (average of three) | Adequate strategies to understand participant ‘receipt’ (understanding and internalisation) of behaviour change content not in place | Dissonance. Quantitative data show us that most people attend; qualitative data show our lack of understanding about whether or not they understand or can use the intervention skills |
| 14 | 4 × weekly foundation and three ‘booster’ sessions; one-to-one telephone support calls made (‘dose delivered’) | All foundation sessions were delivered; one booster session did not run. Not all support contacts were delivered. Facilitators found it difficult to contact some participants; however, there was incidence of non-compliance (such as first attempt to contact not being personalised and failure to conduct contacts) | Interviews with facilitators indicate that sessions were delivered, but a large number of support contact attempts were unsuccessful | Partial agreement. Qualitative research shows that support contact metrics must be interpreted with caution |
| 15 | Eligible people were invited to attend the STEPWISE programme (‘reach’) | 414 out of 1223 potentially eligible service users were randomised. Estimates of potentially eligible service users varied significantly between trusts for which data were available (1233–12,968). Target vs. actual recruitment at these sites was 40 out of 36, 50 out of 45 and 42 out of 35, respectively | – | Silence. Qualitative data did not speak to reach |
| Selected intermediate outcomes (see Outcomes and estimation and Perceived effectiveness) | ||||
| 16 | Physical activity | Accelerometry shows no statistically significant difference between the intervention and control groups | Qualitative research participants claimed to have achieved changes in physical activity | Dissonance. Data types suggest different outcomes |
| 17 | Nutrition | DINE shows no statistically significant difference between the intervention and control groups | Qualitative research participants claimed to have achieved dietary changes | Dissonance. Data types suggest different outcomes |
| 18 | Weight | No statistically significant difference between the intervention and control groups | Qualitative research participants claimed to have achieved weight loss; for most, this was not borne out by quantitative evidence, or the weight loss was not clinically significant | Dissonance. Data types suggest different outcomes |
Completeness assessment
The quantitative research contributed information to 14 out of 18 logic model components, whereas the qualitative components contributed to 16. This was expected: some components were not amenable to investigation in quantitative terms.
Brief summary
The numbers relate to rows in Table 37. The study was not run in naturalistic conditions, meaning that national (1, 11) and local (6) infrastructure for quality assurance was not in place. Training resources were adequate (2). Most health professionals were motivated, but neither co-ordination resource nor senior leadership were adequate at some trusts (3). The STEPWISE course materials were popular with participants; professionals doubted their sustainability (4). When offered the STEPWISE trial, half of the people with schizophrenia were motivated to participate (5).
Training and fidelity assessments were conducted (7). Facilitators often felt that programme management (8) and case management (10) were under-resourced and, as a result, stressful. There was some evidence of clinical gatekeepers barring entry to eligible people, but we do not think that this was widespread (9).
Lapses in fidelity included giving insufficient time to answer questions and giving, rather than eliciting, solutions from participants (12). STEPWISE has no reliable mechanisms by which facilitators can investigate whether or not participants understand or can use the intervention (13). 24 Scheduled sessions went ahead but, when support contacts were attempted, they were not always successful (14). During the trial period, we reached a small fraction of those who were potentially eligible in the study areas (15).
Although many participants claimed to have achieved important changes in physical activity levels, nutrition and weight, quantitative data showed that this was true only for a minority and that any differences between trial groups could not be explained by receipt of the STEPWISE intervention.
Chapter 6 Results of the health economic analysis
Health-related quality of life
The EQ-5D-5L utility scores (Table 38) at the 3-month follow-up point were slightly higher in the intervention group (0.81 in the intervention group vs. 0.79 in the control group); however, at the 12-month point, there was no difference between the tariff values of the two groups (0.793 in the intervention group vs. 0.794 in the control group). At baseline, the EQ-5D-5L score was higher in the intervention group than in the control group, which has a negative effect on the cost-effectiveness calculations that consider the level of QoL (measured by the tariff) before the intervention. The total QALYs were very similar over the 12-month period, with a slight advantage for the intervention group.
| Time point | Trial arm, mean (SD) | All, mean (SD) | |
|---|---|---|---|
| Treatment | Control | ||
| Baseline | 0.79 (0.20) | 0.78 (0.19) | 0.79 (0.19) |
| 3 months | 0.81 (0.16) | 0.79 (0.21) | 0.80 (0.19) |
| 12 months | 0.79 (0.24) | 0.79 (0.24) | 0.79 (0.24) |
| Total QALYs | 0.81 (0.012) | 0.79 (0.015) | 0.80 (0.010) |
Short Form questionnaire-36 items questionnaire and Short Form questionnaire-6 Dimensions measure of quality of life
Short Form questionnaire-6 Dimensions scores, in contrast to the EQ-5D scores, were slightly higher in the control group (0.01 tariff points) than in the intervention group at baseline. Tariff scores also appear to be 0.02 tariff points higher in the intervention group at the 3- and 12-month follow-up periods. There is still only a very small advantage in the QALYs of the intervention group when using the SF-36 questionnaire to calculate a SF-6D QALY (Table 39). It is of interest that the tariffs using the SF-6D are substantially lower than those obtained from the EQ-5D, but this is in line with earlier findings. 128
| Time point | Trial arm, mean (SD) | All, mean (SD) | |
|---|---|---|---|
| Treatment | Control | ||
| Baseline | 0.61 (0.005) | 0.62 (0.005) | 0.62 (0.004) |
| 3 months | 0.63 (0.005) | 0.61 (0.006) | 0.62 (0.004) |
| 12 months | 0.63 (0.006) | 0.61 (0.005) | 0.62 (0.004) |
| Total QALYs | 0.63 (0.005) | 0.61 (0.006) | 0.62 (0.004) |
Costs
Intervention costs
For the base case, the unit cost of two facilitators providing the intervention was provided, assuming that a group of four participants attended the intervention and the cost of facilitators was based on the wages of a mental health nurse. In this case, the cost of the intervention is around £35.20 per patient per hour and £88 for a full 2.5-hour session. The mean cost of the intervention is £565 for the base case. The maximum cost of the intervention, in the base-case scenario, is £616 for seven full sessions and £283.20 for 24 support contacts at a price of £1.18 per minute for 10 minutes.
Health and social care costs and lost work/education
Table 40 shows the number and percentage of service users accessing each type of care, the mean number of contacts with the service for those using it, and the mean cost of the service across all participants. Less frequently visited health-care professionals were combined into one category: other health-care professionals. This category includes drug advisors, other counsellors, home treatment teams, outreach workers, intervention therapists, occupational therapists, drug service facilities and community mental health support workers. The costs of social care were combined into another category, including day care, drop-in centre, self-help group, leisure groups, adult classes and other types of care. The number of contacts for the intervention is shown separately for each time point and represents the number of full sessions attended in the case of the intervention group. However, the cost calculations for other health-care services take into account patients attending part of a session.
| Category of service use | Health and social care service use and productivity losses during the 3 months pre randomisation | |||||
|---|---|---|---|---|---|---|
| Trial arm, n (%) of participants using services | Trial arm, mean (SD) contacts for those using services | Trial arm, mean (SD) costs (£) for all participants (2015–16 pounds sterling) | ||||
| Treatment | Control | Treatment | Control | Treatment | Control | |
| GP | 142 (68) | 138 (67) | 2 (0.2) | 3 (0.2) | 83 (9) | 90 (9) |
| Psychiatrist | 127 (61) | 122 (60) | 2 (0.2) | 2 (0.2) | 94 (14) | 89 (10) |
| Psychologist | 25 (12) | 27 (13) | 5 (0.8) | 4 (0.7) | 67 (19) | 53 (13) |
| Other doctor | 16 (8) | 14 (7) | 2 (0.3) | 3 (0.8) | 8 (3) | 10 (4) |
| Mental health nurse | 121 (58) | 121 (59) | 6 (0.8) | 4 (0.3) | 172 (38) | 137 (22) |
| Social worker | 41 (20) | 35 (17) | 4 (0.5) | 5 (0.9) | 52 (14) | 91 (30) |
| Other health-care professionala | 104 (50) | 99 (48) | 5 (0.7) | 4 (0.5) | 308 (55) | 196 (28) |
| Inpatient (days) | 6 (3) | 12 (6) | 28 (37) | 28 (30) | 324 (183) | 658 (242) |
| Medication | 206 (99) | 199 (97) | N/A | N/A | 408 (26) | 397 (29) |
| Other health and social care servicesb | 76 (37) | 69 (34) | 14.8 (2.0) | 12.3 (1.4) | 308 (55) | 195 (28) |
| Informal care (hours per week) | 117 (56) | 125 (61) | 10.4 (1.1) | 10.8 (1.5) | 912 (1597) | 1019 (2218) |
| Lost employment (days) | 27 (13) | 26 (13) | 2.6 (1.4) | 6.3 (2.8) | 29 (223) | 66 (453) |
| Lost education (days) | 25 (12) | 13 (63) | 5.4 (3.3) | 1.9 (1.6) | 54 (34) | 10 (9) |
| Police | 3 (14) | 6 (29) | 1.7 (0.7) | 1.2 (0.2) | 2 (1) | 1 (1) |
| Total health and social careb | 208 (100) | 202 (99) | 1623 (236) | 1802 (260) | ||
| Total societal | 208 (100) | 205 (100) | 2619 (258) | 2900 (315) | ||
| Category of service use | Health and social care service use and productivity losses during the 3 months post randomisation | |||||
| Trial arm, n (%) using services | Trial arm, mean (SE) contacts for those using services | Trial arm, mean (SE) costs (£) for all participants (2015–16 pounds sterling) | ||||
| Treatment | Control | Treatment | Control | Treatment | Control | |
| GP | 96 (46) | 112 (54) | 2 (0.2) | 2 (0.1) | 83 (9) | 90 (10) |
| Psychiatrist | 86 (41) | 90 (43) | 3 (1.1) | 1.2 (0.3) | 94 (14) | 89 (10) |
| Psychologist | 15 (7) | 16 (8) | 4 (0.9) | 6 (1.1) | 67 (19) | 53 (13) |
| Other doctor | 15 (7) | 14 (7) | 2.4 (0.9) | 1.6 (0.4) | 7 (3) | 10 (4) |
| Mental health nurse | 88 (42) | 98 (47) | 6 (1.1) | 5 (0.5) | 172 (39) | 136 (22) |
| Social worker | 27 (13) | 29 (14) | 9 (3) | 4 (0.9) | 52 (14) | 91 (30) |
| Other health-care professionala | 62 (30) | 67 (32) | 2 (0.4) | 3 (0.7) | 304 (55) | 195 (28) |
| Inpatient (days) | 7 (3) | 8 (4) | 18 (32) | 7 (10) | 238 (157) | 112 (57) |
| Medication | 177 (86) | 168 (85) | N/A | N/A | 159 (21) | 175 (24) |
| Other health and social care servicesb | 82 (40) | 46 (23) | 15.0 (1.9) | 13.2 (1.4) | 304 (55) | 195 (28) |
| Informal care (hours per week) | 106 (52) | 114 (58) | 13.3 (1.8) | 12.2 (2.1) | 1038 (2264) | 1018 (2671) |
| Lost employment (days) | 5 (2) | 11 (6) | 1.6 (0.9) | 6.9 (3.4) | 19 (11) | 86 (44) |
| Lost education (days) | 3 (1) | 1 (0.5) | 1.25 (0.8) | 0.8 (0.8) | 6 (4) | 3 (3) |
| Police | 7 (3) | 5 (2) | 1.3 (0.3) | 2.0 (0.6) | 3 (2) | 2 (1) |
| Intervention | 174 (85) | – | 2.83 (0.1) | – | 305 (7) | – |
| Total health and social careb | 205 (100) | 195 (98) | 1640 (197) | 1133 (100) | ||
| Total societal | 205 (100) | 198 (100) | 2599 (248) | 2135 (214) | ||
| Category of service use | Health and social care service use and productivity losses between 3 and 12 months post randomisation | |||||
| Trial arm, n (%) using services | Trial arm, mean (SE) contacts for those using services | Trial arm, mean (SE) costs (£) for all participants (2015–16 pounds sterling) | ||||
| Treatment | Control | Treatment | Control | Treatment | Control | |
| GP | 128 (62) | 133 (64) | 4 (0.4) | 5 (0.5) | 132 (15) | 150 (19) |
| Psychiatrist | 124 (60) | 116 (56) | 3 (0.3) | 2 (0.2) | 123 (14) | 111 (13) |
| Psychologist | 29 (14) | 22 (11) | 11 (3) | 9 (2) | 176 (56) | 92 (27) |
| Other doctor | 24 (12) | 22 (11) | 2 (0.4) | 3 (0.5) | 15 (4) | 19 (6) |
| Mental health nurse | 97 (47) | 102 (49) | 6 (1.1) | 5 (0.5) | 107 (30) | 270 (73) |
| Social worker | 30 (14) | 31 (15) | 9 (3) | 4 (1) | 75 (22) | 107 (30) |
| Other health-care professionala | 69 (33) | 79 (38) | 8 (2) | 8 (2) | 390 (95) | 413 (136) |
| Inpatient (days) | 18 (8) | 14 (7) | 20 (7) | 25 (8) | 670 (251) | 607 (249) |
| Medication | 204 (98) | 195 (97) | N/A | N/A | 1080 (67) | 1033 (82) |
| Other health and social care servicesb | 51 (25) | 59 (29) | 43.7 (8.2) | 33.3 (4.8) | 390 (95) | 413 (135) |
| Informal care (hours per week) | 90 (43) | 99 (49) | 13.2 (1.9) | 10.9 (1.3) | 1038 (2264) | 1018 (2671) |
| Lost employment (days) | 12 (6) | 14 (7) | 6.9 (3.6) | 20.8 (10.8) | 77 (42) | 237 (128) |
| Lost education | 3 (1) | 4 (2) | 1.8 (1.1) | 2.0 (1.2) | 7 (5) | 7 (4) |
| Police | 7 (3) | 6 (3) | 2.7 (0.8) | 1.8 (0.4) | 28 (8) | 4 (2) |
| Intervention | 174 (85) | – | 1.43 (0.1) | – | 273 (13) | – |
| Total health and social careb | 208 (100) | 200 (99.5) | 3616 (353) | 3319 (387) | ||
| Total societal | 208 (100) | 201 (100) | 8734 (898) | 8170 (863) | ||
At baseline, the most frequently consulted health professionals were GPs (68% in the intervention arm and 67% in the control arm), psychiatrists (61% in the intervention arm and 60% in the control arm) and mental health nurses (58% in the intervention arm and 60% in the control arm). At baseline, 20% of participants in the intervention group and 17% in the control group accessed social care. Fifty per cent of participants in the intervention group accessed other health-care professionals and 49% in the control group did so.
At the 3-month follow-up, the most frequently consulted health professionals were still GPs (46% in the intervention arm and 54% in the control arm), psychiatrists (41% in the intervention arm and 43% in the control arm) and mental health nurses (42% in the intervention arm and 47% in the control arm). At the 3-month follow-up, 13% of participants in the intervention group and 14% of those in the control group (a slightly lower proportion than at baseline) accessed social care. Thirty per cent of participants in the intervention group and 32% in the control group accessed other health-care professionals. Overall, the level of participants reporting use of all of these categories of services was slightly lower in both groups.
At the 12-month follow-up, the most frequently consulted health professionals remained GPs (reported by 62% in the intervention group and 64% in the control group), psychiatrists (reported by 60% in the intervention group and 56% in the control group) and mental health nurses (reported by 47% in the intervention group and 56% in the control group). At 12 months, 14% of participants in the intervention group and 15% in the control group accessed social care. According to self-reported data, 33% of participants in the intervention group and 38% in the control group accessed other health-care professionals. At baseline, 43% of participants in the intervention and 49% in the control group group reported receiving informal, largely unpaid, care. The same percentages also reported this at the 3-month follow-up. At the 12-month follow-up, 65% of the intervention group and 59% of the control group reported receiving informal care.
Table 40 also includes productivity losses, which are the cost of lost work and education time of participants in the study and informal care received from carers. As informal carers are largely unpaid, productivity losses relating to informal care indicate that the amount of money that could have been gained by working during the time that informal care is provided (i.e. the opportunity cost of caring for the service user). At baseline, 56% of participants in the intervention group and 61% in the control group were employed. By the end of the study, these figures had fallen slightly to 43% in the intervention group and 49% in the control group. At baseline, 13% of participants reported that they were working and missing work because of illness in the intervention group and 13% reported this in the control group. This was reported at similar levels (12% in the intervention group and 14% in the control group) at the 12-month follow-up. At baseline, 12% of participants in the intervention group and 13% in the control group attended further education, mostly further education colleges. At baseline, the proportion of participants who reported that they were unable to attend further education college because of illness was 4% in the intervention group and 2% in the control group. At the 12-month follow-up, the percentage of participants in further education and missing one or more days of education was 2% in both the intervention group and the control group.
Health and social care costs included the costs of medicines and of visits to NHS professionals in primary and community care and inpatient settings, as well as social care costs. Over the follow-up period, the mean total health and social care costs were £5255 in the intervention group and £4453 in the control group. Societal costs were calculated using police costs, productivity losses from lost education and employment and informal care costs. The mean total societal costs were £11,332 in the intervention group and £10,305 in the control group. The cost differences were partly attributable to the intervention, but were also caused by greater use of other services.
The differences in health and social care and societal costs are not significant. For health and social care costs, the difference (after controlling for baseline) was £870.39 (p-value = 0.132, bootstrapped CI –£262 to £2003). For societal costs, the difference (after controlling for baseline) was £1295.37 (p-value = 0.33, bootstrapped CI –£1329 to £3919).
Cost-effectiveness analysis
The net benefit approach was used in order to produce cost-effectiveness planes and CEACs. Multiple imputation (chained equations) was applied to the cost and QoL data.
Cost-effectiveness results based on the EuroQol-5 Dimensions, five-level version
The incremental costs and QALYs are shown in Table 41. The ICER from the health-care perspective is £246,921 per QALY and from the societal perspective is £367,543 per QALY.
| Outcome: QALYs from the EQ-5D-5L | Treatment vs. control |
|---|---|
| Incremental effect | 0.0035234 |
| Incremental health-care cost | £870 |
| ICER (health care) | £246,921 |
| Incremental societal cost | £1295 |
| ICER (societal) | £367,543 |
The bootstrapped replications of the difference in health and social care costs range from £433 to £1392. The bootstrapped replications of the difference in EQ-5D QALYs range from –0.012 to 0.013 (Figure 26). It is clear that the intervention is cost-increasing and results in better or worse outcomes to a similar extent. The line shown on the scatterplot represents the NICE threshold, below which the replicates should fall for the intervention to be cost-effective. No replications fall below this line. The probability that the STEPWISE intervention is cost-effective is 0% at the £20,000 threshold set by NICE, as shown by Figure 27. The probability that the intervention is cost-effective is still only 17% at a threshold of £200,000 when the analysis is conducted from the health-care perspective.
FIGURE 26.
Cost-effectiveness plane for EQ-5D QALYs from the health and social care perspective.
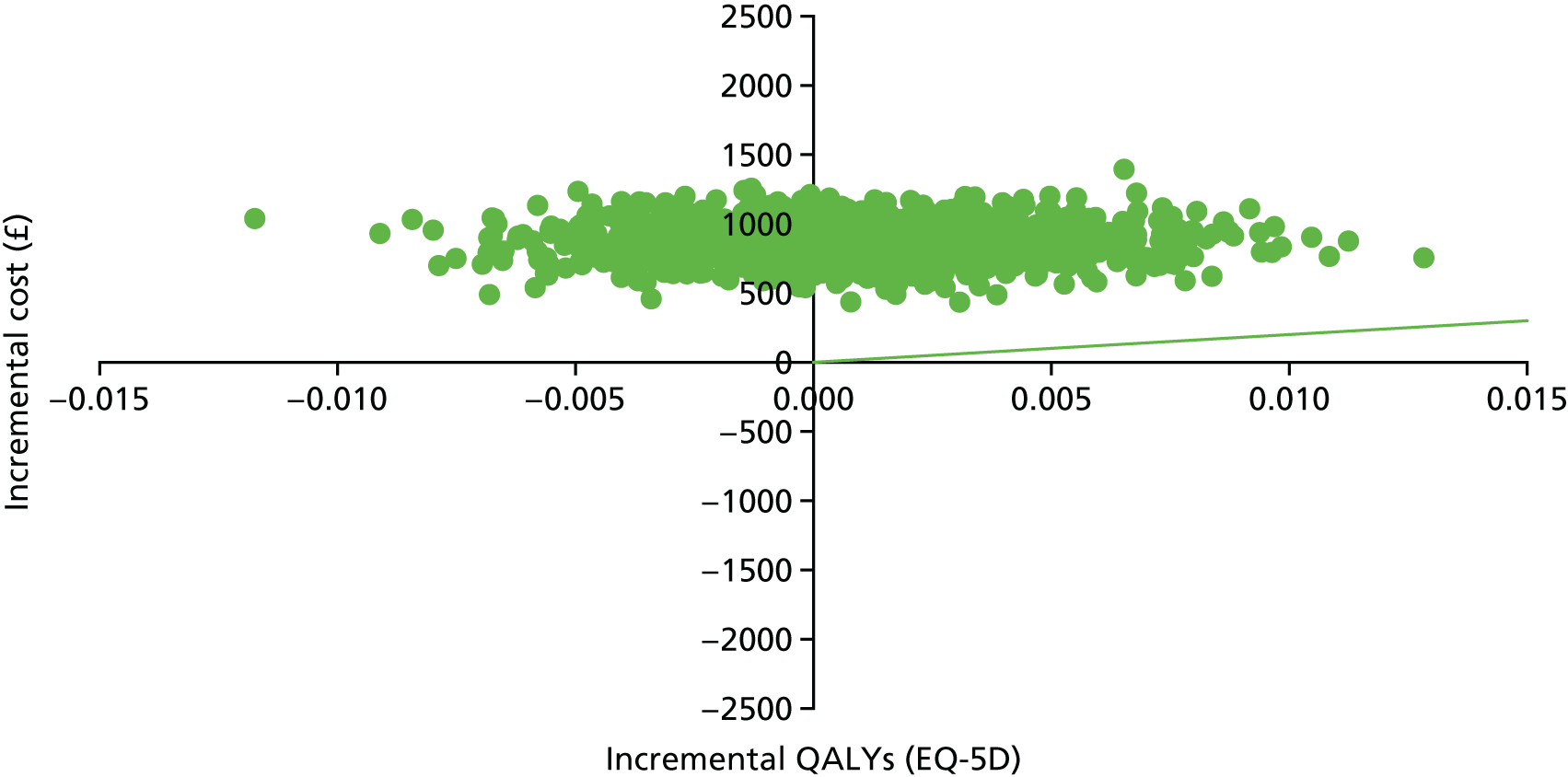
FIGURE 27.
Cost-effectiveness acceptability curve for EQ-5D QALYs from the health and social care perspective.
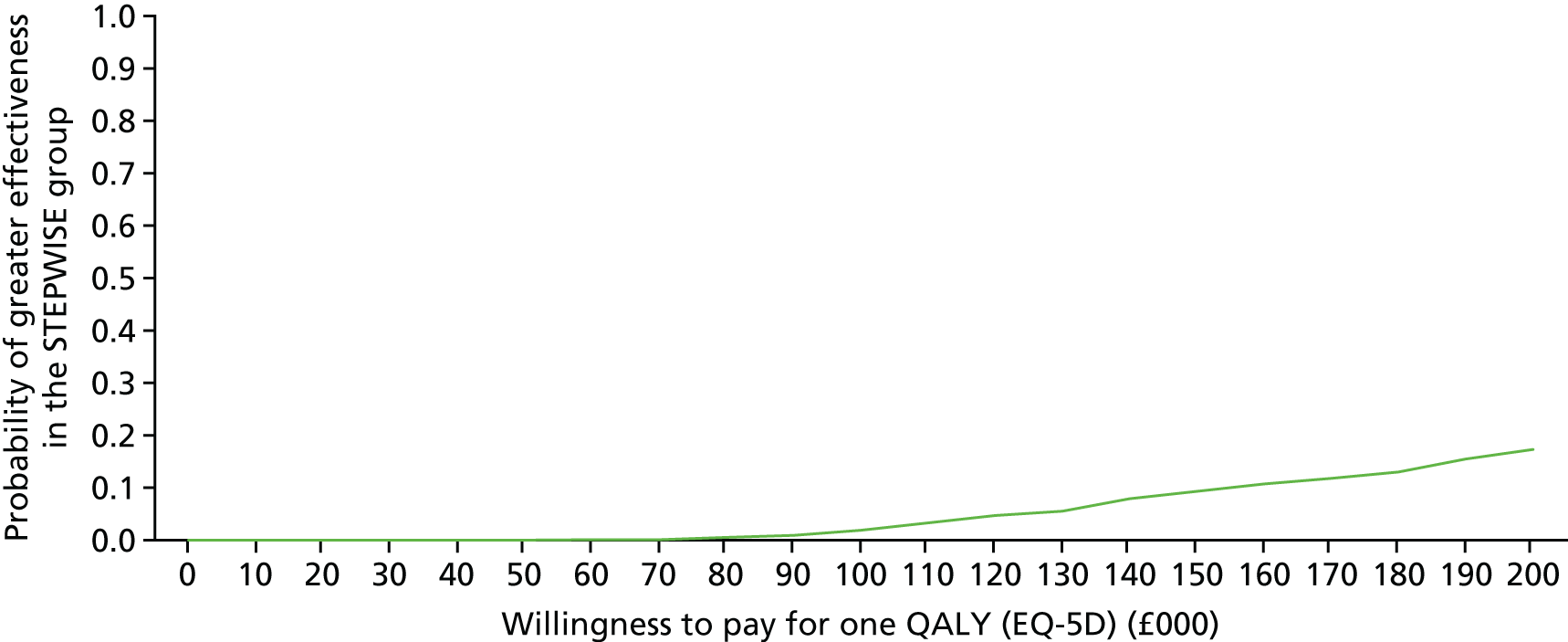
The ICERs for the societal perspective showed similar results to those for the health-care perspective. The bootstrapped replications of the difference in societal costs range from £192 to £2382. The bootstrapped replications of the difference in EQ-5D QALYs range from –0.012 to 0.013 (Figure 28). Again, the intervention results in higher costs and approximately equal likelihoods of better or worse outcomes. We have not shown an ICER line, as NICE does not take a societal perspective. However, the probability that the STEPWISE intervention is cost-effective is 0% at a £20,000 threshold (Figure 29). The probability that the intervention is cost-effective is still only 7% at a threshold of £200,000 when the analysis is conducted from the societal perspective.
FIGURE 28.
Cost-effectiveness plane for EQ-5D QALY from the societal perspective.
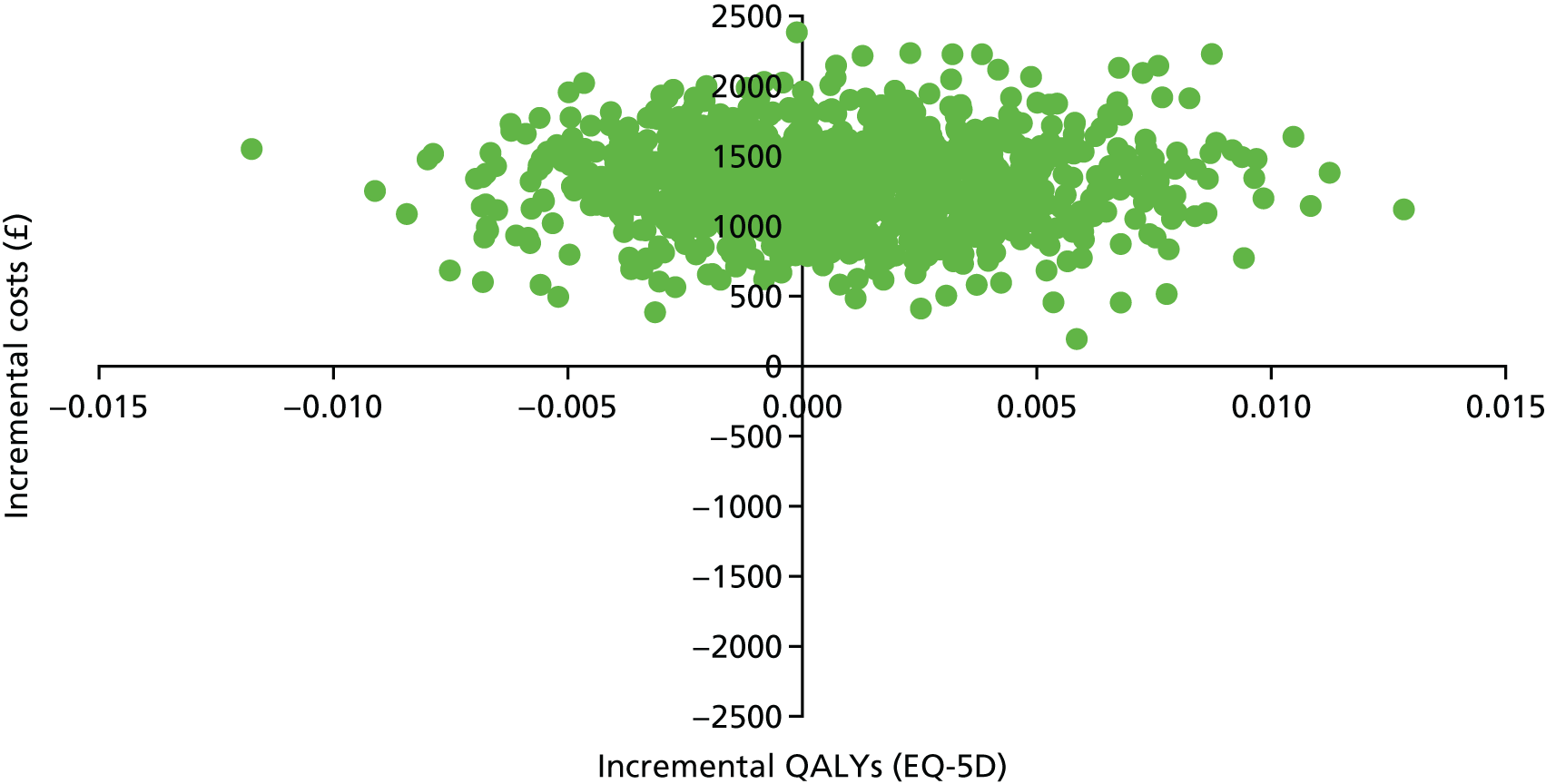
FIGURE 29.
Cost-effectiveness acceptability curve for EQ-5D QALY from the health and social care perspective.
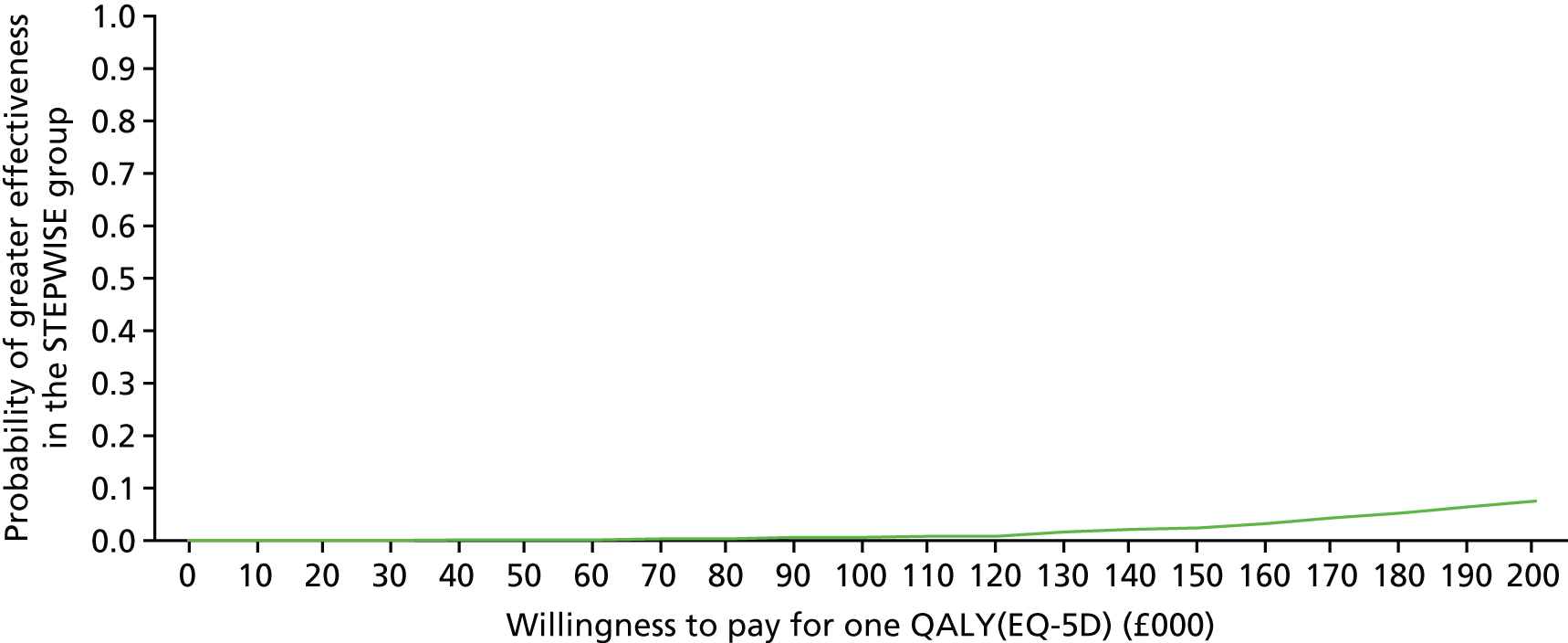
Cost-effectiveness results based on the Short Form questionnaire-6 Dimensions
Health and social care perspective
The bootstrapped replications of the difference in health and social care costs range from £433 to £1392. A total of 1000 bootstrapped replications of both the costs and the QALYs were performed. The bootstrapped replications of the difference in SF-6D QALYs range from 0.003 to 0.012 QALYs (Figure 30). Table 42 shows the SF-36 QALY outcomes at 1 year. It is clear that the intervention is cost-increasing and results in better or worse outcomes to a similar extent. The line shown on the scatterplot represents the NICE threshold, below which the replicates need to fall for the intervention to be cost-effective. No replications fall below this line. The probability that the STEPWISE intervention is cost-effective is therefore, 0% at the £20,000 per QALY threshold set by NICE, as shown by Figure 31. The probability that the intervention is cost-effective is 50% at a threshold of £110,000 per QALY and 99% at a threshold of £200,000 per QALY when the analysis is conducted from the health-care perspective.
FIGURE 30.
Cost-effectiveness plane for SF-6D QALYs from the health and social care perspective.
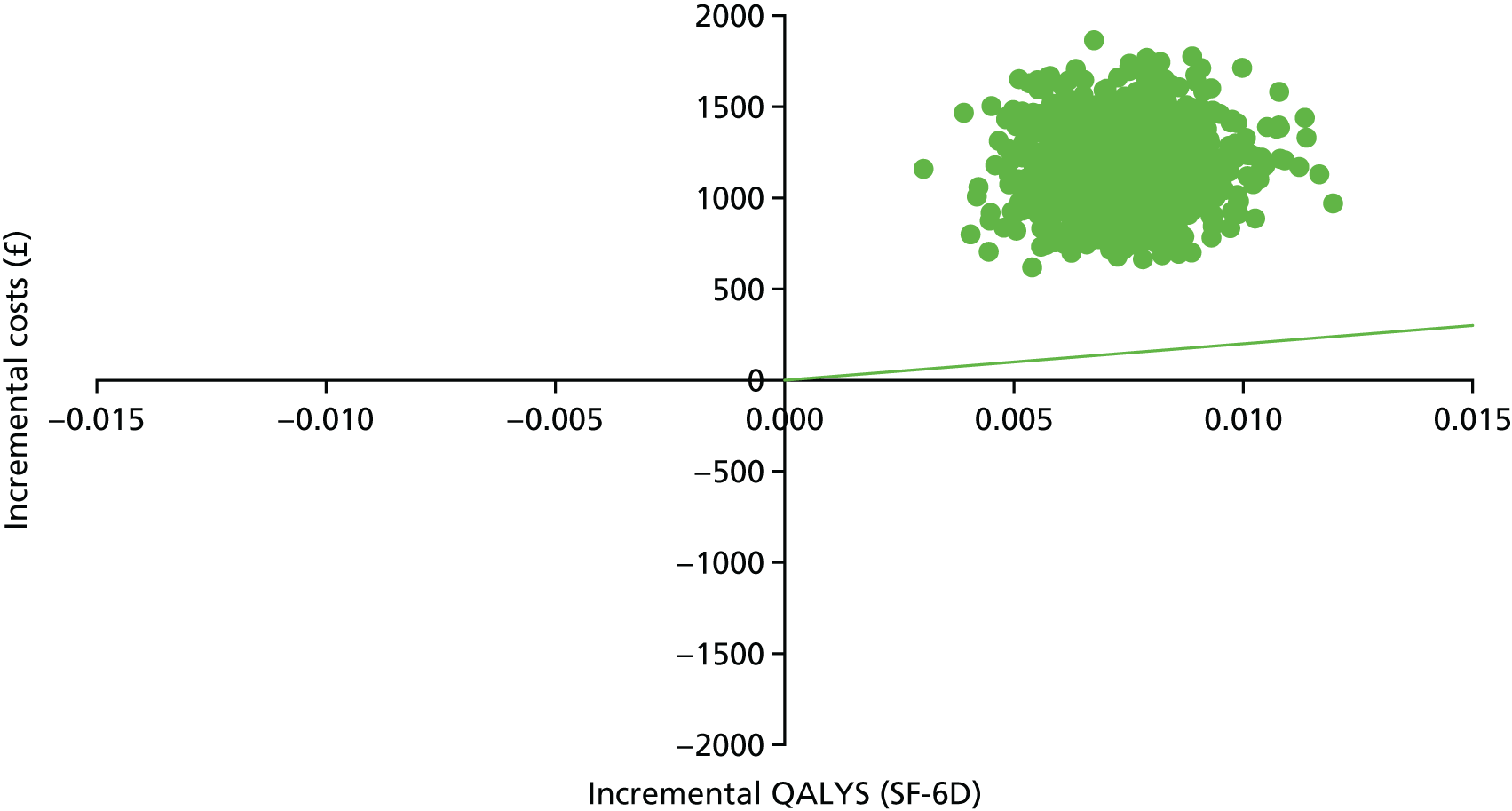
| Outcome: QALYs from the SF-6D | Treatment vs. control |
|---|---|
| Incremental effect | 0.0148241 |
| Incremental health-care cost | £870 |
| ICER (health care) | £58,688 |
| Incremental societal cost | £1295 |
| ICER (societal) | £87,358 |
FIGURE 31.
Cost-effectiveness acceptability curve for SF-6D QALYs from the health and social care perspective.
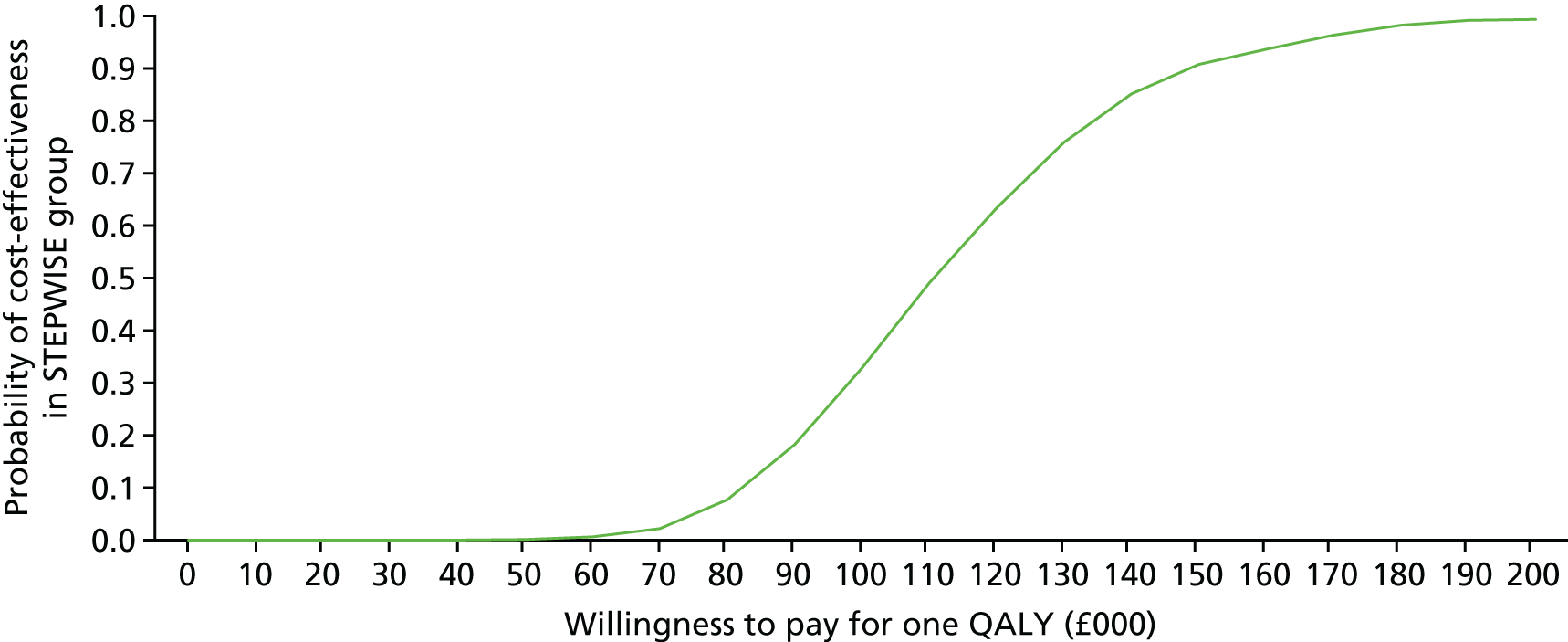
Societal perspective
The bootstrapped replications of the difference in societal costs range from £403 to £1667. The bootstrapped replications of the difference in SF-6D QALYs range from 0.003 to 0.012 QALYs.
The probability that the STEPWISE intervention is cost-effective is 0% at the £20,000 threshold set by NICE, as shown by Figure 32. The probability that the intervention is cost-effective is 74% at a threshold of £200,000 when the analysis is conducted from the societal perspective (Figure 33).
FIGURE 32.
Cost-effectiveness plane for SF-6D QALYs from the societal perspective.
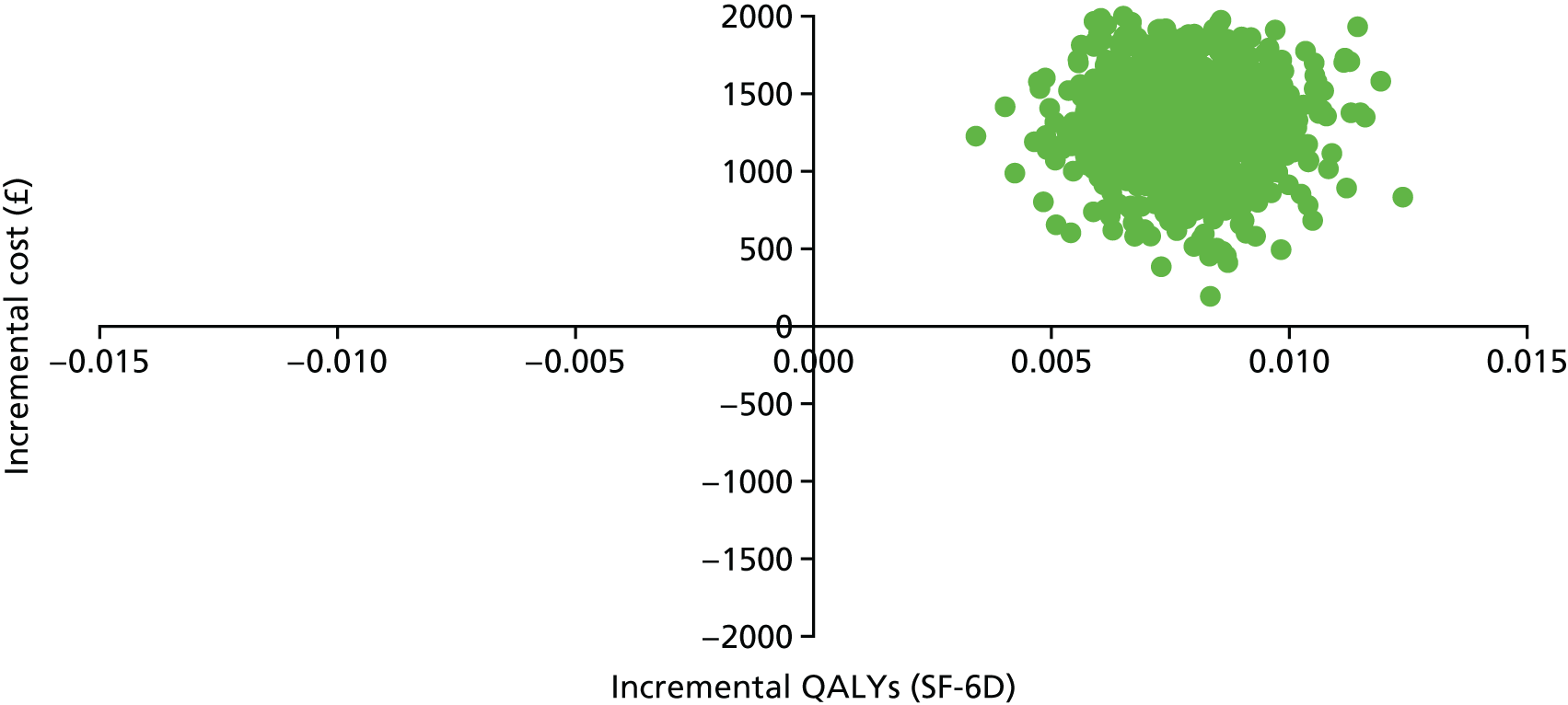
FIGURE 33.
Cost-effectiveness acceptability curve for SF-6D QALYs from the societal perspective.
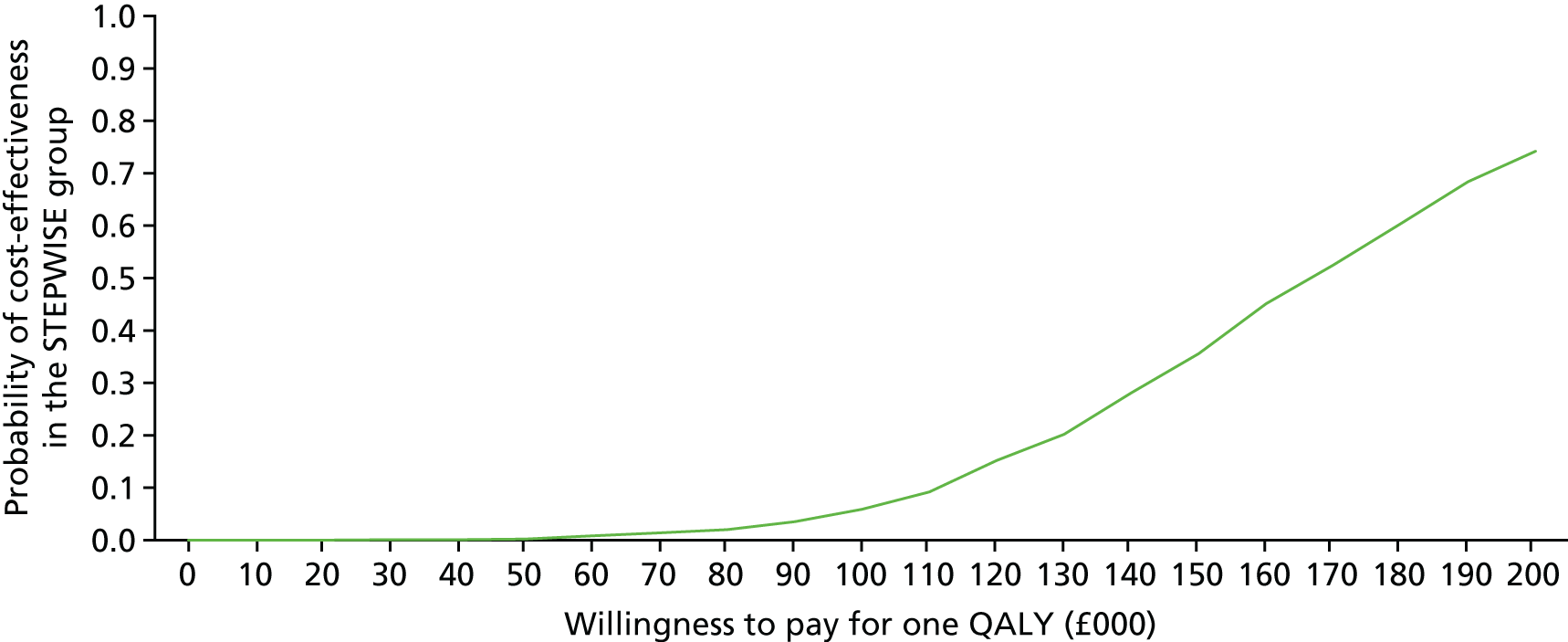
Sensitivity analysis
Table 43 shows the different outcomes for the ICER and the optimal strategy if the costs of the intervention and the group size attending the intervention are varied in the health and social care perspective. The table also shows how varying the cost of informal care and lost employment days affects the outcomes if the cost of the intervention is held constant at the price of a group of four patients receiving the course from two mental health nurses. Attendance of sessions was low, with only 53.6% of patients attending more than three main sessions and one or more booster sessions. Therefore, the base case assumed an attendance of four patients rather than the six planned for in the study design.
| Health-care perspective (EQ-5D) | |||||
|---|---|---|---|---|---|
| Profession | Group size | Unit cost, £ (per hour) | Incremental | ||
| Cost (£) | Benefit | ICER (£) | |||
| Mental health nurse | 4 | 35 | 870 | 0.0035234 | 246,921 |
| Mental health nurse | 6 | 24 | 746 | 0.0035234 | 211,727 |
| Mental health nurse | 8 | 18 | 685 | 0.0035234 | 194,414 |
| Societal perspective (EQ-5D, assuming a group of four patients) | |||||
| Informal care wage rate | Employment wage rate | Unit cost, £ (per hour) | Incremental | ||
| Cost (£) | Benefit | ICER (£) | |||
| Average | Average | 12 | 1295 | 0.0035234 | 367,543 |
| Minimum | Minimum | 7 | 785 | 0.0035234 | 222,796 |
| Health-care worker | Minimum | 20 | 795 | 0.0035234 | 225,634 |
| Health-care perspective (SF-6D) | |||||
| Profession | Group size | Unit cost, £ (per hour) | Incremental | ||
| Cost (£) | Benefit | ICER (£) | |||
| Mental health nurse | 4 | 35 | 870 | 0.0148241 | 58,688 |
| Mental health nurse | 6 | 24 | 746 | 0.0148241 | 50,323 |
| Mental health nurse | 8 | 18 | 685 | 0.0148241 | 46,209 |
| Societal perspective (SF-6D, assuming a group of four patients) | |||||
| Informal care wage rate | Employment wage rate | Unit cost, £ (per hour) | Incremental | ||
| Cost (£) | Benefit | ICER (£) | |||
| Average | Average | 12 | 1295 | 0.0148241 | 87,358 |
| Minimum | Minimum | 7 | 785 | 0.0148241 | 52,954 |
| Health-care worker | Minimum | 20 | 795 | 0.0148241 | 53,629 |
Chapter 7 Discussion
Conduct of the trial
The STEPWISE trial was a complex trial, which faced many challenges. Previous studies have suggested that the recruitment and retention of people with schizophrenia are difficult. In addition to this generic problem, the STEPWISE trial faced the additional challenge of recruiting in short waves because of the nature of the group intervention. We recognised that, if recruitment took too long, those who were recruited first would have to wait for a group to start and may lose interest by the time the intervention started. We also recognised the importance of delivering the intervention before the 3-month follow-up visits. Consequently, a concentrated period of recruitment was needed at each site to overcome this.
The STEPWISE trial was undertaken in 10 locations across England, covering both the north and south of England, rural and urban settings and mixed ethnic populations. Although this had the strength of representing the diversity of the NHS, it posed logistical challenges for the conduct of the trial, requiring robust organisation at sites and the Sheffield CTRU.
In STEPWISE, we used the methodology designed to develop interventions for people with, or at risk of, diabetes mellitus. At the outset, it was unclear whether or not it would be possible to transfer this process to a different disease area, namely schizophrenia. The intervention development phase showed that this was achievable, but it took 3 months longer than originally anticipated.
Despite the challenges of recruitment, the trial recruited 414 participants (original target: 396) in just over 1 year, which was almost 3 months quicker than planned. This illustrates the level of interest for such interventions among people with schizophrenia and mental health care professionals. Previous studies have indicated that people with psychotic illness have the same level of interest in their physical health, but struggle to prioritise this over other issues, such as their mental illness.
Following successful recruitment, 341 participants (81.6%) completed the trial. This retention was similar to our original estimates and other intervention studies for people with schizophrenia. 55 A total of 20 participants were lost to follow-up, 48 withdrew consent and three died. The reason for withdrawal of consent was often the worsening of mental illness precluding attendance at research assessments.
A strength of the STEPWISE trial was the use of an objective primary outcome measure, which could not be greatly affected by knowledge of the intervention. 129 Although participants were not blinded, the STEPWISE assessors were blinded and largely remained blind during follow-up, further reducing the risk of observer bias. 130
As a result, the STEPWISE trial is the largest trial of weight management in severe mental illness of its kind. It was adequately powered to answer the hypothesis we posed at its outset. Furthermore, through the use of sensitivity analyses, even under extreme assumptions about missing data, the results of the trial would be unchanged.
Summary of the findings
The STEPWISE intervention was neither clinically effective nor cost-effective over the 12-month trial period. Both groups lost an average of 0.5 kg, suggesting that there may have been a small benefit to participation in the trial, given the known trajectory of weight gain for people taking antipsychotics,131 but there was no difference in weight change between groups. To achieve weight reduction, the intervention needed to promote changes in dietary intake, physical activity or both. The baseline data confirmed that the trial participants were eating a diet that was high in refined carbohydrates and low in fibre. In addition, the accelerometry data indicated that they were physically inactive, with levels of activity similar to older adults from the general population aged 75–79 years. 132 The imprudent diet and physical inactivity provided substantial opportunity for change, but there was no sustained behaviour change in either of these crucial behaviours. Furthermore, the intervention did not lead to changes in other biomedical outcomes, psychiatric well-being, QoL or obesity perception.
The estimated cost of the STEPWISE programme was £565, but the intervention was not cost-effective, owing to the relatively small effect of the intervention on the (EQ-5D-5L) QoL measure (a QALY difference of around 0.0035 between the treatment and control groups after controlling for baseline utility scores) and increased costs of other services. This results in an ICER of > £200,000 in the base-case analysis.
The lack of effect on weight contrasts with previous reports that suggested that lifestyle interventions could help people with severe mental illness to lose weight. The STEPWISE study drew heavily from the experience published in the meta-analysis by Caemmerer et al. ,12 which reviewed 17 studies and included 810 people receiving antipsychotics. This paper found that lifestyle interventions led to significant weight reduction, in association with concomitant improvements in cardiovascular risk factors. However, most of the studies were of short duration, with most lasting for 12–16 weeks, and included a small number of participants (median 53, range 15–110).
The 2014 NICE guidance for psychosis and schizophrenia in adults14 included 24 studies and came to similar conclusions. Both reviews included studies that had recruited on the basis of antipsychotic treatment rather than diagnosis, and so significant numbers of participants had other types of mental illness. This is important, as the strategies needed to promote weight loss in people with schizophrenia may be different. NICE comments that few studies examined weight change beyond 6 months, and only six included > 100 participants. With relevance to the NHS, no study was conducted in the UK. There was a moderate risk of bias for most studies and there was substantial heterogeneity of effect size. The evidence review concluded that lifestyle interventions were effective in reducing and maintaining body weight in the short term, but, without longer-term data, effects beyond 6 months were unknown. Despite the limitations in the evidence, based on expert opinion, NICE recommends that mental health-care services should offer a combined healthy eating and physical activity programme to people with severe mental illness, particularly if they are taking antipsychotics. 14
The length of follow-up of the STEPWISE trial allowed a longer-term perspective, which addressed some of the shortfalls of earlier studies. Over the course of the STEPWISE trial, several other long-term studies examining the effect of lifestyle interventions were published and are included in the most recent systematic review. 15 Six studies reported that interventions lasting for more than 1 year showed a more consistent weight reduction (SMD = −0.24, 95% CI −0.36 to −0.12), although only two of the six studies recorded a statistically significant weight loss, both of which included substantial numbers of people with other severe mental illnesses.
Given the unexpected results of the STEPWISE trial, we examined why the intervention failed, in order to inform future research and clinical practice. The first question is whether or not the intervention was fit for purpose.
Development and implementation of the intervention
The STEPWISE programme meets most of the Department of Health and Social Care guidelines,17 on which its parent programme, DESMOND,19 was based. It is theory driven (see The intervention’s theory of change) and evidence based (see Intervention development), coproduced with patients and flexible to emerging individual needs (see Intervention development). Its specific aims and objectives are shared with users and it supports self-management attitudes, beliefs, knowledge and skills for the user (see Intervention development). 17 The structured, written curriculum incorporates the assessment of individual learning needs and can be flexibly applied, meeting diverse needs (see Intervention development). It appears to be reliable, valid, relevant and comprehensive and uses different teaching media and supporting materials (see Table 35). However, staff raised questions about its resource efficiency (see Chapter 5, Appraisal work) and the health economic model (see Chapter 6, Cost-effectiveness analysis).
The STEPWISE facilitators demonstrated an understanding of empowerment philosophy133–135 (see Chapter 5, Facilitator views of STEPWISE, Sense-making), but perhaps lacked an understanding of the behaviour change theories that underpinned the intervention. It is possible that a narrow interpretation of empowerment, as autonomous change driven by intrinsic motivation, deprived some participants of more external support that they desired (see Chapter 5, Facilitator views of STEPWISE, Sense-making) and of effective behaviour change techniques such as monitoring by, and feedback from, others (see Chapter 5, Facilitator views of STEPWISE, Appraisal work). If such case-by-case adaptations are a matter of informed choice, then they should not be seen as a departure from empowerment philosophy.
Although a participatory approach to intervention design involved service users, a potentially ‘over-controlled’ intervention,136 in which the work as done was different from the work as imagined,137 may have been prevented by participatory implementation and adaptation during the trial itself. 138–141 Although the developers considered the course to be of low intensity and modest in cost, at a time when NHS mental health trusts were under considerable pressure,142,143 some struggled to release resources, making programme management difficult for front-line staff (see Chapter 5, Facilitator views of STEPWISE, Operational work).
The training was well received, and the delivery of programme content in line with empowerment philosophy principles was evident (see Chapter 5, Activities, Trainers train and periodically assess fidelity of facilitators). A quality assurance programme was developed with relevant assessment criteria. The user experience was described positively in general (see Chapter 5, Participant acceptability), but this was audited as part of this evaluation, not as part of the intervention. Routine auditing of content comprehension, behaviour change and clinical outcomes formed no part of the intervention programme, meaning that facilitators could not appraise its effects (see Chapter 5, Appraisal work). 24,80
Staff tailored the intervention appropriately for individuals, whose views and experiences were mostly valued, which is an important marker of quality. Although the pace of delivery could not always be adjusted to everyone’s needs, many participants found that the duration of support was acceptable, although in some cases it may not have been adequate to develop and apply behaviour change strategies that would lead to lasting benefits. 144
The two successful long-term trials employed interventions that were considerably more intensive than STEPWISE, and a more intensive intervention may have achieved better results. The ACHIEVE study by Daumit et al. 145 included 291 participants who were seen in 10 community psychiatric rehabilitation outpatient programs. The intervention, which involved a combination of group weight management sessions (weekly in the first 6 months, then monthly), monthly individual visits and thrice-weekly group activity classes, achieved a mean weight loss of –3.2 kg over 18 months. The more recent STRIDE study146 included 56 men and 144 women who were randomised to routine care or a 6-monthly weekly group intervention plus six monthly maintenance sessions. STRIDE intervention participants lost 4.4 kg more than control participants from baseline to 6 months (95% CI –6.96 to –1.78 kg), but this difference was reduced to 2.6 kg by 12 months (95% CI –5.14 to –0.07 kg). Further evidence in favour of a more intensive programme comes from a service evaluation of a UK-based weight management programme in which the only predictor of weight loss was the number of sessions attended. 53 Many participants valued the group sessions and the opportunities to interact with others with the same condition; however, there was a breadth of understanding and ability to learn. For some participants, one-to-one sessions, with greater attention to memory support, may have proved more effective. However, intensity may not provide the whole explanation. The Danish CHANGE trial147 randomised 428 people with schizophrenia spectrum disorders and abdominal obesity to 12 months of intensive lifestyle coaching plus care co-ordination plus usual care, or care co-ordination and usual care, or usual care alone. In the lifestyle coaching arm, the participants were offered contact with a team member for 1 year. These personal one-to-one contacts occurred at least once per week and typically lasted for up to 1 hour. Despite the similar intensity to the ACHIEVE and STRIDE studies, neither lifestyle coaching nor care co-ordination reduced body weight or waist circumference.
Another alternative explanation may lie in the diagnoses of the participants. Unlike the STEPWISE and CHANGE studies, which recruited only people with schizophrenia spectrum disorder, ACHIEVE and STRIDE included significant numbers of people with other psychiatric disorders. Only 58.1% of the participants in the ACHIEVE study had schizophrenia or schizoaffective disorder, and one-third of participants had a mood disorder. Only 29% of the STRIDE participants had schizophrenia spectrum disorders.
Given the current funding constraints within the NHS, it is debatable whether or not a more intensive intervention would have been affordable within a UK NHS setting and it is noteworthy that the total contact time in the STEPWISE sessions (17.5 hours) is similar to that recommended by the NICE prevention of diabetes mellitus guidance. 18 Even with the current number of STEPWISE sessions, fewer than one-quarter of participants attended all sessions, with more participants attending core sessions than booster sessions. This problem is not unique to the STEPWISE trial, and the level of engagement is similar to other group-based education programmes. 16,27,148
Although the duration of illness and antipsychotic treatment was not reported in the ACHIEVE and STRIDE studies, differences in these factors may further explain the differences in results. We deliberately set out to include a broad range of people with schizophrenia to represent those in contact with NHS mental health services. Consequently, our participants had a wide spectrum of BMI, ranging from normal weight to morbid obesity. It is possible that, had we restricted our entry to the study to those with a BMI of 25–35 kg/m2, the intervention may have proved more successful. Fifty-eight people had type 2 diabetes mellitus, for whom lifestyle intervention is key to self-management. Although there is overlap between obesity and diabetes mellitus management, there are areas in which this diverges and could lead to different results.
Most STEPWISE participants had a long history of an established psychiatric disorder, and it is possible that different approaches are required depending on disease duration. Weight gain is most rapid during the early phase of psychosis,149,150 and this may be the time when lifestyle approaches are most effective. Although we planned to include individuals shortly after the diagnosis of first episode psychosis, very few participants had been on treatment for < 3 months, because of the need to recruit in waves for the group intervention.
Usual care and the risk of contamination
The trial followed best practice for pragmatic trials by allowing considerable leeway for alternative management strategies in the control arm. 34 The use in combination of the usual care survey,36 the CSRI43 and a weight loss programme CRF ensured that the treatment effect was not diluted by a control arm that was more active than intended. 151,152
We undertook a survey of usual care to ascertain the physical health provision at each site. Although there was considerable variation between sites, none was compliant with the latest NICE guidance. To standardise usual care for control participants, the research team provided each participant with written lifestyle advice. Arguably, this went beyond usual care and it is possible that this brief intervention may have a small beneficial effect for control participants, thus minimising the observed effect of the trial. Participation in the trial may have heightened awareness of the obesity problem and led to changes in behaviour. The reduction in body weight in both arms, as opposed to the expected weight gain, suggests that this may have occurred.
Only 8.6% of participants attended weight loss programmes outside the trial, with a slightly higher proportion in the intervention group. There were a few ad hoc reports of referrals by GPs for diet advice or gym membership for those who were newly identified as having diabetes mellitus or prediabetes mellitus.
Other strengths of the trial
The trial results came as a surprise to investigators working at sites that had experienced benefits at their trust. At the results meeting, the investigators described how health-care professionals had become more engaged in weight management and how trusts had improved physical health monitoring. The STEPWISE programme had addressed both patient and care co-ordinator demand for weight management programmes and, in some cases, led to substantial weight reduction. The investigators also reported that participants overcame their anxiety of group-based sessions and reported the value of sharing experiences with other people with severe mental illness with similar weight problems. The social interaction of the groups undoubtedly had a positive effect for participants. The investigators reported how their own practice and personal lives had changed as a result of participation in the trial. They valued the opportunities for interaction between the clinical and research teams within the trust.
Strengths and weaknesses of the process evaluation
Following guidance,68 we fully explored how context affected implementation and identified – as have others153 – poverty, under-resourced public services and stigmatisation of people with diagnoses of mental illness as key issues. The process evaluation showed that the funding of transport and the patient-focused educational approach was popular with participants, but the scarcity of resources remains a problem for implementation.
The study used direct observation of STEPWISE sessions, the gold standard method for investigating intervention fidelity. 24 Although the intervention developers undertook the fidelity assessment, other aspects of the process evaluation were conducted by evaluators (DH and RG-W) who were sufficiently independent to observe the work of stakeholders critically, as recommended in the relevant guidance. 68 The process evaluation was informed by both programme theory and middle range theory, allowing some investigation of the causal assumptions that underpinned the intervention. However, the use of the Behaviour Change Wheel95 in the analysis of participant transcripts was ‘after the fact’; as it did not inform the topic guide, the associated analysis (see Tables 33 and 34) reflects the participants’ patterns of attention rather than systematic elicitation of information based on all theoretical constructs.
Strengths and weaknesses of the economic analysis
Strengths
The high follow-up rate has generated reliable results; however, the use of the relatively new EQ-5D-5L tariffs to calculate the QALY values may mean that the results are not easily comparable with previous results of other studies using the EQ-5D-3L questionnaire, which gave participants three instead of five options to each question. With the extra options, the EQ-5D-5L is more sensitive to change than EQ-5D-3L.
Limitations
Information on service use was collected with questionnaires, which may limit the data accuracy. However, the CSRI method is a well-established method for collecting service use data and this is a data collection issue, which would affect both groups. We also accept that there may be some recall bias in the 9-month follow-up period between 3 and 12 months post randomisation, whereby patients found the number of appointments difficult to remember. There is no reason to suggest that there would be a difference between the treatment and control groups in this respect.
The EQ-5D is sometimes considered to be insensitive to changes in QoL in people with serious mental illness, in comparison with the SF-6D. 128 That is to say, many patients in the disease area report no problems in each area in the EQ-5D questionnaire, perhaps because the domains are less relevant than in other disease areas. The STEPWISE economic evaluation also used the SF-6D, but found a similarly small QALY difference between the two groups. As the STEPWISE intervention was aimed at improving physical health, we might expect to see more improvement than usual on the EQ-5D, which is more focused on physical symptoms, but this was not the case.
In STEPWISE, all participants received a lifestyle information booklet, which was not costed as part of the study. A nominal value was used to cost e-mail and postcard support contacts, which may not accurately represent the cost of the programme. As we did not have data on the wages or education levels of participants and carers, we used the common approach of taking average, minimum and home carer wage rates, but this may not present an accurate picture of the economic impact of the productivity losses. The benefits of lifestyle behaviour change may take longer to emerge than the intervention tested; however, it is unlikely that longer-term benefits would be observed, given the lack of short-term benefits. 147
Implications for policy-makers, health professionals, people with schizophrenia and their families
The trial has important clinical implication for practice, despite being no closer to finding the best way to manage obesity and weight gain in people with schizophrenia. The STEPWISE trial has focused on lifestyle modification, as have previous studies, but this does not recognise the breadth of factors that contribute to weight gain and obesity. Antipsychotics are associated with significant weight gain, and broader approaches that combine individualised lifestyle modification with tailoring of antipsychotic treatment or co-prescription with drugs that reduce antipsychotic-associated weight gain may be needed.
Currently, NICE guidance recommends that mental health trusts should offer lifestyle interventions to people with psychotic illnesses. 14 Although it is clear that behavioural change to address the challenge of obesity is effective for some people with severe mental illness, STEPWISE has indicated how difficult this is to achieve. Furthermore, the results of our study and the CHANGE study147 suggest that people with schizophrenia may respond differently to lifestyle interventions from people with other severe mental illness. It is also possible that people with schizophrenia require an intervention that is of greater intensity than other populations to achieve weight loss. In addition to our experience, the INTERvention to Encourage ACTivity, Improve Diet, and Reduce Weight Gain (InterACT) study, which evaluated a healthy-living intervention to control weight in people taking antipsychotic medication after a first episode of psychosis, found no difference in weight change between the intervention and usual care groups. 154
The results of this trial suggest that interventions that have been shown to be effective in other populations, such as people with diabetes mellitus, are not necessarily effective in people with schizophrenia. Lifestyle programmes for people with schizophrenia should have proven effectiveness and it needs to be recognised that these may need greater resourcing than for other populations.
Chapter 8 Further research
Further analyses of the STEPWISE data set will be undertaken to understand further what predicts a positive response to the intervention. Further health economic analyses within the project will include a comparison of the EQ-5D-5L QALY results with those gained from the SF-36 questionnaire, which generate SF-6D tariffs, which are then used to calculate a QALY. The final analysis will also include a sensitivity analysis, which varies the cost of employment to the minimum wage rate and the cost of informal care to the minimum wage rate and the cost of a home care worker, as mentioned in the methodology.
STEPWISE recruited limited numbers of people with first episode psychosis, and further research is needed into the timing of the intervention to coincide with the often rapid trajectory of weight gain that characterises the early treatment phase for those with a first episode psychosis; moreover, the nature of the intervention may need to reflect the lifestyles and concerns of this typically young population.
Further research into physical health conditions such as diabetes mellitus in mental health should fully explore the optimal approach to ensure that weight loss or QoL changes are sustained over the follow-up period.
Further research should investigate if:
-
a more intensive lifestyle management programme with longer periods of maintenance support, complemented by objective measures of weight, diet and exercise, delivered by more experienced facilitators or those from different professional backgrounds, is clinically effective and cost-effective
-
a more flexible approach, including both group and one-to-one sessions, is more effective
-
a broader approach incorporating the adjustment of antipsychotic treatments and the use of adjunctive pharmacological interventions may be required
-
it is possible to overcome the barriers to attendance at lifestyle management programmes
-
other formats including family members or carers would be more effective
-
lifestyle management programmes should be tailored on the basis of the duration of psychotic illness; for example, interventions for people with first episode psychosis may need to be different from interventions for people with more established disease
-
a lifestyle intervention should be combined with specific medication review and/or pharmacological approaches to weight management.
Chapter 9 Conclusions
Despite concerns about the feasibility of undertaking this RCT in people with schizophrenia, we recruited and retained participants successfully in the RCT, indicating the level of interest in this topic among both people with schizophrenia and their mental health care professionals. The RCT showed that the STEPWISE intervention was neither clinically effective nor cost-effective.
Current NICE guidance recommends that mental health-care services should offer a combined healthy eating and physical activity programme to people with severe mental illness, particularly if they are taking antipsychotics. 14 It is clear that many mental health trusts are not implementing this guidance, in part because of a lack of clear evidence about how to do so. When we conceived the STEPWISE trial, we hoped to provide this evidence; however, the results of the trial mean that this is not the case.
Although the process evaluation has identified some of the reasons why the intervention was not delivered as effectively as planned, engagement by staff and participants was good and the sessions were valued highly. This indicates that alternative strategies are needed to overcome the clinical challenge of obesity and overweight in this patient population. A broader perspective that takes account of antipsychotic medication and utilises adjunctive therapies for those who are unable to achieve weight loss through lifestyle modification may be needed. Further research is needed to address these questions.
Acknowledgements
We thank the 414 services users who participated in this study. We gratefully acknowledge the hard work, support and advice from Nicholas Bell, Director of Research and Development (Sheffield Health and Social Care NHS Foundation Trust), as Research Sponsor; the research nurses, clinical studies officers and facilitators in the 10 participating NHS trusts for participant screening and data collection, and delivering the intervention; and the data managers at Sheffield CTRU. We acknowledge advice and oversight from the independent members of the Trial Steering Committee – Charles Fox (Chairperson, Former Consultant Physician at Northampton General Hospital), Peter Tyrer (Emeritus Professor in Community Psychiatry – Clinical, Imperial College London), Irene Stratton (Statistician, Gloucestershire Hospitals NHS Foundation Trust), Deborah Hicks (Diabetic Nurse Consultant, Barnet Enfield and Haringey Mental Health Trust) – and service user representatives; and members of the independent Data Monitoring Committee: Irene Cormac (Chairperson, Honorary Consultant Forensic Psychiatrist), John Wilding (Professor of Medicine, University of Liverpool) and Merryn Voysey (Senior Statistician, University of Oxford). We acknowledge Jonathan Mitchell (Consultant Psychiatrist, Sheffield Health and Social Care NHS Foundation Trust) as the PI of the intervention development study; and the NIHR Clinical Research Network for supporting recruitment to the study, and Tees Esk and Wear Valleys NHS Foundation Trust, which supported the study from 1 October 2015.
Contributions of authors
Richard IG Holt (Professor and Honorary Consultant in Diabetes and Endocrinology, Chief Investigator).
Daniel Hind (Assistant Director, Reader), Rebecca Gossage-Worrall (Trial Manager, Research Associate), Mike J Bradburn (Senior Statistician), David Saxon (Research Fellow), Paul McCrone (Health Economist), Tiyi A Morris (Research Assistant), Angela Etherington (Patient and Public Involvement Representative), David Shiers (Carer Representative), Katharine Barnard (Chartered Health Psychologist), Lizzie Swaby (Research Assistant), Charlotte Edwardson (Associate Professor in Physical Activity, Sedentary Behaviour and Health), Marian E Carey (Director, Structured Education Research Portfolio), Melanie J Davies (Professor of Diabetes Medicine), Christopher M Dickens (Professor of Psychological Medicine, PI), Yvonne Doherty (Senior Research Associate), Paul French (Professor of Psychology, PI), Kathryn E Greenwood (Consultant Psychologist and Senior Research Fellow, PI), Sridevi Kalidindi (Consultant Psychiatrist, co-PI), Kamlesh Khunti (Professor of Primary Care Diabetes and Vascular Medicine), Richard Laugharne (Consultant Psychiatrist, PI), John Pendlebury (Community Psychiatric Nurse – retired), Shanaya Rathod (Consultant psychiatrist, PI), Najma Siddiqi (Consultant Psychiatrist, PI), Stephen Wright (Consultant Psychiatrist, PI), Glenn Waller (Professor of Psychology, PI), Fiona Gaughran (Consultant Psychiatrist, co-PI), Janette Barnett (Diabetes Research Nurse) and Alison Northern (Intervention Development Study Project Manager).
The following conceived or designed the work: Richard IG Holt, Daniel Hind, Rebecca Gossage-Worrall, Mike J Bradburn, Paul McCrone, Angela Etherington, David Shiers, Katharine Barnard, Christopher M Dickens, Paul French, Fiona Gaughran, Melanie J Davies, Kamlesh Khunti, Richard Laugharne, John Pendlebury, Shanaya Rathod, Marian E Carey and Yvonne Doherty, on behalf of the STEPWISE Research Group.
The following were involved in the acquisition of data for the work: Daniel Hind, Rebecca Gossage-Worrall, Lizzie Swaby, Marian E Carey, Yvonne Doherty, Janette Barnett and Alison Northern, PIs on behalf of the STEPWISE Research Group.
The following were involved in the analysis of data: Richard IG Holt, Daniel Hind, Rebecca Gossage-Worrall, Mike J Bradburn, David Saxon, Paul McCrone, Tiyi A Morris, Angela Etherington, David Shiers, Katharine Barnard, Lizzie Swaby, Charlotte Edwardson, Marian E Carey, Yvonne Doherty Janette Barnett and Alison Northern, on behalf of the STEPWISE Research Group.
Richard IG Holt, Daniel Hind, Rebecca Gossage-Worrall, Mike J Bradburn, David Saxon, Paul McCrone, Tiyi A Morris, Charlotte Edwardson, Angela Etherington, David Shiers, Katharine Barnard, Lizzie Swaby, Marian E Carey, Yvonne Doherty, Christopher M Dickens, Paul French, Kathryn E Greenwood, Sridevi Kalidindi, Melanie Davies, Kamlesh Khunti, Richard Laugharne, John Pendlebury, Shanaya Rathod, Najma Siddiqi, Stephen Wright, Glenn Waller, Janette Barnett and Alison Northern, on behalf of the STEPWISE Research Group, were involved in the interpretation of data.
Richard IG Holt, Daniel Hind, Rebecca Gossage-Worrall, Mike J Bradburn, Dave Saxon, Paul McCrone, Tiyi A Morris, Charlotte Edwardson, David Shiers, Lizzie Swaby and Marian E Carey drafted the monograph.
Richard IG Holt, Daniel Hind, Rebecca Gossage-Worrall, Mike J Bradburn, David Saxon, Paul McCrone, Tiyi A Morris, Charlotte Edwardson, Angela Etherington, David Shiers, Katharine Barnard, Lizzie Swaby, Marian E Carey, Yvonne Doherty, Christopher M Dickens, Paul French, Kathryn E Greenwood, Sridevi Kalidindi, Melanie Davies, Kamlesh Khunti, Richard Laugharne, John Pendlebury, Shanaya Rathod, Najma Siddiqi, Stephen Wright, Glenn Waller, Janette Barnett and Alison Northern, on behalf of the STEPWISE Research Group, critically revised the work for important intellectual content.
Richard IG Holt, Daniel Hind, Rebecca Gossage-Worrall, Mike J Bradburn, David Saxon, Paul McCrone, Tiyi A Morris, Charlotte Edwardson, Angela Etherington, David Shiers, Katharine Barnard, Lizzie Swaby, Marian E Carey, Yvonne Doherty, Christopher M Dickens, Paul French, Kathryn E Greenwood, Sridevi Kalidindi, Melanie Davies, Kamlesh Khunti, Richard Laugharne, John Pendlebury, Shanaya Rathod, Najma Siddiqi, Stephen Wright, Glenn Waller, Janette Barnett and Alison Northern were involved in the final approval of the version to be published.
All authors agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publications
Gossage-Worrall R, Holt RIG, Barnard K, Carey ME, Davies MJ, Dickens C, et al. STEPWISE – STructured lifestyle Education for People WIth SchizophrEnia: a study protocol for a randomised controlled trial. Trials 2016;17:475.
Swaby L, Hind D, Gossage-Worrall R, Shiers D, Mitchell J, Holt RIG. Adherence to NICE guidance on lifestyle advice for people with schizophrenia: a survey. BJPsych Bull 2017;41:137–44.
Holt RIG, Gossage-Worrall R, Hind D, Bradburn MJ, McCrone P, Morris T, et al. Structured lifestyle education for people with schizophrenia, schizoaffective disorder and first episode psychosis (STEPWISE): randomised controlled trial [published online ahead of print September 25, 2018]. Br J Psychiatry 2018.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010;196:116-21. https://doi.org/10.1192/bjp.bp.109.067512.
- Chang CK, Hayes RD, Perera G, Broadbent MT, Fernandes AC, Lee WE, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0019590.
- Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol 2014;10:425-48. https://doi.org/10.1146/annurev-clinpsy-032813-153657.
- Holt RI, Peveler RC. Obesity, serious mental illness and antipsychotic drugs. Diabetes Obes Metab 2009;11:665-79. https://doi.org/10.1111/j.1463-1326.2009.01038.x.
- Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987–1996. Schizophr Res 2002;55:277-84. https://doi.org/10.1016/S0920-9964(01)00256-0.
- McCreadie R, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, et al. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case–control study. BMJ 1998;317:784-5. https://doi.org/10.1136/bmj.317.7161.784.
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med 1999;29:697-701. https://doi.org/10.1017/S0033291798008186.
- Citrome L, Holt RI, Walker DJ, Hoffmann VP. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig 2011;31:455-82. https://doi.org/10.2165/11589060-000000000-00000.
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 2008;371:1085-97. https://doi.org/10.1016/S0140-6736(08)60486-9.
- Tek C, Kucukgoncu S, Guloksuz S, Woods SW, Srihari VH, Annamalai A. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry 2016;10:193-202. https://doi.org/10.1111/eip.12251.
- Kinon BJ, Kaiser CJ, Ahmed S, Rotelli MD, Kollack-Walker S. Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol 2005;25:255-8. https://doi.org/10.1097/01.jcp.0000161501.65890.22.
- Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res 2012;140:159-68. https://doi.org/10.1016/j.schres.2012.03.017.
- McCreadie RG, Kelly C, Connolly M, Williams S, Baxter G, Lean M, et al. Dietary improvement in people with schizophrenia: randomised controlled trial. Br J Psychiatry 2005;187:346-51. https://doi.org/10.1192/bjp.187.4.346.
- Psychosis and Schizophrenia in Adults: Prevention and Management. London: NICE; 2014.
- Naslund JA, Whiteman KL, McHugo GJ, Aschbrenner KA, Marsch LA, Bartels SJ. Lifestyle interventions for weight loss among overweight and obese adults with serious mental illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2017;47:83-102. https://doi.org/10.1016/j.genhosppsych.2017.04.003.
- Yates T, Davies M, Khunti K. Preventing type 2 diabetes: can we make the evidence work?. Postgrad Med J 2009;85:475-80. https://doi.org/10.1136/pgmj.2008.076166.
- Structured Patient Education in Diabetes: Report from the Patient Education Working Group. London: Department of Health and Social Care; 2005.
- Preventing Type 2 Diabetes – Risk Identification and Interventions for Individuals at High Risk. London: NICE; 2012.
- Khunti K, Gray LJ, Skinner T, Carey ME, Realf K, Dallosso H, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ 2012;344. https://doi.org/10.1136/bmj.e2333.
- Usher K, Park T, Foster K, Buettner P. A randomized controlled trial undertaken to test a nurse-led weight management and exercise intervention designed for people with serious mental illness who take second generation antipsychotics. J Adv Nurs 2013;69:1539-48. https://doi.org/10.1111/jan.12012.
- Mauri M, Simoncini M, Castrogiovanni S, Iovieno N, Cecconi D, Dell’Agnello G, et al. A psychoeducational program for weight loss in patients who have experienced weight gain during antipsychotic treatment with olanzapine. Pharmacopsychiatry 2008;41:17-23. https://doi.org/10.1055/s-2007-992148.
- Bartholomew LK, Parcel GS, Kok G, Gottlieb NH. Planning Health Promotion Programs: An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass; 2011.
- Holt RIG, Gossage-Worrall R, Hind D, Bradburn MJ, McCrone P, Morris T, et al. Structured lifestyle education for people with schizophrenia, schizoaffective disorder and first episode psychosis (STEPWISE): randomised controlled trial. [published online ahead of print September 25 2018]. Br J Psychiatry 2018. https://doi.org/10.1192/bjp.2018.167.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomised controlled trial. Prev Med 2016;84:48-56. https://doi.org/10.1016/j.ypmed.2015.12.012.
- Barnett J, Daly H, Doherty Y, Carey M, Hadjiconstantinou M, Northern A, et al. Structured Lifestyle Education for People with Schizophrenia (STEPWISE): how an established approach in diabetes self-management education enabled successful development of a weight management intervention for people with schizophrenia. Diabet Med 2016;33.
- Glaser BG. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436-45. https://doi.org/10.2307/798843.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-32.
- Gossage-Worrall R, Holt RIG, Barnard K, Carey ME, Davies MJ, Dickens C, et al. STEPWISE – STructured lifestyle Education for People WIth SchizophrEnia: a study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1572-1.
- HTA 12/28/05 STEPWISE: STructured lifestyle Education for People WIth Schizophrenia. Southampton: NIHR Journals Library; 2017.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464-75. https://doi.org/10.1016/j.jclinepi.2008.12.011.
- Ayling K, Brierley S, Johnson B, Heller S, Eiser C. How standard is standard care? Exploring control group outcomes in behaviour change interventions for young people with type 1 diabetes. Psychol Health 2015;30:85-103. https://doi.org/10.1080/08870446.2014.953528.
- Swaby L, Hind D, Gossage-Worrall R, Shiers D, Mitchell J, Holt RIG. Adherence to NICE guidance on lifestyle advice for people with schizophrenia: a survey. BJ Psych Bull 2017;41:137-44. https://doi.org/10.1192/pb.bp.116.054304.
- Craddock M, Asherson P, Owen MJ, Williams J, McGuffin P, Farmer AE. Concurrent validity of the OPCRIT diagnostic system. Comparison of OPCRIT diagnoses with consensus best-estimate lifetime diagnoses. Br J Psychiatry 1996;169:58-63. https://doi.org/10.1192/bjp.169.1.58.
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375-81. https://doi.org/10.1093/fampra/11.4.375.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- McHorney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40-66. https://doi.org/10.1097/00005650-199401000-00004.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Overall J, Gorham D. The brief psychiatric rating scale. Psychol Rep 1962;10:799-812. https://doi.org/10.2466/pr0.1962.10.3.799.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737-44. https://doi.org/10.1001/jama.282.18.1737.
- da Silva IC, van Hees VT, Ramires VV, . Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol 2014;43:1959-68. https://doi.org/10.1093/ije/dyu203.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. https://doi.org/10.1097/00005650-199206000-00002.
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
- Osborn DP, Hardoon S, Omar RZ, Holt RI, King M, Larsen J, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry 2015;72:143-51. https://doi.org/10.1001/jamapsychiatry.2014.2133.
- University Hopsitals of Leicester NHS Trust . Diabetes UK – Know Your Risk of Type 2 Diabetes n.d. https://riskscore.diabetes.org.uk/start (accessed 26 October 2017).
- Devlin N, Shah K, Feng Y, Mulhern B, VanHout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2016;27:7-22. https://doi.org/10.1002/hec.3564.
- Das C, Mendez G, Jagasia S, Labbate LA. Second-generation antipsychotic use in schizophrenia and associated weight gain: a critical review and meta-analysis of behavioral and pharmacologic treatments. Ann Clin Psychiatry 2012;24:225-39.
- Holt RI, Pendlebury J, Wildgust HJ, Bushe CJ. Intentional weight loss in overweight and obese patients with severe mental illness: 8-year experience of a behavioral treatment program. J Clin Psychiatry 2010;71:800-5. https://doi.org/10.4088/JCP.09m05627gre.
- Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes 2005;29:1153-67. https://doi.org/10.1038/sj.ijo.0802982.
- Villeneuve K, Potvin S, Lesage A, Nicole L. Meta-analysis of rates of drop-out from psychosocial treatment among persons with schizophrenia spectrum disorder. Schizophr Res 2010;121:266-70. https://doi.org/10.1016/j.schres.2010.04.003.
- von Hippel PT. Regression with missing ys: an improved strategy for analyzing multiply imputed data. Sociol Methodol 2007;37:83-117. https://doi.org/10.1111/j.1467-9531.2007.00180.x.
- Carpenter JR, Kenward MG, White IR. Sensitivity analysis after multiple imputation under missing at random: a weighting approach. Stat Methods Med Res 2007;16:259-75. https://doi.org/10.1177/0962280206075303.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c869.
- White I. RCTMISS: Stata Module to Analyse a Randomised Controlled Trial (RCT) Allowing for Informatively Missing Outcome Data. Chestnut Hill, MA: Boston College Department of Economics; 2017.
- Linnan L, Steckler A, Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377-94. https://doi.org/10.1177/1049732305285708.
- Creswell JW. Research Design. London: Sage Publications; 2014.
- Carter SM, Little M. Justifying knowledge, justifying method, taking action: epistemologies, methodologies, and methods in qualitative research. Qual Health Res 2007;17:1316-28. https://doi.org/10.1177/1049732307306927.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18380.
- O’Cathain A, Goode J, Drabble SJ, Thomas KJ, Rudolph A, Hewison J. Getting added value from using qualitative research with randomized controlled trials: a qualitative interview study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-215.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0026-y.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Cherryholmes CH. Notes on pragmatism and scientific realism. Educ Res 1992;21:13-7. https://doi.org/10.3102/0013189X021006013.
- Dewey J, Thayer H. Pragmatism: The Classic Writings. Indianapolis, IN: Hackett; 1989.
- Peirce CS, Thayer H. Meaning and Action: A Critical History of Pragmatism. Indianapolis, IN: Hackett; 1984.
- Eakin JM. Educating critical qualitative health researchers in the land of the randomized controlled trial. Qual Inq 2016;22:107-18. https://doi.org/10.1177/1077800415617207.
- O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research. Acad Med 2014;89:1-7. https://doi.org/10.1097/ACM.0000000000000388.
- Yin RK, Yin RK. Case Study Research: Design and Methods. London: Sage Publications; 2014.
- Vroman K, Arthanat S, Stone J, Blouin M. International Encyclopedia of Rehabilitation. Buffalo, NY: Center for International Rehabilitation Research Information and Exchange (CIRRIE); 2010.
- Towards a Common Language for Functioning, Disability and Health ICF. Geneva: WHO; 2002.
- Weissman EM, Moot DM, Essock SM. What do people with schizophrenia think about weight management?. Psychiatr Serv 2006;57:724-5. https://doi.org/10.1176/ps.2006.57.5.724.
- Bradshaw T, Lovell K, Bee P, Campbell M. The development and evaluation of a complex health education intervention for adults with a diagnosis of schizophrenia. J Psychiatr Ment Health Nurs 2010;17:473-86. https://doi.org/10.1111/j.1365-2850.2009.01543.x.
- Leventhal H, Friedman MA, . Does establishing fidelity of treatment help in understanding treatment efficacy? Comment on Bellg. Health Psychol 2004;23:452-6. https://doi.org/10.1037/0278-6133.23.5.452.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Finch TL, Rapley T, Girling M, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-43.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. https://doi.org/10.1037/0033-295X.84.2.191.
- Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychol Health 1998;13:717-33. https://doi.org/10.1080/08870449808407425.
- Marlatt GA, George WH. Relapse prevention: introduction and overview of the model. Br J Addict 1984;79:261-73. https://doi.org/10.1111/j.1360-0443.1984.tb03867.x.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Donaldson S, Lipsey M, Shaw I, Greene J, Mark M. The Handbook of Evaluation: Policies, Programs, and Practices. London: Sage Publications; 2006.
- Coryn CLS, Noakes LA, Westine CD, Schroter DC. A systematic review of theory-driven evaluation practice from 1990 to 2009. Am J Eval 2010;32:199-226. https://doi.org/10.1177/1098214010389321.
- Leeuw FL, Donaldson SI. Theory in evaluation: reducing confusion and encouraging debate. Evaluation 2015;21:467-80. https://doi.org/10.1177/1356389015607712.
- Funnell SC, Rogers PJ. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models. San Francisco, CA: Jossey-Bass; 2011.
- McLaughlin JA, Jordan GB. Logic models: a tool for telling your programs performance story. Eval Program Plann 1999;22:65-72. https://doi.org/10.1016/S0149-7189(98)00042-1.
- Logic Model Development Guide. Battle Creek, MI: WK Kellogg Foundation; 2004.
- Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide To Designing Interventions. Sutton: Silverback Publishing; 2014.
- Skinner TC, Carey ME, Cradock S, Dallosso HM, Daly H, Davies MJ, et al. ‘Educator talk’ and patient change: some insights from the DESMOND (Diabetes Education and Self Management for Ongoing and Newly Diagnosed) randomized controlled trial. Diabet Med 2008;25:1117-20. https://doi.org/10.1111/j.1464-5491.2008.02492.x.
- O’Cathain A, Murphy E, Nicholl J. Why, and how, mixed methods research is undertaken in health services research in England: a mixed methods study. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-85.
- RAND Europe . 36-Item Short Form Survey (SF-36) Scoring Instructions n.d. www.rand.org/health/surveys_tools/mos/36-item-short-form/scoring.html.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- NHS Reference Costs 2015 to 2016. London: Department of Health and Social Care; 2016.
- PSSRU . Client Service Receipt Inventory n.d. www.pssru.ac.uk/csri/client-service-receipt-inventory/ (accessed 1 November 2018).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Earnings and Working Hours. Newport: Office for National Statistics; 2017.
- UK Government . National Minimum Wage and National Living Wage Rates 2017. www.gov.uk/national-minimum-wage-rates (accessed 11 July 2017).
- Police Federation . Constables’ Pay Scales 2016. www.polfed.org/ranks/3277.aspx (accessed 11 July 2017).
- Hargreaves J, Cooper J, Woods E, McKee C. Police Workforce, England and Wales, 31 March 2016 n.d. www.gov.uk/government/statistics/police-workforce-england-and-wales-31-march-2016 (accessed 11 July 2017).
- EuroQol . EQ-5D-5L – EQ-5D 2017. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 11 July 2017).
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2010.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Mental Health Act 1983. London: The Stationery Office; 1983.
- Pfadenhauer LM, Mozygemba K, Gerhardus A, Hofmann B, Booth A, Lysdahl KB, et al. Context and implementation: a concept analysis towards conceptual maturity. Z Evid Fortbild Qual Gesundhwes 2015;109:103-14. https://doi.org/10.1016/j.zefq.2015.01.004.
- Presseau J, Ivers NM, Newham JJ, Knittle K, Danko KJ, Grimshaw JM. Using a behaviour change techniques taxonomy to identify active ingredients within trials of implementation interventions for diabetes care. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0248-7.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660-80. https://doi.org/10.1111/j.1464-0597.2008.00341.x.
- Leventhal H, Brissette I, Leventhal E, Cameron L, Leventhal H. The Self-Regulation of Health and Illness Behaviour. New York, NY: Routledge; 2003.
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviours. New York, NY: Guilford; 1985.
- Feste C, Anderson RM. Empowerment: from philosophy to practice. Patient Educ Couns 1995;26:139-44. https://doi.org/10.1016/0738-3991(95)00730-N.
- Fawcett J, Fawcett J. The Relationship of Theory and Research. Philadelphia, PA: FA Davis; 1999.
- Michie S, West R, Campbell R, Brown J, Gainforth H. ABC of Behaviour Change Theories. London: Silverback Publishing; 2014.
- May CR, Eton DT, Boehmer K, Gallacher K, Hunt K, MacDonald S, et al. Rethinking the patient: using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-281.
- Klein R, Maybin J. Thinking About Rationing. London: The King’s Fund; 2012.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129-36. https://doi.org/10.1126/science.847460.
- Funnell MM, Anderson RM, Arnold MS, Barr PA, Donnelly M, Johnson PD, et al. Empowerment: an idea whose time has come in diabetes education. Diabetes Educ 1991;17:37-41. https://doi.org/10.1177/014572179101700108.
- Skinner TC, Carey ME, Cradock S, Daly H, Davies MJ, Doherty Y, et al. Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND): process modelling of pilot study. Patient Educ Couns 2006;64:369-77. https://doi.org/10.1016/j.pec.2006.04.007.
- Rogers E, Rogers E. Diffusion of Innovations. New York, NY: Free Press; 2003.
- Lawton J, Kirkham J, Rankin D, White DA, Elliott J, Jaap A, et al. Who gains clinical benefit from using insulin pump therapy? A qualitative study of the perceptions and views of health professionals involved in the Relative Effectiveness of Pumps over MDI and Structured Education (REPOSE) trial. Diabet Med 2016;33:243-51. https://doi.org/10.1111/dme.12879.
- Cooper S, Crawford M, Etherington A, Jayakumar S, Lemmey S, Marsh R, et al. Report of the National Audit of Schizophrenia (NAS) 2012. London: Royal College of Psychiatrists; 2012.
- McCrone P, Patel A, Knapp M, Schene A, Koeter M, Amaddeo F, et al. A comparison of SF-6D and EQ-5D utility scores in a study of patients with schizophrenia. J Ment Health Policy Econ 2009;12:27-31.
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696-700. https://doi.org/10.1016/S0140-6736(02)07816-9.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. https://doi.org/10.1001/jama.1995.03520290060030.
- Alvarez-Jiménez M, González-Blanch C, Crespo-Facorro B, Hetrick S, Rodríguez-Sánchez JM, Pérez-Iglesias R, et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs 2008;22:547-62. https://doi.org/10.2165/00023210-200822070-00002.
- Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank study. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0169649.
- Anderson RM. Patient empowerment and the traditional medical model. A case of irreconcilable differences?. Diabetes Care 1995;18:412-15. https://doi.org/10.2337/diacare.18.3.412.
- Anderson RM, Funnell MM, Aikens JE, Krein SL, Fitzgerald JT, Nwankwo R, et al. Evaluating the efficacy of an empowerment-based self-management consultant intervention: results of a two-year randomized controlled trial. Ther Patient Educ 2009;1:3-11. https://doi.org/10.1051/tpe/2009002.
- Skinner TC, Cradock S. Empowerment?: what about the evidence?. Pract Diabetes 2000;17:91-5. https://doi.org/10.1002/(SICI)1528-252X(200005)17:3<91::AID-PDI59>3.0.CO;2-#.
- Hawe P, Shiell A, Riley T. In response to Spillane V, Byrne MC, Byrne M, Leathem CS, O’Malley M, Cupples ME (2007) Monitoring treatment fidelity in a randomized trial of a complex intervention. Journal of Advanced Nursing n.d.;60:343-52. https://doi.org/10.1111/j.1365-2648.2008.04686.x.
- Hollnagel E, Wears R, Hollnagel E, Braithwaite J. Resilient Health Care. Volume 2: The Resilience of Everyday Clinical Work. Farnham: Ashgate; 2015.
- Hawe P, Shiell A, Riley T. Theorising interventions as events in systems. Am J Community Psychol 2009;43:267-76. https://doi.org/10.1007/s10464-009-9229-9.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. https://doi.org/10.1136/bmj.328.7455.1561.
- Hawe P. Lessons from complex interventions to improve health. Annu Rev Public Health 2015;36:307-23. https://doi.org/10.1146/annurev-publhealth-031912-114421.
- Leykum LK, Pugh JA, Lanham HJ, Harmon J, McDaniel RR. Implementation research design: integrating participatory action research into randomized controlled trials. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-69.
- Wise J. Coalition cuts have had toxic effect on UK mental health, say therapists. BMJ 2015;350. https://doi.org/10.1136/bmj.h2090.
- Iacobucci G. Budget cuts have worsened quality of mental healthcare, think tank warns. BMJ 2015;351. https://doi.org/10.1136/bmj.h6121.
- Frost J, Garside R, Cooper C, Britten N. A qualitative synthesis of diabetes self-management strategies for long term medical outcomes and quality of life in the UK. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-348.
- Daumit GL, Dickerson FB, Wang N-Y, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013;368:594-602. https://doi.org/10.1056/NEJMoa1214530.
- Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, et al. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry 2015;172:71-8. https://doi.org/10.1176/appi.ajp.2014.14020173.
- Speyer H, Christian Brix Nørgaard H, Birk M, Karlsen M, Storch Jakobsen A, Pedersen K, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016;15:155-65. https://doi.org/10.1002/wps.20318.
- Biddle SJ, Edwardson CL, Wilmot EG, Yates T, Gorely T, Bodicoat DH, et al. A Randomised Controlled Trial to Reduce Sedentary Time in Young Adults at Risk of Type 2 Diabetes Mellitus: Project STAND (Sedentary Time ANd Diabetes). PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0143398.
- Alvarez-Jiménez M, Hetrick SE, González-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2008;193:101-7. https://doi.org/10.1192/bjp.bp.107.042853.
- Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 2011;68:609-16. https://doi.org/10.1001/archgenpsychiatry.2011.2.
- Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care. Psychosom Med 2011;73:323-35. https://doi.org/10.1097/PSY.0b013e318218e1fb.
- Smelt AF, van der Weele GM, Blom JW, Gussekloo J, Assendelft WJ. How usual is usual care in pragmatic intervention studies in primary care? An overview of recent trials. Br J Gen Pract 2010;60:e305-18. https://doi.org/10.3399/bjgp10X514819.
- Ewart SB, Happell B, Bocking J, Platania-Phung C, Stanton R, Scholz B. Social and material aspects of life and their impact on the physical health of people diagnosed with mental illness. Health Expect 2017;20:984-91. https://doi.org/10.1111/hex.12539.
- Marshall M, Barrowclough C, Drake R, Husain N, Lobban F, Lovell K, et al. The HELPER programme: HEalthy Living and Prevention of Early Relapse – three exploratory randomised controlled trials of phase-specific interventions in first-episode psychosis. Programme Grants Appl Res 2015;3.
Appendix 1 Semistructured interview guide for participants
Appendix 2 Semistructured interview guide for health professionals
Glossary
- Blinded/blinding
- Concealment of group allocation from individuals involved in a randomised controlled trial.
- F20
- The International Classification of Diseases, Tenth Revision, code for schizophrenia.
- F25
- The International Classification of Diseases, Tenth Revision, code for schizoaffective disorder.
- First episode psychosis
- First episode psychosis is defined as 3 years since presentation to mental health services.
- Framework analysis
- A qualitative method, suited for applied policy research.
- Logic model
- A tool used to evaluate the implementation of a programme of care.
- NVivo (QSR International, Warrington, UK)
- A computer software package for qualitative data analysis.
- Stata® (StataCorp LP, College Station, TX, USA)
- A computer software package for statistical analysis.
- Triangulation
- A method used by qualitative researchers to establish trustworthiness by comparing the findings of different methods or perspectives.
List of abbreviations
- B-IPQ
- Brief Illness Perception Questionnaire
- BMI
- body mass index
- BPRS
- Brief Psychiatric Rating Scale
- CACE
- complier-average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CONSORT
- Consolidated Standards of Reporting Trials
- CQUIN
- Commissioning for Quality and Innovation
- CRF
- case report form
- CSRI
- Client Service Receipt Inventory
- CTRU
- Clinical Trials Research Unit
- DESMOND
- Diabetes Education and Self-Management for Ongoing and Newly Diagnosed
- DINE
- Dietary Instrument for Nutrition Education
- DOT
- DESMOND Observation Tool
- ENMO
- Euclidean norm minus one
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GEE
- generalised estimating equation
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- ICD-10
- International Classification of Diseases, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning
- LDC
- Leicester Diabetes Centre
- MAR
- missing at random
- MNAR
- missing not at random
- MVPA
- moderate or vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NIHBCC
- National Institutes for Health Behaviour Change Consortium
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- OPCRIT+
- Operational Criteria Checklist for Psychotic Illness and Affective Illness
- PHQ-9
- Patient Health Questionnaire-9
- PI
- principal investigator
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SMD
- standardised mean difference
- STEPWISE
- STructured lifestyle Education for People WIth SchizophrEnia, schizoaffective disorder and first episode psychosis programme
- TAU
- treatment as usual
- TDF
- theoretical domains framework
