Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/78/01. The contractual start date was in May 2016. The draft report began editorial review in June 2017 and was accepted for publication in February 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Brazzelli et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problems
Brief statement describing the health problem
Endovascular abdominal aortic aneurysm repair (EVAR) was undertaken for the first time by a Ukrainian surgeon, Nicholas Volodos, in 19871 and introduced into wider clinical practice by Juan Parodi in 1991. 2 Since then, EVAR has become the preferred treatment option for abdominal aortic aneurysm (AAA). 3 A typical EVAR device consists of a stent covered with graft material to prevent the leakage of blood out of the device. 4 Although it is less invasive than open surgery, and has a lower perioperative mortality rate, EVAR is associated with complications in the follow-up period, such as different types of endoleaks, stent–graft migration, distortion or kinking of the stent–graft, structural disintegration of the stent–graft and aneurysm expansion, all of which could potentially lead to failure of treatment in the form of an aneurysm rupture. 5–8 Therefore, all patients receiving EVAR are placed on surveillance with a view to identifying complications in time to allow for remedial secondary interventions.
Data from The EUROpean collaborators on Stent–graft Techniques for abdominal aortic Aneurysm Repair (EUROSTAR) registry of 2846 patients treated with EVAR from December 1999 to December 2004 showed a cumulative incidence of secondary interventions of 6.0%, 8.7%, 12% and 14% at 1, 2, 3 and 4 years, respectively. 9,10 It is therefore necessary that patients receive lifelong surveillance following EVAR. The main purpose of surveillance is to detect clinically significant complications, which are often asymptomatic, and to prevent aneurysm rupture. 11 Endoleaks are the most common complications that occur after EVAR. 12–14
Classification of endoleaks
An endoleak, which can be defined as a persistent blood flow within the aneurysm but outside the stent–graft, is the most frequent complication after EVAR, and is noted in approximately 20% of patients at some point during follow-up. Endoleaks vary in size, direction and the rate of blood flow, and they have variable origins. 15 Five categories of endoleaks have been described in the literature in accordance with the source of blood flow (Table 1).
| Endoleak | Origin of blood flow |
|---|---|
| Type I | Attachment site leaks |
| A | Proximal |
| B | Distal |
| C | Iliac occluder |
| Type II | Branch leaks |
| A | Simple (one patent branch) |
| B | Complex (two or more patent branches) |
| Type III | Graft defect |
| A | Junctional leak or modular defect |
| B | Fabric disruption (midgraft hole) |
| Type IV | Fabric porosity (within 30 days of procedure) |
| Type V | Endotension |
| A | With no endoleak |
| B | With sealed endoleak |
| C | With type I or III endoleak discovered at the time of open redo surgery |
| D | With type II endoleak discovered at the time of open redo surgery |
Treatment and prognosis depends on the type of endoleak. Type I endoleaks, which have been reported to occur in as many as 10% of patients after EVAR,17 have blood flow from the stent–graft attachment site as a result of sealing failure and are associated with increased pressure in the aneurysm sac. Type I endoleaks are usually treated at the time of the index operation and require urgent treatment if they present later. The risk of intraoperative as well as a secondary late type I endoleak is higher in anatomically difficult situation. 17,18 Type II endoleaks, which are characterised by retrograde blood flow into the aneurysm, are the most common type of endoleaks after abdominal EVAR and could be noted in as many as 20–30% of patients at 30 days, 18.9% of patients at 1 year and 10% of patients after 1 year. 17 Most of the type II endoleaks run a benign course and hence are dealt with a ‘wait and see’ follow-up approach. In some patients, surveillance monitoring may be increased. Treatment is required if the aneurysm increases in size; often a > 5 mm increase is deemed to be clinically significant. 15,18 Type III endoleaks result from structural defects arising in the stent–graft or modular disconnection, and always require immediate treatment. Structural failure of the device is more likely to happen over time as arterial pulsations and other factors cause repetitive stress on the device. Tears or holes in the fabric of the graft can be hard to detect, but modular disconnections are usually well seen with computed tomography angiography (CTA) and on plain radiography (stent–grafts have radio-opaque markers to allow for the diagnosis of modular distraction or dissociation on radiological examinations). The incidence of type III endoleaks is usually low (with an estimated incidence of 4% beyond 1 year). 17 Type IV endoleaks occur perioperatively or in the early postoperative phase (defined as being within 30 days) as a result of graft fabric porosity. However, with the advent of low-porosity graft fabrics, this type of endoleak is now observed less frequently. An endoleak detected on follow-up imaging should not be considered a type IV endoleak. Type IV endoleaks usually resolve once the coagulation profile returns to normal after the EVAR procedure. Treatment of type IV endoleaks is not usually required, but care should be taken to exclude other types of endoleaks at the point of diagnosis. 15,18–20 Type V endoleaks are a diagnosis of exclusion when no endoleak is actually demonstrable. This refers to the phenomenon of endotension, defined as the persistent or recurrent pressurisation of an aneurysm, which is identified by the continued expansion of the aneurysm sac. Although the exact cause of endotension is not always elucidated, possible causes include slow blood flow that is not visible on current imaging techniques, ultrafiltration of blood through the stent–graft, seroma, infection and the transmission of pressure through the thrombus in seal zones. Type V endoleaks are managed on an individual basis. 15,18
Epidemiology of abdominal aortic aneurysm
Abdominal aortic aneurysm represents a significant health risk in the older population. Studies conducted in the 1990s in Europe and the USA indicated an overall prevalence of 2–4% for men and 1–2% for women. 10,21,22 A prospective population-based study conducted in Oxfordshire, UK, between 2002 and 2014 showed an annual incidence rate per 100,000 population of 55 in men aged 65–74 years; the incidence increased to 112 in men aged 75–84 years and to 298 in those aged ≥ 85 years. 23 Similarly, a systematic literature review published in 2014, which estimated the global and regional incidence and prevalence of AAA in 21 world regions, reported that in 2010 the age-specific annual incidence rate per 100,000 population ranged from 0.83 [95% confidence interval (CI) 0.61 to 1.11] in the 40–44 years age group to 165 (95% CI 152.20 to 178.78) in the 75–79 years age group. 24
In the USA, even though the total number of AAAs remains stable at 45,000 cases per year, the overall use of EVAR has risen sharply in the past 10 years (from 5.2% to 74% of the total number of AAA repairs). 25 In the UK, the 2016 report of the National Vascular Registry (NVR), which was based on information on AAA repairs from 98 NHS organisations (82 in England, five in Wales, nine in Scotland and two in Northern Ireland), showed an increasing trend in the proportion of EVAR procedures, growing from 54% in 2009 to 66% in 2013. This trend appears to have stabilised over the last few years, with EVAR procedures accounting for 69% of the elective AAA repairs in 2015. The total number of elective EVAR repairs submitted to the NVR in 2015 was 2882. The majority of the EVAR procedures performed were in men (89%) and in people aged > 65 years (86%). Similarly, the UK 2015–16 record of the Hospital Episode Statistics indicates that there were 2975 hospital admissions for endovascular insertion of a stent–graft for AAA in England. Of these, 2650 were admissions of male patients and 382 were emergency admissions. The mean age of admitted patients was 76 years.
Current post-endovascular abdominal aortic aneurysm repair surveillance: variation in services and uncertainty about best practice
Surveillance following EVAR is now universally accepted and recommended, even though there are currently no standard regimens. 26 Post-EVAR surveillance should include a measurement of the aortic aneurysm, the identification and classification of endoleaks and the detection of stent–graft deformation and thrombus build-up within the graft. 27,28 The ideal frequency of surveillance is not defined and heterogeneous strategies exist between centres. 8,15,29 A web-based survey of UK surveillance practice conducted among the members of the British Society of Interventional Radiologists (BSIR) in 2011 indicated that imaging protocols comprise routine CTA imaging at 1 month, 6 months, 12 months and annually thereafter. 29 CTA is still considered to be the current reference standard for monitoring aneurysm size and migration and for the detection of endoleaks. 26 CTA scanning, however, does not provide information on the direction of blood flow associated with an endoleak and its frequent use has the disadvantage of exposing the patient to cumulative doses of ionising radiation with a potential lifetime cancer risk, as well as exposing the patient to contrast medium-induced nephrotoxicity. 30–32 The risks associated with the repeated use of CTA have led some investigators to consider revising the current surveillance protocols in order to minimise the radiation dose and to eliminate unnecessary CTA examinations. 12,33–35 The results of the 5-year follow-up of the US Zenith (Cook Inc., Bloomington, IN, USA) trial suggest, for example, that, in patients without an early endoleak, the 6-month surveillance can be safely omitted from the surveillance schedule. 36 Moreover, it has been observed that only 1.4–9% of patients require reintervention as a result of surveillance-detected abnormalities, whereas the majority of reinterventions occur in symptomatic patients with previously normal surveillance assessments. 11,26,37–39 Colour duplex ultrasound (CDU) and, more recently, contrast-enhanced ultrasound (CEU) have been proposed as possible safer alternatives to CTA. 40–43 Some investigators have suggested that CDU/CEU might have a role in situations when CTA is equivocal or when endotension is suspected. 44 It has also been suggested that CDU/CEU could replace CTA for annual surveillance for patients who have not experienced endoleaks or an increase in aneurysmal sac size in the first year after EVAR. 19,36,45,46 It is debatable whether or not CDU or CEU can currently replace CTA in the immediate post-EVAR surveillance period, as complications are more likely in the early postoperative period and CTA provides more precise evaluation of aneurysm morphologic changes, sac diameter, graft anchorage and integrity. 18 A significant increase in aneurysm size, the detection of a new endoleak or cases in which CDU is non-diagnostic because of obesity, gas or the lack of a suitable window, may also prompt further imaging with CTA for clarification. 3,12,36
A survey conducted in 2010 among the 41 clinical centres enrolled in the UK EVAR trial 147 showed that 12 out of 41 centres used CTA as the primary surveillance modality, 14 out of 41 centres used CDU as the primary surveillance modality and 15 out of 41 centres used a combination of CTA and CDU. Similarly, the recently published 15-year follow-up of the UK EVAR trial 148 demonstrated a shift in contemporary practice towards CDU.
Although the original EVAR trial 1 protocol was for annual follow-up using CTA, which was used in the early stages of the trial, in the later stages, many EVAR patients were followed up with CDU. 48 The change from CTA to CDU was partly influenced by the growing concern about the risks associated with radiation exposure. 49
Relevant clinical guidelines
Although there is currently no consensus on the best place for CDU/CEU in the care pathway of surveillance after EVAR, some clinical guidelines allude to a possible role of CDU/CEU within the existing imaging care pathway. In the USA, the Society for Vascular Surgery practice guidelines, published in 2009,19 recommend contrast-enhanced computerised tomography (CT) imaging at 1 month and 12 months during the first year after EVAR. If at 1 month the CT imaging identifies an endoleak or other abnormalities of concern, postoperative imaging at 6 months should be considered to further evaluate the proper exclusion of an aneurysm. If neither an endoleak nor an aneurysm enlargement is detected during the first year of surveillance after EVAR, colour duplex ultrasound may be regarded as a reasonable alternative to CT imaging for postoperative surveillance. The presence of a type II endoleak should initially prompt continued CT surveillance to ascertain whether or not the aneurysm is increasing in size. However, if the aneurysm is shrinking in size or is stable, follow-up with CDU may be an option.
Despite some existing algorithms and guidelines,19,29 there is currently no consensus on the optimal surveillance strategy after EVAR. Current surveillance paradigms in the UK are considerably heterogeneous, with each centre performing its own protocol, which varies in both the timing and the modality of imaging.
Description of technology under assessment
Summary of interventions
Computed tomography angiography
Computed tomography angiography is widely used as an imaging modality for surveillance after EVAR and is considered to be the reference standard imaging test. 11 Multiple-phase CTA is recommended initially, because of the variable flow rates of endoleaks after contrast injection. With multiple-phase CTA, imaging is conducted before the administration of an intravenous iodinated contrast medium, after administration in the arterial phase of contrast circulation as determined by bolus chasing, and in a delayed phase, usually in the portal venous phase of contrast circulation. 15 CTA is quick, widely available and less operator dependent. CTA offers clear vascular and non-vascular imaging, and enables differentiation between true endoleaks and areas of calcification or high attenuation that may mimic an endoleak.
The disadvantages of CTA include the cost of follow-up imaging, radiation exposure (15–31 mSv per study11 compared with 0.014 mSv for a chest radiography),50 the nephrotoxic properties of the contrast medium and occasional allergic reactions to the contrast material. The incidence of contrast-induced nephropathy is estimated to range from 7% to 12%. 32,45,47,51 CTA imaging is therefore unsuitable for use in patients with, or at risk of, significant renal impairment.
Plain radiography
Despite the availability of advanced imaging modalities, plain radiography is still used in many centres in Europe and North America for a general assessment of stent–graft position and integrity,12,52 as well as for evaluating device migration, wire frame fracture, kinking or distortion. 53,54 The European Society for Vascular Surgery recommends using plain radiography in conjunction with CTA for the first 12 months of surveillance and, if no endoleaks are detected, in conjunction with CDU or CEU thereafter. 31 The BSIR survey showed that 20 out of 37 respondents (54%) performed plain films in addition to CTA at the 1-year postoperative follow-up. 29 Contrary to CTA, CDU and CEU, plain radiography has little to no role in surveillance for sac enlargement and the detection of endoleaks. 12 For this reason, plain radiography must be used in conjunction with other imaging modalities and cannot be used as the sole surveillance modality after EVAR. 11
Colour duplex ultrasound
Colour duplex ultrasound offers high levels of endoleak characterisation by delivering information regarding the direction of endoleaks and velocity of blood flow, which is not provided by CTA. CDU can also be used to guide the endovascular treatment of endoleaks, is inexpensive and portable, and avoids exposing the patient to radiation and potentially nephrotoxic contrast agents. The imaging quality of CDU is, however, operator-dependent, and scanning and reporting protocols can vary considerably between institutions. 55 CDU imaging is also affected by patient body habitus and bowel gas and is less able to detect stent–graft defects or migration than CTA.
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound is an evolving imaging modality that provides dynamic examination through the administration of an intravenous contrast agent, which can be followed in real time as it appears within the graft, with endoleaks appearing as a contrast outside the stent–graft, but within the aneurysm. 11
During the last decade, the technique of CEU has changed and the developments include more stable microbubble contrast material, as well as the introduction of a fundamentally different method of generating ultrasound images utilising harmonics, compared with the earlier version of Doppler imaging with contrast material. 56 The contrast agents used in contemporary CEU are stabilised microspheres consisting of sulphur hexafluoride or perfluorocarbon encapsulated by a phospholipid shell. 20,57
Unlike CTA, CEU is safe to use in patients with renal impairment. Like CDU, CEU imaging is operator dependent and, because of its technical requirements and the need to administer a contrast agent, should be conducted by specialist sonographers trained in EVAR surveillance, rather than general sonographers. Obesity and bowel gas can interfere with ultrasound scanning. 29,56 Ultrasound equipment needs to be of adequate standard and equipped with the relevant capabilities, which is often missing in dated equipment.
Purpose of this assessment
The purpose of this appraisal is to assess the current evidence for the clinical effectiveness and cost-effectiveness of imaging strategies using either CDU or CEU alone or in conjunction with plain radiography compared with CTA for the surveillance of EVAR.
Chapter 2 Clinical effectiveness and diagnostic accuracy of endovascular abdominal aortic aneurysm repair surveillance imaging modalities
This chapter reports the assessment of the clinical effectiveness and diagnostic accuracy of imaging strategies using either CDU or CEU alone or in conjunction with plain radiography compared with CTA for the surveillance of EVAR. The methods were prespecified in a research protocol (PROSPERO database CRD42016036475).
Clinical effectiveness
Methods for assessing the outcomes arising from the use of the intervention
We conducted an objective synthesis of the evidence for the clinical effectiveness of imaging strategies using either CDU or CEU alone or in conjunction with plain film X-ray compared with CTA for the surveillance of EVAR. The evidence synthesis was carried out in accordance with the general principles of the Centre for Reviews and Dissemination (CRD)’s guidance for undertaking reviews in health care,58 the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 59 and the National Institute for Health and Care Excellence (NICE)’s guidance on the methods of technology appraisal,60 and it is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 61
Identification of studies
Comprehensive electronic searches were conducted to identify reports of published randomised trials and cohort studies. Highly sensitive search strategies were designed, including appropriate subject headings and text-word terms, to combine the search facets for endovascular aneurysm repair, the imaging modalities under consideration and the study design. The searches were initially undertaken on 25 January 2015 and updated on 5 September 2016, and these included studies published from 1996 in order to reflect the introduction of CEU into clinical practice. There were no language restrictions, but non-English-language reports were excluded because the evidence base containing English-language reports was sufficiently large. Full details of the search strategies are reported in Appendix 1. The databases searched were Ovid MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Ovid MEDLINE (1946 to 5 September 2016), EMBASE (1996 to week 36 2016), Science Citation Index (1997 to 5 September 2016), Scopus’ Articles-in-Press (inception to 5 September 2016), Cochrane Central Register of Controlled Trials [(CENTRAL) issue 3 2016], Cochrane Database of Systematic Reviews [(CDSR) issue 3 2016], Database of Abstracts of Reviews of Effects [(DARE) inception to 25 January 2016] and the Health Technology Assessment (HTA) database (inception to 25 January 2016). The reference lists of all of the included studies were perused for further evidence. Members of the advisory group were contacted for details of additional reports.
Identification of other relevant information, including unpublished data
The World Health Organization’s International Clinical Trials Registry Platform (ICTRP), Current Controlled Trials and Clinical Trials.gov were searched on 27 January 2016 for evidence of ongoing studies.
Eligibility criteria
Studies fulfilling the following criteria were included in this assessment.
Population
Adults undergoing surveillance following EVAR for AAAs.
Setting
Secondary or tertiary care settings.
Interventions
Contrast-enhanced ultrasound or CDU, used either alone or in conjunction with plain radiography for long-term surveillance following EVAR.
Colour duplex ultrasound
Colour duplex ultrasound is inexpensive, portable and avoids exposing the patient to radiation and potentially nephrotoxic contrast agents; however, the imaging quality of CDU is dependent on the quality of the machine and the thoroughness of the examination. Similarly, CDU image reporting is operator dependent and scanning protocols can vary considerably between institutions. 55 CDU imaging is also affected by patient habitus and bowel gas and is less able to detect graft defects or migration than CTA.
Contrast-enhanced ultrasound
There is evidence that the use of contrast enhancement increases the sensitivity of ultrasound surveillance. 62 The main advantage of CEU is better classification of endoleaks as a result of dynamic visualisation of the direction of blood into the aneurysm sac. 43,52 As with CDU, CEU is operator dependent, with scan quality and scanning protocols varying considerably between centres. Unlike CTA, CEU is safe to use in patients with renal impairment. The use of an intravenous contrast agent and the presence of a clinician for its administration make CEU more expensive than CDU. At present in the UK, CEU is not as widely available as CDU. 29
Plain radiography
The European Society for Vascular Surgery recommends using plain radiography in conjunction with CTA for the first 12 months of surveillance, and, if no endoleaks are detected, in conjunction with CDU or CEU thereafter. 29,31 In contrast to CTA, CDU and CEU, plain radiography has little to no role in surveillance for sac enlargement and the detection of endoleaks. 12
Comparator
Computed tomography angiography
Computed tomography angiography is the most widely used imaging modality for surveillance after EVAR and is considered to be the reference standard imaging test. 11 CTA is quick, widely available and less operator dependent, and it is not affected by body habitus. CTA offers clear vascular and non-vascular imaging and enables differentiation between true endoleaks and areas of calcification or high attenuation that may mimic an endoleak. Disadvantages include the cost of CTA follow-up, radiation exposure11 and nephrotoxic properties of the contrast medium. 32,45,47,51
Outcomes
Studies providing data on any of the following outcomes (using any measure) were considered to be suitable for inclusion:
-
Clinical and surgical outcomes –
-
incidence and type of complications (e.g. all types of endoleaks, migration, kinking and fracture), as defined by the authors of the relevant selected studies
-
reintervention rate
-
incidence and type of secondary interventions.
-
Adverse effects and harms associated with a specific mode of surveillance (imaging modality) were also taken into consideration (e.g. contrast-induced nephropathy).
Study design
We considered randomised controlled trials (RCTs), non-randomised comparative studies and/or prospective and retrospective cohort studies of different surveillance imaging modalities, regimens and follow-up strategies.
Exclusion criteria
Studies not fulfilling the prespecified criteria and the following types of reports were excluded:
-
preclinical and biological studies
-
case reports
-
reports investigating technical aspects of the imaging modalities used for surveillance after EVAR
-
editorials and opinions.
Data extraction and management
Two reviewers (PS and MS or CR and MS) independently screened the titles and abstracts of all citations identified by the search strategies. Full-text copies of all of the potentially relevant studies were retrieved and assessed independently by the two reviewers for eligibility using a screening form developed ad hoc for the purpose of this assessment (see Appendix 2). Any disagreements during study selection were resolved by discussion or in consultation with a third reviewer (MB).
A data extraction form was specifically designed and piloted for the purpose of this assessment (see Appendix 2). Detailed information on study design, characteristics of the participants, settings, characteristics of the interventions and outcome measures was extracted. Data extraction was carried out by three reviewers (PS, CR and MS). One reviewer completed the data extraction and a second reviewer cross-checked the extracted data for errors or inaccuracies. There were no disagreements between reviewers.
Quality assessment strategy
The methodological quality of the included studies was independently assessed by two reviewers (PS, CR or MS). Disagreements were resolved by consensus or arbitration with a third reviewer (MB). Studies were not excluded on the basis of their methodological quality. We assessed the risk of bias of non-randomised studies using a 17-item checklist that we developed for NICE through the Review Body for Interventional Procedures [(ReBIP) see Appendix 3]. The ReBIP checklist was adapted from several sources, including the NHS CRD guidance for conducting or commissioning systematic reviews,58 Verhagen et al. ,63 Downs and Black64 and the Generic Appraisal Tool for Epidemiology (GATE). 65 The four italicised questions of the checklist used to evaluate the risk of bias of comparative studies were disregarded for all but the two included comparative studies. 66,67 Individual items within the checklist were rated as ‘yes’, ‘no’ or ‘unclear’ so that a rating of ‘yes’ denoted the optimal rating for methodological quality. We did not assess the quality of abstracts, as the word limit for abstracts is usually insufficient to make informed judgements about the potential risk of bias of the reported study.
Method of analysis/synthesis
The summary results and baseline characteristics from eligible studies have been described, tabulated and demonstrated by graphs using methods that are appropriate for the types of measurements reported by the included studies. We had planned a formal meta-analysis and metaregression of outcome data from the included studies; however, this was not possible, owing to the lack of comparative studies. The outcome data have been summarised descriptively.
Results of the evidence synthesis
Quantity and source of the evidence
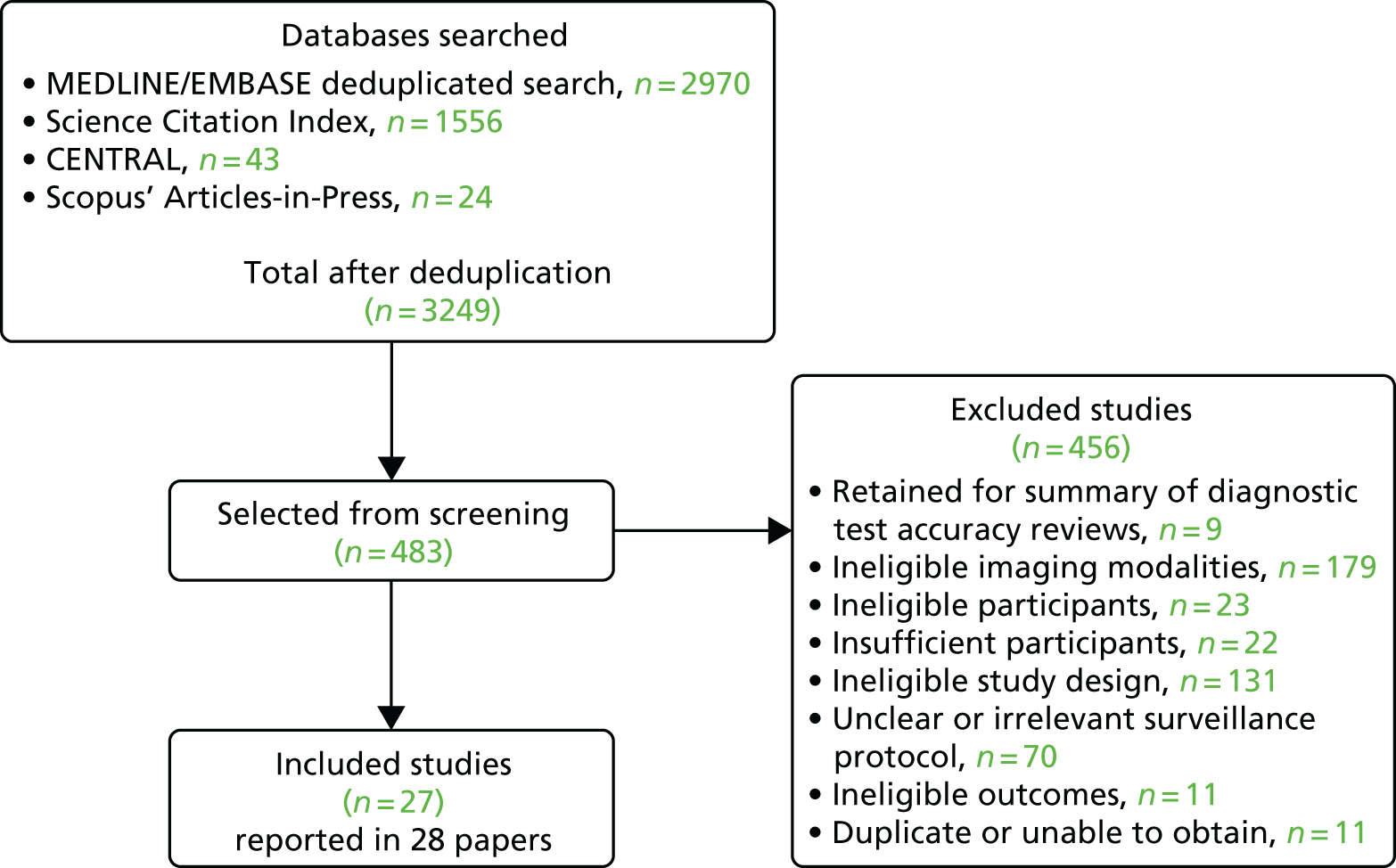
The original primary searches and subsequent updates retrieved a total of 3249 records after deduplication. After reviewing the titles and abstracts, 456 records were subsequently excluded. Full-text copies of 483 potentially relevant reports were obtained and screened for inclusion, of which 27 were deemed to be eligible for inclusion. This comprised 24 full-text papers (two non-randomised comparative studies and 22 cohort studies) and three abstracts (all cohort studies). Figure 1 shows the flow diagram of the study selection process. Appendix 4 lists all of the studies included in this assessment, and Appendix 5 lists the studies excluded after full-text scrutiny together with the reasons for their exclusion. Studies were excluded if they failed to meet one or more of the specified inclusion criteria with regard to study design, participants, intervention or outcomes.
FIGURE 1.
Flow diagram of the study selection process.
Quality assessment of included studies
Non-randomised comparative cohort studies
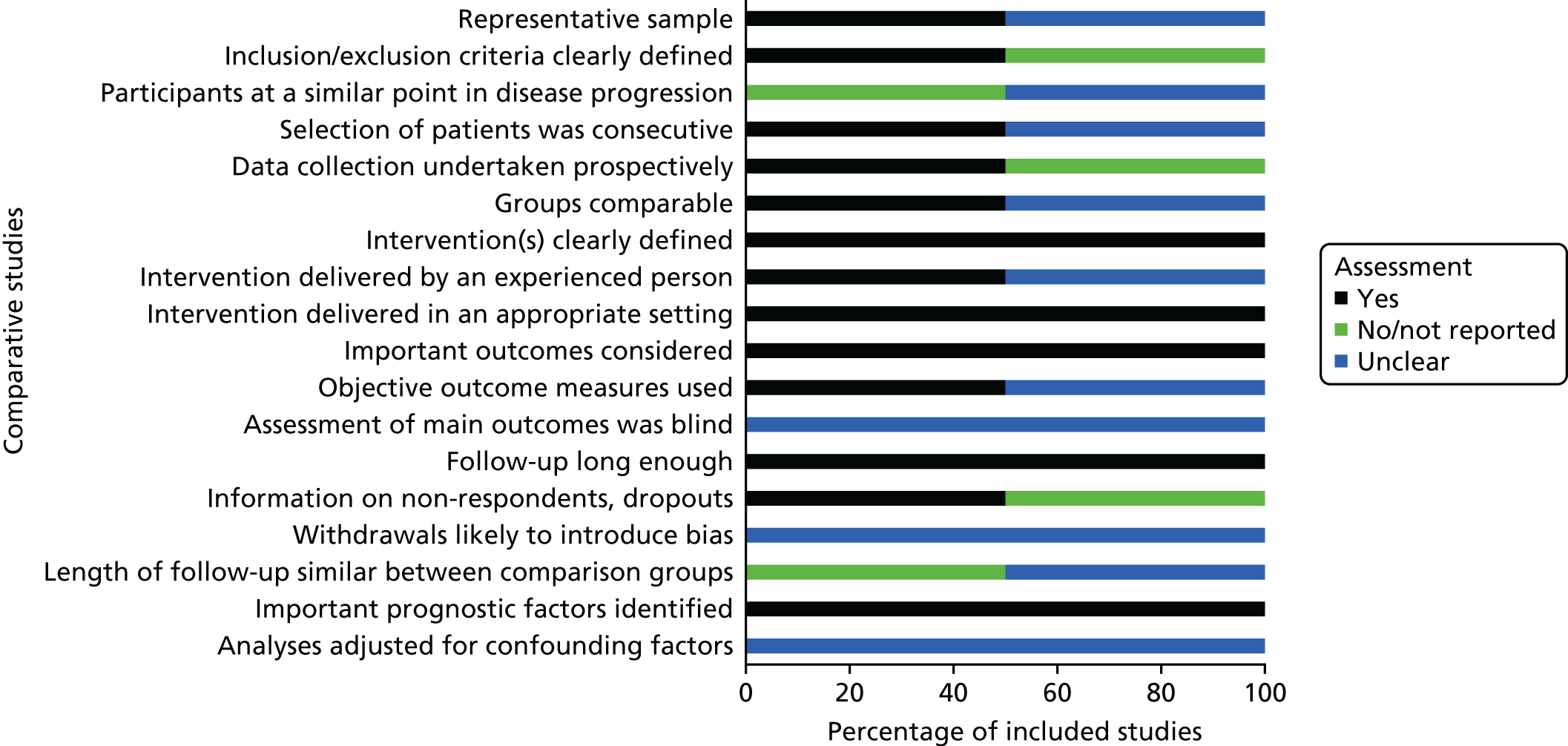
The results of the methodological quality assessment for the two non-randomised comparative cohort studies66,67 indicated that the Chisci et al. 66 study was of moderate methodological quality, whereas the Nyheim et al. 67 study was of poor methodological quality, mainly because over half of the ReBIP checklist items were rated as having an ‘unclear’ risk of bias. 67 In particular, it was unclear if the patients were taken from a representative sample – at a similar point in their disease progression – or selected consecutively, and if the study groups were comparable. 67 The study groups in the Chisci et al. 66 study were comparable, but we noted that the length of the follow-up period was not similar between the study groups. In both studies, it was unclear whether or not the outcomes were assessed blindly or if the authors had adjusted for confounding factors. Figure 2 summarises the results of the methodological assessment of the two non-randomised comparative studies.
Cohort studies
The 22 cohort studies published in full were of mixed quality (Figure 3). 40,41,68–87 The individual study-level results are detailed in Appendix 6. For the majority of studies, over half or more of the ReBIP criteria were not met, or the information provided in the studies was insufficient to determine if the criteria were met, and were, therefore, judged as being of low or moderate quality. Three cohort studies were deemed to be of higher quality as they met all the ReBIP criteria. 76,84,87 Three of the included cohort studies were published only as abstracts88–90 and therefore were not quality assessed.
FIGURE 3.
Quality assessment for non-comparative cohort studies. N/A, not applicable.
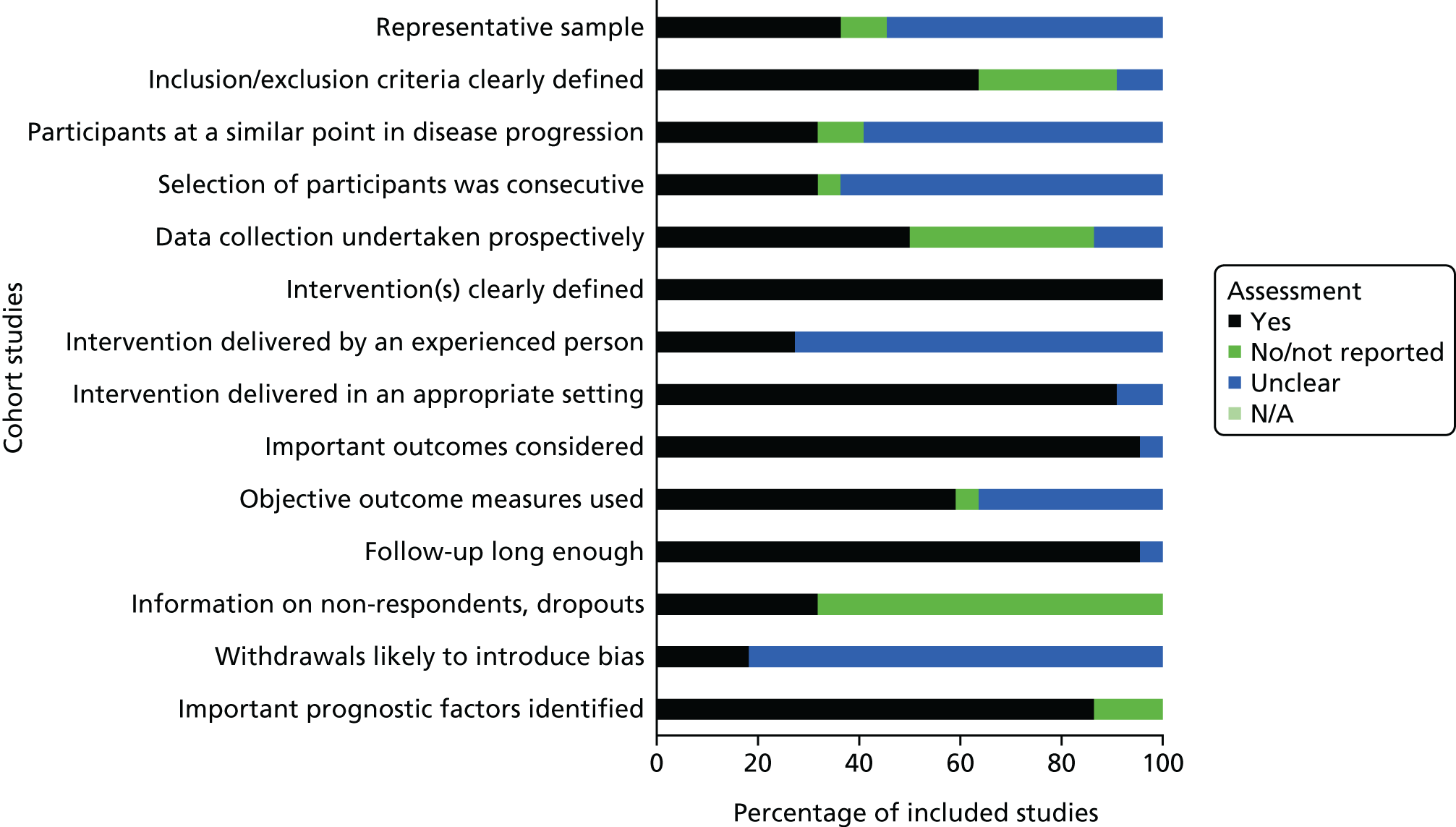
Participants were selected consecutively and were a representative sample in just over one-third of the 22 cohort studies. Similarly, the majority of studies undertook prospective data collection (see Appendix 6), clearly defined the intervention and clinical setting and considered long-term outcomes. The majority of the cohort studies (85%) were not clear in their reporting of participant dropouts and withdrawals and over one-third did not clearly report their inclusion/exclusion criteria.
Study characteristics of all included studies
Details of all of the included studies, including baseline characteristics of participants, description of the adopted surveillance strategy (imaging modality and frequency) and clinical outcomes, are described in the subsequent text and Table 2, and are presented in Appendix 8.
| Participants’ characteristics | Total | Studies | |
|---|---|---|---|
| Comparative | Cohort | ||
| Total enrolled, n | 9596 | 1282 | 8314 |
| Total analysed, n | 7946 | 750 | 7196 |
| Number lost to follow-up, n (%)a | 1650 (17.2) | 532 (41.5) | 1118 (13.4) |
| Number of men, n (%)b | 5399 (67.9) | 663 (88.4) | 4856 (86.0) |
| Range of mean age (years) | 68.7–77.5 | 74–77.5 | 68.7–76.6 |
| Range of aneurysm diameter (mm)c | 51.6–64 | 61–64 | 51.6–59 |
| Comorbidities, n (%)d | N = 5918 | N = 1613 | N = 4225 |
| Hypertension | 1602 (27.4) | 523 (32.4) | 1079 (25.5) |
| Cardiovascular diseasee | 1468 (25.1) | 449 (27.8) | 1019 (24.1) |
| Cerebrovascular disease | 181 (3.1) | NR | 181 (4.3) |
| Hyperlipidaemia | 988 (16.9) | 417 (25.9) | 571 (13.5) |
| Respiratory diseasef | 169 (2.9) | NR | 169 (4.0) |
| Diabetes | 649 (11.1) | 224 (13.9) | 425 (10.1) |
| Smoking | 773 (13.2) | NR | 773 (18.3) |
| Other | 8 (0.1) | NR | 8 (0.2) |
| Type/terminology of AAA, n (%) | |||
| AAA (no additional description supplied) | 6770 (85.2) | 514 (68.5) | 6256 (86.9) |
| Infrarenal AAA | 842 (11.3) | 0 (0) | 842 (11.7) |
| Iliac artery aneurysmg | 295 (3.7) | 236 (31.5) | 59 (0.8) |
| Ruptured AAA | 39 (0.5) | 0 (0) | 39 (0.5) |
Country
Nine of the included studies were conducted in the USA,40,69–71,73,80,81,87,88 six studies were conducted in Italy,66,75,76,83,86,89 three studies were conducted in Germany,68,77,84 three studies were conducted in the UK,41,78,82 two studies were conducted in France,72,85 one study was conducted in the Czech Republic,79 one study was conducted in Norway67 and one study was conducted throughout Europe. 74 The location was not reported in one study. 90
Setting
Surveillance following EVAR took place largely at hospitals, vascular centres and tertiary referral centres. Two studies were conducted in two centres each,68,74 one study involved 33 centres81 and the remaining 24 studies were conducted in a single centre.
Length of follow-up
The study duration ranged from 340,68,76,84,86 to 16 years. 89 The longest median length of follow-up was 68 months (range 1–144 months),90 whereas the shortest median length of follow-up was 23.4 months. 72 Mean follow-up ranged between 14 months [interquartile range (IQR) 7–27 months; range 1–46 months]83 and 55 months [standard deviation (SD) 36 months]. 85 Seven studies had mean or median follow-up assessment periods that were > 36 months. 41,66–68,78,85,90
Participants
A total of 7946 participants were assessed among the 27 included studies. The characteristics of the patients’ aneurysm type varied. The majority of studies (17/27 studies with a total of 6770 participants) did not specify the type of AAA or reported only that participants had ‘abdominal aortic aneurysm’. 40,41,67,69,72–74,76–82,86–90 In four studies, participants (total of 898) were reported to have infrarenal AAAs;70,71,84,85 in two studies participants (total of 295) were reported to have iliac artery aneurysms;66,83 in two other studies, participants (total of 195) were reported to have asymptomatic aneurysms;77,79 in three studies, participants (total of 45) were reported to have symptomatic aneurysms;75,77,79 and, in two studies, participants (total of 39) were reported to have ruptured AAAs. 68,75
Surveillance imaging and frequency
We did not identify any studies that compared a surveillance protocol based on CEU with one based on CDU.
Non-randomised comparative cohort studies
Of the two included non-randomised comparative studies, the study by Chisci et al. 66 compared a surveillance strategy based on CDU and CTA 1 month after EVAR and every 6 months thereafter, with a strategy based on CDU and CTA 1 month after EVAR and CDU and radiography every 6 months thereafter. The study by Nyheim et al. 67 compared a conventional surveillance protocol consisting of CTA, CDU and plain radiography at 1, 6 and 12 months and annually thereafter with a simplified surveillance protocol based on CDU and plain radiography at 6–8 weeks, CTA/CDU/plain radiography at 1 year and CDU and plain radiography annually thereafter.
Cohort studies
Among the included 25 cohort studies, the majority (22/25 studies) reported surveillance protocols based on mixed CDU and CTA imaging. Only three studies included CEU with or without CDU as a part of their surveillance strategy. 75,76,86 Of these, one study included CEU used alone at 6 months and in combination with CTA annually thereafter,75 one study used CEU along with CTA and CDU at 1, 3, 6 and 12 months and annually thereafter86 and, in the remaining study, the use of CEU instead of CDU was restricted to selective cases only. 76 Two of the three studies did not report the technique,76,86 and one reported the use of SonoVue [sulphur hexafluoride microbubbles (Bracco UK, High Wycombe, UK)]. 75
There was significant heterogeneity with regard to the modality of imaging, the timing of imaging and the duration of surveillance among the included cohort studies (Table 3). Depending on the type and frequency of imaging, the included cohort studies were broadly categorised into the following six surveillance protocols:
-
Early and mid-term CTA and/or CDU and long-term CDU surveillance – eight studies (CTA and/or CDU then CDU).
-
The eight studies varied in their early and medium-term surveillance; however, all studies used CDU for the annual long-term surveillance after EVAR. Six studies used a combination of CTA and CDU for early surveillance after EVAR (1-month or 3-month follow-up). 41,77,80,82,89,90 Four studies assessed patients at 6 months. 76,80,89,90 Two of these studies used CDU for the 6-month follow-up,76,89 whereas two studies used both CTA and CDU. 79,90 Of the two studies that used CDU at 6 months, one study reported the use of CEU alongside CDU for selective cases only. 76 One study assessed patients using CTA at 1 and 12 months and CDU annually thereafter. 40
-
Computed tomography angiography scans were performed in case of abnormalities in three studies40,41,82 and plain abdominal radiography was used as a part of the surveillance protocols in two studies. 41,82
-
-
Early CTA, mid-term CDU and long-term CTA surveillance – two studies (CTA then CDU then CTA).
-
Combination of CTA and CDU throughout surveillance – 10 studies (CTA and CDU).
-
Ten studies used CTA and/or CDU for both short- and long-term surveillance after EVAR. The frequency of imaging was broadly similar between the surveillance protocols, with most of the studies using imaging at 1 month, 6 months, 12 months and annually thereafter (see Table 3). 70–72,79,81,83–85,87,88 Six of these studies included the use of radiography alongside CTA and CDU for the surveillance examinations following EVAR. 70,72,81,83,85,87
-
-
Colour duplex ultrasound-based surveillance – three studies.
-
Combination of CTA and CEU/CDU throughout surveillance – one study (CTA and CDU and CEU).
-
In one study, participants underwent CTA, CEU and CDU surveillance at 1, 3, 6 and 12 months after EVAR and annually thereafter. 86
-
-
Early CTA, mid-term CEU and long-term CTA or CEU surveillance – one study (CTA then CEU then CTA or CEU).
-
In one study, the surveillance protocol after EVAR included CTA at 1 month, CEU at 6 months and yearly examinations with either CTA or CEU thereafter. 75
-
| Study, first author (year of publication) | Surveillance frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 1 month | 1 month | 3 months | 6 months | Every 6 months | 12 months | Annually thereafter | 18 months | 24 months | Annually thereafter | |
| Early and mid-term CTA and/or CDU and long-term CDU surveillance | ||||||||||
| Chaer (2009)40 | CTA | CTA | CDU (CTA selectively) | |||||||
| Fargion (2016)89 | CTA and CDU | CDU | ||||||||
| Freyrie (2014)76 | CDU | CTA | CDU (CEU selectively) | CTA | CDU | |||||
| Ghotbi (2010)77 | CDU | CTA and CDU | CTA and CDU | CDU | ||||||
| Harrison (2011)41 | CTA and CDU | CDU and AXR (CTA selectively) | CDU and AXR (CTA selectively) | |||||||
| Kray (2015)80 | CTA and CDU | CTA and CDU | CTA and duplex ultrasound | CDU | ||||||
| Mazzaccaro (2011)90 | CTA and CDU | CTA and CDU | CDU | CDU | ||||||
| Oshin (2010)82 | CTA and CDU and AXR | CDU and AXR (CTA selectively) | ||||||||
| Early CTA, mid-term CDU and long-term CTA surveillance | ||||||||||
| Bisdas (2014)68 | CTA | CDU | CTA | CTA | ||||||
| Donas (2016)74 | CTA | CDU | CTA | CTA | ||||||
| Combination of CTA and CDU throughout surveillance | ||||||||||
| Bush (2001)70 | CTA | CTA and CDU and radiography | CTA and CDU and radiography | CTA and CDU and radiography | ||||||
| Carroccio (2002)71 | CTA and CDU | CTA and CDU | CTA and CDU | CTA and CDU | CTA and CDU | |||||
| Cochennec (2007)72 | CTA and CDU and radiography | CTA and CDU and radiography | CTA and CDU and radiography | CTA and CDU and radiography | ||||||
| Dominguez (2010)88 | CTA and CDU | CTA and CDU | CTA and CDU | |||||||
| Köcher (2004)79 | CTA and CDU | CTA and CDU | CTA and CDU | CTA and CDU | ||||||
| Meier (2001)81 | CTA and CDU and radiography | CTA and CDU and radiography | CTA and CDU and radiography | CTA and CDU and radiography | ||||||
| Parlani (2002)83 | CTA and CDU and AXR | CDU and AXR | CDU and AXR | CDU and AXR | CTA | |||||
| Schunn (2000)84 | CTA and/or CDU (every 6 to 12 months) | |||||||||
| Soler (2015)85 | CTA and CDU and AXR | CTA and CDU and AXR | CTA and CDU and AXR | CTA and CDU and AXR | CTA and CDU and AXR | |||||
| Wolf (2002)87 | CTA and CDU and AXR | CTA and CDU and AXR | CTA and CDU and AXR | |||||||
| CDU-only surveillance | ||||||||||
| Blom (2012)69 | CDU | CDU | CDU | |||||||
| Collins (2007)73 | CDU | CDU (CTA selectively) | CDU (CTA selectively) | |||||||
| Karthikesalingam (2012)78 | CDU | CDU | CDU | CDU (at 9 months) | CDU | CDU annually thereafter | ||||
| Combination of CTA and CEU/CDU throughout surveillance | ||||||||||
| Stella (2009)86 | CTA and CDU and CEU | CTA and CDU and CEU | CTA and CDU and CEU | CTA and CDU and CEU | CTA and CDU and CEU | |||||
| Early CTA, midterm CEU and long term CTA or CEU surveillance | ||||||||||
| Fossaceca (2013)75 | CTA | CEU | CTA/CEU | |||||||
Assessment of outcomes and follow-up
Of the 27 included studies, > 90% reported data on reinterventions and on the incidence and type of clinical complications, 85% reported mortality data and 37% reported changes in aneurysm diameter. In total, 20 studies reported the incidence of type I endoleaks,40,41,66–68,73–77,79,82–85,87–91 18 studies reported the incidence of type II endoleaks,40,41,66–68,73,75–77,79,80,82–85,88–90 10 studies reported the incidence of type III endoleaks,66–68,74–76,79,83,85,86 10 studies reported the incidence of limb occlusion40,41,66,69,72,74,78,82,84,85 and 12 studies reported the rate of aneurysm rupture41,66,74,76,77,80,82,83,85–87,90 (see Appendix 9). Other complications reported in the included studies were thrombosis (seven studies68,71,75,76,79,86,90), infection (seven studies66–68,70,74,79,85), stenosis (five studies41,74–76,79), migration (six studies66,67,72,76,77,79), ischaemia (five studies66,68,72,88,92) and kinking (three studies41,72,78). All of the outcomes were measured at different time points after EVAR and during surveillance using various imaging modalities. When reported, the definitions of complications varied among the included studies.
Results of individual studies
The results of the included studies in terms of type and rate of EVAR-related clinical complications, reintervention rates and types of secondary procedure performed, changes in aneurysm diameter and mortality rates are presented in Appendices 10–13.
Results of non-randomised comparative cohort studies
The two non-randomised comparative studies included a total of 750 participants. Both studies used CTA along with CDU, but the timing of imaging varied between the two studies. The results from two comparative studies are shown in Table 4 and described in the text below.
| Outcomes | Study, first author (year of publication) | |||||||
|---|---|---|---|---|---|---|---|---|
| Chisci (2012)66 | Nyheim (2013)67 | |||||||
| Time point | Protocol | p-value | Time point | Protocol | p-value | |||
| I: CTA, CDU at 1 month and every 6 months thereafter (N = 376) | II: CTA, CDU at 1 month and CDU every 6 months thereafter (N = 341) | I: CTA, CDU at 1, 6 and 12 months and annually thereafter (N = NR) | II: CDU at 6–8 weeks, CT/CDU at 1 year and CDU yearly thereafter (N = 56) | |||||
| Reintervention | ||||||||
| Number (%) of secondary interventions | During 3 years | 68 (18.1) | 56 (16.4) | 0.625 | – | – | – | – |
| < 30 days | 17 (4.5) (two asymptomatic and 15 symptomatic) | 11 (3.2) (one asymptomatic and 10 symptomatic) | 0.602 | – | – | – | – | |
| > 30 days | 51 (13.6) (31 asymptomatic and 20 symptomatic) | 45 (13.2) (24 asymptomatic and 21 symptomatic) | 0.621 | > 30 days | NR | 14 (25) | – | |
| Secondary intervention free survival (%) | At 3 years | 82 | 83.5 | 0.876 | – | – | – | – |
| Conversion to open repair, n (%) | Not specified | 3 (0.8) | 1 (0.3) | 0.626 | – | – | – | – |
| Mortality | ||||||||
| Number (%) of participants who died (all cause) | During 3 years | 8 (2.1) | 6 (1.8) | 0.932 | < 30 days | NR | 0 | – |
| – | – | – | – | > 30 days | NR | 9 (16) | ||
| Number of participants who died (AAA related) | – | – | – | – | > 30 days | NR | 0 | |
| Overall survival rate (%) | At 3 years | 83 | 84 | 0.764 | – | – | – | |
| Freedom from AAA-related mortality (%) | At 3 years | 94.9 | 95.6 | 0.814 | – | – | – | – |
| EVAR-related adverse events (only symptomatic data for Chisci et al.66), n (%) | ||||||||
| Type I endoleak | During 3 years | 7 (1.9) | 5 (1.5) | NR | – | – | – | – |
| A (proximal) | < 30 days | 2 (0.5) | 1 (0.3) | 1.000 | < 30 days | NR | 2 (3.6) | – |
| > 30 days | 4 (1.1) | 4 (1.2) | 1.000 | – | – | – | – | |
| B (distal) | During 3 years | 1 (0.3) | 0 | 1.00 | – | – | – | – |
| Type II endoleak | 57 (15.2) | 45 (13.2) | 0.519 | < 30 days | NR | 9 (16) | – | |
| – | – | – | – | At 6 months | NR | 1 (1.8) | – | |
| Type III endoleak | During 3 years | 3 (0.8) | 3 (0.9) | 1.000 | – | – | – | – |
| < 30 days | 0 | 1 (0.3) | 1.000 | < 30 days | NR | 1 (1.8) | – | |
| > 30 days | 3 (0.8) | 2 (0.6) | 1.000 | – | – | – | – | |
| Graft migration | > 1 cm; during 3 years | 2 (0.5) | 1 (0.3) | 0.565 | > 10 mm; > 30 days | NR | 4 (7.1) | – |
| Graft kinking | > 30 days | 5 (1.3) | 10 (3.0) | 0.050 | – | – | – | – |
| Limb occlusion | During 3 years | 10 (2.6) | 8 (2.3) | 0.977 | < 30 days | NR | 2 (3.6) | – |
| – | – | – | – | > 30 days | NR | 0 | – | |
| Limb ischaemia | During 3 years | 5 (2.7) | 2 (0.6) | NR | – | – | – | – |
| < 30 days | 2 (0.5) | 0 | 0.501 | – | – | – | – | |
| > 30 days | 3 (0.8) | 2 (0.6) | 1.000 | – | – | – | – | |
| Aneurysm rupture | > 30 days | 2 (0.5) | 1 (0.3) | 1.00 | – | – | – | – |
| Graft infection | During 3 years | 0 | 0 | – | < 30 days | NR | 2 (3.6) | – |
| Bowel ischaemia | During 3 years | 2 (0.5) | 0 | 0.501 | – | – | – | – |
| < 30 days | 1 (0.3) | 0 | 1.000 | – | – | – | – | |
| > 30 days | 1 (0.3) | 0 | 1.000 | – | – | – | – | |
| Aneurysm diameter/sac size | > 5-mm increase during 3 years | 54 (14.4) | 43 (12.6) | 0.565 | > 5-mm increase within > 30 days | NR | 6 (10.8) | – |
| Reduction in mean aneurysm diameter | – | – | – | – | During 3 years | 9 mm | – | |
The study by Chisci et al. 66 compared CTA and CDU surveillance at 1 month after EVAR and every 6 months thereafter (protocol I; 376 participants) with CTA and CDU at 1 month after EVAR and CDU and radiography every 6 months thereafter (protocol II; 341 participants) and reported outcomes on reintervention rates, clinical complications, mortality and aneurysm diameter. The proportion of participants who required reintervention was similar between the two protocols (18.1% vs. 16.4%; p = 0.625). There was no evidence of a difference between the two protocols with regard to early reintervention and late reintervention rates. Similarly, the incidence of type Ia and Ib endoleak, type II endoleak, type III endoleak, graft migration, limb occlusion, limb ischaemia, aneurysm rupture, graft infection and bowel ischaemia was similar between the two protocols (Table 5). A higher proportion of graft kinking was picked up by protocol II compared with protocol I (3.0% vs. 1.3%; p = 0.050). Mortality was similar between the two protocols (2.1% vs. 1.8%; p = 0.932) and there was no evidence of a difference in the proportion of participants with permanent (> 30% over baseline) renal impairment (8.8% vs. 8.5%; p = 0.997).
| Study information | Surveillance protocols | |||||
|---|---|---|---|---|---|---|
| Early and mid-term CTA and/or CDU and long-term CDU surveillance | Early CTA, mid-term CDU and long-term CTA surveillance | Early CTA and CDU, mid-term CDU and long-term CTA surveillance | CDU-based surveillance | Combination of CTA and CEU and CDU throughout surveillance | Early CTA, mid-term CEU and long-term CTA or CEU surveillance | |
| Number of studies | 8 | 2 | 10 | 3 | 1 | 1 |
| Total enrolled, n | 2701 | 405 | 4000 | 886 | 100 | 222 |
| Total analysed, n | 1821 | 401 | 3766 | 886 | 100 | 222 |
| Follow-up (months) | Mean 20 | Mean 24.6 | Mean 14.6 | Mean 22.3 | Mean 23.2 | Mean 29.6 |
| Outcomes, % (number of studies reporting each outcome) | ||||||
| All-cause mortality |
Early: 0–1.2 (3) Late: 0–19.7 (3) |
17.2–28.6 (2) Early: 0.8 (1) |
Early: 0.5–7.7 (4) Late: 3.9–42 (8) |
4.4 (1) | 6 | 6.8 |
| Aneurysm-related mortality | 0.5–0.8 (2) | 0.2 (1) | 0.4 (1) | NR | NR | 0 |
| Reintervention | 1.1–11.5 (6) (NC = 2)a | 9.5–15.6 (2) | 2.9–23.8 (9); NC (1)a | 10.1 (1) | 6 | 10.8 |
| Clinical complications | ||||||
| Type I endoleak | 0–7.9 (5) | 1.8–3.1 (2) |
Early: 0.8–8.3 (3) Late: 1.8–7.7 (4) |
NC (1)a | 2 | 1.8 |
| Type II endoleak | 0.5–13 (6) | 1.5–24.8 (2) | 1–24.8 (5) | NC (1)a | 26 | 24.8 |
| Type III endoleak | 0 (1) | 0.4–1.6 (2) | 0–0.8 (4) | NR | 0 | 0.45 |
| Thrombosis | 0.6–5.6 (2) | NR | 2.5–4.5 (3) | NR | 4 | 4.5 |
| Aneurysm rupture | 0–1.3 (5) | 0.8 (1) | 0–0.6 (3) | NR | 0/100 | NR |
| Limb occlusion | 0–1.1 (3) | 3.1–3.7 (2) | 5.3–7.2 (2); NC (1)a | 0–0.4 (2) | NR | NR |
| Kinking | 0.5 (1) | NR | NR | 7.5 (1) | NR | NR |
| Migration | 1 (1) | NR | NR | NR | NR | NR |
| Infection | NR | 0.4–0.8 (2) | 0–2 (2) | NR | NR | NR |
| Ischaemia | NR | 0.4 (1) | 0.2 (1) | NR | NR | NR |
| Stenosis | NR | 4.7 (1) | 0.5 (1) | NR | NR | 0.4 |
The study by Nyheim et al. 67 compared a conventional surveillance protocol consisting of CTA, CDU and plain radiography at 1, 6 and 12 months and annually thereafter (participant numbers not reported), with a simplified surveillance protocol of CDU and plain radiography at 6–8 weeks, CTA/CDU/plain radiography at 1 year and CDU and plain radiography annually thereafter (56 participants), but failed to provide suitable comparative data. Data on reintervention rates, mortality rates and aneurysm diameter were available for the simplified protocol only. The number of participants who died (16%) or required reintervention (25%) was fairly high. In general, the rate of complications picked up by a surveillance protocol based on CDU soon after EVAR, CTA/CDU at 1 year and CDU annually thereafter was higher than that in the study by Chisci et al. 66
Results of cohort studies
Reintervention and complication rates
Eighteen studies reported the number of participants requiring reintervention for various complications (see Appendix 11). 40,41,68,70,72,74–80,83,85–88,90 The proportion of participants who required reintervention ranged from 1.1% during a mean follow-up of 24 months40 to 23.8% in a cohort that included high-risk patients with hostile neck anatomy during a mean follow-up of 32 months. 85 Five studies did not provide a breakdown of the type of reintervention or the type of complication that required reintervention. 69,80,83,88,90 Six studies reported the total number of reintervention procedures performed during surveillance and are described below in accordance with the type of surveillance protocol. 69,71,73,82,85,89 In particular, three of these studies reported the total number of graft limbs that required an intervention. 69,71,82
Reintervention after EVAR was mainly indicated for a type I endoleak in < 1%75 to 8.3% of participants,79 for a type II endoleak in < 1%41,79 to 13.1% of participants,89 for a type III endoleak in < 1%68,79,85 and 1.6% of participants,74 for limb occlusion in < 1%72,78,79 to 7.2% of participants and for thrombosis/stenosis in < 1%41 to 10.7% of participants. 85 In ≤ 1% of the participants for each report, reintervention was needed for aneurysm rupture, infection, graft angulation, ischaemia, haematoma, false aneurysm, endotension, migration and kinking. 41,68,70,74–76,85,86 In one study, a high proportion of the participants (8.3%) who were detected with primary endoleaks were treated during the early postoperative follow-up. 79 Another study reported that reintervention was required in 13.6% of participants for the repair of any endoleaks during a mean follow-up of 15.8 months (range 1–48 months). 87
Overall aneurysm diameter
Eleven of the studies reported various data on aneurysm shrinkage/expansion (see Appendix 12). 40,41,68,74,76,79,81,83,85–87 The observed average aneurysm size decrease was 4.3 mm83 to 15 mm. 40 In studies assessing aneurysm shrinkage, > 50% of participants were reported to have aneurysm shrinkage during follow-up. 68,74,76,79,83,85 It is worth noting that the definitions of decreased aneurysm size and the axis of diameter measured varied among the included studies.
Overall mortality
Overall, 19 cohort studies reported the number of deaths during surveillance after EVAR (see Appendix 13). 40,41,68,70,72–77,79,80,83–88,90 The all-cause mortality rate ranged from 0% during a 12-month follow-up80 to 42% during a mean follow-up of 54.8 months. 85 It is worth noting that the study that reported the highest all-cause mortality (42%) focused on high-risk patients, some of whom presented with features of hostile neck anatomy. Two studies reported that no deaths occurred during follow-up. 77,80 Early mortality rate (< 30 postoperative days) ranged from 0.5%74 to 7.6%. 75 With regard to the study that reported the highest postoperative mortality rate (7.6%; 17 patients), it is worth noting that 13 out of the 17 patients underwent EVAR as an urgent procedure, whereas 4 out of the 17 patients underwent EVAR as an elective procedure. 75 Aneurysm-related deaths occurred in < 1% of the participants in four studies. 40,41,68,83 Three studies reported no aneurysm-related deaths. 70,75,87
Results in accordance with the type of surveillance protocols
The 25 included cohort studies (22 published in full40,41,68–87 and three abstracts88–90) assessed a total of 7196 participants. There was considerable heterogeneity among the included cohort studies in terms of imaging modalities, frequency of imaging, length of follow-up and outcome measures.
The outcomes from the included cohort studies are presented according to the six broad surveillance protocols we described before (see Study characteristics of all included studies). Table 5 presents a summary of the results of the included cohort studies in terms of mortality, reintervention and complication rates.
1. Early and mid-term computed tomography angiography and/or colour duplex ultrasound and long-term colour duplex ultrasound surveillance = eight studies (computed tomography angiography and/or colour duplex ultrasound then colour duplex ultrasound)
Table 6 details the results of the eight cohort studies, with a total of 1821 patients, that used CTA and CDU for the short- and mid-term surveillance and CDU for the long-term surveillance following EVAR.
| Characteristic | Study, first author (year of publication) | |||||||
|---|---|---|---|---|---|---|---|---|
| aChaer (2009)40 | Fargion (2016)89 | Freyrie (2014)76 | Ghotbi (2010)77 | Harrison (2011)41 | Kray (2015)80 | Mazzaccaro (2011)90 | Oshin (2010)82 | |
| Follow-up, months (range) | Mean 24 (1–48) | Median 30 (1–168) | Mean 32.9 ± 23.3 (1–77) | Mean 20 (NR) | Median 36 (12–57) | Up to 12 months’ follow-up | Median 68 (1–144) | Median 24 (NR) |
| All-cause mortality, n/N (%) | 5/184 (2.7) | NR | 2/177 (1.1) at 30 days | 0/100 (0) at 30 days |
25/219 (11.7) at 12 months AAA related: 1/194 (0.5) |
0/191 (0) at 12 months |
6/488 (1.2) at 30 days 77/391 (19.7) at > 30 days AAA related: 3/391 (0.8) |
NR |
| Reintervention rate, n/N (%) | 2/184 (1.1) | 47/289 (16.3) procedures | 20/177 (11.3) at 45 months | 6/100 (6) | 9/194 (4.6) at 12 months | 13/191 (6.8) at > 6 months | 45/391 (11.5) | 11/583 (1.8) limbs |
| Clinical complications n/N (%) | ||||||||
| Type I endoleak | 2/184 (1.1) | 9/289 (3.1) procedures | 2/177 (1.1) |
0/100 (0) at 3 months 0/100 (0) at 12 months |
1/194 (0.5) | NR | 31/391 (7.9) | NR |
| Type II endoleak | 1/184 (0.5) | 38/289 (13.1) procedures | 23/177 (13.0) |
15/100 (15) at 3 months 7/100 (7) at 12 months |
4/194 (2.1) |
17/191 (8.9) at 1 month 18/191 (9.4) at 6 months |
3/391 (0.8) | NR |
| Type III endoleak | NR | NR | 0/177 (0) | NR | NR | NR | NR | NR |
| Thrombosis | NR | NR | 10/177 (5.6) | NR | NR | NR |
3/488 (0.6) at 30 days 8/391 (2.0) |
NR |
| Limb occlusion | 0/184b (0) | NR | 2/177c (1.1) | NR | 2/194 (1.0) | NR | NR | 11/583 (1.8) procedures |
| Kinking | NR | NR | NR | NR | 1/194 (0.5) | NR | NR | NR |
| Aneurysm rupture | 0/184 (0) | NR | 2/177 (1.1) | NR | 1/194 (0.5) | 0/191 (0) at 6 months | 5/391 (1.3) | NR |
| Migration | NR | NR | 0/177 (0) | 1/100 (1.0) at 24 months | NR | NR | NR | NR |
| Stenosis | NR | NR | 1/177 (0.6) | NR | 1/194 (0.5) | NR | NR | NR |
Among studies that used CDU and/or CTA for the short- and mid-term surveillance and CDU for the long-term surveillance after EVAR, reintervention was initiated in 1.1% of participants at a mean follow-up length of 24 months40 to ≈11% of participants at a median follow-up length of 68 months. 76,90 Only four studies provided a breakdown of the type of reintervention or reported the proportion of participants with complications who required reintervention. Reinterventions were performed for type Ia endoleaks in 0.6%76 to 1% of participants,77 for type Ib endoleaks in ≈2% of participants,40,76 for type II endoleaks in 1.1% of participants,41,76 for thrombosis in 5.6% of participants,76 for stenosis, haematoma and kinking in 0.5% of participants,41,76 for aortic rupture in 1.1% of participants,76 for occlusion in 1% of participants77 and for migration in 1.5% of participants. 41 Two studies82,89 provided information on the total number of reinterventions. In one study, among 289 participants who were followed up for a median of 30 months, a total of 47 reinterventions were required for the treatment of nine type I endoleaks and 38 type II endoleaks. 89 In another study, among a total of 583 limbs at risk in 295 patients treated with EVAR, 11 stent–graft limb occlusions (1.8%) were identified over a median follow-up length of 24 months, and eight of these required secondary intervention. 82
The proportion of participants with type I endoleaks ranged from 0%77 to 7.9%90 in five studies that reported this information,40,41,76,77,90 although the proportion of participants with type II endoleaks ranged from 0.5%40 to 13%76 in six studies. 40,41,76,77,80,90 No incidence of type III endoleaks was reported. Two studies76,90 reported the proportion of participants with thrombosis and the rate was fairly high in one study (5.6% at a median follow-up length of 32 months)76 compared with the other study (2.0% at a median follow-up length of 68 months). 90 Data from five studies showed that aneurysm rupture occurred in up to 1.3% of participants. 40,41,76,80,90 Less than 1% of participants experienced limb occlusion (≈1%),41,76 kinking (0.5%),41 stenosis (0.5–0.6%)41,76 and migration (1%). 77
Three of the studies that used CDU for the long-term surveillance after EVAR reported data on aneurysm shrinkage/expansion. 40,41,76 Two studies observed an average decrease in aneurysm size of 10 mm76 and 15 mm,40 respectively. One study reported that around 73% of participants showed an aneurysm shrinkage of > 5 mm. 76 Another study reported an aneurysm expansion of ≈1%. 41
Of the eight studies that used CDU and CTA short- and mid-term surveillance after EVAR and then CDU for the following examinations, six studies reported data on mortality. Of these, three studies reported data on early mortality (< 30 days)76,77,90 and three studies reported data on late mortality (> 30 days). 40,41,80 With regard to early mortality, no deaths occurred in one study77 and the proportions of participants who died were similar in the other two studies (1.1%76 and 1.2%,90 respectively). Mortality rates of > 30 days ranged from 0% at 1 year80 to 19.7% during a median follow-up length of 68 months. 90
Data from two studies indicate that < 1% of participants died as a result of aneurysm-related complications. 41,90 The overall survival rate was 86.2% at 3 years in one study76 and 32% at 12 years in another study. 90
2. Early computed tomography angiography, mid-term colour duplex ultrasound and long-term computed tomography angiography surveillance – two studies (computed tomography angiography then colour duplex ultrasound then computed tomography angiography)
The results from the two studies that used CTA immediately after EVAR, CDU at 6 months and CTA at 12 months and annually thereafter are presented in Table 7. The studies included a total of 401 patients.
| Characteristic | Study, first author (year of publication) | |
|---|---|---|
| Bisdas (2014)68 | Donas (2016)74 | |
| Follow-up (months) | Median 42 (IQR 31–50) | Mean 24.6 (SD 17.4) range 0–61 |
| All-cause mortality, n/N (%) | 78/273 (28.6) |
22/128 (17.2) at mean follow-up 1/128 (0.8) at 30 days |
| Aneurysm-related mortality, n/N (%) | 1/273 (0.4) | NR |
| Reintervention rate, n/N (%) | 26/273 (9.5) | 20/128 (15.6) |
| Clinical complications, n/N (%) | ||
| Type I endoleak | 5/273 (1.8) | 4/128 (3.1) |
| Type II endoleak | 4/273 (1.5) | NR |
| Type III endoleak | 1/273 (0.4) at 10 months | 2/128 (1.6) |
| Limb occlusion | 10/273 (3.7) | 4/128 (3.1) |
| Aneurysm rupture | NR | 1/128 (0.8) |
| Infection | 1/273a (0.4) | 1/128 (0.8) |
| Ischaemia | 1/273 (0.4) | NR |
| Stenosis | NR | 6/128 (4.7) |
The proportion of participants who required reintervention was 9.5% in one study (median follow-up length of 42 months)68 and 15.6% in the other study (mean follow-up length of 24.6 months). 74 In both studies, secondary procedures were undertaken mainly for treating limb occlusion (≈4% of participants),68,74 stenosis (4.7% of participants)74 and type I endoleak (1.8% of participants). 68
Data from the two studies indicate that the presence of a type I endoleak was observed in 1.8%68 and 3.1%74 of participants and the presence of a type III endoleak was observed in 0.3%68 and 1.6% of participants. 74 In one study, the proportion of participants with a type II endoleak was 1.5%. 68 In both studies, a similar proportion of participants had limb occlusion (3.7% in one study68 and 3.1% in the other study74). Other complications, such as infection68,74 and ischaemia,68 were observed in < 1% of participants across the studies. One study reported aneurysm rupture in 0.8% of participants and stenosis in 4.7% of participants. 74
Aneurysm shrinkage was observed in > 50% of participants in both studies. The definitions of aneurysm shrinkage varied between studies despite the availability of reporting standards. The average decrease in aneurysm size was 9 mm (IQR 3–15 mm) at a median follow-up length of 42 months in one study68 and ≈4 mm at a mean follow-up length of 24.6 months in the other. 74
The proportion of deaths was 17.2% (mean follow-up length of 24.6 months) in one study74 and 28.6% in the other study (median follow-up length of 42 months). 68 One study reported an early mortality rate (< 30 days) of 0.8%. 74 The rate of aneurysm-related death was 0.4% in one study. 68 The overall survival rate at 5 years was 67% in one study. 68
3. Combination of computed tomography angiography and colour duplex ultrasound throughout surveillance after endovascular abdominal aortic aneurysm repair = 10 studies (computed tomography angiography and colour duplex ultrasound)
Table 8 shows the results from the 10 cohort studies, with a total of 3766 patients, that used a combination of CTA and CDU for surveillance after EVAR. 70–72,79,81,83–85,87,88 All but one study81 reported data that could be tabulated. The frequency of imaging was broadly similar between the studies with follow-up imaging carried out at 1 month, 6 months, 12 months and annually thereafter in most of them.
| Characteristic | Study, first author (year of publication) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bush (2001)70 | Carroccio (2002)71 | Cochennec (2007)72 | Dominguez (2010)88 | Köcher (2004)79 | Meier (2001)81 | Parlani (2002)83 | aSchunn (2000)84 | Soler (2015)85 | Wolf (2002)87 | |
| Mean follow-up (months) | 14.6 ± 12.4 | 20 ± 9 (range 2–54) | 28 (NR) | NR | 20.7 (range 2–60) | 23.2 (range 2.0–78.8) | 14 (IQR 7–27, range 1–46) | 18 (length of follow-up 46) | 54.8 ± 35.9 | 15.8 ± 11.3 (range 1–48) |
| Mortality, n/N (%) | 12/104 (11.5) | NR | 18/460 (3.9) | 22/1378 (1.6) at 30 days | 13/120 (10.8) | NR |
Late mortality: 21/336 (6.3) AAA related: 1/336 (0.4) |
1/190 (0.5) at 30 days | 83/197 (42) |
< 30 days: 2/154 (1.3) > 30 days: 25/154 (16.2) |
| Reintervention rate, n/N (%) | 3/104 (2.9) | 26/702 (3.7) procedures | 33/460 (7.2) | 273/1378 (19.8) | 16/120 (13.3) | NR | 19/336 (5.6) | 31/190 (16.3) | 47/197 (23.8) | 23/154 (15.0) |
| Clinical complications, n/N (%) | ||||||||||
| Any endoleak | 21/154 (13.7) | |||||||||
| Type I | 18/104 (17.3) at 1 month | NR | NR | 106/1378 (7.7) | Total early: 10/120 (8.3) [A 7/120 (5.8); B 3/120 (2.5)] | NR | 4/366 (1.1) at 30 days | 32/190 (16.8) | Reintervention for 21 endoleaks | 1/154 (0.6) |
| Type II | 329/1378 (5.7) | 9/120 (7.5) | NR | 22/366 (6.0) at 30 days | 32/190 (16.8) | |||||
| Type III | Early: 1/120 (0.8) | NR | 1/366 (0.3) at 30 days | |||||||
| Thrombosis | 26/702 limbs | 3/120 (2.5) | NR | |||||||
| Limb occlusion |
33/460 (7.2) at follow-up 9/460 (2.0) at week 1; 14/360 (3.9) at 1 month; 23/460 (5.0) at 6 months; 30/460 (6.5) at 36 months |
NR |
10/190 (5.3) at 30 days |
|||||||
| Aneurysm rupture | NR | 1/366 (0.3) at 30 days | Late: 1/154 (0.6) | |||||||
| Infection |
1/104 (≈1) at 3 months 2/104 (≈2) at 26 months |
0/120 | ||||||||
| Stenosis | ||||||||||
All but one study84 provided information on the proportion of participants requiring reintervention. This ranged from 2.9% (mean follow-up length of 14 months)70 to 23.8% of participants (mean follow-up length of 31.9 months). 85 Reinterventions (see Appendix 11) for type I endoleaks occurred in 1%86 to 8.3% of participants,79 for type II endoleaks in 1.7% of participants,79 for any type of endoleak in approximately 14% of participants,87 for limb occlusion in 7.2% of participants,72 for thrombosis in 2% of participants,79,86 for infection in 1% of participants,70 for hook fracture in 2% of participants,70 for migration in 0.6% of participants87 and for ischaemia in 1% of participants. 86 One study reported that endoleaks (any type of endoleak) were observed at 1 month in approximately 17% of participants, but they did not seem to require reintervention throughout the follow-up period. 70
Two studies provided information on the type of secondary procedures undertaken. 71,85 In one of these studies, which assessed a total of 351 participants, secondary procedures were performed for 26 limb occlusions out of 702 limbs evaluated. 71 In the other study, which assessed a total of 197 participants, 70 secondary procedures were performed to repair 12 type Ia endoleaks, nine type Ib endoleaks, 29 type II endoleaks, two type III endoleaks, one endotension, 29 stenosis/occlusions, three infections and three ruptures. 85
Surveillance strategies based on the use of CTA and CDU picked up a type I endoleak in 1.1%83 to 8.3% of participants79 1 month after EVAR. Type III endoleaks were identified in < 1% of participants75,79,83 and type II endoleaks were detected in 5.7%88 to 7.5%79 of participants at 30 days. One per cent of these type II endoleaks were detected immediately after EVAR (< 30 days). 83 In one study, thrombosis was detected in 2.5% of participants during a mean follow-up length of ≈20 months,79 whereas in another study, the proportion of participants with limb occlusion was reported to increase during the follow-up period (by 2% in the first week after EVAR, 3.9% at 1 month, 5% at 6 months and 6.5% within 3 years). 72 Infection was reported in 2% of participants in one study. 70 The results of two studies indicate that aneurysm rupture occurred in <1% of participants. 83,87
Five studies reported information on the aneurysm diameter. 79,81,83,85,87 A decrease in aneurysm diameter was detected in > 50% of participants after EVAR. 79,83,85 It is worth noting, however, that the definitions of aneurysm size shrinkage and the duration of the follow-up period varied among studies. In one study,79 the proportion of participants with shrinkage (i.e. a decrease in aneurysm diameter) increased as the length of follow-up doubled (58.6% at > 12 months’ follow-up and 67.4% at > 24 months’ follow-up). One study reported a mean aneurysm shrinkage of 7.3 mm during a mean follow-up length of 23 months. 81 In another study, there was no change in orthogonal and transverse aneurysm diameter during a mean follow-up length of 15.8 months. 87
All but one study71 reported information on mortality. The late mortality rate (> 30 postoperative days) ranged from 3.9%72 during a mean follow-up length of 28 months to 42% during a mean follow-up length of 54.8 months. 85 The mortality rate assessed within 30 days of EVAR ranged from 0.5%84 to 7.7%. 83 One study reported a proportion of aneurysm-related deaths of 0.4% at a mean follow-up length of 14 months. 83
4. Colour duplex ultrasound-based surveillance (three studies)
Three studies with a total of 886 patients used exclusively CDU-based imaging for surveillance after EVAR. One of these three studies also used CTA, but for selective cases only. 73
In one study, 10% of participants required a secondary intervention for the treatment of limb occlusion (0.4%) and limb outflow impairment (7.5%). 78 Kinking was observed in 7.5% of participants, but no reintervention was required.
Across the three studies, the rate of reinterventions ranged from 2%69 to 9% (type I endoleaks = 2%; type II endoleaks = 7%). 73
None of the studies reported on aneurysm shrinkage.
One study reported a mortality rate of 4.4% during a 5-year follow-up period. 73
5. Combination of computed tomography angiography and contrast-enhanced ultrasound/colour duplex ultrasound throughout surveillance = one study (computed tomography angiography and colour duplex ultrasound and contrast-enhanced ultrasound)
In one study with a total of 100 participants, CTA, CEU and CDU were used at 1, 3, 6 and 12 months after EVAR and annually thereafter. The mean duration of the follow-up period was 23.2 months. 86
Reinterventions were needed for participants with iliac limb thrombosis (2%), type I endoleaks (1%), external artery iliac occlusion (2%) and spinal cord ischaemia (1%). The two reinterventions for the external iliac artery occlusion occurred at 1 month and 8 months.
A type I endoleak was detected in three patients (3%): one on 2 days postoperatively, one at 4 months and one at 6 months. Within 24 months, type II endoleaks were detected in 26 patients (26%). At 6 months, four patients (4%) showed signs of thrombosis. No patients had aneurysm ruptures at any point during the follow-up period.
The mean baseline aneurysm diameter was 55.2 mm and ranged from 45 to 99 mm. During the follow-up period, an increase in aneurysm diameter (of 6 mm) was observed in two patients (2%). The diameter of the aneurysm was unchanged in 98 patients (98%).
Six patients died of all-cause mortality during the follow-up period (mean 23.3 months).
6. Early computed tomography angiography, mid-term contrast-enhanced ultrasound and long-term computed tomography angiography or contrast-enhanced ultrasound surveillance = one study (computed tomography angiography then contrast-enhanced ultrasound then computed tomography angiography or contrast-enhanced ultrasound)
In one study with a total of 222 patients, surveillance after EVAR was based on CTA at 1 month, CEU at 6 months and yearly examinations with either CTA or CEU thereafter. The mean duration of the follow-up period was 29.6 months. 75
A total of 24 participants (10.8%) required interventions during the follow-up period and three participants required interventions within 30 days. The majority of the interventions were required because of thrombosis (10 participants) and type II endoleaks (eight participants). The rest of the reinterventions were for the treatment of type Ia and type III endoleaks combined (three participants), type Ib endoleaks (two participants) and infection (one participant). Details of the reinterventions for the three patients who suffered complications within the first 30 days were not reported.
Type I endoleaks occurred in four participants (1.8%), type II endoleaks occurred in 55 participants (24.8%) and type III endoleaks occurred in one participant (0.45%). Of the 55 type II endoleaks, eight were treated and 47 were managed conservatively with CEU follow-up. Stenosis occurred in one participant (0.4%) and thrombosis occurred in 10 participants (4.5%).
The study did not report on aneurysm diameter.
Within 30 days postoperatively, 17 people (7.7%) died. During the follow-up period (mean 29.6 months), 14 of the remaining 205 participants (6.8%) died.
Summary of clinical effectiveness
The evidence for this assessment derives from two non-randomised comparative studies and 25 cohort studies assessing various surveillance protocols after EVAR based on a combination of CTA and CDU or CEU. Of the two included non-randomised comparative studies, one was judged to be of moderate methodological quality, whereas the other study was considered to be of poor quality. The majority of the cohort studies were judged to be of low or moderate methodological quality.
The study duration ranged from 3 years to 16 years among the included studies and the mean length of follow-up ranged from 14 months (IQR 7–27 months; range 1–46 months) to 54.8 months (SD 35.9 months). The characteristics of the participants and the type of aneurysm varied between the studies.
The majority of the included studies assessed EVAR surveillance protocol based on a combination of CTA and CDU imaging throughout the follow-up period. Only two studies included CEU as the main imaging modality and one other study used CEU, but only in selective cases. We did not identify any studies comparing surveillance protocols based on CEU with those based on CDU.
Non-randomised comparative studies
The two non-randomised comparative studies assessed a total of 750 participants (694 participants in one study and 56 participants in the other), and compared a CTA and CDU surveillance protocol with a simplified protocol based on the use of CDU for long-term surveillance after EVAR. The timing of imaging varied between studies, and one of these did not provide suitable data for statistical comparisons. It is worth noting that the largest comparative study, which assessed a total of 694 participants, reported that there was no evidence of a difference between the two surveillance groups in terms of reintervention rate, clinical incidences, mortality and adverse effects, including renal impairment.
Cohort studies
Twenty-five cohort studies assessed a total of 7196 participants. There was considerable heterogeneity in terms of frequency of imaging modalities, duration of the follow-up period, outcome measures, definition of outcomes used (e.g. the definition of decreased aneurysm size) and the time points at which the outcomes were assessed. Owing to the observed heterogeneity between studies, it was not deemed to be appropriate to provide a statistical summary of the outcomes considered. We decided to group the studies according to their similarity in terms of type and frequency of imaging modalities for surveillance after EVAR and created six different surveillance categories (see Table 5). We tabulated and narratively summarised the results for each of these categories.
All but one of the included studies included CDU as part of their surveillance protocols. The remaining study followed up patients using CEU and/or CTA. In the majority of the studies (n = 10), surveillance after EVAR was based on the use of CTA and CDU throughout the follow-up period. Eight studies used CDU for long-term surveillance after EVAR and CTA and/or CDU for early and mid-term surveillance. Two studies used CTA for the long-term surveillance after EVAR (CTA at discharge, CDU at 6 months and then CTA at 12 months and annually thereafter). Two studies included CEU, together with CTA, as part of their surveillance strategies, and three studies adopted a surveillance protocol based exclusively on the use of CDU.
Overall, in the assessed cohort studies, the proportion of participants requiring reintervention after EVAR ranged from 1.1% during a mean follow-up period of 24 months to 23.8% in a cohort that included high-risk patients with hostile neck anatomy who were followed up for a mean length of 32 months. For the cohort studies that provided information on the type of complications requiring treatment, a reintervention was required mainly for the treatment of limb occlusion (< 1–7.2% of participants), thrombosis/stenosis (< 1–5.6% of participants), type II endoleaks (< 1–3.6% of participants), type I endoleaks (< 1–3.1% of participants) and type III endoleaks (< 1–1.6%). The studies that used a protocol based on assessments with CTA and/or CDU throughout the follow-up period showed the highest proportion of participants (range 2.9–23.8%) who required reintervention for complications after EVAR, including type I endoleaks, type II endoleaks, type III endoleaks, thrombosis, limb occlusion, infection and aneurysm rupture. It is worth noting that the study that reported the highest proportion of participants requiring reinterventions (23.8%) focused on high-risk patients, some of whom presented with features of hostile neck anatomy and had the longest follow-up period (mean length 54.8 months). Only limited data were available from studies using CEU as part of their surveillance protocol or studies based exclusively on CDU.
Across the included studies, all-cause mortality ranged from 2.7% (during a mean follow-up length of 24 months) to 42% in a cohort that included a proportion of high-risk patients with hostile neck anatomy (during a mean follow-up length of 54.8 months). Aneurysm-related deaths occurred in < 1% of the participants in four studies. All-cause mortality was generally higher among surveillance strategies that used CTA for early and long-term surveillance after EVAR. One study based on long-term CDU surveillance (median follow-up length of 68 months; range 1–144 months) reported a higher mortality rate and a higher proportion of participants who required reintervention.
The current evidence from the literature assessing the effect of surveillance after EVAR does not show a consistent paradigm. The type of imaging modalities, frequency of imaging and length of follow-up vary considerably between surveillance protocols. Therefore, no firm conclusions can be drawn with regard to the optimal surveillance strategy after EVAR.
Summary of published endovascular abdominal aortic aneurysm repair registries data
Data from relevant registries are summarised in Table 9 and the results are described in the following text. It is worth pointing out that data from existing clinical registries and databases are not organised in accordance with the imaging modalities used for post-EVAR surveillance.
| Characteristic | Registry | |||||||
|---|---|---|---|---|---|---|---|---|
| ENGAGE | EUROSTAR | KPSGR | ASERNIP-S | Vascunet database | The west of Scotland Anaconda Registry | RETA | Lifeline Registry of Endovascular Aneurysm Repair | |
| Number of publications | 5 | 16 | 5 | 4 | 1 | 4 | 2 | 4 |
| Study dates | March 2009–January 2017 | 1996–2006 | Since 2000 and ongoing | Between November 1999 and May 2001; January 2009 and May 2013; ongoing | 2005–2009 | June 2005–September 2009 | January 1996–March 2000 | 1999–2004 |
| Study centres | Multinational, 79 centres in 30 countries | European countries | 17 Kaiser Permanente Northern California medical centres, USA | Australia | Nine countries (Denmark, Hungary, Italy, Norway, Sweden, UK, Australia, Finland and Switzerland) | Three hospitals in the west of Scotland, UK | UK | USA |
| Surveillance protocol | In accordance with standard practice at each clinical site, with the exception of the requirement for 30-day and 1-year imaging | Post-EVAR protocol varied within the centres. Most frequently, CT examinations were used during follow-up at 1, 6, 12, 18 and 24 months and annually thereafter | Post-EVAR protocol varied within the centres. Patients generally received a CT scan at 1 month and then usually every 6–12 months, depending on the clinical scenario | No standard protocol. Postoperatively up to 30 days, at 3 months, 6 months, 12 months and then on an annual basis | Varied depending on country | CT and abdominal radiography at discharge, 1 month, 6, 12 months and annually thereafter | Not specified | Not specified; follow-up up to 5 years |
The Endurant Stent Graft Natural Selection Global Postmarket Registry
The Endurant Stent Graft Natural Selection Global Postmarket Registry (ENGAGE) is a prospective, multinational, long-term post-market study of the real-world use of the Endurant stent–graft system (Medtronic, Santa Rosa, CA, USA) for infrarenal AAA repair. The registry, which used only minimal selection criteria to obtain a more realistic representation of the current clinical practice, commenced in March 2009 and ended in January 2017. Patients with unruptured infrarenal AAAs who underwent elective EVAR were recruited from 79 clinical centres in 30 different countries. A minimum of five consecutive patients were enrolled from each centre. An EVAR surveillance protocol was carried out in accordance with the standard practice at each clinical site, with the exception of the requirement for 30-day and 1-year imaging.
Five publications reporting data from the ENGAGE registry were identified in the literature93–97 (Table 10). The study by Tang et al. ,93 which compared the 12 outcomes after repair of AAA with bifurcated versus aorto-uni-iliac configuration of the Endurant stent–graft, used data collected in the ENGAGE registry from March 2009 to August 2010. Among the total of 1172 participants in this study, 1089 (92.9%) received bifurcated device stent–graft repair and 83 (7.1%) were treated with an aorto-uni-iliac femorofemoral bypass. The study by Stokmans et al. 94 reported data from 1266 participants from March 2008 to April 2011. Both of these studies reported similar proportions of participants requiring secondary intervention at 1 month (1.5%94 and 0.9%93) and at 12 months (4.6%94 and 4.9%93). Reinterventions were needed for the repair of type I and type III endoleaks in 1.2% of participants in the Stokmans et al. study94 and in 0.6% of participants at 12 months in the Tang et al. study. 93 In the Stokmans et al. study,94 at 12 months, secondary procedures were performed in 2.0% of participants for occlusion/stenosis/kinking and in 0.6% of participants for persistent type II endoleaks. Overall, at 1 month, the detection rates of type I endoleaks, type II endoleaks, type III endoleaks, type IV endoleaks, graft occlusion, graft kinking and graft stenosis were similar in both of these studies (see Table 10). The occurrence of other complications, including bowel ischaemia, myocardial infarction, renal failure and stroke, was similar in both studies. In both studies, the all-cause mortality rate was 1.3% at 1 month and ≈8.5% at 1 year. In the Tang et al. study,93 the proportion of participants who died from aneurysm-related causes was 1.2% at 1 month and 1.5% at 1 year. 93 In the Stokmans et al. study,94 the 1-year assessment showed an overall survival rate of 91.6% (SD 1.4%) and an aneurysm-related survival rate of 98.8% (SD 0.5%).
| Characteristic | Study, first author (year of publication) | ||||||
|---|---|---|---|---|---|---|---|
| Stokmans (2012)94 | Tang (2013)93 | Karthikesalingam (2015);95 and Bastos Goncalves (2015)97 | Faure (2015)96 | ||||
| Study dates | March 2008–April 2011 | March 2009–August 2010 | March 2009–April 2011 | March 2009–April 2011 | |||
| Mean length of follow-up (months) | 12 | 29.9 (range 24.0–36.8) | 18 | ||||
| Mean (SD) aneurysm diameter (mm) | 60.3 (11.7) | Median 58 (IQR 54–65) | |||||
| Mean age (years) | 73.1 (SD 8.1; range 43–93) | 73.1 (SD 8.1; range 43–93) | Median 74 (IQR 79–68) | ||||
| Total number of participants | 1266 | 1172 | 1263 | 1143 | |||
| Outcomes | At 1 month, N = 1151 | At 1 month, N = 1262 (ITT) | At 1 year, N = 500 | 1 month, N = 1089 | 1 year, N = 325 | ||
| Stent–graft kinking, n (%) | 20 (1.7) | 18 (1.6) | 0 | ||||
| Stent–graft occlusion, n (%) | 23 (2) | 19 (1.7) | 1 (0.3) | 39 [(92.9) 42 in total]; 13 (31.0) within 30 days and 30 (71.4) within 6 months | |||
| Stent–graft stenosis, n (%) | 16 (1.4) | 13 (1.2) | 3 (0.9) | ||||
| Stent–graft migration, n | 0 | 0 | |||||
| Endoleak, n (%) | 138 (12) | ||||||
| Type I | 16 (1.4) | 10 (0.9) | 0 | 18 [(1.4) Bastos Goncalves et al.97] | |||
| Type II | 114 (10) | 102 (9.3) | 19 (5.8) | ||||
| Type III | 2 (0.2) | 2 (0.2) | 1 (0.3) | ||||
| Type IV | 1 (0.09) | 0 | 0 | ||||
| Type I and/or III | 17 (1.5) | 12 (1.1) | 1 (0.3) | ||||
| Undetermined | 7 (0.6) | 7 (0.6) | 0 | ||||
| All-cause mortality, n (%) | 16 (1.3) | 42 (8.4) | 14 (1.3) | 28 (8.6) | |||
| Procedure-related mortality (up to 30 days) | 3/325 (0.9) | 4/325 (1.2) | |||||
| Aneurysm-related mortality (up to 30 days) | – | 1 (0.2) | 4/325 (1.2) | 5/325 (1.5) | |||
| Bowel ischaemia, n (%) | 3 (0.2) | 2 (0.4) | 2 (0.2) | 0 | |||
| Myocardial infarction, n (%) | 14 (1.1) | 9 (1.8) | 12 (1.1) | 7 (2.2) | |||
| Renal failure, n (%) | 4 (0.3) | 5 (1.0) | 3 (0.3) | 5 (1.5) | |||
| Stroke, n (%) | 2 (0.1) | 2 (0.4) | 2 (0.2) | 2 (0.6) | |||
| Respiratory failure, n (%) | – | 1 (0.2) | |||||
| Conversion to open repair, n (%) | 3 (0.2) | – | |||||
| Secondary interventions, n (%) | 19 (1.5) | 23 (4.6) | 3/325 (0.9) | 16/325 (4.9) | 12 [(1%) for type I endoleaks] | ||
| Endovascular (occlusion, stenosis or kinking) | 8 (0.6) | 10 (2.0) | |||||
| Endovascular (type I/III endoleak) | 4 (0.3) | 6 (1.2) | 0/325 | 2/325 (0.6) | |||
| Open bypass procedure | 6 (0.5) | 5 (1.0) | |||||
| Other | 1 (0.07) | ||||||
| Endovascular (persistent type II endoleak) | – | 3 (0.6) | |||||
| Aneurysm rupture | – | 0 | 0/325 | 1/325 (0.3%) | |||
| Overall survival rate, % (SD) | 91.6 (1.4) at 1 year | ||||||
| Aneurysm-related survival rate, % (SD) | 98.8 (0.5) at 1 year | ||||||
| Aneurysm size | Increased by ≥ 5 mm (2.8%); stable (55.9%); decreased by ≥ 5 mm (41.3%) at 1 year | ||||||
| Freedom from limb occlusion, % (SD) | 97.9 (0.3) at 2 years | ||||||
The study by Karthikesalingam et al. 95 used data from the ENGAGE registry on reintervention and engraft complications at 3 years to predict whether patients would be at a low risk or a high risk of complications after EVAR based on the international validated St George’s Vascular Institute score. 95 Overall, there were 107.6 type I endoleaks, 209.8 type II endoleaks and 86.3 type III endoleaks per 100 patient-years of follow-up (affecting 4.5%, 19.6% and 0.4% of participants, respectively). Aneurysm expansion that was greater than 5 mm was observed in 90.1 participants per 100 patient-years of follow-up (affecting 14.8% of participants). The study by Faure et al. 96 reported 97.9% freedom from limb occlusion at 2 years.
The EUROpean collaborators on Stent–graft Techniques for abdominal aortic Aneurysm Repair registry
The EUROSTAR registry was established in 1996 to collate and analyse data from patients who underwent endovascular treatment for AAAs. The EUROSTAR standardised case record forms were used to collect data. Information on patients with AAA enlargements but without detectable endoleaks (known as endotension), patients who had an elective treatment for AAAs and patients with suitable vascular anatomy for implantation of a stent–graft were collected from various centres in different European countries. The EUROSTAR registry is no longer active (patient enrolment was closed in November 2006).
Sixteen publications reporting data from the EUROSTAR registry were identified in the literature. 5,9,91,98–110 Post-EVAR protocols varied considerably between centres. In most centres, CTA examinations were used at 1, 6, 12, 18 and 24 months after EVAR and annually thereafter. Other imaging modalities used for EVAR surveillance were CDU, CEU and magnetic resonance imaging. It is worth noting, however, that these publications were based on data collected from 1996 to 2006, when CDU and CEU were not in much use in clinical practice. The results from each EUROSTAR report are outlined in Table 11.
| Study, first author (year of publication) | Study dates | Total number of participants | Mean length of follow-up | Summary of the major findings that are relevant to the review |
|---|---|---|---|---|
| Buth (2003)91 | 1996–NR | 2272 | NR |
In total, 297 (12%) participants had type I or type III endoleaks Overall 2-year survival was 90% in the entire cohort A total of 0.6% (n = 15) of the participants had a rupture of their aneurysm at a mean of 16 months’ follow-up, and 5.4% of participants reported an increase in the size of the aneurysm Secondary intervention was needed in 54% (n = 160) of those with type I and III endoleaks compared with 6% (n = 118) of those without endoleaks Type I and type III endoleaks were associated with an increased frequency of open conversion (11% vs. 0.8%) or risk of rupture of the aneurysm (3.4% vs. 0.25%) compared with those without endoleaks |
| Cuypers [(2011)99 reprinted article]; Cuypers [(1999)110 original article] | 1994–98 | 899 | Median 6.2 months (range 0–48 months) |
At 18 months, cumulative patient survival was 88% and persistent endoleak-free survival was 79% During follow-up, procedure- or device-related complications occurred in 7–14% of patients, 0.7% of patients (n = 6) had aneurysm rupture and reintervention was needed in 4–4.7% of patients in each 3-month follow-up interval |
| Harris (2000)5 | 1996–2000 | 2464 | 12.19 months (SD 12.3 months) |
There were 0.6% of patients (n = 14) with a confirmed rupture of their aneurysms. The cumulative rate of rupture was ≈1% per year The death rate at 30 days was 3.2% (n = 79) At 1 month, an endoleak was identified in 8.3% of patients (n = 140/1688) Significant risk factors for rupture included proximal type I endoleak, type III endoleak, graft migration and kinking. Significant risk factors for late conversion were proximal or distal type I endoleak, type III endoleak, type II endoleak, graft migration and kinking |
| Hobo (2006)9 | 1999–2004 | 2864 | 23 months (SD 12 months, range 1–60 months) | Secondary intervention was required in 8.7% of patients (n = 247) at a mean of 12 months after EVAR. The cumulative incidence of secondary intervention was 6.0%, 8.7%, 12% and 14% at 1, 2, 3 and 4 years, respectively. The most frequent reasons for secondary procedures were type I endoleak (n = 144), type II endoleak (n = 370), type III endoleak (n = 101), thrombosis/stenosis (n = 100) and migration/kinking (n = 113) |
| Koole (2011)98 | 1996–2006 | 6337 | 24.6 months (range 1–120 months) | Aneurysm rupture and aneurysm-related mortality occurred in 0.4% of patients (n = 26) and 2.5% of patients (n = 162), respectively. At 7 years, 95.9% of patients had freedom from rupture. A total of 1.3% (n = 83) had conversion to open AAA repair |
| Laheij (2000)100 | 1996–99 | 1023 | 20 months |
In total, 18% of participants (n = 186) needed secondary intervention, occurring at a mean of 14 months after initial EVAR. The rates of freedom from intervention at 1, 3 and 4 years were 89%, 67% and 62%, respectively The 3-year cumulative survival rate of patients was 90% (n = 41) in those without a secondary intervention and 85% (n = 13) in those who had secondary intervention |
| Leurs (2004)103 | 1996–2004 | 676 | 13.5 months (range 1–60 months) |
Results were presented for those with an aneurysm diameter that was < 5.5 cm [(n = 300) group A] and > 5.5 cm [(n = 376) group B] Device migration (0% vs. 2%), type I and type III endoleak (2% vs. 4%) occurred more frequently in those with a larger aneurysm The overall death rate after 3 years of follow-up was significantly higher in group B participants (4% vs. 14%; p = 0.0025). Aneurysm-related mortality at 3 years was significantly higher in group B (0.3% vs. 3%; p = 0.02) |
| Leurs (2005)105 | 1996–2004 | 4433 | Evaluation of the determinants and consequences of surveillance completeness. Results were presented based on patients who attended all scheduled visits compared with those who came infrequently | |
| Leurs (2006)104 | 1999–2005 | 3499 | Analysis of clinical outcomes was based on infrarenal neck length. Overall results not presented | |
| Leurs (2007)102 | 1998–2005 | 213 | 18 months (SD 16.1 months, range 1–60 months) |
In total, 12% of participants (n = 25) needed secondary intervention occurring at a mean of 8 months after initial EVAR. The rates of freedom from intervention at 1 and 2 years were 86% and 83%, respectively The 2-year cumulative survival rate was 85% in participants without secondary intervention and 58% in those who had secondary procedures Complications, including migration, occlusion/stenosis and type I and type III endoleak, occurred more frequently in those who needed a secondary intervention |
| Leurs (2007)101 | 1994–99 | 1190 | 3820 person-years of follow-up |
Overall, all-cause death and aneurysm-related death occurred in 19.9% and 3.0% of the participants, respectively. In total, 7.1% of participants had conversion to open repair and 2.4% of participants had aneurysm rupture during the follow-up period. The most frequently occurring procedure-related complications were endoleak (13 cases per 100 patient-years), stenosis/thrombosis (4.6 cases per 100 patient-years), and stent migration (4.3 cases per 100 patient-years) |
| Peppelenbosch (2004)106 | 1998–2002 | 4392 | Outcomes were presented for three groups defined by the preoperative diameter of the aneurysm. Overall results not presented | |
| Szmidt (2007)107 | 1998–2006 | 445 | Case studies of three patients | |
| van Marrewijk (2004)108 | 1996–2002 | 3595 | 15 months (range 0–72 months) |
Analysis of risk factors for type II endoleak and adverse events Overall, 55% of participants with type II endoleak had reintervention after EVAR along with aneurysmal growth compared with 15% of patients without any endoleak (p < 0.0001) |
| Vallabhaneni (2001)109 | 1996–2000 | 2862 | Median 12 month (range 0–72 months) |
The mortality rate at 30 days was 2.9% (n = 85). The cumulative survival rate at 48 months was 77.1% Late rupture of the aneurysm occurred in 14 out of 2464 participants for an annual cumulative rate of 1% Late conversion to open repair occurred in 41 out of 2862 participants for an annual cumulative rate of 2.1% |
The Kaiser Permanente Endovascular Stent Graft Registry
The Kaiser Permanente Endovascular Stent Graft Registry (KPSGR) is a prospective registry that makes use of electronic medical records to track device utilisation and to appraise short- and long-term EVAR outcomes. The data collection started in 2000 and is ongoing. Patients with endovascular repair of AAAs were identified from a retrospective review of EVARs performed at 17 Kaiser Permanente Northern California medical centres in the USA.
Five publications reported data from the KPSGR. 111–116 No standardised post-EVAR surveillance protocol existed during the data collection period. In general, patients received CTA at the 1-month follow-up and then every 6–12 months depending on the clinical scenario.
In three studies, the proportion of participants requiring reintervention was 10.3%,115 10.8%112 and 15% (median of 32.2 months’ follow-up), respectively. 116 The study by Hye et al. 112 reported an overall reintervention rate of 10.8%. Of the reinterventions, 4.6% were for endoleaks, 1.7% were for stenosis, 1.5% were for thrombosis, 1.3% were for occlusion, 1.6% were for device malfunction, 1.3% were for haematoma/seroma, 0.6% were for pseudoaneurysm, 0.6% were for abdominal compartment syndrome, 0.5% were for infection and 0.4% were for rupture. In the study by Walker et al. ,114 aneurysm rupture occurred in 1.2% of participants during a median follow-up length of 32.2 months (IQR 14.2–52.8 months). Aneurysm-related mortality was 0.6% at 1 month and 0.8% at 1 year, whereas all-cause mortality was 14.3% at 1 year. In the study by Anthony et al. ,115 all-cause mortality was 1.2% at the 1-month follow-up.
Australian Safety and Efficacy Register of New Interventional Procedures – Surgical
The Australian Safety and Efficacy Register of New Interventional Procedures – Surgical (ASERNIP-S) is a national collection of data for the evaluation of EVAR. An audit containing information on patients who had a Zenith graft repair for AAA between November 1999 and May 2001 was managed and published by the ASERNIP-S.
No standardised EVAR surveillance protocols were specified. Postoperative follow-up was carried out at 30 days, 3 months, 6 months, 12 months and annually thereafter.
The 787 Zenith graft patients enrolled in the audit were followed up until 2008. Technical success was 93.5% at 30 days. During the 7-year follow-up period, reinterventions were required in 13.5% of participants. Overall, 4.2% of participants developed type I endoleaks, 14% developed type II endoleaks, < 2% experienced kinking, stenosis, migration or thrombosis and < 1% developed type III endoleaks or infection.
All-cause mortality was 0.5% at 1 month, 32% at 5 years and 44% at 7 years. During the follow-up period (7 years after EVAR) 4.4% of participants (35/787) died from aneurysm-related causes. Ten of these deaths (1.5%) were due to ruptured aneurysms.
A recent publication by Fitridge et al. ,117 which combined data from a total of 1647 patients from two ASERNIP-S audits of EVAR (from 1999 to 2001 and from 2009 to 2013), reported a 1-year survival rate of 93.7% (1544/1647) and a 30-day survival rate of 98.4% (1620/1647).
Vascunet database
The Vascunet registry collected data from national and regional vascular registries in Australia, Denmark, Finland, Hungary, Italy, Norway, Sweden, Switzerland and the UK on primary AAA repairs performed between 2005 and 2009.
A total of 31,427 intact AAA repairs were assessed. The overall perioperative mortality rate (in-hospital or within 30 days) was 2.8% and was stable over time. The perioperative mortality rate varied from 1.6% (95% CI 1.3% to 1.8%) in Italy to 4.1% (95% CI 2.4% to 7.0%) in Finland. A total of 7040 ruptured AAA repairs were identified. The overall perioperative mortality rate was 31.6% (95% CI 30.6% to 32.8%), which decreased over time. 118
Registry of Endovascular Treatment of Abdominal Aortic Aneurysms
The Registry of Endovascular Treatment of Abdominal Aortic Aneurysms was established in January 1996 to collect data from 41 centres that initially undertook EVAR in the UK. 119,120 The data for the first 1000 cases submitted to the registry were published in 2005 by Thomas et al. 119 Overall, the mortality rate was 11% at 1 year and 8% at 5 years. The cumulative risk of rupture was 2% at 5 years. Complications related to the aneurysm or device occurred in 13% of participants at 1 year and in 16% of participants at 5 years. The most common complications were endoleaks or graft migration during a mean follow-up length of 3.1 years (range 30 days to 5 years). The cumulative freedom from endoleak was 88% at 1 year and 68% at 5 years. The cumulative freedom from secondary procedures was 87% at 1 year and 62% at 5 years.
Lifeline Registry of Endovascular Aneurysm Repair
The Lifeline Registry of Endovascular Aneurysm Repair was established in 1998 to evaluate the long-term outcomes of endovascular treatment for patients with AAAs. Four publications have reported outcomes of EVAR based on the data submitted to the Lifeline Registry of Endovascular Aneurysm Repair from 1999 to 2004. 121–124 Outcome data from 2664 endograft patients were published in 2005. 124 The overall survival rate was 74% at 4 years, 66% at 5 years and 52% at 6 years. A survival analysis conducted using 6-year data revealed freedom from aneurysm rupture in 99% of patients who had undergone EVAR, freedom from aneurysm-related death in 98% of patients and freedom from surgical conversion in 95% of patients. Most secondary interventions (85%) were performed < 30 days after EVAR. Freedom from secondary interventions was 84% at 1 year and 78% at 5 years.
Anaconda Registry
The Anaconda Registry was a prospective database of clinical outcomes of 106 consecutive patients who underwent endoluminal repair of AAAs using the Anaconda endograft (Vascunet, Inchinnan, UK) in three hospitals in the west of Scotland between 2005 and 2009. Four publications based on data from the Anaconda Registry were identified in the literature. 76,86,92,125 Three of these publications were included in the review of clinical effectiveness evidence. 76,86,92 During a mean follow-up period of 2 years, 9.4% of participants died from causes other than aneurysm. There were no aneurysm-related deaths. Type II endoleaks were detected through CTA scanning at 1, 6, 12, 24, 36 and 48 months in 8.4%, 4.8%, 7.2%, 7.8%, 11.1% and 0% of patients, respectively. There were no type I, III or IV endoleaks. Five cases of endograft limb thrombosis were observed during follow-up. Four of these cases were treated by femorofemoral crossover grafting without any further complications. Follow-up CTA detected hypogastric artery occlusion in three other patients. All three patients remained asymptomatic with no further intervention required. 125
Diagnostic performance of imaging modalities for surveillance after endovascular abdominal aortic aneurysm repair
The scoping literature searches identified a number of published systematic reviews assessing the diagnostic test accuracy of the imaging modalities considered for the purpose of this assessment. Therefore, we adopted a pragmatic approach and conducted an overview of reviews126 in order to obtain appropriate estimates of diagnostic test accuracy to populate the economic model. The reviews included in the overview were used as a source of existing evidence, but were not formally updated.
Methods for assessing the diagnostic test accuracy of colour duplex ultrasound and contrast-enhanced ultrasound versus computed tomography angiography
Identification of studies
The literature searches for the clinical effectiveness review were sufficiently broad that they retrieved nine relevant diagnostic test accuracy reviews. 3,62,127–133 Therefore, specific searching to identify additional reviews was more focused but included appropriate subject headings and text word terms. To combine the search facets for endovascular aneurysm repair, the imaging modalities under consideration and diagnostic reviews, MEDLINE and EMBASE were searched from 1996 until March 2016, whereas the CDSR and DARE were searched on 29 March 2016 without date restrictions. The search strategies are reproduced in Appendix 1.
Eligibility criteria
We included systematic reviews of diagnostic test accuracy that compared imaging surveillance with CDU and/or CEU in participants who have undergone EVAR for AAA. CTA, despite not demonstrating perfect accuracy, is generally considered to be the reference standard for surveillance imaging after EVAR. To be eligible for inclusion, reviews had to report on the sensitivity and specificity of CDU and/or CDU for the detection of endoleaks and/or other relevant clinical complications.
Data extraction and management
Two reviewers (PS and CR) independently screened the titles and abstracts of all citations identified by the search strategies. Full-text copies of all potentially relevant studies were retrieved and assessed for eligibility independently by the two reviewers. Any disagreements during study selection were resolved by discussion or in consultation with a third reviewer (MB). A data extraction form was specifically designed and piloted for the purpose of this assessment (see Appendix 2). Detailed information on study design, participant characteristics, study settings, characteristics of the index tests and reference standard and estimates of accuracy was extracted. One reviewer completed the data extraction form (CR) and a second reviewer (MS) cross-checked the extracted data for possible errors or inaccuracies. There was no disagreement between the reviewers.
Quality assessment strategy
The risk of bias of included reviews was assessed using both the Assessment of Multiple Systematic Reviews (AMSTAR) tool for the assessment of the methodological quality of systematic reviews134 and the recommendations of the York CRD. 58 The included reviews were independently assessed by two reviewers (PS, CR or MS). Disagreements were resolved by consensus or arbitration with a third reviewer (MB). We did not assess the quality of the abstracts, as the word limit for abstracts is usually insufficient to make informed judgements about the potential risk of bias of reported reviews.
Quantity and quality of the evidence
Characteristics of the included reviews
The literature searches retrieved a total of 48 records, and, after deduplication, 45 reports were available for full-text screening. Nine reviews met the inclusion criteria and were included in this assessment. No additional reviews were identified. Table 12 shows the characteristics of the identified reviews. Eight of the included reviews were published in full, but one131 was available as an abstract and was consequently excluded from the risk-of-bias assessment. The number of studies included in the reviews ranged from 8 to 35, the number of participants ranged from 259 to 4654, and the number of paired scans ranged from 639 to 5343. The review by Cantisani et al. 129 included two literature reviews, which are also included in this overview,62,132 and the review by Howard et al. 131 included one literature review, but did not provide its bibliographic details. Three reviews assessed both CDU and CEU versus CTA,62,132,133 two reviews130,131 assessed CEU versus CTA and one review130 provided sensitivity and specificity estimates for CEU.
| Study, first author (year of publication) | Aim | Databases searched | Number of included studies | Total number of participants/paired scans | Surveillance imaging modality |
|---|---|---|---|---|---|
| Ashoke (2005)127 | To synthesise available evidence regarding the diagnostic accuracy of CDU vs. CTA for the detection and classification of endoleaks after aortic endografting | MEDLINE, EMBASE, PubMed, BioMed, CENTRAL, Business Information Database System and Ingenta from 1991 to 2004 | 10 |
711 participants 1355 paired scans |
CDU and CTA |
| Bevis (2012)128 | To review the accuracy of CDU compared with CTA for endoleak detection | MEDLINE, Google Scholar (Google Inc., Mountain View, CA, USA) and the Current Controlled Trials register from 1998 to 2011 | 29 | 5343 paired scans | CDU and CTA |
| Cantisani (2015)129 | To present a comprehensive overview of the use of CEU for post-EVAR surveillance | MEDLINE, EMBASE and The Cochrane Library from 1998 to 2015 | 8 |
> 259 patients > 1191 paired scans |
CDU, CEU and CTA |
| Chung (2015)130 | To assess the accuracy of CEU vs. CTA for the detection of endoleaks during post-EVAR surveillance | PubMed, EMBASE and The Cochrane Library from 1997 to 2013 | 8 |
454 patients 639 paired scans |
CEU and CTA |
| Howard (2011)131 | To assess the role of CEU for EVAR surveillance and endoleak detection | NR | 11 | NR | CEU and CTA |
| Karanikola (2014)3 | To review the current literature for the effectiveness and safety of CDU compared with CTA for post-EVAR surveillance | PubMed, MEDLINE, Ovid, EMBASE and The Cochrane Library from 1995 to 2013 | 35 | 4525 patients | CDU and CTA |
| Karthikesalingam (2012)132 | To review the diagnostic accuracy of CEU and CDU, focusing on the detection of clinically relevant type I and type III endoleaks | EMBASE, MEDLINE, the Current Controlled Trials register, DARE and The Cochrane Library from 1996 until 2012 | 31 | 4654 paired scans | CDU, CEU and CTA |
| Mirza (2010)62 | To determine the diagnostic accuracy of CDU and CEU vs. CTA for endoleak detection | EMBASE, MEDLINE, Current Controlled Trials register, DARE and the Cochrane Controlled Trials Register from 1996 to 2009 | 21 |
2886 patients 2895 paired scans |
CDU, CEU and CTA |
| Sun (2006)133 | To investigate the diagnostic accuracy of CDU vs. CTA for the detection of endoleaks and aneurysm sac measurements | PubMed and MEDLINE from 1991 to 2005 | 21 | 1534 patients | CDU, CEU and CTA |
Quality assessment of the diagnostic test performance reviews
The eight reviews of diagnostic test accuracy published in full were of mixed methodological quality. 3,62,127–130,132,133 The majority of the included reviews were considered to have searched major relevant bibliographic data sources, conducted hand-searching of references and provided example of key text words,3,62,127,128,130,132 specified their inclusion/exclusion criteria62,127,128,130,132,133 and provided sufficient information on the characteristics of included studies. 3,62,127,130,132,133 However, only one study provided information on the inclusion of grey literature and on a priori design,127 two reported on duplicated selection and extraction,62,127 two provided a list of excluded studies,130,133 one assessed the presence of publication bias62 and only two used the results of the risk-of-bias assessment to draw conclusions. 62,132 A potential conflict of interest was assessed in half of the included reviews. 62,130,132,133 Of the eight reviews published in full, one, by Cantisani et al. ,129 was rated as being at a high risk of bias because, for the majority of the AMSTAR and CRD criteria, the information was unclear or not reported (Figures 4 and 5). Four reviews were considered to be of good methodological quality. 62,127,130,132 The three remaining reviews were considered to be of moderate quality. 3,128,133
FIGURE 4.
Risk-of-bias assessment using the CRD criteria. N/A, not applicable.
FIGURE 5.
Risk-of-bias assessment using the AMSTAR criteria. N/A, not applicable.
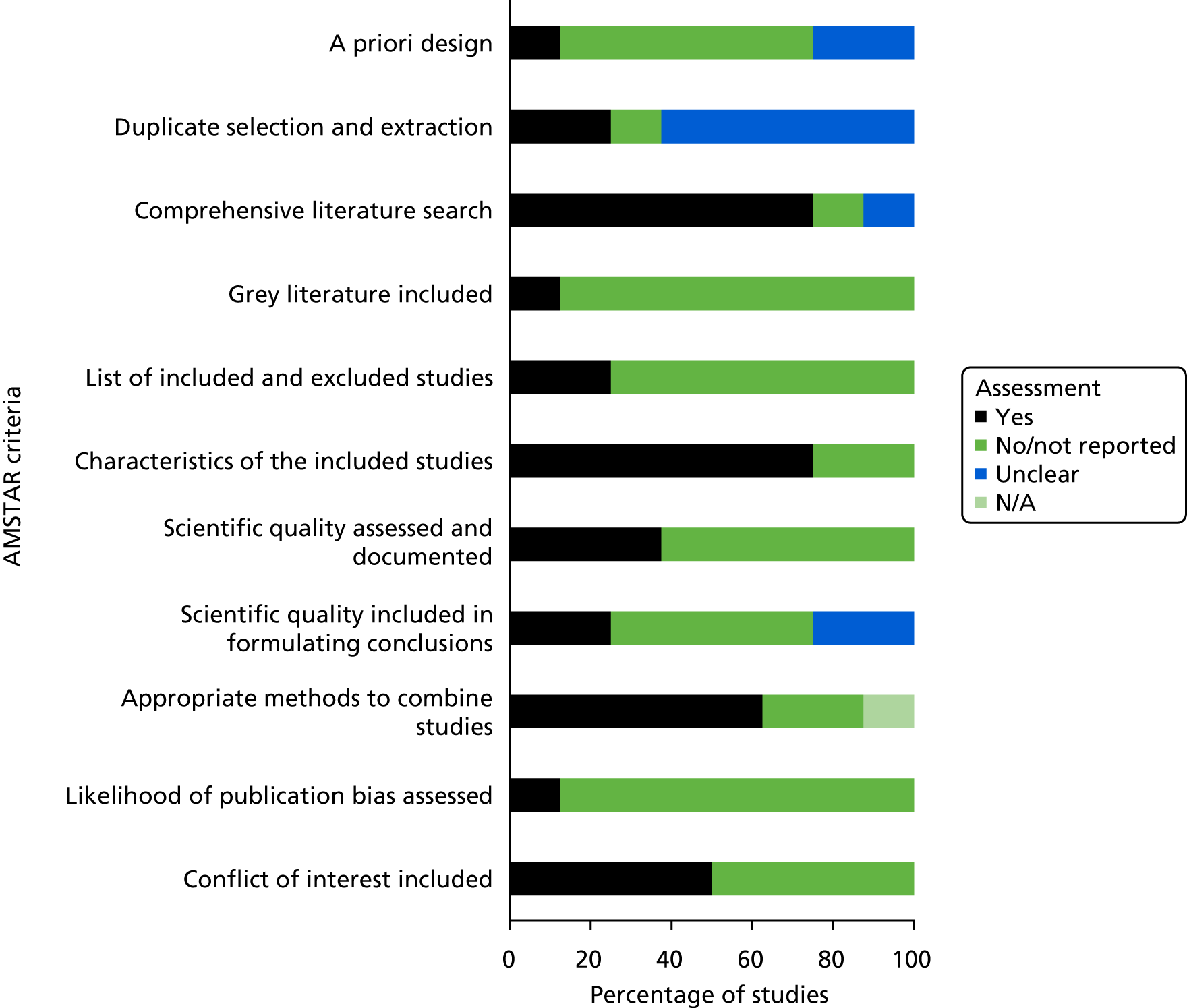
Assessment of diagnostic test performance
All of the included reviews assessed the diagnostic test accuracy of CDU and/or CEU versus CTA for the identification of endoleaks during post-EVAR surveillance. Sun et al. 133 also assessed the accuracy of CDU for aneurysm sac measurements. Six reviews provided pooled estimates of accuracy for CDU,62,127–129,132,133 five reviews provided these for CEU,62,130–133 and two reviews reported the same accuracy estimates for CTA. 129,132 The systematic review by Ashoke et al. 127 provided estimates of accuracy for CDU based on eight published data sources and also combined the results of the published data with two unpublished data sources. The review by Karanikola et al. 3 did not combine estimates of test accuracy because of the heterogeneity observed between the included studies.
The pooled estimates of test accuracy for detecting all types of endoleaks are presented in Table 13. The lowest reported sensitivity estimate for CDU was 62%128 and the highest was 96%;127 the lowest specificity estimate was 90%129 and the highest was 97%. 129 The lowest reported sensitivity estimate for CEU was 81%133 and the highest was 98%;62,131 the lowest specificity estimate for CEU was 78%130 and the highest was 88%. 62 Two reviews128,132 also reported the test accuracy estimates categorised by type of endoleak (Table 14 provides further details). The review conducted by Chung et al. 130 also presented a narrative summary of the results of the included studies, indicating that the majority of endoleaks detected or missed by CEU were characterised as type II endoleaks.
| Study, first author (year of publication) | Imaging modality, % (95% CI) | Number of studies included in the review | |||||
|---|---|---|---|---|---|---|---|
| CTA | CDU | CEU | |||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | ||
| Ashoke (2005)127 | 96 (52 to 87) | 91 (87 to 95) | 10 (eight published andtwo unpublished) | ||||
| 67.4 (47.5 to 87.3) | 92.2 (88.3 to 96.1) | Eight published | |||||
| Bevis (2012)128 | 62 (58 to 65) | 94 (93 to 94) | 29 | ||||
| Cantisani (2015)129 | Reports pooled data from Karthikesalingam et al.132 | 62–83 (NR) | 90–97 (NR) | Reports pooled data from Mirza et al.62 | 8 | ||
| Chung (2015)130 | 91 (87 to 95) | 78 (74 to 82) | 8 | ||||
| Howard [(2011)131 abstract] | 98 (95% CI NR) | 11 | |||||
| Karanikola (2014)3 | Pooled estimates not reported because of the observed heterogeneity between studies | 35 | |||||
| Karthikesalingam (2012)132 | 70 (53 to 82) | 98 (94 to 100) | 74 (62 to 83) | 94 (90 to 97) | 96 (85 to 99) | 85 (76 to 92) | 31 |
| Mirza (2010)62 | 77 (64 to 86) | 94 (88 to 97) | 98 (90 to 99) | 88 (78 to 94) | 21 | ||
| Sun (2006)133 | 66* (52 to 81) | 93 (89 to 97) | 81* (52 to 100) | 82 (68 to 97) | 21 | ||
| Study, first author (year of publication) | Imaging modality, % (95% CI) | Number of studies providing data | |||||
|---|---|---|---|---|---|---|---|
| CTA | CDU | CEU | |||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | ||
| Karthikesalingam (2012)132 | |||||||
| Types I and III endoleaks | 83 (40 to 97) | 100 (97 to 100) | 99 (25 to 100) | 100 (98 to 100) |
13 on CDU Eight on CEU |
||
| Accuracy, n/N (%) | |||||||
| Bevis (2012)128 | 29 | ||||||
| Type I endoleaks | 43/51 (84) | ||||||
| Type II endoleaks | 126/228 (55) | ||||||
| Type III endoleaks | 6/10 (60) | ||||||
| Type IV endoleaks | 1/2 (50) | ||||||
Of the three reviews assessing the diagnostic test accuracy of both CDU and CEU,62,132,133 two62,132 were rated as being at a low risk of bias. 62,132 In particular, the results of the review by Karthikesalingam et al. ,132 which included more recent literature searches and provided estimates of accuracy by type of endoleak, were used to populate the economic model.
Despite its limitations, CTA is still considered to be the best current imaging modality for the detection of endoleaks and clinical complications after EVAR. However, it is worth pointing out that the technique of ultrasound, and in particular of CEU, has fundamentally changed during the last decade. Many studies included in the identified reviews predate the most recent technical improvements. A mixture of early- and late-generation ultrasound machines and different CTA phases were used in the primary studies included in the identified systematic reviews. The impact of this ‘technological heterogeneity’ on the reported pooled estimates of accuracy is unclear. Two reviews attempted to address this issue. Mirza et al. 62 conducted a sensitivity analysis in which studies published prior to 2003 were excluded in order to assess the potential confounding effect of CEU equipment being more modern than CDU equipment. Similar sensitivity and specificity estimates were obtained for CDU and CEU. Chung et al. 130 reported that CEU studies that utilised second-generation contrast agents [i.e. SonoVue and Optison (Amersham Health, Princeton, NJ, USA)] had excellent sensitivity estimates compared with CEU studies that utilised first-generation contrast agents. They also observed that two studies that used both generations of contrast agents demonstrated good sensitivity for the detection of type II endoleaks. References for these two studies were, however, not given. The authors concluded that CEU was as accurate as CTA in detecting endoleaks when studies that utilised first-generation contrast agents were omitted from the analyses. 130 These results are in line with the claim of some investigators that more recent data seem to suggest that the specificity of CEU is higher than that of CTA (Professor Srinivasa Rao Vallabhaneni, Royal Liverpool University Hospital, 2017, personal communication). It is therefore possible that the performance of single-phase CTA as the current accepted reference test was not good enough to assess the accuracy of modern CEU. To take this into account in the economic model, we have conducted sensitivity analyses using different sensitivity and specificity estimates to reflect the recent technological improvements of imaging modalities for surveillance after EVAR.
Chapter 3 Assessment of cost-effectiveness
The evidence of the cost-effectiveness of using CDU or CEU alone or in conjunction with CTA for the surveillance of adults after EVAR was explored in the health economic component of this assessment. A two-step approach was used: (1) a systematic review of economic evaluations to retrieve any readily available evidence on cost-effectiveness, followed by (2) a de novo decision-analytic model to synthesise the available evidence on effectiveness, health-care resources used and costs. Review of the cost-effectiveness studies reports the systematic review of cost-effectiveness studies and Economic analysis with a newly developed decision model focuses on the economic model exercise.
Review of the cost-effectiveness studies
In order to summarise the available evidence on cost-effectiveness, we conducted a systematic literature review to identify studies that reported an economic evaluation of surveillance strategies for adult individuals after an EVAR intervention that included CDU and/or CEU compared with CTA.
Methods for review of the cost-effectiveness studies
Search strategy
Comprehensive search strategies were designed to identify economic evaluations of surveillance after EVAR (see Appendix 1). Searches were undertaken on 29 March 2016 and updated on 5 September 2016. The following databases were searched: NHS Economic Evaluation Database (from inception to 31 March 2015), the HTA Database (from inception to 5 September 2016) and MEDLINE In-Process and Epub Ahead of Print (from 1946 to 5 September 2016), EMBASE (from 1947 to week 36 2016) and Research Papers in Economics (from inception to 5 September 2016). The websites of HTA organisations were consulted for additional reports. The reference lists of all included studies were scanned, and appropriate experts were contacted for details of additional reports of cost-effectiveness.
The titles and abstracts of all citations identified by the search strategies for economic evaluations were screened for inclusion by a health economist (RH). The full-text papers of potentially relevant studies were retrieved and formally assessed for inclusion. Any uncertainty regarding study selection was discussed with the review team.
Inclusion and exclusion criteria
The inclusion criteria required the studies to be full economic evaluations (i.e. to consider the costs and effects of more than one strategy) in order to be included in the review. No restrictions were imposed on the way in which costs and/or effects were calculated. In addition, the study should compare post-EVAR surveillance strategies with at least one of the relevant diagnostic tests (e.g. CDU, CEU and CTA).
Either RCTs or decision-model economic evaluations were included. Studies that did not meet the inclusion criteria but reported relevant data that could inform the de novo economic model (e.g. costs, quality of life, model structure, probabilities) were retained for further consultation.
Data extraction
Data were extracted from the included studies using a prespecified data extraction form. The following information was sought:
-
Background information, such as the research question, study design, intervention and comparator details.
-
Characteristics of the study population (e.g. age, setting, inclusion criteria, exclusion criteria).
-
Costing methodology, in particular the perspective, year, currency and the discount rate applied.
-
Methodology used for the analysis of costs, effectiveness and uncertainty.
-
Mean costs and outcomes, incremental costs and outcomes for the differences between groups and incremental cost-effectiveness ratios (ICERs). The results were reported when uncertainty was explored (e.g. 95% CI from the bootstrap analysis).
-
Study strengths and limitations, as reported by the study authors.
-
Conclusions and suggestions for further research, as reported by the study authors.
Quality assessment of the included studies
Cohort-based studies were assessed for quality against the British Medical Journal checklist for referees of economic evaluations. 135 When possible, the results were assessed from the NHS perspective. No decision-modelling studies matching the inclusion criteria were identified and therefore no studies were appraised against the NICE reference case. 60,136
Data synthesis
No data synthesis was attempted, and a summary of the study characteristics, the costing methods used and the quality assessment of each study is provided.
Results of the review of cost-effectiveness studies
After deduplication of the records, 278 abstracts were screened for suitability. Seven studies were selected for full-text assessment, with only five of these studies meeting the inclusion criteria. 41,45,66,137,138 Two studies were conducted in the USA45,137 and three studies were conducted in Europe (in Italy,66 Ireland138 and the UK41). All of these studies considered the effects of crossing from CTA to CDU plus plain radiography as first-line surveillance test. The studies estimated the difference in the number of CTAs required by the new surveillance strategy and the replaced strategy.
Beeman et al. 45 attempted to determine the cost savings and outcome differences of moving from both CTA and CDU imaging at 2 weeks, 6 months and 12 months after discharge and yearly thereafter (group 1 – before 1 July 2004) to CDU imaging as the sole surveillance test after the 2-week scans (group 2 – after 1 July 2004), with CTA being conducted only if any problem was detected by CDU. The authors analysed data on 82 patients for group 1 and on 117 patients for group 2. The clinical outcomes included the number of endoleaks detected and the measurement of the AAA sac diameter. The average length of the follow-up period was 3.5 years (range 0–9 years) and 1.6 years (range 6 months–4 years) for groups 1 and 2, respectively. The authors used hospital charges for CTA and CDU and noted that the decreased charges of US$1595 per patient per year (2008 prices) or US$198 per patient per year using Medicare reimbursements were realised by eliminating CTA surveillance in group 2. Moreover, the sensitivity of CDU for detecting endoleaks was 0.71 and the specificity was 0.99, whereas the sensitivity of CTA was 0.731 and the specificity was 0.991. The authors could not find any difference in aneurysm sac diameters measured by CDU and CTA when these scans were performed within 1 month of each other in group 1. Although the authors do not advocate eliminating CTA from the surveillance protocols, they state that its use should be limited to those circumstances in which it could provide other details about problems first detected by the ultrasound examination.
Bendick et al. 137 evaluated the use of an ultrasound contrast agent to enhance imaging for stent–graft surveillance and compared the costs of this technique with those of CTA. Data on the first 40 patients received in their vascular laboratory were analysed. The follow-up examinations ranged from 1 to 24 months after graft placement, with a mean follow-up time of 13.7 ± 6.1 months. Clinical outcomes included type I or type II endoleaks and costs were calculated using hospital charges with average costs per study that were equal to US$2779 for CTA, US$525 for CDU (including contrast) and US$147 for plain film abdominal radiography. No details of the price year were stated. The authors reported a sensitivity to the presence of any endoleaks of 53% (8/15) for CDU, 93% (14/15) for CEU and 73% (11/15) for CTA. The average 3-year charges per patient were US$22,232 and US$5376 for CTA-based surveillance and surveillance using CDU (including contrast) plus radiography, respectively (a saving of US$16,856 per patient over 3 years). The authors concluded that the technique of duplex ultrasound with an ultrasound contrast agent should become the method of choice for stent–graft surveillance if the promising early results shown in their present series can be demonstrated in a larger patient population.
Chisci et al. 66 evaluated whether or not the imaging modality of surveillance influenced the detection of these conditions affecting the rate of asymptomatic secondary interventions (i.e. endoleaks, AAA expansion, migration, graft infection, graft thrombosis, conversion to open repair, postoperative renal impairment, bowel ischaemia and myocardial infarction). The authors followed a cohort of individuals for whom the follow-up protocol was changed at a given date. Protocol I, performed from January 2003 to December 2006, consisted of CDU plus CTA at 1 month after the procedure and every 6 months thereafter. Protocol II, performed from January 2007 to June 2010, included CDU plus CTA at 1 month after operation, CDU plus plain radiography every 6 months thereafter and CTA carried out during follow-up only for specific conditions. The authors analysed data for 376 individuals in protocol I and 341 individuals in protocol II with a mean follow-up of 1148 days (range 1–3204 days) and 942 days (range 1–1512 days), respectively (p < 0.001). On the 3-year analysis, the authors reported that protocol I cost approximately €3000, whereas protocol II cost approximately €1000; this was a threefold reduction in overall costs for protocol II (p < 0.0001). However, there were no details of the costing method used or the cost categories included in this analysis. The authors concluded that the detection rate of asymptomatic secondary interventions following EVAR is not affected by the type of surveillance imaging and that a surveillance schedule based primarily on CDU and radiography appears to be justified.
Gray et al. 138 retrospectively reviewed the CDU and CTA scans of all 145 patients who underwent EVAR at the Mater Misericordiae University Hospital, Ireland, from 1 June 2003 to 1 July 2010 and compared their results for endoleak detection and determination of residual sac size. The authors’ aim was to assess the cost savings obtained if CDU was employed as a first-line surveillance tool following EVAR and to compare CDU and CTA in terms of efficacy. A total of 484 scans (68%) from 114 patients (78.6%) were available for comparison. The hospital protocol for patients after EVAR included CDU and CTA scans of the aorta within 7 days of surgery. After discharge, all patients underwent CDU at 1 month and then CDU and CTA at 6 months, 12 months and annually thereafter, provided that there was no documented endoleak on either the CDU or CTA. The costs of CTA (€500 per scan) and of radiography (€85) were considered in the costing calculations (expressed in 2010 prices). However, no details of the unit cost sources were reported. The authors found that CDU was 100% sensitive and 95.7% specific in the detection of endoleaks, with a positive predictive value of 28.7% and a negative predictive value of 100%. Furthermore, no statistically significant difference between the two imaging modalities was detected for the determination of residual AAA sac diameter. The authors hypothesised that a reduction in costs resulted from a change in protocol for the year 2010. Adopting a protocol with CDU and abdominal radiography as the first-line surveillance tool would result in a reduction in the number of postoperative CTA scans from 235 to 36. This would equate to a reduction in expenditure from €117,500 to €34,915 (a saving of €82,585). The authors concluded that CDU combined with plain abdominal radiography could safely replace CTA as the primary long-term imaging modality, resulting in a significant cost saving without the loss of scan accuracy.
The only study conducted in the UK41 was a retrospective review of a prospectively maintained database of all patients undergoing elective, standard EVAR at a large tertiary referral centre (Royal Liverpool University Hospital). As with the other studies, the authors assessed the efficacy of a modified post-EVAR surveillance protocol based, primarily, on CDU and radiography, with CTA triggered only by significant findings on the CDU scan or radiography. The study included patients who had their EVAR operation between 1 August 2005 and April 2009, for whom at least 1 year’s post-surgical follow-up data were available. The primary outcome measure considered was aneurysm rupture, whereas the secondary outcome measures included the requirement for secondary intervention and the number of CTA scans avoided, from which the radiation dose reduction and cost savings were calculated. The costs were expressed in 2010–11 prices and NHS tariffs were used to cost the tests (radiography, €35.71; CDU, €187.47; CTA, €269.61; exchange rate: £1 = €1.18). 41 The authors analysed data on 194 patients who underwent a total of 606 sets of surveillance imaging: 194 sets at 1 month (radiography, CDU and CTA) and 412 per protocol sets thereafter (radiography and CDU). No patient presented with ruptures or aneurysm-related complications that were not identified by the modified surveillance protocol. The authors obtained the number of tests performed in the modified protocol and compared this with those that would have been performed for the same group had the protocol not been modified: 412 tests would have been conducted for the follow-up period that would have costed €14,711 for radiography, €77,326 for CDU and €111,078 for CTA. With the modified protocol, the number of tests and costs for radiography and CDU remained the same. However, only 71 CTA scans were conducted, with a cost of €19,142, which was a saving of €91,936. The authors concluded that follow-up after EVAR primarily based on CDU and radiography was feasible and safe, and reduced the use of CTA substantially, with consequent reductions in exposure to ionising radiation or an intravascular contrast medium, and costs.
Summary
Five studies met the inclusion criteria. All of these compared a surveillance strategy based on CDU or CEU with a CTA-based strategy. All of the studies assessed the reduction in costs as a result of the smaller number of CTA scans performed in the modified protocol. Only cohort studies were identified in the searches. However, the studies by Beeman et al. 45 and Chisci et al. 66 compared cohorts before and after the protocol changes took place. In the other studies, an economic analysis was conducted on the basis of the resources required (i.e. the number of CTA scans performed) if a hypothetical alternative protocol were to be used. Although all of the studies41,45,66,137,138 fairly agree on the clinical outcomes of interest (i.e. endoleaks, AAA size and the need for secondary interventions), the reporting of costs and the cost method was disparate; although one study gave details of the cost categories, the unit cost used, the sources and the price year used, another study reported only the final cost calculations.
The only study from the UK that could inform NHS policy was the study conducted by Harrison et al. 41 However, neither this nor any of the non-UK studies used a preference-based measure of effectiveness. Moreover, judging from the number of scans used in the authors’ cost calculations, the follow-up period considered was just over 2 years. This time horizon might not be long enough to consider all of the costs and consequences that are relevant for the question posed. As such, a new economic model was developed to assess the relative efficiency of CDU or CEU in the surveillance of individuals after EVAR. This is reported in the next section.
Economic analysis with a newly developed decision model
None of the available economic evaluations from the systematic review provided a definite answer on the cost-effectiveness of the use of CDU or CEU compared with that of CTA from the NHS perspective. Therefore, a de novo economic model was developed. The aim of the economic model was to assess the relative efficiency of surveillance strategies that used CEU or CDU alone or in combination with CTA.
Methods
Care pathways
Care pathways were discussed and developed within the project management group and the project advisory group meetings. It was agreed that surveillance involves the search for information about abnormalities that are relevant to the disease. It was also agreed that, once there is any indication of an abnormality, the patient status changes and the surveillance stops. After this, the following steps are then part of diagnostic investigations and/or eventual treatment. Hence, surveillance applies only to those individuals who are deemed to have no detected EVAR-related abnormalities and, as such, the model considers those patients who were regarded as not having an EVAR-related complication (e.g. at 6 months post surgery).
Five surveillance strategies were agreed:
-
annual CTA scan plus plain radiography
-
annual CDU scan plus plain radiography
-
annual CEU scan plus plain radiography
-
colour duplex ultrasound scan together with CTA scan and plain radiography at 1 year, followed by CDU scan and plain radiography on an annual basis
-
contrast-enhanced ultrasound scan together with CTA scan and plain radiography at 1 year, followed by CEU scan and plain radiography on an annual basis.
A positive test result in any of the surveillance strategies would trigger either further diagnostic investigations or treatment. This part of the decision model was identical for all of the strategies.
The economic model
A Markov model approach was selected for the decision-analytic model exercise. 135 Markov models have Markov states in which individuals spend a period of time, which is named a ‘cycle’. At the end of each cycle, the individuals can remain in their current Markov state or move to another state. The probabilities of moving to other Markov states or remaining in the current state are named ‘transition probabilities’. Individuals in the model would accrue costs and benefits (e.g. life-years) depending on the time spent in each Markov state and the interventions and/or events modelled within each Markov state. Markov models are particularly suitable to model recurrent issues and chronic diseases. They allow the incorporation of health states to reflect the movement of patients during surveillance, further diagnosis and treatment. In the current study, model states reflect the underlying condition (e.g. post-EVAR state with known or unknown complications), together with the decision on treatment (e.g. reintervention after EVAR). In all of these models, an absorbing state is included, in which all individuals would end up if the model was run for a sufficiently long period of time (e.g. Markov death state).
Description of the Markov model and the model structure
The model overall state-transition diagram is reported in Appendix 14. A simpler, schematic representation of the Markov model is shown in Figures 6–9. In these figures, circles represent the Markov states, whereas squares represent an event that occurs within a Markov state (e.g. an emergency procedure). Arrows show the direction of the possible transitions in the model. Unless specified, individuals can remain in a Markov state for more than one cycle. Eight Markov states are considered in the model:
-
normal (no residual EVAR complication)
-
abnormal Ia (intervention required)
-
abnormal Ib (intervention required – endoleak)
-
abnormal II (no intervention required)
-
enhanced follow-up (normal)
-
elective surgery (one cycle, temporary state)
-
enhanced follow-up (abnormal II)
-
death.
FIGURE 6.
Schematic diagram of the surveillance for EVAR Markov model; underlying condition.
FIGURE 7.
Schematic diagram of the surveillance for EVAR Markov model (from abnormal Ia, intervention needed Markov state). FN, false negative; TN, true negative; TP, true positive.
FIGURE 8.
Schematic diagram of the surveillance for EVAR Markov model (from abnormal II, no intervention needed Markov state). FN, false negative; TN, true negative; TP, true positive.
FIGURE 9.
Schematic diagram of the surveillance for EVAR Markov model (from normal, no residual EVAR complications Markov state). FP, false positive; TN, true negative; TP, true positive.
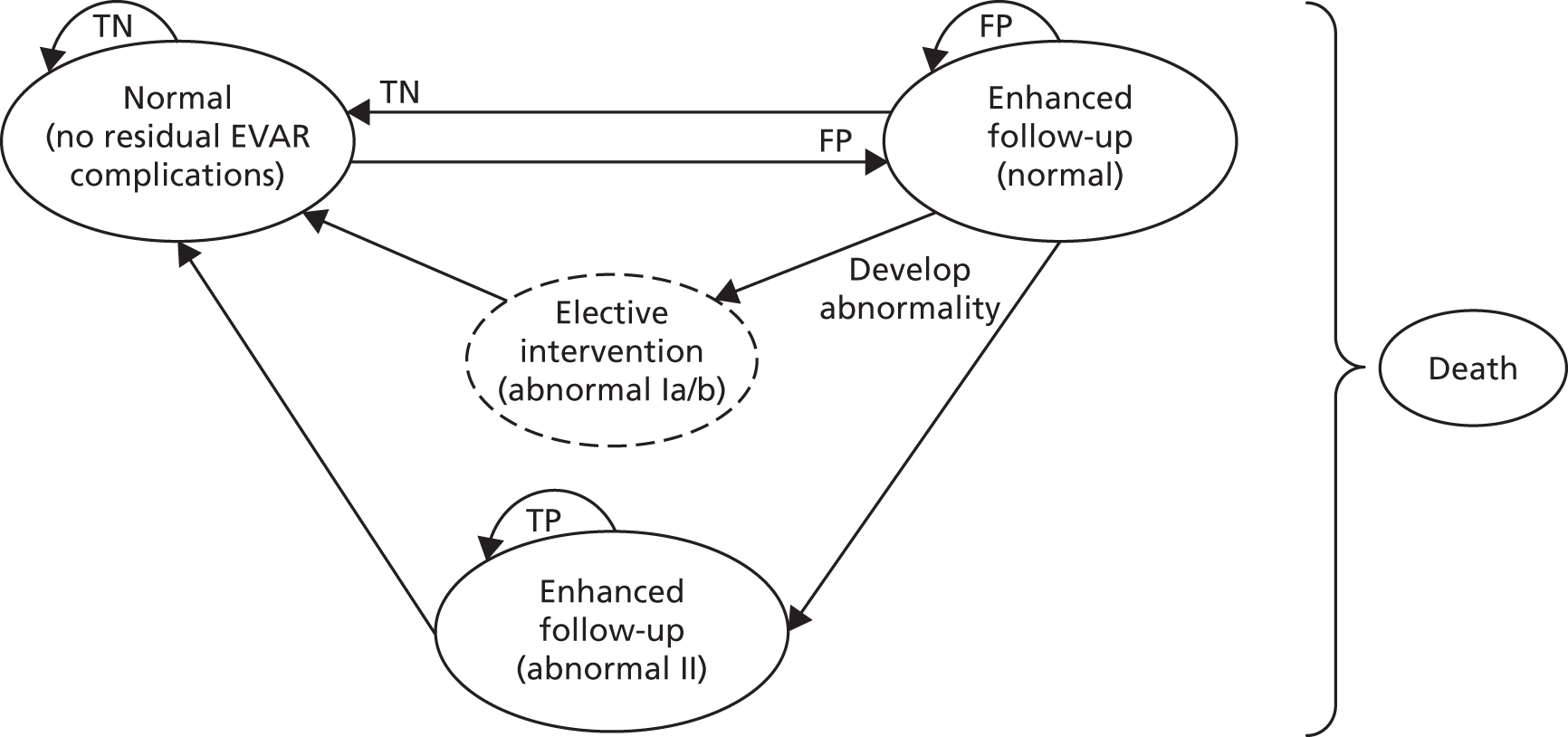
Figure 6 shows the four Markov states that reflect the underlying condition but are yet to be detected (‘undiagnosed’ states 2–4 above). Figures 7–9 show one of these four Markov states representing the underlying condition together with the Markov states that individuals can move to (e.g. those states that result from a diagnosis – the ’diagnosed’ side of the figure – correctly or not). The performance of a surveillance strategy in this model is given by the correct identification of those individuals with an abnormal condition and the corresponding transfer of those individuals into the true-positive states on the right sides of Figures 7–9.
All individuals start in the ‘normal (no residual EVAR complication)’ health state and can develop abnormalities as the model runs (see Figure 6). The surveillance strategies aimed to detect a variety of conditions and complications . These were generically described as ‘abnormalities’ and were divided into two categories: conditions that trigger an elective intervention and conditions that, on clinical assessment, necessitate closer follow-up (e.g. additional 6-month CTA scans). The first category was subdivided into two: abnormal 1a includes non-endoleak-triggered interventions (e.g. limb occlusions, graft infections) and abnormal 1b counts for the endoleak-prompted interventions (e.g. type I, III and IV, type II or endotension with sac expansion > 5 mm). Examples of patients with conditions within the close follow-up group (abnormal II) are those with type II endoleaks with a sac expansion of < 5 mm in 6 months or with limbs with kinking or partial thrombosis.
These abnormal Markov states are tunnel states. In tunnel states, individuals go through the health states in a defined sequence. The rationale behind these tunnel states for individuals with an abnormality is to count the length of time for which the individuals have had the abnormality. Thus, the probability of experiencing a rupture as a result of, for instance, a type I or III endoleak will increase as length of time for which the individual has had the endoleak increases (i.e. the length of time that the individual remains in the undetected health state). Finally, individuals can move from any health state to the absorbing death state (presented, for simplicity, at the side of the figures).
Once an abnormal condition has been identified, individuals move to another Markov state, in which they can be treated (see Figure 7) or followed up more closely (see Figure 8). Figure 7 shows the pathway of individuals who developed a non-endoleak abnormality that would require elective surgery. If the condition is undetected through surveillance (a false-negative result), the person remains in the abnormal Ia state. This person can experience an event and, as a result, go through an emergency intervention. If the situation is resolved, the person would move back to the original normal state. If the underlying condition is detected through surveillance (a true-positive result), the individual would move to the elective surgery state. This is a temporary state and individuals can remain within this state only for one cycle (to have surgery and subsequently move on to another health state). Again, once the surgical intervention is successful, the individual moves back to the original normal state. Moreover, individuals can move to the absorbing death state from any of the other health states as a result of an emergency (e.g. rupture), surgery (e.g. hospital mortality) or other comorbidities (general population mortality). A similar structure was followed for individuals who developed an endoleak abnormality that required an elective intervention (abnormal Ib Markov health state).
Figure 8 shows the pathway for the abnormal II health state. Undetected individuals (a false-negative result) will remain in this state (i.e. represented by the back arrow from the state). These people have the risk of experiencing an event that would trigger an emergency intervention. If the abnormality is detected (a true-positive result), the individuals will move to enhanced follow-up, which is defined as 6-month visits at which a CTA-based assessment is conducted. If the patient is stable and no further interventions (e.g. elective surgery) are decided after 2 years (four model cycles), the patient returns to the surveillance pathway (e.g. annual check-ups based on the original surveillance test – CDU, CEU or CTA).
The model also includes a false-positive state for those individuals without an abnormal condition (see Figure 9) but with a positive test result [e.g. enhanced follow-up (normal)]. If the individual developed an abnormality while under enhanced follow-up, they would either move straight to elective intervention (e.g. for abnormal Ia or Ib) or remain under enhanced follow-up but within a different Markov state (e.g. for abnormal II).
Individuals can suffer an event between surveillance visits. This is considered in the model to be an event within a Markov state and is shown in Figures 6–9 as a square (e.g. emergency procedure). The model assumes that individuals who survive an unsuccessful second intervention could undergo a third intervention. However, a pragmatic assumption based on small probabilities was made and individuals can either have a successful third intervention or die.
Parameter estimates used in the economic model
The parameter estimates required to populate the economic model were obtained from the results of the clinical effectiveness search, which was supplemented by structured and focused searches (e.g. of EVAR trials with a longer follow-up period). When no suitable data resulted from these searches, expert opinion was sought. Probabilities gives details of the probabilities, unit costs and utility weights used in the model. Also provided within this section are details of the probability distributions used for the probabilistic sensitivity analysis.
Probabilities
The model starts with the whole cohort in the normal state, and therefore no prevalence data were necessary. The annual incidence of abnormalities was developed from data reported by Tang et al. ,93 based on ENGAGE (Table 15). The authors report 1-year data, excluding the first 31 days after EVAR. From a total of 325 patients, 25 abnormalities were reported (20 endoleaks, one stent–graft occlusion, three stent–graft stenoses and one other event related to the stent–graft). These data were initially used to obtain the proportions of cases within each of the model abnormal subgroups (graft occlusion for abnormal Ia, types I and III endoleaks for abnormal Ib, and type II endoleak for abnormal II). However, in consultation with the experts in the project advisory group, these figures were revised, as it was believed that a higher proportion of abnormalities Ia and Ib are currently seen in UK practice (Professor Srinivasa Rao Vallabhaneni, and Dr Russell Jamieson, NHS Grampian, 2017, personal communication). Beta distributions were used to assess the uncertainty around the central point estimates.
| Variable | Value | Probability distribution | Source |
|---|---|---|---|
| Annual incidence of abnormal cases | 0.04 | Beta(13,312) | Tang et al.93 |
| Proportion of abnormal Ia subgroup from all abnormal individuals | 0.15 | Beta(15,85) | Based on expert opinion |
| Proportion of abnormal Ib subgroup from all abnormal individuals | 0.15 | Beta(15,85) | Based on expert opinion |
| Proportion of abnormal II subgroup from all abnormal individuals | 0.70 | Beta(70,30) | Based on expert opinion |
Unfortunately, the systematic review of clinical effectiveness could not identify any studies that directly compared the performance of alternative CDU and CEU surveillance strategies. Therefore, test performance data were used to feed the model. Test sensitivity and specificity (Table 16) were obtained from the systematic review by Karthikesalingam et al. 132 Alternative data were available (see Diagnostic performance of imaging modalities for surveillance after endovascular abdominal aortic aneurysm repair in Chapter 2). However, Karthikesalingam et al. 132 is the only review reporting sensitivity and specificity for all three tests (CDU, CEU and CTA), and the quality assessment of all of these diagnostic review studies resulted in the Karthikesalingam et al. 132 review being deemed to be of higher quality. Beta distributions were used to address uncertainty around the central parameter values.
| Variable | Value | Probability distribution | Source |
|---|---|---|---|
| CDU sensitivity | 0.74 | Beta(48.8,17.2) | Karthikesalingam et al.132 |
| CDU specificity | 0.94 | Beta(164.5,10.5) | Karthikesalingam et al.132 |
| CEU sensitivity | 0.96 | Beta(28,1.2) | Karthikesalingam et al.132 |
| CEU specificity | 0.85 | Beta(64.3,11.3) | Karthikesalingam et al.132 |
| CTA sensitivity | 0.70 | Beta(26.1,11.2) | Karthikesalingam et al.132 |
| CTA specificity | 0.98 | Beta(81.1,1.7) | Karthikesalingam et al.132 |
| Proportion of individuals adhering to surveillance visits | 0.93 | Uniform(0.5,1) | Assumption based on expert opinion |
The probability of having a reintervention and the risk of rupture and mortality are reported in Table 17. The probability of having a reintervention was developed from Tang et al. 93 Disregarding the first month post EVAR, 13 individuals out of 319 had a secondary procedure. The proportion of successful secondary procedures was based on the proportion of individuals free of a secondary intervention in the EVAR 1 trial – 15-year follow-up data. 48 The model allows for individuals with an unsuccessful surgery to go to a third procedure. We found no data to inform the model parameters related to the third intervention (e.g. the probability of having a third intervention and the proportion of successful interventions) and therefore the data as for the second intervention were applied.
| Variable | Value | Probability distribution | Source |
|---|---|---|---|
| Probability of having a reintervention | 0.020 | Beta(13,312) | Tang et al.93 |
| Probability of not having a third intervention | 0.860 | Beta(541,85) | Patel et al.48 |
| Probability of rupture (type I or III endoleak) | 0.0102 | Beta(3,294) | Developed from Buth et al.91 |
| Probability of rupture (type I or III endoleak) after 1 year | 0.1 | Uniform(0.1,0.2) | Moll et al.31 |
| Probability of rupture (type I or III endoleak) after 2 years | 0.3 | Uniform(0.3,0.3) | Moll et al.31 |
| Probability of rupture (type II endoleak) | 0.0018 | Beta(3.5,1971.5) | Developed from Buth et al.91 |
| Probability of rupture (no endoleaks) | 0.0018 | Beta(3.5,1971.5) | Developed from Buth et al.91 |
| Mortality | |||
| Elective surgery | 0.007 | Beta(12,598) | Based on expert opiniona |
| Emergency surgical intervention (rupture) | 0.32 | Beta(36.6,77.9) | Antoniou et al.139 |
| Rupture | 0.16 | Beta(30,160) | Antoniou et al.139 |
| Age- and gender-specific general population mortality for the UK | Various | Not applicable | National Life Tables, UK146 2013–2015. Office for National Statistics |
The risk of rupture for undetected endoleaks was based on an analysis of early data from the EUROSTAR registry. Buth et al. 91 conducted two cohort analyses comparing a cohort of people with type I and type III endoleaks (n = 297) with those who had never experienced an endoleak (n = 1975), and a cohort of people with type II endoleaks (n = 320) with those who had never experienced an endoleak (n = 3275). The authors state that the cumulative rate of rupture from type I and type III endoleaks was 4% at 2 years compared with 0.7% for those who had never experienced an endoleak. Moreover, the number of late ruptures in patients with type II endoleaks was not significantly different from the number of late ruptures in those who had never experienced an endoleak. The risk of rupture for individuals with type I and type III endoleaks after 1 year was adjusted based on data reported by Moll et al. 31 The authors report the risk of rupture according to aneurysm size, based on population studies. To calculate the risk in the model, an aneurysm size of 55 mm at baseline was assumed, together with a growth rate of 5 mm per cycle. These rupture risks were applied to the undetected abnormal Ib group in the model only.
Table 17 also reports the mortality data assumed in the model. Age- and gender-specific general population mortality rates were applied to the cohort. The risk of surgical death in an elective setting was based on expert opinion (Professor Srinivasa Rao Vallabhaneni and Dr Russell Jamieson, personal communication). The main event that surveillance is trying to avoid is the aneurysm rupture and its associated high mortality rate. The risk of death from a rupture was calculated based on the systematic review and meta-analysis of late ruptures by Antoniou et al. 139 The authors identified 11 studies (case series) that reported a total of 190 ruptures: 30 patients were managed with palliative care or died before surgery. Moreover, the authors reported a perioperative mortality rate of 32% (95% CI 24% to 41%).
Costs
Unit costs were obtained from NHS Reference Costs 2015 to 2016140 (Table 18). The unit costs for ultrasound tests (with and without contrast) of < 20 minutes’ duration reported in the NHS reference costs are surprisingly similar. Moreover, the stated average unit cost for an ultrasound with contrast was lower than an ultrasound without contrast. Therefore, the unit cost for a vascular ultrasound scan was used to cost CDU and CEU tests. The clinical experts noted that clinical staff (i.e. a consultant radiologist) should be present to administer the contrast agent for CEU. In addition, CEU includes a contrast agent [i.e. sulfur hexafluoride or perfluorocarbon encapsulated by a phospholipid shell (SonoVue)] with an associated cost of £46 for a 10-vial box (Mr Craig Rore, Grampian Medicines Information Centre, 2017, personal communication). Therefore, the unit cost of CEU was adjusted to add the cost of 30 minutes (Professor Srinivasa Rao Vallabhaneni, personal communication) of a medical consultation (based on a cost per hour of £135)141 plus £4.60 for the cost of the contrast. Furthermore, the cost of a CT scan of one area, with pre and post contrast, was used for CTA. Notably, NHS Reference Costs 2015 to 2016140 does not report the unit cost for a plain radiography. The cost of a plain radiography is absorbed within other cost categories (e.g. outpatient visit) because of its relatively high volume and low cost. As plain radiography was considered in all of the strategies in a similar manner (on an annual basis), an executive decision was made and the unit cost of plain radiography was not incorporated in the model. If a surveillance test outcome was indeterminate, a further assessment was assumed (i.e. with CTA) and the cost of a visit was added to the cost of the subsequent test (i.e. non-admitted face-to-face attendance, follow-up – vascular surgery).
| Variable | Cost (£) | Probability distribution | Source |
|---|---|---|---|
| CDU | 57.53 | Gamma(6.23,0.11) | RD47Z – vascular ultrasound scan – NHS Reference Costs 2015 to 2016 (main schedule)140 |
| CEU | 57.53 | Gamma(6.23,0.11) | RD47Z – vascular ultrasound scan – NHS Reference Costs 2015 to 2016 (main schedule)140 |
| CTA | 118.53 | Gamma(13.67,0.12) | RD22Z – CT scan of one area, with pre and post contrast – NHS Reference Costs 2015 to 2016 (main schedule)140 |
| Additional cost of CEU | 72.10 | Uniform(49.6,94.6) | 30 minutes of medical consultation (based on an hourly cost of £135 – PSSRU hospital-based doctors with qualifications) plus contrast agent at £46 for 10 vials (one vial used per test per person). Probability distribution based on assumption141 |
| Further assessment visit | 140.21 | Gamma(8.38,0.06) | WF01A – non-admitted face-to-face attendance, follow-up – vascular surgery – NHS Reference Costs 2015 to 2016140 |
| EVAR intervention (elective) | 11,925.16 | Gamma(5.99,0) | Weighted average codes YR04Z and YR03Z (AAA endovascular and complex endovascular elective repair) – NHS Reference Costs 2015 to 2016140 |
| Other surgical procedures (elective) | 12,707.99 | Gamma(4.03,0) | Percutaneous transluminal embolectomy or thrombolysis (weighted average codes YR23A and YQ11B plus YR12Z – percutaneous and open procedures plus stent) – elective inpatient – NHS Reference Costs 2015 to 2016140 |
| EVAR intervention (emergency) | 20,675.86 | Gamma(4.3,0.0002) | AAA endovascular and complex endovascular repair (weighted average categories YR04Z and YR03Z – non-elective) and VB01Z (emergency medicine) and ASS02 (ambulance) – NHS Reference Costs 2015 to 2016140 |
| Other surgical procedures (emergency) | 18,681.87 | Gamma(3.65,0.0002) | Percutaneous transluminal embolectomy or thrombolysis (weighted average for codes YR23A and YQ11B plus YR12Z – percutaneous and open procedures plus stent) – non-elective inpatient and VB01Z (emergency medicine) and ASS02 (ambulance) – NHS Reference Costs 2015 to 2016140 |
Endovascular AAA repair reintervention was costed as a weighted average of the unit costs for elective EVAR repair (complex and non-complex). The cost of percutaneous transluminal embolectomy or thrombolysis was used as the cost of other procedures for the abnormal Ia group. Emergency procedures were costed assuming non-elective categories plus the cost of emergency medicine (i.e. any investigation with category 5 treatment) and ambulance service (i.e. see and treat and convey).
Utility weights
Population-based utility weights for patients aged ≥ 74 years were assumed for individuals after EVAR (Table 19). These utility weights were calculated using the equation provided by Ara and Brazier142 [i.e. EuroQol-5 Dimensions (EQ-5D) = 0.9508566 + 0.0212126 × (1, if males, or 0, if females) – 0.0002587 × age – 0.0000332 × age2]. 142 The rationale behind this is that the condition is mostly asymptomatic and, as such, would have no effect on the individual’s quality of life. Interestingly, this utility weight is of a similar value to the one reported by Brown et al. 143 on the EVAR 1 RCT for baseline EQ-5D score [mean 0.75 (SD 0.22); mean age 74 years]. The utility decrement for those going into any reintervention was developed from the EVAR 1 trial. 143 This decrement was calculated as a proportional reduction from baseline until the first year post EVAR, when patients are assumed to be back to the population-based utility weight.
| Variable | Value | Probability distribution | Source |
|---|---|---|---|
| All health states – at the start (74-year-old male cohort) | 0.77 | Not applicable | Age- and gender-specific general population EQ-5D score142 |
| Surgery QALY weight decrement | 2.22 | Beta(4.7,209.4) | Developed from Brown et al.143 (EVAR 1 RCT) as difference from baseline |
Base-case analyses
The model base-case analysis was run for a cohort of 74-year-old men for a lifetime time horizon. A 6-month cycle length was defined. The analysis was conducted from the NHS and Personal Social Services perspective. Costs were expressed in 2015–16 Great British pounds and effectiveness was expressed in quality-adjusted life-years (QALYs). Costs and QALYs were discounted at an annual rate of 3.5%. 60 The cost-effectiveness analysis results are reported using ICERs. 60 ICERs are calculated as the ratio of the difference in expected costs between two alternative strategies to the difference in expected QALYs. This ratio measures the additional costs that would have to be paid in order to obtain an extra unit of effectiveness (i.e. an extra QALY). A probabilistic sensitivity analysis was conducted, in which 10,000 iterations were run. The stability of results was verified by examining the probabilistic results for a lower number of iterations (e.g. 1000). The probabilistic analysis results are reported using cost-effectiveness acceptability curves (CEACs). 144,145 These curves show the probability that a particular strategy is cost-effective at alternative values of willingness to pay for an extra QALY.
Assessment of uncertainty (sensitivity analysis)
A number of sensitivity analyses were conducted to address the uncertainty in this economic evaluation (one-way, two-way, threshold, scenario and probabilistic sensitivity analyses).
Approximately 90% of EVAR procedures in the UK are conducted in males (see Epidemiology of abdominal aortic aneurysm). Therefore, the base-case analysis was run for a male cohort. Gender-specific data were not available and the only differing data for men and women were general population mortality rates and utility weight. Female utility weight for 74-year-olds is lower (0.75) than that for males (mean 0.77), but mortality data show a longer life expectancy that could result in a longer time for benefits, but also costs. Therefore, a further analysis was conducted using these data, to observe the effect of longer life expectancy in the model results. In addition, one-way sensitivity analyses were conducted on all cost categories (e.g. cost of tests, visits and surgery), test diagnosis sensitivity and specificity, incidence of abnormalities, adherence to surveillance and mortality as a result of an unexpected event (rupture) and emergency intervention. Ranges to run these analyses were informed by the lower- and upper-unit cost quartiles published in NHS Reference Costs 2015 to 2016140 (cost variables), the 95% CI reported by Karthikesalingam et al. 132 (sensitivity and specificity) and, for those variables for which there were no published data available, by expert opinion (e.g. adherence to surveillance).
Given the base-case and sensitivity analyses results, a threshold analysis was conducted for the cost of CEU, which explored the value that would make CEU cost-effective. Two scenario analyses were developed; the first assumes that the information from the CEU test is perfect, that is, sensitivity and specificity are equal to 1, plus no indeterminate or inconclusive results. This scenario corresponds to the notion that CEU could be the present reference standard.
A further scenario analysis was implemented, which assumed a cohort with a higher proportion (50%) of individuals belonging to abnormal Ib group together with a higher overall incidence for any abnormality (up to 10% per 6-month cycle). This scenario explored the effects of monitoring only those individuals at high risk of developing abnormalities.
The base-case and selected sensitivity analyses results are presented in Results. The full sensitivity analysis results are reported in Appendix 15.
Results
In Table 20, the base-case analysis results are reported. Annual follow-up with CDU only is the strategy with the lowest expected cost, followed by CTA only and CEU only. The strategies with higher expected costs are those that use CDU or CEU in conjunction with CTA at the start. In Table 21, the strategy expected costs are disaggregated into three cost categories: costs of surgical procedures, costs of surveillance visits and costs of tests. Consistently throughout the alternative strategies, surgical costs represent a higher proportion of the total costs. For the strategies involving CTA, the costs of the test represent over 30% of the total costs. The costs of visits were considered only in the case of a reassessment, and therefore these represent the lowest proportion in all of the surveillance strategies (i.e. between 6% and 13%).
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.00150 | Dominated |
| CEU | 4709 | 919 | 6.5594 | 0.00622 | 147,626 |
| CDU and CTA, then CDU | 4732 | 22 | 6.5543 | –0.00510 | Dominated |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.00032 | 2,912,177 |
| Strategy | Cost of, £ (%) | Total cost, £ (%) | ||
|---|---|---|---|---|
| Surgery only | Visits only | Test only | ||
| CDU | 2423 (64) | 477 (13) | 890 (23) | 3791 (100) |
| CTA | 2400 (63) | 229 (6) | 1198 (31) | 3828 (100) |
| CEU | 3146 (67) | 585 (12) | 978 (21) | 4709 (100) |
| CDU and CTA, then CDU | 2430 (51) | 441 (9) | 1861 (39) | 4732 (100) |
| CEU and CTA, then CEU | 3148 (56) | 550 (10) | 1945 (34) | 5644 (100) |
The CTA-only strategy produces the lowest number of expected QALYs (see Table 20). This can be explained by the relatively low sensitivity of CTA that was assumed in the model. As such, the CDU-only strategy dominates the CTA-only strategy (i.e. CDU has lower expected costs and a higher number of expected QALYs than CTA only). Moreover, adding CTA to CDU or CEU at the start results in more QALYs than using only one imaging modality. However, either these strategies are dominated (i.e. CDU and CTA, then CDU) or the incremental cost for an additional QALY is well above the often-accepted cost-effectiveness threshold [£30,00060 (i.e. CEU and CTA, then CEU)]. Furthermore, CEU-based strategies result in a higher number of expected QALYs than all of the other strategies, although the ICER to adopt any of the CEU-based strategies is well above the £30,000 threshold. 60
Figure 10 shows the cost-effectiveness plane for the base-case analysis. For ease of interpretation, square data markers were used for CDU-based strategies, triangle data markers were used for CEU-based strategies and dots were used for CTA-only strategies. It can be clearly observed that the CEU-based strategies produce more QALYs, but at higher expected costs, than the CDU-based strategies. Furthermore, it is of note that the CDU plus CTA and then annual CDU strategy is dominated by the CEU-only strategy. However, the ICERs to move to any of these strategies (either CDU and CTA, then CDU or CEU only) from CDU only are well above the usual cost-effectiveness threshold.
FIGURE 10.
Base-case cost-effectiveness results: men.
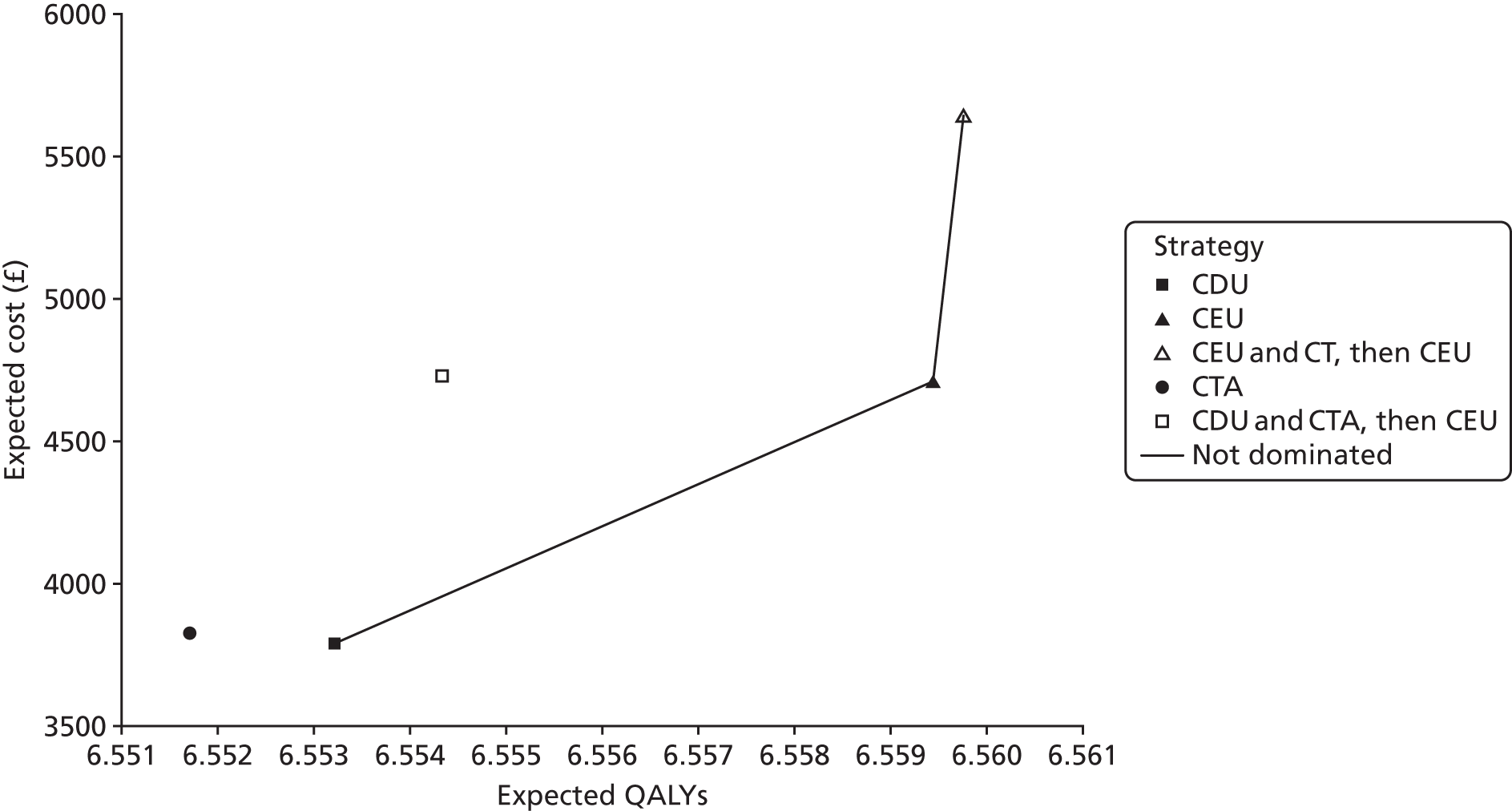
Table 22 shows the probabilistic sensitivity analysis results for the base case. Annual follow-up with CDU only has the highest probability of being cost-effective for any value of willingness to pay for an extra QALY below £50,000 (i.e. a probability of > 58%). Surveillance with CTA only has a probability of between 32% and 42% of being cost-effective at values of willingness to pay for an extra QALY of between £10,000 and £50,000. Adding CTA to CDU or CEU has a zero probability of being cost-effective at any willingness-to-pay values. Finally, as surveillance with CEU only produces more expected QALYs, this strategy has a growing probability of being cost-effective at increasing willingness-to-pay values. However, at £50,000, its chance of being cost-effective is just 4.1%. Figure 11 presents the CEACs. It is worth noting that the probability of CDU being cost-effective stabilises at around 60% for high values of willingness to pay for a QALY. At higher values than those reported in the figure, surveillance with CEU increases its chances of being cost-effective compared with CDU- and CTA-only strategies (i.e. 27% at £100,000 – data not shown). Finally, the ICERs calculated with the probabilistic analysis were lower than the deterministic base-case analysis reported in Table 20 (i.e. ICER for CEU with respect to CDU: £129,700 and for CEU and CTA; then CEU strategy with respect to CEU only: £2,479,000). However, these ICERs are all well above the usual threshold used in the UK (e.g. £30,000).
| Strategy | Proportion cost-effective at alternative willingness-to-pay for a QALY threshold (%) | ||||
|---|---|---|---|---|---|
| £10,000 | £20,000 | £30,000 | £40,000 | £50,000 | |
| CDU | 58.0 | 60.5 | 62.6 | 63.5 | 63.8 |
| CTA | 42.0 | 39.2 | 36.6 | 34.6 | 32.1 |
| CEU | 0.1 | 0.2 | 0.8 | 1.9 | 4.1 |
| CDU and CTA, then CDU | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CEU and CTA, then CEU | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
FIGURE 11.
Cost-effectiveness acceptability curves, base case: men.
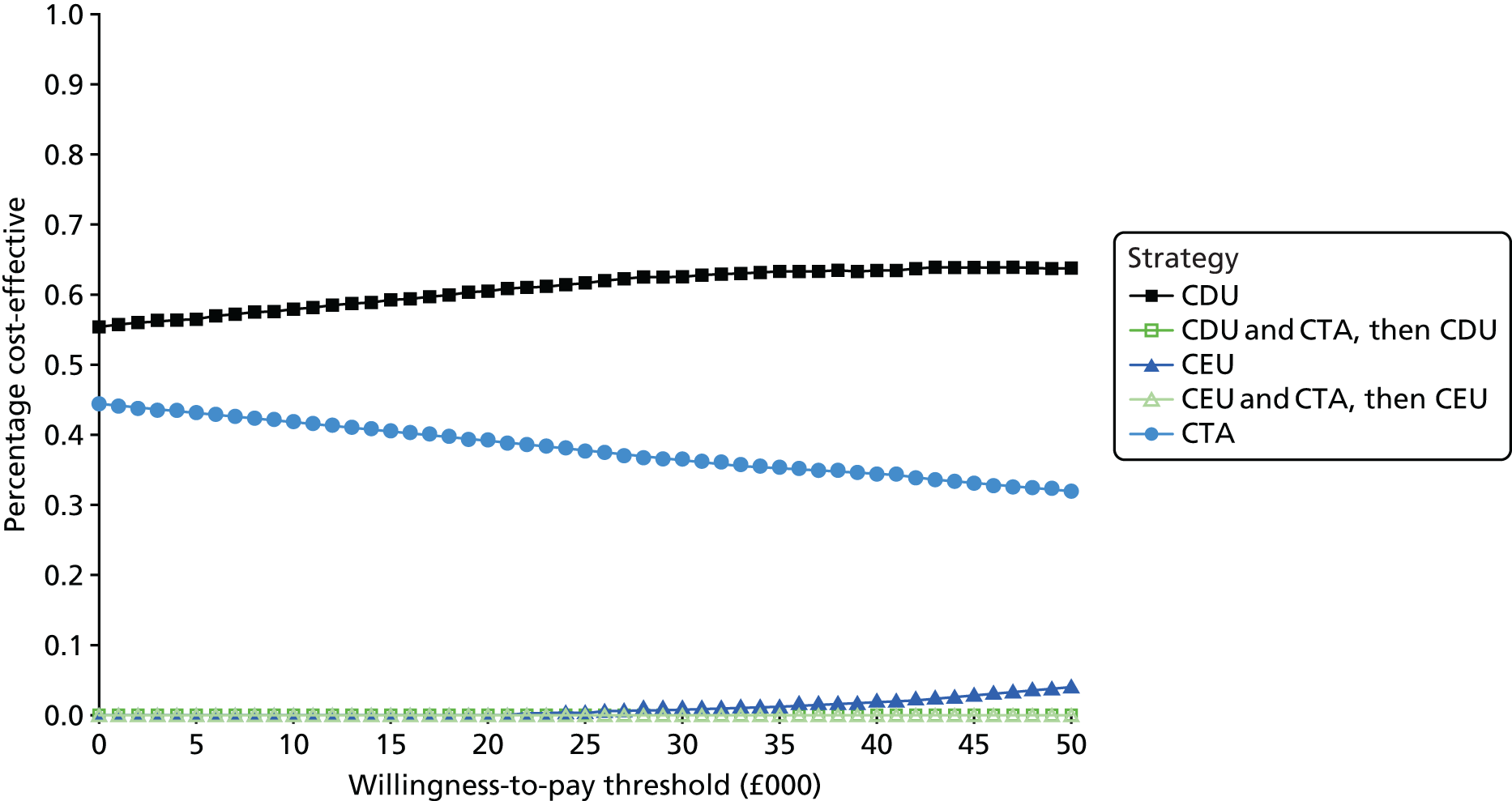
Sensitivity analysis results
Base-case analysis for women
Table 23 shows the results of the base-case analysis for women. General population mortality data146 for women were used for this analysis, as well as the utility weight for women > 74 years of age (i.e. mean 0.75), using the methods provided by Ara and Brazier 2010. 142 Expected costs and QALYs for women are generally higher than for males, reflecting the longer life expectancy of women. Overall, the results are very similar to those of the base-case analysis for men, with CDU having the lowest expected cost, followed by CTA only and surveillance with CTA only being dominated by surveillance with CDU only. CEU-based strategies have ICERs that are well above the often-used willingness to pay for an extra QALY threshold. 60 Owing to the similarity of these results to those for the males model run, all other sensitivity analyses were conducted using data for only males.
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| CDU | 4327 | 7.1460 | – | ||
| CTA | 4367 | 40 | 7.1442 | –0.0018 | Dominated |
| CEU | 5372 | 1046 | 7.1536 | 0.0075 | 138,707 |
| CDU and CTA, then CDU | 5393 | 20 | 7.1473 | –0.0063 | Dominated |
| CEU and CTA, then CEU | 6431 | 1059 | 7.1539 | 0.0004 | 2,926,141 |
One-way sensitivity analysis results for the unit cost of the CDU test are reported in Table 24. The upper quartile for a vascular ultrasound in NHS Reference Costs 2015–16140 is £70. For this reason, a range up to £80 was used in an attempt to include other plausible values. The base-case unit cost for a CDU test was £58; thus, for any values below this, the base-case results hold. At a CDU unit cost of £80, CDU is more costly than CTA, and, therefore, CTA becomes cost-effective. CEU only improves its cost-effectiveness compared with CDU as the unit cost for CDU increases. However, the ICERs for CEU-based strategies are still above the £30,000 threshold, at a unit cost of £80 for a CDU test.
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 55 | CDU | 3769 | 6.5532 | |||
| CTA | 3828 | 58 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 940 | 6.5594 | 0.0062 | 151,067 | |
| CDU and CTA, then CDU | 4710 | 1 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 60 | CDU | 3812 | 6.5532 | |||
| CTA | 3828 | 16 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 898 | 6.5594 | 0.0062 | 144,267 | |
| CDU and CTA, then CDU | 4753 | 43 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 70 | CTA | 3828 | 6.5517 | |||
| CDU | 3896 | 69 | 6.5532 | 0.0015 | 45,611 | |
| CEU | 4709 | 813 | 6.5594 | 0.0062 | 130,667 | |
| CDU and CTA, then CDU | 4837 | 128 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 75 | CTA | 3828 | 6.5517 | |||
| CDU | 3939 | 111 | 6.5532 | 0.0015 | 73,755 | |
| CEU | 4709 | 771 | 6.5594 | 0.0062 | 123,867 | |
| CDU and CTA, then CDU | 4879 | 170 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 80 | CTA | 3828 | 6.5517 | |||
| CDU | 3981 | 153 | 6.5532 | 0.0015 | 101,899 | |
| CEU | 4709 | 728 | 6.5594 | 0.0062 | 117,067 | |
| CDU and CTA, then CDU | 4922 | 212 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 |
Table 25 presents the results for the one-way sensitivity analysis for the unit cost of CEU. The lower quartile reported in NHS Reference Costs 2015 to 2016140 for a vascular ultrasound was £39; thus, a lower value was used in order to consider other lower plausible values. In addition to the £72.10 for the contrast agent and the extra staff involved (who remained unchanged for the present one-way sensitivity analysis), the ultrasound cost for the CEU base-case analysis was £58, so any values above this would result in CEU being less cost-effective. The base-case results are robust to changes in the cost of the CEU test. A threshold analysis was conducted, and, owing to CEU being more sensitive but less specific, CEU needs to be slightly cheaper than CDU in order to become cost-effective.
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 20 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4394 | 603 | 6.5594 | 0.0062 | 96,889 | |
| CDU and CTA, then CDU | 4732 | 338 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5328 | 935 | 6.5598 | 0.0003 | 2,912,607 | |
| 30 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4478 | 687 | 6.5594 | 0.0062 | 110,408 | |
| CDU and CTA, then CDU | 4732 | 254 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5412 | 935 | 6.5598 | 0.0003 | 2,912,493 | |
| 40 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4562 | 771 | 6.5594 | 0.0062 | 123,927 | |
| CDU and CTA, then CDU | 4732 | 170 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5496 | 935 | 6.5598 | 0.0003 | 2,912,378 | |
| 50 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4646 | 855 | 6.5594 | 0.0062 | 137,446 | |
| CDU and CTA, then CDU | 4732 | 86 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5581 | 935 | 6.5598 | 0.0003 | 2,912,264 |
Test performance
Two-way sensitivity analyses were conducted for sensitivity and specificity for each compared test. Figure 12 presentd the results for CDU. The figure shows the strategy with the highest net benefit at £30,000 per QALY according to alternative values of sensitivity and specificity for CDU. The value ranges used were broader than the 95% CIs reported by Karthikesalingam et al. 132 (i.e. sensitivity, 95% CI 0.62 to 0.83; specificity, 95% CI 0.90 to 0.97), in order to explore the effects of alternative plausible figures. At low levels of sensitivity and specificity for CDU, the imaging strategy that has the highest net benefit is CTA. Surveillance with CDU only has the highest net benefit at 93% specificity (or higher), regardless of the CDU sensitivity.
FIGURE 12.
Two-way sensitivity analysis: CDU test sensitivity and specificity (net benefit, willingness-to-pay threshold of 30,000).
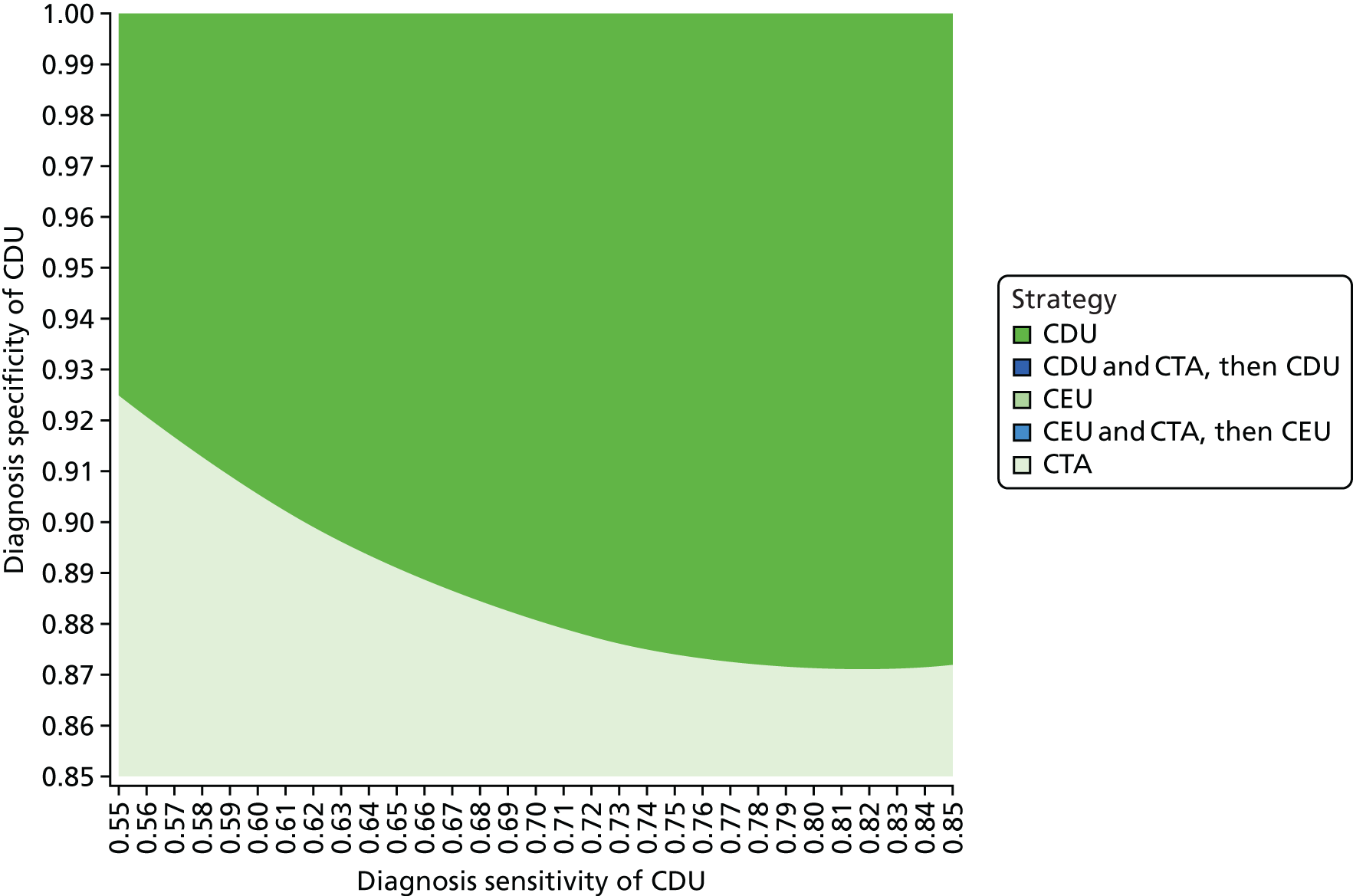
Perfect information from contrast-enhanced ultrasound
There was an indication from the clinical experts that the CEU test might have become the reference standard. A scenario analysis was conducted, assuming perfect information from CEU. That is, it was assumed that sensitivity and specificity were equal to 100% and that no indeterminate or inconclusive results were possible. Moreover, a threshold analysis was conducted to explore the difference in cost between CEU and CDU that would make CEU cost-effective. The results of this analysis are reported in Table 26 and show that, if the test performance from CEU is assumed to be perfect, a cost difference of up to £55 between CEU and CDU could make CEU cost-effective at a threshold value of willingness to pay for a QALY of £30,000. Larger cost differences would shift the ICER above the frequently used cost-effectiveness threshold (i.e. £30,000).
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 40 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 3943 | 152 | 6.5614 | 0.0082 | 18,682 | |
| CDU and CTA, then CDU | 4732 | 788 | 6.5543 | –0.0070 | Dominated | |
| CEU and CTA, then CEU | 4947 | 1004 | 6.5614 | 0.0000 | > 29 million | |
| 50 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4027 | 237 | 6.5614 | 0.0082 | 28,978 | |
| CDU and CTA, then CDU | 4732 | 704 | 6.5543 | –0.0070 | Dominated | |
| CEU and CTA, then CEU | 5032 | 1004 | 6.5614 | 0.0000 | > 29 million | |
| 55 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4069 | 279 | 6.5614 | 0.0082 | 34,126 | |
| CDU and CTA, then CDU | 4732 | 662 | 6.5543 | –0.0070 | Dominated | |
| CEU and CTA, then CEU | 5074 | 1004 | 6.5614 | 0.0000 | > 29 million | |
| 60 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4111 | 321 | 6.5614 | 0.0082 | 39,274 | |
| CDU and CTA, then CDU | 4732 | 620 | 6.5543 | –0.0070 | Dominated | |
| CEU and CTA, then CEU | 5116 | 1004 | 6.5614 | 0.0000 | > 29 million | |
| 70 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4195 | 405 | 6.5614 | 0.0082 | 49,569 | |
| CDU and CTA, then CDU | 4732 | 536 | 6.5543 | –0.0070 | Dominated | |
| CEU and CTA, then CEU | 5200 | 1004 | 6.5614 | 0.0000 | > 29 million |
High-risk patient group
A relatively more sensitive test can be beneficial when a higher proportion of individuals have the condition under study. A scenario analysis was considered in which half of the abnormal individuals belonged to the abnormal Ib group (e.g. types I and III endoleaks, plus other conditions necessitating elective intervention). Table 27 presents the results from a one-way sensitivity analysis for the incidence of any abnormality. The base-case analysis assumed circa 4% incidence per 6-month cycle. In the present analysis, this incidence of abnormalities implies that 2% of abnormalities are type Ib. Alternatively, the percentages in Table 27 could be broadly interpreted as the annual incidence of Ib abnormalities. To facilitate the comparison between CDU- and CEU-only strategies, in Table 27 the ICER for the CEU-only strategy has been calculated with respect to the CDU-only strategy and not the strategy with an immediately lower cost (i.e. CDU and CTA, then CDU). The results show that CEU is more cost-effective than CDU when the incidence of group Ib abnormalites is > 6% per year (3% incidence per 6-month cycle, which corresponds to 6% in Table 27).
| Value | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 4% | CDU | 5966 | 6.5018 | |||
| CTA | 5991 | 25 | 6.4961 | –0.0057 | Dominated | |
| CDU and CTA, then CDU | 6918 | 952 | 6.5057 | 0.0038 | 248,084 | |
| CEU | 6929 | 963 | 6.5258 | 0.0240 | 40,126 | |
| CEU and CTA, then CEU | 7875 | 946 | 6.5270 | 0.0011 | 849,127 | |
| 5% | CDU | 6902 | 6.4818 | |||
| CTA | 6919 | 17 | 6.4751 | –0.0068 | Dominated | |
| CDU and CTA, then CDU | 7854 | 952 | 6.4866 | 0.0047 | 200,797 | |
| CEU | 7910 | 1008 | 6.5105 | 0.0287 | 35,167 | |
| CEU and CTA, then CEU | 8852 | 942 | 6.5119 | 0.0014 | 683,732 | |
| 6% | CDU | 7748 | 6.4635 | |||
| CTA | 7757 | 9 | 6.4557 | –0.0078 | Dominated | |
| CDU and CTA, then CDU | 8701 | 953 | 6.4691 | 0.0056 | 169,359 | |
| CEU | 8801 | 1053 | 6.4964 | 0.0329 | 31,965 | |
| CEU and CTA, then CEU | 9740 | 939 | 6.4980 | 0.0016 | 573,576 | |
| 7% | CDU | 8516 | 6.4465 | |||
| CTA | 8517 | 2 | 6.4378 | –0.0087 | Dominated | |
| CDU and CTA, then CDU | 9470 | 954 | 6.4530 | 0.0065 | 146,967 | |
| CEU | 9612 | 1096 | 6.4833 | 0.0368 | 29,756 | |
| CEU and CTA, then CEU | 10,548 | 936 | 6.4852 | 0.0019 | 494,974 | |
| 8% | CTA | 9209 | 6.4212 | |||
| CDU | 9215 | 6 | 6.4308 | 0.0095 | 642 | |
| CDU and CTA, then CDU | 10,172 | 957 | 6.4381 | 0.0073 | 130,221 | |
| CEU | 10,353 | 1138 | 6.4712 | 0.0404 | 28,159 | |
| CEU and CTA, then CEU | 11,287 | 934 | 6.4733 | 0.0021 | 436,083 | |
| 9% | CTA | 9840 | 6.4059 | |||
| CDU | 9853 | 14 | 6.4162 | 0.0103 | 1316 | |
| CDU and CTA, then CDU | 10,813 | 959 | 6.4244 | 0.0082 | 117,233 | |
| CEU | 11,031 | 1178 | 6.4599 | 0.0437 | 26,964 | |
| CEU and CTA, then CEU | 11,964 | 932 | 6.4623 | 0.0024 | 390,326 | |
| 10% | CTA | 10,417 | 6.3916 | |||
| CDU | 10,437 | 21 | 6.4026 | 0.0110 | 1884 | |
| CDU and CTA, then CDU | 11,400 | 963 | 6.4116 | 0.0090 | 106,873 | |
| CEU | 11,655 | 1217 | 6.4494 | 0.0467 | 26,045 | |
| CEU and CTA, then CEU | 12,586 | 931 | 6.4520 | 0.0026 | 353,758 |
Summary of cost-effectiveness
This chapter reported on a systematic review of economic evaluations and a model-based economic evaluation of alternative strategies to monitor individuals after an EVAR intervention. Similarly to the systematic review of clinical effectiveness, only cohort studies were identified in the systematic review of economic evaluations. Five studies met the inclusion criteria. Although two studies45,66 compared outcomes before and after a surveillance protocol took place, the three remaining studies hypothesised the resource use implications (i.e. the number of CTA scans performed) of moving to surveillance strategies, using ultrasound as first-line test. Only one study gave full details of the cost calculations. Moreover, none of the studies assessed the relative efficiency of CDU and CEU, which is addressed by the current assessment. As such, the studies identified were unsuitable to fully inform the study question posed and therefore unlikely to help decision-making in the UK. Thus, a new model was developed following UK methodological guidelines. 60
The developed model included five strategies. Three of these were CTA, CDU or CEU used on an annual basis, and the two other strategies considered CTA in addition to CDU or CEU for the first surveillance visit, with CTA scans being conducted afterwards only if further investigations were needed. Plain radiography was included in all of the strategies as part of the surveillance assessment.
The model base-case results show that, once the primary EVAR surgical complications have been discarded, surveillance with CDU as a first-line test becomes the less expensive option. This strategy is less expensive and produces more expected QALYs than a strategy that uses CTA only. Adding CTA to CDU in the first surveillance visits is not worthwhile. Moreover, surveillance strategies based on CEU result in more expected QALYs, but are also more expensive, and the ICERs are well above the usual threshold used in the UK (i.e. £30,000). The our base-case probabilistic analyses show that the CDU-only strategy has a probability of being cost-effective of between 57% and 64%, depending on the cost-effectiveness threshold (e.g. 62% at £30,000).
Extensive sensitivity analyses were conducted (see Appendix 15), with the base-case results being robust for the great majority of these. The sensitivity analysis showed that a CEU-only strategy could become cost-effective at very high rates of test sensitivity and specificity (e.g. when it was assumed to produce perfect information – sensitivity and specificity of 100% and no indeterminate results). Even in this case, the cost difference between CDU and CEU should not be above £55 for CEU to be cost-effective at a £30,000 threshold of willingness to pay for an additional QALY.
As risk stratification of patients might become a feasible option, a further sensitivity analysis was conducted to explore the effect of using these surveillance strategies in a very high-risk group only. Incidence rates of > 2% per 6-month cycle were considered for the abnormal Ib group (i.e. types I and III endoleaks, together with type II endoleaks with sac expansion > 5 mm and other conditions commonly detected by non-radiography imaging modalities). At an annual incidence rate of 7% for this group, CEU-only surveillance becomes cost-effective with an ICER of £29,756 with respect to CDU-only surveillance. It is worth noting that, although an incidence of 7% for type I or type III endoleaks is unlikely to be observed in clinical practice, an incidence of 6% for type II endoleaks with sac expansion is perhaps possible.
We can conclude that the use of CDU as a first-line test for the surveillance of individuals after EVAR is cost-effective, with a probability of > 58% at the usual cost-effectiveness threshold used in the UK. 60 The analysis results are driven by the interplay of the test performance data (i.e. sensitivity and specificity) and the cost of the test. Lower unit costs together with higher specificity are needed for CEU to become cost-effective.
Chapter 4 Discussion and conclusions
Statement of principal findings
Clinical effectiveness
To our knowledge, this is the first assessment that considers the effectiveness and cost-effectiveness of CTA, CDU and CEU for surveillance after EVAR. The clinical evidence base for this assessment consists of two non-randomised comparative studies (with a total of 750 participants) and 25 observational cohort studies (with a total of 7196 participants), assessing various surveillance protocols after EVAR. The surveillance protocols were based on the use of either CDU and/or CEU in combination with CTA.
The majority of the included studies assessed EVAR surveillance protocols based on a combination of CDU and CTA. Three studies used CDU as the main imaging modality for surveillance after EVAR, two studies used CEU as the main imaging modality and one study used CEU in selected cases only. There were no studies that compared CDU surveillance with CEU surveillance.
The risk of bias was rated as being high or moderate for the majority of the included studies, with only three cohort studies rated as being at a low risk of bias according to the prespecified criteria for the risk-of-bias assessment (ReBIP checklist). 76,84,87
There was considerable heterogeneity among the included studies in terms of the surveillance protocols (imaging modality, frequency of imaging, duration of follow-up, reported outcomes, definition of clinical outcomes – for example, the definition of decreased aneurysm size, the axis of the diameter measured and the time points at which outcomes were assessed). Owing to the observed clinical heterogeneity, it was deemed to be inappropriate to perform a statistical synthesis of the reported outcomes.
We conducted a narrative synthesis of the main clinical findings and grouped studies according to their similarities in terms of modality and frequency of imaging. A combination of CTA and CDU was the most commonly implemented surveillance strategy. Studies that used a combination of CTA and CDU for surveillance after EVAR were published between 2001 and 2010. The second most common surveillance strategy involved CTA and/or CDU for early and mid-term assessments and CDU for long-term surveillance after EVAR. Studies assessing this type of strategy were published more recently, between 2009 and 2016.
This may indicate a growing trend towards a CDU-based surveillance. It is worth noting that one study that followed up 494 patients who underwent EVAR using CTA and CDU for early and mid-term imaging assessments and CDU for long-term surveillance reported the highest mortality and reintervention rates. 90 However, any comparisons between cohort studies are tentative, owing to the observed clinical heterogeneity. In this particular case, it is difficult to ascertain whether or not the reported high mortality and reintervention rates were observed because of the length of the follow-up period (12 years), the characteristics of the patient population or the imaging modalities used for surveillance.
Three of the included cohort studies were conducted in the UK. 41,78,82 Evidence from these studies was considered to be of moderate methodological quality, as the studies did not satisfy all of the criteria of the ReBIP checklist. One of these studies used CDU exclusively for surveillance after EVAR,78 whereas the other two studies used a strategy based on a combination of CDU and CTA. 41,82 In particular, Harrison et al. ,41 who followed up a total of 194 patients using a combination of CDU and CTA for early surveillance and CDU for long-term surveillance after EVAR, reported a mortality rate of 13% at 12 months. In contrast, a non-UK-based study that assessed 494 EVAR patients using a similar surveillance strategy reported 19.7% mortality during a median follow-up of 68 months. 90 In general, the proportion of patients requiring reintervention in the study by Harrison et al. 41 was similar to that reported by other non-UK-based studies that used a combination of CTA and CDU for early surveillance and CDU for long-term surveillance. The study by Karthikesalingam et al. 78 used CDU at 1.5, 3, 5, 9, 12 and 18 months and annually thereafter to assess the role of peak systolic velocity provided by CDU for the prediction of limb complications in a cohort of 478 EVAR patients. The authors found that serial increases in the peak systolic velocity recorded during CDU surveillance were associated with an increased risk of stent–graft limb complications. 78
The proportion of patients with type I endoleaks identified by a surveillance strategy based on early and mid-term CTA and/or CDU and long-term CDU was comparable to that identified by a surveillance strategy based on a combination of CTA and CDU throughout the follow-up period (range 0–7.9% vs. 0.8–8.3%). No type III endoleaks were reported in the eight cohort studies that used early and mid-term CTA and/or CDU and long-term CDU for the surveillance after EVAR. It is worth noting that all but one study76 were rated as being at a high or moderate risk of bias. The study by Freyrie et al. ,76 which was the only study that was rated as being at a low risk of bias in this surveillance category, reported two cases (1.1%) of type I endoleak, 23 cases (13%) of type II endoleak and no cases of type III or IV endoleak during a mean follow-up of 33 months.
Detection of limb occlusion was lower among cohort studies that used CDU for long-term surveillance after EVAR (range 0–1.1%) than cohort studies that used either CTA for long-term surveillance (range 3.1–3.7%) or a combination of CTA and CDU throughout surveillance (range 5.3–7.2%). This is, however, only an observation and not a causal association.
The study by Chaer et al. 40 evaluated the safety of long-term CDU for surveillance after EVAR. One hundred and eighty-four patients with shrinking or stable aneurysms who received CTA at 1 and 12 months after EVAR were followed up annually with CDU for up to 4 years. Freedom from endoleaks was 96% and freedom from secondary interventions was 95% at 4 years. The authors concluded that CDU-based surveillance after EVAR is safe in patients with stable aneurysms.
Similarly, the comparative study by Chisci et al. ,66 which compared CDU and CTA 1 month after EVAR and every 6 months afterwards (protocol I, 367 patients) with CDU and CTA 1 month after EVAR and CDU and radiography every 6 months afterwards (protocol II, 341 participants), reported no significant differences between the two surveillance strategies during the course of the study (3-year follow-up) in terms of reinterventions, clinical complications and mortality. The authors concluded that the current post-EVAR surveillance protocol could be simplified by adopting CDU as the main follow-up imaging modality and restricting the use of CTA to selective cases, when adverse events are suspected.
Cost-effectiveness
This assessment is the first model-based economic evaluation to consider the role of CDU, CEU and CTA for post-EVAR surveillance. Only the study by Bendick et al. 137 provided a head-to-head comparison of the three imaging modalities as first-line surveillance using data from a cohort of 40 individuals and considered both costs and clinical outcomes. All of the other economic evaluation studies identified in the systematic review compared the use of CTA as first-line monitoring with CDU only, with CTA being used after CDU for selected cases only, to provide further diagnostic information. Moreover, all of the studies assessed the reduction in costs as a result of the fewer number of CTA scans conducted in the ultrasound-based protocols. The model considered a broader measure of effectiveness based on a preference-based measure of utility in accordance with the UK economic evaluation methodological guidelines,60 as well as a lifetime time horizon with all relevant consequences from the NHS perspective. None of the retrieved studies attempted an incremental analysis of the costs and clinical outcomes. For this reason, any comparison between the study’s results and those of the earlier economic evaluations should be conducted with caution.
The model results show that a surveillance strategy based on CDU as the imaging modality of choice becomes the strategy with the lowest expected costs, in addition to producing more QALYs than a strategy based exclusively on CTA. By comparison, although a surveillance strategy based exclusively on CEU would generate more QALYs, it would be more expensive, and the ICER would be well above the usual threshold used in the UK (i.e. £30,000). In addition, the base-case probabilistic analysis shows that a CDU-only strategy would have a probability of being cost-effective of between 57% and 64%, depending on the cost-effectiveness threshold (e.g. 62% at £30,000). Adding CTA to CDU or CEU in the first annual surveillance visit is not worthwhile, as it generates more QALYs but at a very high cost per QALY.
The base-case results, which show that CDU is the least expensive option, are in general agreement with those of previous economic evaluations that reported savings because fewer CTA tests were conducted in ultrasound-based protocols. 41,45,66,137,138 However, although Bendick et al. 137 reported savings for a 3-year cost comparison between CEU- and CTA-based protocols, the model results indicate a higher expected cost for a CEU-only strategy than for a CTA-only strategy. This can be explained by the relatively lower specificity assumed for CEU in the economic model, which generates a higher proportion of false-positive results. These false-positive results will trigger further testing for a period of up to 2 years.
Extensive sensitivity analyses were conducted (see Appendix 15), with the base-case results being robust for the great majority of these. The sensitivity analysis showed that a CEU-only strategy could become cost-effective at very high rates of test sensitivity and specificity (e.g. when it was assumed to produce perfect information – sensitivity and specificity of 100% and no indeterminate results) and with a difference in the cost of CEU and CDU of < £55.
A further sensitivity analysis considered higher incidence rates for the abnormal Ib group (e.g. types I and III endoleaks and other abnormalities commonly detected by non-radiography imaging modalities). Annual incidence rates of 4% and above were used in this analysis. Compared with CDU-only surveillance, CEU-only surveillance becomes cost-effective, with an ICER of £29,756, when the annual incidence rate for this group is 7%. Although in clinical practice it is unlikely that an incidence rate of 7% for type I or type III endoleaks would be observed, an incidence rate of 6% for type II endoleaks with sac expansion is perhaps possible.
The interplay of sensitivity, specificity and unit costs of the test drives the results in the study’s model. For instance, a lower unit cost for CEU helps to make the CEU-based strategies relatively more cost-effective; however, cost on its own will not make a CEU-only strategy a cost-effective option. A higher specificity is also necessary in order to reduce the expected cost of the strategy as a result of the follow-up of individuals without an abnormality. In addition, a higher sensitivity triggers further interventions (e.g. secondary interventions for the abnormal Ia and Ib groups and further monitoring for the abnormal II group). As such, although these interventions might result in higher expected QALYs, they also add to the expected costs, with an uncertain final effect on cost-effectiveness.
The majority of patients in the cohort considered in the model will have no further need for subsequent interventions. Furthermore, in the base-case analysis, 70% of patients with an abnormality correspond to the abnormal II group. Because a large proportion of patients are elderly with multiple comorbidities, there are instances when a secondary intervention, which is considered to be technically indicated based on surveillance imaging, may not be carried out because the risk associated with the intervention is considered prohibitively high. Furthermore, in the cost–utility analysis, there is no benefit attributed to the reassurance that the abnormality is minor or from any information provided by the test. From the point of view of the economic model, following up individuals for whom no further interventions are possible just adds to the expected cost of the strategy, with no effects on QALYs. Future research should explore more broadly the effects of the information generated by the surveillance strategies and incorporate this within the economic analysis.
Uncertainties from the assessment
Clinical effectiveness
The clinical evidence identified for this assessment demonstrates that surveillance practice after EVAR is currently heterogeneous and the most effective method for surveillance has yet to be established.
Since the advent of EVAR, CTA has been the main imaging modality for long-term surveillance. A survey of current clinical practice after EVAR published by Uthoff et al. 147 in 2012, which involved 674 respondents from 52 countries worldwide, found that CTA was the imaging modality used most often for standard surveillance. A CTA scan at 1 year was scheduled by 64.5% of the respondents.
The use of CTA presents important drawbacks, including exposure to ionising radiation, which may result in an increased risk of cancer. 30 Moreover, the intravenous iodinated contrast medium used in CTA may damage renal function over time and increase the risk of contrast nephropathy. A study by Mitchell et al. ,148 published in 2010, found that the incidence of contrast-induced nephropathy was 11% among a cohort of 633 patients who received contrast-enhanced CT in the emergency department. Six patients with contrast-induced nephropathy developed severe renal failure and four (0.6%) died. 148 In most EVAR patients, these risks could be eliminated or reduced by modifying the surveillance protocol and limiting the number of CTA scans. 11,26,35,149
As the main purpose of surveillance is to identify complications and direct treatments, most surveillance protocols involve CTA scans at 1, 6 and 12 months and annually thereafter; however, some investigators have challenged the utility of the 6-month CTA in patients with a normal 1-month CTA. 46 The authors of the 5-year US Zenith multicentre trial have proposed a reduced surveillance protocol, with no 6-month CTA, for patients without early endoleaks. 36 According to the European Society for Vascular Surgery 2010 guideline,31 CTA and radiography should be used to categorise patients with and without endoleaks. Patients without an endoleak should be followed up with CTA at 12 months and with CDU and plain radiography thereafter, whereas those with a type II endoleak should receive CTA at 6 and 12 months and annual CTA and plain radiography thereafter. 31 Similarly, the Society for Vascular Surgery practice guidelines recommend CTA at 1 and 12 months during the first year after EVAR. CTA at 6 months is added to the surveillance schedule only if the 1-month CTA identifies an endoleak or other abnormalities of concern. 19 In the survey of current clinical practice after EVAR published by Uthoff et al. 147 in 2012, 48.6% of the 674 respondents agreed that, in the absence of an endoleak or AAA sac enlargement after initial CTA, no further CTA follow-up at 6 months is required.
Although the current trend is to reduce the frequency of CTA for early surveillance after EVAR or replace it with other imaging modalities, there is limited information on the optimal duration of long-term surveillance and if annual surveillance should continue indefinitely. A systematic review published by Nordon et al. 26 in 2010, assessed the rates of secondary interventions in 32 studies (17,987 EVAR patients) and reported the evidence in favour of a modified EVAR practice. The authors have observed a mean time to secondary intervention of approximately 1–1.5 years and have suggested that patients who have completed 3 years of surveillance without detection of endoleaks or sac enlargement can be discharged from standard surveillance. 26
Some investigators and clinical guidelines now recommend annual post-EVAR surveillance with CDU if the first annual CTA does not demonstrate an endoleak or residual sac enlargement. 12,19,73,150 Compared with CTA, CDU is less invasive, less expensive, easily available and less risky as it does not require the use of a contrast agent and does not expose the patient to repeated radiation. A number of studies and systematic reviews have confirmed the role of CDU in the evaluation of endoleaks. 42,62,73,78,129,151–154 CDU can be regarded as a feasible and safer alternative to CTA, especially in patients with a stable aneurysm. Indeed, the number of centres using CDU seems to be increasing. In the survey by Uthoff et al. 147 published in 2012, the use of CDU during surveillance was reported by 36.3% of centres. High-volume, experienced centres were more likely to opt for CDU surveillance after 1 year than less experienced centres with fewer cases. Moreover, centres with a lot of EVAR experience were more likely to favour ultrasound for the follow-up of type II endoleaks. 147 Similarly, a UK telephone survey administered to 41 centres with 10 years’ experience in EVAR showed that 14 out of 41 (34.1%) centres used CDU as the primary surveillance modality. 155
In general, the evidence identified for this assessment showed no significant differences in terms of reinterventions and clinical complications between strategies based on the use of CDU for long-term surveillance after EVAR and those based on the use of CTA or CTA and CDU; however, the identified studies were clinically heterogeneous and any attempt to compare surveillance strategies should be considered to be tentative.
Current evidence on the use of CEU is limited, and CEU technology has evolved considerably over the past decade. A number of studies in the literature have reported a high accuracy of CEU in comparison with single and biphasic CTA. 57,156,157 A systematic review published in 2015,130 which assessed the accuracy of CEU versus CTA for the detection of endoleaks during post-EVAR surveillance, concluded that, compared with CTA, CEU that utilises second-generation contrast agents is a highly sensitive modality for the detection of endoleaks and especially for the detection of type II endoleaks. Similarly, a study of 539 patients published by Millen et al. 43 in 2013, suggests that CEU may be useful for the resolution of clinical uncertainties that arise from conventional imaging modalities, especially in the classification of endoleaks.
There is growing evidence that the majority of reinterventions post EVAR are triggered by symptoms and are independent of standard surveillance. 39 Among the cohort studies included in this assessment, the proportion of patients requiring reintervention during surveillance ranged from 1.1% during a mean follow-up of 24 months40 to 23.8% in a cohort of high-risk patients who presented with hostile neck anatomy after a mean follow-up of 32 months,85 indicating that the risk of reintervention was not homogeneous and was related to patients’ characteristics. Karthikesalingam et al. ,39 who followed a cohort of 553 patients for a median follow-up period of 31 months (range 1–97 months) and assessed the extent to which surveillance after EVAR triggers reinterventions, found that 5.1% of asymptomatic patients underwent reintervention prompted exclusively by surveillance imaging, whereas 8.3% of patients presented symptomatically. Black et al. 37 assessed the number of secondary interventions after EVAR among 417 patients and reported that reinterventions were performed in 31 (7.4%), of whom only six (1.4% (6/417) had abnormalities that were detected by standard surveillance. Similarly, Dias et al. 38 found that the majority of follow-up CTA scans post EVAR did not lead to reintervention, and only 9.3% of asymptomatic patients (26/279) underwent a secondary procedure based on imaging findings detected by routine surveillance. The systematic review by Nordon et al. ,26 which assessed secondary interventions after EVAR from 32 papers and included a total of 17,987 cases, reported that surveillance practice alone initiated a secondary intervention in only 1.4–9% of cases.
It is possible that current surveillance practice is poorly targeted and that most patients do not benefit from an unstratified surveillance programme that does not take into account the individual risk of developing complications. 11 There is a growing interest in risk-stratified surveillance, whereby the frequency of imaging is directed by the preoperative risk of complications. Risk factors for early and late complications post EVAR have been documented. 158–160 Such an approach would allow more intense surveillance regimes to be targeted to those patients with greater risk, with less frequent surveillance in patients at low risk (Alan Karthikesalingham, St. George’s Vascular Institute, St. George’s University of London, 2016, personal communication).
Schanzer et al. 161 assessed a large population of US Medicare beneficiaries (19,962 patients) who underwent EVAR between 2001 and 2008 and found that 50% of patients were lost to annual imaging follow-up by 5 years post EVAR. For the subset of patients with 8 years of follow-up, substantive declines in imaging follow-up continued, with only 37% undergoing an imaging study between year 6 and year 8. 161 In the UK, Karthikesalingam and Holt,162 as part of the Multicentre Post-EVAR Surveillance Evaluation Study (EVAR-SCREEN), assessed 1539 patients who underwent EVAR in 10 EVAR-SCREEN collaborator centres. Five years after EVAR, 39.7% of patients remained compliant with the surveillance programme, whereas 21.4% were deliberately removed from surveillance. The authors reported that, compared with 131 compliant patients, non-compliant patients were more likely to undergo reintervention (5-year freedom from reintervention was 76.6% vs. 62.7% in compliant and non-compliant patients, respectively), but demonstrated similar all-cause mortality rates (5-year survival rate of 65.6% vs. 54.7% in compliant and non-compliant patients, respectively). 162 These findings suggest that patients who undergo EVAR should receive appropriate information and counselling about the lifelong risk of complications and the need for annual imaging surveillance. UK centres that have adopted a comprehensive informative approach towards EVAR patients have reported satisfactory compliance rates (Professor Srinivasa Rao Vallabhaneni, 2016, personal communication). These findings also highlight that the current challenge facing EVAR surveillance is the frequency/timing of imaging, which currently is not targeted to patients’ risk of developing complications. It is possible that current imaging surveillance is performed too frequently for low-risk patients and not frequently enough for high-risk patients.
Patient perspectives of endovascular abdominal aortic aneurysm repair surveillance
We invited two lay patient representatives to join the advisory group for this assessment. We sought their opinion on receiving surveillance following EVAR to better understand the patient experience of undergoing surveillance. Both representatives were men who had received EVAR between 6 and 3 years prior to joining the advisory group and had received 6-monthly CTA or CDU surveillance. Both men indicated that they had no preference for the type of imaging modality they received; however, they felt that continuity of the professional conducting and interpreting the results of the imaging procedure was important, to give them reassurance that they were receiving adequate monitoring and that their test results were being correctly interpreted. Both men stated that they would welcome more information about the reasons underlying the frequency of their surveillance schedule, with one man stating that he would be willing to undergo more frequent assessments, either for the added reassurance given by a normal surveillance imaging result or for the reassurance that any abnormality would be detected (and treated) early. It is, however, possible that other patients would feel more anxious by the prospect of more intense surveillance regimens. These concerns highlight the potential need to ensure that patients have a greater understanding of the purpose of their surveillance programme after EVAR, as well as of the required frequency of imaging. Travel to surveillance appointments was mentioned as a possible constraint, as the men lived between 8 and 16 miles from their nearest surveillance centre. Both men felt that it would be impossible to attend surveillance appointments solely by public transport and, therefore, relied upon travel by car for all or part of the distance. Practical issues, like travel limitations (especially for elderly people), could explain poor compliance with EVAR surveillance in some cases.
Cost-effectiveness
With regard to the reported economic model and cost-effectiveness analyses, there are a number of limitations that are worth mentioning. Both the identification and the selection of the input data were challenging. In order to identify the relative effect of different surveillance strategies, it was necessary to model a baseline situation (e.g. the cost and consequences of a situation without surveillance). Unfortunately, data to model the natural history of the disease were scarce. For this reason, the attention was turned to studies that analysed registry data. When particular input data were available from more than one source, the newest study was selected in an attempt to capture the technical developments of the modalities under consideration. Moreover, in the economic model, it was assumed that the imaging modalities differentiate the same conditions, that is, the tests identify a proportion of ‘abnormalities’ according to test sensitivity and specificity data. Once the overall abnormality proportion was defined, this proportion was divided among the abnormal Ia, Ib and II groups in the same proportions, regardless of the original test performed. In addition, the performance data used (i.e. sensitivity and specificity) were based on the detection of endoleaks (all types) that was reported by Karthikesalingam et al. 132 Unfortunately, there were no sensitivity and specificity data available by type of abnormality and test, and therefore this is a limitation of the analysis.
A further assumption in the model is the perfect identification of certain abnormalities by plain radiography. In effect, all individuals developing a type Ia abnormality (e.g. non-endoleak needing elective intervention) are assumed to be correctly identified in the next surveillance visits through a plain radiography. Unfortunately, there were no data to inform the test performance for plain radiography in this context. Moreover, although plain radiography shows graft migration and kinking, the abnormal Ia group includes graft infection and limb thrombosis, which will not be seen on plain radiography. In fact, plain radiography in the model is always conducted alongside another imaging test. Therefore, the underlying assumption is that the conditions in the abnormal Ia group are perfectly identified by either plain radiography or the imaging test. A corollary of this is that the differences between the surveillance strategies are a result of the test performances for the abnormal Ib and II subgroups. This is in line with the project brief, as its main interest was related to the detection and management of endoleaks.
There are a number of structural assumptions in the study‘s model. First, no patients present with symptoms between surveillance visits. Individuals presenting with symptoms make surveillance less worthwhile. Hence, the assumption in the study’s model works in favour of surveillance. However, up to 8% of individuals could present with symptoms in a non-emergency situation (Professor Srinivasa Rao Vallabhaneni, 2016, personal communication), and the magnitude of the effect of this assumption on the model results is limited. Second, the model did not include a ‘do nothing’ alternative. Although this is recommended by a number of economic evaluation methodological guidelines,60 a no-surveillance strategy was regarded as unacceptable and unrealistic for the UK context. Third, none of the strategies considered a partial use of CEU in a search for further information if the results from the CDU test were inconclusive. This is a limitation of the present analysis and material for further research. Fifth, all strategies considered surveillance on an annual basis. This was agreed with the clinical advisors, as annual surveillance frequency was deemed to be the only acceptable option. Finally, there was no risk stratification of the cohort. An alternative model could consider different surveillance strategies with differing visit frequencies, as well as alternative test arrangements, depending on the patient’s risk of developing complications.
The model might overestimate overall survival for this patient group compared with the results of the EVAR 1 trial (EVAR trial arm). 48 A higher overall survival rate would make any surveillance programme relatively more attractive, as people would enjoy the benefits for a longer period of time. The EVAR 1 trial includes individuals who are under surveillance. As a result, the lower mortality rate in the study’s model might correspond to events and conditions that cannot be avoided through a surveillance programme. Therefore, our model might overestimate the overall expected costs and QALYs because of a higher overall survival rate; the relative effect of this issue on the modelled strategies is ultimately unclear, although it is believed to be small in magnitude.
Conclusions
The current evidence assessing the effect of surveillance after EVAR is very heterogeneous, with surveillance protocols based on different imaging modalities, frequency of imaging and length of follow-up. Consequently, no firm conclusion can be drawn with regard to the optimal surveillance strategy after EVAR. There is a need to improve current surveillance protocols to reduce radiation exposure, risk of contrast nephropathy and costs, while ensuring that the patients are adequately followed up to minimise their risk of secondary complications, especially aneurysm rupture. CDU may be a safe alternative to CTA, with CTA reserved for abnormal or inconclusive CDU cases that require further investigation. Further research is required, however, to validate the safety of modified surveillance protocols after EVAR based on the use of CDU and/or CEU. Access to modern equipment and highly experienced operators remains a crucial requirement for the adoption of CDU surveillance. The study’s economic evaluation shows that CDU is the most cost-effective option for post-EVAR surveillance, with a 63% probability of being cost-effective at a threshold of willingness to pay per QALY of £30,000. Surveillance strategies based on CEU produce more QALYs, but are also more expensive, and might be cost-effective only for higher-risk patient groups.
Suggested research priorities
-
Further research is needed to assess the value of targeted surveillance (i.e. patients with a greater risk of complications may receive more frequent surveillance, whereas those with uncomplicated EVAR may undergo less frequent assessments or be discharged from surveillance). A large, multicentre trial with an extended follow-up period (over many years) would be required to answer the question of the optimal surveillance strategy after EVAR; therefore, the identification of high-risk EVAR patients mandating close follow-up may be a more realistic recommendation.
-
If surveillance is to be targeted, is ultrasound-based surveillance (CDU and/or CEU) satisfactory for all patient groups, or are there groups for which CTA is required to avoid excessive risk?
-
The criteria for identifying patients who are at a high risk of complications (e.g. use of validated score systems, risk prediction models) require further investigation.
-
There is a need to clarify the role of plain radiography as part of EVAR surveillance. If CTA is to be performed less frequently or avoided, should plain radiography be mandatory or reserved for patients with abnormalities on ultrasound imaging?
-
Future research should explore more broadly the effects of the information generated by the imaging modalities used for surveillance and incorporate this within the economic analyses.
Various aspects of EVAR surveillance may also warrant further consideration, for example it would be useful if:
-
some indication of patients’ compliance with surveillance could be documented across centres in order to identify best surveillance practice and ensure that surveillance protocols are engaging with patients
-
national clinical registries and databases could consider recording data on complications and mortality after EVAR according to the imaging modalities used for surveillance.
Acknowledgements
The authors are grateful to Marion Campbell (Dean of Research and Professor of Health Services Research, College of Life Sciences & Medicine, University of Aberdeen), Christopher Burton (Professor of Primary Medical Care, University of Sheffield), Matt Waltham [Aortic Endovascular Product Manager, W L Gore & Associates (UK) Ltd], Sam Waton (Manager, NVR, UK) and Rachel Sanchez (Manager, Science and Technology Development, Cook Medical, Bjaeverskov, Denmark), for providing clinical and methodological guidance, as well as information about relevant clinical registries and databases, as members of the advisory group for this assessment; to Charles Officer and James Lister for sharing their experience as patient representatives; to Alan Karthikesalingam (National Institute for Health Research Academic Clinical Lecturer, St George’s University Hospitals NHS Foundation Trust Vascular Institute, London, UK), for providing comments on the research protocol and useful information about current EVAR practice in the UK; to Christine Clark (Ultrasound Manager, Inpatient X-ray Department, Aberdeen Royal Infirmary), for providing information on the way in which ultrasound tests are conducted in clinical practice; to Craig Rore (Lead Pharmacist, Grampian Medicines Information Centre, Aberdeen Royal Infirmary), for providing the price of a CEU contrast agent; and to Lara Kemp, for her secretarial support and patience.
Contributions of authors
Miriam Brazzelli (Senior Research Fellow) oversaw and co-ordinated all aspects of this assessment.
Rodolfo Hernández (Health Economist) reviewed the evidence on the cost-effectiveness of the relevant imaging modalities used for EVAR surveillance, developed the economic model and conducted the cost-effectiveness analyses.
Pawana Sharma and Clare Robertson (Research Fellows) reviewed and summarised the current evidence on the clinical effectiveness of imaging strategies for EVAR surveillance.
Michal Shimonovich (Research Assistant) contributed to the data extraction process and to the assessment of the risk of bias of included studies with assistance from Clare Robertson and Miriam Brazzelli.
Graeme MacLennan (Senior Statistician) provided statistical support.
Cynthia Fraser (Senior Information Specialist) developed and ran the literature searches and provided information support throughout the assessment.
Russell Jamieson (Consultant Vascular Surgeon, NHS Grampian, Aberdeen, UK) and Srinivasa Rao Vallabhaneni (Professor of Vascular Surgery, Consultant Vascular and Endovascular Surgeon, Regional Vascular Unit, Royal Liverpool University Hospital, Liverpool, UK) provided expert advice on the clinical aspects of this assessment and clinical guidance.
All authors contributed to the writing of this report and approved its final version.
Data-sharing statement
Most available data are contained within the report. All queries should be submitted to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Volodos NL, Karpovich IP, Shekhanin VE, Troian VI, Iakovenko LF. A case of distant transfemoral endoprosthesis of the thoracic artery using a self-fixing synthetic prosthesis in traumatic aneurysm. Grudn Khir 1988;6:84-6.
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5:491-9. https://doi.org/10.1007/BF02015271.
- Karanikola E, Dalainas I, Karaolanis G, Zografos G, Filis K. Duplex ultrasound versus computed tomography for the postoperative follow-up of endovascular abdominal aortic aneurysm repair. Where do we stand now?. Int J Angiol 2014;23:155-64. https://doi.org/10.1055/s-0034-1387925.
- Ilyas S, Shaida N, Thakor AS, Winterbottom A, Cousins C. Endovascular aneurysm repair (EVAR) follow-up imaging: the assessment and treatment of common postoperative complications. Clin Radiol 2015;70:183-96. https://doi.org/10.1016/j.crad.2014.09.010.
- Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg 2000;32:739-49. https://doi.org/10.1067/mva.2000.109990.
- Politz JK, Newman VS, Stewart MT. Late abdominal aortic aneurysm rupture after AneuRx repair: a report of three cases. J Vasc Surg 2000;31:599-606. https://doi.org/10.1067/mva.2000.105612.
- Schepens MA, van den Berg JC, Moll FL, Dossche KM, Heijmen RH. AneuRx stent–graft failure four years after TAA exclusion. J Endovasc Ther 2004;11:49-52. https://doi.org/10.1177/152660280401100106.
- Sharma P, Kyriakides C. Surveillance of patients post-endovascular aneurysm repair. Postgrad Med J 2007;83:750-3. https://doi.org/10.1136/pgmj.2007.062851.
- Hobo R, Buth J. EUROSTAR Collaborators . Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg 2006;43:896-902. https://doi.org/10.1016/j.jvs.2006.01.010.
- Eliason JL, Upchurch GR. Endovascular abdominal aortic aneurysm repair. Circulation 2008;117:1738-44. https://doi.org/10.1161/CIRCULATIONAHA.107.747923.
- Tse DM, Tapping CR, Patel R, Morgan R, Bratby MJ, Anthony S, et al. Surveillance after endovascular abdominal aortic aneurysm repair. Cardiovasc Intervent Radiol 2014;37:875-88. https://doi.org/10.1007/s00270-014-0916-z.
- Walker TG, Kalva SP, Yeddula K, Wicky S, Kundu S, Drescher P, et al. Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol 2010;21:1632-55. https://doi.org/10.1016/j.jvir.2010.07.008.
- Katzen BT, MacLean AA. Complications of endovascular repair of abdominal aortic aneurysms: a review. Cardiovasc Intervent Radiol 2006;29:935-46. https://doi.org/10.1007/s00270-005-0191-0.
- Liaw JV, Clark M, Gibbs R, Jenkins M, Cheshire N, Hamady M. Update: complications and management of infrarenal EVAR. Eur J Radiol 2009;71:541-51. https://doi.org/10.1016/j.ejrad.2008.05.015.
- Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 2007;243:641-55. https://doi.org/10.1148/radiol.2433051649.
- Veith FJ, Baum RA, Ohki T, Amor M, Adiseshiah M, Blankensteijn JD, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 2002;35:1029-35. https://doi.org/10.1067/mva.2002.123095.
- Cao P, De Rango P, Verzini F, Parlani G. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg 2010;51:53-69.
- Rand T, Uberoi R, Cil B, Munneke G, Tsetis D. Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR). Cardiovasc Intervent Radiol 2013;36:35-4. https://doi.org/10.1007/s00270-012-0439-4.
- Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009;50:2-49. https://doi.org/10.1016/j.jvs.2009.07.002.
- Partovi S, Kaspar M, Aschwanden M, Lopresti C, Madan S, Uthoff H, et al. Contrast-enhanced ultrasound after endovascular aortic repair-current status and future perspectives. Cardiovasc Diagn Ther 2015;5:454-63. https://doi.org/10.3978/j.issn.2223-3652.2015.09.04.
- Bengtsson H, Bergqvist D, Sternby NH. Increasing prevalence of abdominal aortic aneurysms. A necropsy study. Eur J Surg 1992;158:19-23.
- McFarlane MJ. The epidemiologic necropsy for abdominal aortic aneurysm. JAMA 1991;265:2085-8. https://doi.org/10.1001/jama.1991.03460160063030.
- Howard DP, Banerjee A, Fairhead JF, Handa A, Silver LE, Rothwell PM. Oxford Vascular Study . Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br J Surg 2015;102:907-15. https://doi.org/10.1002/bjs.9838.
- Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE, et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart 2014;9:159-70. https://doi.org/10.1016/j.gheart.2013.12.009.
- Dua A, Kuy S, Lee CJ, Upchurch GR, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg 2014;59:1512-17. https://doi.org/10.1016/j.jvs.2014.01.007.
- Nordon IM, Karthikesalingam A, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Secondary interventions following endovascular aneurysm repair (EVAR) and the enduring value of graft surveillance. Eur J Vasc Endovasc Surg 2010;39:547-54. https://doi.org/10.1016/j.ejvs.2009.11.002.
- Geller SC. Society of Interventional Radiology Device Forum . Imaging guidelines for abdominal aortic aneurysm repair with endovascular stent grafts. JVIR 2003;14:S263-4.
- Kranokpiraksa P, Kaufman JA. Follow-up of endovascular aneurysm repair: plain radiography, ultrasound, CT/CT angiography, MR imaging/MR angiography, or what?. J Vasc Interv Radiol 2008;19:27-36. https://doi.org/10.1016/j.jvir.2008.03.009.
- Patel A, Edwards R, Chandramohan S. Surveillance of patients post-endovascular abdominal aortic aneurysm repair (EVAR). A web-based survey of practice in the UK. Clin Radiol 2013;68:580-7. https://doi.org/10.1016/j.crad.2012.11.019.
- Brenner DJ, Hall EJ. Computed tomography – an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. https://doi.org/10.1056/NEJMra072149.
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011;41:1-58. https://doi.org/10.1016/j.ejvs.2010.09.011.
- Walsh SR, Tang TY, Boyle JR. Renal consequences of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2008;15:73-82. https://doi.org/10.1583/07-2299.1.
- Iezzi R, Cotroneo AR, Filippone A, Di Fabio F, Quinto F, Colosimo C, et al. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images effective for endoleak detection?. Radiology 2006;241:915-21. https://doi.org/10.1148/radiol.2413050959.
- Macari M, Chandarana H, Schmidt B, Lee J, Lamparello P, Babb J. Abdominal aortic aneurysm: can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose?. Radiology 2006;241:908-14. https://doi.org/10.1148/radiol.2413051571.
- Kirkpatrick VE, Wilson SE, Williams RA, Gordon IL. Surveillance computed tomographic arteriogram does not change management before 3 years in patients who have a normal post-EVAR study. Ann Vasc Surg 2014;28:831-6. https://doi.org/10.1016/j.avsg.2013.09.017.
- Sternbergh WC, Greenberg RK, Chuter TA, Tonnessen BH. Zenith Investigators . Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg 2008;48:278-84. https://doi.org/10.1016/j.jvs.2008.02.075.
- Black SA, Carrell TW, Bell RE, Waltham M, Reidy J, Taylor PR. Long-term surveillance with computed tomography after endovascular aneurysm repair may not be justified. Br J Surg 2009;96:1280-3. https://doi.org/10.1002/bjs.6732.
- Dias NV, Riva L, Ivancev K, Resch T, Sonesson B, Malina M. Is there a benefit of frequent CT follow-up after EVAR?. Eur J Vasc Endovasc Surg 2009;37:425-30. https://doi.org/10.1016/j.ejvs.2008.12.019.
- Karthikesalingam A, Holt PJ, Hinchliffe RJ, Nordon IM, Loftus IM, Thompson MM. Risk of reintervention after endovascular aortic aneurysm repair. Br J Surg 2010;97:657-63. https://doi.org/10.1002/bjs.6991.
- Chaer RA, Gushchin A, Rhee R, Marone L, Cho JS, Leers S, et al. Duplex ultrasound as the sole long-term surveillance method post-endovascular aneurysm repair: a safe alternative for stable aneurysms. J Vasc Surg 2009;49:845-9. https://doi.org/10.1016/j.jvs.2008.10.073.
- Harrison GJ, Oshin OA, Vallabhaneni SR, Brennan JA, Fisher RK, McWilliams RG. Surveillance after EVAR based on duplex ultrasound and abdominal radiography. Eur J Vasc Endovasc Surg 2011;42:187-92. https://doi.org/10.1016/j.ejvs.2011.03.027.
- Manning BJ, O’Neill SM, Haider SN, Colgan MP, Madhavan P, Moore DJ. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg 2009;49:60-5. https://doi.org/10.1016/j.jvs.2008.07.079.
- Millen A, Canavati R, Harrison G, McWilliams RG, Wallace S, Vallabhaneni SR, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg 2013;58:18-23. https://doi.org/10.1016/j.jvs.2012.12.057.
- Napoli V, Bargellini I, Sardella SG, Petruzzi P, Cioni R, Vignali C, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233:217-25. https://doi.org/10.1148/radiol.2331031767.
- Beeman BR, Doctor LM, Doerr K, McAfee-Bennett S, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging alone is sufficient for midterm endovascular aneurysm repair surveillance: a cost analysis study and prospective comparison with computed tomography scan. J Vasc Surg 2009;50:1019-24. https://doi.org/10.1016/j.jvs.2009.06.019.
- Go MR, Barbato JE, Rhee RY, Makaroun MS. What is the clinical utility of a 6-month computed tomography in the follow-up of endovascular aneurysm repair patients?. J Vasc Surg 2008;47:1181-7. https://doi.org/10.1016/j.jvs.2008.01.056.
- EVAR trial participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurym (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. https://doi.org/10.1016/S0140-6736(05)66627-5.
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. EVAR trial investigators . Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. https://doi.org/10.1016/S0140-6736(16)31135-7.
- Motaganahalli R, Martin A, Feliciano B, Murphy MP, Slaven J, Dalsing MC. Estimating the risk of solid organ malignancy in patients undergoing routine computed tomography scans after endovascular aneurysm repair. J Vasc Surg 2012;56:929-37. https://doi.org/10.1016/j.jvs.2012.02.061.
- Ionising Radiation: Dose Comparisons. London: PHE; 2011.
- Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 1989;320:143-9. https://doi.org/10.1056/NEJM198901193200303.
- Picel AC, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. AJR Am J Roentgenol 2014;203:W358-72. https://doi.org/10.2214/AJR.13.11736.
- Chabbert V, Otal P, Bouchard L, Soula P, Van TT, Kos X, et al. Midterm outcomes of thoracic aortic stent–grafts: complications and imaging techniques. J Endovasc Ther 2003;10:494-50. https://doi.org/10.1177/152660280301000314.
- Fearn S, Lawrence-Brown MM, Semmens JB, Hartley D. Follow-up after endovascular aortic aneurysm repair: the plain radiograph has an essential role in surveillance. J Endovasc Ther 2003;10:894-901. https://doi.org/10.1177/152660280301000508.
- Sato DT, Goff CD, Gregory RT, Robinson KD, Carter KA, Herts BR, et al. Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound scan versus computed tomography scan. J Vasc Surg 1998;28:657-63. https://doi.org/10.1016/S0741-5214(98)70091-6.
- Clevert DA, Minaifar N, Kopp R, Stickel M, Meimarakis G, Sommer W, et al. Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS). A pictorial comparison with CTA. Clin Hemorheol Microcirc 2009;41:151-68. https://doi.org/10.3233/CH-2009-1160.
- Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, et al. Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg 2006;43:259-64. https://doi.org/10.1016/j.jvs.2005.09.045.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. http://handbook-5-1.cochrane.org/ (accessed May 2017).
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009;3:e123-30.
- Mirza TA, Karthikesalingam A, Jackson D, Walsh SR, Holt PJ, Hayes PD, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg 2010;39:418-28. https://doi.org/10.1016/j.ejvs.2010.01.001.
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41. https://doi.org/10.1016/S0895-4356(98)00131-0.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. https://doi.org/10.1136/jech.52.6.377.
- Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, et al. The GATE frame: critical appraisal with pictures. Evid Based Med 2006;11:35-8. https://doi.org/10.1136/ebm.11.2.35.
- Chisci E, Setacci F, Iacoponi F, de Donato G, Cappelli A, Setacci C. Surveillance imaging modality does not affect detection rate of asymptomatic secondary interventions following EVAR. Eur J Vasc Endovasc Surg 2012;43:276-81. https://doi.org/10.1016/j.ejvs.2011.11.020.
- Nyheim T, Staxrud LE, Rosen L, Slagsvold CE, Sandbaek G, Jørgensen JJ. Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans?. Acta Radiol 2013;54:54-8. https://doi.org/10.1258/ar.2012.110291.
- Bisdas T, Weiss K, Eisenack M, Austermann M, Torsello G, Donas KP. Durability of the Endurant stent graft in patients undergoing endovascular abdominal aortic aneurysm repair. J Vasc Surg 2014;60:1125-31. https://doi.org/10.1016/j.jvs.2014.04.070.
- Blom AS, Troutman D, Beeman B, Yarchoan M, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging to detect limb stenosis or kinking of endovascular device. J Vasc Surg 2012;55:1577-80. https://doi.org/10.1016/j.jvs.2011.12.058.
- Bush RL, Lumsden AB, Dodson TF, Salam AA, Weiss VJ, Smith RB, et al. Mid-term results after endovascular repair of the abdominal aortic aneurysm. J Vasc Surg 2001;33:70-6. https://doi.org/10.1067/mva.2001.111740.
- Carroccio A, Faries PL, Morrissey NJ, Teodorescu V, Burks JA, Gravereaux EC, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg 2002;36:679-84. https://doi.org/10.1016/S0741-5214(02)00117-9.
- Cochennec F, Becquemin JP, Desgranges P, Allaire E, Kobeiter H, Roudot-Thoraval F. Limb graft occlusion following EVAR: clinical pattern, outcomes and predictive factors of occurrence. Eur J Vasc Endovasc Surg 2007;34:59-65. https://doi.org/10.1016/j.ejvs.2007.01.009.
- Collins JT, Boros MJ, Combs K. Ultrasound surveillance of endovascular aneurysm repair: a safe modality versus computed tomography. Ann Vasc Surg 2007;21:671-5. https://doi.org/10.1016/j.avsg.2007.07.009.
- Donas KP, Torsello GB, Piccoli G, Pitoulias GA, Torsello GF, Bisdas T, et al. The PROTAGORAS study to evaluate the performance of the Endurant stent graft for patients with pararenal pathologic processes treated by the chimney/snorkel endovascular technique. J Vasc Surg 2016;63:1-7. https://doi.org/10.1016/j.jvs.2015.07.080.
- Fossaceca R, Guzzardi G, Cerini P, Di Terlizzi M, Malatesta E, Filice L, et al. Endovascular treatment of abdominal aortic aneurysms: 6 years of experience at a single centre. Radiol Med 2013;118:616-32. https://doi.org/10.1007/s11547-012-0905-8.
- Freyrie A, Gallitto E, Gargiulo M, Faggioli G, Bianchini Massoni C, Mascoli C, et al. Results of the endovascular abdominal aortic aneurysm repair using the Anaconda aortic endograft. J Vasc Surg 2014;60:1132-9. https://doi.org/10.1016/j.jvs.2014.04.073.
- Ghotbi R, Sotiriou A, Mansur R. New results with 100 Excluder cases. J Cardiovasc Surg 2010;51:475-80.
- Karthikesalingam A, Kumar S, Anandarajah JJ, Hinchliffe RJ, Poloniecki JD, Thompson MM, et al. Predictive value of peak systolic velocity for the development of graft limb complications after endovascular aneurysm repair. J Endovasc Ther 2012;19:428-33. https://doi.org/10.1583/11-3739MR.1.
- Köcher M, Utíkal P, Koutná J, Bachleda P, Buriánková E, Herman M, et al. Endovascular treatment of abdominal aortic aneurysms – 6 years of experience with Ella stent–graft system. Eur J Radiol 2004;51:181-8. https://doi.org/10.1016/S0720-048X(03)00165-7.
- Kray J, Kirk S, Franko J, Chew DK. Role of type II endoleak in sac regression after endovascular repair of infrarenal abdominal aortic aneurysms. J Vasc Surg 2015;61:869-74. https://doi.org/10.1016/j.jvs.2014.11.003.
- Meier GH, Parker FM, Godziachvili V, Demasi RJ, Parent FN, Gayle RG. Endotension after endovascular aneurysm repair: the Ancure experience. J Vasc Surg 2001;34:421-6. https://doi.org/10.1067/mva.2001.117145.
- Oshin OA, Fisher RK, Williams LA, Brennan JA, Gilling-Smith GL, Vallabhaneni SR, et al. Adjunctive iliac stents reduce the risk of stent–graft limb occlusion following endovascular aneurysm repair with the Zenith stent–graft. J Endovasc Ther 2010;17:108-14. https://doi.org/10.1583/09-2854.1.
- Parlani G, Zannetti S, Verzini F, De Rango P, Carlini G, Lenti M, et al. Does the presence of an iliac aneurysm affect outcome of endoluminal AAA repair? An analysis of 336 cases. Eur J Vasc Endovasc Surg 2002;24:134-8. https://doi.org/10.1053/ejvs.2002.1669.
- Schunn CD, Krauss M, Heilberger P, Ritter W, Raithel D. Aortic aneurysm size and graft behavior after endovascular stent–grafting: clinical experiences and observations over 3 years. J Endovasc Ther 2000;7:167-76. https://doi.org/10.1177/152660280000700301.
- Soler RJ, Bartoli MA, Mancini J, Lerussi G, Thevenin B, Sarlon-Bartoli G, et al. Aneurysm sac shrinkage after endovascular repair: predictive factors and long-term follow-up. Ann Vasc Surg 2015;29:770-9. https://doi.org/10.1016/j.avsg.2014.12.016.
- Stella A, Freyrie A, Gargiulo M, Faggioli GL. The advantages of Anaconda endograft for AAA. J Cardiovasc Surg 2009;50:145-52.
- Wolf YG, Tillich M, Lee WA, Fogarty TJ, Zarins CK, Rubin GD. Changes in aneurysm volume after endovascular repair of abdominal aortic aneurysm. J Vasc Surg 2002;36:305-9. https://doi.org/10.1067/mva.2002.126085.
- Dominguez I, Mehta M, Roddy SP, Clement Darling R, Sternbach Y, Taggert JB, et al. A prospective evaluation of the impact of balloon-expandable Palmaz stent placement in aortic neck during EVAR. J Vasc Surg 2010;52. https://doi.org/10.1016/j.jvs.2010.06.134.
- Fargion A, Masciello F, Melani A, Pratesi G, Pulli R, Dorigo W, et al. The influence of the timing of onset of type II endoleak on the late outcomes of endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2016;63. https://doi.org/10.1016/j.jvs.2016.03.193.
- Mazzaccaro D, Settembrini AM, Malacrida G, Stegher S, Occhiuto MT, Sorba F, et al. Endovascular aneurysm repair: experience of 12 years in a single institution. Interact Cardiovasc Thorac Surg 2011;12.
- Buth J, Harris PL, Van Marrewijk C, Fransen G. Endoleaks during follow-up after endovascular repair of abdominal aortic aneurysm. Are they all dangerous?. J Cardiovasc Surg 2003;44:559-66.
- Freyrie A, Testi G, Faggioli GL, Gargiulo M, Giovanetti F, Serra C, et al. Ring-stents supported infrarenal aortic endograft fits well in abdominal aortic aneurysms with tortuous anatomy. J Cardiovasc Surg 2010;51:467-74.
- Tang T, Sadat U, Walsh S, Hayes PD. ENGAGE Investigators . Comparison of the Endurant bifurcated endograft vs. aortouni-iliac stent–grafting in patients with abdominal aortic aneurysms: experience from the ENGAGE registry. J Endovasc Ther 2013;20:172-81. https://doi.org/10.1583/1545-1550-20.2.172.
- Stokmans RA, Teijink JA, Forbes TL, Böckler D, Peeters PJ, Riambau V, et al. Early results from the ENGAGE registry: real-world performance of the Endurant stent graft for endovascular AAA repair in 1262 patients. Eur J Vasc Endovasc Surg 2012;44:369-75. https://doi.org/10.1016/j.ejvs.2012.07.005.
- Karthikesalingam A, Vidal-Diez A, De Bruin JL, Thompson MM, Hinchliffe RJ, Loftus IM, et al. International validation of a risk score for complications and reinterventions after endovascular aneurysm repair. Br J Surg 2015;102:509-15. https://doi.org/10.1002/bjs.9758.
- Faure EM, Becquemin JP, Cochennec F. ENGAGE collaborators . Predictive factors for limb occlusions after endovascular aneurysm repair. J Vasc Surg 2015;61:1138-45.e2. https://doi.org/10.1016/j.jvs.2014.11.084.
- Bastos Goncalves F, Hoeks SE, Teijink JA, Moll FL, Castro JA, Stolker RJ, et al. Risk factors for proximal neck complications after endovascular aneurysm repair using the Endurant stentgraft. Eur J Vasc Endovasc Surg 2015;49:156-62. https://doi.org/10.1016/j.ejvs.2014.10.003.
- Koole D, Moll FL, Buth J, Hobo R, Zandvoort HJ, Bots ML, et al. Annual rupture risk of abdominal aortic aneurysm enlargement without detectable endoleak after endovascular abdominal aortic repair. J Vasc Surg 2011;54:1614-22. https://doi.org/10.1016/j.jvs.2011.06.095.
- Cuypers P, Buth J, Harris PL, Gevers E, Lahey R. Realistic expectations for patients with stent–graft treatment of abdominal aortic aneurysms. Results of a European multicentre registry. Eur J Vasc Endovasc Surg 2011;42:S63-71. https://doi.org/10.1016/j.ejvs.2011.06.012.
- Laheij RJ, Buth J, Harris PL, Moll FL, Stelter WJ, Verhoeven EL. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg 2000;87:1666-73. https://doi.org/10.1046/j.1365-2168.2000.01661.x.
- Leurs LJ, Buth J, Laheij RJ. Long-term results of endovascular abdominal aortic aneurysm treatment with the first generation of commercially available stent grafts. Arch Surg 2007;142:33-41. https://doi.org/10.1001/archsurg.142.1.33.
- Leurs LJ, Harris PL, Buth J. EUROSTAR Collaborators . Secondary interventions after elective endovascular repair of degenerative thoracic aortic aneurysms: results of the European collaborators registry (EUROSTAR). J Vasc Interv Radiol 2007;18:491-5. https://doi.org/10.1016/j.jvir.2007.01.018.
- Leurs LJ, Hobo R, Buth J. EUROSTAR Collaborators . The multicenter experience with a third-generation endovascular device for abdominal aortic aneurysm repair. A report from the EUROSTAR database. J Cardiovasc Surg 2004;45:293-300.
- Leurs LJ, Kievit J, Dagnelie PC, Nelemans PJ, Buth J. EUROSTAR Collaborators . Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2006;13:640-8. https://doi.org/10.1583/06-1882.1.
- Leurs LJ, Laheij RJ, Buth J. EUROSTAR Collaborators . What determines and are the consequences of surveillance intensity after endovascular abdominal aortic aneurysm repair?. Ann Vasc Surg 2005;19:868-75. https://doi.org/10.1007/s10016-005-7751-2.
- Peppelenbosch N, Buth J, Harris PL, van Marrewijk C, Fransen G. EUROSTAR Collaborators . Diameter of abdominal aortic aneurysm and outcome of endovascular aneurysm repair: does size matter? A report from EUROSTAR. J Vasc Surg 2004;39:288-97. https://doi.org/10.1016/j.jvs.2003.09.047.
- Szmidt J, Galazka Z, Rowinski O, Nazarewski S, Jakimowicz T, Pietrasik K, et al. Late aneurysm rupture after endovascular abdominal aneurysm repair. Interact Cardiovasc Thorac Surg 2007;6:490-4. https://doi.org/10.1510/icvts.2007.152447.
- van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J. EUROSTAR Collaborators . Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg 2004;27:128-37. https://doi.org/10.1016/j.ejvs.2003.10.016.
- Vallabhaneni SR, Harris PL. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aortic aneurysm repair. Eur J Radiol 2001;39:34-41. https://doi.org/10.1016/S0720-048X(01)00340-0.
- Cuypers P, Buth J, Harris PL, Gevers E, Lahey R. Realistic expectations for patients with stent–graft treatment of abdominal aortic aneurysms. Results of a European multicentre registry. Eur J Vasc Endovasc Surg 1999;17:507-16. https://doi.org/10.1053/ejvs.1999.0836.
- Candell L, Tucker LY, Goodney P, Walker J, Okuhn S, Hill B, et al. Early and delayed rupture after endovascular abdominal aortic aneurysm repair in a 10-year multicenter registry. J Vasc Surg 2014;60:1146-52. https://doi.org/10.1016/j.jvs.2014.05.046.
- Hye RJ, Inui TS, Anthony FF, Kiley ML, Chang RW, Rehring TF, et al. A multiregional registry experience using an electronic medical record to optimize data capture for longitudinal outcomes in endovascular abdominal aortic aneurysm repair. J Vasc Surg 2015;61:1160-6. https://doi.org/10.1016/j.jvs.2014.12.055.
- Walker J, Tucker LY, Goodney P, Candell L, Hua H, Okuhn S, et al. Adherence to endovascular aortic aneurysm repair device instructions for use guidelines has no impact on outcomes. J Vasc Surg 2015;61:1151-9. https://doi.org/10.1016/j.jvs.2014.12.053.
- Walker J, Tucker LY, Goodney P, Candell L, Hua H, Okuhn S, et al. Type II endoleak with or without intervention after endovascular aortic aneurysm repair does not change aneurysm-related outcomes despite sac growth. J Vasc Surg 2015;62:551-61. https://doi.org/10.1016/j.jvs.2015.04.389.
- Anthony F, Kiley ML, Mays K, Javines J, Hye R, Chang R, et al. Aligning information technology with longitudinal outcomes surveillance: a multiregional vascular registry experience. Circulation 2013;128.
- Chang RW, Goodney P, Tucker LY, Okuhn S, Hua H, Rhoades A, et al. Ten-year results of endovascular abdominal aortic aneurysm repair from a large multicenter registry. J Vasc Surg 2013;58:324-32. https://doi.org/10.1016/j.jvs.2013.01.051.
- Fitridge RA, Boult M, de Loryn T, Cowled P, Barnes M. Predictors of 1-year survival after endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2016;51:528-34. https://doi.org/10.1016/j.ejvs.2015.12.019.
- Mani K, Lees T, Beiles B, Jensen LP, Venermo M, Simo G, et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a Vascunet report. Eur J Vasc Endovasc Surg 2011;42:598-607. https://doi.org/10.1016/j.ejvs.2011.06.043.
- Thomas SM, Beard JD, Ireland M, Ayers S. Vascular Society of Great Britain and Ireland . Results from the prospective Registry of Endovascular Treatment of Abdominal Aortic Aneurysms (RETA): mid term results to five years. Eur J Vasc Endovasc Surg 2005;29:563-70. https://doi.org/10.1016/j.ejvs.2005.03.012.
- Thomas SM, Gaines PA, Beard JD. Vascular Surgical Society of Great Britain and Ireland . Short-term (30-day) outcome of endovascular treatment of abdominal aortic aneurism: results from the prospective Registry of Endovascular Treatment of Abdominal Aortic Aneurism (RETA). Eur J Vasc Endovasc Surg 2001;21:57-64. https://doi.org/10.1053/ejvs.2000.1268.
- Lifeline Registry of Endovascular Aneurysm Repair Steering Committee . Lifeline Registry of Endovascular Aneurysm Repair: registry data report. J Vasc Surg 2002;35:616-20. https://doi.org/10.1067/mva.2002.122232.
- Lifeline Registry of EVAR Publications Committee . Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg 2005;42:1-10. https://doi.org/10.1016/j.jvs.2005.05.012.
- Anonymous . Lifeline Registry of Endovascular Aneurysm Repair: registry data report. J Vasc Surg 2002;35:616-20. https://doi.org/10.1067/mva.2002.122232.
- Siami FS. Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg 2005;42:1-10. https://doi.org/10.1016/j.jvs.2005.05.012.
- Majumder B, Urquhart G, Edwards R, Irshad K, Velu R, Reid DB. Early clinical experience with the Anaconda re-deployable endograft in 106 patients with abdominal aortic aneurism: the west of Scotland Anaconda registry. Scott Med J 2012;57:61-5. https://doi.org/10.1258/smj.2012.012001.
- Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-15.
- Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther 2005;12:297-305. https://doi.org/10.1583/04-1479R.1.
- Bevis PM, Cooper DG. Duplex ultrasound for surveillance after endovascular repair of abdominal aortic aneurysm. Ital J Vasc Endovasc Surg 2012;19:237-43.
- Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, et al. EVAR: benefits of CEUS for monitoring stent–graft status. Eur J Radiol 2015;84:1658-65. https://doi.org/10.1016/j.ejrad.2015.07.001.
- Chung J, Kordzadeh A, Prionidis I, Panayiotopoulos Y, Browne T. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound 2015;18:91-9. https://doi.org/10.1007/s40477-014-0154-x.
- Howard JM, Ezwawah O, Guiney M, Ryan M, McEniff N. Contrast-enhanced ultrasound versus CT angiography for the detection of endoleak in patients post-EVAR: an evidence based radiology approach. CardioVasc Intervent Radiol 2011;34:567-8.
- Karthikesalingam A, Al-Jundi W, Jackson D, Boyle JR, Beard JD, Holt PJ, et al. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br J Surg 2012;99:1514-23. https://doi.org/10.1002/bjs.8873.
- Sun Z. Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol 2006;17:759-64. https://doi.org/10.1097/01.RVI.0000217944.36738.02.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-10.
- Drummond MF, Sculpher M, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Bendick PJ, Zelenock GB, Bove PG, Long GW, Shanley CJ, Brown OW. Duplex ultrasound imaging with an ultrasound contrast agent: the economic alternative to CT angiography for aortic stent graft surveillance. Vasc Endovascular Surg 2003;37:165-70. https://doi.org/10.1177/153857440303700302.
- Gray C, Goodman P, Herron CC, Lawler LP, O’Malley MK, O’Donohoe MK, et al. Use of colour duplex ultrasound as a first line surveillance tool following EVAR is associated with a reduction in cost without compromising accuracy. Eur J Vasc Endovasc Surg 2012;44:145-50. https://doi.org/10.1016/j.ejvs.2012.05.008.
- Antoniou GA, Georgiadis GS, Antoniou SA, Neequaye S, Brennan JA, Torella F, et al. Late rupture of abdominal aortic aneurysm after previous endovascular repair: a systematic review and meta-analysis. J Endovasc Ther 2015;22:734-44. https://doi.org/10.1177/1526602815601405.
- NHS Reference Costs 2015 to 2016. London: DHSC; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16090.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- National Life Tables, UK: 2013–2015. Newport: ONS; 2016.
- Uthoff H, Peña C, Katzen BT, Gandhi R, West J, Benenati JF, et al. Current clinical practice in postoperative endovascular aneurysm repair imaging surveillance. J Vasc Interv Radiol 2012;23:1152-9.e6. https://doi.org/10.1016/j.jvir.2012.06.003.
- Mitchell AM, Jones AE, Tumlin JA, Kline JA. Incidence of contrast-induced nephropathy after contrast-enhanced computed tomography in the outpatient setting. Clin J Am Soc Nephrol 2010;5:4-9. https://doi.org/10.2215/CJN.05200709.
- Schlösser FJ, Gusberg RJ, Dardik A, Lin PH, Verhagen HJ, Moll FL, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted?. Eur J Vasc Endovasc Surg 2009;37:15-22. https://doi.org/10.1016/j.ejvs.2008.10.011.
- Bargellini I, Cioni R, Napoli V, Petruzzi P, Vignali C, Cicorelli A, et al. Ultrasonographic surveillance with selective CTA after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 2009;16:93-104. https://doi.org/10.1583/08-2508.1.
- Fletcher J, Saker K, Batiste P, Dyer S. Colour Doppler diagnosis of perigraft flow following endovascular repair of abdominal aortic aneurysm. Int Angiol 2000;19:326-30.
- Sandford RM, Bown MJ, Fishwick G, Murphy F, Naylor M, Sensier Y, et al. Duplex ultrasound scanning is reliable in the detection of endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2006;32:537-41. https://doi.org/10.1016/j.ejvs.2006.05.013.
- Wolf YG, Johnson BL, Hill BB, Rubin GD, Fogarty TJ, Zarins CK. Duplex ultrasound scanning versus computed tomographic angiography for postoperative evaluation of endovascular abdominal aortic aneurysm repair. J Vasc Surg 2000;32:1142-8. https://doi.org/10.1067/mva.2000.109210.
- Zannetti S, De Rango P, Parente B, Parlani G, Verzini F, Maselli A, et al. Role of duplex scan in endoleak detection after endoluminal abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2000;19:531-5. https://doi.org/10.1053/ejvs.1999.1033.
- Karthikesalingam A, Page AA, Pettengell C, Hinchliffe RJ, Loftus IM, Thompson MM, et al. Heterogeneity in surveillance after endovascular aneurysm repair in the UK. Eur J Vasc Endovasc Surg 2011;42:585-90. https://doi.org/10.1016/j.ejvs.2011.06.053.
- Giannoni MF, Fanelli F, Citone M, Cristina Acconcia M, Speziale F, Gossetti B. Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up. Interact Cardiovasc Thorac Surg 2007;6:359-62. https://doi.org/10.1510/icvts.2006.137265.
- Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2009;49:552-60. https://doi.org/10.1016/j.jvs.2008.10.008.
- AbuRahma AF, Campbell J, Stone PA, Nanjundappa A, Jain A, Dean LS, et al. The correlation of aortic neck length to early and late outcomes in endovascular aneurysm repair patients. J Vasc Surg 2009;50:738-48. https://doi.org/10.1016/j.jvs.2009.04.061.
- Sternbergh WC, Carter G, York JW, Yoselevitz M, Money SR. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg 2002;35:482-6. https://doi.org/10.1067/mva.2002.119506.
- Antoniou GA, Georgiadis GS, Antoniou SA, Kuhan G, Murray D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J Vasc Surg 2013;57:527-38. https://doi.org/10.1016/j.jvs.2012.09.050.
- Schanzer A, Messina LM, Ghosh K, Simons JP, Robinson WP, Aiello FA, et al. Follow-up compliance after endovascular abdominal aortic aneurysm repair in Medicare beneficiaries. J Vasc Surg 2015;61:16-22.e1. https://doi.org/10.1016/j.jvs.2014.06.006.
- Karthikesalingam A, Holt PJ. EVAR-Screen Collaborators . Multicentre Post-EVAR Surveillance Evaluation Study (EVAR-SCREEN). Eur J Vasc Endovasc Surg 2016;52. https://doi.org/10.1016/j.ejvs.2016.05.025.
Appendix 1 Search strategy
Clinical effectiveness
Databases
EMBASE (1996 to week 36 2016), Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 5 September 2016).
Last date of search: 5 September 2016.
Search strategy
-
Endoleak/di [Diagnosis]
-
endoleak/ or endoleak?.tw,kw.
-
evar.tw,kw.
-
(endovascular adj5 repair? adj5 abdominal).tw,kw.
-
or/2-4
-
Ultrasonography/ use ppez
-
Echography/ use emef
-
(duplex adj2 (ultrasound or ultrasono$)).tw.
-
Ultrasonography, Doppler, Duplex/ use ppez
-
Doppler echography/ use emef
-
(contrast enhanced adj2 (ultrasound or ultrasono$)).tw
-
(cdu or ceu).tw,kw.
-
Tomography, X-Ray Computed/ use ppez
-
Multidetector Computed Tomography/
-
Computer Tomography Scanner/ use emef
-
(computed adj3 tomograph$).tw.
-
Endoleak/us [Ultrasonography]
-
5 and (6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16)
-
1 or 17 or 18
-
Aortic Aneurysm, Abdominal/ use ppez
-
Abdominal Aorta Aneurysm/ use emef
-
endovascular procedures/ use ppez
-
endovascular surgery/ use emef
-
evar.tw,kw.
-
(endovasc$ adj5 repair?).tw.
-
(20 or 21) and (22 or 23 or 24 or 25)
-
26 or endovascular aneurysm repair/
-
exp Epidemiological Monitoring/ use ppez
-
Patient monitoring/ use emef
-
surveillance.tw,kw.
-
monitor$.tw,kw.
-
27 and (28 or 29 or 30 or 31)
-
19 or 32
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomi?ed.ab.
-
randomization/ use emef
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
(chang$ or evaluat$ or reviewed or baseline).tw.
-
major clinical study/ use emef
-
exp clinical trial/ use emef
-
comparative study/
-
follow-up studies/
-
time factors/
-
(prospective$ or retrospective$).tw.
-
(cohort$ or case series).tw.
-
(compare$ or compara$).tw.
-
(registry or registries or register?).tw.
-
(anaconda or eurostar or karbase or lifeline or renu or swedvasc or uk reta or vascunet).tw.
-
or/34-53
-
33 and 54
-
55 not (editorial or letter or comment or case reports).pt.
-
limit 56 to yr=1996-2016
-
remove duplicates from 57
Databases
Science Citation Index (1997 to 5 September 2016).
Web of Knowledge ISI (http://wok.mimas.ac.uk/).
Last date of search: 5 September 2016.
Search strategy
-
TS=endoleak*
-
TS=evar
-
TS=(endovascular N/5 repair* N/5 abdominal)
-
#1 OR #2 OR #3
-
TS=(duplex NEAR/3 (ultrasound OR ultrasono*))
-
TS=(‘contrast enhanced’ NEAR/3 (ultrasound OR ultrasono*))
-
TS=(CDU OR CEU)
-
TS=(Computed NEAR/3 tomograph*)
-
#5 OR #6 OR #7 OR #8
-
#4 AND #9
-
TS=(abdominal NEAR/5 aort* NEAR/5 aneurysm*)
-
TS=evar
-
TS=(endovascular NEAR/5 repair*) Indexes=SCI-EXPANDED, IC Timespan=1997-2016
-
#11 AND (#12 OR #13)
-
TS=monitor*
-
TS=surveillance
-
#16 OR #15
-
#17 AND #14
-
#18 OR #10
Database
The Cochrane Library: Issue 3 2016 [CENTRAL, CDSR, DARE (www3.interscience.wiley.com/)].
Last date of search: 5 September 2016.
Search strategy
-
MeSH (medical subject heading) descriptor: [Endoleak] explode all trees and with qualifier(s): [Diagnosis – DI]
-
MeSH descriptor: [Endoleak] explode all trees
-
endoleak*:ti,ab,kw (Word variations have been searched)
-
(endovascular near/5 repair* near/5 abdominal):ti,ab,kw (Word variations have been searched)
-
#2 or #3 or #4
-
MeSH descriptor: [Ultrasonography] this term only
-
(duplex near/2 (ultrasound or ultrasono*))
-
MeSH descriptor: [Ultrasonography, Doppler, Duplex] this term only
-
(contrast enhanced near/2 (ultrasound or ultrasono*))
-
cdu or ceu:ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Tomography, X-Ray Computed] this term only
-
MeSH descriptor: [Multidetector Computed Tomography] this term only
-
MeSH descriptor: [Endoleak] explode all trees and with qualifier(s): [Ultrasonography - US]
-
#5 and (#6 or #7 or #8 or #8 or #9 OT #10 or #11 or #12)
-
#1 or #13 or #14
-
MeSH descriptor: [Aortic Aneurysm, Abdominal] this term only
-
MeSH descriptor: [Endovascular Procedures] this term only
-
evar or (endovasc* near/5 repair*):ti,ab,kw (Word variations have been searched)
-
#16 and (#17 or #18)
-
MeSH descriptor: [Epidemiological Monitoring] explode all trees
-
(surveillance or monitor*):ti,ab,kw (Word variations have been searched)
-
#19 and (#20 or #21)
-
#15 or #22
Database
Scopus’ Articles-In-Press (www.scopus.com/).
Last date of search: 5 September 2016.
Search strategy
( TITLE-ABS-KEY ( endoleak* ) AND DOCTYPE ( ip ) ) OR ( TITLE-ABS-KEY ( abdominal aortic aneurysm* ) AND DOCTYPE ( ip ) ) ) AND ( TITLE-ABS-KEY ( surveillance OR monitor* OR ultraso* OR tomograph* ) AND DOCTYPE ( ip ) ).
Database
Clinical Trials (http://clinicaltrials.gov/ct/gui/c/r).
Date searched: 27 January 2016.
Search strategy
Abdominal Aortic Aneurysm And endoleak.
Database
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Date searched: 27 January 2016.
Search strategy
Abdominal Aortic Aneurysm And endoleak.
Database
The World Health Organization’s ICTRP (www.who.int/ictrp/en/).
Date searched: 27 January 2016.
Search strategy
Abdominal Aortic Aneurysm And endoleak.
Diagnostic reviews
Databases
EMBASE (1996 to week 13 2016), Ovid MEDLINE(R) without Revisions (1996 to week 3 2016), Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations (28 March 2016) Ovid multifile search (https://shibboleth.ovid.com/).
Date searched: 29 March 2016.
Search strategy
-
Endoleak/di [Diagnosis]
-
endoleak/ or endoleak?.tw,kw.
-
evar.tw,kw.
-
(endovascular adj5 repair? adj5 abdominal).tw,kw.
-
or/2-4
-
Ultrasonography/ use medf
-
Echography/ use emef
-
(duplex adj2 (ultrasound or ultrasono$)).tw.
-
Ultrasonography, Doppler, Duplex/ use medf
-
Doppler echography/ use emef
-
(contrast enhanced adj2 (ultrasound or ultrasono$)).tw.
-
(cdu or ceu).tw,kw.
-
Tomography, X-Ray Computed/ use medf
-
Multidetector Computed Tomography/
-
Computer Tomography Scanner/ use emef
-
(computed adj3 tomograph$).tw.
-
Endoleak/us [Ultrasonography]
-
5 and (6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16)
-
‘sensitivity and specificity’/
-
roc curve/
-
receiver operating characteristic/ use emef
-
predictive value of tests/
-
diagnostic errors/ use emef
-
false positive reactions/ use medf
-
false negative reactions/ use medf
-
diagnostic accuracy/ use emef
-
diagnostic value/ use emef
-
du.fs. use medf
-
sensitivity.tw.
-
distinguish$.tw.
-
differentiat$.tw.
-
identif$.tw.
-
detect$.tw.
-
diagnos$.tw.
-
(predictive adj4 value$).tw.
-
accura$.tw.
-
comparison.tw.
-
or/19-37
-
18 and 38
-
1 or 17 or 39
-
systematic$ review$.tw.
-
systematic review/ use emef
-
systematic review as topic/ use emef
-
Meta analysis as topic/
-
meta analysis/ use emef
-
meta analysis.tw,pt.
-
metanalysis.tw.
-
metaanalysis.tw.
-
meta synthesis.tw.
-
metasynthesis.tw
-
Meta regression.tw.
-
metaregression.tw.
-
(synthes$ adj3 (literature or evidence)).tw.
-
(systematic study or systematic studies).tw.
-
evidence based review.tw.
-
comprehensive review.tw.
-
or/41-56
-
review.pt,ti.
-
(medline or pubmed or cochrane or embase or cinahl or psyc?lit or psyc?info).ab.
-
(search adj3 (literature or database? or bibliographic or electronic or internet or computeri?ed)).ab.
-
included studies.ab.
-
(inclusion adj3 studies).ab.
-
((inclusion or selection or predefined or predetermined) adj criteria).ab.
-
(assess$ adj3 (quality or validity)).ab.
-
(select$ adj3 (study or studies)).ab.
-
(data adj3 extract$).ab.
-
extracted data.ab.
-
(data adj2 abstracted).ab.
-
(data adj3 abstraction).ab.
-
or/59-69
-
58 and 70
-
57 or 71
-
(letter or editorial or comment).pt.
-
72 not 73
-
74 and 40
-
remove duplicates from 75 (36)
Databases
Database of Abstracts of Reviews of Effects and CRD (www.crd.york.ac.uk/CRDWeb/).
Date searched: 29 March 2016.
Search strategy
MeSH DESCRIPTOR Endoleak EXPLODE ALL TREES.
Database
The Cochrane Library Issue 1 2016 [CDSR (www3.interscience.wiley.com/)].
Date searched: 29 March 2016.
Search strategy
-
MeSH descriptor: [Endoleak] explode all trees and with qualifier(s): [Diagnosis – DI]
-
MeSH descriptor: [Endoleak] explode all trees
-
endoleak*:ti,ab,kw (Word variations have been searched)
-
(endovascular near/5 repair* near/5 abdominal):ti,ab,kw (Word variations have been searched)
-
#2 or #3 or #4
-
MeSH descriptor: [Ultrasonography] this term only
-
(duplex near/2 (ultrasound or ultrasono*))
-
MeSH descriptor: [Ultrasonography, Doppler, Duplex] this term only
-
(contrast enhanced near/2 (ultrasound or ultrasono*))
-
cdu or ceu:ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Tomography, X-Ray Computed] this term only
-
MeSH descriptor: [Multidetector Computed Tomography] this term only
-
MeSH descriptor: [Endoleak] explode all trees and with qualifier(s): [Ultrasonography - US]
-
#5 and (#6 or #7 or #8 or #8 or #9 OT #10 or #11 or #12)
-
#1 or #13 or #14
Cost-effectiveness
Databases
EMBASE (1996 to week 36 2016), Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 5 September 2016).
Last date of search: 5 September 2016.
Search strategy
-
exp ‘costs and cost analysis’/ use mesz
-
exp economic evaluation/ use emcz
-
economics/
-
health economics/ use emcz
-
exp health care cost/ use emcz
-
exp economics,hospital/ use mesz
-
exp economics,medical/ use mesz
-
economics,pharmaceutical/ use mesz
-
pharmacoeconomics/ use emcz
-
exp models, economic/ use mesz
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp technology assessment, biomedical/
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economic$ or pharmacoeconomic$).tw
-
(price or prices or pricing).tw.
-
budget$.tw.
-
(value adj1 money).tw.
-
(expenditure$ not energy).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
or/1-24
-
(metabolic adj cost).tw.
-
((energy or oxygen) adj (cost or expenditure)).tw.
-
25 not (26 or 27) (1521739)
-
(letter or editorial or note or comment).pt.
-
28 not 29
-
exp animals/ not humans/ use mesz
-
(animal/ or nonhuman/) not exp human/ use emcz
-
30 not (31 or 32)
-
Endoleak/di [Diagnosis]
-
endoleak/ or endoleak?.tw,kw
-
evar.tw,kw)
-
(endovascular adj5 repair? adj5 abdominal).tw,kw.
-
or/35-37
-
Ultrasonography/ use mesz
-
Echography/ use emcz
-
(duplex adj2 (ultrasound or ultrasono$)).tw.
-
Ultrasonography, Doppler, Duplex/ use mesz
-
Doppler echography/ use emcz
-
(contrast enhanced adj2 (ultrasound or ultrasono$)).tw.
-
(cdu or ceu).tw,kw.
-
Tomography, X-Ray Computed/ use mesz
-
Multidetector Computed Tomography/
-
Computer Tomography Scanner/ use emcz
-
(computed adj3 tomograph$).tw.
-
Endoleak/us [Ultrasonography]
-
38 and (39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49)
-
34 or 50 or 51
-
Aortic Aneurysm, Abdominal/ use mesz
-
Abdominal Aorta Aneurysm/ use emcz
-
endovascular procedures/ use mesz
-
endovascular surgery/ use emcz
-
evar.tw,kw.
-
(endovasc$ adj5 repair?).tw
-
(53 or 54) and (55 or 56 or 57 or 58)
-
59 or endovascular aneurysm repair/
-
exp Epidemiological Monitoring/ use mesz
-
Patient monitoring/ use emcz
-
surveillance.tw,kw.
-
monitor$.tw,kw.
-
60 and (61 or 62 or 63 or 64)
-
52 or 65
-
33 and 66
-
Aortic Aneurysm, Abdominal/ec use mesz
-
67 or 68
-
remove duplicates from 69
Databases
NHS Economics Evaluations Database.
Centre for Reviews and Dissemination.
Date of last search: 9 September 2016.
Search strategy
-
MeSH DESCRIPTOR Aortic Aneurysm, Abdominal IN NHSEED
-
MeSH DESCRIPTOR Endoleak IN NHSEED
-
(endoleak*) OR (evar) OR (repair*)
-
(surveillance) OR (monitor*)
-
#2 OR #3 OR #4
-
#1 AND #5
Database
Health Technology Assessment Database [Canadian (www.crd.york.ac.uk/PanHTA/HistoryPage.asp)].
Date of last search: 9 September 2016.
Search strategy
-
MeSH DESCRIPTOR Aortic Aneurysm, Abdominal EXPLODE ALL TREES IN PCHTA 9 Delete
-
MeSH DESCRIPTOR Endoleak EXPLODE ALL TREES IN HTA
-
#1 or #2
Database
Research Papers in Economics (http://repec.org/).
Date of last search: 9 September 2016.
Search strategy
Abdominal aortic aneurysm* or endoleak*.
Appendix 2 Study eligibility and data extraction forms
Full-text screening form
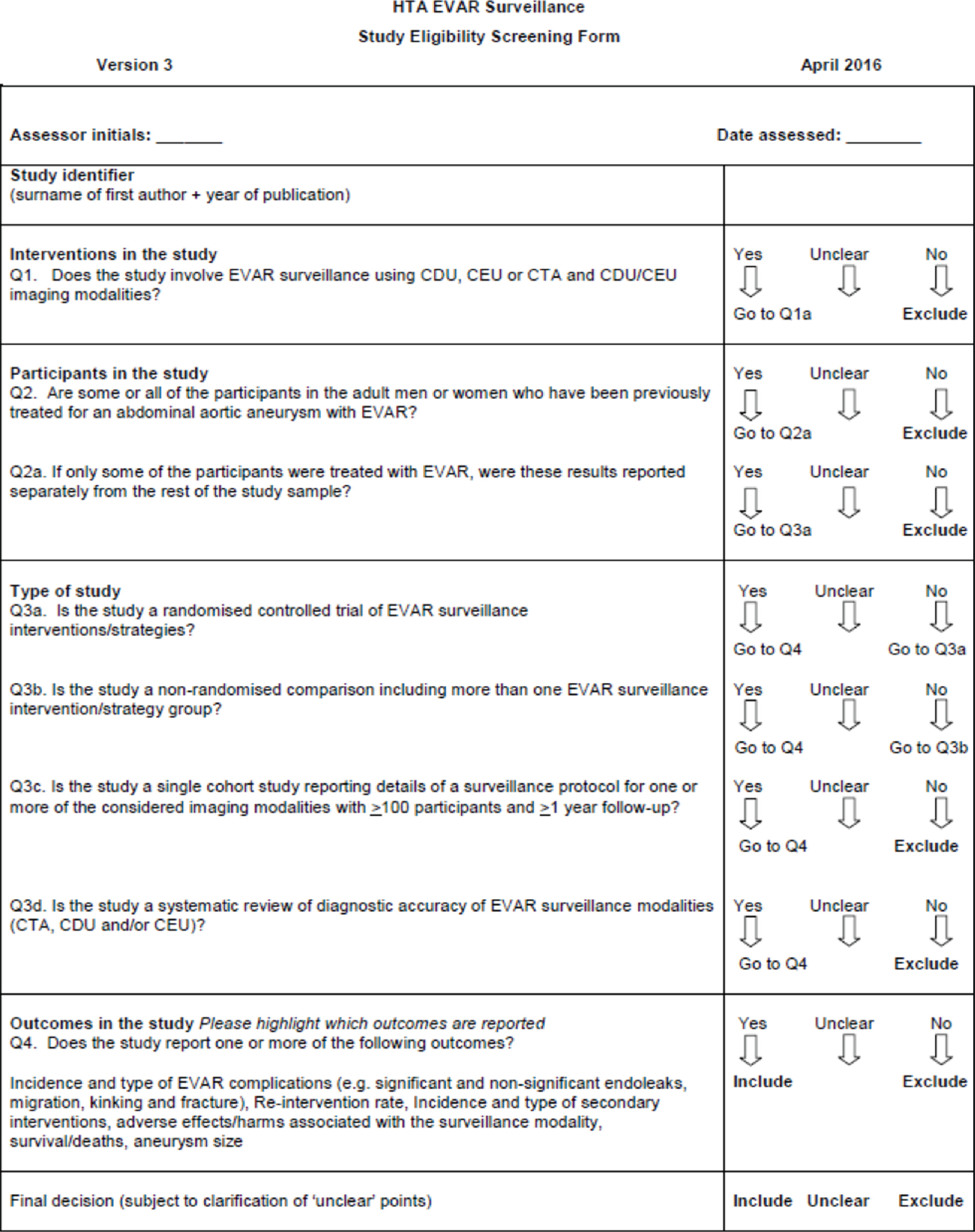
Data extraction form
| Reviewer | ||||||||||||||
| Date | ||||||||||||||
| Administration details | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | ||||||||||||||
| Publication status | ||||||||||||||
| Study IDs of linked reports | ||||||||||||||
| Study aim | ||||||||||||||
| Study details | ||||||||||||||
| Study design | ||||||||||||||
| Setting | ||||||||||||||
| Country | ||||||||||||||
| Number of centres | ||||||||||||||
| Sample identification | ||||||||||||||
| Method of recruitment | ||||||||||||||
| Allocation method | ||||||||||||||
| Study dates | ||||||||||||||
| Duration of the study | ||||||||||||||
| Length of follow-up | ||||||||||||||
| Eligibility criteria | ||||||||||||||
| Inclusion criteria | ||||||||||||||
| Exclusion criteria | ||||||||||||||
| Interventions and comparators | ||||||||||||||
| Details of the intervention | CDU | CEU | CDU and CEU | With plain radiography | Without plain radiography | Other (describe) | ||||||||
|
Frequency of imaging (frequency and duration – e.g. imaging at 1 month, 6 months, 12 months post EVAR) |
||||||||||||||
| Details of the operator, including experience level if reported | ||||||||||||||
| Details of the comparator | CTA multiphase | CTA single phase | CDU/CEU (specify) | With plain radiography | Without plain radiography | Other (describe) | ||||||||
|
Frequency of imaging (frequency and duration – e.g. imaging at 1 month, 6 months, 12 months post EVAR) |
||||||||||||||
| Details of the operator, including experience level if reported | ||||||||||||||
| Outcomes | ||||||||||||||
| Primary outcomes reported | ||||||||||||||
| Secondary outcomes reported | ||||||||||||||
| Adverse events reported | ||||||||||||||
| Other information | ||||||||||||||
| Additional information on intervention and comparators | ||||||||||||||
| Data analysis – did the analysis adjust for any confounding factors [if yes, please state the confounding factor(s)]? How was the confounding factor categorised? | ||||||||||||||
| Source of | ||||||||||||||
| Number of participants, n (%) | Total | Intervention | Comparator | |||||||||||
| Enrolled | ||||||||||||||
| Randomised | ||||||||||||||
| Received treatment | ||||||||||||||
| Post-randomisation exclusions | ||||||||||||||
| Discontinued study | ||||||||||||||
| Lost to follow-up | ||||||||||||||
| Analysed | ||||||||||||||
| Reasons for dropouts | ||||||||||||||
| Pre randomisation | ||||||||||||||
| Post randomisation | ||||||||||||||
| Participant baseline characteristics | Intervention | n | N | Comparator | n | N | Total | n | N | |||||
| Age | ||||||||||||||
| Male/female (%) | ||||||||||||||
| Aneurysm type | ||||||||||||||
| Abdominal aortic (AAA) | ||||||||||||||
| Iliac aortic | ||||||||||||||
| Infrarenal | ||||||||||||||
| Ruptured AAA | ||||||||||||||
| Other (specify) | ||||||||||||||
| BMI | ||||||||||||||
| Renal insufficiency | ||||||||||||||
| None | ||||||||||||||
| Mild | ||||||||||||||
| Moderate | ||||||||||||||
| Severe | ||||||||||||||
| End stage | ||||||||||||||
| Hypertension | ||||||||||||||
| Coronary heart disease | ||||||||||||||
| Ischaemic heart disease | ||||||||||||||
| Cerebrovascular disease | ||||||||||||||
| Hyperlipidaemia | ||||||||||||||
| Diabetes | ||||||||||||||
| Smoking | ||||||||||||||
| Aneurysm diameter/sac size (mm) | ||||||||||||||
| Time since EVAR (months) | ||||||||||||||
| Type of endograph | ||||||||||||||
| Insert name of graft | ||||||||||||||
| Insert name of graft | ||||||||||||||
| Insert name of graft | ||||||||||||||
| Insert name of graft | ||||||||||||||
| Additional information | ||||||||||||||
| Clinical outcomes | Time point | Specify measures (mean %, median %, etc.) and variance (SD, range, 95% CI, etc.) | Intervention | Control | Difference between groups (include type of difference) | p-value | Additional information | |||||||
| Values | Variance | n | Values | Variance | n | |||||||||
| Type of reintervention | ||||||||||||||
| Type of secondary intervention | ||||||||||||||
| Mortality | Time point | Specify measures (mean %, median %, etc.) and variance (SD, range, 95% CI, etc.) | Intervention | Control | Difference between groups (include type of difference) | p-value | Additional information | |||||||
| Values | Variance | n | Values | Variance | n | |||||||||
| Adverse events (EVAR related) | Time point | Specify measures (mean %, median %, etc.) and variance (SD, range, 95% CI, etc.) | Intervention | Control | Difference between groups (include type of difference) | p-value | Additional information | |||||||
| Value | Variance | n | Values | Variance | n | |||||||||
| Type I endoleak – attachment site | ||||||||||||||
| A (proximal) | ||||||||||||||
| B (distal) | ||||||||||||||
| C (iliac occluder) | ||||||||||||||
| Type II endoleak – branch leak | ||||||||||||||
| A (simple – one branch) | ||||||||||||||
| B (complex – two or more branches) | ||||||||||||||
| Type III endoleak – graft defect | ||||||||||||||
| A (junctional leak or modular defect) | ||||||||||||||
| B (fabric disruption/graft hole) | ||||||||||||||
| Type IV endoleak – fabric porosity within 30 days | ||||||||||||||
| Type V endoleak – endotension | ||||||||||||||
| A (no endoleak) | ||||||||||||||
| B (sealed endoleak) | ||||||||||||||
| C (type I or 3 endoleak discovered at the time of open redo surgery) | ||||||||||||||
| D (type II endoleak discovered at the time of open redo surgery) | ||||||||||||||
| Graft migration | ||||||||||||||
| Graft kinking | ||||||||||||||
| Graft stenting | ||||||||||||||
| Limb outflow impairment | ||||||||||||||
| Limb occlusion | ||||||||||||||
| Aneurysm rupture | ||||||||||||||
| Aneurysm diameter/sac size | ||||||||||||||
Appendix 3 Review Body for Interventional Procedures tool for assessing the quality of non-randomised studies
Checklist for the quality assessment of non-randomised studies (comparative and cohort studies)
Items specific to comparative studies are in italic.
Assessor initial:
Date evaluated:
Study ID:
| Criteria | Yes | No | Unclear | Comments |
|---|---|---|---|---|
| 1. Were participants a representative sample selected from a relevant patient population, e.g. randomly selected from those seeking for treatment despite of age, duration of disease, primary or secondary disease and severity of disease? | ||||
| 2. Were the inclusion/exclusion criteria of participants clearly described? | ||||
| 3. Were participants entering the study at a similar point in their disease progression, i.e. severity of disease? | ||||
| 4. Was selection of patients consecutive? | ||||
| 5. Was data collection undertaken prospectively? | ||||
| 6. Were the groups comparable on demographic characteristics and clinical features? | ||||
| 7. Was the intervention (and comparison) clearly defined? | ||||
| 8. Was the intervention undertaken by someone experienced at performing the procedure?a | ||||
| 9. Were the staff, place, and facilities where the patients were treated appropriate for performing the procedure? (e.g. access to back-up facilities in hospital or special clinic) | ||||
| 10. Were any of the important outcomes considered, i.e. on clinical effectiveness, cost-effectiveness, or learning curves? | ||||
| 11. Were objective (valid and reliable) outcome measures used, including satisfaction scale? | ||||
| 12. Was the assessment of main outcomes blind? | ||||
| 13. Was follow-up long enough (≥ 1 year) to detect important effects on outcomes of interest? | ||||
| 14. Was information provided on non-respondents, dropouts?b | ||||
| 15. Were the withdrawals/dropouts having similar characteristics as those completed the study and therefore unlikely to cause bias?c | ||||
| 16. Was length of follow-up similar between comparison groups? | ||||
| 17. Were the important prognostic factors identified, e.g. age, duration of disease, disease severity?d | ||||
| 18. Were the analyses adjusted for confounding factors? |
The same form was adapted to assess the quality of the case series after taking out questions 6, 12, 16 and 18.
Appendix 4 Included primary studies
Clinical effectiveness
Comparative
Chisci E, Setacci F, Iacoponi F, de Donato G, Cappelli A, Setacci C. Surveillance imaging modality does not affect detection rate of asymptomatic secondary interventions following EVAR. Eur J Vascular Endovasc Surg 2012;43:276–81.
Nyheim T, Staxrud LE, Rosen L, Slagsvold CE, Sandbaek G, Jørgensen JJ. Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans? Acta Radiol 2013;54:54–8.
Cohort
Bisdas T, Weiss K, Eisenack M, Austermann M, Torsello G, Donas KP. Durability of the Endurant stent graft in patients undergoing endovascular abdominal aortic aneurysm repair. J Vasc Surg 2014;60:1125–31. https://doi.org/10.1016/j.jvs.2014.04.070
Blom AS, Troutman D, Beeman B, Yarchoan M, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging to detect limb stenosis or kinking of endovascular device. J Vasc Surg 2012;55:1577–80. https://doi.org/10.1016/j.jvs.2011.12.058
Bush RL, Lumsden AB, Dodson TF, Salam AA, Weiss VJ, Smith RB, III, Chaikof EL. Mid-term results after endovascular repair of the abdominal aortic aneurysm. J Vasc Surg 2001;33(Suppl. 2):70–6.
Chaer RA, Gushchin A, Rhee R, Marone L, Cho JS, Leers S, Makaroun MS. Duplex ultrasound as the sole long-term surveillance method post-endovascular aneurysm repair: a safe alternative for stable aneurysms. J Vasc Surg 2009;49:845–9. https://doi.org/10.1016/j.jvs.2008.10.073
Carroccio A, Faries PL, Morrissey NJ, Teodorescu V, Burks JA, Gravereaux EC, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg 2002;36:679–84.
Cochennec F, Becquemin JP, Desgranges P, Allaire E, Kobeiter H, Roudot-Thoraval F. Limb graft occlusion following EVAR: clinical pattern, outcomes and predictive factors of occurrence. Eur J Vasc Endovasc Surg 2007;34:59–65.
Collins JT, Boros MJ, Combs K. Ultrasound surveillance of endovascular aneurysm repair: a safe modality versus computed tomography. Ann Vasc Surg 2007;21:671–5.
Dominguez I, Mehta M, Roddy SP, Clement Darling R, Sternbach Y, Taggert JB, et al. A prospective evaluation of the impact of balloon-expandable palmaz stent placement in aortic neck during EVAR. J Vasc Surg 2010;52:1123.
Donas KP, Torsello GB, Piccoli G, Pitoulias GA, Torsello GF, Bisdas T, et al. The PROTAGORAS study to evaluate the performance of the Endurant stent graft for patients with pararenal pathologic processes treated by the chimney/snorkel endovascular technique. J Vasc Surg 2016;63:1–7. https://doi.org/10.1016/j.jvs.2015.07.080
Fargion A, Masciello F, Melani A, Pratesi G, Pulli R, Dorigo W, Pratesi C. The influence of the timing of onset of type II endoleak on the late outcomes of endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2016;63(Suppl. 1):19S.
Fossaceca R, Guzzardi G, Cerini P, Di Terlizzi M, Malatesta E, Filice L, et al. [Endovascular treatment of abdominal aortic aneurysms: 6 years of experience at a single centre.] Radiol Med 2013;118:616–32. https://doi.org/10.1007/s11547-012-0905-8
Freyrie A, Gallitto E, Gargiulo M, Faggioli G, Bianchini Massoni C, Mascoli C, et al. Results of the endovascular abdominal aortic aneurysm repair using the Anaconda aortic endograft. J Vasc Surg 2014;60:1132–9. https://doi.org/10.1016/j.jvs.2014.04.073
Ghotbi R, Sotiriou A, Mansur R. New results with 100 Excluder cases. J Cardiovasc Surg 2010;51:475–80.
Harrison GJ, Oshin OA, Vallabhaneni SR, Brennan JA, Fisher RK, McWilliams RG. Surveillance after EVAR based on duplex ultrasound and abdominal radiography. Eur J Vasc Endovasc Surg 2011;42:187–92. https://doi.org/10.1016/j.ejvs.2011.03.027
Karthikesalingam A, Kumar S, Anandarajah JJ, Hinchliffe RJ, Poloniecki JD, Thompson MM, Holt PJ. Predictive value of peak systolic velocity for the development of graft limb complications after endovascular aneurysm repair. J Endovasc Ther 2012;19:428–33. https://doi.org/10.1583/11-3739MR.1
Köcher M, Utíkal P, Koutná J, Bachleda P, Buriánková E, Herman M, et al. Endovascular treatment of abdominal aortic aneurysms – 6 years of experience with Ella stent–graft system. Eur J Radiol 2004;51:181–8. https://doi.org/10.1016/S0720-048X(03)00165-7
Kray J, Kirk S, Franko J, Chew DK. Role of type II endoleak in sac regression after endovascular repair of infrarenal abdominal aortic aneurysms. J Vasc Surg 2015;61:869–74. https://doi.org/10.1016/j.jvs.2014.11.003
Mazzaccaro D, Settembrini AM, Malacrida G, Stegher S, Occhiuto MT, Sorba F, et al. Endovascular aneurysm repair: experience of 12 years in a single institution. Interact Cardiovasc Thorac Surg 2011;12:S43.
Meier GH, Parker FM, Godziachvili V, Demasi RJ, Parent FN, Gayle RG. Endotension after endovascular aneurysm repair: the Ancure experience. J Vasc Surg 2001;34:421–6.
Oshin OA, Fisher RK, Williams LA, Brennan JA, Gilling-Smith GL, Vallabhaneni SR, McWilliams RG. Adjunctive iliac stents reduce the risk of stent–graft limb occlusion following endovascular aneurysm repair with the Zenith stent–graft. J Endovasc Ther 2010;17:108–14. https://doi.org/10.1583/09-2854.1
Parlani G, Zannetti S, Verzini F, De Rango P, Carlini G, Lenti M, Cao P. Does the presence of an iliac aneurysm affect outcome of endoluminal AAA repair? An analysis of 336 cases. Eur J Vasc Endovasc Surg 2002;24:134–8.
Schunn CD, Krauss M, Heilberger P, Ritter W, Raithel D. Aortic aneurysm size and graft behavior after endovascular stent–grafting: clinical experiences and observations over 3 years. J Endovasc Ther 2000;7:167–76. https://doi.org/10.1177/152660280000700301
Soler RJ, Bartoli MA, Mancini J, Lerussi G, Thevenin B, Sarlon-Bartoli G, Magnan PE. Aneurysm sac shrinkage after endovascular repair: predictive factors and long-term follow-up. Ann Vasc Surg 2015;29:770–9. https://doi.org/10.1016/j.avsg.2014.12.016
Stella A, Freyrie A, Gargiulo M, Faggioli GL. The advantages of Anaconda endograft for AAA. J Cardiovasc Surg 2009;50:145–52.
Wolf YG, Tillich M, Lee WA, Fogarty TJ, Zarins CK, Rubin GD. Changes in aneurysm volume after endovascular repair of abdominal aortic aneurysm. J Vasc Surg 2002;36:305–9.
Appendix 5 List of excluded studies with rationale
Diagnostic reviews (n = 9)
Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, Powell JT. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther 2005;12:297–305.
Bevis PM, Cooper DG. [Duplex ultrasound for surveillance after endovascular repair of abdominal aortic aneurysm.] Ital J Vasc Endovasc Surg 2012;19:237–43.
Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, et al. EVAR: benefits of CEUS for monitoring stent–graft status. Eur J Radiol 2015;84:1658–65. https://doi.org/10.1016/j.ejrad.2015.07.001
Chung J, Kordzadeh A, Prionidis I, Panayiotopoulos Y, Browne T. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound 2015;18:91–9. https://doi.org/10.1007/s40477-014-0154-x
Howard JM, Ezwawah O, Guiney M, Ryan M, McEniff N. Contrast-enhanced ultrasound versus CT angiography for the detection of endoleak in patients post-EVAR: an evidence based radiology approach. Cardiovasc Intervent Radiol 2011;34:567–8.
Karanikola E, Dalainas I, Karaolanis G, Zografos G, Filis K. Duplex ultrasound versus computed tomography for the postoperative follow-up of endovascular abdominal aortic aneurysm repair. Where do we stand now? Int J Angiol 2014;23:155–64. https://doi.org/10.1055/s-0034-1387925
Karthikesalingam A, Al-Jundi W, Jackson D, Boyle JR, Beard JD, Holt PJ, Thompson MM. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br J Surg 2012;99:1514–23. https://doi.org/10.1002/bjs.8873
Mirza TA, Karthikesalingam A, Jackson D, Walsh SR, Holt PJ, Hayes PD, Boyle JR. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg 2010;39:418–28. https://doi.org/10.1016/j.ejvs.2010.01.001
Sun Z. Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol 2006;17:759–64.
Imaging modalities (n = 179)
Abraham CZ, Chuter TA, Reilly LM, Okuhn SP, Pethan LK, Kerlan RB, et al. Abdominal aortic aneurysm repair with the Zenith stent graft: short to midterm results. J Vasc Surg 2002;36:217–24.
Ahanchi SS, Carroll M, Almaroof B, Panneton JM. Anatomic severity grading score predicts technical difficulty, early outcomes, and hospital resource utilization of endovascular aortic aneurysm repair. J Vasc Surg 2011;54:1266–72. https://doi.org/10.1016/j.jvs.2011.05.019
Albuquerque FC, Tonnessen BH, Noll RE, Cires G, Kim JK, Sternbergh WC. Paradigm shifts in the treatment of abdominal aortic aneurysm: trends in 721 patients between 1996 and 2008. J Vasc Surg 2010;51:1348–52. https://doi.org/10.1016/j.jvs.2010.01.078
Altaf N, Abisi S, Yong Y, Saunders JH, Braithwaite BD, MacSweeney ST. Mid-term results of endovascular aortic aneurysm repair in the young. Eur J Vasc Endovasc Surg 2013;46:315–19. https://doi.org/10.1016/j.ejvs.2013.04.027
Arko FR, Filis KA, Hill BB, Fogarty TJ, Zarins CK. Morphologic changes and outcome following endovascular abdominal aortic aneurysm repair as a function of aneurysm size. Arch Surg 2003;138:651–5. https://doi.org/10.1001/archsurg.138.6.651
Bartoli MA, Thevenin B, Sarlon G, Giorgi R, Albertini JN, Lerussi G, et al. Secondary procedures after infrarenal abdominal aortic aneurysms endovascular repair with second-generation endografts. Ann Vasc Surg 2012;26:166–74. https://doi.org/10.1016/j.avsg.2011.02.047
Becker GJ, Kovacs M, Mathison MN, Katzen BT, Benenati JF, Zemel G, et al. Risk stratification and outcomes of transluminal endografting for abdominal aortic aneurysm: 7-year experience and long-term follow-up. J Vasc Interv Radiol 2001;12:1033–46.
Beckerman WE, Tadros RO, Faries PL, Torres M, Wengerter SP, Vouyouka AG, et al. No major difference in outcomes for endovascular aneurysm repair stent grafts placed outside of instructions for use. J Vasc Surg 2016;64:63–74.e2. https://doi.org/10.1016/j.jvs.2016.01.034
Becquemin JP, Aksoy M, Marzelle J, Roudot-Thoraval F, Desgranges P, Allaire E, Kobeiter H. Abdominal aortic aneurysm sac behavior following Cook Zenith graft implantation: a five-year follow-up assessment of 212 cases. J Cardiovasc Surg 2008;49:199–206.
Biasi L, Ali T, Ratnam LA, Morgan R, Loftus I, Thompson M. Intra-operative DynaCT improves technical success of endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2009;49:288–95. https://doi.org/10.1016/j.jvs.2008.09.013
Biebl M, Hakaim AG, Oldenburg WA, Lau LL, Klocker J, Neuhauser B, et al. Midterm results of a single-center experience with commercially available devices for endovascular aneurysm repair. Mt Sinai J Med 2005;72:127–35.
Blum U, Voshage G, Beyersdorf F, Töllner D, Spillner G, Morgenroth A, et al. Two-center German experience with aortic endografting. J Endovasc Surg 1997;4:137–46.
Bobadilla JL, Suwanabol P, Reeder S, Pozniak M, Tefera G. Clinical utility and safety of noncontrast computed tomography for follow-up after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2010;1:28S–9S.
Bobadilla JL, Suwanabol PA, Reeder SB, Pozniak MA, Bley TA, Tefera G. Clinical implications of non-contrast-enhanced computed tomography for follow-up after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2013;27:1042–8. https://doi.org/10.1016/j.avsg.2012.10.021
Böckler D, Holden A, Thompson M, Hayes P, Krievins D, de Vries JP, Reijnen MM. Multicenter Nellix EndoVascular Aneurysm Sealing system experience in aneurysm sac sealing. J Vasc Surg 2015;62:290–8. https://doi.org/10.1016/j.jvs.2015.03.031
Boult M, Babidge W, Maddern G, Fitridge R, Audit Reference Group. Endoluminal repair of abdominal aortic aneurysm-contemporary Australian experience. Eur J Vasc Endovasc Surg 2004;28:36–40. https://doi.org/10.1016/j.ejvs.2004.03.025
Brown LC, Greenhalgh RM, Powell JT, Thompson SG, EVAR Trial Participants. Use of baseline factors to predict complications and reinterventions after endovascular repair of abdominal aortic aneurysm. Br J Surg 2010;97:1207–17. https://doi.org/10.1002/bjs.7104
Burks JA, Faries PL, Gravereaux EC, Hollier LH, Marin ML. Endovascular repair of abdominal aortic aneurysms: stent–graft fixation across the visceral arteries. J Vasc Surg 2002;35:109–13.
Buth J, Harris PL, Van Marrewijk C, Fransen G. Endoleaks during follow-up after endovascular repair of abdominal aortic aneurysm. Are they all dangerous? J Cardiovasc Surg 2003;44:559–66.
Buth J, Harris PL, van Marrewijk C, Fransen G. The significance and management of different types of endoleaks. Semin Vasc Surg 2003;16:95–102.
Millen A, Canavati R, Harrison G, McWilliams RG, Wallace S, Vallabhaneni SR, Fisher RK. Defining a role for contrast-enhanced ultrasound in EVAR surveillance. J Vasc Surg 2013;58:18–23.
Canavati RN, Harrison G, McWilliams RG, Hargreaves S, Wallace S, Harrison R, et al. Defining a role for contrast-enhanced US in endovascular aneurysm repair surveillance. Cardiovasc Intervent Radiol 2012;35:S221.
Candell L, Tucker LY, Goodney P, Walker J, Okuhn S, Hill B, Chang R. Early and delayed rupture after endovascular abdominal aortic aneurysm repair in a 10-year multicenter registry. J Vasc Surg 2014;60:1146–52. https://doi.org/10.1016/j.jvs.2014.05.046
Cao P, De Rango P, Parlani G, Verzini F, Talent Unidoc Retrospective Italian Study (TAURIS) Group. Durability of abdominal aortic endograft with the Talent Unidoc stent graft in common practice: core lab reanalysis from the TAURIS multicenter study. J Vasc Surg 2009;49:859–65. https://doi.org/10.1016/j.jvs.2008.11.044
Carpenter JP, Endologix Investigators. Multicenter trial of the PowerLink bifurcated system for endovascular aortic aneurysm repair. J Vasc Surg 2002;36:1129–37. https://doi.org/10.1067/mva.2002.129641
Carpenter JP, Anderson WN, Brewster DC, Kwolek C, Makaroun M, Martin J, et al. Multicenter pivotal trial results of the Lifepath System for endovascular aortic aneurysm repair. J Vasc Surg 2004;39:34–43. https://doi.org/10.1016/j.jvs.2003.10.036
Carpenter JP, Endologix Investigators. Midterm results of the multicenter trial of the powerlink bifurcated system for endovascular aortic aneurysm repair. J Vasc Surg 2004;40:849–59.
Carpenter JP. The Powerlink bifurcated system for endovascular aortic aneurysm repair: four-year results of the US multicenter trial. J Cardiovasc Surg 2006;47:239–43.
Carpenter JP. Midterm results of the Powerlink suprarenal bifurcated device pivotal trial. J Vasc Surg 2009;5:S35.
Chuter TA, Faruqi RM, Sawhney R, Reilly LM, Kerlan RB, Canto CJ, et al. Endoleak after endovascular repair of abdominal aortic aneurysm. J Vasc Surg 2001;34:98–105.
Concepcion Rodriguez NA, Riera Del Moral LF, Fernandez Heredero A, Salazar Alvarez A, Cuervo Vidal L, Riera De Cubas L. [Outcome of type II endoleaks after endovascular infrarenal aortoiliac aneurysms repair.] Angiologia 2015;67:167–73.
Couchet G, Pereira B, Carrieres C, Maumias T, Ribal JP, Ben Ahmed S, Rosset E. Predictive factors for type II endoleaks after treatment of abdominal aortic aneurysm by conventional endovascular aneurysm repair. Ann Vasc Surg 2015;29:1673–9. https://doi.org/10.1016/j.avsg.2015.07.007
Dalainas I, Avgerinos E, Klonaris C, Verikokos C, Papapetrou A, Papasideris C, et al. Different patients but same results: mid-term comparison of endovascular repair of abdominal aortic aneurysms with Excluder and Zenith. Interact Cardiovasc Thorac Surg 2009;8:S74.
de Donato G, Setacci F, Bresadola L, Castelli P, Chiesa R, Mangialardi N, et al. Aortic neck evolution after endovascular repair with TriVascular Ovation stent graft. J Vasc Surg 2016;63:8–15. https://doi.org/10.1016/j.jvs.2015.07.099
Deglise S, Qanadli SD, Rizzo E, Ducrey N, Doenz F, Haller C, et al. Long-term follow-up of surgically excluded popliteal artery aneurysms with multi-slice CT angiography and Doppler ultrasound. Eur Radiol 2006;16:1323–30. https://doi.org/10.1007/s00330-005-0034-z
Dias NV, Riva L, Ivancev K, Resch T, Sonesson B, Malina M. Is there a benefit of frequent CT follow-up after EVAR? Eur J Vasc Endovasc Surg 2009;37:425–30. https://doi.org/10.1016/j.ejvs.2008.12.019
Donayre CE, Othman F, Kopchok GE, Khoynezhad A, White RA. Determinants of abdominal aortic aneurysm sac enlargement after endovascular aneurysm repair with a long-term follow-up to 15 years. J Vasc Surg 2012;56:579.
England A, Butterfield JS, McCollum CN, Ashleigh RJ. Endovascular aortic aneurysm repair with the talent stent–graft: outcomes in patients with large iliac arteries. Cardiovasc Intervent Radiol 2008;31:723–7. https://doi.org/10.1007/s00270-008-9318-4
Erben Y, Kalra M, Ricotta JJ, McKusick MA, Bower TC, Oderich GS, et al. Long-term persistent type 2 endoleak following endovascular abdominal aortic aneurysm. J Vasc Surg 2011;54:1543.
Espinosa G, Ribeiro Alves M, Ferreira Caramalho M, Dzieciuchowicz L, Santos SR. A 10-year single-center prospective study of endovascular abdominal aortic aneurysm repair with the Talent stent–graft. J Endovasc Ther 2009;16:125–35. https://doi.org/10.1583/08-2686.1
Espinosa G, Ribeiro M, Riguetti C, Caramalho MF, Mendes WD, Santos SR. Six-year experience with talent stent–graft repair of abdominal aortic aneurysms. J Endovasc Ther 2005;12:35–45.
Faries PL, Brener BJ, Connelly TL, Katzen BT, Briggs VL, Burks JA Jr, et al. A multicenter experience with the Talent endovascular graft for the treatment of abdominal aortic aneurysms. J Vasc Surg 2002;35:1123–8.
Fernandez JD, Craig JM, Garrett HE, Burgar SR, Bush AJ. Endovascular management of iliac rupture during endovascular aneurysm repair. J Vasc Surg 2009;50:1293–9. https://doi.org/10.1016/j.jvs.2009.06.020
Fillinger M, Excluder Bifurcated Endoprosthesis Clinical Investigators. Three-dimensional analysis of enlarging aneurysms after endovascular abdominal aortic aneurysm repair in the Gore Excluder Pivotal clinical trial. J Vasc Surg 2006;43:888–95.
Forbes TL, Harris JR, Lawlor DK, Derose G. Midterm results of the Zenith endograft in relation to neck length. Ann Vasc Surg 2010;24:859–62. https://doi.org/10.1016/j.avsg.2010.05.012
Frego M, Lumachi F, Bianchera G, Pilon F, Scarpa M, Ruffolo C, et al. Risk factors of endoleak following endovascular repair of abdominal aortic aneurysm. A multicentric retrospective study. In Vivo 2007;21:1099–102.
Fulton JJ, Farber MA, Sanchez LA, Godshall CJ, Marston WA, Mendes R, et al. Effect of challenging neck anatomy on mid-term migration rates in AneuRx endografts. J Vasc Surg 2006;44:932–7.
Garg T, Baker LC, Mell MW. Adherence to postoperative surveillance guidelines after endovascular aortic aneurysm repair among Medicare beneficiaries. J Vasc Surg 2015;61:23–7. https://doi.org/10.1016/j.jvs.2014.07.003
Gargiulo M, Gallitto E, Serra C, Freyrie A, Mascoli C, Bianchini Massoni C, et al. Could four-dimensional contrast-enhanced ultrasound replace computed tomography angiography during follow up of fenestrated endografts? Results of a preliminary experience. Eur J Vasc Endovasc Surg 2014;48:536–42. https://doi.org/10.1016/j.ejvs.2014.05.025
Go MR, Barbato JE, Rhee RY, Makaroun MS. What is the clinical utility of a 6-month computed tomography in the follow-up of endovascular aneurysm repair patients? J Vasc Surg 2008;47:1181–6.
Godfrey AD, Morbi AH, Nordon IM. Patient compliance with surveillance following elective endovascular aneurysm repair. Cardiovasc Intervent Radiol 2015;38:1130–6. https://doi.org/10.1007/s00270-015-1073-8
Golledge J, Parr A, Boult M, Maddern G, Fitridge R. The outcome of endovascular repair of small abdominal aortic aneurysms. Ann Surg 2007;245:326–33. https://doi.org/10.1097/01.sla.0000253965.95368.52
Gonzalez L, Barshes NR, Lu RL, Dougherty K, Krajcer Z, Kougias P. Predictors of infrarenal aortic neck diameter changes after endovascular aneurysm repair (EVAR). J Vasc Surg 2013;1:39S.
Greenberg R, Zenith Investigators. The Zenith AAA endovascular graft for abdominal aortic aneurysms: clinical update. Semin Vasc Surg 2003;16:151–7.
Greenberg RK, Chuter TA, Sternbergh WC, Fearnot NE, Zenith Investigators. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg 2004;39:1209–18. https://doi.org/10.1016/j.jvs.2004.02.032
Hale AL, Twomey K, Ewing JA, Langan EM, Cull DL, Gray BH. Impact of sarcopenia on long-term mortality following endovascular aneurysm repair. Vasc Med 2016;21:217–22. https://doi.org/10.1177/1358863X15624025
Hammond CJ, Shah AH, Snoddon A, Patel JV, Scott DJ. Mortality and rates of secondary intervention after EVAR in an unselected population: influence of simple clinical categories and implications for surveillance. Cardiovasc Intervent Radiol 2016;39:815–23. https://doi.org/10.1007/s00270-016-1303-8
Hao B, Lujan R, Nguyen A, Donayre C, Lee L, Wallot I, et al. Impact of endoluminal treatment on small abdominal aortic aneurysm: aneurysm sac regression and secondary interventions with 5 years of follow-up. Vasc Endovascular Surg 2007;41:294–300.
Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg 2000;32:739–49. https://doi.org/10.1067/mva.2000.109990
Harthun NL, Lau CL. The incidence of pulmonary neoplasms discovered by serial CT scanning following endovascular AAA repair. J Vasc Surg 2010;1:32S.
Harthun NL, Lau CL. The incidence of pulmonary neoplasms discovered by serial computed tomography scanning after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011;53:738–41. https://doi.org/10.1016/j.jvs.2010.09.066
Herdrich BJ, Murphy EH, Wang GJ, Jackson BM, Fairman RM, Woo EY. The fate of untreated concomitant suprarenal aortic aneurysms after endovascular aneurysm repair of infrarenal aortic aneurysms. J Vasc Surg 2013;58:1201–6. https://doi.org/10.1016/j.jvs.2013.05.009
Hinchliffe RJ, Macierewicz JA, Hopkinson BR. Endovascular repair of inflammatory abdominal aortic aneurysms. J Endovasc Ther 2002;9:277–81. https://doi.org/10.1177/152660280200900304
Hiramoto JS, Reilly LM, Schneider DB, Sivamurthy N, Rapp JH, Chuter TA. Long-term outcome and reintervention after endovascular abdominal aortic aneurysm repair using the Zenith stent graft. J Vasc Surg 2007;45:461–5.
Hobo R, Buth J, EUROSTAR Collaborators. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg 2006;43:896–902.
Hofer S, Heller G, Derungs U, Knusel P, Furrer M. Patterns of rupture of abdominal aneurysms after endovascular aortic repair (EVAR). Vasa – European Journal of Vascular Medicine 2015:16;44(Suppl. 89):7
Hong C, Heiken JP, Sicard GA, Pilgram TK, Bae KT. Clinical significance of endoleak detected on follow-up CT after endovascular repair of abdominal aortic aneurysm. AJR Am J Roentgenol 2008;191:808–13. https://doi.org/10.2214/AJR.07.3668
Holt PJ, Karthikesalingam A, Patterson BO, Ghatwary T, Hinchliffe RJ, Loftus IM, Thompson MM. Aortic rupture and sac expansion after endovascular repair of abdominal aortic aneurysm. Br J Surg 2012;99:1657–64. https://doi.org/10.1002/bjs.8938
Hosaka A, Kato M, Motoki M, Sugai H, Okubo N. Quantified aortic luminal irregularity as a predictor of complications and prognosis after endovascular aneurysm repair. Medicine 2016;95:e2863. https://doi.org/10.1097/MD.0000000000002863
Hossain S, Steinmetz OK, Corriveau MM, MacKenzie KS. Patency of the contralateral internal iliac artery in aortouni-iliac endografting. J Vasc Surg 2016;63:974–82. https://doi.org/10.1016/j.jvs.2015.10.056
Hye RJ, Inui TS, Anthony FF, Kiley ML, Chang RW, Rehring TF, et al. A multiregional registry experience using an electronic medical record to optimize data capture for longitudinal outcomes in endovascular abdominal aortic aneurysm repair. J Vasc Surg 2015;61:1160–6. https://doi.org/10.1016/j.jvs.2014.12.055
Iwakoshi S, Ichihashi S, Itoh H, Sakaguchi S, Kichikawa K. Long-term outcome after endovascular abdominal aortic aneurysm repair using the Cook Zenith endograft in the Japanese population. Cardiovasc Intervent Radiol 2013;36:S255.
Jones JE, Atkins MD, Brewster DC, Chung TK, Kwolek CJ, LaMuraglia GM, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg 2007;46:1–8.
Jones WB, Taylor SM, Kalbaugh CA, Joels CS, Blackhurst DW, Langan EM, et al. Lost to follow-up: a potential under-appreciated limitation of endovascular aneurysm repair. J Vasc Surg 2007;46:434–40.
Kaladji A, Cardon A, Abouliatim I, Campillo-Gimenez B, Heautot JF, Verhoye JP. Preoperative predictive factors of aneurysmal regression using the reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2012;55:1287–95. https://doi.org/10.1016/j.jvs.2011.11.122
Kalliafas S, Albertini JN, Macierewicz J, Yusuf SW, Whitaker SC, Davidson I, Hopkinson BR. Stent–graft migration after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 2002;9:743–7. https://doi.org/10.1177/152660280200900605
Karthikesalingam A, Attallah O, Ma X, Bahia SS, Thompson L, Vidal-Diez A, et al. An artificial neural network stratifies the risks of reintervention and mortality after endovascular aneurysm repair; a retrospective observational study. PLOS ONE 2015;10:e0129024. https://doi.org/10.1371/journal.pone.0129024
Karthikesalingam A, Vidal-Diez A, De Bruin JL, Thompson MM, Hinchliffe RJ, Loftus IM, Holt PJ. International validation of a risk score for complications and reinterventions after endovascular aneurysm repair. Br J Surg 2015;102:509–15. https://doi.org/10.1002/bjs.9758
Kirkwood ML, Saunders A, Jackson BM, Wang GJ, Fairman RM, Woo EY. Aneurysmal iliac arteries do not portend future iliac aneurysmal enlargement after endovascular aneurysm repair for abdominal aortic aneurysm. J Vasc Surg 2011;53:269–73.
Kirkpatrick VE, Wilson SE, Williams RA, Gordon IL. Surveillance computed tomographic arteriogram does not change management before 3 years in patients who have a normal post-EVAR study. Ann Vasc Surg 2014;28:831–6. https://doi.org/10.1016/j.avsg.2013.09.017
Koole D, Moll FL, Buth J, Hobo R, Zandvoort HJ, Bots ML, et al. Annual rupture risk of abdominal aortic aneurysm enlargement without detectable endoleak after endovascular abdominal aortic repair. J Vasc Surg 2011;54:1614–22. https://doi.org/10.1016/j.jvs.2011.06.095
Laheij RJ, Buth J, Harris PL, Moll FL, Stelter WJ, Verhoeven EL. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg 2000;87:1666–73.
Lalka S, Dalsing M, Cikrit D, Sawchuk A, Shafique S, Nachreiner R, Pandurangi K. Secondary interventions after endovascular abdominal aortic aneurysm repair. Am J Surg 2005;190:787–94.
Lange C, Aasland JK, Ødegård A, Myhre HO. The durability of EVAR – what are the evidence and implications on follow-up? Scand J Surg 2008;97:205–12. https://doi.org/10.1177/145749690809700227
Leurs LJ, Buth J, Laheij RJ. Long-term results of endovascular abdominal aortic aneurysm treatment with the first generation of commercially available stent grafts. Arch Surg 2007;142:33–41.
Leurs LJ, Harris PL, Buth J, EUROSTAR Collaborators. Secondary interventions after elective endovascular repair of degenerative thoracic aortic aneurysms: results of the European collaborators registry (EUROSTAR). J Vasc Interv Radiol 2007;18:491–5.
Leurs LJ, Hobo R, Buth J, EUROSTAR Collaborators. The multicenter experience with a third-generation endovascular device for abdominal aortic aneurysm repair. A report from the EUROSTAR database. J Cardiovasc Surg 2004;45:293–300.
Leurs LJ, Kievit J, Dagnelie PC, Nelemans PJ, Buth J, EUROSTAR Collaborators. Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2006;13:640–8.
Lifeline Registry of Endovascular Aneurysm Repair Steering Committee. Lifeline Registry of Endovascular Aneurysm Repair: registry data report. J Vasc Surg 2002;35:616–20.
Lifeline Registry of EVAR Publications Committee. Lifeline Registry of Endovascular Aneurysm Repair: long-term primary outcome measures. J Vasc Surg 2005;42:1–10.
Loewenthal D, Herzog L, Bulla K, Rogits B, Halloul Z, Pech M, et al. Can early computed tomography after endoluminal stent–graft repair predict the need for reintervention in patients with type II endoleak? Cardiovasc Intervent Radiol 2013;36:S231.
Lomazzi C, Mariscalco G, Piffaretti G, Bacuzzi A, Tozzi M, Carrafiello G, Castelli P. Endovascular treatment of elective abdominal aortic aneurysms: independent predictors of early and late mortality. Ann Vasc Surg 2011;25:299–305. https://doi.org/10.1016/j.avsg.2010.08.001
Lundbom J, Hatlinghus S, Wirsching J, Amundsen S, Staxrud LE, GjŁlberg T, et al. Endovascular treatment of abdominal aortic aneurysms in Norway: the first 100 patients. Eur J Vasc Endovasc Surg 1999;18:506–9. https://doi.org/10.1053/ejvs.1999.0944
Majumder B, Urquhart G, Edwards R, Irshad K, Velu R, Reid DB. Early clinical experience with the Anaconda re-deployable endograft in 106 patients with abdominal aortic aneurism: the west of Scotland Anaconda registry. Scott Med J 2012;57:61–5. https://doi.org/10.1258/smj.2012.012001
Makaroun MS, Tuchek M, Massop D, Henretta J, Rhee R, Buckley C, et al. One year outcomes of the United States regulatory trial of the Endurant stent graft system. J Vasc Surg 2011;54:601–8. https://doi.org/10.1016/j.jvs.2011.03.002
Malas MB, Jordan WD, Cooper MA, Qazi U, Beck AW, Belkin M, et al. Performance of the Aorfix endograft in severely angulated proximal necks in the PYTHAGORAS United States clinical trial. J Vasc Surg 2015;62:1108–17. https://doi.org/10.1016/j.jvs.2015.05.042
Maleux G, Koolen M, Heye S, Heremans B, Nevelsteen A. Mural thrombotic deposits in abdominal aortic endografts are common and do not require additional treatment at short-term and midterm follow-up. J Vasc Interv Radiol 2008;19:1558–62.
Maleux G, Claes H, Van Holsbeeck A, Janssen R, Laenen A, Heye S, et al. Ten years of experience with the Gore Excluder® stent–graft for the treatment of aortic and iliac aneurysms: outcomes from a single center study. Cardiovasc Intervent Radiol 2012;35:498–507. https://doi.org/10.1007/s00270-011-0235-6
Marchiori A, von Ristow A, Guimaraes M, Schönholz C, Uflacker R. Predictive factors for the development of type II endoleaks. J Endovasc Ther 2011;18:299–305. https://doi.org/10.1583/10-3116.1
Maudet A, Daoudal A, Cardon A, Clochard E, Lucas A, Verhoye JP, Kaladji A. Endovascular treatment of infrarenal aneurysms: comparison of the results of second- and third-generation stent grafts. Ann Vasc Surg 2016;34:95–105. https://doi.org/10.1016/j.avsg.2015.12.020
May J, White GH, Harris JP. Endoluminal repair of abdominal aortic aneurysms – state of the art. Eur J Radiol 2001;39:16–21.
May J, White GH, Yu W, Waugh R, Stephen MS, Arulchelvam M, Harris JP. Importance of graft configuration in outcome of endoluminal aortic aneurysm repair: a 5-year analysis by the life table method. Eur J Vasc Endovasc Surg 1998;15:406–11.
May J, White GH, Yu W, Waugh R, Stephen MS, Sieunarine K, et al. Endoluminal repair of abdominal aortic aneurysms: strengths and weaknesses of various prostheses observed in a 4.5-year experience. J Endovasc Surg 1997;4:147–51. https://doi.org/10.1583/1074-6218(1997)004<0147:EROAAA>2.0.CO;2
Mayer D, Pfammatter T, Rancic Z, Hechelhammer L, Wilhelm M, Veith FJ, Lachat M. 10 years of emergency endovascular aneurysm repair for ruptured abdominal aortoiliac aneurysms: lessons learned. Ann Surg 2009;249:510–15. https://doi.org/10.1097/SLA.0b013e31819a8b65
McDonnell CO, Semmens JB, Allen YB, Jansen SJ, Brooks DM, Lawrence-Brown MM. Large iliac arteries: a high-risk group for endovascular aortic aneurysm repair. J Endovasc Ther 2007;14:625–9.
Mehta M, Valdés FE, Nolte T, Mishkel GJ, Jordan WD, Gray B, et al. One-year outcomes from an international study of the Ovation Abdominal Stent Graft System for endovascular aneurysm repair. J Vasc Surg 2014;59:65–73.e1–3. https://doi.org/10.1016/j.jvs.2013.06.065
Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PSK, et al. Midterm outcomes of secondary procedures following endovascular aneurysm repair: a prospective analysis. J Vasc Surg 2009;50:1538.
Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PS, et al. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg 2010;52:1442–9. https://doi.org/10.1016/j.jvs.2010.06.110
Mehta M, Henretta J, Glickman M, Deaton D, Naslund TC, Gray B, et al. Outcome of the pivotal study of the Aptus endovascular abdominal aortic aneurysms repair system. J Vasc Surg 2014;60:275–85. https://doi.org/10.1016/j.jvs.2014.02.017
Melissano G, Bertoglio L, Esposito G, Civilini E, Setacci F, Chiesa R. Midterm clinical success and behavior of the aneurysm sac after endovascular AAA repair with the Excluder graft. J Vasc Surg 2005;42:1052–7.
Mennander A, Pimenoff G, Heikkinen M, Partio T, Zeitlin R, Salenius JP. Nonoperative approach to endotension. J Vasc Surg 2005;42:194–9.
Mertens J, Houthoofd S, Daenens K, Fourneau I, Maleux G, Lerut P, Nevelsteen A. Long-term results after endovascular abdominal aortic aneurysm repair using the Cook Zenith endograft. J Vasc Surg 2011;54:48–57.e2. https://doi.org/10.1016/j.jvs.2010.12.068
Miller A, Marotta M, Scordi-Bello I, Tammaro Y, Marin M, Divino C. Ischemic colitis after endovascular aortoiliac aneurysm repair: a 10-year retrospective study. Arch Surg 2009;144:900–3. https://doi.org/10.1001/archsurg.2009.70
Müller-Wille R, Güntner O, Zeman F, Dollinger M, Hälg C, Beyer LP, et al. The influence of preoperative aneurysmal thrombus quantity and distribution on the development of type II endoleaks with aneurysm sac enlargement after EVAR of AAA. Cardiovasc Intervent Radiol 2016;39:1099–109. https://doi.org/10.1007/s00270-016-1386-2
Nakai M, Ikoma A, Sato H, Sato M, Nishimura Y, Okamura Y. Risk factors associated with late aneurysmal sac expansion after endovascular abdominal aortic aneurysm repair. Diagn Interv Radiol 2015;21:195–201. https://doi.org/10.5152/dir.2014.14308
Nakai M, Sato M, Sato H, Sakaguchi H, Tanaka F, Ikoma A, et al. Midterm results of endovascular abdominal aortic aneurysm repair: comparison of instruction-for-use (IFU) cases and non-IFU cases. Jpn J Radiol 2013;31:585–92. https://doi.org/10.1007/s11604-013-0223-7
Oberhuber A, Schwarz A, Hoffmann MH, Klass O, Orend KH, Mühling B. Influence of different self-expanding stent–graft types on remodeling of the aortic neck after endovascular aneurysm repair. J Endovasc Ther 2010;17:677–84. https://doi.org/10.1583/10-3172.1
Ohki T, Veith FJ, Shaw P, Lipsitz E, Suggs WD, Wain RA, et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms: a note of caution based on a 9-year experience. Ann Surg 2001;234:323–34.
Ohrlander T, Dencker M, Acosta S. Morphological state as a predictor for reintervention and mortality after EVAR for AAA. Cardiovasc Intervent Radiol 2012;35:1009–15. https://doi.org/10.1007/s00270-011-0229-4
Oranen BI, Bos WT, Verhoeven EL, Tielliu IF, Zeebregts CJ, Prins TR, van den Dungen JJAM. Is emergency endovascular aneurysm repair associated with higher secondary intervention risk at mid-term follow-up? J Vasc Surg 2006;44:1156–61.
Park B, Danes S, Drezner AD, Gallagher J, Allmendinger P, Lowe R, et al. Endovascular abdominal aortic aneurysm repair at hartford hospital: a six year experience. Conn Med 2006;70:357–62.
Parlani G, Verzini F, De Rango P, Brambilla D, Coscarella C, Ferrer C, Cao P. Long-term results of iliac aneurysm repair with iliac branched endograft: a 5-year experience on 100 consecutive cases. Eur J Vasc Endovasc Surg 2012;43:287–92. https://doi.org/10.1016/j.ejvs.2011.12.011
Patel MS, Carpenter JP. The value of the initial post-EVAR computed tomography angiography scan in predicting future secondary procedures using the Powerlink stent graft. J Vasc Surg 2010;52:1135–9. https://doi.org/10.1016/j.jvs.2010.06.019
Peppelenbosch N, Buth J, Harris PL, van Marrewijk C, Fransen G, EUROSTAR Collaborators. Diameter of abdominal aortic aneurysm and outcome of endovascular aneurysm repair: does size matter? A report from EUROSTAR. J Vasc Surg 2004;39:288–97. https://doi.org/10.1016/j.jvs.2003.09.047
Pippin K, Hill J, He J, Johnson P. Outcomes of type II endoleaks after endovascular abdominal aortic aneurysm (AAA) repair: a single-center, retrospective study. Clin Imaging 2016;40:875–9. https://doi.org/10.1016/j.clinimag.2016.04.004
Pitoulias GA, Schulte S, Donas KP, Horsch S. Secondary endovascular and conversion procedures for failed endovascular abdominal aortic aneurysm repair: can we still be optimistic? Vascular 2009;17:15–22.
Pitton MB, Scheschkowski T, Ring M, Herber S, Oberholzer K, Leicher-Düber A, et al. Ten-year follow-up of endovascular aneurysm treatment with Talent stent–grafts. Cardiovasc Intervent Radiol 2009;32:906–17. https://doi.org/10.1007/s00270-009-9599-2
Pratesi C, Piffaretti G, Pratesi G, Castelli P, ITalian Excluder Registry Investigators. ITalian Excluder Registry and results of Gore Excluder endograft for the treatment of elective infrarenal abdominal aortic aneurysms. J Vasc Surg 2014;59:52–7.e1. https://doi.org/10.1016/j.jvs.2013.06.067
Ramaiah VG, Westerband A, Thompson C, Ravi R, Rodriguez JA, DiMugno L, et al. The AneuRx stent–graft since FDA approval: single-center experience of 230 cases. J Endovasc Ther 2002;9:464–9. https://doi.org/10.1177/152660280200900413
Resch T, Malina M, Lindblad B, Ivancev K. The evolution of Z stent-based stent–grafts for endovascular aneurysm repair: a life-table analysis of 7.5-year followup. J Am Coll Surg 2002;194(Suppl. 1):74–8.
Rhee RY, Eskandari MK, Zajko AB, Makaroun MS. Long-term fate of the aneurysmal sac after endoluminal exclusion of abdominal aortic aneurysms. J Vasc Surg 2000;32:689–96.
Rydberg J, Lalka S, Johnson M, Cikrit D, Dalsing M, Sawchuk A, Shafique S. Characterization of endoleaks by dynamic computed tomographic angiography. Am J Surg 2004;188:538–43.
Sampram ES, Karafa MT, Mascha EJ, Clair DG, Greenberg RK, Lyden SP, et al. Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm. J Vasc Surg 2003;37:930–7. https://doi.org/10.1067/mva.2003.281
Schanzer A, Greenberg RK, Hevelone N, Robinson WP, Eslami MH, Goldberg RJ, Messina L. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011;123:2848–55. https://doi.org/10.1161/CIRCULATIONAHA.110.014902
Shrivastava V, Mahmood A, Weir G, Clarke M, Wilson L, Williams R, et al. 10 years of Zenith: a single centre experience. Cardiovasc Intervent Radiol 2009;32:284–5.
Sivamurthy N, Schneider DB, Reilly LM, Rapp JH, Skovobogatyy H, Chuter TA. Adjunctive primary stenting of Zenith endograft limbs during endovascular abdominal aortic aneurysm repair: implications for limb patency. J Vasc Surg 2006;43:662–70.
Skibba AA, Evans JR, Greenfield DT, Yoon HR, Katras T, Ouriel K, Rush DS. Management of late main-body aortic endograft component uncoupling and type IIIa endoleak encountered with the Endologix Powerlink and AFX platforms. J Vasc Surg 2015;62:868–75. https://doi.org/10.1016/j.jvs.2015.04.454
Sobocinski J, Maurel B, Delsart P, d’Elia P, Guillou M, Maioli F, et al. Should we modify our indications after the EVAR-2 trial conclusions? Ann Vasc Surg 2011;25:590–7. https://doi.org/10.1016/j.avsg.2010.08.010
Solonynko B, Gałązka Z, Jakimowicz T, Szmidt J. Influence of atheromatous lesions in the ilio-femoral segment on the occurrence of stentgraft thrombosis after endovascular treatment of an abdominal aortic aneurysm. Pol Przegl Chir 2012;84:551–9. https://doi.org/10.2478/v10035-012-0092-2
Sternbergh WC, Greenberg RK, Chuter TA, Tonnessen BH, Zenith Investigators. Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg 2008;48:278–84. https://doi.org/10.1016/j.jvs.2008.02.075
Sternbergh WC, Conners MS, Tonnessen BH, Carter G, Money SR. Aortic aneurysm sac shrinkage after endovascular repair is device-dependent: a comparison of Zenith and AneuRx endografts. Ann Vasc Surg 2003;17:49–53. https://doi.org/10.1007/s10016-001-0334-y
Stolzmann P, Frauenfelder T, Pfammatter T, Peter N, Scheffel H, Lachat M, et al. Endoleaks after endovascular abdominal aortic aneurysm repair: detection with dual-energy dual-source CT. Radiology 2008;249:682–91. https://doi.org/10.1148/radiol.2483080193
Strajina V, Oderich GS, Fatima J, Gloviczki P, Duncan AA, Kalra M, et al. Endovascular aortic aneurysm repair in patients with narrow aortas using bifurcated stent grafts is safe and effective. J Vasc Surg 2015;62:1140–7.e1. https://doi.org/10.1016/j.jvs.2015.07.050
Sun A, Tian X, Zhang N, Xu Z, Deng X, Liu M, Liu X. Does lower limb exercise worsen renal artery hemodynamics in patients with abdominal aortic aneurysm? PLOS ONE 2015;10:e0125121
Szmidt J, Galazka Z, Rowinski O, Nazarewski S, Jakimowicz T, Pietrasik K, et al. Late aneurysm rupture after endovascular abdominal aneurysm repair. Interact Cardiovasc Thorac Surg 2007;6:490–4.
Tanski W, III, Fillinger M. Outcomes of original and low-permeability Gore Excluder endoprosthesis for endovascular abdominal aortic aneurysm repair. J Vasc Surg 2007;45:243–9.
Taudorf M, Jensen LP, Vogt KC, Grønvall J, Schroeder TV, Lönn L. Endograft limb occlusion in EVAR: iliac tortuosity quantified by three different indices on the basis of preoperative CTA. Eur J Vasc Endovasc Surg 2014;48:527–33. https://doi.org/10.1016/j.ejvs.2014.04.018
Timaran CH, Lipsitz EC, Veith FJ, Chuter T, Greenberg RK, Ohki T, et al. Endovascular aortic aneurysm repair with the Zenith endograft in patients with ectatic iliac arteries. Ann Vasc Surg 2005;19:161–6. https://doi.org/10.1007/s10016-004-0157-8
Ting AC, Cheng SW, Ho P, Chan YC, Poon JT, Yiu WK, Cheung GC. Endovascular repair for abdominal aortic aneurysms: the first hundred cases. Hong Kong Med J 2008;14:361–6.
Torsello G, Osada N, Florek HJ, Horsch S, Kortmann H, Luska G, et al. Long-term outcome after Talent endograft implantation for aneurysms of the abdominal aorta: a multicenter retrospective study. J Vasc Surg 2006;43:277–84.
Tran NT, Garland BT, Quiroga E, Starnes B, Hatsukami T. Endoleaks after endovascular repair of ruptured abdominal aortic aneurysm: should they be treated? J Vasc Surg 2012;56:579.
Traul D, Street D, Faught W, Eaton M, Castillo J, Brawner J, Varner L. Endoluminal stent–graft placement for repair of abdominal aortic aneurysms in the community setting. J Endovasc Ther 2008;15:688–94. https://doi.org/10.1583/07-2206.1
Tsang JS, Naughton PA, Wang TT, Keeling AN, Moneley DS, Lee MJ, et al. Endovascular repair of para-anastomotic aortoiliac aneurysms. Cardiovasc Intervent Radiol 2009;32:1165–70. https://doi.org/10.1007/s00270-009-9653-0
Tsilimparis N, Dayama A, Ricotta JJ. Remodeling of aortic aneurysm and aortic neck on follow-up after endovascular repair with suprarenal fixation. J Vasc Surg 2015;61:28–34. https://doi.org/10.1016/j.jvs.2014.06.104
Umscheid T, Stelter WJ. Time-related alterations in shape, position, and structure of self-expanding, modular aortic stent–grafts: a 4-year single-center follow-up. J Endovasc Surg 1999;6:17–32. https://doi.org/10.1583/1074-6218(1999)006<0017:TRAISP>2.0.CO;2
Vallabhaneni SR, Harris PL. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aortic aneurysm repair. Eur J Radiol 2001;39:34–41.
van Herwaarden JA, van de Pavoordt ED, Waasdorp EJ, Albert Vos J, Overtoom TT, Kelder JC, et al. Long-term single-center results with AneuRx endografts for endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2007;14:307–17.
van Keulen JW, de Vries JP, Dekker H, Gonçalves FB, Moll FL, Verhagen HJ, van Herwaarden JA. One-year multicenter results of 100 abdominal aortic aneurysm patients treated with the Endurant stent graft. J Vasc Surg 2011;54:609–15. https://doi.org/10.1016/j.jvs.2011.02.053
van Lammeren GW, Fioole B, Waasdorp EJ, Moll FL, van Herwaarden JA, de Vries JP. Long-term follow-up of secondary interventions after endovascular aneurysm repair with the AneuRx endoprosthesis: a single-center experience. J Endovasc Ther 2010;17:408–15. https://doi.org/10.1583/10-3086.1
van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J, EUROSTAR Collaborators. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg 2004;27:128–37. https://doi.org/10.1016/j.ejvs.2003.10.016
Varetto G, Quaglino S, Benintende E, Castagno C, Garneri P, Bertoldo U, Rispoli P. [Treatment and follow-up of type II endoleak: a four-year experience on 129 patients.] Ital J Vasc Endovasc Surg 2014;21:205–8.
Verzini F, Parlani G, De Rango P, Cieri E, Cao P. EVAR for adverse proximal aortic necks: comparison of midterm CT findings based on core laboratory reanalysis. J Endovasc Ther 2010;17:Ie30-Ie1.
Waduud MA, Choong WL, Ritchie M, Williams C, Yadavali R, Lim S, et al. Endovascular aneurysm repair: is imaging surveillance robust, and does it influence long-term mortality? Cardiovasc Intervent Radiol 2015;38:33–9.
Walker J, Tucker LY, Goodney P, Candell L, Hua H, Okuhn S, et al. Type II endoleak with or without intervention after endovascular aortic aneurysm repair does not change aneurysm-related outcomes despite sac growth. J Vasc Surg 2015;62:551–61.
Wang GJ, Carpenter JP, Endologix Investigators. The Powerlink system for endovascular abdominal aortic aneurysm repair: six-year results. J Vasc Surg 2008;48:535–45. https://doi.org/10.1016/j.jvs.2008.04.031
Ward TJ, Cohen S, Patel RS, Kim E, Fischman AM, Nowakowski FS, et al. Anatomic risk factors for type-2 endoleak following EVAR: a retrospective review of preoperative CT angiography in 326 patients. Cardiovasc Intervent Radiol 2014;37:324–8. https://doi.org/10.1007/s00270-013-0646-7
Wijffels CJ, Van Lammeren GW, Waasdorp EJ, Wille J, Werson DA, Van Den Heuvel DA, De Vries JP. Results of reinterventions for failed endovascular aortic repair: a single-center experience. J Cardiovasc Surg 2014;55:593–600.
Wong S, Greenberg RK, Brown CR, Mastracci TM, Bena J, Eagleton MJ. Endovascular repair of aortoiliac aneurysmal disease with the helical iliac bifurcation device and the bifurcated-bifurcated iliac bifurcation device. J Vasc Surg 2013;58:861–9. https://doi.org/10.1016/j.jvs.2013.02.033
Wu Z, Xu L, Qu L, Raithel D. Seventeen years’ experience of late open surgical conversion after failed endovascular abdominal aortic aneurysm repair with 13 variant devices. Cardiovasc Intervent Radiol 2015;38:53–9. https://doi.org/10.1007/s00270-014-0909-y
Wu CY, Chen H, Gallagher KA, Eliason JL, Rectenwald JE, Coleman DM. Predictors of compliance with surveillance after endovascular aneurysm repair and comparative survival outcomes. J Vasc Surg 2015;62:27–35. https://doi.org/10.1016/j.jvs.2015.02.023
Wyss TR, Dick F, Brown LC, Greenhalgh RM. The influence of thrombus, calcification, angulation, and tortuosity of attachment sites on the time to the first graft-related complication after endovascular aneurysm repair. J Vasc Surg 2011;54:965–71. https://doi.org/10.1016/j.jvs.2011.04.007
Zarins CK, Bloch DA, Crabtree T, Matsumoto AH, White RA, Fogarty TJ. Stent graft migration after endovascular aneurysm repair: importance of proximal fixation. J Vasc Surg 2003;38:1264–72. https://doi.org/10.1016/S0741
Zarins CK, Bloch DA, Crabtree T, Matsumoto AH, White RA, Fogarty TJ. Aneurysm enlargement following endovascular aneurysm repair: AneuRx clinical trial. J Vasc Surg 2004;39:109–17. https://doi.org/10.1016/j.jvs.2003.08.002
Zarins CK, Shaver DM, Arko FR, Schubart PJ, Lengle SJ, Dixon SM. Introduction of endovascular aneurysm repair into community practice: initial results with a new Food and Drug Administration-approved device. J Vasc Surg 2002;36:226–32.
Zarins CK, White RA, Hodgson KJ, Schwarten D, Fogarty TJ.. Endoleak as a predictor of outcome after endovascular aneurysm repair: AneuRx multicenter clinical trial. J Vasc Surg 2000;32:90–107.
Zarins CK, White RA, Moll FL, Crabtree T, Bloch DA, Hodgson KJ, et al. The AneuRx stent graft: four-year results and worldwide experience 2000. J Vasc Surg 2001;33(Suppl. 2):135–45.
Zhou W, Blay E Jr, Varu V, Ali S, Jin MQ, Sun L, Joh JH. Outcome and clinical significance of delayed endoleaks after endovascular aneurysm repair. J Vasc Surg 2014;59:915–20. https://doi.org/10.1016/j.jvs.2013.10.093
Zimmermann H, Rübenthaler J, Rjosk-Dendorfer D, Helck A, Reimann R, Reiser M, Clevert DA. Comparison of portable ultrasound system and high end ultrasound system in detection of endoleaks. Clin Hemorheol Microcirc 2015;63:99–111
Participants (n = 23)
AbuRahma AF, Campbell J, Stone PA, Nanjundappa A, Scott Dean L, Keiffer T, Emmett M. Early and late clinical outcomes of endovascular aneurysm repair in patients with an angulated neck. Vascular 2010;18:93–101. https://doi.org/10.2310/6670.2010.00010
Agu O, Boardley D, Adiseshiah M. Another late complication after endovascular aneurysm repair: aneurysmal degeneration at the iliac artery landing site. Vascular 2008;16:316–20. https://doi.org/10.2310/6670.2008.00065
Amiot S, Haulon S, Becquemin JP, Magnan PE, Lermusiaux P, Goueffic Y, et al. Fenestrated endovascular grafting: the French multicentre experience. Eur J Vasc Endovasc Surg 2010;39:537–44. https://doi.org/10.1016/j.ejvs.2009.12.008
Bos WT, Tielliu IF, Sondakh AO, Vourliotakis G, Bracale UM, Verhoeven EL. Hybrid endograft solution for complex iliac anatomy: Zenith body and Excluder limbs. Vascular 2010;18:136–40. https://doi.org/10.2310/6670.2010.00034
United Kingdom Small Aneurysm Trial Participants, Powell JT, Brady AR, Brown LC, Fowkes FG, Greenhalgh RM, et al. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002;346:1445–52. https://doi.org/10.1056/NEJMoa013527
Chandra V, Gowing R, Peruzarro A, Lee JT. Case-specific endovascular aneurysm repair simulation: a pilot comparison of simulated aneurysm repair with actual live cases. J Vasc Surg 2012;56:579.
Chen CK, Liang IP, Chang HT, Chen WY, Chen IM, Wu MH, et al. Impact on outcomes by measuring tortuosity with reporting standards for thoracic endovascular aortic repair. J Vasc Surg 2014;60:937–44. https://doi.org/10.1016/j.jvs.2014.04.008
D’Andrea C, Piscione F, Iannelli G, Di Tommaso L, D’Anna C, Pierri A, et al. Eight-year outcome after endovascular aortic repair for abdominal and thoracic aortic disease: a single centre experience. Eur Heart J 2009;30:985.
Dindyal S, Brewin M, Thrush A, Birch M, Kyriakides C. Contrast enhanced aortic ultrasonography for EVAR surveillance. J Vasc Intervent Radiol 2011;22:1785.e5.
Dindyal S, Kyriakides C. Duplex ultrasound with the implementation of contrast enhancement in selected cases is satisfactory for EVAR surveillance. J Endovasc Ther 2012;19:844–6. https://doi.org/10.1583/JEVT-12-4060L.1
Donas KP, Lee JT, Lachat M, Torsello G, Veith FJ, PERICLES investigators. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: the PERICLES registry. Ann Surg 2015;262:546–53. https://doi.org/10.1097/SLA.0000000000001405
Du Toit DF, Saaiman JA, Labuschagne BC, Vorster W, Van Beek FJ, Boden BH, Geldenhuys KM. EVAR: critical applied aortic morphology relevant to type-II endoleaks following device enhancement in patients with abdominal aortic aneurysms. Cardiovasc J S Afr 2004;15:170–7.
El Sayed HF, Meier GH, Mendoza B, Sprouse LR, Parent FN, Panneton JM. Aneurysm regression after endovascular aneurysm repair: what should we expect? Vasc Endovascular Surg 2008;42:545–50.
England A, García-Fiñana M, McWilliams RG, Collaborators. Multicenter retrospective investigation into migration of fenestrated aortic stent grafts. J Vasc Surg 2015;62:884–92. https://doi.org/10.1016/j.jvs.2015.04.420
Ferrari M, Adami D, Del Corso A, Berchiolli R, Pietrabissa A, Romagnani F, Mosca F. Laparoscopy-assisted abdominal aortic aneurysm repair: early and middle-term results of a consecutive series of 122 cases. J Vasc Surg 2006;43:695–700.
Hertault A, Maurel B, Pontana F, Martin-Gonzalez T, Spear R, Sobocinski J, et al. Benefits of completion 3D angiography associated with contrast enhanced ultrasound to assess technical success after EVAR. Eur J Vasc Endovasc Surg 2015;49:541–8. https://doi.org/10.1016/j.ejvs.2015.01.010
Hinterseher I, Kuffner H, Berth H, Gäbel G, Bötticher G, Saeger HD, Smelser D. Long-term quality of life of abdominal aortic aneurysm patients under surveillance or after operative treatment. Ann Vasc Surg 2013;27:553–61. https://doi.org/10.1016/j.avsg.2012.05.028
Krauss M, Ritter W, Bär I, Heilberger P, Schunn C, Raithel D. [Imaging of aortic endoprostheses and their complications.] Rofo 1998;169:388–96. https://doi.org/10.1055/s-2007-1015305
Liapis C, Kakisis J, Kaperonis E, Papavassiliou V, Karousos D, Tzonou A, Gogas J. Changes of the infrarenal aortic segment after conventional abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2000;19:643–7. https://doi.org/10.1053/ejvs.1999.1086
Liewald F, Scharrer-Pamler R, Görich J, Kapfer X, Seifarth H, Halter G, Sunder-Plassmann L. Intraoperative, perioperative and late complications with endovascular therapy of aortic aneurysm. Eur J Vasc Endovasc Surg 2001;22:251–6. https://doi.org/10.1053/ejvs.2001.1417
Sveinsson M, Sobocinski J, Resch T, Sonesson B, Dias N, Haulon S, Kristmundsson T. Early versus late experience in fenestrated endovascular repair for abdominal aortic aneurysm. J Vasc Surg 2015;61:895–901. https://doi.org/10.1016/j.jvs.2014.11.007
Svensjö S, Mani K, Björck M, Lundkvist J, Wanhainen A. Screening for abdominal aortic aneurysm in 65-year-old men remains cost-effective with contemporary epidemiology and management. Eur J Vasc Endovasc Surg 2014;47:357–65. https://doi.org/10.1016/j.ejvs.2013.12.023
Wanhainen A, Bylund N, Björck M. Outcome after abdominal aortic aneurysm repair in Sweden 1994–2005. Br J Surg 2008;95:564–70. https://doi.org/10.1002/bjs.6109
Case series with < 100 patients (n = 22)
Ambler GK, Coughlin PA, Hayes PD, Varty K, Gohel MS, Boyle JR. Incidence and outcomes of severe renal impairment following ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2015;50:443–9. https://doi.org/10.1016/j.ejvs.2015.06.024
Arsicot M, Lathelize H, Martinez R, Marchand E, Picquet J, Enon B. Follow-up of aortic stent grafts: comparison of the volumetric analysis of the aneurysm sac by ultrasound and CT. Ann Vasc Surg 2014;28:1618–28. https://doi.org/10.1016/j.avsg.2014.03.034
Bargellini I, Napoli V, Petruzzi P, Cioni R, Vignali C, Sardella SG, et al. Type II lumbar endoleaks: hemodynamic differentiation by contrast-enhanced ultrasound scanning and influence on aneurysm enlargement after endovascular aneurysm repair. J Vasc Surg 2005;41:10–18.
Berdejo GL, Lyon RT, Ohki T, Sanchez LA, Wain RA, Del Valle WN, Veith FJ. Color duplex ultrasound evaluation of transluminally placed endovascular grafts for aneurysm repair. J Vasc Tech 1998;22:201–7.
Fletcher J, Saker K, Batiste P, Dyer S. Colour Doppler diagnosis of perigraft flow following endovascular repair of abdominal aortic aneurysm. Int Angiol 2000;19:326–30.
Giannoni MF, Fanelli F, Citone M, Cristina Acconcia M, Speziale F, Gossetti B. Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up. Interact Cardiovasc Thorac Surg 2007;6:359–62.
Han SM, Patel K, Rowe VL, Perese S, Bond A, Weaver FA. Ultrasound-determined diameter measurements are more accurate than axial computed tomography after endovascular aortic aneurysm repair. J Vasc Surg 2010;51:1381–7. https://doi.org/10.1016/j.jvs.2010.01.033
Holst J, Resch T, Ivancev K, Björses K, Dias N, Lindblad B, et al. Early and intermediate outcome of emergency endovascular aneurysm repair of ruptured infrarenal aortic aneurysm: a single-centre experience of 90 consecutive patients. Eur J Vasc Endovasc Surg 2009;37:413–19. https://doi.org/10.1016/j.ejvs.2008.12.015
Kaladji A, Cardon A, Laviolle B, Heautot JF, Pinel G, Lucas A. Evolution of the upper and lower landing site after endovascular aortic aneurysm repair. J Vasc Surg 2012;55:24–32. https://doi.org/10.1016/j.jvs.2011.07.067
Kalliafas S, Travis SJ, Macierewicz J, Yusuf SW, Whitaker SC, Davidson I, Hopkinson BR. Color duplex ultrasonography of the superior mesenteric artery after placement of endografts with suprarenal stents. Vasc Endovascular Surg 2002;36:29–32. https://doi.org/10.1177/153857440203600106
Kelso RL, Lyden SP, Butler B, Greenberg RK, Eagleton MJ, Clair DG. Late conversion of aortic stent grafts. J Vasc Surg 2009;49:589–95. https://doi.org/10.1016/j.jvs.2008.10.020
Kopp R, Weckbach S, Minaifar N, Meimarakis G, Weidenhagen R, Clevert DA. [Follow-up control after endovascular treatment of infrarenal aortic aneurysms: contrast-enhanced sonography as alternative to CT angiography.] Gefasschirurgie 2008;13:410–6.
Makaroun M, Zajko A, Sugimoto H, Eskandari M, Webster M. Fate of endoleaks after endoluminal repair of abdominal aortic aneurysms with the EVT device. Eur J Vasc Endovasc Surg 1999;18:185–90.
Millen A, Canavati R, Harrison G, McWilliams RG, Wallace S, Vallabhaneni SR, Fisher RK. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg 2013;58:18–23. https://doi.org/10.1016/j.jvs.2012.12.057
Napoli V, Sardella SG, Bargellini I, Petruzzi P, Cioni R, Vignali C, et al. Evaluation of the proximal aortic neck enlargement following endovascular repair of abdominal aortic aneurysm: 3-years experience. Eur Radiol 2003;13:1962–71. https://doi.org/10.1007/s00330-003-1859-y
Pratesi G, Fargion A, Pulli R, Dorigo W, Guidotti A, Barbante M, et al. Long-term durability of endovascular treatment with iliac branch endograft for aorto-iliac aneurysms. J Vasc Surg 2013;1:86S.
Ruppert V, Erz K, Bürklein D, Treitl M, Steckmeier B, Stelter W, Umscheid T. Double tube stent–grafts for infrarenal aortic aneurysm: a new concept. J Endovasc Ther 2007;14:144–9.
Sandford RM, Batchelder AJ, Bown MJ, Sayers RD. Pre-discharge duplex ultrasound scans detect endoleaks not seen on completion angiography after endovascular aneurysm repair. J Endovasc Ther 2010;17:349–53. https://doi.org/10.1583/09-2119.1
Thompson MM, Boyle JR, Hartshorn T, Maltezos C, Nasim A, Sayers RD, et al. Comparison of computed tomography and duplex imaging in assessing aortic morphology following endovascular aneurysm repair. Br J Surg 1998;85:346–50. https://doi.org/10.1046/j.1365-2168.1998.00593.x
Tsolaki E, Zenunaj G, Gresta E, Di Mase S, Mascoli F. Contrast-enhanced ultrasound versus computed tomography angiography in the follow-up of the treatment of abdominal aortic aneurysm with endovascular techniques. J Vasc Ultrasound 2012;36:263–6.
Tutein Nolthenius RP, van Herwaarden JA, van den Berg JC, van Marrewijk C, Teijink JA, Moll FL. Three year single centre experience with the AneuRx aortic stent graft. Eur J Vasc Endovasc Surg 2001;22:257–64. https://doi.org/10.1053/ejvs.2001.1440
Vignali C, Cioni R, Neri E, Petruzzi P, Bargellini I, Sardella S, et al. Endoluminal treatment of abdominal aortic aneurysms. Abdom Imaging 2001;26:461–8.
Diagnostic test accuracy studies (n = 68)
Abbas A, Hansrani V, Sedgwick N, Ghosh J, McCollum CN. 3D contrast enhanced ultrasound for detecting endoleak following endovascular aneurysm repair (EVAR). Eur J Vasc Endovasc Surg 2014;47:487–92. https://doi.org/10.1016/j.ejvs.2014.02.002
AbuRahma AF, Welch CA, Mullins BB, Dyer B. Computed tomography versus color duplex ultrasound for surveillance of abdominal aortic stent–grafts. J Endovasc Ther 2005;12:568–73.
AbuRahma AF. Fate of endoleaks detected by CT angiography and missed by color duplex ultrasound in endovascular grafts for abdominal aortic aneurysms. J Endovasc Ther 2006;13:490–5.
AbuRahma AF. XVI.4 computed tomographic scanning versus duplex ultrasonography for surveillance after endovascular aneurysm repair: Computed tomography is essential and better. Vascular 2005;13:S75.
Badri H, El Haddad M, Ashour H, Nice C, Timmons G, Bhattacharya V. Duplex ultrasound scanning (DUS) versus computed tomography angiography (CTA) in the follow-up after EVAR. Angiology 2010;61:131–6. https://doi.org/10.1177/0003319709348296
Bargellini I, Cioni R, Napoli V, Petruzzi P, Vignali C, Cicorelli A, et al. Ultrasonographic surveillance with selective CTA after endovascular repair of abdominal aortic aneurysm. J Endovasc Ther 2009;16:93–104. https://doi.org/10.1583/08-2508.1
Beeman BR, Doctor LM, Doerr K, McAfee-Bennett S, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging alone is sufficient for midterm endovascular aneurysm repair surveillance: a cost analysis study and prospective comparison with computed tomography scan. J Vasc Surg 2009;50:1019–24. https://doi.org/10.1016/j.jvs.2009.06.019
Bendick PJ, Bove PG, Long GW, Zelenock GB, Brown OW, Shanley CJ. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 2003;37:381–5. https://doi.org/10.1067/mva.2003.17
Bohm B, Heyne J, Seifert S, Rimpler H, Bartel M. [Contrast-enhanced color duplex ultrasound scan in detection of endoleaks following aortic endografting.] Gefasschirurgie 2000;5:225–31.
Cantisani V, Ricci P, Grazhdani H, Napoli A, Fanelli F, Catalano C, et al. Prospective comparative analysis of colour-Doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2011;41:186–92. https://doi.org/10.1016/j.ejvs.2010.10.003
Carrafiello G, Laganà D, Recaldini C, Mangini M, Bertolotti E, Caronno R, et al. Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovasc Intervent Radiol 2006;29:969–74. https://doi.org/10.1007/s00270-005-0267-x
Causey MW, Jayaraj A, Leotta DF, Paun M, Beach KW, Kohler TR, et al. Three-dimensional ultrasonography measurements after endovascular aneurysm repair. Ann Vasc Surg 2013;27:146–53. https://doi.org/10.1016/j.avsg.2012.01.018
Civitello AB, Woodruff A, Mahmood H, Doughtery K, Askins C, Krajcer Z, et al. Contrast enhanced duplex ultrasound improves endoleak detection following endovascular abdominal aortic aneurysm repair. J Am Coll Cardiol 2003;41:39A.
Chisci E, Pigozzi C, Pecchioli A, Romano E, Ercolini L, Michelagnoli S. The role of contrast-enhanced ultrasound in endoleaks surveillance is to define the need of a secondary Intervention. J Vasc Surg 2015;61:199S–200S.
Chisci E, Pecchioli A, Barbanti E, Frosini P, Romano E, Ercolini L, et al. PC098. Three criteria derived from contrast-enhanced ultrasound define when a secondary intervention is needed during endoleaks surveillance. J Vasc Surg 2016;63(Suppl. 1):182S.
Clevert DA, Helck A, D’Anastasi M, Gürtler V, Sommer WH, Meimarakis G, et al. Improving the follow up after EVAR by using ultrasound image fusion of CEUS and MS-CT. Clin Hemorheol Microcirc 2011;49:91–104. https://doi.org/10.3233/CH-2011-1460
Clevert DA, Minaifar N, Weckbach S, Kopp R, Meimarakis G, Clevert DA, Reiser M. Color duplex ultrasound and contrast-enhanced ultrasound in comparison to MS-CT in the detection of endoleak following endovascular aneurysm repair. Clin Hemorheol Microcirc 2008;39:121–32.
Clevert DA, Kopp R. Contrast-enhanced ultrasound for endovascular grafting in infrarenal abdominal aortic aneurysm in a single patient with risk factors for the use of iodinated contrast. J Vasc Interv Radiol 2008;19:1241–5. https://doi.org/10.1016/j.jvir.2008.04.019
Costa P, Bureau Du Colombier P, Lermusiaux P. [Duplex ultrasound detection of type II endoleaks by after endovascular aneurysm repair: interest of contrast enhancement.] J Mal Vasc 2013;38:352–9. https://doi.org/10.1016/j.jmv.2013.08.004
Crosbie IM, Manning B, Haider N, Colgan MP, Madhavan P, Moore D, O’Neill S. PP68 comparison of duplex ultrasound and computed tomography measuring sac size in endovascular abdominal aortic aneurysm repair. J Vasc Surg 2009;1:34S.
d’Audiffret A, Desgranges P, Kobeiter DH, Becquemin JP. Follow-up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: validation with computed tomography. J Vasc Surg 2001;33:42–50.
Demirpolat G, Ozturk N, Parildar M, Posacioğlu H, Tamsel S. Duplex ultrasound evaluation of endoluminally treated aortic aneurysms with emphasis on diameter measurement: a comparison with computed tomography. J Clin Ultrasound 2011;39:263–9. https://doi.org/10.1002/jcu.20802
Dill-Macky MJ, Wilson SR, Sternbach Y, Kachura J, Lindsay T. Detecting endoleaks in aortic endografts using contrast-enhanced sonography. AJR Am J Roentgenol 2007;188:W262–8.
Elkouri S, Panneton JM, Andrews JC, Lewis BD, McKusick MA, Noel AA, et al. Computed tomography and ultrasound in follow-up of patients after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg 2004;18:271–9. https://doi.org/10.1007/s10016-004-0034-5
Franca GJ, Baroncini LAV, de Oliveira A, Vidal EA, Miyamotto M, Toregeani JF, et al. [Evaluation with Doppler vascular ultrasound in postoperative endovascular treatment of abdominal aortic aneurysm: a prospective comparative study with angiotomography.] J Vasc Bras 2013;12:102–9.
Freyrie A, Serra C, Testi G, Rossi C, Mauro R, Faggioli GL, Stella A. [Follow-up of type II endoleaks after endovascular aortic repair: the role of contrast-enhanced ultrasound.] Ital J Vasc Endovasc Surg 2007;14:1–8.
Giannoni MF, Palombo G, Sbarigia E, Speziale F, Zaccaria A, Fiorani P. Contrast-enhanced ultrasound imaging for aortic stent–graft surveillance. J Endovasc Ther 2003;10:208–17. https://doi.org/10.1177/152660280301000208
Gilabert R, Buñesch L, Real MI, García-Criado Á, Burrel M, Ayuso JR, et al. Evaluation of abdominal aortic aneurysm after endovascular repair: prospective validation of contrast-enhanced US with a second-generation US contrast agent. Radiology 2012;264:269–77. https://doi.org/10.1148/radiol.12111528
Golzarian J, Murgo S, Dussaussois L, Guyot S, Said KA, Wautrecht JC, Struyven J. Evaluation of abdominal aortic aneurysm after endoluminal treatment: comparison of color Doppler sonography with biphasic helical CT. AJR Am J Roentgenol 2002;178:623–8. https://doi.org/10.2214/ajr.178.3.1780623
Gray C, Goodman P, Herron CC, Lawler LP, O’Malley MK, O’Donohoe MK, McDonnell CO. Use of colour duplex ultrasound as a first line surveillance tool following EVAR is associated with a reduction in cost without compromising accuracy. Eur J Vasc Endovasc Surg 2012;44:145–50. https://doi.org/10.1016/j.ejvs.2012.05.008
Gürtler VM, Sommer WH, Meimarakis G, Kopp R, Weidenhagen R, Reiser MF, Clevert DA. A comparison between contrast-enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow-up after endovascular aneurysm repair. J Vasc Surg 2013;58:340–5. https://doi.org/10.1016/j.jvs.2013.01.039
Heilberger P, Schunn C, Ritter W, Weber S, Raithel D. Postoperative color flow duplex scanning in aortic endografting. J Endovasc Surg 1997;4:262–71. https://doi.org/10.1583/1074-6218(1997)004<0262:PCFDSI>2.0.CO;2
Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, et al. Contrast-enhanced duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg 2006;43:259–64.
Hodge M, Parker D, Collado E, Broadbent K, Lumsden AB, McCollum CH, et al. Continuous ultrasound contrast infusion as an adjunct to color duplex ultrasound in the assessment of aortic endografts. J Vasc Ultrasound 2007;31:17–21.
Houdek K, Treska V, Certik B, Mirka H, Korcakova E, Molacek J, et al. [Initial experience of follow up of patients after the endovascular treatment of abdominal aortic aneurysms using contrast-enhanced ultrasound.] Cor et Vasa 2015;57:e121–6.
Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2009;49:552–60. https://doi.org/10.1016/j.jvs.2008.10.008
Kamal DM, Steinmetz OK, Obrand DI. The value of duplex ultrasound versus contrast enhanced CT scan in the follow-up of endoluminally repaired abdominal aortic aneurysm: a blinded comparison. Bahrain Med Bull 2008;30:101–7.
Lowe C, Abbas A, Sedgewick N, Armitage J, Rogers S, Smith L, et al. 3D contrast enhanced ultrasound for endoleak detection after EVAR – a new gold standard? Br J Surg 2015;102:9.
Manning BJ, O’Neill SM, Haider SN, Colgan MP, Madhavan P, Moore DJ. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg 2009;49:60–5. https://doi.org/10.1016/j.jvs.2008.07.079
Maioli F, Freyrie A, Testi G, Palumbo N, Serra C, Mauro R, Stella A. Is CEUS a valid alternative to CTA in endoleaks detection? Ital J Vasc Endovasc Surg 2010;17:253–8.
Mazzei MA, Guerrini S, Mazzei FG, Cioffi Squitieri N, Notaro D, de Donato G, et al. Follow-up of endovascular aortic aneurysm repair: Preliminary validation of digital tomosynthesis and contrast enhanced ultrasound in detection of medium- to long-term complications. World J Radiol 2016;8:530–6. https://doi.org/10.4329/wjr.v8.i5.530
McLafferty RB, McCrary BS, Mattos MA, Karch LA, Ramsey DE, Solis MM, Hodgson KJ. The use of color-flow duplex scan for the detection of endoleaks. J Vasc Surg 2002;36:100–4.
McWilliams RG, Martin J, White D, Gould DA, Rowlands PC, Haycox A, et al. Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther 2002;9:170–9. https://doi.org/10.1177/152660280200900206
McWilliams RG, Martin J, White D, Gould DA, Harris PL, Fear SC, et al. Use of contrast-enhanced ultrasound in follow-up after endovascular aortic aneurysm repair. J Vasc Interv Radiol 1999;10:1107–14.
Motta R, Rubaltelli L, Vezzaro R, Vida V, Marchesi P, Stramare R, et al. Role of multidetector CT angiography and contrast-enhanced ultrasound in redefining follow-up protocols after endovascular abdominal aortic aneurysm repair. Radiol Med 2012;117:1079–92. https://doi.org/10.1007/s11547-012-0809-x
Nagre SB, Passman MA, Combs BR, Lowman BG, Jordan WD. Duplex ultrasound graft limb velocity asymmetry predicts endoleak after endovascular aneurysm repair. J Vasc Surg 2010;51:1069.
Nagre SB, Taylor SM, Passman MA, Patterson MA, Combs BR, Lowman BG, Jordan WD. Evaluating outcomes of endoleak discrepancies between computed tomography scan and ultrasound imaging after endovascular abdominal aneurysm repair. Ann Vasc Surg 2011;25:94–100. https://doi.org/10.1016/j.avsg.2010.08.003
Nerlekar R, Warrier R, De Ryke R, Miller R, Hewitt PM, Scott A. A comparative study of ultrasound and computed tomography scan for the follow-up of abdominal aortic aneurysms after endovascular repair. J Vasc Ultrasound 2006;30:81–5.
Oderich GS, Ribeiro M, Hofer J, Wigham J, Cha S, Reis De Souza L, et al. Prospective, nonrandomized study to evaluate endovascular repair of pararenal and thoracoabdominal aortic aneurysms using fenestrated and branched endografts with supraceliac sealing zones. J Vasc Surg 2016;63(Suppl. 1):136S.
Pages S, Favre JP, Cerisier A, Pyneeandee S, Boissier C, Veyret C. Comparison of color duplex ultrasound and computed tomography scan for surveillance after aortic endografting. Ann Vasc Surg 2001;15:155–62. https://doi.org/10.1007/s100160010065
Parent FN, Meier GH, Godziachvili V, LeSar CJ, Parker FM, Carter KA, et al. The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with color duplex ultrasound scan. J Vasc Surg 2002;35:474–81.
Perini P, Sediri I, Midulla M, Delsart P, Gautier C, Haulon S. Contrast-enhanced ultrasound vs. CT angiography in fenestrated EVAR surveillance: a single-center comparison. J Endovasc Ther 2012;19:648–55. https://doi.org/10.1583/JEVT-12-3909R.1
Perini P, Sediri I, Midulla M, Delsart P, Mouton S, Gautier C, et al. Single-centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. Eur J Vasc Endovasc Surg 2011;42:797–802. https://doi.org/10.1016/j.ejvs.2011.09.003
Pfister K, Rennert J, Uller W, Schnitzbauer AA, Stehr A, Jung W, et al. Contrast harmonic imaging ultrasound and perfusion imaging for surveillance after endovascular abdominal aneurysm repair regarding detection and characterization of suspected endoleaks. Clin Hemorheol Microcirc 2009;43:119–28. https://doi.org/10.3233/CH-2009-1226
Raman KG, Missig-Carroll N, Richardson T, Muluk SC, Makaroun MS. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 2003;38:645–51.
Ruiz J, Santos C, Ruiz M. Duplex ultrasonography as predictor of persistent type II endoleak and increased sac diameter after endovascular aneurysm repair. J Vasc Intervent Radiol 2013;24:145.e3.
San Norberto-Garcia EM, Del Blanco-Alonso I, Ibanez-Marana MA, Cenizo-Revuelta N, Brizuela-Sanz JA, Mengibar-Fuentes L, et al. [The diagnostic value of colour Doppler ultrasonography in the clinical monitoring of endovascular abdominal aortic aneurysm repair.] Angiología 2007;59:29–37.
Sandford RM, Bown MJ, Fishwick G, Murphy F, Naylor M, Sensier Y, et al. Duplex ultrasound scanning is reliable in the detection of endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2006;32:537–41.
Sato DT, Goff CD, Gregory RT, Robinson KD, Carter KA, Herts BR, et al. Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound scan versus computed tomography scan. J Vasc Surg 1998;28:657–63.
Schmieder GC, Stout CL, Stokes GK, Parent FN, Panneton JM. Endoleak after endovascular aneurysm repair: duplex ultrasound imaging is better than computed tomography at determining the need for intervention. J Vasc Surg 2009;50:1012–17. https://doi.org/10.1016/j.jvs.2009.06.021
Sorrentino K, Shah PS, Bendick P. Ultrasonographic surveillance of abdominal aortic endovascular aneurysm stent graft repair in a dedicated vascular laboratory. J Diagn Med Sonography 2015;31:40–51.
Ten Bosch JA, Rouwet EV, Peters CT, Jansen L, Verhagen HJ, Prins MH, Teijink JA. Contrast-enhanced ultrasound versus computed tomographic angiography for surveillance of endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol 2010;21:638–43. https://doi.org/10.1016/j.jvir.2010.01.032
Tran K, Fajardo A, Ullery BW, Goltz C, Lee JT. Renal function changes after fenestrated endovascular aneurysm repair. J Vasc Surg 2016;64:273–80.
Ustymowicz A, Janica J, Kowalewski R, Lewszuk A, Lukasiewicz A, Michalak P, Waszczeniuk-Sobotko O. Contrast-enhanced ultrasonography versus computed tomographic angiography in the monitoring of patients after endovascular repair of abdominal aortic aneurysm – preliminary experience. Nucl Med Rev Cent East Eur 2009;12:95–8.
Wolf YG, Johnson BL, Hill BB, Rubin GD, Fogarty TJ, Zarins CK. Duplex ultrasound scanning versus computed tomographic angiography for postoperative evaluation of endovascular abdominal aortic aneurysm repair. J Vasc Surg 2000;32:1142–8.
Yang X, Chen YX, Zhang B, Jiang YX, Liu CW, Zhao RN, et al. Contrast-enhanced ultrasound in detecting endoleaks with failed computed tomography angiography diagnosis after endovascular abdominal aortic aneurysm repair. Chin Med J 2015;128:2491–7. https://doi.org/10.4103/0366-6999.164935
Zannetti S, De Rango P, Parente B, Parlani G, Verzini F, Maselli A, et al. Role of duplex scan in endoleak detection after endoluminal abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2000;19:531–5. https://doi.org/10.1053/ejvs.1999.1033
Other study design (n = 64)
Arko FR, Filis KA, Heikkinen MA, Johnson BL, Zarins CK. Duplex scanning after endovascular aneurysm repair: an alternative to computed tomography. Semin Vasc Surg 2004;17:161–5.
Bakken AM, Illig KA. Long-term follow-up after endovascular aneurysm repair: is ultrasound alone enough? Perspect Vasc Surg Endovasc Ther 2010;22:145–51.
Balm R, Jacobs MJ. Use of spiral computed tomographic angiography in monitoring abdominal aortic aneurysms after transfemoral endovascular repair. Tex Heart Inst J 1997;24:200–3.
Baxter BT, Matsumura J, Curci J, McBride R, Blackwelder WC, Liu X, et al. Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA(3)CT): design of a Phase IIb, placebo-controlled, double-blind, randomized clinical trial of doxycycline for the reduction of growth of small abdominal aortic aneurysm. Contemp Clin Trials 2016;48:91–8. https://doi.org/10.1016/j.cct.2016.03.008
Bhatt S, Dogra VS. Catastrophes of abdominal aorta: sonographic evaluation. Ultrasound Clin 2008;3:83–91.
Bush RL. Contrast-enhanced duplex ultrasound for endoleak detection following EVAR. J Endovasc Ther 2007;14:I5–I6.
Cantisani V, David E, Mauro L, D’Ambrosio F, Clevert DA. CEUS: what is its role in abdominal aortic diseases? Med Ultrason 2015;17:419–21. https://doi.org/10.11152/mu.2013.2066.173.zsp
Carpenter JP. Regarding: ‘The incidence and natural history of type I and II endoleak: a 5-year follow-up assessment with color duplex ultrasound scan’. J Vas Surg 2002;35:595–7.
Carrafiello G, Recaldini C, Laganà D, Piffaretti G, Fugazzola C. Endoleak detection and classification after endovascular treatment of abdominal aortic aneurysm: value of CEUS over CTA. Abdom Imaging 2008;33:357–62. https://doi.org/10.1007/s00261-007-9268-3
Clevert DA, Weckbach S, Kopp R, Meimerakis G, Clevert DA, Jauch KW, Reiser M. Imaging of aortic lesions with color coded duplex sonography and contrast-enhanced ultrasound versus multislice computed tomography (MS-CT) angiography. Clin Hemorheol Microcirc 2008;40:267–79.
Clevert DA, Horng A, Kopp R, Schick K, Meimarakis G, Sommer WH, Reiser M. [Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS).] Radiologe 2009;49:1033–9. https://doi.org/10.1007/s00117-009-1876-1
Clevert DA, Minaifar N, Kopp R, Stickel M, Meimarakis G, Sommer W, Reiser M. Imaging of endoleaks after endovascular aneurysm repair (EVAR) with contrast-enhanced ultrasound (CEUS). A pictorial comparison with CTA. Clin Hemorheol Microcirc 2009;41:151–68. https://doi.org/10.3233/CH-2009-1160
Clevert DA, Helck A, D’Anastasi M, Trumm C, Meimarakis G, Weidenhagen R, et al. [Ultrasound-guided EVAR interventions and follow-up diagnostics using contrast-enhanced ultrasound and image fusion.] Gefasschirurgie 2011;16:490.
Clevert DA, Schick K, Chen MH, Zhu QL, Reiser M. Role of contrast enhanced ultrasound in detection of abdominal aortic abnormalities in comparison with multislice computed tomography. Chin Med J 2009;122:858–64.
de Bruin JL, Karthikesalingam A, Holt PJ, Prinssen M, Thompson MM, Blankensteijn JD, Dutch Randomised Endovascular Aneurysm Management Study Group. Predicting reinterventions after open and endovascular aneurysm repair using the St George’s Vascular Institute score. J Vasc Surg 2016;63:1428.
Deklunder G, Sediri I, Donati T, Boivin V, Gautier C, Haulon S. [Follow up of endovascular abdominal aortic aneurysm repair with contrast ultrasound.] J De Radiol 2009;90:141–7.
Dias NV, Billberg H, Sonesson B, Törnqvist P, Resch T, Kristmundsson T. The effects of combining fusion imaging, low-frequency pulsed fluoroscopy, and low-concentration contrast agent during endovascular aneurysm repair. J Vasc Surg 2016;63:1147–55. https://doi.org/10.1016/j.jvs.2015.11.033
Dill-Macky MJ. Aortic endografts: detecting endoleaks using contrast-enhanced ultrasound. Ultrasound Q 2006;22:49–52.
Dindyal S, Brewin M, Thrush A, Birch M, Kyriakides C. Contrast enhanced aortic ultrasonography – a laboratory phantom to determine the limitations of enhanced and unenhanced ultrasonography scanning for post-operative EVAR surveillance. Br J Surg 2012;99:16.
Dindyal S, Kyriakides C. Duplex ultrasound will reduce costs of EVAR surveillance but the addition of microbubble contrast will improve this further. Eur J Vasc Endovasc Surg 2012;44:522.
Dindyal S, Kyriakides C. Aortic sac diameter measurements are important for EVAR surveillance but can be achieved reliably with ultrasound. Acta Radiol 2013;54:59. https://doi.org/10.1258/ar.2012.120574
Dingemans SA, Jonker FH, Moll FL, van Herwaarden JA. Aneurysm sac enlargement after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2016;31:229–38. https://doi.org/10.1016/j.avsg.2015.08.011
Fillinger MF. Postoperative imaging after endovascular AAA repair. Semin Vasc Surg 1999;12:327–38.
Flynn S, Creedon S, Fitzgerald T, Bourke MG, Sparrow P, Brady A, et al. Open management of complications arising following endovascular repair of aorto-iliac aneurysms: single centre experience. Ir J Med Sci 2015;184(Suppl. 1):411.
Gossetti B, Salvatori F, Irace L, Martinelli O, Benedetti-Valentini F. [Endoleaks after endovascular repair of abdominal aortic aneurysm: detection and management.] Ital J Vasc Endovasc Surg 2005;12:23–31.
Griffin J, Williams RS, Pemberton M. Is duplex ultrasound endoleak surveillance after EVAR cost effective? A comparison with European Society of Vascular Surgery guidelines. Int J Surg 2013;11:734.
Haulon S, Perini P. Response to letter to the editor ‘Re: Single Centre Prospective Comparison between Contrast Enhanced UltraSound and Computed Tomography Angiography after EVAR’. Eur J Vasc Endovasc Surg 2012;43:240–1.
Hellinger JC. Endovascular repair of thoracic and abdominal aortic aneurysms: pre- and postprocedural imaging. Tech Vasc Interv Radiol 2005;8:2–15.
Hermsen K, Chong WK. Ultrasound evaluation of abdominal aortic and iliac aneurysms and mesenteric ischemia. Radiol Clin North Am 2004;42:365–81. https://doi.org/10.1016/j.rcl.2003.12.003
Hiatt MD, Rubin GD. Surveillance for endoleaks: how to detect all of them. Semin Vasc Surg 2004;17:268–78.
Horsch S. SOA XI: EVAR follow-up: selection of imaging modality, frequency, cost-effectiveness, indications for re-intervention. Pathophysiol Haemost Thromb 2008;36:P35–6.
Iezzi R, Cotroneo AR, Basilico R, Simeone A, Storto ML, Bonomo L. Endoleaks after endovascular repair of abdominal aortic aneurysm: value of CEUS. Abdom Imaging 2010;35:106–14. https://doi.org/10.1007/s00261-009-9526-7
Iezzi R, Cotroneo AR, Pirro F, Dattesi R, Storto ML, Bonomo L. Contrast-enhanced ultrasound in the follow-up of patients after endovascular abdominal aortic aneurysm repair (EVAR): a pictorial review. Cardiovasc Intervent Radiol 2010;33:254.
Johnson BL, Harris EJ Jr, Fogarty TJ, Olcott IC, Zarins CK. Color duplex evaluation of endoluminal aortic stent grafts. J Vasc Technol 1998;22:97–104.
Karthikesalingam A, Young M, Powell SA, Morshedian G, Ramachandran V, D’Abate F, et al. The impact of endograft surveillance on a vascular imaging service. Vasc Endovascular Surg 2013;47:92–6. https://doi.org/10.1177/1538574412474497
Kranokpiraksa P, Kaufman JA. Follow-up of endovascular aneurysm repair: plain radiography, ultrasound, CT/CT angiography, MR imaging/MR angiography, or what? J Vasc Interv Radiol 2008;19(Suppl. 6):27–36. https://doi.org/10.1016/j.jvir.2008.03.009
Kuehnl A, Assadian A, Ockert S, Berger H, Eckstein HH. [Detection and treatment monitoring of secondary coeliac trunk endoleak following hybrid open-endovascular aortic aneurysm repair using B-flow and contrast-enhanced ultrasound.] Ultraschall Med 2009;30:494–6. https://doi.org/10.1055/s-0028-1109443
Kurimoto Y, Maruyama R, Ujihira K, Nishioka N, Iba Y, Yamada A, et al. Postoperative 2-day blood pressure management facilitates the shrinkage of abdominal aortic aneurysm following endovascular aortic repair by reducing the incidence rate of type II endoleaks. J Vasc Surg 2016;63(Suppl. 1):19S–20S.
Lane TR, Metcalfe MJ, Narayanan S, Davies AH. Post-operative surveillance after open peripheral arterial surgery. Eur J Vasc Endovasc Surg 2011;42:59–77. https://doi.org/10.1016/j.ejvs.2011.03.023
Laturnus J, Oliveira N, Basto Gonçalves F, Schurink GW, Verhagen H, Jacobs MJ, Mees BM. Towards individualized follow-up protocols after endovascular aortic aneurysm repair. J Cardiovasc Surg 2016;57:242–7.
Lim J, Wolff J, Rodd CD, Cooper DG, Earnshaw JJ. Outcome in men with a screen-detected abdominal aortic aneurysm who are not fit for intervention. Eur J Vasc Endovasc Surg 2015;50:732–6. https://doi.org/10.1016/j.ejvs.2015.07.035
Majd P, Ahmad W, Luebke T, Gawenda M, Brunkwall J. Impairment of erectile function after elective repair of abdominal aortic aneurysm. Vascular 2016;24:37–43. https://doi.org/10.1177/1708538115577290
Matsumura JS, Ryu RK, Ouriel K. Identification and implications of transgraft microleaks after endovascular repair of aortic aneurysms. J Vasc Surg 2001;34:190–7.
Mauro R, Maioli F, Freyrie A, Testi G, Palumbo N, Serra C, Stella A. Is CEUS a valid alternative to CTA in endoleaks detection? Ital J Vasc Endovasc Surg 2010;17:253–8.
Mazon M, Cabrera G, Atares M, Ruiz A, Ripolles T, Lonjedo E. Percutaneous contrast enhanced ultrasound (CEUS)-guided thrombin injection for endoleaks after endovascular repair of abdominal aortic aneurysm: a pictorial review. Cardiovasc Intervent Radiol 2011;34:568.
Nicolau C, Ripollés T. Contrast-enhanced ultrasound in abdominal imaging. Abdom Imaging 2012;37:1–19. https://doi.org/10.1007/s00261-011-9796-8
Pandey N, Litt HI. Surveillance imaging following endovascular aneurysm repair. Semin Intervent Radiol 2015;32:239–48. https://doi.org/10.1055/s-0035-1556878
Patel A, Edwards R, Chandramohan S. Surveillance of patients post-endovascular abdominal aortic aneurysm repair (EVAR). A web-based survey of practice in the UK. Clin Radiol 2013;68:580–7. https://doi.org/10.1016/j.crad.2012.11.019
Pfister K, Kasprzak PM, Apfelbeck H, Kopp R, Janotta M. [The significance of contrast-enhanced ultrasound in vascular surgery.] Zentralbl Chir 2014;139:518–24. https://doi.org/10.1055/s-0033-1351028
Rayt HS, Sandford RM, Salem M, Bown MJ, London NJ, Sayers RD. Conservative management of type 2 endoleaks is not associated with increased risk of aneurysm rupture. Eur J Vasc Endovasc Surg 2009;38:718–23.
Ricotta JJ. Endoleak management and postoperative surveillance following endovascular repair of thoracic aortic aneurysms. J Vasc Surg 2010;52(Suppl. 4):91–9. https://doi.org/10.1016/j.jvs.2010.06.149
Ritter RG, Nelson K, Adili F, Schmitz-Rixen T. [Abdominal aortic aneurysm: screening and surveillance.] Hamostaseologie 2004;24:151–6.
Schmieder GC, Stout CL, Stokes GK, Parent FN, Panneton JM. Endoleak after endovascular aneurysm repair: duplex ultrasound imaging is better than computed tomography at determining the need for intervention. J Vasc Surg 2009;50:1012–17. https://doi.org/10.1016/j.jvs.2009.06.021
Mahajan A, Barber M, Cumbie T, Filardo G, Shutze WP, Sass DM, Shutze W. The impact of aneurysm morphology and anatomic characteristics on long-term survival after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2016;34:75–83. https://doi.org/10.1016/j.avsg.2015.12.022
Sousaris N, McCutcheon J, Barr R. Incidental detection of an aortic stent endoleak with contrast-enhanced sonography. J Ultrasound Med 2014;33:738–40. https://doi.org/10.7863/ultra.33.4.738
Suckow B, Schanzer AS, Hoel AW, Wyers M, Marone LK, Veeraswamy RK, Nolan BW. A national survey of disease-specific knowledge in patients with an abdominal aortic aneurysm. J Vasc Surg 2016;63:1156–62. https://doi.org/10.1016/j.jvs.2015.12.042
Sun Z. Evidence for contrast-enhanced ultrasound in fenestrated EVAR surveillance. J Endovasc Ther 2012;19:656–60. https://doi.org/10.1583/JEVT-12-3909C.1
Tawfick W, Sultan S. Incidence of concomitant malignancy in AAA: 8-year experience. Vascular 2010;18:S65–6.
Teodorescu VJ, Morrissey NJ, Olin JW. Duplex ultrasonography and its impact on providing endograft surveillance. Mt Sinai J Med 2003;70:364–6.
Tuerff SN, Rockman CB, Lamparello PJ, Adelman MA, Jacobowitz GR, Gagne PJ, et al. Are type II (branch vessel) endoleaks really benign? Ann Vasc Surg 2002;16:50–4.
Tse DM, Tapping CR, Patel R, Morgan R, Bratby MJ, Anthony S, Uberoi R. Surveillance after endovascular abdominal aortic aneurysm repair. Cardiovasc Intervent Radiol 2014;37:875–88. https://doi.org/10.1007/s00270-014-0916-z
Uthoff H, Peña C, Katzen BT, Gandhi R, West J, Benenati JF, Geisbüsch P. Current clinical practice in postoperative endovascular aneurysm repair imaging surveillance. J Vasc Interv Radiol 2012;23:1152–9.e6. https://doi.org/10.1016/j.jvir.2012.06.003
van der Vliet JA, Kool LJ, van Hoek F. Simplifying post-EVAR surveillance. Eur J Vasc Endovasc Surg 2011;42:193–4. https://doi.org/10.1016/j.ejvs.2011.04.012
Verhoeven EL, Oikonomou K, Ventin FC, Lerut P, Fernandes EFR, Mendes Pedro L. Is it time to eliminate CT after EVAR as routine follow-up? J Cardiovasc Surg 2011;52:193–8.
Surveillance protocol not stated, mixed or not relevant to research question (n = 70)
AbuRahma AF, Yacoub M, Hass SM, AbuRahma J, Mousa AY, Dean LS, et al. Compliance of postendovascular aortic aneurysm repair imaging surveillance. J Vasc Surg 2016;63:589–95. https://doi.org/10.1016/j.jvs.2015.09.021
Alric P, Hinchliffe RJ, Picot MC, Braithwaite BD, MacSweeney ST, Wenham PW, Hopkinson BR. Long-term renal function following endovascular aneurysm repair with infrarenal and suprarenal aortic stent–grafts. J Endovasc Ther 2003;10:397–405.
Alsac JM, Jouanet C, Mirault T, Julia P, Sapoval M, Messas E, Fabiani JN. Efficacy and cost-effectiveness of reinterventions for type 2 endoleak with enlargement of the aneurysmal sac after endovascular abdominal aortic aneurysm. Arch Cardiovasc Dis Suppl 2013;5:70.
Anthony F, Kiley ML, Mays K, Javines J, Hye R, Chang R, et al. Aligning information technology with longitudinal outcomes surveillance: a multiregional vascular registry experience. Circulation 2013;128:A16031.
Arhuidese I, Locham S, Obeid T, Nejim B, Hicks CW, Malas MB. Racial disparity in the outcome and cost of treatment of abdominal aortic aneurysms in the United States. J Vasc Surg 2016;63(Suppl. 1):S74–5.
Arnaoutoglou E, Kouvelos G, Papa N, Gartzonika K, Milionis H, Koulouras V, Matsagkas M. Prospective evaluation of postimplantation syndrome evolution on patient outcomes after endovascular aneurysm repair for abdominal aortic aneurysm. J Vasc Surg 2016;63:1248–55. https://doi.org/10.1016/j.jvs.2015.11.043
Bastos Gonçalves F, Jairam A, Voûte MT, Moelker AD, Rouwet EV, ten Raa S, et al. Clinical outcome and morphologic analysis after endovascular aneurysm repair using the Excluder endograft. J Vasc Surg 2012;56:920–8. https://doi.org/10.1016/j.jvs.2012.03.263
Bastos Gonçalves F, van de Luijtgaarden KM, Hoeks SE, Hendriks JM, ten Raa S, Rouwet EV, et al. Adequate seal and no endoleak on the first postoperative computed tomography angiography as criteria for no additional imaging up to 5 years after endovascular aneurysm repair. J Vasc Surg 2013;57:1503–11. https://doi.org/10.1016/j.jvs.2012.11.085
Bastos Gonçalves F, Hoeks SE, Teijink JA, Moll FL, Castro JA, Stolker RJ, et al. Risk factors for proximal neck complications after endovascular aneurysm repair using the Endurant stentgraft. Eur J Vasc Endovasc Surg 2015;49:156–62. https://doi.org/10.1016/j.ejvs.2014.10.003
Beeman BR, Murtha K, Doerr K, McAfee-Bennett S, Dougherty MJ, Calligaro KD. Duplex ultrasound factors predicting persistent type II endoleak and increasing AAA sac diameter after EVAR. J Vasc Surg 2010;52:1147–52. https://doi.org/10.1016/j.jvs.2010.06.099
Brunner-Ziegler S, Hammer A, Seidinger D, Willfort-Ehringer A, Koppensteiner R, Steiner S. Longterm evaluation on the impact of thrombus formation on the course of abdominal aortic diameter expansion. Vasa Journal of Vascular Diseases 2013;42:14–15.
Burke Best W, Ahanchi SS, Larion S, Ammar CP, Lavingia KS, Panneton JM. Abdominal Aortic Aneurysm Anatomic Severity Grading Score Predicts Implant-Related Complications, Systemic Complications, and Mortality. Paper presented at the plenary session of the 43rd Annual Symposium of the Society for Clinical Vascular Surgery, Miami, FL, 29 March to 2 April 2015.
Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E, CAESAR Trial Group. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg 2011;41:13–25. https://doi.org/10.1016/j.ejvs.2010.08.026
Chambers JG, Nagre SB, Patterson MA, Taylor SM, Passman MA, Jordan WD. Delayed conversions after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2010;51:1068.
Chang RW, Goodney P, Tucker LY, Okuhn S, Hua H, Rhoades A, et al. Ten-year results of endovascular abdominal aortic aneurysm repair from a large multicenter registry. J Vasc Surg 2013;58:324–32. https://doi.org/10.1016/j.jvs.2013.01.051
Chinsakchai K, Hongku K, Hahtapornsawan S, Wongwanit C, Ruangsetakit C, Sermsathanasawadi N, Mutirangura P. Outcomes of abdominal aortic aneurysm with aortic neck thrombus after endovascular abdominal aortic aneurysm repair. J Med Assoc Thai 2014;97:518–24.
Corriere MA, Feurer ID, Becker SY, Dattilo JB, Passman MA, Guzman RJ, Naslund TC. Endoleak following endovascular abdominal aortic aneurysm repair: implications for duration of screening. Ann Surg 2004;239:800–5.
Cuypers P, Buth J, Harris PL, Gevers E, Lahey R. Realistic expectations for patients with stent–graft treatment of abdominal aortic aneurysms. Results of a European multicentre registry. Eur J Vasc Endovasc Surg 1999;17:507–16. https://doi.org/10.1053/ejvs.1999.0836
Debus ES, Nullen H, Torsello G, Lang W, Flessenkamper I, Hupp T, et al. [Treatment of abdominal aortic aneurysms in Germany. Quality assurance data 2013.] Gefasschirurgie 2014;19:412–21.
Du Toit DF, Saaiman JA, Carpenter JP, Geldenhuys KM. Endovascular aortic aneurysm repair by a multidisciplinary team: lessons learned and six-year clinical update. Cardiovasc J S Afr 2005;16:36–47.
Faure EM, Becquemin JP, Cochennec F, ENGAGE collaborators. Predictive factors for limb occlusions after endovascular aneurysm repair. J Vasc Surg 2015;61:1138–45.e2. https://doi.org/10.1016/j.jvs.2014.11.084
Fitridge RA, Boult M, de Loryn T, Cowled P, Barnes M. Predictors of 1-year survival after endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2016;51:528–34. https://doi.org/10.1016/j.ejvs.2015.12.019
Garg T, Baker LC, Mell MW. Postoperative surveillance and long-term outcomes after endovascular aneurysm repair among Medicare beneficiaries. J Vasc Surg 2014;1:8S.
Georgiadis GS, Trellopoulos G, Antoniou GA, Gallis K, Nikolopoulos ES, Kapoulas KC, et al. Early results of the Endurant endograft system in patients with friendly and hostile infrarenal abdominal aortic aneurysm anatomy. J Vasc Surg 2011;54:616–27. https://doi.org/10.1016/j.jvs.2011.03.235
Gossetti B, Ferri M, Ippoliti A, Verzini F, Irene Group I, Silingardi R. Preliminary results of a multicenter experience with NELLIX system for endovascular aneurysm sealinB. J Vasc Surg 2016;63(Suppl. 1):S36–7.
Grisafi JL, Rahbar R, Nelms J, Detschelt EL, Chess BA, Benckart DH, Muluk SC. Challenging neck anatomy is associated with need for intraoperative endovascular adjuncts during endovascular aortic aneurysm repair (EVAR). Ann Vasc Surg 2011;25:729–34. https://doi.org/10.1016/j.avsg.2011.02.028
Hinchliffe RJ, Goldberg J, Macsweeney ST, Zenith Users Group. A UK multi-centre experience with a second-generation endovascular stent–graft: results from the Zenith Users Group. Eur J Vasc Endovasc Surg 2004;27:51–5. https://doi.org/10.1016/j.ejvs.2003.10.004
Hogg ME, Morasch MD, Park T, Flannery WD, Makaroun MS, Cho JS. Long-term sac behavior after endovascular abdominal aortic aneurysm repair with the Excluder low-permeability endoprosthesis. J Vasc Surg 2011;53:1178–83. https://doi.org/10.1016/j.jvs.2010.11.045
Houbballah R, Majewski M, Becquemin JP. Total sac retraction after endovascular aneurysm repair: 4 years’ follow-up, correlation to treatment success and predictive factors. J Vasc Surg 2010;52:806–7.
Karthikesalingam A, Holt PJ, Hinchliffe RJ, Nordon IM, Loftus IM, Thompson MM. Risk of reintervention after endovascular aortic aneurysm repair. Br J Surg 2010;97:657–63. https://doi.org/10.1002/bjs.6991
Karthikesalingam A, Holt PJ, Vidal-Diez A, Choke EC, Patterson BO, Thompson LJ, et al. Predicting aortic complications after endovascular aneurysm repair. Br J Surg 2013;100:1302–11. https://doi.org/10.1002/bjs.9177
Karthikesalingam A, Page AA, Pettengell C, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. Heterogeneity in surveillance after endovascular aneurysm repair in the UK. Eur J Vasc Endovasc Surg 2011;42:585–90. https://doi.org/10.1016/j.ejvs.2011.06.053
Katsargyris A, Botos B, Oikonomou K, Pedraza de Leistl M, Ritter W, Verhoeven EL. The new C3 Gore Excluder stent–graft: single-center experience with 100 patients. Eur J Vasc Endovasc Surg 2014;47:342–8. https://doi.org/10.1016/j.ejvs.2013.12.015
Kothari AN, Halandras PM, Drescher M, Blackwell RH, Graunke DM, Kliethermes S, et al. Transient postoperative atrial fibrillation after abdominal aortic aneurysm repair increases mortality risk. J Vasc Surg 2016;63:1240–7. https://doi.org/10.1016/j.jvs.2015.12.046
Krol E, Pineda DM, Calligaro KD, Phillips Z, Sheikh MS, Dougherty MJ, et al. The fate of EVAR after 5 years followed up with duplex ultrasound. J Vasc Surg 2015;61:57S.
Leurs LJ, Laheij RJ, Buth J, Collaborators E. What determines and are the consequences of surveillance intensity after endovascular abdominal aortic aneurysm repair? Ann Vasc Surg 2005;19:868–75.
Lo RC, Buck DB, Herrmann J, Hamdan AD, Wyers M, Patel VI, et al. Risk factors and consequences of persistent type II endoleaks. J Vasc Surg 2016;63:895–901. https://doi.org/10.1016/j.jvs.2015.10.088
Loa J, Dubenec S, Cao P, Milner R, Silveira PG, Trimarchi S, et al. The Gore Global Registry for Endovascular Aortic Treatment: objectives and design. Ann Vasc Surg 2016;31:70–6. https://doi.org/10.1016/j.avsg.2015.08.024
Madigan MC, Singh MJ, Chaer RA, Al-Khoury G, Makaroun MS. Failure of type II endoleak treatment may be due to occult type I or III endoleaks. J Vasc Surg 2016;63(Suppl. 1):9S.
Mani K, Lees T, Beiles B, Jensen LP, Venermo M, Simo G, et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a Vascunet report. Eur J Vasc Endovasc Surg 2011;42:598–607. https://doi.org/10.1016/j.ejvs.2011.06.043
Mousa AY, Broce M, Yacoub M, Bates M, AbuRahma A. Significant predictors of survival following endovascular abdominal aortic aneurysm repair. Arterioscler Thromb Vasc Biol 2015;35:35S.
Ogawa Y, Nishimaki H, Osuga K, Ikeda O, Hongo N, Iwakoshi S, et al. A multi-institutional survey of interventional radiology for type II endoleaks after endovascular aortic repair: questionnaire results from the Japanese Society of Endoluminal Metallic Stents and Grafts in Japan. Jpn J Radiol 2016;34:564–71. https://doi.org/10.1007/s11604-016-0558-y
Oikonomou K, Ventin FC, Paraskevas KI, Geisselsoder P, Ritter W, Verhoeven EL. Early follow-up after endovascular aneurysm repair: is the first postoperative computed tomographic angiography scan necessary? J Endovasc Ther 2012;19:151–6.
Oliveira NG, Goncalves FB, De Ruiter Q, Schuurman R, Moll F, De Vries JP, et al. Standard endovascular aneurysm repair in patients with wide proximal aneurysm necks is associated with increased risk of adverse events. J Vasc Surg 2016;63(Suppl. 1):146S.
Oliveira NG, Goncalves FB, Van Rijn MJ, Moll F, Raa ST, Hoeks S, et al. Neck dilatation after endovascular aneurysm repair rarely exceeds the implanted endograft on long-term follow-up. J Vasc Surg 2016;63(Suppl. 1):S20–1.
Park KM, Kim DI, Kim YW, Do YS, Park HS, Park KB. Factors affecting anatomical changes after endovascular abdominal aortic aneurysm repair. Thorac Cardiovasc Surg 2015;63:139–45. https://doi.org/10.1055/s-0034-1387819
Peppelenbosch N, Geelkerken RH, Soong C, Cao P, Steinmetz OK, Teijink JA, et al. Endograft treatment of ruptured abdominal aortic aneurysms using the Talent aortouniiliac system: an international multicenter study. J Vasc Surg 2006;43:1111–23.
Petrik PV, Moore WS. Endoleaks following endovascular repair of abdominal aortic aneurysm: the predictive value of preoperative anatomic factors – a review of 100 cases. J Vasc Surg 2001;33:739–44.
Polo-De Santos M, Luengo-Matos S, Muñoz-Navarro B, Saz-Parkinson Z. Results from the monitoring use programme for endovascular repair of abdominal aortic aneurysms in Spain. Int Angiol 2009;28:181–91.
Qu L, Hetzel G, Raithel D. Seven years’ single center experience of Powerlink unibody bifurcated endograft for endovascular aortic aneurysm repair. J Cardiovasc Surg 2007;48:13–19.
Qu L, Raithel D. Experience with the Endologix Powerlink endograft in endovascular repair of abdominal aortic aneurysms with short and angulated necks. Perspect Vasc Surg Endovasc Ther 2008;20:158–66. https://doi.org/10.1177/1531003508320343
Qu L, Raithel D. From clinical trials to clinical practice: 612 cases treated with the Powerlink stent–graft for endovascular repair of AAA. J Cardiovasc Surg 2009;50:131–7.
Quinney BE, Nagre SB, Taylor SM, Passman MA, Patterson MA, Combs BR, et al. Secondary vascular interventions after EVAR. J Vasc Surg 2010;1:S5–6.
Sarlon G, Lapierre F, Sarlon E, Bartoli MA, Magnan PE, Branchereau A. [Endovascular aneurysm repair follow-up by unenhanced and contrast-enhanced duplex ultrasound.] J Mal Vasc 2009;34:34–43. https://doi.org/10.1016/j.jmv.2008.10.003
Sobocinski J, Chenorhokian H, Maurel B, Midulla M, Hertault A, Le Roux M, et al. The benefits of EVAR planning using a 3D workstation. Eur J Vasc Endovasc Surg 2013;46:418–23. https://doi.org/10.1016/j.ejvs.2013.07.018
Spanos K, Rountas C, Saleptsis V, Athanasoulas A, Fezoulidis I, Giannoukas AD. The association of simple renal cysts with abdominal aortic aneurysms and their impact on renal function after endovascular aneurysm repair. Vascular 2016;24:150–6. https://doi.org/10.1177/1708538115586917
Stokmans RA, Teijink JA, Forbes TL, Böckler D, Peeters PJ, Riambau V, et al. Early results from the ENGAGE registry: real-world performance of the Endurant stent graft for endovascular AAA repair in 1262 patients. Eur J Vasc Endovasc Surg 2012;44:369–75. https://doi.org/10.1016/j.ejvs.2012.07.005
Tang T, Sadat U, Walsh S, Hayes PD, ENGAGE Investigators. Comparison of the Endurant bifurcated endograft vs. aortouni-iliac stent–grafting in patients with abdominal aortic aneurysms: experience from the ENGAGE registry. J Endovasc Ther 2013;20:172–81. https://doi.org/10.1583/1545-1550-20.2.172
Thompson M. Predicting reintervention rates after EVAR for AAA: do all patients need the same surveillance? Cardiovasc Intervent Radiol 2013;36:S169–70.
Tomlinson J, McNamara J, Matloubieh J, Hart J, Singh MJ, Davies MG, et al. Intermediate follow-up after endovascular aneurysm repair: can we forgo CT scanning in certain patients? Ann Vasc Surg 2007;21:663–70.
Väärämäki S, Suominen V, Pimenoff G, Saarinen J, Salenius J. Long-term experience of endovascular aneurysm repair with Zenith prosthesis: diminishing graft-related complications over time. Ann Vasc Surg 2012;26:845–51. https://doi.org/10.1016/j.avsg.2012.01.022
van Zeggeren L, Bastos Gonçalves F, van Herwaarden JA, Zandvoort HJ, Werson DA, Vos JA, et al. Incidence and treatment results of Endurant endograft occlusion. J Vasc Surg 2013;57:1246–54. https://doi.org/10.1016/j.jvs.2012.11.069
Verhoeven EL, Tielliu IF, Prins TR, Zeebregts CJ, van Andringa de Kempenaer MG, Cinà CS, van den Dungen JJ. Frequency and outcome of re-interventions after endovascular repair for abdominal aortic aneurysm: a prospective cohort study. Eur J Vasc Endovasc Surg 2004;28:357–64. https://doi.org/10.1016/j.ejvs.2004.06.013
Verzini F, Isernia G, De Rango P, Simonte G, Parlani G, Loschi D, Cao P. Abdominal aortic endografting beyond the trials: a 15-year single-center experience comparing newer to older generation stent–grafts. J Endovasc Ther 2014;21:439–47. https://doi.org/10.1583/13-4599MR.1
Waasdorp EJ, Sterkenburg A, De Vries JPPM, Vos JA, Zarins CK, Moll FL. The importance of iliac fixation in preventing migration in suprarenal aortic endografts. J Endovasc Ther 2010;17:Ie31.
Waduud MA, Choong W, Lim S, McCormack L. Happily EVAR after? – retrospective analysis of longterm outcomes following endovascular aneurysm repair in Scotland. Int J Surg 2014;12:S112–13.
Waduud M, Ritchie M, Yadavali R, Lim SH, Buchanan F, Choong W, et al. Endovascular aneurysm repair (EVAR) – is imaging surveillance robust and does it influence long-term mortality? J Vasc Interv Radiol 2014;1:S14–15.
Warrier R, Miller R, Bond R, Robertson IK, Hewitt P, Scott A. Risk factors for type II endoleaks after endovascular repair of abdominal aortic aneurysms. ANZ J Surg 2008;78:61–3. https://doi.org/10.1111/j.1445-2197.2007.04378.x
Zandvoort HJ, Gonçalves FB, Verhagen HJ, Werson DA, Moll FL, de Vries JP, van Herwaarden JA. Results of endovascular repair of infrarenal aortic aneurysms using the Endurant stent graft. J Vasc Surg 2014;59:1195–202. https://doi.org/10.1016/j.jvs.2013.12.031
Outcomes not relevant or not reported by modality (n = 11)
Brooke BS, Hoel AW, Beck AW, Austin AJ, Kraiss L, Cronenwett JL, et al. Variation in surveillance imaging after major vascular surgery procedures: are we doing enough to follow our patients? J Vasc Surg 2016;63(Suppl. 1):186S–7S.
Byrne J, Mehta M, Dominguez I, Paty PS, Roddy SP, Feustel P, et al. Does Palmaz XL stent deployment for type 1 endoleak during elective or emergency endovascular aneurysm repair predict poor outcome? A multivariate analysis of 1470 patients. Ann Vasc Surg 2013;27:401–11. https://doi.org/10.1016/j.avsg.2012.10.007
Cao P, Verzini F, Parlani G, Rango PD, Parente B, Giordano G, et al. Predictive factors and clinical consequences of proximal aortic neck dilatation in 230 patients undergoing abdominal aorta aneurysm repair with self-expandable stent–grafts. J Vasc Surg 2003;37:1200–5.
Clevert DA, Gürtler VM, Meimarakis G, D’Anastasi M, Weidenhagen R, Reiser MF, Becker CR. Classification of endoleaks in the follow-up after EVAR using the time-to-peak of the contrast agent in CEUS examinations. Clin Hemorheol Microcirc 2013;55:183–91. https://doi.org/10.3233/CH-131701
Jouhannet C, Alsac JM, Julia P, Sapoval M, El Batti S, Di Primio M, Fabiani JN. Reinterventions for type 2 endoleaks with enlargement of the aneurismal sac after endovascular treatment of abdominal aortic aneurysms. Ann Vasc Surg 2014;28:192–200. https://doi.org/10.1016/j.avsg.2012.10.038
Kontopodis N, Tsetis D, Kehagias E, Daskalakis N, Galanakis N, Ioannou CV. Totally percutaneous endovascular aneurysm repair using the preclosing technique: towards the least invasive therapeutic alternative. Surg Laparosc Endosc Percutan Tech 2015;25:354–7. https://doi.org/10.1097/SLE.0000000000000176
Napoli V, Bargellini I, Sardella SG, Petruzzi P, Cioni R, Vignali C, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233:217–25. https://doi.org/10.1148/radiol.2331031767
Slovut DP, Bacharach JM. Aortic aneurysm repair with endovascular grafts: developing a graft surveillance program. Catheter Cardiovasc Interv 2004;62:252–61. https://doi.org/10.1002/ccd.20075
Sobocinski J, Briffa F, Holt PJ, Martin Gonzalez T, Spear R, Azzaoui R, et al. Evaluation of the Zenith low-profile abdominal aortic aneurysm stent graft. J Vasc Surg 2015;62:841–7. https://doi.org/10.1016/j.jvs.2015.04.452
Troisi N, Torsello G, Donas KP, Austermann M. Endurant stent–graft: a 2-year, single-center experience with a new commercially available device for the treatment of abdominal aortic aneurysms. J Endovasc Ther 2010;17:439–48. https://doi.org/10.1583/10-3090.1
Troutman DA, Chaudry M, Dougherty MJ, Calligaro KD. Endovascular aortic aneurysm repair surveillance may not be necessary for the first 3 years after an initially normal duplex postoperative study. J Vasc Surg 2014;60:558–62.
Duplicate publications (n = 3)
Blom AS, Troutman D, Beeman B, Yarchoan M, Dougherty MJ, Calligaro KD. Duplex ultrasound imaging to detect endovascular aneurysm repair limb stenosis or kinking after midterm follow-up. J Vasc Surg 2011;54:916.
Chisci E, Setacci F, De Donato G, Setacci C. Does the modality of surveillance imaging influence the pick-up rate of asymptomatic secondary interventions following endovascular aortic aneurysm repair (EVAR)? J Vasc Surg 2011;1:S71–2.
Freyrie A, Testi G, Faggioli GL, Gargiulo M, Giovanetti F, Serra C, Stella A. Ring-stents supported infrarenal aortic endograft fits well in abdominal aortic aneurysms with tortuous anatomy. J Cardiovasc Surg 2010;51:467–74.
Not obtained (n = 8)
Carter KA. Color duplex ultrasound for the evaluation of endovascular stent grafts following endovascular repair of abdominal aortic aneurysm. J Vasc Ultrasound 2005;29:137–41.
Huang D, Zhou M, Liu C, Qiao T, Ran F. [Analysis of endoleak in short term after endovascular aneurysm repair for abdominal aortic aneurysms]. Chung-Kuo Hsiu Fu Chung Chien Wai Ko Tsa Chih 2013;27:1355–8.
Jung EM, Krauss M, Ritter W, Bär I. [3D vascular imaging with power mode in planning and controlling percutaneously implanted abdominal aortic stent grafts.] Rofo 2000;172:888–93. https://doi.org/10.1055/s-2000-8365
Maggio D, Udini M, Mazzei R, Palombo D. [Colour duplex scanning using contrast medium in the follow-up of patients given endograft treatment for abdominal aorta aneurysms.] G Ital Chir Vasc 2001;8:33–42.
Nelms C, Carter K, DeMasi R, Meier G. Color duplex ultrasound characteristics: can we predict aortic aneurysm expansion following endovascular repair? J Vasc Ultrasound 2005;29:143–6.
Schlensak C, Doenst T, Uhrmeister P, Spillner G, Beyersdorf F. [Explantation of aortic stent–grafts.] Zentralbl Chir 2001;126:975–9. https://doi.org/10.1055/s-2001-19650
Xiao Y, Tian JM, Sheng J, Jing ZP, Gong J, Li XM. [The follow-up value of multi-slice CT for abdominal aortic aneurysms after endovascular exclusion.] J Intervent Radiol 2007;16:367–70.
Zhang HP, Guo W, Liu XP, Zhang GH, Liang FQ, Yin T, et al. [Endovascular aneurysm repair in high-surgical-risk abdominal aortic aneurysm patients: initial and long-term results.] Zhonghua Wai Ke Za Zhi 2011;49:873–7.
Appendix 6 Quality assessment result of individual included studies
| Study, first author (year of publication) | Representative sample | Inclusion/exclusion criteria clearly defined | Participants at a similar point in disease progression | Selection of patients was consecutive | Data collection undertaken prospectively | Groups comparable | Intervention(s) clearly defined | Intervention delivered by an experienced person | Intervention delivered in an appropriate setting | Important outcomes considered | Objective outcome measures used | Assessment of main outcomes blind | Follow-up long enough | Information on non-respondents, dropouts | Withdrawals likely to introduce bias | Length of follow-up similar between comparison groups | Important prognostic factors identified | Analyses adjusted for confounding factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chisci et al. (2012)66 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 0 |
| Nyheim et al. (2013)67 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 |
| Study, first author (year of publication) | Representative sample | Inclusion/exclusion criteria clearly defined | Participants at a similar point in disease progression | Selection of patients was consecutive | Data collection undertaken prospectively | Intervention(s) clearly defined | Intervention delivered by an experienced person | Intervention delivered in an appropriate setting | Important outcomes considered | Objective outcome measures used | Follow-up long enough | Information on non-respondents, dropouts | Withdrawals likely to introduce bias | Important prognostic factors identified |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bisdas et al. (2014)68 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Blom et al. (2012)69 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Bush et al. (2001)70 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 1 |
| Carroccio et al. (2002)71 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 2 |
| Chaer et al. (2009)40 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 1 |
| Cochennec et al. (2007)72 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 |
| Collins et al. (2007)73 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 2 |
| Donas et al. (2016)74 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 |
| Fossaceca et al. (2013)75 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 |
| Freyrie et al. (2014)76 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Ghotbi et al. (2010)77 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Harrison et al. (2011)41 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Karthikesalingam et al. (2012)78 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Köcher et al. (2004)79 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 1 |
| Kray et al. (2015)80 | 1 | 2 | 1 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Meier et al. (2001)81 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Oshin et al. (2010)82 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Parlani et al. (2002)83 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Schunn et al. (2000)84 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Soler et al. (2015)85 | 2 | 1 | 2 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| Stella et al. (2009)86 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 |
| Wolf et al. (2002)87 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Appendix 7 Review-level quality assessment of the diagnostic test performance systematic reviews
| Study, first author (year of publication) | CRD criteria | ||||
|---|---|---|---|---|---|
| Inclusion/exclusion criteria | Substantial search effort | Validity adequately assessed | Sufficient details of individual studies | Primary studies summarised | |
| Ashoke et al. (2005)127 | 1 | 1 | 1 | 1 | 1 |
| Bevis and Cooper (2012)128 | 1 | 2 | 2 | 2 | 0 |
| Cantisani et al. (2015)129 | 2 | 2 | 2 | 2 | 2 |
| Chung et al. (2015)130 | 1 | 1 | 1 | 1 | 1 |
| Karanikola et al. (2014)3 | 2 | 1 | 2 | 1 | 1 |
| Karthikesalingam et al. (2012)132 | 1 | 1 | 1 | 1 | 1 |
| Mirza et al. (2010)62 | 1 | 1 | 1 | 1 | 1 |
| Sun (2006)133 | 1 | 2 | 0 | 1 | 0 |
| Study, first author (year of publication) | AMSTAR criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A priori design | Duplicate selection and extraction | Comprehensive literature search | Grey literature included | List of included and excluded studies | Characteristics of included studies | Scientific quality assessed and documented | Scientific quality included in formulating conclusions | Appropriate methods to combine studies | Likelihood of publication bias assessed | Conflict of interest included | |
| Ashoke et al. (2005)127 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 1 | 2 | 2 |
| Bevis and Cooper (2012)128 | 0 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cantisani et al. (2015)129 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Chung et al. (2015)130 | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 1 |
| Karanikola et al. (2014)3 | 0 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 3 | 2 | 2 |
| Karthikesalingam et al. (2012)132 | 2 | 0 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Mirza et al. (2010)62 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sun (2006)133 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 1 |
Appendix 8 Characteristics of the included primary studies
| Study, first author (year of publication) | Type of study | Setting | Number of centres | Geographic location | Study duration, mean/median follow-up SD (range unless otherwise noted) | Total analysed | Age (years) | Number of male participants | Frequency | Imaging modality sequence | Aneurysm type (%) | Aneurysm diameter (mm) | Type of endograph |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparative cohort studies | |||||||||||||
| Chisci et al. (2012)66 | Retrospective | Tertiary referral centre | 1 | Italy |
Protocol I: 4 years Protocol II: 3.5 years |
Protocol II: 376 Protocol II: 341 |
Protocol I: 76.8 (± 8.7, range 67–90) Protocol II: 77.7 (± 7.0, range 66–92) p = 0.239 |
Protocol 1: 327 (87%) Protocol II: 286 (84%) p = 0.285 |
Protocol I: 1 month post operation, every 6 months thereafter | Protocol I: CTA, CDU and clinical examination |
Iliac artery aneurysm:p = 0.119 Bilateral iliac aneurysm:p = 0.241 |
Protocol I: 64 (+ 6) Protocol II: 61 (+ 8) p < 0.001 |
Talent (Medtronic, Santa Rosa, CA, USA):p < 0.0001 Zenith:p < 0.0001 Endurant:Anaconda:p < 0.05 Excluder (W L Gore & Associates, Inc., Flagstaff, AZ, USA):p < 0.0001 |
|
Protocol I mean: 1148 days (1–3204 days) Protocol II mean: 942 days (1–1512 days) |
Protocol II: 1 month post operation | Protocol II: CTA, CDU and plain radiography, clinical examination | |||||||||||
| Every 6 months | CDU, plain radiography and clinical examination | ||||||||||||
| Nyheim et al. (2013)67 | Prospective | Vascular centre | 1 | Norway | 5 years | 56 | 74 (range 55–87) | 50 (89.3%) | 6–8 weeks | CDU and plain radiography | AAA: 56 (100) | Median 60 (range 51–80) | Talent stent–graft |
| Median: 41.5 months (2–94 months) | 1 year | CTA, CDU and plain radiography | |||||||||||
| Poor visibility, presence of endoleak, AAA diameter increase on ultrasound or migration on PFA | CTA | ||||||||||||
| Non-comparative cohort studies | |||||||||||||
| Bisdas et al. (2014)68 | Prospective | Outpatient department of hospital and university clinic | 2 | Germany | 3 years | 273 | 73 (± 9) | 246 (90%) | Before discharge, after 1 year, annually up to 5 years | CTA |
Ruptured AAA (2) Symptomatic aneurysms: (7) AAA (91) |
Maximal AAA: 57 (range 40–87) | Endurant stent–graft (100%) |
| Median: 42 months (IQR 30.7–50.7 months) | At 6 months | CDU | |||||||||||
| Blom et al. (2012)69 | Prospective | Hospital | 1 | USA | 12 years | 248 | NR | NR | 1 week, 6 months and annually | Duplex ultrasound | AAA (100) | NR |
Ancure [(Guidant Cardiac and Vascular Division, Menlo Park, CA, USA) 4.8%] AneuRx [(AneuRx, Inc., Sunnyvale, CA, USA) 41.5%] Excluder (16.5%) Endologix [(Endologix Inc., Irvine, CA, USA) 5.2%] Zenith (31.9%) |
| Mean: 22.3 months (1–123 months) | |||||||||||||
| Bush et al. (2001)70 | Retrospective | University hospital | 1 | USA | 5.5 years |
104 High-risk group: 51 Low-risk group: 53 |
High-risk group: 72.2 (± 7.2) Low-risk group: 73.8 (± 7.1) |
NR | 1 month | CEU | Infrarenal AAA: (100) |
High-risk group: 58.2 (± 11.3) Low-risk group: 52.2 (± 11.5) |
EVT/Guidant endograft (Guidant Corp, Menlo Park, CA, USA) 71 (68.3%) AneuRx: 16 (15.4%) Excluder: 17 (16.3%) |
|
High-risk group: Mean 14.6 ± 12.4 months Low-risk group: Mean 17.7 ± 15.0 months |
6 months, 12 months, annually thereafter | CTA, CDU and plain radiography | |||||||||||
| Carroccio et al. (2002)71 | Prospective | Medical centre | 1 | USA | 4 years | 351 | NR | NR | 1, 3, 6, 12 months, annually thereafter | Duplex scan and 3-mm slice CTA | Infrarenal aortic aneurysms: (100) | NR |
AneuRx: 35 (10.0%) Ancure: 8 (2.3%) Gore (W L Gore & Associates, Sunnyvale, CA, USA) 25 (7.1%) Talent: 255 (72.6%) TERAMed (TERAMed Inc., Miami, FL, USA): 10 (2.8%) Vanguard (Boston Scientific, Marlborough, MA, USA): 18 (5.1%) |
| Mean: 20 ± 9 months (2–54 months) | |||||||||||||
| Chaer et al. (2009)40 | Prospective | University medical centre | 1 | USA | 3 years | 184 | 73.9 (± 7.1, range 52.6–96.4) | 159 (86.4%) | Annually | CDU | AAA: (100) | 54 (± 8) |
Ancure: 76 (41.3%) Zenith: 58 (31.5%) Excluder: 39 (21.2%) AneuRx: 7 (3.8%) Lifepath (Edwards Lifesciences, Irvine, CA, USA): 4 (2.2%) |
| Mean: 24 ± 13 months (12–48 months) | 1 month and 12 months, and only selectively thereafter | Helical CT | |||||||||||
| Cochennec et al. (2007)72 | Prospective | Department of vascular surgery at hospital | 1 | France | 10 years | 460 | 72.3 (± 8, range 47–88) | 435 (94.6%) | 1, 6 and 12 months, annually thereafter | Plain radiography, CDU and CTA |
Bifurcated: 369 Aortomonoiliac: 91 |
NR |
Zenith: 310 Vanguard: 60 Gore: 31 AneuRx: 21 Stenford (Stenford Groupe, Valendos S.A., Nanterre, France): 9 Stentor (MinTec, La Ciotat, France): 8 Talent (World Medical Inc., Medtronic Vascular, Sunrise, FL, USA): 7 |
| Mean: 28 months, median: 23.4 months | Clinical symptoms suggesting endograft thrombosis | CT scans or angiography and duplex scan | |||||||||||
| Collins et al. (2007)73 | Retrospective | University department of surgery | 1 | USA | 5 years (NR) | 160 | NR | NR | Every 6 months, first study within 1 month of EVAR | Ultrasound | AAA: (100) | NR |
Ancure: 82 (51.3%) AneuRx: 63 (39.4%) Other: 15 (9.4%) |
| Select cases, enlargement of the AAA sac and evidence of an endoleak | CT | ||||||||||||
| Dominguez et al. (2010)88 (abstract only) | Unclear | NR | NR | USA | 8 years (NR) | 1378 | NR |
EVAR with Palmaz (Cordis Corp, Miami Lakes, Fl, USA) stent: 90 (61.6%) EVAR only: 969 (78.7%) |
1 and 6 months, and every 12 months thereafter | CTA and duplex ultrasound | AAA: (100) | NR | NR |
| Donas et al. (2016)74 | Prospective | Vascular centres | 2 | Europe | 4 years | 128 | 76.6 (± 7.7) | 113 (88.3%) | 6 and 12 months, annually thereafter | CTA | AAA: (100) | 64.8 (± 14.6, range 48–135) | Endurant stent–graft (100%) |
| Mean: 24.6 months ± SD 17.4 months (0–61 months) | |||||||||||||
| Fargion et al. (2016)89 (abstract only) | Retrospective | University hospital | 1 | Italy | 16 years | 289 | NR | NR | Within 3 months post operation | CTA and duplex ultrasound | AAA: (100) | NR | NR |
| Median: 30 months (1–168 months) | Every 6 months | Duplex ultrasound | |||||||||||
| Fossaceca et al. (2013)75 | Retrospective | Hospital | 1 | Italy | 6 years | 222 | 76 (range 54–97) | 213 (95.9%) | 1 month, 12 months | CT (contrast enhanced) |
Urgent cases: Ruptured AAA – 34 (15.3) Symptomatic AAA – 20 (9.0%) Elective cases – 168 (75.7) |
59 (range 40–100) |
Excluder: 85 (38.3%) Zenith: 74 (33.3%) Endurant: 35 (15.8%) Evita (JOTECH GmbH, Hechingen, Germany): 24 (10.8%) Talent: 2 (0.9%0 LeMaitre (LeMaitre Vascular, Inc., Burlington, MA, USA): 1 (0.5%) Aorfix (Lombard Medical, Didcot, UK): 1 (0.5%) |
| Mean: 29.6 months | 6 months | CEU | |||||||||||
| Annually thereafter | Either CT angiography or CEU | ||||||||||||
| Freyrie et al. (2010)92 [secondary study to Freyrie et al. (2014)76] | Prospective | Hospital | NR | Italy | 2 years | 127 | 73.5 ± 6.9 (range 55–89) | 120 (94.4%) | 1, 3, 6 and 12 months and annually thereafter | CTA, CEU and CDU | AAA (100) |
Group A: 58.9 ± 12.8 Group B: 55.0 ± 8.2 |
Anaconda: 127 (100%) |
| Freyrie et al. (2014)76 | Prospective | Hospital | 1 | Italy | 3 years | 177 | 73.3 ± 7.4 (range 47–89) | 167 (94.4%) | Discharge, 6 and 12 months, yearly thereafter | CDU | NR | 55 ± 9.7 (range 45–99) | Anaconda: 177 (100%) |
| Mean: 32.9 ± 23.3 months (1–77 months) | 1 and 12 months | CTA | |||||||||||
| Ghotbi et al. (2010)77 | Unclear | Clinic | 1 | Germany | 4 years | 100 | 74.1 (range 44–91) | 91 (91%) | 1, 3 and 12 months, annually thereafter | CDU |
Asymptomatic aneurysm: 91 (91) Symptomatic aneurysm: 9 (9) |
56.1 (range 45–70) | Excluder: 100 (100%) |
| Mean: 20 months | 3 and 12 months | CTA | |||||||||||
| Harrison et al. (2011)41 | Retrospective | Tertiary referral centre | 1 | UK | 4 years | 194 | 76 (range 47–93) | 165 (85%) | 1 month | Abdominal radiography, CDU and CTA | NR | NR | NR |
| 12 months after EVAR, annually thereafter | Abdominal radiography and CDU | ||||||||||||
| Median: 36 months (12–57 months) | Inadequate DUS, abnormality identified | CTA | |||||||||||
| Karthikesalingam et al. (2012)78 | Retrospective | NR | NR | UK | 6 years | 478 | 75 ± 7 | 425 (88.9%) | 1.5, 3, 6, 9, 12 and 18 months and annually thereafter | CDU | AAA: (100) | 65 ± 13 |
Zenith: 295 (61.7%) Talent: 98 (20.5%) Other: 85 (17.8%) |
| Median: 43 months (1–92 months) | |||||||||||||
| Köcher et al. (2004)79 | Unclear | NR | NR | Czech Republic | 6 years | 120 | 70.7 (range 49–89) | 102 (85.0%) | 3, 6 and 12 months, annually thereafter | CTA and CDU | AAA: 120 (100) |
< 50: 18 (15%) 50–65: 73 (61%) > 65: 29 (24%) |
Ella stent–graft (Ella-CS Hradec Králové, Czech Republic): 120 (100%) |
| Mean: 20.7 months (2–60 months) |
Type I AAA: 6 (5) Type II AAA: 105 (87) Type III AAA: 9 (8) |
||||||||||||
|
Asymptomatic: 104 (86.6) Symptomatic: 16 (13.4) |
|||||||||||||
| Kray et al. (2015)80 | Retrospective | Medical centre | 1 | USA | 4 years | 191 | 74.4 ± 7.4 (range 53–92) | 161 (84.3%) | 1, 6 and 12 months | CTA and CDU | AAA: 191 (100) | NR | Zenith, Excluder and AneuRx |
| Maximum follow-up of 12 months | |||||||||||||
| Mazzaccaro et al. [201190 (abstract only)] | Unclear | NR | NR | NR | 12 years | 391 | 73 (range 49–91) | 363 (92.8%) | 2 months, 12 months and annually thereafter | CDU | AAA: 391 (100) | NR | NR |
| Median: 68 months (1–144 months) | 6 months | CEU | |||||||||||
| Meier et al. (2001)81 | Prospective | Clinic | 33 | USA | 5.5 years | 476 | NR | NR | 3, 6 and 12 months, annually thereafter | CTA, CDU and plain radiography | AAA: 476 (100) |
Major axis diameter: 57.5 ± 9.9 (range 34.8–92.7) Minor axis diameter: 51.6 ± 9.2 (range 30.9–86.4) |
Ancure endograft: 476 (100%) |
| Mean: 23.2 months (2.0–78.8) | |||||||||||||
| Oshin et al. (2010)82 | Retrospective | NR | NR | UK | 8 years | 295 | 75 (± 7) | 261 (88.5%) | 1 month, annually thereafter | Abdominal radiograph, CEU and CDU | AAA: 295 (100) | NR | Zenith: 295 (100%) |
| Maximum follow-up: 27 months (median 24 months) | Duplex ultrasound and radiography (CT selectively) | ||||||||||||
| Parlani et al. (2002)83 | Prospective | NR | 1 | Italy | 4 years | 336 |
Group A: 70 Group B: 71 p = NS |
Group A: 260 (94%) Group B: 56 (95%) p = NS |
1 month, annually thereafter | CTA |
Concomitant iliac aneurysm: 40 (11.9) Bilateral CIA aneurysm: 19 (5.7) AAA: 277 (82.4) |
Group A: 50 (IQR 45 ± 55; range 40 ± 86) Group B: 52 (IQR 48 ± 56; range 40 ± 74) p = NS |
AneuRx: 228 (68%) Anaconda: 27 (8%) Zenith: 2 (0.6%) Excluder Gore-Tex (W L Gore & Associates, Inc., Flagstaff, AZ, USA): 29 (9%) Endologix: 39 (12%) |
| Mean: 14 months (IQR 7–27 months; range 1–46 months) | 1, 6 and 12 months, every 6 months thereafter | Clinical evaluation, abdominal radiography and CDU | |||||||||||
| Schunn et al. (2000)84 | Prospective | Tertiary care | NR | Germany | 3 years | 190 | 68.7 (range 40–87) | 176 (92.6%) | 3–6 months | CEU and CDU (if possible), abdominal radiography | Intrarenal AAA: 190 (100) | NR |
Stentor system (August 1994 to May 1996): 190 (100%) Vanguard aortic stent–graft (May 1996 to July 1997): 190 (100%) |
| Mean:18 months, range to 46 | 6 to 12 months, 1 CTA scan per year in subsequent year | CTA and/or CDU | |||||||||||
| Soler et al. (2015)85 | Retrospective analysis of prospective registry | Centre | 1 | France | 11 years | 197 | 74.8 | 190 (96.4%) | 6, 12, 18 and 24 months, annually thereafter | CTA, plain abdominal radiography, CDU and a standard blood test | Intrarenal AAA: 197 (100). From 2003, EVAR was performed only for the patients who met the high-risk criteria defined by the French Agency for the Safety of Health Products | 56.7 ± 9.4 (range 42–110) |
Zenith: 124 (62.9%) PowerLink (Endologix, Irvine, CA, USA): 35 (17.8%) AneurX: 15 (7.6%) Talent: 9 (4.6%) Lifepath: 6 (3.0%) Home-made: 6 (3.0%) Excluder: 1 (0.5%) Bard device (Bard, Murray Hill, NJ, USA): 1 (0.5%) |
| Mean: 54.8 ± 35.9 months | |||||||||||||
| Stella et al. (2009)86 | Prospective | Hospital | 1 | Italy | 3 years | 100 | 73.9 (range, 55–89) | 94 (94%) | 1, 3, 6 and 12 months, annually thereafter | CTA, CDU and CEU | AAA: 100 (100) | 55.2 ± 3.4 (range 45–99) | NR |
| Mean: 23.2 ± 11.0 months (1.4–38.6 months) | |||||||||||||
| Wolf et al. (2002)87 | Unclear | University hospital | 1 | USA | 3.5 years | 154 | NR | NR | 6 months, 12 months, annually thereafter | CTA, CDU, abdominal radiograph and clinical examination | AAA: 154 (100) | 57.9 ± 9.4a | Bifurcated AneuRx (Medtronic): 154 (100%) |
| Mean: 15.8 ± 11.3 months (1–48 months) | |||||||||||||
Appendix 9 Type of clinical complications reported in the included studies
| Study, first author (year of publication) | Endoleak | Graft | Stenosis | Thrombosis | Limb occlusion | Aneurysm rupture | Infection | Ischaemia | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Type III | Type IV | Migration | Kinking | |||||||
| Comparative cohort studies | ||||||||||||
| Chisci et al. (2012)66 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Nyheim et al. (2013)67 | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Non-comparative cohort studies | ||||||||||||
| Bisdas et al. (2014)68 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Blom et al. (2012)69 | ✗ | |||||||||||
| Bush et al. (2001)70 | ✗ | ✗ | ||||||||||
| Carrocio et al. (2002)71 | ✗ | |||||||||||
| Chaer et al. (2009)40 | ✗ | ✗ | ✗ | |||||||||
| Cochennec et al. (2007)72 | ✗ | ✗ | ✗ | ✗ | ||||||||
| Collins et al. (2007)73 | ✗ | ✗ | ||||||||||
| Dominguez et al. (2010)88 (abstract only) | ✗ | ✗ | ✗ | |||||||||
| Donas et al. (2016)74 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Fargion et al. (2016)89 (abstract only) | ✗ | ✗ | ||||||||||
| Fossaceca et al. (2013)75 | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Freyrie et al. (2014)76 and Freyrie et al. (2010)92 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Ghotbi et al. (2010)77 | ✗ | ✗ | ✗ | ✗ | ||||||||
| Harrison et al. (2011)41 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Karthikesalingam et al. (2012)78 | ✗ | ✗ | ||||||||||
| Köcher et al. (2004)79 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Kray et al. (2015)80 | ✗ | ✗ | ||||||||||
| Mazzaccaro et al. (2011)90 | ✗ | ✗ | ✗ | ✗ | ||||||||
| Oshin et al. (2010)82 | ✗ | ✗ | ||||||||||
| Parlani et al. (2002)83 | ✗ | ✗ | ✗ | ✗ | ||||||||
| Schunn et al. (2000)84 | ✗ | ✗ | ✗ | |||||||||
| Soler et al. (2015)85 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Stella et al. (2009)86 | ✗ | ✗ | ✗ | ✗ | ||||||||
| Wolf et al. (2002)87 | ✗ | ✗ | ||||||||||
Appendix 10 Endovascular abdominal aortic aneurysm repair-related clinical complications
| Study, first author (year of publication) | Time point (follow-up if time point not reported) | Endoleak (specify subtypes, e.g. proximal, distal), n/N | Stenosis, n/N | Thrombosis, n/N | Aneurysm rupture, n/N | Limb occlusion, n/N | Infection, n/N | Ischaemia, n/N | Notes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | Type II | Type III | Type IV | |||||||||
| Comparative cohort studies | ||||||||||||
| Chisci et al. (2012)66 | Protocol I | |||||||||||
| < 30 days | 2/376 |
Migration (> 1 cm): 2/376 Conversion to open repair: 3/376 |
||||||||||
| > 30 days | 5/376 | 57/376 | 3/376 | 2/376 | 10/376 | 0/376 (graft) | 2/376 (bowel) | |||||
| Protocol II | ||||||||||||
| < 30 days | 1/341 |
Migration (> 1 cm): 1/341 Conversion to open repair: 1/341 |
||||||||||
| > 30 days | 4/341 | 45/341 | 3/341 | 1/341 | 8/341 | 0/341 (graft) | 0/341 (bowel) | |||||
| Nyheim et al. (2013)67 | < 30 days | 2/56 (4%) | 9/56 (16%) | 1/56 (2%) | 2/56 | Migration (> 10 mm): 4/56 (7%) (> 30 days) | ||||||
| 6 months | 1/56 (2%) | |||||||||||
| Non-comparative cohort studies | ||||||||||||
| Bisdas et al. (2014)68 | NR [median 42 months (IQR 31–50 months)] | 5/273 (1.8%) | 4/273 (1.5%) | 10/273a (3.7%) | 1/273 [0.4 (groin)] | 1/273 [0.4 (bowel ischaemia)] |
Renal artery occlusion: 1/273 False aneurysm: 1/273 Progression of aneurysmal disease: 1/273 Distal popliteal artery embolisation: 1/273 |
|||||
| 10 months | 1/273 (0.4%) | |||||||||||
| Blom et al. (2012)69 | 46 months (range 1 month–10 years) | 0/248 | ||||||||||
| Bush et al. (2001)70 | 1 month | 18/104 (17.3%) | Endoleak detected by CTA | |||||||||
| 2 months | 1/104 [1.0% (graft)] | |||||||||||
| 26 months | 2/104 [2.0% (hook fracture)] | Data combined for two groups | ||||||||||
| Carroccio et al. (2002)71 | Mean time to occlusion 5.2 ± 6.5 months (range 1 day to 23 months) | 26/702 limbs (3.7%) |
13/26 (50%) identified within 30 days 24/26 (92.3%) identified within 1 year |
|||||||||
| Chaer et al. (2009)40 | NR [mean: 24 ± 13 months (range 12–48 months)] | 2/184 (1.1%) | 1/184 (0.5%) | 0/184 | 0/184 (graft occlusion) | Graft occlusion: 0; one type II endoleak with stable sac size could not be identified on the CTA obtained 3 months later | ||||||
| Cochennec et al. (2007)72 | During follow-up (mean: 28 months) | 33/460 (7.2%) | 9/33 (27%) | |||||||||
| Intraoperative | 2/460 (0.4%) |
Graft migration: 9/460 (1.96) Graft limb kink: 13/460 (2.82) |
||||||||||
| Within the first week | 9/460 (2.0%) | |||||||||||
| Within 1 month | 14/460 (3.0%) | |||||||||||
| Within 6 months | 23/460 (5.0%) | |||||||||||
| Within 3 years | 30/460 (6.5%) | |||||||||||
| NR | Symptomatic: 27 | Acute ischaemia: (n = 9); rest pain: (n = 8); claudication (n = 10) | ||||||||||
| NR | Asymptomatic: 4 | Found on systematic duplex scan | ||||||||||
| Collins et al. (2007)73 | Ultrasound, NR (scans performed every 6 months until AAA sac resolved) | 7/359 scans | 26/359 |
Ultrasound scans: n = 359; CTA scans: n = 35; Combine types I and II:CTA discovered three endoleaks that were not seen with CDU. However, these scans were inadequate because of additional factors Of the 41 endoleaks found on CDU, only 14 were found on CTA |
||||||||
| CTA scans | 9/35 | 9/35 | ||||||||||
| Dominguez et al. (2010)88 | NR (follow-up NR) | 106/1378 (7.7%) | 329/1378 (23.9%) | 29/1378 [2.1% (ischaemic colitis)] |
Data combined for ‘EVAR only’ and ‘EVAR with Palmaz stent’ Stent–graft explant: 16/1378 |
|||||||
| Donas et al. (2016)74 | NR mean: 24.6 (SD 17.4 months; range 0–61 months) | 4/128 (3.1%) | 2/128 (1.6%) | 6/128 [4.7% (high grade)] | 8/128 (6.25%) | 1/128 (0.8%) | ||||||
| 27 months | 1/128 (0.8%) | Procedure-related late aortic rupture caused by the dislocation of iliac limbs and type III endoleaks; endotension, n = 1 | ||||||||||
| Fargion et al. (2016)89 (abstract only) | NR [median: 30 months (range 1–168 months)] | 9/289 | 38/289 | Total number of procedures, n = 289 | ||||||||
| Fossaceca et al. (2013)75 | > 30 days’ follow-up (mean 29.6 months) | 4/222 (1.8%) | 55/222 (24.8%) | 1/222 (0.45%) | 1/222 [0.45% (stent–graft explant)] | 10/222 (4.5%) | Type II endoleaks: eight treated; 47 managed conservatively with CEU follow-up; thrombosis in seven cases at the 1-month follow-up and in three cases at the 6-month follow-up | |||||
| Freyrie et al. (2010)92 | Second postoperative day | 1/127 | ||||||||||
| 6 months | 1/127 | |||||||||||
| 9 months | 2/127 | |||||||||||
| 1 year | 1/127 | |||||||||||
| NR | 1/127 | |||||||||||
| Freyrie et al. (2014)76 | NR [32.9 ± 23.3 months (range 1–77 months)] | 2/177 (1.1%) | 23/177 (13%) | 0/177 | 1/177 [0.6% (iliac leg)] | 10/177 [5.6% (iliac leg, n = 8; external iliac artery, n = 1; endograft, n = 1)] | 2/177 (1.1%) |
Endoleaks observed at completion angiography Renal artery occlusion: 2/177 (12-month follow-up) Migration: 0/177 |
||||
| Ghotbi et al. (2010)77 | Intraoperative | 3/100 (3%) | 24/100 (24%) | 0/100 (0%) | Migration: 1/100 (after 2 years) | |||||||
| After 3 months | 0/100 | 15/100 (15%) | ||||||||||
| After 12 months | 0/100 | 7/100 (7%) | ||||||||||
| Harrison et al. (2011)41 | During 4 years’ follow-up [median: 36 months (range 12–57 months)] | 1/194 (0.5%) | 4/194 (2.1%) | 1/194 (0.5%) | 1/194 (0.5%) | 2/194 (1.0%) |
Kinking: 1/194 (0.5) Indeterminant endoleaks: 3/194 (1.54) |
|||||
| Karthikesalingam et al. (2012)78 | NR [median: 34 months (range 1–92 months)] | 2/478 (0.4%) | Kinking: 36/478 (7.5) | |||||||||
| Köcher et al. (2004)79 | Early complications | 10/120 (8.3%); A: 7/120 (5.8%); B: 3/120 (2.5%) | 1/120 (0.8%) | |||||||||
| During follow-up [mean 20.7 months (range 2–60 months)] | 9/120 (7.5%) | 0/120 | 3/120 (2.5%) | 0/120 (graft infection) | Migration: 0/120 | |||||||
| Kray et al. (2015)80 | 1 month | 17/191 (8.9%) | ||||||||||
| 6 month | 18/191 (9.4%) | 0/191 | ||||||||||
| Meier et al. (2001)81 | 23.2 (range 2.0–78.8 months) | |||||||||||
| Mazzaccaro et al. (2011)90 | Long-term [median: 68 months (range 1–144 months)] | 31/391 (7.9%) | 3/391 (0.8%) | 8/391 (2.0%) | 5 (three fatal)/391 (1.3%) | |||||||
| At 30 days | 3/488 (0.6%) | |||||||||||
| Oshin et al. (2010)82 | 27 months | 11/583 limbs (1.83%) | Stent–graft limb occlusions | |||||||||
| Parlani et al. (2002)83 | At 30 days | 4/366 (1.1%) | 22/36 (6.0%) | 1/366 (0.3%) | 1/366 (0.3%) | Data combined for two groups | ||||||
| Schunn et al. (2000)84 | Intraoperatively or at the first postoperative imaging study | 32/190 (16.8%) | 32/190 (16.8%) | |||||||||
| 30 days | 10/190 (5.3) | |||||||||||
| Soler et al. (2015)85 | NR (mean: 54.8 ± 35.9 months) | 21/197 (10.6%) | 29/197 (14.7%) | 2/197 (1.0%) | 1/197 (0.5%) | 4/197 (2.0%) | Limb occlusion and stenosis: 29/197 (14.7%) | 3/197 (1.5%) | Data combined for two groups | |||
| Stella et al. (2009)86 | NR (mean: 23.3 months) | 0/100 | 0/100 | |||||||||
| Postoperative day 2 | 1/100 (1%) | |||||||||||
| 3 months | 26/100 | |||||||||||
| 4 months | 1/100 (1%) | |||||||||||
| 6 months | 1/100 (1%) | 4/200 (2%) | ||||||||||
| Wolf et al. (2002)87 | Late [mean: 15.8 ± 11.3 months (range 1–48 months)] | 1/154 (0.6%) | 1/154 (0.6%) | |||||||||
Appendix 11 Reintervention and type of secondary procedures performed
| Study, first author (year of publication) | Time point (follow-up if time point not reported) | Total reintervention, n/N | Type of reintervention (specify open, endovascular, revision, secondary/other) | Complication needing reintervention | Number of patients receiving reintervention | Health state (A, B or C) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparative cohort studies | ||||||||||
| Chisci et al. (2012)66 | Protocol I | Protocol II | Protocol I | Protocol II | Secondary interventions, number of interventions = 124 (Protocol I: n = 68; Protocol II: n = 56)a | Protocol I | Protocol II | Protocol I | Protocol II | |
| < 30 days (early secondary intervention) | 2/68 | 1/56 | Type I endoleak | 2 | 1 | |||||
| 0/68 | 1/56 | Type III endoleak | 0 | 1 | ||||||
| 3/68 | 2/56 | Limb occlusion | 3 | 2 | ||||||
| 2/68 | 0/56 | Limb ischaemia | 2 | 0 | ||||||
| 1/68 | 1/56 | Stent–graft limb kink | 1 | 1 | ||||||
| 1/68 | 0/56 | Bowel ischaemia | 1 | 0 | ||||||
| 8/68 | 5/56 | Access site-related problems | 8 | 5 | ||||||
| 1/68 | 0/56 | Blue toe syndrome | 1 | 0 | ||||||
| 0/68 | 1/56 | Renal infarction | 0 | 1 | ||||||
| > 30 days (late secondary intervention) | 5/68 | 4/56 | Type I endoleak | 5 | 4 | |||||
| 21/68 | 17/56 | Type II endoleak | 21 | 17 | ||||||
| 1/68 | 1/56 | Impending rupture, type II endoleak | 1 | 1 | ||||||
| 3/68 | 2/56 | Type III endoleak | 3 | 2 | ||||||
| 2/68 | 4/56 | Limb occlusion | 2 | 4 | ||||||
| 3/68 | 2/56 | Limb ischaemia | 3 | 2 | ||||||
| 5/68 | 10/56 | Stent–graft limb kink | 5 | 10 | ||||||
| 2/68 | 1/56 | Rupture | 2 | 1 | ||||||
| 1/68 | 0/56 | Bowel ischaemia | 1 | 0 | ||||||
| 5/68 | 4/56 | Access site-related problems | 5 | 4 | ||||||
| Nyheim 201367 | > 30 days | 14/56 (25%) patients | Secondary interventions | Endoleaks | 7 | |||||
| Endotension | 5 | |||||||||
| Migration | 2 | |||||||||
| Non-comparative cohort studies | ||||||||||
| Bisdas et al. (2014)68 | NR [median 42 months (IQR 31–50 months)] | 26/273 (9.5%) patients | Explanation of the endograft and open repair (open) | Type Ia endoleak | 1 | A (1) | ||||
| NR | Proximal cuff (NR) | Type Ia endoleak | 1 | A (1) | ||||||
| NR | Chimney endografting and use of Onyx (Endovascular Inc., Plymouth, MN, USA) (endovascular) | Type Ia endoleak | 1 | A (1) | ||||||
| NR | Iliac side branch device (NR) | Type Ib endoleak and progression of aneurysmal disease distal | 2 | A (1) | ||||||
| NR | Embolisation of the inferior mesentric artery (NR) | Type II endoleak | 3 | A (2) | ||||||
| NR | Open repair (open) | Type II endoleak | 1 | A (2) | ||||||
| 10 months | Implantation of an additional endurant limb (endovascular) | Type III endoleak | 1 | A (1) | ||||||
| NR | Iliac-to-renal bypass [no dialysis (NR)] | Renal artery occlusion | 1 | A (1) | ||||||
| NR | Thrombectomy and stenting (NR) | Limb occlusion | 6 | A (1/2) | ||||||
| NR | Crossover bypass (NR) | Limb occlusion | 4 | A (1/2) | ||||||
| NR | Hemicolectomy (NR) | Bowel ischaemia | 1 | A (1) | ||||||
| NR | Overstitch of the common femoral artery (NR) | False aneurysm | 1 | A (1) | ||||||
| NR | Thrombectomy, distal extension of the iliac limb with Advanta V12 stent–graft (Atrium Europe, Mijdrecht, the Netherlands) (NR) | Distal popliteal artery embolisation | 1 | A (1) | ||||||
| NR | Vacuum-assisted closure device (NR) | Groin infection | 1 | Not a surveillance issue | ||||||
| NR | Early reintervention (< 1 year) (NR) | NR | 13 | Inadequate information | ||||||
| NR | Late secondary procedures [> 4 years (NR)] | NR | 4b | Inadequate information | ||||||
| Blom et al. (2012)69 | At mean follow-up [46 months (range, 1 month–10 years)] | 12/496 limbs (2.4%) required intervention in 248 patients | NR | NR | NR | Inadequate information | ||||
| Bush et al. (2001)70 | 2 months | 3/104 (2.9%) patients | Late conversion (NR) | Graft infection | 1 | A (1/2) | ||||
| 26 months | Late conversion (NR) | Hook fracture | 2 | A (1/2) | ||||||
| Carroccio et al. (2002)71 | NR [mean: 20 ± 9 months (range 2–54 months)] | 26/702 limbs (3.7%) in 351 patients | Thrombolysis and stent [endovascular (NR)] | Limb occlusion | 2 | A (1) | ||||
| Axillary femoral bypass (NR) | 1 | A (1/2) | ||||||||
| Femorofemoral bypass (NR) | 13 | A (1/2) | ||||||||
| Axillary bifemoral bypass (NR) | 2 | A (1/2) | ||||||||
| Observation (NR) | 8 | |||||||||
| cChaer et al. (2009)40 | NR [mean: 24 ± 13 months (range 12–48 months)] | 2/184 (1.1%) patients | Limb extension (secondary intervention) | Type Ib endoleak | 2 | A (1/2) | ||||
| Cochennec et al. (2007)72 | NR (mean: 28 months; median: 23.4 months) | 33/460 (7.2%) patients | Thrombectomy and stent (endovascular) | Limb occlusion | 3 | A (1/2) | ||||
| NR | Thrombolysis and stent (endovascular) | NR | 6 | A (1) | ||||||
| NR | Femorofemoral bypass (NR) | NR | 19 | A (1/2) | ||||||
| NR | Axillofemoral bypass (NR) | NR | 3 | A (1/2) | ||||||
| NR | Conservative (NR) | NR | 2 | B | ||||||
| Collins et al. (2007)73 | NR (follow-up NR) | 33/359 (9.2%) procedures | NR | Type I endoleak | 7 | A1 | ||||
| NR | NR | Type II endoleak | 26 | A2 | ||||||
| Dominguez et al. (2010)88 (abstract) | NR (follow-up NR) | 273/1378 (19.8%) patients | Secondary intervention (NR) | NR | NR | Inadequate information | ||||
| Donas et al. (2016)74 | NR [24.6 ± SD 17.4 months (range 0–61 months)] | 20/128 (15.6%) patients | Endovascular management (NR) | High-grade stenosis of renal chimney | 6 | Not a complication of standard EVAR | ||||
| 2 months | Endovascular management (NR) | Chimney graft occlusion (majority of the occlusions were identified during the first 2 months) | 4 | Not a complication of standard EVAR | ||||||
| 45 days post operation | Ileorenal extra-anatomic bypass (NR) | NR | 1 | A1 | ||||||
| NR | Conservative treatment (NR) | NR | 1 | B | ||||||
| NR | Surgical ligation of the aneurysm sac (NR) | Endotension | 1 | A2 | ||||||
| 2.5 and 4 years | Transformation of single to multiple chimneys and tube placement (NR) | Type Ia endoleak | 2 | A1 | ||||||
| NR | Distal iliac limb extension (NR) | Type Ib endoleak | 1 | A1 | ||||||
| NR | Surgical conversion (NR) | Type Ib endoleak and infection | 1 | A (1/2) | ||||||
| 27 months | Iliac limb placement (NR) | Type III endoleak | 2 | A1 | ||||||
| NR | Endovascular management (NR) | Inadvertent coverage of the superior mesentric artery | 1 | Not a surveillance issue | ||||||
| Fargion et al. (2016)89 (abstract only) | NR [median: 30 months (range 1–168 months)] | 47/289 (16.3%) procedures | Reintervention (type NR) | Type II endoleak with significant sac enlargement | 38 | A2 | ||||
| Up to 3 months | NR | NR | 17 | Inadequate information | ||||||
| > 3 months | NR | NR | 21 | Inadequate information | ||||||
| NR | Reintervention, as type I developed after reintervention owing to type II endoleak (NR) | Type I endoleak | 9 | A1 | ||||||
| Up to 3 months | NR | NR | 5 | Inadequate information | ||||||
| More than 3 months | NR | NR | 4 | Inadequate information | ||||||
| Fossaceca et al. (2013)75 | NR (mean: 29.6 months) | 24/222 (10.8%) patients | Fibrinolysis (NR) | Thrombosis | 10 | A (1) | ||||
| Iliac extension (NR) | Type Ib endoleak | 2 | A (1) | |||||||
| Cuff (NR) | Type Ia and III endoleaks | 3 | A (1) | |||||||
| Thrombin injections (NR) | Type II endoleak | 8 | A (2) | |||||||
| Stent–graft removal (NR) | Infection | 1 | A (1/2) | |||||||
| Up to 30 days | 3/222 (1.4%) patients | Surgical conversion (NR) | NR | 3 | A (1/2) | |||||
| Freyrie et al. (2010)92 | 6 months | 3/127 (2.4%) patients | Iliac extension (NR) | Type Ib endoleak | 1 | A (1) | ||||
| 9 months | Thrombolytic therapy and percutaneous angioplasty (NR) | Iliac limb thrombosis | 1 | A (1/2) | ||||||
| 9 months | Surgical conversion (NR) | Iliac limb thrombosis | 1 | A (1/2) | ||||||
| Freyrie et al. (2014)76 | 0, 0, 4, 4, 4, 4, 6, 23 and 31 months | 20/177 (11.3%) procedures | Percutaneous balloon angioplasty iliac leg and stenting of external iliac artery or adjunctive urokinase (Urokinase medac, Medac Pharma GmbH, Wedel, Germany) and percutaneous balloon angioplasty stenting of external iliac artery (endovascular) | Iliac leg stenosis/thrombosis | 9 | A (2) | ||||
| 6 months | Renal artery chimney (endovascular) | Type Ia endoleak | 1 | A (1) | ||||||
| 9 months | Conversion (open repair) | Iliac leg thrombosis | 1 | A (1/2) | ||||||
| 32 and 36 months | Conversion (open repair) | Contained aortic rupture | 2 | A (1) | ||||||
| 33 months | Iliac leg surgical repair (open repair) | Type Ib endoleak | 1 | A (1) | ||||||
| 7, 35 and 45 months | Iliac leg extension (endovascular) | Type Ib endoleak | 3 | A (1) | ||||||
| 22 and 35 months | Inferior mesentric artery clipping (open repair) | Type II endoleak and AAA sac enlargement | 2 | A (2) | ||||||
| 0 months | Surgical drainage (open repair) | Retroperitoneal haematoma | 1 | A (1/2) | ||||||
| Ghotbi et al. (2010)77 | NR (mean: 20 months) | 6/100 (6%) patients | Stentangioplasty of the iliac artery (NR) | Plication at the distal end of the endoprosthesis | 3 | A (2) | ||||
| NR | Thrombectomy and TEA of the inguinal artery (NR) | Occlusion of iliac artery | 1 | A (1/2) | ||||||
| NR | Proximal banding (NR) | NR | 1 | A (1) | ||||||
| NR | Proximal cuff implantation (NR) | Migration and type I endoleak | 1 | A (1) | ||||||
| Harrison et al. (2011)41 | Up to first year of follow-up [median: 36 months (range 12–57 months)] | 9/194 (4.6%) | Embolisation (NR) | Type II endoleak | 1 | A (2) | ||||
| Iliac angioplasty (NR) | Stenosis | 1 | A (2) | |||||||
| Iliac stent (NR) | Kinked graft | 2 | A (2) | |||||||
| Open revision (NR) | Graft migration | 3 | A (1) | |||||||
| Bridging stent (NR) | Limb dislocation | 1 | A (1) | |||||||
| Stent (NR) | Graft angulation | 1 | A (2) | |||||||
| Karthikesalingam et al. (2012)78 | NR [median: 34 months (range 1–92 months)] | 38/478 patients (7.9%) | NR | Limb outflow impairment | 36 | B | ||||
| NR | Limb occlusion | 2 | A (1/2) | |||||||
| Köcher et al. (2004)79 | Early reinterventions [mean 20.7 months (range 2–60 months)] | 11/120 (9.2%) patients | Additional stent–graft | Type Ia | 2 | A (1) | ||||
| Extra large Palmaz stent | Type Ia | 1 | A (1) | |||||||
| Surgical conversion | Type Ia | 1 | A (1) | |||||||
| Surgical banding neck | Type Ia | 3 | A (1/2) | |||||||
| Spontaneous seal (NR) | Type Ib | 1 | C | |||||||
| Additional stent–graft | Type Ib | 1 | A (1) | |||||||
| Surgical conversion | Type Ib | 1 | A (1) | |||||||
| Endovascular conversion | Type IIIa | 1 | A (1) | |||||||
| During follow-up, NR | 5/120 (4.2%) patients | Laparoscopic clipping (NR) | Type II endoleaks | 2 | A (2) | |||||
| Femorofemoral crossover bypass | Thrombosis | 3 | A (1/2) | |||||||
| Kray et al. (2015)80 | Up to 6 months (maximum follow-up of 12 months) | 0/191 (0%) patients | NR | NR | NR | Inadequate information | ||||
| > 6 months | 13/191 (6.8%) | NR | NR | NR | Inadequate information | |||||
| Mazzaccaro et al. (2011)90 | Long-term [median: 68 months (range 1–144 months)] | 45/391 (11.5%) patients | NR | NR | NR | Inadequate information | ||||
| Oshin et al. (2010)82 | 27 months | 11/583 limbs (1.8%) | Conservative management | Stent–graft limb occlusions | 3 | C | ||||
| Femorofemoral crossover graft | 7 | A | ||||||||
| Mechanical thrombectomy and secondary adjunctive stenting | 1 | A | ||||||||
| Parlani et al. (2002)83 | NR [mean: 14 months (IQR 7–27 months; range 1–46 months)] | 19/336 (5.6%) patients | NR | NR | NR | Inadequate information | ||||
| Schunn et al. (2000)84 | Early (< 7 days) | 14/190 (7.4%) | Conversion to conventional transabdominal repair | Malpositioned graft | 4 | |||||
| Prosthetic defect | 5 | |||||||||
| Endoleak | 2 | |||||||||
| Occlusion and endoleak | 1 | |||||||||
| Arterial disruption | 2 | |||||||||
| Late [mean: 20.9 months (range 1.7–35.6 months)] | 17/190 (8.9%) | Prosthetic defect | 1 | |||||||
| Endoleak | 11 | |||||||||
| Occlusion and endoleak | 3 | |||||||||
| Distal secondary aneurysm | 2 | |||||||||
| dSoler et al. (2015)85 | During follow-up (mean: 31.9 months) | 70 procedures 47/197 patients (23.8%) | NR | Type Ia endoleak | 11 | A (1) | ||||
| NR | ||||||||||
| NR | NR | Type Ib endoleak | 7 | A (1) | ||||||
| NR | NR | Type II endoleak | 24 | A (2) | ||||||
| NR | NR | Type III endoleak | 1 | A (1) | ||||||
| NR | NR | Endotension | 1 | A (2) | ||||||
| NR | NR | Stenosis and occlusions | 21 | A (1/2) | ||||||
| NR | NR | Infection | 2 | A (1/2) | ||||||
| NRe | NR | Rupture | 3 | A (1) | ||||||
| Stella et al. (2009)86 | 4 months | 6/100 (6%) patients | Iliac extension (NR) | Type I endoleak | 1 | A (1) | ||||
| Up to 6 months | Thrombolytic therapy (unsuccessful) and surgical conversion (NR) | Iliac limb thrombosis | 1 | A (1/2) | ||||||
| Thrombolytic therapy and percutaneous angioplasty iliac limbs (NR) | Iliac limb thrombosis | 1 | A (1) | |||||||
| 1 and 8 months | Thrombectomy (NR) | External iliac artery obstructions | 2 | A (1/2) | ||||||
| NR [mean: 23.2 ± 11.0 months (range 1.4–38.6 months)] | Endograft extended to both external iliac arteries and surgical revascularisation of the right hypogastric artery (NR) | Spinal cord ischaemia | 1 | A (1) | ||||||
| Wolf et al. (2002)87 | During follow-up period [mean: 15.8 ± 11.3 months (range 1–48 months)] | 23/154 (15.0%) patients | NR | Presence of endoleak with expanding or non-shrinking aneurysm | 21 | A (2) | ||||
| NR | NR | Volume increased without a demonstrable endoleak | 1 | A (2) | ||||||
| NR | NR | Migration occurred and proximal fixation appeared to be insecure | 1 | A (1) | ||||||
Appendix 12 Results on aneurysm shrinkage, enlargement and stability, as reported in cohort studies
| Study, first author (year of publication) | Total number of patients | Mean/median follow-up ± SD (range, unless specified otherwise) | Aneurysm diameter/sac size (mm) | Change | Other indicators | |
|---|---|---|---|---|---|---|
| At baseline | At last follow-up | |||||
| Comparative cohort studies | ||||||
| Nyheim et al. (2013)67 | 56 | Median: 41.5 months (2–94 months) | 57 (range 30–87) | NR | NR | Identified increased diameter (≥ 5 mm) without evidence of an endoleak: 6/56 |
| Non-comparative cohort studies | ||||||
| Bisdas et al. (2014)68 | 273 | Median: 42 months (IQR 30.7–50.7 months) | NR | NR |
AAA shrinkage Median: 9 mm (IQR 3–15 mm) |
Aneurysm shrinkage of > 5 mm: 158/273 (57.8%) |
|
Chaer et al. (2009)40 (CTA-US) |
184 | Mean: 24 ± 13 months (1–4 years) | Mean: 54 ± 8 | Mean: 39 ± 11 | Mean AAA diameter decreased by 15 mm | NR |
| Donas et al. (2016)74 | 128 | Mean: 24.6 ± 17.4 months (0–61 months) | Mean: 64.8 ± 14.6 (range 48–135) | Mean: 60.1 ± 16.3a | NR |
Aneurysm shrinkage: 87/128 (68%) Stable aneurysm: 29/128 (23%) Aneurysm enlargement: 12/128 (9%) |
|
Freyrie et al. (2014)76 Ultrasound and CTA-ultrasound |
177 | Mean: 32.9 ± 23.3 months (1–77 months) | Mean: 55 ± 9.7 (range 45–99) | NR |
AAA shrinkage Mean: 10 ± 8.7 (range –10 to 44) |
Aneurysm shrinkage: 130/177 (73.4%) Stable aneurysm: 40/177 (22.6%) Aneurysm enlargement of > 5 mm: 7/177 (4%) |
|
Harrison et al. (2011)41 Ultrasound and CTA-ultrasound |
194 | Median: 36 months (12–57 months) | NR | NR | NR | Aneurysm expansion: 2/194 (≈1%) |
| Köcher et al. (2004)79 | 120 | Mean: 20.7 months (2–60 months) | NR | NR | NR | Follow-up of > 12 months:
|
| Meier et al. (2001)81 | 476 | Mean: 23.2 months (2–78.8 months) | Major axis diameter:
|
NR | AAA shrinkage: Mean: –7.3 ± 9.2 (range –50.6 to 32.6) | Rate of overall aneurysm contraction: (–3.75 mm/year) |
| Parlani et al. (2002)83 | 366 | Mean: 14 months (IQR 7–27; range 1–46 months) |
Group A: 50 (IQR 45 ± 55; range 40 ± 86); group B: 52 (IQR 48 ± 56; range 40 ± 74) p = NS |
NR | AAA diameter
|
Decrease of diameter of > 2 mm: 182/366 (56%) Unchanged diameter: 127/366 (39%) Increase of > 2 mm: 21/366 (6%) Of the 77 iliac aneurysms treated without immediate death and/or conversion: |
| Soler et al. (2015)85 | 197 | Mean: 54.8 ± 35.9 months |
Group A: 55.8 mm Group B: 57.7 mm Population: 56.7 mm |
NR | NR | Reduction of ≥ 10 mm of the maximum aneurysmal diameter after EVAR in 51.8% of the patients |
| Stella et al. (2009)86 | 100 | Mean: 23.2 ± 11.0 months (1.4–38.6 months) | Mean: 55.2 ± 3.4 (range 45–99) | NR | NR |
Diameter of AAA was unchanged: 98/100 (98%) Increase in aneurysmal sack of 6 mm: 2/100 (2%) |
| Wolf et al. (2002)87 | 154 | Mean: 15.8 ± 11.3 months (1–48 months) | 57.9 ± 9.4b | 58.3 ± 8.9 |
Overall change in transverse diameter after endovascular repair: –0.29 mm/month ± 0.73 Absolute changes in transverse diameter during mean follow-up of 7 ± 3 months (range 3–24 months) after endovascular repair No endoleak: –2.7 mm ± 4.5 Endoleak present: 1.0 mm ± 3.9 |
|
Appendix 13 Mortality rates reported in the included cohort studies
| Study, first author (year of publication) | Time point (follow-up if time point not specified) | Mortality, n/N (%) | Survival | Notes | ||
|---|---|---|---|---|---|---|
| All-cause | AAA-related | Overall rate | Disease-free rate, n/N (%) | |||
| Comparative cohort studies | ||||||
| Chisci et al. (2012)66 | Protocol I | |||||
| 30 days | 8/376 (2.1) | |||||
| 3 years | 64/376 (17) | 19/376 (5.1) | 357/376 (94.9) | |||
| Protocol II | ||||||
| 30 days | 6/341 (1.8) | |||||
| 3 years | 55/341 (16.1) | 15/341 (4.4) | 326/341 (95.6) | |||
| Nyheim et al. (2012)67 | > 30 days | 9/56 (16) | All died of other causes | |||
| Non-comparative cohort studies | ||||||
| Bisdas et al. (2014)68 | Median: 42 months (IQR 31–50 months) | 78/273 (28.6) | 1/273 (0.4) |
All-cause mortality (including AAA-related mortality): cardiac (n = 29), carcinoma (n = 13), pulmonary (n = 14), sepsis (n = 6), stroke (n = 4), suicide (n = 1), unknown (n = 10) AAA related: technical failure to advance the endograft. The patient was denied open repair because of severe heart insufficiency and the aneurysm ruptured 2 weeks later |
||
| 3 years post operation | 77% | |||||
| 4 years post operation | 73% | |||||
| 5 years post operation | 67% | |||||
| Bush et al. (2001)70 |
High-risk group: mean 14.6 months (± 12.4 months) Low-risk group: mean 17.7 months (± 15.0 months) |
12/104 (11.5) | 0/104 (0) | NR | NR | All-cause mortality: conversion from endovascular to open repair (n = 1), aborted procedure and severe coronary artery disease (n = 1), successful endovascular repair without evidence of postoperative endoleak (n = 1), severe heart failure (n = 1). None of the reported late deaths were related to the initial endovascular procedure, device failure or late aneurysm rupture |
| Up to 30 days post operation | 5/104 (4.8) | |||||
| Chaer et al. (2009)40 | Mean: 24 months (± 13 months, range 1–4 years) | 5/184 (2.7) | 1/184 (0.5) | All-cause mortality (including AAA-related mortality): deaths from lung cancer (n = 2), acute coronary event with post-infarction heart failure and a prolonged stay in the coronary care unit (n = 2); AAA related: (n = 1) | ||
| Cochennec et al. (2007)72 | Mean: 28 months; median: 23.4 months | 18/460 (3.9) | NR | NR | ||
| Collins et al. (2007)73 | NR (study duration 5 years) | 7/160 (4.4) | NR | NR | ||
| aDominguez et al. (2010)88 | Up to 30 day post operation | 22/1378 (1.6) | NR | NR | ||
| Donas et al. (2016)74 | Up to 30 day post operation | 1/128 (0.8) | NR | NR | Cause of death: cardiac decompensation | |
| Mean: 24.6 months, ± SD 17.4 months (range 0–61 months) | 22/128 (17.2) | NR | NR | All-cause mortality: cardiac insufficiency and tumour as major causes | ||
| Fossaceca et al. (2013)75 | Up to 30 days post operation | 17/222 (7.7) | NR | NR | All-cause mortality: heart failure, respiratory failure and aspiration pneumoniab | |
| During follow-up (mean: 29.6 months) | 14/205 (6.8) | 0/205 (%) | NR | NR | Unrelated to aneurysm | |
| Freyrie et al. (2014)76 | Up to 30 days post operation | 2/177 (1.1) | NR | NR | ||
| 3 years | 86.2% | |||||
| Ghotbi et al. (2010)77 | > 30 days post operation | 0/100 (0) | ||||
| Harrison et al. (2011)41 | 1 year post operation | 25/194 (12.9) | 1/194 (0.5) |
All-cause mortality (includes AAA-related mortality): ischaemic heart disease (n = 6), malignancy (n = 10), gastrointestinal disease (n = 2), respiratory illness (n = 3), cerebrovascular accident (n = 2), renal failure (n = 1) AAA-related mortality: (n = 1) |
||
| Köcher et al. (2004)79 | Perioperative | 4/120 (3.3) | ||||
| During follow-up [mean: 20.7 months (range 2–60 months)] | 13/120 (10.8) | All-cause mortality: cardiac, pulmonary or malignancyc | ||||
| Kray et al. (2015)80 | Up to 12 months’ follow-up | 0/191d (0) | ||||
| Mazzaccaro et al. (2011)90 | Up to 30 days post operation | 6/488 (1.2) | ||||
| > 30 days post operation | 77/391 (19.7) | |||||
| 144 months | 32.80 ( ± 4.4) | |||||
| Parlani et al. (2002)83 | Perioperative | 4/336 (1.2) | 1/336 (0.4) | All-cause mortality (including AAA-related mortality): congestive heart failure in a patient with severe respiratory and cardiac disease (n = 1), pulmonary oedema (n = 1), massive haemorrhage from intraprocedural aortic rupture requiring immediate conversion to open repair (n = 1), and sepsis in a patient affected by chronic leukaemia and tender AAA (n = 1) | ||
| Late mortality | 21/336 (6.3) | Not related to the endovascular procedure | ||||
| Schunn et al. (2000)84 | Up to 30 days post operation | 1/190 (0.5) | Succumbed to retroperitoneal haemorrhage attributable to an unrecognised iliac artery puncture | |||
| Soler et al. (2015)85 | Mean 54.8 months ( ± 35.9 months) | 83/197 (42) |
Group 1 (diameter reduction of ≥ 10 mm during follow-up): 34/102 Group 2 (diameters were increased, stable, or reduced by < 10 mm during follow-up): 49/95 (p = 0.0144) |
|||
| 5 years post operation |
Group 1: 71% Group 2: 58.7% p < 0.0001 |
|||||
| Stella et al. (2009)86 | Mean: 23.3 months | 6/100 (6) | Data given for 1–24 months; rates given for clinical success | |||
| 24 months follow-up | 87.90% | |||||
| Wolf et al. (2002)87 | < 30 days post operation | 2/154 (1.3) | Both died of myocardial infarction | |||
| > 30 days | 25/154 (16.2) | 0/154 (0) | None of the deaths were aneurysm related; post-mortem examinations were performed in six cases | |||
Appendix 14 State-transition diagram for the surveillance after endovascular abdominal aortic aneurysm repair Markov model
FIGURE 13.
State-transition diagram for the surveillance after EVAR Markov model. FP, false positive; TP, true positive.
The whole cohort starts at the ‘Normal (no residual EVAR complications)’ Markov state. The arrows in the model show possible transitions from each state. Arrows from and to the same Markov state have not been drawn for simplicity. Individuals can remain in any of the Markov states for more than one cycle. The exception to this is the ‘TP – surgery (elective)’ state, as this is a one-cycle tunnel state. Arrows to the ‘Death’ Markov states have also been omitted for simplicity. Age-adjusted general population mortality has been accounted for, and individuals can move from any Markov state to the ‘Death (general population mortality)’ state. In addition, individuals with EVAR-related abnormalities can die as a result of EVAR-related complications, moving to the ‘Death (EVAR related)’ Markov state.
Appendix 15 Economic evaluation sensitivity analyses results
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 50 | CDU | 3484 | 6.5532 | |||
| CTA | 3680 | 197 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4333 | 849 | 6.5594 | 0.0062 | 136,513 | |
| CDU and CTA, then CDU | 4448 | 115 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5290 | 957 | 6.5598 | 0.0003 | 2,981,660 | |
| 100 | CDU | 3654 | 6.5532 | |||
| CTA | 3762 | 108 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4542 | 888 | 6.5594 | 0.0062 | 142,673 | |
| CDU and CTA, then CDU | 4605 | 64 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5486 | 945 | 6.5598 | 0.0003 | 2,943,149 | |
| 150 | CDU | 3824 | 6.5532 | |||
| CTA | 3844 | 20 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4750 | 926 | 6.5594 | 0.0062 | 148,832 | |
| CDU and CTA, then CDU | 4762 | 12 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5682 | 932 | 6.5598 | 0.0003 | 2,904,637 | |
| 200 | CTA | 3925 | 6.5517 | |||
| CDU | 3994 | 69 | 6.5532 | 0.0015 | 45,791 | |
| CDU and CTA, then CDU | 4920 | 925 | 6.5543 | 0.0011 | 827,921 | |
| CEU | 4959 | 39 | 6.5594 | 0.0051 | 7666 | |
| CEU and CTA, then CEU | 5878 | 920 | 6.5598 | 0.0003 | 2,866,125 | |
| 250 | CTA | 4007 | 6.5517 | |||
| CDU | 4165 | 157 | 6.5532 | 0.0015 | 104,639 | |
| CDU and CTA, then CDU | 5077 | 912 | 6.5543 | 0.0011 | 816,205 | |
| CEU | 5167 | 91 | 6.5594 | 0.0051 | 17,739 | |
| CEU and CTA, then CEU | 6075 | 907 | 6.5598 | 0.0003 | 2,827,613 |
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 80 | CTA | 3438 | 6.5517 | |||
| CDU | 3660 | 221 | 6.5532 | 0.0015 | 147,221 | |
| CEU | 4549 | 264 | 6.5594 | 0.0051 | 51,682 | |
| CDU and CTA, then CDU | 4285 | 625 | 6.5543 | 0.0011 | 559,439 | |
| CEU and CTA, then CEU | 5169 | 620 | 6.5598 | 0.0003 | 1,932,376 | |
| 90 | CTA | 3539 | 6.5517 | |||
| CDU | 3694 | 154 | 6.5532 | 0.0015 | 102,632 | |
| CEU | 4590 | 190 | 6.5594 | 0.0051 | 37,132 | |
| CDU and CTA, then CDU | 4401 | 707 | 6.5543 | 0.0011 | 632,756 | |
| CEU and CTA, then CEU | 5292 | 702 | 6.5598 | 0.0003 | 2,186,672 | |
| 100 | CTA | 3640 | 6.5517 | |||
| CDU | 3728 | 87 | 6.5532 | 0.0015 | 58,044 | |
| CEU | 4632 | 115 | 6.5594 | 0.0051 | 22,582 | |
| CDU and CTA, then CDU | 4517 | 789 | 6.5543 | 0.0011 | 706,074 | |
| CEU and CTA, then CEU | 5415 | 783 | 6.5598 | 0.0003 | 2,440,968 | |
| 110 | CTA | 3741 | 6.5517 | |||
| CDU | 3762 | 20 | 6.5532 | 0.0015 | 13,455 | |
| CEU | 4674 | 41 | 6.5594 | 0.0051 | 8032 | |
| CDU and CTA, then CDU | 4633 | 871 | 6.5543 | 0.0011 | 779,392 | |
| CEU and CTA, then CEU | 5539 | 865 | 6.5598 | 0.0003 | 2,695,263 | |
| 120 | CDU | 3796 | 6.5532 | |||
| CTA | 3843 | 47 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4715 | 920 | 6.5594 | 0.0062 | 147,807 | |
| CDU and CTA, then CDU | 4749 | 33 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5662 | 947 | 6.5598 | 0.0003 | 2,949,559 | |
| 130 | CDU | 3830 | 6.5532 | |||
| CTA | 3944 | 114 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4757 | 927 | 6.5594 | 0.0062 | 149,039 | |
| CDU and CTA, then CDU | 4865 | 108 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5785 | 1028 | 6.5598 | 0.0003 | 3,203,854 | |
| 140 | CDU | 3864 | 6.5532 | |||
| CTA | 4045 | 181 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4799 | 935 | 6.5594 | 0.0062 | 150,271 | |
| CDU and CTA, then CDU | 4981 | 182 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5909 | 1110 | 6.5598 | 0.0003 | 3,458,150 | |
| 150 | CDU | 3898 | 6.5532 | |||
| CTA | 4146 | 248 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4841 | 943 | 6.5594 | 0.0062 | 151,503 | |
| CDU and CTA, then CDU | 5097 | 256 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 6032 | 1191 | 6.5598 | 0.0003 | 3,712,445 |
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 6000 | CDU | 3163 | 6.5532 | |||
| CTA | 3217 | 54 | 6.5517 | –0.0015 | Dominated | |
| CEU | 3997 | 834 | 6.5594 | 0.0062 | 134,071 | |
| CDU and CTA, then CDU | 4097 | 100 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 4930 | 932 | 6.5598 | 0.0003 | 2,905,650 | |
| 8000 | CDU | 3375 | 6.5532 | |||
| CTA | 3423 | 48 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4238 | 863 | 6.5594 | 0.0062 | 138,646 | |
| CDU and CTA, then CDU | 4311 | 74 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5171 | 933 | 6.5598 | 0.0003 | 2,907,853 | |
| 10,000 | CDU | 3587 | 6.5532 | |||
| CTA | 3629 | 43 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4478 | 891 | 6.5594 | 0.0062 | 143,222 | |
| CDU and CTA, then CDU | 4526 | 48 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5412 | 934 | 6.5598 | 0.0003 | 2,910,056 | |
| 12,000 | CDU | 3799 | 6.5532 | |||
| CTA | 3835 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4718 | 920 | 6.5594 | 0.0062 | 147,798 | |
| CDU and CTA, then CDU | 4740 | 21 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5653 | 935 | 6.5598 | 0.0003 | 2,912,260 | |
| 14,000 | CDU | 4011 | 6.5532 | |||
| CTA | 4041 | 31 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4959 | 5 | 6.5594 | 0.0051 | 960 | |
| CDU and CTA, then CDU | 4954 | 943 | 6.5543 | 0.0011 | 843,974 | |
| CEU and CTA, then CEU | 5894 | 935 | 6.5598 | 0.0003 | 2,914,463 | |
| 16,000 | CDU | 4222 | 6.5532 | |||
| CTA | 4247 | 25 | 6.5517 | –0.0015 | Dominated | |
| CEU | 5199 | 31 | 6.5594 | 0.0051 | 6106 | |
| CDU and CTA, then CDU | 5168 | 945 | 6.5543 | 0.0011 | 845,943 | |
| CEU and CTA, then CEU | 6135 | 936 | 6.5598 | 0.0003 | 2,916,666 |
| Value (£) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 2000 | CDU | 2950 | 6.5532 | |||
| CTA | 2988 | 38 | 6.5517 | –0.0015 | Dominated | |
| CEU | 3864 | 914 | 6.5594 | 0.0062 | 146,834 | |
| CDU and CTA, then CDU | 3891 | 27 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 4798 | 934 | 6.5598 | 0.0003 | 2,911,629 | |
| 6000 | CDU | 3264 | 6.5532 | |||
| CTA | 3302 | 38 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4180 | 915 | 6.5594 | 0.0062 | 147,130 | |
| CDU and CTA, then CDU | 4205 | 25 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5114 | 934 | 6.5598 | 0.0003 | 2,911,834 | |
| 10,000 | CDU | 3578 | 6.5532 | |||
| CTA | 3615 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4495 | 917 | 6.5594 | 0.0062 | 147,426 | |
| CDU and CTA, then CDU | 4519 | 23 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5430 | 935 | 6.5598 | 0.0003 | 2,912,039 | |
| 14,000 | CDU | 3892 | 6.5532 | |||
| CTA | 3929 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4811 | 919 | 6.5594 | 0.0062 | 147,722 | |
| CDU and CTA, then CDU | 4833 | 22 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5746 | 935 | 6.5598 | 0.0003 | 2,912,243 | |
| 18,000 | CDU | 4206 | 6.5532 | |||
| CTA | 4243 | 36 | 6.5517 | –0.0015 | Dominated | |
| CEU | 5127 | 921 | 6.5594 | 0.0062 | 148,018 | |
| CDU and CTA, then CDU | 5147 | 20 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 6062 | 935 | 6.5598 | 0.0003 | 2,912,448 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.5 | CDU | 3791 | 4.4668 | |||
| CTA | 3828 | 37 | 4.4657 | –0.0011 | Dominated | |
| CEU | 4709 | 919 | 4.4712 | 0.0044 | 208,846 | |
| CDU and CTA, then CDU | 4732 | 22 | 4.4676 | –0.0036 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 4.4714 | 0.0002 | 4,212,790 | |
| 0.6 | CDU | 3791 | 5.3602 | |||
| CTA | 3828 | 37 | 5.3589 | –0.0013 | Dominated | |
| CEU | 4709 | 919 | 5.3654 | 0.0053 | 174,038 | |
| CDU and CTA, then CDU | 4732 | 22 | 5.3611 | –0.0043 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 5.3657 | 0.0003 | 3,510,658 | |
| 0.7 | CDU | 3791 | 6.2535 | |||
| CTA | 3828 | 37 | 6.2520 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.2597 | 0.0062 | 149,176 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.2546 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.2600 | 0.0003 | 3,009,136 | |
| 0.8 | CDU | 3791 | 7.1469 | |||
| CTA | 3828 | 37 | 7.1452 | –0.0017 | Dominated | |
| CEU | 4709 | 919 | 7.1539 | 0.0070 | 130,529 | |
| CDU and CTA, then CDU | 4732 | 22 | 7.1481 | –0.0058 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 7.1543 | 0.0004 | 2,632,994 | |
| 0.9 | CDU | 3791 | 8.0402 | |||
| CTA | 3828 | 37 | 8.0383 | –0.0019 | Dominated | |
| CEU | 4709 | 919 | 8.0482 | 0.0079 | 116,026 | |
| CDU and CTA, then CDU | 4732 | 22 | 8.0416 | –0.0065 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 8.0486 | 0.0004 | 2,340,439 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.0 | CDU | 3791 | 6.5541 | |||
| CTA | 3828 | 37 | 6.5526 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5604 | 0.0063 | 145,617 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5552 | –0.0052 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5607 | 0.0003 | 2,893,195 | |
| 0.5 | CDU | 3791 | 6.5539 | |||
| CTA | 3828 | 37 | 6.5524 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5602 | 0.0063 | 146,064 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5550 | –0.0052 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5605 | 0.0003 | 2,897,445 | |
| 1.0 | CDU | 3791 | 6.5537 | |||
| CTA | 3828 | 37 | 6.5522 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5600 | 0.0063 | 146,514 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5548 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5603 | 0.0003 | 2,901,707 | |
| 2.0 | CDU | 3791 | 6.5533 | |||
| CTA | 3828 | 37 | 6.5518 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5595 | 0.0062 | 147,423 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5544 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5599 | 0.0003 | 2,910,269 | |
| 4.0 | CDU | 3791 | 6.5525 | |||
| CTA | 3828 | 37 | 6.5510 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5587 | 0.0062 | 149,274 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5536 | –0.0050 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5590 | 0.0003 | 2,927,545 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0 | CDU | 3791 | 6.5539 | |||
| CTA | 3828 | 37 | 6.5524 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5601 | 0.0062 | 147,541 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5550 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5604 | 0.0003 | 2,910,951 | |
| 1 | CDU | 3791 | 6.5536 | |||
| CTA | 3828 | 37 | 6.5521 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5598 | 0.0062 | 147,579 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5547 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5601 | 0.0003 | 2,911,503 | |
| 2 | CDU | 3791 | 6.5533 | |||
| CTA | 3828 | 37 | 6.5518 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5595 | 0.0062 | 147,618 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5544 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,055 | |
| 3 | CDU | 3791 | 6.5530 | |||
| CTA | 3828 | 37 | 6.5515 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5592 | 0.0062 | 147,656 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5541 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5595 | 0.0003 | 2,912,607 | |
| 4 | CDU | 3791 | 6.5527 | |||
| CTA | 3828 | 37 | 6.5512 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5589 | 0.0062 | 147,695 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5538 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5592 | 0.0003 | 2,913,159 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 20 | CDU | 3781 | 6.5515 | |||
| CTA | 3817 | 36 | 6.5499 | –0.0016 | Dominated | |
| CEU | 4702 | 921 | 6.5582 | 0.0067 | 137,783 | |
| CDU and CTA, then CDU | 4722 | 20 | 6.5527 | –0.0055 | Dominated | |
| CEU and CTA, then CEU | 5637 | 935 | 6.5585 | 0.0003 | 2,718,603 | |
| 40 | CDU | 3733 | 6.5430 | |||
| CTA | 3766 | 33 | 6.5409 | –0.0022 | Dominated | |
| CEU | 4668 | 935 | 6.5520 | 0.0090 | 103,821 | |
| CDU and CTA, then CDU | 4675 | 7 | 6.5446 | –0.0074 | Dominated | |
| CEU and CTA, then CEU | 5602 | 934 | 6.5525 | 0.0005 | 2,040,700 | |
| 60 | CDU | 3685 | 6.5345 | |||
| CTA | 3715 | 30 | 6.5318 | –0.0027 | Dominated | |
| CEU | 4633 | 6 | 6.5458 | 0.0093 | 603 | |
| CDU and CTA, then CDU | 4628 | 942 | 6.5365 | 0.0020 | 477,727 | |
| CEU and CTA, then CEU | 5567 | 934 | 6.5464 | 0.0006 | 1,633,664 | |
| 80 | CDU | 3637 | 6.5260 | |||
| CTA | 3664 | 26 | 6.5228 | –0.0032 | Dominated | |
| CEU | 4599 | 18 | 6.5396 | 0.0113 | 1624 | |
| CDU and CTA, then CDU | 4580 | 943 | 6.5284 | 0.0024 | 399,585 | |
| CEU and CTA, then CEU | 5532 | 934 | 6.5403 | 0.0007 | 1,362,184 | |
| 100 | CDU | 3590 | 6.5176 | |||
| CTA | 3613 | 23 | 6.5138 | –0.0038 | Dominated | |
| CEU | 4564 | 31 | 6.5335 | 0.0132 | 2346 | |
| CDU and CTA, then CDU | 4533 | 944 | 6.5203 | 0.0027 | 343,576 | |
| CEU and CTA, then CEU | 5497 | 933 | 6.5343 | 0.0008 | 1,168,209 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 10 | CDU | 3856 | 6.5648 | |||
| CTA | 3898 | 42 | 6.5640 | –0.0008 | Dominated | |
| CEU | 4756 | 900 | 6.5679 | 0.0031 | 293,910 | |
| CDU and CTA, then CDU | 4796 | 40 | 6.5654 | –0.0025 | Dominated | |
| CEU and CTA, then CEU | 5691 | 935 | 6.5680 | 0.0002 | 5,644,541 | |
| 20 | CDU | 3826 | 6.5595 | |||
| CTA | 3866 | 39 | 6.5584 | –0.0011 | Dominated | |
| CEU | 4735 | 909 | 6.5640 | 0.0045 | 201,888 | |
| CDU and CTA, then CDU | 4767 | 32 | 6.5604 | –0.0037 | Dominated | |
| CEU and CTA, then CEU | 5670 | 935 | 6.5643 | 0.0002 | 3,956,617 | |
| 30 | CDU | 3797 | 6.5543 | |||
| CTA | 3834 | 37 | 6.5528 | –0.0014 | Dominated | |
| CEU | 4714 | 917 | 6.5602 | 0.0059 | 154,478 | |
| CDU and CTA, then CDU | 4737 | 24 | 6.5553 | –0.0049 | Dominated | |
| CEU and CTA, then CEU | 5648 | 935 | 6.5605 | 0.0003 | 3,046,161 | |
| 40 | CDU | 3767 | 6.5490 | |||
| CTA | 3802 | 35 | 6.5472 | –0.0018 | Dominated | |
| CEU | 4692 | 925 | 6.5564 | 0.0074 | 125,567 | |
| CDU and CTA, then CDU | 4708 | 16 | 6.5503 | –0.0061 | Dominated | |
| CEU and CTA, then CEU | 5627 | 934 | 6.5568 | 0.0004 | 2,476,565 | |
| 50 | CDU | 3737 | 6.5438 | |||
| CTA | 3771 | 33 | 6.5416 | –0.0021 | Dominated | |
| CEU | 4671 | 933 | 6.5526 | 0.0088 | 106,096 | |
| CDU and CTA, then CDU | 4679 | 8 | 6.5453 | –0.0072 | Dominated | |
| CEU and CTA, then CEU | 5605 | 934 | 6.5530 | 0.0004 | 2,086,592 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 40 | CDU | 2554 | 6.5240 | |||
| CTA | 2568 | 14 | 6.5224 | –0.0016 | Dominated | |
| CEU | 2976 | 11 | 6.5319 | 0.0070 | 1587 | |
| CDU and CTA, then CDU | 2965 | 410 | 6.5249 | 0.0009 | 449,694 | |
| CEU and CTA, then CEU | 3385 | 409 | 6.5323 | 0.0003 | 1,263,460 | |
| 50 | CDU | 2817 | 6.5316 | |||
| CTA | 2835 | 18 | 6.5299 | –0.0017 | Dominated | |
| CEU | 3337 | 9 | 6.5399 | 0.0073 | 1205 | |
| CDU and CTA, then CDU | 3329 | 512 | 6.5326 | 0.0010 | 503,094 | |
| CEU and CTA, then CEU | 3847 | 510 | 6.5402 | 0.0003 | 1,458,998 | |
| 60 | CDU | 3061 | 6.5381 | |||
| CTA | 3083 | 22 | 6.5363 | –0.0017 | Dominated | |
| CEU | 3678 | 4 | 6.5463 | 0.0072 | 617 | |
| CDU and CTA, then CDU | 3674 | 613 | 6.5392 | 0.0011 | 564,002 | |
| CEU and CTA, then CEU | 4288 | 610 | 6.5467 | 0.0004 | 1,692,335 | |
| 70 | CDU | 3293 | 6.5436 | |||
| CTA | 3319 | 26 | 6.5419 | –0.0017 | Dominated | |
| CEU | 4004 | 711 | 6.5515 | 0.0079 | 90,203 | |
| CDU and CTA, then CDU | 4006 | 2 | 6.5447 | –0.0068 | Dominated | |
| CEU and CTA, then CEU | 4713 | 709 | 6.5519 | 0.0004 | 1,974,710 | |
| 80 | CDU | 3515 | 6.5483 | |||
| CTA | 3545 | 31 | 6.5466 | –0.0017 | Dominated | |
| CEU | 4317 | 803 | 6.5556 | 0.0073 | 110,298 | |
| CDU and CTA, then CDU | 4327 | 10 | 6.5494 | –0.0061 | Dominated | |
| CEU and CTA, then CEU | 5125 | 808 | 6.5559 | 0.0003 | 2,322,223 | |
| 90 | CDU | 3728 | 6.5522 | |||
| CTA | 3764 | 35 | 6.5506 | –0.0015 | Dominated | |
| CEU | 4620 | 892 | 6.5587 | 0.0065 | 137,508 | |
| CDU and CTA, then CDU | 4639 | 19 | 6.5533 | –0.0054 | Dominated | |
| CEU and CTA, then CEU | 5526 | 905 | 6.5590 | 0.0003 | 2,758,785 | |
| 100 | CDU | 3935 | 6.5554 | |||
| CTA | 3975 | 40 | 6.5540 | –0.0014 | Dominated | |
| CEU | 4914 | 979 | 6.5609 | 0.0056 | 176,167 | |
| CDU and CTA, then CDU | 4944 | 30 | 6.5565 | –0.0045 | Dominated | |
| CEU and CTA, then CEU | 5916 | 1002 | 6.5612 | 0.0003 | 3,321,498 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.5 | CDU | 3552 | 6.5421 | |||
| CTA | 3828 | 276 | 6.5517 | 0.0096 | 28,822 | |
| CEU | 4709 | 882 | 6.5594 | 0.0077 | 114,115 | |
| CDU and CTA, then CDU | 4506 | 678 | 6.5447 | –0.0070 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.6 | CDU | 3654 | 6.5474 | |||
| CTA | 3828 | 173 | 6.5517 | 0.0043 | 40,032 | |
| CEU | 4709 | 882 | 6.5594 | 0.0077 | 114,115 | |
| CDU and CTA, then CDU | 4603 | 775 | 6.5492 | –0.0025 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.7 | CDU | 3753 | 6.5517 | |||
| CTA | 3828 | 75 | 6.5517 | 0.0000 | Dominated | |
| CEU | 4709 | 14 | 6.5594 | 0.0064 | 2121 | |
| CDU and CTA, then CDU | 4696 | 943 | 6.5530 | 0.0013 | 720,056 | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.75 | CDU | 3800 | 6.5536 | |||
| CTA | 3828 | 28 | 6.5517 | –0.0019 | Dominated | |
| CEU | 4709 | 909 | 6.5594 | 0.0059 | 154,976 | |
| CDU and CTA, then CDU | 4741 | 31 | 6.5546 | –0.0048 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.8 | CTA | 3828 | 6.5517 | |||
| CDU | 3847 | 19 | 6.5552 | 0.0035 | 5367 | |
| CEU | 4709 | 863 | 6.5594 | 0.0042 | 205,467 | |
| CDU and CTA, then CDU | 4784 | 75 | 6.5561 | –0.0033 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.85 | CTA | 3828 | 6.5517 | |||
| CDU | 3892 | 64 | 6.5567 | 0.0050 | 12,831 | |
| CEU | 4709 | 817 | 6.5594 | 0.0027 | 301,755 | |
| CDU and CTA, then CDU | 4827 | 118 | 6.5574 | –0.0020 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.9 | CTA | 3828 | 6.5517 | |||
| CDU | 3937 | 109 | 6.5581 | 0.0063 | 17,162 | |
| CEU | 4709 | 773 | 6.5594 | 0.0014 | 558,213 | |
| CDU and CTA, then CDU | 4869 | 160 | 6.5586 | –0.0009 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 1.0 | CTA | 3828 | 6.5517 | |||
| CDU | 4023 | 195 | 6.5602 | 0.0085 | 22,844 | |
| CEU | 4709 | 687 | 6.5594 | –0.0008 | Dominated | |
| CDU and CTA, then CDU | 4950 | 928 | 6.5605 | 0.0002 | 4,263,080 | |
| CEU and CTA, then CEU | 5644 | 693 | 6.5598 | –0.0007 | Dominated |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.8 | CTA | 3828 | 6.5517 | |||
| CDU | 3967 | 140 | 6.5532 | 0.0015 | 92,448 | |
| CEU | 4709 | 742 | 6.5594 | 0.0062 | 119,382 | |
| CDU and CTA, then CDU | 4916 | 207 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.85 | CTA | 3828 | 6.5517 | |||
| CDU | 3904 | 77 | 6.5532 | 0.0015 | 50,833 | |
| CEU | 4709 | 805 | 6.5594 | 0.0062 | 129,466 | |
| CDU and CTA, then CDU | 4850 | 141 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.9 | CTA | 3828 | 6.5517 | |||
| CDU | 3841 | 14 | 6.5532 | 0.0015 | 9018 | |
| CEU | 4709 | 868 | 6.5594 | 0.0062 | 139,553 | |
| CDU and CTA, then CDU | 4784 | 75 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.95 | CDU | 3778 | 6.5532 | |||
| CTA | 3828 | 50 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 931 | 6.5594 | 0.0062 | 149,645 | |
| CDU and CTA, then CDU | 4718 | 9 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 1.0 | CDU | 3715 | 6.5532 | |||
| CTA | 3828 | 113 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 57 | 6.5594 | 0.0051 | 11,152 | |
| CDU and CTA, then CDU | 4652 | 937 | 6.5543 | 0.0011 | 838,696 | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0 | CDU | 3419 | 6.5535 | |||
| CTA | 3828 | 408 | 6.5517 | –0.0018 | Dominated | |
| CEU | 4709 | 270 | 6.5594 | 0.0048 | 55,755 | |
| CDU and CTA, then CDU | 4439 | 1020 | 6.5546 | 0.0011 | 960,112 | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 5 | CDU | 3523 | 6.5534 | |||
| CTA | 3828 | 305 | 6.5517 | –0.0017 | Dominated | |
| CEU | 4709 | 189 | 6.5594 | 0.0049 | 38,426 | |
| CDU and CTA, then CDU | 4520 | 998 | 6.5545 | 0.0011 | 926,163 | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 10 | CDU | 3626 | 6.5534 | |||
| CTA | 3828 | 202 | 6.5517 | –0.0016 | Dominated | |
| CEU | 4709 | 108 | 6.5594 | 0.0050 | 21,587 | |
| CDU and CTA, then CDU | 4602 | 976 | 6.5544 | 0.0011 | 893,091 | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 15 | CDU | 3729 | 6.5533 | |||
| CTA | 3828 | 99 | 6.5517 | –0.0016 | Dominated | |
| CEU | 4709 | 26 | 6.5594 | 0.0051 | 5220 | |
| CDU and CTA, then CDU | 4683 | 954 | 6.5544 | 0.0011 | 860,869 | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 20 | CTA | 3828 | 6.5517 | |||
| CDU | 3832 | 4 | 6.5532 | 0.0015 | 2939 | |
| CEU | 4709 | 877 | 6.5594 | 0.0063 | 140,201 | |
| CDU and CTA, then CDU | 4764 | 55 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 25 | CTA | 3828 | 6.5517 | |||
| CDU | 3935 | 107 | 6.5531 | 0.0014 | 77,897 | |
| CEU | 4709 | 774 | 6.5594 | 0.0063 | 121,991 | |
| CDU and CTA, then CDU | 4845 | 136 | 6.5542 | –0.0052 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 30 | CTA | 3828 | 6.5517 | |||
| CDU | 4038 | 211 | 6.5530 | 0.0013 | 163,168 | |
| CEU | 4709 | 671 | 6.5594 | 0.0064 | 104,271 | |
| CDU and CTA, then CDU | 4927 | 217 | 6.5542 | –0.0053 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.8 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4569 | 778 | 6.5552 | 0.0020 | 384,006 | |
| CDU and CTA, then CDU | 4732 | 162 | 6.5543 | –0.0009 | Dominated | |
| CEU and CTA, then CEU | 5512 | 942 | 6.5561 | 0.0009 | 1,097,985 | |
| 0.85 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4614 | 823 | 6.5567 | 0.0035 | 234,124 | |
| CDU and CTA, then CDU | 4732 | 118 | 6.5543 | –0.0024 | Dominated | |
| CEU and CTA, then CEU | 5554 | 940 | 6.5574 | 0.0007 | 1,407,843 | |
| 0.9 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4658 | 867 | 6.5581 | 0.0048 | 179,185 | |
| CDU and CTA, then CDU | 4732 | 74 | 6.5543 | –0.0037 | Dominated | |
| CEU and CTA, then CEU | 5595 | 937 | 6.5586 | 0.0005 | 1,881,847 | |
| 0.95 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4701 | 910 | 6.5592 | 0.0060 | 151,514 | |
| CDU and CTA, then CDU | 4732 | 31 | 6.5543 | –0.0049 | Dominated | |
| CEU and CTA, then CEU | 5636 | 935 | 6.5596 | 0.0003 | 2,682,568 | |
| 1.0 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4743 | 11 | 6.5602 | 0.0059 | 1879 | |
| CDU and CTA, then CDU | 4732 | 941 | 6.5543 | 0.0011 | 841,931 | |
| CEU and CTA, then CEU | 5675 | 933 | 6.5605 | 0.0002 | 4,286,486 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.75 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4835 | 104 | 6.5594 | 0.0051 | 20,296 | |
| CDU and CTA, then CDU | 4732 | 941 | 6.5543 | 0.0011 | 841,931 | |
| CEU and CTA, then CEU | 5775 | 940 | 6.5598 | 0.0003 | 2,930,454 | |
| 0.8 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CDU and CTA, then CDU | 4732 | 941 | 6.5543 | 0.0011 | 841,931 | |
| CEU | 4772 | 41 | 6.5594 | 0.0051 | 7968 | |
| CEU and CTA, then CEU | 5710 | 937 | 6.5598 | 0.0003 | 2,921,324 | |
| 0.85 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5594 | 0.0062 | 147,626 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.9 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4646 | 855 | 6.5594 | 0.0062 | 137,498 | |
| CDU and CTA, then CDU | 4732 | 85 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5578 | 932 | 6.5598 | 0.0003 | 2,903,015 | |
| 0.95 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4583 | 792 | 6.5594 | 0.0062 | 127,355 | |
| CDU and CTA, then CDU | 4732 | 149 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5512 | 929 | 6.5598 | 0.0003 | 2,893,836 | |
| 1.0 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4520 | 729 | 6.5594 | 0.0062 | 117,196 | |
| CDU and CTA, then CDU | 4732 | 212 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5446 | 926 | 6.5598 | 0.0003 | 2,884,641 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4405 | 614 | 6.5606 | 0.0073 | 83,663 | |
| CDU and CTA, then CDU | 4732 | 327 | 6.5543 | –0.0062 | Dominated | |
| CEU and CTA, then CEU | 5409 | 1003 | 6.5607 | 0.0001 | 9,142,747 | |
| 5 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4490 | 699 | 6.5603 | 0.0071 | 99,125 | |
| CDU and CTA, then CDU | 4732 | 242 | 6.5543 | –0.0059 | Dominated | |
| CEU and CTA, then CEU | 5474 | 984 | 6.5604 | 0.0002 | 5,970,958 | |
| 10 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4574 | 783 | 6.5600 | 0.0067 | 116,170 | |
| CDU and CTA, then CDU | 4732 | 158 | 6.5543 | –0.0056 | Dominated | |
| CEU and CTA, then CEU | 5539 | 965 | 6.5602 | 0.0002 | 4,334,894 | |
| 15 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4659 | 868 | 6.5596 | 0.0064 | 135,141 | |
| CDU and CTA, then CDU | 4732 | 73 | 6.5543 | –0.0053 | Dominated | |
| CEU and CTA, then CEU | 5605 | 946 | 6.5599 | 0.0003 | 3,340,291 | |
| 20 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4743 | 11 | 6.5593 | 0.0050 | 2305 | |
| CDU and CTA, then CDU | 4732 | 941 | 6.5543 | 0.0011 | 841,931 | |
| CEU and CTA, then CEU | 5670 | 927 | 6.5596 | 0.0003 | 2,674,165 | |
| 25 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4828 | 96 | 6.5590 | 0.0046 | 20,782 | |
| CDU and CTA, then CDU | 4732 | 941 | 6.5543 | 0.0011 | 841,931 | |
| CEU and CTA, then CEU | 5735 | 908 | 6.5594 | 0.0004 | 2,198,543 | |
| 30 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4912 | 181 | 6.5586 | 0.0043 | 42,443 | |
| CDU and CTA, then CDU | 4732 | 941 | 6.5543 | 0.0011 | 841,931 | |
| CEU and CTA, then CEU | 5801 | 889 | 6.5591 | 0.0005 | 1,843,151 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.5 | CTA | 3581 | 6.5395 | |||
| CDU | 3749 | 168 | 6.5516 | 0.0121 | 13,922 | |
| CEU | 4672 | 923 | 6.5584 | 0.0069 | 134,033 | |
| CDU and CTA, then CDU | 4690 | 18 | 6.5526 | –0.0058 | Dominated | |
| CEU and CTA, then CEU | 5609 | 937 | 6.5589 | 0.0005 | 1,986,663 | |
| 0.6 | CTA | 3708 | 6.5463 | |||
| CDU | 3770 | 62 | 6.5524 | 0.0061 | 10,196 | |
| CEU | 4691 | 921 | 6.5590 | 0.0065 | 140,583 | |
| CDU and CTA, then CDU | 4711 | 20 | 6.5535 | –0.0054 | Dominated | |
| CEU and CTA, then CEU | 5626 | 936 | 6.5594 | 0.0004 | 2,374,667 | |
| 0.7 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5594 | 0.0062 | 147,626 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 0.8 | CDU | 3811 | 6.5540 | |||
| CTA | 3941 | 130 | 6.5559 | 0.0019 | 67,435 | |
| CEU | 4728 | 786 | 6.5599 | 0.0040 | 197,736 | |
| CDU and CTA, then CDU | 4752 | 24 | 6.5551 | –0.0048 | Dominated | |
| CEU and CTA, then CEU | 5661 | 933 | 6.5601 | 0.0003 | 3,702,825 | |
| 0.9 | CDU | 3832 | 6.5547 | |||
| CTA | 4049 | 217 | 6.5591 | 0.0044 | 49,742 | |
| CEU | 4746 | 697 | 6.5603 | 0.0012 | 566,664 | |
| CDU and CTA, then CDU | 4772 | 26 | 6.5559 | –0.0045 | Dominated | |
| CEU and CTA, then CEU | 5678 | 932 | 6.5605 | 0.0002 | 4,974,254 | |
| 1.0 | CDU | 3852 | 6.5554 | |||
| CTA | 4151 | 299 | 6.5614 | 0.0060 | 50,171 | |
| CEU | 4764 | 613 | 6.5607 | –0.0007 | Dominated | |
| CDU and CTA, then CDU | 4792 | 641 | 6.5566 | –0.0048 | Dominated | |
| CEU and CTA, then CEU | 5695 | 1545 | 6.5608 | –0.0005 | Dominated |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0.5 | CDU | 4023 | 6.5536 | |||
| CTA | 4753 | 730 | 6.5531 | –0.0005 | Dominated | |
| CEU | 4931 | 908 | 6.5596 | 0.0060 | 151,652 | |
| CDU and CTA, then CDU | 5089 | 157 | 6.5550 | –0.0046 | Dominated | |
| CEU and CTA, then CEU | 5923 | 991 | 6.5600 | 0.0004 | 2,582,245 | |
| 0.6 | CDU | 3962 | 6.5534 | |||
| CTA | 4548 | 586 | 6.5526 | –0.0009 | Dominated | |
| CEU | 4878 | 916 | 6.5595 | 0.0061 | 150,596 | |
| CDU and CTA, then CDU | 5007 | 129 | 6.5547 | –0.0048 | Dominated | |
| CEU and CTA, then CEU | 5867 | 989 | 6.5599 | 0.0004 | 2,761,378 | |
| 0.7 | CDU | 3907 | 6.5533 | |||
| CTA | 4340 | 433 | 6.5522 | –0.0012 | Dominated | |
| CEU | 4827 | 919 | 6.5595 | 0.0061 | 149,598 | |
| CDU and CTA, then CDU | 4927 | 100 | 6.5545 | –0.0049 | Dominated | |
| CEU and CTA, then CEU | 5807 | 980 | 6.5598 | 0.0003 | 2,886,831 | |
| 0.8 | CDU | 3860 | 6.5533 | |||
| CTA | 4141 | 281 | 6.5519 | –0.0014 | Dominated | |
| CEU | 4780 | 920 | 6.5595 | 0.0062 | 148,725 | |
| CDU and CTA, then CDU | 4852 | 72 | 6.5544 | –0.0050 | Dominated | |
| CEU and CTA, then CEU | 5747 | 966 | 6.5598 | 0.0003 | 2,948,485 | |
| 0.9 | CDU | 3819 | 6.5532 | |||
| CTA | 3959 | 140 | 6.5518 | –0.0015 | Dominated | |
| CEU | 4739 | 920 | 6.5594 | 0.0062 | 148,029 | |
| CDU and CTA, then CDU | 4783 | 44 | 6.5544 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5688 | 950 | 6.5598 | 0.0003 | 2,948,570 | |
| 0.95 | CDU | 3801 | 6.5532 | |||
| CTA | 3875 | 74 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4720 | 919 | 6.5594 | 0.0062 | 147,760 | |
| CDU and CTA, then CDU | 4750 | 30 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5660 | 940 | 6.5598 | 0.0003 | 2,929,045 | |
| 1.0 | CDU | 3784 | 6.5532 | |||
| CTA | 3797 | 13 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4702 | 918 | 6.5594 | 0.0062 | 147,549 | |
| CDU and CTA, then CDU | 4719 | 17 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5633 | 931 | 6.5598 | 0.0003 | 2,899,056 |
| Value (%) | Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|---|
| 0 | CDU | 3791 | 6.5532 | |||
| CTA | 3828 | 37 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5594 | 0.0062 | 147,626 | |
| CDU and CTA, then CDU | 4732 | 22 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5644 | 935 | 6.5598 | 0.0003 | 2,912,177 | |
| 10 | CDU | 3791 | 6.5532 | |||
| CTA | 4045 | 254 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5594 | 0.0062 | 147,626 | |
| CDU and CTA, then CDU | 4739 | 30 | 6.5543 | –0.0051 | Dominated | |
| CEU and CTA, then CEU | 5651 | 941 | 6.5597 | 0.0003 | 3,152,095 | |
| 20 | CDU | 3791 | 6.5532 | |||
| CTA | 4262 | 472 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5594 | 0.0062 | 147,626 | |
| CDU and CTA, then CDU | 4747 | 38 | 6.5543 | –0.0052 | Dominated | |
| CEU and CTA, then CEU | 5658 | 948 | 6.5597 | 0.0003 | 3,430,618 | |
| 30 | CDU | 3791 | 6.5532 | |||
| CTA | 4480 | 689 | 6.5517 | –0.0015 | Dominated | |
| CEU | 4709 | 919 | 6.5594 | 0.0062 | 147,626 | |
| CDU and CTA, then CDU | 4754 | 45 | 6.5543 | –0.0052 | Dominated | |
| CEU and CTA, then CEU | 5665 | 955 | 6.5597 | 0.0003 | 3,757,877 |
FIGURE 14.
Two-way sensitivity analysis: CEU sensitivity and specificity (based on net benefit, willingness-to-pay threshold of £30,000).
FIGURE 15.
Two-way sensitivity analysis: CTA sensitivity and specificity (based on net benefit, willingness-to-pay threshold of £30,000).
List of abbreviations
- AAA
- abdominal aortic aneurysm
- AMSTAR
- Assessment of Multiple Systematic Reviews
- ASERNIP-S
- Australian Safety and Efficacy Register of New Interventional Procedures – Surgical
- BSIR
- British Society of Interventional Radiologists
- CDSR
- Cochrane Database of Systematic Reviews
- CDU
- colour duplex ultrasound
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CEU
- contrast-enhanced ultrasound
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- CT
- computerised tomography
- CTA
- computed tomography angiography
- DARE
- Database of Abstracts of Reviews of Effects
- ENGAGE
- Endurant Stent Graft Natural Selection Global Postmarket Registry
- EQ-5D
- EuroQol-5 Dimensions
- EUROSTAR
- The EUROpean collaborators on Stent–graft Techniques for abdominal aortic Aneurysm Repair
- EVAR
- endovascular abdominal aortic aneurysm repair
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- IQR
- interquartile range
- ITT
- intention to treat
- KPNC
- Kaiser Permanente Northern California
- KPSGR
- Kaiser Permanente Endovascular Stent Graft Registry
- MeSH
- medical subject heading
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NVR
- National Vascular Registry
- PSV
- peak systolic velocity
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ReBIP
- Review Body for Interventional Procedures
- SD
- standard deviation