Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/03/25. The contractual start date was in October 2014. The draft report began editorial review in January 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Susanna Esposito reports grants and personal fees from GlaxoSmithKline (GSK) plc (GSK House, Middlesex, UK), grants and personal fees from Pfizer Inc. (New York, NY, USA), grants and personal fees from Sanofi Pasteur MSD [Sanofi Pasteur (Lyon France) and Merck Sharp & Dohme Corp. (MSD, Kenilworth, NJ, USA)], grants from DuPage Medical Group (DMG, Downers Grove, IL, USA), personal fees from Valeas S.p.A. (Milan, Italy), and grants and personal fees from Vifor Pharma (Bern, Switzerland), outside the submitted work. Emma Goodall reports personal fees from GSK outside the submitted work. Wim Janssens reports grants from Instituut voor Innovatie door Wetenschap en Technologie (IWT)–Vlaanderen and from Laboratoires SMB (Brussels, Belgium) during the conduct of the study. David Mauger reports funding from the National Heart, Lung, and Blood Institute, MA, USA. Rachel Neale reports grants from the National Institutes of Health and the Medical Research Council during the conduct of the study. Judy R Rees reports that a use patent is held by Dartmouth College and Dr John A Baron for calcium as a chemopreventive agent. Dr Baron is not an author on this paper but is the principal investigator of the parent study from which the study by Rees (Rees JR, Hendricks K, Barry EL, Peacock JL, Mott LA, Sandler RS, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis 2013;57:1384–92) was conducted. The patent was previously licensed by Pfizer (with royalties), but has not been licensed for about 5 years. Judy R Rees is not involved in the patent and the patent does not involve vitamin D.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Martineau et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Acute respiratory infections (ARIs) are a major cause of global morbidity and mortality, responsible for 10% of ambulatory and emergency department visits in the USA1 and an estimated 2.65 million deaths worldwide in 2013. 2 Viral ARI precipitate the majority of acute exacerbations of asthma and chronic obstructive pulmonary disease (COPD),3 which represent the major cause of morbidity and mortality in people with these conditions. 4,5
Observational studies report consistent independent associations between low serum concentrations of 25-hydroxyvitamin D [25(OH)D], the major circulating vitamin D metabolite, and susceptibility to ARI6,7 and acute exacerbations of asthma;8,9 such observational studies have yielded more conflicting results for the outcomes of COPD exacerbation. 10–12 The observation that 25(OH)D supports induction of antimicrobial peptides in response to both viral and bacterial stimuli13–15 suggests a potential mechanism by which vitamin D-inducible protection against these outcomes may be mediated. Vitamin D metabolites have also been reported to induce other innate antimicrobial effector mechanisms, including autophagy and synthesis of reactive nitrogen intermediates and reactive oxygen intermediates. 16 In addition, vitamin D metabolites have been reported to induce anti-inflammatory activity via multiple mechanisms, including induction of the regulatory cytokine interleukin (IL) 1017 and inhibition of the pro-inflammatory cytokine IL-17A. 18 We have also recently shown that 25(OH)D attenuates rhinovirus-induced expression of the genes encoding intercellular adhesion molecule 1 (ICAM-1, a cell surface glycoprotein that acts as the cellular receptor for major group rhinoviruses) and platelet-activating factor receptor (PAFR, a G-protein coupled receptor implicated in adhesion of Streptococcus pneumoniae to respiratory epithelial cells). 19 These findings suggest possible mechanisms by which vitamin D may enhance resistance to rhinovirus infection and reduce risk of secondary bacterial infection in vitamin D-deficient individuals.
These epidemiological and in vitro data have prompted numerous randomised controlled trials (RCTs) to determine whether or not vitamin D supplementation can decrease the risk of ARI and acute exacerbations of asthma and COPD. For the outcome of ARI, a total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date, of which two report statistically significant protective effects20,21 and three report no statistically significant effects. 22–24 All but one of these aggregate data meta-analyses22 reported significant heterogeneity of effect between primary trials. For the outcome of asthma exacerbation, a total of four aggregate data meta-analyses incorporating data from up to nine primary trials have been conducted to date, of which three report statistically significant protective effects23,25,26 and one reports no statistically significant effects. 27 The most recent of these – and the one incorporating data from the most studies – reported a high degree of heterogeneity of effect between trials for the outcome of study-defined asthma exacerbation. 26 We are not aware of any published meta-analyses investigating effects of vitamin D supplementation on the risk of COPD exacerbation, which may reflect the fact that only three primary trials investigating this question have been published to date. 28–30
When heterogeneity of effect is present, it may have arisen as a result of intertrial variation in participant characteristics and in dosing regimens, either of which may modify the effects of vitamin D supplementation on immunity to respiratory pathogens. 31 Subgroup analyses within primary trials suggest that COPD patients with lower baseline vitamin D status may derive greater clinical benefit from supplementation than those with higher baseline status. 28,29 Moreover, participant characteristics such as age and body mass index have been reported to modify the 25(OH)D response to vitamin D supplementation. 32,33 Administration of large boluses of vitamin D has been associated with reduced efficacy for non-classical effects20 and, in some cases, increased risk of adverse outcomes. 34 Although study-level factors are amenable to exploration via aggregate data meta-analysis of published data, potential effect modifiers operating at an individual level, such as baseline vitamin D status, can only be explored using individual participant data (IPD) meta-analysis. This is because subgroups are not consistently disaggregated in trial reports, and consistent adjustments for potential confounders cannot be applied. 35 In order to identify factors that might modify effects of vitamin D supplementation on the risk of ARI and acute exacerbations of asthma and COPD, we undertook a meta-analysis of IPD from RCTs that had investigated these outcomes. The results of some of these analyses have been published elsewhere. 36–38
Chapter 2 Research questions
-
What is the overall effect of vitamin D supplementation on the risk of:
-
acute respiratory infections, incorporating events classified as upper respiratory infections (URIs), lower respiratory infections (LRIs) and ARIs of unclassified location (i.e. infection of the upper and/or lower respiratory tract)
-
upper respiratory infections and LRIs, analysed separately
-
emergency department attendance and/or hospital admission for ARI
-
use of antimicrobials for treatment of ARI
-
work/school absence as a result of ARI
-
severe exacerbations of asthma
-
severe exacerbations of COPD
-
serious adverse events
-
potential adverse reactions to vitamin D (hypercalcaemia and renal stones)
-
mortality (related to ARI/respiratory failure, infection and all-cause) and to identify factors modifying this effect?
-
-
Do the following factors modify the effect of vitamin D supplementation on the risk of ARI?
-
baseline vitamin D status [serum 25(OH)D concentration of < 25 nmol/l vs. ≥ 25 nmol/l]
-
dosing regimen [daily or weekly administration of vitamin D without bolus dosing vs. administration of a regimen including at least one bolus dose of ≥ 30,000 international units (IU) of vitamin D]
-
dose size (daily equivalent < 800 IU vs. 800–1999 IU vs. ≥ 2000 IU of vitamin D)
-
age (≤ 1 year vs. 1.1–15.9 years vs. 16–65 years vs. > 65 years)
-
body mass index (< 25 kg/m2 vs. ≥ 25 kg/m2)
-
presence versus absence of respiratory comorbidity (asthma, COPD)
-
influenza vaccination status.
-
Chapter 3 Methods
Protocol and registration
Methods were prespecified in a protocol that was registered with the PROSPERO International Prospective Register of Systematic Reviews [www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013953 accessed 1 May 2018]. Research Ethics Committee approval to conduct this meta-analysis was not required in the UK; local ethics permission to contribute de-identified IPD from primary trials was required and obtained for studies by Camargo et al. 39 (the Ethics Review Committee of the Mongolian Ministry of Health), Murdoch et al. 40 (Southern Health and Disability Ethics Committee, reference URB/09/10/050/AM02), Rees et al. 41 (Committee for the Protection of Human Subjects, Dartmouth College, NH, USA; Protocol no 24381), Tachimoto et al. 42 (Ethics Committee of the Jikei University School of Medicine, reference 26-333: 7839), Tran et al. 43 (QIMR Berghofer Medical Research Institute Human Research Ethics Committee, reference number P1570) and Urashima et al. 44,45 (Ethics Committee of the Jikei University School of Medicine, reference 26-333: 7839).
Patient and public involvement
Two patient and public involvement (PPI) representatives were involved in development of the research question and the choice of outcome measures specified in the study protocol through discussion with the investigators. When possible, results of this systematic review and meta-analysis will be disseminated to individual participants via the principal investigators of each trial (e.g. via e-mail to participants who have requested updates on how their data are being used). PPI representatives and participants in primary trials are thanked for their contributions in the Acknowledgements.
Eligibility criteria
For the IPD meta-analysis of ARI outcomes, randomised, double-blind, placebo-controlled trials of supplementation with vitamin D3 or vitamin D2 of any duration were eligible for inclusion if they had been approved by a Research Ethics Committee and if data on incidence of ARI were collected prospectively and prespecified as an efficacy outcome. The last requirement was imposed to minimise misclassification bias (prospectively designed instruments to capture these events were deemed more likely to be sensitive and specific for this outcome). Studies reporting results of long-term follow-up of primary RCTs were excluded.
For the IPD meta-analysis of asthma exacerbation, randomised, double-blind, placebo-controlled trials of supplementation with vitamin D3 or vitamin D2 in patients with asthma were eligible for inclusion if they had been approved by a Research Ethics Committee and if data on incidence of asthma exacerbation were reported.
For the IPD meta-analysis of COPD exacerbation, randomised, double-blind, placebo-controlled trials of supplementation with vitamin D3 or vitamin D2 in patients with COPD were eligible for inclusion if they had been approved by a Research Ethics Committee and if data on incidence of COPD exacerbation were reported.
Study identification and selection
Two investigators (ARM and DAJ) searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, ClinicalTrials.gov and the International Standard Randomised Controlled Trials Number (ISRCTN) registry using the electronic search strategies described in Appendix 1. Searches were regularly updated up to and including 31 December 2015 for the ARI analysis, 26 October 2016 for the analysis of asthma exacerbations and 31 July 2017 for the analysis of COPD exacerbations. No language restrictions were imposed. These searches were supplemented by searching review articles and reference lists of trial publications. Collaborators were asked if they knew of any additional trials. Three investigators (ARM, CAC and DAJ) determined which trials met the eligibility criteria; disagreements were resolved by consensus. References were managed in EndNote X5 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA].
Data collection processes
Individual participant data were requested from the principal investigator for each eligible trial and the terms of collaboration were specified in a data transfer agreement, signed by representatives of the data provider and the recipient (Queen Mary University of London). Data were de-identified at source prior to transfer via e-mail. On receipt, three investigators (DAJ, RLH and LG) assessed data integrity by performing internal consistency checks and by attempting to replicate results of analyses that were published in trial reports. Study authors were contacted to provide missing data and to resolve queries arising from these integrity checks. Once queries had been resolved, clean data were uploaded to the main study database, which was held in Stata® IC version12 (StataCorp LP, College Station, TX, USA).
Data relating to study characteristics were extracted for the following variables: setting, eligibility criteria, details of intervention and control regimens, study duration and case definitions for ARI. IPD were extracted for the following variables, when available: baseline data were requested for age, sex, cluster ID (cluster randomised trials only), racial/ethnic origin, influenza vaccination status, history of asthma, history of COPD, weight, height (adults and children able to stand) or length (infants), serum 25(OH)D concentration, study allocation (vitamin D vs. placebo) and details of any stratification or minimisation variables. For all trials, follow-up data were requested for serum 25(OH)D concentration at final follow-up, duration of follow-up, number and nature of serious adverse events, number of adverse reactions (incident hypercalcaemia or renal stones) and end-trial status (completed vs. withdrew vs. lost to follow-up vs. died). For trials contributing IPD to the ARI analysis, follow-up data were also requested for the total number of ARIs, URIs and LRIs experienced during the trial, time from first dose of study medication to first ARI/URI/LRI if applicable, total number of courses of antibiotics taken for ARI during the trial and total number of days off work or school as a result of ARI symptoms during the trial. For trials contributing IPD to the analysis of severe asthma exacerbation, follow-up data were also requested for the total number of asthma exacerbations experienced during the trial that were treated with systemic corticosteroids and the time from the first dose of study medication to the first such exacerbation, if applicable. For trials contributing IPD to the analysis of severe COPD exacerbation, follow-up data were also requested for the total number of COPD exacerbations experienced during the trial that were treated with systemic corticosteroids and/or antibiotics and the time from the first dose of study medication to the first such exacerbation if applicable.
Risk-of-bias assessment for individual studies
We used the Cochrane Collaboration Risk of Bias tool46 to assess the following variables: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; completeness of outcome data; evidence of selective outcome reporting; and other potential threats to validity. Study quality was assessed independently by two investigators (ARM and DAJ), except for the three trials by Martineau et al. ,29,47,48 which were assessed by Carlos A Camargo Jr. Discrepancies were resolved by consensus.
Definition of outcomes
The primary outcome of the meta-analysis was incidence of ARI, incorporating events classified as URIs, LRIs and ARIs of unclassified location (i.e. infection of the upper and/or lower respiratory tract). Secondary outcomes were incidence of URI and LRI, analysed separately; incidence of emergency department attendance and/or hospital admission for ARI; use of antimicrobials for treatment of ARI; work/school absence as a result of ARI; incidence of severe asthma exacerbation, defined as a worsening of asthma symptoms resulting in treatment with systemic corticosteroids; incidence of severe COPD exacerbation, defined as a worsening of symptoms resulting in treatment with systemic corticosteroids and/or antibiotics; incidence and nature of serious adverse events; incidence of potential adverse reactions to vitamin D (hypercalcaemia and renal stones); and mortality (related to ARI/respiratory failure, infection and all-cause).
Synthesis methods
Data were analysed by Lauren Greenberg and Richard L Hooper. Our IPD meta-analysis approach followed published guidelines. 35 Initially, all studies were reanalysed separately; the original authors were asked to confirm the accuracy of this reanalysis when it had been performed previously, and any discrepancies were resolved. We then performed both one-step and two-step IPD meta-analysis using a random-effects model adjusted for age, sex and study duration to obtain the pooled intervention effect with a 95% confidence interval (CI). We did not adjust for other covariates because missing values for some participants would have led to their exclusion from statistical analyses. In the one-step approach, IPD from all studies were modelled simultaneously while accounting for the clustering of participants within studies. In the two-step approach, IPD were first analysed for each separate study independently to produce an estimate of the treatment effect for that study; these data were then synthesised in a second step. 35 For one-step IPD meta-analysis, heterogeneity was assessed by calculation of the standard deviation of random effects; for two-step IPD meta-analysis, heterogeneity was summarised using the I2 statistic. The number needed to treat (NNT) for an additional beneficial outcome was calculated using the Visual Rx NNT calculator (www.nntonline.net/visualrx/) when meta-analysis of dichotomous outcomes revealed a statistically significant beneficial effect of allocation to vitamin D versus placebo.
Exploration of variation in effects
In order to explore the causes of heterogeneity and identify factors modifying the effects of vitamin D supplementation on ARI risk, we performed prespecified subgroup analyses by extending the one-step meta-analysis framework to include treatment–covariate interaction terms. Subgroups were defined according to baseline vitamin D status [serum 25(OH)D concentration of < 25 nmol/l vs. ≥ 25 nmol/l], vitamin D dosing regimen (daily or weekly administration without bolus dosing vs. administration of a regimen including at least one bolus dose of ≥ 30,000 IU of vitamin D), dose size (daily equivalent < 800 IU vs. 800–1999 IU vs. ≥ 2000 IU), age (≤ 1 year vs. 1.1–15.9 years vs. 16–65 years vs. > 65 years), body mass index (< 25 kg/m2 vs. ≥ 25 kg/m2), presence versus absence of asthma or COPD and previous influenza vaccination status. Interaction analyses were adjusted for potential confounders (age, sex and study duration) to ensure that reported subgroup effects were independent. The 25 nmol/l cut-off point for baseline 25(OH)D concentration in subgroup analyses was selected on the basis that it is the threshold for vitamin D deficiency defined by the UK Department of Health and Social Care49 and the level below which participants in clinical trials have experienced the most consistent benefits of supplementation. 50 In order to minimise the chance of type I error arising from multiple analyses, significance was inferred only when p-values for treatment–covariate interaction terms were < 0.05.
Quality assessment across studies
For the primary analysis, the likelihood of publication bias was investigated through the construction of a contour-enhanced funnel plot. 51 We used the five Grading of Recommendations Assessment, Development and Evaluation (GRADE) considerations [(1) (study limitations, (2) consistency of effect, (3) imprecision, (4) indirectness and (5) publication bias]52 to assess the quality of the body of evidence contributing to analyses of the primary efficacy outcome and major safety outcome of our meta-analysis.
Additional analyses
For the ARI analysis, we conducted sensitivity analyses excluding IPD from trials in which ARI was a secondary outcome (as opposed to a primary or coprimary outcome) and in which risk of bias was assessed as being unclear. We also conducted a responder analysis in participants randomised to the intervention arm of included studies for whom end-study 25(OH)D concentration data were available, comparing risk of ARI in those who attained a serum concentration of 25(OH)D of ≥ 75 nmol/l with that in those who did not.
Chapter 4 Results
Study selection and individual participant data obtained
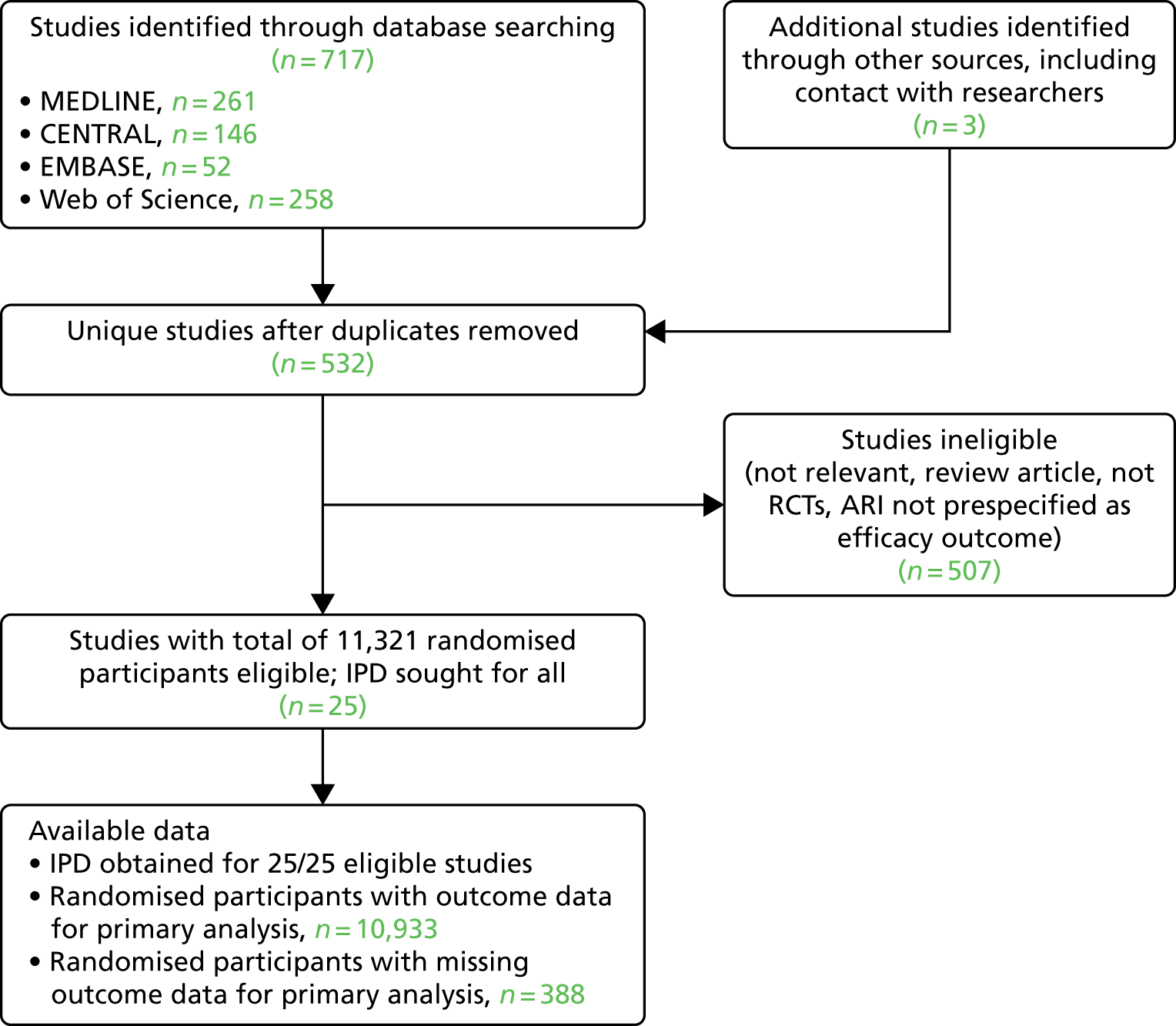
For the ARI analysis, our search identified a total of 532 unique studies that were assessed for eligibility; of these, 25 studies with a total of 11,321 randomised participants fulfilled eligibility criteria. IPD were sought and obtained for all 25 studies. Outcome data were obtained for 10,933 out of 11,321 (96.6%) of the randomised participants in these 25 studies (Figure 1).
FIGURE 1.
The PRISMA flow diagram: ARI analysis. CENTRAL, Cochrane Central Register of Controlled Trials; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
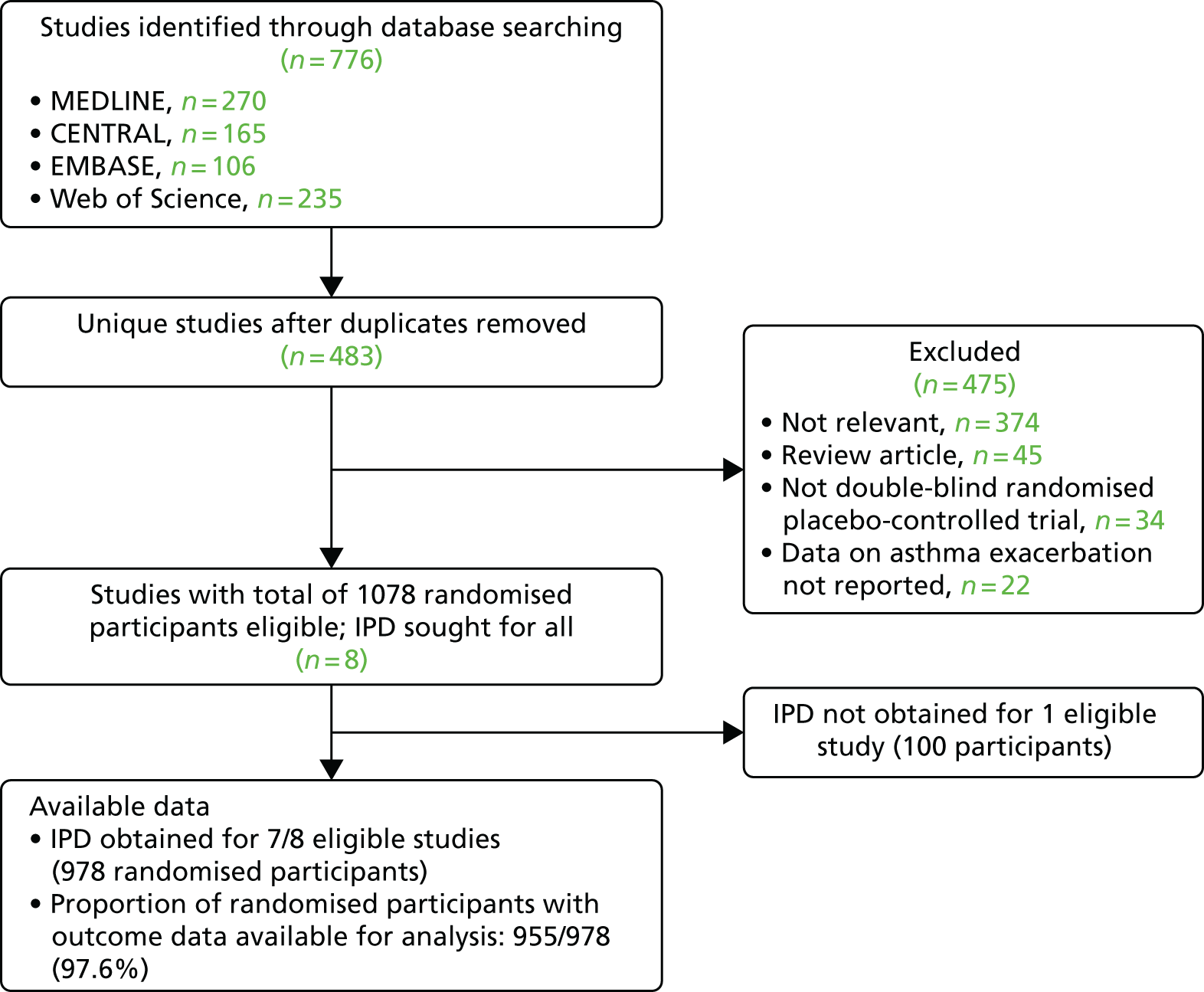
For the analysis of severe asthma exacerbations, our search identified a total of 483 unique studies that were assessed for eligibility; of these, eight studies with a total of 1078 randomised participants fulfilled eligibility criteria. IPD were obtained for seven of the eight studies. Outcome data were obtained for 955 out of 978 (97.6%) of the randomised participants in these seven studies (Figure 2).
FIGURE 2.
The PRISMA flow diagram: asthma exacerbation analysis. CENTRAL, Cochrane Central Register of Controlled Trials; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
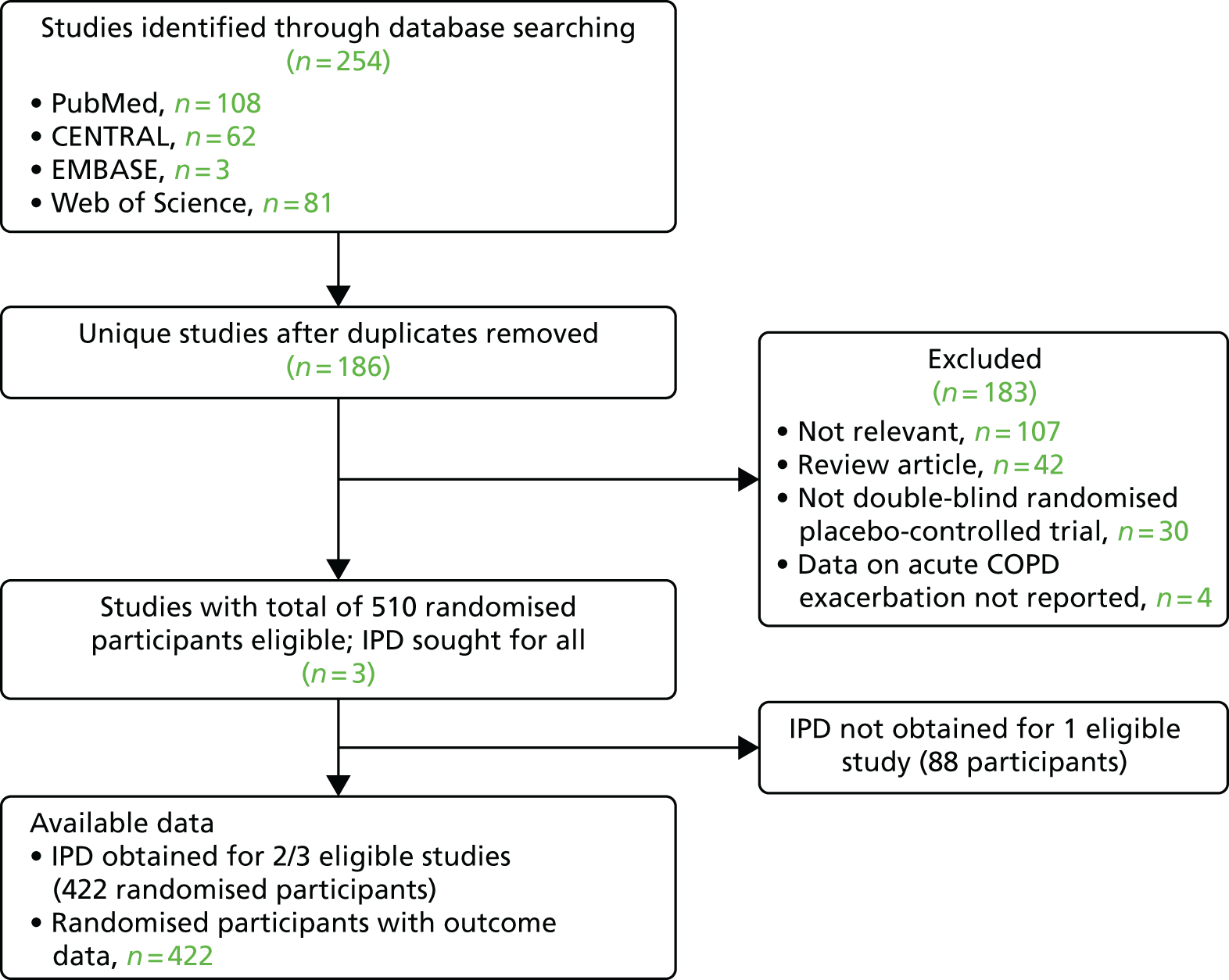
For the analysis of severe COPD exacerbations, our search identified a total of 254 unique studies that were assessed for eligibility; of these, three studies with a total of 510 randomised participants fulfilled eligibility criteria. IPD were obtained for two of the three studies. Outcome data were obtained for 422 out of 422 (100%) of the randomised participants in these two studies (Figure 3).
FIGURE 3.
The PRISMA flow diagram: COPD exacerbation analysis. CENTRAL, Cochrane Central Register of Controlled Trials; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study and participant characteristics
Characteristics of studies contributing data to this meta-analysis and their participants are presented in Table 1.
| First author and year | Setting | Participants | Mean age (years) (SD) [range] | Male : female | 25(OH)D assay, EQA scheme | Mean baseline 25(OH)D concentration (nmol/l) (SD) [range] | Baseline 25(OH)D concentration < 25 nmol/l (%) | Intervention : control | Oral dose of vitamin D3, intervention arm | Control | Study duration | Outcome | ARI definition | n entering analysis/N randomised (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li-Ng 200953 | USA | Healthy adults | 57.9 (13.6) [21.4–80.6] | 34 : 128 | RIA (DiaSorin, Saluggia, Italy) | 63.7 (25.5) [16.0–156.0] | 3/150 (2.0) | 84 : 78 | 50 µg daily | Placebo | 3 months | ARI (1y) | URI: ≥ 2 URI symptoms in absence of allergy symptoms | 157/162 (96.9) |
| Urashima 201045 | Japan | Schoolchildren | 10.2 (2.3) [6.0–15.0) | 242 : 188 | – | Not determined | – | 217 : 213 | 30 µg daily | Placebo | 4 months | ARI (1y), asthma exacerbation. (2y in subset) | URI: influenza A/B diagnosed by RIDT or RIDT-negative ILI | 334/430 (77.7), ARI; 99/110, asthma exacerbation |
| Manaseki-Holland 201054 | Afghanistan | Pre-school children with pneumonia | 1.1 (0.8) [0.1–3.3] | 257 : 196 | – | Not determined | – | 224 : 229 | 2.5-mg bolus, once | Placebo | 3 months | ARI (2y) | LRI: repeat episode of pneumonia – age-specific tachypnoea without wheeze | 453/453 (100.0) |
| Laaksi 201055 | Finland | Military conscripts | 19.1 (0.6) [18.0–21.0] | 164 : 0 | EIA [Immunodiagnostic Systems Holdings plc (IDS), The Boldons, UK; Octeia®] | 75.9 (18.7) [41.9–129.0] | 0/73 (0.0) | 80 : 84 | 10 µg daily | Placebo | 6 months | ARI (1y) | ARI: medical record diagnosis | 164/164 (100.0) |
| Majak 201156 | Poland | Children with asthma | 10.9 (3.3) [6.0–17.0] | 32 : 16 | RIA (BioSource Europe, S.A., Nivelles, Belgium) | 88.9 (38.2) [31.5–184.7] | 0/48 (0.0) | 24 : 24 | 12.5 µg daily | Placebo | 6 months | Asthma exacerbation (1y), ARI (2y) | ARI: self-report | 48/48 (100.0), asthma exacerbation and ARI |
| Trilok Kumar 201157 | India | Low-birthweight infants | 0.1 (0.0) [0.0–0.3] | 970 : 1109 | – | Not determined | Not determined | 1039 : 1040 | 35 µg weekly | Placebo | 6 months | ARI (2y) | ARI: medical record diagnosis of events causing hospitalisation | 2064/2079 (99.3) |
| Lehouck 201228 | Belgium | Adults with COPD | 67.9 (8.3) [48.0–86.0] | 145 : 37 | RIA (DiaSorin) | 49.8 (29.2) [9.0–159.7] | 31/182 (17.0) | 91 : 91 | 2.5-mg bolus monthly | Placebo | 1 year | COPD exacerbation (1y), ARI (2y) | URI: self-report | 175/182 (96.2), ARI; 180/182 COPD exacerbation |
| Manaseki-Holland 201258 | Afghanistan | Infants | 0.5 (0.3) [0.0–1.0] | 1591 : 1455 | – | Not determined | Not determined | 1524 : 1522 | 2.5-mg bolus once every 3 months | Placebo | 1.5 years | ARI (1y) | LRI: pneumonia confirmed by chest radiograph | 3011/3046 (98.9) |
| Camargo 201239 | Mongolia | Third/fourth grade schoolchildren | 10.0 (0.9) [7.0–12.7] | 129 : 118 | LC-MS/MS | 18.9 (9.7) [3.3–61.2] | 192/245 (78.4) | 143 : 104 | 7.5 µg daily | Placebo | 7 weeks | ARI (2y) | ARI: parent-reported ‘chest infections or colds’ | 244/247 (98.8) |
| Murdoch 201240 | New Zealand | Healthy adults | 48.1 (9.7) [18.0–67.6] | 81 : 241 | LC-MS/MS | 72.1 (22.1) [13.0–142.0] | 5/322 (1.6) | 161 : 161 | 2 × 5-mg bolus monthly, then 2.5-mg bolus monthly | Placebo | 1.5 years | ARI (1y) | URI: assessed with symptom score | 322/322 (100.0) |
| Bergman 201259 | Sweden | Adults with increased susceptibility to ARI | 53.1 (13.1) [20.0–77.0] | 38 : 102 | CLA (DiaSorin) | 49.3 (23.2) [8.0–135.0] | 15/131 (11.45) | 70 : 70 | 100 µg daily | Placebo | 1 year | ARI (2y) | URI: assessed with symptom score | 124/140 (88.6) |
| Marchisio 201360 | Italy | Children with recurrent acute otitis media | 2.8 (1.0) [1.3–4.8] | 64 : 52 | CLA (DiaSorin) | 65.3 (17.3) [24.7–120.6] | 2/116 (1.7) | 58 : 58 | 25 µg daily | Placebo | 6 months | ARI (1y) | URI: doctor-diagnosed acute otitis media | 116/116 (100.0) |
| Rees 201341 | USA | Adults with previous colorectal adenoma | 61.2 (6.6) [47.1–77.9] | 438 : 321a | RIA (IDS) | 62.5 (21.3) [30.2–171.6] | 0/759 (0.0) | 399 : 360 | 25 µg daily | Placebo | 13 months (average) | ARI (2y) | URI: assessed from daily symptom diary | 759/759 (100.0) |
| Tran 201443 | Australia | Healthy older adults | 71.7 (6.9) [60.3–85.2] | 343 : 301 | CLA (DiaSorin) | 41.7 (13.5) [12.6–105.0] | 66/643 (10.3) | 430 : 214 | 0.75-mg bolus vs. 1.5-mg bolus monthly | Placebo | 1 year | ARI (2y) | URI: self-reported cold | 594/644 (92.2) |
| Goodall 201461 | Canada | Healthy university students | 19.6 (2.2) [17.0–33.0] | 218 : 382 | – | Not determined | – | 300 : 300 | 0.25 mg weekly (factorial with gargling) | Placebo | 8 weeks | ARI (1y) | URI: self-reported cold | 492/600 (82.0) |
| Urashima 201444 | Japan | High school students | 16.5 (1.0) [15.0–18.0] | 162 : 85 | – | Not determined | – | 148 : 99 | 50 µg daily | Placebo | 2 months | ARI (1y) | URI: influenza A diagnosed by RIDT or RIDT-negative ILI | 247/247 (100.0) |
| Grant 201562 | New Zealand | Pregnant women and offspring | unborn |
0 : 260 (mothers) 121 : 128 (offspring) |
LC-MS/MS | 54.8 (25.8) [8.0–128.0] | 30/200 (15.0) |
173 : 87 (mothers) 164 : 85 (offspring) |
Mothers: 25 µg vs. 50 µg daily; infants: 10 µg vs. 20 µg daily | Placebo | 9 months (3 months in pregnancy and 6 months in infancy) | ARI (2y) | ARI: doctor-diagnosed ARI precipitating primary care consultation | 236/260 (90.8) |
| Martineau 201529 (ViDiCO) | UK | Adults with COPD | 64.7 (8.5) [40.0–85.0] | 144 : 96 | LC-MS/MS | 46.1 (25.7) [0.0–160.0] | 50/240 (20.8) | 122 : 118 | 3-mg bolus once every 2 months | Placebo | 1 year | ARI and COPD exacerbation (Co1y) | URI: assessed from daily symptom diary | 240/240 (100.0), ARI and COPD exacerbation |
| Martineau 201547 (ViDiAs) | UK | Adults with asthma | 47.9 (14.4) [16.0–78.0] | 109 : 141 | LC-MS/MS | 49.6 (24.7) [0.0–139.0] | 36/250 (14.4) | 125 : 125 | 3-mg bolus once every 2 months | Placebo | 1 year | ARI and asthma exacerbation (Co1y) | URI: assessed from daily symptom diary | 250/250 (100.0), ARI and asthma exacerbation |
| Martineau 201548 (ViDiFlu) | UK | Older adults and their carers | 67.1 (13.0) [21.4–94.0] | 82 : 158 | LC-MS/MS | 42.9 (23.0) [0.0–128.0] | 60/240 (25.0) | 137 : 103 |
Older adults: 2.4-mg bolus once every 2 months + 10 µg daily Carers: 3 mg once every 2 months |
Older adults: placebo + 10 µg daily Carers: placebo |
1 year | ARI (1y) | URI and LRI, both assessed from daily symptom diary | 240/240 (100.0) |
| Simpson 201563 | Australia | Healthy adults | 32.2 (12.2) [18.0–52.0] | 14 : 20 | LC-MS/MS | 67.9 (23.0) [32.0–132.0] | 0/33 (0.0) | 18 : 16 | 0.5 mg weekly | Placebo | 17 weeks | ARI (1y) | ARI assessed with symptom score | 34/34 (100.0) |
| Dubnov-Raz 201564 | Israel | Adolescent swimmers with vitamin D insufficiency | 15.2 (1.6) [12.9–18.6] | 34 : 20 | RIA (DiaSorin) | 60.4 (11.9) [28.0–74.6] | 0/54 (0.0) | 27 : 27 | 50 µg daily | Placebo | 12 weeks | ARI (1y) | URI assessed with symptom score | 25/54 (46.3) |
| Castro 201465/Denlinger 201666 | USA | Adults with asthma | 39.2 (12.9) [18.0–85.0] | 130 : 278 | CLA (DiaSorin) | 47.0 (16.9) [10.0–74.6] | 55/408 (13.5) | 201 : 207 | 2.5-mg bolus then 100 µg daily | Placebo | 28 weeks | Asthma exacerbation (2y), ARI (2y) | URI assessed with symptom score | 408/408 (100.0), ARI and asthma exacerbation |
| Tachimoto 201642 | Japan | Children with asthma | 9.9 (2.3) [6.0–15.0] | 50 : 39 | RIA (DiaSorin) | 74.9 (24.6) [20.0–187.2] | 1/89 (1.1) | 54 : 35 | 20 µg daily, first 2 months | Placebo | 6 months | Asthma exacerbation (2y), ARI (2y) | URI: assessed with symptom score | 89/89 (100.0), ARI and asthma exacerbation |
| Kerley 201667 | Ireland | School children with asthma | 8.6 (2.8) [5.0–15.0] | 24 : 15 | LC-MS/MS | 54.4 (17.4) [26–92] | 0/39 (0.0) | 17 : 22 | 50 µg daily | Placebo | 15 weeks | Asthma exacerbation (2y) | n/a | 39/51 (76.5) |
| Jensen 201668 | Canada | Preschool children with asthma | 2.9 (1.1) [1.6–5.5) | 7 : 15 | LC-MS/MS | 64.2 (14.0) | 0/22 (0.0) | 11 : 11 | 2.5-mg bolus then 10 µg daily | 10 µg daily | 6 months | Asthma exacerbation (2y) | n/a | 22/22 (100.0) |
| Ginde 201769 | USA | Institutionalised older adults | 80.7 (9.9) [60.0–95.0] | 45 : 62 | LC-MS/MS | 57.3 (22.7) [11.7–106.1] | 12/107 (11.2) | 55 : 52 | 2.5-mg bolus monthly + ≤ 25 µg per day equivalent | Placebo + 10–25 µg per day equivalent | 1 year | ARI (1y) | ARI: medical record diagnosis | 107/107 (100.0) |
Trials were conducted in 15 different countries on four continents and enrolled participants of both sexes from birth to 95 years of age. Baseline serum 25(OH)D concentrations were determined in 21 out of 27 trials: the mean baseline 25(OH)D concentration ranged from 18.9 to 88.9 nmol/l. All studies administered oral vitamin D3 to participants in the intervention arm: this was given as monthly to once every 3 months bolus doses in seven studies, as weekly doses in three studies, as a daily dose in 13 studies, and as a combination of bolus and daily doses in four studies. Study duration ranged from 7 weeks to 1.5 years. Incidence of ARI was a primary or coprimary outcome for 14 studies, and a secondary outcome for 11 studies. Incidence of asthma exacerbation was a primary or coprimary outcome for two studies and a secondary outcome for five studies. Incidence of COPD exacerbation was a primary or coprimary outcome for two studies; no study included this as a secondary outcome.
The integrity of the IPD was confirmed by replication of the primary analyses in published papers when applicable. The process of checking IPD identified three minor errors in published reports. For the 2012 trial by Manaseki-Holland et al. ,58 the correct number of repeat episodes of chest radiograph-confirmed pneumonia was 134, rather than 138 as reported. For the trial by Dubnov-Raz et al. ,64 the number of patients randomised to the intervention arm was 27, rather than 28 as reported. For the trial by Laaksi et al. ,55 the proportion of men randomised to placebo who did not experience any ARI was 30 out of 84, rather than 30 out of 80 as reported.
Risk of bias within studies
Details of the risk-of-bias assessment are provided in Table 2.
| First author and year | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Li-Ng 200953 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Urashima 201045 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Manaseki-Holland 201054 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Laaksi 201055 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Majak 201156 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Trilok Kumar 201157 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Lehouck 201228 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Manaseki-Holland 201258 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Camargo 201239 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Murdoch 201240 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Bergman 201259 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Marchisio 201360 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Rees 201341 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tran 201443 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Goodall 201461 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Urashima 201444 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Grant 201562 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Martineau 201529 (ViDiCO) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Martineau 201547 (ViDiAs) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Martineau 201548 (ViDiFlu) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Simpson 201563 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Dubnov-Raz 201564 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Denlinger 201666 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tachimoto 201642 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Kerley 201667 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Jensen 201668 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ginde 201769 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
All but three trials were assessed as being at a low risk of bias for all aspects assessed. Three trials were assessed as being at an unclear risk of bias owing to high rates of loss to follow-up. In the trial by Dubnov-Raz et al. ,64 52% of participants did not complete all symptom questionnaires. In the trial by Laaksi et al. ,55 37% of randomised participants were lost to follow-up. In the trial by Kerley et al. ,67 24% of randomised participants were lost to follow-up.
Overall results: acute respiratory infection incidence
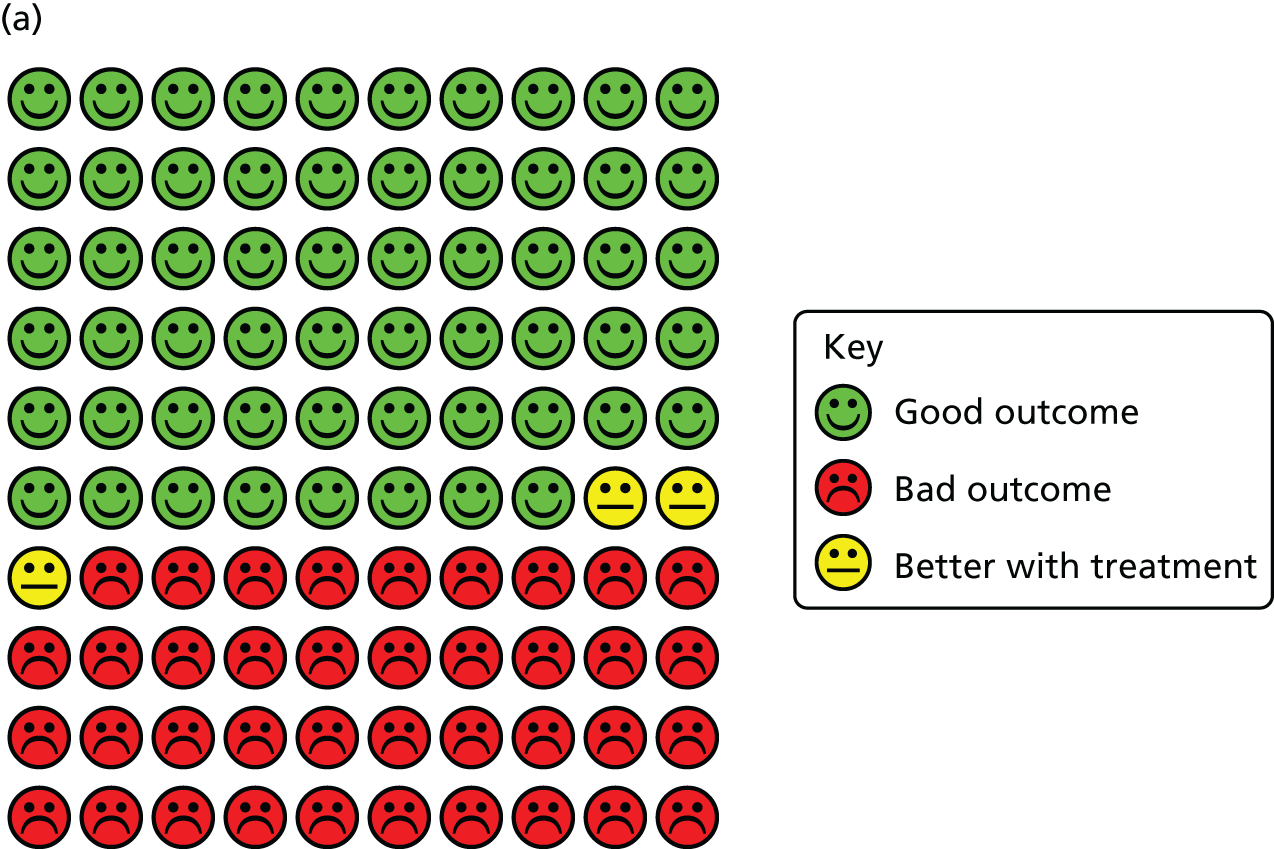
The results of the one-step IPD meta-analysis testing the effects of vitamin D on the proportion of all participants experiencing at least one ARI, adjusting for age, sex and study duration, are presented in Table 3. Vitamin D supplementation resulted in a statistically significant reduction in the proportion of participants experiencing at least one ARI [adjusted odds ratio (aOR) 0.88, 95% CI 0.81 to 0.96, p = 0.003; p for heterogeneity < 0.001; NNT 33, 95% CI 20 to 101; 10,933 participants in 25 studies; Cates plot, Figure 4].
| Subgroup | Number of trialsa | Proportion with ≥ 1 ARI subgroup (%) | aOR (95% CI)b | p-value | p-value for interaction | |
|---|---|---|---|---|---|---|
| Control | Intervention | |||||
| Overall | 25 | 2204/5225 (42.2) | 2303/5708 (40.3) | 0.88 (0.81 to 0.96) | 0.003 | – |
| Baseline 25(OH)D (nmol/l) | ||||||
| < 25 | 14 | 137/249 (55.0) | 117/289 (40.5) | 0.58 (0.40 to 0.82) | 0.002 | 0.01 |
| ≥ 25 | 19 | 1027/1639 (62.7) | 1179/1995 (59.1) | 0.89 (0.77 to 1.04) | 0.15 | |
| Dosing regimen type | ||||||
| Bolus dose ≥ 30,000 IU given | 10 | 994/2786 (35.7) | 1097/3014 (36.4) | 0.97 (0.86 to 1.10) | 0.67 | 0.05 |
| Bolus dose not given | 15 | 1210/2439 (49.6) | 1206/2694 (44.8) | 0.81 (0.72 to 0.91) | < 0.001 | |
| Daily dose equivalent (IU) | ||||||
| < 800 | 5 | 629/1321 (47.6) | 619/1435 (43.1) | 0.80 (0.68 to 0.94) | 0.006 | 0.12 |
| 800–1999.9 | 9 | 945/2796 (33.8) | 1023/3077 (33.2) | 0.90 (0.79 to 1.01) | 0.08 | |
| ≥ 2000 | 11 | 630/1108 (56.9) | 661/1196 (55.3) | 0.98 (0.81 to 1.18) | 0.84 | |
| Age (years) | ||||||
| ≤ 1 | 4 | 832/2744 (30.3) | 854/2827 (30.2) | 0.94 (0.83 to 1.06) | 0.33 | 0.61 |
| 1.1–15.9 | 8 | 241/513 (47.0) | 194/566 (34.3) | 0.60 (0.46 to 0.77) | < 0.001 | |
| 16–65 | 17 | 854/1459 (58.5) | 885/1592 (55.6) | 0.93 (0.79 to 1.10) | 0.41 | |
| > 65 | 11 | 277/509 (54.4) | 370/723 (51.2) | 0.86 (0.67 to 1.09) | 0.21 | |
| Body mass index (kg/m2) | ||||||
| < 25 | 19 | 972/1943 (50.0) | 956/2074 (46.1) | 0.85 (0.74 to 0.97) | 0.02 | 0.29 |
| ≥ 25 | 17 | 659/1039 (63.4) | 754/1235 (61.1) | 0.95 (0.79 to 1.14) | 0.58 | |
| Comorbidity: asthma | ||||||
| No | 11 | 518/1008 (51.4) | 520/1101 (47.2) | 0.82 (0.68 to 0.99) | 0.04 | 0.48 |
| Yes | 11 | 296/534 (55.4) | 285/542 (52.6) | 0.95 (0.73 to 1.25) | 0.73 | |
| Comorbidity: COPD | ||||||
| No | 7 | 477/763 (62.5) | 493/791 (62.3) | 1.00 (0.80 to 1.26) | 0.98 | 0.38 |
| Yes | 6 | 122/230 (53.0) | 120/238 (50.4) | 0.84 (0.57 to 1.24) | 0.38 | |
| Influenza vaccination | ||||||
| No | 10 | 255/373 (68.4) | 253/407 (62.2) | 0.74 (0.52 to 1.03) | 0.08 | 0.51 |
| Yes | 10 | 564/779 (72.4) | 577/826 (69.9) | 0.86 (0.68 to 1.09) | 0.22 | |
FIGURE 4.
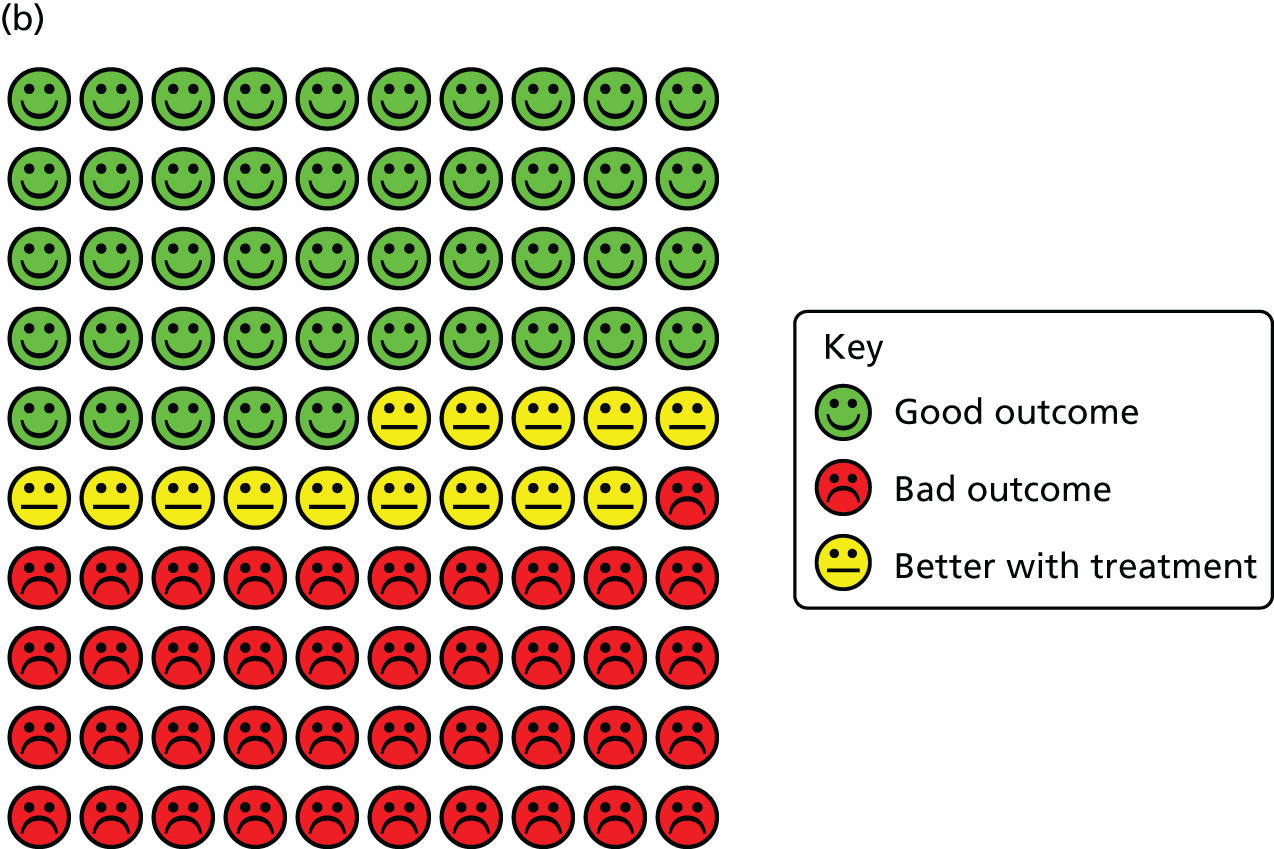
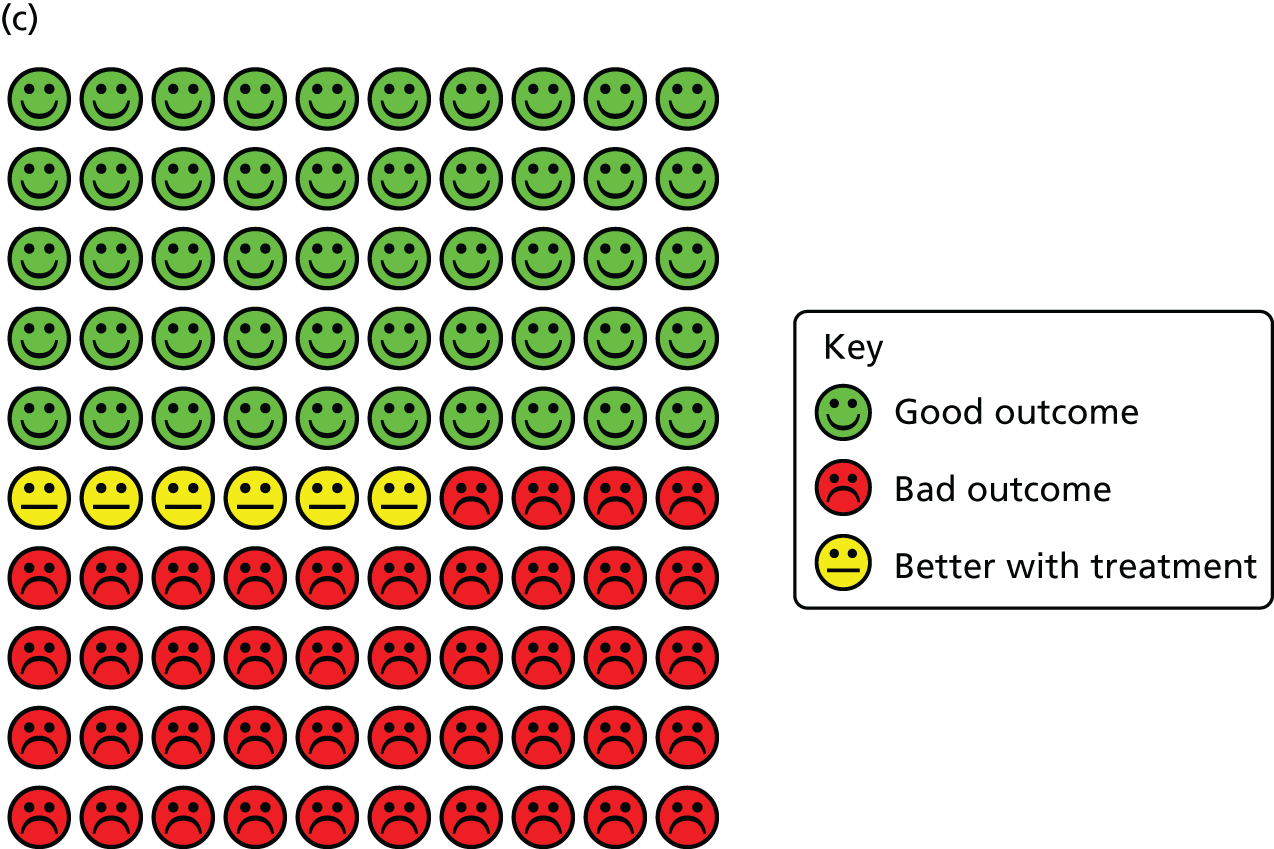
Cates plots illustrating reduction in risk of ARI with vitamin D supplementation, irrespective of dosing frequency. (a) All participants, irrespective of baseline vitamin D status; (b) participants with baseline serum 25(OH)D concentration of < 25 nmol/l; and (c) participants receiving daily or weekly vitamin D supplementation regimens without any additional bolus doses.
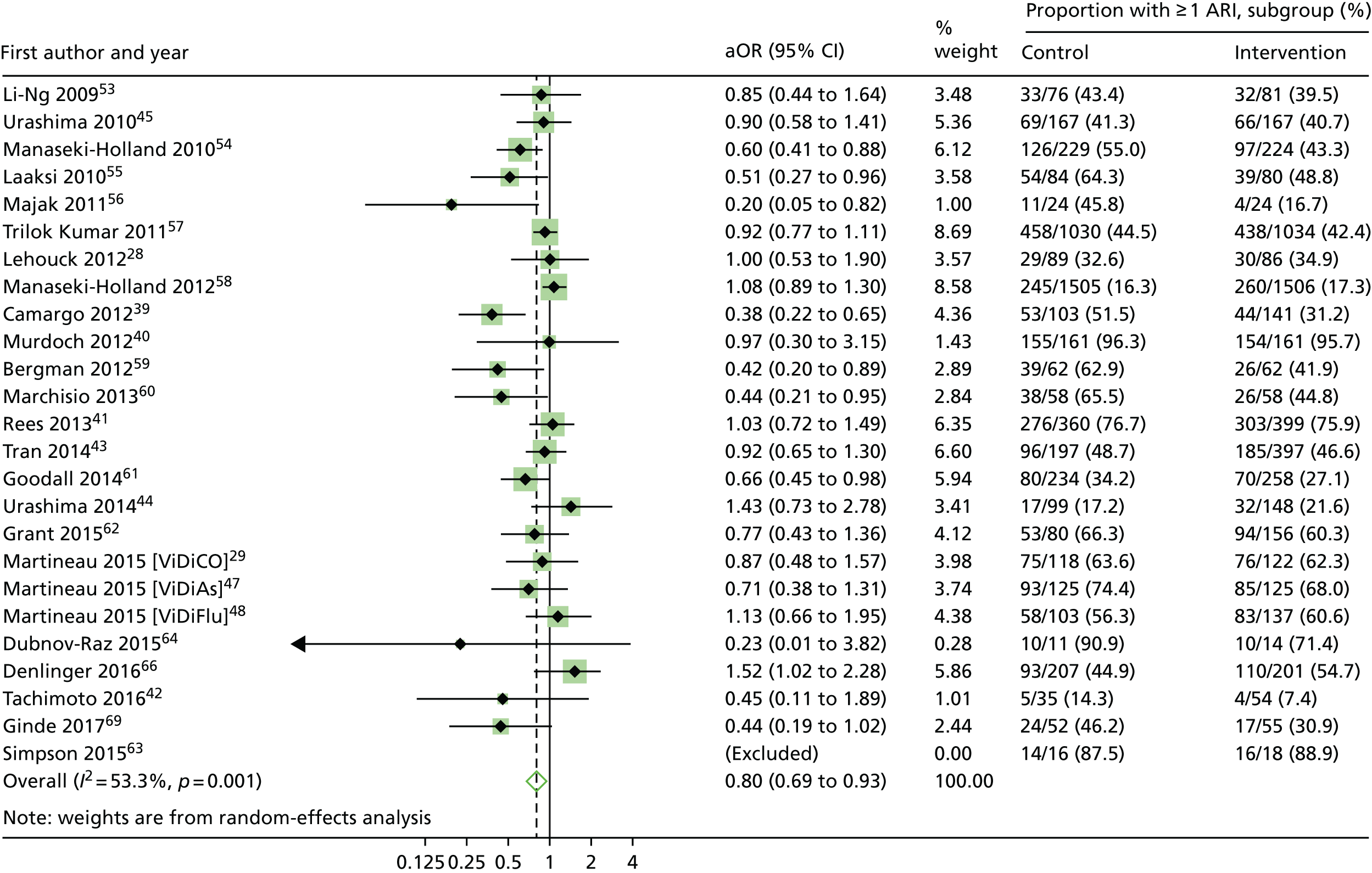
Statistically significant protective effects of vitamin D were also seen for the one-step analyses of ARI rate [adjusted incidence rate ratio (aIRR) 0.96, 95% CI 0.92 to 0.997; p = 0.04 and p for heterogeneity < 0.001] in 10,703 participants in 25 studies (Table 4). However, the protective effects of vitamin D were not seen in the analysis of time to first ARI [adjusted hazard ratio (aHR) 0.95, 95% CI 0.89 to 1.01; p = 0.09 and p for heterogeneity < 0.001] in 9108 participants in 18 studies (Table 5). Two-step analyses also showed consistent effects for the proportion of participants experiencing at least one ARI (aOR 0.80, 95% CI 0.69 to 0.93; p = 0.004 and p for heterogeneity = 0.001) in 10,899 participants in 24 studies (Figure 5), ARI rate (aIRR 0.91, 95% CI 0.84 to 0.98; p = 0.018 and p for heterogeneity < 0.001) in 10,703 participants in 25 studies, and time to first ARI (aHR 0.92, 95% CI 0.85 to 1.00; p = 0.051 and p for heterogeneity = 0.14) in 9108 participants in 18 studies. This evidence was assessed as being of high quality.
| Subgroup | Number of trials | Number of individuals | Rate of ARI per participant-year, subgroup | aIRR (95% CI)a | p-value | p-value for Interaction | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| Overall | 25 | 10,703 | 1.15 | 1.13 | 0.96 (0.92 to 0.997) | 0.04 | – |
| Baseline 25(OH)D (nmol/l) | |||||||
| < 25 | 14 | 509 | 2.15 | 1.67 | 0.78 (0.66 to 0.93) | 0.004 | 0.02 |
| ≥ 25 | 19 | 3458 | 2.12 | 1.91 | 0.95 (0.90 to 1.00) | 0.04 | |
| Dosing regimen type | |||||||
| Bolus dose ≥ 30,000 IU given | 10 | 5595 | 0.73 | 0.76 | 0.99 (0.94 to 1.05) | 0.83 | 0.11 |
| Bolus dose not given | 15 | 5133 | 2.23 | 2.09 | 0.93 (0.88 to 0.98) | 0.008 | |
| Subgroup | Number of trials | Number of individuals | Median time (days) to first ARI, subgroup (IQR) | aHR (95% CI)a | p-value | p-value for Interaction | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| Overall | 18 | 9108 | 452 (79 to –) | 502 (81 to –) | 0.95 (0.89 to 1.01) | 0.09 | – |
| Baseline 25(OH)D (nmol/l) | |||||||
| < 25 | 10 | 229 | 159 (56 to –) | 172 (74 to –) | 0.92 (0.66 to 1.28) | 0.62 | 0.61 |
| ≥ 25 | 12 | 2231 | 104 (41 to 280) | 110 (40 to 328) | 0.97 (0.88 to 1.06) | 0.48 | |
| Dosing regimen type | |||||||
| Bolus dose ≥ 30,000 IU given | 8 | 4795 | – (121 to –) | – (117 to –) | 0.98 (0.89 to 1.08) | 0.74 | 0.30 |
| Bolus dose not given | 10 | 4313 | 138 (57 to 331) | 153 (61 to 351) | 0.91 (0.84 to 0.99) | 0.04 | |
FIGURE 5.
Two-step IPD meta-analysis, proportion of participants experiencing at least one ARI. Data from the trial by Simpson et al. 63 were not included in this two-step meta-analysis, as an estimate for the effect of the intervention in the study could not be obtained in the regression model because of the small sample size.
Overall results: asthma exacerbation
One-step IPD meta-analysis testing the effects of vitamin D on the rate of asthma exacerbations requiring treatment with systemic corticosteroids revealed a statistically significant protective effect of the intervention (aIRR 0.74, 95% CI 0.56 to 0.97; p = 0.03; 955 participants in seven studies). Two-step IPD meta-analysis revealed a similar effect size (aIRR 0.69, 95% CI 0.52 to 0.92, p = 0.01; p for heterogeneity = 0.56; 719 participants in four studies). Consistent trends were seen for analysis of the proportion of participants experiencing at least one asthma exacerbation requiring treatment with systemic corticosteroids in both one-step analysis (aOR 0.75, 95% CI 0.51 to 1.09; p = 0.13; 955 participants in seven studies) and two-step analysis (aOR 0.69, 95% CI 0.46 to 1.02, p = 0.06; p for heterogeneity = 0.74; 719 participants in four studies). Similarly, trends towards a delay to first exacerbation with vitamin D versus placebo were seen in both one-step analysis (aHR 0.78, 95% CI 0.55 to 1.10; p = 0.16; 868 participants in five studies) and two-step analysis (aHR 0.74, 95% CI 0.52 to 1.05, p = 0.09; p for heterogeneity = 0.58; 680 participants in three studies).
Overall results: chronic obstructive pulmonary disease exacerbation
One-step IPD meta-analysis testing the effects of vitamin D on the rate of COPD exacerbations requiring treatment with antibiotics and/or systemic corticosteroids did not reveal a statistically significant protective effect of the intervention overall (aIRR 0.95, 95% CI 0.82 to 1.10; p = 0.50; 422 participants in two studies). Two-step IPD meta-analysis revealed a similar estimate (aIRR 0.96, 95% CI 0.83 to 1.10; p = 0.54; 422 participants in two studies). Consistent results were seen for analysis of the proportion of participants experiencing at least one study-defined exacerbation in both one-step IPD meta-analysis (aOR 0.80, 95% CI 0.51 to 1.25; p = 0.32; 422 participants in two studies) and two-step IPD meta-analysis (aOR 0.79, 95% CI 0.50 to 1.24; p = 0.31; 422 participants in two studies). Similarly, no statistically significant effects of the intervention on time to first exacerbation were seen for one-step IPD meta-analysis (aHR 0.95, 95% CI 0.75 to 1.21; p = 0.67; 422 participants in two studies) or two-step IPD meta-analysis (aHR 0.96, 95% CI 0.76 to 1.22; p = 0.75; 422 participants in two studies) overall.
Subgroup analyses: acute respiratory infection incidence
In order to explore reasons for heterogeneity, subgroup analyses were conducted to investigate whether or not the effects of vitamin D supplementation on ARI risk differed according to baseline vitamin D status, dosing frequency, dose size, age, body mass index, the presence or absence of comorbidity (asthma or COPD) or influenza vaccination status. Race/ethnicity was not investigated as a potential effect-modifier, as data for this variable were missing for 3680 out of 10,933 (34%) participants, and power for subgroup analyses was limited by small numbers in many racial/ethnic subgroups that could not be meaningfully combined. Similarly, baseline data relating to environmental exposure to particulate matter, nutritional supplement use and vitamin D-related genotype were unavailable or available only in a very small number of studies, precluding investigation of these factors as potential effect modifiers. The results are presented in Overall results: acute respiratory infection incidence (see Table 3). Subgroup analysis revealed a strong protective effect of vitamin D supplementation among individuals at a baseline circulating 25(OH)D concentration level of < 25 nmol/l (aOR 0.58, 95% CI 0.40 to 0.82; NNT 8, 95% CI 5 to 21; 538 participants in 14 studies; within subgroup, p = 0.002; Cates plot, see Figure 4), and no statistically significant effect among those at a baseline 25(OH)D concentration level of ≥ 25 nmol/l (aOR 0.89, 95% CI 0.77 to 1.04; 3634 participants in 19 studies; within subgroup, p = 0.15; for interaction, p = 0.01). This evidence was assessed as being of high quality.
A meta-analysis of data from trials in which vitamin D was administered using a daily or weekly regimen without additional bolus doses revealed a protective effect of vitamin D against ARI (aOR 0.81, 95% CI 0.72 to 0.91; NNT 20, 95% CI 13 to 43; 5133 participants in 15 studies; within subgroup, p < 0.001; Cates plot, see Figure 4). No such protective effect was seen among participants in trials in which at least one bolus dose of vitamin D was administered (aOR 0.97, 95% CI 0.86 to 1.10; 5800 participants in 10 studies; within subgroup, p = 0.67; for interaction, p = 0.05). This evidence was assessed as being of high quality. The p-values for interaction were > 0.05 for all other potential effect modifiers investigated. For both of these subgroup analyses, broadly consistent effects were observed for event rate analysis (see Table 4) and survival analysis (see Table 5).
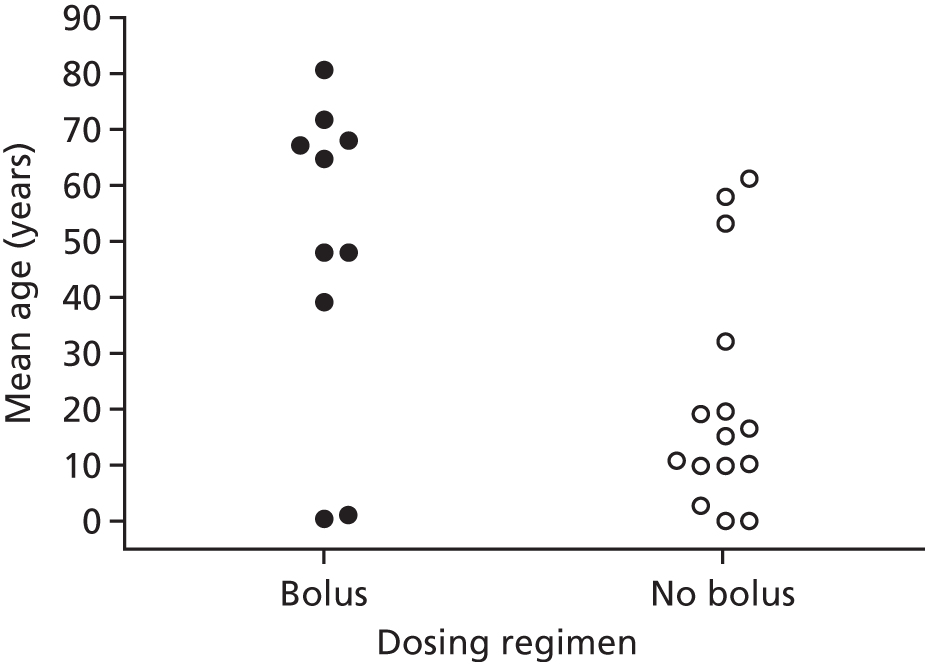
Having identified two potential factors that modified the influence of vitamin D supplementation on the risk of ARI (i.e. baseline vitamin D status and dosing frequency), we then proceeded to investigate whether or not these factors were acting as independent effect modifiers, and whether or not they were confounded by each other or by another potential effect modifier, such as age. Dot plots revealed a trend towards lower median baseline serum 25(OH)D concentration and higher median age for studies employing bolus versus daily or weekly dosing (Figures 6 and 7). In order to establish which of these potential effect modifiers was acting independently, we repeated the analysis to include treatment–covariate interaction terms for baseline vitamin D status, dosing frequency and age. In this model, interaction terms for baseline vitamin D status and dosing frequency were statistically significant (p = 0.01 and p = 0.004, respectively), but the interaction term for age was not statistically significant (p = 0.20), consistent with the hypothesis that baseline vitamin D status and dosing frequency, but not age, independently modified the effect of vitamin D supplementation on ARI risk.
FIGURE 6.
Mean baseline serum 25(OH)D concentration at enrolment by dosing regimen. Bolus, studies in which a bolus dose of ≥ 30,000 IU of vitamin D was given in the intervention arm; No bolus, studies in which vitamin D was administered daily or weekly without administration of a bolus dose.
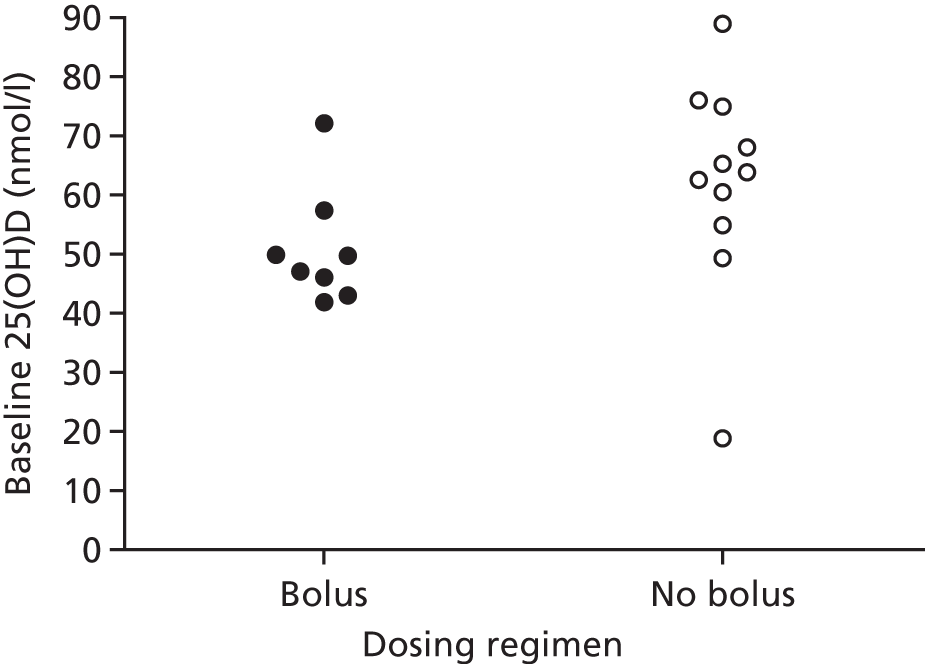
FIGURE 7.
Mean age at enrolment by dosing regimen. Bolus, studies in which a bolus dose of ≥ 30,000 IU of vitamin D was given in the intervention arm; No bolus, studies in which vitamin D was administered daily or weekly without administration of a bolus dose.
We then proceeded to stratify the subgroup analysis presented in Table 3 according to dosing frequency, in order to provide a ‘cleaner’ look at the results of the subgroup analyses under the assumption that administration of bolus doses was ineffective. The results of this exploratory analysis are presented in Table 6.
| Subgroup | Bolus | Daily or weekly | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of trialsa | Proportion with ≥ 1 ARI subgroup (%) | aOR (95% CI)b | p-value | p-value for interaction | Number of trialsa | Proportion with ≥ 1 ARI subgroup (%) | aOR (95% CI)b | p-value | p-value for interaction | |||
| Control | Intervention | Control | Intervention | |||||||||
| Overall | 10 | 994/2786 (35.7) | 1097/3014 (36.4) | 0.97 (0.86 to 1.10) | 0.67 | N/A | 15 | 1210/2439 (49.6) | 1206/2694 (44.8) | 0.81 (0.72 to 0.91) | 0.001 | N/A |
| Baseline 25(OH)D (nmol/l) | ||||||||||||
| < 25 | 8 | 73/142 (51.4) | 77/162 (47.5) | 0.82 (0.51 to 1.33) | 0.43 | 0.42 | 6 | 64/107 (59.8) | 40/127 (31.5) | 0.30 (0.17 to 0.53) | < 0.001 | 0.006 |
| ≥ 25 | 8 | 550/910 (60.4) | 663/1121 (59.1) | 1.02 (0.83 to 1.24) | 0.87 | 11 | 477/729 (65.4) | 516/874 (59.0) | 0.75 (0.60 to 0.95) | 0.02 | ||
| Daily dose equivalent (IU) | ||||||||||||
| < 800 | 0 | N/A | N/A | N/A | N/A | 0.56 | 5 | 629/1321 (47.6) | 619/1435 (43.1) | 0.80 (0.68 to 0.94) | 0.006 | 0.82 |
| 800–1999.9 | 3 | 467/1931 (24.2) | 542/2127 (25.5) | 0.95 (0.81 to 1.10) | 0.50 | 6 | 478/865 (55.3) | 481/950 (50.6) | 0.81 (0.66 to 1.01) | 0.06 | ||
| ≥ 2000 | 7 | 527/855 (61.6) | 555/887 (62.6) | 1.03 (0.83 to 1.28) | 0.81 | 4 | 103/253 (40.7) | 106/309 (34.3) | 0.85 (0.58 to 1.24) | 0.39 | ||
| Age (years) | ||||||||||||
| ≤ 1 | 2 | 321/1634 (19.6) | 322/1637 (19.7) | 0.99 (0.83 to 1.19) | 0.93 | 0.72 | 2 | 511/1110 (46.0) | 532/1190 (44.7) | 0.91 (0.77 to 1.08) | 0.30 | 0.37 |
| 1.1–15.9 | 1 | 50/100 (50.0) | 35/93 (37.6) | 0.62 (0.35 to 1.11) | 0.11 | 7 | 191/413 (46.2) | 159/473 (33.6) | 0.59 (0.45 to 0.79) | < 0.001 | ||
| 16–65 | 8 | 432/678 (63.7) | 466/716 (65.1) | 1.15 (0.90 to 1.48) | 0.27 | 9 | 422/781 (54.0) | 419/876 (47.8) | 0.79 (0.63 to 0.99) | 0.04 | ||
| > 65 | 8 | 191/374 (51.1) | 274/568 (48.2) | 0.85 (0.65 to 1.12) | 0.25 | 3 | 86/135 (63.7) | 96/155 (61.9) | 0.88 (0.52 to 1.52) | 0.66 | ||
| Body mass index (kg/m2) | ||||||||||||
| < 25 | 8 | 215/372 (57.8) | 231/417 (55.4) | 1.01 (0.72 to 1.40) | 0.97 | 0.70 | 11 | 757/1571 (48.2) | 725/1657 (43.8) | 0.82 (0.71 to 0.95) | 0.009 | > 0.99 |
| ≥ 25 | 8 | 406/677 (60.0) | 509/867 (58.7) | 1.00 (0.80 to 1.25) | 0.98 | 9 | 253/358 (70.7) | 245/367 (66.8) | 0.83 (0.59 to 1.17) | 0.30 | ||
| Asthma | ||||||||||||
| No | 5 | 303/484 (62.6) | 323/523 (61.8) | 0.95 (0.71 to 1.28) | 0.75 | 0.40 | 6 | 215/524 (41.0) | 197/578 (34.1) | 0.74 (0.58 to 0.95) | 0.02 | 0.40 |
| Yes | 4 | 224/371 (60.4) | 232/364 (63.7) | 1.18 (0.85 to 1.65) | 0.32 | 7 | 72/163 (44.2) | 53/178 (29.8) | 0.60 (0.37 to 0.98) | 0.04 | ||
| COPD | ||||||||||||
| No | 5 | 410/632 (64.9) | 436/656 (66.5) | –c | –c | –c | 2 | 67/131 (51.1) | 57/135 (42.2) | –c | –c | –c |
| Yes | 4 | 117/223 (52.5) | 119/231 (51.5) | –c | –c | –c | 2 | 5/7 (71.4) | 1/7 (14.3) | –c | –c | –c |
| Influenza vaccination | ||||||||||||
| No | 5 | 119/163 (73.0) | 121/178 (68.0) | –c | –c | –c | 5 | 136/210 (64.8) | 132/229 (57.6) | –c | –c | –c |
| Yes | 5 | 286/396 (72.2) | 294/421 (69.8) | 5 | 278/383 (72.6) | 283/405 (69.9) | ||||||
This analysis reveals that daily or weekly administration of vitamin D was associated with an even greater degree of protection against ARI among participants with baseline circulating 25(OH)D concentrations of < 25 nmol/l than in the unstratified analysis (aOR 0.30, 95% CI 0.17 to 0.53; NNT 4, 95% CI 3 to 7; 234 participants in six studies; within subgroup, p < 0.001; Cates plot, Figure 8). Moreover, administration of daily or weekly vitamin D also protected against ARI among participants with higher baseline 25(OH)D concentrations (aOR 0.75, 95% CI 0.60 to 0.95; NNT 15, 95% CI 9 to 86; 1603 participants in six studies; within subgroup, p = 0.02; Cates plot, see Figure 8). The p-value for interaction for this subgroup analysis was 0.006, indicating that protective effects of daily or weekly vitamin D supplementation were significantly greater in the subgroup of participants with profound vitamin D deficiency. No other statistically significant interaction was seen; notably, bolus-dose vitamin D supplementation did not offer any protection against ARI even when administered to those with circulating 25(OH)D concentrations of < 25 nmol/l (aOR 0.82, 95% CI 0.51 to 1.33; 304 participants in eight studies; within subgroup, p = 0.43).
FIGURE 8.
Cates plot illustrating reduction in risk of ARI with daily or weekly vitamin D supplementation without additional bolus doses. (a) Participants with baseline serum 25(OH)D concentrations of < 25 nmol/l; and (b) participants with baseline serum 25(OH)D concentrations of ≥ 25 nmol/l.
Subgroup analyses: asthma exacerbation
Subgroup analyses were conducted to investigate whether or not the effects of vitamin D supplementation on the rate of asthma exacerbations requiring treatment with systemic corticosteroids differed according to baseline vitamin D status, age, body mass index, administration of bolus doses of vitamin D, amount of vitamin D administered and concomitant use of inhaled corticosteroids. The results are presented in Table 7. Vitamin D supplementation reduced the rate of asthma exacerbations requiring treatment with systemic corticosteroids among individuals with baseline circulating 25(OH)D concentrations of < 25 nmol/l (aIRR 0.33, 95% CI 0.11 to 0.98; 92 participants in three studies; within subgroup, p = 0.046) and in those with baseline 25(OH)D concentrations of ≥ 25 nmol/l (aIRR 0.77, 95% CI 0.58 to 1.03; 764 participants in six studies within subgroup, p = 0.08). The treatment–covariate interaction term (ratio of aIRRs) for this subgroup analysis was 0.56 (95% CI 0.20 to 1.52; for interaction, p = 0.25). The p-values for interaction for all other subgroup analyses were also > 0.05.
| Subgroup | Number of trialsa | Number of individuals | Event rate per participant-year subgroup | aIRR (95% CI)b | p-value | p-value for interaction | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| Overall | 7 | 955 | 121/284.7 (0.43) | 85/286.6 (0.30) | 0.74 (0.56 to 0.97) | 0.03 | – |
| Baseline 25(OH)D (nmol/l) | |||||||
| < 25 | 3 | 92 | 14/33.0 (0.42) | 6/32.2 (0.19) | 0.33 (0.11 to 0.98) | 0.046 | 0.25 |
| ≥ 25 | 6 | 764 | 107/233.8 (0.46) | 79/240.2 (0.33) | 0.77 (0.58 to 1.03) | 0.08 | |
| Age (years) | |||||||
| < 16 | 5 | 290 | 26/57.6 (0.45) | 19/61.8 (0.31) | 0.64 (0.34 to 1.20) | 0.16 | 0.56 |
| ≥ 16 | 3 | 665 | 95/227.2 (0.42) | 66/224.7 (0.29) | 0.70 (0.51 to 0.97) | 0.03 | |
| Sex | |||||||
| Female | 7 | 547 | 80/163.6 (0.49) | 47/167.7 (0.28) | 0.61 (0.43 to 0.88) | 0.008 | 0.17 |
| Male | 7 | 408 | 41/121.1 (0.34) | 38/118.7 (0.32) | 0.91 (0.58 to 1.42) | 0.67 | |
| Body habitus | |||||||
| Not overweight | 7 | 381 | 38/110.5 (0.34) | 26/104.5 (0.25) | 0.91 (0.55 to 1.51) | 0.71 | 0.31 |
| Overweightc | 7 | 574 | 83/174.3 (0.48) | 59/182.0 (0.32) | 0.68 (0.49 to 0.95) | 0.02 | |
| Bolus-dose vitamin D given | |||||||
| No | 4 | 275 | 13/53.8 (0.24) | 10/58.9 (0.17) | 0.65 (0.26 to 1.63) | 0.36 | 0.49 |
| Yes | 3 | 680 | 108/230.9 (0.47) | 75/227.6 (0.33) | 0.71 (0.52 to 0.95) | 0.02 | |
| Daily dose equivalent (IU) | |||||||
| < 2000 | 4 | 258 | 13/52.1 (0.25) | 10/58.6 (0.17) | 0.62 (0.26 to 1.44) | 0.26 | 0.78 |
| ≥ 2000 | 3 | 697 | 108/232.7 (0.46) | 75/228.0 (0.33) | 0.73 (0.54 to 0.98) | 0.03 | |
| Inhaled corticosteroids | |||||||
| No | 4 | 92 | 1/18.8 (0.05) | 4/26.1 (0.15) | 1.11 (0.07 to 18.40) | 0.94 | 0.19 |
| Yes | 5 | 764 | 120/248.0 (0.48) | 81/246.3 (0.33) | 0.71 (0.54 to 0.95) | 0.02 | |
| Study duration (months) | |||||||
| < 6 | 2 | 138 | 13/25.0 (0.52) | 9/19.4 (0.46) | 0.50 (0.18 to 1.37) | 0.18 | 0.62 |
| ≥ 6 | 5 | 816 | 108/259.8 (0.42) | 76/267.2 (0.28) | 0.72 (0.53 to 0.96) | 0.03 | |
Subgroup analyses: chronic obstructive pulmonary disease exacerbation
Subgroup analyses were conducted to investigate whether or not the effects of vitamin D supplementation on the rate of study-defined COPD exacerbation differed according to baseline vitamin D status, COPD severity, inhaled corticosteroid requirement at baseline and body mass index. The results are presented in Table 8. Vitamin D supplementation reduced the rate of COPD exacerbations among individuals with baseline circulating 25(OH)D concentrations of < 25 nmol/l (aIRR 0.56, 95% CI 0.39 to 0.81; 81 participants in two studies; within subgroup, p = 0.002) but not in those with baseline 25(OH)D concentrations of ≥ 25 nmol/l (aIRR 1.05, 95% CI 0.89 to 1.23; 341 participants in two studies; within subgroup, p = 0.65).
| Subgroup | Number of trials | Number of individuals | Event rate per participant-year, group subgroup | aIRR (95% CI)a | p-value | p-value for interaction | |
|---|---|---|---|---|---|---|---|
| Control | Intervention | ||||||
| Overall | 2 | 422 | 374/189.75 (1.97) | 364/193.67 (1.88) | 0.95 (0.82 to 1.10) | 0.50 | |
| Baseline 25(OH)D (nmol/l) | |||||||
| < 25 | 2 | 81 | 76/35.87 (2.12) | 46/39.45 (1.16) | 0.56 (0.39 to 0.81) | 0.002 | 0.003 |
| ≥ 25 | 2 | 341 | 298/153.88 (1.94) | 318/154.23 (2.06) | 1.05 (0.89 to 1.23) | 0.56 | |
| COPD severity | |||||||
| GOLD stage 1/2 | 2 | 223 | 123/100.82 (1.22) | 130/104.25 (1.25) | 1.03 (0.81 to 1.32) | 0.79 | 0.45 |
| GOLD stage 3/4 | 2 | 199 | 251/88.93 (2.82) | 234/89.42 (2.62) | 0.92 (0.77 to 1.10) | 0.37 | |
| Concomitant inhaled corticosteroid at baseline | |||||||
| No | 2 | 112 | 55/45.31 (1.21) | 55/53.53 (1.03) | 0.91 (0.62 to 1.32) | 0.61 | 0.70 |
| Yes | 2 | 310 | 319/144.44 (2.21) | 309/140.14 (2.20) | 0.97 (0.83 to 1.14) | 0.72 | |
| Body mass index (kg/m2) | |||||||
| < 25 | 2 | 199 | 200/97.92 (2.04) | 172/82.07 (2.10) | 0.99 (0.81 to 1.21) | 0.91 | 0.70 |
| ≥ 25 | 2 | 223 | 174/91.83 (1.89) | 192/111.60 (1.72) | 0.92 (0.75 to 1.13) | 0.42 | |
The treatment–covariate interaction term (ratio of aIRRs) for this subgroup analysis was 1.85 (95% CI 1.24 to 2.75; for interaction, p = 0.003). The p-values for interaction for other subgroup analyses were > 0.05.
Secondary outcomes: efficacy
Results of one-step IPD meta-analysis of secondary outcomes are presented in Table 9.
| Outcome | Number of trials | Proportion with ≥ 1 event subgroup (%) | aOR (95% CI)a | p-value | |
|---|---|---|---|---|---|
| Control | Intervention | ||||
| URI | 19 | 1656/3286 (50.4) | 1807/3733 (48.4) | 0.93 (0.83 to 1.03) | 0.15 |
| LRI | 9 | 542/3285 (16.5) | 561/3413 (16.4) | 0.96 (0.83 to 1.10) | 0.52 |
| Hospitalisation or emergency department attendance as a result of ARI | 11 | 47/3886 (1.2) | 40/3986 (1.0) | 0.83 (0.54 to 1.27) | 0.39 |
| Use of antimicrobials for treatment of ARI | 9 | 397/983 (40.4) | 413/1121 (36.8) | 0.84 (0.69 to 1.03) | 0.10 |
| Work/school absence as a result of ARI | 7 | 321/632 (50.8) | 319/684 (46.6) | 0.87 (0.69 to 1.09) | 0.22 |
| Serious adverse event of any cause | 25 | 216/5371 (4.0) | 221/5853 (3.8) | 0.98 (0.80 to 1.20) | 0.83 |
| Death as a result of ARI/respiratory failure | 25 | 7/5330 (0.1) | 6/5802 (0.1) | 0.70 (0.23 to 2.20) | 0.55 |
| Death as a result of any infection | 25 | 15/5338 (0.3) | 16/5812 (0.3) | 0.95 (0.46 to 1.99) | 0.90 |
| Death as a result of any cause | 25 | 48/5371 (0.9) | 56/5853 (1.0) | 1.39 (0.85 to 2.27) | 0.18 |
| Hypercalcaemia | 14 | 9/1739 (0.5) | 12/2111 (0.6) | –b | –b |
| Renal stones | 14 | 4/1707 (0.2) | 2/2134 (0.1) | –b | –b |
When all studies were analysed together, no statistically significant effect of vitamin D was seen on the proportion of participants with at least one URI, LRI, hospitalisation or emergency department attendance for ARI, use of a course of antimicrobials for ARI, work/school absence as a result of ARI, severe asthma exacerbation or severe COPD exacerbation. When this analysis was stratified by dosing frequency in an exploratory analysis, a borderline significant protective effect of daily or weekly vitamin D supplementation against URI was seen (aOR 0.88, 95% CI 0.78 to 1.00; 4483 participants in 11 studies, p = 0.05; Table 10).
| Outcome | Bolus dosing | Daily or weekly dosing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of trials | Proportion with ≥ 1 event subgroup (%) | aOR (95% CI)a | p-value | Number of trials | Proportion with ≥ 1 event subgroup (%) | aOR (95% CI)a | p-value | |||
| Control | Intervention | Control | Intervention | |||||||
| URI | 8 | 606/1052 (57.6) | 730/1284 (56.9) | 1.03 (0.86 to 1.24) | 0.72 | 11 | 1050/2234 (47.0) | 1077/2449 (44.0) | 0.88 (0.78 to 1.00) | 0.05 |
| LRI | 4 | 424/1889 (22.4) | 427/1922 (22.2) | 0.96 (0.82 to 1.13) | 0.60 | 5 | 118/1396 (8.5) | 134/1491 (9.0) | 0.98 (0.75 to 1.28) | 0.88 |
| Use of antimicrobials for treatment of ARI | 4 | 201/348 (57.8) | 203/367 (55.3) | 0.79 (0.56 to 1.10) | 0.16 | 5 | 196/635 (30.9) | 210/754 (27.9) | 0.87 (0.67 to 1.13) | 0.31 |
| Work/school absence as a result of ARI | 4 | 219/409 (53.5) | 196/411 (47.7) | 0.78 (0.59 to 1.04) | 0.10 | 3 | 102/223 (45.7) | 123/273 (45.1) | 1.03 (0.71 to 1.48) | 0.88 |
| Severe asthma exacerbation | 3 | 73/343 (21.3) | 57/337 (16.9) | 0.72 (0.49 to 1.07) | 0.11 | 4 | 8/140 (5.7) | 6/146 (4.1) | 0.73 (0.19 to 2.85) | 0.65 |
| Severe COPD exacerbation | 2 | 140/207 (67.6) | 133/213 (62.4) | 0.77 (0.50 to 1.20) | 0.25 | 0 | – | – | – | – |
| Serious adverse event of any cause | 10 | 107/2822 (3.8) | 115/3070 (3.8) | 1.00 (0.74 to 1.35) | 0.99 | 15 | 109/2549 (4.3) | 106/2783 (3.8) | 0.97 (0.73 to 1.30) | 0.86 |
| Death as a result of any cause | 10 | 29/2822 (1.0) | 35/3070 (1.1) | 1.29 (0.71 to 2.35) | 0.40 | 15 | 19/2549 (0.7) | 21/2783 (0.8) | –b | –b |
| Death as a result of ARI/respiratory failure | 10 | 4/2797 (0.1) | 3/3038 (0.1) | 0.61 (0.12 to 3.02) | 0.54 | 15 | 3/2533 (0.1) | 3/2765 (0.1) | –b | –b |
| Death as a result of any infection | 10 | 8/2801 (0.3) | 5/3040 (0.2) | 0.55 (0.17 to 1.80) | 0.32 | 15 | 7/2537 (0.3) | 11/2773 (0.4) | –b | –b |
| Hospitalisation or emergency department attendance as a result of ARI | 6 | 4/2081 (0.2) | 6/2124 (0.3) | –b | –b | 5 | 43/1805 (2.4) | 34/1862 (1.8) | –b | –b |
| Hypercalcaemia | 8 | 8/1062 (0.8) | 11/1303 (0.8) | –b | –b | 6 | 1/677 (0.1) | 1/808 (0.1) | –b | –b |
| Renal stones | 6 | 0/764 (0.0) | 1/1011 (0.1) | –b | –b | 8 | 4/943 (0.4) | 1/1123 (0.1) | –b | –b |
Secondary outcomes: safety
Administration of vitamin D did not influence the risk of serious adverse events of any cause (aOR 0.98, 95% CI 0.80 to 1.20) or death attributable to any cause (aOR 1.39, 95% CI 0.85 to 2.27) (see Table 9). Instances of potential adverse reactions to vitamin D were rare. Hypercalcaemia was detected in 21 out of 3850 (0.5%) and renal stones were diagnosed in 6 out of 3841 (0.2%); both events were equally represented in the intervention and control arms (see Table 9). Stratification of this analysis by dosing frequency did not reveal any statistically significant increase in the risk of adverse events with either bolus or daily or weekly supplementation (see Table 10).
Risk of bias across studies
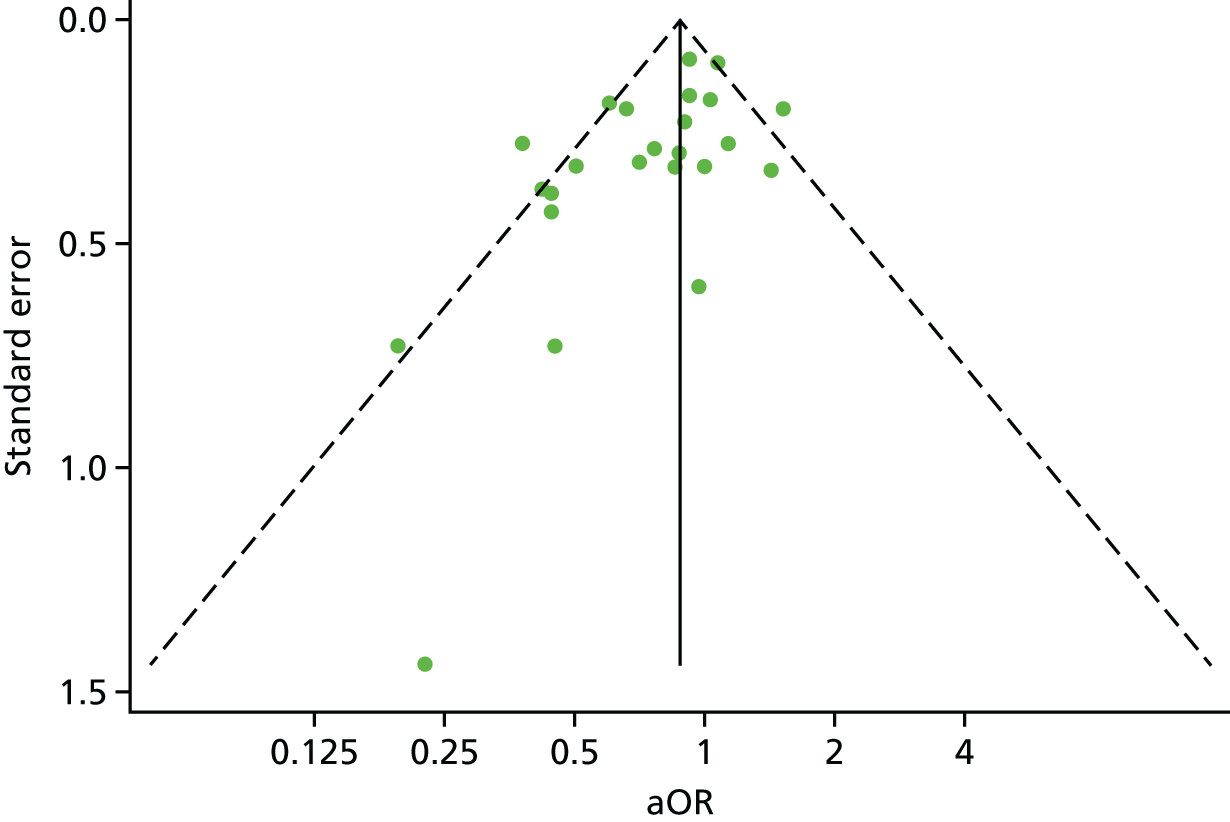
A funnel plot for the proportion of participants experiencing at least one ARI showed a degree of asymmetry, raising the possibility that small trials showing adverse effects of vitamin D may not have been included in the meta-analysis (Figure 9).
FIGURE 9.
Funnel plot with pseudo 95% confidence limits for IPD meta-analysis of proportion of participants experiencing at least one ARI.
Responder analyses
The results of responder analyses for the outcome of the proportion of participants with at least one ARI are presented in Table 11. Among participants randomised to the intervention arm of included studies for whom end-study 25(OH)D concentration data were available, no difference in risk of ARI was observed between those who attained a serum 25(OH)D concentration of ≥ 75 nmol/l and those who did not.
| 25(OH)D status | Number of trials | Impact on ARI | Ratio | p-value |
|---|---|---|---|---|
| Proportion with ≥ 1 ARI (%) | aOR (95% CI)a | |||
| Intervention, end-study 25(OH)D level of < 75 nmol/l | 18 | 542/1120 (48.4) | 1 | – |
| Intervention, end-study 25(OH)D level of ≥ 75 nmol/l | 18 | 784/1291 (60.7) | 0.96 (0.78 to 1.18) | 0.68 |
| Median time (days) to first ARI (IQR) | aHR (95% CI) | |||
| Intervention, end-study 25(OH)D level of < 75 nmol/l | 11 | 190 (63, –)b | 1 | – |
| Intervention, end-study 25(OH)D level of ≥ 75 nmol/l | 12 | 102 (39–312) | 1.02 (0.88 to 1.19) | 0.76 |
| Rate of ARI per participant-year | aIRR (95% CI) | |||
| Intervention, end-study 25(OH)D level of < 75 nmol/l | 18 | 1.51 | 1 | – |
| Intervention, end-study 25(OH)D level of ≥ 75 nmol/l | 18 | 2.04 | 1.01 (0.94 to 1.10) | 0.72 |
Sensitivity analyses
A meta-analysis using IPD of the proportion of participants experiencing at least one ARI, excluding two trials assessed as being at an unclear risk of bias,55,64 revealed protective effects of vitamin D supplementation consistent with the main analysis (aOR 0.82, 95% CI 0.70 to 0.95; 10,744 participants; p = 0.01). Sensitivity analyses for the same outcome, restricted to the 14 trials that investigated ARI as a primary or coprimary outcome, also revealed protective effects of vitamin D supplementation consistent with the main analysis (aOR 0.82, 95% CI 0.68 to 1.00; 5739 participants; p = 0.05).
Chapter 5 Discussion
Principal findings
We report the results of the first IPD meta-analysis of RCTs of vitamin D to prevent ARI. In the study population as a whole, vitamin D supplementation reduced the risk of experiencing at least one ARI. Subgroup analysis revealed that daily or weekly vitamin D supplementation without additional bolus doses protected against ARI, while regimens containing large bolus doses did not. Among those receiving daily or weekly vitamin D, protective effects of vitamin D were strongest in individuals with profound vitamin D deficiency at baseline, although those with higher baseline 25(OH)D concentrations also experienced benefit. This evidence was assessed as being of high quality, using the GRADE criteria. 52 As baseline vitamin D status and use of bolus doses varied significantly between studies, our results suggest that the high degree of heterogeneity between trials may be at least partly attributable to these factors. Administration of vitamin D was safe: potential adverse reactions were very rare, and the risk of such events was the same among both participants randomised to intervention arms and those randomised to control arms.
Why might administration of a bolus dose of vitamin D be ineffective for prevention of ARI? One explanation relates to potentially adverse effects of wide fluctuations in circulating 25(OH)D concentrations, which are seen following administration of bolus doses but not with daily or weekly supplementation. Vieth70 has proposed that high circulating 25(OH)D concentrations following bolus dosing may chronically dysregulate activity of the enzymes responsible for synthesis and degradation of the active vitamin D metabolite 1,25-dihydroxyvitamin D, resulting in decreased concentrations of this metabolite in extrarenal tissues. Such an effect could attenuate the ability of 25(OH)D to support protective immune responses to respiratory pathogens. Increased efficacy of vitamin D supplementation in individuals with lower baseline vitamin D status is more readily explicable, based on the principle that individuals who are the most deficient in a micronutrient will be the most likely to respond to its replacement.
One question raised by our results relates to whether or not vitamin D supplementation will be more beneficial in reducing ARI risk for individuals or for the population as a whole. A targeted approach aimed at individuals would involve testing baseline vitamin D status and offering supplements to those who are deficient. This approach would be likely to result in relatively good adherence (motivation to take a supplement will be higher if an individual knows that they are deficient), but it will be costly and it may not reach a large proportion of the people who stand to benefit. Making additional vitamin D available to the population as a whole in an untargeted fashion (e.g. via food fortification) has some inefficiencies, in that some vitamin D-replete individuals will receive extra vitamin D unnecessarily. However, the strategy also has potential advantages, in that it would provide superior coverage to a ‘test-and-treat’ approach. The relative merits of the two strategies need to be formally evaluated with a health economic analysis, as suggested in Future research.
Our study also investigated the effects of vitamin D supplementation on the risk of acute exacerbations of asthma and COPD. Vitamin D supplementation reduced the rate of asthma exacerbation requiring treatment with systemic corticosteroids overall; non-statistically significant trends towards protection were also seen when the outcome of asthma exacerbation was analysed as the proportion of participants with at least one event and the time to first event. Subgroup analyses for this outcome revealed a trend towards greater protection in participants with a baseline 25(OH)D concentration level of < 25 nmol/l than in those with a higher baseline 25(OH)D concentration level; however, the p-value for interaction for this subgroup analysis was 0.25, indicating that we found no statistically significant evidence to implicate baseline vitamin D status as an effect modifier. By contrast, vitamin D supplementation had no statistically significant effect on the risk of COPD exacerbation requiring treatment with antibiotics and/or systemic corticosteroids overall. Subgroup analyses for this outcome revealed a strong protective effect in individuals with baseline 25(OH)D concentrations of < 25 nmol/l, but no protective effect among individuals with higher baseline 25(OH)D concentration levels. The p-value for interaction for this subgroup analysis was 0.003, indicating that baseline vitamin D status modifies the effects of vitamin D supplementation on the risk of COPD exacerbation.
Strengths and limitations
Our study has several strengths. We obtained IPD for all 25 trials identified by our search, the proportion of randomised participants with missing outcome data was small (3.4%), participants with diverse characteristics in multiple settings were represented and 25(OH)D concentration levels were measured using validated assays in laboratories that participated in external quality-assessment schemes. Our findings therefore have a high degree of internal and external validity. Moreover, the subgroup effects we report fulfil published ‘credibility criteria’ relating to study design, analysis and context. 71 Specifically, the relevant effect modifiers were specified a priori and measured at baseline, p-values for interaction remained significant after adjustment for potential confounders and subgroup effects were consistent when analysed as proportions and event rates. Survival analysis revealed consistent trends that did not attain statistical significance, possibly owing to lack of power (fewer studies contributed data to survival analyses than to analyses of proportions and event rates). As discussed above, the concepts that vitamin D supplementation may be (1) more effective when given to those with lower baseline 25(OH)D concentration levels and (2) less effective when bolus doses are administered are also biologically plausible. Although the results are consistent with the hypothesis that baseline vitamin D status and dosing regimen independently modify effects of vitamin D supplementation, we cannot exclude the possible influence of other effect modifiers linked to these two factors. The risk of residual confounding by other effect modifiers is increased for analyses in which relatively few trials are represented within a subgroup, for example when subgroup analyses were stratified by dosing regimen. We therefore suggest caution when interpreting the results in Tables 6 and 10.
Our study has some limitations. One explanation for the degree of asymmetry seen in the funnel plot (see Figure 9) is that some small trials showing adverse effects of vitamin D may have escaped our attention. With regard to the potential for missing data, we made strenuous efforts to identify published and (at the time) unpublished data, as illustrated by the fact that our meta-analysis includes data from 25 studies, which is 10 more than the largest aggregate data meta-analysis in the field. 24 However, if one or two small trials showing large adverse effects of vitamin D were to emerge, we do not anticipate that they would greatly alter the results of the one-step IPD meta-analysis, as any negative signal from a modest number of additional participants would probably be diluted by the robust protective signal generated from analysis of data from nearly 11,000 participants. A second limitation is that our power to detect effects of vitamin D supplementation was limited for some subgroups [e.g. individuals with baseline 25(OH)D concentration of < 25 nmol/l on bolus dosing regimens] and for some secondary outcomes (e.g. incidence of LRI). Null and borderline significant results for analyses of these outcomes may have arisen as a consequence of type II error. Additional RCTs investigating the effects of vitamin D on the risk of ARI and exacerbation of asthma and COPD are ongoing, and inclusion of data from these studies in future meta-analyses has the potential to increase statistical power to test for subgroup effects. However, all three of the largest such studies for ARI prevention (NCT01169259, ACTRN12611000402943 and ACTRN12613000743763) are being conducted in populations in which profound vitamin D deficiency is rare, and two are using intermittent bolus dosing regimens (ACTRN12611000402943 and ACTRN12613000743763): their results are therefore unlikely to alter our finding of benefit in very deficient individuals, or in those receiving daily or weekly regimens. A third potential limitation relates to the fact that data relating to adherence to study medication were not available for all subjects. However, inclusion of non-adherent participants would bias the results of our intention-to-treat analysis towards the null: thus, we conclude that effects of vitamin D in those who are fully adherent to supplementation will be no less than those reported for the study population overall. Our definition of ARI was wide, incorporating both URI and LRI and, consequently, our overall findings cannot necessarily be generalised to specific ARIs (e.g. those confined to a specific anatomical site or caused by a single pathogen). Finally, we caution that virological, microbiological and/or radiological confirmation was obtained for a minority of ARI events. ARI is often a clinical diagnosis in practice, however, and, as all studies were double-blind and placebo-controlled, differences in incidence of events between study arms cannot be attributed to observation bias.
Future research
Incorporation of additional IPD from ongoing trials in the field has the potential to increase the statistical power of subgroup analyses; this IPD meta-analysis should therefore be updated when a significant new body of data has accumulated. Given the major impact of ARIs on economic productivity and health-care use, our findings are likely to influence the economic case for the introduction of vitamin D fortification of foods in the UK. Economic models of the cost-effectiveness of vitamin D fortification in the UK should therefore be updated to take account of the previously unappreciated protective effects of vitamin D against ARIs. Such models will need to be populated with accurate data regarding vitamin D intake and the prevalence of vitamin D deficiency in the general population and in subpopulations deemed to be at particular risk of vitamin D deficiency, intake of vitamin D-fortifiable foods and drinks in these populations, attitudes towards different fortification strategies (mandatory vs. voluntary) and the costs of adverse health outcomes such as fractures, falls and ARIs that could be reduced by realising improvements in population vitamin D status. Such modelling would also need to take into account differential profiles of URI compared with LRI in terms of frequency, severity and heath-care costs.
Conclusions
Our synthesis of the current evidence suggests that vitamin D supplementation can prevent ARI, broadly defined. We identified that the greatest potential benefit is for those individuals who are very deficient in vitamin D. Those receiving daily or weekly supplementation without additional bolus doses also experienced particular benefit. Our results add to the body of evidence supporting the introduction of public health measures, such as food fortification, to improve vitamin D status in settings in which profound vitamin D deficiency is common.
Acknowledgements
We thank all the people who participated in the primary RCTs, the teams who performed them and our PPI representatives, Charanjit Patel and Jane Gallagher, for comments on study design and drafts of this manuscript.
Contributions of authors
Adrian R Martineau (Professor of Respiratory Infection and Immunity) led the funding application, assessed the eligibility of studies for inclusion, was directly involved in the acquisition of data for the work, designed statistical analyses in consultation with authors contributing IPD and wrote the first draft of the report.
David A Jolliffe (Postdoctoral Research Fellow) assessed the eligibility of studies for inclusion, designed statistical analyses in consultation with authors contributing IPD and undertook statistical analyses.
Lauren Greenberg (Medical Statistician) designed statistical analyses in consultation with authors contributing IPD and undertook statistical analyses.
John F Aloia (Professor of Medicine), Peter Bergman (Associate Professor), Gal Dubnov-Raz (Consultant Paediatrician), Susanna Esposito (Professor of Paediatrics), Davaasambuu Ganmaa (Assistant Professor), Adit A Ginde (Professor of Emergency Medicine), Emma C Goodall (Assistant Professor), Cameron C Grant (Associate Professor), Wim Janssens (Professor of Pneumonology), Megan E Jensen (Postdoctoral Clinical Researcher), Conor P Kerley (Postdoctoral Clinical Researcher), Ilkka Laaksi (Chief Administrative Medical Officer), Semira Manaseki-Holland (Senior Clinical Lecturer), David Mauger (Professor of Public Health Sciences and Statistics), David R Murdoch (Professor of Pathology), Rachel Neale (Associate Professor), Judy R Rees (Assistant Professor), Steve Simpson Jr (Postdoctoral Research Fellow), Iwona Stelmach (Professor of Paediatric Allergy), Geeta Trilok Kumar (Associate Professor) and Mitsuyoshi Urashima (Professor of Molecular Epidemiology) were all directly involved in the acquisition of data for the work.
Carlos A Camargo Jr (Professor of Emergency Medicine, Medicine and Epidemiology) was a co-applicant in the funding application, assessed the eligibility of studies for inclusion and was directly involved in the acquisition of data for the work.
Christopher J Griffiths (Professor of Primary Care) was a co-applicant in the funding application and was directly involved in the acquisition of data for the work.
Richard L Hooper (Reader in Medical Statistics) assisted in the funding application, designed statistical analyses in consultation with authors contributing IPD and undertook statistical analyses.
All authors critically revised the manuscript for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Publications
Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017;5:881–90.
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583.
Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials [published online ahead of print January 11 2019]. Thorax 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009;302:758-66. https://doi.org/10.1001/jama.2009.1163.
- Global Burden of Disease (GBD) Mortality and Causes of Death Collaborators Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. https://doi.org/10.1016/S0140-6736(14)61682-2.
- Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc 2005;2:150-6. https://doi.org/10.1513/pats.200502-018AW.
- The Global Asthma Report 2014. Auckland: Global Asthma Network; 2014.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. https://doi.org/10.1016/S0140-6736(07)61380-4.
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect 2006;134:1129-40. https://doi.org/10.1017/S0950268806007175.
- Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 2013;136:321-9. https://doi.org/10.1016/j.jsbmb.2012.11.017.
- Brehm JM, Acosta-Pérez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2012;186:140-6. https://doi.org/10.1164/rccm.201203-0431OC.
- Salas NM, Luo L, Harkins MS. Vitamin D deficiency and adult asthma exacerbations. J Asthma 2014;51:950-5. https://doi.org/10.3109/02770903.2014.930883.
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 2009;179:630-6. https://doi.org/10.1164/rccm.200810-1576PP.
- Kunisaki KM, Niewoehner DE, Connett JE. COPD Clinical Research Network . Vitamin D levels and risk of acute exacerbations of chronic obstructive pulmonary disease: a prospective cohort study. Am J Respir Crit Care Med 2012;185:286-90. https://doi.org/10.1164/rccm.201109-1644OC.
- Quint JK, Donaldson GC, Wassef N, Hurst JR, Thomas M, Wedzicha JA. 25-hydroxyvitamin D deficiency, exacerbation frequency and human rhinovirus exacerbations in chronic obstructive pulmonary disease. BMC Pulm Med 2012;12. https://doi.org/10.1186/1471-2466-12-28.
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181:7090-9. https://doi.org/10.4049/jimmunol.181.10.7090.
- Olliver M, Spelmink L, Hiew J, Meyer-Hoffert U, Henriques-Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J Infect Dis 2013;208:1474-81. https://doi.org/10.1093/infdis/jit355.
- Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015;7:4240-70. https://doi.org/10.3390/nu7064240.
- Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol 2011;7:337-45. https://doi.org/10.1038/nrendo.2010.226.
- Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 2006;116:146-55. https://doi.org/10.1172/JCI21759.
- Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol 2013;132:297-304.e3. https://doi.org/10.1016/j.jaci.2013.03.037.
- Greiller CL, Suri R, Jolliffe DA, Kebadze T, Hirsman AG, Griffiths CJ, et al. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells [published online ahead of print November 23 2018]. J Steroid Biochem Mol Biol 2018.
- Bergman P, Lindh AU, Björkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0065835.
- Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother 2012;3:300-3. https://doi.org/10.4103/0976-500X.103685.
- Mao S, Huang S. Vitamin D supplementation and risk of respiratory tract infections: a meta-analysis of randomized controlled trials. Scand J Infect Dis 2013;45:696-702. https://doi.org/10.3109/00365548.2013.803293.
- Xiao L, Xing C, Yang Z, Xu S, Wang M, Du H, et al. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. Br J Nutr 2015;114:1026-34. https://doi.org/10.1017/S000711451500207X.
- Vuichard Gysin D, Dao D, Gysin CM, Lytvyn L, Loeb M. Effect of vitamin D3 supplementation on respiratory tract infections in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0162996.
- Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0136841.
- Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD011511.pub2.
- Luo J, Liu D, Liu CT. Can vitamin D supplementation in addition to asthma controllers improve clinical outcomes in patients with asthma? A meta-analysis. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000002185.
- Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012;156:105-14. https://doi.org/10.7326/0003-4819-156-2-201201170-00004.
- Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2015;3:120-30. https://doi.org/10.1016/S2213-2600(14)70255-3.
- Zendedel A, Gholami M, Anbari K, Ghanadi K, Bachari EC, Azargon A. Effects of vitamin D intake on FEV1 and COPD exacerbation: a randomized clinical trial study. Glob J Health Sci 2015;7:243-8. https://doi.org/10.5539/gjhs.v7n4p243.
- Martineau AR. Bolus-dose vitamin D and prevention of childhood pneumonia. Lancet 2012;379:1373-5. https://doi.org/10.1016/S0140-6736(12)60405-X.
- Steenhoff AP, Schall JI, Samuel J, Seme B, Marape M, Ratshaa B, et al. Vitamin D3 supplementation in Batswana children and adults with HIV: a pilot double blind randomized controlled trial. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0117123.
- Waterhouse M, Tran B, Armstrong BK, Baxter C, Ebeling PR, English DR, et al. Environmental, personal, and genetic determinants of response to vitamin D supplementation in older adults. J Clin Endocrinol Metab 2014;99:E1332-40. https://doi.org/10.1210/jc.2013-4101.
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815-22. https://doi.org/10.1001/jama.2010.594.
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340. https://doi.org/10.1136/bmj.c221.
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356. https://doi.org/10.1136/bmj.i6583.
- Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med 2017;5:881-90. https://doi.org/10.1016/S2213-2600(17)30306-5.
- Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials [published online ahead of print January 11 2019]. Thorax 2019.
- Camargo CA, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012;130:e561-7. https://doi.org/10.1542/peds.2011-3029.
- Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 2012;308:1333-9. https://doi.org/10.1001/jama.2012.12505.
- Rees JR, Hendricks K, Barry EL, Peacock JL, Mott LA, Sandler RS, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis 2013;57:1384-92. https://doi.org/10.1093/cid/cit549.
- Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy 2016;71:1001-9. https://doi.org/10.1111/all.12856.
- Tran B, Armstrong BK, Ebeling PR, English DR, Kimlin MG, van der Pols JC, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr 2014;99:156-61. https://doi.org/10.3945/ajcn.113.063271.
- Urashima M, Mezawa H, Noya M, Camargo CA. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: a randomized controlled trial. Food Funct 2014;5:2365-70. https://doi.org/10.1039/c4fo00371c.
- Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010;91:1255-60. https://doi.org/10.3945/ajcn.2009.29094.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015;70:451-7. https://doi.org/10.1136/thoraxjnl-2014-206449.
- Martineau AR, Hanifa Y, Witt KD, Barnes NC, Hooper RL, Patel M, et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax 2015;70:953-60. https://doi.org/10.1136/thoraxjnl-2015-206996.
- Department of Health Report on Health and Social Subjects, No. 49. Nutrition and Bone Health with Particular Reference to Calcium and Vitamin D. London: HMSO; 1998.
- Reid IR. Towards a trial-based definition of vitamin D deficiency. Lancet Diabetes Endocrinol 2016;4:376-7. https://doi.org/10.1016/S2213-8587(16)00079-6.
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991-6. https://doi.org/10.1016/j.jclinepi.2007.11.010.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect 2009;137:1396-404. https://doi.org/10.1017/S0950268809002404.
- Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 2010;15:1148-55. https://doi.org/10.1111/j.1365-3156.2010.02578.x.
- Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamäki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis 2010;202:809-14. https://doi.org/10.1086/654881.
- Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol 2011;127:1294-6. https://doi.org/10.1016/j.jaci.2010.12.016.
- Trilok Kumar G, Sachdev HS, Chellani H, Rehman AM, Singh V, Arora H, et al. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ 2011;342. https://doi.org/10.1136/bmj.d2975.
- Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012;379:1419-27. https://doi.org/10.1016/S0140-6736(11)61650-4.
- Bergman P, Norlin AC, Hansen S, Rekha RS, Agerberth B, Björkhem-Bergman L, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001663.
- Marchisio P, Consonni D, Baggi E, Zampiero A, Bianchini S, Terranova L, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J 2013;32:1055-60. https://doi.org/10.1097/INF.0b013e31829be0b0.
- Goodall EC, Granados AC, Luinstra K, Pullenayegum E, Coleman BL, Loeb M, et al. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: a randomized controlled trial. BMC Infect Dis 2014;14. https://doi.org/10.1186/1471-2334-14-273.
- Grant CC, Kaur S, Waymouth E, Mitchell EA, Scragg R, Ekeroma A, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial. Acta Paediatr 2015;104:396-404. https://doi.org/10.1111/apa.12819.
- Simpson SJ, van der Mei I, Stewart N, Blizzard L, Tettey P, Taylor B. Weekly cholecalciferol supplementation results in significant reductions in infection risk among the vitamin D deficient: results from the CIPRIS pilot RCT. BMC Nutrition 2015;1:1-10.
- Dubnov-Raz G, Rinat B, Hemilä H, Choleva L, Cohen AH, Constantini NW. Vitamin D supplementation and upper respiratory tract infections in adolescent swimmers: a randomized controlled trial. Pediatr Exerc Sci 2015;27:113-19. https://doi.org/10.1123/pes.2014-0030.
- Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 2014;311:2083-91. https://doi.org/10.1001/jama.2014.5052.
- Denlinger LC, King TS, Cardet JC, Craig T, Holguin F, Jackson DJ, et al. Vitamin D supplementation and the risk of colds in patients with asthma. Am J Respir Crit Care Med 2016;193:634-41. https://doi.org/10.1164/rccm.201506-1169OC.
- Kerley CP, Hutchinson K, Cormican L, Faul J, Greally P, Coghlan D, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol 2016;27:404-12. https://doi.org/10.1111/pai.12547.
- Jensen ME, Mailhot G, Alos N, Rousseau E, White JH, Khamessan A, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1483-1.
- Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, et al. High dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc 2017;65:496-503. https://doi.org/10.1111/jgs.14679.
- Vieth R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res 2009;29:3675-84.
- Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340. https://doi.org/10.1136/bmj.c117.
Appendix 1 Electronic search strategy
The full electronic search strategy for MEDLINE is presented.
Cochrane highly sensitive search strategy for identifying randomised controlled trials
#1. randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]
#2. animals [mh] NOT humans [mh]
#3. #1 NOT #2
Terms specific to vitamin D
#4. Vitamin D OR vitamin D2 OR vitamin D3 OR cholecalciferol OR ergocalciferol OR alphacalcidol OR alfacalcidol OR calcitriol OR paricalcitol OR doxerocalciferol
Terms specific to acute respiratory infection
#5. Acute Respiratory Infection OR Upper Respiratory Infection OR Lower Respiratory Infection OR Respiratory Tract Infection OR Common Cold OR Sinusitis OR Pharyngitis OR Laryngitis OR Laryngotracheobronchitis OR Tonsillitis OR peritonsillar abscess OR Croup OR Epiglottitis OR supraglottitis OR Otitis Media OR Pneumonia OR Bronchopneumonia OR Bronchitis OR Pleurisy OR Pleuritis
Terms specific to asthma
#6 Asthma OR bronchial hyperreactivity OR bronchial hyper-reactivity OR respiratory hypersensitivity OR reactive airway
Terms specific to chronic obstructive pulmonary disease
#7 Chronic obstructive pulmonary disease OR COPD OR COAD OR emphysema OR chronic bronchitis OR AECB OR AECOPD
Combination of terms to identify randomised controlled trials of vitamin D for the prevention of acute respiratory infection
#3 AND #4 AND #5
Combination of terms to identify randomised controlled trials of vitamin D conducted in patients with asthma
#3 AND #4 AND #6
Combination of terms to identify randomised controlled trials of vitamin D conducted in patients with chronic obstructive pulmonary disease
#3 AND #4 AND #7
List of abbreviations
- 25(OH)D
- 25-hydroxyvitamin D
- aHR
- adjusted hazard ratio
- aIRR
- adjusted incidence rate ratio
- aOR
- adjusted odds ratio
- ARI
- acute respiratory infection
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- IL
- interleukin
- IPD
- individual participant data
- IU
- international unit
- LRI
- lower respiratory infection
- NNT
- number needed to treat
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- URI
- upper respiratory infection
