Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/101/02. The contractual start date was in December 2012. The final report began editorial review in August 2017 and was accepted for publication in February 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Anthony P Morrison reports personal fees from the provision of training workshops in cognitive–behavioural therapy (CBT) for psychosis and royalties from books on the topic, outside the submitted work. Andrew Gumley reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (grant number 13/15/04) outside the submitted work. Douglas Turkington reports personal fees from Insight–CBT partnership (Insight Healthcare, Newcastle upon Tyne), outside the submitted work. Gemma Shields reports grants from NIHR during the conduct of the study. Graeme MacLennan reports grants from the NIHR HTA programme during the conduct of the study. Hamish J MacLeod reports that he occasionally provides CBT for psychosis workshops and receives fees for this work. John Norrie reports personal fees from the NIHR Editors Board and grants from NIHR HTA General Board Deputy Chairperson, outside the submitted work, and has membership of the HTA Funding Boards Policy Group and Pre-Exposure Prophylaxis Impact Review Panel. Linda Davies reports grants from the NIHR HTA programme during the conduct of the study. Paul French has membership of the HTA prioritisation Panel. Paul Hutton reports that he sits on an Expert Steering Group for Professor Jill Stavert’s Centre for Mental Health and Incapacity Law Rights and Policy at Edinburgh Napier University, and that he is a member of a committee developing National Institute for Health and Care Excellence (NICE) guidelines on supporting decision-making for people who may lack mental capacity. Robert Dudley reports receiving a NIHR Comprehensive Local Research Network Greenshoots award to fund time to support his contribution to the FOCUS (Focusing on Clozapine Unresponsive Symptoms) trial, royalties from Guilford Press and personal fees from Trinity College Dublin, outside the submitted work. Samantha Bowe reports personal fees from Pennine Care NHS Foundation Trust and personal fees from Cheshire & Wirral Partnership NHS Foundation Trust, outside the submitted work. Thomas RE Barnes reports personal fees from Sunovion (Marlborough, MA, USA) and Otsuka (Tokyo, Japan)/Lundbeck (Copenhagen, Denmark), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Morrison et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Psychosis and schizophrenia
The term psychosis is used to refer to a mental health problem that can involve changes to a person’s perceptions and/or to their thoughts. A person who has experience of psychosis may report perceptual changes such as hearing a voice that another person cannot hear, or seeing something that another person cannot see. Perceptual changes may also occur to a person’s taste, smell and/or bodily sensations. Such perceptual changes are typically referred to as hallucinations. A person experiencing psychosis may also report beliefs that others around them consider unusual, that are out of keeping with their social or cultural background and that are lacking rational grounds (often referred to as delusions). Common delusional beliefs include feelings of being persecuted, ideas of reference and feelings of importance. 1 Persecutory beliefs are thought to be the most commonly occurring of the range of delusional beliefs. 2 Experiences of hallucinations and delusions can be distressing and confusing and can have a negative impact on functioning. In addition, a person with experience of psychosis may report changes in their ability to concentrate or may communicate in a manner that is hard for other people to understand, which is commonly referred to as thought disorder. In both clinical practice and research, delusions, hallucinations and thought disorder are typically referred to as positive symptoms of psychosis. In addition, people who experience psychosis may report flat affect (blunted affect), a decrease in verbal output and verbal expressiveness, loss of motivation (including social withdrawal) and loss of enjoyment. 3 These experiences are often referred to as negative symptoms. This term was originally proposed because these changes refer to the loss or absence of usually present functions or characteristics. 4–6 Estimates suggest that 15–20% of people who receive a schizophrenia diagnosis will experience persistent negative symptoms. 7,8
Psychosis is considered to exist on a continuum,9 from the occasional occurrence of psychotic phenomena in the general population9–12 to persistent and frequent psychotic symptoms resulting in distress and, in many cases, the need for care from a mental health service. Someone who experiences psychosis and presents to mental health services for care may receive a schizophrenia spectrum diagnosis. Experiences of psychosis are often categorised using a diagnostic classification system, namely the International Classification of Diseases, Tenth Revision (ICD-10),13 or the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). 14 Schizophrenia spectrum diagnoses include schizophrenia, schizoaffective disorder, delusional disorder and schizophreniform disorder. However, there has been considerable debate over the use of such diagnostic terms and classification systems. 15 One argument is that these classification systems suggest that psychosis difficulties are dichotomous rather than continuum based. 9 In addition, concerns have been raised regarding the reliability of the diagnostic classification systems used. 16 The diagnostic label of schizophrenia has become stigmatising, with intrinsic negative associations about prognosis17 leading some countries to drop the term schizophrenia. 18 The use of diagnostic terminology has, therefore, been contentious and Murray,19 in 2017, highlighted the burgeoning evidence that schizophrenia is not a dichotomous condition, but rather the severe end of a continuum. Murray19 expects to see the end of the concept of schizophrenia in the future. However, diagnosis and diagnostic terminology is commonly utilised throughout both clinical practice and research. For the purpose of this report, we wish to acknowledge this debate and, throughout, we will adopt respectful terminology. When reviewing the literature relevant to the Focusing on Clozapine Unresponsive Symptoms (FOCUS) trial, we use the terms psychosis and schizophrenia, depending on the sample of participants recruited to the studies being referred to. In relation to the sample of participants included in the FOCUS trial, we refer to the population as people who meet the criteria for a schizophrenia spectrum diagnosis or people who meet the criteria for clozapine-resistant schizophrenia (CRS).
Prevalence rates and the personal, social and economic impact of schizophrenia
A first episode of psychosis typically occurs in young adults; a review of the literature on the age at onset of mental health disorders by Kessler et al. 20 found that, for those with a schizophrenia diagnosis, the age at onset is usually between 15 and 35 years of age. Reports of the lifetime prevalence in the literature vary from 0.12 to 1.6 per 100 persons. 21 In the UK, a systematic review22 commissioned by the Department of Health and Social Care reported that the incidence rate for all clinically relevant psychosis diagnoses is 31.72 per 100,000 persons per year, and for schizophrenia is 15 per 100,000 persons per year; the incidence of the latter being the same as the international incidence rate for schizophrenia reported by McGrath et al. 23
Although the prevalence of schizophrenia is relatively low, the personal, social and economic costs are considerable and the management of schizophrenia is among one of the largest health challenges globally as well as for the UK NHS. For example, Kirkbride et al. 22 report that, in 2009, the estimated economic costs for services and society attributable to broadly defined schizophrenia amounted to £8.8B. The majority of the costs were attributable to lost employment (47%) and service costs (40%). Using disability-adjusted life-years lost as a measure of the overall number of years lost as a result of ill health, disability or early death, schizophrenia was ranked eighth among mental health diagnoses and brain disorders in Europe in 2010. 24
Considerable health inequalities are reported for people who have a schizophrenia diagnosis, with increased risk of serious diseases, such as diabetes mellitus, cardiovascular disease (CVD), a human immunodeficiency virus (HIV) infection and respiratory problems. 25 The prevalence of CVD among those with a schizophrenia diagnosis is estimated to be 75%, compared with an estimated 50% in the general population. 26 Of great concern is the mortality risk associated with schizophrenia. A recent systematic review and meta-analysis27 of the literature on potential life lost and life expectancy in schizophrenia found that, on average, people with a diagnosis of schizophrenia die 14.5 years earlier than those in the general population. The same authors27 report that the average life expectancy for people with a schizophrenia diagnosis is 64.7 years (for men and women combined); among men only, the average life expectancy is 8 years shorter than in the general population. The estimated risk of suicide in the general population is 1%, in comparison with 10% in those with a schizophrenia diagnosis. 26 In addition to the health inequalities outlined above, people with a schizophrenia diagnosis face widespread public stigma and discrimination, representing further inequalities and a major challenge to recovery. The incidence of anticipated, experienced and internalised stigma is high among people with psychosis. 28,29 A large-scale survey of people with schizophrenia diagnoses across 27 different countries found that nearly half of the respondents felt at a disadvantage as a result of having a diagnosis of schizophrenia, because of stigma. 29 Nearly half of the sample reported interpersonal/social difficulties with making or keeping friends and around one-quarter identified feeling at a disadvantage in terms of finding and keeping work and in relation to their personal safety. 29
Given the significant personal, societal and economic costs associated with schizophrenia, the treatment and care provided for people who meet the criteria for schizophrenia spectrum diagnoses should be a priority for policy-makers, commissioners and service providers.
Treatment options for people with a schizophrenia diagnosis
In this section of the report, we will consider the evidence base for treatments for people with a schizophrenia diagnosis considering both pharmacological and psychological interventions. We will begin the section with a consideration of the efficacy of antipsychotic medication, with a particular emphasis on treatment options for people with a schizophrenia diagnosis who have a poor response to antipsychotic medication. We will outline the psychological interventions recommended by the National Institute for Health and Care Excellence (NICE), with a specific emphasis on cognitive–behavioural therapy (CBT), given that this is the intervention evaluated by the FOCUS trial. We will consider the efficacy of CBT in conjunction with antipsychotic medication with a particular emphasis on efficacy for CRS.
Antipsychotic medication
The NICE guideline30 for psychosis and schizophrenia in adults recommends, for people with a first episode of psychosis, an acute exacerbation or recurrence of psychosis or schizophrenia, that oral antipsychotic medication be offered. The NICE guideline30 does not make a specific recommendation for the type of antipsychotic offered, that is first-generation antipsychotics (FGAs) or atypical/second-generation antipsychotics (SGAs). The range of antipsychotic medications in both of these classes differ in their psychopharmacological properties, efficacy and adverse effect profiles. 31 A recent systematic review and meta-analysis32 of placebo-controlled antipsychotic medication trials identified a total of 167 double-blind randomised controlled trials (RCTs) with a total sample of 28,102 people with a schizophrenia diagnosis. Meta-analysis of the data found a small to medium effect size (ES) for overall efficacy of 0.47, but this reduced to a small ES of 0.38 when looking at rigorous trials. Leucht et al. 32 reported that 23% of the participants who received antipsychotic medication had a ‘good’ response (defined as ≥ 50% change on the primary outcome), compared with 14% in the placebo group, with an absolute difference of 9%. Although the percentage is greater for those who receive antipsychotic medication, the authors32 conclude that this is still only a minority of participants who have a good response. With regard to the superiority of FGAs versus SGAs, a meta-analysis31 of studies comparing FGAs and SGAs found that four SGAs demonstrated superiority over FGAs in reducing overall symptoms for people with a schizophrenia diagnosis, with small to moderate ESs; specifically, these were amisulpride (ES = 0.31), clozapine (ES = 0.52), olanzapine (ES = 0.28) and risperidone (Risperdal, McGregor Cory Ltd) (ES = 0.13). However, direct comparisons of FGAs with SGAs in large, rigorously conducted, publicly funded RCTs33,34 have found no differences in efficacy, leading some to conclude that the class of ‘atypical’ antipsychotics has been fabricated for marketing purposes and has no basis in science or clinical practice. 35
Antipsychotic medication is the mainstay of treatment for people who meet the criteria for a schizophrenia diagnosis; however, it has been argued that evidence from meta-analyses demonstrates that the superiority of antipsychotic medication over placebo has been overestimated. 36 In addition, antipsychotics have a wide range of side effects, such as metabolic effects (including weight gain), cardiovascular effects, hyperprolactinaemia, antimuscarinic side effects (dry mouth, blurred vision and cognitive impairment), sexual dysfunction and movement disorders. 37 The adverse effects of antipsychotic medication are associated with increased stigma, physical morbidity and mortality, poor adherence and reduced quality of life. 37 A systematic review38 of the effects of antipsychotic drugs on brain volume concluded that some of the structural brain changes found in people with a schizophrenia diagnosis may be the result of antipsychotic medication. The headline result from the largest study34 to date to compare FGAs with SGAs was that 74% of participants discontinued their study medication before 18 months, which indicates issues of tolerability and adherence to antipsychotic medication.
Morrison et al. 36 argue that the adverse effects of antipsychotic medication have been underestimated, and authors of a recent review39 of antipsychotic medication as a treatment for people with a schizophrenia diagnosis concluded that ‘we still remain a long way from being able to recommend with precision, specific treatments for individual patients, in terms of the clinical response and lack of adverse events’ (Lally and MacCabe39).
A choice of treatments for people with experience of psychosis or with a schizophrenia diagnosis and an evidence base for these treatments is, therefore, imperative.
Treatment-resistant schizophrenia
It is common for the symptoms experienced by someone with a diagnosis of schizophrenia to become progressively more unresponsive to medication with subsequent relapses. 40 Findings from a recent systematic review conducted by Kennedy et al. 41 indicate a 60% failure rate to achieve a response to treatment with standard antipsychotic medication after 23 weeks. The persistence of symptoms after adequate treatment with antipsychotic medication is commonly referred to in the literature and clinical practice as ‘treatment-resistant’ schizophrenia (TRS); for comparison with the literature, we will utilise the terminology ‘people with experience of TRS’ throughout this report. Global estimates of the prevalence of TRS would suggest that 7.8 million people worldwide are experiencing TRS. 42 As outlined in Prevalence rates and the personal, social and economic impact of schizophrenia, people with a schizophrenia diagnosis frequently experience health inequalities; for the TRS group, these health inequalities are inflated. The mean quality of life score for those who meet TRS criteria is 20% lower than for people with a diagnosis of schizophrenia whose symptoms are considered to be in remission. 41 Moreover, in comparison with other mental health diagnoses that are considered to be ‘severe and enduring’, those with TRS have worse outcomes in terms of both symptoms, as measured by the Positive and Negative Syndrome Scale (PANSS), and social/functional outcomes, with TRS being a predictor for poor community functioning. 43 For the person experiencing TRS, the continued presence of symptoms represents a barrier to recovery and improvements in quality of life, and increased personal social/economic costs. 41 In sum, the importance of identifying effective treatments for this group cannot be overstated.
In defining criteria for TRS, many researchers and clinicians have referred to the following conceptualisation of TRS, outlined by Kane et al. :44 two periods of treatment with different antipsychotics at an adequate dose for ≥ 4 weeks without a ≥ 20% reduction in symptoms. Since the publication of the Kane et al. 44 study, there have been numerous investigations of treatments for people with TRS; however, these studies often use different definitions of TRS. A systematic review45 of the TRS literature highlights the fact that the definition of TRS has been inconsistent across numerous clinical trials for TRS, and it is argued that the lack of clarity regarding the definition of TRS is a limiting factor for translating the research findings into clinical practice. It also increases heterogeneity across the studies and, therefore, reduces the conclusions that can be drawn from meta-analyses. 45 Furthermore, the international guidelines utilised by clinicians working in the field use varying definitions of TRS (e.g. NICE guidelines30). In the UK, NICE does not specify a duration of treatment of an episode that it deems adequate, unlike the American Psychiatric Association, which specifies a duration of ≥ 6 weeks. In response, the Treatment Response and Resistance in Psychosis (TRRIP) working group was convened to develop criteria for treatment resistance in schizophrenia. This work represents an important development in research and practice in the field of TRS. Howes et al. 45 note that, of the 42 studies identified as eligible for their systematic review, only two, from the same research group, used identical TRS criteria. Based on the systematic review conducted by Howes et al. ,45 an online survey of the TRRIP group members (identifying agreements and disagreements) and meetings of the TRRIP group members, the following criteria for TRS have been recommended: (1) current symptoms of a minimum duration and severity determined by a standardised rating scale that includes both positive and negative symptoms, that is, the Brief Psychiatric Rating Scale (BPRS) or PANSS; (2) moderate or worse functional impairment; (3) prior treatment of at least two different antipsychotics, each for a minimum duration and dosage; (4) systematic monitoring of adherence and meeting of minimum adherence criteria; (5) at least one prospective treatment trial; and (6) criteria that clearly separate responsive from treatment-resistant patients. 45
The NICE guideline30 treatment recommendation for people diagnosed with TRS is to review diagnosis and adherence to medication, ensure that the medication prescription is at an adequate dose for the correct period of time, consider possible causes of non-response, such as substance misuse and physical health problems, and offer psychological intervention [CBT and family intervention (FI)]. For those who experience persistent symptoms of schizophrenia following adequate treatment with at least two different antipsychotic drugs (at least one of which should be a non-clozapine SGA), a trial of clozapine should be offered. 30 Clozapine is a SGA and it is currently considered to be the treatment of choice for people with TRS,46 as demonstrated by the NICE guideline recommendation. 30 However, clozapine has a number of adverse effects and, in comparison with FGAs, clozapine is associated with more frequent haematological problems, drowsiness, hypersalivation and temperature increases. 47 The most dangerous adverse effect associated with clozapine is agranulocytosis, which is a haematological disorder of the white blood cells that help fight infection. The unwanted side effects of clozapine can prevent the optimal dose of clozapine being reached or tolerated over time, and data from a cohort study in the UK showed that 45% of people discontinued treatment with clozapine within the first 2 years of the drug being initiated. 48 Results of the same study also suggest that tolerability and adverse drug reactions play a key role in patient-led decisions to discontinue clozapine: in just over half of cases, the reason given for discontinuation was adverse drug reactions, with sedation being the most frequently reported. 48
The use of clozapine as a treatment for TRS grew in popularity following the highly influential double-blind study of clozapine versus chlorpromazine conducted by Kane et al. 44 Since this study, there has been an increased number of RCTs comparing clozapine with FGAs, paving the way for subsequent meta-analyses of these trials. Essali et al. 47 conducted a systematic review and meta-analysis of trials comparing clozapine with FGAs, identifying a total of 42 eligible trials with 3950 participants. Although there was no difference in mortality or employment status between those on clozapine and those receiving FGAs, clozapine was superior in relation to clinical improvements, reduced relapse rates and greater reduction in BPRS scores. Although the meta-analysis conducted by Essali et al. 47 suggests superiority of clozapine over FGA, the authors note problems of heterogeneity with the data and the high risk of bias across the studies. 47
There has been some debate regarding the efficacy of clozapine in comparison with other antipsychotic medications. Two meta-analyses49,50 of clozapine versus treatment with standard antipsychotic medication were published in 2016. Both meta-analyses found that clozapine was no more effective in the long term for total psychotic symptoms than other antipsychotic medications. However, Siskind et al. 49 report that for positive symptoms clozapine is superior to other antipsychotic medications in both the short and the long term. Given the absence of superiority over other antipsychotic medications for total symptoms in the long term, Siskind et al. 49 recommend that, for patients who do not respond to clozapine by 6 months, other antipsychotic medications with lower side effect profiles should be considered. The network meta-analysis by Samara et al. ,50 which integrated all published and unpublished single- and double-blind RCTs of all antipsychotic medications for TRS, found that there was little evidence of efficacy of antipsychotic medications other than clozapine, haloperidol, olanzapine and risperidone. The authors50 concluded that there is, however, insufficient evidence to determine which of these medications are most effective for people with TRS, commenting that ‘The most surprising finding was that clozapine was not significantly more efficacious than most other drugs’ (Samara et al. 50) and arguing that there is a need for blinded studies of antipsychotic medication for TRS. 50 Howes et al. 45 note that the conclusions that can be drawn from the network meta-analysis by Samara et al. 50 may be limited by the heterogeneity across the studies included in the review. Clearly, it is challenging when two meta-analyses with similar research questions are published within the same year, making it difficult for clinicians, researchers and commissioners to interpret the data; however, clozapine remains the mainstay of treatment for TRS.
Clozapine-resistant schizophrenia
Around 30–40% of people who trial clozapine will experience a poor response to this medication. 51 Moreover, the range of adverse effects from clozapine means that the optimal dose may not always be reached or clozapine may not be tolerated long term. In both the research literature and clinical practice, a person who experiences a poor response to clozapine is typically said to have ‘clozapine-resistant schizophrenia’ (CRS) and, for this reason, we use that term in this report. CRS is defined as the persistence of symptoms after treatment with clozapine for ≥ 12 weeks at a stable dose of ≥ 400 mg per day, unless the dose was limited by side effects. 52
The most frequent approach to the treatment of CRS is to augment clozapine with another antipsychotic medication. 53,54 This is an approach taken frequently in clinical practice. 53,54 Clozapine has low antidopaminergic properties and, therefore, is often combined with an antipsychotic medication that has dopaminergic properties. 53 There is some evidence of small but significant benefits of clozapine augmentation with a second antipsychotic,54,55 but studies are scarce. 56 There is some indication that augmentation with risperidone may have adverse effects, as evidence from the Cochrane review57 comparing risperidone with placebo suggests that adding risperidone to clozapine treatment leads to reduced functioning. Not only are antipsychotic augmentation studies infrequent, but their results are highly heterogeneous, which limits the conclusions that can be drawn from meta-analyses. 54 Many of the studies to date are subject to detection bias, with concealment of allocation unclear in eight of the studies included in the meta-analysis of antipsychotic augmentation conducted by Taylor et al. 55 With this representing just over 50% of the studies included in the review, findings may be compromised by the risk of detection bias. Moreover, several of the studies included in the systematic review conducted by Porcelli et al. 54 were rated as being of low quality, with the mean quality assessment score across the 24 studies being 5.43 points (SD 1.88 points, range 3–8 points), with 0 points being the minimum score and 9 points being the maximum score.
A systematic review and meta-analysis conducted by Taylor et al. 55 identified 14 RCTs, with a total sample of 734 participants, that compared clozapine plus a second antipsychotic with clozapine plus placebo for ≥ 6 weeks’ duration. Augmentation with a second antipsychotic was found to have a small but significant effect over placebo, with an ES of 0.239. The long-term adverse effects of clozapine augmentation with a second antipsychotic are unclear from the Taylor et al. 55 review because 11 of the 14 trials followed up participants for only ≤ 10 weeks. Potential long-term adverse effects include hyperprolactinaemia and increased striatal dopamine blockade. 55 There is scarce evidence to answer the question of which combination strategy of clozapine and another antipsychotic medication is more effective. Sommer et al. 56 conducted a systematic review of 29 randomised, double-blind, placebo-controlled trials of clozapine augmentation with a second drug, including augmentation with drugs from a different class (i.e. not an antipsychotic). Of these, 10 trials evaluated the efficacy of augmentation with another antipsychotic and included amisulpride, aripiprazole, haloperidol, risperidone and sulpiride. Only clozapine augmented with sulpiride proved superior to placebo in reducing symptom severity, and this finding was from one small trial (with a sample size of n = 28).
The most recent review of clozapine augmentation with another antipsychotic medication was carried out by Barber et al. 58 The aim was to evaluate the efficacy and tolerability (in terms of side effects) of clozapine combined with various antipsychotic medications. The search yielded a limited number of studies, which were of low quality and high heterogeneity. In total, five studies met the inclusion criteria, yielding a total of 309 participants. Findings from the review demonstrated that there are a very limited number of studies that can indicate the superiority of one clozapine combination strategy over another, and the evidence that is available is of low quality. The current evidence does not allow a specific clozapine augmentation strategy to be recommended; individual pragmatic trials may be indicated, but, given the increased risk of adverse effects from polypharmacy, augmentation with a second antipsychotic should be discontinued if the benefits do not outweigh the risks.
An alternative strategy that has been evaluated is augmenting clozapine with another medication of a different class, namely benzodiazepines or antidepressants. In a review, Dold and Leucht53 argued that there is currently insufficient evidence for this approach, although they do recognise that targeted use of augmentation may be indicated in specific cases, such as the use of medication to target agitation.
In summary, the literature regarding the efficacy of treatments for TRS indicates that a significant proportion of people will experience CRS and continue to experience persistent difficulties. The evidence base for treatments for CRS is sparse59 and augmentation strategies with a second antipsychotic demonstrate small effects. 55
Psychological interventions
Psychological therapies for people with psychosis have been extensively evaluated in recent years. Clinical trials and subsequent meta-analyses have evaluated individual and group treatments (including CBT, supportive counselling, befriending, narrative therapies and psychodynamic approaches), FIs (individual or multifamily) and art therapies (including music therapies, dance therapy and art therapy). After thoroughly reviewing the evidence base, the NICE guideline30 currently recommends that all people with experience of psychosis or with a schizophrenia diagnosis should be offered CBT and FI, and for those who experience TRS or CRS it is recommended that the care team review the person’s engagement with and use of both of these psychological treatments. 30
Cognitive–behavioural therapy
The generic cognitive model60 has been applied to our understanding and treatment of schizophrenia. This cognitive model suggests that the way in which we interpret events has consequences for how we feel and behave, and that such interpretations are often maintained by unhelpful thinking biases and behavioural responses. Cognitive models of psychosis and psychotic experiences suggest that it is the way in which people interpret and respond to psychotic phenomena that accounts for distress and disability, rather than the psychotic experiences themselves. 61–63
Key elements of CBT include a shared, individualised formulation of the problem, which can include consideration of life events that may contribute to the development and/or maintenance of psychosis, such as trauma and deprivation; evaluating unhelpful thoughts; and conducting behavioural experiments. 64 Morrison et al. 64 place emphasis on the importance of CBT being conducted via a strong therapeutic relationship for people who experience psychosis and schizophrenia, the use of normalising information, collaboration between the client and the therapists, and therapy being based on a client’s problem list and idiosyncratic goals.
The importance of delivering CBT in an empowering and recovery-orientated manner has been highlighted in a 2016 article by Brabban et al. ,65 who suggest 10 key considerations to ensure that CBT is delivered ethically, in a manner that is recovery orientated and promotes therapeutic relationships: (1) collaboration, (2) use of everyday language, (3) acknowledging the historical and developmental context of the client’s difficulties, namely adverse life experiences, so as not to minimise the impact of these, (4) evaluating rather than challenging beliefs, (5) applying caution with use of the stress vulnerability model of psychosis and schizophrenia, (6) validating the client’s experience using a cognitive formulation, (7) delivering hope to the client, (8) offering informed choice about engaging with CBT, (9) ensuring that CBT training is extensive and specialist and (10) ensuring that there is access to continued supervision.
Cognitive–behavioural therapy has been shown to have small to moderate effects when delivered in combination with antipsychotic medication, with several meta-analyses showing support for this approach. 66–68 The most conservative ES estimate for total symptoms is 0.33, demonstrating small but significant effects of CBT for psychosis over treatment as usual (TAU); however, the ES for total symptoms reduces to 0.15 for studies with a low risk of bias from masking. 69 The same meta-analysis69 reports small ESs for positive symptoms (ES = 0.25) and negative symptoms (ES = 0.13). A 2014 meta-analysis conducted by Turner et al. ,70 which compared CBT for psychosis with other psychological therapies, found that, across the 48 included studies, CBT was more efficacious in improving overall and positive symptoms of psychosis than in improving other psychological therapies. van der Gaag et al. 71 note that there has been a focus on positive and negative symptoms in meta-analyses of CBT; however, CBT is a formulation-based approach that aims not necessarily to reduce the frequency or severity of positive and negative symptoms, but rather to help service users make sense of distressing hallucinatory experiences and delusional beliefs, with the aim of reducing distress and increasing coping. In a meta-analysis of treatment effects of individually tailored case-formulation CBT on auditory hallucinations and delusions, van der Gaag et al. 71 found modest and significant ESs for auditory hallucinations at the end of treatment (ES = 0.44), and this increased when contrasted with active treatment (ES = 0.49) and for blinded studies (ES = 0.46). Although modest significant ESs were found for delusions at the end of treatment (ES = 0.36), these ESs lost significance when (1) contrasted with active treatment and (2) the ES was reduced for blinded studies (ES = 0.24). Findings from the meta-analysis conducted by van der Gaag et al. 71 suggest that CBT can be effective in treating auditory hallucinations, but that the evidence for treating delusions is less robust.
Although meta-analyses suggest small to moderate ESs for CBT, in comparison with other psychological approaches, there remains debate in the literature about CBT’s value for psychosis and schizophrenia. 69,72 In particular, McKenna,72 in the 2014 Maudsley Debate, suggest that the meta-analysis carried out by NICE in 2009 for the schizophrenia guideline was methodologically flawed, leading to an increased chance of type I error and the probability that any positive findings were as a result of chance. In addition, Jauhar et al. 69 suggest that the conclusions regarding efficacy of CBT are mistaken, because the most large, well-conducted trials have failed to demonstrate a significant effect at the end of treatment, and the supportive meta-analyses overestimate the effects from smaller, low-quality trials. They also argue that their finding of ‘non-significant effects on positive symptoms in a relatively large set of 21 masked studies also suggests that claims that CBT is effective against these symptoms of the disorder are no longer tenable’. 69
Cognitive–behavioural therapy for treatment-resistant schizophrenia
In relation to TRS, the efficacy of CBT has been evaluated in a number of RCTs with some encouraging evidence; key details of these studies are presented below. Tarrier et al. 73 conducted one of the earliest trials of CBT for TRS using a three-arm RCT design in which participants were randomly allocated to CBT, supportive counselling or TAU. In total, 87 eligible participants were randomised, and those who received CBT exhibited significantly greater improvements in positive symptoms at the 3-month assessment than those who received supportive counselling or TAU. Because the authors did not provide further definition of ‘stable medication’, it is difficult to establish whether or not this group would meet strict TRS criteria. Augmenting clozapine with CBT was first evaluated by Pinto et al. 74 In their RCT74 of CBT with social skills training compared with supportive therapy as an adjunct to clozapine, 41 treatment-resistant participants who had recently started taking clozapine were recruited. Treatment resistance was defined as non-response to at least two antipsychotic medication trials, each ≥ 6 weeks in duration, at a dose of > 600 mg per day of chlorpromazine equivalents. At the end of treatment, both the CBT plus social skills training and supportive counselling groups showed statistically significant improvement in total BPRS score and positive and negative symptom ratings. However, comparisons between the groups showed that, post intervention, participants who had received CBT plus social skills training had lower total BPRS score and lower negative symptoms scores than participants who had received supportive therapy. 74 This study74 provided preliminary evidence for augmenting clozapine with a psychological intervention; however, as this study is non-blind there is a risk of bias that limits the conclusions that be drawn from the findings. In another RCT of CBT for TRS, Kuipers et al. 75 found that the CBT group had a significant improvement on the BPRS, as defined by a 25% reduction in the BPRS score; however, significant differences were not observed between the CBT and control groups on any of the other clinical outcomes. Findings from the study75 also suggested that CBT was considered an acceptable treatment, because participants had a low drop-out rate from therapy (11%), and 80% of those who received CBT expressed high levels of satisfaction with the intervention. Sensky et al. 76 recruited 90 participants to a RCT comparing CBT with befriending for people with TRS. For this study, treatment resistance was defined as the persistence of symptoms resulting in distress or dysfunction for ≥ 6 months despite an adequate trial of antipsychotic medication. 76 Analysis of the data demonstrated no significant difference between the groups at the end of treatment, but an effect was observed at follow-up for positive and negative symptom ratings and depression for the CBT group. A 5-year follow-up study77 of these participants indicated some evidence for the medium-range effectiveness of CBT: participants who received CBT had significantly lower overall symptom severity scores than those in the befriending group. A three-arm RCT78 of CBT versus supportive psychotherapy (SP) versus TAU, for people with a schizophrenia diagnosis who experienced persistent delusions and/or hallucinations after treatment with antipsychotic medication for 6 months, found greater improvement in PANSS total score among those allocated to CBT. In addition, the results of the study demonstrated that those in the psychological intervention arms (CBT or SP) experienced a greater reduction in the severity of delusions and that more people in the CBT arm than in the SP and TAU arms achieved a ≥ 25% reduction in PANSS scores. 78 Although the study indicates promise for the acceptability of CBT for this group, and promise for the effects of CBT on total PANSS score and delusion severity, this was a small study with between 19 and 23 participants in each arm. Valmaggia et al. 79 carried out a small RCT of manualised CBT for psychosis versus a time-matched control intervention of supportive counselling for people with TRS, matching the Kane et al. 44 definition. Sixty-two participants were randomised and the between-group analyses at the end of treatment demonstrated no significant difference between the CBT and supportive counselling groups on positive, negative or general subscale of the PANSS, or the delusion subscale of the Psychotic Symptom Rating Scale (PSYRATS). There was however, a significant improvement in the CBT group on two items of the PSYRATS voices subscale (the physical characteristics and interpretation of voices). These differences were not sustained at follow-up, indicating some short-term effects of CBT on voices for the TRS group.
To date, there have been no published meta-analyses of CBT specifically for TRS. However, a meta-analysis of CBT and FIs for people with a diagnosis of schizophrenia reported that the majority of the studies included in the review included participants who would appear to meet TRS criteria, that is, they were prescribed an antipsychotic medication and had a long duration of illness (DI), and concluded that CBT may be useful for those with TRS. 66 A more recent systematic review80 of the literature on interventions for TRS found 13 studies investigating CBT for TRS. This review included any paper in which the authors had considered the participants to be experiencing TRS, and so did not follow a strictly defined definition of TRS, with the result that the samples included are likely to be highly heterogeneous; this raises the question of whether or not the studies included are reflective of intervention studies for the TRS group. In addition, closer inspection of the CBT studies included in the review80 reveals that the CBT intervention usually targeted specific symptoms of psychosis (i.e. command hallucinations81 and auditory hallucinations82) or was aimed at improving outcomes that were not directly related to symptoms, such as therapeutic alliance. 83
Cognitive–behavioural therapy for clozapine-resistant schizophrenia
To date, only one study has examined the efficacy of CBT for CRS. 84 In this controlled trial, treatment resistance was evaluated using Kane et al. ’s44 criteria and, to meet trial inclusion criteria, participants were required to have taken clozapine for ≥ 6 months without improvement of symptoms of psychosis. Twenty-two participants who met the inclusion criteria were allocated to either CBT (n = 10) or befriending (n = 12). CBT was found to be significantly more effective in reducing BPRS total score, PANSS total score and PANSS general psychopathology subscale score at the end of treatment and at the 6-month follow-up. However, an effect was not found for the reduction of positive symptoms. Although the result of the study by Barretto et al. 84 is encouraging, there are significant methodological limitations. The sample size was very small, limiting the power of the study to detect an effect of CBT for positive symptoms. Moreover, the study design is limited by the absence of randomisation.
Predictors of response to cognitive–behavioural therapy
In addition to understanding the overall efficacy of CBT relative to standard treatment, a further important research consideration is determining predictors of response to CBT, to better understand who will benefit from CBT. In determining who will have a good response to CBT, secondary analyses of CBT trials have indicated that patient characteristics including sex, ethnicity and baseline symptom severity may moderate the outcomes of CBT for people with a schizophrenia diagnosis. 85–88 More specifically in relation to TRS, studies of CBT in this group have indicated that fewer recent hospital admissions,89 greater cognitive flexibility concerning delusions89 and less severe symptoms on allocation73 are associated with a better response to CBT. The DI has been shown to be associated with response to CBT,90–92 and this has also been demonstrated in TRS groups. 73 Similarly, insight at baseline has been shown to be associated with good outcomes in CBT for psychosis. 93
It is likely that the way in which events are appraised will be dependent on the experiences a person has had in life and the way in which they view themselves and other people. 63 Experience of traumatic life events, such as abuse, could lead to the development of a view that other people are threatening, causing later experiences to be interpreted in this light. 62,63 Research in the general population that has found an association between negative life events and unusual beliefs or perceptual experiences has provided support for this view. 94 There is increasing evidence of a link between abuse and psychosis95 as well as other types of traumatic or difficult life experiences and psychosis, for example being held hostage,96 living in highly urbanised areas,97 refugee migrant status,98 low social capital99 and racial discrimination. 100 A 2012 meta-analysis101 of 41 studies found a significant relationship between adversity in childhood and risk of psychosis later in life. The types of traumatic childhood experience that were included in the review101 were emotional, physical and sexual abuse; neglect; bullying; and death of a parent. It was found that, apart from parental death, each of these factors was significantly related to psychosis. Loss of a parent was also found to be significantly related to psychosis when the data from one paper with outlying results were removed from the analysis. This review,101 therefore, concluded that the experience of trauma in childhood is strongly related to an increased risk of psychosis. Specific types of traumatic experience have also been found to relate to specific psychotic experiences. It has been found that sexual abuse is related to voice-hearing, whereas growing up in care is related to experience of paranoia. 102 A longitudinal study103 found that experience of psychosis in children aged 12 years was particularly associated with traumatic events, characterised by intention to harm. This suggests that it could be the perception of threat that is of significance to the development of psychotic experiences. 103 Experiences, such as those described above, could lead to beliefs that others are dangerous, and increase the likelihood that future experiences will be interpreted as threatening. 2 Use of a longitudinal formulation within CBT can, therefore, provide validation of the experiences that an individual has suffered and make sense of current experiences in the context of a traumatic history. 104 This process can also foster hope of recovery by identifying specific areas in which change strategies could be applied. 104
Cognitive difficulties, that is, difficulties with attention, memory and working memory, are frequently experienced by people with a schizophrenia diagnosis. 105,106 Working memory has been described as a system for temporarily storing information while using it to complete tasks involving cognitive function, such as problem-solving. 107 A large meta-analysis investigating working memory in schizophrenia consistently found that participants with a diagnosis of schizophrenia performed worse than control groups on a range of tests of working memory. 108 Furthermore, it has been found that those individuals with a longer-term diagnosis and receiving antipsychotic medication are likely to be the most seriously affected. For example, it has been shown that participants with a greater DI demonstrate the poorest performance on working memory tasks. 108 In relation to spatial working memory, it was found that participants’ performances significantly worsened after receiving clozapine for just 17 weeks. 109 It has previously been demonstrated that anticholinergic drugs, including clozapine, affect memory performance. 110
It has been proposed that neurocognitive deficits in people with a diagnosis of depression are likely to predict outcome from CBT. 111 The same is likely to be true for people with a schizophrenia diagnosis, given that CBT relies on skills, such as memory and generating alternative hypotheses. 112 Neurocognition is also known to be associated with functional outcomes, such as social skills and ability to perform daily activities. 113 Neuropsychological impairment has been found to be predictive of poorer outcome among participants receiving Cognitive Behavioural Social Skills Training. 112 It was hypothesised that neuropsychological inpairment could be related to poorer attendance and reduced engagement with homework in this group. Memory difficulties could make engaging with homework tasks, a factor thought to improve outcome, more problematic. 114 However, few studies have formally evaluated the impact of neurocognitive variables on outcome with CBT, and a clear relationship has not been identified. 115
Although some researchers have endeavoured to identify who has a good response to CBT, the current evidence for predictors of a good response to CBT is limited and the findings are often unreliable because of insufficient statistical power, with very few findings surviving replication. There is a clear benefit to understanding how best to target CBT at those who are most likely to respond, and further research is required.
Important outcomes for trials
A further criticism of these studies is the absent or limited focus on outcome measures that service users consider meaningful and important to their recovery. A review116 of 24 measures commonly used to evaluate psychosis and mood disorders found that service user preference was for measures that were patient rated rather than clinician rated and that evaluated side effects of both pharmacological and psychological interventions; interestingly, measures of social functioning were rated particularly low because of the assumptions made about ‘good’ social functioning. However, it is interesting to note that the PANSS, a commonly used outcome measure, was rated as the most acceptable of the psychosis outcome measures. 116 Although this suggests that measuring these symptom-based domains is important to service users, there is also clear evidence that recovery-orientated outcomes are a priority. 117 A recovery-orientated model of care engenders values of hope, independence and control over one’s life following a mental health problem, connection with a self-identity and having meaning to life and being able to take responsibility for one’s recovery. 117 The emphasis is not on the remission or absence of symptoms. 118 A qualitative study119 investigating how service users with experience of psychosis or schizophrenia diagnoses defined recovery identified three key themes: (1) rebuilding self, (2) rebuilding life and (3) hope for a better future. Within these key themes, processes of recovery included understanding oneself, empowerment, participation in life activities and social support, understanding a personal process for change and a personal desire for change. Importantly, Pitt et al. 119 emphasised that recovery is a journey, not a linear process with a clear end point. A 2011 systematic review120 of the literature on personal recovery from a mental health problem identified a total of 97 papers; synthesis of the findings from these papers resulted in five key processes of recovery: (1) connectedness, (2) hope and optimism for the future, (3) identity, (4) meaning and (5) empowerment.
It has also been argued, especially in relation to the evaluation of psychological therapies for people with psychosis, that affective processes or emotional distress or dysfunction should be the outcomes that are evaluated in trials. For example, CBT for psychosis trials have been criticised as inappropriately conceiving CBT as a quasi-neuroleptic on the basis of adopting methodologies designed to evaluate antipsychotic medication, including use of psychiatric symptoms as the primary outcome rather than affective dysfunction. 121
The importance of recovery-orientated services and treatments for people with experience of psychosis or for people with a schizophrenia diagnosis is in the NICE guideline,30 which emphasises the importance of recovery-orientated values in the treatment of psychosis and schizophrenia. The Schizophrenia Commission report122 makes a call for all mental health services working with people with experience of psychosis and schizophrenia to work in a person-centered approach that embraces the interests and opinions, as well as the strengths and aspirations of the person with psychosis.
Arguably, the lack of specific focus on outcome measures that evaluate these domains, which are important to service users, limits any conclusions that can be drawn from previous treatment studies, both psychological and pharmacological. Similarly, trials that use symptom-focused measures, such as the PANSS, often fail to demonstrate clinically significant change, even if treatments demonstrate statistical superiority. This has led to attempts to define clinically significant response,123 and meta-analyses of trials often use a > 50% improvement on the PANSS as an operational definition of a good outcome. 32
Summary
To summarise, there is clear evidence from the CBT trials that people with TRS and CRS can be engaged in CBT, and that CBT can have small to moderate effects on overall symptoms and may be particularly beneficial for auditory hallucinations. However, it has been highlighted in a 2014 meta-analysis69 that the large and methodologically robust trials of CBT for psychosis have not demonstrated a significant advantage of CBT for either symptoms or relapse, and to date there have been no large high-quality trials of CBT for people with CRS. Moreover, CBT trials have been criticised for poor reporting of adverse effects,124 and future trials should report adverse effects as an outcome. Klingberg et al. 125,126 have provided a useful template for assessing adverse effects that includes death caused by suicide, suicide attempt, suicidal crisis [as defined in the Calgary Depression Rating Scale for Schizophrenia (CDSS), item 8, rating 2] and severe symptomatic exacerbation, defined by the Clinical Global Impression (CGI) scale, which includes ratings of illness severity, changes in overall clinical status, and therapeutic effects. In addition, further research is needed to identify factors that predict a good outcome from CBT.
Rationale for the research/trial aims and objectives
The objectives of this RCT were to provide evidence of the clinical effectiveness and cost-effectiveness of CBT for people with CRS and to utilise baseline data from the RCT to develop a risk model that identifies factors that predict good outcome from CBT. Using the patient-level data available from the trial, the objectives for the economic evaluation were to:
-
estimate the costs of health and social care in the intervention and TAU groups, and assess whether or not there were differences between the groups
-
estimate the quality-adjusted life-years (QALYs) of participants in the intervention and TAU groups, and assess whether or not there were differences between groups
-
assess whether or not any additional benefit is worth any additional cost.
The research objectives of this RCT were to test the following hypotheses:
-
In people with a diagnosis of a schizophrenia spectrum disorder who have an inadequate response to or are unable to tolerate clozapine, CBT plus TAU will lead to improvement in psychotic symptoms, measured using a psychiatric interview (PANSS) over a 21-month follow-up period compared with TAU alone.
-
Cognitive–behavioural therapy plus TAU will lead to improved quality of life and user-defined recovery compared with TAU alone.
-
Cognitive–behavioural therapy plus TAU will lead to a reduction in affective symptoms and negative symptoms compared with TAU alone.
-
Cognitive–behavioural therapy plus TAU will be cost-effective compared with TAU alone.
Chapter 2 Methods
Trial design
The FOCUS trial was a parallel-group, randomised, outcome-blinded evaluation (PROBE) to evaluate the addition of a standardised CBT intervention to TAU for individuals who are unable to tolerate or have had an inadequate response to clozapine. The comparison group received TAU only. The trial was intended to be a definitive, pragmatic clinical effectiveness and cost-effectiveness trial. It was conducted over 4 years across five sites within the UK, with recruitment commencing on 1 January 2013 and ending on 1 June 2015. The follow-up phase for the trial ended in February 2017. A copy of the full ethics-approved trial protocol can be found on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/1010102#/. In addition, the study protocol has been published in a peer-reviewed journal. 127
Role of funding source
The FOCUS trial was funded as a result of a National Institute for Health Research (NIHR) Health Technology Assessment-commissioned call. The call specified the design in PICO (population, intervention, comparator and outcome) terms, requiring that the population be patients with schizophrenia who had not responded to an adequate dose of clozapine or were unable to tolerate it, the intervention was CBT, the comparator was TAU and the outcome was psychiatric symptoms.
Approval
The National Research Ethics Service (NRES) Committee North West – Lancaster (reference 12/NW/0520) approved the FOCUS trial. Ethics approval was granted on 13 August 2012. The trial was also registered on the International Standard Randomised Controlled Trial Number (ISRCTN) clinical trial registry (reference ISRCTN99672552). The trial was registered on 29 November 2012, before recruitment was started in January 2013.
Trial sites
The study was conducted in secondary care mental health services (community mental health, residential rehabilitation and inpatient settings) at five UK centres. These were (1) Manchester, (2) Edinburgh, (3) Glasgow, (4) Newcastle upon Tyne and (5) Southampton.
Participants
A total of 487 participants were recruited across the five sites between 1 January 2013 and 1 June 2015. The Manchester site recruited 108 of the total participants, Southampton recruited 105, Edinburgh recruited 94, Newcastle upon Tyne recruited 92 and Glasgow recruited 88. Participants were recruited from a range of services and settings including community mental health teams (CMHTs), early intervention teams, recovery teams and inpatient services.
Inclusion and exclusion criteria
Participants were eligible to take part in the FOCUS trial if they were considered to have had an inadequate response to a trial of clozapine treatment, specifically treatment with clozapine at a stable dose of ≥ 400 mg (unless limited by tolerability) for ≥ 12 weeks, or, if currently augmented with a second antipsychotic, for ≥ 12 weeks, without remission of psychotic symptoms. This criterion was selected as a review of medication trials found 400 mg to be the minimum dose necessary for effective treatment with clozapine. 128 Other clinical trials looking at CRS have employed the same criteria. 52 Alternatively, participants were eligible for the trial if they had discontinued clozapine in the preceding 2 years because of side effects, lack of efficacy or a problem identified during routine blood monitoring appointments.
Participants were also required to have been given an ICD-10 diagnosis on the schizophrenia spectrum, or to meet criteria for an Early Intervention in Psychosis (EIP) service.
To be included, participants were also required to achieve a minimum total score on the PANSS of 58 (equivalent to a CGI scale of at least mild difficulties),129 as well as a score of ≥ 4 on items for delusions or hallucinations, or of ≥ 5 for items on suspiciousness or grandiosity, to ensure that symptoms of psychosis had not remitted. The research assistant (RA) assessed this at baseline. Participants had to be aged ≥ 16 years and have an identified care co-ordinator or consultant psychiatrist. In additional, participants were required to be competent and willing to provide written informed consent to take part.
Exclusion criteria were having a primary diagnosis of substance or alcohol dependence if this could be the cause of the psychotic experiences, having a diagnosis of developmental disability or organic impairment and being non-English-speaking. Individuals who were currently receiving or had received structured CBT from a qualified psychological therapist within the preceding 12 months were also excluded from the trial. This was operationalised as CBT delivered in line with the NICE guidelines30 for the treatment of psychosis and schizophrenia as ≥ 16 sessions of CBT that is delivered in line with a CBT treatment manual. 30
Data collection
In accordance with the approved protocol, potential participants were initially informed about the study by a member of their care team and, if they expressed interest, were asked to consent to being contacted by the FOCUS trial research team. If they did so, a member of the research team briefly described the study and sent the participant information sheet (PIS) by post. The individual was then given a minimum of 24 hours to consider the information. Following this, the RA arranged to meet the participant at a place of their choosing; in the majority of cases this was the participant’s own home. Some preferred to meet within a mental health service or, if there were any possible risk issues, a meeting at a NHS site would be arranged. Participants who were current inpatients were visited on the ward. The RA talked through the PIS with the individual and ensured that the information was understood by asking the participant to reflect it back to them. When both the RA and the participant were satisfied that all the information about the trial had been provided and understood, the participant was asked to sign the consent form. The RA then read through each point and the participant initialled the boxes provided if they agreed to the information. Both the RA and the participant signed their names underneath.
The RA would then commence the baseline assessment. In the majority of cases, this was conducted across two visits, but this was at the participant’s preference. On average, to complete all assessment measures in full would take approximately 2 hours. The assessment would begin with the PANSS interview and then move on to the self-report measures (outlined below). Each participant was also provided with a personalised crisis card at the baseline assessment. This included contact details for their care team and general practitioner (GP) as well as other helpline numbers, such as the Samaritans. Finally, participants were compensated with £10 for their time and contribution to the research process.
Face-to-face follow-up assessments were completed at 9 and 21 months. The participant was contacted by telephone and an appointment was arranged. Ongoing consent was confirmed with participants at each follow-up. The RA conducted a PANSS interview and asked the participant to complete the self-report measures at each of these time points. Participants were compensated £10 for their time and contribution to the research process at these time points.
Follow-up assessments were completed by telephone at 3, 6, 13 and 17 months. These telephone assessments focused only on obtaining health economics data – no clinical outcome data were collected. The participants were sent £5 gift vouchers in the post on completion of these follow-ups. ‘Keeping in touch’ cards were also posted to the participant on two occasions between these telephone calls.
Outcome measures
Primary outcome
Although there is considerable debate regarding the most appropriate or important outcome measures (e.g. whether to focus on specific psychiatric symptoms or broader recovery and quality of life), the FOCUS trial was funded as a result of a commissioned call that specified the PICO. The commissioned call specified the important outcome as psychiatric symptoms and, therefore, PANSS,130 a reliable and valid, semistructured interview to assess the severity of symptoms associated with psychosis, was chosen as the primary outcome. It is widely used as the primary outcome measure in studies of treatments for people with a schizophrenia diagnosis and research indicates that a 15-point change on the total PANSS score translates to minimal clinical improvement. 123 This allows comparison with other published trials and inclusion of these results in any future systematic reviews and meta-analyses of treatment evidence. PANSS has 30 items that are scored between 1 (absent) and 7 (extreme), and includes seven items that map on to the positive symptoms (such as hallucinations and delusions), seven items relating to negative symptoms (such as blunted affect and emotional withdrawal) and 16 items assessing general psychopathology (such as anxiety and depression). This three-factor model of PANSS was originally proposed by Kay et al. 130 However, multiple-factor structures have been suggested for PANSS, including the original three-factor model, a four-factor model and, more commonly, a five-factor model. 131 Using confirmatory factor analysis on a large data set (n = 5769), van der Gaag et al. 131 tested the fit of 25 published five-factor models; the results indicated that it was not possible to find a fit of these models. Further analysis of the same data set using a 10-fold cross-validation identified a five-factor model with the following subscales: (1) positive, (2) negative, (3) agitation–excitement, (4) depression–anxiety and (5) cognitive. 132 This model of PANSS was used for the FOCUS trial. As the PANSS has a 1 (absent) to 7 (severe) rating scale, each participant is allocated a minimum score of 30 even if they have no symptomology. As noted by Leucht et al. ,133 this poses a significant challenge to understanding percentage change on PANSS, as percentage change is underestimated if 30 minimum points are not subtracted from the total score before calculating percentage change. Therefore, for the analysis of PANSS percentage change for the FOCUS trial, we rescaled the PANSS as recommended by Leucht et al. 133 The commissioned call specified that the minimum duration of follow-up should be 12 months. The primary outcome was therefore specified as PANSS total score at 12-month follow-up from the end of the 9-month treatment window.
Secondary outcomes
Positive and negative symptoms
These were measured by PANSS as described in the preceding section.
Hallucinations and delusions
The PSYRATS134 is a semistructured interview consisting of 12 items assessing aspects of voice-hearing, such as frequency, volume, distress and disruption caused, and six items assessing aspects of unusual beliefs, such as preoccupation, distress and disruption. All items are scored from 0 to 4, with higher scores indicating greater severity. Both sections include cognitive and emotional subscales, and the voices section also includes a physical subscale.
Recovery
The Process of Recovery Questionnaire (QPR) was developed in collaboration with service users to assess recovery from psychosis. 135 A shortened 15-item version was used here. 136 Participants respond using a five-point scale ranging from ‘disagree strongly’ to ‘agree strongly’. Items include ‘I feel better about myself’ and relate to the preceding 7 days.
Social and occupational function
The Personal and Social Performance (PSP) scale137 assesses functioning in four key areas: (1) socially useful activities, (2) personal and social relations, (3) self-care and (4) disturbing and aggressive behaviour. A score is allocated out of 100, with higher scores indicating better functioning.
Depression
The CDSS138 is a structured interview measure with nine items. The items include assessment of hopelessness, feelings of guilt and suicidal ideation. For each section, the assessor can score the client between a score of zero (absent) and three (severe). Therefore, possible scores range from 0 to 27. The measure was incorporated into the PANSS interview during the assessment of depression.
Anxiety
The Anxious Thoughts Inventory (AnTI)139 is a 22-item, self-report questionnaire designed to measure aspects of worry. Each question is scored from one (almost never) to four (almost always). The measure has a three-factor structure comprising (1) social worry, (2) health worry and (3) meta-worry. The seven-item meta-worry scale only was included in the FOCUS trial. This subscale includes statements, such as ‘I worry that I cannot control my thoughts as well as I would like to’.
Substance use
The Alcohol Use Disorders Identification Test (AUDIT) was developed by the World Health Organization (WHO). It consists of 10 questions relating to alcohol use, with cut-off scores to identify hazardous drinking levels. Scores range from 0 to 4 on each item, with total AUDIT scores ranging from 0 to 40. 140 The higher the score, the more severe the alcohol use-related problems. AUDIT has been found to be reliable when used with participants with first-episode psychosis. 140
The Drug Abuse Screening Test (DAST)141 consists of 10 items relating to recent drug use. Participants are asked to provide a dichotomous yes/no response to such questions as ‘Are you always able to stop using drugs when you want to?’. DAST has been found to reliably identify substance abuse issues in participants with first-episode psychosis. 140
Clinical Global Impression
The CGI consists of three items, each scored on a seven-point scale. The RA was required to rate the severity of the participant’s current difficulties from one (not at all ill) to seven (extremely ill). This was completed at all time points. At 9 and 21 months only, the RA also rated change in the participant’s presentation since baseline. This was rated from one (very much improved) to seven (very much worse). In addition, at each time point, the participant was asked to rate the perceived severity of their own difficulties from one (no mental health problems) to seven (very severe mental health problems).
Measurement of adverse events and effects
To ensure a thorough review of adverse events (AEs) and effects, we used a number of methods to identify and report AEs including Health Research Authority (HRA) standard operating procedure (SOP), guidance from our Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) and guidance recommended by Klingberg et al. 126 and a bespoke patient-rated adverse effects measure, developed for the FOCUS trial. Each will be outlined in more detail.
The HRA requires all non-Clinical Trials of an Investigational Medicinal Product (CTIMPs) to report the following AEs, when the chief investigator considers the event related and unexpected: death, is life-threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, consists of a congenital anomaly or birth defect and is otherwise considered medically significant by the investigator. In addition to this list of AEs, our DMEC and TSC advised that self-harm and harm to others also be included. All such events were reported by RAs and therapists to the chief investigator. As per HRA policy, serious adverse events (SAEs) were reported to the Research Ethics Committee (REC) if they were deemed by the chief investigator to be related to trial proceedings and unexpected. To minimise the potential for bias, all AEs were also reviewed by an independent clinician who was a member of the independent DMEC. If the independent clinician considered the event both related and unexpected, then it was reported to the REC.
In addition to the above, for the purpose of the trial, we also defined adverse effects in the trial protocol in line with Klingberg et al. 125,126 as:
-
death caused by suicide
-
suicide attempt
-
suicidal crisis (explicit plan for serious suicidal activity without suicide attempt) as defined in CDSS, item 8, rating 2)
-
severe symptomatic exacerbation, defined by the CGI, which includes ratings of illness severity, changes in overall clinical status, and therapeutic effects. A rating of CGI 2 as six or more and CGI 1 as six or more would be regarded as a severe AE.
In order to better evaluate the adverse effects of trial participation, the adverse effects measure was developed for the FOCUS trial. Participants rated 27 statements on a five-point scale from ‘not at all’ to ‘very much’. Statements included ‘taking part took up too much time’ and ‘I did not like or feel I could trust the FOCUS team members’. A free-text box was also provided for participants to record any additional details about their experience of taking part in the FOCUS trial. This measure was either provided following the final assessment, or at the point of withdrawal for participants who left the trial early. The measure was completed anonymously and was optional.
Other measures including psychological processes
Appraisals of voices
The Interpretation of Voices Inventory (IVI)142 is a 26-item measure consisting of cognitive and metacognitive appraisals of voice-hearing. The IVI has three subscales that relate to (1) positive beliefs about voices, (2) metaphysical beliefs and (3) beliefs about loss of control. Participants respond on a four-point scale from ‘not at all’ to ‘very much’ to indicate how much they endorse each belief. Items include ‘they will take over my mind’ and ‘they are a sign that I am evil’.
Appraisals of paranoia
The Beliefs about Paranoia Scale143 contains 18 items relating to paranoia, such as ‘my paranoid thoughts worry me’ and ‘paranoia is normal’. The scale has been found to have three subscales, namely (1) negative beliefs about paranoia, (2) beliefs about paranoia as a survival strategy and (3) normalising beliefs. The three-factor structure has been validated in a large clinical sample. 144
Beliefs about self and others
The Brief Core Schema Scale (BCSS)145 is a 24-item measure assessing beliefs about self and others. It consists of four subscales: (1) positive beliefs about self, (2) negative beliefs about self, (3) positive beliefs about others and (4) negative beliefs about others. Participants respond ‘yes’ or ‘no’ to a question about whether or not they endorse each belief and then, if they reply ‘yes’, state how much they believe this on a scale from 1 (believe it slightly) to 4 (believe it totally).
Working memory
The letter–number span (LNS)146 was completed at baseline and 9 months only and was read aloud to the participant by the RA. In this test, a participant is presented with a string of letters and numbers and asked to respond by reciting first the numbers in ascending numerical order and then the letters in alphabetical order. The sequences provided begin with two items (e.g. D-6) and increase until they are seven items long (e.g. C-7-G-4-Q-1-S). There are four sequences of each length and the test is stopped when the participant answers all four of any one length incorrectly. The highest possible score is 24.
Attachment
The Psychosis Attachment Measure (PAM-SR)147 is a 16-item measure of adult attachment styles that was developed specifically for use with individuals with psychosis. The PAM-SR has two subscales relating to anxious attachment and avoidant attachment styles. It has been found to be a reliable and valid measure. 147
Stigma
The Internalised Stigma of Mental Illness (ISMI) scale148 assesses the individual’s experience of stigma. It consists of 29 items, each rated on a four-point scale between strongly disagree and strongly agree. The measure includes items such as ‘others think that I can’t achieve much in life because I have a mental illness’. It has five subscales – (1) alienation, (2) stereotype endorsement, (3) perceived discrimination, (4) social withdrawal and (5) stigma resistance – and has been found to be reliable and valid. 148
Childhood trauma
The Childhood Trauma Questionnaire (CTQ)149 was designed to retrospectively assess childhood trauma. It has 28 items on a five-point scale, ranging from ‘never true’ to ‘very often true’. It consists of five subscales – (1) physical abuse, (2) emotional abuse, (3) sexual abuse, (4) emotional neglect and (5) physical neglect – and is thought to be a reliable and valid measure. 149 This measure was administered at 9 months only and delivered in line with a protocol developed in collaboration with members of a service user reference group for managing any distress that could arise from completing this measure. Participants were all offered a list of support services in relation to experience of abuse and offered a follow-up telephone call for the next day.
Semistructured clinical interview for psychosis subgroups
The semistructured clinical interview for psychosis subgroups (SCIPS)150 assesses areas of life and events before the onset of psychotic symptoms. The items cover psychosocial factors and comorbid conditions that have been proven to be associated with psychosis to allow for the classification of a specified subgroup: traumatic, drug related, anxiety or stress sensitivity. SCIPS was administered at 12-month follow-up (21 months) for participants who reached this time point by October 2015; all other participants completed SCIPS at the end-of-treatment assessment (9 months).
Demographic characteristics were captured for each participant at baseline. These included years in full-time education, ethnicity, the participant’s estimate of their DI and duration of untreated psychosis (DUP). Participants were also asked if they considered themselves to be experiencing mental health problems. If they agreed with this, they were asked to rate the degree to which they felt their difficulties to be caused by biological/genetic origins or by life stress/problems or experiences. At each assessment time point, a record was taken of a participant’s current medication. This included dose and duration of time taking clozapine or duration of time since discontinuation of clozapine and the reason for discontinuation. Augmentation with a second antipsychotic was also recorded, as well as other medications for both mental and physical health.
Unless specified above, each outcome measure was administered at baseline and subsequently at 9 and 21 months by RAs who were trained in the use of all the instruments and scales.
Participants were offered choices regarding the length of the assessments, including the option of breaks and multiple visits.
Economic assessment
Health economics
At each face-to-face assessment, and additionally at assessment by telephone at 3, 6, 13 and 17 months, an economic patient questionnaire (an assessment of health service receipt) was completed with each participant. See Chapter 5 for more detail regarding this measure. For those participants who received psychiatric inpatient care during the trial, a psychiatric hospital record was also completed by screening their medical records for services received while hospitalised.
The EuroQoL-5 Dimensions (EQ-5D)151 health questionnaire assesses health outcomes and can be used across a range of health conditions. Participants are asked to rate each item in relation to their health on that day. The items rated are mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Participants then rate their health that day between 0 (the worst health imaginable) and 100 (the best health imaginable). The EQ-5D has been found to have acceptable validity among participants with a schizophrenia diagnosis. 152
Trial interventions
The intervention to be assessed was CBT. This was delivered by appropriately qualified psychological therapists over a 9-month period. Therapists were employed at NHS band 7, which is the starting point for most psychological and nurse therapists and is representative of CBT delivered within NHS services. Participants were offered up to 30 hours of CBT on an approximately weekly basis during the treatment window. The sessions were provided on an individual basis. Most therapy appointments were delivered in the participants’ own homes to help increase the acceptability and accessibility of the intervention.
The CBT was based on a specific cognitive model63 and was manualised. 104 For a more detailed outline of the intervention, see Pyle et al. 127 The specific CBT interventions used were dependent on the individual formulation, but had to be consistent with the intervention model. The range of permissible interventions was provided in published treatment manuals. 64,153 The therapy was flexible, but aimed to involve four phases: (1) assessment, engagement and formulation, (2) change strategies, (3) longitudinal aspects and (4) consolidation. Key milestones were included in each phase. The aims of CBT were to reduce distress (particularly that associated with psychotic symptoms) and to improve quality of life, often by changing the impact of psychotic experiences and beliefs. The CBT was delivered in a collaborative relationship between the participant and therapist and addressed the problems and goals that were agreed between the participant and therapist. Therefore, target of treatment could include positive symptoms of psychosis, social relational issues and issues of comorbidity including anxiety and depression. Key therapeutic principles included formulation, normalisation, collaboration and evaluation of the client’s appraisals of and responses to psychological phenomena.
There was an emphasis on encouraging participants to undertake between-session practice, as research evaluating components of CBT as mechanisms for change suggests that CBT is more effective if between-session tasks are used. 154
By the third session it was expected that there would be a shared list of problems and goals, and shared formulation. It was expected that, by session 12, there would be a shared longitudinal formulation. Milestones regarding formulation were important given the evidence to suggest that formulation is a core component of CBT. 154
Fidelity to the treatment manual
The therapists received weekly CBT-style clinical supervision from the central site to ensure fidelity to the treatment protocol. Additional fortnightly clinical supervision was provided by clinical supervisors, or research site leads, to deal with site-specific clinical issues and ensure that local governance arrangements were followed. All central and local clinical supervisors had expertise and appropriate training in CBT.
To ensure fidelity to the treatment protocol and assess the quality of the therapy delivered, trial therapists regularly submitted audio-recordings of FOCUS trial therapy sessions. These sessions were rated on the Cognitive Therapy Scale – Revised (CTS-R) by the therapist’s central supervisor and detailed feedback was provided. Emphasis was placed on therapists submitting sessions across a range of participants, including sessions that they may be finding difficult as a result of obstacles (e.g. thought disorder). At quarterly therapist training days, group-based CTS-R therapy session ratings were carried out. Therapy sessions were attended by trial therapists, clinical supervisors, the principal investigator and site leads. All trial therapists received a group CTS-R rating at least once.
Fidelity to the treatment protocol was also monitored by analysing data on the content of the CBT sessions. Following every CBT session, based on the treatment protocol, therapists completed a record sheet designed to capture the key elements of CBT (e.g. agenda setting, homework setting/review) and what specific CBT strategies were used with the client (e.g. developing a maintenance formulation, specific cognitive, behavioural and meta-cognitive change strategies). Data were analysed to see if the milestones in the treatment protocol were being met (e.g. a maintenance formulation by session 4) and to check for any site differences in fidelity to the treatment protocol. At each therapist training day, the analysis of the fidelity data were presented and discussed.
The control condition was TAU. Referrers were not asked to withhold any treatment. All participants were required to have an allocated keyworker or care co-ordinator and, therefore, should have been receiving regular outpatient follow-up from a multidisciplinary team within secondary mental health services or from an inpatient setting.
Research assistant training and supervision
All RAs received initial training in the outcome measures from the FOCUS trial co-applicants and the trial manager. Initial training on PANSS was delivered by a PANSS Institute certified trainer who was a co-applicant on the FOCUS trial (Professor Thomas Barnes). RAs were required to complete role plays of the PANSS interview and be observed by a senior clinician before conducting the PANSS interview with participants. In addition, RAs were required to demonstrate a minimum interclass correlation co efficient (ICC) of 0.80 on the PANSS gold standard rating provided by Professor Barnes. In addition to training on the outcome measures, RAs were also given training from senior clinicians in clinical risk assessment, and conducting clinical assessment and supporting service users who disclose trauma and abuse (given the use of the CTQ at the end-of-treatment assessment). All RAs were required to complete Good Clinical Practice training from NIHR.
All RAs were supervised by the trial manager on a weekly basis. As the FOCUS trial was a multisite RCT, this supervision was done over the telephone, except in Manchester where it was completed face to face. The agenda for trial management supervision had a specific focus on the recruitment and retention of participants, data quality and assurance (in particular a check on the accuracy of PANSS scores) and a review of blind breaks, withdrawals and SAEs. Trial management supervision provided an opportunity to problem-solve recruitment and retention difficulties, assurance with data quality and systematic reporting of blind breaks and evaluation of how to minimise future breaks and systematic reporting of withdrawals and SAEs. All RAs attended a fortnightly group conference with the trial manager to share learning and best practice regarding recruitment and to ensure consistency across the sites in scoring the primary outcome measure. In addition, RAs received local clinical supervision from the principal investigator at their site. This covered clinical assessment and risk management, compliance with local NHS policy and procedure, and time management.
Research assistant PANSS consensus days were held on 14 occasions over the lifetime of the trial. At PANSS consensus days, the RAs were required to independently rate a PANSS interview. The ICC across all RAs’ ratings was calculated for each PANSS consensus day. The mean ICC was 0.83 [standard deviation (SD) 0.06] using single measures and 0.96 (SD 0.04) using average measures, demonstrating a good level of inter-rater reliability across the FOCUS trial assessors.
Randomisation and blinding
Randomisation (at the individual level) was independent and concealed, using randomised permuted blocks of random size (blocks of four or six) and stratified by site. The Centre for Healthcare Randomised Trials (CHaRT), Health Services Research Unit, University of Aberdeen, a fully registered (registration number 007) UK Clinical Research Collaboration Clinical Trials Unit (CTU), provided advice regarding the development of the randomisation algorithms and was also consulted regarding the web-based technology. Randomisation was undertaken using OpenCDMS (Open Clinical Data Management System), a web-based system developed with the Mental Health Research Network that has been successfully used in several multisite trials. Utilising this web-based technology ensured that randomisation was centralised, preventing the investigators who were enrolling participants from predicting the randomisation sequence and, therefore, avoiding selection bias. Assessors were blind to treatment allocation. Masking was maintained using a wide range of measures, such as separate offices for therapists and researchers; protocols for answering phones, message taking and secretarial support; separate diaries; and security for electronic randomisation information. When accidental blind breaks did occur, these were reported to the trial manager, and, when possible, a second RA who remained blind to allocation independently rated the assessment. The DMEC and TSC regularly monitored blind breaks by each centre, and implemented corrective action if necessary. Outcome analyses were repeated, excluding those participants for whom a blind break had occurred, to determine the robustness of the findings. Following baseline assessment, eligible participants were randomised within 2 working days. Each randomised allocation was made known to the trial manager (in order to monitor adherence to the randomisation algorithm), the trial administrator and trial therapists by e-mail. The allocation was also made known to the participant by letter, sent by the trial administrator. Blinding of the allocation code was maintained for RAs until all outcome measures for all participants had been collected.
Patient and public involvement
Two co-applicant members of the trial management group are employed as user–researchers (SS and RB). Both contributed to the development of the study protocol, and to oversight of the study during the lifetime of the trial through regular attendance at trial management meetings and involvement with training staff. These two co-applicants also wrote the end-of-study information sheet for participants. In addition, both the trial DMEC and TSC include a service user representative. All trial-specific materials, such as PISs, were developed with patient and public input from the Psychosis Research Unit Service User Reference Group (SURG), the members of which all have experience of psychosis. A key example of the SURG members’ valuable contribution was the recommendation for a standardised protocol for managing distress arising from sensitive disclosures during trial assessments. This included offering a telephone contact within 48 hours of assessments to check on well-being. Along with the key patient and public involvement (PPI) contributions described above, Rory Byrne and Suzy Syrett along with a third user-researcher (Caroline Asher) also undertook an add-on qualitative study to evaluate trial participants’ experiences of CBT. All qualitative interviews, transcriptions and analyses for this study were user led.
Change to the protocol
During the lifetime of the trial, some aspects of the original protocol were changed. All changes were approved by the DMEC, TSC, funder and REC. A summary of these changes can be found in Table 1.
| Details of the protocol amendment | Protocol version | Date of approval by the REC |
|---|---|---|
| Change of inclusion criteria specifically relating to clozapine augmentation with another antipsychotic medication from ‘Treatment for ≤ 24 weeks at a stable dose of ≥ 400 mg of clozapine a day, unless the size of the dose was limited by side effects, without remission of psychotic symptoms, or have discontinued clozapine because of adverse reactions (including agranulocytosis) or lack of efficacy in the past 24 months’ to ‘Treatment of clozapine at a stable dose of ≥ 400 mg or more (unless limited by tolerability) for ≤ 12 weeks, or if currently augmented with a second antipsychotic that this has been given for at least 12 weeks, without remission of psychotic symptoms, or have discontinued clozapine because of adverse reactions (including agranulocytosis) or lack of efficacy in the past 24 months’. The number of items in the economic patient questionnaire was reduced (time use items removed as these were not necessary for the economic evaluation). The version of the EQ-5D was updated to the most current five-point version and the QPR was reduced to the 15-item version (following publication of a confirmatory factor analysis indicating that the 15-item version was a better model for this measure) | V2, 1 November 2012 | 4 December 2012 |
| Changes to the protocol that related to (1) notifying teams of the outcome of randomisation and (2) reflecting the change in the CTU from Glasgow to Aberdeen | V3, 4 February 2013 | 21 March 2013 |
| Inclusion of three measures: (1) CGI scale (patient and rater version), (2) measure of memory (Maryland LNS) and (3) the adverse effects measure (A and B for completers and early discontinuation). Addition of a nested qualitative study in the trial to assess the acceptability of CBT | V4, 18 August 2013 | 2 September 2013 |
| Addition of the SCIPS and £10 token of appreciation for the participants who take part in the nested qualitative study | V5, 19 March 2014 | 9 May 2014 |
| Removal of Heinrichs Quality-of-Life Scale. Owing to an administrative error, when the battery of assessments was initially collated, the Heinrichs Scale was taken from the appendix of the original publication, which only contained four items, instead of 21 items. This mistake was not identified and, therefore, at the time the amendment was submitted only four items had been administered to FOCUS trial participants. There were a number of other secondary outcome measures that related conceptually to quality of life, including the Personal, Social and Performance Subscale, the QPR and the EQ-5D. It was agreed with the independent DMEC, TSC and funder that we should remove this measure. Change to the protocol also included inviting participants at the end of the final assessment (21 months) who were at the Manchester site to take part in an experimental study looking at the impact of manipulating response styles on distress and frequency of words detected in an ambiguous auditory task. In addition, three self-report measures of perseverative thinking, rumination and anxiety were added at the final assessment (21 months) for the Newcastle upon Tyne site only | V6, 30 April 2015 | 20 May 2015 |
| Addition of a nested qualitative study with the trial therapists to explore the therapists’ experiences and views of delivering Cognitive Behavioural Therapy for Psychosis (CBTp) on the FOCUS trial | V7, 27 June 2016 | 26 July 2016 |
Statistical methods and trial analysis
Ground rules for the statistical analysis
The trial analysis followed a statistical analysis plan (SAP), which was agreed by the DMEC. The SAP was published on the CTU’s website in advance of pre access to data and, therefore, pre analysis and can be found at http://w3.abdn.ac.uk/hsru/chart/publicfiles/sapfocus.pdf (accessed 23 November 2018).
The main analyses were based on the intention-to-treat (ITT) (i.e. analyse as randomised) principle and took place after full recruitment and follow-up. Baseline and follow-up data were summarised using appropriate descriptive statistics and graphical summaries. Statistical significance was at the two-sided 5% level with corresponding confidence intervals (CIs) derived. There was no adjustment for secondary outcome CIs for multiple testing. All analysis was done using Stata® version 14 software (StataCorp LP, College Station, TX, USA).
Sample size
The FOCUS trial was designed to estimate treatment effects across a range of outcomes, in addition to psychiatric symptoms. Therefore, we powered the study to detect a generic ES of 0.33. A sample size of 194 participants per group was required to provide 90% power to detect a difference in means using a t-test with a significance level of 5%. To allow a drop-out rate of 20%, 485 participants (97 per site) were required.
Primary and secondary outcome analyses
The primary outcome, PANSS total score assessed at 21 months, was analysed using a linear model with adjustment for prespecified baseline covariates (baseline score, sex and age) and including a random effect for site. Treatment effects over time were explored using repeated-measures mixed-effects models. A sensitivity analysis of missing PANSS data was conducted; these models explored the robustness of the treatment estimates, imputing missing outcome data using multiple imputation. 155 If baseline PANSS data were missing, data were imputed with the centre specific mean.
Secondary outcomes were analysed in a similar way using a linear model and adjusted for prespecified baseline covariates; for the CGI improvement score, no adjustment for baseline score was made as this was not collected.
Planned subgroup analysis
Subgroup analyses explored the potential moderating effect of covariates through the use of treatment-by-subgroup interactions. The subgroups explored were as follows:
-
age
-
sex
-
positive and negative core beliefs from the BCSS
-
working memory using the LNS
-
trauma in childhood using CTQ collected at 9 months
-
substance use from DAST
-
alcohol use from AUDIT
-
difficulty with abstract thinking using item N5 on the PANSS questionnaire
-
conceptual disorganisation using item P2 on the PANSS questionnaire
-
duration of illness
-
duration of untreated psychosis
-
age at onset of psychotic symptoms
-
dose of clozapine
-
number of antipsychotic drugs prescribed
-
attachment using the attachment avoidance subscale taken from PAM-SR
-
psychosis subgroups.
All subgroups were captured at baseline unless otherwise stated.
Risk modelling
We modelled response to treatment, defined as change in PANSS total score from baseline to 21 months, using a general linear model. The baseline covariates included age, age at onset, DI, duration of untreated psychosis, number of antipsychotics, dose of clozapine, PANSS items on conceptual disorganisation and difficulty in abstract thinking, sex, memory and treatment allocation. We explored the impact of missing data at 21 months using a range of strategies, for example using 9-month data if available and multiple imputation based on observed covariates.
Complier-average causal effect analysis
We used instrumental variable methods to estimate the complier-average causal effect (CACE) to explore the impact of compliance with allocated treatment on effect estimates. We considered six or more sessions as a measure of compliance. Randomisation was used as the instrumental variable.
Timing and frequency of analysis
A single principal analysis was carried out when the final participant reached the 21-month time point.
Economic evaluation methods
Aims and objectives
The economic evaluation was a within-trial analysis using patient-level data collected during baseline and follow-up FOCUS trial time points. The aim was to estimate the cost-effectiveness of usual care plus CBT, relative to usual care alone, for people unable to tolerate, or with an inadequate response to, clozapine, in a UK secondary care setting.
Using the patient-level data available from the trial, the objectives for the economic evaluation were to:
-
estimate the costs of health and social care in the intervention and TAU groups and assess whether or not there were differences between groups
-
estimate the QALYs of participants in the intervention and TAU groups and assess whether or not there were differences between groups
-
assess whether or not any additional benefit is worth any additional cost.
Descriptive analysis and data manipulation was conducted using IBM SPSS (Statistical Product and Service Solutions) Statistics version 23 (IBM Corporation, Armonk, NY, USA). The main statistical analyses and estimation of net benefit statistics and cost-effectiveness analysis were conducted using Stata® version 13.
Intervention and comparator, study sample, time horizon and perspective
The study sample comprised all of the participants randomised in the trial. The time horizon of the economic evaluation was 21 months (in line with the final trial follow-up). Costs, QALYs and the secondary outcome measures were estimated from baseline to the end of the scheduled follow-up, to estimate the incremental cost-effectiveness of the CBT intervention. The perspectives for the primary analysis were health and social care service providers (costs) and service users (health benefits). Costs and outcomes were discounted at a rate of 3.5%, in line with UK guidelines. 156
Measure of health benefit
Quality-adjusted life-years were the measure of health benefit used in the primary analysis, on the premise that the intervention and comparator would have a differential impact on participants’ overall health status. QALYs were estimated from the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), and associated utility tariffs157 completed at baseline, 9 months and end of scheduled follow-up (21 months). Resulting health status profiles were converted to utility values using the most recently published utility tariffs for the EQ-5D-5L for a UK population. 157 These utility values represent the weight of preference for each health state of a sample of 912 adults in England.
The EQ-5D has been shown to be a valid and responsive measure of health in people with psychosis. 152,158 The QALY and the EQ-5D are the measures recommended for economic evaluations by NICE. 156 However, the EQ-5D is not a condition-specific measure, and so may miss differences in symptoms that are important to service users. Accordingly, the measure of health benefit was varied in the sensitivity analysis.
Total QALYs were estimated as follows:
Here, U = utility value and t = time between assessments. The time between assessments is the time from baseline data collection to 21-month follow-up; this varied by participant. QALY calculations also accounted for mortality during the trial period.
Resource use and costs
Direct costs of health-care services used by trial participants were estimated for the primary analysis. The total direct costs of service use for each trial arm were estimated by summing the costs of each resource used to provide health and social care. Data on the resources used for each participant were collected using a tailored service use questionnaire at baseline and follow-up (3, 6, 9, 13, 17 and 21 months). The questionnaire was adapted from those used successfully in previous large mental health-based integrated clinical and economic trials, including two trials of first- and second-generation antipsychotic drugs in people with severe schizophrenia. 159,160 In addition, it was anticipated that use of psychiatric inpatient care would be a key component of total cost. Accordingly, data on psychiatric hospital admissions were also collected by case note review. When there were discrepancies between case note and patient reports on admission or length of stay, the case note review data were used. This was based on the assumption that the patient report data may be subject to problems of participant recall.
Services covered by the questionnaire included hospital inpatient stays (psychiatric and non-psychiatric), hospital outpatient visits, primary care services (e.g. GP), community care services (e.g. CMHT) and accident and emergency services.
The cost of providing CBT in the intervention arm was added to the costs of other services used by participants to estimate the total costs of TAU plus CBT. The number of CBT sessions attended by each participant was recorded. The protocol specified that participants would receive up to 30 hours of CBT (rather than a set number of sessions) as shorter sessions may be more appropriate for some people. CBT sessions were usually delivered at home; thus, the cost of a home-based CBT session was applied to the number of sessions to calculate a per-participant cost of CBT.
The unit costs of NHS and social care services were derived from national average unit cost data. These unit costs are published annually in the NHS reference costs database,161 and in the Unit Costs of Health and Social Care document published by the Personal Social Services Research Unit (PSSRU), University of Kent. 162–164 The price year was 2016 and costs are presented in Great British pounds (GBP).
Missing data
Analysis of the economic data was based on ITT principles, namely that outcome data for all trial participants were included in the analysis regardless of whether or not the participant completed the planned treatment. Missing data occurred as a result of both loss to follow-up and incomplete data collection.
Single imputation was used to impute values for missing baseline data. 165 Multiple imputation (MI) was used to impute missing cost and utility data and passively calculate missing total cost and QALYs for each participant. MI was used for the primary and sensitivity economic analyses. MI of both costs and QALYs is increasingly recognised as an appropriate approach to deal with missing observation and missing follow-up data. 166 Missing cost and utility data were treated as missing at random. Missing values were imputed for each time point, rather than as total values covering the whole follow-up period. To ensure that all available data were used, we imputed values by health-care category for costs (inpatient, outpatient and primary/community care) and utility, rather than as total costs or QALYs.
Imputations were conducted in Stata® version 13, using predictive mean matching and sequential chained equations. The choice of independent variables for the imputation regression models was based on initial descriptive analyses and regression analyses. These were used to identify key baseline and follow-up variables (e.g. age, sex, PANSS score) that were significantly associated with either costs or outcomes. These initial analyses were informed by published literature. 159,160
Primary analysis
The incremental cost-effectiveness ratio (ICER) was the primary measure of interest for the economic evaluation. Rather than considering costs and health outcomes separately, the ICER is a joint measure of both. It is calculated by dividing the difference in costs (net costs) by the difference in QALYs (net QALYs) between any two interventions. For this analysis, the ICER represents the additional cost of CBT per additional QALY gained compared with usual care.
Regression analysis was used to estimate the net costs and QALYs of CBT. Key covariates were included in the regression models to control for factors that may influence costs or QALYs. The covariates for these analyses were identified using the approach outlined for the MI described in Missing data.
The estimates of net costs and health outcomes from the regression were bootstrapped156 to simulate 10,000 pairs of incremental cost and QALY outcomes of the FOCUS trial intervention. This captures the relationship between costs and QALYs and looks at how the pairs of net costs and QALYs are distributed on the cost-effectiveness plane. This allowed parameter uncertainty to be captured in our economic evaluation and enabled the undertaking of cost-effectiveness acceptability analysis, which is recommended by NICE for health technology appraisals. 156
The ICER measures the cost per QALY gained by an intervention, which then raises the question of whether or not the additional cost to service providers of a QALY is economically acceptable. To help address this, the ICER can be compared with benchmark or threshold values of how much decision-makers may be willing to pay to gain 1 additional QALY. This is analogous to placing a monetary value on 1 QALY. However, in the UK there is no universally agreed cost-effectiveness threshold value. One commonly reported threshold in the UK, from NICE, is approximately £20,000 to £30,000 per QALY. 156 However, some argue that this may have decreased in recent years as expenditure has been constrained. 167 In 2013, the threshold was re-estimated to be £18,317 per QALY (taking into account expenditure breakdown and mortality), although this was noted to be variable depending on other factors (e.g. disease category and primary care trust). 168 In February 2015, this estimate was revised to ≈£13,000 per QALY. 169 Recognising this lack of consensus, the monetary value of our simulated QALYs used a mid-estimate threshold value of £15,000 per QALY gained. This was varied from £0 to £30,000 to reflect a range of hypothetical thresholds for decision-makers’ willingness-to-pay for an additional QALY [willingness-to-pay thresholds (WTPTs)], from nothing (i.e. they are interested only in the lowest-cost option) to £30,000.
Each of the net QALY estimates from the bootstrap simulation results was revalued by multiplying it by a WTPT. Using these revalued QALY estimates, a net benefit statistic for each pair of simulated net costs and net outcomes was produced as:
where O is the net outcome score and C is the net cost. This process was repeated for the WTPT values of interest to generate a cost-effectiveness acceptability curve. For the simulated net cost and QALY pairs, the cost-effectiveness acceptability curve shows the probability that the intervention is cost-effective for each WTPT value (i.e. provides a positive net benefit). This probability varies at different ICER threshold values. For example, if decision-makers are willing to pay more for an additional QALY, the additional health benefits from an intervention would become more valuable. A cost-effectiveness acceptability curve was used to plot the proportion of bootstrapped simulations in which the net benefit of an intervention is equal to or greater than zero for each WTPT value.
Sensitivity analysis
Sensitivity analyses were used to test the impact of the study design on the ICER and results of the cost-effectiveness acceptability analysis. Table 2 details these.
| Parameters | Rationale | Measure |
|---|---|---|
| Complete-case analysis | If the level of missing observations for the cost and QALY measures is high (> 10%), then MI to estimate these data is more open to bias and imprecision. Complete-case analysis may also be biased and the subsample may not be representative of all trial participants. These factors make it important to analyse both sets of data and assess whether or not the results indicate similar conclusions | Costs and QALYs for participants with complete data at 21-month follow-up |
| Time horizon | It was anticipated that results may vary at different time horizons, depending on when (if any) effect of CBT occurred and how long this was sustained for | Baseline to 9 months costs and QALYs |
| Measure of health benefits | The EQ-5D-5L is a general measure of health, recommended for use in economic evaluations to calculate a generate QALY. However, there is debate about whether or not this is sensitive to clinically relevant changes in mental health. Accordingly, the impact on the results of using mental health-specific measures was assessed. The alternative health benefit measures used were clinically relevant improvements on the PANSS and QPR. The PANSS is a commonly used scale for measuring symptoms in schizophrenia, and the primary outcome of the trial. The QPR captures items of recovery that are important to people with a psychosis diagnosis | The PANSS and QPR were used to estimate whether or not participants had a clinically relevant improvement in symptoms or recovery at the 25% and 50% levels129 |
| Utility value set to estimate QALYs | Until recently, utility scores for the EQ-5D-5L were calculated using a crosswalk method that mapped values from the three-level version (van Hout et al.170). In 2016, a new value set, specific to the five-level version, was released (Devlin et al.157) and was used for the primary analysis. The crosswalk value set provides a link between the three- and five-level versions of the EQ-5D, allowing some comparison between studies | The crosswalk value set was used to estimate QALYs (van Hout et al.170) |
| Inclusion of indirect costs/benefits of employment | Health measures may not fully reflect the impact of treatment on non-health aspects that are of benefit to participants or society. Employment and productive activity is one such area. To minimise participant burden, limited information was collected at follow-up about whether the participant was employed, engaged in other productive activity or unemployed. Detailed information about type of paid or unpaid employment or time spent in different productive activities was not collected. Accordingly, a measure of whether or not the participant was engaged in any productive activity was used to estimate the net cost per person employed | Whether or not the participant was in paid or unpaid employment, education or training at the 21-month follow-up assessment |
Chapter 3 Baseline results
Reliability of outcome measures
The reliability of all outcome measures (total scores and subscales) at baseline were assessed using Cronbach’s alpha reliability statistic. The reliability statistics can be seen in Table 3. The reliability of all measures was acceptable (as indicated by α ≥ 0.7) except for PANSS positive, PANSS excitement, PANSS emotional distress and ISMI scale stigma resistance.
| Measure | α |
|---|---|
| PANSS total | 0.78 |
| PANSS positive | 0.58 |
| PANSS negative | 0.73 |
| PANSS disorganised | 0.75 |
| PANSS excitement | 0.51 |
| PANSS emotional distress | 0.63 |
| CDSS | 0.77 |
| AnTi | 0.84 |
| QPR | 0.93 |
| AUDIT | 0.80 |
| DAST | 0.80 |
| PSYRATS – delusion | 0.86 |
| PSYRATS – auditory hallucinations | 0.95 |
| PSYRATS unusual beliefs – cognitive | 0.82 |
| PSYRATS unusual beliefs – emotional | 0.85 |
| PSYRATS voices – cognitive | 0.75 |
| PSYRATS voices – emotional | 0.94 |
| PSYRATS voices – physical | 0.94 |
| PSYRATS voices – loudness | 0.87 |
| ISMI scale alienation | 0.84 |
| ISMI scale stereotype endorsement | 0.73 |
| ISMI scale discrimination experience | 0.83 |
| ISMI scale social withdrawal | 0.87 |
| ISMI scale stigma resistance | 0.68 |
| ISMI scale total | 0.92 |
Participant baseline characteristics
Trial recruitment
In total, 487 participants were recruited from five centres (Table 4): 242 were randomised to CBT and 245 were randomised to TAU. The referral pathway by the different service type for the participants randomised overall and by centre is shown in Appendix 1. The highest recruiter across all centres was the CMHTs, followed by the clozapine clinic. Participants were recruited to the trial between 1 January 2013 and 31 May 2015 and followed up to March 2017. The trajectory of recruitment from all centres is shown in Figure 1.
| Centre | Participants, n (%) | |||||
|---|---|---|---|---|---|---|
| Eligible (N = 565) | Ineligible (N = 47) | Declined (N = 31) | Randomised (N = 487) | Randomised to CBT (N = 242) | Randomised to TAU (N = 245) | |
| Manchester | 129 (22.8) | 11 (23.4) | 10 (32.3) | 108 (22.2) | 54 (22.3) | 54 (22.0) |
| Southampton | 121 (21.4) | 10 (21.3) | 6 (19.4) | 105 (21.6) | 52 (21.5) | 53 (21.6) |
| Newcastle | 109 (19.3) | 8 (17.0) | 9 (29.0) | 92 (18.9) | 46 (19.0) | 46 (18.8) |
| Edinburgh | 100 (17.7) | 6 (12.8) | 2 (6.5) | 92 (18.9) | 46 (19.0) | 46 (18.8) |
| Glasgow | 106 (18.8) | 12 (25.5) | 4 (12.9) | 90 (18.5) | 44 (18.2) | 46 (18.8) |
FIGURE 1.
Recruitment over time.
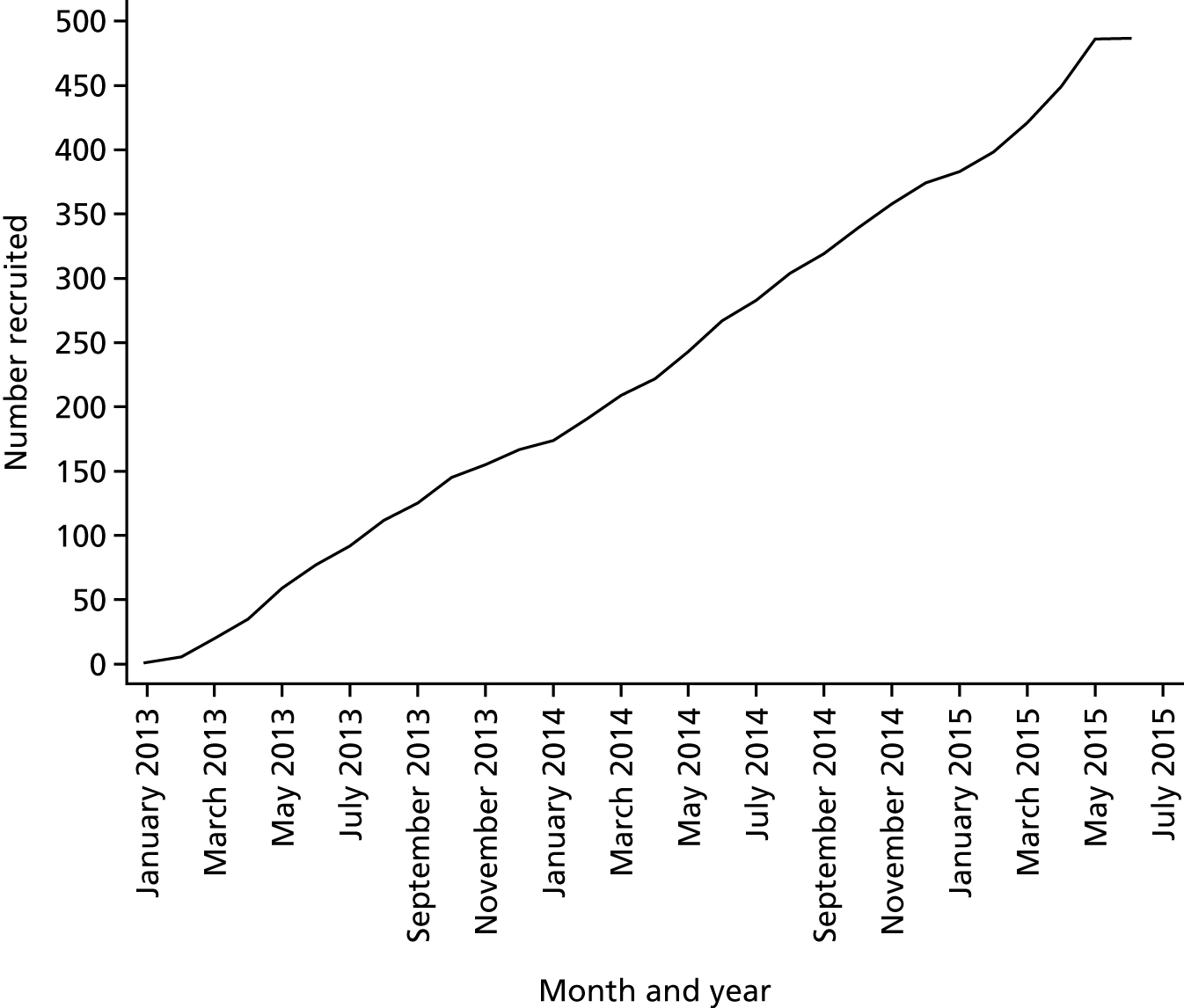
Participant flow
Figure 2 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial. Of the patients, 898 were identified as potentially eligible for inclusion in the trial and were referred, and of these 565 were found to be eligible at the referral stage. Of the 78 patients excluded from the trial, 47 were found to be ineligible and 31 declined. Details of the reasons for patients being ineligible at the referral stage and before randomisation are shown in Appendix 1. All randomised participants completed the baseline questionnaires. In the CBT arm, 230 participants received treatment; further detail on the number of sessions attended is in Chapter 5. At 21 months, 425 participants (87.2%) were included in the primary analysis: there were 10 deaths, 36 participants withdrew from the trial and declined to provide outcome data and a further six were not assessed as they were not contactable at follow-up.
FIGURE 2.
The CONSORT flow diagram. Reprinted from Lancet Psychiatry, Volume 5, Morrison et al. , Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): an assessor-blinded, randomised controlled trial, Pages 633–43, © 2018, with permission from Elsevier. 171
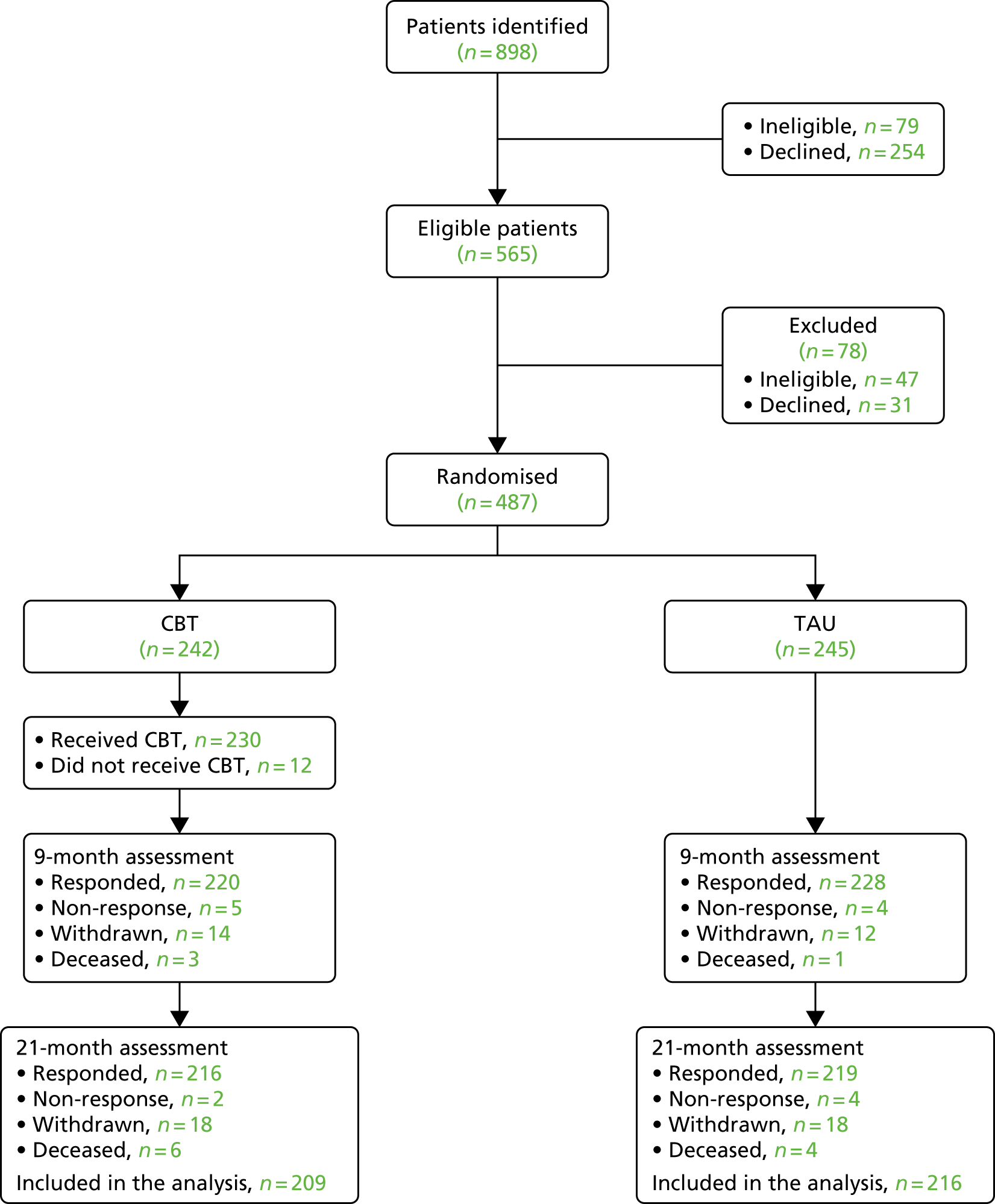
Baseline characteristics
Baseline characteristics are shown in Table 5. The treatment groups were well balanced. The mean age of participants was 42 years in the CBT group and 43 years in the TAU group; > 70% of participants were male, the majority were unemployed (203 participants in the CBT group and 204 in the TAU group) and 71% were living independently. The median duration of untreated psychosis was 9 months [interquartile range (IQR) 1–24 months] in the CBT group and 18 months (IQR 2–48 months) in the TAU group, and the median DI was 216 months (IQR 132–300 months) and 240 months (IQR 144–300 months) in the CBT and TAU groups, respectively. The mean PANSS total score, the primary outcome, was 82.8 points (SD 13.7 points) in the CBT group and 83.3 points (SD 14.0 points) in the TAU group. The PANSS positive and negative scores were also similar in both groups.
| Characteristic | Trial arm | |
|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |
| Age (to the closest year), mean (SD) | 42.2 (10.7) | 42.8 (10.4) |
| Sex, n (%) | ||
| Male | 176 (72.7) | 173 (70.6) |
| Female | 66 (27.3) | 72 (29.4) |
| Ethnicity, n (%) | ||
| White | 222 (91.7) | 222 (90.6) |
| Asian | 9 (3.7) | 4 (1.6) |
| Black | 5 (2.1) | 3 (1.2) |
| Mixed | 4 (1.7) | 12 (4.9) |
| Other | 2 (0.8) | 3 (1.2) |
| Refused to answer | 0 (0.0) | 1 (0.4) |
| Employment, n (%) | ||
| Paid (full or part time) | 10 (4.1) | 10 (4.1) |
| Voluntary | 14 (5.8) | 16 (6.5) |
| Education or training | 9 (3.7) | 5 (2.0) |
| Other unpaid activity | 6 (2.5) | 8 (3.3) |
| Unemployed | 203 (83.9) | 204 (83.3) |
| Missing | 0 (0.0) | 2 (0.8) |
| Residential status, n (%) | ||
| Inpatient | 17 (7.0) | 16 (6.5) |
| Rehabilitation ward | 13 (5.4) | 8 (3.3) |
| Support accommodation | 39 (16.1) | 45 (18.4) |
| Independent living | 172 (71.1) | 174 (71.0) |
| Missing | 1 (0.4) | 2 (0.8) |
| Years in full-time education, median (25th, 75th percentile); n | 12 (11, 14); 223 | 12 (11, 14); 229 |
| Duration of untreated psychosis (months), median (25th, 75th percentile); n | 8 (1, 24); 195 | 18 (2, 48); 203 |
| Duration of illness (months), median (25th, 75th percentile); n | 216 (132, 300); 227 | 240 (144, 300); 231 |
| Primary outcome, mean (SD) | ||
| PANSS total | 82.8 (13.7) | 83.3 (14.0) |
| Secondary outcomes, mean (SD); n | ||
| PANSS positive | 24.7 (5.9); 242 | 25.2 (5.7); 245 |
| PANSS negative | 19.3 (6.1); 242 | 19.4 (6.4); 245 |
| PANSS disorganised | 24.7 (6.5); 242 | 24.8 (6.6); 245 |
| PANSS excitement | 18.0 (4.5); 242 | 17.9 (4.3); 245 |
| PANSS emotional distress | 27.0 (5.6); 242 | 27.4 (5.6); 245 |
| CDSS | 7.1 (4.8); 233 | 7.4 (4.7); 238 |
| AnTI | 18.2 (4.8); 226 | 18.9 (4.9); 236 |
| PSYRATS – delusion | 14.3 (5.7); 218 | 14.9 (5.3); 236 |
| PSYRATS – auditory hallucinations | 21.1 (14.1); 214 | 24.9 (12.6); 200 |
| PSYRATS unusual beliefs – cognitive | 9.6 (3.8); 221 | 9.9 (3.5); 240 |
| PSYRATS unusual beliefs – emotional | 4.7 (2.6); 227 | 5.0 (2.4); 238 |
| PSYRATS voices – cognitive | 3.9 (2.8); 224 | 4.5 (2.5); 213 |
| PSYRATS voices – emotional | 4.7 (3.1); 232 | 5.4 (2.8); 222 |
| PSYRATS voices – physical | 5.5 (3.8); 232 | 6.2 (3.4); 223 |
| PSYRATS voices – loudness | 2.5 (1.5); 229 | 2.6 (1.4); 239 |
| PSP | 49.2 (15.5); 242 | 48.3 (13.5); 245 |
| QPR | 48.5 (11.4); 216 | 47.4 (11.1); 228 |
| AUDIT | 4.3 (6.0); 230 | 3.5 (5.4); 234 |
| DAST | 0.7 (1.4); 224 | 0.7 (1.5); 231 |
| Severity CGI | 4.8 (0.9); 158 | 4.8 (0.8); 162 |
| Participant severity CGI | 3.9 (1.4); 152 | 4.0 (1.6); 157 |
| EQ-5D-5L utility | 0.740 (0.201); 223 | 0.703 (0.225); 230 |
| Diagnosis at baseline, n (%) | ||
| Schizophrenia | 209 (86.4) | 218 (89.0) |
| Schizoaffective | 28 (11.6) | 20 (8.2) |
| Delusional disorder | 2 (0.8) | 5 (2.0) |
| Unspecified psychosis not attributable to a substance or known physiological condition | 2 (0.8) | 1 (0.4) |
| Missing | 1 (0.4) | 1 (0.4) |
Table 6 describes the baseline medication for the trial population; > 90% of participants in both arms were prescribed clozapine. The length of time on clozapine was the same in both groups (median 60 months, IQR 24–120 months). In the CBT group, 19 participants discontinued clozapine, and, in the TAU group, 24 discontinued it, with side effects given as the main reason in both groups. Other antipsychotic medication was taken by 106 participants (43.8%) in the CBT group and 103 (42.0%) in the TAU group. Participants also took other medication, listed in Table 6.
| Medication | Trial arm | |
|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |
| Prescribed clozapine, n (%) | 223 (92.1) | 221 (90.2) |
| Length of time on clozapine (months), median (25th, 75th percentile); n | 60 (24, 120); 218 | 60 (24, 120); 216 |
| Clozapine dose (mg), median (25th, 75th centile); n | 400 (300, 525); 221 | 400 (300, 500); 221 |
| Discontinued clozapine, n (%) | 19 (7.9) | 24 (9.8) |
| Length of time discontinued (months), median (IQR); n | 9 (5–13); 19 | 13 (3–20); 24 |
| Reasons for discontinuing clozapine, n (%) | ||
| Side effects | 16 (84.2) | 23 (95.8) |
| Lack of efficacy | 3 (15.8) | 1 (4.2) |
| Taking other antipsychotic medication, n (%) | ||
| None | 136 (56.2) | 142 (58.0) |
| One | 99 (40.9) | 95 (38.8) |
| Two | 7 (2.9) | 7 (2.9) |
| Three | 0 (0.0) | 1 (0.4) |
| Other medication,a n (%) | ||
| None | 85 (35.1) | 81 (33.1) |
| Antidepressants | 113 (46.7) | 129 (52.7) |
| Other mental health medication | 52 (21.5) | 35 (14.3) |
| Benzodiazepines | 27 (11.2) | 30 (12.2) |
| Medication for the side effects of antipsychotics | 27 (11.2) | 24 (9.8) |
| Unknown medication | 2 (0.8) | 2 (0.8) |
Chapter 4 Outcome and results
Primary outcome
The PANSS total and subscale scores at each time point are described in Table 7. Figure 3 shows the profile for the two treatment groups over the study period. Treatment effect estimates are also included in Table 7, based on 425 participants for whom at least one follow-up measurement was available. At 9 months, the total PANSS score was lower in the CBT arm (mean –2.40, 95% CI –4.79 to –0.02; p = 0.049), with a standardised ES of 0.16. The mean difference at 21 months was –0.89 (95% CI –3.32 to 1.55; p = 0.475), with a standardised ES of 0.06. At 9 months, the subscale PANSS positive score was lower in the CBT arm (mean –1.56, 95% CI –2.53 to –0.59; p = 0.002), with a standardised ES of 0.24; PANSS excitement was lower in the CBT arm (mean –1.18, 95% CI –1.85 to 0.50; p = 0.001), with a standardised ES of 0.28, and PANSS emotional distress was lower in the CBT arm (mean –1.08, 95% CI –2.02 to –0.13; p = 0.025), with a standardised ES of 0.17. For PANSS negative and PANSS disorganised, there was no evidence of a difference at either time point. Sensitivity analysis using MI gave similar results (see Appendix 2). Figure 4 shows the site difference and within-site differences for total PANSS score at 9 and 21 months. At 21 months, the Manchester site recorded a greater effect in total PANSS score in favour of CBT.
| Time point | Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Cronbach’s alpha | |
|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||
| PANSS total | ||||||
| Baseline | 82.8 (13.7); 242 | 83.3 (14.0); 245 | ||||
| 9 months | 75.2 (15.5); 218 | 77.8 (14.6); 224 | –2.40 | –4.79 to –0.02 | 0.049 | 0.16 |
| 21 months | 73.0 (16.7); 209 | 74.1 (14.8); 216 | –0.89 | –3.32 to 1.55 | 0.475 | 0.06 |
| PANSS positive | ||||||
| Baseline | 24.7 (5.9); 242 | 25.2 (5.7); 245 | ||||
| 9 months | 21.7 (6.6); 218 | 23.6 (6.2); 225 | –1.56 | –2.53 to –0.59 | 0.002 | 0.24 |
| 21 months | 21.3 (7.0); 209 | 22.5 (6.1); 216 | –0.85 | –1.84 to 0.15 | 0.095 | 0.13 |
| PANSS negative | ||||||
| Baseline | 19.3 (6.1); 242 | 19.4 (6.4); 245 | ||||
| 9 months | 18.1 (7.0); 220 | 18.6 (6.7); 227 | –0.49 | –1.48 to 0.49 | 0.327 | 0.07 |
| 21 months | 17.8 (6.8); 211 | 17.5 (6.1); 216 | 0.29 | –0.72 to 1.29 | 0.578 | 0.05 |
| PANSS disorganised | ||||||
| Baseline | 24.7 (6.5); 242 | 24.8 (6.6); 245 | ||||
| 9 months | 23.2 (6.4); 218 | 23.1 (6.0); 225 | –0.01 | –0.91 to 0.88 | 0.975 | 0.00 |
| 21 months | 22.7 (6.6); 210 | 22.4 (6.2); 216 | 0.14 | –0.78 to 1.05 | 0.770 | 0.02 |
| PANSS excitement | ||||||
| Baseline | 18.0 (4.5); 242 | 17.9 (4.3); 245 | ||||
| 9 months | 16.2 (4.1); 220 | 17.4 (4.2); 228 | –1.18 | –1.85 to –0.50 | 0.001 | 0.28 |
| 21 months | 15.4 (3.9); 210 | 15.9 (4.0); 216 | –0.57 | –1.26 to 0.12 | 0.106 | 0.15 |
| PANSS emotional distress | ||||||
| Baseline | 27.0 (5.6); 242 | 27.4 (5.6); 245 | ||||
| 9 months | 24.1 (6.2); 220 | 25.4 (6.3); 228 | –1.08 | –2.02 to –0.13 | 0.025 | 0.17 |
| 21 months | 23.4 (6.6); 210 | 24.0 (6.0); 216 | –0.27 | –1.24 to 0.70 | 0.583 | 0.04 |
FIGURE 3.
Profile of PANSS total scores.
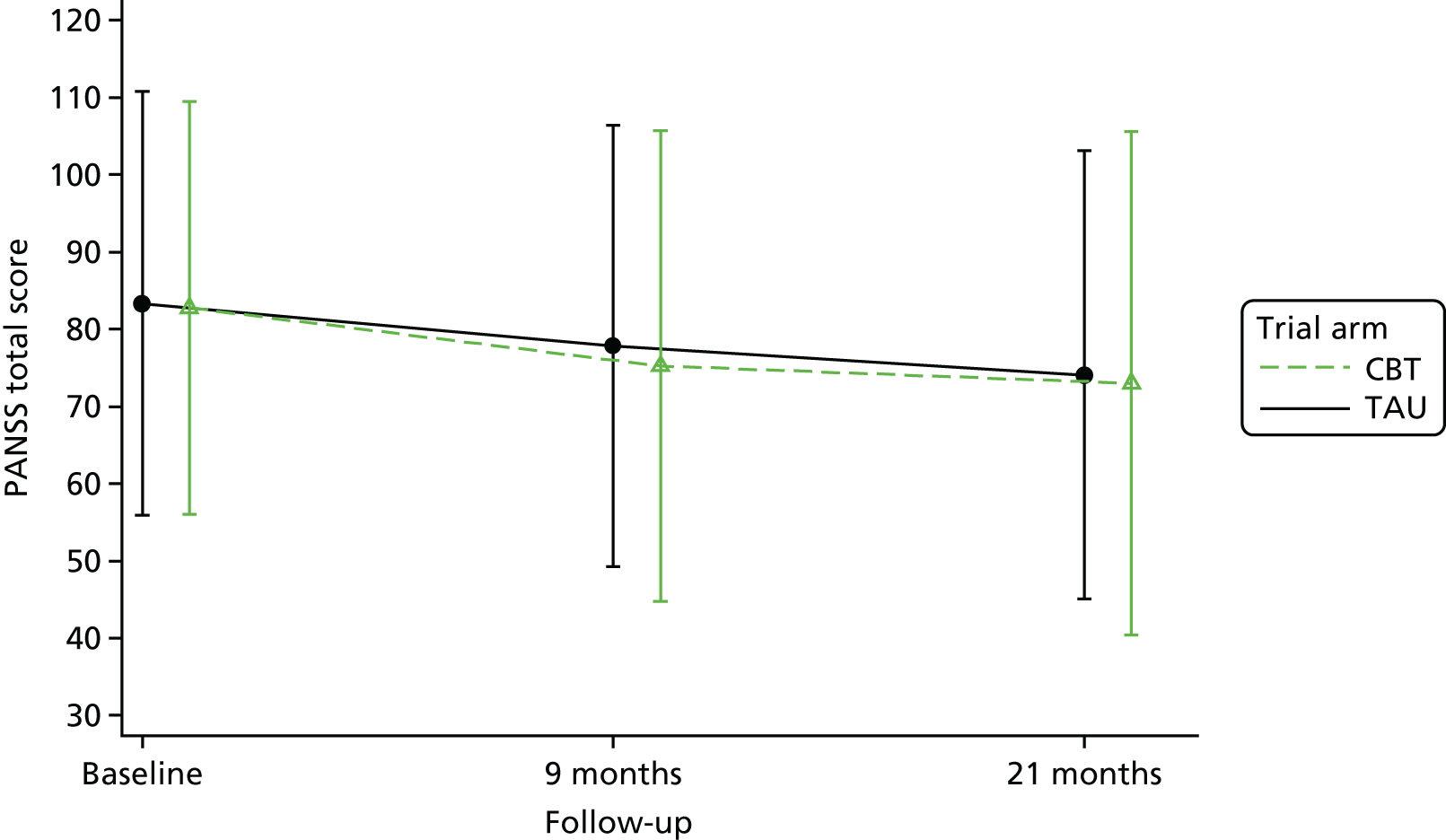
FIGURE 4.
Site differences for PANSS total scores. (a) 9 months; and (b) 21 months.
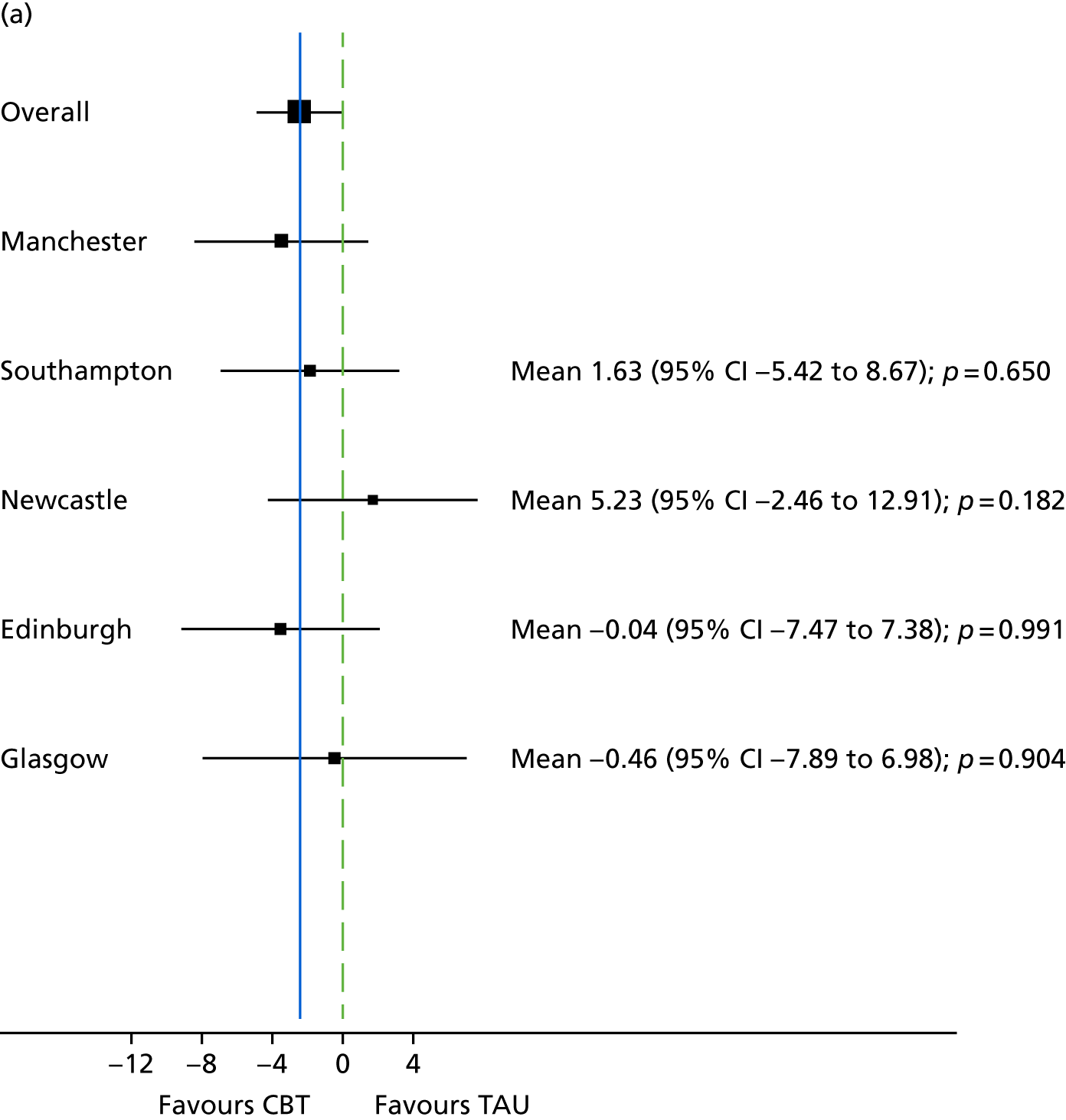
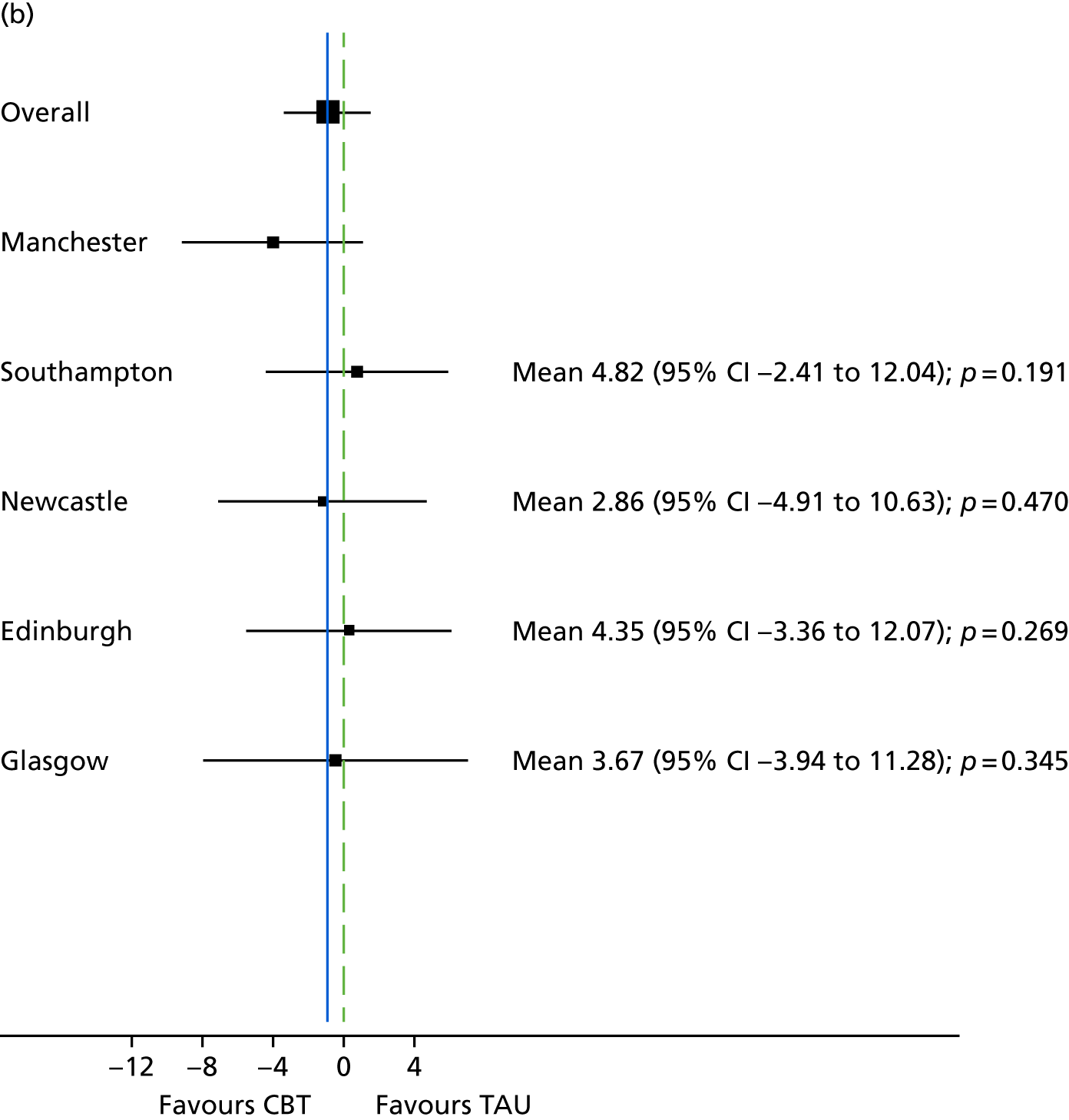
At the 9-month assessment, a total of 51 blind breaks had occurred. However, 23 of these cases were transferred to a new, independent assessor, meaning that 28 assessments were unblind at the 9-month assessment. At the 21-month assessment, the number of breaks that had occurred was 55. However, 35 of these cases were transferred to a new, independent assessor, meaning that 20 assessments were unblind at the 21-month assessment. Outcome analyses for PANSS were repeated excluding those participants for whom a blind break had occurred; the results are presented in Table 8.
| Time point | Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | ||||
| Baseline | 82.8 (13.7); 242 | 83.3 (14.0); 245 | |||
| 9 months | 75.6 (15.5); 194 | 77.7 (14.5); 222 | –2.21 | –4.66 to 0.24 | 0.078 |
| 21 months | 73.4 (16.7); 193 | 73.8 (14.6); 214 | –0.48 | –2.96 to 2.00 | 0.704 |
The percentage of improvement in PANSS total is shown in Table 9. At 9 months, 16 participants (6.6%) in the CBT arm and 11 (4.5%) in the TAU arm had > 50% improvement; the number needed to treat (NNT) was 42. At 21 months, 28 participants (11.6%) in the CBT arm and 14 (5.7%) in the TAU arm had > 50% improvement, and the NNT was 15.
| Improvement | Trial arm, n (%) | NNT | 95% CI | |
|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||
| > 25% | ||||
| 9 months | 68 (28.1) | 57 (23.3) | 18 | NNTH 37 to ∞ to NNTB 8 |
| 21 months | 80 (33.1) | 82 (33.5) | 318 | NNTH 11 to ∞ to NNTB 11 |
| > 50% | ||||
| 9 months | 16 (6.6) | 11 (4.5) | 42 | NNTH 48 to ∞ to NNTB 15 |
| 21 months | 28 (11.6) | 14 (5.7) | 15 | NNTB 8 to NNTB 81 |
| > 75% | ||||
| 9 months | 2 (0.8) | 2 (0.8) | 4070 | NNTH 57 to ∞ to NNTB 56 |
| 21 months | 4 (1.7) | 2 (0.8) | 102 | NNTH 78 to ∞ to NNTB 31 |
Table 10 shows the effect of time on PANSS total for the sample as a whole. The analysis was adjusted for randomised treatment, age, sex and centre. Table 10 shows a reduction in PANSS total score of 5.26 at 9 months compared with baseline and a reduction in PANSS total score of 9.1 at 21 months compared with baseline.
| Time point | Effect estimate | 95% CI | p-value |
|---|---|---|---|
| 9 months | –5.26 | –7.84 to –2.67 | < 0.001 |
| 21 months | –9.1 | –11.71 to –6.48 | < 0.001 |
Secondary outcomes
The PSYRATS subscales at 9 and 21 months are shown in Table 11. At 9 months there was a mean difference in favour of CBT for auditory hallucinations (–2.56, 95% CI –4.87 to –0.26; p = 0.029) and physical voices (–0.58, 95% CI –1.11 to –0.04; p = 0.034). Both subscales were similar in both groups at 21 months. At 21 months the mean difference between groups on emotional unusual beliefs was –0.53 (95% CI –1.05 to –0.00; p = 0.049). For the remainder of the PSYRATS subscales, there was no evidence of a difference between the two treatments at 9 or 21 months. Other secondary outcomes were similar with the exceptions of QPR and CGI (Table 12).
| Time point | Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | ||||
| PSYRATS auditory hallucinations | |||||
| Baseline | 21.1 (14.1); 214 | 24.9 (12.6); 200 | |||
| 9 months | 17.8 (14.2); 185 | 22.4 (13.4); 192 | –2.56 | –4.87 to –0.26 | 0.029 |
| 21 months | 17.1 (14.2); 179 | 20.3 (14.4); 182 | –1.38 | –3.75 to 0.99 | 0.255 |
| PSYRATS delusion | |||||
| Baseline | 14.3 (5.7); 218 | 14.9 (5.3); 236 | |||
| 9 months | 12.2 (6.8); 200 | 13.2 (6.7); 216 | –0.42 | –1.61 to 0.77 | 0.493 |
| 21 months | 11.4 (7.1); 193 | 12.7 (6.8); 203 | –0.76 | –1.98 to 0.46 | 0.224 |
| PSYRATS unusual beliefs – cognitive | |||||
| Baseline | 9.6 (3.8); 221 | 9.9 (3.5); 240 | |||
| 9 months | 8.2 (4.5); 201 | 8.8 (4.3); 216 | –0.24 | –1.01 to 0.54 | 0.551 |
| 21 months | 7.8 (4.7); 194 | 8.5 (4.4); 205 | –0.35 | –1.14 to 0.44 | 0.385 |
| PSYRATS unusual beliefs – emotional | |||||
| Baseline | 4.7 (2.6); 227 | 5.0 (2.4); 238 | |||
| 9 months | 3.9 (2.9); 206 | 4.4 (2.9); 219 | –0.29 | –0.79 to 0.22 | 0.269 |
| 21 months | 3.6 (3.0); 199 | 4.3 (2.9); 206 | –0.53 | –1.05 to –0.00 | 0.049 |
| PSYRATS voices – cognitive | |||||
| Baseline | 3.9 (2.8); 224 | 4.5 (2.5); 213 | |||
| 9 months | 3.4 (2.8); 193 | 4.0 (2.7); 204 | –0.32 | –0.82 to 0.17 | 0.195 |
| 21 months | 3.3 (2.9); 187 | 3.8 (2.8); 187 | –0.17 | –0.68 to 0.34 | 0.514 |
| PSYRATS voices – emotional | |||||
| Baseline | 4.7 (3.1); 232 | 5.4 (2.8); 222 | |||
| 9 months | 4.2 (3.3); 202 | 5.0 (3.0); 208 | –0.43 | –0.95 to 0.08 | 0.101 |
| 21 months | 4.1 (3.3); 199 | 4.6 (3.3); 197 | –0.03 | –0.55 to 0.50 | 0.914 |
| PSYRATS voices – physical | |||||
| Baseline | 5.5 (3.8); 232 | 6.2 (3.4); 223 | |||
| 9 months | 4.7 (3.8); 208 | 5.7 (3.6); 209 | –0.58 | –1.11 to –0.04 | 0.034 |
| 21 months | 4.4 (3.6); 201 | 5.1 (3.8); 198 | –0.30 | –0.85 to 0.24 | 0.279 |
| PSYRATS voices – loudness | |||||
| Baseline | 2.5 (1.5); 229 | 2.6 (1.4); 239 | |||
| 9 months | 2.0 (1.6); 206 | 2.3 (1.6); 219 | –0.22 | –0.50 to 0.06 | 0.120 |
| 21 months | 1.9 (1.7); 199 | 2.3 (1.6); 206 | –0.28 | –0.57 to 0.01 | 0.056 |
| Time point | Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | ||||
| CDSS | |||||
| Baseline | 7.1 (4.8); 233 | 7.4 (4.7); 238 | |||
| 9 months | 6.3 (4.5); 210 | 6.8 (4.8); 215 | –0.54 | –1.31 to 0.23 | 0.168 |
| 21 months | 6.0 (4.4); 202 | 6.6 (5.1); 205 | –0.50 | –1.28 to 0.29 | 0.212 |
| AnTI | |||||
| Baseline | 18.2 (4.8); 226 | 18.9 (4.9); 236 | |||
| 9 months | 17.5 (5.2); 189 | 18.0 (5.0); 206 | –0.07 | –0.88 to 0.73 | 0.856 |
| 21 months | 16.9 (5.1); 180 | 18.1 (5.0); 193 | –0.60 | –1.44 to 0.24 | 0.160 |
| PSP | |||||
| Baseline | 49.2 (15.5); 242 | 48.3 (13.5); 245 | |||
| 9 months | 53.2 (14.6); 213 | 50.9 (13.9); 224 | 1.90 | –0.31 to 4.11 | 0.093 |
| 21 months | 51.5 (15.2); 206 | 51.4 (14.7); 214 | 0.18 | –2.07 to 2.44 | 0.872 |
| QPR | |||||
| Baseline | 48.5 (11.4); 216 | 47.4 (11.1); 228 | |||
| 9 months | 50.9 (11.6); 181 | 48.7 (11.1); 194 | 1.88 | –0.03 to 3.79 | 0.053 |
| 21 months | 52.0 (9.6); 165 | 49.1 (11.7); 185 | 2.03 | 0.04 to 4.01 | 0.045 |
| AUDIT | |||||
| Baseline | 4.3 (6.0); 230 | 3.5 (5.4); 234 | |||
| 9 months | 4.4 (6.0); 194 | 3.5 (5.7); 209 | 0.69 | –0.17 to 1.56 | 0.116 |
| 21 months | 4.6 (6.5); 190 | 3.2 (5.0); 193 | 0.80 | –0.09 to 1.69 | 0.079 |
| DAST | |||||
| Baseline | 0.7 (1.4); 224 | 0.7 (1.5); 231 | |||
| 9 months | 0.7 (1.7); 153 | 0.9 (1.7); 173 | –0.13 | –0.43 to 0.18 | 0.409 |
| 21 months | 0.6 (1.3); 170 | 0.6 (1.3); 181 | 0.12 | –0.17 to 0.41 | 0.417 |
| Condition improvement CGIa | |||||
| 9 months | 3.3 (1.1); 141 | 3.3 (1.1); 157 | –0.04 | –0.50 to 0.42 | 0.822 |
| 21 months | 3.2 (0.9); 131 | 3.5 (1.0); 147 | –0.33 | –0.54 to -0.11 | 0.013 |
| Severity CGI | |||||
| Baseline | 4.8 (0.9); 158 | 4.8 (0.8); 162 | |||
| 9 months | 4.2 (1.0); 207 | 4.3 (1.1); 213 | –0.09 | –0.30 to 0.12 | 0.395 |
| 21 months | 4.1 (1.0); 208 | 4.2 (1.0); 212 | –0.03 | –0.24 to 0.18 | 0.772 |
| Participant severity CGI | |||||
| Baseline | 3.9 (1.4); 152 | 4.0 (1.6); 157 | |||
| 9 months | 3.6 (1.7); 197 | 3.7 (1.5); 186 | 0.06 | –0.27 to 0.39 | 0.729 |
| 21 months | 3.7 (1.5); 193 | 3.7 (1.6); 210 | 0.12 | –0.22 to 0.46 | 0.483 |
| EQ-5D-5L utility | |||||
| Baseline | 0.740 (0.201); 223 | 0.703 (0.225); 230 | |||
| 9 months | 0.760 (0.223); 187 | 0.721 (0.254); 205 | 0.035 | –0.004 to 0.073 | 0.079 |
| 21 months | 0.773 (0.204); 180 | 0.730 (0.223); 189 | 0.028 | –0.012 to 0.068 | 0.170 |
As indicated in Table 13, chi-squared analysis did not indicate any significant difference between the groups regarding access to education, employment or training at either 9 or 21 months.
| Employment status | Trial arm, n (%) | p-value | |
|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | ||
| 9 months | |||
| NEET | 180 (74.4) | 186 (75.9) | |
| EET | 41 (16.9) | 42 (17.1) | 0.774 |
| 21 months | |||
| NEET | 162 (66.9) | 175 (71.4) | |
| EET | 50 (12.4) | 41 (16.7) | 0.499 |
Compliance with treatment
The median number of CBT sessions attended was 23, and 213 out of 242 participants (88%) attended at least six sessions, which was the minimum number of sessions needed to be classified as having received CBT (Table 14). Treatment effects from a CACE analysis, adjusting for compliance at 9 months and the primary and secondary outcomes, are shown in Appendix 2. We repeated this analysis using the actual number of sessions attended; results are presented in Table 15 for 9 months and Table 16 for 21 months. For every extra session attended, the PANSS total score resulted in a difference of –0.12 (95% CI –0.24 to 0.00; p = 0.059) at 9 months, which suggests that attending more CBT sessions was beneficial in the short term.
| Number of sessions | CBT arm (N = 242), n (%) |
|---|---|
| 0 | 12 (5.0) |
| 1–5 | 17 (7.0) |
| 6–10 | 20 (8.3) |
| 11–20 | 48 (19.8) |
| 21–30 | 124 (51.2) |
| > 31 | 21 (8.7) |
| Median | 23 |
| 25th centile | 13 |
| 75th centile | 28 |
| Outcome | Mean difference | 95% CI | p-value |
|---|---|---|---|
| PANSS total | –0.12 | –0.24 to 0.00 | 0.059 |
| PANSS positive | –0.02 | –0.08 to 0.03 | 0.347 |
| PANSS negative | –0.08 | –0.13 to –0.03 | 0.001 |
| PANSS disorganised | –0.00 | –0.05 to 0.05 | 0.997 |
| PANSS excitement | –0.06 | –0.10 to –0.02 | 0.001 |
| PANSS emotional distress | –0.06 | –0.10 to –0.01 | 0.028 |
| CDSS | –0.13 | –0.25 to –0.01 | 0.040 |
| AnTI | –0.02 | –0.08 to 0.04 | 0.506 |
| PSYRATS – auditory hallucinations | –0.01 | –0.05 to 0.03 | 0.566 |
| PSYRATS – delusion | –0.01 | –0.04 to 0.01 | 0.283 |
| PSYRATS unusual beliefs – cognitive | –0.02 | –0.04 to 0.01 | 0.206 |
| PSYRATS unusual beliefs – emotional | –0.02 | –0.05 to 0.01 | 0.114 |
| PSYRATS voices – cognitive | –0.03 | –0.06 to –0.00 | 0.038 |
| PSYRATS voices – emotional | –0.01 | –0.03 to 0.00 | 0.112 |
| PSYRATS voices – physical | –0.03 | –0.07 to 0.01 | 0.151 |
| PSYRATS voices – loudness | –0.00 | –0.04 to 0.04 | 0.942 |
| PSP | 0.10 | –0.02 to 0.21 | 0.091 |
| QPR | 0.10 | –0.00 to 0.19 | 0.061 |
| AUDIT | 0.03 | –0.01 to 0.08 | 0.127 |
| DAST | –0.01 | –0.02 to 0.01 | 0.415 |
| Severity CGI | –0.00 | –0.02 to 0.01 | 0.417 |
| Participant severity CGI | 0.00 | –0.01 to 0.02 | 0.669 |
| Condition improvement CGI | –0.02 | –0.03 to –0.01 | 0.003 |
| EQ-5D-5L | 0.002 | –0.000 to 0.004 | 0.094 |
| Instrument | Mean difference | 95% CI | p-value |
|---|---|---|---|
| PANSS total | –0.04 | –0.16 to 0.08 | 0.547 |
| PANSS positive | 0.02 | –0.03 to 0.06 | 0.527 |
| PANSS negative | –0.04 | –0.09 to 0.01 | 0.101 |
| PANSS disorganised | 0.01 | –0.04 to 0.05 | 0.745 |
| PANSS excitement | –0.02 | –0.06 to 0.01 | 0.145 |
| PANSS emotional distress | –0.01 | –0.06 to 0.03 | 0.565 |
| CDSS | –0.02 | –0.06 to 0.02 | 0.263 |
| AnTI | –0.03 | –0.07 to 0.01 | 0.156 |
| PSYRATS – auditory hallucinations | –0.06 | –0.17 to 0.05 | 0.310 |
| PSYRATS – delusion | –0.03 | –0.09 to 0.02 | 0.231 |
| PSYRATS unusual beliefs – cognitive | –0.02 | –0.05 to 0.02 | 0.396 |
| PSYRATS unusual beliefs – emotional | –0.02 | –0.05 to 0.00 | 0.050 |
| PSYRATS voices – cognitive | –0.01 | –0.03 to 0.02 | 0.551 |
| PSYRATS voices – emotional | –0.00 | –0.03 to 0.02 | 0.969 |
| PSYRATS voices – physical | –0.01 | –0.04 to 0.01 | 0.267 |
| PSYRATS voices – loudness | –0.01 | –0.03 to 0.00 | 0.057 |
| PSP | –0.00 | –0.12 to 0.11 | 0.941 |
| QPR | 0.09 | 0.01 to 0.18 | 0.036 |
| AUDIT | 0.03 | –0.01 to 0.08 | 0.107 |
| DAST | 0.00 | –0.01 to 0.02 | 0.488 |
| Severity CGI | –0.00 | –0.01 to 0.01 | 0.752 |
| Participant severity CGI | 0.01 | –0.01 to 0.02 | 0.502 |
| Condition improvement CGI | –0.00 | –0.01 to 0.01 | 0.757 |
| EQ-5D-5L | 0.00 | –0.00 to 0.00 | 0.152 |
Session record data were analysed to determine if therapy milestones were achieved. The percentage of participants allocated to CBT with whom therapy milestones were achieved is shown in Table 17.
| Milestones | CBT, n/N (%) |
|---|---|
| Problem and goals identified during sessions 1–4 | 221/242 (91.3) |
| Maintenance formulation developed during sessions 1–4 | 185/242 (76.4) |
| If maintenance formulation was not developed during sessions 1–4, it was developed after | 26/57 (45.6) |
| Longitudinal formulation developed during sessions 1–16 | 111/242 (45.9) |
| If longitudinal formulation was not developed during sessions 1–16, it was developed after | 21/131 (16.0) |
| Change strategies were used during sessions 1–5 | 200/242 (82.6) |
| If change strategies were not used during sessions 1–5, they were used in sessions 6–10 | 15/42 (35.7) |
| Homework set during sessions 1–5 | 219/242 (90.5) |
| If homework was set during sessions 1–5, it was completed | 181/219 (82.6) |
Fidelity to the CBT model was evaluated using 57 audio-recordings of therapy sessions. Table 18 provides descriptive statistics for the total fidelity ratings.
| Fidelity indicator | CTS-R fidelity (N = 57) |
|---|---|
| Mean (SD) | 43.11 (7.56) |
| Range | 29.75–60.50 |
| Number (%) achieving a pass on the CTS-R | 45 (78.9) |
Subgroup analyses
Results from the subgroup analysis are reported in Figures 5 and 6 for PANSS total. Results from the subgroup analysis for the secondary outcomes QPR, PSP and PSYRATS – auditory hallucinations are in Appendix 2. There was no evidence that the treatment effect was moderated by any of the specified subgroups. Sensitivity analysis for the subgroup LNS including participants who refused at least one question is shown in Appendix 2. Overall, there was no evidence of a statistical difference for any of the subgroups on PANSS total at either time point.
FIGURE 5.
Comparison of CBT and TAU by subgroups. (a) Age, age of onset and sex at 9 months; (b) age, age of onset and sex at 21 months; (c) DI (years), DUP (years), antipsychotic and clozapine daily dose at 9 months; (d) DI (years), DUP (years), antipsychotic and clozapine daily dose at 21 months; (e) difficulty with abstract thinking and conceptual disorganisation at 9 months; (f) difficulty with abstract thinking and conceptual disorganisation at 21 months; (g) childhood trauma at 9 months; (h) childhood trauma at 21 months; (i) AUDIT, DAST and PAM-SR attachment avoidance at 9 months; (j) AUDIT, DAST and PAM-SR attachment avoidance at 21 months; (k) BCSS at 9 months; and (l) BCSS at 21 months.
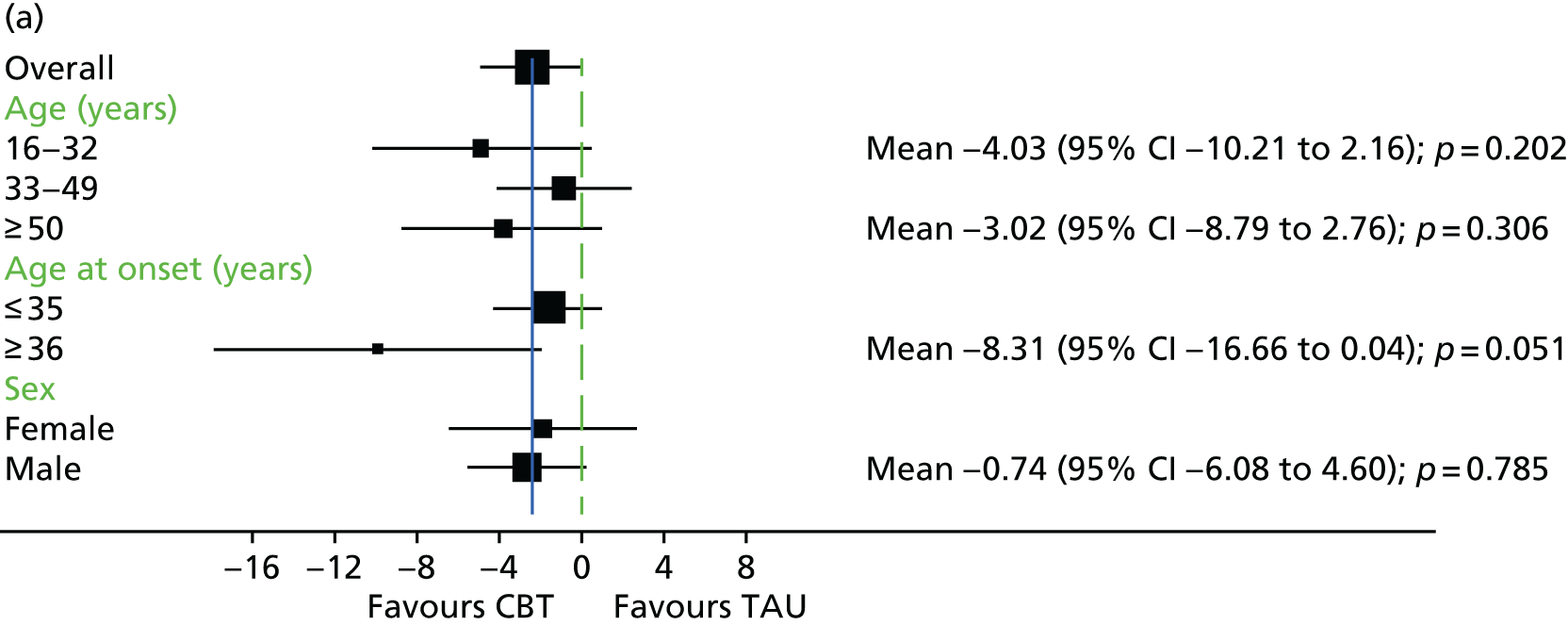
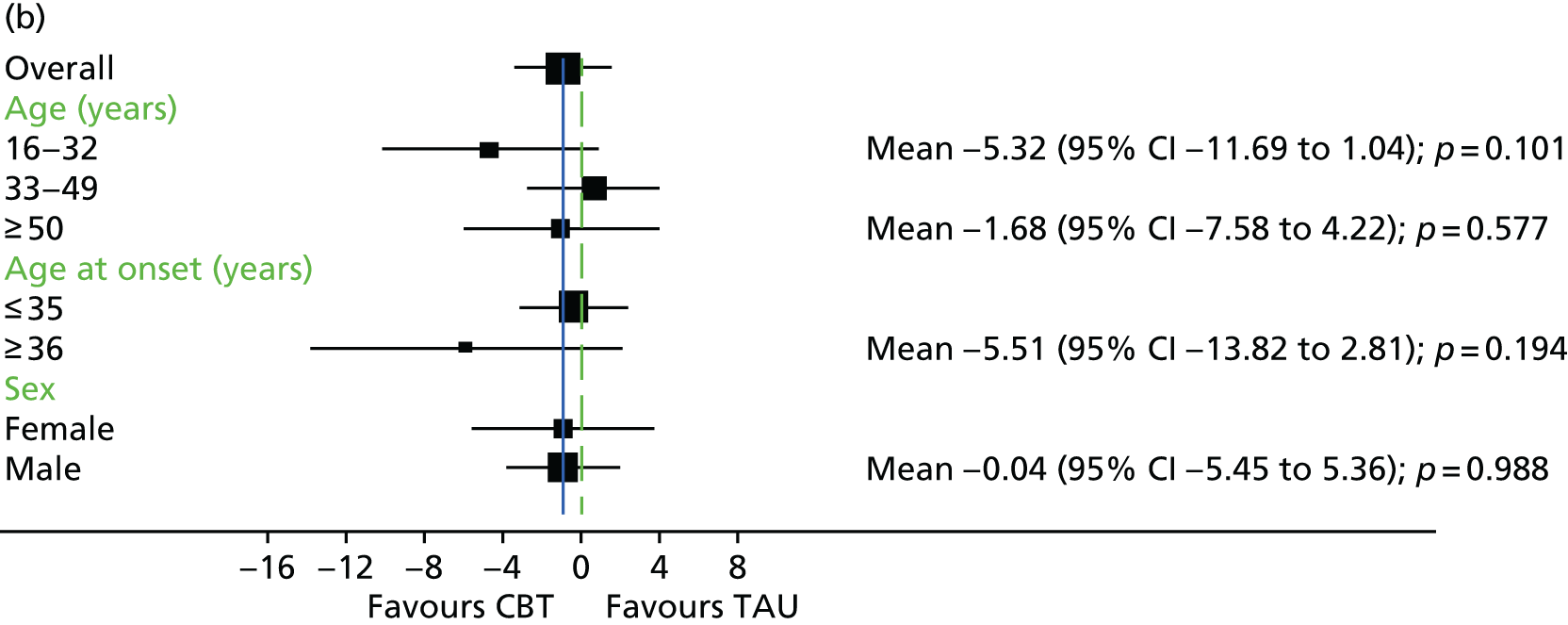
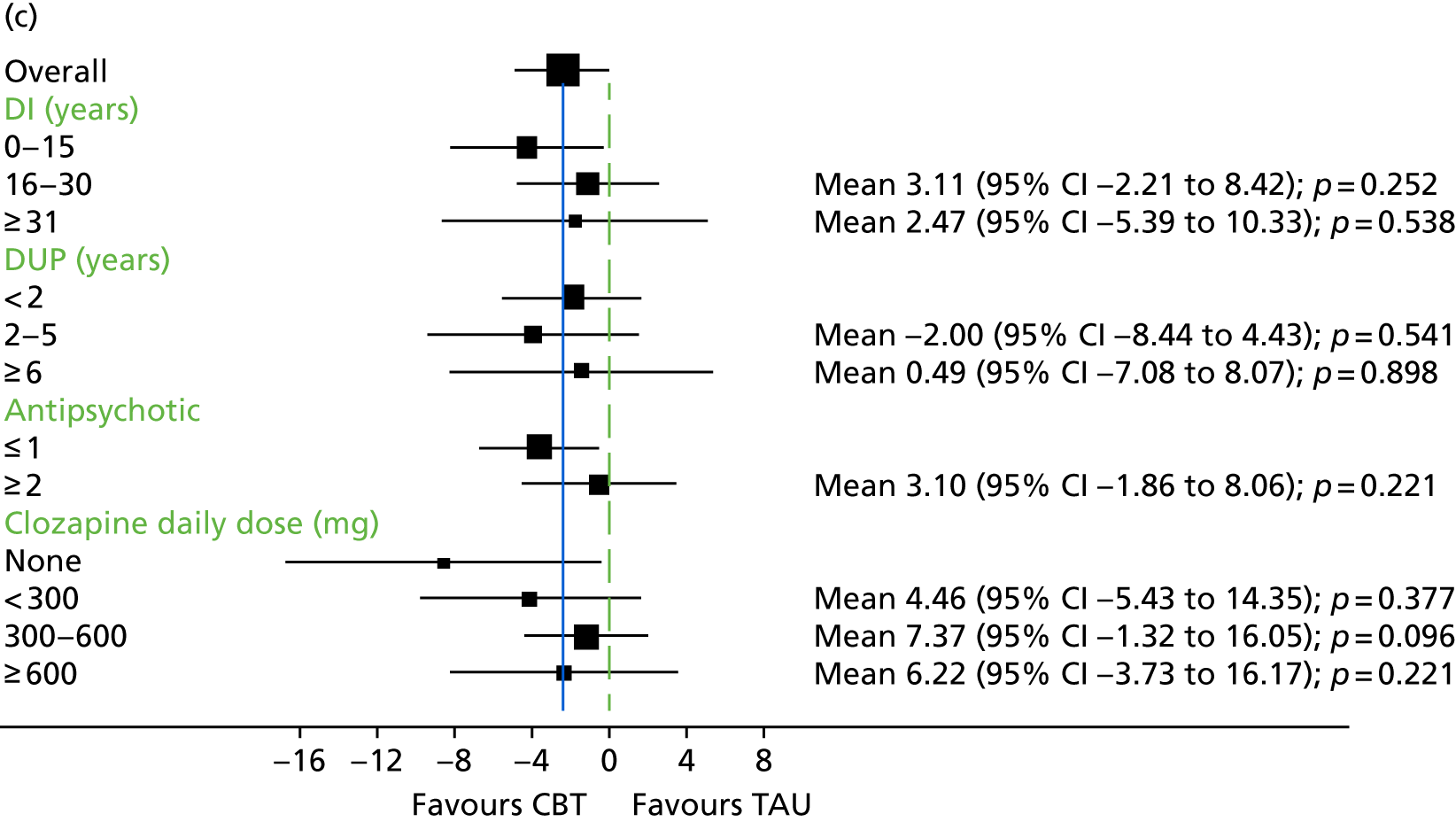
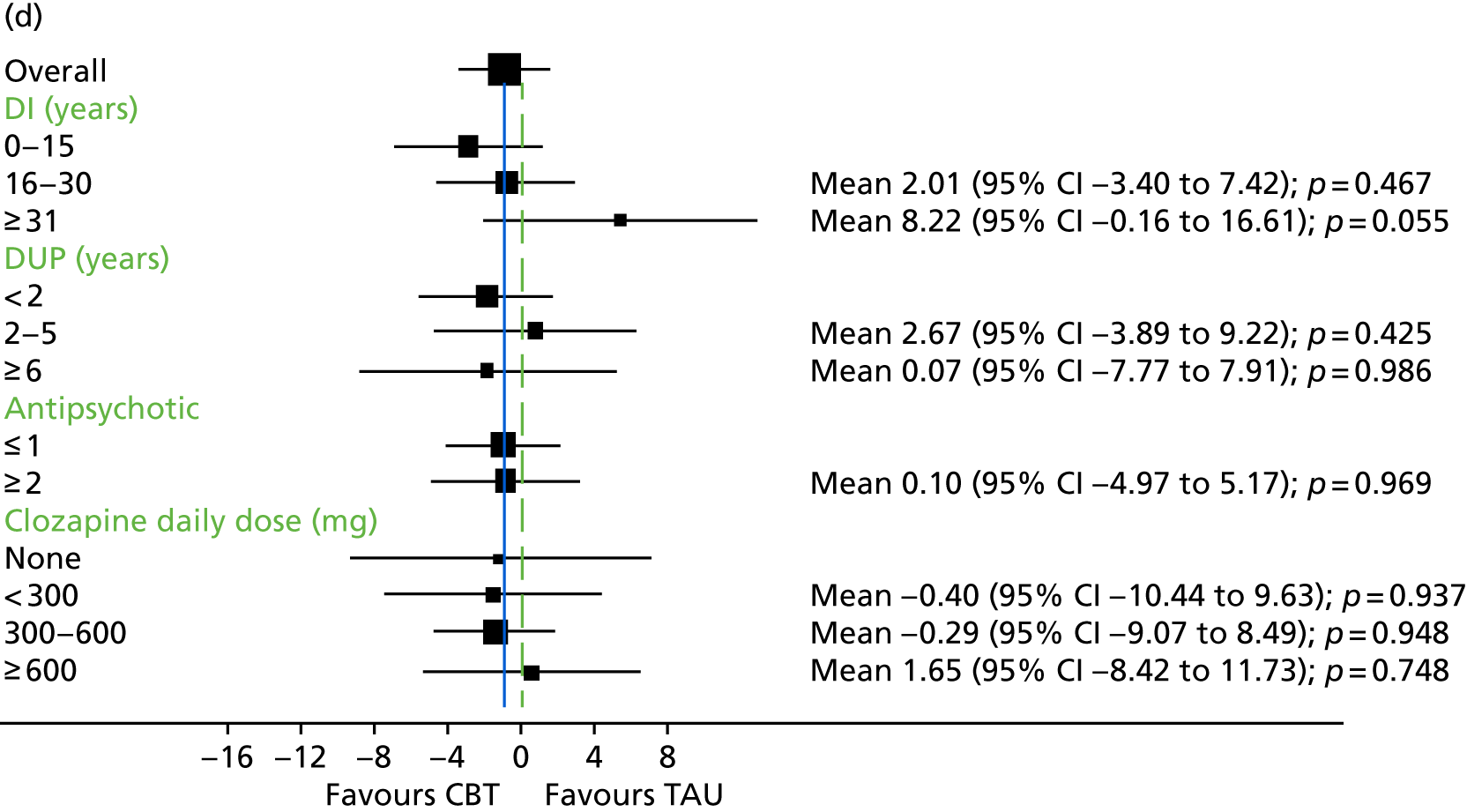
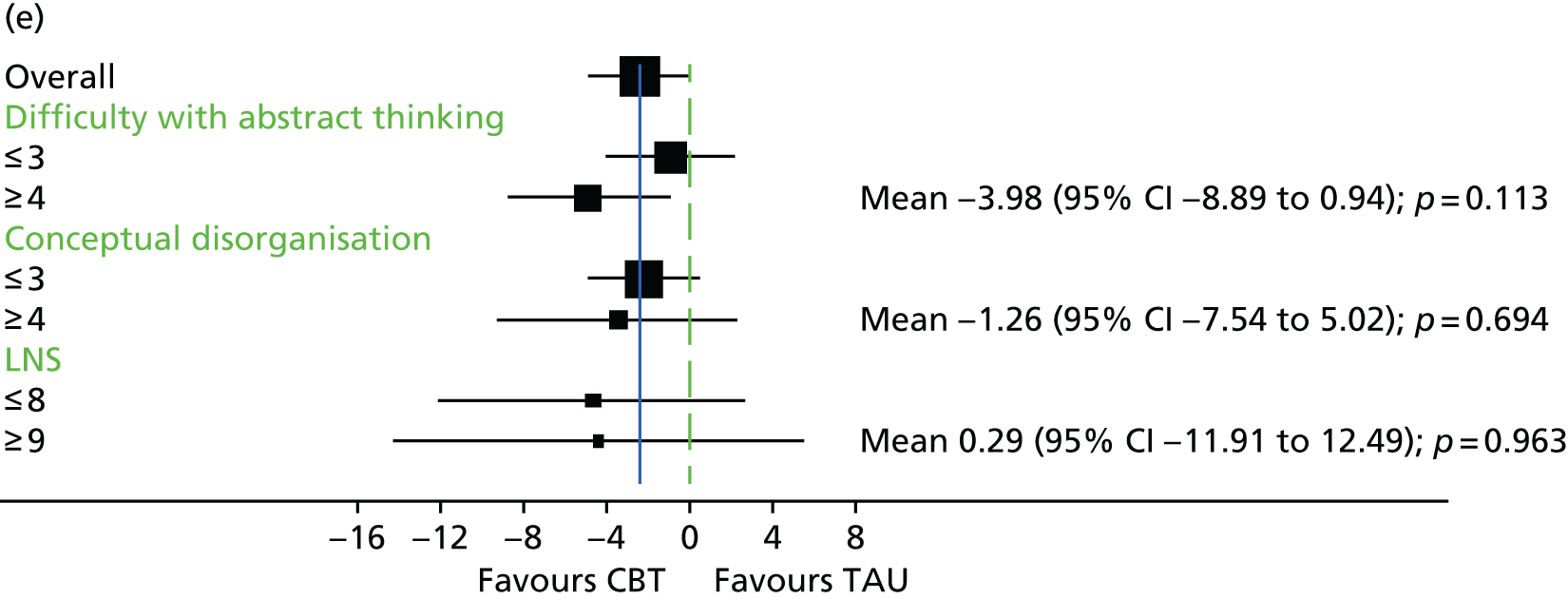
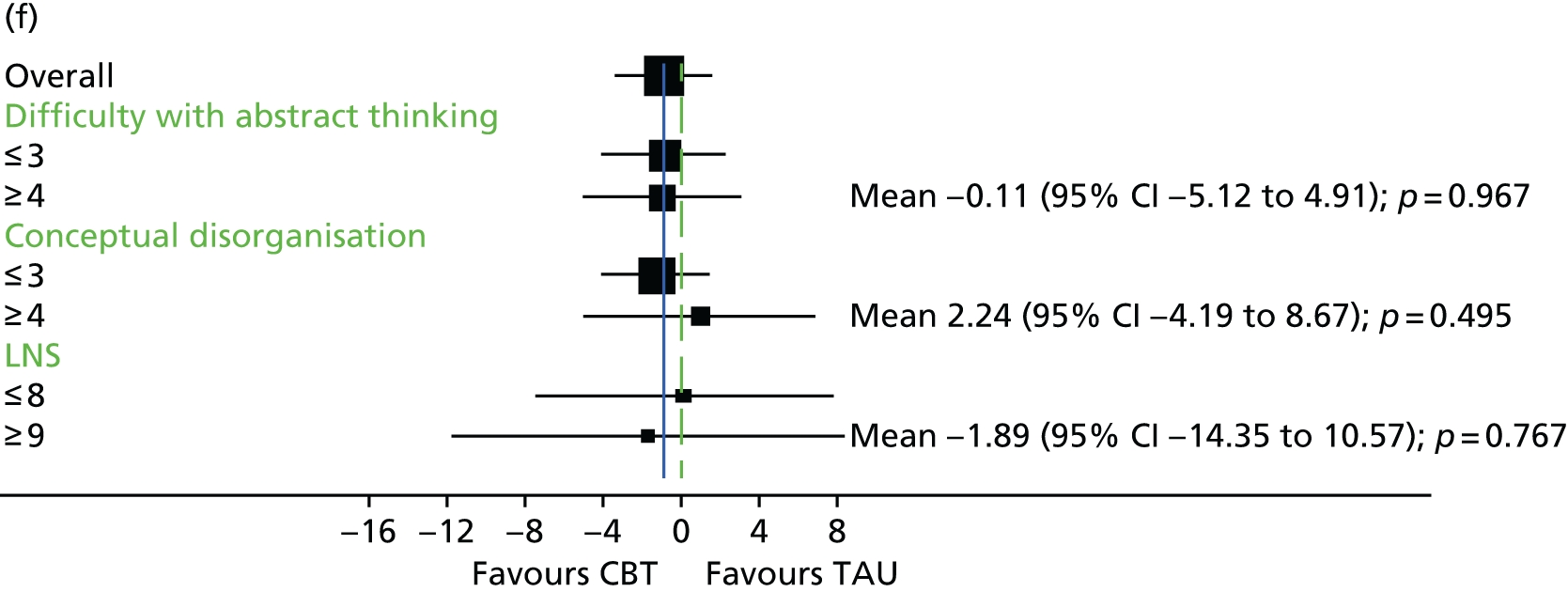
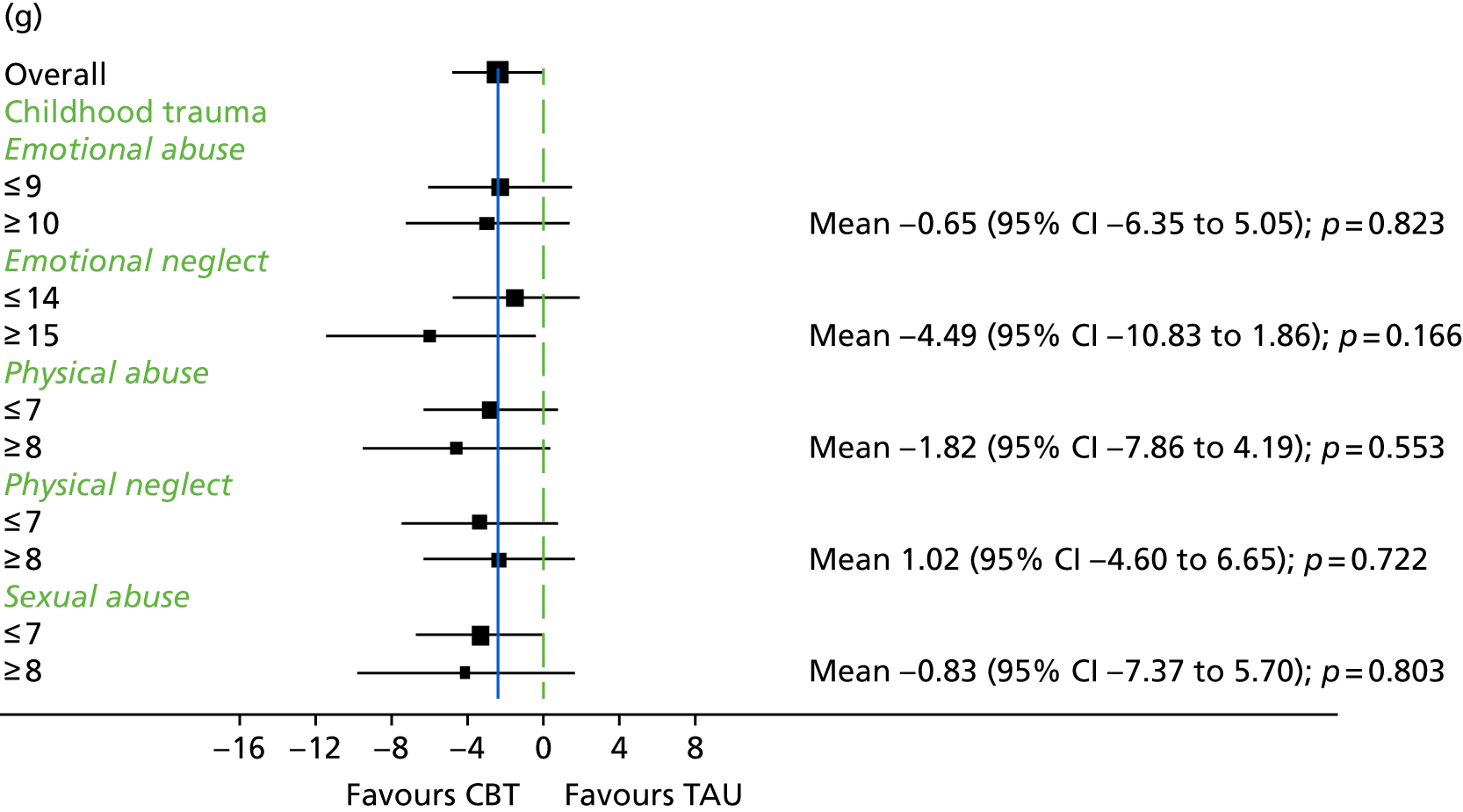
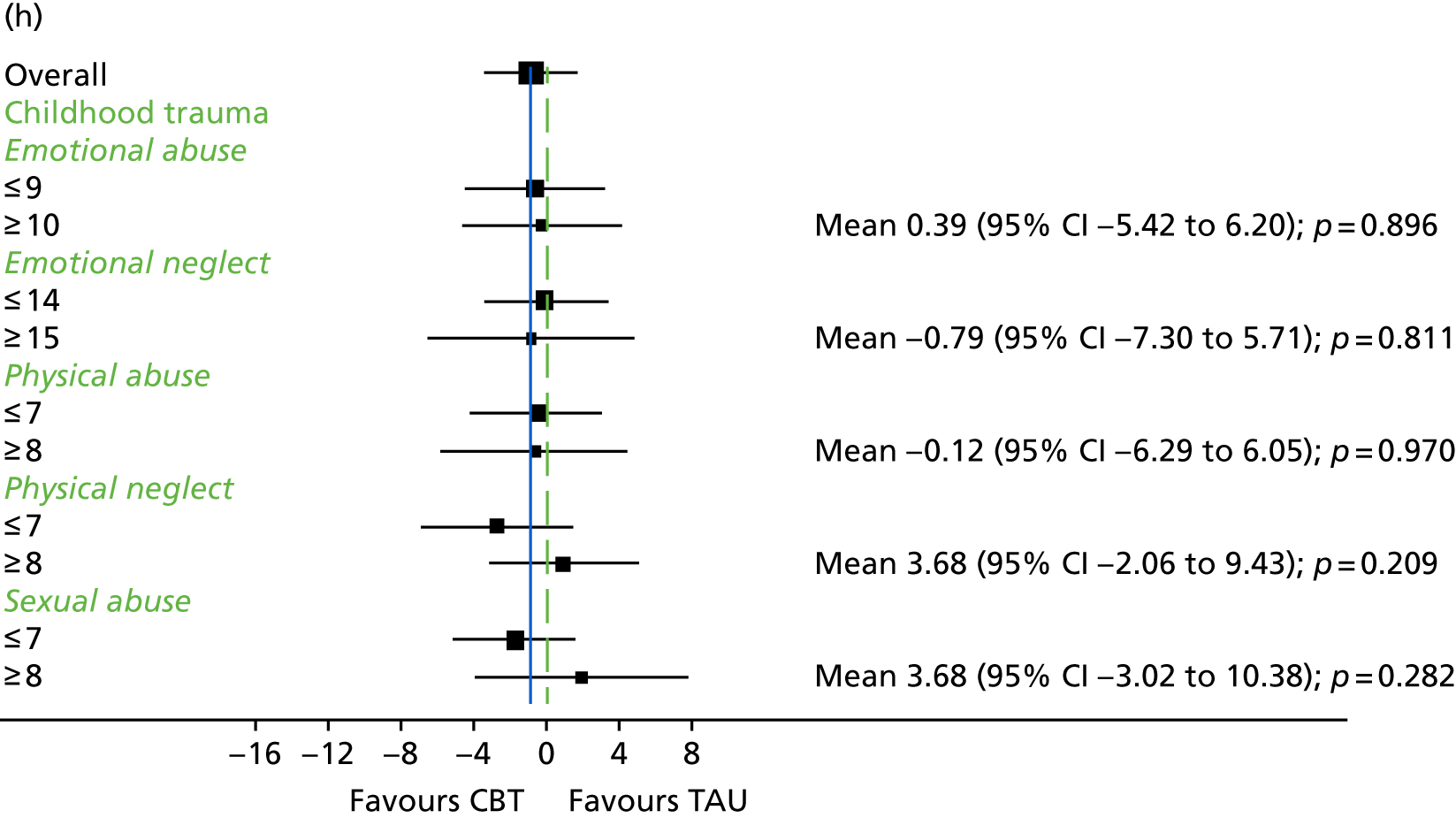
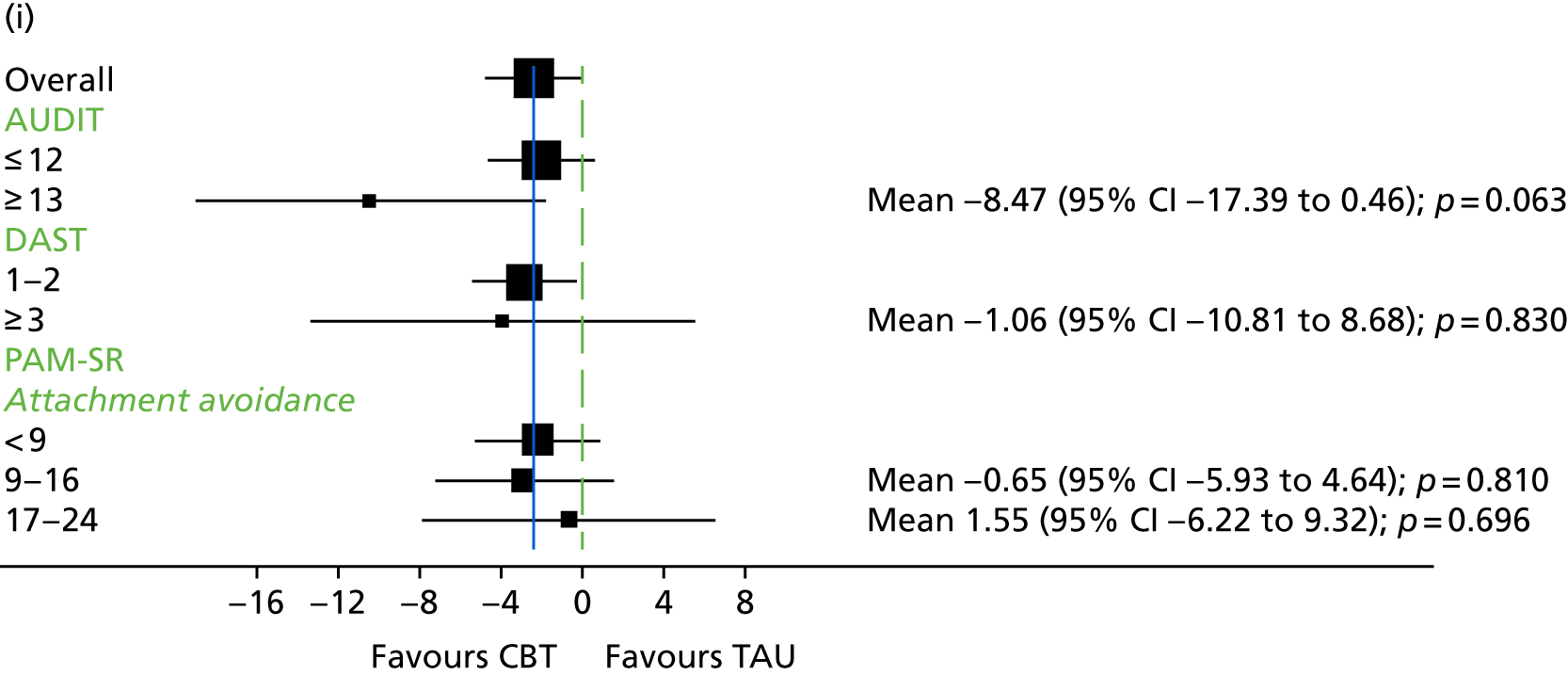
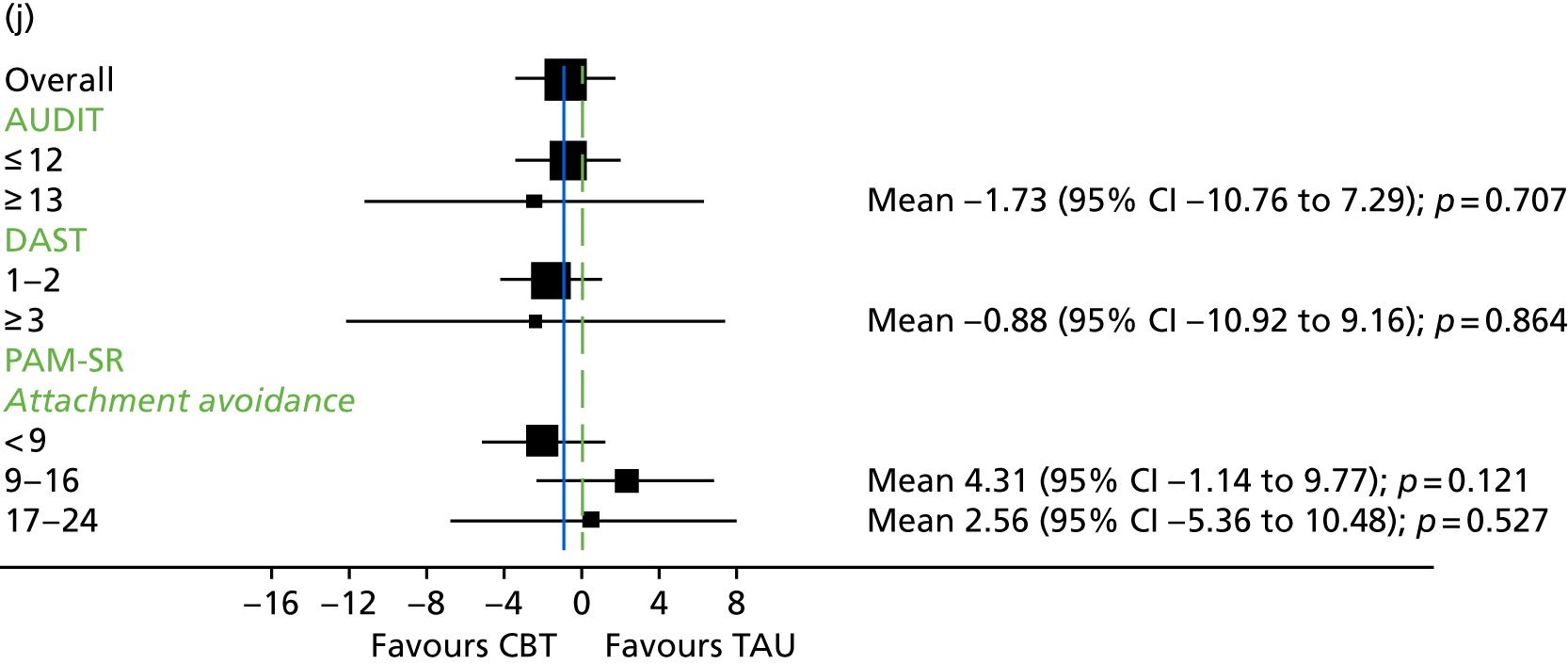
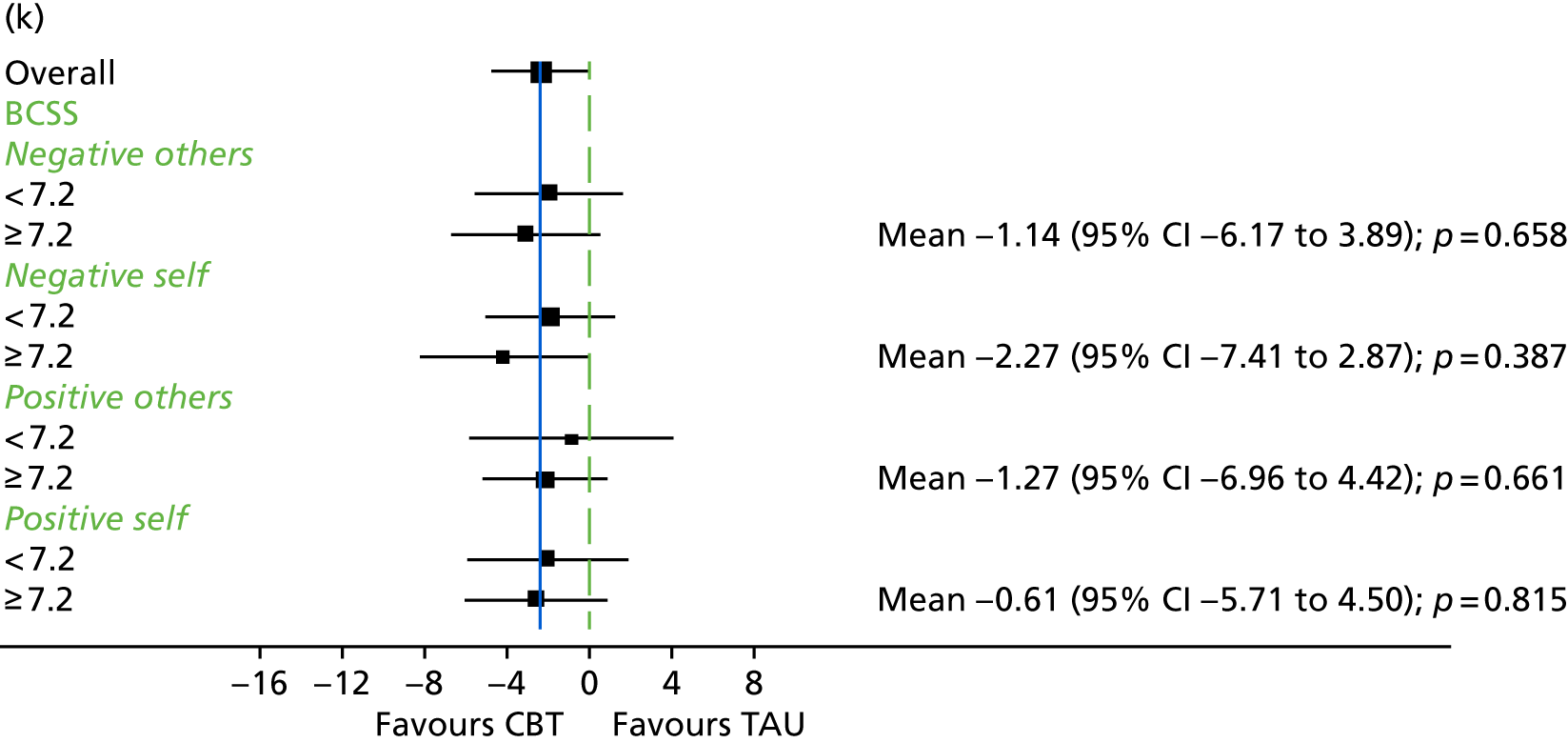
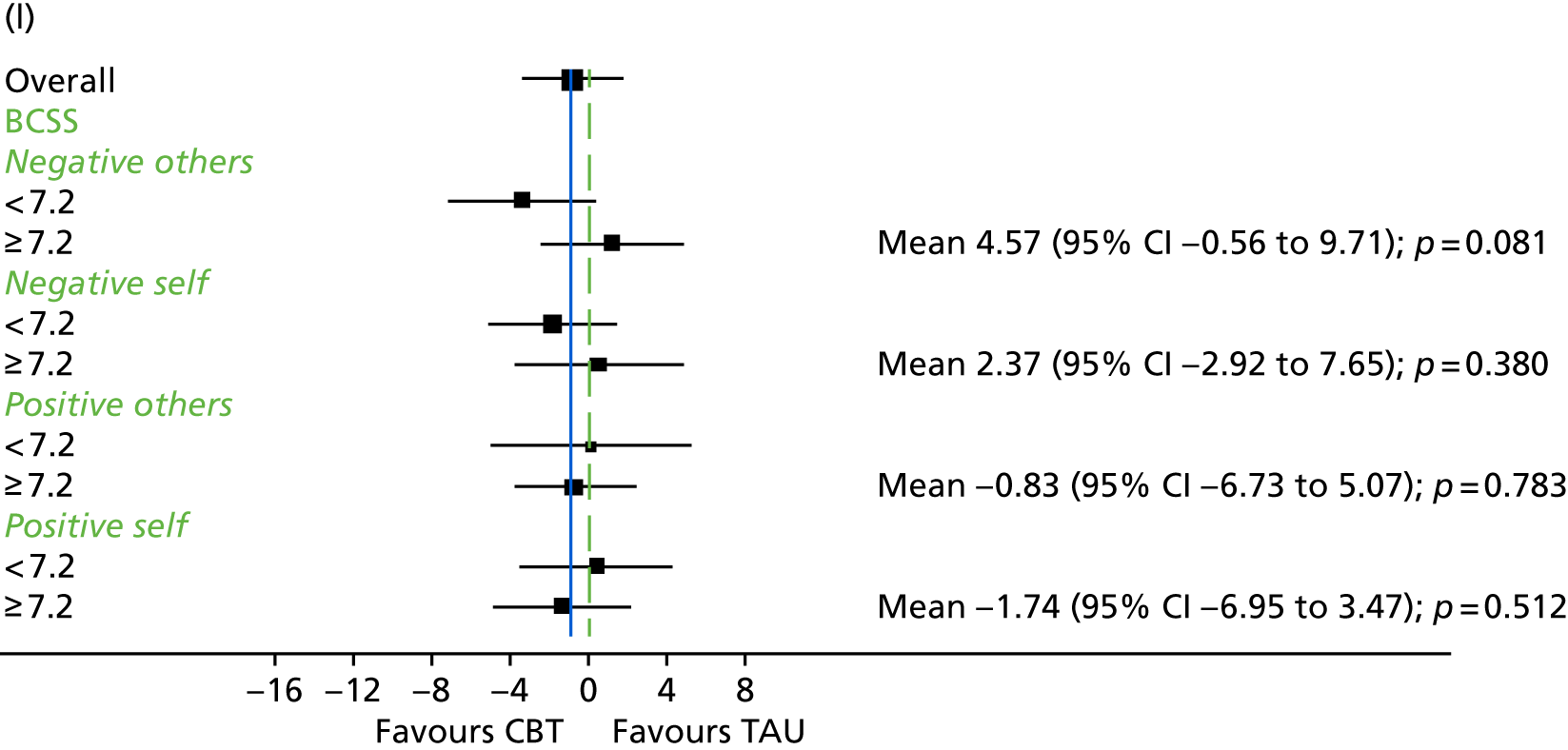
FIGURE 6.
Comparison of CBT and TAU by psychosis subgroup. (a) 9 months; and (b) 21 months.
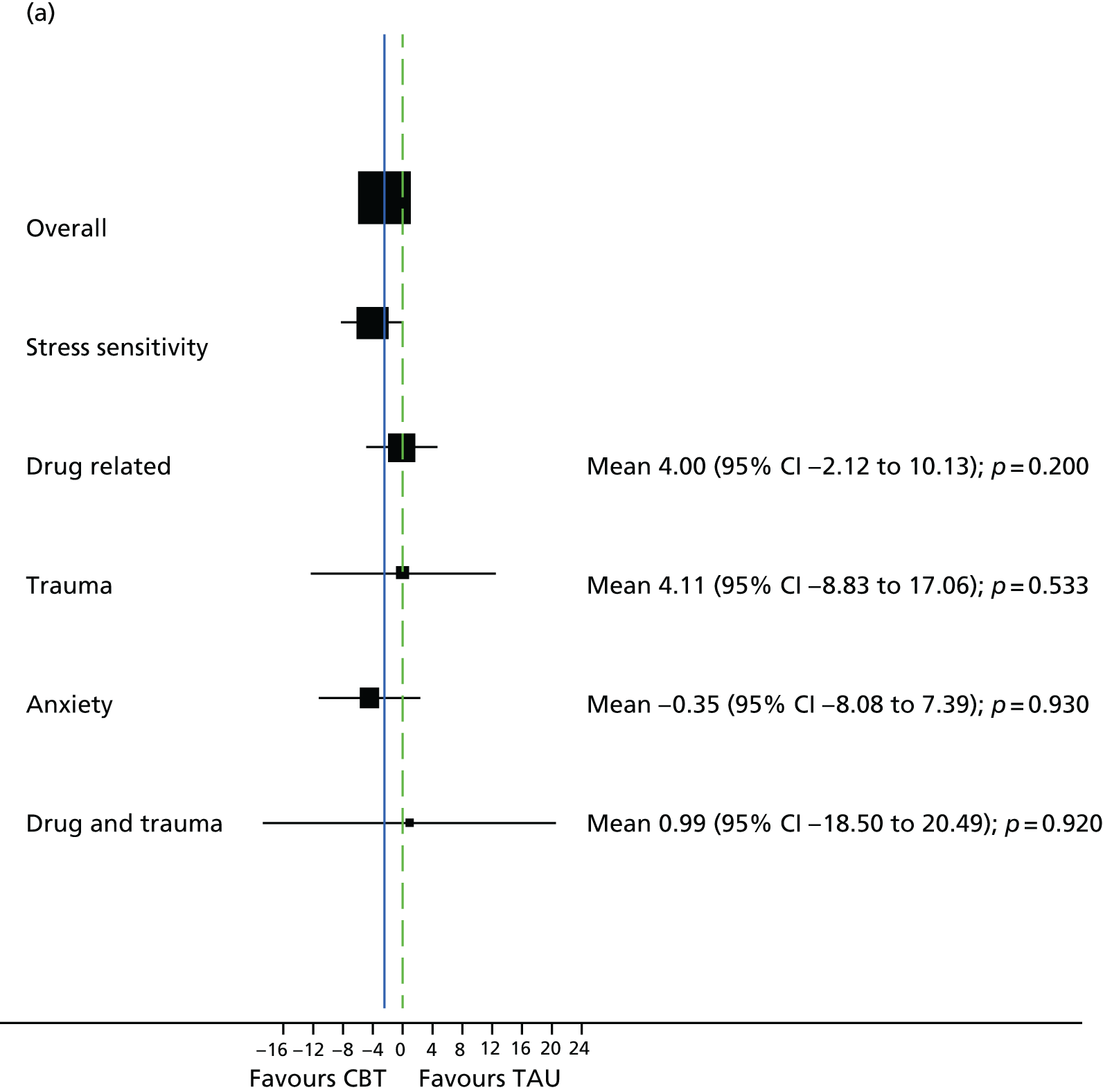
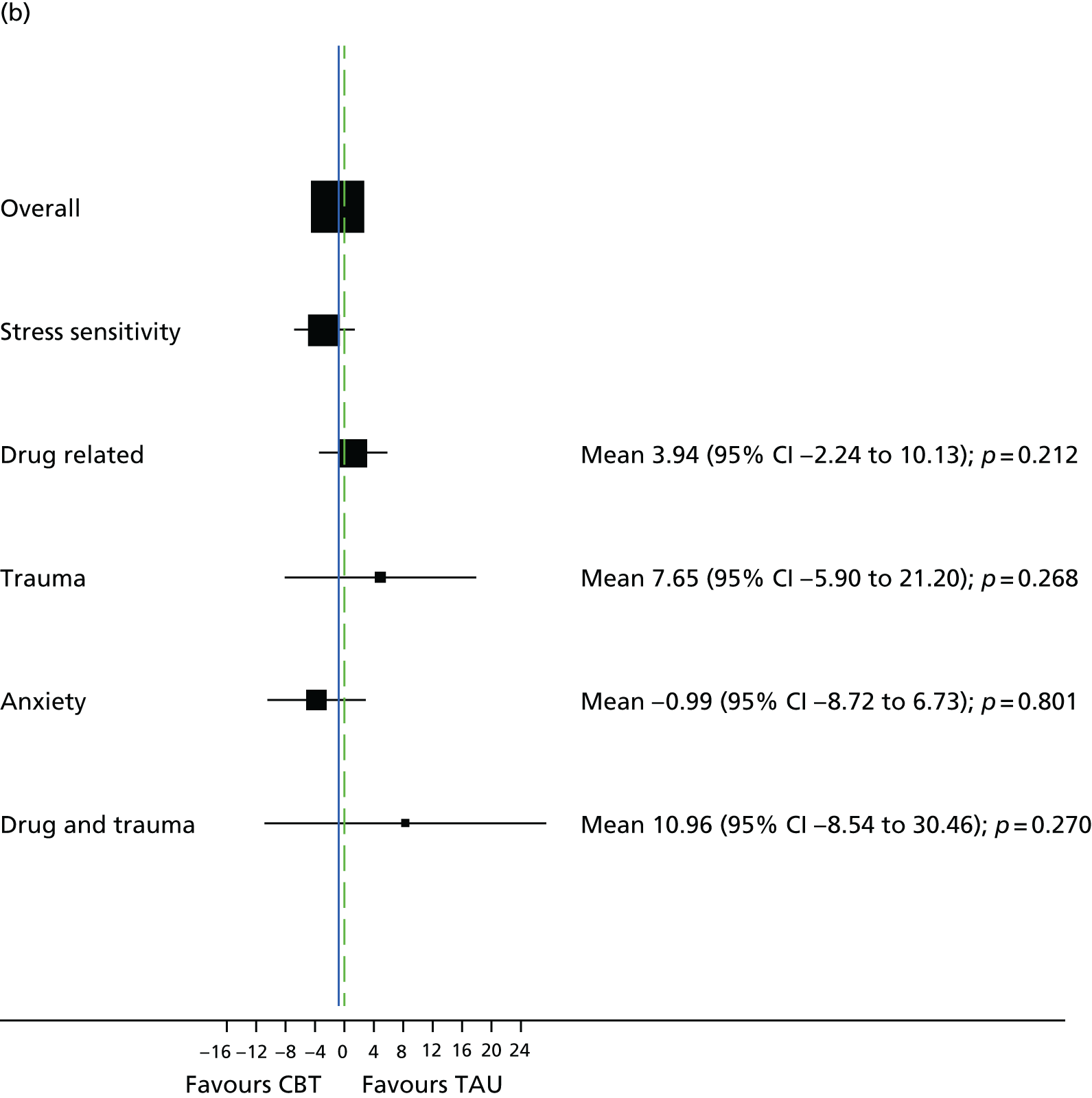
For subgroup analyses of QPR (see Appendix 2), there was evidence of a significant interaction effect at 9 months for the BCSS subscale ‘negative others’, which scored < 7.2, with those subscales that scored ≥ 7.2 (interaction effect –4.96, 95% CI –8.94 to –0.98; p = 0.015). All other subgroups showed no evidence of a difference. Appendix 2 shows the subgroup analysis for PSP. There was evidence of a significant interaction effect for participants with a DI of ≥ 31 years with participants with a DI of 0–15 years at 9 months (interaction effect –9.03, 95% CI –16.62 to –1.98; p = 0.013). For the CTQ physical abuse subscale, there was evidence of a difference at 9 months for participants scoring ≥ 8 with those scoring ≤ 7 (interaction effect 6.29, 95% CI 0.80 to 11.78; p = 0.025).
In the subgroup analyses for PSYRATS – auditory hallucinations (see Appendix 2), there was evidence of a difference for participants with a DI of between 16 and 30 years and those with a DI of between 0 and 15 years at 9 months (interaction effect –7.33, 95% CI –12.41 to –2.25; p = 0.005). For AUDIT, these was evidence of a difference for participants with a score of ≤ 12 and for those with a score of ≤ 12 (interaction) (interaction effect –9.76, 95% CI –18.40 to –1.12; p = 0.027). Furthermore, there was evidence of a difference for PAM-SR attachment avoidance at 9 months for those who scored 17–24 compared with those who scored 1–9 in favour of TAU (interaction effect 8.56, 95% CI 0.73 to 16.30; p = 0.032).
Adverse events and potential unwanted side effects of trial participation
In total, three participants experienced an AE that was deemed to be related to the trial or unclear if related; these AEs were reported to the National Research Ethics Committee (Table 19). Two of the affected participants were in the CBT arm (unclear if related or not) and one was in the TAU arm (related); one AE was categorised as life-threatening and resulted in self-harm, one was an involuntary hospitalisation to a psychiatric hospital and one was self-harm that required treatment at an accident and emergency department.
| Adverse events and effects | Trial arm, n (%) | Odds ratio | 95% CI | p-value | |
|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | ||||
| SAEs | |||||
| Participants who had a trial-related SAE | 2 (0.8) | 1 (0.4) | |||
| Life-threatening/resulted in self-harm | 1 | ||||
| Involuntary hospitalisation | 1 | ||||
| Self-harm that required treatment at A&E | 1 | ||||
| Any AEs | |||||
| Participants who had an AE | 107 (44.2) | 104 (42.4) | 1.09 | 0.81 to 1.46 | 0.58 |
| Total number of AEs | 143 | 120 | |||
| Detailsa | |||||
| Death | 6 | 4 | |||
| Voluntary hospitalisation | 33 | 24 | |||
| Involuntary hospitalisation | 10 | 14 | |||
| Prolongation of hospitalisation | 4 | 2 | |||
| Risk to others | 2 | 0 | |||
| Self-harm | 27 | 6 | |||
| Suicide attempt | 2 | 3 | |||
| Suicidal crisis (CDSS item 8, rating 2) (n/N) | |||||
| 9 months | 12/215 (5.6) | 14/224 (6.3) | 0.90 | 0.40 to 1.10 | 0.79 |
| 21 months | 9/209 (4.3) | 7/214 (3.3) | 1.35 | 0.49 to 3.73 | 0.56 |
| Severe symptomatic exacerbation (n/N) | |||||
| CGI severity ≥ 6 | |||||
| 9 months | 18/207 (8.7) | 25/213 (11.7) | 0.69 | 0.36 to 1.33 | 0.27 |
| 21 months | 19/208 (9.1) | 17/212 (8.0) | 1.16 | 0.57 to 2.33 | 0.69 |
| CGI improvement ≥ 6b | |||||
| 9 months | 0/131 | 5/147 (3.4) | 0.06 | ||
| 21 months | 3/141 (2.1) | 6/157 (3.8) | 0.45 | 0.10 to 2.02 | 0.30 |
| Deterioration in PANSS total (n/N) | |||||
| > 25% | |||||
| 9 months | 22/218 (10.1) | 28/224 (12.5) | 0.75 | 0.41 to 1.38 | 0.35 |
| 21 months | 15/209 (7.2) | 21/216 (9.7) | 0.68 | 0.33 to 1.37 | 0.28 |
| > 50% | |||||
| 9 months | 6/218 (2.8) | 7/224 (3.1) | 0.77 | 0.25 to 2.43 | 0.66 |
| 21 months | 8/209 (3.8) | 8/216 (3.7) | 0.90 | 0.32 to 2.55 | 0.84 |
| > 75% | |||||
| 9 months | 2/218 (0.9) | 1/224 (0.4) | 1.78 | 0.15 to 20.95 | 0.65 |
| 21 months | 1/209 (0.5) | 3/216 (1.4) | 0.26 | 0.03 to 2.73 | 0.26 |
There were 107 participants (44.2%) in the CBT arm and 104 (42.4%) in the TAU arm who reported at least one AE or adverse effect; there was no significant difference between the groups in the number of people with at least one AE or adverse effect (p = 0.58) (see Table 19). The main reasons were voluntary hospitalisation to a psychiatric hospital, self-harm, CGI severity of > 6 and > 25% deterioration on the PANSS. In the CBT group, 22 of the 27 reported self-harm events were from the same participant. There were six deaths in the CBT arm and four in the TAU arm; all were deemed to be unrelated to the study.
At 9 months, 22 participants (9%) in the CBT arm and 28 (11%) in the TAU arm had a deterioration in PANSS total score of > 25%. The number of deteriorations at > 50% and > 75% was similar in both groups at 9 and 21 months.
The frequency of participants responding ‘quite a lot’ or ‘very much’ to each of the items on the bespoke adverse effects measure developed for the FOCUS trial can be found in Appendix 2. There were no significant differences between the groups for any of the 27 items in the adverse effects measure.
Internalised stigma of mental illness
Levels of ISMI subscales for the whole sample at baseline are reported in Table 20. For the ISMI alienation scale, 201 participants (41%) had a severe level, and 225 participants (46%) had moderate levels of stigma resistance.
| ISMI subscales | Participants, n (%) (N = 487) | ISMI scale levels, n (%) | |||
|---|---|---|---|---|---|
| Minimal | Low | Moderate | Severe | ||
| ISMI alienation | 435 (89.3) | 65 (13.3) | 95 (19.5) | 126 (25.9) | 201 (41.3) |
| ISMI stereotype endorsement | 435 (89.3) | 186 (38.2) | 149 (30.6) | 76 (15.6) | 76 (15.6) |
| ISMI discrimination experience | 434 (89.1) | 104 (21.4) | 99 (20.3) | 155 (31.8) | 129 (26.5) |
| ISMI social withdrawal | 435 (89.3) | 76 (15.6) | 122 (25.1) | 142 (29.2) | 147 (30.2) |
| ISMI stigma resistance | 433 (88.9) | 46 (9.4) | 85 (17.5) | 225 (46.2) | 131 (26.9) |
Figure 7 shows the profile for the ISMI subscales for the two treatment groups over the study period and Table 21 shows the treatment effects. At 9 months, ISMI discrimination experience was lower in the CBT arm than the TAU arm (–0.13, 95% CI –0.25 to –0.01; p = 0.029). For the subgroup analysis on the PANSS total, only stigma resistance at 9 months showed evidence of a significant interaction effect (Figure 8).
FIGURE 7.
Profile of ISMI subscales. (a) ISMI alienation; (b) ISMI sterotype endorsement; (c) ISMI discrimination experience; (d) ISMI social withdrawal; (e) ISMI stigma resistance; and (f) ISMI total.
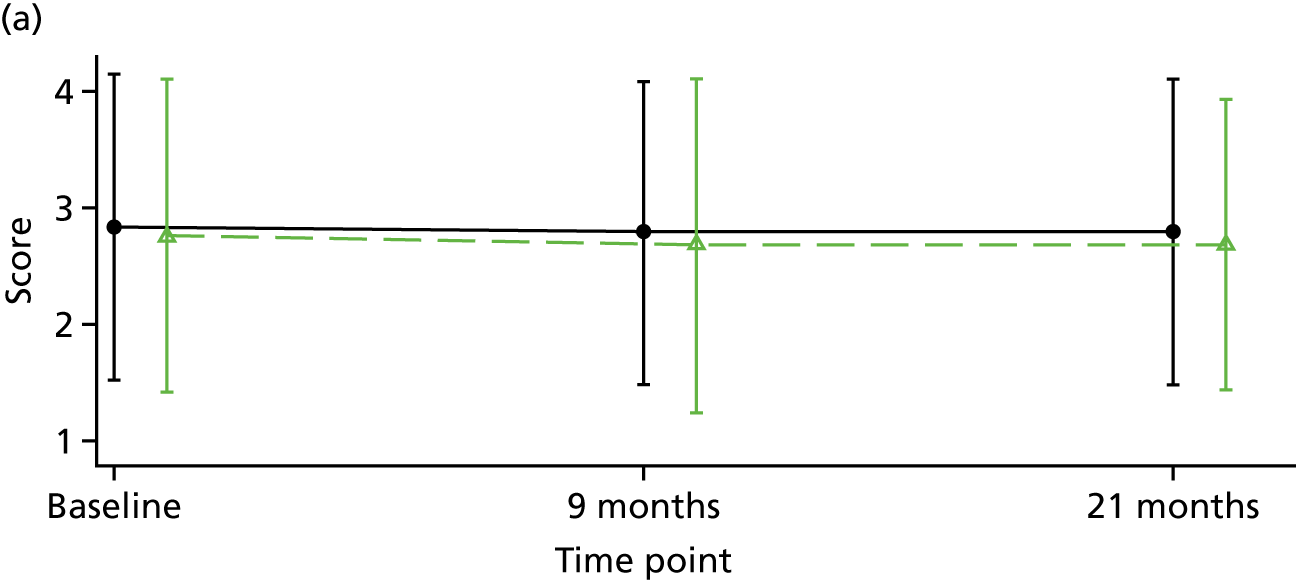
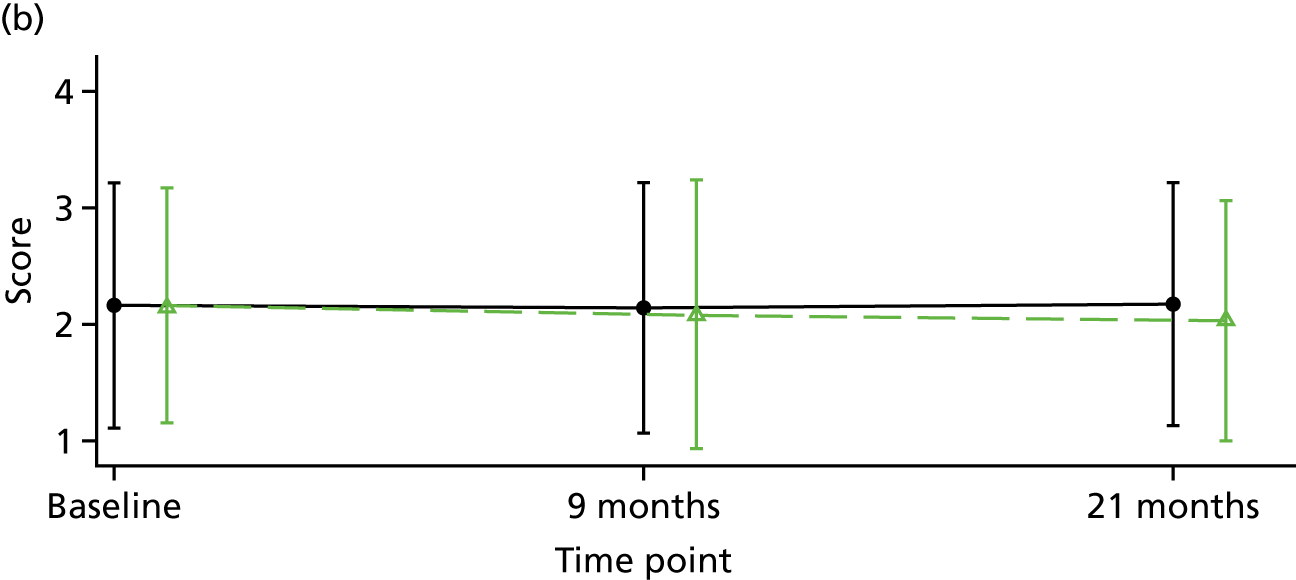
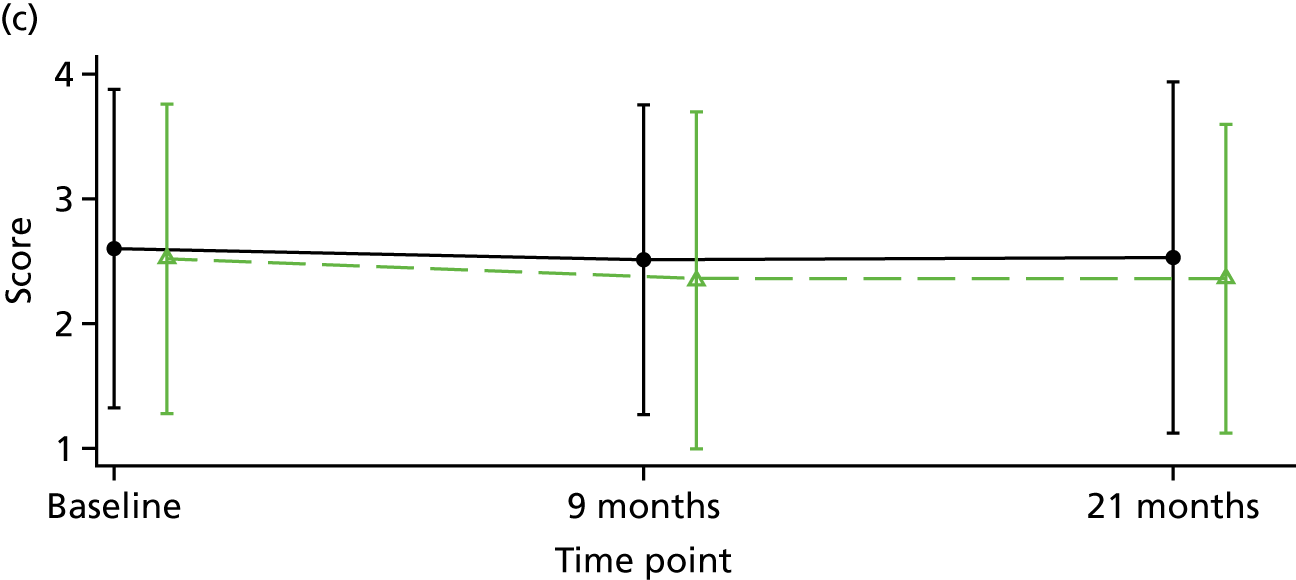
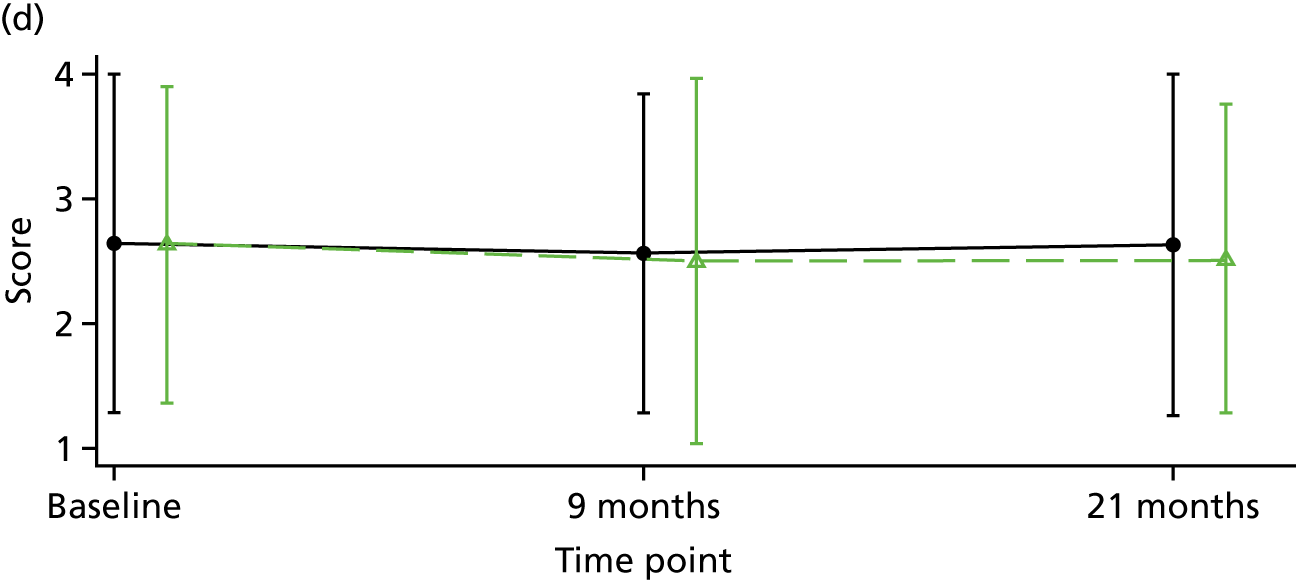
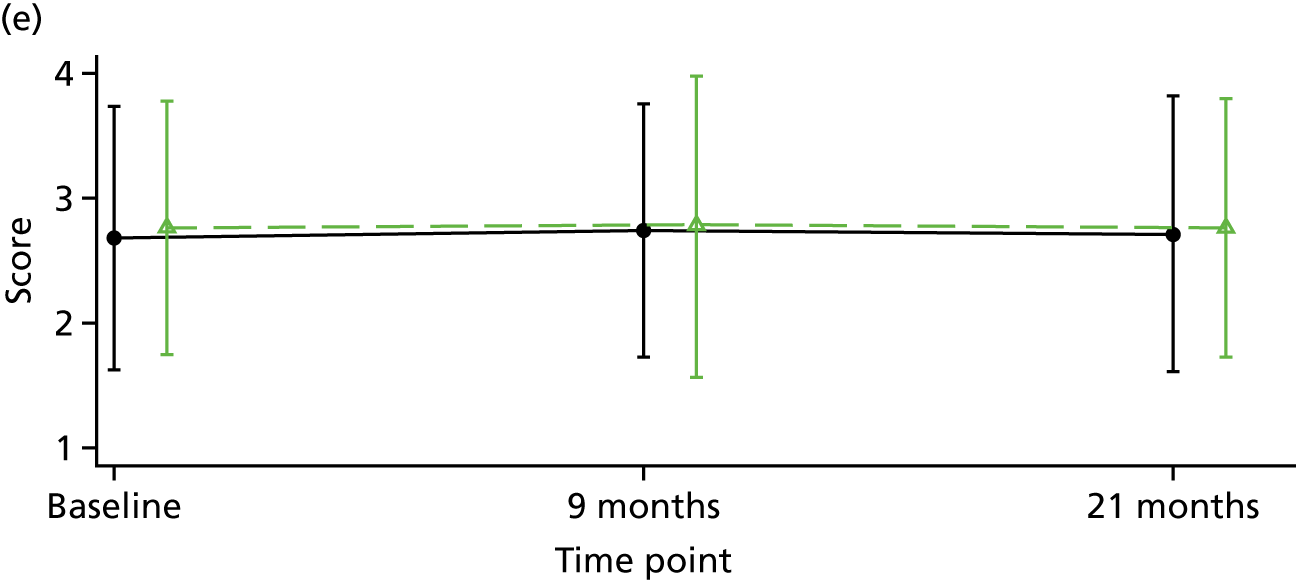
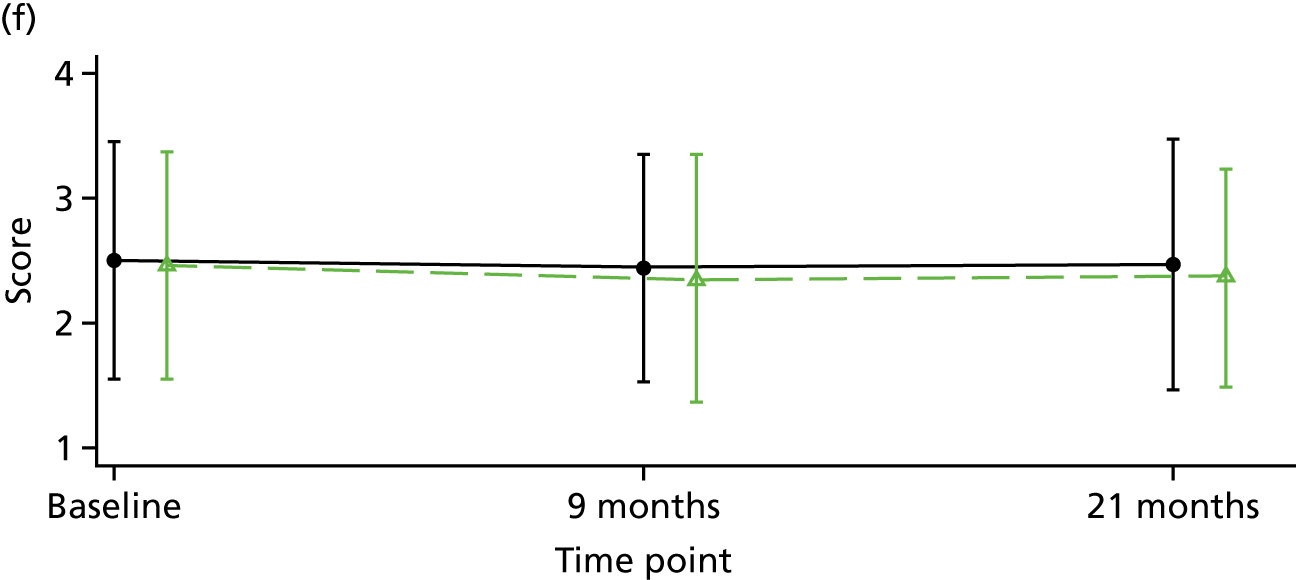
| ISMI subscale | Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | |
|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | ||||
| Alienation | |||||
| Baseline | 2.76 (0.69); 218 | 2.84 (0.68); 217 | |||
| 9 months | 2.68 (0.74); 179 | 2.79 (0.67); 198 | –0.08 | –0.19 to 0.03 | 0.169 |
| 21 months | 2.68 (0.64); 164 | 2.80 (0.68); 180 | –0.04 | –0.16 to 0.08 | 0.513 |
| Stereotype endorsement | |||||
| Baseline | 2.16 (0.51); 218 | 2.16 (0.54); 217 | |||
| 9 months | 2.08 (0.59); 178 | 2.14 (0.55); 198 | –0.06 | –0.16 to 0.03 | 0.177 |
| 21 months | 2.03 (0.53); 164 | 2.17 (0.54); 180 | –0.08 | –0.17 to 0.02 | 0.128 |
| Discrimination experience | |||||
| Baseline | 2.52 (0.63); 218 | 2.60 (0.66); 216 | |||
| 9 months | 2.34 (0.69); 177 | 2.50 (0.64); 197 | –0.13 | –0.25 to –0.01 | 0.029 |
| 21 months | 2.36 (0.63); 163 | 2.52 (0.72); 180 | –0.05 | –0.18 to 0.07 | 0.397 |
| Social withdrawal | |||||
| Baseline | 2.62 (0.65); 218 | 2.64 (0.69); 217 | |||
| 9 months | 2.50 (0.74); 177 | 2.56 (0.65); 198 | –0.07 | –0.19 to 0.05 | 0.241 |
| 21 months | 2.52 (0.63); 164 | 2.63 (0.70); 180 | –0.10 | –0.23 to 0.02 | 0.095 |
| Stigma resistance | |||||
| Baseline | 2.77 (0.51); 217 | 2.68 (0.53); 216 | |||
| 9 months | 2.78 (0.61); 177 | 2.74 (0.52); 198 | 0.02 | –0.09 to 0.12 | 0.747 |
| 21 months | 2.76 (0.53); 163 | 2.71 (0.56); 180 | 0.02 | –0.09 to 0.13 | 0.681 |
| Total | |||||
| Baseline | 2.46 (0.46); 218 | 2.50 (0.48); 217 | |||
| 9 months | 2.36 (0.51); 179 | 2.44 (0.46); 198 | –0.07 | –0.14 to 0.01 | 0.090 |
| 21 months | 2.37 (0.44); 165 | 2.48 (0.51); 180 | –0.04 | –0.12 to 0.04 | 0.303 |
FIGURE 8.
The PANSS total subgroups. (a) Alienation, stereotype endorsement and discrimination experience at 9 months; (b) alienation, stereotype endorsement and discrimination experience at 21 months; (c) social withdrawal and stigma resistance at 9 months; and (d) social withdrawal and stigma resistance at 21 months.
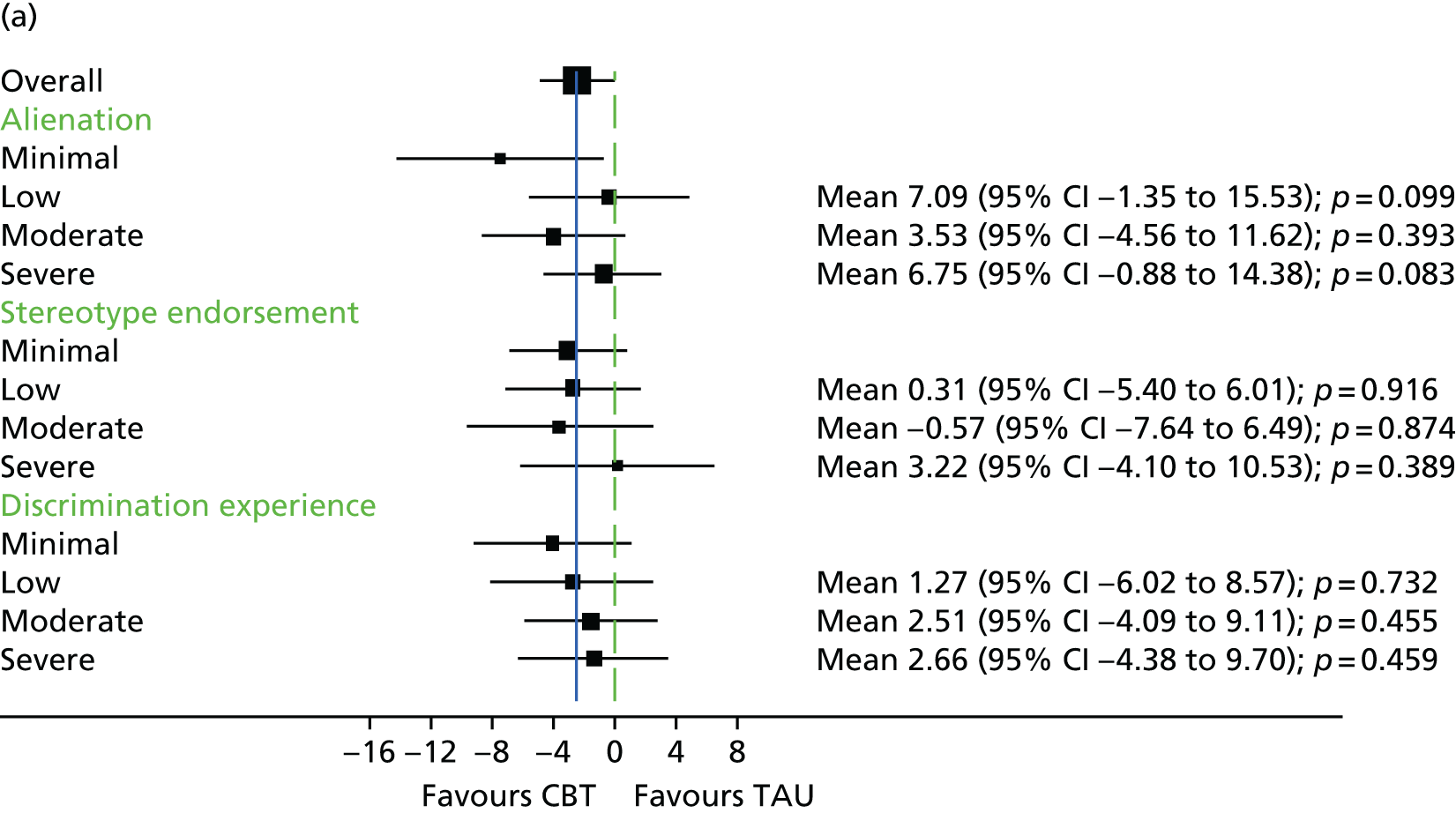
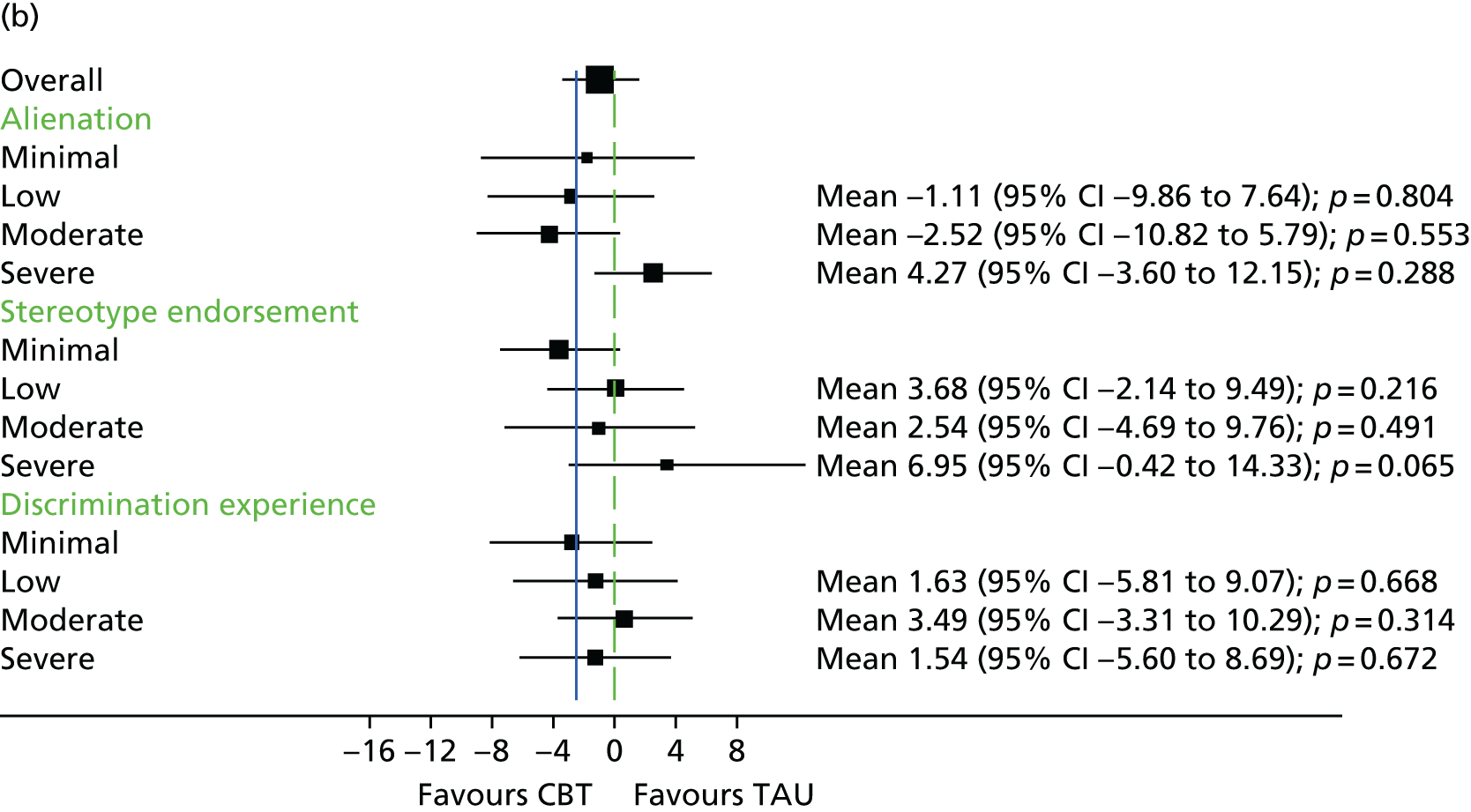
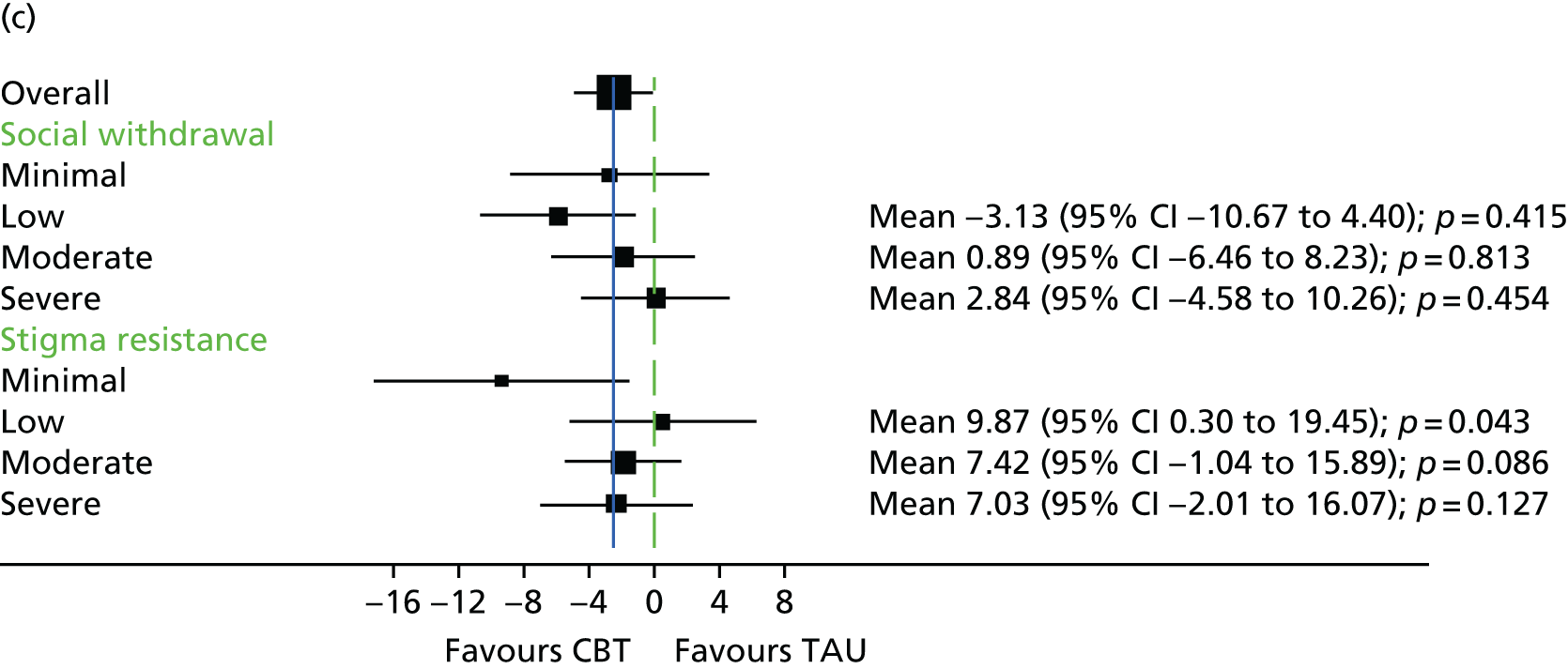
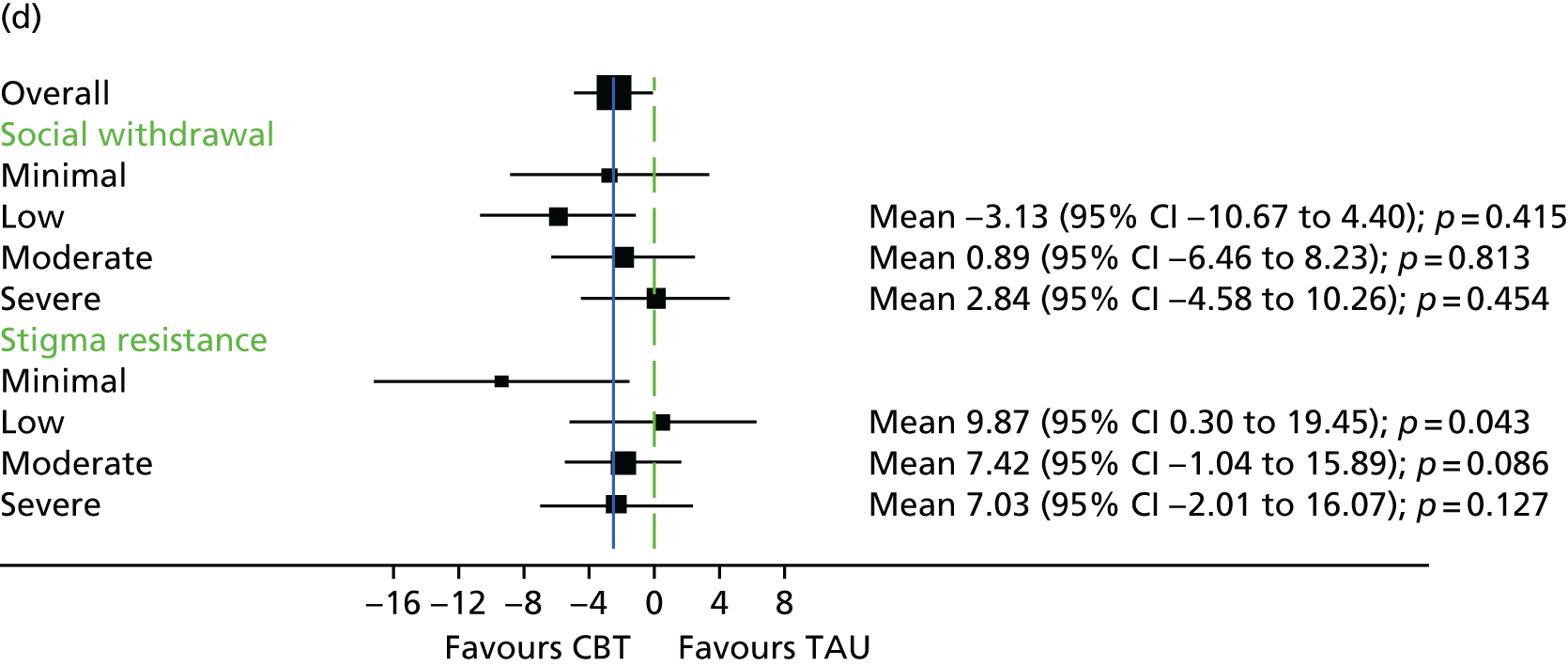
Chapter 5 Economic evaluation results
Chapter overview
This chapter describes the results of the economic evaluation conducted as part of the FOCUS trial of CBT compared with TAU for people who cannot tolerate or have had an inadequate response to clozapine. The economic evaluation uses service use and health status data collected in the RCT to compare the costs and QALYs of CBT with those of TAU, and estimate the cost per QALY gained. The analysis used the viewpoint of NHS health and social care service providers (costs) and patients (health benefits) for the 21-month follow-up period of the trial. Full methods are provided in Chapter 2.
Missing cost and utility data
Table 22 summarises the number of participants with complete cost or utility data at baseline and at 9- and 21-month follow-up. Overall, complete cost and QALY data at 9-month follow-up were available for 126 out of 245 participants (51%) in the TAU arm, compared with 114 out of 242 participants (47%) in the CBT group. At 21-month follow-up, complete cost and QALY data were available for 93 out of 245 participants (38%) in the TAU arm, compared with 76 out of 242 participants (31%) in the CBT group.
| Study follow-up | Available data, n (%) | |||
|---|---|---|---|---|
| CBT arm (N = 242) | TAU arm (N = 245) | |||
| Cost | Utility | Cost | Utility | |
| Baseline overall | 195 (81) | 223 (92) | 195 (80) | 230 (94) |
| Hospital inpatient stay (psychiatric) | 235 (97) | Not relevant | 236 (96) | Not relevant |
| Hospital inpatient stay (other) | 202 (83) | Not relevant | 205 (84) | Not relevant |
| Hospital outpatient, day and A&E care | 237 (98) | Not relevant | 239 (98) | Not relevant |
| Primary, community and social care | 232 (96) | Not relevant | 238 (97) | Not relevant |
| Baseline to 9 months | 135 (56) | 187 (77) | 144 (59) | 205 (84) |
| Hospital inpatient stay (psychiatric) | 214 (88) | Not relevant | 215 (88) | Not relevant |
| Hospital inpatient stay (other) | 150 (62) | Not relevant | 155 (63) | Not relevant |
| Hospital outpatient, day and A&E care | 178 (74) | Not relevant | 182 (74) | Not relevant |
| Primary, community and social care | 168 (69) | Not relevant | 175 (71) | Not relevant |
| Baseline to 21 months | 103 (43) | 180 (74) | 110 (45) | 189 (77) |
| Hospital inpatient stay (psychiatric) | 203 (84) | Not relevant | 195 (80) | Not relevant |
| Hospital inpatient stay (other) | 122 (50) | Not relevant | 120 (49) | Not relevant |
| Hospital outpatient, day and A&E care | 153 (63) | Not relevant | 145 (59) | Not relevant |
| Primary, community and social care | 139 (57) | Not relevant | 137 (56) | Not relevant |
Baseline clinical and demographic characteristics
Sociodemographic characteristics for the participants were reported in Table 5. The data suggested that the two groups were similar at baseline.
Table 23 reports key baseline demographic and clinical characteristics that are statistically significantly associated with either baseline utility or baseline cost data (Kendall’s τ-coefficient).
| Demographic or clinical characteristic | Kendall’s τ-coefficient; p-value (n) | |
|---|---|---|
| Cost | Utility | |
| EQ VAS | NSS | 0.346; < 0.001 (450) |
| Age | NSS | –0.177; < 0.001 (453) |
| Years of full-time education | NSS | 0.072; 0.043 (419) |
| Gender | NSS | –0.089; 0.022 (453) |
| Ethnicity (white British vs. non-white British) | NSS | 0.105; 0.007 (453) |
| Consider themselves to be experiencing mental health problems | NSS | –0.156; < 0.001 (444) |
| Duration of illness in months | 0.083; 0.020 (366) | –0.129; < 0.001 (426) |
| Impact of health on socially useful activities, including work and study | NSS | –0.143; < 0.001 (453) |
| Impact of health on personal and social relationships | NSS | –0.107; 0.006 (453) |
| Taking clozapine | 0.131; 0.002 (390) | –0.087; 0.024 (453) |
| Taking antidepressant medication | NSS | –0.150; < 0.001 (453) |
| Taking benzodiazepine medication | NSS | –0.077; 0.047 (453) |
| Total PSP score | –0.070; 0.042 (390) | 0.124; < 0.001 (453) |
| Total PANSS score | NSS | –0.189; < 0.001 (453) |
| Total QPR score | NSS | 0.328; < 0.001 (426) |
| Total CDSS score | 0.113; 0.002 (376) | –0.308; < 0.001 (439) |
| Study centre | NSS | 0.115; 0.001 (453) |
Stepwise linear regression (SPSS version 22), using all the characteristics in Table 23, was used to identify the key characteristics to include in the MI of missing data and as covariates for the primary and sensitivity analyses.
Table 24 shows the results of the regression analyses for the characteristics that were statistically associated with utility (p ≤ 0.10) and included as covariates in the analyses. QALYs are estimated from baseline and follow-up utilities. The EuroQol Visual Analogue Scale (EQ VAS) score was used to adjust for any differences in participants’ reported health status at baseline. The EQ VAS is included in the EQ-5D instrument as an alternative measure of self-reported health. None of the baseline characteristics identified in Table 24 was associated with baseline cost. The characteristics listed in Table 24 that were statistically significantly associated with cost were included in the MI of missing data and as covariates for the analyses. The baseline cost categories were also included in the MI models, whereas total baseline cost was included as a covariate in the regression models to estimate net costs. 159,160
| Baseline characteristic | Utility (n = 412, adjusted R2 = 0.39, p < 0.001) | |
|---|---|---|
| Coefficient, SE (95% CI) | p-value | |
| Age | 0.004, 0.001 (–0.005 to –0.002) | < 0.001 |
| Number of benzodiazepine medications | –0.053, 0.023 (–0.099 to –0.007) | 0.023 |
| PANSS | –0.002, 0.001 (–0.003 to –0.001) | 0.006 |
| QPR | 0.005, 0.001 (0.003 to 0.007) | < 0.001 |
| CDSS | –0.008, 0.002 (–0.013 to –0.004) | < 0.001 |
| Taking clozapine | –0.052, 0.030 (–0.110 to 0.006) | 0.080 |
| EQ VAS | 0.002, < 0.001 (0.001 to 0.003) | < 0.001 |
| Constant | 0.733, 0.079 (0.577 to 0.888) | < 0.001 |
Descriptive analysis of health status, utility, quality-adjusted life-years and cost for participants with complete cost and quality-adjusted life-year data
Health status, utility and quality-adjusted life-years
Table 25 reports the percentage of people with no problems on each of the EQ-5D health domains at each follow-up assessment. A breakdown, reporting across all levels and domains of the EQ-5D-5L, is provided in Appendix 3.
| EQ-5D health states | Trial arm, n (%) | |
|---|---|---|
| CBT (N = 76) | TAU (N = 93) | |
| Baseline | ||
| No problem with mobility | 55 (72) | 50 (54) |
| No problem with self-care | 48 (63) | 64 (69) |
| No problem with usual activities | 27 (26) | 32 (34) |
| No problem with pain/discomfort | 43 (57) | 38 (41) |
| No problem with anxiety/depression | 9 (12) | 12 (13) |
| 9-month assessment | ||
| No problem with mobility | 54 (71) | 47 (51) |
| No problem with self-care | 55 (72) | 60 (65) |
| No problem with usual activities | 41 (54) | 40 (43) |
| No problem with pain/discomfort | 47 (62) | 42 (45) |
| No problem with anxiety/depression | 17 (22) | 11 (12) |
| 21-month assessment | ||
| No problem with mobility | 50 (66) | 49 (53) |
| No problem with self-care | 55 (72) | 51 (55) |
| No problem with usual activities | 38 (50) | 36 (39) |
| No problem with pain/discomfort | 47 (62) | 38 (41) |
| No problem with anxiety/depression | 17 (22) | 15 (16) |
Table 26 reports the EQ VAS scores and the EQ-5D-5L utility scores at each follow-up assessment. The utility values are reported for the crosswalk system,170 developed to map the EQ-5D-5L to the EQ-5D, three-level version and the new utility value set estimated specifically for the EQ-5D-5L. 157 The new utility value set was used for the primary analysis and the crosswalk system was included in one of the sensitivity analyses.
| Measure | Trial arm, mean, SE (95% CI) | |
|---|---|---|
| CBT (n = 76) | TAU (n = 93) | |
| EQ VAS values | ||
| Baseline | 58, 2 (54 to 63) | 59, 2 (55 to 64) |
| 9-month assessment | 65, 2 (60 to 69) | 57, 2 (53 to 62) |
| 21-month assessment | 65, 2 (61 to 69) | 58, 2 (53 to 62) |
| EQ-5D utility values, crosswalk system, sensitivity analysis | ||
| Baseline | 0.647, 0.028 (0.592 to 0.703) | 0.566, 0.030 (0.508 to 0.625) |
| 9-month assessment | 0.706, 0.028 (0.655 to 0.765) | 0.597, 0.030 (0.538 to 0.657) |
| 21-month assessment | 0.717, 0.027 (0.665 to 0.772) | 0.592, 0.028 (0.537 to 0.648) |
| EQ-5D utility values, new value set, primary analysis | ||
| Baseline | 0.728, 0.024 (0.680 to 0.776) | 0.668, 0.025 (0.619 to 0.7170) |
| 9-month assessment | 0.774, 0.024 (0.727 to 0.824) | 0.686, 0.028 (0.630 to 0.741) |
| 21-month assessment | 0.780, 0.025 (0.731 to 0.831) | 0.682, 0.026 (0.631 to 0.733) |
The length of follow-up (days) and QALYs (derived from the EQ-5D) are presented in Table 27 for participants with complete cost and QALY data. The number of days of follow-up is similar in both groups at both the 9- and 21-month follow-ups.
| Assessment point | Trial arm, mean, SE (95% CI) | |
|---|---|---|
| CBT (n = 76) | TAU (n = 93) | |
| Days of follow-up | ||
| Baseline to 9 months | 288, 3 (282 to 294) | 288, 3 (282 to 294) |
| Baseline to 21 months | 647, 3 (642 to 653) | 644, 2 (640 to 648) |
| QALYs | ||
| Baseline to 9 months | 0.59, 0.02 (0.56 to 0.63) | 0.53, 0.02 (0.49 to 0.57) |
| Baseline to 21 months (discounted) | 1.31, 0.04 (1.24 to 1.38) | 1.16, 0.04 (1.08 to 1.24) |
Costs
Appendix 3 reports unit costs and costs of the different types of service used at each assessment point for participants with complete cost and QALY data. Service use and costs for the 3 months prior to baseline,from baseline to 9 months and from baseline to 21 months are summarised in Tables 28 and 29. Overall, the large standard errors (SEs) and wide 95% CIs indicate that there is a relatively high level of variation in costs between participants. There appear to be differences between the comparator and CBT groups at 9 and 21 months. However, the 95% CIs overlap, suggesting that any differences could be attributable to chance rather than being statistically significantly different.
| Service type | Trial arm, mean, SE (95% CI) | |
|---|---|---|
| CBT (n = 76) | TAU (n = 93) | |
| 3 months prior to baseline | ||
| Hospital inpatient admission (psychiatric) | 0.01, 0.01 (< 0.001 to 0.04) | 0.03, 0.02 (0 to 0.07) |
| Hospital inpatient admission (non-psychiatric) | 0.03, 0.02 (< 0.001 to 0.06) | 0.02, 0.02 (< 0.001 to 0.05) |
| Hospital outpatient, day and emergency care | 0.97, 0.20 (0.58 to 1.37) | 1.17, 0.23 (0.72 to 1.63) |
| Other community and social care | 13.73, 1.52 (10.69 to 16.76) | 15.97, 3.2 (9.61 to 22.32) |
| Baseline to 3 months | ||
| Hospital inpatient admission (psychiatric) | 0 | 0 |
| Hospital inpatient admission (non-psychiatric) | 0.01, 0.01 (< 0.001 to 0.04) | 0.02, 0.02 (< 0.001 to 0.06) |
| Hospital outpatient, day and emergency care | 0.92, 0.17 (0.58 to 1.26) | 1.32, 0.22 (0.88 to 1.77) |
| Other community and social care | 12.88, 1.42 (10.06 to 15.70) | 14.87, 3.24 (8.44 to 21.31) |
| 3–6 months | ||
| Hospital inpatient admission (psychiatric) | 0 | 0 |
| Hospital inpatient admission (non-psychiatric) | 0.01, 0.01 (< 0.001 to 0.04) | 0.06, 0.03 (0.01 to 0.12) |
| Hospital outpatient, day and emergency care | 0.91, 0.18 (0.55 to 1.26) | 1.46, 0.25 (0.97 to 1.96) |
| Other community and social care | 10.16, 1.13 (7.91 to 12.41) | 14.25, 2.01 (10.25 to 18.25) |
| 6–9 months | ||
| Hospital inpatient admission (psychiatric) | 0 | 0 |
| Hospital inpatient admission (non-psychiatric) | 0.03, 0.03 (< 0.001 to 0.08) | 0.05, 0.02 (0.01 to 0.10) |
| Hospital outpatient, day and emergency care | 1.34, 0.31 (0.73 to 1.95) | 1.28, 0.22 (0.85 to 1.71) |
| Other community and social care | 10.68, 1.02 (8.66 to 12.71) | 15.90, 2.60 (10.73 to 21.07) |
| 9–13 months | ||
| Hospital inpatient admission (psychiatric) | 0 | 0 |
| Hospital inpatient admission (non-psychiatric) | 0.03, 0.02 (< 0.001 to 0.06) | 0.04, 0.03 (< 0.001 to 0.09) |
| Hospital outpatient, day and emergency care | 1.24, 0.22 (0.79 to 1.68) | 1.31, 0.20 (0.91 to 1.71) |
| Other community and social care | 14.69, 1.72 (11.27 to 18.11) | 16.40, 1.97 (12.48 to 20.32) |
| 13–17 months | ||
| Hospital inpatient admission (psychiatric) | 0 | 0 |
| Hospital inpatient admission (non-psychiatric) | 0 | 0.04, 0.02 (0 to 0.09) |
| Hospital outpatient, day and emergency care | 1.37, 0.29 (0.78 to 1.95) | 1.40, 0.22 (0.96 to 1.83) |
| Other community and social care | 18.03, 2.62 (12.80 to 23.25) | 17.42, 2.67 (12.11 to 22.73) |
| 17–21 months | ||
| Hospital inpatient admission (psychiatric) | 0 | 0 |
| Hospital inpatient admission (non-psychiatric) | 0.05, 0.03 (0 to 0.10) | 0.05, 0.03 (0 to 0.11) |
| Hospital outpatient, day and emergency care | 1.29, 0.22 (0.85 to 1.73) | 2.02, 0.42 (1.19 to 2.85) |
| Other community and social care | 17.22, 2.64 (11.96 to 22.49) | 16.71, 2.19 (12.37 to 21.05) |
| Costs of services used | Trial arm, mean cost (£), SE (95% CI) | |
|---|---|---|
| CBT (n = 76) | TAU (n = 93) | |
| 3 months prior to baseline | ||
| Hospital inpatient admission (psychiatric) | 63, 63 (0 to 187) | 308, 264 (0 to 829) |
| Hospital inpatient admission (non-psychiatric) | 0 | 0 |
| Hospital outpatient, day and emergency care | 45, 17 (12 to 78) | 62, 22 (19 to 104) |
| General practice, community and social | 704, 89 (529 to 879) | 616, 63 (491 to 741) |
| Total cost | 718, 100 (521 to 915) | 661, 68 (527 to 795) |
| Baseline to 9 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 234, 170 (0 to 610) | 290, 123 (16 to 468) |
| Hospital outpatient, day and emergency care | 125, 27 (67 to 173) | 215, 42 (130 to 299) |
| General practice, community and social | 1568, 135 (1260 to 1805) | 1899, 195 (1511 to 2294) |
| Total cost | 1927, 251 (1255 to 2184) | 2404, 249 (1841 to 2827) |
| Baseline to 21 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 506, 216 (88 to 1034) | 497, 178 (108 to 837) |
| Hospital outpatient, day and emergency care | 295, 63 (152 to 406) | 434, 80 (271 to 594) |
| General practice, community and social | 3834, 424 (2886 to 4613) | 4345, 484 (3383 to 5329) |
| Total cost | 4635, 529 (3241 to 5204) | 5277, 581 (4026 to 6417) |
The mean use, length and costs of the CBT intervention are summarised in Table 30. In the intervention arm, 230 out of 242 participants (95%) received treatment and provided data on the number of CBT sessions attended. Some information on CBT session length was available for nearly half of participants in the CBT arm (113/242; 47%); complete data on CBT session length for all attended sessions were available for 14 out of 242 participants (6%). The unit cost per session for CBT reported by the PSSRU (£97 per 55-minute session) was used to calculate the cost of CBT for each participant with data on the number of sessions. Those participants allocated to the intervention group but who did not receive CBT were allocated a CBT treatment cost of zero.
| Item | Mean (SE) | Range | 95% CI |
|---|---|---|---|
| All participants receiving one or more CBT sessions (n/N = 230/242) | |||
| Number of sessions | 21 (1) | 1–46 | 20 to 23 |
| Session length (minutes) (n/N = 113/242) | 51 (1) | 5–76 | 48 to 54 |
| Average cost per participant receiving one or more CBT sessions (£) | 2038 (58) | 95–4370 | 1924 to 2152 |
| Average cost per participant allocated to CBT (n/N = 242/242) (£) | 1937 (62) | 0–4370 | 1815 to 2059 |
Table 31 summarises the total cost per person, including the costs of CBT for participants in the intervention arm of the trial, for the 3 months prior to baseline, from baseline to 9 months and from baseline to 21 months. Appendix 3 presents the total cost per person for each assessment point. Although the total costs from baseline to 21-month follow-up appear higher for those participants in the CBT group, the variance is high and the 95% CIs overlap, suggesting that any apparent differences may be attributable to chance.
| Assessment | Trial arm, mean cost (£), SE (95% CI) | |
|---|---|---|
| CBT (n = 76) | TAU (n = 93) | |
| 3 months prior to baseline | 718, 100 (521 to 915) | 661, 68 (527 to 795) |
| Baseline to 9-month follow-up | 4197, 254 (3485 to 4451) | 2404, 249 (1841 to 2827) |
| Baseline to 21-month follow-up | 7073, 527 (5478 to 7463) | 5468, 581 (4026 to 6417) |
Descriptive analysis of utility, quality-adjusted life-years and cost for all participants, using multiple imputation data
Table 32 reports the utility values and QALYs for all participants, using the MI data. As with the complete-case analysis, utility increases from baseline to end of scheduled follow-up in both groups. The data in Table 32 indicate that the number of QALYs is similar in each group at 9 months. There appears to be a trend towards a difference in total QALYs between the two groups at 21 months. However, these data are not adjusted for any differences in the characteristics or utility of participants at baseline. Although the QALY estimates include baseline utility, this may not fully capture the impact of differences in baseline utility on utility at follow-up.
| Utility and QALYs, all participants, multiple imputation data | Trial arm, mean, SE (95% CI) | |
|---|---|---|
| CBT (n = 242) | TAU (n = 245) | |
| Utility | ||
| Baseline (single imputation) | 0.734, 0.013 (0.708 to 0.760) | 0.704, 0.014 (0.675 to 0.732) |
| 9 months | 0.769, 0.014 (0.741 to 0.796) | 0.723, 0.016 (0.691 to 0.755) |
| 21 months | 0.781, 0.013 (0.756 to 0.808) | 0.740, 0.014 (0.712 to 0.768) |
| QALYs | ||
| Baseline to 9 months | 0.61, 0.01 (0.59 to 0.63) | 0.57, 0.01 (0.55 to 0.60) |
| Baseline to 21 months (discounted) | 1.32, 0.02 (1.28 to 1.36) | 1.24, 0.02 (1.19 to 1.28) |
Costs
Appendix 3 reports the average costs by cost category at each assessment point estimated for all participants, from the MI data. The costs by category are summarised in Table 33 for the 3 months prior to baseline, from baseline to 9 months and from baseline to 21 months. One point to note is that the costs at follow-up are higher in the MI data than in the complete-case data. This is mainly because complete cost and QALY data at 21-month follow-up were not available for participants who were inpatients during scheduled follow-up. In contrast, the mean costs estimated from the MI data do include the cost of inpatient stays in psychiatric hospitals. The pooled available case data indicate that the mean cost of an inpatient psychiatric hospital stay was £9525 (SE £1447, 95% CI £6681 to £12,368; n = 429) from baseline to 9-month follow-up and £19,095 (SE £2962, 95% CI £13,271 to £24,919; n = 389) from baseline to 21-month follow-up. However, participants for whom complete data were available at 21 months did not have a psychiatric hospital inpatient stay. This suggests that the subsample of participants with complete data was not representative of the full sample.
| Costs of services used | Trial arm, mean cost (£), SE (95% CI) | |
|---|---|---|
| CBT (n = 242) | TAU (n = 245) | |
| 3 months prior to baseline | ||
| Hospital inpatient admission (psychiatric) | 2170, 515 (1158 to 3183) | 2581, 553 (1494 to 3668) |
| Hospital inpatient admission (non-psychiatric) | 48, 25 (0 to 97) | 44, 24 (0 to 92) |
| Hospital outpatient, day and emergency care | 95, 19 (57 to 133) | 95, 21 (54 to 135) |
| General practice, community and social | 796, 65 (668 to 924) | 830, 78 (677 to 982) |
| Total cost | 959, 69 (823 to 1095) | 1011, 102 (810 to 1212) |
| Baseline to 9 months | ||
| Hospital inpatient admission (psychiatric) | 9099, 1891 (5383 to 12,814) | 8104, 1744 (4678 to 11,530) |
| Hospital inpatient admission (non-psychiatric) | 182, 71 (42 to 322) | 269, 84 (102 to 436) |
| Hospital outpatient, day and emergency care | 178, 23 (133 to 224) | 304, 44 (218 to 391) |
| General practice, community and social | 1972, 180 (1619 to 2325) | 2190, 160 (1874 to 2505) |
| Total cost (including CBT intervention) | 13,368, 1884 (9667 to 17,069) | 10,867, 1769 (7390 to 14,344) |
| Baseline to 21 months | ||
| Hospital inpatient admission (psychiatric) | 17,492, 3587 (10,443 to 24,541) | 15,482, 3340 (8919 to 22,045) |
| Hospital inpatient admission (non-psychiatric) | 589, 140 (313 to 866) | 573, 146 (287 to 860) |
| Hospital outpatient, day and emergency care | 492, 72 (351 to 633) | 601, 74 (456 to 746) |
| General practice, community and social | 4882, 416 (4065 to 5698) | 4842, 315 (4221 to 5462) |
| Total cost (including CBT intervention) | 25,392, 3638 (18,244 to 32,540) | 21,499, 3399 (14,820 to 28,178) |
Overall, the MI results indicate some variation between participants and between the allocation groups in total costs at 9- and 21-month follow-ups. The 95% CIs for the two groups overlap, suggesting that there are no statistically significant differences in costs.
Primary analysis of incremental costs, quality-adjusted life-years and incremental cost-effectiveness ratio
Table 34 shows the net costs and QALYs of CBT compared with TAU at 21 months of follow-up for the primary and sensitivity analyses. Appendix 3 reports the full results of the regression analyses used to adjust for baseline covariates. A linear regression model was used to estimate net QALYs and alternative measures of health benefit and a generalised linear model with gamma log distribution was used for the costs, to account for the skewed distribution of costs towards zero. The data from each of the primary and sensitivity analyses were bootstrapped to generate 10,000 pairs of net cost and net QALY estimates.
| Analysis | Net cost (£), SE (95% CI); p-value | Net QALYs, SE (95% CI); p-value |
|---|---|---|
| Primary analysis, n = 487 | 5378, 9382 (–13,010 to 23,766); 0.566 | 0.052, 0.025 (0.003 to 0.103); 0.038 |
| Sensitivity analyses | ||
| Complete-case analysis, n = 169 | 2531, 782 (998 to 4065); 0.001 | 0.153, 0.046 (0.062 to 0.243); 0.001 |
| 9-month time horizon, n = 487 | 3851, 8977 (–13,744 to 21,447); 0.67 | 0.023, 0.013 (–0.003 to 0.049); 0.077 |
| Crosswalk value set used to estimate utility values | 5378, 9382 (–13,010 to 23,766); 0.566 | 0.074, 0.028 (0.019 to 0.130) p; 0.009 |
| Clinically relevant improvement, PANSS (25%) | 5378, 9382 (–13,010 to 23,766); 0.566 | –0.066, 0.206 (–0.470 to 0.339); 0.750 |
| Clinically relevant improvement, PANSS (50%) | 5378, 9382 (–13,010 to 23,766); 0.566 | 0.245, 0.277 (–0.298 to 0.788); 0.376 |
| Clinically relevant improvement, QPR (25%) | 5378, 9382 (–13,010 to 23,766); 0.566 | 0.426, 0.272 (–0.107 to 0.958); 0.117 |
| Clinically relevant improvement, QPR (50%) | 5378, 9382 (–13,010 to 23,766); 0.566 | –0.104, 0.415 (–0.918 to 0.710); 0.802 |
| Engaged in productive activity | 5378, 9382 (–13,010 to 23,766); 0.566 | 0.318, 0.249 (–0.169 to 0.805); 0.201 |
The primary analysis indicates that CBT is associated with a net gain in QALYs and a net cost. The net QALY gain is statistically significant. In contrast, the net cost is associated with a high level of variability and is not statistically significant. The additional net cost associated with CBT is attributable to the costs of delivering the intervention and the higher costs of care overall. Excluding the cost of CBT, the net cost of care for the intervention was £2123 (SE £4595, 95% CI –£6883 to £11,129; p = 0.644); however, the difference in cost between CBT and TAU was not statistically significantly.
It is important to consider the joint uncertainty associated with the pairs of net cost and QALY estimates, because these are combined to estimate the ICER, which is the overall outcome measure for the economic evaluation. This uncertainty is illustrated in Figure 9 with a scatterplot of the 10,000 pairs of net cost and QALYs, from the bootstrapped data, in the form of a cost-effectiveness plane. The cost-effectiveness plane demonstrates that the majority of pairs of net cost and QALYs are to the right-hand side of the horizontal axis, indicating that the CBT intervention is associated with a net gain in QALYs and health benefit. However, the majority of pairs also lie in the top half of the vertical axis, indicating a net cost to CBT.
FIGURE 9.
Cost-effectiveness plane of 10,000 pairs of net costs and QALYs: bootstrapped MI data.
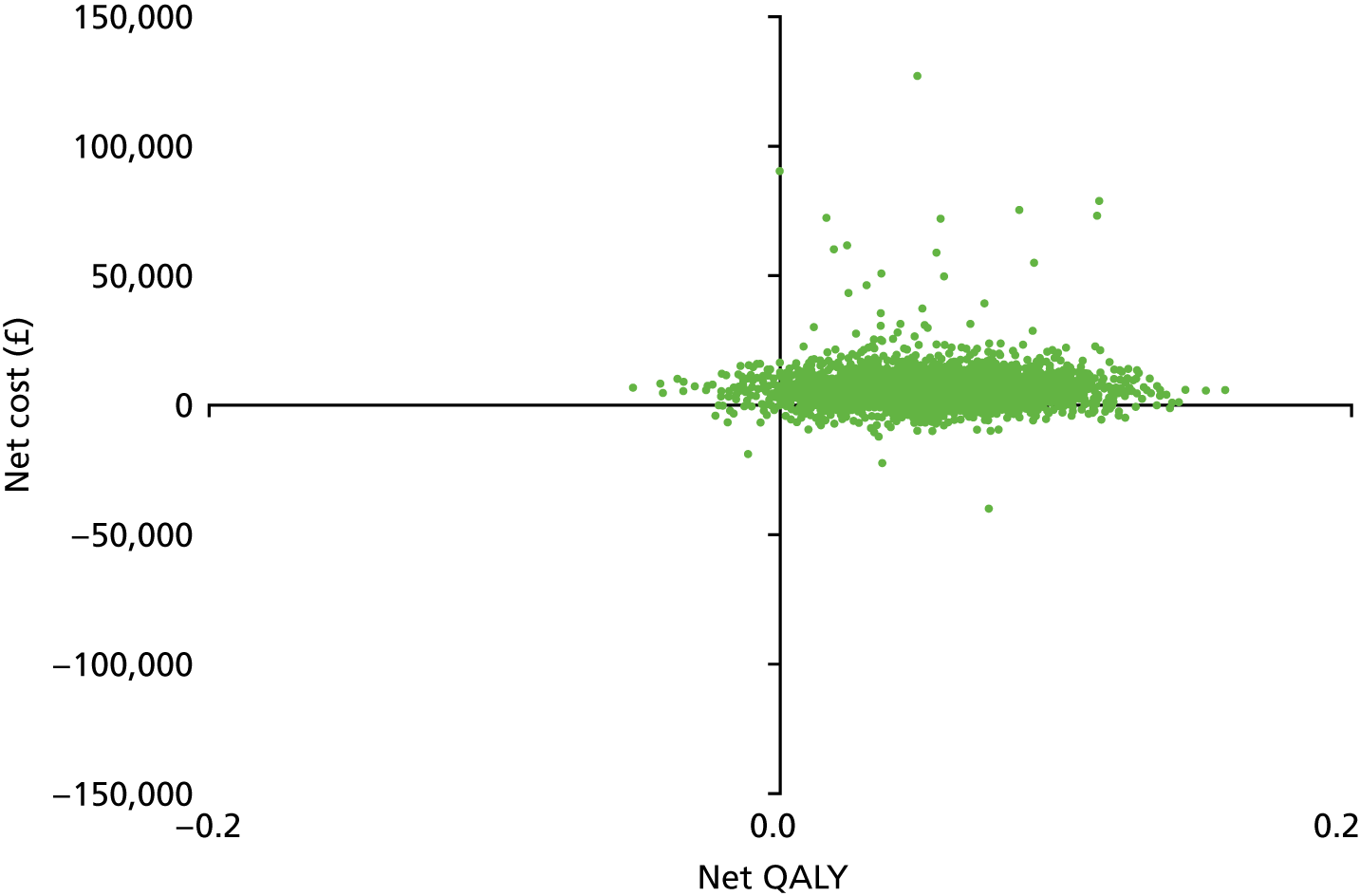
Table 35 reports the ICER, the likelihood that CBT is cost-effective at a WTPT of £15,000 and the estimated net monetary benefit statistic of CBT, when compared with TAU. There is no universally agreed monetary value to attach to QALYs. Therefore, the simulated net QALYs were revalued using a range of values that a decision-maker may be willing to pay to gain 1 QALY, ranging from £0 to £30,000. This was based on the range of willingness-to-pay values historically used in NICE decisions. 167,172 This approach takes into account uncertainty about the amount that decision-makers would be willing to pay to gain 1 additional QALY from the CBT intervention. The impact of different WTPT values on the likelihood that CBT is cost-effective is shown in the cost-effectiveness acceptability curve in Figure 10.
| Analysis | ICER (£) | Probability that CBT is cost-effective (WTPT = £15,000 to gain 1 QALY) | Net monetary benefit statistic, mean (£), SE (2.5th, 97.5th percentiles) |
|---|---|---|---|
| Primary analysis | 103,423 | 0.13 | –5414, 53 (–14,184, 3483) |
| Sensitivity analyses | |||
| Complete-case analysis | 16,542 | 0.40 | –280, 11 (–2700, 1775) |
| 9-month time horizon | 167,435 | 0.08 | –3801, 36 (–8788, 1159) |
| Crosswalk value set used to estimate utility values | 72,676 | 0.14 | –5093, 50 (–13,935, 3780) |
| Clinically relevant improvement, PANSS (25%) | Dominated by TAU | 0.09 | –7236, 59 (–17,710, 2976) |
| Clinically relevant improvement, PANSS (50%) | 21,951 | 0.35 | –2498, 89 (–20,636, –265) |
| Clinically relevant improvement, QPR (25%) | 12,624 | 0.54 | 392, 56 (–17,249, 3391) |
| Clinically relevant improvement, QPR (50%) | Dominated by TAU | 0.15 | –7690, 59 (–28,345, –7709) |
| Engaged in productive activity | 16,912 | 0.43 | –1274, 68 (–18,547, 2147) |
FIGURE 10.
Cost-effectiveness acceptability curve: primary analysis.
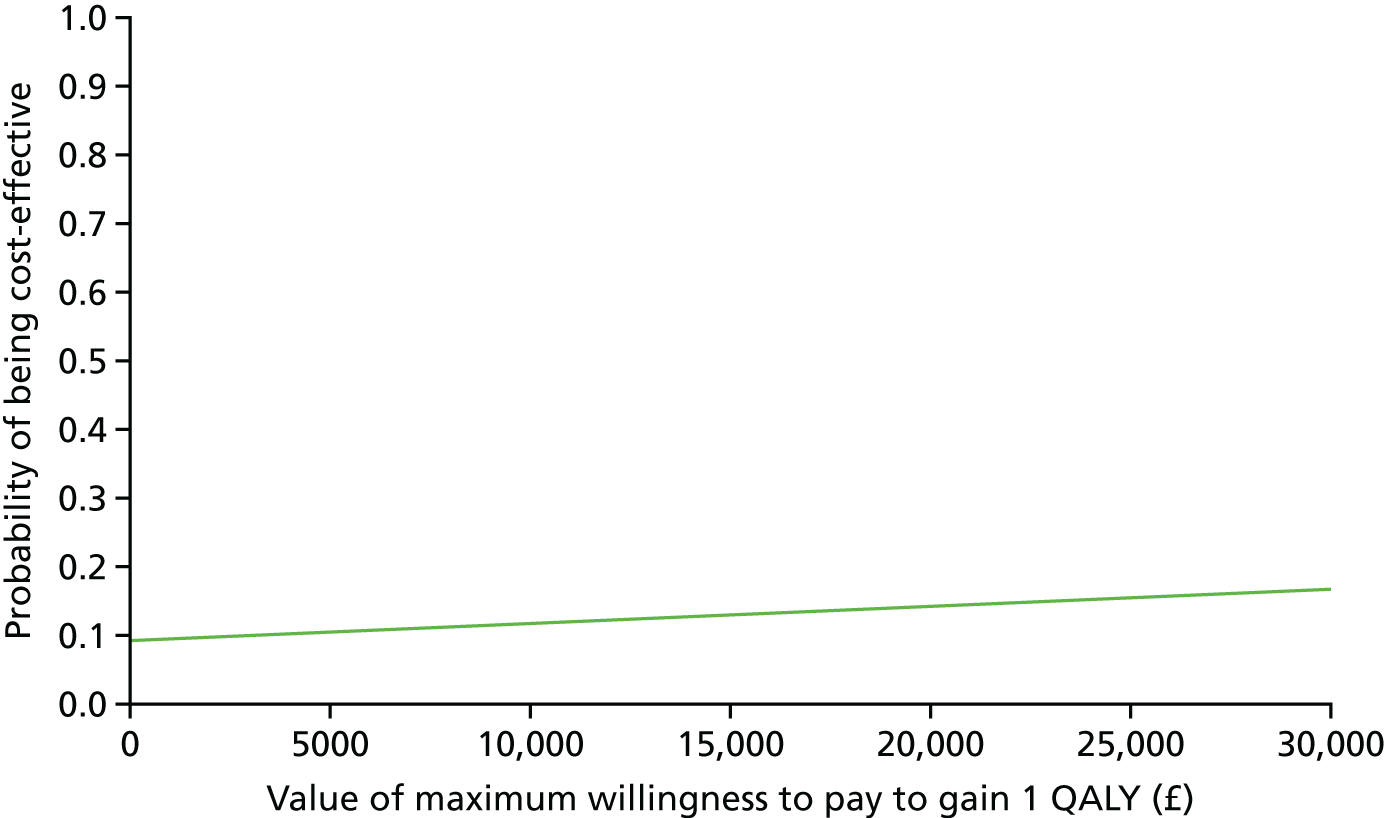
The primary analysis indicates that, although CBT is associated with higher health benefits than TAU in terms of QALYs, the additional costs mean that it is unlikely to be cost-effective if decision-makers are willing to pay £15,000 to gain 1 QALY. The probability that CBT is cost-effective (in this analysis of 10,000 net cost and QALY pair estimates) is 0.13, or 13% (see Table 35). If decision-makers are willing to pay £30,000 to gain 1 QALY, the chance that CBT is cost-effective is < 50%, at 17% (see Figure 10).
Sensitivity analyses of incremental costs, quality-adjusted life-years and incremental cost-effectiveness ratio
The cost-effectiveness acceptability curves in Figures 11 and 12 show the sensitivity analyses for the complete-case analysis, 9-month time horizon, QALYs estimated from crosswalk utility values (see Figure 11) and the alternative health benefit measures (see Figure 12). These indicate that CBT has a likelihood of being cost-effective of < 50%. This is the case for a WTPT of up to £30,000 per QALY gained (or, for the alternative health benefit measures, a WTPT of up to £30,000 per person with a clinically relevant improvement gained). The exception is the analysis using ≥ 25% improvement in QPR. In this case, CBT had a 54% chance of being cost-effective.
FIGURE 11.
Cost-effectiveness acceptability curve: sensitivity analysis 1.
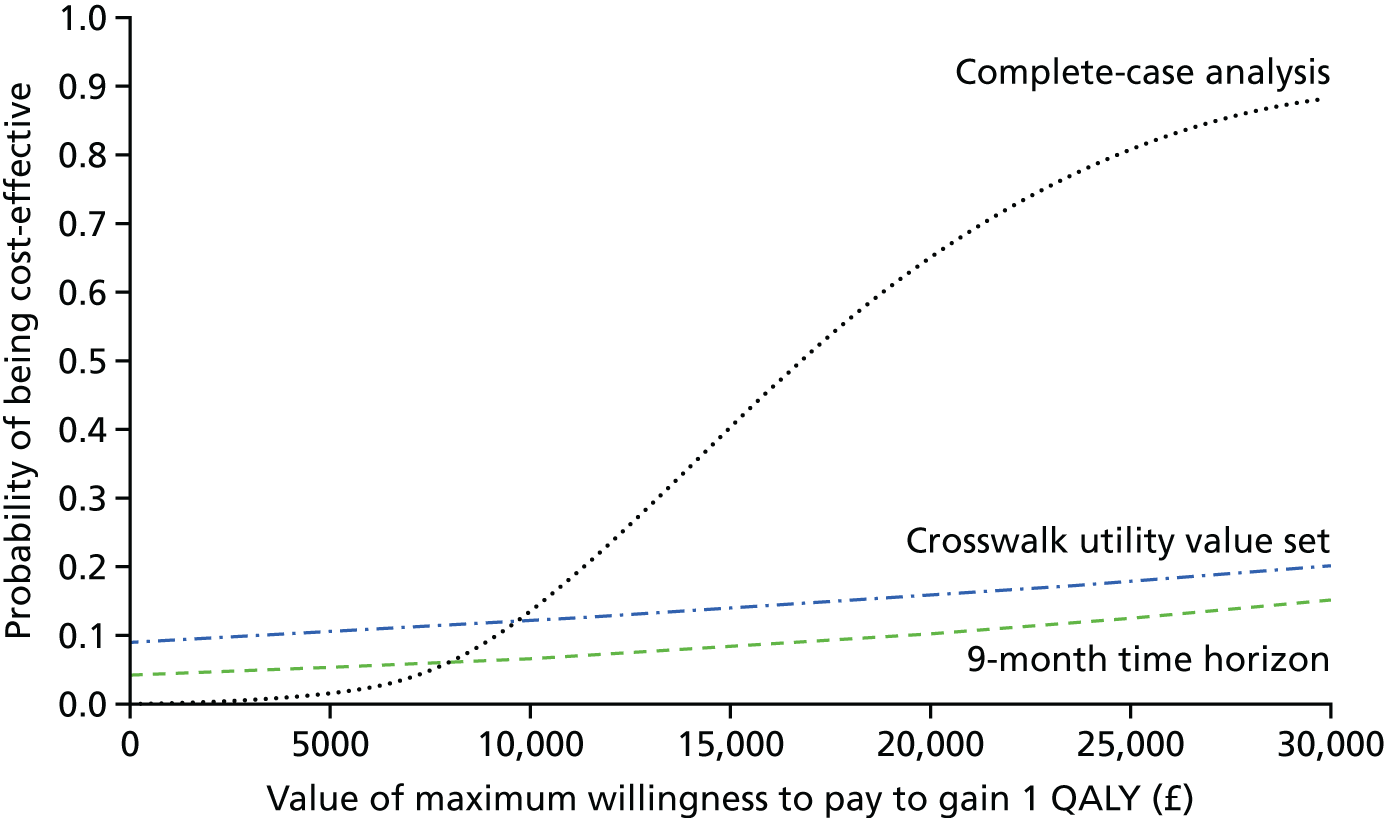
FIGURE 12.
Cost-effectiveness acceptability curve: sensitivity analysis 2.
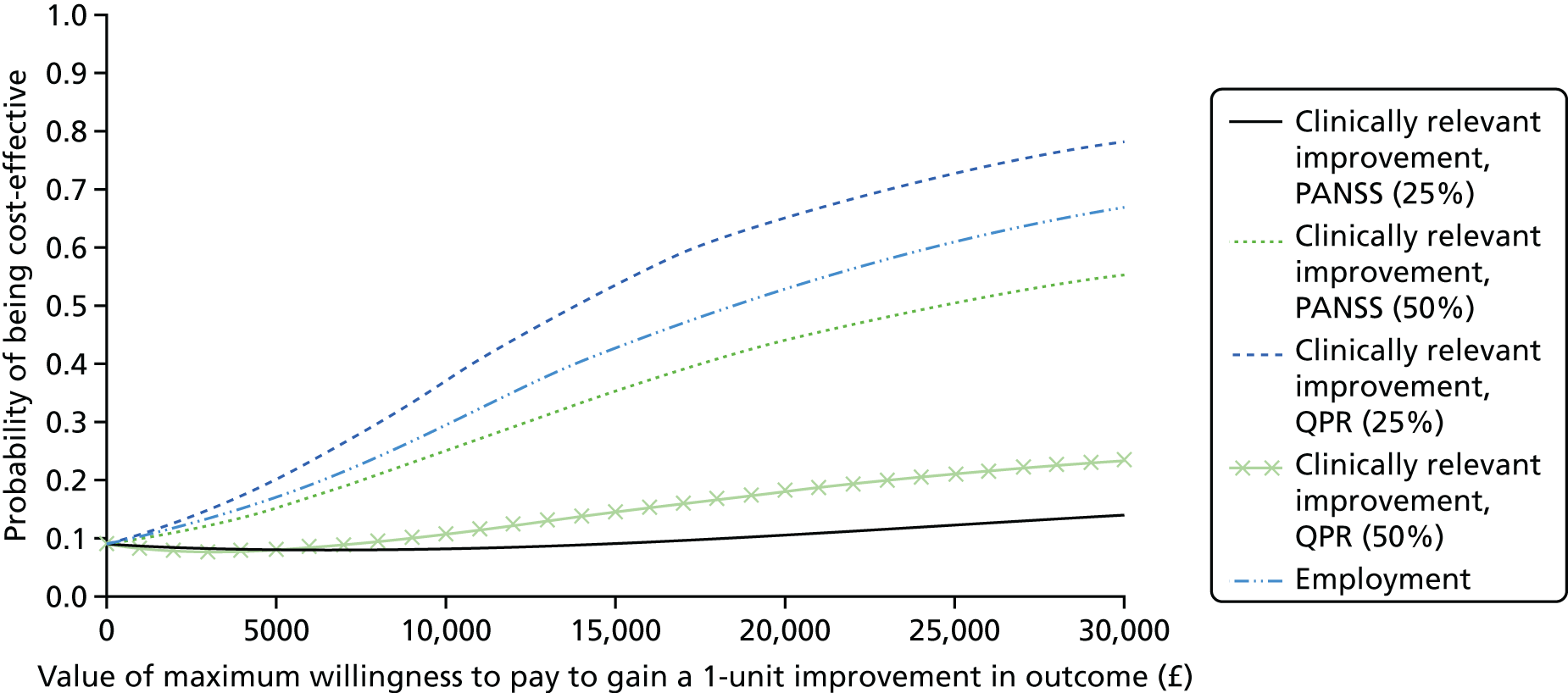
The complete-case analysis suggests that there is a 40% chance that CBT is cost-effective if decision-makers are willing to pay up to £15,000 to gain 1 QALY. This rises to an 89% chance of being cost-effective if decision-makers are willing to pay up to £30,000 to gain 1 QALY. However, the complete-case analysis consists of the subset of participants with complete data on costs and QALYs over the 21-month follow-up period. These participants did not use inpatient psychiatric hospital care over the 21-month follow-up period.
Chapter 6 Discussion
Summary
The FOCUS trial is, to our knowledge, the first definitive RCT to evaluate the long-term clinical effectiveness and cost-effectiveness of CBT in comparison with TAU for people who meet the criteria for CRS. In addition, we utilised baseline data to develop a risk model of factors that may predict a good outcome from CBT. The FOCUS trial was methodologically robust with a low risk of bias.
No effect on the primary outcome of PANSS total score was found at the 21-month assessment and the CIs around estimated treatment effect rule out the hypothesised ES. Many of the secondary outcomes were not found to be significant; however, there was a significant effect of CBT over TAU for the main secondary outcome of PANSS total at the end of treatment (9-month assessment) and an encouraging NNT for achieving a good outcome on the PANSS total score at 21 months. The results also indicated small but significant effects of CBT over TAU for self-rated recovery, emotional distress associated with delusions and CGI improvement at long-term follow-up, and at the end of treatment on PANSS total score, positive symptoms, emotional distress and auditory hallucinations. With 88% of those who were allocated to CBT having six sessions or more, it is clear that CBT is acceptable to the majority of those who are offered it. We did not find a difference between CBT and TAU in reportable SAEs or in the number of participants who had one or more AE. The number of reportable SAEs and the absence of any significant difference between the groups on non-reportable AEs suggest that CBT is a safe treatment for this population.
The results of the risk modelling did not reveal any factors that predict a good response to CBT. There was no evidence that the treatment effects of CBT varied over any of the subgroups investigated in the FOCUS trial population for PANSS total score or the PANSS subscales.
Overall, CBT was associated with a net cost and net QALY gain compared with TAU. The additional cost for the CBT group was the result of higher use of health and social care services by participant as well as the additional cost of the CBT intervention. There was a high level of variance in the costs of different services and total costs, and the differences in cost between the two groups were not statistically significant. This indicates uncertainty about whether the CBT group incurred higher costs than the TAU group or whether the difference found was attributable to chance. Nevertheless, the cost-effectiveness acceptability analysis indicated a low likelihood that CBT was cost-effective, in the primary and sensitivity analyses (probability of < 50%). There are a number of limitations that increase the uncertainty of the results, which are discussed in Strengths and limitations.
Findings in context
The results of the FOCUS trial showed no lasting effect of CBT for people who meet the criteria for CRS on total symptoms as measured by PANSS. However, there was a statistically significant impact on overall health for those who received CBT in comparison with those who received TAU. Clinically significant improvement on PANSS total score has been defined in meta-analyses of antipsychotic medication as ≥ 50% improvement in the PANSS total score (rescaled) from baseline;32 our NNT for a good improvement in PANSS total score was 15. A recent meta-analysis of all double-blind, placebo-controlled RCTs of antipsychotic medications, except clozapine, found a NNT of eight for ≥ 50% improvement on PANSS. 32 More specifically, for clozapine the NNT has been reported as eight. 47 However, the participants in the FOCUS trial had experienced a poor response to treatment with both standard antipsychotic medication and clozapine and, therefore, our NNT of 15 is encouraging, particularly as this was at long-term follow-up.
In addition, the effect of CBT at end of treatment on PANSS total, PANSS subscales (positive symptoms, emotional distress and excitement) and auditory hallucinations demonstrates that CBT can change symptoms of psychosis in the short term. The reasons for the short-term effect of CBT are unclear; however, given the long DI experienced by our sample, it is possible that a greater number of CBT sessions or longer duration of the treatment window may be required to yield a long-term benefit. It could also be argued that the effects seen at the end of treatment are a result of the non-specific aspects of therapy, such as the therapeutic relationship, rather than the active components of CBT. Therapeutic alliance has been shown to contribute to both improvement and deterioration in PANSS scores for CBT and other psychological therapies, such as supportive counselling. 173 However, not all FOCUS trial participants completed CBT over the full 9-month treatment window and, therefore, it is not possible to say with any confidence that the effects of CBT seen at the end of treatment were indicative that it works only while treatment is ongoing.
The ES of CBT in this trial is similar to those reported by meta-analyses of CBT for psychosis trials that are deemed to be methodically robust. 67,69 The authors of the most current meta-analysis have proposed that the field of CBT for psychosis research is lacking evidence from a large and methodologically robust trial. 69 Prior to the FOCUS trial, there had been no definitive trials of CBT for CRS. The FOCUS trial has clearly addressed this need for the CRS population and also addressed methodological limitations of previous trials. Jauhar et al. 69 reported an ES of 0.15 from trials at a low risk of masking bias and 0.62 from trials at a high risk of bias. For the FOCUS trial, the risk of this particular bias is low, with only 6.17% of assessments at 9 months (end of treatment) being conducted by an unmasked assessor and our ES at the end of treatment being 0.16, consistent with the pooled estimate from low-risk trials reported by Jauhar et al. 69
With regard to other outcomes, meta-analyses have shown very small ESs for trials with a low risk of bias from masking for positive symptoms. 67,69 Jauhar et al. 69 found, at the end of treatment, an ES of 0.08 for positive symptoms and concluded, therefore, that it is not justified to propose that CBT is effective for positive symptoms. However, the end-of-treatment results for the FOCUS trial indicate an ES of 0.24 and, although this is a small effect, this finding comes from a methodologically robust trial and is encouraging as it indicates that for positive symptoms, CBT can have small short-term benefits for people who meet criteria for CRS, while in receipt of treatment. This finding is particularly encouraging given that our participants have experienced positive symptoms with a poor response to antipsychotic medication and their DI, on average, was long. This finding also demonstrates, in line with psychological models of psychosis, that hallucinations and delusions can change in response to consideration of the person’s interpretation of these experiences and their associated behavioural responses.
Cognitive–behavioural models of psychosis and schizophrenia recognise the link between cognitions, emotions and behaviours; the CBT delivered in this trial permitted work on emotional distress and dysfunction, including prioritising depression and anxiety (if the participant prioritised this as part of their problem list or goals for therapy). Given the role of emotion in psychosis, it has been argued that CBT for psychosis trials should include emotional distress as a secondary outcome121 and the results of the FOCUS trial indicate that CBT can have small but significant effects at the end of treatment for this important secondary outcome.
There were also some small long-term treatment effects at the 21-month assessment, including for self-rated recovery and an encouraging NNT of 15 to achieve a good response (> 50% improvement on PANSS at 21 months). The primary health economics analysis also suggested that CBT is associated with higher health benefits than TAU in terms of QALYs. Service users advocate that outcomes used in research and clinical services should refocus to ones of personal recovery goals, rather than the reduction or absence of symptoms, which has traditionally been the key outcome advocated by clinicians. 118 UK policy places an emphasis on recovery-orientated outcomes and services for people who experience psychosis and schizophrenia30 and, for this reason, our finding that CBT can have a lasting effect on self-rated recovery is arguably more important for service users and services.
The primary health economics analysis uses the EQ-5D-5L and new value set rather than the earlier, interim, crosswalk value set. The crosswalk value set mapped utility values from the older EQ-5D-3L measure to the EQ-5D-5L. 170 The new set of utility values157 is intended to replace the crosswalk system. The data for the new value set were collected using a different methodology, at a different time and on a different sample to the original utility value set used for the EQ-5D-3L and the crosswalk value set. This means that differences in utility between the crosswalk and new value set may reflect differences in methods, time and sample rather than underlying preferences and/or changes in preferences over time. Thus, the crosswalk-derived values were used to facilitate comparison between utility values estimated using the older three-level version of the EQ-5D and the more recent five-level version.
Table 36 compares the UK population norms for the EQ-5D-3L utility values and those of the FOCUS trial participants using the EQ-5D-5L and crosswalk value set. This indicates that FOCUS trial participants have lower utility values across all age group, and, overall, than the sample of the general population used to generate the population norms. 174
| EQ-5D utility values | Age (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 18–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | ≥ 75 | All ages | |
| Population norms, EQ-5D-3L | ||||||||
| Mean | 0.940 | 0.927 | 0.911 | 0.847 | 0.799 | 0.779 | 0.726 | 0.856 |
| SE | 0.007 | 0.006 | 0.007 | 0.011 | 0.012 | 0.012 | 0.015 | 0.004 |
| 25th percentile | 0.97 | 0.85 | 0.85 | 0.80 | 0.73 | 0.69 | 0.66 | 0.8 |
| 75th percentile | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 95% CI | Not available | Not available | Not available | Not available | Not available | Not available | Not available | Not available |
| FOCUS trial participants, EQ–5D-5L and crosswalk values, n = 453 | ||||||||
| Mean | 0.721 | 0.723 | 0.643 | 0.604 | 0.466 | 0.662 | No cases | 0.631 |
| SE | 0.057 | 0.019 | 0.018 | 0.025 | 0.040 | 0.065 | No cases | 0.012 |
| 25th percentile | 0.62 | 0.63 | 0.54 | 0.46 | 0.20 | 0.56 | No cases | 0.50 |
| 75th percentile | 0.88 | 0.85 | 0.82 | 0.82 | 0.70 | 0.76 | No cases | 0.84 |
| 95% CI | 0.600 to 0.842 | 0.685 to 0.761 | 0.607 to 0.679 | 0.554 to 0.654 | 0.385 to 0.547 | 0.508 to 0.816 | No cases | 0.607 to 0.655 |
The utility values for FOCUS trial participants were similar to those reported in other studies. Barton et al. 158 reported EQ-5D-3L baseline utility values of 0.657 (n = 32) to 0.693 (n = 36), whereas Crawford et al. 175 reported EQ-5D-3L utility values of 0.664 to 0.699 (n = 409). The pooled baseline mean utility for participants with severe schizophrenia requiring a change in management in the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) (unpublished analysis of utility data from the CUtLASS trial) was 0.628 (SE 0.017, 95% CI 0.595 to 0.661; n = 361). 159,160 Similarly, another large study,159 also using the EQ-5D-3L, looked at the use of antipsychotics in a population with schizophrenia and found that the baseline mean utility was 0.61 to 0.67 (SD 0.29 to 0.33; n = 118). Finally, a recent large study176 (n = 275) of schizophrenia reported higher values using the EQ-5D-5L, with reported baseline utility values between 0.74 and 0.76.
There is limited evidence about the relative cost-effectiveness of CBT in people with psychosis and schizophrenia. We identified three economic evaluations158,177,178 published since 2010 that used broadly similar methods, in a UK setting. Barton et al. 158 compared social recovery-orientated CBT with usual care (defined as active case management), over 9 months’ follow-up. The authors concluded that social recovery-orientated CBT was cost-effective in 54% of scenarios at a WTPT of £20,000 per QALY. 158 A UK evaluation of CBT combined with motivational care, using a societal perspective and a time frame of 18 months, concluded that the probability of the intervention being cost-effective was 69%, even if decision-makers are not willing to pay to gain a 1-point increase in the Global Assessment of Functioning. 177 McCrone et al. 178 evaluated an early intervention service for people with psychosis, which included low-dose medication regimens, CBT, family therapy and vocational rehabilitation. This study found that the intervention did not increase costs and was likely to be cost-effective (probability of 76%) even if decision-makers are not willing to pay to gain an additional person who makes a full or partial vocational recovery. 178
A fourth study179 compared cognitive remediation therapy plus usual care with usual care alone and found it likely to be cost-effective from a NHS and social care perspective but at a limited time horizon of 40 weeks.
Although using different time horizons and health benefit measures, the findings of these studies differ from the findings of this study, which indicates that CBT is not likely to be cost-effective. All identified studies had limitations, including missing data, small sample sizes and challenges controlling for other medications/treatments received outside the trial intervention. In addition, the participants in this study were people unable to tolerate, or with an inadequate response to, clozapine. This may mean that they are less likely to respond to CBT than people who are not treatment resistant or treatment intolerant. Although CBT was associated with a net gain in QALYs, the costs of CBT were not offset by reduced use of other health and social care services; the participants in the CBT group used more services and had higher costs than the TAU group. However, the greater use of health and social care services found in the CBT group may reflect a desirable outcome for this population who typically have a high level of unidentified and unmet need for health and social care. 180 There is evidence that the physical health needs of this population are severely neglected, often as a result of discrimination by health-care providers. 180,181 This can arise if health-care providers attribute physical sympyoms to a person’s mental health condition, often referred to as ‘diagnostic overshadowing’. 181 Moreover, the fear and shame associated with the public stigmatisation of psychosis and schizophrenia often results in feelings of disempowerment and avoidance of help-seeking for health-care needs. 182 The reduction of stigma and discrimination has been identified as a key target for improving the physical health care of people with a serious mental illness. 183 The early mortality associated with these unmet needs indicates an area in which improvements in health and social care are needed. It is suggested that one approach to making such improvements could be to utilise interventions that encourage service users with serious mental illness, such as CRS, to become empowered to seek support for their physical health care. 183 The findings of this trial indicate that participants who received CBT had greater use of the health-care services that people who meet the criteria for CRS have an equal right to access. Increasing the overall level of physical and mental health care for CRS patients may reduce the differences in costs between the TAU and CBT groups in this trial, potentially improving the relative cost-effectiveness of CBT.
Strengths and limitations
The FOCUS trial provides high-quality evidence regarding the clinical effectiveness and cost-effectiveness of CBT for this population, with a low risk of bias across a number of domains. Our trial was pre-registered and our a priori SAP was published online and in the public domain. Compared with other trials that have evaluated CBT, the FOCUS trial had many methodological strengths that reduced the potential for bias: randomisation was implemented via a web-based platform centrally and independently, resulting in concealed allocation; outcome assessors were blinded and there were few instances of unblinding; a high proportion of participants received their allocated intervention; and there was low attrition for the primary outcome. Furthermore, the FOCUS trial was adequately powered to detect a clinically meaningful difference at 21-month follow-up. Data from the CONSORT diagram (see Figure 2) indicate a representative sample of people who meet CRS criteria. Of people identified for the study, 72% agreed to meet with the research team and, of those, 94.5% agreed to take part in the study. Although there is a subgroup of people who met the CRS criteria who refused to meet with the study team, overall the majority of people referred were engaged. Moreover, the services we recruited from were typical of services for CRS, that is CMHTs, clozapine clinics, psychiatrists and inpatient settings.
The FOCUS trial had strong patient and public involvement (PPI) in the conception of the study design and oversight of the study throughout, with PPI representation on the trial management committee, the Trial Steering Committee and the Data Monitoring and Ethics Committee. The trial documentation was reviewed by two co-applicants, who provided PPI representation, interpreted the results and wrote the participant summary sheet.
The RAs and therapist received training before the trial commenced, or, if they were appointed during the trial, received training before they started to see participants. They received regular supervision throughout the lifetime of the trial to ensure that assessments were conducted reliably and CBT was delivered with fidelity to the model. The small treatment effects should be routinely replicable in the NHS, because almost all of our therapists were band 7, which is the lowest NHS band for psychological therapists. The frequency with which FOCUS trial therapists received supervision is likely to be greater for FOCUS trial therapists than in the NHS; however, it could be argued that for the CRS population, in whom there is a greater degree of complexity, more frequent supervision may be indicated. One limitation to the design of the study was the choice of TAU as the comparator. Although this was specified in the commissioned call by the funder as the comparator, we recognise that a control group that does not include non-specific therapeutic factors, such as contact time, does not allow us to exclude the possibility that any observed change was not attributable to the non-specific therapeutic aspects of CBT.
When placing the above findings in context, it is important to note that, although the effects at the end of treatment and long-term follow-up were statistically significant, the changes observed on the outcome measures are unlikely to reach a threshold of clinically significant change. The NNT of 15 to achieve a good response on our primary outcome, a 50% reduction in PANSS score, at 21 months is encouraging. NNT is reported for 25%, 50% and 75% change in PANSS score from baseline and it is widely accepted that a 50% improvement in PANSS score is a good outcome. 32 Clinicians consider NNT to be a useful way to interpret the findings of trials. 184 However, there are limitations to representing the outcome data for this trial as NNT; the amount of change on PANSS from baseline is dependent on the starting point, that is the baseline score, and the use of percentage change to generate the NNT ignores the absolute benefit. The NNT should be presented with CIs; however, when there is no treatment effect, generating an accurate CI is problematic. 185 In the case of the FOCUS trial, the treatment effect on PANSS at 21 months was limited. Therefore, although NNT has been reported here to aid interpretation by clinicians, who regularly use this statistic, its interpretation should be undertaken with caution, with the caveat that the most efficient and least biased way to analyse the trial data is using our primary analysis of a linear mixed model adjusting for baseline. It should also be noted that NNT is one of a number of statistical tests carried out, and multiple hypothesis testing may lead to type I error, inflating the chance of finding a positive result.
The economic analysis was subject to the same strengths and limitations discussed for the clinical evaluation of effectiveness in terms of trial design, trial sample and length of follow-up.
In line with NICE recommendations,156 the measure of health benefit used for the primary analysis was the QALY, estimated from the published EQ-5D utility values. This enables comparison between different disorders, which is useful for policy-makers and commissioners, who have to consider the distribution of limited budgets between different health-care services. However, the EQ-5D is a generic health status measure that may not be sufficiently sensitive to identify important clinical changes in participants’ mental health. The measure covers five domains (mobility, self-care, usual activity, pain and distress and anxiety and depression), but does not include specific symptoms that may be important to service users. This means that the measure could underestimate the benefits of a successful intervention. The descriptive analyses indicated that the EQ-5D health status measure and associated utility index correlated well with the clinical measures used in the trial (see Appendix 3). In addition, the sensitivity analyses using clinical measures of improvement in mental health symptoms (PANSS) and indicators of recovery (QPR) also found that CBT was not likely to be cost-effective.
As planned prior to the end of data collection, the new value set was used to estimate utility values from the EQ-5D-5L. More recently, however, NICE advised that the crosswalk mapping system, developed as an interim method of estimating utility values, should be used. 186 This is to allow time for further work to explore and understand differences between two approaches before the NICE methods guidance is updated. Accordingly, we re-estimated the results using the older crosswalk system as one of the sensitivity analyses. The results of this did not differ substantially from the primary analysis.
There were differences between the CBT and TAU groups in utility scores at baseline, although these differences were not statistically significant. The lower score in TAU participants may reflect the lower overall health status of people in the TAU group. This was accounted for, to some extent, by including the baseline utility value in the calculation of total QALYs between baseline and the end of follow-up. In addition, the EQ-5D thermometer or EQ VAS score at baseline was included as a covariate in the analyses to account for possible differences in overall health and utility between the groups.
The proportion of participants with complete-case data (34%) makes imputation of missing data essential but difficult. In addition, none of the participants for whom complete cost and QALY data were available at 21-month follow-up had psychiatric inpatient stays. In contrast, the available case data at each assessment indicated that this service was used by participants. This suggests that the group of participants with complete cost and QALY data may not be representative of the study sample as a whole. To strengthen the robustness of the results, available data were used at each assessment point to impute cost categories and EQ-5D domains and passively impute total costs and QALYs. The level of missing data for psychiatric inpatient stays was ≤ 20% at baseline and at 9- and 21-month follow-up, which, in part, reflects the use of hospital case note review for participants reporting any psychiatric hospital inpatient stay. Psychiatric inpatient stay was the key cost component of the total cost. In the available and multiple imputation data, it accounted for approximately 70% of the total cost.
With the exception of non-psychiatric hospital stay, the level of missing data for the other cost categories at baseline and 9-month follow-up was ≈30%. However, it was higher (37–44%) at 21-month follow-up. Constraints on researcher time meant that it was not feasible to review primary and community care or non-psychiatric hospital case notes. The level of missing utility data was lower: < 10% at baseline and < 30% at 21-month follow-up.
The greater number of complete data for each cost category means that our approach of imputing cost categories for each assessment point mitigates the overall impact of missing data to some extent (in terms of both bias and imprecision). Nevertheless, the large number of missing cost data at 21-month follow-up means that the results must be interpreted with caution. The extensive uncertainty around estimates from all analysis reflects this.
The primary analysis for the economic evaluation was limited to direct costs, from NHS and social care perspectives. In this population, effective interventions may also affect whether or not a person is employed and the subsequent earnings and benefits they receive. The trial design needed to balance complete and detailed assessment of outcomes and service use with minimisation of participant burden when possible. It was felt to be important to collect detailed service use data at 3-monthly intervals to minimise problems with participant recall. Accordingly, limited information was collected about paid and unpaid work and activities. This covered whether the participant was participating in paid or unpaid/voluntary employment, education or training, other productive activity or was unemployed. The data suggested that there were no statistically significant differences between groups in the proportion of participants engaged in any productive activity or participating in paid employment.
If CBT improves overall health and/or mental health symptoms and recovery, then this may reduce the need for informal carers (e.g. family members), potentially offsetting some of the costs of formal care and CBT. Experience in previous trials indicated a number of barriers to collecting data about the use of informal carers and the time spent by carers. These included incomplete reporting of whether or not participants had family members or friends who they considered to be informal carers. In addition, informed consent was required from participants to contact any informal carers identified, and from informal carers to collect this information. The data collected from informal carers were also incomplete in a number of cases. These factors meant that complete data were available only for a small proportion of participants. 159,160 Combined with the need to collect detailed formal care-use data, it was felt to be outside the scope of this trial to collect information about the use of informal care. This means that, if CBT reduced or increased the use of informal care, our estimates would underestimate or overestimate the relative cost-effectiveness of CBT.
The economic evaluation was limited to the choice of intervention and comparator included within the trial. Although this leads to a robust comparison, it may not reflect the full range of interventions that are available within a clinical setting. For example, other economic evaluations identified looked at a wider range of psychological therapies in a population with schizophrenia, including cognitive remediation therapy, group art therapy and body psychotherapy. 179,187,188 A modelling study, informed by a network meta-analysis of trial data, would have the potential to compare costs and outcomes across a wider range of interventions. However, in this treatment-resistant population there are likely to be limited data to inform the model structure and populate the model.
Although PANSS is commonly used as a primary outcome measure in treatment trials for people who meet criteria for a schizophrenia diagnosis (both pharmacological and psychological), it may not have been the most appropriate outcome for this trial for a number of reasons. Although PANSS has been rated favourably by service users,116 the same study also found that, overall, service users have a preference for self-rated measures. In addition, it has been argued that distinctions between pharmacological and psychological interventions should be made clearer in that psychological interventions, such as CBT, are not a quasi-neuroleptic. Therefore, outcome measures should reflect the target of the specific intervention. 121 For CBT this could be positive symptoms,67 but it may be more appropriate to consider the reduction of emotional distress given that this is a primary aim of CBT, or self-rated recovery, given the emphasis in CBT on working with the client’s own problem list and goals.
The analysis adjusting for the measure of working memory at baseline needs to be interpreted with caution for two reasons. First, the response rate at baseline was poor because the assessment was introduced 9 months into the recruitment window; a small proportion of data was expected to be missing given that the working-memory assessment was introduced 9 months into the recruitment window. Burden may also explain missing data for this measure. There were a relatively high number of measures in the assessment battery; more specifically, in relation to the working-memory measure, the participant was required to recall and reorder sequences of letters and numbers from a string length of two to seven, and this may have been too burdensome. Second, we proposed a sensitivity analysis using our own algorithm for scoring the measure that was not described by the author.
Although the RAs were blind to allocation, it is not possible to blind participants from the treatment allocation and mask the receipt of CBT when the control is TAU. It could be argued, therefore, that the treatment effect, because it was so small, might be an artefact of the design.
Our inclusion criteria for CRS had been employed in a previous study of clozapine augmentation with a second antipsychotic. 52 This did not include tests to determine, for each potential participant, whether or not there were any periods of less than full clozapine adherence, that an adequate plasma clozapine level (350 ng/ml) had been achieved consistently for an adequate time or substance use (that may have adversely influenced the effectiveness of the clozapine treatment). It could be argued that such tests were necessary to establish clozapine unresponsiveness; however, given the existing precedent for our inclusion criteria, it was considered unrealistic and impracticable to apply criteria relating to the adequacy of the clozapine trial for each patient being screened for eligibility, which involved checks and investigations that are not routinely done in clinical practice and which, for many patients, could prove to be difficult if not impossible to definitively establish whether or not they were met.
Implications for public health/treatment services
The results of the FOCUS trial suggest that CBT should not be offered routinely with the aim of achieving lasting symptom reduction in people who meet the criteria for CRS. However, the finding that CBT has short-term effects for symptoms and long-term effects for self-recovery, and had a NNT of 15 for good response on our primary outcome, suggests that services could consider offering CBT as a pragmatic individual trial. This may be particularly indicated when the service user’s goals relate to acutely distressing positive symptoms and voices or longer-term recovery-orientated goals. The economic evaluation indicates that participants in the CBT group had a net gain in overall health as measured by QALYs. The CBT group also used more services over the course of the trial. Whether this was attributable to chance or to the intervention is unclear. The economic analysis of the participants with complete cost and QALY data also indicates that there may be participants for whom CBT is more likely to be cost-effective. One feature of those with complete cost and QALY data was that they had lower costs in the 3 months prior to the baseline assessment. As outlined, it was not possible to identify any subgroups of participants who had a good outcome from CBT and, therefore, if offering a pragmatic trial of CBT, it should be considered for all service users who meet the criteria for CRS. In addition, the uptake of CBT by FOCUS trial participants provides an optimistic message for the ability to engage people whose experiences are considered ‘treatment resistant’.
A commonly used approach to the treatment of CRS is to augment clozapine with a second antipsychotic. 53 The treatment ES for augmentation with a second antipsychotic has been shown to be in the small but significant range. The ESs found in the FOCUS trial for CBT are also very similar to those found for pharmacological augmentation with a second antipsychotic. 55 However, the FOCUS trial provides a more rigorous test of the effects of a treatment for CRS, with a lower risk of bias than the few, small, short-duration, low-quality trials that contribute to the meta-analyses of augmentation with antipsychotics. In addition to the methodological limitations of the clozapine augmentation trials, the current evidence base cannot provide a clear indication from the literature to indicate which clozapine combination strategy is superior. 58 The adverse effect profile for CBT is also likely to be favourable when compared with the likely cardiovascular risks associated with multiple antipsychotic medications. Therefore, for service users who are reluctant to consider pharmacological augmentation because of the likely side effect burden of polypharmacy, the provision of CBT may be indicated.
A pragmatic trial of CBT may be more effective if offered earlier in the course of clozapine use, when treatment resistance is first established and when DI is shorter, given the personal and social functioning subgroup analysis, which indicated that participants with a shorter DI had greater benefits from CBT in this domain. This may be particularly important as entrenched symptoms may persist for longer and respond more slowly to CBT, which is similar to the use of medication. A person may need to attend more sessions of CBT to derive maximum benefit, given that results of the CACE analysis indicated a potential dose response: for every session of CBT attended, there was a 0.11 reduction in the PANSS total score.
The FOCUS trial recruited people who met the criteria for CRS and, for this reason, the small and short-term effect on symptoms does not mean that CBT will not have stronger effects for less severe or earlier psychosis populations. The NICE guideline for the treatment of psychosis and schizophrenia30 recommends that EIP services routinely offer CBT and family intervention for people with a first episode of psychosis, and meta-analysis of the effects of CBT for this population has demonstrated that it can reduce symptom severity. 189
Finally, it is important to note that the FOCUS trial has an optimistic message for people who meet the criteria for CRS: both the CBT and TAU groups recovered during the trial period to a degree that, on average, approaches clinical significance. Given the DI, poor response to medication and lack of social opportunities (such as employment) among FOCUS trial participants, the finding that, on average, each group had a 10-point improvement in PANSS total scores is a positive one.
Implications for future research
Clearly, this is a population of people who have significant unmet need in relation to their physical and mental health and, therefore, future research to develop and evaluate more effective interventions is required. This could include pharmacological (although issues of physical health and side effects are likely to be important) and other new interventions of either a psychological or social nature. Given the current criticism of CBT for psychosis trials, future RCTs of psychological or social interventions for this population should also demonstrate high methodological rigour and a low risk of bias, as this will help to identify populations most likely to benefit from this intervention.
More specifically, in relation to CBT for this population, approaches to analysis, such as trajectory analysis, may identify responders to CBT; however, it was not possible to use this analytical approach in the FOCUS trial because of the limited number of time points at which our data were collected. There is no research to date to indicate whether or not there are any active ingredients of CBT for the CRS population, and, in the FOCUS trial, a number of techniques were utilised within therapy. Therefore, from the FOCUS trial data, it should be explored if there are any specific therapeutic techniques that result in a good outcome from CBT. Previous research indicates that there is a greater treatment effect from CBT for people considered at risk of developing psychosis if therapy involves between-session tasks and formulation,154 and mediation analyses of the FOCUS trial data may reveal components of therapy as predictors of response.
The mean health and social care costs for the 3 months prior to the baseline assessment were lower in the analysis of participants with complete cost and QALY data (£686, 95% CI £572 to £801; n = 169) than the MI analysis of all participants (£985, 95% CI £863 to £1107; n = 487). This indicates that, for whatever reason, these participants were making less use of services. In addition, the analysis indicated that participants in the CBT group used more health and social care services than those in the TAU group, particularly towards the end of the follow-up period. Future research could explore the reasons for high and low service use and whether it indicates higher need for care, or whether CBT helps participants and/or health-care professionals better recognise the need for care.
The economic evaluation was limited to the choice of intervention and comparator included within the trial. Although this leads to a robust comparison, it may not reflect the full range of interventions that are available within a clinical setting. A modelling study, informed by a network meta-analysis of trial data, would have the potential to compare costs and outcomes across a wider range of interventions. This could include comparisons of TAU plus CBT with TAU plus other forms of counselling, supportive care or psychosocial therapy interventions or with TAU plus pharmacological augmentation. However, in this treatment-resistant population, there are likely to be limited data to inform the model structure and populate the model.
Conclusions
Cognitive–behavioural therapy for people who meet the criteria for CRS has small but significant improvements for symptoms of psychosis in the short term, but there is not a lasting effect in the long term (except in relation to self-rated recovery). CBT is a highly acceptable treatment with little evidence of adverse effects for this population. Finally, psychotic symptoms in CRS improved in both groups to a clinically significant extent over 21 months, suggesting that long-term recovery is possible.
Acknowledgements
Thank you to all the participants who agreed to take part in the trial. This study was supported by NHS Research Scotland, through the Chief Scientist Office, the Scottish Mental Health Research Network and the Mental Health Research Network. We are grateful to the Psychosis Research Unit SURG for their consultation regarding the design of the trial and contribution to the developments of trial-related materials. We are grateful to our Independent Trial Steering Committee (Max Birchwood, Daniel Freeman, Rod Taylor, Julia Renton, David Shiers, Yvonne Thomas and Kathryn Harney) and Independent Data Monitoring and Ethics Committee (Richard Bentall, Sabine Landau, Emmanuelle Peters and Tim Rawcliffe) for providing oversight of the trial. We are also grateful to the many researchers, network staff and trial therapists who supported the study, including Elizabeth Murphy, Vicky Brooks, Natasha Holden, Ann Steele, Nicola Chapman, Lisa Wood, Lucy Carter, Rachel Sellers, Eilish Burke, Savas Akgonul, Mary Shinner, Yvonne Slater, Elizabeth Graves, Helen Mander, Trevor Munro-Clarke, Kate Shirvell, Carolyn Asher, Liesbeth Tip, Jane Owens, Shauneen Porter, Clare Jackson, Susan Irving, Caoimhe Clarke, Helen Whitehill, Rachel Allan, Suzan Aydinlar, Mairi Spanswick, Jacqueline McTaggart, James Courtley, Laura McCartney, Toyah Lebert, Maggie Douglas-Bailey, Anna Cummings and Helen Spencer.
The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Government Health Directorate.
Contributions of authors
Anthony P Morrison (Professor of Clinical Psychology) planned the study, contributed to the application for funding, made substantial contributions to the design of the trial protocol, the SAP, managed the trial as chief investigator, contributed to writing and critically read the manuscript.
Melissa Pyle (Research Trial Manager) made substantial contribution to the development of the trial protocol, as well as overall management of the trial and data management and wrote the first draft of the manuscript.
Andrew Gumley (Professor of Psychological Therapy) contributed to the application for funding, made a substantial contribution to the design of the trial and protocol and critically read the manuscript.
Matthias Schwannauer (Professor of Clinical Psychology) contributed to the application for funding, made substantial contribution to the design of the trial and protocol and critically read the manuscript.
Douglas Turkington (Professor of Psychosocial Psychiatry) contributed to the application for funding, made substantial contribution to the design of the trial and protocol and critically read the manuscript.
Graeme MacLennan (Director and Professor, The Centre for Healthcare Randomised Trials) made substantial contribution to the development of the trial design and protocol, as well as the SAP, conducted the analysis and critically read the manuscript.
John Norrie (Professor of Medical Statistics and Trial Methodology) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, the SAP, and critically read the manuscript.
Jemma Hudson (Trial Statistician) made substantial contribution to the development of the trial design and protocol, as well as the SAP, conducted the analysis and critically read the manuscript.
Samantha Bowe (Clinical Psychologist) made substantial contribution to the development of the trial design and protocol, and critically read the manuscript.
Paul French (Mental Health Clinical Lead for Greater Manchester and East Cheshire Strategic Clinical Network, NHS England North Clinical Lead for EIP and Honorary Professor) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, and the SAP, and critically read the manuscript.
Paul Hutton (Associate Professor of Therapeutic Interventions and Honorary Consultant Clinical Psychologist) made substantial contribution to the development of the trial design and protocol, and critically read the manuscript.
Rory Byrne (User-led Researcher) contributed to the application for funding, made substantial contribution to the design of the trial and protocol and critically read the manuscript.
Suzy Syrett (User-led Researcher) contributed to the application for funding, made substantial contribution to the design of the trial and protocol and critically read the manuscript.
Robert Dudley (Consultant Clinical Psychologist and Associate Clinical Lecturer) made substantial contribution to the trial protocol and critically read the manuscript.
Hamish J McLeod (Senior Lecturer in Mental Health and Wellbeing) made substantial contribution to the development of the trial design and protocol, and critically read the manuscript.
Helen Griffiths (Clinical Psychologist and Lecturer in Clinical Psychology) made substantial contribution to the development of the trial design and protocol, and critically read the manuscript.
Thomas RE Barnes (Professor of Clinical Psychiatry) contributed to the application for funding, made substantial contribution to the design of the trial and protocol and critically read the manuscript.
Linda Davies (Professor of Health Economics) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, the health economic analysis plan, and critically read the manuscript.
Gemma Shields (Health Economist) made substantial contribution to the health economics SAP, conducted the health economics analysis, conducted the health economics section of the manuscript and critically read the manuscript as a whole.
Deborah Buck (Health Economist) made substantial contribution to the health economics SAP, conducted the health economics analysis, conducted the health economics section of the manuscript and critically read the manuscript as a whole.
Sarah Tully (Assistant Research Psychologist) contributed to the trial protocol, significantly contributed to writing the manuscript and critically read the manuscript.
David Kingdon (Professor of Mental Health Care Delivery) contributed to the application for funding, made substantial contribution to the design of the trial and protocol, and critically read the manuscript.
All authors read and approved the final manuscript.
Publications
Pyle M, Norrie J, Schwannauer M, Kingdon D, Gumley A, Tukington D, et al. Design and protocol for the Focusing on Clozapine Unresponsive Symptoms (FOCUS) trial: a randomised controlled trial. BMC Psychiatry 2016;16:1–12.
Morrison AP, Pyle M, Gumley A, Schwannauer M, Turkington D, MacLennan G, et al. Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): an assessor-blinded, randomised controlled trial. Lancet Psychiatry 2018;5:633–43.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Suhail K, Cochrane R. Effect of culture and environment on the phenomenology of delusions and hallucinations. Int J Soc Psychiatry 2002;48:126-38. https://doi.org/10.1177/002076402128783181.
- Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol 2002;41:331-47. https://doi.org/10.1348/014466502760387461.
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 2006;32:214-19. https://doi.org/10.1093/schbul/sbj053.
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry 1982;39:784-8. https://doi.org/10.1001/archpsyc.1982.04290070020005.
- Crow TJ. Positive and negative schizophrenic symptoms and the role of dopamine. Br J Psychiatry 1980;137:383-6.
- Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 2009;10:48-5. https://doi.org/10.1038/nrn2536.
- Bobes J, Arango C, Garcia-Garcia M, Rejas J. CLAMORS Study Collaborative Group . Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry 2010;71:280-6. https://doi.org/10.4088/JCP.08m04250yel.
- Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull 2007;33:1013-22. https://doi.org/10.1093/schbul/sbl057.
- Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev 2001;21:1125-41. https://doi.org/10.1016/S0272-7358(01)00103-9.
- Peters E, Ward T, Jackson M, Morgan C, Charalambides M, McGuire P, et al. Clinical, socio-demographic and psychological characteristics in individuals with persistent psychotic experiences with and without a ‘need for care’. World Psychiatry 2016;15:41-52. https://doi.org/10.1002/wps.20301.
- van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population?. Schizophr Res 2000;45:11-20. https://doi.org/10.1016/S0920-9964(99)00224-8.
- van Os J. Is there a continuum of psychotic experiences in the general population?. Epidemiol Psychiatr Sci 2003;12:242-52. https://doi.org/10.1017/S1121189X00003067.
- The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
- Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Washington, DC: American Psychiatric Association; 2013.
- van Os J. ‘Schizophrenia’ does not exist. BMJ 2016;352. https://doi.org/10.1136/bmj.i375.
- Bentall RP. Deconstructing the concept of ’schizophrenia’. J Ment Health 1993;2:223-38. https://doi.org/10.3109/09638239309003768.
- McGorry PD. The concept of recovery and secondary prevention in psychotic disorders. Aust N Z J Psychiatry 1992;26:3-17. https://doi.org/10.3109/00048679209068305.
- Sato M. Renaming schizophrenia: a Japanese perspective. World Psychiatry 2006;5:53-5.
- Murray RM. Mistakes I have made in my research career. Schizophr Bull 2017;43:253-6. https://doi.org/10.1093/schbul/sbw165.
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007;20:359-64. https://doi.org/10.1097/YCO.0b013e32816ebc8c.
- Goldner EM, Hsu L, Waraich P, Somers JM. Prevalence and incidence studies of schizophrenic disorders: a systematic review of the literature. Can J Psychiatry 2002;47:833-43. https://doi.org/10.1177/070674370204700904.
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, Boydell J, et al. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0031660.
- McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med 2004;2. https://doi.org/10.1186/1741-7015-2-13.
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:655-79. https://doi.org/10.1016/j.euroneuro.2011.07.018.
- DE Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52-77. https://doi.org/10.1002/j.2051-5545.2011.tb00014.x.
- Hennekens CH. Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. J Clin Psychiatry 2007;68:4-7. https://doi.org/10.4088/JCP.0507e12.
- Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 2017;4:295-301. https://doi.org/10.1016/S2215-0366(17)30078-0.
- Brohan E, Elgie R, Sartorius N, Thornicroft G. GAMIAN-Europe Study Group . Self-stigma, empowerment and perceived discrimination among people with schizophrenia in 14 European countries: the GAMIAN-Europe study. Schizophr Res 2010;122:232-8. https://doi.org/10.1016/j.schres.2010.02.1065.
- Thornicroft G, Brohan E, Rose D, Sartorius N, Leese M. INDIGO Study Group . Global pattern of experienced and anticipated discrimination against people with schizophrenia: a cross-sectional survey. Lancet 2009;373:408-15. https://doi.org/10.1016/S0140-6736(08)61817-6.
- Psychosis and Schizophrenia in Adults: Prevention and Management. London: NICE; 2014.
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 2009;373:31-4. https://doi.org/10.1016/S0140-6736(08)61764-X.
- Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry 2017;174:927-42. https://doi.org/10.1176/appi.ajp.2017.16121358.
- Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006;63:1079-87. https://doi.org/10.1001/archpsyc.63.10.1079.
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23. https://doi.org/10.1056/NEJMoa051688.
- Kendall T. The rise and fall of the atypical antipsychotics. Br J Psychiatry 2011;199:266-8. https://doi.org/10.1192/bjp.bp.110.083766.
- Morrison AP, Hutton P, Shiers D, Turkington D. Antipsychotics: is it time to introduce patient choice?. Br J Psychiatry 2012;201:83-4. https://doi.org/10.1192/bjp.bp.112.112110.
- Haddad PM, Sharma SG. Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs 2007;21:911-36. https://doi.org/10.2165/00023210-200721110-00004.
- Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 2010;40:1409-22. https://doi.org/10.1017/S0033291709992297.
- Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull 2015;114:169-79. https://doi.org/10.1093/bmb/ldv017.
- Barnes T, Buckley P, Schulz S, Hirsch S, Weinberger D. Schizophrenia. Oxford: Blackwell Publishing; 2003.
- Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol 2014;29:63-76. https://doi.org/10.1097/YIC.0b013e32836508e6.
- Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev 2008;30:1-14. https://doi.org/10.1093/epirev/mxn011.
- Iasevoli F, Giordano S, Balletta R, Latte G, Formato MV, Prinzivalli E, et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog Neuropsychopharmacol Biol Psychiatry 2016;65:34-48. https://doi.org/10.1016/j.pnpbp.2015.08.010.
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45:789-96. https://doi.org/10.1001/archpsyc.1988.01800330013001.
- Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am J Psychiatry 2017;174:216-29. https://doi.org/10.1176/appi.ajp.2016.16050503.
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 2012;13:318-78. https://doi.org/10.3109/15622975.2012.696143.
- Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD000059.pub2.
- Legge SE, Hamshere M, Hayes RD, Downs J, O’Donovan MC, Owen MJ, et al. Reasons for discontinuing clozapine: a cohort study of patients commencing treatment. Schizophr Res 2016;174:113-19. https://doi.org/10.1016/j.schres.2016.05.002.
- Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2016;209:385-92. https://doi.org/10.1192/bjp.bp.115.177261.
- Samara MT, Dold M, Gianatsi M, Nikolakopoulou A, Helfer B, Salanti G, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry 2016;73:199-210. https://doi.org/10.1001/jamapsychiatry.2015.2955.
- Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001;158:518-26. https://doi.org/10.1176/appi.ajp.158.4.518.
- Honer WG, Thornton AE, Chen EY, Chan RC, Wong JO, Bergmann A, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med 2006;354:472-82. https://doi.org/10.1056/NEJMoa053222.
- Dold M, Leucht S. Pharmacotherapy of treatment-resistant schizophrenia: a clinical perspective. Evid Based Ment Health 2014;17:33-7. https://doi.org/10.1136/eb-2014-101813.
- Porcelli S, Balzarro B, Serretti A. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol 2012;22:165-82. https://doi.org/10.1016/j.euroneuro.2011.08.005.
- Taylor DM, Smith L, Gee SH, Nielsen J. Augmentation of clozapine with a second antipsychotic – a meta-analysis. Acta Psychiatr Scand 2012;125:15-24. https://doi.org/10.1111/j.1600-0447.2011.01792.x.
- Sommer IE, Begemann MJ, Temmerman A, Leucht S. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull 2012;38:1003-11. https://doi.org/10.1093/schbul/sbr004.
- Rattehalli RD, Zhao S, Li BG, Jayaram MB, Xia J, Sampson S. Risperidone versus placebo for schizophrenia. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD006918.pub3.
- Barber S, Olotu U, Corsi M, Cipriani A. Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia. Cochrane Database Syst Rev 2017;3. https://doi.org/10.1002/14651858.CD006324.pub3.
- Keating D, McWilliams S, Schneider I, Hynes C, Cousins G, Strawbridge J, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013881.
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1979.
- Chadwick P, Birchwood M. The omnipotence of voices. A cognitive approach to auditory hallucinations. Br J Psychiatry 1994;164:190-201. https://doi.org/10.1192/bjp.164.2.190.
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med 2001;31:189-95. https://doi.org/10.1017/S0033291701003312.
- Morrison AP. The interpretation of intrusions in psychosis: an integrative cognitive approach to hallucinations and delusions. Behav Cogn Psychother 2001;29:257-76. https://doi.org/10.1017/S1352465801003010.
- Morrison A, Renton J, Dunn H, Williams S, Bentall R. Cognitive Therapy for Psychosis: a Formulation-Based Approach. Hove: Brunner–Routledge; 2004.
- Brabban A, Byrne R, Longden E, Morrison AP. The importance of human relationships, ethics and recovery-orientated values in the delivery of CBT for people with psychosis. Psychosis 2016;9:157-66. https://doi.org/10.1080/17522439.2016.1259648.
- Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Orbach G, et al. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002;32:763-82. https://doi.org/10.1017/S0033291702005895.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523-37. https://doi.org/10.1093/schbul/sbm114.
- Zimmermann G, Favrod J, Trieu VH, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res 2005;77:1-9. https://doi.org/10.1016/j.schres.2005.02.018.
- Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014;204:20-9. https://doi.org/10.1192/bjp.bp.112.116285.
- Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry 2014;171:523-38. https://doi.org/10.1176/appi.ajp.2013.13081159.
- van der Gaag M, Valmaggia LR, Smit F. The effects of individually tailored formulation-based cognitive behavioural therapy in auditory hallucinations and delusions: a meta-analysis. Schizophr Res 2014;156:30-7. https://doi.org/10.1016/j.schres.2014.03.016.
- McKenna P, Kingdon D. Has cognitive behavioural therapy for psychosis been oversold?. BMJ 2014;348. https://doi.org/10.1136/bmj.g2295.
- Tarrier N, Yusupoff L, Kinney C, McCarthy E, Gledhill A, Haddock G, et al. Randomised controlled trial of intensive cognitive behaviour therapy for patients with chronic schizophrenia. BMJ 1998;317:303-7. https://doi.org/10.1136/bmj.317.7154.303.
- Pinto A, Pia SL, Mennella R, Giorgio D, DeSimone L. Rehab rounds: cognitive–behavioral therapy and clozapine for clients with treatment-refractory schizophrenia. Psychiatri Serv 1999;50:901-4. https://doi.org/10.1176/ps.50.7.901.
- Kuipers E, Garety P, Fowler D, Dunn G, Bebbington P, Freeman D, et al. London-East Anglia randomised controlled trial of cognitive-behavioural therapy for psychosis. I: effects of the treatment phase. Br J Psychiatry 1997;171:319-27. https://doi.org/10.1192/bjp.171.4.319.
- Sensky T, Turkington D, Kingdon D, Scott JL, Scott J, Siddle R, et al. A randomized controlled trial of cognitive–behavioral therapy for persistent symptoms in schizophrenia resistant to medication. Arch Gen Psychiatry 2000;57:165-72. https://doi.org/10.1001/archpsyc.57.2.165.
- Turkington D, Sensky T, Scott J, Barnes TR, Nur U, Siddle R, et al. A randomized controlled trial of cognitive-behavior therapy for persistent symptoms in schizophrenia: a five-year follow-up. Schizophr Res 2008;98:1-7. https://doi.org/10.1016/j.schres.2007.09.026.
- Durham RC, Guthrie M, Morton RV, Reid DA, Treliving LR, Fowler D, et al. Tayside-Fife clinical trial of cognitive–behavioural therapy for medication-resistant psychotic symptoms. Results to 3-month follow-up. Br J Psychiatry 2003;182:303-11. https://doi.org/10.1192/bjp.182.4.303.
- Valmaggia LR, van der Gaag M, Tarrier N, Pijnenborg M, Slooff CJ. Cognitive–behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication. Randomised controlled trial. Br J Psychiatry 2005;186:324-30. https://doi.org/10.1192/bjp.186.4.324.
- Sinclair D, Adams CE. Treatment resistant schizophrenia: a comprehensive survey of randomised controlled trials. BMC Psychiatry 2014;14. https://doi.org/10.1186/s12888-014-0253-4.
- Birchwood M, Peters E, Tarrier N, Dunn G, Lewis S, Wykes T, et al. A multi-centre, randomised controlled trial of cognitive therapy to prevent harmful compliance with command hallucinations. BMC Psychiatry 2011;11. https://doi.org/10.1186/1471-244X-11-155.
- Haddock G, Slade PD, Bentall RP, Reid D, Faragher EB. A comparison of the long-term effectiveness of distraction and focusing in the treatment of auditory hallucinations. Br J Med Psychol 1998;71:339-49. https://doi.org/10.1111/j.2044-8341.1998.tb00996.x.
- Hayward P, David A, Green N, Rabe-Hesketh S, Haworth E, Thompson N, et al. Promoting therapeutic alliance in clozapine users: an exploratory randomized controlled trial. Clin Schizophr Relat Psychoses 2009;3:127-32. https://doi.org/10.3371/CSRP.3.3.1.
- Barretto EM, Kayo M, Avrichir BS, Sa AR, Camargo Md, Napolitano IC, et al. A preliminary controlled trial of cognitive behavioral therapy in clozapine-resistant schizophrenia. J Nerv Ment Dis 2009;197:865-8. https://doi.org/10.1097/NMD.0b013e3181be7422.
- Allott K, Alvarez-Jimenez M, Killackey EJ, Bendall S, McGorry PD, Jackson HJ. Patient predictors of symptom and functional outcome following cognitive behaviour therapy or befriending in first-episode psychosis. Schizophr Res 2011;132:125-30. https://doi.org/10.1016/j.schres.2011.08.011.
- Brabban A, Tai S, Turkington D. Predictors of outcome in brief cognitive behavior therapy for schizophrenia. Schizophr Bull 2009;35:859-64. https://doi.org/10.1093/schbul/sbp065.
- Lincoln TM, Rief W, Westermann S, Ziegler M, Kesting ML, Heibach E, et al. Who stays, who benefits? Predicting dropout and change in cognitive behaviour therapy for psychosis. Psychiatry Res 2014;216:198-205. https://doi.org/10.1016/j.psychres.2014.02.012.
- Rathod S, Kingdon D, Smith P, Turkington D. Insight into schizophrenia: the effects of cognitive behavioural therapy on the components of insight and association with sociodemographics – data on a previously published randomised controlled trial. Schizophr Res 2005;74:211-19. https://doi.org/10.1016/j.schres.2004.07.003.
- Garety P, Fowler D, Kuipers E, Freeman D, Dunn G, Bebbington P, et al. London-East Anglia randomised controlled trial of cognitive-behavioural therapy for psychosis. II: predictors of outcome. Br J Psychiatry 1997;171:420-6. https://doi.org/10.1192/bjp.171.5.420.
- Drury V, Birchwood M, Cochrane R, MacMillan F. Cognitive therapy and recovery from acute psychosis: a controlled trial. I. Impact on psychotic symptoms. Br J Psychiatry 1996;169:593-601. https://doi.org/10.1192/bjp.169.5.593.
- Morrison AP, Renton JC, Williams S, Dunn H, Knight A, Kreutz M, et al. Delivering cognitive therapy to people with psychosis in a community mental health setting: an effectiveness study. Acta Psychiatr Scand 2004;110:36-44. https://doi.org/10.1111/j.1600-0447.2004.00299.x.
- Morrison AP, Turkington D, Wardle M, Spencer H, Barratt S, Dudley R, et al. A preliminary exploration of predictors of outcome and cognitive mechanisms of change in cognitive behaviour therapy for psychosis in people not taking antipsychotic medication. Behav Res Ther 2012;50:163-7. https://doi.org/10.1016/j.brat.2011.12.001.
- Naeem F, Kingdon D, Turkington D. Predictors of response to cognitive behaviour therapy in the treatment of schizophrenia: a comparison of brief and standard interventions. Cognit Ther Res 2008;32:651-6. https://doi.org/10.1007/s10608-008-9186-x.
- Johns LC, Cannon M, Singleton N, Murray RM, Farrell M, Brugha T, et al. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry 2004;185:298-305. https://doi.org/10.1192/bjp.185.4.298.
- Manning C, Stickley T. Childhood abuse and psychosis; a critical review of the literature. J Res Nurs 2009;14:531-47. https://doi.org/10.1177/1744987109347045.
- Siegel RK. Hostage hallucinations. Visual imagery induced by isolation and life-threatening stress. J Nerv Ment Dis 1984;172:264-72. https://doi.org/10.1097/00005053-198405000-00003.
- Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull 2012;38:1118-23. https://doi.org/10.1093/schbul/sbs096.
- Hollander AC, Dal H, Lewis G, Magnusson C, Kirkbride JB, Dalman C. Refugee migration and risk of schizophrenia and other non-affective psychoses: cohort study of 1.3 million people in Sweden. BMJ 2016;352. https://doi.org/10.1136/bmj.i1030.
- Kirkbride JB, Jones PB, Ullrich S, Coid JW. Social deprivation, inequality, and the neighborhood-level incidence of psychotic syndromes in East London. Schizophr Bull 2014;40:169-80. https://doi.org/10.1093/schbul/sbs151.
- Janssen I, Hanssen M, Bak M, Bijl RV, de Graaf R, Vollebergh W, et al. Discrimination and delusional ideation. Br J Psychiatry 2003;182:71-6. https://doi.org/10.1192/bjp.182.1.71.
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull 2012;38:661-71. https://doi.org/10.1093/schbul/sbs050.
- Bentall RP, Wickham S, Shevlin M, Varese F. Do specific early-life adversities lead to specific symptoms of psychosis? A study from the 2007 the Adult Psychiatric Morbidity Survey. Schizophr Bull 2012;38:734-40. https://doi.org/10.1093/schbul/sbs049.
- Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A. Childhood trauma and children’s emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. Am J Psychiatry 2011;168:65-72. https://doi.org/10.1176/appi.ajp.2010.10040567.
- Morrison AP. A manualised treatment protocol to guide delivery of evidence-based cognitive therapy for people with distressing psychosis: learning from clinical trials. Psychosis 2017;9:1-11. https://doi.org/10.1080/17522439.2017.1295098.
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat 2006;2:531-6. https://doi.org/10.2147/nedt.2006.2.4.531.
- Goldberg TE, Green MF, Davis KL, Charney D, Coyle JT, Nemeroff C. Neuropsychopharmacology: the Fifth Generation of Progress. Brentwood, CA: American College of Neuropsychopharmacology; 2002.
- Baddeley A. Working Memory. Oxford: Clarendon Press; 1986.
- Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med 2009;39:889-905. https://doi.org/10.1017/S0033291708004558.
- McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, et al. The effects of clozapine and risperidone on spatial working memory in schizophrenia. Am J Psychiatry 2005;162:1013-16. https://doi.org/10.1176/appi.ajp.162.5.1013.
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry 2004;161:116-24. https://doi.org/10.1176/appi.ajp.161.1.116.
- James IA, Reichelt FK, Carlsonn P, McAnaney A. Cognitive behavior therapy and executive functioning in depression. J Cogn Psychother 2008;22:210-8. https://doi.org/10.1891/0889-8391.22.3.210.
- Granholm E, McQuaid JR, Link PC, Fish S, Patterson T, Jeste DV. Neuropsychological predictors of functional outcome in Cognitive Behavioral Social Skills Training for older people with schizophrenia. Schizophr Res 2008;100:133-43. https://doi.org/10.1016/j.schres.2007.11.032.
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ’right stuff’?. Schizophr Bull 2000;26:119-36. https://doi.org/10.1093/oxfordjournals.schbul.a033430.
- Glaser NM, Kazantzis N, Deane FP, Oades LG. Critical issues in using homework assignments within cognitive–behavioral therapy for schizophrenia. J Ration Emot Cogn Behav Ther 2000;18:247-61. https://doi.org/10.1023/A:1007888615752.
- Kurtz MM. Neurocognition as a predictor of response to evidence-based psychosocial interventions in schizophrenia: what is the state of the evidence?. Clin Psychol Rev 2011;31:663-72. https://doi.org/10.1016/j.cpr.2011.02.008.
- Crawford MJ, Robotham D, Thana L, Patterson S, Weaver T, Barber R, et al. Selecting outcome measures in mental health: the views of service users. J Ment Health 2011;20:336-46. https://doi.org/10.3109/09638237.2011.577114.
- Andresen R, Oades L, Caputi P. The experience of recovery from schizophrenia: towards an empirically validated stage model. Aust N Z J Psychiatry 2003;37:586-94. https://doi.org/10.1046/j.1440-1614.2003.01234.x.
- Bellack AS. Scientific and consumer models of recovery in schizophrenia: concordance, contrasts, and implications. Schizophr Bull 2006;32:432-42. https://doi.org/10.1093/schbul/sbj044.
- Pitt L, Kilbride M, Nothard S, Welford M, Morrison AP. Researching recovery from psychosis: a user-led project. Psychiatrist 2007;31:55-60.
- Leamy M, Bird V, Le Boutillier C, Williams J, Slade M. Conceptual framework for personal recovery in mental health: systematic review and narrative synthesis. Br J Psychiatry 2011;199:445-52. https://doi.org/10.1192/bjp.bp.110.083733.
- Birchwood M, Trower P. The future of cognitive–behavioural therapy for psychosis: not a quasi-neuroleptic. Br J Psychiatry 2006;188:107-8. https://doi.org/10.1192/bjp.bp.105.014985.
- The Abandoned Illness: a Report from the Schizophrenia Commission. London: Rethink Mental Illness; 2012.
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006;31:2318-25. https://doi.org/10.1038/sj.npp.1301147.
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behavior therapy versus other psychosocial treatments for schizophrenia. Schizophr Bull 2012;38:908-10. https://doi.org/10.1093/schbul/sbs090.
- Klingberg S, Wittorf A, Meisner C, Wölwer W, Wiedemann G, Herrlich J, et al. Cognitive behavioural therapy versus supportive therapy for persistent positive symptoms in psychotic disorders: the POSITIVE Study, a multicenter, prospective, single-blind, randomised controlled clinical trial. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-123.
- Klingberg S, Herrlich J, Wiedemann G, Wölwer W, Meisner C, Engel C, et al. Adverse effects of cognitive behavioral therapy and cognitive remediation in schizophrenia: results of the treatment of negative symptoms study. J Nerv Ment Dis 2012;200:569-76. https://doi.org/10.1097/NMD.0b013e31825bfa1d.
- Pyle M, Norrie J, Schwannauer M, Kingdon D, Gumley A, Turkington D, et al. Design and protocol for the Focusing on Clozapine Unresponsive Symptoms (FOCUS) trial: a randomised controlled trial. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-0983-6.
- Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208. https://doi.org/10.1097/01.jcp.0000117422.05703.ae.
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophr Res 2005;79:231-8. https://doi.org/10.1016/j.schres.2005.04.008.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- van der Gaag M, Cuijpers A, Hoffman T, Remijsen M, Hijman R, de Haan L, et al. The five-factor model of the Positive and Negative Syndrome Scale I: confirmatory factor analysis fails to confirm 25 published five-factor solutions. Schizophr Res 2006;85:273-9. https://doi.org/10.1016/j.schres.2006.04.001.
- van der Gaag M, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res 2006;85:280-7. https://doi.org/10.1016/j.schres.2006.03.021.
- Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr Bull 2010;36:461-2. https://doi.org/10.1093/schbul/sbq016.
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med 1999;29:879-89. https://doi.org/10.1017/S0033291799008661.
- Neil ST, Kilbride M, Pitt L, Nothard S, Welford M, Sellwood W, et al. The Questionnaire about the Process of Recovery (QPR): a measurement tool developed in collaboration with service users. Psychosis 2009;1:145-55. https://doi.org/10.1080/17522430902913450.
- Law H, Neil ST, Dunn G, Morrison AP. Psychometric properties of the Questionnaire about the Process of Recovery (QPR). Schizophr Res 2014;156:184-9. https://doi.org/10.1016/j.schres.2014.04.011.
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101:323-9.
- Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res 1992;6:201-8. https://doi.org/10.1016/0920-9964(92)90003-N.
- Wells A. A multi-dimensional measure of worry: development and preliminary validation of the Anxious Thoughts Inventory. Anxiety Stress Coping 1994;6:289-99. https://doi.org/10.1080/10615809408248803.
- Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry 2008;53:26-33. https://doi.org/10.1177/070674370805300105.
- Skinner HA. The drug abuse screening test. Addict Behav 1982;7:363-71. https://doi.org/10.1016/0306-4603(82)90005-3.
- Morrison AP, Wells A, Nothard S. Cognitive and emotional predictors of predisposition to hallucinations in non-patients. Br J Clin Psychol 2002;41:259-70. https://doi.org/10.1348/014466502760379127.
- Morrison AP, Gumley AI, Schwannauer M, Campbell M, Gleeson A, Griffin E, et al. The Beliefs about Paranoia Scale: preliminary validation of a metacognitive approach to conceptualizing paranoia. Behav Cogn Psychother 2005;33:153-64. https://doi.org/10.1017/S1352465804001900.
- Murphy EK, Tully S, Pyle M, Gumley AI, Kingdon D, Schwannauer M, et al. The Beliefs about Paranoia Scale: confirmatory factor analysis and tests of a metacognitive model of paranoia in a clinical sample. Psychiatry Res 2017;248:87-94. https://doi.org/10.1016/j.psychres.2016.11.012.
- Fowler D, Freeman D, Smith B, Kuipers E, Bebbington P, Bashforth H, et al. The Brief Core Schema Scales (BCSS): psychometric properties and associations with paranoia and grandiosity in non-clinical and psychosis samples. Psychol Med 2006;36:749-59. https://doi.org/10.1017/S0033291706007355.
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 1997;54:159-65. https://doi.org/10.1001/archpsyc.1997.01830140071013.
- Berry K, Wearden A, Barrowclough C, Liversidge T. Attachment styles, interpersonal relationships and psychotic phenomena in a non-clinical student sample. Pers Individ Differ 2006;41:707-18. https://doi.org/10.1016/j.paid.2006.03.009.
- Ritsher JB, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res 2003;121:31-49. https://doi.org/10.1016/j.psychres.2003.08.008.
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151:1132-6. https://doi.org/10.1176/ajp.151.8.1132.
- Kinoshita Y, Kingdon D, Kinoshita K, Sarafudheen S, Umadi D, Dayson D, et al. A semi-structured clinical interview for psychosis sub-groups (SCIPS): development and psychometric properties. Soc Psychiatry Psychiatr Epidemiol 2012;47:563-80. https://doi.org/10.1007/s00127-011-0357-9.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- Bobes J, García-Portilla P, Sáiz PA, Bascarán T, Bousoño M. Quality of life measures in schizophrenia. Eur Psychiatry 2005;20:313-17. https://doi.org/10.1016/S0924-9338(05)80182-8.
- Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. New York, NY: Guilford Press; 2005.
- Flach C, French P, Dunn G, Fowler D, Gumley AI, Birchwood M, et al. Components of therapy as mechanisms of change in cognitive therapy for people at risk of psychosis: analysis of the EDIE-2 trial. Br J Psychiatry 2015;207:123-9. https://doi.org/10.1192/bjp.bp.114.153320.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. https://doi.org/10.1136/bmj.d40.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. OHE Research Paper 16/01. London: Office of Health Economics; 2016.
- Barton GR, Hodgekins J, Mugford M, Jones PB, Croudace T, Fowler D. Measuring the benefits of treatment for psychosis: validity and responsiveness of the EQ-5D. Br J Psychiatry 2009;195:170-7. https://doi.org/10.1192/bjp.bp.108.057380.
- Davies LM, Lewis S, Jones PB, Barnes TR, Gaughran F, Hayhurst K, et al. Cost-effectiveness of first- v. second-generation antipsychotic drugs: results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. Br J Psychiatry 2007;191:14-22. https://doi.org/10.1192/bjp.bp.106.028654.
- Davies LM, Barnes TR, Jones PB, Lewis S, Gaughran F, Hayhurst K, et al. A randomized controlled trial of the cost-utility of second-generation antipsychotics in people with psychosis and eligible for clozapine. Value Health 2008;11:549-62. https://doi.org/10.1111/j.1524-4733.2007.00280.x.
- Department of Health and Social Care . NHS Reference Costs: National Schedule of Reference Costs 2015–2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- Claxton K, Martin S, Soares MO, Rice N, Spackman DE, Hinde S, et al. Methods for the Estimation of the NICE Cost Effectiveness Threshold. York: Centre for Health Economics, University of York; 2013.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Morrison AP, Pyle M, Gumley A, Schwannauer M, Turkington D, MacLennan G, et al. Cognitive behavioural therapy in clozapine-resistant schizophrenia (FOCUS): an assessor-blinded, randomised controlled trial. Lancet Psychiatry 2018;5:633-43. https://doi.org/10.1016/S2215-0366(18)30184-6.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. https://doi.org/10.1136/bmj.329.7459.224.
- Goldsmith LP, Lewis SW, Dunn G, Bentall RP. Psychological treatments for early psychosis can be beneficial or harmful, depending on the therapeutic alliance: an instrumental variable analysis. Psychol Med 2015;45:2365-73. https://doi.org/10.1017/S003329171500032X.
- Janssen B, Szende A, Szende A, Janssen B, Cabases J. Self-Reported Population Health: an International Perspective Based on EQ-5D. The Netherlands: Springer; 2014.
- Crawford MJ, Killaspy H, Barnes TR, Barrett B, Byford S, Clayton K, et al. Group art therapy as an adjunctive treatment for people with schizophrenia: a randomised controlled trial (MATISSE). Health Technol Assess 2012;16. https://doi.org/10.3310/hta16080.
- Priebe S, Pavlickova H, Eldridge S, Golden E, McCrone P, Ockenden N, et al. Effectiveness of one-to-one volunteer support for patients with psychosis: protocol of a randomised controlled trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011582.
- Haddock G, Barrowclough C, Tarrier N, Moring J, O’Brien R, Schofield N, et al. Cognitive–behavioural therapy and motivational intervention for schizophrenia and substance misuse. 18-month outcomes of a randomised controlled trial. Br J Psychiatry 2003;183:418-26. https://doi.org/10.1192/bjp.183.5.418.
- McCrone P, Craig TK, Power P, Garety PA. Cost-effectiveness of an early intervention service for people with psychosis. Br J Psychiatry 2010;196:377-82. https://doi.org/10.1192/bjp.bp.109.065896.
- Patel A, Knapp M, Romeo R, Reeder C, Matthiasson P, Everitt B, et al. Cognitive remediation therapy in schizophrenia: cost-effectiveness analysis. Schizophr Res 2010;120:217-24. https://doi.org/10.1016/j.schres.2009.12.003.
- Lawrence D, Kisely S. Inequalities in healthcare provision for people with severe mental illness. J Psychopharmacol 2010;24:61-8. https://doi.org/10.1177/1359786810382058.
- Thornicroft G. Physical health disparities and mental illness: the scandal of premature mortality. Br J Psychiatry 2011;199:441-2. https://doi.org/10.1192/bjp.bp.111.092718.
- Corrigan PW, Larson JE, Rüsch N. Self-stigma and the ‘why try’ effect: impact on life goals and evidence-based practices. World Psychiatry 2009;8:75-81. https://doi.org/10.1002/j.2051-5545.2009.tb00218.x.
- De Hert M, Cohen D, Bobes J, Cetkovich-Bakmas M, Leucht S, Ndetei DM, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry 2011;10:138-51. https://doi.org/10.1002/j.2051-5545.2011.tb00036.x.
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452-4. https://doi.org/10.1136/bmj.310.6977.452.
- Altman DG. Confidence intervals for the number needed to treat. BMJ 1998;317:1309-12. https://doi.org/10.1136/bmj.317.7168.1309.
- Position Statement on the use of the EQ-5D-5L Valuation Set. London: NICE; 2017.
- Crawford MJ, Killaspy H, Barnes TR, Barrett B, Byford S, Clayton K, et al. Group art therapy as an adjunctive treatment for people with schizophrenia: multicentre pragmatic randomised trial. BMJ 2012;344. https://doi.org/10.1136/bmj.e846.
- Priebe S, Savill M, Wykes T, Bentall R, Lauber C, Reininghaus U, et al. Clinical effectiveness and cost-effectiveness of body psychotherapy in the treatment of negative symptoms of schizophrenia: a multicentre randomised controlled trial. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20110.
- Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. Early intervention services, cognitive–behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry 2010;197:350-6. https://doi.org/10.1192/bjp.bp.109.074526.
Appendix 1 Recruitment information
| Service setting | Centre, n (%) | |||||
|---|---|---|---|---|---|---|
| All (N = 487) | Manchester (N = 108) | Southampton (N = 105) | Newcastle (N = 92) | Edinburgh (N = 92) | Glasgow (N = 90) | |
| CMHT | 224 (46.0) | 24 (22.2) | 69 (65.7) | 69 (75.0) | 24 (26.1) | 38 (42.2) |
| Clozapine clinic | 129 (26.5) | 20 (18.5) | 19 (18.1) | 8 (8.7) | 39 (42.4) | 43 (47.8) |
| MHRN | 46 (9.4) | 44 (40.7) | 2 (1.9) | 0.00 | 0.00 | 0.00 |
| Inpatient unit | 42 (8.6) | 4 (3.7) | 1 (1.0) | 7 (7.6) | 24 (26.1) | 6 (6.7) |
| Research trial | 9 (1.8) | 2 (1.9) | 4 (3.8) | 0.00 | 3 (3.3) | 0.00 |
| EIP | 8 (1.6) | 3 (2.8) | 1 (1.0) | 4 (4.3) | 0.00 | 0.00 |
| Supported accommodation | 8 (1.6) | 2 (1.9) | 4 (3.8) | 0.00 | 1 (1.1) | 1 (1.1) |
| Psychiatrist | 8 (1.6) | 6 (5.6) | 1 (1.0) | 1 (1.1) | 0.00 | 0.00 |
| Assertive outreach | 6 (1.2) | 0.00 | 3 (2.9) | 2 (2.2) | 0.00 | 1 (1.1) |
| Forensic services | 2 (0.4) | 0.00 | 0.00 | 1 (1.1) | 0.00 | 1 (1.1) |
| Psychologist | 2 (0.4) | 2 (1.9) | 0.00 | 0.00 | 0.00 | 0.00 |
| Voluntary/third sector | 2 (0.4) | 1 (0.9) | 0.00 | 0.00 | 1 (1.1) | 0.00 |
| Older adults’ mental health service | 1 (0.2) | 0.00 | 1 (1.0) | 0.00 | 0.00 | 0.00 |
| Ineligible reasons | n (%) |
|---|---|
| After patients identified | N = 79 |
| No current positive symptoms of psychosis or problems | 21 (26.6) |
| Dose of clozapine < 400 mg and not limited because of side effects | 16 (20.3) |
| Lacking capacity to consent | 9 (11.4) |
| Current CBT or CBT in previous year | 8 (10.1) |
| Never taken clozapine | 7 (8.9) |
| Diagnosis not schizophrenia spectrum | 7 (8.9) |
| Discontinued clozapine > 2 years ago | 6 (7.6) |
| Moved out of area | 2 (2.5) |
| Diagnosis of a developmental disability | 1 (1.3) |
| Not English speaking | 1 (1.3) |
| Deceased before consented | 1 (1.3) |
| After patients found to be eligible | N = 47 |
| Below threshold on PANSS | 40 (85.1) |
| Diagnosis not schizophrenia spectrum | 2 (4.3) |
| Lacking capacity to consent | 2 (4.3) |
| Dose of clozapine < 400 mg and not limited because of side effects | 1 (2.1) |
| No current positive symptoms of psychosis or problems | 1 (2.1) |
| Unable to complete the baseline assessment | 1 (2.1) |
Appendix 2 Additional analyses
| PANSS subscale | Mean difference | 95% CI | p-value |
|---|---|---|---|
| PANSS total | |||
| 9 months | –2.50 | –4.98 to –0.02 | 0.048 |
| 21 months | –0.63 | –3.22 to 1.96 | 0.634 |
| PANSS positive | |||
| 9 months | –1.64 | –2.65 to –0.63 | 0.002 |
| 21 months | –0.74 | –1.80 to 0.33 | 0.174 |
| PANSS negative | |||
| 9 months | –0.43 | –1.44 to 0.59 | 0.408 |
| 21 months | 0.28 | –0.77 to 1.32 | 0.604 |
| PANSS disorganised | |||
| 9 months | 0.05 | –0.88 to 0.98 | 0.915 |
| 21 months | 0.22 | –0.75 to 1.19 | 0.657 |
| PANSS excitement | |||
| 9 months | –1.14 | –1.84 to –0.44 | 0.001 |
| 21 months | –0.51 | –1.25 to 0.23 | 0.176 |
| PANSS emotional distress | |||
| 9 months | –1.04 | –2.02 to –0.05 | 0.040 |
| 21 months | –0.10 | –1.14 to 0.94 | 0.853 |
| Mean difference | 95% CI | p-value | |
|---|---|---|---|
| PANSS | |||
| Total | –2.50 | –5.10 to 0.11 | 0.060 |
| Positive | –0.52 | –1.60 to 0.56 | 0.348 |
| Negative | –1.67 | –2.68 to –0.67 | 0.001 |
| Disorganised | –0.00 | –0.97 to 0.97 | 0.997 |
| Excitement | –1.28 | –2.05 to –0.50 | 0.001 |
| Emotional distress | –1.17 | –2.21 to –0.13 | 0.028 |
| CDSS | –0.59 | –1.41 to 0.22 | 0.152 |
| AnTI | –0.03 | –0.90 to 0.83 | 0.942 |
| PSYRATS | |||
| Delusion | –0.42 | –1.67 to 0.82 | 0.506 |
| Auditory hallucinations | –2.62 | –5.12 to –0.12 | 0.040 |
| Unusual beliefs – cognitive | –0.24 | –1.05 to 0.58 | 0.566 |
| Unusual beliefs – emotional | –0.29 | –0.83 to 0.24 | 0.283 |
| Voices – cognitive | –0.34 | –0.87 to 0.19 | 0.205 |
| Voices – emotional | –0.45 | –1.01 to 0.11 | 0.114 |
| Voices – physical | –0.63 | –1.23 to –0.04 | 0.038 |
| Voices – loudness | –0.24 | –0.54 to 0.06 | 0.112 |
| PSP | 2.05 | –0.33 to 4.43 | 0.091 |
| QPR | 2.02 | –0.10 to 4.14 | 0.062 |
| AUDIT | 0.72 | –0.20 to 1.64 | 0.127 |
| DAST | –0.15 | –0.53 to 0.22 | 0.414 |
| Severity CGI | –0.09 | –0.32 to 0.13 | 0.418 |
| Participant severity CGI | 0.08 | –0.27 to 0.42 | 0.669 |
| Condition improvement CGI | –0.35 | –0.58 to –0.11 | 0.004 |
| EQ-5D-5L | 0.036 | –0.006 to 0.078 | 0.094 |
| Mean difference | 95% CI | p-value | |
|---|---|---|---|
| PANSS | |||
| Total | –0.87 | –3.69 to 1.96 | 0.548 |
| Positive | 0.36 | –0.76 to 1.48 | 0.527 |
| Negative | –0.95 | –2.10 to 0.19 | 0.102 |
| Disorganised | 0.17 | –0.87 to 1.22 | 0.745 |
| Excitement | –0.56 | –1.32 to 0.20 | 0.146 |
| Emotional distress | –0.33 | –1.47 to 0.80 | 0.565 |
| CDSS | –0.51 | –1.41 to 0.38 | 0.263 |
| AnTI | –0.68 | –1.61 to 0.26 | 0.155 |
| PSYRATS | |||
| Delusion | –0.84 | –2.22 to 0.54 | 0.232 |
| Auditory hallucinations | –1.37 | –4.01 to 1.27 | 0.310 |
| Unusual beliefs – cognitive | –0.39 | –1.28 to 0.51 | 0.397 |
| Unusual beliefs – emotional | –0.58 | –1.16 to 0.00 | 0.050 |
| Voices – cognitive | –0.17 | –0.75 to 0.40 | 0.551 |
| Voices – emotional | –0.01 | –0.60 to 0.58 | 0.969 |
| Voices – physical | –0.33 | –0.91 to 0.25 | 0.267 |
| Voices – loudness | –0.32 | –0.64 to 0.01 | 0.057 |
| PSP | –0.10 | –2.85 to 2.64 | 0.941 |
| QPR | 2.27 | 0.15 to 4.39 | 0.036 |
| AUDIT | 0.84 | –0.18 to 1.87 | 0.107 |
| DAST | 0.10 | –0.18 to 0.38 | 0.488 |
| Severity CGI | –0.04 | –0.26 to 0.19 | 0.752 |
| Participant severity CGI | 0.12 | –0.23 to 0.48 | 0.502 |
| Condition improvement CGI | –0.04 | –0.32 to 0.23 | 0.757 |
| EQ-5D-5L | 0.03 | –0.01 to 0.07 | 0.151 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Age (years) | ||||||||
| 9 months | ||||||||
| 16–32 | 53.3 (12.1); 40 | 50.6 (9.6); 40 | 0.41 | –3.67 to 4.50 | 0.842 | –2.33 | –7.16 to 2.51 | 0.345 |
| 33–49 | 51.1 (10.1); 97 | 48.7 (10.8); 105 | 2.74 | 0.16 to 5.33 | 0.038 | |||
| ≥ 50 | 48.3 (13.8); 44 | 47.0 (12.9); 49 | 1.23 | –2.70 to 5.16 | 0.540 | –1.51 | –6.22 to 3.19 | 0.528 |
| 21 months | ||||||||
| 16–32 | 52.5 (8.4); 92 | 49.4 (11.0); 104 | 2.52 | –0.10 to 5.14 | 0.059 | |||
| 33–49 | 54.6 (9.2); 38 | 50.9 (11.9); 32 | 0.80 | –3.66 to 5.26 | 0.724 | –1.72 | –6.89 to 3.45 | 0.514 |
| ≥ 50 | 47.9 (11.8); 35 | 47.2 (12.7); 49 | 1.78 | –2.39 to 5.94 | 0.403 | –0.74 | –5.67 to 4.18 | 0.767 |
| Age at onset (years) | ||||||||
| 9 months | ||||||||
| ≤ 35 | 52.2 (10.8); 148 | 49.0 (10.9); 169 | 2.45 | 0.40 to 4.51 | 0.019 | |||
| ≥ 36 | 46.7 (14.3); 22 | 43.0 (12.8); 16 | 2.96 | –3.24 to 9.16 | 0.349 | 0.51 | –6.02 to 7.05 | 0.878 |
| 21 months | ||||||||
| ≤ 35 | 52.6 (9.0); 141 | 49.4 (11.5); 159 | 1.71 | –0.41 to 3.83 | 0.114 | |||
| ≥ 36 | 47.7 (14.5); 15 | 42.1 (12.5); 15 | 6.01 | –0.60 to 12.61 | 0.075 | 4.30 | –2.65 to 11.24 | 0.226 |
| Sex | ||||||||
| 9 months | ||||||||
| Female | 52.2 (12.1); 51 | 46.8 (12.9); 59 | 4.82 | 1.27 to 8.37 | 0.008 | |||
| Male | 50.3 (11.4); 130 | 49.5 (10.2); 135 | 0.70 | –1.56 to 2.95 | 0.546 | –4.13 | –8.33 to 0.08 | 0.054 |
| 21 months | ||||||||
| Female | 51.6 (10.2); 48 | 50.0 (11.7); 53 | 0.92 | –2.80 to 4.64 | 0.628 | |||
| Male | 52.1 (9.4); 117 | 48.7 (11.7); 132 | 2.45 | 0.12 to 4.79 | 0.039 | 1.53 | –2.85 to 5.92 | 0.493 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| DI (years) | ||||||||
| 9 months | ||||||||
| 0–15 | 51.8 (12.2); 79 | 48.8 (11.3); 68 | 2.20 | –0.81 to 5.22 | 0.153 | |||
| 16–30 | 50.7 (10.8); 75 | 48.8 (10.5); 89 | 1.60 | –1.27 to 4.47 | 0.275 | –0.60 | –4.77 to 3.56 | 0.777 |
| ≥ 31 | 53.7 (10.2); 16 | 47.3 (13.3); 28 | 6.51 | 0.66 to 12.35 | 0.029 | 4.30 | –2.27 to 10.87 | 0.199 |
| 21 months | ||||||||
| 0–15 | 52.5 (10.3); 75 | 47.7 (12.6); 59 | 2.52 | –0.66 to 5.70 | 0.120 | |||
| 16–30 | 52.1 (8.8); 68 | 49.2 (11.0); 85 | 2.45 | –0.53 to 5.42 | 0.107 | –0.08 | –4.43 to 4.28 | 0.972 |
| ≥ 31 | 50.4 (11.5); 13 | 49.7 (12.4); 30 | 0.49 | –5.60 to 6.59 | 0.874 | –2.03 | –8.89 to 4.83 | 0.562 |
| DUP (years) | ||||||||
| 9 months | ||||||||
| 0–2 | 51.6 (11.4); 99 | 48.2 (10.5); 79 | 2.00 | –0.76 to 4.76 | 0.156 | |||
| 2–5 | 51.7 (8.6); 28 | 48.8 (11.8); 59 | 1.33 | –3.01 to 5.66 | 0.548 | –0.67 | –5.80 to 4.47 | 0.799 |
| ≥ 6 | 49.5 (11.8); 23 | 47.3 (11.9); 27 | 4.97 | –0.27 to 10.21 | 0.063 | 2.97 | –2.96 to 8.90 | 0.326 |
| 21 months | ||||||||
| 0–2 | 52.7 (9.5); 90 | 48.0 (11.7); 76 | 2.27 | –0.60 to 5.15 | 0.121 | |||
| 2–5 | 52.9 (10.4); 28 | 49.7 (12.0); 54 | 1.65 | –2.68 to 5.97 | 0.456 | –0.63 | –5.82 to 4.56 | 0.812 |
| ≥ 6 | 51.3 (9.1); 20 | 47.5 (11.1); 23 | 6.26 | 0.65 to 11.87 | 0.029 | 3.99 | –2.33 to 10.30 | 0.216 |
| Number of antipsychotic drugs at baseline | ||||||||
| 9 months | ||||||||
| One or less | 51.9 (11.0); 109 | 48.2 (11.5); 123 | 2.52 | 0.07 to 4.98 | 0.044 | |||
| Two or more | 49.3 (12.3); 72 | 49.6 (10.6); 71 | 0.98 | –2.07 to 4.04 | 0.528 | –1.54 | –5.47 to 2.39 | 0.442 |
| 21 months | ||||||||
| One or less | 53.2 (9.1); 97 | 49.6 (11.8); 114 | 1.82 | –0.76 to 4.40 | 0.167 | |||
| Two or more | 50.3 (10.1); 68 | 48.3 (11.5); 71 | 2.51 | –0.59 to 5.60 | 0.113 | 0.69 | –3.34 to 4.72 | 0.738 |
| Clozapine daily dose (mg) | ||||||||
| 9 months | ||||||||
| None | 50.3 (10.4); 15 | 47.9 (12.7); 17 | 5.01 | –1.41 to 11.42 | 0.126 | |||
| < 300 | 54.5 (8.2); 25 | 47.7 (10.0); 41 | 5.23 | 0.52 to 9.93 | 0.030 | 0.22 | –7.75 to 8.19 | 0.957 |
| 300–600 | 49.8 (12.5); 106 | 49.4 (11.1); 103 | 0.41 | –2.15 to 2.98 | 0.752 | –4.59 | –11.50 to 2.31 | 0.192 |
| ≥ 600 | 51.7 (11.3); 35 | 47.9 (12.1); 33 | 2.15 | –2.22 to 6.51 | 0.335 | –2.86 | –10.62 to 4.90 | 0.470 |
| 21 months | ||||||||
| None | 50.0 (14.8); 15 | 51.0 (13.7); 15 | 0.50 | –6.02 to 7.01 | 0.882 | |||
| < 300 | 55.0 (7.7); 24 | 49.1 (12.6); 43 | 4.12 | –0.61 to 8.85 | 0.088 | 3.62 | –4.43 to 11.68 | 0.378 |
| 300–600 | 51.3 (9.2); 94 | 49.2 (11.5); 97 | 1.18 | –1.49 to 3.85 | 0.386 | 0.69 | –6.36 to 7.73 | 0.849 |
| ≥ 600 | 52.7 (9.0); 32 | 47.9 (10.0); 30 | 3.65 | –1.00 to 8.31 | 0.124 | 3.16 | –4.85 to 11.17 | 0.440 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Difficulty with abstract thinking (PANSS) | ||||||||
| 9 months | ||||||||
| 0–4 | 51.6 (10.9); 114 | 48.7 (11.6); 124 | 2.18 | –0.20 to 4.57 | 0.073 | |||
| ≥ 4 | 49.7 (12.8); 67 | 48.7 (10.4); 70 | 1.32 | –1.88 to 4.52 | 0.418 | –0.86 | –4.86 to 3.14 | 0.673 |
| 21 months | ||||||||
| 0–4 | 52.7 (9.7); 103 | 49.0 (11.2); 121 | 2.61 | 0.12 to 5.09 | 0.040 | |||
| ≥ 4 | 50.8 (9.4); 62 | 49.3 (12.5); 64 | 0.89 | –2.41 to 4.19 | 0.597 | –1.72 | –5.85 to 2.42 | 0.416 |
| Conceptual disorganisation (PANSS) | ||||||||
| 9 months | ||||||||
| 0–4 | 50.3 (11.6); 153 | 48.0 (11.0); 159 | 2.19 | 0.10 to 4.27 | 0.040 | |||
| ≥ 4 | 53.9 (11.3); 28 | 51.9 (11.2); 35 | 0.43 | –4.38 to 5.25 | 0.860 | –1.75 | –7.00 to 3.50 | 0.513 |
| 21 months | ||||||||
| 0–4 | 51.8 (9.7); 138 | 48.7 (11.8); 151 | 2.00 | –0.17 to 4.18 | 0.071 | |||
| ≥ 4 | 53.1 (9.6); 27 | 50.7 (11.2); 34 | 1.96 | –2.88 to 6.81 | 0.427 | –0.04 | –5.35 to 5.27 | 0.988 |
| LN | ||||||||
| 9 months | ||||||||
| ≤ 8 | 52.4 (11.9); 23 | 44.6 (14.6); 17 | 5.42 | –0.59 to 11.44 | 0.077 | |||
| ≥ 9 | 54.6 (9.9); 14 | 50.9 (14.1); 11 | 3.02 | –4.34 to 10.38 | 0.421 | –2.40 | –11.86 to 7.05 | 0.618 |
| 21 months | ||||||||
| ≤ 8 | 51.0 (8.6); 20 | 51.3 (9.5); 15 | –1.11 | –7.51 to 5.28 | 0.733 | |||
| ≥ 9 | 51.7 (9.6); 13 | 52.1 (10.4); 12 | –0.58 | –7.94 to 6.78 | 0.877 | 0.53 | –9.19 to 10.26 | 0.914 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Emotional abuse | ||||||||
| 9 months | ||||||||
| ≤ 9 | 49.2 (11.9); 80 | 48.6 (10.8); 83 | 1.54 | –1.35 to 4.43 | 0.296 | |||
| ≥ 10 | 52.5 (11.7); 55 | 46.8 (11.2); 67 | 3.46 | 0.03 to 6.88 | 0.048 | 1.92 | –2.57 to 6.40 | 0.402 |
| 21 months | ||||||||
| ≤ 9 | 50.7 (9.2); 75 | 49.5 (11.0); 76 | 1.62 | –1.37 to 4.61 | 0.289 | |||
| ≥ 10 | 53.2 (9.9); 48 | 47.5 (12.0); 65 | 3.07 | –0.45 to 6.60 | 0.087 | 1.46 | –3.17 to 6.08 | 0.537 |
| Emotional neglect | ||||||||
| 9 months | ||||||||
| ≤ 14 | 51.2 (11.6); 104 | 49.8 (9.9); 106 | 2.04 | –0.48 to 4.57 | 0.112 | |||
| ≥ 15 | 47.7 (12.7); 34 | 42.6 (12.3); 40 | 2.66 | –1.71 to 7.04 | 0.233 | 0.62 | –4.43 to 5.67 | 0.810 |
| 21 months | ||||||||
| ≤ 14 | 52.0 (9.2); 97 | 50.7 (10.7); 96 | 1.83 | –0.78 to 4.44 | 0.170 | |||
| ≥ 15 | 50.3 (10.6); 27 | 42.8 (11.6); 41 | 4.20 | –0.40 to 8.79 | 0.074 | 2.37 | –2.92 to 7.66 | 0.380 |
| Physical abuse | ||||||||
| 9 months | ||||||||
| ≤ 7 | 51.0 (11.5); 93 | 48.5 (10.8); 92 | 2.92 | 0.19 to 5.64 | 0.036 | |||
| ≥ 8 | 49.6 (13.5); 42 | 46.4 (11.3); 56 | 2.20 | –1.67 to 6.07 | 0.265 | –0.72 | –5.46 to 4.02 | 0.767 |
| 21 months | ||||||||
| ≤ 7 | 51.7 (8.6); 85 | 49.7 (11.0); 92 | 2.11 | –0.65 to 4.88 | 0.134 | |||
| ≥ 8 | 51.7 (11.1); 39 | 45.8 (12.0); 47 | 3.64 | –0.46 to 7.75 | 0.082 | 1.53 | –3.43 to 6.48 | 0.546 |
| Physical neglect | ||||||||
| 9 months | ||||||||
| ≤ 7 | 49.9 (11.7); 72 | 47.6 (11.0); 72 | 2.63 | –0.50 to 5.75 | 0.100 | |||
| ≥ 8 | 50.9 (12.4); 64 | 47.9 (11.0); 79 | 2.37 | –0.81 to 5.55 | 0.144 | –0.26 | –4.72 to 4.21 | 0.911 |
| 21 months | ||||||||
| ≤ 7 | 50.6 (8.1); 64 | 49.2 (11.8); 70 | 1.04 | –2.18 to 4.27 | 0.526 | |||
| ≥ 8 | 52.9 (10.6); 58 | 47.9 (11.2); 72 | 4.15 | 0.87 to 7.43 | 0.013 | 3.11 | –1.49 to 7.70 | 0.186 |
| Sexual abuse | ||||||||
| 9 months | ||||||||
| ≤ 7 | 50.4 (11.8); 102 | 48.2 (10.1); 110 | 2.25 | –0.31 to 4.81 | 0.085 | |||
| ≥ 8 | 50.6 (13.1); 31 | 46.9 (13.3); 40 | 3.75 | –0.75 to 8.25 | 0.103 | 1.50 | –3.68 to 6.68 | 0.571 |
| 21 months | ||||||||
| ≤ 7 | 52.1 (9.0); 94 | 48.9 (11.0); 104 | 3.01 | 0.38 to 5.64 | 0.025 | |||
| ≥ 8 | 50.3 (11.3); 29 | 47.1 (12.6); 37 | 1.36 | –3.29 to 6.01 | 0.568 | –1.66 | –7.00 to 3.69 | 0.544 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| AUDIT | ||||||||
| 9 months | ||||||||
| ≤ 12 | 51.3 (11.8); 160 | 48.5 (11.1); 177 | 2.03 | 0.03 to 4.03 | 0.047 | |||
| ≥ 13 | 46.1 (10.6); 17 | 48.5 (13.7); 11 | 0.83 | –6.07 to 7.73 | 0.813 | –1.20 | –8.38 to 5.99 | 0.744 |
| 21 months | ||||||||
| ≤ 12 | 52.1 (9.9); 147 | 48.8 (11.8); 166 | 1.76 | –0.31 to 3.83 | 0.095 | |||
| ≥ 13 | 51.5 (6.8); 13 | 51.7 (12.0); 14 | 1.14 | –5.71 to 8.00 | 0.744 | –0.61 | –7.77 to 6.54 | 0.866 |
| DAST | ||||||||
| 9 months | ||||||||
| 1–2 | 51.1 (11.6); 160 | 48.3 (10.9); 175 | 1.89 | –0.08 to 3.87 | 0.060 | |||
| ≥ 3 | 52.0 (5.8); 9 | 49.8 (16.2); 11 | 4.92 | –3.64 to 13.48 | 0.260 | 3.02 | –5.77 to 11.81 | 0.501 |
| 21 months | ||||||||
| 1–2 | 52.2 (9.7); 146 | 48.4 (11.6); 168 | 2.45 | 0.40 to 4.49 | 0.019 | |||
| ≥ 3 | 49.7 (11.0); 7 | 56.9 (10.2); 9 | –5.60 | –14.93 to 3.74 | 0.240 | –8.06 | –17.63 to 1.51 | 0.099 |
| PAM-SR attachment avoidance | ||||||||
| 9 months | ||||||||
| < 9 | 54.3 (10.2); 116 | 51.3 (9.9); 113 | 2.96 | 0.58 to 5.34 | 0.015 | |||
| 9–16 | 43.5 (12.0); 49 | 43.7 (11.5); 66 | –0.47 | –3.92 to 2.98 | 0.788 | –3.43 | –7.62 to 0.76 | 0.109 |
| 17–24 | 48.7 (9.4); 16 | 50.5 (11.8); 15 | –1.03 | –8.10 to 6.05 | 0.776 | –3.99 | –11.45 to 3.48 | 0.295 |
| 21 months | ||||||||
| < 9 | 54.3 (9.0); 106 | 51.2 (10.6); 109 | 2.89 | 0.44 to 5.33 | 0.021 | |||
| 9–16 | 46.0 (9.6); 42 | 44.5 (13.0); 62 | 0.16 | –3.46 to 3.78 | 0.931 | –2.73 | –7.10 to 1.65 | 0.222 |
| 17–24 | 52.5 (7.7); 17 | 52.8 (6.9); 14 | 0.52 | –7.02 to 8.06 | 0.892 | –2.36 | –10.29 to 5.56 | 0.559 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Negative others | ||||||||
| 9 months | ||||||||
| < 7.2 | 54.0 (10.4); 93 | 48.6 (10.0); 79 | 4.42 | 1.65 to 7.18 | 0.002 | |||
| ≥ 7.2 | 47.0 (12.0); 72 | 48.5 (11.9); 103 | –0.54 | –3.39 to 2.31 | 0.709 | –4.96 | –8.94 to –0.98 | 0.015 |
| 21 months | ||||||||
| < 7.2 | 53.4 (9.4); 87 | 49.5 (11.3); 71 | 2.78 | –0.10 to 5.66 | 0.059 | |||
| ≥ 7.2 | 50.2 (10.1); 66 | 48.4 (12.4); 101 | 1.79 | –1.14 to 4.73 | 0.231 | –0.98 | –5.10 to 3.13 | 0.639 |
| Negative self | ||||||||
| 9 months | ||||||||
| < 7.2 | 54.5 (10.1); 106 | 50.8 (9.7); 111 | 2.61 | 0.17 to 5.04 | 0.036 | |||
| ≥ 7.2 | 45.4 (11.4); 61 | 44.7 (12.1); 72 | 1.61 | –1.61 to 4.83 | 0.327 | –1.00 | –5.04 to 3.05 | 0.629 |
| 21 months | ||||||||
| < 7.2 | 55.0 (8.4); 99 | 51.7 (10.4); 107 | 2.38 | –0.11 to 4.88 | 0.061 | |||
| ≥ 7.2 | 46.6 (9.5); 51 | 44.5 (12.7); 68 | 1.86 | –1.54 to 5.25 | 0.284 | –0.53 | –4.75 to 3.69 | 0.806 |
| Positive others | ||||||||
| 9 months | ||||||||
| < 7.2 | 46.5 (11.2); 36 | 45.1 (11.7); 59 | 0.77 | –3.08 to 4.62 | 0.694 | |||
| ≥ 7.2 | 52.1 (11.5); 130 | 50.2 (10.4); 124 | 2.11 | –0.16 to 4.39 | 0.068 | 1.34 | –3.13 to 5.81 | 0.557 |
| 21 months | ||||||||
| < 7.2 | 47.3 (11.6); 29 | 46.3 (12.5); 63 | 0.97 | –3.14 to 5.07 | 0.644 | |||
| ≥ 7.2 | 53.0 (9.2); 121 | 50.4 (11.2); 111 | 2.06 | –0.32 to 4.43 | 0.089 | 1.09 | –3.64 to 5.83 | 0.651 |
| Positive self | ||||||||
| 9 months | ||||||||
| < 7.2 | 45.9 (12.4); 68 | 44.2 (11.4); 86 | 2.72 | –0.22 to 5.66 | 0.069 | |||
| ≥ 7.2 | 54.6 (9.6); 97 | 52.2 (9.4); 96 | 1.64 | –0.98 to 4.27 | 0.219 | –1.08 | –5.03 to 2.87 | 0.592 |
| 21 months | ||||||||
| < 7.2 | 47.2 (10.1); 63 | 45.0 (11.7); 81 | 2.45 | –0.58 to 5.48 | 0.114 | |||
| ≥ 7.2 | 55.2 (8.3); 88 | 52.0 (11.1); 90 | 1.92 | –0.81 to 4.65 | 0.168 | –0.53 | –4.61 to 3.56 | 0.801 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| 9 months | ||||||||
| Stress sensitivity | 51.1 (11.7); 62 | 47.7 (9.5); 67 | 3.18 | 0.00 to 6.36 | 0.050 | |||
| Drug related | 51.5 (10.1); 57 | 49.8 (10.0); 48 | 1.62 | –1.85 to 5.09 | 0.359 | –1.56 | –6.27 to 3.15 | 0.517 |
| Trauma | 47.7 (15.2); 7 | 55.5 (15.9); 4 | –12.90 | –24.96 to –0.84 | 0.036 | –16.08 | –28.56 to –3.61 | 0.012 |
| Anxiety | 47.3 (14.9); 20 | 47.6 (12.7); 32 | 1.13 | –4.08 to 6.34 | 0.671 | –2.05 | –8.16 to 4.05 | 0.510 |
| Drug and trauma | 40.3 (8.0); 3 | 36.0 (21.5); 4 | 11.84 | –2.00 to 25.68 | 0.094 | 11.84 | –2.00 to 25.68 | 0.094 |
| 21 months | ||||||||
| Stress sensitivity | 52.4 (9.0); 61 | 48.6 (10.2); 68 | 2.44 | –0.75 to 5.63 | 0.134 | |||
| Drug related | 52.6 (9.3); 52 | 51.2 (9.9); 46 | 1.77 | –1.82 to 5.36 | 0.335 | –0.67 | –5.48 to 4.13 | 0.783 |
| Trauma | 46.0 (9.6); 7 | 47.6 (25.0); 5 | –9.26 | –20.23 to 1.70 | 0.098 | –11.71 | –23.12 to –0.29 | 0.044 |
| Anxiety | 50.5 (12.7); 17 | 47.1 (13.9); 31 | 4.99 | –0.54 to 10.52 | 0.077 | 2.55 | –3.84 to 8.94 | 0.434 |
| Drug and trauma | 37.5 (14.8); 2 | 49.8 (8.4); 4 | –2.00 | –17.23 to 13.23 | 0.797 | –4.44 | –20.02 to 11.14 | 0.576 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Age (years) | ||||||||
| 9 months | ||||||||
| 16–32 | 57.0 (13.0); 48 | 50.6 (14.3); 41 | 5.86 | 0.94 to 10.77 | 0.019 | 4.21 | –1.55 to 9.96 | 0.152 |
| 33–49 | 53.8 (14.9); 114 | 51.7 (13.2); 124 | 1.65 | –1.35 to 4.65 | 0.281 | |||
| ≥ 50 | 48.3 (14.2); 51 | 49.6 (15.1); 59 | –0.66 | –5.09 to 3.77 | 0.770 | –2.31 | –7.67 to 3.05 | 0.399 |
| 21 months | ||||||||
| 16–32 | 52.2 (15.5); 109 | 52.1 (15.2); 120 | –0.10 | –3.15 to 2.96 | 0.951 | |||
| 33–49 | 53.5 (15.3); 47 | 54.7 (13.3); 38 | –0.60 | –5.65 to 4.44 | 0.815 | –0.51 | –6.41 to 5.39 | 0.866 |
| ≥ 50 | 47.8 (14.3); 50 | 47.8 (14.0); 56 | 1.25 | –3.26 to 5.76 | 0.588 | 1.34 | –4.11 to 6.80 | 0.629 |
| Age at onset (years) | ||||||||
| 9 months | ||||||||
| ≤ 35 | 53.8 (14.6); 178 | 51.5 (13.7); 191 | 1.89 | –0.53 to 4.31 | 0.126 | |||
| ≥ 36 | 52.0 (13.9); 23 | 44.8 (14.5); 20 | 4.13 | –3.04 to 11.29 | 0.259 | 2.24 | –5.33 to 9.81 | 0.562 |
| 21 months | ||||||||
| ≤ 35 | 52.1 (15.4); 169 | 52.3 (14.5); 181 | –0.21 | –2.69 to 2.27 | 0.869 | |||
| ≥ 36 | 47.9 (14.5); 24 | 44.5 (15.3); 20 | 1.35 | –5.73 to 8.43 | 0.709 | 1.56 | –5.95 to 9.06 | 0.684 |
| Sex | ||||||||
| 9 months | ||||||||
| Female | 56.5 (13.3); 59 | 53.9 (14.9); 64 | 2.66 | –1.52 to 6.84 | 0.212 | |||
| Male | 52.0 (14.9); 154 | 49.7 (13.4); 160 | 1.59 | –1.02 to 4.20 | 0.231 | –1.07 | –6.00 to 3.86 | 0.671 |
| 21 months | ||||||||
| Female | 54.8 (15.0); 58 | 56.2 (14.8); 62 | –0.90 | –5.13 to 3.34 | 0.678 | |||
| Male | 50.2 (15.2); 148 | 49.5 (14.2); 152 | 0.62 | –2.05 to 3.29 | 0.647 | 1.52 | –3.48 to 6.52 | 0.552 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| DI (years) | ||||||||
| 9 months | ||||||||
| 0–15 | 55.4 (13.4); 92 | 49.7 (13.8); 76 | 4.80 | 1.22 to 8.39 | 0.009 | |||
| 16–30 | 54.0 (15.2); 87 | 51.2 (14.0); 102 | 1.57 | –1.81 to 4.94 | 0.363 | –3.24 | –8.17 to 1.69 | 0.198 |
| ≥ 31 | 44.0 (12.8); 22 | 52.5 (14.2); 33 | –4.50 | –10.87 to 1.88 | 0.167 | –9.30 | –16.62 to –1.98 | 0.013 |
| 21 months | ||||||||
| 0–15 | 53.0 (16.3); 91 | 51.3 (14.3); 73 | 1.28 | –2.36 to 4.91 | 0.491 | |||
| 16–30 | 51.6 (14.2); 83 | 52.3 (15.8); 98 | –1.16 | –4.61 to 2.29 | 0.510 | –2.44 | –7.45 to 2.57 | 0.341 |
| ≥ 31 | 44.4 (13.7); 19 | 49.4 (12.7); 30 | –1.58 | –8.37 to 5.21 | 0.649 | –2.85 | –10.56 to 4.85 | 0.468 |
| DUP (years) | ||||||||
| 9 months | ||||||||
| 0–2 | 53.1 (14.9); 112 | 50.8 (14.3); 94 | 1.76 | –1.52 to 5.04 | 0.292 | |||
| 2–5 | 55.4 (15.2); 35 | 49.7 (13.9); 62 | 5.32 | 0.34 to 10.30 | 0.036 | 3.56 | –2.41 to 9.52 | 0.243 |
| ≥ 6 | 51.2 (14.2); 26 | 51.5 (13.7); 31 | 0.93 | –5.33 to 7.18 | 0.771 | –0.84 | –7.90 to 6.22 | 0.816 |
| 21 months | ||||||||
| 0–2 | 51.6 (15.6); 110 | 51.6 (15.0); 89 | –0.33 | –3.67 to 3.01 | 0.846 | |||
| 2–5 | 53.6 (16.5); 34 | 52.5 (14.1); 60 | 0.99 | –4.06 to 6.05 | 0.700 | 1.32 | –4.75 to 7.40 | 0.669 |
| ≥ 6 | 47.2 (13.4); 25 | 47.5 (13.6); 29 | 0.84 | –5.58 to 7.26 | 0.798 | 1.17 | –6.06 to 8.40 | 0.751 |
| Number of antipsychotic drugs at baseline | ||||||||
| 9 months | ||||||||
| One or less | 52.1 (14.5); 134 | 50.6 (14.4); 142 | 1.57 | –1.22 to 4.36 | 0.270 | |||
| Two or more | 55.1 (14.5); 79 | 51.4 (13.0); 82 | 2.45 | –1.20 to 6.10 | 0.189 | 0.87 | –3.73 to 5.48 | 0.710 |
| 21 months | ||||||||
| One or less | 50.4 (15.6); 129 | 51.5 (14.6); 136 | –0.33 | –3.18 to 2.53 | 0.823 | |||
| Two or more | 53.2 (14.5); 77 | 51.3 (14.9); 78 | 1.04 | –2.67 to 4.76 | 0.582 | 1.37 | –3.33 to 6.07 | 0.567 |
| Clozapine daily dose (mg) | ||||||||
| 9 months | ||||||||
| None | 52.1 (11.4); 17 | 46.3 (14.4); 23 | 5.13 | –2.23 to 12.49 | 0.172 | |||
| < 300 | 56.4 (16.6); 31 | 53.2 (16.0); 46 | 0.80 | –4.55 to 6.15 | 0.769 | –4.32 | –13.44 to 4.79 | 0.352 |
| 300–600 | 52.3 (14.5); 129 | 50.4 (13.3); 120 | 1.98 | –0.93 to 4.89 | 0.182 | –3.15 | –11.06 to 4.77 | 0.436 |
| ≥ 600 | 54.4 (14.4); 36 | 52.7 (12.2); 35 | 1.93 | –3.52 to 7.38 | 0.488 | –3.20 | –12.38 to 5.98 | 0.495 |
| 21 months | ||||||||
| None | 46.3 (13.7); 17 | 49.0 (16.5); 21 | –2.27 | –9.77 to 5.24 | 0.554 | |||
| < 300 | 54.1 (18.4); 30 | 53.6 (16.5); 46 | –1.98 | –7.37 to 3.42 | 0.473 | 0.29 | –8.97 to 9.54 | 0.951 |
| 300–600 | 50.0 (14.9); 124 | 50.6 (14.3); 113 | 0.11 | –2.87 to 3.09 | 0.943 | 2.37 | –5.70 to 10.45 | 0.565 |
| ≥ 600 | 56.9 (12.7); 35 | 52.6 (12.0); 34 | 4.94 | –0.59 to 10.47 | 0.080 | 7.21 | –2.14 to 16.55 | 0.131 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Difficulty with abstract thinking (PANSS) | ||||||||
| 9 months | ||||||||
| 0–4 | 54.6 (14.4); 126 | 51.5 (13.5); 142 | 1.38 | –1.46 to 4.21 | 0.341 | |||
| ≥ 4 | 51.3 (14.7); 87 | 49.9 (14.6); 82 | 2.72 | –0.84 to 6.29 | 0.135 | 1.35 | –3.22 to 5.91 | 0.564 |
| 21 months | ||||||||
| 0–4 | 53.8 (16.4); 122 | 51.9 (14.8); 135 | 0.60 | –2.29 to 3.49 | 0.684 | |||
| ≥ 4 | 48.1 (12.7); 84 | 50.6 (14.5); 79 | –0.40 | –4.04 to 3.24 | 0.830 | –1.00 | –5.66 to 3.66 | 0.674 |
| Conceptual disorganisation (PANSS) | ||||||||
| 9 months | ||||||||
| 0–4 | 53.9 (14.1); 178 | 52.6 (13.1); 182 | 1.49 | –0.94 to 3.92 | 0.230 | |||
| ≥ 4 | 49.6 (16.4); 35 | 43.5 (14.9); 42 | 3.66 | –1.63 to 8.94 | 0.175 | 2.17 | –3.66 to 8.00 | 0.466 |
| 21 months | ||||||||
| 0–4 | 52.9 (15.2); 170 | 52.7 (14.4); 177 | 0.73 | –1.75 to 3.21 | 0.563 | |||
| ≥ 4 | 44.8 (13.7); 36 | 45.4 (14.9); 37 | –2.27 | –7.68 to 3.14 | 0.410 | –3.00 | –8.97 to 2.96 | 0.323 |
| LN | ||||||||
| 9 months | ||||||||
| ≤ 8 | 50.9 (12.2); 25 | 44.6 (14.8); 23 | 7.05 | 0.27 to 13.82 | 0.041 | |||
| ≥ 9 | 50.7 (16.4); 17 | 56.2 (12.8); 11 | –7.48 | –16.49 to 1.54 | 0.104 | –14.52 | –25.75 to –3.29 | 0.011 |
| 21 months | ||||||||
| ≤ 8 | 47.2 (12.2); 24 | 51.6 (14.3); 23 | –3.03 | –9.91 to 3.85 | 0.388 | |||
| ≥ 9 | 56.5 (16.3); 15 | 57.9 (18.5); 11 | –2.46 | –11.70 to 6.77 | 0.601 | 0.57 | –10.90 to 12.04 | 0.922 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Emotional abuse | ||||||||
| 9 months | ||||||||
| ≤ 9 | 55.0 (14.3); 89 | 50.6 (13.5); 88 | 2.77 | –0.70 to 6.25 | 0.118 | |||
| ≥ 10 | 53.0 (15.2); 63 | 51.7 (15.3); 75 | 3.33 | –0.63 to 7.30 | 0.100 | 0.56 | –4.73 to 5.85 | 0.836 |
| 21 months | ||||||||
| ≤ 9 | 52.8 (15.5); 84 | 51.5 (15.1); 88 | –0.34 | –3.86 to 3.19 | 0.851 | |||
| ≥ 10 | 50.4 (14.3); 59 | 52.8 (15.7); 75 | –0.46 | –4.51 to 3.58 | 0.823 | –0.13 | –5.51 to 5.25 | 0.964 |
| Emotional neglect | ||||||||
| 9 months | ||||||||
| ≤ 14 | 54.1 (14.8); 113 | 52.7 (13.2); 114 | 1.67 | –1.39 to 4.73 | 0.285 | |||
| ≥ 15 | 53.5 (14.0); 39 | 48.2 (16.4); 47 | 4.90 | –0.11 to 9.92 | 0.055 | 3.23 | –2.64 to 9.11 | 0.281 |
| 21 months | ||||||||
| ≤ 14 | 52.0 (15.3); 109 | 53.8 (15.6); 114 | –1.39 | –4.48 to 1.70 | 0.379 | |||
| ≥ 15 | 51.0 (14.2); 35 | 49.5 (15.4); 46 | 1.35 | –3.85 to 6.55 | 0.610 | 2.74 | –3.31 to 8.79 | 0.375 |
| Physical abuse | ||||||||
| 9 months | ||||||||
| ≤ 7 | 53.1 (14.4); 102 | 52.9 (13.2); 103 | 0.49 | –2.72 to 3.71 | 0.765 | |||
| ≥ 8 | 55.1 (14.7); 49 | 48.9 (15.4); 60 | 6.78 | 2.34 to 11.23 | 0.003 | 6.29 | 0.80 to 11.78 | 0.025 |
| 21 months | ||||||||
| ≤ 7 | 51.6 (14.7); 97 | 53.0 (15.7); 103 | –1.03 | –4.28 to 2.23 | 0.536 | |||
| ≥ 8 | 50.6 (15.3); 46 | 51.4 (15.0); 59 | –0.30 | –4.85 to 4.24 | 0.896 | 0.72 | –4.87 to 6.32 | 0.800 |
| Physical neglect | ||||||||
| 9 months | ||||||||
| ≤ 7 | 55.5 (13.8); 77 | 53.1 (13.6); 75 | 3.47 | –0.29 to 7.23 | 0.071 | |||
| ≥ 8 | 52.2 (15.3); 73 | 49.6 (14.7); 91 | 2.12 | –1.52 to 5.76 | 0.254 | –1.35 | –6.59 to 3.89 | 0.614 |
| 21 months | ||||||||
| ≤ 7 | 53.1 (14.5); 74 | 53.3 (15.1); 78 | 0.60 | –3.16 to 4.36 | 0.755 | |||
| ≥ 8 | 50.5 (15.5); 68 | 51.3 (15.8); 87 | –0.94 | –4.69 to 2.81 | 0.622 | –1.54 | –6.85 to 3.77 | 0.570 |
| Sexual abuse | ||||||||
| 9 months | ||||||||
| ≤ 7 | 53.5 (14.8); 114 | 52.0 (14.8); 115 | 1.61 | –1.40 to 4.62 | 0.293 | |||
| ≥ 8 | 55.7 (14.6); 34 | 48.7 (12.0); 48 | 6.53 | 1.42 to 11.63 | 0.012 | 4.91 | –1.01 to 10.84 | 0.104 |
| 21 months | ||||||||
| ≤ 7 | 52.1 (14.2); 109 | 53.5 (15.2); 116 | –1.08 | –4.12 to 1.96 | 0.486 | |||
| ≥ 8 | 51.1 (15.9); 32 | 48.5 (15.4); 46 | 1.70 | –3.55 to 6.95 | 0.525 | 2.78 | –3.28 to 8.85 | 0.368 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| AUDIT | ||||||||
| 9 months | ||||||||
| ≤ 12 | 53.6 (14.6); 185 | 50.9 (13.8); 202 | 2.64 | 0.30 to 4.98 | 0.027 | |||
| ≥ 13 | 52.8 (13.1); 20 | 47.9 (13.7); 14 | –0.45 | –8.48 to 7.58 | 0.912 | –3.09 | –11.46 to 5.28 | 0.469 |
| 21 months | ||||||||
| ≤ 12 | 51.6 (15.7); 176 | 51.8 (14.7); 192 | 0.19 | –2.21 to 2.59 | 0.874 | |||
| ≥ 13 | 51.4 (11.7); 19 | 47.3 (13.3); 15 | –0.56 | –8.52 to 7.39 | 0.890 | –0.76 | –9.07 to 7.56 | 0.858 |
| DAST | ||||||||
| 9 months | ||||||||
| 1–2 | 53.8 (14.4); 185 | 50.9 (13.8); 197 | 2.22 | –0.12 to 4.57 | 0.063 | |||
| ≥ 3 | 48.2 (17.1); 12 | 46.4 (13.4); 16 | 0.98 | –7.80 to 9.75 | 0.827 | –1.25 | –10.34 to 7.85 | 0.788 |
| 21 months | ||||||||
| 1–2 | 52.1 (15.2); 178 | 51.3 (14.9); 189 | 0.73 | –1.67 to 3.13 | 0.550 | |||
| ≥ 3 | 46.1 (14.9); 11 | 50.2 (13.0); 14 | –4.35 | –13.62 to 4.91 | 0.357 | –5.08 | –14.66 to 4.49 | 0.298 |
| PAM-SR attachment avoidance | ||||||||
| 9 months | ||||||||
| < 9 | 55.5 (14.4); 128 | 53.9 (13.5); 126 | 0.91 | –1.93 to 3.75 | 0.529 | |||
| 9–16 | 50.7 (13.8); 59 | 46.9 (13.2); 75 | 3.75 | –0.20 to 7.70 | 0.063 | 2.84 | –2.04 to 7.71 | 0.254 |
| 17–24 | 47.8 (15.3); 26 | 47.7 (14.8); 23 | 0.41 | –6.07 to 6.89 | 0.901 | –0.50 | –7.58 to 6.58 | 0.889 |
| 21 months | ||||||||
| < 9 | 54.4 (15.4); 125 | 54.9 (14.5); 124 | –0.88 | –3.76 to 1.99 | 0.547 | |||
| 9–16 | 46.3 (14.0); 54 | 46.8 (14.1); 70 | –0.16 | –4.27 to 3.96 | 0.940 | 0.72 | –4.30 to 5.75 | 0.778 |
| 17–24 | 48.0 (13.8); 27 | 46.0 (11.8); 20 | 3.91 | –2.78 to 10.60 | 0.252 | 4.79 | –2.49 to 12.08 | 0.197 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Negative others | ||||||||
| 9 months | ||||||||
| < 7.2 | 55.4 (14.3); 104 | 53.3 (13.7); 88 | 0.91 | –2.43 to 4.26 | 0.592 | |||
| ≥ 7.2 | 51.5 (14.7); 84 | 50.0 (13.7); 117 | 2.22 | –1.09 to 5.52 | 0.189 | 1.30 | –3.41 to 6.01 | 0.588 |
| 21 months | ||||||||
| < 7.2 | 54.4 (15.0); 99 | 52.5 (15.8); 84 | 0.66 | –2.76 to 4.08 | 0.705 | |||
| ≥ 7.2 | 48.5 (15.4); 85 | 51.5 (14.2); 111 | –1.66 | –4.99 to 1.68 | 0.330 | –2.32 | –7.10 to 2.47 | 0.343 |
| Negative self | ||||||||
| 9 months | ||||||||
| < 7.2 | 54.6 (14.8); 121 | 52.2 (14.7); 125 | 0.57 | –2.36 to 3.50 | 0.702 | |||
| ≥ 7.2 | 50.8 (13.0); 68 | 49.7 (12.0); 81 | 3.42 | –0.36 to 7.21 | 0.076 | 2.85 | –1.94 to 7.65 | 0.244 |
| 21 months | ||||||||
| < 7.2 | 54.6 (15.8); 116 | 53.2 (15.0); 121 | 0.02 | –2.96 to 3.00 | 0.990 | |||
| ≥ 7.2 | 46.1 (13.1); 66 | 49.1 (14.2); 76 | –0.55 | –4.43 to 3.32 | 0.780 | –0.57 | –5.47 to 4.33 | 0.819 |
| Positive others | ||||||||
| 9 months | ||||||||
| < 7.2 | 50.3 (15.6); 44 | 47.6 (13.3); 69 | 2.22 | –2.19 to 6.62 | 0.324 | |||
| ≥ 7.2 | 54.1 (13.8); 144 | 53.3 (13.7); 136 | 0.87 | –1.85 to 3.59 | 0.530 | –1.35 | –6.54 to 3.84 | 0.611 |
| 21 months | ||||||||
| < 7.2 | 48.0 (15.7); 39 | 47.4 (14.5); 69 | –0.27 | –4.85 to 4.30 | 0.906 | |||
| ≥ 7.2 | 52.5 (15.4); 144 | 54.6 (14.4); 128 | –1.44 | –4.20 to 1.32 | 0.306 | –1.17 | –6.52 to 4.19 | 0.669 |
| Positive self | ||||||||
| 9 months | ||||||||
| < 7.2 | 49.6 (14.0); 81 | 49.4 (13.3); 96 | –0.38 | –3.86 to 3.10 | 0.831 | |||
| ≥ 7.2 | 56.2 (14.3); 108 | 53.0 (14.1); 108 | 3.07 | –0.06 to 6.20 | 0.054 | 3.45 | –1.24 to 8.14 | 0.149 |
| 21 months | ||||||||
| < 7.2 | 48.8 (15.0); 76 | 50.2 (14.8); 92 | –2.07 | –5.65 to 1.51 | 0.257 | |||
| ≥ 7.2 | 53.4 (15.7); 106 | 53.3 (14.8); 103 | 0.61 | –2.57 to 3.79 | 0.705 | 2.69 | –2.11 to 7.48 | 0.273 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| 9 months | ||||||||
| Stress sensitivity | 54.3 (14.3); 74 | 49.9 (14.6); 76 | 3.74 | 0.01 to 7.47 | 0.049 | |||
| Drug related | 54.8 (14.0); 61 | 51.4 (12.6); 53 | 0.59 | –3.70 to 4.89 | 0.786 | –3.15 | –8.83 to 2.53 | 0.277 |
| Trauma | 46.9 (17.4); 10 | 42.6 (14.4); 7 | 7.17 | –4.09 to 18.44 | 0.212 | 3.43 | –8.45 to 15.31 | 0.571 |
| Anxiety | 53.5 (14.9); 22 | 54.7 (15.3); 36 | 1.59 | –4.61 to 7.78 | 0.616 | –2.16 | –9.39 to 5.07 | 0.559 |
| Drug and trauma | 44.3 (11.0); 3 | 54.5 (5.7); 4 | –3.15 | –21.04 to 14.74 | 0.730 | –3.15 | –21.04 to 14.74 | 0.730 |
| 21 months | ||||||||
| Stress sensitivity | 53.8 (14.4); 72 | 51.9 (15.1); 75 | 1.24 | –2.53 to 5.01 | 0.519 | |||
| Drug related | 53.3 (16.3); 60 | 51.2 (13.7); 54 | –0.48 | –4.77 to 3.80 | 0.825 | –1.72 | –7.43 to 3.98 | 0.553 |
| Trauma | 41.8 (14.5); 8 | 45.6 (20.9); 7 | –1.39 | –13.23 to 10.45 | 0.818 | –2.63 | –15.07 to 9.81 | 0.678 |
| Anxiety | 51.9 (15.0); 24 | 52.7 (15.9); 35 | 2.86 | –3.20 to 8.93 | 0.354 | 1.62 | –5.51 to 8.76 | 0.655 |
| Drug and trauma | 32.7 (7.5); 3 | 53.0 (7.0); 4 | –9.57 | –27.06 to 7.92 | 0.284 | –10.81 | –28.71 to 7.09 | 0.236 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Age (years) | ||||||||
| 9 months | ||||||||
| 16–32 | 17.8 (13.5); 41 | 22.0 (13.8); 37 | –1.66 | –6.72 to 3.41 | 0.522 | 0.88 | –5.02 to 6.78 | 0.770 |
| 33–49 | 17.5 (14.7); 102 | 23.0 (13.0); 105 | –2.54 | –5.59 to 0.52 | 0.104 | |||
| ≥ 50 | 18.2 (14.2); 42 | 21.5 (14.1); 50 | –3.43 | –8.24 to 1.38 | 0.162 | –0.90 | –6.60 to 4.81 | 0.758 |
| 21 months | ||||||||
| 16–32 | 16.8 (14.5); 98 | 20.0 (14.7); 105 | –1.11 | –4.22 to 2.01 | 0.485 | |||
| 33–49 | 15.8 (12.8); 41 | 19.4 (13.4); 29 | 0.71 | –4.73 to 6.15 | 0.798 | 1.82 | –4.44 to 8.08 | 0.569 |
| ≥ 50 | 19.3 (14.6); 40 | 21.5 (14.4); 48 | –3.46 | –8.37 to 1.45 | 0.167 | –2.35 | –8.17 to 3.46 | 0.428 |
| Age at onset (years) | ||||||||
| 9 months | ||||||||
| ≤ 35 | 17.4 (14.2); 157 | 22.7 (13.3); 163 | –2.95 | –5.46 to –0.44 | 0.021 | |||
| ≥ 36 | 17.3 (14.0); 19 | 21.8 (14.8); 18 | –3.16 | –10.90 to 4.58 | 0.423 | –0.21 | –8.37 to 7.94 | 0.959 |
| 21 months | ||||||||
| ≤ 35 | 17.0 (14.1); 153 | 20.7 (14.1); 153 | –1.02 | –3.60 to 1.56 | 0.438 | |||
| ≥ 36 | 16.5 (14.9); 19 | 19.3 (14.9); 18 | –5.56 | –13.28 to 2.16 | 0.158 | –4.54 | –12.70 to 3.61 | 0.275 |
| Sex | ||||||||
| 9 months | ||||||||
| Female | 19.1 (15.2); 54 | 25.4 (12.5); 54 | –3.54 | –7.69 to 0.61 | 0.095 | |||
| Male | 17.2 (13.8); 131 | 21.3 (13.6); 138 | –2.15 | –4.89 to 0.60 | 0.125 | 1.39 | –3.57 to 6.35 | 0.583 |
| 21 months | ||||||||
| Female | 16.5 (15.6); 51 | 22.0 (14.0); 54 | –3.87 | –8.12 to 0.38 | 0.074 | |||
| Male | 17.4 (13.6); 128 | 19.6 (14.5); 128 | –0.28 | –3.11 to 2.55 | 0.846 | 3.59 | –1.51 to 8.68 | 0.167 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| DI (years) | ||||||||
| 9 months | ||||||||
| 0–15 | 19.1 (13.5); 79 | 20.2 (13.8); 65 | 1.08 | –2.67 to 4.83 | 0.573 | |||
| 16–30 | 15.2 (14.7); 80 | 25.3 (12.3); 90 | –6.25 | –9.70 to –2.80 | < 0.001 | –7.33 | –12.41 to –2.25 | 0.005 |
| ≥ 31 | 19.9 (13.9); 17 | 19.8 (14.5); 26 | –4.13 | –10.77 to 2.51 | 0.223 | –5.21 | –12.84 to 2.42 | 0.181 |
| 21 months | ||||||||
| 0–15 | 16.4 (13.8); 78 | 18.7 (13.9); 60 | 0.09 | –3.75 to 3.94 | 0.962 | |||
| 16–30 | 16.9 (14.8); 78 | 22.1 (13.9); 87 | –1.99 | –5.52 to 1.55 | 0.272 | –2.08 | –7.29 to 3.13 | 0.434 |
| ≥ 31 | 19.2 (13.1); 16 | 19.5 (15.4); 24 | –4.56 | –11.54 to 2.41 | 0.199 | –4.66 | –12.62 to 3.30 | 0.251 |
| DUP (years) | ||||||||
| 9 months | ||||||||
| 0–2 | 18.7 (14.1); 97 | 22.1 (14.3); 86 | –1.36 | –4.62 to 1.89 | 0.412 | |||
| 2–5 | 11.3 (12.1); 33 | 22.9 (12.9); 52 | –7.06 | –11.93 to –2.19 | 0.005 | –5.69 | –11.53 to 0.14 | 0.056 |
| ≥ 6 | 16.4 (14.6); 25 | 23.8 (13.1); 28 | –8.17 | –14.29 to –2.05 | 0.009 | –6.81 | –13.72 to 0.11 | 0.054 |
| 21 months | ||||||||
| 0–2 | 16.7 (13.9); 95 | 21.0 (14.3); 78 | –1.69 | –5.05 to 1.67 | 0.325 | |||
| 2–5 | 11.5 (13.4); 29 | 20.0 (14.2); 49 | –6.20 | –11.32 to –1.07 | 0.018 | –4.51 | –10.62 to 1.61 | 0.149 |
| ≥ 6 | 18.3 (14.4); 25 | 22.8 (13.8); 27 | –3.27 | –9.58 to 3.05 | 0.311 | –1.58 | –8.71 to 5.55 | 0.665 |
| Number of antipsychotic drugs at baseline | ||||||||
| 9 months | ||||||||
| One or less | 15.8 (14.1); 109 | 21.4 (13.6); 114 | –2.75 | –5.74 to 0.24 | 0.071 | |||
| Two or more | 20.6 (14.0); 76 | 23.9 (13.1); 78 | –2.49 | –6.01 to 1.04 | 0.167 | 0.26 | –4.36 to 4.89 | 0.911 |
| 21 months | ||||||||
| One or less | 15.1 (14.0); 108 | 18.8 (14.3); 113 | –0.56 | –3.59 to 2.47 | 0.718 | |||
| Two or more | 20.1 (14.0); 71 | 22.8 (14.1); 69 | –2.92 | –6.65 to 0.82 | 0.126 | –2.36 | –7.17 to 2.46 | 0.338 |
| Clozapine daily dose (mg) | ||||||||
| 9 months | ||||||||
| None | 19.1 (15.7); 15 | 23.3 (14.6); 21 | –1.94 | –9.37 to 5.50 | 0.610 | |||
| < 300 | 10.9 (13.8); 23 | 22.3 (13.4); 39 | –6.92 | –12.71 to –1.13 | 0.019 | –4.98 | –14.38 to 4.42 | 0.299 |
| 300–600 | 18.2 (14.1); 113 | 22.3 (13.2); 99 | –3.03 | –6.05 to –0.01 | 0.049 | –1.10 | –9.11 to 6.92 | 0.788 |
| ≥ 600 | 20.2 (13.6); 34 | 22.4 (13.8); 33 | 1.70 | –3.82 to 7.23 | 0.546 | 3.64 | –5.62 to 12.89 | 0.441 |
| 21 months | ||||||||
| None | 20.3 (15.3); 16 | 23.5 (13.4); 20 | –1.98 | –9.40 to 5.44 | 0.601 | |||
| < 300 | 11.6 (11.9); 24 | 21.1 (13.9); 37 | –4.81 | –10.55 to 0.94 | 0.101 | –2.83 | –12.18 to 6.53 | 0.554 |
| 300–600 | 17.6 (14.5); 109 | 20.1 (14.5); 94 | –1.05 | –4.18 to 2.08 | 0.511 | 0.93 | –7.11 to 8.98 | 0.820 |
| ≥ 600 | 18.2 (13.7); 30 | 18.1 (15.2); 31 | 1.19 | –4.64 to 7.02 | 0.689 | 3.17 | –6.27 to 12.61 | 0.510 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Difficulty with abstract thinking (PANSS) | ||||||||
| 9 months | ||||||||
| 0–4 | 16.9 (14.5); 112 | 21.2 (13.5); 126 | –2.38 | –5.27 to 0.51 | 0.106 | |||
| ≥ 4 | 19.1 (13.8); 73 | 24.7 (13.0); 66 | –2.77 | –6.60 to 1.06 | 0.156 | –0.39 | –5.18 to 4.40 | 0.874 |
| 21 months | ||||||||
| 0–4 | 16.5 (14.4); 109 | 19.8 (14.1); 120 | –2.04 | –4.99 to 0.92 | 0.177 | |||
| ≥ 4 | 18.1 (13.8); 70 | 21.4 (14.8); 62 | –0.00 | –3.97 to 3.96 | 0.998 | 2.03 | –2.91 to 6.97 | 0.420 |
| Conceptual disorganisation (PANSS) | ||||||||
| 9 months | ||||||||
| 0–4 | 18.2 (14.3); 160 | 22.6 (13.3); 160 | –2.53 | –5.02 to –0.03 | 0.047 | |||
| ≥ 4 | 15.0 (14.1); 25 | 21.3 (13.9); 32 | –2.94 | –8.90 to 3.03 | 0.335 | –0.41 | –6.87 to 6.05 | 0.901 |
| 21 months | ||||||||
| 0–4 | 17.7 (14.3); 150 | 20.6 (14.3); 153 | –1.43 | –4.02 to 1.16 | 0.280 | |||
| ≥ 4 | 14.3 (13.4); 29 | 18.8 (15.0); 29 | –1.08 | –6.91 to 4.75 | 0.717 | 0.35 | –6.03 to 6.73 | 0.914 |
| LN | ||||||||
| 9 months | ||||||||
| ≤ 8 | 17.7 (13.1); 23 | 24.4 (14.0); 19 | –1.01 | –7.41 to 5.38 | 0.756 | |||
| ≥ 9 | 17.8 (14.9); 16 | 22.4 (9.9); 9 | –4.80 | –14.43 to 4.83 | 0.328 | –3.79 | –15.22 to 7.64 | 0.516 |
| 21 months | ||||||||
| ≤ 8 | 19.5 (14.0); 21 | 21.5 (15.4); 17 | 3.51 | –3.10 to 10.13 | 0.298 | |||
| ≥ 9 | 20.1 (13.2); 16 | 23.3 (9.9); 9 | –3.04 | –12.08 to 6.00 | 0.510 | –6.55 | –17.65 to 4.55 | 0.247 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Emotional abuse | ||||||||
| 9 months | ||||||||
| ≤ 9 | 17.2 (14.5); 79 | 23.5 (12.7); 75 | –3.88 | –7.39 to –0.37 | 0.030 | |||
| ≥ 10 | 18.8 (14.6); 53 | 24.3 (12.2); 67 | –1.49 | –5.55 to 2.56 | 0.470 | 2.39 | –2.98 to 7.75 | 0.383 |
| 21 months | ||||||||
| ≤ 9 | 17.2 (13.7); 78 | 19.1 (14.3); 74 | 0.30 | –3.25 to 3.84 | 0.870 | |||
| ≥ 10 | 19.2 (14.6); 52 | 23.4 (13.4); 64 | –2.16 | –6.28 to 1.96 | 0.304 | –2.46 | –7.90 to 2.98 | 0.376 |
| Emotional neglect | ||||||||
| 9 months | ||||||||
| ≤ 14 | 18.0 (14.7); 96 | 23.0 (12.5); 98 | –3.60 | –6.75 to –0.46 | 0.025 | |||
| ≥ 15 | 16.1 (14.5); 36 | 25.6 (12.3); 43 | –1.55 | –6.59 to 3.49 | 0.546 | 2.05 | –3.86 to 7.97 | 0.497 |
| 21 months | ||||||||
| ≤ 14 | 17.7 (13.7); 97 | 19.8 (13.9); 95 | –0.71 | –3.88 to 2.46 | 0.660 | |||
| ≥ 15 | 16.5 (15.5); 32 | 24.7 (13.0); 42 | –2.93 | –8.17 to 2.30 | 0.273 | –2.22 | –8.33 to 3.89 | 0.476 |
| Physical abuse | ||||||||
| 9 months | ||||||||
| ≤ 7 | 16.8 (14.7); 87 | 23.4 (12.4); 93 | –4.04 | –7.36 to –0.72 | 0.017 | |||
| ≥ 8 | 18.0 (15.0); 44 | 25.3 (12.7); 50 | –2.08 | –6.61 to 2.45 | 0.367 | 1.96 | –3.63 to 7.55 | 0.492 |
| 21 months | ||||||||
| ≤ 7 | 16.8 (14.0); 87 | 19.7 (14.0); 90 | –0.69 | –4.06 to 2.68 | 0.689 | |||
| ≥ 8 | 17.9 (14.8); 41 | 24.3 (13.2); 48 | –2.91 | –7.55 to 1.73 | 0.219 | –2.22 | –7.94 to 3.49 | 0.446 |
| Physical neglect | ||||||||
| 9 months | ||||||||
| ≤ 7 | 17.0 (15.1); 65 | 24.6 (12.0); 67 | –5.13 | –8.93 to –1.34 | 0.008 | |||
| ≥ 8 | 17.5 (14.0); 65 | 23.9 (12.7); 76 | –1.88 | –5.61 to 1.86 | 0.325 | 3.26 | –2.04 to 8.55 | 0.228 |
| 21 months | ||||||||
| ≤ 7 | 17.5 (14.4); 66 | 21.0 (13.3); 67 | –0.98 | –4.80 to 2.83 | 0.613 | |||
| ≥ 8 | 17.2 (14.1); 61 | 21.8 (14.4); 73 | –2.27 | –6.08 to 1.55 | 0.244 | –1.28 | –6.66 to 4.09 | 0.640 |
| Sexual abuse | ||||||||
| 9 months | ||||||||
| ≤ 7 | 16.7 (14.3); 96 | 23.4 (12.7); 100 | –3.85 | –7.02 to –0.68 | 0.017 | |||
| ≥ 8 | 19.7 (15.1); 32 | 25.3 (11.9); 43 | –2.04 | –7.12 to 3.05 | 0.433 | 1.81 | –4.16 to 7.79 | 0.552 |
| 21 months | ||||||||
| ≤ 7 | 16.8 (14.1); 97 | 19.4 (14.1); 99 | –0.42 | –3.61 to 2.76 | 0.795 | |||
| ≥ 8 | 18.9 (13.8); 29 | 26.0 (12.4); 39 | –4.58 | –10.00 to 0.84 | 0.098 | –4.15 | –10.43 to 2.12 | 0.194 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| AUDIT | ||||||||
| 9 months | ||||||||
| ≤ 12 | 18.2 (14.4); 160 | 21.9 (13.6); 173 | –1.88 | –4.33 to 0.57 | 0.132 | |||
| ≥ 13 | 16.2 (12.8); 18 | 31.4 (5.9); 12 | –11.64 | –19.93 to –3.35 | 0.006 | –9.76 | –18.40 to –1.12 | 0.027 |
| 21 months | ||||||||
| ≤ 12 | 17.8 (14.2); 155 | 20.0 (14.3); 164 | –0.75 | –3.26 to 1.76 | 0.558 | |||
| ≥ 13 | 15.2 (12.8); 16 | 26.8 (12.9); 12 | –7.76 | –16.34 to 0.82 | 0.076 | –7.01 | –15.95 to 1.92 | 0.124 |
| DAST | ||||||||
| 9 months | ||||||||
| 1–2 | 17.5 (14.3); 161 | 23.0 (13.1); 171 | –3.59 | –6.03 to –1.16 | 0.004 | |||
| ≥ 3 | 19.6 (10.6); 10 | 20.0 (15.7); 12 | 4.83 | –4.73 to 14.39 | 0.322 | 8.42 | –1.43 to 18.27 | 0.094 |
| 21 months | ||||||||
| 1–2 | 17.3 (14.0); 156 | 21.0 (14.2); 160 | –2.05 | –4.55 to 0.44 | 0.107 | |||
| ≥ 3 | 15.2 (12.5); 9 | 19.5 (16.1); 12 | –0.77 | –11.27 to 9.73 | 0.885 | 1.28 | –9.50 to 12.06 | 0.816 |
| PAM-SR attachment avoidance | ||||||||
| 9 months | ||||||||
| < 9 | 14.9 (13.9); 114 | 21.5 (12.7); 108 | –4.21 | –7.19 to –1.23 | 0.006 | |||
| 9–16 | 23.8 (13.0); 50 | 26.3 (12.3); 68 | –0.97 | –4.94 to 3.00 | 0.632 | 3.24 | –1.72 to 8.21 | 0.201 |
| 17–24 | 19.0 (14.8); 21 | 12.4 (16.8); 16 | 4.35 | –2.88 to 11.58 | 0.239 | 8.56 | 0.73 to 16.39 | 0.032 |
| 21 months | ||||||||
| < 9 | 15.3 (13.6); 114 | 19.6 (13.6); 104 | –2.06 | –5.08 to 0.96 | 0.181 | |||
| 9–16 | 23.9 (13.7); 43 | 23.6 (14.4); 62 | 0.13 | –4.15 to 4.42 | 0.951 | 2.20 | –3.05 to 7.44 | 0.412 |
| 17–24 | 13.5 (14.2); 22 | 12.3 (16.0); 16 | 1.62 | –5.59 to 8.83 | 0.660 | 3.68 | –4.14 to 11.51 | 0.356 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| Negative others | ||||||||
| 9 months | ||||||||
| < 7.2 | 15.7 (14.0); 90 | 23.4 (12.0); 72 | –4.55 | –8.19 to –0.92 | 0.014 | |||
| ≥ 7.2 | 20.5 (13.6); 75 | 23.1 (13.5); 104 | –1.75 | –5.15 to 1.66 | 0.314 | 2.80 | –2.19 to 7.80 | 0.271 |
| 21 months | ||||||||
| < 7.2 | 16.6 (13.8); 89 | 21.9 (13.3); 69 | –2.48 | –6.17 to 1.20 | 0.186 | |||
| ≥ 7.2 | 18.7 (14.4); 73 | 19.9 (14.7); 96 | –0.91 | –4.46 to 2.64 | 0.616 | 1.57 | –3.55 to 6.70 | 0.547 |
| Negative self | ||||||||
| 9 months | ||||||||
| < 7.2 | 16.4 (13.9); 105 | 21.5 (12.8); 105 | –1.47 | –4.66 to 1.71 | 0.365 | |||
| ≥ 7.2 | 20.0 (14.2); 60 | 25.3 (13.1); 74 | –5.36 | –9.16 to –1.57 | 0.006 | –3.89 | –8.86 to 1.07 | 0.125 |
| 21 months | ||||||||
| < 7.2 | 15.4 (13.4); 100 | 19.7 (13.7); 99 | –1.34 | –4.59 to 1.92 | 0.422 | |||
| ≥ 7.2 | 20.8 (14.4); 59 | 22.7 (14.6); 68 | –1.86 | –5.80 to 2.08 | 0.355 | –0.53 | –5.65 to 4.59 | 0.840 |
| Positive others | ||||||||
| 9 months | ||||||||
| < 7.2 | 16.6 (14.9); 38 | 24.2 (13.2); 59 | –1.97 | –6.68 to 2.73 | 0.411 | |||
| ≥ 7.2 | 18.4 (13.9); 127 | 22.5 (12.8); 118 | –3.42 | –6.31 to –0.52 | 0.021 | –1.44 | –6.96 to 4.07 | 0.608 |
| 21 months | ||||||||
| < 7.2 | 15.5 (13.9); 35 | 22.5 (14.9); 60 | –3.28 | –8.10 to 1.53 | 0.182 | |||
| ≥ 7.2 | 18.2 (14.0); 125 | 19.7 (13.6); 108 | –0.38 | –3.38 to 2.62 | 0.804 | 2.90 | –2.76 to 8.57 | 0.315 |
| Positive self | ||||||||
| 9 months | ||||||||
| < 7.2 | 19.2 (15.1); 67 | 23.6 (13.5); 85 | –3.06 | –6.71 to 0.58 | 0.099 | |||
| ≥ 7.2 | 17.1 (13.3); 98 | 22.3 (12.7); 92 | –2.95 | –6.25 to 0.34 | 0.079 | 0.11 | –4.80 to 5.02 | 0.965 |
| 21 months | ||||||||
| < 7.2 | 18.7 (14.5); 71 | 21.5 (14.3); 81 | –2.24 | –5.89 to 1.41 | 0.229 | |||
| ≥ 7.2 | 17.1 (13.7); 89 | 20.1 (13.9); 85 | –0.44 | –3.92 to 3.04 | 0.805 | 1.80 | –3.24 to 6.85 | 0.484 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| 9 months | ||||||||
| Stress sensitivity | 16.1 (14.2); 60 | 23.0 (13.3); 65 | –4.17 | –8.05 to –0.28 | 0.035 | |||
| Drug related | 15.8 (13.2); 56 | 22.7 (13.5); 44 | –4.05 | –8.57 to 0.48 | 0.079 | 0.12 | –5.82 to 6.06 | 0.969 |
| Trauma | 31.1 (2.7); 9 | 18.9 (18.2); 7 | 6.81 | –3.62 to 17.23 | 0.201 | 10.98 | –0.18 to 22.13 | 0.054 |
| Anxiety | 12.9 (14.4); 22 | 21.4 (13.6); 33 | –5.20 | –11.33 to 0.93 | 0.096 | –1.03 | –8.27 to 6.20 | 0.779 |
| Drug and trauma | 33.0 (1.4); 2 | 25.3 (12.3); 3 | 19.71 | 0.45 to 38.98 | 0.045 | 19.71 | 0.45 to 38.98 | 0.045 |
| 21 months | ||||||||
| Stress sensitivity | 19.6 (13.8); 63 | 22.2 (14.4); 65 | –0.56 | –4.46 to 3.34 | 0.778 | |||
| Drug related | 14.5 (12.6); 57 | 18.8 (13.5); 44 | –2.02 | –6.50 to 2.47 | 0.378 | –1.46 | –7.38 to 4.46 | 0.629 |
| Trauma | 21.3 (16.8); 7 | 22.1 (15.9); 7 | –5.11 | –16.18 to 5.95 | 0.365 | –4.55 | –16.32 to 7.21 | 0.448 |
| Anxiety | 8.9 (13.6); 22 | 20.4 (14.4); 30 | –8.60 | –14.79 to –2.41 | 0.006 | –8.04 | –15.33 to –0.75 | 0.031 |
| Drug and trauma | 34.3 (7.5); 3 | 9.3 (18.5); 4 | 31.39 | 13.46 to 49.31 | 0.001 | 31.95 | 13.62 to 50.28 | 0.001 |
| Trial arm, mean (SD); n | Mean difference | 95% CI | p-value | Interaction effect | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |||||||
| PANSS total | ||||||||
| 9 months | ||||||||
| ≤ 8 | 75.8 (13.9); 29 | 82.5 (15.0); 27 | –2.31 | –7.82 to 3.20 | 0.412 | |||
| ≥ 9 | 75.5 (17.0); 69 | 76.5 (14.2); 75 | –2.34 | –7.32 to 2.63 | 0.356 | –0.04 | –7.48 to 7.41 | 0.992 |
| 21 months | ||||||||
| ≤ 8 | 76.8 (18.4); 26 | 78.9 (16.4); 27 | –1.12 | –6.73 to 4.49 | 0.696 | |||
| ≥ 9 | 72.9 (18.2); 67 | 74.5 (14.7); 72 | –2.37 | –7.48 to 2.74 | 0.364 | –1.25 | –8.86 to 6.36 | 0.748 |
| QPR | ||||||||
| 9 months | ||||||||
| ≤ 8 | 52.6 (11.7); 24 | 44.7 (13.7); 20 | 3.53 | –0.76 to 7.81 | 0.107 | |||
| ≥ 9 | 50.9 (11.5); 55 | 49.5 (9.9); 64 | 1.32 | –2.26 to 4.90 | 0.469 | –2.21 | –7.79 to 3.38 | 0.439 |
| 21 months | ||||||||
| ≤ 8 | 51.4 (8.7); 21 | 50.7 (8.8); 19 | –0.04 | –4.47 to 4.38 | 0.985 | |||
| ≥ 9 | 52.4 (10.4); 50 | 49.8 (11.1); 62 | 0.45 | –3.25 to 4.16 | 0.811 | 0.50 | –5.28 to 6.27 | 0.866 |
| PSP | ||||||||
| 9 months | ||||||||
| ≤ 8 | 51.2 (12.1); 26 | 45.2 (14.2); 26 | 7.08 | 1.93 to 12.22 | 0.007 | |||
| ≥ 9 | 52.2 (15.2); 69 | 51.4 (14.4); 73 | –1.90 | –6.54 to 2.73 | 0.421 | –8.98 | –15.92 to –2.04 | 0.011 |
| 21 months | ||||||||
| ≤ 8 | 48.0 (12.6); 25 | 51.4 (13.5); 27 | –0.11 | –5.32 to 5.10 | 0.967 | |||
| ≥ 9 | 52.1 (15.6); 65 | 50.0 (15.6); 71 | 1.71 | –3.02 to 6.44 | 0.479 | 1.82 | –5.23 to 8.87 | 0.613 |
| PSYRATS – auditory hallucination | ||||||||
| 9 months | ||||||||
| ≤ 8 | 16.9 (13.3); 24 | 23.8 (14.1); 22 | –0.75 | –5.71 to 4.22 | 0.768 | |||
| ≥ 9 | 15.0 (13.9); 58 | 22.9 (12.7); 64 | –4.10 | –8.21 to 0.01 | 0.051 | –3.35 | –9.76 to 3.06 | 0.305 |
| 21 months | ||||||||
| ≤ 8 | 18.6 (14.3); 22 | 20.2 (15.4); 19 | 2.84 | –2.16 to 7.85 | 0.266 | |||
| ≥ 9 | 15.1 (13.8); 57 | 22.1 (13.4); 61 | –4.60 | –8.90 to –0.30 | 0.036 | –7.44 | –14.02 to –0.86 | 0.027 |
| Item | Whole sample (N = 279), n (%) | Trial arm, n (%) | p-value | ||||
|---|---|---|---|---|---|---|---|
| CBT (N = 131) | TAU (N = 148) | ||||||
| Quite a lot | Very much | Quite a lot | Very much | Quite a lot | Very much | ||
| 1 | 50 (17.9) | 20 (7.2) | 23 (17.6) | 8 (6.1) | 27 (18.2) | 12 (8.1) | 0.61 |
| 2 | 0 (0.0) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.4) | 0.18 |
| 3 | 8 (2.9) | 2 (0.7) | 4 (3.1) | 0 (0.0) | 4 (2.7) | 2 (1.4) | 0.65 |
| 4 | 6 (2.2) | 1 (0.4) | 3 (2.3) | 0 (0.0) | 3 (2.0) | 1 (0.7) | 0.83 |
| 5 | 4 (1.4) | 1 (0.4) | 2 (1.5) | 1 (0.8) | 2 (1.4) | 0 (0.0) | 0.56 |
| 6 | 1 (0.4) | 1 (0.4) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0.93 |
| 7 | 9 (3.2) | 5 (1.8) | 4 (3.1) | 5 (3.8) | 5 (3.4) | 0 (0.0) | 0.18 |
| 8 | 20 (7.2) | 12 (4.3) | 10 (7.6) | 8 (6.1) | 10 (6.8) | 4 (2.7) | 0.26 |
| 9 | 4 (1.4) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 2 (1.4) | 0 (0.0) | 0.90 |
| 10 | 7 (2.5) | 1 (0.4) | 3 (2.3) | 0 (0.0) | 4 (2.7) | 1 (0.7) | 0.59 |
| 11 | 9 (3.2) | 0 (0.0) | 5 (3.8) | 0 (0.0) | 4 (2.7) | 0 (0.0) | 0.60 |
| 12 | 2 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.4) | 0 (0.0) | 0.18 |
| 13 | 2 (0.7) | 2 (0.7) | 1 (0.8) | 0 (0.0) | 1 (0.7) | 2 (1.4) | 0.38 |
| 14 | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0.29 |
| 15 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.35 |
| 16 | 3 (1.1) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.49 |
| 17 | 2 (0.7) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.93 |
| 18 | 3 (1.1) | 2 (0.7) | 1 (0.8) | 0 (0.0) | 2 (1.4) | 2 (1.4) | 0.22 |
| 19 | 18 (6.5) | 2 (0.7) | 7 (5.3) | 0 (0.0) | 11 (7.4) | 2 (1.4) | 0.23 |
| 20 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.35 |
| 21 | 9 (3.2) | 2 (0.7) | 5 (3.8) | 1 (0.8) | 4 (2.7) | 1 (0.7) | 0.61 |
| 22 | 4 (1.4) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 2 (1.4) | 0 (0.0) | 0.90 |
| 23 | 5 (1.8) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 4 (2.7) | 0 (0.0) | 0.22 |
| 24 | 14 (5.0) | 3 (1.1) | 8 (6.1) | 0 (0.0) | 6 (4.1) | 3 (2.0) | 0.99 |
| 25 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0.35 |
| 26 | 2 (0.7) | 1 (0.4) | 1 (0.8) | 1 (0.8) | 1 (0.7) | 0 (0.0) | 0.49 |
| 27a | 15 (5.4) | 5 (1.8) | 12 (9.2) | 1 (0.8) | 3 (2.0) | 4 (2.7) | 0.09 |
Appendix 3 Economic analyses
| EQ-5D health states | Trial arm, n (%) | |
|---|---|---|
| CBT (N = 76) | TAU (N = 93) | |
| Mobility | ||
| No problems | 55 (72) | 50 (54) |
| Slight problems | 12 (16) | 19 (20) |
| Moderate problems | 5 (7) | 15 (16) |
| Severe problems | 4 (5) | 8 (9) |
| Extreme problems | 0 (0) | 1 (1) |
| Self-care | ||
| No problems | 48 (63) | 64 (69) |
| Slight problems | 15 (20) | 15 (16) |
| Moderate problems | 10 (13) | 11 (12) |
| Severe problems | 3 (4) | 3 (3) |
| Extreme problems | 0 | 0 |
| Usual activities | ||
| No problems | 27 (35) | 32 (34) |
| Slight problems | 19 (25) | 22 (24) |
| Moderate problems | 21 (28) | 29 (31) |
| Severe problems | 7 (9) | 8 (9) |
| Extreme problems | 2 (3) | 2 (2) |
| Pain and distress | ||
| No problems | 43 (57) | 38 (41) |
| Slight problems | 17 (22) | 21 (23) |
| Moderate problems | 11 (15) | 16 (17) |
| Severe problems | 4 (5) | 12 (13) |
| Extreme problems | 1 (1) | 6 (6) |
| Anxiety and depression | ||
| No problems | 9 (12) | 12 (13) |
| Slight problems | 25 (33) | 20 (22) |
| Moderate problems | 22 (29) | 34 (36) |
| Severe problems | 15 (20) | 16 (17) |
| Extreme problems | 5 (6) | 11 (12) |
| EQ-5D health states | Trial arm, n (%) | |
|---|---|---|
| CBT (N = 76) | TAU (N = 93) | |
| Mobility | ||
| No problems | 54 (71) | 47 (50) |
| Slight problems | 12 (16) | 21 (23) |
| Moderate problems | 5 (6.5) | 14 (15) |
| Severe problems | 5 (6.5) | 11 (12) |
| Extreme problems | 0 (0) | 0 (0) |
| Self-care | ||
| No problems | 55 (72) | 60 (65) |
| Slight problems | 9 (12) | 18 (19) |
| Moderate problems | 10 (13) | 10 (11) |
| Severe problems | 2 (3) | 5 (5) |
| Extreme problems | 0 (0) | 0 (0) |
| Usual activities | ||
| No problems | 41 (54) | 40 (43) |
| Slight problems | 12 (16) | 17 (18) |
| Moderate problems | 18 (24) | 26 (28) |
| Severe problems | 4 (5) | 10 (11) |
| Extreme problems | 1 (1) | 0 (0) |
| Pain and distress | ||
| No problems | 47 (62) | 42 (45) |
| Slight problems | 12 (16) | 20 (22) |
| Moderate problems | 13 (17) | 16 (17) |
| Severe problems | 3 (4) | 11 (12) |
| Extreme problems | 1 (1) | 4 (4) |
| Anxiety and depression | ||
| No problems | 17 (22) | 11 (12) |
| Slight problems | 20 (26) | 28 (30) |
| Moderate problems | 24 (32) | 32 (34) |
| Severe problems | 11 (15) | 12 (13) |
| Extreme problems | 4 (5) | 10 (11) |
| EQ-5D health states | Trial arm, n (%) | |
|---|---|---|
| CBT (N = 76) | TAU (N = 93) | |
| Mobility | ||
| No problems | 50 (66) | 49 (53) |
| Slight problems | 6 (8) | 16 (17) |
| Moderate problems | 14 (18) | 16 (17) |
| Severe problems | 6 (8) | 10 (11) |
| Extreme problems | 0 (0) | 2 (2) |
| Self-care | ||
| No problems | 55 (72) | 51 (55) |
| Slight problems | 12 (16) | 30 (32) |
| Moderate problems | 7 (9) | 6 (7) |
| Severe problems | 2 (3) | 4 (4) |
| Extreme problems | 0 (0) | 2 (2) |
| Usual activities | ||
| No problems | 38 (50) | 36 (39) |
| Slight problems | 22 (29) | 20 (21) |
| Moderate problems | 13 (17) | 29 (31) |
| Severe problems | 3 (4) | 6 (7) |
| Extreme problems | 0 (0) | 2 (2) |
| Pain and distress | ||
| No problems | 47 (62) | 38 (41) |
| Slight problems | 13 (17) | 20 (21.5) |
| Moderate problems | 10 (13) | 20 (21.5) |
| Severe problems | 5 (7) | 12 (13) |
| Extreme problems | 1 (1) | 3 (3) |
| Anxiety and depression | ||
| No problems | 17 (22) | 15 (16) |
| Slight problems | 23 (30) | 18 (20) |
| Moderate problems | 26 (34) | 41 (44) |
| Severe problems | 8 (11) | 14 (15) |
| Extreme problems | 2 (3) | 5 (5) |
| Service type | Unit costs (£) | Source |
|---|---|---|
| Inpatient costs | ||
| Psychiatric ward | 381.55 | NHS Reference Costs 161 |
| Emergency/crisis centre | 414.06 | NHS Reference Costs 161 |
| General medical ward | 538.63 | NHS Reference Costs 161 |
| Alcohol-treatment ward | 334.28 | NHS Reference Costs 161 |
| Drug-treatment ward | 334.28 | NHS Reference Costs 161 |
| Respite care | 389.16 | NHS Reference Costs 161 |
| Maternity | 919.45 | NHS Reference Costs 161 |
| Outpatient and day visits | ||
| Psychiatric | 105.08 | NHS Reference Costs 161 |
| Hospital alcohol service | 101.46 | NHS Reference Costs 161 |
| Hospital substance use service | 101.46 | NHS Reference Costs 161 |
| A&E | 146.86 | NHS Reference Costs 161 |
| Day hospital | 120.61 | NHS Reference Costs 161 |
| Psychotherapy | 199.06 | NHS Reference Costs 161 |
| Clinical psychology/psychology | 144.70 | NHS Reference Costs 161 |
| Colonoscopy | 455.82 | NHS Reference Costs 161 |
| General diagnostic test | 37.30 | NHS Reference Costs 161 |
| Eating disorders | 52.92 | NHS Reference Costs 161 |
| Stroke | 170.60 | NHS Reference Costs 161 |
| Maxillofacial | 118.90 | NHS Reference Costs 161 |
| Neurosurgery | 205.98 | NHS Reference Costs 161 |
| Hepatology | 255.35 | NHS Reference Costs 161 |
| Clozapine clinic | 3.37 | NHS Reference Costs 161 |
| Crisis team | 148 | NHS Reference Costs 161 |
| Anticoagulant clinic | 26.26 | NHS Reference Costs 161 |
| Oncology | 151.12 | NHS Reference Costs 161 |
| Clinical oncology | 126.60 | NHS Reference Costs 161 |
| Haematology | 160.58 | NHS Reference Costs 161 |
| General surgery | 130.06 | NHS Reference Costs 161 |
| Cataract | 123.98 | NHS Reference Costs 161 |
| Spinal unit | 280.03 | NHS Reference Costs 161 |
| Obstetrics | 127.54 | NHS Reference Costs 161 |
| Plastic surgery | 99.95 | NHS Reference Costs 161 |
| Ear, nose and throat | 96.87 | NHS Reference Costs 161 |
| Radiography | 37.30 | NHS Reference Costs 161 |
| Neurology | 175.60 | NHS Reference Costs 161 |
| Diabetic | 159.31 | NHS Reference Costs 161 |
| Sexual health | 84.11 | NHS Reference Costs 161 |
| Drug services | 21.30 | NHS Reference Costs 161 |
| Endocrinology | 157.74 | NHS Reference Costs 161 |
| Ophthalmology | 90.64 | NHS Reference Costs 161 |
| Trauma and orthopaedics | 117.01 | NHS Reference Costs 161 |
| Gynaecology | 133.01 | NHS Reference Costs 161 |
| Gastroenterology | 136.57 | NHS Reference Costs 161 |
| Mammogram | 37.30 | NHS Reference Costs 161 |
| MRI | 147.25 | NHS Reference Costs 161 |
| Occupational therapy | 65.85 | NHS Reference Costs 161 |
| Orthotics | 57.76 | NHS Reference Costs 161 |
| Radiology | 84.52 | NHS Reference Costs 161 |
| Physiotherapist | 48.33 | NHS Reference Costs 161 |
| CT scan | 107.04 | NHS Reference Costs 161 |
| Ultrasound scan | 52.55 | NHS Reference Costs 161 |
| Midwifery | 75.15 | NHS Reference Costs 161 |
| Diagnostic biopsy | 30.77 | NHS Reference Costs 161 |
| Urology | 105.19 | NHS Reference Costs 161 |
| Audiology | 58.33 | NHS Reference Costs 161 |
| Dermatologist | 101.63 | NHS Reference Costs 161 |
| Dentist | 124.14 | NHS Reference Costs 161 |
| Pain management | 139.12 | NHS Reference Costs 161 |
| Nephrology | 150.78 | NHS Reference Costs 161 |
| Respiratory | 154.77 | NHS Reference Costs 161 |
| Dietitian | 71.17 | NHS Reference Costs 161 |
| General | 116.92 | NHS Reference Costs 161 |
| Podiatry | 42.84 | NHS Reference Costs 161 |
| Electrocardiography | 162.09 | NHS Reference Costs 161 |
| Endoscopy | 259.73 | NHS Reference Costs 161 |
| Lung function | 115.59 | NHS Reference Costs 161 |
| Knee surgery | 130.06 | NHS Reference Costs 161 |
| Gastric-band management | 157.75 | NHS Reference Costs 161 |
| Abscess draining | 121.60 | NHS Reference Costs 161 |
| Simple blood test | 3.37 | NHS Reference Costs 161 |
| Rheumatology | 142.74 | NHS Reference Costs 161 |
| Gender reassignment | 309.75 | NHS Reference Costs 161 |
| Orthopaedics | 117.01 | NHS Reference Costs 161 |
| Fracture clinic | 117.01 | NHS Reference Costs 161 |
| Gall bladder pre surgery | 201.25 | NHS Reference Costs 161 |
| Cardiology | 127.67 | NHS Reference Costs 161 |
| Clinical neurophysiology | 215.59 | NHS Reference Costs 161 |
| Group therapy | 46.72 | NHS Reference Costs 161 |
| Primary, community and social care | ||
| GP, surgery visit | 36.00 | PSSRU 2016164 |
| GP, home visit | 115.32 | PSSRU 2014,162 updated to 2016 prices164 |
| GP (telephone call) | 36.00 | PSSRU 2016164 |
| Practice nurse (at surgery) | 11.11 | Estimated from average time per visit (PSSRU 2015163) and cost per minute (PSSRU 2016164) |
| Blood test/clozapine clinic | 3.37 | NHS Reference Costs 161 |
| Psychiatrist | 105.08 | NHS Reference Costs 161 |
| Psychologist | 144.70 | NHS Reference Costs 161 |
| Alcohol or drug treatment or rehabilitation service | 52.00 (per hour) | PSSRU164 |
| District nurse | 38 | NHS Reference Costs 161 |
| Community psychiatric nurse/case manager | 77.24 | NHS Reference Costs 161 |
| Social worker | 79.00 (per hour) | PSSRU164 |
| Occupational therapist | Individual: 78.54 (per hour) | NHS Reference Costs 161 |
| Group: 46.72 | ||
| Voluntary counsellor | 32.00 (per hour) | PSSRU164 |
| Home help/care worker | 24.00 (per hour) | PSSRU164 |
| Advocacy worker | 58.00 (per hour) | PSSRU164 |
| Anticoagulant clinic | 26.26 | NHS Reference Costs 161 |
| Assertive outreach team | 55.00 | PSSRU164 |
| Day care | 32.00–34.00 | PSSRU164 |
| Community mental health team | 38.00 (per hour) | PSSRU164 |
| Community rehabilitation team | 78.31 | NHS Reference Costs 161 |
| Crisis team | 39.00 (per hour) | PSSRU164 |
| Dentist | 121.00 | PSSRU164 |
| Debt advice | 270.00 | PSSRU164 |
| Dermatologist | 101.63 | NHS Reference Costs 161 |
| Dietitian | 81.32 | NHS Reference Costs 161 |
| Diabetes mellitus clinic | 70.59 | NHS Reference Costs 161 |
| Gender identity | 309.75 | NHS Reference Costs 161 |
| Housing support – council | 22.97 | PSSRU164 |
| Mindfulness | 14.00 | PSSRU164 |
| One-to-one therapy | 78.95 | NHS Reference Costs 161 |
| Podiatrist | 42.39 | NHS Reference Costs 161 |
| Physiotherapy | 48.94 | NHS Reference Costs 161 |
| Support group | 17.00 | PSSRU164 |
| Sexual health | 84.11 | NHS Reference Costs 161 |
| Support worker | 21.94 (per hour) | PSSRU 2015, inflated163 |
| Service type | Trial arm, mean cost (£), SE (95% CI) | |
|---|---|---|
| CBT (N = 76) | TAU (N = 93) | |
| 3 months prior to baseline | ||
| Hospital inpatient admission (psychiatric) | 63, 63 (0 to 187) | 308, 264 (0 to 829) |
| Hospital inpatient admission (non-psychiatric) | 0, 0 (0 to 0) | 0, 0 (0 to 0) |
| Hospital outpatient, day and emergency care | 45, 17 (11 to 78) | 62, 22 (19 to 104) |
| General practice, community and social care | 704, 89 (529 to 879) | 616, 63 (491 to 741) |
| Baseline to 3 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 50, 50 (0 to 148) | 23, 23 (0 to 69) |
| Hospital outpatient, day and emergency care | 34, 9 (17 to 51) | 69, 18 (33 to 106) |
| General practice, community and social care | 604, 60 (486 to 723) | 577, 67 (445 to 710) |
| 3–6 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 99, 99 (0 to 297) | 122, 59 (5 to 238) |
| Hospital outpatient, day and emergency care | 31, 8 (14 to 48) | 81, 23 (35 to 127) |
| General practice, community and social care | 457, 54 (350 to 564) | 558, 60 (438 to 677) |
| 6–9 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 85, 85 (0 to 254) | 145, 65 (15 to 275) |
| Hospital outpatient, day and emergency care | 60, 18 (23 to 97) | 64, 16 (33 to 95) |
| General practice, community and social care | 507, 53 (400 to 613) | 764, 125 (515 to 1013) |
| 9–13 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 21, 16 (0 to 53) | 46, 29 (0 to 105) |
| Hospital outpatient, day and emergency care | 59, 19 (22 to 96) | 55, 14 (26 to 83) |
| General practice, community and social care | 731, 120 (492 to 970) | 970, 268 (438 to 1502) |
| 13–17 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 0, 0 (0 to 0) | 81, 59 (0 to 200) |
| Hospital outpatient, day and emergency care | 50, 18 (14 to 86) | 46, 9 (28 to 65) |
| General practice, community and social care | 807, 106 (597 to 1017) | 832, 143 (549 to 1116) |
| 17–21 months | ||
| Hospital inpatient admission (psychiatric) | No cases using inpatient care | No cases using inpatient care |
| Hospital inpatient admission (non-psychiatric) | 269, 148 (0 to 564) | 98, 56 (0 to 209) |
| Hospital outpatient, day and emergency care | 71, 16 (39 to 104) | 134 to 48 (38 to 230) |
| General practice, community and social care | 867, 171 (526 to 1208) | 802, 117 (569 to 1034) |
| Assessment period | Trial arm, mean cost (£), SE (95% CI) | |
|---|---|---|
| CBT (N = 76) | TAU (N = 93) | |
| 3 months prior to baseline | 718, 100 (521 to 915) | 661, 68 (527 to 795) |
| Baseline to 3 months | 688, 83 (523 to 853) | 670, 77 (517 to 823) |
| 3–6 months | 587, 116 (356 to 819) | 761, 91 (581 to 941) |
| 6–9 months | 652, 113 (427 to 876) | 973, 147 (681 to 1265) |
| 9–13 months | 811, 128 (557 to 1065) | 1071, 275 (525 to 1616) |
| 13–17 months | 857, 108 (642 to 1072) | 959, 165 (632 to 1287) |
| 17–21 months | 1208, 222 (765 to 1651) | 1034, 136 (763 to 1305) |
| Baseline to 9-month follow-up | 1927, 251 (1255 to 2184) | 2404, 250 (1841 to 2827) |
| Baseline to 21-month follow-up | 4635, 529 (3241 to 5204) | 5277, 581 (4026 to 6417) |
| Service type | Trial arm, mean cost (£), SE (95% CI) | |
|---|---|---|
| CBT (N = 242) | TAU (N = 245) | |
| 3 months prior to baseline (single imputation) | ||
| Hospital inpatient admission (psychiatric) | 2170, 515 (1158 to 3183) | 2581, 553 (1494 to 3668) |
| Hospital inpatient admission (non-psychiatric) | 48, 25 (0 to 97) | 44, 24 (0 to 92) |
| Hospital outpatient care | 95, 19 (57 to 133) | 95, 21 (54 to 135) |
| General practice, community and social care | 796, 65 (668 to 924) | 830, 78 (677 to 981) |
| Baseline to 3 months | ||
| Hospital inpatient admission (psychiatric) | 2607, 588 (1451 to 3762) | 2802, 627 (1569 to 4035) |
| Hospital inpatient admission (non-psychiatric) | 57, 30 (0 to 117) | 56, 39 (0 to 135) |
| Hospital outpatient care | 56, 11 (34 to 77) | 93, 19 (55 to 131) |
| General practice, community and social care | 692, 68 (558 to 825) | 715, 71 (575 to 855) |
| 3–6 months | ||
| Hospital inpatient admission (psychiatric) | 2932, 615 (1724 to 4139) | 2889, 630 (1651 to 4126) |
| Hospital inpatient admission (non-psychiatric) | 44, 35 (0 to 113) | 59, 30 (0 to 120) |
| Hospital outpatient care | 59, 12 (36 to 83) | 87, 19 (50 to 125) |
| General practice, community and social care | 650, 71 (510 to 789) | 593, 49 (497 to 689) |
| 6–9 months | ||
| Hospital inpatient admission (psychiatric) | 3561, 834 (1921 to 5200) | 2414, 591 (1253 to 3575) |
| Hospital inpatient admission (non-psychiatric) | 81, 44 (0 to 169) | 154, 60 (36 to 271) |
| Hospital outpatient care | 63, 11 (41 to 85) | 124, 21 (82 to 166) |
| General practice, community and social care | 631, 73 (488 to 774) | 881, 99 (686 to 1077) |
| 9–13 months | ||
| Hospital inpatient admission (psychiatric) | 2391, 597 (1219 to 3564) | 2345, 598 (1170 to 3519) |
| Hospital inpatient admission (non-psychiatric) | 56, 29 (0 to 113) | 86, 51 (0 to 186) |
| Hospital outpatient care | 155, 52 (13 to 217) | 92, 32 (30 to 154) |
| General practice, community and social care | 1017, 142 (738 to 1295) | 958, 128 (706 to 1210) |
| 13–17 months | ||
| Hospital inpatient admission (psychiatric) | 2931, 693 (1569 to 4292) | 2246, 587 (1094 to 3399) |
| Hospital inpatient admission (non-psychiatric) | 131, 70 (0 to 269) | 64, 39 (0 to 140) |
| Hospital outpatient care | 95, 19 (57 to 133) | 86, 20 (47 to 125) |
| General practice, community and social care | 1093, 121 (854 to 1331) | 969, 113 (743 to 1195) |
| 17–21 months | ||
| Hospital inpatient admission (psychiatric) | 3706, 876 (1985 to 5427) | 3348, 814 (1748 to 4948) |
| Hospital inpatient admission (non-psychiatric) | 242, 97 (51 to 433) | 176, 78 (20 to 331) |
| Hospital outpatient care | 121, 22 (78 to 164) | 141, 27 (87 to 195) |
| General practice, community and social care | 977, 105 (770 to 1183) | 901, 91 (722 to 1080) |
| Model details | |||||
|---|---|---|---|---|---|
| Number of imputations | 10 | ||||
| Number of observations | 487 | ||||
| Average RVI | 0.02 | ||||
| Largest FMI | 0.00 | ||||
| DF adjustment: large sample | |||||
| Minimum | 7481.84 | ||||
| Average | 121,678.36 | ||||
| Maximum | 438,186.39 | ||||
| Model F-test | |||||
| Equal FMI | F (6, 170,398.3) = 9.99 | ||||
| Within-VCE type: OIM | Probability > F = 0.0000 | ||||
| Model results | |||||
| Net cost | Coefficient | SE | t-value | p-value | 95% CI |
| CBT | 0.22 | 0.18 | 1.21 | 0.226 | –0.14 to 0.58 |
| Baseline cost | 0.00 | 0.00 | 1.25 | 0.213 | 0.00 to 0.00 |
| DI | 0.00 | 0.00 | –0.31 | 0.758 | 0.00 to 0.00 |
| Taking clozapine | 0.09 | 0.32 | 0.29 | 0.775 | –0.54 to 0.72 |
| Baseline PSP score | –0.05 | 0.01 | –7.11 | 0.000 | –0.06 to –0.03 |
| Baseline CDSS score | –0.04 | 0.02 | –2.15 | 0.032 | –0.07 to 0.00 |
| Constant | 12.04 | 0.49 | 24.65 | 0.000 | 11.08 to 13.00 |
| Model details | |||||
|---|---|---|---|---|---|
| Number of imputations | 10 | ||||
| Number of observations | 487 | ||||
| Average RVI | 0.08 | ||||
| Largest FMI | 0.20 | ||||
| Complete DF | 478 | ||||
| DF adjustment: small sample | |||||
| Minimum | 151.20 | ||||
| Average | 328.53 | ||||
| Maximum | 439.92 | ||||
| Model F-test | F (8, 455.1) = 20.88 | ||||
| Equal FMI | 0.0000 | ||||
| Within-VCE type: OLS | 151.20 | ||||
| Model results | |||||
| Net cost | Coefficient | SE | t-value | p-value | 95% CI |
| CBT | 0.053 | 0.027 | 1.96 | 0.050 | 0.000 to 0.106 |
| Age | –0.006 | 0.001 | –4.58 | 0.000 | –0.009 to –0.004 |
| Taking clozapine | 0.036 | 0.050 | 0.73 | 0.468 | –0.062 to 0.133 |
| Number of benzodiazepines | –0.036 | 0.038 | –0.93 | 0.351 | –0.111 to 0.040 |
| Baseline CDSS score | –0.001 | 0.001 | –0.81 | 0.420 | –0.003 to 0.001 |
| Baseline QPR score | –0.013 | 0.003 | –3.980 | 0.000 | –0.020 to –0.007 |
| Baseline EQ VAS score | 0.005 | 0.001 | 3.350 | 0.001 | 0.002 to 0.008 |
| Complete case analysis | Net cost (£), SE (95% CI); p-value | Net QALYs, SE (95% CI) |
|---|---|---|
| Unadjusted for baseline covariates (N = 169) | ||
| Baseline to 9 months | 1793, 359 (1085 to 2501); p < 0.001 | 0.06, 0.03 (0.005 to 0.11); p = 0.031 |
| Baseline to 21 months | 1628, 800 (48 to 3208); p = 0.043 | 0.15, 0.05 (0.04 to 0.26); p = 0.006 |
| Adjusted for baseline covariates (N = 169) | ||
| Baseline to 9 months | 3151, 562 (2050 to 4253); p < 0.001 | 0.05, 0.02 (0.01 to 0.10); p = 0.020 |
| Baseline to 21 months | 2872, 715 (1471 to 4274); p < 0.001 | 0.15, 0.05 (0.06 to 0.25); p = 0.002 |
List of abbreviations
- AE
- adverse event
- AnTI
- Anxious Thoughts Inventory
- AUDIT
- Alcohol Use Disorders Identification Test
- BCSS
- Brief Core Schema Scale
- BPRS
- Brief Psychiatric Rating Scale
- CACE
- complier-average causal effect
- CBT
- cognitive–behavioural therapy
- CDSS
- Calgary Depression Scale for Schizophrenia
- CGI
- Clinical Global Impression
- CI
- confidence interval
- CMHT
- community mental health team
- CONSORT
- Consolidated Standards of Reporting Trials
- CRS
- clozapine-resistant schizophrenia
- CTQ
- Childhood Trauma Questionnaire
- CTS-R
- Cognitive Therapy Scale – Revised
- CTU
- Clinical Trials Unit
- CVD
- cardiovascular disease
- DAST
- Drug Abuse Screening Test
- DI
- duration of illness
- DMEC
- Data Monitoring and Ethics Committee
- DUP
- duration of untreated psychosis
- EIP
- Early Intervention in Psychosis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ VAS
- EuroQol Visual Analogue Scale
- ES
- effect size
- FGA
- first-generation antipsychotic
- FI
- family intervention
- FOCUS
- Focusing on Clozapine Unresponsive Symptoms
- GP
- general practitioner
- HRA
- Health Research Authority
- ICC
- interclass correlation coefficient
- ICD-10
- International Classification of Diseases, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISMI
- Internalised Stigma of Mental Illness
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- IVI
- Interpretation of Voices Inventory
- LNS
- letter–number span
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- NRES
- National Research Ethics Service
- PAM-SR
- Psychosis Attachment Measure self-report
- PANSS
- Positive and Negative Syndrome Scale
- PICO
- population, intervention, comparator and outcome
- PIS
- participant information sheet
- PPI
- patient and public involvement
- PSP
- Personal and Social Performance
- PSSRU
- Personal Social Services Research Unit
- PSYRATS
- Psychotic Symptom Rating Scale
- QALY
- quality-adjusted life-year
- QPR
- Questionnaire about the Process of Recovery
- RA
- research assistant
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SCIPS
- semistructured clinical interview for psychosis subgroups
- SD
- standard deviation
- SE
- standard error
- SGA
- second-generation antipsychotic
- SP
- supportive psychotherapy
- SURG
- Service User Reference Group
- TAU
- treatment as usual
- TRRIP
- Treatment Response and Resistance in Psychosis
- TRS
- treatment-resistant schizophrenia
- TSC
- Trial Steering Committee
- WTPT
- willingness-to-pay threshold
