Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 16/51/19. The protocol was agreed in November 2016. The assessment report began editorial review in July 2017 and was accepted for publication in April 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jonathan Wadsley has received personal fees from Sanofi Genzyme (Cambridge, MA, USA), Ipsen (Paris, France), Bayer AG (Leverkusen, Germany), Baxalta (Shire Pharmaceuticals, London, UK), Eisai Co. Ltd (Tokyo, Japan) and Eli Lilly and Company (Basingstoke, UK), grants and personal fees from AstraZeneca plc (Cambridge, UK), non-financial support from Swedish Orphan Biovitrum AB (Stockholm, Sweden), and personal fees and non-financial support from Novartis International AG (Basel, Switzerland) and Celgene Corporation (Summit, NJ, USA) outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Tappenden et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Incidence and prevalence
Thyroid cancer is the most common malignant endocrine tumour, but represents only ≈1% of all malignancies. 1,2 The disease is more common in women than in men. According to Cancer Research UK, 3404 new diagnoses of thyroid cancer were reported in England in 2014: 966 cases (28%) were in men and 2438 cases (72%) were in women. 1 The age-standardised incidence rate of thyroid cancer is reported to be 7 per 100,000 women and 3 per 100,000 men. 1 The UK incidence rate is the 11th lowest in Europe for men and the 15th lowest in Europe for women. The median age at diagnosis is approximately 50 years. 3,4
There are four main types of thyroid cancer: (1) papillary, (2) follicular, (3) medullary and (4) anaplastic. Papillary and follicular thyroid cancer are the most common types of thyroid cancer and account for > 90% of all cases. 3 Medullary thyroid cancer (MTC), the disease type considered in this report, develops from the parafollicular cells (also known as C cells) and commonly presents as a mass in the neck. 2 MTC is very rare and accounts for ≈5% of all thyroid cancers,2 although a lower frequency has been quoted by the American Thyroid Association guidelines. 5 Anaplastic cancers, thyroid lymphomas and metastases to thyroid from other primary tumours are rarer than MTC; anaplastic thyroid cancer accounts for ≈2% of all thyroid cancers. 3 MTC is reported to account for 3% of all thyroid cancers in adults and 10% of all thyroid cancers in children. 2 Based on 2014 estimates of disease incidence,1 the number of new cases of MTC in England in any year would be ≈170 individuals (5% of 3404).
There are four types of MTC: (1) sporadic, (2) multiple endocrine neoplasia (MEN) 2 (formerly MEN 2A), (3) MEN 3 (formerly MEN 2B) and (4) familial MTC. Incidence rates for each type differ by age and sex. 1 Approximately 75% of MTC cases are sporadic in nature, whereas the remaining 25% are genetically determined (MEN 2, MEN 3 and familial MTC). 2,3 The RE-arranged during Transfection (RET) oncogene is central to the development of sporadic and hereditary MTC. 5 Germline testing of the RET oncogene mutation is recommended for all confirmed cases of MTC to establish the possible hereditary basis for the disease within an individual and to facilitate the identification of family members who might be at risk. 2 Almost all patients with MEN 2, MEN 3 and familial MTC have germline RET mutation, whereas approximately 40–50% of patients with sporadic MTC have somatic RET mutations. 2,5 Only germline RET mutation testing is routinely undertaken in the NHS.
Diagnosis and management
In > 75% of cases, patients with MTC will typically present with a lump in the neck (which may represent a thyroid or lymph node mass) or distant metastases. 2 The lumps are not usually associated with other symptoms but may occasionally cause dysphagia (difficulty or discomfort in swallowing) or dysphonia (difficulty in speaking). 2,6 Symptoms might also relate to the effect of metastases, especially diarrhoea, flushing, dyspnoea and bone pain.
Diagnosis is usually made by either fine-needle aspiration cytology of a thyroid nodule or lymph node or core needle biopsy with ultrasound guidance, alongside biochemical investigations of serum-based biomarkers, especially calcitonin (CTN). 2,3,5,7 CTN is the major product secreted by C cells:5 CTN levels of > 100 pg/ml are considered to have a 100% positive predictive value for the presence of MTC. 2,3
The disease is staged and, if appropriate, surgery is performed (usually total thyroidectomy and central compartment node dissection, with or without lateral neck dissection). 2,8,9 Patients with MTC may be classified into three groups: (1) patients with localised disease without evidence of metastases, in whom surgical cure is possible; (2) patients with metastatic disease limited to the neck, in whom surgical cure might be possible but is not always achieved; and (3) patients with distant metastasis, that is the disease has spread outside the neck and in whom surgery is not curative. 3 The only curative treatment for MTC is complete surgical resection, but lymph node or systemic metastases are present at initial diagnosis in around half of MTC cases5 and resection is sometimes incomplete because of extensive lateral spread. 3,4 Patients with unresectable locally advanced or metastatic MTC were the focus of this review. For these patients, the treatment options are limited because MTC is relatively unresponsive to conventional doses of radiation therapy and to all tested chemotherapeutic regimens (see Impact of health problem and Current service provision). 2,3,5 Therefore, patients with symptomatic and progressive disease, according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria,10 are the principal candidates for systemic treatment. 6
Prognosis
Compared with other advanced solid tumours, MTC can be relatively indolent, but it can sometimes be aggressive; data indicate that survival is influenced by age and stage at diagnosis. 4,5,11 It has been reported that patients who are < 40 years of age at the time of diagnosis have a significantly higher adjusted survival rate than older patients,4,12 and 10-year survival rates are reported to be up to 100% for stage I disease, that is, if tumours are confined to the thyroid gland. 4,5,9,13 In the absence of progressive and symptomatic disease, health-related quality of life (HRQoL) can be maintained for months or years. 2,6 However, reported 10-year survival rates decrease to ≈75% with regional disease spread3,14 and range from 21 to 40% for subjects with metastatic disease at diagnosis. 2,3,5 Distant metastases, which can affect multiple organs, most commonly the liver, lungs and bones, are reported to be present in between 7% and 23% of MTC cases at diagnosis. 3,6 Just under half of all patients with sporadic MTC will present with stage III or IV (advanced) disease. 5
Calcitonin and, to a lesser extent, carcinoembryonic antigen (CEA) are used as biological markers of post-operative MTC burden, progression and survival. 15 CEA levels are not specific to MTC and are less sensitive and less reliable than CTN for diagnosis; however, when measured alongside CTN, they are considered to be potentially useful in assessing disease progression. 5,15 Certain levels of CEA might indicate regional spread to draining lymph nodes or more distant spread to non-regional lymph nodes, but are particularly important as an indicator of disease progression. 3,5 Studies16–20 have indicated that patients with CTN and CEA doubling times of ≤ 24 months have more progressive disease and a reduced survival time compared with patients with CTN and CEA doubling times of > 24 months. A 2005 study16 reported 5- and 10-year survival rates of 25% and 8%, respectively, in MTC patients with post-operative CTN doubling times of < 6 months, compared with 92% and 37%, respectively, in patients with doubling times of between 6 and 24 months. In the same study,16 the 10-year survival rate for patients with CTN doubling times of > 24 months was 100%.
Impact of the health problem
Significance for patients
There is little published research concerning the impact of MTC on patients’ HRQoL. As noted in the Ipsen (Paris, France) submission to the National Institute for Health and Care Excellence (NICE),21 most of the available HRQoL evidence is derived from studies of patients with other more common types of thyroid cancer. MTC is associated with a number of symptoms that may impair patients’ HRQoL, including the presence of a thyroid mass (usually a non-tender thyroid nodule or diffuse thyroid enlargement), cervical lymphadenopathy, airway compromise, pain, dysphagia and dysphonia. Diarrhoea is commonly seen in patients with advanced MTC as a result of hormonal excess caused by increased CTN secretion from the parafollicular cells; this may be debilitating and can lead to problems with nutrition. Distant metastases may result in additional symptoms including spinal cord compression, bone fracture, bronchial obstruction and pain. 5 Debilitating symptoms associated with MTC (e.g. severe diarrhoea) may lead to workplace absence and lost productivity.
Significance for the NHS
Medullary thyroid cancer is a very rare disease and, for many patients, surgery can be curative; hence, the population of patients with advanced or metastatic MTC eligible for treatment with vandetanib and cabozantinib is very small. However, given the list prices of the drugs and the lack of effective alternative treatments, the cost per patient treated may be considerable. Both vandetanib (Caprelsa®; Cambridge, MA, USA) and cabozantinib (Cometriq®; Ipsen, Paris, France) are also associated with additional monitoring costs. The Summary of Product Characteristics (SmPC) for vandetanib22 states the following:
An ECG [electrocardiography], and levels of serum potassium, calcium and magnesium and thyroid stimulating hormone (TSH) should be obtained at baseline, at 1, 3, 6 and 12 weeks after starting treatment and every 3 months for at least a year thereafter. This schedule should apply to the period after dose reduction due to QTc [corrected QT interval] prolongation and after dose interruption for more than two weeks. ECGs and blood tests should also be obtained as clinically indicated during this period and afterwards. Frequent ECG monitoring of the QTc interval should be continued.
Serum potassium, serum magnesium and serum calcium should be kept within normal range to reduce the risk of ECG QTc prolongation. Additional monitoring of QTc, electrolytes and renal function are required especially in case of diarrhoea, increase in diarrhoea/dehydration, electrolyte imbalance and/or impaired renal function. If QTc increases markedly but stays below 500 msec, cardiologist advice should be sought.
European Medicines Agency, vandetanib SmPC. © EMA [1995–2018]. Reproduced with permission from the European Medicines Agency22
The SmPC for cabozantinib23 also recommends close monitoring during the first 8 weeks of treatment:
As most events can occur early in the course of treatment, the physician should evaluate the patient closely during the first eight weeks of treatment to determine if dose modifications are warranted. Events that generally have early onset include hypocalcaemia, hypokalaemia, thrombocytopenia, hypertension, palmarplantar erythrodysaesthesia syndrome (PPES), and gastrointestinal (GI) events (abdominal or mouth pain, mucosal inflammation, constipation, diarrhoea, vomiting).
European Medicines Agency, cabozantinib SmPC. © EMA [1995–2018]. Reproduced with permission from the European Medicines Agency23
One of the clinical advisors to the assessment group (AG) noted that although cardiac toxicity is less for cabozantinib than vandetanib, electrocardiographic monitoring may also be required.
Current service provision
Clinical guidelines
There are no clinical guidelines for the management of MTC in the UK. A NICE quality standard for head and neck cancer has recently been published;24 however, this does not include the management of MTC.
Current National Institute for Health and Care Excellence technology appraisal guidance
There is currently no NICE technology appraisal guidance for interventions for the treatment of unresectable locally advanced or metastatic MTC.
Current service cost
The current cost of managing MTC is uncertain. However, MTC is a very rare disease, with an estimated annual incidence for England of around 170 new patients. 1 Prescribing data from the Cancer Drugs Fund (CDF) indicate that in 2016 (confidential information has been removed) new patients received vandetanib and (confidential information has been removed) new patients received cabozantinib (Professor Peter Clark, Chairperson of CDF, 2017, personal communication). The data from 2015 indicate very similar prescribing levels, with (confidential information has been removed) new patients starting vandetanib and (confidential information has been removed) patients starting cabozantinib (Professor Peter Clark, personal communication). Based on current prescribing levels, the cost of treating new MTC patients with cabozantinib and vandetanib for 1 year (assuming full dose and excluding any discontinuation) is approximately £1.96M.
Variation in services and uncertainty about best practice
Clinical advisors to the AG noted that although the indications set out in the marketing authorisations for cabozantinib and vandetanib22,23 relate to patients with progressive disease, this may be determined on the basis of radiographic evidence or the presence of symptomatic disease. They also noted that, elsewhere in Europe, clinicians often initiate treatment earlier on the basis of imaging, whereas clinicians in the UK tend to consider symptomatic progression as the more important time point at which to initiate palliative treatment.
The SmPCs for both vandetanib and cabozantinib state that ‘For patients in whom Rearranged during Transfection (RET) mutation status is not known or is negative, a possible lower benefit should be taken into account before individual treatment decision’ (p. 2) (© EMA [1995–2018]. Reproduced with permission from the European Medicines Agency). 22,23 Clinical advisors to the AG noted that all patients should have an assessment of their germline RET status to check if their disease is sporadic or genetic. This is, however, different from checking if the tumour expresses RET (somatic RET mutation testing). In the UK, it is not routine practice to check the tumour (either primary or metastases) for RET mutations. Although clinicians do not currently have routine access to mutation analysis, this may change in the future. The clinical advisors warned that the RET status of the primary thyroid cancer may not reflect the mutation landscape in the metastases and that it would be inadvisable to base recommendations about the use of vandetanib and cabozantinib in the NHS on RET mutation status without a full and accurate picture of the significance of somatic RET status. Furthermore, the clinicians commented that the thyroid primary may have been removed many years before metastases develop; hence, at the time of relapse, the mutation analysis may no longer be accurate. In addition, as cabozantinib and vandetanib have multiple targets, although a patient may be RET mutation negative in the metastases, they may still obtain a treatment response by virtue of other mutations that are targeted by the individual drug received.
Current treatment pathway
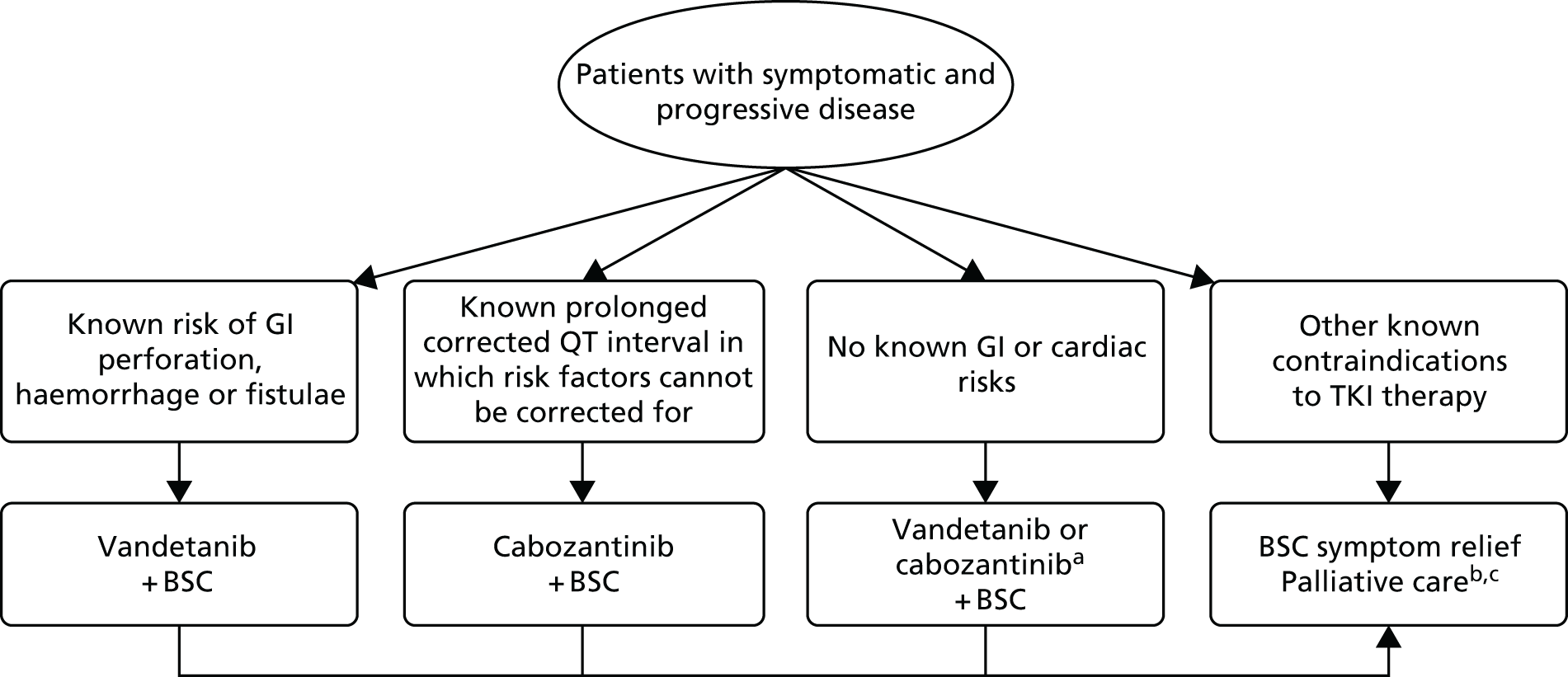
A summary of the treatment pathway, as developed by the AG, is presented in Figure 1. For patients who are ineligible to receive cabozantinib or vandetanib, treatment is likely to comprise palliative treatments. Both cabozantinib and vandetanib are currently available on the CDF as first-line treatments for unresectable, locally advanced or metastatic MTC. 25 The CDF indication for each therapy is the same, as shown in Box 1.
FIGURE 1.
Current treatment pathway for adults with symptomatic and progressive MTC. a, Patient may switch to other TKI if intolerant or severe AEs are experienced within 3 months. Note that the vandetanib licence is in aggressive and symptomatic disease, whereas the licence for cabozantinib is for progressive, unresectable locally advanced or metastatic MTC. b, May include palliative surgery, palliative radiotherapy and/or treatments for bone pain. c, Nuclear medicine therapies, such as MIBG/dotatate, may be considered in some patients. AE, adverse event; BSC, best supportive care; GI, gastrointestinal; MIBG, iodine-123 metaiodobenzylguanidine; TKI, tyrosine kinase inhibitor.
Cabozantanib and vandetanib are the first-line treatments of MTC when all of the following criteria are met:
-
A consultant specialist specifically trained and accredited in the use of systemic anticancer therapy prescribes application and first cycle of systemic anticancer therapy.
-
Unresectable, locally advanced or metastatic MTC, confirmed histologically.
-
First-line indication.
-
Progressive, symptomatic disease.
-
For cabozantinib: no history of tyrosine kinase therapy unless intolerant of vandetanib within 3 months of starting it and, on vandetanib, toxicity that cannot be managed by dose delay or dose modification and absence of disease progression.
-
For vandetanib: no history of tyrosine kinase therapy unless intolerant of cabozantinib within 3 months of starting it and, on cabozantanib, toxicity that cannot be managed by dose delay or dose modification and absence of disease progression.
Description of technology under assessment
Interventions considered in the scope of this report
This assessment includes two interventions: cabozantinib and vandetanib.
Cabozantinib
Cabozantinib has an European Union (EU) marketing authorisation for the treatment of adult patients with progressive, unresectable locally advanced or metastatic MTC. The SmPC for cabozantinib23 states that for patients in whom RET mutation status is not known or is negative, a possible lower benefit should be taken into account before an individual treatment decision. Cabozantinib is administered orally at a recommended dose of 140 mg once daily, taken as one 80-mg capsule and three 20-mg capsules. Treatment should continue until the patient is no longer clinically benefiting from therapy or until unacceptable toxicity occurs. 23 Cabozantinib is available in packs of (1) 80 × 20-mg capsules, (2) 28 × 20-mg capsules and 28 × 80-mg capsules or (3) 84 × 20-mg capsules and 28 × 80-mg capsules. The list price for cabozantinib is £4800 per pack. A confidential Patient Access Scheme (PAS) has been proposed for cabozantinib.
Vandetanib
Vandetanib has an EU marketing authorisation for the treatment of aggressive and symptomatic MTC in patients with unresectable locally advanced or metastatic disease (including children and adolescents aged ≥ 5 years). 22 The SmPC for vandetanib22 states that, for patients in whom RET mutation is not known or is negative, a possible lower benefit should be taken into account before an individual treatment decision. Vandetanib is administered orally at a recommended dose of 300 mg once a day. Vandetanib may be administered until disease progression or until the benefits of treatment continuation no longer outweigh its risk, taking into account the severity of adverse events (AEs) in relation to the degree of clinical stabilisation of the tumour status. 22 Vandetanib is available in packs of (1) 30 × 100-mg tablets (cost per pack of £2500) and (2) 30 × 300-mg tablets (cost per pack of £5000). A confidential PAS has also been proposed for vandetanib.
Mode of action
Cabozantinib
Cabozantinib is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs) implicated in tumour growth and angiogenesis, pathological bone remodelling and metastatic progression of cancer. Cabozantinib was evaluated for its inhibitory activity against a variety of kinases and was identified as an inhibitor of MET (hepatocyte growth factor receptor protein) and vascular endothelial growth factor (VEGF) receptors. In addition, cabozantinib inhibits other tyrosine kinases including RET, the GAS6 receptor (AXL), the stem cell factor receptor (KIT) and FMS-like tyrosine kinase-3. 23
Vandetanib
Vandetanib is a potent inhibitor of VEGF receptor-2 (VEGFR-2) (also known as kinase insert domain-containing receptor), epidermal growth factor receptor (EGFR) and RET tyrosine kinases. Vandetanib is also a submicromolar inhibitor of vascular endothelial receptor-3 tyrosine kinase. Vandetanib inhibits VEGF-stimulated endothelial cell migration, proliferation, survival and new blood vessel formation in in vitro models of angiogenesis. In addition, vandetanib inhibits epidermal growth factor (EGF)-stimulated EGF RTK in tumour cells and endothelial cells. Vandetanib inhibits EGFR-dependent cell proliferation and cell survival in vitro. Vandetanib also inhibits both wild type and the majority of mutated, activated forms of RET, and significantly inhibits the proliferation of MTC cell lines in vitro. In vivo vandetanib administration reduced tumour cell-induced angiogenesis, tumour vessel permeability, tumour microvessel density, and inhibited tumour growth of a range of human xenograft tumour models in athymic mice. Vandetanib also inhibited the growth of MTC xenograft tumours in vivo. The precise mechanism of action of vandetanib in locally advanced or metastatic MTC is unknown. 22
Current usage in the NHS
As noted in Current service cost, both cabozantinib and vandetanib are currently available for use through the CDF. Given the rarity of MTC, total prescribing rates of these products are low. In 2016, (confidential information has been removed) new patients were prescribed cabozantinib or vandetanib through the CDF.
Chapter 2 Definition of the decision problem
This assessment evaluates the clinical effectiveness and cost-effectiveness of cabozantinib and vandetanib within their marketing authorisations for treating unresectable or metastatic MTC. Vandetanib holds an EU marketing authorisation for the treatment of aggressive and symptomatic MTC in patients with unresectable locally advanced or metastatic MTC. Vandetanib is indicated in adults, adolescents and children aged ≥ 5 years. 22 Cabozantinib holds an EU marketing authorisation for the treatment of adult patients with progressive, unresectable locally advanced or metastatic MTC. 23 The SmPCs for each product state that, for patients in whom RET mutation status is not known or is negative, a possible lower benefit should be taken into account before an individual treatment decision. 22,23
Decision problem
In line with the final NICE scope,26 the decision problem is specified as follows.
Population
-
Adults with unresectable locally advanced or metastatic MTC.
In December 2016, the marketing authorisation for vandetanib was extended to include adolescents and children aged ≥ 5 years;22 this population is beyond the scope of this appraisal. 26 Clinical advisors to the AG note that the incidence of unresectable locally advanced or metastatic MTC in children and adolescents aged ≥ 5 years is expected to be extremely low.
Interventions
-
Cabozantinib (oral).
-
Vandetanib (oral).
Relevant comparators
Cabozantinib and vandetanib were compared with:
-
each other
-
best supportive care (BSC).
Outcomes
The following outcomes are included in this assessment:
-
overall survival (OS)
-
progression-free survival (PFS)
-
response rates
-
adverse effects of treatment
-
HRQoL.
Although response rates were not included in the final NICE scope,26 this outcome has been included in this assessment as it is a clinically relevant end point in the key trials considered in this report. 27,28
Subgroups
The final NICE scope26 states that ‘If the evidence allows subgroups according to RET mutation status will be considered.’ Based on the guidance of the clinical advisors to the AG (see Chapter 1, Variation in services and uncertainty about best practice), RET mutation status has not been considered within the health economic analysis presented in this report.
Overall aims and objectives of assessment
The aims of the assessment are to:
-
evaluate the clinical effectiveness and safety of cabozantinib and vandetanib within their marketing authorisations for treating unresectable locally advanced or metastatic MTC
-
estimate the incremental cost-effectiveness of cabozantinib and vandetanib compared with each other and BSC
-
identify key areas for primary research
-
estimate the overall cost of these treatments in England.
Chapter 3 Assessment of clinical effectiveness
This section presents a summary and critique of relevant studies on the efficacy and safety of cabozantinib and vandetanib for the treatment of unresectable locally advanced or metastatic MTC. The systematic review was conducted and reported following the general principles outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and checklist29 and the Centre for Reviews and Dissemination (CRD) guidance. 30 The protocol for this review has been registered with, and is available from, the PROSPERO database (registration number CRD42016050403). 31
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for reviewing effectiveness
Inclusion criteria
The inclusion criteria for the reviews are described in Table 1. These criteria are in accordance with the decision problem set out in the final NICE scope. 26
| Element | Criteria | |
|---|---|---|
| Inclusion | Exclusion | |
| Population | Participants with unresectable locally advanced or metastatic MTC, aged ≥ 18 years. Studies with populations broader than unresectable locally advanced or metastatic MTC will be considered only if data for the relevant study population are available and are reported separately | Studies conducted in paediatric populations |
| Interventions |
|
|
| Comparators | Interventions will be compared with each other and against BSC (including locally ablative treatments, such as radiotherapy) | |
| Outcomes | The following outcomes will be included in the assessment:
|
|
| Study design | RCTs are to be included in the clinical effectiveness systematic review. If no relevant RCTs are identified for an intervention, non-randomised comparative studies will be considered for inclusion. Non-randomised comparative studies are also to be included, when necessary, as a source of additional evidence (e.g. regarding AEs related to the interventions) | Pre-clinical or biological studies, as well as studies of animal models, will be excluded. The following publication types will not be considered for inclusion in the review and synthesis, although the reference lists of reviews and guidelines will be checked for additional relevant trials: narrative reviews, systematic reviews, clinical guidelines, editorials, letters, opinion pieces and abstracts with insufficient details to assess study quality or results |
| Language | Searches were not limited by language | N/A |
Searches
A comprehensive literature search was undertaken to systematically identify randomised controlled trials (RCTs) and systematic reviews (for the identification of additional trials) of the clinical effectiveness of cabozantinib and vandetanib for the treatment of unresectable locally advanced or metastatic MTC.
The following electronic databases were searched from inception to November 2016:
-
MEDLINE – via Ovid, 1946 to present.
-
MEDLINE In-Process & Other Non-Indexed Citations – via Ovid, 1946 to present.
-
MEDLINE Epub Ahead of Print – via Ovid, 1946 to present.
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) – via EBSCOhost, 1982 to present.
-
EMBASE – via Ovid, 1980 to present.
-
Cochrane Database of Systematic Reviews – via Wiley Online Library, 1996 to present.
-
Cochrane Central Register of Controlled Trials (CENTRAL) – via Wiley Online Library, 1995 to present.
-
Database of Abstracts of Reviews of Effects – via Wiley Online Library, 1995 to 2015.
-
Health Technology Assessment Database (HTA) – via Wiley Online Library, 1995 to present.
-
Web of Science [Science Citation Index (SCI)] – via Clarivate Analytics (formerly Thomson Reuters), 1900 to present.
-
Conference Proceedings Citation Index (CPCI) – via Clarivate Analytics (formerly Thomson Reuters), 1990 to present.
To identify ongoing or recently completed studies, trial registers were searched using the International Clinical Trials Registry Portal of the World Health Organization (WHO),32 which regularly compiles and updates data from > 15 clinical trial registers.
Searches were not limited by language or publication date and were not restricted to published research only. Search terms included medical subject heading (MeSH) terms and free-text synonyms for MTC combined with a RCT or systematic review study design filter. The search strategy was designed to be deliberately broad to capture all intervention studies within the MTC population, that is, studies of cabozantinib and vandetanib, as well as additional evidence for possible comparators, including BSC and radiotherapy, as such studies may be used to inform indirect comparisons. The MEDLINE search strategy is presented in Appendix 1.
To identify additional studies, reference lists of relevant studies, systematic reviews, clinical guidelines and submissions to regulatory authorities and advisory bodies [All Wales Medicines Strategy Group (AWMSG), Scottish Medicines Consortium (SMC), European Medicines Agency (EMA) and the US Food and Drug Administration] were examined. In addition, company submissions (CSs) to NICE related to the interventions within the scope of this review were examined. Citation searches of key included studies using the Web of Science database were also conducted. Clinical advisors to the AG provided advice on whether or not any relevant studies were missing from the search results.
A comprehensive database of relevant published and unpublished articles was constructed using EndNote version 8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] software.
Study selection and data extraction
Following standard systematic review processes, two reviewers (CC and EK) independently screened all titles and abstracts using the eligibility criteria outlined in Table 1; full papers were retrieved for any publication that was deemed by a reviewer to be potentially includable. The two reviewers independently screened all full texts to identify studies that satisfied the inclusion criteria. Any discrepancies between reviewers were resolved through discussion. Results were reported in text, tables and a PRISMA flow chart. Data extraction was performed by one reviewer (CC) and was independently checked for errors against the original and published trial reports by the second reviewer (EK). Any discrepancies were resolved through discussion. Results were reported in text and tables.
Quality assessment
For the RCT evidence, critical appraisal of included trials was conducted by one reviewer (CC) using the Cochrane Risk of Bias tool;33 this was checked by a second reviewer (EK) and any discrepancies were resolved through discussion.
Evidence synthesis
Details of the included RCTs, including population characteristics, interventions, comparators and outcomes, were tabulated and discussed in a narrative review. On account of the small number of included studies, with just one study contributing evidence for each of the interventions, pairwise meta-analysis was not appropriate. In the absence of direct evidence comparing cabozantinib with vandetanib, a network meta-analysis (NMA) was performed using the ZETA trial EU-label and Efficacy of XL184 (Cabozantinib) in Advanced Medullary Thyroid Cancer (EXAM) trial intention-to-treat (ITT) populations (see Network meta-analysis).
Results
Quantity and quality of research available
The details of the study selection process are outlined in the PRISMA flow chart (Figure 2). The search identified 1581 references after deduplication, of which 1516 were excluded because they did not satisfy the eligibility criteria. The full texts of 65 studies were retrieved to assess eligibility; 38 of these studies were excluded for the following reasons: absence of a control arm (n = 17), review (n = 6), letter/commentary (n = 6), wrong population (n = 5), wrong intervention (n = 2), animal study (n = 1) and a duplicate (n = 1). A list of excluded full papers, with reasons, is provided in Appendix 2. The excluded studies included two single-arm studies of vandetanib in children and adolescents with unresectable locally advanced or metastatic MTC as a result of MEN 2 (one published study34 and one ongoing study35). These studies may be relevant to the extension to the marketing authorisation for vandetanib;22 however, this population is beyond the scope of this appraisal.
FIGURE 2.
PRISMA flow chart.
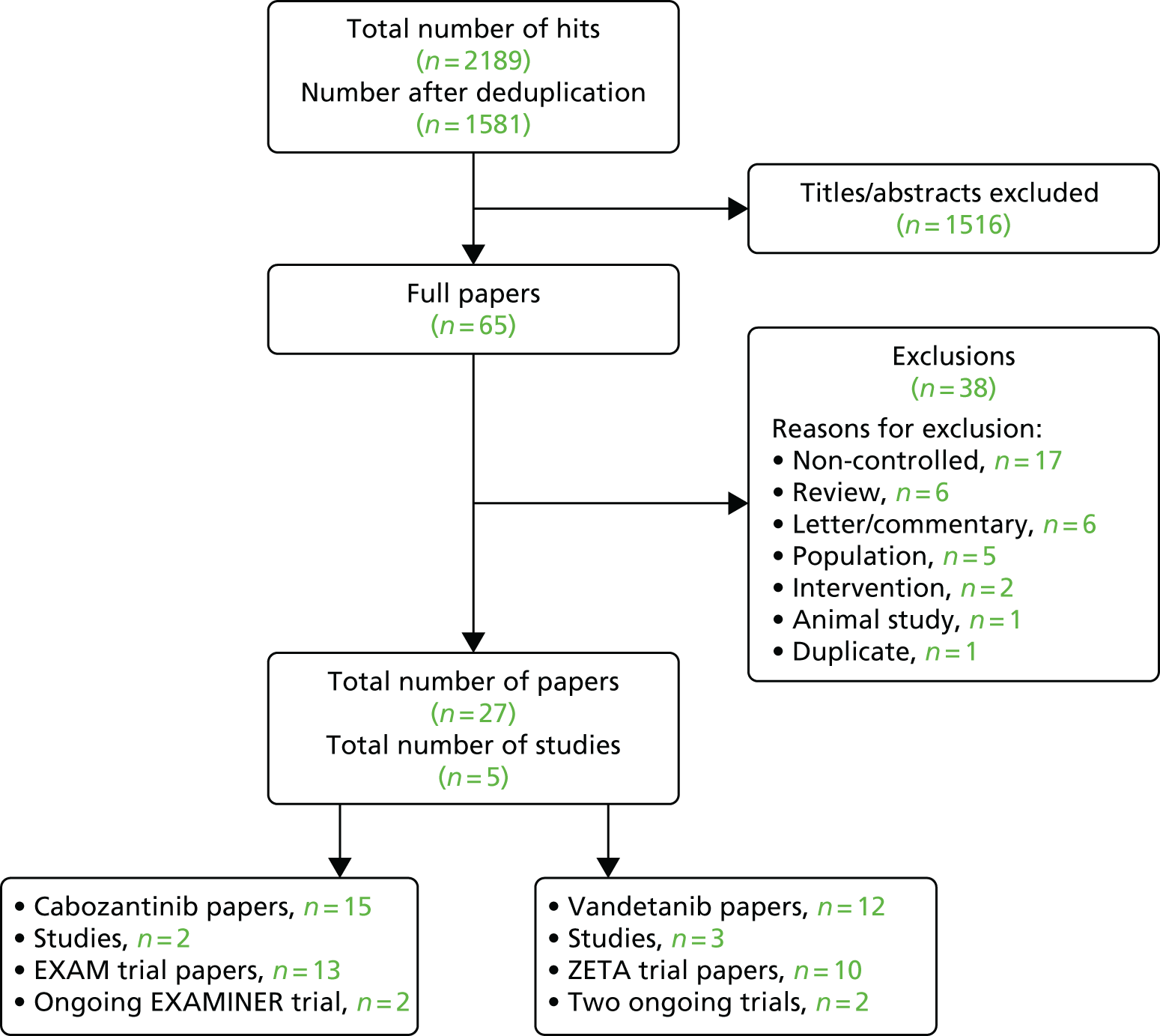
There were five potentially relevant controlled trials of comparator interventions, principally other tyrosine kinase inhibitors (TKIs), one of which ended prematurely because of recruitment issues;36 the remaining four studies37–40 are ongoing. There is also one published retrospective study41 comparing MTC patients who received radioactive iodine therapy with those whose did not. As a result, there was no appropriate additional controlled trial evidence of other potential comparators to cabozantinib or vandetanib (e.g. radiotherapy) that could may have been used to inform a NMA.
The final result was 27 publications and protocols relating to five RCTs. For cabozantinib, this included 13 publications28,42–53 relating to the Phase III EXAM trial,28,42 which compared 140 mg per day of cabozantinib with placebo, and two publications54,55 relating to the ongoing EXAMINER trial,54 which compares 140 mg per day with 60 mg per day of cabozantinib, and seeks to recruit 188 participants (expected completion date: March 2019). 55 For vandetanib, this included 10 publications18,27,56–63 relating to the Phase III ZETA trial,27,56 which compares 300 mg per day of vandetanib with placebo, and two publications64,65 relating to two ongoing vandetanib trials: one trial64 comparing 300 mg per day with 150 mg per day of vandetanib, and one65 comparing vandetanib with vandetanib plus bortezomib (Velcade®, Takeda, Osaka, Japan).
No additional relevant papers or studies were identified from the reference lists of included studies or reviews, or from citation searching of the key publications for the EXAM or ZETA trials. The clinical advisors to the AG were satisfied that no other relevant studies were missing.
The two pivotal Phase III trials, EXAM and ZETA, were international, multicentre, placebo-controlled trials. The characteristics of the EXAM and ZETA trials are presented in Table 2.
| Study | Trial | |
|---|---|---|
| EXAM28 (carbozantinib) | ZETA27 (vandetanib) | |
| Design | International (including Europe), multicentre, Phase III, parallel-group, double-blind RCT | International (including Europe), multicentre, Phase III, parallel-group, double-blind RCT |
| Follow-up | 13.9 months (median); range 3.6–32.5 months | 24 months (median) |
| Populationa |
|
|
| Intervention | 140 mg of cabozantinib (free-base equivalent), taken orally once per day until either intolerable toxicity or disease progression as per mRECIST. Dose holds and up to two dose level reductions (to a minimum dose of 60 mg per day) were allowed | 300 mg of vandetanib taken orally once per day until disease progression |
| Comparator | Placebo | Placebo |
| Outcomes |
|
|
The clinical evidence submitted to NICE by the manufacturers of cabozantinib21 and vandetanib66 included data from six studies. All of these studies were identified by the search for this review, but only four studies satisfied the review eligibility criteria: for cabozantinib, the EXAM trial and ongoing EXAMINER trial,54 and for vandetanib, the ZETA trial and the ongoing NCT01496313 trial. 64 The submissions also included data from a Phase I, non-controlled, single-arm cabozantinib, dose-escalation trial, which included a subset of relevant MTC patients;67,68 a controlled study to assess the addition of an outreach programme to vandetanib treatment;69 and two ‘real-world’, non-controlled, single-arm vandetanib studies. 70–72 All of these studies were identified by the search but were excluded from this review because they did not satisfy the eligibility criteria; either they were single-arm cohort studies without a control group or the intervention evaluated in the trial did not relate to either cabozantinib or vandetanib (see Appendix 2).
The inclusion and exclusion criteria of the two trials were virtually identical, with the exception that the cabozantinib EXAM trial participants were required to have radiographic evidence of progressive disease (PD) at baseline. This was not an eligibility criterion for the vandetanib ZETA trial as the percentage of participants with ‘aggressive and symptomatic disease’ at baseline is reported to be 56% (186/331). 57 The cabozantinib trial had a median follow-up of 13.9 months, compared with 24 months for the vandetanib trial. The two trials had common primary (PFS) and secondary [OS, objective response rate (ORR), RET mutation status, CTN and CEA levels] end points. The cabozantinib trial assessed quality of life using the MD Anderson Symptom Inventory for thyroid cancer (MDASI-Thy), whereas the vandetanib trial also assessed disease control rate, and measured quality of life using the Functional Assessment of Cancer Therapy – General (FACT-G) tool and time to worsening of pain (TWP). It is noteworthy that the MDASI-Thy and TWP were both listed in the protocols but were not reported in the publications of the EXAM trial [only in the clinical study reports (CSRs)], whereas the FACT-G was not listed in any publication of the ZETA trial, but its results were reported in the Sanofi CS. 66
The definitions of PFS used in the trials were similar (i.e. the time from random assignment to the date of disease progression or death) and both trials employed a central committee to confirm investigator assessments. However, the EXAM trial used the modified RECIST (mRECIST) criteria10 and employed a blinded independent review committee, whereas the ZETA trial used the standard RECIST criteria, and it is unclear whether or not the central review was blinded.
The EXAM and ZETA trials had 330 and 331 participants, respectively (Table 3). Both trials randomised patients 2 : 1 to receive the active drug or placebo, respectively. In terms of baseline characteristics, the two arms of the cabozantinib EXAM trial are generally well balanced with the possible exceptions of Eastern Cooperative Oncology Group (ECOG) performance status of 0 (56.2% in the cabozantinib arm vs. 50.5% in the placebo arm), the proportion who had received prior systemic therapy for MTC (37% in the cabozantinib arm vs. 42% in the placebo arm) and positive RET mutation status (46.1% in the cabozantinib arm vs. 52.3% in the placebo arm), indicating that the control group might have had more severe disease. RET mutation status was unknown in 39% of participants as a result of missing sequence data or the presence of a mutation of unknown significance. 28 The two arms of the vandetanib ZETA trial were also generally well balanced, albeit with higher proportions of participants in the control arm than the treatment arm also potentially having more severe disease on account of a WHO performance status of 1–2 (42% for the placebo arm vs. 33% for the vandetanib arm) and having involvement of two or more organs (92% for the placebo arm vs. 87% for the vandetanib arm).
| Participant characteristics | Trial | |||
|---|---|---|---|---|
| EXAM28 (N = 330) | ZETA27 (N = 331) | |||
| Cabozantinib, 140 mg (n = 219) | Placebo (n = 111) | Vandetanib, 300 mg (n = 231) | Placebo (n = 100) | |
| Male, n (%) | 151 (69) | 70 (63) | 134 (58) | 56 (56) |
| Age (years), median (range) | 55 (20–86) | 55 (21–79) | 51a (NR) | 53a (NR) |
| Disease type, n (%) | ||||
| Hereditary | 12 (6) | 8 (7) | 28 (12) | 5 (5) |
| Sporadic or unknown | 207b (95) | 103 (93) | 203 (88) | 95 (95) |
| Locally advanced | NR | 14 (6) | 3 (3) | |
| Metastatic | NR | 217 (94) | 97 (97) | |
| RET mutation status, n (%) | ||||
| Positive | 101 (46) | 58 (52) | 137 (59) | 50 (50) |
| Negative | 31 (14) | 10 (9) | 2 (1) | 6 (6) |
| Unknown | 87 (40) | 43 (39) | 92 (40) | 44 (44) |
| Performance status, n (%) (ECOG/WHO) | ||||
| 0 | 123 (56) | 56 (51) | 154 (67) | 58 (58) |
| 1 or 2 | 95 (43) | 55 (50) | 77 (33) | 42 (42) |
| Number of organs involvedc | ||||
| 0 or 1 | 28 (13) | 15 (14) | 29 (13) | 8 (8) |
| ≥ 2 | 191 (87) | 96 (87) | 202 (87) | 92 (92) |
| Prior systemic therapy for MTC | 81 (37) | 47 (42) | 90 (39) | 42 (42) |
| Prior thyroidectomy | 201 (92) | 104 (94) | NR | NR |
| Prior anticancer therapy | 85 (39) | 48 (43) | NR | NR |
| Prior TKI, n (%) | ||||
| Yes | 44 (20) | 24 (22) | NR | NR |
| No | 171 (78) | 86 (78) | NR | NR |
| Unknown | 4 (2) | 1 (1) | NR | NR |
Comparing the two trials, the vandetanib ZETA trial included substantially greater proportions of participants with hereditary disease (12% in the vandetanib arm vs. 6% in the cabozantinib intervention arm) and participants with a performance status of 0 (67% in the vandetanib arm vs. 56% in the cabozantinib arm). However, the principal difference between the EXAM and ZETA trial populations concerns the presence of PD: participants in the EXAM trial were required to have evidence of PD, whereas participants in the ZETA trial were not. The two ITT populations are therefore sufficiently different to invalidate a standard indirect comparison.
In both trials, participants discontinued study treatment if there was evidence of disease progression or toxicity. The ZETA trial, however, also permitted treatment continuation or treatment switching post progression. 27 During the randomised phase, if there was disease progression based on investigator assessment, participants discontinued study treatment, but were offered the opportunity to receive vandetanib post progression as unblinded open-label treatment until normal discontinuation criteria applied (e.g. toxicity or progression). 27 In the vandetanib arm during the randomised stage of the trial, 120 out of 231 (52%) participants discontinued treatment because of progression or toxicity (compared with 55% in the cabozantinib trial28), but 44 of these 120 (37%) participants continued to receive vandetanib in the open-label phase. In the placebo arm of the ZETA trial, 71 out of 99 (72%) discontinued ‘treatment’ because of progression or toxicity (compared with 86% in the cabozantinib trial), and 58 of these 71 (82%) participants then switched to receive vandetanib in the open-label phase. All efficacy and safety data reported subsequently are subject to bias because of treatment switching, unless otherwise stated. This raises issues of confounding for some of the outcome data from the ZETA trial.
The marketing authorisation for vandetanib states that it is indicated ‘for the treatment of aggressive and symptomatic medullary thyroid cancer (MTC) in patients with unresectable locally advanced or metastatic disease’. 22 The terms ‘aggressive’ and ‘symptomatic’ are not defined in the licence, but were defined post hoc. The Sanofi CS for vandetanib66 presents PFS and OS outcome data from post hoc analyses on two preplanned subpopulations within the ZETA trial (and, as such, are more restrictive than the overall population recruited to this trial):
-
Patients with unresectable, locally advanced or metastatic MTC and whose disease is ‘progressive and symptomatic’ (defined as having ‘documented progression 12 months prior to enrolment and at least one of the following symptoms at baseline: pain score > 4, ≥ 10 mg/day opioid use, diarrhoea, flushing, fatigue, pain, nausea, dysphagia, dysphonia, respiratory symptoms, and weight loss.’57 This corresponds to the ‘EU-label’ or ‘progressive and symptomatic’ population (n = 186) referred to in the Sanofi CS. 66 In the post hoc analyses conducted by the company, the data reported by Kreissl et al. 57 could not be replicated exactly, and the number reported is n = 190 for PFS and n = 189 for OS data in the Sanofi CS (see the Sanofi CS,66 appendix 6, tables 5 and 7, respectively). Numbers from the published Kreissl et al. 57 analyses are used throughout the clinical-effectiveness section, whereas the cost-effectiveness section is based on the slightly larger subgroup defined for the purposes of the NICE submission.
-
Patients with unresectable, locally advanced or metastatic MTC whose disease is ‘progressive and symptomatic’ (as above) and is ‘aggressive’, that is, with CTN and CEA doubling times of < 24 months from screening. This is the so-called ‘restricted EU-label population’ [n = (confidential information has been removed)] presented in the Sanofi CS. The Sanofi CS claims that ‘This population closely reflects UK clinical practice for TKI treatment’ (Sanofi CS,66 pp. 11 and 54). However, clinical advice received by the AG suggests that CTN and CEA monitoring would not usually inform decisions about whether or not to commence TKI therapy, as this is principally determined by radiographic evidence of progression and symptoms.
The data presented for these groups are partly unpublished (only the PFS and ORR data for the EU-label population are published)57 and are reported here because they are used to inform the health economic model developed by the AG. The baseline characteristics of these subgroups are presented in Table 4, together with the comparable baseline data for the EXAM trial ITT population. Despite the EXAM trial ITT population being ‘progressive’ and the EU-label ZETA trial population being ‘progressive and symptomatic’, clinical advice received by the AG confirmed that these two populations were comparable.
| Participant characteristics | Trial | |||||
|---|---|---|---|---|---|---|
| EXAM28 ‘progressive’ (N = 330) | ZETA | |||||
| EU label, ‘progressive and symptomatic’ (N = 186) | Restricted EU label, ‘progressive, symptomatic and with CTN/CEA criteria’ (confidential information has been removed) | |||||
| Intervention | Cabozantinib, 140 mg (n = 219) | Placebo (n = 111) | Vandetanib, 300 mg (n = 126) | Placebo (n = 60) | (Confidential information has been removed) | (Confidential information has been removed) |
| Male (%) | 69 | 69 | 63 | 65 | (Confidential information has been removed) | (Confidential information has been removed) |
| Age (years), median | 55 | 55 | 53.1 | 53.9 | (Confidential information has been removed) | (Confidential information has been removed) |
| Disease type (%) | ||||||
| Hereditary | 6 | 7 | 8.7 | 3.3 | (Confidential information has been removed) | (Confidential information has been removed) |
| Sporadic | 95 | 93 | 50.8 | 46.7 | (Confidential information has been removed) | (Confidential information has been removed) |
| Locally advanced | NR | NR | 5.6 | 1.7 | (Confidential information has been removed) | (Confidential information has been removed) |
| Metastatic | NR | NR | 94.4 | 98.3 | (Confidential information has been removed) | (Confidential information has been removed) |
| RET mutation status (%) | ||||||
| Positive | 46.1 | 52.3 | 59.5 | 50.0 | (Confidential information has been removed) | (Confidential information has been removed) |
| Negative | 13.2 | 9.0 | 0.8 | 10.0 | (Confidential information has been removed) | (Confidential information has been removed) |
| Unknown | 39.7 | 38.7 | 39.7 | 40.0 | (Confidential information has been removed) | (Confidential information has been removed) |
| Prior systemic therapy for MTC | 37 | 42 | 35.7 | 48.3 | (Confidential information has been removed) | (Confidential information has been removed) |
It should also be noted that, among the EU-label population, (confidential information has been removed) of patients in the intervention group continued to receive vandetanib in the open-label phase, whereas (confidential information has been removed) of patients in the placebo arm ‘crossed over’ to receive open-label vandetanib (see Sanofi clarification response,73 question 3). In the restricted EU-label population, (confidential information has been removed) of patients in the intervention group continued to receive vandetanib in the open-label phase, whereas (confidential information has been removed) of patients in the placebo arm ‘crossed over’ to receive open-label vandetanib (Sanofi CS,66 pp. 17 and 63). All efficacy and safety data reported subsequently for this group are subject to bias because of treatment switching, unless otherwise stated. This raises issues of confounding for some of the trial data, including for the restricted EU-label population.
The risk of bias in the EXAM and ZETA trials was assessed using the Cochrane Risk of Bias Tool (Table 5). These assessments made use of the protocols (published and unpublished), the trial publications and unpublished CSRs for each trial.
| Risk of bias | Criteria | Trial | |
|---|---|---|---|
| EXAM (cabozantinib)28 | ZETA (vandetanib)27 | ||
| Selection bias | Random-sequence generation and allocation concealment | Unclear
|
Unclear
|
| Performance bias | Blinding of participants and personnel | Low
|
Moderate to high
|
| Detection bias | Blinding of outcome assessment | Low
|
Moderate
|
| Attrition bias | Incomplete outcome data | Low
|
Low
|
| Reporting bias | Selective reporting | Moderate
|
Moderate
|
| Other bias | Any important concerns about bias not addressed above | Moderate
|
Moderate
|
The AG considers the EXAM trial to be of generally good quality, being assessed as having a low risk of performance, detection and attrition bias on account of measures to ensure blinding and the management of dropouts. The EXAM trial is at unclear risk of selection bias because full details of the randomisation and allocation concealment processes were absent from the documents identified from the searches, or from those made available during this appraisal. It was at moderate risk of reporting bias on account of the failure to report the results of some outcomes in published documents, and at moderate risk of other bias owing to potential conflicts of interest and the failure to control for the possible treatment effect modifier of CTN and CEA doubling times.
Overall, the AG considers that the ZETA trial was at moderate to high risk of bias across most domains. As with the EXAM trial, the likelihood of attrition bias was considered to be low and the risk of selection bias was unclear. However, there was a moderate risk of reporting and other bias because of the presence of selective reporting and some potential conflicts of interest, although post hoc analyses were conducted on the potential treatment effect modifier of CTN and CEA doubling times. In contrast to the EXAM trial, performance bias and detection bias were assessed as being of moderate to high risk because there was a lack of detail on blinding procedures and certain outcomes, and their results were potentially confounded by the inclusion of patients switching to open-label treatment within the analysis.
Assessment of effectiveness
In the EXAM trial, at the data cut-off point (15 June 2011), the median duration of follow-up was 13.9 months. At this time point, 98 out of 219 (45%) participants in the cabozantinib arm were still receiving blinded study treatment, whereas only 15 out of 111 (14%) participants in the placebo arm were still receiving blinded study treatment. 28 In the ZETA trial, at the data cut-off point (July 2009), the median duration of follow-up was 24 months. At this time point, 111 out of 231 (48%) participants in the vandetanib arm were still receiving blinded study treatment, whereas only 28 out of 100 (28%) participants in the placebo arm were still receiving blinded study treatment. 27
Progression-free survival
Both pivotal trials reported PFS as their primary outcome using similar definitions and based on tumour measurements performed at screening and every 12 weeks. Both treatments resulted in a significantly reduced risk of progression. For cabozantinib, the hazard ratio (HR) for PFS was reported to be 0.28 [95% confidence interval (CI) 0.19 to 0.40; p < 0.001] by central review and 0.29 (95% CI 0.21 to 0.42; p < 0.001) by investigator read28,43 (Table 6). For vandetanib, the HR for PFS was reported to be 0.46 (95% CI 0.31 to 0.69; p < 0.001) by central review of all patients (ITT population), 0.28 (95% CI 0.18 to 0.42; p < 0.001) by central review excluding open-label patients, and (confidential information has been removed).
| Assessed by | Trial arm | HR (95% CI; p-value) | |
|---|---|---|---|
| Cabozantinib (n = 219) | Placebo (n = 111) | ||
| EXAM trial, (n = 330)28 | |||
| Central review | 11.2 | 4.0 | 0.28 (0.19 to 0.40; < 0.001) |
| Investigator | 13.8 | 3.1 | 0.29 (0.21 to 0.42; < 0.001) |
| Vandetanib (n = 231) | Placebo (n = 100) | ||
| ZETA trial ITT population (n = 331)27 | |||
| Central review (ITT population)a | 30.5 | 19.3b | 0.46 (0.31 to 0.69; < 0.001) |
| Central review (excluding open-label)a | 32.4 | 16.4b | b0.28 (0.18 to 0.42; < 0.001c) |
| Investigator (all patients, ITT population) | 22.3 | 8.3b | 0.40 (0.27 to 0.58; < 0.001) |
| Vandetanib (n = 126) | Placebo (n = 60) | ||
| ZETA trial EU-label population (n = 186)57,66 | |||
| Central review (all patients)a,b | 28.0 | 16.4 | 0.47 (0.29 to 0.77; 0.0024) |
| Central review (excluding open-label)a,d | 30.1 | 11.1 | 0.32 (0.19 to 0.54; < 0.0001) |
| Investigatord | 22.1 | 8.3 | 0.33 (0.2 to 0.53;e 0.0226) |
| Vandetanib (confidential information has been removed) | Placebo (confidential information has been removed) | ||
| ZETA trial restricted EU-label population (confidential information has been removed) | |||
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
In the post hoc analysis, PFS was also calculated for the EU-label (n = 186) and restricted EU-label (confidential information has been removed) populations. For the vandetanib EU-label population, the HR for PFS was reported to be 0.47 (95% CI 0.29 to 0.77; p = 0.0024) by central review66 and 0.33 (95% CI 0.20 to 0.53; p = 0.0226) by investigator read. 57 The HR by central review but excluding open-label patients57 was reported to be 0.32 (95% CI 0.19 to 0.54; p < 0.001). According to the Sanofi CS (p. 55),66 the median PFS for the restricted EU-label group was (confidential information has been removed) in the placebo arm compared with (confidential information has been removed) in the vandetanib arm (confidential information has been removed).
The investigator-read risk of progression, compared with placebo, for the comparable EXAM trial (n = 331) and ZETA trial EU-label (n = 186) populations was HR 0.29 (95% CI 0.21 to 0.42; p < 0.001) for cabozantinib and HR 0.33 (95% CI 0.2 to 0.53; p = 0.0226) for vandetanib, respectively.
The proportion of randomised patients progressing was similar in the treatment and placebo groups across the two trials. The EXAM trial publication (i.e. Elisei et al. )28 states that 57 out of 219 (26%) participants randomised to cabozantinib had progressed at follow-up compared with 67 out of 111 (60%) participants in the placebo group. The ZETA trial publication27 reported data on 124 participants who progressed: 73 out of 231 (32%) participants randomised to vandetanib had progressed (previously reported as 37% at 24 months58) and 51 out of 100 (51%) participants randomised to placebo had progressed.
In the EXAM trial, the Kaplan–Meier estimates for the proportion of participants alive and progression free at 1 year were reported to be 47.3% for cabozantinib compared with 7.2% for placebo. 28 In the ZETA trial, the proportion of participants in the ITT population alive and progression free at 6 months was reported to be 91% for vandetanib compared with 74% for placebo. 59
Subgroup analyses according to prespecified subgroups were conducted for PFS for both cabozantinib and vandetanib. For both interventions, all subgroups demonstrated a beneficial effect with treatment (HR < 1.0), although 95% CIs indicated non-statistically significant treatment effects for some small subgroups, as may be expected. Subgroups considered included sex, performance status, and number of previous anticancer regimens or other TKIs received and response to those therapies. 27,28,43,44,77 The Ipsen CS21 for cabozantinib reported that PFS was also prolonged in a subgroup of cabozantinib patients (n = 34) who had received prior vandetanib (median PFS was 12.8 months for cabozantinib and 2.8 months for placebo, and ORR was 28%, when prior vandetanib use reported). PFS for cabozantinib was also consistent across subgroups according to age and the presence of bone metastases28 and PFS for vandetanib was not sensitive to ethnicity. 27
Subgroup analyses based on RET mutation status (as specified in the final NICE scope26) were also conducted for the EXAM trial. Details of the number of participants in each of these groups within the EXAM trial are presented in Tables 7 and 8. The data demonstrate that cabozantinib was associated with a beneficial effect compared with placebo for all subgroups tested (see Tables 7 and 8) although the treatment effect was not statistically significant at the 95% level (p = 0.21) for the RET-negative subgroup, and PFS improvement was least pronounced in the small subset of RET mutation-negative participants who were also RAt Sarcoma (RAS) mutation negative. 45,46
| Mutation status | Patients, n (%) | ||
|---|---|---|---|
| Total (N = 330) | Cabozantinib arm (N = 219) | Placebo arm (N = 111) | |
| RET mutation subgroup | |||
| Positive | NR (51.2) | 46.1 (48.9) | 52.3 (55.9) |
| Negative | NR (13.9) | 14.2 (16.0) | 9.0 (9.9) |
| Unknown | NR (34.8) | 39.7 (35.2) | 38.7 (34.2) |
| RET M918T status | |||
| Positive | NR (38.2) | 34.2 (37.0) | 38.7 (40.5) |
| Negative | NR (32.4) | 30.6 (34.2) | 27.0 (28.8) |
| Unknown | NR (29.4) | 35.2 (28.8) | 34.2 (30.6) |
| Mutation status | Trial arm | HR (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Cabozantinib | Placebo | |||||
| n | Median PFS (weeks) | n | Median PFS (weeks) | |||
| RET positive | 107 | 60 | 62 | 20 | 0.23 (0.14 to 0.38) | < 0.0001 |
| RET negative | 35 | 25 | 11 | 23 | 0.53 (0.19 to 1.50) | 0.2142 |
| RET unknown | 77 | 48 | 38 | 13 | 0.30 (0.16 to 0.57) | 0.0001 |
| RET M918T positive | 81 | 61 | 45 | 17 | 0.15 (0.08 to 0.28) | < 0.0001 |
| RAS positive | 13 | 47 | 3 | 8 | 0.15 (0.02 to 1.10) | 0.0317 |
| RET negative and RAS negative | 22 | 24 | 8 | 23 | 0.88 (0.24 to 3.22) | 0.8330 |
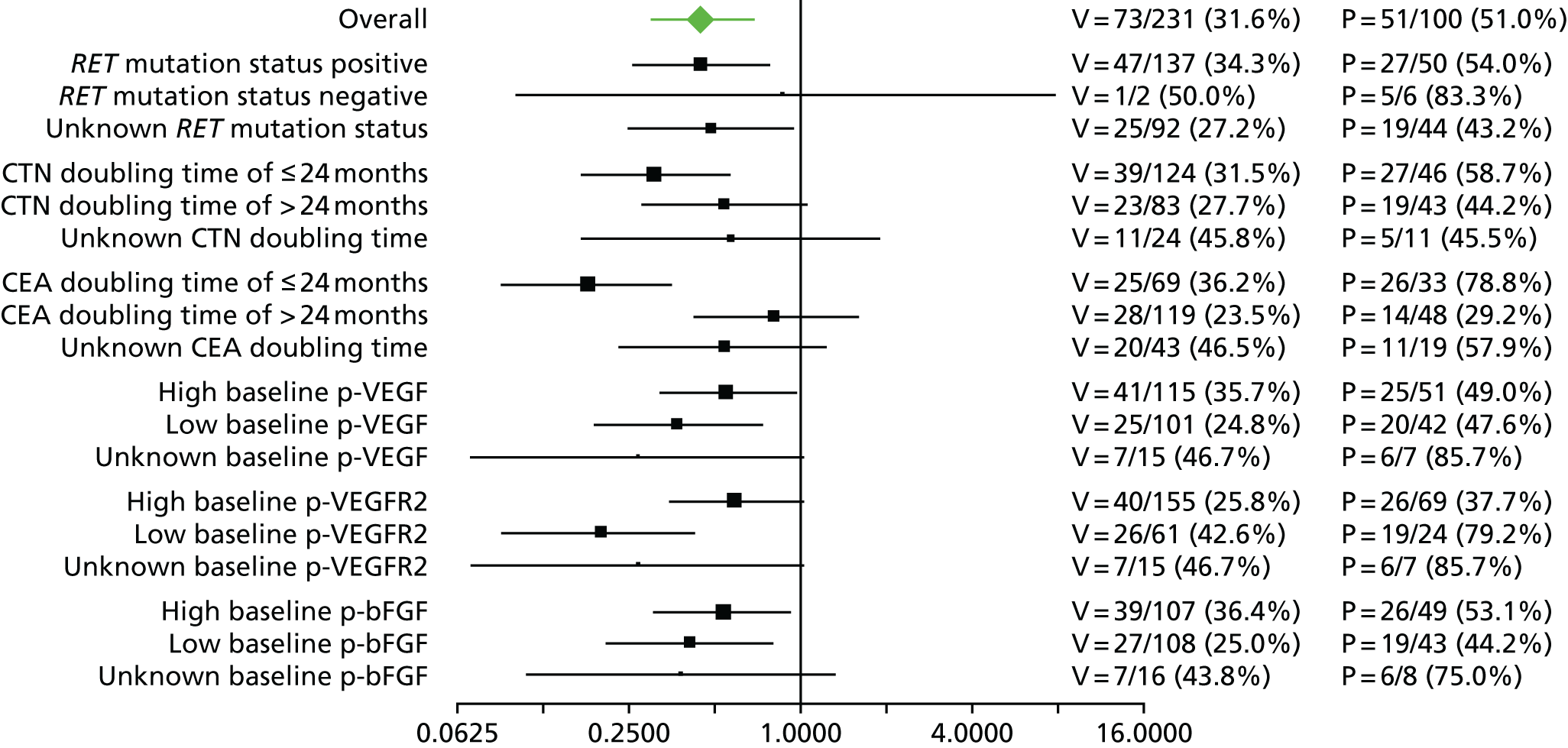
With respect to vandetanib, the Sanofi CS states that, ‘subgroups relating to two different definitions for “aggressive disease” were included in a pre-specified subgroup analysis: calcitonin (CTN) doubling time (DT) ≤ 24 months and CEA DT ≤ 24 months’ (Sanofi CS,66 section 4.3, p. 45). Subgroup analyses by these criteria were reported in this CS66 and the unpublished CSR. 74 These found that all subgroups demonstrated a beneficial effect on PFS (HR < 1.0), with a statistically significant treatment effect observed between patients with a CTN doubling time of ≤ 24 months and patients with a CEA doubling time of ≤ 24 months (Figure 3).
Overall survival
The authors of the EXAM trial paper28 reported that there was no statistically significant difference between cabozantinib and placebo based on an interim analysis. According to a 2015 abstract,47 the EXAM trial was designed with 80% power to detect a HR of 0.667 for the secondary end point of OS. A final analysis was conducted after 218 deaths (the trial required 217 deaths for the analysis28) at a median follow-up of 52.4 months. 47 The estimated median OS was 26.6 months for cabozantinib compared with 21.1 months for placebo (stratified HR 0.85, 95% CI 0.64 to 1.12), which was not statistically significantly different (p = 0.241; Table 9). 47
| Treatment | Placebo | HR (95% CI; p-value) |
|---|---|---|
| EXAM trial arm (N = 330)47 | ||
| Cabozantinib (n = 219) | Placebo (n = 111) | |
| 26.6 | 21.1 | 0.85 (0.64 to 1.12; 0.2409) |
| ZETA ITT population (N = 331)27 | ||
| Vandetanib (n = 231) | Placebo (n = 100) | |
| NR | NR | 0.99 (0.72 to 1.38; 0.9750) |
| aEU-label population (N = 189)78 | ||
| Vandetanib (n = 126) | Placebo (n = 60) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Restricted EU-label population (confidential information has been removed)a | ||
| Vandetanib (confidential information has been removed) | Placebo (confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
For the 215 (65%) participants with known positive or negative RET mutations in the EXAM trial,45 median OS was 31.6 months in the cabozantinib arm compared with 24.8 months in the placebo arm (HR 0.79, 95% CI 0.54 to 1.17; p = 0.240). 79 For the 126 participants with known RET M918T-positive mutations, median OS was 44.3 months for cabozantinib compared with 18.9 months for placebo (HR 0.60, 95% CI 0.38 to 0.94; p = 0.026). 47,79 Subgroups of participants lacking RET mutations or lacking RET M918T showed no increase in OS. 47,79 The secondary end point of improved OS was not met because the difference between arms was not statistically significant in the ITT population. 47
The data on OS from the ZETA trial were immature: a non-significant interim result was reported (HR 0.89, 95% CI 0.48 to 1.65; p-value not reported),27 as well as the intention to conduct a final analysis when 50% of participants had died. The number of participants who had died at the data cut-off point (31 July 2009) was reported in the published CSR:27 32 out of 231 (14%) participants in the vandetanib arm compared with 16 out of 100 (16%) participants in the placebo arm (p = 0.711527; and Sanofi CS,66 p. 49). In the final analysis set (data cut-off point of 7 September 2015), there remained no survival benefit: 50% of participants randomised to vandetanib had died compared with 52% of participants randomised to placebo (HR 0.99, 95% CI 0.72 to 1.38; p = 0.975), although the placebo group included participants who had crossed over to vandetanib in the unblinded stage of the trial, thereby potentially confounding these results (Sanofi CS,66 p. 49).
For the ZETA trial’s EU-label population, the estimated median OS was (confidential information has been removed) for vandetanib compared with (confidential information has been removed) for placebo (confidential information has been removed).
According to the Sanofi CS66 (p. 55 and table 20), the median OS for the restricted EU-label group was (confidential information has been removed) in the placebo arm compared with (confidential information has been removed) in the vandetanib arm (confidential information has been removed).
Response rate
The end point of ORR was reported in both trials, including complete and partial response, and was determined using the stated RECIST criteria27,28 In the EXAM trial (n = 312 for this outcome), no participant had a complete response. Twenty-eight per cent of participants had a partial response in the cabozantinib arm compared with 0% in the placebo arm (p < 0.001), with a median estimated duration of response of 14.6 months (95% CI 11.1 to 17.5 months)28 and similar rates for RET mutation-positive and -negative subgroups. 43,44
In the full publication of the ZETA trial27 (n = 331 for this outcome), the ORR was 45% in the vandetanib group compared with 13% in the placebo group (p < 0.001), with a predicted median duration of response of 22 months. Within an earlier abstract,60 the odds ratio (OR) was reported to be 5.4 compared with placebo (95% CI 2.99 to 10.79; p < 0.0001). It should be noted that 12 out of 13 participants in the placebo group had a response only when they switched to vandetanib in the open-label phase of the trial. 27,58 The OR was reported to be 45.7% (p < 0.0001) compared with placebo for the EU-label patients (n = 186) in the ZETA trial before any switching occurred. 57 The Sanofi CS66 (table 24, p. 67) states that 43.7% of these participants had a response in this vandetanib group (n = 126), compared with (confidential information has been removed) in the restricted EU-label vandetanib group (confidential information has been removed). Small numbers of RET-negative participants were deemed to render findings from the subgroup analysis of the EU-label group inconclusive, although other analyses did suggest that M918T mutation-positive participants had a better response to vandetanib than M918T mutation-negative patients. 27 The Sanofi CS66 (p. 51) also stated that higher proportions of participants with a CTN or CEA doubling time of < 24 months (47% and 54%, respectively) achieved ORR than participants with a CTN or CEA doubling time of ≥ 24 months (40% and 37%, respectively).
Calcitonin and carcinoembryonic antigen response
Serum levels of CTN and CEA are recognised indicators of tumour burden and prognosis. 15–17 In both the EXAM and ZETA trials, CTN and CEA were evaluated from serum samples at baseline and, at the most, every 12 weeks after initiation of treatment, to coincide with radiological tumour assessments; response was calculated as a percentage change compared with baseline. 27,28 In the EXAM trial, the cabozantinib and placebo groups did not have statistically significantly different baseline levels of CTN or CEA, but at 12 weeks’ follow-up, evaluated participants in the cabozantinib group had statistically significantly better responses than those in the placebo group: levels of both biomarkers decreased in the treatment group and increased in the placebo group (Table 10). 28,48,49
| Time point | Biomarkers | Trial arm, mean (SD) | p-value | |
|---|---|---|---|---|
| Cabozantinib | Placebo | |||
| Baseline | CTN (n = 330), pmol/l | 6370 (11,332) | 8846 (15,722) | 0.27a |
| CEA (n = 330), µg/l | 736 (3555) | 1108 (5168) | 0.58a | |
| Percentage change, mean (SD) | ||||
| Week 12 | CTN (n = 201) | –45.2 (60.71) | 57.3 (115.4) | < 0.001 |
| CEA (n = 241) | –23.7 (58.21) | 88.7 (182) | < 0.001 | |
In the ZETA trial, higher, statistically significant percentages of participants receiving vandetanib achieved a CTN and CEA response (69% and 52%, respectively) than participants receiving placebo (3% for CTN and 2% for CEA). 27,66
Lesion size
Lesion size was only measured and reported within the EXAM trial. To be included, participants needed measurable disease at baseline and at least one subsequent assessment. 28 A total of 180 out of 219 cabozantinib participants and 89 out of 111 placebo participants satisfied these criteria. Ninety-four per cent of these cabozantinib participants and 27% of these placebo participants had a detectable decrease in target lesion size. 28 Elisei et al. 28 also noted that there was a ‘generally linear relationship’ in the reductions in lesion size and both CTN and CEA levels.
MD Anderson Symptom Inventory – Thyroid
The MDASI-Thy module was the only patient-reported outcome measure used in the EXAM trial and data on this outcome were reported only in the unpublished CSR. 76 Data were also provided by the company at the request of the AG. The analysis was exploratory and was evaluated at screening and every 12 weeks (±5 days) to disease progression, coinciding with tumour assessments. The tool measured clinical symptoms, such as pain, fatigue, nausea, diarrhoea and mood, with higher scores indicating more symptoms. The CSR reported (section 11.4.4.2) that, although no formal statistical testing had been performed, in terms of change from baseline to the data cut-off point, there was no apparent difference between the treatment arms. However, it was stated that there were data for only 75% of participants at week 12, with declining numbers for subsequent assessments. 76
Functional Assessment of Cancer Therapy – General, and time to worsening of pain
The FACT-G and TWP outcomes were only measured and reported for the ZETA trial; the details and results appear in the published and unpublished CSR,27,74 although data were also provided by Sanofi at the request of the AG. The CSR74 states that quality of life was measured using the FACT-G instrument and that, overall, scores between the two arms were similar. TWP was a composite end point, derived from opioid analgesic use and the worst pain item of the Brief Pain Inventory. The ZETA trial reported a significantly longer median TWP for vandetanib (7.85 months) than placebo (3.25 months: HR 0.61, 95% CI 0.43 to 0.87; p = 0.0062) in the published CSR. 27 In the EU-label population, TWP was 11.1 months in the vandetanib arm compared with 3.4 months in the placebo arm (HR 0.62; 95% CI 0.39 to 0.99; p = 0.45). 66
Safety outcomes
In order to be considered for safety outcomes, participants had to receive at least one dose of the study drug. 27,28
Any adverse event
The EXAM trial safety data were taken from the trial publications or the EXAM Final Analysis Set of August 2014, which was provided in the Ipsen CS21 for cabozantinib (median follow-up of 10.8 months). The ZETA trial safety data were taken from the final Safety Analysis Set, provided in the Sanofi CS for vandetanib66 and the unpublished CSR of 201174 (median total exposure was 90.1 weeks for vandetanib compared with 39.9 weeks for placebo). Seven participants were missing from the EXAM safety population data; therefore, there were 214 participants for cabozantinib, rather than 219, in the ITT population, and 109 participants for placebo rather than 111.
Adverse events were very common in both trials. Overall, 100% of participants were affected by at least one AE in the cabozantinib arm of the EXAM trial, and 99.6% of participants were affected by at least one AE in the vandetanib arm of the ZETA trial, 96% of which were attributed to vandetanib by the investigator. 27 Both trials reported many AEs affecting ≥ 10% and < 20% of participants. Some of these AEs were dry skin, insomnia, abdominal pain, dermatitis acneiform, cough, nasopharyngitis, prolonged ECG QT [as defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE80)], alopecia, pain in extremity, dyspnoea, arthralgia, dizziness, oral pain, dry mouth, dysphagia, cough, muscle spasms, dyspepsia, erythema and glossodynia. 27,28
Given their high frequency, only the most common AEs, that is, those affecting ≥ 20% of participants in any trial arm, are presented in Table 11. The most common AEs for cabozantinib were diarrhoea (63%), hand–foot syndrome (HFS) (50%), decreased weight (48%), decreased appetite (46%), nausea (43%) and fatigue (41%). 28
| AE | Trial (% with event) | |||
|---|---|---|---|---|
| EXAM | ZETA | |||
| 10.8 months’ follow-up (median)a | 90.1 weeks’ follow-upb | 39.9 weeks’ follow-upb | ||
| Cabozantinib (n = 214) | Placebo (n = 109) | Vandetanib (n = 231) | Placebo (n = 99) | |
| Overall | 100a | 95a | 9727 | 9127 |
| Diarrhoea | 63 | 33 | 56 | 26 |
| HFS | 50 | 2 | – | – |
| Decreased weight | 48 | 10 | 10 | 9 |
| Decreased appetite | 46 | 16 | 21 | 12 |
| Nausea | 43 | 21 | 33 | 16 |
| Fatigue | 41 | 28 | 24 | 23 |
| Dysgeusia | 34 | 6 | – | – |
| Hair colour changes | 34 | 1 | – | – |
| Hypertension | 33 | 5 | 32 | 5 |
| Stomatitis | 29 | 3 | – | – |
| Constipation | 27 | 6 | – | – |
| Haemorrhage | 25 | 16 | – | – |
| Vomiting | 24 | 2 | 14 | 7 |
| Mucosal inflammation | 23 | 4 | – | – |
| Asthenia | 21 | 15 | 14 | 11 |
| Dysphonia | 20 | 9 | – | – |
| Rash | 19 | 10 | 45 | 11 |
| Headache | 18 | 8 | 26 | 9 |
| Acne | – | – | 20 | 5 |
| Back pain | 15 | 11 | 9 | 20 |
Similarly, the most common AEs for vandetanib were diarrhoea (56%), decreased appetite (21%), nausea (33%) and fatigue (24%). In addition, there was a high incidence of rash (45%), hypertension (32%) and headache (26%), but low or no incidence of HFS. 27,58 Hypertension is a known AE for TKIs. 81,82 The incidence of diarrhoea in patients receiving vandetanib treatment for MTC appears to be similar to that reported for patients receiving vandetanib treatment for other cancers,83 but the rates of any grade or high-grade severity rash and hypertension appear to be higher for vandetanib in MTC patients than in most other cancer patients,84,85 which might be attributable to longer treatment duration. 85
It should be noted that patients with MTC have a substantial disease burden. This is demonstrated by the AEs and comorbidities in the placebo arm and baseline data for EXAM and ZETA trial participants (see Table 11), and especially those in the EXAM trial, with radiographic evidence of PD (n = 330); for example, percentages of participants with reported symptoms at baseline were pain in 46.1%, diarrhoea in 39.7%, fatigue in 25.8% and dysphonia in 23%. 50 Most symptoms were of grade 1 or 2 severity.
Grade 3 or higher adverse events, and serious adverse events
The adverse events, grade 3 or higher, reported for ≥ 2% of participants are presented in Table 12. The most common grade 3 or higher AEs for cabozantinib were diarrhoea (16%), HFS (13%), fatigue (9%), hypertension (8%), asthenia (6%), and decreased weight (5%) and appetite (5%). 28,43 These appear to be consistent with other anti-VEGF TKIs and the open-label cabozantinib studies. 86–89 However, it should be noted that the incidence and severity of HFS reported in the EXAM trial are lower than those reported in other cabozantinib trials for the treatment of other solid malignancies. 90
| Adverse event | Trial (% with event) | |||
|---|---|---|---|---|
| EXAM | ZETA | |||
| 10.8 months’ follow-up (median)a | 90.1 weeks’ follow-upb | 39.9 weeks’ follow-upb | ||
| Cabozantinib (n = 214) | Placebo (n = 109) | Vandetanib (n = 231) | Placebo (n = 99) | |
| Overall | 69 (78a) | 33 | 55 (CSR, Langmuir and Yver19); 61 (Kreissl et al.57) | 24 (CSR and Kreissl et al.57) |
| Diarrhoea | 16 | 2 | 11 | 2 |
| HFS | 13 | 0 | – | – |
| Fatigue | 9 | 3 | 6 | 1 |
| Hypertension | 8 | 1 | 9 | 0 |
| Asthenia | 6 | 2 | 3 | 1 |
| Decreased weight | 5 | 0 | – | – |
| Decreased appetite | 5 | 1 | 4 | 0 |
| Dysphagia | 4 | 1 | – | – |
| Abdominal pain | 3 | 1 | – | – |
| Haemorrhage | 3 | 1 | – | – |
| Dyspnoea | 2 | 10 | 1 | 3 |
| Back pain | 2 | 1 | 0 | 3 |
| Mucosal inflammation | 3 | 0 | – | – |
| Vomiting | 2 | 1 | – | – |
| Rash | 1 | 0 | 4 | 1 |
| Headache | 1 | 0 | – | – |
| Syncope | – | – | 0 | 2 |
| Prolonged ECG QT | – | – | 8 | 1 |
The most common grade 3 or higher AEs for vandetanib were diarrhoea (11%), hypertension (9%), fatigue (6%) and decreased appetite (4%) as well as rash (4%) and prolonged ECG QT (8%). An exploratory study of a subset of the ZETA trial participants has indicated potential benefits of vandetanib in terms of weight and muscle loss. 61–63 This study also identified significant toxicities in the presence of higher mean vandetanib plasma concentration, the most frequent toxicities being asthenia grade 3 (36%), prolongation of the corrected QT interval (QTc) (25%), and cutaneous symptoms (11%). 62 Vandetanib is one of only two TKIs (the other being sunitinib) identified as being associated with prolonged QTc. 91
Serious adverse events (SAEs), as defined by the National Cancer Institute’s CTCAE,80 affected more participants receiving cabozantinib (42.1%28 or 53%,21 depending on the source) than placebo (22.9%28 or 24%,21 depending on the source) in the EXAM trial. 21,28 The overall incidence of any SAE in the ZETA trial was 31% in the vandetanib arm and 13% in the placebo arm. 27 SAEs that occurred in ≥ 2% of participants in any arm of the EXAM or ZETA trials were mucosal inflammation, hypocalcaemia, pulmonary embolism, hypertension and diarrhoea.
Grade 5 AEs occurring within 30 days of the last dose were reported in more cabozantinib participants than placebo participants (7.9% and 7.3%, respectively). 28 A number of these grade 5 AEs were specified as being related to cabozantinib: fistula, respiratory failure, haemorrhage, sepsis/multiorgan failure, sudden death, cardiopulmonary failure and ‘death, not other specified.’ At 52.4 months’ follow-up, the most common SAEs (≥ 2%) were pneumonia (4.2% of those receiving cabozantinib experienced this event), pulmonary embolism (3.3%), mucosal inflammation (2.8%), hypocalcaemia (2.8%), and hypertension, dysphagia, dehydration and lung abscess (2.3% each). 92
Adverse events leading to discontinuation or dose interruption/reduction
Adverse events leading to dose reductions/interruptions and/or discontinuation of treatment were reported for both trials (Table 13). There were similar proportions of participants across the two trials who discontinued because of AEs (16% or 23% for cabozantinib and 12% for vandetanib); however, there was a higher percentage of participants experiencing AEs, leading to dose interruption or reduction on cabozantinib (65%) than on vandetanib (35%). 27,28 A later abstract detailing this outcome for the EXAM trial reported that dose reduction to manage AEs was performed for 82% of participants treated with cabozantinib,55 which increased again to 87% in the final analysis. 21 The percentages of participants experiencing AEs leading to dose interruption (17%) or discontinuation (8%) were also higher in the placebo arm of the cabozantinib trial28 than in the placebo arm of the vandetanib trial (3% for dose interruption and 3% for discontinuation). High rates of dose reduction and discontinuation have also been reported for a retrospective study of 15 patients with progressive MTC on cabozantinib. 77
| Trial | Trial arm (%) | |
|---|---|---|
| EXAM | Cabozantinib (n = 214) | Placebo (n = 109) |
| Dose interruption because of AE28 | 65 | 17 |
| Discontinuation because of AE28 | 16 (23a) | 8 (9a) |
| Dose interruption or reduction | 87 | 22 |
| Dose reductiona | 79s | 9 |
| ZETA | Vandetanib (n = 231) | Placebo (n = 99) |
| Dose interruptionb | 47 | 15 |
| Discontinuation because of AEs27 | 12 | 3 |
| Dose interruption or reduction | 49 | 15 |
| Dose reduction27 | 35 | 3 |
| EU-label population only (Sanofi CS, table 33)b | Vandetanib (n = 126) | Placebo (n = 60) |
| Discontinuation because of AEs | 12 | 2 |
| Dose reduction | 33 | 3 |
Deaths
In the EXAM trial, at the data cut-off point, 30% of participants (65/214) had died in the cabozantinib arm compared with 28% (30/109) in the placebo arm. Twenty-three per cent (15/65) of deaths in the cabozantinib arm were attributable to AEs, compared with 20% (6/30) in the placebo arm;28 other deaths were attributable to disease progression. Full details of the AEs leading to death were not reported. 28 By the final analysis (August 2014), the figures had increased to 65% (138/214) in the cabozantinib arm and 70% (76/109) in the placebo arm, with deaths deemed to be treatment related remaining at 4–5% for cabozantinib and 1% for placebo at both the interim analysis and the final analysis. 21
During the randomised phase of the ZETA trial, five participants who received vandetanib experienced AEs that led to death. Reasons given were aspiration pneumonia, respiratory arrest, respiratory failure, staphylococcal sepsis, and, in one participant, arrhythmia and acute cardiac failure. Instances of gastroenteritis and gastrointestinal haemorrhage led to death in two participants in the placebo group. 27 The number of deaths reported at the safety follow-up was 10 (4.3%) in the vandetanib group and 6 (6.1%) in the placebo group, although two of the deaths in the vandetanib group did not have MTC as either the primary or secondary cause; no such deaths were recorded in the placebo group. 74
Supplementary safety evidence
The Sanofi CS66 also presented safety data from two additional published studies69,71 and one ongoing study;64 the data from this third, ongoing study are unpublished. The findings on the most frequent AEs and SAEs, and the incidence and type of AEs, were all similar to the ZETA trial for the 300-mg vandetanib dose. Dose interruption and reduction rates were also similar, except for higher rates in a trial arm that included additional monitoring through an outreach programme. 69 Only the ‘real world’ study of 68 MTC patients treated with vandetanib in France71 had a markedly higher incidence of death (42% compared with ≤ 12% in the other studies for the 300-mg vandetanib dose) and AE-related discontinuations (27% compared with ≤ 15%) than the other study69 or the ZETA trial. These trials had a similar or shorter duration of follow-up than the ZETA trial, but were not subject to potential confounding because of treatment switching.
Network meta-analysis
Justification for conducting a network meta-analysis
In the absence of head-to-head evidence comparing cabozantinib with vandetanib, an indirect comparison using a NMA was considered. An indirect comparison has previously been published as an abstract93 and is presented in the Ipsen CS;21 however, owing to the differences between the ITT population of the EXAM and ZETA trials, this analysis was not deemed appropriate for formal consideration within this assessment. The validity of the NMA depends on the assumption that there is no difference in the distribution of trial-level treatment effect modifiers between the populations in the two trials. This is unlikely to be the case for the ITT populations of the ZETA and EXAM trials in particular, because participants in the EXAM trial had confirmed disease progression, whereas the ZETA trial recruited a broader population of participants with no requirement for established disease progression. HRs for the effectiveness of vandetanib compared with placebo for investigator-assessed PFS in the ZETA trial were reported for the symptomatic and progressive subgroup (n = 186, HR 0.33, 95% CI 0.20 to 0.53) and the full analysis set excluding symptomatic and progressive participants (n = 139, HR 0.49, 95% CI 0.27 to 0.58) within the Sanofi CS. 66 This suggests that progression may be a treatment-effect modifier, with a greater treatment effect observed for the subgroup with confirmed progression (though a statistically significant difference between the two groups cannot be inferred).
Despite differences in the ITT populations, the AG considered a NMA based on the EU-label subgroup of the ZETA population to be appropriate. There was a marked difference in the median PFS in the control groups of the two studies [EXAM trial: 4.0 months, ZETA trial EU-label population: 16.4 months (by central review)]; however, differences in baseline characteristics of the included studies as a result of differences in study protocols are to be expected and do not invalidate an indirect comparison. For a NMA to be valid, it is important that there is not an imbalance in treatment-effect modifiers. Clinical advisors to the AG identified severity of disease as an important potential treatment-effect modifier. Information on ECOG/WHO performance status at baseline was not available for the ZETA trial EU-label population, so balance across the two studies could not be assessed, and there is no clear evidence to demonstrate the balance of this potential treatment-effect modifier. However, subgroup analyses indicated consistent treatment effects according to performance status at baseline for both interventions,27,28 and clinical advice received by the AG suggested that the ZETA trial EU-label and EXAM trial ITT populations could be considered to be broadly comparable. Therefore, on the basis of clinical advice, and since there was no evidence to invalidate indirect comparison, a NMA was considered to be justified.
Methods for the network meta-analysis
The NMA was conducted by the AG to provide an indirect comparison between cabozantinib and vandetanib for central-read PFS and investigator-read PFS. For OS, the HRs for both treatment groups are confounded by treatment switching; therefore, a NMA was not conducted for this outcome, as it would not provide a meaningful comparison.
The network diagram is presented in Figure 4 and data contributing to the NMA are presented in Table 14. Analyses were conducted using a Bayesian random-effects model, as described by Dias et al. 94 Given that there is potential heterogeneity between the trials, a random effects model was considered to be the most appropriate so that this uncertainty is appropriately reflected in the estimated treatment effects. There was insufficient information to estimate the between-study variance from the data alone, hence a weakly informative prior was used for this parameter (log-normal –2.56, 1.742 based on the recommendation in Turner et al. 95), which has a median of 0.08 and 95% range of 0.003 to 2.34 on the untransformed scale. This prior was also truncated such that the ratio of the upper and lower 95% CI of the prior does not exceed 10, based on evidence from Speigelhalter et al. 96 and Smith et al. 97 that the between-study treatment effects are unlikely to vary by more than an order of magnitude.
FIGURE 4.
Network diagram for the NMA.
| Trial | Treatment | Comparator | PFS HR (95% CI) | |
|---|---|---|---|---|
| Investigator read | Central read | |||
| EXAM (n = 330), Elisei et al.28 | Cabozantinib | Placebo | 0.29 (0.21 to 0.42) | 0.28 (0.19 to 0.40) |
| ZETA EU label (n = 186), Kreissl et al.57 | Vandetanib | Placebo | 0.33 (0.20 to 0.53) | 0.47 (0.29 to 0.77) |
Analyses were conducted in the freely available software packages WinBUGS98 (MRC Biostatistics Unit, Cambridge, UK) and R (The R Foundation for Statistical Computing, Vienna, Austria) using the R2WinBUGS interface package. Convergence to the target posterior distributions was assessed using the Gelman–Rubin statistic, as modified by Brooks and Gelman,99 for two chains with different initial values. A burn-in of 50,000 iterations of the Markov chain was used with a further 20,000 iterations retained to estimate parameters. There was no evidence of high autocorrelation between successive iterations of the Markov chain.
It should be noted that the results from the NMA are not used to inform the health economic model developed by the AG (see Chapter 4, Independent assessment group model). The NMA utilises HRs, which are averaged estimates of treatment effect, and their use in the health economic model would be appropriate only if the hazards are proportional over the entire extrapolation period. However, the AG’s health economic model considers a broader range of parametric functions, not all of which conform to the proportional hazards assumption; hence the use of HRs from the NMA would not be appropriate. Instead, estimation of the treatment effects and baseline model is conducted using the same parametric model type (see Time to event analysis using individual patient data), conforming to the recommendation in Guyot et al. 100
Results of the network meta-analysis
The results of the NMA are shown in Figure 5 for investigator-read PFS and Figure 6 for central-read PFS. Based on investigator-read PFS, the results of the two treatments are broadly similar [vandetanib vs. cabozantinib, HR 1.14, 95% credible interval (CrI) 0.41 to 3.09]. The magnitude of the treatment effect is more favourable towards cabozantinib when the comparison is based on central-read PFS (HR 1.68, 95% CrI 0.61 to 4.62); however, the difference between the two interventions is not statistically significant.
FIGURE 5.
Results of the NMA for investigator-read PFS. PrI, prediction interval.
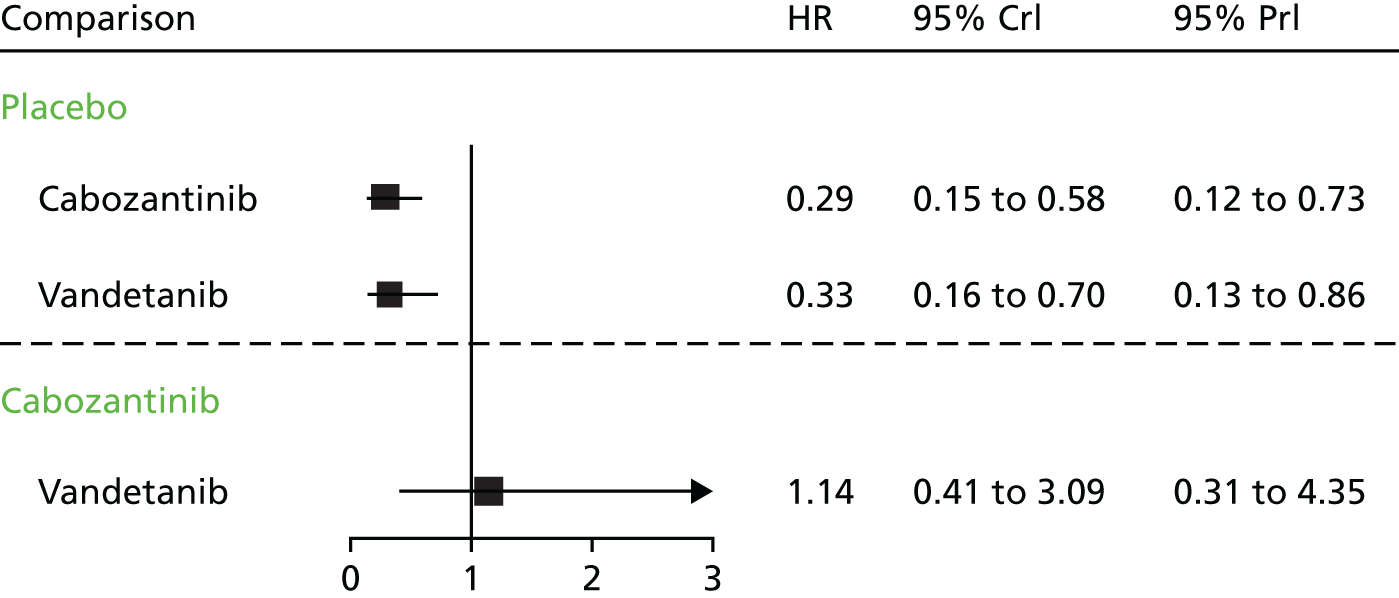
FIGURE 6.
Results of the NMA for central-read PFS. PrI, prediction interval.
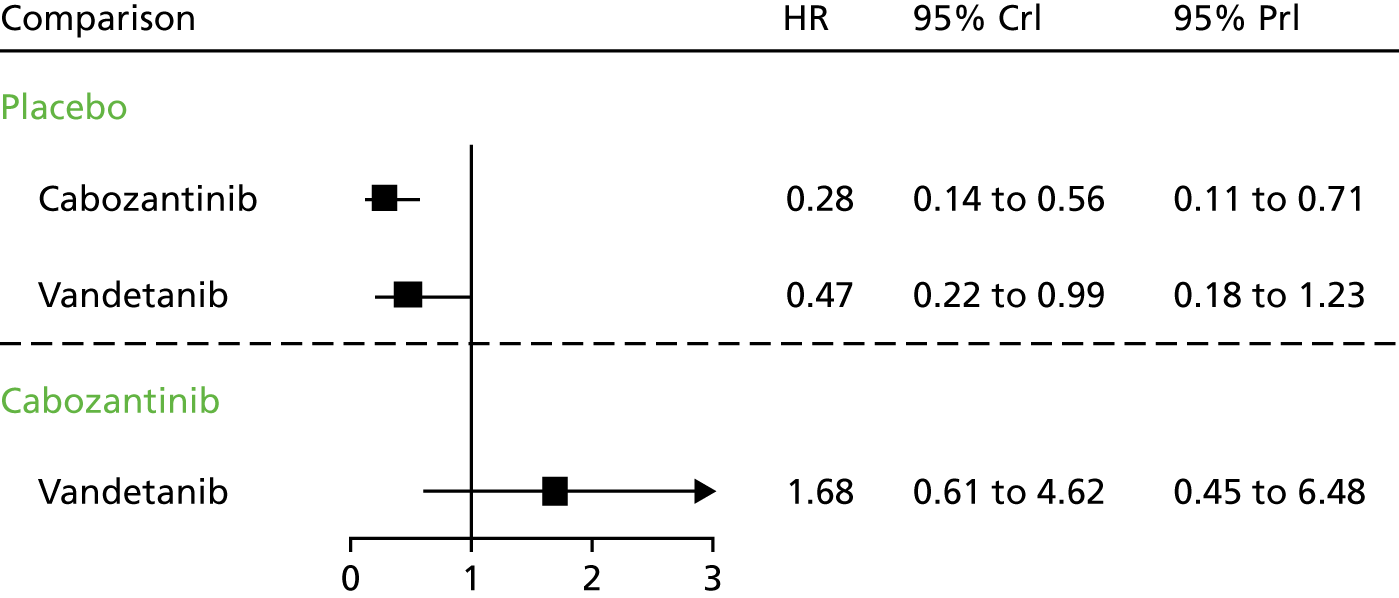
Discussion
The systematic review of the clinical effectiveness evidence identified two placebo-controlled RCTs. The EXAM trial evaluated the efficacy and safety of cabozantinib in patients with unresectable locally advanced or metastatic and progressive MTC (n = 330). The ZETA trial evaluated the efficacy and safety of vandetanib in patients with unresectable locally advanced or metastatic MTC (n = 331). The EXAM trial was deemed to be at low risk of bias across most domains (although the risk of selection bias was unclear because the method of randomisation was not explicitly reported). In contrast, the ZETA trial was rated as being at moderate to high risk of bias across a number of domains; in particular, the method of randomisation was not described and several outcomes were confounded by the inclusion of individuals who had switched to open-label treatment.
The two trials assessed different populations. The EXAM trial (n = 330) included only patients with unresectable locally advanced or metastatic and progressive MTC, whereas the ZETA trial inclusion criteria (n = 331) did not specify the requirement for patients to have ‘progressive’ disease; therefore, the ITT population in the ZETA trial generally had less severe disease (there were more participants with potentially indolent disease). The more progressive and severe disease of EXAM trial participants is evidenced by the between-trial baseline differences in performance status (see Table 3) and the relatively shorter duration of PFS for the participants in the placebo arm of the EXAM trial. However, a published abstract57 and the Sanofi CS66 provided data on a subgroup of the ZETA ITT population, that is, those with ‘progressive and symptomatic disease’ (n = 186) – the EU-label population. Despite slight differences in definition (e.g. the explicit requirement for defined symptoms in the ZETA trial EU-label population subgroup), clinical advice received by the AG confirmed that the EXAM trial and ZETA trial ‘progressive and symptomatic’ (EU-label) populations are comparable. Clinical advice also confirmed that these populations reflect patients who are likely to present in clinical practice in England. The Sanofi CS66 also presented data on a restricted EU-label subgroup from the ZETA trial (confidential information has been removed), which was composed of ‘progressive and symptomatic’ patients who also had ‘aggressive’ disease, defined by CTN and CEA doubling times of < 24 months. CTN and CEA doubling times are acknowledged prognostic factors for MTC15–17 and were not controlled for in the EXAM trial. However, clinical advice received by the AG suggests that these biomarkers are unlikely to be relevant in the presence of other criteria indicating PD (e.g. RECIST criteria and symptoms), and, although they might be used to determine whether or not treatment is still working, they would not be used to inform decisions about whether or not to initiate TKI treatment.
In terms of efficacy, both cabozantinib and vandetanib significantly improved PFS compared with placebo. For the principal comparison between the EXAM trial ITT population and the ZETA trial EU-label population, PFS was similar for cabozantinib (investigator-read HR 0.29, 95% CI 0.21 to 0.42; p < 0.001, central-read HR 0.28, 95% CI 0.19 to 0.40; p < 0.001) and vandetanib [investigator-read HR 0.33 (95% CI 0.2 to 0.53; p = 0.0226), central-read, excluding participants switching treatments, HR 0.47 (95% CI 0.29 to 0.77; p = 0.0024), including open-label populations HR 0.32 (95% CI 0.19 to 0.54; p < 0.001), see Progression-free survival)]. The difference in PFS between vandetanib and placebo was (confidential information has been removed) for the restricted EU-label population (confidential information has been removed). 66 Subgroup analyses demonstrated a favourable treatment effect for all subgroup categories. The publications and CSs also presented data for PFS based on RET mutation status, but clinical advice received by the AG indicated that germline RET mutation status testing is conducted in the NHS in England only for the purpose of identifying patients with hereditary MTC. Somatic and other RET mutation testing is not routinely undertaken to inform treatment choices. Subgroup analyses reported in the Sanofi CS66 and the unpublished ZETA trial CSR showed that participants with a CTN or CEA doubling time of < 24 months had a PFS response to vandetanib that was more pronounced than that of participants with a doubling time of > 24 months and those in whom the doubling time is unknown. 66,74
The NMA suggests that the PFS effects for the two treatments are broadly similar (vandetanib vs. cabozantinib PFS HR 1.14, 95% CrI 0.41 to 3.09). The magnitude of the treatment effect is more favourable towards cabozantinib when the comparison is based on central-read PFS rather than investigator-read PFS (HR 1.68, 95% CrI 0.61 to 4.62), but the difference between the two interventions was not statistically significant. In the absence of direct evidence comparing the two interventions, the results of the NMA provide a useful comparison but should be interpreted with caution for the following reasons. Owing to the sparsity of the network, it was necessary to use a weakly informative prior for the between-study variance. This was considered to be more realistic than assuming that the between-study heterogeneity would be zero (i.e. taking a fixed-effects approach); however, the results are subject to the suitability of the prior and the resulting CrIs and prediction intervals are relatively wide, representing genuine uncertainty in the network. Furthermore, the NMA utilises HRs, which are averaged estimates of treatment effect, and ignore any potential treatment-by-time interaction. Alternative methods that allow the relative treatment effects to vary over time have been proposed, including the use of fractional polynomials. 101 The AG did not deem this approach to be necessary as the results of the NMA are used to judge the comparative effectiveness of the interventions over the observed trial period and have not been used to inform the health economic model (see Chapter 4, Independent assessment group model).
Based on the available trial evidence, there was no significant survival benefit in terms of OS for either cabozantinib or vandetanib compared with placebo, although the data from the vandetanib ZETA trial were confounded by treatment switching. In the EXAM trial, the estimated median OS was 26.6 months for cabozantinib compared with 21.1 months for placebo (stratified HR 0.85, 95% CI 0.64 to 1.12; p = 0.241). 47 Within this study, the only significant difference in OS was found for 126 participants with known RET M918T-positive mutations: the median OS was 44.3 months for cabozantinib compared with 18.9 months for placebo (HR 0.60, 95% CI 0.38 to 0.94; p = 0.026). In the ZETA trial, the reported OS for the ITT population was 50% for vandetanib compared with 52% for placebo (HR 0.99, 95% CI 0.72 to 1.38; p = 0.975), although the placebo group included participants who had switched to vandetanib in the open-label stage of the trial, thus potentially confounding these results. 66 According to the Sanofi CS,66 the median OS for the restricted EU-label group was (confidential information has been removed) in the placebo arm compared with (confidential information has been removed) in the vandetanib arm (confidential information has been removed).
Both cabozantinib (p < 0.001) and vandetanib (ITT group, p < 0.001; EU-label group, p < 0.0001) demonstrated significant benefits compared with placebo in terms of ORR, as determined by the RECIST criteria. Both cabozantinib (p < 0.001) and vandetanib (p < 0.001) also demonstrated significantly better CTN and CEA response rates than placebo.
The two trials conducted exploratory assessments of participants’ quality of life using instruments that evaluated various criteria, including symptoms: the MDASI-Thy in the EXAM trial and the FACT-G in the ZETA trial. However, when assessed, no difference was found between the treatment or placebo arms in either trial; this covers both baseline and follow-ups. Clinical advice received by the AG suggested that these tools did not necessarily capture symptomatic benefit produced by improved PFS or response on treatment.
Both cabozantinib and vandetanib produced frequent AEs. Based on the EXAM trial, the most common AEs for cabozantinib were diarrhoea (63%), HFS (50%), decreased weight (48%) and appetite (46%), nausea (43%) and fatigue (41%). The most common AEs for vandetanib were diarrhoea (56%), decreased appetite (21%), nausea (33%) and fatigue (24%); in addition, there was a high incidence of rash (45%), hypertension (32%) and headache (26%), and low or no incidence of HFS. Hypertension is a known AE of TKIs. 81,82 The incidence rates of rash and hypertension appear to be higher for vandetanib in MTC patients than in most other cancer patients,84,85 which might be attributable to a longer treatment duration. 85
The most common grade 3 or higher AEs for cabozantinib, as reported from the EXAM trial, were diarrhoea (16%), HFS (13%), fatigue (9%), hypertension (8%), asthenia (6%), and decreased weight (5%) and appetite (5%). These appear to be consistent with other anti-VEGF TKIs and the open-label cabozantinib studies. The most common grade 3 or higher AEs for vandetanib, as reported for the ITT population from the ZETA trial, were diarrhoea (11%), hypertension (9%), fatigue (6%) and decreased appetite (4%); however, rash (4%) and prolonged ECG QT (8%) were also common. An exploratory study also identified significant toxicities in the presence of higher mean vandetanib plasma concentration, the most frequent toxicities being asthenia grade 3 (36%), prolongation of the QTc interval (25%) and cutaneous symptoms (11%). 62 Vandetanib is one of only two TKIs (the other being sunitinb) identified as being particularly associated with prolonged QTc interval. 91
Similar proportions of participants across the two trials discontinued treatment because of AEs (16% for cabozantinib and 12% for vandetanib), but a higher percentage of participants on cabozantinib experienced AEs leading to dose interruption or reduction (65%) than on vandetanib (35%). A later abstract55 detailing this outcome for the EXAM trial reported that dose reduction to manage AEs was performed for 82% of participants treated with cabozantinib, which increased again to 87% in the final analysis. The percentages of participants experiencing AEs leading to dose interruption or discontinuation were also higher in the placebo arm of the cabozantinib EXAM trial (17% for dose interruption and 8% for discontinuation) than in the vandetanib ZETA trial (3% for dose interruption and 3% for discontinuation). High rates of dose reduction and discontinuation have also been reported for a retrospective study of 15 patients with progressive MTC on cabozantinib. 77 The authors of the EXAM trial28 acknowledged the high rate of dose interruption with 140 mg of cabozantinib: the EXAMINER trial54 has therefore been developed to assess the efficacy and safety of a lower dose of cabozantinib (60 mg) compared with the current standard dose (140 mg).
Finally, in the EXAM trial, up to 5% of deaths were reported as being treatment related for cabozantinib and 1% for placebo. 21 During the randomised phase of the ZETA trial, 2% of participants who received vandetanib (5/231) experienced AEs leading to death. The reasons given were aspiration pneumonia, respiratory arrest, respiratory failure, staphylococcal sepsis, and, in one participant, arrhythmia and acute cardiac failure. 27 Instances of gastroenteritis and gastrointestinal haemorrhage led to deaths in two participants in the placebo group. 27
Chapter 4 Assessment of cost-effectiveness
This chapter presents a systematic review of existing economic evaluations of treatments for locally advanced or metastatic MTC, and a summary and critique of economic analyses submitted by the manufacturers of vandetanib and cabozantinib, together with details of the methods and results of a de novo health economic analysis undertaken by the AG.
Systematic review of existing cost-effectiveness evidence
Review of existing economic evaluations: methods
A comprehensive search was undertaken to systematically identify economic evaluations of treatments for locally advanced or metastatic MTC and studies reporting on the HRQoL of patients with locally advanced or metastatic thyroid cancer (including MTC as well as other commoner forms of thyroid cancer). In anticipation of the likely lack of relevant evidence, the AG’s search strategy was designed to be intentionally broad.
The following electronic databases were searched from inception to 3 November 2016:
-
MEDLINE – via Ovid, 1946 to present
-
MEDLINE In-Process & Other Non-Indexed Citations – via Ovid, 1946 to present
-
MEDLINE Epub Ahead of Print – via Ovid, 1946 to present
-
CINAHL – via EBSCOhost, 1982 to present
-
EMBASE – via Ovid, 1980 to present
-
HTA database, 1995 to present
-
NHS Economic Evaluation Database (NHS EED), 1995 to 2015
-
Web of Science (SCI) – via Clarivate Analytics (formerly Thomson Reuters) 1899 to present
-
CPCI – via Clarivate Analytics (formerly Thomson Reuters) 1990 to present.
The search strategy comprised MeSH or Emtree Thesaurus terms and free-text synonyms for ‘thyroid cancer’. Searches were translated across databases and were not limited by either language or publication date. The search strategies are presented in Appendix 1. Search filters designed to identify economic evaluations and HRQoL studies were applied in MEDLINE and other databases, when appropriate. Reference and citation searching of included papers was also undertaken.
Potentially includable studies were sifted by title and abstract by one reviewer (PT). In keeping with the breadth of the search strategy, the inclusion criteria were also defined broadly and the sifting process followed an inclusive approach in order to maximise sensitivity. Given that the cost-effectiveness search also identified studies relating to health utilities (e.g. those used within models), and the HRQoL search also identified health economic evaluation studies, the results of both searches were sifted together using a common set of inclusion criteria (Box 2). Although the inclusion criteria for the review of existing economic evaluation studies were specific to MTC, HRQoL studies were considered to be potentially includable if they were undertaken in patients with MTC or other types of thyroid cancer (papillary, follicular, Hürthle cell carcinoma).
-
Comparative economic evaluations of interventions for the treatment of locally advanced or metastatic MTC.
-
Studies reporting preference-based health utilities relating to any type of thyroid cancer.
-
Studies evaluating diagnostic/staging interventions, for example FNAB (unless the study specifically mentions utilities for advanced/metastatic disease or reports QALYs).
-
Partial economic analyses, for example costing studies.
-
Editorials.
-
Reviews.
-
Clinical studies that do not report costs.
-
Letters and commentaries.
-
Non-English language.
FNAB, fine-needle aspiration biopsy; QALY, quality-adjusted life-year.
Review of existing economic evaluations: results
Figure 7 presents the study selection results. Before deduplication, the searches yielded 3161 citations (HRQoL search, n = 1282 studies; economic evaluation search, n = 1879 citations). Following the initial sift, 3057 of these studies were excluded. Full texts of the remaining 104 potentially includable studies were retrieved for further examination. However, none of these studies contained an economic evaluation of treatments for MTC; hence all studies were excluded from the review. In addition, none of these studies reported health utilities for patients with locally advanced or metastatic MTC. One study102 reported health utilities for patients with radioactive iodine-refractory differentiated thyroid cancer; this study is discussed in further detail in Independent assessment group model and Health-related quality of life.
FIGURE 7.
Study selection results.
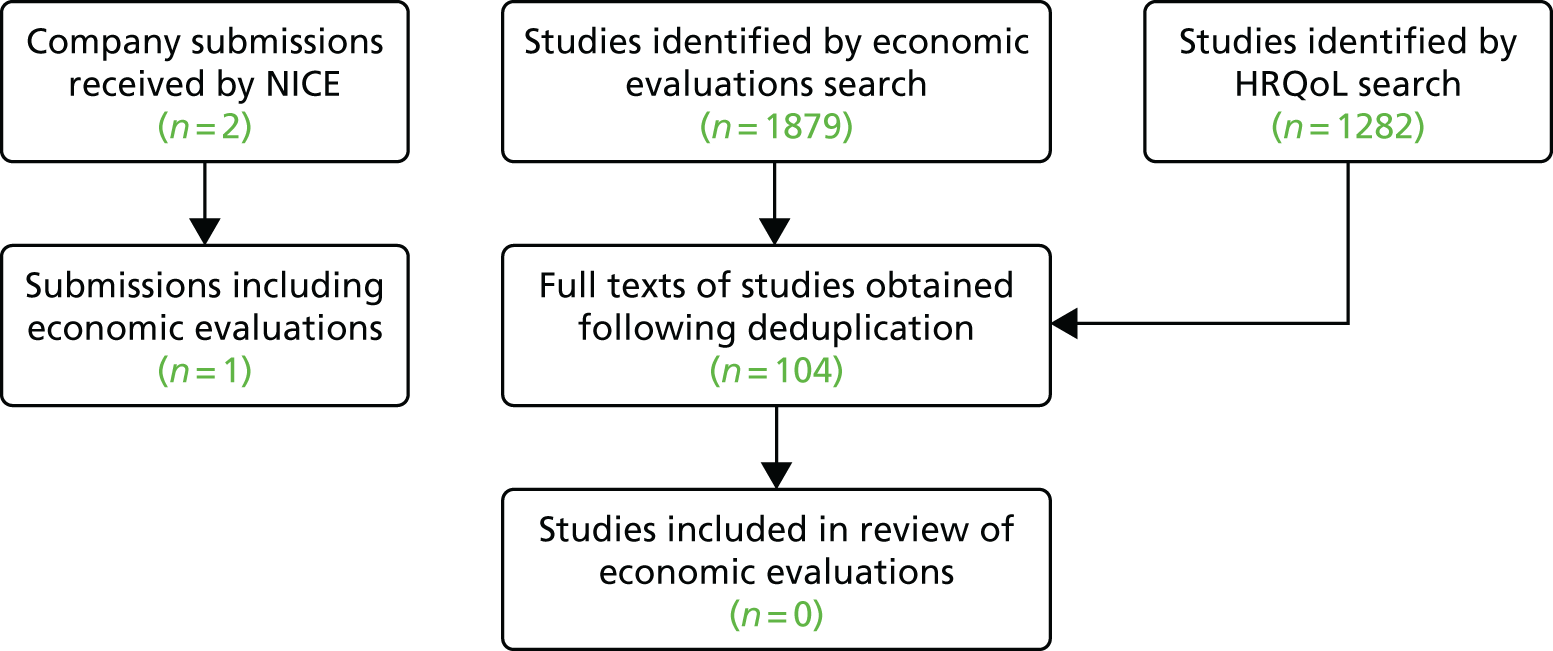
Review of models submitted by the companies
The Sanofi submission66 includes a health economic evaluation of vandetanib for the treatment of locally advanced or metastatic MTC, together with a fully executable health economic model. The Ipsen submission21 does not include any economic evidence for this appraisal.
Scope of the Sanofi economic evaluation
The Sanofi CS66 presents the methods and results of a model-based economic evaluation of vandetanib for the treatment of MTC, based largely on analyses of a subgroup of the ZETA trial. The scope of the company’s model is summarised in Table 15. The model assesses the incremental cost-effectiveness of vandetanib versus BSC over a lifetime (20-year) time horizon from the perspective of the NHS. Cost-effectiveness is expressed in terms of the incremental cost per quality-adjusted life-year (QALY) gained. The population considered within the company’s model relates to the restricted EU-label population, that is, patients with aggressive and symptomatic unresectable locally advanced or metastatic MTC, defined as progressive (documented progression within 12 months prior to enrolment) and symptomatic (at least one symptom at baseline, including pain score of > 4, ≥ 10 days of opioid use, diarrhoea, flushing, fatigue, pain, nausea, dysphagia, dysphonia, respiratory symptoms, weight loss) plus CTN and CEA doubling times within 24 months of screening. 66 The AG noted that this population is narrower than the indication permitted by the EMA marketing authorisation for vandetanib;22 a health economic analysis of the broader licensed population is not presented within the CS. 66 Costs and health outcomes are discounted at a rate of 3.5% per annum. The company’s economic analysis includes a PAS that takes the form of a simple price discount for vandetanib. The results presented within this report use the list price for vandetanib; the results of the Sanofi model including the PAS are presented within a separate confidential appendix to this report. Costs were valued at 2015/16 prices.
| Model component | Description |
|---|---|
| Population | The restricted EU-label population for vandetanib: patients with aggressive and symptomatic unresectable locally advanced or metastatic MTC defined as progressive (documented progression within 12 months prior to enrolment) and symptomatic (at least one symptom at baseline, including pain score of > 4, ≥ 10 days of opioid use, diarrhoea, flushing, fatigue, pain, nausea, dysphagia, dysphonia, respiratory symptoms, weight loss) plus CTN and CEA doubling times within 24 months of screening |
| Intervention | 300 mg per daya of vandetanib [with post-progression continuation of vandetanib in (confidential information has been removed) of participants] |
| Comparator | BSC [with switch to 300 mg per day of vandetanib post progression in (confidential information has been removed) of patients] |
| Analysis type | Cost–utility analysis |
| Economic outcome | Incremental cost per QALY gained |
| Perspective | NHS |
| Time horizon | 20 years (lifetime) |
| Discount rate | 3.5% per annum for health outcomes and costs |
It is important to note from the outset that a substantial proportion of participants (confidential information has been removed) in the restricted EU-label population who were allocated to the placebo arm of the ZETA trial switched to open-label vandetanib (either post progression or in any participant following a protocol amendment in January 2010, see the Sanofi clarification response,73 question A2). In addition, a proportion of participants (confidential information has been removed) in the restricted EU-label population who were allocated to the intervention group continued to receive open-label vandetanib following disease progression. Although the company attempted to adjust for treatment switching using the rank-preserving structural failure time (RPSFT) method, this was not successful (see the Sanofi CS,66 pp. 98–9); hence, the estimates of OS for both modelled treatment groups are unadjusted and remain potentially confounded by the use of open-label vandetanib. As the potential impact of open-label vandetanib use could not be addressed, the company’s model includes the estimated costs of post-progression vandetanib use within both the intervention and comparator treatment groups. The economic comparison made by the company’s model is, therefore, vandetanib, including continued use in some participants post progression, versus BSC with vandetanib use in most participants post progression. The AG notes that this may not be useful for decision-making; the same issue also applies to the two pairwise comparisons of vandetanib versus BSC undertaken using the AG model (see Independent assessment group model).
The AG also notes that two errors were identified within the company’s original submitted model, which related to (1) the duration over which QALY losses owing to AEs are applied and (2) inputs relating to the proportion of participants who discontinued vandetanib prior to disease progression (see Critical appraisal of the economic analysis presented by Sanofi). All results presented within this report include corrections to these errors.
Sanofi model structure
The economic analysis presented by Sanofi takes the form of a cohort-level partitioned survival model implemented using the discretely integrated condition event (DICE) simulation methodology103 (Figure 8). The model includes three health states: (1) progression free, (2) post progression and (3) dead. The model operates as follows. At any time t, the probability that a participant allocated to treatment group k is alive is given by S(t)OS_k, whereas the probability that a participant allocated to treatment group k is alive and progression free is given by S(t)PFS_k. The probability that a participant is alive following disease progression is calculated as the difference between the two survivor functions: S(t)OS_k – S(t)PFS_k for any time t. Given the presence of censoring, parametric survivor functions were fitted to Kaplan–Meier curves for OS and PFS from the ITT/safety populations of the ZETA trial, including adjustment for two covariates: (1) ‘SympProg’ (presence of symptomatic and progressive disease) and (2) ‘BiomarkerChg’ (CEA and CTN doubling times of ≤ 24 months). Weibull functions were selected to model both OS and PFS, assuming independent (non-proportional) hazards between treatment groups. The DICE routine is evaluated using a monthly cycle length over a 20-year lifetime horizon and includes a half-cycle correction to account for the timing of events.
The model assumes that health utility is determined by the presence/absence of disease progression, with higher utilities applied to the progression-free state. In addition, a once-only QALY loss is applied to each group to account for the incidence of grade 3/4 AEs.
The model includes the following resource costs: (1) vandetanib drug acquisition costs, (2) monitoring for participants receiving vandetanib, (3) BSC costs, (4) palliative care costs and (5) costs associated with managing AEs.
The model employs the following structural assumptions:
-
Health-related quality of life is determined according to the presence/absence of disease progression and the incidence of grade 3/4 AEs.
-
Progression-free survival and OS are modelled using Weibull functions assuming independent (non-proportional) hazards.
-
Survival models were fitted to the overall ITT population for PFS and the safety population for OS, including covariate adjustments to reflect the characteristics of the restricted EU-label population.
-
No adjustment is made to account for logical inconsistencies [i.e. when S(t)PFS > S(t)OS].
-
The modelling of costs and health outcomes includes the level of open-label vandetanib use (either post progression or in any patient following the January 2010 protocol amendment73) observed in the ZETA trial.
-
Adverse events are assumed to affect both costs and HRQoL. According to the Sanofi CS,66 AE impacts on HRQoL apply only during the first month of the time horizon. This aspect of the model is subject to a programming error (see Critical appraisal of the economic analysis presented by Sanofi) and was corrected by the company in its clarification response73 (question A18).
-
Palliative care costs are assumed to be incurred only during the final month of life.
Evidence used to inform the company’s model
Table 16 summarises the evidence used to parameterise the company’s model. The derivation of these parameters and their evidence sources are discussed in further detail in the following sections.
| Parameter group | Evidence source | |
|---|---|---|
| PFS | Parametric survival models fitted to ZETA trial ITT population PFS data, and subsequently adjusted by setting the coefficients for covariates ‘SympProg’ and ‘BiomarkerChg’ to 100%66 | |
| OS | Parametric survival models fitted to ZETA trial safety population OS data, and subsequently adjusted by setting the coefficients for covariates ‘SympProg’ and ‘BiomarkerChg’ to 100% | |
| Health utilities |
Progression-free state: the FACT-G scores for the progression-free state observed in the ZETA trial were mapped to the EQ-5D-3L instrument using the algorithm reported by Dobrez et al. 104 Post-progression state: calculated using utility multiplier (0.766) for post progression vs. pre progression from SG study of societal preferences for advanced melanoma health states reported by Beusterien et al. 105 Disutility due to AEs: disutility for any grade 3/4 AE taken from Beusterien et al. ’s105 advanced melanoma SG study |
|
| Time spent receiving vandetanib | Vandetanib group | BSC group |
| (a) Pre progression | (b) Pre progression
|
|
(c) Post progression
|
(d) Post progression
|
|
| Probability of receiving vandetanib while in post-progression state | Based on observed continuation proportion in the vandetanib group of the restricted EU-label population from the ZETA trial (confidential information has been removed)66 | Based on observed switching proportion in the placebo group of the restricted EU-label population from the ZETA trial (confidential information has been removed)66 |
| Vandetanib acquisition cost | Sanofi CS66 | |
| Monitoring resource use | Resource use related to ECGs and phlebotomy during the first and subsequent years of use based on the SmPC for vandetanib22 | |
| AE incidence | Grade 3/4 AEs observed within full safety population of the ZETA trial66,74 | |
| BSC resource use | Assumption | |
| AE management costs | NHS Reference Costs 2015/16 106 | |
| BSC costs | NHS Reference Costs 2015/16 106 | |
| Palliative chemotherapy costs | NHS Reference Costs 2015/16 106 | |
| Palliative care costs | Curtis and Burns107 | |
Overall survival
Overall survival was defined as the time from randomisation to death or the last date at which the subject was known to be alive. 66 The analyses of OS used individual patient data (IPD) for all participants who received randomised treatment (the safety population) including follow-up to the data cut-off point of 7 September 2015. As noted in Scope of the Sanofi economic evaluation, the Sanofi CS66 states that although attempts were made to adjust for treatment switching using the RPSFT method, these were unsuccessful (Sanofi CS,66 pp. 98–9). Therefore, the OS data used in the model remain subject to potential confounding as they include data relating to the use of open-label vandetanib in both treatment groups. With respect to this issue, the company states: ‘the OS data are more likely to show the impact of treatment with immediate vs delayed vandetanib, rather than be a true comparison of vandetanib vs placebo’. (Sanofi CS,66 p. 63). Parametric survival models (Weibull, log-normal, log-logistic, exponential and gamma functions) were fitted to the available data including two covariates – (1) ‘SympProg’ (presence of symptomatic and progressive disease) and (2) ‘BiomarkerChg’ (CEA and CTN doubling times of ≤ 24 months) – using the LIFEREG procedure in SAS® (SAS Institute Inc., Cary, NC, USA. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.). In order to reflect the restricted EU-label population within the model, the coefficients for both covariates were set equal to 100%. Statistical goodness-of-fit was assessed using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). The CS states that the plausibility of the long-term projections for each model was also assessed, although the CS does not provide details about who undertook this assessment or whether or not any external data were used to inform these judgements. The company’s subsequent clarification response states that assessments of clinical plausibility involved an expert clinician, the statistical consultants and the modelling team (Sanofi clarification response,73 question A15).
The observed and predicted OS curves are presented in Figure 17 in Appendix 3, based on the comparison presented in both the Sanofi CS66 and the model. As the CS includes only a comparison of the Weibull function against the empirical Kaplan–Meier data, the AG digitised the Kaplan–Meier data and plotted the predictions of the covariate-adjusted Weibull, log-normal and log-logistic OS functions for the purposes of comparison. The AG considers this comparison of observed and predicted OS to be inappropriate as the population represented by the observed Kaplan–Meier data is not the same as the population reflected by the modelled functions (the observed data reflect the safety population with the CTN/CEA biomarker but without aggressive and progressive disease; see Critical appraisal of the economic analysis presented by Sanofi). The corresponding AIC/BIC statistics for all five parametric models are presented in Table 40 in Appendix 3.
With respect to the vandetanib group, the AIC and BIC were lowest for the log-normal model, whereas for the placebo group, the AIC and BIC were lowest for the gamma model. The CS states that the Weibull function was selected for use in the base-case analysis as, in this instance, this function ‘matches human mortality better in the long term’ (Sanofi CS,66 p. 105). The impact of uncertainty surrounding the choice of parametric function for PFS and OS was partially explored in the sensitivity analyses.
Progression-free survival
Progression-free survival was defined as the time from randomisation to documented progression based on central review or death. 66 The Sanofi CS66 (p. 101) notes that, although the use of central-read PFS is subject to confounding because of treatment switching, using this end point mirrors the per-protocol end points of the ZETA trial. The analyses of PFS used IPD for all randomised participants available at the date of the initial data cut-off point, as reported in the original CSR of 6 July 2011. 74 As with the company’s analysis of OS, parametric survival models (Weibull, log-normal, log-logistic, exponential and gamma functions) were fitted to the available PFS data including two covariates: (1) ‘SympProg’ (presence of symptomatic and progressive disease) and (2) ‘BiomarkerChg’ (CEA and CTN doubling times of ≤ 24 months) using the LIFEREG procedure in SAS. In order to reflect the restricted EU-label population, the coefficients for both covariates were set equal to 100%. Statistical goodness-of-fit was assessed using the AIC and BIC. The CS states that the plausibility of the long-term projections for each model was also assessed; the company’s clarification response states that this exercise involved an expert clinician, the statistical consultants and the modelling team (Sanofi clarification response,73 question A15).
The observed and predicted PFS curves are presented in Figure 18 in Appendix 3, based on the observed central review PFS Kaplan–Meier curves for the restricted EU-label population presented in figure 6 of the CS (see Sanofi CS,66 p. 56). As the CS includes only a comparison of the Weibull function against the empirical Kaplan–Meier PFS curves, the AG digitised the Kaplan–Meier data and plotted the predictions of the covariate-adjusted Weibull, log-normal and log-logistic PFS functions for the purposes of comparison. The AG notes that the Kaplan–Meier curves used to compare model-predicted with observed PFS within the Sanofi CS and those presented in the company’s model differ considerably; the reasons for these differences are unclear. The corresponding AIC/BIC statistics for all five parametric models are presented in Table 41 in Appendix 3.
The AIC and BIC were lowest for the log-normal model for the vandetanib group, whereas the AIC and BIC were lowest for the exponential model for the placebo group. The CS states that ‘As there is no clear, clinical expectation for the PFS over the long-term, Weibull was also selected in the base case for consistency’ (Sanofi CS,66 p. 105). The impact of uncertainty surrounding the choice of parametric function for PFS and OS was partially explored in the sensitivity analyses.
Health-related quality of life
The health utility values applied in the Sanofi model are summarised in Table 42 in Appendix 3. The ZETA trial assessed HRQoL using the FACT-G instrument;74 the trial did not include the use of a preference-based HRQoL instrument. Within the model, the health utility score associated with the progression-free state was estimated by mapping FACT-G scores for participants who were progression free in the ZETA trial to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), using a published ordinary least squares algorithm reported by Dobrez et al. 104 This mapping exercise produced a mean utility score for the progression-free state of 0.84.
The Sanofi CS66 notes that in the ZETA trial, post-progression FACT-G data were available for only 62 participants (27%). Rather than applying the mapping approach used for the progression-free state, the health utility score for the post-progression state was instead estimated using a utility multiplier for the states of post progression versus pre progression derived from a general population standard gamble study of societal preferences for advanced melanoma states reported by Beusterien et al. 105 In this study, the ratio of progressive disease utility to stable disease utility was 0.766 (0.59/0.77); applying this multiplier to the company’s estimated utility score for the progression-free state leads to an estimated post-progression utility score of 0.64 (0.84 × 0.766). The disutility associated with any grade 3/4 AEs was also derived from the Beusterien et al. 105 advanced melanoma valuation study (disutility = –0.11). The same disutility was assumed to apply to each type of AE.
Time spent receiving vandetanib
Table 43 in Appendix 3 presents the percentage of time spent receiving each dose level of vandetanib during the progression-free period divided by the total pre-progression time on treatment, calculated from data for the restricted EU-label population. 66,78 This distribution is applied within the vandetanib group to determine the amount of time spent receiving treatment in the progression-free state. The Sanofi CS66 (p. 103) notes that ‘Patients whose cancer had not yet progressed were allowed, nevertheless, to discontinue treatment. These treatment discontinuations were addressed by applying the relevant proportion to the patients not having progressed in each cycle (21.9%)’. This value was later corrected by the company [corrected value = (confidential information has been removed)]. Although the wording of the CS implies that all participants start treatment on vandetanib and a proportion of participants subsequently discontinue treatment during each cycle, this discontinuation parameter is instead applied as a fixed proportion of participants in the progression-free state who do not receive vandetanib (and, therefore, do not incur any costs of vandetanib treatment). The appropriateness of this parameter is unclear. The distribution of vandetanib use, shown Table 43 in Appendix 3, is also applied in the post-progression state for the proportions of participants who switch to or continue to receive vandetanib post progression in each treatment group, albeit without the vandetanib discontinuation parameter. As a consequence, participants receive more vandetanib per cycle during the post-progression phase than in the pre-progression phase; it is unclear whether this reflects an error or an unreasonable assumption.
Probability of receiving vandetanib in the post-progression state
Based on the experience of the ZETA trial66,78 (specifically with respect to the restricted EU-label population), the model assumes that (confidential information has been removed) of participants in the vandetanib group continue to receive vandetanib post progression, whereas (confidential information has been removed) of participants in the BSC group cross over to receive vandetanib post progression. Clinical advisors to the AG noted that the use of vandetanib post progression does not reflect usual clinical practice in England.
Vandetanib acquisition cost
The acquisition costs of vandetanib are summarised in Table 44 in Appendix 3, based on the current prices listed in the British National Formulary. 108
Monitoring costs
Resource use estimates were based on the monitoring regimen detailed in the SmPC for vandetanib. 22 Unit costs were derived from NHS Reference Costs 2015/16106 (see Table 45 in Appendix 3). Owing to the inclusion of the costs associated with post-progression vandetanib use in the BSC group, these monitoring costs are applied in both groups (to the proportion of participants who initially receive/continue vandetanib in the intervention group and to the proportion of participants who switch from BSC to vandetanib in the comparator group). Although the monitoring costs are presented in the CS as being dependent on the time since starting treatment, this time dependence is captured only in the progression-free state for the intervention group. The lower ‘subsequent years’ cost is applied to the proportion of participants continuing or switching to vandetanib post progression (see Sanofi CS,66 p. 111). The company states that this approach was deemed to be conservative (see Sanofi clarification response,73 question A20), although the AG notes that the impact on the incremental cost-effectiveness ratio (ICER) is likely to be small.
Adverse event management costs
The company’s model includes any grade 3/4 AEs that occurred in ≥ 2% of participants in either treatment group. Table 46 in Appendix 3 presents the grade 3/4 AE incidence rate and associated management costs included in the company’s model. The incidence of any grade 3/4 AEs was taken from the safety population of the ZETA trial27 (derived directly from the Wells et al. 27 trial publication). Unit costs associated with the management of AEs were derived from NHS Reference Costs 2015/16. 106 In response to a request for clarification from the AG, the company clarified that the AE data for the safety population were used because the equivalent data for the restricted EU-label population were not available at the time of the submission (see Sanofi clarification response,73 question A11). The model applies the total AE cost once during the first model cycle. The AG notes that all NHS reference cost codes assume that a participant is treated in an elective inpatient setting; given that these costs are associated with the management of AEs (i.e. non-elective), this is inappropriate but is likely to have only a negligible impact on the model results.
Palliative care costs
The company’s model includes a cost of £5775 for palliative care derived from the Personal Social Services Research Unit (PSSRU)107 and £827 for palliative chemotherapy given at the end of life, based on NHS Reference Costs 2015/16. 106 This cost is applied for the last month before death. As these costs are common to both groups, and because virtually all participants die within the time horizon (> 98.7% of participants), the only differences in these costs between the two treatment groups are as a result of discounting.
Model evaluation methods
The headline results presented in the Sanofi CS66 are based on the deterministic version of the model. Uncertainty surrounding model parameters was explored using deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA). The company’s probabilistic results were estimated from 1000 Monte Carlo samples. Uncertainty was represented using tornado diagrams, cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs).
Sanofi model results
Sanofi central estimates of cost-effectiveness (excluding Patient Access Scheme, including error corrections)
Table 17 presents the company’s base-case estimates of cost-effectiveness using the list price for vandetanib. Based on the probabilistic version of the company’s model, vandetanib is expected to generate an additional 1.34 QALYs at an additional cost of £42,215 compared with BSC; the ICER for vandetanib versus BSC is expected to be £31,546 per QALY gained. The deterministic version of the model produces a slightly higher ICER of £31,731 per QALY gained.
| Option | Absolute | Incremental | |||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ICER (£) | |
| Probabilistic model | |||||
| Vandetaniba | 3.53 | 181,130 | 1.34 | 42,215 | 31,546 |
| BSCa | 2.19 | 138,915 | – | – | – |
| Deterministic model | |||||
| Vandetaniba | 3.49 | 175,316 | 1.36 | 43,024 | 31,731 |
| BSCa | 2.13 | 132,292 | – | – | – |
Figure 9 presents the CEACs for vandetanib and BSC, generated by the AG using the corrected version of the Sanofi model. The CEAC indicates that, assuming willingness-to-pay (WTP) thresholds of £20,000 and £30,000 per QALY gained, the probability that vandetanib produces more net benefit than BSC is approximately 0.33 and 0.48, respectively.
FIGURE 9.
Cost-effectiveness acceptability curves generated using the Sanofi model: vandetanib vs. BSC (redrawn by the AG).
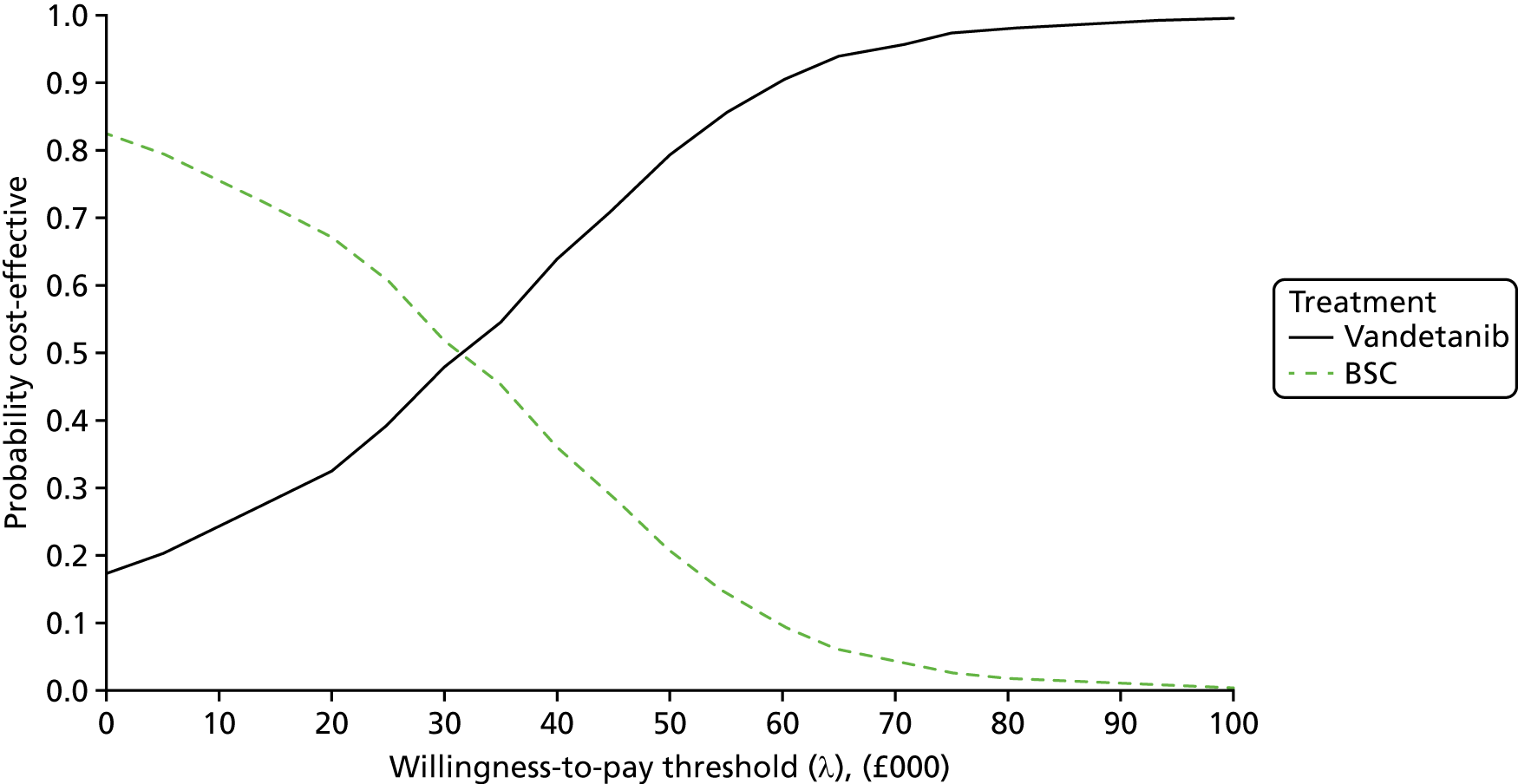
Sanofi’s deterministic sensitivity analysis results
Figure 10 presents the results of the company’s DSAs. The most influential parameters (of those assessed by the company) relate to the probability of vandetanib continuation beyond progression, the probability of treatment switching in the BSC group and the vandetanib discontinuation parameter applied to the vandetanib group during the progression-free phase. The use of the log-logistic and log-normal functions for PFS and OS (analyses not shown in Figure 10) did not have a substantial impact on the ICER for vandetanib versus BSC (log-normal PFS and OS ICER = £37,227 per QALY gained; log-logistic PFS and OS ICER = £28,879 per QALY gained). It should be noted that a higher proportion of vandetanib participants are alive at 20 years (> 8%) using these functions rather than the Weibull model (< 2%).
FIGURE 10.
The DSA results generated using the Sanofi model (reproduced from the Sanofi model). Note: tornado plot shows absolute change to base-case ICER.
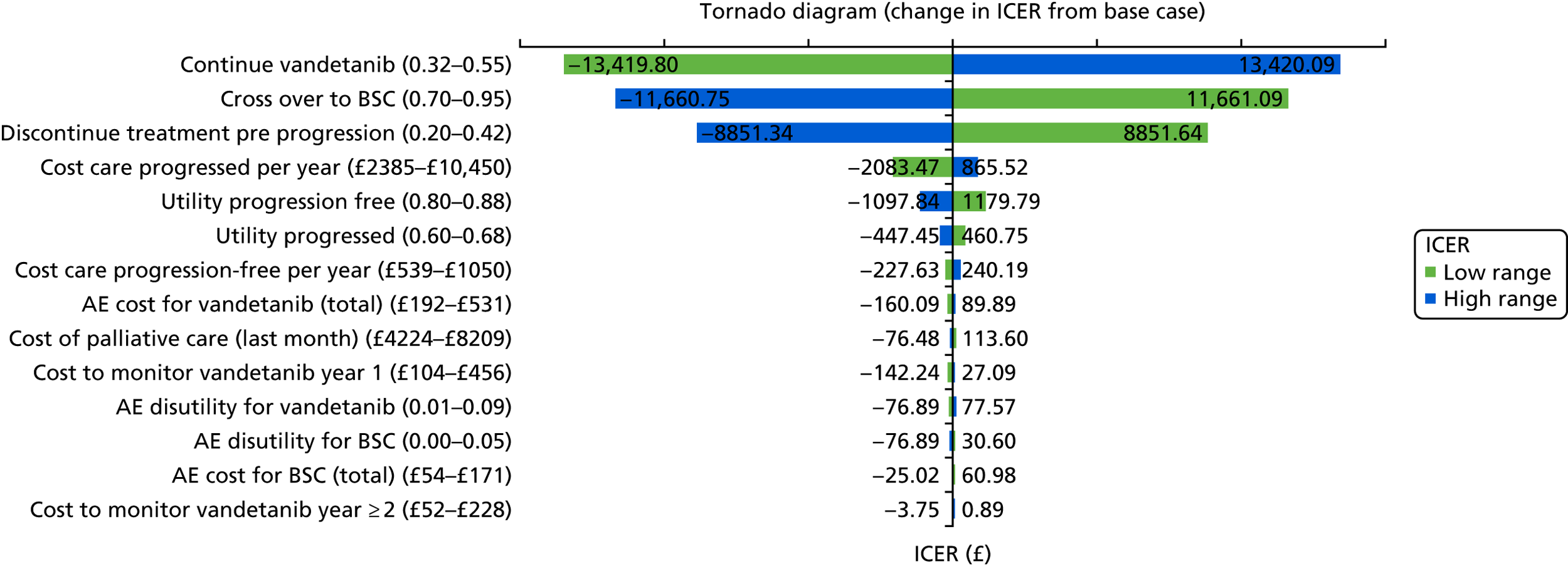
Critical appraisal of the economic analysis presented by Sanofi
Methods for reviewing the company’s economic evaluation and health economic model
The AG adopted a number of approaches to explore, interrogate and critically appraise the economic evaluation submitted by Sanofi and the underlying health economic model on which this was based. These approaches included the following:
-
an assessment of the extent to which the model adheres to the NICE Reference Case109
-
consideration of key items contained in published economic evaluation and health economic modelling checklists110,111 to critically appraise the model and associated analysis
-
scrutiny of the model and discussion of issues identified among the members of the AG
-
double-programming of the deterministic version of the Sanofi model to fully assess the logic of the company’s model structure, to draw out any unwritten assumptions and to identify any apparent errors in the implementation of the model
-
examination of the correspondence between the description of the model reported within the CS66 and the executable model
-
replication of the base-case results, PSA and scenario analysis presented within the Sanofi CS66
-
when possible, checking of the Sanofi model parameter values against the original data sources
-
the use of expert clinical input to judge the clinical credibility of the company’s economic evaluation and the assumptions underpinning the model.
Adherence of the company’s economic analysis to the National Institute for Health and Care Excellence Reference Case
Table 18 summarises the extent to which the economic analysis submitted by Sanofi adheres to the NICE Reference Case. 109
| Element | Reference Case | AG’s comments |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | The analysis is partially in line with the decision problem set out in the final NICE scope. The two key deviations are as follows:
|
| Comparator(s) | As listed in the scope developed by NICE | The company’s model compares vandetanib with BSC. However, estimates of OS are not adjusted for continued post-progression vandetanib use or switching from placebo to vandetanib post progression, or any pre-progression open-label vandetanib use permitted following the January 2010 protocol amendment to the ZETA trial. Cabozantinib is not considered within the economic analysis. Locally ablative therapies are not explicitly considered as comparators |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | The model includes direct health effects |
| Perspective on costs | NHS and PSS | The Sanofi model adopts a NHS perspective. PSS costs are not explicitly considered |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | The economic evaluation takes the form of a cost–utility analysis. Results are presented in terms of the incremental cost per QALY gained for vandetanib vs. BSC |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | A lifetime (20-year) time horizon is adopted |
| Synthesis of evidence on health effects | Based on systematic review | The company did not undertake a systematic review of clinical effectiveness evidence |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Within the progression-free state, health utility was estimated by mapping from the FACT-G collected in the ZETA trial to the EQ-5D. The health utility multiplier for the post-progression state and for the disutility associated with AEs was based on a SG study of societal preferences for advanced melanoma states reported by Beusterien et al.105 A disutility for any grade 3/4 AE is included based on Beusterien et al.105 |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | No equity weighting is applied |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Resource use estimates were based on data from the ZETA trial, expert opinion and assumptions. Unit costs were taken from NHS Reference Costs 2015/16106 |
| Discount rate | The same annual rate for both costs and health effects (currently 3.5%) | Costs and health outcomes are discounted at a rate of 3.5% per annum |
The two main deviations from the NICE Reference Case109 concern the exclusion of cabozantinib as a comparator and the population considered within the economic analysis (the restricted EU-label population). The AG also notes that the clinical evidence and health utilities were not identified using systematic review methods. These issues are discussed further in Critical appraisal of the economic analysis presented by Sanofi.
Model verification
The AG reproduced the deterministic version of the company’s DICE model using a simple partitioned survival approach implemented in Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA). Table 47 in Appendix 3 compares the results generated by the company’s submitted model and the AG’s double-programmed model [including corrections detailed in critical appraisal point 6 (see Box 3)]. As shown in the table, the results generated by the two models are very similar. The AG is confident that the model has been implemented by the company as intended.
Summary of main issues identified within the critical appraisal
Box 3 presents a summary of the main issues surrounding the company’s health economic analysis. These issues are discussed in further detail in the following sections.
-
Relevance of the restricted EU-label population.
-
Failure to adjust for continued vandetanib use, and BSC switching to vandetanib, post progression.
-
Likely overestimation of costs of vandetanib use in post-progression state.
-
Questionable implementation of the vandetanib discontinuation parameter.
-
Robustness of covariate-adjusted survival modelling to reflect the restricted EU-label population.
-
Technical programming errors.
-
Concerns regarding health utility parameters.
-
Limited exploration of uncertainty surrounding survivor functions.
-
Concerns regarding costings.
Relevance of the restricted EU-label population
The company’s health economic analysis is limited to the restricted EU-label population, based on the argument that this reflects the current use of vandetanib in clinical practice in England. In response to a request for clarification from the AG, the company stated that:
In developing the submission, we consulted with two UK clinical experts to discuss management of MTC in practice. Factors which determined the need for systemic treatment were speed of progression, tumour burden/size and symptoms. CTN/CEA doubling are known markers of poor prognosis and more aggressive disease. Sanofi Genzyme re-analysed the ZETA trial population and considered the patients who were symptomatic, had progressed within 12 months and with CTN/CEA doubling < 24 months most closely reflected UK clinical expert opinion. This approach is within the intent of the EU label where benefit outweighs the risk by using local clinical approaches to identify those most in need of treatment.
Reproduced with permission from Sanofi Genzyme, response to clarification questions,73 question A3
However, clinical advisors to the AG disagree with this assertion and instead suggest that in clinical practice vandetanib is used in patients with symptomatic and progressive disease irrespective of CEA/CTN biomarker levels. The clinical advisors also noted that CTN is an unstable measure and that the presence of disease progression (which is likely to also be accompanied by symptomatic disease) is more useful for informing treatment decisions. The advisors further noted that, although CEA and CTN are routinely measured in patients with MTC, these biomarkers are typically used to monitor patients while they are receiving treatment (to assess whether or not treatment is working), rather than to determine whether or not treatment should be initiated. The clinical advisors also noted that patients with symptomatic and progressive disease are also likely to have CEA/CTN doubling times of ≤ 24 months. As noted previously, the CS does not contain a health economic analysis of vandetanib within the broader population indicated by its marketing authorisation. 22 The clinical advisors did, however, agree that the company’s interpretation of what constitutes ‘progressive and symptomatic’ disease (see Chapter 3) is clinically appropriate.
Failure to adjust for continued vandetanib use and best supportive care switching to vandetanib post progression
The Sanofi CS states that although attempts were made to account for treatment switching in the ZETA trial using the RPSFT method, these were reported to have been unsuccessful. In response to a request for clarification, the company stated that:
RPSFT failed to undo bias as the method looks for the effect sizes needed so that the two survival curves match if they are given the same treatment, if the curves never separate, or don’t separate enough because crossover happens too early or before sufficient events occur in placebo (as was the case in ZETA), the curves will match up with effects very close to the null. This was the result obtained in the analyses.
Reproduced with permission from Sanofi Genzyme, response to clarification questions,73 question A2
Based on the company’s description, it seems likely that the RPSFT model did work as it would be expected to given its assumptions, but the company describes the approach as failing as it showed a null treatment effect. The company’s clarification response also provides further details regarding other treatment-switching adjustment methods considered by the company (the iterative parameter estimation, inverse probability-of-censoring weights and two-stage methods); however, these methods were not implemented. Consequently, the OS data for the BSC group remain subject to potential confounding because of treatment switching. Clinical advisors to the AG noted that the continued use of vandetanib beyond disease progression does not reflect usual clinical practice in England; hence, the survival outcomes observed in the intervention group reflect an atypical treatment pathway. One clinical advisor suggested that if imaging showed a mixed response with the largest or most symptomatic/problematic lesions being stable and some other lesions progressing, vandetanib may still be continued; however, the advisor did note that this scenario is uncommon. Consequently, the AG’s notes that the results generated by the company’s model may not be meaningful for the purposes of decision-making.
Likely overestimation of costs of vandetanib use in post-progression state
The company’s model includes a single progression event that corresponds to the partition between the progression-free and post-progression health states. As a result, patients who receive vandetanib post progression in either the intervention or the comparator group are assumed to continue to do so until death. In reality, these patients could experience a second progression event prior to death and such progression would be likely to trigger a clinical decision to discontinue vandetanib. This is not reflected in the company’s model. The AG accepts that, owing to the failure of the treatment-switching adjustment attempts, it is reasonable to include the costs of the drug in both groups; however, assuming that all post-progression treatment continues indefinitely will probably lead to the overestimation of the costs of vandetanib in both groups. This bias strongly favours the intervention group, as a considerably higher proportion of patients receive vandetanib post progression in the BSC group than in the intervention group [proportion of patients on treatment post progression: BSC (confidential information has been removed) vs. vandetanib (confidential information has been removed); post-progression drug costs: BSC £106,331 vs. vandetanib £68,490]. Removing the costs of vandetanib received post progression in both groups increases the deterministic ICER from £31,731 per QALY gained to £59,740 per QALY gained. This same concern also applies to the pairwise comparisons of vandetanib versus BSC undertaken using the AG model.
Questionable implementation of the vandetanib discontinuation parameter
Although the company’s model includes dose reductions (including treatment interruptions) for participants receiving vandetanib in both groups as per the ZETA trial (see Table 43 in Appendix 3), a further discontinuation parameter is also applied, but only to those participants in the vandetanib group during the progression-free phase. This parameter is applied as a fixed proportion of participants who incur no vandetanib costs (confidential information has been removed) during any model cycle, whilst participants in the intervention group are progression free. As a consequence of this parameter, together with the long post-progression phase (see critical appraisal point 3), the pre-progression vandetanib acquisition costs in the intervention group are lower than the post-progression vandetanib costs in the BSC group (vandetanib pre-progression drug costs, £75,767; BSC post-progression drug costs, £106,331). This lacks face validity and it is unclear whether or not the company’s omission of this parameter from post-progression cost calculations was intentional. Setting this parameter equal to zero increases the ICER from £31,731 to £57,266 per QALY gained.
Robustness of covariate-adjusted survival modelling to reflect the restricted EU-label population
The Sanofi CS66 (p. 57) states that ‘it was not possible to fit a parametric regression model to the observed K–M data . . . due to relatively sparse data in the restricted population producing K–M curves with long steps would lead to inaccurate estimates of the median survival function when extrapolated for the economic model’. Instead, the company used the ITT and safety data sets for PFS and OS, respectively, and fitted curves including covariates for symptomatic and progressive disease and for the CEA/CTN biomarker. The AG considers that it would have been more appropriate to fit parametric functions directly to the data relating to the population of interest [the restricted EU-label population, vandetanib group (confidential information has been removed), placebo group (confidential information has been removed)] as these are the most relevant data available to estimate PFS and OS in this subgroup. Although the CS explains that the Kaplan–Meier curves feature large steps between events because of the small sample size, it is not clear that this would lead to more inaccurate estimates of median survival in the restricted EU-label population than those produced by fitting a covariate-adjusted model to the broader EU-label population. It should be noted that the model fit statistics (AIC/BIC) presented by the company reflect how well each parametric model with covariates fits the data observed for the entire ITT/safety population, and so the model with lowest AIC/BIC does not necessarily indicate the best fit to the population of interest.
The AG has further concerns regarding the company’s interpretation of their covariate-adjusted survival modelling. Figure 9 of the Sanofi CS66 presents a comparison of the covariate-adjusted Weibull OS model against the empirical Kaplan–Meier curves from the ZETA trial (see Figure 17 in Appendix 3) and states that:
These parameterised curves appear to underestimate the benefit of vandetanib in the CTN/CEA doubling population from the ITT dataset (figure 7), even without undoing crossover. There is uncertainty regarding how well this function would fit the ‘true’ survival curves in the CTN/CEA doubling population from the EU label dataset with cross over undone.
Sanofi CS,66 figure 9 (p. 59)
However, the comparison of predicted and observed OS probabilities represented in this comparison relates to two different populations: the covariate-adjusted Weibull model relates to the restricted EU-label population, whereas the observed Kaplan–Meier curves relate to the ZETA trial ITT population with CEA and CTN doubling times of ≤ 24 months (excluding the progressive population characteristics). Figure 19 in Appendix 3 shows the company’s Weibull OS model fitted against the relevant Kaplan–Meier curve for the restricted EU-label subgroup (plotted by the AG). As shown in the figure, the company’s Weibull model does not provide a good visual fit to either the vandetanib or the BSC group data.
Technical programming errors
According to the CS66 (p. 107), the disutility for AEs was intended to be applied during the first cycle only (1-month duration). However, the DICE event used to calculate disutilities in each group does not include a time adjustment; hence, this disutility is applied to the whole first year of the model. This reflects a programming error that exaggerates the QALY loss in both groups; given that the incidence of AEs is higher for vandetanib, the error produces a small bias in favour of the BSC comparator group. This issue was later corrected by the company in its clarification response73 (question A18). During the appraisal process, the company also highlighted a further error relating to the vandetanib discontinuation parameter; this was originally reported to be (confidential information has been removed) but was later corrected to (confidential information has been removed). Correcting these two errors reduces the company’s original deterministic ICER from £40,363 to £31,731 per QALY gained.
The AG also notes that the model does not include any adjustment for logical inconsistency (i.e. when the cumulative survival probability for PFS is greater than that for OS at a given time point). This does not affect the company’s deterministic base-case Weibull functions for OS and PFS. However, this issue is evident in scenarios in which other parametric functions are used (e.g. if the log-normal function is used for PFS and the Weibull function is used for OS). This leads to a situation whereby the health state population of the post-progression state becomes negative (see Table 48 in Appendix 3). This issue could have been resolved by conditioning the PFS survivor function to be equal to or lower than the OS survivor function.
Concerns regarding health utility parameters
The CS does not include details of a systematic review of utility estimates in MTC or other types of thyroid cancer. The means through which the company identified the Beusterien et al. 105 study, which is used to inform the post-progression utility multiplier and the disutility for grade 3/4 AEs, are unclear from the Sanofi CS. 66 The AG also notes that the Beusterien et al. 105 study relates to advanced melanoma health states, hence its relevance to MTC is unclear. Although the Sanofi CS66 (p. 114) states that there are ‘insufficient data available for alternative estimates’, such statements are difficult to qualify without undertaking a formal systematic review of the available evidence. However, as shown in the company’s DSAs, these parameters do not have a marked impact on the cost-effectiveness of vandetanib within the restricted EU-label population (see Figure 10).
Limited exploration of uncertainty surrounding survivor functions
The CS includes only limited consideration of uncertainty surrounding the range of potentially plausible survivor functions for PFS or OS. Although a number of parametric functions were fitted to the available data for PFS and OS, only the impact of the log-logistic and log-normal functions for both PFS and OS (together) were explored within the company’s DSAs (see Sanofi model results). It should also be noted that although the company’s executable model includes the parameters for five alternative survivor functions, only the Weibull, log-logistic and log-normal curves can be selected as options. The reasons for this are unclear.
Concerns regarding costings
Clinical advisors to the AG noted several concerns regarding the company’s cost assumptions:
-
Monitoring costs. Although the company’s model includes the costs associated with ECGs to monitor patients while receiving vandetanib, these costs should also include consultant-/nurse-led outpatient appointments (typically at a frequency of around 12 consultant-led visits and four nurse-led visits per year).
-
Best supportive care costs in post-progression state. The company’s assumption of 36.5 outpatient appointments per year (one appointment every 10 days) while patients are receiving BSC is unrealistically high. Clinical advisors to the AG suggested that a more reasonable estimate would be around six appointments per year.
-
Costs of managing AEs. Clinical advisors to the AG believe that the costs of managing some of the grade 3/4 events included in the company’s model are implausibly high. As noted in Evidence used to inform the company’s model, the unit costs assumed for these events all assume that the episode is elective, which is, by definition, incorrect. The clinical advisors suggested that the incidence of prolonged QT interval, hypertension, decreased appetite and rash would most likely be managed by discontinuing vandetanib. Hypertension would probably require the prescription of antihypertensive drugs.
Discussion of existing evidence relating to the cost-effectiveness of cabozantinib and vandetanib for the treatment of locally advanced or metastatic medullary thyroid cancer
The systematic review of existing economic evaluations did not identify any relevant published studies. The manufacturer of cabozantinib did not submit any economic evidence relating to this product. The manufacturer of vandetanib (Sanofi) submitted a de novo model-based health economic evaluation of vandetanib versus BSC in the restricted EU-label population (patients with symptomatic and progressive disease with CEA/CTN doubling times of ≤ 24 months). An economic analysis for the broader licensed population was not presented. The corrected version of the company’s submitted model suggests that the probabilistic ICER for vandetanib versus BSC is approximately £31,546 per QALY gained. The AG notes several concerns relating to the company’s submitted model, in particular (1) the questionable relevance of the restricted EU-label population to current clinical practice, (2) the failure to adjust for open-label vandetanib use in both treatment groups, (3) the likely overestimation of the costs of vandetanib use in the post-progression state, (4) questionable assumptions regarding the amount of vandetanib received and (5) concerns regarding the robustness of the company’s covariate-adjusted survival modelling to reflect the restricted EU-label population. The AG considers it probable that the ICER for vandetanib is considerably higher than the estimates presented within the Sanofi CS.
Independent assessment group model
Model scope
The scope of the AG’s analysis is summarised in Table 19. The AG’s analyses are presented across two populations of patients with locally advanced or metastatic MTC: (1) patients with progressive and symptomatic disease (the EU-label population for vandetanib) and (2) the restricted EU-label population for vandetanib. Within the broader symptomatic and progressive population, pairwise economic comparisons are made for cabozantinib versus BSC based on the ITT population of the EXAM trial28 (AG analysis 1) and for vandetanib versus BSC based on the post hoc EU-label (symptomatic and progressive) subgroup of the ZETA trial66,78 (AG analysis 2). It should be noted that these analyses are limited in that they do not include all relevant treatment options. As the AG did not have access to the underlying IPD (including data on relevant covariates) from the ZETA trial, it was not possible to implement statistical adjustments to account for open-label vandetanib use in either treatment group, or to adjust for other potential baseline imbalances in the subgroup. Consequently, the comparison of vandetanib versus BSC is subject to potential confounding. To provide more meaningful estimates of the cost-effectiveness of vandetanib and cabozantinib, two sets of fully incremental analyses of all options are also presented. The first of these (AG analysis 3) uses the EXAM trial data for cabozantinib and BSC and applies the PFS treatment effect for vandetanib versus placebo from the ZETA trial EU-label subgroup to the EXAM trial placebo group baseline; OS is assumed to be the same for both TKIs (equivalent to the cabozantinib arm in the EXAM trial). The second incremental analysis (AG analysis 4) assumes that PFS and OS outcomes for vandetanib are equivalent to those for cabozantinib. Although these incremental analyses necessarily reflect potentially strong assumptions concerning transferable/equivalent treatment effects between vandetanib and cabozantinib, they are not subject to potential confounding caused by post-progression vandetanib use within the clinical data. A further pairwise comparison (AG analysis 5) that evaluates vandetanib versus BSC within the restricted EU-label population (patients with symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months) is also presented as equivalent covariate data were not available from the EXAM trial, cabozantinib could not be included within this analysis. Across all five sets of analyses, cost-effectiveness is evaluated in terms of the incremental cost per QALY gained from the perspective of the NHS and Personal Social Services (PSS) over a 20-year (lifetime) horizon. Costs and health outcomes were discounted at a rate of 3.5% per annum. 109 Costs were valued at 2016/17 prices.
| Model scope | Population | |
|---|---|---|
| EU-label: symptomatic and progressive MTC | Restricted EU-label: symptomatic and progressive MTC with CEA/CTN doubling time of ≤ 24 months | |
| Intervention(s) | Vandetanib | Vandetanib |
| Cabozantinib | ||
| Comparator | BSC | BSC |
| Outcomes | Incremental cost per QALY gained | Incremental cost per QALY gained |
| Economic comparisons | AG analysis 1: pairwise economic evaluation of cabozantinib vs. BSC in the EXAM trial ITT population | AG analysis 5: pairwise economic evaluation of vandetanib vs. BSC in the ZETA trial restricted EU-label population |
| AG analysis 2: pairwise economic evaluation of vandetanib vs. BSC in the ZETA trial EU-label population | ||
| AG analysis 3: fully incremental analysis based on EXAM trial ITT population with vandetanib PFS treatment effect applied to EXAM trial placebo baseline; vandetanib OS assumed to be equivalent to cabozantinib OS | ||
| AG analysis 4: fully incremental analysis based on EXAM trial ITT population assuming PFS and OS are equivalent for vandetanib and cabozantinib | ||
| Perspective | NHS and PSSa | NHS and PSSa |
| Time horizon | 20 years | 20 years |
| Cycle length | 1 month | 1 month |
| Discount rate | 3.5% for health outcomes and costs | 3.5% for health outcomes and costs |
Model structure
The structure of the AG model is presented in Figure 11. As shown in the diagram, the AG model structure is broadly similar to that adopted within the Sanofi model (see Sanofi model structure). The AG model adopts a partitioned survival approach, based on three health states: (1) progression free, (2) post progression and (3) dead. For any time, t, the probability that a patient is alive and progression free is given by the cumulative survival probability for PFS, whereas the probability that a patient is alive is given by the cumulative survival probability for OS. The probability that a patient is in the post-progression state at any time t is given by the difference between the cumulative survival probabilities for PFS and OS. The model includes an adjustment for logical inconsistency, whereby, if the probability of PFS is greater than that of OS, PFS is constrained to reflect the lower OS probability. As with the Sanofi model, HRQoL is defined according to the presence or absence of disease progression and a separate QALY loss is applied to account for the incidence of grade 3/4 AEs during the first model cycle. The model includes costs associated with drug acquisition, health-state costs incurred while receiving cabozantinib and vandetanib [consultant-led outpatient visits, nurse-led outpatient visits, ECG, blood tests and computerised tomography (CT) scans], costs associated with managing grade 3/4 AEs, BSC-related costs [consultant-led outpatient visits, CT scans, magnetic resonance imaging (MRI) scans, specialist palliative care visits, palliative radiotherapy, palliative surgery and bisphosphonates for bone metastases] and end-of-life care costs.
FIGURE 11.
The AG model structure. N/A, not applicable. a, Applies only to patients not receiving vandetanib/cabozantinib. b, Applies only to open-label vandetanib costs within pairwise comparisons of vandetanib vs. BSC.
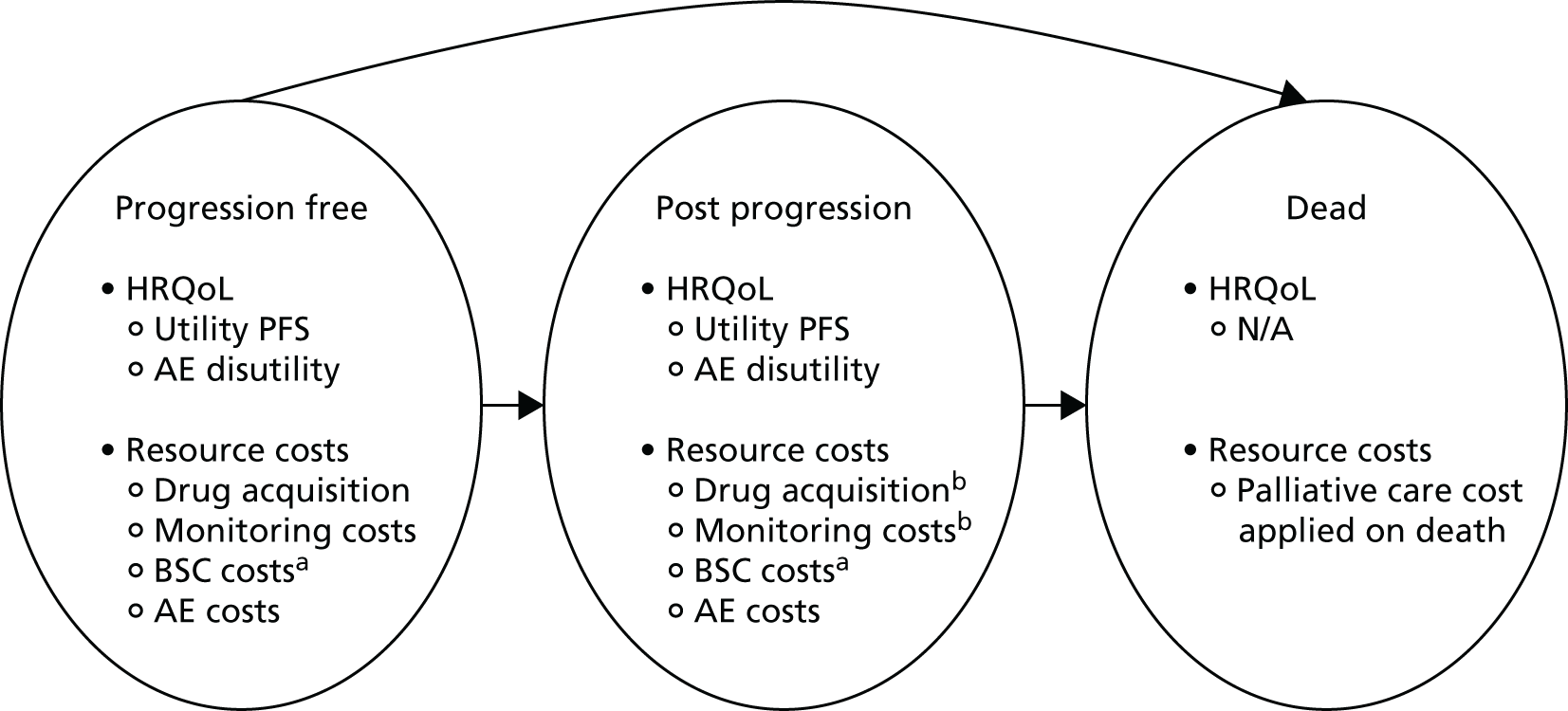
The model employs the following structural assumptions:
-
Health-related quality of life is assumed to be determined according to the presence/absence of disease progression and the incidence of grade 3/4 AEs.
-
The model includes an adjustment to account for logical inconsistencies [i.e. when S(t)PFS>S(t)OS].
-
In the pairwise comparisons of vandetanib versus BSC (see Table 19, AG analyses 2 and 5), the modelling of costs and health outcomes includes the level of treatment switching and continued vandetanib use post progression observed in the ZETA trial subgroups. This was included as the company’s attempts to adjust for treatment switching and treatment continuation post progression were reported to have been unsuccessful (see Critical appraisal of the economic analysis presented by Sanofi).
-
Grade 3/4 AEs are assumed to affect both costs and HRQoL. Health losses resulting from AEs are assumed to be transient and resolved quickly: a QALY loss is applied during the first model cycle only (1-month duration).
-
As patients receiving BSC, by definition, have progressed disease, the costs associated with BSC are assumed to be the same in both the progression-free and post-progression states.
-
Health state resource use (including additional TKI monitoring requirements) incurred during the progression-free period are assumed to differ between the three treatment options.
-
Palliative care costs are incurred only during the final month of life.
Evidence used to inform the model’s parameters
Summary of evidence sources used to inform the assessment group model
Table 20 summarises the evidence sources used to inform the AG’s health economic model. These evidence sources are discussed in further detail in the subsequent sections.
| Parameter group | Evidence source |
|---|---|
| PFS | Pairwise comparisons of TKI vs. BSC (AG analyses 1, 2 and 5)
|
Incremental comparison of all options including a differential PFS treatment effect between vandetanib and cabozantinib (AG analysis 3)
|
|
Incremental comparison of all options assuming equivalent effectiveness for TKIs (AG analysis 4)
|
|
| OS | Pairwise comparisons of TKI vs. BSC (AG analyses 1, 2 and 5)
|
Incremental comparisons of all options (AG analyses 3 and 4)
|
|
| Health utilities | Progression-free and post-progression health states
|
Disutility due to AEs
|
|
| Time spent receiving vandetanib | Based on the proportion of PFS time spent on each dose level (or interrupted treatment) for relevant subgroup in the ZETA trial.66,73,78 Vandetanib dose distribution also applied to post-progression vandetanib use (in AG analyses 2 and 5 only). Includes vandetanib pre-progression discontinuation parameter in both progression-free and post-progression states |
| Time spent receiving cabozantinib | Based on the proportion of PFS time spent on each dose level (or interrupted treatment) within the EXAM trial28 |
| Probability of receiving vandetanib while in post-progression state | Treatment switching/continuation proportions observed in relevant subgroups of the ZETA trial.66,73 Vandetanib dose distribution also applied to post-progression use |
| Drug acquisition costs | BNF108 |
| AE incidence | Derived from EXAM and ZETA trial publications27,28 |
| Health state resource use | Personal communication: Dr Jon Wadsley (Weston Park Hospital, Sheffield, 2017) and Dr Laura Moss (Velindre Cancer Centre, Cardiff, 2017) |
| BSC resource use | Personal communication: Dr Jon Wadsley and Dr Laura Moss |
| Health state unit costs | NHS Reference Costs 2015/16 106 |
| AE management costs | NHS Reference Costs 2015/16.106 Weighted mean of all non-elective excess bed-days |
| BSC costs | NHS Reference Costs 2015/16 106 |
| Palliative care and palliative chemotherapy costs | NHS Reference Costs 2015/16,106 and Curtis and Burns107 |
Time-to-event analysis using individual patient data
Table 21 summarises the use of the time-to-event data from the ZETA and EXAM trials within the AG model.
| Outcome | Population | ||||
|---|---|---|---|---|---|
| EU-label: symptomatic and progressive MTC | Restricted EU-label: symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months | ||||
| AG analysis 1: cabozantinib vs. BSC (pairwise) | AG analysis 2: vandetanib vs. BSC (pairwise) | AG analysis 3: all options – vandetanib PFS treatment effect from joint model | AG analysis 4: all options – cabozantinib and vandetanib equivalent | AG analysis 5: vandetanib vs. BSC (pairwise) | |
| PFS | |||||
| Cabozantinib PFS | Cabozantinib arm, EXAM ITT | N/A | Cabozantinib arm, EXAM ITT | Cabozantinib arm, EXAM ITT | N/A |
| Vandetanib PFS | N/A | Vandetanib arm, ZETA EU label | Treatment effect from ZETA EU label applied to EXAM placebo arm | Assumed same as cabozantinib arm, EXAM ITT | Vandetanib arm, ZETA restricted EU label |
| BSC PFS | Placebo arm, EXAM ITT | Placebo arm, ZETA EU label | Placebo arm, EXAM ITT | Placebo arm, EXAM ITT | Placebo arm, ZETA restricted EU label |
| OS | |||||
| Cabozantinib OS | Cabozantinib arm, EXAM ITT | N/A | Cabozantinib arm, EXAM ITT | Cabozantinib arm, EXAM ITT | N/A |
| Vandetanib OS | N/A | Vandetanib arm, ZETA EU label | Assumed same as cabozantinib arm, EXAM ITT | Assumed same as cabozantinib arm, EXAM ITT | Vandetanib arm, ZETA restricted EU label |
| BSC OS | Placebo arm, EXAM ITT | Placebo arm, ZETA EU label | Placebo arm, EXAM ITT | Placebo arm, EXAM ITT | Placebo arm, ZETA restricted EU label |
| Treatment switching | |||||
| Includes switching/continued vandetanib costs? | N/A | Yes | No | No | Yes |
The comparison of cabozantinib with placebo was based on IPD relating to the full population of the EXAM trial (cabozantinib, n = 219; placebo, n = 111). These data were supplied by Ipsen for both PFS and OS. 112
The comparison of vandetanib with placebo was based on post hoc subgroups of participants enrolled in the ZETA trial: the EU-label population [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed) for PFS; placebo, n = (confidential information has been removed) for OS] and the restricted EU-label population [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed)]. Owing to concerns regarding the intellectual property rights of the patient-level data set, Sanofi was unable to provide the original IPD collected during the trial. Instead, Kaplan–Meier curves for each population and outcome were provided by Sanofi. 73 The supplied Kaplan–Meier curves were digitised using Engauge Digitizer113 and IPD were then reconstructed from the digitised curves using the algorithm reported by Guyot et al. 114 This method maps the digitised curves back to time-to-event data by finding numerical solutions to the inverted Kaplan–Meier equations. There are four variations on the method depending on the amount of information supplied. For both of the ZETA trial subgroups (EU label and restricted EU label) and outcomes (PFS and OS), both the number at risk tables and the total numbers of events were supplied by Sanofi, thereby allowing the most accurate variation of the algorithm to be used. In addition, as the sample sizes of the subgroups are fairly small and there are a small number of events that can be readily identified from the Kaplan–Meier survival curves, the resulting reconstructed IPD are likely to provide a good approximation of the original data set.
Methods for time-to-event analysis
For each data set, model selection was conducted following the process described in the NICE Decision Support Unit Technical Support Document No. 14. 115,116 Log-cumulative hazard plots were produced to assess the type of hazards observed in the trial to help inform which types of parametric function may be considered appropriate. For all analyses except for AG analysis 4, individual models were fitted to the data for each treatment group, thereby avoiding unnecessarily restrictive assumptions of proportional hazards or constant acceleration factors. The AIC and BIC were examined to assess the comparative internal validity of competing models. The final choice of models for the economic analysis was made on the basis of fit to the observed data as well as consideration of the clinical plausibility of competing candidate models, based on judgements elicited from one clinical expert (JW). The final model selections used to inform the health economic model are presented in this report (see Table 23).
To inform the fully incremental analyses of cabozantinib, vandetanib and BSC (AG analysis 3), a single parametric model with a covariate indicating treatment arm was considered for PFS in the EU-label population of the ZETA trial. As discussed in Chapter 3, Quantity and quality of research available and Justification for conducting a network meta-analysis, this population is considered to be broadly comparable to that of the EXAM trial. Fitting a combined model provides a treatment effect for vandetanib compared with placebo (either a HR or constant acceleration factor, depending on the parametric model). This can then be applied to the baseline model (taken to be the placebo arm in the EXAM trial) to approximate the absolute effect for a vandetanib treatment group in the chosen baseline population. The estimated HR from the NMA (see Chapter 3, Network meta-analysis) was not used as it is generally recommended that estimation of the treatment effects and baseline follows a consistent modelling procedure. 100 Furthermore, the use of HRs would not be appropriate for the accelerated failure time models as these do not make the assumption of proportional hazards.
Curve fitting was conducted in R using the flexsurv package. The muhaz package was used to estimate the empirical hazard function. Exponential, Weibull, Gompertz, log-normal, log-logistic, gamma and generalised gamma models were considered. The more flexible generalised F-distribution was also considered; however, for some of the analyses, the model-fitting algorithm failed to converge. In these cases, the AG considered that the generalised F-distribution model would not be appropriate. The goodness-of-fit information is provided for all considered models.
Cabozantinib versus best supportive care, EXAM trial intention-to-treat population (used in the assessment group’s analyses 1, 3 and 4)
Progression-free survival
The analysis of PFS for cabozantinib versus placebo was based on IPD from the full population of the EXAM trial (cabozantinib, n = 219; placebo, n = 111; see Figure 20 in Appendix 4) provided by Ipsen. Empirical diagnostic plots are provided in Figure 21 in Appendix 4. Visual inspection of the empirical hazard function plot indicates potentially different behaviours between the two treatment arms. Visual inspection of the log–log plot of cumulative survival versus time indicates that the exponential model may not be appropriate as the gradient is not close to 1.0; the remaining standard parametric models were deemed suitable for consideration.
Measures of comparative internal validity are presented in Table 49 in Appendix 4. Plots of the fitted models against the empirical Kaplan–Meier PFS curves are presented in Figures 22 (cabozantinib) and 23 (placebo) in Appendix 4. For the placebo arm, the log-logistic model provided the best fit to the observed data according to both the AIC and BIC (AIC = 308.71; BIC = 314.13), although the log-normal model also provided a good fit to the data (AIC = 311.48; BIC = 316.90). For the cabozantinib arm, the Weibull model provided the best fit according to both the AIC and BIC (AIC = 579.70; BIC = 586.48), although the BIC was similar for several models.
Overall survival
The analysis of OS for cabozantinib versus placebo was based on IPD from the full population of the EXAM trial (cabozantinib, n = 219; placebo, n = 111; see Figure 24 in Appendix 4) provided by Ipsen. Log-cumulative hazard plots are provided in Figure 25, Appendix 4. Visual inspection of the empirical hazard function indicates that the observed hazard is approximately constant for both trial arms, and visual inspection of the log–log plot of cumulative survival versus time indicates a gradient of approximately 1.0, suggesting that the exponential model may be appropriate in this case.
Measures of comparative internal validity are presented in Table 49 in Appendix 4. Plots of the fitted models against the empirical Kaplan–Meier OS curves are presented in Figures 26 (cabozantinib) and Figure 27 (placebo) in Appendix 4. Based on AIC and BIC statistics for the placebo arm, the log-logistic and exponential models provided the best fit (log-logistic AIC = 708.31, BIC = 713.73; exponential AIC = 709.58, BIC = 712.29). Findings were similar for the cabozantinib arm: the log-logistic model provided the best fit to the observed data based on the AIC (1343.69) and the exponential model provided the best fit based on the BIC (1348.42).
Vandetanib versus best supportive care, ZETA trial EU-label population (used in the assessment group’s analysis 2)
Progression-free survival
The analysis of PFS for vandetanib versus placebo was based on Kaplan–Meier curves for the EU-label population of the ZETA trial [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed)]. The Kaplan–Meier curves provided by Sanofi73 are presented in Figure 28 in Appendix 4. The number of observed events was (confidential information has been removed) in the vandetanib arm and (confidential information has been removed) in the placebo arm (Sanofi CS appendices,78 table 5, p. 51). The replicated Kaplan–Meier curves appear consistent with the reported data (see Figure 29 in Appendix 4): the replicated median PFS time of (confidential information has been removed) months for placebo is close to the value reported from the observed data (median 16.4, n = 60 from Kriessl et al. 57). Median PFS was not reached for the vandetanib arm.
Log cumulative hazard plots are presented in Figure 30 in Appendix 4. Visual inspection of the empirical hazard function indicates that the observed hazard is approximately constant for both trial arms, and visual inspection of the log–log plot of cumulative survival versus time indicates a gradient of approximately 1.0 for the placebo arm, thereby suggesting that the exponential model may be an appropriate model choice.
Measures of comparative internal validity are presented in Table 50 in Appendix 4. Plots of the fitted models against the empirical PFS data are presented in Figures 31 (vandetanib) and 32 (placebo) in Appendix 4. For the placebo arm, the exponential model provided the best fit to the observed data based on both the AIC and the BIC (AIC = 296.49, BIC = 298.58). For the vandetanib arm, the gamma model provided the best fit to the observed data based on both AIC and BIC (AIC = 467.93, BIC = 473.66); however, differences in the goodness-of-fit statistics across models were generally small, giving little justification to discriminate between models on this basis.
Overall survival
The analysis of OS for vandetanib was based on Kaplan–Meier curves for the EU-label population of the ZETA trial [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed)]. The Kaplan–Meier curves provided by the company are shown in Figure 33 in Appendix 4; the number of events observed was (confidential information has been removed) in the vandetanib arm and (confidential information has been removed) in the placebo arm (Sanofi CS appendices,78 table 7, p. 53). The replicated Kaplan–Meier curves appear consistent with the reported data (see Figure 34 in Appendix 4): the replicated median OS times of (confidential information has been removed) months for placebo and (confidential information has been removed) months for vandetanib are close to the estimates reported from the observed data (placebo median = (confidential information has been removed); vandetanib median = (confidential information has been removed), from Kreissl et al. 57).
Log-cumulative hazard plots are provided in Figure 35 in Appendix 4. Visual inspection of the empirical hazard function indicates that the observed hazard is approximately constant for both trial arms, and visual inspection of the log–log plot of cumulative survival versus time indicates a gradient of approximately 1.0 for both treatment models, thereby suggesting that the exponential model may be appropriate.
Measures of comparative internal validity are presented in Table 50 in Appendix 4. Plots of the fitted models against the empirical Kaplan–Meier OS curves are presented in Figures 36 (vandetanib) and 37 (placebo) in Appendix 4. For the placebo arm, the exponential model provided the best fit to the observed data (AIC = 421.65, BIC = 423.73). For the vandetanib arm, the log-normal model provided the best fit to the observed data (AIC = 847.27, BIC = 853.01); however, differences in the AIC and BIC were generally small, thereby giving little justification to discriminate between models on this basis.
Vandetanib versus best supportive care, restricted EU-label population, ZETA trial (used in the assessment group’s analysis 5)
Progression-free survival
The analysis of PFS for vandetanib versus placebo was based on Kaplan–Meier curves for the restricted EU-label population of the ZETA trial [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed)]. The Kaplan–Meier curves provided by Sanofi are shown in Figure 38 in Appendix 4; the number of progression events observed was (confidential information has been removed) in the vandetanib arm and (confidential information has been removed) in the placebo arm. The replicated Kaplan–Meier curves appear to be consistent with the reported data (see Figure 39 in Appendix 4): the replicated median PFS times of (confidential information has been removed) months for the placebo arm and (confidential information has been removed) months for the vandetanib arm are close to the estimates reported from the observed data [placebo median = (confidential information has been removed) months, vandetanib median = (confidential information has been removed) months (from the Sanofi CS,78 appendix 6)].
Log-cumulative hazard plots are presented in Figure 40 in Appendix 4. Measures of comparative internal validity are presented in Table 51 in Appendix 4. Plots of the fitted models against the empirical Kaplan–Meier PFS curves are presented in Figures 41 (vandetanib) and 42 (placebo) in Appendix 4. For the placebo arm, the log-logistic model provided the best fit to the observed data based on the AIC (89.55), whereas the exponential model provided the best fit based on the BIC (90.54). For the vandetanib arm, the log-normal model provided the best fit based on the AIC (132.60), whereas the exponential model provided the best fit based on the BIC (134.30); however, differences in the AIC and BIC statistics were generally small, thereby giving little justification to discriminate between models on this basis.
Overall survival
The analysis of OS for vandetanib was based on Kaplan–Meier curves for the restricted EU-label population within the ZETA trial [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed)]. The Kaplan–Meier curves provided by Sanofi are shown in Figure 43 in Appendix 4; the number of progression events observed was (confidential information has been removed) in the vandetanib arm and (confidential information has been removed) in the placebo arm. The replicated Kaplan–Meier curves appear consistent with the reported estimates (see Figure 44 in Appendix 4): the median PFS times of (confidential information has been removed) months for placebo and (confidential information has been removed) months for vandetanib are close to the estimates reported from the observed data (placebo median = (confidential information has been removed) months, vandetanib median = (confidential information has been removed) months, from the Sanofi CS,78 appendix 6).
Log-cumulative hazard plots are provided in Figure 45 in Appendix 4. Measures of comparative internal validity are presented in Table 51 in Appendix 4. Plots of the fitted models against the empirical Kaplan–Meier OS curves are presented in Figures 46 (vandetanib) and 47 (placebo) in Appendix 4. For the placebo arm, the Gompertz model provided the best fit to the observed data based on both the AIC and BIC (AIC = 150.44, BIC = 152.11). For the vandetanib arm, the exponential model provided the best fit to the observed data based on both the AIC and the BIC (AIC = 212.75, BIC = 214.21).
Combined model used to estimate progression-free survival treatment effect for vandetanib and best supportive care (used in assessment group analysis 3)
The analysis of PFS for vandetanib versus placebo, used to inform AG analysis 3, utilised the Kaplan–Meier curves for the ZETA trial EU-label population [vandetanib, n = (confidential information has been removed); placebo, n = (confidential information has been removed)]; these curves were provided by Sanofi and reconstructed by the AG as described in the previous sections.
Visual inspection of the log–log plot of cumulative survival versus time suggests that the proportional hazards assumption may be considered valid for the observed period, and the use of a single model with a covariate indicating treatment group is therefore appropriate.
Measures of comparative internal validity are presented in Table 52 in Appendix 4. The log-normal model provided the best fit to the observed data based on both the AIC and BIC (AIC = 764.25, BIC = 773.99). Figure 48 in Appendix 4 presents plots of the reconstructed survival data for both the placebo and vandetanib groups.
Within the health economic model, the treatment effect covariate (shown in Table 52 in Appendix 4) is applied to the baseline model (taken to be the placebo arm in the EXAM trial ITT population) in order to approximate the absolute effect for a vandetanib treatment group in the chosen baseline population.
For parametric models in the proportional hazards family (exponential, Weibull, Gompertz), the estimated treatment effect represents a HR. For parametric models in the accelerated failure time family (log-normal, log-logistic, gamma, generalised gamma and generalised F), the estimated treatment effect represents an acceleration factor. These parameters are applied to the survivor function of the baseline proportional hazards/accelerated failure time model as follows.
Proportional hazards models
Given a survivor function for the placebo arm, Sp(t), and a HR, r, for treatment (vandetanib) compared with placebo, the survivor function for the vandetanib arm, SV(t), is obtained using:
Further detail can be found in Collett. 117
Accelerated failure time models
Given an acceleration factor of θ in the treatment arm (vandetanib) compared with placebo, the survivor function for the vandetanib arm is given by:
where θ = exp(–βx) and β is the coefficient on the analysis scale. Applying the coefficients presented in Appendix 4, Table 52, we have:
If θ > 1, then events in the treatment arm happen more quickly than in the control arm (assuming a negative outcome, this favours the control). If θ < 1, then events in the treatment arm happen less quickly than in the control arm (assuming a negative outcome, this favours the treatment).
Model selection
The clinical plausibility of the competing survivor functions for each analysis was assessed using clinical opinion. Clinical advisors were asked to select their preferred model(s) on the basis of visual fit to the data within the observed trial period and the clinical plausibility of the extrapolated portion of each curve. Clinicians were allowed to select more than one preferred model and were asked to provide justification for their preferences. The responses from the first clinical advisor are presented in Table 22. The second clinical advisor felt unable to complete the model selection exercise. The AG’s selected base-case survivor functions for each analysis are presented in Table 23.
| Population | Advisor number 1 (JW) | |
|---|---|---|
| Preferred curve | Justificationa | |
| EU-label population: symptomatic and progressive MTC | ||
| EXAM trial ITT, PFS, cabozantinib | Log-logistic | There is a tail to account for small proportion of patients with extended PFS but best fit at earlier time points |
| EXAM trial ITT, PFS, placebo | Log-logistic | Appears to most closely fit observed data |
| EXAM trial ITT, OS, cabozantinib | Log-logistic or log-normal | Good fit with observed data at early time points and both allow for a small proportion of long-term survivors |
| EXAM trial ITT, OS, placebo | Gompertz, log-logistic or log-normal | All have good fit at early time points and allow for possibility of long-term survival for a small number of patients |
| ZETA trial EU label, PFS, vandetanib | Log-logistic | Good fit at early time points and allows for a small proportion of long-term PFS patients |
| ZETA trial EU label, PFS, placebo | Log-logistic, log-normal, Gompertz | Good fit at early time points and allow for small proportion of patients without progression at later time points |
| ZETA trial EU label, OS, vandetanib | Log-normal or log-logistic | Appears to give best fit to early data |
| ZETA trial EU label, OS, placebo | Log-logistic | Good fit with early data and allows for a small proportion of long-term survivors |
| Restricted EU-label population: symptomatic and progressive MTC with CEA/CTN doubling time of ≤ 24 months | ||
| ZETA trial EU label, PFS, vandetanib | Log-logistic, log-normal and Gompertz | Allow for a small but realistic proportion of long-term survivors – too many long-term PF patients with exponential model |
| ZETA trial EU label, PFS, placebo | Log-normal, log-logistic, Gompertz | Close fit to early data and realistic, small number of longer-term PF survivors |
| ZETA trial EU label, OS, vandetanib | Log-logistic, log-normal, Gompertz | Good fit with early data and realistic number of longer-term survivors |
| ZETA trial EU label, OS, placebo | Gompertz | Closest fit to early data and realistic upper limit of 100 months OS for this poor-prognosis group |
| Population | Selected curve | Justification |
|---|---|---|
| Cabozantinib vs. BSC, EXAM trial ITT population (used in AG analyses 1, 3 and 4) | ||
| EXAM trial ITT, PFS, cabozantinib | Log-logistic | Selected based on clinical justification of long-term survivors. The AIC and BIC for the log-logistic function are higher than the best-fitting model (Weibull). It should be noted that outcomes predicted by the log-logistic function are more favourable than those of the Weibull model |
| EXAM trial ITT, PFS, placebo | Log-logistic | Selected based on clinical opinion and on the basis of consistency with the model used for the intervention group. There is a cluster of models that appear to provide a very similar visual fit to the data during the observed period of the trial. The log-logistic is also the best-fitting model in terms of the AIC and BIC |
| EXAM trial ITT, OS, cabozantinib | Log-logistic | Log-logistic and log-normal provide a similar fit. The log-logistic is the best-fitting model in terms of the AIC (the exponential provides the best fit based on the BIC) |
| EXAM trial ITT, OS, placebo | Log-logistic | Clinician’s selected models (log-logistic, Gompertz and log-normal) all provide a similar visual fit to the data. Log-logistic is the best-fitting model in terms of AIC and is consistent with the choice of model used for the intervention group |
| Vandetanib vs. BSC, ZETA trial, EU-label population (used in AG analysis 2) | ||
| ZETA trial EU label, PFS, vandetanib | Log-logistic | Reflects clinician’s choice, justified in terms of the proportion of long-term survivors. The gamma model gives the best fit in terms of both AIC and BIC but the log-logistic is very similar |
| ZETA trial EU label, PFS, placebo | Log-logistic | Clinicians’ choices (log-logistic, log-normal and Gompertz) are within a cluster of very similar models. The log-logistic model does not provide the best AIC or BIC (the best-fitting model is the exponential); however, the differences between the three candidate curves are small. Log-logistic was the model selected on basis of consistency with the intervention arm |
| ZETA trial EU label, OS, vandetanib | Log-logistic | Of the two candidate curves (log-logistic and log-normal), the log-normal model provides best fit to observed data. The log-logistic model was selected for consistency with the comparator arm and is very similar in terms of AIC/BIC |
| ZETA trial EU label, OS, placebo | Log-logistic | Reflects clinician’s choice, justified in terms of the proportion of long-term survivors |
| Vandetanib vs. BSC, ZETA trial, restricted EU-label population (used in AG analysis 5) | ||
| ZETA trial restricted EU label, PFS, vandetanib | Log-normal | Predicted outcomes are very similar for all three candidate models (log logistic, log normal and Gompertz). The log-normal model was selected because it had the lowest AIC among the competing candidate models |
| ZETA trial restricted EU label, PFS, placebo | Log-normal | Log-normal selected for consistency with the intervention arm, and very similar to log-logistic model in terms of AIC |
| ZETA trial restricted EU label, OS, vandetanib | Gompertz | Selected on basis of consistency with comparator arm |
| ZETA trial restricted EU label, OS, placebo | Gompertz | Models selected on basis of clinical justification (proportion of long-term survivors). Gompertz model has best AIC/BIC |
Health-related quality of life
The AG’s systematic searches for HRQoL evidence identified only one published study102 that reports health utilities for states of progression free and post progression in patients with thyroid cancer. In this study, the authors developed vignettes for seven health states based on the results of a previous qualitative study118 in differentiated thyroid cancer. These states included (1) stable/no response, (2) response (partial and complete), (3) progressive disease, (4) stable/no response with grade 3 diarrhoea, (5) stable/no response with grade 3 fatigue, (6) stable/no response with grade 3 HFS and (7) stable/no response with grades 1 and 2 alopecia. A total of 100 members of the UK general public participated in time trade-off (TTO) interviews to value the defined health states. Utility scores were estimated directly from the raw interview response data and using regression analyses. The results of the TTO valuations are presented in Table 24.
| Health state | Mean utility (observed, no adjustment) | 95% CI |
|---|---|---|
| Best state: stable/no response | 0.80 | 0.77 to 0.84 |
| Response to therapy | 0.86 | 0.83 to 0.89 |
| Progressive disease | 0.50 | 0.45 to 0.56 |
| Diarrhoea | 0.42 | 0.36 to 0.48 |
| Fatigue | 0.72 | 0.67 to 0.77 |
| HFS | 0.52 | 0.46 to 0.58 |
| Alopecia | 0.75 | 0.71 to 0.79 |
Owing to the lack of published evidence relating to the HRQoL associated with thyroid cancer states, the AG also explored the health utility values considered within previous thyroid cancer drug submissions to the SMC and the AWMSG. Table 25 summarises the health utilities assumed within these submissions.
| Body | Drug | Indication | Health utility values |
|---|---|---|---|
| SMC | Lenvatinib119 | Adult patients with progressive, locally advanced or metastatic, differentiated (papillary/follicular/Hürthle cell) thyroid carcinoma, refractory to radioactive iodine | Derived from Fordham et al.102 |
| Stable disease: 0.80 | |||
| Response: 0.86 | |||
| Progressive disease: 0.50 | |||
| Utility decrements of –0.042 for lenvatinib and –0.117 for sorafenib applied for AEs (diarrhoea, fatigue, HFS, alopecia) | |||
| SMC | Sorafenib120 | Patients with progressive, locally advanced or metastatic, differentiated thyroid carcinoma, refractory to radioactive iodine | Utilities derived from EQ-5D data from the DECISION study121 |
| Sorafenib, progression free: 0.72 | |||
| BSC, progression free: 0.80 | |||
| Post progression (both groups): 0.64 | |||
| SMC | Cabozantinib122 | Adult patients with progressive, unresectable locally advanced or metastatic MTC | Published trial data in thyroid cancer (not specified) in which SF-36 outcomes had been converted to utilities by mapping to EQ-5D and converting to SF-6D values for the non-progressed and progressed states |
| Progression free: 0.796 | |||
| Post progression: 0.624 | |||
| AWMSG | Vandetanib123 | Patients with aggressive and symptomatic unresectable locally advanced or metastatic MTC | FACT-G scores collected in the ZETA trial mapped to TTO values. Pre- and post-progression utility values not reported |
| Disutilities for AEs based on Beusterien et al.105 (values of −0.11 and −0.13 assumed) | |||
| AWMSG | Cabozantinib124 | Adult patients with progressive, unresectable, locally advanced or metastatic MTC | For the base-case analysis, utility values were taken from two published studies in thyroid cancer, albeit in patients with less severe disease than the progressive MTC population (sources and values not specified) |
| Utility decrements for AEs were derived from the published literature (also not specified) |
The health utilities assumed in the AG’s base-case analysis are summarised in Table 53 in Appendix 4. Health utilities associated with the absence/presence of disease progression were based on the study reported by Fordham et al. ,102 as this study specifically relates to thyroid cancer states, and health utilities were valued using a preference-based measure (TTO). 102 The disutility associated with grade 3/4 AEs was based on the lower value reported by Beusterien et al. 105 (disutility = –0.11). Uncertainty surrounding these parameters was modelled using beta distributions. Alternative utility values based on the cabozantinib122 and the sorafenib120 SMC submissions are explored within the sensitivity analyses.
Adverse event rates
The probability of experiencing grade 3/4 AEs was taken directly from the EXAM and ZETA trial publications (each based on the ITT study populations, see Table 54 in Appendix 4). 27,28 Within the incremental comparisons (AG analyses 3 and 4), the AE rates for the BSC group were assumed to reflect those observed in the placebo group of the EXAM trial. AEs were assumed to have a duration of 1 month.
Treatment switching/continuation parameters (assessment group analyses 2 and 5 only)
As noted in Scope of the Sanofi economic evaluation, Sanofi applied the RPSFT approach in an attempt to adjust for the high level of treatment switching that occurred within the ZETA trial. 66 However, the company’s attempts were reported to have been unsuccessful; hence, the available OS data for vandetanib that are used in the pairwise comparisons of vandetanib versus BSC in the symptomatic and progressive MTC population and the restricted EU-label MTC population remain subject to potential confounding (AG analyses 2 and 5). In order to allow for a fairer comparison, the AG included the costs associated with treatment switching and vandetanib continuation post progression in the pairwise analyses of vandetanib versus BSC. The number of participants who received vandetanib post progression in each arm of each subgroup of the ZETA trial was provided by Sanofi (see Table 55 in Appendix 4).
Resource use and costs
Drug acquisition
Table 56 in Appendix 4 presents the drug acquisition costs for cabozantinib and vandetanib based on their current list prices. 108 As shown in the table, the cost of cabozantinib is the same for all dose packs. Both vandetanib and cabozantinib have separate agreed PAS schemes. The results of the AG’s economic analysis including the PAS discounts for vandetanib and cabozantinib are presented in a separate confidential appendix to this report.
Time spent receiving cabozantinib and vandetanib
Table 57 in Appendix 4 presents the proportion of PFS time spent receiving each dose of cabozantinib within the EXAM trial. 112 Table 58 in Appendix 4 presents the proportion of PFS time spent receiving each dose of vandetanib within the ZETA trial subgroups. 66,73 As these data are multinomial in nature, uncertainty was modelled using a Dirichlet distribution with minimally informative priors.
The model also includes a further parameter to reflect those participants who discontinued vandetanib prior to disease progression [(confidential information has been removed) in the restricted EU-label population and 22.31% in the broader EU-label population]. Although these participants could have discontinued treatment at any time, assuming that they incur no drug costs (i.e. discontinued at day 0) is likely to bias the model in favour of vandetanib (see Critical appraisal of the economic analysis presented by Sanofi, critical appraisal point 4). In contrast to the assumption taken within the Sanofi model, the AG assumed that these participants incur half of the total cost of vandetanib during the progression-free phase (hence the discontinuation parameter was divided by 2). Uncertainty surrounding this parameter was modelled using a beta distribution.
Cost of managing grade 3/4 adverse events
The cost associated with managing grade 3/4 AEs was assumed to require a single non-elective bed-day. The unit cost per AE was assumed to reflect the weighted mean cost of a non-elective excess bed-day, based on the NHS Reference Costs 2015/16106 (mean cost £298.41). Uncertainty surrounding this parameter was modelled using a normal distribution, assuming that the standard error (SE) was equal to 15% of the mean (SE £44.76).
Best supportive care costs
Resource use for patients receiving cabozantinib, vandetanib and BSC was estimated using expert opinion (Dr Jon Wadsley and Dr Laura Moss, personal communication) (see Tables 59 and 60 in Appendix 4). Clinical advice received by the AG suggested that the resource use associated with BSC is likely to be the same for both the pre-progression and post-progression states as these patients have, by definition, progressed disease. Conversely, total health-state resource use associated with cabozantinib and vandetanib was assumed to be time dependent in order to account for the monitoring requirements associated with the TKIs. With respect to the pairwise comparisons of vandetanib versus BSC (AG analyses 2 and 5), patients who switch from BSC to vandetanib post progression are assumed to incur the ‘subsequent years’ costs for vandetanib; this assumption was also made in the Sanofi model.
One clinical expert (JW) provided resource use estimates (central estimates, minimum and maximum), which were then verified and augmented with additional components by a second clinical expert (LM). As the elicited information relates to ranges and some of the distributions are highly skewed, uncertainty surrounding these parameters was represented using triangular distributions. The experts’ central estimates were taken to be the mode of the distribution; means were calculated as:
The numbers of ECGs, CT scans, and blood tests were not associated with uncertain ranges and were thus held as fixed values within the probabilistic analysis.
Cost of palliative care
The costs associated with palliative care and palliative chemotherapy are applied at the point of death to all patients. These costs were based on the same data used in the Sanofi model,66 which were, in turn, derived from the NHS Reference Costs 2015/16106 and the PSSRU. 107 A total cost of £6602.52 is applied per patient.
Unit costs
Table 61 in Appendix 4 summarises the unit costs included in the AG model.
Model evaluation methods
Uncertainty was evaluated using PSA and DSA. PSA was undertaken using simple Monte Carlo sampling methods (2000 samples). The choice of distribution assumed for each parameter group is summarised in Table 62 in Appendix 4. The results of the PSA are presented as CEACs. DSAs were undertaken to explore the impact of alternative assumptions regarding discount rates, choices of parametric survivor functions, disutilities associated with AEs, and resource use and cost assumptions.
Model validation
The AG adopted a number of approaches to ensure the credibility of the model. These included scrutiny of the implemented model coding and formulae by two modellers, black-box testing, double-programming of the deterministic base case for all pairwise comparisons, checking the accuracy of all model inputs against the original sources, consultation with clinical experts, peer review of the model assumptions by clinical experts and peer review of the report by two third-party modellers (see Acknowledgements).
Assessment group model results
This section presents the results based on the AG model for each of the five sets of analyses.
Analysis 1: EU-label population (symptomatic and progressive medullary thyroid cancer), cabozantinib versus best supportive care (pairwise)
Table 26 presents the results of the pairwise comparison of cabozantinib versus BSC within the EU-label (symptomatic and progressive) MTC population. Disaggregated life-years gained (LYGs), QALYs and costs are presented in Table 63 in Appendix 5. Based on the probabilistic version of the AG model (assuming the log-logistic function for both PFS and OS), cabozantinib is expected to generate 0.48 additional QALYs at an additional cost of £72,734 compared with BSC; the ICER for cabozantinib versus BSC is expected to be £150,874 per QALY gained. The deterministic version of the model (based on point estimates of parameters) produces similar results (deterministic ICER = £148,169 per QALY gained). The disaggregated results show that a considerable amount of the OS gain in both groups is accrued in the post-progression state.
| Option | Absolute | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model | |||||
| Cabozantinib | 2.28 | 88,527 | 0.48 | 72,734 | 150,874 |
| BSC | 1.79 | 15,793 | – | – | – |
| Deterministic model | |||||
| Cabozantinib | 2.27 | 87,960 | 0.49 | 72,287 | 148,169 |
| BSC | 1.79 | 15,672 | – | – | – |
Figure 12 presents CEACs for the pairwise comparison of cabozantinib versus BSC within the EU-label (symptomatic and progressive) MTC population. Assuming a WTP threshold (λ) of £30,000 per QALY gained, the probability that cabozantinib produces more net benefit than BSC is zero.
FIGURE 12.
Analysis 1: EU-label population (symptomatic and progressive MTC), cabozantinib vs. BSC (pairwise), CEACs (PFS = log-logistic, OS = log-logistic for both options).
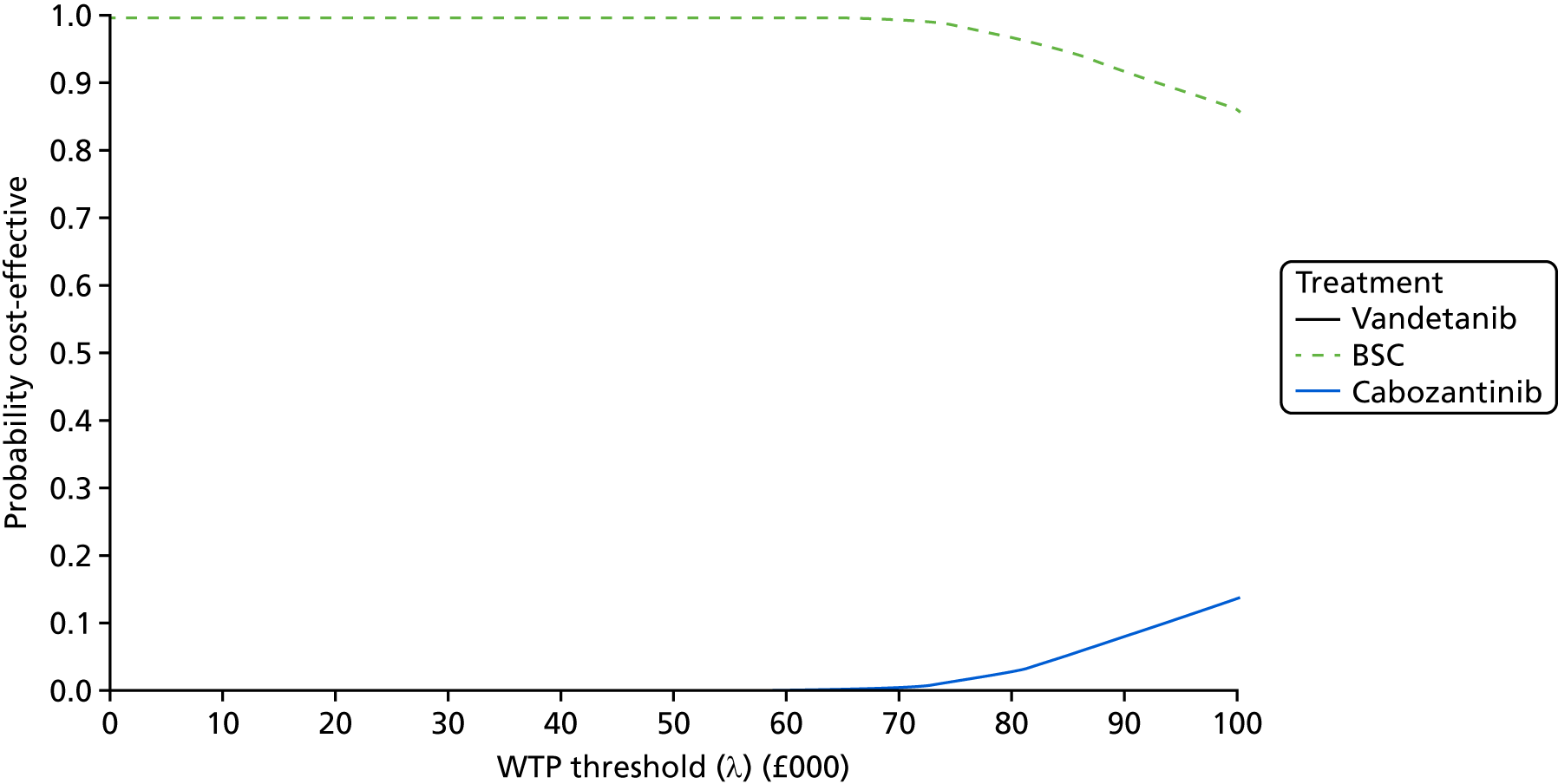
Table 27 presents the results of the DSAs for the pairwise comparison of cabozantinib versus BSC within the EU-label (symptomatic and progressive) MTC population. As shown in the table, the ICER remains in excess of £135,000 per QALY gained across all scenarios. The alternative scenarios regarding health utilities, AE impacts and health state resource use do not have a marked impact on the cost-effectiveness of cabozantinib. The exclusion of dose reductions for cabozantinib increases the ICER to £174,297 per QALY gained. The choice of survivor functions for PFS and OS produces ICERs for cabozantinib versus BSC in the range £138,259 to £239,141 per QALY gained; the curves used in the AG’s base-case analysis (PFS = log-logistic, OS = log-logistic) are close to the most favourable scenario.
| Scenario | Incremental | ICER (£) | |
|---|---|---|---|
| QALYs | Costs (£) | ||
| Base case | 0.49 | 72,287 | 148,169 |
| Undiscounted health outcomes and costs | 0.57 | 77,243 | 135,531 |
| Sanofi CS utilities | 0.47 | 72,287 | 154,582 |
| DECISION study utilities | 0.43 | 72,287 | 166,890 |
| Cabozantinib SMC utilities | 0.44 | 72,287 | 165,816 |
| AE disutility doubled | 0.48 | 72,287 | 150,159 |
| AE disutility halved | 0.49 | 72,287 | 147,194 |
| AE management costs doubled | 0.49 | 72,498 | 148,601 |
| AE management costs halved | 0.49 | 72,182 | 147,954 |
| Health state resource use doubled | 0.49 | 72,959 | 149,546 |
| Health state resource use halved | 0.49 | 71,951 | 147,481 |
| No cabozantinib dose reductions | 0.49 | 85,034 | 174,297 |
| Curve choice | |||
| PFS – exponential; OS – exponential | 0.45 | 71,195 | 158,030 |
| PFS – exponential; OS – Weibull | 0.42 | 71,012 | 170,550 |
| PFS – exponential; OS – Gompertz | 0.31 | 70,525 | 227,293 |
| PFS – exponential; OS – log-normal | 0.47 | 71,298 | 150,146 |
| PFS – exponential; OS – log-logistic | 0.46 | 71,251 | 153,284 |
| PFS – exponential; OS – gamma | 0.43 | 71,061 | 166,964 |
| PFS – Weibull; OS – exponential | 0.38 | 55,213 | 147,111 |
| PFS – Weibull; OS – Weibull | 0.34 | 55,035 | 161,300 |
| PFS – Weibull; OS – Gompertz | 0.24 | 54,530 | 232,034 |
| PFS – Weibull; OS – log-normal | 0.40 | 55,345 | 138,424 |
| PFS – Weibull; OS – log-logistic | 0.39 | 55,297 | 141,864 |
| PFS – Weibull; OS – gamma | 0.35 | 55,093 | 157,191 |
| PFS – Gompertz; OS – exponential | 0.36 | 52,776 | 147,369 |
| PFS – Gompertz; OS – Weibull | 0.32 | 52,593 | 162,336 |
| PFS – Gompertz; OS – Gompertz | 0.22 | 52,105 | 239,141 |
| PFS – Gompertz; OS – log-normal | 0.38 | 52,879 | 138,259 |
| PFS – Gompertz; OS – log-logistic | 0.37 | 52,831 | 141,855 |
| PFS – Gompertz; OS – gamma | 0.33 | 52,642 | 157,984 |
| PFS – log-normal; OS – exponential | 0.46 | 70,719 | 152,833 |
| PFS – log-normal; OS – Weibull | 0.43 | 70,551 | 164,542 |
| PFS – log-normal; OS – Gompertz | 0.32 | 70,024 | 217,141 |
| PFS – log-normal; OS – log-normal | 0.49 | 70,909 | 145,511 |
| PFS – log-normal; OS – log-logistic | 0.48 | 70,834 | 148,443 |
| PFS – log-normal; OS – gamma | 0.44 | 70,617 | 161,210 |
| PFS – log-logistic; OS – exponential | 0.47 | 72,176 | 152,470 |
| PFS – log-logistic; OS – Weibull | 0.44 | 72,008 | 163,867 |
| PFS – log-logistic; OS – Gompertz | 0.33 | 71,481 | 214,567 |
| PFS – log-logistic; OS – log-normal | 0.50 | 72,342 | 145,282 |
| PFS – log-logistic; OS – log-logistica | 0.49 | 72,287 | 148,169 |
| PFS – log-logistic; OS – gamma | 0.45 | 72,070 | 160,627 |
| PFS – gamma; OS – exponential | 0.39 | 57,437 | 147,094 |
| PFS – gamma; OS – Weibull | 0.36 | 57,260 | 160,678 |
| PFS – gamma; OS – Gompertz | 0.25 | 56,743 | 226,874 |
| PFS – gamma; OS – log-normal | 0.42 | 57,582 | 138,733 |
| PFS – gamma; OS – log-logistic | 0.41 | 57,535 | 142,051 |
| PFS – gamma; OS – gamma | 0.37 | 57,318 | 156,755 |
Analysis 2: EU-label population (symptomatic and progressive MTC), vandetanib versus best supportive care (pairwise)
Table 28 presents the results of the pairwise comparison of vandetanib versus BSC within the EU-label (symptomatic and progressive) MTC population. It should be noted that this analysis is subject to potential confounding as a result of the open-label use of vandetanib in the ZETA trial; hence, post-progression vandetanib costs are included for both treatment groups. Disaggregated LYGs, QALYs and costs are presented in Table 64 in Appendix 5. Based on the probabilistic version of the AG model (assuming the log-logistic function for both PFS and OS), vandetanib is expected to generate 0.23 additional QALYs at an additional cost of £79,745 compared with BSC; the ICER for vandetanib versus BSC is expected to be £352,508 per QALY gained. The deterministic version of the model yields a lower ICER of £336,896 per QALY gained. The disaggregated results indicate that, based on the log-logistic model, OS is expected to be higher in the BSC group than in the vandetanib group: this is likely to be a consequence of confounding as a result of open-label vandetanib use in the placebo group. It is also noteworthy that, based on the selected OS functions, a similar proportion of patients in each group (11–12%) are predicted to still be alive at 20 years as a consequence of the flattening of the tails of the modelled curves; additional analyses undertaken by the AG indicate that the ICER for vandetanib versus BSC remains stable over longer time horizons (the ICER using a 30-year time horizon, excluding any general population mortality constraints, is £345,284 per QALY gained).
| Option | Absolute | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model | |||||
| Vandetanib | 4.02 | 255,677 | 0.23 | 79,745 | 352,508 |
| BSC | 3.79 | 175,932 | – | – | – |
| Deterministic model | |||||
| Vandetanib | 4.02 | 255,114 | 0.23 | 79,044 | 336,896 |
| BSC | 3.78 | 176,070 | – | – | – |
Figure 13 presents CEACs for the pairwise comparison of vandetanib versus BSC within the EU-label (symptomatic and progressive) MTC population. Assuming a WTP threshold (λ) of £30,000 per QALY gained, the probability that vandetanib produces more net benefit than BSC is approximately 0.01.
FIGURE 13.
Analysis 2: EU-label population (symptomatic and progressive MTC), vandetanib vs. BSC (pairwise), CEACs (PFS = log-logistic, OS = log-logistic for both options).
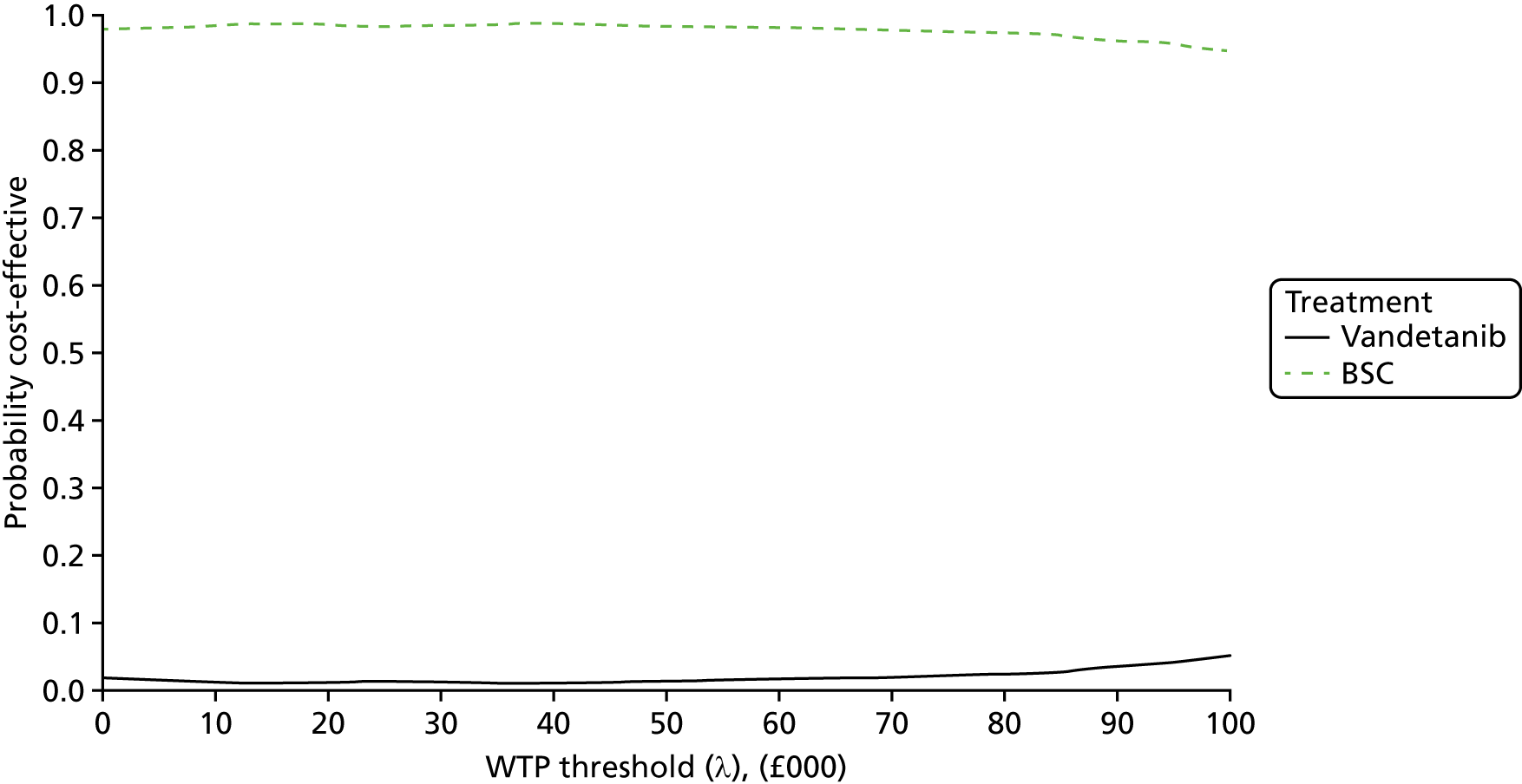
Table 29 presents the results of the DSAs for the pairwise comparison of vandetanib versus BSC within the EU-label (symptomatic and progressive) MTC population. Across the range of DSAs considered, the ICERs for vandetanib versus BSC remain above £123,000 per QALY gained. In several scenarios in which the Gompertz function is used to model PFS, vandetanib is expected to be dominated by BSC. The DSAs indicate that the choice of utility values used in the base-case analysis produces a considerably more favourable ICER for vandetanib versus BSC than the alternative sources identified. The scenarios surrounding health state resource use assumptions do not substantially alter the ICER; however, the exclusion of post-progression vandetanib costs in both groups produces a marked increase in the ICER for vandetanib (ICER = £752,136 per QALY gained). In addition, setting the vandetanib discontinuation parameter equal to zero leads to an increase in the ICER for vandetanib (ICER = £378,272 per QALY gained). The choice of survival curves produces ICERs for vandetanib versus BSC ranging from £123,723 per QALY gained to dominated; the parametric survivor functions selected for use in the AG’s base case do not represent the most optimistic case for vandetanib, nor do they represent they least favourable.
| Scenario | Incremental | ICER (£) | |
|---|---|---|---|
| QALYs | Costs (£) | ||
| Base case | 0.23 | 79,044 | 336,896 |
| Undiscounted health outcomes and costs | 0.25 | 81,248 | 320,133 |
| Sanofi CS utilities | 0.10 | 79,044 | 822,117 |
| DECISION study utilities | 0.05 | 79,044 | 1,532,109 |
| Cabozantinib SMC utilities | 0.07 | 79,044 | 1,161,487 |
| AE disutility doubled | 0.23 | 79,044 | 340,951 |
| AE disutility halved | 0.24 | 79,044 | 334,904 |
| AE management costs doubled | 0.23 | 79,134 | 337,283 |
| AE management costs halved | 0.23 | 78,998 | 336,702 |
| Post-progression vandetanib costs excluded | 0.23 | 176,468 | 752,136 |
| Vandetanib discontinuation parameter equal to zero | 0.23 | 88,751 | 378,272 |
| Health state resource use doubled | 0.23 | 80,593 | 343,500 |
| Health state resource use halved | 0.23 | 78,269 | 333,593 |
| No vandetanib dose reductions | 0.23 | 85,802 | 365,703 |
| Curve choice | |||
| PFS – exponential; OS – exponential | 0.46 | 59,484 | 130,328 |
| PFS – exponential; OS – Weibull | 0.46 | 62,545 | 137,196 |
| PFS – exponential; OS – Gompertz | 0.59 | 72,938 | 123,723 |
| PFS – exponential; OS – log-normal | 0.39 | 49,372 | 128,083 |
| PFS – exponential; OS – log-logistic | 0.37 | 49,310 | 134,230 |
| PFS – exponential; OS – gamma | 0.43 | 60,268 | 139,406 |
| PFS – Weibull; OS – exponential | 0.22 | 37,245 | 165,924 |
| PFS – Weibull; OS – Weibull | 0.22 | 40,327 | 179,916 |
| PFS – Weibull; OS – Gompertz | 0.36 | 50,707 | 141,776 |
| PFS – Weibull; OS – log-normal | 0.15 | 27,155 | 176,631 |
| PFS – Weibull; OS – log-logistic | 0.14 | 27,093 | 199,768 |
| PFS – Weibull; OS – gamma | 0.20 | 38,051 | 189,697 |
| PFS – Gompertz; OS – exponential | –0.08 | 53,486 | Dominated |
| PFS – Gompertz; OS – Weibull | –0.08 | 56,486 | Dominated |
| PFS – Gompertz; OS – Gompertz | 0.07 | 64,762 | 969,254 |
| PFS – Gompertz; OS – log-normal | –0.15 | 43,375 | Dominated |
| PFS – Gompertz; OS – log-logistic | –0.17 | 43,313 | Dominated |
| PFS – Gompertz; OS – gamma | –0.11 | 54,271 | Dominated |
| PFS – log-normal; OS – exponential | 0.39 | 97,481 | 249,691 |
| PFS – log-normal; OS – Weibull | 0.39 | 100,596 | 257,665 |
| PFS – log-normal; OS – Gompertz | 0.53 | 110,381 | 209,110 |
| PFS – log-normal; OS – log-normal | 0.32 | 87,433 | 273,140 |
| PFS – log-normal; OS – log-logistic | 0.30 | 87,371 | 289,324 |
| PFS – log-normal; OS – gamma | 0.37 | 98,325 | 267,980 |
| PFS – log-logistic; OS – exponential | 0.32 | 89,180 | 275,834 |
| PFS – log-logistic; OS – Weibull | 0.32 | 92,278 | 285,560 |
| PFS – log-logistic; OS – Gompertz | 0.46 | 101,633 | 218,981 |
| PFS – log-logistic; OS – log-normal | 0.25 | 79,106 | 312,992 |
| PFS – log-logistic; OS – log-logistica | 0.23 | 79,044 | 336,896 |
| PFS – log-logistic; OS – gamma | 0.30 | 90,002 | 300,416 |
| PFS – gamma; OS – exponential | 0.28 | 41,060 | 147,850 |
| PFS – gamma; OS – Weibull | 0.28 | 44,151 | 159,114 |
| PFS – gamma; OS – Gompertz | 0.41 | 54,525 | 132,686 |
| PFS – gamma; OS – log-normal | 0.21 | 30,979 | 149,603 |
| PFS – gamma; OS – log-logistic | 0.19 | 30,917 | 163,617 |
| PFS – gamma; OS – gamma | 0.25 | 41,875 | 164,911 |
Analysis 3: EU-label population (symptomatic and progressive MTC), fully incremental analysis of all options using vandetanib progression-free survival treatment effect from combined model, central estimates of cost-effectiveness
Table 30 presents the results of the fully incremental analysis of all options within the EU-label (symptomatic and progressive) MTC population based on the EXAM trial baseline, together with the PFS treatment effect derived from the EU-label population of the ZETA trial. It should be noted that this analysis assumes that OS for vandetanib is equal to that of cabozantinib, which, given the increased hazard rate/acceleration factor for PFS, may be seen to be optimistic for vandetanib. Disaggregated LYGs, QALYs and costs are presented in Table 65 in Appendix 5. Based on the probabilistic version of the model (assuming the log-logistic function for both PFS and OS), the ICER for vandetanib versus BSC is expected to be £138,405 per QALY gained, whilst the ICER for cabozantinib versus vandetanib is expected to be £195,593 per QALY gained. The deterministic version of the model produces similar results (vandetanib vs. BSC ICER = £134,817 per QALY gained; cabozantinib vs. vandetanib ICER = £195,053 per QALY gained). The disaggregated results indicate that a considerable amount of the OS gain for all options is accrued in the post-progression state.
| Option | Absolute | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model | |||||
| Cabozantinib | 2.28 | 88,527 | 0.11 | 20,559 | 195,593 |
| Vandetanib | 2.17 | 67,968 | 0.38 | 52,175 | 138,405 |
| BSC | 1.79 | 15,793 | – | – | – |
| Deterministic model | |||||
| Cabozantinib | 2.27 | 87,960 | 0.11 | 21,094 | 195,053 |
| Vandetanib | 2.16 | 66,866 | 0.38 | 51,193 | 134,817 |
| BSC | 1.79 | 15,672 | – | – | – |
Figure 14 presents CEACs for the pairwise comparison of cabozantinib, vandetanib and BSC within the EU-label (symptomatic and progressive) MTC population, including the PFS treatment effect for vandetanib from the ZETA trial. Assuming a WTP threshold (λ) of £30,000 per QALY gained, the probability that either cabozantinib or vandetanib produces more net benefit than BSC is zero.
FIGURE 14.
Analysis 3: EU-label population (symptomatic and progressive MTC), fully incremental analysis of all options using vandetanib PFS treatment effect from combined model, CEACs (PFS = log-logistic, OS = log-logistic for all options).
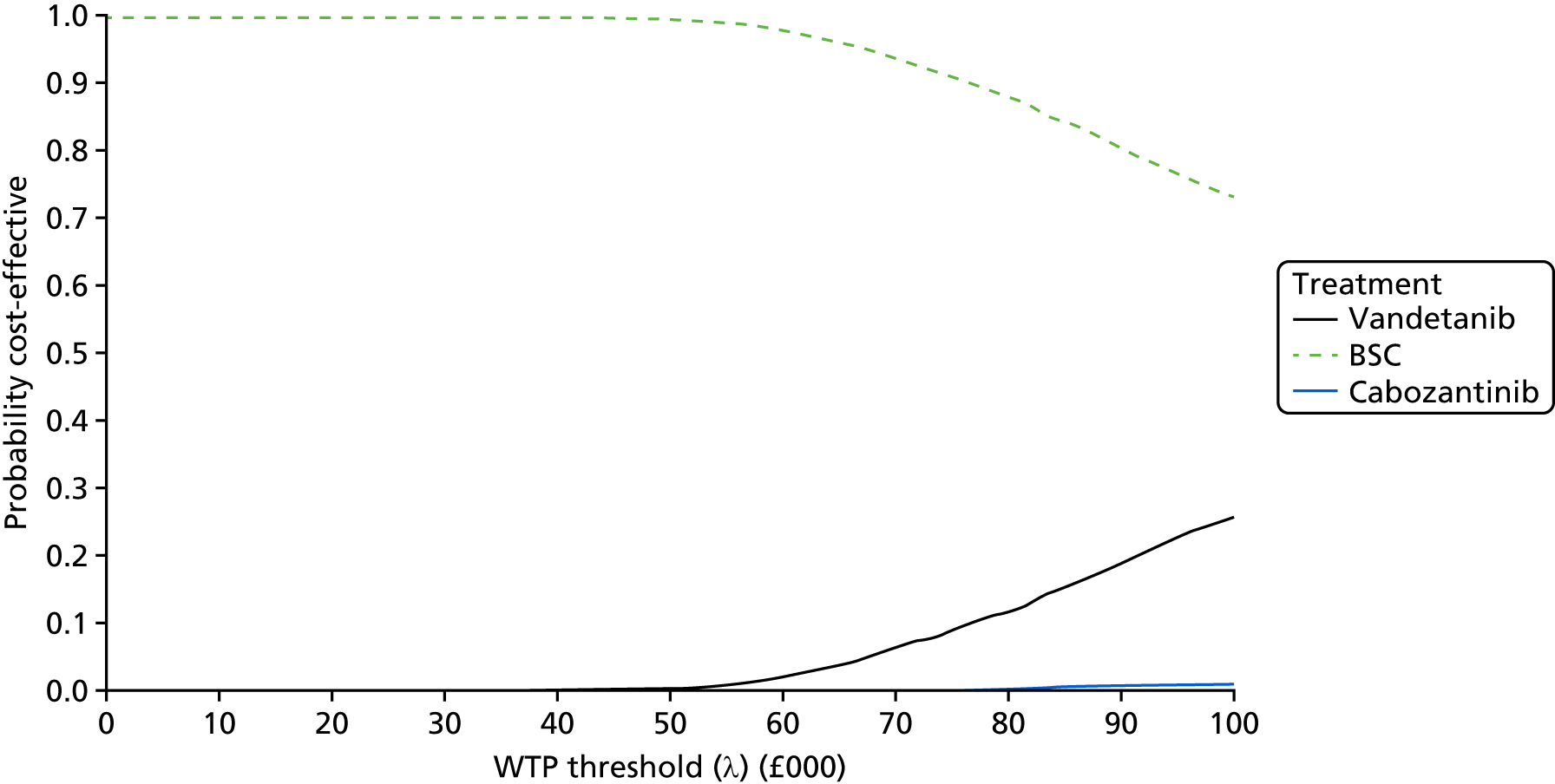
Table 31 presents the results of the DSAs for the fully incremental analyses of cabozantinib, vandetanib and BSC within the EU-label (symptomatic and progressive) MTC population, including the PFS treatment effect for vandetanib from the ZETA trial. Across the range of DSAs considered, the ICERs for vandetanib remain above £85,000 per QALY gained, whilst the ICERs for cabozantinib remain above £148,000 per QALY gained. In several scenarios in which the Gompertz function is used to model OS, vandetanib is ruled out of the analysis because of extended dominance. The DSAs indicate that the choice of utility values used in the base-case analysis produces a considerably more favourable ICER for cabozantinib than for the alternative sources identified. The scenarios surrounding alternative health state resource use assumptions do not substantially alter the ICER. Setting the vandetanib discontinuation parameter equal to zero leads to a situation in which vandetanib is ruled out because of extended dominance; the ICER for cabozantinib versus BSC is estimated to be £148,169 per QALY gained. The choice of survival curves produces ICERs for vandetanib in the range £85,217 per QALY gained to extendedly dominated and ICERs for cabozantinib in the range £180,985 to £239,141 per QALY gained. The parametric survivor functions selected for use in the AG’s base case do not represent the most optimistic case for either drug, nor are they the least favourable.
| Scenario | ICER (£) (vs. next-best comparator) | |
|---|---|---|
| Cabozantinib | Vandetanib | |
| Base case | 195,053 (vs. vandetanib) | 134,817 (vs. BSC) |
| Undiscounted health outcomes and costs | 192,555 (vs. vandetanib) | 119,397 (vs. BSC) |
| Sanofi CS utilities | 298,889 (vs. vandetanib) | 128,932 (vs. BSC) |
| DECISION study utilities | 379,753 (vs. vandetanib) | 135,577 (vs. BSC) |
| Cabozantinib SMC utilities | 351,244 (vs. vandetanib) | 136,191 (vs. BSC) |
| AE disutility doubled | 203,651 (vs. vandetanib) | 135,495 (vs. BSC) |
| AE disutility halved | 191,021 (vs. vandetanib) | 134,480 (vs. BSC) |
| AE management costs doubled | 196,428 (vs. vandetanib) | 134,980 (vs. BSC) |
| AE management costs halved | 194,366 (vs. vandetanib) | 134,735 (vs. BSC) |
| Vandetanib discontinuation parameter equal to zero | 148,169 (vs. BSC) | Extended dominance |
| Health state resource use doubled | 173,521 (vs. vandetanib) | 142,718 (vs. BSC) |
| Health state resource use halved | 205,819 (vs. vandetanib) | 130,866 (vs. BSC) |
| No vandetanib or cabozantinib dose reductions | 273,909 (vs. vandetanib) | 145,927 (vs. BSC) |
| Curve choice | ||
| PFS – exponential; OS – exponential | 204,220 (vs. vandetanib) | 147,531 (vs. BSC) |
| PFS – exponential; OS – Weibull | 204,220 (vs. vandetanib) | 162,113 (vs. BSC) |
| PFS – exponential; OS – Gompertz | 227,293 (vs. BSC) | Extended dominance |
| PFS – exponential; OS – log-normal | 204,220 (vs. vandetanib) | 138,620 (vs. BSC) |
| PFS – exponential; OS – log-logistic | 204,220 (vs. vandetanib) | 142,141 (vs. BSC) |
| PFS – exponential; OS – gamma | 204,220 (vs. vandetanib) | 157,880 (vs. BSC) |
| PFS – Weibull; OS – exponential | 197,918 (vs. vandetanib) | 133,290 (vs. BSC) |
| PFS – Weibull; OS – Weibull | 197,908 (vs. vandetanib) | 150,033 (vs. BSC) |
| PFS – Weibull; OS – Gompertz | 232,034 (vs. BSC) | Extended dominance |
| PFS – Weibull; OS – log-normal | 197,873 (vs. vandetanib) | 123,454 (vs. BSC) |
| PFS – Weibull; OS – log-logistic | 197,873 (vs. vandetanib) | 127,303 (vs. BSC) |
| PFS – Weibull; OS – gamma | 197,895 (vs. vandetanib) | 145,084 (vs. BSC) |
| PFS – Gompertz; OS – exponential | 207,886 (vs. vandetanib) | 135,751 (vs. BSC) |
| PFS – Gompertz; OS – Weibull | 207,886 (vs. vandetanib) | 152,470 (vs. BSC) |
| PFS – Gompertz; OS – Gompertz | 239,141 (vs. BSC) | Extended dominance |
| PFS – Gompertz; OS – log-normal | 207,886 (vs. vandetanib) | 125,894 (vs. BSC) |
| PFS – Gompertz; OS – log-logistic | 207,886 (vs. vandetanib) | 129,755 (vs. BSC) |
| PFS – Gompertz; OS – gamma | 207,886 (vs. vandetanib) | 147,537 (vs. BSC) |
| PFS – log-normal; OS – exponential | 204,639 (vs. vandetanib) | 142,355 (vs. BSC) |
| PFS – log-normal; OS – Weibull | 204,672 (vs. vandetanib) | 155,650 (vs. BSC) |
| PFS – log-normal; OS – Gompertz | 217,141 (vs. BSC) | Extended dominance |
| PFS – log-normal; OS – log-normal | 204,981 (vs. vandetanib) | 134,340 (vs. BSC) |
| PFS – log-normal; OS – log-logistic | 204,897 (vs. vandetanib) | 137,538 (vs. BSC) |
| PFS – log-normal; OS – gamma | 204,722 (vs. vandetanib) | 151,833 (vs. BSC) |
| PFS – log-logistic; OS – exponential | 194,919 (vs. vandetanib) | 139,808 (vs. BSC) |
| PFS – log-logistic; OS – Weibull | 194,936 (vs. vandetanib) | 153,657 (vs. BSC) |
| PFS – log-logistic; OS – Gompertz | 214,567 (vs. BSC) | Extended dominance |
| PFS – log-logistic; OS – log-normal | 195,113 (vs. vandetanib) | 131,503 (vs. BSC) |
| PFS – log-logistic; OS – log-logistica | 195,053 (vs. vandetanib) | 134,817 (vs. BSC) |
| PFS – log-logistic; OS – gamma | 194,966 (vs. vandetanib) | 149,667 (vs. BSC) |
| PFS – gamma; OS – exponential | 180,990 (vs. vandetanib) | 97,633 (vs. BSC) |
| PFS – gamma; OS – Weibull | 180,990 (vs. vandetanib) | 122,911 (vs. BSC) |
| PFS – gamma; OS – Gompertz | 226,874 (vs. BSC) | Extended dominance |
| PFS – gamma; OS – log-normal | 180,985 (vs. vandetanib) | 85,217 (vs. BSC) |
| PFS – gamma; OS – log-logistic | 180,985 (vs. vandetanib) | 89,881 (vs. BSC) |
| PFS – gamma; OS – gamma | 180,989 (vs. vandetanib) | 114,798 (vs. BSC) |
Analysis 4: EU-label population (symptomatic and progressive medullary thyroid cancer), cabozantinib and vandetanib assumed equivalent
Table 32 presents the results of the fully incremental analysis of all options within the EU-label (symptomatic and progressive) MTC population, assuming equivalent PFS and OS outcomes for cabozantinib and vandetanib, using time-to-event data from the EXAM trial. Disaggregated LYGs, QALYs and costs are presented in Table 66 in Appendix 5. Based on the probabilistic version of the model (assuming the log-logistic function for both PFS and OS), cabozantinib is expected to be dominated; this is a consequence of the more favourable grade 3 or higher AE profile and the slightly lower total relative dose intensity-adjusted drug costs for vandetanib. The probabilistic ICER for vandetanib versus BSC is estimated to be £144,841 per QALY gained. The deterministic version of the model produces a similar result (deterministic ICER = £142,279 per QALY gained). The disaggregated results indicate that a considerable proportion of the total OS gain for all options is accrued in the post-progression state.
| Option | Absolute | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model | |||||
| Vandetanib | 2.28 | 86,276 | 0.49 | 70,482 | 144,841 |
| Cabozantinib | 2.28 | 88,527 | – | – | Dominated |
| BSC | 1.79 | 15,793 | – | – | – |
| Deterministic model | |||||
| Vandetanib | 2.28 | 85,736 | 0.49 | 70,063 | 142,279 |
| Cabozantinib | 2.27 | 87,960 | – | – | Dominated |
| BSC | 1.79 | 15,672 | – | – | – |
Figure 15 presents CEACs for the pairwise comparison of vandetanib versus BSC within the EU-label (symptomatic and progressive) MTC population for the analysis in which PFS and OS outcomes are assumed to be equivalent for both drugs. Assuming a WTP threshold (λ) of £30,000 per QALY gained, the probability that either cabozantinib or vandetanib produces more net benefit than BSC is zero.
FIGURE 15.
Analysis 4: EU-label population (symptomatic and progressive MTC), cabozantinib and vandetanib assumed equivalent, CEACs (PFS = log-logistic, OS = log-logistic for all options).
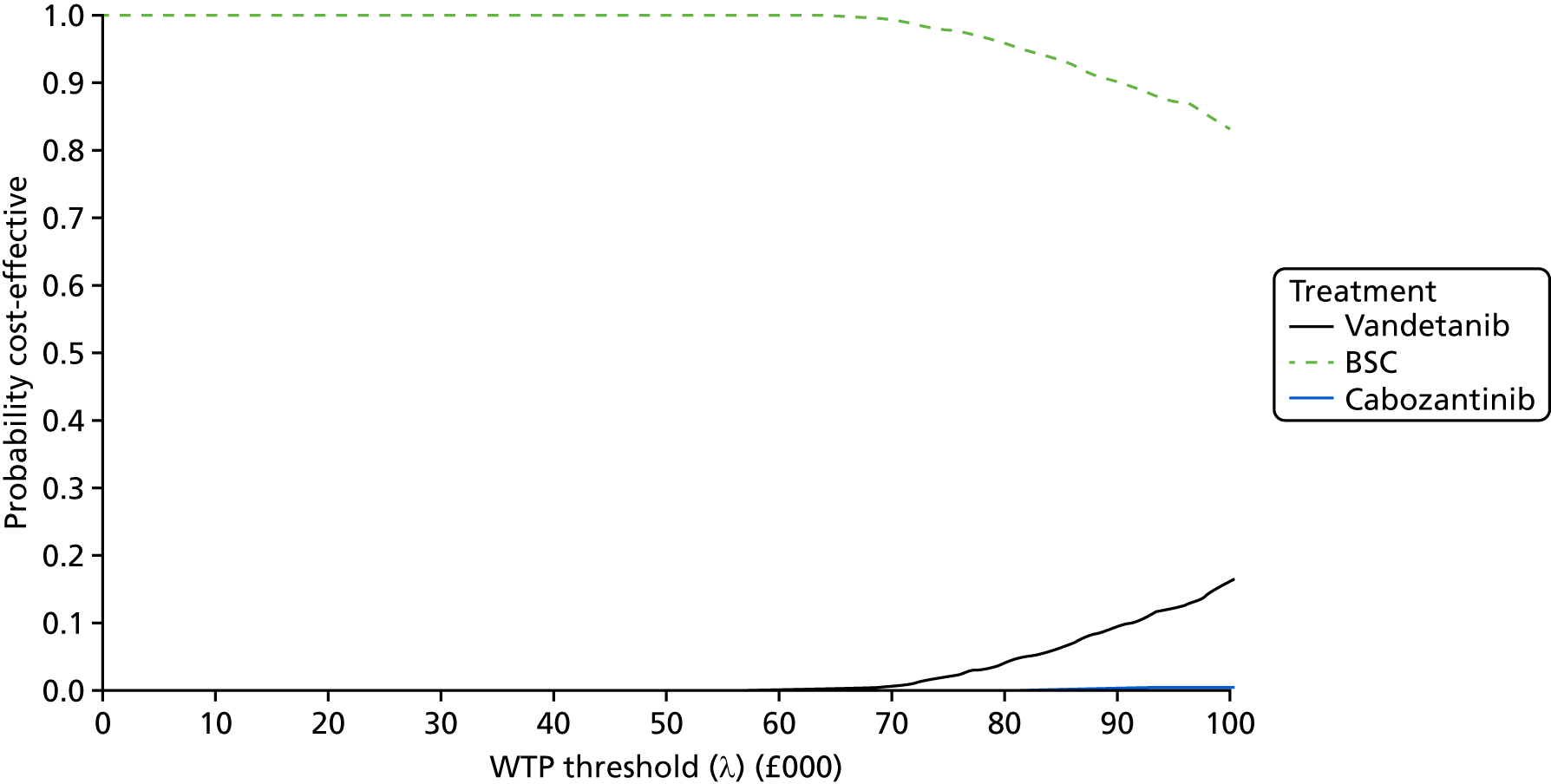
Table 33 presents the results of the DSAs for the fully incremental analysis of all options based on the assumption of equivalent PFS and OS outcomes for cabozantinib and vandetanib, using time-to-event outcome data from the EXAM trial. Cabozantinib remains dominated across all scenarios, except for the scenario in which the vandetanib discontinuation parameter is set equal to zero. In this scenario, the ICER for cabozantinib versus BSC is estimated to be £148,169 per QALY gained, whilst the ICER for vandetanib versus cabozantinib is estimated to be in excess of £1.35M per QALY gained. Across the remaining scenarios, the ICER for vandetanib versus BSC remains > £130,000 per QALY gained. The DSAs indicate that the choice of utility values and assumptions regarding AE impacts and health state resource use do not have a marked impact on the conclusions of the analysis. The choice of survival curves produces ICERs for vandetanib versus BSC in the range of £132,998 to £227,918 per QALY gained; the parametric survivor functions selected for use in the AG’s base case are close to the most favourable scenario for vandetanib.
| Scenario | ICER (£) (vs. next-best comparator) | |
|---|---|---|
| Cabozantinib | Vandetanib | |
| Base case | Dominated | 142,279 (vs. BSC) |
| Undiscounted health outcomes and costs | Dominated | 130,280 (vs. BSC) |
| Sanofi CS utilities | Dominated | 148,377 (vs. BSC) |
| DECISION study utilities | Dominated | 160,069 (vs. BSC) |
| Cabozantinib SMC utilities | Dominated | 159,049 (vs. BSC) |
| AE disutility doubled | Dominated | 142,831 (vs. BSC) |
| AE disutility halved | Dominated | 142,005 (vs. BSC) |
| AE management costs doubled | Dominated | 142,405 (vs. BSC) |
| AE management costs halved | Dominated | 142,217 (vs. BSC) |
| Vandetanib discontinuation parameter equal to zero | 148,169 (vs. BSC) | 1,354,088 (vs. cabozantinib) |
| Health state resource use doubled | Extended dominance | 148,745 (vs. BSC) |
| Health state resource use halved | Dominated | 139,047 (vs. BSC) |
| No vandetanib or cabozantinib dose reductions | Dominated | 154,164 (vs. BSC) |
| Curve choice | ||
| PFS – exponential; OS – exponential | Dominated | 151,561 (vs. BSC) |
| PFS – exponential; OS – Weibull | Dominated | 163,420 (vs. BSC) |
| PFS – exponential; OS – Gompertz | Dominated | 216,938 (vs. BSC) |
| PFS – exponential; OS – log-normal | Dominated | 144,080 (vs. BSC) |
| PFS – exponential; OS – log-logistic | Dominated | 147,058 (vs. BSC) |
| PFS – exponential; OS – gamma | Dominated | 160,026 (vs. BSC) |
| PFS – Weibull; OS – exponential | Dominated | 141,362 (vs. BSC) |
| PFS – Weibull; OS – Weibull | Dominated | 154,796 (vs. BSC) |
| PFS – Weibull; OS – Gompertz | Dominated | 221,301 (vs. BSC) |
| PFS – Weibull; OS – log-normal | Dominated | 133,120 (vs. BSC) |
| PFS – Weibull; OS – log-logistic | Dominated | 136,386 (vs. BSC) |
| PFS – Weibull; OS – gamma | Dominated | 150,910 (vs. BSC) |
| PFS – Gompertz; OS – exponential | Dominated | 141,640 (vs. BSC) |
| PFS – Gompertz; OS – Weibull | Dominated | 155,804 (vs. BSC) |
| PFS – Gompertz; OS – Gompertz | Dominated | 227,918 (vs. BSC) |
| PFS – Gompertz; OS – log-normal | Dominated | 132,998 (vs. BSC) |
| PFS – Gompertz; OS – log-logistic | Dominated | 136,411 (vs. BSC) |
| PFS – Gompertz; OS – gamma | Dominated | 151,689 (vs. BSC) |
| PFS – log-normal; OS – exponential | Dominated | 146,684 (vs. BSC) |
| PFS – log-normal; OS – Weibull | Dominated | 157,787 (vs. BSC) |
| PFS – log-normal; OS – Gompertz | Dominated | 207,458 (vs. BSC) |
| PFS – log-normal; OS – log-normal | Dominated | 139,734 (vs. BSC) |
| PFS – log-normal; OS – log-logistic | Dominated | 142,517 (vs. BSC) |
| PFS – log-normal; OS – gamma | Dominated | 154,630 (vs. BSC) |
| PFS – log-logistic; OS – exponential | Dominated | 146,363 (vs. BSC) |
| PFS – log-logistic; OS – Weibull | Dominated | 157,175 (vs. BSC) |
| PFS – log-logistic; OS – Gompertz | Dominated | 205,085 (vs. BSC) |
| PFS – log-logistic; OS – log-normal | Dominated | 139,536 (vs. BSC) |
| PFS – log-logistic; OS – log-logistica | Dominated | 142,279 (vs. BSC) |
| PFS – log-logistic; OS – gamma | Dominated | 154,103 (vs. BSC) |
| PFS – gamma; OS – exponential | Dominated | 141,316 (vs. BSC) |
| PFS – gamma; OS – Weibull | Dominated | 154,181 (vs. BSC) |
| PFS – gamma; OS – Gompertz | Dominated | 216,482 (vs. BSC) |
| PFS – gamma; OS – log-normal | Dominated | 133,382 (vs. BSC) |
| PFS – gamma; OS – log-logistic | Dominated | 136,532 (vs. BSC) |
| PFS – gamma; OS – gamma | Dominated | 150,469 (vs. BSC) |
Analysis 5: restricted EU-label population (symptomatic and progressive medullary thyroid cancer with carcinoembryonic antigen/calcitonin doubling times of ≤ 24 months), vandetanib versus best supportive care (pairwise)
Table 34 presents the results of the pairwise comparison of vandetanib versus BSC for the restricted EU-label population (symptomatic and progressive MTC plus CEA/CTN doubling times of ≤ 24 months). Disaggregated LYGs, QALYs and costs are presented in Table 67 in Appendix 5. This analysis closely reflects the economic analysis presented within the Sanofi CS,66 but includes survival models fitted directly to the observed data for the ZETA trial restricted EU-label subgroup, alternative assumptions regarding the vandetanib discontinuation parameter, different health state costs and different utility values. It should also be noted that this analysis is subject to potential confounding as a result of the open-label use of vandetanib; hence, post-progression vandetanib costs are included in both treatment groups. Based on the probabilistic version of the AG model (assuming the log-normal function for PFS and the Gompertz function for OS), vandetanib is expected to generate 1.61 additional QALYs at an additional cost of £107,780 compared with BSC; the ICER for vandetanib versus BSC is expected to be £66,779 per QALY gained. The deterministic version of the model yields a slightly lower ICER of £65,184 per QALY gained. The disaggregated results indicate that the majority of the incremental OS gain for vandetanib is accrued in the progression-free state. It is also noteworthy that, based on the selected Gompertz OS function, ≈12% of the vandetanib cohort are still alive at 20 years (indicated by the tail of the modelled curve). Additional analyses undertaken by the AG indicate that the ICER for vandetanib versus BSC is similar over longer time horizons (the ICER using a 30-year time horizon, excluding any general population mortality constraints, is £63,357 per QALY gained). However, the AG considers that the level of survival at 20 years may be an overestimate, and that the true ICER for vandetanib may therefore be > £67,000 per QALY gained. The impact of assuming alternative OS functions is explored within the sensitivity analyses (see Table 35).
| Option | Absolute | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| QALYs | Costs (£) | QALYs | Costs (£) | ||
| Probabilistic model | |||||
| Vandetanib | 3.45 | 204,539 | 1.61 | 107,780 | 66,779 |
| BSC | 1.83 | 96,759 | – | – | – |
| Deterministic model | |||||
| Vandetanib | 3.46 | 205,457 | 1.64 | 106,762 | 65,184 |
| BSC | 1.82 | 98,695 | – | – | – |
Figure 16 presents CEACs for the pairwise comparison of vandetanib versus BSC within the restricted EU-label MTC population. Assuming a WTP threshold (λ) of £30,000 per QALY gained, the probability that vandetanib produces more net benefit than BSC is approximately 0.02.
FIGURE 16.
Analysis 5: restricted EU-label population (symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months), vandetanib vs. BSC (pairwise), CEACs (PFS = log-normal, OS = Gompertz for both options).
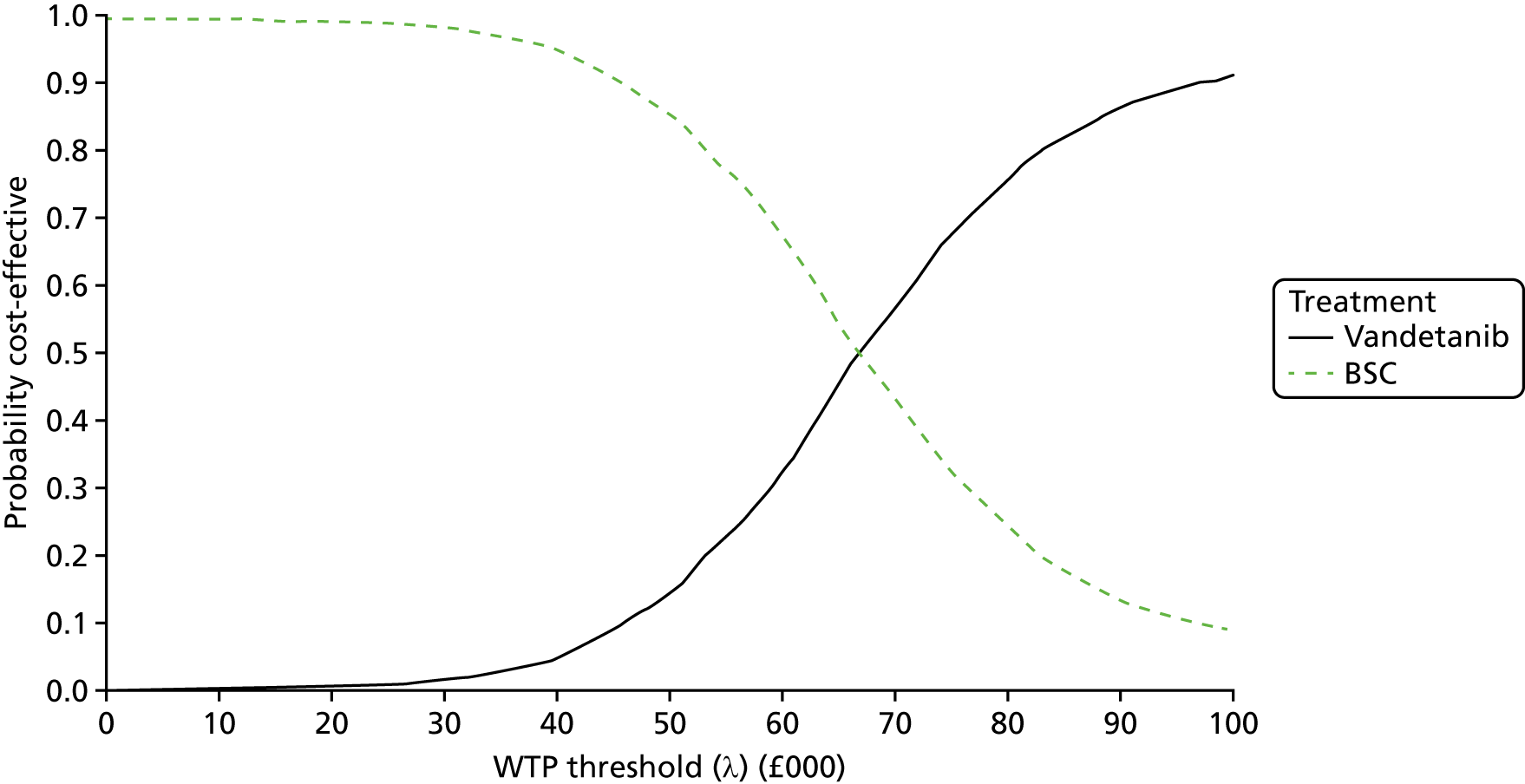
Table 35 presents the results of the DSAs for the pairwise comparison of vandetanib versus BSC within the restricted EU-label population. As shown in the table, the ICER remains > £51,000 per QALY gained across all scenarios. The DSAs indicate that the choice of utility values used in the base-case analysis produces a slightly less favourable ICER for vandetanib versus BSC within this population compared with the alternative sources identified. The alternative assumptions regarding health state resource use and AEs do not have a marked impact on the cost-effectiveness of vandetanib. In this population, excluding the post-progression vandetanib costs increases the ICER to £84,438 per QALY gained. Setting the vandetanib discontinuation parameter equal to zero increases the ICER to £76,352 per QALY gained. The choice of survival curves produces ICERs for vandetanib versus BSC in the range of £51,194 to £71,128 per QALY gained; the curves used in the AG’s base-case analysis (PFS = log-normal, OS = Gompertz) represent neither the most favourable nor the least favourable scenario for vandetanib within the restricted EU-label population.
| Scenario | Incremental | ICER (£) | |
|---|---|---|---|
| QALYs | Costs (£) | ||
| Base case | 1.64 | 106,762 | 65,184 |
| Undiscounted health outcomes and costs | 2.23 | 137,406 | 61,584 |
| Sanofi CS utilities | 1.76 | 106,762 | 60,576 |
| DECISION study utilities | 1.69 | 106,762 | 63,186 |
| Cabozantinib SMC utilities | 1.68 | 106,762 | 63,683 |
| AE disutility doubled | 1.64 | 106,762 | 65,295 |
| AE disutility halved | 1.64 | 106,762 | 65,128 |
| AE management costs doubled | 1.64 | 106,853 | 65,239 |
| AE management costs halved | 1.64 | 106,717 | 65,156 |
| Post-progression vandetanib costs excluded | 1.64 | 138,298 | 84,438 |
| Vandetanib discontinuation parameter equal to zero | 1.64 | 125,054 | 76,352 |
| Health state resource use doubled | 1.64 | 115,552 | 70,551 |
| Health state resource use halved | 1.64 | 102,367 | 62,500 |
| No vandetanib dose reductions | 1.64 | 116,928 | 71,390 |
| Curve choice | |||
| PFS – exponential; OS – exponential | 1.30 | 81,931 | 63,007 |
| PFS – exponential; OS – Weibull | 1.30 | 82,041 | 63,165 |
| PFS – exponential; OS – Gompertz | 1.50 | 90,264 | 60,296 |
| PFS – exponential; OS – log-normal | 1.28 | 73,914 | 57,821 |
| PFS – exponential; OS – log-logistic | 1.06 | 56,920 | 53,857 |
| PFS – exponential; OS – gamma | 1.27 | 80,262 | 63,172 |
| PFS – Weibull; OS – exponential | 1.25 | 77,205 | 61,602 |
| PFS – Weibull; OS – Weibull | 1.25 | 77,316 | 61,765 |
| PFS – Weibull; OS – Gompertz | 1.45 | 85,538 | 58,993 |
| PFS – Weibull; OS – log-normal | 1.23 | 69,188 | 56,193 |
| PFS – Weibull; OS – log-logistic | 1.01 | 52,195 | 51,687 |
| PFS – Weibull; OS – gamma | 1.22 | 75,537 | 61,739 |
| PFS – Gompertz; OS – exponential | 1.40 | 99,812 | 71,119 |
| PFS – Gompertz; OS – Weibull | 1.41 | 99,165 | 70,439 |
| PFS – Gompertz; OS – Gompertz | 1.61 | 106,531 | 66,060 |
| PFS – Gompertz; OS – log-normal | 1.38 | 91,856 | 66,516 |
| PFS – Gompertz; OS – log-logistic | 1.16 | 74,863 | 64,564 |
| PFS – Gompertz; OS – gamma | 1.38 | 97,861 | 71,128 |
| PFS – log-normal; OS – exponential | 1.44 | 98,830 | 68,718 |
| PFS – log-normal; OS – Weibull | 1.44 | 98,899 | 68,821 |
| PFS – log-normal; OS – Gompertza | 1.64 | 106,762 | 65,184 |
| PFS – log-normal; OS – log-normal | 1.42 | 90,824 | 64,128 |
| PFS – log-normal; OS – log-logistic | 1.19 | 73,831 | 61,791 |
| PFS – log-normal; OS – gamma | 1.41 | 97,169 | 68,989 |
| PFS – log-logistic; OS – exponential | 1.44 | 100,247 | 69,779 |
| PFS – log-logistic; OS – Weibull | 1.44 | 99,816 | 69,348 |
| PFS – log-logistic; OS – Gompertz | 1.64 | 107,120 | 65,132 |
| PFS – log-logistic; OS – log-normal | 1.41 | 92,230 | 65,198 |
| PFS – log-logistic; OS – log-logistic | 1.19 | 75,237 | 63,056 |
| PFS – log-logistic; OS – gamma | 1.41 | 98,433 | 69,923 |
| PFS – gamma; OS – exponential | 1.25 | 76,695 | 61,206 |
| PFS – gamma; OS – Weibull | 1.25 | 76,806 | 61,368 |
| PFS – gamma; OS – Gompertz | 1.45 | 85,028 | 58,651 |
| PFS – gamma; OS – log-normal | 1.23 | 68,678 | 55,789 |
| PFS – gamma; OS – log-logistic | 1.01 | 51,685 | 51,194 |
| PFS – gamma; OS – gamma | 1.22 | 75,027 | 61,334 |
Budget impact analysis
Table 36 presents a budget impact analysis for cabozantinib and vandetanib based on year-on-year drug acquisition costs predicted using the AG model. The budget impact analysis makes the following assumptions:
-
The analysis considers only the acquisition costs of the drugs; other resource use components are excluded.
-
The analysis includes prevalent (surviving) and incident (new) patients.
-
Cumulative costs for surviving patients remaining progression free and on treatment (based on the log-logistic PFS models) are considered over a period of 10 years. The costs of post-progression vandetanib use are excluded from the analysis.
-
The analysis assumes a constant eligible incident population of (confidential information has been removed) MTC patients per year, based on the current use of the drugs on the CDF.
-
The maximum annual budget impact is calculated using the total incident and prevalent cohort at 10 years.
| Cohort year | Cohort year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Budget impact – cabozantinib, symptomatic and progressive MTC population (based on EXAM trial ITT PFS population, log-logistic model) | |||||||||||
| Entry year | 1 | 1,293,225 | 488,370 | 214,984 | 118,396 | 74,784 | 51,564 | 37,756 | 28,878 | 22,828 | 18,518 |
| 2 | – | 1,293,225 | 488,370 | 214,984 | 118,396 | 74,784 | 51,564 | 37,756 | 28,878 | 22,828 | |
| 3 | – | – | 1,293,225 | 488,370 | 214,984 | 118,396 | 74,784 | 51,564 | 37,756 | 28,878 | |
| 4 | – | – | – | 1,293,225 | 488,370 | 214,984 | 118,396 | 74,784 | 51,564 | 37,756 | |
| 5 | – | – | – | – | 1,293,225 | 488,370 | 214,984 | 118,396 | 74,784 | 51,564 | |
| 6 | – | – | – | – | – | 1,293,225 | 488,370 | 214,984 | 118,396 | 74,784 | |
| 7 | – | – | – | – | – | – | 1,293,225 | 488,370 | 214,984 | 118,396 | |
| 8 | – | – | – | – | – | – | – | 1,293,225 | 488,370 | 214,984 | |
| 9 | – | – | – | – | – | – | – | – | 1,293,225 | 488,370 | |
| 10 | – | – | – | – | – | – | – | – | – | 1,293,225 | |
| Total annual cost | 1,293,225 | 1,781,595 | 1,996,579 | 2,114,975 | 2,189,759 | 2,241,323 | 2,279,080 | 2,307,958 | 2,330,786 | 2,349,304 | |
| Budget impact: vandetanib, symptomatic and progressive MTC population (based on ZETA trial EU-label subgroup PFS, log-logistic model) | |||||||||||
| Entry year | 1 | 1,465,575 | 1,087,458 | 775,968 | 568,666 | 432,204 | 339,574 | 274,328 | 226,761 | 191,027 | 163,483 |
| 2 | – | 1,465,575 | 1,087,458 | 775,968 | 568,666 | 432,204 | 339,574 | 274,328 | 226,761 | 191,027 | |
| 3 | – | – | 1,465,575 | 1,087,458 | 775,968 | 568,666 | 432,204 | 339,574 | 274,328 | 226,761 | |
| 4 | – | – | – | 1,465,575 | 1,087,458 | 775,968 | 568,666 | 432,204 | 339,574 | 274,328 | |
| 5 | – | – | – | – | 1,465,575 | 1,087,458 | 775,968 | 568,666 | 432,204 | 339,574 | |
| 6 | – | – | – | – | – | 1,465,575 | 1,087,458 | 775,968 | 568,666 | 432,204 | |
| 7 | – | – | – | – | – | – | 1,465,575 | 1,087,458 | 775,968 | 568,666 | |
| 8 | – | – | – | – | – | – | – | 1,465,575 | 1,087,458 | 775,968 | |
| 9 | – | – | – | – | – | – | – | – | 1,465,575 | 1,087,458 | |
| 10 | – | – | – | – | – | – | – | – | – | 1,465,575 | |
| Total annual cost | 1,465,575 | 2,553,033 | 3,329,001 | 3,897,667 | 4,329,872 | 4,669,446 | 4,943,774 | 5,170,534 | 5,361,561 | 5,525,045 | |
The maximum annual budget impact for cabozantinib within the symptomatic and progressive population is expected to be ≈£2.35M. The maximum budget impact for vandetanib within the symptomatic and progressive population is expected to be ≈£5.53M; the costs of vandetanib in the restricted EU-label population are expected to be lower.
Discussion
The AG’s systematic review of existing economic evaluations did not identify any relevant published studies.
The manufacturer of cabozantinib did not submit any economic evidence relating to this product.
The manufacturer of vandetanib submitted a de novo model-based health economic evaluation of vandetanib versus BSC in the restricted EU-label population (symptomatic and progressive MTC plus CTN/CEA doubling times of ≤ 24 months). An economic analysis for the broader licensed population was not presented. The corrected version of Sanofi’s partitioned survival model suggests that the probabilistic ICER for vandetanib versus BSC is approximately £31,546 per QALY gained. The AG notes several concerns relating to the company’s submitted model, in particular (1) the questionable relevance of the restricted EU-label population to current clinical practice, (2) the failure to adjust for open-label vandetanib use in both treatment groups, (3) the likely overestimation of the costs of vandetanib use in the post-progression state, (4) questionable assumptions regarding the amount of vandetanib received and (5) concerns regarding the robustness of the company’s covariate-adjusted survival modelling to reflect the restricted EU-label population. The AG considers that the ICER for vandetanib is likely to be considerably higher than the estimates presented within the Sanofi CS. 66
In the light of concerns regarding the economic analysis submitted by Sanofi and the absence of any economic evidence for cabozantinib, the AG developed a de novo health economic model. The AG model was evaluated across five sets of analyses from the perspective of the NHS and PSS over a lifetime horizon. Four sets of analyses of cabozantinib and/or vandetanib versus BSC were undertaken in the EU-label (symptomatic and progressive) MTC population and one set of analyses of vandetanib versus BSC was undertaken in the restricted EU-label population (symptomatic and progressive MTC with CTN/CEA doubling times of ≤ 24 months). Costs and health outcomes were discounted at a rate of 3.5% per annum. Costs were valued at 2016/17 prices. The AG model used a partitioned survival approach based on three health states: (1) progression free, (2) post progression and (3) dead. Costs and health utilities were assumed to differ according to the presence/absence of disease progression. The model parameters were informed by analyses of IPD from the EXAM trial, replicated IPD from the ZETA trial, the submissions from Sanofi and Ipsen and data contained within subsequent clarification responses, as well as published literature, standard reference cost sources and expert judgement. The results of the AG’s economic analysis are summarised in Table 37.
| Analysis number | Description | Probabilistic ICER (£ per QALY gained) | Probability of being cost-effective at λ = £30,000 per QALY gained | ICER range (£ per QALY gained) from alternative parametric survivor functions |
|---|---|---|---|---|
| 1 | Pairwise economic evaluation of cabozantinib vs. BSC in the EXAM trial ITT population | 150,874 | Cabozantinib: 0.00 | 138,259–239,141 |
| 2 | Pairwise economic evaluation of vandetanib vs. BSC in the ZETA trial EU-label population | 352,508 | Vandetanib: 0.01 | 123,723 to dominated |
| 3 | Fully incremental analysis based on EXAM trial ITT population with vandetanib PFS treatment effect applied to EXAM trial placebo baseline; vandetanib OS assumed to be equivalent to cabozantinib OS | Vandetanib vs. BSC: 138,405 | Vandetanib: 0.00 | Vandetanib vs. next-best comparator: 85,217 to extendedly dominated |
| Cabozantinib vs. vandetanib: 195,593 | Cabozantinib: 0.00 | Cabozantinib vs. next-best comparator: 180,985–239,141 | ||
| 4 | Fully incremental analysis based on EXAM trial ITT population assuming PFS and OS are equivalent for vandetanib and cabozantinib | Cabozantinib = dominated | Cabozantinib: 0.00 | Cabozantinib: dominated to dominated |
| Vandetanib vs. BSC: 144,841 | Vandetanib: 0.00 | Vandetanib: 132,998–227,918 | ||
| 5 | Pairwise economic evaluation of vandetanib vs. BSC using ZETA trial restricted EU-label population | 66,779 | Vandetanib: 0.02 | 51,194–71,128 |
Assessment group analysis 1: EU-label population (symptomatic and progressive medullary thyroid cancer), pairwise economic evaluation of cabozantinib versus best supportive care
Based on the AG’s probabilistic model (assuming the log-logistic function for both PFS and OS), the ICER for cabozantinib versus BSC is expected to be £150,874 per QALY gained. The DSAs indicate that the AG’s base case is close to the most favourable scenario.
Assessment group analysis 2: EU-label population (symptomatic and progressive medullary thyroid cancer), pairwise economic evaluation of vandetanib versus best supportive care
Based on the probabilistic version of the AG model (assuming the log-logistic function for both PFS and OS), the ICER for vandetanib versus BSC is expected to be £352,508 per QALY gained. The DSAs indicate that the AG’s base case does not represent the most optimistic case for vandetanib, nor does it reflect the most pessimistic scenario.
Assessment group analysis 3: EU-label population (symptomatic and progressive medullary thyroid cancer), fully incremental analysis, vandetanib progression-free survival treatment effect applied to EXAM trial placebo baseline, vandetanib overall survival assumed equivalent to cabozantinib overall survival
Within this analysis, the ICER for vandetanib versus BSC is expected to be £138,405 per QALY gained, whilst the ICER for cabozantinib versus vandetanib is expected to £195,593 per QALY gained. The DSAs indicate that the AG’s base case represents neither the most favourable nor the least favourable scenario for either drug.
Assessment group analysis 4: EU-label population (symptomatic and progressive medullary thyroid cancer), fully incremental analysis, progression-free and overall survival outcomes assumed equivalent for vandetanib and cabozantinib
Based on the probabilistic version of the model (assuming the log-logistic function for both PFS and OS), cabozantinib is expected to be dominated; this is a consequence of the more favourable grade 3 or higher AE profile and the slightly lower total relative dose intensity-adjusted drug costs for vandetanib. The probabilistic ICER for vandetanib versus BSC is expected to be £144,841 per QALY gained. The DSAs indicate that the AG’s base case represents one of the more favourable scenarios for vandetanib.
Assessment group analysis 5: restricted EU-label population (symptomatic and progressive medullary thyroid cancer plus carcinoembryonic antigen/calcitonin doubling times of ≤ 24 months), pairwise economic evaluation of vandetanib versus best supportive care
Based on the probabilistic version of the AG model (assuming the log-normal function for PFS and the Gompertz function for OS), the ICER for vandetanib versus BSC is expected to be £66,779 per QALY gained. The DSAs indicate that the AG’s base case represents neither a highly favourable nor a highly unfavourable scenario for vandetanib.
Table 38 highlights the key differences between the AG model and the Sanofi model. Although the two models are very similar in terms of their structure and definition of parameters, the key differences between the analyses relate to (1) the scope of the economic comparisons, (2) the time-to-event data used to inform the analyses (covariate-adjusted ITT/safety data set vs. actual subgroup data), (3) the source of health utility values, (4) assumptions regarding the costs associated with BSC and (5) assumptions regarding the costs of vandetanib in patients who discontinue therapy prior to disease progression.
| Element of economic analysis | Model | |
|---|---|---|
| Sanofi | AG | |
| Comparisons | Vandetanib vs. BSC | Cabozantinib vs. BSC |
| Vandetanib vs. BSC | ||
| Full incremental analysis of all options | ||
| Trial evidence used to inform time-to-event outcomes | ZETA trial ITT/safety population | EXAM trial ITT, ZETA trial EU label, ZETA trial restricted EU label |
| Structure | Partitioned survival model. No adjustment for logical inconsistency | Partitioned survival model. Includes adjustment for logical inconsistency |
| Survival modelling approach | Covariate-adjusted survivor functions fitted to ITT/safety data set | Survivor functions fitted directly to data for relevant populations |
| Health state utilities | Mapped utilities for progression-free state, decrement for post progression based on Beusterien et al.105 | Health state utilities derived from Fordham et al.102 |
| Costing approach | Different costs for BSC in progression-free and post-progression states | Same costs for BSC in progression-free and post-progression states. Additional resource use components included for patients receiving TKIs and for those receiving BSC |
| Vandetanib discontinuation parameter | Applied in full only to the pre-progression vandetanib group | Half of total value applied to all patients receiving vandetanib in progression-free and post-progression states (when applicable) |
Chapter 5 Assessment of factors relevant to the NHS and other parties
Additional monitoring requirements
Vandetanib and cabozantinib are associated with additional monitoring requirements, particularly during the first 3 months after initiating treatment (see Chapter 1, Significance for the NHS) These additional monitoring requirements impose additional costs on the NHS over and above the costs of drug acquisition. However, given the small population of MTC patients eligible to receive vandetanib and cabozantinib, these additional resource requirements are expected to be negligible.
Current availability of cabozantinib and vandetanib for medullary thyroid cancer
Both vandetanib and cabozantinib are currently available for the treatment of symptomatic and progressive MTC through the CDF. The current CDF recommendations for each TKI allow for the use of the other TKIs for patients in whom toxicity occurs, provided that (1) switching to the other TKI takes place within 3 months of starting the initial TKI, (2) the toxicity cannot be managed by dose delay or dose modification and (3) the patient has not experienced disease progression on the initial TKI. In addition, given the different AE profiles of cabozantinib and vandetanib and special warnings listed within their SmPCs,22,23 some patients will not be able to receive both therapies. The clinical advisors to the AG consider that there is value in having access to both TKIs for this reason.
End-of-life considerations
The end-of-life supplementary advice from NICE109 should be applied in the following circumstances and when the criteria referred to below are satisfied:
-
the treatment is indicated for patients with a short life expectancy, usually < 24 months and
-
there is sufficient evidence to indicate that the treatment offers an extension to life at least 3 additional months, compared with current NHS treatments.
Table 39 presents the undiscounted LYGs predicted by the AG’s base-case model (see Chapter 4, Time to event analysis using individual patient data). As shown in the table, the expected mean survival in the placebo group of the EXAM trial and the subgroups of the ZETA trial is > 24 months. This conclusion remains consistent irrespective of the choice of parametric model used to represent OS. However, it should be noted that the analyses of the OS data for the ZETA trial subgroups remain confounded by open-label vandetanib use; hence, the true survival duration in this population is unknown. The analyses suggest that the criterion relating to > 3 months life extension is likely to be met for cabozantinib in the EU-label (symptomatic and progressive) MTC population and for vandetanib within the restricted EU-label population (symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months).
| Outcome | Trial population/subgroup | |||||
|---|---|---|---|---|---|---|
| EXAM safety population | ZETA symptomatic and progressive | ZETA symptomatic and progressive with CEA/CTN biomarker | ||||
| Cabozantinib | BSC | Vandetanib | BSC | Vandetanib | BSC | |
| AG base-case OS (undiscounted LYGs) | 4.49 | 3.91 | 7.32 | 7.58 | 6.50 | 3.34 |
| Incremental OS gain (undiscounted LYGs) | 0.59 | –0.27 | 3.16 | |||
Chapter 6 Discussion
Statement of principal findings
The systematic review of the clinical effectiveness evidence identified two relevant placebo-controlled RCTs: (1) the EXAM trial, which evaluated cabozantinib (n = 330), and (2) the ZETA trial, which evaluated vandetanib. The EXAM trial was deemed to be at low risk of bias across most domains, whereas the ZETA trial was deemed to be at moderate to high risk of bias across a number of domains. The two trials assessed different populations (the ZETA trial inclusion criteria did not specify ‘progressive’ disease), but the ZETA trial did include a subgroup with ‘progressive and symptomatic disease’ (n = 186), which formed the ‘EU-label’ population. This group was considered to be comparable to the EXAM ITT population. In terms of efficacy, both cabozantinib and vandetanib significantly improved PFS compared with placebo. In the absence of direct evidence comparing the two interventions, a NMA was performed, which suggested that the results of the two treatments were broadly similar in terms of PFS, although these findings must be treated with caution because of the sparsity of the network.
Both cabozantinib and vandetanib also demonstrated significant benefits compared with placebo in terms of ORR, as determined by RECIST criteria. However, there was no significant OS benefit for either cabozantinib or vandetanib compared with placebo, although the data from the vandetanib trial were subject to potential confounding due to open-label vandetanib use in both groups. The two trials also conducted exploratory assessments of patients’ quality of life using instruments that evaluated various criteria, but no difference was found between the treatment or placebo arms at follow-up in either trial. Clinical advice received by the AG suggested that these tools did not necessarily capture symptomatic benefits produced by improved PFS or response to treatment. Both cabozantinib and vandetanib produced frequent AEs, with similar types and rates of grade 3 or higher AEs, except for higher rates of HFS (13%) for cabozantinib, and prolonged ECG QT (8%) for vandetanib. Similar proportions of patients across the two trials discontinued treatment because of AEs, but a higher percentage of patients experienced AEs leading to dose interruption or reduction on cabozantinib than on vandetanib.
Based on the AG’s probabilistic analysis of cabozantinib versus placebo in the EU-label (symptomatic and progressive) MTC population, the ICER for cabozantinib versus BSC is expected to be £150,874 per QALY gained. Within the EU-label (symptomatic and progressive) MTC population of the ZETA trial, the AG’s probabilistic analysis suggests that the ICER for vandetanib versus BSC is expected to be £352,508 per QALY gained. The fully incremental analysis of cabozantinib, vandetanib and BSC based on the EXAM ITT population and the vandetanib PFS treatment effect from the ZETA trial suggests that the ICER for vandetanib versus BSC is expected to be £138,405 per QALY gained, whilst the ICER for cabozantinib versus vandetanib is expected to be £195,593 per QALY gained. Within the fully incremental analysis in which the PFS and OS outcomes for vandetanib were assumed to be equivalent to the cabozantinib group outcomes in the EXAM trial, cabozantinib is expected to be dominated, whilst the ICER for vandetanib versus BSC is expected to be £144,841 per QALY gained. Within the restricted EU-label population (symptomatic and progressive MTC plus CEA/CTN doubling times of ≤ 24 months), the ICER for vandetanib versus BSC is expected to be £66,779 per QALY gained.
The AG’s economic analysis suggests that NICE’s criteria109 for life-extending therapies given at the end of life are not met for cabozantinib in the EU-label population (symptomatic and progressive MTC) or for vandetanib in either the EU-label population or the restricted EU-label population (symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months).
Strengths and limitations of the assessment
The key strengths of this assessment are as follows:
-
The AG’s economic evaluation includes fully incremental analyses of cabozantinib, vandetanib and BSC within the symptomatic and progressive MTC population.
-
The health economic model developed by the AG uses a simple partitioned survival approach that directly uses the available data on PFS and OS from the EXAM and ZETA trials. This model structure is very similar to that used within the Sanofi model.
-
The AG’s economic analysis includes a thorough assessment of uncertainty surrounding the impact of using alternative parametric functions for PFS and OS based on models fitted directly to data for the relevant population/subgroup under consideration. This is particularly important given that the choice of parametric functions has been informed by only one clinical expert; it is possible that other clinical experts may have selected different preferred curves.
The main weaknesses of the assessment are largely a consequence of weaknesses and gaps in the clinical evidence base:
-
The AG did not have access to IPD from the ZETA trial; instead, PFS and OS outcomes were replicated using a published algorithm. Although the accuracy of this replication is likely to be good, this process may have introduced a small loss of accuracy relative to using the IPD directly.
-
The ITT populations for the EXAM and ZETA trials are notably different. The analyses of the ZETA trial subgroups have been defined post hoc and may be subject to confounding because of differences in baseline characteristics.
-
The OS data for the ZETA trial are subject to potential confounding due to open-label vandetanib use. Sanofi’s attempts to adjust OS estimates using the RPSFT approach were reported to be unsuccessful. As a consequence, the pairwise economic comparisons of vandetanib versus BSC (presented by both Sanofi and the AG) may be of limited relevance for decision-making. Conversely, the AG’s incremental analyses make potentially strong assumptions concerning transferable/equivalent treatment effects between vandetanib and cabozantinib.
-
The systematic review of HRQoL evidence did not identify any relevant published health valuation studies relating specifically to the MTC population.
Uncertainties
The key uncertainties associated with this evaluation are as follows:
-
Quality-of-life gains as a result of PFS and related symptom management. These have not been adequately explored in the literature.
-
The comparative clinical effectiveness and cost-effectiveness of cabozantinib and vandetanib compared with each other and compared with BSC.
-
The incremental OS benefits associated with vandetanib in patients with symptomatic and progressive MTC and in patients with the additional CEA/CTN biomarker. Other outcomes, for example safety, are also subject to potential confounding.
-
Treatment duration in patients who discontinue TKI therapy prior to disease progression.
-
The impact of locally advanced or metastatic MTC on HRQoL, as measured using a preference-based utility instrument.
-
The relative AE profiles of vandetanib and cabozantinib within the symptomatic and progressive MTC population.
Other relevant factors
The number of patients who would be eligible for these treatments is very small. In 2016, (confidential information has been removed) patients initiated treatment using cabozantinib [n = (confidential information has been removed)] or vandetanib [n = (confidential information has been removed)].
Chapter 7 Conclusions
The systematic review of the clinical effectiveness evidence identified two relevant placebo-controlled RCTs: (1) the EXAM trial, which evaluated cabozantinib (n = 330); and (2) the ZETA trial, which evaluated vandetanib (n = 331). The two trials assessed different MTC populations (the ZETA trial inclusion criteria did not specify ‘progressive’ disease), but the ZETA trial did include a subgroup with ‘progressive and symptomatic disease’ (n = 186), which formed the ‘EU-label’ population. This group was considered to be comparable to the EXAM ITT population. Both cabozantinib and vandetanib demonstrated significant benefits compared with placebo in terms of PFS and appear to be broadly similar in terms of efficacy, although neither drug has demonstrated significant OS benefit compared with placebo. Both cabozantinib and vandetanib produced frequent AEs, with substantial proportions of patients experiencing AEs that led to dose interruption or reduction.
Based on the AG’s probabilistic analysis of cabozantinib versus placebo in the EU-label (symptomatic and progressive) MTC population, the ICER for cabozantinib versus BSC is expected to be £150,874 per QALY gained. Within the EU-label (symptomatic and progressive) MTC population of the ZETA trial, the AG’s probabilistic analysis suggests that the ICER for vandetanib versus BSC is expected to be £352,508 per QALY gained. The fully incremental analysis of cabozantinib, vandetanib and BSC based on the EXAM ITT population and the vandetanib PFS treatment effect from the ZETA trial suggests that the ICER for vandetanib versus BSC is expected to be £138,405 per QALY gained, whilst the ICER for cabozantinib versus vandetanib is expected to be £195,593 per QALY gained. Within the fully incremental analysis in which the PFS and OS outcomes for vandetanib were assumed to be equivalent to the cabozantinib group outcomes in the EXAM trial, cabozantinib is expected to be dominated, whilst the ICER for vandetanib versus BSC is expected to be £144,841 per QALY gained. Within the restricted EU-label population (symptomatic and progressive MTC plus CEA/CTN doubling times of ≤ 24 months), the ICER for vandetanib versus BSC is expected to be £66,779 per QALY gained.
The AG’s economic analysis suggests that NICE’s criteria109 for life-extending therapies given at the end of life are not met for cabozantinib in the EU-label population (symptomatic and progressive MTC) or for vandetanib in either the EU-label population or the restricted EU-label population (symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months).
The AG’s economic analysis suggests that the maximum annual budget impact for cabozantinib within the symptomatic and progressive population is expected to be ≈£2.35M. The maximum budget impact for vandetanib within the symptomatic and progressive population is expected to be ≈£5.53M; the costs of vandetanib in the restricted EU-label population are expected to be lower.
Implications for service provision
The implications for service provision are minimal owing to the rarity of the disease and the current availability of both therapies through the CDF.
Suggested research priorities
-
Primary research comparing the long-term clinical benefits of cabozantinib and vandetanib within relevant subgroups.
-
Analyses of existing evidence from the ZETA trial to investigate the impact of adjusting for open-label vandetanib use using appropriate statistical methods.
-
Studies assessing the impact of MTC on HRQoL using a preference-based measure, such as the EuroQol-5 Dimensions (EQ-5D).
Acknowledgements
We would like to thank Hazel Squires, School of Health and Related Research (ScHARR), and Tristan Snowsill, Peninsula Technology Assessment Group (PenTAG), for providing comments on the draft report.
We also thank Andrew Rawdin, ScHARR, for providing help in checking the AG model and Andrea Shippam, Programme Manager, ScHARR, for providing administrative support and in preparing and formatting the report.
We thank Sanofi and Ipsen for providing data to support the appraisal.
Contributions of authors
Paul Tappenden (Professor of Health Economic Modelling) acted as the project lead, critiqued the health economic analysis submitted by Sanofi and developed the independent AG model.
Christopher Carroll (Reader in Systematic Review and Evidence Synthesis) undertook the systematic review of clinical effectiveness and safety evidence.
Jean Hamilton (Research Fellow) conducted the statistical analysis.
Eva Kaltenthaler (Professor of Health Technology Assessment) undertook the systematic review of clinical effectiveness and safety evidence.
Ruth Wong (Information Specialist) undertook the electronic searches.
Jonathan Wadsley (Consultant Clinical Oncologist), Laura Moss (Consultant Oncologist) and Sabapathy Balasubramanian (Consultant Surgeon and Honorary Senior Lecturer) provided clinical advice throughout the appraisal.
All authors were involved in drafting and commenting on the final report.
Publications
Tappenden P, Carroll C, Hamilton J, Kaltenthaler E, Wong R, Wadsley J, et al. Cabozantinib and Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer. Technology Assessment Report: Final Report to the National Institute for Health and Care Excellence. Sheffield: University of Sheffield; 2017.
Data-sharing statement
Access to the model developed within this study can be requested from the corresponding author. Data that is not in the public domain cannot be shared further owing to the nature of this study. All queries should be submitted to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Thyroid Cancer Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/thyroid-cancer (accessed 27 March 2017).
- Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol 2014;81:1-122. https://doi.org/10.1111/cen.12515.
- Ernani V, Kumar M, Chen AY, Owonikoko TK. Systemic treatment and management approaches for medullary thyroid cancer. Cancer Treat Rev 2016;50:89-98. https://doi.org/10.1016/j.ctrv.2016.09.006.
- Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006;107:2134-42. https://doi.org/10.1002/cncr.22244.
- Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. https://doi.org/10.1089/thy.2014.0335.
- Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JW. European Thyroid Association Task Force . 2012 European Thyroid Association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J 2012;1:5-14. https://doi.org/10.1159/000336977.
- Bugalho MJ, Santos JR, Sobrinho L. Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. J Surg Oncol 2005;91:56-60. https://doi.org/10.1002/jso.20269.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual. New York, NY: Springer; 2010.
- Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. Clin Endocrinol 1998;48:265-73. https://doi.org/10.1046/j.1365-2265.1998.00392.x.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. https://doi.org/10.1016/j.ejca.2008.10.026.
- Esfandiari NH, Hughes DT, Yin H, Banerjee M, Haymart MR. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab 2014;99:448-54. https://doi.org/10.1210/jc.2013-2942.
- Saad MF, Ordonez NG, Rashid RK, Guido JJ, Hill CS, Hickey RC, et al. Medullary carcinoma of the thyroid. A study of the clinical features and prognostic factors in 161 patients. Medicine 1984;63:319-42. https://doi.org/10.1097/00005792-198411000-00001.
- Ito Y, Miyauchi A, Yabuta T, Fukushima M, Inoue H, Tomoda C, et al. Alternative surgical strategies and favorable outcomes in patients with medullary thyroid carcinoma in Japan: experience of a single institution. World J Surg 2009;33:58-66. https://doi.org/10.1007/s00268-008-9795-2.
- Cupisti K, Simon D, Dotzenrath C, Goretzki PE, Röher HD. Results of selective venous site-specific catheterization in occult C-cell carcinoma of the thyroid gland. Langenbecks Arch Chir 1997;382:295-301.
- Grande E, Santamaría Sandi J, Capdevila J, Navarro González E, Zafón Llopis C, Ramón Y, et al. Consensus on management of advanced medullary thyroid carcinoma on behalf of the Working Group of Thyroid Cancer of the Spanish Society of Endocrinology (SEEN) and the Spanish Task Force Group for Orphan and Infrequent Tumors (GETHI). Clin Transl Oncol 2016;18:769-75. https://doi.org/10.1007/s12094-015-1465-x.
- Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF. GTE Study Group . Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 2005;90:6077-84. https://doi.org/10.1210/jc.2005-0044.
- Laure Giraudet A, Al Ghulzan A, Aupérin A, Leboulleux S, Chehboun A, Troalen F, et al. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol 2008;158:239-46. https://doi.org/10.1530/EJE-07-0667.
- Chatal JF, Kraeber-Bodéré F, Goldenberg DM, Barbet J. Treatment of metastatic medullary thyroid cancer with vandetanib: need to stratify patients on basis of calcitonin doubling time. J Clin Oncol 2012;30. https://doi.org/10.1200/JCO.2012.42.3160.
- Langmuir PB, Yver A. Vandetanib for the treatment of thyroid cancer. Clin Pharmacol Ther 2012;91:71-80. https://doi.org/10.1038/clpt.2011.272.
- Meijer JA, le Cessie S, van den Hout WB, Kievit J, Schoones JW, Romijn JA, et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin Endocrinol 2010;72:534-42. https://doi.org/10.1111/j.1365-2265.2009.03666.x.
- Cabozantinib and Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer. Slough: Ipsen Ltd; 2017.
- Vandetanib (Caprelsa) – Summary of Product Characteristics. London: European Medicines Agency; 2012.
- Cabozantinib (Cometriq) – Summary of Product Characteristics. London: European Medicines Agency; 2014.
- Head and Neck Cancer. London: NICE; 2017.
- National Cancer Drugs Fund List. Leeds: NHS England; 2017.
- Cabozantinib and Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer – Final Scope. London: NICE; 2016.
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. https://doi.org/10.1200/JCO.2011.35.5040.
- Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639-46. https://doi.org/10.1200/JCO.2012.48.4659.
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) . Welcome to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Website! n.d. www.prisma-statement.org (accessed 25 October 2018).
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Carroll C, Kaltenhaler E, Wong R, Hamilton J. Cabozantinib and Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=50403 (accessed 9 August 2018).
- World Health Organization (WHO) . International Clinical Trials Registry Platform n.d. http://apps.who.int/trialsearch/ (accessed 2 November 2016).
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res 2013;19:4239-48. https://doi.org/10.1158/1078-0432.CCR-13-0071.
- ClinicalTrials.gov . Vandetanib to Treat Children and Adolescents With Medullary Thyroid Cancer 2007. https://clinicaltrials.gov/ct2/show/NCT00514046 (accessed 25 October 2018).
- ClinicalTrials.gov . Efficacy and Safety Study of Sorafenib to Treat Advanced Medullary Thyroid Carcinoma (SUMMIT) 2013. https://clinicaltrials.gov/ct2/show/NCT01736878 (accessed 25 October 2018).
- ClinicalTrials.gov . Pasireotide & Everolimus in Adult Patients With Radioiodine-Refractory Differentiated &Amp; Medullary Thyroid Cancer 2011. https://clinicaltrials.gov/ct2/show/NCT01270321 (accessed 25 October 2018).
- ClinicalTrials.gov . SOM230 Alone or in Combination With RAD001 in Patients With Medullary Thyroid Cancer 2012. https://clinicaltrials.gov/ct2/show/NCT01625520 (accessed 25 October 2018).
- ClinicalTrials.gov . Nintedanib (BIBF1120) in Thyroid Cancer 2013. https://clinicaltrials.gov/ct2/show/NCT01788982 (accessed 25 October 2018).
- ClinicalTrials.gov . Study of Anlotinib in Patients With Medullary Thyroid Carcinoma (ALTER01031) 2015. https://clinicaltrials.gov/ct2/show/NCT02586350 (accessed 25 October 2018).
- Meijer JA, Bakker LE, Valk GD, de Herder WW, de Wilt JH, Netea-Maier RT, et al. Radioactive iodine in the treatment of medullary thyroid carcinoma: a controlled multicenter study. Eur J Endocrinol 2013;168:779-86. https://doi.org/10.1530/EJE-12-0943.
- ClinicalTrials.gov . Efficacy of XL184 (Cabozantinib) in Advanced Medullary Thyroid Cancer 2008. https://clinicaltrials.gov/ct2/show/NCT00704730 (accessed 25 October 2018).
- Schoffski P, Elisei R, Müller S, Brose MS, Shah MH, Licitra LF, et al. An international, double-blind, randomized, placebo-controlled Phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol 2012;30.
- Shah M, Elisei R, Muller S, Schoffski P, Brose MS, Licitra L, et al. Clinical activity of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (PTS): subgroup analysis in the phase 3 study (exam). Thyroid 2012;22.
- Sherman SI, Clary DO, Elisei R, Schlumberger MJ, Cohen EE, Schöffski P, et al. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 2016;122:3856-64. https://doi.org/10.1002/cncr.30252.
- Sherman SI, Cohen EEW, Schoffski P, Elisei R, Schlumberger M, Wirth LJ, et al. Efficacy of cabozantinib (Cabo) in medullary thyroid cancer (MTC) patients with RAS or RET mutations: results from a Phase III study. J Clin Oncol 2013;31.
- Schlumberger M, Elisei R, Müller S, Schoffski P, Brose MS, Shah MH, et al. Final overall survival analysis of EXAM, an international, double-blind, randomized, placebo-controlled phase III trial of cabozantinib (Cabo) in medullary thyroid carcinoma (MTC) patients with documented RECIST progression at baseline. J Clin Oncol 2015;33.
- Elisei R, Müller S, Schöffski P, Brose M, Shah M, Licitra L, et al. Clinical and biochemical activity in the exam trial, a phase 3 study of cabozantinib (XL184) in patients with hereditary and non-hereditary medullary thyroid carcinoma (MTC). Eur Thyroid J 2012;1.
- Brose MS, Elisei R, Schlumberger M, Muller S, Schoffski P, Shah M, et al. Correlative biomarker analysis in the exam trial, a phase 3 study of cabozantinib (XL184) in patients (PTS) with medullary thyroid carcinoma (MTC). Thyroid 2012;22:A48-A9.
- Schöffski P, Cohen E, Bentzien F, Sherman S. Progressive, metastatic medullary thyroid cancer: baseline symptoms and disease characteristics among patients enrolled in the EXAM trial. Eur J Cancer 2013;49.
- Cohen EEW, Elisei R, Schlumberger MJ, Mueller SP, Schöffski P, Brose M, et al. Clinical activity and pharmacokinetics (pk) of cabozantinib (XL184) in patients with progressive medullary thyroid carcinoma (MTC). Ann Oncol 2012;23:154-5.
- Brose MS, Sherman SI, Schoffski P, Elisei R, Schlumberger M, Wirth L, et al. Correlative analyses of RET and RAS mutations in a Phase 3 study of cabozantinib in patients (pts) with progressive, metastatic medullary thyroid cancer (MTC). Thyroid 2013;23.
- Sherman S, Elisei R, Mueller S, Schoffski P, Brose MS, Shah MH, et al. The impact of RET and RAS mutation status on overall survival in the exam trial, a Phase 3 study of cabozantinib (cabo) in patients (pts) with progressive, metastatic medullary thyroid cancer (MTC). Thyroid 2015;25:A21-2.
- ClinicalTrials.gov . A Study of Two Different Doses of Cabozantinib (XL184) in Progressive, Metastatic Medullary Thyroid Cancer 2013. https://clinicaltrials.gov/ct2/show/NCT01896479 (accessed 25 October 2018).
- Yaron Y, Alvarez-Escola C, Capdevila J, De La Fouchardiere C, Eriksson B, Gross D, et al. EXAMINER: a randomized, double-blind study to evaluate the efficacy and safety of cabozantinib at two dose levels in patients with progressive, metastatic medullary thyroid cancer (MTC). Thyroid 2015;25:A126-A7.
- ClinicalTrials.gov . An Efficacy Study Comparing ZD6474 to Placebo in Medullary Thyroid Cancer 2006. https://clinicaltrials.gov/ct2/show/NCT00410761 (accessed 25 October 2018).
- Kreissl M, Hauch O, Webster A, Schlumberger M. Efficacy and safety of vandetanib in aggressive and symptomatic medullary thyroid cancer (MTC)-post-HOC analysis from the ZETA trial (NCT00410761). Eur Thyroid J 2014;3.
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in locally advanced or metastatic medullary thyroid cancer (MTC): a randomized, double-blind Phase III trial (zeta). Ann Oncol 2010;21.
- Wells SA, Robinson BG, Gagel RF. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind Phase III trial (vol 30, pg 134, 2012). J Clin Oncol 2012;30:134-41. https://doi.org/10.1200/JCO.2011.35.5040.
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib (VAN) in locally advanced or metastatic medullary thyroid cancer (MTC): a randomized, double-blind Phase III trial (ZETA). J Clin Oncol 2010;28. https://doi.org/10.1200/jco.2010.28.15_suppl.5503.
- Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab 2013;98:2401-8. https://doi.org/10.1210/jc.2013-1115.
- Massicotte M, Borget I, Baracos VE, Leboulleux S, Eric B, Merad M, et al. Effects of vandetanib on body composition in patients with advanced medullary thyroid carcinoma (MTC): results from a placebo-controlled study. Thyroid 2012;22.
- Massicotte M, Brassard M, Claude-Desroches M, Borget I, Bonichon F, Giraudet A, et al. Off-label tyrosine kinase inhibitor treatments in patients with metastatic thyroid carcinomas: a study of the tuthyref network. Thyroid 2012;22.
- ClinicalTrials.gov . To Compare The Effects of Two Doses of Vandetanib In Patients With Advanced Medullary Thyroid Cancer 2011. https://clinicaltrials.gov/ct2/show/NCT01496313 (accessed 25 October 2018).
- ClinicalTrials.gov . A Targeted Phase I II Trial of ZD6474 (Vandetanib; ZACTIMA) Plus the Proteasome Inhibitor, Bortezomib (Velcade), in Adults With Solid Tumors With a Focus on Hereditary or Sporadic, Locally Advanced or Metastatic Medullary Thyroid Cancer (MTC) 2009. https://clinicaltrials.gov/ct2/show/NCT00923247 (accessed 25 October 2018).
- Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer (ID56): Evidence Submission to NICE. Oxford: Genzyme Therapeutics; 2017.
- Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660-6. https://doi.org/10.1200/JCO.2010.32.4145.
- ClinicalTrials.gov . Study of XL184 (Cabozantinib) in Adults With Advanced Malignancies 2013. https://clinicaltrials.gov/ct2/show/NCT00215605 (accessed 25 October 2018).
- Bastholt L, Kreissl MC, Führer D, Maia AL, Locati LD, Maciel L, et al. Effect of an outreach programme on vandetanib safety in medullary thyroid cancer. Eur Thyroid J 2016;5:187-94. https://doi.org/10.1159/000448919.
- Chougnet C, Schlumberger M, Isabelle B. Efficacy and toxicity of vandetanib for advanced medullary thyroid cancer treatment, the French experience. Eur Thyroid 2014;3:77-8.
- Chougnet CN, Borget I, Leboulleux S, de la Fouchardiere C, Bonichon F, Criniere L, et al. Vandetanib for the treatment of advanced medullary thyroid cancer outside a clinical trial: results from a French cohort. Thyroid 2015;25:386-91. https://doi.org/10.1089/thy.2014.0361.
- ClinicalTrials.gov . Observational Study to Evaluate Vandetanib in RET – + Patients With Metastatic Medullary Thyroid Cancer 2013. https://clinicaltrials.gov/ct2/show/NCT01945762 (accessed 25 October 2018).
- Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer – Response to Clarification Questions. Oxford: Sanofi Genzyme; 2017.
- AstraZeneca . Clinical Study Report. An International, Phase III, Randomized, Double-Blinded, Placebo-Controlled, Multi-Center Study to Assess the Efficacy of ZD6474 Versus Placebo in Subjects With Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer 2011.
- AstraZeneca . Clinical Study Report. An International, Randomised, Double-Blind, Two-Arm Study to Evaluate the Safety and Efficacy of Vandetanib 150 and 300 Mg Day in Patients With Unresectable Locally Advanced or Metastatic Medullary Thyroid Carcinoma With Progressive or Symptomatic Disease 2014.
- Exelixis . Clinical Study Report. Xl184-301. An International, Randomized, Doubleblinded, Phase 3 Efficacy Study of Xl184 Versus Placebo In Subjects With Unresectable, Locally Advanced, or Metastatic Medullary Thyroid Cancer 2012.
- Tiedje V, Locati LD, Kroiss M, Frank-Raue K, Garcia A, Kreissl M, et al. Cabozantinib therapy in medullary thyroid carcinoma patients outside a clinical trial. Thyroid 2015;25.
- Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer (ID56): Evidence Submission to NICE – Appendices. Oxford: Genzyme Therapeutics; 2017.
- Sherman SI, Elisei R, Müller S, Schöffski P, Brose MS, Shah MH, et al. The impact of RET and RAS mutation status on overall survival in the exam trial, a phase 3 study of cabozantinib (CABO) in patients (pts) with progressive, metastatic medullary thyroid cancer (MTC). Thyroid 2015;25:A21-A2.
- Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Rockville, MD: NCI; 2016.
- Liu B, Ding F, Liu Y, Xiong G, Lin T, He D, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget 2016;7:67661-73. https://doi.org/10.18632/oncotarget.11813.
- Zhang X, Shao Y, Wang K. Incidence and risk of hypertension associated with cabozantinib in cancer patients: a systematic review and meta-analysis. Expert Rev Clin Pharmacol 2016;9:1109-15. https://doi.org/10.1080/17512433.2016.1190269.
- Huo Z, Yu S, Hong S, Cao X, Xiu L, Liao Z, et al. A systematic review and meta-analysis of the risk of diarrhea associated with vandetanib treatment in carcinoma patients. Onco Targets Ther 2016;9:3621-31. https://doi.org/10.2147/OTT.S96830.
- Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME. Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:1125-33. https://doi.org/10.1210/jc.2011-2677.
- Qi WX, Min DL, Shen Z, Sun YJ, Lin F, Tang LN, et al. Risk of venous thromboembolic events associated with VEGFR-TKIs: a systematic review and meta-analysis. Int J Cancer 2013;132:2967-74. https://doi.org/10.1002/ijc.27979.
- Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010;28:2323-30. https://doi.org/10.1200/JCO.2009.25.0068.
- Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol 2009;27:3794-801. https://doi.org/10.1200/JCO.2008.18.7815.
- Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 2008;26:4708-13. https://doi.org/10.1200/JCO.2007.15.9566.
- de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, de Vries MM, Links TP, Lips CJ, et al. A Phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:3466-9. https://doi.org/10.1210/jc.2007-0649.
- Belum VR, Serna-Tamayo C, Wu S, Lacouture ME. Incidence and risk of hand–foot skin reaction with cabozantinib, a novel multikinase inhibitor: a meta-analysis. Clin Exp Dermatol 2016;41:8-15. https://doi.org/10.1111/ced.12694.
- Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK. QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer 2015;112:296-305. https://doi.org/10.1038/bjc.2014.564.
- Schlumberger M, Catargi B, Deandreis D, Zerdoud S, Rusu D, Bardet S, et al. Impact on the thyroid hormone withdrawal method on patients’ quality of life at the time of radioiodine administration. Thyroid 2015;25:A135-A6.
- Rinciog C, Myrén KJ, Aldén M, Diamantopoulos A, LeReun C. An indirect treatment comparison of cabozantinib verse vandetanib in progressive medullary thyroid cancer (MTC). Value Health 2014;17:A616-7. https://doi.org/10.1016/j.jval.2014.08.2173.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. https://doi.org/10.1177/0272989X12458724.
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818-27. https://doi.org/10.1093/ije/dys041.
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluations. Chichester: John Wiley and Sons, Ltd; 2004.
- Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med 1995;14:2685-99. https://doi.org/10.1002/sim.4780142408.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37. https://doi.org/10.1023/A:1008929526011.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comp Graph Stat 1998;7:434-55.
- Guyot P, Welton NJ, Ouwens MJ, Ades AE. Survival time outcomes in randomized, controlled trials and meta-analyses: the parallel universes of efficacy and cost-effectiveness. Value Health 2011;14:640-6. https://doi.org/10.1016/j.jval.2011.01.008.
- Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-61.
- Fordham BA, Kerr C, de Freitas HM, Lloyd AJ, Johnston K, Pelletier CL, et al. Health state utility valuation in radioactive iodine-refractory differentiated thyroid cancer. Patient Prefer Adher 2015;9:1561-72. https://doi.org/10.2147/PPA.S90425.
- Caro JJ. Discretely integrated condition event (DICE) simulation for pharmacoeconomics. PharmacoEconomics 2016;34:665-72. https://doi.org/10.1007/s40273-016-0394-z.
- Dobrez D, Cella D, Pickard AS, Lai JS, Nickolov A. Estimation of patient preference-based utility weights from the functional assessment of cancer therapy – general. Value Health 2007;10:266-72. https://doi.org/10.1111/j.1524-4733.2007.00181.x.
- Beusterien KM, Szabo SM, Kotapati S, Mukherjee J, Hoos A, Hersey P, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer 2009;101:387-9. https://doi.org/10.1038/sj.bjc.6605187.
- Department of Health and Social Care . NHS Reference Costs 2015 16 2015. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 4 February 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- British National Formulary. London; 2016.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – modeling studies. Value Health 2003;6:9-17. https://doi.org/10.1046/j.1524-4733.2003.00234.x.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Cabozantinib and Vandetanib for Treating Unresectable Locally Advanced or Metastatic Medullary Thyroid Cancer. Company Evidence Submission to the National Institute for Health and Care Excellence – Response to Clarification Questions. Slough: Ipsen Ltd; 2017.
- Mitchell M, Muftakhidinov B, Winchen T, Jędrzejewski-Szmek Z. The Gitter Badger . Engauge-Digitizer: Support for Smaller Monitors 2016. http://doi.org/10.5281/zenodo.61108.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-9.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. https://doi.org/10.1177/0272989X12472398.
- Latimer N. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trials – Extrapolation with Patient-level Data. Sheffield: University of Sheffield; 2013.
- Collett D. Modelling Survival Data in Medical Research. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015.
- Gallop K, Kerr C, Simmons S, McIver B, Cohen EE. A qualitative evaluation of the validity of published health utilities and generic health utility measures for capturing health-related quality of life (HRQL) impact of differentiated thyroid cancer (DTC) at different treatment phases. Qual Life Res 2015;24:325-38. https://doi.org/10.1007/s11136-014-0776-7.
- Scottish Medicines Consortium (SMC) . Detailed Advice – Lenvatinib 4 Mg and 10 Mg Hard Capsules (Lenvima®) SMC No. 1179 16 2016.
- Scottish Medicines Consortium (SMC) . Detailed Advice – Sorafenib 200 Mg Film-Coated Tablets (Nexavar®) SMC No. (1055 15) 2015.
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319-28. https://doi.org/10.1016/S0140-6736(14)60421-9.
- Scottish Medicines Consortium (SMC) . Cabozantinib 20 Mg and 80 Mg Hard Capsules (Cometriq®) SMC No. (1022 15) 2015.
- All Wales Therapeutics and Toxicology Centre . AWMSG Secretariat Assessment Report. Vandetanib (Caprelsa®) 100 Mg and 300 Mg Film-Coated Tablets 2014.
- All Wales Therapeutics and Toxicology Centre . AWMSG Secretariat Assessment Report. Cabozantinib (Cometriq®) 20 Mg and 80 Mg Hard Capsules 2014.
Appendix 1 Literature search strategies
Clinical effectiveness studies
Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Date range searched: 1946 to present.
Date searched: 2 November 2016.
| # | Searches |
|---|---|
| 1 | exp Thyroid Neoplasms/ |
| 2 | exp Goiter, Nodular/ |
| 3 | (thyr?oid* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. |
| 4 | Thyroid Gland/ |
| 5 | exp Neoplasms/ |
| 6 | 4 and 5 |
| 7 | or/1-3,6 |
| 8 | exp Carcinoma, medullary/ |
| 9 | (medullary or MTC).mp. |
| 10 | 8 or 9 |
| 11 | 7 and 10 |
| 12 | Randomized controlled trials as Topic/ |
| 13 | Randomized controlled trial/ |
| 14 | Random allocation/ |
| 15 | randomized controlled trial.pt. |
| 16 | Double blind method/ |
| 17 | Single blind method/ |
| 18 | Clinical trial/ |
| 19 | exp Clinical Trials as Topic/ |
| 20 | controlled clinical trial.pt. |
| 21 | clinical trial$.pt. |
| 22 | multicenter study.pt. |
| 23 | or/12-22 |
| 24 | (clinic$ adj25 trial$).ti,ab. |
| 25 | ((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw. |
| 26 | Placebos/ |
| 27 | Placebo$.tw. |
| 28 | (allocated adj2 random).tw. |
| 29 | or/24-28 |
| 30 | 23 or 29 |
| 31 | Case report.tw. |
| 32 | Letter/ |
| 33 | Historical article/ |
| 34 | 31 or 32 or 33 |
| 35 | exp Animals/ |
| 36 | Humans/ |
| 37 | 35 not (35 and 36) |
| 38 | 34 or 37 |
| 39 | 30 not 38 |
| 40 | meta-analysis/ |
| 41 | meta-analysis as topic/ |
| 42 | (meta analy* or metanaly* or metaanaly*).ti,ab. |
| 43 | ((systematic* or evidence*) adj3 (review* or overview*)).ti,ab. |
| 44 | (reference list* or bibliograph* or hand search* or manual search* or relevant journals).ab. |
| 45 | (search strategy or search criteria or systematic search or study selection or data extraction).ab. |
| 46 | (search* adj4 literature).ab. |
| 47 | (medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or scie nce citation index or bids or cancerlit).ab. |
| 48 | cochrane.jw. |
| 49 | ((multiple treatment* or indirect or mixed) adj2 comparison*).ti,ab. |
| 50 | or/40-49 |
| 51 | 39 or 50 |
| 52 | 11 and 51 |
Cost-effectiveness studies
Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Date range searched: 1946 to present.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| 1 | exp Thyroid Neoplasms/ |
| 2 | exp Goiter, Nodular/ |
| 3 | (thyr?oid* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. |
| 4 | Thyroid Gland/ |
| 5 | exp Neoplasms/ |
| 6 | 4 and 5 |
| 7 | or/1-3,6 |
| 8 | exp “Costs and Cost Analysis”/ |
| 9 | Economics/ |
| 10 | exp Economics, Hospital/ |
| 11 | exp Economics, Medical/ |
| 12 | Economics, Nursing/ |
| 13 | exp models, economic/ |
| 14 | Economics, Pharmaceutical/ |
| 15 | exp “Fees and Charges"/ |
| 16 | exp Budgets/ |
| 17 | budget$.tw. |
| 18 | ec.fs. |
| 19 | cost$.ti. |
| 20 | (cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab. |
| 21 | (economic$ or pharmacoeconomic$ or pharmaco-economic$).ti. |
| 22 | (price$ or pricing$).tw. |
| 23 | (financial or finance or finances or financed).tw. |
| 24 | (fee or fees).tw. |
| 25 | (value adj2 (money or monetary)).tw. |
| 26 | quality-adjusted life years/ |
| 27 | (qaly or qalys).af. |
| 28 | (quality adjusted life year or quality adjusted life years).af. |
| 29 | or/8-28 |
| 30 | 7 and 29 |
EMBASE
Date range searched: 1974 to 1 November 2016.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| 1 | exp thyroid tumor/ |
| 2 | exp nodular goiter/ |
| 3 | (thyr?oid* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. |
| 4 | thyroid gland/ |
| 5 | exp neoplasm/ |
| 6 | 4 and 5 |
| 7 | or/1-3,6 |
| 8 | Socioeconomics/ |
| 9 | Cost benefit analysis/ |
| 10 | Cost effectiveness analysis/ |
| 11 | Cost of illness/ |
| 12 | Cost control/ |
| 13 | Economic aspect/ |
| 14 | Financial management/ |
| 15 | Health care cost/ |
| 16 | Health care financing/ |
| 17 | Health economics/ |
| 18 | Hospital cost/ |
| 19 | (fiscal or financial or finance or funding).tw. |
| 20 | Cost minimization analysis/ |
| 21 | (cost adj estimate$).mp. |
| 22 | (cost adj variable$).mp. |
| 23 | (unit adj cost$).mp. |
| 24 | or/8-23 |
| 25 | 7 and 24 |
Web of Science Core Collection
Science Citation Index Expanded (1900–).
Conference Proceedings Citation Index – Science (1990–).
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| #1 | TOPIC: ((thyr*oid* NEAR/5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*))) |
| #2 | TS=(cost* and (effective* or utilit* or benefit* or minimi*)) OR TS=(cost*) OR TI=(economic* or pharmacoeconomic* or pharmaco-economic*) OR TS=(price* or pricing*) OR TS=(financial or finance or finances or financed) OR TS=(fee or fees) OR TS=(value and (money or monetary)) OR TS=(economic*) OR TS=(economic* and (hospital or medical or nursing or pharmaceutical)) OR TS=(“quality adjusted life year” or “quality adjusted life years”) OR TS=(qaly or qalys) OR TS=(budget*) |
| #3 | #2 AND #1 |
Cochrane Database of Systematic Reviews: Wiley Online Library
Health Technology Assessment database: Wiley Online Library.
NHS Economic Evaluation Database: Wiley Online Library.
Date range searched: 1995–2015.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| #1 | MeSH descriptor: [Thyroid Neoplasms] explode all trees |
| #2 | MeSH descriptor: [Goiter, Nodular] explode all trees |
| #3 | (thyr*oid* near/5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)):ti,ab,kw |
| #4 | MeSH descriptor: [Thyroid Gland] this term only |
| #5 | MeSH descriptor: [Neoplasms] explode all trees |
| #6 | #4 and #5 |
| #7 | 30-#3,#6 |
Cumulative Index to Nursing and Allied Health Literature
Date range searched: 1982 to present.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| S1 | (MH “Thyroid Neoplasms+”) |
| S2 | (thyr?oid* N5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)) |
| S3 | (MH “Thyroid Gland”) |
| S4 | (MH “Neoplasms+”) |
| S5 | S3 AND S4 |
| S6 | S1 OR S2 OR S5 |
| S7 | (MH “Costs and Cost Analysis+”) |
| S8 | (MH “Economics”) |
| S9 | (MH “Economics, Pharmaceutical”) |
| S10 | (MH “Fees and Charges+”) |
| S11 | (MH “Budgets”) |
| S12 | budget* |
| S13 | cost* |
| S14 | AB cost* and (effective* or utilit* or benefit* or minimi*) |
| S15 | TI economic* or pharmacoeconomic* or pharmaco-economic* |
| S16 | price* or pricing* |
| S17 | financial or finance or finances or financed |
| S18 | fee or fees |
| S19 | value and (money or monetary) |
| S20 | qaly or qalys |
| S21 | quality adjusted life year or quality adjusted life years |
| S22 | S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 |
| S23 | S6 AND S22 |
Quality-of-life studies
Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Date range searched: 1946 to present.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| 1 | exp Thyroid Neoplasms/ |
| 2 | exp Goiter, Nodular/ |
| 3 | (thyr?oid* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. |
| 4 | Thyroid Gland/ |
| 5 | exp Neoplasms/ |
| 6 | 4 and 5 |
| 7 | or/1-3,6 |
| 8 | “Quality of Life”/ |
| 9 | (qol or (quality adj2 life)).ab,ti. |
| 10 | (value adj2 (money or monetary)).tw. |
| 11 | value of life/ |
| 12 | quality adjusted life year/ |
| 13 | quality adjusted life.tw. |
| 14 | (qaly$ or qald$ or qale$ or qtime$).tw. |
| 15 | disability adjusted life.tw. |
| 16 | daly$.tw. |
| 17 | health status indicators/ |
| 18 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 19 | (sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 20 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 21 | (sf6D or sf 6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).tw. |
| 22 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 23 | (euroqol or euro qol or eq5d or eq 5d).tw. |
| 24 | (hql or hqol or h qol or hrqol or hr qol).tw. |
| 25 | (hye or hyes).tw. |
| 26 | health$ year$ equivalent$.tw. |
| 27 | health utilit$.tw. |
| 28 | (hui or hui1 or hui2 or hui3).tw. |
| 29 | disutilit$.tw. |
| 30 | rosser.tw. |
| 31 | (quality adj2 wellbeing).tw. |
| 32 | qwb.tw. |
| 33 | (willingness adj2 pay).tw. |
| 34 | standard gamble$.tw. |
| 35 | time trade off.tw. |
| 36 | time tradeoff.tw. |
| 37 | tto.tw. |
| 38 | letter.pt. |
| 39 | editorial.pt. |
| 40 | comment.pt. |
| 41 | 38 or 39 or 40 |
| 42 | or/8-37 |
| 43 | 42 not 41 |
| 44 | 7 and 43 |
EMBASE
Date range searched: 1974 to 1 November 2016.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| 1 | exp thyroid tumor/ |
| 2 | exp nodular goiter/ |
| 3 | (thyr?oid* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. |
| 4 | thyroid gland/ |
| 5 | exp neoplasm/ |
| 6 | 4 and 5 |
| 7 | or/1-3,6 |
| 8 | socioeconomics/ |
| 9 | quality adjusted life year/ |
| 10 | quality adjusted life.tw. |
| 11 | (qaly$ or qald$ or qale$ or qtime$).tw. |
| 12 | disability adjusted life.tw. |
| 13 | daly$.tw. |
| 14 | health survey/ |
| 15 | (sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw. |
| 16 | (sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw. |
| 17 | (sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw. |
| 18 | (sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).tw. |
| 19 | (sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw. |
| 20 | (euroqol or euro qol or eq5d or eq 5d).tw. |
| 21 | (hql or hqol or h qol or hrqol or hr qol).tw. |
| 22 | (hye or hyes).tw. |
| 23 | health$ year$ equivalent$.tw. |
| 24 | health utilit$.tw. |
| 25 | (hui or hui1 or hui2 or hui3).tw. |
| 26 | disutili$.tw. |
| 27 | rosser.tw. |
| 28 | quality of wellbeing.tw. |
| 29 | qwb.tw. |
| 30 | willingness to pay.tw. |
| 31 | standard gamble$.tw. |
| 32 | time trade off.tw. |
| 33 | time tradeoff.tw. |
| 34 | tto.tw. |
| 35 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 |
| 36 | 7 and 35 |
Web of Science Core Collection
Science Citation Index Expanded (1900–).
Conference Proceedings Citation Index – Science (1990–).
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| #1 | TOPIC: ((thyr*oid* NEAR/5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*))) |
| #2 | TS=(qol or “quality of life” or “quality adjusted life” or qaly* or qald* or qale* or qtime* or “disability adjusted life" or daly*) |
| #3 | TS=(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six) OR TS=(sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six) OR TS=(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve) OR TS=(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen) OR TS=(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty) |
| #4 | TS=(euroqol or euro qol or eq5d or eq 5d or hql or hqol or h qol or hrqol or hr qol or disutilit* or rosser “quality of wellbeing” or qwb or “willingness to pay” or “standard gamble*” or “time trade off” or “time tradeoff” or tto) |
| #5 | #4 OR #3 OR #2 |
| #6 | #5 AND #1 |
Cochrane Database of Systematic Reviews: Wiley Online Library
Health Technology Assessment database: Wiley Online Library.
NHS Economic Evaluation Database: Wiley Online Library.
Date range searched: 1995–2015.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| #1 | MeSH descriptor: [Thyroid Neoplasms] explode all trees |
| #2 | MeSH descriptor: [Goiter, Nodular] explode all trees |
| #3 | (thyr*oid* near/5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)):ti,ab,kw |
| #4 | MeSH descriptor: [Thyroid Gland] this term only |
| #5 | MeSH descriptor: [Neoplasms] explode all trees |
| #6 | #4 and #5 |
| #7 | {or #1-#3, #6} |
Cumulative Index to Nursing and Allied Health Literature
Date range searched: 1982 to present.
Date searched: 3 November 2016.
| # | Searches |
|---|---|
| S1 | (MH “Thyroid Neoplasms+”) |
| S2 | (thyr?oid* N5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)) |
| S3 | (MH “Thyroid Gland”) |
| S4 | (MH “Neoplasms+”) |
| S5 | S3 AND S4 |
| S6 | S1 OR S2 OR S5 |
| S7 | (MH “Quality of Life”) |
| S8 | TI ( qol or (quality N2 life) ) or AB ( qol or (quality N2 life) ) |
| S9 | TI value and TI ( money or monetary ) or AB value and AB ( money or monetary ) |
| S10 | (MH “Economic Value of Life”) |
| S11 | (MH “Quality-Adjusted Life Years”) |
| S12 | TI ( qaly* or qald* or qale* or qtime* ) or AB ( qaly* or qald* or qale* or qtime* ) |
| S13 | TI disability adjusted life or AB disability adjusted life |
| S14 | TI daly* or AB daly* |
| S15 | (MH “Health Status Indicators”) |
| S16 | TI ( sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six ) or AB ( sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shorform thirtysix or shortform thirty six or short form thirtysix or short form thirty six ) |
| S17 | TI ( sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six ) or AB ( sf 6 or sf6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six ) |
| S18 | TI quality adjusted life or AB quality adjusted life |
| S19 | TI ( sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve ) or AB ( sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve ) |
| S20 | TI ( sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen ) or AB ( sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortfrom sixteen or short form sixteen ) |
| S21 | TI ( sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty ) or AB ( sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty ) |
| S22 | TI ( euroqol or euro qol or eq5d or eq 5d ) or AB ( euroqol or euro qol or eq5d or eq 5d ) |
| S23 | TI ( hql or hqol or h qol or hrqol or hr qol ) or AB ( hql or hqol or h qol or hrqol or hr qol ) |
| S24 | TI ( hye or hyes ) or AB ( hye or hyes ) |
| S25 | TI health* year* equivalent* or AB health* year* equivalent* |
| S26 | TI health utilit* or AB health utilit* |
| S27 | TI ( hui or hui1 or hui2 or hui3 ) or AB ( hui or hui1 or hui2 or hui3 ) |
| S28 | TI disutilit* or AB disutilit* |
| S29 | TI rosser or AB rosser |
| S30 | TI quality N2 wellbeing or AB quality N2 wellbeing |
| S31 | TI qwb or AB qwb |
| S32 | TI willingness N2 pay or AB willingness N2 pay |
| S33 | TI standard gamble* or AB standard gamble* |
| S34 | TI time trade off or AB time trade off |
| S35 | TI time tradeoff or AB time tradeoff |
| S36 | TI tto or AB tto |
| S37 | PT letter |
| S38 | PT editorial |
| S39 | PT comment |
| S40 | S37 or S38 or S39 |
| S41 | S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 |
| S42 | S41 NOT S40 |
| S43 | S6 AND S42 |
Appendix 2 Excluded studies with reasons
Single-arm studies
Anagnostou E, Saltiki K, Vasiliou V, Tsigkos C, Papanastasiou L, Alevizaki M, et al. Experience from the administration of tyrosine kinase inhibitors (TKI) in patients with metastatic progressive medullary thyroid carcinoma (MTC) in a referral centre in Greece. Eur Thyroid J 2016;5:75. https://doi.org/10.1159/000447416
Chougnet C, Schlumberger M, Isabelle B. Efficacy and toxicity of vandetanib for advanced medullary thyroid cancer treatment, the French experience. Eur Thyroid 2014;3:77–8. https://doi.org/10.1159/000365244
Chougnet CN, Borget I, Leboulleux S, de la Fouchardiere C, Bonichon F, Criniere L, et al. Vandetanib for the treatment of advanced medullary thyroid cancer outside a clinical trial: results from a French cohort. Thyroid 2015;25:386–91. https://doi.org/10.1089/thy.2014.0361
Kurzrock R, Atkins J, Wheler J, Fu S, Naing A, Busaidy N, et al. Tumor marker and measurement fluctuations may not reflect treatment efficacy in patients with medullary thyroid carcinoma on long-term RET inhibitor therapy. Ann Oncol 2013;24:2256–61. https://doi.org/10.1093/annonc/mdt177
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660–6. https://doi.org/10.1200/JCO.2010.32.4145
Marquez Fernandez E, Marmesat Rodas B, Quesada Sanz MP, Guerra Estévez D, Villanueva Jiménez P. Use of vandetanib in medullary thyroid cancer. Int J Clin Pharm 2016;38:587. https://doi.org/10.1007/s11096-015-0240-y
ClinicalTrials.gov. NCT00098345. Efficacy and Tolerability of ZD6474 in Patients with Thyroid Cancer. 2004. URL: https://clinicaltrials.gov/ct2/show/NCT00098345 (accessed 25 October 2018).
ClinicalTrials.gov. NCT00358956. A Study to Assess ZD6474 (ZACTIMA™) Monotherapy in Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00358956 (accessed 25 October 2018).
ClinicalTrials.gov. NCT01661179. Evaluate the Safety and Tolerability of Vandetanib in Japanese Patients with Medullary Thyroid Carcinoma. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01661179 (accessed 25 October 2018).
ClinicalTrials.gov. NCT01683110. Expanded Access of Cabozantinib in Medullary Thyroid Cancer. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01683110 (accessed 25 October 2018).
ClinicalTrials.gov. NCT01945762. Observational Study to Evaluate Vandetanib in RET –/+ Patients with Metastatic Medullary Thyroid Cancer. 2013. URL: https://clinicaltrials.gov/ct2/show/NCT01945762 (accessed 25 October 2018).
Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2664–71. https://doi.org/10.1210/jc.2009-2461
Takahashi S, Tomomatsu J, Okamoto T, Horiuchi K, Tsuji A, Ito Y, et al. Safety and tolerability of vandetanib in Japanese patients (PTS) with medullary thyroid cancer (MTC): a phase I/II open-label study. Thyroid 2015;25:A273. https://doi.org/10.1089/thy.2015.29004
Tiedje V, Locati LD, Kroiss M, Frank-Raue K, Garcia A, Kreissl M, et al. Cabozantinib therapy in medullary thyroid carcinoma patients outside a clinical trial. Thyroid 2015;25:A266. https://doi.org/10.1089/thy.2015.29004
Tiedje V, Ting S, Walter RF, Herold T, Worm K, Badziong J, et al. Prognostic markers and response to vandetanib therapy in sporadic medullary thyroid cancer patients. Eur J Endocrinol 2016;175:173–80. https://doi.org/10.1530/EJE-16-0252
Wells SA, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 2010;28:767–72. https://doi.org/10.1200/JCO.2009.23.6604
Zhang L, Li S, Zhang Y, Zhan J, Zou BY, Smith R, et al. Pharmacokinetics and tolerability of vandetanib in Chinese patients with solid, malignant tumors: an open-label, Phase I, rising multiple-dose study. Clin Ther 2011;33:315–27. https://doi.org/10.1016/j.clinthera.2011.04.005
Reviews
Belum VR, Serna-Tamayo C, Wu S, Lacouture ME. Incidence and risk of hand–foot skin reaction with cabozantinib, a novel multikinase inhibitor: a meta-analysis. Clin Exp Dermatol 2016;41:8–15. https://doi.org/10.1111/ced.12694
Huo Z, Yu S, Hong S, Cao X, Xiu L, Liao Z, et al. A systematic review and meta-analysis of the risk of diarrhea associated with vandetanib treatment in carcinoma patients. Onco Targets Ther 2016;9:3621–31. https://doi.org/10.2147/OTT.S96830
Qi WX, Shen Z, Lin F, Sun YJ, Min DL, Tang LN, et al. Incidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trials. Br J Clin Pharmacol 2013;75:919–30. https://doi.org/10.1111/j.1365-2125.2012.04417.x
Rinciog C, Myrén KJ, Aldén M, Diamantopoulos A, LeReun C. An indirect treatment comparison of cabozantinib verse vandetanib in progressive medullary thyroid cancer (MTC). Value Health 2014;17:A616–7. https://doi.org/10.1016/j.jval.2014.08.2173
Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME. Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:1125–33. https://doi.org/10.1210/jc.2011-2677
Thornton K, Kim G, Maher VE, Chattopadhyay S, Tang S, Moon YJ, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res 2012;18:3722–30. https://doi.org/10.1158/1078-0432.CCR-12-0411
Letters/commentaries
Anonymous. Vandetanib (Caprelsa) for medullary thyroid cancer. Med Lett Drugs Ther 2012;54:3–4.
Anonymous. Vandetanib: too dangerous in medullary thyroid cancer. Pres Int 2012;21:233.
Baudry C, Paepegaey AC, Groussin L. Reversal of Cushing’s syndrome by vandetanib in medullary thyroid carcinoma. N Engl J Med 2013;369:584–6. https://doi.org/10.1056/NEJMc1301428
Chatal JF, Kraeber-Bodéré F, Goldenberg DM, Barbet J. Treatment of metastatic medullary thyroid cancer with vandetanib: need to stratify patients on basis of calcitonin doubling time. J Clin Oncol 2012;30:2165. https://doi.org/10.1200/JCO.2012.42.3160
Fuerst M. Medullary thyroid cancer: cabozantinib extends PFS in patients with RET or RAS mutations. Oncology Times 2013;35:11–12.
Susman E. Cabozantinib linked to worrisome weight loss. Oncology Times 2015;37:55–6.
Population
Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res 2013;19:4239–48. https://doi.org/10.1158/1078-0432.CCR-13-0071
ClinicalTrials.gov. NCT00514046. Vandetanib to Treat Children and Adolescents with Medullary Thyroid Cancer. 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00514046 (accessed 25 October 2018).
ClinicalTrials.gov. NCT01709435. Cabozantinib S-Malate in Treating Younger Patients with Recurrent or Refractory Solid Tumors. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01709435 (accessed 25 October 2018).
ClinicalTrials.gov. NCT02867592. Cabozantinib-s-malate in Treating Younger Patients with Recurrent, Refractory, or Newly Diagnosed Sarcomas, Wilms tumor, or Other Rare Tumors. 2016. URL: https://clinicaltrials.gov/ct2/show/NCT02867592 (accessed 25 October 2018).
Nguyen L, Holland J, Ramies D, Mamelok R, Benrimoh N, Ciric S, et al. Effect of renal and hepatic impairment on the pharmacokinetics of cabozantinib. J Clin Pharmacol 2016;56:1130–40. https://doi.org/10.1002/jcph.714
Intervention
Bastholt L, Kreissl MC, Führer D, Maia AL, Locati LD, Maciel L, et al. Effect of an outreach programme on vandetanib safety in medullary thyroid cancer. Eur Thyroid J 2016;5:187–94. https://doi.org/10.1159/000448919
ClinicalTrials.gov. NCT02088645. 177Lu-PP-F11N for Receptor Targeted Therapy and Imaging of Metastatic Thyroid Cancer. 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02088645 (accessed 25 October 2018).
Animal study
Lin H, Jiang X, Zhu H, Jiang W, Dong X, Qiao H, et al. 2ME2 inhibits the activated hypoxia-inducible pathways by cabozantinib and enhances its efficacy against medullary thyroid carcinoma. Tumour Biol 2016;37:381–91. https://doi.org/10.1007/s13277-015-3816-1
Appendix 3 Additional tables and figures relating to the Sanofi model
FIGURE 17.
(Confidential information has been removed.)
| Model | Statistic | |
|---|---|---|
| AIC | BIC | |
| Vandetanib | ||
| Weibull | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-normal | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-logistic | (Confidential information has been removed) | (Confidential information has been removed) |
| Exponentiala | (Confidential information has been removed) | (Confidential information has been removed) |
| Gammaa | (Confidential information has been removed) | (Confidential information has been removed) |
| Placebo | ||
| Weibull | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-normal | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-logistic | (Confidential information has been removed) | (Confidential information has been removed) |
| Exponentiala | (Confidential information has been removed) | (Confidential information has been removed) |
| Gammaa | (Confidential information has been removed) | (Confidential information has been removed) |
FIGURE 18.
(Confidential information has been removed.)
| Model | Statistic | |
|---|---|---|
| AIC | BIC | |
| Vandetanib | ||
| Weibull | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-normal | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-logistic | (Confidential information has been removed) | (Confidential information has been removed) |
| Exponentiala | (Confidential information has been removed) | (Confidential information has been removed) |
| Gammaa | (Confidential information has been removed) | (Confidential information has been removed) |
| Placebo | ||
| Weibull | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-normal | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-logistic | (Confidential information has been removed) | (Confidential information has been removed) |
| Exponentiala | (Confidential information has been removed) | (Confidential information has been removed) |
| Gammaa | (Confidential information has been removed) | (Confidential information has been removed) |
| Health state | Value | Source |
|---|---|---|
| Progression free | 0.84 | FACT-G mapped to EQ-5D using Dobrez et al.104 |
| Post progression | 0.64 | Derived by applying progressive disease to stable disease multiplier from Beusterien et al.105 to pre-progression utility from ZETA trial FACT-G mapping exercise |
| Disutility any grade 3/4 AE | –0.11 | Beusterien et al.105 |
| Dose | % of PFS time receiving vandetaniba |
|---|---|
| 300 mg (full dose) | 66.3 |
| 200-mg dose | 16.5 |
| 100-mg dose | 15.5 |
| Interrupted | 1.7 |
| Intervention: vandetanib tablets | Cost (£) | |
|---|---|---|
| Per pack (30 tablets) | Annual (assuming full dose) | |
| 300 mg | 5000.00 | 60,875.00 |
| 100 mg | 2500.00 | 30,437.50 |
| Resource item | Unit cost (£) | Frequency/year | Total cost (£) | ||
|---|---|---|---|---|---|
| Year 1 | Subsequent years | Year 1 | Subsequent years | ||
| EY51Z ECG monitoring or stress testing (directly accessed diagnostic services) | 40.00 | 8 | 4 | 320.00 | 160.00 |
| DAPS04 Clinical biochemistry; DAPS08 Phlebotomy; DAPS05 Haematology | 7.00 | 8 | 4 | 56.00 | 28.00 |
| DAPS09 Other (thyroid-stimulating hormone) | 3.00 | 8 | 4 | 24.00 | 12.00 |
| AE type | Unit cost (£) | Vandetanib | BSC | NHS Reference Cost 2015/16106 HRG code |
|---|---|---|---|---|
| Diarrhoea | 1102.00 | 11% | 2% | FZ91M: non-malignant gastrointestinal tract disorders without interventions, with a CC score of 0–2 |
| Hypertension | 982.00 | 9% | 0% | EB04Z: hypertension |
| ECG QT prolonged | 1014.00 | 8% | 1% | EB07E: arrhythmia or conduction disorders, with a CC score of 0–3 |
| Fatigue | 0.00 | 6% | 1% | N/A |
| Decreased appetite | 1512.00 | 4% | 0% | FZ49H: nutritional disorders without interventions, with a CC score of 0 or 1 |
| Rash | 1078.00 | 4% | 1% | JD07 K: skin disorders without interventions, with a CC score of 0 or 1 |
| Asthenia | 0.00 | 3% | 1% | N/A |
| Dyspnoea | 896.00 | 1% | 3% | DZ19 N: other respiratory disorders without interventions, with a CC score of 0–4 |
| Back pain | 1510.00 | 0% | 3% | HC32 K: low back pain without interventions, with a CC score of 0–2 |
| Syncope | 1067.00 | 0% | 2% | EB08E: syncope or collapse, with a CC score of 0–3 |
| Weighted AE cost (£) | – | 413.42 | 136.48 | – |
| Outcome | Model | |||||
|---|---|---|---|---|---|---|
| Company | AG’s double programmed | |||||
| Vandetanib | Placebo | Incremental | Vandetanib | Placebo | Incremental | |
| LYGs | 4.84 | 3.10 | 1.74 | 4.84 | 3.10 | 1.74 |
| PFLYGsa | 2.07 | 0.77 | 1.30 | 2.07 | 0.77 | 1.30 |
| QALYs | 3.49 | 2.13 | 1.36 | 3.49 | 2.13 | 1.36 |
| Treatment costs, pre progression (£) | 75,766.71 | 0.00 | 75,766.71 | 75,817.76 | 0.00 | 75,817.76 |
| Treatment costs, post progression (£) | 68,490.03 | 106,330.94 | –37,840.91 | 68,490.35 | 106,317.39 | –37,827.04 |
| Monitoring costs (£) | 653.86 | 385.80 | 268.06 | 646.21 | 385.75 | 260.46 |
| AE costs (£) | 409.32 | 136.48 | 272.84 | 409.32 | 136.48 | 272.84 |
| Cost of BSC (£) | 24,506.37 | 19,521.81 | 4984.56 | 24,506.45 | 19,519.65 | 4986.80 |
| Palliative care costs (£) | 5489.93 | 5916.92 | –426.99 | 5574.17 | 6004.49 | –430.31 |
| Total costs (£) | 175,316.22 | 132,291.95 | 43,024.27 | 175,444.26 | 132,363.76 | 43,080.50 |
| ICER (£) | – | – | 31,730.99 | – | – | 31,636.28 |
FIGURE 19.
(Confidential information has been removed.)
| Year | Group | |||||
|---|---|---|---|---|---|---|
| BSC | Vandetanib | |||||
| OS | PFS | PPS state population (OS minus PFS) | OS | PFS | PPS state population (OS minus PFS) | |
| 0 | 1.000 | 1.000 | 0.000 | 1.000 | 1.000 | 0.000 |
| 1 | 0.768 | 0.322 | 0.446 | 0.886 | 0.737 | 0.149 |
| 2 | 0.575 | 0.171 | 0.404 | 0.760 | 0.516 | 0.244 |
| 3 | 0.424 | 0.107 | 0.317 | 0.640 | 0.378 | 0.262 |
| 4 | 0.310 | 0.074 | 0.236 | 0.533 | 0.287 | 0.246 |
| 5 | 0.224 | 0.054 | 0.170 | 0.439 | 0.225 | 0.214 |
| 6 | 0.162 | 0.041 | 0.121 | 0.359 | 0.180 | 0.179 |
| 7 | 0.116 | 0.032 | 0.084 | 0.291 | 0.147 | 0.144 |
| 8 | 0.082 | 0.026 | 0.056 | 0.235 | 0.121 | 0.114 |
| 9 | 0.058 | 0.021 | 0.037 | 0.188 | 0.102 | 0.086 |
| 10 | 0.041 | 0.017 | 0.024 | 0.150 | 0.086 | 0.064 |
| 11 | 0.029 | 0.015 | 0.014 | 0.119 | 0.074 | 0.045 |
| 12 | 0.020 | 0.012 | 0.008 | 0.094 | 0.064 | 0.030 |
| 13 | 0.014 | 0.011 | 0.003 | 0.074 | 0.055 | 0.019 |
| 14 | 0.010 | 0.009 | 0.001 | 0.058 | 0.049 | 0.009 |
| 15 | 0.007 | 0.008 | –0.001 | 0.045 | 0.043 | 0.002 |
| 16 | 0.005 | 0.007 | –0.002 | 0.035 | 0.038 | –0.003 |
| 17 | 0.003 | 0.006 | –0.003 | 0.027 | 0.034 | –0.007 |
| 18 | 0.002 | 0.006 | –0.004 | 0.021 | 0.030 | –0.009 |
| 19 | 0.002 | 0.005 | –0.003 | 0.016 | 0.027 | –0.011 |
| 20 | 0.001 | 0.004 | –0.003 | 0.012 | 0.024 | –0.012 |
Appendix 4 The Assessment group’s model: time-to-event analysis and other model inputs
FIGURE 20.
EXAM trial ITT population PFS.
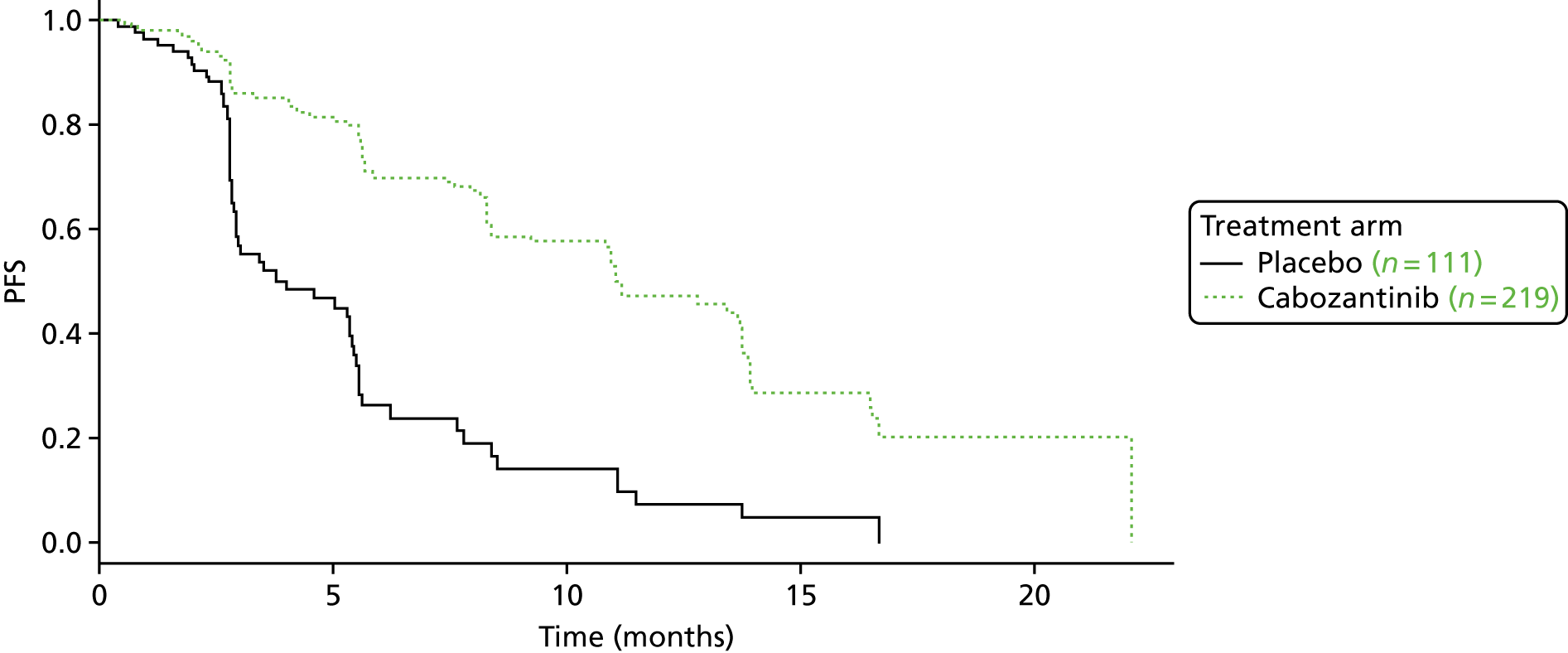
FIGURE 21.
ITT EXAM trial standard diagnostic plots for PFS. (a) Empirical hazard function plot; (b) plot for Weibull and exponential; (c) plot for log-logistic; and (d) plot for log-normal.
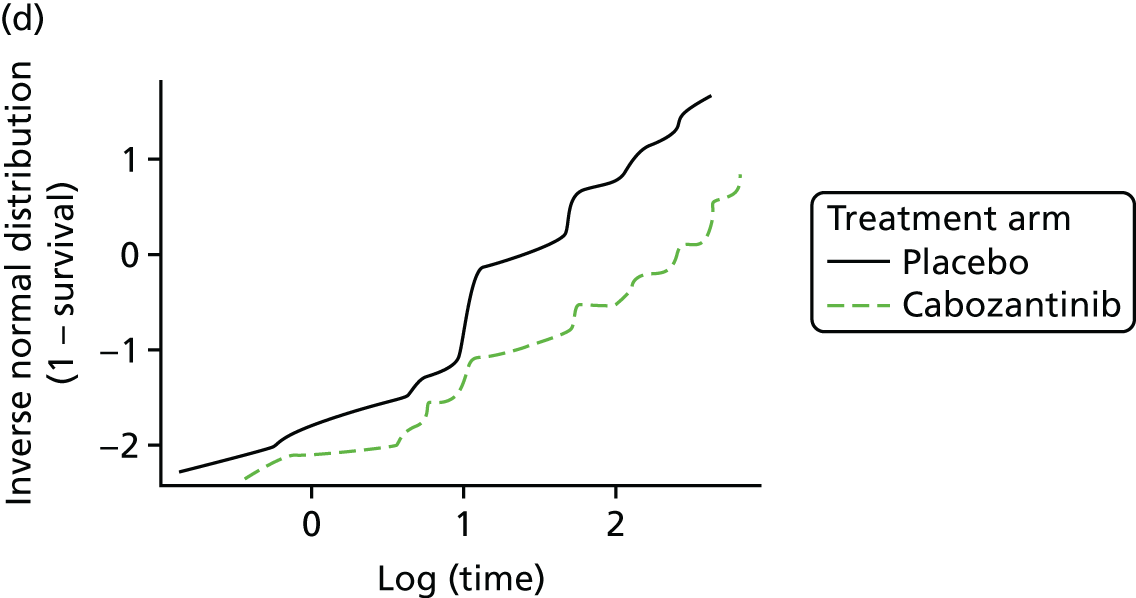
| Model fit statistic | Treatment arm | |||
|---|---|---|---|---|
| Placebo | Cabozantinib | |||
| AIC | BIC | AIC | BIC | |
| PFS | ||||
| Exponential | 338.71 | 341.42 | 599.32 | 602.71 |
| Weibull | 320.19 | 325.61 | 579.70 | 586.48 |
| Gompertz | 333.52 | 338.94 | 582.76 | 589.54 |
| Log-normal | 311.48 | 316.90 | 584.68 | 591.46 |
| Log-logistic | 308.71 | 314.13 | 583.59 | 590.37 |
| Gamma | 314.44 | 319.86 | 580.06 | 586.84 |
| Generalised gamma | 313.16 | 321.28 | 581.68 | 591.85 |
| Generalised F | Failed to converge | Failed to converge | 583.69 | 597.24 |
| OS | ||||
| Exponential | 709.58 | 712.29 | 1345.03 | 1348.42 |
| Weibull | 711.35 | 716.77 | 1346.97 | 1353.75 |
| Gompertz | 709.88 | 715.29 | 1346.48 | 1353.26 |
| Log normal | 708.80 | 714.22 | 1344.34 | 1351.12 |
| Log-logistic | 708.31 | 713.73 | 1343.69 | 1350.47 |
| Gamma | 711.54 | 716.95 | 1346.76 | 1353.54 |
| Generalised gamma | 710.22 | 718.34 | 1345.03 | 1355.19 |
| Generalised F | 712.18 | 723.01 | 1347.03 | 1360.59 |
FIGURE 22.
EXAM trial ITT population, PFS, cabozantinib group (extrapolation up to 10 years).
FIGURE 23.
EXAM trial ITT population, PFS, placebo group (extrapolation up to 10 years).
FIGURE 24.
EXAM trial ITT population OS.
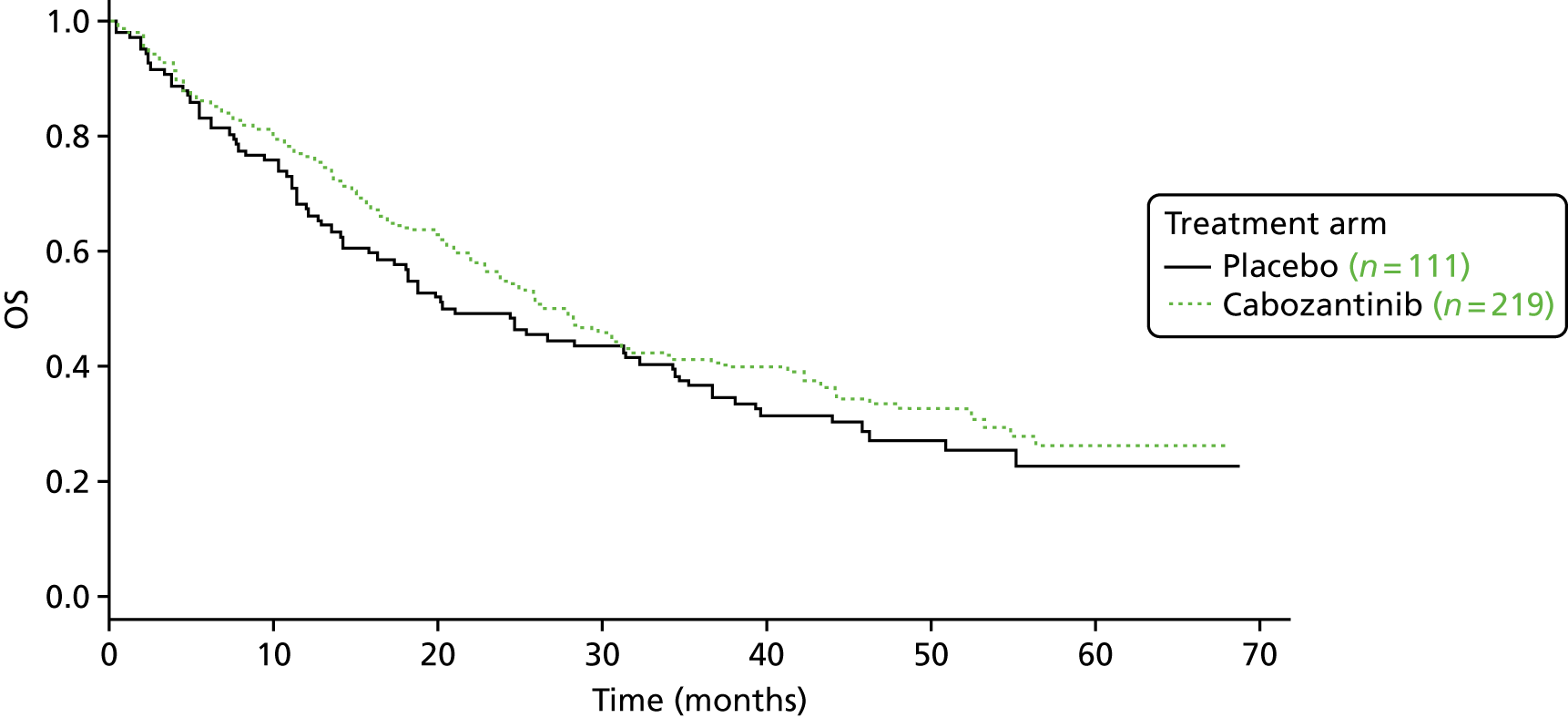
FIGURE 25.
EXAM trial ITT population, standard diagnostic plots for OS. (a) Empirical hazard function plot; (b) plot for Weibull and exponential; (c) plot for log-logistic; and (d) plot for log-normal.
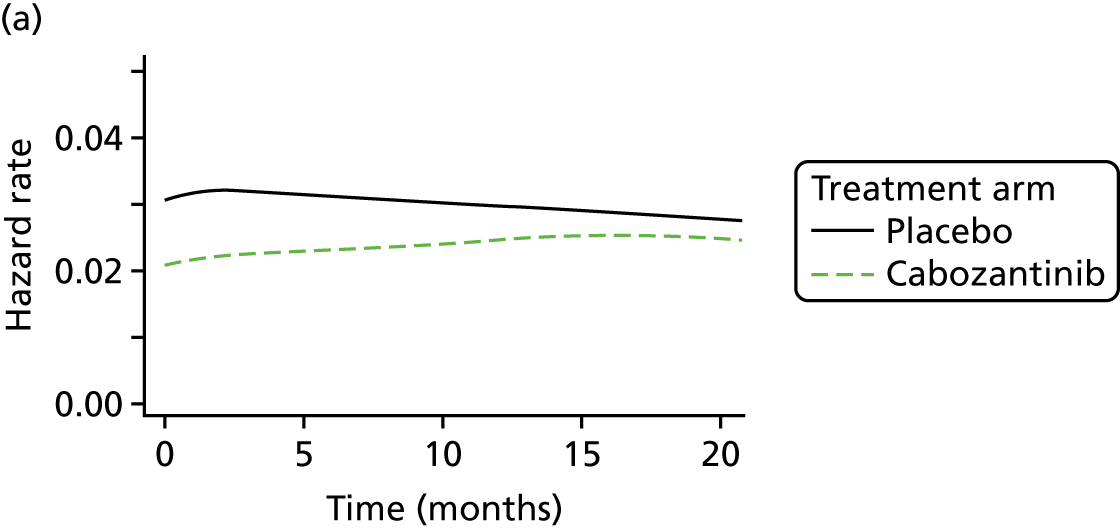
FIGURE 26.
EXAM trial ITT population, OS, cabozantinib group (extrapolation up to 20 years).
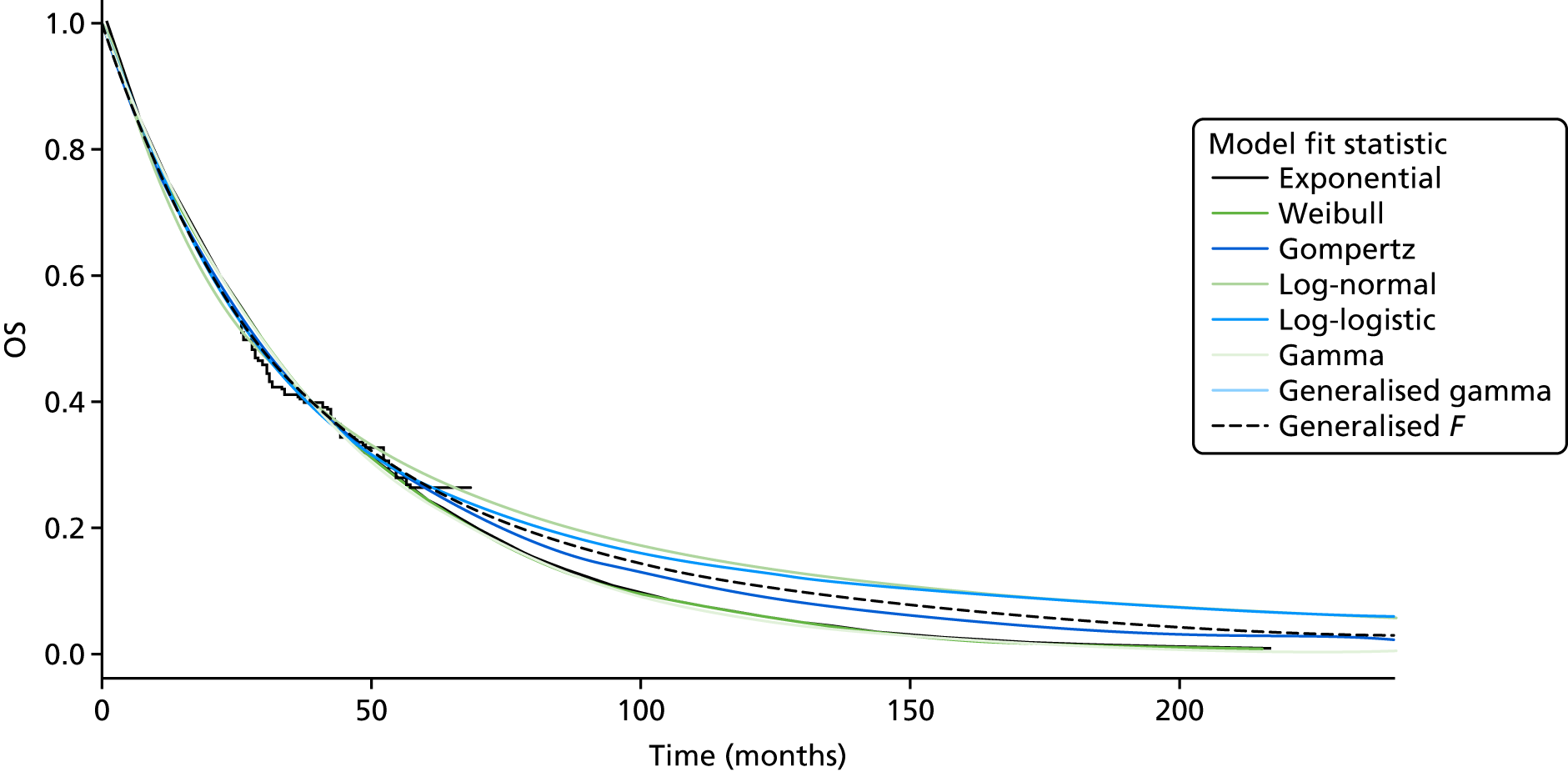
FIGURE 27.
EXAM trial ITT population, OS, placebo group (extrapolation up to 20 years).
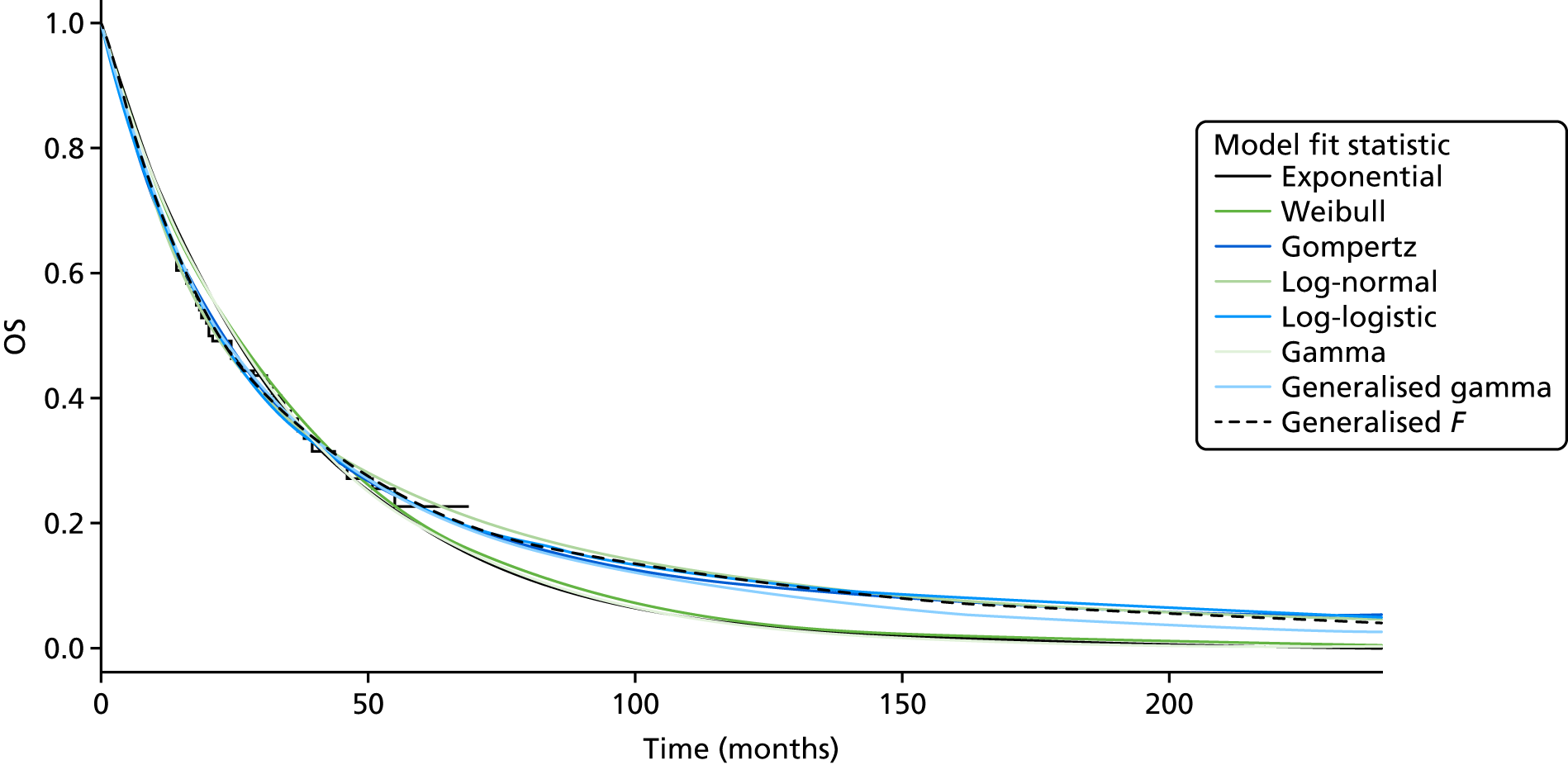
FIGURE 28.
(Confidential information has been removed.)
FIGURE 29.
(Confidential information has been removed.)
FIGURE 30.
(Confidential information has been removed.)
| Model fit statistic | Treatment arm | |||
|---|---|---|---|---|
| Placebo | Vandetanib | |||
| AIC | BIC | AIC | BIC | |
| PFS | ||||
| Exponential | 296.49 | 298.58 | 471.89 | 474.76 |
| Weibull | 298.48 | 302.67 | 467.96 | 473.69 |
| Gompertz | 298.05 | 302.24 | 468.95 | 474.69 |
| Log-normal | 296.85 | 301.04 | 468.52 | 474.26 |
| Log-logistic | 296.80 | 300.99 | 468.57 | 474.31 |
| Gamma | 298.43 | 302.62 | 467.93 | 473.66 |
| Generalised gamma | 298.76 | 305.05 | 469.92 | 478.53 |
| Generalised F | 300.24 | 308.62 | Failed to converge | Failed to converge |
| OS | ||||
| Exponential | 421.65 | 423.73 | 851.75 | 854.62 |
| Weibull | 422.13 | 426.29 | 851.32 | 857.05 |
| Gompertz | 422.37 | 426.52 | 853.57 | 859.31 |
| Log-normal | 425.21 | 429.36 | 847.27 | 853.01 |
| Log-logistic | 423.24 | 427.39 | 847.62 | 853.36 |
| Gamma | 422.21 | 426.37 | 850.40 | 856.14 |
| Generalised gamma | 424.11 | 430.34 | 849.20 | 857.80 |
| Generalised F | 425.97 | 434.28 | 850.91 | 862.38 |
FIGURE 31.
(Confidential information has been removed.)
FIGURE 32.
(Confidential information has been removed.)
FIGURE 33.
(Confidential information has been removed.)
FIGURE 34.
(Confidential information has been removed.)
FIGURE 35.
(Confidential information has been removed.)
FIGURE 36.
(Confidential information has been removed.)
FIGURE 37.
(Confidential information has been removed.)
FIGURE 38.
(Confidential information has been removed.)
FIGURE 39.
(Confidential information has been removed.)
FIGURE 40.
(Confidential information has been removed.)
| Model fit statistic | Treatment arm | |||
|---|---|---|---|---|
| Placebo | Vandetanib | |||
| AIC | BIC | AIC | BIC | |
| PFS | ||||
| Exponential | 89.71 | 90.54 | 132.83 | 134.30 |
| Weibull | 91.64 | 93.31 | 134.63 | 137.56 |
| Gompertz | 91.48 | 93.14 | 134.79 | 137.72 |
| Log-normal | 89.62 | 91.29 | 132.60 | 135.53 |
| Log-logistic | 89.55 | 91.22 | 133.60 | 136.53 |
| Gamma | 91.43 | 93.10 | 134.44 | 137.38 |
| Generalised gamma | 91.57 | 94.07 | 133.70 | 138.10 |
| Generalised F | 92.83 | 96.16 | 135.70 | 141.56 |
| OS | ||||
| Exponential | 152.90 | 153.74 | 212.75 | 214.21 |
| Weibull | 153.02 | 154.69 | 214.74 | 217.67 |
| Gompertz | 150.44 | 152.11 | 214.23 | 217.16 |
| Log-normal | 158.84 | 160.51 | 212.96 | 215.89 |
| Log-logistic | 158.34 | 160.00 | 213.19 | 216.12 |
| Gamma | 153.95 | 155.62 | 214.68 | 217.61 |
| Generalised gamma | 152.19 | 154.69 | 214.92 | 219.32 |
| Generalised F | 154.19 | 157.52 | 216.92 | 222.79 |
FIGURE 41.
(Confidential information has been removed.)
FIGURE 42.
(Confidential information has been removed.)
FIGURE 43.
(Confidential information has been removed.)
FIGURE 44.
(Confidential information has been removed.)
FIGURE 45.
(Confidential information has been removed.)
FIGURE 46.
(Confidential information has been removed.)
FIGURE 47.
(Confidential information has been removed.)
| Model fit statistic | PH/AFT | Model fit | Treatment effect | |||
|---|---|---|---|---|---|---|
| AIC | BIC | β | SE (β) | HR/AFT | ||
| Exponential | PH | 768.38 | 774.87 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Weibull | PH | 767.30 | 777.04 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Gompertz | PH | 768.80 | 778.54 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-normal | AFT | 764.25 | 773.99 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Log-logistic | AFT | 764.57 | 774.31 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Gamma | AFT | 766.55 | 776.29 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Generalised gamma | AFT | 766.09 | 779.08 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
FIGURE 48.
(Confidential information has been removed.)
| Health state | Mean (95% CI) | Beta distribution parameters | Source | |
|---|---|---|---|---|
| α | β | |||
| Progression free | 0.80 (0.77 to 0.84) | 400.61 | 100.15 | Fordham et al.102 |
| Post progression | 0.50 (0.45 to 0.56) | 158.24 | 158.24 | |
| Disutility AEs | –0.11 (SE 0.02) | 26.81 | 216.94 | Beusterien et al.105 |
| Treatment arm | Pairwise comparison | Incremental comparisons: all options (AG analyses 3 and 4) | |
|---|---|---|---|
| Cabozantinib vs. BSC (AG analysis 1) | Vandetanib vs. BSC (AG analyses 2 and 5) | ||
| Cabozantinib | 0.94 | N/A | 0.94 |
| Vandetanib | N/A | 0.45 | 0.45 |
| Placebo | 0.24 | 0.14 | 0.24 |
| Parameter | Population | |||||
|---|---|---|---|---|---|---|
| EU-label: symptomatic and progressive MTC | Restricted EU-label: symptomatic and progressive MTC with CEA/CTN doubling time of ≤ 24 months | |||||
| Proportion | Continued PP | Not continued PP | Proportion | Continued PP | Not continued PP | |
| Proportion of vandetanib group continuing vandetanib PP | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Proportion of BSC group switching to vandetanib PP | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Item | Cost (£) | |
|---|---|---|
| Per pack | Annual at full dose | |
| Cabozantinib, 84 × 20-mg capsules (two-level dose reduction) | 4800.00 | 62,614.29 |
| Cabozantinib, 28 × 20-mg and 28 × 80-mg capsule combination (one-level dose reduction) | 4800.00 | 62,614.29 |
| Cabozantinib, 84 × 20-mg and 28 × 80-mg capsule combination (full dose) | 4800.00 | 62,614.29 |
| Vandetanib, 30 × 300-mg tablets | 5000.00 | 60,875.00 |
| Vandetanib, 30 × 100-mg tablets | 2500.00 | 30,437.50 |
| Dose | Mean proportion | Dirichlet parameters | |
|---|---|---|---|
| Days on dose | Total PFS days | ||
| Cabozantinib, 140 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cabozantinib, 100 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cabozantinib, 60 mg | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cabozantinib, interrupted dose | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Dose | Mean proportion of PFS time spent on specified dose | Dirichlet parameters | |
|---|---|---|---|
| Days on dose | Total PFS days | ||
| EU-label population: symptomatic and progressive MTC | |||
| Vandetanib, 300 mg | 0.73 | 76,994.70 | 106,105.13 |
| Vandetanib, 200 mg | 0.13 | 13,806.45 | 106,105.13 |
| Vandetanib, 100 mg | 0.13 | 13,550.78 | 106,105.13 |
| Vandetanib, interrupted dose | 0.02 | 1753.20 | 106,105.13 |
| Restricted EU-label population: symptomatic and progressive MTC with CEA/CTN doubling times of ≤ 24 months | |||
| Vandetanib, 300 mg | 0.66 | 13,769.93 | 20,746 |
| Vandetanib, 200 mg | 0.17 | 3433.35 | 20,746 |
| Vandetanib, 100 mg | 0.15 | 3214.20 | 20,746 |
| Vandetanib, interrupted dose | 0.02 | 328.73 | 20,746 |
| Resource item | Visits/items per year |
|---|---|
| Progression-free and post-progression states | |
| Consultant outpatient visits | 6 (range 2–12) |
| CT scans | 2 (range 0–4) |
| MRI scan | 1 (range 0–2) |
| Community palliative care support | 12 (range 0–20) |
| Palliative radiotherapy | 2 (fixed) |
| Bisphosphonates for bone metastases | 0.6 (fixed)a |
| Palliative surgery | 0.03 (fixed) |
| Resource item | Treatment | |||
|---|---|---|---|---|
| Cabozantinib | Vandetanib | |||
| Year 1 | Subsequent yearsa | Year 1 | Subsequent yearsa | |
| Consultant-led outpatient visits | 12 (range 4–16) | 6 (range 4–12) | 12 (range 4–16) | 6 (range 4–12) |
| Nurse-led outpatient visits | 4 (range 0–6) | 6 (range 0–6) | 4 (range 0–6) | 6 (range 0–6) |
| ECG | 0 | 0 | 12 | 6 |
| Blood tests | 12 | 6 | 12 | 6 |
| CT scan | 4 | 4 | 4 | 4 |
| Unit | Cost (£) | SE (£) | Source |
|---|---|---|---|
| Consultant-led outpatient visit (medical oncology) | 162.84 | 6.48 | NHS Reference Costs 2015/16,106 consultant-led, non-admitted face-to-face attendance, follow-up, WF01A |
| Nurse-led outpatient (medical oncology) | 99.97 | 8.46 | NHS Reference Costs 2015/16,106 non-consultant-led, non-admitted face-to-face attendance, follow-up, WF01A |
| CT scan | 136.50 | 7.13 | NHS Reference Costs 2015/16,106 outpatient, complex CT scan, RD28Z |
| MRI scan | 161.93 | 3.68 | NHS Reference Costs 2015/16,106 outpatient, MRI scan of two or three areas, without contrast, RD04Z |
| ECG | 207.98 | 29.16 | NHS Reference Costs 2015/16,106 outpatient (medical oncology), ECG monitoring or stress testing, EY51Z |
| Blood test | 3.37 | 0.26 | NHS Reference Costs 2015/16,106 directly accessed pathology, phlebotomy, DAPS08 |
| Palliative care nurse visit | 91.83 | 4.81 | NHS Reference Costs 2015/16,106 specialist nursing, palliative/respite care, adult, face to face, N21AF |
| Palliative radiotherapy (per fraction) | 104.77 | 7.47 | NHS Reference Costs 2015/16,106 outpatient, deliver a fraction of treatment on a megavoltage machine, SC22Z |
| Palliative surgery | 3363.82 | 70.08 | NHS Reference Costs 2015/16,106 elective inpatient, thyroid procedures with a CC score of 0 or 1, KA09E |
| Bisphosphonates for bone metastases (4 mg per 100-ml infusion bags)a | 150.00 | N/A | BNF,108 Zerlinda 4 mg per 100-ml infusion bags [Actavis UK Ltd (now Accord Healthcare Ltd, Barnstaple, UK)] |
| Palliative care (last month of life) | 5775.52 | 866.33b | PSSRU,107 palliative care costs (assumes equal weighting between child and adult inpatient and outpatient) |
| Palliative chemotherapy (last month of life) | 827.00 | 124.05b | Sanofi CS66 (based on NHS Reference Costs 2015/16,106) other, procure chemotherapy drugs for regimens in band 1–10, SB01Z–10Z |
| Cost of managing AEs | 298.41 | 44.76b | NHS Reference Costs 2015/16,106 weighted mean of all non-elective excess bed-days, AA22C–YR55Z |
| Parameter group | Distribution | Comments |
|---|---|---|
| Time-to-event outcomes (PFS and OS) | Normal/multivariate normal | Sampled via Cholesky decomposition using variance–covariance matrices for each parametric model |
| Vandetanib PFS treatment effect (AG analysis 3 only) | Normal (log-scale) | Treatment effect parameters (HRs and acceleration factors) derived from joint models fitted to ZETA trial subgroup data |
| Grade 3/4 AE rates | Beta | Distribution parameters based on total number of AEs reported in ITT population |
| Vandetanib switching/continuation parameters | Beta | Distribution parameters based on numbers continuing/not continuing in ZETA trial subgroups |
| Health state utilities | Beta | Derived using method of moments |
| Disutility for grade 3/4 AEs | Beta | Derived using method of moments |
| Drug dose distributions for cabozantinib and vandetanib | Dirichlet | Includes minimally informative priors, specified in days |
| Proportion of patients discontinuing vandetanib prior to progression | Beta | Distribution parameters based on observed data for ZETA subgroups |
| BSC resource use (outpatient visits, CT scans, MRI scans and community palliative care support)a | Triangular | Distribution selected to reflect expert’s beliefs |
| Vandetanib and cabozantinib health state resource useb | Triangular | Distribution selected to reflect expert’s beliefs |
| Drug acquisition costs | Fixed | – |
| Unit costs | Normal | SE derived from interquartile ranges |
| Palliative care costs | Normal | SE assumed to be 15% of mean |
| AE costs | Normal | SE assumed to be 15% of mean |
Appendix 5 The Assessment group’s model: disaggregated results
| Outcomes (undiscounted) | Treatment | |
|---|---|---|
| Cabozantinib | BSC | |
| LYGs | 4.49 | 3.91 |
| LYGs in progression-free state | 1.39 | 0.45 |
| LYGs in post-progression state | 3.10 | 3.46 |
| Total QALYs | 2.66 | 2.09 |
| Total QALYs in progression-free state | 1.10 | 0.36 |
| Total QALYs in post-progression state | 1.55 | 1.73 |
| Total cost (£) | 95,307.00 | 18,063.00 |
| Total cost in progression-free state (£) | 79,788.00 | 1417.00 |
| Total cost in post-progression state (£) | 15,519.00 | 16,647.00 |
| Modelled probability of being alive at 20 years | 0.06 | 0.05 |
| Outcomes (undiscounted) | Treatment | |
|---|---|---|
| Vandetanib | BSC | |
| LYGs | 7.32 | 7.58 |
| LYGs in progression-free state | 4.00 | 2.70 |
| LYGs in post-progression state | 3.32 | 4.89 |
| Total QALYs | 4.85 | 4.60 |
| Total QALYs in progression-free state | 3.20 | 2.16 |
| Total QALYs in post-progression state | 1.66 | 2.44 |
| Total cost (£) | 305,003.00 | 223,755.00 |
| Total cost in progression-free state (£) | 216,263.00 | 8131.00 |
| Total cost in post-progression state (£) | 88,740.00 | 215,624.00 |
| Modelled probability of being alive at 20 years | 0.11 | 0.12 |
| Outcomes (undiscounted) | Treatment | ||
|---|---|---|---|
| Cabozantinib | Vandetanib | BSC | |
| LYGs | 4.49 | 4.49 | 3.91 |
| LYGs in progression-free state | 1.39 | 0.96 | 0.45 |
| LYGs in post-progression state | 3.10 | 3.54 | 3.46 |
| Total QALYs | 2.66 | 2.53 | 2.09 |
| Total QALYs in progression-free state | 1.10 | 0.76 | 0.36 |
| Total QALYs in post-progression state | 1.55 | 1.77 | 1.73 |
| Total cost (£) | 95,307.00 | 71,105.00 | 18,063.00 |
| Total cost in progression-free state (£) | 79,788.00 | 54,284.00 | 1417.00 |
| Total cost in post-progression state (£) | 15,519.00 | 16,820.00 | 16,647.00 |
| Modelled probability of being alive at 20 years | 0.06 | 0.06 | 0.05 |
| Outcomes (undiscounted) | Treatment | ||
|---|---|---|---|
| Cabozantinib | Vandetanib | BSC | |
| LYGs | 4.49 | 4.49 | 3.91 |
| LYGs in progression-free state | 1.39 | 1.39 | 0.45 |
| LYGs in post-progression state | 3.10 | 3.10 | 3.46 |
| Total QALYs | 2.66 | 2.66 | 2.09 |
| Total QALYs in progression-free state | 1.10 | 1.11 | 0.36 |
| Total QALYs in post-progression state | 1.55 | 1.55 | 1.73 |
| Total cost (£) | 95,307.00 | 92,909.00 | 18,063.00 |
| Total cost in progression-free state (£) | 79,788.00 | 77,390.00 | 1417.00 |
| Total cost in post-progression state (£) | 15,519.00 | 15,519.00 | 16,647.00 |
| Modelled probability of being alive at 20 years | 0.06 | 0.06 | 0.05 |
| Outcomes (undiscounted) | Treatment | |
|---|---|---|
| Vandetanib | BSC | |
| LYGs | 6.50 | 3.34 |
| LYGs in progression-free state | 3.15 | 0.97 |
| LYGs in post-progression state | 3.35 | 2.37 |
| Total QALYs | 4.19 | 1.96 |
| Total QALYs in progression-free state | 2.52 | 0.78 |
| Total QALYs in post-progression state | 1.67 | 1.18 |
| Total cost (£) | 245,641.00 | 108,236.00 |
| Total cost in progression-free state (£) | 161,051.00 | 2956.00 |
| Total cost in post-progression state (£) | 84,591.00 | 105,279.00 |
| Modelled probability of being alive at 20 years | 0.12 | 0.00 |
Glossary
- Calcitonin
- A hormone produced by the parafollicular cells (C cells) of the thyroid gland.
- Carcinoembryonic antigen
- A protein that might appear in the blood of people who have certain types of cancer.
- Extended dominance
- A situation in which the incremental cost-effectiveness ratio for a given treatment alternative is higher than that of the next most effective (non-dominated) comparator.
- Medullary thyroid cancer
- A rare type of thyroid cancer that originates from the parafollicular cells (also called C cells) of the thyroid.
- Meta-analysis
- A statistical method by which the results of a number of studies are pooled to give a combined summary statistic.
- Network meta-analysis
- A meta-analysis in which multiple treatments are compared using both direct comparisons of interventions within randomised controlled trials and indirect comparisons across trials, based on a common comparator.
- Partitioned survival model
- A model in which individuals reside in one of a series of mutually exclusive and jointly exhaustive health states. State membership is determined fully by a series of independently modelled non-mutually exclusive survival curves. A survival curve must be specified for each alive health state that describes time from model start (i.e. patient entry in to the model) to transiting to any health state that is further along the sequence.
- Simple dominance
- A situation in which an intervention is less effective and more expensive than its comparator.
List of abbreviations
- AE
- adverse event
- AG
- assessment group
- AIC
- Akaike information criterion
- AWMSG
- All Wales Medicines Strategy Group
- BIC
- Bayesian information criterion
- BSC
- best supportive care
- CDF
- Cancer Drugs Fund
- CEA
- carcinoembryonic antigen
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index of Nursing and Allied Health Literature
- CPCI
- Conference Proceedings Citation Index
- CrI
- credible interval
- CS
- company submission
- CSR
- clinical study report
- CT
- computerised tomography
- CTCAE
- Common Terminology Criteria for Adverse Events
- CTN
- calcitonin
- DICE
- discretely integrated condition event
- DSA
- deterministic sensitivity analysis
- ECG
- electrocardiography
- ECOG
- Eastern Cooperative Oncology Group
- EGF
- epidermal growth factor
- EMA
- European Medicines Agency
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQoL-5 Dimensions, three-level version
- EU
- European Union
- EXAM
- Efficacy of XLI84 (Cabocatinib) in Advanced Medullary Thyroid Cancer
- FACT-G
- Functional Assessment of Cancer Therapy – General
- HFS
- hand–foot syndrome
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- ITT
- intention to treat
- LYG
- life-year gained
- MDASI-Thy
- MD Anderson Symptom Inventory for thyroid cancer
- MEN
- multiple endocrine neoplasia
- MeSH
- medical subject heading
- MRI
- magnetic resonance imaging
- MTC
- medullary thyroid cancer
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NR
- not reported
- OR
- odds ratio
- ORR
- objective response rate
- OS
- overall survival
- PAS
- Patient Access Scheme
- PD
- progressive disease
- PFS
- progression-free survival
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QTc
- corrected QT interval
- RAS
- RAt Sarcoma
- RCT
- randomised controlled trial
- RECIST
- Response Evaluation Criteria in Solid Tumours
- RET
- REarranged during Transfection
- RPSFT
- rank-preserving structural failure time
- RTK
- receptor tyrosine kinase
- SAE
- serious adverse event
- ScHARR
- School of Health and Related Research
- SCI
- Science Citation Index
- SE
- standard error
- SMC
- Scottish Medicines Consortium
- SmPC
- Summary of Product Characteristics
- TKI
- tyrosine kinase inhibitor
- TTO
- time trade-off
- TWP
- time to worsening of pain
- VEGF
- vascular endothelial growth factor
- WHO
- World Health Organization
- WTP
- willingness to pay
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.